Vented emergency wound dressings
Shulman , et al.
U.S. patent number RE48,007 [Application Number 16/144,170] was granted by the patent office on 2020-05-26 for vented emergency wound dressings. This patent grant is currently assigned to Medical Devices, Inc.. The grantee listed for this patent is Medical Devices, Inc.. Invention is credited to Jahan Azziz, Stephen Shulman, Stuart Shulman.







| United States Patent | RE48,007 |
| Shulman , et al. | May 26, 2020 |
Vented emergency wound dressings
Abstract
A wound dressing provides a tenacious occlusive seal against the skin of a wearer, even in the presence of excessive blood or heavy perspiration. The preferred embodiment combines an adhesive backing layer with a hydrogel island providing superior hydrophilic gel adhesion. The product performs under extreme temperatures (i.e., 32-140.degree. F.), and may be used to hold other dressings in place. The preferred embodiments are entirely translucent, and include a large pull-tab for easy removal. Two dressings may be packaged in a pliable re-sealable protective pouch, thereby forming a portable "kit." Different "vented" embodiments are disclosed. A preferred structure includes a cover layer peripherally bonded to backing and hydrogel layers having a central aperture therethrough. The cover layer has one or more vent holes, and pressure is released through the central aperture and out the vent hole(s).
| Inventors: | Shulman; Stephen (Ann Arbor, MI), Shulman; Stuart (Ann Arbor, MI), Azziz; Jahan (Ann Arbor, MI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | Medical Devices, Inc. (Ann
Arbor, MI) |
||||||||||
| Family ID: | 1000004656630 | ||||||||||
| Appl. No.: | 16/144,170 | ||||||||||
| Filed: | September 27, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | Issue Date | ||
|---|---|---|---|---|---|
| 12748008 | Mar 26, 2010 | ||||
| 61915198 | Dec 12, 2013 | ||||
| 61163613 | Mar 26, 2009 | ||||
| Reissue of: | 14247884 | Apr 8, 2014 | 9452088 | Sep 27, 2016 | |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61F 13/0213 (20130101); A61F 13/0263 (20130101); A61F 13/0213 (20130101); A61F 2013/00676 (20130101); A61F 17/00 (20130101) |
| Current International Class: | A61F 13/00 (20060101); A61F 13/02 (20060101); A61F 17/00 (20060101) |
| Field of Search: | ;602/41,42,47,48,52,54,57-59 ;604/304-307,122 |
References Cited [Referenced By]
U.S. Patent Documents
| 3521632 | July 1970 | Graham |
| 4904247 | February 1990 | Therriault et al. |
| 4909244 | March 1990 | Quarfoot et al. |
| 4920158 | April 1990 | Murray et al. |
| 4948575 | August 1990 | Cole et al. |
| 5013769 | May 1991 | Murray et al. |
| 5052381 | October 1991 | Gilbert et al. |
| 5059424 | October 1991 | Cartmell et al. |
| 5076265 | December 1991 | Wokalek |
| 5106362 | April 1992 | Gilman |
| 5106629 | April 1992 | Cartmell et al. |
| 5154706 | October 1992 | Cartmell et al. |
| 5160322 | November 1992 | Scheremet et al. |
| 5195977 | March 1993 | Pollitt |
| 5204110 | April 1993 | Cartmell et al. |
| 5263922 | November 1993 | Sova et al. |
| 5423736 | June 1995 | Cartmell et al. |
| 5429589 | July 1995 | Cartmell et al. |
| 5447492 | September 1995 | Cartmell et al. |
| 5465735 | November 1995 | Patel |
| 5476443 | December 1995 | Cartmell et al. |
| 5478333 | December 1995 | Asherman, Jr. |
| 5480717 | January 1996 | Kundel |
| 5489262 | February 1996 | Cartmell et al. |
| 5501661 | March 1996 | Cartmell et al. |
| 5556375 | September 1996 | Ewall |
| 5603946 | February 1997 | Constantine |
| 5637080 | June 1997 | Geng |
| 5674346 | October 1997 | Kundel |
| 5674523 | October 1997 | Cartmell et al. |
| 5679371 | October 1997 | Tanihara et al. |
| 5762620 | June 1998 | Cartmell et al. |
| 5788682 | August 1998 | Maget |
| 5804213 | September 1998 | Rolf |
| 5846214 | December 1998 | Makuuchi et al. |
| 5902600 | May 1999 | Woller et al. |
| 5973221 | October 1999 | Collyer et al. |
| 5977428 | November 1999 | Bozigian et al. |
| 6040493 | March 2000 | Cooke et al. |
| 6406712 | June 2002 | Rolf |
| 6861067 | March 2005 | McGhee et al. |
| 7265256 | September 2007 | Artenstein |
| 7429687 | September 2008 | Kauth et al. |
| 7504549 | March 2009 | Castellani et al. |
| 7615674 | November 2009 | Asherman |
| 2001/0031370 | October 2001 | Kundel |
| 2003/0204174 | October 2003 | Cisko |
| 2004/0013715 | January 2004 | Wnek et al. |
| 2005/0214376 | September 2005 | Faure et al. |
| 2005/0256437 | November 2005 | Silcock et al. |
| 2006/0015053 | January 2006 | Crisp |
| 2006/0200063 | September 2006 | Munro et al. |
| 2006/0253079 | November 2006 | McDonough et al. |
| 2006/0264796 | November 2006 | Flick et al. |
| 2007/0082036 | April 2007 | Dixon |
| 2008/0009802 | January 2008 | Lambino et al. |
| 2008/0033377 | February 2008 | Kauth et al. |
| 2008/0064998 | March 2008 | Gregory et al. |
| 2008/0091152 | April 2008 | Asherman |
| 2008/0234726 | September 2008 | Biddle et al. |
| 2012/0078153 | March 2012 | Russell et al. |
Attorney, Agent or Firm: Posa; John G. Belzer PC
Parent Case Text
REFERENCE TO RELATED APPLICATIONS
This application claims priority from U.S. Provisional Patent Application Ser. No. 61/915,198, filed Dec. 12, 2013, and is also a continuation-in-part of U.S. patent application Ser. No. 12/748,008, filed Mar. 26, 2010, which claims priority from U.S. Provisional Patent Application Ser. No. 61/163,613, filed Mar. 26, 2009, the entire content of all of which is incorporated herein by reference.
Claims
We claim:
1. A wound dressing, comprising: a backing layer having a periphery, a front surface .[.with an adhesive layer adapted for patient contact.]. and a back surface .[.facing away from the patient.].; a .[.preformed.]. hydrogel pad having a periphery.Iadd., the hydrogel pad being .Iaddend.attached to the front surface of the backing layer, the hydrogel pad and backing layer both further including aligned central apertures; .[.the periphery of the hydrogel pad being less than that of the backing layer such that a ring of the adhesive layer remains exposed around the hydrogel pad;.]. a cover .[.peripherally.]. bonded directly to the back surface of the backing layer so as to create an expandable pocket between the cover and the back surface of the backing layer; wherein the cover includes at least one vent .[.hole through the cover.]. .Iadd.passage .Iaddend.such that pressure from the wound temporarily expands the pocket, enabling liquids or gasses from the wound to be vented through the apertures, through the pocket, and out the at least one vent .[.hole.]. .Iadd.passage .Iaddend.to atmosphere; and after the pressure is relieved or with negative pressure, the cover falls back onto the backing layer, thereby eliminating the pocket between the cover and the back surface of the backing layer and maintaining a seal around the wound.
2. The wound dressing of claim 1, wherein the backing layer with the hydrogel pad attached thereto are temporarily held against a .[.larger.]. release layer.
3. The wound dressing of claim 1, wherein the backing layer is a polyethylene tape.
4. The wound dressing of claim 1, further including .[.an adhesive-free tab.]. .Iadd.a pull-tab on the backing layer to assist in removing the wound dressing from the release layer.Iaddend..
5. The wound dressing of claim 1, further including a pouch to protect the dressing, thereby providing a portable kit.
.Iadd.6. The wound dressing of claim 1, having a shape that is circular..Iaddend.
.Iadd.7. The wound dressing of claim 1, having a shape that is rectangular..Iaddend.
.Iadd.8. The wound dressing of claim 1, having a width in the range of 3 to 6 inches..Iaddend.
.Iadd.9. The wound dressing of claim 1, having a length in the range of 4 to 8 inches..Iaddend.
.Iadd.10. A wound dressing, comprising: a backing layer having a periphery, a front surface adapted for patient contact and a back surface facing away from the patient; a hydrogel pad having a periphery attached to the front surface of the backing layer, the hydrogel pad and backing layer both further including aligned central apertures; the periphery of the hydrogel pad being less than that of the backing layer; a cover having an inner surface bonded directly to the back surface of the backing layer, with the periphery of the cover being larger that the aligned central apertures of the hydrogel pad and the backing layers, thereby forming a pocket between the back surface of the backing layer and the inner surface of the cover; wherein the pocket formed between the back surface of the backing layer and the inner surface of the cover communicates with at least one passage enabling liquids or gasses from the wound to be vented through the apertures and the pocket to atmosphere; and wherein, after the pressure is relieved or with negative pressure, the cover falls back onto the backing layer, thereby eliminating the pocket between the cover and the back surface of the backing layer and maintaining a seal around the wound..Iaddend.
.Iadd.11. The wound dressing of claim 10, wherein the backing layer with the hydrogel pad attached thereto are temporarily held against a release layer..Iaddend.
.Iadd.12. The wound dressing of claim 11, further including a pull-tab on the backing layer to assist in removing the wound dressing from the release layer..Iaddend.
.Iadd.13. The wound dressing of claim 10, further including a pouch to protect the dressing, thereby providing a portable kit..Iaddend.
.Iadd.14. The wound dressing of claim 10, wherein the wound dressing is circular..Iaddend.
.Iadd.15. The wound dressing of claim 10, wherein the wound dressing is rectangular..Iaddend.
.Iadd.16. The wound dressing of claim 10, having a width in the range of 3 to 6 inches..Iaddend.
.Iadd.17. The wound dressing of claim 10, having a length in the range of 4 to 8 inches..Iaddend.
Description
FIELD OF THE INVENTION
This invention relates generally to wound dressings and, in particular, to a wound dressing that provides a unique combination of a flexible backing material and hydrogel to provide a safe and effective patch for emergency situations, including battlefield applications.
BACKGROUND OF THE INVENTION
There are instances when an emergency wound dressing may be required not only to stop bleeding, but also to block the transfer of gasses to or from the lungs. Bullet wounds in battlefield situations represent one such need. Until now, however, there are few if any bandages that will remain affixed to a recipient given heat and moisture from weather, sweating, and so forth.
SUMMARY OF THE INVENTION
This invention is a wound dressing that uses a novel combination of materials to provide a tenacious occlusive seal, even in the presence of excessive blood or heavy perspiration. The preferred embodiment combines an adhesive backing layer with a hydrogel island providing superior hydrophilic gel adhesion. The product performs under extreme temperatures (i.e., 32-140.degree. F.), and may be used to hold other dressings in place. The preferred embodiments are entirely translucent, and include a large pull-tab for easy removal. One or two dressings may be packaged in a pliable re-sealable protective pouch.
Different "vented" embodiments are disclosed. A preferred structure includes a cover layer peripherally bonded to backing and hydrogel layers having a central aperture therethrough. The cover layer has one or more vent holes, and pressure is released through the central aperture and out the vent hole(s).
BRIEF DESCRIPTION OF THE DRAWINGS
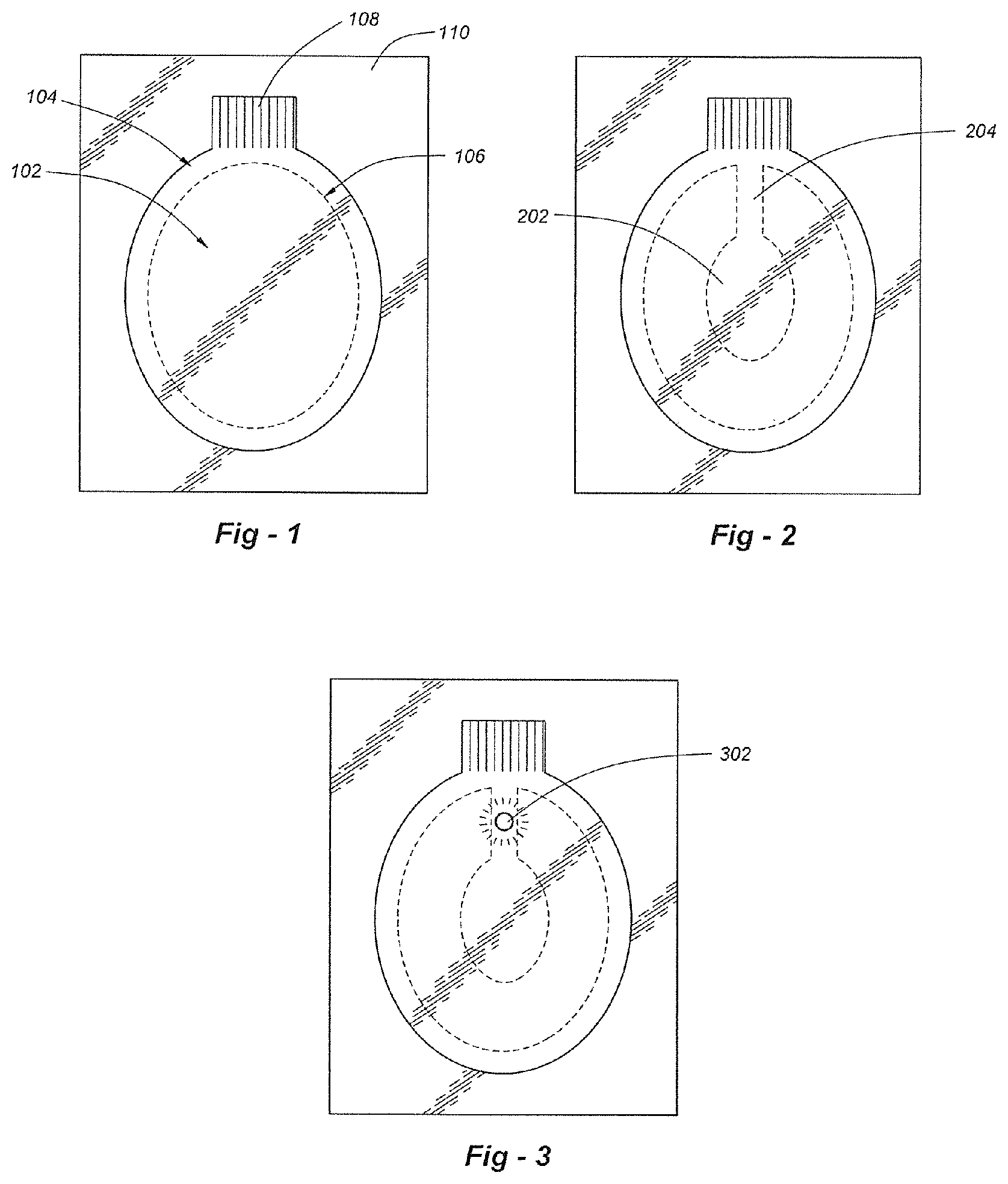
FIG. 1 is a plan view drawing that illustrates a preferred embodiment of the invention;
FIG. 2 is a drawing that depicts a vented structure;
FIG. 3 is a drawing that depicts a vented structure with an active status indicator;
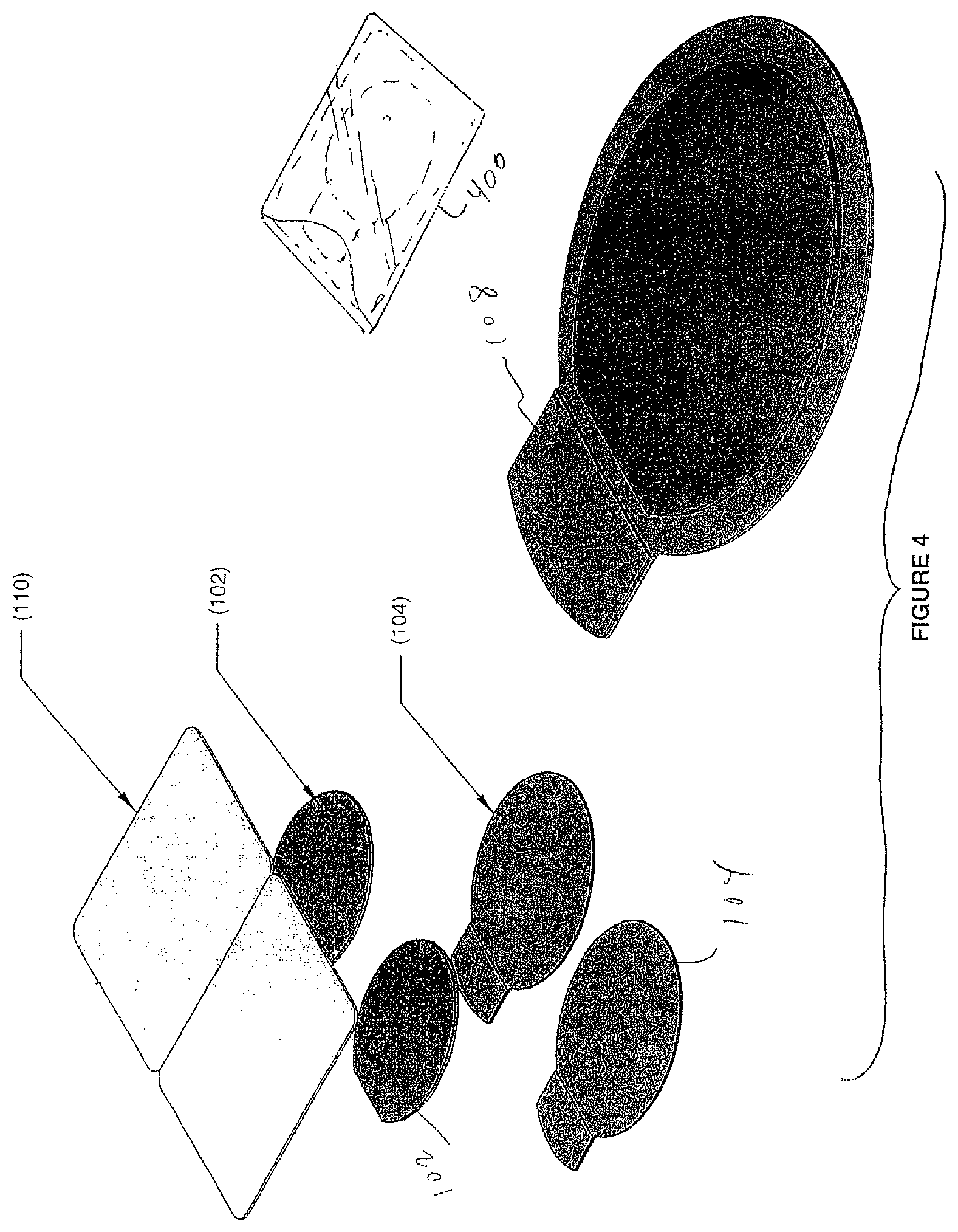
FIG. 4 is a partially exploded view of a wound dressing assembly according to the invention;
FIG. 5 is a plan view drawing that shows one set of dimensions.
FIG. 6 is an exploded view drawing that illustrates a vented embodiment of the invention seen from a bottom perspective;
FIG. 7 is a not-to-scale cross section depicting the way in which the venting structure operates;
FIG. 8 is a detail drawing with applicable dimensions;
FIG. 9 shows details of a pouch structure;
FIG. 10 depicts an embodiment of the invention utilizing four vent holes; and
FIG. 11 illustrates an optional valve structure.
DETAILED DESCRIPTION OF THE INVENTION
This invention is a wound dressing that uses a novel combination of materials to provide a tenacious occlusive seal, even in the presence of excessive blood or heavy perspiration. FIG. 1 is a plan view drawing that illustrates a preferred embodiment of the invention, including a backing layer 104 and a pre-formed hydrogel pad 102. The backing layer 104 is preferably a flexible polyethylene "tape" (i.e., 3.0 mil) having a hypoallergenic pressure-sensitive water-resistant adhesive such as an acrylic adhesive 500. The hydrogel pad 102 may be obtained from Katecho, Inc. of Des Moines, Iowa (product code KM-10). The hydrogel material may be obtained from Katecho, Inc. of Des Moines, Iowa (product code KM-10). The backing material may be obtained from 3M Corp. of St. Paul, Minn. Other vendors are possible.
The hydrogel pad 102 preferably has a periphery 106 spaced apart from the periphery of the backing layer 104 in all dimensions to expose the adhesive layer. An adhesive-free tab 108, which may be embossed, is used to release the pad from a release liner 110. The release liner 110 extends beyond all sides of the backing layer 104, as shown in FIGS. 1-5. The release liner may be a 3.0 mil PET layer. In terms of dimensions, the dressing has a preferred width on the order of 3 to 6 inches and a preferred length on the order of 4 to 8 inches. Other forms besides ovals may be used, including circles, squares and rectangles. Materials other than polyethylene may be used for the backing layer 104 so long as the adhesive and combined system is sufficiently tenacious in terms of adherence to the skin.
FIG. 4 is a partially exploded view of a wound dressing assembly according to the invention, and FIG. 5 is a plan view drawing that shows one set of dimensions. Two systems are shown, enabling the dressings to be folded against one another and placed in a pouch 400 which may be re-sealable, thereby forming a dressing/carrier "kit."
On occasion, the wound dressing may need to vent pressure built up from a chest cavity, for example. To address this, one or more vents such as 204 may be provided as shown in FIG. 2. As pressure builds around a wound in region 202, it will be released through vent 204. As a further alternative, a passive or active status sensor may be used to indicate a user condition or to show that pressure release has occurred. As shown in FIG. 3, such a monitor 302 may be passive, using dyes or other materials which change color in the presence of oxygen or other gasses, for example. The detector may be active, for example, using an electronic pressure sensor, an acoustic sensor (to detect lung sound) or resistive sensor (to detect perspiration or breathing) and a visual indicator such as an LED. The light may have different colors to convey status (i.e., green =OK; yellow =concern; red =emergency).
In all embodiments, beneficial or therapeutic substances may be added to the hydrogel layer. For example, a clotting agent such as calcium carbonate may be added to aid in clotting, and/or an antimicrobial such as chlorhexidine digluconate may be used. Such substances may be in the range of 1-5% or thereabout.
FIG. 6 is an exploded view drawing that illustrates an alternative vented embodiment of the invention seen from a bottom perspective. The article comprises a backing layer shown at 604 and the hydrogel pad is shown at 602. As with other embodiments disclosed herein, the backing layer is preferably a flexible polyethylene "tape" (i.e., 3.0 mil) having a hypoallergenic pressure-sensitive adhesive. The hydrogel material may be obtained from Katecho, Inc. of Des Moines, Iowa (product code KM-10). The backing material may be obtained from 3M Corp. of St. Paul, Minn. as Part No. 1526. Other material and different vendors are possible.
In the embodiment of FIG. 6, the entire bottom surface of the backing layer 604 is coated with an adhesive shown by the hatching, and the hydrogel pad 602, which is preformed into the shape depicted, is adhered to the backing layer using the adhesive layer, the hydrogel itself, or both. Once adhered, the hydrogel forms an island having a periphery spaced apart from the periphery of the backing layer 104 in all dimensions to expose the adhesive layer. An adhesive-free tab 608 may be provided to release the dressing from a release liner 610. The release liner may be a 3.0 mil PET layer.
In terms of dimensions, the dressing of FIGS. 6, 8 has a preferred width on the order of 3 to 6 inches and a preferred length on the order of 4 to 8 inches. Other shapes besides ovals may be used, including circles, squares and rectangles. Materials other than polyethylene may be used for the backing layer 604 so long as the adhesive and combined system is sufficiently tenacious. A set of applicable dimensions are provided in FIG. 8. Two dressings may be folded against one another and placed in a pouch shown in FIG. 9 which may be re-sealable, thereby forming a dressing/carrier "kit"
Returning to FIG. 6, both the hydrogel layer 602 and backing layer 604 include a central aperture 612 which may be round with a preferred diameter in the range of 0.5 to 1.5 inches. Other dimensions and shapes including oval may be used. On the outer side of the backing layer, facing away from the recipient, there is attached a cover 614 having vent holes 616, 618. The cover 614 is peripherally bonded to the outer surface of the backing layer along line 620. The cover 614 may be the same material as the backing material, 3M No. 1526, and may have roughly the same outer dimensions as the hydrogel pad. The cover 614 may be attached to the backing layer with or without an adhesive, using thermal welding, for example. The Any appropriate peripheral adhesive may be used, including UV-cured products.
FIG. 7 is a not-to-scale cross section depicting the way in which the venting structure operates. As can be seen, if pressure builds up from wound 701 on patient 700, the gasses (or liquids) can flow through aperture 612 and out to atmosphere through vent holes 616, 618. When this happens, the entire cover 614 may pull slightly away from the backing material 604, temporarily creating a pocket 703 that is depressurized through the vent holes. After the pressure is relieved or with negative pressure, the cover 614 will typically fall back onto backing layer 604, thereby maintaining a seal around the wound.
FIG. 10 illustrates a preferred embodiment of the invention using four vent holes 1102, 1104, 1106, 1108 and dimensions. As a further embodiment, as opposed to an open central aperture through the hydrogel and backing layers leading to a pocket formed with the cover layer, a one-way bicuspid or tricuspid valve of the type shown in FIG. 11. The apex of the valve would be oriented away from the recipient, which would open when pressurized and close following pressurization to maintain cleanliness.
* * * * *
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.