Recombinant production of mixtures of antibodies
Van Berkel , et al. Dec
U.S. patent number RE47,770 [Application Number 15/158,543] was granted by the patent office on 2019-12-17 for recombinant production of mixtures of antibodies. This patent grant is currently assigned to Merus N.V.. The grantee listed for this patent is Merus N.V.. Invention is credited to Abraham Bout, Ronald Hendrik Brus, Ton Logtenberg, Patricius H. C. Van Berkel.






View All Diagrams
| United States Patent | RE47,770 |
| Van Berkel , et al. | December 17, 2019 |
Recombinant production of mixtures of antibodies
Abstract
Provided is methods for producing mixtures of antibodies from a single host cell clone, wherein, a nucleic acid sequence encoding a light chain and nucleic acid sequences encoding different heavy chains are expressed in a recombinant host cell. The recombinantly produced antibodies in the mixtures according to the invention suitably comprise identical light chains paired to different heavy chains capable of pairing to the light chain, thereby forming functional antigen-binding domains. Mixtures of the recombinantly produced antibodies are also provided by the invention. Such mixtures can be used in a variety of fields.
| Inventors: | Van Berkel; Patricius H. C. (Berkel en Rodenrijs, NL), Brus; Ronald Hendrik (Voorschoten, NL), Logtenberg; Ton (Utrecht, NL), Bout; Abraham (Leiden, NL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | Merus N.V. (Utrecht,
NL) |
||||||||||
| Family ID: | 68807854 | ||||||||||
| Appl. No.: | 15/158,543 | ||||||||||
| Filed: | May 18, 2016 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | Issue Date | ||
|---|---|---|---|---|---|
| 12221021 | Apr 19, 2011 | 7927834 | |||
| 11593279 | Sep 30, 2008 | 7429486 | |||
| 11039767 | Aug 28, 2007 | 7262028 | |||
| PCT/EP03/07690 | Jul 15, 2003 | ||||
| 60397066 | Jul 18, 2002 | ||||
| Reissue of: | 12932719 | Mar 4, 2011 | 9303081 | Apr 5, 2016 | |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C07K 16/30 (20130101); C07K 16/2851 (20130101); C07K 16/32 (20130101); C07K 16/2833 (20130101); C07K 16/10 (20130101); C07K 16/00 (20130101); C07K 16/2851 (20130101); C07K 16/2803 (20130101); C07K 16/32 (20130101); C07K 16/30 (20130101); A61K 39/39558 (20130101); C07K 16/10 (20130101); C12P 21/005 (20130101); C07K 16/2896 (20130101); C07K 16/2803 (20130101); C07K 16/2833 (20130101); C07K 16/00 (20130101); C07K 2317/515 (20130101); C12N 2795/00041 (20130101); C07K 2317/31 (20130101); C07K 2317/622 (20130101); C07K 2317/626 (20130101); C07K 2317/732 (20130101); Y02P 20/582 (20151101); C07K 2317/21 (20130101); C07K 2319/30 (20130101); C07K 2317/73 (20130101); C07K 2317/12 (20130101); C07K 2317/31 (20130101); C07K 2317/734 (20130101); C07K 2317/51 (20130101); C07K 2317/50 (20130101); C07K 2317/56 (20130101); A61K 2039/507 (20130101) |
| Current International Class: | C07K 16/18 (20060101); C07K 16/32 (20060101); C07K 16/28 (20060101); A61K 39/395 (20060101); C12P 21/00 (20060101); C07K 16/10 (20060101); C07K 16/30 (20060101); C07K 16/00 (20060101) |
| Field of Search: | ;424/130.1,133.1,134.1,135.1,141.1,801,809 ;530/387.1,387.3,866,867 ;435/69.6 |
References Cited [Referenced By]
U.S. Patent Documents
| 4399216 | August 1983 | Axel et al. |
| 4599311 | July 1986 | Kawasaki |
| 4634665 | January 1987 | Axel et al. |
| 4801687 | January 1989 | Ngo |
| 4816567 | March 1989 | Cabilly et al. |
| 4868103 | September 1989 | Stavrianopoulos et al. |
| 4937190 | June 1990 | Palmenberg et al. |
| 5030002 | July 1991 | North |
| 5137809 | August 1992 | Loken et al. |
| 5151504 | September 1992 | Croze |
| 5179017 | January 1993 | Axel et al. |
| 5223409 | June 1993 | Ladner et al. |
| 5385839 | January 1995 | Stinski |
| 5627037 | May 1997 | Ward et al. |
| 5631169 | May 1997 | Lakowicz et al. |
| 5641640 | June 1997 | Hanning |
| 5667988 | September 1997 | Barbas et al. |
| 5667998 | September 1997 | Dougherty et al. |
| 5733779 | March 1998 | Reff |
| 5770429 | June 1998 | Lonberg et al. |
| 5772997 | June 1998 | Hudziak et al. |
| 5783186 | July 1998 | Arakawa et al. |
| 5789208 | August 1998 | Sharon |
| 5789215 | August 1998 | Berns et al. |
| 5827690 | October 1998 | Meade et al. |
| 5830698 | November 1998 | Reff et al. |
| 5834237 | November 1998 | Jacobs et al. |
| 5849500 | December 1998 | Breitling et al. |
| 5885827 | March 1999 | Wabl et al. |
| 5888789 | March 1999 | Rodriguez |
| 5939598 | August 1999 | Kucherlapati et al. |
| 5965371 | October 1999 | Marasco et al. |
| 6004940 | December 1999 | Marasco et al. |
| 6054297 | April 2000 | Carter et al. |
| 6080560 | June 2000 | Russell et al. |
| 6114598 | September 2000 | Kucherlapati et al. |
| 6180357 | January 2001 | Young et al. |
| 6207446 | March 2001 | Szostak et al. |
| 6265150 | July 2001 | Terstappen et al. |
| 6291740 | September 2001 | Bremel et al. |
| 6303341 | October 2001 | Hiatt et al. |
| 6335163 | January 2002 | Sharon |
| 6570061 | May 2003 | Rajewsky et al. |
| 6586251 | July 2003 | Economides et al. |
| 6596541 | July 2003 | Murphy et al. |
| 7067284 | June 2006 | Barbas et al. |
| 7105348 | September 2006 | Murphy et al. |
| 7183076 | February 2007 | Arathoon et al. |
| 7262028 | August 2007 | Van Berkel et al. |
| 7329530 | February 2008 | Houtzager et al. |
| 7429486 | September 2008 | Van Berkel et al. |
| 7491516 | February 2009 | Collinson et al. |
| 7579446 | August 2009 | Bakker et al. |
| 7642228 | January 2010 | Carter et al. |
| 7696330 | April 2010 | Meulen et al. |
| 7740852 | June 2010 | Bakker et al. |
| 7777010 | August 2010 | Logtenberg |
| 7858086 | December 2010 | Geuijen et al. |
| 7901919 | March 2011 | Houtzager et al. |
| 7919257 | April 2011 | Hoogenboom et al. |
| 7927834 | April 2011 | Van Berkel et al. |
| 7932360 | April 2011 | Van Berkel et al. |
| 7960518 | June 2011 | Throsby et al. |
| 7968092 | June 2011 | Throsby et al. |
| 8052974 | November 2011 | Throsby et al. |
| 8106170 | January 2012 | Ter Meulen et al. |
| 8148497 | April 2012 | Bakker et al. |
| 8192927 | June 2012 | Van Den Brink et al. |
| 8211431 | July 2012 | Throsby et al. |
| 8241631 | August 2012 | Throsby et al. |
| 8268756 | September 2012 | Logtenberg et al. |
| 8470327 | June 2013 | Throsby et al. |
| 8592562 | November 2013 | Kannan et al. |
| 8911738 | December 2014 | Throsby et al. |
| 9012371 | April 2015 | Logtenberg et al. |
| 9248181 | February 2016 | De Kruif et al. |
| 9248182 | February 2016 | De Kruif et al. |
| 9303081 | April 2016 | Van Berkel et al. |
| 9358286 | June 2016 | De Kruif et al. |
| 9738701 | August 2017 | Hoogenboom et al. |
| 2002/0088016 | July 2002 | Bruggeman |
| 2002/0138857 | September 2002 | Ghayur |
| 2003/0039958 | February 2003 | Holt et al. |
| 2003/0077739 | April 2003 | Simmons et al. |
| 2003/0091561 | May 2003 | van de Winkel et al. |
| 2003/0093820 | May 2003 | Green et al. |
| 2003/0096225 | May 2003 | Logtenberg |
| 2003/0194403 | October 2003 | van de Winkel et al. |
| 2003/0207346 | November 2003 | Arathoon et al. |
| 2003/0215914 | November 2003 | Houtzager et al. |
| 2003/0219829 | November 2003 | Logtenberg et al. |
| 2003/0224408 | December 2003 | Hoogenboom et al. |
| 2005/0014261 | January 2005 | Houtzager et al. |
| 2005/0037001 | February 2005 | Germeraad et al. |
| 2005/0037427 | February 2005 | Houtzager et al. |
| 2005/0170398 | August 2005 | Van Berkel et al. |
| 2006/0015949 | January 2006 | Lonberg |
| 2006/0015957 | January 2006 | Lonberg |
| 2006/0088520 | April 2006 | Germeraad et al. |
| 2006/0117699 | June 2006 | Di Trapani |
| 2006/0160184 | July 2006 | Hoogenboom et al. |
| 2006/0177437 | August 2006 | Houtzager et al. |
| 2006/0205077 | September 2006 | Schwenk et al. |
| 2006/0257397 | November 2006 | Throsby et al. |
| 2006/0292634 | December 2006 | Houtzager et al. |
| 2007/0054362 | March 2007 | Van Berkel et al. |
| 2007/0059766 | March 2007 | Logtenberg |
| 2007/0178552 | August 2007 | Arathoon et al. |
| 2007/0280945 | December 2007 | Stevens et al. |
| 2008/0070799 | March 2008 | Bakker et al. |
| 2008/0241166 | October 2008 | Tomlinson et al. |
| 2009/0017521 | January 2009 | Houtzager et al. |
| 2009/0054254 | February 2009 | Throsby et al. |
| 2009/0130652 | May 2009 | Throsby et al. |
| 2009/0181855 | July 2009 | Vasquez et al. |
| 2009/0182127 | July 2009 | Kjaergaard et al. |
| 2009/0263864 | October 2009 | Van Berkel et al. |
| 2010/0015133 | January 2010 | Igawa et al. |
| 2010/0069614 | March 2010 | Houtzager et al. |
| 2010/0146647 | June 2010 | Logtenberg et al. |
| 2010/0172917 | July 2010 | Ter Meulen et al. |
| 2010/0286374 | November 2010 | Kannan et al. |
| 2010/0297153 | November 2010 | Geuijen et al. |
| 2010/0310572 | December 2010 | Bakker et al. |
| 2010/0310586 | December 2010 | Dolcetti et al. |
| 2010/0331527 | December 2010 | Davis et al. |
| 2011/0177073 | July 2011 | Van Berkel et al. |
| 2011/0195454 | August 2011 | McWhirter et al. |
| 2011/0268739 | November 2011 | Throsby et al. |
| 2012/0021409 | January 2012 | McWhirter et al. |
| 2012/0039898 | February 2012 | Throsby et al. |
| 2012/0058907 | March 2012 | Logtenberg et al. |
| 2012/0076794 | March 2012 | Throsby et al. |
| 2012/0093823 | April 2012 | Van Den Brink et al. |
| 2012/0141493 | June 2012 | Throsby et al. |
| 2012/0177637 | July 2012 | Hoogenboom et al. |
| 2012/0192300 | July 2012 | Babb et al. |
| 2012/0276115 | November 2012 | Van Den Brink et al. |
| 2012/0315278 | December 2012 | Throsby et al. |
| 2013/0115208 | May 2013 | Ho et al. |
| 2013/0145484 | June 2013 | Logtenberg et al. |
| 2013/0336981 | December 2013 | de Kruif et al. |
| 2014/0072579 | March 2014 | De Kruif et al. |
| 2014/0120096 | May 2014 | Bakker et al. |
| 2014/0140999 | May 2014 | De Kruif et al. |
| 2014/0314755 | October 2014 | Logtenberg et al. |
| 2014/0317766 | November 2014 | Logtenberg et al. |
| 2015/0139996 | May 2015 | De Kruif et al. |
| 2015/0196637 | July 2015 | De Kruif et al. |
| 2016/0238600 | August 2016 | Hoogenboom et al. |
| 2016/0319320 | November 2016 | Van Berkel et al. |
| 2018/0094289 | April 2018 | Van Berkel et al. |
| 2018/0112247 | April 2018 | Van Berkel et al. |
| 2003250074 | Feb 2004 | AU | |||
| 2 405 961 | Nov 2001 | CA | |||
| 2405961 | Nov 2001 | CA | |||
| 1341364 | Jun 2002 | CA | |||
| 2445255 | Oct 2002 | CA | |||
| 2114353 | Jan 2006 | CA | |||
| 0120694 | Oct 1984 | EP | |||
| 0314161 | May 1989 | EP | |||
| 0402029 | Dec 1990 | EP | |||
| 0445625 | Sep 1991 | EP | |||
| 0 469 897 | Feb 1992 | EP | |||
| 0469025 | Feb 1992 | EP | |||
| 0469897 | Feb 1992 | EP | |||
| 0481790 | Apr 1992 | EP | |||
| 171142 | Jul 1992 | EP | |||
| 0523949 | Jan 1993 | EP | |||
| 469025 | Aug 1995 | EP | |||
| 0814159 | Dec 1997 | EP | |||
| 0724639 | Jan 2001 | EP | |||
| 666868 | Apr 2002 | EP | |||
| 1349234 | Oct 2003 | EP | |||
| 1399575 | Mar 2004 | EP | |||
| 1439234 | Nov 2004 | EP | |||
| 1 325 932 | Apr 2005 | EP | |||
| 1325932 | Apr 2005 | EP | |||
| 1870459 | Dec 2007 | EP | |||
| 2147594 | Jan 2010 | EP | |||
| 2817875 | Jun 2002 | FR | |||
| 5-68599 | Mar 1993 | JP | |||
| 8116978 | May 1996 | JP | |||
| 2001523971 | Nov 2001 | JP | |||
| 2004008214 | Aug 2003 | JP | |||
| 20048218 | Jan 2004 | JP | |||
| 2004-524841 | Aug 2004 | JP | |||
| 2006-109711 | Apr 2006 | JP | |||
| 2006-515503 | Jun 2006 | JP | |||
| 2008-538912 | Nov 2008 | JP | |||
| 2010-505418 | Feb 2010 | JP | |||
| 2010-512749 | Apr 2010 | JP | |||
| 2011508604 | Mar 2011 | JP | |||
| 2011-525808 | Sep 2011 | JP | |||
| 2013004215 | Jan 2013 | JP | |||
| 5749161 | May 2015 | JP | |||
| 2236127 | Sep 2004 | RU | |||
| 9002809 | Mar 1990 | WO | |||
| WO 90/02809 | Mar 1990 | WO | |||
| 9004036 | Apr 1990 | WO | |||
| 9012878 | Nov 1990 | WO | |||
| 9100906 | Jan 1991 | WO | |||
| 9100906 | Jan 1991 | WO | |||
| 9108216 | Jun 1991 | WO | |||
| 9117271 | Nov 1991 | WO | |||
| 9201047 | Jan 1992 | WO | |||
| 9203918 | Mar 1992 | WO | |||
| 9209690 | Jun 1992 | WO | |||
| 9215679 | Sep 1992 | WO | |||
| 9218619 | Oct 1992 | WO | |||
| 9220791 | Nov 1992 | WO | |||
| 9301288 | Jan 1993 | WO | |||
| 9312227 | Jun 1993 | WO | |||
| 9402602 | Feb 1994 | WO | |||
| 9402610 | Feb 1994 | WO | |||
| 9404667 | Mar 1994 | WO | |||
| 9423046 | Oct 1994 | WO | |||
| 9425591 | Nov 1994 | WO | |||
| 9517085 | Jun 1995 | WO | |||
| 9517500 | Jun 1995 | WO | |||
| 9520401 | Aug 1995 | WO | |||
| 9627011 | Sep 1996 | WO | |||
| 9630498 | Oct 1996 | WO | |||
| 9742313 | Nov 1997 | WO | |||
| 9747739 | Dec 1997 | WO | |||
| 9815627 | Apr 1998 | WO | |||
| 9815833 | Apr 1998 | WO | |||
| 9839416 | Sep 1998 | WO | |||
| 9841645 | Sep 1998 | WO | |||
| 9824893 | Nov 1998 | WO | |||
| 9824923 | Nov 1998 | WO | |||
| 9850431 | Nov 1998 | WO | |||
| 9852976 | Nov 1998 | WO | |||
| 9920749 | Apr 1999 | WO | |||
| 99156894 | Apr 1999 | WO | |||
| 9923221 | May 1999 | WO | |||
| 9926569 | Jun 1999 | WO | |||
| 9936569 | Jul 1999 | WO | |||
| WO 99/26569 | Jul 1999 | WO | |||
| 9945962 | Sep 1999 | WO | |||
| 1999050657 | Oct 1999 | WO | |||
| 9964582 | Dec 1999 | WO | |||
| 44777 | Aug 2000 | WO | |||
| 0063403 | Oct 2000 | WO | |||
| 0070023 | Nov 2000 | WO | |||
| 0071694 | Nov 2000 | WO | |||
| WO 00/71694 | Nov 2000 | WO | |||
| 0076310 | Dec 2000 | WO | |||
| WO 00/76310 | Dec 2000 | WO | |||
| 0100245 | Jan 2001 | WO | |||
| 0119394 | Mar 2001 | WO | |||
| 0127279 | Apr 2001 | WO | |||
| 0132901 | May 2001 | WO | |||
| 0148485 | Jul 2001 | WO | |||
| 0164929 | Sep 2001 | WO | |||
| 0188132 | Nov 2001 | WO | |||
| 188132 | Nov 2001 | WO | |||
| 0218948 | Mar 2002 | WO | |||
| 236789 | May 2002 | WO | |||
| 0243478 | Jun 2002 | WO | |||
| 0246233 | Jun 2002 | WO | |||
| WO 02/43478 | Jun 2002 | WO | |||
| WO 02/46233 | Jun 2002 | WO | |||
| 2059297 | Aug 2002 | WO | |||
| 02066630 | Aug 2002 | WO | |||
| 02074969 | Sep 2002 | WO | |||
| WO 02/074969 | Sep 2002 | WO | |||
| 02096948 | Dec 2002 | WO | |||
| WO 02/096948 | Dec 2002 | WO | |||
| 03002609 | Jan 2003 | WO | |||
| 03004704 | Jan 2003 | WO | |||
| WO 03/004704 | Jan 2003 | WO | |||
| WO 03/0026909 | Jan 2003 | WO | |||
| 03016501 | Feb 2003 | WO | |||
| WO 03/016501 | Feb 2003 | WO | |||
| 3033670 | Apr 2003 | WO | |||
| 00048306 | Jun 2003 | WO | |||
| 03046560 | Jun 2003 | WO | |||
| WO 03/046560 | Jun 2003 | WO | |||
| WO 03/048306 | Jun 2003 | WO | |||
| 03102157 | Dec 2003 | WO | |||
| 3106674 | Dec 2003 | WO | |||
| 03106684 | Dec 2003 | WO | |||
| WO 03/0102157 | Dec 2003 | WO | |||
| WO 03/106684 | Dec 2003 | WO | |||
| 2004009618 | Jan 2004 | WO | |||
| 2004061104 | Jul 2004 | WO | |||
| 2004003211 | Aug 2004 | WO | |||
| 2004106375 | Dec 2004 | WO | |||
| 2004106375 | Dec 2004 | WO | |||
| 2005068622 | Jul 2005 | WO | |||
| WO 2005/068622 | Jul 2005 | WO | |||
| 2005118635 | Dec 2005 | WO | |||
| 2006028936 | Mar 2006 | WO | |||
| 2006106905 | Oct 2006 | WO | |||
| 2006117699 | Nov 2006 | WO | |||
| 2006117699 | Nov 2006 | WO | |||
| 2007110205 | Oct 2007 | WO | |||
| 2007117410 | Oct 2007 | WO | |||
| 2007147901 | Dec 2007 | WO | |||
| 2008054606 | May 2008 | WO | |||
| 2008076379 | Jun 2008 | WO | |||
| 2008119353 | Oct 2008 | WO | |||
| 2009051974 | Apr 2009 | WO | |||
| 2009080251 | Jul 2009 | WO | |||
| 2009080252 | Jul 2009 | WO | |||
| 2009080253 | Jul 2009 | WO | |||
| 2009089004 | Jul 2009 | WO | |||
| 2009098596 | Aug 2009 | WO | |||
| 2009157771 | Dec 2009 | WO | |||
| 2010084197 | Jul 2010 | WO | |||
| 2010129304 | Nov 2010 | WO | |||
| 2011028952 | Mar 2011 | WO | |||
| 2011028953 | Mar 2011 | WO | |||
| 2011097603 | Aug 2011 | WO | |||
| 2011143545 | Nov 2011 | WO | |||
| 2012020096 | Feb 2012 | WO | |||
| 2012023053 | Feb 2012 | WO | |||
| 2012058768 | May 2012 | WO | |||
| 2012131555 | Oct 2012 | WO | |||
| 2012141798 | Oct 2012 | WO | |||
Other References
|
AL. Joyner, Gene Targeting: A Practical Approach, The Practical Approach Series, 2005, (196 pages), Second Edition, Oxford University Press. cited by applicant . Abstract dated Jul. 9, 2012, EP12175544. cited by applicant . Abstract, "Recombinant Production of Mixtures of Antibodies", Reference No. P61090EP20, at least as early as Oct. 1, 2010, 1 page. cited by applicant . Allen, Ligand-targeted therapeutics in anticancer therapy, Nat. Rev. Cancer, 2002, 2:750-783, Abstract only. cited by applicant . Almagro et al., Humanization of antibodies, Frontiers in Bioscience, Jan. 1, 2008, pp. 1619-1633, vol. 13. cited by applicant . Appeal Brief under 37 C.F.R. .sctn. 41.37 filed by Brenda Herschbach Jarrell, U.S. Appl. No. 13/948,818, dated Jul. 20, 2015, 26 pages. cited by applicant . U.S. Appl. No. 11/645,238, sharing common inventors, available on the U.S. Patent Office website. cited by applicant . Annexes in respect of a request for a change from Merus B.V. to Merus N.V. dated May 19, 2016 (English version). cited by applicant . Annexes in respect of a request for a change from Merus B.V. to Merus N.V. dated May 27, 2016 (Dutch version). cited by applicant . Acknowledgement of receipt from European Patent Office for EP 10186063.3 dated May 20, 2016. cited by applicant . Acknowledgement of receipt of European Patent Office regarding EP 10186063.3 dated Jun. 6, 2016, 2 pages. cited by applicant . Amendment, Australian patent application No. 2009263082, Jan. 23, 2014, 22 pages. cited by applicant . Advice of receipt to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Registration No. of item RD119029438NL, Mar. 14, 2016, one page. cited by applicant . Advice of receipt to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Registration No. of item RD118911257NL, May 25, 2016, one page. cited by applicant . Auxiliary Request 13 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 13, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 14 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 14, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 2 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 2, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 3 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 3, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 4 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 4, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 5 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 5, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 6 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 6, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 7 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, seven pages. cited by applicant . Auxiliary Request 7, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 8 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, seven pages. cited by applicant . Auxiliary Request 8, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 9 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, nine pages. cited by applicant . Auxiliary Request 9, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Aya Jakobovits, The long-awaited magic bullets: therapeutic human monoclonal antibodies from transgenic mice, Exp. Opin. Invest. Drugs, 1998, pp. 607-614, vol. 7, No. 4, Ashley Publications Ltd. cited by applicant . U.S. Appl. No. 15/090,505, sharing common inventors, available on the U.S. Patent Office website. cited by applicant . Approved Judgement in Regeneron Pharmaceuticals Inc.vs Kymab Limited and Novo Nordisk A/S, Case No: HP-2013-000001/HP-2014-000001 for Hearing dates: Nov. 18-20, 23-27,30 and Dec. 7 & 8, 2015. cited by applicant . Arai et al., Antibody responses induced by immunization with a Japanese rabies vaccine detennined by neutralization test and enzyme-linked immunosorbert assay, Vaccine, Jun. 2002, pp. 2448-2453, vol. 7, No. 20(19-20). cited by applicant . Attaelmannan, Mohammed et al., "Understanding and Identifying Monoclonal Gammopathies," Clinical Chemistry, vol. 46(88):1230-1238 (2000). cited by applicant . Atwell et al., Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library1, J. Mol. Biol., Jul. 4, 1997, pp. 26-35, vol. 270, Issue 1, Abstract only. cited by applicant . Aucouturier et al., Monoclonal lg L Chain and L Chain V Domain Fragment Crystallization in Myeloma-Associated Fanconi's Syndrome, The Journal of Immunology, Apr. 15, 1993, pp. 3561-3568, vol. 150, No. 8. cited by applicant . Auerbach et al., Angiogenesis Assays: A Critical Overview, Clin. Chemistry, Jan. 2003, pp. 32 40, vol. 49, No. 1. cited by applicant . Australian Office Action for Application No. 2009263082, 8 pages, dated Mar. 18, 2014. cited by applicant . Auxiliary Request 1 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 1, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 10 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, nine pages. cited by applicant . Auxiliary Request 10, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Auxiliary Request 11 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 11, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, three pages. cited by applicant . Auxiliary Request 12 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, Five pages. cited by applicant . Auxiliary Request 12, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, three pages. cited by applicant . Auxiliary request 5, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 1, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 2, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 3, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 6, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Arnold et al., Development of B-1 Cells: Segregation of Phosphatidyl Choline-specific B Cells to the B-1 Population Occurs After Immunoglobulin Gene Expression, J. Exp. Med., May 1994, pp. 1585-1595, vol. 179, The Rockfeller University Press. cited by applicant . Auxiliary request 4, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 1 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 2 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 3 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 5 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Auxiliary request 6 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013 three pages. cited by applicant . Auxiliary request 4 (amendments indicated), EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Campbell et al., Sheep cloned by nuclear transfer from a cultured cell line, Nature, Mar. 7, 1996, pp. 64-66, vol. 380, Nature Publishing Group. cited by applicant . Birchmeier et. al., Met, metastasis, motility and more, Nat. Rev. Mol. Cell Biol., Dec. 2003, pp. 915-925, vol. 4, Abstract only. cited by applicant . Bogen, Bjarne et al., "A rearranged lambda 2 light gene chain retards but does not exclude kappa and lambda 1 expression," Eur. J. Immunol., vol. 21:2391-2395 (1991). cited by applicant . Brady et al., Rapid specific amplification of rat antibody cDNA from nine hybridomas in the presence of myeloma light chains, Journal of Immunological Methods, Aug. 31, 2006, pp. 61-67, vol. 315. cited by applicant . Bruggemann et al., A Repertoire of Monoclonal Antibodies with Human Heavy Chains from Transgenic Mice, Proc. Natl. Acad. Sci., Sep. 1989, pp. 6709-6713, vol. 86, USA. cited by applicant . Burger et al., An integrated strategy for the process development of a recombinant antibody-cytokine fusion protein expressed in BHK cells, Appl. Microbiol. Biotechnol., Sep. 1999, pp. 345-353, vol. 52, Issue 3, Abstract only. cited by applicant . Brief communication in opposition proceedings for EP application 10186063.3 dated May 31, 2016, one page. cited by applicant . Brief communication in Opposition proceedings in EP 10186063.3 dated May 26, 2016. cited by applicant . Communication from the European Patent Office to Isenbruck Bosl Forschler LLP regarding change to Merus N.V. dated Jun. 7, 2016. cited by applicant . Brief Communication from European Patent Office to Isenbruck Bosl Forschler LLP regarding EP 10186063.3 dated Jun. 13, 2016. cited by applicant . Brief Communication from European Patent Office to JA Kemp regarding EP 10186063.3 dated Jun. 13, 2016. cited by applicant . Brief Communication from European Patent Office to JA Kemp regarding EP 10186063.3 dated Jun. 7, 2016. cited by applicant . Brief Communication from European Patent Office to Isenbruck Bosl Forschler LLP regarding EP 10186063.3 dated Jun. 10, 2016 about Oral proceedings on Jun. 22, 2016. cited by applicant . Brief communication from European Patent Office to JA Kemp about Opposition--Oral proceedings on Jun. 22, 2016. cited by applicant . Correspondence from A. Bentham of J A Kemp to The European Patent Office regarding the reply to the Patentees response to Opposition, EP Application No. 09075279.1, Aug. 20, 2015, eight pages. cited by applicant . Cao et al., Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models, Proc. Natl. Acad. Sci. U.S.A., Jun. 19, 2001, pp. 7443-7448, vol. 98, No. 13. cited by applicant . Carmack et al, Influence of a Vkappa8 L Chain Transgene on Endogenous Rearrangements and the Immune Response to the HA(SB) Determinant on Influenza Virus The Journal of Immunology, 1991, vol. 147, No. 6, pp. 2024-2033. cited by applicant . Carter et al., Humanization of anti-p185her2 antibody for human cancer therapy, PNAS, 1992, pp. 4285-4289, vol. 89. cited by applicant . Carter, Paul, "Bispecific human lgG by design," Journal of Immunological Methods, vol. 248:7-15 (2001). cited by applicant . Cho et al., Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab, Nature, Feb. 13, 2003, pp. 756-760, vol. 421, Abstract only. cited by applicant . Chothia, et al., Canonical Structures for the Hypervariable Regions of Immunoglobulins, J. Mol. Biol., Aug. 20, 1987, pp. 901-917, vol. 196, Issue 4. cited by applicant . Casellas et al., Contribution of Receptor Editing to the Antibody Repertoire, Science, Feb. 23, 2001, pp. 1541-1544, vol. 291, Issue 5508. cited by applicant . Castelli et al., HLA-DP4, the Most Frequent HLA II Molecule, Defines a New Supertype of Peptide-Binding Specificity, J. Immunol., Dec. 15, 2002, pp. 6928-6934, vol. 169, No. 12. cited by applicant . Cherrington et al., New paradigms for the treatment of cancer: The role of anti-angiogenesis agents, Adv. Cancer. Res., 2000, pp. 1-38, vol. 79, Abstract only. cited by applicant . Cheung et al., A recombinant human Fab expressed in Escherichia coli neutralizes rabies virus, J. Virol., Nov. 1992, pp. 6714-6720, vol. 66, No. 11. cited by applicant . Christophe Sirac; Sirac et al. (2006) Role of the monoclonal kappa chain V domain and reversibility of renal damage in a transgenic model of acquired Fanconi syndrome, Blood 108:536-543. cited by applicant . Claims dated Jul. 9, 2012, EP12175544. cited by applicant . Claims, Replacement pp. 125-129, Reference No. P61090EP20, at least as early as Oct. 1, 2010, 5 pages. cited by applicant . Conn et al., Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line, Proc. Natl. Acad. Sci. U.S.A., Feb. 1, 1990, pp. 1323-1327, vol. 87, No. 4. cited by applicant . Correspondence from C.M. Jansen of V.O. to the European Patent Office regarding change of correspondence, EP Application No. 10186063.3 and EP Patent No. 2314629, Dec. 17, 2015, one page. cited by applicant . Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding change of correspondence, EP Application No. 10186063.3 and EP Patent No. 2314629, Jan. 8, 2016, one page. cited by applicant . Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding the Oral Proceedings on Jun. 22, 2016, EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 16, 2016, one page. cited by applicant . Correspondence from A Bentham of J A Kemp to The European Patent Office regarding inquiry on status of opposition, EP Application No. 09075279.1, Nov. 17, 2015, one page. cited by applicant . Correspondence from C.M. Jansen of V.O. to European Patent Office regarding the Registration of the Association and change of address, reference No. RvE/E100EPEP, Sep. 29, 2015, one page. cited by applicant . Correspondence from A. Bentham of J A Kemp to The European Patent Office regarding request to change date of Oral Proceedings, EP Patent No. 2147594, Jan. 29, 2016, two pages. cited by applicant . Correspondence from Fritz Lahrtz of Isenbruck Bosl Hoschler LLP to European Patent Office regarding request for Postponement of Oral Proceedings, EP Application No. 09075279.1 and EP Patent No. 2147594, Feb. 1, 2016, two pages. cited by applicant . Correspondence from A. Bentham of J A Kemp to The European Patent Office regarding possible dates for Oral Proceedings, EP Patent No. 2147594, Feb. 15, 2016, one page. cited by applicant . Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding change of name for Proprietor, EP Application No. 09075279.1 and EP Patent No. 2147594, May 30, 2016, one page. cited by applicant . Bitter et al., Expression and Secretion Vectors for Yeast, in Methods in Enzymology, Ed. Wu & Grossman, Acad. Press, N.Y. 153:516 544 (1987) Abstract only. cited by applicant . Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding change of representation, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 8, 2016, one page. cited by applicant . Cascalho et al., A Quasi-Monoclonal Mouse, Science, Jun. 14, 1996, pp. 1649-1652, vol. 272. cited by applicant . Claims (amendments indicated), European Patent Application No. 09075279.1, Dec. 22, 2011, Reference No. P85231EP00, five pages. cited by applicant . Correspondence from A Bentham of J A Kemp to European Patent Office regarding an opposition, EP Patent No. 2147594, Aug. 11, 2014, one page. cited by applicant . Correspondence from C.M. Jansen of V.O. to European Patent Office regarding change of representation, EP Patent No. 2147594, Dec. 17, 2015, one page. cited by applicant . EP Priority Document of EP Application No. 02077953.4, "Recombinant Production of Mixtures of Antibodies", submitted in International Application No. PCT/EP03/07690, Sep. 5, 2003, 140 pages. cited by applicant . EP Priority Document of International Application No. PCT/EP03/50201, "Recombinant Production of Mixtures of Antibodies", submitted in International Application No. PCT/EP03/07690, Sep. 1, 2003, 168 pages. cited by applicant . EP Priority Document of International Application No. PCT/EP2003/07690, "Recombianant Production of Mixtures of Antibodies", Oct. 25, 2010, 186 pages. cited by applicant . EPO Acknowledgement of receipt dated Aug. 20, 2012, EP12175544.1. cited by applicant . EPO Acknowledgement of receipt dated Dec. 17, 2015, EP12175544.1. cited by applicant . EPO Acknowledgement of receipt dated Jul. 9, 2012, Application No. EP12175544.1. cited by applicant . EPO Acknowledgement of Receipt of the Notice of Opposition against EP Application No. 10186063.3 and EP Patent No. 2314629, date of receipt Jul. 15, 2014, three pages. cited by applicant . EPO Acknowledgement of Receipt of the submission by the proprietor, EP Application No. 10186063.3 and EP Patent No. 2314629, date of receipt Feb. 24, 2015, one page. cited by applicant . EPO Acknowledgement of Receipt of the submission by the proprietor, EP Application No. 10186063.3 and EP Patent No. 2314629, date of receipt Oct. 16, 2014, one page. cited by applicant . EPO Acknowledgement of receipt, Acknowledgement of Receipt, Application No. 10186063.3 and EP Patent No. 2314629, Nov. 27, 2015, one page. cited by applicant . EPO Acknowledgement of receipt, request, Application No. 10186063.3, Dec. 17, 2015, one page. cited by applicant . EPO Annexes in respect of a request for a change dated May 30, 2016, EP12175544.1. cited by applicant . EPO Brief Communication regarding the Opposition against EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 27, 2015, EPO Form 2911O 01.12, one page. cited by applicant . EPO Brief Communication regarding the Opposition againts EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 24, 2014, EPO Form 2911O 01.12, one page. cited by applicant . EPO Client Database System--clean up dated Apr. 23, 2013, EP12175544.1. cited by applicant . EPO Communication concerning the registration of amendments relating to entries pertaining to the applicant/the proprietor dated Jun. 20, 2016, EP12175544.1. cited by applicant . EPO Communication of a notice of opposition (R. 79(1) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Aug. 22, 2014, EPO Form 2317A, 12.07, one page. cited by applicant . EPO Communication of a notice of opposition for EP Application No. 10186063.3 and EP Patent No. 2314629, Jul. 21, 2014 EPO Form 2316, 01.12, one page. cited by applicant . EPO Communication of amended entries concerning the representation dated Dec. 23, 2015, EP12175544.1. cited by applicant . EPO Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 8, 2015, EPO Form 2548, 08.13, one page. cited by applicant . EPO Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 039075279.1 and Patent No. 2147594, Oct. 8, 2015, EPO Form 2548 08.13, one page. cited by applicant . EPO Communication of further notices of opposition pursuant to Rule 79(2) EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Aug. 22, 2014, EPO Form 2318, 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of letter regarding request for extension of time, EP Application No. 09075279.1, date of receipt Oct. 16, 2014, one page. cited by applicant . EPO Brief Communication regarding the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Oct. 22, 2014, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of letter regarding reply to opposition, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Apr. 2, 2015, one page. cited by applicant . EPO Brief Communication regarding the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Apr. 13, 2015, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of letter regarding reply patentee's response to opposition, EP Application No. 09075279.1 and Patent No. 02147594, date of receipt Aug. 20, 2015, one page. cited by applicant . EPO Acknowledgement of receipt of letter regarding in vivo data, EP Application No. 09075279.1, date of receipt Jun. 13, 2013, one page. cited by applicant . EPO Brief Communication regarding the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Aug. 25, 2015, EPO Form 2911O 01.12, one page. cited by applicant . EPO Brief Communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP regarding the telephone conversation on the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Mar. 16, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of request to change date of oral proceedings, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Jan. 29, 2016, one page. cited by applicant . EPO Authorization of Johan Renew regarding Oral Proceedings, EP Application No. 09075279.1, Dec. 2, 2015, one page. cited by applicant . EPO Brief Communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP regarding the Opposition and Oral Proceedings, EP Application No. 09075279.1 and Patent No. 2147594, Feb. 9, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO Brief Communication to Andrew Bentham of J A Kemp regarding the Opposition and Oral Proceedings, EP Application No. 09075279.1 and Patent No. 2147594, Feb. 9, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of possible dates for oral proceedings, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Feb. 15, 2016, one page. cited by applicant . EPO Brief Communication to Andrew Bentham of J A Kemp regarding the telephone conversation on the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Mar. 16, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO Brief Communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP regarding the Oral Proceedings on Oct. 13, 2016, EP Application No. 09075279.1 and Patent No. 2147594, Mar. 22, 2016, EPO Form 2310A 12.07, one page. cited by applicant . EPO Brief Communication to Andrew Bentham of J A Kemp regarding the Oral Proceedings on Oct. 13, 2016, EP Application No. 09075279.1 and Patent No. 2147594, Mar. 22, 2016, EPO Form 2310A 12.07, one page. cited by applicant . EPO Acknowledgement of receipt of executed acknowledgment, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Apr. 12, 2016, one page. cited by applicant . EPO Brief Communication to Andrew Bentham of J A Kemp regarding the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Jun. 14, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of letter of inquiry, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Nov. 17, 2015, one page. cited by applicant . EPO Brief Communication regarding the Opposition, EP Application No. 09075279.1 and Patent No. 2147594, Dec. 9, 2015, EPO Form 2911O 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of change of representation, EP Application No. 09075279.1 and Patent No. 2147594, date of receipt Dec. 17, 2015, one page. cited by applicant . EPO Acknowledgement of receipt of Notice of Opposition, EP Application No. 09075279.1 and EP Patent No. 2147594, date of receipt Aug. 11, 2014, two pages. cited by applicant . EPO Communication of a Notice of Opposition, EP Application No. 09075279.1 and EP Patent No. 2147594, Aug. 20, 2014, EPO Form 2316 01.12, one page. cited by applicant . EPO Acknowledgement of receipt of claim requests, EP Application No. 09075279.1, date of receipt Apr. 23, 2013, two pages. cited by applicant . EPO Acknowledgement of receipt of written submissions, EP Application No. 09075279.1, date of receipt Apr. 24, 2013, one page. cited by applicant . EP Application No. 09075279.1 with annotations, Aug. 3, 2010, 170 pages. cited by applicant . EPO Acknowledgement of receipt of letter regarding French and German translated claims, EP Application No. 09075279.1, date of receipt Sep. 2, 2013, one page. cited by applicant . EPO Acknowledgement of receipt of letter regarding request to hold application, EP Application No. 09075279.1, date of receipt Sep. 3, 2013, one page. cited by applicant . EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Brief Communication regarding Oral proceedings on Jun. 22, 2016 at 10:00 in S2.1, EP Application No. 10186063.3 and EP Patent No. 2314629, Apr. 26, 2016, EPO Form 2911O 01.12, one page. cited by applicant . EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Jan. 12, 2016, EPO Form 2548 08.13, one page. cited by applicant . EPO Communication to J A Kemp, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 19, 2015, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication to V.O., Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 19, 2015, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication under rule 71(3) EPC, EP Application No. 10186063.3, Jun. 17, 2013, EPO Form 2004C, 04.12TRI, 196 pages. cited by applicant . EPO Communication, Annex to EPO Form 2004, Communication pursuant to Rule 71(3) EPC, Bibliographical data of EP Application No. 10186063.3, Jun. 5, 2013, EPO Form 2056, 11.08, 1 page. cited by applicant . EPO Communication pursuant to Article 94(3) EPC, Application No. 10186063.3, Dec. 12, 2011, EPO Form 2001, 12.10CSX, 5 pages. cited by applicant . EPO Communication pursuant to Article 94(3) EPC, Application No. 10186063.3, Jun. 11, 2012, EPO Form 2001, 12.10CSX, 3 pages. cited by applicant . EPO Communication pursuant to Rule 55 EPC, EP Application No. 10186063.3, Nov. 25, 2010, EPO Form 1047A, 11.09, 1 page. cited by applicant . EPO Communication pursuant to Rules 70(2) and 70a(2) EPC and reference to Rule 39(1) EPC, EP Application No. 10186063.3, May 2, 2011, EPO Form 1082, 04.10, 2 pages. cited by applicant . EPO Communication pursuant to the Decision of the President of the European Patent Office on the filing of priority document, EP Application No. 10186063.3, Oct. 21, 2010, EPO Form 1195, 04.09 PRIO, 1 page. cited by applicant . EPO Communication regarding Applicant Address Change, EP Application No. 10186063.3, Jan. 26, 2012, EPO FOrm 2544, 04.10, 1 page. cited by applicant . EPO Communication regarding important information concerning oral proceedings, at least as early as Nov. 19, 2015, EPO Form 2043 02.09, three pages. cited by applicant . EPO Communication regarding opposition, EP Application No. 10186063.3, Nov. 19, 2015, EPO Form 2906 01.91TRI, 11 pages. cited by applicant . EPO Communication regarding The oral proceedings dated Jun. 22, 2016, EP Application No. 10186063.3, EPO Form 2341 09.14, one page. cited by applicant . EPO Communication regarding the preparation for oral proceedings--Instructions to Support Service dated Nov. 11, 2015, EP Application No. 10186063.3 and EP Patent No. 2314629, EPO Form 2040 12.01TRI, two pages. cited by applicant . EPO communication to Andrew Bentham of J A Kemp, Brief Communication regarding EPO Form 2548 of Jan. 12, 2016, EP Application No. 10186063.3 and EP Patent No. 2314629, Jan. 12, 2016, EPO Form 29100O 01.12, two pages. cited by applicant . EPO communication to Andrew Bentham of J A Kemp, Brief Communication regarding Oral Proceedings on Jun. 22, 2016 and the Letter from the proprietor of the patent of Feb. 16, 2016, EP Application No. 10186063.3 and EP Patent No. 2314629, Mar. 7, 2016, EPO Form 2310A 12.07, two pages. cited by applicant . EPO communication to Andrew Bentham of J A Kemp, Brief Communication regarding Oral Proceedings on Jun. 22, 2016 at 10:00 in S2.1., EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 25, 2016, EPO Form 29100O 01.12, one page. cited by applicant . EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Brief Communication regarding letter dated Feb. 16, 2016, EP Application No. 10186063.3 and EP Patent No. 2314629, Mar. 7, 2016, EPO Form 2310A 12.07, one page. cited by applicant . EPO Communication of further notices of opposition Rule 79(2) EPC, EP Application No. 09075279.1 and Patent No. 2147594, Sep. 25, 2014, EPO Form 2318 01.12, one page. cited by applicant . EPO Communication regarding Submission in opposition proceedings, Request for extension of time, EP Application No. 09075279.1 and Patent No. 2147594, Oct. 16, 2014, two pages. cited by applicant . EPO Communication regarding Extension of time limit pursuant to Rule 132 EPC, EP Application No. 09075279.1 and Patent No. 2147594, Oct. 22, 2014, one page. cited by applicant . EPO Communication regarding Submission in opposition proceedings, Reply of the patent proprietor to the notice(s) of opposition, EP Application No. 09075279.1 and Patent No. 2147594, Apr. 2, 2015, two pages. cited by applicant . EPO communication to Andrew Bentham of J A Kemp, Brief Communication regarding EPO Form 2548 of Jan. 12, 2016, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 12, 2016, EPO Form 29100O 01.12, two pages. cited by applicant . EPO Communication regarding important information concerning oral proceedings, at least as early as Jan. 19, 2016, EPO Form 2043 02.09, three pages. cited by applicant . EPO Communication regarding Preliminary, Non-binding Opinion of the Opposition Division, EP Application No. 09075279.1, Jan. 19, 2016, EPO Form 2906 01.91TRI, 11 pages. cited by applicant . EPO Communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 09075279.1 and EP Patent No. 2147594,Jan. 19, 2016, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication, After communication under Rule 71(3) EPC (IGRA) but before decision to grant (EPO Form 2006A), EP Application No. 09075279.1, Sep. 5, 2013, EPO Form 2092C 04.12, two pages. cited by applicant . EPO Communication pursuant to Rule 114(2) EPC, EP Application No. 09075279.1, Oct. 10, 2013, EPO Form 2022 12.07, one page. cited by applicant . EPO Communication pursuant to Rule 114(2) EPC, EP Application No. 09075279.1 and Patent No. 2147594, May 8, 2012, EPO Form 2022 12.07, one page. cited by applicant . EPO Communication pursuant to Rule 114(2) EPC, EP Application No. 09075279.1 and Patent No. 2147594, Jun. 14, 2013, EPO Form 2022 12.07, one page. cited by applicant . EPO Communication pursuant to Rule 114(2) EPC, EP Application No. 09075279.1 and Patent No. 2147594, Jul. 2, 2013, EPO Form 2022 12.07, one page. cited by applicant . EPO Communication to Andrew Bentham of J A Kemp, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 19, 2016, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication regarding the cancelling of the Summons for Oral Proceedings dated Oct. 13, 2016, EP Application No. 09075279.1, Mar. 17, 2016, EPO Form 2088 06.14, one page. cited by applicant . EPO Communication regarding important information concerning oral proceedings, at least as early as Mar. 22, 2016, EPO Form 2043 02.09, three pages. cited by applicant . EPO Communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 22, 2016, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication to Andrew Bentham of J A Kemp, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 22, 2016, EPO Form 2936 08.10, one page. cited by applicant . EPO Communication regarding the entries pertaining to the applicant / the proprietor (R. 143(1)(f) EPC), EP Application No. 09075279.1 and EP Patent 2147594, Jun. 13, 2016, EPO Form 2544 03.14, two pages. cited by applicant . EPO Communication to Fritz Lahrtz of Isenbruck Bosl Forschler LLP, Refund of fees, EP Application No. 09075279.1 and Patent No. 2147594, Jun. 15, 2016, EPO Form 2907 04.14, one page. cited by applicant . EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 12, 2016, EPO Form 2548 08.13, one page. cited by applicant . EPO Communication under Rule 71(3) EPC, EP Application No. 09075279.1, Sep. 2, 2013, EPO Form 2004C 06.13TRI, five pages. cited by applicant . EPO Communication, Annex to EPO Form 2004, Communication pursuant to Rule 71(3) EPC, EP Application No. 09075279.1, Sep. 2, 2013, EPO Form 2056, two pages. cited by applicant . EPO Communication of notices of opposition (R. 79(1) EPC), EP Application No. 09075279.1 and Patent No. 2147594, Sep. 25, 2014, EPO Form 2317A 12.07, one page. cited by applicant . EPO Communication pursuant to Article 94(3) EPC, EP Application No. 09075279.1, Jun. 29, 2012, EPO Form 2001 12.10CSX, six pages. cited by applicant . EPO Communication pursuant to Rule 114(2) EPC, EP Application No. 09075279.1 and Patent No. 2147594, Nov. 5, 2012, EPO Form 2022 12.07, one page. cited by applicant . EPO Communication regarding Preparation for oral Proceeding--Instructions to Support Service, EP Application No. 09075279.1, Feb. 5, 2013, EPO Form 2040 12.07TRI, two pages. cited by applicant . EPO Communication to Martin Hatzmann of Vereenigde, Summons to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1, Mar. 6, 2013, EPO form 2008 12.12, one page. cited by applicant . EPO Communication to Martin Hatzmann of Vereenigde, Acknowledgement of receipt of the document specified above, EP Application No. 09075279.1, Mar. 6, 2013, EPO Form 2936 08.10, one page. cited by applicant . EPO communication to Martin Hatzmann of V.O., Brief Communication regarding the letter of Apr. 23, 2013, EP Application No. 09075279.1 and EP Patent No. 2147594, May 22, 2013, EPO Form 2008A 12.07, one page. cited by applicant . EPO communication, Client Database System (CDS)--clean up, EP Application No. 10186063.3, Apr. 23, 2013, EPO Form 2596C, 04.08, 1 page. cited by applicant . EPO Communication, EP Application No. 10186063.3, Mar. 3, 2011, EPO Form 1507N, 08.10, 1 page. cited by applicant . EPO communication, Maintenance / Change of date / Cancellation of oral proceedings arranged for: Jun. 22, 2016 at 10.00 hrs, EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 22, 2016, EPO Form 2088 06.14, two pages. cited by applicant . EPO Communication, Summons to J A Kemp to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 19, 2015, EPO Form 2310 12.14, one page. cited by applicant . EPO Communication, Summons to V.O. to attend oral proceedings pursant to Rule 115(1) EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 19, 2015, EPO Form 2310 12.14, one page. cited by applicant . EPO Decision to grant a European patent pursuant to Article 97(1) EPC, EP Application No. 10186063.3, Sep. 19, 2013, EPO Form 2006A, 12.07, 2 pages. cited by applicant . EPO Extension of time limit pursuant to Rule 132 EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 24, 2014, EPO Form 2944C, 06.12, one page. cited by applicant . EPO General enquiry dated Jun. 16, 2016, EP12175544.1. cited by applicant . EPO Information on Search Strategy dated Jun. 30, 2016, EP12175544.1. cited by applicant . EPO Invitation to remedy deficiencies dated Aug. 31, 2012, EP12175544.1. cited by applicant . EPO Invitation to remedy deficiencies pursuant to Rule 30(3) EPC / Rule 163(3) EPC, EP Application No. 10186063.3, Nov. 23, 2010, EPO Form 1128, 05.10, 3 pages. cited by applicant . EPO Letter accompanying subsequently filed items dated Aug. 20, 2012, EP12175544.1. cited by applicant . EPO Letter accompanying subsequently filed items dated Dec. 17, 2015, EP12175544.1. cited by applicant . EPO Letter accompanying subsequently filed items, Document concerning representation filed by C.M. Jansen of V. O., .EP Application No. 10186063.3, Dec. 17, 2015, one page. cited by applicant . EPO Letter accompanying subsequently filed items, Documents filed during examination procedure and Letter dealing with Oral proceedings filed by David Power of J A Kemp, EP Application No. 10186063.3, May 20, 2016, one page. cited by applicant . EPO Model-Sheet dated Oct. 29, 2012, EP12175544.1. cited by applicant . EPO Notification of European Publication Number and Information on the application of Article 67(3) EPC, EP Application No. 10186063.3, dated Mar. 3, 2011, EPO Form 1133, 05.10, 1 page. cited by applicant . EPO Notification of European publication number dated Jan. 16, 2013, EP12175544.1. cited by applicant . EPO Partial description filed in response to formal objections dated Aug. 20, 2012, EP12175544.1. cited by applicant . EPO communication, Preparation for oral proceedings--Instruction to Support Service, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 14, 2016, EPO Form 2040 12.07TRI, two pages. cited by applicant . EPO Communication, Summons to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 19, 2016, EPO Form 2310 12.14, one page. cited by applicant . EPO Communication, Summons to Andrew Bentham of J A Kemp to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 19, 2016, EPO Form 2310 12.14, one page. cited by applicant . EPO Communication, Consultation by telephone with the applicant / representative, EP Application No. 09075279.1, Oct. 9, 2013, EPO Form 2036 12.07TRI, one page. cited by applicant . EPO Communication, Result of consultation, EP Application No. 09075279.1, Oct. 14, 2013, EPO Form 2049A 12.07TRI, two pages. cited by applicant . EPO Communication, Decision to grant a European patent pursuant to Article 97(1) EPC, EP Application No. 09075279.1, Oct. 17, 2013, EPO Form 2006A 12.07, two pages. cited by applicant . EPO Communication, Transmission of the certificate for a European patent pursuant to Rule 74 EPC, EP Application No. 09075279.1, Nov. 13, 2013, EPO Form 2047 12.07, one page. cited by applicant . EPO Communication, Notice of Opposition to a European Patent, EP Application No. 09075279.1 and EP Patent No. 2147594, Aug. 11, 2014, EPO Form 2300E, eight pages. cited by applicant . EPO Request for change of applicant's representation dated Dec. 17, 2015, EP12175544.1. cited by applicant . EPO Request for change of applicant's representation dated Dec., 22, 2015, EP12175544.1. cited by applicant . EPO Request for change of applicant's representative dated Sep. 29, 2015, EP12175544.1. cited by applicant . EPO Request for grant of a European patent dated Jul. 9, 2012, Application No. EP12175544.1. cited by applicant . EPO Request for recordation of a transfer dated May 30, 2016, EP12175544.1. cited by applicant . EPO Request for recording a change in name of representative dated Apr. 2, 2013, EP12175544.1. cited by applicant . EPO Search has started dated Jun. 15, 2016, EP12175544.1. cited by applicant . EPO Sequence Listing dated Oct. 29, 2012, EP12175544.1. cited by applicant . EPO Communication, Minutes of the oral proceedings before the Examining Division, EP Application No. 09075279.1 and Patent No. 2147594, May 23, 2013, EPO Form 2009.1 12.07TRI, two pages. cited by applicant . EPO Communication, Provision of a copy of the minutes in accordance with Rule 124(4) EPC, EP Application No. 09075279.1, Aug. 8, 2013, EPO Form 2042 12.07TRI, one page. cited by applicant . EPO communication, Maintenance / Change of date / Cancellation of oral proceedings arranged for: Jun. 22, 2016 at 10.00 hrs, EP Application No. 09075279.1 and EP patent No. 2147594, Feb. 4, 2016, EPO Form 2088 06.14, two pages. cited by applicant . EPO Communication, Summons to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 22, 2016, EPO Form 2310 12.14, one page. cited by applicant . EPO Communication, Summons to Andrew Bentham of J A Kemp to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 22, 2016, EPO Form 2310 12.14, one page. cited by applicant . EPO Payment of fees and expenses dated May 30, 2016, EP12175544.1. cited by applicant . EPO Payment of fees and expenses dated Oct. 29, 2012, EP12175544.1. cited by applicant . EPO Reply to the invitation to remedy deficiencies dated Oct. 29, 2012, EP12175544.1. cited by applicant . EPO Communication, Minutes, EP Application No. 09075279.1, Aug. 8, 2013, EPO Form 2906 01.91TRI, 25 pages. cited by applicant . EPO communication, Maintenance / Change of date / Cancellation of oral proceedings arranged for: May 23, 2013 at 10.00 hrs, EP Application No. 19075279.1, Apr. 25, 2013, EPO Form 2088 04.10, two pages. cited by applicant . EPO Communication, Annex to Summons to attend oral proceedings pursuant to Rule 115(1) EPC, EP Application No. 09075279.1, Mar. 6, 2013, EPO form 2906 01.91TRI, six pages. cited by applicant . EPO communication, Client Database System (CDS)--clean up, EP Application No. 19075279.1, Apr. 23, 2013, EPO Form 2596C, 04.08, 1 page. cited by applicant . EPO communication, Executed Maintenance / Change of date / Cancellation of oral proceedings arranged for: May 23, 2013 at 10.00 hrs, EP Application No. 19075279.1, May 14, 2013, EPO Form 2088 04.10, two pages. cited by applicant . EPO communication, EP Application No. 19075279.1, at least as early as May 22, 2013, EPO Form 2906 01.91TRI, one page. cited by applicant . Fussenegger et al., Genetic optimization of recombinant glycoprotein production by mammalian cells, Reviews, Tibtech, Jan. 1999, pp. 35-42, vol. 17. cited by applicant . European Patent Office Communication for Application No. 09075279.1 dated Nov. 5, 2012. cited by applicant . EPO Submission in opposition proceedings, Acknowledgement of Receipt filed by David Power of J A Kemp, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 20, 2015, two pages. cited by applicant . EPO Submission in opposition proceedings, Reply of the patent proprietor to the notice(s) of opposition, EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 24, 2015, two pages. cited by applicant . EPO Submission in opposition proceedings, Request for extension of time, EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 16, 2014, two pages. cited by applicant . EPO Transmission of the certificate for a European patent pursuant to Rule 74 EPC, EP Application No. 10186063.3, Oct. 18, 2013, EPO Form 2047, 12.07, 1 page. cited by applicant . Esposito. Gloria et al.. "Phage display of a human antibody against Clostridium tetani toxin," Gene, vol. 148:167-168 (1994). cited by applicant . European Search Opinion, EP Application No. 10186063.3, at least as early as Mar. 24, 2011, EPO Form 1703, 01,91TRI, 3 pages. cited by applicant . GenBank Accession No. ABA26122.1, Immunoglobulin light chain variable region, partial [Homo sapiens], 2005, 1 page. cited by applicant . European Search Report for EP Application No. 10186063, Mar. 16, 2011, EPO Form 1503, 03.82 (P04CO1), 2 pages. cited by applicant . Ewert et al., Biophysical properties of human antibody variable domains, J. Mol. Biol., Jan. 17, 2003, pp. 531-553, vol. 325, Iss. 3. cited by applicant . F.T. Wunderlich (2004), "Generation of inducible Cre systems for conditional gene inactivation in mice," Inauguraldissertation zur Erlangung des Doktorgrades der Mathematisch Naturwissenschaftlichen Fakultat der Universitat zu Koln; on the World Wide Web at deposit.ddb.de/cgi.bin/dokserv?idn=97557230x&dok_var=d1&dok_ext=pdf&filen- ame= 97557230x.pd. cited by applicant . Fecteau, Jessie F. et al., "A New Memory CD27 IgG+ B Cell Population in Peripheral Blood Expressing VH Genes with Low Frequency of Somatic Mutation," The Journal of Immunology, vol. 177:3728-3736 (2006). cited by applicant . Fendly et al., Characterization of Murine Monoclonal Antibodies Reactive to Either the Human Epidermal Growth Factor Receptor or HER2/neu Gene Product, Cancer Research, Mar. 1, 1990, pp. 1550-1558, vol. 50. cited by applicant . Ferrara, N., Vascular endothelial growth factor: molecular and biological aspects., Curr. Top. Microbiol. Immunol., 1999, 237:1-30. cited by applicant . Flavell et al., "Therapy of human T-cell acute lymphoblastic leukaemia with a combination of anti-CD7 and anti-CD38-SAPORIN immunotoxins is significantly better than therapy with each individual immunotoxin", British Journal of Cancer, vol. 84, No. 4, 2001, pp. 571-578. cited by applicant . Folkman, Angiogenesis in cancer, vascular, rheumatoid and other disease, J. Nat. Med., 1995, pp. 27-31, vol. 1, Abstract only. cited by applicant . Franklin et al., Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex, Cancer Cell, Apr. 2004, pp. 317-328, vol. 5, issue 4. cited by applicant . Giddings et al., Transgenic plants as factories for biopharmaceuticals, Nature Biotechnology, 2000, pp. 1151-1155, vol. 18, Nature America Inc. cited by applicant . G. Neufeld et al., Vascular endothelial growth factor (VEGF) and its receptors, FASEB J., Jan. 1999, pp. 9-22, vol. 13, No. 1. cited by applicant . Galun et al., Clinical evaluation (Phase I) of a combination of two human monoclonal antibodies to HBV: Safety and antiviral properties., Hepatology, Mar. 2002, pp. 673-679, vol. 35, Issue 3. cited by applicant . Gascan et al., Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by Interleukin-4 and a signal provided by activated CD4C T cell clones. J Exp Med. 1991;173:747-750. cited by applicant . GenBank Accession No. M87478, "Human rearranged IgK mRNA VJC region," 1 page. (1994). cited by applicant . Gerbert et al., Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation, J Biol. Chem., Nov. 13, 1998, pp. 30336-30343, vol. 273. cited by applicant . Gerstner et al., Sequence Plasticity in the Antigen-binding Site of a Therapeutic Anti-HER2 Antibody, J. Mol. Biol., Aug. 30 2002, pp. 851-862, vol. 321, issue 5, Elsevier, Abstract only. cited by applicant . Fritz Lahrtz of Isenbruck Bosl Hoschler LLP communication to EPO, Executed Acknowledgement, EP Application No. 09075279.1 and EP Patent No. 2147594, Jan. 25, 2016, EPO Form 2936 08.10, one page. cited by applicant . Fritz Lahrtz of Isenbruck Bosl Hoschler LLP communication to EPO, Executed Acknowledgement, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 29, 2016, EPO Form 2936 08.10, one page. cited by applicant . Gluzman, SV40-transformed simian cells support the replication of early SV40 mutants, Cell, Jan. 1981, pp. 175-182, vol. 23, Issue 1, Abstract only. cited by applicant . Gonzales-Fernandez et al., Analysis of somatic hypennutation in mouse Peyer's patches using immunoglobulin K lightchain transgenes, Proc. Natl. Acad. Sci., Nov. 1993, pp. 9862-9866, vol. 90. cited by applicant . Goyenechea et al., Modifying the sequence of an immunoglobulin V -gene alters the resulting pattern of hypermutation, Proc. Natl. Acad. Sci. 1996, pp. 13979-13984, vol. 93. cited by applicant . Goyenechea, Beatriz et al., "Cells strongly expressing Igk transgenes show clonal recruitment of hypermutation: a role for both MAR and the enhancers," The EMBO Journal, vol. 16(13):3987-3994 (1997). cited by applicant . Green et al., Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs, Nat. Genet., 1994, pp. 13-21, vol. 7, Abstract only. cited by applicant . Griffiths et al., Isolation of high affinity human antibodies directly from large synthetic repertoires, EMBO J., Jul. 15, 1994, pp. 3245-3260, vol. 13, No. 14. cited by applicant . Hochedlinger, Konrad et al., "Monoclonal mice generated by nuclear transfer from mature B and T donor cells," Nature, vol. 415:1 035-1 038 (2002). cited by applicant . Holliger et al., "Diabodies": Small bivalent and bispecitic antibody fragments, Proc. Natl. Acad. Sci., Jul. 1993, pp. 5444-5448, vol. 90. cited by applicant . Homig-Holzel et al., Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis, J. Exp. Med., 2008, pp. 1317-1329, vol. 205, No. 6. cited by applicant . Griffiths, et al., Human anti-self antibodies with high specificity from phage display libraries, EMBO J., Feb. 1993, pp. 725-734, vol. 12, No. 2. cited by applicant . Heintges et al., Cloning, Bacterial Expression and Sequencing of Human Antibody Fragments Against Hepatitis C Virus NS3 by Phage Display of a Combinatorial Phagemid Library, Hepatology, 1998, p. 497, vol. 28, No. 4. cited by applicant . Hengstschlager et al., A lambda 1 trans gene under the control of a heavy chain promoter and enhancer does not undergo somatic hypennutation, Eur. J. Immunol. 1994, pp. 1649-1656, vol. 24. cited by applicant . Hiatt et al., "Production of antibodies in transgenic plants", Department of Molecular Biology, Letters to Nature, vol. 342, Nov. 2, 1989, pp. 76-78. cited by applicant . Hardy et al., B Cell Development Pathways, Annu. Rev. Immunol., 2001, pp. 595-621, vol. 19. cited by applicant . German Translation of claims for EP Application No. 09075279.1, at least as early as Sep. 2, 2013, four pages. cited by applicant . French Translation of claims for EP Application No. 09075279.1, at least as early as Sep. 2, 2013, three pages. cited by applicant . Executed Acknowledgement of receipt of the document specified above, EP Application No. 09075279.1, Mar. 7, 2013, EPO Form 2936 08.10, one page. cited by applicant . Letter accompanying subsequently filed items regarding translations of claims, EP Application No. 10186063.3, Sep. 6, 2013, 13 pages. cited by applicant . Letter from Mr. Andrew Bentham of JA Kemp to European Patent Office dated Jul. 15, 2014, accompanying subsequently filed items, one page. cited by applicant . Letter from Mr. C.M. Jansen of V.O. Patents & Trademarks to European Patent Office, Regarding Registration of the Association and change of address, Sep. 29, 2015, one page. cited by applicant . Letter from Mr. T.J. Elmore of V.O. Patents & Trademarks to European Patent Office, at least as early as Oct. 16, 2014, accompanying subsequently filed items, one page. cited by applicant . Li et al., Stable expression of three genes from a tricistronic retroviral vector containing a picornavirus and 9-nt cellular internal ribosome entry site elements, J. Virol. Methods, Feb. 2004, pp. 137-144, vol. 115, Issue 2. cited by applicant . Lie, Y.S. et al., "Advances in quantitative PCR technology: 5' nuclease assays," Curr. Opin. Biotechnol., vol. 9 (1):43-48 (1998). cited by applicant . Lindhofer et al., Preferential Species-Restricted Heavy/Light Chain Pairing in Rat/Mouse Quadromas, Journal of Immunology, 1995, pp. 219-225, vol. 155. cited by applicant . Letter from European Patent Office to Mr. Andrew Bentham of JA Kemp dated Jun. 6, 2016, accompanying subsequently filed items, one page. cited by applicant . Letter regarding the opposition procedure (no time limit) dated Jun. 20, 2016 from Isenbruck Bosl Forschler LLP to European Patent Office, 1 page. cited by applicant . Letter from JA Kemp to The European Patent Office regarding Oral Proceedings scheduled for Jun. 22, 2016. cited by applicant . List of references in Opposition to Merus B.V.'s EP 2 314 29 B1, Consolidated List of Documents, undated, one page. cited by applicant . Letter submitting declarations of Peter Hudson and Robert Brink dated Jun. 2, 2015, Australian Application No. 2009263082, 1 page. cited by applicant . Letter accompanying subsequently filed items regarding revocation procedure, EP Application No. 09075279.1, Aug. 20, 2015, one page. cited by applicant . Letter accompanying subsequently filed items regarding examination, EP Application No. 09075279.1, Jun. 13, 2013, one page. cited by applicant . Main Request with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Main Request, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . Mao, Xiaohong et al., "Activation of EGFP expression by ere-mediated excision in a new ROSA26 reporter mouse strain," Blood, vol. 97(1 ):324-326 (2001). cited by applicant . Marks et al., By-passing immunization: Human antibodies from V-gene libraries displayed on phage, J. Mol. Biol., Dec. 5, 1991, pp. 581-597, vol. 222, Issue 3, Abstract only. cited by applicant . Marvin, J.S., et al., Recombinant approaches to IgG-like bispecific antibodies, Acta Pharmacologica Sinica, Jun. 2005, pp. 649-658, vol. 26. cited by applicant . Little et al., Human antibody libraries in Escherichia coli, Journal of Biotechnology, 1995, pp. 187-195, vol. 41, Elsevier. cited by applicant . McCafferty et al., Antibody Engineering, PAS, 2002, 178 pages, Oxford University Press. cited by applicant . Melvyn Little, Antibodies for Immunotherapy, Cambridge University Press, 2009. 23 pages. cited by applicant . Mendel et al., The Angiogenesis Inhibitor SU5416 Has Long-lasting Effects on Vascular Endothelial Growth Factor Receptor Phosphorylation and Function, Clin. Cancer Res., Dec. 2000, pp. 4848-4858, vol. 6. cited by applicant . Mendez et al., Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice, Nature Genetics. 1997. pp. 146-156. vol. 15. cited by applicant . Merus, "MeMo--the ingenious mouse, for improved antibody therapeutics," www.merus.nl, 3 pages (2011). cited by applicant . Meyer et al., the Igk 3'-enhancer triggers gene expression in early B lymphocytes but its activity in enhanced on B cell activation, Int. Immunol., 1996, pp. 1561-1568, vol. 8, No. 10. cited by applicant . Main request, EP Application No. 09075279.1, Reference No. P85261EP00, Apr. 23, 2013, three pages. cited by applicant . Lofgren et al., Comparing Elisa and Surface Plasmon Resonance for Assessing Clinical Immunogenicity of Panitumumab, .J Immunol., 2007, pp. 7467-7472, vol. 178. cited by applicant . Lonberg et al., Human antibodies from transgenic animals, Nature Biotechnology, Sep. 1, 2005, pp. 1117-1125, vol. 23, No. 9, Nature Publishing Group, New York, NY, US. cited by applicant . Lonberg, Nils et al., "Antigen-specific human antibodies from mice comprising four distinct genetic modificaitons," Nature, vol. 368:856-859 (1994). cited by applicant . Lu et al., Acquired antagonistic activity of a bispecific diabody directed against two different epitopes on vascular endothelial growth factor receptor 2, J. Immunol. Methods, Nov. 19, 1999, pp. 159-171, vol. 230. cited by applicant . Lu et al., Complete Inhibition of Vascular Endothelial Growth Factor (VEGF) Activities with a Bifunctional Diabody Directed against Both VEGF Kinase Receptors, fms-like Tyrosine Kinase Receptor and Kinase Insert Domain-containing Receptor, Cancer Res., Oct. 1, 2001, pp. 7002-7008, vol. 61. cited by applicant . Lu et al., Identification of the Residues in the Extracellular Region of KDR Important for Interaction with Vascular Endothelial Growth Factor and Neutralizing Anti-KDR Antibodies, J. Biol. Chem., May 12, 2000, pp. 14321-14330, vol. 275. cited by applicant . Ma et al., Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants, Eur. J. Immunol., 1994, p. 131-138, vol. 24. cited by applicant . Macdonald et al., Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes, PNAS, 2013, (6 Pages), Early Edition. cited by applicant . Letter accompanying subsequently filed items regarding Document concerning representation, EP Application No. 09075279.1, Submitted by C.M. Jansen of V.O., Dec. 17, 2015, one page. cited by applicant . Meyer, Kerstin B. et al., "The importance of the 3'-enhancer region in immunoglobulin kappa gene expression," Nucleic Acids Research, vol. 18(19):5609-5615 (1990). cited by applicant . Middendorp et al., Cellular Maturation Defects in Bruton's Tyrosine Kinase-Deficient Immature B Cells Are Amplified by Premature B Cell Receptor Expression and Reduced by Receptor Editing, J. Immunol., Feb. 1, 2004, pp. 1371-1379, vol. 172, No. 3. cited by applicant . Middendorp et al., Impaired Precursor B Cell Differentiation in Bruton's Tyrosine Kinase-Deficient Mice, J. Immunol., Mar. 15, 2002, pp. 2695-2703, vol. 168 No. 6. cited by applicant . Letter accompanying subsequently filed items regarding acknowledgement, EP Application No. 09075279.1, Submitted by David Power of J A Kemp, Apr. 12, 2016, one page. cited by applicant . Letter accompanying subsequently filed items regarding German and French translation of the claims, EP Application No. 09075279.1, Sep. 2, 2013, two pages. cited by applicant . Letter disclosing in vivo data dated Jun. 13, 2013, European patent application No. 09075279.1, 2 pages. cited by applicant . Letter accompanying subsequently filed items regarding oral proceedings, EP Application No. 09075279.1, Apr. 24, 2013, one page. cited by applicant . Letter accompanying subsequently filed items regarding documents filed during examination procedure, EP Application No. 09075279.1, Sep. 3, 2013, one page. cited by applicant . Letter accompanying subsequently filed items regarding amended claims with clean and annotated copies, EP Application No. 09075279.1, Apr. 23, 2013, 2 pages. cited by applicant . Peeters et al., Production of antibodies and antibody fragments in plants, Vaccine, Mar. 21, 2001, pp. 2756-2761, vol. 19, Issues 17-19, Elsevier. cited by applicant . Pelanda et al., a prematurely expressed Ig(kappa) transgene, but not V(kappa)J(kappa) gene segment targeted into the Ig(kappa) locus, can rescue B cell development in lambdaS-deficient mice, Immunity, Sep. 1996, pp. 229-239, vol. 5, No. 3. cited by applicant . Peled, Jonathan U. et al., "The Biochemistry of Somatic Hypermutation," Annu. Rev. Immunol., vol. 26:481-511 (2008). cited by applicant . Perrin et al., In vitro rabies vaccine potency appraisal by Elisa: advant of the immunocapture method with a neutralizing anti-glycoprotein monoclonal antibody, Biologicals, Oct. 1990, pp. 321-330, vol. 18(4). cited by applicant . Persic, L. et al. An integrated vector system for the eukaryotic expression of antibodies or their fragments after selection from phage display libraries, Gene, Mar. 10, 1997, pp. 9-18, vol. 187, Issue 1. cited by applicant . Pollock et al., "Transgenic milk as a method for the production of recombinant antibodies", Elsevier, Journal of Immunological Methods, 231 (1999), pp. 147-157. cited by applicant . Popov, Andrei V. et al., "A Human Immunoglobulin lambda Locus Is Similarly Well Expressed in Mice and Humans," J. Exp. Med., vol. 189(10):1611-1619 (1999). cited by applicant . Presta et al., Engineering of therapeutic antibodies to minimize immunogenicity and optimize function, Advanced Drug Delivery Reviews, Aug. 7, 2006, pp. 640-656, vol. 58, No. 5-6, Elsevier BV, Amsterdam, NL. cited by applicant . Opposition Filed Against European Patent No. EP 2 314 629 B1 (European Patent Application No. 10186063.3) in the Name of Merus B.V., Declaration of Dr. Joel Martin, May 18, 2016, 13 pages. cited by applicant . Padlan et al., A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand-binding properties, Mol. Immunol., 1991, pp. 489-498, vol. 28, Abstract only. cited by applicant . Pasqualucci et al., BCL-6 mutations in normal germinal center B cells: evidence of somatic hypermutation acting outside Ig loci, Proc. Natl. Acad. Sci. USA, Sep. 1998, pp. 11816-11821, vol. 95. cited by applicant . Pau et al, The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines, Vaccine, 2001, pp. 2716-2721, vol. 19. cited by applicant . Paul Carter, "Bispecific human IgG by design", Elsevier, Journal of Immunological Methods, vol. 248, 2001, pp. 7-15. cited by applicant . PCT International Preliminary Examination Report, PCT/EP03/07690, dated Nov. 29, 2004. cited by applicant . PCT International Preliminary Report on Patentability, PCT/NL2009/050381 dated Jan. 5, 2011. cited by applicant . PCT International Search Report, PCT/NL2009/050381 dated Dec. 7, 2009. cited by applicant . Payment of fees and expenses for EP Application No. 10186063.3 dated May 27, 2016, one page. cited by applicant . Phelps et al., Expression and Characterization of a Chimeric Bifunctional Antibody with Therapeutic Applications, The Journal of Immunology, Aug. 15, 1990, pp. 1200-1204, vol. 145, No. 4. cited by applicant . Notice of Opposition, Australian application No. 2009263082, Jun. 20, 2014, 1 page. cited by applicant . Opposition Summary, Australian Application No. 2009263082, May 18, 2015, 11 pages. cited by applicant . Nicholson et al., Antibody Repertoires of Four- and Five-Feature Translocus Mice Carrying Human Immunoglobulin Heavy Chain and kappa and lambda Light Chain Yeast Artificial Chromosomes, The Journal of Immunology, 1999, pp. 6898-6906, vol. 163. cited by applicant . Nissim et al., Antibody fragments from a `single pot` phage display library as immunochemical the reagents, The EMBO Journal, 1994, pp. 692-698, vol. 13. No. 3. cited by applicant . Norderhaug et al., Balanced expression of single subunits in a multisubunit proteins, achieved by cell fusion of individual transfectants, European Journal of Biochemistry, 2002, pp. 3205-3210, vol. 269. cited by applicant . Notice of Opposition dated Jul. 8, 2016, EP2264163 10010741.6. cited by applicant . Notice of Opposition to a European patent, EP Patent No. 2314629, EP Application No. 10186063.3, Jul. 14, 2014, EPO Form 2300E, Q40114EP, 8 pages. cited by applicant . Notification to EPO regarding Applicant Address Change, EP Application No. 10186063.3, Jan. 4, 2012, 1 page. cited by applicant . Notification to EPO regarding Request for recording a change in name of representative, EP Application No. 10186063.3, Mar. 23, 2013, 3 pages. cited by applicant . Novobrantseva et al., Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice, J. Exp. Med., Jan. 4, 1999, pp. 75-88, vol. 189, No. 1. cited by applicant . Nowakowski et al., Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody, PNAS, Aug. 20, 2002, pp. 11346-11350, vol. 99, No. 17. cited by applicant . Odegard et al., Targeting of somatic hypermutation, Nature Reviews, Immunology, Aug. 2006, pp. 573-583, vol. 6, No. 8. cited by applicant . NCBI, Aucouturier et al., Monoclonal IgL Claim and L chain V domain fragment crystallization in myeloma-associated Fanconi's syndrome, <http://www.ncbi.nlm.nib/gov/nuccore/M87478, at least as early as Apr. 25, 2012. cited by applicant . Nemazee, David, "Receptor editing in lymphocyte development and central tolerance," Nature, vol. 6(10):728-740 (2006). cited by applicant . Neuberger, M.S. et al., "Isotype exclusion and transgene down-regulation in immunoglobulin-lambda transgenic mice," Nature, vol. 338:350-352 (1989). cited by applicant . Ngo, T.-H., et al, Identification of functional synergism between monoclonal antibodies. Application to the enhancement of plasminogen activator inhibitor-1 neutralizing effects, FEBS Letters, 1997, pp. 373-376, vol. 416. cited by applicant . Min Soo Kim et al., Comparative Analyses of Complex Formation and Binding Sites between Human Tumor Necrosis Factor-alpha and its Three Antagonists Elucidate their Different Neutralizing Mechanisms, JMB, Dec. 14, 2007, pp. 1374-1388, vol. 374, Issue 5. cited by applicant . Mirick et al., A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies: not four letter words, Q. Nucl. Med. Mol. Imaging, Dec. 2004, pp. 251-257, vol. 48, No. 4. cited by applicant . Morimoto et al., High level expression of a human rabies virus-neutralizing monoclonal antibody by a rhabdovirus-based vector, J. Immunol. Methods, Jun. 2001, pp. 199-206, vol. 1, No. 252(1-2). cited by applicant . Morrison et al., Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains, Proc. Natl. Acad. Sci. USA, Nov. 1, 1984, pp. 6851-6855, vol. 81, No. 21. cited by applicant . Mostoslavsky et al., "Asynchronous replication and allelic exclusion in the immune system," Nature (2001) 414:221-225, Abstract only. cited by applicant . Murphy et al., Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice, PNAS, 2013, 6 pages, Early Edition. cited by applicant . Murphy, "Chapter 8: The Development and Survival of Lymphocytes", Janeway's Immunobiology, Eight Edition, Jul. 24, 2011, pp. 275-290. cited by applicant . Nagle, "Regeneron helps make Sanofi Velcolmmue to its `weak` pipline", Outsourcing-Pharma.com, Dec. 3, 2007, two pages, William Reed Business Media SAS. cited by applicant . Nahta et al., The HER-2-Targeting Antibodies Trastuzumab and Pertuzumab Synergistically Inhibit the Survival of Breast Cancer Cells, Cancer Research, Apr. 1, 2004, pp. 2343-2346, vol. 64. cited by applicant . Prak, Eline Lunning, Light Chain Replacement: a new model for antibody gene rearrangement, J. Exp. Med., Aug. 1995, pp. 541-548, vol. 182, the Rockefeller University Press. cited by applicant . Statement of Fact and Argument in Support of Opposition filed against EP Patent No. 2314629, at least as early as Jul. 15, 2014, 30 pages. cited by applicant . Priority Document dated Oct. 27, 2009, EP12175544. cited by applicant . Queen et al., A humanized antibody that binds to the interleukin 2 receptor, Proc. Natl. Acad. Sci. U.S.A., Dec. 1, 1989, pp. 10029-10033, vol. 86, No. 24. cited by applicant . Spillner et al., Paratope-based protein identification by antibody and peptide phage display, Analytical Biochemistry, 2003, pp. 96-104, vol. 321, Academic Press. cited by applicant . Radic et al., Ig H and L chain contributions to autoimmune specificities, The Journal of Immunology, Jan. 1, 1991, pp. 176-182, vol. 146, No. 1, The American Association of Immunologists. cited by applicant . Rajewsky et al., Conditional gene targeting, J Clin Invest, Aug. 1, 1996, pp. 600-603, vol. 98, No. 3. cited by applicant . Refund of Fees, EP Application No. 10186063.3, Jun. 4, 2011, EPO Form 2907, 12.07, 1 page. cited by applicant . Refund of Fees, EP Application No. 10186063.3, Nov. 17, 2010, EPO Form 2907, 12.07, 1 page. cited by applicant . Reply to Communication under Rule 79(1) EPC, EP Application No. 101860633 and EP Patent No. 2314629, Feb. 24, 2015, 20 pages. cited by applicant . Request for Grant of a European Patent for EP Application No. 101860633, Oct. 1, 2010, 6 pages. cited by applicant . Response to Communication pursuant to Article 94(3) EPC, EP Application No. 10186063.3, Dec. 21, 2011, 13 pages. cited by applicant . Response to Communication pursuant to Article 94(3) EPC, EP Application No. 10186063.3, Jul. 19, 2012, 45 pages. cited by applicant . Response to the Summons to attend Oral Proceedings dated Nov. 29, 2015 and in preparation of the Hearing of Jun. 22, 2016, from Isenbruck Bosl Forschler LLP to European Patent Office dated May 20, 2016. cited by applicant . Request for recordal of change of Proprietor from Merus B.V. to Merus N.V. filed by Isenbruck Bosl Forschler LLP with European Patent Office dated May 27, 2016, 2 pages. cited by applicant . Second Declaration by David Tarlington dated Oct. 15, 2015, Australian patent application No. 2009263082, 24 pages. cited by applicant . Second Declaration of Anthony L. DeFranco dated Oct. 18, 2015, Australian application No. 2009263082, 31 pages. cited by applicant . Sirac et al., "Toward Understanding Renal Fanconi Syndrome: Step by Step Advances through Experimental Models", Contributions to Nephrology, Experimental Models of Renal Fanconi Syndrome, vol. 169, 2011, pp. 247-261. cited by applicant . Sanger et al., DNA sequencing with chain-terminating inhibitors, PNAS, Dec. 1, 1997, pp. 5463-5467, vol. 74, No. 12. cited by applicant . Sasaki, Yoshiteru et al., "Canonical NF-kB Activity, Dispensable forB Cell Development, Replaces BAFF-Receptor Signals and Promotes B Cell Proliferation upon Activation," Immunity, vol. 24:729-739 (2006). cited by applicant . Schnieke et al., Human Factor IX Transgenic Sheep Produced by Transfer of Nuclei from Transfected Fetal Fibroblasts, Science, Dec. 19, 1997, pp. 2130-2133, vol. 278, Issue 5346. cited by applicant . Scott, Christopher Thomas, "Mice with a human touch," Nature Biotechnology, vol. 25:1075-1077 (2007). cited by applicant . Response to Communication pursuant to Rules 70(2) and 70a(2) EPC and reference to Rule 39(1) EPC, EP Application No. 10186063.3, Oct. 17, 2011, 16 pages. cited by applicant . Response to Invitation to remedy deficiencies pursuant to Rule 30(3) EPC / Rule 163(3) EPC, EP Application No. 10186063.3, Jan. 27, 2011, 2 pages. cited by applicant . Retter, Marc W. et al., "Receptor Editing Occurs Frequently during Normal B Cell Development," J. Exp. Med., vol. 188(7):1231-1238 (1998). cited by applicant . Rickert, Robert C. et al., "B lymphocyte-specific, Cre-mediated mutagenesis in mice," Nucleic Acids Research, vol. 25(6)1317-1318 (1997). cited by applicant . Roberts and Szostak, RNA-peptide fusions for the in vitro selection of peptides and proteins, Proc. Natl. Acad. Sci. U.S.A., Nov. 1997, pp. 12297-12302, vol. 94. cited by applicant . Second Declaration of Peter Hudson, Jun. 2, 2015, 81 pages. cited by applicant . Rong et al., Tumorigenesis induced by coexpression of human hepatocyte growth factor and the human met protooncogene leads to high levels of expression of the ligand and receptor, Cell Growth Differ., Jul. 1993, pp. 563-569, vol. 4, No. 7. cited by applicant . Rong et al., Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor, Mol. Cell Biol., Nov. 1992, pp. 5152-5158, vol. 12, No. 11. cited by applicant . Simmons et al., Expression of full-length immunoglobulins in Escherichia coli: rapid and efficient production of aglycosylated antibodies, J. Immunol. Methods, May 1, 2002, pp. 133-147, vol. 263. cited by applicant . Sirac et al., Role of the monoclonal kappa chain V domain and reversibility of renal damage in a transgenic model of acquired Fanconi syndrome, Blood, Jul. 15, 2006, pp. 536-543, vol. 108, No. 2. cited by applicant . Sjolander and Urbaniczky, Integrated fluid handling system for biomolecular interaction analysis, Anal. Chem., 1991, pp. 2338-2345, vol. 63, No. 20, Abstract only. cited by applicant . Smith, G.P., Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science, Jun. 14, 1985, pp. 1315-1317, vol. 228, Issue 4705, Abstract only. cited by applicant . Smith-Gill et al., Contributions of immunoglobulin heavy and light chains to antibody specificity for lysozyme and two haptens, Journal of Immunology, Dec. 15, 1987, pp. 4135-4144, vol. 139, No. 12., Baltimore, MD, US. cited by applicant . Specification of International Application No. PCT/EP03/07690, "Recombinant Production of Mixtures of Antibodies", at least as early as Oct. 1, 2010, 122 pages. cited by applicant . Srinivas et al., Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus, BMC Dev. Biol., Mar. 27, 2001, vol. 1 :4. cited by applicant . Sharpe et al., Somatic hypermutation of immunoglobulin kappa may depend on sequences 3' of C kappa and occurs on passengertransgenes, The EMBO.Ioumal. 1991. pp. 2139-2145, vol. 10, No. 8. cited by applicant . Shvarts et al., A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling, Genes Dev., Mar. 15, 2002, pp. 681-686, vol. 16(6). cited by applicant . Sidhu et al., Phage display for selection of novel binding peptides, Methods Enzymol., 2000 328:333 363. cited by applicant . Second Declaration of Robert Brink, Jun. 2, 2015, 38 pages. cited by applicant . Segal et al., Introduction: bispecific antibodies, Journal of Immunological Methods, 2001, pp. 1-6, vol. 248, Elsevier. cited by applicant . Seibler et al., Rapid generation of inducible mouse mutants, Nucleic Acids Res., Feb. 15, 2003, e12, vol. 31, No. 4. cited by applicant . Sequence Listing, at least as early as Oct. 1, 2010, 18 pages. cited by applicant . Sequence Listing, Reference No. P61909EP20, Jan. 27, 2011, 12 pages. cited by applicant . Response to communication pursuant Article 94(3) EPC, EP Application No. 09075279.1, Sep. 11, 2012, Reference No. P85231EP00, eleven pages. cited by applicant . Statement of Fact and Arguments in Support of Opposition dated Jul. 15, 2014 for EP 2 314 629 B1. cited by applicant . Statement of Facts and Arguments in support of Opposition, EP Application No. 09075279.1 and EP Patent No. 2147594, at least as early as Aug. 11, 2014, 46 pages. cited by applicant . Statement of Grounds and Particulars submitted in opposition to Australian Patent Application 2009263082, filed Sep. 22, 2014, 35 pages. cited by applicant . Stevens, Sean, "Human Antibody Discovery, Veloclmmune- A novel platform," Pharma Focus Asia, Issue 8, pp. 72-74 (2008). cited by applicant . Strelkauskas et al., Human Monoclonal Antibody: 2. Simultaneous Expression of IgG and IgM with Similar Binding Specificities by a Human Hybrid Clone, Hybridoma, 1987, pp. 479-487, vol. 6, No. 5, Mary Ann Lieber, Inc., Publishers. cited by applicant . Tada et al., Expression and characterization of a chimeric bispecific antibody against fibrin and against urokinase-type plasminogen activator, Journal of Biotechnology, 1994, pp. 157-174, vol. 33. cited by applicant . Verma et al., Antibody engineering: Comparison of bacterial, yeast, insect and mammalian expression systems, Journal of Immunological Methods, 1998, pp. 165-181, vol. 216. cited by applicant . Third Party Observation for application No. EP20090075279, Anonymous, at least as early as Sep. 5, 2013, seven pages. cited by applicant . Third Party Observations Against European Parent Application No. 09075279.1 in the Name of Merus BV, 3 pages, dated Jul. 1, 2013. cited by applicant . Third Party Observations for Application No. EP09075279.1, 6 pages, dated Oct. 25, 2012. cited by applicant . Third Party Observations Under Article 115 EPC Against European Parent Application No. 09075279.1 in the name of Merus B.V., dated Apr. 25, 2012. 6 pages. cited by applicant . Third Party Observations Under Article 115 EPC Against European Parent Application No. 09075279.1 in the name of Merus B.V., dated Oct. 25, 2012. 6 pages. cited by applicant . Thomas et al., Site-Directed Mutagenesis by Gene Targeting in Mouse Embryo-Derived Stem Cells, Cells, Nov. 6, 1987, pp. 503-512, vol. 51. cited by applicant . Throsby, Isolation and Characterization of Human Monoclonal Antibodies from Individuals Infected with West Nile Virus, J. Virol., Jul. 2006, pp. 6982-6992, vol. 80, No. 14. cited by applicant . Torres et al., "Chapter 10: LoxP-containing transgenes", Laboratory Protocols for Conditional Gene Targeting, 1997, pp. 42-53, Oxford University Press Inc., New York, USA. cited by applicant . U.S. Priority Document of U.S. Appl. No. 60/397,066, "Recombinant Production of Mixtures of Antibodies", submitted in International Application No. PCT/EP03/07690, Sep. 1, 2003, 140 pages. cited by applicant . Urlaub et al., Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity, PNAS, Jul. 1, 1980, pp. 4216-4220, vol. 77, No. 7. cited by applicant . V.O. communication to EPO, Executed Acknowledgement of receipt of EPO Form 2310 and 2043, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 20, 2015, EPO Form 2936 08.10, one page. cited by applicant . Vajdos et al., Comprehensive Functional Maps of the Antigen-binding Site of an Anti-ErbB2 Antibody Obtained with Shotgun Scanning Mutagenesis, J. Mol. Biol., Jul. 5, 2002, pp. 415-428, vol. 320, issue 2, Elsevier. cited by applicant . van den Beucken et al., Building novel binding ligands to B7.1 and B7.2 based on human antibody single variable light chain domains, J. Mol. Biol., Jul. 13, 2001, pp. 591-601, vol. 310, Issue 3, Abstract only. cited by applicant . van der Heijden et al., Structural and functional studies on a unique linear neutralizing antigenic site (G5) of the rabies virus glycoprotein, J. Gen. Virol., Aug. 1993, pp. 1539-1545, vol. 74, Issue 8. cited by applicant . Vaughan et al., "Human Antibodies with Sub-nanomolar Affinities Isolated from a Large Non-immunized Phage Display Library", Nature Biotechnology, vol. 14, Mar. 1996, pp. 309-314. cited by applicant . Weiner, et al., Abstract, Fully human therapeutic monoclonal antibodies, Journal of Immunotherapy, Jan. 1, 2006, pp. 1-9, vol. 29, No. 1, Lippincott Williams & Wilkins, Hagerstown, MD, US. cited by applicant . Wen et al., Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: Preclinical studies in myeloma, Cancer Gene Therapy, 2001,pp. 361-370, vol. 8 No. 5. cited by applicant . Wildt et al., "Analysis of Heavy and Light Chain Pairings Indicates that Receptor Editing Shapes the Human Antibody Repertoire", Journal of Molecular Biology, vol. 285, 1999, pp. 895-901. cited by applicant . Wilmut et al., Basic techniques for transgenesis, Journals of Reproduction and Fertility, 1991, pp. 265-275, vol. 43, Journals of Reproduction & Fertility Ltd. cited by applicant . Wilmut et al., Viable offspring derived from fetal and adult mammalian cells, Nature, Feb. 27, 1997, pp. 810-813, vol. 385, Issue 6619. cited by applicant . Winter et al., Insertion of 2 kb of bacteriophage DNA between an immunoglobulin promoter and leader exon stops somatic hypermutation in a kappa trans gene, Molecular Immunology, Apr. 1997, pp. 359-366, vol. 34, No. 5. cited by applicant . Winter et al., Making antibodies by phage display technology, Annu. Rev. Immunol., Apr. 1994, pp. 433-455, vol. 12, Abstract only. cited by applicant . Xiang, Yougui et al., "The Downstream Transcriptional Enhancer, Ed, Positively Regulates Mouse Igk Gene Expression and Somatic Hypermutation," J. Immunol., vol. 180(10):6725-6732 (2008). cited by applicant . Xu et al, "Deletion of the Ig kappa light chain intronic enhancer/matrix attachment region impairs but does not abolish V kappa J kappa rearrangement," Immunity (1996) 4:377-385. cited by applicant . Taylor, Lisa D. et al., "A transgenic mouse that expresses a diversity of human sequence heavy and light chain immunoglobulins," Nucleic Acids Research, vol. 20(23):6287-6295 (1992). cited by applicant . Taylor, Lisa D. et al., "Human immunoglobulin transgenes undergo rearrangement, somatic mutation and class switching in mice that lack endogenous IgM," International Immunology, vol. 6(4):579-591 (1994). cited by applicant . Thiebe et al., The variable genes and gene families of the mouse immunoglobulin ? locus, European Journal of Immunology, Jul. 1999, pp. 2072-2081, vol. 29, Issue 7. cited by applicant . Third Party Observation for Application No. 2009263082, 25 pages, dated Oct. 21, 2013. cited by applicant . Szabo et al., Surface plasmon resonance and its use in biomolecular interaction analysis (BIA), Curr. Opin. Struct. Biol., Oct. 1995, pp. 699-705, vol. 5, Issue 5, Abstract only. cited by applicant . Tan et al., "Superhumanized" Antibodies: Reduction of Immunogenic Potential by Complementarity-Determining Region Grafting with Human Germline Sequences: Application to an Anti-CD281, J. Immunol., Jul. 15, 2002, pp. 1119-1125, vol. 169, No. 2. cited by applicant . Storb et al., Immunoglobulin transgenes as targets for somatic hypermutation, Int. J. Dev. Biol., 1998, pp. 977-982, vol. 42(7). cited by applicant . Storb, Ursula et al., "Transgenic Mice with mu and kappa Genes Encoding Antiphosphorylcholine Antibodies," J. Exp. Med., vol. 164:627-641 (1986). cited by applicant . Submission in opposition proceedings by Andrew Bentham, EP Application No. 09075279.1 and Patent No. 2147594, Nov. 17, 2015, two pages. cited by applicant . Yang, X.W. et al., "Homologous recombination based modification in Escherichia coil and germline transmission in transgenic mice of a bacterial artificial chromosome," Nat. Biotechnol., vol. 15(9):859-865 (1997). cited by applicant . Submission in opposition proceedings by Andrew Bentham, EP Application No. 09075279.1 and Patent No. 2147594, Jan. 29, 2016, two pages. cited by applicant . Summons to Attend Oral Proceedings, EP Patent No. 2147594, at least as early as Feb. 1, 2016, five pages. cited by applicant . Submission in opposition proceedings by Andrew Bentham, Letter providing alternated dates for Oral Proceedings, EP Application No. 09075279.1 and Patent No. 2147594, Feb. 15, 2016, one page. cited by applicant . Third Party Observations Against European Parent Application No. 09075279.1 in the name of Merus BV, at least as early as Sep. 5, 2013, four pages. cited by applicant . Zahn Zabel et al., Development of stable cell lines for production or regulated expression using matrix attachment regions, J. Biotechnology, Apr. 27, 2001, pp. 29-42, vol. 87, Issue 1. cited by applicant . Zhu et al., Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library, Cancer Res., Aug. 1998, pp. 3209-3214, vol. 58, No. 15. cited by applicant . Zhu et al., Remodeling domain interfaces to enhance heterodimer formation, Protein Science, Apr. 1997, pp. 781-788, vol. 6, Issue 4. cited by applicant . Zhu et. al., Inhibition of Tumor Growth and Metastasis by Targeting Tumor-Associated Angiogenesis with Antagonists to the Receptors of Vascular Endothelial Growth Factor, Invest. New Drugs, Aug. 1999, pp. 195-212, vol. 17, Issue 3, Abstract only. cited by applicant . Zou et al., Cre-loxP-mediated gene replacement: a mouse strain producing humanized antibodies, Current Biology, 1994, pp. 1099-1103, vol. 4. cited by applicant . ImMunoGeneTics Information System, for analysed sequence CHEB VK, http://www.imgt.org/IMGT vguesVvguest, at least as early as Apr. 25, 2012. cited by applicant . Inlay et al., Essential roles of the kappa light chain intronic enhancer and 3' enhancer in kappa rearrangement and demethylation, Nat Immunol., Apr. 22, 2002, pp. 463-468, vol. 3, Abstract only. cited by applicant . Inlay et al., Roles of the Ig kappa light chain intronic and 3' enhancers in Igk somatic hypermutation, J. Immunol., 2006, pp. 1146-1151, vol. 177(2). cited by applicant . International Preliminary Report on Patentability and Written Opinion for Application No. PCT/NL2009/050381, 11 pages, dated Jan. 5, 2011. cited by applicant . International Search Report for Application No. PCT/NL2009/050381, 5 pages, dated Dec. 7, 2009. cited by applicant . J A Kemp communication to EPO, Executed Acknowledgement of receipt of EPO Form 2310 and 2043, EP Application 10186063.3 and EP Patent No. 2314629, Nov. 25, 2015, EPO Form 2936 08.10, one page. cited by applicant . Jain et al., Engineering antibodies for clinical applications, Trends in Biotechnol., Jul. 2007, pp. 307-316, vol. 25, Issue 7. cited by applicant . Kong et al., A lambda 3' Enhancer Drives Active and Untemplated Somatic Hypermutation of a lambda1 Trans gene, The Journal ofImmunology, 1998, pp. 294-301, vol. 161. cited by applicant . Koochekpour et. al., Met and Hepatocyte Growth Factor/Scatter Factor Expression in Human Gliomas, Cancer Res., Dec. 1, 1997, pp. 5391-5398, vol. 57. cited by applicant . Kramer et al., A novel helper phage that improves phage display selection efficiency by preventing the amplification of phages without recombinant protein, Nucleic Acids Res., 2003, e59, vol. 31, No. 11. cited by applicant . Kunkel et al., Rapid and efficient site-specific mutagenesis without phenotypic selection, Methods in Enzymol., 1987, pp. 367-382, vol. 154. cited by applicant . Kwaks et al., Employing epigenetics to augment the expression of therapeutic proteins in mammalian cells, Trends in Biotechnology, Mar. 1, 2006, pp. 137-142, vol. 24, No. 3, Elsevier Publications, Cambridge, GB. cited by applicant . Kwaks et al., Identification of anti-repressor elements that confer high and stable protein production in mammalian cells, Nature Biotechnology, 2003, pp. 553-558, vol. 269. cited by applicant . Lang, A.B. et al, Immunotherapy with Human Monoclonal Antibodies, Journal of Immunology, Jul. 1993, pp. 466-472, vol. 151, No. 13. cited by applicant . Larrick et al., Producing proteins in transgenic plants and animals, Current Opinion in Biotechnology, Aug. 1, 2001, pp. 411-418, vol. 12, Issue 4. cited by applicant . Lazar et al., A molecular immunology approach to antibody humanization and functional optimization, Mol Immunol.,Mar. 2007, pp. 1986-1998, vol. 44, Issue 8. cited by applicant . Jakobovits et al., Analysis of homozygous mutant chimeric mice: deletion of the immunoglobulin heavy-chain joining region blocks B-cell development and antibody production, Proc. Natl. Acad. Sci. U.S.A., Mar. 1993, pp. 2551-2555, vol. 90. cited by applicant . Jakobovits et al., From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice, Nature Biotechnology, Oct. 2007, pp. 1134-1143, vol. 25, No. 10. cited by applicant . Jakobovits et al., Germ-line transmission and expression of a human-derived yeast artificial chromosome, Nature, Mar. 18, 1993, pp. 255-258, vol. 362, Abstract only. cited by applicant . Jeffers et al., Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network, Mol. Cell. Biol., Mar. 1996, pp. 1115-1125, vol. 16, No. 3. cited by applicant . Jolly et al. Rapid methods for the analysis of immunoglobulin gene hypennutation: application to transgenic and gene targeted mice, Nucleic Acids Research, 1997, pp. 1913-1919, vol. 25, No. 10. cited by applicant . Jones et al., "High-Level Expression of Recombinant IgG in the Human Cell Line PER.C6", Biotechnology Progress, vol. 19, 2003, pp. 163-168. cited by applicant . Jones et al., Replacing the complementarity-determining regions in a human antibody with those from a mouse, Nature May 29, 1986, pp. 522-525, vol. 321, Abstract only. cited by applicant . Kabat, et al. (1991) Sequences of Proteins of Immunological Interest, Fifth Edition, U.S. Department of Health and Human Services, NIH Publication No. 91 3242 Abstract only. cited by applicant . Kakitani et al., "A novel transgenic chimaeric mouse system for the rapid functional evaluation of genes encoding secreted proteins," Nucleic Acids Research (2005) 33(9):e85. cited by applicant . Kasprzyk et al., Therapy of an Animal Model of Human Gastric Cancer Using a Combination of Anti-erbB-2 Monoclonal Antibodies, Cancer Research, May 15, 1992, pp. 2271-2776, vol. 52. cited by applicant . Kaufman et al., Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary DNA gene, J. Mol. Biol., Aug. 25, 1982, pp. 601-621, vol. 159, Issue 4, Abstract only. cited by applicant . Klagsbrun et al., Vascular endothelial growth factor and its receptors, Cytokine Rev., Oct. 1996, pp. 259-270, vol. 7, Issue 3, Abstract only. cited by applicant . Kling, Jim, Big Pharma vies for mice, Nature Biotechnology, Jun. 1, 2007, pp. 613-614, vol. 25. cited by applicant . Klitz et al., New HLA haplotype frequency reference standards: High-resolution and large sample typing of HLA-DR DQ haplotypes in a sample of European Americans, Tissue Antigens, pp. 296-307, vol. 62, Issue 4, Abstract only. cited by applicant . Klohn, Peter-Christian et al., "IBC's 23rd Annual Antibody Engineering, 10th Annual Antibody Therapeutics International Conferences and the 2012 Annual Meeting of the Antibody Society," mAbs, vol. 5(2):178-201 (2013). cited by applicant . Klotz et al., Somatic Hypermutation of a lambda, Transgene Under the Control of the lambda, Enhancer or the Heavy Chain Intron Enhancer, The Journal of Immunology, 1996. pp. 4458-4463. vol. 157. cited by applicant . Kohler and Milstein, Continuous cultures of fused cells secreting antibody of predefined specificity, Nature, Aug. 7, 1975, pp. 495-497, vol. 256. cited by applicant . JA Kemp to The European Patent Office of Final Written Submissions for Oral Proceedings scheduled for Jun. 22, 2016 in Opposition to Merus B.V.'s EP 2 314 629 B1 dated May 20, 2016. cited by applicant . Janeway, The Development and Survival of Lymphocytes, Chapter 8, Immunobiology, 1999, pp. 275-290. cited by applicant . Hwang et al., Immunogenicity of engineered antibodies, Methods, May 2005, pp. 3-10, vol. 36, Issue 1. cited by applicant . Ignatovich et al., Dominance of intrinsic genetic factors in shaping the human immunoglobulin Vlambda repertoire, J. Mol. Biol., Nov. 26, 1999, pp. 457-465, vol. 294, Issue 2. cited by applicant . Hoogenboom et al., Selecting and screening recombinant antibody libraries, Nat. Biotechnol., Sep. 7, 2005, pp. 1105-1116, vol. 23, Abstract only. cited by applicant . Hoogenboom, Designing and optimizing library selection strategies for generating high-affinity antibodies, Trends Biotechnol., 1997, pp. 62-70, vol. 15, Issue 2, Abstract only. cited by applicant . Hoogenboom, et al., Natural and designer binding sites made by phage display technology, Immunol. Today, Aug. 1, 2000, pp. 371-378, vol. 21, Issue 8, Abstract only. cited by applicant . Hudziak et al., p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor, Mol. Cell. Biol., Mar. 1989, pp. 1165-1172, vol. 9. No. 3. cited by applicant . Huls, G., et al., Antitumor Immune Effector Mechanisms Recruited by Phage Display-derived Fully Human IgG1 and IgA1 Monoclonal Antibodies, Cancer Research, Nov. 15, 1999, pp. 5778-5784, vol. 59. cited by applicant . Judgement in Preliminary Relief Proceedings of Aug. 14, 2015 with English translation, Case No. C/09/480452/ KG ZA 15-9, 33 pages. cited by applicant . Hoogenboom et al., By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro, J Mol Biol., Sep. 20, 1992, pp. 381-388, vol. 227, Issue 2, Abstract only. cited by applicant . Hoogenboom et al., Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains, Nucl. Acids Res., 1991, pp. 4133-4137, vol. 19, Issue 15. cited by applicant . Japan Patent Office, Final Notice of Reasons for Rejection, Japanese Patent Application No. 2011-516168,dated Jul. 28, 2014, six pages. cited by applicant . Japan Patent Office, Notice of Allowance, Japanese Patent Application No. 2011-516168, dated Apr. 13, 2015, four pages. cited by applicant . Sirac et al., Light chain inclusion permits terminal B cell differentation and does not necessarily result in autoreactivity, PNAS, May 16, 2006, pp. 7747-7752, vol. 103, No. 20. cited by applicant . Isenbruck Bosl Horschler LLP to European Patent Office, Documents filed by Proprietor, Response to the summons to attend oral proceedings scheduled for Oct. 28, 2016 and to the preliminary opinion of the Opposition Division dated Jan. 19, 2016, EP 2147594 / 09075279.1-1405, dated Aug. 26, 2016, 32 pages. cited by applicant . EPO Communication to J A Kemp, Submission in opposition proceedings made following summons to attend oral proceedings, Patent No. EP 2147594, Application No. EP09075279.1, dated Aug. 26, 2016, two pages. cited by applicant . Janeway et al., Chapter 3: Structure of the Antibody Molecule and the Immunoglobulin Genes, ImmunoBiology The Immune System in Health and Disease, Fourth Edition, 1999, pp. 90-108, Elsevier Science Ltd/Garland Publishing. cited by applicant . Declaration of Profressor Anthony DeFranco, European Patent No. 2147594 B1, European Patent Application No. 09075279.1, dated Aug. 24, 2016, 23 pages. cited by applicant . Declaration of Prof. Ton Logtenberg dated Sep. 15, 2015 filed in U.S Appl. No. 13/750,753, four pages. cited by applicant . Sequence Alignment and Declaration of Dr. John McWhirter, European Patent Application No. 09075289.1, European Patent No. 2147594 B1, dated Aug. 2, 2016, four pages. cited by applicant . Lefranc, Marie-Paule, Nomenclature of the Human Immunoglobulin Kappa (IGK) Genes, Exp Clin Immunogenet, 2001, pp. 161-174, vol. 18, Karger. cited by applicant . J A Kemp to European Patent Office, Final Written Submissions Oral Proceedings Scheduled for Oct. 28, 2016, Opposition to Merus N.V.'s EP2147594 dated Aug. 26, 2016, 40 pages. cited by applicant . Second Declaration of Ton Logtenberg Under 37 C.F.R. 1.132, U.S. Appl. No. 13/750,753 dated Dec. 18, 2015, ten pages. cited by applicant . Third Party Observation for application No. EP20120783456, Anonymous, Jun. 16, 2016, three pages. cited by applicant . Opposition Filed Against European Patent No. 2147594 (European Patent Application No. 09075279.1) in the Name of Merus N.V., Declaration of Professor Anthony DeFranco, dated Aug. 24, 2016, 23 pages. cited by applicant . Japan Patent Office, Notice of Reasons for Rejection, Japanese Patent Application No. 2011-516168, dated Oct. 15, 2013, four pages. cited by applicant . Japan Patent Office, Third Party Observation, Japanese Patent Application No. 2011-516168, May 9, 2014, 14 pages. cited by applicant . Third Party Observation for U.S. Appl. No. 15/140,321, filed Sep. 2, 2016, two pages. cited by applicant . Japan Patent Office, Opposition against Patent, JP Patent. No. 5749161, Jan. 15, 2016, 55 pages. cited by applicant . EPO Acknowledgement of receipt--Opposition proceedings in relation to EP09075279.1 dated Aug. 26, 2016, two pages. cited by applicant . Canadian Intellectual Property Office, Office Action, Application No. 2729095, dated Nov. 10, 2015, eight pages. cited by applicant . Borden Ladner Gervais LLP to Canadian Patent Office, Response to Official Action of Nov. 10, 2015, Application No. 2729095, dated May 10, 2016, 12 pages. cited by applicant . Third Party Opposition filed in Canadian Intellectual Property Office, Application No. 2729095, dated Sep. 16, 2015, 15 pages. cited by applicant . Borden Ladner Gervais LLP in the Canadian Patent Office, Voluntary Amendment, Application No. 2729095, dated May 12, 2016, two pages. cited by applicant . Japan Patent Office, Notification of Third Party Observation, Japanese Patent Application No. 2011-516168, May 20, 2014, one page. cited by applicant . Third Party citation for application No. 14163642.3, 3 pages, dated Jan. 29, 2016. cited by applicant . Shiga International Patent Office to Japan Patent Office, Amendments to claims made in response to notice of reasons for rejection, Japanese Patent Application No. 2011-516168, dated Jan. 5, 2015, three pages. cited by applicant . Shiga International Patent Office to Japan Patent Office, Amendments to claims made in response to notice of reasons for rejection, Japanese Patent Application No. 2011-516168, dated Jan. 14, 2014, three pages. cited by applicant . Japan Patent Office, Registration Fee Payment, Japanese Patent Application No. 2011-516168, dated May 13, 2015, one page. cited by applicant . Shiga International Patent Office to Japan Patent Office, Remarks in response to notice of reasons for rejection, Japanese Patent Application No. 2011-516168, dated Jan. 5, 2015, 16 pages. cited by applicant . Shiga International Patent Office to Japan Patent Office, Remarks in response to notice of reasons for rejection, Japanese Patent Application No. 2011-516168, dated Jan. 14, 2014, six pages. cited by applicant . Japan Patent Office, Certificate of Patent, Japanese Patent. No. 5749161, Japanese Application No. 2011-516168, dated May 22, 2015. cited by applicant . Japan Patent Office, As-Filed english language application, Japanese Patent Application No. 2015-097258, dated May 12, 2015, 218 pages. cited by applicant . Japan Patent Office, Request for Substantive Examination, Japanese Patent Application No. 2015-097258, dated Jun. 1, 2015, 1 page. cited by applicant . Japan Patent Office, As-Filed Application, Japanese Patent Application No. 2015-097258, dated May 13, 2015, 270 pages. cited by applicant . Japan Patent Office, Official Action, Japan Patent Application No. 2015-097258, dated Mar. 31, 2016, seven pages. cited by applicant . Canadian Patent Office, Completion Requirement, Submission of Sequence Listing , CA Application No. 2729095, dated Mar. 9, 2011, one page. cited by applicant . Canadian Intellectual Property Office to Blake Cassels & Graydon LLP, Protest Confirmation, Canadian Patent Application No. 2729095, dated Apr. 16, 2014, one page. cited by applicant . Canadian Intellectual Property Office, General Correspondence Form, CA Application No. 2729095, PCT Application No. PCT/NL2009/050381, Dec. 22, 2010, three pages. cited by applicant . Canadian Patent Office, Information Letter, Foreign and non-patent references, CA Applicaiton No. 2729095, Mar. 9, 2011, two pages. cited by applicant . Japan Patent Office, Acknowledgement of receipt, Japanese Patent Application No. 2015-097258, dated May 12, 2015, 1 page. cited by applicant . Canadian Intellectual Property Office to Borden Ladner Gervais LLP, Requisition by the Examiner, CA Application No. 2729095, Jun. 11, 2014, three pages. cited by applicant . Canadian Patent Office, Response to the Office Action dated Jun. 11, 2014, CA Application No. 2729095, Dec. 10, 2014, 24 pages. cited by applicant . Canadian Intellectual Property Office to Borden Ladner Gervais LLP, Requisition by the Examiner, CA Application No. 2729095, Apr. 16, 2013, seven pages. cited by applicant . Canadian Patent Office, Response to the Examiner's Report dated Apr. 16, 2013, CA Application No. 2729095, Oct. 15, 2013, 20 pages. cited by applicant . Canadian Patent Office, Voluntary Amendment , CA Application No. 2729095, dated Dec. 5, 2011, thirteen pages. cited by applicant . Canadian Intellectual Property Office to Borden Ladner Gervais LLP, Advisement of protest filed, CA Application No. 2729095, Nov. 2, 2015, one page. cited by applicant . Canadian Patent Office, Statement and Declaration Under Rule 37, CA Application No. 2729095, Dec. 22, 2010, one page. cited by applicant . Canadian Patent Office, Statement of Support , CA Application No. 2729095, Mar. 9, 2011, one page. cited by applicant . Ritchie et al., Allelic exclusion of control of endogenous immunoglobin gene rearrangement in kappa transgenic mice, Nature, Dec. 1984, pp. 517-520, vol. 312, Nature Publishing Group. cited by applicant . Atwell, S. et al, Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library, J Mol Biol, 1997, 270(1), 26-35. cited by applicant . Baeuerle, PA., Reinhard, C., Bispecific T-cell engaging antibodies for cancer therapy, Cancer Res., 2009, 69(12), 1941-4944. cited by applicant . Bendig, MM., The production of foreign proteins in mammalian cells, Genet Eng., 1988, (7):91-127. cited by applicant . Bogan, AA., Thorn KS., Anatomy of hot spots in protein interfaces, J Mol Biol., 1998, 280(1), 1-9. cited by applicant . Bostrom, J., et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site, Science, 2009, 323(5921), 1610-1614. cited by applicant . Capelle, MA et al., Spectroscopic characterization of antibodies adsorbed to aluminium adjuvants: correlation with antibody vaccine immunogenicity, 2005, Vaccine, 23(14), 1686-1694. cited by applicant . Carter, P. et al, Toward the production of bispecific antibody fragments for clinical applications, J Hematother, 1995, 4(5), 463-470. cited by applicant . Coligan JE, Commonly used detergents, Curr protoc Protein sci, 2001, Appendix 1. cited by applicant . Davies, J. et al., Antibody VH domains as Small Recognition Units, Biotechnology, 1995, 13(5), 475-479. cited by applicant . Davis, JH. et al, SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies, 2010, Protein Eng Des Sel., 23(4), 195-202. cited by applicant . de Kruif, J. et al, Generation of stable cell clones expressing mixtures of human antibodies, 2010, 106(5), 741-750. cited by applicant . de Vries, SJ. et al, The Haddock web server for data- driven biomolecular docking, 2010, 5(5):883-897. cited by applicant . Deisenhofer, Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution, 1981, 20(9), 2361-2370. cited by applicant . Demeule, B. Characterization of protein aggregation: the case of a therapeutic immunoglobulin, Biochim Biophys Acta, 2007, 1774(1),146-153. cited by applicant . Demeule, Detection and characterization of protein aggregates by fluorescence microscopy, 2007, Int J Pharm, 329(1-2):37-45. cited by applicant . Ellerson; JR: et al, Structure and function of immunoglobulin domains. III. Isolation and characterization of a fragment corresponding to the Cgamma2 homology region of human immunoglobin G1, J Immunol., 1976, 116(2), 510-517. cited by applicant . Farnan, D, Moreno GT, Multiproduct high-resolution monoclonal antibody charge variant separations by pH gradient ion-exchange chromatography, Anal Chem, 2009, 81(21), 8846-8857. cited by applicant . Gunasekaran, K. et al., Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG, JBC, 2010, 285(25), 19637-19646. cited by applicant . Hendsch, ZS. et al., Preferential heterodimer formation via undercompensated electrostatic interactions, J Am Chem Soc, 2001,123(6),1264-1265. cited by applicant . Idusogie, EE. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc, 2000, J Immunol., 164(8), 4178-4184. cited by applicant . Ionescu, RM. et al, Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies, 2008, J Pharm Sci., 2008, 97(4), 1414-1426. cited by applicant . Kabat, EA., Wu, TT, Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites, J Immunol., 1991,147(5), 1709-1719. cited by applicant . Kumar, R, Shieh, BH, The second PDZ domain of INAD is a type I domain involved in binding to eye protein kinase C. Mutational analysis and naturally occurring variants, J Biol Chem., 2001, 276(27), 24971-24977. cited by applicant . Lakowicz, Jr, Principles of fluorescence spectroscopy, 2nd edition, Kluwer Academic/Plenum Publisher, 2006. cited by applicant . Lee, B, Richards,FM: The interpretation of protein structures: estimation of static accessibility., J Mol Biol, 1971, 55(3), 379-400. cited by applicant . Marvin, JS. et al., Redesigning an antibody fragment for faster association with its antigen, Biochemistry, 2003,42(23), 7077-7083. cited by applicant . McPhee, F. et al., Engineering human immunodeficiency virus 1 protease heterodimers as macromolecular inhibitors of viral maturation, PNAS, 1996, 93(21),11477-11481. cited by applicant . Merus, Press Release (www.merus.nl), Jan. 7, 2013, 2 pages. cited by applicant . Merus, Press Release (www.merus.nl), Jun. 17, 2013, 3 pages. cited by applicant . Miller S., Protein-protein recognition and the association of immunoglobulin constant domains, J Mol Biol, 1990, 216 (4), 965-973. cited by applicant . Nieba, L. et al., Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment, Protein Eng., 1997, 10(4), 435-444. cited by applicant . Nohaile; MJ. et al., Altering dimerization specificity by changes in surface electrostatics, Proc Natl Acad Sci USA, 2001, 98(6), 3109-3114. cited by applicant . Padlan, EA, X-ray crystallography of antibodies, Adv Protein Chem, 1996, 49, 57-133. cited by applicant . Papadea, EA. and Check, IJ, Human immunoglobulin G and immunoglobulin G subclasses: biochemical, genetic, and clinical aspects, Crit Rev Clin Lab Sci, 1989, 27(1), 27-58. cited by applicant . Raffen, R. et al., Reengineering immunoglobulin domain interactions by introduction of charged residuesProtein Eng., 1998, 11(4), 303-309. cited by applicant . Ridgway, JB. et al., `Knobs-into-holes` engineering of antibody CH3 domains for heavy chain heterodimerization, Protein Eng, 1996, 9(7), 617-621. cited by applicant . Sal-Man, N. and Shai, Y., Arginine mutations within a transmembrane domain of Tar, an Escherichia coli aspartate receptor, can drive homodimer dissociation and heterodimer association in vivo, Biochem J, 2005, 385(Pt 1):29-36. cited by applicant . Schaefer, G. et al, A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies, Cancer Cell, 2011, 20(4), 472-486. cited by applicant . Schiffer, M. et al., Analysis of immunoglobulin domain interactions. Evidence for a dominant role of salt bridges, J Mol Biol, 1988, 203(3),799-802. cited by applicant . Selzer, T. et al., Rational design of faster associating and tighter binding protein complexes, Nat Struct Biol, 2000, 7(7), 537-541. cited by applicant . Sheinerman, FB. et al., Electrostatic aspects of protein- protein interactions, Curr Opin Struc Biol, 2000, 10(2),153-159. cited by applicant . Sinha, N. et al., Differences in electrostatic properties at antibody-antigen binding sites: implications for specificity and cross-reactivity, Biophys. J., 2002, 83(6), 2946-2968. cited by applicant . Sinha, N. and Smith-Gill, SJ., Electrostatics in protein binding and function, Curr Protein Pept Sci, 2002, 3(6),601-614. cited by applicant . Tahallah, N. et al, The effect of the source pressure on the abundance of ions of noncovalent protein assemblies in an electrospray ionization orthogonal time-of-flight instrument, Rapid Commun Mass Spectrom., 2001, 15(8):596-601. cited by applicant . Van Rhenen, A. et al., The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells, Blood, 2007, 110(7), 2659-2666. cited by applicant . Zhao, X., et.al., Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia, Haematologica, 2010, 95(1), 71-. cited by applicant . McGinnes, K., B-lineage colonies from normal, human bone marrow are initiated by B cells and their progenitors, Blood, 1991, 77(5), 961-970. cited by applicant . Mostoslavsky et al., "Asynchronous replication and allelic exclusion in the immune system," Nature (2001) 414:221-225. cited by applicant . Murakami, T. et al, Splenic CD19-CD35+B220+ cells function as an inducer of follicular dendritic cell network formation, Blood, 2007,110(4), 1215-1224. cited by applicant . Murphy, Chapter 6: Antigen Presentation to T Lymphocytes, Janeway's Immunobiology, Eighth Edition, 2012, 31 pages. cited by applicant . Nelson, Al. et al., Development trends for human monoclonal antibody therapeutics, Nat Rev Drug Discov, 2010, 9 (10), pp. 767-774. cited by applicant . Nikolic, T. et al, A subtraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics, Eur J Immunol., 2002, 32(3), 686-692. cited by applicant . O'Brien, RL., Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice, Nature, 1987, 326 (6111), 405-409. cited by applicant . Opponent's submissions filed on Jan. 15, 2016 (oppo JP5749161). cited by applicant . Opponent's (REGN) submissions filed on Oct. 19, 2016 in--AU10. cited by applicant . Orban, PC. et al, Tissue- and site--specific DNA recombination in transgenic mice, Proc Natl Acad Sci U S A, 1992, 89 (15), 6861-6865. cited by applicant . Padlan et al., A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand-binding properties, Mol. Immunot, 1991, pp. 489-498, vol. 28. cited by applicant . Phan, TG., High affinity germinal center B cells are actively selected into the plasma cell compartment, J Exp Med., 2006; 203(11); 2419-2424. cited by applicant . Retter, MW., Nemazee, D., Receptor editing: genetic reprogramming of autoreactive lymphocytes, Cell Biochem Biophys., 1999, 31(1), 81-88. cited by applicant . Roitt, Immunology, Moscow, 2000. cited by applicant . Shaffer, Al. Et al., In vivo occupancy of the kappa light chain enhancers in primary pro- and pre-B cells: a model for kappa locus activation, Immunity, 1997, 6(2), 131-143. cited by applicant . Singer et al., Genes & Genomes A Changing Perspective, University Science Books, Mill Valley, California, 1991, 134-145. cited by applicant . Smith, EJ. et al., A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeysSci Rep., 2015, 5: 17943. cited by applicant . Soriano, P., Generalized lacZ expression with the ROSA26 Cre reporter strain, Nat Genet. 1999;21(1), 70-71. cited by applicant . Submissions filed by applicant on Oct. 19, 2016 in--AU10. cited by applicant . Submissions filed by applicant on Jun. 9, 2016 in--AU10. cited by applicant . Weiner, et al., Fully human therapeutic monoclonal antibodies, Journal of Immunotherapy, Jan. 1, 2006, pp. 1-9, vol. 29, No. 1, Lippincott Williams & Wilkins. cited by applicant . Yang, SY. Et al, Control of gene conversion and somatic hypermutation by immunoglobulin promoter and enhancer sequences, J Exp Med., 2006, 203(13), 2919-2928. cited by applicant . Yarilin, Fundamentals of Immunology, Moscow, 1999. cited by applicant . Yoshio-Hoshino, N. et al., Establishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumor, Cancer Res., 2007, 67(3), 871-875. cited by applicant . Zou, YR. et al, Generation of a mouse strain that produces immunoglobulin kappa chains with human constant regions, Science, 1993, 262(5137), 1271-1274. cited by applicant . Inlay et al., Essential roles of the kappa light chain intronic enhancer and 3' enhancer in kappa rearrangement and demethylation, Nat Immunol., Apr. 22, 2002, pp. 463-468, vol. 3. cited by applicant . Arnold, LW., et al., Development of B-1 cells: segregation of phosphatidyl choline- specific B cells to the B-1 population occurs after immunoglobulin gene expression, J Exp Med., 1994;179(5),1585-1595. cited by applicant . Attaelmannan, M., Understanding and identifying monoclonal gammopathies, Clin Chem., 2000, 46(8 Pt 2), 1230-1238. cited by applicant . Aucouturier et al., Monocloanl Ig L Chain and L Chain V Domain Fragment Crystallization in Myelloma-Associated Fanconi's Syndrome, and Aucouturier et al. Sequence alignment, the Journal of Immunology, 1993, 3561-3568. cited by applicant . Betz, AG. Elements regulating somatic hypermutation of an immunoglobulin kappa gene: critical role for the intron enhancer/matrix attachment region, Cell, 1994, 77(2), 239-248. cited by applicant . Cheong et al., Affinity Enhancement of Bispecific Antibody Against Two Different Epitopes in the Same Antigen, Biochemical and Biophysical Research Communications, vol. 173, No. 3, 1990, pp. 795-800. cited by applicant . Conrath K.E. et al., Emergence and evolution of functional heavy-chain antibodies in Camelidae.Development & Comparative Immunology., 2003, 27(2), 87-103. cited by applicant . Davies, J. Riechmann, L, Antibody VH domains as small recognition units, Biotechnology (N Y), 1995, 13(5), 475-479. cited by applicant . De Chiara 2009, Chapter 16 of Gene Knockout Protocols: 2nd Ed, vol. 530, Humana Press, 311-324. cited by applicant . Decision of UK High Court of Justice (REGN against Kymab Limited; Novo Nordisk) dated Feb. 2, 2016. cited by applicant . Decision of US District Court about U.S. Pat. No. 8,502,018, REGN vs. Menus B.V., dated Feb. 11, 2015. cited by applicant . Decl. Robert Brink (1st) Apr. 2015. cited by applicant . Decl. Robert Brink (2nd) Jun. 2015. cited by applicant . Decl. Robert Brink (4th), Oct. 19, 2016 (--AU10). cited by applicant . Decl. Anthony De Franco (1st) Dec. 2014. cited by applicant . Decl. Anthony De Franco (2nd) Oct. 2015. cited by applicant . Decl. Anthony De Franco (3rd) Apr. 10, 2016 (against--AU10). cited by applicant . Decl. Anthony De Franco (4th) Oct. 18, 2016 (against--AU10). cited by applicant . Decl. Anthony De Franco filed in Aug. 2016 (-EP). cited by applicant . Decl. Christopher Carl Goodnow (1st) Oct. 2015. cited by applicant . Decl. Christopher Carl Goodnow (2nd), Apr. 10, 2016 against--AU10. cited by applicant . Decl. Peter Hudson (1st) May 2015. cited by applicant . Decl. Peter Hudson (2nd) Jun. 2015. cited by applicant . Declaration of Prof. Ton Logtenberg dated Sep. 15, 2015 filed in U.S. Appl. No. 131/50,753, four pages. cited by applicant . Decl. John McWhirter incl. Sequence Alignment filed on Aug. 2, 2016. cited by applicant . Decl. David Tarlinton (2nd) Oct 2015. cited by applicant . Desmet et al., Fast and accurate side-chain topology and energy refinement (Faster) as a new method for protein structure optimization, Proteins, Jul. 1, 2002, pp. 31-43, vol. 48, Issue 1. cited by applicant . Desmet et al., Anchor profiles of HLA-specific peptides: Analysis by a novel affinity scoring method and experimental validation, Proteins, Jan. 1, 2005, pp. 53-69, vol. 58. cited by applicant . Fecteau, JF. et al., A new memory CD27- IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation, J Immunol., 2006, 177(6), 3728-3736. cited by applicant . Gen Bank Acc. No. DQ187586-1 2005. cited by applicant . Gen Bank Acc. No. X59315 (human Ig kappa LC variable region). cited by applicant . Matsuda, F. et al, The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus, J. Exp. Med., 1998, 188 (11), 2151-2162. cited by applicant . Green et al., Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs, Nat. Genet., 1994, pp. 13-21, vol. 7. cited by applicant . Hardy, R., Hayakawa, K., B cell development pathways, Annu Rev Immunol., 2001, 19, 595-621. cited by applicant . Hengstschlager, M. et al, a lambda 1 transgene under the control of a heavy chain promoter and enhancer does not undergo somatic hypermutation., Eur J Immuno1., 1994, 24(7), 1649-1656. cited by applicant . Hoogenboom et al., Selecting and screening recombinant antibody libraries, Nat. Biotechnol., Sep. 7, 2005, pp. 1105-1116, vol. 23. cited by applicant . Jakobovits A., The long-awaited magic bullets: therapeutic human monoclonal antibodies from transgenic mice, Expert Opinion Investigating Drugs, 1998, 7(4), 607-614. cited by applicant . McCafferty; Hoogenboom; Chiswell: Antibody engineering : a practical approach, 1996, Oxford University press. cited by applicant . Jones et al., Replacing the complementarity-determining regions in a human antibody with those from a mouse, Nature, May 29, 1986, pp. 522-525, vol. 321. cited by applicant . Kim, MS. et al., Comparative analyses of complex formation and binding sites between human tumor necrosis factor-alpha and its three antagonists elucidate their different neutralizing mechanisms, J Mol Biol., 2007, 374(5), 1374-1388. cited by applicant . Kitamura D., A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene, Nature, 1991, 350(6317), 423-426. cited by applicant . Klotz, EL. Storb, U, Somatic hypermutation of a lambda 2 transgene under the control of the lambda enhancer or the heavy chain intron enhancer, J Immunol, 1996, 157(10), 4458-4463. cited by applicant . Kontermann,RE, Dual targeting strategies with bispecific antibodies, 2012, mAbs 4(2), pp. 182-197. cited by applicant . Kroesen et al., Bispecific antibodies for treatment of cancer in experimental animal models and man, Department of Clinical Immunology, 1998 pp. 105-129. cited by applicant . Little, M., Recombinant antibodies for immunotherapy, chapter 7; 8; 2009, Cambridge Univ. Press. cited by applicant . Lonberg, N., Human antibodies from transgenic animals, Nat Biotechnol., 2005, 23(9), 1117-1125. cited by applicant . Lonberg, N., Fully human antibodies from transgenic mouse and phage display platforms, Curr Opin Immunol, 2008, 20(4), pp. 450-459. cited by applicant . Macdonald, LE. et al, Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes, Proc Natl Acad Sci USA, 2014, 111(14):5147-5152. cited by applicant . Mao, X. et al., Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain, Blood, 2001, 97(1), 324-6. cited by applicant . Communication of further notices of opposition, Aug. 23, 2016. cited by applicant . Acknowledgement of receipt, Jul. 13, 2016. cited by applicant . Murphy, Statement of, Exhibit Murphy 1, Mar. 18, 2015, Defendant's Exhibit DX145, Case No. 14-CV-1650 (KBF). cited by applicant . Declaration from Professor Allen Bradley in Respect of the opposition to EP2264163 filed by Kymab Limited, dated Jul. 7, 2016, with curriculum vitae. cited by applicant . Statement of Dr. Anne Corcoran dated Jul. 8, 2016 with listing of Literature Cited and curriculum vitae. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Jan. 2, 2016, between Regeneron Pharmaceuticals Inc., Claimant and Kymab Limited and Novo Nordisk A/S, Defendants, Mr. Justice Henry Carr, Approved Judgment. cited by applicant . United States District Court Southern District of New York, Regeneron Pharmaceuticals, Inc., Plaintiff v. Merus B.V., Defendant, 14 Civ. 1650 (KBF) Opinion & Order (Claim Construction) dated Nov. 21, 2014. cited by applicant . United States District Court Southern District of New York, Regeneron Pharmaceuticals, Inc., Plaintiff v. Merus B.V., Defendant, 14 Civ. 1650 (KBF) Opinion & Order dated Nov. 2, 2015. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Oct. 6, 2015, Expert Report of Adrian Francis Stewart, Report relates to a patent owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 2, 2015, Second Expert Report of Adrian Francis Stewart, Report relates to a patent owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 17, 2015, Third Expert Report of Adrian Francis Stewart, Report relates to a patent owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Oct. 6, 2015, First Expert Report of Professor Sir Martin Evans FRS Ph.D., Report relates to a patent owned by Regeneron Pharmaceuticals, Inc., Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . Annex 1 to First Expert Report of Professor Sir Martin Evans FRS Ph.D., undated. cited by applicant . Annex 2 to First Expert Report of Professor Sir Martin Evans FRS Ph.D., undated. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 3, 2015, Second Expert Report of Professor Sir Martin Evans FRS Ph.D., Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 12, 2015, Third Expert Report of Professor Sir Martin Evans FRS Ph.D., Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 3, 2015, Second Witness Statement of A. J. Murphy, Report relates to patent owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 15, 2015, Third Witness Statement of Andrew Joseph Murphy, Report relates to patent owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Oct. 6, 2015, Expert Report of Professor Jonathan Charles Howard, Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 3, 2015, Second Expert Report of Professor Jonathan Charles Howard, Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . In The High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Oct. 6, 2015, First Expert Report of Professor Hiddie L Ploegh, Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . In the High Court of Justice, Chancery Division, Patents Court, Case No. HP-2013-000001/HP-2014-000001 dated Nov. 3, 2015, Second Expert Report of Professor Hiddie L Ploegh, Report relates to patents owned by Regeneron Pharmaceuticals, Inc., EP 1 360 287 and 2 264 163. cited by applicant . Andrew, Simon dated Mar. 2, 2011, 9 pages. cited by applicant . Notice of Opposition with statement and facts dated Oct. 14, 2015. cited by applicant . Abuin et al., Recycling Selectable Markers in Mouse Embryonic Stem Cells, Molecular and Cellular Biology, 6 pages, Apr. 1996, pp. 1851-1856, vol. 16. No. 4, American Society of Microbiology. cited by applicant . Askew et al., Site-Directed Point Mutations in Embryonic Stem Cells: a Gene-Targeting Tag- and Exchange Strategy, Molecular and Cellular Biology, 10 pages, Jul. 1993, pp. 4115-4124 vol. 13, No. 7, American Society of Microbiology. cited by applicant . Bagchi et al., CHD5 Is a Tumor Supp.ressor at Human 1p36, Cell, 17 pages,Feb. 9, 2007, pp. 459-475, vol. 128, No. 3, Elsevier Inc. cited by applicant . Blair et al., The Liberation of Embryonic Stem Cells, PLoS Genetics, 6 pages, Apr. 2011,pp. 1-6, vol. 7, No. 4. cited by applicant . Bolland et al., Antisense intergenic transcription in V (D) J recombination, Nature Immunology, 8 pages, Apr. 25, 2004, pp. 630-637, vol. 5, No. 6, Nature Publishing Group. cited by applicant . Bono et al., VH Gene Segments in the Mouse and Human Genomes, JMB, 3 pages, Sep. 3, 2004, pp. 131-143, vol. 342, No. 1, Elsevier Ltd . . . . cited by applicant . Bradley et al., Embryonic stem cells: proliferation and differentiation, Cell Biology, 6 pages, 1990, pp. 1013-1017, Current Biology Ltd . . . . cited by applicant . Bruggemann, Transgenic Animals: generation and use, 6 pages, 1997, pp. 397-402, OPA. cited by applicant . Chevillard et al., A Three-Megabase Yeast Artificial Chromosome Contig Spanning the C57BL Mouse Igh Locus, The Journal of Immunology, 8 pages, Jun. 1, 2002, pp. 5659-5666 vol. 168, No. 11 The American Association of Immunology. cited by applicant . Ceary et al , Disruption of an imprinted gene cluster by a targeted chromosomal translocation in mice, Nature Genetics, 5 pages, Aug. 20, 2001, pp. 78-82, vol. 29, No. 1, Nature Publishing Group. cited by applicant . Corcoran et al., The interleukin-7 receptor a chain transmits distinct signals for proliferation and differentiation during a lymphopoiesis, The EMBO Journal, 9 pages, Apr. 15, 1996, pp. 1924-1932, vol. 15, No. 8, Oxford University Press. cited by applicant . Davis et al., A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice, Genes & Development, 12 pages, Apr. 7, 1993, pp. 671-682, vol. 7, No. 4, Cold Spring Harbor Laboratory Press. cited by applicant . Deng et al., Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus, Mol Cell Biol., Aug. 12, 1992, 3365-3371, vol. 12, No. 8, Medline. cited by applicant . Ebert et al., The Distal VH Gene Cluster of the Igh Locus Contains Distinct Regulatory Elements with Pax5 Transcription Fator-Dependent Activity in Pro-B Cells, Immunity Articles,13 pages, Feb. 25, 2011, pp. 175-187, vol. 34, No. 2, Elsevier, Inc . . . . cited by applicant . Fedorov et al., A comparison of the germline potential of differently aged ES cell lines and their transfected descendants, Transgenic Research, 9 pages, May 6, 1997, pp. 223-231, vol. 6, No. 3 Chapman & Hall. cited by applicant . Gu, et al., Independent Control of Immunoglobulin Switch Recombination at Individual Switch Regions Evidenced through Cre-loxP Mediated Gene Targeting, Cell, 10 pages, Jun. 18, 1993, pp. 1155-1164, vol. 73, No. 6, Cell Press. cited by applicant . Haines et al., Germline diversity of the expressed BALB/c VhJ558 gene family, Molecular Immunology, 10 pages, May 22, 2001, pp. 9-18 vol. 38, No. 1, Elsevier Science Ltd. . . . cited by applicant . Hansen et al., Large-scale gene trapp.ing in C57BL/6N mouse embryonic stem cells, Genome Research, 10 pages, 2008, pp. 1670-1679, vol. 18, Cold Spring Harbor Laboratory Press. cited by applicant . Herault et al., Engineering chromosomes in mice through targeted meiotic recombination (TAMERE), Nature Genetics, 4 pages, Dec. 20, 1998, pp. 381-384, vol. 20, Nature America Inc . . . . cited by applicant . Hong et al., Long targeting arms do not increase the efficiency of homologous recombination in the beta-globin locus of murine embryonic stem cells, Red Cells, 3 pages, Aug. 15, 2003, pp. 1531-1533, vol. 102, No. 4, The American Society of Hematology. cited by applicant . Huang et al., Association of telomere length with authentic pluripotency of ES/iPS cells, Cell Research, 13 pages, Feb. 2011, pp. 779-792, vol. 21, No. 5. cited by applicant . Johnston et al., Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region, The Journal of Immunology, 13 pages, Apr. 1, 2006, vol. 176, No. 7, The American Society of Immunology. cited by applicant . Joyner, 2007, previously submitted. cited by applicant . Karu et al., Recombinant Antibody Technology, ILAR Journal, 10 pages, 1995, pp. 132-141, vol. 37, No. 3, Oxford Journals. cited by applicant . Keane et al., Mouse genomic variation and its effect on phenotypes and gene regulation, Nature International Weekly Journal of Science, 6 pages, Sep. 15, 2011, pp. 289-294, vol. 477 Macmillan Publishers Limited. cited by applicant . Kuehn et al., A potential animal model for Lesch Nyhan syndrome through introduction of HPRT mutations into mice, Letters to Nature, 4 pages, Mar. 19, 1987, pp. 295-298, vol. 326, Nature Publishing Group. cited by applicant . Kuroiwa et al., Sequential targeting of the genes encoding immunoglobulin .mu. and prion protein in cattle, Nature Genetics, 5 pages, Jun. 6, 2004, pp. 775-780, vol. 36, No. 7, Nature Publishing Group. cited by applicant . Kwaks et al., 2003, previously submitted. cited by applicant . Lee et al., Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery, Nature Biotechnology, 8 days, Mar. 16, 2014, pp. 356-363, vol. 32, No. 4, Nature America Inc . . . . cited by applicant . Lewis et al., A common Human .beta. Globin Splicing Mutation Modeled in Mice, Blood Journal, 5 pages, Mar. 15, 1998, pp. 2152-2156, vol. 91, No. 6, The American Society of Hematology. cited by applicant . Liang et al., Extensive genomic copy number variation in embryonic stem cell, pnas, 4 pages, Nov. 11, 2008, pp. 17453-17456, vol. 105, No. 45, Genetic Society of America. cited by applicant . Lioudmila et al, Global Gene Expression profiling reveals similarities and differences among mouse pluripotent stem cells of different orgin and strains, Development Biology, 14 pages, Oct. 4, 2007, pp. 446-459, vol. 307, No. 2, NIH Public Access. cited by applicant . Liu, et al., Embryonic Lethality and Tumorigenesis Caused by Segmental Aneuploidy on Mouse Chromosome 11,14 pages, Nov. 1998, pp. 1155-1168, vol. 150, No. 3, The Genetic Society of America. cited by applicant . Liu, et al., Trisomy Eight in ES Cells Is a Common Potential Problem in Gene Targeting and Interferes With Germ Line Transmission, Development Dynamics, 7 pages, May 1997, pp. 85-91, vol. 209, No. 1, Wiley Liss, Inc. cited by applicant . MacDonald et al., 2014, previously submitted. cited by applicant . Matzuk et al., a- Inhibin is a tumor-supp.ressor gene with gonadal specificity in mice, Nature, 7 pages, Nov. 26, 1992, pp. 313-319, vol. 360, Nature Publishing Group. cited by applicant . McMahon et al., The Wnt-1 (int-1)Proto-Oncogene Is Required for Development of a Large Region of the Mouse Brain, Cell, 13 pages, Sep. 21, 1990, pp. 1073-1085, vol. 62, No. 6, Cell Press. cited by applicant . Miller, Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis, Mechanisms of Development, 19 pages, Jan. 21, 1999, pp. 3-21, vol. 82, No. 1-2, Elsevier Inc. cited by applicant . Murphy et al., 2014, previously submitted. cited by applicant . Nagy et al., Derivation of completely cell culture-derived mice from early-passage embryonic stem cells, Development Biology, 5 pages, Sep. 15, 1993, pp. 8424-8428, vol. 90, No. 18, Proc Natl Acad Sci U S A . . . . cited by applicant . Nakatani et al., Abnormal Behavior in a Chromosome Engineered Mouse Model for Human 15q11-13 Duplication Seen in Austin, Cell, 12 pages, Jun. 26, 2009, pp. 1235-1246, vol. 137, No. 7, Elsevier Inc. cited by applicant . Nobrega et al., Megabase deletions of gene deserts result in viable mice, Nature, 6 pages, Oct. 21, 2004, pp. 988-993 vol. 431, Nature Publishing Group. cited by applicant . Pawlitzky et al., Identification of a Candidate Regulatory Element within the 5' Flanking Region of the Mouse Igh Locus Defined by Pro-B Cell-Specific Hypersensitivity Associated with Binding of PU.1, Pax5, and E2A, The Journal of Immunology, 13 pages, Jun. 1, 2006, pp. 6839-6851, vol. 176, No. 11, The American Association of Immunology. cited by applicant . Perlot et al., Analysis of Mice Lacking DNasel Hypersensitive Sites at the 5' End of the IgH Locus, PLoS One, 11 pages, Nov. 15, 2010, pp. 1-10, vol. 5, No. 11. cited by applicant . Prosser et al., A resource of vectors and ES cells for targeted deletion of MicroRNAs in mice, Nature Biotechnology, 6 pages, Aug. 7, 2011, pp. 840-845, vol. 29, No. 9, Nature America Inc. cited by applicant . Ramirez-Solis et al., Chromosome engineering in mice, Nature, 5 pages, Dec. 14, 1995, pp. 720-724, vol. 378, Nature Publishing Group. cited by applicant . Reh et al., Gene Targeting by Homologous Recombination, eLS, 11 pages, Apr. 15, 2014, pp. 1-10, vol. 10, No. 2, John Wiley & Sons Ltd . . . . cited by applicant . Retter et al., Sequences and Characterization of the Ig Heavy Chain Constant and Partial Variable Region of the Mouse Strain 129S1, The Journal of Immunology, 9 pages, Aug. 15, 2007, pp. 2419-2427, vol. 179, No. 4, The American Association of Immunologists. cited by applicant . Ringrose et al., Quantitative comparison of DNA Looping in vitro and in vivo: chromatin increases effective DNA flexibility at short distances, The EMBO Journal, 12 pages, Dec. 1, 1999, pp. 6630-6641, vol. 18, No. 23, European Molecular Biology Organization. cited by applicant . Shen et al., A General Method to Modify BACs to Generate Large Recombinant DNA Fragments, Molecular Biotechnology, 6 pages, Nov. 3, 2005, pp. 181-186, vol. 31, No. 3, Humana Press Inc. cited by applicant . Skarnes et al., A conditional knock out resource for the genome--wide study of mouse gene function, Nature, 6 pages, Jun. 16, 2011, pp. 337-342, vol. 474, MacMillan Publishers. cited by applicant . Smith et al., A site-directed chromosomal translocation induced in embryonic stem cells by Cre-IoxP recombination, Nature Genetics, 10 pages, Apr. 1995, pp. 376-385, vol. 9, No. 4, Nature Publishing Group. cited by applicant . Stacy et al., Use of Double Replacement Gene Targeting to Replace the Murine a-Lactalbumin Gene with Its Human Counterpart in Embryonic Stem Cells and Mice, Molecular and Cellular Biology,8 pages, Feb. 1994, pp. 1009-1016, vol. 14, No. 2, American Society for Microbiology. cited by applicant . Storb et al., Ig gene expression and regulation in Ig transgenic mice, Immunoglobin Genes, 19 pages, 1995, pp. 345-363, Elsevier Ltd . . . . cited by applicant . Takahashi et al., Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors, Cell, 4 pages, Aug. 25, 2006, pp. 663-676, vol. 126, No. 4, Elsevier, Inc. cited by applicant . Taki et al., Targeted insertion of a Variable Region Gene into the Immunoglobulin Heavy Chain Locus, Science, 4 pages, Nov. 19, 1993, pp. 1268-1271, vol. 262, No. 5137. cited by applicant . Toyooka et al., Identification and characterization of subpopulations in undifferentiated ES Cell Culture, Development, 10 pages, Mar. 2008, pp. 909-918 vol. 135, No. 5. cited by applicant . Valenzuela et al., High Through put engineering of the mouse genome coupled with high resolution expression analysis, 13 pages, Nature Biotechnology, May 5, 2003, pp. 652-659, vol. 21, No. 6, Nature Publishing Group. cited by applicant . Wallace et al., Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence, Cell, 12 pages, Jan. 12, 2007, pp. 197-209, vol. 128, No. 1, Elsevier Inc . . . . cited by applicant . Wu et al., A protocol for constructing gene targeting vectors: generating knockout mice for the cadherin family and beyond, Natures Protocol, 20 pages, May 29, 2008, pp. 1056-1076, vol. 3, No. 6, Nature Publishing. cited by applicant . Yu et al., A mouse model of Down syndrome trisomic for all human chromosome 21 synthetic regions, Human Molecular Genetics, 12 pages May 12, 2010, pp. 1-12, Oxford University Press. cited by applicant . Zambrowicz et al., Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells, Nature, 4 pages Apr. 9, 1998, pp. 608-611, vol. 392, Nature Macmillan Publishing. cited by applicant . Zheng et al., Engineering a mouse balancer chromosome, Nature Genetics, 4 pages, Aug. 1999, pp. 375-378, vol. 22, No. 4, Nature America Inc. cited by applicant . Opponents Initial Supplementary Submissions, Australia Oct. 5, 2016, 7 pages. cited by applicant . Letter with Fee, Australia, May 18, 2015, 1 page. cited by applicant . Acknowledgment of Receipt of Notice of Opposition from the APO, Jun. 23, 2014, 1 page. cited by applicant . Annexure PH-4 referred to in Peter Hudson Jun. 2, 2015 Declaration, 37 pages--Part 1. cited by applicant . Annexure PH-4 referred to in Peter Hudson Jun. 2, 2015 Declaration, 37 pages--Part 2. cited by applicant . Applicant response to post hearing submission, Australia, Sep. 16, 2016. cited by applicant . Section 27 Notice, Australia, Oct. 31, 2013, 25 pages. cited by applicant . Section 27 Notice, Australia, Mar. 18, 2014, 8 pages. cited by applicant . Applicant request for extension of time, Australia, May 18, 2015, 6 pages. cited by applicant . Lai et al., Mouse Cell Surface Antigens: Nomenclature and Immunophenotyping, The Journal of Immunology, 1998, 9 pages, pp. 3861-3868, The American Association of Immunologists. cited by applicant . Opponent Objects to the Allowability of the Ext, Australia, May 4, 2015, 3 pages. cited by applicant . Applicant Written Submission, Australia, Sep. 6, 2016, 49 pages. cited by applicant . Abedi, M.R. et al., Green, fluorescent protein as a scaffold for intracellular presentation of peptides, Nucleic Acids Res., 1998, 26(2), 623-630. cited by applicant . Akerstrom, B. et al., On the interaction between single chain Fv antibodies and bacterial immunoglobulin-binding proteins, J Immunol Methods., 1994, 177(1-2), 151-163. cited by applicant . Alber, T., Kawasaki, G., Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae, J Mol Appl Genet., 1982, 1(5), 419-434. cited by applicant . Ammerer, G., Expression of genes in yeast using the ADCI promoter, Methods Enzymol., 1983, 101, 192-201. cited by applicant . Antica, M. et al., Thymic stem cells in mouse bone marrow, Blood, 1994, 84(1), 111-117. cited by applicant . Appel RD, et al., A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server, 1994, Trends Biochem. Sci., 19, 258-260. cited by applicant . Aranda, A., Pascual, A., Nuclear hormone receptors and gene expression, Physiol Rev., 2001, 81(3), 1269-1304. cited by applicant . Barbas, CF. et al., Assembly of combinatorial antibody libraries on phage surfaces: the gene III site, Proc Natl Acad Sci U S A, 1991, 88(18), 7978-7982. cited by applicant . Barnes, LM. et al, Characterization of the stability of recombinant protein production in the GS-NS0 expression system, Biotechnol Bioeng., 2001, 73(4), 261-270. cited by applicant . Bebbington, CR. et al, High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker, Biotechnology (N Y), 1992, 10(2), 169-175. cited by applicant . Bell, AC. et al., Insulators and boundaries: versatile regulatory elements in the eukaryotic genome, Science, 2001, 291 (5503), 447-450. cited by applicant . Bertagnolli, M., Herrmann, S., IL-7 supports the generation of cytotoxic T lymphocytes from thymocytes. Multiple lymphokines required for proliferation and cytotoxicity, J Immunol., 1990,145(6), 1706-1712. cited by applicant . Bertagnolli, MM. et al., IL-4- supported induction of cytolytic T lymphocytes requires IL-2 and IL-6, Cell Immunol., 1991, 133(2), 327-341. cited by applicant . Bertagnolli, MM. et al., IL-12 augments antigen-dependent proliferation of activated T lymphocytes, J Immunol., 1992, 149(12), 3778-3783. cited by applicant . Bhardwaj, N. et al., Influenza virus--infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells, J Clin Invest., 1994; 94(2), 797-807. cited by applicant . Binz, H.K. et al., Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins, J Mol Biol., 2003, 332(2), 489-503. cited by applicant . Bode et al. 2001, Int. J. Gene Ther. Mol. Biol. 6:33-46. cited by applicant . Boder, ET., Wittrup, KD., Yeast surface display for screening combinatorial polypeptide libraries, Nat Biotechnol., 1997, 15(6), 553-557. cited by applicant . Bowman, MR. et al., The cloning of CD70 and its identification as the ligand for CD27, J Immunol., 1994, 152(4), 1756-1761. cited by applicant . Brezinsky, SC. Et al., A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods, 2003, 277(1-2),141-155. cited by applicant . Brink MF, et al., Developing efficient strategies for the generation of transgenic cattle which produce biopharmaceuticals in milk, Theriogenology, 2000, 53(1), 139-148. cited by applicant . Broach; JR. et al., Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene, Gene, 1979, 8(1), 121-133. cited by applicant . Chan, A., Mak, TW., Genomic organization of the T cell receptor, Cancer Detect Prev., 1989,14(2), 261-267. cited by applicant . Chesnut, J. et al., Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody, Journal of Immunological Methods, 1996 pp. 17-27. cited by applicant . Clackson, T. et al., Making antibody fragments using phage display libraries, Nature, 1991, 352(6336), 624-628. cited by applicant . Cockett MI, Bet al., High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamate synthetase gene amplification, Biotechnology, 1990, 8(7), 662-667. cited by applicant . Corsaro, CM., Pearson, ML., Enhancing the efficiency of DNA--mediated gene transfer in mammalian cells, Somatic Cell Genet., 1981, 7(5), 603-616. cited by applicant . Darzynkiewicz, Z. et al., Features of apoptotic cells measured by flow cytometry, Cytometry, 1992,13(8), 795-808. cited by applicant . de Vries, P. et al., The effect of recombinant mast cell growth factor on purified murine hematopoietic stem cells, J Exp Med, 1991, 173(5), 1205-1211. cited by applicant . de Jong, G., Mammalian artificial chromosome pilot production facility: large-scale isolation of functional satellite DNA--based artificial chromosomes, Cytometry, 1999, 35(2), 129-133. cited by applicant . Declaration of Joel Martin filed May 18, 2016 in EP2314629B. cited by applicant . Desmyter, A. et al., Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme, Nat Struct Biol., 1996, 3(9), 803-811. cited by applicant . Dumoulin, M. et al., Single-domain antibody fragments with high conformational stability, Protein Sci., 2002, 11(3), 500-515. cited by applicant . Dumoulin, M. et al., A camelid antibody fragment inhibits the formation of amyloid fibrils by human lysozyme, Nature, 2003, 424(6950), 783-788. cited by applicant . Eren, R. et al., Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees, Hepatology, 2000, 32(3), 588-596. cited by applicant . Ezzell, C., Magic bullets fly again, Sci Am., 2001, 285(4), 34-41. cited by applicant . Feige, U. et. al., Anti-interleukin-1 and anti-tumor necrosis factor--alpha synergistically inhibit adjuvant arthritis in Lewis rats, Cell Mol Life Sci., 2000, 57(10), 1457-1470. cited by applicant . Fine, JS. et al., Interleukin-10 enhances gamma delta T cell development in the murine fetal thymus, Cell Immunol., 1994, 155(1), 111-122. cited by applicant . Fishwild DM, et al., High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice, Nat Biotechnol, 1996, 14(7), 845-851. cited by applicant . Frenken, LG. et al., Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae, J Biotechnol., 2000, 78(1), 11-21. cited by applicant . Frykman, S. et al, Quantitating secretion rates of individual cells: design of secretion assays, Biotechnol Bioeng., 1998,59(2), 214-226. cited by applicant . Galy, AH. et al., Delineation of T- progenitor cell activity within the CD34+ compartment of adult bone marrow, Blood, 1995, 85(10), 2770- 2778. cited by applicant . Gan, W. et al, Functional characterization of the internal ribosome entry site of elF4G mRNA, J Biol Chem., 1998, 273 (9), 5006-5012. cited by applicant . Garber, K. Biotech industry faces new bottleneck, Nat Biotechnol., 2001, 19(3), 184-185. cited by applicant . Garnick, RL., Peptide mapping for detecting variants in protein products, Dev Biol Stand., 1992, 76, 117-130. cited by applicant . Garrard, LJ. et al., Fab assembly and enrichment in a monovalent phage display system, Biotechnology (N Y), 1991, 9 (12), 1373-1377. cited by applicant . Gelpi, E., Biomedical and biochemical applications of liquid chromatography-mass spectrometry, J Chromatogr A, 1995, 703(1-2), 59-80, Abstract Only. cited by applicant . Ghetie, M-A., et al., Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells, Proc Natl Acad Sci U S A, 1997, 94(14), 7509-7514. cited by applicant . Gorczyca, W. et al., DNA strand breaks occurring during apoptosis--their early insitu detection by the terminal deoxynucleotidyl transferase and nick translation assays and prevention by serine protease inhibitors, Int J Oncol., 1992, 1(6), 639-648. cited by applicant . Gorczyca, W. et al., Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays, Cancer Res., 1993, 53(8), 1945-1951. cited by applicant . Gorczyca, W. et al., Induction of DNA strand breaks associated with apoptosis during treatment of leukemias, Leukemia, 1993, 7(5), 659-670. cited by applicant . Gorman, C., Bullock, C., Site--specific gene targeting for gene expression in eukaryotes, Curr Opin Biotechnol., 2000, 11(5), 455-460. cited by applicant . Graham, FL., van der Eb,AJ., A new technique for the assay of infectivity of human adenovirus 5 DNA, Virology, 1973, 52(2), 456-467. cited by applicant . Gram, H. et al., In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library, Proc Natl Acad Sci U S A, 1992, 89(8), 3576-3580. cited by applicant . Graslund, T. et al., Integrated strategy for selective expanded bed ion-exchange adsorption and site--specific protein processing using gene fusion technology, J Biotechnol., 2002, 96(1), 93-102. cited by applicant . Gray, F. et al., Secretion capture and report web: use of affinity derivatized agarose microdroplets for the selection of hybridoma cells, J Immunol Methods, 1995, 182(2), 155-163. cited by applicant . Greenberger, JS. et al., Demonstration of permanent factor--dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines, Proc Natl Acad Sci U S A, 1983, 80(10), 2931-2935. cited by applicant . Groeneveld EH., Burger Eh., Bone morphogenetic proteins in human bone regeneration, Eur J Endocrinol., 2000, 142 (1), 9-21. cited by applicant . Grosveld, F., Activation by locus control regions?, Curr Opin Genet Dev., 1999, 9(2),152-157. cited by applicant . Guery, JC, Adorini, L., Dendritic cells are the most efficient in presenting endogenous naturally processed self-epitopes to class II--restricted T cells, J Immunol., 1995, 154(2), 536-544. cited by applicant . Hamers-Casterman, C. et al., Naturally occurring antibodies devoid of light chains, Nature, 1993, 363(6428), 446-448. cited by applicant . Hanes, J. et al., Picomolar affinity antibodies from a fully synthetic naive library selected and evolved by ribosome display, Nat Biotechnol., 2000, 18(12), 1287-1292. cited by applicant . Hanes, J. et al., Selecting and evolving functional proteins in vitro by ribosome display, Methods Enzymol., 2000, 328, 404-430. cited by applicant . Harjunpaa, A., et al, Rituximab (anti- CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms, Scand J Immunol., 2000, 51(6), 634-641. cited by applicant . Hawkins, RE. et al., Selection of phage antibodies by binding affinity. Mimicking affinity maturation, J Mol Biol., 1992, 226(3), 889-896. cited by applicant . Hay, BN. et al., Bacteriophage cloning and Escherichia coli expression of a human IgM Fab, Hum Antibodies Hybridomas, 1992, 3(2), 81-85. cited by applicant . Hiatt A, et al., Production of antibodies in transgenic plants, Nature, 1989, 342(6245), 76-78. cited by applicant . Hitzeman, RA. et al., Isolation and characterization of the yeast 3- phosphoglycerokinase gene (PGK) by an immunological screening technique, J Biol Chem., 1980, 255(24), 12073-12080. cited by applicant . Holmes, P., Al-Rubeai, M., Improved cell line development by a high throughput affinity capture surface display technique to select for high secretors, J Immunol Methods, 1999, 230(1-2), 141-147. cited by applicant . Holt, L.J. et al., Domain antibodies: proteins for therapy, Trends Biotechnol., 2003, 21(11), 484-490. cited by applicant . Hooper, D., "Rabies Virus," In: Manual of Clinical Laboratory Immunology, Part II, 5 ed., N.R. Rose (Ed.), ASM Press, Wash. D.C. pp. 755-760, (1997). cited by applicant . Houshmand, H. et al., Use of bacteriophage T7 displayed peptides for determination of monoclonal antibody specificity and biosensor analysis of the binding reaction, Anal Biochem., 1999, 268(2), 363-370. cited by applicant . Houston, M.E., Jr. et al., Use of a conformationally restricted secondary structural element to display peptide libraries: 3 two- stranded alpha-helical coiled-coil stabilized by lactam bridges, J Mol Biol., 1996, 262(2), 270-282. cited by applicant . Huang, AY. et al., Role of bone marrow-derived cells in presenting MHC class I--restricted tumor antigens, Science, 1994, 264(5161), 961-965. cited by applicant . Huls, G. A., et al., A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments, Nat Biotechnol., 1999, 17(3), 276-281. cited by applicant . Huse, WD. et al., Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda, Science, 1989, 246(4935), 1275-1281. cited by applicant . Hynes, RO., Cell adhesion: old and new questions, Trends Cell Biol., 1999, 9(12), M33-37. cited by applicant . Inaba, K. et al., Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC--restricted T cells in situ, J Exp Med., 1990, 172(2), 631-640. cited by applicant . Inaba, M. et al., Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells, J Exp Med., 1991, 173(3), 549-559. cited by applicant . Itoh, N. et al., The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis, Cell, 1991, 66(2), 233-243. cited by applicant . Jespers LS, et al., Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen, Biotechnology (N Y), 1994, 12(9), 899-903. cited by applicant . Johansson, BM. et al., Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development, Mol Cell Biol., 1995, 15(1), 141-151. cited by applicant . Jonasson, P. et al., Genetic design for facilitated production and recovery of recombinant proteins in Escherichia coli, Biotechnol Appl Biochem., 2002, 35(Pt 2), 91-105. cited by applicant . Jones, D., et al., High-level expression of recombinant IgG in the human cell line PER.CX , Biotechnol Prog, 2003, 19 (1), 163-168. cited by applicant . Keller, G. et al., Hematopoietic commitment during embryonic stem cell differentiation in culture, Mol Cell Biol., 1993, 13(1), 473-486. cited by applicant . Kelley et al, Antigen Binding Thermodynamics and Antiproliferative Effects of Chimeric and Humanized anti-p185HER2 Anitbody Fab Fragments, 1992 Biochemistry 31:5435-5441. cited by applicant . Kim SJ, et al., Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase--mediated gene amplification and their stability in the absence of selective pressure, Biotechnol Bioeng., 1998, 58(1), 73-84. cited by applicant . Klagsbrun, M., D'Amore PA.,Vascular endothelial growth factor and its receptors, Cytokine Growth Factor Rev., 1996, 7(3), 259 270. cited by applicant . Kohler, G., Milstein, C., Continuous cultures of fused cells secreting antibody of predefined specificity, Nature, 1975, 256(5517), 495-497. cited by applicant . Koide, A. et al., The fibronectin type III domain as a scaffold for novel binding proteins, J Mol Biol., 1998, 284(4), 1141-1151. cited by applicant . Koopman G, et al., Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis, The Blood Journal, 1994, pp. 1415-1420. cited by applicant . Korndorfer, IP. et al., Crystallographic analysis of an "anticalin" with tailored specificity for fluorescein reveals high structural plasticity of the lipocalin loop region, Proteins, 2003, 53(1), 121-129. cited by applicant . Korndorfer, IP. et al., Structural mechanism of specific ligand recognition by a lipocalin tailored for the complexation of digoxigenin, J Mol Biol., 2003, 330(2), 385-396. cited by applicant . Kruse PF and Patterson MK (eds) Tissue Culture. Methods and Applications, 1973, Academic Press, New York, no pages provided. cited by applicant . Ku J. et al., Alternate protein frameworks for molecular recognition, Proc Natl Acad Sci U S A, 1995, 92(14), 6552-6556. cited by applicant . Kuhlman, B. et al, Design of a novel globular protein fold with atomic--level accuracy, Science, 2003, 302(5649), 1364-1368. cited by applicant . Letter of Protest filed by Regeneron against U.S. Appl. No. 15/158,543, filed Oct. 14, 2016. cited by applicant . Lobato MN., Rabbitts, TH., Intracellular antibodies and challenges facing their use as therapeutic agents, Trends Mol Med., 2003, 9(9), 390-396. cited by applicant . Lucas, BK. et al, High-level production of recombinant proteins in CHO cells using a dicistronic DHFR intron expression vector, Nucleic Acids Res., 1996, 24(9), 1774-1779. cited by applicant . Macatonia, SE et al, Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro, J Exp Med., 1989,169(4), 1255-1264. cited by applicant . Macatonia, SE. et al., Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+T cells, J Immunol., 1995, 154(10), 5071-5079. cited by applicant . Macejak, DG., Sarnow, P., Internal initiation of translation mediated by the 5' leader of a cellular mRNA, Nature, 1991, 353(6339), 90-94. cited by applicant . Manen, D. et al., A sensitive reporter gene system using bacterial luciferase based on a series of plasmid cloning vectors compatible with derivatives of pBR322, Gene, 1997, 186(2), 197-200. cited by applicant . Marasco, WA., Intrabodies as antiviral agents, Curr Top Microbiol Immunol., 2001, 260, 247-270. cited by applicant . Marks, JD., Deciphering antibody properties that lead to potent botulinum neurotoxin neutralization, Mov Disord., 2004, 19 Suppl 8, S101-108. cited by applicant . Massengale, WT et al., CD20--negative relapse of cutaneous B--cell lymphoma after anti-CD20 monoclonal antibody therapy, J Am Acad Dermatol, 2002, 46(3), 441- 443. cited by applicant . Mattheakis, LC. et al., An in vitro polysome display system for identifying ligands from very large peptide libraries, Proc Nati Acad Sci U S A, 1994, 91(19), 9022-9026. cited by applicant . Mayer, MP., A new set of useful cloning and expression vectors derived from pBlueScript, Gene, 1995, 163(1), 41-46. cited by applicant . McBurney, MW. et al., Evidence for repeat-induced gene silencing in cultured Mammalian cells: inactivation of tandem repeats of transfected genes, Exp Cell Res., 2002, 274(1), 1-8. cited by applicant . McClanahan, T. et al., Hematopoietic growth factor receptor genes as markers of lineage commitment during in vitro development of hematopoietic cells, Blood, 1993, 81(11), 2903-2915. cited by applicant . McConnell, S.J., Hoess, Rh., Tendamistat as a scaffold for conformationally constrained phage peptide libraries, J Mol Biol., 1995, 250(4), 460-470. cited by applicant . Muyldermans, S., Single domain camel antibodies: current status, J Biotechnol., 2001, 74(4), 277-302. cited by applicant . Nair, S. et al., Induction of primary, antiviral cytotoxic, and proliferative responses with antigens administered via dendritic cells, J Virol., 1993, 67(7), 4062-4069. cited by applicant . Nanbru, C. et al., Alternative translation of the proto-oncogene c--myc by an internal ribosome entry site, J Biol Chem., 1997, 272(51), 32061-32066. cited by applicant . Neumann, E., Gene transfer into mouse lyoma cells by electroporation in high electric fields, EMBO J.,1982, 1(7), 841-845. cited by applicant . Nord, K. et al., A combinatorial library of an alpha-helical bacterial receptor domain, Protein Eng., 1995, 8(6), 601-608. cited by applicant . Nord, K. et al., Recombinant human factor VIII-specific affinity ligands selected from phage-displayed combinatorial libraries of protein A, Eur J Biochem., 2001, 268(15), 4269-4277. cited by applicant . Office Action Response in U.S. Appl. No. 12/932,719 dated Aug. 10, 2013 filed in protest against U.S. App. No. 15/158,543. cited by applicant . Office Action Response in U.S. Appl. No. 12/932,719 dated Feb. 27, 2012 filed in protest against U.S. Appl. No. 15/158,543. cited by applicant . Office Action Response in U.S. Appl. No. 12/932,719 dated Nov. 6, 2014 filed in protest against U.S. Appl. No. 15/158,543. cited by applicant . Oh, SK., et al., Homeotic gene Antennapedia mRNA contains 5'--noncoding sequences that confer translational initiation by internal ribosome binding, Genes Dev., 1992, 6(9), 1643-1653. cited by applicant . Patel AK, Boyd, PN., An improved assay for antibody dependent cellular cytotoxicity based on time resolved fluorometry, J Immunol Methods, 1995, 184(1), 29-38. cited by applicant . PI?ckthun, A. et al, In vitro selection and evolution of proteins. In: Adv. Prot. Chem., F.M. Richards et al, Eds, Academic Press, San Diego, 2001, vol. 55, 367-403. cited by applicant . Porgador, A. et al., Bone marrow--generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes, J Exp Med., 1995, 182(1), 255-260. cited by applicant . Rebar, EJ. et al., Phage display methods for selecting zinc finger proteins with novel DNA-binding specificities, Methods Enzymol., 1996, 267, 129-149. cited by applicant . Rees, S. et al, Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein, Biotechniques, 1996, 20(1), 102-4, 106, 108-10. cited by applicant . Reiter, Y. et al., An antibody single--domain phage display library of a native heavy chain variable region: isolation of functional single-domain VH molecules with a unique interface, J Mol Biol., 1999, 290(3), 685-698. cited by applicant . Repp, R. et al., Phase I clinical trial of the bispecific antibody MDX-H210 (anti-FcgammaRI .times. anti-HER- 2/neu) in combination with Filgrastim (G-CSF) for treatment of advanced breast cancer, Br J Cancer, 2003,89(12), 2234-2243. cited by applicant . Riechmann, L., Winter, G., Novel folded protein domains generated by combinatorial shuffling of polypeptide segments, Proc Natl Acad Sci U S A, 2000, 97(18), 10068-10073. cited by applicant . Roitt, I.M. et al., Anti-idiotypes as surrogate antigens: structural considerations, Immunol Today, 1985, 6(9), 265-267. cited by applicant . Rosenberg A., e al., T7Select Phage Display System: A Powerful New Protein Display System Based on Bacteriophage T7, 1996, Innovations 6, 1-6. cited by applicant . Rottgen, P., Collins, J. et al., A human pancreatic secretory trypsin inhibitor presenting a hypervariable highly constrained epitope via monovalent phagemid display, Gene, 1995, 164(2), 243-250. cited by applicant . Sambrook, Fritsch and Maniatis, Molecular Cloning: A Laboratory Manual, 2nd edition, 1989. cited by applicant . Santini, C. et al., Efficient display of an HCV cDNA expression library as C-terminal fusion to the capsid protein D of bacteriophage lambda, J Mol Biol., 1998, 282(1), 125-135. cited by applicant . Schaffitzel, C. et al., Ribosome display: an in vitro method for selection and evolution of antibodies from libraries, J Immunol Methods, 1999, 231(1-2), 119-135. cited by applicant . Schaffitzel,C. et al., In vitro selection and evolution of protein--ligand interactions by ribosome display. In: Protein-Protein Interactions. A Molecular Cloning Manual, E. Golemis, Ed., Cold Spring Harbor Laboratory Press, New York, 2001, pp. 535-567. cited by applicant . Schlehuber et al., Tuning ligand affinity, specificity, and folding stability of an engineered lipocalin variant--a so-called "anticalin"--using a molecular random approach, Biophysical Chemistry 96 (2002) 213-228. cited by applicant . Schoonjans et al., A new model for intermediate molecular weight recombinant bispecific and trispecific antibodies by efficient heterodimerization of single chain variable domains through fusion to a Fab-chain, Biomolecular Engineering 17 (2001) 193-202. cited by applicant . Shields, RL, et al., High resolution mapping of the binding site on human IgGI for FcgRl, FcgRII, FcgRIII and FcRn and design of IgGI variants with improved binding to the FcgR, J Biol Chem., 2001, 276(9), 6591-6604. cited by applicant . Smith, CA., Rennick, DM., Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3, Proc Natl Acad Sci U S A, 1986, 83(6), 1857-1861. cited by applicant . Smith, GP. et al., Small binding proteins selected from a combinatorial repertoire of knottins displayed on phage, J Mol Biol., 1998, 27, 277(2), 317-332. cited by applicant . Spiridon CI, et al., Tartgeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo, Clin Cancer Res., 2002, 8(6), 1720-1730. cited by applicant . Stein, I., et al., Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia, Mol Cell Biol., 1998, 18(6), 3112-3119. cited by applicant . Stijlemans, B. et al., Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm, J Biol Chem., 2004, 279(2), 1256-1261. cited by applicant . Stoneley, M., et al., C-Myc 5' untranslated region contains an internal ribosome entry segment, Oncogene, 1998 , 16 (3), 423-428. cited by applicant . Strelkauskas, AJ. Et al., Human monoclonal antibody: 2. Simultaneous expression of IgG and IgM with similar binding specificities by a human hybrid clone, Hybridoma, 1987, 6(5), 479-487. cited by applicant . Struhl, K. et al., High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules, Proc Natl Acad Sci U S A, 1979, 76(3), 1035-1039. cited by applicant . (Page 1) EPO Form 2906 regarding Patent Application No. 10 186 063.3 dated Jul. 27, 2016, indicating the description needs to be brought in conformity with the claims, 1 page. cited by applicant . (Pages 2-3) EPO Document regarding Patent Application No. 10 186 0633 dated Jul. 27, 2016, Communication pursuant to Article 101(1) and Rule 81(2) to (3) EPC 2 pages. cited by applicant . (Page 4) The communication was printed for and notified to each of the representatives/parties, regarding EP Application 10186063.3, at least as early as Jul. 27, 2016, 1 page. cited by applicant . (Page 5-6) Letter from Isenbruck to the European Patent Office dated Jun. 20, 2016, indicating Ton Logtenberg will not be in attendance at the oral proceedings, 2 pages. cited by applicant . (Page 7) EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated Jun. 13, 2016, 1 page. cited by applicant . (Page 8) EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated Jun. 10, 2016, 1 page. cited by applicant . (Pages 9-61) Deed of Conversion and Amendment of the Articles of Association for Mews B.V. (new name: Merus N. V.), first in Dutch and then in English (Dutch version previously submitted without English translation). cited by applicant . (Page 62) EPO Payment of fees and expenses for EP Application 10186063.3 dated May 27, 2016, 1 page. cited by applicant . (Page 63-64) Letter dated May 27, 2016, accompanying the Deed of Conversion and Amendment, and Form 1010, 2 pages. cited by applicant . (Page 65) EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated May 26, 2016, 1 page. cited by applicant . (Page 66-70) Main Request with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 71-75) Auxiliary Request 1 with annotations, EP Patent. No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 76-80) Auxiliary Request 2, EP Patent. No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted). cited by applicant . (Pages 81-85) Auxiliary Request 3 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 86-90) Auxiliary Request 4 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 91-95) Auxiliary Request 5 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted). cited by applicant . (Pages 96-100) Auxiliary Request 6 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 101-107) Auxiliary Request 7 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, seven pages (previously submitted); (pp. 108-114) Auxiliary Request 8 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, seven pages (previously submitted). cited by applicant . (Pages 115-123) Auxiliary Request 9 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, nine pages (previously submitted); (pp. 124-132) Auxiliary Request 10 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, nine pages (previously submitted); (pp. 133-137) Auxiliary Request 11 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . (Pages 138-142) Auxiliary Request 12 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 143-147) Auxiliary Request 13 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages; (pp. 148-152) Auxiliary Request 14 with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . (Pages 153-157) Auxiliary Request 1, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . (Pages 158-162) Auxiliary Request 2, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages; (previously submitted); (pp. 163-167) Auxiliary Request 4, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 173-177); Auxiliary Request 5, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted). cited by applicant . (Pages 178-182) Auxiliary Request 6, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 183-197) Auxiliary Request 7, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 188-192); Auxiliary Request 8, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted). cited by applicant . (Pages 193-197) Auxiliary Request 9, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 198-202) Auxiliary Request 10, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 203-205) Auxiliary Request 11, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, three pages (previously submitted). cited by applicant . (Pages 206-208); Auxiliary Request 12, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, three pages (previously submitted); (pp. 209-213); Auxiliary Request 13, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 214-218) Auxiliary Request 14, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages. cited by applicant . (Pages 219-225) Logtenberg, Prof. Ton Declaration of, CEO, Merus B.V., dated May 4, 2016, 7 pages (previously submitted); (pp. 226-251) Appeal Brief under 37 C.F.R. .sctn. 41.37 filed by Brenda Herschbach Jarrell, U.S. Appl. No. 13/948,818, filed Jul. 20, 2015, 26 pages with Claims Appendix (previously submitted). cited by applicant . (Pages 252-267) Response to the Summons to attend Oral Proceedings dated Nov. 29, 2015 and in preparation of the Hearing of Jun. 22, 2016, from Isenbruck Bosl Forschler LLP to European Patent Office dated May 20, 2016. cited by applicant . (Pages 268-272) Main Request with annotations, EP Patent No. 2314629B1, Reference No. M70120EPEIN FLZ, May 20, 2016, five pages (previously submitted); (pp. 273-279) EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Brief Communication regarding Oral proceedings on Jun. 22, 2016 at 10:00 in S2.1, EP Application No. 10186063.3 and EP Patent No. 2314629, Apr. 26, 2016, (previously submitted). cited by applicant . (Page 280) Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding the Oral Proceedings on Jun. 22, 2016, EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 16, 2016, one page (previously submitted); (p. 281) EPO communication to Fritz Lahrtz of Isenbruck Bosl Hoschler LLP, Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Jan. 12, 2016, EPO Form 2548 08.13, one page (previsously submitted). cited by applicant . (Page 282) Correspondence from Dr. Fritz Lahrtz of Isenbruck Bosl Forschler LLP to the European Patent Office regarding change of correspondence, EP Application No. 10186063.3 and EP Patent No. 2314629, Jan. 8, 2016, one page (Previously submitted); (p. 283) EPO Acknowledgement of receip, Application No. 10186063.3, Dec. 17, 2015, one page; (previously submitted). cited by applicant . (Page 284-285) EPO Letter accompaning subsequently filed items, Document concerning representation filed by C. M. Jansen of V.O., EP Application No. 10186063.3, Dec. 17, 2015, two pages (previously submited); (pp. 286-287) EPO Communication to J A Kemp, Acknowledgement of receipt of EPO Forms 2310 and 2043, EP Application No. 10186063.3 and EP Patent No. 2314629, Nov. 19, 2015, EPO 2936 08.10, one page; (previously submitted). cited by applicant . (Pages 288-298) EPO Communication regarding opposition, EP Application No. 10186063.3, Nov. 19, 2015 EPO Form 2906 01.91TRI with Consolidated list o documents, 11 pages (previously submitted). cited by applicant . (Pages 299-301) EPO Communication regarding important information concerning oral proceedings, requesting information by Apr. 20, 2016, EPO Form 2043 02.09, three pages (previously submited). cited by applicant . (Page 302-303) EPO Communication in preparation for oral preceeding dated Jun. 22, 2016, EP Application No. 10186063.3, EPO Form 2040, two pages. cited by applicant . (Page 304-305) Summons to Attend Oral Proceedings, EP Application No. 10186063.3, dated Nov. 19, 2015, two pages. cited by applicant . (Page 306) EPO Communication of amended entries concerning the representative (R. 143(1)(h) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 8, 2015, EPO Form 2548, 08.13, one page (previously submitted); (p. 307) Correspondence from C.M. Jansen of V.O. to European Patent Office regarding the Registration of the Association and change of addres, reference No. RvE/E100EPEP, Sep. 29, 2015, one page (previously submitted). cited by applicant . (Page 308) EPO Acknowledgement of Receipt of the submission by the proprietor, EP Application No. 10186063.3 and EP Patent No. 2314629, date of receipt Feb. 24, 2015, one page (previously submitted). cited by applicant . (Pages 309-310) EPO Communicaton regarding Submission in opposition proceedings, Reply of the patent proprietor to the notice(s) of opposition, EP Application No. 1018606.3 and Patent No. 2314629, Oct. 16, 2013, two pages. cited by applicant . (Pages 311-330) Reply to Communication under Rule 79(1) EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Feb. 24, 2015, 20 pages (previously submitted). cited by applicant . (Page 331) EPO Extension of time limit pursuant to Rule 132 EPC, EP Application No. 10186063.3 and EP Patent No. 2314629, Oct. 24, 2014, EPO Form 2944C, 06.12, one page. cited by applicant . (Page 332-335) EPO Communication regarding Submission in opposition proceedings, Request for extension of time, EP Application No. 10186063.3 and U.S. Patent No. 2314629, Oct. 16, 2014, four pages. cited by applicant . (Page 336) EPO Communication of a notice of opposition (R. 79(1) EPC), EP Application No. 10186063.3 and EP Patent No. 2314629, Aug. 22, 2014, EPO Form 2317A, 12.07, one page (previously submitted). cited by applicant . (Page 337) EPO Communication of a notice of opposition EP Application No. 10186063.3 and EP Patent No. 2314629 Jul. 21, 2014, EPO Form 2316, one page. cited by applicant . Roitt, et al., Really Essential Medical Immunology, pp. 23-35, 17 pages. cited by applicant . Takai, Y. et al., Requirement for three distinct lymphokines for the induction of cytotoxic T lymphocytes from thymocytes, J Immunol., 1986,137(11), 3494-3500. cited by applicant . Takai, Y. et al., B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes, J Immunol., 1988, 140(2), 508-512. cited by applicant . Tanha, J. et al.,Selection by phage display of llama conventional V(H) fragments with heavy chain antibody V(H)H properties, J Immunol Methods, 2002, 263(1-2), 97-109. cited by applicant . Teaching of U.S. Appl. No. 12/589,181 (MeMo), submitted in U.S. Appl. No. 12/589,181, filed Jun. 20, 2012. cited by applicant . Thomassen ,Y. et al, Large-scale production of VHH antibody fragments by Saccharomyces cerevisiae, 2002, Enzyme Microb. Technol., 30, 273-278. cited by applicant . Thotakura, NR., Blithe, DL., Glycoprotein hormones: glycobiology of gonadotrophins, thyrotrophin and free alpha subunit, Glycobiology, 1995, 5(1), 3-10. cited by applicant . Toki, J. et al., Analyses of T-cell differentiation from hemopoietic stem cells in the GO phase by an in vitro method, Proc Natl Acad Sci U S A, 1991, 88(17), 7548-7551. cited by applicant . Transue, TR. et al., Camel single--domain antibody inhibits enzyme by mimicking carbohydrate substrate, Proteins, 1998, 32(4), 515-522. cited by applicant . Urlaub, G. et al., Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity, Proc Natl Acad Sci U S A, 1980, 77(7), 4216-4220. cited by applicant . Vagner, S., et al, Alternative translation of human fibroblast growth factor 2 mRNA occurs by internal entry of ribosomes, Mol Cell Biol., 1995, 15(1), 35-44. cited by applicant . Valenzuela, DM., High-throughput engineering of the mouse genome coupled with high-resolution expression analysis, Nat Biotechnol, 2003, 21(6), 652-659. cited by applicant . Van der Vuurst de Vries A, Logtenberg T, Dissecting the human peripheral B-cell compartment with phage display-derived antibodies, Immunology, 1999, 98(1), 55-62. cited by applicant . Wang, G. et al, A T cell--independent antitumor response in mice with bone marrow cells retrovirally transduced with an antibody/Fc-gamma chain chimeric receptor gene recognizing a human ovarian cancer antigen, Nat Med., 1998, 4 (2), 168-172. cited by applicant . Weinberger, O. et al., Cellular interactions in the generation of cytolytic T lymphocyte responses: role of la-positive splenic adherent cells in presentation in H-2 antigen, Proc Natl Acad Sci U S A, 1980,77(10), 6091-6095. cited by applicant . Weinberger, O. et al, Cellular interactions in the generation of cytolytic T lymphocyte responses. Analysis of the helper T cell pathway, Eur J Immunol., 1981, 11(5), 405-411. cited by applicant . WHO Technical Series Report, 1994, vol. 848, p. 8. cited by applicant . Wigler, M. et al.,Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor, Cell, 1978, 14 (3), 725-731. cited by applicant . Wilson TJ, Kola I., The LoxP/CRE system and genome modification, Methods Mol Biol., 2001, 158, 83-94. cited by applicant . Wright A, Morrison SL., Effect of glycosylation on antibody function: implications for genetic engineering, Trends Biotechnol., Jan. 1997;15(1):26-32. cited by applicant . Ye, X., et al., Ultrabithorax and Antennapedia 5' untranslated regions promote developmentally regulated internal translation initiation, Mol. Cell Biol., 1997, 17(3), 1714-17121. cited by applicant . Yelverton E, et al., Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli, Science, 1983, 219(4585), 614-620. cited by applicant . Yoo EM et al., Structural requirements for polymeric immunoglobulin assembly and association with J chain, J Biol Chem., 1999, 274(47), 33771-33777. cited by applicant . Zacharchuk, CM. Et al., Programmed T lymphocyte death. Cell activation- and steroid-induced pathways are mutually antagonistic, J Immunol., 1990, 145(12), 4037-4045. cited by applicant . Zamai et al., Optimal detection of apoptosis by flow cytometry depends on cell morphology, Cytometry, 1993, 14(8), 891-897. cited by applicant . Zou, YR. et al. Cre-loxP-mediated gene replacement: a mouse strain producing humanized antibodies, Curr Biol, 1994 4(12), 1099-1103 cited by applicant . Jun. 20, 2016 Communication of the registration of a transfer or change of name and/or address, 2 pages. cited by applicant . Jun. 16, 2016 General enquiry, 1 page. cited by applicant . Jun. 15, 2016 Search started, 1 page. cited by applicant . May 30, 2016 Annexes in respect of a request for a change, 53 pages. cited by applicant . May 30, 2016 Payment of fees and costs, 1 page. cited by applicant . May 30, 2016 Request for change of name--applicant, 1 page. cited by applicant . Dec. 23, 2015 Communication of amended entries concerning the representative, 1 page. cited by applicant . Dec. 22, 2015 Request for change of applicant's representative, 2 pages. cited by applicant . Dec. 17, 2015 (Electronic) Receipt, 1 page. cited by applicant . Dec. 17, 2015 Letter accompanying subsequently filed items, 1 page. cited by applicant . Dec. 17, 2015 Request for change of applicant's representative, 1 page. cited by applicant . Oct. 8, 2015 Communication of amended entries concerning the representative, 1 page. cited by applicant . Sep. 29, 2015 Request for change of applicant's representative, 1 page. cited by applicant . Apr. 23, 2013 CDS Clean up--amended data concerning the representative for the applicant, 1 page. cited by applicant . Apr. 2, 2013 Document concerning representation, 3 pages. cited by applicant . Jan. 6, 2013 Notification of forthcoming publication, 2 pages. cited by applicant . Oct. 29, 2012 Non-scannable object, 1 page. cited by applicant . Oct. 29, 2012 Reply to the invitation to remedy deficiencies, 2 pages. cited by applicant . Oct. 29, 2012 Sequence listing, 76 pages. cited by applicant . Aug. 31, 2012 Deficiencies in sequence listing, 2 pages. cited by applicant . Aug. 20, 2012 (Electronic) Receipt, 1 page. cited by applicant . Aug. 20, 2012 (Partial) description filed in response to formal objections, 8 pages. cited by applicant . Aug. 20, 2012 Drawings, 79 pages. cited by applicant . Aug. 20, 2012 Letter accompanying subsequently filed items, 1 page. cited by applicant . Jul. 20, 2012 Deficiencies in application documents--annex B and C, 4 pages. cited by applicant . Jul. 9, 2012 Abstract, 1 page. cited by applicant . Jul. 9, 2012 Acknowledgement of receipt of electronic submission of the request for grant of a European patent, 2 pages. cited by applicant . Jul. 9, 2012 Claims, 6 pages. cited by applicant . Jul. 9, 2012 Description, 87 pages. cited by applicant . Jul. 9, 2012 Designation of inventor Daniel, 1 page. cited by applicant . Jul. 9, 2012 Designation of inventor Erwin, 1 page. cited by applicant . Jul. 9, 2012 Designation of inventor Ton, 1 page. cited by applicant . Jul. 9, 2012 Designation of inventor Mark, 1 page. cited by applicant . Jul. 9, 2012 Drawings, 72 pages. cited by applicant . Jul. 9, 2012 Request for grant of a European patent (divisional application), 6 pages. cited by applicant . Oct. 27, 2009 Priority document, 72 pages. cited by applicant . Aug. 8, 2016 Invitation to confirm maintenance of the application and to correct deficiencies in the Written Opinion/amend application, 2 pages. cited by applicant . Jul. 13, 2016 Refund of fees, 1 page. cited by applicant . Jun. 30, 2016 Communication regarding the transmission of the European search report, 1 page. cited by applicant . Jun. 30, 2016 European search opinion, 6 pages. cited by applicant . Jun. 30, 2016 European search report, 9 pages. cited by applicant . Jun. 30, 2016 Information on Search Strategy, 1 page. cited by applicant . Japan, Third Party Observation 2011-516168, 14 pages. cited by applicant . Japan, Argument, Jun. 21, 2016, 15 pages. cited by applicant . Japan, declaration of Ton Logtenberg, Sep. 15, 2015, 5 pages. cited by applicant . Japan, English translation and Opponents counter arguments, 25 pages. cited by applicant . Japan, Declaration of Peter Hudson, Jun. 17, 2016, 15 pages. cited by applicant . Japan, IMGT/LIGM-DB sequence, Jul. 26, 2016, 13 pages. cited by applicant . Japan, Information Sheet for Submitted Publications, 3 pages. cited by applicant . Japan, Notification 084747, 1 pages. cited by applicant . Japan, Opponents Counterargument 2016-700031, 19 pages. cited by applicant . Japan, Notice of Reasons for Revocation, Mar. 17, 2016, 8 pages. cited by applicant . Allen, Ligand-targeted therapeutics in anticancer therapy, Nat. Rev. Cancer, 2002, 2:750-783. cited by applicant . Folkman, Angiogenesis in cancer, vascular, rheumatoid and other disease, J. Nat. Med., 1995, pp. 27-31, vol. 1. cited by applicant . Gerstner et al., Sequence Plasticity in the Antigen-binding Site of a Therapeutic Anti-HER2 Antibody, J. Mol. Biol., Aug. 30, 2002, pp. 851-862, vol. 321, issue 5, Elsevier. cited by applicant . Gluzman, SV40-transformed simian cells support the replication of early SV40 mutants, Cell, Jan. 1981, pp. 175-182, vol. 23, Issue 1. cited by applicant . Hoogenboom et al., By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro, J Mol Biol., Sep. 20, 1992, pp. 381-388, vol. 227, Issue 2. cited by applicant . Hoogenboom, Designing and optimizing library selection strategies for generating high-affinity antibodies, Trends Biotechnol., 1997, pp. 62-70, vol. 15, Issue 2. cited by applicant . Kaufman et al., Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary DNA gene, J. Mol. Biol., Aug. 25, 1982, pp. 601-621, vol. 159, Issue 4. cited by applicant . Bitter et al., Expression and Secretion Vectors for Yeast, in Methods in Enzymology, Ed. Wu & Grossman, Acad. Press. N.Y. 153:516 544 (1987). cited by applicant . Cherrington et al., New paradigms for the treatment of cancer: The role of anti-angiogenesis agents, Adv. Cancer. Res., 2000, pp. 1-38, vol. 79. cited by applicant . Zhu et. al., Inhibition of Tumor Growth and Metastasis by Targeting Tumor-Associated Angiogenesis with Antagonists to the Receptors of Vascular Endothelial Growth Factor, Invest. New Drugs, Aug. 1999, pp. 195-212, vol. 17, Issue 3. cited by applicant . EP, Instructions to the EPO to amend the application, Sep. 29, 2014, 7 pages. cited by applicant . Votice of Third Party Submission filed with the U.S. Patent Office on Aug. 29, 2016 in U.S. Appl. No. 15/140,321, 2 pages. cited by applicant . Concise Description of Relevance in Third Party Submission filed with the U.S. Patent Office on Aug. 29, 2016 in U.S. Appl. No. 15/140,321, 46 pages. cited by applicant . Concise Description of Relevance in Third Party Submission filed with the U.S. Patent Office on Aug. 29, 2016 in U.S. Appl. No. 15/140,321, 6 pages. cited by applicant . Third-Party Submission filed with the U.S. Patent Office on Aug. 29, 2016 in U.S. Appl. No. 15/140,321, 4 pages. cited by applicant . Documents listed in the Third-Party Submission include the following: U.S. Pat. No. 7,262,028 (previously submitted); Merchant et al., 1998 (previously submitted); Declaration of Dr. Joel Martin executed May 18, 2016 (previously submitted); U.S. Pat. No. 9,248,182 (previously submitted); WO 1998/050431 (previously submitted); Carter, 2001; WO 1999/045962 (previously submitted); Ritchie et al., 1984 (previously submitted); WO 02/066630 (previously submitted). cited by applicant . Canadian Intellectual Property Office--office action for Application No. 2,729,095 held by Merus B.V. dated Nov. 10, 2015 listing references considered: D8--Sirac et al., 2006 (previously submitted); D10--WO 2006/117699 (previously submitted); D12--WO 2004/106375 (previously submitted); D13--WO 02/066630 (previously submitted); D14--US 2007/0280945 (previously submitted). cited by applicant . D15-WO 2008/076379 (previously submitted); D16-WO 2008/054606 (previously submitted); D17--DeFrancesco et al., 2007 (listed separately below); D18--Scott, et al., 2007 (previously submitted); D19--Nagle, 2007 (previously submitted); Examination Search Report lists Family Members EP2147594B1 and AU2009263082B9. cited by applicant . DeFrancesco et al., Big Pharma vies for mice, Nature Biotechnology, 25/6, pp. 613-614, Jun. 2007. cited by applicant . Response to office action for Canadian Application No. 2,729,095 dated May 10, 2016, 12 pages. cited by applicant . Third-Party Opposition dated Sep. 16, 2015, for Canadian Application No. 2,729,095, and Protest and Submission of Prior Art, which lists the following documents D8, D9, D10, D11, D12, D13, D14, D15, D16, D17, D18, D19, D20. cited by applicant . The Third-Party Opposition of Sep. 16, 2015, indicates the following attachments: 1) Second Protest (13 pages); 2) D8--Sirac et al., 2006, 9 pages (previously submitted); 3) D9--US20060015957 (299 pages); 4) D10--WO 2006117699 (79 pages) (previously submitted); 5) D11--WO 2004009618 (186 pp.); 6) D12--WO 2004106375 (189 pages) (previously submitted); 7) D13--WO 20066630 (74 pages) (previously submitted). cited by applicant . 8) D14--US 20070280945 (71 pages) (previously submitted); 9) D15--WO2008076379 (37 pages) (previously submitted); 10) D16--WO 2008054606 (30 pages) (previously submitted); 11) D17--New in Brief 2007 (2 pages) (previously submitted); 12) D18--Scott et al., 2007 (3 pages.) (previously submitted); 13) D19--Nagle et al., 2007 (2 pages) (previously submitted) and 14) D20--Sirac et al., 2011 (15 pages) (previously submitted). cited by applicant . Voluntary Amendment filed by Borden Ladner Gervais LLP dated May 12, 2016 in Canadian Application No. 2,729,095, 2 pages. cited by applicant . Correspondence from the Canadian Intellectual Property Office in Canadian Application No. 2,729,095 to Borden Ladner Gervais LLP dated Apr. 16, 2014, advising that a protest has been filed by Blake Cassels & Graydon LLP, 1 page. cited by applicant . Correspondence from the Canadian Intellectual Property Office in Canadian Application No. 2,729,095 to Blake, Cassels & Graydon LLP dated Apr. 16, 2014, regarding filed protest, 1 page. cited by applicant . Protest and Submission of Prior Art submitted by Blake, Cassels & Graydon LLP dated Apr. 8, 2014, indicates the following attachments: 1) Protest and Submission of Prior Art (13 pages); 2) D8--Sirac et al., 2006, 9 pages (previously submitted); 3) D9--Aucouturier et al. (8 pages) (previously submitted); D10--GenBank M87478 (1 page) (previously submitted); D11--Sequence Alignment of GenBank (7 pages) (previously submitted); D12--de Wildt (7 pages) (previously submitted); D13--US 20060015957 (299 pages) (previously submitted); D14--WO 2004106375. cited by applicant . D17--WO 9850431 (70 pages) (previously submitted); D18--WO 02066630 (74 pages) (previously submitted); D19--JS 20070280945 (71 pages.) (previously submitted); D20--WO 2008076379 (37 pages) (previously submitted); D21--WO 2008054606 (30 pages) (previously submitted); D22--NIB 2007 (2 pages) (previously submitted); 23--Scott et al., 2007 (3 pages) (previously submitted); and D24--Nagle et al., 2007 (2 pages) (previously submitted). cited by applicant . Protest and Submission of Prior Art submitted by Blake, Cassels & Graydon LLP dated Sep. 16, 2015, indicates the following attachments: D8--Sirac et al., 2006, 9 pages (previously submitted); D9--US 20060015957 (299 pages) (previously submitted); D10--WO 2006117699 (previously submitted); D11--WO 2004009618 (previously submitted); D12--WO 2004106375 (previously submitted). cited by applicant . D13--WO 02066630 (previously submitted); D14--US 20070280945 (previously submitted); D15--WO 2008076379 (previously submitted); D16--WO 2008054606 (previously submitted); D17--News in Brief Article (previously submitted); D18--Scott, 2007 (previously submitted); D19, Nagle, 2007 (previously submitted); D20, Sirac et al., 2011. cited by applicant . Waterhouse et al., Combinatorial infection and in vivo recombination: strategy for making large phage antibody repertoires, Nucleic Acids Research 21(9), 1993, pp. 2265-2266. cited by applicant . Houldsworth et al., Comparative Genomic Hybridization: An Overview, American Journal of Pathology, vol. 145, Dec. 1994. cited by applicant . Jessen et al (1998) Modification of bacterial artificial chromosomes through Chi-stimulated homologous recombination and its application in zebrafish transgenesis. Proc. Natl. Acad. Sci. USA 95:5121-5126. cited by applicant . Muyrers et al., Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Research, 1999, 27(6):1555-1557. cited by applicant . Narayanan et al., Efficient and precise engineering of a 200 kb beta-globin human/bacterial artificial chromosome in E. coli DH1OB using an inducible homologous recombination system, Gene Therapy, 1999, 6:442-447. cited by applicant . Soukharev et al., Segmental genomic replacement in embryonic stem cells by double lox targeting, Nucleic Acids Research, 1999, pp. e21, vol. 27, No. 18. cited by applicant . Appeal Briefs filed with the U.S. Appl. No. 13/948,818 at least as early as Jul. 17, 2015, available on the U.S. Patent Office website. cited by applicant . U.S. Appl. No. 60/244,665, filed Oct. 31, 2000, available on the U.S. Patent Office website. cited by applicant . Chu, 66 F.3d, 292--Case Summary and Opinion; United States Court of Appeals for the Federal Circuit, Sep. 14, 1995. cited by applicant . Moldenhauer et al., Bispecific antibodies from hybrid hybridoma, in R.E. Kontermann (ed): Bispecific antibodies, Berlin Heidelberg, Springer Verlag, 2011, pp. 29-46. cited by applicant . Staerz et al., Hybrid hybridoma producing a bispecific monoclonal antibody that can focus effector T-cell activity, Proc Natl Acad Sci U S A, 1986, vol. 83(5) pp. 1453-1457. cited by applicant . Description of relevance of Third Party Submission in U.S. Appl. No. 15/140,321 dated Feb. 10, 2017. cited by applicant . Description of relevance of Third Party Submission in U.S. Appl. No. 15/090,505 dated Feb. 24, 2017. cited by applicant . Materials from examination--submission in response to attend oral proceedings of a European Patent Application No. 09075279.1, Apr. 23, 2013. cited by applicant . Online Response of Regeneron Pharmaceuticals, Inc. for European Patent Application No. 12173456.0 dated Apr. 12, 2013. cited by applicant . Janeway's Immunobioloby, Murphy, Travers, Walport eds, Seventh Edition, 2008 (pp. 266-267). cited by applicant . Janeway's Immunobiology, Murphy, Travers, Walport eds, Seventh Edition, 2008 (pp. 144-155). cited by applicant . Mead G.P. et al., Poster, Detection of Bence Jones myeloma and monitoring of myeloma chemotherapy using immunoassays specific for free immunoglobulin light chains, Clinical Laboratory, 2003, vol. 49, No. 1-2, 2003, p. 25-27. cited by applicant . Roebroek, Anton J. et al., Mutant Lrp1 Knock-In mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain on LRP1 for normal fetal development, Molecular and Cellular Biology, 2006, vol. 26, No. 2, p. 605-616. cited by applicant . Shmerling, D. et al., Strong and ubiquitous expression of transgenes targeted into the beta-actin locus by Cre/lox cassette replacement, Genesis: The Journal of Genetics and Development, 2005, vol. 42, No. 4, p. 229-235. cited by applicant . Toledo, F, et al., RMCE-ASAP: a gene targeting method for ES and somatic cells to accelerate phenotype analyses, Nucleic Acids Research, 2006, vol. 34 No. 13, pp. e92-1. cited by applicant . Brief Communication regarding EP Application 10186063.3, dated Oct. 24, 2014, 1 page. cited by applicant . Communication of further notices of opposition pursuant to Rule 79(2) EPC, dated Aug. 22, 2014, 1 page. cited by applicant . Correspondence from S. van Doom of Vereenigde to the European Patent Office in response to the communication pursuant to Article 94(3) EPC, European Patent Application No. 09075279.1, Dec. 22, 2011, five pages. cited by applicant . Correspondence from S. van Doom to European Patent Office regarding request to hold application, EP Application No. 09075279.1, Sep. 3, 2013, one page. cited by applicant . Correspondence from S.T. van Doom of V.O. to European Patent Office in response to Communication under Rule 79 (1) EPC, EP Application No. 09075279.1 and Patent. No. 2147594, Apr. 2, 2015, 32 pages. cited by applicant . Correspondence from S.T. van Doom to European Patent Office regarding written submissions in response to the summons to attend oral proceedings dated Mar. 6, 2013, EP Application No. 09075279.1, Apr. 23, 2013, 15 pages. cited by applicant . Correspondence from S.T. van Doom of V.O. to European Patent Office regarding in vivo data, EP Application No. 09075279.1, Jun. 13, 2013, one page. cited by applicant . Correspondence from S.T. van Doom to European Patent Office regarding written submissions filed Apr. 23, 2013, EP Application No. 09075279.1, Apr. 24, 2013, one page. cited by applicant . Correspondence from T.J. Elmore of V.O. to European Patent Office regarding request for extension of time, EP Application No. 09075279.1 and Patent. No. 2147594, Oct. 16, 2014, one page. cited by applicant . Cvetkovic, B., et al., "Appropriate Tissue- and Cell-specific Expression of a Single Copy Human Angiotensinogen Transgene Specifically Targeted Upstream of the Hprt Locus by Homologous Recombination," Journal of Biological Chemistry 275(2):1073-1078, American Society for Biochemistry and Molecular Biology, United States (Jan. 2000 ). cited by applicant . Dammacco F., et al., "Immunoglobulin Secretion by Peripheral Blood and Bone Marrow B Cells in Patients With Multiple Myeloma. Studies by the Reverse Haemolytic Plaque Assay," Clinical & Experimental Immunology 57(3):743-751, Blackwell Scientific Publications, England (Sep. 1984). cited by applicant . David Power of J A Kemp communication to EPO, Executed Acknowledgement, EP Application No. 09075279.1 and EP Patent No. 2147594, Mar. 22, 2016, EPO Form 2936 08.10, one page. cited by applicant . Davies, N.P., et al., "Creation of Mice Expressing Human Antibody Light Chains by Introduction of a Yeast Artificial Chromosome Containing the Core Region of the Human Immunoglobulin Kappa Locus," Biotechnology 11(8):911-914, Nature Publishing Group (Aug. 1993). cited by applicant . De Graaf, M., et al., "Expression of scFvs and scFv Fusion Proteins in Eukaryotic Cells," Antibody Phage Display Methods and Protocals 178:379-387, Methods in Molecular Biology (2002). cited by applicant . De Haard, H.J., et al., "A Large Non-immunized Human Fab Fragment Phage Library That Permits Rapid Isolation and Kinetic Analysis of High Affinity Antibodies," Journal of Biological Chemistry 274(26):18218-18230, American Society for Biochemistry and Molecular Biology, United States (Jun. 1999). cited by applicant . De Kruif, J., et al., "Selection and Application of Human Single Chain Fv Antibody Fragments from a Semi-Synthetic Phage Antibody Display Library with Designed CDR3 Regions," Journal of Molecular Biology 248(1):97-105, Elsevier, England (Apr. 1995). cited by applicant . Decl. Andrew Murphy in the Matter of Australian Patent Application No. 2009263082 (the Opposed Application) in the name of Merus B.v. (the Applicant)--and--Opposition thereto by RegeneronPharmaceuticals, Inc. (the Opponent, Dated Dec. 19, 2014, 18 pages. cited by applicant . Declaration of Prof. Ton Logtenberg, CEO, Merus B.V., European Patent No. EP 2 314 629 B1, May 4, 2016, seven pages. cited by applicant . Deed of Conversion and Amendment of the Articles of Association for Merus BV (new name: Merus N.V.), May 19, 2016, 27 pages. cited by applicant . Deficiencies in application documents dated Jul. 20, 2012, EP12175544.1. cited by applicant . Description dated Jul. 9, 2012, EP12175544. cited by applicant . Designation of Inventor Bout Abraham, User Reference No. P61090EP20, at least as early as Oct. 1, 2010, 1 page. cited by applicant . Designation of Inventor Brus Ronald Hendrik Peter, User Reference No. P61090EP20, at least as early as Oct. 1, 2010, 1 page. cited by applicant . Designation of inventor Erwin Houtzager dated Jul. 9, 2012, EP12175544. cited by applicant . Designation of Inventor Logtenberg Ton, User Reference No. P61090EP20, at least as early as Oct. 1, 2010, 1 page. cited by applicant . Designation of inventor Mark Throsby dated Jul. 9, 2012, User Reference: P85261EP10, EP12175544. cited by applicant . Designation of inventor Pinto Rui Daniel dated Jul. 9, 2012, User Reference P85261EP10, EP12175544. cited by applicant . Designation of inventor Ton Logtenberg dated Jul. 9, 2012, User Reference: P85261EP10, EP12175544. cited by applicant . Designation of Inventor Van Berkel Patricius Hendrikus, User Reference No. P61090EP20, at least as early as Oct. 1, 2010, 1 page. cited by applicant . Desmet J., et al., "Computation of the Binding of Fully Flexible Peptides to Proteins With Flexible Side Chains," The FASEB Journal 11(2):164-172, The Federation, United States (Feb. 1997). cited by applicant . Desmet J., et al., "The Dead-end Elimination Theorem and Its Use in Protein Side-chain Positioning," Nature 356(6369):539-542, Nature Publishing Group, England (Apr. 1992). cited by applicant . Dietzschold, B., et al., "Delineation of Putative Mechanisms Involved in Antibody-Mediated Clearance of Rabies Virus From the Central Nervous System," Proceedings of the National Academy of Sciences of the United States of America 89(15):7252-7256, National Academy of Sciences, United States (Aug. 1992). cited by applicant . Dinnyes et al., "Somatic Cell Nuclear Transfer: Recent Progress and Challenges", Cloning and Stem Cells, vol. 4, No. 1, 2002, pp. 81-90. cited by applicant . Documents titled Oppostion to Merus B.V.'s EP 2 314 629 B1 Consolidated List of Documents filed by All Parties, listing of US patents and applications, foreign patents and non-patent literature, at least as early as Jun. 6, 2016, 1 page, (all documents on the Consolidated List have been or are being submitted on Information Disclosure Statements in the currently pending U.S. Patent Application). cited by applicant . Drawings, at least as early as Oct. 1, 2010, 33 pages. cited by applicant . Drawings continued dated Jul. 9, 2012, EP12175544. cited by applicant . Drawings dated Aug. 20, 2012, EP12175544.1. cited by applicant . Drew Murphy Statement dated Sep. 8, 2015, (5 page). cited by applicant . ECACC deposit, Deposit Ref. 96022940 dated Feb. 29, 1996. cited by applicant . ECACC deposit, Deposit Reference 03041601 dated Apr. 16, 2003. cited by applicant . Eggan, K., et al., "Hybrid Vigor, Fetal Overgrowth, and Viability of Mice Derived by Nuclear Cloning and Tetraploid Embryo Complementation," Proceedings of the National Academy of Sciences of the United States of America 98(11):6209-6214, National Academy of Sciences, United States (May 2001). cited by applicant . Eigenbrot, C., et al., "X-ray Structures of the Antigen-binding Domains From Three Variants of Humanized Anti-p185her2 Antibody 4d5 and Comparison With Molecular Modeling," Journal of Molecular Biology 229(4):969-995, Elsevier, England (Feb. 1993). cited by applicant . English translation of Deed of Conversion and Amendment of the Articles of Association for Merus BV (new name: Merus N.V.), May 19, 2016, 26 pages. cited by applicant . EP Acknowledgement of Receipt for EP Application No. 10186063.3, Sep. 6, 2013, 1 page. cited by applicant . EP Acknowledgement of Receipt for Request for Grant of EP Application No. 10186063.3, Oct. 1, 2010, 2 pages. cited by applicant . EPO Acknowledgement of receipt for EP Application 10186063.3, dated Jun. 6, 2016, 2 pages. cited by applicant . EPO Acknowledgement of receipt for EP Application 10186063.3, dated May 20, 2016, 2 pages. cited by applicant . EPO Acknowledgement of receipt for EP Application 10186063.3, dated Nov. 27, 2015, 1 page. cited by applicant . EPO Acknowledgement of receipt for EP Application 10186063.3 regarding submission in opposition proceedings, lated Nov. 27, 2015, 2 pages. cited by applicant . EPO Application No. 10186063.3, dated Jun. 6, 2016, Letter accompanying subsequently filed items, including the following: 1) comments on patentees subs., 2) consolidated document list, 3) Phelps, 4)Fussenegger, 5) Tada, and 6) Verma (non-patent literature documents previously submitted individually). cited by applicant . EPO Application No. 10186063.3, dated Jun. 6, 2016, Letter accompanying subsequently filed items, including the following: 1) Final Written Submissions for Oral Proceedings Scheduled for Jun. 22, 2016; 2) Huls; 3) Jones; 3) U.S. Pat. No. 9,248,182; 3) PCT Publication WO 02/18948 A2 ; PCT Publication WO 00/63403 ; (US Patent, PCT Publications and Non-patent literature documents previously submitted individually). cited by applicant . EPO Brief Communication regarding EP Application 10186063.3, dated Feb. 27, 2015, 1 page. cited by applicant . EPO Brief Communication regarding EP Application 10186063.3, dated Jan. 12, 2016, 1 page. cited by applicant . EPO Brief Communication regarding EP Application 10186063.3, dated Jun. 7, 2016, 1 page. cited by applicant . EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated Jun. 13, 2016 regarding Oral proceedings on Jun. 22, 2016, 1 page. cited by applicant . EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated Jun. 21, 2016 regarding Oral proceedings on Jun. 22, 2016, 1 page. cited by applicant . EPO Brief Communication regarding the Opposition against EP Application 10186063.3, dated May 31, 2016 regarding Oral proceedings on Jun. 22, 2016. cited by applicant . EPO Communication of amended entries concerning the representative, regarding EP Application 10186063.3, dated Jan. 12, 2016, 1 page. cited by applicant . EPO Communication, Payment of fees and expenses, EP Application No. 09075279.1, May 30, 2016, EPO Form 1010 03.15, one page. cited by applicant . EPO Communication regarding EP Application 10186063.3, dated Jun. 7, 2016, 3 page. cited by applicant . EPO Summons to attend oral proceedings pursuant to Rule 115(1) EPC, dated Nov. 19, 2015. cited by applicant . Kruif, D.J., et al., "Human Immunoglobulin Repertoires Against Tetanus Toxoid Contain a Large and Diverse Fraction of High-affinity Promiscuous V(H) Genes," Journal of Molecular Biology 387(3):548-558, Elsevier, England (Apr. 2009). cited by applicant . Kruif, J.D., et al., "Rapid Selection of Cell Subpopulation-Specific Human Monoclonal Antibodies from a Synthetic Phage Antibody Library," Proceedings of the National Academy of Sciences 92(9):3938-3942, National Academy of Sciences, United States (Apr. 1995). cited by applicant . McCafferty, et al., Antibody Engineering: A Practical Approach (Practical Approach Series), vol. 169, Irl Press (1996). cited by applicant . Nemazee, D.,, "Receptor Editing in B Cells," Advances in immunology 74:89-126, Academic Press, United States (2000). cited by applicant . Office Action dated Aug. 27, 2014, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 20 pages. cited by applicant . Office Action dated Mar. 11, 2014, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 14 pages. cited by applicant . Office Action dated May 2, 2012, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 11 pages. cited by applicant . Office Action dated May 5, 2015, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 15 pages. cited by applicant . Office Action dated May 9, 2013, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 11 pages. cited by applicant . Office Action dated Sep. 27, 2011, in U.S. Appl. No. 12/932,719 Hendrikus Van Berkel. et al., filed Mar. 4, 2011, 7 pages. cited by applicant . Office Action dated Aug. 21, 2017, in U.S. Appl. No. 15/090,505 Hendrikus Van Berkel. et al.,filed Apr. 4, 2016, 17 pages. cited by applicant . Office Action dated May 1, 2018, in United States Patent Application No. 15/090,505 Hendrikus Van Berkel. et al.,filed Apr. 4, 2016, 17 pages. cited by applicant . Office Action dated Feb. 15, 2018, in U.S. Appl. No. 15/855,258 Hendrikus Van Berkel. et al.,filed Dec. 27, 2017 19 pages. cited by applicant . US. Appl. No. 15/140,321, Method for Selecting a Single Cell Expressing a hetergeneous combination of antibodies, filed Apr. 27, 2016. cited by applicant . Beaudette-Zlatanova, B.C., et al., "B Cells and Dendritic Cells from VK8 Light Chain Transgenic Mice Activate MRL-lpr/gld CD4+ T Cells," J. Immunol 177:45-52, American Association of Immunologists, United States (2006). cited by applicant . Marvin, J.S., et al., Acta Pharmacologica Sinica, 16(6): 649-658, 2005. cited by examiner . Ngo, T.-H., et al, FEBS Letters, 416: 373-376, 1997. cited by examiner . Lang, A.B. et al, Journal of Immunology, 151(13): 466-472, 1993. cited by examiner . Huls, G., et al. Cancer Research, 59: 5778-5784, 1999. cited by examiner . Roholt et al, Antibodies of Limited Heterogeneity: L. Chains of a Single Mobility, Immunochemistry, Pergamon Press, 1970, vol. 7, pp. 329-340. cited by applicant . Morimoto et al., Abstract, High level expression of a human rabies virus-neutralizing monoclonal antibody by a rhabdovirus-based vector, J. Immunol. Methods, Jun. 2001, pp. 199-206, vol. 1, No. 252(1-2). cited by applicant . Arai et al., Abstract, Antibody responses induced by immunization with a Japanese rabies vaccine determined by neutralization test and enzyme-linked immunosorbert assay, Vaccine, Jun. 2002, pp. 2448-2453, vol. 7, No. 20(19-20). cited by applicant . Perrin et al., Abstract, In vitro rabies vaccine potency appraisal by ELISA: advant of the immunocapture method with a neutralizing anti-glycoprotein monoclonal antibody, Biologicals, Oct. 1990, pp. 321-330, vol. 18(4). cited by applicant . Boel et al., Functional human monoclonal antibodies of all isotypes constructed from phage display library-derived single-chain Fv antibody fragments, Journal of Immunological Methods, 2000, pp. 153-166, vol. 239. cited by applicant . Burioni et al., Nonneutralizing Human Antibody Fragments against Hepatitis C Virus E2 Glycoprotein Modulate Neutralization of Binding Activity of Human Recombinant Fabs, Abstract, Virology, Sep. 2001, pp. 29-35, vol. 288, No. 1. cited by applicant . Champion et al., Abstract, The development of monoclonal human rabies virus-neutralizing antibodies as a substitute for pooled human immune globulin in the prophylactic treatment of rabies virus exposure, Abstract, Journal of Immunological Methods, Feb. 2000, pp. 81-90, vol. 235, No. 1-2, Elsevier Science Publishers B.V., Amsterdam, NL. cited by applicant . Chen et al., Abstract, Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen, Journal of Molecular Biology, Nov. 5, 1999, pp. 865-881, vol. 293, No. 4. cited by applicant . De Kruif et al., Rapid selection of cell subpopulation-specific human monoclonal antibodies from a synthetic phage antibody library, Proc. Natl. Acad. Sci., USA, Apr. 1995, pp. 3938-3942, vol. 92. cited by applicant . Figini et al., In Vitro Assembly of Repertoires of Antibody Chains on the Surface of Phage by Renaturation, Journal of Molecular Biology, 1994, pp. 68-78, vol. 239. cited by applicant . Franconi et al., Functional expression in bacteria and plants of an scFv antibody fragment against tospoviruses, Immunotechnology, 1999, pp. 189-201, vol. 4. cited by applicant . French, et al., Cancer Research, 1991, pp. 2353-2361, vol. 51. cited by applicant . Friedenson et al., Immunoglobulin G Antibodies from an Individual Rabbit in Which Several Heavy Chain Variants Are Paired with One Light Chain Sequence, The Journal of Biological Chemistry, 1973, pp. 7073-7079, vol. 248, No. 20. cited by applicant . Holliger et al., "Diabodies": Small bivalent and bispecific antibody fragments, Proc. Natl. Acad. Sci., Jul. 1993, pp. 6444-6448, vol. 90. cited by applicant . Hoogenboom et al., Antibody phage display technology and its applications, Immunotechnology, 1998, pp. 1-20, vol. 4. cited by applicant . Huse et al., Purification of antibodies by affinity chromatography, Journal of Biochemical and Biophysical Methods, 2002, pp. 217-231, vol. 51. cited by applicant . Kang et al., Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces, Proc. Natl. Acad. Sci., May 1991, pp. 4363-4366, vol. 88. cited by applicant . Kortt et al., Dimeric and trimeric antibodies: high avidity scFvs for cancer targeting, Biomol. Eng., Oct. 15, 2001, pp. 95-108, vol. 18, No. 3. cited by applicant . Krebs et al., High-throughput generation and engineering of recombinant human antibodies, Journal of Immunological Methods, 2001, pp. 67-84, vol. 254. cited by applicant . Kwaks et al., Identification of anti-repressor elements that confer high and stable protein production in mammalian cells, Nature Biotechnology, 2003, pp. 553-558, vol. 21. cited by applicant . Lekkerkerker, Phage antibodies against human dendritic cell subpopulations obtained by flow cytometry-based selection on freshly isolated cells, Journal of Immunological Methods, 1999, pp. 53-63, vol. 231. cited by applicant . Lindhofer et al., Preferential Species-Restricted Heavy/Light Chain Pairing in Rat/Mouse Quadromas, 1995, pp. 219-225, vol. 155. cited by applicant . Lu et al., Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy, Abstract, International Journal of Cancer, Jan. 20, 2002, pp. 393-399, vol. 97, No. 3. cited by applicant . Merchant et al., An efficient route to human bispecific IgG, Nature Biotechnology, Jul. 1998, pp. 677-681, vol. 16. cited by applicant . Morrison, Sherie L., Transfectomas Provide Novel Chimeric Antibodies, Science, Sep. 20, 1985, pp. 1202-1207, vol. 229. cited by applicant . Norderhaug et al., Balanced expression of single subunits in a multisubunit proteins, achieved by cell fusion of individual transfectants, European Journal of Biochemistry, 2002, pp. 3205-10, vol. 269. cited by applicant . Office Action for U.S. Appl. No. 11/490,545 dated Jul. 30, 2008. cited by applicant . Office Action for U.S. Appl. No. 11/490,545 dated Jan. 13, 2010. cited by applicant . Office Action for U.S. Appl. No. 11/490,545 dated Mar. 25, 2008. cited by applicant . Office Action for U.S. Appl. No. 11/490,545 dated May 29, 2009. cited by applicant . Pau et al, The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines, Vaccine, 2001, pp. 2716-2712, vol. 19. cited by applicant . PCT International Preliminary Examination Report, PCT/EP03/07690, dated Nov. 11, 2004. cited by applicant . PCT International Search Report, PCT/EP03/07690, dated Apr. 16, 2004. cited by applicant . PCT International Search Report, PCT/NL2004/000386 dated Nov. 23, 2004. cited by applicant . PCT International Search Report, PCT/NL2005/000036, dated Jan. 19, 2005. cited by applicant . Sidhu et al., Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions, Abstract, Journal of Molecular Biology, Apr. 23, 2004, pp. 299-310, vol. 338, No. 2. cited by applicant . Sugita, et al., Int. J. Cancer, 1986, pp. 351-357, vol. 37. cited by applicant . Vaughan et al., Human Antibodies with Sub-nanomolar Affinities Isolated from a Large Non-immunized Phage Display Library, Nature Biotechnology, Mar. 1996, pp. 309-314, vol. 14. cited by applicant . Ward et al., Nature, 1989, pp. 544-546, vol. 341. cited by applicant . Warnaar et al., Hybridoma, 1994, pp. 519-526, vol. 13, No. 6. cited by applicant . Conrath et al., Camel Single-domain Antibodies as Modular Building Units in Bispecific and Bivalent Antibody Constructs, The Journal of Biological Chemistry, 2001, pp. 7346-7350, vol. 276, No. 10. cited by applicant . Nguyen et al., Heavy-chain only antibodies derived from dromedary are secreted and displayed by mouse B cells, Immunology, 2003, pp. 93-101, vol. 109. cited by applicant . Tanaka et al., De novo production of diverse intracellular antibody libraries, Nucleic Acids Research, 2003, e23, pp. 1-10, vol. 31, No. 5. cited by applicant . Lenz, et al.; Expression of heterobispecific antibodies by genes transfected into producer hybridoma cells,Gene; 87 Mar. 15, 1990, No. 2; pp. 213-218. cited by applicant . Muyldermans, Reviews in Molecular Biotechnology, 2001, pp. 277-302, vol. 72. cited by applicant . Schmitz et al., Phage Display: A Molecular Tool for the Generation of Antibodies--A Review, Placenta, 2000, pp. S106-S112, Supplement A, Trophoblast Research, vol. 14. cited by applicant . Friedenson, Bernard et al., "Immunoglobulin G Antibodies from an Individual Rabbit in Which Several Heavy Chain Variants are Paired with One Light Chain Sequence," The Journal of Biological Chemistry, Oct. 25, 1973, pp. 7073-7079, vol. 248, No. 20. cited by applicant . Notice of Allowance for U.S. Appl. No. 11/490,545, dated Jun. 15, 2012. cited by applicant . Flavell et al., Systemic Therapy with 3BIT, a Triple Combination Cocktail of Anti--CD19, -CD22, and -CD38--Saporin Immunotoxins, Is Curative of Human B-Cell Lymphoma in Severe Combined Immunodeficient Mice, Cancer Research, Nov. 1997, pp. 4824-4829, vol. 57. cited by applicant . Rojas et al. Phage antibody fragments library combining a single human light chain variable region with immune mouse heavy chain variable regions, Journal of Biotechnology. 2002, pp. 287-298, vol. 94. cited by applicant . European Search Report for European patent application No. 10189886.4 dated Nov. 20, 2012. cited by applicant . Logtenberg, Ton, Antibody cocktails: Next-Generation Biopharmaceuticals with Improved Potency, Trends in Biotechnology, 2007, pp. 390-394, vol. 25, No. 9, Science Direct. cited by applicant . Skerra, Arne, `Anticalins`: A New Class of Engineered Ligand-Binding Proteins with Antibody-Like Properties, 2001, Reviews in Molecular Biotechnology, pp. 257-275, vol. 74, Elsevier. cited by applicant . Communication from copending European patent application No. 05704566.8 dated Jun. 6, 2013. cited by applicant. |
Primary Examiner: Ponnaluri; Padmashri
Attorney, Agent or Firm: Sterne, Kessler, Goldstein & Fox P.L.L.C.
Parent Case Text
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation of co-pending U.S. patent application Ser. No. 12/221,021, filed Jul. 29, 2008 now U.S. Pat. No. 7,927,834, which is a divisional patent application of co-pending application Ser. No. 11/593,279, filed Nov. 6, 2006 now U.S. Pat. No. 7,429,486, which is a divisional patent application of patent application Ser. No. 11/039,767, filed Jan. 18, 2005, now U.S. Pat. No. 7,262,028, issued Aug. 28, 2007, which is a continuation of PCT International Patent Application No. PCT/EP2003/007690, filed on Jul. 15, 2003, designating the United States of America, published in English as International Publication No. WO 2004/009618 A2 on Jan. 29, 2004, which itself claims the benefit of PCT International Patent Application No. PCT/EP03/50201, filed May 27, 2003, European Patent Application No. 02077953.4, filed Jul. 18, 2002, and U.S. Provisional Patent Application Ser. No. 60/397,066, filed Jul. 18, 2002, the contents of the entirety of each of which are incorporated by reference.
Claims
What is claimed is:
1. A composition comprising a mixture of two or three non-identical antibodies and a .[.suitable.]. .Iadd.pharmaceutically acceptable .Iaddend.carrier, wherein two different heavy chains and a common immunoglobulin light chain able to pair with the two different heavy chains are present in the mixture of the two or three non-identical antibodies, and wherein the mixture of two or three non-identical antibodies comprises: a bispecific antibody and at least one monospecific antibody.Iadd., and wherein at least one of the two different heavy chains is modified as compared to wild-type.Iaddend..
2. The composition of claim 1, wherein the mixture comprises a bispecific antibody and two different monospecific antibodies.
3. The composition of claim 1, wherein the two or three non-identical antibodies have differing specificities for the same target antigen.
4. The composition of claim 1, wherein the two or three non-identical antibodies have differing affinity for the same target epitope.
5. The composition of claim 1, wherein the two or three non-identical antibodies bind to different epitopes on the same target antigen.
6. The composition of claim 1, wherein the two or three non-identical antibodies bind to different antigens.
7. The composition of claim 1, wherein the two or three non-identical antibodies are independently selected from the group consisting of IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, IgD, IgE, and IgM.
8. The composition of claim 1, wherein the different immunoglobulin heavy chains are of IgG isotype.
.[.9. A composition comprising a mixture of two or three non-identical antibodies that have been produced in the same host cells, wherein two different heavy chains and a common immunoglobulin light chain are present in the mixture of the two or three non-identical antibodies, wherein the two different heavy chains are able to pair to said common light chain, wherein the two different heavy chains have different binding specificities, and wherein said heavy chains further differ in their constant regions sufficiently so that the amount of bispecific antibodies is decreased as compared to the theoretical percentage of bispecific antibodies, and wherein the mixture of two or three non-identical antibodies comprises two different monospecific antibodies..].
.[.10. A composition comprising a mixture of two or three non-identical antibodies and a suitable carrier, wherein only two different heavy chains are present in the two or three antibodies, wherein a common immunoglobulin light chain able to pair with the two different heavy chains is present in the mixture of the two or three non-identical antibodies, and wherein the mixture of two or three non-identical antibodies comprises: a bispecific antibody and at least one monospecific antibody..].
.[.11. A composition comprising a mixture of two or three non-identical antibodies and a suitable carrier, wherein two different heavy chains are present in the two or three antibodies, wherein only a common immunoglobulin light chain able to pair with the two different heavy chains is present in the mixture of the two or three antibodies, and wherein the mixture of two or three non-identical antibodies comprises: a bispecific antibody and at least one monospecific antibody..].
12. A composition comprising a mixture of two or three non-identical antibodies and a .[.suitable.]. .Iadd.pharmaceutically acceptable .Iaddend.carrier, wherein only two different heavy chains are present in the two or three antibodies, and wherein a common immunoglobulin light chain able to pair with the two different heavy chains is present in the mixture of the two or three non-identical antibodies, .[.and.]. wherein the mixture of two or three non-identical antibodies comprises a monospecific antibody and a bispecific antibody.Iadd., and wherein at least one of the two different heavy chains is modified as compared to wild-type.Iaddend..
13. A composition comprising a mixture of two or three non-identical antibodies and a .[.suitable.]. .Iadd.pharmaceutically acceptable .Iaddend.carrier, wherein two different heavy chains are present in the two or three antibodies, and wherein only a common immunoglobulin light chain able to pair with the two different heavy chains is present in the mixture of the two or three antibodies, .[.and.]. wherein the mixture of two or three non-identical antibodies comprises a monospecific antibody and a bispecific antibody.Iadd., and wherein at least one of the two different heavy chains is modified as compared to wild-type.Iaddend..
14. A composition comprising at least a first antibody and a second antibody, wherein the first and second antibodies have a common light chain, wherein the first antibody comprises two copies of a first heavy chain, wherein the second antibody comprises one copy of the first heavy chain and one copy of a second heavy chain, .[.and.]. wherein the first heavy chain and the second heavy chain are different from one another.Iadd., and wherein at least one of the two different heavy chains is modified as compared to wild-type.Iaddend..
.Iadd.15. The composition of claim 1, wherein the mixture comprises three non-identical antibodies..Iaddend.
.Iadd.16. The composition of claim 1, wherein the common light chain is identical in each light chain/heavy chain pair of the two or three non-identical antibodies..Iaddend.
.Iadd.17. The composition of claim 1, wherein the common light chain is the only light chain present in the composition..Iaddend.
.Iadd.18. The composition of claim 1, wherein the two different heavy chains differ in their variable region..Iaddend.
.Iadd.19. The composition of claim 1, wherein the two different heavy chains differ in both the variable region and constant region..Iaddend.
.Iadd.20. The composition of claim 1, wherein the mixture of two or three non-identical antibodies has been produced in the same host cells..Iaddend.
.Iadd.21. The composition of claim 1, wherein: the mixture comprises three non-identical antibodies; the common light chain is identical in each light chain/heavy chain pair of the two or three non-identical antibodies; the common light chain is the only light chain present in the composition; the three non-identical antibodies have been produced in the same host cells; and the two different heavy chains differ in their variable region..Iaddend.
.Iadd.22. The composition of claim 1, wherein the mixture of two or three non-identical antibodies has been isolated from the host cells which produce the antibodies..Iaddend.
.Iadd.23. The composition of claim 1, wherein the light chain is able to pair with one of the two heavy chains present in the mixture..Iaddend.
.Iadd.24. The composition of claim 1 wherein the two or three non-identical antibodies have a different affinity for the same target epitope..Iaddend.
.Iadd.25. The composition of claim 24 wherein the different affinity is analyzed by surface plasmon resonance..Iaddend.
.Iadd.26. The composition of claim 1, wherein the two or three non-identical antibodies have been isolated separately or as a mixture from a culture of the host cell..Iaddend.
Description
STATEMENT ACCORDING TO 37 C.F.R. .sctn.1.52(e)(5)--SEQUENCE LISTING SUBMITTED ON COMPACT DISC
Pursuant to 37 C.F.R. .sctn.1.52(e)(1)(iii), a compact disc containing an electronic version of the Sequence Listing has been submitted concomitant with this application, the contents of which are hereby incorporated by reference. A second compact disc is submitted and is an identical copy of the first compact disc. The discs are labeled "copy 1" and "copy 2," respectively, and each disc contains one file entitled "0079WO000RD.ST25.txt" which is 27 KB and created on Mar. 4, 2011.
TECHNICAL FIELD
The invention relates generally to the field of biotechnology, and more particularly, to the field of medicine and the production of antibodies, and even more particularly, to the production of mixtures of antibodies.
BACKGROUND
The essential function of the immune system is the defense against infection. The humoral immune system combats molecules recognized as non-self, such as pathogens, using immunoglobulins. These immunoglobulins, also called antibodies, are raised specifically against the infectious agent, which acts as an antigen, upon first contact (Roitt, Essential Immunology, Blackwell Scientific Publications, fifth edition, 1984; all references cited herein are incorporated in their entirety by reference). Antibodies are multivalent molecules comprising heavy (H) chains and light (L) chains joined with interchain disulfide bonds. Several isotypes of antibodies are known, including IgG1, IgG2, IgG3, IgG4, IgA, IgD, IgE, and IgM. An IgG contains two heavy and two light chains. Each chain contains constant (C) and variable (V) regions, which can be broken down into domains designated C.sub.H1, C.sub.H2, C.sub.H3, V.sub.H, and C.sub.L, V.sub.L (FIG. 1). Antibody binds to antigen via the variable region domains contained in the Fab portion and, after binding, can interact with molecules and cells of the immune system through the constant domains, mostly through the Fc portion.
B-lymphocytes can produce antibodies in response to exposure to biological substances like bacteria, viruses and their toxic products. Antibodies are generally epitope-specific and bind strongly to substances carrying these epitopes. The hybridoma technique (Kohler and Milstein, 1975) makes use of the ability of B-cells to produce monoclonal antibodies to specific antigens and to subsequently produce these monoclonal antibodies by fusing B-cells from mice exposed to the antigen of interest to immortalized murine plasma cells. This technology resulted in the realization that monoclonal antibodies produced by hybridomas could be used in research, diagnostics and therapies to treat different kinds of diseases like cancer and auto-immune-related disorders.
Because antibodies that are produced in mouse hybridomas can induce strong immune responses in humans, it has been appreciated in the art that antibodies required for successful treatment of humans needed to be less immunogenic or, preferably, non-immunogenic. For this to be done, murine antibodies were first engineered by replacing the murine constant regions with human constant regions (referred to as chimeric antibodies). Subsequently, domains between the complementarity-determining regions (CDRs) in the variable domains, the so-called framework regions, were replaced by their human counterparts (referred to as humanized antibodies). The final stage in this humanization process has been the production of fully human antibodies.
In the art, bispecific antibodies, which have binding specificities for two different antigens, have also been described. These are generally used to target a therapeutic or diagnostic moiety, for instance, T-cell, a cytotoxic trigger molecule, or a chelator that binds a radionuclide, that is recognized by one variable region of the antibody to a cell that is recognized by the other variable region of the antibody, for instance, a tumor cell (for bispecific antibodies, see Segal et al., 2001).
One very useful method known in the art to obtain fully human monoclonal antibodies with desirable binding properties, employs phage display libraries. This is an in vitro, recombinant DNA-based, approach that mimics key features of the humoral immune response (for phage display methods, see, e.g., C. F. Barbas III et al., Phage Display, A laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 2001). For the construction of phage display libraries, collections of human monoclonal antibody heavy- and light-chain variable region genes are expressed on the surface of bacteriophage particles, usually in single-chain Fv (scFv) or in Fab format. Large libraries of antibody fragment-expressing phages typically contain more than 10.sup.9 antibody specificities and may be assembled from the immunoglobulin V regions expressed in the B lymphocytes of immunized or non-immunized individuals.
Alternatively, phage display libraries may be constructed from immunoglobulin variable regions that have been partially assembled or rearranged in vitro to introduce additional antibody diversity in the library (semi-synthetic libraries) (De Kruif et al., 1995b). For example, in vitro-assembled variable regions contain stretches of synthetically produced, randomized or partially randomized DNA in those regions of the molecules that are important for antibody specificity.
The genetic information encoding the antibodies identified by phage display can be used for cloning the antibodies in a desired format, for instance, IgG, IgA or IgM, to produce the antibody with recombinant DNA methods (Boel et al., 2000).
An alternative method to provide fully human antibodies uses transgenic mice that comprise genetic material encoding a human immunoglobulin repertoire (Fishwild et al., 1996; Mendez et al., 1997). Such mice can be immunized with a target antigen and the resulting immune response will produce fully human antibodies. The sequences of these antibodies can be used in recombinant production methods.
Production of monoclonal antibodies is routinely performed by use of recombinant expression of the nucleic acid sequences encoding the H and L chains of antibodies in host cells (see, e.g., EP0120694; EP0314161; EP0481790; U.S. Pat. No. 4,816,567; WO 00/63403, the contents of the entirety of each which are incorporated herein by reference).
To date, many different diseases are being treated with either humanized or fully human monoclonal antibodies. Products based on monoclonal antibodies that are currently approved for use in humans include HERCEPTIN.TM. (trastuzumab, anti-Her2/Neu), REOPRO.TM. (abciximab, anti-Glycoprotein IIB/IIIA receptor), MYLOTARG.TM. (gemtuzumab, anti-CD33), RITUXAN.TM. (Rituximab, anti-CD20), SIMULECT.TM. (basiliximab, anti-CD25), REMICADE.TM. (infliximab, anti-TNF), SYNAGIS.TM. (palivizumab, anti-RSV), ZENAPAX.TM. (daclizumab, IL2-receptor), and CAMPATH.TM. (alemtuzumab, anti-CD52). Despite these successes, there is still room for new antibody products and for considerable improvement of existing antibody products.
The use of monoclonal antibodies in cancer treatment has shown that so-called "antigen-loss tumor variants" can arise, making the treatment with the monoclonal antibody less effective. Treatment with the very successful monoclonal antibody RITUXIMAB.RTM. (anti-CD20) has, for instance, shown that antigen-loss escape variants can occur, leading to relapse of the lymphoma (Massengale et al., 2002). In the art, the potency of monoclonal antibodies has been increased by fusing them to toxic compounds, such as radionuclides, toxins, cytokines, and the like. Each of these approaches, however, has its limitations, including technological and production problems and/or high toxicity.
Furthermore, it appears that the gain in specificity of monoclonal antibodies compared to traditional undefined polyclonal antibodies, comes at the cost of loss of efficacy. In vivo, antibody responses are polyclonal in nature, i.e., a mixture of antibodies is produced because various B-cells respond to the antigen, resulting in various specificities being present in the polyclonal antibody mixture. Polyclonal antibodies can also be used for therapeutic applications, for instance, for passive vaccination or for active immunotherapy, and currently are usually derived from pooled serum from immunized animals or from humans who recovered from the disease. The pooled serum is purified into the proteinaceous or gamma globulin fraction, so named because it contains predominantly IgG molecules.
Polyclonal antibodies that are currently used for treatment include anti-rhesus polyclonal antibodies, gamma globulin for passive immunization, anti-snake venom polyclonal (Cro-Fab), THYMOGLOBULIN.TM. for allograft rejection, anti-digoxin to neutralize the heart drug digoxin, and anti-rabies polyclonal antibodies. In currently marketed therapeutic antibodies, an example of the higher efficacy of polyclonal antibodies compared to monoclonal antibodies can be found in the treatment of acute transplant rejection with anti-T-cell antibodies. The monoclonal antibodies on the market (anti-CD25 BASILIXIMAB.RTM.) are less efficacious than a rabbit polyclonal antibody against thymocytes (THYMOGLOBULIN.TM.) (press releases dated Mar. 12, Apr. 29, and Aug. 26, 2002, on sangstat.com). The use of pooled human sera, however, potentially bears the risk of infections with viruses such as HIV or hepatitis, with toxins such as lipopolysaccharide, with proteinaceous infectious agents such as prions, and with unknown infectious agents. Furthermore, the supply that is available is limited and insufficient for widespread human treatments. Problems associated with the current application of polyclonal antibodies derived from animal sera in the clinic include a strong immune response of the human immune system against such foreign antibodies. Therefore, such polyclonals are not suitable for repeated treatment or for treatment of individuals that were injected previously with other serum preparations from the same animal species.
The art describes the idea of the generation of animals with a human immunoglobulin repertoire, which can subsequently be used for immunization with an antigen to obtain polyclonal antibodies against this antigen from the transgenic animals (WO 01/19394, the entirety of which is incorporated herein by reference). However, many technological hurdles still will have to be overcome before such a system is a practical reality in larger animals than mice and it will take years of development before such systems can provide the polyclonal antibodies in a safe and consistent manner in sufficient quantities. Moreover, antibodies produced from pooled sera, whether being from human or animal origin, will always comprise a high amount of unrelated and undesired specificities, as only a small percentage of the antibodies present in a given serum will be directed against the antigen used for immunization. It is, for instance, known that in normal, i.e., non-transgenic, animals, about 1% to 10% of the circulating immunoglobulin fraction is directed against the antigen used for hyper-immunization; hence, the vast majority of circulating immunoglobulins is not specific.
One approach towards expression of polyclonal antibody libraries has been described (WO 95/20401; U.S. Pat. Nos. 5,789,208 and 6,335,163, the contents of the entirety of each of which are incorporated herein by reference). A polyconal library of Fab antibody fragments is expressed using a phage display vector and selected for reactivity towards an antigen. To obtain a sub-library of intact polyconal antibodies, the selected heavy and light chain-variable region gene combinations are transferred en mass as linked pairs to a eukaryotic-expression vector that provides constant region genes. Upon transfection of this sub-library into myeloma cells, stable clones produce monoclonal antibodies that can be mixed to obtain a polyclonal antibody mixture. While in theory it would be possible to obtain polyclonal antibodies directly from a single recombinant production process using this method by culturing a mixed population of transfected cells, potential problems would occur concerning the stability of the mixed cell population and, hence, the consistency of the produced polyclonal antibody mixture. The control of a whole population of different cells in a pharmaceutically acceptable large-scale process (i.e., industrial) is a daunting task. It would seem that characteristics, such as growth rates of the cells and production rates of the antibodies, should remain stable for all of the individual clones of the non-clonal population in order to keep the ratio of antibodies in the polyclonal antibody mixture more or less constant.
SUMMARY OF THE INVENTION
Disclosed are means and methods for producing a mixture of antibodies in recombinant hosts.
In one aspect, provided is a method of producing a mixture of antibodies in a recombinant host, the method comprising expressing in a recombinant host cell a nucleic acid sequence or nucleic acid sequences encoding at least one light chain and at least three different heavy chains that are capable of pairing with at least one light chain. A further aspect is the elimination of the production of potentially non-functional light-heavy chain pairing by using pre-selected combinations of heavy and light chains. It has been recognized that phage display libraries built from a single light chain and many different heavy chains can encode antibody fragments with very distinct binding properties. This feature can be used to find different antibodies having the same light chain but different heavy chains, against the same target or different targets, wherein a target can be a whole antigen or an epitope thereof. Such different targets may, for instance, be on the same surface (e.g., cell or tissue). Such antibody fragments obtained by phage display can be cloned into vectors for the desired format, e.g., IgG, IgA or IgM, and the nucleic acid sequences encoding these formats can be used to transfect host cells. In one approach, H and L chains can be encoded by different constructs that, upon transfection into a cell wherein they are expressed, give rise to intact Ig molecules. When different H chain constructs are transfected into a cell with a single L chain construct, H and L chains will be assembled to form all possible combinations. However, in contrast to approaches where different light chains are expressed, such as for the production of bispecific antibodies, this method will result only in functional binding regions. It would be particularly useful when the host, for example, a single cell line, is capable of expressing acceptable levels of recombinant antibodies without the necessity to first amplify in the cell the nucleic acid sequences encoding the antibodies. The advantage is that cell lines with only a limited copy number of the nucleic acids are expected to be genetically more stable, because there will be less recombination between the sequences encoding the heavy chains, than in cell lines where a multitude of these copies is present. A cell line suitable for use in these methods is the human cell line PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins). Using this method, a mixture of antibodies with defined specificities can be produced from a single cell clone in a safe, controlled, and consistent manner.
In certain embodiments, provided is a method for producing a mixture of antibodies in a recombinant host, the method comprising expressing a nucleic acid sequence or nucleic acid sequences encoding at least one light chain and at least three different heavy chains that are capable of pairing with at least one light chain in a recombinant host cell. In certain embodiments, the recombinant host cell comprises a nucleic acid sequence encoding a common light chain that is capable of pairing with at least three different heavy chains, such that the produced antibodies comprise a common light chain. Those of skill in the art will recognize that "common" also refers to functional equivalents of the light chain of which the amino acid sequence is not identical. Many variants of the light chain exist wherein mutations (deletions, substitutions, additions) are present that do not materially influence the formation of functional binding regions.
Further provided is a composition comprising a mixture of recombinantly produced antibodies, wherein at least three different heavy chain sequences are represented in the mixture. In certain embodiments, the light chains of such mixtures have a common sequence. The mixture of antibodies can be produced by the method according to the invention. Preferably, the mixture of antibodies is more efficacious than the individual antibodies it comprises. More preferably, the mixture acts synergistically in a functional assay.
Further provided is a recombinant host cell for producing mixtures of antibodies and methods for making such host cells.
Independent clones obtained from the transfection of nucleic acid sequences encoding a light chain and more than one heavy chain may express the different antibodies in the mixture at different levels. It is another aspect to select a clone using a functional assay for the most potent mixture of antibodies. Further provides a method for identifying at least one host cell clone that produces a mixture of antibodies, wherein the mixture of antibodies has a desired effect according to a functional assay, the method comprising: (i) providing a host cell with nucleic acid sequences encoding at least one light chain and nucleic acid sequences encoding at least two different heavy chains, wherein the heavy and light chains are capable of pairing with each other; (ii) culturing at least one clone of the host cell under conditions conducive to expression of the nucleic acid sequences; (iii) screening at least one clone of the host cell for production of a mixture of antibodies having the desired effect by a functional assay; and (iv) identifying at least one clone that produces a mixture of antibodies having the desired effect. This method, as used herein, can be performed using high-throughput procedures if desired. The clones identified by the method can be used to produce antibody mixtures.
In certain embodiments, further provided are transgenic non-human animals and transgenic plants or transgenic plant cells capable of expressing mixtures of antibodies and mixtures of antibodies produced by these.
In certain embodiments, further provided are pharmaceutical compositions comprising a mixture of recombinantly produced antibodies and a suitable carrier.
In certain embodiments, further provided are mixtures of antibodies for use in the treatment or diagnosis and for the preparation of a medicament for use in the treatment or diagnosis of a disease or disorder in a human or animal subject.
In certain embodiments, further provided is a method for producing a mixture of antibodies comprising different isotypes from a single host cell clone.
In certain embodiments, further provided is a method for identifying a mixture of antibodies having a desired effect in a functional assay.
In certain embodiments, further provided is a method for producing a mixture of antibodies that are capable of binding to a target, the method comprising: i) bringing a phage library comprising antibodies into contact with material comprising a target, ii) at least one step of selecting phages binding to the target, iii) identifying at least two phages that comprise antibodies binding to the target, wherein at least two antibodies comprise a common light chain, iv) introducing a nucleic acid sequence encoding the light chain and a nucleic acid sequence or sequences encoding the heavy chains of at least two antibodies into a host cell, v) culturing a clone of the host cell under conditions conducive to expression of the nucleic acid sequences.
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 is a schematic representation of an antibody. The heavy and light chains are paired via interchain disulfide bonds (dotted lines). The heavy chain can be either of the .alpha., .gamma., .mu., .delta. or isotype. The light chain is either .lamda. or .kappa.. An antibody of IgG1 isotype is shown.
FIG. 2 is a schematic representation of a bispecific monoclonal antibody. A bispecific antibody contains two different functional F(Ab) domains, indicated by the different patterns of the V.sub.H-V.sub.L regions.
FIGS. 3A and 3B show a sequence alignment of V.sub.L (FIG. 3A) and V.sub.H (FIG. 3B) of K53, UBS-54 and 02-237. The DNA sequence of common V.sub.L of UBS54 and K53 is SEQ ID NO:1, while the amino acid sequence is given as SEQ ID NO:2. DNA sequences of V.sub.L of 02-237, V.sub.H of UBS54, K53 and 02-237 are SEQ ID NOS:3, 5, 7 and 9, respectively, while the amino acid sequences are given in SEQ ID NOS:4, 6, 8 and 10, respectively.
FIG. 4 is an overview of plasmids pUBS3000Neo and pCD46_3000 (Neo).
FIG. 5, Panel A, shows the isoelectric focusing (IEF) of transiently expressed pUBS3000Neo, pCD46_3000(Neo) and a combination of both. In Panel B, the upper part shows a schematic representation of the expected molecules when a single light chain and a single heavy chain are expressed in a cell, leading to monoclonal antibodies UBS-54 or K53. The lower part under the arrow shows a schematic representation of the combinations produced when both heavy chains and the common light chain are co-expressed in a host cell, with theoretical amounts when both heavy chains are expressed at equal levels and pair to each other with equal efficiency. The common light chain is indicated with the vertically striped bars.
FIG. 6 is a schematic representation of a possible embodiment of the method according to the invention (see, e.g., Example 9). At (1), introduction of nucleic acid sequences encoding one light chain and three different heavy chains capable of pairing to the common light chain to give functional antibodies into host cells is shown; at (2), selection of stable clones; (3) shows clones can be screened for, for instance, expression levels, binding; at (4), clones are expanded; and at (5), production of functional mixtures of antibodies is shown. Some or all of steps 2-5 could be performed simultaneously or in a different order.
FIGS. 7A and 7B show the sequence of V.sub.H and V.sub.L of phages directed against CD22 (clone B28), CD72 (clone II-2) (FIG. 7A), and HLA-DR (class II; clone I-2) (FIG. 7B). DNA sequences of .[.V.sub.L.]. .Iadd.V.sub.H .Iaddend.of clones B28, II-2 and I-2 are SEQ ID NOS:11, 13 and 15, respectively, while the amino acid sequences are SEQ ID NOS:12, 14 and 16, respectively. DNA sequence of the common light chain of these clones is SEQ ID NO:17, while the amino acid sequence is SEQ ID NO:18.
FIG. 8 is a map of pUBS54-IgA (pCRU-L01 encoding human IgA1 against EPCAM).
FIG. 9 shows dimeric bispecific IgA with a single light chain (indicated by horizontally striped bar). The method of the invention will produce a mixture of forms wherein different heavy chains can be paired. Only the most simple form is depicted in this schematic representation. A J-chain is shown to join the two monomers.
FIG. 10 is a pentameric multispecific IgM with a single light chain (indicated by horizontally striped bars). The method of the invention will produce a mixture of many different forms, wherein different heavy chains can be paired. Only the most simple form is depicted in this schematic representation when five different heavy chains are expressed with a single light chain and all five different heavy chains are incorporated in the pentamer and paired to the same heavy chain. Pentamers with less specificities can also be formed by incorporation of less than five different heavy chains. Hexamers can also be obtained, especially when the J-chain is not expressed.
FIG. 11 depicts expression of a mixture of human IgG isotypes consisting of a common light chain but with different binding specificities in a single cell to avoid the formation of bispecific antibodies. The different binding specificities are indicated by the different colors of the V.sub.H sequences. The common light chain is indicated with the vertically striped bars. The IgG1 isotype is indicated with the grey Fc and the IgG3 isotype is indicated with the black Fc part.
FIGS. 12A-12E depict DNA and protein sequences of variable domains of heavy chains of K53 (FIG. 12A), UBS54 (FIG. 12C) and 02-237 (FIG. 12B) IgG (SEQ ID NOS:7, .[.9.]. .Iadd.5 .Iaddend.and .[.5.]. .Iadd.9.Iaddend., respectively) and light chains (SEQ ID NOS:1 and 3, respectively, for K53/UBS54 (FIG. 12D) and 02-237 IgG (FIG. 12E)).
FIG. 13 shows alignment of the variable sequences of the heavy chains of K53, 02-237 and UBS54 (SEQ ID NOS:7, 9, and 5, respectively). CDR1, CDR2 and CDR3 regions are indicated in bold.
FIG. 14 is a BIACORE.TM. (surface plasmon resonance) analysis of K53 and 02-237. Affinity-purified human CD46 from LS174T cells was coupled (640 RU) to CM5 chips (BIACORE BR-1000-14.TM.). Binding of 1000 (A), 500 (B), 250 (C), 125 (D), 63 (E), 31 (F), 16 (G), 8 (H) or 0 (I) nM 02-237 or K53 purified from stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines to the CD46 was monitored using a BIACORE 3000.TM. system at 37.degree. C. Using this experimental set-up, a K.sub.d of 9.1.times.10.sup.7 and 2.2.times.10.sup.8 was found for K53 and 02-237, respectively.
FIG. 15 shows binding of K53 and 02-237 to LS174T cells. Serial dilutions of purified 02-237 (.box-solid.), K53 (*) and the negative control GBSIII (.diamond.) conjugated to biotin were incubated with LS147T cells pre-incubated with normal human serum to block Fc.gamma. receptor interaction. Binding (MFI, ordinate) was determined by FACS after incubation with streptavidin-conjugated phycoerythrin.
FIG. 16A is an SDS-PAGE analysis of purified IgG fractions. Three .mu.g purified IgG was analyzed on a non-reduced 4-20% NUPAGE.RTM. gel (NOVEX) according to recommendations of the manufacturer. Proteins were visualized by staining with colloidal blue (NOVEX Cat. No LC6025) according to recommendations of the manufacturer. Clone identity is indicated on top of the SDS-PAGE. Each gel contains a control, which is either purified 02-237 or K53. FIGS. 16B and 16C are continuations of the gel in FIG. 16A.
FIG. 16D is an SDS-PAGE analysis of purified IgG fractions. Three .mu.g purified IgG was analyzed on a reduced 4-20% NUPAGE.RTM. gel according to recommendations of the manufacturer. Proteins were visualized by staining with colloidal blue (NOVEX cat. No LC6025) according to recommendations of the manufacturer. Clone identity is indicated on top of the SDS-PAGE. Each gel contains a control, which is either purified 02-237 or K53. NR, Non-reduced; R, reduced. FIGS. 16E and 16F are continuations of the gel in FIG. 16D.
FIG. 17A shows an IEF analysis of purified IgG fractions. Ten .mu.g purified IgG was analyzed on an Isogel 3-10 gel (BMA) according to recommendations of the manufacturer. Proteins were visualized by staining with colloidal blue according to recommendations of the manufacturer. Clone identity is indicated on top of the IEF. Each gel contains a control, consisting of a 1:1:1 mixture of 02-237, K53 and UBS54. FIGS. 17B through 17D are continuations of the gel in FIG. 17A.
FIG. 18 is an IEF analysis of polyclonal mixtures 241, 280, 282, 361 and 402 in comparison to single K53, 02-237 and UBS54. Ten .mu.g purified IgG was analyzed on an Isogel 3-10 gel (BMA) according to recommendations of the manufacturer. Proteins were visualized by staining with colloidal blue according to recommendations of the manufacturer. IgG identity is indicated on top of the IEF.
FIG. 19 contains mass chromatograms of CDR3 peptides of K53, 02-237, UBS54 and the two unique light chain peptides L1-K53/UBS54 and L1-237 in IgG fraction Poly1-280. On the right-hand side of each mass chromatogram, the isotopic pattern of the peptide is shown. The doubly charged ion at m/z 1058.98 (Mw 2115.96 Da) results from peptide H11-K53. The doubly charged ion at m/z 1029.96 (Mw 2057.92 Da) results from peptide H11-02-237. The triply charged ion at m/z 770.03 (Mw 2307.09 Da) results from peptide H9-UBS54. The doubly charged ion at m/z 1291.08 (Mw 2580.16 Da) results from peptide L1-K53/UBS54. The doubly charged ion at m/z 1278.11 (Mw 2554.22 Da) results from peptide L1-02-237.
Purified IgG was dissolved in a 0.1% RAPIGEST.TM. (Waters) in 50 mM NH.sub.4HCO.sub.3. The disulfides were reduced using 1 M DTT (1,4-dithio-DL-threitol), followed by incubation at 65.degree. C. for 30 minutes. Then, for alkylation of all sulfhydryl groups, 1 M iodoacetamide was added, followed by incubation at room temperature for 45 minutes in the dark. Alkylation was stopped by addition of 1 M DTT. The buffer was exchanged to 25 mM NH.sub.4HCO.sub.3, pH 7.5. Finally, the antibodies were digested overnight at 37.degree. C. by addition of a freshly prepared trypsin solution in 25 mM NH.sub.4HCO.sub.3. The peptide mixture was analyzed by LC-MS. The LC-system consisted of a Vydac reversed-phase C18 column that was eluted by applying a gradient of solvent A (5/95/1 acetonitrile, water, glacial acetic acid v/v/v) and solvent B (90/10/1 acetonitrile, water, glacial acetic acid v/v/v). The LC was on-line coupled to a Q-TOF2 mass spectrometer (Micromass), equipped with an electrospray source operated at 3 kV. Mass spectra were recorded in a positive ion mode from m/z 50 to 1500 at a cone voltage of 35V. The instrumental resolution of >10,000 enabled unambiguous determination of the charge and, therefore, the mass of most ions up to at least +7. In this way, all peptides were identified according to their molecular weight. The amino acid sequence of the peptide was confirmed by MS/MS-experiments. MS/MS spectra were recorded in a positive ion mode from m/z 50-2000 with collision energy between 20 and 35 eVolts.
FIG. 20 is a BIACORE.TM. (surface plasmon resonance) analysis of polyclonal 280. Affinity-purified human CD46 from LS174T cells was coupled (640 RU) to CM5 chips (BIACORE BR-1000-14.TM.). Binding of 1000 (A), 500 (B), 250 (C), 125 (D), 63 (E), 31 (F), 16 (G), 8 (H) or 0 (I) nM Poly1-280 to CD46 was monitored using a BIACORE 3000.TM. system at 37.degree. C.
FIG. 21 is an IEF analysis of sub-clones from clones poly 1-241, poly 1-280 and poly 1-402 producing a mixture of antibodies.
Panel A contains clones poly 1-241 and poly 1-280. Lane 1 contains a pI marker (Amersham, Cat. No. 17-0471-01). Lane 2 contains isolated IgG from the parent clone poly 1-241 (as in FIG. 18). Lanes 3, 4 and 5, respectively, contain isolated IgG from three independent sub-clones derived from poly 1-241 by limiting dilution. Lane 6 contains isolated IgG from the parent clone poly 1-280 (as in FIG. 18). Lanes 7, 8 and 9, respectively, contain isolated IgG from three independent sub-clones derived from poly 1-280 by limiting dilution.
Panel B contains clone poly 1-402. Lanes 1 and 7 contain a pI marker. Lane 2 contains isolated IgG from the parent clone poly 1-402 (as in FIG. 18). Lanes 3, 4 and 5, respectively, contain isolated IgG from three independent sub-clones derived from poly 1-402 by limiting dilution. Lane 6 contains a control (a 1:1:1 mixture of 02-237, K53 and UBS54).
FIG. 22 is a fluorescence activated cell sorting (FACS) analysis of mixtures of antibodies produced from sub-clones of poly 1-241 (A), poly 1-280 (B) and poly 1-402 (C). Binding of the mixtures of antibodies to cells transfected with cDNA of CD46, EpCAM, or a negative control (CD38), was determined with FACS analysis. Mean fluorescent intensity (MFI) is shown for the various parent clones and three independent sub-clones of each. Control antibodies GBS-III (negative control), anti-CD72 (02-004; negative control) and the single antibodies UBS54, 02-237 and K53 are also included.
DETAILED DESCRIPTION OF THE INVENTION
Provided is a method for producing a mixture of antibodies in a recombinant host, the method comprising expressing, in a recombinant host cell, a nucleic acid sequence or nucleic acid sequences encoding at least one light chain and at least three different heavy chains that are capable of pairing with at least one light chain. In certain embodiments, the light and heavy chains form functional antigen-binding domains when paired. A functional antigen-binding domain is capable of specifically binding to an antigen.
In certain embodiments, the method for producing a mixture of antibodies further comprises the step of recovering the antibodies from the cell or the host cell culture to obtain a mixture of antibodies suitable for further use.
In certain embodiments, a method is provided for production of a mixture of antibodies, the method comprising expressing in a recombinant host cell a nucleic acid sequence encoding a common light chain and nucleic acid sequence or sequences encoding at least three different heavy chains that are capable of pairing with the common light chain, such that the antibodies that are produced comprise common light chains. In one aspect, the common light chain is identical in each light chain/heavy chain pair.
The term "antibody," as used herein, means a polypeptide containing one or more domains that bind an epitope on an antigen, where such domains are derived from, or have sequence identity with, the variable region of an antibody. The structure of an antibody is schematically represented in FIG. 1. Examples of antibodies according to the invention include full length antibodies, antibody fragments, bispecific antibodies, immunoconjugates, and the like. An antibody, as used herein, may be isotype IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, IgD, IgE, IgM, and the like, or a derivative of these. Antibody fragments include Fv, Fab, Fab', F(ab').sub.2 fragments, and the like. Antibodies according to the invention can be of any origin, including murine, of more than one origin, e.g., chimeric, humanized, or fully human antibodies. Immunoconjugates comprise antigen-binding domains and a non-antibody part such as a toxin, a radiolabel, an enzyme, and the like.
An "antigen-binding domain" preferably comprises variable regions of a heavy and a light chain and is responsible for specific binding to an antigen of interest. Recombinant antibodies are prepared by expressing both a heavy and a light chain in a host cell. Similarly, by expressing two chains with their respective light chains (or a common light chain), wherein each heavy chain/light chain has its own specificity, so-called "bispecific" antibodies can be prepared. "Bispecific antibodies" comprise two non-identical heavy-light chain combinations (FIG. 2), and both antigen-binding regions of a bispecific antibody may recognize different antigens or different epitopes on an antigen. "Epitope" means a moiety of an antigen to which an antibody binds. A single antigen may have multiple epitopes.
A "common light chain," refers to light chains which may be identical or have amino acid sequence differences. Common light chains may comprise mutations which do not alter the specificity of the antibody when combined with the same heavy chain without departing from the scope of the invention. It is, for instance, possible within the scope of the definition of common light chains as used herein, to prepare or find light chains that are not identical but still functionally equivalent, e.g., by introducing and testing conservative amino acid changes, changes of amino acids in regions that do not or only partly contribute to binding specificity when paired with the heavy chain, and the like. In an exemplary embodiment, provided is the use of a common light chain, one identical light chain, to combine with different heavy chains to form antibodies with functional antigen-binding domains. The use of one common light chain avoids the formation of heterodimers in which pairing of light and heavy chains results in antigen-binding domains that are not functional or, in other words, which are not capable of binding to the target antigen or antigens. The use of a common light chain and two heavy chains has been proposed (Merchant et al., 1998; WO 98/50431, the entirety of which are incorporated herein by reference) for a different purpose, viz., to increase the formation of functional bispecific antibodies at the expense of antibody mixture complexity. These publications teach a method for preferentially producing one defined and desired bispecific antibody, thereby minimizing the complexity of the produced mixture. Hence, Merchant specifically teaches to prevent the production of monospecific antibodies because these are undesired byproducts in the process for bispecific antibody production described in those publications. Clearly, there is no teaching in the prior art to prepare a complex mixture of antibodies from a recombinant host cell avoiding the formation of non-functional binding domains or the benefits thereof, let alone how. In the method according to the invention, at least three different heavy chains that are capable of pairing with the common light chain are expressed. In other embodiments, the host cell, as used herein, is provided with nucleic acid sequences encoding for 4, 5, 6, 7, 8, 9, 10, or more, heavy chains capable of pairing with the common light chain, to increase the complexity of the produced mixture of antibodies.
"Different heavy chains," according to the invention, may differ in the variable region and have the same constant region. In other embodiments, where it is clear from the context, they may have the same variable region and differ in the constant region, e.g., be of a different isotype. The use of a mixture of antibodies having different constant regions, such as the Fc-portion, may be advantageous if different arms of the immune system are to be mobilized in the treatment of the human or animal body. In yet other embodiments, also to be clear from the context, both the variable and the constant regions may differ.
A "mixture of antibodies," according to the invention, comprises at least two non-identical antibodies, but may comprise 3, 4, 5, 6, 7, 8, 9, 10, or more, different antibodies and may resemble a polyclonal or at least an oligoclonal antibody mixture with regard to complexity and number of functional antigen-binding molecules. The mixtures produced according to the invention usually will comprise bispecific antibodies. If desired, formation of monospecific antibodies in the mixture can be favored over the formation of bispecific antibodies.
When n heavy chains and one common light chain are expressed, as used herein, in a host cell at equal levels, the theoretical percentage of bispecific antibodies produced by the method according to the invention is (1-1/n).times.100%. The total number of different antibodies in the mixture produced by the method according to the invention is theoretically n+{(n.sup.2-n)/2}, of which (n.sup.2-n/2) are bispecific antibodies. Distortion of the ratio of expression levels of the different heavy chains may lead to values deviating from the theoretical values. The amount of bispecific antibodies can also be decreased, compared to these theoretical values, if all heavy chains do not pair with equal efficiency. It is, for instance, possible to engineer the heavy chains, for example, by introducing specific and complementary interaction surfaces between selected heavy chains, to promote homodimer pairing over heterodimer pairing, contrary to what has been proposed by Merchant, supra. Heavy chains may also be selected so as to minimize heterodimer formation in the mixture. A special form of this embodiment involves heavy chains of two or more different isotypes (e.g., IgG1, IgG3, IgA). When heavy chains of different isotype are expressed in the same host cell in accordance with the invention and one light chain that can pair to these heavy chains, the amount of bispecific antibodies will be reduced, possibly to very low or even undetectable levels. Thus, when bispecific antibodies are less desirable, it is possible to produce a mixture of antibodies according to the invention, wherein a nucleic acid sequence encoding a common light chain and nucleic acid sequences encoding at least two different heavy chains with a different variable region capable of pairing to the common light chain are expressed in a recombinant host, and wherein the heavy chains further differ in their constant regions sufficiently to reduce or prevent pairing between the different heavy chains. The mixtures of antibodies may be produced from a clone that was derived from a single host cell, i.e., from a population of cells containing the same recombinant nucleic acid sequences.
It will be understood that the different heavy chains can be encoded on separate nucleic acid molecules, but may also be present on one nucleic acid molecule comprising different regions encoding at least three heavy chains. The nucleic acid molecules usually encode precursors of the light and/or heavy chains, which, when expressed, are secreted from the host cells, thereby becoming processed to yield the mature form. These and other aspects of expressing antibodies in a host cell are well known to those having ordinary skill in the art.
A "recombinant host cell," as used herein, is a cell comprising one or more so-called transgenes, i.e., recombinant nucleic acid sequences not naturally present in the cell. These transgenes are expressed in the host cell to produce recombinant antibodies encoded by these nucleic acid sequences when these cells are cultured under conditions conducive to expression of nucleic acid sequences. The host cell, as used herein, can be present in the form of a culture from a clone that is derived from a single host cell wherein the transgenes have been introduced. To obtain expression of nucleic acid sequences encoding antibodies, it is well known to those skilled in the art that sequences capable of driving such expression can be functionally linked to the nucleic acid sequences encoding the antibodies.
"Functionally linked" is meant to describe that the nucleic, acid sequences encoding the antibody fragments or precursors thereof is linked to the sequences capable of driving expression such that these sequences can drive expression of the antibodies or precursors thereof.
Useful expression vectors are available in the art, for example, the pcDNA vector series of Invitrogen. Where the sequence encoding the polypeptide of interest is properly inserted with reference to sequences governing the transcription and translation of the encoded polypeptide, the resulting expression cassette is useful to produce the polypeptide of interest, referred to as expression. Sequences driving expression may include promoters, enhancers and the like, and combinations thereof. These should be capable of functioning in the host cell, thereby driving expression of the nucleic acid sequences that are functionally linked to them. Promoters can be constitutive or regulated and can be obtained from various sources, including viruses, prokaryotic or eukaryotic sources, or artificially designed. Expression of nucleic acids of interest may be from the natural promoter or derivative thereof or from an entirely heterologous promoter. Some well-known and much-used promoters for expression in eukaryotic cells comprise promoters derived from viruses, such as adenovirus, for instance, the E1A promoter, promoters derived from cytomegalovirus (CMV), such as the CMV immediate early (IE) promoter, promoters derived from Simian Virus 40 (SV40), and the like. Suitable promoters can also be derived from eukaryotic cells, such as methallothionein (MT) promoters, elongation factor 1.alpha. (EF-1.alpha.) promoter, an actin promoter, an immunoglobulin promoter, heat shock promoters, and the like. Any promoter or enhancer/promoter capable of driving expression of the sequence of interest in the host cell is suitable in the invention. In one embodiment, the sequence capable of driving expression comprises a region from a CMV promoter, preferably the region comprising nucleotides -735 to +95 of the CMV immediate early gene enhancer/promoter. The skilled artisan will be aware that the expression sequences used in the invention may suitably be combined with elements that can stabilize or enhance expression, such as insulators, matrix attachment regions, STAR elements (WO 03/004704, the entirety of which is incorporated herein by reference), and the like. This may enhance the stability and/or levels of expression.
Protein production in recombinant host cells has been extensively described, e.g., in Current Protocols in Protein Science, 1995, Coligan J. E., Dunn B. M., Ploegh H. L., Speicher D. W., Wingfield P. T., ISBN 0-471-11184-8; Bendig, 1988, the entirety of which is incorporated herein by reference. Culturing a cell is done to enable it to metabolize, grow, divide, and/or produce recombinant proteins of interest. This can be accomplished by methods well known to persons skilled in the art and includes, but is not limited to, providing nutrients for the cell. The methods comprise growth adhering to surfaces, growth in suspension, or combinations thereof. Several culturing conditions can be optimized by methods well known in the art to optimize protein production yields. Culturing can be done, for instance, in dishes, roller bottles or in bioreactors, using batch, fed-batch, continuous systems, hollow fiber, and the like. In order to achieve large-scale (continuous) production of recombinant proteins through cell culture, it is preferred in the art to have cells capable of growing in suspension and it is preferred to have cells capable of being cultured in the absence of animal- or human-derived serum or animal- or human-derived serum components. Thus, purification is easier and safety is enhanced due to the absence of additional animal or human proteins derived from the culture medium, while the system is also very reliable as synthetic media are the best in reproducibility.
"Host cells," according to the invention, may be any host cell capable of expressing recombinant DNA molecules, including bacteria such as Escherichia (e.g., E. coli), Enterobocter, Salmonella, Bacillus, Pseudomonas, Streptomyces, yeasts such as S. cerevisiae, K. lactis, P. pastoris, Candida, or yarrowia, filamentous fungi such as Neurospora, Aspergillus oryzae, Aspergillus nidulans and Aspergillus niger, insect cells such as Spodoptera frugiperda SF-9 or SF-21 cells, mammalian cells such as Chinese hamster ovary (CHO) cells, BHK cells, mouse cells including SP2/0 cells and NS-0 myeloma cells, primate cells such as COS and Vero cells, MDCK cells, BRL 3A cells, hybridomas, tumor cells, immortalized primary cells, human cells such as W138, HepG2, HeLa, HEK293, HT1080 or embryonic retina cells such as PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins), and the like. Often, the expression system of choice will involve a mammalian cell expression vector and host so that the antibodies are appropriately glycosylated. A human cell line, preferably PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins), can advantageously be used to obtain antibodies with a completely human glycosylation pattern. The conditions for growing or multiplying cells (see, e.g., Tissue Culture, Academic Press, Kruse and Paterson, editors (1973), the entirety of which is incorporated herein by reference) and the conditions for expression of the recombinant product may differ somewhat and optimization of the process is usually performed to increase the product yields and/or growth of the cells with respect to each other, according to methods generally known to one of ordinary skill in the art.
In general, principles, protocols, and practical techniques for maximizing the productivity of mammalian cell cultures can be found in Mammalian Cell Biotechnology: a Practical Approach (M. Butler, ed., IRL Press, 1991), the entirety of which is incorporated herein by reference. Expression of antibodies in recombinant host cells has been extensively described in the art (see, e.g., EP0120694; EP0314161; EP0481790; EP0523949; U.S. Pat. No. 4,816,567; WO 00/63403, the entirety of which are incorporated herein by reference). The nucleic acid molecules encoding the light and heavy chains may be present as extrachromosomal copies and/or stably integrated into the chromosome of the host cell. With regard to stability of production, the latter is preferred.
The antibodies are expressed in the cells according to the invention and may be recovered from the cells or, preferably, from the cell culture medium, by methods generally known to persons skilled in the art. Such methods may include precipitation, centrifugation, filtration, size-exclusion chromatography, affinity chromatography, cation- and/or anion-exchange chromatography, hydrophobic interaction chromatography, and the like. For a mixture of antibodies comprising IgG molecules, protein A- or protein G-affinity chromatography can be suitably used (see, e.g., U.S. Pat. Nos. 4,801,687 and 5,151,504, the entirety of which are incorporated herein by reference).
In one embodiment, at least two antibodies from the mixture produced according to the invention comprise a heavy-light chain dimer having different specificities and/or affinities. The specificity determines which antigen or epitope thereof is bound by the antibody. The affinity is a measure for the strength of binding to a particular antigen or epitope. Specific binding is defined as binding with an affinity (K.sub.a) of at least 5.times.10.sup.4 liter/mole, more preferably, 5.times.10.sup.5, even more preferably, 5.times.10.sup.6, and still more preferably, 5.times.10.sup.7, or more. Typically, monoclonal antibodies may have affinities which go up to 10.sup.10 liter per mole or even higher. The mixture of antibodies produced according to the invention may contain at least two antibodies that bind to different epitopes on the same antigen molecule and/or may contain at least two antibodies that bind to different antigen molecules present in one antigen-comprising mixture. Such an antigen-comprising mixture may be a mixture of partially or wholly purified antigens, such as toxins, membrane components and proteins, viral envelope proteins, or it may be a healthy cell, a diseased cell, a mixture of cells, a tissue or mixture of tissues, a tumor, an organ, a complete human or animal subject, a fungus or yeast, a bacteria or bacterial culture, a virus or virus stock, or combinations of these, and the like. Unlike monoclonal antibodies that are able to bind to a single antigen or epitope only, the mixture of antibodies according to the invention may, therefore, have many of the advantages of a polyclonal or oligoclonal antibody mixture.
In a preferred embodiment, the host cell according to the method of the invention is capable of high-level expression of human immunoglobulin, i.e., at least 1 picograms per cell per day, preferably, at least 10 picograms per cell per day and, even more preferably, at least 20 picograms per cell per day or more without the need for amplification of the nucleic acid molecules encoding the heavy and light chains in the host cell.
Preferably, host cells according to the invention contain in their genome between one and ten copies of each recombinant nucleic acid to be expressed. In the art, amplification of the copy number of the nucleic acid sequences encoding a protein of interest in, e.g., CHO cells can be used to increase expression levels of the recombinant protein by the cells (see, e.g., Bendig, 1988; Cockett et al., 1990; U.S. Pat. No. 4,399,216, the entirety of which are incorporated herein by reference). This is currently a widely used method. However, a significant time-consuming effort is required before a clone with a desired high copy number and high expression levels has been established and, moreover, clones harboring very high copy numbers (up to hundreds) of the expression cassette often are unstable (e.g., Kim et al., 1998, the entirety of which is incorporated herein by reference). It is, therefore, a preferred embodiment of the invention to use host cells that do not require such amplification strategies for high-level expression of the antibodies of interest. This allows fast generation of stable clones of host cells that express the mixture of antibodies according to the invention in a consistent manner. We provide evidence that host cells according to the invention can be obtained, sub-cloned and further propagated for at least around 30 cell divisions (population doublings) while expressing the mixture of antibodies according to the invention in a stable manner, in the absence of selection pressure. Therefore, in certain aspects, the methods of the invention include culturing the cells for at least 20, preferably 25, more preferably 30, population doublings and, in other aspects, the host cells according to the invention have undergone at least 20, preferably 25, more preferably 30, population doublings and are still capable of expressing a mixture of antibodies according to the invention. Also provided is a culture of cells producing a mixture of immunoglobulins from a single cell, the mixture comprising at least three different heavy chains. Also provided is a culture of cells producing at least three different monospecific immunoglobulins from a single cell. In certain exemplary aspects, the culture produces the mixture or at least three different monospecific immunoglobulins in a single cell for more than 20, preferably more than 25, more preferably, more than 30 population doublings.
Preferably, host cells according to the method are derived from human retina cells that have been immortalized or transformed with adenoviral E1 sequences. A particularly preferred host cell according to methods of the invention is PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) as deposited under ECACC no. 96022940, or a derivative thereof. PER.C6.RTM.-derived clones can be generated fast, usually contain a limited number of copies (about 1-10) of the transgene, and are capable of high-level expression of recombinant antibodies (Jones et al., 2003, the entirety of which is incorporated herein by reference). Therefore, such clones are expected to maintain a stable copy number over many generations, which is an advantage in the production of biopharmaceuticals. PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) cells have been extensively characterized and documented, demonstrating good process of scaling up, suspension growth and growth factor independence. Furthermore, PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) can be incorporated into a suspension in a highly reproducible manner, making it particularly suitable for large-scale production. In this regard, the PER.C6.RTM. cell line (human retina cells that express adenovirus E1A and E1B proteins) has been characterized for bioreactor growth, where it can grow to very high densities. The use of PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) for recombinant production of antibodies has been described in detail in publication WO 00/63403 and in (Jones et al., 2003, the entirety of which is incorporated herein by reference).
Also provided is a mixture of antibodies obtainable by a method described herein. Such mixtures of antibodies are expected to be more effective than the sole components it comprises, in analogy to polyclonal antibodies usually being more effective than monoclonal antibodies to the same target. Such mixtures can be prepared against a variety of target antigens or epitopes.
It certain embodiments, provided is a recombinant host cell comprising a nucleic acid sequence encoding a light chain and a nucleic acid sequence or nucleic acid sequences encoding at least three different heavy chains of an antibody, wherein the light chain and heavy chains are capable of pairing, preferably to form a functional binding domain. The paired heavy and light chains form functional antigen-binding regions against the target antigen or target antigens. The host cells are useful in the described methods. They can be used to produce mixtures of antibodies.
In certain embodiments, provided is a composition comprising a mixture of recombinantly produced antibodies, wherein at least three different heavy chain sequences are represented in the mixture of recombinant antibodies. Monoclonal antibodies are routinely produced by recombinant methods. Also disclosed are mixtures of antibodies useful for diagnosis or treatment in various fields. In certain embodiments, the compositions of the invention comprise mixtures of at least three different heavy chains paired to light chains in the form of antibodies. Preferably, the light chains of the antibodies in the mixtures have a common light chain. The mixtures may comprise bispecific antibodies. The mixtures may be produced from a clone that was derived from a single host cell, e.g., from a population of cells containing the same recombinant nucleic acid sequences. The mixtures can be obtained by methods according to the invention or be produced by host cells according to the invention. In other embodiments, the number of heavy chains represented in the mixture is 4, 5, 6, 7, 8, 9, 10, or more. The optimal mixture for a certain purpose may be determined empirically by methods known to one of ordinary skill in the art or by methods provided by the invention. Such compositions according to the invention may have several of the advantages of a polyclonal antibody mixture, without the disadvantages usually inherently associated with polyclonal antibody mixtures, because of the manner in which they are produced. It is furthermore expected that the mixture of antibodies is more efficacious than separate monoclonal antibodies. Therefore, the dosage and, hence, the production capacity required may be less for the mixtures of antibodies according to the invention than for monoclonal antibodies.
It has, for instance, been described that although no single monoclonal antibody to botulinum neurotoxin (BoNT/A) significantly neutralized toxin, a combination of three such monoclonal antibodies (oligoclonal antibody) neutralized 450,000 50% lethal doses of BoNTI A, a potency 90 times greater than human hyperimmune globulin (Nowakowski et al. 2002, the entirety of which is incorporated herein by reference). This result demonstrates that oligoclonal mixtures of antibodies comprising only two to three different specificities may have very high potency.
Furthermore, the chances of a mixture herein losing its activity due to target or epitope loss are reduced, when compared to a single monoclonal antibody. In particular embodiments, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more of the antibodies present in the mixture according to the invention have different specificities. Different specificities may be directed to different epitopes on the same antigen and/or may be directed to different antigens present in one antigen-comprising mixture. A composition as described herein may also further comprise 2, 3, 4, 5, 6, 7, 8, 9, 10, or more antibodies having different affinities for the same epitope. Antibodies with differing affinities for the same epitope may, for instance, be generated by methods of affinity maturation known to one of ordinary skill in the art.
In a particularly preferred embodiment, the composition according to the invention has an effect that is greater than the effect of each individual monospecific antibody present in the composition. The effect can be measured in a functional assay. A "functional assay," as used herein, is an assay that can be used to determine one or more desired parameters of the antibody or the mixture of antibodies subject to the assay conditions. Suitable functional assays may be binding assays, apoptosis assays, antibody-dependent cellular cytotoxicity (ADCC) assays, complement-dependent cytotoxicity (CDC) assays, inhibition of cell growth or proliferation (cytostatic effect) assays, cell-killing (cytotoxic effect) assays, cell-signaling assays, assays for measuring inhibition of binding of pathogen to target cell, assays to measure the secretion of vascular endothelial growth factor (VEGF) or other secreted molecules, assays for bacteriostasis, bactericidal activity, neutralization of viruses, assays to measure the attraction of components of the immune system to the site where antibodies are bound, including in situ hybridization methods, labeling methods, and the like. Clearly, also in vivo assays, such as animal models, including mouse tumor models, models of auto-immune disease, virus-infected or bacteria-infected rodent or primate models, and the like, can be used for this purpose. The efficacy of a mixture of antibodies according to the invention can be compared to individual antibodies in such models by methods generally known to one of ordinary skill in the art.
In certain embodiments, provided is a method for identifying at least one host cell clone that produces a mixture of antibodies, wherein the mixture of antibodies has a desired effect according to a functional assay, the method comprising (i) providing a host cell comprising a nucleic acid sequence encoding at least one light chain and nucleic acid sequence or sequences encoding at least two different heavy chains, wherein the heavy and light chains are capable of pairing with each other; (ii) culturing at least one clone of the host cell under conditions conducive to expression of nucleic acid sequences; (iii) screening at least one clone of the host cell for production of a mixture of antibodies having the desired effect by a functional assay; and (iv) identifying at least one clone that produces a mixture of antibodies having the desired effect. Preferably, the host cell comprises a nucleic acid sequence encoding a common light chain that is capable of pairing with at least two different heavy chains, such that produced antibodies comprise common light chains, as described above. In specific embodiments, culturing in step (ii) and screening in step (iii) of the method is performed with at least two clones. The method may optionally include an assay for measuring the expression levels of the antibodies that are produced, which assay may be during or after step (ii) according to the method, or later in the procedure. Such assays are well known to one of ordinary skill in the art and include protein concentration assays, immunoglobulin-specific assays such as ELISA, RIA, DELFIA, and the like. In particular embodiments of the method according to the invention, the host cell comprises nucleic acid sequence or sequences encoding at least 3, 4, 5, 6, 7, 8, 9, 10, or more, heavy chains capable of pairing with at least one light chain. Functional assays useful for the method according to the invention may be assays for apoptosis, ADCC, CDC, cell killing, inhibition of proliferation, virus neutralization, bacterial opsonization, receptor-mediated signaling, cell signaling, bactericidal activity, and the like. Useful screening assays for anti-cancer antibodies have, for instance, been described in U.S. Pat. No. 6,180,357, the entirety of which is incorporated herein by reference. Such assays may also be used to identify a clone according to the method of the invention. It is, for instance, possible to use enzyme-linked immunosorbent assays (ELISAs) for the testing of antibody binding to their target. Using such assays, it is possible to screen for antibody mixtures that most avidly bind the target antigen (or mixture of target antigens against which the mixture of antibodies is to be tested). Another possibility that can be explored is to directly screen for cytotoxicity or cytostatic effects. It is possible that upon such a different screen, other or the same clones producing mixtures of antibodies will be chosen than with the ELISA mentioned above. The screening for cell killing or cessation of growth of cancerous cells may be suitably used according to the invention. Cell death can be measured by various endpoints, including the absence of metabolism or the denaturation of enzymes. In one possible embodiment of the invention, the assay is conducted by focusing on cytotoxic activity toward cancerous cells as an endpoint. For this assay, a live/dead assay kit, for example, the LIVE/DEAD.RTM. Viability/Cytotoxicity Assay Kit (L-3224) by Molecular Probes (Eugene, Oreg.), can suitably be used. Other methods of assessing cell viability, such as tryspan blue exclusion, .sup.51Cr release, Calcein-AM, ALAMAR BLUE.TM., LDH activity, and similar methods, can also be used. The assays may also include screening of the mixture of antibodies for specificity to the desired antigen-comprising tissue. The antibodies according to the invention may have a limited tissue distribution. It is possible to include testing the mixtures of antibodies against a variety of cells, cell types, or tissues, to screen for mixtures of antibodies that preferably bind to cells, cell types or tissues of interest.
Irrespective of a functional assay as described above, also disclosed herein are ways to determine the identity of the antibodies expressed by a clone, using methods such as iso-electric focusing (IEF), mass-spectrometry (MS), and the like. In certain embodiments, therefore, provided is use of MS and/or IEF in selecting a clone that expresses a mixture of antibodies according to the invention.
When monoclonal antibodies are produced by recombinant host cells, a screening step is usually performed to assess expression levels of the individual clones that were generated. The addition of more heavy chains to produce mixtures adds a level of complexity to the production of antibodies. When host cells are transfected with nucleic acid molecules encoding the light and heavy chains that will form the mixture of antibodies desired, independent clones may arise containing the same genetic information but, nevertheless, differing in expression levels, thereby producing different ratios of the encoded antibodies, giving rise to different mixtures of antibodies from the same genetic repertoire. The method according to the invention is useful for identifying a clone that produces an optimal mixture for a certain purpose.
The culturing and/or screening according to steps (ii) and (iii), respectively, may be suitably performed using high-throughput procedures, optionally in an automated fashion. Clones can, for instance, be cultured in 96-well plates or other multi-well plates, e.g., in arrayed format, and screened for production of a desired mixture. Robotics may be suitably employed for this purpose. Methods to implement high-throughput culturing and assays are generally available and known to one of ordinary skill in the art. It will also be clear that for this method according to the invention, it is beneficial to use host cells capable of high-level expression of proteins, without the need for amplification of the nucleic acid encoding the proteins in the cell. In one embodiment, the host cell is derived from a human embryonic retinoblast cell that has been immortalized or transformed by adenoviral E1 sequences. In a preferred embodiment, the cell is derived from PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins). This cell line has already been shown to be amenable to high-throughput manipulations, including culturing (WO 99/64582, the entirety of which is incorporated herein by reference).
In specific embodiments of the invention, the mixture of antibodies according to the method of identifying at least one host cell according to the invention comprises at least 2, 3, 4, 5, 6, 7, 8, 9, 10, or more, antibodies having different specificities and/or affinities.
A potential advantage of the method will be that it will allow exploring many possible combinations simultaneously, the combinations inherently including the presence of bispecific antibodies in the produced mixture. Therefore, more combinations can be tested than by just mixing purified known monoclonal antibodies, both in number of combinations and in ratios of presence of different antibodies in these combinations.
The clone that has been identified by the method according to the invention can be used for producing a desired mixture of antibodies. In certain embodiments, provided is a method of producing a mixture of antibodies, the method comprising culturing a host cell clone identified by the method of identifying at least one host cell clone that produces a mixture of antibodies according to the invention, culturing being under conditions conducive to expression of the nucleic acid molecules encoding at least one light chain and at least two different heavy chains. The produced antibodies may be recovered from the host cells and/or from the host cell culture, for example, from the culture medium. The mixture of antibodies can be recovered according to a variety of techniques known to one of ordinary skill in the art.
In certain embodiments, provided is a mixture of antibodies obtainable by the method according to the invention described above. The mixtures can be used for a variety of purposes, such as in the treatment or diagnosis of disease, and may replace, or be used in addition to, monoclonal or polyclonal antibodies.
The methods according to the invention may suitably use nucleic acid molecules for encoding the antibodies, which nucleic acid molecules have been obtained by any suitable method, including in vivo, e.g., immunization, methods or in vitro, for instance, antibody display methods (A. Pluckthun et al., In vitro selection and evolution of proteins, in Adv. Prot. Chem., F. M. Richards et al., Eds, Academic Press, San Diego, 2001, vol. 55:367-403, the entirety of which is incorporated herein by reference), such as phage display, ribosome display or mRNA display (C. Schaffitzel et al., In vitro selection and evolution of protein-ligand interactions by ribosome display, in Protein-Protein Interactions, A Molecular Cloning Manual, E. Golemis, Ed., Cold Spring Harbor Laboratory Press, New York, 2001, pp. 535-567, the entirety of which is incorporated herein by reference), and yeast display (e.g., WO 99/36569, the entirety of which is incorporated herein by reference). Methods of identifying antibodies to a certain target, which target may be a known antigen or an unknown antigen present in an antigenic mixture, by phage display are known to one of ordinary skill in the art. In general, a library of phages that express an antigen-binding domain or derivative thereof on their surface, the antigen-binding domain encoded by genetic material present in the phages, is incubated with the antigen or antigen mixture of interest, after which binding of a sub-population of the phages that display antigen-binding sites binding to the desired antigen is obtained whereas the non-binding phages are discarded. Such selection steps may be repeated one, two, or more times to obtain a population of phages that are more or less specific for the antigen of interest. Phage display methods to obtain antibodies, parts or derivatives thereof have been extensively described in C. F. Barbas III et al., Phage Display, A laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 2001, the entirety of which is incorporated herein by reference. The library used for such screening may be generated by using the genetic information of one or more light chains, combined with genetic information encoding a plurality of heavy chains. The library described by De Kruif et al. (1995b), the entirety of which is incorporated herein by reference, comprises seven light chains, the entirety of which is incorporated herein by reference. Therefore, in a panel of phages binding to a target, which can, e.g., be obtained by methods described in De Kruif et al. (supra), and U.S. Pat. No. 6,265,150 (the entirety of which is incorporated herein by reference), not more than seven different light chains will be represented and, if the panel is large enough, several phages with the same light chain coupled to unrelated heavy chains may be found. Such phages can be used to obtain the nucleic acid molecules useful in the methods according to the invention.
In certain embodiments, provided is a method for producing a mixture of antibodies to a target, the method comprising i) bringing an antibody display library comprising antibodies or antibody fragments into contact with material comprising a target, ii) at least one step of selecting antibodies or antibody fragments binding to the target, iii) identifying at least two antibodies or antibody fragments binding to the target, wherein at least two antibodies or antibody fragments comprise a common light chain, iv) introducing a nucleic acid sequence encoding the light chain and a nucleic acid sequence or nucleic acid sequences encoding the heavy chains of at least two antibodies into a host cell, v) culturing a clone of the host cell under conditions conducive to expression of nucleic acid sequences. The antibody display library may be a phage display library, a ribosome display library, an mRNA display library, or a yeast display library. Steps i) and ii) may optionally be repeated one or more times.
The nucleic acid sequences encoding the antibodies obtained by the phage display, ribosome display or yeast display method may be converted to encode any desired antibody format such as IgG1, IgG2, IgG3, IgG4, IgA, IgM, IgD, IgE, before introducing them into a host cell, using standard molecular cloning methods and means known to one of ordinary skill in the art (e.g., described in Boel et al., 2000, the entirety of which is incorporated herein by reference).
It will be clear to one of ordinary skill in the art that libraries in which only one light chain is represented are especially useful in light of the invention, since all antibodies that can be obtained from such a library will have a common light chain that is functional in binding target antigen with each of the heavy chains. In other words, in accordance with the methods of the invention, the formation of non-functional light chain-heavy chain dimers is avoided. Phage antibody display libraries having extensive H chain repertoires and unique or very few L chain sequences have been disclosed in the art (Nissim et al., 1994; Vaughan et al., 1996, the entirety of which are incorporated herein by reference). In general, the specificity of an antibody appears to be determined to a large extent by its heavy chain. It is even possible to screen for and identify light chains that do not contribute significantly to binding of the antibody, which light chains also could be suitably used according to the invention. It may also be possible to follow the teachings of the invention but use one heavy chain and vary the light chains. However, the use of a common light chain and different heavy chains appears preferable and the following observations support the idea that the specificity of an antibody appears to be dominated by its heavy chain sequence. In the process of receptor editing, a mechanism of B-cells to monitor if their immunoglobulin receptor encodes a potentially harmful auto-antibody, B-cells expressing an auto-antibody replace the expressed heavy chain with another heavy chain while retaining the expressed light chain. Thus, a new antibody specificity is generated that does not encode an auto-antibody. This shows that a single light chain can successfully dimerize with multiple heavy chains to form different antibody specificities (Nemazee, 2000; Casellas et al., 2001, the entirety of which are incorporated herein by reference). Series of transfected cell lines using a single heavy chain gene with different light chain genes have been reported, the antibodies produced to a large extent maintaining their specificity, regardless of the light chain (Radic et al., 1991, the entirety of which is incorporated herein by reference).
Different antibodies have been obtained from a library that has been constructed using a single light chain (Nissim et al., 1994). Several antibodies have been obtained from the library described by De Kruif et al. (1995, the entirety of which is incorporated herein by reference), which was constructed using seven light chains, that have the same light chain but different specificities (see, e.g., Example 1: antibodies binding to EpCAM and to CD46, described in WO 01/48485 and WO 02/18948, respectively, the entirety of which are incorporated herein by reference).
Besides screening a phage library against a target, it will also be possible to start with an antibody that has already proven its merits and use the light chain of this antibody in the preparation of a library of heavy chains combined with this particular light chain only, according to methods known to one of ordinary skill in the art, such as phage display. Using this strategy, a monoclonal antibody can be used to obtain a mixture of antibodies according to the invention, functionally resembling a polyclonal or oligoclonal antibody to the same target. Alternatively, a method reminiscent of the method described by Jespers et al. (1994, the entirety of which is incorporated herein by reference) to obtain a human antibody based on a functional rodent antibody can be used. The heavy chain of a known antibody of non-human origin is first cloned and paired as a template chain with a repertoire of human light chains for use in phage display, after which the phages are selected for binding to the antigen or mixture of antigens. The selected light chain is, in turn, paired with a repertoire of human heavy chains displayed on a phage and the phages are selected again to find several heavy chains that, when paired with the light chain, are able to bind to the antigen or mixture of antigens of interest. This enables creating a mixture of human antibodies against a target for which thus far only a non-human monoclonal antibody is described. It is possible that a mixture according to the invention already has beneficial functional effects when the individual antibodies do not have high affinities for the target, whereas high affinities are often required for monoclonal antibodies to be effective. This would have the advantage that affinity maturation may be required in less instances for methods and mixtures according to the invention than when an approach with monoclonal antibodies is envisaged.
The heavy and light chain coding sequences can be introduced simultaneously or consecutively into the host cell. It is also an aspect to prepare a host cell comprising a recombinant nucleic acid encoding a light chain of an antibody. Such a cell can, for instance, be obtained by transfection of the nucleic acid and, optionally, a clone can be identified that has a high expression of the light chain. An established clone may then be used to add genetic information encoding 2, 3, 4, 5, 6, 7, 8, 9, 10, or more, heavy chains of the invention by introducing the nucleic acid molecules encoding these into cells of the clone that already contains the light chain. The nucleic acid molecules encoding the heavy chains may be introduced into the host cell concomitantly. It is, of course, also possible to introduce them consecutively, for instance, by using different selection markers, which can be advantageous if not all heavy chains can be introduced simultaneously because the cells do not take up enough copies of recombinant nucleic acid molecules. Methods to introduce recombinant nucleic acid molecules into host cells are well known to one of ordinary skill in the art and include transfection, electroporation, calcium phosphate precipitation, virus infection, and the like. One of ordinary skill in the art has several possibilities to introduce more vectors with nucleic acid sequences of interest into the same host cell, see, e.g., Sambrook, Fritsch and Maniatis, Molecular Cloning: A Laboratory Manual, 2.sup.nd edition, 1989; Current Protocols in Molecular Biology, Ausubel F. M., et al., eds, 1987; the series Methods in Enzymology (Academic Press, Inc.), the entirety of which are incorporated herein by reference.
Suitable dominant selection markers for introducing nucleic acids into eukaryotic host cells, as used herein, may be G418 or neomycin (geneticin), hygromycin or mycophenolic acid, puromycin, and the like, for which genes encoding resistance are available on expression vectors. Further possibilities include, for instance, the use of vectors containing DHFR genes or glutamate synthetase to select in the presence of methotrexate in a DHFR.sup.- cell or the absence of glutamine in a glutamine auxotroph, respectively. The use of expression vectors with different selection markers enables subsequent transfections with heavy chain sequences of interest into the host cell, which already stably contains other heavy chains introduced previously by use of other selection markers. It is also possible to use selection markers that can be used more than once, for instance, when containing mutations, introns, or weakened promoters that render them concentration-dependent (e.g., EP0724639; WO 01/32901; U.S. Pat. No. 5,733,779, the entirety of which are incorporated herein by reference). Alternatively, a selection marker may be re-used by deleting it from the host cell after use, for example, by site-specific recombination. A selectable marker located between sequences recognized by a site-specific recombinase, for example, lox-sites or FRT-sites, is used for the generation of the first stable transfectant (for Cre-lox site-specific recombination, see, Wilson and Kola, 2001, the entirety of which is incorporated herein by reference). Subsequently, the selectable marker is excised from the host cell DNA by the matching site-specific recombinase, for example, Cre or Flp. A subsequent transfection can suitably use the same selection marker.
Different host cell clones each comprising the genetic information encoding a different light chain may be prepared. If the antibodies are identified by an antibody display method, it is thus possible to prepare several host cells, each comprising one light chain present in the antibody display library. After identifying antibodies that bind to a target using antibody display, the nucleic acid molecules encoding the heavy chains can be introduced into the host cell containing the common light chain that is capable of pairing to the heavy chains. It is, therefore, an aspect to provide a method for making a host cell for production of a mixture of antibodies, the method comprising the steps of: introducing into the host cell a nucleic acid sequence encoding a light chain and nucleic acid sequence or sequences encoding 3, 4, 5, 6, 7, 8, 9, 10, or more, different heavy chains that are capable of pairing with the light chain, wherein the nucleic acid molecules are introduced consecutively or simultaneously. It is, of course, also possible to introduce at least two of the nucleic acid molecules simultaneously, and introduce at least one other of the nucleic acid molecules consecutively.
In yet another aspect, a method is provided for making a recombinant host cell for production of a mixture of antibodies, the method comprising the step of: introducing a nucleic acid sequence or nucleic acid sequences encoding 2, 3, 4, 5, 6, 7, 8, 9, 10, or more, different heavy chains into a recombinant host cell comprising a nucleic acid sequence encoding a light chain capable of pairing with at least two of the heavy chains.
If it appears that a recombinant host cell of the invention does not express sufficient light chain to dimerize with all of the expressed at least two heavy chains, extra copies of the nucleic acid molecules encoding the light chain may be transfected into the cell.
Besides random integration after transfection, methods to integrate the transgenes in predetermined positions of the genome resulting in favorable expression levels can also be used according to the invention. Such methods may, for instance, employ site-specific integration by homologous recombination (see, e.g., WO 98/41645, the entirety of which is incorporated herein by reference) or make use of site-specific recombinases (Gorman and Bullock, 2000, the entirety of which is incorporated herein by reference).
It is yet another aspect to provide a transgenic non-human mammal or a transgenic plant comprising a nucleic acid sequence encoding a light chain and a nucleic acid sequence or nucleic acid sequences encoding at least two different heavy chains that are capable of pairing with the light chain, wherein the nucleic acid sequences encoding the light and heavy chains are under the control of a tissue-specific promoter. Promoters in plants may also be non-tissue specific and general gene-expression elements, such as the CaMV .sup.35S promoter and nopaline synthase polyA addition site, can also be used. The light chain is a common light chain according to the invention. In specific embodiments, the transgenic animal or plant according to the invention comprises 3, 4, 5, 6, 7, 8, 9, 10, or more, heavy chain sequences. Besides cell culture as a production system for recombinant proteins, the art also discloses the use of transgenic animals, transgenic plants and, for instance, transgenic chickens to produce proteins in the eggs, and the like to produce recombinant proteins of interest (Pollock et al., 1999; Larrick and Thomas, 2001; WO 91/08216, the entirety of which are incorporated herein by reference). These usually comprise the recombinant gene or genes encoding one or more proteins of interest in operable association with a tissue-specific promoter. It has, for instance, been shown that recombinant antibodies can be produced at high levels in the milk of transgenic animals that contain the nucleic acids encoding a heavy and a light chain behind a mammary gland-specific promoter (e.g., Pollock et al., 1999; WO 95/17085, the entirety of which are incorporated herein by reference). Particularly useful in this respect are cows, sheep, goats, pigs, rabbits, mice, and the like, which can be milked to obtain antibodies. Useful promoters are the casein promoters, such as the .beta.-casein promoter, the .alpha.S1-casein promoter, the whey acidic protein (WAP) promoter, the .beta.-lactoglobulin promoter, the .alpha.-lactalbumin promoter, and the like. Production of biopharmaceutical proteins in the milk of transgenic mammals has been extensively described (e.g., Pollock et al., 1999, the entirety of which is incorporated herein by reference). Besides mammary gland-specific promoters, other tissue-specific promoters may be used, directing the expression to the blood, urine, saliva, and the like. The generation of transgenic animals comprising recombinant nucleic acid molecules has been extensively documented and may include micro-injection of oocytes (see, e.g., Wilmut and Clark, 1991, the entirety of which is incorporated herein by reference), nuclear transfer after transfection (e.g., Schnieke et al., 1997, the entirety of which is incorporated herein by reference), infection by recombinant viruses (e.g., U.S. Pat. No. 6,291,740, the entirety of which is incorporated herein by reference), and the like. Nuclear transfer and cloning methods for mammalian cells are known to one of ordinary skill in the art, and are, for example, described in Campbell et al., 1996; Wilmut et al., 1997; Dinnyes et al., 2002; WO 95/17500; and WO 98/39416, the entirety of which are incorporated herein by reference. It is possible to clone animals and to generate lines of animals that are genetically identical, which renders it possible for a person skilled in the art to create such a line once an individual animal producing the desired mixture of antibodies has been identified. Alternatively, classical breeding methods can be used to generate transgenic offspring. Strategies for the generation of transgenic animals for production of recombinant proteins in milk are described in Brink et al., 2000, the entirety of which is incorporated herein by reference.
Transgenic plants or plant cells producing antibodies have also been described (Hiatt et al., 1989; Peeters et al., 2001, the entirety of which are incorporated herein by reference) and useful plants for this purpose include corn, maize, tobacco, soybean, alfalfa, rice, and the like. Constitutive promoters that can, for instance, be used in plant cells are the CaMV 35S and 19S promoters and Agrobacterium promoters nos and ocs. Other useful promoters are light-inducible promoters such as rbcS. Tissue-specific promoters can, for instance, be seed-specific, such as promoters from zein, napin, beta-phaseolin, ubiquitin, or tuber-specific, leaf-specific (e.g., useful in tobacco), root-specific, and the like. It is also possible to transform the plastid organelle by homologous recombination to express proteins in plants.
Methods and means for expression of proteins in recombinant plants or parts thereof, or recombinant plant cell culture, are known to one of ordinary skill in the art and have been, for instance, described in Giddings et al., 2000; WO 01/64929; WO 97/42313; U.S. Pat. Nos. 5,888,789, 6,080,560 (for practical guidelines, see Methods In Molecular Biology vol. 49 "Plant Gene Transfer And Expression Protocols," H. Jones, 1995), the entirety of which are incorporated herein by reference. Other transgenic systems for producing recombinant proteins have also been described, including the use of transgenic birds to produce recombinant proteins in eggs (e.g., WO 97/47739, the entirety of which is incorporated herein by reference) and the use of transgenic fish (e.g., WO 98/15627, the entirety of which is incorporated herein by reference), and can be used in combination with the teachings of the invention to obtain mixtures of antibodies. It is also possible to use an in vitro transcription/translation or in vitro translation system for the expression of mixtures of antibodies according to the invention. It will be clear to one of ordinary skill in the art that the teachings of the current invention will allow producing mixtures of antibodies in systems where recombinant nucleic acids encoding the light chain and heavy chains can be introduced and expressed. Preferably, such systems are able to produce antibodies encoded by nucleic acid sequences, without the use of amplification of nucleic acid sequences in the systems. In another aspect, a cell from a transgenic non-human animal or a transgenic plant according to the invention is provided. Such cells can be used to generate the animals or plants according to the invention, using techniques known to one of ordinary skill in the art, such as nuclear transfer or other known methods of cloning whole organisms from single cells. The cells according to the invention may also be obtained by introducing the light and at least two heavy chain sequences into isolated cells of non-human animals or plants, which cells are capable of becoming part of a transgenic animal or plant. Particularly useful for such purposes are embryonic stem cells. These can contribute to the germ line and, therefore, the genetic information introduced into such cells can be passed to future generations. In addition, plant cell cultures of cotton, corn, tomato, soybean, potato, petunia, and tobacco can be utilized as hosts when transformed with the nucleic acid molecules encoding the light chain and the heavy chains, for instance, by use of the plant-transforming bacterium A. tumefaciens or by particle bombardment or by infecting with recombinant plant viruses.
In certain embodiments, provided is a pharmaceutical composition comprising a mixture of recombinantly produced antibodies and a suitable carrier, wherein at least two different heavy chains are represented in the mixture of recombinantly produced antibodies. Pharmaceutically acceptable carriers as used herein are exemplified, but not limited to, adjuvants, solid carriers, water, buffers, or other carriers used in the art to hold therapeutic components, or combinations thereof. In particular embodiments, 3, 4, 5, 6, 7, 8, 9, 10, or more, different heavy chains are represented in the mixture. The mixture can be obtained by mixing recombinantly produced monoclonal antibodies, but may also be obtained by methods according to the invention. The mixture may, therefore, comprise a common light chain for the antibodies. The mixture may comprise bispecific antibodies. The mixture may be produced from a clone that was derived from a single host cell, e.g., from a population of cells containing the same recombinant nucleic acid molecules. The term "recombinantly produced" as used herein refers to production by host cells that produce antibodies encoded by recombinant nucleic acids introduced in such host cells or ancestors thereof. It does not, therefore, include the classical method of producing polyclonal antibodies, whereby a subject is immunized with an antigen or antigen-comprising mixture, after which the antibodies produced by this subject are recovered from the subject, for example, from the blood.
In certain embodiments, provided is a mixture of antibodies wherein at least two heavy chains are represented for use in the treatment or diagnosis of a human or animal subject. In another aspect, provided is the use of a mixture of antibodies wherein at least two different heavy chains are represented for the preparation of a medicament for use in the treatment or diagnosis of a disease or disorder in a human or animal subject. In particular embodiments, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more, heavy chains are represented in the mixture. The mixtures of antibodies may be mixtures of antibodies according to the invention or obtained by methods according to the invention. Antibodies present in the mixture may preferably comprise a common light chain. The mixtures may comprise bispecific antibodies and may be recombinantly produced from a clone that was derived from a single host cell, i.e., from a population of cells containing the same recombinant nucleic acid molecules. The targets may be used to screen an antibody display library, as described supra, to obtain 2, 3, 4, 5, 6, 7, 8, 9, 10, or more, antibodies comprising a common light chain that bind to the target and produce a mixture of these according to the teachings of the invention. Virtually any area of medicine where monoclonal antibodies can be used is amenable for the use of the mixtures according to the invention. This can, e.g., include treatment of auto-immune diseases and cancer, including solid tumors of the brain, head, neck, breast, prostate, colon, lung, and the like, as well as hematologic tumors, such as B-cell tumors. Neoplastic disorders which can be treated with the mixtures according to the invention include leukemias, lymphomas, sarcomas, carcinomas, neural cell tumors, squamous cell carcinomas, germ cell tumors, metastases, undifferentiated tumors, seminomas, melanomas, myelomas, neuroblastomas, mixed cell tumors, neoplasias caused by infectious agents, and other malignancies. Targets for the antibody mixtures may include, but are not limited to, the HER-2/Neu receptor, other growth factor receptors (such as VEGFR1 and VEGFR2 receptors), B-cell markers (such as CD19, CD20, CD22, CD37, CD72, etc.), T-cell markers (such as CD3, CD25, etc.), other leukocyte cell surface markers (such as CD33 or HLA-DR, etc.), cytokines (such as TNF), interleukins, receptors for these cytokines (such as members of the TNF receptor family), and the like. It is anticipated that the use of such mixtures of antibodies in the treatment of cancerous tissues or other complex multi-antigen-comprising cells such as microorganisms or viruses will give rise to less occurrence of epitope-loss escape variants than the use of single monoclonal antibodies. Several treatments nowadays use polyclonal mixtures of antibodies, which are derived from immunized humans or animals. These treatments may be replaced by use of the mixtures according to the invention. Use of these mixtures can also include use in graft-versus-host rejections known in the art of transplantation, e.g., by use of anti-thymocyte antibodies. It is anticipated that the mixtures of antibodies are superior to monoclonal antibodies in the treatment of complex antigens or antigen-comprising mixtures such as bacteria or viruses. Therefore, use according to the invention can also include use against strains of bacteria and fungi, e.g., in the treatment of infectious diseases due to pathogenic bacteria such as multi-drug-resistant S. aureus and the like, fungi such as Candida albicans and Aspergillus species, yeast and the like. The mixtures according to the invention may also be used for post exposure prophylaxis against viruses, such as members of the genus Lyssavirus, e.g., rabies virus, or for therapeutic or prophylactic use against viruses such as Varicella-Zoster Virus, Adenoviruses, Respiratory Syncitium Virus, Human Immunodeficiency Virus, Human Metapneumovirus, influenza virus, West Nile Virus, the virus causing Severe Acute Respiratory Syndrome (SARS), and the like. Mixtures according to the inventions can also be used to protect against agents, both bacteria and viruses, and against toxic substances that are potential threats of biological warfare. Therefore, use according to the invention can also include use against strains of bacteria such as Bacillus anthracis, Clostridium botulinum toxin, Clostridium perfringens epsilon toxin Yersinia Pestis, Francisella tulariensis, Coxiella burnetii, Brucella species, Staphylococcus enterotoxin B, or against viruses such as Variola major, alpha viruses causing meningoencephalitis syndromes (EEEV, VEEV, and WEEV), viruses known to cause hemorrhagic fevers such as Ebola, Marburg and Junin virus or against viruses such as Nipah virus, Hantaviruses, Tick borne encephalitis virus and Yellow fever virus or against toxins, for example, ricin toxin from Ricinus communis and the like. Use of the mixtures according to the invention can also include use against unicellular or multicellular parasites. Recombinant mixtures of antibodies according to the invention may become a safe alternative to polyclonal antibodies obtained from pools of human sera for passive immunization or from sera of hyper-immunized animals. The mixtures may be more efficacious than recombinant monoclonal antibodies in various therapeutic applications, including cancer, allergy, viral diseases, chronic inflammation, and the like.
It has been described that homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells (Ghetie et al., 1997, the entirety of which is incorporated herein by reference). Possibly, when antibodies against receptors or other surface antigens on target cells, such as tumor cells or infectious microorganisms, are produced according to the invention, the bispecific antibodies present in mixtures according to the invention may also cross-link different receptors or other antigens on the surface of target cells and, therefore, such mixtures may be very suitable for killing such cells. Alternatively, when bispecific antibodies are less desirable, the invention also provides methods to recombinantly produce mixtures of antibodies comprising mainly monospecific antibodies. It has been described that the efficacy of treatment with Rituximab.TM. (anti-CD20 monoclonal antibody) was increased when anti-CD59 antibodies were added (Herjunpaa et al., 2000, the entirety of which is incorporated herein by reference).
Therefore, it is thought that inclusion of antibodies against CD59 in a mixture comprising anti-tumor antibodies in the form of B-cell receptor-recognizing antibodies increases the sensitivity of tumor cells to complement attack. It has also been shown that a triple combination cocktail of anti-CD19, anti-CD22, and anti-CD38-saporin immunotoxins is much more effective than the individual components in the treatment of human B-cell lymphoma in an immunodeficient mouse model (Flavell et al., 1997, the entirety of which is incorporated herein by reference). Many other combinations may also be feasible and can be designed by one of ordinary skill in the art. In general, the use of antibody mixtures that are capable of recognizing multiple B-cell epitopes will likely decrease the occurrence of escape variants.
Another possible target is a transmembrane tyrosine kinase receptor, encoded by the Her-2/Neu (ErbB2) proto-oncogene (see, e.g., U.S. Pat. Nos. 5,772,997 and 5,783,186 for anti-Her2 antibodies, the entirety of which are incorporated herein by reference). Her-2 is overexpressed on 30% of highly malignant breast cancers and successful antibodies against this target marketed under the name HERCEPTIN.TM. (Trastuzumab) have been developed. It has been shown that targeting multiple Her-2 epitopes with a mixture of monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo (Spiridon et al., 2002, the entirety of which is incorporated herein by reference). Her-2 may, therefore, be a good target for antibody mixtures according to the invention. Antibodies useful for this purpose can be obtained by methods described in the invention, including antibody display methods.
Human antibodies are capable of eliciting effector function via binding to immunoglobulin receptors on immune effector cells. Human IgG and, in particular, IgG1 and IgG3, fix complement to induce CDC and interact with Fey receptors to induce antibody-dependent cell-mediated cytotoxicity (ADCC), phagocytosis, endocytosis, induction of respiratory burst and release of inflammatory mediators and cytokines. Human IgA interacts with Fc.alpha.R, also resulting in efficient activation of ADCC and phagocytosis of target cells. Hence, due to the differential distribution of Fc.gamma.R and Fc.alpha.R on peripheral blood cells (Huls et al., 1999, the entirety of which is incorporated herein by reference), using a mixture of antibodies directed against the target and consisting of both IgG and IgA would potentially maximize the recruitment and activation of different immune effector cells. Such a mixture of both IgG and IgA could be obtained by producing the IgG and IgA monoclonal antibody in a separate production process using two distinct production cell lines, but could also be obtained from a single cell line producing both the IgG and the IgA monoclonal antibody. This would have the advantage that only a single production process has to be developed. Thus, when different heavy chains are mentioned, heavy chains differing in their constant regions are also encompassed in the invention. The principle of using a common light chain can also be used for the production of a mixture of isotypes from a host cell. Therefore, certain embodiments of the invention provide a method for producing a mixture of antibodies comprising different isotypes from a host cell, the method comprising the step of: culturing a host cell comprising a nucleic acid sequence encoding a light chain and nucleic acid sequences encoding at least two heavy chains of different isotype that are capable of pairing with the light chain, under conditions conducive to expression of the nucleic acid sequences. According to this aspect, different heavy chains may have identical variable regions and only differ in their constant regions (i.e., be of different isotype and have the same specificity). In a particular embodiment, the isotypes comprise at least an IgG and an IgA and/or IgM, preferably IgG1 or IgG3 and IgA. Other combinations of IgG1, IgG2, IgG3 and IgG4 can also be used. In these embodiments, bispecific antibodies will not be produced because the variable regions are the same.
In other embodiments of this aspect, not only the constant regions of the heavy chains may differ, but also the variable regions, thereby giving rise to different specificities paired with the same light chain. When bispecific antibodies are not desired for a given purpose, for example, because the mixtures of antibodies are less efficacious because of the presence of the bispecific antibodies, it is possible to use at least two heavy chains combined with the common light chain according to the invention wherein the heavy chains differ sufficient in their constant regions to reduce or prevent pairing between the different heavy chains, for example, by using heavy chains of different isotypes, such as an IgG1 and an IgG3 (see FIG. 11 for a schematic representation). It is anticipated that the heavy chains of different isotype will pair much less efficient, if at all, compared to the same heavy chains. Alternatively, it is also possible to engineer the different heavy chains in their constant region such that homodimerization is favored over heterodimerization, e.g., by introducing self-complementary interactions (see, e.g., WO 98/50431 for possibilities, such as "protuberance-into-cavity" strategies (see, WO 96/27011, the entirety of which is incorporated herein by reference)). It is, therefore, another aspect to provide a method for producing a mixture of antibodies in a recombinant host, the method including the step of: expressing in a recombinant host cell a nucleic acid sequence encoding a common light chain and nucleic acid sequences encoding at least two different heavy chains that differ in the variable region and that are capable of pairing with the common light chain, and wherein the heavy chains further differ in their constant regions sufficiently to reduce or prevent pairing between the different heavy chains. In one embodiment, the heavy chains are of different isotype. In specific embodiments, 3, 4, 5, 6, 7, 8, 9, 10, or more, different heavy chains are expressed. Mixtures of antibodies obtainable by this method are also embodied in the invention. Such mixtures will comprise mainly monospecific antibodies.
The teachings herein can also be used to obtain novel multispecific antibodies or mixtures thereof. Therefore, in another aspect, provided is a method for producing a mixture of antibodies comprising dimeric IgA isotype {(IgA).sub.2} antibodies in a recombinant host, wherein at least part of the dimeric IgA antibodies have different binding regions in each of the IgA sub-units, the method comprising the step of: expressing in a recombinant host cell a nucleic acid sequence encoding a common light chain and nucleic acid sequences encoding at least two different heavy chains of IgA isotype capable of pairing to the common light chain, wherein the different heavy chains differ in their variable region. Dimerization of the IgA molecules can be enhanced by co-expressing J-chain (Yoo et al., 1999, the entirety of which is incorporated herein by reference). Dimeric IgA antibodies have two specificities (see FIG. 9 for a schematic representation of one possible form produced and present in the mixture).
In certain embodiments, provided is a method for producing a mixture of antibodies comprising an IgM antibody having at least two different specificities, the method comprising expressing in a recombinant host cell a nucleic acid sequence encoding a common light chain and nucleic acid sequences encoding at least two different heavy chains of IgM isotype, wherein the heavy chains are capable of pairing to the common light chain and form functional antigen-binding regions. Up to five specificities can be comprised in an IgM pentamer in the presence of a J-chain and up to six in an IgM hexamer in the absence of a J-chain (Yoo et al., 1999). Therefore, in specific embodiments, 3, 4, 5, or 6 IgM heavy chains are co-expressed with the common light chain according to this aspect. See FIG. 10 for a schematic representation of one of the possible forms that can be produced and present in the mixture according to this aspect, when five different heavy chains are expressed with a common light chain. Also provided is for IgA dimers, IgM pentamers or hexamers having at least two different specificities. These molecules can be produced from a clone of a single host cell according to the invention. Such molecules harboring antigen-binding regions with different specificities can bind different epitopes on the same antigen, different antigens on one cell, or different antigens on different cells, thereby cross-linking the antigens or cells.
In certain embodiments, provided is a method for identifying a mixture of antibodies having a desired effect in a functional assay, the method comprising i) adding a mixture of antibodies in a functional assay, and ii) determining the effect of the mixture in the assay, wherein the mixture of antibodies comprises antibodies having a common light chain. In a preferred embodiment, the mixture is comprised in a composition of the invention.
Also provided is a method for recombinant expression of one or more proteins in a single host cell, wherein at least four different polypeptides are expressed in the single host cell. Each polypeptide is independently expressed and may be under control of a heterologous promoter. The protein or proteins may be isolated separately or as a mixture from a culture of the host cell. Preferably, the host cell of this embodiment is a human cell and/or may be derived from a retina cell, more preferably a cell comprising adenovirus E1 sequences in its genome, most preferably a PER.C6.RTM. cell (human retina cells that express adenovirus E1A and E1B proteins).
EXAMPLES
The following examples are provided to illustrate the invention and are not to be construed in any way to limit the scope of the invention. The practice of this invention will employ, unless otherwise indicated, conventional techniques of immunology, molecular biology, microbiology, cell biology, and recombinant DNA, which are within the skill of the art. See, e.g., Sambrook, Fritsch and Maniatis, Molecular Cloning: A Laboratory Manual, 2.sup.nd edition, 1989; Current Protocols in Molecular Biology, F. M. Ausubel, et al., eds, 1987; the series Methods in Enzymology (Academic Press, Inc.); PCR2: A Practical Approach, M. J. MacPherson, B. D. Hams, G. R. Taylor, eds, 1995; Antibodies: A Laboratory Manual, Harlow and Lane, eds, 1988, the entirety of which are incorporated herein by reference.
Example 1
Production of a Mixture of Monoclonal Antibodies with a Common Light Chain and Two Different Heavy Chain-Variable Regions in a Single Cell
Clone UBS-54 and Clone K53 were previously isolated by selections on the colorectal cell line SW40 (Huls et al., 1999) and on a heterogeneous mixture of mononuclear cells of a patient with multiple myeloma (WO 02/18948, the entirety of which is incorporated herein by reference), respectively, with a semi-synthetic library (de Kruif et al., 1995b). Further studies revealed that clone UBS-54 and K53 bound to the EP-CAM homotypic adhesion molecule (Huls et al., 1999) and the membrane cofactor protein CD46 (WO 02/18948), respectively. DNA sequencing of the clones revealed that they were unique in the Heavy chain CDRs, but that they contained an identical light chain sequence (FIG. 3). The V.sub.H and V.sub.L of clones UBS-54 and K53 were inserted into an expression vector containing the HAVT20 leader sequence and all the coding sequences for the constant domains of a human IgG1 with a Kappa alight chain by a method essentially as described (Boel et al., 2000), which resulted in plasmids pUBS3000Neo and pCD46_3000(Neo) (FIG. 4). These plasmids were transiently expressed, either alone or in combination in PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins). In brief, each 80 cm.sup.2 flask was transfected by incubation for four hours with 140 .mu.l lipofectamine+10 .mu.g DNA (either pUBS3000Neo, pCD46_3000 (Neo) or 10 .mu.g of both) in serum-free DMEM medium at 37.degree. C. After four hours this was replaced with DMEM+10% FBS and the cells were grown overnight at 37.degree. C. Cells were then washed with PBS and the medium was replaced with Excell 525 medium (JRH Bioscience). The cells were allowed to grow at 37.degree. C. for six days, after which the cell culture supernatant was harvested. Human IgG-specific ELISA analysis (described in WO 00/63403, the entirety of which is incorporated herein by reference) indicated that IgG was present at approximately 10 .mu.g/ml for all flasks containing expression plasmids. No IgG1 was present in a control flask which was not transfected with expression plasmid.
Human IgG from each supernatant was subsequently purified using Protein A-affinity chromatography (Hightrap Protein A HP, cat. no. 1-040203) according to standard procedures, following recommendations of the manufacturer (Amersham Biosciences). After elution, samples were concentrated in a Microcon YM30 concentrator (Amicon) and buffer exchanged to 10 mM sodium phosphate, pH 6.7. Twelve .mu.g of purified IgG was subsequently analyzed on Isoelectric-focusing gels (Serva Pre-cast IEF gels, pH range 3-10, cat. no. 42866). The samples were loaded on the low pH side and after focusing, stained with colloidal blue (FIG. 5). Lane 1 shows transiently expressed K53, Lane 2 shows transiently expressed UBS-54 and Lane 3 shows the IgG sample of the cells in which both antibodies were co-transfected. Clearly, K53 and UBS-54 each have a unique pI profile and the sample from the co-transfection showed other unique isoforms, with the major isoform having a pI in between those of K53 and UBS-54. This is also anticipated on the basis of the theoretic pI when calculated with the ProtParam tool provided on the Expasy homepage (expasy.ch; Appel et al., 1994, the entirety of which is incorporated herein by reference). K53 and UBS-54 have a theoretic pI of 8.24 and 7.65, respectively, whereas an isoform representing a heterodimer of one UBS-54 heavy chain and one K53 heavy chain has a theoretical pI of 8.01. Assembly of such a heterodimer can only occur when a single cell translates both the heavy chain of K53 and the heavy chain of UBS-54 and assembles these into a full length IgG molecule together with the common light chain.
Therefore, this experiment shows that it is possible to express two unique human IgG molecules in a single cell and that a heterodimer consisting of these two unique binding specificities is also efficiently formed.
Example 2
Production of a Mixture of Antibodies Against Human B-Cell Markers in a PER.C6.RTM. Cell Line (Human Retina Cells that Express Adenovirus E1A and E1B Proteins)-Derived Clone
A method for producing a mixture of antibodies according to the invention, using expression in a recombinant host cell of a single light chain and three different heavy chains capable of pairing to the single light chain to form functional antibodies, is exemplified herein and is schematically shown in FIG. 6. Phages encoding antibodies capable of binding proteins present on human B-cells, i.e., CD22, CD72 and Major Histocompatibility Complex (MHC) class II (further referred to as HLA-DR) were previously isolated from a semi-synthetic phage library (de Kruif et al., 1995; van der Vuurst de Vries & Logtenberg, 1999, the entirety of which is incorporated herein by reference). DNA sequencing of the V.sub.H and V.sub.L sequences of the phages clone B28 (anti-CD22), clone I-2 (anti-HLA-DR) and clone II-2 (anti-CD72) revealed that they all contain a unique V.sub.H sequence but a common light chain sequence (V.lamda.3) with an identical CDR region (FIG. 7).
The V.sub.H and V.sub.L sequences of clones B28, I-1 and II-2 are cloned behind the HAVT20 leader sequences of an expression plasmid comprising a heavy chain. An example of such a plasmid is pCRU-K01 (contains kappa heavy chain sequences that can be easily interchanged for lambda heavy chain sequences if desired by a person skilled in the art), as deposited at the ECACC under number 03041601. The cloning gives rise to plasmids encoding a full length human IgG1 with binding specificities for CD22, CD72 and HLA-DR. These plasmids will further be referred to as pCRU-CD22, pCRU-CD72 and pCRU-HLA-DR, respectively.
Stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines are generated, according to methods known to one of ordinary skill in the art (see, e.g., WO 00/63403), the cell lines expressing antibodies encoded by genetic information on either pCRU-CD22, pCRU-CD72 or pCRU-HLA-DR and a cell line expressing antibodies encoded by all three plasmids. Therefore, PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) are seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) or T80 flasks with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections are performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with either 1-2 .mu.g pCRU-CD22, 1-2 .mu.g pCRU-CD72, 1-2 .mu.g pCRU-HLA-DR or 1 .mu.g of a mixture of pCRU-CD22, pCRU-CD72 and pCRU-HLA-DR. As a control for transfection efficiency, a few dishes are transfected with a LacZ control vector, while a few dishes will not be transfected and serve as negative controls.
After four to five hours, cells are washed twice with DMEM and given fresh medium without selection. The next day, the medium is replaced with fresh medium containing 500 .mu.g/ml G418. Cells are refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies are visible and from each transfection, at least 300 are picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. At this stage, cells are frozen (at least one, but usually four vials per sub-cultured colony) and production levels of recombinant human IgG antibody are determined in the supernatant using an ELISA specific for human IgG1 (described in WO 00/63403). Also, at this stage, G418 is removed from the culture medium and never re-applied again. For a representative number of colonies, larger volumes will be cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A affinity chromatography according to standard procedures. Purified human IgG1 from the various clones is analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the targets CD22, CD72 and HLA-DR using cell transfectants expressing these human antigens on their cell surface (transfectants expressing CD72 and HLA-DR have been described by van der Vuurst-de Vries and Logtenberg, 1999; a CD22 transfectant has been prepared according to similar standard procedures in PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)).
Colonies obtained from the co-transfection with pCRU-CD22, pCRU-CD72 and pCRU-HLA-DR are screened by PCR on genomic DNA for the presence or absence of each of the three constructs. The identity of the PCR products is further confirmed by DNA sequencing.
Next, it is demonstrated that a clonal cell line accounts for the production of each of the three binding specificities, i.e., proving that a single cell is able to produce a mixture of more than two functional human IgGs. Therefore, a limited number of colonies, which screened positive for the production of each of the three binding specificities (both by PCR at the DNA level as well as in the specified binding assays against CD22, CD72 and HLA-DR), are subjected to single cell sorting using a fluorescence-activated cell sorter (FACS) (Becton & Dickinson FACS VANTAGE SE.TM. (high-performance, high-speed cell sorter)). Alternatively, colonies are seeded at 0.3 cells/well to guarantee clonal outgrowth. Clonal cell populations, hereafter designated as sub-clones, are refreshed once a week with fresh medium. Sub-clones are grown and transferred from 96-well plates via 24- and 6-well plates to T25 flasks. At this stage, sub-clones are frozen (at least one, but usually four vials per sub-clone) and production levels of recombinant human IgG1 antibody are determined in the supernatant using a human IgG1-specific ELISA. For a representative number of sub-clones, larger volumes are cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A-affinity chromatography according to standard procedures.
Purified human IgG1 from the various sub-clones is subsequently analyzed as described above for human IgG1 obtained from the parental clones, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to the targets CD22, CD72 and HLA-DR. Sub-clones will also be screened by PCR on genomic DNA for the presence or absence of each of the three constructs pCRU-CD22, pCRU-CD72 and pCRU-HLA-DR. The identity of the PCR products is further confirmed by DNA sequencing.
Other methods such as Southern blot and/or FISH can also be used to determine whether each of the three constructs are present in the clonal cell line.
Sub-clones that are proven to be transgenic for each of the three constructs are brought into culture for an extensive period to determine whether the presence of the transgenes is stable and whether expression of the antibody mixture remains the same, not only in terms of expression levels, but also for the ratio between the various antibody isoforms that are secreted from the cell. Therefore, the sub-clone culture is maintained for at least 25 population doubling times, either as an adherent culture or as a suspension culture. At every four to six population doublings, a specific production test is performed using the human IgG-specific ELISA and larger volumes are cultured to obtain the cell pellet and the supernatant. The cell pellet is used to assess the presence of the three constructs in the genomic DNA, either via PCR, Southern blot and/or FISH. The supernatant is used to purify the recombinant human IgG1 fraction as described supra. Purified human IgG1 obtained at the various population doublings is analyzed as described, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to the targets CD22, CD72 and HLA-DR using cell transfectants expressing these antigens.
Example 3
Screening of Clones Expressing Multiple Human IgGs for the Most Potent Mixture of Functional Human IgGs
Functionality of the antibody mixture is analyzed in cell-based assays to determine whether the human IgG1 mixture inhibits proliferation and/or induces apoptosis of B-cell lines, such as, for example, Ramos. Other cell lines can also be used. In addition, the antibody mixtures are analyzed for their potential to induce antibody-dependent cellular toxicity and complement-dependent cytotoxicity of, for example, Ramos cells.
In each of the following experiments, the functionality of the antibody mixture recognizing the targets CD22, CD72 and HLA-DR is analyzed and can be compared to each of the individual IgG1 antibodies and to an equimolar combination of the three individual IgG1 specificities.
To assess the ability of the antibody mixtures to inhibit the proliferation of Ramos cells, these cells are incubated in 96-well plates (0.1-1.0.times.10.sup.5/ml) with several concentrations (5-20 .mu.g/ml) of the antibody mixtures against CD22, CD72 and HLA-DR for 24 hours. The proliferation of the cells is measured by .sup.3H-thymidine incorporation during another 16 hours of culture. Inhibition of growth is determined by plotting the percentage of .sup.3H-thymidine incorporation compared to untreated cells (taken as 100% reference value).
To analyze apoptosis induction of Ramos cells, these cells are stimulated in 48-well plates (0.2-1.0.times.10.sup.6/ml) with several concentrations (5-20 .mu.g/ml) of the antibody mixtures against the targets CD22, CD72 and HLA-DR for 24 or 48 hours. After the incubation period, the phosphatidyl serine exposure on apoptotic cells is analyzed (G. Koopman et al., 1994, the entirety of which is incorporated herein by reference). Therefore, the cells are harvested, washed twice with PBS and are incubated at RT for 10 minutes with 100 .mu.l FITC-labeled annexin V (Caltag) diluted 1:25 in annexin V-binding buffer (Caltag). Prior to the analysis of the samples by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, Calif.), propidium iodide (PI)(Sigma) is added to a final concentration of 5 .mu.g/ml to distinguish necrotic cells (ginexin V-/PI+) from apoptotic cells (annexin V+/PI-, early apoptotic cells; annexin V+/PI+, late apoptotic cells).
In an alternative assay, apoptosis is induced by cross-linking the antibody mixtures against CD22, CD72 and HLA-DR on the cell surface of Ramos cells with 25 .mu.g/ml of F(ab)2 of goat-anti-human (Fc-specific) polyclonal antibodies (Jackson Immunoresearch Laboratories, West Grove, Pa.) during the incubation period.
In another alternative assay, apoptosis is induced by incubating the Ramos cells with several concentrations (5-20 .mu.g/ml) of the antibody mixtures against CD22, CD72 and HLA-DR while co-incubating them with the chemosensitizing agents doxorubicin (Calbiochem) or dexamethasone (UMCU, Utrecht, NL).
Antibody-Dependent Cellular Cytotoxicity (ADCC) of the antibody mixtures is analyzed using peripheral blood mono-nuclear cells as effector cells in a standard .sup.51Cr release assay (Huls et al., 1999). To this purpose, 1-3.times.10.sup.6 Ramos cells are labeled with 100 .mu.Ci (Amersham, Buckinghamshire, UK) for one hour at 37.degree. C. After three washes with medium, the Ramos target cells are plated in U bottom 96-well plates at 5.times.10.sup.3 cells/well. Peripheral blood mononuclear cells that are obtained from healthy donors by Ficoll-Hypaque density gradients are then added to each well at effector:target ratios ranging from 80:1 to 10:1 in triplicate. The cells are incubated at 37.degree. C. in the presence of various concentrations of the antibody mixtures (5-20 .mu.g/ml) in a final volume of 200 .mu.l.
After four hours of incubation, part of the supernatant is harvested and .sup.51Cr release is measured. The percentage of specific lysis is calculated using the following formula: % specific lysis=([experimental cpm-spontaneous cpm]/[maximal cpm-spontaneous cpm].times.100%). Maximal .sup.51Cr release is determined by adding triton X-100 to a final concentration of 1% to the target cells and spontaneous release is determined after incubation of the target cells with medium alone.
Complement-dependent cytotoxicity is determined in a similar assay. Instead of the effector cells, now 50 .mu.l human serum is added to the target cells. Subsequently, the assay is performed in the same manner.
Alternatively, ADCC and CDC of the antibody mixtures is determined using a Europium release assay (Patel and Boyd, 1995, the entirety of which is incorporated herein by reference) or using an LDH release assay (Shields et al., 2001, the entirety of which is incorporated herein by reference).
Example 4
Use of Phage Display to Isolate Multiple Phages with an Identical V.sub.L Sequence Against a Predefined Target (Her-2) and Production in a Recombinant Host Cell of a Mixture of Antibodies Capable of Binding this Target
Phages displaying scFv fragments capable of binding multiple epitopes present on the same protein, for example, the epidermal growth factor receptor Her-2, can be isolated from a semi-synthetic phage library (de Kruif et al., 1995a, b). It is possible to identify several of such phages and select the ones comprising the same light chain sequence for further use according to the invention. The semi-synthetic library is formed by mixing seven sub-libraries that each contain a different light chain (de Kruif et al., 1995a, b). It is, therefore, particularly practical to use such a sub-library, containing only one light chain and many heavy chains, for screening so that multiple antibodies with an identical V.sub.L sequence are obtained and further used for expressing the antibody mixtures according to the invention.
For the selection of phages against Her-2, several fusion proteins are generated comprising different parts of the extra-cellular domain of Her-2 that are fused to the CH2 and CH3 domains of human IgG 1. For this purpose, a pcDNA3.1zeo-expression vector (Invitrogen) has been constructed that contains in its multiple cloning region an XhoI restriction site in the hinge region in frame prior to the CH2 and CH3 domains of human IgG1. Using a Her-2 cDNA clone as a template, PCR fragments are generated using standard molecular biology techniques known to a person skilled in the art. These fragments consist of a unique 5' restriction site, a start codon followed by a eukaryotic leader sequence that is linked in frame to either the total extracellular (EC) domain of Her-2 or to a part of the EC domain of Her-2 that is followed in frame by an XhoI restriction site. These PCR fragments are subsequently cloned in frame with the CH2-CH3 IgG1 region into the pcDNA3.1zeo-expression vector. In addition to the fusion protein containing the total EC domain of Her-2, several smaller fusion proteins are generated containing non-overlapping fragments of the Her-2 EC domain. These constructs encoding the Her-2-Ig fusion proteins are used for transient transfection of 293T cells using the lipofectamine reagent (Gibco). Five days after transfection, the supernatants of the 293T cells are harvested and Her-2-Ig fusion proteins are purified using protein A-affinity chromatography according to standard procedures.
Her-2-Ig fusion proteins containing non-overlapping fragments of the Her-2 EC domain are coated for two hours at 37.degree. C. onto the surface of MAXISORP.TM. (polystyrene based modified surface with a high affinity for polar groups) plastic tubes (Nunc) at a saturating concentration (0.5-5 .mu.g/ml). The tubes are blocked for one hour in 2% fat-free milk powder dissolved in PBS (MPBS). Simultaneously, 500 .mu.l (approximately 10.sup.13 cfu) of a semi-synthetic phage display library (a sub-library according to the terminology used above) in which only one V.kappa.1 light chain is represented (prepared as described by De Kruif et al. (1995a, b) and referenced therein), is added to two volumes of 4% MPBS. In addition, human serum is added to a final concentration of 15% and blocking is allowed to proceed for 30 to 60 minutes. The Her-2-Ig-coated tubes are emptied and the blocked phage library is added. The tube is sealed and rotated slowly for one hour, followed by two hours of incubation without rotation. The tubes are emptied and washed ten times in PBS containing 0.1% Tween-20, followed by washing five times in PBS. One ml glycine-HCL, 0.05 M, pH 2.2 is added, and the tube is rotated slowly for ten minutes. The eluted phages are added to 500 .mu.l 1 M Tris-HCl pH 7.4. To this mixture, 3.5 ml of exponentially growing XL-1 blue bacterial culture is added. The tubes are incubated for 30 minutes at 37.degree. C. without shaking. Subsequently, the bacteria are plated on 2TY agar plates containing ampicillin, tetracycline and glucose. After overnight incubation of the plates at 37.degree. C., the colonies are scraped from the plates and used to prepare an enriched phage library, essentially as described by De Kruif et al. (1995a). Briefly, scraped bacteria are used to inoculate 2TY medium containing ampicillin, tetracycline and glucose and are grown at 37.degree. C. to an OD.sub.600nm of .about.0.3 Helper phages are added and allowed to infect the bacteria after which the medium is changed to 2TY containing ampicillin, tetracycline and kanamycin. Incubation is continued overnight at 30.degree. C. The next day, the bacteria are removed from the 2TY medium by centrifugation, after which the phages are precipitated using polyethylene glycol 6000/NaCl. Finally, the phages are dissolved in a small volume of PBS-1% BSA, filter-sterilized and used for a next round of selection. The selection/re-infection procedure is performed twice. After the second round of selection, individual E. coli colonies are used to prepare monoclonal phage antibodies. Essentially, individual colonies are grown to log phase and infected with helper phages, after which phage antibody production is allowed to proceed overnight. Phage antibody containing supernatants are tested in ELISA for binding activity to Her-2-total EC-Ig coated 96-well plates.
Selected phage antibodies that are obtained in the screen described above are validated by ELISA for specificity. For this purpose, Her-2-Ig fusion proteins containing non-overlapping fragments of the Her-2 EC domain are coated to Maxisorp ELISA plates. After coating, the plates are blocked in 2% MPBS. The selected phage antibodies are incubated in an equal volume of 4% MPBS. The plates are emptied, washed once in PBS, after which the blocked phages are added. Incubation is allowed to proceed for one hour, the plates are washed in PBS 0.1% Tween-20 and bound phages are detected using an anti-M13 antibody conjugated to peroxidase. The procedure is performed simultaneously using a control phage antibody directed against thyroglobulin (De Kruif et al. 1995a, b), which serves as a negative control.
In another assay, the selected phage antibodies are analyzed for their ability to bind BT474 human breast cancer cells that express Her-2. For flow cytometry analysis, phage antibodies are first blocked in an equal volume of 4% MPBS for 15 minutes at 4.degree. C. prior to the staining of the BT474 cells. The binding of the phage antibodies to the cells is visualized using a biotinylated anti-M13 antibody (Santa Cruz Biotechnology) followed by streptavidin-phycoerythrin (Caltag).
Alternatively, phage antibodies recognizing multiple epitopes on Her-2 are selected using a method based upon competition of phage binding to Her-2 with binding of the well-characterized murine anti-Her-2 antibodies HER50, HER66 and HER70 (Spiridon et al., 2002, the entirety of which is incorporated herein by reference). To this purpose, 2.times.10.sup.6 BT474 cells are incubated at 4.degree. C. with approximately 10'' cfu (0.5 ml) of a semi-synthetic phage display library in which only one V.kappa.1 light chain is represented, prepared as described supra, and blocked with two volumes of medium containing 10% of FBS. The mixture is slowly rotated at 4.degree. C. for two hours in a sealed tube.
Subsequently, non-bound phages are removed by two washes with 50 ml of cold medium containing 10% FBS. Hereafter, phages recognizing multiple epitopes on Her-2 are eluted by resuspending the BT474 cells in 1 ml of cold medium containing saturating concentrations (5-20 .mu.g/ml) of the HER50, HER66 and HER70 murine anti-Her-2 antibodies. The cells are left on ice for 10 minutes, spun down and the supernatant containing the anti-Her-2 phage antibodies is used to reinfect XL1-Blue cells as described supra.
From the panel of Her-2-specific phage antibodies generated by the screens described above, three phage antibodies are selected that recognize three different non-overlapping epitopes on the Her-2 protein.
The V.sub.H sequences and the unique V.kappa.1 light chain sequence of these clones, provisionally designated V.kappa.1HER2-1, V.kappa.1HER2-2 and V.kappa.1HER2-3, are cloned behind the HAVT20 leader sequences of expression plasmid pCRU-K01 (ECACC deposit 03041601), or a similar expression plasmid, to obtain plasmids encoding a full-length human IgG1-.kappa. with binding specificities for Her-2. These plasmids are provisionally designated as pCRU-V.kappa.1HER2-1, pCRU-V.kappa.1HER2-2 and pCRU-V.kappa.1HER2-3, respectively.
Stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines are generated, according to methods known to one of ordinary skill in the art, the cell lines expressing antibodies encoded by genetic information on either pCRU-V.kappa.1HER2-1, pCRU-V.kappa.1HER2-2 or pCRU-V.kappa.1HER2-3 and a cell line expressing antibodies encoded by all three plasmids. Therefore, PER.C6.RTM. cells are seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) or T80 flasks with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections are performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with either 1-2 .mu.g pCRU-V.kappa.1HER2-1, 1-2 .mu.g pCRU-V.kappa.1HER2-2, 1-2 .mu.g pCRU-V.kappa.1HER2-3 or 1 .mu.g of a mixture of pCRU-V.kappa.1HER2-1, pCRU-V.kappa.1HER2-2 and pCRU-V.kappa.1HER2-3. As a control for transfection efficiency, a few dishes are transfected with a LacZ control vector, while a few dishes are not transfected and serve as negative controls.
After five hours, cells are washed twice with DMEM and re-fed with fresh medium without selection. The next day, medium is replaced with fresh medium containing 500 .mu.g/ml G418. Cells are refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies are visible and from each transfection, at least 300 are picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. At this stage, cells are frozen (at least one, but usually four vials per sub-cultured colony) and production levels of recombinant human IgG antibody are determined in the supernatant using an ELISA specific for human IgG1. Also, at this stage, G418 is removed from the culture medium and never re-applied again. For a representative number of colonies, larger volumes are cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A-affinity chromatography according to standard procedures. Purified human IgG1 from the various clones is analyzed on SDS-PAGE, Iso-electric focusing (IEF), assayed binding to Her-2-Ig fusion proteins by ELISA, and analyzed for binding to Her-2 on the surface of BT474 cells by flow cytometry.
Clones obtained from the co-transfection of pCRU-V.kappa.1HER2-1, pCRU-V.kappa.1HER2-2 and pCRU-V.kappa.1HER2-3 are screened by PCR on genomic DNA for the presence or absence of each of the three constructs. The identity of the PCR products is further confirmed by DNA sequencing.
Next, it is demonstrated that a clonal cell line accounts for the production of each of the three binding specificities. Therefore, a limited number of colonies, which screened positive for the production of each of the three binding specificities (both by PCR at the DNA level as well as in the specified binding assays against Her-2), are subjected to single cell sorting using a fluorescence-activated cell sorter (FACS) (Becton & Dickinson FACS VANTAGE SE.TM.). Alternatively, colonies are seeded at 0.3 cells/well to guarantee clonal outgrowth.
Clonal cell populations, hereafter designated as sub-clones, are refreshed once a week with fresh medium. Sub-clones are grown and transferred from 96-well plates via 24- and 6-well plates to T25 flasks. At this stage, sub-clones are frozen (at least one, but usually four vials per sub-clone) and production levels of recombinant human IgG1 antibody are determined in the supernatant using a human IgG1-specific ELISA. For a representative number of sub-clones, larger volumes are cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A-affinity chromatography according to standard procedures.
Purified human IgG1 from the various sub-clones is subsequently analyzed as described above for human IgG1 obtained from the parental clones, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to Her-2. Sub-clones will also be screened by PCR on genomic DNA for the presence or absence of each of the three constructs pCRU-V.kappa.1HER2-1, pCRU-V.kappa.1HER2-2 and pCRU-V.kappa.1HER2-3. The identity of the PCR products is further confirmed by DNA sequencing.
Other methods such as Southern blot and/or FISH can also be used to determine whether each of the three constructs are present in the clonal cell line.
Sub-clones that are proven to be transgenic for each of the three constructs are brought into culture for an extensive period to determine whether the presence of the transgenes is stable and whether expression of the antibody mixture remains the same, not only in terms of expression levels, but also for the ratio between the various antibodies that are secreted from the cell. Therefore, the sub-clone culture is maintained for at least 25 population doubling times, either as an adherent culture or as a suspension culture. At every four to six population doublings, a specific production test is performed using the human IgG-specific ELISA and larger volumes are cultured to obtain the cell pellet and the supernatant. The cell pellet is used to assess the presence of the three constructs in the genomic DNA, either via PCR, Southern blot and/or FISH. The supernatant is used to purify the recombinant human IgG1 fraction as described supra. Purified human IgG1 obtained at the various population doublings is analyzed as described, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to Her-2 by ELISA and by flow cytometry using BT474 cells.
Functionality of the antibody mixture of anti-Her-2 antibodies is analyzed in cell-based assays to determine whether the human IgG1 mixture inhibits proliferation and/or induces apoptosis of BT474 cells. In addition, the antibody mixtures are analyzed for their potential to induce antibody-dependent cellular toxicity and complement-dependent cytotoxicity of BT474 cells.
In each of the experiments described below, the functionality of the antibody mixture recognizing Her-2 can be analyzed and compared to each of the individual IgG1 antibodies and to an equimolar combination of the three individual monospecific IgG1 molecules.
To assess the ability of the antibody mixtures to inhibit the proliferation of BT474 cells, these cells are allowed to adhere overnight in 96-well plates (1.5.times.10.sup.5/well) and are subsequently incubated with several concentrations (5-20 .mu.g/ml) of the antibody mixtures against Her-2 for 72 hours. The proliferation of the cells is measured by .sup.3H-thymidine incorporation during the last six hours of culture. Inhibition of growth is determined by plotting the percentage of .sup.3H-thymidine incorporation compared with untreated cells (taken as 100% reference value).
To analyze apoptosis induction of BT474 cells, these cells are allowed to adhere overnight in 48-well plates (2.5.times.10.sup.5/wellin 1 ml) and are subsequently incubated with several concentrations (5-20 .mu.g/ml) of the antibody mixtures against Her-2 for four hours. Hereafter, the cells are harvested by trypsinization, washed twice with PBS and incubated at RT for ten minutes with 100 .mu.l FITC-labeled annexin V (Caltag) diluted 1:25 in annexin V-binding buffer (Caltag). Prior to the analysis of the samples by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, Calif.) propidium iodide (PI) (Sigma) is added to a final concentration of 5 .mu.g/ml to distinguish necrotic cells (annexin V.sup.-/PI.sup.+) from apoptotic cells (annexin V.sup.+/PI.sup.-, early apoptotic cells; annexin V.sup.+/PI.sup.+, late apoptotic cells).
Antibody-Dependent Cellular Cytotoxicity of the antibody mixtures is analyzed using peripheral blood mononuclear cells as effector cells and BT474 cells as target cells in a standard .sup.51Cr release assay as described supra (Huls et al., 1999). Complement-dependent cytotoxicity is determined in a similar assay. Instead of the effector cells, now 50 .[..mu.A.]. .Iadd..mu.L .Iaddend.human serum is added to the target cells. Subsequently, the assay is performed as described supra.
Alternatively, ADCC and CDC of the antibody mixtures is determined using a Europium release assay (Patel and Boyd, 1995) or using an LDH release assay (Shields et al., 2001).
The functionality of the antibody mixtures against Her-2 is also tested using in vivo animal models, such as, for instance, described in Spiridon et al., 2002.
Example 5
Expression of Different Functional Human IgGs in the Milk of Transgenic Animals
The V.sub.H and .[.V.sub.H.]. .Iadd.V.sub.L .Iaddend.sequences of phages against proteins present on human B-cells, i.e., CD22 (clone B28), CD72 (clone II-2) and HLA-DR (clone I-2) (FIG. 7) are cloned into expression plasmid pBC1 (as provided in the pBC1 Mouse Milk Expression System, Invitrogen Life Technologies) to obtain mammary gland- and lactation-specific expression of these human IgG molecules in transgenic animals, according to the manufacturer's instructions. These mammary gland-specific expression vectors encoding the antibody sequences for anti-CD22, anti-CD72 and anti-HLA-DR, are introduced into the murine germline according to the manufacturer's instructions. Obtained pups are screened for the presence of each of the three constructs by PCR on DNA isolated from the tail. Pups, either male or female, confirmed for being transgenic for each of the three antibodies, are weaned and matured. Female transgenic mice are fertilized at the age of 6-8 weeks and milk samples are obtained at several time points after gestation. Male transgenic mice are mated with non-transgenic females and female transgenic offspring (as determined with PCR as described above) is mated and milked as described above for the female transgenic founders. Whenever needed, female or male transgenic founders are mated for another generation to be able to obtain sufficient amounts of transgenic milk for each founder line. Transgenic milk is analyzed for the presence of human IgG with a human IgG-specific ELISA, which does not cross-react with mouse IgG or other mouse milk components. Human IgG is purified from transgenic mouse milk using Protein A-affinity chromatography according to standard procedures. Purified human IgG is analyzed on SDS-PAGE, Iso-electric focusing and binding on the targets CD22, CD72 and HLA-DR. Functionality of the antibody mixture is analyzed as described supra.
Example 6
Production of an IgA/IgG Mixture Against a Predefined Target in a PER.C6.RTM. (Human Retina Cells that Express adenovirus E1A and E1B Proteins)-Derived Clone
The V.sub.H-V.sub.L sequences of the phage UBS-54 directed against the homotypic adhesion molecule EP-CAM (Huls et al., 1999) was not only cloned into a vector encoding the constant domains of a human IgG1 with Kappa light chain (expression vector pUBS3000Neo), but also into an expression vector encoding the constant domains of a human IgA1 with Kappa light chain (expression vector pUBS54-IgA, FIG. 8). Hence, antibodies derived from pUBS3000Neo and pUBS54-IgA do bind to the same epitope on EPCAM. The only differences antibodies derived from pUBS3000Neo and pUBS54-IgA are in the sequences encoding the constant domains of the heavy chain, resulting in either an IgG1 or IgA1 isotype. The Kappa light chain sequences of these two vectors are identical.
Stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines expressing antibodies encoded by genetic information on pUBS3000Neo and pUBS54-IgA are generated by procedures well known to persons skilled in the art. Therefore, PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) are seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) or T80 flasks with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections are performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with either 1-2 .mu.g pUBS3000.Neo and pUBS54-IgA. As a control for transfection efficiency, a few dishes are transfected with a LacZ control vector, while a few dishes are not transfected and serve as negative controls.
After four to five hours, cells are washed twice with DMEM and given fresh medium without selection. The next day, medium is replaced with fresh medium containing 500 .mu.g/ml G418. Cells are refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies are visible and from each transfection, at least 300 are picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. At this stage, cells are frozen (at least one, but usually four vials per sub-cultured colony) and production levels of recombinant human IgG and human IgA antibody are determined in the supernatant using an ELISA specific for human IgG1 as well as an ELISA specific for human IgA. Also, at this stage, G418 is removed from the culture medium and never re-applied again. For a representative number of colonies, larger volumes are cultured to purify the recombinant human IgG1 and human IgA fraction from the conditioned supernatant using, for instance, a combination of Protein L- or LA-affinity chromatography, cation exchange chromatography, hydrophobic interaction chromatography and gel filtration. Purified human immunoglobulins from the various clones are analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the target EPCAM using cell lines having a high expression of this molecule. The clones will also be screened by PCR on genomic DNA for the presence or absence of pUBS3000Neo and pUBS54-IgA. The identity of the PCR products is further confirmed by DNA sequencing.
A limited number of clones, which are screened positive for the production of both EPCAM IgG1 and EPCAM IgA, are subjected to single cell sorting using a fluorescence-activated cell sorter (FACS) (Becton Dickinson FACS VANTAGE SE.TM.). Alternatively, colonies are seeded at 0.3 cells/well to guarantee clonal outgrowth. Clonal cell populations, hereafter designated as sub-clones, are refreshed once a week with fresh medium. Sub-clones are grown and transferred from 96-well plates via 24- and 6-well plates to T25 flasks. At this stage, sub-clones are frozen (at least one, but usually four vials per sub-clone) and production levels of recombinant human IgG1 and IgA antibody are determined in the supernatant using a human IgG1-specific ELISA and a human IgA-specific ELISA. For a representative number of sub-clones, larger volumes are cultured to purify the recombinant human IgG1 and human IgA1 fraction from the conditioned supernatant using, for instance, a combination of Protein L- or LA-affinity chromatography, cation exchange chromatography, hydrophobic interaction chromatography and gel filtration. Purified human immunoglobulins from the various clones are analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the target EPCAM using cell lines having a high expression of this molecule.
Sub-clones will also be screened by PCR on genomic DNA for the presence or absence of pUBS3000Neo and pUBS54-IgA. The identity of the PCR products is further confirmed by DNA sequencing.
Other methods such as Southern blot and/or FISH may also be used to determine whether both constructs are present in the clonal cell line.
Example 7
Production of a human IgG1/IgG3 Mixture Against Multiple Targets in a Clonal PER.C6.RTM. Cell Line (Human Retina Cells that Express Adenovirus E1A and E1B Proteins)
Phage clone UBS-54 and Clone K53 (FIG. 3) were obtained as described in Example 1. The V.sub.H and V.sub.L of clone UBS-54 was inserted into an expression vector containing the HAVT20 leader sequence and all the coding sequences for the constant domains of a human IgG1 with a Kappa light chain by a method essentially as described (Boel et al., 2000). The resulting plasmid was designated as pUBS3000Neo (FIG. 4). It will be clear that expression vectors containing heavy chain constant domains of any desired isotype can be constructed by routine methods of molecular biology, using the sequences of these regions that are all available in the art. The V.sub.H and V.sub.L sequences of Phage clone K53 are cloned into an expression vector containing the HAVT20 leader sequence and all the coding sequences for the constant domains of a heavy chain of a human IgG3 with a Kappa light chain by a method essentially as described (Boel et al., 2000). This expression vector is designated as pK53IgG3.
These plasmids are transiently expressed, either alone or in combination, in PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins). In brief, each 80 cm.sup.2 flask is transfected by incubation for four hours with 140 .mu.l lipofectamine+10 .mu.g DNA (either pUBS3000Neo, pK53IgG3 or 10 .mu.g of both) in serum-free DMEM medium at 37.degree. C. After four hours, this is replaced with DMEM+10% FBS and the cells are grown overnight at 37.degree. C. Cells are then washed with PBS and the medium is replaced with Excell 525 medium (JRH Bioscience). The cells are allowed to grow at 37.degree. C. for six days, after which the cell culture supernatant is harvested. Human IgG-specific ELISA analysis, i.e., measuring all IgG sub-types, is done to determine the IgG concentration in transfected and non-transfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins). Human IgG from each supernatant is subsequently purified using Protein A-affinity chromatography (Hightrap Protein A HP, cat. no. 1-040203) according to standard procedures, following recommendations of the manufacturer (Amersham Biosciences). After elution, samples are concentrated in a Microcon YM30 concentrator (Amicon) and buffer exchanged to 10 mM sodium phosphate, pH 6.7. Samples are analyzed for binding to the targets EPCAM and CD46 using cell lines having a high expression of these molecules such as LS174T cells. Twelve .mu.g of purified IgG, either transiently expressed UBS-54 IgG1, K53 IgG3 or IgG from the cells in which both antibodies were co-transfected, is subsequently analyzed on iso-electric-focusing gels (Serva Pre-cast IEF gels, pH range 3-10, cat. no. 42866). Samples are loaded on the low pH side and, after focusing, stained with colloidal blue. The pI values of the major isoforms for each sample are determined to illustrate whether there has been expression of UBS-54 IgG1, K53 IgG3 or bispecific heterodimers, depending on how the cells were transfected. The identification of heterodimers would indicate that single cells have translated both the IgG3 heavy chain of K53 and the IgG1 heavy chain of UBS-54 and assembled these into a full-length IgG molecule together with the common light chain.
The absence of bispecific heterodimers indicates that it is possible to translate both the IgG3 heavy chain of K53 and the IgG1 heavy chain of UBS-54 in single cells, but that these do not assemble into a full-length IgG molecule together with the common light chain, i.e., there is preferential binding of IgG1 and IgG3 heavy chains. This could, however, also be explained by the lack of co-expression of UBS-54 IgG1 and K53 IgG3. Therefore, stable clonal cell lines expressing both pUBS3000Neo and pK53IgG3 are generated by procedures as such well known to persons skilled in the art. PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) are seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) or T80 flasks with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections are performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with either 1-2 .mu.g pUBS3000Neo, pK53IgG3 or both. As a control for transfection efficiency, a few dishes are transfected with a LacZ control vector, while a few dishes will be not transfected and serve as negative controls.
After four to five hours, cells are washed twice with DMEM and given fresh medium without selection. The next day, medium is replaced with fresh medium containing 500 .mu.g/ml G418. Cells are refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies are visible and from each transfection, at least 300 are picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. At this stage, cells are frozen (at least one, but usually four vials per sub-cultured colony) and production levels of recombinant human IgG antibody are determined in the supernatant using an ELISA specific for all sub-types of human IgG. Also, at this stage, G418 is removed from the culture medium and never re-applied again. For a representative number of colonies, larger volumes are cultured to purify the recombinant human IgG from the conditioned supernatant using Protein A-affinity chromatography (Hightrap Protein A HP, cat. no. 1-040203) according to standard procedures, following recommendations of the manufacturer (Amersham Biosciences). Purified human immunoglobulins from the various clones are analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the targets EPCAM and CD46 using cell lines having a high expression of these molecules such as LS174T cells. The clones are also screened by PCR on genomic DNA for the presence or absence of pUBS3000Neo and pK53IgG3. The identity of the PCR products is further confirmed by DNA sequencing.
A limited number of clones, which are screened positive for the production of both EPCAM IgG1 and K53 IgG3, are subjected to single cell sorting using a fluorescence-activated cell sorter (FACS) (Becton Dickinson FACS VANTAGE SE.TM.). Alternatively, colonies are seeded at 0.3 cells/well to guarantee clonal outgrowth. Clonal cell populations, hereafter designated as sub-clones, are refreshed once a week with fresh medium. Sub-clones are grown and transferred from 96-well plates via 24- and 6-well plates to T25 flasks. At this stage, sub-clones are frozen (at least one, but usually four vials per sub-clone) and production levels of recombinant human IgG antibody are determined in the supernatant using a human IgG-specific ELISA. For a representative number of sub-clones, larger volumes are cultured to purify the'recombinant human IgG fraction from the conditioned supernatant usings Protein A-affinity chromatography (Hightrap Protein A HP, cat. no. 1-040203) according to standard procedures, following recommendations of the manufacturer (Amersham Biosciences). Purified human immunoglobulins from the various clones are analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the targets EPCAM and CD46 using cell lines having a high expression of this molecules, such as, for instance, LS174T cells, or transfectants expressing these molecules.
Sub-clones are also screened by PCR on genomic DNA for the presence or absence of pUBS3000Neo and pK53IgG3. The identity of the PCR products is further confirmed by DNA sequencing.
Other methods such as Southern blot and/or FISH may also be used to determine whether both constructs are present in the clonal cell line.
Once the clonal sub-clones are available and confirmed positive for the expression of both UBS-54 IgG1 and K53 IgG3, the presence of functional K53 and UBS-54 shows that it is possible to generate a mixture of functional IgGs with different isotypes with the common light chain in a single cell. Analysis of the expression of bispecific antibodies binding both EpCAM and CD46 will reveal to what extent the different heavy chains having a different sub-type will pair, which will influence the amount of bispecific antibodies produced. It is expected that no or very low levels of bispecific antibodies will be found in this case.
Example 8
Selection of Phage Carrying Single Chain Fv Fragments Specifically Recognizing Rabies Virus Glyco Protein (RVGP) Using RVGP-Ig Fusion Protein, and Expression of Mixtures of Antibodies Against the Rabies Virus
This example describes the production of mixtures of antibodies against the rabies virus as another potential target. As an antigen, the Rabies Virus Glycoprotein (RVGP) is chosen, but other rabies antigens may be chosen or included as well for this purpose. Several monoclonal antibodies recognizing RVGP have already been described in the art, and polyclonal antibodies have been recognized to be useful in treatment of rabies infections as well (e.g., EP0402029; EP0445625, the entirety of which are incorporated herein by reference).
Antibody fragments are selected using antibody phage display libraries and MAbstract.TM. technology, essentially as described in U.S. Pat. No. 6,265,150 and in WO 98/15833, the entirety of which is incorporated herein by reference. All procedures are performed at room temperature unless stated otherwise. The sequence of RVGP is available to one of ordinary skill in the art for cloning purposes (e.g., Yelverton et al., 1983, the entirety of which is incorporated herein by reference). An RVGP-Ig fusion protein consisting of whole RVGP fused genetically to the CH2 and CH3 domains of human IgG1 is produced using vector pcDNA3.1 Zeo-CH2-CH3 expressed in PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) and coated for two hours at 37.degree. C. onto the surface of MAXISORP.TM. (polystyrene based modified surface with a high affinity for polar groups) plastic tubes (Nunc) at a concentration of 1.25 .mu.g/ml. The tubes are blocked for one hour in 2% fat-free milk powder dissolved in PBS (MPBS). Simultaneously, 500 .mu.l (approximately 10.sup.13 cfu) of a phage display library expressing single chain Fv fragments (scFvs) essentially prepared as described by De Kruif et al. (1995a, b) and references therein, is added to two volumes of 4% MPBS. In this experiment, selections are performed using fractions of the original library constructed using only one single variable light chain gene species (e.g., a "V.kappa.1"-library). In addition, human serum is added to a final concentration of 15% and blocking is allowed to proceed for 30 to 60 minutes. The RVGP-Ig-coated tubes are emptied and the blocked phage library is added. The tube is sealed and rotated slowly for one hour, followed by two hours of incubation without rotation. The tubes are emptied and washed ten times in PBS containing 0.1% Tween-20, followed by washing five times in PBS. One ml glycine-HCL, 0.05 M, pH 2.2 is added, and the tube is rotated slowly for ten minutes. The eluted phages are added to 500 .mu.l 1 M Tris-HCl pH 7.4. To this mixture, 3.5 ml of exponentially growing XL-1 blue bacterial culture is added. The tubes are incubated for 30 minutes at 37.degree. C. without shaking. Then, the bacteria are plated on 2TY agar plates containing ampicillin, tetracycline and glucose. After overnight incubation of the plates at 37.degree. C., the colonies are scraped from the plates and used to prepare an enriched phage library, essentially as described by De Kruif et al. (1995a, b). Briefly, scraped bacteria are used to inoculate 2TY medium containing ampicillin, tetracycline and glucose and grown at a temperature of 37.degree. C. to an OD.sub.600nm of .about.0.3. Helper phages are added and allowed to infect the bacteria, after which the medium is changed to 2TY containing ampicillin, tetracycline and kanamycin. Incubation is continued overnight at 30.degree. C. The next day, the bacteria are removed from the 2TY medium by centrifugation, after which the phages are precipitated using polyethylene glycol 6000/NaCl. Finally, the phages are dissolved in a small volume of PBS-1% BSA, filter-sterilized and used for a next round of selection. The selection/re-infection procedure is performed twice.
After the second round of selection, individual E. coli colonies are used to prepare monoclonal phage antibodies. Essentially, individual colonies are grown to log-phase and infected with helper phages, after which phage antibody production is allowed to proceed overnight. Phage antibody-containing supernatants are tested in ELISA for binding activity to human RVGP-Ig coated 96-well plates.
Selected phage antibodies that are obtained in the screen described above are validated in ELISA for specificity. For this purpose, human RVGP-Ig is coated to Maxisorp ELISA plates. After coating, the plates are blocked in 2% MPBS. The selected phage antibodies are incubated in an equal volume of 4% MPBS. The plates are emptied, washed once in PBS, after which the blocked phages are added. Incubation is allowed to proceed for one hour, the plates are washed in PBS 0.1% Tween-20 and bound phages are detected using an anti-M13 antibody conjugated to peroxidase. As a control, the procedure is performed simultaneously using a control phage antibody directed against thyroglobulin (De Kruif et al. 1995a, b), which serves as a negative control.
The phage antibodies that bind to human RVGP-Ig are subsequently tested for binding to human serum IgG to exclude the possibility that they recognized the Fc part of the fusion protein.
In another assay, the phage antibodies are analyzed for their ability to bind PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) that express RVGP. To this purpose, PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) are transfected with a plasmid carrying a cDNA sequence encoding RVGP or with the empty vector and stable transfectants are selected using standard techniques known to a person skilled in the art (e.g., J. E. Coligan et al. (2001), Current Protocols In Protein Science, volume I, John Wiley & Sons, Inc. New York, the entirety of which is incorporated herein by reference). For flow cytometry analysis, phage antibodies are first blocked in an equal volume of 4% MPBS for 15 minutes at 4.degree. C. prior to the staining of the RVGP- and control-transfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins). The blocked phages are added to a mixture of unlabeled control-transfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) and RGVP-transfected PER.C6.RTM. cells that have been labeled green using a lipophylic dye (PKH67, Sigma). The binding of the phage antibodies to the cells is visualized using a biotinylated anti-M13 antibody (Santa Cruz Biotechnology), followed by streptavidin-phycoerythrin (Caltag). Anti RVGP scFv selectively stains the PER.C6.RTM. RVGP transfectant while they do not bind the control transfectant.
An alternative way of screening for phages carrying single chain Fv fragments specifically recognizing human RVGP, is by use of RVGP-transfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins).
PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) expressing membrane-bound RVGP are produced as described supra. Phage selection experiments are performed as described supra, using these cells as target. A fraction of the phage library comprised of scFv phage particles using only one single scFv species (500 .[..mu.A.]. .Iadd..mu.L.Iaddend., approximately 10.sup.13 cfu) is blocked with 2 ml RPMI/10% FCS/1% NHS for 15 minutes at RT. Untransfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) (.about.10.times.10.sup.6 cells) are added to the PER.C6-RVGP cells (.about.1.0.times.10.sup.6 cells). This mixture is added to the blocked light chain restricted phage library and incubated for 2.5 hours while slowly rotating at 4.degree. C. Subsequently, the cells are washed twice and were resuspended in 500 .[..mu.l.]. .Iadd..mu.L .Iaddend.RPMI/10% FCS and incubated with a murine anti-RVGP antibody (Becton Dickinson) followed by a phycoerythrin (PE)-conjugated anti-mouse-IgG antibody (Caltag) for 15 minutes on ice. The cells are washed once and transferred to a 4 ml tube. Cell sorting is performed on a FACSvantage fluorescence-activated cell sorter (Becton Dickinson) and RVGP (PE positive) cells are sorted. The sorted cells are spun down, the supernatant is saved and the bound phages are eluted from the cells by resuspending the cells in 500 .mu.l 50 mM Glycin pH2.2 followed by incubation for five minutes at room temperature. The mixture is neutralized with 250 .mu.l 1 M Tris-HCl pH 7.4 and added to the rescued supernatant. Collectively, these phages are used to prepare an enriched phage library as described above. The selection/re-infection procedure is performed twice. After the second round of selection, monoclonal phage antibodies are prepared and tested for binding to RVGP-PER.C6.RTM. cells and untransfected PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) as described supra. Phages that are positive on RVGP-transfected cells are subsequently tested for binding to the RVGP-IgG fusion protein in ELISA as described supra.
The selected scFv fragments are cloned in a human IgG1 format, according to methods known in the art (e.g., Boel et al., 2000). To this purpose, the V.sub.L fragment shared by the selected scFv is PCR amplified using oligos that add appropriate restriction sites. A similar procedure is used for the V.sub.H genes. Thus, modified genes are cloned in expression pCRU-K01 (ECACC deposit 03041601), which results in expression vectors encoding a complete huIgG1 heavy chain and a complete human light chain gene having the same specificity as the original phage clone. By this method, three different heavy chains are cloned into separate expression vectors, while only one of the vectors needs to comprise the common light chain sequence. These expression vectors are provisionally designated pCRU-RVGP-1, pCU-RVGP-2, and pCRU-RVGP-3. Alternatively, these three vectors may lack DNA encoding the V.sub.L region, which can then be encoded in a fourth, separate expression vector not encoding a heavy chain. It is also possible to have V.sub.L sequences present in all three or two of the three vectors comprising the different V.sub.H sequences.
Stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines are generated, according to methods known to one of ordinary skill in the art (see, e.g., WO 00/63403), the cell lines expressing antibodies encoded by genetic information on either pCRU-RVGP-1, pCRU-RVGP-2 or pCRU-RVGP-3 and a cell line expressing antibodies encoded by all three plasmids. Therefore, PER.C60 cells are seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) or T80 flasks with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections are performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with either 1-2 .mu.g pCRU-RVGP-1, 1-2 .mu.g pCRU-RVGP-2, 1-2 .mu.g pCRU-RVGP-3 or 1 .mu.g of a mixture of pCRU-RVGP-1, pCRU-RVGP-2 and pCRU-RVGP-3. As a control for transfection efficiency, a few dishes are transfected with a LacZ control vector, while a few dishes will not be transfected and serve as negative controls.
After four to five hours, cells are washed twice with DMEM and given fresh medium without selection. The next day, the medium is replaced with fresh medium containing 500 .mu.g/ml G418. Cells are refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies are visible and from each transfection, at least 300 are picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. At this stage, cells are frozen (at least one, but usually four vials per sub-cultured colony) and production levels of recombinant human IgG antibody are determined in the supernatant using an ELISA specific for human IgG1 (described in WO 00/63403). Also, at this stage, G418 is removed from the culture medium and never re-applied again. For a representative number of colonies, larger volumes will be cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A-affinity chromatography according to standard procedures. Purified human IgG1 from the various clones is analyzed on SDS-PAGE, Iso-electric focusing (IEF) and binding to the target RVGP using an RVGP PER.C6-transfectant described above.
Colonies obtained from the co-transfection with pCRU-RVGP-1, pCRU-RVGP-2 and pCRU-RVGP-3 are screened by PCR on genomic DNA for the presence or absence of each of the three constructs. The identity of the PCR products is further confirmed by DNA sequencing.
A limited number of colonies, which screened positive for the production of each of the three binding specificities (both by PCR at the DNA level as well as in the specified binding assays against RVGP), are subjected to single cell sorting using a fluorescence-activated cell sorter (FACS) (Becton & Dickinson FACS VANTAGE SE.TM.).
Alternatively, colonies are seeded at 0.3 cells/well to guarantee clonal outgrowth. Clonal cell populations, hereafter designated as sub-clones, are refreshed once a week with fresh medium. Sub-clones are grown and transferred from 96-well plates via 24- and 6-well plates to T25 flasks. At this stage, sub-clones are frozen (at least one, but usually four vials per sub-clone) and production levels of recombinant human IgG1 antibody are determined in the supernatant using a human IgG1-specific ELISA. For a representative number of sub-clones, larger volumes are cultured to purify the recombinant human IgG1 fraction from the conditioned supernatant using Protein A-affinity chromatography according to standard procedures.
Purified human IgG1 from the various sub-clones is subsequently analyzed as described above for human IgG1 obtained from the parental clones, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to the target RVGP.
Sub-clones are also screened by PCR on genomic DNA for the presence or absence of each of the three constructs pCRU-RVGP-1, pCRU-RVGP-2 and pCRU-RVGP-3. The identity of the PCR products is further confirmed by DNA sequencing.
Other methods such as Southern blot and/or FISH can also be used to determine whether each of the three constructs are present in the clonal cell line.
Sub-clones that are proven to be transgenic for each of the three constructs are brought into culture for an extensive period to determine whether the presence of the transgenes is stable and whether expression of the antibody mixture remains the same, not only in terms of expression levels, but also for the ratio between the various antibody isoforms that are secreted from the cell. Therefore, the sub-clone culture is maintained for at least 25 population doubling times, either as an adherent culture or as a suspension culture. At every four to six population doublings, a specific production test is performed using the human IgG-specific ELISA and larger volumes are cultured to obtain the cell pellet and the supernatant. The cell pellet is used to assess the presence of the three constructs in the genomic DNA, either via PCR, Southern blot and/or FISH. The supernatant is used to purify the recombinant human IgG1 fraction as described supra. Purified human IgG1 obtained at the various population doublings is analyzed as described, i.e., by SDS-PAGE, Iso-electric focusing (IEF) and binding to the target RVGP.
The efficacy of the antibody mixtures against rabies is tested in in vitro cell culture assays where the decrease in spread of rabies virus is measured, as well as in in vivo animal models infected by rabies. Such models are known to one of ordinary skill in the art and are, e.g., described in EP0402029.
Example 9
Production of a Mixture of Antibodies with a Common Light Chain and Three Different Heavy Chain-Variable Regions in a Single Cell
A method for producing a mixture of antibodies according to the invention using expression in a recombinant host cell of a single light chain and three different heavy chains capable of pairing to the single light chain to form functional antibodies, is exemplified herein and is schematically shown in FIG. 6.
Human IgGs UBS54 and K53 against the EP-CAM homo-typic adhesion molecule (Huls et al., 1999) and the membrane cofactor protein CD46 (WO 02/18948), respectively, are described in Example 1. Another clone that was identified to bind to cofactor protein CD46 was clone 02-237 (sequence of V.sub.H provided in FIG. 12, SEQ ID NO:10). DNA sequencing of this clone revealed that it contained the same light chain as UBS54 and K53 but a unique heavy chain-variable sequence (see alignment in FIG. 3). As a result, the CDR3 of the heavy chain of 02-237 differs at four positions from that of K53 (see alignment in FIG. 13). The heavy and light chain-variable sequences of phage 02-237 were cloned into the expression plasmid pCRU-K01 (pCRU-K01 is deposited at the European Collection of Cell Cultures (ECACC) under number 03041601), which contains the heavy and light chain constant domains for an IgG 1 antibody.
The resulting plasmid was designated pgG102-237. Due to the cloning strategy followed, the resulting N-terminus of the light chain of 02-237 as encoded by pgG102-237 differed slightly from the N-terminus of UBS54 and K53 as present by pUBS3000Neo, pCD46_3000(Neo), respectively (FIG. 3). Plasmid pgG102-237 was transiently produced in human 293 (T) cells or stably in PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins). It appeared that purified 02-237 IgG had a much higher affinity for purified CD46 (FIG. 14) than K53 IgG, i.e., the affinity had increased from 9.1.times.10.sup.-7 M to 2.2.times.10.sup.-8 M for K53 and 02-237, respectively. Also, 02-237 bound much better to CD46 on human colon carcinoma LS174T cells than K53 (FIG. 15).
Stable PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins)-derived cell lines expressing a combination of the plasmids pUBS3000Neo, pCD46_3000 (Neo) and pgG102-237 encoding human IgG 02-237 were generated according to methods known as such to one of ordinary skill in the art (see, e.g., WO 00/63403). Therefore, PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) were seeded in DMEM plus 10% FBS in tissue culture dishes (10 cm diameter) with approximately 2.5.times.10.sup.6 cells per dish and kept overnight under their normal culture conditions (10% CO.sub.2 concentration and 37.degree. C.). The next day, transfections were performed in separate dishes at 37.degree. C. using Lipofectamine (Invitrogen Life Technologies) according to standard protocols provided by the manufacturer, with 2 .mu.g of an equimolar mixture of pUBS3000Neo, pCD46_3000(Neo) and pgG102-237. As negative control for selection, a few dishes were not transfected.
After four to five hours, cells were washed twice with DMEM and given fresh medium without selection. The next day, medium was replaced with fresh medium containing 500 .mu.g/ml G418. Cells were refreshed every two or three days with medium containing the same concentrations of G418. About 20 to 22 days after seeding, a large number of colonies were visible and about 300 were picked and grown via 96-well plates and/or 24-well plates via 6-well plates to T25 flasks. During sub-culturing, production levels of recombinant human IgG antibody were determined in the supernatant using an ELISA specific for human IgG1 (described in WO 00/63403). About 25% of all colonies appeared to be positive in this highly specific assay. The production levels measured at this stage were comparable to the levels when a single IgG is expressed in PER.C6.RTM. cells (human retina cells that express adenovirus E1A and E1B proteins) (expression of a single IgG described in Jones et al., 2003). It is important to stress that these high expression levels were obtained without any methods for amplification of the transgene and that they occur at a low copy number of the transgene.
The 30 best producing colonies were frozen down in vials and the 19 highest producing clones were selected for purification of the IgG (Table 1). They were sub-cultured in T80 flasks and human IgG from each clone was subsequently purified using Protein A-affinity chromatography. Therefore, 15 to 25 ml of conditioned medium was loaded on a 5 ml Protein A FF Sepharose column (Amersham Biosciences). The column was washed with 4 mM phosphate buffered saline, pH 7.4 (PBS) before elution with 0.1 M citrate pH 3.0. The eluted fraction was subsequently desalted on a Sephadex G25 Fine HiPrep Desalting column (Amersham Biotech) to PBS. The concentration of the purified IgG fraction was determined by absorbance measurement at 280 nm using a coefficient of 1.4 for a 0.1% (w/v) solution (Table 1).
The purified IgG samples were analyzed on non-reduced and reduced SDS-PAGE and IEF. Non-reduced SDS-PAGE (FIG. 16A) showed that all IgG samples migrated comparable to the control K53 or 02-237 as an assembled, intact IgG molecule of approximately 150 kDa. On reduced SDS-PAGE (FIG. 16B), the IgG samples migrated as heavy and light chains of about 50 and 25 kDa, respectively, comparable to the heavy and light chain of the control K53 or 02-237.
On IEF, the purified IgG fractions were first compared to a mixture of equal amounts of K53, UBS54 and 02-237 (FIG. 17). Clearly, some of the samples contained isoforms with a unique pI profile when compared to the mixture containing purified K53, UBS54 and 02-237. Some major unique isoforms have a pI in between the pI of K53 and 02-237 on one hand and UBS54 on the other hand. This is also anticipated on the basis of the theoretic pI when calculated with the Prot-Param tool provided on the Expasy homepage (expasy.ch; Appel et al., 1994). K53, 02-237 and UBS54 have a theoretic pI of 8.24, 8.36 and 7.65, respectively, whereas an isoform representing a heterodimer of one UBS54 heavy chain and one K53 heavy chain, has a theoretical pI of 8.01. Assembly, of such a heterodimer can only occur when a single cell translates both the heavy chain of K53 and the heavy chain of UBS54 and assembles these into a full-length IgG molecule together with the common light chain. Hence, these results suggest that certain clones at least express two functional antibodies. To confirm the unique identity of some of the isoforms, samples of the most interesting clones were run in parallel with K53, UBS54 and 02-237, either alone or in a mixture (FIG. 18). This furthermore showed that some clones expressed at least two antibodies (241, 282, 361). Moreover, it provided evidence that some clones express all three functional antibodies (280 and 402).
To confirm that the clones expressed IgG mixtures comprising all three heavy chains, peptide mapping (Garnick, 1992; Gelpi, 1995, the entirety of which are incorporated herein by reference) was used to analyze the polyclonal IgG fraction. We previously employed peptide mapping to recover 99% of the protein sequence of K53.
Based on the protein sequence provided in FIG. 12, the mass of the theoretical tryptic peptides of K53, UBS54 and 02-237 was calculated (Table II and III). A few unique peptides for each IgG could be identified, for instance, the CDR3 peptides for K53, 02-237 and UBS54 with a Mw of 2116.05, 2057.99 and 2307.15 Da, respectively. Next, a tryptic digest of Poly1-280 was prepared and this was analyzed using LC-MS (FIG. 19).
Peptides with Mw of 2116, 2057 and 2308 Da, representing the unique CDR3 peptides of K53, 02-237 and UBS54, respectively, were detected. The precise amino acid sequence of these peptides (as listed in Table III) was confirmed by MS-MS analysis (Tables IV, V and VI). The presence of the two unique N-terminal light chain peptides with Mw of 2580 and 2554 Da, respectively, was also confirmed. The peptide mapping data unequivocally showed that a mixture of antibodies comprising a common light chain and three different heavy chains was expressed by PER.C6.RTM. (human retina cells that express adenovirus E1A and E1B proteins) clone Poly1-280. Also, clones 055, 241 and 402 were screened by peptide mapping. Clones 241 and 402 were confirmed positive for all three heavy chain sequences, whereas clone 055 only showed expression of the heavy chains of K53 and 02-237, and not of UBS54. This confirms the IEF screening (FIG. 18) where no UBS54-related band was seen in sample 055.
Poly1-280 was analyzed by BIACORE.TM. (surface plasmon resonance) for binding to CD46 (FIG. 20). The affinity of poly1-280 for CD46 was 2.1.times.10.sup.-8 M, which shows that the IgG mixture contains CD46-binding molecules having the same affinity as 02-237 IgG alone.
Taken together, this experiment shows that it is possible to express a mixture of functional IgG molecules comprising three unique heavy chains in a single cell and that next to the homodimers, heterodimers consisting of two binding specificities are also formed. Furthermore, the frequency of clones expressing three different heavy chains suggests that it will also be possible to obtain clones expressing at least 4, 5, or more, heavy chains, using the same procedure. In the case where it would be difficult to obtain clones expressing higher numbers of heavy chains, a clone expressing at least three heavy chains according to the invention can be used to introduce more heavy chains in a separate round of transfection, for instance by using a different selection marker.
Next, it was demonstrated that a single cell is able to produce a mixture of more than two functional human IgGs. Therefore, clones 241, 280 and 402, which were screened positive for the production of each of the three IgGs, both by IEF and MS, were subjected to limiting dilution, i.e., seeded at 0.3 cells/well in 96-well plates to guarantee clonal outgrowth.
Clonal cell populations, hereafter designated as sub-clones, were refreshed once a week with fresh medium. Sub-clones were grown and transferred from 96-well plates via 24- and 6-well plates, T25, T80 and T175 flasks. At the T80 stage, sub-clones were frozen. Production levels of recombinant human IgG1 antibody were determined in the supernatant using a human IgG1-specific ELISA. For each parental clone, three sub-clones were chosen and cultured in a few T175 flasks to obtain sufficient conditioned medium for purification using Protein A-affinity chromatography as described above.
Purified human IgG1 from the sub-clones was subsequently analyzed as described above for human IgG1 obtained from the parental clone by iso-electric focusing (IEF). The result is shown in FIG. 21. Sub-clones from clone poly 1-241 each have the same pattern, but differ from the parental clone in that they appear to miss certain bands.
Sub-clones from clone poly 1-280 all appear to differ from each other and from the parental clone. Patterns obtained by IEF for sub-clones from parental clone poly 1-402 are identical for all three sub-clones and the parent clone.
From these data, it can be concluded that clone 402 is stably producing a mixture of antibodies. This demonstrates that it is feasible to produce a mixture of antibodies according to the invention from a single cell clone. The clones have undergone about 25 population doublings (cell divisions) from the transfection procedure up to the first analysis (shown in FIG. 18) under selection pressure and, from that point on, have undergone about 30 population doublings during the sub-cloning procedure in the absence of selection pressure before the material analyzed in FIG. 21 was harvested. Therefore, the production of a mixture of antibodies from a clone from a single cell can be stable over at least 30 generations.
Purified IgG1 from the parental 241, 280 and 402 clones, and sub-clones, were also analyzed for binding reactivity towards the CD46 and EpCAM antigens. To this end, cDNA of EpCAM, CD46, and control antigen CD38 were cloned into expression vectors pcDNA (Invitrogen). These vectors were transfected into CHO (dhfr-) cells using Fugene (Roche) according to the protocol supplied by the manufacturer. Cells were cultured in Iscove's medium containing 10% FBS and HT supplement (Gibco). After culturing for two days, cells were harvested by trypsinization and suspended in PBS-1% BSA (PBSB) for use in FACS analysis.
Purified IgG1 of the clones producing the mixtures of antibodies and control IgG1 samples of anti-GBSIII, an anti-CD72 antibody (02-004), as well as antibodies from anti-EpCAM clone UBS54 and anti-CD46 clones K53 and 02-237, were diluted in PBSB to a concentration of 20 .mu.g IgG1/ml. Twenty .mu.l of each was added to 200,000 transfected cells and incubated on ice for one hour. Thereafter, cells were washed once in ice-cold PBSB. Bound IgG was then detected using incubation with goat-anti-human IgG-biotin followed by streptavidin-PE. After a final washing step, cells were suspended in PBSB containing 1 .mu.g/ml propidium iodide. The samples were analyzed on a FACS (FACSvantage, Becton Dickinson). Live cells were gated and Mean Fluorescent Intensities (MFI) were calculated from the FACS plots. The results are represented in FIG. 22. As expected, UBS54 bound selectively to EpCAM-transfected cells and 02-237 and K53 bound selectively to CD46 transfectants, while unrelated antibodies did not bind to these transfectants.
The results demonstrate that binding activities towards both EpCAM and CD46 were present in the purified IgG1 preps of most clones expressing a mixture of antibodies according to the invention, demonstrating that a mixture of functional antibodies was produced by sub-clones that have undergone more than 30 cell divisions and that result from a single cell. In sub-clone 280-015, binding patterns towards CD46 and EpCAM were similar as in the parent clone poly 1-280, in contrast to the other clones.
It should be stated that the quantitative aspect of this assay is not completely clear. Routine screening, for example, by a functional test, can be used to find a clone with the desired expression profile. Quantitative aspects may also be included in such screens. Such screening allows for the identification of desired clones, which express the mixture of antibodies with a given functionality in a quantitatively stable manner.
All references, including publications, patents, and patent applications, cited herein are hereby incorporated by reference to the same extent as if each reference were individually and specifically indicated to be incorporated by reference and were set forth in its entirety herein.
TABLE-US-00001 TABLE I Overview of the clones used for purification of IgG. Purification Screening Conc. Clone ELISA in feed Purified Polyl- (.mu.g/ml) (.mu.g/ml) (mg) 209 6.1 98 1.37 233 10.0 53 0.75 234 8.0 51 0.71 241 6.6 91 1.42 250 12.5 117 2.10 280 6.3 36 0.80 282 8.5 67 1.48 289 8.2 33 0.64 304 7.2 161 3.91 320 6.3 43 0.83 322 15.2 168 3.27 340 6.0 109 2.64 361 10.4 71 1.73 379 9.5 78 1.75 402 39.9 135 3.14 022 16.2 83 1.69 040 7.8 67 1.43 048 6.5 43 0.94 055 11 55 1.04
TABLE-US-00002 TABLE II Tryptic peptides of the variable domains of the light chain of K53/UBS54 and 02-237. Monoisotopic Monoisotopic First Last M.sub.W (Da) M.sub.W (Da) Peptide AA.sup.1) AA K53/UBS54 02-237 L1 1 24 2580.31.sup.(2) 2554.28.sup.(2) L2 25 59 4039.02 4039.02 L3 60 66 700.35 700.35 L4 67 79 1302.61 1302.61 L5 80 82 374.23 374.23 L6 83 107 2810.29.sup.(2) 2810.29.sup.(2) L7 108 111 487.30 487.30 L8 112 112 174.11 174.11 .sup.1)AA, amino acid .sup.(2)One Cysteine residue alkylated
TABLE-US-00003 TABLE III Tryptic peptides of variable domains of heavy chains of K53, 02-237 and UBS54. K53 02-237 UBS54 A B C D A B C D A B C D H1 1 12 1267.68 H1 1 12 1267.68 H1 1 12 1267.68 H2 13 19 685.41 H2 13 19 685.41 H3 20 23 492.24 H3 20 23 492.24 H3 20 23 492.24 H4 24 38 1693.81 H4 24 38 1693.81 H5 39 63 2783.28 H5 39 63 2783.28 H6 64 67 472.28 H6 64 67 472.28 H7 68 84 1906.87 H7 68 84 1906.87 H8 85 87 374.23 H8 85 87 374.23 -- -- -- -- H9 88 98 1319.55 H9 88 98 1319.55 -- -- -- -- Key: A: peptide B: first amino acid C: last amino acid D: monoisotopic M.sub.w (Da) Remarks: 1) for H1, amino acid residue 1 is a pyroglutamic acid 2) peptides H3 and H9 from K53 and 02-237, and peptides H3 and H8 of UBS54 contain one alkylated cysteine residue 3) Unique peptides that can be used to confirm the presence of the respective IgGs are indicated in bold italics
TABLE-US-00004 TABLE IV MS/MS-data of CDR3 peptide (H11) of K53, obtained by collision induced dissociation of doubly charged m/z 1059.06. Ion m/z Ion m/z Y''.sub.1 147.12 B.sub.1 n.d. Y''.sub.2 248.18 B.sub.2 157.10 Y''.sub.3 335.21.sup.(1) B.sub.3 304.18 Y''.sub.4 406.25 B.sub.4 419.22 Y''.sub.5 507.30 B.sub.5 582.31 Y''.sub.6 594.33 B.sub.6 768.38 Y''.sub.7 693.40 B.sub.7 825.39 Y''.sub.8 794.46 B.sub.8 953.43 Y''.sub.9 893.54 B.sub.9 n.d. Y''.sub.10 1006.63 B.sub.10 n.d. Y''.sub.11 1107.67 B.sub.11 1224.65 Y''.sub.12 1164.68 B.sub.12 1323.68 Y''.sub.13 1292.81 B.sub.13 1424.79 Y''.sub.14 1349.77 B.sub.14 1523.86 Y''.sub.15 1535.85 B.sub.15 n.d. Y''.sub.16 1698.95 B.sub.16 n.d. Y''.sub.17 1813.95 B.sub.17 1782.96 Y''.sub.18 1960.97 B.sub.18 n.d. Y''.sub.19 n.d..sup.(2) B.sub.19 n.d. .sup.(1)Underlined m/z-values are main peaks in the MS/MS-spectrum. .sup.(2)n.d. is not detected.
TABLE-US-00005 TABLE V MS/MS-data of CDR3 peptide (H11) of 02-237, obtained by collision induced dissociation of doubly charged m/z 1030.02. Ion m/z Ion m/z Y''.sub.1 147.12 B.sub.1 n.d. Y''.sub.2 248.18 B.sub.2 189.09 Y''.sub.3 335.20 B.sub.3 n.d. Y''.sub.4 406.24 B.sub.4 451.22 Y''.sub.5 493.30 B.sub.5 n.d. Y''.sub.6 580.32 B.sub.6 n.d. Y''.sub.7 679.40 B.sub.7 n.d. Y''.sub.8 780.44 B.sub.8 n.d. Y''.sub.9 879.53 B.sub.9 n.d. Y''.sub.10 992.60 B.sub.10 n.d. Y''.sub.11 1093.65 B.sub.11 n.d. Y''.sub.12 1150.67 B.sub.12 n.d. Y''.sub.13 1278.80 B.sub.13 n.d. Y''.sub.14 1335.80 B.sub.14 n.d. Y''.sub.15 1521.83 B.sub.15 n.d. Y''.sub.16 1608.90 B.sub.16 n.d. Y''.sub.17 1724.00 B.sub.17 n.d. Y''.sub.18 n.d. B.sub.18 n.d. Y''.sub.19 n.d. B.sub.19 n.d. .sup.1Underlined m/z-values are main peaks in the MS/MS-spectrum. .sup.2n.d. is not detected.
TABLE-US-00006 TABLE VI MS/MS-data of CDR3 peptide (H9) of UBS54, obtained by collision induced dissociation of triply charged m/z 770.09. Ion m/z Ion m/z Y''.sub.1 n.d. B.sub.1 n.d. Y''.sub.2 248.17 B.sub.2 213.17 Y''.sub.3 335.20 B.sub.3 360.16 Y''.sub.4 406.25 B.sub.4 473.27 Y''.sub.5 507.30 B.sub.5 610.32 Y''.sub.6 594.33 B.sub.6 773.41 Y''.sub.7 693.42 B.sub.7 959.48 Y''.sub.8 794.45 B.sub.8 1016.50 Y''.sub.9 893.53 B.sub.9 1144.57 Y''.sub.10 1006.64 B.sub.10 1201.59 Y''.sub.11 1107.67 B.sub.11 1302.68 Y''.sub.12 1164.68 B.sub.12 1415.72 Y''.sub.13 n.d. B.sub.13 1514.78 Y''.sub.14 n.d. B.sub.14 n.d. Y''.sub.15 n.d. B.sub.15 n.d. Y''.sub.16 n.d. B.sub.16 n.d. Y''.sub.17 n.d. B.sub.17 n.d. Y''.sub.18 n.d. B.sub.18 n.d. Y''.sub.19 n.d. B.sub.19 n.d. Y''.sub.20 n.d. B.sub.20 n.d. .sup.1Underlined m/z-values are main peaks in the MS/MS-spectrum. .sup.2n.d is not detected.
REFERENCES
Appel R. D., Bairoch A. and Hochstrasser D. F. (1994) A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem. Sci. 19:258-260. Bendig M. M. (1988) The production of foreign proteins in mammalian cells. Genet. Eng. 7:91-127. Boel E., Verlaan S., Poppelier M. J., Westerdaal N. A., Van Strijp J. A. and Logtenberg T. (2000) Functional human monoclonal antibodies of all isotypes constructed from phage display library-derived single-chain Fv antibody fragments. J. Immunol. Methods 239:153-166. Brink M. F., Bishop M. D. and Pieper F. R. (2000) Developing efficient strategies for the generation of transgenic cattle which produce biopharmaceuticals in milk. Theriogenology 53:139-148. Campbell K. H., McWhir J., Ritchie W. A. and Wilmut I. (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380:64-66. Casellas R., Shih T. A., Kleinewietfelt M., Rakoniac J., Nemazee D., Rajewski K. and Nussenzweig M. C. (2001) Contribution of receptor editing to the antibody repertoire. Science 291:1541-1544. Cockett M. I., Bebbington C. R. and Yarranton G. T. (1990) High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamate synthetase gene amplification. Bio/technology 8:662-667. De Kruif J., Terstappen L., Boel E. and Logtenberg T. (1995a) Rapid selection of cell sub-population-specific human monoclonal antibodies from a synthetic phage antibody library. Proc. Natl. Acad. Sci. U.S.A. 92:3938 De Kruif J., Boel E. and Logtenberg T. (1995b) Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J. Mol. Biol. 248:97 Dinnyes A., De Sousa P., King T. and Wilmut I. (2002) Somatic cell nuclear transfer: recent progress and challenges. Cloning Stem Cells 4:81-90. Flavell D. J., Noss A., Pulford K. A., Ling N. and Flavell S. U. (1997) Systemic therapy with 3BIT, a triple combination cocktail of anti-CD19, -CD22, and -CD38-saporin immunotoxins, is curative of human B-cell lymphoma in severe combined immunodeficient mice. Cancer Res. 57:4824-4829. Fishwild D. M., O'Donnell S. L., Bengoechea T., Hudson D. V., Harding F., Bernhard S. L., Jones D., Kay R. M., Higgins K. M., Schramm S. R. and Lonberg N. (1996) High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 14:845-51. Garnick R L. (1992) Peptide mapping for detecting variants in protein products. Develop. Biol. Standard 76:117-130. Gelpi E. (1995) Biomedical and biochemical applications of liquid chromatography-mass spectrometry. J. Chromatography A 703:59-80. Ghetie M.-A., Podar E. M., Ilgen A., Gordon B. E., Uhr J. W. and Vitetta E S. (1997) Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc. Natl. Acad. Sci. U.S.A. 94:7509-7514. Giddings G., Allison G., Brooks D. and Carter A. (2000) Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 18:1151-1155. Gorman C. and Bullock C. (2000) Site-specific gene targeting for gene expression in eukaryotes. Curr. Opin. Biotechnol. 11:455-460. Hiatt A., Cafferkey R. and Bowdish K. (1989) Production of antibodies in transgenic plants. Nature 342:76-78. Huls G. A., Heijnen I. A., Cuomo M. E., Koningsberger J. C., Wiegman L., Boel E., van der Vuurst de Vries A. R., Loyson S. A., Helfrich W., van Berge Henegouwen G. P., van Meijer M., de Kruif J. and Logtenberg T. (1999) A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nat. Biotechnol. 17:276-281. Jespers L. S., Roberts A., Mahler S. M., Winter G. and Hoogenboom H. R. (1994) Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology (N.Y.) 12:899-903. Jones D., Kroos N., Anema R., Van Montfort B., Vooys A., Van Der Kraats S., Van Der Helm E., Smits S., Schouten J., Brouwer K., Lagerwerf F., Van Berkel P., Opstelten D-J., Logtenberg T. and Bout A. (2003) High-level expression of recombinant IgG in the human cell line PER.C6.RTM.. Biotechnol. Prog. 19, 163-168. Kim S. J., Kim N. S., Ryu C. J., Hong H. J. and Lee G. M. (1998) Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnol. Bioeng. 58:73-84. Kohler G. and Millstein C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T. and van Oers M. H. (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415-1420. Larrick J. W. and Thomas D. W. (2001) Producing proteins in transgenic plants and animals. Curr. Opin. Biotechnol. 12:411-418. Massengale W. T., McBurney E. and Gurtler J. (2002) CD20-negative relapse of cutaneous B-cell lymphoma after anti-CD20 monoclonal antibody therapy. J. Am. Acad. Dermatol. 46:441-443. Mendez M. J., Green L. L., Corvalan J. R., Jia X. C., Maynard-Currie C. E., Yang X. D., Gallo M. L., Louie D. M., Lee D. V., Erickson K. L., Luna J., Roy C. M., Abderrahim H., Kirschenbaum F., Noguchi M., Smith D. H., Fukushima A., Hales J. F., Klapholz S., Finer M. H., Davis C. G., Zsebo K. M. and Jakobovits A. (1997) Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat. Genet. 15:146-56. Merchant A. M., Zhu Z., Yuan J. Q., Goddard A., Adams C. W., Presta L. G. and Carter P. (1998) An efficient route to human bispecific IgG. Nat. Biotech. 16:677-681. Nemazee D. (2000) Receptor editing in B cells. Adv. Immunol. 74:89-126. Nissim A., Hoogenboom H. R., Tomlinson I. M., Flynn G., Midgley C., Lane D. and Winter G. (1994) Antibody fragments from a "single pot" phage display library as immunological reagents. EMBO. J. 13:692-698. Nowakowski A., Wang C., Powers D. B., Amersdorfer P., Smith T. J., Montgomery V. A., Sheridan R., Blake R., Smith L. A. and Marks J. D. (2002) Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 99:11346-11350. Patel A. K. and Boyd P. N. (1995) An improved assay for antibody-dependent cellular cytotoxicity based on time resolved fluorometry. Journal of Immunological Methods 184:29-38. Peeters K., De Wilde C., De Jaeger G., Angenon G. and Depicker A. (2001) Production of antibodies and antibody fragments in plants. Vaccine 19:2756-2761. Pollock D. P., Kutzko J. P., Birck-Wilson E., Williams J. L., Echelard Y. and Meade H. M. (1999) Transgenic milk as a method for the production of recombinant antibodies. J. Immunol. Methods 231:147-157. Radic M. C., Mascelli M. A., Shan H. and Weigert M. (1991) Ig H and L chain contributions to auto-immune specificities. J. Immunol. 146:176-182. Schnieke A. E., Kind A. J., Ritchie W. A., Mycock K., Scott A. R., Ritchie M., Wilmut I., Colman A. and Campbell K. H. (1997) Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science 278:2130-2133. Segal D. M., Weiner G. J. and Weiner L. M. (2001) Introduction: bispecific antibodies. J. Immunol. Methods 248:1-6. Shields R. L., Namenuk A. K., Hong K., Gloria Meng Y., Rae J., Biggs J., Xie D., Lai J., Stadlen A., Li B., Fox J. A. and Presta L. G. (2001) High resolution mapping of the binding site on human IgG1 for FcgRI, FcgRII, FcgRIII and FcRn and design of IgG1 variants with improved binding to the FcgR. J. Biol. Chem. 276:6591-6604. Spiridon C. I., Ghetie M. A., Uhr J., Marches R., Li J. L., Shen G. L. and Vitetta E. S. (2002) Targeting multiple her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin. Cancer Res. 8:1720-1730. Van der Vuurst de Vries A. and Logtenberg T. (1999) Dissecting the human peripheral B-cell compartment with phage display-derived antibodies. Immunology 98:55-62. Vaughan T. J., Williams A. J., Pritchard K., Osbourn J. K., Pope A. R., Earnshaw J. C., McCafferty J., Hodits R. A., Wilton J. and Johnson K. S. (1996) Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotech. 14:309-314. Wilmut I. and Clark A. J. (1991) Basic techniques for transgenesis. J. Reprod. Fertil. Suppl. 43:265-275. Wilmut I., Schnieke A. E., McWhir J., Kind A. J. and Campbell K. H. (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385:810-813. Wilson T. J. and Kola I. (2001) The LoxP/CRE system and genome modification. Methods Mol. Biol. 158:83-94. Yelverton E., Norton S., Obijeski J. F. and Goeddel D. V. (1983) Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science 219:614-620. Yoo E. M., Coloma M. J., Trinh K. R., Nguyen T. Q., Vuong L. U., Morrison S. L. and Chintalacharuvu K. R. (1999) Structural requirements for polymeric immunoglobulin assembly and association with J chain. J. Biol. Chem. 274:33771-33777.
SEQUENCE LISTINGS
1
181333DNAArtificialVL sequence of UBS54 (anti-EpCAM) and K53 (anti-CD46)CDS(1)..(333) 1gaa att gag ctc act cag tct cca ctc tcc ctg ccc gtc acc cct gga 48Glu Ile Glu Leu Thr Gln Ser Pro Leu Ser Leu Pro Val Thr Pro Gly1 5 10 15gag ccg gcc tcc atc tcc tgc agg tct agt cag agc ctc ctg cat agt 96Glu Pro Ala Ser Ile Ser Cys Arg Ser Ser Gln Ser Leu Leu His Ser 20 25 30aat gga tac aac tat ttg gat tgg tac ctg cag aag cca ggg cag tct 144Asn Gly Tyr Asn Tyr Leu Asp Trp Tyr Leu Gln Lys Pro Gly Gln Ser 35 40 45cca cag ctc ctg atc tat ttg ggt tct aat cgg gcc tcc ggg gtc cct 192Pro Gln Leu Leu Ile Tyr Leu Gly Ser Asn Arg Ala Ser Gly Val Pro 50 55 60gac agg ttc agt ggc agt gga tca ggc aca gat ttt aca ctg aaa atc 240Asp Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile65 70 75 80agc aga gtg gag gct gag gat gtt ggg gtt tat tac tgc atg caa gct 288Ser Arg Val Glu Ala Glu Asp Val Gly Val Tyr Tyr Cys Met Gln Ala 85 90 95cta caa act ttc act ttc ggc cct ggg acc aag gtg gag atc aaa 333Leu Gln Thr Phe Thr Phe Gly Pro Gly Thr Lys Val Glu Ile Lys 100 105 1102111PRTArtificialVL sequence of UBS54 (anti-EpCAM) and K53 (anti-CD46) 2Glu Ile Glu Leu Thr Gln Ser Pro Leu Ser Leu Pro Val Thr Pro Gly1 5 10 15Glu Pro Ala Ser Ile Ser Cys Arg Ser Ser Gln Ser Leu Leu His Ser 20 25 30Asn Gly Tyr Asn Tyr Leu Asp Trp Tyr Leu Gln Lys Pro Gly Gln Ser 35 40 45Pro Gln Leu Leu Ile Tyr Leu Gly Ser Asn Arg Ala Ser Gly Val Pro 50 55 60Asp Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile65 70 75 80Ser Arg Val Glu Ala Glu Asp Val Gly Val Tyr Tyr Cys Met Gln Ala 85 90 95Leu Gln Thr Phe Thr Phe Gly Pro Gly Thr Lys Val Glu Ile Lys 100 105 1103333DNAArtificialVL sequence of 02-237 (anti-CD46)CDS(1)..(333) 3gac atc gtg atg act cag tct cca ctc tcc ctg ccc gtc acc cct gga 48Asp Ile Val Met Thr Gln Ser Pro Leu Ser Leu Pro Val Thr Pro Gly1 5 10 15gag ccg gcc tcc atc tcc tgc agg tct agt cag agc ctc ctg cat agt 96Glu Pro Ala Ser Ile Ser Cys Arg Ser Ser Gln Ser Leu Leu His Ser 20 25 30aat gga tac aac tat ttg gat tgg tac ctg cag aag cca ggg cag tct 144Asn Gly Tyr Asn Tyr Leu Asp Trp Tyr Leu Gln Lys Pro Gly Gln Ser 35 40 45cca cag ctc ctg atc tat ttg ggt tct aat cgg gcc tcc ggg gtc cct 192Pro Gln Leu Leu Ile Tyr Leu Gly Ser Asn Arg Ala Ser Gly Val Pro 50 55 60gac agg ttc agt ggc agt gga tca ggc aca gat ttt aca ctg aaa atc 240Asp Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile65 70 75 80agc aga gtg gag gct gag gat gtt ggg gtt tat tac tgc atg caa gct 288Ser Arg Val Glu Ala Glu Asp Val Gly Val Tyr Tyr Cys Met Gln Ala 85 90 95cta caa act ttc act ttc ggc cct ggg acc aag gtg gag atc aaa 333Leu Gln Thr Phe Thr Phe Gly Pro Gly Thr Lys Val Glu Ile Lys 100 105 1104111PRTArtificialVL sequence of 02-237 (anti-CD46) 4Asp Ile Val Met Thr Gln Ser Pro Leu Ser Leu Pro Val Thr Pro Gly1 5 10 15Glu Pro Ala Ser Ile Ser Cys Arg Ser Ser Gln Ser Leu Leu His Ser 20 25 30Asn Gly Tyr Asn Tyr Leu Asp Trp Tyr Leu Gln Lys Pro Gly Gln Ser 35 40 45Pro Gln Leu Leu Ile Tyr Leu Gly Ser Asn Arg Ala Ser Gly Val Pro 50 55 60Asp Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile65 70 75 80Ser Arg Val Glu Ala Glu Asp Val Gly Val Tyr Tyr Cys Met Gln Ala 85 90 95Leu Gln Thr Phe Thr Phe Gly Pro Gly Thr Lys Val Glu Ile Lys 100 105 1105345DNAArtificialVH sequence of UBS54 (anti-EpCAM)CDS(1)..(345) 5cag gtg cag ctg gtg cag tct ggg gct gag gtg aag aag cct ggg tcc 48Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ser1 5 10 15tcg gtg agg gtc tcc tgc aag gct tct gga ggc acc ttc agc agc tat 96Ser Val Arg Val Ser Cys Lys Ala Ser Gly Gly Thr Phe Ser Ser Tyr 20 25 30gct atc agc tgg gtg cga cag gcc cct gga caa ggg ctt gag tgg atg 144Ala Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45gga ggg atc atc cct atc ttt ggt aca gca aac tac gca cag aag ttc 192Gly Gly Ile Ile Pro Ile Phe Gly Thr Ala Asn Tyr Ala Gln Lys Phe 50 55 60cag ggc aga gtc acg att acc gcg gac gaa tcc acg agc aca gcc tac 240Gln Gly Arg Val Thr Ile Thr Ala Asp Glu Ser Thr Ser Thr Ala Tyr65 70 75 80atg gag ctg agc agc ctg aga tct gag gac acg gct gtg tat tac tgt 288Met Glu Leu Ser Ser Leu Arg Ser Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95gca aga gac ccg ttt ctt cac tat tgg ggc caa ggt acc ctg gtc acc 336Ala Arg Asp Pro Phe Leu His Tyr Trp Gly Gln Gly Thr Leu Val Thr 100 105 110gtc tcg aca 345Val Ser Thr 1156115PRTArtificialVH sequence of UBS54 (anti-EpCAM) 6Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ser1 5 10 15Ser Val Arg Val Ser Cys Lys Ala Ser Gly Gly Thr Phe Ser Ser Tyr 20 25 30Ala Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Gly Ile Ile Pro Ile Phe Gly Thr Ala Asn Tyr Ala Gln Lys Phe 50 55 60Gln Gly Arg Val Thr Ile Thr Ala Asp Glu Ser Thr Ser Thr Ala Tyr65 70 75 80Met Glu Leu Ser Ser Leu Arg Ser Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Asp Pro Phe Leu His Tyr Trp Gly Gln Gly Thr Leu Val Thr 100 105 110Val Ser Thr 1157354DNAArtificialVH sequence of K53 (anti-CD46)CDS(1)..(354) 7cag gtg cag ctg gtg cag tct ggg gct gag gtg aag aag cct ggg gcc 48Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15tca gtg aag gtc tcc tgc aag gct tct ggt tac acc ttt acc agc tat 96Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Ser Tyr 20 25 30ggt atc agc tgg gtg cga cag gcc cct gga caa ggg ctt gag tgg atg 144Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45gga tgg atc agc gct tac aat ggt aac aca aac tat gca cag aag ctc 192Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60cag ggc aga gtc acc atg acc aca gac aca tcc acg agc aca gcc tac 240Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Ser Thr Ala Tyr65 70 75 80atg gag ctg agg agc ctg aga tct gac gac acg gcc gtg tat tac tgt 288Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95gca agg ggc atg atg agg ggt gtg ttt gac tac tgg ggc caa ggt acc 336Ala Arg Gly Met Met Arg Gly Val Phe Asp Tyr Trp Gly Gln Gly Thr 100 105 110ctg gtc acc gtc tcg aca 354Leu Val Thr Val Ser Thr 1158118PRTArtificialVH sequence of K53 (anti-CD46) 8Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Ser Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Ser Thr Ala Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Met Met Arg Gly Val Phe Asp Tyr Trp Gly Gln Gly Thr 100 105 110Leu Val Thr Val Ser Thr 1159354DNAArtificialVH sequence of 02-237 (anti-CD46)CDS(1)..(354) 9cag gtg cag ctg gtg cag tct ggg gct gag gtg aag aag cct ggg gcc 48Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15tca gtg aag gtc tcc tgc aag gct tct ggt tac acc ttt acc agc tat 96Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Ser Tyr 20 25 30ggt atc agc tgg gtg cga cag gcc cct gga caa ggg ctt gag tgg atg 144Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45gga tgg atc agc gct tac aat ggt aac aca aac tat gca cag aag ctc 192Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60cag ggc aga gtc acc atg acc aca gac aca tcc acg agc aca gcc tac 240Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Ser Thr Ala Tyr65 70 75 80atg gag ctg agg agc ctg aga tct gac gac acg gcc gtg tat tac tgt 288Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95gca agg ggc ttt ccg cgt acg tcg ttt gac tcc tgg ggc cag ggc acc 336Ala Arg Gly Phe Pro Arg Thr Ser Phe Asp Ser Trp Gly Gln Gly Thr 100 105 110ctg gtg acc gtc tcc tca 354Leu Val Thr Val Ser Ser 11510118PRTArtificialVH sequence of 02-237 (anti-CD46) 10Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Ser Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Ser Thr Ala Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Phe Pro Arg Thr Ser Phe Asp Ser Trp Gly Gln Gly Thr 100 105 110Leu Val Thr Val Ser Ser 11511369DNAArtificialVH sequence of clone B28 (anti-CD22 phage)CDS(1)..(369) 11atg gcc gag gtg cag ctg gtg gag tct ggg gga ggt gtg gta cgg cct 48Met Ala Glu Val Gln Leu Val Glu Ser Gly Gly Gly Val Val Arg Pro1 5 10 15gga ggg tcc ctg aga ctc tcc tgt gca gcc tct gga ttc acc ttt gat 96Gly Gly Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp 20 25 30gat tat ggc atg agc tgg gtc cgc caa gct cca ggg aag ggg ctg gag 144Asp Tyr Gly Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu 35 40 45tgg gtc tct ggt att aat tgg aat ggt ggt agc aca ggt tat gca gac 192Trp Val Ser Gly Ile Asn Trp Asn Gly Gly Ser Thr Gly Tyr Ala Asp 50 55 60tct gtg aag ggc cga ttc acc atc tcc aga gac aac gcc aag aac tcc 240Ser Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser65 70 75 80ctg tat ctg caa atg aac agt ctg aga gcc gag gac acg gcc gtg tat 288Leu Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr 85 90 95tac tgt gca aga ggc ttt ctt cgt ttt gct tcc tcc tgg ttt gac tat 336Tyr Cys Ala Arg Gly Phe Leu Arg Phe Ala Ser Ser Trp Phe Asp Tyr 100 105 110tgg ggc caa ggt acc ctg gtc acc gtc tcg aga 369Trp Gly Gln Gly Thr Leu Val Thr Val Ser Arg 115 12012123PRTArtificialVH sequence of clone B28 (anti-CD22 phage) 12Met Ala Glu Val Gln Leu Val Glu Ser Gly Gly Gly Val Val Arg Pro1 5 10 15Gly Gly Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp 20 25 30Asp Tyr Gly Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu 35 40 45Trp Val Ser Gly Ile Asn Trp Asn Gly Gly Ser Thr Gly Tyr Ala Asp 50 55 60Ser Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser65 70 75 80Leu Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr 85 90 95Tyr Cys Ala Arg Gly Phe Leu Arg Phe Ala Ser Ser Trp Phe Asp Tyr 100 105 110Trp Gly Gln Gly Thr Leu Val Thr Val Ser Arg 115 12013369DNAArtificialVH sequence of clone II-2 (anti-CD72 phage)CDS(1)..(369) 13atg gcc cag gtg cag ctg gtg cag tct ggg gct gag gtg aag aag cct 48Met Ala Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro1 5 10 15ggg gcc tca gtg aag gtt tcc tgc aag gca tct gga tac acc ttc acc 96Gly Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr 20 25 30agc tac tat atg cac tgg gtg cga cag gcc cct gga caa ggg ctt gag 144Ser Tyr Tyr Met His Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu 35 40 45tgg atg gga ata atc aac cct agt ggt ggt ggc aca agc tac gca cag 192Trp Met Gly Ile Ile Asn Pro Ser Gly Gly Gly Thr Ser Tyr Ala Gln 50 55 60aag ttc cag ggc aga gtc acc atg acc agg gac acg tcc acg agc aca 240Lys Phe Gln Gly Arg Val Thr Met Thr Arg Asp Thr Ser Thr Ser Thr65 70 75 80gtc tac atg gag ctg agc agc ctg aga tct gag gac acg gcc gtg tat 288Val Tyr Met Glu Leu Ser Ser Leu Arg Ser Glu Asp Thr Ala Val Tyr 85 90 95tac tgt gca aga gac tac tat gtt acg tat gat tcc tgg ttt gac tcc 336Tyr Cys Ala Arg Asp Tyr Tyr Val Thr Tyr Asp Ser Trp Phe Asp Ser 100 105 110tgg ggc caa ggt acc ctg gtc acc gtc tcg aga 369Trp Gly Gln Gly Thr Leu Val Thr Val Ser Arg 115 12014123PRTArtificialVH sequence of clone II-2 (anti-CD72 phage) 14Met Ala Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro1 5 10 15Gly Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr 20 25 30Ser Tyr Tyr Met His Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu 35 40 45Trp Met Gly Ile Ile Asn Pro Ser Gly Gly Gly Thr Ser Tyr Ala Gln 50 55 60Lys Phe Gln Gly Arg Val Thr Met Thr Arg Asp Thr Ser Thr Ser Thr65 70 75 80Val Tyr Met Glu Leu Ser Ser Leu Arg Ser Glu Asp Thr Ala Val Tyr 85 90 95Tyr Cys Ala Arg Asp Tyr Tyr Val Thr Tyr Asp Ser Trp Phe Asp Ser 100 105 110Trp Gly Gln Gly Thr Leu Val Thr Val Ser Arg 115 12015360DNAArtificialVH sequence of clone I-2 (anti-class II phage)CDS(1)..(360) 15atg gcc gag gtg cag ctg gtg gag tct ggg gga ggc ttg gta cag cct 48Met Ala Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro1 5 10 15ggc agg tcc ctg aga ctc tcc tgt gca gcc tct gga ttc acc ttt gat 96Gly Arg Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp 20 25 30gat tat gcc atg cac tgg gtc cgg caa gct cca ggg aag ggc ctg gag 144Asp Tyr Ala Met His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu 35 40 45tgg gtc tca ggt att agt tgg aat agt ggt agc ata ggc tat gcg gac 192Trp Val Ser Gly Ile Ser Trp Asn Ser Gly Ser Ile Gly Tyr Ala Asp 50 55 60tct gtg aag ggc cga ttc acc atc tcc aga gac aac gcc aag aac tcc 240Ser Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser65 70 75 80ctg tat ctg caa atg aac agt ctg aga gct gag gac acg gcc gtg tat 288Leu Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr 85 90 95tac tgt gca agg gac ctt tat ctt gcg cat ttt gac tac tgg ggc caa 336Tyr Cys Ala Arg Asp Leu Tyr Leu Ala His Phe Asp Tyr Trp Gly Gln
100 105 110ggt acc ctg gtc acc gtc tcg aga 360Gly Thr Leu Val Thr Val Ser Arg 115 12016120PRTArtificialVH sequence of clone I-2 (anti-class II phage) 16Met Ala Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro1 5 10 15Gly Arg Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp 20 25 30Asp Tyr Ala Met His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu 35 40 45Trp Val Ser Gly Ile Ser Trp Asn Ser Gly Ser Ile Gly Tyr Ala Asp 50 55 60Ser Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser65 70 75 80Leu Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr 85 90 95Tyr Cys Ala Arg Asp Leu Tyr Leu Ala His Phe Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Arg 115 12017336DNAArtificialcommon VL sequence of clones B28 (anti-CD22 phage), II-2 (anti-CD72 phage) and I-2 (anti-class II phage)CDS(1)..(336) 17tcg tct gag ctg act cag gac cct gct gtg tct gtg gcc ttg gga cag 48Ser Ser Glu Leu Thr Gln Asp Pro Ala Val Ser Val Ala Leu Gly Gln1 5 10 15aca gtc agg atc aca tgc caa gga gac agc ctc aga agc tat tat gca 96Thr Val Arg Ile Thr Cys Gln Gly Asp Ser Leu Arg Ser Tyr Tyr Ala 20 25 30agc tgg tac cag cag aag cca gga cag gcc cct gta ctt gtc atc tat 144Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Leu Val Ile Tyr 35 40 45ggt aaa aac aac cgg ccc tca ggg atc cca gac cga ttc tct ggc tcc 192Gly Lys Asn Asn Arg Pro Ser Gly Ile Pro Asp Arg Phe Ser Gly Ser 50 55 60agc tca gga aac aca gct tcc ttg acc atc act ggg gct cag gcg gaa 240Ser Ser Gly Asn Thr Ala Ser Leu Thr Ile Thr Gly Ala Gln Ala Glu65 70 75 80gat gag gct gac tat tac tgt aac tcc cgg gac agc agt ggt aac cat 288Asp Glu Ala Asp Tyr Tyr Cys Asn Ser Arg Asp Ser Ser Gly Asn His 85 90 95gtg gta ttc ggc gga ggg acc aag ctg acc gtc cta ggt gcg gcc gca 336Val Val Phe Gly Gly Gly Thr Lys Leu Thr Val Leu Gly Ala Ala Ala 100 105 11018112PRTArtificialcommon VL sequence of clones B28 (anti-CD22 phage), II-2 (anti-CD72 phage) and I-2 (anti-class II phage) 18Ser Ser Glu Leu Thr Gln Asp Pro Ala Val Ser Val Ala Leu Gly Gln1 5 10 15Thr Val Arg Ile Thr Cys Gln Gly Asp Ser Leu Arg Ser Tyr Tyr Ala 20 25 30Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Leu Val Ile Tyr 35 40 45Gly Lys Asn Asn Arg Pro Ser Gly Ile Pro Asp Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly Asn Thr Ala Ser Leu Thr Ile Thr Gly Ala Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Asn Ser Arg Asp Ser Ser Gly Asn His 85 90 95Val Val Phe Gly Gly Gly Thr Lys Leu Thr Val Leu Gly Ala Ala Ala 100 105 110
* * * * *
References
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

D00013
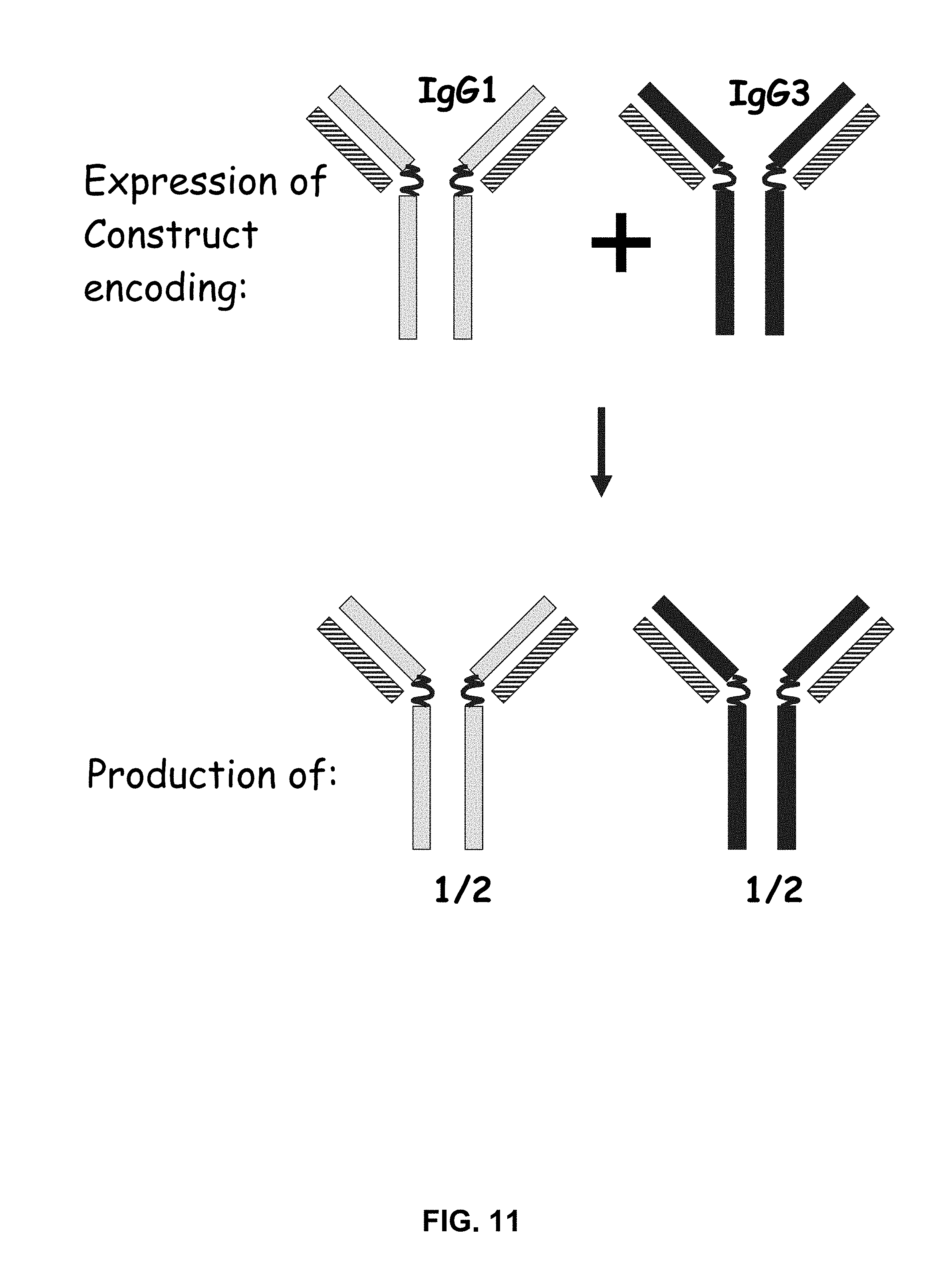
D00014

D00015

D00016

D00017

D00018

D00019

D00020
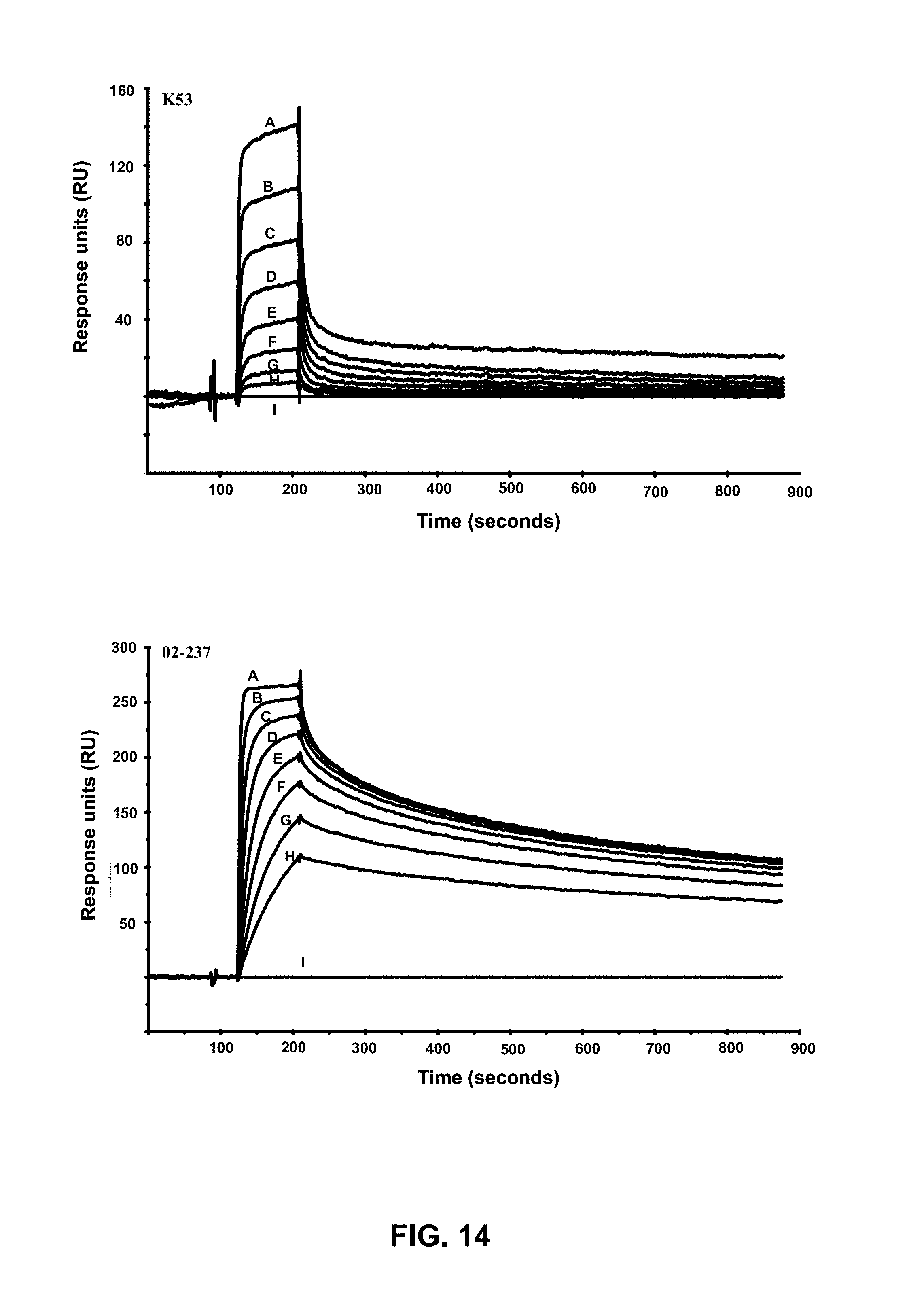
D00021
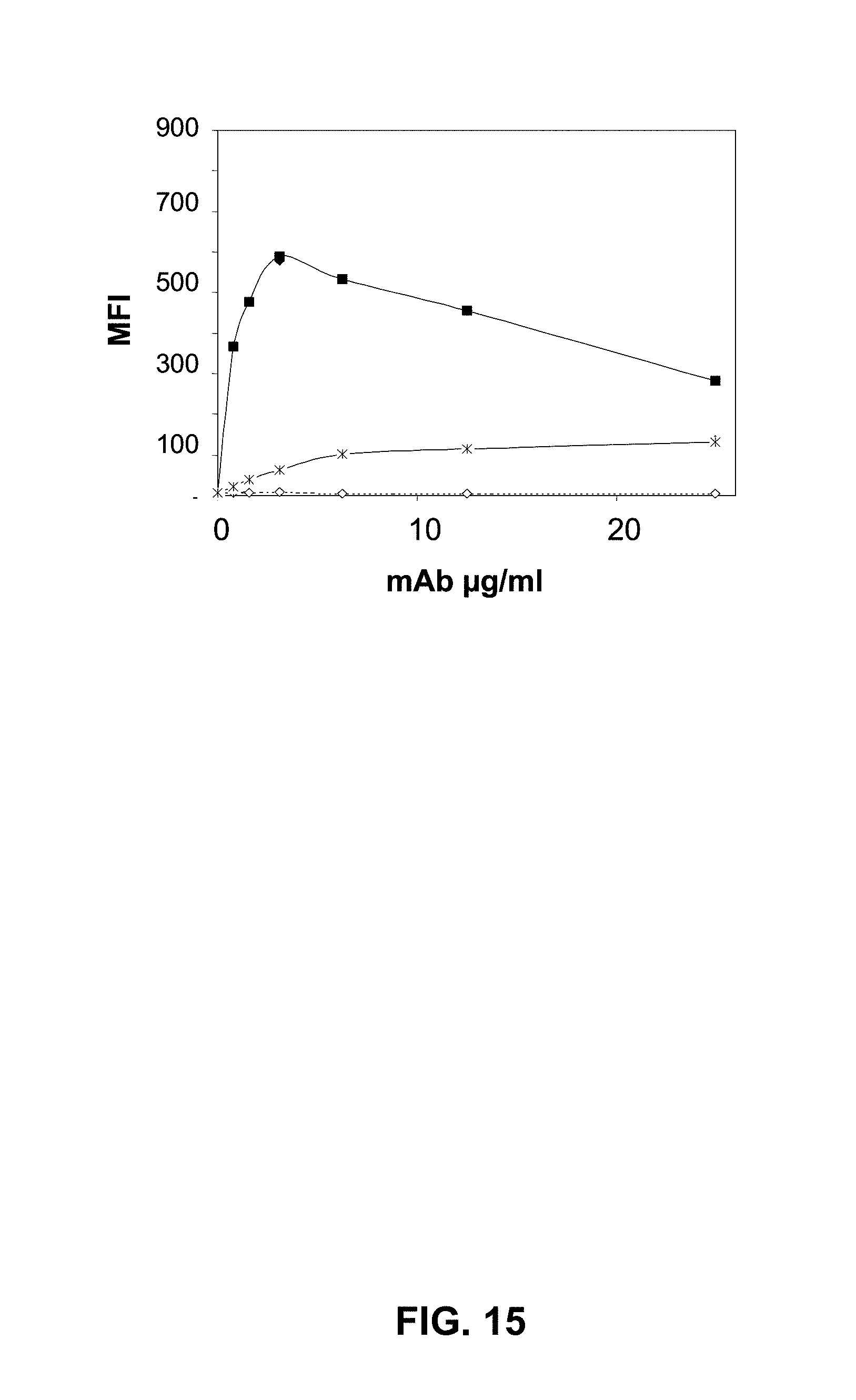
D00022
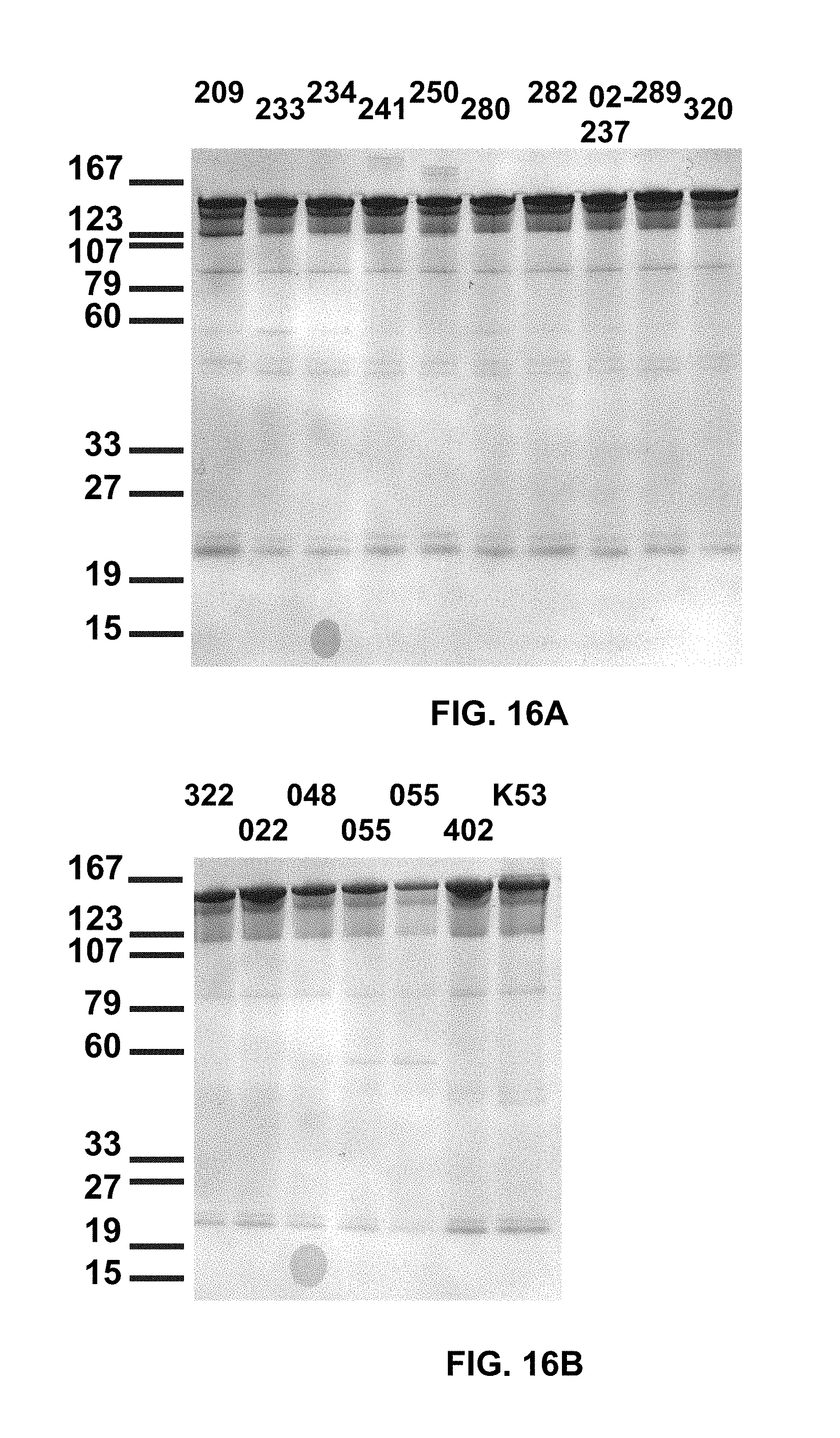
D00023
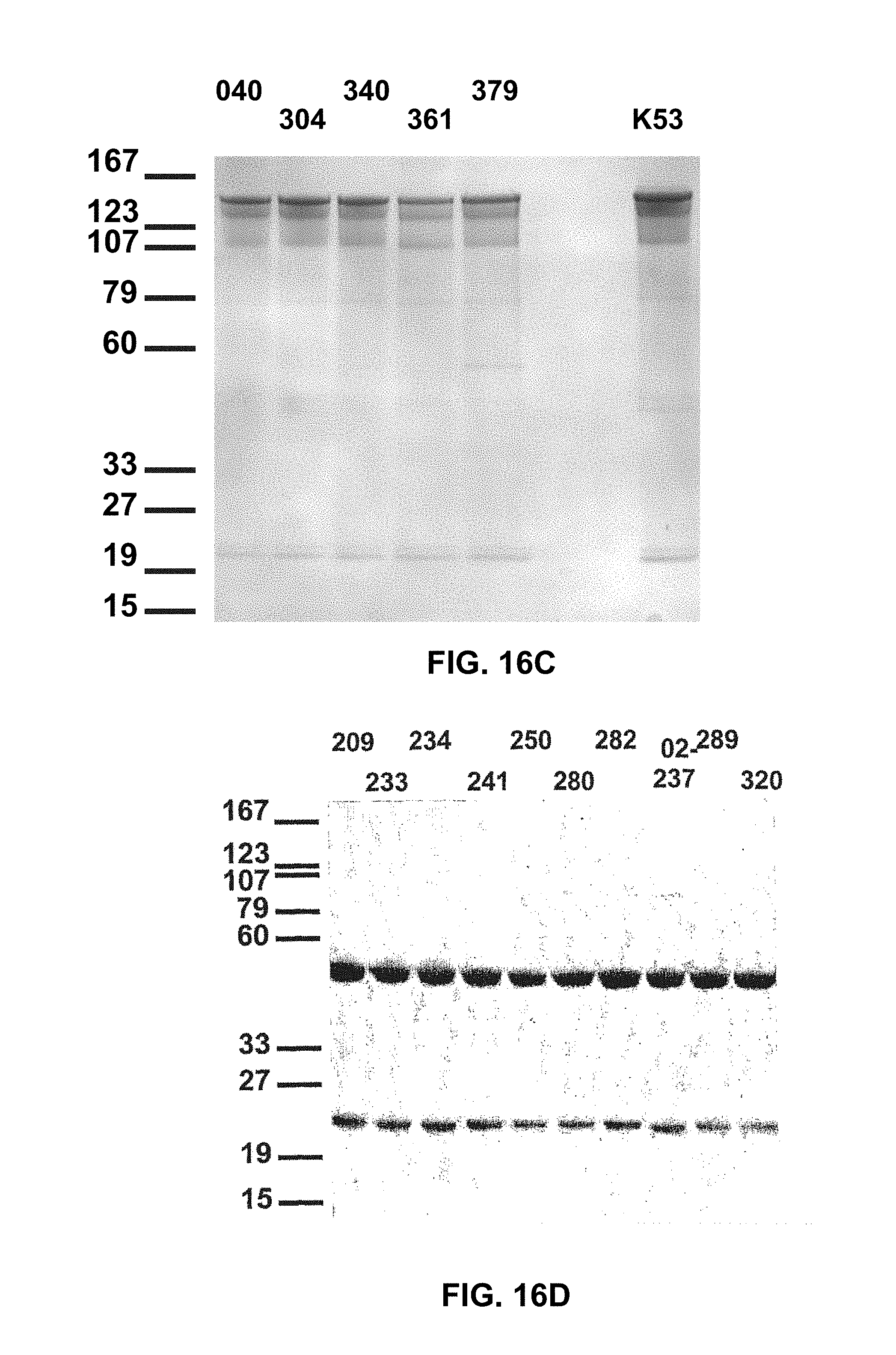
D00024
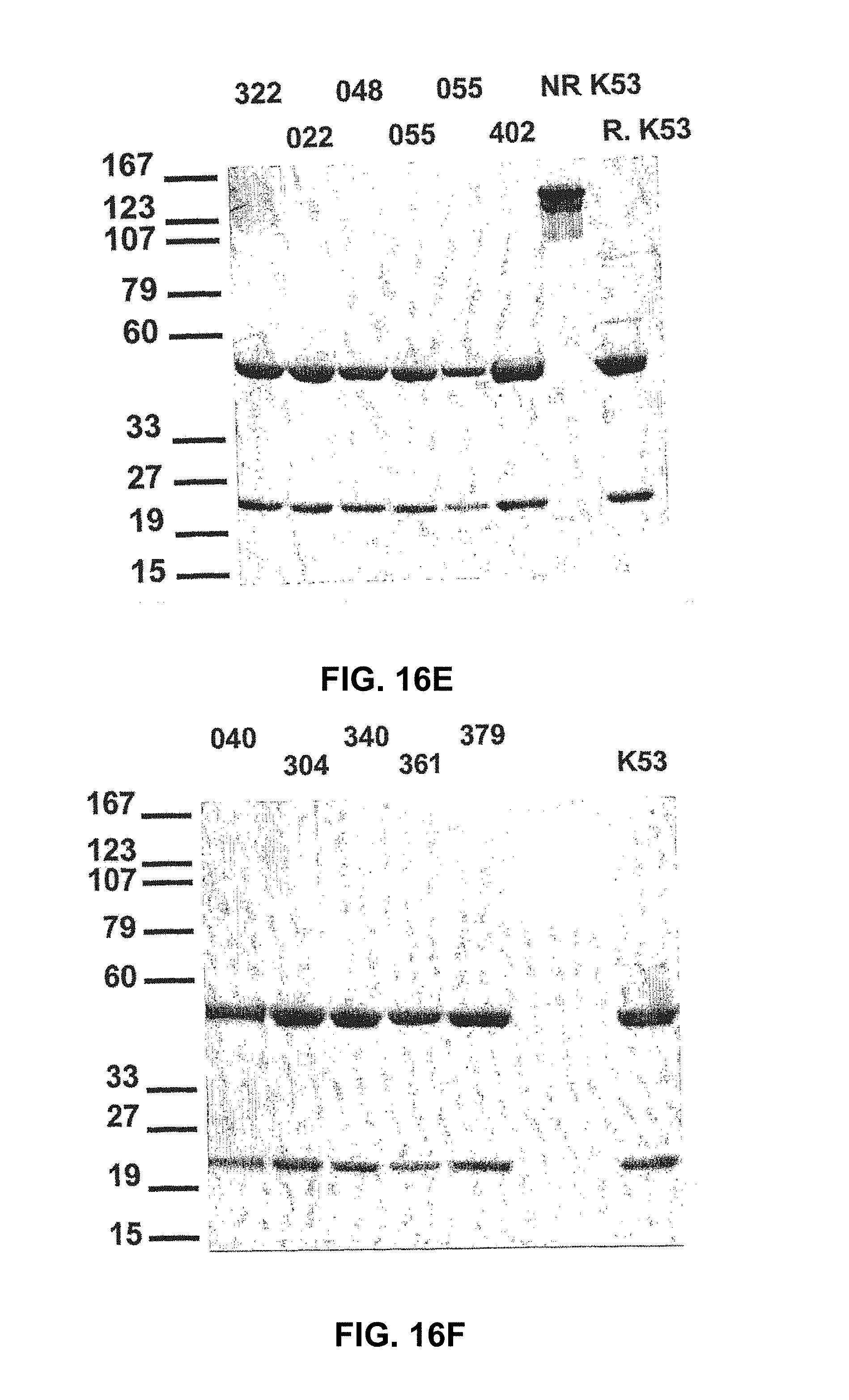
D00025
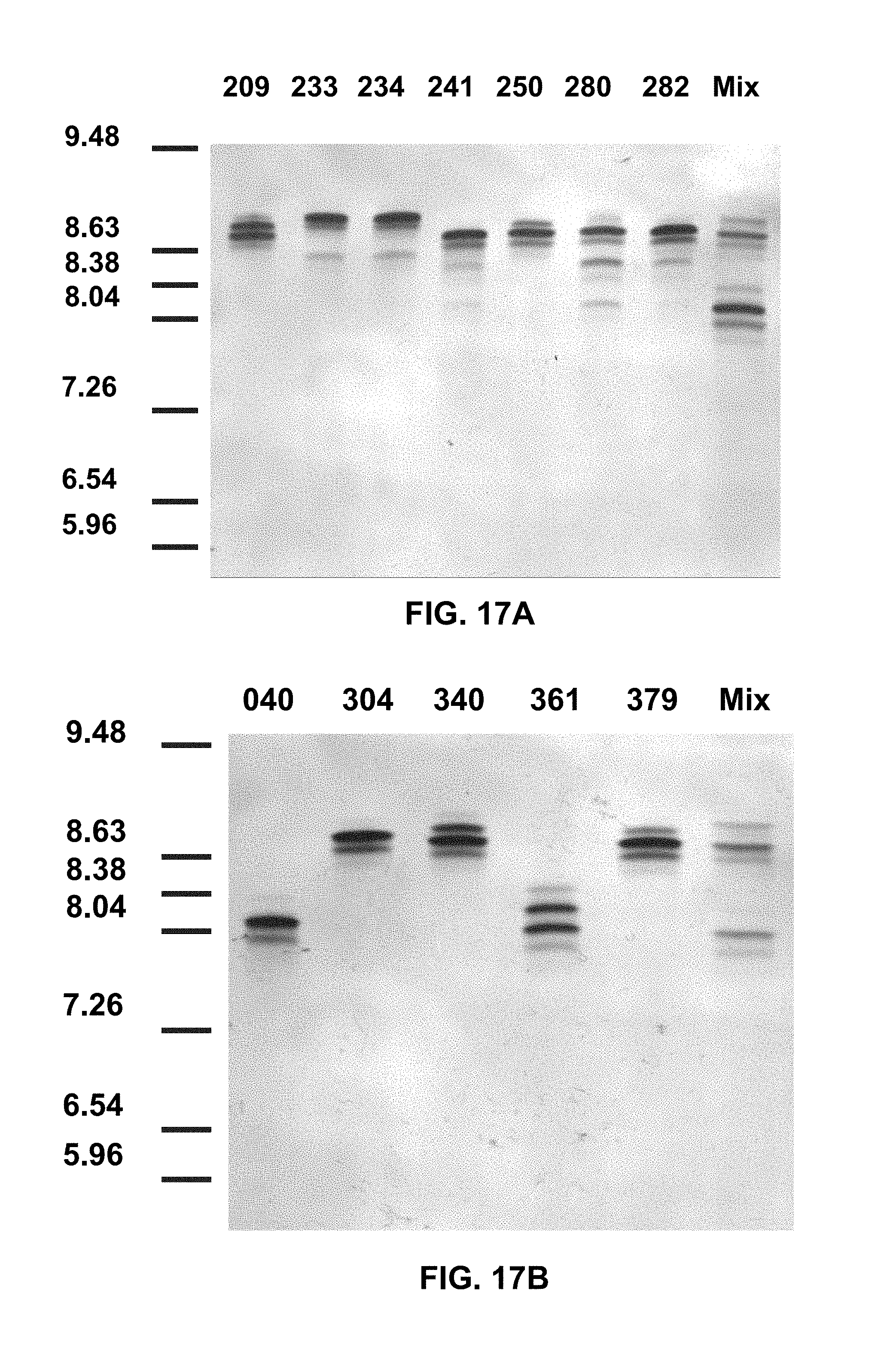
D00026

D00027
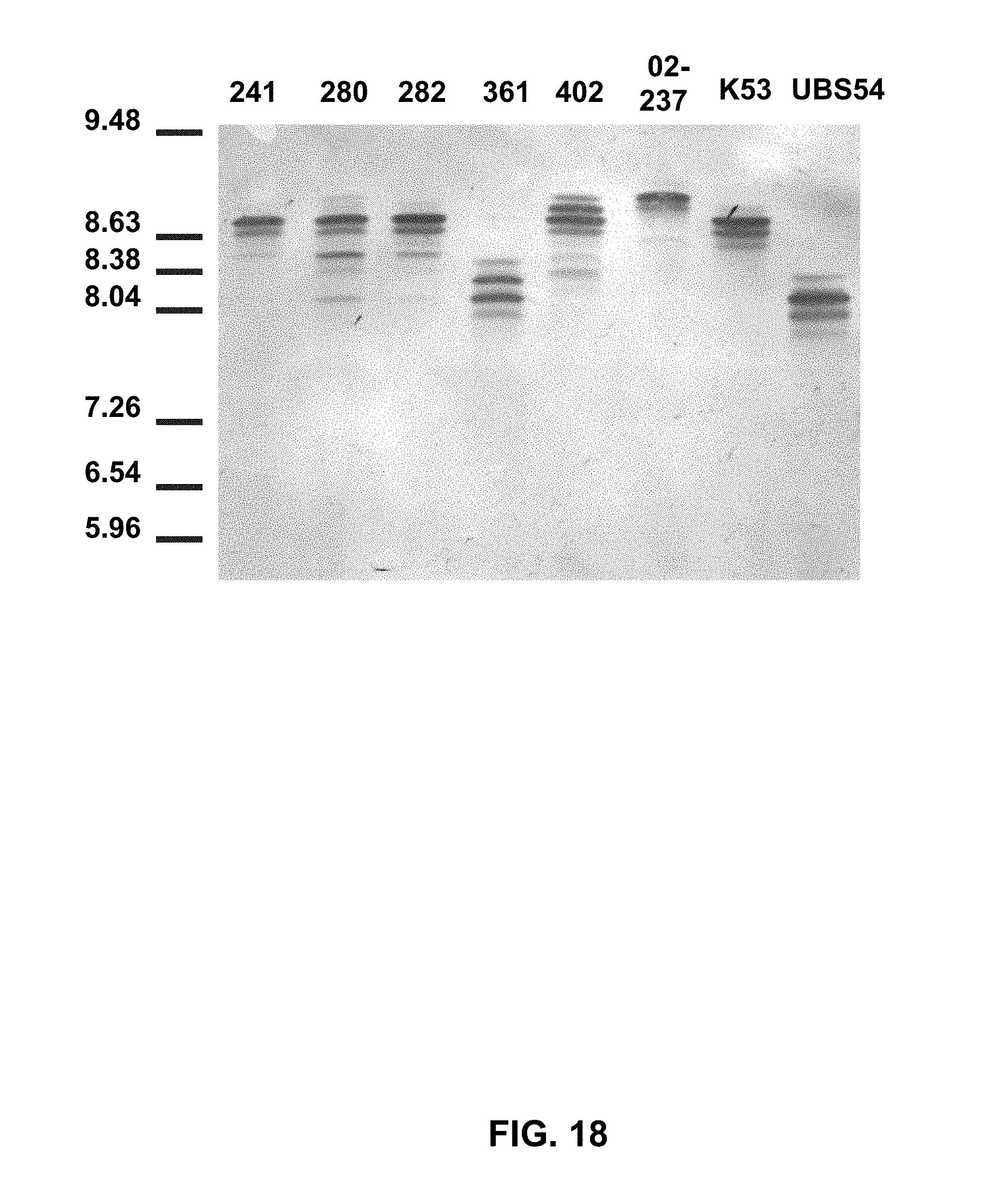
D00028

D00029
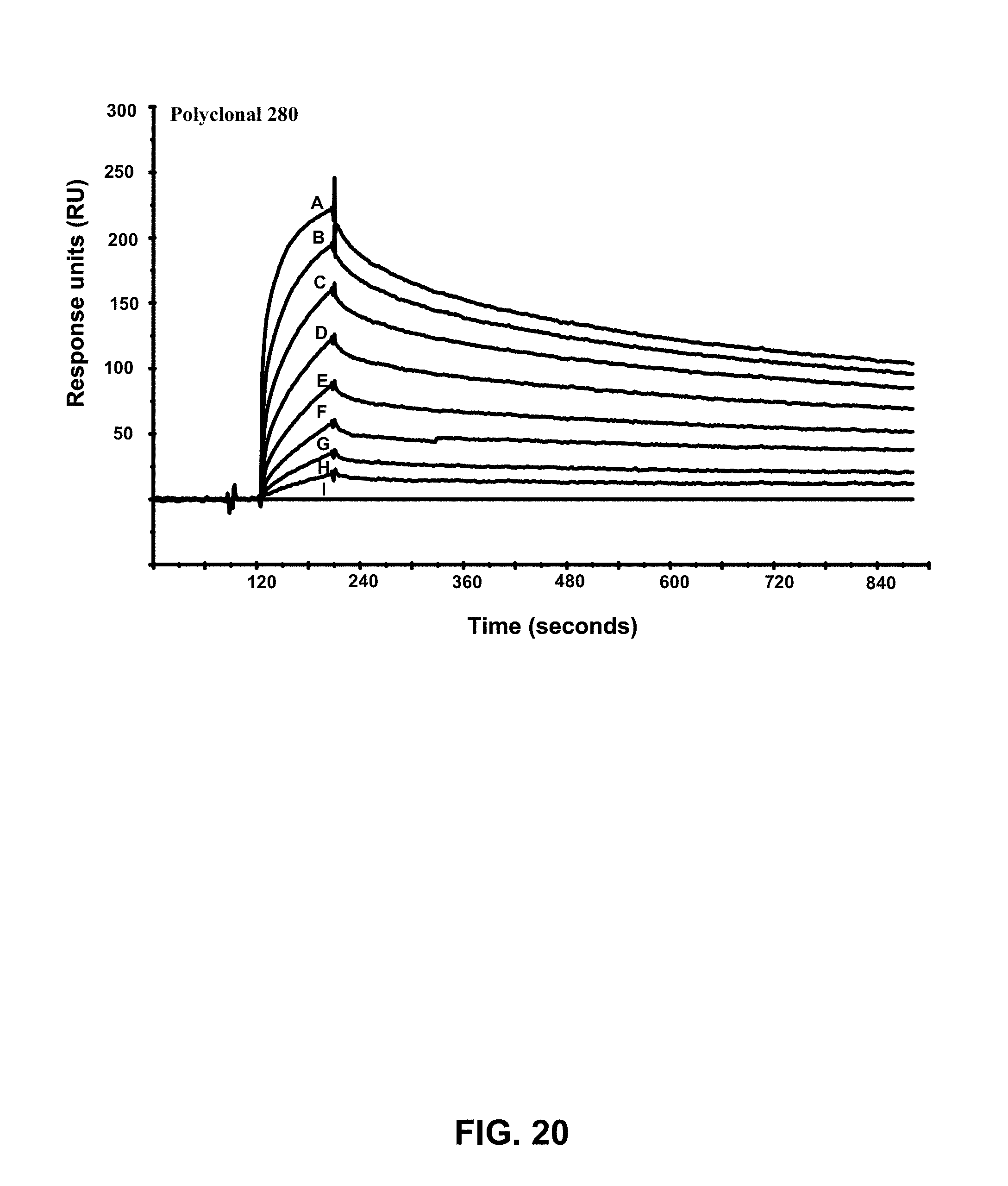
D00030
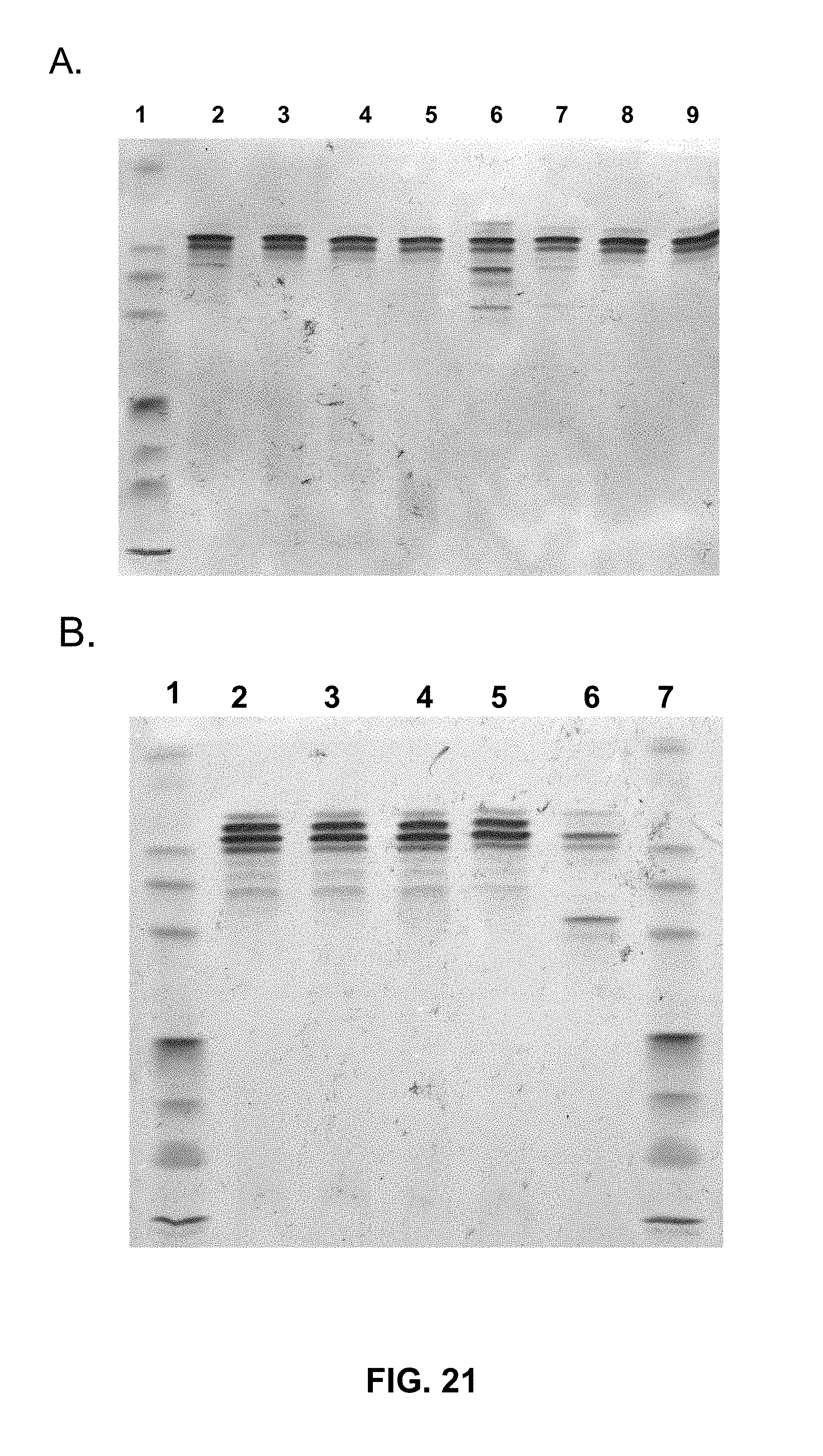
D00031
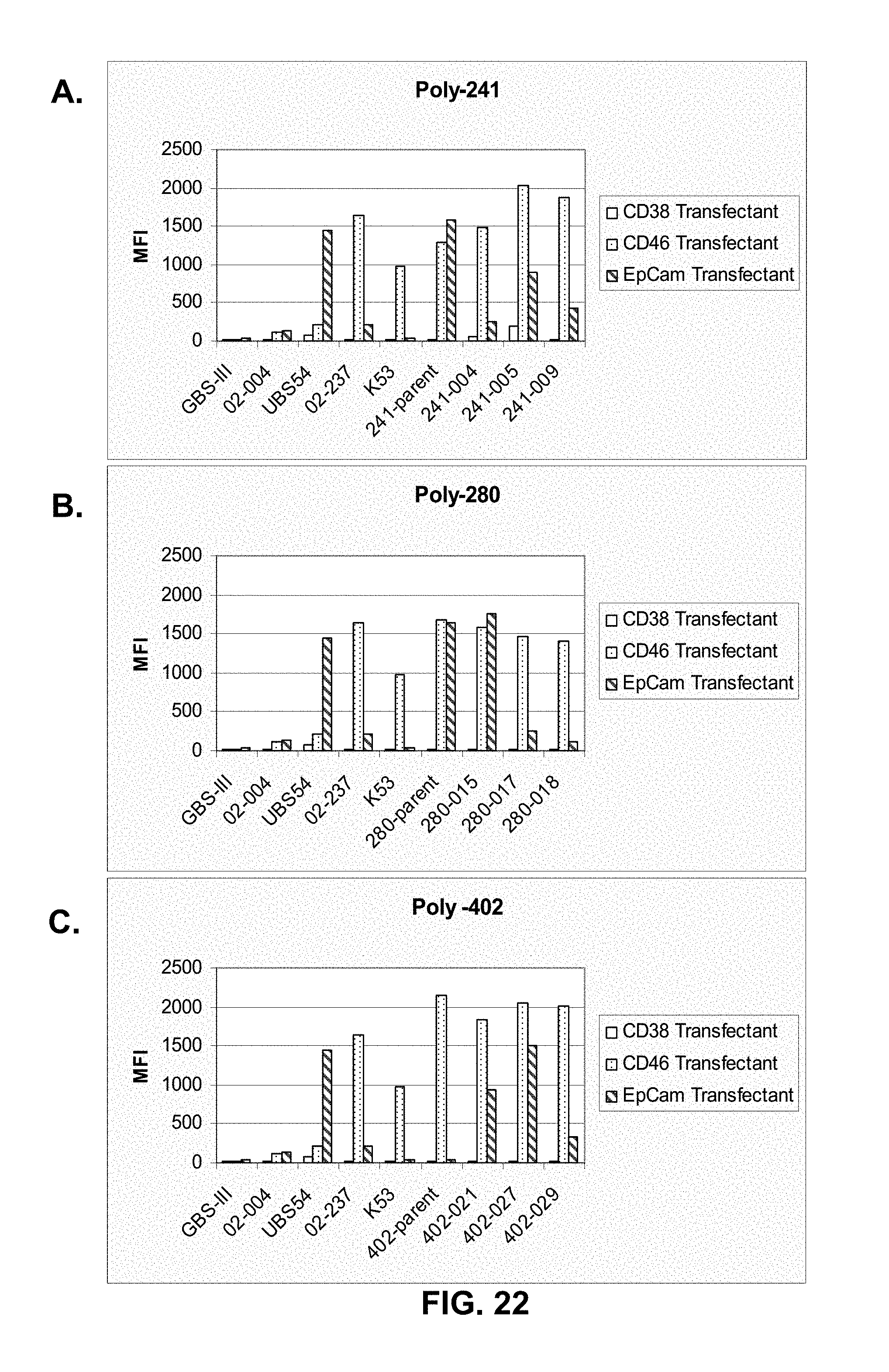
P00001
P00002
P00003

P00004

P00005
P00006
P00007
P00008
P00009

P00010
P00011
P00012
P00013

P00014
P00015

P00016
P00017
P00018

P00019

P00020
P00021
P00022
P00023
P00024
P00025
P00026

P00027
P00028

P00029

P00030

P00031

P00032
P00033

P00034
P00035

P00036

P00037

P00038
P00039

P00040

P00041
P00042

P00043

P00044

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.