Implant placement guide
Smith , et al. Fe
U.S. patent number D840,030 [Application Number D/566,823] was granted by the patent office on 2019-02-05 for implant placement guide. This patent grant is currently assigned to Intarcia Therapeutics, Inc.. The grantee listed for this patent is Intarcia Therapeutics, Inc.. Invention is credited to Michael R. Cole, Jay S. Smith, Amy K. Whitson.
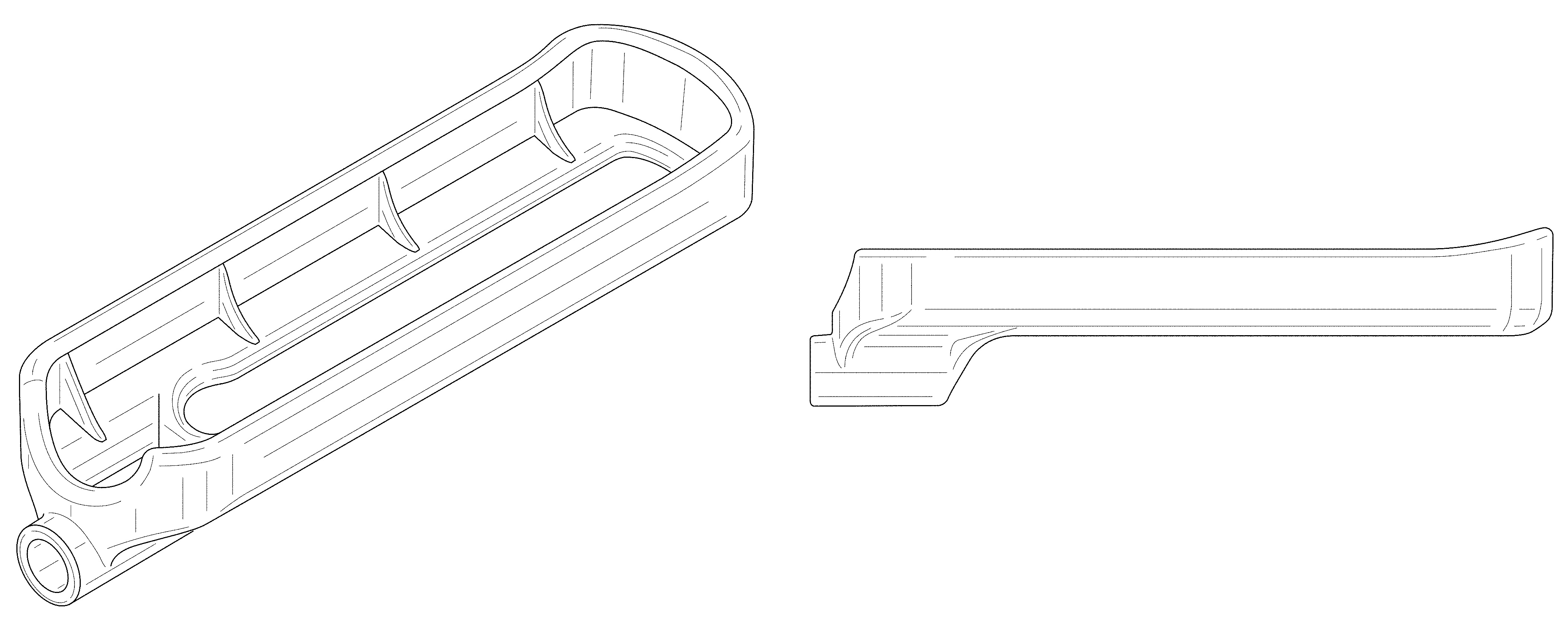

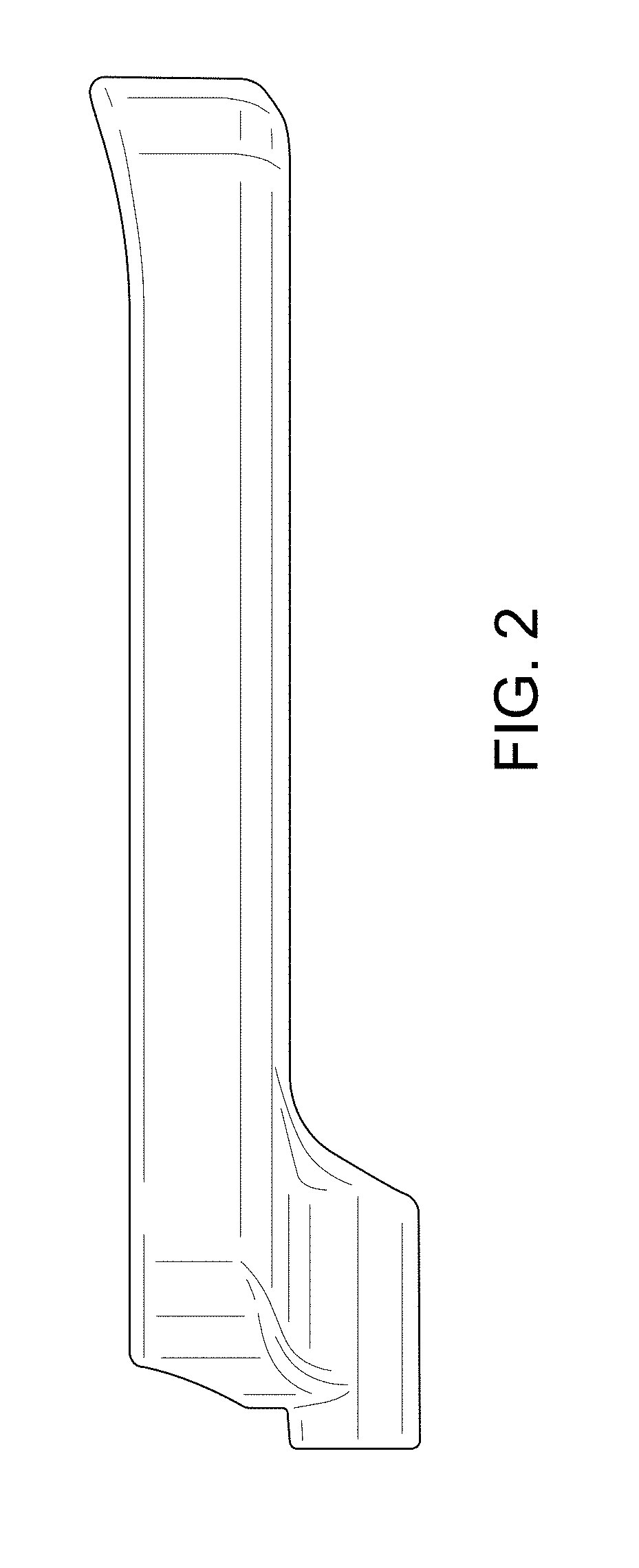
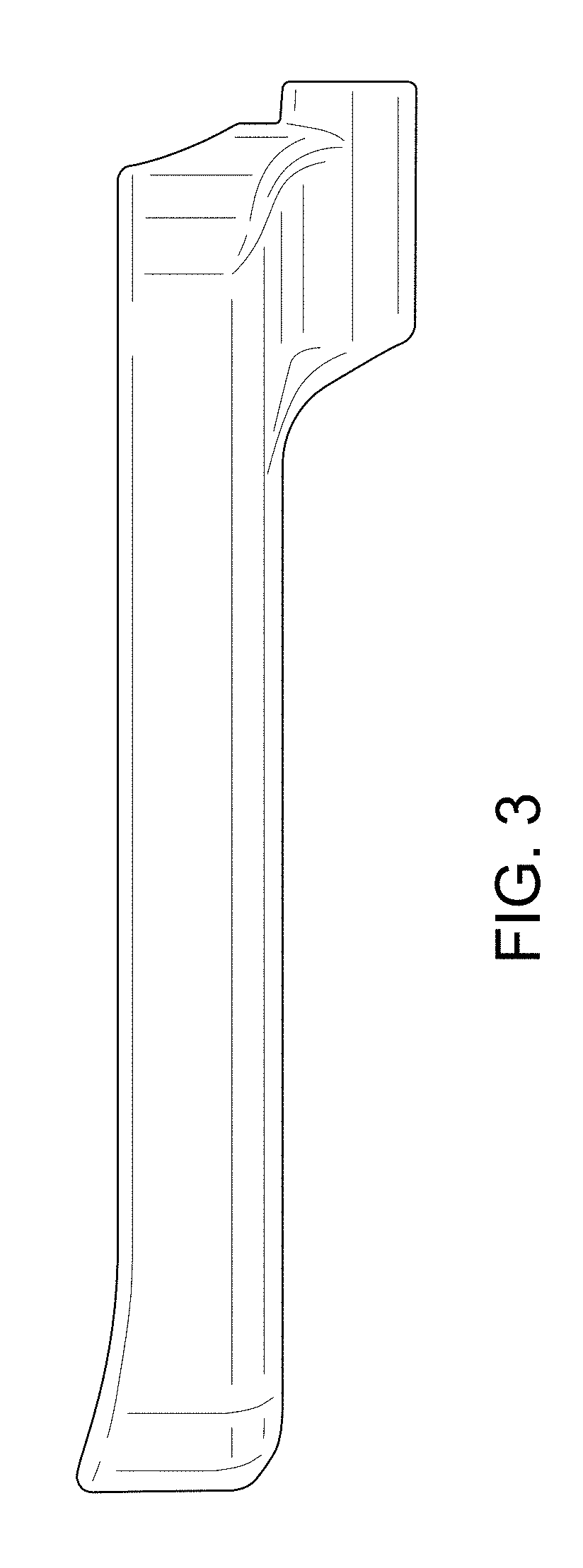
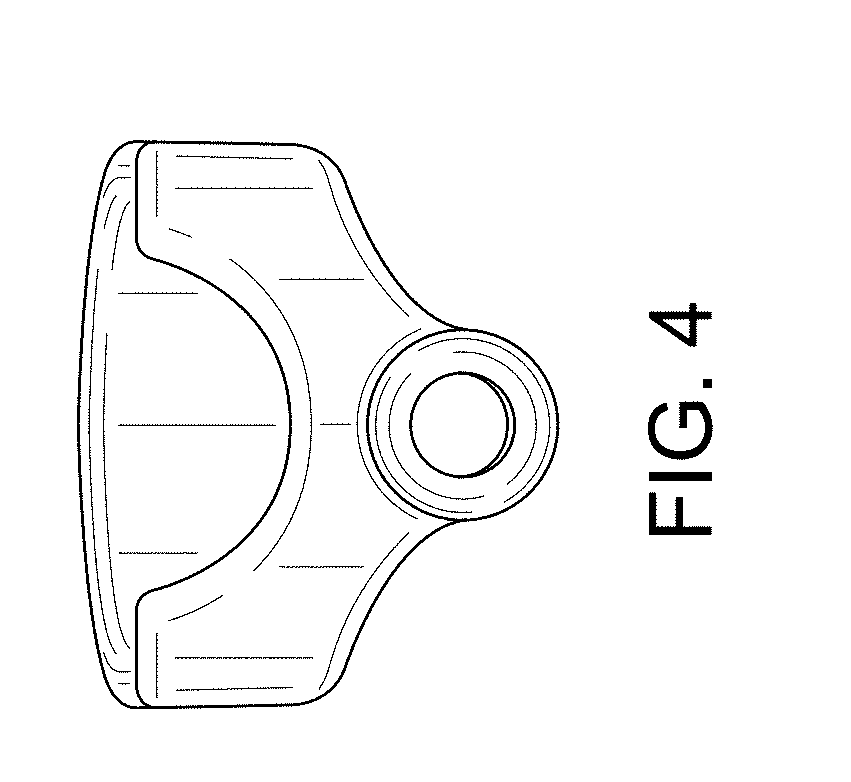


| United States Patent | D840,030 |
| Smith , et al. | February 5, 2019 |
Implant placement guide
Claims
CLAIM The ornamental design for an implant placement guide, as shown and described.
| Inventors: | Smith; Jay S. (Wellesley Hills, MA), Cole; Michael R. (Stratham, NH), Whitson; Amy K. (Columbus, OH) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | Intarcia Therapeutics, Inc.
(Boston, MA) |
||||||||||
| Appl. No.: | D/566,823 | ||||||||||
| Filed: | June 2, 2016 |
| Current U.S. Class: | D24/140; D24/133 |
| Current International Class: | 2402 |
| Field of Search: | ;D24/140,231,133,146,147,155,169,141 |
References Cited [Referenced By]
U.S. Patent Documents
| 1655158 | January 1928 | Muir |
| 2110208 | March 1938 | Eggert |
| 2168437 | August 1939 | Buercklin |
| 2531724 | November 1950 | Cevasco |
| 2643653 | June 1953 | Heidrich |
| D179537 | January 1957 | Floyd |
| 3016895 | January 1962 | Sein |
| 3025991 | March 1962 | Gillon |
| 3122162 | February 1964 | Sands |
| 35232906 | August 1970 | Vrancken et al. |
| 3538916 | November 1970 | Groff et al. |
| 3620216 | November 1971 | Szymanski |
| 3625214 | December 1971 | Higuchi |
| 3632768 | January 1972 | Bergy et al. |
| 3669104 | June 1972 | Wyatt et al. |
| 3691090 | September 1972 | Kitajima et al. |
| D226915 | May 1973 | Huggins |
| 3732865 | May 1973 | Higuchi et al. |
| 3737337 | June 1973 | Sonnoring et al. |
| 3766915 | October 1973 | Rychlik |
| 3773919 | November 1973 | Boswell et al. |
| 3797492 | March 1974 | Place |
| 3869549 | March 1975 | Geller |
| 3891570 | June 1975 | Fukushima et al. |
| D236035 | July 1975 | Ciencewicki |
| 3960757 | June 1976 | Morishita et al. |
| 3987790 | October 1976 | Eckenhoff et al. |
| 3995631 | December 1976 | Higuchi et al. |
| 3995632 | December 1976 | Nakano et al. |
| 4008719 | February 1977 | Theeuwes et al. |
| 4034756 | July 1977 | Higuchi et al. |
| 4078060 | March 1978 | Benson et al. |
| 4105030 | August 1978 | Kercso |
| 4111201 | September 1978 | Theeuwes |
| 4111202 | September 1978 | Theeuwes |
| 4111203 | September 1978 | Theeuwes |
| 4203439 | May 1980 | Theeuwes |
| 4211771 | July 1980 | Witkowski et al. |
| 4221862 | September 1980 | Naito et al. |
| 4223674 | September 1980 | Fluent et al. |
| 4243030 | January 1981 | Lynch et al. |
| D258837 | April 1981 | Spranger |
| D259458 | June 1981 | Fuller |
| 4305927 | December 1981 | Theeuwes et al. |
| 4310516 | January 1982 | Chang et al. |
| 4340054 | July 1982 | Michaels |
| 4350271 | September 1982 | Eckenhoff |
| 4373527 | February 1983 | Fischell |
| 4376118 | March 1983 | Daher |
| 4384975 | May 1983 | Fong |
| 4389330 | June 1983 | Tice et al. |
| D271990 | December 1983 | Lyne |
| 4439196 | March 1984 | Higuchi |
| 4444498 | April 1984 | Heinemann |
| 4451253 | May 1984 | Harman |
| 4455143 | June 1984 | Theeuwes et al. |
| 4455145 | June 1984 | Theeuwes |
| 4474572 | October 1984 | McNaughton et al. |
| 4530840 | July 1985 | Tice et al. |
| 4552561 | November 1985 | Eckenhoff et al. |
| 4588614 | May 1986 | Lauchenauer |
| 4594108 | June 1986 | Greminger, Jr. et al. |
| 4597753 | July 1986 | Turley |
| 4609374 | September 1986 | Ayer |
| 4639244 | January 1987 | Rizk et al. |
| 4655462 | April 1987 | Balsells |
| 4673405 | June 1987 | Guittard et al. |
| 4675184 | June 1987 | Hasegawa et al. |
| 4695623 | September 1987 | Stabinsky |
| 4727138 | February 1988 | Goeddel et al. |
| 4734284 | March 1988 | Terada et al. |
| 4737437 | April 1988 | Gutseil, Jr. et al. |
| 4743449 | May 1988 | Yoshida et al. |
| 4753651 | June 1988 | Eckenhoff |
| 4762791 | August 1988 | Goeddel et al. |
| 4765989 | August 1988 | Wong et al. |
| 4783337 | November 1988 | Wong et al. |
| 4818517 | April 1989 | Kwee et al. |
| 4820267 | April 1989 | Harman |
| 4820638 | April 1989 | Swetly et al. |
| 4826144 | May 1989 | Balsells |
| 4830344 | May 1989 | Balsells |
| 4834708 | May 1989 | Pillari |
| 4840896 | June 1989 | Reddy et al. |
| 4845196 | July 1989 | Cowling |
| 4847079 | July 1989 | Kwan |
| 4851228 | July 1989 | Zentner et al. |
| 4865845 | September 1989 | Eckenhoff et al. |
| 4871094 | October 1989 | Gall et al. |
| 4873080 | October 1989 | Brickl et al. |
| 4874388 | October 1989 | Wong et al. |
| 4876781 | October 1989 | Balsells |
| 4882166 | November 1989 | Graham et al. |
| 4885166 | December 1989 | Meyer et al. |
| 4886668 | December 1989 | Haslam et al. |
| 4892778 | January 1990 | Theeuwes et al. |
| 4893795 | January 1990 | Balsells |
| 4897471 | January 1990 | Stabinsky |
| 4907788 | March 1990 | Balsells |
| 4915366 | April 1990 | Balsells |
| 4915949 | April 1990 | Wong et al. |
| 4915954 | April 1990 | Ayer et al. |
| 4917887 | April 1990 | Hauptmann et al. |
| 4917895 | April 1990 | Lee et al. |
| 4923805 | May 1990 | Reddy et al. |
| 4927687 | May 1990 | Nuwayser |
| 4929554 | May 1990 | Goeddel et al. |
| 4931285 | June 1990 | Edgren et al. |
| 4934666 | June 1990 | Balsells |
| 4940465 | July 1990 | Theeuwes et al. |
| 4940588 | July 1990 | Sparks et al. |
| 4952402 | August 1990 | Sparks et al. |
| 4957119 | September 1990 | de Nijs |
| 4961253 | October 1990 | Balsells |
| 4964204 | October 1990 | Balsells |
| 4969884 | November 1990 | Yum |
| 4974821 | December 1990 | Balsells |
| 4976966 | December 1990 | Theeuwes et al. |
| 4994028 | February 1991 | Leonard et al. |
| 5004689 | April 1991 | Fiers et al. |
| 5006346 | April 1991 | Theeuwes et al. |
| 5019382 | May 1991 | Cummins, Jr. |
| 5019400 | May 1991 | Gombotz et al. |
| 5023088 | June 1991 | Wong et al. |
| 5024842 | June 1991 | Edgren et al. |
| 5030216 | July 1991 | Theeuwes et al. |
| 5034229 | July 1991 | Magruder et al. |
| 5053014 | October 1991 | Van Heugten |
| 5057318 | October 1991 | Magruder et al. |
| 5059423 | October 1991 | Magruder et al. |
| 5066436 | November 1991 | Komen et al. |
| 5071642 | December 1991 | Lahr et al. |
| 5072070 | December 1991 | Balsells |
| 5079388 | January 1992 | Balsells |
| 5090962 | February 1992 | Landry et al. |
| 5091188 | February 1992 | Haynes |
| 5108078 | April 1992 | Balsells |
| 5110596 | May 1992 | Magruder et al. |
| 5112614 | May 1992 | Magruder et al. |
| 5113938 | May 1992 | Clayton |
| 5117066 | May 1992 | Balsells |
| D326718 | June 1992 | Maxwell |
| 5118666 | June 1992 | Habener |
| 5120306 | June 1992 | Gosselin |
| 5120712 | June 1992 | Habener |
| 5120832 | June 1992 | Goeddel et al. |
| 5122128 | June 1992 | Cardinal et al. |
| 5122377 | June 1992 | Miller |
| 5126142 | June 1992 | Ayer et al. |
| 5126147 | June 1992 | Silvestri et al. |
| 5134244 | July 1992 | Balsells |
| 5137727 | August 1992 | Eckenhoff |
| D329278 | September 1992 | Gallup |
| 5147295 | September 1992 | Stewart |
| 5151093 | September 1992 | Theeuwes et al. |
| 5160122 | November 1992 | Balsells |
| 5160743 | November 1992 | Edgren et al. |
| 5161806 | November 1992 | Balsells |
| 5180591 | January 1993 | Margruder et al. |
| 5190765 | March 1993 | Jao et al. |
| 5192273 | March 1993 | Bierman |
| 5203849 | April 1993 | Balsells |
| 5204108 | April 1993 | Illum |
| 5207752 | May 1993 | Sorenson et al. |
| 5209746 | May 1993 | Balaban et al. |
| 5213809 | May 1993 | Wright et al. |
| 5213810 | May 1993 | Steber |
| 5219572 | June 1993 | Sivaramakrishnan |
| 5221278 | June 1993 | Linkwitz et al. |
| 5223265 | June 1993 | Wong |
| 5225205 | July 1993 | Orsolini |
| 5231176 | July 1993 | Goeddel et al. |
| 5234424 | August 1993 | Yum et al. |
| 5234692 | August 1993 | Magruder et al. |
| 5234693 | August 1993 | Magruder et al. |
| 5234695 | August 1993 | Hobbs et al. |
| 5250026 | October 1993 | Ehrlich et al. |
| 5252338 | October 1993 | Jao et al. |
| 5260069 | November 1993 | Chen |
| D342855 | January 1994 | Butler, II |
| 5276926 | January 1994 | Lopez |
| 5278151 | January 1994 | Korb et al. |
| 5279544 | January 1994 | Gross et al. |
| 5279554 | January 1994 | Turley |
| 5279555 | January 1994 | Lifshey |
| 5279608 | January 1994 | Cherif Cheikh |
| 5284655 | February 1994 | Bogdansky et al. |
| 5288501 | February 1994 | Nurnberg et al. |
| 5288502 | February 1994 | Mcginity et al. |
| 5290271 | March 1994 | Jemberg |
| 5300079 | April 1994 | Niezink et al. |
| 5300302 | April 1994 | Tachon et al. |
| 5308348 | May 1994 | Balaban et al. |
| 5312335 | May 1994 | McKinnon et al. |
| 5312389 | May 1994 | Theeuwes et al. |
| 5312390 | May 1994 | Wong |
| 5318558 | June 1994 | Linkwitz et al. |
| 5318780 | June 1994 | Viegas et al. |
| 5320616 | June 1994 | Magndu et al. |
| 5324280 | June 1994 | Wong et al. |
| 5336057 | August 1994 | Fukuda et al. |
| 5336505 | August 1994 | Ng et al. |
| 5348544 | September 1994 | Sweeney et al. |
| 5352662 | October 1994 | Brooks et al. |
| 5368588 | November 1994 | Bettinger |
| 5368863 | November 1994 | Eckenhoff et al. |
| 5371089 | December 1994 | Rattan |
| 5374620 | December 1994 | Clark et al. |
| 5385738 | January 1995 | Yamahira et al. |
| 5385887 | January 1995 | Yim et al. |
| 5395319 | March 1995 | Hirsch et al. |
| 5407609 | April 1995 | Tice et al. |
| D358644 | May 1995 | Park |
| 5411951 | May 1995 | Mitchell |
| 5413572 | May 1995 | Wong |
| 5413672 | May 1995 | Hirotsuji et al. |
| 5424286 | June 1995 | Eng |
| 5428024 | June 1995 | Chu et al. |
| 5429602 | July 1995 | Hauser |
| 5439688 | August 1995 | Orsolini et al. |
| 5443459 | August 1995 | Wong et al. |
| 5445829 | August 1995 | Paradissis et al. |
| 5456679 | October 1995 | Balaban et al. |
| 5458888 | October 1995 | Chen |
| 5464929 | November 1995 | Bezwada et al. |
| 5472708 | December 1995 | Chen |
| 5473564 | December 1995 | Wantier et al. |
| 5484403 | January 1996 | Yoakum et al. |
| 5486365 | January 1996 | Takado et al. |
| 5498255 | March 1996 | Wong et al. |
| 5511355 | April 1996 | Dingler |
| 5512293 | April 1996 | Landrau et al. |
| 5512549 | April 1996 | Chen et al. |
| 5514110 | May 1996 | Teh |
| 5529914 | June 1996 | Hubbell et al. |
| 5531736 | July 1996 | Wong et al. |
| 5540665 | July 1996 | Mercado et al. |
| 5540912 | July 1996 | Roorda et al. |
| 5541172 | July 1996 | Labrie et al. |
| 5542682 | August 1996 | Goldstein et al. |
| 5543156 | August 1996 | Roorda et al. |
| 5545618 | August 1996 | Buckley et al. |
| 5556642 | September 1996 | Kobayashi et al. |
| 5557318 | September 1996 | Gabriel |
| 5558637 | September 1996 | Allonen et al. |
| 5569289 | October 1996 | Yoon |
| 5571525 | November 1996 | Roorda et al. |
| 5574008 | November 1996 | Johnson et al. |
| 5574137 | November 1996 | Gray et al. |
| 5580578 | December 1996 | Oshlack et al. |
| 5589167 | December 1996 | Cleland et al. |
| 5595751 | January 1997 | Bezwada |
| 5595759 | January 1997 | Wright et al. |
| 5597579 | January 1997 | Bezwada et al. |
| 5602010 | February 1997 | Hauptmann et al. |
| 5605688 | February 1997 | Himmler et al. |
| 5607687 | March 1997 | Bezwada et al. |
| 5609885 | March 1997 | Rivera et al. |
| 5613954 | March 1997 | Nelson et al. |
| 5614221 | March 1997 | Fjellstrom |
| 5614492 | March 1997 | Habener |
| 5618552 | April 1997 | Bezwada et al. |
| 5620698 | April 1997 | Bezwada et al. |
| 5620705 | April 1997 | Dong et al. |
| 5630796 | May 1997 | Bellhouse et al. |
| 5633011 | May 1997 | Dong et al. |
| 5635213 | June 1997 | Nystrom et al. |
| 5639477 | June 1997 | Maruyama et al. |
| 5639640 | June 1997 | Reddy et al. |
| 5645850 | July 1997 | Bezwada et al. |
| 5648088 | July 1997 | Bezwada et al. |
| 5650173 | July 1997 | Ramstack et al. |
| 5654008 | August 1997 | Herbert et al. |
| 5654010 | August 1997 | Johnson et al. |
| 5656297 | August 1997 | Bernstein et al. |
| 5656299 | August 1997 | Kino et al. |
| 5658593 | August 1997 | Orly et al. |
| 5660847 | August 1997 | Magruder et al. |
| 5660858 | August 1997 | Parikh et al. |
| 5660861 | August 1997 | Jao et al. |
| 5667803 | September 1997 | Johnson et al. |
| 5668170 | September 1997 | Gyory |
| 5672549 | September 1997 | Minami et al. |
| 5676942 | October 1997 | Testa et al. |
| 5686097 | November 1997 | Taskovich et al. |
| 5688801 | November 1997 | Mesens et al. |
| 5690925 | November 1997 | Gray et al. |
| 5690952 | November 1997 | Magruder et al. |
| 5695463 | December 1997 | Cherif-Cheikh |
| 5697113 | December 1997 | Shatz |
| 5697914 | December 1997 | Brimhall |
| 5698213 | December 1997 | Jamiolkowski et al. |
| 5700486 | December 1997 | Canal et al. |
| 5700583 | December 1997 | Jamiolkowski et al. |
| 5703200 | December 1997 | Bezwada et al. |
| 5707644 | January 1998 | Illum |
| 5711967 | January 1998 | Juch |
| 5713847 | February 1998 | Howard, III et al. |
| 5718922 | February 1998 | Herrero-Vanrell |
| 5725497 | March 1998 | Woodruff et al. |
| 5728088 | March 1998 | Margruder et al. |
| 5728396 | March 1998 | Peery et al. |
| 5733572 | March 1998 | Unger et al. |
| 5736159 | April 1998 | Chen et al. |
| 5738845 | April 1998 | Imakawa |
| 5747058 | May 1998 | Tipton et al. |
| 5756450 | May 1998 | Lorenz et al. |
| 5767251 | June 1998 | Reddy et al. |
| 5770231 | June 1998 | Mesens et al. |
| 5782396 | July 1998 | Mastri et al. |
| 5792477 | August 1998 | Rickey et al. |
| 5795591 | August 1998 | Lee et al. |
| 5795779 | August 1998 | McCormick et al. |
| 5807876 | September 1998 | Armistead et al. |
| 5810769 | September 1998 | Schlegel et al. |
| 5814323 | September 1998 | Lyle |
| D399821 | October 1998 | Tyneski |
| 5817129 | October 1998 | Labrecque et al. |
| 5827297 | October 1998 | Boudjema |
| 5830501 | November 1998 | Dong et al. |
| 5836935 | November 1998 | Ashton et al. |
| 5843891 | December 1998 | Sherman |
| 5844017 | December 1998 | Jamiolkowslci et al. |
| 5851451 | December 1998 | Takechi et al. |
| 5858746 | January 1999 | Hubbell et al. |
| 5859150 | January 1999 | Jamiolkowski et al. |
| 5861166 | January 1999 | Eckenhoff |
| 5871770 | February 1999 | Margruder et al. |
| 5871778 | February 1999 | Kino et al. |
| 5874388 | February 1999 | Hsu |
| 5876746 | March 1999 | Jona et al. |
| 5882676 | March 1999 | Lee et al. |
| D408917 | April 1999 | Hacker |
| 5904935 | May 1999 | Eckenhoff et al. |
| 5906599 | May 1999 | Kaldany |
| 5906816 | May 1999 | Soos et al. |
| 5906830 | May 1999 | Farinas et al. |
| 5908621 | June 1999 | Glue et al. |
| 5916598 | June 1999 | Rickey et al. |
| 5922253 | July 1999 | Herbert et al. |
| 5928666 | July 1999 | Farinas et al. |
| 5932547 | August 1999 | Stevenson et al. |
| 5938654 | August 1999 | Wong et al. |
| 5939286 | August 1999 | Johnson et al. |
| 5942223 | August 1999 | Bazer et al. |
| 5942253 | August 1999 | Gombotz et al. |
| 5945126 | August 1999 | Thanoo et al. |
| 5948430 | September 1999 | Zerbe et al. |
| 5951521 | September 1999 | Mastrototaro et al. |
| 5958909 | September 1999 | Habener |
| D415073 | October 1999 | Meehan |
| 5962023 | October 1999 | Jamiolkowski et al. |
| 5965168 | October 1999 | Mesens et al. |
| 5972370 | October 1999 | Eckenhoff et al. |
| 5972373 | October 1999 | Yajima et al. |
| 5976109 | November 1999 | Heruth |
| 5980945 | November 1999 | Ruiz |
| 5981719 | November 1999 | Woiszwillo et al. |
| 5984890 | November 1999 | Gast et al. |
| 5985305 | November 1999 | Peery et al. |
| 5989463 | November 1999 | Tracy et al. |
| 5997527 | December 1999 | Gumucio et al. |
| 5997902 | December 1999 | Maruyama et al. |
| 6007805 | December 1999 | Foster et al. |
| 6017545 | January 2000 | Modi |
| 6022561 | February 2000 | Carlsson et al. |
| 6023802 | February 2000 | King |
| 6029361 | February 2000 | Newman |
| 6053927 | April 2000 | Hamas |
| 6056718 | May 2000 | Funderburk et al. |
| 6060450 | May 2000 | Soos et al. |
| 6069133 | May 2000 | Carlo et al. |
| 6074377 | June 2000 | Sanfilippo, II |
| 6074660 | June 2000 | Jamiolkowski et al. |
| 6074673 | June 2000 | Guillen |
| 6100346 | August 2000 | Jamiolkowski et al. |
| 6110503 | August 2000 | Rickey et al. |
| D430671 | September 2000 | Shute |
| 6113938 | September 2000 | Chen et al. |
| 6113947 | September 2000 | Cleland et al. |
| 6120787 | September 2000 | Gustafsson et al. |
| 6124261 | September 2000 | Stevenson et al. |
| 6124281 | September 2000 | Lewis et al. |
| 6127520 | October 2000 | Ueda et al. |
| 6129761 | October 2000 | Hubbell |
| 6130200 | October 2000 | Brodbeck et al. |
| 6132420 | October 2000 | Dionne et al. |
| 6133249 | October 2000 | Hills |
| 6133429 | October 2000 | Davis et al. |
| 6146139 | November 2000 | Harrison, III |
| 6147168 | November 2000 | Jamiolkowski et al. |
| 6156331 | December 2000 | Peery et al. |
| 6172046 | January 2001 | Albrecht |
| 6174547 | January 2001 | Dong et al. |
| 6177096 | January 2001 | Zerbe et al. |
| 6183461 | February 2001 | Matsuura et al. |
| 6187095 | February 2001 | Labrecque et al. |
| 6190350 | February 2001 | Davis et al. |
| 6190700 | February 2001 | Okada et al. |
| 6190702 | February 2001 | Takada et al. |
| 6191102 | February 2001 | DiMarchi et al. |
| 6204022 | March 2001 | Johnson et al. |
| 6217393 | April 2001 | Pellet et al. |
| 6217906 | April 2001 | Gumucio et al. |
| 6217908 | April 2001 | Mathiowitz et al. |
| 6218431 | April 2001 | Schoen et al. |
| 6224894 | May 2001 | Jamiolkowski et al. |
| 6235712 | May 2001 | Stevenson et al. |
| 6245349 | June 2001 | Yiv et al. |
| 6245357 | June 2001 | Edgren et al. |
| 6248112 | June 2001 | Gambale et al. |
| 6251435 | June 2001 | Jamiolkowski et al. |
| D445975 | July 2001 | Stratford |
| 6258377 | July 2001 | New et al. |
| 6261584 | July 2001 | Peery et al. |
| 6268343 | July 2001 | Knudsen et al. |
| 6270700 | August 2001 | Ignatious |
| 6270787 | August 2001 | Ayer |
| 6277413 | August 2001 | Sankaram |
| 6283949 | September 2001 | Roorda |
| 6284264 | September 2001 | Zerbe et al. |
| 6284727 | September 2001 | Kim et al. |
| 6287295 | September 2001 | Chen et al. |
| 6284725 | December 2001 | Coolidge et al. |
| 6329336 | December 2001 | Bridon et al. |
| 6331311 | December 2001 | Brodbeck et al. |
| 6372218 | April 2002 | Cummins, Jr. |
| 6372256 | April 2002 | Jamiolkowski et al. |
| 6375978 | April 2002 | Kleiner et al. |
| D456776 | May 2002 | Lindekugel |
| 6395282 | May 2002 | Kende et al. |
| 6395292 | May 2002 | Peery et al. |
| 6402716 | June 2002 | Ryoo et al. |
| 6403655 | June 2002 | Bezwada et al. |
| 6419952 | July 2002 | Wong et al. |
| 6428517 | August 2002 | Hochman et al. |
| 6433144 | August 2002 | Morris et al. |
| 6436091 | August 2002 | Harper et al. |
| 6447522 | September 2002 | Gambale et al. |
| 6451974 | September 2002 | Hansen |
| 6458385 | October 2002 | Jamiolkowski et al. |
| 6458387 | October 2002 | Scott et al. |
| 6458924 | October 2002 | Knudsen et al. |
| 6461605 | October 2002 | Cutler et al. |
| 6464688 | October 2002 | Harper et al. |
| 6468961 | October 2002 | Brodbeck et al. |
| 6471688 | October 2002 | Harper et al. |
| 6472512 | October 2002 | LaFleur et al. |
| 6478768 | November 2002 | Kneer |
| 6485706 | November 2002 | McCoy et al. |
| 6495164 | December 2002 | Ramstack et al. |
| 6506724 | January 2003 | Hiles et al. |
| 6508808 | January 2003 | Carr et al. |
| 6514500 | February 2003 | Bridon et al. |
| 6514517 | February 2003 | Jamilolkowski et al. |
| 6524305 | February 2003 | Peterson et al. |
| 6528093 | March 2003 | Kamei et al. |
| 6528486 | March 2003 | Larsen et al. |
| D472896 | April 2003 | Peiker |
| 6541021 | April 2003 | Johnson et al. |
| 6544239 | April 2003 | Kinsey et al. |
| 6544252 | April 2003 | Theeuwes et al. |
| 6547250 | April 2003 | Noble |
| 6551613 | April 2003 | Dong et al. |
| 6569420 | May 2003 | Chen et al. |
| 6572890 | June 2003 | Faour et al. |
| 6579851 | June 2003 | Goeke et al. |
| 6589157 | July 2003 | Fontayne et al. |
| 6592508 | July 2003 | Ravins et al. |
| 6592887 | July 2003 | Zerbe et al. |
| 6593295 | July 2003 | Bridon et al. |
| 6607529 | August 2003 | Jones et al. |
| 6626863 | September 2003 | Berler |
| 6635268 | October 2003 | Peery et al. |
| 6645192 | November 2003 | Kenison et al. |
| 6667061 | December 2003 | Rarnstack et al. |
| 6670368 | December 2003 | Breault et al. |
| 6673767 | January 2004 | Brodbeck et al. |
| 6682522 | January 2004 | Carr et al. |
| 6703225 | March 2004 | Kojima et al. |
| 6703359 | March 2004 | Young et al. |
| 6706689 | March 2004 | Coolidge et al. |
| 6709671 | March 2004 | Zerbe et al. |
| 6720407 | April 2004 | Hughes et al. |
| 6730328 | May 2004 | Maskiwicz et al. |
| 6767887 | July 2004 | Hoffmann et al. |
| 6821949 | November 2004 | Bridon et al. |
| 6833256 | December 2004 | Pontzer et al. |
| 6835194 | December 2004 | Johnson et al. |
| 6840931 | January 2005 | Peterson et al. |
| 6849708 | February 2005 | Habener |
| 6849714 | February 2005 | Bridon et al. |
| 6858576 | February 2005 | Young et al. |
| 6872700 | March 2005 | Young et al. |
| 6875748 | April 2005 | Manthorpe et al. |
| 6887470 | May 2005 | Bridon et al. |
| 6887849 | May 2005 | Bridon et al. |
| 6899887 | May 2005 | Ayer |
| 6899898 | May 2005 | Albayrak |
| 6902744 | June 2005 | Kolterman et al. |
| 6903186 | June 2005 | Dong |
| 6913767 | July 2005 | Cleland et al. |
| 6923800 | August 2005 | Chen et al. |
| 6924264 | August 2005 | Prickett et al. |
| 6939556 | September 2005 | Lautenbach |
| 6956026 | October 2005 | Beeley et al. |
| 6960192 | November 2005 | Flaherty et al. |
| 6969702 | November 2005 | Bertilsson et al. |
| 6976981 | December 2005 | Ayer |
| 6989366 | January 2006 | Beeley et al. |
| 6992065 | January 2006 | Okumu |
| 6997922 | February 2006 | Theeuwes et al. |
| 7008439 | March 2006 | Janzen et al. |
| 7014636 | March 2006 | Gilbert |
| 7022674 | April 2006 | DeFelippis et al. |
| 7041646 | May 2006 | Pan et al. |
| 7074423 | July 2006 | Fereira et al. |
| 7084243 | August 2006 | Glaesner et al. |
| 7101567 | September 2006 | Sano et al. |
| 7101843 | September 2006 | Glaesner et al. |
| 7112335 | September 2006 | Lautenbach |
| 7115569 | October 2006 | Beeley et al. |
| 7138375 | November 2006 | Beeley et al. |
| 7138486 | November 2006 | Habener et al. |
| 7141547 | November 2006 | Rosen et al. |
| 7144863 | December 2006 | DeFelippis et al. |
| 7153825 | December 2006 | Young |
| 7157555 | January 2007 | Beeley et al. |
| 7163688 | January 2007 | Peery et al. |
| 7163697 | January 2007 | Hanes et al. |
| 7199217 | April 2007 | DiMarchi et al. |
| 7205409 | April 2007 | Pei et al. |
| 7207982 | April 2007 | Dionne et al. |
| 7214206 | May 2007 | Rue et al. |
| 7241457 | July 2007 | Chen et al. |
| 7258869 | August 2007 | Berry et al. |
| D555589 | November 2007 | Hussaini |
| 7297761 | November 2007 | Beeley et al. |
| 7316680 | January 2008 | Gilbert |
| 7393827 | July 2008 | Nadler |
| 7407499 | August 2008 | Trautman |
| 7442682 | October 2008 | Kitaura et al. |
| 7456254 | November 2008 | Wright et al. |
| 7459432 | December 2008 | Cowley et al. |
| 7521423 | April 2009 | Young et al. |
| 7563871 | July 2009 | Wright et al. |
| 7589169 | September 2009 | Bolotin |
| 7604647 | October 2009 | Chen |
| 7612176 | November 2009 | Wright et al. |
| 7632256 | December 2009 | Mosler et al. |
| 7635463 | December 2009 | Bolotin et al. |
| D608447 | January 2010 | Meyer |
| 7655254 | February 2010 | Dennis et al. |
| 7655257 | February 2010 | Peery et al. |
| 7666835 | February 2010 | Bloom et al. |
| 7682356 | March 2010 | Alessi et al. |
| 7727519 | June 2010 | Moran |
| 7731947 | June 2010 | Eliaz et al. |
| 7736665 | June 2010 | Patel et al. |
| 7741269 | June 2010 | Young et al. |
| 7766924 | August 2010 | Bombard et al. |
| 7790140 | September 2010 | Bolotin |
| 7825091 | November 2010 | Bloom et al. |
| 7829109 | November 2010 | Chen et al. |
| 7833543 | November 2010 | Gibson et al. |
| 7879028 | February 2011 | Alessi et al. |
| 7879794 | April 2011 | Berry et al. |
| 7919109 | April 2011 | Berry et al. |
| D638478 | May 2011 | Block |
| 7928065 | June 2011 | Rohloff et al. |
| 7964183 | June 2011 | Eliaz et al. |
| 8039432 | October 2011 | Bridon et al. |
| 7959938 | November 2011 | Lautenbach et al. |
| 8048438 | November 2011 | Berry et al. |
| 8052996 | November 2011 | Lautenbach et al. |
| 8058233 | November 2011 | Cowley et al. |
| 8101576 | January 2012 | Bloom |
| 8114430 | February 2012 | Rohloff et al. |
| 8114437 | February 2012 | Rohloff et al. |
| 8158150 | April 2012 | Lautenbach et al. |
| 8173150 | May 2012 | Berry et al. |
| 8202836 | June 2012 | Moore et al. |
| 8206745 | June 2012 | Rohloff et al. |
| 8211467 | July 2012 | Rohloff et al. |
| 8217001 | July 2012 | Cowley et al. |
| 8231859 | July 2012 | Bolotin et al. |
| 8251946 | August 2012 | Bardy |
| 8257682 | September 2012 | Bolotin et al. |
| 8257691 | September 2012 | Eliaz et al. |
| 8262667 | September 2012 | Silver et al. |
| 8263545 | September 2012 | Bloom |
| 8263736 | September 2012 | Berry |
| 8268341 | September 2012 | Berry |
| 8273365 | September 2012 | Lautenbach et al. |
| 8273713 | September 2012 | Pittner et al. |
| D669589 | October 2012 | Delaey |
| 8277776 | October 2012 | Boictin et al. |
| 8278267 | October 2012 | Weyer et al. |
| 8288338 | October 2012 | Alessi et al. |
| 8298561 | October 2012 | Alessi et al. |
| 8299025 | October 2012 | Alessi et al. |
| 8343140 | January 2013 | Alessi et al. |
| 8367095 | February 2013 | Lautenbach et al. |
| 8372424 | February 2013 | Berry et al. |
| D678889 | March 2013 | Chiu |
| 8398967 | March 2013 | Eliaz et al. |
| 8440226 | May 2013 | Rohloff et al. |
| 8454552 | June 2013 | Bardy |
| 8460694 | June 2013 | Rohloff et al. |
| 8470353 | June 2013 | Lautenbach et al. |
| 8556861 | October 2013 | Tsals |
| 8722037 | May 2014 | Veenstra et al. |
| 8747412 | June 2014 | Bae et al. |
| 8801700 | August 2014 | Alessi et al. |
| 8815802 | August 2014 | Kalthoff et al. |
| 8845726 | September 2014 | Tebbe |
| 8858621 | October 2014 | Oba |
| 8865202 | October 2014 | Zerbe et al. |
| 8888745 | November 2014 | Van Der Graaf et al. |
| 8926595 | January 2015 | Alessi et al. |
| 8940316 | January 2015 | Alessi et al. |
| 8992961 | March 2015 | Berry et al. |
| 8992962 | March 2015 | Lautenbach et al. |
| 9044209 | June 2015 | Dayton et al. |
| 9078900 | July 2015 | Kuzma et al. |
| 9095553 | August 2015 | Rohloff et al. |
| 9241722 | January 2016 | Yu |
| D750764 | March 2016 | DeSocio |
| 9332995 | May 2016 | Russo |
| 9526763 | December 2016 | Rohloff et al. |
| 9539200 | January 2017 | Lautenbach |
| 9572889 | February 2017 | Alessi et al. |
| D789539 | June 2017 | Kleiner |
| D789540 | June 2017 | Gyorgy |
| 9682127 | June 2017 | Alessi et al. |
| RE46577 | October 2017 | Collins |
| D805199 | December 2017 | Chen |
| D810289 | February 2018 | Jutila |
| 9889085 | February 2018 | Alessi et al. |
| 9931133 | April 2018 | Mirza |
| D816843 | May 2018 | Lewis |
| D819820 | June 2018 | Radau |
| 2001/0012511 | August 2001 | Bezwada et al. |
| 2001/0021377 | September 2001 | Jamiolkowski et al. |
| 2001/0021822 | September 2001 | Ayer |
| 2001/0022974 | September 2001 | Ayer |
| 2001/0026793 | October 2001 | Jamiolkowski et al. |
| 2001/0027311 | October 2001 | Chen et al. |
| 2001/0031940 | October 2001 | Loos |
| 2001/0031790 | November 2001 | Beisswenger |
| 2001/0036472 | November 2001 | Wong et al. |
| 2001/0040326 | November 2001 | Balczun |
| 2002/0001631 | January 2002 | Okumu |
| 2002/0004481 | January 2002 | Cleland et al. |
| 2002/0012818 | January 2002 | Ruppi et al. |
| 2002/0034532 | March 2002 | Brodbeck et al. |
| 2002/0037309 | March 2002 | Jaworowicz et al. |
| 2002/0048600 | April 2002 | Bhatt et al. |
| 2002/0077599 | June 2002 | Wojcik |
| 2002/0098180 | July 2002 | Lei |
| 2002/0136848 | September 2002 | Yoshii et al. |
| 2002/0137666 | September 2002 | Beeley et al. |
| 2002/0141985 | October 2002 | Pittner et al. |
| 2002/0197185 | December 2002 | Jamiolkowski et al. |
| 2002/0197235 | December 2002 | Moran |
| 2003/0007992 | January 2003 | Gibson et al. |
| 2003/0032947 | February 2003 | Harper et al. |
| 2003/0040699 | February 2003 | Talling |
| 2003/0044467 | March 2003 | Brodbeck et al. |
| 2003/0045454 | March 2003 | Okumu et al. |
| 2003/0059376 | March 2003 | Libbey et al. |
| 2003/0059471 | March 2003 | Compton et al. |
| 2003/0060425 | March 2003 | Ahlem et al. |
| 2003/0078618 | April 2003 | Fey et al. |
| 2003/0097121 | June 2003 | Babcock et al. |
| 2003/0104063 | June 2003 | Babcock et al. |
| 2003/0108608 | June 2003 | Berry et al. |
| 2003/0108609 | June 2003 | Berry et al. |
| 2003/0113380 | June 2003 | Ramstack et al. |
| 2003/0114837 | June 2003 | Peterson et al. |
| 2003/0118660 | June 2003 | Rickey et al. |
| 2003/0135153 | July 2003 | Hagemeier |
| 2003/0138403 | July 2003 | Drustrup |
| 2003/0138491 | July 2003 | Tracy et al. |
| 2003/0157178 | August 2003 | Chen et al. |
| 2003/0170289 | September 2003 | Chen et al. |
| 2003/0180364 | September 2003 | Chen et al. |
| 2003/0186858 | October 2003 | Arentsen |
| 2003/0191099 | October 2003 | Bohlmann et al. |
| 2003/0211974 | November 2003 | Brodbeck et al. |
| 2003/0215515 | November 2003 | Truong-Le et al. |
| 2003/0220617 | November 2003 | Dickerson |
| 2003/0233101 | December 2003 | Lubock et al. |
| 2004/0001689 | January 2004 | Goldsmith et al. |
| 2004/0001889 | January 2004 | Chen et al. |
| 2004/0002442 | January 2004 | Pan et al. |
| 2004/0022859 | February 2004 | Chen et al. |
| 2004/0024068 | February 2004 | Chen et al. |
| 2004/0024069 | February 2004 | Chen et al. |
| 2004/0029784 | February 2004 | Hathaway |
| 2004/0039376 | February 2004 | Peery et al. |
| 2004/0047888 | March 2004 | Kenison et al. |
| 2004/0097906 | May 2004 | Fereira et al. |
| 2004/0101557 | May 2004 | Gibson et al. |
| 2004/0102762 | May 2004 | Gilbert |
| 2004/0115236 | June 2004 | Chan et al. |
| 2004/0142867 | July 2004 | Oi et al. |
| 2004/0142902 | July 2004 | Stmijker-Boudier |
| 2004/0151753 | August 2004 | Chen et al. |
| 2004/0157951 | August 2004 | Wolf |
| 2004/0198654 | October 2004 | Glaesner et al. |
| 2004/0199140 | October 2004 | Rue |
| 2004/0209801 | October 2004 | Brand et al. |
| 2004/0215133 | October 2004 | Weber et al. |
| 2004/0224903 | November 2004 | Berry et al. |
| 2004/0225113 | November 2004 | LaFleur et al. |
| 2004/0243106 | December 2004 | Ayer |
| 2004/0265273 | December 2004 | Li et al. |
| 2004/0266683 | December 2004 | Hathaway et al. |
| 2004/0266692 | December 2004 | Young et al. |
| 2005/0004557 | January 2005 | Russell |
| 2005/0008661 | January 2005 | Fereira et al. |
| 2005/0009742 | January 2005 | Bertilsson et al. |
| 2005/0010196 | January 2005 | Fereira et al. |
| 2005/0070883 | March 2005 | Brown et al. |
| 2005/0070927 | March 2005 | Feinberg |
| 2005/0079200 | April 2005 | Rathenow et al. |
| 2005/0079202 | April 2005 | Chen et al. |
| 2005/0010942 | May 2005 | Eliaz et al. |
| 2005/0095284 | May 2005 | Trautman |
| 2005/0101943 | May 2005 | Ayer et al. |
| 2005/0106214 | May 2005 | Chen |
| 2005/0112188 | May 2005 | Eliaz et al. |
| 2005/0118206 | June 2005 | Luk et al. |
| 2005/0118221 | June 2005 | Blakely et al. |
| 2005/0131386 | June 2005 | Freeman et al. |
| 2005/0131389 | June 2005 | Peterson et al. |
| 2005/0143749 | June 2005 | Zalenski et al. |
| 2005/0175701 | August 2005 | Pan et al. |
| 2005/0201980 | September 2005 | Moran |
| 2005/0215475 | September 2005 | Ong et al. |
| 2005/0216087 | September 2005 | Zucherman et al. |
| 2005/0266087 | December 2005 | Junnarkar et al. |
| 2005/0271702 | December 2005 | Wright et al. |
| 2005/0276856 | December 2005 | Fereira et al. |
| 2005/0281879 | December 2005 | Chen et al. |
| 2006/0013879 | January 2006 | Brodbeck et al. |
| 2006/0014678 | January 2006 | Cowley et al. |
| 2006/0030526 | February 2006 | Liu et al. |
| 2006/0069029 | March 2006 | Kolterman et al. |
| 2006/0073182 | April 2006 | Wong et al. |
| 2006/0084604 | April 2006 | Kitaura et al. |
| 2006/0084922 | April 2006 | Botha |
| 2006/0094652 | May 2006 | Levy et al. |
| 2006/0094693 | May 2006 | Aziz et al. |
| 2006/0106399 | May 2006 | Taras et al. |
| 2006/0141040 | June 2006 | Chen et al. |
| 2006/0142234 | June 2006 | Chen et al. |
| 2006/0160736 | July 2006 | Nadler |
| 2006/0178304 | August 2006 | Juul-Mortensen et al. |
| 2006/0193918 | August 2006 | Rohloff et al. |
| 2006/0216242 | September 2006 | Rohloff et al. |
| 2006/0224145 | October 2006 | Gills |
| 2006/0233841 | October 2006 | Brodbeck et al. |
| 2006/0246138 | November 2006 | Rohloff et al. |
| 2006/0251618 | November 2006 | Dennis et al. |
| 2006/0263433 | November 2006 | Ayer et al. |
| 2006/0264890 | November 2006 | Moberg et al. |
| 2006/0280795 | December 2006 | Penhasi et al. |
| 2006/0293232 | December 2006 | Levy et al. |
| 2007/0027105 | February 2007 | Junnarkar et al. |
| 2007/0141102 | June 2007 | De Graaff et al. |
| 2007/0149011 | June 2007 | Kent et al. |
| 2007/0166352 | July 2007 | Wright et al. |
| 2007/0207958 | September 2007 | Bridon et al. |
| 2007/0248572 | October 2007 | Moran et al. |
| 2007/0281024 | December 2007 | Lautenbach et al. |
| 2008/0020016 | January 2008 | Li et al. |
| 2008/0038316 | February 2008 | Wong et al. |
| 2008/0064636 | March 2008 | Bloom et al. |
| 2008/0065090 | March 2008 | Scribner et al. |
| 2008/0091176 | April 2008 | Alessi et al. |
| 2008/0110515 | May 2008 | Angelosanto et al. |
| 2008/0112994 | May 2008 | Junnarkar et al. |
| 2008/0200383 | August 2008 | Jennings et al. |
| 2008/0207512 | August 2008 | Roth et al. |
| 2008/0208194 | August 2008 | Bickenbach |
| 2008/0226625 | September 2008 | Berry et al. |
| 2008/0226689 | September 2008 | Berry et al. |
| 2008/0260838 | October 2008 | Hokenson et al. |
| 2008/0260840 | October 2008 | Alessi et al. |
| 2008/0269725 | October 2008 | Deem et al. |
| 2008/0312157 | December 2008 | Levy et al. |
| 2009/0012463 | January 2009 | Beelen et al. |
| 2009/0022727 | January 2009 | Houston et al. |
| 2009/0036364 | February 2009 | Levy et al. |
| 2009/0042781 | February 2009 | Petersen et al. |
| 2009/0074734 | March 2009 | Rottiers |
| 2009/0087408 | April 2009 | Berry et al. |
| 2009/0125030 | May 2009 | Tebbe |
| 2009/0156474 | June 2009 | Roth et al. |
| 2009/0163447 | June 2009 | Maggio |
| 2009/0186817 | July 2009 | Ghosh et al. |
| 2009/0202481 | August 2009 | Li et al. |
| 2009/0202608 | August 2009 | Alessi et al. |
| 2009/0209460 | August 2009 | Young et al. |
| 2009/0210019 | August 2009 | Kim et al. |
| 2009/0215694 | August 2009 | Kolterman et al. |
| 2009/0234392 | September 2009 | Dziedzic |
| 2009/0247463 | October 2009 | Wright et al. |
| 2009/0254143 | October 2009 | Tweden et al. |
| 2009/0286723 | November 2009 | Levy et al. |
| 2009/0312246 | December 2009 | Baron et al. |
| 2010/0092566 | April 2010 | Alessi et al. |
| 2010/0094252 | April 2010 | Wengreen et al. |
| 2010/0105627 | April 2010 | Salama et al. |
| 2010/0144621 | June 2010 | Kim et al. |
| 2010/0185184 | July 2010 | Alessi et al. |
| 2010/0297209 | November 2010 | Rohloff et al. |
| 2010/0298807 | November 2010 | Jansen et al. |
| 2010/0298840 | November 2010 | Schwartz |
| 2010/0331868 | December 2010 | Bardy |
| 2011/0076317 | March 2011 | Alessi et al. |
| 2011/0091527 | April 2011 | Moonen et al. |
| 2011/0104111 | May 2011 | Rohloff et al. |
| 2011/0152181 | June 2011 | Alsina-Fernandez et al. |
| 2011/0152182 | June 2011 | Alsina-Fernandez et al. |
| 2011/0160708 | June 2011 | Berry et al. |
| 2011/0166554 | July 2011 | Alessi et al. |
| 2011/0264077 | October 2011 | Rohloff et al. |
| 2011/0306549 | December 2011 | Tatarkiewicz et al. |
| 2012/0178687 | July 2012 | Alessi et al. |
| 2012/0208755 | August 2012 | Leung |
| 2012/0265232 | October 2012 | Surbone |
| 2012/0303045 | November 2012 | Cooper et al. |
| 2013/0030417 | January 2013 | Alessi |
| 2013/0034210 | February 2013 | Rohloff et al. |
| 2013/0052237 | February 2013 | Eliaz et al. |
| 2013/0296661 | November 2013 | Bornzin et al. |
| 2013/0324977 | December 2013 | Vanderpool |
| 2014/0058409 | February 2014 | Bratlie |
| 2014/0058425 | February 2014 | Porat |
| 2014/0121741 | May 2014 | Bennett et al. |
| 2014/0148837 | May 2014 | Levitan |
| 2014/0163057 | June 2014 | Patel et al. |
| 2014/0236162 | August 2014 | Barongan |
| 2014/0257272 | September 2014 | Clark, III et al. |
| 2014/0324067 | October 2014 | Emken |
| 2014/0378900 | December 2014 | Alessi et al. |
| 2015/0111818 | January 2015 | Alessi et al. |
| 2015/0057227 | February 2015 | Leung |
| 2015/0133791 | May 2015 | Sato et al. |
| 2015/0231062 | August 2015 | Lautenbach et al. |
| 2015/0231256 | August 2015 | Berry et al. |
| 2015/0297509 | October 2015 | Schwarz |
| 2015/0359553 | December 2015 | Harnisch |
| 2016/0022582 | January 2016 | Alessi et al. |
| 2016/0030337 | February 2016 | Kuzma et al. |
| 2016/0354115 | December 2016 | Smith |
| 2016/0354305 | December 2016 | Alessi et al. |
| 2017/0056476 | March 2017 | Rohloff et al. |
| 2017/0079906 | March 2017 | Alessi et al. |
| 2017/0119854 | May 2017 | Alessi et al. |
| 2017/0119855 | May 2017 | Berry et al. |
| 2017/0181964 | June 2017 | Lautenbach et al. |
| 2017/0252409 | September 2017 | Leung |
| 2017/0273706 | September 2017 | Mirza |
| 2017/0319470 | November 2017 | Eliaz et al. |
| 2017/0319662 | November 2017 | Berry et al. |
| 2017/0348392 | December 2017 | Rohloff et al. |
| 2017/0368145 | December 2017 | Alessi et al. |
| 2018/0009871 | January 2018 | Blackwell et al. |
| 2018/0193059 | July 2018 | Bae |
| 000000635692 | Sep 1936 | DE | |||
| 0079405 | May 1983 | EP | |||
| 0254394 | Jan 1988 | EP | |||
| 0295411 | Dec 1988 | EP | |||
| 0304107 | Feb 1989 | EP | |||
| 0368339 | May 1990 | EP | |||
| 0373867 | Jun 1990 | EP | |||
| 0431942 | Jun 1991 | EP | |||
| 0486959 | May 1992 | EP | |||
| 0497575 | Aug 1992 | EP | |||
| 0521586 | Jan 1993 | EP | |||
| 0596161 | May 1994 | EP | |||
| 0379147 | Sep 1994 | EP | |||
| 0627231 | Dec 1994 | EP | |||
| 0631794 | Jan 1995 | EP | |||
| 0729747 | May 1997 | EP | |||
| 0771817 | May 1997 | EP | |||
| 0596161 | Feb 1998 | EP | |||
| 0841359 | May 1998 | EP | |||
| 0767689 | Jun 1999 | EP | |||
| 1046399 | Oct 2000 | EP | |||
| 1084703 | Mar 2001 | EP | |||
| 1300129 | Apr 2003 | EP | |||
| 1300173 | Apr 2003 | EP | |||
| 1300129 | May 2003 | EP | |||
| 1323450 | Jul 2003 | EP | |||
| 1600187 | Jan 2009 | EP | |||
| 2133073 | Dec 2009 | EP | |||
| 2020990 | Sep 2010 | EP | |||
| 640907 | Jul 1928 | FR | |||
| 2616665 | Dec 1988 | FR | |||
| 1049104 | Nov 1966 | GB | |||
| 1518683 | Jul 1978 | GB | |||
| 2138298 | Nov 1986 | GB | |||
| 2501400 | Oct 2013 | GB | |||
| 2501400 | Oct 2013 | GB | |||
| H02124814 | May 1990 | JP | |||
| H07196479 | Aug 1995 | JP | |||
| 1997/509346 | Sep 1997 | JP | |||
| 9241153 | Sep 1997 | JP | |||
| 11-100353 | Apr 1999 | JP | |||
| 2006/213727 | Aug 2006 | JP | |||
| 8901004 | Nov 1990 | NL | |||
| 9000948 | Nov 1990 | NL | |||
| 9100160 | Aug 1992 | NL | |||
| 9100160 | Aug 1992 | NL | |||
| 592113 | Aug 2012 | NZ | |||
| 200634060 | Oct 2006 | TW | |||
| WO 1989/003678 | May 1989 | WO | |||
| WO-1990/013285 | Nov 1990 | WO | |||
| WO-1990/013361 | Nov 1990 | WO | |||
| WO-1990/013780 | Nov 1990 | WO | |||
| WO 91/07160 | May 1991 | WO | |||
| WO-1992/019241 | Nov 1992 | WO | |||
| WO 93/06819 | Apr 1993 | WO | |||
| WO 93/06821 | Apr 1993 | WO | |||
| WO 93/008832 | May 1993 | WO | |||
| WO 93/09763 | May 1993 | WO | |||
| WO 93/23083 | Nov 1993 | WO | |||
| WO 94/09743 | May 1994 | WO | |||
| WO-1994/010982 | May 1994 | WO | |||
| WO 94/21262 | Sep 1994 | WO | |||
| WO 95/01167 | Jan 1995 | WO | |||
| WO 95/09006 | Apr 1995 | WO | |||
| WO 95/09007 | Apr 1995 | WO | |||
| WO 1995/013799 | May 1995 | WO | |||
| WO 95/34285 | Dec 1995 | WO | |||
| WO 96/001134 | Jan 1996 | WO | |||
| WO 96/003116 | Feb 1996 | WO | |||
| WO-1996/036317 | Nov 1996 | WO | |||
| WO 96/39142 | Dec 1996 | WO | |||
| WO 96/40049 | Dec 1996 | WO | |||
| WO 96/40139 | Dec 1996 | WO | |||
| WO 96/40355 | Dec 1996 | WO | |||
| WO-1996/040049 | Dec 1996 | WO | |||
| WO 97/15289 | May 1997 | WO | |||
| WO 97/15296 | May 1997 | WO | |||
| WO 97/28181 | Aug 1997 | WO | |||
| WO-1997/031943 | Sep 1997 | WO | |||
| WO-1997/044039 | Nov 1997 | WO | |||
| WO 97/46204 | Dec 1997 | WO | |||
| WO 97/47339 | Dec 1997 | WO | |||
| WO 98/00152 | Jan 1998 | WO | |||
| WO 98/00157 | Jan 1998 | WO | |||
| WO 98/00158 | Jan 1998 | WO | |||
| WO 98/02169 | Jan 1998 | WO | |||
| WO-1997/041837 | Feb 1998 | WO | |||
| WO-1998/007412 | Feb 1998 | WO | |||
| WO 98/13091 | Apr 1998 | WO | |||
| WO 98/13092 | Apr 1998 | WO | |||
| WO 98/16250 | Apr 1998 | WO | |||
| WO 98/17315 | Apr 1998 | WO | |||
| WO 98/20930 | May 1998 | WO | |||
| WO 98/27960 | Jul 1998 | WO | |||
| WO 98/027962 | Jul 1998 | WO | |||
| WO 98/27963 | Jul 1998 | WO | |||
| WO 98/030231 | Jul 1998 | WO | |||
| WO 98/32463 | Jul 1998 | WO | |||
| WO-1998/030231 | Jul 1998 | WO | |||
| WO 98/42317 | Oct 1998 | WO | |||
| WO 98/47487 | Oct 1998 | WO | |||
| WO 98/51282 | Nov 1998 | WO | |||
| WO 98/58698 | Dec 1998 | WO | |||
| WO 99/03453 | Jan 1999 | WO | |||
| WO 99/04767 | Feb 1999 | WO | |||
| WO 99/004768 | Feb 1999 | WO | |||
| WO-1999/012549 | Mar 1999 | WO | |||
| WO 99/16419 | Apr 1999 | WO | |||
| WO 99/025728 | May 1999 | WO | |||
| WO 99/29306 | Jun 1999 | WO | |||
| WO 99/033446 | Jul 1999 | WO | |||
| WO 99/33449 | Jul 1999 | WO | |||
| WO 99/39700 | Aug 1999 | WO | |||
| WO 99/040788 | Aug 1999 | WO | |||
| WO 99/044659 | Sep 1999 | WO | |||
| WO 99/062501 | Dec 1999 | WO | |||
| WO 99/064061 | Dec 1999 | WO | |||
| WO 00/013663 | Mar 2000 | WO | |||
| WO 00/029206 | May 2000 | WO | |||
| WO 00/038652 | Jul 2000 | WO | |||
| WO 00/039280 | Jul 2000 | WO | |||
| WO 00/040273 | Jul 2000 | WO | |||
| WO 00/041548 | Jul 2000 | WO | |||
| WO 00/045790 | Aug 2000 | WO | |||
| WO 00/054745 | Sep 2000 | WO | |||
| WO-2000/059476 | Oct 2000 | WO | |||
| WO 00/066138 | Nov 2000 | WO | |||
| WO 00/067728 | Nov 2000 | WO | |||
| WO-2000/066087 | Nov 2000 | WO | |||
| WO-2001/019345 | Mar 2001 | WO | |||
| WO 01/28631 | Apr 2001 | WO | |||
| WO-2001/028525 | Apr 2001 | WO | |||
| WO 01/043528 | Jun 2001 | WO | |||
| WO 01/051041 | Jul 2001 | WO | |||
| WO 01/68168 | Sep 2001 | WO | |||
| WO 01/78683 | Oct 2001 | WO | |||
| WO 02/028366 | Apr 2002 | WO | |||
| WO 02/036072 | May 2002 | WO | |||
| WO 02/043800 | Jun 2002 | WO | |||
| WO 02/045752 | Jun 2002 | WO | |||
| WO 02/47716 | Jun 2002 | WO | |||
| WO 02/067895 | Sep 2002 | WO | |||
| WO 02/069983 | Sep 2002 | WO | |||
| WO 02/76344 | Oct 2002 | WO | |||
| WO 02/085428 | Oct 2002 | WO | |||
| WO 03/000230 | Jan 2003 | WO | |||
| WO 03/007981 | Jan 2003 | WO | |||
| WO 03/011892 | Feb 2003 | WO | |||
| WO 03/024357 | Mar 2003 | WO | |||
| WO 03/024503 | Mar 2003 | WO | |||
| WO-2003/020245 | Mar 2003 | WO | |||
| WO 03/030923 | Apr 2003 | WO | |||
| WO 03/041684 | May 2003 | WO | |||
| WO 03/041757 | May 2003 | WO | |||
| WO 03/053400 | Jul 2003 | WO | |||
| WO-2003/066585 | Aug 2003 | WO | |||
| WO 03/072113 | Sep 2003 | WO | |||
| WO 03/072133 | Sep 2003 | WO | |||
| WO 04/002565 | Jan 2004 | WO | |||
| WO 2004/026106 | Apr 2004 | WO | |||
| WO-2004/034975 | Apr 2004 | WO | |||
| WO-2004/035754 | Apr 2004 | WO | |||
| WO-2004/035762 | Apr 2004 | WO | |||
| WO 2004/036186 | Apr 2004 | WO | |||
| WO 04/046338 | Jun 2004 | WO | |||
| WO 04/052336 | Jun 2004 | WO | |||
| WO 04/056338 | Jul 2004 | WO | |||
| WO 04/089335 | Oct 2004 | WO | |||
| WO 2004/089458 | Oct 2004 | WO | |||
| WO-2004/103342 | Dec 2004 | WO | |||
| WO 05/048930 | Jun 2005 | WO | |||
| WO 05/048952 | Jun 2005 | WO | |||
| WO 05/102293 | Nov 2005 | WO | |||
| WO 2005/102293 | Nov 2005 | WO | |||
| WO-2005/110425 | Nov 2005 | WO | |||
| WO 06/017772 | Feb 2006 | WO | |||
| WO 06/023526 | Mar 2006 | WO | |||
| WO 2006/077242 | Jul 2006 | WO | |||
| WO 2006/077250 | Jul 2006 | WO | |||
| WO 06/081279 | Aug 2006 | WO | |||
| WO 06/083761 | Aug 2006 | WO | |||
| WO 06/084139 | Aug 2006 | WO | |||
| WO 06/086727 | Aug 2006 | WO | |||
| WO 06/101815 | Sep 2006 | WO | |||
| WO 06/111169 | Oct 2006 | WO | |||
| WO-2006/131730 | Dec 2006 | WO | |||
| WO 07/024700 | Mar 2007 | WO | |||
| WO 07/056681 | May 2007 | WO | |||
| WO 07/075534 | Jul 2007 | WO | |||
| WO 07/084460 | Jul 2007 | WO | |||
| WO 2007/082889 | Jul 2007 | WO | |||
| WO 07/133778 | Nov 2007 | WO | |||
| WO 07/140416 | Dec 2007 | WO | |||
| WO 08/021133 | Feb 2008 | WO | |||
| WO-2008/041245 | Apr 2008 | WO | |||
| WO 08/061355 | May 2008 | WO | |||
| WO-2008/086086 | Jul 2008 | WO | |||
| WO 08/133908 | Nov 2008 | WO | |||
| WO 08/134425 | Nov 2008 | WO | |||
| WO 09/109927 | Sep 2009 | WO | |||
| WO-2009/143285 | Nov 2009 | WO | |||
| WO 2010/045169 | Apr 2010 | WO | |||
| WO 2013/004983 | Jan 2013 | WO | |||
| WO 2013/184235 | Dec 2013 | WO | |||
Other References
|
Croxatto. "Clinical profile of Implanon: a single-rod etonogestrel contraceptive implant." Eur J Contracept Reprod Health Care. Sep. 2000; 5 Suppl 2:21-8. cited by applicant . Implanon_Prescribing Information. Manufactured for Merck Sharp & Dohme Corp. Copyright .COPYRGT. 2006, 2009, 2012 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc. Revised Mar. 2016. cited by applicant . Nexplanon_Prescribing Information. Manufactured for Merck Sharp & Dohme Corp. Copyright .COPYRGT. 2011 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc. Revised Mar. 2016. cited by applicant . Probuphine_Prescribing Information. Distributed by Braeburn Pharmaceuticals, Inc. Revised May 2016. cited by applicant . International Search Report and Written Opinion for PCT Application No. PCT/US2016/035602 dated Nov. 29, 2016. cited by applicant . Adolf, "Human interferon omega-a review," Mult. Sclr. 1:S44-47 (1995). cited by applicant . Costantino et al., "Protein Spray Freeze Drying. 2. Effect of Formulation Variables on particle Size and Stability," J. Pharm. Sci. 91:388-395 (2002). cited by applicant . Henry et al., "Comparing ITCA 650, continuous subcutaneous delivery of exenatide via Duros.RTM. device, vs. twice daily exenatide injections in metformin-treated type 2 diabetes," oral presentation at the 46th Annual Meeting of the European Association for the Study of Diabetes in Stockholm, Sweden , 21 pages (Sep. 20-24, 2010). cited by applicant . Huggins et al., "Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses," Antimicrobial Agents & Chemotherapy, 26(4):476-480 (1984). cited by applicant . Ishiwata et al., "Clinical effects of the recombinant feline interferon-omega on experimental parvovirus infection in beagle dogs," J. Vet. Med. Sci. 60(8):911-917 (1998). cited by applicant . Johnson et al., "How interferons fight disease," Sci. Am. 270(5):68-75 (May 1994). cited by applicant . Lublin et al., "Defining the clinical course of multiple sclerosis: results of an international survey," Neurology. 46:907-911 (1996). cited by applicant . Madsbad, "Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)--preclinical and clinical results," Best Practice & Research Clinical Endocrinology & Metabolism 23:463-77 (2009). cited by applicant . Nielsen, "Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes," Drug Discovery Today 10(10):703-710 (May 15, 2005). cited by applicant . Patti et al., "Natural interferon-b treatment of relapsing-remitting and secondary-progressive multiple sclerosis patients: two-year study," Acta. Neurol. Scand. 100:283-289 (1999). cited by applicant . Paty et al., "Interferon beta-1 b is effective in relapsing-remitting multiple sclerosis," Neurology 43:662-667 (1993). cited by applicant . PCT International Search Report for PCT/US2009/000916, 4 pages (dated Aug. 12, 2009). cited by applicant . "Intarcia Therapeutics Announces Final Results from a Phase 2 Study of Injectable Omega Interferon plus Ribavirin for the Treatment of Hepatitis C Genotype-1," NLV Partners Press Coverage Portfolio News (Apr. 12, 2007) (Press Release). cited by applicant . Quianzon et al., "Lixisenatide-Once-daily Glucagon-like Peptide-1 Diabetes," US Endocrinology 7(2):104-109 (2011). cited by applicant . Ratner et al., "Dose-dependent effects of the one-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metfmmin: a randomized, double-blind, placebo-controlled trial," Diabetic Medicine 27(9):1024-1032 (Sep. 2010). cited by applicant . Roberts et al., "The Evolution of the Type I Interferonsl," J. Interferon Cytokine Res. 18(10):805-816 (Oct. 1998). cited by applicant . Rohloff et al., "DUROS Technology Delivers Peptides and Proteins at Consistent Rate Continuously for 3 to 12 Months," J. Diabetes Sci. & Tech., 2(3):461-467 (May 1, 2008). cited by applicant . "Sequence Listings for International Patent Application Publication No. WO2009109927, WIPO Patentscope", http://patentscope.wipoint/search/docservicepdf_pct/id00000008776887, 1 page (last visited Nov. 14, 2012). cited by applicant . Shire et al., "Challenges in the Development of High Protein Concentration Formulations," J. Pharm. Sci. 93:1390-1402 (2004). cited by applicant . Smith, "Peripheral Neuro-hormones as a Strategy to Treat Obesity," oral presentation at the 2007 Cardiometabolic Health Congress in Boston, MA, pp. 1-35 (Sep. 26-29, 2007). cited by applicant . Written Opinion for International Patent Application No. PCT/US2009/005629 (corresponding to U.S. Appl. No. 12/587,946), 5 pages (dated Apr. 15, 2011). cited by applicant . Zhang et al., "Efficacy observations of different dosages of interferon to treat 150 Hepatitis B carriers," Current Physician 2(12):45-46 (1997). cited by applicant . Pratley et al., "Targeting Incretins in Type 2 Diabetes: Role of GLP-1 Receptor Agonists and DPP-4 Inhibitors," Rev. Diabet. Stud., 5(2):73-94 (2008). cited by applicant . Gonzalez, et al., "Hemoglobin Alc: A Reliable and Accurate Test for Diabetes Care? A Prospective Study in Mexico," Salud Publica Mex 55:462-468 (2013). cited by applicant . Ahn et al., "A New Approach to Search for the Bioactive Confirmation of Glucagon: Positional Cyclization Scanning" Journal of Medicinal Chemistry, vol. 44, No. 19, (2001): 3109-3116. cited by applicant . "Abstracts 2007," Diabetologia Clinical & Experimental Diabetes & Metabolism, Springer, Berlin, Germany, vol. 50 S243 (Aug. 21, 2007) (paragraph [0586]) (XP002538652). cited by applicant . Jetschmann et al., "Open-label rising-dose study of omega interferon in IFN-naive patients with chronic hepatitis C," Gastroenterology 122:A278-A347 (Apr. 1, 2002) (Abstract M1454). cited by applicant . Bray, "Gut Signals and Energy Balance: Ghrelin, Peptide YY, Leptin, and Amylin," (Dec. 19, 2007) (slides and transcript for presentation at Medscape CME). cited by applicant . "Implantable infusion pumps: technology poised for takeoff," BBI Newsletter 17(12):209211 (Dec. 1994). cited by applicant . "Search Report and Written Opinion" dated Dec. 19, 2008 for PCT/US2008/005235, filed Apr. 22, 2008. cited by applicant . Adamson et al., "Phase I trial and pharmacokinetic study of all-trans-retinoic acid administered on an intermittent schedule in combination with interferon-alpha2a in pediatric patients with refractory cancer," J. Clin. Oncol. 15(11):3330-3337 (Nov. 1997). cited by applicant . Adolf et al., "Monoclonal antibodies and enzyme immunoassays specific for human interferon (IFN) .omega.1: evidence that IFN-.omega.1 is a component of human leukocyte IFN," Virology 175(2):410-471 (Apr. 1990). cited by applicant . Adolf et al., "Antigenic structure of human interferon .omega.1 (Interferon .alpha.ll 1): comparison with other human interferons," J. Gen. Virol. 68(6):1669-1676 (Jun. 1987). cited by applicant . Adolf et al., "Purification and characterization of natural human interferon .omega.l," J. Bio. Chem. 265(16):9290-9295 (Jun. 1990). cited by applicant . Adolf et al., "Human interferon .omega.l: isolation of the gene, expression in Chinese hamster ovary cells and characterization of the recombinant protein," Biochim. Biophys. Acta 108(9):167-174 (Jun. 1991). cited by applicant . Andrx Pharmaceuticals, LLC, ANDA for Concerta.RTM. Extended-Release Tablets, 6 pages (correspondence dated Sep. 6, 2005). cited by applicant . ASTM International, Annual Book of ASTM Standards, 8.02:208-211, 584-587 (1984). cited by applicant . Ansel et al., "Dosage Form Design: Pharmaceutical and Formulation Considerations," Pharmaceutical Dosage Forms and Drug Delivery Systems, Ch. 3 at 87-92 (7th ed. Lippincott Williams & Wilkins 1999). cited by applicant . Ansel et al., "Modified-Release Dosage Forms and Drug Delivery Systems," Pharmaceutical Dosage Forms and Drug Delivery Systems, Ch. 8 at 229-243 (7th ed. Lippincott Williams & Wilkins 1999). cited by applicant . Aulitzky, " Successful Treatment of Metastatic Renal Cell Carcinoma With a Biologically Active Dose of Recombinant Interferon-Gama," Journal of Clinical Oncology 7(12):1875-1884 (1989). cited by applicant . Hauck, "Engineer's Guide to Plastics," Materials Engineering 5(72):38-45 (Jul. 17, 1972). cited by applicant . Bailon et al., "Rational Design of a Potent, Long-lasting Form of Interferon: A 40 kDa Branched Polyethylene Glycol-conjugated Interferon Alpha-2a for the Treatment of Hepatitis C," Bioconjugate Chemistry 12(2):195-202 (2001). cited by applicant . Bakan et al., "Physicochemical Characterization of a Synthetic Lipid Emulsion for Hepatocyte-Selective Delivery of Lipophilic Compounds: Application to Polyiodinated triglycerides as Contrast Agents for Computed Tomography," J. Pharm. Sci., 85(9):908-914 (1996). cited by applicant . Bakhtiar et al, "Taking Delivery," Soap Perfumery & Cosmetics 76(3):59-65 (2003) (liposomes in cosmetic delivery systems). cited by applicant . Balkwill,F., "Interferons," Lancet 1(8646):1060-1063 (May 1989). cited by applicant . Bauer et al., "Non-aqueous emulsions as vehicles for capsule fillings," Drug Dev. & Industrial Pharmacy 10(5):699-712 (1984). cited by applicant . Bekkering et al., "Estimation of early hepatitis C viral clearance in patients receiving daily interferon and ribavirin therapy using a mathematical model," Hepatology 33(2):419-423 (Feb. 2001). cited by applicant . Bell et al., "Hamster preproglucagon contains the sequence of glucagon and two related peptides," Nature 302:716-718 (1983). cited by applicant . Bell et al, "Impact of moisture on thermally induced denaturation and decomposition of lyophilized bovine somatotropin," Drug Delivery Research & Dev. Biopolymers, (35):201209 (1995). cited by applicant . Bertoncello et al., "Haematopoietic radioprotection by Cremophor EL: a polyethoxylated castor oil," Int. J. Radiat. Biol. 67(1):57-64 (1995). cited by applicant . Bohlinder et al., "Use and characteristics of a novel lipid particle-forming matrix as a drug-carrier system," Euro. J. Pharm. Sci. 2(4):271-279 (1994). cited by applicant . Bolinger et al., "Recombinant interferon .gamma. for treatment of chronic granulomatous disease and other disorders," Clin. Pharm. 11(10):834-850 (Oct. 1992). cited by applicant . Bonkovsky et al., "Outcomes research in chronic viral hepatitis C: effects of interferon therapy," Can. J. Gastroenterol. 14(Supp. B):21B-29B (Jul.-Aug. 2000). cited by applicant . Borden et al., "Second-generation interferons for cancer: clinical targets," Semin. Cancer Biol. 10(2):125-144 (Apr. 2000). cited by applicant . Boue et al., "Antiviral and antiluteolytic activity of recombinant bovine IFN-.omega.1 obtained from Pichia pastoris," J. Interferon & Cytokine Res. 20:677-683 (2000). cited by applicant . Buckwold et. al. "Antiviral activity of CHO-SS cell-derived human omega interferon and other human interferons against HCV RNA replicons and related viruses," Antiviral Res. 73(2):118-25 (Feb. 2007) (Epub Sep. 11, 2006). cited by applicant . Cantor, "Theory of lipid monolayers comprised of mixtures of flexible and stiff amphiphiles in anthermal solvents: fluid phase coexistence," J. Chem. Physics 104(20):8082-8095 (1996). cited by applicant . CAS Number: 56-81-5 (Nov. 16, 1984). cited by applicant . Chang et al., "Biodegradable polyester implants and suspension injection for sustained release of a cognitive enhancer," Pharm. Tech. 20(1):80-84 (1996). cited by applicant . Chapman et al., "Physical Studies of Phospholipids. VI. Thermotropic and Lyotropic Mesomorphism of Some 1,2-Diacylphosphatidylcholines (lecithins)," Chem. & Physics of Lipids 1(5):445-475 (1967). cited by applicant . Chaumeil, "Micronization: a method of improving the bioavailability of poorly soluble drugs," Methods & Findings in Experimental & Clinical Pharmacology 20(3):211-215 (1998). cited by applicant . Clark et al., "The diabetic Zucker fatty rat," Proc. Soc. Exp. Biol. 173(1):68-75 (1983). cited by applicant . Condino-Neto, "Interferon-.gamma. improves splicing efficiency of CYBB gene transcripts in an interferon responsive variant of chronic granulomatous disease due to a splice site consensus region mutation," Blood 95(11):3548-3554 (Jun. 2000). cited by applicant . Darney, "Subdermal progestin implant contraception," Current Opinion in Obstetrics & Gynecology 3:470-476 (1991). cited by applicant . Das et al., "Reviewing Antisense Oligonucleotide Therapy: Part 2, Delivery Issues," BioPharm, 2(11):44-51 (1999). cited by applicant . Dash et al., "Therapeutic applications of implantable drug delivery systems," Journal of Pharmacological and Toxicological Methods, 40(1):1-12 (1998). cited by applicant . Davis et al., "Durability of viral response to interferon alone or in combination with oral ribavirin in patients with chronic hepatitis C," Prog. Abstr. 50th Annu. Mtg. Postgrad. Courses Am. Assn. Study Liver Dis., Dallas, TX (Nov. 5-9, 1999)(Abstract 570). cited by applicant . Deacon et al., "GLP-1-(9-36) amide reduces blood glucose in anesthetized pigs by a mechanism that does not involve insulin secretion," Am. J. Physiol. Endocrinol. Metab., 282:E873-E879 (2002). cited by applicant . Desai et al., "Protein structure in the lyophilized state: a hydrogen isotope exchange/NMR study with bovine pancreatic trypsin inhibitor," J. Am. Chem. Soc. 116(21):9420-9422 (1994). cited by applicant . Di Marco et al., "Combined treatment of relapse of chronic hepatitis C with high-dose .alpha.-2B interferon plus ribavirin for 6 or 12 months," Prog. Abstr. 50th Annu. Mtg. Postgrad. Courses Am. Assn. Study Liver Dis., Dallas, TX (Nov. 5-9, 1999)(Abstract 569). cited by applicant . Dorr et al., "Phase I-II trial of interferon-alpha 2b by continuous subcutaneous infusion over 28 days," J. Interferon Res. 8:717-725 (1988). cited by applicant . Uhlig et al., "The electro-osmotic actuation of implantable insulin micropumps," J. Biomed. Materials Res. 17:931-943 (1983). cited by applicant . Efendic et al., "Overview of incretin hormones," Honn. Metab. Res., 36(11-12):742-746 (2004). cited by applicant . Eissele et al., "Rat gastric somatostatin and gastrin release: interactions of exendin-4 and truncated glucagon-like peptide-1 (GLP-1) amide," Life Sci., 55(8):629-634 (1994). cited by applicant . Elias et al., "Infusional Interleukin-2 and 5-fluorouracil with subcutaneous interferon-.alpha. for the treatment of patients with advanced renal cell carcinoma: a southwest oncology group Phase II study," Cancer 89(3):597-603 (Aug. 2000). cited by applicant . Eng et al., "Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas," J. Biol. Chem., 267(11):7402-7405 (1992). cited by applicant . Eng et al., "Purification and structure of exendin-3, a new pancreatic secretagogue isolated from Heloderma horridum venom," J. Biol. Chem., 265(33):20259-20262 (1990). cited by applicant . Eppstein et al., "Biological activity of liposome-encapsulated murine interferon .gamma. is mediated by a cell membrane receptor," PNAS USA 82:3688-3692 (1985). cited by applicant . Eros et al., "Multiple phase emulsions as controlled drug delivery therapeutic systems," Proc.-Conf. Colloid Chem. 193-196 (1993). cited by applicant . Fang et al., "The impact of baseline liver histology on virologic response to interferon .alpha.-2b.+-..rho. ribavirin therapy in patients with chronic hepatitis C," Prog. Abstr. 50th Annu. Mtg. Postgrad. Courses Am. Assn. Study Liver Dis., Dallas, TX (Nov. 5-9, 1999)(Abstract 572). cited by applicant . Felker et al., "The Rate of Transfer of Unesterified Cholesterol from Rat Erythrocytes to Emulsions Modeling Nascent Triglyceride-Rich Lipoproteins and Chylomicrons Depends on the Degree of Fluidity of the Surface," J. Nutritional Biochem. 4(1):630-634 (1993). cited by applicant . Ferenci et al, "Combination of interferon (IFN) induction therapy and ribavirin in chronic hepatitis C," Prog. Abstr. Dig. Dis. Week 2000, San Diego, CA (May 21-24, 2000) (Abstract 977). cited by applicant . Fontaine et al., "Recovery from chronic hepatitis C in long-term responders to ribarivin plus interferon .beta.," Lancet 356(9223):41 (Jul. 2000). cited by applicant . Franchetti et al., "Furanfurin and Thiophenfurin: Two Novel Tiazofurin Analogues. Synthesis, Structure, Antitumor Activity, and Interactions with Inosine Monophosphate Dehydrogenase," J. Medicinal Chem. 38(19):3829-3837 (1995). cited by applicant . Fujii et al., "Effect of phosphatidylcholine on Skin Permeation of Indomethacin from gel prepared with Liquid Paraffin and Hydrogenated Phospholipid," Int'l J. Pharmaceutics 222(1):57-64 (2001). cited by applicant . Fujii et al., "Enhancement of skin permeation of miconazole by phospholipid and dodecyl 2-(N, N-dimethylamino) propionate (DDAIP)," Int'l J. Pharmaceutics 234(1-2):121-128 (2002). cited by applicant . Luft et al., "Electro-osmotic valve for the controlled administration of drugs," Med. & Biological Engineering & Computing 45-50 (Jan. 1978) (non-English with English abstract). cited by applicant . Gan To Kagaku Ryoho, "Phase II study of recombinant leukocyte A interferon (Ro22-8181) in malignant brain tumors," Cancer & Chemotherapy 12(4):913-920 (Apr. 1985) (non-English with English abstract). cited by applicant . Gappa et al., "Juvenile laryngeal papillomatosis--a case report," Pneurnologie 45(11):936-938 (Nov. 1991) (XP009079028) (non-English with English abstract). cited by applicant . Gause et al., "Phase I study of subcutaneously administered interleukin-2 in combination with interferon alfa-2a in patients with advanced cancer," J. Clin. Oncol. 14(8):2234-2241 (Aug. 1996). cited by applicant . Ghiglione et al., "How glucagon-like is glucagon-like peptide-1?" Diabetologia 27:599-600 (1984). cited by applicant . Glue et al., "A dose-ranging study of Peg-intron and ribavirin in chronic hepatitis C--safety, efficacy, and virological rationale," Prog. Abstr. 50th Annu. Mtg. Postgrad. Courses Am. Assn. Study Liver Dis., Dallas, TX(Nov. 5-9, 1999)(Abstract 571). cited by applicant . Goke et al., "Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells," J. Biol. Chem., 268(26):19650-19655 (1993). cited by applicant . Gonzales et al., "Randomized controlled trial including an initial 4-week `induction` period during one year of high-dose interferon .alpha.-2B treatment for chronic hepatitis C," Prog. Abstr. Dig. Dis. Week 2000, San Diego, CA (May 21-24, 2000) (Abstract 975). cited by applicant . Gosland et al., "A phase I trial of 5-day continuous infusion cisplatin and interferon alpha," Cancer Chemother. Pharrnacol. 37(1-2):39-46 (1995). cited by applicant . Grant et al., "Combination therapy with interferon-.alpha. plus N-acetyl cysteine for chronic hepatitis C: a placebo controlled double-blind multicentre study," J. Med. Virol. 61(4):439-442 (Aug. 2000). cited by applicant . Gutniak et al., "Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus," N. Engl. J. Med., 326(20):1316-1322 (1992). cited by applicant . Hageman, "The Role of Moisture in Protein Stability, " Drug Dev. & Ind. Pharm. 14(14):2047-2070 (1988). cited by applicant . Heathcote et al., "Peginterferon alfa-2a in Patients With Chronic Hepatitis C and Cirrhosis," New England J. Med. 343(23) 1673-1680 (2000). cited by applicant . Heim et al., "Intracellular signaling and antiviral effects of interferons," Dig. Liver Dis. 32(3):257-263 (Apr. 2000). cited by applicant . Heinrich et al., "Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid," Endocrinol., 115:2176-2181 (1984). cited by applicant . Hellstrand et al., "Histamine and cytokine therapy," Acta Oncol. 37(4):347-353 (1998). cited by applicant . Hellstrand et al., "Histamine and the response to IFN-.alpha. in chronic hepatitis C," Interferon Cytokine Res. 18(1):21-22 (Jan. 1998). cited by applicant . Hellstrand et al., "Histamine in immunotherapy of advanced melanoma: a pilot study," Cancer Immunol Immunother. 39(6):416-419 (Dec. 1994). cited by applicant . Hisatomi et al., "Toxicity of polyoxyethylene hydrogenated castor oil 60 (HCO-60) in experimental animals," J. Toxicol. Sci., 18(3):1-9 (1993). cited by applicant . Hodoshima et al., "Lipid nanoparticles for delivering antitumor drugs," International Journal of Pharmaceutics, 146(1):81-92 (1997). cited by applicant . Hoffmann-La Roche Inc., Pegasys.RTM. (peginterferon alfa-2a), 15 pages (2002). cited by applicant . Horton et al., "Antitumor effects of interferon-omega: in vivo therapy of human tumor xenografts in nude mice" Cancer Res 59(16):4064-4068 (Aug. 1999). cited by applicant . Hubel et al., "A phase I/II study of idarubicin, dexamethasone and interferon-alpha (1-Dexa) in patients with relapsed or refractory multiple myeloma" Leukemia 11 Suppl 5:S47-S51 (Dec 1997). cited by applicant . Iacobelli et al., "A phase I study of recombinant interferon-alpha administered as a seven-day continuous venous infusion at circadian-rhythm modulated rate in patients with cancer," Am. J. Clin. Oncol. 18(1):27-31 (1995). cited by applicant . IFNB Multiple Sclerosis Study Group, "Interferon.beta.-1b is effective in relapsing-remitting multiple sclerosis," Neurology 43(4):655-667 (Apr. 1993). cited by applicant . Intermune.RTM. Inc., Infergen.RTM. (Interferon alfacon-1), 5 pages (2002). cited by applicant . "Introduction to Antibodies", http://www.chemicon.com/resource/ANT101/a1.asp, 8 pages (retrieved May 2, 2007). cited by applicant . Isaacs et al., "Virus interference. I. The interferon," Pro. R. Soc. Lond. B. Biol. Sci. 147:258-267 (1957). cited by applicant . Jain et M., "Controlled delivery of drugs from a novel injectable in situ formed biodegradable PLGA microsphere system," J. Microencapsulation 17(3):343-362 (2000). cited by applicant . Jordan et al., "Guidelines for Antiemetic Treatment of Chemotherapy-Induced Nausea and Vomiting: Past, Present and Future Recommendations," The Oncologist 12(9):11431150 (2007). cited by applicant . Kabalnov et al., "Macroemulsion type and stability of alkane-water-phospholipid systems," Abstracts of Papers, Part 1, 210th ACS National Meeting, 0-8412-3222-9, American Chemical Society, Chicago, IL (Aug. 20-24, 1995) (Abstract only). cited by applicant . Kabalnov et al., "Phospholipids as Emulsion Stabilizers.2. Phase Behavior Versus Emulsion Stability," Journal of Colloid and Interface Science 184(1):227-235 (1996). cited by applicant . Khalili et M., "Interferon and ribavirin versus interferon and amantadine in interferon nonresponders with chronic hepatitis C," Am. J. Gastroenterol. 95(5):1284-1289 (May 2000). cited by applicant . Kildsig et al., "Theoretical Justification of Reciprocal Rate Plots in Studies of Water Vapor Transmission through Films," J. Pharma. Sci. 29(11):1634-01637 (Nov. 17, 1970). cited by applicant . Kirkwood et al., "Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684," J. Clin. Oncol. 14(1):7-17 (1996). cited by applicant . Kita et al., "Characterization of a polyethylene glycol conjugate of recombinant human interferon-.gamma.," Drug Des. Deliv. 6(3):157-0167 (Sep. 1990). cited by applicant . Knepp et al, "Identification of antioxidants for prevention of peroxide-mediated oxidation of recombinant human ciliary neurotrophic factor and recombinant human nerve growth factor," J. Pharm. Sci. Tech. 50(3):163-171 (1996). cited by applicant . Knepp et al., "Stability of nonaqueous suspension formulations of plasma derived factor IX and recombinant human alpha interferon at elevated temperatures," Phanna. Res. 15(7):1090-1095 (1998). cited by applicant . Knobler et al., "Systemic .alpha.-interferon therapy of multiple sclerosis," Neurology 34(10):1273-1279 (Oct. 1984). cited by applicant . Kovacevic et al., "Treatment of chronic viral hepatitis B in secondary membranoproliferative glomerulonephritis using recombinant .alpha.-2 interferon," Maksic Dj Vojnosanit. Pregl. 57(2):235-240 (Mar.-Apr. 2000) (non-English with English abstract). Abs. cited by applicant . Kracke et al., "Mx proteins in blood leukocytes for monitoring interferon .beta.-lb therapy in patients with MS," Neurology 54(1):193-199 (Jan. 2000). cited by applicant . Kronenberger et al., "Influence of interferon-.alpha. on CD82-expression in HCV-positive patients," Prog. Abstr. Dig. Dis. Week 2000, San Diego, CA (May 21-24, 2000) (Abstract 976). cited by applicant . Krown et al., "Interferons and interferon inducers in cancer treatment," Semin. Oncol. 13(2):207-217 (1986). cited by applicant . Kubes et al., "Cross-species antiviral and antiproliferative activity of human interferon-.omega.," J. Interferon Res. 14:57-59 (1994). cited by applicant . Kunzi et al., "Role of interferon-stimulated gene ISG-15 in the interferon-.omega.-mediated inhibition of human immunodeficiency virus replication," J. Interferon Cytokine Res. 16(11):919-927 (Nov. 1996). cited by applicant . Larsson, "Stability of emulsions formed by polar lipids," Progress in the Chemistry of Fats and Other Lipids 16:163-0169 (1978). cited by applicant . Lee et al., "Dynamics of hepatitis C virus quasispecies turnover during interferon-A treatment," Prog. Abstr. Dig. Dis. Week 2000, San Diego, CA (May 21-24, 2000) (Abstract 974). cited by applicant . Lee, "Therapy of hepatitis C: interferon alfa-2A trials," Hepatology 26: 89S-95S (Sep. 1997) (XP000981288). cited by applicant . Lopez et al., "Manunalian pancreatic preproglucagon contains three glucagon-related peptides," Proc. Natl. Acad. Sci. USA, 80(18):5485-5489 (1983). cited by applicant . Lukaszewski et al., "Pegylated .alpha. interferon is an effective treatment for virulent Venezuelan equine encephalitis virus and has profound effects on host immune response to infection," J. Virol. 74(11):5006-5015 (Jun. 2000). cited by applicant . Lund et al., "Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem," Proc. Natl. Acad. Sci. USA, 79(2):345-349 (1982). cited by applicant . Lundberg, "A submicron lipid emulsion coated with amphipathic polyethylene glycol for parenteral administration of paclitaxel (Taxol)," J. Pharm. & Pharmacol. 49(1):16-21 (1997). cited by applicant . Magnuson et al. "Enhanced recovery of a secreted mammalian protein from suspension culture of genetically modified tobacco cells," Protein Expression & Purification 7:220-228 (1996). cited by applicant . Malley et al., "Chronic Toxicity and Oncogenicity of N-Methylpyrrolidone (Nmp) in Rats and Mice by Dietary Administration," Drug Chem Toxicol. 24(4):315-38 (Nov. 2001). cited by applicant . Manning et al, "Stability of protein pharmaceuticals," Pharm. Res. 6(11):903-918 (1989). cited by applicant . Marincola et al., "Combination therapy with interferon alfa-2a and interleukin-2 for the treatment of metastatic cancer," J. Clinical Oncol. 13(5):1110-1122 (1995) (XP009078965). cited by applicant . Massey, "Interaction of vitamin E with saturated phospholipid bilayers," Biochem. & Biophys. Res. Comms. 106(3):842-847 (1982). cited by applicant . McHutchison et al., "Interferon .alpha.-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C," N. Engl J Med. 339(21):1485-1492 (Nov. 1998). cited by applicant . McHutchison et al., "Open-label phase 1B study of hepatitis C viral dynamics with omega interferon treatment," Hepatology 34(4):A333 (Oct. 1, 2001) (XP004716177) (Abstract Only). cited by applicant . Meier et al., "The glucagon-like peptide-1 metabolite GLP-1-(9-36) amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans," Am. J. Physiol. Endocrinol. Metab., 290(6):E1118-E1123 (2006). cited by applicant . Merad et al., "Generation of monocyte-derived dendritic cells from patients with renal cell cancer: modulation of their functional properties after therapy with biological response modifiers (IFN-.alpha. plus IL-2 and IL-12)," J. Immunother. 23(3):369-378 (May-Jun. 2000). cited by applicant . Milella et al., "Neutralizing antibodies to recombinant .alpha.-interferon and response to therapy in chronic hepatitis C infection," Liver 13(3):146-150 (Jun. 1993). cited by applicant . Mohler, "Primer on electrodeposited coatings," Materials Engineering 5:38-45 (1972). cited by applicant . Mojsov, "Structural requirements for biological activity of glucagon-like peptide-I," Int. J. Peptide Protein Research, 40:333-343 (1992). cited by applicant . Morgan, "Structure and Moisture Permeability of Film-Forming Poloyers," Ind. Eng. Chem. 45(10):2296-2306 (1953). cited by applicant . Motzer et al., "Phase I trial of 40-kd branched pegylated interferon alfa-2a for patients with advanced renal cell carcinoma," J. Clinical Oncol. 19(5):1312-1319 (2001). cited by applicant . Nauck et al., "Normalization of fasting glycaemia by intravenous GLP-1 ([7-36 amide] or [7-37]) in type 2 diabetic patients," Diabet. Med., 15(11):937-945(1998). cited by applicant . Neumann et al., "Hepatitis C Viral Dynamics in Vivo and the Antiviral Efficacy of Interferon-alpha Therapy," Science 282:103-107 (Dec. 1998). cited by applicant . Nieforth et al., "Use of an indirect pharmacodynamic stimulation model of MX protein induction to compare in vivo activity of interferon-.alpha.-2a and a polyethylene glycol-modified derivative in healthy subjects," Clin. Pharmacol. Ther. 59(6):636-646 (Jun. 1996). cited by applicant . Norden et al., "Physicochemical characterization of a drug-containing phospholipid-stabilized o / w emulsion for intravenous administration," Eur. J. Phann. Sci. 13(4).393-401 (2001). cited by applicant . Olaso et al., "Early prediction of lack of response to treatment with interferon and interferon plus ribavirin using biochemical and virological criteria in patients with chronic hepatitis C," Esp. Quimioter. 12(3):220-228 (Sep. 1999) (non-English with English abstract). cited by applicant . Ortiz et al., "A differential scanning calorimetry study of the interaction of .alpha.-tocopherol with mixtures of phospholipids," Biochim et Biophys Acta 898(2):214-222 (1987). cited by applicant . Panitch, "Interferons in multiple sclerosis," Drugs 44(6):946-962 (Dec. 1992). cited by applicant . Patzelt et al., "Identification and processing of proglucagon in pancreatic islets," Nature, 282:260-266 (1979). cited by applicant . Peterson et al., "Zucker Diabetic Fatty Rat as a Model for Non-insulin-dependent Diabetes Mellitus," ILAR Journal, 32(3):16-19 (1990). cited by applicant . Peterson et al., "Neuropathic complications in the Zucker diabetic fatty rat (ZDF/Drt-fa)," Frontiers in diabetes research. Lessons from Animal Diabetes III, Shafrir, E. (ed), pp. 456-458, Smith-Gordon, London (1990). cited by applicant . Pimstone et al., "High dose (780 MIU/52 weeks) interferon monotherapy is highly effective treatment for hepatitis C," Prog. Abstr. Dig. Dis. Week 2000, San Diego, CA (May 21-24, 2000) (Abstract 973). cited by applicant . Plauth et al, "Open-label phase II study of omega interferon in previously untreated HCV infected patients," Hepatology 34(4):A331 (Oct. 1, 2001) (XP004716169) (Abstract Only). cited by applicant . Plauth et al, "Open-label study of omega interferon in previously untreated HCV-infected patients," J. Hepatology 36(Supp. 1):125 (Apr. 2002) (XP002511882) (Abstract Only). cited by applicant . Pohl et al., "Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. Relationship to vasoactive intestinal polypeptide/pituitary adenylate cyclase activating polypeptide and glucagon-like peptide 1 and evidence against the existence of mammalian homologues," J. Biol. Chem., 273(16):9778-9784 (1998). cited by applicant . Poynard et al., "Is an `a la carte` combined interferon .alpha. 2b plus ribavirin possible for the first line treatment in patients with chronic hepatitis C," Hepatology 31(1):211-218 (Jan. 2000). cited by applicant . Poynard et al., "Randomized trial of interferon .alpha. 2b plus ribavirin for 48 weeks or for 24 weeks versus interferon .alpha. 2b plus placebo for 48 weeks for the treatment of chronic infection with hepatitis C virus," Lancet 352(9138):1426-1432 (Oct. 1998). cited by applicant . "Intarcia Presents Positive ITCA 650 Phase 2 Study Results for Type 2 Diabetes at EASD," Intarcia Therapeutics, Inc. (Sep. 22, 2010) (Press Release). cited by applicant . Quesada et al., "Interferons in Hematological Malignancies", eds. Baron et al., U. Tex. 487-495 (1987). cited by applicant . Quintanar-Guerrero et al., "Applications of the ion-pair concept to hydrophilic substances with special emphasis on peptides," Pharm. Res. 14(2):119-127 (1997). cited by applicant . Rajkumar et al., "Phase I evaluation of radiation combined with recombinant interferon alpha-2a and BCNU for patients with high-grade glioma," Int'l J. Radiat. Oncol. Biol. Phys. 40(2):297-302 (Jan. 15, 1998). cited by applicant . Roche Pharmaceuticals, Roferon.RTM.-A (Interferon alfa-2a, recombinant), 22 pages (2003). cited by applicant . Roff et al., "Handbook of Common Polymers", Cleveland Rubber Co. 72 pages (1971). cited by applicant . Rogers et al., "Permeability Valves," Ind. & Eng. Chem. 49(11):1933-1936 (Nov. 17, 1957). cited by applicant . Roman et al., "Cholestasis in the rat by means of intravenous administration of cyclosporine vehicle, Cremophor EL," Transplantation 48(4):554-558 (1989). cited by applicant . Roth et al., "High Dose Etretinate and Interferon-alpha--A Phase I Study in Squamous Cell Carcinomas and Transitional Cell Carcinomas," Acta Oncol. 38(5):613-617 (1999). cited by applicant . Roth et al., "Combination therapy with amylin and peptide YY[3-36] in obese rodents: anorexigenic synergy and weight loss additivity," Endocrinol. 148(12):6054-61 (Dec. 2007). cited by applicant . Schepp et al., "Exendin-4 and exendin-(9-39)NH2: agonist and antagonist, respectively, at the rat parietal cell receptor for glucagon-like peptide-1-(7-36)NH2," Eur. J. Pharmacol., 269(2):183-191 (1994). cited by applicant . Schering Corp., Intron.RTM. A for Injection, 6 pages (2001). cited by applicant . Schering Corp., PEG-Intron.TM. (Peginterferon alfa-2b) Powder for Injection, 29 pages (2003). cited by applicant . Schmalfub et al., "Modification of drug penetration into human skin using microemulsions," J. Controlled Release 46(3):279-285 (1997). cited by applicant . Sen et al., "The interferon system: a bird's eye view of its biochemistry," J. Biol. Chem. 267(8):5017-5020 (Mar. 1992). cited by applicant . Shiffman et al., "A decline in HCV-RNA level during interferon or interferon/ribavirin therapy in patients with virologic nonresponse is associated with an improvement in hepatic histology," Prog. Abstr. 50th Annu. Mtg. Postgrad. Courses Am. Assn. Study Liver Dis., Dallas, TX (Nov. 5-9, 1999) (Abstract 567). cited by applicant . Shima et al., "Serum total bile acid level as a sensitive indicator of hepatic histological improvement in chronic hepatitis C patients responding to interferon treatment," J. Gastroenterol. Hepato). 15(3):294-299 (Mar. 2000). cited by applicant . Shiratori et al., "Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy," Ann. Int. Med. 132(7):517-524 (Apr. 2000). cited by applicant . Simon et al., "A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon .beta.1a," Neurology 55(2):185-192 (Jul. 2000). cited by applicant . Sparks et al., "Lipoprotein alterations in 10- and 20-week-old Zucker diabetic fatty rats: hyperinsulinemic versus insulinopenic hyperglycemia," Metabolism, 47(11):1315-1324 (1998). cited by applicant . Sulkowski et al., "Pegylated Interferon Alfa-2A (Pegasys.TM.) and Ribavirin Combination Therapy for Chronic Hepatitis C: A Phase II Open-Label Study," Gastroenterology 118(4, Supp. 2) (2000) (Abstract 236). cited by applicant . Sulkowski et al., "Peginterferon-.alpha.-2a (40kD) and ribavirin in patients with chronic hepatitis C: a phase II open label study," Biodrugs 16(2):105-109 (2002). cited by applicant . Talpaz et al., "Phase I study of polyethylene glycol formulation of interferon alpha-2B (Schering 54031) in Philadelphia chromosome-positive chronic myelogenous leukemia," Blood 98(6):1708-1713 (2001). cited by applicant . Talsania et al., "Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice," Endocrinology 146(9):3748-56 ( Sep. 2005). cited by applicant . Tanaka et al., "Effect of interferon therapy on the incidence of hepatocellular carcinoma and mortality of patients with chronic hepatitis C: a retrospective cohort study of 738 patients," Int. J. Cancer 87(5):741-749 (Sep. 2000). cited by applicant . Tong et al., "Prediction of response during interferon .alpha. 2b therapy in chronic hepatitis C patients using viral and biochemical characteristics: a comparison," Hepatology 26(6):1640-01645 (Dec. 1997). cited by applicant . Touza Rey et al., "The clinical response to interferon-.gamma. in a patient with chronic granulomatous disease and brain abscesses due to Aspergillus fumigatus," Ann. Med. Int. 17(2):86-87 (Feb. 2000). cited by applicant . Trudeau et al., "A phase I study of recombinant human interferon alpha-2b combined with 5-fluorouracil and cisplatin in patients with advanced cancer," Cancer Chemother. Pharmacol. 35(6):496-500 (1995). cited by applicant . Tseng et al., "Glucose-dependent insulinotropic peptide: structure of the precursor and tissue-specific expression in rat," PNAS USA, 90(5):1992-1996 (1993). cited by applicant . Tsung et al., "Preparation and Stabilization of Heparin/Gelatin Complex Coacervate Microcapsules," J. Pharm. Sci. 86(5):603-7 (May 1997). cited by applicant . Unniappan et al., "Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY," Diabetologia; Clinical and Experimental Diabetes and Metabolism 49(8):1915-1923 (Jun. 27, 2006). cited by applicant . Yokes et al., "A phase I trial of concomitant chemoradiotherapy with cisplatin dose intensification and granulocyte-colony stimulating factor support for advanced malignancies of the chest," Cancer Chemother. Pharmacol. 35(4):304-312 (1995). cited by applicant . Vrabec, "Tympanic membrane perforations in the diabetic rat: a model of impaired wound healing," Otolaryngol. Head Neck Surg., 118(3 Pt. 1):304-308 (1998). cited by applicant . Wang et al., "Preferential interaction of .alpha.-tocopherol with phosphatidylcholines in mixed aqueous dispersions of phosphatidylcholine and phosphatidylethanolamine," Eur. J. Biochem. 267(21):6362-6368 (2000). cited by applicant . Wang et al., "Ripple phases induced by .alpha.-tocopherol in saturated diacylphosphatidylcholines," Archives of Biochem. & Biophys. 377(2):304-314 (2000). cited by applicant . Wang et al., "The distribution of .alpha.-tocopherol in mixed aqueous dispersions of phosphatidylcholine and phosphattidylethanolamine," Biochimica et Biophysica Acta-Biomembranes 1509(1-2):361-372 (2000). cited by applicant . Wang et al, "Parenteral formulations of proteins and peptides: stability and stabilizers," J. Parenter. Sci. Technol. 42(2S):S4-S26 (1988). cited by applicant . Weinstock-Guttman et al., "What is new in the treatment of multiple sclerosis?" Drugs 59(3):401-410 (Mar. 2000). cited by applicant . Weissmann et al., "The interferon genes," Prog. Nucleic Acid Res. Mol. Biol. 33:251-300 (1986). cited by applicant . Wright et al., "Preliminary experience with .alpha.-2b-interferon therapy of viral hepatitis in liver allograft recipients," Transplantation 53(1):121-123 (Jan. 1992). cited by applicant . Young et al., "Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta)," Diabetes, 48(5):1026-1034 (1999). cited by applicant . Younossi et al., "The role of amantadine, rimantadine, ursodeoxycholic acid, and NSAIDs, alone or in combination with .alpha. interferons, in the treatment of chronic hepatitis C," Semin. Liver Dis. 19(Supp. 1):95-102 (1999). cited by applicant . Yu et al., "Preparation, characterization, and in vivo evaluation of an oil suspension of a bovine growth hormone releasing factor analog," J. Pharm. Sci. 85(4):396-401 (1996). cited by applicant . Zeidner et al., "Treatment of FeLV-induced immunodeficiency syndrome (feLV-FAIDS) with controlled release capsular implantation of 2',3'-dideoxycytidine," Antivir. Res. 11(3):147-0160 (Apr. 1989). cited by applicant . Zein, "Interferons in the management of viral hepatitis," Cytokines Cell Mol. Ther. 4(4):229-241 (Dec. 1998). cited by applicant . Zeuzem et al., "Peginterferon Alfa-2a in Patients with Chronic Hepatitis C," New Engl J. Med. 343(23):1666-1672 (2000). cited by applicant . Zeuzem et al., "Hepatitis C virus dynamics in vivo: effect of ribavirin and interferon .alpha. on viral turnover," Hepatology 28(1):245-252 (Jul. 1998). cited by applicant . Zhang et al., "Report on Large Dosage Interferon to Treat 30 Cases of Viral Encephalitis," J. Clinical Pediatrics 14(2):83-84 (1996). cited by applicant . Zhang et al, "A new strategy for enhancing the stability of lyophilized protein: the effect of the reconstitution medium on keratinocyte growth factor," Pharm. Res. 12(10):1447-1452 (1995). cited by applicant . Zheng et al. "Therapeutic Effect of Interferon Varied Dose in Treating Virus Encephalitis," Beijing Med. J. 13(2):80-81 (1998). cited by applicant . Ziesche et al., "A preliminary study of long-term treatment with interferon .gamma.-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis," New Engl. J. Med. 341(17):1264-1269 (Oct. 1999). cited by applicant . Sanofi-Aventis U.S. LLC, Prescribing Information for Adlyxin.RTM. (Lixisenatide) Injection, for Subcutaneous Use, rev. Jul. 2016, 31 pages. cited by applicant . Amylin Pharmaceuticals, Inc., Prescribing Information for Byetta.RTM. (Exenatide) Injection, rev. Oct. 2009, 34 pages. cited by applicant . Astrazeneca Pharmaceuticals LP, Prescribing Information for Bydureon.RTM. (Exenatide Extended-Release for Injectable Suspension), rev. Mar. 2015, 60 pages. cited by applicant . Novo Nordisk A/S, Prescribing Information for Victoza.RTM. (Liraglut de [rDNA Origin] Injection), Solution for Subcutaneous Use, v. 1, Jan. 2010, 23 pages. cited by applicant . Glaxosmithkline LLC, Prescribing Information for Tanzeum.RTM. (Albiglutide) for Injection, for Subcutaneous Use, rev. Jun. 2014, 55 pages. cited by applicant . Eli Lilly & Company, Prescribing Information for Trulicity.RTM. (Dulaglutide) Injection, for Subcutaneous Use, rev. Mar. 2015, 19 pages. cited by applicant . Glumetza Brochure 2009, 13 Pages. cited by applicant . Erowid,"Introduction to the Federal Controlled Substance Analog Act" 2001, 4 pages. cited by applicant . Li et al. "Glucagon-Like Peptide-I Receptor Agonists Versus Insulin Glargine for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" in Current Therapeutic Research, vol. 71, No. 4, Aug. 2010. cited by applicant . Palmeri et al., "5-Fluorouracil and recombinant .alpha.-interferon-2a in the treatment of advanced colorectal carcinoma: a dose optimization study," J. Chemotherapy 2(5):327330 (Oct. 1990). cited by applicant . Akers, et al., "Formulation Design and Development of Parenteral Suspensions," Journal of Parenteral Science & Technology, 41(3): 88-96 (1987). cited by applicant . Alonso, et al., "Determinants of Release Rate of Tetanus Vaccine from Polyester Microspheres," Pharmaceutical Research, 10(7):945-953 (1993). cited by applicant . Beck, et al., "Poly(dl-lactide-co-glycolide)/norethisterone microcapsules: An injectable biodegradable contraceptive," Biology of Reproduction, 28(1): 186-195 (1983). cited by applicant . Bodmeier and McGinity, "Solvent selection in the preparation of poly(dl-lactide) microspheres prepared by the solvent evaporation method," International Journal of Pharmaceutics, 43(1-2): 179-186 (Apr. 1988). cited by applicant . Cha and Pitt, "A one-week subdermal delivery system for I-methadone based on biodegradable microcapsules," Journal of Controlled Release, 7: 69-78 (1988). cited by applicant . Cha and Pitt, "The acceleration of degradation-controlled drug delivery from polyester microspheres," Journal of Controlled Release, 8: 259-265 (1989). cited by applicant . Cohen, et al., "Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres," Pharmaceutical Research, 8(6): 713-720 (1991). cited by applicant . Conti, et al., "Use of polylactic acid for the preparation of microparticulate drug delivery systems," Journal of Microencapsulation, 9(2): 153-166 (1992). cited by applicant . Hodgman, et al., Eds., Handbook of Chemistry and Physics, 35th Edition, 1024-1025 (1953). cited by applicant . Jalil and Nixon, "Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: Problems associated with preparative techniques and release properties," Journal of Microencapsulation, 7(3): 297-325 (Jul.-Sep. 1990). cited by applicant . Lee and Timasheff, "The stabilization of proteins by sucrose," J. Biological Chem., 256(14): 7193-7201 (Jul. 1981). cited by applicant . Li, et al., "Prediction of solvent removal profile and effect on properties for peptide-loaded PLGA microspheres prepared by solvent extraction/evaporation method," Journal of Controlled Release, 37: 199-214 (1995). cited by applicant . Maa and Hsu, "Liquid-liquid emulsification by static mixers for use in microencapsulation," Journal of Microencapsulation, 13(4): 419-433 (Jul.-Aug. 1996). cited by applicant . Maulding, et al., "Biodegradable microcapsules: Acceleration of polymeric excipient hydrolytic rate by incorporation of a basic medicament," Journal of Controlled Release, 3: 103-117 (1986). cited by applicant . Mehta, et al.,"Peptide containing microspheres from low molecular weight and hydrophilic poly(d,l-lactide-co-glycolide)," Journal of Controlled Release, 41: 249-257 (1996). cited by applicant . Sah, et al., "A novel method of preparing PLGA microcapsules utilizing methylethyl ketone," Pharmaceutical Research, 13(3): 360-367 (1996). cited by applicant . Sato, et al., "Porous biodegradable microspheres for controlled drug delivery. I. Assessment of processing conditions and solvent removal techniques," Pharmaceutical Research, 5(1): 21-30 (1988). cited by applicant . Szayna, et al., "Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats," Endocrinology, 141(6): 1936-1941 (2000). cited by applicant . Thomasin, et al., "A contribution to overcoming the problem of residual solvents in biodegradable microspheres prepared by coacervation," Eur. J. Pharm. Biopharm., 42(1): 16-24 (1996). cited by applicant . Van Santbrink and Fauser, "Urinary follicle-stimulating hormone for normogonadotropic colomiphene-resistant anovulatory infertility: Prospective, randomized comparison between low dose step-up and step-down dose regimens," J. Clin. Endocrin. Metab., 82(11): 3597-3602 (1997). cited by applicant . Tracy et al., "Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheresin vivo and in vitro." Biomaterials. 20(11:): 1057-1062 (1999). cited by applicant . Ertl et al., "Poly (DL-lactide-co-glycolide) microspheres as carriers for peptide vaccines," Vaccine 14(9):879-885.(1996). cited by applicant . Thompson et al., "Biodegradable microspheres as a delivery system for rismorelin porcine, a porcine-growth-hormone-releasing hormone," Journal of Controlled Release 43(1):9-22 (1997). cited by applicant. |
Primary Examiner: Simmons; Ian
Assistant Examiner: Johnson; Karra S
Attorney, Agent or Firm: Moriarty; Gordon R. Trinque; Brian C. Lathrop Gage LLP
Description
FIG. 1 is a perspective view of our design for an implant placement guide;
FIG. 2 is a first side view of the design for the implant placement guide of FIG. 1;
FIG. 3 is a second side view of the design for the implant placement guide of FIG. 1;
FIG. 4 is a front view of the design for the implant placement guide of FIG. 1;
FIG. 5 is a back view of the design for the implant placement guide of FIG. 1;
FIG. 6 is a top view for the design for the implant placement guide of FIG. 1; and,
FIG. 7 is a bottom view for the design of the implant placement guide of FIG. 1.
* * * * *
References
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.