Copper-iron Alloy Electroplating Solution And Electroplating Method Using The Same
HORI; Masao
U.S. patent application number 16/434632 was filed with the patent office on 2020-12-10 for copper-iron alloy electroplating solution and electroplating method using the same. This patent application is currently assigned to JCU INTERNATIONAL, INC.. The applicant listed for this patent is JCU INTERNATIONAL, INC.. Invention is credited to Masao HORI.
| Application Number | 20200385882 16/434632 |
| Document ID | / |
| Family ID | 1000004259049 |
| Filed Date | 2020-12-10 |
| United States Patent Application | 20200385882 |
| Kind Code | A1 |
| HORI; Masao | December 10, 2020 |
COPPER-IRON ALLOY ELECTROPLATING SOLUTION AND ELECTROPLATING METHOD USING THE SAME
Abstract
Provided are a copper-iron alloy electroplating solution containing divalent copper ions, trivalent iron ions, and an organic compound having a carboxy group, and a technique for producing a copper-iron alloy through a method other than a melting method by an electroplating method using the copper-iron alloy electroplating solution.
| Inventors: | HORI; Masao; (Wixom, MI) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | JCU INTERNATIONAL, INC. Wixom MI |
||||||||||
| Family ID: | 1000004259049 | ||||||||||
| Appl. No.: | 16/434632 | ||||||||||
| Filed: | June 7, 2019 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C25D 3/58 20130101; C25D 3/562 20130101 |
| International Class: | C25D 3/56 20060101 C25D003/56; C25D 3/58 20060101 C25D003/58 |
Claims
1. A copper-iron alloy electroplating solution, comprising divalent copper ions, trivalent iron ions, and an organic compound having a carboxy group.
2. The copper-iron alloy electroplating solution according to claim 1, wherein a source of the divalent copper ions is cupric sulfate or cupric chloride.
3. The copper-iron alloy electroplating solution according to claim 1, wherein a source of the trivalent iron ions is ferric sulfate or ferric chloride.
4. The copper-iron alloy electroplating solution according to claim 1, wherein the organic compound having a carboxy group is ethylenediaminetetraacetic acid, sodium gluconate, or citric acid.
5. The copper-iron alloy electroplating solution according to claim 1, wherein the solution has a pH in a range of from 4 to 11.
6. The copper-iron alloy electroplating solution according to claim 1, wherein a molar ratio of copper ions/iron ions is in a range of from 0.1 to 0.8.
7. A copper-iron alloy electroplating method, comprising electroplating a material to be plated in the copper-iron alloy electroplating solution according to claim 1, thereby forming a copper-iron alloy film on the material to be plated.
8. A copper-iron alloy film obtained by the copper-iron alloy electroplating method according to claim 7.
Description
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] The present invention relates to a copper-iron alloy electroplating solution and an electroplating method using the same.
Background Art
[0002] A copper-iron alloy has properties of both copper and iron, and therefore is expected to be used for a structural material, a thermally conductive material, a magnetic material, an electrically conductive spring raw material, an electrically conductive material, etc.
[0003] However, copper and iron have different specific gravities, and therefore, it is difficult to uniformly alloy these together, and a melting method is known as a technique for producing a copper-iron alloy for the time being (for example, JP-A-2016-216758). However, a copper-iron alloy produced by a melting method has difficulty in processing, and use thereof is limited.
SUMMARY OF THE INVENTION
[0004] In view of this, an object of the present invention is to provide a technique for producing a copper-iron alloy by a method other than the melting method.
[0005] The present inventors made intensive studies for achieving the above object, and as a result, they found that a copper-iron alloy can be electroplated using an electroplating solution prepared by combining copper ions and iron ions both having a specific valence, and thus completed the invention.
[0006] That is, the present invention is directed to a copper-iron alloy electroplating solution containing divalent copper ions, trivalent iron ions, and an organic compound having a carboxy group.
[0007] Further, the present invention is directed to a copper-iron alloy electroplating method including electroplating a material to be plated in the above-mentioned copper-iron alloy electroplating solution, thereby forming a copper-iron alloy film on the material to be plated.
[0008] In addition, the present invention is directed to a copper-iron alloy film obtained by the above-mentioned electroplating method.
[0009] By using the copper-iron alloy electroplating solution of the present invention, a copper-iron alloy film can be formed by plating, and therefore can be used for materials or applications to which it could not be applied so far.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The patent or application file contains at least one drawing executed in color. Copies of this patent or patent application publication with color drawing(s) will be provided by the Office upon request and payment of the necessary fee.
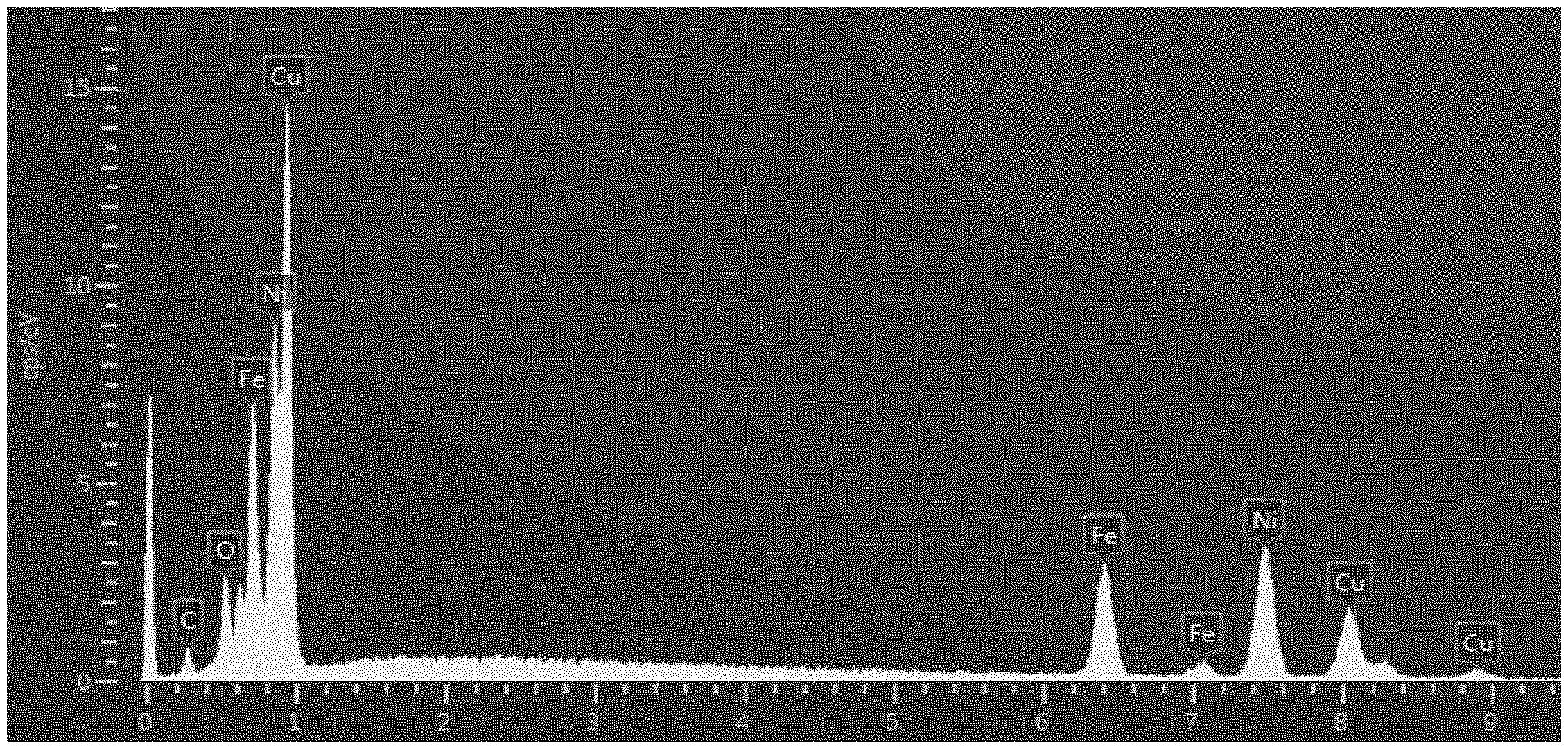
[0011] FIG. 1 shows an EDS spectrum of a copper-iron alloy film obtained in Example 1.
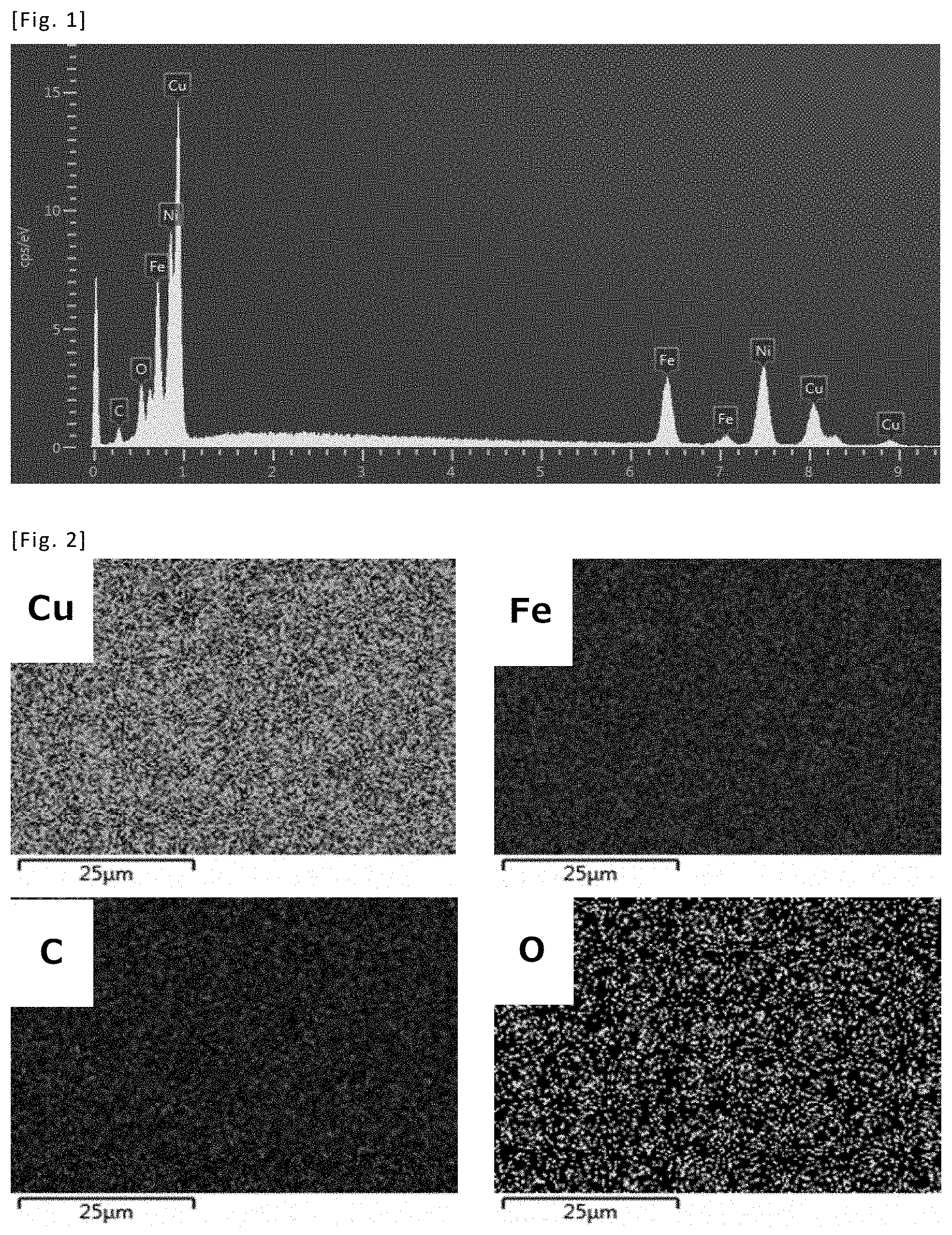
[0012] FIG. 2 shows EDS elemental mapping of the copper-iron alloy film obtained in Example 1.
DETAILED DESCRIPTION OF THE INVENTION
[0013] The copper-iron alloy electroplating solution of the present invention (hereinafter referred to as "the plating solution of the present invention") contains divalent copper ions, trivalent iron ions, and an organic compound having a carboxy group.
[0014] The content of the divalent copper ions in the plating solution of the present invention is not particularly limited, but is, for example, from 0.05 to 5 mass % (hereinafter simply referred to as "%"), preferably from 0.1 to 2.5%.
[0015] A source of the divalent copper ions is not particularly limited, but examples thereof include sulfates such as cupric sulfate and chlorides such as cupric chloride.
[0016] The content of the trivalent iron ions in the plating solution of the present invention is not particularly limited, but is, for example, from 0.1 to 20%, preferably from 0.25 to 10%.
[0017] A source of the trivalent iron ions is not particularly limited, but examples thereof include sulfates such as ferric sulfate and chlorides such as ferric chloride.
[0018] In the plating solution of the present invention, the ratio of copper ions/iron ions is not particularly limited, but is preferably from 0.05 to 0.95, more preferably from 0.1 to 0.8.
[0019] The organic compound having a carboxy group contained in the plating solution of the present invention is not particularly limited, but examples thereof include ethylenediaminetetraacetic acid, carboxylic acids such as gluconic acid and citric acid, and alkali metal salts of carboxylic acids such as sodium gluconate and sodium citrate. Among these, ethylenediaminetetraacetic acid, sodium gluconate, and citric acid are preferred. Among these organic compounds having a carboxy group, one type or two or more types, preferably two or more types may be used. The content of the organic compound having a carboxy group in the plating solution of the present invention is not particularly limited, but is, for example, from 2 to 25%, preferably from 5 to 10%.
[0020] The plating solution of the present invention is preferably a solution composed only of the above-mentioned essential components, and more preferably a chloride bath containing chlorides as the source of copper ions and the source of iron ions, a sulfate bath containing sulfates as the source of copper ions and the source of iron ions, or the like, however, an electrically conductive salt such as potassium chloride, sodium chloride, potassium sulfate, or sodium sulfate, a surfactant such as polyethylene glycol or polypropylene glycol, a pH buffer agent such as boric acid or sodium borate, or the like may be further incorporated therein.
[0021] The pH of the plating solution of the present invention is not particularly limited, but is, for example, from 4 to 11. In the adjustment of this pH, an alkaline substance such as sodium hydroxide or an acidic substance such as hydrochloric acid may be used.
[0022] As preferred embodiments of the plating solution of the present invention, the following solutions are exemplified.
<Chloride Bath 1>
[0023] Ferric chloride: 2.5 to 10 g/L as iron
[0024] Cupric chloride: 1.25 to 5 g/L as copper
[0025] Sodium gluconate: 40 to 100 g/L
[0026] Disodium ethylenediaminetetraacetate: 15 to 60 g/L
[0027] pH: 7 to 11
<Chloride Bath 2>
[0028] Ferric chloride: 2.5 to 10 g/L as iron
[0029] Cupric chloride: 1.25 to 5 g/L as copper
[0030] Sodium gluconate: 40 to 100 g/L
[0031] Citric acid: 1 to 50 g/L
[0032] pH: 4 to 7
<Sulfate Bath>
[0033] Ferric sulfate: 2.5 to 10 g/L as iron
[0034] Copper sulfate: 1.25 to 5 g/L as copper
[0035] Sodium gluconate: 40 to 100 g/L
[0036] Disodium ethylenediaminetetraacetate: 15 to 60 g/L
[0037] pH: 7 to 11
[0038] A method for preparing the plating solution of the present invention is not particularly limited, and for example, the above-mentioned components may be added and mixed in water, and if necessary, the pH may be adjusted.
[0039] A method for electroplating a material to be plated using the plating solution of the present invention is not particularly limited, and for example, a material to be plated may be electroplated in the plating solution of the present invention under conditions at a bath temperature of 10 to 70.degree. C. using an anode of copper, iron, carbon, stainless steel, iridium oxide, or the like, and at a current density of 0.25 to 4 A/dm.sup.2, preferably at a bath temperature of 30 to 60.degree. C. using an anode of carbon, iridium oxide, or the like, and at a current density of 0.5 to 3 A/dm.sup.2. By doing this, a copper-iron alloy film is formed on the material to be plated. Whether or not this copper-iron alloy film is formed can be confirmed by analysis such as EDS or X-ray fluorescence.
[0040] The material to be plated that can be plated in the plating solution of the present invention is not particularly limited as long as it can be plated, however, examples thereof include metals such as brass, copper, nickel, stainless steel, and aluminum, and resins such as ABS, nylon, polypropylene (PP), polybutylene terephthalate (PBT), and polyphenylene sulfide (PPS).
[0041] The thus obtained copper-iron alloy film of the present invention has properties of thermal conductivity, magnetism, and electrical conductivity. The composition of this copper-iron alloy film can be adjusted by changing the ratio of copper ions/iron ions in the plating solution of the present invention, the pH of the plating solution, and the temperature of the plating solution, and therefore is not particularly limited, but is, for example, as follows: copper: 5 to 97.9%, iron: 1.5 to 94.4%, O: 0.3 to 15%, and C: 0.3 to 15%, preferably, copper: 10 to 96%, iron: 2 to 88%, O: 1 to 10%, and C: 1 to 10%. Incidentally, when the composition of the copper-iron alloy film of the present invention is calculated only in terms of metals, the composition is as follows: copper: 10 to 97.5% and iron: 2.5 to 90%, preferably copper: 20 to 95% and iron: 5 to 80%.
[0042] Since the copper-iron alloy film of the present invention has properties of thermal conductivity, magnetism, and electrical conductivity, the film can be utilized for a structural material, a thermally conductive material, a magnetic material, an electrically conductive spring raw material, an electrically conductive material, etc. in the same manner as a copper-iron alloy film produced by a method other than conventionally known plating.
EXAMPLE
[0043] Hereinafter, the present invention will be described in detail with reference to Examples, however, the invention is by no means limited to these Examples.
Examples 1 to 7 and Comparative Example 1
[0044] Copper-Iron Alloy Electroplating:
[0045] Copper-iron alloy electroplating solutions were prepared by dissolving respective components shown in the following Table 1 in water. In each of these copper-iron alloy electroplating solutions, a test piece (one obtained by nickel plating a brass flat plate) was placed, and electroplated for 5 minutes under conditions shown in Table 1.
TABLE-US-00001 TABLE 1 Plating solution composition and Example Example Example Example Example Example Example Comparative plating conditions 1 2 3 4 5 6 7 Example 1 Ferric chloride 35 35 35 -- 35 35 25 -- hexahydrate g/L Ferric sulfate -- -- -- 25 -- -- -- -- n-hydrate g/L Ferrous sulfate g/L -- -- -- -- -- -- -- 25 Cupric chloride g/L 7.5 7.5 7.5 -- 2.5 15.0 5 -- Copper sulfate g/L -- -- -- 5 -- -- -- 5 0.5 mol/L EDTA 200 -- -- 150 200 200 200 solution ml/L Citric acid g/L -- 30 30 -- -- -- Sodium gluconate g/L 80 80 80 60 80 80 60 60 Plating solution pH* 10 4.5 8.5 8.4 10 10 6.8 4.5 Bath temperature .degree. C. 50 50 50 45 50 50 50 45 Current density A/dm.sup.2 1 1 1 1 1 1 1 1 Cu content in film % 67.8 47.8 25.4 65.8 54.7 84.9 31.9 96.7 Fe content in film % 24.7 38.6 55.5 27.7 34.8 8.75 48.6 0 O content in film % 3.86 7.03 9.53 3.23 6.46 4.29 9.59 2.01 C content in film % 3.64 6.57 9.57 3.27 4.04 2.06 9.91 1.29 *adjusted with sodium hydroxide
[0046] The contents of copper and iron in each film after plating were determined by EDS. These results are also shown in Table 1. Further, the results of EDS of the test piece of Example 1 are shown in FIG. 1 (spectrum) and FIG. 2 (elemental mapping).
[0047] From the above results, a copper-iron alloy film was obtained only using a plating solution containing divalent copper ions and trivalent iron ions. Further, from the result of EDS, it could be confirmed that the alloy is uniform.
[0048] The present invention can be utilized for producing a copper-iron alloy film, and a structural material, a thermally conductive material, a magnetic material, an electrically conductive spring raw material, an electrically conductive material, etc. using the film.
* * * * *
D00000

D00001

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.