Root-preferential And Stress Inducible Promoter And Uses Thereof
SAIDI; Younousse ; et al.
U.S. patent application number 17/077510 was filed with the patent office on 2021-02-11 for root-preferential and stress inducible promoter and uses thereof. The applicant listed for this patent is BASF AGRICULTURAL SOLUTIONS SEED, US LLC. Invention is credited to Bart DEN BOER, Celine MOUCHEL, Stephane PIEN, Younousse SAIDI.
| Application Number | 20210040493 17/077510 |
| Document ID | / |
| Family ID | 1000005170189 |
| Filed Date | 2021-02-11 |




| United States Patent Application | 20210040493 |
| Kind Code | A1 |
| SAIDI; Younousse ; et al. | February 11, 2021 |
ROOT-PREFERENTIAL AND STRESS INDUCIBLE PROMOTER AND USES THEREOF
Abstract
The present invention relates to the field of agriculture. In particular, the invention provides a promoter, a recombinant gene, plants comprising the recombinant genes and a method to improve yield of a cotton plant under stress conditions.
| Inventors: | SAIDI; Younousse; (De Pinte, BE) ; DEN BOER; Bart; (Merelbeke, BE) ; MOUCHEL; Celine; (De Pinte, BE) ; PIEN; Stephane; (Bergisch Gladbach, DE) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 1000005170189 | ||||||||||
| Appl. No.: | 17/077510 | ||||||||||
| Filed: | October 22, 2020 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15750695 | Feb 6, 2018 | 10844390 | ||
| PCT/EP2016/067152 | Jul 19, 2016 | |||
| 17077510 | ||||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C12N 15/8271 20130101; C12N 15/8227 20130101 |
| International Class: | C12N 15/82 20060101 C12N015/82 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Aug 7, 2015 | EP | 15180269.1 |
| Sep 14, 2015 | EP | 15185056.7 |
| Sep 14, 2015 | EP | 15185057.5 |
Claims
1-19. (canceled)
20. A recombinant gene comprising: (a) a root-preferential promoter; (b) a nucleic acid sequence encoding an Annexin protein; and (c) optionally, a transcription termination and polyadenylation sequence.
21. The recombinant gene of claim 20, wherein the root-preferential promoter is the Pbtg-26GhD10 promoter.
22. The recombinant gene of claim 20, wherein the nucleic acid encoding an Annexin protein comprises: (i) a nucleotide sequence of SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (ii) a nucleotide sequence at least 80% identical to SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (iii) a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (iv) a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13, SEQ ID NO: 15 or SEQ ID NO: 17; (v) a nucleotide sequence encoding the amino acid sequence having 80% identity with SEQ ID NO: 13, SEQ ID NO: 15 or SEQ ID NO: 17; or (vi) a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains.
23. A method to increase yield of a plant under a stress condition, comprising: regenerating a plant from plant cells comprising the recombinant gene of claim 20, wherein the increase is compared to a control plant.
24. The method of claim 23, wherein said plant is cotton, soybean or wheat.
25. The method of claim 23, wherein the stress condition is drought stress.
26. The method of claim 23, wherein the stress condition occurs during the plant reproductive stage.
27. The method of claim 23, wherein the stress condition occurs on field-grown plants.
28. The method of claim 23, wherein the yield is lint yield.
29. The method of claim 23, wherein the yield is seed yield.
30. The method of claim 23, wherein the yield is increased by at least 5%.
31. The method of claim 23, wherein the yield increase is more consistently obtained compared to a method to increase the yield of a plant under a stress condition comprising regenerating a plant comprising plant cells comprising (a) a constitutive plant expressible promoter, (b) a nucleic acid sequence encoding an Annexin protein; and (c) and optionally, a transcription termination and polyadenylation sequence, wherein the nucleic acid encoding an Annexin protein comprises: ((i) a nucleotide sequence of SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (ii) a nucleotide sequence at least 80% identical to SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (iii) a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12, SEQ ID NO: 14 or SEQ ID NO: 16; (iv) a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13, SEQ ID NO: 15 or SEQ ID NO: 17; (v) a nucleotide sequence encoding the amino acid sequence having 80% identity with SEQ ID NO: 13, SEQ ID NO: 15 or SEQ ID NO: 17; or (vi) a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains.
32. The method of claim 31, wherein the constitutive plant expressible promoter is a CaMV35S promoter.
33. The method of claim 31, wherein the transcription termination and polyadenylation sequence is functional in plants.
34. A plant cell comprising the recombinant gene of claim 20.
35. A plant comprising the plant cell of claim 34.
36. A plant part obtainable from the plant of claim 35.
37. The plant cell of claim 34, which is cotton, wheat or soybean.
38. The plant of claim 35, which is cotton, wheat or soybean.
39. The recombinant gene of claim 20, wherein the transcription termination and polyadenylation sequence is functional in plants.
Description
FIELD OF THE INVENTION
[0001] The present invention relates to the field of agricultural biotechnology, more specifically to the use of a transgene to improve plant yield under stress conditions. In particular, a method is provided to express a gene encoding an Annexin from Brassica juncea in plants to improve yield under drought stress condition. A stress induced root-preferential promoter is provided as well as an expression cassette for regulating the expression of Annexin preferentially in the roots under stress conditions.
BACKGROUND OF THE INVENTION
[0002] In recent years the phenomenon of global warming and its effect on crop plant production has become a crucial issue. Solving this problem at the plant level is almost exclusively a question of coping with plant stress. International agricultural and environmental research institutions as well as companies now re-discover plant stress as a major component of the effect of global warming on local and global food production. Research to meet these challenges involves widely diverging disciplines such as atmospheric sciences, soil science, plant physiology, biochemistry, genetics, plant breeding, molecular biology and agricultural engineering.
[0003] Abiotic plant environmental stress constitutes a major limitation to crop production. The major plant environmental stresses of contemporary economic importance worldwide are water stress including drought and flooding, cold (chilling and freezing), heat, salinity, water logging, soil mineral deficiency, soil mineral toxicity and oxidative stress. These factors are not isolated but also interrelated and influencing each other.
[0004] A major challenge in agriculture practice and research today is thus how to cope with plant environmental stress in an economical and an environmentally sustainable approach. In view of the already existing regions exposed to abiotic stress conditions in the world and the ongoing climate change, the provision of transgenic plants conferring resistance on at least one kind of abiotic stress is still a major goal in order to achieve a satisfying nutritional situation also in regions exposed to such abiotic stress in the world.
[0005] Cotton (Gossypium spp.) is the world's most important natural textile fiber and is also a significant oilseed crop. Cotton production provides income for approximately 250 million families, and approximately 150 countries are involved in cotton import and export. Its economic impact is estimated to be approximately $500 billion/year worldwide. World consumption of cotton fiber is approximately 115 million bales or approximately 27 million metric tons per year (National Cotton Council, www.cotton.org, 2006). The genus Gossypium is relatively complex and includes approximately 45 diploid (2n=2x=26) and five tetraploid (2n=4x=52) species, all exhibiting disomic patterns of inheritance. Diploid species (2n=26) fall into eight genomic groups (A-G, and K). The African Glade, comprising the A, B, E, and F gcnomcs, occurs naturally in Africa and Asia, while the D genome Glade is indigenous to the Americas. A third diploid Glade, including C, G, and K, is found in Australia. All 52 chromosome species, including Gossypium hirsutum and Gossypium barbadense, are classic natural allotetraploids that arose in the New World from interspecific hybridization between an A genome-like ancestral African species and a D genome-like American species. The closest extant relatives of the original tetraploid progenitors are the A genome species Gossypium herbaceum (A1) and Gossypium arboreum (A2) and the D genome species Gossypium raimondii (D5) `Ulbrich`. Polyploidization is estimated to have occurred 1 to 2 million years ago, giving rise to five extant allotetraploid species. Interestingly, the A genome species produce spinnable fiber and are cultivated on a limited scale, whereas the D genome species do not. More than 95% of the annual cotton crop worldwide is G. hirsutum, Upland or American cotton, and the extra-long staple or Pima cotton (G. barbadense) accounts for less than 2% (National Cotton Council, www.cotton.org, 2006).
[0006] In addition to its importance for the textile industry, cotton agriculture also provides cottonseed to feed livestock, dairy cattle and poultry while cottonseed oil is used for food products like cooking oil (National Cotton Council, www.cotton.org, 2016).
[0007] Although cotton plants are naturally very drought tolerant compared to other crops and are mostly grown without irrigation (Cotton Today) cotton yield is severely affected by periods of drought especially at peak flowering, with each day of drought stress reducing lint yield by more than 18 kg/ha (Gibb et al. 2013, WATERpak section 3.1, p 117-126).
[0008] Soybean (Glycine max (L.) Merrill) is the world's leading source of vegetable oil and protein meal. The oil extracted from soybeans is used for cooking oil, margarine, and salad dressings. Soybean oil is composed of saturated, monounsaturated and polyunsaturated fatty acids. It has a typical composition of 11% palmitic, 4% stearic, 25% oleic, 50% linoleic and 9% linolenic fatty acid content ("Economic Implications of Modified Soybean Traits Summary Report", Iowa Soybean Promotion Board and American Soybean Association Special Report 92S, May 1990). Changes in fatty acid composition for improved oxidative stability and nutrition are constantly sought after. Industrial uses of soybean oil which is subjected to further processing include ingredients for paints, plastics, fibers, detergents, cosmetics, lubricants and biodiesel fuel. Soybean oil may be split, inter-esterified, sulfurized, epoxidized, polymerized, ethoxylated, or cleaved. Designing and producing soybean oil derivatives with improved functionality and improved oliochemistry is a rapidly growing field. The typical mixture of triglycerides is usually split and separated into pure fatty acids, which are then combined with petroleum-derived alcohols or acids, nitrogen, sulfonates, chlorine, or with fatty alcohols derived from fats and oils.
[0009] Soybean is also used as a food source for both animals and humans. Soybean is widely used as a source of protein for animal feeds for poultry, swine and cattle. During processing of whole soybeans, the fibrous hull is removed and the oil is extracted. The remaining soybean meal is a combination of carbohydrates and approximately 50% protein. For human consumption soybean meal is made into soybean flour which is processed to protein concentrates used for meat extenders or specialty pet foods. Production of edible protein ingredients from soybean offers a healthier, less expensive replacement for animal protein in meats as well as in dairy-type products. Whole soybeans are an excellent source of protein and dietary fiber. Soy protein is the only vegetable with a complete protein as it contains all eight amino acids essential for human health. Most soybeans are processed, or "crushed" into soybean meal and oil. Most of the soybean meal that is crushed is further processed into animal feed with the balance used to make soy flour and proteins. Of the oil fraction, most is consumed as edible oil, the rest is used for industrial products such as fatty acids, soaps, inks, hydraulic oil, grease, biodiesel, solvent, plastics and other products. Food uses of soybeans include traditional soy-foods such as tofu and soymilk as well as meat analogs and soy-based yogurts.
[0010] Soybeans grow on a variety of soils and a wide range of climates, and most soybeans are produced in the United States, Brazil, Argentina, China and India. A given area of land planted with soybeans can produce much more protein than land planted with other crops, or if the land were used to raise cattle. Soybean is however particularly sensitive to pests like nematodes which can cause yield losses of more than 30% in heavily infested field. Combined with drought the impact of the nematode infection increases dramatically and can lead to complete yield loss.
[0011] Many genes involved in stress response mechanisms in plants have been described in the art and some were demonstrated to confer some level of stress tolerance to the plant. For example, such genes encode antioxidant enzymes, synthetic genes of osmolytes, molecular chaperones like HSPs, enzymes involved in the production of plant hormones like abscisic acid (reviewed for example in Hu et al. 2014. Annu. Rev. Plant Biol. 65:715-41).
[0012] Despite the numerous reports of genes conferring abiotic stress tolerance in plants, few actually report a yield improvement in field condition. This limited success, reviewed for example in Cominelli et al. 2012, New Biotechnol, dx.doi.org/10.1016/j.nbt.2012.11.001, Lawlor 2013, J Exp Bot, Vol 64(l):83-108 and Tardieu 2012, J. Exp. Bot, Vol 63(l):25-31, is mainly attributed to agronomically unrealistic stress conditions and timing of stress application. Furthermore growth conditions are well controlled in laboratory or greenhouse experiments while field grown plants experience varying conditions and rarely a single stress. Genes conferring actual yield improvement under stress in field conditions are thus limited and the transferability of stress tolerance identified in laboratories or greenhouse experiments to field conditions is not straightforward.
[0013] To date, genes known to confer drought, salt or osmotic stress tolerance in cotton are the Arabidopsis vacuolar pyrophosphatase gene AVP1 (Pasapula et al. 2011, Plant Biotechnology Journal 9:88-99), the Arabidopsis EDT1/HDG11 gene involved in ABA signaling (Yu et al., 2016, Plant Biotechnol J, 14(1):72-84), the Arabidopsis LOSS gene involved in ABA biosynthesis (Yue et al., 2012, J. Exp Bot, 63(10): 3741-3748), IPT gene involved in cytokinin biosynthesis (Kuppu et al. 2013, PLoS ONE 8(5): e64190), the rice SNAC1 gene (Liu et al. 2014, PLoS ONE 9(1): e86895), the annexin 1 gene from Brassica juncea AnnBj1 or Gossypium hirsutum GhAnn1 (Divya et al. 2010, Plant Mol. Biol. 73:293-308 and Zhang et al. 2015, Plant Mol. Biol., 87: 47-67 respectively) and the heat shock protein gene GHSP26 from Gossypium arboreum (Maqbool et al. 2009, Biotechnol. Prog. 26(1): 21-25). Of those, only AVP1 was shown to confer a yield increase under stress in the field.
[0014] Annexins (ANN) form a multigene family and have been so far identified in both plant and animal kingdom. They encode calcium-dependent membrane binding proteins involved in the calcium dependent polar growth of cells like root hairs, pollen and cotton fibers. Annexins are defined by their highly conserved fold consisting of four or more repeats of a so-called annexin-repeat domain signature sequence (Barton et al. 1991, Eur. J. Biochem. 198: 749-760).
[0015] Annexins are ubiquitously expressed and their expression level is modulated by environmental stimuli like light, gravity, abiotic stresses and wounding suggesting a role in mediating stress response. Such role is supported by the discovery that ANN1s from Arabidopsis thaliana and Brassica juncea were found to have peroxidase activity (Gorecka et al., 2005, Biochem Biophys Res Commun, 336(3):868-875, Divya et al., 2010, Plant Mol. Biol. 73:293-308), therefore able to act as cellular antioxidant.
[0016] The involvement of ANN1 in stress response was studied in Arabidopsis, tobacco, and cotton. Loss of function analysis of Atann1 and Atann4 mutants indicated an increased sensitivity to salt and osmotic stress as well as a reduced germination rate and growth following abscisic acid treatment (US application 2005/089872). Cantero et al. (2006, Plant Physiology and Biochemistry, 44: 13-24) showed that AtANN1 is upregulated by cold, heat, drought and salt stress. The Arabidopsis Atann1 knockout mutant accumulates more reactive oxygen species and is more sensitive to severe drought stress than wild type. Arabidopsis plants overexpressing AtANN1 were found more drought tolerant as they could resurrect from a severe desiccation (Konopka-Postupolska et al., 2009, Plant Physiology, 150: 1394-1410). Furthermore, rice plants expressing AtANN1 constitutively or preferentially in the green tissues had an increased yield under both optimal and drought stress conditions (US application 2010/0170011).
[0017] Arabidopsis lines engineered to overexpress the Lotus Annexinl (NnAnnl) were found to have an improved germination rate under heat stress (patent application CN102229662).
[0018] The Annexinl gene of Brassica juncea (AnnBj1) is induced by ABA, salt and peroxide treatments (Jami et al., 2009, Plant Physiology and Biochemistry 47: 977-990). Tobacco plants constitutively expressing AnnBj1 were shown to be more tolerant to drought (mannitol), salt and oxidative stress in survival assays at the seedling stage (Jami et al., 2008, Plant Physiology and Biochemistry, 46: 1019-1030). Similarly cotton plants constitutively expressing AnnBj1 had increased tolerance to salt, osmotic and oxidative stress at the seedling stage (Divya et al., 2010, Plant Mol. Biol. 73: 293-308). Furthermore, these transgenic plants were shown to maintain normal seed development and fiber quality when grown under salt stress (Divya et al., 2010, Plant Mol. Biol. 73:293-308).
[0019] In cotton, GhAnn1 expression is induced upon treatment with ABA, peroxide, salt and PEG (Zhang et al., 2015, Plant Mol. Biol. 87: 47-67). Germination and seedling growth of cotton plants overexpressing GhAnn1 was studied under various stresses. Overexpressing lines germinated faster and showed better seedling growth than wild type when subjected to salt or drought (PEG) stress, indicating a better stress tolerance (Zhang et al., 2015, Plant Mol. Biol. 87: 47-67).
[0020] Even though the prior art described an improved germination and early growth in cotton under various abiotic stresses, and yield increase in rice, the prior art does not reveal an increase of cotton fiber yield. There remains thus a need to increase cotton yield (lint yield and seed yield) under drought stress in field condition. To that end, appropriate expression (spatial, temporal) is also required. There also remains a need to obtain a more consistent increase in yield in plants, particularly under drought conditions, particularly in field conditions.
[0021] Genetic modification of plants to alter and/or improve phenotypic characteristics (such as productivity or quality) relies on the availability of a means to drive and to control gene expression as required. Indeed, genetic modification relies on the availability and use of suitable promoters which are effective in plants and which regulate transcription so as to give the desired effect(s) in the transgenic plant.
[0022] For numerous applications in plant biotechnology it is required to express the transgenes in a tissue-preferential and/or an inducible manner to avoid the undesirable effects the transgene expression could cause in other tissues or at times it is not required.
[0023] Root-preferential promoters are useful for expressing or down-regulating genes preferentially in the roots to get the desired function or effect, such as improving the resistance to soil-borne pathogens or root pathogens, improving tolerance to abiotic stress, such as temperature, water or salt stress, broadening the range of soils in which the plant may grow, altering root architecture, such as root density, or root strength, altering or improving nutrient uptake and/or nutrient use, modifying the interaction between the roots and above-ground biomass, or modifying metabolic pathways in the root.
[0024] Examples of root-preferential promoters include the RB7 promoter from Nicotiana tabacum (U.S. Pat. Nos. 5,459,252 and 5,750,386); the ARSK1 promoter from Arabidopsis thaliana (Hwang and Goodman (1995) Plant J 8:37-43), the MR7 promoter from Zea mays (U.S. Pat. No. 5,837,848), the ZRP2 promoter of Zea mays (U.S. Pat. No. 5,633,363), and the MTL promoter from Zea mays (U.S. Pat. Nos. 5,466,785 and 6,018,099), the pLTP and TIP2-3 promoters from Sorghum bicolor (WO2014/164399A1 and WO2014/159113A1 respectively), Class-H-Patatin-Promotor (Koster-Topfer et al., Mol. Gen. Genet. 219 (1989), 390-396), Agropinsynthase-Promotor (ags) (Inoguchi et at., Plant Phys. 149 (1996), 73-78), AKT1 promoter (Lagarde et al., Plant J. 9 (1996), 195-203), and TobRB7 promoter (Yamamoto ct al., Plant Cell 3 (1991), 371-382).
[0025] Stress-inducible promoters are useful for expressing or down-regulating genes specifically in stressful conditions to get the desired function or effect, such as improving the tolerance to abiotic stress, such as temperature, water or salt stress.
[0026] Examples of abiotic stress inducible promoters include the drought-inducible rd29a promoter from Arabidopsis thaliana (Yamaguchi-Shinozaki et al. 1993, Mol. Gen. Genet., 236: 331-340), the heat-inducible HSP81.1 from Arabidopsis thaliana (Takahashi et al., 1992, Plant Physiol., 99: 383-390), and the drought-inducible rab17 promoter from Zea mays (Morran et al., 2011, Plant Biotechnology Journal, 9: 230-249).
[0027] Few promoters combining both the tissue specificity and the stress inducibility have been isolated. Examples of such promoters include the shoot specific and drought stress inducible HPR1 promoter of Arabidopsis (Wang et al., 2009, Molecular Plant, 2(1): 191-200), the shoot specific and salt and drought stress inducible AISAP promoter from Aeluropus littoralis (Saad ct al., 2011, Transgenic Rcs, 20: 1003-1018), the root specific and osmotic stress responsive EgTIP2 promoter of Eucalyptus grandis (Rodrigues et al., 2013, Plant Science, 213: 106-113) and the salt-inducible and root epidermis specific btg-26 promoter from Brassica napus (WO 2001/055433, US2005044585A1), also demonstrated to be functional in barley (Good et al., 2007, Can J. Bot. 85: 252-262) and the promoter of its orthologous gene from rice OsANT1 (U.S. Pat. No. 7,982,093).
[0028] There is a need for further promoters conferring tissue-specificity, stress-inducibility or both, particularly promoters controlling stress-induced and/or root-preferential expression in plants, such as Gossypium plants, Glycine plants and Triticum plants.
[0029] It is an objective of the present invention to increase yield in plants, such as increasing cotton yield including lint yield or seed yield under drought stress in field condition. It is another objective to obtain a more consistent increase in yield in plants, such as cotton lint yield or cotton seed yield. These and other problems are solved as hereinafter described, particularly in the different embodiments, examples and claims. Also provided is a Gossypium promoter for stress-induced and/or root-preferential expression of genes of interest in plants.
SUMMARY OF THE INVENTION
[0030] In one aspect, the invention provides an isolated nucleic ac id comprising root-preferential and stress-inducible promoter activity selected from the group consisting of (a) a nucleic acid comprising a nucleotide sequence of SEQ ID NO: 7 or a functional fragment thereof; (b) a nucleic acid comprising a nucleotide sequence having at least about 95% sequence identity to SEQ ID NO: 7 or a functional fragment thereof; and (c) the nucleic acid of a functional promoter hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 7, or a functional fragment thereof; wherein said functional fragment comprises at the 400 bp sequence upstream of the transcription start of SEQ ID NO: 7.
[0031] A further embodiment provides a recombinant gene comprising the nucleic acid according to the invention operably linked to a heterologous nucleic acid sequence encoding an expression product of interest, and optionally a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plant cells. In a further embodiment, said expression product of interest is an RNA capable of modulating the expression of a gene or is a protein.
[0032] Yet another embodiment provides a host cell, such as an E. coli cell, an Agrobacterium cell, a yeast cell, or a plant cell, comprising the isolated nucleic acid according to the invention, or the recombinant gene according to the invention.
[0033] In a further embodiment, a plant is provided comprising the recombinant gene according to the invention. A further embodiment provides plant parts and seeds obtainable from the plant according to the invention. These plant parts and seeds comprise the recombinant gene described above. In another embodiment, the plants, plant parts or seeds according to the invention are cotton, soybean or wheat plants, plant parts or seeds. It can also be expected that this promoter would be functional in other dicotyledonous and monocotyledonous plants.
[0034] Yet another embodiment provides a method of producing a transgenic plant comprising the steps of (a) introducing or providing the recombinant gene according to the invention to a plant cell to create transgenic cells; and (b) regenerating transgenic plants from said transgenic cell.
[0035] Further provided are methods of effecting root-preferential, stress-inducible, and combined root-preferential and stress-inducible expression of a nucleic acid comprising introducing the recombinant gene according to the invention into the genome of a plant, or providing the plant according to the invention. Also provided is a method for altering biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, or yield of a plant, said method comprising introducing the recombinant gene according to the invention into the genome of a plant, or providing the plant according to the invention. In another embodiment, said plant is a cotton, a soybean or a wheat plant.
[0036] Also provided is the use of the isolated nucleic acid according to the invention to regulate expression of an operably linked nucleic acid in a plant, and the use of the isolated nucleic acid according to the invention, or the recombinant gene according to the invention to alter biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, or yield of a plant. In a further embodiment, said plant is a cotton, a soybean or a wheat plant.
[0037] Yet another embodiment provides a method of producing food, feed, or an industrial product comprising (a) obtaining the plant or a part thereof, according to the invention; and (b) preparing the food, feed or industrial product from the plant or part thereof. In another embodiment, said food or feed is oil, meal, ground or crushed seeds, soybean flakes, grain, starch, flour or protein, or said industrial product is biofuel, fiber, industrial chemicals, a pharmaceutical or a nutraceutical. Such food, feed or industrial products contain the root-preferential, stress-inducible and stress-induced root-preferential promoter described herein.
[0038] In another aspect, the invention provides a recombinant gene comprising (a) a plant expressible promoter selected from the group consisting of i) root-preferential promoter, ii) stress-inducible promoter and iii) stress-induced root-preferential promoter, (b) a nucleic acid sequence encoding an Annexin protein (c) and optionally, a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants.
[0039] In a further embodiment the root preferential promoter is the Pbtg-26GhD10 promoter.
[0040] In another embodiment the nucleic acid sequence encoding an Annexin protein comprises (a) a nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; (b) a nucleotide sequence at least 80% identical to SEQ ID NO: 12 or SEQ ID NO: 14 (c) a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14, (d) a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13 or SEQ ID NO: 15 (e) a nucleotide sequence encoding the amino acid sequence having at least 80% identity with SEQ ID NO: 13 or SEQ ID NO: 15, (f) a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains.
[0041] In yet another aspect, the invention provides a method to increase the yield, such as fiber yield and seed yield, of a plant, such as a cotton, a soybean or a wheat plant, compared to a control plant under stress condition comprising (a) providing to cells of said plant a recombinant gene comprising (i) a heterologous plant expressible promoter, (ii) a nucleic acid sequence encoding an Annexin protein (iii) and optionally, a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants, and (b) regenerating said plant.
[0042] In a further embodiment the heterologous plant expressible promoter is selected from the group consisting of a) a root-preferential promoter, b) a stress-inducible promoter and c) a stress-induced root-preferential promoter. In another embodiment said promoter is the Pbtg26-GhD10 promoter.
[0043] In a further embodiment the heterologous plant expressible promoter is a constitutive promoter. In another embodiment said promoter is the Cauliflower Mosaic Virus CaMV35S promoter.
[0044] In another embodiment the nucleic acid sequence encoding an Annexin protein comprises (a) a nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; (b) a nucleotide sequence at least 80% identical to SEQ ID NO: 12 or SEQ ID NO: 14 (c) a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14, (d) a nucleotide sequence encoding an amino acid sequence of SEQ ID NO: 13 or SEQ ID NO: 15 (e) a nucleotide sequence encoding an amino acid sequence having at least 80% identity with SEQ ID NO: 13 or SEQ ID NO: 15, (f) a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains.
[0045] Further embodiments disclose the stress as a drought stress, occurring during the plant reproductive stage, on field-grown plants.
[0046] Another embodiment provides a method to increase yield of a plant. In a further embodiment, said plant is a cotton, a soybean or a wheat plant.
[0047] The present invention provides a method to increase lint yield and a method to increase seed yield. In a further embodiment the increased yield compared to a control plant is at least 5%.
[0048] According to the present invention, the method provided more consistently increased yield when the promoter used is selected from the group of root-preferential, stress-inducible or stress-induced root-preferential promoters, preferentially the Pbtg-26GhD10 promoter, compared to when the promoter used is a constitutive plant expressible promoter, preferentially the CaMV35S promoter.
[0049] The invention further provides plants, plant parts or plants cells comprising the provided recombinant gene. In a specific embodiment, the plant, plant part or plant cell is cotton, soybean or wheat.
BRIEF DESCRIPTION OF THE FIGURES
[0050] FIG. 1: alignment of promoter regions of the btg-26 gene from subgenome A and from subgenome D of Gossypium hirsutum. The nucleotide sequence of the promoter of subgenome A (upper sequence) corresponds to the nucleotide sequence of SEQ ID NO: 4 from position 472 to position 1486. The nucleotide sequence of promoter of subgenome D (lower sequence) corresponds to the nucleotide sequence of SEQ ID NO: 5 from position 2067 to position 3089. Differences in nucleotide sequence are indicated by gray boxes. Nucleotides which do not have a corresponding nucleotide in the other promoter region are indicated by dashes in the nucleotide sequence missing the nucleotides. The first nucleotide of each promoter fragment of ca. 0.6 kb is underlined. The predicted TATA box is double underlined while the transcription initiation start is wave-underlined. Predicted ABA-responsive element-like binding site motifs (ABRE-like motifs) are framed. Nucleotides between brackets are replaced by the sequence ACC in the T-DNAs due to the creation of a Ncol restriction site required for the cloning of the promoters. The translation START codon is indicated in bold. Overall identity between the two promoter regions is of about 78% sequence identity. Sequence identity between the ca. 600 bp upstream of the translation start of the two promoters is about 94%.
[0051] FIG. 2: Alignment of the amino acid sequence of different Annexin proteins. Amino acid residues conserved in all proteins are indicated by an asterisk. Conserved amino-acid substitutions are indicated by a column. Annexin-repeat domains are underlined. The 17 aminoacid endonexin fold region with its characteristic KGhGTDEXXLIpILApR motifs are framed. The conserved Histidinc residue for peroxidase activity is indicated in bold on a grey background. The conserved phospholipid binding sites (Tryptophan residues) are indicated in bold on a yellow background. The type II calcium binding sequences (GXGTD motifs) are highlighted in green. AnnBj1: Annexinl protein from Brassica juncea (SEQ ID NO: 13); AtAnn1: Annexinl protein from Arabidopsis thaliana (SEQ ID NO: 17); GhAnn1: Annexinl protein from Gossypium hirsutum (SEQ ID NO: 15). Amino-acids which do not have a corresponding nucleotide in the other protein sequence are indicated by dashes in the amino-acid sequence missing the amino-acids. Overall identity between AnnBj1 and AtAnn1 is about 91%, between AnnBj1 and GhAnn1 about 70% and between AtAnn1 and GhAnn1 about 72%.
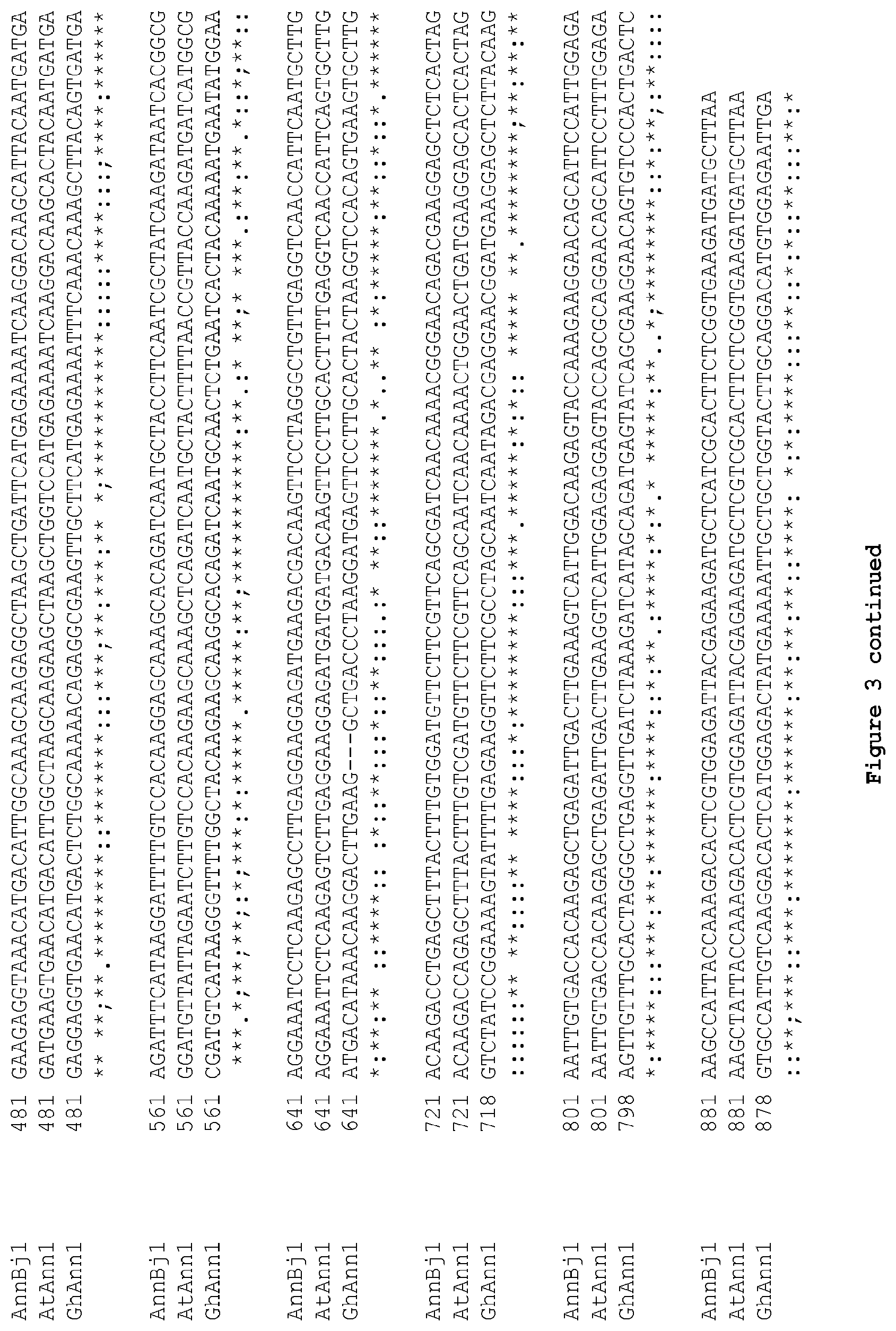
[0052] FIG. 3: Alignment of the nucleotide sequence of different Annexin coding sequences. AnnBj1: Annexinl coding sequence from Brassica juncea (SEQ ID NO: 12); AtAnn1: Annexinl coding sequence from Arabidopsis thaliana (SEQ ID NO: 16); GhAnn1: Annexinl coding sequence from Gossypium hirsutum (SEQ ID NO: 14). Nucleotides conserved in all three sequences are indicated with an asterisk. Nucleotides conserved only between the AnnBj1 and AtAnn1 sequences are indicated with a column. Nucleotides conserved only between the AnnBj1 and GhAnn1 sequences are indicated with a semi-column. Nucleotides conserved only between the AtAnn1 and GhAnn1 sequences arc indicated with a point. Nucleotides which do not have a corresponding nucleotide in the other nucleotide sequence are indicated by dashes in the nucleotide sequence missing the nucleotides. Overall identity between the coding sequences is about 70% between AtAnn1 and GhAnn1 and between AnnBj1 and GhAnn1 and is about 89% between AnnBj1 1 and AtAnn1.
DETAILED DESCRIPTION OF THE INVENTION
[0053] The present invention is based on the surprising discovery that the expression of an Annexin in a plant lead to an increased yield under stress condition in the field compared to their respective control plants. Furthermore, the promoter sequence from the D genome of Gossypium hirsutum Pbtg-26GhD10 was found to exhibit stress-induced root-preferential promoter activity in plants. It was moreover discovered that the yield increase obtained when expressing an Annexin is more consistently obtained when the Annexin is expressed under control of a stress-induced root-preferential promoter than when the expression of the same Annexin is under control of a constitutive promoter.
[0054] In a first aspect, the invention provides an isolated nucleic acid comprising root-preferential and stress-inducible promoter activity selected from the group consisting of (a) a nucleic acid comprising a nucleotide sequence of SEQ ID NO: 7 or a functional fragment thereof; (b) a nucleic acid comprising a nucleotide sequence having at least about 95% sequence identity to SEQ ID NO: 7, or a functional fragment thereof; and (c) the nucleic acid of a functional promoter capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 7, or a functional fragment thereof; wherein said functional fragment comprises at least the 400 bp sequence upstream of the transcription start of SEQ ID NO: 7.
Promoter
[0055] SEQ ID NO: 7 represents the ca. 1 kb long sequence of the btg-26Gh-D promoter upstream of the translation start of Gossypium hirsutum. SEQ ID NO: 7 is a preferred promoter fragment in this invention, however alternative functional fragments may be used. Such functional fragment would preferably be longer than 600, longer than 700, longer than 800 or even longer than 900 consecutive nucleotides upstream of the transcription start site (SEQ ID No 7 nucleotide position 755) or be longer than 700, longer than 800, longer than 900 or even longer than 1000 consecutive nucleotides upstream of the translation start site (FIG. 1 nucleotide positions 1022-1024) and promote transcription of an operably linked nucleic acid preferentially in the roots and in a stress-inducible manner. A promoter fragment according to the invention may thus comprise a nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 351 to the nucleotide at position 755. Alternatively, a promoter fragment according to the invention may thus comprise a nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 251 to the nucleotide at position 755. Yet another promoter fragment according to the invention may thus comprise a nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 151 to the nucleotide at position 755. Still another promoter fragment according to the invention may thus comprise a nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 51 to the nucleotide at position 755. Yet another promoter fragment according to the invention may thus comprise a nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 1 to the nucleotide at position 755.
[0056] The nucleic acid comprising the stress-induced root-preferential promoter activity according to the invention may also be comprised in a larger DNA molecule.
[0057] "Root-preferential promoter activity" in the context of this invention means the promoter activity is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher in roots than in other tissues. In other words, in root-preferential promoter activity, transcription of the nucleic acid operably linked to the promoter of the invention in the roots is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher than in other tissues. In other words, the root-preferential promoter controls root-preferential expression of the nucleic acid operably linked to the root-preferential promoter. "Root-preferential promoter activity" encompasses "stress-induced root-preferential promoter activity".
[0058] "Stress-inducible promoter activity" means the promoter activity is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher when the plant or plant part is subjected to environmental stress than in control condition. In other words, in stress-inducible promoter activity, transcription of the nucleic acid operably linked to the promoter of the invention is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher when the plant or plant part is subjected to stress than in control condition. In other words, the stress-inducible promoter controls stress-inducible expression of the nucleic acid operably linked to the stress-inducible promoter. "Stress-inducible promoter activity" encompasses "stress-induced root-preferential promoter activity".
[0059] "Stress induced root-preferential promoter activity" means the promoter activity is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher in the roots compared to other plant tissues when the plant or plant part is subjected to environmental stress. In other words, in stress-induced root-preferential promoter activity, transcription of the nucleic acid operably linked to the promoter of the invention is at least 2 times, or at least 5 times, or at least 10 times, or at least 20 times or even at least 100 times higher in the root tissues than in other plant tissues when the plant or plant part is subjected to stress. In other words, the stress-induced root-preferential promoter controls stress-induced root-preferential expression of the nucleic acid operably linked to the stress-induced root-preferential promoter.
[0060] As used herein, "promoter" means a region of DNA sequence that is essential for the initiation of transcription of DNA, resulting in the generation of an RNA molecule that is complementary to the transcribed DNA; this region may also be referred to as a "5' regulatory region". Promoters are usually located upstream of the coding sequence to be transcribed and have regions that act as binding sites for RNA polymerase II and other proteins such as transcription factors (trans-acting protein factors that regulate transcription) to initiate transcription of an operably linked gene. Promoters may themselves contain sub-elements (i.e. promoter motifs) such as cis-elements or enhancer domains that regulate the transcription of operably linked genes. The promoters of this invention may be altered to contain "enhancer DNA" to assist in elevating gene expression. As is known in the art, certain DNA elements can be used to enhance the transcription of DNA. These enhancers often are found 5' to the start of transcription in a promoter that functions in eukaryotic cells, but can often be inserted upstream (5') or downstream (3') to the coding sequence. In some instances, these 5' enhancer DNA elements are introns. Among the introns that are useful as enhancer DNA are the 5' introns from the rice actin 1 gene (see U.S. Pat. No. 5,641,876), the rice actin 2 gene, the maize alcohol dehydrogenase gene, the maize heat shock protein 70 gene (see U.S. Pat. No. 5,593,874), the maize shrunken 1 gene, the light sensitive 1 gene of Solanum tuberosum, the Arabidopsis histone 4 intron and the heat shock protein 70 gene of Petunia hybrida (see U.S. Pat. No. 5,659,122). Thus, as contemplated herein, a promoter or promoter region includes variations of promoters derived by inserting or deleting regulatory regions, subjecting the promoter to random or site-directed mutagenesis, etc. The activity or strength of a promoter may be measured in terms of the amounts of RNA it produces, or the amount of protein accumulation in a cell or tissue, relative to a promoter whose transcriptional activity has been previously assessed.
[0061] A promoter as used herein may thus include sequences downstream of the transcription start, such as sequences coding the 5' untranslated region (5' UTR) of the RNA or introns located downstream of the transcription start. A promoter fragment according to the invention may comprise its own 5'UTR comprising the nucleotide sequence of SEQ ID No: 7 from nucleotide 756 to nucleotide 1022. As experimentally demonstrated the last three nucleotides from the 5'UTR of the herein described promoters can be exchanged for other nucleotides to create a convenient restriction enzyme recognition site. Thus a promoter fragment according to the invention may comprise its own 5'UTR comprising the nucleotide sequence of SEQ ID No: 7 from nucleotide 756 to nucleotide 1019. In combination with the above described promoter fragments, a promoter fragment according to the invention may thus comprise the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 351 to the nucleotide at position 1019, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 251 to the nucleotide at position 1019 or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 151 to the nucleotide at position 1019, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 51 to the nucleotide at position 1019, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 1 to the nucleotide at position 1019 such as the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 351 to the nucleotide at position 1022, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 251 to the nucleotide at position 1022 or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 151 to the nucleotide at position 1022, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 51 to the nucleotide at position 1022, or the nucleotide sequence of SEQ ID No: 7 from the nucleotide at position 1 to the nucleotide at position 1022. Alternatively, 5'UTR fragments from other genes may be used.
[0062] Promoter activity for a functional promoter fragment in roots and promoter activity for a functional promoter fragment under stress may be determined by those skilled in the art, for example using analysis of RNA accumulation produced from the nucleic acid which is operably linked to the promoter as described herein, whereby the nucleic acid which is operably linked to the promoter can be the nucleic acid which is naturally linked to the promoter, i.e. the endogenous gene of which expression is controlled by the promoter.
[0063] The RNA accumulation, or levels of RNA, such as mRNA, can be measured either at a single time point or at multiple time points and as such the fold increase can be average fold increase or an extrapolated value derived from experimentally measured values. As it is a comparison of levels, any method that measures mRNA levels can be used. In a preferred aspect, the tissue or organs compared are root tissues with other tissues of the organism. In another preferred aspect, multiple tissues or organs are compared. A preferred multiple comparison is root tissue compared with 1 or 2 tissues or organs selected from the group consisting of leaves and stems or leaf tissue under stress. Another preferred multiple comparison is tissues or organs under stress condition compared with tissues or organs under control condition. As used herein, examples of plant organs arc fiber, leaf, root, etc. and example of tissues are leaf primordia, shoot apex, vascular tissue, etc.
[0064] The root-preferential, stress-inducible or stress-induced root-preferential expression capacity of the identified or generated fragment of the promoter can be conveniently tested by operably linking such DNA molecules to a nucleotide sequence encoding an easy scorable marker, e.g. a beta-glucuronidase gene, introducing such a recombinant gene into a plant and analyzing the expression pattern of the marker in roots as compared with the expression pattern of the marker in other parts of the plant. Other candidates for a marker (or a reporter gene) are chloramphenicol acetyl transferase (CAT) and proteins with fluorescent properties, such as green fluorescent protein (GFP) from Aequora victoria. To define a minimal promoter region, a DNA segment representing the promoter region is removed from the 5' region of the gene of interest and operably linked to the coding sequence of a marker (reporter) gene by recombinant DNA techniques well known to the art. The reporter gene is linked downstream of the promoter, so that transcripts initiating at the promoter proceed through the reporter gene. Reporter genes generally encode proteins, which are easily measured, including, but not limited to, chloramphenicol acetyl transferase (CAT), beta-glucuronidase (GUS), green fluorescent protein (GFP), beta-galactosidase (beta-GAL), and luciferase. The expression cassette containing the reporter gene under the control of the promoter can be introduced into an appropriate cell type by transfection techniques well known to the art. To assay for the reporter protein, cell lysates are prepared and appropriate assays, which are well known in the art, for the reporter protein are performed. The level of enzyme activity corresponds to the amount of enzyme that was made, which in turn reveals the level of expression and the root-specific functionality from the promoter or promoter fragment of interest. This level of expression can also be compared to other promoters to determine the relative strength of the promoter under study. Once activity and functionality is confirmed, additional mutational and/or deletion analyses may be employed to determine the minimal region and/or sequences required to initiate transcription. Thus, sequences can be deleted at the 5' end of the promoter region and/or at the 3' end of the promoter region, and nucleotide substitutions introduced. These constructs are then again introduced in cells and their activity and/or functionality determined.
[0065] The activity or strength of a promoter may be measured in terms of the amount of mRNA or protein accumulation it specifically produces, relative to the total amount of mRNA or protein. The promoter preferably expresses an operably linked nucleic acid sequence at a level greater than about 0.1%, about 0.2%, about 0.5%, more preferably greater than about 1% of the total mRNA. Alternatively, the activity or strength of a promoter may be expressed relative to a well-characterized promoter (for which transcriptional activity was previously assessed).
[0066] It will herein further be clear that equivalent root-preferential, stress-inducible and stress-induced root-preferential promoters can be isolated from other Gossypium plants carrying the D genome like for example Gossypium raimondii, Gossypium barbadense and Gossypium darwinii. To this end, orthologous promoter fragments may be isolated from other plants using SEQ ID NO: 7 or a functional fragment having at least 600 consecutive nucleotides thereof as a probe and identifying nucleotide sequences from these other plants which hybridize under the herein described hybridization conditions. By way of example, a promoter of the invention may be used to screen a genomic library of a crop or plant of interest to isolate corresponding promoter sequences according to techniques well known in the art. Thus, a promoter sequence of the invention may be used as a probe for hybridization with a genomic library under medium to high stringency conditions. As an alternative equivalent promoters can be isolated using the coding sequences of the genes controlled by the promoters of SEQ TD NO: 7 to screen a genomic library (e.g. by hybridization or in silico) of a crop of interest. When sufficient identity between the coding sequences is obtained (for example, higher than 95% identity), promoter regions can be isolated upstream of the orthologous genes.
[0067] Suitable to the invention are nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity which comprise a nucleotide sequence having at least 95%, or at least 98% or at least 99% sequence identity to the herein described promoters and promoter regions or functional fragments thereof and are also referred to as variants. The term "variant" with respect to the transcription regulating nucleotide sequence SEQ ID NO: 7 of the invention is intended to mean substantially similar sequences. Naturally occurring allelic variants such as these can be identified with the use of well-known molecular biology techniques, as, for example, with polymerase chain reaction (PCR) and hybridization techniques as herein outlined before. Variant nucleotide sequences also include synthetically derived nucleotide sequences, such as those generated, for example, by using site-directed mutagenesis of SEQ ID NO: 7. Generally, nucleotide sequence variants of the invention will have generally at least 80%, e.g. 81%, 82%, 83%, 84%, at least 85%, e.g. 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, to 98% and 99% nucleotide sequence identity to the native (wild type or endogenous) nucleotide sequence or a functional fragment thereof. Derivatives of the DNA molecules disclosed herein may include, but are not limited to, deletions of sequence, single or multiple point mutations, alterations at a particular restriction enzyme site, addition of functional elements, or other means of molecular modification which may enhance, or otherwise alter promoter expression. Techniques for obtaining such derivatives are well-known in the art (see, for example, J. F. Sambrook, D. W. Russell, and N. Irwin (2000) Molecular Cloning: A Laboratory Manual, 3.sup.rd edition Volumes 1, 2, and 3. Cold Spring Harbor Laboratory Press). For example, one of ordinary skill in the art may delimit the functional elements within the promoters disclosed herein and delete any non-essential elements. Functional elements may be modified or combined to increase the utility or expression of the sequences of the invention for any particular application. Those of skill in the art are familiar with the standard resource materials that describe specific conditions and procedures for the construction, manipulation, and isolation of macromolecules (e.g., DNA molecules, plasmids, etc.), as well as the generation of recombinant organisms and the screening and isolation of DNA molecules.
[0068] As used herein, the term "percent sequence identity" refers to the percentage of identical nucleotides between two segments of a window of optimally aligned DNA. Optimal alignment of sequences for aligning a comparison window are well-known to those skilled in the art and may be conducted by tools such as the local homology algorithm of Smith and Waterman (Waterman, M. S. Introduction to Computational Biology: Maps, sequences and genomes. Chapman & Hall. London (1995), the homology alignment algorithm of Needleman and Wunsch (J. MoI. Biol., 48:443-453 (1970), the search for similarity method of Pearson and Lipman (Proc. Natl. Acad. Sci., 85:2444 (1988), and preferably by computerized implementations of these algorithms such as GAP, BESTFIT, FASTA, and TFASTA available as part of the GCG (Registered Trade Mark), Wisconsin Package (Registered Trade Mark from Accelrys Inc., San Diego, Calif.). An "identity fraction" for aligned segments of a test sequence and a reference sequence is the number of identical components that are shared by the two aligned sequences divided by the total number of components in the reference sequence segment, i.e., the entire reference sequence or a smaller defined part of the reference sequence. Percent sequence identity is represented as the identity fraction times 100. The comparison of one or more DNA sequences may be to a full-length DNA sequence or a portion thereof, or to a longer DNA sequence.
[0069] A nucleic acid comprising a nucleotide sequence having at least about 95% sequence identity to SEQ ID NO: 7 can thus be a nucleic acid comprising a nucleotide sequence having at least about 95%, or at least about 98%, 99% or 100% sequence identity to SEQ ID NO: 7.
[0070] A "functional fragment" of a nucleic acid comprising root-preferential and stress-inducible promoter denotes a nucleic acid comprising a stretch of the nucleic acid sequences of SEQ ID NO: 7, or of the nucleic acid having at least 95% sequence identity to SEQ ID NO: 7 which is at least 400 bp and still exerts the desired function, i.e. which has root-preferential and stress inducible promoter activity. Assays for determining root-preferential promoter activity are provided herein. Preferably, the functional fragment of the root-preferential and stress-inducible promoter contains the conserved promoter motifs, such as, for example, conserved promoter motifs as described in DoOP (doop.abc.hu, databases of Orthologous Promoters, Barta E. et al. (2005) Nucleic Acids Research Vol. 33, D86-D90). A functional fragment may be a fragment of at least about 400 pb, at least about 500 bp, at least about 600 bp, at least about 700 bp, at least about 800 bp, at least about 900 bp from the transcription start site or at least about 600 bp, at least about 700 bp, at least about 800 bp, at least about 900 bp, at least about 1000 bp from the translation start site.
[0071] A nucleic acid comprising the nucleotide sequence of SEQ ID NO: 7 which further comprises insertion, deletion, substitution of at least 1 nucleotide up to 20 nucleotides, at least 1 nucleotide up to 15 nucleotides, at least 1 nucleotide up to 10 nucleotides, at least 1 nucleotide up to 5 nucleotides, at least 1 nucleotide up to 4 nucleotides, at least 1 nucleotide up to 3 nucleotides, or even at least 1 nucleotide up to 2 nucleotides may cover at least about 600 bp, at least about 700 bp, at least about 800 bp, at least about 900 bp from the transcription start site or at least about 800 bp, at least about 900 bp, at least about 1000 bp from the translation start site.
[0072] A number of putative response elements were identified on the promoter sequence disclosed herein. The search was limited to stress-responsive elements. Four ABA-responsive element-like (ABRE-like) motifs were identified at the nucleotide positions 186 to 193, 192 to 199, 223 to 230 and 235 to 242 in SEQ ID NO: 7.
[0073] Variants of the promoter described herein include those which comprise the identified ABRE-like motifs, but have otherwise been modified to delete nucleotide stretches within the sequence which are not needed for the promoter to be functional in root-preferential and stress-inducible manner. For example, any nucleotide stretch located between the motifs and/or between the transcriptional start and the first motif may be at least partially deleted to result in a shorter nucleotide sequence than the about 1 kb sequence of SEQ ID NO: 7.
[0074] Other nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity can be identified using methods known in the art. Such nucleotide sequence may be identified and isolated by hybridization under stringent conditions using as probes a nucleic acid comprising the nucleotide sequences of SEQ ID NO: 7 or part thereof. Other nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity may also be obtained by DNA amplification using oligonucleotides specific for the sequences according to the invention as primers, such as but not limited to oligonucleotides comprising or consisting of about 20 to about 50 consecutive nucleotides from any one of the nucleotide sequences of SEQ ID NO: 7 or its complement. Other nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity can be identified in silico using Basic Local Alignment Search Tool (BLAST) homology search with other nucleotide or amino acid sequences. Functionality of the identified nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity can be validated using the methods described herein. Other nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity may also be identified by identification of gene sequences orthologous to the gene sequences of the endogenous coding sequences of the genes controlled by the promoters of the invention, and isolating and testing the promoter sequences upstream of these orthologous homologous coding sequences.
[0075] The promoters according to the invention can further be used to create hybrid promoters, i.e. promoters containing (parts of) one or more of the promoters(s) of the current invention and (parts of) other promoter which can be newly identified or known in the art. Such hybrid promoters may have optimized tissue specificity or expression level.
[0076] As used herein, "plant-expressible promoter" means a region of DNA sequence that is essential for the initiation of transcription in a plant cell. This includes any promoter of plant origin, but also any promoter of non-plant origin which is capable of directing transcription in a plant cell, i. e. certain promoters of viral or bacterial origin such as the CaMV35S, the subterranean clover virus promoter No 4 or No 7 (WO9606932) or T-DNA gene promoters and the like.
[0077] In a further embodiment the plant expressible promoter is a constitutive promoter. In another embodiment the promoter is the Cauliflower Mosaic Virus CaMV35S promoter.
[0078] Other examples of constitutive promoters include the promoter from the actin gene (McElroy et al. (1990) Plant Cell 2: 163-171), the CaMV19S promoter (Nilsson et al. (1997) Physiol. Plant. 100: 456-462), the GOS2 promoter (de Pater et al. (1992) Plant. J. 2(6): 837-44), the promoter from ubiquitin gene (Christensen et al. (1992) Plant Mol. Biol. 18: 675-689), the promoter from rice cyclophilin gene (Buchholz et al. (1994) Plant. Mol. Biol. 25(5): 837-43), the promoter from the maize H3 histone gene (Lepetit et al. (1992) Mol. Gen. Genet. 231: 276-285) or the promoter from the actin 2 gene (An et al. (1996) Plant J. 10(1): 107-121).
Recombinant Genes
[0079] A further embodiment provides a recombinant gene comprising the nucleic acid having stress-induced root-preferential promoter activity described above operably linked to a heterologous nucleic acid sequence encoding an expression product of interest, and optionally a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in Gossypium plant cells. In a further embodiment, said expression product of interest an RNA capable of modulating the expression of a gene or is a protein.
[0080] The phrase "operably linked" refers to the functional spatial arrangement of two or more nucleic acid regions or nucleic acid sequences. For example, a promoter region may be positioned relative to a nucleic acid sequence such that transcription of a nucleic acid sequence is directed by the promoter region. Thus, a promoter region is "operably linked" to the nucleic acid sequence. "Functionally linked" is an equivalent term.
[0081] The term "expression product" refers to a product of transcription. Said expression product can be the transcribed RNA. It is understood that the RNA which is produced is a biologically active RNA. Said expression product can also be a peptide, a polypeptide, or a protein, when said biologically active RNA is an mRNA and said protein is produced by translation of said mRNA.
[0082] Alternatively, the heterologous nucleic acid, operably linked to the promoters of the invention, may also code for an RNA capable of modulating the expression of a gene. Said RNA capable of modulating the expression of a gene can be an RNA which reduces expression of a gene. Said RNA can reduce the expression of a gene for example through the mechanism of RNA-mediated gene silencing.
[0083] Said RNA capable of modulating the expression of a gene can be a silencing RNA downregulating expression of a target gene. As used herein, "silencing RNA" or "silencing RNA molecule" refers to any RNA molecule, which upon introduction into a plant cell, reduces the expression of a target gene. Such silencing RNA may e.g. be so-called "antisense RNA", whereby the RNA molecule comprises a sequence of at least 20 consecutive nucleotides having 95% sequence identity to the complement of the sequence of the target nucleic acid, preferably the coding sequence of the target gene. However, antisense RNA may also be directed to regulatory sequences of target genes, including the promoter sequences and transcription termination and polyadenylation signals. Silencing RNA further includes so-called "sense RNA" whereby the RNA molecule comprises a sequence of at least 20 consecutive nucleotides having 95% sequence identity to the sequence of the target nucleic acid. Other silencing RNA may be "unpolyadenylated RNA" comprising at least 20 consecutive nucleotides having 95% sequence identity to the complement of the sequence of the target nucleic acid, such as described in WO01/12824 or U.S. Pat. No. 6,423,885 (both documents herein incorporated by reference). Yet another type of silencing RNA is an RNA molecule as described in WO03/076619 (herein incorporated by reference) comprising at least 20 consecutive nucleotides having 95% sequence identity to the sequence of the target nucleic acid or the complement thereof, and further comprising a largely-double stranded region as described in WO03/076619 (including largely double stranded regions comprising a nuclear localization signal from a viroid of the Potato spindle tuber viroid-type or comprising CUG trinucleotide repeats). Silencing RNA may also be double stranded RNA comprising a sense and antisense strand as herein defined, wherein the sense and antisense strand are capable of base-pairing with each other to form a double stranded RNA region (preferably the said at least 20 consecutive nucleotides of the sense and antisense RNA are complementary to each other). The sense and antisense region may also be present within one RNA molecule such that a hairpin RNA (hpRNA) can be formed when the sense and antisense region form a double stranded RNA region. hpRNA is well-known within the art (see e.g WO99/53050, herein incorporated by reference). The hpRNA may be classified as long hpRNA, having long, sense and antisense regions which can be largely complementary, but need not be entirely complementary (typically larger than about 200 bp, ranging between 200-1000 bp). hpRNA can also be rather small ranging in size from about 30 to about 42 bp, but not much longer than 94 bp (see WO04/073390, herein incorporated by reference). Silencing RNA may also be artificial micro-RNA molecules as described e.g. in WO2005/052170, WO2005/047505 or US 2005/0144667, or ta-siRNAs as described in WO2006/074400 (all documents incorporated herein by reference). Said RNA capable of modulating the expression of a gene can also be an RNA ribozyme.
[0084] Said RNA capable of modulating the expression of a gene can modulate, preferably downregulate, the expression of other genes (i.e. target genes) comprised within the roots or even of genes present within a pathogen or pest that feeds upon the roots of the transgenic plant such as a virus, fungus, insect, nematode, bacteria. An example of pest control using gene silencing is described, for example, in WO2007/080127.
[0085] The nucleic acid sequence heterologous to the promoters according to the invention may generally be any nucleic acid sequence effecting increased, altered (e.g. in a different organ) or reduced level of transcription of a gene for which such expression modulation is desired. The nucleic acid sequence can for example encode a protein of interest. Exemplary genes for which an increased or reduced level of transcription may be desired in the roots are e.g. nucleic acids that can provide an agriculturally or industrially important feature in roots. Suitable heterologous nucleic acid sequences of interest include nucleic acids modulating expression of genes conferring resistance to root pests, like nematodes, and diseases, stress tolerance genes, genes encoding proteins involved in cell expansion and cell division genes involved in nutrient uptake, genes involved in metabolism or nutrient assimilation, genes encoding transport proteins, such as nitrate transporters including NRT transport proteins, ammonium transporters including AMT proteins, and the like.
[0086] Examplary genes for which an increased or reduced level of transcription may be desired upon stress are e.g. genes encoding protection factors of macromolecules (LEA proteins, chaperones), key enzymes for osmolyte biosynthesis (proline, sugars), detoxification enzymes (e.g. Super Oxide Dismutase), water channels or transporters, transcription factors (for example DREB2, AREB, MYC, bZIP, NAC) or genes involved in hormone signaling or biosynthesis (examples of relevant hormones are ABA, brassinosteroid, cytokinin, ethylene). Genes for nematode resistance are also of relevance (e.g., WO 1995/020669, WO 2001/051627, WO 2008/139334, WO 2008/095972, WO 2006/085966, WO 2003/033651, WO 1999/060141, WO 1998/012335, WO 1996/030517, WO 1993/018170, WO2008/095886, WO2008/095887, WO2008/095888, WO2008/095889, WO2008/095910, WO2008/095911, WO2008/095916, WO2008/095919, WO2008/095969, WO2008/095970, WO2008/095972, WO2008/110522, WO2008/139334, WO2008/152008, WO2010/077858, WO 2010/091230, WO 2010/102172, WO 2010/106163, WO2011/082217, WO2011/003783, WO 2011/014749, WO 2007/147029, WO 2014/003769, WO 2010/077858.
[0087] A "transcription termination and polyadenylation region" as used herein is a sequence that controls the cleavage of the nascent RNA, whereafter a poly(A) tail is added at the resulting RNA 3' end, functional in plant cells. Transcription termination and polyadenylation signals functional in plant cells include, but are not limited to, 3'nos, 3'35S, 3'his and 3'g7.
[0088] The term "protein" interchangeably used with the term "polypeptide" as used herein describes a group of molecules consisting of more than 30 amino acids, whereas the term "peptide" describes molecules consisting of up to 30 amino acids. Proteins and peptides may further form dimers, trimers and higher oligomers, i.e. consisting of more than one (poly)peptide molecule. Protein or peptide molecules forming such dimers, trimers etc. may be identical or non-identical. The corresponding higher order structures arc, consequently, termed homo- or heterodimers, homo- or heterotrimers etc. The terms "protein" and "peptide" also refer to naturally modified proteins or peptides wherein the modification is effected e.g. by glycosylation, acetylation, phosphorylation and the like. Such modifications are well known in the art.
[0089] The term "heterologous" refers to the relationship between two or more nucleic acid or protein sequences that are derived from different sources. For example, a promoter is heterologous with respect to an operably linked DNA region, such as a coding sequence if such a combination is not normally found in nature. In addition, a particular sequence may be "heterologous" with respect to a cell or organism into which it is inserted (i.e. does not naturally occur in that particular cell or organism). For example, the recombinant gene disclosed herein is a heterologous nucleic acid.
[0090] The term "recombinant gene" refers to any artificial gene that contains: a) DNA sequences, including regulatory and coding sequences that are not found together in nature, or b) sequences encoding parts of proteins not naturally adjoined, or c) parts of promoters that are not naturally adjoined. Accordingly, a recombinant gene may comprise regulatory sequences and coding sequences that are derived from different sources, or comprise regulatory sequences, and coding sequences derived from the same source, but arranged in a manner different from that found in nature.
[0091] In another aspect, the invention provides a recombinant gene comprising (a) a plant expressible promoter selected from the group consisting of i. root-preferential promoter, ii. stress-inducible promoter and iii. Stress-induced root-preferential promoter, (b) a nucleic acid sequence encoding an Annexin protein (c) and optionally, a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants.
[0092] Any of the promoters and heterologous nucleic acid sequences described above may be provided in a recombinant vector. A recombinant vector typically comprises, in a 5' to 3' orientation: a promoter to direct the transcription of a nucleic acid sequence and a nucleic acid sequence. The recombinant vector may further comprise a 3' transcriptional terminator, a 3' polyadenylation signal, other untranslated nucleic acid sequences, transit and targeting nucleic acid sequences, selectable markers, enhancers, and operators, as desired. The wording "5' UTR" refers to the untranslated region of DNA upstream, or 5' of the coding region of a gene and "3' UTR" refers to the untranslated region of DNA downstream, or 3' of the coding region of a gene. Means for preparing recombinant vectors are well known in the art. Methods for making recombinant vectors particularly suited to plant transformation are described in U.S. Pat. Nos. 4,971,908, 4,940,835, 4,769,061 and 4,757,011. Typical vectors useful for expression of nucleic acids in higher plants are well known in the art and include vectors derived from the tumor-inducing (Ti) plasmid of Agrobacterium tumefaciens. One or more additional promoters may also be provided in the recombinant vector. These promoters may be operably linked, for example, without limitation, to any of the nucleic acid sequences described above. Alternatively, the promoters may be operably linked to other nucleic acid sequences, such as those encoding transit peptides, selectable marker proteins, or antisense sequences. These additional promoters may be selected on the basis of the cell type into which the vector will be inserted. Also, promoters which function in bacteria, yeast, and plants are all well taught in the art. The additional promoters may also be selected on the basis of their regulatory features. Examples of such features include enhancement of transcriptional activity, inducibility, tissue specificity, and developmental stage-specificity.
[0093] The recombinant vector may also contain one or more additional nucleic acid sequences. These additional nucleic acid sequences may generally be any sequences suitable for use in a recombinant vector. Such nucleic acid sequences include, without limitation, any of the nucleic acid sequences, and modified forms thereof, described above. The additional structural nucleic acid sequences may also be operably linked to any of the above described promoters. The one or more structural nucleic acid sequences may each be operably linked to separate promoters. Alternatively, the structural nucleic acid sequences may be operably linked to a single promoter (i.e. a single operon).
Annexins
[0094] Suitable for the invention are nucleic acids, encoding an Annexin protein, which comprise a nucleotide sequence having at least 40%, at least 50%, or at least 60%, or at least 70%, or at least 80%, or at least 85%, or at least 90%, or at least 95%, or at least 98% sequence identity to the herein described gene and arc also referred to as variants. The term "variant" with respect to the nucleotide sequences SEQ ID NO: 12 and SEQ ID NO: 14 of the invention is intended to mean substantially similar sequences. Naturally occurring allelic variants such as these can be identified with the use of well-known molecular biology techniques, as, for example, with polymerase chain reaction (PCR) and hybridization techniques as herein outlined before. Variant nucleotide sequences also include synthetically derived nucleotide sequences, such as those generated, for example, by using site-directed mutagenesis of any one of SEQ ID NOs 12 or 14. Generally, nucleotide sequence variants of the invention will have at least 40%, 50%, 60%, to 70%, e.g., preferably 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, to 79%, generally at least 80%, e.g., 81% to 84%, at least 85%, e.g., 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, to 98% and 99% nucleotide sequence identity to the native (wild type or endogenous) nucleotide sequence. Derivatives of the DNA molecules disclosed herein may include, but are not limited to, deletions of sequence, single or multiple point mutations, alterations at a particular restriction enzyme site, addition of functional elements, or other means of molecular modification. Techniques for obtaining such derivatives are well-known in the art (see, for example, J. F. Sambrook, D. W. Russell, and N. Irwin (2000) Molecular Cloning: A Laboratory Manual, 3.sup.rd edition Volumes 1, 2, and 3. Cold Spring Harbor Laboratory Press). Those of skill in the art are familiar with the standard resource materials that describe specific conditions and procedures for the construction, manipulation, and isolation of macromolecules (e.g., DNA molecules, plasmids, etc.), as well as the generation of recombinant organisms and the screening and isolation of DNA molecules.
[0095] The term "percent sequence identity" is used in this section as defined above.
[0096] A nucleic acid comprising a nucleotide sequence having at least 80% sequence identity to SEQ ID NO: 12 or SEQ ID NO: 14 can thus be a nucleic acid comprising a nucleotide sequence having at least 80%, or at least 85%, or at least 90%, or at least 95%, or at least 98%, or 100% sequence identity to SEQ ID NO: 12 or SEQ ID NO: 14 respectively.
[0097] In a preferred embodiment, the nucleic acid sequence, encoding an Annexin protein, comprises (a) a nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; (b) a nucleotide sequence at least 80% identical to SEQ ID NO: 12 or SEQ ID NO: 14 (c) a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14, (d) a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13 or SEQ ID NO: 15 (e) a nucleotide sequence encoding an amino acid sequence having 80% identity with SEQ ID NO: 13 or SEQ ID NO: 15, (f) a nucleotide sequence encoding a protein comprising four or more annexin-repeat domains.
[0098] SEQ ID NO: 12 represents the nucleotide sequence of the AnnBj1 gene, SEQ ID NO: 14 represents the nucleotide sequence of the GhAnn1 gene, SEQ ID NO: 13 represents the amino-acid sequence of the AnnBj1 protein and SEQ ID NO: 15 represents the amino-acid sequence of the GhAnn1 protein.
[0099] Annexins are defined by their highly conserved fold consisting of four or more repeats of a so-called annexin-repeat domain signature sequence. The annexin-repeat domains are known in the Pfam database as PF00191, in the Interpro database as IPR001464, and in the smart database as SM00335. Each repeat consists of a five-helix bundle. An endonexin fold can be identified at the start of each repeat. Calcium binding occurs in type II binding sites established in the endonexin fold regions of the first and fourth repeats. Further particular amino-acid are essential to the Annexin function: a tryptophan and a histidine in the first endonexin fold, a tryptophan at the end of the first annexin repeat domain and an isoleucine-arginine-isoleucine in the third endonexin fold (FIG. 2).
[0100] Though the invention preferably uses nucleic acid encoding the AnnBj1 from Brassica juncea, nucleic acids encoding other Annexins can be used. Examples of such proteins are: Q67EX8, EOZQA2, D2JYA7, A0A078HJR9, X2JGY9, A0A078CEH0, D9J167, M4F009, D7KLX8, Q9SYTO, D2JYA6, ROGQT5, A0A078HYS1, M4F6Q4, A0A087G1V1, A0A078DROO, V4LGL7, A0A061FNB4, A0A067GQH6, V4T729, M4N630, 082090, S5GFP3, P93157, A9PA39, G3E7M9, M4MZO2, M5XRZ4, A9PH68, A0A059BS83, A0A067KYU4, M4FEQ6, K9JGF9, I3SZS2, A0A067LQJ4, B9RJJ1, 022341, S5G971, A0A059B7W7, A0A061DJJ7, A5BTZ8, G7KB73, A0A059B7M2, A0A059B7C5, Q9XEN8, A0A068TXQ7, K4BSR4, A0A059B8A3, A0A059B8Z0, Q42657, M5W098, Q9SB88, Q9ZRU7, P93158, A0A059BRT1, 024131, B9HFG8, MOZNV9, B9H529, C6TFT8, I3RZY7, M4MX74, Q69DC2, LOAU94, LOASQ7, V7B5 V0, Q9M3H3, O81536, O24132, M4MX50, A0A068TYU6, ROG7 S3, 081535, A0A022R8D3, A0A067ERS2, A0A078FJE4, M4E6E2, I3Y171, Q2XTE7, V7CRX1, A5B479, Q9XEE2, Q42922, A9X4R2, D7MT72, A0A072TF84, V4MJ15, A0A078H8V3, W9QYY2, M4ESWO, A0A087GE86, X2JPM6, Q9LX07, V4VZP8, B3TLY9, Q4ABP7, A0A078BZL8, A9X4R1, V4KSN9, B7U9R9.
[0101] Furthermore, it is clear that variants of Annexin proteins, wherein one or more amino acid residues have been deleted, substituted or inserted, can also be used to the same effect in the methods according to the invention, provided that the Annexin repeat domains are not affected by the deletion, substitution or insertion of amino-acid. These variant Annexin proteins may have about 95% sequence identity to any one of the herein mentioned Annex in proteins.
[0102] Examples of substitutions are the conservative substitutions, i.e. substitutions of one amino-acid by another having similar physiochemical properties. These substitutions arc known not to affect the structure of a protein. Such substitutions are achieved by replacing one aminoacid by another aminoacid belonging to the same group as follows: Group 1: Cysteine (C);
Group 2: Phenylalanine (F), Tryptophan (W) and Tyrosine (Y);
Group 3: Histidine (H), Lysing K) and Arginine (R);
[0103] Group 4: Aspartic acid (D), Glutamic acid (E), Asparagine (N) and Glutamine (Q);
Group 5: Isoleucine (I), Leucine (L), Methionine (M) and Valine (V);
Group 6: Alanine (A), Glycine (G), Proline (P), Serine (S) and Threonine (T).
Host Cells and Plants
[0104] Yet other embodiments provide a host cell, such as an E. coli cell, an Agrobacterium cell, a yeast cell, or a plant cell, comprising the isolated nucleic acid according to the invention, or the recombinant genes according to the invention.
[0105] Other nucleic acid sequences may also be introduced into the host cell along with the promoter and structural nucleic acid sequence, e. g. also in connection with the vector of the invention. These other sequences may include 3' transcriptional terminators, 3' polyadenylation signals, other untranslated nucleic acid sequences, transit or targeting sequences, selectable markers, enhancers, and operators. Preferred nucleic acid sequences of the present invention, including recombinant vectors, structural nucleic acid sequences, promoters, and other regulatory elements, are described above.
[0106] In further embodiments, a plant is provided comprising any of the recombinant genes according to the invention. A further embodiment provides plant parts and seeds obtainable from the plant according to the invention. These plant parts and seeds comprise the recombinant genes described above. In another embodiment, the plants, plant parts or seeds according to the invention are cotton, soybean or wheat plants, plant parts or seeds.
[0107] The plant cell or plant comprising any of the recombinant gene according to the invention can be a plant cell or a plant comprising a recombinant gene of which either the promoter, or the heterologous nucleic acid sequence operably linked to said promoter, are heterologous with respect to the plant cell. Such plant cells or plants may be transgenic plant in which the recombinant gene is introduced via transformation. Alternatively, the plant cell of plant may comprise the promoter according to the invention derived from the same species operably linked to a nucleic acid which is also derived from the same species, i.e. neither the promoter nor the operably linked nucleic acid is heterologous with respect to the plant cell, but the promoter is operably linked to a nucleic acid to which it is not linked in nature. A recombinant gene can be introduced in the plant or plant cell via transformation, such that both the promoter and the operably linked nucleotide are at a position in the genome in which they do not occur naturally. Alternatively, the promoter according to the invention can be integrated in a targeted manner in the genome of the plant or plant cell upstream of an endogenous nucleic acid encoding an expression product of interest, i.e. to modulate the expression pattern of an endogenous gene. The promoter that is integrated in a targeted manner upstream of an endogenous nucleic acid can be integrated in cells of a plant species from which it is originally derived, or in cells of a heterologous plant species. Alternatively, a heterologous nucleic acid can be integrated in a targeted manner in the genome of the plant or plant cell downstream of the promoter according to the invention, such that said heterologous nucleic acid is expressed root-preferentially and is stress-inducible. Said heterologous nucleic acid is a nucleic acid which is heterologous with respect to the promoter, i.e. the combination of the promoter with said heterologous nucleic acid is not normally found in nature. Said heterologous nucleic acid may be a nucleic acid which is heterologous to said plant species in which it is inserted, but it may also naturally occur in said plant species at a different location in the plant genome. Said promoter or said heterologous nucleic acid can be integrated in a targeted manner in the plant genome via targeted sequence insertion, using, for example, the methods as described in WO2005/049842.
[0108] "Plants" encompasses "monocotyledonous plants" and "dicotyledonous plants".
[0109] "Monocotyledonous plants", also known as "monocot plants" or "monocots" are well known in the art and are plants of which the seed typically has one cotyledon. Examples of monocotyledons plants are grasses, such as meadow grass (blue grass, Poa), forage grass such as festuca, lolium, temperate grass, such as Agrostis, and cereals, e.g., wheat, oats, rye, barley, rice, sorghum, and maize (corn).
[0110] "Dicotyledonous plants", also known as "dicot plants" or "dicots" are well known in the art and are plants of which the seed typically has two cotyledons. Examples of families of dicotyledonous plants are Brassicaceae, Solanaceae, Fabaceae, Malvaceae.
[0111] "Malvaceae" as used herein refers to plants belonging to the family of Malvaceae plants, also called mallows family. Examples of Malvaceae are, but are not limited to, Gossypium species, such as Gossypium hirsutum, Gossypium barbadense, Gossypium arboreum and Gossypium herbaceum or progeny from crosses of such species with other species or crosses between such species.
[0112] "Cotton" or "cotton plant" as used herein can be any variety useful for growing cotton. The most commonly used cotton varieties are Gossypium barbadense, G. hirsutum, G. arboreum and G. herbaceum. Further varieties include G. africanum and G. raimondii. Also included are progeny from crosses of any of the above species with other species or crosses between such species.
[0113] The following is a non-limiting list of cotton genotypes which can be used for transformation: Coker 312, Coker310, Coker 5Acala SJ-5, GSC25110, Siokra 1-3, T25, GSA75, Acala SJ2, Acala SJ4, Acala SJ5, Acala SJ-C1, Acala B1644, Acala B1654-26, Acala B1654-43, Acala B3991, Acala GC356, Acala GC510, Acala GAM1, Acala C1, Acala Royale, Acala Maxxa, Acala Prema, Acala B638, Acala B1810, Acala B2724, Acala B4894, Acala B5002, Acala 1517-88, Acala 1517-91, Acala 1517-95, non Acala "picker" Siokra, "stripper" variety FC2017, Coker 315, STONEVILLE 506, STONEVILLE 825, STONEVILLE 324, STONEVILLE 453, STONEVILLE 474, STONEVILLE KC 311, STONEVILLE LA 887, STONEVILLE 4145, STONEVILLE 4288, STONEVILLE 4498, STONEVILLE 4554, STONEVILLE 4747, STONEVILLE 4946, STONEVILLE 5032, STONEVILLE 5115, STONEVILLE 5289, STONEVILLE 5445, STONEVILLE 5458, STONEVILLE 6182, STONEVILLE 6448, Daytona, Cobalt, DP20, DP20B, DP NUCOTN 33B, DP NUCOTN 35B, DP41, DP50, DP51, DP61, DP90, DP77, DP161, DP340, DP357, DP358, DP360, DP744, DP0912, DP0920, DP0924, DP0935, DP0949, DP0920, DP1028, DP1034, DP1044, DP1050, DP1133, DP1137, DP1212, DP1219, DP1252, DP1311, DP1321, DP1359, DP1410, DP1441, DP1454, DP5409, DP5415, DP5461, DP5690, DP5816, MON/DP 09R303, MON/DP 09R549, MON/DP 09R550, MON/DP 09R555, MON/DP 09R573, MON/DP 09R605, MON/DP 09R615, MON/DP 09R619, MON/DP 09R621, MON/DP 09R623, MON/DP 09R627, MON/DP 09R643, MON/DP 09R796, MON/DP 09R999, MON/DP 10R013, MON/DP 10R020, MON/DP 10R030, MON/DP 10R051, MON/DP 10R052, MON/DP 11R112, MON/DP 11R124, MON/DP 11R130, MON/DP 11R136, MON/DP 11R154, MON/DP 11R158, MON/DP 11R159, MON/DP 12R224, MON/DP 12R242, MON/DP 12R244, MON/DP 12R249, MON/DP 12R251, 12R254, MON/DP 13R310, MON/DP 13R348, MON/DP 13R352, MON/DP 14R1455, MON/DP 14R1456, DP Suregrow, Suregrow 125, Suregrow 248, Suregrow 404, Suregrow 501, Surcgrow 1001, DES119, McN235, HBX87, HBX191, HBX107, FC 3027, CHEMBRED A1, CHEMBRED A2, CHEMBRED A3, CHEMBRED A4, CHEMBRED B1, CHEMBRED B2, CHEMBRED B3, CHEMBRED C1, CHEMBRED C2, CHEMBRED C3, CHEMBRED C4, CHEMBRED CB407, PAYMASTER 145, HS26, HS46, Hyperformer 44, Hyperformer HS46, SICALA, PIMA S6 ORO BLANCO PIMA, PIMA S7, HA01, HA02, HA03, HA04, HA05, HA195, HA211, HA195, HA222, White PIMA, PHY72, PHY222, PHY333, PHY339, PHY367, PHY375, PHY417, PHY427, PHY495, PHY499, PHY565, PHY575, PHY725, PHY755, PHY800, PHY802, PHY804, PHY805, PHY811, PHY830, FM5013, FM5015, FM5017, FM989, FM832, FM966, FM958, FM989, FM958, FM832, FM991, FM819, FM800, FM960, FM966, FM981, FM1320, FM1740, FM1773, FM1830, FM1845, FM1880, FM1900, FM1944, FM2007, FM2011, FM2322, FM2324, FM2334, FM2484, FM2989, FM5035, FM5044, FM5045, FM5013, FM5015, FM5017, FM5024, FM8270, FM9058, FM9160, FM9170, FM9180, FM9250 and plants with genotypes derived thereof.
[0114] "Fabaceae" as used herein refers to the plant commonly known as the legume, pea, or bean family plants. Examples of Fabaceac arc, but arc not limited to, Glycine max (soybean), Phaseolus (beans), Pisum sativum (pea), Cicer arietinum (chickpeas), Medicago sativa (alfalfa), Arachis hypogaca (peanut), Lathyrus odoratus (sweet pea), Ceratonia siliqua (carob), and Glycyrrhiza glabra (liquorice).
[0115] "Plant parts" as used herein are parts of the plant, which can be cells, tissues or organs, such as seeds, severed parts such as roots, leaves, flowers, pollen, fibers etc.
[0116] The plants according to the invention may additionally contain an endogenous or a transgene, which confers herbicide resistance, such as the bar or pat gene, which confer resistance to glufosinate ammonium (Liberty.RTM., Basta.RTM. or Ignite.RTM.) [EP 0 242 236 and EP 0 242 246 incorporated by reference]; or any modified EPSPS gene, such as the 2mEPSPS gene from maize [EPO 508 909 and EP 0 507 698 incorporated by reference], or glyphosate acetyltransferase, or glyphosate oxidoreductase, which confer resistance to glyphosate (RoundupReady.RTM.), or bromoxynitril nitrilase to confer bromoxynitril tolerance, or any modified AHAS gene, which confers tolerance to sulfonylureas, imidazolinones, sulfonylaminocarbonyltriazolinones, triazolopyrimidines or pyrimidyl(oxy/thio)benzoates, such as oilseed rape imidazolinone-tolerant mutants PM1 and PM2, currently marketed as Clearfield.RTM. canola. Further, the plants according to the invention may additionally contain an endogenous or a transgene which confers increased oil content or improved oil composition, such as a 12:0 ACP thioesteraseincrease to obtain high laureate, which confers pollination control, such as such as barnase under control of an anther-specific promoter to obtain male sterility, or barstar under control of an anther-specific promoter to confer restoration of male sterility, or such as the Ogura cytoplasmic male sterility and nuclear restorer of fertility.
[0117] The plants or seeds of the plants according to the invention may be further treated with a chemical compound, such as a chemical compound selected from the following lists: Herbicides: Diuron, Fluometuron, MSMA, Oxyfluorfen, Prometryn, Trifluralin, Carfentrazone, Clethodim, Fluazifop-butyl, Glyphosate, Norflurazon, Pendimethalin, Pyrithiobac-sodium, Trifloxysulfuron, Tepraloxydim, Glufosinate, Flumioxazin, Thidiazuron; cotton insecticides such as Acephate, Aldicarb, Chlorpyrifos, Cypermethrin, Deltamethrin, Abamectin, Acetamiprid, Emamectin Benzoate, Imidacloprid, Indoxacarb, Lambda-Cyhalothrin, Spinosad, Thiodicarb, Gamma-Cyhalothrin, Spiromesifen, Pyridalyl, Flonicamid, Flubendiamide, Triflumuron, Rynaxypyr, Beta-Cyfluthrin, Spirotetramat, Clothianidin, Thiamethoxam, Thiacloprid, Dinctofuran, Flubendiamide, Cyazypyr, Spinosad, Sp inotoram, gamma Cyhalothrin, 4-[[(6-Chlorpyridin-3-yemethyl] (2,2-difluorethyl)amino]furan-2(5H)-on, Thiodicarb, Avermcctin, Flonicamid, Pyridalyl, Spiromesifen, Sulfoxaflor; and cotton fungicides such as Azoxystrobin, Bixafen, Boscalid, Carbendazim, Chlorothalonil, Copper, Cyproconazole, Difenoconazole, Dimoxystrobin, Epoxiconazole, Fenamidone, Fluazinam, Fluopyram, Fluoxastrobin, Fluxapyroxad, Iprodione, Isopyrazam, Isotianil, Mancozeb, Maneb, Metominostrobin, Penthiopyrad, Picoxystrobin, Propineb, Prothioconazole, Pyraclostrobin, Quintozene, Tebuconazole, Tetraconazole, Thiophanate-methyl, Trifloxystrobin, Clopyralid, Diclofop, Ethametsulfuron, Fluazifop, Metazachlor, Quinmerac, Quizalofop. Fungicides/PGRs: Azoxystrobin, N-[9-(dichloromethylene)-1,2,3,4-tetrahydro-1,4-methanonaphthalen-5-yl]-3- -(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (B enzovindiflupyr, B enzodiflupyr), Bixafen, B oscalid, Carbendazim, Carboxin, Chlormequat-chloride, Coniothryrium minitans, Cyproconazole, Cyprodinil, Difenoconazole, Dimethomorph, Dimoxystrobin, Epoxiconazole, Famoxadone, Fluazinam, Fludioxonil, Fluopicolide, Fluopyram, Fluoxastrobin, Fluquinconazole, Flusilazole, Fluthianil, Flutriafol, Fluxapyroxad, Iprodione, Isopyrazam, Mefenoxam, Mepiquat-chloride, Metalaxyl, Metconazole, Metominostrobin, Paclobutrazole, Penflufen, Penthiopyrad, Picoxystrobin, Prochloraz, Prothioconazole, Pyraclostrobin, Sedaxane, Tebuconazole, Tetraconazole, Thiophanate-methyl, Thiram, Triadimenol, Trifloxystrobin, Bacillus firmus, Bacillus firmus strain 1-1582, Bacillus subtilis, Bacillus subtilis strain GB03, Bacillus subtilis strain QST 713, Bacillus pumulis, Bacillus. pumulis strain GB34. Insecticides: Acetamiprid, Aldicarb, Azadirachtin, Carbofuran, Chlorantraniliprole (Rynaxypyr), Clothianidin, Cyantraniliprole (Cyazypyr), (beta-)Cyfluthrin, gamma-Cyhalothrin, lambda-Cyhalothrin, Cypermethrin, Deltamethrin, Dimethoate, Dinetofuran, Ethiprole, Flonicamid, Flubendiamide, Fluensulfone, Fluopyram, Flupyradifurone, tau-Fluvalinate, Imicyafos, Imidacloprid, Metaflumizone, Methiocarb, Pymetrozine, Pyrifluquinazon, Spinetoram, Spinosad, Spirotetramate, Sulfoxaflor, Thiacloprid, Thiamethoxam, 1-(3-chloropyridin-2-yl)-N-[4-cyano-2-methyl-6-(methylcarbamoyephenyl]-3-- {[5-(trifluoromethyl)-2H-tetrazol-2-yl] methyl}-1H-pyrazole-5-carb oxamide, 1-(3-chloropyridin-2-yl)-N-[4-cyano-2-methyl-6-(methylcarbamoyl)- phenyl]-3-{[5-(trifluoromethyl)-1H-tetrazol-1-yl]methyl}-1H-pyrazole-5-car- boxamide, 1-{2-fluoro-4-methyl-5-[(2,2,2-trifluorethyl)sulfinyl] phenyl}-3-(trifluoromethyl)-1H-1,2,4-triazol-5-amine, (1E)-N-[(6-chloropyridin-3-yl)methyl]-N'-cyano-N-(2,2-difluoroethyeethani- midamide, Bacillus firmus, Bacillus firmus strain 1-1582, Bacillus subtilis, Bacillus subtilis strain GB03, Bacillus subtilis strain QST 713, Metarhizium anisopliae F52.
[0118] Whenever reference to a "plant" or "plants" according to the invention is made, it is understood that also plant parts (cells, tissues or organs, seed pods, seeds, severed parts such as roots, leaves, flowers, pollen, etc.), progeny of the plants which retain the distinguishing characteristics of the parents, such as seed obtained by selfing or crossing, e.g. hybrid seed (obtained by crossing two inbred parental lines), hybrid plants and plant parts derived there from are encompassed herein, unless otherwise indicated.
[0119] In some embodiments, the plant cells of the invention as well as plant cells generated according to the methods of the invention, may be non-propagating cells.
[0120] The obtained plants according to the invention can be used in a conventional breeding scheme to produce more plants with the same characteristics or to introduce the same characteristic in other varieties of the same or related plant species, or in hybrid plants. The obtained plants can further be used for creating propagating material. Plants according to the invention can further be used to produce gametes, seeds (including crushed seeds and seed cakes), seed oil, fibers, yarn, embryos, either zygotic or somatic, progeny or hybrids of plants obtained by methods of the invention. Seeds obtained from the plants according to the invention are also encompassed by the invention.
[0121] "Creating propagating material", as used herein, relates to any means know in the art to produce further plants, plant parts or seeds and includes inter alia vegetative reproduction methods (e.g. air or ground layering, division, (bud) grafting, micropropagation, stolons or runners, storage organs such as bulbs, corms, tubers and rhizomes, striking or cutting, twin-scaling), sexual reproduction (crossing with another plant) and asexual reproduction (e.g. apomixis, somatic hybridization).
Methods and Uses
[0122] Yet other embodiments provide a method of producing a transgenic plant comprising the steps of (a) introducing or providing any of the recombinant genes according to the invention to a plant cell to create transgenic cells; and (b) regenerating transgenic plants from said transgenic cell.
[0123] "Introducing" in connection with the present application relates to the placing of genetic information in a plant cell or plant by artificial means. This can be effected by any method known in the art for introducing RNA or DNA into plant cells, protoplasts, calli, roots, tubers, seeds, stems, leaves, seedlings, embryos, pollen and microspores, other plant tissues, or whole plants. "Introducing" also comprises stably integrating into the plant's genome. Introducing the recombinant gene can be performed by transformation or by crossing with a plant obtained by transformation or its descendant (also referred to as "introgression").
[0124] The term "providing" may refer to introduction of an exogenous DNA molecule to a plant cell by transformation, optionally followed by regeneration of a plant from the transformed plant cell. The term may also refer to introduction of the recombinant DNA molecule by crossing of a transgenic plant comprising the recombinant DNA molecule with another plant and selecting progeny plants which have inherited the recombinant DNA molecule or transgene. Yet another alternative meaning of providing refers to introduction of the recombinant DNA molecule by techniques such as protoplast fusion, optionally followed by regeneration of a plant from the fused protoplasts.
[0125] The recombinant gene may be introduced into a plant cell by methods well-known in the art.
[0126] The term "transformation" herein refers to the introduction (or transfer) of nucleic acid into a recipient host such as a plant or any plant parts or tissues including plant cells, protoplasts, calli, roots, tubers, seeds, stems, leaves, fibers, seedlings, embryos and pollen. Plants containing the transformed nucleic acid sequence are referred to as "transgenic plants". Transformed, transgenic and recombinant refer to a host organism such as a plant into which a heterologous nucleic acid molecule (e.g. an expression cassette or a recombinant vector) has been introduced. The nucleic acid can be stably integrated into the genome of the plant.
[0127] As used herein, the phrase "transgenic plant" refers to a plant having a nucleic acid stably integrated into a genome of the plant, for example, the nuclear or plastid genomes. In other words, plants containing transformed nucleic acid sequence are referred to as "transgenic plants" and includes plants directly obtained from transformation and their descendants (Tx generations). Transgenic and recombinant refer to a host organism such as a plant into which a heterologous nucleic acid molecule (e.g. the promoter, the recombinant gene or the vector as described herein) has been introduced. The nucleic acid can be stably integrated into the genome of the plant.
[0128] It will be clear that the methods of transformation used arc of minor relevance to the current invention. Transformation of plants is now a routine technique. Advantageously, any of several transformation methods may be used to introduce the nucleic acid/gene of interest into a suitable ancestor cell. Transformation methods include the use of liposomes, electroporation, chemicals that increase free DNA uptake, injection of the DNA directly into the plant, particle gun bombardment, transformation using viruses or pollen and microprojection. Methods may be selected from the calcium/polyethylene glycol method for protoplasts (Krens et al. (1982) Nature 296: 72-74; Negrutiu et al. (1987) Plant. Mol. Biol. 8: 363-373); electroporation of protoplasts (Shillito et al. (1985) Bio/Technol. 3: 1099-1102); microinjection into plant material (Crossway et al. (1986) Mol. Gen. Genet. 202: 179-185); DNA or RNA-coated particle bombardment (Klein et al. (1987) Nature 327: 70) infection with (non-integrative) viruses and the like.
[0129] Methods to transform cotton plants are also well known in the art. Agrobacterium-mediated transformation of cotton has been described e.g. in US patent 5.004.863 or in US patent 6.483.013 and cotton transformation by particle bombardment is reported e.g. in WO 92/15675. Other suitable cotton transformation methods are disclosed e.g. in WO 00071733 and U.S. Pat. No. 5,159,135, which disclosures are incorporated by reference herein as if fully set forth. Methods to transform soybean arc described e.g. in WO2014/150449.
[0130] Different transformation systems could be established for various cereals: the electroporation of tissue, the transformation of protoplasts and the DNA transfer by particle bombardment in regenerable tissue and cells (for an overview see Jane, Euphytica 85 (1995), 35-44). The transformation of wheat has been described several times in literature (for an overview see Maheshwari, Critical Reviews in Plant Science 14 (2) (1995), 149-178, Nehra et al., Plant J. 5 (1994), 285-297). Yuji Ishida et al. 2015, Methods in Molecular Biology, 1223: 189-198 describes a recent method to obtain transgenic wheat plants.
[0131] The recombinant DNA molecules according to the invention may be introduced into plants in a stable manner or in a transient manner using methods well known in the art. The recombinant genes may be introduced into plants, or may be generated inside the plant cell as described e.g. in EP 1339859.
[0132] Further provided are methods of effecting root-preferential, stress-inducible and stress-induced root-preferential expression of a nucleic acid comprising introducing a recombinant gene according to the invention that comprise a promoter having root-preferential, stress-inducible or stress-induced root-preferential promoter activity into the genome of a plant, or providing the plant according to the invention. Also provided is a method for altering biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, nematode resistance or yield of a plant, comprising introducing the recombinant gene according to the invention into the genome of a plant, or providing the plant according to the invention. In another embodiment, said plant is a cotton, a soybean or a wheat plant.
[0133] Also provided is the use of the isolated nucleic acid according to the invention to regulate expression of an operably linked nucleic acid in a plant, and the use of the isolated nucleic acid according to the invention, or the recombinant gene comprising the nucleic acid having root-preferential, stress-inducible and stress-induced root-preferential promoter activity to alter biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, or yield in a plant. In a further embodiment, said plant is a cotton, a soybean or a wheat plant. Also provided is the use of the isolated nucleic acid according to the invention to identify other nucleic acids comprising root-preferential, stress-inducible or stress-induced root-preferential promoter activity.
[0134] Yet another embodiment provides a method of producing food, feed, or an industrial product comprising (a) obtaining the plant or a part thereof, according to the invention; and (b) preparing the food, feed or industrial product from the plant or part thereof. In another embodiment, said food or feed is oil, meal, ground or crushed seeds, soybean flakes, grain, starch, flour or protein, or said industrial product is biofuel, fiber, industrial chemicals, a pharmaceutical or a nutraceutical. Such food, feed or industrial products contain the root-preferential, stress-inducible and stress-induced root-preferential promoter described herein.
[0135] In another embodiment, the invention provides a method to increase the yield, such as fiber yield and seed yield, of a plant, such as a cotton, a soybean plant and a wheat plant compared to a control plant under stress condition comprising (a) providing to cells of said plant a recombinant gene comprising (i) a heterologous plant expressible promoter, (ii) a nucleic acid sequence encoding an Annexin protein and (iii) optionally a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants, and (b) regenerating the plant.
[0136] In further embodiments, the stress is a drought stress, occurring during the plant reproductive stage, on field-grown plants.
[0137] The present invention provides a method to increase lint yield and a method to increase seed yield. In a further embodiment the increase yield compared to a control plant is at least 5%.
[0138] "Control plant" as used herein refers to a plant genetically resembling the tested plant but not carrying the recombinant gene, such as wild type plants or null segregant plants.
[0139] Furthermore, the disclosed method is expected to yield similar results in other plant species. Particularly, it is expected to increase yield in corn under drought stress under field conditions. It may also lead to a yield increase in Brassica napus under stress condition in the field.
[0140] According to the present invention, the method provided more consistently increased yield when said plant expressible promoter is a root-preferential, stress-inducible or stress-induced root-preferential promoter, preferentially the Pbtg-26GhD10 promoter, compared to when said plant expressible promoter is a constitutive promoter, preferentially the CaMV35S promoter.
[0141] The phrase "more consistently increase yield" as used in this application means that a larger proportion of the obtained plants display the increased yield when using the root-preferential, stress-inducible or stress-induced root-preferential promoter compared to the proportion of plants displaying an increased yield when using a constitutive promoter. For example the proportion may be increased by at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90% or even 100%.
[0142] Furthermore, the disclosed method is expected to yield similar results in other plant species. Particularly, it is expected to more consistently increase yield in corn under drought stress under field conditions. It may also lead to a more consistent yield increase in Brassica napus under stress condition in the field.
[0143] The transformed plant cells and plants obtained by the methods described herein may be further used in breeding procedures well known in the art, such as crossing, selfing, and backcrossing. Breeding programs may involve crossing to generate an F1 (first filial) generation, followed by several generations of selfing (generating F2, F3, etc.). The breeding program may also involve backcrossing (BC) steps, whereby the offspring is backcrossed to one of the parental lines, termed the recurrent parent.
[0144] Accordingly, also disclosed herein is a method for producing plants comprising the recombinant gene disclosed herein comprising the step of crossing the plant disclosed herein with another plant or with itself and selecting for offspring comprising said recombinant gene.
[0145] The transformed plant cells and plants obtained by the methods disclosed herein may also be further used in subsequent transformation procedures, e. g. to introduce a further recombinant gene.
Stress and Yield Definitions
[0146] Yield as used herein can comprise yield of the plant or plant part which is harvested, such as lint, biomass, or seed, including seed oil content, seed weight, seed number. Increased yield can be increased yield per plant, and increased yield per surface unit of cultivated land, such as yield per hectare. Yield can be increased by modulating, for example, water uptake in the roots, or indirectly by increasing the tolerance to biotic and abiotic stress conditions.
[0147] "Stress" refers to non-optimal environmental conditions such as biotic stress and abiotic stress.
[0148] Abiotic stress tolerance as used herein can comprise resistance to environmental stress factors such as drought, flood, extreme (high or low) temperatures, soil salinity or heavy metals, hypoxia, anoxia, osmotic stress, oxidative stress, low nutrient levels such as nitrogen or phosphorus.
[0149] Biotic stress tolerance as used herein can comprise pest resistance, such as resistance or fungal, bacterial, bacterial or viral pathogens or nematodes or insects.
[0150] Drought as used in the present application relates to the shortage or absence of water available to a plant for a specified time. Such shortage or absence of water may last only a few days such as at least or up to 2, at least or up to 3, at least or up to 4, at least or up to 5, at least or up to 6, at least or up to 7, at least or up to 8, at least or up to 9, at least or up to 10, at least or up to 15 or at least or up to 20 days. It may as well be for a longer period such as at least or up to 3 weeks, at least or up to 4 weeks, at least or up to 5 weeks, at least or up to 6 weeks, at least or up to 2 months, at least or up to 3 months, at least or up to 4 months, at least or up to 5 months or at least or up to 6 months. In some areas of the world, drought may even last longer than 6 month, such as 7, 8, 9, 10, 11, 12, 15, 18 or 24 months.
[0151] Drought stress may be applied to the plant simply by depriving it of or reducing its water supply, either by placing them in a naturally drought exposed region or by reducing water supply in the field. For example, the water supply may be reduced by at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90% or even 100% for a desired time falling within those described above in connection with drought stress.
General Definitions
[0152] "Isolated nucleic acid", used interchangeably with "isolated DNA" as used herein refers to a nucleic acid not occurring in its natural genomic context, irrespective of its length and sequence. Isolated DNA can, for example, refer to DNA which is physically separated from the genomic context, such as a fragment of genomic DNA. Isolated DNA can also be an artificially produced DNA, such as a chemically synthesized DNA, or such as DNA produced via amplification reactions, such as polymerase chain reaction (PCR) well-known in the art. Isolated DNA can further refer to DNA present in a context of DNA in which it does not occur naturally. For example, isolated DNA can refer to a piece of DNA present in a plasmid. Further, the isolated DNA can refer to a piece of DNA present in another chromosomal context than the context in which it occurs naturally, such as for example at another position in the genome than the natural position, in the genome of another species than the species in which it occurs naturally, or in an artificial chromosome.
[0153] Hybridization occurs when the two nucleic acid molecules anneal to one another under appropriate conditions. Nucleic acid hybridization is a technique well known to those of skill in the art of DNA manipulation. The hybridization property of a given pair of nucleic acids is an indication of their similarity or identity. Another indication that two nucleic acid sequences are substantially identical is that the two molecules hybridize to each other under stringent conditions. The phrase "hybridizing specifically to" refers to the binding, duplexing, or hybridizing of a molecule only to a particular nucleotide sequence under stringent conditions when that sequence is present in a complex mixture (e.g., total cellular) DNA or RNA. "Bind(s) substantially" refers to complementary hybridization between a probe nucleic acid and a target nucleic acid and embraces minor mismatches that can be accommodated by reducing the stringency of the hybridization media to achieve the desired detection of the target nucleic acid sequence. "Stringent hybridization conditions" and "stringent hybridization wash conditions" in the context of nucleic acid hybridization experiments such as Southern and Northern hybridization are sequence dependent, and are different under different environmental parameters. An example of highly stringent wash conditions is 0.15 M NaCl at 72.degree. C. for about 15 minutes. An example of stringent wash conditions is a 0.2.times.SSC wash at 65.degree. C. for 15 minutes. Stringent conditions may also be achieved with the addition of destabilizing agents such as formamide. In general, a signal to noise ratio of 2 X (or higher) than that observed for an unrelated probe in the particular hybridization assay indicates detection of a specific hybridization. Nucleic acids that do not hybridize to each other under stringent conditions are still substantially identical if the proteins that they encode are substantially identical. This occurs, e.g., when a copy of a nucleic acid is created using the maximum codon degeneracy permitted by the genetic code.
[0154] The phrases "DNA", "DNA sequence," "nucleic acid sequence," "nucleic acid molecule" "nucleotide sequence" and "nucleic acid" refer to a physical structure comprising an orderly arrangement of nucleotides. The DNA sequence or nucleotide sequence may be contained within a larger nucleotide molecule, vector, or the like. In addition, the orderly arrangement of nucleic acids in these sequences may be depicted in the form of a sequence listing, figure, table, electronic medium, or the like.
[0155] As used herein "comprising" is to be interpreted as specifying the presence of the stated features, integers, steps or components as referred to, but does not preclude the presence or addition of one or more features, integers, steps or components, or groups thereof. Thus, e.g., a nucleic acid or protein comprising a sequence of nucleotides or amino acids, may comprise more nucleotides or amino acids than the actually cited ones, i.e., be embedded in a larger nucleic acid or protein. A recombinant gene comprising a nucleic acid which is functionally or structurally defined, may comprise additional DNA regions etc. However, in context with the present disclosure, the term "comprising" also includes "consisting of".
[0156] The sequence listing contained in the file named "BCS15-2008WO_ST25.txt", which is 118 kilobytes (size as measured in Microsoft Windows.RTM.), contains 31 sequences SEQ ID NO: 1 through SEQ ID NO: 31 is filed herewith by electronic submission and is incorporated by reference herein.
[0157] In the description and examples, reference is made to the following sequences:
SEQUENCE LISTING
[0158] SEQ ID NO: 1 nucleotide sequence of the T-DNA Pbtg-26Bn::GUS.
[0159] SEQ ID NO: 2 nucleotide sequence of the KVA07-32 primer.
[0160] SEQ ID NO: 3 nucleotide sequence of the KVA07-34 primer.
[0161] SEQ ID NO: 4 nucleotide sequence of the A-genome variant of btg-26Gh.
[0162] SEQ ID NO: 5 nucleotide sequence of the D-genome variant of btg-26Gh.
[0163] SEQ ID NO: 6 nucleotide sequence of the ca. 1 kb long promoter of the A-genome variant of Pbtg-26Gh.
[0164] SEQ ID NO: 7 nucleotide sequence of the ca. 1 kb long promoter of the D-genome variant of Pbtg-26Gh.
[0165] SEQ ID NO: 8 nucleotide sequence of the T-DNA Pbtg-26GhA0.6::GUS.
[0166] SEQ TD NO: 9 nucleotide sequence of the T-DNA Pbtg-26GhA10::GUS.
[0167] SEQ ID NO: 10 nucleotide sequence of the T-DNA Pbtg-26GhD0.6::GUS.
[0168] SEQ ID NO: 11 nucleotide sequence of the T-DNA Pbtg-26GhD10::GUS.
[0169] SEQ ID NO: 12: nucleotide sequence of AnnBj1 SEQ ID NO: 13: amino acid sequence of AnnBj1 SEQ ID NO: 14: nucleotide sequence of GhAnn1.
[0170] SEQ ID NO: 15: amino acid sequence of GhAnn1.
[0171] SEQ ID NO: 16: nucleotide sequence of AtAnn1.
[0172] SEQ ID NO: 17: amino acid sequence of AtAnn1.
[0173] SEQ ID NO: 18: nucleotide sequence of the T-DNA P35S::AnnBj1
[0174] SEQ ID NO: 19: nucleotide sequence of the T-DNA Pbtg-26GhD10::AnnBj1
[0175] SEQ ID NO: 20: qRT-PCR forward primer AnnBj1.
[0176] SEQ ID NO: 21: qRT-PCR reverse primer AnnBj1
[0177] SEQ ID NO: 22: qRT-PCR forward primer PP2A.
[0178] SEQ ID NO: 23: qRT-PCR reverse primer PP2A.
[0179] SEQ ID NO: 24: nucleotide sequence of Axmi196.
[0180] SEQ TD NO: 25: amino acid sequence of Axmi196.
[0181] SEQ ID NO: 26: nucleotide sequence of Axmi031.
[0182] SEQ ID NO: 27: amino acid sequence of Axmi031.
[0183] SEQ ID NO: 28: nucleotide sequence of Axmi277.
[0184] SEQ ID NO: 29: amino acid sequence of Axmi277.
[0185] SEQ ID NO: 30: nucleotide sequence of Axn-2.
[0186] SEQ ID NO: 31: amino acid sequence of Axn-2.
EXAMPLES
Example 1--Generation of Expression Constructs with the Pbtg-26Bn Promoter of Brassica Napus Operably Linked to the GUS Reporter Gene (Pbtg-26Bn::GUS)
[0187] The promoter sequence of the Brassica napus htg-26 promoter (EMBL accession number 577096, 5' to 3' position 4474 to 4148 of SEQ ID NO:1), the GUS gene (.beta.-glucuronidase) with intron (5' to 3' position 4101 to 2101 of SEQ ID NO: 1) and a fragment of the 3' untranslated region (UTR) of the CaMV 35S gene (5' to 3' position 2031 to 1827 of SEQ ID NO: 1) were assembled in a vector which contains the bar selectable marker cassette (position 1720 to 56 of SEQ ID NO: 1) to result in the T-DNA Pbtg-26Bn::GUS (SEQ ID NO: 1).
Example 2--Generation of Transgenic Plants Comprising Pbtg-26Bn::GUS
[0188] In a next step the recombinant vector comprising the expression cassette of example 1, i. e. Pbtg-26Bn::GUS, was used to stably transform Gossypium hirsutum Coker 312 using the embryogenic callus transformation protocol.
Example 3--Expression Pattern of Pbtg-26Bn::GUS in Gossypium hirsutum
[0189] .beta.-glucuronidase activity of plants transformed with btg-26Bn::GUS was monitored in planta with the chromogenic substrate X-Gluc (5-bromo-4-Chloro-3-indolyl-.beta.-D-glucuronic acid) during corresponding activity assays (Jefferson R A et al (1987) EMBO J. 20; 6 (13):3901-7). For determination of promoter activity plant tissue was dissected, embedded, stained and analyzed as described (e.g., Pien S. et al (2001) PNAS 98(20):11812-7). Thus, the activity of beta-glucuronidase in the transformed plants was witnessed by the presence of the blue color due to the enzymatic metabolism of the substrate X-Gluc.
[0190] After growing the progeny of independent TO plants under optimal growing conditions plants were examined for GUS expression. From these plants leaf samples from the first pair of leaves, root samples and stems were taken and tested for GUS reporter gene expression (e.g., Pien S. et al (2001) PNAS 98(20):11812-7).
[0191] Surprisingly, the Brassica napus promoter was unable to confer root-preferential expression in Gossypium hirsutum (see table 1 for result) although it was demonstrated in the prior art that this promoter retained its root preferential activity even in the more distant species barley and therefore suggested a high degree of conservation throughout dicotyledons and monocotyledons.
Example 4--Isolation of the Gossypium hirsutum a and D-Subgenome Specific Alleles Encoding the Orthologous Genes of Btg-26 from Brassica napus
[0192] The coding sequences of the btg-26 genes from Brassica napus (EMBL accession number 577096), rice (EMBL accession number AF32358) and Arabidopsis thaliana (At1g54100) were used to blast against a Gossypium hirsutum genomic sequence database. Based on the obtained result, a 656 bp probe was amplified using the primer KVA07-32 (SEQ ID NO:2) and KVA07-34 (SEQ ID NO:3) to screen a BAC library containing genomic DNA clones of Gossypium hirsutum FiberMax variety. The nucleotide sequence of genomic fragments for each of the allelic variants were identified and are represented in SEQ ID NO:4 (A genome) and SEQ ID NO:5 (D genome).
[0193] For the A-genome variant (SEQ ID NO:4), a TATA box could be identified at positions 1986 to 1999; a transcription initiation site at position 1219. The 5' untranslated leader extends from nucleotide 1219 to 1483; the translation initiation codon is located at positions 1484 to 1486.
[0194] For the D-genome variant (SEQ ID NO:5), a TATA box could be identified at positions 2789 to 2803; a transcription initiation site at position 2822. The 5' untranslated leader extends from nucleotide 2822 to 3089; the translation initiation codon is located at positions 3090 to 3092.
[0195] FIG. 1 shows an alignment of the nucleotide sequence of the first ca. 1 kb of the A and D promoters. Strikingly the two promoter fragments share about 78% sequence identity while the first 600 bp are nearly identical, sharing about 94% sequence identity.
Example 5--Generation of Expression Constructs with the Pbtg-26Gh Promoters of Gossypium hirsutum Operably Linked to the GUS Reporter Gene (Pbtg-26GhA0.6::GUS, Pbtg-26GhA10::GUS, Pbtg-26GhD0.6::GUS, Pbtg-26GhD10::GUS)
[0196] The promoter short sequence (ca. 600 bp) of the Gossypium hirsutum btg-26 promoter from the A subgenome (Pbtg-26GhA0.6, 5' to 3' position 4650 to 4086 of SEQ ID NO:8), the GUS gene with intron (5' to 3' position 4082 to 2082 of SEQ ID NO: 8) and a fragment of the 3' untranslated region (UTR) of the CaMV 35S gene (5' to 3' position 2012 to 1808 of SEQ ID NO: 8) were assembled in a vector which contains the bar selectable marker cassette (position 1720 to 56 of SEQ ID NO: 8) to result in the T-DNA Pbtg-26GhA0.6::GUS (SEQ ID NO: 8).
[0197] The promoter long sequence (ca. 1 kb) of the Gossypium hirsutum btg-26 promoter from the A subgenome (Pbtg-26GhA10, 5' to 3' position 5094 to 4086 of SEQ ID NO:9), the GUS gene with intron (5' to 3' position 4082 to 2082 of SEQ ID NO: 9) and a fragment of the 3' untranslated region (UTR) of the CaMV 35S gene (5' to 3' position 2012 to 1808 of SEQ ID NO: 9) were assembled in a vector which contains the bar selectable marker cassette (position 1720 to 56 of SEQ ID NO: 9) to result in the T-DNA Pbtg-26GhA10::GUS (SEQ ID NO: 9).
[0198] The promoter short sequence (ca. 600 bp) of the Gossypium hirsutum btg-26 promoter from the D subgenome (Pbtg-26GhD0.6, 5' to 3' position 4654 to 4083 of SEQ ID NO:10), the GUS gene with intron (5' to 3' position 4082 to 2082 of SEQ ID NO: 10) and a fragment of the 3' untranslated region (UTR) of the CaMV 35S gene (5' to 3' position 2012 to 1808 of SEQ ID NO: 10) were assembled in a vector which contains the bar selectable marker cassette (position 1720 to 56 of SEQ ID NO: 10) to result in the T-DNA Pbtg-26GhD0.6::GUS (SEQ ID NO: 10).
[0199] The promoter long sequence (ca. 1 kb) of the Gossypium hirsutum btg-26 promoter from the D subgenoine (Pbtg-26GhD10, 5' to 3' position 5104 to 4083 of SEQ ID NO:11), the GUS gene with intron (5' to 3' position 4082 to 2082 of SEQ ID NO: 11) and a fragment of the 3' untranslated region (UTR) of the CaMV 35S gene (5' to 3' position 2012 to 1808 of SEQ ID NO: 11) were assembled in a vector which contains the bar selectable marker cassette (position 1720 to 56 of SEQ ID NO: 11) to result in the T-DNA Pbtg-26GhD10::GUS (SEQ ID NO: 11).
Example 6--Generation of Transgenic Plants Comprising the Different Pbtg-26Gh::GUS Cassettes
[0200] In a next step the recombinant vector comprising the expression cassettes of example 5, i.e. Pbtg-26GhA0.6::GUS, Pbtg-26GhA10::GUS, Pbtg-26GhD0.6::GUS and Pbtg-26GhD10::GUS, were used to stably transform Gossypium hirsutum coker 312 using the embryogenic callus transformation protocol.
[0201] The recombinant vector comprising the expression cassette Pbtg-26GhD10::GUS is used to stably transform wheat using the method described in Yuji Ishida et al. 2015, Methods in Molecular Biology, 1223: 189-198.
[0202] The recombinant vector comprising the expression cassette Pbtg-26GhD10::GUS is used to stably transform soybean using the method described in the patent application WO2014/150449.
Example 7--Expression Pattern of the Different Pbtg-26Gh::GUS in Gossypium hirsutum
[0203] .beta.-glucuronidase activity of plants transformed with Pbtg-26GhA0.6::GUS, Pbtg-26GhA10::GUS, Pbtg-26GhD0.6::GUS and Pbtg-26GhD10::GUS was monitored as described in example 3.
[0204] Table 1 shows the average expression profile of all events produced per construct in the selected tissues (roots, leaves and stems). Intensity of the staining was quantified on a scale from 0 to 5, 0 corresponding to the absence of staining.
[0205] It was unexpectedly observed that only the long version of the pbtg-26D promoter lead to a preferential expression of GUS in the roots. Indeed the short and long promoter fragments from the A subgenome as well as the short promoter fragment from the D subgenome drive similar expression levels in all tissues tested, with the short D promoter and long A promoter driving a slightly lower expression in the roots.
TABLE-US-00001 TABLE 1 Nb of events Expression level Promoter tested Root tissues Leaf tissues Stem tissues Pbtg-26GhA0.6 5 3.0 2.8 3.0 Pbtg-26GhA10 7 2.7 3.7 3.3 Pbtg-26GhD0.6 8 1.7 2.4 2.4 Pbtg-26GhD10 16 4.6 1.3 2.2 Pbtg-26Bn 8 3.7 3.4 3.6
[0206] It can further be concluded from these results that the shorter promoter fragment of D does not influence the root-preferential activity of the longer promoter fragment (SEQID NO: 7) and may for example be replaced by the sequence of the short A promoter in the nucleotide sequence of SEQ ID NO: 7 without affecting its activity.
Example 8--Sequence Analysis of the Pbtg-26GhD10 Promoter
[0207] FIG. 1 shows the nucleotide sequence of the Pbtg-26 promoters from Gossypium hirsutum annotated with the predicted CIS elements relevant for stress-inducible expression as well as the position of the TATA box and the transcription initiation site.
[0208] Four ABA responsive-like motifs (ABRE-like) could be predicted from the btg-26 promoter of the D sub-genome but these motifs are not conserved in the promoter sequence from the A sub-genome. These motifs suggest that only the promoter sequence from the D sub-genome is capable to respond to stress.
Example 9--Stress Inducibility of the Cotton Endogenous BTG-26D Gene
[0209] Gossypium hirsutum plants from the Cocker variety were grown in a growth chamber and were watered until the 2-leaf developmental stage. Leaf samples were then collected in triplicates on the last day of watering (control samples) and after 7 days without watering (D7 samples).
[0210] RNA was extracted using the Sigma plant RNA extraction kit and analyzed by sequencing. Table 2 shows the expression values obtained for the BTG-26D endogenous gene in the different samples. BTG-26D is significantly induced by the drought stress and its expression level is ca. 1.9 times higher after 7 days of drought than in the control condition. As predicted the BTG26-D promoter therefore has stress-inducible activity as demonstrated with the application of drought stress. The functionality of the ABRE-like elements in the promoter is thus confirmed.
TABLE-US-00002 TABLE 2 Replicate 1 Replicate 2 Replicate 3 Average SD control 43.5 28.3 47.0 39.6 9.9 D7 95.7 72 57 74.9 19.5
Example 10--Assessment of the Promoter Activity of the Pbtg-26GhD10 Promoter in Soybean and Wheat
[0211] .beta.-glucuronidase activity of soybean and wheat plants transformed with Pbtg-26GhD10::GUS is monitored as described in example 3.
[0212] Results indicate that the promoter Pbtg-26GhD10 has root-preferential promoter activity in soybean. They also indicate that the promoter has stress-inducible promoter activity in Soybean. Furthermore, the promoter has stress-induced root-preferential promoter activity in soybean.
[0213] Results indicate that the promoter Pbtg-26GhD10 has root-preferential promoter activity in wheat. They also indicate that the promoter has stress-inducible promoter activity in wheat. Furthermore, the promoter has stress-induced root-preferential promoter activity in wheat.
Example 11--Construction of a Recombinant Gene Encoding an Annexin for Root-Preferential and for Constitutive Expression in Cotton Cells
[0214] A DNA molecule having the nucleic acid sequence according to SEQ ID NO: 12 was synthesized by Entelechon GmbH.
[0215] Using standard recombinant DNA techniques, the constitutive promoter region CaMV35S according to the sequence from nucleotide position 89 to 506 of SEQ ID NO: 18, the 5'UTR sequence including the leader sequence of the chlorophyll a/b binding protein gene of Petunia hybrid according to the sequence from nucleotide position 511 to 568 of SEQ ID NO: 18, the DNA fragment coding for AnnBj laccording to the sequence SEQ ID NO: 12 or to the sequence from nucleotide position 577 to 1530 of SEQ ID NO: 18, and the 3' untranslated sequence of the Arabidopsis thaliana histone H4 gene according to the sequence from nucleotide position 1542 to 2202 of SEQ ID NO: 18 were assembled in a vector which contains the 2mepsps selectable marker cassette (position 2252 to 6080 of SEQ ID NO: 18) to result in the T-DNA P35S::AnnBj1 (SEQ ID NO: 18).
[0216] Using standard recombinant DNA techniques, the root-preferential promoter region Pbtg-26GhD10 according to the sequence from nucleotide position 89 to 1107 of SEQ ID NO: 19, the DNA fragment coding for AnnBj1 according to the sequence SEQ ID NO: 12 or to the sequence from nucleotide position 1111 to 2064 of SEQ ID NO: 19, and the 3' untranslated sequence of the Arabidopsis thaliana histone H4 gene according to the sequence from nucleotide position 2076 to 2736 of SEQ ID NO: 19 were assembled in a vector which contains the 2mepsps selectable marker cassette (position 2786 to 6614 of SEQ ID NO: 19) to result in the T-DNA Pbtg-26GhD10::AnnBj1 (SEQ ID NO: 19).
Example 12--Generation of Transgenic Cotton Plants Expressing AnnBj1
[0217] The T-DNA vectors from the Example 11 were introduced into Agrobacterium tumefaciens strains containing a helper Ti-plasmid and used in cotton transformation essentially as described in WO00/71733. Homozygous plants and their null segregants were further analyzed as described in the following Examples.
Example 13--Seed and Lint Yield Assessment of Transgenic Cotton Plants Expressing AnnBj1 in Field Trial
[0218] Field trials were performed in the United States on 12 events from the transformation with the T-DNA Pbtg-26GhD10::AnnBj1 and 15 events from the transformation with the T-DNA P35S::AnnBj1 using a split plot design with 3 blocks. The events were allocated to the whole-plot within a block and the zygosity (Homozygous and Null) were allocated to the sub-block within the whole-plot.
[0219] Deficient irrigation treatment was applied from squaring stage. Typical agronomic inputs for conventionally grown cotton for the area, following best local agronomic practices were applied.
[0220] The parameters scored were lint yield and seed cotton yield. The obtained data were analysed using linear mixed model and AsREML software (Gilmour et al. 1999). The fixed part of the model consists on the main effect of the event, main effect of the zygosity and their interaction. The random terms of the model were block, whole-plot and sub-block affects to adjust for field heterogeneity.
[0221] Under control condition, no seed yield nor lint yield was observed for the tested events. However, under drought stress, the expression of AnnBj1 lead to an increased seed and/or lint yield. The results for the drought condition are shown in Table 3.
[0222] Out of the 12 events tested for Pbtg-26GhD10::AnnBj1, 9 have an increased seed yield compared to their null segregant of at least 5%, meaning that the three quarters of the events produced display the positive effect of the transgene. In comparison, out of the 15 P35S::AnnBj1 events, only 8 have a yield increase of at least 5% compared to their null segregant, meaning that half of the events produced display the positive effect of the transgene. Expressing the AnnBj1 gene under control of the Pbtg-26GhD10 promoter as opposed to the constitutive promoter thus results in obtaining 50% more events with an at least 5% seed yield increase compare to their nulls.
[0223] Regarding the lint yield, 8 out of the 12 events produced with the transformation with the T-DNA Pbtg-26GhD10::AnnBj1 have an increased yield of at least 5% compared to their nulls, i.e. three quarter of the events display the positive effect of the transgene. In contrast, only 7 of the 15 events tested from the transformation with the T-DNA 35S::AnnBj1 have an increased yield of at least 5% compared to their nulls, i.e. half of the events display the positive effect of the transgene. Expressing the AnnBj1 gene under control of the Pbtg-26GhD10 promoter as opposed to the constitutive promoter thus results in obtaining 50% more events with an at least 5% seed yield increase compare to their nulls.
[0224] In conclusion, constitutive expression of AnnBj1 in cotton results in both a seed yield and a lint yield increase of at least 5% compared to the respective null segregants. Stress-induced root-preferential expression of AnnBj1 in cotton results in both a seed yield and a lint yield increase of at least 5% compared to the respective null segregants. Furthermore, when using the root-preferential promoter Pbtg-26GhD10 in cotton, the effect of the AnnBj1 overexpression is more penetrant with more events displaying the improved yield compared to when using the constitutive promoter 35S under drought stress conditions in the field.
TABLE-US-00003 TABLE 3 % yield increase of homozygous over their respective null segregant - drought stress condition. % seed % lint Independent yield yield T-DNA Events increase increase Pbtg-26GhD10::AnnBj1 1 -5.69 -5.09 2 13.96 15.63 3 -15.75 -15.67 4 5.43 5.72 5 7.68 7.87 6 20.35 23.83 7 20.24 25.84 8 12.79 11.97 9 8.52 9.87 10 18.17 -0.65 11 13.51 15.52 12 -5.25 -3.92 P35S::AnnBj1 1 -4.18 -4.43 2 18.59 20.41 3 -74.25 -75.13 4 13.98 15.82 5 -1.65 -1.20 6 12.72 12.72 7 28.78 28.25 8 5.74 6.26 9 4.02 4.70 10 9.33 9.56 11 16.98 19.43 12 6.20 4.74 13 -3.48 -1.52 14 1.16 4.69 15 0.16 0.07
Example 14--Seed and Lint Yield Assessment of Selected Transgenic Cotton Plants from Example 13 in Field Trial
[0225] Field trials were performed again on 6 events from the transformation with the T-DNA Pbtg-26GhD10::AnnBj1 and 4 events from the transformation with the T-DNA P35S::AnnBj1 Events were selected based on their performance in the previous field trial. The field trial was designed, run and the results analyzed as described in Example 13.
[0226] Under control condition, no significant yield penalty was observed for the tested events. However, under drought stress, the expression of AnnBj1 lead to an increased lint yield compared to the wild type Coker plants. The results for the drought condition arc shown in Table 4.
TABLE-US-00004 TABLE 4 % lint yield increase of homozygous over the wild type Coker - drought stress condition. The result of the first year field trial are added as reference. % lint yield % lint yield increase to increase to Independent wild type, wild type, T-DNA Events year 1 year 2 Pbtg-26GhD10::AnnBj1 2 111% 114% 4 103% 113% 6 116% 121% 8 121% 123% 9 110% 118% 11 116% 118% P35S::AnnBj1 2 111% 115% 9 111% 116% 11 118% 118% 14 113% 115%
Example 15--Stress-Induced Root-Preferential Promoter Activity of the Pbtg-26GhD10 Promoter in Cotton
[0227] To further confirm the expression pattern conferred by the Pbtg-26GhD10 promoter, cotton seeds from 8 events containing the Pbtg-26GhD10::AnnBj1 transgene and wild type cotton seeds were surface sterilized, sown and grown in vitro either on control media or media containing 250 mM mannitol (i.e. stress media). Mannitol is well known in the art to mimick drought stress. Root and leaf tissues were collected at respectively 16 and 27 days after sowing from the plants grown on control media and stress media.
[0228] RNA from the sampled root and leaf tissues were extracted using the Spectrum plant total RNA kit from Sigma with protocol A. The gene PP2A was used as a reference gene. Q-RT PCR were performed and analysed using the method described in the manual of Applied Biosystems with the primers SEQ ID NO: 20 and SEQ ID NO: 21 for the AnnBj1 transcript and SEQ ID NO: 22 and SEQ ID NO: 23 for the PP2A transcript. Table 5 shows the obtained results.
TABLE-US-00005 TABLE 5 Control media Stress media Fold change Event 2-[deltaCt] 2-[deltaCt] stress over number value stdev value stdev control media Leaf 2 0.91 0.31 0.29 0.03 0.32 4 2.17 0.43 0.60 0.13 0.28 6 0.75 0.20 0.25 0.06 0.33 8 1.66 0.67 1.01 0.59 0.61 9 2.32 0.64 1.38 0.50 0.60 11 1.10 0.92 0.49 0.03 0.45 root 2 0.11 0.03 0.82 0.55 7.68 4 0.22 0.08 1.09 0.29 4.89 6 0.06 0.02 0.85 0.08 13.72 8 0.10 0.01 4.35 1.73 45.08 9 1.62 0.64 8.05 0.56 4.97 11 0.08 0.01 2.11 0.87 25.83
[0229] Although the expression level in the leaf was not increased by the stress treatment applied, the expression in the root is increased at least 4 fold in the stress condition compared to control condition. The Pbtg-26GhD10 promoter therefore has, under high stress, a stress-induced root-preferential promoter activity.
[0230] A similar experiment was performed with a lower concentration of mannitol (200 mM instead of 250 mM) on the same events. The events carrying two copies of the Pbtg-26GhD10::AnnBj1 transgene also confirmed the stress-induced root-preferential promoter activity of the Pbtg-26GhD10 promoter under milder stress condition.
Example 16--Construction of a Recombinant Gene Encoding Nematode Resistance Genes for Stress-Induced Root-Preferential Expression in Soybean Cells
[0231] Using standard recombinant DNA techniques, the stress-induced root-preferential promoter region Pbtg-26GhD10 as described above, the DNA fragment coding for Axmi196 according to the sequence SEQ ID NO: 24 are assembled in a vector which contains a selectable marker cassette to result in the T-DNA Pbtg-26GhD10::Axmi196.
[0232] The stress-induced root-preferential promoter region Pbtg-26GhD10 as described above, the DNA fragment coding for Axmi031 according to the sequence SEQ ID NO: 26 are assembled in a vector which contains a selectable marker cassette to result in the T-DNA Pbtg-26GhD10::Axmi031.
[0233] The stress-induced root-preferential promoter region Pbtg-26GhD10 as described above, the DNA fragment coding for Axmi277 according to the sequence SEQ ID NO: 28 are assembled in a vector which contains a selectable marker cassette to result in the T-DNA Pbtg-26GhD10::Axmi277.
[0234] The stress-induced root-preferential promoter region Pbtg-26GhD10 as described above, the DNA fragment coding for Axn-2 according to the sequence SEQ ID NO: 30 are assembled in a vector which contains a selectable marker cassette to result in the T-DNA Pbtg-26GhD10::Axn-2.
Example 17--Generation of Transgenic Soybean Plants Expressing Nematode Resistance Genes
[0235] The T-DNA vectors from the Example 16 are introduced into Agrobacterium tumefaciens strains containing a helper Ti-plasmid and used in soybean transformation essentially as described in the patent application WO2014/150449. Homozygous plants and their null segregants are further analyzed as described in the following Examples.
Example 18--Assessment of the Nematode Resistance of Transgenic Soybean Plants Expressing Axmi196, Axmi031, Axmi277 or Axn-2 Under Control of the Pbtg-26GhD10 Promoter
[0236] The nematode resistance of the transgenic plants was assessed according to the method described in WO 2011/014749, WO 2007/147029, WO 2014/003769, WO 2010/077858.
[0237] In conclusion, the promoter Pbtg-26GhD10 can be used in soybean to confer biotic stress tolerance, like nematode resistance.
[0238] Preferred embodiments are summarized in the following paragraphs: [0239] 1. An isolated nucleic acid having root-preferential, stress-inducible or stress-induced root-preferential promoter activity selected from the group consisting of: [0240] a. a nucleic acid comprising a nucleotide sequence of SEQ ID NO: 7 or a functional fragment thereof comprising the nucleotide sequence of SEQ ID NO: 7 from nucleotide position 351 to nucleotide position 755; [0241] b. a nucleic acid comprising a nucleotide sequence having at least about 95% sequence identity to SEQ ID NO: 7, or a functional fragment thereof; and [0242] c. the nucleic acid of a functional promoter capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 7, or a functional fragment thereof. wherein said functional fragment comprises at least about 400 consecutive nucleotides upstream of the transcription start of SEQ ID NO: 7. [0243] 2. A recombinant gene comprising the nucleic acid according to paragraph 1 operably linked to a heterologous nucleic acid sequence encoding an expression product of interest, and optionally a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants. [0244] 3. The recombinant gene according to paragraph 2, wherein the expression product of interest is an RNA molecule capable of modulating the expression of a gene or is a protein. [0245] 4. A host cell comprising the isolated nucleic acid according to paragraph 1, or the recombinant gene according to paragraph 2 or 3. [0246] 5. The host cell of paragraph 4 which is an E. coli cell, an Agrobacterium cell, yeast cell, or a plant cell. [0247] 6. A plant comprising the recombinant gene of paragraph 2 or 3, preferably stably integrated in the genome of said plant. [0248] 7. Plant parts and seeds obtainable from the plant according to paragraph 6 which comprise the recombinant gene according to paragraph 2 or paragraph 3. [0249] 8. The plant or plant cell or plant part or seed according to any one of paragraphs 5 to 7, which is a cotton plant, or a cotton plant cell or cotton plant or cotton seed. [0250] 9. The plant or plant cell or plant part or seed according to any one of paragraphs 5 to 7, which is a soybean plant, or a soybean plant cell or soybean plant part or soybean seed. [0251] 10. The plant or plant cell or plant part or seed according to any one of paragraphs 5 to 7, which is a wheat plant, or a wheat plant cell or wheat plant part or wheat seed. [0252] 11. Method of producing a transgenic plant comprising the steps of: [0253] a. introducing or providing the recombinant gene according to paragraph 2 or 3 to a plant cell to create transgenic cells; and [0254] b. regenerating transgenic plants from said transgenic cell. [0255] 12. Method of effecting root-preferential expression of a nucleic acid comprising introducing the recombinant gene according to paragraph 2 or 3 into the genome of a plant, or providing the plant according to paragraph 6. [0256] 13. Method of effecting stress-inducible expression of a nucleic acid comprising introducing the recombinant gene according to paragraph 2 or 3 into the genome of a plant, or providing the plant according to paragraph 6. [0257] 14. Method of effecting stress-induced expression of a nucleic acid preferentially in the roots comprising introducing the recombinant gene according to paragraph 2 or 3 into the genome of a plant, or providing the plant according to paragraph 6. [0258] 15. Method for altering biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, or yield of a plant, said method comprising introducing the recombinant gene according to paragraph 2 or 3 into the genome of a plant, or providing the plant according to paragraph 6. [0259] 16. Use of the isolated nucleic acid according to paragraph 1 to regulate expression of an operably linked nucleic acid in a plant. [0260] 17. Use of the isolated nucleic acid according to paragraph 1, or the recombinant gene according to paragraph 2 or 3 to alter biotic or abiotic stress tolerance, root architecture, nutrient use efficiency, or yield in a plant. [0261] 18. Use of the isolated nucleic acid according to paragraph 1 to identify other nucleic acids comprising root-preferential promoter activity. [0262] 19. Use of the isolated nucleic acid according to paragraph 1 to identify other nucleic acids comprising stress-inducible promoter activity. [0263] 20. Use of the isolated nucleic acid according to paragraph 1 to identify other nucleic acids comprising stress-induced root-preferential promoter activity. [0264] 21. The method according to any one of paragraphs 11 to 15, or the use according to paragraph 17 to 19, wherein said plant is a cotton plant. [0265] 22. The method according to any one of paragraphs 11 to 15, or the use according to paragraph 17 to 19, wherein said plant is a soybean plant. [0266] 23. The method according to any one of paragraphs 11 to 15, or the use according to paragraph 17 to 19, wherein said plant is a wheat plant. [0267] 24. A method of producing food, feed, or an industrial product comprising [0268] a) obtaining the plant or a part thereof, of any one of paragraphs 6 to 10; and [0269] b) preparing the food, feed or industrial product from the plant or part thereof. [0270] 25. The method of paragraph 24 wherein [0271] a) the food or feed is oil, meal, grain, starch, flour or protein; or [0272] b) the industrial product is biofuel, fiber, industrial chemicals, a pharmaceutical or a nutraceutical. [0273] 26. A recombinant gene comprising: [0274] (a) a plant expressible promoter selected from [0275] i. root-preferential promoter; [0276] ii. stress-inducible promoter; or [0277] iii. stress-induced root-preferential promoter; [0278] (b) a nucleic acid sequence encoding an Annexin protein; [0279] (c) and optionally, a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants; [0280] 27. The recombinant gene of paragraph 26, wherein said plant expressible promoter is the Pbtg-26 GhD10 promoter. [0281] 28. The recombinant gene of paragraph 26 or 27, wherein said nucleic acid encoding an Annexin protein comprises: [0282] a. a nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; [0283] b. a nucleotide sequence at least 80% identical to SEQ ID NO: 12 or SEQ ID NO: 14; [0284] c. a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; [0285] d. a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13 or SEQ ID NO: 15; [0286] e. a nucleotide sequence encoding an amino acid sequence having 80% identity with SEQ ID NO: 13 or SEQ ID NO: 15; [0287] f. a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains. [0288] 29. A method to increase the yield of a plant under stress condition comprising: [0289] a. providing to cells of said plant a recombinant gene comprising: [0290] i. a heterologous plant expressible promoter; [0291] ii. a nucleic acid sequence encoding an Annexin protein; [0292] iii. and optionally, a transcription termination and polyadenylation sequence, preferably a transcription termination and polyadenylation region functional in plants; [0293] b. regenerating said plant; wherein the increase in yield is compared to the yield in a control plant. [0294] 30. The method of paragraph 29, wherein said plant expressible promoter is selected from the group consisting of: [0295] a. a root-preferential promoter; [0296] b. a stress-inducible promoter; and [0297] c. a stress-induced root-preferential promoter. [0298] 31. The method of paragraph 29 or 30, wherein said plant expressible promoter is the Pbtg-26GhD10 promoter. [0299] 32. The method of paragraph 29, wherein said plant expressible promoter is a constitutive promoter. [0300] 33. The method of paragraph 29 or 32, wherein said plant expressible promoter is the CaMV35S promoter. [0301] 34. The method of any one of paragraphs 29 to 33, wherein said nucleic acid encoding an Annexin protein comprises: [0302] a. a nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO: 14; [0303] b. a nucleotide sequence at least 80% identical to SEQ ID NO: 12 or SEQ ID NO: 14; [0304] c. a nucleotide sequence of a nucleic acid capable of hybridizing under stringent conditions to the nucleotide sequence of SEQ ID NO: 12 or SEQ ID NO 14; [0305] d. a nucleotide sequence encoding the amino acid sequence of SEQ ID NO: 13 or SEQ ID NO: 15; [0306] e. a nucleotide sequence encoding an amino acid sequence having 80% identity with SEQ ID NO: 13 or SEQ ID NO: 15; [0307] f. a nucleotide sequence encoding a protein comprising four or more annexin-repeated domains. [0308] 35. The method of any one of paragraphs 29 to 34, wherein said plant is cotton. [0309] 36. The method of any one of paragraphs 29 to 34, wherein said plant is soybean. [0310] 37. The method of any one of paragraphs 29 to 34, wherein said plant is wheat. [0311] 38. The method of any one of paragraphs 29 to 37, wherein said stress condition is drought stress. [0312] 39. The method of any one of paragraphs 29 to 37, wherein said stress condition is occurring during the plant reproductive stage. [0313] 40. The method of any one of paragraphs 29 to 37, wherein said stress condition is occurring on field-grown plants. [0314] 41. The method of any one of paragraphs 29 to 35, wherein said plant is cotton and said yield is lint yield. [0315] 42. The method of any one of paragraphs 29 to 37, wherein said yield is seed yield. [0316] 43. The method of any one of paragraphs 29 to 42, wherein said yield is increased by at least 5%. [0317] 44. The method of paragraph 43, wherein the yield increased is more consistently obtained with the method of paragraphs 30 or 31 compared to the method of paragraphs 32 or 33. [0318] 45. A plant cell comprising a recombinant gene as defined in any one of paragraphs 26 to 28. [0319] 46. A plant consisting essentially of the plant cells of paragraph 45. [0320] 47. Plant parts or seeds obtainable from the plant according to paragraph 46. [0321] 48. The plant, plant cell, plant part or seed according to any one of paragraphs 45 to 47, which is a cotton plant, cotton plant cell, cotton plant part or cotton seed. [0322] 49. The plant, plant cell, plant part or seed according to any one of paragraphs 45 to 47, which is a soybean plant, soybean plant cell, soybean plant part or soybean seed. [0323] 50. The plant, plant cell, plant part or seed according to any one of paragraphs 45 to 47, which is a wheat plant, wheat plant cell, wheat plant part or wheat seed.
Sequence CWU 1
1
3314556DNAArtificial SequenceT-DNA Pbtg-26Bn::GUS 1cggcaggata
tattcaattg taaatggctc catggcgatc gctctagagg atctgcgatc 60tagtaacata
gatgacaccg cgcgcgataa tttatcctag tttgcgcgct atattttgtt
120ttctatcgcg tattaaatgt ataattgcgg gactctaatc ataaaaaccc
atctcataaa 180taacgtcatg cattacatgt taattattac atgcttaacg
taattcaaca gaaattatat 240gataatcatc gcaagaccgg caacaggatt
caatcttaag aaactttatt gccaaatgtt 300tgaacgatct gcttcggatc
ctagaacgcg tgatctcaga tctcggtgac gggcaggacc 360ggacggggcg
gtaccggcag gctgaagtcc agctgccaga aacccacgtc atgccagttc
420ccgtgcttga agccggccgc ccgcagcatg ccgcgggggg catatccgag
cgcctcgtgc 480atgcgcacgc tcgggtcgtt gggcagcccg atgacagcga
ccacgctctt gaagccctgt 540gcctccaggg acttcagcag gtgggtgtag
agcgtggagc ccagtcccgt ccgctggtgg 600cggggggaga cgtacacggt
cgactcggcc gtccagtcgt aggcgttgcg tgccttccag 660gggcccgcgt
aggcgatgcc ggcgacctcg ccgtccacct cggcgacgag ccagggatag
720cgctcccgca gacggacgag gtcgtccgtc cactcctgcg gttcctgcgg
ctcggtacgg 780aagttgaccg tgcttgtctc gatgtagtgg ttgacgatgg
tgcagaccgc cggcatgtcc 840gcctcggtgg cacggcggat gtcggccggg
cgtcgttctg ggtccatggt tatagagaga 900gagatagatt tatagagaga
gactggtgat ttcagcgtgt cctctccaaa tgaaatgaac 960ttccttatat
agaggaaggg tcttgcgaag gatagtggga ttgtgcgtca tcccttacgt
1020cagtggagat gtcacatcaa tccacttgct ttgaagacgt ggttggaacg
tcttcttttt 1080ccacgatgct cctcgtgggt gggggtccat ctttgggacc
actgtcggca gaggcatctt 1140gaatgatagc ctttccttta tcgcaatgat
ggcatttgta ggagccacct tccttttcta 1200ctgtcctttc gatgaagtga
cagatagctg ggcaatggaa tccgaggagg tttcccgaaa 1260ttatcctttg
ttgaaaagtc tcaatagccc tttggtcttc tgagactgta tctttgacat
1320ttttggagta gaccagagtg tcgtgctcca ccatgttgac gaagattttc
ttcttgtcat 1380tgagtcgtaa aagactctgt atgaactgtt cgccagtctt
cacggcgagt tctgttagat 1440cctcgatttg aatcttagac tccatgcatg
gccttagatt cagtaggaac taccttttta 1500gagactccaa tctctattac
ttgccttggt ttatgaagca agccttgaat cgtccatact 1560ggaatagtac
ttctgatctt gagaaatatg tctttctctg tgttcttgat gcaattagtc
1620ctgaatcttt tgactgcatc tttaaccttc ttgggaaggt atttgatctc
ctggagattg 1680ttactcgggt agatcgtctt gatgagacct gctgcgtagg
aacgcggccg ctgtacaggg 1740cccgggcata tggcgcgtta gggataacag
ggtaattacg tattaattaa ggcgcgtcct 1800gcaggaagct tgcatgcctg
caggtcactg gattttggtt ttaggaatta gaaattttat 1860tgatagaagt
attttacaaa tacaaataca tactaagggt ttcttatatg ctcaacacat
1920gagcgaaacc ctataagaac cctaattccc ttatctggga actactcaca
cattattctg 1980gagaaaaata gagagagata gatttgtaga gagagactgg
tgatttttgc gccgggtacc 2040gagctcggta gcaattcccg aggctgtagc
cgacgatggt gcgccaggag agttgttgat 2100tcattgtttg cctccctgct
gcggtttttc accgaagttc atgccagtcc agcgtttttg 2160cagcagaaaa
gccgccgact tcggtttgcg gtcgcgagtg aagatccctt tcttgttacc
2220gccaacgcgc aatatgcctt gcgaggtcgc aaaatcggcg aaattccata
cctgttcacc 2280gacgacggcg ctgacgcgat caaagacgcg gtgatacata
tccagccatg cacactgata 2340ctcttcactc cacatgtcgg tgtacattga
gtgcagcccg gctaacgtat ccacgccgta 2400ttcggtgatg ataatcggct
gatgcagttt ctcctgccag gccagaagtt ctttttccag 2460taccttctct
gccgtttcca aatcgccgct ttggacatac catccgtaat aacggttcag
2520gcacagcaca tcaaagagat cgctgatggt atcggtgtga gcgtcgcaga
acattacatt 2580gacgcaggtg atcggacgcg tcgggtcgag tttacgcgtt
gcttccgcca gtggcgcgaa 2640atattcccgt gcaccttgcg gacgggtatc
cggttcgttg gcaatactcc acatcaccac 2700gcttgggtgg tttttgtcac
gcgctatcag ctctttaatc gcctgtaagt gcgcttgctg 2760agtttccccg
ttgactgcct cttcgctgta cagttctttc ggcttgttgc ccgcttcgaa
2820accaatgcct aaagagaggt taaagccgac agcagcagtt tcatcaatca
ccacgatgcc 2880atgttcatct gcccagtcga gcatctcttc agcgtaaggg
taatgcgagg tacggtagga 2940gttggcccca atccagtcca ttaatgcgtg
gtcgtgcacc atcagcacgt tatcgaatcc 3000tttgccacgc aagtccgcat
cttcatgacg accaaagcca gtaaagtaga acggtttgtg 3060gttaatcagg
aactgttcgc ccttcactgc cactgaccgg atgccgacgc gaagcgggta
3120gatatcacac tctgtctggc ttttggctgt gacgcacagt tcatagagat
aaccttcacc 3180cggttgccag aggtgcggat tcaccacttg caaagtcccg
ctagtgcctt gtccagttgc 3240aaccacctgt tgatccgcat cacgcagttc
aacgctgaca tcaccattgg ccaccacctg 3300ccagtcaaca gacgcgtggt
tacagtcttg cgcgacatgc gtcaccacgg tgatatcgtc 3360cacccaggtg
ttcggcgtgg tgtagagcat tacgctgcga tggattccgg catagttaaa
3420gaaatcatgg aagtaagact gctttttctt gccgttttcg tcggtaatca
ccattcccgg 3480cgggatagtc tgccagttca gttcgttgtt cacacaaacg
gtgatacctg cacatcaaca 3540aattttggtc atatattaga aaagttataa
attaaaatat acacacttat aaactacaga 3600aaagcaattg ctatatacta
cattctttta ttttgaaaaa aatatttgaa atattatatt 3660actactaatt
aatgataatt attatatata tatcaaaggt agaagcagaa acttacgtac
3720acttttcccg gcaataacat acggcgtgac atcggcttca aatggcgtat
agccgccctg 3780atgctccatc acttcctgat tattgaccca cactttgccg
taatgagtga ccgcatcgaa 3840acgcagcacg atacgctggc ctgcccaacc
tttcggtata aagacttcgc gctgatacca 3900gacgttgccc gcataattac
gaatatctgc atcggcgaac tgatcgttaa aactgcctgg 3960cacagcaatt
gcccggcttt cttgtaacgc gctttcccac caacgctgat caattccaca
4020gttttcgcga tccagactga atgcccacag gccgtcgagt tttttgattt
cacgggttgg 4080ggtttctaca ggacggacca tggcgagatc aactaagacg
aagggggcgg cagagcagcg 4140aggatcctta aggatatcaa acagtgatta
aagaatcaat cacctctctt agtattatcg 4200atggtatcaa agaatggagt
tttccgatta tacgtttgat cagagagtct ttataagagg 4260aagtgagtga
gagagagaga gagagagaga gacgtgtacg acttgtaagt aaagttggaa
4320cagcgattgg accgaaaacg aaagctgagc tgttgtgtca atcccccttt
tggcttgatt 4380cactcggatc atatgaagtt ttgtcacgtg tcctacgttt
ctttttccgg gtcgggatat 4440accccgatta ggatccgttg acctgcaggt
cgacatttat cgaattcgag ctcgagttaa 4500ggcgcgccgt tgcaggtcga
cggccgagta ctggcaggat atataccgtt gtaatt 4556220DNAArtificial
Sequenceprimer KVA07-32 2tgtagttgac cagctacttg 20319DNAArtificial
Sequenceprimer KVA07-34 3ttaacacccg acgtgtttc 1942164DNAGossypium
hirsutum 4ttagaatata atgattaatt agaaaaaaaa ttataaaact tctatgtaag
cataaaatta 60attctagtaa atataaagca taatgactta tttgaccatt tatttaattt
tttacatatt 120ttagttattg tggttttttt tgggtcaaat tacctgaata
aatgaaaaag tgaacattta 180attaattttt taaaatttaa aaattaatta
aatgctgaca tgccatctat attagaatcc 240acatgtatgc tatgttagta
aagttaataa acatgaactt ttccatccat tttaggatga 300tttgacaaaa
actacaaatt taaggactaa aaaagataat tttttttaaa taatttgttt
360agtttattat tataggtcta gatcaaaaag tctaataaaa catttgaagc
aaaataaaaa 420aaaacatagc aacaaaaata aaataaaact aaaacaacct
ctagaaactt catccaaaaa 480aagcacttca gattattggt gataccaaat
ggccaaaccc ttcctgacca aaacaatcgt 540gaagaatgtg acaggagggg
agattgaggt tatctttgaa tggatagatg aaatcctcca 600tccaaagagt
aggacctaat atgtagaaac tcatggcagt tgcatgagtt gattgcatga
660gttgaaggtc cgatgctgtt aaatctatgg ctcttaaatg tgcatcttct
acctagaaac 720aatcttctaa gcttttcatc ccttctttcg ccattgcatg
cgcgacggta tttcctcctc 780tggttatgaa gttaaaacga tatgttaaga
agtttcgaga taatgcattc atatcccagg 840taatcggtct aatttcataa
tagtaaaata ggcgaaaaat taaatgaaaa actaaaataa 900ttcttttata
aaattggagg gtaaaaaaaa ttattatgcc taaatataac acatgttata
960aatactcata agacgaaaaa gttaaaaaat tacaaaggaa aggacctgat
tggagcaata 1020tgataatata gggacttgtt taaaatgttt taaagtttag
gacttattta gagtatcacc 1080catgatttgg tataataaat aaaaaatcag
atgagagagc cacctcatga aaaagacaag 1140aacattacgt gtgatccatt
gcagaagagg ataaagtatg gacaaaattt atagatataa 1200tcttgtacat
cccccatacg tcacggctct gttcagatca taggccgaaa aggcctccgt
1260ctgtctcagt cctctactta aggtactctt ctctctctcc ttccacatca
actttaacat 1320tttacttcct ctctctacct ttgttactca agaaaaagca
atgtattaga gatcgagttc 1380atgatgaatt attaaaaacc tttcctctgt
ttttgtatat atttcggttg gattttgaag 1440gaaacttctt tttttccttt
tttttttgtg tgtaattgca gagatgggtt tttcaaggaa 1500agagtacgag
ttcttgagcg agatcggatt gagttctggc aatttgggat gttttgtgaa
1560tggcacctgg aaaggaagtg gccctgtggt ttctactctt aatcctgcca
ataatcaggt 1620tctatttttt agaatgtttt gttttttcat gctggataag
atgggtttat tattaattgt 1680ggtgagactg atgggattgt ctttctgaga
aaataacata cattcaatct catcgtattt 1740ttccgacttg gatcctcctc
tataaatctt taaaataaac caatgttaag acttgcgctt 1800ttatggagtg
gcagtattct tggttttaat ttttagcttt tactcatatg ctgtaggttt
1860tcttttattt ttgtttctgc attttaaaga tattattaat gttttacaaa
cacacttgct 1920agttgctgag tactaaacta cttgatccag gcatttagaa
actattagtt tcttcactcc 1980tataatttta gtattattta aatgttgctt
ttgcagaaaa ttgccgaagt tagtgaggct 2040tccatccaag actatgagga
agggatgcaa gcttgcagtg aagcagcaaa gatttggatg 2100caggtaagat
ggacggatac catactctta aacttaatat ttcggttgga aatttacatt 2160tctt
216454086DNAGossypium hirsutum 5tctcgagaaa ggcatgattg aattgtaccg
catgtctaga catttctaaa agagaacatt 60tagaacctca ctttgccaca taccgaaatt
atctgtgcca tcgaagatct ccactactga 120tctcgcccac atggacgatg
ttggggctat tgatgttgta gatgtggatc attttggatt 180ttccaccatt
tcagctataa tatttcaata tgctaaagga aatctatgct gatgtggaag
240attagtttaa actgcaacca tagagcatac ttcgaataac cttcggctct
aatacccctt 300gttgtgcaaa tagggtaaaa aggagtaaat tattgtacta
gaaaaccaca ctaggtttag 360tttccaagaa agattagata ggtcacaaac
ggtcctattt aaaacccaac ctcctaaata 420gaatctccta caatgtaatt
taatagcaca atatatcact acaattatac tctataaaaa 480attgaaagat
aaataaaaat aataaagaac acccgaaagt tcacgatgtt cgacaaatta
540tgcctacgtc atcgaacact accaaatata ttcattataa aagatacaag
tgaagaataa 600aaaaagagaa aaaatttacc ttattaagaa aatgacaatt
ttggatgatt caaaggtgga 660gaaacaaccc tatttatagt agcataaatt
ctccacaatc ttgtatctca catctaaaac 720aaccctattc attgcatatt
gaattatagc caagggccaa ttgtgaatta accctttttt 780aaactacata
caattataaa acttataaaa ttacttaaac atatatttct cttttcgtct
840atttataaat aaacataaat aaaataggta aaaacttaaa taaatcagta
taaattttag 900ttacaataat ttaattatta aaaattaagt attattaatt
aagtatggta aataaatttt 960tacatttcat gtattataga atttattcat
aaatttaaaa agaaaagaaa aagaatttta 1020taaatttaat ataaatgatt
atattttaat aatttaattg ctaaaagtaa tttaaattag 1080tatagaaatt
attatatatt tattgcattt tataaaatta atgcaattaa ataatttcta
1140attaattata aatcttaaaa gtagtttaat tataaaacca attgttattt
agggtaatta 1200tttgatatat cattagtgga gttttttaga ctccacaagc
gggttgagca tgtgacaagt 1260gtcatattaa tttatgtaat tagctttttc
ttcttttact acatcctata atacactaat 1320caaatcctta attcacttgt
gaagtctaaa acactctatt ggcggtgtat caaataattt 1380ttctattatt
tgtatttatc tataaaaata aagattaaaa tatgccttag gtccctgtac
1440ttttcataaa tttgaaattt aatctctata tttttatttt caagaattta
gtccctctac 1500tttccagatt ttaaaattca agtccaattg ttaatgctat
taattttttg ttaaatttgt 1560tggtgtgaca ttttgaaata gaaaaaaaat
gctcacttga tagaaatgta actaaaaaaa 1620tatgttataa taaacttgca
tttaacagaa taatcttaaa agtgataaca attggacttg 1680aatttgaaat
ctaaaaagta ggaaactaaa ttcctaaaaa ttaaagtaca tagtctaact
1740tccaaattta ccacgagtac aaggagttaa gacatatttt aaccaaaaat
aaatgagtaa 1800tgaataagaa gaaaaagaac accaattcga aacttagaga
taacattatt gggaaggaca 1860acatcgaacc tcgaactata acatcaatga
aaaatataca cgattactat tcgtaaaaca 1920ttatagaagt ttcatggtta
gaggttgaga cgtaaatttt atttatttat tttacttggt 1980tggtgatcgc
ctcttgacgt accaactgta acacccctaa cccgtattac gtcgcctaaa
2040caaggttaag gagtattacc ggacaaatgg aatagaaaaa ccattcaaat
catacattaa 2100tacaaacata ctcaaatttc attcaaatac atccataatg
ttccttaatt gagccctaga 2160ggccctaaaa atattaaaga aacaattcgg
gactgaatcg aaaacatttg gaaaatttag 2220gaaaaagttg aaaaatttgg
tctgtagggg tcacatggcc gtgtcaacat tcgaaatagg 2280tacagacagt
cgtgtcttag cccgtgtcca tgccagtgta acttattgac ttgggtcaca
2340ggtctaagct tttcatccct tctttcgcca ttgcatgtgc gacagtgttt
cctcctctag 2400ctatgaattt aaaacaatag tgctaagaag ctttgagata
atgctttcac ataatttcat 2460aatagtaaaa gaggcaaaaa attaaatgaa
aagctaaaat aatttttttt ataaaattga 2520agggcaaaaa aaatcatcat
gcctaaatat aaaacatgtt ataaatactc ataagacgaa 2580aaagttaaaa
aattacaaag gaaaggacct gattggagca gtatgataat atagggactt
2640gtttaaaatg ttttaaagtt taggacttat ttagagtatc acccatgatt
tggtataata 2700aataaaaaat cagatgagag agccacctca tgaaaaagac
aagaacatta cgtgtgatcc 2760attgcagaag aggataaagt atggacaaaa
tttataaata taatcttgta catcccccat 2820acgtcacggc tcttttcaga
tcataggccg aaaaggcctc agtctgtctc agtcctctac 2880ttaaggtact
cttctctctc tccttcgaca tcaacttcaa catattactt tcgctctctt
2940cctttggtac tcaagaaaaa gcaaggtaat agagatcgag ttcatgatga
attattaaaa 3000acctttcctc tgtttttgta tatattttgg ttggattttg
aaggaaactt cttttttttc 3060cctttttgtg tgtgtgtgca attgcagaga
tgggtttttc aaggaaagag tacgagttct 3120tgagcgagat cggattgagt
tctggcaatt tgggatgttt tgtgaatggc acctggaaag 3180gaagtggccc
tgtggtttct actcttaatc ctgcccataa tcaggttcta tttttaagaa
3240tgttttgttt ttttatgcta gataagatgg gtttattatt aattgtggtg
agactgatag 3300aattgtcttt ctgagaaaat aacttacatt caatctcatc
gtatttttcc gacttggatc 3360ctcctctata aatctttaaa ataaaccaat
gttaagactt gctcttctat tgagtggcag 3420tattcttggt tttaattttt
agcttttact catatgctgc aggttttctc ttatttttgc 3480ttctgcattt
taaagataat agtaatgttt tacaaacaca cttgctagtt actgagtact
3540aaactacttg atccagtcat ttagaaacta ttagtttctt cactcctata
attttagtat 3600tatttcaatg ttgcttttgc agaaaattgc cgaagttagt
gaggcttcca tccaagacta 3660tgaggaaggg atgcaagctt gcagtgaagc
agcaaagatt tggatgcagg taagatggac 3720ggataccata ctcttaaact
ttatatttcg gttggaaatt tacatttctt aatgccattt 3780ttacttctct
gaacctttta tgctttattt attgtttcag gttccagccc ctaagagagg
3840tgacatagtt cgacaaatag gtgatgcatt gagatccaaa ctacagcagc
ttggccgcct 3900tgtttctctt gagatgggaa aaattcttcc cgaaggaatt
ggggaagttc aagtatgtta 3960tgcggccttg tcactgttat cacattggtc
tctttgcata taatatgtca taaggcagcc 4020catattgaac tacatgagcc
atttagcatt tggttgagtt agtttaaata ttttcttcaa 4080tcttca
408661009DNAGossypium hirsutum 6atccaaaaaa agcacttcag attattggtg
ataccaaatg gccaaaccct tcctgaccaa 60aacaatcgtg aagaatgtga caggagggga
gattgaggtt atctttgaat ggatagatga 120aatcctccat ccaaagagta
ggacctaata tgtagaaact catggcagtt gcatgagttg 180attgcatgag
ttgaaggtcc gatgctgtta aatctatggc tcttaaatgt gcatcttcta
240cctagaaaca atcttctaag cttttcatcc cttctttcgc cattgcatgc
gcgacggtat 300ttcctcctct ggttatgaag ttaaaacgat atgttaagaa
gtttcgagat aatgcattca 360tatcccaggt aatcggtcta atttcataat
agtaaaatag gcgaaaaatt aaatgaaaaa 420ctaaaataat tcttttataa
aattggaggg taaaaaaaat tattatgcct aaatataaca 480catgttataa
atactcataa gacgaaaaag ttaaaaaatt acaaaggaaa ggacctgatt
540ggagcaatat gataatatag ggacttgttt aaaatgtttt aaagtttagg
acttatttag 600agtatcaccc atgatttggt ataataaata aaaaatcaga
tgagagagcc acctcatgaa 660aaagacaaga acattacgtg tgatccattg
cagaagagga taaagtatgg acaaaattta 720tagatataat cttgtacatc
ccccatacgt cacggctctg ttcagatcat aggccgaaaa 780ggcctccgtc
tgtctcagtc ctctacttaa ggtactcttc tctctctcct tccacatcaa
840ctttaacatt ttacttcctc tctctacctt tgttactcaa gaaaaagcaa
tgtattagag 900atcgagttca tgatgaatta ttaaaaacct ttcctctgtt
tttgtatata tttcggttgg 960attttgaagg aaacgtcttt ttttcctttt
ttttttgtgt gtaattgca 100971022DNAGossypium hirsutum 7atggaataga
aaaaccattc aaatcataca ttaatacaaa catactcaaa tttcattcaa 60atacatccat
aatgttcctt aattgagccc tagaggccct aaaaatatta aagaaacaat
120tcgggactga atcgaaaaca tttggaaaat ttaggaaaaa gttgaaaaat
ttggtctgta 180ggggtcacat ggccgtgtca acattcgaaa taggtacaga
cagtcgtgtc ttagcccgtg 240tccatgccag tgtaacttat tgacttgggt
cacaggtcta agcttttcat cccttctttc 300gccattgcat gtgcgacagt
gtttcctcct ctagctatga atttaaaaca atagtgctaa 360gaagctttga
gataatgctt tcacataatt tcataatagt aaaagaggca aaaaattaaa
420tgaaaagcta aaataatttt ttttataaaa ttgaagggca aaaaaatcat
catgcctaaa 480tataaaacat gttataaata ctcataagac gaaaaagtta
aaaaattaca aaggaaagga 540cctgattgga gcagtatgat aatataggga
cttgtttaaa atgttttaaa gtttaggact 600tatttagagt atcacccatg
atttggtata ataaataaaa aatcagatga gagagccacc 660tcatgaaaaa
gacaagaaca ttacgtgtga tccattgcag aagaggataa agtatggaca
720aaatttataa atataatctt gtacatcccc catacgtcac ggctcttttc
agatcatagg 780ccgaaaaggc ctcagtctgt ctcagtcctc tacttaaggt
actcttctct ctctccttcg 840acatcaactt caacatatta ctttcgctct
cttcctttgg tactcaagaa aaagcaaggt 900aatagagatc gagttcatga
tgaattatta aaaacctttc ctctgttttt gtatatattt 960tggttggatt
ttgaaggaaa cttctttttt ttcccttttt gtgtgtgtgt gcaattgcaa 1020cc
102284734DNAArtificial SequenceT-DNA Pbtg-26GhA0.6::GUS 8cggcaggata
tattcaattg taaatggctc catggcgatc gctctagagg atctgcgatc 60tagtaacata
gatgacaccg cgcgcgataa tttatcctag tttgcgcgct atattttgtt
120ttctatcgcg tattaaatgt ataattgcgg gactctaatc ataaaaaccc
atctcataaa 180taacgtcatg cattacatgt taattattac atgcttaacg
taattcaaca gaaattatat 240gataatcatc gcaagaccgg caacaggatt
caatcttaag aaactttatt gccaaatgtt 300tgaacgatct gcttcggatc
ctagaacgcg tgatctcaga tctcggtgac gggcaggacc 360ggacggggcg
gtaccggcag gctgaagtcc agctgccaga aacccacgtc atgccagttc
420ccgtgcttga agccggccgc ccgcagcatg ccgcgggggg catatccgag
cgcctcgtgc 480atgcgcacgc tcgggtcgtt gggcagcccg atgacagcga
ccacgctctt gaagccctgt 540gcctccaggg acttcagcag gtgggtgtag
agcgtggagc ccagtcccgt ccgctggtgg 600cggggggaga cgtacacggt
cgactcggcc gtccagtcgt aggcgttgcg tgccttccag 660gggcccgcgt
aggcgatgcc ggcgacctcg ccgtccacct cggcgacgag ccagggatag
720cgctcccgca gacggacgag gtcgtccgtc cactcctgcg gttcctgcgg
ctcggtacgg 780aagttgaccg tgcttgtctc gatgtagtgg ttgacgatgg
tgcagaccgc cggcatgtcc 840gcctcggtgg cacggcggat gtcggccggg
cgtcgttctg ggtccatggt tatagagaga 900gagatagatt tatagagaga
gactggtgat ttcagcgtgt cctctccaaa tgaaatgaac 960ttccttatat
agaggaaggg tcttgcgaag gatagtggga ttgtgcgtca tcccttacgt
1020cagtggagat gtcacatcaa tccacttgct ttgaagacgt ggttggaacg
tcttcttttt 1080ccacgatgct cctcgtgggt gggggtccat ctttgggacc
actgtcggca gaggcatctt 1140gaatgatagc ctttccttta tcgcaatgat
ggcatttgta ggagccacct tccttttcta 1200ctgtcctttc gatgaagtga
cagatagctg ggcaatggaa tccgaggagg tttcccgaaa 1260ttatcctttg
ttgaaaagtc tcaatagccc tttggtcttc tgagactgta tctttgacat
1320ttttggagta gaccagagtg tcgtgctcca ccatgttgac gaagattttc
ttcttgtcat 1380tgagtcgtaa aagactctgt atgaactgtt cgccagtctt
cacggcgagt tctgttagat 1440cctcgatttg aatcttagac tccatgcatg
gccttagatt cagtaggaac taccttttta 1500gagactccaa tctctattac
ttgccttggt ttatgaagca agccttgaat cgtccatact 1560ggaatagtac
ttctgatctt gagaaatatg tctttctctg tgttcttgat gcaattagtc
1620ctgaatcttt
tgactgcatc tttaaccttc ttgggaaggt atttgatctc ctggagattg
1680ttactcgggt agatcgtctt gatgagacct gctgcgtagg aacgcggccg
ctgtacaggg 1740cccgggcata tggcgcgtta gggataacag ggtaattacg
tattaattaa ggcgcgtcct 1800gcaggtcact ggattttggt tttaggaatt
agaaatttta ttgatagaag tattttacaa 1860atacaaatac atactaaggg
tttcttatat gctcaacaca tgagcgaaac cctataagaa 1920ccctaattcc
cttatctggg aactactcac acattattct ggagaaaaat agagagagat
1980agatttgtag agagagactg gtgatttttg cgccgggtac cgagctcggt
agcaattccc 2040gaggctgtag ccgacgatgg tgcgccagga gagttgttga
ttcattgttt gcctccctgc 2100tgcggttttt caccgaagtt catgccagtc
cagcgttttt gcagcagaaa agccgccgac 2160ttcggtttgc ggtcgcgagt
gaagatccct ttcttgttac cgccaacgcg caatatgcct 2220tgcgaggtcg
caaaatcggc gaaattccat acctgttcac cgacgacggc gctgacgcga
2280tcaaagacgc ggtgatacat atccagccat gcacactgat actcttcact
ccacatgtcg 2340gtgtacattg agtgcagccc ggctaacgta tccacgccgt
attcggtgat gataatcggc 2400tgatgcagtt tctcctgcca ggccagaagt
tctttttcca gtaccttctc tgccgtttcc 2460aaatcgccgc tttggacata
ccatccgtaa taacggttca ggcacagcac atcaaagaga 2520tcgctgatgg
tatcggtgtg agcgtcgcag aacattacat tgacgcaggt gatcggacgc
2580gtcgggtcga gtttacgcgt tgcttccgcc agtggcgcga aatattcccg
tgcaccttgc 2640ggacgggtat ccggttcgtt ggcaatactc cacatcacca
cgcttgggtg gtttttgtca 2700cgcgctatca gctctttaat cgcctgtaag
tgcgcttgct gagtttcccc gttgactgcc 2760tcttcgctgt acagttcttt
cggcttgttg cccgcttcga aaccaatgcc taaagagagg 2820ttaaagccga
cagcagcagt ttcatcaatc accacgatgc catgttcatc tgcccagtcg
2880agcatctctt cagcgtaagg gtaatgcgag gtacggtagg agttggcccc
aatccagtcc 2940attaatgcgt ggtcgtgcac catcagcacg ttatcgaatc
ctttgccacg caagtccgca 3000tcttcatgac gaccaaagcc agtaaagtag
aacggtttgt ggttaatcag gaactgttcg 3060cccttcactg ccactgaccg
gatgccgacg cgaagcgggt agatatcaca ctctgtctgg 3120cttttggctg
tgacgcacag ttcatagaga taaccttcac ccggttgcca gaggtgcgga
3180ttcaccactt gcaaagtccc gctagtgcct tgtccagttg caaccacctg
ttgatccgca 3240tcacgcagtt caacgctgac atcaccattg gccaccacct
gccagtcaac agacgcgtgg 3300ttacagtctt gcgcgacatg cgtcaccacg
gtgatatcgt ccacccaggt gttcggcgtg 3360gtgtagagca ttacgctgcg
atggattccg gcatagttaa agaaatcatg gaagtaagac 3420tgctttttct
tgccgttttc gtcggtaatc accattcccg gcgggatagt ctgccagttc
3480agttcgttgt tcacacaaac ggtgatacct gcacatcaac aaattttggt
catatattag 3540aaaagttata aattaaaata tacacactta taaactacag
aaaagcaatt gctatatact 3600acattctttt attttgaaaa aaatatttga
aatattatat tactactaat taatgataat 3660tattatatat atatcaaagg
tagaagcaga aacttacgta cacttttccc ggcaataaca 3720tacggcgtga
catcggcttc aaatggcgta tagccgccct gatgctccat cacttcctga
3780ttattgaccc acactttgcc gtaatgagtg accgcatcga aacgcagcac
gatacgctgg 3840cctgcccaac ctttcggtat aaagacttcg cgctgatacc
agacgttgcc cgcataatta 3900cgaatatctg catcggcgaa ctgatcgtta
aaactgcctg gcacagcaat tgcccggctt 3960tcttgtaacg cgctttccca
ccaacgctga tcaattccac agttttcgcg atccagactg 4020aatgcccaca
ggccgtcgag ttttttgatt tcacgggttg gggtttctac aggacggacc
4080atggttgcaa ttacacacaa aaaaaaaagg aaaaaaagac gtttccttca
aaatccaacc 4140gaaatatata caaaaacaga ggaaaggttt ttaataattc
atcatgaact cgatctctaa 4200tacattgctt tttcttgagt aacaaaggta
gagagaggaa gtaaaatgtt aaagttgatg 4260tggaaggaga gagagaagag
taccttaagt agaggactga gacagacgga ggccttttcg 4320gcctatgatc
tgaacagagc cgtgacgtat gggggatgta caagattata tctataaatt
4380ttgtccatac tttatcctct tctgcaatgg atcacacgta atgttcttgt
ctttttcatg 4440aggtggctct ctcatctgat tttttattta ttataccaaa
tcatgggtga tactctaaat 4500aagtcctaaa ctttaaaaca ttttaaacaa
gtccctatat tatcatattg ctccaatcag 4560gtcctttcct ttgtaatttt
ttaacttttt cgtcttatga gtatttataa catgtgttat 4620atttaggcat
aataattttt tttaccctcc gcggccgcag aattcgagct cgagttaagg
4680cgcgccgttg caggtcgacg gccgagtact ggcaggatat ataccgttgt aatt
473495177DNAArtificial SequenceT-DNA Pbtg-26GhA10::GUS 9cggcaggata
tattcaattg taaatggctc catggcgatc gctctagagg atctgcgatc 60tagtaacata
gatgacaccg cgcgcgataa tttatcctag tttgcgcgct atattttgtt
120ttctatcgcg tattaaatgt ataattgcgg gactctaatc ataaaaaccc
atctcataaa 180taacgtcatg cattacatgt taattattac atgcttaacg
taattcaaca gaaattatat 240gataatcatc gcaagaccgg caacaggatt
caatcttaag aaactttatt gccaaatgtt 300tgaacgatct gcttcggatc
ctagaacgcg tgatctcaga tctcggtgac gggcaggacc 360ggacggggcg
gtaccggcag gctgaagtcc agctgccaga aacccacgtc atgccagttc
420ccgtgcttga agccggccgc ccgcagcatg ccgcgggggg catatccgag
cgcctcgtgc 480atgcgcacgc tcgggtcgtt gggcagcccg atgacagcga
ccacgctctt gaagccctgt 540gcctccaggg acttcagcag gtgggtgtag
agcgtggagc ccagtcccgt ccgctggtgg 600cggggggaga cgtacacggt
cgactcggcc gtccagtcgt aggcgttgcg tgccttccag 660gggcccgcgt
aggcgatgcc ggcgacctcg ccgtccacct cggcgacgag ccagggatag
720cgctcccgca gacggacgag gtcgtccgtc cactcctgcg gttcctgcgg
ctcggtacgg 780aagttgaccg tgcttgtctc gatgtagtgg ttgacgatgg
tgcagaccgc cggcatgtcc 840gcctcggtgg cacggcggat gtcggccggg
cgtcgttctg ggtccatggt tatagagaga 900gagatagatt tatagagaga
gactggtgat ttcagcgtgt cctctccaaa tgaaatgaac 960ttccttatat
agaggaaggg tcttgcgaag gatagtggga ttgtgcgtca tcccttacgt
1020cagtggagat gtcacatcaa tccacttgct ttgaagacgt ggttggaacg
tcttcttttt 1080ccacgatgct cctcgtgggt gggggtccat ctttgggacc
actgtcggca gaggcatctt 1140gaatgatagc ctttccttta tcgcaatgat
ggcatttgta ggagccacct tccttttcta 1200ctgtcctttc gatgaagtga
cagatagctg ggcaatggaa tccgaggagg tttcccgaaa 1260ttatcctttg
ttgaaaagtc tcaatagccc tttggtcttc tgagactgta tctttgacat
1320ttttggagta gaccagagtg tcgtgctcca ccatgttgac gaagattttc
ttcttgtcat 1380tgagtcgtaa aagactctgt atgaactgtt cgccagtctt
cacggcgagt tctgttagat 1440cctcgatttg aatcttagac tccatgcatg
gccttagatt cagtaggaac taccttttta 1500gagactccaa tctctattac
ttgccttggt ttatgaagca agccttgaat cgtccatact 1560ggaatagtac
ttctgatctt gagaaatatg tctttctctg tgttcttgat gcaattagtc
1620ctgaatcttt tgactgcatc tttaaccttc ttgggaaggt atttgatctc
ctggagattg 1680ttactcgggt agatcgtctt gatgagacct gctgcgtagg
aacgcggccg ctgtacaggg 1740cccgggcata tggcgcgtta gggataacag
ggtaattacg tattaattaa ggcgcgtcct 1800gcaggtcact ggattttggt
tttaggaatt agaaatttta ttgatagaag tattttacaa 1860atacaaatac
atactaaggg tttcttatat gctcaacaca tgagcgaaac cctataagaa
1920ccctaattcc cttatctggg aactactcac acattattct ggagaaaaat
agagagagat 1980agatttgtag agagagactg gtgatttttg cgccgggtac
cgagctcggt agcaattccc 2040gaggctgtag ccgacgatgg tgcgccagga
gagttgttga ttcattgttt gcctccctgc 2100tgcggttttt caccgaagtt
catgccagtc cagcgttttt gcagcagaaa agccgccgac 2160ttcggtttgc
ggtcgcgagt gaagatccct ttcttgttac cgccaacgcg caatatgcct
2220tgcgaggtcg caaaatcggc gaaattccat acctgttcac cgacgacggc
gctgacgcga 2280tcaaagacgc ggtgatacat atccagccat gcacactgat
actcttcact ccacatgtcg 2340gtgtacattg agtgcagccc ggctaacgta
tccacgccgt attcggtgat gataatcggc 2400tgatgcagtt tctcctgcca
ggccagaagt tctttttcca gtaccttctc tgccgtttcc 2460aaatcgccgc
tttggacata ccatccgtaa taacggttca ggcacagcac atcaaagaga
2520tcgctgatgg tatcggtgtg agcgtcgcag aacattacat tgacgcaggt
gatcggacgc 2580gtcgggtcga gtttacgcgt tgcttccgcc agtggcgcga
aatattcccg tgcaccttgc 2640ggacgggtat ccggttcgtt ggcaatactc
cacatcacca cgcttgggtg gtttttgtca 2700cgcgctatca gctctttaat
cgcctgtaag tgcgcttgct gagtttcccc gttgactgcc 2760tcttcgctgt
acagttcttt cggcttgttg cccgcttcga aaccaatgcc taaagagagg
2820ttaaagccga cagcagcagt ttcatcaatc accacgatgc catgttcatc
tgcccagtcg 2880agcatctctt cagcgtaagg gtaatgcgag gtacggtagg
agttggcccc aatccagtcc 2940attaatgcgt ggtcgtgcac catcagcacg
ttatcgaatc ctttgccacg caagtccgca 3000tcttcatgac gaccaaagcc
agtaaagtag aacggtttgt ggttaatcag gaactgttcg 3060cccttcactg
ccactgaccg gatgccgacg cgaagcgggt agatatcaca ctctgtctgg
3120cttttggctg tgacgcacag ttcatagaga taaccttcac ccggttgcca
gaggtgcgga 3180ttcaccactt gcaaagtccc gctagtgcct tgtccagttg
caaccacctg ttgatccgca 3240tcacgcagtt caacgctgac atcaccattg
gccaccacct gccagtcaac agacgcgtgg 3300ttacagtctt gcgcgacatg
cgtcaccacg gtgatatcgt ccacccaggt gttcggcgtg 3360gtgtagagca
ttacgctgcg atggattccg gcatagttaa agaaatcatg gaagtaagac
3420tgctttttct tgccgttttc gtcggtaatc accattcccg gcgggatagt
ctgccagttc 3480agttcgttgt tcacacaaac ggtgatacct gcacatcaac
aaattttggt catatattag 3540aaaagttata aattaaaata tacacactta
taaactacag aaaagcaatt gctatatact 3600acattctttt attttgaaaa
aaatatttga aatattatat tactactaat taatgataat 3660tattatatat
atatcaaagg tagaagcaga aacttacgta cacttttccc ggcaataaca
3720tacggcgtga catcggcttc aaatggcgta tagccgccct gatgctccat
cacttcctga 3780ttattgaccc acactttgcc gtaatgagtg accgcatcga
aacgcagcac gatacgctgg 3840cctgcccaac ctttcggtat aaagacttcg
cgctgatacc agacgttgcc cgcataatta 3900cgaatatctg catcggcgaa
ctgatcgtta aaactgcctg gcacagcaat tgcccggctt 3960tcttgtaacg
cgctttccca ccaacgctga tcaattccac agttttcgcg atccagactg
4020aatgcccaca ggccgtcgag ttttttgatt tcacgggttg gggtttctac
aggacggacc 4080atggttgcaa ttacacacaa aaaaaaaagg aaaaaaagac
gtttccttca aaatccaacc 4140gaaatatata caaaaacaga ggaaaggttt
ttaataattc atcatgaact cgatctctaa 4200tacattgctt tttcttgagt
aacaaaggta gagagaggaa gtaaaatgtt aaagttgatg 4260tggaaggaga
gagagaagag taccttaagt agaggactga gacagacgga ggccttttcg
4320gcctatgatc tgaacagagc cgtgacgtat gggggatgta caagattata
tctataaatt 4380ttgtccatac tttatcctct tctgcaatgg atcacacgta
atgttcttgt ctttttcatg 4440aggtggctct ctcatctgat tttttattta
ttataccaaa tcatgggtga tactctaaat 4500aagtcctaaa ctttaaaaca
ttttaaacaa gtccctatat tatcatattg ctccaatcag 4560gtcctttcct
ttgtaatttt ttaacttttt cgtcttatga gtatttataa catgtgttat
4620atttaggcat aataattttt tttaccctcc aattttataa aagaattatt
ttagtttttc 4680atttaatttt tcgcctattt tactattatg aaattagacc
gattacctgg gatatgaatg 4740cattatctcg aaacttctta acatatcgtt
ttaacttcat aaccagagga ggaaataccg 4800tcgcgcatgc aatggcgaaa
gaagggatga aaagcttaga agattgtttc taggtagaag 4860atgcacattt
aagagccata gatttaacag catcggacct tcaactcatg caatcaactc
4920atgcaactgc catgagtttc tacatattag gtcctactct ttggatggag
gatttcatct 4980atccattcaa agataacctc aatctcccct cctgtcacat
tcttcacgat tgttttggtc 5040aggaagggtt tggccatttg gtatcaccaa
taatctgaag tgcttttttt ggatgcggcc 5100gcgaattcga gctcgagtta
aggcgcgccg ttgcaggtcg acggccgagt actggcagga 5160tatataccgt tgtaatt
5177104737DNAArtificial SequenceT-DNA Pbtg-26GhD0.6::GUS
10cggcaggata tattcaattg taaatggctc catggcgatc gctctagagg atctgcgatc
60tagtaacata gatgacaccg cgcgcgataa tttatcctag tttgcgcgct atattttgtt
120ttctatcgcg tattaaatgt ataattgcgg gactctaatc ataaaaaccc
atctcataaa 180taacgtcatg cattacatgt taattattac atgcttaacg
taattcaaca gaaattatat 240gataatcatc gcaagaccgg caacaggatt
caatcttaag aaactttatt gccaaatgtt 300tgaacgatct gcttcggatc
ctagaacgcg tgatctcaga tctcggtgac gggcaggacc 360ggacggggcg
gtaccggcag gctgaagtcc agctgccaga aacccacgtc atgccagttc
420ccgtgcttga agccggccgc ccgcagcatg ccgcgggggg catatccgag
cgcctcgtgc 480atgcgcacgc tcgggtcgtt gggcagcccg atgacagcga
ccacgctctt gaagccctgt 540gcctccaggg acttcagcag gtgggtgtag
agcgtggagc ccagtcccgt ccgctggtgg 600cggggggaga cgtacacggt
cgactcggcc gtccagtcgt aggcgttgcg tgccttccag 660gggcccgcgt
aggcgatgcc ggcgacctcg ccgtccacct cggcgacgag ccagggatag
720cgctcccgca gacggacgag gtcgtccgtc cactcctgcg gttcctgcgg
ctcggtacgg 780aagttgaccg tgcttgtctc gatgtagtgg ttgacgatgg
tgcagaccgc cggcatgtcc 840gcctcggtgg cacggcggat gtcggccggg
cgtcgttctg ggtccatggt tatagagaga 900gagatagatt tatagagaga
gactggtgat ttcagcgtgt cctctccaaa tgaaatgaac 960ttccttatat
agaggaaggg tcttgcgaag gatagtggga ttgtgcgtca tcccttacgt
1020cagtggagat gtcacatcaa tccacttgct ttgaagacgt ggttggaacg
tcttcttttt 1080ccacgatgct cctcgtgggt gggggtccat ctttgggacc
actgtcggca gaggcatctt 1140gaatgatagc ctttccttta tcgcaatgat
ggcatttgta ggagccacct tccttttcta 1200ctgtcctttc gatgaagtga
cagatagctg ggcaatggaa tccgaggagg tttcccgaaa 1260ttatcctttg
ttgaaaagtc tcaatagccc tttggtcttc tgagactgta tctttgacat
1320ttttggagta gaccagagtg tcgtgctcca ccatgttgac gaagattttc
ttcttgtcat 1380tgagtcgtaa aagactctgt atgaactgtt cgccagtctt
cacggcgagt tctgttagat 1440cctcgatttg aatcttagac tccatgcatg
gccttagatt cagtaggaac taccttttta 1500gagactccaa tctctattac
ttgccttggt ttatgaagca agccttgaat cgtccatact 1560ggaatagtac
ttctgatctt gagaaatatg tctttctctg tgttcttgat gcaattagtc
1620ctgaatcttt tgactgcatc tttaaccttc ttgggaaggt atttgatctc
ctggagattg 1680ttactcgggt agatcgtctt gatgagacct gctgcgtagg
aacgcggccg ctgtacaggg 1740cccgggcata tggcgcgtta gggataacag
ggtaattacg tattaattaa ggcgcgtcct 1800gcaggtcact ggattttggt
tttaggaatt agaaatttta ttgatagaag tattttacaa 1860atacaaatac
atactaaggg tttcttatat gctcaacaca tgagcgaaac cctataagaa
1920ccctaattcc cttatctggg aactactcac acattattct ggagaaaaat
agagagagat 1980agatttgtag agagagactg gtgatttttg cgccgggtac
cgagctcggt agcaattccc 2040gaggctgtag ccgacgatgg tgcgccagga
gagttgttga ttcattgttt gcctccctgc 2100tgcggttttt caccgaagtt
catgccagtc cagcgttttt gcagcagaaa agccgccgac 2160ttcggtttgc
ggtcgcgagt gaagatccct ttcttgttac cgccaacgcg caatatgcct
2220tgcgaggtcg caaaatcggc gaaattccat acctgttcac cgacgacggc
gctgacgcga 2280tcaaagacgc ggtgatacat atccagccat gcacactgat
actcttcact ccacatgtcg 2340gtgtacattg agtgcagccc ggctaacgta
tccacgccgt attcggtgat gataatcggc 2400tgatgcagtt tctcctgcca
ggccagaagt tctttttcca gtaccttctc tgccgtttcc 2460aaatcgccgc
tttggacata ccatccgtaa taacggttca ggcacagcac atcaaagaga
2520tcgctgatgg tatcggtgtg agcgtcgcag aacattacat tgacgcaggt
gatcggacgc 2580gtcgggtcga gtttacgcgt tgcttccgcc agtggcgcga
aatattcccg tgcaccttgc 2640ggacgggtat ccggttcgtt ggcaatactc
cacatcacca cgcttgggtg gtttttgtca 2700cgcgctatca gctctttaat
cgcctgtaag tgcgcttgct gagtttcccc gttgactgcc 2760tcttcgctgt
acagttcttt cggcttgttg cccgcttcga aaccaatgcc taaagagagg
2820ttaaagccga cagcagcagt ttcatcaatc accacgatgc catgttcatc
tgcccagtcg 2880agcatctctt cagcgtaagg gtaatgcgag gtacggtagg
agttggcccc aatccagtcc 2940attaatgcgt ggtcgtgcac catcagcacg
ttatcgaatc ctttgccacg caagtccgca 3000tcttcatgac gaccaaagcc
agtaaagtag aacggtttgt ggttaatcag gaactgttcg 3060cccttcactg
ccactgaccg gatgccgacg cgaagcgggt agatatcaca ctctgtctgg
3120cttttggctg tgacgcacag ttcatagaga taaccttcac ccggttgcca
gaggtgcgga 3180ttcaccactt gcaaagtccc gctagtgcct tgtccagttg
caaccacctg ttgatccgca 3240tcacgcagtt caacgctgac atcaccattg
gccaccacct gccagtcaac agacgcgtgg 3300ttacagtctt gcgcgacatg
cgtcaccacg gtgatatcgt ccacccaggt gttcggcgtg 3360gtgtagagca
ttacgctgcg atggattccg gcatagttaa agaaatcatg gaagtaagac
3420tgctttttct tgccgttttc gtcggtaatc accattcccg gcgggatagt
ctgccagttc 3480agttcgttgt tcacacaaac ggtgatacct gcacatcaac
aaattttggt catatattag 3540aaaagttata aattaaaata tacacactta
taaactacag aaaagcaatt gctatatact 3600acattctttt attttgaaaa
aaatatttga aatattatat tactactaat taatgataat 3660tattatatat
atatcaaagg tagaagcaga aacttacgta cacttttccc ggcaataaca
3720tacggcgtga catcggcttc aaatggcgta tagccgccct gatgctccat
cacttcctga 3780ttattgaccc acactttgcc gtaatgagtg accgcatcga
aacgcagcac gatacgctgg 3840cctgcccaac ctttcggtat aaagacttcg
cgctgatacc agacgttgcc cgcataatta 3900cgaatatctg catcggcgaa
ctgatcgtta aaactgcctg gcacagcaat tgcccggctt 3960tcttgtaacg
cgctttccca ccaacgctga tcaattccac agttttcgcg atccagactg
4020aatgcccaca ggccgtcgag ttttttgatt tcacgggttg gggtttctac
aggacggacc 4080atggttgcaa ttgcacacac acacaaaaag ggaaaaaaaa
gaagtttcct tcaaaatcca 4140accaaaatat atacaaaaac agaggaaagg
tttttaataa ttcatcatga actcgatctc 4200tattaccttg ctttttcttg
agtaccaaag gaagagagcg aaagtaatat gttgaagttg 4260atgtcgaagg
agagagagaa gagtacctta agtagaggac tgagacagac tgaggccttt
4320tcggcctatg atctgaaaag agccgtgacg tatgggggat gtacaagatt
atatttataa 4380attttgtcca tactttatcc tcttctgcaa tggatcacac
gtaatgttct tgtctttttc 4440atgaggtggc tctctcatct gattttttat
ttattatacc aaatcatggg tgatactcta 4500aataagtcct aaactttaaa
acattttaaa caagtcccta tattatcata ctgctccaat 4560caggtccttt
cctttgtaat tttttaactt tttcgtctta tgagtattta taacatgttt
4620tatatttagg catgatgatt tttttgccct tcaagcggcc gcgaattcga
gctcgagtta 4680aggcgcgccg ttgcaggtcg acggccgagt actggcagga
tatataccgt tgtaatt 4737115187DNAArtificial SequenceT-DNA
Pbtg-26GhD10::GUS 11cggcaggata tattcaattg taaatggctc catggcgatc
gctctagagg atctgcgatc 60tagtaacata gatgacaccg cgcgcgataa tttatcctag
tttgcgcgct atattttgtt 120ttctatcgcg tattaaatgt ataattgcgg
gactctaatc ataaaaaccc atctcataaa 180taacgtcatg cattacatgt
taattattac atgcttaacg taattcaaca gaaattatat 240gataatcatc
gcaagaccgg caacaggatt caatcttaag aaactttatt gccaaatgtt
300tgaacgatct gcttcggatc ctagaacgcg tgatctcaga tctcggtgac
gggcaggacc 360ggacggggcg gtaccggcag gctgaagtcc agctgccaga
aacccacgtc atgccagttc 420ccgtgcttga agccggccgc ccgcagcatg
ccgcgggggg catatccgag cgcctcgtgc 480atgcgcacgc tcgggtcgtt
gggcagcccg atgacagcga ccacgctctt gaagccctgt 540gcctccaggg
acttcagcag gtgggtgtag agcgtggagc ccagtcccgt ccgctggtgg
600cggggggaga cgtacacggt cgactcggcc gtccagtcgt aggcgttgcg
tgccttccag 660gggcccgcgt aggcgatgcc ggcgacctcg ccgtccacct
cggcgacgag ccagggatag 720cgctcccgca gacggacgag gtcgtccgtc
cactcctgcg gttcctgcgg ctcggtacgg 780aagttgaccg tgcttgtctc
gatgtagtgg ttgacgatgg tgcagaccgc cggcatgtcc 840gcctcggtgg
cacggcggat gtcggccggg cgtcgttctg ggtccatggt tatagagaga
900gagatagatt tatagagaga gactggtgat ttcagcgtgt cctctccaaa
tgaaatgaac 960ttccttatat agaggaaggg tcttgcgaag gatagtggga
ttgtgcgtca tcccttacgt 1020cagtggagat gtcacatcaa tccacttgct
ttgaagacgt ggttggaacg tcttcttttt 1080ccacgatgct cctcgtgggt
gggggtccat ctttgggacc actgtcggca gaggcatctt 1140gaatgatagc
ctttccttta tcgcaatgat ggcatttgta ggagccacct tccttttcta
1200ctgtcctttc gatgaagtga cagatagctg ggcaatggaa tccgaggagg
tttcccgaaa 1260ttatcctttg ttgaaaagtc tcaatagccc tttggtcttc
tgagactgta tctttgacat 1320ttttggagta gaccagagtg tcgtgctcca
ccatgttgac gaagattttc ttcttgtcat 1380tgagtcgtaa aagactctgt
atgaactgtt cgccagtctt cacggcgagt tctgttagat 1440cctcgatttg
aatcttagac tccatgcatg gccttagatt cagtaggaac taccttttta
1500gagactccaa tctctattac ttgccttggt ttatgaagca agccttgaat
cgtccatact 1560ggaatagtac ttctgatctt gagaaatatg tctttctctg
tgttcttgat gcaattagtc 1620ctgaatcttt tgactgcatc tttaaccttc
ttgggaaggt atttgatctc ctggagattg 1680ttactcgggt agatcgtctt
gatgagacct gctgcgtagg aacgcggccg ctgtacaggg 1740cccgggcata
tggcgcgtta gggataacag ggtaattacg tattaattaa ggcgcgtcct
1800gcaggtcact
ggattttggt tttaggaatt agaaatttta ttgatagaag tattttacaa
1860atacaaatac atactaaggg tttcttatat gctcaacaca tgagcgaaac
cctataagaa 1920ccctaattcc cttatctggg aactactcac acattattct
ggagaaaaat agagagagat 1980agatttgtag agagagactg gtgatttttg
cgccgggtac cgagctcggt agcaattccc 2040gaggctgtag ccgacgatgg
tgcgccagga gagttgttga ttcattgttt gcctccctgc 2100tgcggttttt
caccgaagtt catgccagtc cagcgttttt gcagcagaaa agccgccgac
2160ttcggtttgc ggtcgcgagt gaagatccct ttcttgttac cgccaacgcg
caatatgcct 2220tgcgaggtcg caaaatcggc gaaattccat acctgttcac
cgacgacggc gctgacgcga 2280tcaaagacgc ggtgatacat atccagccat
gcacactgat actcttcact ccacatgtcg 2340gtgtacattg agtgcagccc
ggctaacgta tccacgccgt attcggtgat gataatcggc 2400tgatgcagtt
tctcctgcca ggccagaagt tctttttcca gtaccttctc tgccgtttcc
2460aaatcgccgc tttggacata ccatccgtaa taacggttca ggcacagcac
atcaaagaga 2520tcgctgatgg tatcggtgtg agcgtcgcag aacattacat
tgacgcaggt gatcggacgc 2580gtcgggtcga gtttacgcgt tgcttccgcc
agtggcgcga aatattcccg tgcaccttgc 2640ggacgggtat ccggttcgtt
ggcaatactc cacatcacca cgcttgggtg gtttttgtca 2700cgcgctatca
gctctttaat cgcctgtaag tgcgcttgct gagtttcccc gttgactgcc
2760tcttcgctgt acagttcttt cggcttgttg cccgcttcga aaccaatgcc
taaagagagg 2820ttaaagccga cagcagcagt ttcatcaatc accacgatgc
catgttcatc tgcccagtcg 2880agcatctctt cagcgtaagg gtaatgcgag
gtacggtagg agttggcccc aatccagtcc 2940attaatgcgt ggtcgtgcac
catcagcacg ttatcgaatc ctttgccacg caagtccgca 3000tcttcatgac
gaccaaagcc agtaaagtag aacggtttgt ggttaatcag gaactgttcg
3060cccttcactg ccactgaccg gatgccgacg cgaagcgggt agatatcaca
ctctgtctgg 3120cttttggctg tgacgcacag ttcatagaga taaccttcac
ccggttgcca gaggtgcgga 3180ttcaccactt gcaaagtccc gctagtgcct
tgtccagttg caaccacctg ttgatccgca 3240tcacgcagtt caacgctgac
atcaccattg gccaccacct gccagtcaac agacgcgtgg 3300ttacagtctt
gcgcgacatg cgtcaccacg gtgatatcgt ccacccaggt gttcggcgtg
3360gtgtagagca ttacgctgcg atggattccg gcatagttaa agaaatcatg
gaagtaagac 3420tgctttttct tgccgttttc gtcggtaatc accattcccg
gcgggatagt ctgccagttc 3480agttcgttgt tcacacaaac ggtgatacct
gcacatcaac aaattttggt catatattag 3540aaaagttata aattaaaata
tacacactta taaactacag aaaagcaatt gctatatact 3600acattctttt
attttgaaaa aaatatttga aatattatat tactactaat taatgataat
3660tattatatat atatcaaagg tagaagcaga aacttacgta cacttttccc
ggcaataaca 3720tacggcgtga catcggcttc aaatggcgta tagccgccct
gatgctccat cacttcctga 3780ttattgaccc acactttgcc gtaatgagtg
accgcatcga aacgcagcac gatacgctgg 3840cctgcccaac ctttcggtat
aaagacttcg cgctgatacc agacgttgcc cgcataatta 3900cgaatatctg
catcggcgaa ctgatcgtta aaactgcctg gcacagcaat tgcccggctt
3960tcttgtaacg cgctttccca ccaacgctga tcaattccac agttttcgcg
atccagactg 4020aatgcccaca ggccgtcgag ttttttgatt tcacgggttg
gggtttctac aggacggacc 4080atggttgcaa ttgcacacac acacaaaaag
ggaaaaaaaa gaagtttcct tcaaaatcca 4140accaaaatat atacaaaaac
agaggaaagg tttttaataa ttcatcatga actcgatctc 4200tattaccttg
ctttttcttg agtaccaaag gaagagagcg aaagtaatat gttgaagttg
4260atgtcgaagg agagagagaa gagtacctta agtagaggac tgagacagac
tgaggccttt 4320tcggcctatg atctgaaaag agccgtgacg tatgggggat
gtacaagatt atatttataa 4380attttgtcca tactttatcc tcttctgcaa
tggatcacac gtaatgttct tgtctttttc 4440atgaggtggc tctctcatct
gattttttat ttattatacc aaatcatggg tgatactcta 4500aataagtcct
aaactttaaa acattttaaa caagtcccta tattatcata ctgctccaat
4560caggtccttt cctttgtaat tttttaactt tttcgtctta tgagtattta
taacatgttt 4620tatatttagg catgatgatt tttttgccct tcaattttat
aaaaaaaatt attttagctt 4680ttcatttaat tttttgcctc ttttactatt
atgaaattat gtgaaagcat tatctcaaag 4740cttcttagca ctattgtttt
aaattcatag ctagaggagg aaacactgtc gcacatgcaa 4800tggcgaaaga
agggatgaaa agcttagacc tgtgacccaa gtcaataagt tacactggca
4860tggacacggg ctaagacacg actgtctgta cctatttcga atgttgacac
ggccatgtga 4920cccctacaga ccaaattttt caactttttc ctaaattttc
caaatgtttt cgattcagtc 4980ccgaattgtt tctttaatat ttttagggcc
tctagggctc aattaaggaa cattatggat 5040gtatttgaat gaaatttgag
tatgtttgta ttaatgtatg atttgaatgg tttttctatt 5100ccatgcggcc
gcgaattcga gctcgagtta aggcgcgccg ttgcaggtcg acggccgagt
5160actggcagga tatataccgt tgtaatt 518712954DNABrassica juncea
12atggcgactc ttaaggtttc ttcttctgtt ccttctccct ctgaagatgc tgagcaattg
60aaaagcgcat ttgatggatg gggtaccaac gaggaattga tcatatcaat cttggctcac
120agaagtgctg aacagaggaa gctgatcagg caaacatacc atgaatcctt
tggagaggat 180cttcttaaga gtcttgagaa ggaacttaca agcgacttcg
agagagccat cttgctctgg 240actcttgaac cgggtgaacg tgatgcctta
ttggttaatg aagctaccaa aagatggact 300tcaagcaacc aagtgcttat
ggaagtagct tgcactagga cctctacgca gcttcttcac 360gctaggcaag
cttaccacgc tcgcttcaag aagtctattg aagaggatgt cgctcaccac
420accaccggtg acttcagaaa gcttttggtt tctcttgtta gctcatacag
gtacgaaggg 480gaagaggtaa acatgacatt ggcaaagcaa gaggctaagc
tgattcatga gaaaatcaag 540gacaagcatt acaatgatga agatttcata
aggattttgt ccacaaggag caaagcacag 600atcaatgcta ccttcaatcg
ctatcaagat aatcacggcg aggaaatcct caagagcctt 660gaggaaggag
atgaagacga caagttccta gggctgttga ggtcaaccat tcaatgcttg
720acaagacctg agctttactt tgtggatgtt cttcgttcag cgatcaacaa
aacgggaaca 780gacgaaggag ctctcactag aattgtgacc acaagagctg
agattgactt gaaagtcatt 840ggacaagagt accaaagaag gaacagcatt
ccattggaga aagccattac caaagacact 900cgtggagatt acgagaagat
gctcatcgca cttctcggtg aagatgatgc ttaa 95413317PRTBrassica juncea
13Met Ala Thr Leu Lys Val Ser Ser Ser Val Pro Ser Pro Ser Glu Asp1
5 10 15Ala Glu Gln Leu Lys Ser Ala Phe Asp Gly Trp Gly Thr Asn Glu
Glu 20 25 30Leu Ile Ile Ser Ile Leu Ala His Arg Ser Ala Glu Gln Arg
Lys Leu 35 40 45Ile Arg Gln Thr Tyr His Glu Ser Phe Gly Glu Asp Leu
Leu Lys Ser 50 55 60Leu Glu Lys Glu Leu Thr Ser Asp Phe Glu Arg Ala
Ile Leu Leu Trp65 70 75 80Thr Leu Glu Pro Gly Glu Arg Asp Ala Leu
Leu Val Asn Glu Ala Thr 85 90 95Lys Arg Trp Thr Ser Ser Asn Gln Val
Leu Met Glu Val Ala Cys Thr 100 105 110Arg Thr Ser Thr Gln Leu Leu
His Ala Arg Gln Ala Tyr His Ala Arg 115 120 125Phe Lys Lys Ser Ile
Glu Glu Asp Val Ala His His Thr Thr Gly Asp 130 135 140Phe Arg Lys
Leu Leu Val Ser Leu Val Ser Ser Tyr Arg Tyr Glu Gly145 150 155
160Glu Glu Val Asn Met Thr Leu Ala Lys Gln Glu Ala Lys Leu Ile His
165 170 175Glu Lys Ile Lys Asp Lys His Tyr Asn Asp Glu Asp Phe Ile
Arg Ile 180 185 190Leu Ser Thr Arg Ser Lys Ala Gln Ile Asn Ala Thr
Phe Asn Arg Tyr 195 200 205Gln Asp Asn His Gly Glu Glu Ile Leu Lys
Ser Leu Glu Glu Gly Asp 210 215 220Glu Asp Asp Lys Phe Leu Gly Leu
Leu Arg Ser Thr Ile Gln Cys Leu225 230 235 240Thr Arg Pro Glu Leu
Tyr Phe Val Asp Val Leu Arg Ser Ala Ile Asn 245 250 255Lys Thr Gly
Thr Asp Glu Gly Ala Leu Thr Arg Ile Val Thr Thr Arg 260 265 270Ala
Glu Ile Asp Leu Lys Val Ile Gly Gln Glu Tyr Gln Arg Arg Asn 275 280
285Ser Ile Pro Leu Glu Lys Ala Ile Thr Lys Asp Thr Arg Gly Asp Tyr
290 295 300Glu Lys Met Leu Ile Ala Leu Leu Gly Glu Asp Asp Ala305
310 31514951DNAGossypium hirsutum 14atggccactc ttacagtgcc
cacgacagtt ccttcagtgt ctgaagattg tgaacaacta 60agaaaagcct tttcaggatg
gggaactaat gagggcttaa tcatagatat attgggtcac 120agaaatgcgg
agcaacgaaa cttgattcga aaaacctacg ctgaaaccta tggagaggat
180ctcctcaagg cactagacaa ggagctctcg aatgactttg agaggctggt
tctgctttgg 240gctcttgatc ctgctgaacg tgatgccctt ttggctaatg
aagccaccaa aaggtggact 300tcaagaaatc aagtccttat ggaaatagcc
tgcacaaggt ctgccaacca actgcttcac 360gcaaggcagg cttatcatgc
tcgttataag aagtcgcttg aagaggacgt tgctcatcac 420acgactgggg
acttccgtaa gctcctccta cctctagtga gttcatacag atatgaggga
480gaggaggtga acatgactct ggcaaaaaca gaggcgaagt tgcttcatga
gaaaatttca 540aacaaagctt acagtgatga cgatgtcata agggttttgg
ctacaagaag caaggcacag 600atcaatgcaa ctctgaatca ctacaaaaat
gaatatggaa atgacataaa caaggacttg 660aaggctgacc ctaaggatga
gttccttgca ctactaaggt ccacagtgaa gtgcttggtc 720tatccggaaa
agtattttga gaaggttctt cgcctagcaa tcaatagacg aggaacggat
780gaaggagctc ttacaagagt tgtttgcact agggctgagg ttgatctaaa
gatcatagca 840gatgagtatc agcgaaggaa cagtgtccca ctgactcgtg
ccattgtcaa ggacactcat 900ggagactatg aaaaattgct gctggtactt
gcaggacatg tggagaattg a 95115316PRTGossypium hirsutum 15Met Ala Thr
Leu Thr Val Pro Thr Thr Val Pro Ser Val Ser Glu Asp1 5 10 15Cys Glu
Gln Leu Arg Lys Ala Phe Ser Gly Trp Gly Thr Asn Glu Gly 20 25 30Leu
Ile Ile Asp Ile Leu Gly His Arg Asn Ala Glu Gln Arg Asn Leu 35 40
45Ile Arg Lys Thr Tyr Ala Glu Thr Tyr Gly Glu Asp Leu Leu Lys Ala
50 55 60Leu Asp Lys Glu Leu Ser Asn Asp Phe Glu Arg Leu Val Leu Leu
Trp65 70 75 80Ala Leu Asp Pro Ala Glu Arg Asp Ala Leu Leu Ala Asn
Glu Ala Thr 85 90 95Lys Arg Trp Thr Ser Arg Asn Gln Val Leu Met Glu
Ile Ala Cys Thr 100 105 110Arg Ser Ala Asn Gln Leu Leu His Ala Arg
Gln Ala Tyr His Ala Arg 115 120 125Tyr Lys Lys Ser Leu Glu Glu Asp
Val Ala His His Thr Thr Gly Asp 130 135 140Phe Arg Lys Leu Leu Leu
Pro Leu Val Ser Ser Tyr Arg Tyr Glu Gly145 150 155 160Glu Glu Val
Asn Met Thr Leu Ala Lys Thr Glu Ala Lys Leu Leu His 165 170 175Glu
Lys Ile Ser Asn Lys Ala Tyr Ser Asp Asp Asp Val Ile Arg Val 180 185
190Leu Ala Thr Arg Ser Lys Ala Gln Ile Asn Ala Thr Leu Asn His Tyr
195 200 205Lys Asn Glu Tyr Gly Asn Asp Ile Asn Lys Asp Leu Lys Ala
Asp Pro 210 215 220Lys Asp Glu Phe Leu Ala Leu Leu Arg Ser Thr Val
Lys Cys Leu Val225 230 235 240Tyr Pro Glu Lys Tyr Phe Glu Lys Val
Leu Arg Leu Ala Ile Asn Arg 245 250 255Arg Gly Thr Asp Glu Gly Ala
Leu Thr Arg Val Val Cys Thr Arg Ala 260 265 270Glu Val Asp Leu Lys
Ile Ile Ala Asp Glu Tyr Gln Arg Arg Asn Ser 275 280 285Val Pro Leu
Thr Arg Ala Ile Val Lys Asp Thr His Gly Asp Tyr Glu 290 295 300Lys
Leu Leu Leu Val Leu Ala Gly His Val Glu Asn305 310
31516954DNAArabidopsis thaliana 16atggcgactc ttaaggtttc tgattctgtt
cctgctcctt ctgatgatgc tgagcaattg 60agaaccgctt ttgaaggatg gggtacgaac
gaggacttga tcatatcaat cttggctcac 120agaagtgctg aacagaggaa
agtcatcagg caagcatacc acgaaaccta cggcgaagac 180cttctcaaga
ctcttgacaa ggagctctct aacgatttcg agagagctat cttgttgtgg
240actcttgaac ccggtgagcg tgatgcttta ttggctaatg aagctacaaa
aagatggact 300tcaagcaacc aagttcttat ggaagttgct tgcacaagga
catcaacgca gctgcttcac 360gctaggcaag cttaccatgc tcgctacaag
aagtctcttg aagaggacgt tgctcaccac 420actaccggtg acttcagaaa
gcttttggtt tctcttgtta cctcatacag gtacgaagga 480gatgaagtga
acatgacatt ggctaagcaa gaagctaagc tggtccatga gaaaatcaag
540gacaagcact acaatgatga ggatgttatt agaatcttgt ccacaagaag
caaagctcag 600atcaatgcta cttttaaccg ttaccaagat gatcatggcg
aggaaattct caagagtctt 660gaggaaggag atgatgatga caagttcctt
gcacttttga ggtcaaccat tcagtgcttg 720acaagaccag agctttactt
tgtcgatgtt cttcgttcag caatcaacaa aactggaact 780gatgaaggag
cactcactag aattgtgacc acaagagctg agattgactt gaaggtcatt
840ggagaggagt accagcgcag gaacagcatt cctttggaga aagctattac
caaagacact 900cgtggagatt acgagaagat gctcgtcgca cttctcggtg
aagatgatgc ttaa 95417317PRTArabidopsis thaliana 17Met Ala Thr Leu
Lys Val Ser Asp Ser Val Pro Ala Pro Ser Asp Asp1 5 10 15Ala Glu Gln
Leu Arg Thr Ala Phe Glu Gly Trp Gly Thr Asn Glu Asp 20 25 30Leu Ile
Ile Ser Ile Leu Ala His Arg Ser Ala Glu Gln Arg Lys Val 35 40 45Ile
Arg Gln Ala Tyr His Glu Thr Tyr Gly Glu Asp Leu Leu Lys Thr 50 55
60Leu Asp Lys Glu Leu Ser Asn Asp Phe Glu Arg Ala Ile Leu Leu Trp65
70 75 80Thr Leu Glu Pro Gly Glu Arg Asp Ala Leu Leu Ala Asn Glu Ala
Thr 85 90 95Lys Arg Trp Thr Ser Ser Asn Gln Val Leu Met Glu Val Ala
Cys Thr 100 105 110Arg Thr Ser Thr Gln Leu Leu His Ala Arg Gln Ala
Tyr His Ala Arg 115 120 125Tyr Lys Lys Ser Leu Glu Glu Asp Val Ala
His His Thr Thr Gly Asp 130 135 140Phe Arg Lys Leu Leu Val Ser Leu
Val Thr Ser Tyr Arg Tyr Glu Gly145 150 155 160Asp Glu Val Asn Met
Thr Leu Ala Lys Gln Glu Ala Lys Leu Val His 165 170 175Glu Lys Ile
Lys Asp Lys His Tyr Asn Asp Glu Asp Val Ile Arg Ile 180 185 190Leu
Ser Thr Arg Ser Lys Ala Gln Ile Asn Ala Thr Phe Asn Arg Tyr 195 200
205Gln Asp Asp His Gly Glu Glu Ile Leu Lys Ser Leu Glu Glu Gly Asp
210 215 220Asp Asp Asp Lys Phe Leu Ala Leu Leu Arg Ser Thr Ile Gln
Cys Leu225 230 235 240Thr Arg Pro Glu Leu Tyr Phe Val Asp Val Leu
Arg Ser Ala Ile Asn 245 250 255Lys Thr Gly Thr Asp Glu Gly Ala Leu
Thr Arg Ile Val Thr Thr Arg 260 265 270Ala Glu Ile Asp Leu Lys Val
Ile Gly Glu Glu Tyr Gln Arg Arg Asn 275 280 285Ser Ile Pro Leu Glu
Lys Ala Ile Thr Lys Asp Thr Arg Gly Asp Tyr 290 295 300Glu Lys Met
Leu Val Ala Leu Leu Gly Glu Asp Asp Ala305 310
315186217DNAArtificial SequenceT-DNA P35S::AnnBj1 18aattacaacg
gtatatatcc tgccagtact gggccccctc gagggcgatc gctacgtacc 60tgcaggcccg
ggttaattaa gcggccgcaa catggtggag cacgacactc tcgtctactc
120caagaatatc aaagatacag tctcagaaga ccaaagggct attgagactt
ttcaacaaag 180ggtaatatcg ggaaacctcc tcggattcca ttgcccagct
atctgtcact tcatcaaaag 240gacagtagaa aaggaaggtg gcacctacaa
atgccatcat tgcgataaag gaaaggctat 300cgttcaagat gcccctgccg
acagtggtcc caaagatgga cccccaccca cgaggagcat 360cgtggaaaaa
gaagacgttc caaccacgtc ttcaaagcaa gtggattgat gtgatatctc
420cactgacgta agggatgacg cacaatccca ctatccttcg caagaccctt
cctctatata 480aggaagttca tttcatttgg agaggactcg agctcatttc
tctattactt cagccataac 540aaaagaactc ttttctcttc ttattaaacc
aaaaccatgg cgactcttaa ggtttcttct 600tctgttcctt ctccctctga
agatgctgag caattgaaaa gcgcatttga tggatggggt 660accaacgagg
aattgatcat atcaatcttg gctcacagaa gtgctgaaca gaggaagctg
720atcaggcaaa cataccatga atcctttgga gaggatcttc ttaagagtct
tgagaaggaa 780cttacaagcg acttcgagag agccatcttg ctctggactc
ttgaaccggg tgaacgtgat 840gccttattgg ttaatgaagc taccaaaaga
tggacttcaa gcaaccaagt gcttatggaa 900gtagcttgca ctaggacctc
tacgcagctt cttcacgcta ggcaagctta ccacgctcgc 960ttcaagaagt
ctattgaaga ggatgtcgct caccacacca ccggtgactt cagaaagctt
1020ttggtttctc ttgttagctc atacaggtac gaaggggaag aggtaaacat
gacattggca 1080aagcaagagg ctaagctgat tcatgagaaa atcaaggaca
agcattacaa tgatgaagat 1140ttcataagga ttttgtccac aaggagcaaa
gcacagatca atgctacctt caatcgctat 1200caagataatc acggcgagga
aatcctcaag agccttgagg aaggagatga agacgacaag 1260ttcctagggc
tgttgaggtc aaccattcaa tgcttgacaa gacctgagct ttactttgtg
1320gatgttcttc gttcagcgat caacaaaacg ggaacagacg aaggagctct
cactagaatt 1380gtgaccacaa gagctgagat tgacttgaaa gtcattggac
aagagtacca aagaaggaac 1440agcattccat tggagaaagc cattaccaaa
gacactcgtg gagattacga gaagatgctc 1500atcgcacttc tcggtgaaga
tgatgcttaa ggcgcgcccc cgatccgcgt ttgtgttttc 1560tgggtttctc
acttaagcgt ctgcgtttta cttttgtatt gggtttggcg tttagtagtt
1620tgcggtagcg ttcttgttat gtgtaattac gctttttctt cttgcttcag
cagtttcggt 1680tgaaatataa atcgaatcaa gtttcacttt atcagcgttg
ttttaaattt tggcattaaa 1740ttggtgaaaa ttgcttcaat tttgtatcta
aatagaagag acaacatgaa attcgacttt 1800tgacctcaaa tcttcgaaca
tttatttcct gatttcacga tggatgagga taacgaaagg 1860gcggttccta
tgtccgggaa agttcccgta gaagacaatg agcaaagcta ctgaaacgcg
1920gacacgacgt cgcattggta cggatatgag ttaaaccgac tcaattcctt
tattaagaca 1980taaaccgatt ttggttaaag tgtaacagtg agctgatata
aaaccgaaac aaaccggtac 2040aagtttgatt gagcaacttg atgacaaact
tcagaatttt ggttattgaa tgaaaatcat 2100agtctaatcg taaaaaatgt
acagaagaaa agctagagca gaacaaagat tctatattct 2160ggttccaatt
tatcatcgct ttaacgtccc tcagatttga tcggggaatt cgatatcatt
2220accctgttat ccctaaagct tattaatgtt tgtcgaggag aaatatgagt
cgaggcatgg 2280atacactaag ttcccctgaa gtgagcatga tctttgatgc
tgagatgatt cccagagcaa 2340gatagtttgt gctgcaagtg acacaattgt
aatgaaacca ccactcaacg aatttacttg 2400tggctttgac atgtcgtgtg
ctctgtttgt atttgtgagt gccggttggt aattattttt 2460gttaatgtga
ttttaaaacc tcttatgtaa atagttactt tatctattga agtgtgttct
2520tgtggtctat agtttctcaa agggaaatta aaatgttgac atcccattta
caattgataa 2580cttggtatac acaaactttg taaatttggt gatatttatg
gtcgaaagaa ggcaataccc 2640attgtatgtt ccaatatcaa tatcaatacg
ataacttgat aatactaaca tatgattgtc 2700attgtttttc cagtatcaat
atacattaag ctactacaaa attagtataa atcactatat 2760tataaatctt
tttcggttgt aacttgtaat tcgtgggttt ttaaaataaa
agcatgtgaa 2820aattttcaaa taatgtgatg gcgcaatttt attttccgag
ttccaaaata ttgccgcttc 2880attaccctaa tttgtggcgc cacatgtaaa
acaaaagacg attcttagtg gctatcactg 2940ccatcacgcg gatcactaat
atgaaccgtc gattaaaaca gatcgacggt ttatacatca 3000ttttattgta
cacacggatc gatatctcag ccgttagatt taatatgcga tctgattgct
3060caaaaaatag actctccgtc tttgcctata aaaacaattt cacatctttc
tcacccaaat 3120ctactcttaa ccgttcttct tcttctacag acatcaattt
ctctcgactc tagaggatcc 3180aagcttatcg atttcgaacc cctcaggcga
agaacaggta tgatttgttt gtaattagat 3240caggggttta ggtctttcca
ttacttttta atgttttttc tgttactgtc tccgcgatct 3300gattttacga
caatagagtt tcgggttttg tcccattcca gtttgaaaat aaaggtccgt
3360cttttaagtt tgctggatcg ataaacctgt gaagattgag tctagtcgat
ttattggatg 3420atccattctt catcgttttt ttcttgcttc gaagttctgt
ataaccagat ttgtctgtgt 3480gcgattgtca ttacctagcc gtgtatcgag
aactagggtt ttcgagtcaa ttttgcccct 3540tttggttata tctggttcga
taacgattca tctggattag ggttttaagt ggtgacgttt 3600agtattccaa
tttcttcaaa atttagttat ggataatgaa aatccccaat tgactgttca
3660atttcttgtt aaatgcgcag atcacaatgg cttcgatctc ctcctcagtc
gcgaccgtta 3720gccggaccgc ccctgctcag gccaacatgg tggctccgtt
caccggcctt aagtccaacg 3780ccgccttccc caccaccaag aaggctaacg
acttctccac ccttcccagc aacggtggaa 3840gagttcaatg tatgcaggtg
tggccggcct acggcaacaa gaagttcgag acgctgtcgt 3900acctgccgcc
gctgtctatg gcgcccaccg tgatgatggc ctcgtcggcc accgccgtcg
3960ctccgttcca ggggctcaag tccaccgcca gcctccccgt cgcccgccgc
tcctccagaa 4020gcctcggcaa cgtcagcaac ggcggaagga tccggtgcat
ggccggcgcc gaggagatcg 4080tgctgcagcc catcaaggag atctccggca
ccgtcaagct gccggggtcc aagtcgcttt 4140ccaaccggat cctcctactc
gccgccctgt ccgaggggac aacagtggtt gataacctgc 4200tgaacagtga
ggatgtccac tacatgctcg gggccttgag gactcttggt ctctctgtcg
4260aagcggacaa agctgccaaa agagctgtag ttgttggctg tggtggaaag
ttcccagttg 4320aggatgctaa agaggaagtg cagctcttct tggggaatgc
tggaatcgca atgcggtcct 4380tgacagcagc tgttactgct gctggtggaa
atgcaactta cgtgcttgat ggagtaccaa 4440gaatgaggga gagacccatt
ggcgacttgg ttgtcggatt gaagcagctt ggtgcagatg 4500ttgattgttt
ccttggcact gactgcccac ctgttcgtgt caatggaatc ggagggctac
4560ctggtggcaa ggtcaagctg tctggctcca tcagcagtca gtacttgagt
gccttgctga 4620tggctgctcc tttggctctt ggggatgtgg agattgaaat
cattgataaa ttaatctcca 4680ttccgtacgt cgaaatgaca ttgagattga
tggagcgttt tggtgtgaaa gcagagcatt 4740ctgatagctg ggacagattc
tacattaagg gaggtcaaaa atacaagtcc cctaaaaatg 4800cctatgttga
aggtgatgcc tcaagcgcaa gctatttctt ggctggtgct gcaattactg
4860gagggactgt gactgtggaa ggttgtggca ccaccagttt gcagggtgat
gtgaagtttg 4920ctgaggtact ggagatgatg ggagcgaagg ttacatggac
cgagactagc gtaactgtta 4980ctggcccacc gcgggagcca tttgggagga
aacacctcaa ggcgattgat gtcaacatga 5040acaagatgcc tgatgtcgcc
atgactcttg ctgtggttgc cctctttgcc gatggcccga 5100cagccatcag
agacgtggct tcctggagag taaaggagac cgagaggatg gttgcgatcc
5160ggacggagct aaccaagctg ggagcatctg ttgaggaagg gccggactac
tgcatcatca 5220cgccgccgga gaagctgaac gtgacggcga tcgacacgta
cgacgaccac aggatggcga 5280tggctttctc ccttgccgcc tgtgccgagg
tccccgtcac catccgggac cctgggtgca 5340cccggaagac cttccccgac
tacttcgatg tgctgagcac tttcgtcaag aattaagctc 5400tagaactagt
ggatcccccg atccgcgttt gtgttttctg ggtttctcac ttaagcgtct
5460gcgttttact tttgtattgg gtttggcgtt tagtagtttg cggtagcgtt
cttgttatgt 5520gtaattacgc tttttcttct tgcttcagca gtttcggttg
aaatataaat cgaatcaagt 5580ttcactttat cagcgttgtt ttaaattttg
gcattaaatt ggtgaaaatt gcttcaattt 5640tgtatctaaa tagaagagac
aacatgaaat tcgacttttg acctcaaatc ttcgaacatt 5700tatttcctga
tttcacgatg gatgaggata acgaaagggc ggttcctatg tccgggaaag
5760ttcccgtaga agacaatgag caaagctact gaaacgcgga cacgacgtcg
cattggtacg 5820gatatgagtt aaaccgactc aattccttta ttaagacata
aaccgatttt ggttaaagtg 5880taacagtgag ctgatataaa accgaaacaa
accggtacaa gtttgattga gcaacttgat 5940gacaaacttc agaattttgg
ttattgaatg aaaatcatag tctaatcgta aaaaatgtac 6000agaagaaaag
ctagagcaga acaaagattc tatattctgg ttccaattta tcatcgcttt
6060aacgtccctc agatttgatc gggaaaccaa aacgtcgtga gacagtttgg
ttaactataa 6120cggtcctaag gtagcgatcg aggcattacg gcattacggc
actcgcgagg gtccgaattc 6180gagcatggag ccatttacaa ttgaatatat cctgccg
6217196751DNAArtificial SequenceT-DNA Pbtg-26GhD10::AnnBj1
19aattacaacg gtatatatcc tgccagtact gggccccctc gagggcgatc gctacgtacc
60tgcaggcccg ggttaattaa gcggccgcat ggaatagaaa aaccattcaa atcatacatt
120aatacaaaca tactcaaatt tcattcaaat acatccataa tgttccttaa
ttgagcccta 180gaggccctaa aaatattaaa gaaacaattc gggactgaat
cgaaaacatt tggaaaattt 240aggaaaaagt tgaaaaattt ggtctgtagg
ggtcacatgg ccgtgtcaac attcgaaata 300ggtacagaca gtcgtgtctt
agcccgtgtc catgccagtg taacttattg acttgggtca 360caggtctaag
cttttcatcc cttctttcgc cattgcatgt gcgacagtgt ttcctcctct
420agctatgaat ttaaaacaat agtgctaaga agctttgaga taatgctttc
acataatttc 480ataatagtaa aagaggcaaa aaattaaatg aaaagctaaa
ataatttttt ttataaaatt 540gaagggcaaa aaaatcatca tgcctaaata
taaaacatgt tataaatact cataagacga 600aaaagttaaa aaattacaaa
ggaaaggacc tgattggagc agtatgataa tatagggact 660tgtttaaaat
gttttaaagt ttaggactta tttagagtat cacccatgat ttggtataat
720aaataaaaaa tcagatgaga gagccacctc atgaaaaaga caagaacatt
acgtgtgatc 780cattgcagaa gaggataaag tatggacaaa atttataaat
ataatcttgt acatccccca 840tacgtcacgg ctcttttcag atcataggcc
gaaaaggcct cagtctgtct cagtcctcta 900cttaaggtac tcttctctct
ctccttcgac atcaacttca acatattact ttcgctctct 960tcctttggta
ctcaagaaaa agcaaggtaa tagagatcga gttcatgatg aattattaaa
1020aacctttcct ctgtttttgt atatattttg gttggatttt gaaggaaact
tctttttttt 1080ccctttttgt gtgtgtgtgc aattgcaacc atggcgactc
ttaaggtttc ttcttctgtt 1140ccttctccct ctgaagatgc tgagcaattg
aaaagcgcat ttgatggatg gggtaccaac 1200gaggaattga tcatatcaat
cttggctcac agaagtgctg aacagaggaa gctgatcagg 1260caaacatacc
atgaatcctt tggagaggat cttcttaaga gtcttgagaa ggaacttaca
1320agcgacttcg agagagccat cttgctctgg actcttgaac cgggtgaacg
tgatgcctta 1380ttggttaatg aagctaccaa aagatggact tcaagcaacc
aagtgcttat ggaagtagct 1440tgcactagga cctctacgca gcttcttcac
gctaggcaag cttaccacgc tcgcttcaag 1500aagtctattg aagaggatgt
cgctcaccac accaccggtg acttcagaaa gcttttggtt 1560tctcttgtta
gctcatacag gtacgaaggg gaagaggtaa acatgacatt ggcaaagcaa
1620gaggctaagc tgattcatga gaaaatcaag gacaagcatt acaatgatga
agatttcata 1680aggattttgt ccacaaggag caaagcacag atcaatgcta
ccttcaatcg ctatcaagat 1740aatcacggcg aggaaatcct caagagcctt
gaggaaggag atgaagacga caagttccta 1800gggctgttga ggtcaaccat
tcaatgcttg acaagacctg agctttactt tgtggatgtt 1860cttcgttcag
cgatcaacaa aacgggaaca gacgaaggag ctctcactag aattgtgacc
1920acaagagctg agattgactt gaaagtcatt ggacaagagt accaaagaag
gaacagcatt 1980ccattggaga aagccattac caaagacact cgtggagatt
acgagaagat gctcatcgca 2040cttctcggtg aagatgatgc ttaaggcgcg
cccccgatcc gcgtttgtgt tttctgggtt 2100tctcacttaa gcgtctgcgt
tttacttttg tattgggttt ggcgtttagt agtttgcggt 2160agcgttcttg
ttatgtgtaa ttacgctttt tcttcttgct tcagcagttt cggttgaaat
2220ataaatcgaa tcaagtttca ctttatcagc gttgttttaa attttggcat
taaattggtg 2280aaaattgctt caattttgta tctaaataga agagacaaca
tgaaattcga cttttgacct 2340caaatcttcg aacatttatt tcctgatttc
acgatggatg aggataacga aagggcggtt 2400cctatgtccg ggaaagttcc
cgtagaagac aatgagcaaa gctactgaaa cgcggacacg 2460acgtcgcatt
ggtacggata tgagttaaac cgactcaatt cctttattaa gacataaacc
2520gattttggtt aaagtgtaac agtgagctga tataaaaccg aaacaaaccg
gtacaagttt 2580gattgagcaa cttgatgaca aacttcagaa ttttggttat
tgaatgaaaa tcatagtcta 2640atcgtaaaaa atgtacagaa gaaaagctag
agcagaacaa agattctata ttctggttcc 2700aatttatcat cgctttaacg
tccctcagat ttgatcgggg aattcgatat cattaccctg 2760ttatccctaa
agcttattaa tgtttgtcga ggagaaatat gagtcgaggc atggatacac
2820taagttcccc tgaagtgagc atgatctttg atgctgagat gattcccaga
gcaagatagt 2880ttgtgctgca agtgacacaa ttgtaatgaa accaccactc
aacgaattta cttgtggctt 2940tgacatgtcg tgtgctctgt ttgtatttgt
gagtgccggt tggtaattat ttttgttaat 3000gtgattttaa aacctcttat
gtaaatagtt actttatcta ttgaagtgtg ttcttgtggt 3060ctatagtttc
tcaaagggaa attaaaatgt tgacatccca tttacaattg ataacttggt
3120atacacaaac tttgtaaatt tggtgatatt tatggtcgaa agaaggcaat
acccattgta 3180tgttccaata tcaatatcaa tacgataact tgataatact
aacatatgat tgtcattgtt 3240tttccagtat caatatacat taagctacta
caaaattagt ataaatcact atattataaa 3300tctttttcgg ttgtaacttg
taattcgtgg gtttttaaaa taaaagcatg tgaaaatttt 3360caaataatgt
gatggcgcaa ttttattttc cgagttccaa aatattgccg cttcattacc
3420ctaatttgtg gcgccacatg taaaacaaaa gacgattctt agtggctatc
actgccatca 3480cgcggatcac taatatgaac cgtcgattaa aacagatcga
cggtttatac atcattttat 3540tgtacacacg gatcgatatc tcagccgtta
gatttaatat gcgatctgat tgctcaaaaa 3600atagactctc cgtctttgcc
tataaaaaca atttcacatc tttctcaccc aaatctactc 3660ttaaccgttc
ttcttcttct acagacatca atttctctcg actctagagg atccaagctt
3720atcgatttcg aacccctcag gcgaagaaca ggtatgattt gtttgtaatt
agatcagggg 3780tttaggtctt tccattactt tttaatgttt tttctgttac
tgtctccgcg atctgatttt 3840acgacaatag agtttcgggt tttgtcccat
tccagtttga aaataaaggt ccgtctttta 3900agtttgctgg atcgataaac
ctgtgaagat tgagtctagt cgatttattg gatgatccat 3960tcttcatcgt
ttttttcttg cttcgaagtt ctgtataacc agatttgtct gtgtgcgatt
4020gtcattacct agccgtgtat cgagaactag ggttttcgag tcaattttgc
cccttttggt 4080tatatctggt tcgataacga ttcatctgga ttagggtttt
aagtggtgac gtttagtatt 4140ccaatttctt caaaatttag ttatggataa
tgaaaatccc caattgactg ttcaatttct 4200tgttaaatgc gcagatcaca
atggcttcga tctcctcctc agtcgcgacc gttagccgga 4260ccgcccctgc
tcaggccaac atggtggctc cgttcaccgg ccttaagtcc aacgccgcct
4320tccccaccac caagaaggct aacgacttct ccacccttcc cagcaacggt
ggaagagttc 4380aatgtatgca ggtgtggccg gcctacggca acaagaagtt
cgagacgctg tcgtacctgc 4440cgccgctgtc tatggcgccc accgtgatga
tggcctcgtc ggccaccgcc gtcgctccgt 4500tccaggggct caagtccacc
gccagcctcc ccgtcgcccg ccgctcctcc agaagcctcg 4560gcaacgtcag
caacggcgga aggatccggt gcatggccgg cgccgaggag atcgtgctgc
4620agcccatcaa ggagatctcc ggcaccgtca agctgccggg gtccaagtcg
ctttccaacc 4680ggatcctcct actcgccgcc ctgtccgagg ggacaacagt
ggttgataac ctgctgaaca 4740gtgaggatgt ccactacatg ctcggggcct
tgaggactct tggtctctct gtcgaagcgg 4800acaaagctgc caaaagagct
gtagttgttg gctgtggtgg aaagttccca gttgaggatg 4860ctaaagagga
agtgcagctc ttcttgggga atgctggaat cgcaatgcgg tccttgacag
4920cagctgttac tgctgctggt ggaaatgcaa cttacgtgct tgatggagta
ccaagaatga 4980gggagagacc cattggcgac ttggttgtcg gattgaagca
gcttggtgca gatgttgatt 5040gtttccttgg cactgactgc ccacctgttc
gtgtcaatgg aatcggaggg ctacctggtg 5100gcaaggtcaa gctgtctggc
tccatcagca gtcagtactt gagtgccttg ctgatggctg 5160ctcctttggc
tcttggggat gtggagattg aaatcattga taaattaatc tccattccgt
5220acgtcgaaat gacattgaga ttgatggagc gttttggtgt gaaagcagag
cattctgata 5280gctgggacag attctacatt aagggaggtc aaaaatacaa
gtcccctaaa aatgcctatg 5340ttgaaggtga tgcctcaagc gcaagctatt
tcttggctgg tgctgcaatt actggaggga 5400ctgtgactgt ggaaggttgt
ggcaccacca gtttgcaggg tgatgtgaag tttgctgagg 5460tactggagat
gatgggagcg aaggttacat ggaccgagac tagcgtaact gttactggcc
5520caccgcggga gccatttggg aggaaacacc tcaaggcgat tgatgtcaac
atgaacaaga 5580tgcctgatgt cgccatgact cttgctgtgg ttgccctctt
tgccgatggc ccgacagcca 5640tcagagacgt ggcttcctgg agagtaaagg
agaccgagag gatggttgcg atccggacgg 5700agctaaccaa gctgggagca
tctgttgagg aagggccgga ctactgcatc atcacgccgc 5760cggagaagct
gaacgtgacg gcgatcgaca cgtacgacga ccacaggatg gcgatggctt
5820tctcccttgc cgcctgtgcc gaggtccccg tcaccatccg ggaccctggg
tgcacccgga 5880agaccttccc cgactacttc gatgtgctga gcactttcgt
caagaattaa gctctagaac 5940tagtggatcc cccgatccgc gtttgtgttt
tctgggtttc tcacttaagc gtctgcgttt 6000tacttttgta ttgggtttgg
cgtttagtag tttgcggtag cgttcttgtt atgtgtaatt 6060acgctttttc
ttcttgcttc agcagtttcg gttgaaatat aaatcgaatc aagtttcact
6120ttatcagcgt tgttttaaat tttggcatta aattggtgaa aattgcttca
attttgtatc 6180taaatagaag agacaacatg aaattcgact tttgacctca
aatcttcgaa catttatttc 6240ctgatttcac gatggatgag gataacgaaa
gggcggttcc tatgtccggg aaagttcccg 6300tagaagacaa tgagcaaagc
tactgaaacg cggacacgac gtcgcattgg tacggatatg 6360agttaaaccg
actcaattcc tttattaaga cataaaccga ttttggttaa agtgtaacag
6420tgagctgata taaaaccgaa acaaaccggt acaagtttga ttgagcaact
tgatgacaaa 6480cttcagaatt ttggttattg aatgaaaatc atagtctaat
cgtaaaaaat gtacagaaga 6540aaagctagag cagaacaaag attctatatt
ctggttccaa tttatcatcg ctttaacgtc 6600cctcagattt gatcgggaaa
ccaaaacgtc gtgagacagt ttggttaact ataacggtcc 6660taaggtagcg
atcgaggcat tacggcatta cggcactcgc gagggtccga attcgagcat
6720ggagccattt acaattgaat atatcctgcc g 67512021DNAArtificial
Sequenceprimer 20tgaccacaag agctgagatt g 212122DNAArtificial
Sequenceprimer 21ctccacgagt gtctttggta at 222220DNAArtificial
Sequenceprimer 22gatccttgtg gaggagtgga 202320DNAArtificial
Sequenceprimer 23gcgaaacagt tcgacgagat 20244323DNABacillus
thuringiensis 24atgacaacaa taaatgaatt atatccggct gtaccttata
atgtactggc atatgctcca 60ccacttaatt tagctgattc gacaccatgg ggtcaaatag
ttgttgctga tgcaattaaa 120gaagcttggg ataattttca aaaatatggt
gtattagatt taacagctat aaatcaaggg 180tttgatgatg caaatacagg
ttcttttagt tatcaagctt taatacaaac tgttttgggt 240attataggta
caattggtat gacagttcct gtggctgctc catttgcagc tacagcgcct
300attattagtt tatttgtagg atttttttgg cctaaaaaag ataagggacc
acaattaatc 360gatataattg ataaagaaat taaaaaatta ttagataagg
aattaggaga gcaaaaacgt 420aatgatttag ttagtgcttt aaatgagatg
caagagggag caaatgagtt aagtgatatt 480atgactaatg cactttttga
aggtactata cagggaaatg ttgttactaa tgataaccct 540caaggtaaaa
ggcgaactcc taaagctcca acagttagtg attatgagaa tgtttattcg
600gcatattttg tggaacatgt ggattttaga aacaaaatat ctacgtttct
tactggttct 660tatgatctta tagcactccc attatatgca ttagcaaaaa
caatggagct ttcattgtat 720caatcattta ttaattttgc taataaatgg
atggattttg tatatacaaa agcaattaat 780gaatcagcaa ctgatgatat
gaaaagagat tatcaagcga gatacaatac tcaaaaaagt 840aatttagctg
tacaaaaaac acaattgatt aacaaaatta aagatggtac agatgctgtt
900atgaaagttt ttaaagatac caataattta ccttcaatag gtactaataa
attagcagta 960aatgctcgta ataagtatat tagggcctta caaataaatt
gtttagattt agttgctttg 1020tggcctggct tatatccaga tgaatatctt
ttaccattac aattagataa aacacgtgtt 1080gtattttctg atacaatggg
acctgatgaa acacatgatg gtcaaatgaa agttttaaat 1140atattagact
caactacaag ttataaccat caagatatag gaataagtac aactcaagat
1200gtaaattctt tattatttta tccaagaaaa gaactgttag aattagattt
tgctaaatat 1260atttcatcta gtagtcgttt ttgggtttat ggatttggct
taaaatattc agatgataac 1320ttttatagat atggtgataa cgatccaagc
agtgatttta aacctgcata taagtggttt 1380acgaaaaatt cccagttcga
aaaccttcct acttatggaa atcctactcc tattactaat 1440ttaaatgcta
aaactcaagt aacttcttat cttgatgcat taatatatta tatagacgga
1500ggaactaatc tatataataa tgcgattctt catgatacag ggggttatat
tccgggatat 1560ccaggtgtag aaggatatgg tatgagtaat aatgaacctt
tagcaggaca aaaattaaat 1620gctttatatc ctataaaagt ggaaaatgta
agtggttcac aaggaaaatt aggaacaata 1680gcagcttatg ttcctttaaa
tttacaacca gaaaatatta ttggtgatgc tgatccgaat 1740acaggttttc
cccttaatgt aattaaagga tttccatttg aaaaatatgg acctgattat
1800gagggacgag gaatttcggt tgtaaaagaa tggataaatg gtgcaaatgc
tgtaaaattg 1860tctccaggtc aatcagttgg ggtacaaatt aaaaatataa
caaaacaaaa ttatcaaatt 1920cgtactcgtt atgcaagtaa taacagtaat
caagtatatt ttaatgtaga tccaggtgga 1980tcaccattat ttgcacaatc
agtaacattt gaatctacaa caaatgttac aagtggccaa 2040caaggcgaaa
atggtagata tacattaaaa actatttttt ctggtaatga tctacttaca
2100gtagaaatcc ctgttggaaa tttttatgtg catgttacga ataaaggatc
ttctgatatc 2160tttttagatc gtcttgagtt ttctacagtt ccttcatatg
ttatatattc aggtgattat 2220gatgctacag gtacagatga tgtcttattg
tcagatccac atgagtattt ttatgatgtc 2280atagtgaatg gtactgctag
tcattctagt gcagctactt ctatgaattt gctcaataaa 2340ggaaccgtag
taagaagcat tgatattcca ggtcactcaa cgtcttattc tgtacagtat
2400tcagttccag aaggatttga tgaagttaga attctcagtt ctcttccgga
tattagtgga 2460actataagag tagaatctag taaaccacct gtatttaaga
atgatggtaa tagtggtgat 2520ggtggtaata ctgaatataa ttttaatttt
gatttatcag gattgcaaga tactgggctt 2580tattctggta aacttaaatc
tggtattcgt gtgcaaggta attacactta cacaggtgct 2640ccatctttaa
atctggttgt ttacagaaat aatagtgttg tatccacttt tccagtaggt
2700tctccttttg atatcactat aacaacagaa actgataagg ttatcctttc
attacaacct 2760caacatgggt tggcaacagt tactggtact ggcacaataa
caattcctaa tgataaatta 2820gcaattgttt atgataagtt atttaaatta
ccacatgatt tagaaaatat aagaatacaa 2880gtaaatgcat tattcatatc
gagtacacaa aatgaattag ctaaagaagt aaatgaccat 2940gatattgaag
aagttgcatt gaaagtagat gcattatcgg atgaagtatt tggaaaagag
3000aaaaaagaat tacgtaaact ggtcaatcaa gcgaaacgtt taagtaaagc
acgaaacctt 3060ctggtaggag gcaattttga taattgggaa gcttggtata
aaggaaaaga agttgcaaga 3120gtatctgatc atgaattatt gaagagtgat
catgtattat taccgcctcc aactatgtat 3180ccatcctata tatatcaaaa
agtagaagaa acaaaattaa agccaaatac tcgttatatg 3240atttctggtt
tcatcgcaca tgcggaagat ttagaaattg tggtttctcg ttatgggcaa
3300gaagtaagga aaatagtgca agttccatat ggagaagctt tcccattaac
atccaatgga 3360tcaatttgtt gtacaccaag ttttagacgt gatggaaaac
tatcagatcc acatttcttt 3420agttatagta ttgatgtagg tgaactggat
atgacggcag gtccaggtat tgaattggga 3480cttcgtattg tagatcgatt
aggaatggcc cgtgtaagta atttagaaat tcgtgaagat 3540cgttctttaa
cagcaaatga aatacgaaaa gtgcaacgta tggcaagaaa ttggagaacc
3600gaatatgaga aagaacgtgc agaagtaaca gcattaattg aacctgtatt
aaaccaaatc 3660aatgcgttat atgaaaatgg agattggaat ggttctattc
gttcagatat ttcgtactac 3720gatatagaat ctattgtatt accaacatta
ccaagattac gtcattggtt tgttcctgat 3780atgttaactg aacatggaaa
tatcatgaat cgattcgaag aagcattaaa tcgtgcttat 3840acacagctgg
aaggaaatac actattgcat aacggtcatt ttacaacaga tgcggtaaat
3900tggatgatac aaggagatgc acatcaggta atattagaag atggtagacg
tgtattacga 3960ttaccagact ggtcttcgag tgtatcccaa acaattgaaa
tcgagaaatt tgatccagat 4020aaagaataca acttagtatt tcatgcgcaa
ggagaaggaa cggttacgtt ggagcatgga 4080gaaaaaacaa aatatataga
aacgcataca catcattttg cgaattttac aacatcacaa 4140agtcaaggaa
ttacgtttga atcgaataag gtgaccgtgg aaatttcttc agaagatggg
4200gaattattgg tagatcatat cgcacttgtg gaagttccta tgtttaacaa
gaatcaaatg 4260gtcaatgaaa atagagatgt aaatataaat agcaatacaa
atatgaataa tagcaataat 4320caa 4323251024PRTBacillus
thuringiensis
25Met Thr Thr Ile Asn Glu Leu Tyr Pro Ala Val Pro Tyr Asn Val Leu1
5 10 15Ala Tyr Ala Pro Pro Leu Asn Leu Ala Asp Ser Thr Pro Trp Gly
Gln 20 25 30Ile Val Val Ala Asp Ala Ile Lys Glu Ala Trp Asp Asn Phe
Gln Lys 35 40 45Tyr Gly Val Leu Asp Leu Thr Ala Ile Asn Gln Gly Phe
Asp Asp Ala 50 55 60Asn Thr Gly Ser Phe Ser Tyr Gln Ala Leu Ile Gln
Thr Val Leu Gly65 70 75 80Ile Ile Gly Thr Ile Gly Met Thr Val Pro
Val Ala Ala Pro Phe Ala 85 90 95Ala Thr Ala Pro Ile Ile Ser Leu Phe
Val Gly Phe Phe Trp Pro Lys 100 105 110Lys Asp Lys Gly Pro Gln Leu
Ile Asp Ile Ile Asp Lys Glu Ile Lys 115 120 125Lys Leu Leu Asp Lys
Glu Leu Gly Glu Gln Lys Arg Asn Asp Leu Val 130 135 140Ser Ala Leu
Asn Glu Met Gln Glu Gly Ala Asn Glu Leu Ser Asp Ile145 150 155
160Met Thr Asn Ala Leu Phe Glu Gly Thr Ile Gln Gly Asn Val Val Thr
165 170 175Asn Asp Asn Pro Gln Gly Lys Arg Arg Thr Pro Lys Ala Pro
Thr Val 180 185 190Ser Asp Tyr Glu Asn Val Tyr Ser Ala Tyr Phe Val
Glu His Val Asp 195 200 205Phe Arg Asn Lys Ile Ser Thr Phe Leu Thr
Gly Ser Tyr Asp Leu Ile 210 215 220Ala Leu Pro Leu Tyr Ala Leu Ala
Lys Thr Met Glu Leu Ser Leu Tyr225 230 235 240Gln Ser Phe Ile Asn
Phe Ala Asn Lys Trp Met Asp Phe Val Tyr Thr 245 250 255Lys Ala Ile
Asn Glu Ser Ala Thr Asp Asp Met Lys Arg Asp Tyr Gln 260 265 270Ala
Arg Tyr Asn Thr Gln Lys Ser Asn Leu Ala Val Gln Lys Thr Gln 275 280
285Leu Ile Asn Lys Ile Lys Asp Gly Thr Asp Ala Val Met Lys Val Phe
290 295 300Lys Asp Thr Asn Asn Leu Pro Ser Ile Gly Thr Asn Lys Leu
Ala Val305 310 315 320Asn Ala Arg Asn Lys Tyr Ile Arg Ala Leu Gln
Ile Asn Cys Leu Asp 325 330 335Leu Val Ala Leu Trp Pro Gly Leu Tyr
Pro Asp Glu Tyr Leu Leu Pro 340 345 350Leu Gln Leu Asp Lys Thr Arg
Val Val Phe Ser Asp Thr Met Gly Pro 355 360 365Asp Glu Thr His Asp
Gly Gln Met Lys Val Leu Asn Ile Leu Asp Ser 370 375 380Thr Thr Ser
Tyr Asn His Gln Asp Ile Gly Ile Ser Thr Thr Gln Asp385 390 395
400Val Asn Ser Leu Leu Phe Tyr Pro Arg Lys Glu Leu Leu Glu Leu Asp
405 410 415Phe Ala Lys Tyr Ile Ser Ser Ser Ser Arg Phe Trp Val Tyr
Gly Phe 420 425 430Gly Leu Lys Tyr Ser Asp Asp Asn Phe Tyr Arg Tyr
Gly Asp Asn Asp 435 440 445Pro Ser Ser Asp Phe Lys Pro Ala Tyr Lys
Trp Phe Thr Lys Asn Ser 450 455 460Gln Phe Glu Asn Leu Pro Thr Tyr
Gly Asn Pro Thr Pro Ile Thr Asn465 470 475 480Leu Asn Ala Lys Thr
Gln Val Thr Ser Tyr Leu Asp Ala Leu Ile Tyr 485 490 495Tyr Ile Asp
Gly Gly Thr Asn Leu Tyr Asn Asn Ala Ile Leu His Asp 500 505 510Thr
Gly Gly Tyr Ile Pro Gly Tyr Pro Gly Val Glu Gly Tyr Gly Met 515 520
525Ser Asn Asn Glu Pro Leu Ala Gly Gln Lys Leu Asn Ala Leu Tyr Pro
530 535 540Ile Lys Val Glu Asn Val Ser Gly Ser Gln Gly Lys Leu Gly
Thr Ile545 550 555 560Ala Ala Tyr Val Pro Leu Asn Leu Gln Pro Glu
Asn Ile Ile Gly Asp 565 570 575Ala Asp Pro Asn Thr Gly Phe Pro Leu
Asn Val Ile Lys Gly Phe Pro 580 585 590Phe Glu Lys Tyr Gly Pro Asp
Tyr Glu Gly Arg Gly Ile Ser Val Val 595 600 605Lys Glu Trp Ile Asn
Gly Ala Asn Ala Val Lys Leu Ser Pro Gly Gln 610 615 620Ser Val Gly
Val Gln Ile Lys Asn Ile Thr Lys Gln Asn Tyr Gln Ile625 630 635
640Arg Thr Arg Tyr Ala Ser Asn Asn Ser Asn Gln Val Tyr Phe Asn Val
645 650 655Asp Pro Gly Gly Ser Pro Leu Phe Ala Gln Ser Val Thr Phe
Glu Ser 660 665 670Thr Thr Asn Val Thr Ser Gly Gln Gln Gly Glu Asn
Gly Arg Tyr Thr 675 680 685Leu Lys Thr Ile Phe Ser Gly Asn Asp Leu
Leu Thr Val Glu Ile Pro 690 695 700Val Gly Asn Phe Tyr Val His Val
Thr Asn Lys Gly Ser Ser Asp Ile705 710 715 720Phe Leu Asp Arg Leu
Glu Phe Ser Thr Val Pro Ser Tyr Val Ile Tyr 725 730 735Ser Gly Asp
Tyr Asp Ala Thr Gly Thr Asp Asp Val Leu Leu Ser Asp 740 745 750Pro
His Glu Tyr Phe Tyr Asp Val Ile Val Asn Gly Thr Ala Ser His 755 760
765Ser Ser Ala Ala Thr Ser Met Asn Leu Leu Asn Lys Gly Thr Val Val
770 775 780Arg Ser Ile Asp Ile Pro Gly His Ser Thr Ser Tyr Ser Val
Gln Tyr785 790 795 800Ser Val Pro Glu Gly Phe Asp Glu Val Arg Ile
Leu Ser Ser Leu Pro 805 810 815Asp Ile Ser Gly Thr Ile Arg Val Glu
Ser Ser Lys Pro Pro Val Phe 820 825 830Lys Asn Asp Gly Asn Ser Gly
Asp Gly Gly Asn Thr Glu Tyr Asn Phe 835 840 845Asn Phe Asp Leu Ser
Gly Leu Gln Asp Thr Gly Leu Tyr Ser Gly Lys 850 855 860Leu Lys Ser
Gly Ile Arg Val Gln Gly Asn Tyr Thr Tyr Thr Gly Ala865 870 875
880Pro Ser Leu Asn Leu Val Val Tyr Arg Asn Asn Ser Val Val Ser Thr
885 890 895Phe Pro Val Gly Ser Pro Phe Asp Ile Thr Ile Thr Thr Glu
Thr Asp 900 905 910Lys Val Ile Leu Ser Leu Gln Pro Gln His Gly Leu
Ala Thr Val Thr 915 920 925Gly Thr Gly Thr Ile Thr Ile Pro Asn Asp
Lys Leu Ala Ile Val Tyr 930 935 940Asp Lys Leu Phe Lys Leu Pro His
Asp Leu Glu Asn Ile Arg Ile Gln945 950 955 960Val Asn Ala Leu Phe
Ile Ser Ser Thr Gln Asn Glu Leu Ala Lys Glu 965 970 975Val Asn Asp
His Asp Ile Glu Glu Val Ala Leu Lys Val Asp Ala Leu 980 985 990Ser
Asp Glu Val Phe Gly Lys Glu Lys Lys Glu Leu Arg Lys Leu Val 995
1000 1005Asn Gln Ala Lys Arg Leu Ser Lys Ala Arg Asn Leu Leu Val
Gly 1010 1015 1020Gly261024DNABacillus thuringiensis 26atggattgta
atttacaatc acaacaaaat attccatata atgtattagc aataccagta 60tctaatgtta
attcgttgac tgatacagtt ggagatttaa aaaaagcatg ggaagaattt
120caaaaaactg gttctttttc attaacagct ttacaacaag gattttctgc
ttcacaagga 180ggaacattca attatttaac attactacaa tcaggaatat
cattagctgg ttcttttgtt 240cctggaggta cttttgtagc acctattatt
aatatggtta ttggttggtt atggccacat 300aaaaacaaaa atgcggatac
agaaaattta ataaatttaa ttgattcaga aattcaaaaa 360caattaaaca
aagctttatt agatgcagat agaaatgagt ggagctctta tttagaatct
420atatttgatt cttcaaataa cctaaatggt gcaattgtag atgcacagtg
gtcaggcact 480gtaaatacta caaatagaac actaagaaat ccaacagaat
cagattatac aaatgttgtt 540acaaatttta ttgcagcgga tggtgacatt
gcaaataatg aaaatcacat aatgaatggc 600aactttgacg tagctgcagc
accttatttt gttataggag caacagcacg ttttgcagca 660atgcaatctt
atattaaatt ttgtaatgct tggattgata aagttggatt gagtgacgca
720cagcttacta cacaaaaggc taatttagat cgcacgaaac aaaatatgcg
taatgcaatt 780cttaactata cacaacaagt tatgaaagtt tttaaagatt
ccaaaaatat gcctacaata 840ggtactaata aatttagtgt tgatacctat
aatgtatata ttaaaggaat gacattaaat 900gttttagata ttgtagcaat
atggccttca ttatatccag atgattatac ttcacaaaca 960gccttagaac
aaacacgtgt cactttttca aatatggttg gccaagaaga aggtacagat 1020ggaa
1024271024PRTBacillus thuringiensis 27Met Asp Cys Asn Leu Gln Ser
Gln Gln Asn Ile Pro Tyr Asn Val Leu1 5 10 15Ala Ile Pro Val Ser Asn
Val Asn Ser Leu Thr Asp Thr Val Gly Asp 20 25 30Leu Lys Lys Ala Trp
Glu Glu Phe Gln Lys Thr Gly Ser Phe Ser Leu 35 40 45Thr Ala Leu Gln
Gln Gly Phe Ser Ala Ser Gln Gly Gly Thr Phe Asn 50 55 60Tyr Leu Thr
Leu Leu Gln Ser Gly Ile Ser Leu Ala Gly Ser Phe Val65 70 75 80Pro
Gly Gly Thr Phe Val Ala Pro Ile Ile Asn Met Val Ile Gly Trp 85 90
95Leu Trp Pro His Lys Asn Lys Asn Ala Asp Thr Glu Asn Leu Ile Asn
100 105 110Leu Ile Asp Ser Glu Ile Gln Lys Gln Leu Asn Lys Ala Leu
Leu Asp 115 120 125Ala Asp Arg Asn Glu Trp Ser Ser Tyr Leu Glu Ser
Ile Phe Asp Ser 130 135 140Ser Asn Asn Leu Asn Gly Ala Ile Val Asp
Ala Gln Trp Ser Gly Thr145 150 155 160Val Asn Thr Thr Asn Arg Thr
Leu Arg Asn Pro Thr Glu Ser Asp Tyr 165 170 175Thr Asn Val Val Thr
Asn Phe Ile Ala Ala Asp Gly Asp Ile Ala Asn 180 185 190Asn Glu Asn
His Ile Met Asn Gly Asn Phe Asp Val Ala Ala Ala Pro 195 200 205Tyr
Phe Val Ile Gly Ala Thr Ala Arg Phe Ala Ala Met Gln Ser Tyr 210 215
220Ile Lys Phe Cys Asn Ala Trp Ile Asp Lys Val Gly Leu Ser Asp
Ala225 230 235 240Gln Leu Thr Thr Gln Lys Ala Asn Leu Asp Arg Thr
Lys Gln Asn Met 245 250 255Arg Asn Ala Ile Leu Asn Tyr Thr Gln Gln
Val Met Lys Val Phe Lys 260 265 270Asp Ser Lys Asn Met Pro Thr Ile
Gly Thr Asn Lys Phe Ser Val Asp 275 280 285Thr Tyr Asn Val Tyr Ile
Lys Gly Met Thr Leu Asn Val Leu Asp Ile 290 295 300Val Ala Ile Trp
Pro Ser Leu Tyr Pro Asp Asp Tyr Thr Ser Gln Thr305 310 315 320Ala
Leu Glu Gln Thr Arg Val Thr Phe Ser Asn Met Val Gly Gln Glu 325 330
335Glu Gly Thr Asp Gly Ser Leu Arg Ile Tyr Asn Thr Phe Asp Ser Phe
340 345 350Ser Tyr Gln His Ser Pro Ile Pro Asn Asn Asn Val Asn Leu
Ile Ser 355 360 365Tyr Tyr Asn Asp Glu Leu Gln Asn Leu Glu Leu Gly
Val Tyr Thr Pro 370 375 380Pro Lys Lys Gly Ser Gly Tyr Ser Tyr Pro
Tyr Gly Phe Val Leu Asn385 390 395 400Tyr Ala Asn Ser Lys Tyr Lys
Tyr Gly Asp Ser Asn Asp Pro Glu Ser 405 410 415Leu Gly Gly Leu Ser
Thr Leu Ser Ala Pro Ile Gln Gln Val Asn Ala 420 425 430Ala Thr Gln
Asn Ser Lys Tyr Leu Asp Gly Glu Ile Leu Asn Gly Ile 435 440 445Gly
Ala Ser Leu Pro Gly Tyr Cys Thr Thr Gly Cys Ser Pro Thr Glu 450 455
460Pro Pro Phe Ser Cys Thr Ser Thr Ala Asn Gly Tyr Lys Ala Ser
Cys465 470 475 480Asn Pro Ser Asp Thr Asn Gln Lys Ile Asn Ala Leu
Tyr Pro Phe Thr 485 490 495Gln Ala Asn Val Lys Gly Asn Thr Gly Lys
Leu Gly Val Leu Ala Ser 500 505 510Leu Val Ser Tyr Asp Leu Asn Pro
Lys Asn Val Phe Gly Glu Leu Asp 515 520 525Ser Asp Thr Asn Asn Val
Ile Leu Lys Gly Ile Pro Ala Glu Lys Gly 530 535 540Tyr Phe Pro Asn
Asn Ala Arg Pro Thr Val Val Lys Glu Trp Ile Asn545 550 555 560Gly
Ala Ser Ala Val Pro Leu Asp Ser Gly Asn Thr Leu Phe Met Thr 565 570
575Ala Thr Asn Leu Thr Ala Thr Gln Tyr Arg Ile Arg Ile Arg Tyr Ala
580 585 590Asn Pro Asn Ser Asn Thr Gln Ile Gly Val Arg Ile Thr Gln
Asn Gly 595 600 605Ser Leu Ile Ser Ser Ser Asn Leu Thr Leu Tyr Ser
Thr Thr Asp Met 610 615 620Asn Asn Thr Leu Pro Leu Asn Val Tyr Val
Ile Gly Glu Asn Gly Asn625 630 635 640Tyr Thr Leu Gln Asp Leu Tyr
Asn Thr Thr Asn Val Leu Ser Thr Gly 645 650 655Asp Ile Thr Leu Gln
Ile Thr Gly Gly Asp Gln Lys Ile Phe Ile Asp 660 665 670Arg Ile Glu
Phe Val Pro Thr Met Pro Val Pro Gly Asn Thr Asn Asn 675 680 685Asn
Asn Gly Asn Asn Asn Gly Asn Asn Asn Pro Pro His His Val Cys 690 695
700Ala Ile Ala Gly Thr Gln Gln Ser Cys Ser Gly Pro Pro Lys Phe
Glu705 710 715 720Gln Val Ser Asp Leu Glu Lys Ile Thr Thr Gln Val
Tyr Met Leu Phe 725 730 735Lys Ser Ser Pro Tyr Glu Glu Leu Ala Leu
Glu Val Ser Ser Tyr Gln 740 745 750Ile Ser Gln Val Ala Leu Lys Val
Met Ala Leu Ser Asp Glu Leu Phe 755 760 765Cys Glu Glu Lys Asn Val
Leu Arg Lys Leu Val Asn Lys Ala Lys Gln 770 775 780Leu Leu Glu Ala
Ser Asn Leu Leu Val Gly Gly Asn Phe Glu Thr Thr785 790 795 800Gln
Asn Trp Val Leu Gly Thr Asn Ala Tyr Ile Asn Tyr Asp Ser Phe 805 810
815Leu Phe Asn Gly Asn Tyr Leu Ser Leu Gln Pro Ala Ser Gly Phe Phe
820 825 830Thr Ser Tyr Ala Tyr Gln Lys Ile Asp Glu Ser Thr Leu Lys
Pro Tyr 835 840 845Thr Arg Tyr Lys Val Ser Gly Phe Ile Gly Gln Ser
Asn Gln Val Glu 850 855 860Leu Ile Ile Ser Arg Tyr Gly Lys Glu Ile
Asp Lys Ile Leu Asn Val865 870 875 880Pro Tyr Ala Gly Pro Leu Pro
Ile Thr Ala Asp Ala Ser Ile Thr Cys 885 890 895Cys Ala Pro Glu Ile
Gly Gln Cys Asp Gly Glu Gln Ser Asp Ser His 900 905 910Phe Phe Asn
Tyr Ser Ile Asp Val Gly Ala Leu His Pro Glu Leu Asn 915 920 925Pro
Gly Ile Glu Ile Gly Leu Lys Ile Val Gln Ser Asn Gly Tyr Ile 930 935
940Thr Ile Ser Asn Leu Glu Ile Ile Glu Glu Arg Pro Leu Thr Glu
Met945 950 955 960Glu Ile Gln Ala Val Asn Arg Lys Asn Gln Lys Trp
Glu Arg Glu Lys 965 970 975Leu Leu Glu Cys Ala Ser Ile Ser Glu Leu
Leu Gln Pro Ile Ile Asn 980 985 990Gln Ile Asp Ser Leu Phe Lys Asp
Gly Asn Trp Tyr Asn Asp Ile Leu 995 1000 1005Pro His Val Thr Tyr
Gln Asp Leu Lys Asn Ile Ile Ile Pro Glu 1010 1015
1020Leu283549DNABacillus thuringiensis 28atggattgta atttacgatc
gcaacaaaat attccatata atgtattagc aacacaagca 60tctaatctta gtcagtttac
tgatatagct gaaggtgtaa aaaaagcatg ggcagaattt 120caaaaaactg
gatctttttc attagaagct ttaaaacaag gatttaatgc agcacaggga
180ggaacattca attatttagc attactacaa tcaggaatat cattagctgg
ttcttttgtc 240cctggaggtt cttttgtagc acccattgtt aatatggtta
ttggttggtt atggccaaat 300aaaaacaaaa cagcggatac agaaaattta
ataaaattaa ttgatgaaga aattcaaaaa 360caattaaaca aagccttatt
agaacaagac aaaaacaatt ggacctcttt tttagaaagt 420atatttgatg
tttcaaatac agtgaataat gcaattatag atgcacagtg gtcaggtact
480gtagatgata caaatagaca actaaaaact ccaacaacat cagattataa
aaatgttgtt 540gaaaaatttg attcagcgga tactgcaatt ataactaatg
aaaatcaaat aatgaacggc 600aactttgacg tagctgcatc atcctatttt
gttataggag caacattacg tcttgcatta 660tttcaatctt atattaaatt
ttgtaatcat tggattgata cagttggatt tgattcagat 720aattataata
cacaaaaggc taatttagct cgtacaaaac aaactatgcg tactacaatt
780aatgattata cacaaaaaat tatgaaagtt tttaaaaatt ccgacaatat
gcctacaatg 840ggtactaata aatttagtgt tgatgcttat aatgcatata
ttaaaggaat aacattaaat 900gttttagata tagtatcaac gtggccctca
ttatatccaa atgattatac ttcacaaaca 960aagttagaac aaacacgtat
cattttttca aatatgattg gacaacaaga agctatagat 1020ggaaccgtaa
caatttacga tacttttgat tctgataatt ataaacataa accaatacct
1080aataataatg ttaatttact ttcttatttt actgatgaat tacaaaatat
acaactcgca 1140ctatatacag ctcctcctaa acacaaaagt gatactcggg
atagctatac gtatccttat 1200ggatttattt taaattacca aaatagcaaa
tataaatatg gcgataacga accagtgatt 1260tcaaacacaa tatctgcacc
tatacaacaa attaatgcag caactcaata cactcaatat 1320atagatggag
aaagtatcaa tggcattggc gcatatttac ctggttattg ttctacagat
1380tgttcagaaa taactcctcc ttttgcttgc
acttctaacg ataaaaataa gagctatgga 1440gcaagctgta atagcgtata
ttctagtcaa aaaatgaatg ctttatatcc ttttacacaa 1500actaatgtac
caggaaacca ggggaaatta ggagtactgg caagttatgt tccatatgat
1560ttaaatccta aaaatatatt tggtgaagta gatccagata caaataatat
tatcttaaaa 1620ggaattcctg cagaaaaagg ctatttttct aattatacgc
gacctactgt tgtaaaagaa 1680tggattaatg gtgcaaatgc tgtatcactt
tattcaggaa atactttatt tatagtcgct 1740acgaatataa cagctactca
atataaaatt agaatacgtt atgcaaatcc aaattcagat 1800actgaaatca
gtgtacaaat tacacaaaat aattctctat tacacagtga tacaataaca
1860tttcatagta ctactgattc aaatatgaat aataatttat cacaaaatgt
atatgttaca 1920ggggaaaatg gaaattatac acttctagat ttatatgata
ctactaatgt tttatcaaca 1980ggagatatta cattacaaat tacaggagga
agtcaagaaa tatttattga tcgaatagaa 2040tttattccta ctgcgcctgc
gcctgctcct actaacgaca ataacaatcc ccctttccac 2100ggttgtttaa
tagctggtga acaacaactt tgttctggac cacctaaatt tgaacaatta
2160agtgatttag aaaaaattac aacacaagta tatatgttat tcaaatcttc
ttcatatgaa 2220gaattagatc caaaagtttc tagctatcaa attaatcaag
tcgcattgaa agttatgtca 2280ctatctgatg aaatgttttg tgaagaaaaa
agattgttac gaaaattagt caataaagca 2340aaacagttag tagaagcacg
taacttacta gtaggtggaa gttttgatac acttcaaaat 2400tggttacttg
gaacaaatgc tactataaat tatgattcgt ttttatttaa tggaaattat
2460ttattcttac aaccagcaag tggatttttc tcatcttatg cttatcaaaa
aataaatgag 2520tcaaaattaa aatcatatac acgatataaa gtttctggat
tcattggaca aagtaatcaa 2580gtagaactta ttatttctcg ttatggaaaa
gaaattaata aaatattaaa tatttcatat 2640gcagggcctc ttcctattac
ttctaataca tcaacaactt gttgtgcacc aaatataggt 2700caatgtaatg
aagagcaatc taattctcat ttcttcagct atagcatcga tgtaggtgaa
2760ctttaccccg aattaaatcc tggcattgaa tttggtcttc gtattgtgga
accaaatagt 2820tatatgacaa ttagtaattt agaaattatt gaagaacgtt
cacttacaga aatggaaatt 2880caaacaatca aacgaaaaga tcaaaaatgg
aaaaaagaaa tacttcaaga gtgtgcaaat 2940attaacgaac ttttacaacc
aattatagat aaagtcgatt cattattcaa agatgccgac 3000tggtatggtc
agattcttcc tcatatcaca tatcaaaatc taaaaaatat tgtattacct
3060gaattaccta aattgagaca ttggtttata aacgatcttg caggtgaata
ttatgaaatt 3120gaacaaaaga tccagcaagc tctaaaacat gcatttagac
aattagacga aagaaattta 3180atccacaacg gtcactttac agctaactta
atagattggc aaacagaagg taatgcccaa 3240atgaaaatat tagaaaatgg
tgctctcgca gcacaactct tgtcttggga ttctagtatt 3300tcacaatctt
taaatatatt agactttgat gaggataaag catataaact tcgtgtatat
3360gctcaaggaa gcggaacaat ccaatttaaa aactgtgaag atgaaaccat
ccaatttaat 3420acaaactcat tcacatataa agaaaaaata ttctatttcg
atactccatc aattaactta 3480caaatacaat cagaaggttc taatttcgtt
ataagtagta tcgagctcat tgaattatca 3540gcagacgaa
3549291024PRTBacillus thuringiensis 29Met Asp Cys Asn Leu Arg Ser
Gln Gln Asn Ile Pro Tyr Asn Val Leu1 5 10 15Ala Thr Gln Ala Ser Asn
Leu Ser Gln Phe Thr Asp Ile Ala Glu Gly 20 25 30Val Lys Lys Ala Trp
Ala Glu Phe Gln Lys Thr Gly Ser Phe Ser Leu 35 40 45Glu Ala Leu Lys
Gln Gly Phe Asn Ala Ala Gln Gly Gly Thr Phe Asn 50 55 60Tyr Leu Ala
Leu Leu Gln Ser Gly Ile Ser Leu Ala Gly Ser Phe Val65 70 75 80Pro
Gly Gly Ser Phe Val Ala Pro Ile Val Asn Met Val Ile Gly Trp 85 90
95Leu Trp Pro Asn Lys Asn Lys Thr Ala Asp Thr Glu Asn Leu Ile Lys
100 105 110Leu Ile Asp Glu Glu Ile Gln Lys Gln Leu Asn Lys Ala Leu
Leu Glu 115 120 125Gln Asp Lys Asn Asn Trp Thr Ser Phe Leu Glu Ser
Ile Phe Asp Val 130 135 140Ser Asn Thr Val Asn Asn Ala Ile Ile Asp
Ala Gln Trp Ser Gly Thr145 150 155 160Val Asp Asp Thr Asn Arg Gln
Leu Lys Thr Pro Thr Thr Ser Asp Tyr 165 170 175Lys Asn Val Val Glu
Lys Phe Asp Ser Ala Asp Thr Ala Ile Ile Thr 180 185 190Asn Glu Asn
Gln Ile Met Asn Gly Asn Phe Asp Val Ala Ala Ser Ser 195 200 205Tyr
Phe Val Ile Gly Ala Thr Leu Arg Leu Ala Leu Phe Gln Ser Tyr 210 215
220Ile Lys Phe Cys Asn His Trp Ile Asp Thr Val Gly Phe Asp Ser
Asp225 230 235 240Asn Tyr Asn Thr Gln Lys Ala Asn Leu Ala Arg Thr
Lys Gln Thr Met 245 250 255Arg Thr Thr Ile Asn Asp Tyr Thr Gln Lys
Ile Met Lys Val Phe Lys 260 265 270Asn Ser Asp Asn Met Pro Thr Met
Gly Thr Asn Lys Phe Ser Val Asp 275 280 285Ala Tyr Asn Ala Tyr Ile
Lys Gly Ile Thr Leu Asn Val Leu Asp Ile 290 295 300Val Ser Thr Trp
Pro Ser Leu Tyr Pro Asn Asp Tyr Thr Ser Gln Thr305 310 315 320Lys
Leu Glu Gln Thr Arg Ile Ile Phe Ser Asn Met Ile Gly Gln Gln 325 330
335Glu Ala Ile Asp Gly Thr Val Thr Ile Tyr Asp Thr Phe Asp Ser Asp
340 345 350Asn Tyr Lys His Lys Pro Ile Pro Asn Asn Asn Val Asn Leu
Leu Ser 355 360 365Tyr Phe Thr Asp Glu Leu Gln Asn Ile Gln Leu Ala
Leu Tyr Thr Ala 370 375 380Pro Pro Lys His Lys Ser Asp Thr Arg Asp
Ser Tyr Thr Tyr Pro Tyr385 390 395 400Gly Phe Ile Leu Asn Tyr Gln
Asn Ser Lys Tyr Lys Tyr Gly Asp Asn 405 410 415Glu Pro Val Ile Ser
Asn Thr Ile Ser Ala Pro Ile Gln Gln Ile Asn 420 425 430Ala Ala Thr
Gln Tyr Thr Gln Tyr Ile Asp Gly Glu Ser Ile Asn Gly 435 440 445Ile
Gly Ala Tyr Leu Pro Gly Tyr Cys Ser Thr Asp Cys Ser Glu Ile 450 455
460Thr Pro Pro Phe Ala Cys Thr Ser Asn Asp Lys Asn Lys Ser Tyr
Gly465 470 475 480Ala Ser Cys Asn Ser Val Tyr Ser Ser Gln Lys Met
Asn Ala Leu Tyr 485 490 495Pro Phe Thr Gln Thr Asn Val Pro Gly Asn
Gln Gly Lys Leu Gly Val 500 505 510Leu Ala Ser Tyr Val Pro Tyr Asp
Leu Asn Pro Lys Asn Ile Phe Gly 515 520 525Glu Val Asp Pro Asp Thr
Asn Asn Ile Ile Leu Lys Gly Ile Pro Ala 530 535 540Glu Lys Gly Tyr
Phe Ser Asn Tyr Thr Arg Pro Thr Val Val Lys Glu545 550 555 560Trp
Ile Asn Gly Ala Asn Ala Val Ser Leu Tyr Ser Gly Asn Thr Leu 565 570
575Phe Ile Val Ala Thr Asn Ile Thr Ala Thr Gln Tyr Lys Ile Arg Ile
580 585 590Arg Tyr Ala Asn Pro Asn Ser Asp Thr Glu Ile Ser Val Gln
Ile Thr 595 600 605Gln Asn Asn Ser Leu Leu His Ser Asp Thr Ile Thr
Phe His Ser Thr 610 615 620Thr Asp Ser Asn Met Asn Asn Asn Leu Ser
Gln Asn Val Tyr Val Thr625 630 635 640Gly Glu Asn Gly Asn Tyr Thr
Leu Leu Asp Leu Tyr Asp Thr Thr Asn 645 650 655Val Leu Ser Thr Gly
Asp Ile Thr Leu Gln Ile Thr Gly Gly Ser Gln 660 665 670Glu Ile Phe
Ile Asp Arg Ile Glu Phe Ile Pro Thr Ala Pro Ala Pro 675 680 685Ala
Pro Thr Asn Asp Asn Asn Asn Pro Pro Phe His Gly Cys Leu Ile 690 695
700Ala Gly Glu Gln Gln Leu Cys Ser Gly Pro Pro Lys Phe Glu Gln
Leu705 710 715 720Ser Asp Leu Glu Lys Ile Thr Thr Gln Val Tyr Met
Leu Phe Lys Ser 725 730 735Ser Ser Tyr Glu Glu Leu Asp Pro Lys Val
Ser Ser Tyr Gln Ile Asn 740 745 750Gln Val Ala Leu Lys Val Met Ser
Leu Ser Asp Glu Met Phe Cys Glu 755 760 765Glu Lys Arg Leu Leu Arg
Lys Leu Val Asn Lys Ala Lys Gln Leu Val 770 775 780Glu Ala Arg Asn
Leu Leu Val Gly Gly Ser Phe Asp Thr Leu Gln Asn785 790 795 800Trp
Leu Leu Gly Thr Asn Ala Thr Ile Asn Tyr Asp Ser Phe Leu Phe 805 810
815Asn Gly Asn Tyr Leu Phe Leu Gln Pro Ala Ser Gly Phe Phe Ser Ser
820 825 830Tyr Ala Tyr Gln Lys Ile Asn Glu Ser Lys Leu Lys Ser Tyr
Thr Arg 835 840 845Tyr Lys Val Ser Gly Phe Ile Gly Gln Ser Asn Gln
Val Glu Leu Ile 850 855 860Ile Ser Arg Tyr Gly Lys Glu Ile Asn Lys
Ile Leu Asn Ile Ser Tyr865 870 875 880Ala Gly Pro Leu Pro Ile Thr
Ser Asn Thr Ser Thr Thr Cys Cys Ala 885 890 895Pro Asn Ile Gly Gln
Cys Asn Glu Glu Gln Ser Asn Ser His Phe Phe 900 905 910Ser Tyr Ser
Ile Asp Val Gly Glu Leu Tyr Pro Glu Leu Asn Pro Gly 915 920 925Ile
Glu Phe Gly Leu Arg Ile Val Glu Pro Asn Ser Tyr Met Thr Ile 930 935
940Ser Asn Leu Glu Ile Ile Glu Glu Arg Ser Leu Thr Glu Met Glu
Ile945 950 955 960Gln Thr Ile Lys Arg Lys Asp Gln Lys Trp Lys Lys
Glu Ile Leu Gln 965 970 975Glu Cys Ala Asn Ile Asn Glu Leu Leu Gln
Pro Ile Ile Asp Lys Val 980 985 990Asp Ser Leu Phe Lys Asp Ala Asp
Trp Tyr Gly Gln Ile Leu Pro His 995 1000 1005Ile Thr Tyr Gln Asn
Leu Lys Asn Ile Val Leu Pro Glu Leu Pro 1010 1015
1020Lys303094DNABacillus thuringiensis 30tccgacgacg ttgcagtttc
caacatggca tcagcaccat acgctatcac gggcattcca 60actaccagag cccctgatgg
agccctcccg cttcgtcaag agattgatgc ttggtctgcg 120aacccagcca
atgttgacca ggtgaactta tatctccagg cgcttgctgc tttccaacag
180ttgcctgcga cagataagct ctcttacttc cagattgctg ggattcatgg
ggagcctttt 240atcccgtggg atgagaatac cagtcctaat ccaagatcta
ggtggagagg atattgtaca 300catgcatcaa tcctcttccc aacatggcat
cggccgtatc tcgctgtctt cgagcaaatc 360cttcattcga ttatgcagcg
aattgcggca gcatatccag accaagagct tcgaacccga 420tatcagactg
ccgcagaagc attccgtatt ccatactggg acagtgcaca acttaaggaa
480cgtgggggca gaagatcctt gaacgttcct tacctttgca ccttgcctac
tgttcaagtc 540ttcactccta cttccgctgg agatactatc aggccttttg
aaactattga taatcccttg 600tacagctaca aatttgtcac cacacaagga
attactagtt tccaagacca ggatggaaat 660ttctttccat tcgcaaacgc
gatgggaact tcccgctatc caccacaata caattctcgc 720gaccccaccg
tttcttctca gtggaccaat ggattcgttg ataacgactc gatcacggag
780gcactacgga atctgagttc tcttggtgag gacgtttacc gatcattcac
gaccagcaat 840tatgcctggt actctagcac ccaacaatca aatcccccag
cgcccaacag ctaccaatct 900ctcgaatcga ttcacaatga aatccacggc
atcacaggag ggggtggaca tatgagctgg 960aatacagttt catcttttga
tcctattttc tggctccacc actgcaacgt ggatcgtctg 1020tttgccatct
ggcaagctat ctacgctgat accggccgat atcctgatgc ttggtttaat
1080gcacaatcag cacaacttcg agacgaacga ggaacttggt cgattgctgc
aggttctcgc 1140gaaaatgctg acactccact agctccattc cataaggacg
acagaggcag cgtctacaat 1200tccaatgacg tccgcaattg gactaggttt
ggctcttcgt accctgaatt gcaaccatgg 1260cttcctcaat accgagattc
cactggtgaa tttaacgcaa cgctatatcg taacgatgtt 1320gttgcacagg
tcaccgactt gtattcgcga gtcagaaggc gtgtccagaa cactcaagtt
1380ccacgaaatc gcctttttgc tgccacccag accggcaccc agacattcca
aggcagttcc 1440gctactgcag gcgggtcgtt tgcggcccca ccgacaacac
aagggcccgg tcagcagttg 1500caatttggtc cccctccttc cggcgggcaa
caggccttcg cccctccacc aacagtccaa 1560gcccaagccc agtctcaagg
acaaccattc accccgccaa cgacgctgcc cactcaggga 1620cagcaattta
cctctcctcc tcctcaaact gctcagggcc aacagttccc acccccgccg
1680actcagcagc aacagttctc gccgccgccg actcatcagc agcaattcgc
ccctcctcct 1740acgcaggagc acggacaggc ggttacgtca ccacctgcac
agacacaatt ctcccctccg 1800ccaactcagg cattctcgcc gccaccgact
ggtgattccc acggacagca gtttactcca 1860cagccgcaac agcaattcac
tccacaaccg caacagcaac agcaacagca atttgcgcct 1920ccccagcaag
gaccaggcgg ccatacccca cagggacagc atagctctcc accacccaag
1980aaaagcggcc tcagtggcct tatgtcctct gctaaactgc actttggtga
agcccttact 2040gcaggccgtg aagccgctca aggccaccag cagcctgtac
aacagcatca acagcccact 2100cacactccag gaaaccctgg cagcagtggt
actgctcttg ctactaaatt tggtggtatt 2160attggaggcg gtattcatat
ggcccaagaa cgtcttggtt ctaagaagca gccgggccaa 2220cctggaaccc
gtggtattga tgacgaacct ggtcaagaag gagaattgag ccgtggattc
2280ggtgatatga gcttgggcca acaaagtttc ggctcaggag agtcgcttac
ttaccacgaa 2340tacgatgcaa acatccgatt tgagagattc gacctcggtg
gtcgtccatt cacagtccac 2400atcttccttg gagacttcaa cccggaccca
gcaacttgga tgtgggacaa gaatcgtgtc 2460ggtggaatct ataactttgt
cgccggtgtt cagcgtggag acggaagcgc ttgctccaac 2520tgcgaaactc
aatcccagga ccacactatc gttacgggtc aggtgtctct cactaacgcc
2580cttcttgacg acgttgaaga ctcagcaaat ggcttgaata gcctgattcc
cgaggaggtt 2640atcccgtatt tgcaacgaca tctgcactgg cgtatcactg
acccgaatgg aagggagatc 2700ccacgccaga gcctcaatac cttaaagatc
tctgttgttg aatgttccgc caccatttca 2760aacaaccccg gcgagctcac
ccaatatggg gatcacagag tcttggacat agttactgaa 2820ggtcgtccgg
ctggcaaagc ggctggcgat ggttactaaa aaaaatctag tgaacccttt
2880cagcatattg cacgcagatt gctgttttgt ttgttttatg tagggcattc
gaattcgacg 2940accctgaaat ttgcttcacg agcattaaat cagagaggga
aatagtgaat attaaccgct 3000gggcgagcgt cttttcatgt ttatgtactt
aggcagttgc ctgtttttgc tggaatatat 3060tttaattgag tcccaaaaaa
aaaaaaaaaa aaaa 309431360PRTBacillus thuringiensis 31Met Arg Ile
Arg Arg Asn Gln Ser Thr Leu Ser His Asn Glu Arg Leu1 5 10 15Ala Phe
Thr Asn Ala Val Leu Glu Leu Lys Arg Arg Pro Ser Arg Leu 20 25 30Pro
Met Ser Leu Gly Ser Thr Ser Arg Tyr Asp Asp Tyr Val Tyr Trp 35 40
45His Leu Gln Ser Met Glu Asn Gln Thr Ser Thr Thr Pro Gly Trp Ala
50 55 60His Arg Gly Pro Ala Phe Leu Pro Trp His Arg Tyr Tyr Leu Asn
Gln65 70 75 80Phe Glu Glu Asp Leu Gln Arg Ile Asp His Thr Val Thr
Leu Pro Tyr 85 90 95Trp Asp Trp Thr Val Asp Asn Ser Thr Asp Ser Ser
Val Pro Gly Ser 100 105 110Pro Trp Thr Asp Asp Phe Met Gly Gly Asp
Gly Asp Pro Thr Gln Glu 115 120 125Tyr Thr Val Thr Thr Gly Pro Phe
Thr Gly Asp Asn Trp Lys Leu Thr 130 135 140Leu Phe Asp His His Glu
Asn Glu Pro His Asn Ala Arg Leu Arg Arg145 150 155 160Gln Leu Gly
Thr Thr Leu Asn Ala Ser Gly Asn Thr Ile Ser Ile Asn 165 170 175Leu
Pro Thr Asp Ser Glu Val Gln Asn Cys Leu Leu Glu Thr Pro Tyr 180 185
190Tyr Val Ser Pro Trp Arg Ala Gly Gln Asp Val Asn Gln Pro Ala Leu
195 200 205Asn Pro Thr Lys Pro Ser Phe Cys Asn Arg Leu Glu Gly Trp
Tyr Gly 210 215 220Ala Gly Ser Ile His Asn Lys Val His Val Trp Val
Ala Gly Ala Thr225 230 235 240Glu Gly Ser Met Ile Trp Met Ser Ser
Pro Asn Asp Pro Val Phe Phe 245 250 255Leu His His Ala Asn Ile Asp
Arg Leu Trp Val Gln Trp Gln Ala Asn 260 265 270Asn Pro Asn Glu Gly
Tyr His Pro Thr Gly Asn Gly Asn Glu Val Gly 275 280 285Pro Thr Gly
His Asn Leu Asn Asp Ser Met Asn Pro Trp Gly Arg Lys 290 295 300Val
Thr Pro Asn Asn Val Leu Asn His Tyr Ser Leu Gly Tyr Thr Tyr305 310
315 320Asp Thr Asp Ser Thr Pro Leu Ser Glu Ile Phe Met His Thr Phe
Asn 325 330 335Leu Lys Ile Arg Lys Glu Lys Gln Ile Lys Asp Gly His
Phe Gly Leu 340 345 350Ser Gln Glu Asp Leu Asp Lys Leu 355
3603217PRTArtificial Sequencedegenerate
sequencemisc_feature(8)..(9)Xaa can be any naturally occurring
amino acid 32Lys Gly His Gly Thr Asp Glu Xaa Xaa Leu Ile Pro Ile
Leu Ala Pro1 5 10 15Arg335PRTArtificial SequenceGXGTD
motifmisc_feature(2)..(2)Xaa can be any naturally occurring amino
acid 33Gly Xaa Gly Thr Asp1 5
References
D00001

D00002

D00003

D00004

D00005

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.