Tertiary Hydroxyl Functional Alkoxysilanes and Methods for Preparing Thereof
Damke; Jan-Erik ; et al.
U.S. patent application number 16/898579 was filed with the patent office on 2020-09-24 for tertiary hydroxyl functional alkoxysilanes and methods for preparing thereof. The applicant listed for this patent is Henkel AG & Co. KGaA. Invention is credited to Rok Brisar, Jan-Erik Damke, Johann Klein, Esteban Mejia.
| Application Number | 20200299314 16/898579 |
| Document ID | / |
| Family ID | 1000004944819 |
| Filed Date | 2020-09-24 |
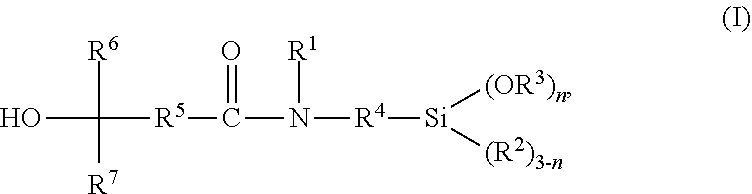
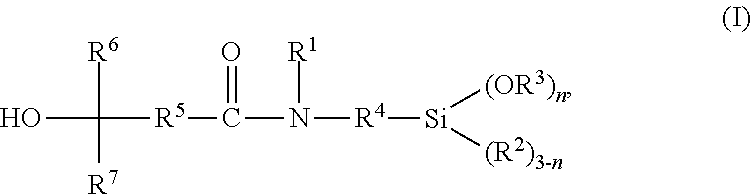







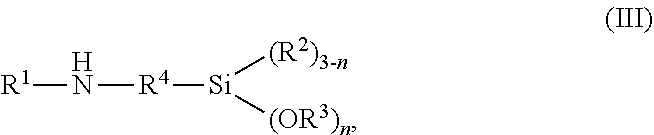
View All Diagrams
| United States Patent Application | 20200299314 |
| Kind Code | A1 |
| Damke; Jan-Erik ; et al. | September 24, 2020 |
Tertiary Hydroxyl Functional Alkoxysilanes and Methods for Preparing Thereof
Abstract
Disclosed is a tertiary hydroxyl functional alkoxysilane of the general formula (I) ##STR00001## wherein R.sup.1 is selected from the group consisting of hydrogen and a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; R.sup.2 and R.sup.3 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; R.sup.4 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; R.sup.5 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; R.sup.6 and R.sup.7 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; and n is 1, 2 or 3, a method for preparing thereof, and the use of the tertiary hydroxyl functional alkoxysilane of the general formula (I).
| Inventors: | Damke; Jan-Erik; (Duesseldorf, DE) ; Klein; Johann; (Duesseldorf, DE) ; Brisar; Rok; (Rostock, DE) ; Mejia; Esteban; (Rostock, DE) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 1000004944819 | ||||||||||
| Appl. No.: | 16/898579 | ||||||||||
| Filed: | June 11, 2020 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| PCT/EP2018/083721 | Dec 6, 2018 | |||
| 16898579 | ||||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C07F 7/1892 20130101; C08L 101/02 20130101 |
| International Class: | C07F 7/18 20060101 C07F007/18; C08L 101/02 20060101 C08L101/02 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Dec 12, 2017 | EP | 17206708.4 |
Claims
1. A tertiary hydroxyl functional alkoxysilane of the general formula (I) ##STR00009## wherein R.sup.1 is selected from the group consisting of hydrogen and a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; R.sup.2 and R.sup.3 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; R.sup.4 is selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; R.sup.5 is selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; R.sup.6 and R.sup.7 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; and n is 1, 2 or 3.
2. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.1 is selected from hydrogen or a C.sub.1-C.sub.8 alkyl residue.
3. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.2 and R.sup.3 are independently selected from a linear or branched, substituted or unsubstituted, C.sub.1-C.sub.20 alkyl or C.sub.6-C.sub.18 aryl residue.
4. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.2 and R.sup.3 are independently selected from a linear or branched, substituted or unsubstituted, methyl, ethyl, or n-propyl residue; and n is 3.
5. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.4 is selected from a linear or branched, substituted or unsubstituted, C.sub.1-C.sub.20 alkylene.
6. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.4 is selected from a linear or branched, substituted or unsubstituted, methylene, ethylene, 1,3-propylene, 2-methyl-1,3-propylene, 1,4-butylene, 3-methyl-1,4-butylene, or 3,3-dimethyl-1,4-butylene residue.
7. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.5 is selected from a linear or branched, substituted or unsubstituted, C.sub.1-C.sub.20 alkylene.
8. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.5 is selected from a linear or branched, substituted or unsubstituted, methylene, ethylene or 1,3-propylene, 2-methyl-1,3-propylene, 1,4-butylene, 3-methyl-1,4-butylene, or 3,3-dimethyl-1,4-butylene residue.
9. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.6 and R.sup.7 are independently from one another selected from a linear or branched, substituted or unsubstituted C.sub.1-C.sub.20 alkyl, alkenyl, or alkynyl, or C.sub.6-C.sub.18 aryl residue.
10. The tertiary hydroxyl functional alkoxysilane according to claim 1, wherein R.sup.6 and R.sup.7 are independently from one another selected from a linear or branched, substituted or unsubstituted a C.sub.1-C.sub.8 alkyl or C.sub.1-C.sub.8 alkenyl residue.
11. A method for preparing the tertiary hydroxyl functional alkoxysilane according to claim 1, comprising reacting at least one di-substituted lactone compound and at least one aminosilane having at least one primary amino group or secondary amino group.
12. The method according to claim 11, wherein the di-substituted lactone compound has the general formula (II) ##STR00010## wherein R.sup.5 is selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; and R.sup.6 and R.sup.7 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms.
13. The method according to claim 11, wherein the aminosilane is an aminoalkylenealkoxysilane having the general formula (III) ##STR00011## wherein R.sup.1 is selected from the group consisting of hydrogen and a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; R.sup.2 and R.sup.3 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms; and R.sup.4 is selected from a linear or branched, substituted or unsubstituted, hydrocarbon residue having 1 to 20 carbon atoms.
14. The method according to claim 11, wherein the reaction is carried out at a temperature in the range of from -50 to 200.degree. C.
15. The method according to claim 11, wherein the reaction is carried out in the presence of a Lewis acid catalyst.
16. A material selected from an adhesion promoter, a urethane coupling agent, an end-capping agent, a surface treatment agent, a water scavenger, a fiber treatment agent, a paint additive, and/or a monomer for a polymer preparation comprising the tertiary hydroxyl functional alkoxysilane of the general formula (I) according to claim 1.
17. An end-capping agent for a moisture curable composition comprising the tertiary hydroxyl functional alkoxysilane of the general formula (I) according to claim 1.
Description
[0001] The present invention relates to a stable tertiary hydroxyl functional alkoxysilane and a method for preparing thereof. The present invention also relates to the use of the obtained hydroxyl functional alkoxysilane as an ingredient in adhesives, sealants and coatings, in construction, industrial applications or in consumer products.
[0002] One of the common silane agent for moisture-curable compositions is primary amine-functionalized alkoxysilanes, which are extremely reactive towards many electrophiles like for example: isocyanates, aldehydes and anhydrides. This makes them difficult to handle and store. Furthermore, fast and highly exothermic reactions impose processing and safety difficulties in the larger scale production of the prepolymers. High reaction rates also result in a low reaction selectivity and oligomerization.
[0003] The stability of the --OH group in the presence of alkoxysilanes is poor as was shown by many authors. Rossmy and Koerner were one of the first who showed the self-dealcoholization reaction undergone by OH-containing alkoxysilanes. They used this to their advantage in order to prepare cyclic alkoxysilanes (also called siloxacycloalkenes) (Die Makromolekulare Chemie 1964, 73, 85-108 and Die Makromolekulare Chemie 1966, 97, 241-247). They prepared primary hydroxyl functional alkoxysilanes by transesterification reaction, which formed 5- or 6-membered ring by eliminating alcohol. They propose that the primary alcohol is not stable in the presence of alkoxysilane and therefore tends to cyclize.
[0004] Trost and Ball (Journal of the American Chemical Society, 2005, 127, 17644-17655) investigated alkyne hydrosilylation catalyzed by a ruthenium catalyst. They also confirmed that secondary hydroxyl groups will perform alcohol exchange in the presence of alkoxysilanes, despite the fact that the secondary alcohol is less nucleophilic.
[0005] Tertiary hydroxyl functional fluorine containing alkoxysilanes were prepared by Semenov et al (Ladilina, E. Y., Lyubova, T. S., Kuznetsova, O. V., Klapshin, Y. P., Baten'kin, M. A., Sidorenko, K. V., Glukhova, T. A., Gorshkov, O. N., Polymer Science Series B, 2015, 57, 150-158). Aminoalkylalkoxysilane was reacted with hexafluoroacetone to obtain a tertiary alcohol, which was unstable in the presence of ethoxysilane. They showed that even the tertiary hydroxyl functionality can preform the self-dealcoholization reaction at ambient conditions. Prepared cyclic siloxacycloalkenes were used as a low reflective index coating for solar cells or similar, since the prepared polymers exhibit good thermal and mechanical stability and self-cleaning properties.
[0006] US 2007/0055036 A1 discloses the preparation of silane functional compound, which is synthesized by a hydrosilylation reaction of allylic alcohol and alkylalkoxysiliane. The self-dealcoholization reaction is induced by heating to produce cyclic silanes in high yields.
[0007] JP 2014001152 A describes the preparation of silane coupling agents for surface treatment applications. Epoxides are ring-opened by aminosilanes to produce --OH functional alkoxysilane as intermediates. Since the hydroxyl functionality is not stable, a siloxacycloalkene compound is formed as a final product.
[0008] Attempts to obtain hydroxyl functionalized silanes by reacting aminosilanes with epoxides are also disclosed in WO 2011/081409 A2. The compound is prepared by a reaction of propylene oxide and ethylene oxide and aminosilane at 80.degree. C. The obtained mixture contains (hydroxyisopropyl)aminopropyltriethoxysilane and bis-(hydroxyisopropyl)aminopropyltriethoxysilane in different ratios. The end-cappers produced with this method contain different ratios of primary and secondary alcohols which points out the poor reaction selectivity.
[0009] EP 2852649 A1, EP 2832757 A1, and EP 2268650 A1 disclose polymers containing silane groups based on hydroxysilanes obtained by reacting lactides or unsubstituted or monosubstituted lactones with aminosilanes.
[0010] Another method for producing hydroxyl functional alkoxysilanes was described by Narayan et al. (Yuya Tachibana, Xiangke Shi, Daniel Graiver, Ramani Narayan, Silicone, 4, 167-174). Aminosilane was reacted with ethyl carbonate to produce the primary hydroxyl functionality. They reported that partial condensation was unavoidable even in the absence of any catalyst.
[0011] Therefore, a need still exists for providing hydroxyl functional alkoxysilanes which can overcome the stability issue.
[0012] The object of this invention is to provide a stable hydroxyl functional alkoxysilanes and a method for the preparation thereof.
[0013] It has been found that the tertiary hydroxyl functional alkoxysilanes having the general formula (I) according to the present invention are significantly less nucleophilic and are therefore considerably less reactive than the standard systems, allowing a better reaction control and a higher storage stability.
[0014] The present invention provides tertiary hydroxyl functional silanes having the general formula (I)
##STR00002##
wherein [0015] R.sup.1 is selected from the group consisting of hydrogen and a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; [0016] R.sup.2 and R.sup.3 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; [0017] R.sup.4 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; [0018] R.sup.5 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; [0019] R.sup.6 and R.sup.7 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms; and [0020] n is 1, 2 or 3.
[0021] As used herein, the singular forms "a", "an" and "the" include plural referents unless the context clearly dictates otherwise.
[0022] The term "at least one," as used herein, means 1 or more, i.e., 1, 2, 3, 4, 5, 6, 7, 8, 9, or more. With reference to an ingredient, the indication refers to the type of ingredient and not to the absolute number of molecules. "At least one polymer" thus means, for example, at least one type of polymer, i.e., that one type of polymer or a mixture of several different polymers may be used.
[0023] The terms "comprising" and "comprises" as used herein are synonymous with "including", "includes", "containing" or "contains", and are inclusive or open-ended and do not exclude additional, non-recited members, elements or method steps.
[0024] When amounts, concentrations, dimensions and other parameters are expressed in the form of a range, a preferable range, an upper limit value, a lower limit value or preferable upper and limit values, it should be understood that any ranges obtainable by combining any upper limit or preferable value with any lower limit or preferable value are also specifically disclosed, irrespective of whether the obtained ranges are clearly mentioned in the context.
[0025] The words "preferred" and "preferably" are used frequently herein to refer to embodiments of the disclosure that may afford particular benefits, under certain circumstances. However, the recitation of one or more preferable or preferred embodiments does not imply that other embodiments are not useful and is not intended to exclude those other embodiments from the scope of the disclosure.
[0026] R.sup.1 in the general formula (I) is selected from the group consisting of hydrogen and a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkyl or C.sub.6-C.sub.18 aryl residue, which may be interrupted by at least one heteroatom. In preferred embodiments, R.sup.1 is hydrogen or selected from a C.sub.1-C.sub.8 alkyl residue, more preferably a methyl, ethyl or n-propyl residue, most preferably, R.sup.1 is hydrogen.
[0027] R.sup.2 and R.sup.3 in the general formula (I) are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkyl or C.sub.6-C.sub.18 aryl residue, more preferably a C.sub.1-C.sub.8 alkyl residue, which may be interrupted by at least one heteroatom. Particularly preferably R.sup.2 and R.sup.3 in the general formula (I) are same or different and are, independent from one another, selected from a methyl, ethyl, or n-propyl residue, most preferably a methyl residue.
[0028] According to an embodiment of the present invention, R.sup.2 is selected from a hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkyl, wherein one or more carbon atom(s) are substituted with at least one heteroatoms, preferably selected from O or N. Preferably the carbon atom in alpha position to Si is substituted with O or N.
[0029] R.sup.4 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkylene, more preferably a C.sub.1-C.sub.8 alkylene residue, which may be interrupted by at least one heteroatom. R.sup.4 is particularly preferably selected from a methylene, ethylene, 1,3-propylene, 2-methyl-1,3-propylene, 1,4-butylene, 3-methyl-1,4-butylene, or 3,3-dimethyl-1,4-butylene residue, most preferably 1,3-propylene residue.
[0030] R.sup.5 is selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkylene, more preferably a C.sub.1-C.sub.8 alkylene residue, which may be interrupted by at least one heteroatom. R.sup.5 is particularly preferably selected from methylene, ethylene or 1,3-propylene, 2-methyl-1,3-propylene, 1,4-butylene, 3-methyl-1,4-butylene, or 3,3-dimethyl-1,4-butylene residue, most preferably ethylene or 1,3-propylene residue.
[0031] R.sup.6 and R.sup.7 are same or different and are, independent from one another, selected from a linear or branched, substituted or unsubstituted hydrocarbon residue having 1 to 20 carbon atoms, preferably a C.sub.1-C.sub.20 alkyl, alkenyl, or alkynyl, or C.sub.6-C.sub.18 aryl residue, more preferably a C.sub.1-C.sub.8 alkyl residue, particularly preferably a methyl, ethyl, or n-hexyl residue, or a C.sub.1-C.sub.8 alkenyl residue, which may be interrupted by at least one heteroatom.
[0032] n is 1, 2 or 3, preferably 2 or 3, more preferably 3.
[0033] The term "substituted hydrocarbon residue," as used in this connection, means that one or more carbon atoms and/or hydrogen atom(s) of the hydrocarbon residues are replaced by heteroatoms or functional groups. Heteroalkyl groups in which one or more carbon atoms are replaced by heteroatoms, particularly selected from O, S, N, and/or Si, are obtained by the replacement of one or more carbon atoms by heteroatoms. Examples of such heteroalkyl groups are, without limitation, methoxymethyl, ethoxyethyl, propoxypropyl, methoxyethyl, isopentoxypropyl, ethylaminoethyl, trimethoxypropylsilyl, etc. Functional groups that can replace the hydrogen atoms are selected particularly from .dbd.O, .dbd.S, --OH, --SH, --NH.sub.2 --NO.sub.2, --CN, --F, --Cl, --Br, --I, --OCN, --NCO, C.sub.3-8 cycloalkyl, C.sub.6-14 aryl, a 5-10-membered heteroaryl ring, in which 1 to 4 ring atoms independently are nitrogen, oxygen, or sulfur, and a 5-10-membered heteroalicyclic ring, in which 1 to 3 ring atoms are independently nitrogen, oxygen, or sulfur.
[0034] As used herein, a "C.sub.1-C.sub.20 alkyl" or "C.sub.1-C.sub.8 alkyl" residue refers to a monovalent group that contains from 1 to 20 or from 1 to 8 carbons atoms, that is a radical of an alkane and includes linear and branched organic groups. Examples of alkyl residues include, but are not limited to: methyl; ethyl; propyl (or n-propyl); isopropyl; n-butyl; isobutyl; sec-butyl; tert-butyl; n-pentyl; n-hexyl; n-heptyl; and, 2-ethylhexyl. In the present invention, such alkyl residues may be unsubstituted or may be substituted with one or more substituents, such as halo, preferably fluoro or chloro, nitro, cyano, amido, amino, sulfonyl, sulfinyl, sulfanyl, sulfoxy, urea, thiourea, sulfamoyl, sulfamide and hydroxy, and may optionally be interrupted by at least one heteroatom. The halogenated derivatives of the exemplary hydrocarbon residues listed above may, in particular, be mentioned as examples of suitable substituted alkyl residues.
[0035] As used herein, a "C.sub.6-C.sub.18 aryl" residue is used alone or as part of a larger moiety--as in "aralkyl residue"--refers to optionally substituted, monocyclic, bicyclic and tricyclic ring systems in which the monocyclic ring system is aromatic or at least one of the rings in a bicyclic or tricyclic ring system is aromatic. The aryl residue may be optionally interrupted by at least one heteroatom. The bicyclic and tricyclic ring systems include benzofused 2-3 membered carbocyclic rings. Exemplary aryl residues include, but are not limited to: phenyl; indenyl; naphthalenyl, tetrahydronaphthyl, tetrahydroindenyl; tetrahydroanthracenyl; and, anthracenyl. A phenyl residue is preferred.
[0036] The term "alkenyl", as used herein, refers to an alkenyl residue which comprises at least two carbon atoms and at least one carbon-carbon double bond, e.g., ethenyl, propenyl, butenyl, or pentenyl and structural isomers thereof such as 1- or 2-propenyl, 1-, 2-, or 3-butenyl, etc. Alkenyl residues can be substituted or unsubstituted. If they are substituted, the substituents are as defined above. The alkenyl residue comprises linear or branched hydrocarbon chains.
[0037] The term "alkynyl," as used herein, refers to an alkynyl residue which comprises at least two carbon atoms and at least one carbon-carbon triple bond, e.g., ethynyl (acetylene), propynyl, or butynyl, and structural isomers thereof as described above. Alkynyl residues can be substituted or unsubstituted. If they are substituted, the substituents are as defined above.
[0038] The term "C.sub.1-C.sub.20 alkylene" or "C.sub.1-C.sub.8 alkylene" residue refers to a divalent group that contains from 1 to 20 or 1 to 8 carbon atoms, that is a radical of an alkane and includes linear, branched organic or cyclic groups, which groups may be unsubstituted or substituted and may optionally be interrupted by at least one heteroatom. In general, a preference for alkylene groups containing from 1-20 carbon atoms (C.sub.1-C.sub.20 alkylene)--for example substituted, unsubstituted, interrupted or un-interrupted alkylene groups containing from 1 to 8 carbon atoms (C.sub.1-C.sub.8 alkylene)--should be noted. Where the term "C.sub.1-C.sub.8 alkylene group" is used to define the component A herein, it is particularly preferred for said alkylene group to be uninterrupted.
[0039] Where mentioned, the expression "interrupted by at least one heteroatom" means that the main chain of a residue comprises, as a chain member, at least one atom that differs from carbon atom, preferably oxygen, sulfur, fluorine, nitrogen, or chloride.
[0040] The present invention further provides a method for preparing the tertiary hydroxyl functional silane having the general formula (I) as defined herein, comprising reacting at least one di-substituted lactone compound and at least one aminosilane having at least one primary or secondary amino group. The tertiary hydroxyl functional silane having the general formula (I) is obtained by ring opening of di-substituted lactone(s) with aminosilane(s).
[0041] In preferred embodiments, the di-substituted lactone has the general
##STR00003##
wherein R.sup.5, R.sup.6 and R.sup.7 are the same as defined for the general formula (I) above.
[0042] In preferred embodiments, the aminosilane is an aminoalkylenealkoxysilane having the general formula (III)
##STR00004##
wherein R.sup.1 to R.sup.4 and n are the same as defined for the general formula (I) above.
[0043] Preferably, the aminoalkylenealkoxysilane is selected from the group consisting of gamma-aminopropyltrimethoxysilane, gamma-aminopropyltriethoxysilane, gamma-aminopropylmethyldiethoxysilane, gamma-aminopropylmethyldimethoxysilane, gamma-aminopropyltriisopropoxysilane, gamma-aminopropylmethyldiisopropoxysilane, alpha-aminomethyltriethoxysilane, alpha-aminomethyltrimethoxysilane, alpha-aminomethyldiethoxymethylsilane, alpha-aminomethyldimethoxymethylsilane, alpha-am inomethyltriisopropoxysilane, alpha-aminomethyldiisopropoxymethylsilane gamma-am inopropylsilanetriol, gamma-aminopropylmethylsilanediol, gamma-(2-aminoethyl)aminopropylsilanetriol, gamma-(2-aminoethyl)aminopropyltrimethoxysilane, gamma-(2-aminoethyl)aminopropylmethyldimethoxysilane, gamma-(2aminoethyl)aminopropyltriethoxysilane, gamma-(2-aminoethyl)aminopropylmethyldiethoxysilane, gamma-(2-aminoethyl)aminopropyltriisopropoxysilane, gamma-(6-aminohexyl)aminopropyltrimethoxysilane, gamma-(6-aminohexyl)aminopropyltrimethoxysilane, gamma-(2-aminoethyl)aminopropyltriisopropoxy, gamma-(2-aminoethyl)aminopropylmethyldiisopropoxy, gamma-(N-ethylamino)-2-methylpropyltrimethoxysilane, N-phenyl-gamma-aminopropylmethyldimethoxysilane, N-benzyl-gamma-am inopropyltrimethoxysilane, N-benzyl-gamma-aminopropyltriethoxysilane, N-benzyl-gamma-aminopropylmethyldimethoxysilane, N-vinylbenzyl-gamma-am inopropyltriethoxysilane, N-methyl-gamma-aminopropyltriethoxysilane, N-methyl-gamma-aminopropylmethyldimethoxysilane, N-methyl-gam ma-aminopropyltrimethoxysilane, N-ethyl-gamma-am inopropyltriethoxysilane, N-ethyl-gamma-aminopropylmethyldimethoxysilane, N-ethyl-gamma-aminopropyltrimethoxysilane, N-propyl-gamma-aminopropyltriethoxysilane, N-propyl-gamma-aminopropylmethyldimethoxysilane, N-propyl-gamma-aminopropyltrimethoxysilane, N-butyl-gamma-am inopropyltriethoxysilane, N-butyl-gamma-aminopropylmethyldimethoxysilane, N-butyl-gamma-am inopropyltrimethoxysilane, N-cyclohexylaminomethyltriethoxysilane, N-cyclohexylam inomethyltriethoxysilane, N-cyclohexylaminomethyltrimethoxysilane, N-cyclohexylam inomethylmethyldiiethoxysilane, N-cyclohexylaminomethyldiethoxymethylsilane, N-phenylaminomethyltrimethoxysilane, (2-aminoethyl)aminomethyltrimethoxysilane, N,N'-bis[3-(trimethoxysilyl)propyl]ethylenediamine, delta-aminoneohexyltrimethoxysilane, N-beta-(am inoethyl)-gamma-aminopropylmethyldimethoxysilane and deltaaminoneohexylmethyldimethoxysilane, or mixtures thereof.
[0044] Synthesis of hydroxyl functional silane having the general formula (I) can be conducted at a broad range of temperatures, e.g., from -50 to 200.degree. C., preferably -10 to 180.degree. C., more preferably 23 to 100.degree. C., most preferably 30 to 60.degree. C. The reaction is performed preferably under argon or nitrogen atmosphere.
[0045] In preferred embodiments, the molar ratio of the di-substituted lactone compound reaction and the aminosilane is from 0.8 to 1.3, more preferably from 1 to 1.2. If the lactone compound is added in excess, the unreacted lactone compounds are removed after the reaction using a vacuum or remain in the final product as a mixture.
[0046] The reaction can be carried out in the presence of a catalyst in order to increase the reaction rates. The catalyst can be selected from a Lewis acid, preferably a metal-containing compound or a Main group derivative, more preferably organoaluminium compound, such as triethylaluminium.
[0047] The catalyst can be added from 0.001 to 5 mol %, preferably from 0.01 to 3 mol %, more preferably from 0.5 to 2 mol %, relative to the mol % of the amine functionality of the aminoalkoxysilane.
[0048] The reaction can be conducted with or without a solvent. Preferable solvents are water-free polar solvents like toluene, acetonitrile, tetrahydrofuran, ethylene glycol, dimethyl ether, diethyl ether, benzene, ethyl acetate, propylene carbonate, ethylene carbonate, isopropanol, butanol, ethylene glycol, n-propanol, ethanol, methanol, chloroform, chloromethane, preferably in dichloromethane. Before the product is used, for example, for preparation of curable compositions, it is preferable to remove the solvent by distillation.
[0049] Reaction time can vary from 0.5 to 12 hours, preferably from 1 to 5 hours, more preferably from 1 to 3 hours.
[0050] The above-defined method has been found to produce the hydroxyl functional alkoxysilane of the general formula (I) at high yields in a one-step reaction at mild conditions. The method according to the present invention is energy-efficient, since no purification of the product is needed; therefore the amount of produced waste is minimized.
[0051] The present invention further relates to the use of the tertiary hydroxyl functional alkoxysilane of the general formula (I) as defined herein as an adhesion promoter, urethane coupling agent, end-capping agent (also called "endcappers") for moisture-curable compositions, surface treatment agent, water scavenger, fiber treatment agent, paint additive, and/or a monomer for polymer preparations.
[0052] In principle in the present invention, all features listed within the context of the present text, particularly the embodiments, proportional ranges, components and other features of the composition according to the invention, of the method according to the invention and of the use according to the invention identified as preferred and/or special, can be implemented in all possible and not mutually exclusive combinations, with combinations of features identified as preferred and/or special also being regarded as preferred and/or special.
[0053] The following examples are used to explain the invention; however, the invention is not limited thereto.
EXAMPLES
Examples 1 to 3: Preparation of Tertiary Hydroxyl Functional Methoxysilanes
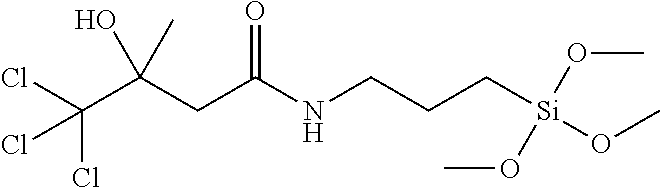
[0054] In a dry round bottom flask under argon atmosphere 5 g (27.9 mmol) of (3-aminopropyl)trimethoxysilane (AMMO) was stirred at 50.degree. C. 0.28 ml of 1M triethylaluminium solution in hexane was slowly added (0.279 mmol, 1 mol %). Afterwards 27.9 mmol of the di-substituted lactone listed in Table 1 was added and vigorously stirred for 3 hours. The following compounds were obtained as colorless liquids in 96-98% purity.
Example 1
##STR00005##
[0056] .sup.1H NMR (400 MHz, Chloroform-d) .delta. 6.44 (s, 1H), 3.54 (s, 4H), 3.18 (q, J=7.0, Hz, 1H), 2.28 (t, J=7.5, Hz, 1H), 1.82-1.65 (m, 1H), 1.63-1.52 (m, 1H), 1.45-1.38 (m, 1H), 1.25 (s, 3H), 1.11 (s, 2H), 0.85 (t, J=1.3 Hz, 1H), 0.66-0.57 (m, 1H); .sup.13C NMR (101 MHz, CDCl.sub.3) .delta.=174.13, 71.51, 50.46, 42.54, 41.93, 36.69, 31.81, 29.89, 26.47, 23.99, 22.63, 22.57, 14.00, 6.46; .sup.29Si NMR (79 MHz, CDCl.sub.3) .delta.=-42.27.
Example 2
##STR00006##
[0058] .sup.1H NMR (400 MHz, Chloroform-d) .delta. 6.51 (s, OH), 5.22 (qd, J=6.1, 1.7 Hz, 1H), 3.45 (s, 4H), 3.11 (q, J=7.0, Hz, 1H), 2.21 (dd, J=6.2, 3.8 Hz, 1H), 2.04-1.85 (m, 2H), 1.77-1.56 (m, 1H), 1.55-1.44 (m, 1H), 1.39 (dt, J=11.2, 4.2 Hz, 1H), 1.05 (d, J=1.9 Hz, 1H), 0.84 (td, J=7.5, 2.1 Hz, 1H), 0.53 (m, 1H); .sup.13C NMR (101 MHz, CDCl.sub.3) .delta.=174.12, 131.54, 128.91, 71.30, 50.39, 42.36, 41.92, 36.76, 30.93, 26.25, 22.60, 21.81, 20.37, 14.22, 6.43; .sup.29Si NMR (79 MHz, CDCl.sub.3) .delta.=-42.28.
Example 3
##STR00007##
[0060] .sup.1H NMR (400 MHz, Chloroform-d) .delta. 6.83 (s, OH), 3.57 (s, 6H), 3.31-3.19 (m, 1H), 3.04-2.59 (m, 1H), 1.71-1.59 (m, 3H), 0.70-0.61 (m, 1H); .sup.13C NMR (101 MHz, CDCl.sub.3) .delta.=171.16, 107.83, 81.59, 50.61, 41.97, 40.80, 23.35, 22.41, 6.56; .sup.29Si NMR (79 MHz, CDCl.sub.3) .delta.=-42.48.
Comparative Examples 1 and 2: Preparation of Secondary Hydroxyl Functional Methoxysilanes
[0061] In a dry round bottom flask under argon atmosphere 5 g (27.9 mmol) of (3-aminopropyl)trimethoxysilane (AMMO) was stirred at 50.degree. C. 0.28 ml of 1M triethylaluminium solution in hexane was slowly added (0.279 mmol, 1 mol %). Afterwards 27.9 mmol of the mono-substituted lactone listed in Table 1 was added and vigorously stirred for 3 hours.
[0062] A yellowish and viscous following product from Comparative Example 1 was obtained.
##STR00008##
[0063] .sup.1H NMR (400 MHz, Chloroform-d) .delta. 6.55 (s, OH), 3.51 (s, 5H), 3.19-3.12 (m, 1H), 2.32-2.26 (m, 1H), 1.84-1.71 (m, 1H), 1.65-1.49 (m, 2H), 1.37 (m, 2H), 0.86 (t, J=7.0 Hz, 1H), 0.63-0.55 (m, 1H); .sup.13C NMR (101 MHz, CDCl.sub.3) .delta.=173.95, 70.74, 50.46, 41.92, 39.78, 33.10, 32.81, 22.62, 18.86, 14.03, 6.45; .sup.29Si NMR (79 MHz, CDCl.sub.3) .delta.=-42.22.
[0064] The NMR of the product from Comparative Example 2 showed a highly crosslinked structure, which cannot be used further.
Comparative Example 3: Preparation of Primary Hydroxyl Functional Methoxysilane
[0065] In a dry round bottom flask under argon atmosphere 5 g (27.9 mmol) of (3-aminopropyl)trimethoxysilane (AMMO) was stirred at 50.degree. C. 0.28 ml of 1M triethylaluminium solution in hexane was slowly added (0.279 mmol, 1 mol %). Afterwards 27.9 mmol of .delta.-valerolactone was added and vigorously stirred for 3 hours. A yellow highly viscous product was obtained. The NMR of the product of Comparative Example 3 showed a highly crosslinked structure, which cannot be used further.
Testing the Stability of Prepared Silanes
[0066] After the preparation of the hydroxyl functional silanes as described above, a round bottom flask with the sample under the nitrogen atmosphere was placed in the heating oven at 50.degree. C. for 8 days. A small amount of sample for the NMR analysis was withdrawn from the flask immediately before putting it the oven and after 2, 5 and 8 days. The degree of crosslinking and consequently the purity was assessed based the integration of the .sup.29Si NMR spectra. The peak at around -42 ppm corresponded to the non-hydrolyzed trimethoxysilane, the peak at around -41 ppm corresponded to self-dealcoholized product and the peaks below the value of -44 ppm correspond to the mono-, di- or three-hydrolized (oligomerized or crosslinked) silane. It was determined that only the silanes with purity higher than 90% after 8 days at 50.degree. C. suffice the standards for further applications.
TABLE-US-00001 TABLE 1 Purity comparison of the hydroxyl functional silanes Purity (%) Right after 2 5 8 Lactone reaction days days days Example 1 4-methyl decalactone 98 98 98 97 Example 2 4-hydroxyl-4-methyl-7- 96 95 94 92 cis-decene gamma lactone Example 3 4-methyl-4- 97 94 93 91 (trichloromethyl)- 2-oxetanone Comp. gamma-heptalactone 92 88 85 82 Example 1 Comp. gamma-valerolactone Completely crosslinked Example 2 Comp. delta-valerolactone Completely crosslinked Example 3
* * * * *
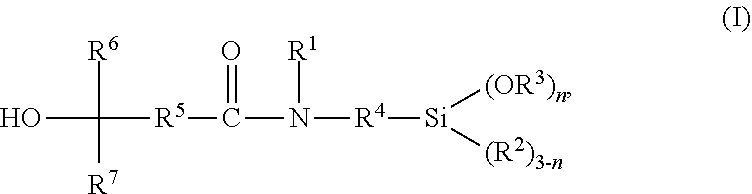
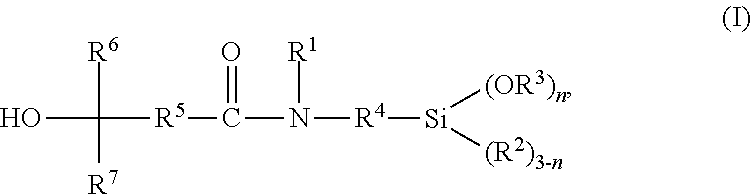








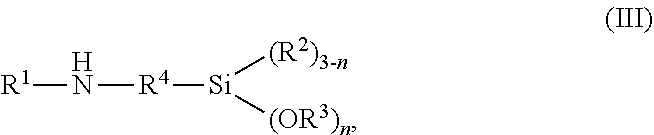
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.