Manufacturing Method for Protein Crimped Staple
Sato; Akito ; et al.
U.S. patent application number 16/965661 was filed with the patent office on 2021-02-11 for manufacturing method for protein crimped staple. This patent application is currently assigned to Spiber Inc.. The applicant listed for this patent is Spiber Inc.. Invention is credited to Yunosuke Abe, Akito Sato.
| Application Number | 20210040649 16/965661 |
| Document ID | / |
| Family ID | 1000005223715 |
| Filed Date | 2021-02-11 |






| United States Patent Application | 20210040649 |
| Kind Code | A1 |
| Sato; Akito ; et al. | February 11, 2021 |
Manufacturing Method for Protein Crimped Staple
Abstract
An object of the present invention is to provide a method for efficiently manufacturing a protein crimped staple from protein filaments at a low cost. A manufacturing method for a protein crimped staple according to the present invention includes: a) preparing an artificial fibroin filament containing a modified fibroin; b) cutting the artificial fibroin filament to obtain an artificial fibroin staple; and c) performing crimping by bringing the artificial fibroin filament into contact with an aqueous medium to crimp the artificial fibroin filament before the cutting or bringing the artificial fibroin staple into contact with an aqueous medium to crimp the artificial fibroin staple after the cutting.
| Inventors: | Sato; Akito; (Tsuruoka-shi, Yamagata, JP) ; Abe; Yunosuke; (Tsuruoka-shi, Yamagata, JP) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | Spiber Inc. Tsuruoka-shi, Yamagata JP |
||||||||||
| Family ID: | 1000005223715 | ||||||||||
| Appl. No.: | 16/965661 | ||||||||||
| Filed: | January 31, 2019 | ||||||||||
| PCT Filed: | January 31, 2019 | ||||||||||
| PCT NO: | PCT/JP2019/003478 | ||||||||||
| 371 Date: | July 29, 2020 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | D02G 3/24 20130101; D06M 11/05 20130101; D01F 4/02 20130101 |
| International Class: | D01F 4/02 20060101 D01F004/02; D02G 3/24 20060101 D02G003/24; D06M 11/05 20060101 D06M011/05 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Jan 31, 2018 | JP | 2018-015122 |
Claims
1. A manufacturing method for a protein crimped staple, comprising: a) preparing an artificial fibroin filament containing a modified fibroin; b) cutting the artificial fibroin filament to obtain an artificial fibroin staple; and c) performing crimping by bringing the artificial fibroin filament into contact with an aqueous medium to crimp the artificial fibroin filament before the cutting or bringing the artificial fibroin staple into contact with an aqueous medium to crimp the artificial fibroin staple after the cutting.
2. The manufacturing method for a protein crimped staple according to claim 1, wherein a shrinkage rate after drying of the artificial fibroin filament defined by the following expression is more than 7%, shrinkage rate after drying={1-(length of artificial fibroin filament after being brought into contact with aqueous medium and subsequently dried/length of artificial fibroin filament before being brought into contact with aqueous medium)}.times.100 (%).
3. The manufacturing method for a protein crimped staple according to claim 1, wherein a shrinkage rate when wetted of the artificial fibroin filament defined by the following expression is 2% or more, shrinkage rate when wetted={1-(length of artificial fibroin filament when wetted by being brought into contact with aqueous medium/length of artificial fibroin filament after being spun and before being brought into contact with aqueous medium)}.times.100 (%).
4. The manufacturing method for a protein crimped staple according to claim 1, wherein the modified fibroin is a modified spider silk fibroin, and the artificial fibroin filament is an artificial spider silk fibroin filament.
5. The manufacturing method for a protein crimped staple according to claim 1, wherein the aqueous medium used in the performing crimping is a liquid or gas containing water and having a temperature of 10.degree. C. to 230.degree. C.
6. The manufacturing method for a protein crimped staple according to claim 1, wherein the performing crimping further includes performing drying after the bringing the artificial fibroin filament or the artificial fibroin staple into contact with the aqueous medium.
7. The manufacturing method for a protein crimped staple according to claim 1, wherein the aqueous medium used in the performing crimping contains a volatile solvent.
Description
TECHNICAL FIELD
[0001] The present invention relates to a manufacturing method for a protein crimped staple and particularly relates to a manufacturing method for a crimped staple of an artificial fibroin including a modified fibroin.
BACKGROUND ART
[0002] A protein fiber, unlike a synthetic fiber, has biodegradability and low energy consumption for production and processing, and thus demands for various fields are expected to increase in response to the recent increase in environmental consciousness.
[0003] As the natural protein fiber, a filament such as silk and a staple such as wool are known. The former has a supple texture, and the latter has a soft feel and a heat retaining property, each of which has own characteristics thereof.
[0004] Recently, attempts have been made to process a protein fiber and apply the protein fiber to a wider range of applications. For example, in Patent Literatures 1 and 2, a method for manufacturing a long-fiber nonwoven fabric or a long-fiber crimped yarn by crimping the natural silk filament has been proposed. Further, the manufacturing of a protein crimped staple from protein filaments and obtaining spun yarn, nonwoven fabric, and the like using the protein crimped staple have also been studied in some fields.
[0005] As a method for obtaining a protein crimped staple from protein filaments, for example, a method of cutting a silk crimped filament crimped by the crimping processing method disclosed in the above patent literatures can be considered.
CITATION LIST
Patent Literature
[0006] [Patent Literature 1] Japanese Unexamined Patent Publication No. 2006-207069
[0007] [Patent Literature 2] Japanese Unexamined Patent Publication No. H9-119033
SUMMARY OF INVENTION
Technical Problem
[0008] However, since in the crimping method described in Patent Literature 1, a protein filament is crimped by a mechanical processing method such as an indentation method, in a case where a protein crimped staple is manufactured using this crimping method, there have been problems that a dedicated crimping device is required and the processing cost is high. Further, in the crimping method described in Patent Literature 2, it is necessary to perform a pretreatment for imparting a non-twisted latent crimping property to the natural silk prior to the crimping process. Therefore, in a case where this crimping method is used for manufacturing the protein crimped staple, there have been problems that the number of processes is increased and the productivity is inevitably decreased.
[0009] The present invention has been made in consideration of the above problems, and an object of the present invention is to provide a method for efficiently manufacturing a protein crimped staple from protein filaments at low cost.
Solution to Problem
[0010] The present invention relates to, for example, each of the following inventions.
[0011] [1] A manufacturing method for a protein crimped staple, including:
[0012] a) preparing an artificial fibroin filament containing a modified fibroin;
[0013] b) cutting the artificial fibroin filament to obtain an artificial fibroin staple; and
[0014] c) performing crimping by bringing the artificial fibroin filament into contact with an aqueous medium to crimp the artificial fibroin filament before the cutting or bringing the artificial fibroin staple into contact with an aqueous medium to crimp the artificial fibroin staple after the cutting.
[0015] [2] The manufacturing method for a protein crimped staple [1], in which a shrinkage rate after drying of the artificial fibroin filament defined by the following expression is more than 7%.
Shrinkage rate after drying={1-(length of artificial fibroin filament after being brought into contact with aqueous medium and subsequently dried/length of artificial fibroin filament before being brought into contact with aqueous medium)}.times.100 (%).
[0016] [3] The manufacturing method for a protein crimped staple according to [1] or [2], in which a shrinkage rate when wetted of the artificial fibroin filament defined by the following expression is 2% or more.
Shrinkage rate when wetted={1-(length of artificial fibroin filament when wetted by being brought into contact with aqueous medium/length of artificial fibroin filament before being brought into contact with aqueous medium)}.times.100 (%).
[0017] [4] The manufacturing method for a protein crimped staple according to any one of [1] to [3], in which the modified fibroin is a modified spider silk fibroin, and the artificial fibroin filament is an artificial spider silk fibroin filament.
[0018] [5] The manufacturing method for a protein crimped staple according to any one of [1] to [4], in which the aqueous medium used in the performing crimping is a liquid or gas containing water and having a temperature of 10.degree. C. to 230.degree. C.
[0019] [6] The manufacturing method for a protein crimped staple according to any one of [1] to [5], in which the performing crimping further includes performing drying after the bringing the artificial fibroin filament or the artificial fibroin staple into contact with the aqueous medium.
[0020] [7] The manufacturing method for a protein crimped staple according to any one of [1] to [6], in which the aqueous medium used in the performing crimping contains a volatile solvent.
Advantageous Effects of Invention
[0021] In the manufacturing method for a protein crimped staple of the present invention, by adopting a simple and unique crimping process of simply bring a raw material protein fiber into contact with an aqueous medium without using a dedicated crimping device, a protein crimped staple can be easily and efficiently manufactured at low cost.
BRIEF DESCRIPTION OF DRAWINGS
[0022] FIG. 1 is a schematic diagram illustrating one example of a domain sequence of a modified fibroin.
[0023] FIG. 2 is a schematic diagram illustrating one example of a domain sequence of a modified fibroin.
[0024] FIG. 3 is a schematic diagram illustrating one example of a domain sequence of a modified fibroin.
[0025] FIG. 4 is an explanatory view schematically illustrating one example of a spinning device for manufacturing a protein filament.
[0026] FIG. 5 is a photograph of a protein crimped staple obtained in Example 1.
[0027] FIG. 6 is another photograph of a protein crimped staple obtained in Example 1.
[0028] FIG. 7 is a photograph of a protein uncrimped staple obtained in Comparative Example.
DESCRIPTION OF EMBODIMENTS
[0029] A manufacturing method for a protein crimped staple according to one aspect of the present invention includes a process a, a process b, and a process c, each of which will be described below, and the process b and the process c are in no particular order. That is, the method may be performed in an order of the process a, the process b, and the process c, or in an order of the process a, the process c, and the process b.
[0030] <Process a>
[0031] The process is a process of preparing an artificial fibroin filament containing a modified fibroin. Here, the filament (also referred to as "long fiber") and the staple (also referred to as "short fiber") are obvious to those skilled in the art.
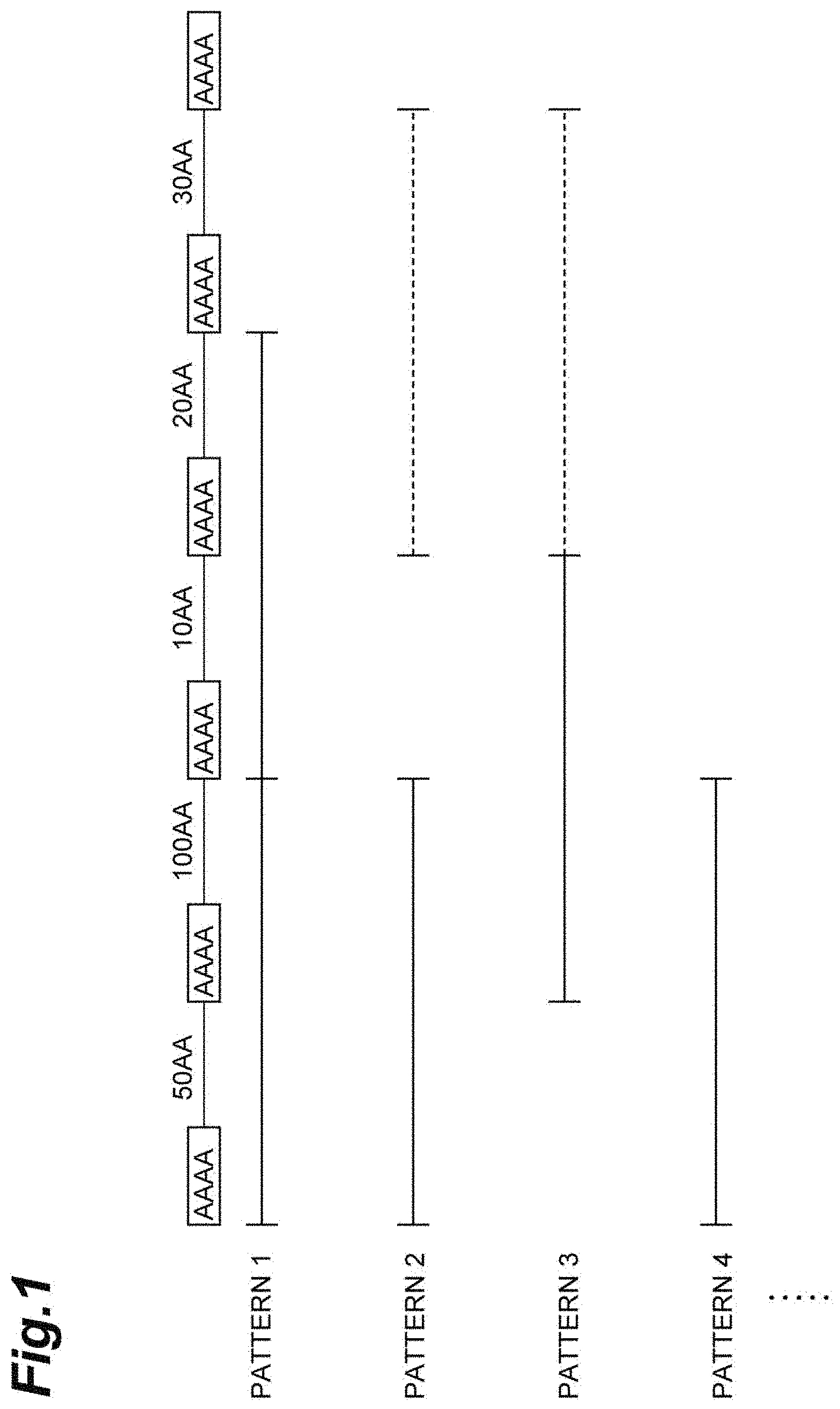
[0032] (Modified Fibroin)
[0033] The modified fibroin according to the present embodiment is a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif. In the modified fibroin, an amino acid sequence (an N-terminal sequence and a C-terminal sequence) may be further added to either or both of the N-terminal side and the C-terminal side of the domain sequence. The
[0034] N-terminal sequence and the C-terminal sequence, although not limited thereto, are typically regions that do not have repetitions of amino acid motifs characteristic of fibroin and consist of amino acids of about 100 residues.
[0035] The term "modified fibroin" in the present specification means an artificially produced fibroin (an artificial fibroin). The modified fibroin may be a fibroin in which the domain sequence is different from the amino acid sequence of a fibroin derived from the natural fibroin or may be the same as the amino acid sequence of a fibroin derived from the natural fibroin. The "fibroin derived from the natural fibroin" referred to in the present specification is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif
[0036] As the "modified fibroin", an amino acid sequence of a fibroin derived from the natural fibroin may be directly used, a fibroin whose amino acid sequence has been modified based on an amino acid sequence of a fibroin derived from the natural fibroin (for example, a fibroin whose amino acid sequence has been modified by altering a cloned gene sequence of a fibroin derived from the natural fibroin) may be used, or a fibroin artificially designed and synthesized independently of a fibroin derived from the natural fibroin (for example, a fibroin having a desired amino acid sequence by chemically synthesizing a nucleic acid encoding the designed amino acid sequence) may be used, as long as it has the amino acid sequence specified in the present embodiment.
[0037] The term "domain sequence" in the present specification refers to an amino acid sequence which produces a crystalline region (typically, corresponds to (A).sub.n motif of an amino acid sequence) and an amorphous region (typically, corresponds to REP of an amino acid sequence) peculiar to fibroin and means an amino acid sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif Here, the (A).sub.n motif represents an amino acid sequence mainly including alanine residues, and the number of amino acid residues in the (A).sub.n motif is 2 to 27. The number of amino acid residues in the (A).sub.n motif may be an integer of 2 to 20, 4 to 27, 4 to 20, 8 to 20, 10 to 20, 4 to 16, 8 to 16, or 10 to 16. Further, the proportion of the number of alanine residues with respect to the total number of amino acid residues in the (A).sub.n motif may be 40% or more, 60% or more, 70% or more, 80% or more, 83% or more, 85% or more, 86% or more, 90% or more, 95% or more, or 100% (meaning that the (A).sub.n motif is composed of only alanine residues). In a plurality of (A).sub.n motifs present in the domain sequence, at least seven of the (A).sub.n motif may be composed of only alanine residues. REP represents an amino acid sequence composed of 2 to 200 amino acid residues. The REP may represent an amino acid sequence composed of 10 to 200 amino acid residues. m represents an integer of 2 to 300 and may be an integer of 10 to 300. The plurality of (A).sub.n motifs may have the same amino acid sequence or amino acid sequences different from each other. The plurality of REPs may have the same amino acid sequence or amino acid sequences different from each other.
[0038] The modified fibroin can be obtained, for example, by carrying out the modification of an amino acid sequence equivalent to the substitution, deletion, insertion and/or addition of one or a plurality of amino acid residues with respect to, for example, a cloned gene sequence of a fibroin derived from the natural fibroin. The substitution, deletion, insertion, and/or addition of an amino acid residue may be carried out by methods well known to those skilled in the art, such as site-directed mutagenesis. Specifically, the modifications may be carried out by methods described in literature such as Nucleic Acid Res. 10, 6487 (1982) and Methods in Enzymology, 100, 448 (1983).
[0039] The fibroin derived from the natural fibroin is a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif, and specifically, for example, a fibroin produced by insects or spiders.
[0040] Examples of the fibroin produced by insects include silk proteins produced by silkworms such as Bombyx mori, Bombyx mandarina, Antheraea yamamai, Anteraea pernyi, Eriogyna pyretorum, Philosamia Cynthia ricini, Samia cynthia, Caligula japonica, Antheraea mylitta, and Antheraea assama; and hornet silk proteins discharged by larvae of Vespa simillima xanthoptera.
[0041] A more specific example of the fibroin produced by insects includes a silkworm fibroin L chain (GenBank Accession No. M76430 (base sequence), AAA27840.1 (amino acid sequence)).
[0042] Examples of the fibroin produced by spiders include spider silk proteins produced by spiders belonging to the genus Araneus such as i Araneus ventricosus, Araneus diadematus, Araneus pinguis, Araneus pentagrammicus and Araneus nojimai, spiders belonging to the genus Neoscona such as Neoscona scylla, Neoscona nautica, Neoscona adianta and Neoscona scylloides, spiders belonging to the genus Pronus such as Pronous minutes, spiders belonging to the genus Cyrtarachne such as Cyrtarachne bufo and Cyrtarachne inaequalis, spiders belonging to the genus Gasteracantha such as Gasteracantha kuhli and Gasteracantha mammosa, spiders belonging to the genus Ordgarius such as Ordgarius hobsoni and Ordgarius sexspinosus, spiders belonging to the genus Argiope such as Argiope amoena, Argiope minuta and Argiope bruennich, spiders belonging to the genus Arachnura such as Arachnura logio, spiders belonging to the genus Acusilas such as Acusilas coccineus, spiders belonging to the genus Cyrtophora such as Cyrtophora moluccensis, Cyrtophora exanthematica and Cyrtophora unicolor, spiders belonging to the genus Poltys such as Poltys illepidus, spiders belonging to the genus Cyclosa such as Cyclosa octotuberculata, Cyclosa sedeculata, Cyclosa vallata and Cyclosa atrata, and spiders belonging to the genus Chorizopes such as Chorizopes nipponicus; and spider silk proteins produced by spiders belonging to the genus Tetragnatha such as Tetragnatha praedonia, Tetragnatha maxillosa, Tetragnatha extensa and Tetragnatha squamata, spiders belonging to the genus Leucauge such as Leucauge magnifica, Leucauge blanda and Leucauge subblanda, spiders belonging to the genus Nephila such as Nephila clavata and Nephila pilipes, spiders belonging to the genus Menosira such as Menosira ornata, spiders belonging to the genus Dyschiriognatha such as Dyschiriognatha tenera, spiders belonging to the genus Latrodectus such as Latrodectus mactans, Latrodectus hasseltii, Latrodectus geometricus and Latrodectus tredecimguttatus, and spiders belonging to the family Tetragnathidae such as spiders belonging to the genus Euprosthenops. Examples of spider silk proteins include traction yarn proteins such as MaSp (MaSp1 and MaSp2) and ADF (ADF3 and ADF4), and MiSp (MiSp1 and MiSp2).
[0043] More specific examples of the spider silk protein produced by spiders include fibroin-3 (adf-3) [derived from Araneus diadematus] (GenBank Accession No. AAC47010 (amino acid sequence), U47855 (base sequence)), fibroin-4 (adf-4) [derived from Araneus diadematus] (GenBank Accession No. AAC47011 (amino acid sequence), U47856 (base sequence)), dragline silk protein spidroin 1 [derived from Nephila clavipes] (GenBank Accession No. AAC04504 (amino acid sequence), U37520 (base sequence)), major ampullate spidroin 1 [derived from Latrodectus hesperus] (GenBank Accession No. ABR68856 (amino acid sequence), EF595246 (base sequence)), dragline silk protein spidroin 2 [derived from Nephila clavata] (GenBank Accession No. AAL32472 (amino acid sequence), AF441245 (base sequence)), major ampullate spidroin 1 [derived from Euprosthenops australis] (GenBank Accession No. CAJ00428 (amino acid sequence), AJ973155 (base sequence)) and major ampullate spidroin 2 [Euprosthenops australis] (GenBank Accession No. CAM32249.1 (amino acid sequence), AM490169 (base sequence)), minor ampullate silk protein 1 [Nephila clavipes] (GenBank Accession No. AAC14589.1 (amino acid sequence), minor ampullate silk protein 2 [Nephila clavipes] (GenBank Accession No. AAC14591.1 (amino acid sequence)), and minor ampullate spidroin-like protein [Nephilengys cruentata] (GenBank Accession No. ABR37278.1 (amino acid sequence)).
[0044] As a further specified example of the fibroin derived from the natural fibroin, a fibroin whose sequence information is registered in NCBI GenBank may be mentioned. For example, sequences thereof may be confirmed by extracting sequences in which spidroin, ampullate, fibroin, "silk and polypeptide", or "silk and protein" is described as a keyword in DEFINITION among sequences containing INV as DIVISION in sequence information registered in NCBI GenBank, sequences in which a specific character string of a product is described from CDS, or sequences in which a specific character string is described from SOURCE to TISSUE TYPE.
[0045] The modified fibroin may be a modified silk fibroin (a modified silk protein obtained by modifying an amino acid sequence of a silk protein produced by silkworm), and a modified spider silk fibroin (a modified spider silk protein obtained by modifying an amino acid sequence of a spider silk protein produced by spiders). Among them, a modified spider silk fibroin is preferably used.
[0046] The specific examples of the modified fibroin include a modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider, a modified fibroin having a reduced content of the glycine residue, a modified fibroin having a reduced content of the (A).sub.n motif, and a modified fibroin having a reduced content of the glycine residue and a reduced content of the (A).sub.n motif
[0047] As the modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider, a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m is mentioned. In the modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider, the number of amino acid residues of the (A).sub.n motif is preferably an integer of 3 to 20, more preferably an integer of 4 to 20, still more preferably an integer of 8 to 20, even more preferably an integer of 10 to 20, even further more preferably an integer of 4 to 16, particularly preferably an integer of 8 to 16, and most preferably an integer of 10 to 16. In the modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider, the number of amino acid residues constituting REP in Formula 1 is preferably 10 to 200 residues, more preferably 10 to 150 residues, and still more preferably 20 to 100 residues, and even more preferably 20 to 75 residues. In the modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider, the number of residues in Formula 1: [(A).sub.n motif--REP].sub.m is preferably 40% or more, more preferably 60% or more, and still more preferably 70% or more with respect to the total number of amino acid residues.
[0048] The modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider may be a polypeptide including an amino acid sequence unit represented by Formula 1: [(A).sub.n motif--REP].sub.m, and being an amino acid sequence in which the C-terminal sequence has the amino acid sequence set forth in any of SEQ ID NOs: 14 to 16 or an amino acid sequence in which the C-terminal sequence has an amino acid sequence having 90% or more homology with the amino acid sequence set forth in any of SEQ ID NOs: 14 to 16.
[0049] The amino acid sequence set forth in SEQ ID NO: 14 is identical to the amino acid sequence consisting of 50 amino acid residues at the C-terminal of the amino acid sequence of ADF3 (GI: 1263287, NCBI). The amino acid sequence set forth in SEQ ID NO: 15 is identical to the amino acid sequence obtained by removing 20 residues from the C-terminal of the amino acid sequence set forth in SEQ ID NO: 14. The amino acid sequence set forth in SEQ ID NO: 16 is identical to the amino acid sequence obtained by removing 29 residues from the C-terminal of the amino acid sequence set forth in SEQ ID NO: 14.
[0050] More specific examples of the modified fibroin derived from a large spinal canal thread protein produced in the major ampullate gland of a spider include a modified fibroin including (1-i) the amino acid sequence set forth in SEQ ID NO: 17 or (1-ii) the amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 17. The sequence identity is preferably 95% or more.
[0051] The amino acid sequence set forth in SEQ ID NO: 17 is an amino acid sequence obtained by approximately doubling repeating regions from the first repeating region to the 13th repeating region and performing mutation so that translation is terminated at the 1154th amino acid residue in an amino acid sequence obtained by adding the amino acid sequence (SEQ ID NO: 18) consisting of a start codon, a His10 tag, and a recognition site for HRV3C protease (human rhinovirus 3C protease) to the N-terminal of ADF3. The C-terminal amino acid sequence of the amino acid sequence set forth in SEQ ID NO: 17 is identical to the amino acid sequence set forth in SEQ ID NO: 16.
[0052] The modified fibroin of (1-i) may consist of the amino acid sequence set forth in SEQ ID NO: 17.
[0053] The domain sequence of the modified fibroin having a reduced content of the glycine residue has an amino acid sequence with a reduced content of the glycine residue, as compared with a fibroin derived from the natural fibroin. It can be said that the modified fibroin has an amino acid sequence equivalent to an amino acid sequence in which at least one or a plurality of glycine residues in REP are substituted with other amino acid residues, as compared with a fibroin derived from the natural fibroin.
[0054] The domain sequence of the modified fibroin having a reduced content of the glycine residue may have an amino acid sequence equivalent to an amino acid sequence in which one glycine residue in at least one or the plurality of motif sequences, at least one of which is selected from GGX and GPGXX (where G represents a glycine residue, P represents a proline residue, and X represents an amino acid residue other than glycine) in REP, is substituted with other amino acid residue, as compared with a fibroin derived from the natural fibroin.
[0055] In the modified fibroin having a reduced content of the glycine residue, the proportion of the motif sequences in which the above-described glycine residue is substituted with other amino acid residue may be 10% or more with respect to the entire motif sequences.
[0056] The modified fibroin having a reduced content of the glycine residue may include a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m and have an amino acid sequence in which z/w is 30% or more, 40% or more, 50% or more, or 50.9% or more, in a case where the total number of amino acid residues consisting of XGX (where G represents a glycine residue and X represents an amino acid residue other than glycine) included in all REPs in the sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence, is denoted by z, and the total number of amino acid residues in the amino acid sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence, is denoted by w. The proportion of the number of alanine residues with respect to the total number of amino acid residues in the (A).sub.n motif is 83% or more, preferably 86% or more, more preferably 90% or more, still more preferably 95% or more, and even still more preferably 100% (which means that the (A).sub.n motif consists of only alanine residues).
[0057] In the modified fibroin having a reduced content of the glycine residue, the content proportion of an amino acid sequence consisting of XGX is preferably increased by substituting one glycine residue in GGX motif with other amino acid residue. In the modified fibroin having a reduced content of the glycine residue, the content proportion of an amino acid sequence consisting of GGX in the domain sequence is preferably 30% or less, more preferably 20% or less, still more preferably 10% or less, even still more preferably 6% or less, still further preferably 4% or less, and particularly preferably 2% or less. The content proportion of an amino acid sequence consisting of GGX in a domain sequence can be calculated by the same method as the following method for calculating the content proportion (z/w) of the amino acid sequence consisting of XGX.
[0058] The calculation method of z/w will be described in more detail. First, in a fibroin (a modified fibroin or a fibroin derived from the natural fibroin) including a domain sequence represented by Formula 1: ([(A).sub.n motif--REP].sub.m], the amino acid sequence consisting of XGX is extracted from all REPs included in a sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence. The total number of amino acid residues constituting XGX is z. For example, in a case where 50 amino acid sequences consisting of XGX (without overlap) are extracted, z is 50.times.3=150. Further, for example, in a case where there exists an X (a central X) contained in two XGXs, as in the case of an amino acid sequence consisting of XGXGX, the calculation is performed by subtracting the overlapping portion (in the case of XGXGX, it is counted as 5 amino acid residues). w is the total number of amino acid residues included in the sequence excluding a sequence from the (A).sub.n motif located closest to the C terminal side to the C terminal of the domain sequence from the domain sequence. For example, in the case of the domain sequence illustrated in FIG. 1, w is 4+50+4+100+4+10+4+20+4+30=230 (the (A).sub.n motif located closest to the C-terminal side is excluded.). Next, z/w (%) can be calculated by dividing z by w.
[0059] In the modified fibroin having a reduced content of the glycine residue, z/w is preferably 50.9% or more, more preferably 56.1% or more, still more preferably 58.7% or more, even still more preferably 70% or more, and still further preferably 80% or more. The upper limit of z/w is not particularly limited, but, for example, it may be 95% or less.
[0060] The modified fibroin having a reduced content of the glycine residue cab be obtained by, for example, modifying a cloned fibroin derived from the natural fibroin gene sequence so that at least a part of a base sequence encoding a glycine residue is substituted with other amino acid residue to encode other amino acid residue. In this case, one glycine residue in GGX motif and GPGXX motif may be selected as the glycine residue to be modified or may be substituted so that z/w is 50.9% or more. Alternatively, a modified fibroin may also be obtained, for example, by designing an amino acid sequence satisfying the above-described aspect based on the amino acid sequence of a fibroin derived from the natural fibroin and chemically synthesizing a nucleic acid encoding the designed amino acid sequence. In any case, with respect to the amino acid sequence of a fibroin derived from the natural fibroin, in addition to the modification corresponding to the substitution of the glycine residue in REP with other amino acid residue, further modification of amino acid sequence corresponding to substitution, deletion, insertion and/or addition of one or a plurality of amino acid residues may be carried out.
[0061] The other amino acid residue described above is not particularly limited as long as it is an amino acid residue other than glycine residue, but it is preferably a hydrophobic amino acid residue such as valine (V) residue, leucine (L) residue, isoleucine (I) residue, methionine (M) residue, proline (P) residue, phenylalanine (F) residue, and tryptophan (W) residue, or a hydrophilic amino acid residues such glutamine (Q) residue, asparagine (N) residue, serine (S) residue, lysine (K) residue, and glutamic acid (E) residue, more preferably valine (V) residue, leucine (L) residue, isoleucine (I) residue, and glutamine (Q) residue, and still more preferably glutamine (Q) residue.
[0062] A more specific example of the modified fibroin having a reduced content of the glycine residues includes a modified fibroin including an amino acid sequence having (2-i) the amino acid sequence set forth in SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12, or (2-ii) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12.
[0063] The modified fibroin of (2-i) will be described. The amino acid sequence set forth in SEQ ID NO: 3 is obtained by substituting all GGXs in REP of the amino acid sequence set forth in SEQ ID NO: 1 equivalent to a fibroin derived from the natural fibroin with GQX. The amino acid sequence set forth in SEQ ID NO: 4 is obtained by deleting one of every two (A).sub.n motifs from the N-terminal side to the C-terminal side in the amino acid sequence set forth in SEQ ID NO: 3 and further inserting one [(A).sub.n motif--REP] just before the C-terminal sequence. The amino acid sequence set forth in SEQ ID NO: 10 is obtained by inserting two alanine residues at the C-terminal side of each (A).sub.n motif of the amino acid sequence set forth in SEQ ID NO: 4, and further substituting a part of glutamine (Q) residues with serine (S) residues and deleting a part of amino acids on the N-terminal side so that the molecular weight thereof is approximately the same as that of SEQ ID NO: 4. The amino acid sequence set forth in SEQ ID NO: 12 is an amino acid sequence obtained by adding a His tag to the C-terminal of a sequence obtained by repeating, four times, a region of 20 domain sequences (where several amino acid residues on the C-terminal side of the region are substituted) present in the amino acid sequence set forth in SEQ ID NO: 9.
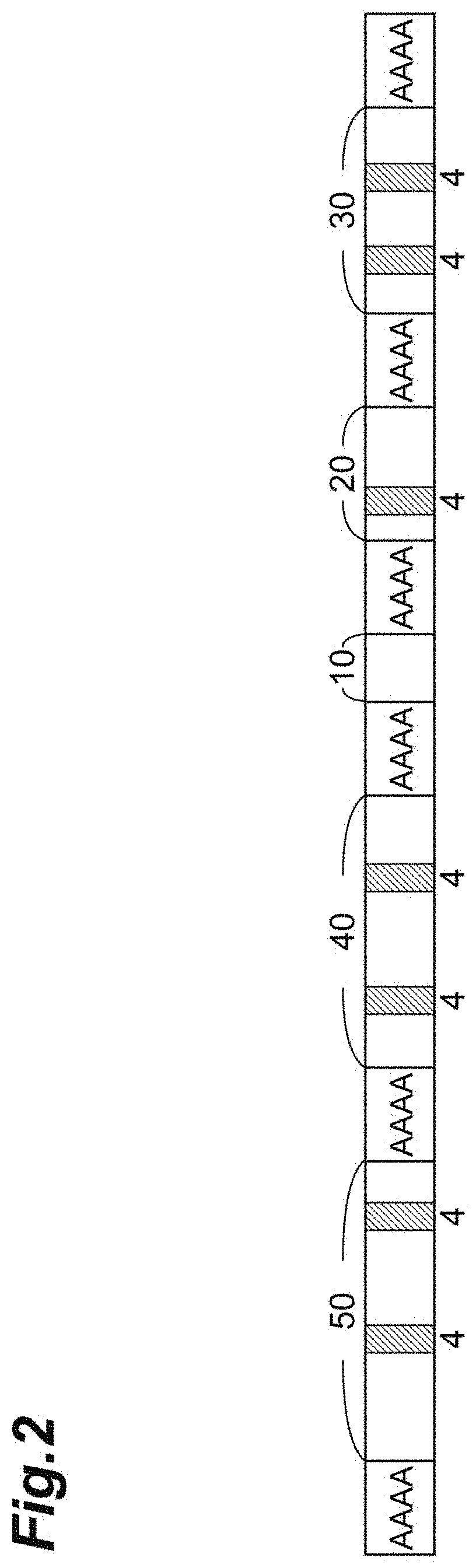
[0064] The value of z/w in the amino acid sequence set forth SEQ ID NO: 1 (corresponds to a fibroin derived from the natural fibroin) is 46.8%. The values of z/w in the amino acid sequence set forth in SEQ ID NO: 3, the amino acid sequence set forth in SEQ ID NO: 4, the amino acid sequence set forth in SEQ ID NO: 10, and the amino acid sequence set forth in SEQ ID NO: 12 are respectively 58.7%, 70.1%, 66.1%, and 70.0%. In addition, the values of x/y with a Giza ratio (described later) of 1:1.8 to 11.3 in the amino acid sequences set forth in SEQ ID NO: 1, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, and SEQ ID NO: 12 are respectively 15.0%, 15.0%, 93.4%, 92.7%, and 89.3%.
[0065] The modified fibroin of (2-i) may consist of the amino acid sequence set forth in SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12.
[0066] The modified fibroin of (2-ii) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12. The modified fibroin of (2-ii) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0067] The modified fibroin of (2-ii) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12, and in a case where the total number of amino acid residues in the amino acid sequence consisting of XGX (where G represents a glycine residue and X represents an amino acid residue other than glycine) included in REP is z, and the total number of amino acid residues in REP in the domain sequence is w, z/w is preferably 50.9% or more.
[0068] The above-described modified fibroin may include a tag sequence at either or both of the N-terminal and C-terminal. This makes it possible to isolate, immobilize, detect, and visualize the modified fibroin.
[0069] The tag sequence may be, for example, an affinity tag utilizing specific affinity (binding property, affinity) with another molecule. As a specific example of the affinity tag, a histidine tag (a His tag) can be mentioned. The His tag is a short peptide in which about 4 to 10 histidine residues are arranged and has a property of specifically binding to a metal ion such as nickel, and thus it can be used for isolation of a modified fibroin by a chelating metal chromatography. A specific example of the tag sequence may be an amino acid sequence set forth in SEQ ID NO: 5 (an amino acid sequence including a His tag).
[0070] In addition, a tag sequence such as glutathione-S-transferase (GST) that specifically binds to glutathione or a maltose binding protein (MBP) that specifically binds to maltose can also be used.
[0071] Further, an "epitope tag" utilizing an antigen-antibody reaction can also be used. By adding a peptide (an epitope) showing antigenicity as a tag sequence, an antibody against the epitope can be bound. Examples of the epitope tag include an HA (peptide sequence of hemagglutinin of influenza virus) tag, a myc tag, and a FLAG tag. The modified fibroin can be easily purified with high specificity by utilizing an epitope tag.
[0072] It is also possible to use a tag sequence which can be cleaved with a specific protease. By treating a protein adsorbed via the tag sequence with a protease, it is also possible to recover a modified fibroin cleaved from the tag sequence.
[0073] A more specific example of the modified fibroin including a tag sequence may be a modified fibroin including (2-iii) the amino acid sequence set forth in SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13, or (2-iv) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
[0074] The amino acid sequences set forth in SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, and SEQ ID NO: 13 are respectively amino acid sequences obtained by adding the amino acid sequence (including a His tag and a hinge sequence) set forth in SEQ ID NO: 5 to the N-terminal of the amino acid sequences set forth in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, and SEQ ID NO: 12.
[0075] The modified fibroin of (2-iii) may consist of the amino acid sequence set forth in SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
[0076] The modified fibroin of (2-iv) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. The modified fibroin of (2-iv) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0077] The modified fibroin of (2-iv) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13, and in a case where the total number of amino acid residues in the amino acid sequence consisting of XGX (where G represents a glycine residue and X represents an amino acid residue other than glycine) included in REP is z, and the total number of amino acid residues in REP in the domain sequence is w, z/w is preferably 50.9% or more.
[0078] The above-mentioned modified fibroin may include a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of a host. The sequence of the secretory signal can be appropriately set depending on the type of the host.
[0079] The domain sequence of a modified fibroin having a reduced content of the (A).sub.n motif has an amino acid sequence with a reduced content of the (A).sub.n motif, as compared with a fibroin derived from the natural fibroin. It can be said that the domain sequence of the modified fibroin has an amino acid sequence equivalent to an amino acid sequence in which at least one or a plurality of (A).sub.n motifs are deleted, as compared with a fibroin derived from the natural fibroin.
[0080] The modified fibroin having a reduced content of the (A).sub.n motif may have an amino acid sequence equivalent to an amino acid sequence in which 10% to 40% of the (A).sub.n motifs are deleted from a fibroin derived from the natural fibroin.
[0081] The domain sequence of the modified fibroin having a reduced content of the (A).sub.n motif may have an amino acid sequence equivalent to an amino acid sequence obtained by deleting one of every one to three (A).sub.n motifs from the N-terminal side to the C-terminal side, as compared with a fibroin derived from the natural fibroin.
[0082] The domain sequence of the modified fibroin having a reduced content of the (A).sub.n motif may have an amino acid sequence equivalent to an amino acid sequence obtained by repeating deletion of at least two consecutive (A).sub.n motifs and deletion of one (A).sub.n motif in this order from the N-terminal side to the C-terminal side, as compared with a fibroin derived from the natural fibroin.
[0083] The domain sequence of the modified fibroin having a reduced content of the (A).sub.n motif may have an amino acid sequence equivalent to an amino acid sequence obtained by deleting one of every two (A).sub.n motifs from the N-terminal side to the C-terminal side.
[0084] The modified fibroin having a reduced content of the (A).sub.n motif may include a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m, and in a case where the number of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other is sequentially compared from the N-terminal side to the C-terminal side and then the number of amino acid residues of one REP having a small number of amino acid residues is set to 1, the maximum total value of the number of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other, in which the ratio of the number of amino acid residues of the other REP is 1.8 to 11.3, is denoted by x, and the total number of amino acid residues in the domain sequence is denoted by y, the modified fibroin may have an amino acid sequence in which x/y is 20% or more, 30% or more, 40% or more, or 50% or more. The proportion of the number of alanine residues with respect to the total number of amino acid residues in the (A).sub.n motif is 83% or more, preferably 86% or more, more preferably 90% or more, still more preferably 95% or more, and even still more preferably 100% (which means that the (A).sub.n motif consists of only alanine residues).
[0085] The method for calculating x/y will be described in more detail with reference to FIG. 1. FIG. 1 illustrates a domain sequence obtained by removing an N-terminal sequence and a C-terminal sequence from a modified fibroin. The domain sequence has a sequence of, from the N-terminal side (left side), (A).sub.n motif--first REP (50 amino acid residues)--(A).sub.n motif--second REP (100 amino acid residues)--(A).sub.n motif--third REP (10 amino acid residues)--(A).sub.n motif--fourth REP (20 amino acid residues)--(A).sub.n motif--fifth REP (30 amino acid residues)--(A).sub.n motif sequence.
[0086] Two [(A).sub.n motif--REP] units adjacent to each other are sequentially selected from the N-terminal side toward the C-terminal side so that the units are not overlapped with each other. In this case, an unselected [(A).sub.n motif--REP] unit may be present. In FIG. 1, pattern 1 (comparison of first REP and second REP, and comparison of third REP and fourth REP), pattern 2 (comparison of first REP and second REP, and comparison of fourth REP and fifth REP), pattern 3 (comparison of second REP and third REP, and comparison of fourth REP and fifth REP), and pattern 4 (comparison of first REP and second REP). There are other selection methods other than these methods.
[0087] Subsequently, for each pattern, the number of amino acid residues of each REP in two selected [(A).sub.n motif--REP] units adjacent to each other is compared. The comparison is performed by determining the ratio of the number of amino acid residues of one REP to the number of amino acid residues of the other REP having the smaller number of amino acid residues so that the number of amino acid residues in the other REP is set to 1. For example, in the case of comparing the first REP (50 amino acid residues) and the second REP (100 amino acid residues), when the first REP having the smaller number of amino acid residues is set to 1, the ratio of the number of amino acid residues of the second REP is 100/50=2. Similarly, in the case of comparing the fourth REP (20 amino acid residues) and the fifth REP (30 amino acid residues), when the fourth REP having the smaller number of amino acid residues is set to 1, the ratio of the number of amino acid residues of the fifth REP is 30/20=1.5.
[0088] In FIG. 1, in a case where one group of [(A).sub.n motif--REP] units having the smaller number of amino acid residues is set to 1, the other group in which the ratio of the number of amino acid residues is 1.8 to 11.3 is indicated by a solid line. Hereinafter, this ratio is referred to as a Giza ratio. In a case where one group of [(A).sub.n motif--REP] units having the smaller number of amino acid residues is set to 1, the other group in which the ratio of the number of amino acid residues is less than 1.8 or more than 11.3 is indicated by a broken line.
[0089] In each pattern, the total numbers of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other indicated by solid lines are added (not only the number of REPs but also the number of the amino acid residues in the (A).sub.n motif are added.) Then, the added total values are compared, and the total value (maximum value of the total values) of the pattern having the maximum total value is denoted by x. In the example illustrated in FIG. 1, the total value of the pattern 1 is the maximum.
[0090] Next, x/y (%) can be calculated by dividing x by y which is the total number of the amino acid residues of the domain sequence.
[0091] In the modified fibroin having a reduced content of the (A).
[0092] motif, x/y is preferably 50% or more, more preferably 60% or more, still more preferably 65% or more, even still more preferably 70% or more, still further preferably 75% or more, and particularly preferably 80% or more. The upper limit of x/y is not particularly limited, but for example, it may be 100% or less. In a case where the Giza ratio is 1:1.9 to 11.3, x/y is preferably 89.6% or more. In a case where the Giza ratio is 1:1.8 to 3.4, x/y is more preferably 77.1% or more. In a case where the Giza ratio is 1:1.9 to 8.4, x/y is still more preferably 75.9% or more. In a case where the Giza ratio is 1:1.9 to 4.1, x/y is even still more preferably 64.2% or more.
[0093] In a case where the modified fibroin having a reduced content of the (A).sub.n motif is a modified fibroin in which at least seven (A).sub.n motifs present in the domain sequence are composed of only alanine residues, x/y is preferably 46.4% or more, more preferably 50% or more, still more preferably 55% or more, even still more preferably 60% or more, still further preferably 70% or more, and particularly preferably 80% or more. The upper limit of x/y is not particularly limited as long as it is 100% or less.
[0094] The modified fibroin having a reduced content of the (A).sub.n motif can be obtained, for example, by deleting one or a plurality sequences encoding (A).sub.n motif from a cloned gene sequence of a fibroin derived from the natural fibroin so that x/y is 64.2% or more. Alternatively, the modified fibroin having a reduced content of the (A).sub.n motif may also be obtained, for example, by designing an amino acid sequence equivalent to an amino acid sequence obtained by deleting one or a plurality (A).sub.n motifs so that x/y is 64.2% or more based on the amino acid sequence of a fibroin derived from the natural fibroin and chemically synthesizing a nucleic acid encoding the designed amino acid sequence. In any case, with respect to the amino acid sequence of a fibroin derived from the natural fibroin, in addition to the modification corresponding to the deletion of the (A).sub.n motif, further modification of amino acid sequence equivalent to substitution, deletion, insertion and/or addition of one or a plurality of amino acid residues may be carried out.
[0095] A more specific example of the modified fibroin having a reduced content of the (A).sub.n motif includes a modified fibroin including an amino acid sequence having (3-i) the amino acid sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12, or (3-ii) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12.
[0096] The modified fibroin of (3-i) will be described. The amino acid sequence set forth in SEQ ID NO: 2 is obtained by deleting one of every two(A).sub.n motifs from the N-terminal side to the C-terminal side in the amino acid sequence set forth in SEQ ID NO: 1 equivalent to a fibroin derived from the natural fibroin and by further inserting one [(A).sub.n motif--REP] just before the C-terminal sequence. The amino acid sequence set forth in SEQ ID NO: 4 is obtained by substituting all GGXs in REP of the amino acid sequence set forth in SEQ ID NO: 2 with GQX. The amino acid sequence set forth in SEQ ID NO: 10 is obtained by inserting two alanine residues at the C-terminal side of each (A).sub.n motif of the amino acid sequence set forth in SEQ ID NO: 4, and further substituting a part of glutamine (Q) residues with serine (S) residues and deleting a part of amino acids on the N-terminal side so that the molecular weight thereof is approximately the same as that of SEQ ID NO: 4. The amino acid sequence set forth in SEQ ID NO: 12 is an amino acid sequence obtained by adding a His tag to the C-terminal of a sequence obtained by repeating, four times, a region of 20 domain sequences (where several amino acid residues on the C-terminal side of the region are substituted) present in the amino acid sequence set forth in SEQ ID NO: 9.
[0097] The value of x/y with a Giza ratio of 1:1.8 to 11.3 in the amino acid sequence set forth in SEQ ID NO: 1 (equivalent to a fibroin derived from the natural fibroin) is 15.0%. Both the values of x/y in the amino acid sequence set forth in SEQ ID NO: 2 and the value of x/y in the amino acid sequence set forth in SEQ ID NO: 4 are 93.4%. The value of x/y in the amino acid sequence set forth in SEQ ID NO: 10 is 92.7%. The value of x/y in the amino acid sequence set forth in SEQ ID NO: 12 is 89.3%. The values of z/w in the amino acid sequences set forth in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, and SEQ ID NO: 12 are respectively 46.8%, 56.2%, 70.1%, 66.1%, and 70.0%.
[0098] The modified fibroin of (3-i) may consist of the amino acid sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12.
[0099] The modified fibroin of (3-ii) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12. The modified fibroin of (3-ii) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0100] The modified fibroin of (3-ii) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12, and in a case where the number of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other is sequentially compared from the N-terminal side to the C-terminal side, then the number of amino acid residues of one REP having a small number of amino acid residues is set to 1, and the maximum total value of the added numbers of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other, in which the ratio (1:1.8 to 11.3 as a Giza ratio) of the number of amino acid residues of the other REP is 1.8 to 11.3, is denoted by x, and the total number of amino acid residues in the domain sequence is denoted by y, x/y is preferably 64.2% or more.
[0101] The modified fibroin described above may include a tag sequence described above at either or both of the N-terminal and C-terminal
[0102] A more specific example of the modified fibroin including a tag sequence may be a modified fibroin including (3-iii) the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13, or (2-iv) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
[0103] The amino acid sequences set forth in SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 11, and SEQ ID NO: 13 are respectively amino acid sequences obtained by adding the amino acid sequence (including a His tag) set forth in SEQ ID NO: 5 to the N-terminal of the amino acid sequences set forth in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 10, and SEQ ID NO: 12.
[0104] The modified fibroin of (3-iii) may consist of the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13.
[0105] The modified fibroin of (3-iv) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13. The modified fibroin of (3-iv) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0106] The modified fibroin of (3-iv) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, or SEQ ID NO: 13, and in a case where the number of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other is sequentially compared from the N-terminal side to the C-terminal side, then the number of amino acid residues of one REP having a small number of amino acid residues is set to 1, the maximum total value of the added numbers of amino acid residues of two [(A).sub.n motif--REP] units adjacent to each other, in which the ratio of the number of amino acid residues of the other REP is 1.8 to 11.3, is denoted by x, and the total number of amino acid residues in the domain sequence is denoted by y, x/y is preferably 64.2% or more.
[0107] The above-mentioned modified fibroin may include a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of a host. The sequence of the secretory signal can be appropriately set depending on the type of the host.
[0108] The domain sequence of the modified fibroin having a reduced content of the glycine residue and (A).sub.n motif has an amino acid sequence having not only a reduced content of the (A).sub.n motif but also having a reduced content of the glycine residue, as compared with a fibroin derived from the natural fibroin. It can be said that the domain sequence of the modified fibroin has an amino acid sequence equivalent to an amino acid sequence in which at least one or a plurality of (A).sub.n motifs are deleted and further at least one or a plurality of glycine residues in REP are substituted with other amino acid residues, as compared with a fibroin derived from the natural fibroin. That is, the modified fibroin is a modified fibroin having both of the characteristics of a modified fibroin having a reduced content of the glycine residue and the characteristics of a modified fibroin having a reduced content of the (A).sub.n motif Specific aspects and the like are as described for a modified fibroin having a reduced content of the glycine residue and a modified fibroin having a reduced content of the (A).sub.n motif
[0109] A more specific example of the modified fibroin having a reduced content of the glycine residue and (A).sub.n motif includes a modified fibroin including an amino acid sequence having (4-i) the amino acid sequence set forth in SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12, or (4-ii) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12. Specific aspects of the modified fibroin including the amino acid sequence set forth in SEQ ID NO: 4, SEQ ID NO: 10, or SEQ ID NO: 12 are as described above.
[0110] A domain sequence of a modified fibroin according to another embodiment may have an amino acid sequence locally containing a region with a high hydropathy index equivalent to an amino acid sequence in which one or a plurality of amino acid residues in REP are substituted with amino acid residues with a high hydropathy index and/or one or a plurality of amino acid residues with a high hydropathy index are inserted into REP, as compared with a fibroin derived from the natural fibroin.
[0111] It is preferable that the region locally having high hydropathy index is composed of two to four consecutive amino acid residues.
[0112] It is more preferable that the amino acid residues with a high hydropathy index are selected from isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M), and alanine (A).
[0113] The modified fibroin according to the present embodiment may further include an amino acid sequence equivalent to an amino acid sequence in which one or a plurality of amino acid residues are substituted, deleted, inserted and/or added, as compared with a fibroin derived from the natural fibroin, in addition to the modification of the amino acid sequence in which one or a plurality of amino acid residues in REP are substituted with amino acid residues with a high hydropathy index and/or one or a plurality of amino acid residues with a high hydropathy index are inserted into REP, as compared with a fibroin derived from the natural fibroin of the present embodiment.
[0114] The modified fibroin according to the present embodiment may be obtained by, with respect to a cloned gene sequence of a fibroin derived from the natural fibroin, substituting one or a plurality of hydrophilic amino acid residues in REP (for example, amino acid residues having a negative hydropathy index) with a hydrophobic amino acid residue (for example, amino acid residues having a positive hydropathy index), and/or inserting one or a plurality of hydrophobic amino acid residues into REP. Further, for example, the modified fibroin may also be obtained by, for example, designing an amino acid sequence equivalent to an amino acid sequence in which with respect to the amino acid sequence of a fibroin derived from the natural fibroin, one or a plurality of hydrophilic amino acid residues in REP are substituted with hydrophobic amino acid residues and/or one or a plurality of hydrophobic amino acid residues are inserted into REP, and chemically synthesizing a nucleic acid encoding the designed amino acid sequence. In any case, with respect to the amino acid sequence a fibroin derived from the natural fibroin, in addition to the modification corresponding to the substitution of one or a plurality of hydrophilic amino acid residues in REP with hydrophobic amino acid residues and/or insertion of one or a plurality of hydrophobic amino acid residues into REP, further modification of amino acid sequence equivalent to substitution, deletion, insertion and/or addition of one or a plurality of amino acid residues may be carried out.
[0115] Further, a modified fibroin according to another embodiment may include a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m and have an amino acid sequence in which p/q is 6.2% or more, in a case where in all REPs included in a sequence excluding a sequence from an (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence, the total number of amino acid residues contained in a region where an average value of hydropathy indices of the four consecutive amino acid residues is 2.6 or more is denoted by p, and the total number of amino acid residues contained in the sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence is denoted by q.
[0116] Regarding the hydropathy index of amino acid residues, known indices from (Hydropathy index: Kyte J, & Doolittle R (1982)"A simple method for displaying the hydropathic character of a protein", J. Mol. Biol., 157, pp. 105-132) may be used as a reference. Specifically, the hydropathy index (hereinafter, also referred to as "HI") of each amino acid is as shown in Table 1 below.
TABLE-US-00001 TABLE 1 Amino acid HI Isoleucine (Ile) 4.5 Valine (Val) 4.2 Leucine (Leu) 3.8 Phenylalanine (Phe) 2.8 Cysteine (Cys) 2.5 Methionine (Met) 1.9 Alanine (Ala) 1.8 Glycine (Gly) -0.4 Threonine (Thr) -0.7 Serine (Ser) -0.8 Tryptophan (Trp) -0.9 Tyrosine (Tyr) -1.3 Proline (Pro) -1.6 Histidine (His) -3.2 Asparagine (Asn) -3.5 Aspartic acid (Asp) -3.5 Glutamine (Gln) -3.5 Glutamic acid (Glu) -3.5 Lysine (Lys) -3.9 Arginine (Arg) -4.5
[0117] The calculation method of p/q will be described in more detail. In the calculation, the sequence (hereinafter, also referred to as "sequence A") excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence represented by Formula 1: [(A).sub.n motif--REP]. is used. First, in all REPs included in the sequence A, average values of hydropathy indices of the four consecutive amino acid residues are calculated. The average value of the hydropathy indices is obtained by dividing the total sum of HI of each of the amino acid residues contained in the four consecutive amino acid residues by 4 (the number of amino acid residues). The average value of the hydropathy indices is obtained for all of the four consecutive amino acid residues (each of the amino acid residues is used for calculating the average value 1 to 4 times). Next, a region where the average value of the hydropathy indices of the four consecutive amino acid residues is 2.6 or more is specified. Even in a case where a plurality of certain amino acid residues correspond to the "four consecutive amino acid residues having an average value of the hydropathy indices of 2.6 or more", the amino acid residue is counted as one amino acid residue in the region. The total number of amino acid residues included in the region is denoted by p. The total number of amino acid residues included in the sequence A is denoted by q.
[0118] For example, in a case where the "four consecutive amino acid residues whose average value of the hydropathy indices is 2.6 or more" are extracted from 20 places (without overlap), in the region where the average value of the hydropathy indices of the four consecutive amino acid residues is 2.6 or more, the number of the four consecutive amino acid residues (without overlap) is 20, and thus p is 20.times.4=80. In addition, for example, in a case where two of the "four consecutive amino acid residues having an average value of the hydropathy indices of 2.6 or more" overlap by only one amino acid residue, in the region where the average value of the hydropathy indices of the four consecutive amino acid residues is 2.6 or more, the number of amino acid residues being included is 7 (p=2.times.4-1=7. "-1" corresponds to the subtraction of the overlapping portion). For example, in the case of the domain sequence shown in FIG. 2, since the number of the "four consecutive amino acid residues having an average value of the hydropathy indices of 2.6 or more", which do not overlap, is 7, p is 7.times.4=28. Further, for example, in the case of the domain sequence illustrated in FIG. 2, q is 4+50+4+40+4+10+4+20+4+30=170 (the (A).sub.n motif present closest to the C-terminal side can not be included). Next, p/q (%) can be calculated by dividing p by q. In the case of FIG. 2, p/q (%) is 28/170=16.47%.
[0119] In the modified fibroin according to the present embodiment, p/q is preferably 6.2% or more, more preferably 7% or more, still more preferably 10% or more, even still more preferably 20% or more, and still further preferably 30% or more. The upper limit of p/q is not particularly limited, but for example, it may be 45% or less.
[0120] The modified fibroin according to the present embodiment may be obtained by, for example, modifying an amino acid sequence of a cloned a fibroin derived from the natural fibroin to an amino acid sequence locally containing a region locally having a high hydropathy index by substituting one or a plurality of hydrophilic amino acid residues in REP (for example, amino acid residues having a negative hydropathy index) with hydrophobic amino acid residues (for example, amino acid residues having a positive hydropathy index), and/or inserting one or a plurality of hydrophobic amino acid residues into REP, so that the p/q condition is satisfied. Alternatively, the modified fibroin may also be obtained, for example, by designing an amino acid sequence satisfying the p/q condition based on the amino acid sequence of a fibroin derived from the natural fibroin and chemically synthesizing a nucleic acid encoding the designed amino acid sequence. In any case, in addition to the modification corresponding to the substitution of one or a plurality of amino acid residues in REP with amino acid residues with a high hydropathy index and/or insertion of one or a plurality of amino acid residues with a high hydropathy index into REP, as compared with the amino acid sequence of a fibroin derived from the natural fibroin, further modification corresponding to substitution, deletion, insertion, and/or addition of one or a plurality of amino acid residues may be carried out.
[0121] The amino acid residue with a high hydropathy index is preferably isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M), and alanine (A), and more preferably valine (V), leucine (L), and isoleucine (I), but is not particularly limited thereto.
[0122] Another specific example of the modified fibroin may be a modified fibroin including (5-iii) the amino acid sequence set forth in SEQ ID NO: 20, SEQ ID NO: 22, or SEQ ID NO: 23, or (5-ii) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 20, SEQ ID NO: 22, or SEQ ID NO: 23.
[0123] The modified fibroin of (5-i) will be described. The amino acid sequence set forth in SEQ ID NO: 19 is obtained by deleting a part of the amino acid sequence of the consecutive alanine residues in the (A).sub.n motif of a fibroin derived from the natural fibroin so that the number of the consecutive alanine residues in the (A).sub.n motif is five. The amino acid sequence set forth in SEQ ID NO: 20 is obtained by inserting an amino acid sequence consisting of three amino acid residues (VLI) at two sites for each REP with respect to the amino acid sequence set forth in SEQ ID NO: 19, and deleting a part of the amino acids on the C-terminal side therefrom so that the molecular weight thereof is approximately the same as that of the amino acid sequence set forth in SEQ ID NO: 19. The amino acid sequence set forth in SEQ ID NO: 21 is obtained by inserting two alanine residues at the C-terminal side of each (A).sub.n motif with respect to the amino acid sequence set forth in SEQ ID NO: 19, and further substituting a part of glutamine (Q) residues with serine (S) residues and deleting a part of amino acids on the C-terminal side so that the molecular weight thereof is approximately the same as that of the amino acid sequence set forth in SEQ ID NO: 19. The amino acid sequence set forth in SEQ ID NO: 22 is obtained by inserting an amino acid sequence consisting of three amino acid residues (VLI) at one site for each REP with respect to the amino acid sequence set forth in SEQ ID NO: 21. The amino acid sequence set forth in SEQ ID NO: 23 is obtained by inserting an amino acid sequence consisting of three amino acid residues (VLI) at two sites for each REP with respect to the amino acid sequence set forth in SEQ ID NO: 21.
[0124] The modified fibroin of (5-i) may consist of the amino acid sequence set forth in SEQ ID NO: 20, SEQ ID NO: 22, or SEQ ID NO: 23.
[0125] The modified fibroin of (5-ii) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 20, SEQ ID NO: 22, or SEQ ID NO: 23. The modified fibroin of (5-ii) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0126] The modified fibroin of (5-ii) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 20, SEQ ID NO: 22, or SEQ ID NO: 23, and preferably has an amino acid sequence in which p/q is 6.2% or more, in a case where in all REPs included in a sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence, the total number of amino acid residues contained in a region where an average value of hydropathy indices of the four consecutive amino acid residues is 2.6 or more is denoted by p, and the total number of amino acid residues contained in the sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence is denoted by q.
[0127] The above-described modified fibroin may include a tag sequence at either or both of the N-terminal and C-terminal
[0128] A more specific example of the modified fibroin including a tag sequence may be a modified fibroin including (5-iii) the amino acid sequence set forth in SEQ ID NO: 24, SEQ ID NO: 25, or SEQ ID NO: 26, or (5-iv) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 24, SEQ ID NO: 25, or SEQ ID NO: 26.
[0129] The amino acid sequences set forth in SEQ ID NO: 24, SEQ ID NO: 25, and SEQ ID NO: 26 are respectively amino acid sequences obtained by adding the amino acid sequence (including a His tag and a hinge sequence) set forth in SEQ ID NO: 5 to the N-terminal of the amino acid sequences set forth in SEQ ID NO: 20, SEQ ID NO: 22, and SEQ ID NO: 23.
[0130] The modified fibroin of (5-iii) may consist of the amino acid sequence set forth in SEQ ID NO: 24, SEQ ID NO: 25, or SEQ ID NO: 26.
[0131] The modified fibroin of (5-iv) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 24, SEQ ID NO: 25, or SEQ ID NO: 26. The modified fibroin of (5-iv) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m. The sequence identity is preferably 95% or more.
[0132] The modified fibroin of (5-iv) preferably has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 24, SEQ ID NO: 25, or SEQ ID NO: 26, and preferably has an amino acid sequence in which p/q is 6.2% or more, in a case where in all REPs included in a sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence, the total number of amino acid residues contained in a region where an average value of hydropathy indices of the four consecutive amino acid residues is 2.6 or more is denoted by p, and the total number of amino acid residues contained in the sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence is denoted by q.
[0133] The above-mentioned modified fibroin may include a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of a host. The sequence of the secretory signal can be appropriately set depending on the type of the host.
[0134] A modified fibroin according to another embodiment has an amino acid sequence with a reduced content of the glutamine residue, as compared with a fibroin derived from the natural fibroin.
[0135] It is preferable that the modified fibroin according to the present embodiment includes at least one motif selected from GGX motif and GPGXX motif in the amino acid sequence of REP.
[0136] In a case where the modified fibroin according to the present embodiment includes a GPGXX motif in REP, a GPGXX motif content rate is usually 1% or more, may be 5% or more, and is preferably 10% or more. The upper limit of the GPGXX motif content rate is not particularly limited, may be 50% or less, and may be 30% or less.
[0137] In the present specification, the "GPGXX motif content rate" is a value calculated by the following method.
[0138] In a fibroin (a fibroin derived from the natural fibroin) including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif, in a case where the number obtained by tripling the total number of the GPGXX motifs included in all REPs included in a sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence (that is, equivalent to the total number of G and P in the GPGXX motifs) is denoted by s, and the total number of amino acid residues in all REPs excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence and further excluding (A).sub.n motifs is denoted by t, the GPGXX motif content rate is calculated as s/t.
[0139] For the calculation of the GPGXX motif content rate, the "sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence" is used to exclude the effect occurring due to the fact that the "sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal from the domain sequence" (sequence equivalent to REP) may include a sequence that is not correlated with the sequence characteristics of fibroin, which influences the calculation result of the GPGXX motif content rate in a case where m is small (that is, in case a where the domain sequence is short). In a case where a "GPGXX motif" is located at the C-terminal of REP, it is treated as "GPGXX motif" even in a case where "XX" is, for example, "AA".
[0140] FIG. 3 is a schematic diagram showing a domain sequence of a modified fibroin. The calculation method of the GPGXX motif content rate will be specifically described with reference to FIG. 3. First, in a domain sequence of a modified fibroin (which is an [(A).sub.n motif--REP].sub.m--(A).sub.n motif] type) illustrated in FIG. 3, since all REPs are included in the "sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence" (in FIG. 3, shown as "region A"), the number of GPGXX motifs for calculating s is 7, and s is 7.times.3=21. Similarly, since all REPs are included in the "sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence" (in FIG. 1, shown as "region A"), t which is the total number of amino acid residues in all REPs excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence and further excluding (A).sub.n motifs, is 50+40+10+20+30=150. Next, s/t (%) can be calculated by dividing s by t and is 21/150=14.0% in the case of the modified fibroin of FIG. 3.
[0141] In the modified fibroin according to the present embodiment, a glutamine residue content rate is preferably 9% or less, more preferably 7% or less, still more preferably 4% or less, and particularly preferably 0%.
[0142] In the present specification, the "glutamine residue content rate" is a value calculated by the following method.
[0143] In a fibroin (a modified fibroin or a fibroin derived from the natural fibroin) including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif, in a case where the total number of glutamine residues included in all REPs included in a sequence (sequence equivalent to "region A" in FIG. 3) excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence is denoted by u, and the total number of amino acid residues in all REPs excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence and further excluding (A).sub.n motifs is denoted by t, the glutamine residue content rate is calculated as u/t. For the calculation of the glutamine residue content rate, the "sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence" is used for the same reason described above.
[0144] The domain sequence of the modified fibroin according to the present embodiment may include an amino acid sequence equivalent to an amino acid sequence in which one or a plurality of glutamine residues in REP are deleted or substituted with other amino acid residues, as compared with a fibroin derived from the natural fibroin.
[0145] The "other amino acid residue" may be an amino acid residue other than a glutamine residue but is preferably an amino acid residue having a higher hydropathy index than that of a glutamine residue. The hydropathy indices of amino acid residues are as shown in Table 1.
[0146] As shown in Table 1, amino acid residues having a higher hydropathy index than a glutamine residue include an amino acid residue selected from isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M), alanine (A), glycine (G), threonine (T), serine (S), tryptophan (W), tyrosine (Y), proline (P) and histidine (H). Among these, an amino acid residue selected from isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M), and alanine (A) is more preferable, and an amino acid residue selected from isoleucine (I), valine (V), leucine (L), and phenylalanine (F) is still more preferable.
[0147] In the modified fibroin according to the present embodiment, the hydrophobicity of REP is preferably -0.8 or more, more preferably -0.7 or more, still more preferably 0 or more, even still more preferably 0.3 or more, and particularly preferably 0.4 or more. The upper limit of the hydrophobicity of REP is not particularly limited, may be 1.0 or less, and may be 0.7 or less.
[0148] In the present specification, the "hydrophobicity of REP" is a value calculated by the following method.
[0149] In a fibroin (a modified fibroin or a fibroin derived from the natural fibroin) including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A).sub.n motif--REP].sub.m--(A).sub.n motif, in a case where the total sum of the hydropathy index of each amino acid residue included in all REPs included in a sequence (sequence equivalent to "region A" in FIG. 1) excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence is denoted by v, and the total number of amino acid residues in all REPs excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence and further excluding (A).sub.n motifs is denoted by t, the hydrophobicity is calculated as v/t. For the calculation of the hydrophobicity of REP, the "sequence excluding a sequence from the (A).sub.n motif located closest to the C-terminal side to the C-terminal of the domain sequence from the domain sequence" is used for the same reason described above.
[0150] The domain sequence of the modified fibroin according to the present embodiment may further include an amino acid sequence equivalent to an amino acid sequence in which one or a plurality of amino acid residues are substituted, deleted, inserted and/or added, in addition to the modification of the amino acid sequence in which one or a plurality of glutamine residues in REP are deleted and/or one or a plurality of glutamine residues in REP are substituted with other amino acid residues, as compared with a fibroin derived from the natural fibroin.
[0151] The modified fibroin according to the present embodiment can be obtained by, for example, with respect to a cloned gene sequence of a fibroin derived from the natural fibroin, deleting one or a plurality of glutamine residues in REP and/or by substituting one or a plurality of glutamine residues in REP with other amino acid residues. Further, for example, the modified fibroin may also be obtained by designing an amino acid sequence equivalent to an amino acid sequence in which with respect to the amino acid sequence of a fibroin derived from the natural fibroin, one or a plurality of glutamine residues in REP are deleted and/or one or a plurality of glutamine residues in REP are substituted with other amino acid residues, and chemically synthesizing a nucleic acid encoding the designed amino acid sequence.
[0152] A more specific example of the modified fibroin according to the embodiment of the present invention may be a modified fibroin including (6-i) the amino acid sequence set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, or SEQ ID NO: 39, or (6-ii) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, or SEQ ID NO: 39.
[0153] The modified fibroin of (6-i) will be described.
[0154] The amino acid sequence (Met-PRT410) set forth in SEQ ID NO: 4 is a modified amino acid sequence obtained by changing the number of the consecutive alanine residues in the (A).sub.n motif to five, or the like, so as to improve productivity, based on the base sequence and amino acid sequence of Nephila clavipes (GenBank Accession No.: P46804.1, GI: 1174415) which is a fibroin derived from the natural fibroin. However, since Met-PRT410 has no modification of glutamine residue (Q), the glutamine residue content rate thereof is the same as the glutamine residue content of a fibroin derived from the natural fibroin.
[0155] The amino acid sequence (M_PRT888) set forth in SEQ ID NO: 27 is obtained by substituting all QQs in Met-PRT410 (SEQ ID NO: 4) with VLs.
[0156] The amino acid sequence (M_PRT965) set forth in SEQ ID NO: 28 is obtained by substituting all QQs in Met-PRT410 (SEQ ID NO: 4) with TSs and substituting the remaining Qs with As.
[0157] The amino acid sequence (M_PRT889) set forth in SEQ ID NO: 29 is obtained by substituting all QQs in Met-PRT410 (SEQ ID NO: 4) with VLs and substituting the remaining Qs with Is.
[0158] The amino acid sequence (M_PRT916) set forth in SEQ ID NO: 30 is obtained by substituting all QQs in Met-PRT410 (SEQ ID NO: 4) with VIs and substituting the remaining Qs with Ls.
[0159] The amino acid sequence (M_PRT918) set forth in SEQ ID NO: 31 is obtained by substituting all QQs in Met-PRT410 (SEQ ID NO: 4) with VFs and substituting the remaining Qs with Is.
[0160] The amino acid sequence (M_PRT525) set forth in SEQ ID NO: 37 is obtained by, with respect to Met-PRT410 (SEQ ID NO: 4), inserting two alanine residues in a region (A5) in which alanine residues are consecutive, and by deleting two domain sequences at the C-terminal side and substituting 13 glutamine residues (Q) with serine residues (S) or prolines (P) so that the molecular weight thereof is approximately the same as that of Met-PRT410.
[0161] The amino acid sequence (M_PRT699) set forth in SEQ ID NO: 38 is obtained by substituting all QQs in M PRT525 (SEQ ID NO: 37) with VLs.
[0162] The amino acid sequence (M PRT698) set forth in SEQ ID NO: 39 is obtained by substituting all QQs in M PRT525 (SEQ ID NO: 37) with VLs and substituting the remaining Qs with Is.
[0163] The glutamine residue content rate of any of the amino acid sequences set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, and SEQ ID NO: 39 is 9% or less (Table 2).
TABLE-US-00002 TABLE 2 Glutamine residue GPGXX motif Hydrophobicity Modified fibroin content rate content rate of REP Met-PRT410 17.7% 27.9% -1.52 (SEQ ID NO: 4) M PRT888 6.3% 27.9% -0.07 (SEQ ID NO: 27) M_PRT965 0.0% 27.9% -0.65 (SEQ ID NO: 28) M_PRT889 0.0% 27.9% 0.35 (SEQ ID NO: 29) M_PRT916 0.0% 27.9% 0.47 (SEQ ID NO: 30) M_PRT918 0.0% 27.9% 0.45 (SEQ ID NO: 31) M_PRT525 13.7% 26.4% -1.24 (SEQ ID NO: 37) M_PRT699 3.6% 26.4% -0.78 (SEQ ID NO: 38) M_PRT698 0.0% 26.4% -0.03 (SEQ ID NO: 39)
[0164] The modified fibroin of (6-i) may consist of the amino acid sequence set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, or SEQ ID NO: 39.
[0165] The modified fibroin of (6-ii) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, or SEQ ID NO: 39. The modified fibroin of (6-ii) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A). motif--REP].sub.m--(A).sub.n motif. The sequence identity is preferably 95% or more.
[0166] The modified fibroin of (6-ii) preferably has the glutamine residue content rate of 9% or less. In addition, the modified fibroin of (6-ii) preferably has the GPGXX motif content rate of 10% or more.
[0167] The above-described modified fibroin may include a tag sequence at either or both of the N-terminal and C-terminal. This makes it possible to isolate, immobilize, detect, and visualize the modified fibroin.
[0168] A more specific example of the modified fibroin including a tag sequence may be a modified fibroin including (6-iii) the amino acid sequence set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, or SEQ ID NO: 41, or (6-iv) an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, or SEQ ID NO: 41.
[0169] The amino acid sequences set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, and SEQ ID NO: 41 are respectively amino acid sequences obtained by adding the amino acid sequence (including a His tag and a hinge sequence) set forth in SEQ ID NO: 5 to the N-terminal of the amino acid sequences set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 38, and SEQ ID NO: 39. Since only the tag sequence is added to the N-terminal, the glutamine residue content rate are not changed, and any of the amino acid sequences set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, and SEQ ID NO: 41 has the glutamine residue content rate of 9% or less (Table 3).
TABLE-US-00003 TABLE 3 Glutamine residue GPGXX motif Hydrophobicity Modified fibroin content rate content rate of REP PRT888 6.3% 27.9% -0.07 (SEQ ID NO: 32) PRT965 0.0% 27.9% -0.65 (SEQ ID NO: 33) PRT889 0.0% 27.9% 0.35 (SEQ ID NO: 34) PRT916 0.0% 27.9% 0.47 (SEQ ID NO: 35) PRT918 0.0% 27.9% 0.45 (SEQ ID NO: 36) PRT699 3.6% 26.4% -0.78 (SEQ ID NO: 40) PRT698 0.0% 26.4% -0.03 (SEQ ID NO: 41)
[0170] The modified fibroin of (6-iii) may consist of the amino acid sequence set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, or SEQ ID NO: 41.
[0171] The modified fibroin of (6-iv) includes an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 32, SEQ ID NO: 33, SEQ ID NO: 34, SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 40, or SEQ ID NO: 41. The modified fibroin of (6-iv) is also a protein including a domain sequence represented by Formula 1: [(A).sub.n motif--REP].sub.m or Formula 2: [(A). motif--REP].sub.m--(A).sub.n motif. The sequence identity is preferably 95% or more.
[0172] The modified fibroin of (6-iv) preferably has the glutamine residue content rate of 9% or less. In addition, the modified fibroin of (6-iv) preferably has the GPGXX motif content rate of 10% or more.
[0173] The above-mentioned modified fibroin may include a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of a host. The sequence of the secretory signal can be appropriately set depending on the type of the host.
[0174] (Manufacturing Method for Modified Fibroin)
[0175] The modified fibroin (hereinafter, also simply referred to as "protein") according to the present embodiment can be produced, for example, by expressing a nucleic acid in a host transformed with an expression vector having the nucleic acid sequence encoding the modified fibroin and one or a plurality of regulatory sequences operably linked to the nucleic acid sequence.
[0176] The producing method for a nucleic acid encoding a modified fibroin is not particularly limited. For example, the nucleic acid is produced by cloning a gene encoding the natural fibroin by amplification with polymerase chain reaction (PCR) or the like and modifying the gene by a genetic engineering method, by chemically synthesizing the nucleic acid. The method for chemically synthesizing a nucleic acid is not particularly limited, and for example, the gene can be chemically synthesized by a method in which oligonucleotides are automatically synthesized by AKTA oligopilot plus 10/100 (GE Healthcare Japan Corporation) or the like and are linked by PCR or the like, based on the amino acid sequence information of the protein obtained from the NCBI web database or the like. In this case, in order to facilitate purification and/or confirmation of the protein, a nucleic acid may be synthesized such that a protein having an amino acid sequence obtained by adding an amino acid sequence consisting of a start codon and a His10 tag to the N-terminal of the above amino acid sequence is encoded.
[0177] The regulatory sequence is a sequence (for example, a promoter, an enhancer, a ribosome binding sequence, or a transcription termination sequence) that controls the expression of a modified fibroin in a host, and can be appropriately selected depending on the type of the host. As a promoter, an inducible promoter that functions in a host cell and is capable of inducing the expression of a modified fibroin may be used. An inducible promoter is a promoter that can control transcription by the presence of an inducer (an expression inducer), the absence of a repressor molecule, or physical factors such as an increase or decrease in temperature, osmotic pressure, or pH value.
[0178] The type of the expression vector such as a plasmid vector, a viral vector, a cosmid vector, a fosmid vector, or an artificial chromosome vector can be appropriately selected depending on the type of the host. As the expression vector, an expression vector that can autonomously replicate in a host cell or can be incorporated into a chromosome of a host and which contains a promoter at a position capable of transcribing the nucleic acid that encodes a protein is suitably used.
[0179] Both prokaryotes and eukaryotes such as yeast, filamentous fungi, insect cells, animal cells, and plant cells can be suitably used as a host.
[0180] Preferred examples of the prokaryotic host cells include bacteria belonging to the genus Escherichia, the genus Brevibacillus, the genus Serratia, the genus Bacillus, the genus Microbacterium, the genus Brevibacterium, the genus Corynebacterium, and the genus Pseudomonas. Examples of microorganisms belonging to the genus Escherichia include Escherichia coli. Examples of the microorganisms belonging to the genus Brevibacillus include Brevibacillus agri. Examples of microorganisms belonging to the genus Serratia include Serratia liquefaciens. Examples of microorganisms belonging to the genus Bacillus include Bacillus subtilis. Examples of microorganisms belonging to the genus Microbacterium include Microbacterium ammoniaphilum. Examples of microorganisms belonging to the genus Brevibacterium include Brevibacterium divaricatum. Examples of microorganisms belonging to the genus Corynebacterium include Corynebacterium ammoniagenes. Examples of microorganisms belonging to the genus Pseudomonas include Pseudomonas putida.
[0181] In a case where a prokaryote is used as a host, examples of a vector into which a nucleic acid encoding a protein is introduced include pBTrp2 (manufactured by Boehringer Mannheim), pGEX (manufactured by Pharmacia), pUC18, pBluescriptll, pSupex, pET22b, pCold, pUB110, and pNCO2 (Japanese Unexamined Patent Publication No. 2002-238569).
[0182] Examples of eukaryotic hosts include yeast and filamentous fungi (mold and the like). Examples of yeasts include yeasts belonging to the genus Saccharomyces, the genus Pichia, and the genus Schizosaccharomyces. Examples of filamentous fungi include filamentous fungi belonging to the genus Aspergillus, the genus Penicillium, and the genus Trichoderma.
[0183] In a case where a eukaryote is used as a host, examples of the vector into which a nucleic acid encoding a modified fibroin is introduced include YEp13 (ATCC37115) and YEp24 (ATCC37051). As a method for introducing an expression vector into the above host cell, any method can be used as long as the method introduces DNA into the host cell. Examples thereof include a method using calcium ions [Proc. Natl. Acad. Sci. USA, 69, 2110 (1972)], electroporation method, spheroplast method, protoplast method, lithium acetate method, and competent method.
[0184] As for the method for expressing a nucleic acid using a host transformed with an expression vector, secretory production, fusion protein expression, or the like, in addition to the direct expression, can be carried out according to the method described in Molecular Cloning, 2nd edition.
[0185] The modified fibroin can be produced, for example, by culturing a host transformed with the expression vector in a culture medium, producing and accumulating the protein in the culture medium, and then collecting the modified fibroin from the culture medium. The method for culturing a host in a culture medium can be carried out according to a method commonly used for culturing a host.
[0186] In the case where the host is a prokaryote such as Escherichia coli or a eukaryote such as yeast, any of a natural medium and a synthetic medium may be used as a culture medium of the host as long as the medium contains a carbon source, a nitrogen source, inorganic salts and the like which can be utilized by the host and the medium can be used for efficiently culturing the host.
[0187] As the carbon source, any carbon source that can be utilized by the transformed microorganism may be used. Examples of the carbon source that can be utilized include carbohydrates such as glucose, fructose, sucrose, and molasses, starch and starch hydrolyzates containing them, organic acids such as acetic acid and propionic acid, and alcohols such as ethanol and propanol. Examples of the nitrogen source that can be utilized include ammonium salts of inorganic or organic acids such as ammonia, ammonium chloride, ammonium sulfate, ammonium acetate, and ammonium phosphate, other nitrogen-containing compounds, peptone, meat extract, yeast extract, corn steep liquor, casein hydrolyzate, soybean cake and soybean cake hydrolyzate, and various fermented microbial cells and digested products thereof Examples of the inorganic salt that can be utilized include potassium dihydrogen phosphate, dipotassium phosphate, magnesium phosphate, magnesium sulfate, sodium chloride, ferrous sulfate, manganese sulfate, copper sulfate, and calcium carbonate.
[0188] Culture of a prokaryote such as Escherichia coli or a eukaryote such as yeast can be carried out under aerobic conditions such as shaking culture or deep aeration stirring culture. The culture temperature is, for example, 15.degree. C. to 40.degree. C. The culture time is usually 16 hours to 7 days. It is preferable to maintain the pH of the culture medium during the culture at 3.0 to 9.0. The pH of the culture medium can be adjusted using an inorganic acid, an organic acid, an alkali solution, urea, calcium carbonate, ammonia, or the like.
[0189] In addition, antibiotics such as ampicillin and tetracycline may be added to the culture medium as necessary during the culture. In a case of culturing a microorganism transformed with an expression vector using an inducible promoter as a promoter, an inducer may be added to the medium as necessary. For example, in a case of culturing a microorganism transformed with an expression vector using a lac promoter, isopropyl-.beta.-D-thiogalactopyranoside or the like is used, and in a case of culturing a microorganism transformed with an expression vector using a trp promoter, indole acrylic acid or the like may be added to the medium.
[0190] The expressed protein can be isolated and purified by a commonly used method. For example, in a case where the protein is expressed in a dissolved state in cells, the host cells are recovered by centrifugation after the completion of the culture, suspended in an aqueous buffer solution, and then disrupted using an ultrasonicator, a French press, a Manton-Gaulin homogenizer, a Dyno-Mill, or the like to obtain a cell-free extract. From the supernatant obtained by centrifuging the cell-free extract, a purified preparation can be obtained by a method commonly used for protein isolation and purification, that is, a solvent extraction method, a salting-out method using ammonium sulfate or the like, a desalting method, a precipitation method using an organic solvent, an anion exchange chromatography method using a resin such as diethylaminoethyl (DEAE)-Sepharose or DIAION HPA-75 (manufactured by Mitsubishi Kasei Kogyo Kabushiki Kaisha), an cation exchange chromatography method using a resin such as S-Sepharose FF (manufactgured by Pharmacia Corporation), a hydrophobic chromatography method using a resin such as butyl sepharose or phenyl sepharose, a gel filtration method using a molecular sieve, an affinity chromatography method, a chromatofocusing method, or an electrophoresis method such as isoelectric focusing or the like, using the above methods singly or in combination thereof
[0191] In addition, in a case where the protein is expressed to form an insoluble body in the cell, similarly, the host cells are recovered, disrupted and centrifuged to recover the insoluble body of the protein as a precipitated fraction. The recovered insoluble body of the protein can be solubilized with a protein denaturing agent. After this operation, a purified preparation of the protein can be obtained by the same isolation and purification method as described above. In a case where the protein is secreted extracellularly, the protein can be recovered from the culture supernatant. That is, a culture supernatant is obtained by treating the culture by a technique such as centrifugation, and a purified preparation can be obtained from the culture supernatant by using the same isolation and purification method as described above.
[0192] (Manufacturing Method for Artificial Fibroin Filament)
[0193] An artificial fibroin filament (hereinafter, sometimes referred to as "artificial protein filament" or simply "protein fiber") may include other protein or a contaminant as long as it includes the modified fibroin as the main component. The artificial fibroin filament is preferably an artificial spider silk fibroin filament. The artificial fibroin filament can be manufactured by a known spinning method. That is, for example, in a case of manufacturing a protein filament including a modified fibroin as the main component, first, the modified fibroin produced according to the above-mentioned method is added to a solvent such as dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), formic acid, or hexafluoroisopropanol (HFIP), as necessary, together with an inorganic salt as a dissolution accelerator and dissolved to prepare a doping liquid. Then, using the doping liquid, spinning can be performed by a known spinning method such as wet-type spinning, dry-type spinning, dry-wet-type spinning, or melt spinning to obtain the protein filament. A preferred spinning method is wet-type spinning and dry-wet-type spinning
[0194] FIG. 3 is an explanatory view schematically illustrating one example of a spinning device for manufacturing a protein filament. A spinning device 10 shown in FIG. 3 is an example of a spinning device for dry-wet-type spinning and includes an extrusion device 1, an undrawn yarn manufacturing device 2, a wet heat drawing device 3, and a drying device 4.
[0195] A spinning method using the spinning device 10 will be described. First, a doping liquid 6 stored in a storage tank 7 is pushed out from a spinneret 9 by a gear pump 8. In the laboratory scale, the doping liquid may be filled in a cylinder and extruded from a nozzle using a syringe pump. Next, the extruded doping liquid 6 is supplied into a coagulation liquid 11 in a coagulation liquid bath 20 via an air gap 19, the solvent is removed, the modified fibroin is coagulated, and a fibrous coagulate is formed. Then, the fibrous coagulate is supplied into a warm water 12 in a drawing bath 21 and is drawn. A drawing ratio is determined according to a speed ratio of a supply nip roller 13 to a withdrawing nip roller 14. Thereafter, the drawn fibrous coagulate is supplied to a drying device 4 and dried in a yarn path 22, and the protein filament 36 is obtained as a wound yarn body 5. Reference signs 18a to 18g indicate yarn guides.
[0196] The coagulation liquid 11 may be any solvent that can be desolvated, and examples thereof include lower alcohols having 1 to 5 carbon atoms such as methanol, ethanol, and 2-propanol, and acetone. The coagulation liquid 11 may appropriately contain water. The temperature of the coagulation liquid 11 is preferably 0.degree. C. to 30.degree. C. In a case where a syringe pump having a nozzle with a diameter of 0.1 to 0.6 mm is used as the spinneret 9, the extrusion speed is preferably 0.2 to 6.0 mL/hour per hole and more preferably 1.4 to 4.0 mL/hour. The distance that the coagulated modified fibroin passes through the coagulation liquid 11 (substantially, the distance from the yarn guide 18a to the yarn guide 18b) may be a length that allows efficient desolvation, for example, 200 to 500 mm. The withdrawing speed of the undrawn yarn may be, for example, 1 to 20 m/min and preferably 1 to 3 m/min. The residence time in the coagulation liquid 11 may be, for example, 0.01 to 3 minutes and preferably 0.05 to 0.15 minutes. In addition, drawing (pre-drawing) may be performed in the coagulation liquid 11. The coagulation liquid bath 20 may be provided in multiple stages, and the drawing may be performed in each stage or in a specific stage as necessary.
[0197] As the drawing performed in a case of obtaining the protein filament, for example, a pre-drawing performed in the coagulation liquid bath 20 and a wet heat drawing performed in the drawing bath 21 are employed, and a dry heat drawing is also employed.
[0198] The wet heat drawing can be performed in warm water, in a solution obtained by adding an organic solvent or the like to warm water, or in heated steam. The temperature may be, for example, 50.degree. C. to 90.degree. C. and preferably 75.degree. C. to 85.degree. C. In the wet heat drawing, the undrawn yarn (or pre-drawn yarn) can be drawn, for example, by 1 to 10 times and preferably by 2 to 8 times.
[0199] The dry heat drawing can be performed using an electric tubular furnace, a dry heat plate, or the like. The temperature may be, for example, 140.degree. C. to 270.degree. C. and preferably 160.degree. C. to 230.degree. C. In the dry heat drawing, the undrawn yarn (or pre-drawn yarn) can be drawn, for example, by 0.5 to 8 times and preferably by 1 to 4 times.
[0200] The wet heat drawing and the dry heat drawing may be performed independently or in combination or may be performed in multiple stages. That is, the wet heat drawing and the dry heat drawing can be performed in a suitable combination, for example, in a manner in which a first stage drawing is performed by wet heat drawing and a second stage drawing is performed by dry heat drawing or in a manner in which the first stage drawing is performed by wet heat drawing, the second stage drawing is performed by wet heat drawing, and a third stage drawing is performed by dry heat drawing.
[0201] The lower limit value of the final drawing ratio with respect to the undrawn yarn (or pre-drawn yarn) is preferably any of more than 1 time, 2 times or more, 3 times or more, 4 times or more, 5 times or more, 6 times or more, 7 times or more, 8 times or more, or 9 times or more, and the upper limit value is preferably 40 times or less, 30 times or less, 20 times or less, 15 times or less, 14 times or less, 13 times or less 12 times or less, 11 times or less, or 10 times or less.
[0202] The length of the protein filament obtained by the above method can be suitably adjusted depending on the spinning conditions and is preferably more than 1,500 in, and may be 10,000 in or more, 15,000 in or more, or 20,000 in or more.
[0203] The artificial fibroin filament preferably has a shrinkage rate (shrinkage rate after drying) of more than 7% and may have a shrinkage rate of 10% or more, 15% or more, 25% or more, 32% or more, 40% or more, 48% or more, 56% or more, 64% or more, or 72% or more, when the artificial fibroin filament has been brought into contact with an aqueous medium to be described later (for example, water having a temperature lower than the boiling point of the water) and then dried. The shrinkage rate after drying is usually 80% or less. Here, the shrinkage rate after drying of the fibroin filament is defined by the following expression.
Shrinkage rate after drying={1-(length of artificial fibroin filament after being brought into contact with aqueous medium and subsequently dried/length of artificial fibroin filament before being brought into contact with aqueous medium)}.times.100 (%)
[0204] In addition, the artificial fibroin filament may have a shrinkage rate (shrinkage rate when wetted) of, for example, 2% or more and may have a shrinkage rate of 2.5% or more, 3% or more, 3.5% or more, 4% or more, 4.5% or more, 5% or more, 5.5% or more, or 6% or more, when the artificial fibroin filament has been brought into a wet state by bringing the artificial fibroin filament into contact with an aqueous medium to be described later (for example, water having a temperature lower than the boiling point of the water). The upper limit of the shrinkage rate when wetted is not particularly limited but is 80% or less, 60% or less, 40% or less, 20% or less, 10% or less, 7% or less, 6% or less, 5% or less, 4% or less, or 3% or less. Here, the shrinkage rate when wetted can be calculated by the following expression.
Shrinkage rate when wetted={1-(length of artificial fibroin filament when wetted by being brought into contact with aqueous medium/length of artificial fibroin filament after being spun and before being brought into contact with aqueous medium)}.times.100 (%).
[0205] <Process b>
[0206] The process b (cutting process) is a process of cutting the artificial fibroin filament to obtain an artificial fibroin staple. Here, in a case where the process b is performed before process c, the artificial fibroin filament to be cut is an artificial fibroin filament before being crimped, and in a case where the process b is performed after the process c, the artificial fibroin filament to be cut is an artificial fibroin filament after being crimped.
[0207] The cutting can be performed using any device capable of cutting a protein fiber. An example of such a cutting device includes a tabletop-type fiber cutting machine (s/NO. IT-160201-NP-300).
[0208] The length of the staple is not particularly limited, but is preferably 20 mm or more, and may be 20 to 140 mm, 70 to 140 mm, or 20 to 70 mm.
[0209] <Process c>
[0210] The process c (crimping process) is a process of bringing the artificial fibroin filament or the artificial fibroin staple into contact with an aqueous medium and then crimping the artificial fibroin filament or the artificial fibroin staple (hereinafter sometimes referred to as "water crimping"). Here, in a case where the process c is performed before the process b, the artificial fibroin filament is crimped by being brought into contact with water to be curled, and in a case where the process c is performed after the process b, the artificial fibroin staple obtained by cutting the artificial fibroin filament is crimped by being brought into contact with water to be curled.
[0211] By being brought into contact with an aqueous medium, the artificial fibroin filament or the artificial fibroin staple can be crimped without depending on the external force. The aqueous medium is a medium of a liquid or gas (steam) containing water (including steam). The aqueous medium may be water or a mixed liquid of water and a hydrophilic solvent. Further, as the hydrophilic solvent, for example, a volatile solvent such as ethanol and methanol or a vapor thereof can be used. The aqueous medium may be a mixed liquid of water and a volatile solvent such as ethanol or methanol and is preferably water or a mixed liquid of water and ethanol. By using an aqueous medium containing a volatile solvent or a vapor thereof, it is possible to improve the drying speed after the water crimping, and further there is a possibility of imparting a soft texture to the crimped staple finally obtained. The ratio of water to the volatile solvent or the vapor thereof is not particularly limited, and, for example, water:volatile solvent or vapor thereof may be 10:90 to 90:10 by mass ratio. The proportion of water is preferably 30% by mass or more and may be 40% by mass or 50% by mass or more. In a case where the aqueous medium is a liquid, it is preferable to disperse an oil agent in the aqueous medium. In this case, the water crimping and the oil agent adhering can be performed at the same time. As the oil agent, any oil agent can be used as long it is a known oil agent used for general purposes including process passability and function impartability, such as an antistatic property, a friction reduction property, a flexibility imparting property, and a water repellency imparting property. The amount of the oil agent is not particularly limited and may be, for example, 1% to 10% by mass or 2% to 5% by mass with respect to the total amount of the oil agent and the aqueous medium.
[0212] The aqueous medium is preferably a liquid or gas containing water and having a temperature of 10.degree. C. to 230.degree. C. The temperature of the aqueous medium may be 10.degree. C. or higher, 25.degree. C. or higher, 40.degree. C. or higher, 60.degree. C. or higher, or 100.degree. C. or higher, and may be 230.degree. C. or lower, 120.degree. C. or lower, or 100.degree. C. or lower. More specifically, when the aqueous medium is a gas (steam), the temperature of the aqueous medium is preferably 100 to 230.degree. C., more preferably 100 to 120.degree. C. In a case where the steam of the aqueous medium is 230.degree. C. or less, the heat denaturation of the protein filament can be prevented. In a case where the aqueous medium is a liquid, the temperature of the aqueous medium is preferably 10.degree. C. or higher, 25.degree. C. or higher, or 40.degree. C. or higher from the viewpoint of efficiently imparting crimpness, and is preferably 60.degree. C. or lower from the viewpoint of highly maintaining fiber strength of the protein filament.
[0213] The time of bringing into contact with the aqueous medium is not particularly limited, but may be 30 seconds or longer, 1 minute or longer, or 2 minutes or longer, and preferably 10 minutes or shorter from the viewpoint of productivity. In the case of steam, it is considered that a high shrinkage rate can be obtained in a short time in comparison with a liquid. The contact with the aqueous medium may be carried out under normal pressure or under reduced pressure (for example, vacuum).
[0214] As a method for bringing into contact with the aqueous medium, a method of immersing an artificial fibroin filament or artificial fibroin staple in the aqueous medium, a method of spraying the steam of the aqueous medium to an artificial fibroin filament or artificial fibroin staple, and a method of exposing an artificial fibroin filament or an artificial fibroin staple to the environment filled with the steam of the aqueous medium. In a case where the aqueous medium is steam, bringing an artificial fibroin filament or an artificial fibroin staple into contact with the aqueous medium can be performed using a general steam setting device. Specific examples of the steam setting device include a device of product name: FMSA type steam setter (manufactured by Fukushin Kyougyo Co., Ltd.) and a device of a product name: EPS-400 (manufactured by Tsujii Dyeing Machine Manufacturing Co., Ltd.). The specific example of the method for crimping an artificial fibroin filament or an artificial fibroin staple by using the steam of the aqueous medium includes a method of accommodating an artificial fibroin filament or an artificial fibroin staple in a predetermined accommodating chamber and bringing the artificial fibroin filament or the artificial fibroin staple into contact with steam, while introducing the steam of the aqueous medium into the accommodating chamber and adjusting the temperature inside the accommodating chamber to the above-described predetermined temperature (for example, 100.degree. C. to 230.degree. C.).
[0215] The crimping process of an artificial fibroin filament or an artificial fibroin staple by contact with the aqueous medium is preferably performed in a state in which no tensile force is applied to the artificial fibroin filament or the artificial fibroin staple (not tensioned in the fiber axis direction) or in a state in which a predetermined amount of tensile force is applied (tensioned by a predetermined amount in the fiber axis direction). At this time, the degree of crimpness can be controlled by adjusting the tensile force applied to the artificial fibroin filament or the artificial fibroin staple. Examples of the adjusting method for the tensile force applied to the artificial fibroin filament or the artificial fibroin staple include, a method in which the artificial fibroin filaments or the artificial fibroin staples are, for example, hung by weighting units having various weights, and the loads applied to the filaments and staples are adjusted, a method in which both ends of the filament or staple are fixed in a state in which the filament or the staple are slackened, and the amount of slackness are changed variously, and a method in which the filament is wound around a wound body such as a paper tube or a bobbin so that the winding force is suitably changed (tightening force on the paper tube or a bobbin).
[0216] Further, the artificial fibroin filament or the artificial fibroin staple may be dried after being brought into contact with the aqueous medium (for example, water having a temperature lower than the boiling point of the water). The drying method is not particularly limited, and the drying may be natural drying, hot air drying, or hot roller drying. The drying temperature is not particularly limited and may be, for example, 20.degree. C. to 150.degree. C., preferably 40.degree. C. to 120.degree. C., and more preferably 60.degree. C. to 100.degree. C.
[0217] The artificial fibroin filament or the artificial fibroin staple after crimping and then drying preferably has a shrinkage rate (shrinkage rate after drying) of more than 7% and may have a shrinkage rate of 10% or more, 15% or more, 25% or more, 32% or more, 40% or more, 48% or more, 56% or more, 64% or more, or 72% or more. The shrinkage rate after drying is usually 80% or less. Here, the shrinkage rate after drying can be calculated by the following expression.
Shrinkage rate after drying={1-(length of artificial fibroin filament or artificial fibroin staple after crimping and then drying/length of artificial fibroin filament or artificial fibroin staple before being brought into contact with aqueous medium)}.times.100 (%)
[0218] In addition, the artificial fibroin filament or the artificial fibroin staple may have a shrinkage rate (shrinkage rate when wetted) of, for example, 2% or more and may have a shrinkage rate of 2.5% or more, 3% or more, 3.5% or more, 4% or more, 4.5% or more, 5% or more, 5.5% or more, or 6% or more, when the artificial fibroin filament has been brought into a wet state by bringing the artificial fibroin filament into contact with an aqueous medium (or example, water having a temperature lower than the boiling point of the water). The upper limit of the shrinkage rate when wetted is not particularly limited but is 80% or less, 60% or less, 40% or less, 20% or less, 10% or less, 7% or less, 6% or less, 5% or less, 4% or less, or 3% or less. Here, the shrinkage rate when wetted can be calculated by the following expression.
Shrinkage rate when wetted={1-(length of artificial fibroin filament or artificial fibroin staple when wetted by being brought into contact with water/length of artificial fibroin filament after being spun and before being brought into contact with water)}.times.100 (%).
[0219] The process b and the process c may be performed in a batch system or a continuous system. In the case of a batch system, for example, the artificial fibroin staple obtained by cutting the artificial fibroin filament is put into a container containing an aqueous medium at an appropriate temperature, and after being brought into contact therewith for a certain time, and then taken out and dried. In the case of a continuous system, for example, while sending out the filament from a bobbin around which the artificial fibroin filament wound, after being immersed in an aqueous medium at an appropriate temperature, the filament is dried by blowing hot air or sending the filament out on a hot roller, and then cut continuously.
[0220] Use applications for protein crimped staple
[0221] The obtained protein crimped staple is a single fiber having a soft feel and can be used for manufacturing a composite material such as spun yarn, nonwoven fabric, and a composite.
[0222] A predetermined amount of the artificial fibroin fiber can be shrunk when first brought into contact with an aqueous medium after spinning Since the protein crimped staple obtained by the manufacturing method of the present invention has already been brought into contact with moisture (aqueous medium), it is possible to suppress the dimensional change (shrinkage) of the staple due to moisture absorption during storage after manufacturing the staple or during the manufacturing process for a product using the staple.
EXAMPLES
[0223] Hereinafter, the present invention will be described more specifically based on Examples. However, the present invention is not limited to the following Examples.
[0224] <Manufacturing example of artificial spider silk protein (artificial spider silk fibroin) filament>
[0225] (1) Preparation of Plasmid Expressing Strain
[0226] Based on the base sequence and the amino acid sequence of a fibroin (GenBank Accession No.: P46804.1, GI: 1174415) derived from Nephila clavipes, a modified fibroin having the amino acid sequence set forth in SEQ ID NO: 13 (hereinafter, also referred to as "PRT799") was designed. The amino acid sequence set forth in SEQ ID NO: 13 has an amino acid sequence obtained by substituting, inserting, and deleting an amino acid residue for the purpose of improving productivity with respect to the amino acid sequence of the fibroin derived from Nephila clavipes, and furthermore, the amino acid sequence set forth in SEQ ID NO: 5 (tag sequence and hinge sequence) is added to the N-terminal of the sequence.
[0227] Next, a nucleic acid encoding PRT799 was synthesized. In the nucleic acid, an Ndel site was added to the 5' end and an EcoRI site was added downstream of the stop codon. The nucleic acid was cloned into a cloning vector (pUC118). Thereafter, the nucleic acid was enzymatically cleaved by treatment with Ndel and EcoRI, and then recombinated into a protein expression vector pET-22b (+) to obtain an expression vector.
[0228] (2) Expression of Protein
[0229] Escherichia coli BLR (DE3) was transformed with a pET22b(+) expression vector including the nucleic acid encoding a protein having the amino acid sequence set forth in SEQ ID NO: 13. The transformed Escherichia coli was cultured in 2 mL of an LB medium containing ampicillin for 15 hours. The culture solution was added to 100 mL of a seed culture medium (Table 4) containing ampicillin so that the OD600 was 0.005. While maintaining the temperature of the culture solution at 30.degree. C., flask culturing was carried out (for about 15 hours) until the OD600 reached 5, thereby obtaining a seed culture solution.
TABLE-US-00004 TABLE 4 Seed culture medium Reagent Concentration (g/L) Glucose 5.0 KH.sub.2PO.sub.4 4.0 K.sub.2HPO.sub.4 9.3 Yeast Extract 6.0 Ampicillin 0.1
[0230] The seed culture solution was added to a jar fermenter containing 500 mL of a production medium (Table 5) so that the OD600 was 0.05. The culture was carried out while keeping the culture solution temperature at 37.degree. C. and controlling the pH constant at 6.9. Further, the concentration of dissolved oxygen in the culture solution was maintained at 20% of the dissolved oxygen saturation concentration.
TABLE-US-00005 TABLE 5 Production medium Reagent Concentration (g/L) Glucose 12.0 KH.sub.2PO.sub.4 9.0 MgSO.sub.4.cndot.7H.sub.2O 2.4 Yeast Extract 15 FeSO.sub.4.cndot.7H.sub.2O 0.04 MnSO.sub.4.cndot.5H.sub.2O 0.04 CaCl.sub.2.cndot.2H.sub.2O 0.04 GD-113 (anti-foaming agent) 0.1(mL/L)
[0231] Immediately after glucose in the production medium was completely consumed, a feed solution (455 g/lL of glucose and 120 g/lL of Yeast Extract) was added at a rate of 1 mL/min. The culture was carried out while keeping the culture solution temperature at 37.degree. C. and controlling the pH constant at 6.9. Further, the concentration of dissolved oxygen in the culture solution was maintained at 20% of the dissolved oxygen saturation concentration, and the culture was carried out for 20 hours. Thereafter, 1 M isopropyl-P-thiogalactopyranoside
[0232] (IPTG) was added to the culture solution to a final concentration of 1 mM to induce the expression of the modified fibroin. 20 hours after the addition of IPTG, the culture solution was centrifuged to recover the bacterial cell pellet. SDS-PAGE was carried out using bacterial cell pellets prepared from the culture solution before the addition of IPTG and after the addition of IPTG, and the expression of the target modified fibroin was checked by the IPTG addition-dependent appearance of a band equivalent to the size of the target modified fibroin.
[0233] (3) Purification of Protein
[0234] The bacterial cell pellet recovered 2 hours after the addition of IPTG was washed with a 20 mM Tris-HCl buffer solution (pH 7.4). The bacterial cell pellet after washing was suspended in 20 mM Tris-HCl buffer solution (pH 7.4) containing about 1 mM PMSF, and the cell suspension was disrupted with a high-pressure homogenizer (manufactured by GEA Niro Soavi SpA). The disrupted cells were centrifuged to obtain a precipitate. The obtained precipitate was washed with a 20 mM Tris-HCl buffer solution (pH 7.4) until the obtained precipitate became highly pure. The precipitate after washing was suspended in 8 M guanidine buffer solution (8 M guanidine hydrochloride, 10 mM sodium dihydrogen phosphate, 20 mM NaCl, 1 mM Tris-HCl, pH 7.0) so that the concentration of the suspension was 100 mg/mL, and dissolved by stirring with a stirrer at 60.degree. C. for 30 minutes. After dissolution, dialysis was carried out in water using a dialysis tube (cellulose tube 36/32 manufactured by Sanko Junyaku Co., Ltd.). The white protein aggregate obtained after dialysis was recovered by centrifugation, the water content was removed with a lyophilizer, and the lyophilized powder was recovered to obtain the modified spider silk fibroin "PRT799".
[0235] (4) Manufacturing of Protein Filament
[0236] The above-described modified fibroin (PRT799) was added to DMSO such that the concentration was 24% by mass, and then LiCl was added thereto as a dissolution accelerator such that the concentration was 4.0% by mass. Then, the modified fibroin was dissolved for 3 hours using a shaker to obtain a DMSO solution of the modified fibroin. Dust and bubbles in the obtained DMSO solution were removed to obtain a doping liquid. The solution viscosity of the doping liquid was 5,000 cP (centipoise) at 90.degree. C.
[0237] Known dry-wet-type spinning was performed using the doping liquid obtained as described above and the spinning device 10 shown in FIG. 4, and the artificial spider silk fibroin fiber was wound around a bobbin. Here, the dry-wet-type spinning was performed under the following conditions.
[0238] Temperature of coagulation liquid (methanol): 5.degree. C. to 10.degree. C.
[0239] Drawing ratio: 4.52 times
[0240] Drying temperature: 80.degree. C.
Example 1
[0241] An artificial spider silk protein staple was prepared by bundling a plurality of artificial spider silk filaments had been obtained in the manufacturing example of an artificial spider silk protein and had been wound around a bobbin, and by cutting the filament into a length of 40 mm by a table-top type fiber cutting machine. The prepared artificial spider silk protein staple was crimped by being immersed in water at 40.degree. C. for 1 minute to be curled and then dried at 40.degree. C. for 18 hours to obtain a crimped staple. The obtained crimped staple is shown in FIG. 5. The shrinkage rate of the artificial spider silk protein staple when immersed in water was 50%.
Example 2
[0242] An artificial spider silk protein staple was prepared by bundling a plurality of artificial spider silk filaments had been obtained in the manufacturing example of an artificial spider silk protein and had been wound around a bobbin, and by cutting the filament into a length of 40 mm by a table-top type fiber cutting machine. A commercially available antistatic oil agent was dispersed in water to a concentration of 1% by weight (concentration in the oil agent dispersion liquid) to obtain an oil agent dispersion liquid. The prepared artificial spider silk protein staple was crimped by being immersed in an oil agent dispersion liquid at 20.degree. C. for 1 minute to be curled and then dried at 40.degree. C. for 18 hours to obtain a crimped staple. The obtained crimped staple is shown in FIG. 6. The shrinkage rate of the artificial spider silk protein staple when immersed in the oil agent dispersion liquid was 50%.
Comparative Example
[0243] An artificial spider silk protein filament was simply cut to obtain an uncrimped staple. A photograph of the obtained uncrimped staple is shown in FIG. 7.
Example 3
[0244] An artificial spider silk protein staple was prepared by bundling a plurality of artificial spider silk filaments had been obtained in the manufacturing example of an artificial spider silk protein and had been wound around a bobbin, and by cutting the filament into a length of 40 mm by a table-top type fiber cutting machine. The prepared artificial spider silk protein staple was crimped by being immersed in a liquid mixture of water and methanol (50% by mass of a methanol concentration) at 20.degree. C. for 1 minute to be curled and then dried at 40.degree. C. for 18 hours to obtain a crimped staple.
[0245] The touch feeling of each of the crimped staple of Example 1 obtained by immersing in 100% water and the crimped staple of Example 3 obtained by immersing in the liquid mixture of water and methanol was confirmed by a sensitivity response test. It has been found that the crimped staple of Example 3 obtained by immersing in the liquid mixture of water and methanol has a softer feel.
REFERENCE SIGNS LIST
[0246] 1 . . . extrusion device, 2 . . . undrawn yarn manufacturing device, 3 . . . wet heat drawing device, 4 . . . drying device, 6 . . . doping liquid, 10 . . . spinning device, 20 . . . coagulation liquid bath, 21 . . . drawing bath, 36 . . . protein filament.
Sequence CWU 1
1
411597PRTArtificial SequenceMet-PRT313 1Met Gly Pro Gly Gly Gln Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala Gly Gly Asn
Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly 20 25 30Gly Ser Ala Ala Ala
Ala Ala Gly Gly Tyr Gly Pro Gly Gly Gln Gly 35 40 45Pro Gly Gln Gln
Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro 50 55 60Gly Gly Tyr
Gly Pro Gly Gly Gln Gly Pro Ser Ala Ser Ala Ala Ala65 70 75 80Ala
Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Ala Ala 85 90
95Ala Ala Ala Gly Gly Tyr Gly Pro Gly Gly Gln Gly Pro Gly Gln Gln
100 105 110Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly
Ser Gly 115 120 125Pro Gly Gln Gln Gly Pro Tyr Gly Ser Ala Ala Ala
Ala Ala Gly Pro 130 135 140Gly Ser Gly Gly Tyr Gly Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Ala145 150 155 160Ala Ala Ala Ala Gly Pro Gly
Gly Tyr Gly Pro Gly Gly Gln Gly Pro 165 170 175Ser Ala Ser Ala Ala
Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly 180 185 190Gly Tyr Gly
Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly 195 200 205Ser
Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gly Ser Ala Ala 210 215
220Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Tyr225 230 235 240Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gly Gln
Gly Pro Tyr Gly 245 250 255Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly
Gly Tyr Gly Tyr Gly Pro 260 265 270Gly Gly Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala 275 280 285Gly Gly Asn Gly Pro Gly
Ser Gly Gly Tyr Gly Pro Gly Gln Gln Gly 290 295 300Pro Gly Gly Ser
Ala Ala Ala Ala Ala Gly Pro Gly Gly Gln Gly Pro305 310 315 320Tyr
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro 325 330
335Gly Gly Gln Gly Pro Gly Gly Tyr Gly Pro Gly Ser Ser Ala Ala Ala
340 345 350Ala Ala Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ser
Ser Ala 355 360 365Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Gln Gln
Gly Pro Tyr Gly 370 375 380Pro Gly Gly Ser Ala Ala Ala Ala Ala Gly
Gly Tyr Gln Gln Gly Pro385 390 395 400Gly Gly Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala 405 410 415Gly Pro Gly Gly Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala 420 425 430Ala Ala Gly
Pro Gly Gly Tyr Gly Pro Gly Gly Gln Gly Pro Ser Ala 435 440 445Ser
Ala Ala Ala Ala Ala Gly Gly Tyr Gly Ser Gly Pro Gly Gly Tyr 450 455
460Gly Pro Tyr Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala Gly Pro
Gly465 470 475 480Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala 485 490 495Ala Ala Ala Gly Gly Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro 500 505 510Gly Gly Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly Gly Tyr Gly 515 520 525Pro Gly Ala Ser Ala Ala
Ala Ala Ala Gly Gly Asn Gly Pro Gly Ser 530 535 540Gly Gly Tyr Gly
Pro Gly Gln Gln Gly Pro Gly Gly Ser Ala Ala Ala545 550 555 560Ala
Ala Gly Gly Tyr Gln Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly 565 570
575Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln
580 585 590Gly Pro Gly Ala Ser 5952590PRTArtificial
SequenceMet-PRT399 2Met Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala1 5 10 15Ala Ala Ala Gly Gly Asn Gly Pro Gly Ser Gly
Gln Gln Gly Pro Gly 20 25 30Gly Ser Gly Gly Tyr Gly Pro Gly Gly Gln
Gly Pro Gly Gln Gln Gly 35 40 45Pro Gly Ser Ser Ala Ala Ala Ala Ala
Gly Pro Gly Gly Tyr Gly Pro 50 55 60Gly Gly Gln Gly Pro Ser Ala Ser
Ala Ala Ala Ala Ala Gly Pro Gly65 70 75 80Ser Gly Gln Gln Gly Pro
Gly Ala Ser Gly Gly Tyr Gly Pro Gly Gly 85 90 95Gln Gly Pro Gly Gln
Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala 100 105 110Gly Gly Tyr
Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Ser Ala 115 120 125Ala
Ala Ala Ala Gly Pro Gly Ser Gly Gly Tyr Gly Gln Gly Pro Tyr 130 135
140Gly Pro Gly Ala Ser Gly Pro Gly Gly Tyr Gly Pro Gly Gly Gln
Gly145 150 155 160Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Ser Gly
Gln Gln Gly Pro 165 170 175Gly Gly Tyr Gly Pro Tyr Ala Ser Ala Ala
Ala Ala Ala Gly Gly Tyr 180 185 190Gly Ser Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gly Ser Gly 195 200 205Ser Gly Gln Gln Gly Pro
Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala 210 215 220Ala Ala Ala Gly
Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ser Ser225 230 235 240Ala
Ala Ala Ala Ala Gly Gly Tyr Gly Tyr Gly Pro Gly Gly Gln Gly 245 250
255Pro Tyr Gly Pro Gly Ala Ser Gly Gly Asn Gly Pro Gly Ser Gly Gly
260 265 270Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gly Ser Ala Ala Ala
Ala Ala 275 280 285Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala
Ser Ala Ala Ala 290 295 300Ala Ala Gly Gly Tyr Gly Pro Gly Gly Gln
Gly Pro Gly Gly Tyr Gly305 310 315 320Pro Gly Ser Ser Gly Pro Gly
Gly Gln Gly Pro Tyr Gly Pro Gly Ser 325 330 335Ser Ala Ala Ala Ala
Ala Gly Gly Tyr Gly Pro Gly Gln Gln Gly Pro 340 345 350Tyr Gly Pro
Gly Gly Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gln Gln 355 360 365Gly
Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly 370 375
380Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala
Gly385 390 395 400Pro Gly Gly Tyr Gly Pro Gly Gly Gln Gly Pro Ser
Ala Ser Ala Ala 405 410 415Ala Ala Ala Gly Gly Tyr Gly Ser Gly Pro
Gly Gly Tyr Gly Pro Tyr 420 425 430Gly Pro Gly Gly Ser Gly Pro Gly
Ser Gly Gln Gln Gly Gln Gly Pro 435 440 445Tyr Gly Pro Gly Ala Ser
Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro 450 455 460Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala465 470 475 480Gly
Pro Gly Ser Gly Gly Tyr Gly Pro Gly Ala Ser Gly Gly Asn Gly 485 490
495Pro Gly Ser Gly Gly Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gly Ser
500 505 510Ala Ala Ala Ala Ala Gly Gly Tyr Gln Gln Gly Pro Gly Gly
Gln Gly 515 520 525Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala
Gly Gly Tyr Gly 530 535 540Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Gly Ser Gly Ser545 550 555 560Gly Gln Gln Gly Pro Gly Gln
Gln Gly Pro Tyr Ala Ser Ala Ala Ala 565 570 575Ala Ala Gly Pro Gly
Ser Gly Gln Gln Gly Pro Gly Ala Ser 580 585 5903597PRTArtificial
SequenceMet-PRT380 3Met Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala1 5 10 15Ala Ala Ala Gly Gln Asn Gly Pro Gly Ser Gly
Gln Gln Gly Pro Gly 20 25 30Gln Ser Ala Ala Ala Ala Ala Gly Gln Tyr
Gly Pro Gly Gln Gln Gly 35 40 45Pro Gly Gln Gln Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala Gly Pro 50 55 60Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro Ser Ala Ser Ala Ala Ala65 70 75 80Ala Ala Gly Pro Gly Ser
Gly Gln Gln Gly Pro Gly Ala Ser Ala Ala 85 90 95Ala Ala Ala Gly Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln 100 105 110Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly 115 120 125Pro
Gly Gln Gln Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly Pro 130 135
140Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser
Ala145 150 155 160Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro 165 170 175Ser Ala Ser Ala Ala Ala Ala Ala Gly Ser
Gly Gln Gln Gly Pro Gly 180 185 190Gln Tyr Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala Gly Gln Tyr Gly 195 200 205Ser Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala 210 215 220Ala Ala Ala Gly
Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr225 230 235 240Ala
Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly 245 250
255Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Tyr Gly Pro
260 265 270Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala Ala 275 280 285Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro
Gly Gln Gln Gly 290 295 300Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Pro Gly Gln Gln Gly Pro305 310 315 320Tyr Gly Pro Gly Ala Ser Ala
Ala Ala Ala Ala Gly Gln Tyr Gly Pro 325 330 335Gly Gln Gln Gly Pro
Gly Gln Tyr Gly Pro Gly Ser Ser Ala Ala Ala 340 345 350Ala Ala Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala 355 360 365Ala
Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Tyr Gly 370 375
380Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln Gly
Pro385 390 395 400Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala
Ala Ala Ala Ala 405 410 415Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala 420 425 430Ala Ala Gly Pro Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Ser Ala 435 440 445Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr 450 455 460Gly Pro Tyr Gly
Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly Pro Gly465 470 475 480Ser
Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala 485 490
495Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
500 505 510Gly Gln Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln
Tyr Gly 515 520 525Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Asn
Gly Pro Gly Ser 530 535 540Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro
Gly Gln Ser Ala Ala Ala545 550 555 560Ala Ala Gly Gln Tyr Gln Gln
Gly Pro Gly Gln Gln Gly Pro Tyr Gly 565 570 575Pro Gly Ala Ser Ala
Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln 580 585 590Gly Pro Gly
Ala Ser 5954590PRTArtificial SequenceMet-PRT410 4Met Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala
Gly Gln Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly 20 25 30Gln Ser
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly 35 40 45Pro
Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro 50 55
60Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly65
70 75 80Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly
Gln 85 90 95Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala Ala Ala
Ala Ala 100 105 110Gly Gln Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln
Tyr Gly Gln Gly Pro Tyr 130 135 140Gly Pro Gly Ala Ser Gly Pro Gly
Gln Tyr Gly Pro Gly Gln Gln Gly145 150 155 160Pro Ser Ala Ser Ala
Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro 165 170 175Gly Gln Tyr
Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr 180 185 190Gly
Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly 195 200
205Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala
210 215 220Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ser Ser225 230 235 240Ala Ala Ala Ala Ala Gly Gln Tyr Gly Tyr Gly
Pro Gly Gln Gln Gly 245 250 255Pro Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln 260 265 270Tyr Gly Pro Gly Gln Gln Gly
Pro Gly Gln Ser Ala Ala Ala Ala Ala 275 280 285Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala 290 295 300Ala Ala Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr Gly305 310 315
320Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser
325 330 335Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro 340 345 350Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gln Gln 355 360 365Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Gly Pro Gly 370 375 380Gln Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly385 390 395 400Pro Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 405 410 415Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr 420 425 430Gly
Pro Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro 435 440
445Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro
450 455 460Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala
Ala Ala465 470 475 480Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala
Ser Gly Gln Asn Gly 485 490 495Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln Ser 500 505 510Ala Ala Ala Ala Ala Gly Gln
Tyr Gln Gln Gly Pro Gly Gln Gln Gly 515 520 525Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly 530 535 540Ser Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser545 550 555
560Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala
565 570 575Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser
580 585 590512PRTArtificial SequenceHisTag 5Met His His His His His
His Ser Ser Gly Ser Ser1 5 106608PRTArtificial SequencePRT313 6Met
His His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Gly1 5 10
15Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gly
20 25 30Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gly Ser Ala Ala
Ala 35 40 45Ala Ala Gly Gly Tyr Gly Pro Gly Gly Gln Gly Pro Gly Gln
Gln Gly 50 55 60Pro
Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Gly Tyr Gly Pro65 70 75
80Gly Gly Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly
85 90 95Ser Gly Gln Gln Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Gly 100 105 110Tyr Gly Pro Gly Gly Gln Gly Pro Gly Gln Gln Gly Pro
Gly Ser Ser 115 120 125Ala Ala Ala Ala Ala Gly Gly Tyr Gly Ser Gly
Pro Gly Gln Gln Gly 130 135 140Pro Tyr Gly Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly Gly Tyr145 150 155 160Gly Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 165 170 175Pro Gly Gly Tyr
Gly Pro Gly Gly Gln Gly Pro Ser Ala Ser Ala Ala 180 185 190Ala Ala
Ala Gly Ser Gly Gln Gln Gly Pro Gly Gly Tyr Gly Pro Tyr 195 200
205Ala Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly Ser Gly Pro Gly Gln
210 215 220Gln Gly Pro Tyr Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala
Gly Ser225 230 235 240Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr
Ala Ser Ala Ala Ala 245 250 255Ala Ala Gly Pro Gly Gly Gln Gly Pro
Tyr Gly Pro Gly Ser Ser Ala 260 265 270Ala Ala Ala Ala Gly Gly Tyr
Gly Tyr Gly Pro Gly Gly Gln Gly Pro 275 280 285Tyr Gly Pro Gly Ala
Ser Ala Ala Ala Ala Ala Gly Gly Asn Gly Pro 290 295 300Gly Ser Gly
Gly Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gly Ser Ala305 310 315
320Ala Ala Ala Ala Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala
325 330 335Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Gly Gln
Gly Pro 340 345 350Gly Gly Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala
Ala Gly Pro Gly 355 360 365Gly Gln Gly Pro Tyr Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala Gly 370 375 380Gly Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gly Ser Ala385 390 395 400Ala Ala Ala Ala Gly
Gly Tyr Gln Gln Gly Pro Gly Gly Gln Gly Pro 405 410 415Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gly Gln 420 425 430Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly 435 440
445Gly Tyr Gly Pro Gly Gly Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala
450 455 460Ala Gly Gly Tyr Gly Ser Gly Pro Gly Gly Tyr Gly Pro Tyr
Gly Pro465 470 475 480Gly Gly Ser Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Gln Gln Gly 485 490 495Gln Gly Pro Tyr Gly Pro Gly Ala Ser
Ala Ala Ala Ala Ala Gly Gly 500 505 510Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gly Ser Ala Ala 515 520 525Ala Ala Ala Gly Pro
Gly Ser Gly Gly Tyr Gly Pro Gly Ala Ser Ala 530 535 540Ala Ala Ala
Ala Gly Gly Asn Gly Pro Gly Ser Gly Gly Tyr Gly Pro545 550 555
560Gly Gln Gln Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala Gly Gly Tyr
565 570 575Gln Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala
Ser Ala 580 585 590Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly Ala Ser 595 600 6057601PRTArtificial SequencePRT399 7Met
His His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Gly1 5 10
15Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gly
20 25 30Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gly Ser Gly Gly
Tyr 35 40 45Gly Pro Gly Gly Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser
Ser Ala 50 55 60Ala Ala Ala Ala Gly Pro Gly Gly Tyr Gly Pro Gly Gly
Gln Gly Pro65 70 75 80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Gln Gln Gly 85 90 95Pro Gly Ala Ser Gly Gly Tyr Gly Pro Gly
Gly Gln Gly Pro Gly Gln 100 105 110Gln Gly Pro Gly Ser Ser Ala Ala
Ala Ala Ala Gly Gly Tyr Gly Ser 115 120 125Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser Gly
Gly Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser145 150 155 160Gly
Pro Gly Gly Tyr Gly Pro Gly Gly Gln Gly Pro Ser Ala Ser Ala 165 170
175Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly Gly Tyr Gly Pro
180 185 190Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly Ser Gly
Pro Gly 195 200 205Gln Gln Gly Pro Tyr Gly Pro Gly Gly Ser Gly Ser
Gly Gln Gln Gly 210 215 220Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala Gly Pro225 230 235 240Gly Gly Gln Gly Pro Tyr Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala 245 250 255Gly Gly Tyr Gly Tyr
Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly 260 265 270Ala Ser Gly
Gly Asn Gly Pro Gly Ser Gly Gly Tyr Gly Pro Gly Gln 275 280 285Gln
Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala Gly Pro Gly Gly Gln 290 295
300Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gly
Tyr305 310 315 320Gly Pro Gly Gly Gln Gly Pro Gly Gly Tyr Gly Pro
Gly Ser Ser Gly 325 330 335Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly
Ser Ser Ala Ala Ala Ala 340 345 350Ala Gly Gly Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Gly 355 360 365Ser Ala Ala Ala Ala Ala
Gly Gly Tyr Gln Gln Gly Pro Gly Gly Gln 370 375 380Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Pro Gly Gly Gln Gly Pro Tyr385 390 395 400Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gly Tyr Gly 405 410
415Pro Gly Gly Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Gly
420 425 430Tyr Gly Ser Gly Pro Gly Gly Tyr Gly Pro Tyr Gly Pro Gly
Gly Ser 435 440 445Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr
Gly Pro Gly Ala 450 455 460Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly
Pro Gly Gln Gln Gly Pro465 470 475 480Tyr Gly Pro Gly Gly Ser Ala
Ala Ala Ala Ala Gly Pro Gly Ser Gly 485 490 495Gly Tyr Gly Pro Gly
Ala Ser Gly Gly Asn Gly Pro Gly Ser Gly Gly 500 505 510Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gly Ser Ala Ala Ala Ala Ala 515 520 525Gly
Gly Tyr Gln Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly 530 535
540Ala Ser Ala Ala Ala Ala Ala Gly Gly Tyr Gly Ser Gly Pro Gly
Gln545 550 555 560Gln Gly Pro Tyr Gly Pro Gly Gly Ser Gly Ser Gly
Gln Gln Gly Pro 565 570 575Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala
Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Gln Gln Gly Pro Gly Ala
Ser 595 6008608PRTArtificial SequencePRT380 8Met His His His His
His His Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln 20 25 30Asn Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala Ala 35 40 45Ala Ala
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly 50 55 60Pro
Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro65 70 75
80Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly
85 90 95Ser Gly Gln Gln Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Gln 100 105 110Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Gly Ser Ser 115 120 125Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly
Pro Gly Gln Gln Gly 130 135 140Pro Tyr Gly Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly Gln Tyr145 150 155 160Gly Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 165 170 175Pro Gly Gln Tyr
Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 180 185 190Ala Ala
Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro Tyr 195 200
205Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln
210 215 220Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala
Gly Ser225 230 235 240Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr
Ala Ser Ala Ala Ala 245 250 255Ala Ala Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Pro Gly Ser Ser Ala 260 265 270Ala Ala Ala Ala Gly Gln Tyr
Gly Tyr Gly Pro Gly Gln Gln Gly Pro 275 280 285Tyr Gly Pro Gly Ala
Ser Ala Ala Ala Ala Ala Gly Gln Asn Gly Pro 290 295 300Gly Ser Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser Ala305 310 315
320Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala
325 330 335Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro 340 345 350Gly Gln Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala
Ala Gly Pro Gly 355 360 365Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala Gly 370 375 380Gln Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gln Ser Ala385 390 395 400Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly Pro 405 410 415Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln 420 425 430Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly 435 440
445Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala
450 455 460Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr
Gly Pro465 470 475 480Gly Gln Ser Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Gln Gln Gly 485 490 495Gln Gly Pro Tyr Gly Pro Gly Ala Ser
Ala Ala Ala Ala Ala Gly Gln 500 505 510Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gln Ser Ala Ala 515 520 525Ala Ala Ala Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser Ala 530 535 540Ala Ala Ala
Ala Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro545 550 555
560Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly Gln Tyr
565 570 575Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala
Ser Ala 580 585 590Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly Ala Ser 595 600 6059601PRTArtificial SequencePRT410 9Met
His His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10
15Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln
20 25 30Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gln Ser Gly Gln
Tyr 35 40 45Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser
Ser Ala 50 55 60Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro65 70 75 80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Gln Gln Gly 85 90 95Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln 100 105 110Gln Gly Pro Gly Ser Ser Ala Ala
Ala Ala Ala Gly Gln Tyr Gly Ser 115 120 125Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser Gly
Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser145 150 155 160Gly
Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala 165 170
175Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro
180 185 190Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly
Pro Gly 195 200 205Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser
Gly Gln Gln Gly 210 215 220Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala Gly Pro225 230 235 240Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala 245 250 255Gly Gln Tyr Gly Tyr
Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly 260 265 270Ala Ser Gly
Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln 275 280 285Gln
Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln 290 295
300Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln
Tyr305 310 315 320Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro
Gly Ser Ser Gly 325 330 335Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ser Ser Ala Ala Ala Ala 340 345 350Ala Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Gln 355 360 365Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Gln Gln Gly Pro Gly Gln Gln 370 375 380Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr385 390 395 400Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly 405 410
415Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln
420 425 430Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly
Gln Ser 435 440 445Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr
Gly Pro Gly Ala 450 455 460Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro465 470 475 480Tyr Gly Pro Gly Gln Ser Ala
Ala Ala Ala Ala Gly Pro Gly Ser Gly 485 490 495Gln Tyr Gly Pro Gly
Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln 500 505 510Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala 515 520 525Gly
Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly 530 535
540Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly
Gln545 550 555 560Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly
Gln Gln Gly Pro 565 570 575Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala
Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Gln Gln Gly Pro Gly Ala
Ser 595 60010565PRTArtificial SequenceMet-PRT468 10Met Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala
Ala Ala Gly Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly 20 25 30Pro Gly
Gln Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 35 40 45Gln
Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly 50 55
60Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala65
70 75 80Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser
Gly 85 90 95Gln Tyr Gly Pro Gly Gln Gln
Gly Pro Gly Gln Gln Gly Pro Gly Ser 100 105 110Ser Ala Ala Ala Ala
Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly 115 120 125Gln Gln Gly
Pro Tyr Gly Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro 130 135 140Gly
Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly145 150
155 160Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala
Ala 165 170 175Ala Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly
Gln Tyr Gly 180 185 190Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala
Gly Ser Tyr Gly Ser 195 200 205Gly Pro Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Gln Ser Gly Ser Gly 210 215 220Gln Gln Gly Pro Gly Gln Gln
Gly Pro Tyr Ala Ser Ala Ala Ala Ala225 230 235 240Ala Ala Ala Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser 245 250 255Ala Ala
Ala Ala Ala Ala Ala Gly Ser Tyr Gly Tyr Gly Pro Gly Gln 260 265
270Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser
275 280 285Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Pro Ser Ala
Ala Ala 290 295 300Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Pro Gly Ala305 310 315 320Ser Ala Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Pro Gly Gln Gln 325 330 335Gly Pro Gly Gln Tyr Gly Pro
Gly Ser Ser Gly Pro Gly Gln Gln Gly 340 345 350Pro Tyr Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser 355 360 365Tyr Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala 370 375 380Ala
Ala Ala Ala Ala Gly Ser Tyr Gln Gln Gly Pro Gly Gln Gln Gly385 390
395 400Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr
Gly 405 410 415Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro
Gly Gln Tyr 420 425 430Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala
Ala Ala Ala Ala Ala 435 440 445Ala Gly Ser Tyr Gly Ser Gly Pro Gly
Gln Tyr Gly Pro Tyr Gly Pro 450 455 460Gly Gln Ser Gly Pro Gly Ser
Gly Gln Gln Gly Gln Gly Pro Tyr Gly465 470 475 480Pro Gly Ala Ser
Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro 485 490 495Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala 500 505
510Ala Ala Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln
515 520 525Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly
Pro Gly 530 535 540Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Gln Gln545 550 555 560Gly Pro Gly Ala Ser
56511576PRTArtificial SequencePRT468 11Met His His His His His His
Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 20 25 30Gly Ser Asn Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Gln Ser Gly 35 40 45Gln Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser 50 55 60Ser Ala Ala
Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly65 70 75 80Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro 85 90
95Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly
100 105 110Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala Ala
Ala Ala 115 120 125Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln
Gln Gly Pro Tyr 130 135 140Gly Ser Ala Ala Ala Ala Ala Ala Ala Gly
Pro Gly Ser Gly Gln Tyr145 150 155 160Gly Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Gly Pro Gly Gln Tyr Gly 165 170 175Pro Gly Gln Gln Gly
Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala 180 185 190Gly Ser Gly
Gln Gln Gly Pro Gly Gln Tyr Gly Pro Tyr Ala Ser Ala 195 200 205Ala
Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln Gln 210 215
220Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro
Gly225 230 235 240Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala
Ala Ala Gly Pro 245 250 255Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser
Ser Ala Ala Ala Ala Ala 260 265 270Ala Ala Gly Ser Tyr Gly Tyr Gly
Pro Gly Gln Gln Gly Pro Tyr Gly 275 280 285Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro 290 295 300Gly Gln Gln Gly
Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly305 310 315 320Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala 325 330
335Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr
340 345 350Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly
Pro Gly 355 360 365Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr
Gly Pro Gly Gln 370 375 380Gln Gly Pro Tyr Gly Pro Gly Pro Ser Ala
Ala Ala Ala Ala Ala Ala385 390 395 400Gly Ser Tyr Gln Gln Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly 405 410 415Ala Ser Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala 420 425 430Ala Ala Ala
Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln 435 440 445Gly
Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly 450 455
460Ser Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly
Pro465 470 475 480Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala 485 490 495Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly
Pro Gly Gln Gln Gly Pro 500 505 510Tyr Gly Pro Gly Pro Ser Ala Ala
Ala Ala Ala Ala Ala Gly Pro Gly 515 520 525Ser Gly Gln Tyr Gly Pro
Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser 530 535 540Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Gly Pro Ser Ala Ala Ala545 550 555 560Ala
Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser 565 570
575122364PRTArtificial SequenceMet-PRT799 12Met Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala Gly Gln
Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly 20 25 30Gln Ser Gly Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly 35 40 45Pro Gly Ser
Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro 50 55 60Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly65 70 75
80Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly Gln
85 90 95Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala
Ala 100 105 110Gly Gln Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Tyr
Gly Gln Gly Pro Tyr 130 135 140Gly Pro Gly Ala Ser Gly Pro Gly Gln
Tyr Gly Pro Gly Gln Gln Gly145 150 155 160Pro Ser Ala Ser Ala Ala
Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro 165 170 175Gly Gln Tyr Gly
Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr 180 185 190Gly Ser
Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly 195 200
205Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala
210 215 220Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ser Ser225 230 235 240Ala Ala Ala Ala Ala Gly Gln Tyr Gly Tyr Gly
Pro Gly Gln Gln Gly 245 250 255Pro Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln 260 265 270Tyr Gly Pro Gly Gln Gln Gly
Pro Gly Gln Ser Ala Ala Ala Ala Ala 275 280 285Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala 290 295 300Ala Ala Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr Gly305 310 315
320Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser
325 330 335Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro 340 345 350Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gln Gln 355 360 365Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Gly Pro Gly 370 375 380Gln Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly385 390 395 400Pro Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 405 410 415Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr 420 425 430Gly
Pro Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro 435 440
445Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro
450 455 460Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala
Ala Ala465 470 475 480Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala
Ser Gly Gln Asn Gly 485 490 495Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln Ser 500 505 510Ala Ala Ala Ala Ala Gly Gln
Tyr Gln Gln Gly Pro Gly Gln Gln Gly 515 520 525Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly 530 535 540Ser Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser545 550 555
560Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala
565 570 575Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser
Gly Gln 580 585 590Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala Ala Gly Gln 595 600 605Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro
Gly Gln Ser Gly Gln Tyr 610 615 620Gly Pro Gly Gln Gln Gly Pro Gly
Gln Gln Gly Pro Gly Ser Ser Ala625 630 635 640Ala Ala Ala Ala Gly
Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro 645 650 655Ser Ala Ser
Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly 660 665 670Pro
Gly Ala Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 675 680
685Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser
690 695 700Gly Pro Gly Gln Gln Gly Pro Tyr Gly Ser Ala Ala Ala Ala
Ala Gly705 710 715 720Pro Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr
Gly Pro Gly Ala Ser 725 730 735Gly Pro Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala 740 745 750Ala Ala Ala Ala Gly Ser Gly
Gln Gln Gly Pro Gly Gln Tyr Gly Pro 755 760 765Tyr Ala Ser Ala Ala
Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly 770 775 780Gln Gln Gly
Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly785 790 795
800Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Pro
805 810 815Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala
Ala Ala 820 825 830Gly Gln Tyr Gly Tyr Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Pro Gly 835 840 845Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly
Gln Tyr Gly Pro Gly Gln 850 855 860Gln Gly Pro Gly Gln Ser Ala Ala
Ala Ala Ala Gly Pro Gly Gln Gln865 870 875 880Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr 885 890 895Gly Pro Gly
Gln Gln Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly 900 905 910Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala 915 920
925Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln
930 935 940Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln Gly Pro Gly
Gln Gln945 950 955 960Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly
Gln Gln Gly Pro Tyr 965 970 975Gly Pro Gly Ala Ser Ala Ala Ala Ala
Ala Gly Pro Gly Gln Tyr Gly 980 985 990Pro Gly Gln Gln Gly Pro Ser
Ala Ser Ala Ala Ala Ala Ala Gly Gln 995 1000 1005Tyr Gly Ser Gly
Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln 1010 1015 1020Ser Gly
Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro 1025 1030
1035Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln
1040 1045 1050Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala
Ala Gly 1055 1060 1065Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser
Gly Gln Asn Gly 1070 1075 1080Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln 1085 1090 1095Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln 1100 1105 1110Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 1115 1120 1125Gln Tyr Gly
Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly 1130 1135 1140Gln
Ser Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr 1145 1150
1155Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
1160 1165 1170Pro Gly Ala Ser Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ala Ser 1175 1180 1185Ala Ala Ala Ala Ala Gly Gln Asn Gly Pro Gly
Ser Gly Gln Gln 1190 1195 1200Gly Pro Gly Gln Ser Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro 1205 1210 1215Gly Gln Gln Gly Pro Gly Ser
Ser Ala Ala Ala Ala Ala Gly Pro 1220 1225 1230Gly Gln Tyr Gly Pro
Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 1235 1240 1245Ala Ala Ala
Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser 1250 1255 1260Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro 1265 1270
1275Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro
1280 1285 1290Gly Gln Gln Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala
Gly Pro 1295 1300 1305Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly
Pro Gly Ala Ser 1310 1315 1320Gly Pro Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro Ser Ala Ser 1325 1330 1335Ala Ala Ala Ala Ala Gly Ser
Gly Gln Gln Gly Pro Gly Gln Tyr 1340 1345 1350Gly Pro Tyr Ala Ser
Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser 1355 1360 1365Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser 1370 1375 1380Gly
Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala 1385 1390
1395Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser
1400 1405 1410Ser Ala Ala Ala Ala
Ala Gly Gln Tyr Gly Tyr Gly Pro Gly Gln 1415 1420 1425Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly 1430 1435 1440Ser
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser Ala 1445 1450
1455Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
1460 1465 1470Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly
Gln Gln 1475 1480 1485Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly
Pro Gly Gln Gln 1490 1495 1500Gly Pro Tyr Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala Gly Gln 1505 1510 1515Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gln Ser Ala 1520 1525 1530Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly 1535 1540 1545Pro Tyr Gly
Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr 1550 1555 1560Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr 1565 1570
1575Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala
1580 1585 1590Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr
Gly Pro 1595 1600 1605Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly
Gln Gly Pro Tyr 1610 1615 1620Gly Pro Gly Ala Ser Ala Ala Ala Ala
Ala Gly Gln Tyr Gly Pro 1625 1630 1635Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Gln Ser Ala Ala Ala Ala 1640 1645 1650Ala Gly Pro Gly Ser
Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln 1655 1660 1665Asn Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro 1670 1675 1680Gly
Gln Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln Gly Pro 1685 1690
1695Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala
1700 1705 1710Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro
Tyr Gly 1715 1720 1725Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro
Gly Gln Gln Gly 1730 1735 1740Pro Tyr Ala Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly Gln 1745 1750 1755Gln Gly Pro Gly Ala Ser Gly
Gln Gln Gly Pro Tyr Gly Pro Gly 1760 1765 1770Ala Ser Ala Ala Ala
Ala Ala Gly Gln Asn Gly Pro Gly Ser Gly 1775 1780 1785Gln Gln Gly
Pro Gly Gln Ser Gly Gln Tyr Gly Pro Gly Gln Gln 1790 1795 1800Gly
Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala 1805 1810
1815Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser
1820 1825 1830Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly 1835 1840 1845Ala Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly
Pro Gly Gln Gln 1850 1855 1860Gly Pro Gly Ser Ser Ala Ala Ala Ala
Ala Gly Gln Tyr Gly Ser 1865 1870 1875Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Ser Ala Ala Ala Ala Ala 1880 1885 1890Gly Pro Gly Ser Gly
Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly 1895 1900 1905Ala Ser Gly
Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser 1910 1915 1920Ala
Ser Ala Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly 1925 1930
1935Gln Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr
1940 1945 1950Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Gln Ser 1955 1960 1965Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly
Pro Tyr Ala Ser 1970 1975 1980Ala Ala Ala Ala Ala Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro 1985 1990 1995Gly Ser Ser Ala Ala Ala Ala
Ala Gly Gln Tyr Gly Tyr Gly Pro 2000 2005 2010Gly Gln Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly 2015 2020 2025Pro Gly Ser
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 2030 2035 2040Ser
Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly 2045 2050
2055Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly
2060 2065 2070Gln Gln Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly
Pro Gly 2075 2080 2085Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala 2090 2095 2100Gly Gln Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gln 2105 2110 2115Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln 2120 2125 2130Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly 2135 2140 2145Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly 2150 2155 2160Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala 2165 2170
2175Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr
2180 2185 2190Gly Pro Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly
Gln Gly 2195 2200 2205Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala
Ala Gly Gln Tyr 2210 2215 2220Gly Pro Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Gln Ser Ala Ala 2225 2230 2235Ala Ala Ala Gly Pro Gly Ser
Gly Gln Tyr Gly Pro Gly Ala Ser 2240 2245 2250Gly Gln Asn Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln 2255 2260 2265Gly Pro Gly
Gln Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln 2270 2275 2280Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala 2285 2290
2295Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro
2300 2305 2310Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro
Gly Gln 2315 2320 2325Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser 2330 2335 2340Gly Gln Gln Gly Ser Ser Val Asp Lys
Leu Ala Ala Ala Leu Glu 2345 2350 2355His His His His His His
2360132375PRTArtificial SequencePRT799 13Met His His His His His
His Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln 20 25 30Asn Gly Pro Gly
Ser Gly Gln Gln Gly Pro Gly Gln Ser Gly Gln Tyr 35 40 45Gly Pro Gly
Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala 50 55 60Ala Ala
Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro65 70 75
80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
85 90 95Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly
Gln 100 105 110Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln
Tyr Gly Ser 115 120 125Gly Pro Gly Gln Gln Gly Pro Tyr Gly Ser Ala
Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser Gly Gln Tyr Gly Gln Gly
Pro Tyr Gly Pro Gly Ala Ser145 150 155 160Gly Pro Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala 165 170 175Ala Ala Ala Ala
Gly Ser Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro 180 185 190Tyr Ala
Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly 195 200
205Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly
210 215 220Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala
Gly Pro225 230 235 240Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala 245 250 255Gly Gln Tyr Gly Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly 260 265 270Ala Ser Gly Gln Asn Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Gln 275 280 285Gln Gly Pro Gly Gln
Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln 290 295 300Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr305 310 315
320Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly
325 330 335Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala
Ala Ala 340 345 350Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Pro Gly Gln 355 360 365Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln
Gln Gly Pro Gly Gln Gln 370 375 380Gly Pro Tyr Gly Pro Gly Ala Ser
Gly Pro Gly Gln Gln Gly Pro Tyr385 390 395 400Gly Pro Gly Ala Ser
Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly 405 410 415Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln 420 425 430Tyr
Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser 435 440
445Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala
450 455 460Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro465 470 475 480Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly 485 490 495Gln Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln 500 505 510Tyr Gly Pro Gly Gln Gln Gly
Pro Gly Gln Ser Ala Ala Ala Ala Ala 515 520 525Gly Gln Tyr Gln Gln
Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly 530 535 540Ala Ser Ala
Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln545 550 555
560Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro
565 570 575Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
Pro Gly 580 585 590Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Gln
Gly Pro Tyr Gly 595 600 605Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Gln Asn Gly Pro Gly Ser 610 615 620Gly Gln Gln Gly Pro Gly Gln Ser
Gly Gln Tyr Gly Pro Gly Gln Gln625 630 635 640Gly Pro Gly Gln Gln
Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly 645 650 655Pro Gly Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 660 665 670Ala
Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly 675 680
685Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser
690 695 700Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly
Gln Gln705 710 715 720Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly
Pro Gly Ser Gly Gln 725 730 735Tyr Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Gly Pro Gly Gln Tyr 740 745 750Gly Pro Gly Gln Gln Gly Pro
Ser Ala Ser Ala Ala Ala Ala Ala Gly 755 760 765Ser Gly Gln Gln Gly
Pro Gly Gln Tyr Gly Pro Tyr Ala Ser Ala Ala 770 775 780Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr785 790 795
800Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly
805 810 815Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln
Gly Pro 820 825 830Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gly Tyr 835 840 845Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Gly Gln Asn 850 855 860Gly Pro Gly Ser Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Gly Gln865 870 875 880Ser Ala Ala Ala Ala
Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro 885 890 895Gly Ala Ser
Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln 900 905 910Gly
Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly 915 920
925Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly
930 935 940Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala
Ala Ala945 950 955 960Ala Gly Gln Tyr Gln Gln Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro 965 970 975Gly Ala Ser Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Ala Ser 980 985 990Ala Ala Ala Ala Ala Gly Pro
Gly Gln Tyr Gly Pro Gly Gln Gln Gly 995 1000 1005Pro Ser Ala Ser
Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly 1010 1015 1020Pro Gly
Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly Pro Gly 1025 1030
1035Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala
1040 1045 1050Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly
Pro Tyr 1055 1060 1065Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Pro Gly Ser Gly 1070 1075 1080Gln Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly 1085 1090 1095Gln Tyr Gly Pro Gly Gln Gln
Gly Pro Gly Gln Ser Ala Ala Ala 1100 1105 1110Ala Ala Gly Gln Tyr
Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr 1115 1120 1125Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser 1130 1135 1140Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser 1145 1150
1155Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala
1160 1165 1170Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly
Ala Ser 1175 1180 1185Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser
Ala Ala Ala Ala 1190 1195 1200Ala Gly Gln Asn Gly Pro Gly Ser Gly
Gln Gln Gly Pro Gly Gln 1205 1210 1215Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln Gln Gly 1220 1225 1230Pro Gly Ser Ser Ala
Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly 1235 1240 1245Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly 1250 1255 1260Pro
Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Tyr Gly 1265 1270
1275Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala
1280 1285 1290Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln
Gln Gly 1295 1300 1305Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly Pro
Gly Ser Gly Gln 1310 1315 1320Tyr Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Gly Pro Gly Gln 1325 1330 1335Tyr Gly Pro Gly Gln Gln Gly
Pro Ser Ala Ser Ala Ala Ala Ala 1340 1345 1350Ala Gly Ser Gly Gln
Gln Gly Pro Gly Gln Tyr Gly Pro Tyr Ala 1355 1360 1365Ser Ala Ala
Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln 1370 1375 1380Gln
Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln Gln Gly 1385 1390
1395Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
1400 1405 1410Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala
Ala Ala 1415 1420 1425Ala Ala Gly Gln Tyr Gly Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr 1430 1435 1440Gly Pro Gly Ala Ser Gly Gln Asn Gly
Pro Gly Ser Gly Gln Tyr 1445 1450 1455Gly Pro Gly Gln Gln Gly Pro
Gly Gln Ser Ala Ala Ala Ala Ala 1460 1465 1470Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala 1475 1480 1485Ala Ala Ala
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 1490 1495
1500Tyr Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly
1505 1510 1515Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly
Pro Gly 1520 1525 1530Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala
Ala Ala Ala Ala 1535 1540 1545Gly Gln Tyr Gln Gln Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro 1550 1555 1560Gly Ala Ser Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Ala 1565 1570 1575Ser Ala Ala Ala Ala
Ala Gly Pro Gly Gln Tyr Gly Pro Gly Gln 1580 1585 1590Gln Gly Pro
Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly 1595 1600 1605Ser
Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly 1610 1615
1620Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala
1625 1630 1635Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln
Gln Gly 1640 1645 1650Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala
Ala Gly Pro Gly 1655 1660 1665Ser Gly Gln Tyr Gly Pro Gly Ala Ser
Gly Gln Asn Gly Pro Gly 1670 1675 1680Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln Ser Ala 1685 1690 1695Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly 1700 1705 1710Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr 1715 1720 1725Gly
Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser 1730 1735
1740Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser
1745 1750 1755Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly 1760 1765 1770Ala Ser Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala 1775 1780 1785Ala Ala Ala Gly Gln Asn Gly Pro Gly
Ser Gly Gln Gln Gly Pro 1790 1795 1800Gly Gln Ser Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro Gly Gln 1805 1810 1815Gln Gly Pro Gly Ser
Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln 1820 1825 1830Tyr Gly Pro
Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala 1835 1840 1845Ala
Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln 1850 1855
1860Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser
1865 1870 1875Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro
Gly Gln 1880 1885 1890Gln Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser 1895 1900 1905Gly Gln Tyr Gly Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Pro 1910 1915 1920Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala 1925 1930 1935Ala Ala Ala Gly Ser
Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro 1940 1945 1950Tyr Ala Ser
Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro 1955 1960 1965Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Gln 1970 1975
1980Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala Ala Ala Ala
1985 1990 1995Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser
Ser Ala 2000 2005 2010Ala Ala Ala Ala Gly Gln Tyr Gly Tyr Gly Pro
Gly Gln Gln Gly 2015 2020 2025Pro Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly 2030 2035 2040Gln Tyr Gly Pro Gly Gln Gln
Gly Pro Gly Gln Ser Ala Ala Ala 2045 2050 2055Ala Ala Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser 2060 2065 2070Ala Ala Ala
Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro 2075 2080 2085Gly
Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro 2090 2095
2100Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly
2105 2110 2115Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala
Ala Ala 2120 2125 2130Ala Ala Gly Gln Tyr Gln Gln Gly Pro Gly Gln
Gln Gly Pro Tyr 2135 2140 2145Gly Pro Gly Ala Ser Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro 2150 2155 2160Gly Ala Ser Ala Ala Ala Ala
Ala Gly Pro Gly Gln Tyr Gly Pro 2165 2170 2175Gly Gln Gln Gly Pro
Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln 2180 2185 2190Tyr Gly Ser
Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln 2195 2200 2205Ser
Gly Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro 2210 2215
2220Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro Gly Gln
2225 2230 2235Gln Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala
Ala Gly 2240 2245 2250Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser
Gly Gln Asn Gly 2255 2260 2265Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln 2270 2275 2280Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gln Gln Gly Pro Gly Gln 2285 2290 2295Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 2300 2305 2310Gln Tyr Gly
Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly 2315 2320 2325Gln
Ser Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr 2330 2335
2340Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
2345 2350 2355Ser Ser Val Asp Lys Leu Ala Ala Ala Leu Glu His His
His His 2360 2365 2370His His 23751450PRTAraneus diadematus 14Ser
Gly Cys Asp Val Leu Val Gln Ala Leu Leu Glu Val Val Ser Ala1 5 10
15Leu Val Ser Ile Leu Gly Ser Ser Ser Ile Gly Gln Ile Asn Tyr Gly
20 25 30Ala Ser Ala Gln Tyr Thr Gln Met Val Gly Gln Ser Val Ala Gln
Ala 35 40 45Leu Ala 501530PRTAraneus diadematus 15Ser Gly Cys Asp
Val Leu Val Gln Ala Leu Leu Glu Val Val Ser Ala1 5 10 15Leu Val Ser
Ile Leu Gly Ser Ser Ser Ile Gly Gln Ile Asn 20 25 301621PRTAraneus
diadematus 16Ser Gly Cys Asp Val Leu Val Gln Ala Leu Leu Glu Val
Val Ser Ala1 5 10 15Leu Val Ser Ile Leu 20171154PRTArtificial
Sequencerecombinant spider silk protein ADF3KaiLargeNRSH1 17Met His
His His His His His His His His His Ser Ser Gly Ser Ser1 5 10 15Leu
Glu Val Leu Phe Gln Gly Pro Ala Arg Ala Gly Ser Gly Gln Gln 20 25
30Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly
35 40 45Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Gly Gly
Tyr 50 55 60Gly Pro Gly Ser Gly Gln Gln Gly Pro Ser Gln Gln Gly Pro
Gly Gln65 70 75 80Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala 85 90 95Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Ser
Gly Gln Gln Gly Pro 100 105 110Gly Gly Gln Gly Pro Tyr Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala 115 120 125Ala Gly Gly Asn Gly Pro Gly
Ser Gly Gln Gln Gly Ala Gly Gln Gln 130 135 140Gly Pro Gly Gln Gln
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala145 150 155 160Gly Gly
Tyr Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly 165 170
175Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala
180 185 190Ala Ala Gly Gly Tyr Gly Pro Gly Ser Gly Gln Gly Pro Gly
Gln Gln 195 200 205Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala
Ser Ala Ala Ala 210 215 220Ala Ala Ala Gly Gly Tyr Gly Pro Gly Ser
Gly Gln Gln Gly Pro Gly225 230 235 240Gln Gln Gly Pro Gly Gln Gln
Gly Pro Gly Gly Gln Gly Pro Tyr Gly 245 250 255Pro Gly Ala Ser Ala
Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly 260 265 270Tyr Gly Gln
Gln Gly Pro Gly Gln Gln Gly Pro Gly Gly Gln Gly Pro 275 280 285Tyr
Gly Pro Gly Ala Ser Ala Ala Ser Ala Ala Ser Gly Gly Tyr Gly 290 295
300Pro Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gly
Gln305 310 315 320Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala
Ala Ala Gly Gly 325 330 335Tyr Gly Pro Gly Ser Gly Gln Gln Gly Pro
Gly Gln Gln Gly Pro Gly 340 345 350Gln Gln Gly Pro Gly Gln Gln Gly
Pro Gly Gly Gln Gly Pro Tyr Gly 355 360 365Pro Gly Ala Ser Ala Ala
Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly 370 375 380Ser Gly Gln Gln
Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro385 390 395 400Gly
Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly 405 410
415Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gly
420 425 430Gln Gly Ala Tyr Gly Pro Gly Ala Ser Ala Ala Ala Gly Ala
Ala Gly 435 440 445Gly Tyr Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly
Gln Gln Gly Pro 450 455 460Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Gly Gln Gln Gly Pro Gly465 470 475 480Gln Gln Gly Pro Gly Gln Gln
Gly Pro Gly Gln Gln Gly Pro Tyr Gly 485 490 495Pro Gly Ala Ser Ala
Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly 500 505 510Ser Gly Gln
Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro 515 520 525Gly
Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ala Ser Ala Ala Val Ser 530 535
540Val Ser Arg Ala Arg Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln
Gln545 550 555 560Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Pro Gly 565 570 575Ala Ser Ala Ala Ala Ala Ala Ala Gly Gly
Tyr Gly Pro Gly Ser Gly 580 585 590Gln Gln Gly Pro Ser Gln Gln Gly
Pro Gly Gln Gln Gly Pro Gly Gly 595 600 605Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Ala Gly 610 615 620Gly Tyr Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Gly Gln Gly Pro625 630 635 640Tyr
Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Gly Gly Asn Gly 645 650
655Pro Gly Ser Gly Gln Gln Gly Ala Gly Gln Gln Gly Pro Gly Gln Gln
660 665 670Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Gly Gly Tyr
Gly Pro 675 680 685Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Gly Gly Gln Gly 690 695 700Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala Ala Ala Gly Gly Tyr705 710 715 720Gly Pro Gly Ser Gly Gln Gly
Pro Gly Gln Gln Gly Pro Gly Gly Gln 725 730 735Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Ala Gly Gly 740 745 750Tyr Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly 755 760 765Gln
Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala 770 775
780Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Tyr Gly Gln Gln
Gly785 790 795 800Pro Gly Gln Gln Gly Pro Gly Gly Gln Gly Pro Tyr
Gly Pro Gly Ala 805 810 815Ser Ala Ala Ser Ala Ala Ser Gly Gly Tyr
Gly Pro Gly Ser Gly Gln 820 825 830Gln Gly Pro Gly Gln Gln Gly Pro
Gly Gly Gln Gly Pro Tyr Gly Pro 835 840 845Gly Ala Ser Ala Ala Ala
Ala Ala Ala Gly Gly Tyr Gly Pro Gly Ser 850 855 860Gly Gln Gln Gly
Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly865 870 875 880Gln
Gln Gly Pro Gly Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala 885 890
895Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Ser Gly Gln Gln Gly
900 905 910Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln
Gly Pro 915 920 925Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln
Gln Gly Pro Gly 930 935 940Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly
Gly Gln Gly Ala Tyr Gly945 950 955 960Pro Gly Ala Ser Ala Ala Ala
Gly Ala Ala Gly Gly Tyr Gly Pro Gly 965 970 975Ser Gly Gln Gln Gly
Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro 980 985 990Gly Gln Gln
Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly 995 1000
1005Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser
1010 1015 1020Ala Ala Ala Ala Ala Ala Gly Gly Tyr Gly Pro Gly Ser
Gly Gln 1025 1030 1035Gln Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln
Gly Pro Gly Gly 1040 1045 1050Gln Gly Pro Tyr Gly Pro Gly Ala Ala
Ser Ala Ala Val Ser Val 1055 1060 1065Gly Gly Tyr Gly Pro Gln Ser
Ser Ser Val Pro Val Ala Ser Ala 1070 1075 1080Val Ala Ser Arg Leu
Ser Ser Pro Ala Ala Ser Ser Arg Val Ser 1085 1090 1095Ser Ala Val
Ser Ser Leu Val Ser Ser Gly Pro Thr Lys His Ala 1100 1105 1110Ala
Leu Ser Asn Thr Ile Ser Ser Val Val Ser Gln Val Ser Ala 1115 1120
1125Ser Asn Pro Gly Leu Ser Gly Cys Asp Val Leu Val Gln Ala Leu
1130 1135 1140Leu Glu Val Val Ser Ala Leu Val Ser Ile Leu 1145
11501824PRTArtificial SequenceHis tag and start codon 18Met His His
His His His His His His His His Ser Ser Gly Ser Ser1 5 10 15Leu Glu
Val Leu Phe Gln Gly Pro 2019590PRTArtificial SequenceMet-PRT410
19Met Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1
5 10 15Ala Ala Ala Gly Gln Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro
Gly 20 25 30Gln Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln
Gln Gly 35 40 45Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln
Tyr Gly Pro 50 55 60Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala
Ala Gly Pro Gly65 70 75 80Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly
Gln Tyr Gly Pro Gly Gln 85 90 95Gln Gly Pro Gly Gln Gln Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala 100 105 110Gly Gln Tyr Gly Ser Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly
Pro Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr 130 135 140Gly Pro Gly
Ala Ser Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly145 150 155
160Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro
165 170 175Gly Gln Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
Gln Tyr 180 185 190Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Gln Ser Gly 195 200 205Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly
Pro Tyr Ala Ser Ala Ala 210 215 220Ala Ala Ala Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ser Ser225 230 235 240Ala Ala Ala Ala Ala
Gly Gln Tyr Gly Tyr Gly Pro Gly Gln Gln Gly 245 250 255Pro Tyr Gly
Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln 260 265
270Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala
275 280 285Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala
Ala Ala 290 295 300Ala Ala Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro
Gly Gln Tyr Gly305 310 315 320Pro Gly Ser Ser Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ser 325 330 335Ser Ala Ala Ala Ala Ala Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro 340 345 350Tyr Gly Pro Gly Gln
Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln 355 360 365Gly Pro Gly
Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly 370 375 380Gln
Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly385 390
395 400Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala
Ala 405 410 415Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr
Gly Pro Tyr 420 425 430Gly Pro Gly Gln Ser Gly Pro Gly Ser Gly Gln
Gln Gly Gln Gly Pro 435 440 445Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala Ala Gly Gln Tyr Gly Pro 450 455 460Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Gln Ser Ala Ala Ala Ala Ala465 470 475 480Gly Pro Gly Ser
Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly 485 490 495Pro Gly
Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser 500 505
510Ala Ala Ala Ala Ala Gly Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly
515 520 525Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln
Tyr Gly 530 535 540Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Gln Ser Gly Ser545 550 555 560Gly Gln Gln Gly Pro Gly Gln Gln Gly
Pro Tyr Ala Ser Ala Ala Ala 565 570 575Ala Ala Gly Pro Gly Ser Gly
Gln Gln Gly Pro Gly Ala Ser 580 585 59020612PRTArtificial
SequenceMet-PRT720 20Met Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala Gly Gln Asn Gly Pro Gly Ser
Gly Gln Gln Gly Pro Gly 20 25 30Gln Ser Gly Gln Tyr Gly Pro Gly Gln
Gln Gly Pro Gly Gln Gln Gly 35 40 45Pro Gly Ser Ser Ala Ala Ala Ala
Ala Gly Pro Gly Gln Tyr Val Leu 50 55 60Ile Gly Pro Gly Gln Gln Val
Leu Ile Gly Pro Ser Ala Ser Ala Ala65 70 75 80Ala Ala Ala Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly 85 90 95Gln Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser 100 105 110Ser Ala
Ala Ala Ala Ala Gly Ser Tyr Gly Ser Val Leu Ile Gly Pro 115 120
125Gly Gln Gln Val Leu Ile Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala
130 135 140Gly Pro Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly Pro
Gly Ala145 150 155 160Ser Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln
Gly Pro Ser Ala Ser 165 170 175Ala Ala Ala Ala Ala Gly Ser Gly Gln
Gln Val Leu Ile Gly Pro Gly 180 185 190Gln Tyr Val Leu Ile Gly Pro
Tyr Ala Ser Ala Ala Ala Ala Ala Gly 195 200 205Gln Tyr Gly Ser Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln 210 215 220Ser Gly Ser
Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser225 230 235
240Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Tyr
245 250 255Val Leu Ile Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly
Gln Tyr 260 265 270Gly Tyr Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Gly 275 280 285Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly
Pro Gly Gln Gln Gly Pro 290 295 300Gly Gln Ser Ala Ala Ala Ala Ala
Gly Pro Gly Gln Gln Val Leu Ile305 310 315 320Gly Pro Tyr Val Leu
Ile Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala 325 330 335Gly Gln Tyr
Gly Pro Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro Gly 340 345 350Ser
Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala 355 360
365Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln Val Leu Ile Gly
370 375 380Pro Tyr Val Leu Ile Gly Pro Gly Pro Ser Ala Ala Ala Ala
Ala Gly385 390 395 400Gln Tyr Gln Gln Gly Pro Gly Gln Gln Gly Pro
Tyr Gly Pro Gly Ala 405 410 415Ser Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala 420 425 430Ala Ala Ala Gly Pro Gly Gln
Tyr Val Leu Ile Gly Pro Gly Gln Gln 435 440 445Val Leu Ile Gly Pro
Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr 450 455 460Gly Ser Gly
Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly465 470 475
480Pro Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser
485 490 495Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln Val
Leu Ile 500 505 510Gly Pro Tyr Val Leu Ile Gly Pro Gly Pro Ser Ala
Ala Ala Ala Ala 515 520 525Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Ala Ser Gly Gln Asn Gly 530 535 540Pro Gly Ser Gly Gln Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gln Ser545 550 555 560Ala Ala Ala Ala Ala
Gly Gln Tyr Gln Gln Val Leu Ile Gly Pro Gly 565 570 575Gln Gln Gly
Pro Tyr Val Leu Ile Gly Pro Gly Ala Ser Ala Ala Ala 580 585 590Ala
Ala Gly Pro Gly Ser Gly Gln Gln Val Leu Ile Gly Pro Gly Ala 595 600
605Ser Val Leu Ile 61021565PRTArtificial SequenceMet-PRT468 21Met
Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10
15Ala Ala Ala Ala Ala Gly Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly
20 25 30Pro Gly Gln Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly
Gln 35 40 45Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly
Pro Gly 50 55 60Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala
Ala Ala Ala65 70 75 80Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly Ala Ser Gly 85 90 95Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly
Gln Gln Gly Pro Gly Ser 100 105 110Ser Ala Ala Ala Ala Ala Ala Ala
Gly Ser Tyr Gly Ser Gly Pro Gly 115 120 125Gln Gln Gly Pro Tyr Gly
Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro 130 135 140Gly Ser Gly Gln
Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly145 150 155 160Pro
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 165 170
175Ala Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln Tyr Gly
180 185 190Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr
Gly Ser 195 200 205Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln
Ser Gly Ser Gly 210 215 220Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr
Ala Ser Ala Ala Ala Ala225 230 235 240Ala Ala Ala Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Ser Ser 245 250 255Ala Ala Ala Ala Ala
Ala Ala Gly Ser Tyr Gly Tyr Gly Pro Gly Gln 260 265 270Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser 275 280 285Gly
Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Pro Ser Ala Ala Ala 290 295
300Ala Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly
Ala305 310 315 320Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly
Pro Gly Gln Gln 325 330 335Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser
Gly Pro Gly Gln Gln Gly 340 345 350Pro Tyr Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala Ala Ala Gly Ser 355 360 365Tyr Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala 370 375 380Ala Ala Ala Ala
Ala Gly Ser Tyr Gln Gln Gly Pro Gly Gln Gln Gly385 390 395 400Pro
Tyr Gly Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly 405 410
415Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr
420 425 430Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala
Ala Ala 435 440 445Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln Tyr Gly
Pro Tyr Gly Pro 450 455 460Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln
Gly Gln Gly Pro Tyr Gly465 470 475 480Pro Gly Ala Ser Ala Ala Ala
Ala Ala Ala Ala Gly Ser Tyr Gly Pro 485 490 495Gly Gln Gln Gly Pro
Tyr Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala 500 505 510Ala Ala Gly
Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln 515 520 525Asn
Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly 530 535
540Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln
Gln545 550 555 560Gly Pro Gly Ala Ser 56522592PRTArtificial
SequenceMet-PRT665 22Met Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala Ala Ala Gly Ser Asn Gly Pro
Gly Ser Gly Gln Gln Gly 20 25 30Pro Gly Gln Ser Gly Gln Tyr Gly Pro
Gly Gln Gln Gly Pro Gly Gln 35 40 45Gln Gly Pro Gly Ser Ser Ala Ala
Ala Ala Ala Ala Ala Gly Pro Gly 50 55 60Gln Tyr Val Leu Ile Gly Pro
Gly Gln Gln Gly Pro Ser Ala Ser Ala65 70 75 80Ala Ala Ala Ala Ala
Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly 85 90 95Ala Ser Gly Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly 100 105 110Pro Gly
Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser 115 120
125Val Leu Ile Gly Pro Gly Gln Gln Gly Pro Tyr Gly Ser Ala Ala Ala
130 135 140Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Tyr Gly Gln Gly
Pro Tyr145 150 155 160Gly Pro Gly Ala Ser Gly Pro Gly Gln Tyr Gly
Pro Gly Gln Gln Gly 165 170 175Pro Ser Ala Ser Ala Ala Ala Ala Ala
Ala Ala Gly Ser Gly Gln Gln 180 185 190Val Leu Ile Gly Pro Gly Gln
Tyr Gly Pro Tyr Ala Ser Ala Ala Ala 195 200 205Ala Ala Ala Ala Gly
Ser Tyr Gly Ser Gly Pro Gly Gln Gln Gly Pro 210 215 220Tyr Gly Pro
Gly Gln Ser Gly Ser Gly Gln Gln Gly Pro Gly Gln Gln225 230 235
240Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln
245 250 255Gln Val Leu Ile Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala
Ala Ala 260 265 270Ala Ala Ala Gly Ser Tyr Gly Tyr Gly Pro Gly Gln
Gln Gly Pro Tyr 275 280 285Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro
Gly Ser Gly Gln Tyr Gly 290 295 300Pro Gly Gln Gln Gly Pro Gly Pro
Ser Ala Ala Ala Ala Ala Ala Ala305 310 315 320Gly Pro Gly Gln Gln
Val Leu Ile Gly Pro Tyr Gly Pro Gly Ala Ser 325 330 335Ala Ala Ala
Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln Gly 340 345 350Pro
Gly Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro 355 360
365Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr
370 375 380Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Tyr Gly Pro Gly
Pro Ser385 390 395 400Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gln
Gln Gly Pro Gly Gln 405 410 415Gln Gly Pro Tyr Gly Pro Gly Ala Ser
Gly Pro Gly Gln Gln Gly Pro 420 425 430Tyr Gly Pro Gly Ala Ser Ala
Ala Ala Ala Ala Ala Ala Gly Pro Gly 435 440 445Gln Tyr Val Leu Ile
Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala 450 455 460Ala Ala Ala
Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln Tyr465 470 475
480Gly Pro Tyr Gly Pro Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly
485 490 495Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala
Ala Ala 500 505 510Gly Ser Tyr Gly Pro Gly Gln Gln Val Leu Ile Gly
Pro Tyr Gly Pro 515 520 525Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly Gln 530 535 540Tyr Gly Pro Gly Ala Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln Tyr545 550 555 560Gly Pro Gly Gln Gln
Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala 565 570 575Ala Gly Pro
Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Val Leu Ile 580 585
59023619PRTArtificial SequenceMet-PRT666 23Met Gly Pro Gly Gln Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala Ala Ala
Gly Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly 20 25 30Pro Gly Gln Ser
Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 35 40 45Gln Gly Pro
Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly 50 55 60Gln Tyr
Val Leu Ile Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Ser65 70 75
80Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln
85 90 95Gly Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro
Gly 100 105 110Gln Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala
Ala Gly Ser 115 120 125Tyr Gly Ser Val Leu Ile Gly Pro Gly Gln Gln
Val Leu Ile Gly Pro 130 135 140Tyr Gly Ser Ala Ala Ala Ala Ala Ala
Ala Gly Pro Gly Ser Gly Gln145 150 155 160Tyr Gly Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Gln Tyr 165 170 175Gly Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala 180 185 190Ala Gly
Ser Gly Gln Gln Val Leu Ile Gly Pro Gly Gln Tyr Val Leu 195 200
205Ile Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr
210 215 220Gly Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln
Ser Gly225 230 235 240Ser Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro
Tyr Ala Ser Ala Ala 245 250 255Ala Ala Ala Ala Ala Gly Pro Gly Gln
Gln Val Leu Ile Gly Pro Tyr 260 265 270Val Leu Ile Gly Pro Gly Ser
Ser Ala Ala Ala Ala Ala Ala Ala Gly 275 280 285Ser Tyr Gly Tyr Gly
Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala 290 295 300Ser Gly Gln
Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln305 310 315
320Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln
325 330 335Gln Val Leu Ile Gly Pro Tyr Val Leu Ile Gly Pro Gly Ala
Ser Ala 340 345 350Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly
Gln Gln Gly Pro 355
360 365Gly Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly Gln Gln Gly Pro
Tyr 370 375 380Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly385 390 395 400Pro Gly Gln Gln Val Leu Ile Gly Pro Tyr
Val Leu Ile Gly Pro Gly 405 410 415Pro Ser Ala Ala Ala Ala Ala Ala
Ala Gly Ser Tyr Gln Gln Gly Pro 420 425 430Gly Gln Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Gln Gln 435 440 445Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly 450 455 460Pro Gly
Gln Tyr Val Leu Ile Gly Pro Gly Gln Gln Val Leu Ile Gly465 470 475
480Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser
485 490 495Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly
Pro Gly 500 505 510Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala 515 520 525Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro
Gly Gln Gln Val Leu Ile 530 535 540Gly Pro Tyr Val Leu Ile Gly Pro
Gly Pro Ser Ala Ala Ala Ala Ala545 550 555 560Ala Ala Gly Pro Gly
Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln 565 570 575Asn Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly 580 585 590Pro
Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln 595 600
605Val Leu Ile Gly Pro Gly Ala Ser Val Leu Ile 610
61524623PRTArtificial SequencePRT720 24Met His His His His His His
Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln 20 25 30Asn Gly Pro Gly Ser
Gly Gln Gln Gly Pro Gly Gln Ser Gly Gln Tyr 35 40 45Gly Pro Gly Gln
Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala 50 55 60Ala Ala Ala
Ala Gly Pro Gly Gln Tyr Val Leu Ile Gly Pro Gly Gln65 70 75 80Gln
Val Leu Ile Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro 85 90
95Gly Ser Gly Gln Gln Gly Pro Gly Ala Ser Gly Gln Tyr Gly Pro Gly
100 105 110Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser Ser Ala Ala
Ala Ala 115 120 125Ala Gly Ser Tyr Gly Ser Val Leu Ile Gly Pro Gly
Gln Gln Val Leu 130 135 140Ile Gly Pro Tyr Gly Ser Ala Ala Ala Ala
Ala Gly Pro Gly Ser Gly145 150 155 160Gln Tyr Gly Gln Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Gln 165 170 175Tyr Gly Pro Gly Gln
Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala 180 185 190Gly Ser Gly
Gln Gln Val Leu Ile Gly Pro Gly Gln Tyr Val Leu Ile 195 200 205Gly
Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly 210 215
220Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly
Gln225 230 235 240Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala 245 250 255Gly Pro Gly Gln Gln Val Leu Ile Gly Pro
Tyr Val Leu Ile Gly Pro 260 265 270Gly Ser Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Gly Tyr Gly Pro Gly 275 280 285Gln Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly 290 295 300Ser Gly Gln Tyr
Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala305 310 315 320Ala
Ala Ala Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Tyr Val Leu 325 330
335Ile Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro
340 345 350Gly Gln Gln Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly
Pro Gly 355 360 365Gln Gln Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala
Ala Ala Ala Gly 370 375 380Ser Tyr Gly Pro Gly Gln Gln Val Leu Ile
Gly Pro Tyr Val Leu Ile385 390 395 400Gly Pro Gly Pro Ser Ala Ala
Ala Ala Ala Gly Gln Tyr Gln Gln Gly 405 410 415Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Gln 420 425 430Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro 435 440 445Gly
Gln Tyr Val Leu Ile Gly Pro Gly Gln Gln Val Leu Ile Gly Pro 450 455
460Ser Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro
Gly465 470 475 480Gln Tyr Gly Pro Tyr Gly Pro Gly Gln Ser Gly Pro
Gly Ser Gly Gln 485 490 495Gln Gly Gln Gly Pro Tyr Gly Pro Gly Ala
Ser Ala Ala Ala Ala Ala 500 505 510Gly Ser Tyr Gly Pro Gly Gln Gln
Val Leu Ile Gly Pro Tyr Val Leu 515 520 525Ile Gly Pro Gly Pro Ser
Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly 530 535 540Gln Tyr Gly Pro
Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln545 550 555 560Tyr
Gly Pro Gly Gln Gln Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala 565 570
575Gly Gln Tyr Gln Gln Val Leu Ile Gly Pro Gly Gln Gln Gly Pro Tyr
580 585 590Val Leu Ile Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Pro Gly 595 600 605Ser Gly Gln Gln Val Leu Ile Gly Pro Gly Ala Ser
Val Leu Ile 610 615 62025603PRTArtificial SequencePRT665 25Met His
His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 20 25
30Gly Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gln Ser Gly
35 40 45Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly
Ser 50 55 60Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Val
Leu Ile65 70 75 80Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala
Ala Ala Ala Ala 85 90 95Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly
Ala Ser Gly Gln Tyr 100 105 110Gly Pro Gly Gln Gln Gly Pro Gly Gln
Gln Gly Pro Gly Ser Ser Ala 115 120 125Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Ser Val Leu Ile Gly Pro 130 135 140Gly Gln Gln Gly Pro
Tyr Gly Ser Ala Ala Ala Ala Ala Ala Ala Gly145 150 155 160Pro Gly
Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser 165 170
175Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala
180 185 190Ala Ala Ala Ala Ala Ala Gly Ser Gly Gln Gln Val Leu Ile
Gly Pro 195 200 205Gly Gln Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala
Ala Ala Ala Gly 210 215 220Ser Tyr Gly Ser Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Gln225 230 235 240Ser Gly Ser Gly Gln Gln Gly
Pro Gly Gln Gln Gly Pro Tyr Ala Ser 245 250 255Ala Ala Ala Ala Ala
Ala Ala Gly Pro Gly Gln Gln Val Leu Ile Gly 260 265 270Pro Tyr Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser 275 280 285Tyr
Gly Tyr Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser 290 295
300Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln
Gly305 310 315 320Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly
Pro Gly Gln Gln 325 330 335Val Leu Ile Gly Pro Tyr Gly Pro Gly Ala
Ser Ala Ala Ala Ala Ala 340 345 350Ala Ala Gly Ser Tyr Gly Pro Gly
Gln Gln Gly Pro Gly Gln Tyr Gly 355 360 365Pro Gly Ser Ser Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ser 370 375 380Ser Ala Ala Ala
Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln385 390 395 400Val
Leu Ile Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala 405 410
415Ala Ala Gly Ser Tyr Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Gly
420 425 430Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro
Gly Ala 435 440 445Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln
Tyr Val Leu Ile 450 455 460Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser
Ala Ala Ala Ala Ala Ala465 470 475 480Ala Gly Ser Tyr Gly Ser Gly
Pro Gly Gln Tyr Gly Pro Tyr Gly Pro 485 490 495Gly Gln Ser Gly Pro
Gly Ser Gly Gln Gln Gly Gln Gly Pro Tyr Gly 500 505 510Pro Gly Ala
Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro 515 520 525Gly
Gln Gln Val Leu Ile Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala 530 535
540Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly
Ala545 550 555 560Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly
Pro Gly Gln Gln 565 570 575Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala
Ala Ala Gly Pro Gly Ser 580 585 590Gly Gln Gln Gly Pro Gly Ala Ser
Val Leu Ile 595 60026630PRTArtificial SequencePRT666 26Met His His
His His His His Ser Ser Gly Ser Ser Gly Pro Gly Gln1 5 10 15Gln Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 20 25 30Gly
Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly Gln Ser Gly 35 40
45Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln Gly Pro Gly Ser
50 55 60Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Val Leu
Ile65 70 75 80Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Ser Ala Ser
Ala Ala Ala 85 90 95Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly
Pro Gly Ala Ser 100 105 110Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro
Gly Gln Gln Gly Pro Gly 115 120 125Ser Ser Ala Ala Ala Ala Ala Ala
Ala Gly Ser Tyr Gly Ser Val Leu 130 135 140Ile Gly Pro Gly Gln Gln
Val Leu Ile Gly Pro Tyr Gly Ser Ala Ala145 150 155 160Ala Ala Ala
Ala Ala Gly Pro Gly Ser Gly Gln Tyr Gly Gln Gly Pro 165 170 175Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Gln Tyr Gly Pro Gly Gln Gln 180 185
190Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Gly Gln
195 200 205Gln Val Leu Ile Gly Pro Gly Gln Tyr Val Leu Ile Gly Pro
Tyr Ala 210 215 220Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly
Ser Gly Pro Gly225 230 235 240Gln Gln Gly Pro Tyr Gly Pro Gly Gln
Ser Gly Ser Gly Gln Gln Gly 245 250 255Pro Gly Gln Gln Gly Pro Tyr
Ala Ser Ala Ala Ala Ala Ala Ala Ala 260 265 270Gly Pro Gly Gln Gln
Val Leu Ile Gly Pro Tyr Val Leu Ile Gly Pro 275 280 285Gly Ser Ser
Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Tyr Gly 290 295 300Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly305 310
315 320Pro Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Pro
Ser 325 330 335Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Gln Val
Leu Ile Gly 340 345 350Pro Tyr Val Leu Ile Gly Pro Gly Ala Ser Ala
Ala Ala Ala Ala Ala 355 360 365Ala Gly Ser Tyr Gly Pro Gly Gln Gln
Gly Pro Gly Gln Tyr Gly Pro 370 375 380Gly Ser Ser Gly Pro Gly Gln
Gln Gly Pro Tyr Gly Pro Gly Ser Ser385 390 395 400Ala Ala Ala Ala
Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln Gln Val 405 410 415Leu Ile
Gly Pro Tyr Val Leu Ile Gly Pro Gly Pro Ser Ala Ala Ala 420 425
430Ala Ala Ala Ala Gly Ser Tyr Gln Gln Gly Pro Gly Gln Gln Gly Pro
435 440 445Tyr Gly Pro Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Pro 450 455 460Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro
Gly Gln Tyr Val465 470 475 480Leu Ile Gly Pro Gly Gln Gln Val Leu
Ile Gly Pro Ser Ala Ser Ala 485 490 495Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Ser Gly Pro Gly Gln Tyr 500 505 510Gly Pro Tyr Gly Pro
Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly 515 520 525Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 530 535 540Gly
Ser Tyr Gly Pro Gly Gln Gln Val Leu Ile Gly Pro Tyr Val Leu545 550
555 560Ile Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro
Gly 565 570 575Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly
Pro Gly Ser 580 585 590Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly
Pro Ser Ala Ala Ala 595 600 605Ala Ala Ala Ala Gly Pro Gly Ser Gly
Gln Gln Val Leu Ile Gly Pro 610 615 620Gly Ala Ser Val Leu Ile625
63027593PRTArtificial SequenceM_PRT888 27Met Gly Ser Ser Gly Pro
Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala1 5 10 15Ser Ala Ala Ala Ala
Ala Gly Gln Asn Gly Pro Gly Ser Gly Val Leu 20 25 30Gly Pro Gly Gln
Ser Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly 35 40 45Val Leu Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Gln 50 55 60Tyr Gly
Pro Gly Val Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala65 70 75
80Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ala Ser Gly Gln Tyr Gly
85 90 95Pro Gly Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser Ser Ala
Ala 100 105 110Ala Ala Ala Gly Gln Tyr Gly Ser Gly Pro Gly Val Leu
Gly Pro Tyr 115 120 125Gly Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser
Gly Gln Tyr Gly Gln 130 135 140Gly Pro Tyr Gly Pro Gly Ala Ser Gly
Pro Gly Gln Tyr Gly Pro Gly145 150 155 160Val Leu Gly Pro Ser Ala
Ser Ala Ala Ala Ala Ala Gly Ser Gly Val 165 170 175Leu Gly Pro Gly
Gln Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala 180 185 190Gly Gln
Tyr Gly Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly 195 200
205Gln Ser Gly Ser Gly Val Leu Gly Pro Gly Val Leu Gly Pro Tyr Ala
210 215 220Ser Ala Ala Ala Ala Ala Gly Pro Gly Val Leu Gly Pro Tyr
Gly Pro225 230 235 240Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr
Gly Tyr Gly Pro Gly 245 250 255Val Leu Gly Pro Tyr Gly Pro Gly Ala
Ser Gly Gln Asn Gly Pro Gly 260 265 270Ser Gly Gln Tyr Gly Pro Gly
Val Leu Gly Pro Gly Gln Ser Ala Ala 275 280 285Ala Ala Ala Gly Pro
Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser 290 295 300Ala Ala Ala
Ala Ala Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly305 310 315
320Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly
325 330 335Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Pro
Gly Val 340
345 350Leu Gly Pro Tyr Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Gln 355 360 365Tyr Val Leu Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro
Gly Ala Ser 370 375 380Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala385 390 395 400Ala Ala Gly Pro Gly Gln Tyr Gly
Pro Gly Val Leu Gly Pro Ser Ala 405 410 415Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly Gln Tyr 420 425 430Gly Pro Tyr Gly
Pro Gly Gln Ser Gly Pro Gly Ser Gly Val Leu Gly 435 440 445Gln Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Gln 450 455
460Tyr Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Gln Ser Ala
Ala465 470 475 480Ala Ala Ala Gly Pro Gly Ser Gly Gln Tyr Gly Pro
Gly Ala Ser Gly 485 490 495Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly
Pro Gly Val Leu Gly Pro 500 505 510Gly Gln Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Val Leu Gly Pro Gly 515 520 525Val Leu Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 530 535 540Gln Tyr Gly Ser
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Gln545 550 555 560Ser
Gly Ser Gly Val Leu Gly Pro Gly Val Leu Gly Pro Tyr Ala Ser 565 570
575Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ala
580 585 590Ser28590PRTArtificial SequenceM_PRT965 28Met Gly Pro Gly
Thr Ser Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala Ala
Gly Ala Asn Gly Pro Gly Ser Gly Thr Ser Gly Pro Gly 20 25 30Ala Ser
Gly Ala Tyr Gly Pro Gly Thr Ser Gly Pro Gly Thr Ser Gly 35 40 45Pro
Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Ala Tyr Gly Pro 50 55
60Gly Thr Ser Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly65
70 75 80Ser Gly Thr Ser Gly Pro Gly Ala Ser Gly Ala Tyr Gly Pro Gly
Thr 85 90 95Ser Gly Pro Gly Thr Ser Gly Pro Gly Ser Ser Ala Ala Ala
Ala Ala 100 105 110Gly Ala Tyr Gly Ser Gly Pro Gly Thr Ser Gly Pro
Tyr Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly Pro Gly Ser Gly Ala
Tyr Gly Ala Gly Pro Tyr 130 135 140Gly Pro Gly Ala Ser Gly Pro Gly
Ala Tyr Gly Pro Gly Thr Ser Gly145 150 155 160Pro Ser Ala Ser Ala
Ala Ala Ala Ala Gly Ser Gly Thr Ser Gly Pro 165 170 175Gly Ala Tyr
Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Ala Tyr 180 185 190Gly
Ser Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro Gly Ala Ser Gly 195 200
205Ser Gly Thr Ser Gly Pro Gly Thr Ser Gly Pro Tyr Ala Ser Ala Ala
210 215 220Ala Ala Ala Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro Gly
Ser Ser225 230 235 240Ala Ala Ala Ala Ala Gly Ala Tyr Gly Tyr Gly
Pro Gly Thr Ser Gly 245 250 255Pro Tyr Gly Pro Gly Ala Ser Gly Ala
Asn Gly Pro Gly Ser Gly Ala 260 265 270Tyr Gly Pro Gly Thr Ser Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala 275 280 285Gly Pro Gly Thr Ser
Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala 290 295 300Ala Ala Gly
Ala Tyr Gly Pro Gly Thr Ser Gly Pro Gly Ala Tyr Gly305 310 315
320Pro Gly Ser Ser Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro Gly Ser
325 330 335Ser Ala Ala Ala Ala Ala Gly Ala Tyr Gly Pro Gly Thr Ser
Gly Pro 340 345 350Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Ala Tyr Thr Ser 355 360 365Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro
Gly Ala Ser Gly Pro Gly 370 375 380Thr Ser Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly385 390 395 400Pro Gly Ala Tyr Gly
Pro Gly Thr Ser Gly Pro Ser Ala Ser Ala Ala 405 410 415Ala Ala Ala
Gly Ala Tyr Gly Ser Gly Pro Gly Ala Tyr Gly Pro Tyr 420 425 430Gly
Pro Gly Ala Ser Gly Pro Gly Ser Gly Thr Ser Gly Ala Gly Pro 435 440
445Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Ala Tyr Gly Pro
450 455 460Gly Thr Ser Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala Ala465 470 475 480Gly Pro Gly Ser Gly Ala Tyr Gly Pro Gly Ala
Ser Gly Ala Asn Gly 485 490 495Pro Gly Ser Gly Ala Tyr Gly Pro Gly
Thr Ser Gly Pro Gly Ala Ser 500 505 510Ala Ala Ala Ala Ala Gly Ala
Tyr Thr Ser Gly Pro Gly Thr Ser Gly 515 520 525Pro Tyr Gly Pro Gly
Ala Ser Ala Ala Ala Ala Ala Gly Ala Tyr Gly 530 535 540Ser Gly Pro
Gly Thr Ser Gly Pro Tyr Gly Pro Gly Ala Ser Gly Ser545 550 555
560Gly Thr Ser Gly Pro Gly Thr Ser Gly Pro Tyr Ala Ser Ala Ala Ala
565 570 575Ala Ala Gly Pro Gly Ser Gly Thr Ser Gly Pro Gly Ala Ser
580 585 59029593PRTArtificial SequenceM_PRT889 29Met Gly Ser Ser
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala1 5 10 15Ser Ala Ala
Ala Ala Ala Gly Ile Asn Gly Pro Gly Ser Gly Val Leu 20 25 30Gly Pro
Gly Ile Ser Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro Gly 35 40 45Val
Leu Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Ile 50 55
60Tyr Gly Pro Gly Val Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala65
70 75 80Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ala Ser Gly Ile Tyr
Gly 85 90 95Pro Gly Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser Ser
Ala Ala 100 105 110Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro Gly Val
Leu Gly Pro Tyr 115 120 125Gly Ser Ala Ala Ala Ala Ala Gly Pro Gly
Ser Gly Ile Tyr Gly Ile 130 135 140Gly Pro Tyr Gly Pro Gly Ala Ser
Gly Pro Gly Ile Tyr Gly Pro Gly145 150 155 160Val Leu Gly Pro Ser
Ala Ser Ala Ala Ala Ala Ala Gly Ser Gly Val 165 170 175Leu Gly Pro
Gly Ile Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala 180 185 190Gly
Ile Tyr Gly Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly 195 200
205Ile Ser Gly Ser Gly Val Leu Gly Pro Gly Val Leu Gly Pro Tyr Ala
210 215 220Ser Ala Ala Ala Ala Ala Gly Pro Gly Val Leu Gly Pro Tyr
Gly Pro225 230 235 240Gly Ser Ser Ala Ala Ala Ala Ala Gly Ile Tyr
Gly Tyr Gly Pro Gly 245 250 255Val Leu Gly Pro Tyr Gly Pro Gly Ala
Ser Gly Ile Asn Gly Pro Gly 260 265 270Ser Gly Ile Tyr Gly Pro Gly
Val Leu Gly Pro Gly Ile Ser Ala Ala 275 280 285Ala Ala Ala Gly Pro
Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser 290 295 300Ala Ala Ala
Ala Ala Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro Gly305 310 315
320Ile Tyr Gly Pro Gly Ser Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly
325 330 335Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Ile Tyr Gly Pro
Gly Val 340 345 350Leu Gly Pro Tyr Gly Pro Gly Ile Ser Ala Ala Ala
Ala Ala Gly Ile 355 360 365Tyr Val Leu Gly Pro Gly Val Leu Gly Pro
Tyr Gly Pro Gly Ala Ser 370 375 380Gly Pro Gly Val Leu Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala Ala385 390 395 400Ala Ala Gly Pro Gly
Ile Tyr Gly Pro Gly Val Leu Gly Pro Ser Ala 405 410 415Ser Ala Ala
Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro Gly Ile Tyr 420 425 430Gly
Pro Tyr Gly Pro Gly Ile Ser Gly Pro Gly Ser Gly Val Leu Gly 435 440
445Ile Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Ile
450 455 460Tyr Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ile Ser
Ala Ala465 470 475 480Ala Ala Ala Gly Pro Gly Ser Gly Ile Tyr Gly
Pro Gly Ala Ser Gly 485 490 495Ile Asn Gly Pro Gly Ser Gly Ile Tyr
Gly Pro Gly Val Leu Gly Pro 500 505 510Gly Ile Ser Ala Ala Ala Ala
Ala Gly Ile Tyr Val Leu Gly Pro Gly 515 520 525Val Leu Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly 530 535 540Ile Tyr Gly
Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ile545 550 555
560Ser Gly Ser Gly Val Leu Gly Pro Gly Val Leu Gly Pro Tyr Ala Ser
565 570 575Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Leu Gly Pro
Gly Ala 580 585 590Ser30590PRTArtificial SequenceM_PRT916 30Met Gly
Pro Gly Val Ile Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala
Ala Ala Gly Leu Asn Gly Pro Gly Ser Gly Val Ile Gly Pro Gly 20 25
30Leu Ser Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro Gly Val Ile Gly
35 40 45Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Leu Tyr Gly
Pro 50 55 60Gly Val Ile Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly
Pro Gly65 70 75 80Ser Gly Val Ile Gly Pro Gly Ala Ser Gly Leu Tyr
Gly Pro Gly Val 85 90 95Ile Gly Pro Gly Val Ile Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala 100 105 110Gly Leu Tyr Gly Ser Gly Pro Gly Val
Ile Gly Pro Tyr Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly Pro Gly
Ser Gly Leu Tyr Gly Leu Gly Pro Tyr 130 135 140Gly Pro Gly Ala Ser
Gly Pro Gly Leu Tyr Gly Pro Gly Val Ile Gly145 150 155 160Pro Ser
Ala Ser Ala Ala Ala Ala Ala Gly Ser Gly Val Ile Gly Pro 165 170
175Gly Leu Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Leu Tyr
180 185 190Gly Ser Gly Pro Gly Val Ile Gly Pro Tyr Gly Pro Gly Leu
Ser Gly 195 200 205Ser Gly Val Ile Gly Pro Gly Val Ile Gly Pro Tyr
Ala Ser Ala Ala 210 215 220Ala Ala Ala Gly Pro Gly Val Ile Gly Pro
Tyr Gly Pro Gly Ser Ser225 230 235 240Ala Ala Ala Ala Ala Gly Leu
Tyr Gly Tyr Gly Pro Gly Val Ile Gly 245 250 255Pro Tyr Gly Pro Gly
Ala Ser Gly Leu Asn Gly Pro Gly Ser Gly Leu 260 265 270Tyr Gly Pro
Gly Val Ile Gly Pro Gly Leu Ser Ala Ala Ala Ala Ala 275 280 285Gly
Pro Gly Val Ile Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala 290 295
300Ala Ala Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro Gly Leu Tyr
Gly305 310 315 320Pro Gly Ser Ser Gly Pro Gly Val Ile Gly Pro Tyr
Gly Pro Gly Ser 325 330 335Ser Ala Ala Ala Ala Ala Gly Leu Tyr Gly
Pro Gly Val Ile Gly Pro 340 345 350Tyr Gly Pro Gly Leu Ser Ala Ala
Ala Ala Ala Gly Leu Tyr Val Ile 355 360 365Gly Pro Gly Val Ile Gly
Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly 370 375 380Val Ile Gly Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly385 390 395 400Pro
Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro Ser Ala Ser Ala Ala 405 410
415Ala Ala Ala Gly Leu Tyr Gly Ser Gly Pro Gly Leu Tyr Gly Pro Tyr
420 425 430Gly Pro Gly Leu Ser Gly Pro Gly Ser Gly Val Ile Gly Leu
Gly Pro 435 440 445Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Leu Tyr Gly Pro 450 455 460Gly Val Ile Gly Pro Tyr Gly Pro Gly Leu
Ser Ala Ala Ala Ala Ala465 470 475 480Gly Pro Gly Ser Gly Leu Tyr
Gly Pro Gly Ala Ser Gly Leu Asn Gly 485 490 495Pro Gly Ser Gly Leu
Tyr Gly Pro Gly Val Ile Gly Pro Gly Leu Ser 500 505 510Ala Ala Ala
Ala Ala Gly Leu Tyr Val Ile Gly Pro Gly Val Ile Gly 515 520 525Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Leu Tyr Gly 530 535
540Ser Gly Pro Gly Val Ile Gly Pro Tyr Gly Pro Gly Leu Ser Gly
Ser545 550 555 560Gly Val Ile Gly Pro Gly Val Ile Gly Pro Tyr Ala
Ser Ala Ala Ala 565 570 575Ala Ala Gly Pro Gly Ser Gly Val Ile Gly
Pro Gly Ala Ser 580 585 59031590PRTArtificial SequenceM_ PRT918
31Met Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1
5 10 15Ala Ala Ala Gly Ile Asn Gly Pro Gly Ser Gly Val Phe Gly Pro
Gly 20 25 30Ile Ser Gly Ile Tyr Gly Pro Gly Val Phe Gly Pro Gly Val
Phe Gly 35 40 45Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Pro Gly Ile
Tyr Gly Pro 50 55 60Gly Val Phe Gly Pro Ser Ala Ser Ala Ala Ala Ala
Ala Gly Pro Gly65 70 75 80Ser Gly Val Phe Gly Pro Gly Ala Ser Gly
Ile Tyr Gly Pro Gly Val 85 90 95Phe Gly Pro Gly Val Phe Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala 100 105 110Gly Ile Tyr Gly Ser Gly Pro
Gly Val Phe Gly Pro Tyr Gly Ser Ala 115 120 125Ala Ala Ala Ala Gly
Pro Gly Ser Gly Ile Tyr Gly Ile Gly Pro Tyr 130 135 140Gly Pro Gly
Ala Ser Gly Pro Gly Ile Tyr Gly Pro Gly Val Phe Gly145 150 155
160Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Ser Gly Val Phe Gly Pro
165 170 175Gly Ile Tyr Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
Ile Tyr 180 185 190Gly Ser Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro
Gly Ile Ser Gly 195 200 205Ser Gly Val Phe Gly Pro Gly Val Phe Gly
Pro Tyr Ala Ser Ala Ala 210 215 220Ala Ala Ala Gly Pro Gly Val Phe
Gly Pro Tyr Gly Pro Gly Ser Ser225 230 235 240Ala Ala Ala Ala Ala
Gly Ile Tyr Gly Tyr Gly Pro Gly Val Phe Gly 245 250 255Pro Tyr Gly
Pro Gly Ala Ser Gly Ile Asn Gly Pro Gly Ser Gly Ile 260 265 270Tyr
Gly Pro Gly Val Phe Gly Pro Gly Ile Ser Ala Ala Ala Ala Ala 275 280
285Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
290 295 300Ala Ala Gly Ile Tyr Gly Pro Gly Val Phe Gly Pro Gly Ile
Tyr Gly305 310 315 320Pro Gly Ser Ser Gly Pro Gly Val Phe Gly Pro
Tyr Gly Pro Gly Ser 325 330 335Ser Ala Ala Ala Ala Ala Gly Ile Tyr
Gly Pro Gly Val Phe Gly Pro 340 345 350Tyr Gly Pro Gly Ile Ser Ala
Ala Ala Ala Ala Gly Ile Tyr Val Phe 355 360 365Gly Pro Gly Val Phe
Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly 370 375 380Val Phe Gly
Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly385 390 395
400Pro Gly Ile Tyr Gly Pro Gly Val Phe Gly Pro Ser Ala Ser Ala Ala
405 410 415Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro Gly Ile Tyr Gly
Pro Tyr 420 425 430Gly Pro Gly Ile Ser Gly Pro Gly Ser Gly
Val Phe Gly Ile Gly Pro 435 440 445Tyr Gly Pro Gly Ala Ser Ala Ala
Ala Ala Ala Gly Ile Tyr Gly Pro 450 455 460Gly Val Phe Gly Pro Tyr
Gly Pro Gly Ile Ser Ala Ala Ala Ala Ala465 470 475 480Gly Pro Gly
Ser Gly Ile Tyr Gly Pro Gly Ala Ser Gly Ile Asn Gly 485 490 495Pro
Gly Ser Gly Ile Tyr Gly Pro Gly Val Phe Gly Pro Gly Ile Ser 500 505
510Ala Ala Ala Ala Ala Gly Ile Tyr Val Phe Gly Pro Gly Val Phe Gly
515 520 525Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Ile
Tyr Gly 530 535 540Ser Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro Gly
Ile Ser Gly Ser545 550 555 560Gly Val Phe Gly Pro Gly Val Phe Gly
Pro Tyr Ala Ser Ala Ala Ala 565 570 575Ala Ala Gly Pro Gly Ser Gly
Val Phe Gly Pro Gly Ala Ser 580 585 59032601PRTArtificial
SequencePRT888 32Met His His His His His His Ser Ser Gly Ser Ser
Gly Pro Gly Val1 5 10 15Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala
Ala Ala Ala Gly Gln 20 25 30Asn Gly Pro Gly Ser Gly Val Leu Gly Pro
Gly Gln Ser Gly Gln Tyr 35 40 45Gly Pro Gly Val Leu Gly Pro Gly Val
Leu Gly Pro Gly Ser Ser Ala 50 55 60Ala Ala Ala Ala Gly Pro Gly Gln
Tyr Gly Pro Gly Val Leu Gly Pro65 70 75 80Ser Ala Ser Ala Ala Ala
Ala Ala Gly Pro Gly Ser Gly Val Leu Gly 85 90 95Pro Gly Ala Ser Gly
Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly Val 100 105 110Leu Gly Pro
Gly Ser Ser Ala Ala Ala Ala Ala Gly Gln Tyr Gly Ser 115 120 125Gly
Pro Gly Val Leu Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly 130 135
140Pro Gly Ser Gly Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala
Ser145 150 155 160Gly Pro Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro
Ser Ala Ser Ala 165 170 175Ala Ala Ala Ala Gly Ser Gly Val Leu Gly
Pro Gly Gln Tyr Gly Pro 180 185 190Tyr Ala Ser Ala Ala Ala Ala Ala
Gly Gln Tyr Gly Ser Gly Pro Gly 195 200 205Val Leu Gly Pro Tyr Gly
Pro Gly Gln Ser Gly Ser Gly Val Leu Gly 210 215 220Pro Gly Val Leu
Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly Pro225 230 235 240Gly
Val Leu Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala 245 250
255Gly Gln Tyr Gly Tyr Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly
260 265 270Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro
Gly Val 275 280 285Leu Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly
Pro Gly Val Leu 290 295 300Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala
Ala Ala Ala Gly Gln Tyr305 310 315 320Gly Pro Gly Val Leu Gly Pro
Gly Gln Tyr Gly Pro Gly Ser Ser Gly 325 330 335Pro Gly Val Leu Gly
Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala 340 345 350Ala Gly Gln
Tyr Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Gln 355 360 365Ser
Ala Ala Ala Ala Ala Gly Gln Tyr Val Leu Gly Pro Gly Val Leu 370 375
380Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Val Leu Gly Pro
Tyr385 390 395 400Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro
Gly Gln Tyr Gly 405 410 415Pro Gly Val Leu Gly Pro Ser Ala Ser Ala
Ala Ala Ala Ala Gly Gln 420 425 430Tyr Gly Ser Gly Pro Gly Gln Tyr
Gly Pro Tyr Gly Pro Gly Gln Ser 435 440 445Gly Pro Gly Ser Gly Val
Leu Gly Gln Gly Pro Tyr Gly Pro Gly Ala 450 455 460Ser Ala Ala Ala
Ala Ala Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro465 470 475 480Tyr
Gly Pro Gly Gln Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly 485 490
495Gln Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln
500 505 510Tyr Gly Pro Gly Val Leu Gly Pro Gly Gln Ser Ala Ala Ala
Ala Ala 515 520 525Gly Gln Tyr Val Leu Gly Pro Gly Val Leu Gly Pro
Tyr Gly Pro Gly 530 535 540Ala Ser Ala Ala Ala Ala Ala Gly Gln Tyr
Gly Ser Gly Pro Gly Val545 550 555 560Leu Gly Pro Tyr Gly Pro Gly
Gln Ser Gly Ser Gly Val Leu Gly Pro 565 570 575Gly Val Leu Gly Pro
Tyr Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Val
Leu Gly Pro Gly Ala Ser 595 60033601PRTArtificial SequencePRT965
33Met His His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Thr1
5 10 15Ser Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Ala 20 25 30Asn Gly Pro Gly Ser Gly Thr Ser Gly Pro Gly Ala Ser Gly
Ala Tyr 35 40 45Gly Pro Gly Thr Ser Gly Pro Gly Thr Ser Gly Pro Gly
Ser Ser Ala 50 55 60Ala Ala Ala Ala Gly Pro Gly Ala Tyr Gly Pro Gly
Thr Ser Gly Pro65 70 75 80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro
Gly Ser Gly Thr Ser Gly 85 90 95Pro Gly Ala Ser Gly Ala Tyr Gly Pro
Gly Thr Ser Gly Pro Gly Thr 100 105 110Ser Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala Gly Ala Tyr Gly Ser 115 120 125Gly Pro Gly Thr Ser
Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser
Gly Ala Tyr Gly Ala Gly Pro Tyr Gly Pro Gly Ala Ser145 150 155
160Gly Pro Gly Ala Tyr Gly Pro Gly Thr Ser Gly Pro Ser Ala Ser Ala
165 170 175Ala Ala Ala Ala Gly Ser Gly Thr Ser Gly Pro Gly Ala Tyr
Gly Pro 180 185 190Tyr Ala Ser Ala Ala Ala Ala Ala Gly Ala Tyr Gly
Ser Gly Pro Gly 195 200 205Thr Ser Gly Pro Tyr Gly Pro Gly Ala Ser
Gly Ser Gly Thr Ser Gly 210 215 220Pro Gly Thr Ser Gly Pro Tyr Ala
Ser Ala Ala Ala Ala Ala Gly Pro225 230 235 240Gly Thr Ser Gly Pro
Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala 245 250 255Gly Ala Tyr
Gly Tyr Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro Gly 260 265 270Ala
Ser Gly Ala Asn Gly Pro Gly Ser Gly Ala Tyr Gly Pro Gly Thr 275 280
285Ser Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Thr Ser
290 295 300Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Ala Tyr305 310 315 320Gly Pro Gly Thr Ser Gly Pro Gly Ala Tyr Gly
Pro Gly Ser Ser Gly 325 330 335Pro Gly Thr Ser Gly Pro Tyr Gly Pro
Gly Ser Ser Ala Ala Ala Ala 340 345 350Ala Gly Ala Tyr Gly Pro Gly
Thr Ser Gly Pro Tyr Gly Pro Gly Ala 355 360 365Ser Ala Ala Ala Ala
Ala Gly Ala Tyr Thr Ser Gly Pro Gly Thr Ser 370 375 380Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Thr Ser Gly Pro Tyr385 390 395
400Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ala Tyr Gly
405 410 415Pro Gly Thr Ser Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala
Gly Ala 420 425 430Tyr Gly Ser Gly Pro Gly Ala Tyr Gly Pro Tyr Gly
Pro Gly Ala Ser 435 440 445Gly Pro Gly Ser Gly Thr Ser Gly Ala Gly
Pro Tyr Gly Pro Gly Ala 450 455 460Ser Ala Ala Ala Ala Ala Gly Ala
Tyr Gly Pro Gly Thr Ser Gly Pro465 470 475 480Tyr Gly Pro Gly Ala
Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly 485 490 495Ala Tyr Gly
Pro Gly Ala Ser Gly Ala Asn Gly Pro Gly Ser Gly Ala 500 505 510Tyr
Gly Pro Gly Thr Ser Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala 515 520
525Gly Ala Tyr Thr Ser Gly Pro Gly Thr Ser Gly Pro Tyr Gly Pro Gly
530 535 540Ala Ser Ala Ala Ala Ala Ala Gly Ala Tyr Gly Ser Gly Pro
Gly Thr545 550 555 560Ser Gly Pro Tyr Gly Pro Gly Ala Ser Gly Ser
Gly Thr Ser Gly Pro 565 570 575Gly Thr Ser Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Thr Ser Gly Pro Gly
Ala Ser 595 60034601PRTArtificial SequencePRT889 34Met His His His
His His His Ser Ser Gly Ser Ser Gly Pro Gly Val1 5 10 15Leu Gly Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Ile 20 25 30Asn Gly
Pro Gly Ser Gly Val Leu Gly Pro Gly Ile Ser Gly Ile Tyr 35 40 45Gly
Pro Gly Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser Ser Ala 50 55
60Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro65
70 75 80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Leu
Gly 85 90 95Pro Gly Ala Ser Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro
Gly Val 100 105 110Leu Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly
Ile Tyr Gly Ser 115 120 125Gly Pro Gly Val Leu Gly Pro Tyr Gly Ser
Ala Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser Gly Ile Tyr Gly Ile
Gly Pro Tyr Gly Pro Gly Ala Ser145 150 155 160Gly Pro Gly Ile Tyr
Gly Pro Gly Val Leu Gly Pro Ser Ala Ser Ala 165 170 175Ala Ala Ala
Ala Gly Ser Gly Val Leu Gly Pro Gly Ile Tyr Gly Pro 180 185 190Tyr
Ala Ser Ala Ala Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro Gly 195 200
205Val Leu Gly Pro Tyr Gly Pro Gly Ile Ser Gly Ser Gly Val Leu Gly
210 215 220Pro Gly Val Leu Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala
Gly Pro225 230 235 240Gly Val Leu Gly Pro Tyr Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala 245 250 255Gly Ile Tyr Gly Tyr Gly Pro Gly Val
Leu Gly Pro Tyr Gly Pro Gly 260 265 270Ala Ser Gly Ile Asn Gly Pro
Gly Ser Gly Ile Tyr Gly Pro Gly Val 275 280 285Leu Gly Pro Gly Ile
Ser Ala Ala Ala Ala Ala Gly Pro Gly Val Leu 290 295 300Gly Pro Tyr
Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Ile Tyr305 310 315
320Gly Pro Gly Val Leu Gly Pro Gly Ile Tyr Gly Pro Gly Ser Ser Gly
325 330 335Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala
Ala Ala 340 345 350Ala Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro Tyr
Gly Pro Gly Ile 355 360 365Ser Ala Ala Ala Ala Ala Gly Ile Tyr Val
Leu Gly Pro Gly Val Leu 370 375 380Gly Pro Tyr Gly Pro Gly Ala Ser
Gly Pro Gly Val Leu Gly Pro Tyr385 390 395 400Gly Pro Gly Ala Ser
Ala Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly 405 410 415Pro Gly Val
Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Ile 420 425 430Tyr
Gly Ser Gly Pro Gly Ile Tyr Gly Pro Tyr Gly Pro Gly Ile Ser 435 440
445Gly Pro Gly Ser Gly Val Leu Gly Ile Gly Pro Tyr Gly Pro Gly Ala
450 455 460Ser Ala Ala Ala Ala Ala Gly Ile Tyr Gly Pro Gly Val Leu
Gly Pro465 470 475 480Tyr Gly Pro Gly Ile Ser Ala Ala Ala Ala Ala
Gly Pro Gly Ser Gly 485 490 495Ile Tyr Gly Pro Gly Ala Ser Gly Ile
Asn Gly Pro Gly Ser Gly Ile 500 505 510Tyr Gly Pro Gly Val Leu Gly
Pro Gly Ile Ser Ala Ala Ala Ala Ala 515 520 525Gly Ile Tyr Val Leu
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly 530 535 540Ala Ser Ala
Ala Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro Gly Val545 550 555
560Leu Gly Pro Tyr Gly Pro Gly Ile Ser Gly Ser Gly Val Leu Gly Pro
565 570 575Gly Val Leu Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
Pro Gly 580 585 590Ser Gly Val Leu Gly Pro Gly Ala Ser 595
60035601PRTArtificial SequencePRT916 35Met His His His His His His
Ser Ser Gly Ser Ser Gly Pro Gly Val1 5 10 15Ile Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Gly Leu 20 25 30Asn Gly Pro Gly Ser
Gly Val Ile Gly Pro Gly Leu Ser Gly Leu Tyr 35 40 45Gly Pro Gly Val
Ile Gly Pro Gly Val Ile Gly Pro Gly Ser Ser Ala 50 55 60Ala Ala Ala
Ala Gly Pro Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro65 70 75 80Ser
Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Ile Gly 85 90
95Pro Gly Ala Ser Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro Gly Val
100 105 110Ile Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Gly Leu Tyr
Gly Ser 115 120 125Gly Pro Gly Val Ile Gly Pro Tyr Gly Ser Ala Ala
Ala Ala Ala Gly 130 135 140Pro Gly Ser Gly Leu Tyr Gly Leu Gly Pro
Tyr Gly Pro Gly Ala Ser145 150 155 160Gly Pro Gly Leu Tyr Gly Pro
Gly Val Ile Gly Pro Ser Ala Ser Ala 165 170 175Ala Ala Ala Ala Gly
Ser Gly Val Ile Gly Pro Gly Leu Tyr Gly Pro 180 185 190Tyr Ala Ser
Ala Ala Ala Ala Ala Gly Leu Tyr Gly Ser Gly Pro Gly 195 200 205Val
Ile Gly Pro Tyr Gly Pro Gly Leu Ser Gly Ser Gly Val Ile Gly 210 215
220Pro Gly Val Ile Gly Pro Tyr Ala Ser Ala Ala Ala Ala Ala Gly
Pro225 230 235 240Gly Val Ile Gly Pro Tyr Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala 245 250 255Gly Leu Tyr Gly Tyr Gly Pro Gly Val Ile
Gly Pro Tyr Gly Pro Gly 260 265 270Ala Ser Gly Leu Asn Gly Pro Gly
Ser Gly Leu Tyr Gly Pro Gly Val 275 280 285Ile Gly Pro Gly Leu Ser
Ala Ala Ala Ala Ala Gly Pro Gly Val Ile 290 295 300Gly Pro Tyr Gly
Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Leu Tyr305 310 315 320Gly
Pro Gly Val Ile Gly Pro Gly Leu Tyr Gly Pro Gly Ser Ser Gly 325 330
335Pro Gly Val Ile Gly Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala
340 345 350Ala Gly Leu Tyr Gly Pro Gly Val Ile Gly Pro Tyr Gly Pro
Gly Leu 355 360 365Ser Ala Ala Ala Ala Ala Gly Leu Tyr Val Ile Gly
Pro Gly Val Ile 370 375 380Gly Pro Tyr Gly Pro Gly Ala Ser Gly Pro
Gly Val Ile Gly Pro Tyr385 390 395 400Gly Pro Gly Ala Ser Ala Ala
Ala Ala Ala Gly Pro Gly Leu Tyr Gly 405 410 415Pro Gly Val Ile Gly
Pro Ser Ala Ser Ala Ala Ala Ala Ala Gly Leu 420 425 430Tyr Gly Ser
Gly Pro Gly Leu Tyr Gly Pro Tyr Gly Pro Gly Leu Ser 435 440 445Gly
Pro Gly Ser Gly Val Ile Gly Leu Gly Pro Tyr Gly Pro Gly Ala 450 455
460Ser Ala Ala Ala Ala Ala Gly Leu Tyr Gly Pro Gly Val Ile Gly
Pro465 470 475 480Tyr Gly Pro Gly Leu Ser Ala Ala Ala Ala Ala Gly
Pro Gly Ser Gly 485 490
495Leu Tyr Gly Pro Gly Ala Ser Gly Leu Asn Gly Pro Gly Ser Gly Leu
500 505 510Tyr Gly Pro Gly Val Ile Gly Pro Gly Leu Ser Ala Ala Ala
Ala Ala 515 520 525Gly Leu Tyr Val Ile Gly Pro Gly Val Ile Gly Pro
Tyr Gly Pro Gly 530 535 540Ala Ser Ala Ala Ala Ala Ala Gly Leu Tyr
Gly Ser Gly Pro Gly Val545 550 555 560Ile Gly Pro Tyr Gly Pro Gly
Leu Ser Gly Ser Gly Val Ile Gly Pro 565 570 575Gly Val Ile Gly Pro
Tyr Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Val
Ile Gly Pro Gly Ala Ser 595 60036601PRTArtificial SequencePRT918
36Met His His His His His His Ser Ser Gly Ser Ser Gly Pro Gly Val1
5 10 15Phe Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Ile 20 25 30Asn Gly Pro Gly Ser Gly Val Phe Gly Pro Gly Ile Ser Gly
Ile Tyr 35 40 45Gly Pro Gly Val Phe Gly Pro Gly Val Phe Gly Pro Gly
Ser Ser Ala 50 55 60Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly Pro Gly
Val Phe Gly Pro65 70 75 80Ser Ala Ser Ala Ala Ala Ala Ala Gly Pro
Gly Ser Gly Val Phe Gly 85 90 95Pro Gly Ala Ser Gly Ile Tyr Gly Pro
Gly Val Phe Gly Pro Gly Val 100 105 110Phe Gly Pro Gly Ser Ser Ala
Ala Ala Ala Ala Gly Ile Tyr Gly Ser 115 120 125Gly Pro Gly Val Phe
Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Gly 130 135 140Pro Gly Ser
Gly Ile Tyr Gly Ile Gly Pro Tyr Gly Pro Gly Ala Ser145 150 155
160Gly Pro Gly Ile Tyr Gly Pro Gly Val Phe Gly Pro Ser Ala Ser Ala
165 170 175Ala Ala Ala Ala Gly Ser Gly Val Phe Gly Pro Gly Ile Tyr
Gly Pro 180 185 190Tyr Ala Ser Ala Ala Ala Ala Ala Gly Ile Tyr Gly
Ser Gly Pro Gly 195 200 205Val Phe Gly Pro Tyr Gly Pro Gly Ile Ser
Gly Ser Gly Val Phe Gly 210 215 220Pro Gly Val Phe Gly Pro Tyr Ala
Ser Ala Ala Ala Ala Ala Gly Pro225 230 235 240Gly Val Phe Gly Pro
Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala 245 250 255Gly Ile Tyr
Gly Tyr Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro Gly 260 265 270Ala
Ser Gly Ile Asn Gly Pro Gly Ser Gly Ile Tyr Gly Pro Gly Val 275 280
285Phe Gly Pro Gly Ile Ser Ala Ala Ala Ala Ala Gly Pro Gly Val Phe
290 295 300Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly
Ile Tyr305 310 315 320Gly Pro Gly Val Phe Gly Pro Gly Ile Tyr Gly
Pro Gly Ser Ser Gly 325 330 335Pro Gly Val Phe Gly Pro Tyr Gly Pro
Gly Ser Ser Ala Ala Ala Ala 340 345 350Ala Gly Ile Tyr Gly Pro Gly
Val Phe Gly Pro Tyr Gly Pro Gly Ile 355 360 365Ser Ala Ala Ala Ala
Ala Gly Ile Tyr Val Phe Gly Pro Gly Val Phe 370 375 380Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Val Phe Gly Pro Tyr385 390 395
400Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly
405 410 415Pro Gly Val Phe Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala
Gly Ile 420 425 430Tyr Gly Ser Gly Pro Gly Ile Tyr Gly Pro Tyr Gly
Pro Gly Ile Ser 435 440 445Gly Pro Gly Ser Gly Val Phe Gly Ile Gly
Pro Tyr Gly Pro Gly Ala 450 455 460Ser Ala Ala Ala Ala Ala Gly Ile
Tyr Gly Pro Gly Val Phe Gly Pro465 470 475 480Tyr Gly Pro Gly Ile
Ser Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly 485 490 495Ile Tyr Gly
Pro Gly Ala Ser Gly Ile Asn Gly Pro Gly Ser Gly Ile 500 505 510Tyr
Gly Pro Gly Val Phe Gly Pro Gly Ile Ser Ala Ala Ala Ala Ala 515 520
525Gly Ile Tyr Val Phe Gly Pro Gly Val Phe Gly Pro Tyr Gly Pro Gly
530 535 540Ala Ser Ala Ala Ala Ala Ala Gly Ile Tyr Gly Ser Gly Pro
Gly Val545 550 555 560Phe Gly Pro Tyr Gly Pro Gly Ile Ser Gly Ser
Gly Val Phe Gly Pro 565 570 575Gly Val Phe Gly Pro Tyr Ala Ser Ala
Ala Ala Ala Ala Gly Pro Gly 580 585 590Ser Gly Val Phe Gly Pro Gly
Ala Ser 595 60037565PRTArtificial SequenceM_PRT525 37Met Gly Pro
Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1 5 10 15Ala Ala
Ala Ala Ala Gly Ser Asn Gly Pro Gly Ser Gly Gln Gln Gly 20 25 30Pro
Gly Gln Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln 35 40
45Gln Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly
50 55 60Gln Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala
Ala65 70 75 80Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln Gly Pro Gly
Ala Ser Gly 85 90 95Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly Gln Gln
Gly Pro Gly Ser 100 105 110Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser
Tyr Gly Ser Gly Pro Gly 115 120 125Gln Gln Gly Pro Tyr Gly Ser Ala
Ala Ala Ala Ala Ala Ala Gly Pro 130 135 140Gly Ser Gly Gln Tyr Gly
Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly145 150 155 160Pro Gly Gln
Tyr Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala 165 170 175Ala
Ala Ala Ala Ala Gly Ser Gly Gln Gln Gly Pro Gly Gln Tyr Gly 180 185
190Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser
195 200 205Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Gln Ser Gly
Ser Gly 210 215 220Gln Gln Gly Pro Gly Gln Gln Gly Pro Tyr Ala Ser
Ala Ala Ala Ala225 230 235 240Ala Ala Ala Gly Pro Gly Gln Gln Gly
Pro Tyr Gly Pro Gly Ser Ser 245 250 255Ala Ala Ala Ala Ala Ala Ala
Gly Ser Tyr Gly Tyr Gly Pro Gly Gln 260 265 270Gln Gly Pro Tyr Gly
Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser 275 280 285Gly Gln Tyr
Gly Pro Gly Gln Gln Gly Pro Gly Pro Ser Ala Ala Ala 290 295 300Ala
Ala Ala Ala Gly Pro Gly Gln Gln Gly Pro Tyr Gly Pro Gly Ala305 310
315 320Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro Gly Gln
Gln 325 330 335Gly Pro Gly Gln Tyr Gly Pro Gly Ser Ser Gly Pro Gly
Gln Gln Gly 340 345 350Pro Tyr Gly Pro Gly Ser Ser Ala Ala Ala Ala
Ala Ala Ala Gly Ser 355 360 365Tyr Gly Pro Gly Gln Gln Gly Pro Tyr
Gly Pro Gly Pro Ser Ala Ala 370 375 380Ala Ala Ala Ala Ala Gly Ser
Tyr Gln Gln Gly Pro Gly Gln Gln Gly385 390 395 400Pro Tyr Gly Pro
Gly Ala Ser Gly Pro Gly Gln Gln Gly Pro Tyr Gly 405 410 415Pro Gly
Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr 420 425
430Gly Pro Gly Gln Gln Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala
435 440 445Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln Tyr Gly Pro Tyr
Gly Pro 450 455 460Gly Gln Ser Gly Pro Gly Ser Gly Gln Gln Gly Gln
Gly Pro Tyr Gly465 470 475 480Pro Gly Ala Ser Ala Ala Ala Ala Ala
Ala Ala Gly Ser Tyr Gly Pro 485 490 495Gly Gln Gln Gly Pro Tyr Gly
Pro Gly Pro Ser Ala Ala Ala Ala Ala 500 505 510Ala Ala Gly Pro Gly
Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln 515 520 525Asn Gly Pro
Gly Ser Gly Gln Tyr Gly Pro Gly Gln Gln Gly Pro Gly 530 535 540Pro
Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly Gln Gln545 550
555 560Gly Pro Gly Ala Ser 56538565PRTArtificial SequenceM_PRT699
38Met Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala1
5 10 15Ala Ala Ala Ala Ala Gly Ser Asn Gly Pro Gly Ser Gly Val Leu
Gly 20 25 30Pro Gly Gln Ser Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro
Gly Val 35 40 45Leu Gly Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala
Gly Pro Gly 50 55 60Gln Tyr Gly Pro Gly Val Leu Gly Pro Ser Ala Ser
Ala Ala Ala Ala65 70 75 80Ala Ala Ala Gly Pro Gly Ser Gly Val Leu
Gly Pro Gly Ala Ser Gly 85 90 95Gln Tyr Gly Pro Gly Val Leu Gly Pro
Gly Val Leu Gly Pro Gly Ser 100 105 110Ser Ala Ala Ala Ala Ala Ala
Ala Gly Ser Tyr Gly Ser Gly Pro Gly 115 120 125Val Leu Gly Pro Tyr
Gly Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro 130 135 140Gly Ser Gly
Gln Tyr Gly Gln Gly Pro Tyr Gly Pro Gly Ala Ser Gly145 150 155
160Pro Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro Ser Ala Ser Ala Ala
165 170 175Ala Ala Ala Ala Ala Gly Ser Gly Val Leu Gly Pro Gly Gln
Tyr Gly 180 185 190Pro Tyr Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Ser 195 200 205Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro
Gly Gln Ser Gly Ser Gly 210 215 220Val Leu Gly Pro Gly Val Leu Gly
Pro Tyr Ala Ser Ala Ala Ala Ala225 230 235 240Ala Ala Ala Gly Pro
Gly Val Leu Gly Pro Tyr Gly Pro Gly Ser Ser 245 250 255Ala Ala Ala
Ala Ala Ala Ala Gly Ser Tyr Gly Tyr Gly Pro Gly Val 260 265 270Leu
Gly Pro Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser 275 280
285Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly Pro Ser Ala Ala Ala
290 295 300Ala Ala Ala Ala Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro
Gly Ala305 310 315 320Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr
Gly Pro Gly Val Leu 325 330 335Gly Pro Gly Gln Tyr Gly Pro Gly Ser
Ser Gly Pro Gly Val Leu Gly 340 345 350Pro Tyr Gly Pro Gly Ser Ser
Ala Ala Ala Ala Ala Ala Ala Gly Ser 355 360 365Tyr Gly Pro Gly Val
Leu Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala 370 375 380Ala Ala Ala
Ala Ala Gly Ser Tyr Val Leu Gly Pro Gly Val Leu Gly385 390 395
400Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Val Leu Gly Pro Tyr Gly
405 410 415Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly
Gln Tyr 420 425 430Gly Pro Gly Val Leu Gly Pro Ser Ala Ser Ala Ala
Ala Ala Ala Ala 435 440 445Ala Gly Ser Tyr Gly Ser Gly Pro Gly Gln
Tyr Gly Pro Tyr Gly Pro 450 455 460Gly Gln Ser Gly Pro Gly Ser Gly
Val Leu Gly Gln Gly Pro Tyr Gly465 470 475 480Pro Gly Ala Ser Ala
Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro 485 490 495Gly Val Leu
Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala 500 505 510Ala
Ala Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Ala Ser Gly Gln 515 520
525Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly
530 535 540Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ser Gly
Val Leu545 550 555 560Gly Pro Gly Ala Ser 56539565PRTArtificial
SequenceM_PRT698 39Met Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly
Ala Ser Ala Ala1 5 10 15Ala Ala Ala Ala Ala Gly Ser Asn Gly Pro Gly
Ser Gly Val Leu Gly 20 25 30Pro Gly Ile Ser Gly Ile Tyr Gly Pro Gly
Val Leu Gly Pro Gly Val 35 40 45Leu Gly Pro Gly Ser Ser Ala Ala Ala
Ala Ala Ala Ala Gly Pro Gly 50 55 60Ile Tyr Gly Pro Gly Val Leu Gly
Pro Ser Ala Ser Ala Ala Ala Ala65 70 75 80Ala Ala Ala Gly Pro Gly
Ser Gly Val Leu Gly Pro Gly Ala Ser Gly 85 90 95Ile Tyr Gly Pro Gly
Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser 100 105 110Ser Ala Ala
Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly 115 120 125Val
Leu Gly Pro Tyr Gly Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro 130 135
140Gly Ser Gly Ile Tyr Gly Ile Gly Pro Tyr Gly Pro Gly Ala Ser
Gly145 150 155 160Pro Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro Ser
Ala Ser Ala Ala 165 170 175Ala Ala Ala Ala Ala Gly Ser Gly Val Leu
Gly Pro Gly Ile Tyr Gly 180 185 190Pro Tyr Ala Ser Ala Ala Ala Ala
Ala Ala Ala Gly Ser Tyr Gly Ser 195 200 205Gly Pro Gly Val Leu Gly
Pro Tyr Gly Pro Gly Ile Ser Gly Ser Gly 210 215 220Val Leu Gly Pro
Gly Val Leu Gly Pro Tyr Ala Ser Ala Ala Ala Ala225 230 235 240Ala
Ala Ala Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ser Ser 245 250
255Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Tyr Gly Pro Gly Val
260 265 270Leu Gly Pro Tyr Gly Pro Gly Ala Ser Gly Ile Asn Gly Pro
Gly Ser 275 280 285Gly Ile Tyr Gly Pro Gly Val Leu Gly Pro Gly Pro
Ser Ala Ala Ala 290 295 300Ala Ala Ala Ala Gly Pro Gly Val Leu Gly
Pro Tyr Gly Pro Gly Ala305 310 315 320Ser Ala Ala Ala Ala Ala Ala
Ala Gly Ser Tyr Gly Pro Gly Val Leu 325 330 335Gly Pro Gly Ile Tyr
Gly Pro Gly Ser Ser Gly Pro Gly Val Leu Gly 340 345 350Pro Tyr Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser 355 360 365Tyr
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala 370 375
380Ala Ala Ala Ala Ala Gly Ser Tyr Val Leu Gly Pro Gly Val Leu
Gly385 390 395 400Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Val Leu
Gly Pro Tyr Gly 405 410 415Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala
Ala Gly Pro Gly Ile Tyr 420 425 430Gly Pro Gly Val Leu Gly Pro Ser
Ala Ser Ala Ala Ala Ala Ala Ala 435 440 445Ala Gly Ser Tyr Gly Ser
Gly Pro Gly Ile Tyr Gly Pro Tyr Gly Pro 450 455 460Gly Ile Ser Gly
Pro Gly Ser Gly Val Leu Gly Ile Gly Pro Tyr Gly465 470 475 480Pro
Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Pro 485 490
495Gly Val Leu Gly Pro Tyr Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala
500 505 510Ala Ala Gly Pro Gly Ser Gly Ile Tyr Gly Pro Gly Ala Ser
Gly Ile 515 520 525Asn Gly Pro Gly Ser Gly Ile Tyr Gly Pro Gly Val
Leu Gly Pro Gly 530 535 540Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly
Pro Gly Ser Gly Val Leu545 550 555 560Gly Pro Gly Ala Ser
56540576PRTArtificial SequencePRT699 40Met His His His His His His
Ser Ser Gly Ser Ser Gly Pro Gly Val1 5 10 15Leu Gly Pro Tyr Gly Pro
Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 20 25 30Gly Ser Asn Gly Pro
Gly Ser Gly Val Leu Gly Pro Gly Gln Ser Gly 35 40
45Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser
50 55 60Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro
Gly65 70 75 80Val Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala
Ala Gly Pro 85 90 95Gly Ser Gly Val Leu Gly Pro Gly Ala Ser Gly Gln
Tyr Gly Pro Gly 100 105 110Val Leu Gly Pro Gly Val Leu Gly Pro Gly
Ser Ser Ala Ala Ala Ala 115 120 125Ala Ala Ala Gly Ser Tyr Gly Ser
Gly Pro Gly Val Leu Gly Pro Tyr 130 135 140Gly Ser Ala Ala Ala Ala
Ala Ala Ala Gly Pro Gly Ser Gly Gln Tyr145 150 155 160Gly Gln Gly
Pro Tyr Gly Pro Gly Ala Ser Gly Pro Gly Gln Tyr Gly 165 170 175Pro
Gly Val Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala 180 185
190Gly Ser Gly Val Leu Gly Pro Gly Gln Tyr Gly Pro Tyr Ala Ser Ala
195 200 205Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly
Val Leu 210 215 220Gly Pro Tyr Gly Pro Gly Gln Ser Gly Ser Gly Val
Leu Gly Pro Gly225 230 235 240Val Leu Gly Pro Tyr Ala Ser Ala Ala
Ala Ala Ala Ala Ala Gly Pro 245 250 255Gly Val Leu Gly Pro Tyr Gly
Pro Gly Ser Ser Ala Ala Ala Ala Ala 260 265 270Ala Ala Gly Ser Tyr
Gly Tyr Gly Pro Gly Val Leu Gly Pro Tyr Gly 275 280 285Pro Gly Ala
Ser Gly Gln Asn Gly Pro Gly Ser Gly Gln Tyr Gly Pro 290 295 300Gly
Val Leu Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly305 310
315 320Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala
Ala 325 330 335Ala Ala Ala Gly Ser Tyr Gly Pro Gly Val Leu Gly Pro
Gly Gln Tyr 340 345 350Gly Pro Gly Ser Ser Gly Pro Gly Val Leu Gly
Pro Tyr Gly Pro Gly 355 360 365Ser Ser Ala Ala Ala Ala Ala Ala Ala
Gly Ser Tyr Gly Pro Gly Val 370 375 380Leu Gly Pro Tyr Gly Pro Gly
Pro Ser Ala Ala Ala Ala Ala Ala Ala385 390 395 400Gly Ser Tyr Val
Leu Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly 405 410 415Ala Ser
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala 420 425
430Ala Ala Ala Ala Ala Ala Gly Pro Gly Gln Tyr Gly Pro Gly Val Leu
435 440 445Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser
Tyr Gly 450 455 460Ser Gly Pro Gly Gln Tyr Gly Pro Tyr Gly Pro Gly
Gln Ser Gly Pro465 470 475 480Gly Ser Gly Val Leu Gly Gln Gly Pro
Tyr Gly Pro Gly Ala Ser Ala 485 490 495Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Pro Gly Val Leu Gly Pro 500 505 510Tyr Gly Pro Gly Pro
Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly 515 520 525Ser Gly Gln
Tyr Gly Pro Gly Ala Ser Gly Gln Asn Gly Pro Gly Ser 530 535 540Gly
Gln Tyr Gly Pro Gly Val Leu Gly Pro Gly Pro Ser Ala Ala Ala545 550
555 560Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ala
Ser 565 570 57541576PRTArtificial SequencePRT698 41Met His His His
His His His Ser Ser Gly Ser Ser Gly Pro Gly Val1 5 10 15Leu Gly Pro
Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala Ala Ala Ala 20 25 30Gly Ser
Asn Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ile Ser Gly 35 40 45Ile
Tyr Gly Pro Gly Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser 50 55
60Ser Ala Ala Ala Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly Pro Gly65
70 75 80Val Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly
Pro 85 90 95Gly Ser Gly Val Leu Gly Pro Gly Ala Ser Gly Ile Tyr Gly
Pro Gly 100 105 110Val Leu Gly Pro Gly Val Leu Gly Pro Gly Ser Ser
Ala Ala Ala Ala 115 120 125Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro
Gly Val Leu Gly Pro Tyr 130 135 140Gly Ser Ala Ala Ala Ala Ala Ala
Ala Gly Pro Gly Ser Gly Ile Tyr145 150 155 160Gly Ile Gly Pro Tyr
Gly Pro Gly Ala Ser Gly Pro Gly Ile Tyr Gly 165 170 175Pro Gly Val
Leu Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala 180 185 190Gly
Ser Gly Val Leu Gly Pro Gly Ile Tyr Gly Pro Tyr Ala Ser Ala 195 200
205Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly Ser Gly Pro Gly Val Leu
210 215 220Gly Pro Tyr Gly Pro Gly Ile Ser Gly Ser Gly Val Leu Gly
Pro Gly225 230 235 240Val Leu Gly Pro Tyr Ala Ser Ala Ala Ala Ala
Ala Ala Ala Gly Pro 245 250 255Gly Val Leu Gly Pro Tyr Gly Pro Gly
Ser Ser Ala Ala Ala Ala Ala 260 265 270Ala Ala Gly Ser Tyr Gly Tyr
Gly Pro Gly Val Leu Gly Pro Tyr Gly 275 280 285Pro Gly Ala Ser Gly
Ile Asn Gly Pro Gly Ser Gly Ile Tyr Gly Pro 290 295 300Gly Val Leu
Gly Pro Gly Pro Ser Ala Ala Ala Ala Ala Ala Ala Gly305 310 315
320Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala Ala Ala Ala
325 330 335Ala Ala Ala Gly Ser Tyr Gly Pro Gly Val Leu Gly Pro Gly
Ile Tyr 340 345 350Gly Pro Gly Ser Ser Gly Pro Gly Val Leu Gly Pro
Tyr Gly Pro Gly 355 360 365Ser Ser Ala Ala Ala Ala Ala Ala Ala Gly
Ser Tyr Gly Pro Gly Val 370 375 380Leu Gly Pro Tyr Gly Pro Gly Pro
Ser Ala Ala Ala Ala Ala Ala Ala385 390 395 400Gly Ser Tyr Val Leu
Gly Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly 405 410 415Ala Ser Gly
Pro Gly Val Leu Gly Pro Tyr Gly Pro Gly Ala Ser Ala 420 425 430Ala
Ala Ala Ala Ala Ala Gly Pro Gly Ile Tyr Gly Pro Gly Val Leu 435 440
445Gly Pro Ser Ala Ser Ala Ala Ala Ala Ala Ala Ala Gly Ser Tyr Gly
450 455 460Ser Gly Pro Gly Ile Tyr Gly Pro Tyr Gly Pro Gly Ile Ser
Gly Pro465 470 475 480Gly Ser Gly Val Leu Gly Ile Gly Pro Tyr Gly
Pro Gly Ala Ser Ala 485 490 495Ala Ala Ala Ala Ala Ala Gly Ser Tyr
Gly Pro Gly Val Leu Gly Pro 500 505 510Tyr Gly Pro Gly Pro Ser Ala
Ala Ala Ala Ala Ala Ala Gly Pro Gly 515 520 525Ser Gly Ile Tyr Gly
Pro Gly Ala Ser Gly Ile Asn Gly Pro Gly Ser 530 535 540Gly Ile Tyr
Gly Pro Gly Val Leu Gly Pro Gly Pro Ser Ala Ala Ala545 550 555
560Ala Ala Ala Ala Gly Pro Gly Ser Gly Val Leu Gly Pro Gly Ala Ser
565 570 575
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.