Dual Pdgf/vegf Antagonists
Perlroth; D. Victor ; et al.
U.S. patent application number 16/795450 was filed with the patent office on 2020-08-20 for dual pdgf/vegf antagonists. This patent application is currently assigned to KODIAK SCIENCES INC.. The applicant listed for this patent is KODIAK SCIENCES INC.. Invention is credited to James Aggen, Didier Benoit, Stephen A. Charles, Justin Cohen, Tetsuya Ishino, Laura Lin, Lidia Mosyak, D. Victor Perlroth, William Somers, Wayne To.
| Application Number | 20200262905 16/795450 |
| Document ID | 20200262905 / US20200262905 |
| Family ID | 1000004812677 |
| Filed Date | 2020-08-20 |
| Patent Application | download [pdf] |











View All Diagrams
| United States Patent Application | 20200262905 |
| Kind Code | A1 |
| Perlroth; D. Victor ; et al. | August 20, 2020 |
DUAL PDGF/VEGF ANTAGONISTS
Abstract
The invention provides a dual VEGF/PDGF antagonist comprising a VEGF antagonist linked to a PDGF antagonist. The VEGF antagonist is an antibody to a VEGF or VEGFR or is a VEGFR extracellular trap segment (i.e., a segment from the extracellular region of one or more VEGFR receptors that inhibits binding of at least one VEGFR to at least one VEGF). The PDGF antagonist is an antibody to a PDGF or PDGFR or is a PDGFR extracellular trap segment (i.e., segment from the extracellular region of one or more PDGFRs, which inhibits binding of at least one PDGFR and at least one PDGF). The dual antagonist is preferably conjugated to a half-life extending moiety, such as a HEMA-PC polymer. The dual antagonist is particularly useful for treating wet aged related macular degeneration.
| Inventors: | Perlroth; D. Victor; (Palo Alto, CA) ; Charles; Stephen A.; (Ravenna, OH) ; Aggen; James; (Westwood, MA) ; Benoit; Didier; (San Jose, CA) ; To; Wayne; (San Mateo, CA) ; Mosyak; Lidia; (Newton, MA) ; Lin; Laura; (Weston, MA) ; Cohen; Justin; (Quincy, MA) ; Ishino; Tetsuya; (Boston, MA) ; Somers; William; (Lexington, MA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | KODIAK SCIENCES INC. PALO ALTO CA |
||||||||||
| Family ID: | 1000004812677 | ||||||||||
| Appl. No.: | 16/795450 | ||||||||||
| Filed: | February 19, 2020 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15820325 | Nov 21, 2017 | |||
| 16795450 | ||||
| 14753824 | Jun 29, 2015 | 9840553 | ||
| 15820325 | ||||
| PCT/US2015/038203 | Jun 28, 2015 | |||
| 14753824 | ||||
| 62018579 | Jun 28, 2014 | |||
| 62018579 | Jun 28, 2014 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C07K 2317/31 20130101; C07K 2317/92 20130101; C07K 2319/036 20130101; A61K 38/1866 20130101; C07K 2317/76 20130101; C07K 2317/71 20130101; C07K 16/22 20130101; C07K 2317/526 20130101; C07K 2317/524 20130101; C07K 2317/565 20130101; C07K 16/28 20130101; C07K 16/2863 20130101; A61K 38/1858 20130101; C07K 14/71 20130101; A61K 38/179 20130101; C07K 2317/72 20130101; C07K 2318/10 20130101; C07K 2319/30 20130101; C07K 2317/55 20130101; C07K 2317/522 20130101; A61K 2039/505 20130101 |
| International Class: | C07K 16/22 20060101 C07K016/22; A61K 38/17 20060101 A61K038/17; A61K 38/18 20060101 A61K038/18; C07K 14/71 20060101 C07K014/71; C07K 16/28 20060101 C07K016/28 |
Claims
1. A fusion protein comprising a vascular endothelial growth factor (hereinafter "VEGF") antagonist linked to a platelet-derived growth factor (hereinafter "PDGF") antagonist, wherein the VEGF antagonist is an anti-VEGF antibody, and the PDGF antagonist is a PDGF receptor (hereinafter "PDGFR") extracellular trap segment, wherein the PDGFR extracellular trap segment comprises domains D1-D3 of PDGFR-.beta..
2.-61. (canceled)
Description
INCORPORATION BY REFERENCE TO ANY PRIORITY APPLICATIONS
[0001] Any and all applications for which a foreign or domestic priority claim is identified in the Application Data Sheet as filed with the present application are hereby incorporated by reference under 37 CFR 1.57.
[0002] This application is a continuation of patent application Ser. No. 15/820,325, filed Nov. 21, 2017, which is a divisional of patent application Ser. No. 14/753,824, filed Jun. 29, 2015, the entirety of which is incorporated herein by reference. Patent application Ser. No. 14/753,824 is a continuation of Patent Application Serial No PCT/US2015/038203, filed Jun. 28, 2015, all of which claim full priority benefit of U.S. Provisional Application Ser. No. 62/018,579 filed Jun. 28, 2014, which is incorporated herein by reference in its entirety.
REFERENCE TO SEQUENCE LISTING
[0003] A Sequence Listing submitted as an ASCII text file via EFS-Web is hereby incorporated by reference in accordance with 35 U.S.C. .sctn. 1.52(e). The name of the ASCII text file for the Sequence Listing is 32236617_1.TXT, the date of creation of the ASCII text file is Feb. 19, 2020, and the size of the ASCII text file is 219 KB.
BACKGROUND OF THE INVENTION
Field of the Invention
[0004] Angiogenesis (the formation of blood vessels) occurs throughout an organism's development. Indeed, the first organ in an embryo is a blood vessel. Angiogenesis is also crucial for wound healing, restoring blood flow to damaged tissue. However, improper or dysregulated angiogenesis contributes to or causes many diseases including cancer, psoriasis, arthritis and blindness. Carmeliet P. 2003. Angiogenesis in health and disease. Nature Med 9(6):653-660.
Description of the Related Art
[0005] Age related macular degeneration (AMD) is a leading cause of vision loss and blindness in the elderly. About ten million Americans are afflicted with AMD. The prevalence of AMD in the population increases steadily with age: at 40 years of age only about 2% of the population is affected by AMD but by the age of 80 it is about 25%. Friedman, D. S. et al. 2004. Arch. Ophthalmol. 122:564-572. There are generally two types of AMD: dry and wet.
[0006] Dry AMD is the most common form of the disease. In dry AMD, there is a depletion of the layer of the retinal pigment epithelial cells in the macula. Dry AMD is chronic and generally causes some loss of vision. In severe cases of dry AMD, patients can develop near total blindness. Wet AMD develops in some 10-15% of patients with dry AMD. Wet AMD is characterized by angiogenesis, specifically choroidal neovascularization (CNV). CNV is characterized by the presence of new immature blood vessels which grow towards the outer retina from the choroid. These immature blood vessels leak fluid below and in the retina, causing vision loss and blindness. Wet AMD blindness is typically acute.
[0007] Angiogenesis also plays a crucial role in cancer and tumor formation and maintenance. The recruitment of new blood vessels is an essential component of the metastatic pathway. For many tumors, the vascular density can provide a prognostic indicator of metastatic potential: highly vascular tumors have a higher incidence of metastasis than less vascular tumors.
[0008] Angiogenesis is the result of a complex interplay between growth factors, vascular endothelial cells, extracellular matrix molecules, chemokines and cell signaling molecules. Factors identified as mediators of angiogenesis include: basic and acidic fibroblast growth factor, transforming growth factors .alpha. and .beta. platelet-derived growth factor (PDGF), angiogenin, platelet-derived endothelial cell growth factor, IL8, and vascular endothelial growth factor (VEGF). The role of VEGF in angiogenesis has been extensively reported on.
[0009] It has been shown that VEGF signaling presents a crucial rate limiting step in physiological angiogenesis. VEGF also plays a central role in pathological angiogenesis (e.g., tumor growth). Ferrara N and Davis-Smyth T. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18: 4-25. VEGF is also known to induce vascular leakage. Bates D O and Curry F E. 1997. Vascular endothelial growth factor increases microvascular permeability via a Ca (2+)-dependent pathway. Am J Physiol. 273: H687-H694; Roberts W G and Palade G E. 1995. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 108:2369-2379.
[0010] Anti-VEGF therapeutics have been successfully used to treat wet AMD and cancer. Genentech's anti-VEGF monoclonal antibody bevacizumab (Avastin.RTM.) received FDA approval in 2004 for the treatment of cancer. Anti-VEGF agents have been approved for the treatment of wet AMD. In 2004, the FDA approved Eyetech/Pfizer Macugen.RTM.. Genentech's Lucentis.RTM. was approved in 2006 for wet AMD. Bevacizumab is also used off label for the treatment of wet AMD. In 2011, Regeneron's Eylea.RTM. was approved for treatment of wet AMD.
[0011] Despite the success of anti-VEGF therapeutics, none of them causes regression in the pathological neovascular (NV) tissue. Hence, NV tissue remains despite continued anti-VEGF treatment and can prevent significant vision gain for treated patients. The NV tissue consists of endothelial cells, pericytes and inflammatory cells (i.e., occasional macrophages). The presence of pericytes on capillaries not only leads to NV support and stabilization but promotes endothelial cell survival through chemical signaling and physical interactions including pericyte production of VEGF. This endothelial survival signaling by integrated pericytes is critical and may explain the resistance of the NV tissue to VEGF withdrawal, i.e., lack of NV regression to monotherapy anti-VEGF treatment. In addition, over time the pathological NV tissue can lead to fibrosis and scarring.
[0012] Subretinal scarring develops in nearly half of treated eyes within two years of anti-VEGF therapy. Daniel E, Toth C A, Grunwald J E. 2014. Risk of scar in the comparison of age-related macular degeneration in clinical settings. Retina 32: 1480-1485. Subretinal fibrosis formation can cause permanent dysfunction of the macular system; it causes destruction of photoreceptors, retinal pigment epithelium and choroidal vessels. Ishikawa K, Ram K, Hinton D R. 2015. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Eye Res. xxx: 1-7. While anti-VEGF therapy generally stabilizes or improves visual acuity, scar formation has been identified as one of the causes of loss of visual acuity after treatment. Cohen S Y, Oubraham H, Uzzan J, et al. 2012. Causes of unsuccessful ranibizumab treatment in exudative age-related macular degeneration in clinical settings. Retina 32: 1480-1485.
[0013] PDGF has been reported to play a role in pericyte recruitment, maturation and resistance to anti-VEGF mediated regression. Corneal and choroidal neovascularization animal models have been reported to have demonstrated that administration of agents that block the PDGF-B/PDGFR-.beta. interaction leads to pericyte stripping from the pathological neovasculature. Jo N, Mailhos C, Ju M, et al. 2006. Inhibition of Platelet-Derived Growth Factor B Signaling Enhances the Efficacy of Anti-Vascular Endothelial Growth Factor Therapy in Multiple Models of Ocular Neovascularization. American J Path. 168(6):2036-2053.
[0014] To target both pathways, clinical trials are currently underway in which patients receive two medications: Lucentis.RTM. (an anti-VEGF Fab) and Fovista.TM. a PEGYlated aptamer directed against PDGF by Ophthotech. Fovista is directed against only a single PDGF ligand: PDGF-BB. However, there are many other PDGF ligands: PDGF-AA, PDGF-CC and PDGF-DD. PDGF-DD, for example, has been shown to play a crucial role in ocular angiogenesis. Kumar A, Hou X, Chunsik L, et al. 2010. Platelet-derived Growth Factor-DD Targeting Arrests Pathological Angiogenesis by Modulating Glycogen Synthase Kinase-313 Phosphorylation. J Biol Chem 285(20):15500-15510. Yet Fovista does not interact with PDGF-DD. There is a need in the art for broader based anti-PDGF therapies.
[0015] In addition, aptamer based therapeutics in general have poor pharmacokinetic properties in that aptamers are subject to renal filtration and to serum digestion. While these problems can be somewhat overcome with PEGylation, PEGylation tends to reduce binding to target. Aptamers typically bind with much lower affinity to targets than their antibody counterparts. PEGylation will tend to reduce binding even further. There is, thus, a need in the art for non-aptamer based anti-PDGF therapeutics.
[0016] Current clinical plans for Fovista double the number of injections patients must receive for treatment relative to the currently approved anti-VEGF therapies. Fovista is formulated separately from the anti-VEGF agent so patients must be given two injections instead of one. Moreover the injections cannot be at the same time because of build-up in intraocular pressure caused by a single injection.
[0017] From the view point of both patients and treating physicians, intravitreal injections are not trivial. Many patients experience pain and discomfort from the injection and patient compliance is a serious issue. Common side effects of intravitreal injections include conjunctiva! hemorrhage, eye pain, vitreous floaters, increased intraocular pressure, and intraocular inflammation. Intravitreal injections are associated with relatively rare serious adverse events, including endophthalmitis, retinal detachment and traumatic cataracts.
[0018] There is thus a need in the art for therapies that do not increase the number of intravitreal injections that patients must endure. In addition, current anti-VEGF therapies often require once a month injections. There is also a need for therapies which are needed less frequently than once a month.
SUMMARY OF THE INVENTION
[0019] The invention provides a dual VEGF/PDGF antagonist comprising a VEGF antagonist linked to a PDGF antagonist, wherein the VEGF antagonist (a) is an antibody to a VEGF or VEGFR or (b) is a VEGFR extracellular trap segment and the PDGF antagonist (a) is an antibody to a PDGF or PDGFR or (b) is a PDGFR extracellular trap segment, provided that the VEGF and PDGF antagonists are not both antibodies. Optionally, the VEGF antagonist is an antibody comprising a heavy chain and a light chain and the PDGF antagonist is the PDGFR extracellular trap segment, and the heavy chain of the antibody is fused via a linker to the C-terminus of the PDGFR extracellular trap segment, and the light chain is complexed with the heavy chain. Optionally, the antibody is a Fab fragment. Optionally, the antibody is an intact antibody. Optionally, the PDGF antagonist is an extracellular trap segment of a PDGFR-.alpha. or PDGFR-.beta. receptor and the VEGF antagonist is an antibody to a VEGF. Optionally, the PDGFR extracellular trap segment comprises one or more of domains D1-D5 of PDGFR-.beta.. Optionally, the PDGFR extracellular trap segment comprises domains D1-D3 of PDGFR-.beta.. Optionally, the PDGFR extracellular trap segment comprises amino acids 33 to 314 of SEQ ID NO. 11. Optionally, the VEGF antagonist comprises an anti-VEGF antibody. Optionally, the anti-VEGF antibody is an anti-VEGF-A antibody. Optionally, the PDGFR extracellular trap segment is located C-terminal of the heavy or light chain. Optionally, the PDGFR extracellular trap segment is located N-terminal of the heavy or light chain.
[0020] Optionally, the dual VEGF/PDGF antagonist of further comprising a linker which is located between the PDGFR trap and the anti-VEGF antibody heavy chain. Optionally the linker is GGGGSGGGGS, GG, or GGGGSGGGGSGGGGSGGGGSG.
[0021] Optionally, the anti-VEGF antibody heavy chain comprises CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3: YPYYYGTSHWYFDV. Optionally, the anti-VEGF light chain comprises CDR.sub.L1: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L3: QQYSTVPWT.
[0022] Optionally, the anti-VEGF heavy chain isotype is IgG comprising a CH.sub.1, hinge, CH.sub.2 and CH.sub.3 domains and the light chain isotype is kappa. Optionally the IgG 1 constant domain has the sequence set forth in SEQ ID NO. 17 and the light chain constant region has the sequence set forth in SEQ ID NO. 18.
[0023] Optionally, the IgG 1 constant domain has one or more mutations to reduce effector function. Optionally the mutations are to one or more of the following amino acid positions (EU numbering): E233, L234, L235, G236, G237, A327, A330, and P331. Optionally, the mutations are selected from the group consisting of: E233P, L234V, L234A, L235A, G237A, A327G, A330S and P331S. Optionally, mutations are L234A, L235A and G237A.
[0024] Optionally, the dual VEGF/PDGF antagonist comprises a heavy chain further comprising a cysteine residue added by recombinant DNA technology. Optionally, the cysteine residue is selected from the group consisting of (EU numbering) Q347C and L443C.
[0025] Optionally, the dual VEGF/PDGF antagonist has a heavy chain comprising the amino acid sequence off SEQ ID NO. 9 and the light chain has an amino acid sequence of SEQ ID NO. 10.
[0026] Optionally, the dual VEGF/PDGF antagonist comprises a PDGFR trap extracellular segment comprising one or more of domains D1-D5 of PDGFR-.beta.. Optionally, the PDGFR trap extracellular segment comprises domains D1-D3 of PDGFR-.beta.. Optionally, the PDGFR trap extracellular segment comprises amino acids 33 to 314 of SEQ ID NO. 11.
[0027] Optionally, the dual VEGF/PDGF antagonist comprises a VEGF antagonist, which is an anti-VEGF antibody. Optionally, the antibody is an anti-VEGF-A Fab fragment. Optionally, the PDGFR extracellular trap segment is located C-terminal of the Fab heavy or light chain. Optionally, the PDGFR extracellular trap segment is located N-terminal of the Fab heavy or light chain.
[0028] Optionally, the dual VEGF/PDGF comprises a heavy chain comprising an anti-VEGF-A Fab fragment heavy chain and a light chain comprising an anti-VEGF-A light chain. Optionally, the dual antagonist further comprises a linker which is located between the PDGFR trap and the anti-VEGF Fab fragment heavy chain. Optionally, the linker is selected from group consisting of GGGGSGGGGS, GG, and GGGGSGGGGSGGGGSGGGGSG. Optionally, the anti-VEGF Fab fragment heavy chain comprises CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3: YPYYYGTSHWYFDV. Optionally, the anti-VEGF light chain comprises CDRd: SASQDISNYLN, CDRr2: FTSSLHS and CDRL3: QQYSTVPWT. Optionally, the anti-VEGF heavy chain isotype is IgG 1 comprising a CH.sub.1 domain and the light chain isotype is kappa.
[0029] Any of the dual VEGF/PDGF antagonists can further comprise a half-life extending moiety. Optionally, the half-life extending moiety comprises a polymer, which is PEG or a zwitterionic polymer. Optionally, the zwitterionic polymer comprises a monomer comprising phosphorylcholine. Optionally, the monomer comprises 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. Optionally, the monomer comprises 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC). Optionally, the polymer has 3 or more arms. Optionally, the polymer has 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Optionally, the polymer has 3, 6 or 9 arms. Optionally, the polymer has 9 arms. Optionally, the polymer portion of the conjugate has a peak molecular weight of between 300,000 and 1,750,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 500,000 and 1,000,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 600,000 to 800,000 Da. Optionally, the dual VEGF/PDGF antagonist is covalently bonded to the polymer. Optionally, the polymer is covalently bonded to at least one of an amino group, a hydroxyl group, a sulfhydryl group and a carboxyl group. Optionally, the sulfhydryl group is from a naturally occurring cysteine residue. Optionally, the sulfhydryl group is from a cysteine residue added by recombinant DNA technology. Optionally, the polymer is covalently bonded to the cysteine residue at position 731 of SEQ ID NO. 9.
[0030] Optionally, the VEGF antagonist comprises a VEGFR extracellular trap segment comprising one or more extracellular segments of VEGFR-1, VEGFR-2 and VEGFR-3 and the PDGF antagonist is an anti-PDGF antibody. Optionally, the extracellular segment of VEGFR comprises one or more of domains D1-D7. Optionally, the extracellular segment comprises D2 from VEGFR-1 and D3 from VEGFR-2. Optionally, the D2 is N-terminal to the D3 and further comprises a linker between the domains. Optionally, the PDGF antagonist is an intact antibody. Optionally, the PDGF antagonist is a Fab fragment. Optionally, the anti-PDGFR antibody is humanized 2A 1E2, HuM4 Ts.22, humanized 1B3, humanized 2C5, anti-PDGF-BB, anti-PDGF-DD, anti-PDGF-BB or anti-PDGF-AB. Optionally, the heavy chain is IgG 1 and the light chain is kappa. Optionally, the heavy chain sequence has a cysteine added via recombinant DNA technology the cysteine selected from the groups consisting of Q347C or a L443C. Optionally, the dual VEGF/PDGF antagonist further comprises a half-life extending moiety conjugated to the cysteine. Optionally, the dual VEGF/PDGF antagonist protein has a half-life extending moiety comprising a zwitterionic polymer, the polymer comprising one or more monomer units and wherein at least one monomer unit comprises a zwitterionic group, such as phosphorylcholine. Optionally, the monomer comprises 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. Optionally, the monomer comprises 2-(methacryloyloxyethyl)-2' (trimethylammoniumethyl) phosphate (HEMA-PC). Optionally, the polymer has 3 or more arms. Optionally, the polymer has 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Optionally, the polymer has 3, 6 or 9 arms. Optionally, the polymer has 9 arms. Optionally, the polymer portion of the conjugate has a peak molecular weight of between 300,000 and 1,750,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 500,000 and 1,000,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 600,000 to 800,000 Da.
[0031] In some dual VEGF/PDGF antagonists the PDGF antagonist comprises a PDGF extracellular trap segment comprising one or more extracellular segments of a PDGFR selected from the group consisting of PDGFR-.alpha. and PDGFR-.beta. and the VEGF antagonist is a VEGF extracellular trap segment comprising one or more extracellular segments of a VEGFR selected from the group consisting of VEGFR-1, VEGFR-2 and VEGFR-3. Optionally, the extracellular trap segment of VEGFR comprises one or more of domains D1-D7. Optionally, the extracellular trap segment comprises D2 from VEGFR-1 and D3 from VEGFR-2. Optionally, the D2 is N-terminal to the D3 and further comprises a linker between the domains. Optionally, the PDGFR trap comprises one or more of domains D1-D5 of PDGFR-.beta.. Optionally, the PDGFR trap comprises domains D1-D3 of PDGFR-. Optionally, the PDGFR trap comprises amino acids 33 to 314 of SEQ ID NO. 11. Optionally, the dual VEGF/PDGF antagonist further comprises a linker sequence between the VEGF antagonist and the PDGF antagonist. Optionally, the dual VEGF/PDGF antagonist further comprises a half-life extending moiety. Optionally, the half-life extending moiety comprises a polymer selected from the group consisting of PEG and a zwitterionic polymer. Optionally, the half-life extending moiety comprises a zwitterionic polymer. Optionally, the zwitterionic polymer comprises a monomer comprising phosphorylcholine. Optionally, the monomer comprises 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. Optionally, the monomer comprises 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC). Optionally, the polymer has 3 or more arms. Optionally, the polymer has 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Optionally, the polymer has 3, 6 or 9 arms. Optionally, the polymer portion of the conjugate has a peak molecular weight of between 300,000 and 1,750,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 500,000 and 1,000,000 Da. Optionally, the polymer portion of the conjugate has a peak molecular weight between 600,000 to 800,000 Da. Optionally, the polymer has 9 arms. Optionally, the dual VEGF/PDGF antagonist is covalently bonded to the polymer. Optionally, the polymer is covalently bonded to at least one of an amino group, a hydroxyl group, a sulfhydryl group and a carboxyl group. Optionally, the sulfhydryl group is from a naturally occurring cysteine residue. Optionally, the sulfhydryl group is from a cysteine residue added by recombinant DNA technology.
[0032] Any dual VEGF/PDGF antagonist as described above can be used in treatment or prophylaxis of disease, particularly a neovascular disorder, optionally an ocular neovascular disorder, such as wet age related macular degeneration.
BRIEF DESCRIPTION OF THE DRAWINGS
[0033] FIG. 1: Protein Sequence of human PDGFR-.beta..
[0034] FIG. 2: Protein Sequence of VEGFR-1.
[0035] FIG. 3: Protein Sequence of VEGFR-2.
[0036] FIG. 4: Protein Sequence of VEGFR-3.
[0037] FIG. 5: bevacizumab sequence (DrugBank DBOO 112)
[0038] FIG. 6: ranibizumab (published by Novartis).
[0039] FIGS. 7A, B: Protein Sequence of A. PDGFR.beta.-GS 10-anti-VEGF-A light chain and B. anti-VEGF-A heavy chain.
[0040] FIGS. 8A, B: A. Protein Sequence of PDGFR.beta.-GG-anti-VEGF-A light chain and B. anti-VEGF-A heavy chain.
[0041] FIGS. 9A, B. Protein Sequence of A. PDGFR.beta.-GS 10-anti-VEGF-A heavy chain (wild type Fe) and B. anti-VEGF-A light chain.
[0042] FIGS. 10A, B. Protein Sequence of A. PDGFR.beta.-GG-anti-VEGF-A heavy chain (wild type Fe) and B. anti-VEGF-A light chain.
[0043] FIGS. 11A, B. Protein Sequence of A. anti-VEGF-A heavy chain (wild type Fc)-GS21-PDGFR.beta. and B. anti-VEGF-A light chain.
[0044] FIGS. 12A, B. Protein Sequence of A. PDGFR-.beta.-GS21-anti-VEGF-A heavy chain (Q347C) and B. anti-VEGF-A light chain (TAF347).
[0045] FIGS. 13A, B. Protein Sequence of A. PDGFR-.beta.-GS21-anti-VEGF-A heavy chain (L443C) and B. anti-VEGF-A light chain (TAF443).
[0046] FIGS. 14A, B. Protein Sequence of A. PDGFR.beta.-GS 10-anti-VEGF-A light chain and B. anti-VEGF-A Fab.
[0047] FIGS. 15A, B. Protein Sequence of A. PDGFR-GG-anti-VEGF-A light chain and B. anti-VEGF-A Fab.
[0048] FIGS. 16A, B. Protein Sequence of A. PDGFR.beta.-GS 10-anti-VEGF-A Fab and B. anti-VEGF-A light chain.
[0049] FIGS. 17A, B. Protein Sequence of A. PDGFR.beta.-GG-anti-VEGF-A Fab and B. anti-VEGF-A light chain.
[0050] FIGS. 18A, B. Protein Sequence of A. anti-VEGF-A Fab-GS21-PDGFR.beta. and B. anti-VEGF-A light chain.
[0051] FIGS. 19A, B. Protein Sequence of A. PDGFR.beta.-GS 10-anti-VEGF-A Fab with certain mutations and B. anti-VEGF-A light chain.
[0052] FIGS. 20A, B. Protein Sequence of A. PDGFR.beta.-anti-VEGF-A heavy chain and B. anti-VEGF-A light chain (1a).
[0053] FIGS. 21A, B. Protein Sequence of A. PDGFR-.beta. (D2-D3)-anti-VEGF-A heavy chain and B. anti-VEGF-A light chain (1b).
[0054] FIGS. 22A, B. Protein Sequence of A. PDGFR-.beta. (D1-D3)-anti-VEGF-A Fab and B. anti-VEGF-A light chain (2b).
[0055] FIGS. 23A, B. Protein Sequence of A. PDGFR-.beta.(D2-D3)-6xGS-anti-VEGF-A Fab and B. anti-VEGF-A light chain (2b').
[0056] FIGS. 24A, B. Protein sequence of A. PDGFR-.beta.-6xGS-anti-VEGF-A Fab and B. anti-VEGF-A light chain.
[0057] FIGS. 25A, B: Protein Sequence of A. anti-VEGF-A Fab-6xGS-PDGFR-.beta. (D2-D3) and B. anti-VEGF-A light chain (3).
[0058] FIG. 26 shows the chemical structure of OG 1448.
[0059] FIG. 27 shows Compound L.
[0060] FIG. 28 shows Compound K.
[0061] FIG. 29 shows the synthesis of OG1802 from R3707.
[0062] FIG. 30 shows OG1786.
[0063] FIG. 31 shows the synthesis of OG1546 from OG1550.
[0064] FIG. 32 shows the synthesis of OG1784 from OG1546 and OG1563.
[0065] FIG. 33 shows the synthesis of OG1405 from OG1784.
[0066] FIG. 34 shows the synthesis of OG1785 from OG1405.
[0067] FIG. 35 shows the synthesis of OG1786 from OG1785.
[0068] FIG. 36 shows OG1802.
[0069] FIG. 37 shows a graph of percent Grade IV laser lesions.
[0070] FIG. 38 shows Compound E.
[0071] FIG. 39 depicts OG1448.
[0072] FIG. 40 shows relative angiogenesis using OG1448, Avastin, and an anti-PDGF-BB antibody and various combinations thereof.
[0073] FIG. 41 shows the % grade IV lesions compared to day in the CNV monkey model for the compounds indicated.
[0074] FIG. 42 shows OG1448 ocular pharmacokinetics versus aflibercept and ranibizumab in the rabbit vitreous.
BRIEF DESCRIPTION OF SEQ ID NOS.
[0075] SEQ ID NO. 1 is the protein sequence of PDGFRb-GS 10-LightChain anti-VEGF-A (Bevacizumab).
[0076] SEQ ID NO. 2 is the anti-VEGF-A Bevacizumab heavy chain.
[0077] SEQ ID NO. 3 is protein sequence of PDGFRb-GG-Light Chain anti-VEGF-A
[0078] (Bevacizumab).
[0079] SEQ ID NO. 4 is PDGFR.beta.-GS 10-Heavy Chain-anti-VEGF-A (Bevacizumab).
[0080] SEQ ID NO. 5 is the anti-VEGF-A Bevacizumab light chain.
[0081] SEQ ID NO. 6 is PDGFR-GG-Heavy Chain-anti-VEGF-A (Bevacizumab).
[0082] SEQ ID NO. 7 is anti-VEGF-A Heavy Chain (Bevacizumab)-GS21-PDGFR.beta..
[0083] SEQ ID NO. 8 is the amino acid sequence of the heavy chain trap extracellular segment of TAF347: PDGFR-trap-anti-VEGF-A heavy chain (Q347C).
[0084] SEQ ID NO. 9 is the amino acid sequence of the heavy chain trap extracellular segment of TAF443: PDGFR-.beta. trap-anti-VEGF-A heavy chain (L443C) and SEQ ID NO:10 is the amino acid sequence of the light chain of anti-VEGF-A.
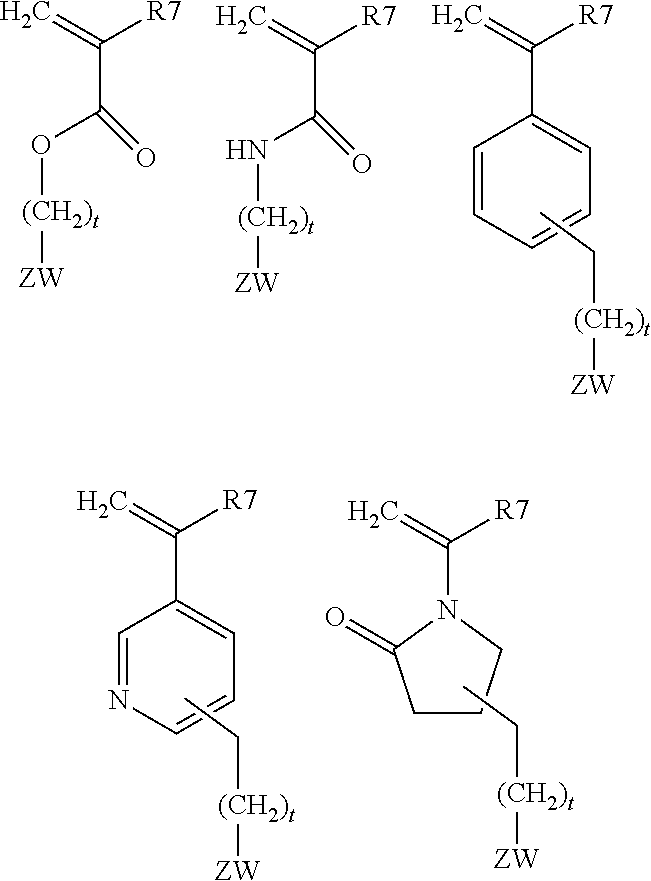
[0085] SEQ ID NO. 11 is human PDGFR-.beta..
[0086] SEQ ID NO. 12 is the ranibizumab light chain.
[0087] SEQ ID NO. 13 is the ranibizumab heavy chain.
[0088] SEQ ID NO. 14 is human VEGFR-1.
[0089] SEQ ID NO. 15 is human VEGFR-2.
[0090] SEQ ID NO. 16 is human VEGFR-3.
[0091] SEQ ID NO. 17 is a human IgG1 constant region.
[0092] SEQ ID NO. 18 is a human kappa light constant region.
[0093] SEQ ID NO. 19 is FIG. 7. PDGFR-GS 10-anti-VEGF-A light chain.
[0094] SEQ ID NO. 20 is FIG. 8. PDGFR-GG-anti-VEGF-A light chain.
[0095] SEQ ID NO. 21 is a Bevacizumab Fab.
[0096] SEQ ID NO. 22 is a PDGFR-.beta.-GS 10-anti-VEGF-A Fab.
[0097] SEQ ID NO. 23 is a PDGFR-.beta.-GG-anti-VEGF-A Fab.
[0098] SEQ ID NO. 24 is an anti-VEGF-A Fab-GS21-PDGFR-.beta..
[0099] SEQ ID NO. 25 is a PDGFR-.beta.-GS 10-anti-VEGF-A Fab with certain mutations.
[0100] SEQ ID NO. 26 is a protein sequence of PDGFR.beta.-anti-VEGF-A heavy chain (1a).
[0101] SEQ ID NO. 27 is a protein sequence of PDGFR-.beta.-(D2-D3)-anti-VEGF-A heavy chain (1b).
[0102] SEQ ID NO. 28 is a protein sequence of PDGFR-.beta. (D2-D3)-anti-VEGF-A Fab (2b).
[0103] SEQ ID NO. 29 is a protein sequence of PDGFR-.beta. (D2-D3)-6xGS-anti-VEGF-A
[0104] SEQ ID NO. 30 is a protein sequence of anti-VEGF-A Fab-6xGS-PDGFR-.beta. (D2-
[0105] SEQ ID NO. 31 is a nucleic acid encoding a heavy chain anti-VEGF-PDGFR fusion.
[0106] SEQ ID NO. 32 is a nucleic acid encoding a light chain anti-VEGF.
[0107] GGGGS (SEQ ID NO. 37), GGGS (SEQ ID NO. 38), GGGES (SEQ ID NO. 39), GGGGSGGGGS (SEQ ID NO. 40) and GGGGSGGGGSGGGGSGGGGSG) (SEQ ID NO. 41).
[0108] Ranibizumab CDRs are: CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3: YPYYYGTSHWYFDV (SEQ ID NOS. 42-44), CDR.sub.L1: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L3: QQYSTVPWT (SEQ ID NOS. 45-47). Bevacizumab CDR.sub.H1 is GYTFTNYGMN (SEQ ID NO. 48) and CDR.sub.H3 is YPHYYGSSHWYFDV (SEQ ID NO:49).
Definitions
[0109] A "neovascular disorder" is a disorder or disease state characterized by altered, dysregulated or unregulated angiogenesis. Examples of neovascular disorders include neoplastic transformation (e.g. cancer) and ocular neovascular disorders including diabetic retinopathy and age-related macular degeneration.
[0110] An "ocular neovascular" disorder is a disorder characterized by altered, dysregulated or unregulated angiogenesis in the eye of a patient. Such disorders include optic disc neovascularization, iris neovascularization, retinal neovascularization, choroidal neovascularization, corneal neovascularization, vitreal neovascularization, glaucoma, pannus, pterygium, macular edema, diabetic retinopathy, diabetic macular edema, vascular retinopathy, retinal degeneration, uveitis, inflammatory diseases of the retina, and proliferative vitreoretinopathy.
[0111] A "polypeptide linker" is a polypeptide comprising two or more amino acid residues joined by peptide bonds that are used to link two polypeptides (e.g., a VH and VL domain or a VH domain and an extracellular trap segment). Examples of such linker polypeptides are well known in the art (see, e.g., Bolliger P, Prospero T, Winter G. 1993. PNAS USA. 90:6444-6448; Poljak R J. 1994. Production and Structure of Diabodies. Structure 2: 1121-1123). Exemplary linkers include G, GG, GGGGS, GGGS, and GGGES, and oligomers of such linkers (e.g., GGGGSGGGGS and GGGGSGGGGSGGGGSGGGGSG).
[0112] Dual antagonists or other biologics described herein are typically provided in isolated form. This means that an antagonist is typically at least 50% w/w pure of interfering proteins and other contaminants arising from its production or purification but does not exclude the possibility that the antagonist is combined with an excess of pharmaceutical acceptable excipient intended to facilitate its use. Sometimes antagonists are at least 60, 70, 80, 90, 95 or 99% w/w pure of interfering proteins and contaminants from production or purification. Often an antagonist is the predominant macromolecular species remaining after its purification.
[0113] The term antibody includes intact antibodies and binding fragments thereof. A binding fragment refers to a molecule other than an intact antibody that comprises a portion of an intact antibody that binds the antigen to which the intact antibody binds. Examples of binding fragments include Fv, Fab', Fab'-SH, F(ab')2; diabodies; linear antibodies; single-chain antibody molecules (e.g. scFv); and multispecific antibodies formed from antibody fragments. scFv antibodies are described in Houston J S. 1991. Methods in Enzymol. 203:46-96. In addition, antibody fragments comprise single chain polypeptides having the characteristics of a VH domain, namely being able to assemble together with a VL domain, or of a VL domain, namely being able to assemble together with a VH domain to a functional antigen binding site and thereby providing the antigen binding property of full length antibodies.
[0114] Specific binding of an antibody, extracellular trap segment or dual antagonist to its target antigen(s) means an affinity of at least 10.sub.6, 10.sub.7, 10.sub.8, 10.sub.9, or 10.sub.10 M.sup.-.sub.1. Specific binding is detectably higher in magnitude and distinguishable from non-specific binding occurring to at least one unrelated target. Specific binding can be the result of formation of bonds between particular functional groups or particular spatial fit (e.g., lock and key type) whereas nonspecific binding is usually the result of van der Waals forces. Specific binding does not however necessarily imply that an antibody or fusion protein binds one and only one target.
[0115] A basic antibody structural unit is a tetramer of subunits. Each tetramer includes two identical pairs of polypeptide chains, each pair having one "light" (about 25 kDa) and one "heavy" chain (about 50-70 kDa). The amino-terminal portion of each chain includes a variable region of about 100 to 110 or more amino acids primarily responsible for antigen recognition.
[0116] This variable region is initially expressed linked to a cleavable signal peptide. The variable region without the signal peptide is sometimes referred to as a mature variable region. Thus, for example, a light chain mature variable region means a light chain variable region without the light chain signal peptide. However, reference to a variable region does not mean that a signal sequence is necessarily present; and in fact signal sequences are cleaved once the antibodies or fusion proteins of the invention have been expressed and secreted. A pair of heavy and light chain variable regions defines a binding region of an antibody. The carboxy-terminal portion of the light and heavy chains respectively defines light and heavy chain constant regions. The heavy chain constant region is primarily responsible for effector function. In IgG antibodies, the heavy chain constant region is divided into CHI, hinge, CH2, and CH3 regions. The CHI region binds to the light chain constant region by disulfide and noncovalent bonding. The hinge region provides flexibility between the binding and effector regions of an antibody and also provides sites for intermolecular disulfide bonding between the two heavy chain constant regions in a tetramer subunit. The CH2 and CH3 regions are the primary site of effector functions and FcR binding.
[0117] Light chains are classified as either kappa or lambda. Heavy chains are classified as gamma, mu, alpha, delta, or epsilon, and define the antibody's isotype as IgG, IgM, IgA, IgD and IgE, respectively. Within light and heavy chains, the variable and constant regions are joined by a "J" segment of about 12 or more amino acids, with the heavy chain also including a "D" segment of about 10 or more amino acids. (See generally, Fundamental Immunology (Paul, W., ed., 2nd ed. Raven Press, N.Y., 1989), Ch. 7) (incorporated by reference in its entirety for all purposes).
[0118] The mature variable regions of each light/heavy chain pair form the antibody binding site. Thus, an intact antibody has two binding sites, i.e., is divalent. In natural antibodies, the binding sites are the same. However, bispecific antibodies can be made in which the two binding sites are different (see, e.g., Songsivilai S, Lachmann P C. 1990. Bispecific antibody: a tool for diagnosis and treatment of disease. Clin Exp Immunol. 79:315-321; Kostelny S A, Cole M S, Tso J Y. 1992. Formation of bispecific antibody by the use of leucine zippers. J Immunol. 148: 1547-1553). The variable regions all exhibit the same general structure of relatively conserved framework regions (FR) joined by three hypervariable regions, also called complementarity determining regions or CDRs. The CDRs from the two chains of each pair are aligned by the framework regions, enabling binding to a specific epitope. From N-terminal to C-terminal, both light and heavy chains comprise the domains FRI, CDR1, FR2, CDR2, FR3, CDR3 and FR4. For convenience, the variable heavy CDRs can be referred to as CDR.sub.H1, CDR.sub.H2 and CDR.sub.H3; the variable light chain CDRs can be referred to as CDR.sub.L1, CDR.sub.L2 and CDR.sub.L3. The assignment of amino acids to each domain is in accordance with the definitions of Kabat E A, et al. 1987 and 1991. Sequences of Proteins of Immunological Interest (National Institutes of Health, Bethesda, Md.) or Chothia C, Lesk A M. 1987. Canonical Structures for the Hypervariable Regions of Immunoglobulins. J Mol Biol 196:901-917; Chothia C, et al. 1989. Conformations of Immunoglobulin Hypervariable Regions. Nature 342:877-883. Kabat also provides a widely used numbering convention (Kabat numbering) in which corresponding residues between different heavy chain variable regions or between different light chain variable regions are assigned the same number. Although Kabat numbering can be used for antibody constant regions, EU numbering is more commonly used, as is the case in this application. Although specific sequences are provided for exemplary dual antagonists, it will be appreciated that after expression of protein chains one to several amino acids at the amino or carboxy terminus of the light and/or heavy chain, particularly a heavy chain C-terminal lysine residue, may be missing or derivatized in a proportion or all of the molecules.
[0119] The term "epitope" refers to a site on an antigen to which an antibody or extracellular trap segment binds. An epitope on a protein can be formed from contiguous amino acids or noncontiguous amino acids juxtaposed by tertiary folding of one or more proteins. Epitopes formed from contiguous amino acids (also known as linear epitopes) are typically retained on exposure to denaturing solvents whereas epitopes formed by tertiary folding (also known as conformational epitopes) are typically lost on treatment with denaturing solvents. An epitope typically includes at least 3, and more usually, at least 5 or 8-10 amino acids in a unique spatial conformation. Methods of determining spatial conformation of epitopes include, for example, x-ray crystallography and 2-dimensional nuclear magnetic resonance. See, e.g., Epitope Mapping Protocols, in Methods in Molecular Biology, Vol. 66, Glenn E. Morris, Ed. (1996).
[0120] Antibodies that recognize the same or overlapping epitopes can be identified in a simple immunoassay showing the ability of one antibody to compete with the binding of another antibody to a target antigen. The epitope of an antibody can also be defined by X-ray crystallography of the antibody (or Fab fragment) bound to its antigen to identify contact residues.
[0121] Alternatively, two antibodies have the same epitope if all amino acid mutations in the antigen that reduce or eliminate binding of one antibody reduce or eliminate binding of the other. Two antibodies have overlapping epitopes if some amino acid mutations that reduce or eliminate binding of one antibody reduce or eliminate binding of the other.
[0122] Competition between antibodies is determined by an assay in which an antibody under test inhibits specific binding of a reference antibody to a common antigen (see, e.g., Junghans et al., Cancer Res. 50: 1495, 1990). A test antibody competes with a reference antibody if an excess of a test antibody (e.g., at least 2.times., 5.times., 10.times., 20.times. or 100.times.) inhibits binding of the reference antibody by at least 50% but preferably 75%, 90% or 99% as measured in a competitive binding assay. Antibodies identified by competition assay (competing antibodies) include antibodies binding to the same epitope as the reference antibody and antibodies binding to an adjacent epitope sufficiently proximal to the epitope bound by the reference antibody for steric hindrance to occur.
[0123] The term "patient" includes human and other mammalian subjects that receive either prophylactic or therapeutic treatment.
[0124] For purposes of classifying amino acids substitutions as conservative or nonconservative, amino acids are grouped as follows: Group I (hydrophobic side chains): met, ala, val, leu, ile; Group II (neutral hydrophilic side chains): cys, ser, thr; Group III (acidic side chains): asp, glu; Group IV (basic side chains): asn, gin, his, lys, arg; Group V (residues influencing chain orientation): gly, pro; and Group VI (aromatic side chains): trp, tyr, phe. Conservative substitutions involve substitutions between amino acids in the same class. Non-conservative substitutions constitute exchanging a member of one of these classes for a member of another.
[0125] Percentage sequence identities are determined with antibody sequences maximally aligned by the Kabat numbering convention for a variable region or EU numbering for a constant region. After alignment, if a subject antibody region (e.g., the entire mature variable region of a heavy or light chain) is being compared with the same region of a reference antibody, the percentage sequence identity between the subject and reference antibody regions is the number of positions occupied by the same amino acid in both the subject and reference antibody region divided by the total number of aligned positions of the two regions, with gaps not counted, multiplied by 100 to convert to percentage. Sequence identities of other sequences can be determined by aligning sequences using algorithms, such as BESTFIT, PASTA, and TFASTA in the Wisconsin Genetics Software Package Release 7.0, Genetics Computer Group, 575 Science Dr., Madison, Wis., using default gap parameters, or by inspection, and the best alignment (i.e., resulting in the highest percentage of sequence similarity over a comparison window).
[0126] Percentage of sequence identity is calculated by comparing two optimally aligned sequences over a window of comparison, determining the number of positions at which the identical residues occurs in both sequences to yield the number of matched positions, dividing the number of matched positions by the total number of positions in the window of comparison (i.e., the window size), and multiplying the result by 100 to yield the percentage of sequence identity.
[0127] Compositions or methods "comprising" one or more recited elements may include other elements not specifically recited. For example, a composition that comprises antibody may contain the antibody alone or in combination with other ingredients.
[0128] The term "antibody-dependent cellular cytotoxicity", or ADCC, is a mechanism for inducing cell death that depends upon the interaction of antibody-coated target cells (i.e., cells with bound antibody) with immune cells possessing lytic activity (also referred to as effector cells). Such effector cells include natural killer cells, monocytes/macrophages and neutrophils. ADCC is triggered by interactions between the Fe region of an antibody bound to a cell and Fey receptors, particularly Fc.gamma.RI and Fc.gamma.RIII, on immune effector cells such as neutrophils, macrophages and natural killer cells. The target cell is eliminated by phagocytosis or lysis, depending on the type of mediating effector cell. Death of the antibody-coated target cell occurs as a result of effector cell activity.
[0129] The term opsonization also known as "antibody-dependent cellular phagocytosis", or ADCP, refers to the process by which antibody-coated cells are internalized, either in whole or in part, by phagocytic immune cells (e.g., macrophages, neutrophils and dendritic cells) that bind to an immunoglobulin Fe region.
[0130] The term "complement-dependent cytotoxicity" or CDC refers to a mechanism for inducing cell death in which an Fe effector domain(s) of a target-bound antibody activates a series of enzymatic reactions culminating in the formation of holes in the target cell membrane. Typically, antigen-antibody complexes such as those on antibody-coated target cells bind and activate complement component C1q which in turn activates the complement cascade leading to target cell death. Activation of complement may also result in deposition of complement components on the target cell surface that facilitate ADCC by binding complement receptors (e.g., CR3) on leukocytes.
[0131] A humanized antibody is a genetically engineered antibody in which the CDRs from a non-human "donor" antibody are grafted into human "acceptor" antibody sequences (see, e.g., Queen, U.S. Pat. Nos. 5,530,101 and 5,585,089; Winter, U.S. Pat. No. 5,225,539, Carter, U.S. Pat. No. 6,407,213, Adair, U.S. Pat. No. 5,859,205 6,881,557, Foote, U.S. Pat. No. 6,881,557). The acceptor antibody sequences can be, for example, a mature human antibody sequence, a composite of such sequences, a consensus sequence of human antibody sequences, or a germline region sequence. Thus, a humanized antibody is an antibody having some or all CDRs entirely or substantially from a donor antibody and variable region framework sequences and constant regions, if present, entirely or substantially from human antibody sequences. Similarly a humanized heavy chain has at least one, two and usually all three CDRs entirely or substantially from a donor antibody heavy chain, and a heavy chain variable region framework sequence and heavy chain constant region, if present, substantially from human heavy chain variable region framework and constant region sequences. Similarly a humanized light chain has at least one, two and usually all three CDRs entirely or substantially from a donor antibody light chain, and a light chain variable region framework sequence and light chain constant region, if present, substantially from human light chain variable region framework and constant region sequences. Other than nanobodies and dAbs, a humanized antibody comprises a humanized heavy chain and a humanized light chain. A CDR in a humanized antibody is substantially from a corresponding CDR in a non-human antibody when at least 85%, 90%, 95% or 100% of corresponding residues (as defined by Kabat) are identical between the respective CDRs. The variable region framework sequences of an antibody chain or the constant region of an antibody chain are substantially from a human variable region framework sequence or human constant region respectively when at least 85, 90, 95 or 100% of corresponding residues defined by Kabat are identical.
[0132] Although humanized antibodies often incorporate all six CDRs (preferably as defined by Kabat) from a mouse antibody, they can also be made with less than all CDRs (e.g., at least 3, 4, or 5 CDRs from a mouse antibody) (e.g., De Pascalis R, Iwahashi M, Tamura M, et al. 2002. Grafting "Abbreviated" Complementary-Determining Regions Containing Specificity-Determining Residues Essential for Ligand Contact to Engineer a Less Immunogenic Humanized Monoclonal Antibody. J Immunol. 169:3076-3084; Vajdos F F, Adams C W, Breece T N, Presta L G, de Vos A M, Sidhu, S S. 2002. Comprehensive functional maps of the antigen-binding site of an anti-ErbB2 antibody obtained with shotgun scanning mutagenesis. J Mol Biol. 320: 415-428; Iwahashi M, Milenic D E, Padlan E A, et al. 1999. CDR substitutions of a humanized monoclonal antibody (CC49): Contributions of individual CDRs to antigen binding and immunogenicity. Mol Immunol. 36:1079-1091; Tamura M, Milenic D E, Iwahashi M, et al. 2000. Structural correlates of an anticarcinoma antibody: Identification of specificity-determining regions (SDRs) and development of a minimally immunogenic antibody variant by retention of SDRs only. J Immunol. 164:1432-1441).
[0133] A chimeric antibody is an antibody in which the mature variable regions of light and heavy chains of a non-human antibody (e.g., a mouse) are combined with human light and heavy chain constant regions. Such antibodies substantially or entirely retain the binding specificity of the mouse antibody, and are about two-thirds human sequence.
[0134] A veneered antibody is a type of humanized antibody that retains some and usually all of the CDRs and some of the non-human variable region framework residues of a non-human antibody but replaces other variable region framework residues that may contribute to B- or T-cell epitopes, for example exposed residues (Padlan E A. 1991. A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand-binding properties. Mol Immunol. 28:489-98) with residues from the corresponding positions of a human antibody sequence. The result is an antibody in which the CDRs are entirely or substantially from a non-human antibody and the variable region frameworks of the non-human antibody are made more human-like by the substitutions. A human antibody can be isolated from a human, or otherwise result from expression of human immunoglobulin genes (e.g., in a transgenic mouse, in vitro or by phage display). Methods for producing human antibodies include the trioma method of Ostberg L, Pursch E. 1983. Human x (mouse x human) hybridomas stably producing human antibodies. Hybridoma 2:361-367; Ostberg, U.S. Pat. No. 4,634,664; and Engleman et al., U.S. Pat. No. 4,634,666, use of transgenic mice including human immunoglobulin genes (see, e.g., Lonberg et al., WO93/12227 (1993); U.S. Pat. Nos. 5,877,397, 5,874,299, 5,814,318, 5,789,650, 5,770,429, 5,661,016, 5,633,425, 5,625,126, 5,569,825, 5,545,806, Nature 148, 1547-1553 (1994), Nature Biotechnology 14, 826 (1996), Kucherlapati, WO 91/10741 (1991) and phage display methods (see, e.g. Dower et al., WO 91/17271 and McCafferty et al., WO 92/01047, U.S. Pat. Nos. 5,877,218, 5,871,907, 5,858,657, 5,837,242, 5,733,743 and 5,565,332.
[0135] "Polymer" refers to a series of monomer groups linked together. A polymer is composed of multiple units of a single monomer (a homopolymer) or different monomers (a heteropolymer). High MW polymers are prepared from monomers that include, but are not limited to, acrylates, methacrylates, acrylamides, methacrylamides, styrenes, vinyl-pyridine, vinyl-pyrrolidone and vinyl esters such as vinyl acetate. Additional monomers are useful in the high MW polymers of the present invention. When two different monomers are used, the two monomers are called "comonomers," meaning that the different monomers are copolymerized to form a single polymer. The polymer can be linear or branched. When the polymer is branched, each polymer chain is referred to as a "polymer arm." The end of the polymer arm linked to the initiator moiety is the proximal end, and the growing-chain end of the polymer arm is the distal end. On the growing chain-end of the polymer arm, the polymer arm end group can be the radical scavenger, or another group.
[0136] "Initiator" refers to a compound capable of initiating a polymerization using the monomers or comonomers of the present invention. The polymerization can be a conventional free radical polymerization or preferably a controlled/"living" radical polymerization, such as Atom Transfer Radical Polymerization (ATRP), Reversible Addition-Fragmentation-Termination (RAFT) polymerization or nitroxide mediated polymerization (NMP). The polymerization can be a "pseudo" controlled polymerization, such as degenerative transfer. When the initiator is suitable for ATRP, it contains a labile bond which can be homolytically cleaved to form an initiator fragment, I, being a radical capable of initiating a radical polymerization, and a radical scavenger, I', which reacts with the radical of the growing polymer chain to reversibly terminate the polymerization. The radical scavenger I' is typically a halogen, but can also be an organic moiety, such as a nitrile. In some embodiments of the present invention, the initiator contains one of more 2-bromoisobutyrate groups as sites for polymerization via ATRP.
[0137] A "chemical linker" refers to a chemical moiety that links two groups together, such as a half-life extending moiety and a protein. The linker can be cleavable or non-cleavable. Cleavable linkers can be hydrolyzable, enzymatically cleavable, pH sensitive, photolabile, or disulfide linkers, among others. Other linkers include homobifunctional and heterobifunctional linkers. A "linking group" is a functional group capable of forming a covalent linkage consisting of one or more bonds to a bioactive agent. Non-limiting examples include those illustrated in Table 1 of WO2013059137 (incorporated by reference).
[0138] The term "reactive group" refers to a group that is capable of reacting with another chemical group to form a covalent bond, i.e. is covalently reactive under suitable reaction conditions, and generally represents a point of attachment for another substance. The reactive group is a moiety, such as maleimide or succinimidyl ester, is capable of chemically reacting with a functional group on a different moiety to form a covalent linkage. Reactive groups generally include nucleophiles, electrophiles and photoactivatable groups.
[0139] "Phosphorylcholine," also denoted as "PC," refers to the following:
##STR00001##
[0140] where * denotes the point of attachment. The phosphorylcholine is a zwitterionic group and includes salts (such as inner salts), and protonated and deprotonated forms thereof.
[0141] "Phosphorylcholine containing polymer" is a polymer that contains phosphorylcholine. "Zwitterion containing polymer" refers to a polymer that contains a zwitterion.
[0142] Poly(acryloyloxyethyl phosphorylcholine) containing polymer refers to a polymer containing 2-(acryloyloxy)ethyl-2-(trimethylammonium)ethyl phosphate (HEA-PC shown below in Example 51) as monomer.
[0143] Poly(methacryloyloxyethyl phosphorylcholine) containing polymer refers to a polymer containing 2-(methacryloyloxy)ethyl-2-(trimethylammonium)ethyl phosphate (HEMA-PC) as monomer.
[0144] "Molecular weight" in the context of the polymer can be expressed as either a number average molecular weight, or a weight average molecular weight or a peak molecular weight. Unless otherwise indicated, all references to molecular weight herein refer to the peak molecular weight. These molecular weight determinations, number average (Mn), weight average (Mw) and peak (Mp), can be measured using size exclusion chromatography or other liquid chromatography techniques. Other methods for measuring molecular weight values can also be used, such as the use of end-group analysis or the measurement of colligative properties (e.g., freezing-point depression, boiling-point elevation, or osmotic pressure) to determine number average molecular weight, or the use of light scattering techniques, ultracentrifugation or viscometry to determine weight average molecular weight. In a preferred embodiment of the present invention, the molecular weight is measured by SEC-MALS (size exclusion chromatography-multi angle light scattering). The polymeric reagents of the invention are typically polydisperse (i.e., number average molecular weight and weight average molecular weight of the polymers are not equal), preferably possessing low polydispersity values of, for example, less than about 1.5, as judged, for example, by the PDI value derived from the SEC-MALS measurement. In other embodiments, the polydispersities (PDI) are more preferably in the range of about 1.4 to about 1.2, still more preferably less than about 1.15, and still more preferably less than about 1.10, yet still more preferably less than about 1.05, and most preferably less than about 1.03.
[0145] The phrase "a" or "an" entity refers to one or more of that entity; for example, a compound refers to one or more compounds or at least one compound. As such, the terms "a" (or "an"), "one or more", and "at least one" can be used interchangeably herein.
[0146] "About" means variation one might see in measurements taken among different instruments, samples, and sample preparations.
[0147] "Protected," "protected form," "protecting group" and "protective group" refer to the presence of a group (i.e., the protecting group) that prevents or blocks reaction of a particular chemically reactive functional group in a molecule under certain reaction conditions. Protecting groups vary depending upon the type of chemically reactive group being protected as well as the reaction conditions to be employed and the presence of additional reactive or protecting groups in the molecule, if any. Suitable protecting groups include those such as found in the treatise by Greene et al., "Protective Groups In Organic Synthesis," 3rd Edition, John Wiley and Sons, Inc., New York, 1999.
[0148] "Alkyl" refers to a straight or branched, saturated, aliphatic radical having the number of carbon atoms indicated. For example, C1-C6 alkyl includes, but is not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, hexyl, etc. Other alkyl groups include, but are not limited to heptyl, octyl, nonyl, decyl, etc. Alkyl can include any number of carbons, such as 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 2-3, 2-4, 2-5, 2-6, 3-4, 3-5, 3-6, 4-5, 4-6 and 5-6. The alkyl group is typically monovalent, but can be divalent, such as when the alkyl group links two moieties together.
[0149] The term "lower" referred to above and hereinafter in connection with organic radicals or compounds respectively defines a compound or radical which can be branched or unbranched with up to and including 7, preferably up to and including 4 and (as unbranched) one or two carbon atoms.
[0150] "Alkylene" refers to an alkyl group, as defined above, linking at least two other groups, i.e., a divalent hydrocarbon radical. The two moieties linked to the alkylene can be linked to the same atom or different atoms of the alkylene. For instance, a straight chain alkylene can be the bivalent radical of --(CH2).sub.n, where n is 1, 2, 3, 4, 5 or 6. Alkylene groups include, but are not limited to, methylene, ethylene, propylene, isopropylene, butylene, isobutylene, sec-butylene, pentylene and hexylene.
[0151] Substituents for the alkyl and heteroalkyl radicals (including those groups often referred to as alkylene, alkenyl, heteroalkylene, heteroalkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl, and heterocycloalkenyl) can be a variety of groups selected from: --OR', .dbd.O, .dbd.NR', .dbd.N--OR'--NR'R'''--SR''-halogen'-SiR'R''R''''--OC(O)R''--C(O)R''--CO.s- ub.2R''--CONR'R'', --OC(O)NR'R'', --NR''C(O)R', --NR'--C(O)NR''R''', --NR''C(O).sub.2R', --NH--C(NH.sub.2).dbd.NH, --NR'C(NH.sub.2).dbd.N H, --NH--C(NH.sub.2).dbd.NR', --S(O)R', --S(OhR', --S(O).sub.2NR'R'', --CN and --NO.sub.2 in a number ranging from zero to (2m'+1), where m' is the total number of carbon atoms in such radical. R', R'' and R'''each independently refer to hydrogen, unsubstituted (C.sub.1-C.sub.8)alkyl and heteroalkyl, unsubstituted aryl, aryl substituted with 1-3 halogens, unsubstituted alkyl, alkoxy or thioalkoxy groups, or aryl-(C.sub.1-C.sub.4) alkyl groups. When R' and R'' are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 5-, 6-, or 7-membered ring. For example, --NR'R'' is meant to include 1-pyrrolidinyl and 4-morpholinyl. The term "alkyl" is include groups such as haloalkyl (e.g., --CF.sub.3 and --CH.sub.2CF.sub.3) and acyl (e.g., --C(O)CH.sub.3, --C(O)CF.sub.3, --C(O)CH.sub.2OCH.sub.3, and the like). Preferably, the substituted alkyl and heteroalkyl groups have from 1 to 4 substituents, more preferably 1, 2 or 3 substituents. Exceptions are those perhalo alkyl groups (e.g., pentafluoroethyl and the like) which are also preferred and contemplated by the present invention.
[0152] Substituents for the alkyl and heteroalkyl radicals (including those groups often referred to as alkylene, alkenyl, heteroalkylene, heteroalkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl, and heterocycloalkenyl) can be one or more of a variety of groups selected from, but not limited to: --OR', .dbd.O, .dbd.NR', .dbd.N--OR''--NR'R'''--SR''-halogen'-SiR'R''R'''--OC(O)R''--C(O)R''--CO.s- ub.2R''--CONR'R'''--OC(O)NR'R'', --NR''C(O)R', --NR'--C(O)NR''R''', --NR''C(OhR', --NR--C(NR'R''R''').dbd.NR'''', --NR--C(NR'R'').dbd.NR''', S(O)R', --S(O).sub.2R', --S(O).sub.2NR'R'', --NRSO.sub.2R', --CN and --NO.sub.2 in a number ranging from zero to (2m'+1), where m' is the total number of carbon atoms in such radical. R', R'', R''' and R'''' each preferably independently refer to hydrogen, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, e.g., aryl substituted with 1-3 halogens, substituted or unsubstituted alkyl, alkoxy or thioalkoxy groups, or arylalkyl groups. When a compound of the invention includes more than one R group, for example, each of the R groups is independently selected as are each R', R'', R''' and R'''' groups when more than one of these groups is present. When R' and R'' are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 5-, 6-, or 7-membered ring. For example, --NR'R'' is meant to include, but not be limited to, 1-pyrrolidinyl and 4-morpholinyl. From the above discussion of substituents, one of skill in the art will understand that the term "alkyl" is meant to include groups including carbon atoms bound to groups other than hydrogen groups, such as haloalkyl (e.g., --CF.sub.3 and --CH.sub.2CF.sub.3) and acyl (e.g., --C(O)CH.sub.3, --C(O)CF.sub.3, --C(O)CH.sub.2OCH.sub.3, and the like).
[0153] "Alkoxy" refers to alkyl group having an oxygen atom that either connects the alkoxy group to the point of attachment or is linked to two carbons of the alkoxy group. Alkoxy groups include, for example, methoxy, ethoxy, propoxy, iso-propoxy, butoxy, 2-butoxy, iso-butoxy, sec-butoxy, tert-butoxy, pentoxy, hexoxy, etc. The alkoxy groups can be further substituted with a variety of substituents described within. For example, the alkoxy groups can be substituted with halogens to form a "halo-alkoxy" group.
[0154] "Carboxyalkyl" means an alkyl group (as defined herein) substituted with a carboxy group. The term "carboxycycloalkyl" means a cycloalkyl group (as defined herein) substituted with a carboxy group. The term alkoxyalkyl means an alkyl group (as defined herein) substituted with an alkoxy group. The term "carboxy" employed herein refers to carboxylic acids and their esters.
[0155] "Haloalkyl" refers to alkyl as defined above where some or all of the hydrogen atoms are substituted with halogen atoms. Halogen (halo) preferably represents chloro or fluoro, but may also be bromo or iodo. For example, haloalkyl includes trifluoromethyl, fluoromethyl, 1,2,3,4,5-pentafluoro-phenyl, etc. The term "perfluoro" defines a compound or radical which has all available hydrogens that are replaced with fluorine. For example, perfluorophenyl refers to 1,2,3,4,5-pentafluorophenyl, perfluoromethyl refers to 1,1,1-trifluoromethyl, and perfluoromethoxy refers to 1,1,1-trifluoromethoxy.
[0156] "Fluoro-substituted alkyl" refers to an alkyl group where one, some, or all hydrogen atoms have been replaced by fluorine.
[0157] "Cytokine" in the context of this invention is a member of a group of protein signaling molecules that may participate in cell-cell communication in immune and inflammatory responses. Cytokines are typically small, water-soluble glycoproteins that have a mass of about 8-35 kDa.
[0158] "Cycloalkyl" refers to a cyclic hydrocarbon group that contains from about 3 to 12, from 3 to 10, or from 3 to 7 endocyclic carbon atoms. Cycloalkyl groups include fused, bridged and spiro ring structures.
[0159] "Endocyclic" refers to an atom or group of atoms which comprise part of a cyclic ring structure.
[0160] "Exocyclic" refers to an atom or group of atoms which are attached but do not define the cyclic ring structure.
[0161] "Cyclic alkyl ether" refers to a 4 or 5 member cyclic alkyl group having 3 or 4 endocyclic carbon atoms and 1 endocyclic oxygen or sulfur atom (e.g., oxetane, thietane, tetrahydrofuran, tetrahydrothiophene); or a 6 to 7 member cyclic alkyl group having 1 or 2 endocyclic oxygen or sulfur atoms (e.g., tetrahydropyran, 1,3-dioxane, 1,4-dioxane, tetrahydrothiopyran, 1,3-dithiane, 1,4-dithiane, 1,4-oxathiane).
[0162] "Alkenyl" refers to either a straight chain or branched hydrocarbon of 2 to 6 carbon atoms, having at least one double bond. Examples of alkenyl groups include, but are not limited to, vinyl, propenyl, isopropenyl, 1-butenyl, 2-butenyl, isobutenyl, butadienyl, 1-pentenyl, 2-pentenyl, isopentenyl, 1,3-pentadienyl, 1,4-pentadienyl, 1-hexenyl, 2-hexenyl, 3-hexenyl, 1,3-hexadienyl, 1,4-hexadienyl, 1,5-hexadienyl, 2,4-hexadienyl, or 1,3,5-hexatrienyl. Alkenyl groups can also have from 2 to 3, 2 to 4, 2 to 5, 3 to 4, 3 to 5, 3 to 6, 4 to 5, 4 to 6 and 5 to 6 carbons. The alkenyl group is typically monovalent, but can be divalent, such as when the alkenyl group links two moieties together.
[0163] "Alkenylene" refers to an alkenyl group, as defined above, linking at least two other groups, i.e., a divalent hydrocarbon radical. The two moieties linked to the alkenylene can be linked to the same atom or different atoms of the alkenylene. Alkenylene groups include, but are not limited to, ethenylene, propenylene, isopropenylene, butenylene, isobutenylene, sec-butenylene, pentenylene and hexenylene.
[0164] "Alkynyl" refers to either a straight chain or branched hydrocarbon of 2 to 6 carbon atoms, having at least one triple bond. Examples of alkynyl groups include, but are not limited to, acetylenyl, propynyl, 1-butynyl, 2-butynyl, isobutynyl, sec-butynyl, butadiynyl, 1-pentynyl, 2-pentynyl, isopentynyl, 1,3-pentadiynyl, 1,4-pentadiynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 1,3-hexadiynyl, 1,4-hexadiynyl, 1,5-hexadiynyl, 2,4-hexadiynyl, or 1,3,5-hexatriynyl. Alkynyl groups can also have from 2 to 3, 2 to 4, 2 to 5, 3 to 4, 3 to 5, 3 to 6, 4 to 5, 4 to 6 and 5 to 6 carbons. The alkynyl group is typically monovalent, but can be divalent, such as when the alkynyl group links two moieties together.
[0165] "Alkynylene" refers to an alkynyl group, as defined above, linking at least two other groups, i.e., a divalent hydrocarbon radical. The two moieties linked to the alkynylene can be linked to the same atom or different atoms of the alkynylene. Alkynylene groups include, but are not limited to, ethynylene, propynylene, butynylene, sec-butynylene, pentynylene and hexynylene.
[0166] "Cycloalkyl" refers to a saturated or partially unsaturated, monocyclic, fused bicyclic or bridged polycyclic ring assembly containing from 3 to 12 ring atoms, or the number of atoms indicated. Monocyclic rings include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cyclooctyl. Bicyclic and polycyclic rings include, for example, norbomane, decahydronaphthalene and adamantane. For example, C.sub.3-8 cycloalkyl includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclooctyl, and norbornane.
[0167] "Cycloalkylene" refers to a cycloalkyl group, as defined above, linking at least two other groups, i.e., a divalent hydrocarbon radical. The two moieties linked to the cycloalkylene can be linked to the same atom or different atoms of the cycloalkylene. Cycloalkylene groups include, but are not limited to, cyclopropylene, cyclobutylene, cyclopentylene, cyclohexylene, and cyclooctylene.
[0168] "Heterocycloalkyl" refers to a ring system having from 3 ring members to about 20 ring members and from 1 to about 5 heteroatoms such as N, O and S. Additional heteroatoms can also be useful, including, but not limited to, B, Al, Si and P. The heteroatoms can also be oxidized, such as, but not limited to, --S(O)-- and --S(Oh-. For example, heterocycle includes, but is not limited to, tetrahydrofuranyl, tetrahydrothiophenyl, morpholino, pyrrolidinyl, pyrrolinyl, imidazolidinyl, imidazolinyl, pyrazolidinyl, pyrazolinyl, piperazinyl, piperidinyl, indolinyl, quinuclidinyl and 1,4-dioxa-8-aza-spiro[4.5]dec-8-yl.
[0169] "Heterocycloalkylene" refers to a heterocyclalkyl group, as defined above, linking at least two other groups. The two moieties linked to the heterocycloalkylene can be linked to the same atom or different atoms of the heterocycloalkylene.
[0170] "Aryl" refers to a monocyclic or fused bicyclic, tricyclic or greater, aromatic ring assembly containing 6 to 16 ring carbon atoms. For example, aryl may be phenyl, benzyl or naphthyl, preferably phenyl. "Arylene" means a divalent radical derived from an aryl group. Aryl groups can be mono-, di- or tri-substituted by one, two or three radicals selected from alkyl, alkoxy, aryl, hydroxy, halogen, cyano, amino, amino-alkyl, trifluoromethyl, alkylenedioxy and oxy-C.sub.2C.sub.3-alkylene; all of which are optionally further substituted, for instance as hereinbefore defined; or 1- or 2-naphthyl; or 1- or 2-phenanthrenyl. Alkylenedioxy is a divalent substitute attached to two adjacent carbon atoms of phenyl, e.g. methylenedioxy or ethylenedioxy. Oxy-C.sub.2C.sub.3-alkylene is also a divalent substituent attached to two adjacent carbon atoms of phenyl, e.g. oxyethylene or oxypropylene. An example for oxy-C.sub.2C.sub.3-alkylene-phenyl is 2,3-dihydrobenzofuran-5-yl.
[0171] Preferred as aryl is naphthyl, phenyl or phenyl mono- or disubstituted by alkoxy, phenyl, halogen, alkyl or trifluoromethyl, especially phenyl or phenyl-mono- or disubstituted by alkoxy, halogen or trifluoromethyl, and in particular phenyl.
[0172] Examples of substituted phenyl groups as R are, e.g. 4-chlorophen-1-yl, 3,4-dichlorophen-1-yl, 4-methoxyphen-1-yl, 4-methylphen-1-yl, 4-aminomethylphen-1-yl, 4-methoxyethylaminomethylphen-1-yl, 4-hydroxyethylaminomethylphen-1-yl, 4-hydroxyethyl-(methyl)-aminomethylphen-1-yl, 3-aminomethylphen-1-yl, 4-N-acetylaminomethylphen-1-yl, 4-aminophen-1-yl, 3-aminophen-1-yl, 2-aminophen-1-yl, 4-phenyl-phen-1-yl, 4-(imidazol-1-yl)-phenyl, 4-(imidazol-1-ylmethyl)-phen-1-yl, 4-(morpholin-1-yl)-phen-1-yl, 4-(morpholin-1-ylmethyl)-phen-1-yl, 4-(2-methoxyethylaminomethyl)-phen-1-yl and 4-(pyrrolidin-1-ylmethyl)-phen-1-yl, 4-(thiophenyl)-phen-1-yl, 4-(3-thiophenyl)-phen-1-yl, 4-(4-methylpiperazin-1-yl)-phen-1-yl, and 4-(piperidinyl)-phenyl and 4-(pyridinyl)-phenyl optionally substituted in the heterocyclic ring.
[0173] "Arylene" refers to an aryl group, as defined above, linking at least two other groups. The two moieties linked to the arylene are linked to different atoms of the arylene. Arylene groups include, but are not limited to, phenylene.
[0174] "Arylene-oxy" refers to an arylene group, as defined above, where one of the moieties linked to the arylene is linked through an oxygen atom. Arylene-oxy groups include, but are not limited to, phenylene-oxy.
[0175] Similarly, substituents for the aryl and heteroaryl groups are varied and are selected from: -halogen, --OR', --OC(O)R', --NR'R'', --SR', --R', --CN, --NO.sub.2, --CO.sub.2R', --CONR'R'', --C(O)R', --OC(O)NR'R'', --NR''C(O)R', --NR''C(OhR', --NR'--C(O)NR''R''', --NH--C(NH.sub.2).dbd.NH, --NR'C(NH.sub.2).dbd.NH, --NH--C(NH.sub.2).dbd.NR', --S(O)R', --S(O).sub.2R', --S(O).sub.2NR'R'', --N.sub.3, --CH(Phh, perfluoro(C.sub.1-C.sub.4)alkoxy, and perfluoro(C.sub.1-C.sub.4)alkyl, in a number ranging from zero to the total number of open valences on the aromatic ring system; and where R', R'' and R''' are independently selected from hydrogen, (C.sub.1-C.sub.8)alkyl and heteroalkyl, unsubstituted aryl and heteroaryl, (unsubstituted aryl)-(C.sub.1C.sub.4)alkyl, and (unsubstituted aryl)oxy-(C.sub.1C.sub.4) alkyl.
[0176] Two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -T-C(O)--(CH.sub.2).sub.q--U--, wherein T and U are independently --NH--, --O--, --CH.sub.2 or a single bond, and q is an integer of from 0 to 2. Alternatively, two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -A-(CH.sub.2).sub.r--B--, wherein A and B are independently --CH.sub.2, --O--, --NH--, --S--, --S(O)--, --S(O).sub.2, --S(O).sub.2NR'-- or a single bond, and r is an integer of from 1 to 3. One of the single bonds of the new ring so formed may optionally be replaced with a double bond. Alternatively, two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula --(CH.sub.2).sub.8--X--(CH.sub.2).sub.t--, where s and t are independently integers of from 0 to 3, and X is --O--, --NR'--, --S--, --S(O)--, --S(O).sub.2--, or --S(O).sub.2NR'--. The substituent R' in --NR'-- and --S(O).sub.2NR'-- is selected from hydrogen or unsubstituted (C.sub.1-C.sub.6)alkyl.
[0177] "Heteroaryl" refers to a monocyclic or fused bicyclic or tricyclic aromatic ring assembly containing 5 to 16 ring atoms, where from 1 to 4 of the ring atoms are a heteroatom each N, O or S. For example, heteroaryl includes pyridyl, indolyl, indazolyl, quinoxalinyl, quinolinyl, isoquinolinyl, benzothienyl, benzofuranyl, furanyl, pyrrolyl, thiazolyl, benzothiazolyl, oxazolyl, isoxazolyl, triazolyl, tetrazolyl, pyrazolyl, imidazolyl, thienyl, or any other radicals substituted, especially mono- or di-substituted, by e.g. alkyl, nitro or halogen. Pyridyl represents 2-, 3- or 4-pyridyl, advantageously 2- or 3-pyridyl. Thienyl represents 2- or 3-thienyl. Quinolinyl represents preferably 2-, 3- or 4-quinolinyl. Isoquinolinyl represents preferably 1-, 3- or 4-isoquinolinyl. Benzopyranyl, benzothiopyranyl represents preferably benzopyranyl or 3-benzothiopyranyl, respectively. Thiazolyl represents preferably 2- or thiazolyl, and most preferred 4-thiazolyl. Triazolyl is preferably 1-, 2- or 5-(1,2,4-triazolyl). Tetrazolyl is preferably 5-tetrazolyl. Preferably, heteroaryl is pyridyl, indolyl, quinolinyl, pyrrolyl, thiazolyl, isoxazolyl, triazolyl, tetrazolyl, pyrazolyl, imidazolyl, thienyl, furanyl, benzothiazolyl, benzofuranyl, isoquinolinyl, benzothienyl, oxazolyl, indazolyl, or any of the radicals substituted, especially mono- or di-substituted.
[0178] The term "heteroalkyl" refers to an alkyl group having from 1 to 3 heteroatoms such as N, O and S. Additional heteroatoms can also be useful, including, but not limited to, B, Al, Si and P. The heteroatoms can also be oxidized, such as, but not limited to, --S(O)-- and --S(O).sub.2--. For example, heteroalkyl can include ethers, thioethers, alkyl-amines and alkyl-thiols.
[0179] The term "heteroalkylene" refers to a heteroalkyl group, as defined above, linking at least two other groups. The two moieties linked to the heteroalkylene can be linked to the same atom or different atoms of the heteroalkylene.
[0180] "Electrophile" refers to an ion or atom or collection of atoms, which may be ionic, having an electrophilic center, i.e., a center that is electron seeking, capable of reacting with a nucleophile. An electrophile (or electrophilic reagent) is a reagent that forms a bond to its reaction partner (the nucleophile) by accepting both bonding electrons from that reaction partner.
[0181] "Nucleophile" refers to an ion or atom or collection of atoms, which may be ionic, having a nucleophilic center, i.e., a center that is seeking an electrophilic center or capable of reacting with an electrophile. A nucleophile (or nucleophilic reagent) is a reagent that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons. A "nucleophilic group" refers to a nucleophile after it has reacted with a reactive group. Non limiting examples include amino, hydroxyl, alkoxy, haloalkoxy and the like.
[0182] "Maleimido" refers to a pyrrole-2,5-dione-1l-yl group having the structure:
##STR00002##
which upon reaction with a sulfhydryl (e.g., a thio alkyl) forms an --S-maleimido group having the structure
##STR00003##
[0183] where " " indicates the point of attachment for the maleimido group and indicates the point of attachment of the sulfur atom the thiol to the remainder of the original sulfhydryl bearing group.
[0184] For the purpose of this disclosure, "naturally occurring amino acids" found in proteins and polypeptides are L-alanine, L-arginine, L-asparagine, L-aspartic acid, L-cysteine, L-glutamine, L-glutamic acid, L-glycine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine, and or L-valine. "Non-naturally occurring amino acids" found in proteins are any amino acid other than those recited as naturally occurring amino acids. Non-naturally occurring amino acids include, without limitation, the D isomers of the naturally occurring amino acids, and mixtures of D and L isomers of the naturally occurring amino acids. Other amino acids, such as N-alpha-methyl amino acids (e.g. sarcosine), 4-hydroxyproline, desmosine, isodesmosine, hydroxylysine, epsilon-N-methyllysine, 3-methylhistidine, although found in naturally occurring proteins, are considered to be non-naturally occurring amino acids found in proteins for the purpose of this disclosure as they are generally introduced by means other than ribosomal translation of mRNA.
[0185] "Linear" in reference to the geometry, architecture or overall structure of a polymer, refers to polymer having a single polymer arm.
[0186] "Branched," in reference to the geometry, architecture or overall structure of a polymer, refers to a polymer having 2 or more polymer "arms" extending from a core structure contained within an initiator. The initiator may be employed in an atom transfer radical polymerization (ATRP) reaction. A branched polymer may possess 2 polymer chains (arms), 3 polymer arms, 4 polymer arms, 5 polymer arms, 6 polymer arms, 7 polymer arms, 8 polymer arms, 9 polymer arms or more. Each polymer arm extends from a polymer initiation site. Each polymer initiation site is capable of being a site for the growth of a polymer chain by the addition of monomers. For example and not by way of limitation, using ATRP, the site of polymer initiation on an initiator is typically an organic halide undergoing a reversible redox process catalyzed by a transition metal compound such as cuprous halide. Preferably, the halide is a bromine.
[0187] "Pharmaceutically acceptable excipient" refers to an excipient that can be included in the compositions of the invention and that causes no significant adverse toxicological effect on the patient and is approved or approvable by the FDA for therapeutic use, particularly in humans. Non-limiting examples of pharmaceutically acceptable excipients include water, NaCl, normal saline solutions, lactated Ringer's, normal sucrose, normal glucose and the like.
[0188] Dual antagonists are administered in an effective regime meaning a dosage, route of administration and frequency of administration that delays the onset, reduces the severity, inhibits further deterioration, and/or ameliorates at least one sign or symptom of a disorder. If a patient is already suffering from a disorder, the regime can be referred to as a therapeutically effective regime. If the patient is at elevated risk of the disorder relative to the general population but is not yet experiencing symptoms, the regime can be referred to as a prophylactically effective regime. In some instances, therapeutic or prophylactic efficacy can be observed in an individual patient relative to historical controls or past experience in the same patient. In other instances, therapeutic or prophylactic efficacy can be demonstrated in a preclinical or clinical trial in a population of treated patients relative to a control population of untreated patients.
[0189] The "biological half-life" of a substance is a pharmacokinetic parameter which specifies the time required for one half of the substance to be removed from a tissue or an organism following introduction of the substance.
[0190] "HEMA-PC" is 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate.
[0191] "TAP" means a PDGFR.beta.-GS 10-anti-VEGF-A heavy chain/anti-VEGF-A light chain wherein amino acids 1-282 of the heavy chain correspond to amino acids 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), fused as a single open reading frame via a glycine-serine linker (GGGGSGGGGS) linked to the N terminus of a bevacizumab heavy chain sequence having the following mutations in the variable region: T28D, N31H, H97Y, S100aT (Ferrara N, Damico L, Shams N, et al. 2006. Development of Ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26(8):859-870); and the following in the Fe region: L234A, L235A, and G237A (EU numbering) (Strohl W R. 2009. Optimization of Fe-mediated effector functions of monoclonal antibodies. Curr Opin in Biotech. 20: 685-691). The light chain is the bevacizumab light chain having an M4L mutation. TAP normally exists as a dimer having two heavy chains and two light chains. TAP may or may not have carbohydrate or other post-translational modifications after being expressed from cells. TAP is also sometimes called TAFwt or TAFWT, which indicates that the molecule in question does not have either the Q347C or L443C mutations in the heavy chain (Fe region) as do TAF347 or TAF443, defined infra.
[0192] "TAF347" is the same as TAP except that it has the Q347C mutation.
[0193] "TAF443" is the same as TAP except that it has the L443C mutation. TAF443 is sometimes referred to herein as OG 1321.
[0194] "OG 1786" is a 9-arm initiator used for polymer synthesis with the structure shown in FIG. 35, which depicts that salt form of OG 1786 with trifluororacetic acid. OG 1786 may be used in accordance with the present invention as other salts or as the free base.
[0195] "OG 1801" is an approximately (+/-15%) 750 kDa polymer (either by Mn or Mp) made using OG 1786 as an intiator for ATRP synthesis using the monomer HEMA-PC.
[0196] "OG 1802" is OG 1801 with a maleimide functionality added and is shown in FIG. 36 wherein each of n.sub.1, n.sub.2, n.sub.3, n.sub.4, n.sub.5, n.sub.6, n.sub.7, n.sub.8, and n.sub.9 is an integer (positive) (from 0 up to about 3000) such that the total molecular weight of the polymer is (Mw) 750,000.+-.15% daltons.
[0197] "OG 1448" is TAF443 conjugated to the OG 1802 biopolymer.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
General
[0198] The present invention provides a dual VEGF/PDGF antagonist comprising a VEGF antagonist linked to a PDGF antagonist. The VEGF antagonist is an antibody to a VEGF or VEGFR or is a VEGFR extracellular trap segment (i.e., a segment from the extracellular region of one or more VEGFR receptors that inhibits binding of at least one VEGFR to at least one VEGF). The PDGF antagonist is an antibody to a PDGF or PDGFR or is a PDGFR extracellular trap segment (i.e., segment from the extracellular region of one or more PDGFRs, which inhibits binding of at least one PDGFR and at least one PDGF). At least one of the antagonists is not an antibody, or put another way, at least one of the antagonists is an extracellular trap segment. Preferably, the dual antagonist includes an antibody antagonist and one extracellular trap segment antagonist. In such a dual antagonist the extracellular trap segment is preferably fused, optionally via a linker to the N-terminus of the antibody heavy chain. The antibody light chain is complexed with the antibody heavy chain in similar manner to that in a natural antibody. Such dual antagonists are preferably provided in the form of conjugates with a half-life extending moiety conjugated to the dual antagonist. Preferably, a cysteine residue is used for conjugation which has been introduced into the antagonist. More preferably, the cysteine residue is at positions 347 or 443 of an IgG 1 heavy chain. It is preferred that the half-life extending moiety is a zwitterionic polymer. Most preferably the zwitterionic polymer is a phosphorylcholine containing polymer.
[0199] Angiogenesis is the process by which new blood vessels are created and plays a crucial role in development (going from embryo to adult) and in wound healing (restoring blood flow to damaged or injured tissue). However, when angiogenesis is dysregulated, it contributes to the pathologies of many disorders, including cancer, psoriasis, arthritis and blindness. Carmeliet P. 2003. Angiogenesis in health and disease. Nature Med 9(6):653-660.
[0200] Abnormal angiogenesis is associated with wet age related macular degeneration (a leading cause of blindness in the elderly) and with cancer. Angiogenesis is characterized by an increase in proliferating endothelial and stromal cells and vasculature with altered morphology. See, generally, Folkman J. 2007. Angiogenesis: an organizing principle for drug discovery?. Nat Rev Drug 6:273-286 and Baluk P, Hashizume H, McDonald D M. 2005. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 15:102-111.
[0201] As mentioned above, neovascularization (NV) is a normal process occurring both in development and in wound healing but can become pathological when angiogenesis is dysregulated and occurs in tissues associated with tumors (cancer), avascular cornea or the subretinal space (wet AMD). The proliferation, invastion and migration of NV vessels is controlled by a complex interplay between growth factors, vascular endothelial cells, extracellular matrix molecules, chemokines and cell signaling molecules.
[0202] NV tissue is composed of endothelial cells (EC), pericytes and inflammatory cells (e.g. macrophages). Pericytes are derived via differentiation from mast cells. The process of neovascularization first involves the formation of angiogenic sprouts composed of EC from existing capillaries into the avascular space. VEGF signaling is understood to be the master switch for this NV process. In this regard, VEGF has been localized in the tip cell fiopodia which leads the angiogenic sprout.
[0203] Following sprout formation, the newly formed vessels are coated by pericytes, leading to maturation of the NV. Pericyte coating of NV leads to stabilization and support of NV both physically and through signaling, including pericyte production of VEGF. Armulik A, Abramsson A, Betsholtz C. 2005. Endothelial/Pericyte Interactions. Circ Res. 97:512-523.
[0204] Approved wet AMD therapies are all directed at the suppression of VEGF signaling. These therapies include pegaptanib (Macugen.RTM.), approved in 2004, Genentech's bevacizumab (Avastin.RTM.), approved in 2004 for cancer, used off label for AMD, Genentech's ranibizumab (Lucentis.RTM.), approved in 2006, and Regeneron's aflibercept (Eylea.RTM.) approved in 2011. Pegaptanib is an aptamer based therapeutic, but with a limited market compared with protein based therapeutics likely due to the limited gains in visual acuity for patients. Bevacizumab is an anti-VEGFA IgG 1 antibody approved for cancer treatment, but is widely used off label for treatment of AMD. Ranibizumab is a Fab which was affinity matured from bevacizumab and is approved for AMD. However, the market for Ranibizumab is substantially undercut by use of the much cheaper bevacizumab. Finally, aflibercept is a VEGF trap, employing a soluble receptor fragment decoy.
[0205] Anti-VEGF monotherapy has not lead to disease-modifying regression of pathological NV. Brown D M, Kaiser P K, Michels M, et al. 2006. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14):1432-1444; Rosenfeld P J, Brown D M, Heier J S, et al. 2006. MARINA study group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419-1431; Regillo C D, Brown D M, Abraham P, et al. 2008. Randomized, double-masked, sham0controlloed trial of ranibizumab for neovacular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 145:239-248. Instead the majority of the efficacy or therapeutic benefit of anti-VEGF therapies is due to their anti-permeability property. Zebrowski B K, Yano S, Liu W, et al. 1999. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 5:3364-3368.
[0206] Because conventional anti-VEGF therapies do not cause regression of pathological NV, visual acuity gains for many patients have been quite limited. Moreover, neovasculature can also lead to subretinal fibrosis which is a cause of blindness in wet AMD patients.
[0207] Subretinal scarring develops in nearly half of treated eyes within two years of anti-VEGF therapy. Daniel E, Toth C A, Grunwald J E. 2014. Risk of scar in the comparison of age-related macular degeneration in clinical settings. Retina 32: 1480-1485. Subretinal fibrosis formation can cause permanent dysfunction of the macular system; it causes destruction of photoreceptors, retinal pigment epithelium and choroidal vessels. Ishikawa K, Ram K, Hinton D R. 2015. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Eye Res. Mar. 13, 2015 Epub 1-7. Although anti-VEGF therapy generally stabilizes or improves visual acuity, scar formation has been identified as one of the causes of loss of visual acuity after treatment. Cohen S Y, Oubraham H, Uzzan J, et al. 2012. Causes of unsuccessful ranibizumab treatment in exudative age-related macular degeneration in clinical settings. Retina 32: 1480-1485.
[0208] Proangiogenic factors are generally upregulated in pathological angiogenesis, including two members of the vascular endothelial growth factor (VEGF) family: VEGF-A and placental growth factor (PGF). VEGF-A and PGF activate quiescent endothelial cells, promote cell proliferation and vascular permeability. VEGF-A has been identified as a major factor in vascular leak in wet AMD. Dvorak H F, Nagy J A, Feng D, Brown L F, Dvorak A M. 1999. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbial Immunol. 237:97-132.
[0209] Platelet derived growth factor "PDGF" signaling plays an important role in NV maturation and in particular to the coating of NV by pericytes. The coating of NV endothelial cells by pericytes begins with EC expression of the paracrine platelet-derived growth factor B, which forms the homodimer PDGF-BB. PDGF-BB is highly retained in the tip cells of the angiogenic sprouts by heparin sulfate proteoglycan. This PDGF-BB is then recognized by the pericyte bound receptor PDGFR-.beta., which initiates the proliferation and migration of pericytes along the growing neovascularization.
[0210] PDGF-DD had also been discovered to play a central role in pathological angiogenesis. Kumar A, Hou X, Chunsik L, et al. 2010. Platelet-derived Growth Factor-DD Targeting Arrests Pathological Angiogenesis by Modulating Glycogen Synthase Kinase-3 Phosphorylation. J Biol Chem 285(20):15500-15510. PDGF-DD overexpression induces blood vessel maturation during angiogenesis. Kong D, Wang Z, Sarkar F H, et al. 2008. Platelet-Derived Growth Factor-D Overexpression Contributes to Epithelial-Mesenchymal Transition of PC3 Prostate Cancer Cells. Stem Cells 26:1425-1435. PDGF-DD is highly expressed in the eye. Ray S, Gao C, Wyatt K, et al. 2005. Platelet-derived Frowth Factor D, Tissue-specific Expression in the Eye, and a Key Role in Control of Lens Epithelial Cell Proliferation. J Biol Chem. 280:8494-8502. Kumar et al. (2010) found that PDGF-DD expression was upregulated during pathological angiogenesis and that inhibition of PDGF-DD signaling decreased choroidal and retinal neovascularization.
[0211] The term "PDGF" as used herein means any member of the class of growth factors that (i) bind to a PDGF receptor such as PDGFR-.beta., or PDGFR-.alpha.; (ii) activates a tyrosine kinase activity associated with the PDGF receptor; and (iii) thereby affects angiogenesis or an angiogenic process. The term "PDGF" generally refers to those members of the class of growth factors that induce DNA synthesis and mitogenesis through the binding and activation of a platelet-derived growth factor cell surface receptor (i.e., PDGFR) on a responsive cell type. PDGFs effect specific biological effects including, for example: directed cell migration (chemotaxis) and cell activation; phospholipase activation; increased phosphatidylinositol turnover and prostaglandin metabolism; stimulation of both collagen and collagenase synthesis by responsive cells; alteration of cellular metabolic activities, including matrix synthesis, cytokine production, and lipoprotein uptake; induction, indirectly, of a proliferative response in cells lacking PDGF receptors; fibrosis and potent vasoconstrictor activity. The term "PDGF" is meant to include both a "PDGF" polypeptide and its corresponding "PDGF" encoding gene or nucleic acid.
[0212] The PDGF family consists of disulfide bonded homo-ffdimers of PDGF-A (Swiss Protein P04085), -B (P01127), -C (Q9NRA1) and -D (Q9GZPO) and the hetero dimer PDGF-AB. The various PDGF isoforms exert their effect by binding to .alpha. and .beta.-tyrosine kinase receptors (PDGFR-.alpha. (P16234) and PDGFR-.beta. (P09619) respectively). See generally U.S. Pat. No. 5,872,218 which is incorporated herein by reference for all purposes. The .alpha. and .beta. receptors are structurally similar: both have extracellular domains with five immunoglobulin (lg) like domains and intracellular domains with a kinase function. PDGF binding occurs mainly through domains 2 and 3 of the receptors and causes dimerization of the receptors. lg like domain 4 is involved in receptor dimerization. Receptor dimerization is a key component of PDGF signaling: receptor dimerization leads to receptor auto-phosphorylation. Auto-phosphorylation in turns causes a conformational change in the receptor and activates the receptor kinase. PDGF-A, --B, --C and -D bind to the two different receptors with different affinities and effects. PDGF-AA, -AB, -BB and -CC induce aa receptor homodimers, PDGF-BB and -DD induced .beta..beta. homodimers and PDGF-AB, -BB, -CC and -DD produce .alpha..beta. receptor heterodimers.
[0213] In terms of function, PDGFR-.alpha. and PDGFR-.beta. appear to have substantially different roles. PDGFR-.alpha. signaling is involved in gastrulation and in development of the cranial and cardiac neural crest, gonads, lung, intestine, skin, CNS and skeleton. PDGFR-.beta. signaling is involved in blood vessel formation and early hematopoiesis. Andrae J, Radiosa G, Betsholtz C. 2008. Role of platelet-derived growth factors in physiology and medicine. Genes Develop 22: 1276-1312. In terms of interaction of the various PDGF ligands with the receptors, PDGF-AA and PDGF-CC exclusively bind to and interact with PDGFR-.alpha.. PDGF-BB and PDGF-AB bind with .alpha. and .beta. receptors. PDGF-DD exclusively interacts with PDGFR-.beta.. Raica M, Cimpean A M. 2010. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceut. 3:572-599.
[0214] Unless otherwise apparent from the context reference to a PDGF means any of PDGF-A, -B, -C and -D in any of the natural isoforms or natural variants or induced variants having at least 90, 95, 98 or 99% sequence identity to a natural form. Preferably, such PDGFs are human PDGFs. Likewise reference to a PDGFR means PDGFR-A (P16234) or PDGFR-B including any natural isoform or natural variant, or an induced variant having at least 90, 95, 98 or 99% or 100% sequence identity to a natural sequences.
[0215] The amino acid sequence of human PDGFR-(UniProtKB/Swiss-Prot: P09619.1) is set forth in FIG. 1, which shows a full-length human PDGFR-.beta. (including the leader sequence), a 1106 amino acid protein. Amino acids 1-32 are part of the leader peptide which is cleaved off in the mature protein. PDGFR-.beta. has five extracellular lg-like domains D 1-D5. Williams A F, Barclay A N. 1988. The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol. 6:381-405. The full-length extracellular region runs from about amino acid 33 to 532, the transmembrane domain from about residue 533 to 553 and the cytoplasmic domain from about residue 554 to 1106. The extracellular region includes five immunoglobulin-like domains, D1-D5. The D1 domain is typically considered to be from about amino acid 33 (Leu) to about 123 (Pro). In accordance with the present invention, D1 may also be from 33 to 122 (Val). D2 is typically considered to be from about 124 (Thr) to about 213 (Ser). In accordance with the present invention, D2 may be 129 (Pro) to 210 (Gln). D3 is typically considered to be from about amino acid 214 (Ile) to 314 (Gly). In accordance with the present invention, D3 may be from 214 (Ile) to 309 (Thr). D4 is typically considered to be from about amino acid 315 (Tyr) to 416 (Pro). D5 is typically considered to be from about amino acid 417 (Val) to 531 (Lys).
[0216] The exact boundaries of the D1-D5 domains can vary depending on how the analysis is done. Preferably, the boundaries vary by 9 amino acids or less. Typically they vary by 7 amino acids of less, more typically by 5 amino acids or less. Usually, boundary variance is 3 amino acids or less. Most typically the boundaries vary by only an amino acid. The essential characteristic of each domain is its ability to bind to its cognate ligands.
[0217] A "PDGF antagonist" or a molecule that "antagonizes PDGF" is an agent that reduces, or inhibits, either partially or fully, at least one activity of a PDGF including its ability to specifically bind to a PDGFR, and consequent cellular responses, such as proliferation. PDGF antagonists include antibodies that specifically bind to a PDGF or PDGFR and extracellular trap segments from a PDGFR.
[0218] One or more portions of a PDGFR-.beta. extracellular receptor sequence can be used as an antagonist for PDGF-PDGFR-.beta. signaling. The term extracellular trap segment refers to a full length extracellular region or any portion thereof, or combination of portions from different PDGF receptors that can antagonize PDGF-PDGFR-beta signaling. Such portions are typically used free of the transmembrane and intracellular sequence of the PDGFR and are consequently referred to as being soluble. The portions antagonize by acting as a trap or decoy for a cognate PDGF. PDGF binds to the soluble PDGFR-.beta. segment trap and is unable to bind to the corresponding membrane bound receptor. Preferably, such traps include one of more of PDGFR-.beta. domains D1-D5. Preferably, the trap contains at least one of D2 and D3. More preferably, the trap contains D1, D2 and D3. More preferably the trap is a contiguous segment corresponding to amino acids 33 to 314 of FIG. 8 which contains D1-D3. PDGFR-alpha likewise includes domains D1 through D5 and extracellular trap segments incorporating corresponding domains of PDGFR-alpha can likewise be used instead of PDGFR-beta.
[0219] Antibodies can also be used as antagonists of PDGFR-.beta., including antibodies which bind to the receptor (e.g., 2A1E2 [U.S. Pat. No. 7,060,271]; HuM4 Ts.22 [U.S. Pat. No. 5,882,644]; or 1B3 or 2C5 [U.S. Pat. No. 7,740,850]), and anti-PDGF antibodies such as anti-PDGF BB, anti-PDGF-DD, anti-PDGF-BB and anti-PDGF-AB.
[0220] "VEGF" or "vascular endothelial growth factor" is a human vascular endothelial growth factor that affects angiogenesis or an angiogenic process. In particular, the term VEGF means any member of the class of growth factors that (i) bind to a VEGF receptor such as VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), or VEGFR-3 (FLT-4); (ii) activates a tyrosine kinase activity associated with the VEGF receptor; and (iii) thereby affects angiogenesis or an angeogenic process.
[0221] The VEGF family of factors is made up of five related glycoproteins: VEGF-A (also known as VPE), --B, --C, -D and PGF (placental growth factor). Of these, VEGF-A is the most well studied and is the target of anti-angiogenic therapy. Ferrara et al, (2003) Nat. Med. 9:669-676. VEGF-A exists as a number of different isotypes which are generated both by alternative splicing and proteolysis: VEGF-A.sub.206, VEGF-A.sub.189, VEGF-A.sub.165, and VEGF-A121. The isoforms differ in their ability to bind heparin and non-signaling binding proteins called neuropilins. The isoforms are all biologically active as dimers.
[0222] The various effects of VEGF are mediated by the binding of a VEGF, e.g., VEGF-A (P15692), -B (P49766), -C (P49767) and -D (Q43915), to receptor tyrosine kinases (RTKs). The VEGF family receptors belong to class V RTKs and each carry seven lg-like domains in the extracellular domain (ECD). In humans, VEGF binds to three types of RTKs: VEGFR-1 (Flt-1) (P17948), VEGFR-2 (KDR, Flk-1) (P935968) and VEGFR-3 (Flt-4) (P35916). A sequence of VEGFR-1 is shown in FIG. 2. Unless otherwise apparent from the context reference to a VEGF means any of VEGF-A, -B, -C, -D, and PGF, in any of the natural isoforms or natural variants or induced variants having at least 90, 95, 98 or 99% or 100% sequence identity to a natural form. Preferably, such VEGFs are human VEGFs. Likewise reference to a VEGFR means any of VEGR-1, R-2 or R-3, including any natural isoform or natural variant, or an induced variant having at least 90, 95, 98 or 99% or 100% sequence identity to a natural sequences.
[0223] The extracellular region runs from about amino acid 27-758, the transmembrane domain from about amino acid 759 to 780 and the intracellular region from about 781-1338. The extracellular region includes seven immunoglobulin-like domains, D1-D7. Domain 1 of VEGFR-1 is from 32 (P) to 128 (I), Domain 2 from 134 (P) to 125 (Q), Domain 3 from 232 (V) to 331 (K), Domain 4 from 333 (F) to 428 (P), Domain 5 is from 431 (Y) to 553 (T), Domain 6 from 558 (G) to 656 (R) and Domain 7 from 662 (Y) to 751 (T). See generally U.S. Pat. No. 8,273,353, incorporated herein by reference for all purposes. The exact boundaries of the domains D1-D7 of VEGFR-1 can vary depending on how the analysis is done. Preferably, the boundaries vary by 9 amino acids or less. Typically they vary by 7 amino acids of less, more typically by 5 amino acids or less. Usually, boundary variance is 3 amino acids or less. Most typically the boundaries vary by only an amino acid.
[0224] The protein sequence of VEGFR-2 is shown below in FIG. 3.
[0225] The extracellular region runs from about residues 20-764, the transmembrane domain from about residues 765-785 and the intracellular domain from about residues 786 to 1356. The extracellular region includes seven immunoglobulin-like domains, D1-D7. Domain 1 of VEGFR-2 is from 32 (P) to 118 (V), Domain 2 is from 124 (P) to 220 (G), Domain 3 is from 226 (V) to 327 (K), Domain 4 is from 329 (F) to 421 (P), Domain 5 is from 424 (G) to 548 (T), Domain 6 is from 553 (I) to 662 (L), and Domain 7 is from 668 (T) to 757 (A). See generally U.S. Pat. No. 8,273,353, incorporated herein by reference for all purposes. The exact boundaries of the domains D1-D7 of VEGFR-2 can vary depending on how the analysis is done. Preferably, the boundaries vary by 9 amino acids or less. Typically they vary by 7 amino acids of less, more typically by 5 amino acids or less. Usually, boundary variance be by 3 amino acids or less. Most typically the boundaries 1 vary by only an amino acid.
[0226] The protein sequence of VEGFR-3 is shown below in FIG. 4. The extracellular region runs from about residues 25-775, the transmembrane domain from about residues 776-796 and the intracellular domain from about residues 797-1363. The extracellular domain includes seven immunoglobulin-like domains D1-D7. Domain 1 of VEGFR-3 is from 30 (P) to 132 (V), Domain 2 is from 138 (P) to 226 (G), Domain 3 is from 232 (I) to 330 (N), Domain 4 is from 332 (F) to 422 (P), Domain 5 is from 425 (H) to 552 (T), Domain 6 is from 557 (G) to 673 (Q), and Domain 7 is from 679 (R) to 768 (S). See generally U.S. Pat. No. 8,273,353, incorporated herein by reference for all purposes. The exact boundaries of the domains D1-D7 of VEGFR-3 can vary depending on how the analysis is done. Preferably, the boundaries vary by 9 amino acids or less. Typically they vary by 7 amino acids of less, more typically by 5 amino acids or less. Usually, boundary variance is 3 amino acids or less. Most typically the boundaries vary by only an amino acid.
[0227] VEGFR-2 is expressed predominately on vascular endothelial cells. VEGFR-1 is also expressed on the vascular endothelium, but in addition is also expressed by a number of other cell types: neutrophils, monocytes, macrophages, mural cells and endothelial progenitor cells. VEGFR-1 has a higher affinity for VEGF-A than does VEGFR-2. However, when VEGFR-1 is bound to VEGF-A in endothelial cells, VEGFR-1 exhibits only very weak tyrosine phosphorylation. Hence, it is believed that the effects of VEGF-A (including its various isoforms) on the vascular endothelium are mediated by the binding of VEGF-A to VEGFR-2.
[0228] PGF and VEGF-B bind only to VEGFR-1. PGF and VEGF-B have been implicated in pathogenic vascular remodeling. Carmeliet P, Moons L, Lutten A, et al. 2001. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 7(5); 575-583. VEGF-C and -D bind with high affinity to VEGFR-3, which is primarily found on lymphatic endothelial cells in the adult. VEGF-C and -D are thought to play a role in regard to Lymphangio genesis.
[0229] A "VEGF antagonist" or a molecule that "antagonizes VEGF" is an agent that reduces, or inhibits, either partially or fully, an activity of a VEGF including its ability to specifically bind to its receptor a VEGFR and consequent cellular responses, such as angiogenesis and cellular proliferation. VEGF antagonists include antibodies specifically binding to a VEGF or a VEGFR or a VEGFR extracellular trap segment.
[0230] The term extracellular trap segment refers to a full length extracellular region or any portion thereof, or combination of portions from different VEGFR receptors that can antagonize signaling between at least one VEGF and VEGFR. Preferably, the extracellular trap segment includes at least one domain from one of VEGFR-1, -2 or -3 defined above, and more preferably at least two contiguous domains, such as D2 and D3. Optionally, an extracellular domain includes at least one domain as defined above from at least two different VEGFRs. A preferred extracellular domain comprises or consists essentially of D2 of VEGFR-1 and D3 of VEGFR-2.
[0231] VEGF antagonist therapies have been approved for the treatment of certain cancers and wet AMD. Bevacizumab (AVASTIN.RTM., Genentech/Roche) is a humanized mouse monoclonal antibody that binds to and neutralizes human VEGF, in particular to all isoforms of VEGF-A and to bioactive proteolytic fragments of VEGF-A. See, e.g., Ferrara N, Hillan K J, Gerber H P, Novotny W. 2004. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 3(5):391-400. Bevacizumab has been approved for the treatment of certain cancers. The protein sequence of the heavy and light chains of bevacizumab (DrugBank DB00112) is shown below in FIG. 5 with CDRs underlined (see also SEQ ID NOs. 2 and 5).
[0232] Bevacizumab variable light chain CDRs are CDR.sub.L1: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L3: QQYSTVPWT. Bevacizumab variable heavy chain CDRs are CDR.sub.H1: GYTFTNYGMN, CDR.sub.H2: WINTYTGEPTY AADFKR, and CDR.sub.H3: YPHYYGSSHWYFDV. CDRs are defined by Kabat except CDR.sub.H1 is the composite Kabat/Chothia definition.
[0233] Another anti-VEGF molecule, derived from the same mouse monoclonal antibody as bevacizumab has been approved as a treatment for wet AMD: ranibizumab (LUCENTIS.RTM., Genentech/Roche). Ranibizumab is an antibody fragment or Fab. Ranibizumab was produced by affinity maturation of the variable heavy and light chains of bevacizumab. The sequence of the heavy and light chains of ranibizumab is shown below (as published by Novartis) in FIG. 6 (see also SEQ ID NOs. 12 and 13):
[0234] Ranibizumab variable light chain CDRs are CDR.sub.L1: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L3: QQYSTVPWT. Ranibizumab variable heavy chain CDRs are CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3: YPYYYGTSHWYFDV.
[0235] Antibodies competing with bevacizumab for binding to VEGF-A or binding to the same epitope on VEGF-A as bevacizumab can also be used.
[0236] Another anti-VEGF therapy is a VEGF Trap. For example, aflibercept (Eylea.RTM., Regeneron), consists of the second lg like domain of VEGFR-1 and the third lg like domain of VEGFR-2 expressed as an in line fusion with the constant region (Fe) of human IgG 1. Papadopoulos N, et al. 2012. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171-185. In theory, aflibercept binds not only VEGF-A, but also VEGF-B and PGF thereby antagonizing their interaction with VEGFR-1.
[0237] In accordance with the present invention, a dual VEGF/PDGF antagonist is provided comprising a VEGF antagonist linked to a PDGF antagonist. The linkage preferably includes a fusion of protein chains to form a hybrid chain formed from components of both antagonists. Alternatively, the components can be joined by chemical cross linking. As an example, of linkage by fusion, if the dual antagonist is formed from an antibody and an extracellular trap segment, then a heavy or light chain of the antibody can be fused to the extracellular trap segment. Preferably, the extracellular trap segment is fused directly or indirectly via a linker to the N-terminus of the antibody heavy or light chain. Whichever chain is not fused to the extracellular trap segment can associate with the chain that is in similar fashion to heavy light chain association in a natural antibody. For example, an exemplary format has an extracellular trap segment fused to the N-terminus of an antibody heavy chain via a linker and the antibody light chain complexed with the antibody heavy chain. The antibody in such a dual antagonist can be an intact antibody or any of the binding fragments described above, such as a Fab fragment. Preferably, in such dual antagonists, the VEGF antagonist is an antibody to VEGF-A, such as bevacizumab or ranibizumab, and the PDGF antagonist is an extracellular trap segment from PDGR-1.
[0238] In an alternative format, the VEGF antagonist and PDGF antagonist are both extracellular trap segments. The two segments can be fused in either orientation with respect to one another, directly or via a linker. That is the VEGFR extracellular trap region can be joined to the N-terminus or the C-terminus of the PDGFR extracellular trap region. The C-terminus of such a fusion protein can be linked to an Fe region of an antibody forming an Fe fusion proteins. In preferred embodiments, the PDGFR is PDGFR-.beta. and the extracellular trap segment comprises one or more of domains D1-D5 of PDGFR-.beta.. More preferably, the extracellular trap segment comprises domains D1-D3 of PDGFR-.beta.. Still more preferably, the extracellular trap segment comprises or consists of amino acids 33 to 314 of SEQ ID NO. 11. In preferred embodiments, the VEGF antagonist is an anti-VEGF antibody, preferably an anti-VEGF-A antibody.
[0239] In dual antagonists having antibody and extracellular trap components fused to one another, the respective components, typically the antibody heavy chain and the extracellular trap segment are separated by a linker sequence. The linker is preferably GGGGSGGGGS, GG, or GGGGSGGGGSGGGGSGGGGSG or an oligomers of any of these. More preferably, the linker is GGGGSGGGGS.
[0240] In accordance with an aspect of the present invention, the anti-VEGF-A antibody heavy chain has at least the following CDR sequences: CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3: YPYYYGTSHWYFDV. Preferably, the anti-VEGF-A light chain has at least the following CDRs: CDR.sub.L2: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L2: QQYSTVPWT. In the case of the anti-VEGF-A antibody heavy chain, it is preferred that its isotype is IgG 1 and has a CH.sub.1, hinge, CH.sub.2 and CH.sub.3 domains. It is also preferred that the light chain isotype is kappa. The constant region of the preferred IgG 1 sequence is set forth in SEQ ID NO. 17. The sequence of the light chain constant region is preferably set forth in SEQ ID NO. 18.
[0241] The IgG 1 domain of the anti-VEGF-A antibody preferably has one or more mutations to reduce or lower effector function. Preferred amino acids to use for effector function reducing mutations include (EU numbering) E233P, L234V, L235, G236, G237, delG236, D270A, K322A, A327G, P329A, A330, A330S, P331S, and P331A, in which the second mentioned amino acid is the mutation. Preferably, the mutations include one or more of the following: E233P, L234V, L234A, L235A, G237A, A327G, A330S and P331S (EU numbering). More preferably, the anti-VEGF-A heavy chain has the following mutations: L234A, L235A and G237A. The number of such mutations relative to a natural human IgG 1 sequence is no more than 10, and preferably no more than 5, 4, 3, 2 or 1.
[0242] Alternatively, the IgG domain can be IgG2, IgG3 or IgG4, preferably, human IgG2, IgG3 or IgG4, or a composite in which a constant regions is formed from more than one of these isotypes (e.g., CH1 region from IgG2 or IgG4, hinge, CH2 and CH3 regions from IgG1). Such domains can contain mutations to reduce effector function at one or more of the EU position mentioned for IgG 1. Human IgG2 and IgG4 have reduced effector functions relative to human IgG 1 and IgG3.
[0243] The anti-VEGF-A heavy chain can also contain a cysteine residue added as a mutation by recombinant DNA technology which can be used to conjugate a half-life extending moiety. Preferably, the mutation is (EU numbering) Q347C and/or L443C. More preferably, the mutation is L443C. Preferably, the stoichiometry of dual antagonist to polymer is 1:1; in other words, a conjugate consists essentially of molecules each comprising one molecule of dual antagonist conjugated to one molecule of polymer.
[0244] A preferred dual antagonist including an antibody to VEGF-A and a PDGFR extracellular trap segment comprises a fusion protein of the antibody heavy chain and the PDGFR extracellular trap segment having the amino acid sequence of SEQ ID NO. 9 and the antibody light chain having the amino acid sequence of SEQ ID NO. 10, or variants thereof including sequences differing each of from SEQ ID NO: 9 and 10 by no more than 10, 9, 8, 7, 6, 5, 4, 3, 2 or 1 amino acids.
[0245] In another aspect of the present invention, a dual VEGF/PDGF antagonist is presented having a PDGF antagonist constituting one or more segments of a PDGFR as described above and a VEGF antagonist constituting an anti-VEGF Fab fragment. For this aspect of the present invention, the PDGFR extracellular trap comprises one or more of domains D1-D5 of PDGFR-.beta.. More preferably, the PDGFR trap constitutes domains D1-D3 of PDGFR--More preferably, the PDGFR trap is amino acids 33 to 314 of SEQ ID NO. 11.
[0246] The PDGFR trap is preferably located C-terminal of the Fab heavy or light chain. The PDGFR trap is also preferentially located N-terminal of the Fab heavy or light chain. Preferably, the dual antagonist includes an anti-VEGF-A Fab fragment heavy chain fused via a linker to a PDGFR extracellular trap segment and an anti-VEGF-A light chain.
[0247] In another aspect of the invention, a dual VEGF/PDGF antagonist is presented wherein the extracellular trap segment binds to one or more of PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC and PDGF-DD. Preferably, the extracellular trap binds PDGF-AB, PDGF-BB and PDGF-DD. Still more preferably, the extracellular trap inhibits PDGF-AB, PDGF-BB and PDGF-DD from binding to any one of PDGFR-.alpha..alpha., PDGFR-.alpha..beta., and PDGFR-.beta..beta. receptors.
[0248] A linker is preferably located between the PDGFR trap and the anti-VEGF Fab fragment heavy chain. Preferably, the linker is selected from group consisting of GGGGSGGGGS, GG, and GGGGSGGGGSGGGGSGGGGSG, and oligomers of any of these. More preferably, the linker is GGGGSGGGGS.
[0249] The anti-VEGF Fab fragment heavy chain preferably has at least the following CDRs: CDR.sub.H1: GYDFTHYGMN, CDR.sub.H2: WINTYTGEPTYAADFKR, and CDR.sub.H3:
[0250] YPYYYGTSHWYFDV. The anti-VEGF-A light chain preferably has at least the following CDRs: CDR.sub.L2: SASQDISNYLN, CDR.sub.L2: FTSSLHS and CDR.sub.L3: QQYSTVPWT.
[0251] A preferred anti-VEGF Fab fragment heavy chain isotype is IgG 1 and comprises a CH.sub.1 domain and the light chain isotype is kappa.
[0252] The dual VEGF/PDGF antagonist can have a half-life extending moiety attached. Preferably the half-life extending moiety is a zwitterionic polymer but PEG or other half-life extenders discussed below can alternatively be used. More preferably, the zwitterionic polymer is formed of monomers having a phosphorylcholine group. Preferably the monomer is 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. More preferably, the monomer is 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC).
[0253] A polymer conjugated to a dual antagonist preferably has at least 2 and more preferably 3 or more arms. Some polymers have 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Still more preferably the polymer has 3, 6 or 9 arms. Most preferably, the polymer has 9 arms. Preferably, the polymer peak molecular weight is between 300,000 and 1,750,000 Da. More preferably, the polymer has a peak molecular weight between 500,000 and 1,000,000 Da. Still more preferably, the polymer has a peak molecular weight between 600,000 to 800,000 Da.
[0254] The polymer can be covalently bonded to the dual antagonist via conjugation. Preferably, the polymer is conjugated to the dual VEGF/PDGF antagonist via a group such as an amino group, a hydroxyl group, a sulfbydryl group or a carboxyl group. The sulfbydryl group can be from a naturally occurring cysteine residue. The sulfbydryl group can also be from a cysteine residue added by recombinant DNA technology.
[0255] In a preferred aspect of the present invention, the polymer is conjugated to the cysteine residue at position 731 of SEQ ID NO. 9, or aligned position of any variants of SEQ ID NO: 9 disclosed herein.
[0256] In another aspect of the present invention, a dual VEGF/PDGF antagonist having a VEGFR trap containing one or more extracellular segments of a VEGFR, such as VEGFR-1, VEGFR-2 or VEGFR-3, fused to an anti-PDGF antibody or Fab fragment heavy or light chain and an anti-PDGF antibody or Fab fragment heavy or light chain not included in fusion.
[0257] In accordance with an aspect of the present invention, the extracellular segment of VEGFR is preferably one or more of domains D1-D7. More preferably, the extracellular segment comprises D2 from VEGFR-1 and D3 from VEGFR-2. Still more preferably, the D2 is N-terminal to the D3 and further comprises a linker between the domains.
[0258] In preferred embodiments of this aspect of the present invention, the PDGF antagonist is an antibody. More preferably, the antibody is selected from the group consisting of humanized 2A 1E2, HuM4 Ts.22, humanized 1B3, humanized 2C5, anti-PDGF-BB, anti-PDGF-DD, anti-PDGF-BB and anti-PDGF-AB. The PDGF antagonist is also preferably a Fab fragment.
[0259] In accordance with this aspect of the present invention, the antibody heavy chain is preferably IgG 1, more preferably human IgG 1 and the light chain is preferably kappa, human kappa. The heavy chain can have a cysteine added via recombinant DNA technology. Preferably, the cysteine is selected from the group consisting of Q347C and L443C. Preferably, there is a half-life extending moiety conjugated to the cysteine.
[0260] Preferably, the half-life extending moiety is a zwitterionic polymer having one or more monomer units and wherein at least one monomer unit has a zwitterionic group. Preferably, the zwitterionic group is phosphorylcholine. The monomer is preferably 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. More preferably, the monomer is 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC).
[0261] In accordance with this aspect of the present invention, the polymer preferably has at least 2 and more preferably 3 or more arms. Some polymers have 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Still more preferably the polymer has 3, 6 or 9 arms. Most preferably, the polymer has 9 arms. In accordance with an aspect of the present invention, the polymer peak molecular weight of between 300,000 and 1,750,000 Da. More preferably, the polymer has a peak molecular weight between 500,000 and 1,000,000 Da. Still more preferably, the polymer has a peak molecular weight between 600,000 to 800,000 Da.
[0262] In accordance with an aspect of the present invention, the polymer is covalently bound to the polymer via conjugation. Preferably, the polymer is conjugated to the dual VEGF/PDGF antagonist via a group selected from the group consisting of an amino group, a hydroxyl group, a sulfhydryl group and a carboxyl group. Preferably, the sulfhydryl group is from a naturally occurring cysteine residue. In other preferred embodiments, the sulfhydryl group is from a cysteine residue added by recombinant DNA technology.
[0263] In preferred aspects of the present invention, the PDGF trap-VEGF trap is conjugated to a half-life extending moiety as discussed with other dual antagonists.
[0264] Preferably, the half-life extending moiety is a zwitterionic polymer having one or more monomer units and wherein at least one monomer unit has a zwitterionic group. Preferably, the zwitterionic group is phosphorylcholine. The monomer is preferably 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate. More preferably, the monomer is 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC).
[0265] In accordance with this aspect of the present invention, the polymer preferably has at least 2 and more preferably 3 or more arms. Some polymers have 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 arms. Still more preferably the polymer has 3, 6 or 9 arms. Most preferably, the polymer has 9 arms. In accordance with an aspect of the present invention, the polymer peak molecular weight of between 300,000 and 1,750,000 Da. More preferably, the polymer has a peak molecular weight between 500,000 and 1,000,000 Da. Still more preferably, the polymer has a peak molecular weight between 600,000 to 800,000 Da.
[0266] In accordance with an aspect of the present invention, the polymer is covalently bound to the polymer via conjugation. Preferably, the polymer is conjugated to the dual VEGF/PDGF antagonist via a group such as an amino group, a hydroxyl group, a sulfbydryl group or a carboxyl group. In some conjugates, the sulfbydryl group is from a naturally occurring cysteine residue. In some conjugates, the sulfbydryl group is from a cysteine residue added by recombinant DNA technology.
[0267] Dual PDGF/VEGF antagonists can be produced by recombinant expression including the production of recombinant DNA by genetic engineering, (ii) introducing recombinant DNA into prokaryotic or eukaryotic cells by, for example and without limitation, transfection, electroporation or microinjection, (iii) cultivating the transformed cells, (iv) expressing dual antagonists, e.g. constitutively or on induction, and (v) isolating the dual antagonist, e.g. from the culture medium or by harvesting the transformed cells, in order to (vi) obtain purified dual antagonist.
[0268] Dual antagonists can be produced by expression in a suitable prokaryotic or eukaryotic host system characterized by producing a pharmacologically acceptable dual antagonist molecule. Examples of eukaryotic cells are mammalian cells, such as CHO, COS, HEK 293, BHK, SK-Hip, and HepG2. Other suitable expression systems are prokaryotic (e.g., coli with pET/BL21 expression system), yeast (Saccharomyces cerevisiae and/or Pichia pastoris systems), and insect cells.
[0269] A wide variety of vectors can be used for the preparation of the dual antagonist and are selected from eukaryotic and prokaryotic expression vectors. Examples of vectors for prokaryotic expression include plasmids such as, and without limitation, preset, pet, and pad, wherein the promoters used in prokaryotic expression vectors include one or more of, and without limitation, lac, trc, trp, recA, or araBAD. Examples of vectors for eukaryotic expression include: (i) for expression in yeast, vectors such as, and without limitation, pAO, pPIC, pYES, or pMET, using promoters such as, and without limitation, AOX 1, GAP, GALl, or AUG 1; (ii) for expression in insect cells, vectors such as and without limitation, pMT, pAc5, pB, pMIB, or pBAC, using promoters such as and without limitation PH, p 10, MT, Ac5, OpIE2, gp64, or polh, and (iii) for expression in mammalian cells, vectors such as, and without limitation, pSVL, pCMV, pRc/RSV, pcDNA3, or pBPV, and vectors derived from, in one aspect, viral systems such as and without limitation vaccinia virus, adeno-associated viruses, herpes viruses, or retroviruses, using promoters such as and without limitation CMV, SV40, EF-1, UbC, RSV, ADV, BPV, and beta-actin.
[0270] The half-life of dual antagonists can be extended by attachment of a "half-life extending moieties" or "half-life extending groups," which terms are herein used interchangeably to refer to one or more chemical groups attached to one or more amino acid site chain functionalities such as --SH, --OH, --COOH, --CONH2, --NH2, or one or more N- and/or 0-glycan structures and that can increase in vivo circulatory half-life of proteins/peptides when conjugated to these proteins/peptides. Examples of half-life extending moieties include polymers described herein, particularly those of zwitterionic monomers, such as HEMA-phosphorylcholine, PEG, biocompatible fatty acids and derivatives thereof, Hydroxy Alkyl Starch (HAS) e.g. Hydroxy Ethyl Starch (HES), Poly Ethylene Glycol (PEG), Poly (Glyx-Sery) (HAP), Hyaluronic acid (HA), Heparosan polymers (HEP), Fleximers, Dextran, Poly-sialic acids (PSA), Fe domains, Transferrin, 25 Albumin, Elastin like (ELP) peptides, XTEN polymers, PAS polymers, PA polymers, Albumin binding peptides, CTP peptides, FcRn binding peptides and any combination thereof.
[0271] In one embodiment a half-life extending moiety can be conjugated to a dual antagonist via free amino groups of the protein using N-hydroxysuccinimide (NHS) esters. Reagents targeting conjugation to amine groups can randomly react to E-amine group of lysines, a-amine group of N-terminal amino acids, and 8-amine group of histidines.
[0272] However, dual antagonists of the present have many amine groups available for polymer conjugation. Conjugation of polymers to free amino groups, thus, might negatively impact the ability of the dual antagonist proteins to bind to VEGF and/or PDGF.
[0273] In another embodiment, a half-life extending moiety is coupled to one or more free SH groups using any appropriate thiol-reactive chemistry including, without limitation, maleimide chemistry, or the coupling of polymer hydrazides or polymer amines to carbohydrate moieties of the dual antagonist after prior oxidation. The use of maleimide coupling is a particularly preferred embodiment of the present invention. Coupling preferably occurs at cysteines naturally present or introduced via genetic engineering.
[0274] Polymers are preferably covalently attached to cysteine residues introduced into dual antagonist by site directed mutagenesis. It is particularly preferred to employ cysteine residues in the Fe portion of the dual antagonist. For preferred sites to introduce cysteine residues into an Fe region see WO 2013/093809, U.S. Pat. No. 7,521,541, WO 2008/020827, U.S. Pat. Nos. 8,008,453, 8,455,622 and US2012/0213705, incorporated herein by reference for all purposes. Particularly preferred cysteine mutations are Q347C and L443C referring to the human IgG heavy chain by EU numbering.
[0275] The invention provides conjugates of dual antagonist and high MW polymers serving as half-life extenders. A preferred conjugate comprises a dual antagonist is coupled to a zwitterionic polymer wherein the polymer is formed from one or more monomer units and wherein at least one monomer unit has a zwitterionic group. Preferably, the zwitterionic group is phosphorylcholine.
[0276] Preferably, one of the monomer units is 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate or 2-(methacryloyloxyethyl)-2' (trimethylammoniumethyl) phosphate (HEMA-PC). In other preferred embodiments, polymer is synthesized from a single monomer which is preferably 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate or 2-(methacryloyloxyethyl)-2' (trimethylammoniumethyl) phosphate.
[0277] Some dual antagonist conjugates have 2 or more preferably 3 or more polymer arms wherein the monomer is HEMA-PC. Preferably, the conjugates have 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 polymer arms wherein the monomer is HEMA-PC. More preferably, the conjugates have 3, 6 or 9 arms. Most preferably, the conjugate has 9 arms.
[0278] Polymer-dual antagonist conjugates preferably have a polymer portion with a molecular weight of between 100,000 and 1,500,000 Da. More preferably the conjugate has a polymer portion with a molecular weight between 500,000 and 1,000,000 Da. Still more preferably the conjugate has a polymer portion with a molecular weight between 600,000 to 800,000 Da. Most preferably the conjugate has a polymer portion with a molecular weight between 600,000 and 850,000 Da and has 9 arms. When a molecular weight is given for a dual VEGF/PDGF antagonist conjugated to a polymer, the molecular weight will be the addition of the molecular weight of the protein, including any carbohydrate moieties associated therewith, and the molecular weight of the polymer.
[0279] In accordance with an aspect of the present invention, a dual VEGF/PDGF antagonist having a HEMA-PC polymer which has a molecular weight measured by Mw of between about 100 kDa and 1500 kDa. More preferably, the molecular weight of the polymer as measured by Mw is between about 500 kDa and 1000 kDa. Still more preferably, the molecular weight of the polymer as measured by Mw is between about 600 kDa to about 900 kDa. Most preferably, the polymer molecular weight as measured by Mw is 750 kDa plus or minus 15%.
[0280] In this aspect of the present invention, the polymer is preferably made from an initiator suitable for ATRP having one or more polymer initiation sites. Preferably, the polymer initiation site has a 2-bromoisobutyrate site. Preferably, the initiator has 3 or more polymer initiation sites. More preferably, the initiator has 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 polymer initiation sites. More preferably, the initiator has 3, 6 or 9 polymer initiation sites. Still more preferably, the initiator has 9 polymer initiation sites. Most preferably, the initiator is OG 1786.
[0281] The invention provides methods for synthesizing a zwitterionic polymer-dual antagonist conjugate, the conjugate having one or more functional agents and one or more polymer arms wherein each of the polymer arms has one or more monomer units wherein at least one of the units has a zwitterion. The method can have the steps of [0282] a. providing an initiator having one or more sites for monomer polymerization and a first linker having an amine group wherein the initiator is a trifluoro acetic acid salt; [0283] b. providing one or more monomers suitable for polymerization wherein at least one of the monomers is zwitterionic; [0284] c. reacting the monomers with the initiator to form one or more polymer arms each corresponding to the sites for monomer polymerization to provide an initiator-polymer conjugate having the first linker with the amine group; [0285] d. providing a second linker having at least second and third reactive groups; [0286] e. coupling one of the second and third reactive groups of the second linker to the amine group of the first linker of the initiator-polymer conjugate to provide a linker-initiator-polymer conjugate having one or more reactive groups that were not used in the coupling step; and [0287] f. coupling one or more functional agents to one or more of the unreacted reactive groups of the linker-initiator-polymer moiety to provide the polymer-functional agent conjugate.
[0288] Prior to the instant invention, the initiator molecule or entity had to contain a deprotectable functional group that would allow coupling of the functional agent. An example of such an initiator having a protected maleimide is shown below:
##STR00004##
[0289] After polymer synthesis, the protected maleimide is deprotected with heat to allow for generation of maleimide which could be used to couple functional agent. If one wanted to vary the nature of the chemical entity in between the maleimide and the polymer initiation site, one would have to synthesize an entire new initiator.
[0290] Each time the initiator is changed or altered in any way, a new scaled up synthesis procedure would have to be developed. Each change in the nature of the initiator molecule can have a wide range of effects on polymer synthesis. However, in accordance with the present invention, a method is presented where the conjugation group (e.g. maleimide) is added after polymer synthesis. This is sometimes referred to as a "snap-on strategy" or "universal polymer strategy. A single initiator moiety can be used for large scale polymer and bioconjugate discovery and development. Thus, conditions can be developed for scaled up optimal polymer synthesis. Such polymer can then be adapted to various types of functional agents by "snapping-on" various types of linkers and functional conjugation chemistries.
[0291] For example, if it is desired to conjugate a larger functional agent to a polymer of the instant invention such as an antibody of even a Fab fragment, a longer linker sequence can be snapped on to the polymer. In contrast, smaller functional agents may call for relatively shorter linker sequences.
[0292] In preferred embodiments of the methods, the initiator has 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 sites for polymer initiation. Preferably, the initiator has 3, 6 or 9 sites for polymer initiation.
[0293] In accordance with an aspect of the present invention, a second linker has second, third, fourth, fifth, and sixth reactive groups. More preferably, a second linker has just second and third reactive groups.
[0294] In accordance with an aspect of the present invention, each polymer arm has from about 20 to about 2000 monomer units. Preferably, each arm has from about 100 to 500 monomer units or from about 500 to 1000 monomer units or from about 1000 to 1500 monomer units or from about 1500 to 2000 monomer units.
[0295] In accordance with an aspect of the present invention, the peak molecular weight of the polymer-functional agent conjugate is about 100,000 to 1,500,000 Da. Preferably, the peak molecular weight of the polymer-functional agent conjugate is about 200,000 to about 300,000 Da, about 400,000 to about 600,000 Da or about 650,000 to about 850,000 Da.
[0296] In accordance with another aspect of the present invention, the first linker is preferably alkyl, substituted alkyl, alkylene, alkoxy, carboxyalkyl, haloalkyl, cycloalkyl, cyclic alkyl ether, alkenyl, alkenylene, alkynyl, alkynylene, cycloalkylene, heterocycloalkyl, heterocycloalkylene, aryl, arylene, arylene-oxy, heteroaryl, amino, amido or any combination thereof. More preferably, the first linker has the formula:
##STR00005##
wherein m is 1 to 10. More preferably, the first linker has the above formula and m is 4.
[0297] In still other aspects of the present invention, the initiator preferably includes a structure selected from group consisting of
##STR00006##
[0298] In preferred embodiments of the present invention, the monomer is selected from the group consisting of
##STR00007##
wherein R7 is H or C.sub.1-6 alkyl and t is 1 to 6.
[0299] More preferably, the monomer is selected from the group consisting of 2-(methacryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate (HEMA-PC) and 2-(acryloyloxyethyl)-2'-(trimethylammoniumethyl) phosphate.
[0300] Most preferably, the monomer is 2-(methacryloyloxyethyl)-2' (trimethylammoniumethyl) phosphate.
[0301] The second linker moiety preferably comprises an activated ester having the structure
##STR00008##
wherein R8 is selected from the group consisting of
##STR00009##
and R9 is
##STR00010##
[0302] wherein p is 1 to 12.
[0303] In more preferred embodiments of the present invention, the polymer has 9 arms, m of R2 is 2-4, R9 is
##STR00011##
and p is 4 to 15. Still more preferably, m is 4 and p is 12.
[0304] When a polymer is to be conjugated via a cysteine (or other specified residue), the polymer can be linked directly or indirectly to the residue (e.g., with an intervening initiator, and or spacer or the like).
[0305] Dual antagonists can be incorporated into a pharmaceutical composition with a pharmaceutically acceptable excipient. Pharmaceutical compositions adapted for oral administration may be presented as discrete units such as capsules, as solutions, syrups or suspensions (in aqueous or non-aqueous liquids; or as edible foams or whips; or as emulsions). Suitable excipients for tablets or hard gelatine capsules include lactose, maize starch or derivatives thereof, stearic acid or salts thereof. Suitable excipients for use with soft gelatine capsules include for example vegetable oils, waxes, fats, semi-solid, or liquid polyols etc. For the preparation of solutions and syrups, excipients which may be used include for example water, polyols and sugars. For the preparation of suspensions oils (e.g. vegetable oils) may be used to provide oil-in-water or water in oil suspensions.
[0306] Pharmaceutical compositions can be adapted for nasal administration wherein the excipient is a solid include a coarse powder having a particle size for example in the range 20 to 500 microns which is administered in the manner in which snuff is taken, i.e. by rapid inhalation through the nasal passage from a container of the powder held close up to the nose. Suitable compositions wherein the excipient is a liquid, for administration as a nasal spray or as nasal drops, include aqueous or oil solutions of the active ingredient. Pharmaceutical compositions adapted for administration by inhalation include fine particle dusts or mists which may be generated by means of various types of metered dose pressurized aerosols, nebulizers or insufflators.
[0307] Pharmaceutical compositions adapted for parenteral administration include aqueous and non-aqueous sterile injection solution which may contain anti-oxidants, buffers, bacteriostats and solutes which render the formulation substantially isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents. Excipients which may be used for injectable solutions include water, alcohols, polyols, glycerine and vegetable oils, for example. The compositions may be presented in unit-dose or multi-dose containers, for example sealed ampoules and vials, and may be stored in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carried, for example water for injections, immediately prior to use. Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets. Pharmaceutical compositions can be substantially isotonic, implying an osmolality of about 250-400 mOsm/kg water.
[0308] The pharmaceutical compositions may contain preserving agents, solubilizing agents, stabilizing agents, wetting agents, emulsifiers, sweeteners, colorants, odorants, salts (substances of the present invention may themselves be provided in the form of a pharmaceutically acceptable salt), buffers, coating agents or antioxidants. They may also contain therapeutically active agents in addition to the substance of the present invention. The pharmaceutical compositions of the invention may be employed in combination with one or more pharmaceutically acceptable excipients. Such excipients may include, but are not limited to, saline, buffered saline (such as phosphate buffered saline), dextrose, liposomes, water, glycerol, ethanol and combinations thereof.
[0309] The dual antagonists and pharmaceutical compositions containing them may be administered in an effective regime for treating or prophylaxis of a patient's disease including, for instance, administration by oral, intravitreal, intravenous, subcutaneous, intramuscular, intraosseous, intranasal, topical, intraperitoneal, and intralesional administration. Parenteral infusions include intramuscular, intravenous, intraarterial, intraperitoneal, or subcutaneous administration or routes among others. In therapy or as a prophylactic, the active agent may be administered to an individual as an injectable composition, for example as a sterile aqueous dispersion, preferably isotonic or substantially isotonic.
[0310] For administration to mammals, and particularly humans, it is expected that the dosage of the active agent is from 0.01 mg/kg body weight, typically around 1 mg/kg. The physician can determine the actual dosage most suitable for an individual which depends on factors including the age, weight, sex and response of the individual, the disease or disorder being treated and the age and condition of the individual being treated. The above dosages are exemplary of the average case. There can, of course, be instances where higher or lower dosages are merited.
[0311] This dosage may be repeated as often as appropriate (e.g., weekly, fortnightly, monthly, quarterly). If side effects develop the amount and/or frequency of the dosage can be reduced, in accordance with normal clinical practice. In one embodiment, the pharmaceutical composition may be administered once every one to thirty days.
[0312] The dual antagonists and pharmaceutical compositions of the invention can be employed alone or in conjunction with other compounds, such as therapeutic compounds or molecules, e.g. anti-inflammatory drugs, analgesics or antibiotics. Such administration with other compounds may be simultaneous, separate or sequential. The components may be prepared in the form of a kit which may comprise instructions as appropriate.
[0313] The dual antagonists and pharmaceutical compositions disclosed herein can be used for treatment or prophylaxis of disease, particularly the ocular diseases or conditions described herein. Although both antagonist modalities within the dual antagonist are believed to contribute to efficacy as discussed above and shown in Example 40 an understanding of mechanism is not required for practice of the invention. Preferably, a dual antagonist is more effective than an equimolar concentration of each antagonist administered alone, or a 1:1 combination of the antagonists administered as separate molecules.
[0314] So used, the conjugates are typically formulated for and administered by ocular, intraocular, and/or intravitreal injection, and/or juxtascleral injection, and/or subretinal injection and/or subtenon injection, and/or superchoroidal injection and/or topical administration in the form of eye drops and/or ointment. Such dual antagonists and compositions can be delivered by a variety of methods, e.g. intravitreally as a device and/or a depot that allows for slow release of the compound into the vitreous, including those described in references such as Intraocular Drug Delivery, Jaffe, Ashton, and Pearson, editors, Taylor & Francis (March 2006). In one example, a device may be in the form of a minipump and/or a matrix and/or a passive diffusion system and/or encapsulated cells that release the compound for a prolonged period of time (Intraocular Drug Delivery, Jaffe, Ashton, and Pearson, editors, Taylor & Francis (March 2006).
[0315] Formulations for ocular, intraocular or intravitreal administration can be prepared by methods and using ingredients known in the art. A main requirement for efficient treatment is proper penetration through the eye. Unlike diseases of the front of the eye, where drugs can be delivered topically, retinal diseases require a more site-specific approach. Eye drops and ointments rarely penetrate the back of the eye, and the blood-ocular barrier hinders penetration of systemically administered drugs into ocular tissue. Accordingly, usually the method of choice for drug delivery to treat retinal disease, such as AMD and CNV, is direct intravitreal injection. Intravitrial injections are usually repeated at intervals which depend on the patient's condition, and the properties and half-life of the drug delivered.
[0316] Therapeutic dual agonists and related conjugates according to the present invention generally are placed into a container having a sterile access port, for example, an intravenous solution bag or vial having a stopper pierceable by a hypodermic injection needle. Such compositions may also be supplied in the form of pre-filled syringes.
[0317] A "stable" formulation is one in which the protein or protein conjugated to a polymer of other half-life extending moiety therein essentially retains its physical stability and/or chemical stability and/or biological activity upon storage. By "stable" is also meant a formulation which exhibits little or no signs of instability, including aggregation and/or deamidation. For example, in accordance with an aspect of the present invention, the formulations provided by the present invention may remain stable for at least two year, when stored as indicated at a temperature of 5-8.degree. C.
[0318] Various analytical techniques for measuring protein stability are available in the art and are reviewed in Peptide and Protein Drug Delivery, 247-301 (Vincent Lee ed., New York, N.Y., 1991) and Jones, 1993 Adv. Drug Delivery Rev. 10: 29-90, for examples. Stability can be measured at a selected temperature for a selected time period. Storage of stable formulations is preferably for at least 6 months, more preferably 12 months, more preferably 12-18 months, and more preferably for 2 or more years.
[0319] A protein, such as an antibody or fragment thereof, "retains its physical stability" in a pharmaceutical formulation if it shows no signs of aggregation, precipitation, deamidation and/or denaturation upon visual examination of color and/or clarity, or as measured by UV light scattering or by size exclusion chromatography.
[0320] A protein "retains its chemical stability" in a pharmaceutical formulation, if the chemical stability at a given time is such that the protein is considered to still retain its biological activity. Chemical stability can be assessed by detecting and quantifying chemically altered forms of the protein. Chemical alteration may involve size modification (e.g., clipping), which can be evaluated using size exclusion chromatography, SDS-PAGE and/or matrix-assisted laser desorption ionization/time-of-flight mass spectrometry (MALDI/TOF MS), for examples. Other types of chemical alteration include charge alteration (e.g., occurring as a result of deamidation), which can be evaluated by ion-exchange chromatography, for example. An antibody "retains its biological activity" in a pharmaceutical formulation, if the biological activity of the antibody at a given time is within about 10% (within the errors of the assay) of the biological activity exhibited at the time the pharmaceutical formulation was prepared as determined in an antigen binding assay, for example.
[0321] A protein-polymer conjugate "retains its chemical stability" the chemical bond between the protein and the polymer is maintained intact, e.g., it is not hydrolyzed or otherwise disrupted. The protein part of the conjugate retains its chemical stability as described above.
[0322] By "isotonic" is meant that the formulation of interest has essentially the same osmotic pressure as human blood or the vitreous for intravitreal injections. Isotonic formulations will generally have an osmotic pressure from about 250 to 400 mOsm. Isotonicity can be measured using a vapor pressure or ice-freezing type osmometer, for example.
[0323] As used herein, "buffer" refers to a buffered solution that resists changes in pH by the action of its acid-base conjugate components. The buffer of this invention has a pH in the range of preferably from about 3.0 to about 8.0; for example from about 4.5 to 8; or about pH 6 to about 7.5; or about 6.0 to about 7.0, or about 6.5-7.0, or about pH 7.0 to about 7.5; or about 7.1 to about 7.4. A pH of any point in between the above ranges is also contemplated.
[0324] "PBS" phosphate buffered saline, Tris based buffers and histidine based buffers are particularly preferred buffers for the instantly invented dual antagonists. In the case of OG 1448, PBS is particularly preferred. More preferably, in the case of OG 1448, the PBS buffer has a pH of 7-8 and the concentration of OG 1448 is from about 10 mg/ml to about 100 mg/ml. Still more preferably, the OG 1448 is from about 25 to about 65 mg/ml and the pH is about 7.4. In the most preferred embodiments of the present invention, the concentration of OG 1448 is 50 mg/ml to 60 mg/ml.
[0325] In preferred embodiments of the present invention, the PBS buffer is made up of at least Na.sub.2HPO.sub.4, KH.sub.2PO.sub.4 and NaCl adjusted so as to provide the appropriate pH. In particularly preferred embodiments of the present invention, the buffer may contain other pharmaceutical excipients such as KCl and other salts, detergents and/or preservatives so as to provide a stable storage solution.
[0326] A "preservative" is a compound which can be included in the formulation to essentially reduce bacterial action therein, thus facilitating the production of a multi-use formulation, for example. Examples of potential preservatives include octadecyldimethylbenzyl ammonium chloride, hexamethonium chloride, benzalkonium chloride (a mixture of alkylbenzyldimethylammonium chlorides in which the alkyl groups are long-chain compounds), and benzethonium chloride. Other types of preservatives include aromatic alcohols such as phenol, butyl and benzyl alcohol, alkyl parabens such as methyl or propyl paraben, catechol, resorcinol, cyclohexanol, 3-pentanol, and m-cresol.
[0327] In accordance with an aspect of the present invention, formulations of dual PDGF/VEGF antagonists according to the present invention to be safe for human use or for animal testing must have sufficiently low levels of endotoxin. "Endotoxin" is lipopolysaccharide (LPS) derived from the cell membrane of Gram-negative bacteria. Endotoxin is composed of a hydrophilic polysaccharide moiety covalently linked to a hydrophobic lipid moiety (lipid A). Raetz C R, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. 1991. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 5(12):2652-2660. Lipid A is responsible for most of the biological activities of endotoxin, i.e., its toxicity. Endotoxins are shed in large amount upon bacterial cell death as well as during growth and division. They are highly heat-stable and are not destroyed under regular sterilizing conditions. Extreme treatments with heat or pH, e.g., 180-250.degree. C. and over 0.1 M of acid or base must be used (Petsch D, Anspach F. 2000. Endotoxin removal from protein solutions. J Biotechnol. 76: 97-119). Such conditions of course would be highly detrimental to biological drugs.
[0328] In the biotech and pharmaceutical industries, it is possible to find endotoxin during both production processes and in final products. As bacteria can grow in nutrient poor media, including water, saline and buffers, endotoxins are prevalent unless precautions are taken. Endotoxin injection into an animal or human causes a wide variety of pathophysiological effects, including endotoxin shock, tissue injury and even death. Ogikubo Y, Ogikubo Y, Narimatsu M, Noda K, Takahashi J, Inotsume M, Tsuchiya M, Tamura Y. 2004. Evaluation of the bacterial endotoxin test for quantifications of endotoxin contamination of porcine vaccines. Biologics 32:88-93.
[0329] Pyrogenic reactions and shock are induced in mammals upon intravenous injection of endotoxin at low concentrations (1 ng/mL) (Fiske J M, Ross A, VanDerMeid RK, McMichael J C, Arumugham. 2001. Method for reducing endotoxin in Moraxella catarrhalis UspA2 protein preparations. J Chrom B. 753:269-278). The maximum level of endotoxin for intravenous applications of pharmaceutical and biologic product is set to 5 endotoxin units (EU) per kg of body weight per hour by all pharmacopoeias (Daneshiam M, Guenther A, Wendel A, Hartung T, Von Aulock S. 2006. In vitro pyrogen test for toxic or immunomodulatory drugs. J Immunol Method 313: 169-175). EU is a measurement of the biological activity of an endotoxin. For example, 100 pg of the standard endotoxin EC-5 and 120 pg of endotoxin from Escherichia coli 0111:B4 have activity of 1 EU (Hirayama C, Sakata M. 2002. Chromatographic removal of endotoxin from protein solutions by polymer particles. J Chrom B 781:419-432). Meeting this threshold level has always been a challenge in biological research and pharmaceutical industry (Berthold W, Walter J. 1994. Protein Purification: Aspects of Processes for Pharmaceutical Products. Biologicals 22: 135-150; Petsch D, Anspach F B. 2000. Endotoxin removal from protein solutions. J Biotech 76:97-119).
[0330] The presence of endotoxin in drugs to be delivered via intravitreal injection is of particular concern. Intravitreal injection of drug (penicillin) was first performed in 1945 by Rycroft. Rycroft B W. 1945. Penicillin and the control of deep intraocular infection. British J Ophthalmol 29 (2): 57-87. The vitreous is a chamber where high level of drug can be introduced and maintained for relatively long periods of time. The concentration of drug that can be achieved via intravitreal injection far exceeds what can be generated by topical administration or by systemic administration (e.g. intravenous).
[0331] One of the most dangerous complications potentially arising from intravitreal injections is endophthalmitis. Endophthalmitis falls into two classes: infectious and sterile. Infectious endophthalmitis is generally cause by bacteria, fungi or parasites. The symptoms of infectious endophthalmitis include severe pain, loss of vision, and redness of the conjunctiva and the underlying episclera. Infectious endophthalmitis requires urgent diagnosis and treatment. Possible treatments include intravitreal injection of antibiotics and pars plana vitrectomy in some cases. Enucleation may be called for to remove a blind and painful eye. See, e.g., Christy N E, Sommer A. 1979. Antibiotic prophylaxis of postoperative endophthalmitis. Ann Ophthalmol 11 (8): 1261-1265.
[0332] Sterile endophthalmitis in contrast does not involve an infectious agent and can be defined as the acute intraocular inflammation of the vitreous cavity that resolves without the need of intravitreal antibiotics and/or vitreoretinal surgery. If a vitreous microbiological study has been done, it needs to be negative culture proven to sustain a diagnosis of sterile endophthalmitis. Marticorena J, Romano V, Gomez-Ulla F. 2012 "Sterile Endophthalmitis after Intravitreal Injections" Med Inflam. 928123.
[0333] It has been observed that intravitreal injection of biological drugs contaminated with endotoxin can result in sterile endophthalmitis. Marticorena, et al. Bevacizumab (Avastin) is approved by the Food and Drug Administration for the treatment of glioblastoma and of metastatic colorectal cancer, advanced nonsquamous non-small-cell lung cancer and metastatic kidney cancer. Bevacizumab is also widely used off label as a treatment for wet AMD. Bevacizumab comes from the manufacturer as a 100 mg/4 ml. This solution cannot be directly used for intravitreal injection and must be compounded by a pharmacist. Clusters of sterile endophthalmitis have been observed and are theorized to be cause by inadvertent contamination of bevacizumab by endotoxin by the compounding pharmacist.
[0334] Given the dire clinical results of intravitreal injection of endotoxin, the total amount of endotoxin that can be given to a patient via intravitreal dosing is highly limited. In accordance with an aspect of the present invention, a solution having a dual VEGF/PDGF antagonist according to the present invention is provided having an endotoxin level that does not exceed 5.0 EU/ml. More preferably, the endotoxin level does not exceed 1.0 EU/ml. Still more preferably, the endotoxin level does not exceed 0.5 EU/ml. Still more preferably, the endotoxin level does not exceed 0.2 EU/ml. In still more preferred embodiments, the endotoxin level does not exceed 0.1, 0.09, 0.08, 0.07, 0.06, 0.05, 0.04, 0.03, 0.02 or 0.01 EU/ml.
[0335] Two commonly used FDA-approved tests for the presence of endotoxin are the rabbit pyrogen test and Limulus Amoebodyte Lysate (LAL) assay (Hoffman S, et al. 2005. International validation of novel pyrogen tests based on human monocytoid cells J. Immunol. Methods 298: 161-173; Ding J L, Ho B A. 2001. New era in pyrogen testing. Biotech. 19:277-281). The rabbit pyrogen test was developed in the 1920s and involves monitoring the temperature rise in a rabbit injected with a test solution. However, use of the rabbit pyrogen test has greatly diminished over the years due to expense and long turnaround time. Much more common is the LAL test. LAL is derived from the blood of a horseshoe crab and clots upon exposure to endotoxin.
[0336] One of the simplest LAL assays is the LAL gel-clot assay. Essentially, the LAL clotting assay is combined with a serial dilution of the sample in question. Formation of the gel is proportional to the amount of endotoxin in the sample. Serial dilutions are prepared from the sample and each dilution assayed for its ability to form LAL gel. At some point a negative reaction is contained. The amount of endotoxin in the original sample can be estimated from the dilution assay.
[0337] Other LAL tests have also been developed, including the turbidimetric LAL assay (Ong K G, Lelan J M, Zeng K F, Barrett G, Aourob M, Grimes C A. 2006. A rapid highly-sensitive endotoxin detection system. Biosensors and Bioelectronics 21:2270-2274) and the chromogenic LAL assay (Haishima Y, Hasegawa C, Yagami T, Tsuchiya T, Matsuda R, Hayashi Y. 2003. Estimation of uncertainty in kinetic-colorimetric assay of bacterial endotoxins. J Pharm Biomed Analysis. 32:495-503). The turbidimetric and chromogenic assays are much more sensitive and quantitative than the simple gel-clot dilution assay.
[0338] The present invention provides a method of reducing the amount of endotoxin in a composition having a dual VEGF/PDGF antagonist, the method having the steps of contacting the composition with an affinity chromatography resin that binds to the dual VEGF/PDGF antagonist; eluting the dual VEGF/PDGF antagonist from the affinity chromatography resin to form an affinity chromatography eluent having the antagonist; contacting the affinity chromatography eluent with an ion-exchange resin that binds the dual VEGF/PDGF antagonist; and eluting the dual VEGF/PDGF antagonist from the ion-exchange resin, wherein the dual VEGF/PDGF antagonist eluted from the ion-exchange resin is substantially free from endotoxin.
[0339] The above method for reducing the amount of endotoxin, or other method or process recited herein, can be performed in the order described in the steps above or it can optionally be performed by varying the order of the steps or even repeating one or more of the steps. In one embodiment, the method of reducing the amount of endotoxin in a composition is performed in the order of the described steps. In some embodiments, the affinity chromatography resin contacting, washing and eluting steps are repeated in the same order more than one time before contacting the affinity chromatography eluent with the ion exchange resin. The method can also include a filtering step using, for example, a 0.1 micron, 0.22 micron, or 0.44 micron filter, that can be performed on either one or more of the eluents removed after each resin binding step.
[0340] In certain instances, the steps of contacting the composition with affinity chromatography resin, washing and eluting the antibody from the affinity chromatography resin can be repeated more than one time before contacting the first eluent with an ion-exchange resin. In one embodiment, the affinity chromatography resin comprises a recombinant Protein A ("rProteinA") resin. One example of a suitable recombinant Protein A resin is rProteinA Sepharose FF.RTM. resin (Amersham, Piscataway, N.J.). In another embodiment, a suitable affinity chromatography resin would comprise a protein G chromatography resin. In other embodiments, a suitable affinity chromatography resin comprises a mixed Protein A/Protein G resin. In other embodiments, a suitable affinity chromatography resin comprises a hydrophobic charge induction resin that comprises a 4-mercaptoethylpyridine ligand such as a MEP HyperCel.RTM. resin (BioSepra, Cergy, Saint Christophe, France).
[0341] In some embodiments, it is preferred that the ion exchange resin comprises an anion-exchange resin. As will be known by the person skilled in the art, ion exchangers may be based on various materials with respect to the matrix as well as to the attached charged groups. For example, the following matrices may be used, in which the materials mentioned may be more or less cross-linked: MacroCap Q (GE Healthcare Biosciences, Piscataway, N.J.), agarose based (such as Sepharose CL-6B.RTM., Sepharose Fast Flow.RTM. and Sepharose High Performance.RTM.), cellulose based (such as DEAE Sephacel.RTM.), dextran based (such as Sephadex.RTM.), silica based and synthetic polymer based. For the anion exchange resin, the charged groups, which are covalently attached to the matrix, may, for example, be diethylaminoethyl, quaternary aminoethyl, and/or quaternary ammonium. It is preferred that the anion-exchange resin comprises a quaternary amine group. An exemplarily anion-exchange resin that has a quaternary amine group for binding the anti-M-CSF antibody is a Q Sepharose.RTM. resin (Amersham, Piscataway, N.J.).
[0342] In other aspects, if the endotoxin levels are higher than desired after subjecting the composition to the aforementioned anion-exchange chromatography step, the composition may in the alternative be subjected to a cation exchange resin. In accordance with this aspect of the present invention, any endotoxin in the composition should have a differential binding to the ion-exchange resin than the protein in question to allow purification of the protein from the endotoxin. In this regard, endotoxin is negatively charged and will generally bind to an anion exchange resin. If both the protein and the endotoxin bind to the anion exchange resin, purification of one from the other may be effectuated by using a salt gradient to elute the two into different fractions. The relative binding of the protein to a particular resin may also be effected by changing the pH of the buffer relative to the pl of the protein. In a preferred aspect of the present invention, cation-exchange chromatography is the sole ion-exchange chromatography employed.
[0343] In accordance with another aspect of the present invention, if the endotoxin levels are too high after the anion exchange resin, the composition may be further subjected to a second ion-exchange step, for example, by contacting the compositions with a cation exchange resin and followed by a wash step, then elution from the ion-exchange resin. In preferred embodiments, the cation exchange resin comprises a sulfonic group for binding. Exemplary cation exchange resins are SP Sepharose.RTM. resin FF (Amersham, Piscataway, N.J.) Porns XS (CEX) (Life Technology, Grand Island, N.Y.).
[0344] In accordance with an aspect of the invention, after the solution of dual PDGF/VEGF antagonist protein is produced having the specified level of endotoxin, there are a number of steps prior to final formulation of the protein. In some embodiments of the present invention, a half-life extending moiety is conjugated to the protein. The conjugate is then formulated into a final drug formulation which is injected into the patients. In some embodiments, the conjugate is again purified on an ion-exchange resin which can preferably be a cation-exchange resin. In other embodiments, the protein is formulated. In all cases, normal laboratory procedures must be employed to prevent the introduction of endotoxin contaminants into the protein sample or into the protein-polymer conjugate.
EXAMPLES
Example 1. Protein Sequence of PDGFR-GS 10-Anti-VEGF-A Light Chain/Anti-VEGF-A Heavy Chain (Wild Type Fe)
[0345] A PDGFR-.beta. trap-anti-VEGF-A light chain/anti-VEGF-A heavy chain was constructed having the sequence set forth below in FIGS. 7A, B. PDGFR-GS 10-anti-VEGF-A light chain amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GGGGSGGGGS and the bevacizumab light chain sequence. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2 (as noted above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs known to those of skill in the art may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The sequence of FIG. 7A is set forth in SEQ ID NO. 19. FIG. 7B shows the bevacizumab heavy chain sequence (SEQ ID NO. 2). The bevacizumab light chain optionally has an M4L mutation (Kabat numbering). The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S100aT (Kabat numbering), L234A, L235A, G237A, Q347C and L443C EU numbering).
Example 2. Protein Sequence of PDGFR.beta.-GG-Anti-VEGF-A Light Chain/Anti-VEGF-A Heavy Chain (Wild Type Fe)
[0346] Another PDGFR-.beta. trap-anti-VEGF-A light chain/anti-VEGF-A heavy chain was constructed having the sequence set forth below in FIGS. 8A, B. FIG. 8A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GG and the bevacizumab light chain sequence. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG (as noted above), GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 8A is set forth in SEQ ID NO. 3. FIG. 8B shows bevacizumab heavy chain sequence (SEQ ID NO. 2). The bevacizumab light chain of FIG. 8A optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S 100aT (Kabat numbering), L234A, L235A, G237A, Q347C and L443C (EU numbering).
Example 3. Protein Sequence of PDGFR.beta.-GS 10-Anti-VEGF-A Heavy Chain (Wild Type Fc)/Anti-VEGF-A Light Chain
[0347] Another PDGFR-.beta. trap-anti-VEGF-A heavy chain (wild type Fc)/anti-VEGF-A light chain was constructed having the sequence set forth in FIGS. 9A, B. FIG. 9A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GGGGSGGGGS and the bevacizumab heavy chain sequence, optionally having Q347C or L443C (EU numbering). Alternatively, the linker may be the GGGGS motif x1, x2 (as noted above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 9A is set forth in SEQ ID NO. 4. The protein of FIG. 9B is the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S1OOaT (Kabat numbering), L234A, L235A, G237A, Q347C and L443C (EU numbering).
Example 4. Protein Sequence of PDGFR-GG-Anti-VEGF-A Heavy Chain (Wild Type Fc)/Anti-VEGF-A Light Chain
[0348] Another PDGFR-.beta. trap-anti-VEGF-A heavy chain (wild type Fc)/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 10A, 10B. FIG. 10A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GG and the bevacizumab heavy chain sequence, optionally having Q347C or L443C. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG (as noted above), GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 10A is set forth in SEQ ID NO. 6. The protein of FIG. 10B is the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S1OOaT (Kabat numbering), L234A, L235A, G237A, Q347C and L443C (EU numbering).
Example 5. Protein Sequence of Anti-VEGF-A Heavy Chain (Wild Type Fc)-GS21-PDGFR/Anti-VEGF-A Light Chain
[0349] A PDGFR-.beta. trap-anti-VEGF-A antibody construct was constructed with the anti-VEGF-A heavy chain being upstream or N-terminal to the PDGFR-.beta. trap having the sequence set forth below in FIGS. 11A, B. FIG. 11A amino acids 1-451 correspond to the bevacizumab heavy chain sequence, optionally having Q347C or L443C, followed by linker sequence GGGGSGGGGSGGGGSGGGGSG. Alternatively, the linker may be the GGGGS motif x1, x2 (as noted above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The linker is followed by amino acid sequences 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). The protein sequence of FIG. 11A is set forth in SEQ ID NO. 7. FIG. 11B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S1OOaT (Kabat numbering), L234A, L235A, G237A, Q347C and L443C (EU numbering).
Example 6. Protein Sequence of PDGFR-GS 10-Anti-VEGF-A Heavy Chain (Q347C)/Anti-VEGF-A Light Chain (TAF347)
[0350] Another PDGFR-.beta. trap-anti-VEGF-A heavy chain (Q347C)/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 12A, B. FIG. 12A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). Immediately following the PDGFR sequence is a 10 amino acid linker GGGGSGGGGS. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. The linker may be combinations of the above. Joined to the carboxyl terminus of the serine residue of the linker is the bevacizumab heavy chain with the following amino acid: T28D, N31H, H97Y, S100aT (Kabat numbering), L234A, L235A, G237A and Q347C (EU numbering). The protein sequence of FIG. 12A is set forth in SEQ ID NO. 8. The protein of FIG. 12B is ranibizumab light chain (bevacizumab w/M4L) (SEQ ID NO. 12).
Example 7. Protein Sequence of PDGFR-GS 10-Anti-VEGF-A Heavy Chain (L443C)/Anti-VEGF-A Light Chain
[0351] Another PDGFR-.beta. trap-anti-VEGF-A heavy chain (L443C))/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 13A, B. FIG. 13A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). Immediately following the PDGFR sequence is a 10 amino acid linker GGGGSGGGGS. Joined to the carboxyl terminus of the serine residue of the linker is the bevacizumab heavy chain with the following amino acid: T28D, N31H, H97Y, S100aT (Kabat numbering), L234A, L235A, G237A and L443C (EU numbering). The TAF443 light chain is the same as bevacizumab except for a M4L change (Kabat numbering). The protein sequence of FIG. 13A is set forth in SEQ ID NO. 9. FIG. 13B shows the ranibizumab light chain (bevacizumab w/M4L) (SEQ ID NO. 12).
Example 8. Protein Sequence of PDGFR.beta.-GS 10-Anti-VEGF-A Light Chain/Anti-VEGF-A Fab
[0352] A PDGFR-.beta. trap-anti-VEGF-A light chain/anti-VEGF-A Fab was constructed having the sequence set forth below in FIGS. 14A, B. FIG. 14A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GGGGSGGGGS and the bevacizumab light chain sequence. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2 (as noted above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 14A is set forth in SEQ ID NO. 1. The protein of FIG. 14B is the bevacizumab Fab (SEQ ID NO. 21). The bevacizumab light chain of FIG. 14A optionally has an M4L mutation. The bevacizumab Fab of the second protein optionally has one or more of the following mutations: T28D, N31H, H97Y, and S1OOaT. The bevacizumab Fab of the second chain optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 9. Protein Sequence of PDGFR.beta.-GG-Anti-VEGF-A Light Chain/Anti-VEGF-A Fab
[0353] A PDGFR-.beta. trap-anti-VEGF-A light chain/anti-VEGF-A Fab was constructed having the sequence set forth below in FIGS. 15A, B. FIG. 15A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619. 1), followed by the linker sequence GG and the bevacizumab light chain sequence. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG (as above), GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 15A is set forth in SEQ ID NO. 3. FIG. 15B shows the heavy chain of bevacizumab Fab (SEQ ID NO. 21). The bevacizumab light chain of FIG. 15A optionally has an M4L mutation (Kabat numbering). The bevacizumab Fab of FIG. 15B optionally has one or more of the following mutations: T28D, N31H, H97Y, and S1OOaT (Kabat numbering). The bevacizumab Fab of FIG. 15B optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 10. Protein Sequence of PDGFR.beta.-GS 10-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain
[0354] Another PDGFR-.beta. trap-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 16A, B. FIG. 16A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GGGGSGGGGS and the bevacizumab Fab sequence. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2 (as above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 16A is set forth in SEQ ID NO. 22. FIG. 16B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S 100aT (Kabat numbering). The heavy chain optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 11. Protein Sequence of PDGFR.beta.-GG-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain
[0355] Another PDGFR-.beta. trap-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 17A, B. FIG. 17A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker sequence GGGGSGGGGS and the bevacizumab Fab sequence. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2 (as above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 17A is set forth in SEQ ID NO. 23. FIG. 17B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S100aT (Kabat numbering). The bevacizumab Fab heavy chain optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 12. Protein Sequence of Anti-VEGF-A Fab-GS21-PDGFR.beta./Anti-VEGF-A Light Chain
[0356] A PDGFR-.beta. trap-anti-VEGF-A antibody construct was constructed with the anti-VEGF-A heavy chain being upstream or N-terminal to the PDGFR-.beta. trap having the sequence set forth below in FIGS. 18A, B. FIG. 18A amino acids 1-231 correspond to the bevacizumab Fab followed by linker sequence GGGGSGGGGSGGGGSGGGGSG. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The linker is followed by amino acid sequences 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). The protein sequence of FIG. 18A is set forth in SEQ ID NO. 24. FIG. 18B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S 100aT. The protein of FIG. 18A optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain of FIG. 18B.
Example 13. Protein Sequence of PDGFR.beta.-GS 10-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain
[0357] Another PDGFR-.beta. trap-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 19A, B. FIG. 19A amino acids 1-282 correspond to 33 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). Immediately following the PDGFR sequence is a 10 amino acid linker GGGGSGGGGS. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2 (as above), x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. Joined to the carboxyl terminus of the serine residue of the linker is the bevacizumab Fab having the mutations T28D, N31H, H97Y, and S1OOaT (Kabat numbering). The protein sequence of FIG. 19A is set forth in SEQ ID NO. 25. The protein of FIG. 19B is the ranibizumab light chain (bevacizumab w/M4L) (SEQ ID NO. 12). The protein of FIG. 19A optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain of FIG. 19B.
Example 14. Protein Sequence of PDGFR.beta.-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain (1a)
[0358] Another PDGFR-.beta. trap-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth in FIGS. 20A, B. FIG. 20A amino acids 1-283 correspond to 32 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the bevacizumab heavy chain. The protein sequence of FIG. 20A is set forth in SEQ ID NO. 26. The protein of FIG. 20B is the bevacizumab light chain sequence (SEQ ID NO. 5). As set forth in this example, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S1OOaT (Kabat numbering), Q347C and L443C (EU numbering).
Example 15. Protein Sequence of PDGFR-.beta. (D2-D3)-Anti-VEGF-A Heavy Chain/Anti-VEGF-A Light Chain (1b)
[0359] Another PDGFR-.beta. trap (D2-D3)-anti-VEGF-A heavy chain/anti-VEGF-A light chain was constructed having the sequence set forth below in FIG. 21A, B. FIG. 21A amino acids 1-190 correspond to 125 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the bevacizumab heavy chain. The protein sequence of FIG. 21A is set forth in SEQ ID NO. 27. As set forth in this example, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. FIG. 21B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, S1OOaT (Kabat numbering), Q347C and L443C (Eu numbering).
Example 16. Protein Sequence of PDGFR-.beta. (D2-D3)-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain (2b)
[0360] Another PDGFR-.beta. trap (D2-D3)-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth in FIGS. 22A, B. FIG. 22A amino acids 1-190 correspond to 125 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the bevacizumab Fab. As set forth in this example, no linker need be used between the PDGFR.beta.-segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. The GGGGSGGGGS linker is particularly preferred. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The sequence of FIG. 22A is set forth in SEQ ID NO. 28. FIG. 22B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S100aT (Kabat numbering). The bevacizumab Fab of FIG. 22A optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain of FIG. 22B.
Example 17. Protein Sequence of PDGFR-.beta. (D2-D3)-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain (2b')
[0361] Another PDGFR-.beta. trap (D2-D3)-6xGS-anti-VEGF-A Fab/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 23A, B. FIG. 23A amino acids 1-190 correspond to 125 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker GGGSGGGGSGGGGSGGGGSGGGGSGGGGS and then by bevacizumab Fab. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs known to those of skill in the art may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The protein sequence of FIG. 23A is set forth in SEQ ID NO. 29. FIG. 23B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S1OOaT (Kabat numbering). The bevacizumab Fab heavy chain optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 18. Protein Sequence of PDGFR-(D2-D3)-Anti-VEGF-A Fab/Anti-VEGF-A Light Chain (2b')
[0362] Another anti-VEGF-A Fab-6xGS-PDGFR-.beta. trap (D2-D3)/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 24A, B. FIG. 24A amino acids 1-190 correspond to 125 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1), followed by the linker GGGSGGGGSGGGGSGGGGSGGGGSGGGGS and then by bevacizumab Fab. Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The sequence of FIG. 24A is set forth in SEQ ID NO. 29. FIG. 24B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain of FIG. 24B optionally has an M4L mutation. The bevacizumab heavy chain of the first protein optionally has one or more of the following mutations: T28D, N31H, H97Y, and S1OOaT (Kabat numbering). The bevacizumab Fab of FIG. 24A optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 19. Protein Sequence of Anti-VEGF-A Fab-6xGS-PDGFR-.beta. (D2-D3)/Anti-VEGF-A Light Chain (3)
[0363] Another anti-VEGF-A Fab-6xGS-PDGFR-.beta. (D2-D3)/anti-VEGF-A light chain was constructed having the sequence set forth below in FIGS. 25A, B. FIG. 25A amino acids 1-231 correspond to bevacizumab Fab, followed by the linker GGGSGGGGSGGGGSGGGGSGGGGSGGGGS and then 125 to 314 of human PDGFR-.beta. (UniProtKB/Swiss-Prot: P09619.1). Optionally, no linker need be used between the PDGFR-.beta. segment and the anti-VEGF segment. Alternatively, the linker may be the GGGGS motif x1, x2, x3, x4, etc. such that the activity of the two proteins is optimized. Other linker motifs may also be used in accordance with the present invention, including G, GG, GGGS and GGGES x1, x2, x3, x4, etc. The linker may be combinations of the above. The sequence of FIG. 25A is set forth in SEQ ID NO. 30. FIG. 25B shows the bevacizumab light chain sequence (SEQ ID NO. 5). The bevacizumab light chain optionally has an M4L mutation. The bevacizumab heavy chain optionally has one or more of the following mutations: T28D, N31H, H97Y, and S1OOaT (Kabat numbering). The PDGFR-.beta. of FIG. 25A optionally has a cysteine moiety added to the C-terminus for conjugating a half-life extending moiety. Preferably, the cysteine moiety is added via SGGGC or CAA. Alternatively, SGGGC or CAA may be added to the C-terminus of the light chain.
Example 20. Production of Dual PDGFR/VEGF Antagonist Protein
[0364] The TAF443 heavy and light chains were cloned into expression plasmids and transfected into CHO cells. Cells were grown up in appropriate media and harvested. TAF443 was purified as follows. 10 L culture medium from CHO cells expressing SEQ ID NOS. 31 and 32 were adjusted with 5% (v/v) 1.1 M HEPES, 0.22 M EDTA, pH 6.7 or 10% 0.55 M Hepes, 0.11M EDTA, 5.5% Triton X-100, pH 6.7, and loaded onto a 167/400 ml Protein A column (2-run) packed with Mab Select Sure resin equilibrated in 50 mM Tris, 150 mM NaCl, pH 7.5 (5-CV). The column was washed with 50 mM Tris, 150 mM NaCl, pH 7.5 (2-CV), 50 mM Tris, 0.5M CaCh, pH 7.5 (5-CV), and then 10 mM Tris, 10 mM NaCl, pH 7.5 (3-CV) before the protein was eluted using 150 mM Glycine, 40 mM NaCl, pH 3.5 (4-CV). Fractions were pooled, adjusted to pH 3.5 using 2M Glycine, pH 2.7, and then neutralized to pH 7 using 2M HEPES, pH 8.0. The Protein A pool was loaded onto a 274 ml TMAE column equilibrated in 50 mM Hepes, 65 mM NaCl, pH 7.0 (5-CV). The column was washed with 50 mM Hepes, 65 mM NaCl, pH 7.0 (3-CV), and then eluted with 50 mM Tris, 200 mM NaCl, pH 7.5 (5-CV). The elution fractions were pooled and buffer exchanged in a 1150 mL Sephadex G-25 Coarse column equilibrated with PBS-CMF, pH 7.2. The pool was filtered, concentrated to >5 mg/ml via 30k MWCO VivaFlow200. The concentrated protein was filtered through a 0.22 um filter, and then characterized by SDS-PAGE, analytical SEC, O.D.280/320, end toxin LAL assay, Protein A ELISA, IEF, and Freeze/Thaw Analysis.
[0365] The table below summarizes the properties of an example batch of purified TAF443.
TABLE-US-00001 TAF443 Purified Lot Characteristics Concentration (UV) 5.69 mg/ml Purity (SEC) 98.6% MW (SDS-PAGE) ~200 kDa (NR) pI (IEF) 4.2-4.5 Endotoxin (LAL) 0.1 EU/mg Protein A (Elisa) <10 ng/ml Final Yield ~700 mg/L(CM)
Example 21. TAP Bi-Functional Molecule Stability at High Concentration in Representative Formulations
[0366] The TAP bi-functionals were concentrated to 50-85 mg/ml in a series of standard formulation buffers ranging from pH 4.5 to 7.5, in the presence of excipients such as sucrose. Aliquots of these samples were stored at room temperature (RT) and 4.degree. C. over a period of 6 weeks, and sampled at time zero and after each subsequent week to measure the percentage of aggregated material by analytical SEC. The effect of pH on aggregation of TAP443 can be seen in the following table.
TABLE-US-00002 % Aggregates Observed in TAF Solution at various pHs over Time Tris pH 7.5 His pH 6.0 His pH 5.5 Lac pH 4.5 Time 0 <1 <1 <1 <2 Day 4 <1 <1 <1 ~3 Week 1 <1 <1 <1 ~4 Week 2 <1 <1 ~2 ~6 Week 4 <1 <1 ~3 ~10 Week 6 <1 <1 ~3 ~10
Example 22. Transfection of Constructs into CHO Cells
[0367] DNA constructs for TAPwt, TAP443 and TAP347 were transfected into CHO-K 1 SV SSI: 3 pools/construct. The normal 3 weeks of recovery was observed in most of the cell lines. However, TAPwt and TAP347 cell lines lagged approximately 1 week behind the other cell lines. Once the pools were established, day 4 for most and day 3 for TAPwt and TAP347, conditioned media samples were run on Octet. 3-day conditioned media for TAPwt and TAP347 showed about 7 mg/ml by Octet. 4-day conditioned media showed about 21 mg/ml for TAP443. Small differences were observed between pools and the pools were used to make pools of pools which were carried forward for protein generation.
Example 23. SEC-MALS of Proteins
[0368] The PDGPR segment of TAP has 7 putative glycosylation sites. The protein appears to be heavily glycosylated from SEC-MALS measurements:
TABLE-US-00003 Construct Protein (kDa) Sugar (kDa) Total (kDa) TAFwt 184 63 247 TAF334 182 62 244 TAF443 187 63 250
[0369] The samples run on SEC-MALS were all greater than 98% pure. The molecular weights measured were reasonable. Some high molecular weight material was observed, probably a tri- to pentamer (data not shown).
Example 24. Thermal Stability of TAP Proteins
[0370] Thermal stability profiles were run of TAFwt, TAF443 and TAF347 in PBS, pH 7.2. Each protein had three peaks (data not shown). The relative positions of the peaks are set forth in the table below:
TABLE-US-00004 Sample T.sub.m1(.degree. C.) T.sub.m2(.degree. C.) T.sub.m3(.degree. C.) TAFwt 58.1 .+-. 0.1 71.9 .+-. 0.1 83.2 .+-. 0.1 TAF347 58.2 .+-. 0.1 71.9 .+-. 0.1 81.7 .+-. 0.1 TAF443 58.2 .+-. 0.1 71.9 .+-. 0.1 84.4 .+-. 0.1
[0371] The stabilities of the proteins over the temperature range are very similar. It is noted however that there are some small changes in T.sub.m3. T.sub.m3 likely corresponds to the CH.sub.3 domain of the antibody domain of the three TAF proteins and the changes reflect the Cys mutations. The low overall stability of the TAF proteins is likely due to unfolding of the PDGFR segment of the proteins.
Example 25. TAP Forced Aggregation
[0372] The percentage of aggregates in a solution of the three TAP proteins as a function of heat was examined (data not shown). Solutions of each of the proteins (TAFwt, TAF347 and TAF443) started to show aggregates starting around 54.degree. C. The percentage of aggregates for each of the proteins increased sharply as the temperature was increased. At 64.degree. C., roughly 40% of each of the TAF proteins constituted aggregates. It is noted that the aggregation starts to occur at the lowest T.sub.m, seemingly corresponding to the unfolding of the PDGFR portion of the protein.
Example 26. TAF443 Thermal Stability as a Function of pH
[0373] The thermal stability of TAF443 was examined at various pHs as set forth in the table below. In non PBS buffers, 4 thermal denaturation peaks are seen:
TABLE-US-00005 Buffer T.sub.m1(.degree. C.) T.sub.m2(.degree. C.) T.sub.m3(.degree. C.) T.sub.m4(.degree. C.) Tris pH 7.5 57 67 74 85.9 His pH 6.0 53.3 62.9 75.4 84.9 Succinate 55.9 66.7 74.9 85.8 Lucentis buffer, 53.9 61.3 75.1 82.9 pH 4.8 PBS pH 7.2 58.2 71.9 84.4
[0374] As can be seen, there is a weak pH dependence. Notably, the T.sub.m2 and T.sub.m3 domain (presumably CH2, Pab) overlap in PBS, but not in other buffers.
Example 27. Affinity of Dual PDGP/VEGP Antagonist Proteins and Conjugates to Targets
[0375] Surface plasmon resonance (SPR) was used to characterize the binding kinetics of recombinant human PDGP-BB (PeproTech, 100-14B) to TAP-WT, TAP-347, TAP-443, TAP443-6A250K, and TAP443-3A250K dual PDGP/VEGP antagonist variants. Initially, an anti-human IgG antibody (GE Healthcare, BR-1008-39) was covalently amine coupled onto all four flow cells of a CM5 carboxymethylated dextran coated sensorchip to a density of about 10,000 resonance units (RUs) following the manufacturer's protocol. Each PDGP/VEGP variant was captured to a level of approximately 150 RUs. The running and sample buffer for the PDGP analysis was HBS-EP+300 mM NaCl (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.4, 300 mM NaCl, 3 mM ethylenediaminetetraacetic acid (EDTA), 0.05% (v/v) Tween-20). A 2-fold serial dilution series of PDGP-BB ranging in concentration from 1 nM to 0.125 nM was injected at a flow rate of 100 .mu.L/minute for a 110 second association with dissociations that varied from 300 to 2700 seconds. The surface was then regenerated with a 30 second pulse of 3M MgCh, a 30 second pulse of an ionic regeneration buffer (0.46M KSCN, 1.83 M MgCl2, 0.92 M urea, and 1.83 M guanidine-HCl pH7.4, Andersson et al., Analytical Chemistry, 1999) and then equilibrated with a 30 second pulse of HBS-EP+300 mM NaCl running buffer.
[0376] Similarly, SPR was used to determine the binding affinities of recombinant human VEGP121 (PeproTech, 100-20A) against the TAP-WT, TAP-347, and TAP-443 dual PDGP/VEGP antagonist variants. The running and sample buffer for the VEGP analysis was HBS-EP+ with a final concentration of 150 mM NaCl. A 2-fold dilution series of VEGP121 ranging in concentration from 100 nM to 12.5 nM was injected at a flow rate of 50 uL/minute for about a 50 second association with dissociations that varied from 300 to 3600 seconds. The surface was then regenerated with a 30 second pulse of 3M MgCh, a 30 second pulse of ionic regeneration buffer (0.46M KSCN, 1.83 M MgCl2, 0.92 M urea, and 1.83 M guanidine-HCl pH7.4, Andersson et al., Analytical Chemistry, 1999), and then equilibrated with a 30 second pulse of HBS-EP+150 mM NaCl running buffer.
[0377] All SPR assays were performed at 25.degree. C. with a data collection rate of 1 Hz using a Biacore T200 instrument (GE Healthcare). The resulting PDGP and VEGP sensorgrams were double referenced using both a control surface and buffer injections. The rate constants were determined by fitting the data to a 1:1 Langmuir model with Biacore T200 evaluation software v2.0 and the equation K.sub.D=ka/k.sub.a.
TABLE-US-00006 Biacore Affinity to PDGF-BB ka kd t1/2 Rmax KD Analyte Ligand (1/Ms) (1/2) (min) (Ru) Chi2/Rmax (pM)* PDGF-A* TAF-WT 7.97E+07 8.01E-05 144.28 15.16 0.16% 1.01 PDGF-B* TAF-WT 8.01E+07 9.19E-05 125.68 15.15 0.20% 1.15 PDGF-C* TAF-WT 8.65E+07 1.03E-04 111.94 15.32 0.15% 1.19 AVG +/- STDEV 1.1 .+-. 0.1 PDGF-A* TAF-347C 4.15E+07 8.41E-05 137.33 13.99 0.86% 2.03 PDGF-B* TAF-347C 5.82E+07 7.87E-05 146.79 13.08 1.05% 1.35 AVG +/- STDEV 1.7 .+-. 0.3 PDGF-A* TAF-443C 3.22E+07 4.96E-05 233.15 13.15 0.81% 1.54 PDGF-B* TAF-443C 5.62E+07 8.76E-05 131.89 12.19 0.96% 1.56 AVG +/- STDEV 1.55 .+-. 0.01 PDGF-A* R3643-6A 7.60E+07 9.46E-05 122.09 8.11 0.41% 1.25 (TAF443- 6A250K) PDGF-B* R3643-6A 5.62E+07 5.85E-05 197.55 8.33 0.30% 1.04 (TAF443- 6A250K) PDGF-C* R3643-6A 3.48E+07 5.13E-05 225.41 8.45 0.80% 1.47 (TAF443- 6A250K) AVG +/- STDEV 1.3 .+-. 0.2 PDGF-A* R3644-3A 5.76E+07 5.92E-05 195.04 8.15 0.31% 1.03 (TAF443- 3A250K) PDGF-B* R3644-3A 2.86E+07 4.96E-05 233.05 8.52 0.60% 1.73 (TAF443- 3A250K) PDGF-C* R3644-3A 4.71E+07 7.52E-05 153.56 8.15 0.49% 1.60 (TAP443- 3A250K) AVG +/- STDEV 1.5 .+-. 0.4 *A, B and C refer to separate runs or measurements concerning the same analyte PDGP-BB
TABLE-US-00007 Biacore Affinity to VEGP121 ka kd T1/2 Rmax KD Analyte* Ligand (1/Ms) (1/s) (min) (RU) Chi2/Rmax (pM) VEGF-A TAF-WT 1.14E+05 2.01E-05 573.89 29.4 0.17% 176.60 VEGF-B TAF-WT 6.85E+05 5.89E-05 196.00 13.90 0.16% 86.03 VEGF-C TAF-WT 1.40E+05 2.96E-05 390.68 27.36 0.17% 212.00 VEGF-D TAF-WT 1.55E+05 2.69E-05 429.78 24.92 0.23% 173.00 AVG/STD DEV 161.96 .+-. 53.46 VEGF-A TAF347 1.37E+05 3.83E-05 301.55 26.57 0.87% 280.30 VEGF-B TAF347 1.42E+05 2.59E-05 446.21 24.56 0.35% 182.40 AVG 231.35 .+-. 48.95 VEGF-A TAF443 1.46E+05 3.20E-05 361.01 25.87 0.75% 219.30 VEGF-B TAF443 1.35E+05 3.10E-05 372.18 25.47 0.31% 229.70 AVG 224.5 .+-. 5.2 *A, B, C, D, refer to different runs of the same analyte (VEGF121).
Example 28. Decapping of TAF443 Prior to Maleimide Conjugation
[0378] The TAF443 Cysteine residue is typically "capped" or oxidized by chemicals in the cell culture media and is not available for conjugation. In this regard, purified TAF443 (OG 1321) is subjected to a decapping (i.e. reducing) procedure to remove the cap and enable the free (i.e. those not involved in Cys-Cys disulfide bonds) cysteine residue to be conjugated to the maleimide functionality of a polymer. Decapping is done by mixing TAP protein with a 30.times. molar excess for 1 hour at 25.degree. C. of the reducing agent TCEP (3,3',3''-Phosphanetriyltripropanoic acid). The reduction reaction with TCEP is monitored by SDS-PAGE. Undenatured TAP runs as a single band at about 250 kDa (about 40 kDa of this weight is carbohydrate). When fully denatured the single 250 kDa band is converted into bands corresponding to the light and heavy chains. Following denaturation, the TAP protein was washed by UFdF using a Pellion XL Ultrafiltration Cassette with 20 mM Tris pH7.5, 150 mM NaCl, 0.5 mM TCEP buffer to remove the cap. The TCEP reagent was then removed in the same UFdF setup with 20 mM Tris pH7.5, 150 mM NaCl. Reduced TAP was allowed to refold using air (ambient Oxygen) which was again followed by SDS-PAGE as an assay.
[0379] A detailed procedure for decapping is as follows:
[0380] 500 mg of OG 1321 was thawed from -80.degree. C. at 4.degree. C. overnight, and warmed up in the 25.degree. C. water bath before mixing with TCEP at 30.times. molar excess. The reaction was incubated in the 25.degree. C. water bath for 1 hour. Samples were taken out at 15, 30, and 60 minutes to run on SDS-PAGE in order to evaluate the reduction completeness. A UFdF cassette with 10 kD MWCO was used to carry out buffer exchange. First buffer exchange step was done with 20 mM Tris pH7.5, 100 mM NaCl, 0.5 mM TCEP for -100.times. to thoroughly remove the cap. A second buffer exchange step was done with 20 mM Tris pH7.5, 100 mM NaCl for -1000.times. for TCEP remove prior to air refolding. The final TCEP concentration in the sample was -0.5 .mu.M. Samples were taken out from both buffer exchange steps for both SDS-PAGE and SEC analyses to evaluate the protein reoxidation status and protein aggregation. After the second buffer exchange step, the OG 1321 was concentrated to -2 mg/ml, 0.22 .mu.m filtered, and allowed to re-oxidize with air at 4.degree. C. Samples were taken out for SDS-PAGE and SEC analyses at different time points to evaluate the re-oxidation status. Re-oxidized OG 1321 was 0.22 .mu.m filtered and further concentrated. Continued to concentrate the sample with VIVACELL 100 30k MWCO spin concentrators to 4-6 mg/ml and sterile filtered the sample. Quantified by OD280.
Example 29. Conjugation of TAF443 to Biopolymer
[0381] TAF443 which is also called OG 1321 was conjugated to polymer OG 1802 (see below) after decapping using a 15.times. excess of polymer in pH 7.5 Tris buffer to produce OG 1448, shown in FIG. 26, showing the chemical structure of OG 1448 which is TAF443 conjugated to biopolymer OG 1802. TAF443 is on the extreme right hand of the molecule shown in the figure, conjugated via the cysteine 443 residue to the 5 member ring. Conjugation was monitored by SDS-PAGE and driven to near completion. Conjugate was purified via anion exchange chromatography and buffer exchanged into the formulation buffer by UF/DF.
[0382] In general, there are three steps involved in the synthesis of OG 1448 from components OG 1802 and OG 1321. Step A: OG 1321 much be reduced or decapped to free up the sulfhydryl groups at cysteine position 443. Although the cysteine position at 443 of the heavy chain of TAP is not believed to be involved in cysteine-cysteine disulfide pairing, this cysteine is typically capped by components of the media and absent reduction is not available to react with maleimide. Step B: reduced TAP is then conjugated to OG1802. Step C: conjugated TAP (OG 1448) is then separated from unconjugated TAP and polymer via chromatography.
[0383] These three general steps are broken down into seven smaller steps in the following table:
TABLE-US-00008 General Step Description IPC Assays Target Range A Step 1: To reduce OG1321 using tris Non-reducing >95% reduction (2-carboxyethyl) phosphine (TCEP). 30x SDS-PAGE molar of TCEP at 25.degree. C. for 1 hour. Step 2: To remove TCEP reducing agent Non-reducing Band shift upon and cap groups using UF/DF. First, wash SDS-PAGE. removal of the with 0.5 mM TCEP in Tris pH 7.5 for a reducing agent. 100-fold volume exchange factor; followed by a 2.sup.nd wash with Tris buffer pH 7.5 for 1,000 fold volume exchange factor to remove the TCEP, targeting final TCEP level lower than 0.5 .mu.M. Step 3: To refold protein to ensure the Non-reducing Band upshift native disulfide pairs are fully oxidized SDS-PAGE upon oxidation of while the internal cysteine residues remain the native reduced. disulfide pairs. UV/Vis for Final protein protein concentration at 6-8 mg/ml. B Step 4: To conjugate OG1321 protein to Non-reducing <20% full length OG1802 biopolymer. Conjugate by SDS-PAGE band remains. mixing the oxidized OG1321 with Analytical AE- <20% unreacted OG1802. The process requires 15x molar HPLC protein at OD 280 of biopolymer to decapped protein and nm. constant mixing. Low temperature at 2-8.degree. C. for 20 hours and overlay the reaction with nitrogen gas to minimize oxidation. C Step 5: To separate OG1448 conjugate Analytical AE- <5% unreacted from the unreacted OG1321 protein, HPLC for protein at OD 180 unreacted OG1802 biopolymer, protein unreacted nm: aggregates and other process contaminants. polymer and <15% unreacted Purify OG1448 using MacroCap Q (AEX). unreacted protein polymer at OD The chromatography is performed at pH 220 nm. 7.5 in 20 mM Tris buffer and eluted using a Non-reducing <5% unreacted NaCl gradient. A pool is made by SDS PAGE protein combining fractions. Step 6: To concentrate the OG1448 and to UV/Vis for OG1448 at 50 exchange the chromatography buffers for protein mg/ml the formulation buffers. The pooled concentration fractions from the previous step are diafiltered and then concentrated by UF/DF to achieve the target OG1448. Step 7: To remove bioburden from the UV/Vis for OG1448 at 50 final product and to dispense into storage protein mg/ml containers. The UF/DF final pool is 0.2 concentration .mu.m filtered into sterile comakers, and pH pH Conductivity pH 7.2-7.5 and conductivity of the final filtrate is established. The drug substance is stored at -20.degree. C.
Example 30. Purification of OG 1448 Via Anion Exchange (Macrocap Q)
[0384] After conjugation of TAF443 to OG 1802 as described above, OG 1448 was purified as follows: After conjugation of TAF443 to OG 1802 as described above, OG 1448 was purified as follows: 2.times.400 ml of Macrocap Q columns were packed according to -3:1 ratio of resin:conjugate. The columns were flushed with 5M of NaCl and equilibrated with 20 mM Tris pH7.5, 20 mM NaCl (equilibration buffer) by syphoning. The conjugation reaction mixture was diluted with 20 mM Tris pH7 0.5 and loaded on the columns. The columns were then chased with the equilibration buffer, and washed with 20 mM Tris pH7.5, 50 mM NaCl (Wash 1) and then 20 mM Tris pH7.5, 100 mM NaCl (Wash 2). Elution was done with 20 mM Tris pH7.5, with step gradient of 150 mM, 200 mM, 220 mM, 250 mM, 300 mM, and 500 mM NaCl. All the column flow-through, washes, and elution were collected in clean bottles for SDS-PAGE and AEX analyses. Elution fractions containing the conjugate were pooled and concentrated using Pellicon XL TFF cassette with 30 kD MWCO and PES membrane. The concentrated pool was then buffer exchanged against 1.times.PBS pH7.4 buffer for -100.times. using the same TFF cassette and transferred to the VIVACELL 100 spin concentrators to further concentrate until the targeted concentration (-30 mg/ml) was achieved. The final conjugate was filtered through a 0.2 .mu.m PES syringe filter for lot release.
Example 31. Reduction of Bacterial Endotoxin
[0385] To reduce levels of endotoxin in the final protein (OG 1321) or conjugate (OG 1448), purification procedures may be employed for either protein or conjugate which utilize cation exchanges in place of anion exchanges. For example, in the above procedure for purifying OG 1321, the anion exchange TMAE resin is employed. In place of TMAE resin, the cation exchange resin CEX may be used. However, in order to use CEX residue the pH of the solution containing the protein in question must be reduced to below the protein's pl. For OG 1321, the pH of the protein solution after the protein A column, is reduced to pH 3.5. The OG 1321 is bound to the Porns XS column at pH5. Then, Porox XS (CEX) can be used to bind and elute the OG1321.
Example 32. Route 1 Synthesis of OG 1802
[0386] A first route for the synthesis of OG 1802 is as follows. First, TFA/amine salt initiator (Compound L) having the structure shown in FIG. 27 was synthesized as follows.
[0387] First, Compound K, having the structure shown in FIG. 28 was synthesized as follows. Into a 200 mL round bottom flask under nitrogen was placed Compound J (OG 1563) (1.9 g, 2.67 mmol, 3.3 equiv)
##STR00012##
and Compound E (0.525 g, 0.81 mmol, 1.0 equiv) (see FIG. 38) followed by dimethylformamide (10 mL) then diisopropylethylamine (2.5 mL, 14.6 mmol, 18 equiv). The flask was cooled to 0.degree. C. using an ice bath. To this was added propylphosphonic anhydride solution (50 wt. % in ethyl acetate, 2.5 mL, 4.04 mmol, 5 equiv) over -6 minutes.
[0388] The reaction was warmed to room temperature and stirred for 15 minutes. The reaction was quenched by adding water (20 mL), saturated aqueous sodium bicarbonate (20 mL) and ethyl acetate (100 mL). The organic layer was separated and the aqueous layer extracted with ethyl acetate (75 mL). The combined organic layers were washed with saturated aqueous sodium bicarbonate (30 mL), 0.5 M aqueous citric acid (40 mL), water (25 mL), and saturated aqueous sodium chloride (40 mL), then dried (sodium sulfate), filtered and concentrated under vacuum. The residue which was used without further purification resulted in 2.0 g (0.80 mmol, 99%) of Compound K.
[0389] 1H NMR (400 MHz DMSO-d6): D D=1.36 (s, 9H, OCCH3), 1.90 (s, 54H, CC(CH3)2Br), 2.31 (t, J=7.2 Hz, 6H, CCH2CH2NH), 2.98 (d, J=5.6 Hz, 6H, CCH2NH), 3.04 (q, J=6.0 Hz, 2H, OCH2CH2NH), 3.18 (s, 2H, OCH2C), 3.3-3.37 (m, 8H, CH2), 3.47-3.55 (m, 12H, CH2), 3.58 (s, 6H, OCH2C), 3.87 (s, 6H, O.dbd.CCH20), 4.27 (s, 18H, CCH2OC=0), 6.74 (br t, 1H, CH2NHC=0), 7.69 (t, J=6.8 Hz, 3H, CH2NHC=0), 7.84 (t, J=6.0 Hz, 3H, CH2NHC=0). LC-MS (ES, m/z): [(M+2H-boc)/2]+ Calcd for (C84H136Br9N7033+2H-Boc)/2=1196.6; Found 1196.6.
[0390] Next Compound L (FIG. 27) was synthesized as follows: into a 100 mL round bottom under nitrogen was added Compound K (2.0 g, 0.8 mmol), dichloromethane (10 mL) followed by trifluoroacetic acid (5 mL). The reaction was stirred at room temperature for 30 minutes.
[0391] The reaction was concentrated under a vacuum. The reaction was diluted using dichloromethane (10 mL) and concentrated under a vacuum. The residue was dissolved using acetonitrile (10 mL), filtered through a syringe filter (Acrodisc CR25, PN 4225T) and loaded onto a preparatory HPLC column and eluted with 60% acetonitrile in water (with 0.1% trifluoroacetic acid) up to 98% acetonitrile (with 0.1% trifluoroacetic acid). The tubes containing product were pooled, concentrated under vacuum, frozen and placed on a lyophilizer. This resulted in 990 mgs (0.4 mmol, 50% over 2 steps) Compound L as a white powder.
[0392] 1H NMR (400 MHz DMSO-d6): D D=1.90 (s, 54H, CC(CH3)2Br), 2.31 (t, J=7.2 Hz, 6H, CCH2CH2NH), 2.97-3.0 (m, 8H, CCH2NH and OCH2CH2NH), 3.17 (s, 2H, OCH2C), 3.3 (q, 6H, CH2CH2NHC=0), 3.4-3.59 (m, 20H, CH2), 3.87 (s, 6H, 0=CCH20), 4.27 (s, 18H, CCH20C=0), 7.69-7.84 (m, 9H, both CH2NHC=0 and NH3+). LC-MS (ES, m/z): [(M+2H)/2]+ Calcd for (C84H136Br9N7033+2H)/2=1196.6; Found 1197.4.
[0393] Next, compound L was used as an initiator to synthesize MPC polymer. Initiator is typically prepared as a stock solution in DMF of about 100 mg/mL. The initiator and the ligand (2,2'-bipyridyl) were introduced into a Schlenk tube. The resultant solution was cooled to -78.degree. C. using a dry ice/acetone mixture, and was degassed under vacuum for 10 min. The tube was refilled under Argon and the catalyst (CuBr unless otherwise indicated), kept under Argon, was introduced into the Schlenck tube (the Molar ratio of atom bromine on the initiator/catalyst (CuBr)/ligand was kept at 1/1/2). The solution became dark brown immediately. The Schlenk tube was sealed and immediately purged by applying a short cycle vacuum/Argon. A solution of HEMA-PC was prepared by mixing a defined quantity of monomer, prepared in a glovebox kept under nitrogen, with 200 proof degassed ethanol. The monomer solution was added drop wise into the Schlenk tube (via cannula) (and homogenized by light stirring), The temperature was maintained at -78.degree. C. A thorough vacuum was applied to the reaction mixture for at least 10 to 15 min. until bubbling from the solution ceased. The tube was then refilled with Argon and warmed to room temperature. The solution was stirred, and as the polymerization proceeded, the solution became viscous. After 3 to 8 hours or just left overnight, the reaction was quenched by direct exposure to air in order to oxidize Cu (I) to Cu (II), the mixture became blue-green in color, and was passed through a silica column in order to remove the copper catalyst. The collected solution was concentrated by rotary evaporation and the resulting mixture was either precipitated with tetrahydrofuran or dialyzed against water followed by freeze drying to yield a free-flowing white powder. The table below sets forth polymer data for polymer employing compound L as an initiator.
TABLE-US-00009 Theor. MW Polymer (kDa) ID No. Initiator Mn(kDa) Mp(kDa) PDI 500 130 L 490 530 1.1 750 150 L 645 750 1.1
[0394] Next, the maleimide Mal-PEG4-PFP ester was snapped on (as set forth in FIG. 29) to the 750 kDa polymer referred to above to provide OG 1802. Into a 20 mL vial was placed Polymer R3707 (750 kDa polymer made using L as initiator, 515 mg) and dissolved using ethanol (4.0 mL) after stirring for 40 minutes. To this was added a 1% solution of 4-methylmorpholine in acetonitrile (22 uL). In a separate vial was dissolved Mal-PEG4-PFP (1.97 mg) in acetonitrile (1.0 mL) and this solution was added to the polymer solution over -2 minute at room temperature and the resulting solution was stirred for overnight. The reaction was diluted with 0.1% aqueous trifluoroacetic acid (2 mL) (pH -5) followed by water (-12 mL), filtered through a syringe filter (Acrodisc Supor, PN 4612) and placed evenly into 3 Amicon centrifuge membrane dialysis tubes (30,000 mwco). The tubes were diluted and mixed with water (-5 mL each), placed into centrifuge (rpm 3200) for 25 minutes. The filtrate is removed for analysis while the retentate is diluted and mixed with water (-10 mL/tube). The centrifuge procedure repeated 5 more times, after which the retentate is removed and placed into a vial. The Amicon membrane tubes were rinsed with water (2.times.-2 mL each tube) and this combined with the retentate. The retentate solution was filtered through a syringe filter (Acrodisc Supor, PN 4612), frozen and placed on a lyophilizer. This resulted in 485 mgs as a white powder.
Example 33. Biacore Binding Studies of TAP (OG 1448 and OG 1321)
[0395] The binding affinity of OG 1448 (and OG 1321) to its intended targets was evaluated via Biacore assay. Binding studies were performed at 25.degree. C. and 37.degree. C. using BioRad Protean XPR36 and Biacore 2000 optical biosensors equipped with GLM (Protean) and CM4 (Biacore) sensor chips and equilibrated with running buffer (10 mM HEPES, 150 mM NaCl, 0.005% Tween-20, 0.2 mg/ml BSA). OG 1448, OG 1321, bevacizumab, aflibercept and anti-PDGF were immobilized to the sensor surface via amine-coupling.
[0396] Binding of the coupled proteins to the ligands was determined by standard methodology. For example, rhVEGFA-165 was tested for binding in a three-fold dilution series starting at 52 nM. rhVEGFA-165 was injected across the surface for five minutes and then the dissociation phase was monitored for >1000 seconds as the surfaces were washed with buffer. The rhVEGFA-165/001448 complex appeared quite stable, as indicated by the apparently flat response during the wash phase (>300 seconds) (data not shown). The dissociation phase for the 52 nM rhVEGFA-165 was monitored for more than 2 hours. No decrease in the binding response over time was observed.
[0397] Similarly, rhPDGF-BB was tested for binding in a three-fold series starting at 11.4 nM. For the rhPDGF-BB/OG 1448 interactions, the rate constants were too fast to be reported with confidence because of mass transport effects. The following K.sub.D constants were observed:
TABLE-US-00010 K.sub.D (pM) OG1321 OG1448 Bevacizumab Aflibercept Anti-PDGF rhVEGFA-165 9.8 .+-. 0.1 5.1 .+-. 0.1 9.6 .+-. 0.8 1.56 .+-. 0.2 (25.degree. C.) rhPDGF-BB 14 .+-. 3 17 .+-. 2 107 .+-. 3 (25.degree. C.)
Example 34. TAP--a Competitive Inhibitor of rhVEGFA-165 Binding to rhVEGFR
[0398] As a measure of its potential potency on anti-VEOP activity, binding activity of TAP (001448 and 001321) to VEOPA-165 was evaluated in a competitive binding assay where TAP, at different concentrations, was competing with immobilized rhVEOPR for binding of rhVEOP. rhVEOPA-165 bound by the immobilized VEOPR was determined by ELISA (data not shown).
[0399] Human VEOPR 1/Pc was coated onto the bottom of 96-well ELISA plates at 1.0 .mu.g/mL. Various concentrations of TAP (001448 and 001321), ranging from 0.39 to 200 nM, were incubated with 0.1 nM of biotinylated VEOPA-165 for 30 min before adding to the ELISA plates. Biotinylated-rhVEOPA-165 bound to VEOPR 1 was detected by streptavidin-HRP and followed by development with HRP substrates. Ranibizumab (Lucentis) and bevacizumab (Avastin) were similarly tested for competitive binding inhibition of VEOPA-165 to VEOPR 1.
[0400] 001321, 001448, ranibizumab and bevacizumab showed similar 1C.sub.S0S in inhibiting the binding of VEOP-165 to rhVEOPR suggesting similar potential potency in anti-VEOP activity. These results suggest that TAP (both 001448 and 001321) can be as potent as the approved agents ranibizumab and bevacizumab, hence, suitable for treating neovascular (i.e., wet) AMD.
TABLE-US-00011 IC.sub.50(nM) OG1321 OG1448 Ranibizumab Bevacizumab Competitive 12.5 .+-. 1.2* 8.5 .+-. 1.1* 12.5 .+-. 1.2* 10.7 .+-. 0.9* binding to rhVEGFA-165 (vs VEGFR) *Mean and SD of at least three trials.
Example 35. 001448--a Competitive Inhibitor of rhVEOPA-165 Binding to rhVEOPR in the Presence of rhPDOP-BB
[0401] To evaluate whether 001448 can bind rhVEOPA-165 in the presence of rhPDOP-BB, i.e., whether rhPDOP-BB binding to the receptor decoy of TAP inhibits the ability of TAP to bind to rhVEGPA-165, a similar binding study to Example 27 was conducted but in the presence of various concentrations of rhPDGP-BB.
[0402] Human VEGPR 1/Pc was coated onto the bottom of 96 well ELISA plates at 1.0 .mu.g/mL. Various concentrations of OG 1448 were incubated with 0.1 nM of rhVEGPA-165 plus rhPDGP-BB at 0.4, 1.2 and 2.0 nM, respectively, for 30 minutes before adding to the ELISA plates. rhVEGPA-165 binding to rhVEGPR 1 was detected by biotinylated anti-VEGPA antibody, 0.4 .mu.g/mL, followed with streptavidin HRP and HRP substrate. OG 1448 was found to have an IC50 (nM) of 10.1. This is quite comparable to the IC50 observed without rhPDGP-BB from example 28. The value for OG 1321 was not determined in this assay but is expected to be similar to OG 1448.
Example 36. TAP--a Competitive Inhibitor of rhPDGP-BB Binding to rhPDGPR
[0403] As a measure of its potential potency of anti-PDGP activity, the binding activity of TAP (OG1448 and 001321) to rhPDGP-BB was evaluated in a competitive binding assay where TAP, at different concentrations, was competing with immobilized PDGPR for binding of rhPDGPBB. rhPDGP-BB bound to immobilized PDGPR was determined by ELISA assay.
[0404] Human PDGPR/Fc was coated onto the bottom of 96-well ELISA plates at 0.4 .mu.g/mL. Various concentrations of OG 1448 and OG 1321, ranging from 1 pM to 20 nM, were incubated with 0.2 nM of rhPDGP-BB for 30 minutes before adding to the ELISA plates. rhPDGP-BB bound to rhPDGPR was detected by biotinylated anti-PDGPBB antibody, 0.4 .mu.g/mL, followed with streptavidin-HRP and HRP substrate.
[0405] OG 1448, OG 1321 and a reference anti-PDGP antibody showed similar IC50s in inhibiting rhPDGPBB binding to PDGPR, as shown in the following table, suggesting highly potent anti-PDGP activity.
TABLE-US-00012 IC50 (pM) OG1321 OG1448 Anti-PDGFBB Competitive Binding 46 .+-. 21* 54 .+-. 21 66 to rhPDGFBB (vs rhPDGFR) *Mean and SD of at least 3 trials.
Example 37. OG 1448--a Competitive Inhibitor of rhPDGP-BB Binding to rhPDGPR in the Presence of rhVEGPA-165
[0406] To evaluate whether OG 1448 can bind rhPDGF-BB in the presence of rhVEGFA-165, a similar competitive inhibition of binding study towards PDGF (as Example 29) was performed in the presence and absence of rhVEGFA-165.
[0407] Human PDGFRb/Fc was coated onto the bottom of 96-well ELISA plates at 0.4 .mu.g/mL. Various concentrations of OG 1448 were incubated with 0.2 nM of PDGFBB and with 0.2 nM of PDGFRb plus rhVEGFA-165 at 0.2 nM, 0.6 nM and 1.0 nM, respectively, for 30 minutes before adding to the ELISA plates. PDGF-BB bind to PDGFRb was detected by biotinylated anti-PEGFBB antibody, 0.4 .mu.g/mL, followed by streptavidin HRP and HRP substrate. The IC50 (pM) in the presence of rhVEGFA-165 (25) was comparable to the figure derived in Example 29. The figure for OG1321 in the presence of rhVEGFA-165 was not determined but is expected to be similar.
Example 38. Inhibition of VEGF-induced Proliferation of Primary Human Retinal Microvascular Endothelial Cells (HRMVEC)
[0408] Endothelial cell proliferation is a crucial step in angiogenesis and hence in the pathogenesis of neovascular AMD. The ability of OG 1448 to antagonize the proliferating action of VEGF on primary human retinal microvascular endothelial cells can be a measure of its bioactivity in treating neovascular AMD.
[0409] HRMVECs were stimulated with 1.3 nM of rhVEGF165-A for 3 days in the presence of various concentrations of TAP (OG 1448 and OG 1321) and reference drugs. Cell proliferation was measured by WST-1 cell proliferation detection reagent. Results are shown in the table below:
TABLE-US-00013 IC50 (nM) OG1321 OG1448 Ranibizumab Bevacizumab Aflibercept Inhibition of 0.43 .+-. 0.05* 0.49 .+-. 0.05* 0.98 .+-. 1.21* 0.81 .+-. 0.32* 0.55 .+-. 0.08* VEGF induced proliferation of HRMVECs *Mean and SD of at least 3 trials.
[0410] OG 1448 and OG 1321 demonstrated an IC50 in this assay comparable to other approved anti-VEGF therapies. These data show that TAP (both OG1448 and 001321) has at least comparable potency to inhibit VEGF-mediated retinal microvascular endothelial cell proliferation activity as ranibizumab, bevacizumab and aflibercept.
Example 39. Inhibition of PDGF-Induced Proliferation of Primary Human Brain Vascular Pericytes (HBVP)
[0411] Pericyte migration and proliferation are crucial events in angiogenesis and hence play important roles in the pathogenesis of neovascular AMD. The ability of TAP (OG 1448 and OG 1321) to antagonize the proliferating action of PDGF on human brain pericytes can be a measure of its effectiveness in treating neovascular AMD.
[0412] HBVPs were stimulated with 2.0 nM of PDGFBB for 3 days in the presence of various concentrations of TAP (OG 1449 and OG 1321) and a reference anti-PDGF-BB antibody (R&D Systems, Catalog # AB-220-NA). Cell proliferation was measured by WST-1 cell proliferation detection reagent.
TABLE-US-00014 IC50 (nM) OG1321 OG1448 Anti-PDGF Inhibition of 5.0 .+-. 2.0* 2.9 .+-. 1.4 5.4 PDGF induced proliferation of HPVPs *Mean and SD of at least 3 trials.
[0413] From the various experiments above comparing OG 1321 (TAF443) to OG 1448 (TAF443 polymer conjugate), it can be seen that conjugation to the HEMA-PC biopolymer does not negatively impact protein activity.
[0414] OG 1448 and OG 1321 show a comparable IC50 to the anti-PDGF antibody.
Example 40. Inhibition of Sprouting in Co-Culture of Human Retinal Microvascular Endothelial Cells (HRMVEC) and Human Mesenchymal Pericytes (HMPs)
[0415] To mimic in vivo conditions where endothelial cells and pericytes coexist in blood vessels and proliferate and migrate together during angiogenesis, events crucial in neovascular AMD, a three dimensional co-culture of HRMVECs and HMPs was established with the goal of evaluating the ability of OG 1448 to inhibit angiogenesis in this complex model.
[0416] Vehicle, Avastin, an anti-PDGF-BB antibody (same as above), Avastin in combination with the anti-PDGF-BB antibody and OG1448 were added to the co-cultures on day 7. On day 14, immunohistochemical staining of CD31 (endothelial cells) and aSMA (pericytes) was used to quantify the lengths of sprouts emanating from established endothelial cell spheroids as compared across the experimental groups.
[0417] OG 1448 was more effective in inhibiting endothelial/pericyte sprouting in HRMVEC-HMP co-culture than Avastin alone or anti-PDGF alone at two different concentrations. Moreover, OG 1448 was also more effective in inhibiting sprouting then a combination of Avastin and the anti-PDGF-BB antibody. This demonstrates that OG 1448 is synergistic relative to Avastin and an anti-PDGF-BB antibody. The results are shown in the table below and in FIG. 40.
TABLE-US-00015 Mean Total Sprout Length S.D. Relative Drug (pix) (pix) Angiogenesis % S.D. % Vehicle 6999 1266 100 18 Avastin-5 nM 4700 722 67 10 Avastin-25 nM 3763 909 54 13 Anti-PDGF- 4924 884 70 13 5 nM Anti-PDGF- 4461 1051 64 15 25 nM Avastin + anti- 5197 948 74 14 PDGF-5 nM Avastin + anti- 4287 822 61 12 PDGF-25 nM OG1448-5 nM 3584 478 51 7 OG1448-25 nM 2933 360 42 5
Example 41. Efficacy of OG 1448 on Inhibition of Laser-Induced Choroidal Neovascularization in Cynomolgus Monkeys
[0418] The in vivo efficacy of OG 1448 was evaluated using the laser-induced choroidal neovascularization (CNV) model in cynomolgus monkeys, a well-recognized primate model of CNV. See, e.g., Nork T M, Dubielzig R R, Christian B J, et al. 2011. Prevention of experimental choroidal neovascularization and resolution of active lesions by VEGF trap in nonhuman primates. Arch Ophthalmol. 129: 1042-1052; Lloyd R L, Harris J, Wadhwa S, Chambers W. 2008. Food and Drug Administration approval process for ophthalmic drugs in the U.S. Curr Opin Ophthalmol. 19:190-194, both of which are hereby incorporated by reference. In this model, laser lesions are placed in the chorioretinal complex in the macula of the monkey eye with evidence of Bruch's membrane breakage. Choroidal neovascularization is developed in two to three weeks. At various time points, fluorescein angiography is used to evaluate the clinically relevant lesions (Grade IV) which show fluorescein leakage beyond the primary lesion. This CNV model has been used extensively for the study of CNV lesions and used as a benchmark for all currently approved treatment for neovascular AMD. In this model, all approved anti-VEGF agents for neovascular AMD are effective in inhibiting the leakage from the clinically relevant Grade IV lesions. The study was conducted at Covance, Madison, Wis.
[0419] In summary, a dose-related response to a single intravitreal injection of OG 1448 at 0.5 or 2.4 mg/eye (calculated based on protein content) was observed in the animals in which CNV lesions were allowed to develop for 14 days before treatment and evaluated at subsequent time points using fluorescein angiography focusing on the clinically relevant Grade IV lesions on the retina/choroid. At 0.5 mg/eye, the beneficial effect on the Grade IV lesions was noticeable (p=0.019; generalized estimating equation [GEE] model; 0.5 mg treatment Group 7 vs PBS injected placebo Group 5). At 2.4 mg/eye OG 1448, a dose (in molar equivalence) within the therapeutic dose of bevacizumab or aflibercept, was highly effective (75% reduction in Grade IV-CNV like lesions on Day 43 from Day 15 versus 27% reduction in the PBS-treated group) (p=0.0007; GEE model; 2.4 mg treatment Group 9 vs PBS injected placebo Group 5) in ameliorating the leakage of Grade IV-CNV lesions.
[0420] OG 1448 shows effectiveness in inhibiting the leakage from the clinically relevant Grade IV lesion in this benchmark CNV model.
[0421] The groups and study design are shown in the following table. The study included groups for tolerability (Groups 1 thorough 4) however for purposes of this patent application only the groups for pharmacological activity and a control group treated with phosphate buffered saline (PBS) injection are shown.
TABLE-US-00016 No. Dose Level Dose of mg/left mg/right Concen- Fe- eye/ eye/ mg/kg/ tration Group males Dose Route dose dose dose (mg/ml) 5 6 Intravitreous.sup.a 0 0 NA 0 6 6 Intravitreous.sup.a 0.24 0.24 NA 5.9 7 6 Intravitreous.sup.b 0.51 0.51 NA 10.2 9 6 Intravitreous.sup.b 2.40 2.40 NA 26.6 NA = not applicable .sup.aat days 1, 15, and 29 (a total of 3 doses): laser on day 8 of the dosing phase. .sup.bonce; laser treatment on 15 days prior to injection
[0422] Two treatment regimens were evaluated. In the prevention regimen, OG 1448 was given intravitreally three times bilaterally at 0.24 mg/eye/dose (dose content was based on protein content; Group 6) or PBS (Group 5) on days 1, 15 and 29 with laser treatment on day 8 of the dosing phase. Fluorescein angiograms on days 15, 21, 30, 37 and 43 of laser treatment (days 22, 28, 37, 44 and 50 of the dosing phase) were used for evaluation of the clinically relevant Grade IV lesions.
[0423] In the treatment regimen (Groups 7 [0.5 mg], 8 [0.5 mg] and 9 [2.4 mg], OG 1448 was administered intravitreally to both eyes of 6 animals at doses of 0.5 mg (Groups 7 and 9) or 2.4 mg/eye (Group 9) 15 days after laser induction when CNV lesions were established. Fluorescein angiograms obtained at Days 15, 21, 30, 37 and 43 of laser treatment were used for evaluation of the clinically relevant Grade IV lesions.
[0424] Using generalized estimating equation (Gee) models (Halekoh, U & Yan J (2006) The R Package geepack for Generalized Estimating Equations Journal of Statistical Software 15, 2, pp 1-11), a dose-related response to OG 1448 was observed in the intervention regimen. At 0.5 mg/eye, the effect was notable as shown by the difference in the percent change in Grade IV lesions as compared to the vehicle control (0.5 mg treatment Group 7 vs PBS injected placebo Group 5; p=0.019, GEE). With 2.4 mg/eye OG 1448 (a dose in molar equivalence within the therapeutic dose of bevacizumab or aflibercept) a 75% reduction in percent change in Grade IV lesions (2.4 mg treatment Group 9 vs PBS injected placebo Group 5; p=0.0007, GEE) was observed on day 43 as compared to a 27% reduction in CNV in the PBS control group. The data from the various experiments in the monkey CNV model are shown in FIG. 41.
[0425] OG 1448 shows dose dependent effectiveness in inhibiting the leakage from the clinically relevant Grade IV lesion in this CNV model. These results are consistent with the studies described above showing activity of OG 1448 against VEGF-mediated angiogenic activities.
Example 42. Tissue Distribution and Pharmacokinetics
[0426] A tissue distribution and pharmacokinetic study using 125I-OG 1448 was conducted using male New Zealand Red White Fl Cross pigmented rabbits. In summary, this study showed a vitreal half-life of 16.1 days for OG 1448 in rabbits, approximately three times that reported for aflibercept (4.5 days) and 5 times that of ranibizumab (2.9 days) (Bakri S J, Snyder M R, Reid J M et al. 2007. Pharmacokinetics of Intravitreal Ranibizumab [Lucentis].
[0427] Ophthalmology 114:2179-2182) with little plasma exposure (approximately 0.2% of that of vitreous exposure) and a plasma half-life of 6.5 days (aflibercept reported 6.5 days) (Struble C, Koehler-Stec E, Zimmer E, and Tu W. 2008. Pharmacokinetics and ocular tissue penetration of VEGF Trap after intravitreal injections in rabbits. EVER; Portorz, Slovenia).
[0428] The purpose of this study was to assess the ocular distribution and pharmacokinetics of non-radiolabeled test articles and radiolabeled test articles following an intravitreal or intravenous dose administration to male New Zealand Red White Fl rabbits. Treatment groups and the study design are shown in the table below:
TABLE-US-00017 Groups and Study Design (Covance study) # of Dose Test Dose Sample Group males Route Article (mg) Collected 1 14 IVT .sup.1251-OG1448 0.25/eye (OU) Blood, ocular tissues 2 2 IV .sup.1251-OG1448 0.25/animal Blood 3 6 IVT OG1448 0.25 (OD) Blood, whole eyes for histology 4 6 IVT OG1448 0.25 (OD) Blood, vitreous humor IVT: intravitreal; IV: intravenous; OU: Both eyes; OD: right eye
[0429] PK parameters were obtained based on radioanalysis. Clearance profiles from vitreous, retina and choroid were similar to one another. This pattern is consistent with other established CNV treatments such as ranibizumab or aflibercept. Set forth in the table below are pharmacokinetic parameters in different ocular tissues after single bilateral intravitreal injection of 0.25 mg .sup.125I-OG 1448.
TABLE-US-00018 Exposure as % of C.sub.MAX T.sub.1/2 AUC.sub.0-.infin. vitreous Matrix (NG Eq./G) (day) (Day*NG EQ./G) exposure Plasma 494 6.48 3,790 0.189 Aqueous humor 5,250 11.6 68,800 3.423 Choroid-RPE 4,170 32.8 134,000 6.667 Iris-ciliary body 12,100 42.6 235,000 11.692 Retina 13,500 30.4 309,000 15.373 Vitreous humor 112,000 16.1 2,010,000 100.00
[0430] The ocular tissue half-life of various VEGF inhibitors is compared with OG 1448 in the table below and in FIG. 42, which suggests that OG 1448 can stay above a pharmaceutically active minimal inhibitory concentration of 0.1 .mu.g/ml for greater than 90 days, as opposed to 30 days for Lucentis and 50 days for Eylea:
TABLE-US-00019 Ocular Tissue Elimination Half Life (Days) Vitreous Retina Choroid Pegaptinib.sup.1 3.5 -- -- Ranibizumab.sup.1 2.9 2.9 -- Aflibercept.sup.1 4.5 5.5 4.8 OG1448.sup.2 16.1 30.5 32.9 .sup.1Based on publicly available data from 28-day rabbit studies: Drolet D W, Nelson J, Tucker CE, et al. 2000. Pharmacokinetics and safety of an anti-vascular endothelial growth factor Aptamer (NX 1828) following injection into the vitreous humor of rhesus monkeys. Pharm Res. 17: 1503-1510; Gaudreault J, Fei D, Beyer J C et al. 2007. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 27: 859-870; Bakri (2007), supra; Struble 2008, supra. .sup.2Based on intravitreal injection of 250 .mu.g in the rabbit eye.
[0431] The study showed a vitreal half-life of 16.1 days for OG 1448 in rabbits, approximately three times the 4.5 day vitreal half-life reported for aflibercept and five times the vitreal half-life of ranibizumab (2.9 days) (Bakri 2007, supra) with low plasma exposure (approximately 0.2% of that of vitreous exposure); the plasma exposure is consistent to that of aflibercept (Sinapis C I, Routsias J G, Sinapis A l, et al. 2011. Pharmacokinetics of intravitreal bevacizumab [Avastin.RTM.] in rabbits. Clinical Ophthalmology 5:697-704). Similar to the reported data for ranibizumab and aflibercept, the vitreal, retinal and choroidal clearance profiles are similar to one another.
Example 43. Toxicology
[0432] Two pilot non-GLP single dose ocular and systemic tolerability studies on OG 1448 were conducted at Covance: (i) a single dose 57-day intravitreal or intravenous tolerability study in pigmented rabbits and (ii) a single dose tolerability study after intravitreal (58-day study) or intravenous (28-day study) administration in cynomolgus monkeys.
[0433] In brief, single dose intravitreal injection of 0.25 mg OG 1448/dose/eye in rabbits was initially well tolerated but was associated with persistent anterior (mild to moderate conjunctiva! hyperemia, mild to moderate aqueous flare and cells) and posterior segment (mild to severe white vitreous cells, mild to moderate vitreous haze and presence of vitreous floaters, and multifocal grey-white subretinal inflammatory foci) inflammation which developed approximately two weeks postdose (or later). This inflammatory response improved with immune-suppressive and anti-inflammatory therapy. The time of onset postdose and response to treatment are consistent with an immune-mediated response typical for intraocularly administered humanized biopharmaceuticals in animals.
[0434] In contrast, a single intravitreal dose at 0.24 or 1.4 mg 001448/dose/eye was well tolerated in cynomolgus monkeys with no adverse finding or evidence of immune reactions ophthalmologically, clinically, and histopathologically.
[0435] In the efficacy study (discussed above), intravitreal injections of 0.24 mg/eye/dose for three times at 14 days apart or a single injection of 0.5 mg/eye/dose were well tolerated with at least 40 days of follow-up as shown on ocular examinations. No immune-related reactions were noted in the eyes of treated animals.
[0436] These studies demonstrate that OG 1448 is well tolerated when administered intravitreally or intravenously at the doses evaluated.
Example 44. Single-Dose Tolerance in Cynomolgus Monkeys
[0437] The purpose of this part of the study was to evaluate tolerability of OG 1448 after intravitreous or intravenous administration in cynomolgus monkeys.
[0438] Ocular and systemic tolerability groups and study design are shown in the table below:
TABLE-US-00020 Group and Study Design No. Dose Level.sup.a,b Dose of .mu.g/left mg/right Concen- fe- Dose eye/ eye/ mg/Kg/ tration Group males Route dose dose dose (mg/ml) 1 3 IVT 0 0.236 NA 5.9 2 3 IVT 0 1.36 NA 27.2 3 2 IV NA NA 0.235 9.4 4 2 IV NA NA 1.41 9.4 IVT = intravitreal; IV = intravenous; NA = not applicable .sup.aThe right eye of animals in Groups 1 and 2 received the test article via intravitreous injection. Animals in Groups 3 and 4 received the test article via colus intravenous injection. .sup.bThe left eye of animals in Groups 1 and 2 animals received vehicle control only (phosphate buffered saline, pH 74).
[0439] Ocular examinations by board certified veterinary ophthalmologists were performed across all four groups predose and (i) for intravitreal groups: on days 3, 8, 15, 29, 43 and 57, and for intravenous groups: on days 3, 8, 15, and 29. Animals were followed with clinical observations and clinical pathology on days 3, 8, 15, 29, 43 and 57 when applicable. Anatomic pathology was also performed--macroscopic observation during necropsy for all animals, and microscopic evaluations for ocular tissues for groups 1 and 2 (day 57) and for a standard list of systemic organs for groups 3 and 4 (day 29).
[0440] There were no adverse or toxicologically meaningful findings in any group. There were no findings in clinical observations and body weight in any group. There were no OG 1448-related macroscopic or microscopic findings from anatomic pathology for any group (ocular tissues for intravitreally injected groups and standard list of organs/tissues for intravenously injected groups).
[0441] Ophthalmic findings for intravitreal administration groups were limited to injection-related events such as mild to moderate and transient presence of aqueous and/or vitreous cells and scars at the site of aqueous humor sampling.
Example 45. Synthesis of Polymer OG 1786
[0442] OG 1786 is the nine-arm initiator for polymer synthesis used as a precursor in the synthesis of OG 1802. Each arm is terminated with a 2-bromoisobutyrate which is capable of initiating polymerization under ATRP. OG1786 is a salt of trifluoro acetic acid (TFA) as shown in FIG. 30. OG 1786 is prepared as follows. First, OG 1550 is reacted with TFA (trifluoro acetic acid) to produce OG 1546 as depicted in FIG. 31.
[0443] In a 1 L round bottom flask equipped with a magnetic stir bar and an addition funnel was added OG 1550 (14.8 g), methyl tert-butyl ether (MTBE) (350 ml) and water (30 ml). The mixture was stirred to dissolve the OG 1550, then cooled in an ice bath. To this mixture was added a solution of trifluoroacetic acid (4.9 ml) in water (90 ml) dropwise over 90 minutes. After addition is complete the mixture was stirred an additional 15 minutes then removed from the ice bath and allowed to warm to room temperature. The mixture was stirred (after removal from the ice bath) for a further 4-5 hours, until tlc showed -5% starting material remaining, and the pH of the aqueous was between 3 and 4 (pH paper).
[0444] The mixture was partitioned. The MTBE layer was washed with water (30 ml). Combine aqueous layers then the aqueous extracted with MTBE (150 ml). This second MTBE phase was washed with water (30 ml). The combined aqueous layers were washed with a third portion of MTBE (100 ml). The third MBTE phase was washed with water (25 ml). The aqueous layers were again combined (-250 ml, pH -4, by pH paper).
[0445] The product was collected by lyophilization. 11.5 g white solid was obtained. This material is extremely hygroscopic, so best handled under nitrogen. The product was confirmed by LCMS.
[0446] The prepared OG 1546 was then reacted with OG 1563 to yield OG 1784 (as depicted in FIG. 32).
[0447] In a 250 ml flask under nitrogen equipped with a stir bar was added OG 1546 (hygroscopic, 9.0 g), followed by N,N-dimethylformamide (110 ml). The mixture was stirred at room temperature until all OG 1546 dissolved (about 15 minutes), then OG 1563 (29.9 g) was added, and the mixture stirred a further 3 minutes until the OG 1563 had also been dissolved. The resulting solution was cooled in an ice bath, and N,N-diisopropylethylamine (37.6 ml) was added over 3 minutes, followed by propylphosphonic anhydride (T3P), 50% in ethyl acetate (34.5 ml) dropwise over 5 minutes (T3P addition is exothermic). After T3P addition was complete, the flask was removed from the cooling bath and allowed to reach room temperature. Samples were then taken at 5 minute intervals for LCMS analysis. The reaction showed very light yellow/tan color.
[0448] After 20 minutes the reaction was cooled again in an ice bath and 5 ml water added. The mixture was then removed from the cooling bath and a further 50 ml water portion added, followed by 50 ml 0.5 M citric acid then isopropylacetate (300 ml). The mixture was partitioned. The aqueous phase (-300 ml) was extracted with additional isopropyl acetate (150 ml). The aqueous phase was AQ 1 for HPLC test. The combined organics were washed with aqueous citric acid (115 ml, 65 mM, which was the mixture of 15 ml of 0.5 M citric acid plus 100 ml water), and the aqueous phase was AQ2 (pH-3). The organic phase was washed with water/saturated sodium chloride (100 ml/25 ml), and the aqueous phase was AQ3 (pH-3). The organic phase was finally washed with saturated sodium chloride (100 ml), and the aqueous phase was AQ4. None of the AQ fractions contained any significant product (data not provided). The organic phase confirmed the product via LCMS. The product was dried over sodium sulfate (80 g), filtered and rinsed with isopropyl acetate (75 ml), and concentrated on a rotary evaporator to a tan oil (33.2 g). The crude was stored overnight under nitrogen.
[0449] The next day the crude was allowed to come to room temperature, then dissolved in acetonitrile/water (46 ml/12 ml) and filtered using an HPLC filter disk (Cole-Parmer PTFE 0.2 .mu.m, product number 02915-20). The filtrate was split into three equal portions and purified in three runs.
[0450] Loaded onto a RediSep Rf Gold C18 column (275 g, SN 69-2203-339, Lot #24126-611Y) equilibrated with 50% acetonitrile/water. The material was eluted at 100 ml/min using the following gradient (solvent A: water, solvent B: acetonitrile). All the relevant fractions were checked by HPLC. The fractions adjudged to be pure enough were pooled (from all three runs) and concentrated (bath temperature kept at about 20.degree. C.) on rotovap, then partitioned between dichloromethane (100 ml) and water (5 ml)/saturated sodium chloride (25 ml). The aqueous was extracted twice more with dichloromethane (2.times.30 ml). The combined organics were dried over sodium sulfate (35 g), filtered, rinsed with DCM (30 ml), and concentrated. The product and purity were confirmed by LCMS methods.
TABLE-US-00021 OG1784 lot R5172 R5228 OG1546 used 5.3 g 9.0 g OG1563 used 17.6 g 29.9 g Isolated yield 53% 58% Purity (a/a 210 nm) 99.3% 100.0%
[0451] Next OG 1405 was prepared from OG 1784 as depicted in FIG. 33. In a 500 ml round bottom flask equipped with a magnetic stir bar was added OG 1784 (20.9 g), followed by dichloromethane (50 ml) then trifluoroacetic acid (20 ml). The mixture was stirred at room temperature and HPLC analysis showed complete deprotection in 23 minutes. The mixture was concentrated on a rotary evaporator, redissolved in dichloromethane (25 ml) and re-concentrated, then redissolved in acetonitrile (25 ml) and re-concentrated. The product was confirmed by LCMS. The material from above (OG 1405, 34.5 g, assume 21.0 g as quantitative yield) was used as a crude oil in the next step. No purification is needed. Next, OG 1405 was reacted with OG 1402 to prepare OG 1785 as set forth in FIG. 34. In a 500 ml flask under nitrogen equipped with a stir bar was placed OG 1402 (5.5 g), followed by acetonitrile (70 ml), then N,N-diisopropylethylamine (26.3 ml) and T3P solution (see above) (7.9 ml). The solution was stirred at room temperature for 30 minutes, then cooled in an ice water bath and a solution of OG 1405 (crude oil from above, 34.5 g) in acetonitrile (70 ml) added. The mixture was warmed to room temperature. After 20 minutes the reaction was cooled in an ice water bath and quenched with water (5 ml). The mixture was then concentrated under vacuum using a rotary evaporator to half volume. Samples were taken for LCMS.
[0452] More water (50 ml), followed by 0.5 M citric acid (75 ml) and isopropyl acetate (175 ml) was added. The mixture was partitioned in 5 minutes. The aqueous was extracted with additional isopropyl acetate (50 mL). The combined organics were washed with aqueous citric acid (0.13 M, 30 ml, consist of 10 ml of 0.5 M citric acid and 20 ml water). The organics were then washed with the mixture of saturated sodium chloride (25 ml) and water (25 ml), then finally washed with the saturated sodium chloride (25 ml). They were then dried over sodium sulfate (124 g), filtered and rinsed with isopropyl acetate (30 ml) and concentrated under rotary evaporator to a tan oil (27.3 g). Samples were taken for LCMS analysis.
[0453] The oil was dissolved in acetonitrile/water (3: 1, 15 ml/5 ml), filtered through an HPLC filter disk (Cole-Parmer PTFE membrane 0.2 .mu.m, product number 02915-20) and split into three equal portions, each of which were individually purified as follows.
[0454] Portions were loaded onto Redi-Sep Gold C18 column (275 g, SN-69-2203-339, Lot 241234-611W) equilibrated at 50% solvent B (acetonitrile)/50% solvent A (water). The material was then purified by reverse phase HPLC with a solvent A: water/solvent B: acetonitrile gradient. Appropriate fractions were pooled and partitioned between dichloromethane (150 ml) and water (5 ml)/saturated sodium chloride (25 ml). The aqueous was extracted twice with dichloromethane (2.times.50 ml). Combined organics were dried over sodium sulfate (60 g), filtered and rinsed with dichloromethane (40 ml) and concentrated. Structure and purity were confirmed by various analytics including LCMS: OG 1785 was isolated as a foamy solid (R5329, 19.0 g, 83% yield, 95.1% purity (a/a 210 nm), stored under nitrogen at 4.degree. C.
[0455] Next, the tert-butyloxycarbonyl protecting group on OG 1785 was removed using trifluoroacetic acid (TFA) to produce OG 1786 as depicted in FIG. 35.
Example 46. Synthesis of Polymer 1801
[0456] Compound OG 1802 is conjugated to a sulfhydryl group of TAF443 to produce OG1448. Polymer OG1801 is made first from the initiator OG1786. OG1801 has an amine functionality, which is more stable (than maleimide) during polymer synthesis. To synthesize polymer OG 1801, a modified version of ATRP is used wherein the copper species (Cu(I)) is generated in situ by adding metallic copper to Cu (II). Starting materials and reagents needed in the reaction are calculated based on batch input of the monomer (HEMA-PC) OG47, as well as the targeted molecular weight (MW).
[0457] Weighed 50 g monomer OG47 in glove box and added 200 mL of degassed EtOH to dissolve the monomer at room temperature; sampled for monomer concentration test. Weighed Cu (II), Bpy, Cu(O) in a 500 mL flask; purged with Argon, while adding monomer solution to the flask; sealed the flask with stopper and vacuumed for 25 min until no bubbles. The reaction changed color gradually from light green to dark green, then to light brown; weighed -200 mg of initiator OG 1786 in glove box, and dissolved in -2000 uL of DMF under room temperature to make 100 mg/mL stock solution; Sampled for initiator concentration and purity test; Added the initiator solution to the flask under Argon. The reaction solution became dark brown and started thickening over time; Sealed the system and let the reaction occur over 2 days.
[0458] OG1801 was then prepared for addition of the maleimide and catalyst (copper) was removed as follows: A prepacked RediSep.RTM. Rf normal phase silica column is used to remove the catalyst. The size of the column is chosen based on the copper amount in the reaction mixture. For instance, a 330 g column (Cat. #69-2203-330, Column size 330 g, CV=443 mL) was used for a 50 g batch of OG 1801. Teflon tubing is used for all the connection as EtOH is the elute solvent.
[0459] After copper removal, transferred all the fractions to a round bottom flask in batches, and evaporated the EtOH by rotary evaporator at 45-50.degree. C. at reduced pressure to dryness. In this step, EtOH volume collected from condensation was monitored to make sure EtOH removal was >90%. The polymer was dissolved in 250 mL of WFI and filtered using a 0.2 um filter. It resulted in a clear to light yellow polymer solution at -150 mg/mL. The solution could be stored at 2-8.degree. C. up to 3 month before use.
Example 47. Synthesis of Polymer OG 1802
[0460] Starting materials and reagents needed in the reaction is calculated based on batch input of OG 1801. The linker is 3-maleimidopropionic acid, NHS ester. Added 30 ml of 0.5 M sodium phosphate (in WFI, pH8) to 50 g polymer solution (-150 mg/mL). Let stir for 1 min; pH was 8.0 by pH paper. Weighed 204.8 mg of linker and dissolved in DMF 4.1 mL to make 50 mg/mL stock sln; Added linker solution dropwise 815 uL per minute to the polymer sln with strong stirring. Took 5 min to added 4095 uL of linker solution. Reacted at room temperature for 30 min. Quenched reaction with 20 mL of 5% acetic acid to achieve a final pH of 5. Filtered the solution using 1 L vacuum filter (0.2 um).
[0461] OG 1802 is then purified as follows: Milipore cross flow cassettes was used for polymer purification in aqueous system. Started with concentrating the polymer solution to 250 mL (-200 mg/mL). Added the fresh WFI from reservoir, and adjusted the flow rate of the fresh WFI feed to the same as the permeate (-2 mL/min). The UF/DF was set up at 2-8.degree. C. overnight. Typically 2.5 L of WFI was used (10.times. volume ratio to the polymer solution). A sample of retente was collected for purity test. The targeted purity was >98%. Filtered the polymer solution by 0.2 .mu.M 1 L filter bottle. The polymer solution could be stored at 2-8.degree. C. for up to 3 month before conjugation.
Example 48. Formulations of OG 1448; Injectability
[0462] 27 0.2 mg/ml and 44.5 mg/ml solutions of OG 1448 were prepared using 1.7 mM KH.sub.2PO.sub.4; 5 mM Na.sub.2HPO.sub.4; 150 mM NaCl in sterile water for injection. The OG 1448 conjugate was concentrated by a Millipore Pellicon XL TFF cartridge (catalog # PXB030A50, EMD Millipore), 30 kD MWCO or VIVACELL 100 spin concentrator (catalog # VC1022, Sartorius), 30 kD MWCO, depending on the volume. The 27.2 mg/ml solution of TAP was injected intravitreally into the monkeys for the efficacy experiments described above through a 30 gauge (G) V2 inch needle. Excessive pressure was not required to push the OG 1448 through the needle. The 44.5 mg/ml solution was tested for injectability in the laboratory and was also capable of being pushed through the needle without excessive pressure by a female operator.
Example 49. Storage Stability
[0463] An ongoing stability study was conducted using OG 1448 reference lot R5606 at 44.5 mg/ml in PBS at pH 7.4 (as described above). Three temperatures were chosen for the study: room temperature (RT), 4.degree. C. and -20.degree. C. Sampling frequency is at 0, 14, 28, 91, 181 and 362 days. Samples were evaluated by SDS-PAGE and analytical AE-HPLC for unreacted and sequestered protein, and potential aggregates. It was observed (data not shown) that OG 1448 demonstrates less than 5% protein impurity by AE-HPLC at all three temperatures up to six months, which is similar to the level at time 0. This study is ongoing.
Example 50. Alternative Phosphorylcholine Polymers
[0464] A HEA-PC polymer was synthesized as described below. HEA-PC (2-(acryloyloxy)ethyl-2-(trimethylammonium)ethyl phosphate), which is an acrylate as opposed to the methacrylate HEMA-PC described above, has the following structure:
##STR00013##
[0465] HEA-PC was polymerized to the initiator shown in Example 23 as compound L.
TABLE-US-00022 Reactant Name Amount MW Initiator Compound L (see above) 1.65 mg 2505.5 Monomer HEA-PC 0.461 g 281.24 Catalyst Cu (I) Bromide 1.2 mg 143.45 Ligand Tris [2- 2.73 mg 230.39 (dimethylamino)ethyl]amine (Me6TREN) Solvent A N,N-Dimethylformamide 21.85 .mu.l 73.09 (DMF) Solvent B Water 0.7 ml 18.02 Solvent C Methanol 0.7 ml 32.04
[0466] Prepared a stock solution of initiator at 200 mg/mL by dissolving 2.2 mg of initiator in 11 .mu.l of dry DMF and a 200 mg/ml solution of ligand by dissolving 4.6 mg of Me6TREN in 23 .mu.L of dry DMF. Dispense 8.25 .mu.l of the stock solution of initiator and 13.6 .mu.l of the ligand into a tube. Degas at -78.degree. C. for 5 mn then refill with Argon and add 1.2 mg of CuBr. Degas and refill with Argon. Add a stock solution of HEA-PC in methanol (weigh out 0.461 g of HEA-PC and dissolve it in 0.5 mL of methanol) to the solution inside the reactor at -78.degree. C. Rinse the vial with 200 .mu.l of methanol and add it inside the reactor at -78.degree. C. and then 0.5 mL of distilled water then another 200 .mu.l of water. Degas thoroughly until no bubbling is seen and all heterogeneity disappears (solid particulates dissolve or disappear). Refill with 4 psi of Argon and let the reaction to proceed at RT for an hour. The reaction was already viscous. The reaction was allowed to proceed for about one hour. A solution of bipyrindine in methanol (5 mg in 0.5 uL) was added. Another 2-3 ml of methanol was added and the catalyst was allowed to oxidize overnight at 4.degree. C. Conversion determined by 1H NMR was estimated to be 94%.
[0467] The next day the polymer was dialyzed and subjected SEC/MALS analysis using Shodex SB806M_HQ column (7.8.times.300 mm) in 1.times.PBS pH 7.4 at 1 ml/min, giving a PDI of 1.157, Mn of 723.5 kDa, Mp of 820.4 kDa and Mw of 837.2 kDa (before dialysis PDI is 1.12, Mn=695 kDa, Mp=778 kDa). Next a maleimide functionality was added to the polymer so that it could be conjugate to a protein, including TAF443.
[0468] Next, the maleimide Mal-PEG4-PFP (see Example 23 above) ester was snapped on to the HEA-PC polymer as shown in Example 23. The resulting maleimide functionalized HEA-PC polymer can then be conjugated to sulfhydryl groups as discussed herein for HEMA-PC polymers.
[0469] An acrylamide PC polymer was also made using the monomer 2-(acrylamyl)ethyl-2-(trimethylammonium)ethyl phosphate (Am-PC), having the following structure:
##STR00014##
[0470] The Am-PC was used for polymerization employing a 3 arm initiator (a TFA salt) having the structure:
##STR00015##
[0471] The synthesis of the Am-PC polymer was conducted as follows:
TABLE-US-00023 Reactant Name/Identity Amount MW Initiator 3-arm initiator (see above) 2.2 mg 885.35 Monomer Am-PC 0.5 g 280.26 Catalyst (I) Copper (I) Bromide 1 mg 143.45 Catalyst (II) Copper (II) Bromide 0.2 mg 223.35 Ligand Tris[2- 3.94 mg 230.39 (dimethylamino)ethyl]amine (Me6TREN) Solvent A N,N-Dimethylformamide 31.7 .mu.l 73.09 (DMF) Solvent B Water 1 ml 18.02 Solvent C Methanol 1 ml 32.04
[0472] A stock solution of ligand at 200 mg/mL was prepared by dissolving 9 mg of Me6TREN in 45 uL of dry DMF. Add 19.7 uL of the stock solution to a reaction vessel. Prepare a stock solution of initiator at 200 mg/mL by dissolving 6.5 mg of material in 32.5 uL of DMF. Add 11 uL of the initiator stock solution to the ligand from above. Degas for 5 mn. Add 1 mg of CuBr. Prepared a stock solution of CuBr.sub.2 at 200 mg/mL by dissolving 4 mg CuBr.sub.2 in 20 .mu.L of DMF. Add 0.5 g of monomer (AmPC) to 1 mL of methanol (slow dissolution/viscous solution), followed by 1 uL of the stock solution of CuBr.sub.2. Add the monomer solution dropwise to the reaction mixture above. Rinse with 1 mL of water. Degas the reaction mixture thoroughly (freeze-thaw). Let the reaction proceed for 24 hours.
[0473] Afterwards the Am-PC polymer may be dialyzed. The molecular weight of the above polymer was determined by SEC/MALS: Mn is 215 kDa, Mp: 250 kDa, PDI is 1.17. Conversion was estimated by 1H NMR to be 94%. A maleimide functionality can be added to the Am-PC polymer as discussed above for HEMA-PC and HEA-PC. Maleimide functionalized Am-PC polymer can be conjugated to a protein, such as TAF443, as described above.
Example 51. Reverse Ellman's Assay for Calculating Free Maleimide in a Compound
[0474] After addition of the maleimide functionality to polymer OG 1801 to form OG 1802 (see above), an Ellman's assay is used to determine the amount of functional maleimide (i.e. conjugatable) in a sample. Thiol converts Ellman's reagent (DTNB) to TNB-then to TNB2-in water at neutral and alkaline pH, which gives off a yellow color (measured at 412 nm). A standard curve is established with cysteine. Since the maleimide reacts with thiol, this assay actually measures the thiol (cysteine) left. The inhibition is calculated as the (original thiol-thiol left after maleimide polymer addition)/(original thiol) and is expressed as a percentage.
[0475] Reagents Employed in Assay: A standard curve was prepared using the cysteine from 62.5 .mu.M to 2 .mu.M. Polymer stock solutions were prepared by dissolving the powder in 1.times.PBS pH7.4 (reaction buffer) and mixing thoroughly. An equal molar of polymer and cysteine solutions were mixed and allowed to react at 27.degree. C. for 30 minutes. The 150 .mu.M of DTNB solution was added into the cysteine standards and polymer/cysteine reactions and the color was developed at 27.degree. C. for 5 minutes. OD at 412 nm was read on the Spectramax plate reader and percent inhibition was calculated with the Softmax Pro software and the cysteine standard curve.
[0476] All patent filings, websites, other publications, accession numbers and the like cited above or below are incorporated by reference in their entirety for all purposes to the same extent as if each individual item were specifically and individually indicated to be so incorporated by reference. If different versions of a sequence are associated with an accession number at different times, the version associated with the accession number at the effective filing date of this application is meant. The effective filing date means the earlier of the actual filing date or filing date of a priority application referring to the accession number if applicable. Likewise if different versions of a publication, website or the like are published at different times, the version most recently published at the effective filing date of the application is meant unless otherwise indicated. Any feature, step, element, embodiment, or aspect of the invention can be used in combination with any other unless specifically indicated otherwise. Although the present invention has been described in some detail by way of illustration and example for purposes of clarity and understanding, it will be apparent that certain changes and modifications may be practiced within the scope of the appended claims.
TABLE-US-00024 SEQUENCES SEQ ID NO. 1 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSDIQMTQSP SSLSASVGDR VTITCSASQD 321 ISNYLNWYQQ KPGKAPKVLI YFTSSLHSGV PSRFSGSGSG TDFTLTISSL QPEDFATYYC QQYSTVPWTF GQGTKVEIKR 401 TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN SQESVTEQDS KDSTYSLSST LTLSKADYEK 481 HKVYACEVTH QGLSSPVTKS FNRGEC SEQ ID NO. 2 1 EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH TCPPCPAPEL 241 LGGPSVFLFP PKPKDTLMIS RTPEVTCVVV DVSHEDPEVK FNWYVDGVEV HNAKTKPREE QYNSTYRVVS VLTVLHQDWL 321 NGKEYKCKVS NKALPAPIEK TISKAKGQPR EPQVYTLPPS REEMTKNQVS LTCLVKGFYP SDIAVEWESN GQPENNYKTT 401 PPVLDSDGSF FLYSKLTVDK SRWQQGNVFS CSVMHEALHN HYTQKSLSLS PGK SEQ ID NO. 3 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGDIQMTQ SPSSLSASVG DRVTITCSAS QDISNYLNWY 321 QQKPGKAPKV LIYFTSSLHS GVPSRFSGSG SGTDFTLTIS SLQPEDFATY YCQQYSTVPW TFGQGTKVEI KRTVAAPSVF 401 IFPPSDEQLK SGTASVVCLL NNFYPREAKV QWKVDNALQS GNSQESVTEQ DSKDSTYSLS STLTLSKADY EKHKVYACEV 481 THQGLSSPVT KSFNRGEC SEQ ID NO. 4 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSEVQLVESG GGLVQPGGSL RLSCAASGYT 321 FTNYGMNWVR QAPGKGLEWV GWINTYTGEP TYAADFKRRF TFSLDTSKST AYLQMNSLRA EDTAVYYCAK YPHYYGSSHW 401 YFDVWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL 481 SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THTCPPCPAP ELLGGPSVFL FPPKPKDTLM ISRTPEVTCV 561 VVDVSHEDPE VKFNWYVDGV EVHNAKTKPR EEQYNSTYRV VSVLTVLHQD WLNGKEYKCK VSNKALPAPI EKTISKAKGQ 641 PREPQVYTLP PSREEMTKNQ VSLTCLVKGF YPSDIAVEWE SNGQPENNYK TTPPVLDSDG SFFLYSKLTV DKSRWQQGNV 721 FSCSVMHEAL HNHYTQKSLS LSPGK SEQ ID NO. 5 1 DIQMTQSPSS LSASVGDRVT ITCSASQDIS NYLNWYQQKP GKAPKVLIYF TSSLHSGVPS RFSGSGSGTD FTLTISSLQP 81 EDFATYYCQQ YSTVPWTFGQ GTKVEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY PREAKVQWKV DNALQSGNSQ 161 ESVTEQDSKD STYSLSSTLT LSKADYEKHK VYACEVTHQG LSSPVTKSFN RGEC SEQ ID NO. 6 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGEVQLVE SGGGLVQPGG SLRLSCAASG YTFTNYGMNW 321 VRQAPGKGLE WVGWINTYTG EPTYAADFKR RFTFSLDTSK STAYLQMNSL RAEDTAVYYC AKYPHYYGSS HWYFDVWGQG 401 TLVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF PEPVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS 481 SSLGTQTYIC NVNHKPSNTK VDKKVEPKSC DKTHTCPPCP APELLGGPSV FLFPPKPKDT LMISRTPEVT CVVVDVSHED 561 PEVKFNWYVD GVEVHNAKTK PREEQYNSTY RVVSVLTVLH QDWLNGKEYK CKVSNKALPA PIEKTISKAK GQPREPQVYT 641 LPPSREEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS DGSFFLYSKL TVDKSRWQQG NVFSCSVMHE 721 ALHNHYTQKS LSLSPGK SEQ ID NO. 7 1 EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH TCPPCPAPEL 241 LGGPSVFLFP PKPKDTLMIS RTPEVTCVVV DVSHEDPEVK FNWYVDGVEV HNAKTKPREE QYNSTYRVVS VLTVLHQDWL 321 NGKEYKCKVS NKALPAPIEK TISKAKGQPR EPQVYTLPPS REEMTKNQVS LTCLVKGFYP SDIAVEWESN GQPENNYKTT 401 PPVLDSDGSF FLYSKLTVDK SRWQQGNVFS CSVMHEALHN HYTQKSLSLS PGGGGGSGGG GSGGGGSGGG GSGLVVTPPG 481 PELVLNVSST FVLTCSGSAP VVWERMSQEP PQEMAKAQDG TFSSVLTLTN LTGLDTGEYF CTHNDSRGLE TDERKRLYIF 561 VPDPTVGFLP NDAEELFIFL TEITEITIPC RVTDPQLVVT LHEKKGDVAL PVPYDHQRGF SGIFEDRSYI CKTTIGDREV 641 DSDAYYVYRL QVSSINVSVN AVQTVVRQGE NITLMCIVIG NEVVNFEWTY PRKESGRLVE PVTDFLLDMP YHIRSILHIP 721 SAELEDSGTY TCNVTESVND HQDEKAINIT VVESG SEQ ID NO. 8 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSEVQLVESG GGLVQPGGSL RLSCAASGYD 321 FTHYGMNWVR QAPGKGLEWV GWINTYTGEP TYAADFKRRF TFSLDTSKST AYLQMNSLRA EDTAVYYCAK YPYYYGTSHW 401 YFDVWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL 481 SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THTCPPCPAP EAAGAPSVFL FPPKPKDTLM ISRTPEVTCV 561 VVDVSHEDPE VKFNWYVDGV EVHNAKTKPR EEQYNSTYRV VSVLTVLHQD WLNGKEYKCK VSNKALPAPI EKTISKAKGQ 641 PREPCVYTLP PSREEMTKNQ VSLTCLVKGF YPSDIAVEWE SNGQPENNYK TTPPVLDSDG SFFLYSKLTV DKSRWQQGNV 721 FSCSVMHEAL HNHYTQKSLS LSPGK SEQ ID NO. 9 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSEVQLVESG GGLVQPGGSL RLSCAASGYD 321 FTHYGMNWVR QAPGKGLEWV GWINTYTGEP TYAADFKRRF TFSLDTSKST AYLQMNSLRA EDTAVYYCAK YPYYYGTSHW 401 YFDVWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL 481 SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THTCPPCPAP EAAGAPSVFL FPPKPKDTLM ISRTPEVTCV 561 VVDVSHEDPE VKFNWYVDGV EVHNAKTKPR EEQYNSTYRV VSVLTVLHQD WLNGKEYKCK VSNKALPAPI EKTISKAKGQ 641 PREPQVYTLP PSREEMTKNQ VSLTCLVKGF YPSDIAVEWE SNGQPENNYK TTPPVLDSDG SFFLYSKLTV DKSRWQQGNV 721 FSCSVMHEAL HNHYTQKSLS CSPGK SEQ ID NO. 10 1 DIQLTQSPSS LSASVGDRVT ITCSASQDIS NYLNWYQQKP GKAPKVLIYF TSSLHSGVPS RFSGSGSGTD FTLTISSLQP 81 EDFATYYCQQ YSTVPWTFGQ GTKVEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY PREAKVQWKV DNALQSGNSQ 161 ESVTEQDSKD STYSLSSTLT LSKADYEKHK VYACEVTHQG LSSPVTKSFN RGEC SEQ ID NO. 11 1 MRLPGAMPAL ALKGELLLLS LLLLLEPQIS QGLVVTPPGP ELVLNVSSTF VLTCSGSAPV VWERMSQEPP QEMAKAQDGT 81 FSSVLTLTNL TGLDTGEYFC THNDSRGLET DERKRLYIFV PDPTVGFLPN DAEELFIFLT EITEITIPCR VTDPQLVVTL 161 HEKKGDVALP VPYDHQRGFS GIFEDRSYIC KTTIGDREVD SDAYYVYRLQ VSSINVSVNA VQTVVRQGEN ITLMCIVIGN 241 EVVNFEWTYP RKESGRLVEP VTDFLLDMPY HIRSILHIPS AELEDSGTYT CNVTESVNDH QDEKAINITV VESGYVRLLG 321 EVGTLQFAEL HRSRTLQVVF EAYPPPTVLW FKDNRTLGDS SAGEIALSTR NVSETRYVSE LTLVRVKVAE AGHYTMRAFH 401 EDAEVQLSFQ LQINVPVRVL ELSESHPDSG EQTVRCRGRG MPQPNIIWSA CRDLKRCPRE LPPTLLGNSS EEESQLETNV 481 TYWEEEQEFE VVSTLRLQHV DRPLSVRCTL RNAVGQDTQE VIVVPHSLPF KVVVISAILA LVVLTIISLI ILIMLWQKKP
561 RYEIRWKVIE SVSSDGHEYI YVDPMQLPYD STWELPRDQL VLGRTLGSGA FGQVVEATAH GLSHSQATMK VAVKMLKSTA 641 RSSEKQALMS ELKIMSHLGP HLNVVNLLGA CTKGGPIYII TEYCRYGDLV DYLHRNKHTF LQHHSDKRRP PSAELYSNAL 721 PVGLPLPSHV SLTGESDGGY MDMSKDESVD YVPMLDMKGD VKYADIESSN YMAPYDNYVP SAPERTCRAT LINESPVLSY 801 MDLVGFSYQV ANGMEFLASK NCVHRDLAAR NVLICEGKLV KICDFGLARD IMRDSNYISK GSTFLPLKWM APESIFNSLY 881 TTLSDVWSFG ILLWEIFTLG GTPYPELPMN EQFYNAIKRG YRMAQPAHAS DEIYEIMQKC WEEKFEIRPP FSQLVLLLER 961 LLGEGYKKKY QQVDEEFLRS DHPAILRSQA RLPGFHGLRS PLDTSSVLYT AVQPNEGDND YIIPLPDPKP EVADEGPLEG 1041 SPSLASSTLN EVNTSSTISC DSPLEPQDEP EPEPQLELQV EPEPELEQLP DSGCPAPRAE AEDSFL SEQ ID NO. 12 1 DIQLTQSPSS LSASVGDRVT ITCSASQDIS NYLNWYQQKP GKAPKVLIYF TSSLHSGVPS RFSGSGSGTD FTLTISSLQP 81 EDFATYYCQQ YSTVPWTFGQ GTKVEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY PREAKVQWKV DNALQSGNSQ 161 ESVTEQDSKD STYSLSSTLT LSKADYEKHK VYACEVTHQG LSSPVTKSFN RGEC SEQ ID NO. 13 1 EVQLVESGGG LVQPGGSLRL SCAASGYDFT HYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP YYYGTSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH L SEQ ID NO. 14 1 MVSYWDTGVL LCALLSCLLL TGSSSGSKLK DPELSLKGTQ HIMQAGQTLH LQCRGEAAHK WSLPEMVSKE SERLSITKSA 81 CGRNGKQFCS TLTLNTAQAN HTGFYSCKYL AVPTSKKKET ESAIYIFISD TGRPFVEMYS EIPEIIHMTE GRELVIPCRV 161 TSPNITVTLK KFPLDTLIPD GKRIIWDSRK GFIISNATYK EIGLLTCEAT VNGHLYKTNY LTHRQTNTII DVQISTPRPV 241 KLLRGHTLVL NCTATTPLNT RVQMTWSYPD EKNKRASVRR RIDQSNSHAN IFYSVLTIDK MQNKDKGLYT CRVRSGPSFK 321 SVNTSVHIYD KAFITVKHRK QQVLETVAGK RSYRLSMKVK AFPSPEVVWL KDGLPATEKS ARYLTRGYSL IIKDVTEEDA 401 GNYTILLSIK QSNVFKNLTA TLIVNVKPQI YEKAVSSFPD PALYPLGSRQ ILTCTAYGIP QPTIKWFWHP CNHNHSEARC 481 DFCSNNEESF ILDADSNMGN RIESITQRMA IIEGKNKMAS TLVVADSRIS GIYICIASNK VGTVGRNISF YITDVPNGFH 561 VNLEKMPTEG EDLKLSCTVN KFLYRDVTWI LLRTVNNRTM HYSISKQKMA ITKEHSITLN LTIMNVSLQD SGTYACRARN 641 VYTGEEILQK KEITIRDQEA PYLLRNLSDH TVAISSSTTL DCHANGVPEP QITWFKNNHK IQQEPGIILG PGSSTLFIER 721 VTEEDEGVYH CKATNQKGSV ESSAYLTVQG TSDKSNLELI TLTCTCVAAT LFWLLLTLFI RKMKRSSSEI KTDYLSIIMD 801 PDEVPLDEQC ERLPYDASKW EFARERLKLG KSLGRGAFGK VVQASAFGIK KSPTCRTVAV KMLKEGATAS EYKALMTELK 881 ILTHIGHHLN VVNLLGACTK QGGPLMVIVE YCKYGNLSNY LKSKRDLFFL NKDAALHMEP KKEKMEPGLE QGKKPRLDSV 961 TSSESFASSG FQEDKSLSDV EEEEDSDGFY KEPITMEDLI SYSFQVARGM EFLSSRKCIH RDLAARNILL SENNVVKICD 1041 FGLARDIYKN PDYVRKGDTR LPLKWMAPES IFDKIYSTKS DVWSYGVLLW EIFSLGGSPY PGVQMDEDFC SRLREGMRMR 1121 APEYSTPEIY QIMLDCWHRD PKERPRFAEL VEKLGDLLQA NVQQDGKDYI PINAILTGNS GFTYSTPAFS EDFFKESISA 1201 PKFNSGSSDD VRYVNAFKFM SLERIKTFEE LLPNATSMFD DYQGDSSTLL ASPMLKRFTW TDSKPKASLK IDLRVTSKSK 1281 ESGLSDVSRP SFCHSSCGHV SEGKRRFTYD HAELERKIAC CSPPPDYNSV VLYSTPPI SEQ ID NO. 15 1 MQSKVLLAVA LWLCVETRAA SVGLPSVSLD LPRLSIQKDI LTIKANTTLQ ITCRGQRDLD WLWPNNQSGS EQRVEVTECS 81 DGLFCKTLTI PKVIGNDTGA YKCFYRETDL ASVIYVYVQD YRSPFIASVS DQHGVVYITE NKNKTVVIPC LGSISNLNVS 161 LCARYPEKRF VPDGNRISWD SKKGFTIPSY MISYAGMVFC EAKINDESYQ SIMYIVVVVG YRIYDVVLSP SHGIELSVGE 241 KLVLNCTART ELNVGIDFNW EYPSSKHQHK KLVNRDLKTQ SGSEMKKFLS TLTIDGVTRS DQGLYTCAAS SGLMTKKNST 321 FVRVHEKPFV AFGSGMESLV EATVGERVRI PAKYLGYPPP EIKWYKNGIP LESNHTIKAG HVLTIMEVSE RDTGNYTVIL 401 TNPISKEKQS HVVSLVVYVP PQIGEKSLIS PVDSYQYGTT QTLTCTVYAI PPPHHIHWYW QLEEECANEP SQAVSVTNPY 481 PCEEWRSVED FQGGNKIEVN KNQFALIEGK NKTVSTLVIQ AANVSALYKC EAVNKVGRGE RVISFHVTRG PEITLQPDMQ 561 PTEQESVSLW CTADRSTFEN LTWYKLGPQP LPIHVGELPT PVCKNLDTLW KLNATMFSNS TNDILIMELK NASLQDQGDY 641 VCLAQDRKTK KRHCVVRQLT VLERVAPTIT GNLENQTTSI GESIEVSCTA SGNPPPQIMW FKDNETLVED SGIVLKDGNR 721 NLTIRRVRKE DEGLYTCQAC SVLGCAKVEA FFIIEGAQEK TNLEIIILVG TAVIAMFFWL LLVIILRTVK RANGGELKTG 801 YLSIVMDPDE LPLDEHCERL PYDASKWEFP RDRLKLGKPL GRGAFGQVIE ADAFGIDKTA TCRTVAVKML KEGATHSEHR 881 ALMSELKILI HIGHHLNVVN LLGACTKPGG PLMVIVEFCK FGNLSTYLRS KRNEFVPYKT KGARFRQGKD YVGAIPVDLK 961 RRLDSITSSQ SSASSGFVEE KSLSDVEEEE APEDLYKDFL TLEHLICYSF QVAKGMEFLA SRKCIHRDLA ARNILLSEKN 1041 VVKICDFGLA RDIYKDPDYV RKGDARLPLK WMAPETIFDR VYTIQSDVWS FGVLLWEIFS LGASPYPGVK IDEEFCRRLK 1121 EGTRMRAPDY TTPEMYQTML DCWHGEPSQR PTFSELVEHL GNLLQANAQQ DGKDYIVLPI SETLSMEEDS GLSLPTSPVS 1201 CMEEEEVCDP KFHYDNTAGI SQYLQNSKRK SRPVSVKTFE DIPLEEPEVK VIPDDNQTDS GMVLASEELK TLEDRTKLSP 1281 SFGGMVPSKS RESVASEGSN QTSGYQSGYH SDDTDTTVYS SEEAELLKLI EIGVQTGSTA QILQPDSGTT LSSPPV SEQ ID NO. 16 1 MQRGAALCLR LWLCLGLLDG LVSGYSMTPP TLNITEESHV IDTGDSLSIS CRGQHPLEWA WPGAQEAPAT GDKDSEDTGV 81 VRDCEGTDAR PYCKVLLLHE VHANDTGSYV CYYKYIKARI EGTTAASSYV FVRDFEQPFI NKPDTLLVNR KDAMWVPCLV 161 SIPGLNVTLR SQSSVLWPDG QEVVWDDRRG MLVSTPLLHD ALYLQCETTW GDQDFLSNPF LVHITGNELY DIQLLPRKSL 241 ELLVGEKLVL NCTVWAEFNS GVTFDWDYPG KQAERGKWVP ERRSQQTHTE LSSILTIHNV SQHDLGSYVC KANNGIQRFR 321 ESTEVIVHEN PFISVEWLKG PILEATAGDE LVKLPVKLAA YPPPEFQWYK DGKALSGRHS PHALVLKEVT EASTGTYTLA 401 LWNSAAGLRR NISLELVVNV PPQIHEKEAS SPSIYSRHSR QALTCTAYGV PLPLSIQWHW RPWTPCKMFA QRSLRRRQQQ 481 DLMPQCRDWR AVTTQDAVNP IESLDTWTEF VEGKNKTVSK LVIQNANVSA MYKCVVSNKV GQDERLIYFY VTTIPDGFTI 561 ESKPSEELLE GQPVLLSCQA DSYKYEHLRW YRLNLSTLHD AHGNPLLLDC KNVHLFATPL AASLEEVAPG ARHATLSLSI 641 PRVAPEHEGH YVCEVQDRRS HDKHCHKKYL SVQALEAPRL TQNLTDLLVN VSDSLEMQCL VAGAHAPSIV WYKDERLLEE 721 KSGVDLADSN QKLSIQRVRE EDAGRYLCSV CNAKGCVNSS ASVAVEGSED KGSMEIVILV GTGVIAVFFW VLLLLIFCNM 801 RRPAHADIKT GYLSIIMDPG EVPLEEQCEY LSYDASQWEF PRERLHLGRV LGYGAFGKVV EASAFGIHKG SSCDTVAVKM 881 LKEGATASEH RALMSELKIL IHIGNHLNVV NLLGACTKPQ GPLMVIVEFC KYGNLSNFLR AKRDAFSPCA EKSPEQRGRF 961 RAMVELARLD RRRPGSSDRV LFARFSKTEG GARRASPDQE AEDLWLSPLT MEDLVCYSFQ VARGMEFLAS RKCIHRDLAA 1041 RNILLSESDV VKICDFGLAR DIYKDPDYVR KGSARLPLKW MAPESIFDKV YTTQSDVWSF GVLLWEIFSL GASPYPGVQI 1121 NEEFCQRLRD GTRMRAPELA TPAIRRIMLN CWSGDPKARP AFSELVEILG DLLQGRGLQE EEEVCMAPRS SQSSEEGSFS 1201 QVSTMALHIA QADAEDSPPS LQRHSLAARY YNWVSFPGCL ARGAETRGSS RMKTFEEFPM TPTTYKGSVD NQTDSGMVLA 1281 SEEFEQIESR HRQESGFSCK GPGQNVAVTR AHPDSQGRRR RPERGARGGQ VFYNSEYGEL SEPSEEDHCS PSARVTFFTD 1361 NSY SEQ ID NO. 17 1 ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT 81 YICNVNHKPS NTKVDKKVEP KSCDKTHTCP PCPAPELLGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS HEDPEVKFNW 161 YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK EYKCKVSNKA LPAPIEKTIS KAKGQPREPQ VYTLPPSRDE 241 LTKNQVSLTC LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW QQGNVFSCSV MHEALHNHYT 321 QKSLSLSPGK SEQ ID NO. 18 1 TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN SQESVTEQDS KDSTYSLSST LTLSKADYEK 81 HKVYACEVTH QGLSSPVTKS FNRGEC SEQ ID NO. 19 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSDIQMTQSP SSLSASVGDR VTITCSASQD 321 ISNYLNWYQQ KPGKAPKVLI YFTSSLHSGV PSRFSGSGSG TDFTLTISSL QPEDFATYYC QQYSTVPWTF GQGTKVEIKR 401 TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN SQESVTEQDS KDSTYSLSST LTLSKADYEK 481 HKVYACEVTH QGLSSPVTKS FNRGEC SEQ ID NO. 21 1 EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH T SEQ ID NO. 22
1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSEVQLVESG GGLVQPGGSL RLSCAASGYT 321 FTNYGMNWVR QAPGKGLEWV GWINTYTGEP TYAADFKRRF TFSLDTSKST AYLQMNSLRA EDTAVYYCAK YPHYYGSSHW 401 YFDVWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL 481 SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THT SEQ ID NO. 23 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGEVQLVE SGGGLVQPGG SLRLSCAASG YTFTNYGMNW 321 VRQAPGKGLE WVGWINTYTG EPTYAADFKR RFTFSLDTSK STAYLQMNSL RAEDTAVYYC AKYPHYYGSS HWYFDVWGQG 401 TLVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF PEPVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS 481 SSLGTQTYIC NVNHKPSNTK VDKKVEPKSC DKTHT SEQ ID NO. 24 1 EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH TGGGGGSGGG 241 GSGGGGSGGG GSGLVVTPPG PELVLNVSST FVLTCSGSAP VVWERMSQEP PQEMAKAQDG TFSSVLTLTN LTGLDTGEYF 321 CTHNDSRGLE TDERKRLYIF VPDPTVGFLP NDAEELFIFL TEITEITIPC RVTDPQLVVT LHEKKGDVAL PVPYDHQRGF 401 SGIFEDRSYI CKTTIGDREV DSDAYYVYRL QVSSINVSVN AVQTVVRQGE NITLMCIVIG NEVVNFEWTY PRKESGRLVE 481 PVTDFLLDMP YHIRSILHIP SAELEDSGTY TCNVTESVND HQDEKAINIT VVESG SEQ ID NO. 25 1 LVVTPPGPEL VLNVSSTFVL TCSGSAPVVW ERMSQEPPQE MAKAQDGTFS SVLTLTNLTG LDTGEYFCTH NDSRGLETDE 81 RKRLYIFVPD PTVGFLPNDA EELFIFLTEI TEITIPCRVT DPQLVVTLHE KKGDVALPVP YDHQRGFSGI FEDRSYICKT 161 TIGDREVDSD AYYVYRLQVS SINVSVNAVQ TVVRQGENIT LMCIVIGNEV VNFEWTYPRK ESGRLVEPVT DFLLDMPYHI 241 RSILHIPSAE LEDSGTYTCN VTESVNDHQD EKAINITVVE SGGGGGSGGG GSEVQLVESG GGLVQPGGSL RLSCAASGYD 321 FTHYGMNWVR QAPGKGLEWV GWINTYTGEP TYAADFKRRF TFSLDTSKST AYLQMNSLRA EDTAVYYCAK YPYYYGTSHW 401 YFDVWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL 481 SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THL SEQ ID NO. 26 1 LVVTPPGPE LVLNVSSTFV LTCSGSAPVV WERMSQEPPQ EMAKAQDGTF SSVLTLTNLT GLDTGEYFCT HNDSRGLETD 80 ERKRLYIFVP DPTVGFLPND AEELFIFLTE ITEITIPCRV TDPQLVVTLH EKKGDVALPV PYDHQRGFSG IFEDRSYICK 160 TTIGDREVDS DAYYVYRLQV SSINVSVNAV QTVVRQGENI TLMCIVIGNE VVNFEWTYPR KESGRLVEPV TDFLLDMPYH 240 IRSILHIPSA ELEDSGTYTC NVTESVNDHQ DEKAINITVV ESGEVQLVES GGGLVQPGGS LRLSCAASGY TFTNYGMNWV 320 RQAPGKGLEW VGWINTYTGE PTYAADFKRR FTFSLDTSKS TAYLQMNSLR AEDTAVYYCA KYPHYYGSSH WYFDVWGQGT 400 LVTVSSASTK GPSVFPLAPS SKSTSGGTAA LGCLVKDYFP EPVTVSWNSG ALTSGVHTFP AVLQSSGLYS LSSVVTVPSS 480 SLGTQTYICN VNHKPSNTKV DKKVEPKSCD KTHTCPPCPA PELLGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP 560 EVKFNWYVDG VEVHNAKTKP REEQYNSTYR VVSVLTVLHQ DWLNGKEYKC KVSNKALPAP IEKTISKAKG QPREPQVYTL 640 PPSREEMTKN QVSLTCLVKG FYPSDIAVEW ESNGQPENNY KTTPPVLDSD GSFFLYSKLT VDKSRWQQGN VFSCSVMHEA 720 LHNHYTQKSL SLSPGK SEQ ID NO. 27 1 VGFLPNDAEE LFIFLTEITE ITIPCRVTDP QLVVTLHEKK GDVALPVPYD HQRGFSGIFE DRSYICKTTI GDREVDSDAY 81 YVYRLQVSSI NVSVNAVQTV VRQGENITLM CIVIGNEVVN FEWTYPRKES GRLVEPVTDF LLDMPYHIRS ILHIPSAELE 161 DSGTYTCNVT ESVNDHQDEK AINITVVESG EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW 241 INTYTGEPTY AADFKRRFTF SLDTSKSTAY LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS 321 VFPLAPSSKS TSGGTAALGC LVKDYFPEPV TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH 401 KPSNTKVDKK VEPKSCDKTH TCPPCPAPEL LGGPSVFLFP PKPKDTLMIS RTPEVTCVVV DVSHEDPEVK FNWYVDGVEV 481 HNAKTKPREE QYNSTYRVVS VLTVLHQDWL NGKEYKCKVS NKALPAPIEK TISKAKGQPR EPQVYTLPPS REEMTKNQVS 561 LTCLVKGFYP SDIAVEWESN GQPENNYKTT PPVLDSDGSF FLYSKLTVDK SRWQQGNVFS CSVMHEALHN HYTQKSLSLS 641 PGK SEQ ID NO. 28 1 VGFLPNDAEE LFIFLTEITE ITIPCRVTDP QLVVTLHEKK GDVALPVPYD HQRGFSGIFE DRSYICKTTI GDREVDSDAY 81 YVYRLQVSSI NVSVNAVQTV VRQGENITLM CIVIGNEVVN FEWTYPRKES GRLVEPVTDF LLDMPYHIRS ILHIPSAELE 161 DSGTYTCNVT ESVNDHQDEK AINITVVESG EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW 241 INTYTGEPTY AADFKRRFTF SLDTSKSTAY LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS 321 VFPLAPSSKS TSGGTAALGC LVKDYFPEPV TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH 401 KPSNTKVDKK VEPKSCDKTH T SEQ ID NO. 29 1 VGFLPNDAEE LFIFLTEITE ITIPCRVTDP QLVVTLHEKK GDVALPVPYD HQRGFSGIFE DRSYICKTTI GDREVDSDAY 81 YVYRLQVSSI NVSVNAVQTV VRQGENITLM CIVIGNEVVN FEWTYPRKES GRLVEPVTDF LLDMPYHIRS ILHIPSAELE 161 DSGTYTCNVT ESVNDHQDEK AINITVVESG GGGSGGGGSG GGGSGGGGSG GGGSGGGGSE VQLVESGGGL VQPGGSLRLS 241 CAASGYTFTN YGMNWVRQAP GKGLEWVGWI NTYTGEPTYA ADFKRRFTFS LDTSKSTAYL QMNSLRAEDT AVYYCAKYPH 321 YYGSSHWYFD VWGQGTLVTV SSASTKGPSV FPLAPSSKST SGGTAALGCL VKDYFPEPVT VSWNSGALTS GVHTFPAVLQ 401 SSGLYSLSSV VTVPSSSLGT QTYICNVNHK PSNTKVDKKV EPKSCDKTHT SEQ ID NO. 30 1 EVQLVESGGG LVQPGGSLRL SCAASGYTFT NYGMNWVRQA PGKGLEWVGW INTYTGEPTY AADFKRRFTF SLDTSKSTAY 81 LQMNSLRAED TAVYYCAKYP HYYGSSHWYF DVWGQGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV 161 TVSWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSCDKTH TGGGSGGGGS 241 GGGGSGGGGS GGGGSGGGGS VGFLPNDAEE LFIFLTEITE ITIPCRVTDP QLVVTLHEKK GDVALPVPYD HQRGFSGIFE 321 DRSYICKTTI GDREVDSDAY YVYRLQVSSI NVSVNAVQTV VRQGENITLM CIVIGNEVVN FEWTYPRKES GRLVEPVTDF 401 LLDMPYHIRS ILHIPSAELE DSGTYTCNVT ESVNDHQDEK AINITVVESG SEQ ID NO: 31 Nucleic acid encoding heavy chain anti-VEGF-PDGFR fusion GACGGATCGGGAGATCTCCCGATCCCCTATGGTGCACTCTCAGTACAATCTGCTCTGATGCCG CATAGTTAAGCCAGTATCTGCTCCCTGCTTGTGTGTTGGAGGTCGCTGAGTAGTGCGCGAGCA AAATTTAAGCTACAACAAGGCAAGGCTTGACCGACAATTGCATGAAGAATCTGCTTAGGGTTA GGCGTTTTGCGCTGCTTCGCGATGTACGGGCCAGATATACGCGTTGACATTGATTATTGACTA GTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAGTTCCGCGTTA CATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCCCATTGACGTCAA TAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTCCATTGACGTCAATGGGTGGAGT ATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATATGCCAAGTACGCCCCCTA TTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGTACATGACCTTATGGGACT TTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTACCATGGTGATGCGGTTTTGGC AGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAGTCTCCACCCCATTG ACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAACAACT CCGCCCCATTGACGCAAATGGGCGGTAGGCGTGTACGGTGGGAGGTCTATATAAGCAGAGCTC TCTGGCTAACTAGAGAACCCACTGCTTACTGGCTTATCGAAATTAATACGACTCACTATAGGG AGACCCAAGCTGGCTAGCGTTTAAACTTAAGCTTGGTACCGAGCTCGGATCCACTAGTCCAGT GTGGTGGAATTCTGCAGATATCCAGCACAGTGGCGGCCGCCatgaaagctgtggtgctggccg tggctctggtcttcctgacagggagccaggctctggtcgtcacacccccggggccagagcttg tcctcaatgtctccagcaccttcgttctgacctgctcgggttcagctccggtggtgtgggaac ggatgtcccaggagcccccacaggaaatggccaaggcccaggatggcaccttctccagcg tgctcacactgaccaacctcactgggctagacacgggagaatacttttgcacccacaatgact cccgggactggagaccgatgagcggaaacggctctacatctttgtgccagatcccaccgtggg cttcctccctaatgatgccgaggaactattcatctttctcacggaaataactgagatcaccat tccatgccgagtaacagacccacagctggtggtgacactgcacgagaagaaaggggacgttgc actgcctgtcccctatgatcaccaacgtggcttttctggtatctttgaggacagaagctacat ctgcaaaaccaccattggggacagggaggtggattctgatgcctactatgtctacagactcca ggtgtcatccatcaacgtctctgtgaacgcagtgcagactgtggtccgccagggtgagaacat caccctcatgtgcattgtgatcgggaatgaggtggtcaacttcgagtggacatacccccgcaa agaaagtgggcggctggtggagccggtgactgacttcctcttggatatgccttaccacatccg
ctccatcctgcacatccccagtgccgagttagaagactcggggacctacacctgcaatgtgac ggagagtgtgaatgaccatcaggatgaaaaggccatcaacatcaccgtggttgagagcggcgg tggtggcggctccggtggaggcggaagcgaggtgcagctggtggaatccggcggaggcctggt ccagcctggcggatccctgagactgtcctgtgccgcctccggctacgacttcacccattacgg catgaactgggtccgacaggcccctggcaagggcctggaatgggtcggatggatcaacaccta caccggcgagcccacctacgccgccgacttcaagcggcggttcaccttctccctggacacctc caagtccaccgcctacctgcagatgaactccctgcgggccgaggacaccgccgtgtactactg cgccaagtacccctactactacggcacctcccactggtacttcgacgtgtggggccagggcac cctggtcaccgtgtcctccgcctctaccaagggcccctccgtgttccctctggccccctccag caagtccacctctggcggcaccgccgctctgggctgcctggtcaaggactacttccccgagcc cgtgaccgtgtcctggaactctggcgccctgacctccggcgtgcacacctttccagccgtgct gcagtcctccggcctgtactccctgtcctccgtcgtgaccgtgccctccagctctctgggcac ccagacctacatctgcaacgtgaaccacaagccctccaacaccaaggtggacaagaaggtgga acccaagtcctgcgacaagacccacacctgtcccccctgccctgcccctgaagcagccggtgc acccagcgtgttcctgttccccccaaagcccaaggacaccctgatgatctcccggacccccga agtgacctgcgtggtggtggacgtgtcccacgaggaccctgaagtgaagttcaattggtacgt ggacggcgtggaagtgcacaatgccaagaccaagcccagagaggaacagtacaactccaccta ccgggtggtgtccgtgctgaccgtgctgcatcaggactggctgaacggcaaagagtacaagtg caaggtctccaacaaggccctgcctgcccccatcgaaaagaccatctccaaggccaagggcca gccccgcgagcctcaggtgtacacactgccacccagccgggaagagatgaccaagaaccaggt ctccctgacctgtctggtcaagggcttctacccctccgatatcgccgtcgaatgggagtccaa cggccagcccgagaacaactacaagaccaccccccctgtgctggactccgacggctcattctt cctgtactccaagctgaccgtggacaagtcccggtggcagcagggcaacgtgttctcctgctc cgtgatgcacgaggccctgcacaaccactacacccagaagtccctgtcctgcagccccggcaa gtgataaTCTAGAGGGCCCGTTTAAACCCGCTGATCAGCCTCGACTGTGCCTTCTAGTTGCCA GCCATCTGTTGTTTGCCCCTCCCCCGTGCCTTCCTTGACCCTGGAAGGTGCCACTCCCACTGT CCTTTCCTAATAAAATGAGGAAATTGCATCGCATTGTCTGAGTAGGTGTCATTCTATTCTGGG GGGTGGGGTGGGGCAGGACAGCAAGGGGGAGGATTGGGAAGACAATAGCAGGCATGCTGGGGA TGCGGTGGGCTCTATGGCTTCTGAGGCGGAAAGAACCAGCTGGGGCTCTAGGGGGTATCCCCA CGCGCCCTGTAGCGGCGCATTAAGCGCGGCGGGTGTGGTGGTTACGCGCAGCGTGACCGCTAC ACTTGCCAGCGCCCTAGCGCCCGCTCCTTTCGCTTTCTTCCCTTCCTTTCTCGCCACGTTCGC CGGCTTTCCCCGTCAAGCTCTAAATCGGGGGCTCCCTTTAGGGTTCCGATTTAGTGCTTTACG GCACCTCGACCCCAAAAAACTTGATTAGGGTGATGGTTCACGTAGTGGGCCATCGCCCTGATA GACGGTTTTTCGCCCTTTGACGTTGGAGTCCACGTTCTTTAATAGTGGACTCTTGTTCCAAAC TGGAACAACACTCAACCCTATCTCGGTCTATTCTTTTGATTTATAAGGGATTTTGCCGATTTC GGCCTATTGGTTAAAAAATGAGCTGATTTAACAAAAATTTAACGCGAATTAATTCTGTGGAAT GTGTGTCAGTTAGGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGCAGAAGTATGCAAAGCATG CATCTCAATTAGTCAGCAACCAGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGCAGAAGTATG CAAAGCATGCATCTCAATTAGTCAGCAACCATAGTCCCGCCCCTAACTCCGCCCATCCCGCCC CTAACTCCGCCCAGTTCCGCCCATTCTCCGCCCCATGGCTGACTAATTTTTTTTATTTATGCA GAGGCCGAGGCCGCCTCTGCCTCTGAGCTATTCCAGAAGTAGTGAGGAGGCTTTTTTGGAGGC CTAGGCTTTTGCAAAAAGCTCCCGGGAGCTTGTATATCCATTTTCGGATCTGATCAAGAGACA GGATGAGGATCGTTTCGCATGATTGAACAAGATGGATTGCACGCAGGTTCTCCGGCCGCTTGG GTGGAGAGGCTATTCGGCTATGACTGGGCACAACAGACAATCGGCTGCTCTGATGCCGCCGTG TTCCGGCTGTCAGCGCAGGGGCGCCCGGTTCTTTTTGTCAAGACCGACCTGTCCGGTGCCCTG AATGAACTGCAGGACGAGGCAGCGCGGCTATCGTGGCTGGCCACGACGGGCGTTCCTTGCGCA GCTGTGCTCGACGTTGTCACTGAAGCGGGAAGGGACTGGCTGCTATTGGGCGAAGTGCCGGGG CAGGATCTCCTGTCATCTCACCTTGCTCCTGCCGAGAAAGTATCCATCATGGCTGATGCAATG CGGCGGCTGCATACGCTTGATCCGGCTACCTGCCCATTCGACCACCAAGCGAAACATCGCATC GAGCGAGCACGTACTCGGATGGAAGCCGGTCTTGTCGATCAGGATGATCTGGACGAAGAGCAT CAGGGGCTCGCGCCAGCCGAACTGTTCGCCAGGCTCAAGGCGCGCATGCCCGACGGCGAGGAT CTCGTCGTGACCCATGGCGATGCCTGCTTGCCGAATATCATGGTGGAAAATGGCCGCTTTTCT GGATTCATCGACTGTGGCCGGCTGGGTGTGGCGGACCGCTATCAGGACATAGCGTTGGCTACC CGTGATATTGCTGAAGAGCTTGGCGGCGAATGGGCTGACCGCTTCCTCGTGCTTTACGGTATC GCCGCTCCCGATTCGCAGCGCATCGCCTTCTATCGCCTTCTTGACGAGTTCTTCTGAGCGGGA CTCTGGGGTTCGAAATGACCGACCAAGCGACGCCCAACCTGCCATCACGAGATTTCGATTCCA CCGCCGCCTTCTATGAAAGGTTGGGCTTCGGAATCGTTTTCCGGGACGCCGGCTGGATGATCC TCCAGCGCGGGGATCTCATGCTGGAGTTCTTCGCCCACCCCAACTTGTTTATTGCAGCTTATA ATGGTTACAAATAAAGCAATAGCATCACAAATTTCACAAATAAAGCATTTTTTTCACTGCATT CTAGTTGTGGTTTGTCCAAACTCATCAATGTATCTTATCATGTCTGTATACCGTCGACCTCTA GCTAGAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAA TTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCT AACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGC TGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTT CCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAA AGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAG GCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCC CCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTAT AAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGC TTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCT GTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCG TTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACG ACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTG CTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCT GCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAA CCACCGCTGGTAGCGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTC AAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAG GGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAA GTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCA GTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCG TGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCGCGAG ACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCA GAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAG TAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGT CACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACAT GATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTA AGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGC CATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTA TGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAA CTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGC TGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTT TCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGG CGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGG GTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTC CGCGCACATTTCCCCGAAAAGTGCCACCTGACGTC SEQ ID NO: 32: Light chain encoding anti-VEGF light chain GACGGATCGGGAGATCTCCCGATCCCCTATGGTGCACTCTCAGTACAATCTGCTCTGATGC CGCATAGTTAAGCCAGTATCTGCTCCCTGCTTGTGTGTTGGAGGTCGCTGAGTAGTGCGCG AGCAAAATTTAAGCTACAACAAGGCAAGGCTTGACCGACAATTGCATGAAGAATCTGCTTA GGGTTAGGCGTTTTGCGCTGCTTCGCGATGTACGGGCCAGATATACGCGTTGACATTGATT ATTGACTAGTTATTAATAGTAATCAATTACGGGGTCATTAGTTCATAGCCCATATATGGAG TTCCGCGTTACATAACTTACGGTAAATGGCCCGCCTGGCTGACCGCCCAACGACCCCCGCC CATTGACGTCAATAATGACGTATGTTCCCATAGTAACGCCAATAGGGACTTTCCATTGACG TCAATGGGTGGAGTATTTACGGTAAACTGCCCACTTGGCAGTACATCAAGTGTATCATATG CCAAGTACGCCCCCTATTGACGTCAATGACGGTAAATGGCCCGCCTGGCATTATGCCCAGT ACATGACCTTATGGGACTTTCCTACTTGGCAGTACATCTACGTATTAGTCATCGCTATTAC CATGGTGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGA TTTCCAAGTCTCCACCCCATTGACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGG ACTTTCCAAAATGTCGTAACAACTCCGCCCCATTGACGCAAATGGGCGGTAGGCGTGTACG GTGGGAGGTCTATATAAGCAGAGCTCTCTGGCTAACTAGAGAACCCACTGCTTACTGGCTT ATCGAAATTAATACGACTCACTATAGGGAGACCCAAGCTGGCTAGCGTTTAAACTTAAGCT TGGTACCGAGCTCGGATCCACTAGTCCAGTGTGGTGGAATTCTGCAGATATCCAGCACAGT GGCGGCCGCCatgggatggagctgtatcatcctcttcttggtggcaacagctacaggcgtg cactccgacatccagctgacccagtccccctccagcctgtccgcctctgtgggcgacagag tgaccatcacctgttccgccagccaggacatctccaactacctgaactggtatcagcagaa gcccggcaaggcccccaaggtgctgatctacttcacctcctccctgcactccggcgtgccc tccagattctccggctctggctccggcaccgactttaccctgaccatctccagcctgcagc ccgaggacttcgccacctactactgccagcagtactccaccgtgccctggaccttcggcca gggcaccaaggtggaaatcaagcggaccgtggccgctccctccgtgttcatcttcccaccc tccgacgagcagctgaagtccggaaccgcctccgtcgtgtgcctgctgaacaacttctacc cccgcgaggccaaggtgcagtggaaggtggacaacgccctgcagagcggcaactcccagga atccgtcaccgagcaggactccaaggacagcacctactccctgtccagcaccctgaccctg tccaaggccgactacgagaagcacaaggtgtacgcctgcgaagtgacccaccagggcctca gctccccagtgaccaagtccttcaaccggggcgagtgctagtaaTCTAGAGGGCCCGTTTA AACCCGCTGATCAGCCTCGACTGTGCCTTCTAGTTGCCAGCCATCTGTTGTTTGCCCCTCC CCCGTGCCTTCCTTGACCCTGGAAGGTGCCACTCCCACTGTCCTTTCCTAATAAAATGAGG
AAATTGCATCGCATTGTCTGAGTAGGTGTCATTCTATTCTGGGGGGTGGGGTGGGGCAGGA CAGCAAGGGGGAGGATTGGGAAGACAATAGCAGGCATGCTGGGGATGCGGTGGGCTCTATG GCTTCTGAGGCGGAAAGAACCAGCTGGGGCTCTAGGGGGTATCCCCACGCGCCCTGTAGCG GCGCATTAAGCGCGGCGGGTGTGGTGGTTACGCGCAGCGTGACCGCTACACTTGCCAGCGC CCTAGCGCCCGCTCCTTTCGCTTTCTTCCCTTCCTTTCTCGCCACGTTCGCCGGCTTTCCC CGTCAAGCTCTAAATCGGGGGCTCCCTTTAGGGTTCCGATTTAGTGCTTTACGGCACCTCG ACCCCAAAAAACTTGATTAGGGTGATGGTTCACGTAGTGGGCCATCGCCCTGATAGACGGT TTTTCGCCCTTTGACGTTGGAGTCCACGTTCTTTAATAGTGGACTCTTGTTCCAAACTGGA ACAACACTCAACCCTATCTCGGTCTATTCTTTTGATTTATAAGGGATTTTGCCGATTTCGG CCTATTGGTTAAAAAATGAGCTGATTTAACAAAAATTTAACGCGAATTAATTCTGTGGAAT GTGTGTCAGTTAGGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGCAGAAGTATGCAAAGCA TGCATCTCAATTAGTCAGCAACCAGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGCAGAAG TATGCAAAGCATGCATCTCAATTAGTCAGCAACCATAGTCCCGCCCCTAACTCCGCCCATC CCGCCCCTAACTCCGCCCAGTTCCGCCCATTCTCCGCCCCATGGCTGACTAATTTTTTTTA TTTATGCAGAGGCCGAGGCCGCCTCTGCCTCTGAGCTATTCCAGAAGTAGTGAGGAGGCTT TTTTGGAGGCCTAGGCTTTTGCAAAAAGCTCCCGGGAGCTTGTATATCCATTTTCGGATCT GATCAAGAGACAGGATGAGGATCGTTTCGCATGATTGAACAAGATGGATTGCACGCAGGTT CTCCGGCCGCTTGGGTGGAGAGGCTATTCGGCTATGACTGGGCACAACAGACAATCGGCTG CTCTGATGCCGCCGTGTTCCGGCTGTCAGCGCAGGGGCGCCCGGTTCTTTTTGTCAAGACC GACCTGTCCGGTGCCCTGAATGAACTGCAGGACGAGGCAGCGCGGCTATCGTGGCTGGCCA CGACGGGCGTTCCTTGCGCAGCTGTGCTCGACGTTGTCACTGAAGCGGGAAGGGACTGGCT GCTATTGGGCGAAGTGCCGGGGCAGGATCTCCTGTCATCTCACCTTGCTCCTGCCGAGAAA GTATCCATCATGGCTGATGCAATGCGGCGGCTGCATACGCTTGATCCGGCTACCTGCCCAT TCGACCACCAAGCGAAACATCGCATCGAGCGAGCACGTACTCGGATGGAAGCCGGTCTTGT CGATCAGGATGATCTGGACGAAGAGCATCAGGGGCTCGCGCCAGCCGAACTGTTCGCCAGG CTCAAGGCGCGCATGCCCGACGGCGAGGATCTCGTCGTGACCCATGGCGATGCCTGCTTGC CGAATATCATGGTGGAAAATGGCCGCTTTTCTGGATTCATCGACTGTGGCCGGCTGGGTGT GGCGGACCGCTATCAGGACATAGCGTTGGCTACCCGTGATATTGCTGAAGAGCTTGGCGGC GAATGGGCTGACCGCTTCCTCGTGCTTTACGGTATCGCCGCTCCCGATTCGCAGCGCATCG CCTTCTATCGCCTTCTTGACGAGTTCTTCTGAGCGGGACTCTGGGGTTCGAAATGACCGAC CAAGCGACGCCCAACCTGCCATCACGAGATTTCGATTCCACCGCCGCCTTCTATGAAAGGT TGGGCTTCGGAATCGTTTTCCGGGACGCCGGCTGGATGATCCTCCAGCGCGGGGATCTCAT GCTGGAGTTCTTCGCCCACCCCAACTTGTTTATTGCAGCTTATAATGGTTACAAATAAAGC AATAGCATCACAAATTTCACAAATAAAGCATTTTTTTCACTGCATTCTAGTTGTGGTTTGT CCAAACTCATCAATGTATCTTATCATGTCTGTATACCGTCGACCTCTAGCTAGAGCTTGGC GTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAAC ATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACAT TAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTA ATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCG CTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGG CGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGG CCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGC CCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGAC TATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCT GCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGC TCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACG AACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCC GGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGG TATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAA CAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTC TTGATCCGGCAAACAAACCACCGCTGGTAGCGGTTTTTTTGTTTGCAAGCAGCAGATTACG CGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGT GGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTA GATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGG TCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTT CATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATC TGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCA ATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCA TCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCG CAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCA TTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAG CGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACT CATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCT GTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCT CTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCAT CATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGT TCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTT CTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAA ATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGT CTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCA CATTTCCCCGAAAAGTGCCACCTGACGTC
Sequence CWU 1
1
561506PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 1Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val
Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala
Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met
Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr
Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn
Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr
Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr
Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu 115 120
125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln
130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys
Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala
Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser
Val Asn Ala Val Gln Thr Val 180 185 190Val Arg Gln Gly Glu Asn Ile
Thr Leu Met Cys Ile Val Ile Gly Asn 195 200 205Glu Val Val Asn Phe
Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg 210 215 220Leu Val Glu
Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile225 230 235
240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr
245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val Asn Asp His Gln Asp
Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val Glu Ser Gly Gly Gly
Gly Gly Ser Gly 275 280 285Gly Gly Gly Ser Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser 290 295 300Ala Ser Val Gly Asp Arg Val Thr
Ile Thr Cys Ser Ala Ser Gln Asp305 310 315 320Ile Ser Asn Tyr Leu
Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro 325 330 335Lys Val Leu
Ile Tyr Phe Thr Ser Ser Leu His Ser Gly Val Pro Ser 340 345 350Arg
Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser 355 360
365Ser Leu Gln Pro Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Tyr Ser
370 375 380Thr Val Pro Trp Thr Phe Gly Gln Gly Thr Lys Val Glu Ile
Lys Arg385 390 395 400Thr Val Ala Ala Pro Ser Val Phe Ile Phe Pro
Pro Ser Asp Glu Gln 405 410 415Leu Lys Ser Gly Thr Ala Ser Val Val
Cys Leu Leu Asn Asn Phe Tyr 420 425 430Pro Arg Glu Ala Lys Val Gln
Trp Lys Val Asp Asn Ala Leu Gln Ser 435 440 445Gly Asn Ser Gln Glu
Ser Val Thr Glu Gln Asp Ser Lys Asp Ser Thr 450 455 460Tyr Ser Leu
Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys465 470 475
480His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro
485 490 495Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 500
5052453PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 2Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Tyr Thr Phe Thr Asn Tyr 20 25 30Gly Met Asn Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Trp Ile Asn Thr Tyr Thr Gly
Glu Pro Thr Tyr Ala Ala Asp Phe 50 55 60Lys Arg Arg Phe Thr Phe Ser
Leu Asp Thr Ser Lys Ser Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val 100 105 110Trp Gly
Gln Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly 115 120
125Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly
130 135 140Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu
Pro Val145 150 155 160Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser
Gly Val His Thr Phe 165 170 175Pro Ala Val Leu Gln Ser Ser Gly Leu
Tyr Ser Leu Ser Ser Val Val 180 185 190Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr Ile Cys Asn Val 195 200 205Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys 210 215 220Ser Cys Asp
Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu225 230 235
240Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr
245 250 255Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Val
Asp Val 260 265 270Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr
Val Asp Gly Val 275 280 285Glu Val His Asn Ala Lys Thr Lys Pro Arg
Glu Glu Gln Tyr Asn Ser 290 295 300Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His Gln Asp Trp Leu305 310 315 320Asn Gly Lys Glu Tyr
Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala 325 330 335Pro Ile Glu
Lys Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro 340 345 350Gln
Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met Thr Lys Asn Gln 355 360
365Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala
370 375 380Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys
Thr Thr385 390 395 400Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe
Leu Tyr Ser Lys Leu 405 410 415Thr Val Asp Lys Ser Arg Trp Gln Gln
Gly Asn Val Phe Ser Cys Ser 420 425 430Val Met His Glu Ala Leu His
Asn His Tyr Thr Gln Lys Ser Leu Ser 435 440 445Leu Ser Pro Gly Lys
4503498PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 3Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val
Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala
Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met
Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr
Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn
Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr
Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr
Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu 115 120
125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln
130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys
Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala
Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser
Val Asn Ala Val Gln Thr Val 180 185 190Val Arg Gln Gly Glu Asn Ile
Thr Leu Met Cys Ile Val Ile Gly Asn 195 200 205Glu Val Val Asn Phe
Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg 210 215 220Leu Val Glu
Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile225 230 235
240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr
245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val Asn Asp His Gln Asp
Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val Glu Ser Gly Gly Gly
Asp Ile Gln Met 275 280 285Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser
Val Gly Asp Arg Val Thr 290 295 300Ile Thr Cys Ser Ala Ser Gln Asp
Ile Ser Asn Tyr Leu Asn Trp Tyr305 310 315 320Gln Gln Lys Pro Gly
Lys Ala Pro Lys Val Leu Ile Tyr Phe Thr Ser 325 330 335Ser Leu His
Ser Gly Val Pro Ser Arg Phe Ser Gly Ser Gly Ser Gly 340 345 350Thr
Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro Glu Asp Phe Ala 355 360
365Thr Tyr Tyr Cys Gln Gln Tyr Ser Thr Val Pro Trp Thr Phe Gly Gln
370 375 380Gly Thr Lys Val Glu Ile Lys Arg Thr Val Ala Ala Pro Ser
Val Phe385 390 395 400Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser
Gly Thr Ala Ser Val 405 410 415Val Cys Leu Leu Asn Asn Phe Tyr Pro
Arg Glu Ala Lys Val Gln Trp 420 425 430Lys Val Asp Asn Ala Leu Gln
Ser Gly Asn Ser Gln Glu Ser Val Thr 435 440 445Glu Gln Asp Ser Lys
Asp Ser Thr Tyr Ser Leu Ser Ser Thr Leu Thr 450 455 460Leu Ser Lys
Ala Asp Tyr Glu Lys His Lys Val Tyr Ala Cys Glu Val465 470 475
480Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys Ser Phe Asn Arg Gly
485 490 495Glu Cys4745PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 4Leu Val Val Thr Pro Pro
Gly Pro Glu Leu Val Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr
Cys Ser Gly Ser Ala Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu
Pro Pro Gln Glu Met Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser
Val Leu Thr Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr
Phe Cys Thr His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75
80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu
85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr
Glu 100 105 110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val
Val Thr Leu 115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val
Pro Tyr Asp His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp
Arg Ser Tyr Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu
Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser
Ser Ile Asn Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg
Gln Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200
205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg
210 215 220Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr
His Ile225 230 235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu
Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val
Asn Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val
Glu Ser Gly Gly Gly Gly Gly Ser Gly 275 280 285Gly Gly Gly Ser Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val 290 295 300Gln Pro Gly
Gly Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Tyr Thr305 310 315
320Phe Thr Asn Tyr Gly Met Asn Trp Val Arg Gln Ala Pro Gly Lys Gly
325 330 335Leu Glu Trp Val Gly Trp Ile Asn Thr Tyr Thr Gly Glu Pro
Thr Tyr 340 345 350Ala Ala Asp Phe Lys Arg Arg Phe Thr Phe Ser Leu
Asp Thr Ser Lys 355 360 365Ser Thr Ala Tyr Leu Gln Met Asn Ser Leu
Arg Ala Glu Asp Thr Ala 370 375 380Val Tyr Tyr Cys Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp385 390 395 400Tyr Phe Asp Val Trp
Gly Gln Gly Thr Leu Val Thr Val Ser Ser Ala 405 410 415Ser Thr Lys
Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser 420 425 430Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe 435 440
445Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly
450 455 460Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr
Ser Leu465 470 475 480Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr 485 490 495Ile Cys Asn Val Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys 500 505 510Val Glu Pro Lys Ser Cys Asp
Lys Thr His Thr Cys Pro Pro Cys Pro 515 520 525Ala Pro Glu Leu Leu
Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys 530 535 540Pro Lys Asp
Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val545 550 555
560Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr
565 570 575Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg
Glu Glu 580 585 590Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His 595 600 605Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 610 615 620Ala Leu Pro Ala Pro Ile Glu Lys
Thr Ile Ser Lys Ala Lys Gly Gln625 630 635 640Pro Arg Glu Pro Gln
Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met 645 650 655Thr Lys Asn
Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro 660 665 670Ser
Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn 675 680
685Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
690 695 700Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val705 710 715 720Phe Ser Cys Ser Val Met His Glu Ala Leu His
Asn His Tyr Thr Gln 725 730 735Lys Ser Leu Ser Leu Ser Pro Gly Lys
740 7455214PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 5Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu
Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Ser Ala Ser
Gln Asp Ile Ser Asn Tyr 20 25 30Leu Asn Trp Tyr Gln Gln Lys Pro Gly
Lys Ala Pro Lys Val Leu Ile 35 40 45Tyr Phe Thr Ser Ser Leu His Ser
Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Gly Ser Gly Thr Asp Phe
Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr
Tyr Tyr Cys Gln Gln Tyr Ser Thr Val Pro Trp 85 90 95Thr Phe Gly Gln
Gly Thr Lys Val Glu Ile Lys Arg Thr Val Ala Ala 100 105 110Pro Ser
Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser Gly 115 120
125Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala
130 135 140Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln Ser Gly Asn
Ser Gln145 150 155 160Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser
Thr Tyr Ser Leu Ser 165 170 175Ser Thr Leu Thr Leu Ser Lys Ala Asp
Tyr Glu Lys His Lys Val Tyr 180 185 190Ala Cys Glu Val Thr His Gln
Gly Leu Ser Ser Pro Val Thr Lys Ser 195 200 205Phe Asn Arg Gly
Glu Cys 2106737PRTArtificial SequenceDescription of Artificial
Sequence Synthetic polypeptide 6Leu Val Val Thr Pro Pro Gly Pro Glu
Leu Val Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly
Ser Ala Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln
Glu Met Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr
Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr
His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg
Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn
Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100 105
110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu
115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp
His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr
Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp Ser
Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile Asn
Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg Gln Gly Glu
Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200 205Glu Val Val
Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg 210 215 220Leu
Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile225 230
235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly
Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val Asn Asp His Gln
Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val Glu Ser Gly Gly
Gly Glu Val Gln Leu 275 280 285Val Glu Ser Gly Gly Gly Leu Val Gln
Pro Gly Gly Ser Leu Arg Leu 290 295 300Ser Cys Ala Ala Ser Gly Tyr
Thr Phe Thr Asn Tyr Gly Met Asn Trp305 310 315 320Val Arg Gln Ala
Pro Gly Lys Gly Leu Glu Trp Val Gly Trp Ile Asn 325 330 335Thr Tyr
Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe Lys Arg Arg Phe 340 345
350Thr Phe Ser Leu Asp Thr Ser Lys Ser Thr Ala Tyr Leu Gln Met Asn
355 360 365Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Lys
Tyr Pro 370 375 380His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val
Trp Gly Gln Gly385 390 395 400Thr Leu Val Thr Val Ser Ser Ala Ser
Thr Lys Gly Pro Ser Val Phe 405 410 415Pro Leu Ala Pro Ser Ser Lys
Ser Thr Ser Gly Gly Thr Ala Ala Leu 420 425 430Gly Cys Leu Val Lys
Asp Tyr Phe Pro Glu Pro Val Thr Val Ser Trp 435 440 445Asn Ser Gly
Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val Leu 450 455 460Gln
Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val Pro Ser465 470
475 480Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val Asn His Lys
Pro 485 490 495Ser Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys Ser
Cys Asp Lys 500 505 510Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu
Leu Leu Gly Gly Pro 515 520 525Ser Val Phe Leu Phe Pro Pro Lys Pro
Lys Asp Thr Leu Met Ile Ser 530 535 540Arg Thr Pro Glu Val Thr Cys
Val Val Val Asp Val Ser His Glu Asp545 550 555 560Pro Glu Val Lys
Phe Asn Trp Tyr Val Asp Gly Val Glu Val His Asn 565 570 575Ala Lys
Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg Val 580 585
590Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly Lys Glu
595 600 605Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala Pro Ile
Glu Lys 610 615 620Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro
Gln Val Tyr Thr625 630 635 640Leu Pro Pro Ser Arg Glu Glu Met Thr
Lys Asn Gln Val Ser Leu Thr 645 650 655Cys Leu Val Lys Gly Phe Tyr
Pro Ser Asp Ile Ala Val Glu Trp Glu 660 665 670Ser Asn Gly Gln Pro
Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val Leu 675 680 685Asp Ser Asp
Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp Lys 690 695 700Ser
Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met His Glu705 710
715 720Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro
Gly 725 730 735Lys7755PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 7Glu Val Gln Leu Val Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser
Cys Ala Ala Ser Gly Tyr Thr Phe Thr Asn Tyr 20 25 30Gly Met Asn Trp
Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Trp Ile
Asn Thr Tyr Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe 50 55 60Lys Arg
Arg Phe Thr Phe Ser Leu Asp Thr Ser Lys Ser Thr Ala Tyr65 70 75
80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Lys Tyr Pro His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp
Val 100 105 110Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser Ala Ser
Thr Lys Gly 115 120 125Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys
Ser Thr Ser Gly Gly 130 135 140Thr Ala Ala Leu Gly Cys Leu Val Lys
Asp Tyr Phe Pro Glu Pro Val145 150 155 160Thr Val Ser Trp Asn Ser
Gly Ala Leu Thr Ser Gly Val His Thr Phe 165 170 175Pro Ala Val Leu
Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val 180 185 190Thr Val
Pro Ser Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val 195 200
205Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys
210 215 220Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro
Glu Leu225 230 235 240Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro
Lys Pro Lys Asp Thr 245 250 255Leu Met Ile Ser Arg Thr Pro Glu Val
Thr Cys Val Val Val Asp Val 260 265 270Ser His Glu Asp Pro Glu Val
Lys Phe Asn Trp Tyr Val Asp Gly Val 275 280 285Glu Val His Asn Ala
Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser 290 295 300Thr Tyr Arg
Val Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu305 310 315
320Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala
325 330 335Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg
Glu Pro 340 345 350Gln Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met
Thr Lys Asn Gln 355 360 365Val Ser Leu Thr Cys Leu Val Lys Gly Phe
Tyr Pro Ser Asp Ile Ala 370 375 380Val Glu Trp Glu Ser Asn Gly Gln
Pro Glu Asn Asn Tyr Lys Thr Thr385 390 395 400Pro Pro Val Leu Asp
Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu 405 410 415Thr Val Asp
Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser 420 425 430Val
Met His Glu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser 435 440
445Leu Ser Pro Gly Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly
450 455 460Gly Gly Ser Gly Gly Gly Gly Ser Gly Leu Val Val Thr Pro
Pro Gly465 470 475 480Pro Glu Leu Val Leu Asn Val Ser Ser Thr Phe
Val Leu Thr Cys Ser 485 490 495Gly Ser Ala Pro Val Val Trp Glu Arg
Met Ser Gln Glu Pro Pro Gln 500 505 510Glu Met Ala Lys Ala Gln Asp
Gly Thr Phe Ser Ser Val Leu Thr Leu 515 520 525Thr Asn Leu Thr Gly
Leu Asp Thr Gly Glu Tyr Phe Cys Thr His Asn 530 535 540Asp Ser Arg
Gly Leu Glu Thr Asp Glu Arg Lys Arg Leu Tyr Ile Phe545 550 555
560Val Pro Asp Pro Thr Val Gly Phe Leu Pro Asn Asp Ala Glu Glu Leu
565 570 575Phe Ile Phe Leu Thr Glu Ile Thr Glu Ile Thr Ile Pro Cys
Arg Val 580 585 590Thr Asp Pro Gln Leu Val Val Thr Leu His Glu Lys
Lys Gly Asp Val 595 600 605Ala Leu Pro Val Pro Tyr Asp His Gln Arg
Gly Phe Ser Gly Ile Phe 610 615 620Glu Asp Arg Ser Tyr Ile Cys Lys
Thr Thr Ile Gly Asp Arg Glu Val625 630 635 640Asp Ser Asp Ala Tyr
Tyr Val Tyr Arg Leu Gln Val Ser Ser Ile Asn 645 650 655Val Ser Val
Asn Ala Val Gln Thr Val Val Arg Gln Gly Glu Asn Ile 660 665 670Thr
Leu Met Cys Ile Val Ile Gly Asn Glu Val Val Asn Phe Glu Trp 675 680
685Thr Tyr Pro Arg Lys Glu Ser Gly Arg Leu Val Glu Pro Val Thr Asp
690 695 700Phe Leu Leu Asp Met Pro Tyr His Ile Arg Ser Ile Leu His
Ile Pro705 710 715 720Ser Ala Glu Leu Glu Asp Ser Gly Thr Tyr Thr
Cys Asn Val Thr Glu 725 730 735Ser Val Asn Asp His Gln Asp Glu Lys
Ala Ile Asn Ile Thr Val Val 740 745 750Glu Ser Gly
7558745PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 8Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val
Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala
Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met
Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr
Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn
Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr
Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr
Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu 115 120
125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln
130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys
Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala
Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser
Val Asn Ala Val Gln Thr Val 180 185 190Val Arg Gln Gly Glu Asn Ile
Thr Leu Met Cys Ile Val Ile Gly Asn 195 200 205Glu Val Val Asn Phe
Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg 210 215 220Leu Val Glu
Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile225 230 235
240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr
245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val Asn Asp His Gln Asp
Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val Glu Ser Gly Gly Gly
Gly Gly Ser Gly 275 280 285Gly Gly Gly Ser Glu Val Gln Leu Val Glu
Ser Gly Gly Gly Leu Val 290 295 300Gln Pro Gly Gly Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Tyr Asp305 310 315 320Phe Thr His Tyr Gly
Met Asn Trp Val Arg Gln Ala Pro Gly Lys Gly 325 330 335Leu Glu Trp
Val Gly Trp Ile Asn Thr Tyr Thr Gly Glu Pro Thr Tyr 340 345 350Ala
Ala Asp Phe Lys Arg Arg Phe Thr Phe Ser Leu Asp Thr Ser Lys 355 360
365Ser Thr Ala Tyr Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
370 375 380Val Tyr Tyr Cys Ala Lys Tyr Pro Tyr Tyr Tyr Gly Thr Ser
His Trp385 390 395 400Tyr Phe Asp Val Trp Gly Gln Gly Thr Leu Val
Thr Val Ser Ser Ala 405 410 415Ser Thr Lys Gly Pro Ser Val Phe Pro
Leu Ala Pro Ser Ser Lys Ser 420 425 430Thr Ser Gly Gly Thr Ala Ala
Leu Gly Cys Leu Val Lys Asp Tyr Phe 435 440 445Pro Glu Pro Val Thr
Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly 450 455 460Val His Thr
Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser Leu465 470 475
480Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln Thr Tyr
485 490 495Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys Val Asp
Lys Lys 500 505 510Val Glu Pro Lys Ser Cys Asp Lys Thr His Thr Cys
Pro Pro Cys Pro 515 520 525Ala Pro Glu Ala Ala Gly Ala Pro Ser Val
Phe Leu Phe Pro Pro Lys 530 535 540Pro Lys Asp Thr Leu Met Ile Ser
Arg Thr Pro Glu Val Thr Cys Val545 550 555 560Val Val Asp Val Ser
His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr 565 570 575Val Asp Gly
Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu 580 585 590Gln
Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His 595 600
605Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys
610 615 620Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys
Gly Gln625 630 635 640Pro Arg Glu Pro Cys Val Tyr Thr Leu Pro Pro
Ser Arg Glu Glu Met 645 650 655Thr Lys Asn Gln Val Ser Leu Thr Cys
Leu Val Lys Gly Phe Tyr Pro 660 665 670Ser Asp Ile Ala Val Glu Trp
Glu Ser Asn Gly Gln Pro Glu Asn Asn 675 680 685Tyr Lys Thr Thr Pro
Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu 690 695 700Tyr Ser Lys
Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn Val705 710 715
720Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr Thr Gln
725 730 735Lys Ser Leu Ser Leu Ser Pro Gly Lys 740
7459745PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 9Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val
Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala
Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met
Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr
Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn
Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr
Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr
Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu 115 120
125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln
130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys
Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala
Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser
Val Asn Ala Val Gln Thr Val
180 185 190Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile
Gly Asn 195 200 205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys
Glu Ser Gly Arg 210 215 220Leu Val Glu Pro Val Thr Asp Phe Leu Leu
Asp Met Pro Tyr His Ile225 230 235 240Arg Ser Ile Leu His Ile Pro
Ser Ala Glu Leu Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val
Thr Glu Ser Val Asn Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn
Ile Thr Val Val Glu Ser Gly Gly Gly Gly Gly Ser Gly 275 280 285Gly
Gly Gly Ser Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val 290 295
300Gln Pro Gly Gly Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Tyr
Asp305 310 315 320Phe Thr His Tyr Gly Met Asn Trp Val Arg Gln Ala
Pro Gly Lys Gly 325 330 335Leu Glu Trp Val Gly Trp Ile Asn Thr Tyr
Thr Gly Glu Pro Thr Tyr 340 345 350Ala Ala Asp Phe Lys Arg Arg Phe
Thr Phe Ser Leu Asp Thr Ser Lys 355 360 365Ser Thr Ala Tyr Leu Gln
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala 370 375 380Val Tyr Tyr Cys
Ala Lys Tyr Pro Tyr Tyr Tyr Gly Thr Ser His Trp385 390 395 400Tyr
Phe Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser Ala 405 410
415Ser Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser
420 425 430Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp
Tyr Phe 435 440 445Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala
Leu Thr Ser Gly 450 455 460Val His Thr Phe Pro Ala Val Leu Gln Ser
Ser Gly Leu Tyr Ser Leu465 470 475 480Ser Ser Val Val Thr Val Pro
Ser Ser Ser Leu Gly Thr Gln Thr Tyr 485 490 495Ile Cys Asn Val Asn
His Lys Pro Ser Asn Thr Lys Val Asp Lys Lys 500 505 510Val Glu Pro
Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro 515 520 525Ala
Pro Glu Ala Ala Gly Ala Pro Ser Val Phe Leu Phe Pro Pro Lys 530 535
540Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val545 550 555 560Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys
Phe Asn Trp Tyr 565 570 575Val Asp Gly Val Glu Val His Asn Ala Lys
Thr Lys Pro Arg Glu Glu 580 585 590Gln Tyr Asn Ser Thr Tyr Arg Val
Val Ser Val Leu Thr Val Leu His 595 600 605Gln Asp Trp Leu Asn Gly
Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys 610 615 620Ala Leu Pro Ala
Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln625 630 635 640Pro
Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met 645 650
655Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro
660 665 670Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu
Asn Asn 675 680 685Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly
Ser Phe Phe Leu 690 695 700Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg
Trp Gln Gln Gly Asn Val705 710 715 720Phe Ser Cys Ser Val Met His
Glu Ala Leu His Asn His Tyr Thr Gln 725 730 735Lys Ser Leu Ser Cys
Ser Pro Gly Lys 740 74510214PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 10Asp Ile Gln Leu Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile
Thr Cys Ser Ala Ser Gln Asp Ile Ser Asn Tyr 20 25 30Leu Asn Trp Tyr
Gln Gln Lys Pro Gly Lys Ala Pro Lys Val Leu Ile 35 40 45Tyr Phe Thr
Ser Ser Leu His Ser Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Gly
Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75
80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Tyr Ser Thr Val Pro Trp
85 90 95Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys Arg Thr Val Ala
Ala 100 105 110Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu
Lys Ser Gly 115 120 125Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe
Tyr Pro Arg Glu Ala 130 135 140Lys Val Gln Trp Lys Val Asp Asn Ala
Leu Gln Ser Gly Asn Ser Gln145 150 155 160Glu Ser Val Thr Glu Gln
Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser 165 170 175Ser Thr Leu Thr
Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val Tyr 180 185 190Ala Cys
Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys Ser 195 200
205Phe Asn Arg Gly Glu Cys 210111106PRTHomo sapiens 11Met Arg Leu
Pro Gly Ala Met Pro Ala Leu Ala Leu Lys Gly Glu Leu1 5 10 15Leu Leu
Leu Ser Leu Leu Leu Leu Leu Glu Pro Gln Ile Ser Gln Gly 20 25 30Leu
Val Val Thr Pro Pro Gly Pro Glu Leu Val Leu Asn Val Ser Ser 35 40
45Thr Phe Val Leu Thr Cys Ser Gly Ser Ala Pro Val Val Trp Glu Arg
50 55 60Met Ser Gln Glu Pro Pro Gln Glu Met Ala Lys Ala Gln Asp Gly
Thr65 70 75 80Phe Ser Ser Val Leu Thr Leu Thr Asn Leu Thr Gly Leu
Asp Thr Gly 85 90 95Glu Tyr Phe Cys Thr His Asn Asp Ser Arg Gly Leu
Glu Thr Asp Glu 100 105 110Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp
Pro Thr Val Gly Phe Leu 115 120 125Pro Asn Asp Ala Glu Glu Leu Phe
Ile Phe Leu Thr Glu Ile Thr Glu 130 135 140Ile Thr Ile Pro Cys Arg
Val Thr Asp Pro Gln Leu Val Val Thr Leu145 150 155 160His Glu Lys
Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln 165 170 175Arg
Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys Lys Thr 180 185
190Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg
195 200 205Leu Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala Val Gln
Thr Val 210 215 220Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile
Val Ile Gly Asn225 230 235 240Glu Val Val Asn Phe Glu Trp Thr Tyr
Pro Arg Lys Glu Ser Gly Arg 245 250 255Leu Val Glu Pro Val Thr Asp
Phe Leu Leu Asp Met Pro Tyr His Ile 260 265 270Arg Ser Ile Leu His
Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr 275 280 285Tyr Thr Cys
Asn Val Thr Glu Ser Val Asn Asp His Gln Asp Glu Lys 290 295 300Ala
Ile Asn Ile Thr Val Val Glu Ser Gly Tyr Val Arg Leu Leu Gly305 310
315 320Glu Val Gly Thr Leu Gln Phe Ala Glu Leu His Arg Ser Arg Thr
Leu 325 330 335Gln Val Val Phe Glu Ala Tyr Pro Pro Pro Thr Val Leu
Trp Phe Lys 340 345 350Asp Asn Arg Thr Leu Gly Asp Ser Ser Ala Gly
Glu Ile Ala Leu Ser 355 360 365Thr Arg Asn Val Ser Glu Thr Arg Tyr
Val Ser Glu Leu Thr Leu Val 370 375 380Arg Val Lys Val Ala Glu Ala
Gly His Tyr Thr Met Arg Ala Phe His385 390 395 400Glu Asp Ala Glu
Val Gln Leu Ser Phe Gln Leu Gln Ile Asn Val Pro 405 410 415Val Arg
Val Leu Glu Leu Ser Glu Ser His Pro Asp Ser Gly Glu Gln 420 425
430Thr Val Arg Cys Arg Gly Arg Gly Met Pro Gln Pro Asn Ile Ile Trp
435 440 445Ser Ala Cys Arg Asp Leu Lys Arg Cys Pro Arg Glu Leu Pro
Pro Thr 450 455 460Leu Leu Gly Asn Ser Ser Glu Glu Glu Ser Gln Leu
Glu Thr Asn Val465 470 475 480Thr Tyr Trp Glu Glu Glu Gln Glu Phe
Glu Val Val Ser Thr Leu Arg 485 490 495Leu Gln His Val Asp Arg Pro
Leu Ser Val Arg Cys Thr Leu Arg Asn 500 505 510Ala Val Gly Gln Asp
Thr Gln Glu Val Ile Val Val Pro His Ser Leu 515 520 525Pro Phe Lys
Val Val Val Ile Ser Ala Ile Leu Ala Leu Val Val Leu 530 535 540Thr
Ile Ile Ser Leu Ile Ile Leu Ile Met Leu Trp Gln Lys Lys Pro545 550
555 560Arg Tyr Glu Ile Arg Trp Lys Val Ile Glu Ser Val Ser Ser Asp
Gly 565 570 575His Glu Tyr Ile Tyr Val Asp Pro Met Gln Leu Pro Tyr
Asp Ser Thr 580 585 590Trp Glu Leu Pro Arg Asp Gln Leu Val Leu Gly
Arg Thr Leu Gly Ser 595 600 605Gly Ala Phe Gly Gln Val Val Glu Ala
Thr Ala His Gly Leu Ser His 610 615 620Ser Gln Ala Thr Met Lys Val
Ala Val Lys Met Leu Lys Ser Thr Ala625 630 635 640Arg Ser Ser Glu
Lys Gln Ala Leu Met Ser Glu Leu Lys Ile Met Ser 645 650 655His Leu
Gly Pro His Leu Asn Val Val Asn Leu Leu Gly Ala Cys Thr 660 665
670Lys Gly Gly Pro Ile Tyr Ile Ile Thr Glu Tyr Cys Arg Tyr Gly Asp
675 680 685Leu Val Asp Tyr Leu His Arg Asn Lys His Thr Phe Leu Gln
His His 690 695 700Ser Asp Lys Arg Arg Pro Pro Ser Ala Glu Leu Tyr
Ser Asn Ala Leu705 710 715 720Pro Val Gly Leu Pro Leu Pro Ser His
Val Ser Leu Thr Gly Glu Ser 725 730 735Asp Gly Gly Tyr Met Asp Met
Ser Lys Asp Glu Ser Val Asp Tyr Val 740 745 750Pro Met Leu Asp Met
Lys Gly Asp Val Lys Tyr Ala Asp Ile Glu Ser 755 760 765Ser Asn Tyr
Met Ala Pro Tyr Asp Asn Tyr Val Pro Ser Ala Pro Glu 770 775 780Arg
Thr Cys Arg Ala Thr Leu Ile Asn Glu Ser Pro Val Leu Ser Tyr785 790
795 800Met Asp Leu Val Gly Phe Ser Tyr Gln Val Ala Asn Gly Met Glu
Phe 805 810 815Leu Ala Ser Lys Asn Cys Val His Arg Asp Leu Ala Ala
Arg Asn Val 820 825 830Leu Ile Cys Glu Gly Lys Leu Val Lys Ile Cys
Asp Phe Gly Leu Ala 835 840 845Arg Asp Ile Met Arg Asp Ser Asn Tyr
Ile Ser Lys Gly Ser Thr Phe 850 855 860Leu Pro Leu Lys Trp Met Ala
Pro Glu Ser Ile Phe Asn Ser Leu Tyr865 870 875 880Thr Thr Leu Ser
Asp Val Trp Ser Phe Gly Ile Leu Leu Trp Glu Ile 885 890 895Phe Thr
Leu Gly Gly Thr Pro Tyr Pro Glu Leu Pro Met Asn Glu Gln 900 905
910Phe Tyr Asn Ala Ile Lys Arg Gly Tyr Arg Met Ala Gln Pro Ala His
915 920 925Ala Ser Asp Glu Ile Tyr Glu Ile Met Gln Lys Cys Trp Glu
Glu Lys 930 935 940Phe Glu Ile Arg Pro Pro Phe Ser Gln Leu Val Leu
Leu Leu Glu Arg945 950 955 960Leu Leu Gly Glu Gly Tyr Lys Lys Lys
Tyr Gln Gln Val Asp Glu Glu 965 970 975Phe Leu Arg Ser Asp His Pro
Ala Ile Leu Arg Ser Gln Ala Arg Leu 980 985 990Pro Gly Phe His Gly
Leu Arg Ser Pro Leu Asp Thr Ser Ser Val Leu 995 1000 1005Tyr Thr
Ala Val Gln Pro Asn Glu Gly Asp Asn Asp Tyr Ile Ile 1010 1015
1020Pro Leu Pro Asp Pro Lys Pro Glu Val Ala Asp Glu Gly Pro Leu
1025 1030 1035Glu Gly Ser Pro Ser Leu Ala Ser Ser Thr Leu Asn Glu
Val Asn 1040 1045 1050Thr Ser Ser Thr Ile Ser Cys Asp Ser Pro Leu
Glu Pro Gln Asp 1055 1060 1065Glu Pro Glu Pro Glu Pro Gln Leu Glu
Leu Gln Val Glu Pro Glu 1070 1075 1080Pro Glu Leu Glu Gln Leu Pro
Asp Ser Gly Cys Pro Ala Pro Arg 1085 1090 1095Ala Glu Ala Glu Asp
Ser Phe Leu 1100 110512214PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 12Asp Ile Gln Leu Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile
Thr Cys Ser Ala Ser Gln Asp Ile Ser Asn Tyr 20 25 30Leu Asn Trp Tyr
Gln Gln Lys Pro Gly Lys Ala Pro Lys Val Leu Ile 35 40 45Tyr Phe Thr
Ser Ser Leu His Ser Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Gly
Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75
80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Tyr Ser Thr Val Pro Trp
85 90 95Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys Arg Thr Val Ala
Ala 100 105 110Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu
Lys Ser Gly 115 120 125Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe
Tyr Pro Arg Glu Ala 130 135 140Lys Val Gln Trp Lys Val Asp Asn Ala
Leu Gln Ser Gly Asn Ser Gln145 150 155 160Glu Ser Val Thr Glu Gln
Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser 165 170 175Ser Thr Leu Thr
Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val Tyr 180 185 190Ala Cys
Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys Ser 195 200
205Phe Asn Arg Gly Glu Cys 21013231PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
13Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Tyr Asp Phe Thr His
Tyr 20 25 30Gly Met Asn Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Gly Trp Ile Asn Thr Tyr Thr Gly Glu Pro Thr Tyr Ala
Ala Asp Phe 50 55 60Lys Arg Arg Phe Thr Phe Ser Leu Asp Thr Ser Lys
Ser Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95Ala Lys Tyr Pro Tyr Tyr Tyr Gly Thr
Ser His Trp Tyr Phe Asp Val 100 105 110Trp Gly Gln Gly Thr Leu Val
Thr Val Ser Ser Ala Ser Thr Lys Gly 115 120 125Pro Ser Val Phe Pro
Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly 130 135 140Thr Ala Ala
Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val145 150 155
160Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe
165 170 175Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser
Val Val 180 185 190Thr Val Pro Ser Ser Ser Leu Gly Thr Gln Thr Tyr
Ile Cys Asn Val 195 200 205Asn His Lys Pro Ser Asn Thr Lys Val Asp
Lys Lys Val Glu Pro Lys 210 215 220Ser Cys Asp Lys Thr His Leu225
230141338PRTHomo sapiens 14Met Val Ser Tyr Trp Asp Thr Gly Val Leu
Leu Cys Ala Leu Leu Ser1 5 10 15Cys Leu Leu Leu Thr Gly Ser Ser Ser
Gly Ser Lys Leu Lys Asp Pro 20 25 30Glu Leu Ser Leu Lys Gly Thr Gln
His Ile Met Gln Ala Gly Gln Thr 35 40 45Leu His Leu Gln Cys Arg Gly
Glu Ala Ala His Lys Trp Ser Leu Pro 50 55 60Glu Met Val Ser Lys Glu
Ser Glu Arg Leu Ser Ile Thr Lys Ser Ala65 70 75 80Cys Gly Arg Asn
Gly Lys Gln Phe Cys Ser Thr Leu Thr Leu Asn Thr 85 90 95Ala Gln Ala
Asn His Thr Gly Phe Tyr Ser Cys Lys Tyr Leu Ala Val
100 105 110Pro Thr Ser Lys Lys Lys Glu Thr Glu Ser Ala Ile Tyr Ile
Phe Ile 115 120 125Ser Asp Thr Gly Arg Pro Phe Val Glu Met Tyr Ser
Glu Ile Pro Glu 130 135 140Ile Ile His Met Thr Glu Gly Arg Glu Leu
Val Ile Pro Cys Arg Val145 150 155 160Thr Ser Pro Asn Ile Thr Val
Thr Leu Lys Lys Phe Pro Leu Asp Thr 165 170 175Leu Ile Pro Asp Gly
Lys Arg Ile Ile Trp Asp Ser Arg Lys Gly Phe 180 185 190Ile Ile Ser
Asn Ala Thr Tyr Lys Glu Ile Gly Leu Leu Thr Cys Glu 195 200 205Ala
Thr Val Asn Gly His Leu Tyr Lys Thr Asn Tyr Leu Thr His Arg 210 215
220Gln Thr Asn Thr Ile Ile Asp Val Gln Ile Ser Thr Pro Arg Pro
Val225 230 235 240Lys Leu Leu Arg Gly His Thr Leu Val Leu Asn Cys
Thr Ala Thr Thr 245 250 255Pro Leu Asn Thr Arg Val Gln Met Thr Trp
Ser Tyr Pro Asp Glu Lys 260 265 270Asn Lys Arg Ala Ser Val Arg Arg
Arg Ile Asp Gln Ser Asn Ser His 275 280 285Ala Asn Ile Phe Tyr Ser
Val Leu Thr Ile Asp Lys Met Gln Asn Lys 290 295 300Asp Lys Gly Leu
Tyr Thr Cys Arg Val Arg Ser Gly Pro Ser Phe Lys305 310 315 320Ser
Val Asn Thr Ser Val His Ile Tyr Asp Lys Ala Phe Ile Thr Val 325 330
335Lys His Arg Lys Gln Gln Val Leu Glu Thr Val Ala Gly Lys Arg Ser
340 345 350Tyr Arg Leu Ser Met Lys Val Lys Ala Phe Pro Ser Pro Glu
Val Val 355 360 365Trp Leu Lys Asp Gly Leu Pro Ala Thr Glu Lys Ser
Ala Arg Tyr Leu 370 375 380Thr Arg Gly Tyr Ser Leu Ile Ile Lys Asp
Val Thr Glu Glu Asp Ala385 390 395 400Gly Asn Tyr Thr Ile Leu Leu
Ser Ile Lys Gln Ser Asn Val Phe Lys 405 410 415Asn Leu Thr Ala Thr
Leu Ile Val Asn Val Lys Pro Gln Ile Tyr Glu 420 425 430Lys Ala Val
Ser Ser Phe Pro Asp Pro Ala Leu Tyr Pro Leu Gly Ser 435 440 445Arg
Gln Ile Leu Thr Cys Thr Ala Tyr Gly Ile Pro Gln Pro Thr Ile 450 455
460Lys Trp Phe Trp His Pro Cys Asn His Asn His Ser Glu Ala Arg
Cys465 470 475 480Asp Phe Cys Ser Asn Asn Glu Glu Ser Phe Ile Leu
Asp Ala Asp Ser 485 490 495Asn Met Gly Asn Arg Ile Glu Ser Ile Thr
Gln Arg Met Ala Ile Ile 500 505 510Glu Gly Lys Asn Lys Met Ala Ser
Thr Leu Val Val Ala Asp Ser Arg 515 520 525Ile Ser Gly Ile Tyr Ile
Cys Ile Ala Ser Asn Lys Val Gly Thr Val 530 535 540Gly Arg Asn Ile
Ser Phe Tyr Ile Thr Asp Val Pro Asn Gly Phe His545 550 555 560Val
Asn Leu Glu Lys Met Pro Thr Glu Gly Glu Asp Leu Lys Leu Ser 565 570
575Cys Thr Val Asn Lys Phe Leu Tyr Arg Asp Val Thr Trp Ile Leu Leu
580 585 590Arg Thr Val Asn Asn Arg Thr Met His Tyr Ser Ile Ser Lys
Gln Lys 595 600 605Met Ala Ile Thr Lys Glu His Ser Ile Thr Leu Asn
Leu Thr Ile Met 610 615 620Asn Val Ser Leu Gln Asp Ser Gly Thr Tyr
Ala Cys Arg Ala Arg Asn625 630 635 640Val Tyr Thr Gly Glu Glu Ile
Leu Gln Lys Lys Glu Ile Thr Ile Arg 645 650 655Asp Gln Glu Ala Pro
Tyr Leu Leu Arg Asn Leu Ser Asp His Thr Val 660 665 670Ala Ile Ser
Ser Ser Thr Thr Leu Asp Cys His Ala Asn Gly Val Pro 675 680 685Glu
Pro Gln Ile Thr Trp Phe Lys Asn Asn His Lys Ile Gln Gln Glu 690 695
700Pro Gly Ile Ile Leu Gly Pro Gly Ser Ser Thr Leu Phe Ile Glu
Arg705 710 715 720Val Thr Glu Glu Asp Glu Gly Val Tyr His Cys Lys
Ala Thr Asn Gln 725 730 735Lys Gly Ser Val Glu Ser Ser Ala Tyr Leu
Thr Val Gln Gly Thr Ser 740 745 750Asp Lys Ser Asn Leu Glu Leu Ile
Thr Leu Thr Cys Thr Cys Val Ala 755 760 765Ala Thr Leu Phe Trp Leu
Leu Leu Thr Leu Phe Ile Arg Lys Met Lys 770 775 780Arg Ser Ser Ser
Glu Ile Lys Thr Asp Tyr Leu Ser Ile Ile Met Asp785 790 795 800Pro
Asp Glu Val Pro Leu Asp Glu Gln Cys Glu Arg Leu Pro Tyr Asp 805 810
815Ala Ser Lys Trp Glu Phe Ala Arg Glu Arg Leu Lys Leu Gly Lys Ser
820 825 830Leu Gly Arg Gly Ala Phe Gly Lys Val Val Gln Ala Ser Ala
Phe Gly 835 840 845Ile Lys Lys Ser Pro Thr Cys Arg Thr Val Ala Val
Lys Met Leu Lys 850 855 860Glu Gly Ala Thr Ala Ser Glu Tyr Lys Ala
Leu Met Thr Glu Leu Lys865 870 875 880Ile Leu Thr His Ile Gly His
His Leu Asn Val Val Asn Leu Leu Gly 885 890 895Ala Cys Thr Lys Gln
Gly Gly Pro Leu Met Val Ile Val Glu Tyr Cys 900 905 910Lys Tyr Gly
Asn Leu Ser Asn Tyr Leu Lys Ser Lys Arg Asp Leu Phe 915 920 925Phe
Leu Asn Lys Asp Ala Ala Leu His Met Glu Pro Lys Lys Glu Lys 930 935
940Met Glu Pro Gly Leu Glu Gln Gly Lys Lys Pro Arg Leu Asp Ser
Val945 950 955 960Thr Ser Ser Glu Ser Phe Ala Ser Ser Gly Phe Gln
Glu Asp Lys Ser 965 970 975Leu Ser Asp Val Glu Glu Glu Glu Asp Ser
Asp Gly Phe Tyr Lys Glu 980 985 990Pro Ile Thr Met Glu Asp Leu Ile
Ser Tyr Ser Phe Gln Val Ala Arg 995 1000 1005Gly Met Glu Phe Leu
Ser Ser Arg Lys Cys Ile His Arg Asp Leu 1010 1015 1020Ala Ala Arg
Asn Ile Leu Leu Ser Glu Asn Asn Val Val Lys Ile 1025 1030 1035Cys
Asp Phe Gly Leu Ala Arg Asp Ile Tyr Lys Asn Pro Asp Tyr 1040 1045
1050Val Arg Lys Gly Asp Thr Arg Leu Pro Leu Lys Trp Met Ala Pro
1055 1060 1065Glu Ser Ile Phe Asp Lys Ile Tyr Ser Thr Lys Ser Asp
Val Trp 1070 1075 1080Ser Tyr Gly Val Leu Leu Trp Glu Ile Phe Ser
Leu Gly Gly Ser 1085 1090 1095Pro Tyr Pro Gly Val Gln Met Asp Glu
Asp Phe Cys Ser Arg Leu 1100 1105 1110Arg Glu Gly Met Arg Met Arg
Ala Pro Glu Tyr Ser Thr Pro Glu 1115 1120 1125Ile Tyr Gln Ile Met
Leu Asp Cys Trp His Arg Asp Pro Lys Glu 1130 1135 1140Arg Pro Arg
Phe Ala Glu Leu Val Glu Lys Leu Gly Asp Leu Leu 1145 1150 1155Gln
Ala Asn Val Gln Gln Asp Gly Lys Asp Tyr Ile Pro Ile Asn 1160 1165
1170Ala Ile Leu Thr Gly Asn Ser Gly Phe Thr Tyr Ser Thr Pro Ala
1175 1180 1185Phe Ser Glu Asp Phe Phe Lys Glu Ser Ile Ser Ala Pro
Lys Phe 1190 1195 1200Asn Ser Gly Ser Ser Asp Asp Val Arg Tyr Val
Asn Ala Phe Lys 1205 1210 1215Phe Met Ser Leu Glu Arg Ile Lys Thr
Phe Glu Glu Leu Leu Pro 1220 1225 1230Asn Ala Thr Ser Met Phe Asp
Asp Tyr Gln Gly Asp Ser Ser Thr 1235 1240 1245Leu Leu Ala Ser Pro
Met Leu Lys Arg Phe Thr Trp Thr Asp Ser 1250 1255 1260Lys Pro Lys
Ala Ser Leu Lys Ile Asp Leu Arg Val Thr Ser Lys 1265 1270 1275Ser
Lys Glu Ser Gly Leu Ser Asp Val Ser Arg Pro Ser Phe Cys 1280 1285
1290His Ser Ser Cys Gly His Val Ser Glu Gly Lys Arg Arg Phe Thr
1295 1300 1305Tyr Asp His Ala Glu Leu Glu Arg Lys Ile Ala Cys Cys
Ser Pro 1310 1315 1320Pro Pro Asp Tyr Asn Ser Val Val Leu Tyr Ser
Thr Pro Pro Ile 1325 1330 1335151356PRTHomo sapiens 15Met Gln Ser
Lys Val Leu Leu Ala Val Ala Leu Trp Leu Cys Val Glu1 5 10 15Thr Arg
Ala Ala Ser Val Gly Leu Pro Ser Val Ser Leu Asp Leu Pro 20 25 30Arg
Leu Ser Ile Gln Lys Asp Ile Leu Thr Ile Lys Ala Asn Thr Thr 35 40
45Leu Gln Ile Thr Cys Arg Gly Gln Arg Asp Leu Asp Trp Leu Trp Pro
50 55 60Asn Asn Gln Ser Gly Ser Glu Gln Arg Val Glu Val Thr Glu Cys
Ser65 70 75 80Asp Gly Leu Phe Cys Lys Thr Leu Thr Ile Pro Lys Val
Ile Gly Asn 85 90 95Asp Thr Gly Ala Tyr Lys Cys Phe Tyr Arg Glu Thr
Asp Leu Ala Ser 100 105 110Val Ile Tyr Val Tyr Val Gln Asp Tyr Arg
Ser Pro Phe Ile Ala Ser 115 120 125Val Ser Asp Gln His Gly Val Val
Tyr Ile Thr Glu Asn Lys Asn Lys 130 135 140Thr Val Val Ile Pro Cys
Leu Gly Ser Ile Ser Asn Leu Asn Val Ser145 150 155 160Leu Cys Ala
Arg Tyr Pro Glu Lys Arg Phe Val Pro Asp Gly Asn Arg 165 170 175Ile
Ser Trp Asp Ser Lys Lys Gly Phe Thr Ile Pro Ser Tyr Met Ile 180 185
190Ser Tyr Ala Gly Met Val Phe Cys Glu Ala Lys Ile Asn Asp Glu Ser
195 200 205Tyr Gln Ser Ile Met Tyr Ile Val Val Val Val Gly Tyr Arg
Ile Tyr 210 215 220Asp Val Val Leu Ser Pro Ser His Gly Ile Glu Leu
Ser Val Gly Glu225 230 235 240Lys Leu Val Leu Asn Cys Thr Ala Arg
Thr Glu Leu Asn Val Gly Ile 245 250 255Asp Phe Asn Trp Glu Tyr Pro
Ser Ser Lys His Gln His Lys Lys Leu 260 265 270Val Asn Arg Asp Leu
Lys Thr Gln Ser Gly Ser Glu Met Lys Lys Phe 275 280 285Leu Ser Thr
Leu Thr Ile Asp Gly Val Thr Arg Ser Asp Gln Gly Leu 290 295 300Tyr
Thr Cys Ala Ala Ser Ser Gly Leu Met Thr Lys Lys Asn Ser Thr305 310
315 320Phe Val Arg Val His Glu Lys Pro Phe Val Ala Phe Gly Ser Gly
Met 325 330 335Glu Ser Leu Val Glu Ala Thr Val Gly Glu Arg Val Arg
Ile Pro Ala 340 345 350Lys Tyr Leu Gly Tyr Pro Pro Pro Glu Ile Lys
Trp Tyr Lys Asn Gly 355 360 365Ile Pro Leu Glu Ser Asn His Thr Ile
Lys Ala Gly His Val Leu Thr 370 375 380Ile Met Glu Val Ser Glu Arg
Asp Thr Gly Asn Tyr Thr Val Ile Leu385 390 395 400Thr Asn Pro Ile
Ser Lys Glu Lys Gln Ser His Val Val Ser Leu Val 405 410 415Val Tyr
Val Pro Pro Gln Ile Gly Glu Lys Ser Leu Ile Ser Pro Val 420 425
430Asp Ser Tyr Gln Tyr Gly Thr Thr Gln Thr Leu Thr Cys Thr Val Tyr
435 440 445Ala Ile Pro Pro Pro His His Ile His Trp Tyr Trp Gln Leu
Glu Glu 450 455 460Glu Cys Ala Asn Glu Pro Ser Gln Ala Val Ser Val
Thr Asn Pro Tyr465 470 475 480Pro Cys Glu Glu Trp Arg Ser Val Glu
Asp Phe Gln Gly Gly Asn Lys 485 490 495Ile Glu Val Asn Lys Asn Gln
Phe Ala Leu Ile Glu Gly Lys Asn Lys 500 505 510Thr Val Ser Thr Leu
Val Ile Gln Ala Ala Asn Val Ser Ala Leu Tyr 515 520 525Lys Cys Glu
Ala Val Asn Lys Val Gly Arg Gly Glu Arg Val Ile Ser 530 535 540Phe
His Val Thr Arg Gly Pro Glu Ile Thr Leu Gln Pro Asp Met Gln545 550
555 560Pro Thr Glu Gln Glu Ser Val Ser Leu Trp Cys Thr Ala Asp Arg
Ser 565 570 575Thr Phe Glu Asn Leu Thr Trp Tyr Lys Leu Gly Pro Gln
Pro Leu Pro 580 585 590Ile His Val Gly Glu Leu Pro Thr Pro Val Cys
Lys Asn Leu Asp Thr 595 600 605Leu Trp Lys Leu Asn Ala Thr Met Phe
Ser Asn Ser Thr Asn Asp Ile 610 615 620Leu Ile Met Glu Leu Lys Asn
Ala Ser Leu Gln Asp Gln Gly Asp Tyr625 630 635 640Val Cys Leu Ala
Gln Asp Arg Lys Thr Lys Lys Arg His Cys Val Val 645 650 655Arg Gln
Leu Thr Val Leu Glu Arg Val Ala Pro Thr Ile Thr Gly Asn 660 665
670Leu Glu Asn Gln Thr Thr Ser Ile Gly Glu Ser Ile Glu Val Ser Cys
675 680 685Thr Ala Ser Gly Asn Pro Pro Pro Gln Ile Met Trp Phe Lys
Asp Asn 690 695 700Glu Thr Leu Val Glu Asp Ser Gly Ile Val Leu Lys
Asp Gly Asn Arg705 710 715 720Asn Leu Thr Ile Arg Arg Val Arg Lys
Glu Asp Glu Gly Leu Tyr Thr 725 730 735Cys Gln Ala Cys Ser Val Leu
Gly Cys Ala Lys Val Glu Ala Phe Phe 740 745 750Ile Ile Glu Gly Ala
Gln Glu Lys Thr Asn Leu Glu Ile Ile Ile Leu 755 760 765Val Gly Thr
Ala Val Ile Ala Met Phe Phe Trp Leu Leu Leu Val Ile 770 775 780Ile
Leu Arg Thr Val Lys Arg Ala Asn Gly Gly Glu Leu Lys Thr Gly785 790
795 800Tyr Leu Ser Ile Val Met Asp Pro Asp Glu Leu Pro Leu Asp Glu
His 805 810 815Cys Glu Arg Leu Pro Tyr Asp Ala Ser Lys Trp Glu Phe
Pro Arg Asp 820 825 830Arg Leu Lys Leu Gly Lys Pro Leu Gly Arg Gly
Ala Phe Gly Gln Val 835 840 845Ile Glu Ala Asp Ala Phe Gly Ile Asp
Lys Thr Ala Thr Cys Arg Thr 850 855 860Val Ala Val Lys Met Leu Lys
Glu Gly Ala Thr His Ser Glu His Arg865 870 875 880Ala Leu Met Ser
Glu Leu Lys Ile Leu Ile His Ile Gly His His Leu 885 890 895Asn Val
Val Asn Leu Leu Gly Ala Cys Thr Lys Pro Gly Gly Pro Leu 900 905
910Met Val Ile Val Glu Phe Cys Lys Phe Gly Asn Leu Ser Thr Tyr Leu
915 920 925Arg Ser Lys Arg Asn Glu Phe Val Pro Tyr Lys Thr Lys Gly
Ala Arg 930 935 940Phe Arg Gln Gly Lys Asp Tyr Val Gly Ala Ile Pro
Val Asp Leu Lys945 950 955 960Arg Arg Leu Asp Ser Ile Thr Ser Ser
Gln Ser Ser Ala Ser Ser Gly 965 970 975Phe Val Glu Glu Lys Ser Leu
Ser Asp Val Glu Glu Glu Glu Ala Pro 980 985 990Glu Asp Leu Tyr Lys
Asp Phe Leu Thr Leu Glu His Leu Ile Cys Tyr 995 1000 1005Ser Phe
Gln Val Ala Lys Gly Met Glu Phe Leu Ala Ser Arg Lys 1010 1015
1020Cys Ile His Arg Asp Leu Ala Ala Arg Asn Ile Leu Leu Ser Glu
1025 1030 1035Lys Asn Val Val Lys Ile Cys Asp Phe Gly Leu Ala Arg
Asp Ile 1040 1045 1050Tyr Lys Asp Pro Asp Tyr Val Arg Lys Gly Asp
Ala Arg Leu Pro 1055 1060 1065Leu Lys Trp Met Ala Pro Glu Thr Ile
Phe Asp Arg Val Tyr Thr 1070 1075 1080Ile Gln Ser Asp Val Trp Ser
Phe Gly Val Leu Leu Trp Glu Ile 1085 1090 1095Phe Ser Leu Gly Ala
Ser Pro Tyr Pro Gly Val Lys Ile Asp Glu 1100 1105 1110Glu Phe Cys
Arg Arg Leu Lys Glu Gly Thr Arg Met Arg Ala Pro 1115 1120 1125Asp
Tyr Thr Thr Pro Glu Met Tyr Gln Thr Met Leu Asp Cys Trp 1130 1135
1140His Gly Glu Pro Ser Gln Arg Pro Thr Phe Ser Glu Leu Val Glu
1145 1150 1155His Leu Gly Asn Leu Leu Gln Ala Asn Ala Gln Gln Asp
Gly Lys 1160 1165 1170Asp Tyr Ile Val Leu Pro Ile Ser Glu Thr Leu
Ser Met Glu Glu 1175 1180 1185Asp Ser Gly Leu Ser Leu Pro Thr Ser
Pro Val Ser Cys Met Glu 1190 1195 1200Glu Glu Glu Val Cys Asp Pro
Lys Phe His Tyr Asp Asn Thr Ala 1205 1210 1215Gly Ile Ser Gln Tyr
Leu Gln Asn Ser Lys Arg Lys Ser Arg Pro 1220
1225 1230Val Ser Val Lys Thr Phe Glu Asp Ile Pro Leu Glu Glu Pro
Glu 1235 1240 1245Val Lys Val Ile Pro Asp Asp Asn Gln Thr Asp Ser
Gly Met Val 1250 1255 1260Leu Ala Ser Glu Glu Leu Lys Thr Leu Glu
Asp Arg Thr Lys Leu 1265 1270 1275Ser Pro Ser Phe Gly Gly Met Val
Pro Ser Lys Ser Arg Glu Ser 1280 1285 1290Val Ala Ser Glu Gly Ser
Asn Gln Thr Ser Gly Tyr Gln Ser Gly 1295 1300 1305Tyr His Ser Asp
Asp Thr Asp Thr Thr Val Tyr Ser Ser Glu Glu 1310 1315 1320Ala Glu
Leu Leu Lys Leu Ile Glu Ile Gly Val Gln Thr Gly Ser 1325 1330
1335Thr Ala Gln Ile Leu Gln Pro Asp Ser Gly Thr Thr Leu Ser Ser
1340 1345 1350Pro Pro Val 1355161363PRTHomo sapiens 16Met Gln Arg
Gly Ala Ala Leu Cys Leu Arg Leu Trp Leu Cys Leu Gly1 5 10 15Leu Leu
Asp Gly Leu Val Ser Gly Tyr Ser Met Thr Pro Pro Thr Leu 20 25 30Asn
Ile Thr Glu Glu Ser His Val Ile Asp Thr Gly Asp Ser Leu Ser 35 40
45Ile Ser Cys Arg Gly Gln His Pro Leu Glu Trp Ala Trp Pro Gly Ala
50 55 60Gln Glu Ala Pro Ala Thr Gly Asp Lys Asp Ser Glu Asp Thr Gly
Val65 70 75 80Val Arg Asp Cys Glu Gly Thr Asp Ala Arg Pro Tyr Cys
Lys Val Leu 85 90 95Leu Leu His Glu Val His Ala Asn Asp Thr Gly Ser
Tyr Val Cys Tyr 100 105 110Tyr Lys Tyr Ile Lys Ala Arg Ile Glu Gly
Thr Thr Ala Ala Ser Ser 115 120 125Tyr Val Phe Val Arg Asp Phe Glu
Gln Pro Phe Ile Asn Lys Pro Asp 130 135 140Thr Leu Leu Val Asn Arg
Lys Asp Ala Met Trp Val Pro Cys Leu Val145 150 155 160Ser Ile Pro
Gly Leu Asn Val Thr Leu Arg Ser Gln Ser Ser Val Leu 165 170 175Trp
Pro Asp Gly Gln Glu Val Val Trp Asp Asp Arg Arg Gly Met Leu 180 185
190Val Ser Thr Pro Leu Leu His Asp Ala Leu Tyr Leu Gln Cys Glu Thr
195 200 205Thr Trp Gly Asp Gln Asp Phe Leu Ser Asn Pro Phe Leu Val
His Ile 210 215 220Thr Gly Asn Glu Leu Tyr Asp Ile Gln Leu Leu Pro
Arg Lys Ser Leu225 230 235 240Glu Leu Leu Val Gly Glu Lys Leu Val
Leu Asn Cys Thr Val Trp Ala 245 250 255Glu Phe Asn Ser Gly Val Thr
Phe Asp Trp Asp Tyr Pro Gly Lys Gln 260 265 270Ala Glu Arg Gly Lys
Trp Val Pro Glu Arg Arg Ser Gln Gln Thr His 275 280 285Thr Glu Leu
Ser Ser Ile Leu Thr Ile His Asn Val Ser Gln His Asp 290 295 300Leu
Gly Ser Tyr Val Cys Lys Ala Asn Asn Gly Ile Gln Arg Phe Arg305 310
315 320Glu Ser Thr Glu Val Ile Val His Glu Asn Pro Phe Ile Ser Val
Glu 325 330 335Trp Leu Lys Gly Pro Ile Leu Glu Ala Thr Ala Gly Asp
Glu Leu Val 340 345 350Lys Leu Pro Val Lys Leu Ala Ala Tyr Pro Pro
Pro Glu Phe Gln Trp 355 360 365Tyr Lys Asp Gly Lys Ala Leu Ser Gly
Arg His Ser Pro His Ala Leu 370 375 380Val Leu Lys Glu Val Thr Glu
Ala Ser Thr Gly Thr Tyr Thr Leu Ala385 390 395 400Leu Trp Asn Ser
Ala Ala Gly Leu Arg Arg Asn Ile Ser Leu Glu Leu 405 410 415Val Val
Asn Val Pro Pro Gln Ile His Glu Lys Glu Ala Ser Ser Pro 420 425
430Ser Ile Tyr Ser Arg His Ser Arg Gln Ala Leu Thr Cys Thr Ala Tyr
435 440 445Gly Val Pro Leu Pro Leu Ser Ile Gln Trp His Trp Arg Pro
Trp Thr 450 455 460Pro Cys Lys Met Phe Ala Gln Arg Ser Leu Arg Arg
Arg Gln Gln Gln465 470 475 480Asp Leu Met Pro Gln Cys Arg Asp Trp
Arg Ala Val Thr Thr Gln Asp 485 490 495Ala Val Asn Pro Ile Glu Ser
Leu Asp Thr Trp Thr Glu Phe Val Glu 500 505 510Gly Lys Asn Lys Thr
Val Ser Lys Leu Val Ile Gln Asn Ala Asn Val 515 520 525Ser Ala Met
Tyr Lys Cys Val Val Ser Asn Lys Val Gly Gln Asp Glu 530 535 540Arg
Leu Ile Tyr Phe Tyr Val Thr Thr Ile Pro Asp Gly Phe Thr Ile545 550
555 560Glu Ser Lys Pro Ser Glu Glu Leu Leu Glu Gly Gln Pro Val Leu
Leu 565 570 575Ser Cys Gln Ala Asp Ser Tyr Lys Tyr Glu His Leu Arg
Trp Tyr Arg 580 585 590Leu Asn Leu Ser Thr Leu His Asp Ala His Gly
Asn Pro Leu Leu Leu 595 600 605Asp Cys Lys Asn Val His Leu Phe Ala
Thr Pro Leu Ala Ala Ser Leu 610 615 620Glu Glu Val Ala Pro Gly Ala
Arg His Ala Thr Leu Ser Leu Ser Ile625 630 635 640Pro Arg Val Ala
Pro Glu His Glu Gly His Tyr Val Cys Glu Val Gln 645 650 655Asp Arg
Arg Ser His Asp Lys His Cys His Lys Lys Tyr Leu Ser Val 660 665
670Gln Ala Leu Glu Ala Pro Arg Leu Thr Gln Asn Leu Thr Asp Leu Leu
675 680 685Val Asn Val Ser Asp Ser Leu Glu Met Gln Cys Leu Val Ala
Gly Ala 690 695 700His Ala Pro Ser Ile Val Trp Tyr Lys Asp Glu Arg
Leu Leu Glu Glu705 710 715 720Lys Ser Gly Val Asp Leu Ala Asp Ser
Asn Gln Lys Leu Ser Ile Gln 725 730 735Arg Val Arg Glu Glu Asp Ala
Gly Arg Tyr Leu Cys Ser Val Cys Asn 740 745 750Ala Lys Gly Cys Val
Asn Ser Ser Ala Ser Val Ala Val Glu Gly Ser 755 760 765Glu Asp Lys
Gly Ser Met Glu Ile Val Ile Leu Val Gly Thr Gly Val 770 775 780Ile
Ala Val Phe Phe Trp Val Leu Leu Leu Leu Ile Phe Cys Asn Met785 790
795 800Arg Arg Pro Ala His Ala Asp Ile Lys Thr Gly Tyr Leu Ser Ile
Ile 805 810 815Met Asp Pro Gly Glu Val Pro Leu Glu Glu Gln Cys Glu
Tyr Leu Ser 820 825 830Tyr Asp Ala Ser Gln Trp Glu Phe Pro Arg Glu
Arg Leu His Leu Gly 835 840 845Arg Val Leu Gly Tyr Gly Ala Phe Gly
Lys Val Val Glu Ala Ser Ala 850 855 860Phe Gly Ile His Lys Gly Ser
Ser Cys Asp Thr Val Ala Val Lys Met865 870 875 880Leu Lys Glu Gly
Ala Thr Ala Ser Glu His Arg Ala Leu Met Ser Glu 885 890 895Leu Lys
Ile Leu Ile His Ile Gly Asn His Leu Asn Val Val Asn Leu 900 905
910Leu Gly Ala Cys Thr Lys Pro Gln Gly Pro Leu Met Val Ile Val Glu
915 920 925Phe Cys Lys Tyr Gly Asn Leu Ser Asn Phe Leu Arg Ala Lys
Arg Asp 930 935 940Ala Phe Ser Pro Cys Ala Glu Lys Ser Pro Glu Gln
Arg Gly Arg Phe945 950 955 960Arg Ala Met Val Glu Leu Ala Arg Leu
Asp Arg Arg Arg Pro Gly Ser 965 970 975Ser Asp Arg Val Leu Phe Ala
Arg Phe Ser Lys Thr Glu Gly Gly Ala 980 985 990Arg Arg Ala Ser Pro
Asp Gln Glu Ala Glu Asp Leu Trp Leu Ser Pro 995 1000 1005Leu Thr
Met Glu Asp Leu Val Cys Tyr Ser Phe Gln Val Ala Arg 1010 1015
1020Gly Met Glu Phe Leu Ala Ser Arg Lys Cys Ile His Arg Asp Leu
1025 1030 1035Ala Ala Arg Asn Ile Leu Leu Ser Glu Ser Asp Val Val
Lys Ile 1040 1045 1050Cys Asp Phe Gly Leu Ala Arg Asp Ile Tyr Lys
Asp Pro Asp Tyr 1055 1060 1065Val Arg Lys Gly Ser Ala Arg Leu Pro
Leu Lys Trp Met Ala Pro 1070 1075 1080Glu Ser Ile Phe Asp Lys Val
Tyr Thr Thr Gln Ser Asp Val Trp 1085 1090 1095Ser Phe Gly Val Leu
Leu Trp Glu Ile Phe Ser Leu Gly Ala Ser 1100 1105 1110Pro Tyr Pro
Gly Val Gln Ile Asn Glu Glu Phe Cys Gln Arg Leu 1115 1120 1125Arg
Asp Gly Thr Arg Met Arg Ala Pro Glu Leu Ala Thr Pro Ala 1130 1135
1140Ile Arg Arg Ile Met Leu Asn Cys Trp Ser Gly Asp Pro Lys Ala
1145 1150 1155Arg Pro Ala Phe Ser Glu Leu Val Glu Ile Leu Gly Asp
Leu Leu 1160 1165 1170Gln Gly Arg Gly Leu Gln Glu Glu Glu Glu Val
Cys Met Ala Pro 1175 1180 1185Arg Ser Ser Gln Ser Ser Glu Glu Gly
Ser Phe Ser Gln Val Ser 1190 1195 1200Thr Met Ala Leu His Ile Ala
Gln Ala Asp Ala Glu Asp Ser Pro 1205 1210 1215Pro Ser Leu Gln Arg
His Ser Leu Ala Ala Arg Tyr Tyr Asn Trp 1220 1225 1230Val Ser Phe
Pro Gly Cys Leu Ala Arg Gly Ala Glu Thr Arg Gly 1235 1240 1245Ser
Ser Arg Met Lys Thr Phe Glu Glu Phe Pro Met Thr Pro Thr 1250 1255
1260Thr Tyr Lys Gly Ser Val Asp Asn Gln Thr Asp Ser Gly Met Val
1265 1270 1275Leu Ala Ser Glu Glu Phe Glu Gln Ile Glu Ser Arg His
Arg Gln 1280 1285 1290Glu Ser Gly Phe Ser Cys Lys Gly Pro Gly Gln
Asn Val Ala Val 1295 1300 1305Thr Arg Ala His Pro Asp Ser Gln Gly
Arg Arg Arg Arg Pro Glu 1310 1315 1320Arg Gly Ala Arg Gly Gly Gln
Val Phe Tyr Asn Ser Glu Tyr Gly 1325 1330 1335Glu Leu Ser Glu Pro
Ser Glu Glu Asp His Cys Ser Pro Ser Ala 1340 1345 1350Arg Val Thr
Phe Phe Thr Asp Asn Ser Tyr 1355 136017330PRTHomo sapiens 17Ala Ser
Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser
Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr 20 25
30Phe Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser
35 40 45Gly Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr
Ser 50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr
Gln Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr
Lys Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys Asp Lys Thr His
Thr Cys Pro Pro Cys 100 105 110Pro Ala Pro Glu Leu Leu Gly Gly Pro
Ser Val Phe Leu Phe Pro Pro 115 120 125Lys Pro Lys Asp Thr Leu Met
Ile Ser Arg Thr Pro Glu Val Thr Cys 130 135 140Val Val Val Asp Val
Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp145 150 155 160Tyr Val
Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu 165 170
175Glu Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu
180 185 190His Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val
Ser Asn 195 200 205Lys Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser
Lys Ala Lys Gly 210 215 220Gln Pro Arg Glu Pro Gln Val Tyr Thr Leu
Pro Pro Ser Arg Asp Glu225 230 235 240Leu Thr Lys Asn Gln Val Ser
Leu Thr Cys Leu Val Lys Gly Phe Tyr 245 250 255Pro Ser Asp Ile Ala
Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn 260 265 270Asn Tyr Lys
Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe 275 280 285Leu
Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn 290 295
300Val Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr
Thr305 310 315 320Gln Lys Ser Leu Ser Leu Ser Pro Gly Lys 325
33018106PRTHomo sapiens 18Thr Val Ala Ala Pro Ser Val Phe Ile Phe
Pro Pro Ser Asp Glu Gln1 5 10 15Leu Lys Ser Gly Thr Ala Ser Val Val
Cys Leu Leu Asn Asn Phe Tyr 20 25 30Pro Arg Glu Ala Lys Val Gln Trp
Lys Val Asp Asn Ala Leu Gln Ser 35 40 45Gly Asn Ser Gln Glu Ser Val
Thr Glu Gln Asp Ser Lys Asp Ser Thr 50 55 60Tyr Ser Leu Ser Ser Thr
Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys65 70 75 80His Lys Val Tyr
Ala Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro 85 90 95Val Thr Lys
Ser Phe Asn Arg Gly Glu Cys 100 10519506PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
19Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val Leu Asn Val Ser Ser1
5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala Pro Val Val Trp Glu
Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met Ala Lys Ala Gln Asp
Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr Asn Leu Thr Gly Leu
Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn Asp Ser Arg Gly Leu
Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp
Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile
Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr Ile Pro Cys Arg Val
Thr Asp Pro Gln Leu Val Val Thr Leu 115 120 125His Glu Lys Lys Gly
Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln 130 135 140Arg Gly Phe
Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys Lys Thr145 150 155
160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg
165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala Val Gln
Thr Val 180 185 190Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile
Val Ile Gly Asn 195 200 205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro
Arg Lys Glu Ser Gly Arg 210 215 220Leu Val Glu Pro Val Thr Asp Phe
Leu Leu Asp Met Pro Tyr His Ile225 230 235 240Arg Ser Ile Leu His
Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys
Asn Val Thr Glu Ser Val Asn Asp His Gln Asp Glu Lys 260 265 270Ala
Ile Asn Ile Thr Val Val Glu Ser Gly Gly Gly Gly Gly Ser Gly 275 280
285Gly Gly Gly Ser Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser
290 295 300Ala Ser Val Gly Asp Arg Val Thr Ile Thr Cys Ser Ala Ser
Gln Asp305 310 315 320Ile Ser Asn Tyr Leu Asn Trp Tyr Gln Gln Lys
Pro Gly Lys Ala Pro 325 330 335Lys Val Leu Ile Tyr Phe Thr Ser Ser
Leu His Ser Gly Val Pro Ser 340 345 350Arg Phe Ser Gly Ser Gly Ser
Gly Thr Asp Phe Thr Leu Thr Ile Ser 355 360 365Ser Leu Gln Pro Glu
Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Tyr Ser 370 375 380Thr Val Pro
Trp Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys Arg385 390 395
400Thr Val Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln
405 410 415Leu Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn
Phe Tyr 420 425 430Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn
Ala Leu Gln Ser 435 440 445Gly Asn Ser Gln Glu Ser Val Thr Glu Gln
Asp Ser Lys Asp Ser Thr 450 455 460Tyr Ser Leu Ser Ser Thr Leu Thr
Leu Ser Lys Ala Asp Tyr Glu Lys465 470 475 480His Lys Val Tyr Ala
Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro 485 490 495Val Thr Lys
Ser Phe Asn Arg Gly Glu Cys 500 50520498PRTArtificial
SequenceDescription of Artificial Sequence Synthetic
polypeptide
20Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val Leu Asn Val Ser Ser1
5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala Pro Val Val Trp Glu
Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met Ala Lys Ala Gln Asp
Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr Asn Leu Thr Gly Leu
Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn Asp Ser Arg Gly Leu
Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp
Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile
Phe Leu Thr Glu Ile Thr Glu 100 105 110Ile Thr Ile Pro Cys Arg Val
Thr Asp Pro Gln Leu Val Val Thr Leu 115 120 125His Glu Lys Lys Gly
Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln 130 135 140Arg Gly Phe
Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys Lys Thr145 150 155
160Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg
165 170 175Leu Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala Val Gln
Thr Val 180 185 190Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile
Val Ile Gly Asn 195 200 205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro
Arg Lys Glu Ser Gly Arg 210 215 220Leu Val Glu Pro Val Thr Asp Phe
Leu Leu Asp Met Pro Tyr His Ile225 230 235 240Arg Ser Ile Leu His
Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys
Asn Val Thr Glu Ser Val Asn Asp His Gln Asp Glu Lys 260 265 270Ala
Ile Asn Ile Thr Val Val Glu Ser Gly Gly Gly Asp Ile Gln Met 275 280
285Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly Asp Arg Val Thr
290 295 300Ile Thr Cys Ser Ala Ser Gln Asp Ile Ser Asn Tyr Leu Asn
Trp Tyr305 310 315 320Gln Gln Lys Pro Gly Lys Ala Pro Lys Val Leu
Ile Tyr Phe Thr Ser 325 330 335Ser Leu His Ser Gly Val Pro Ser Arg
Phe Ser Gly Ser Gly Ser Gly 340 345 350Thr Asp Phe Thr Leu Thr Ile
Ser Ser Leu Gln Pro Glu Asp Phe Ala 355 360 365Thr Tyr Tyr Cys Gln
Gln Tyr Ser Thr Val Pro Trp Thr Phe Gly Gln 370 375 380Gly Thr Lys
Val Glu Ile Lys Arg Thr Val Ala Ala Pro Ser Val Phe385 390 395
400Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser Gly Thr Ala Ser Val
405 410 415Val Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala Lys Val
Gln Trp 420 425 430Lys Val Asp Asn Ala Leu Gln Ser Gly Asn Ser Gln
Glu Ser Val Thr 435 440 445Glu Gln Asp Ser Lys Asp Ser Thr Tyr Ser
Leu Ser Ser Thr Leu Thr 450 455 460Leu Ser Lys Ala Asp Tyr Glu Lys
His Lys Val Tyr Ala Cys Glu Val465 470 475 480Thr His Gln Gly Leu
Ser Ser Pro Val Thr Lys Ser Phe Asn Arg Gly 485 490 495Glu
Cys21231PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 21Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Tyr Thr Phe Thr Asn Tyr 20 25 30Gly Met Asn Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Trp Ile Asn Thr Tyr Thr Gly
Glu Pro Thr Tyr Ala Ala Asp Phe 50 55 60Lys Arg Arg Phe Thr Phe Ser
Leu Asp Thr Ser Lys Ser Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val 100 105 110Trp Gly
Gln Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly 115 120
125Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly
130 135 140Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu
Pro Val145 150 155 160Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser
Gly Val His Thr Phe 165 170 175Pro Ala Val Leu Gln Ser Ser Gly Leu
Tyr Ser Leu Ser Ser Val Val 180 185 190Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr Ile Cys Asn Val 195 200 205Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys 210 215 220Ser Cys Asp
Lys Thr His Thr225 23022523PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 22Leu Val Val Thr Pro Pro
Gly Pro Glu Leu Val Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr
Cys Ser Gly Ser Ala Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu
Pro Pro Gln Glu Met Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser
Val Leu Thr Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr
Phe Cys Thr His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75
80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu
85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr
Glu 100 105 110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val
Val Thr Leu 115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val
Pro Tyr Asp His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp
Arg Ser Tyr Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu
Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser
Ser Ile Asn Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg
Gln Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200
205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg
210 215 220Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr
His Ile225 230 235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu
Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val
Asn Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val
Glu Ser Gly Gly Gly Gly Gly Ser Gly 275 280 285Gly Gly Gly Ser Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val 290 295 300Gln Pro Gly
Gly Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Tyr Thr305 310 315
320Phe Thr Asn Tyr Gly Met Asn Trp Val Arg Gln Ala Pro Gly Lys Gly
325 330 335Leu Glu Trp Val Gly Trp Ile Asn Thr Tyr Thr Gly Glu Pro
Thr Tyr 340 345 350Ala Ala Asp Phe Lys Arg Arg Phe Thr Phe Ser Leu
Asp Thr Ser Lys 355 360 365Ser Thr Ala Tyr Leu Gln Met Asn Ser Leu
Arg Ala Glu Asp Thr Ala 370 375 380Val Tyr Tyr Cys Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp385 390 395 400Tyr Phe Asp Val Trp
Gly Gln Gly Thr Leu Val Thr Val Ser Ser Ala 405 410 415Ser Thr Lys
Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser 420 425 430Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe 435 440
445Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly
450 455 460Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr
Ser Leu465 470 475 480Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr 485 490 495Ile Cys Asn Val Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys 500 505 510Val Glu Pro Lys Ser Cys Asp
Lys Thr His Thr 515 52023515PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 23Leu Val Val Thr Pro Pro
Gly Pro Glu Leu Val Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr
Cys Ser Gly Ser Ala Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu
Pro Pro Gln Glu Met Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser
Val Leu Thr Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr
Phe Cys Thr His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75
80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu
85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr
Glu 100 105 110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val
Val Thr Leu 115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val
Pro Tyr Asp His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp
Arg Ser Tyr Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu
Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser
Ser Ile Asn Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg
Gln Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200
205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg
210 215 220Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr
His Ile225 230 235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu
Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val
Asn Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val
Glu Ser Gly Gly Gly Glu Val Gln Leu 275 280 285Val Glu Ser Gly Gly
Gly Leu Val Gln Pro Gly Gly Ser Leu Arg Leu 290 295 300Ser Cys Ala
Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Gly Met Asn Trp305 310 315
320Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Gly Trp Ile Asn
325 330 335Thr Tyr Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe Lys Arg
Arg Phe 340 345 350Thr Phe Ser Leu Asp Thr Ser Lys Ser Thr Ala Tyr
Leu Gln Met Asn 355 360 365Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr
Tyr Cys Ala Lys Tyr Pro 370 375 380His Tyr Tyr Gly Ser Ser His Trp
Tyr Phe Asp Val Trp Gly Gln Gly385 390 395 400Thr Leu Val Thr Val
Ser Ser Ala Ser Thr Lys Gly Pro Ser Val Phe 405 410 415Pro Leu Ala
Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu 420 425 430Gly
Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser Trp 435 440
445Asn Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val Leu
450 455 460Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val
Pro Ser465 470 475 480Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn
Val Asn His Lys Pro 485 490 495Ser Asn Thr Lys Val Asp Lys Lys Val
Glu Pro Lys Ser Cys Asp Lys 500 505 510Thr His Thr
51524535PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 24Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Tyr Thr Phe Thr Asn Tyr 20 25 30Gly Met Asn Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Trp Ile Asn Thr Tyr Thr Gly
Glu Pro Thr Tyr Ala Ala Asp Phe 50 55 60Lys Arg Arg Phe Thr Phe Ser
Leu Asp Thr Ser Lys Ser Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val 100 105 110Trp Gly
Gln Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly 115 120
125Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly
130 135 140Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu
Pro Val145 150 155 160Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser
Gly Val His Thr Phe 165 170 175Pro Ala Val Leu Gln Ser Ser Gly Leu
Tyr Ser Leu Ser Ser Val Val 180 185 190Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr Ile Cys Asn Val 195 200 205Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys 210 215 220Ser Cys Asp
Lys Thr His Thr Gly Gly Gly Gly Gly Ser Gly Gly Gly225 230 235
240Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Leu Val Val
245 250 255Thr Pro Pro Gly Pro Glu Leu Val Leu Asn Val Ser Ser Thr
Phe Val 260 265 270Leu Thr Cys Ser Gly Ser Ala Pro Val Val Trp Glu
Arg Met Ser Gln 275 280 285Glu Pro Pro Gln Glu Met Ala Lys Ala Gln
Asp Gly Thr Phe Ser Ser 290 295 300Val Leu Thr Leu Thr Asn Leu Thr
Gly Leu Asp Thr Gly Glu Tyr Phe305 310 315 320Cys Thr His Asn Asp
Ser Arg Gly Leu Glu Thr Asp Glu Arg Lys Arg 325 330 335Leu Tyr Ile
Phe Val Pro Asp Pro Thr Val Gly Phe Leu Pro Asn Asp 340 345 350Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu Ile Thr Ile 355 360
365Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu His Glu Lys
370 375 380Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln Arg
Gly Phe385 390 395 400Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys
Lys Thr Thr Ile Gly 405 410 415Asp Arg Glu Val Asp Ser Asp Ala Tyr
Tyr Val Tyr Arg Leu Gln Val 420 425 430Ser Ser Ile Asn Val Ser Val
Asn Ala Val Gln Thr Val Val Arg Gln 435 440 445Gly Glu Asn Ile Thr
Leu Met Cys Ile Val Ile Gly Asn Glu Val Val 450 455 460Asn Phe Glu
Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg Leu Val Glu465 470 475
480Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile Arg Ser Ile
485 490 495Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr Tyr
Thr Cys 500 505 510Asn Val Thr Glu Ser Val Asn Asp His Gln Asp Glu
Lys Ala Ile Asn 515 520 525Ile Thr Val Val Glu Ser Gly 530
53525523PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 25Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val
Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr Cys Ser Gly Ser Ala
Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu Pro Pro Gln Glu Met
Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser Val Leu Thr Leu Thr
Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr Phe Cys Thr His Asn
Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75 80Arg Lys Arg Leu Tyr
Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 85 90 95Pro Asn Asp Ala
Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 100
105 110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr
Leu 115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr
Asp His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser
Tyr Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu Val Asp
Ser Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser Ser Ile
Asn Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg Gln Gly
Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200 205Glu Val
Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg 210 215
220Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr His
Ile225 230 235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu
Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val Asn
Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val Glu
Ser Gly Gly Gly Gly Gly Ser Gly 275 280 285Gly Gly Gly Ser Glu Val
Gln Leu Val Glu Ser Gly Gly Gly Leu Val 290 295 300Gln Pro Gly Gly
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Tyr Asp305 310 315 320Phe
Thr His Tyr Gly Met Asn Trp Val Arg Gln Ala Pro Gly Lys Gly 325 330
335Leu Glu Trp Val Gly Trp Ile Asn Thr Tyr Thr Gly Glu Pro Thr Tyr
340 345 350Ala Ala Asp Phe Lys Arg Arg Phe Thr Phe Ser Leu Asp Thr
Ser Lys 355 360 365Ser Thr Ala Tyr Leu Gln Met Asn Ser Leu Arg Ala
Glu Asp Thr Ala 370 375 380Val Tyr Tyr Cys Ala Lys Tyr Pro Tyr Tyr
Tyr Gly Thr Ser His Trp385 390 395 400Tyr Phe Asp Val Trp Gly Gln
Gly Thr Leu Val Thr Val Ser Ser Ala 405 410 415Ser Thr Lys Gly Pro
Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser 420 425 430Thr Ser Gly
Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe 435 440 445Pro
Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly 450 455
460Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser
Leu465 470 475 480Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly
Thr Gln Thr Tyr 485 490 495Ile Cys Asn Val Asn His Lys Pro Ser Asn
Thr Lys Val Asp Lys Lys 500 505 510Val Glu Pro Lys Ser Cys Asp Lys
Thr His Leu 515 52026735PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 26Leu Val Val Thr Pro Pro
Gly Pro Glu Leu Val Leu Asn Val Ser Ser1 5 10 15Thr Phe Val Leu Thr
Cys Ser Gly Ser Ala Pro Val Val Trp Glu Arg 20 25 30Met Ser Gln Glu
Pro Pro Gln Glu Met Ala Lys Ala Gln Asp Gly Thr 35 40 45Phe Ser Ser
Val Leu Thr Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 50 55 60Glu Tyr
Phe Cys Thr His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu65 70 75
80Arg Lys Arg Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu
85 90 95Pro Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr
Glu 100 105 110Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val
Val Thr Leu 115 120 125His Glu Lys Lys Gly Asp Val Ala Leu Pro Val
Pro Tyr Asp His Gln 130 135 140Arg Gly Phe Ser Gly Ile Phe Glu Asp
Arg Ser Tyr Ile Cys Lys Thr145 150 155 160Thr Ile Gly Asp Arg Glu
Val Asp Ser Asp Ala Tyr Tyr Val Tyr Arg 165 170 175Leu Gln Val Ser
Ser Ile Asn Val Ser Val Asn Ala Val Gln Thr Val 180 185 190Val Arg
Gln Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn 195 200
205Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg
210 215 220Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr
His Ile225 230 235 240Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu
Glu Asp Ser Gly Thr 245 250 255Tyr Thr Cys Asn Val Thr Glu Ser Val
Asn Asp His Gln Asp Glu Lys 260 265 270Ala Ile Asn Ile Thr Val Val
Glu Ser Gly Glu Val Gln Leu Val Glu 275 280 285Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly Ser Leu Arg Leu Ser Cys 290 295 300Ala Ala Ser
Gly Tyr Thr Phe Thr Asn Tyr Gly Met Asn Trp Val Arg305 310 315
320Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Gly Trp Ile Asn Thr Tyr
325 330 335Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe Lys Arg Arg Phe
Thr Phe 340 345 350Ser Leu Asp Thr Ser Lys Ser Thr Ala Tyr Leu Gln
Met Asn Ser Leu 355 360 365Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
Ala Lys Tyr Pro His Tyr 370 375 380Tyr Gly Ser Ser His Trp Tyr Phe
Asp Val Trp Gly Gln Gly Thr Leu385 390 395 400Val Thr Val Ser Ser
Ala Ser Thr Lys Gly Pro Ser Val Phe Pro Leu 405 410 415Ala Pro Ser
Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys 420 425 430Leu
Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser Trp Asn Ser 435 440
445Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val Leu Gln Ser
450 455 460Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val Pro Ser
Ser Ser465 470 475 480Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val Asn
His Lys Pro Ser Asn 485 490 495Thr Lys Val Asp Lys Lys Val Glu Pro
Lys Ser Cys Asp Lys Thr His 500 505 510Thr Cys Pro Pro Cys Pro Ala
Pro Glu Leu Leu Gly Gly Pro Ser Val 515 520 525Phe Leu Phe Pro Pro
Lys Pro Lys Asp Thr Leu Met Ile Ser Arg Thr 530 535 540Pro Glu Val
Thr Cys Val Val Val Asp Val Ser His Glu Asp Pro Glu545 550 555
560Val Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His Asn Ala Lys
565 570 575Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg Val
Val Ser 580 585 590Val Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly
Lys Glu Tyr Lys 595 600 605Cys Lys Val Ser Asn Lys Ala Leu Pro Ala
Pro Ile Glu Lys Thr Ile 610 615 620Ser Lys Ala Lys Gly Gln Pro Arg
Glu Pro Gln Val Tyr Thr Leu Pro625 630 635 640Pro Ser Arg Glu Glu
Met Thr Lys Asn Gln Val Ser Leu Thr Cys Leu 645 650 655Val Lys Gly
Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu Ser Asn 660 665 670Gly
Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser 675 680
685Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg
690 695 700Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met His Glu
Ala Leu705 710 715 720His Asn His Tyr Thr Gln Lys Ser Leu Ser Leu
Ser Pro Gly Lys 725 730 73527643PRTArtificial SequenceDescription
of Artificial Sequence Synthetic polypeptide 27Val Gly Phe Leu Pro
Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr1 5 10 15Glu Ile Thr Glu
Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu 20 25 30Val Val Thr
Leu His Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro 35 40 45Tyr Asp
His Gln Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr 50 55 60Ile
Cys Lys Thr Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala Tyr65 70 75
80Tyr Val Tyr Arg Leu Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala
85 90 95Val Gln Thr Val Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys
Ile 100 105 110Val Ile Gly Asn Glu Val Val Asn Phe Glu Trp Thr Tyr
Pro Arg Lys 115 120 125Glu Ser Gly Arg Leu Val Glu Pro Val Thr Asp
Phe Leu Leu Asp Met 130 135 140Pro Tyr His Ile Arg Ser Ile Leu His
Ile Pro Ser Ala Glu Leu Glu145 150 155 160Asp Ser Gly Thr Tyr Thr
Cys Asn Val Thr Glu Ser Val Asn Asp His 165 170 175Gln Asp Glu Lys
Ala Ile Asn Ile Thr Val Val Glu Ser Gly Glu Val 180 185 190Gln Leu
Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly Ser Leu 195 200
205Arg Leu Ser Cys Ala Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Gly Met
210 215 220Asn Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
Gly Trp225 230 235 240Ile Asn Thr Tyr Thr Gly Glu Pro Thr Tyr Ala
Ala Asp Phe Lys Arg 245 250 255Arg Phe Thr Phe Ser Leu Asp Thr Ser
Lys Ser Thr Ala Tyr Leu Gln 260 265 270Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys Ala Lys 275 280 285Tyr Pro His Tyr Tyr
Gly Ser Ser His Trp Tyr Phe Asp Val Trp Gly 290 295 300Gln Gly Thr
Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly Pro Ser305 310 315
320Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala
325 330 335Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val
Thr Val 340 345 350Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His
Thr Phe Pro Ala 355 360 365Val Leu Gln Ser Ser Gly Leu Tyr Ser Leu
Ser Ser Val Val Thr Val 370 375 380Pro Ser Ser Ser Leu Gly Thr Gln
Thr Tyr Ile Cys Asn Val Asn His385 390 395 400Lys Pro Ser Asn Thr
Lys Val Asp Lys Lys Val Glu Pro Lys Ser Cys 405 410 415Asp Lys Thr
His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly 420 425 430Gly
Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met 435 440
445Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Val Asp Val Ser His
450 455 460Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val
Glu Val465 470 475 480His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln
Tyr Asn Ser Thr Tyr 485 490 495Arg Val Val Ser Val Leu Thr Val Leu
His Gln Asp Trp Leu Asn Gly 500 505 510Lys Glu Tyr Lys Cys Lys Val
Ser Asn Lys Ala Leu Pro Ala Pro Ile 515 520 525Glu Lys Thr Ile Ser
Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val 530 535 540Tyr Thr Leu
Pro Pro Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser545 550 555
560Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu
565 570 575Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr
Pro Pro 580 585 590Val Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser
Lys Leu Thr Val 595 600 605Asp Lys Ser Arg Trp Gln Gln Gly Asn Val
Phe Ser Cys Ser Val Met 610 615 620His Glu Ala Leu His Asn His Tyr
Thr Gln Lys Ser Leu Ser Leu Ser625 630 635 640Pro Gly
Lys28421PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 28Val Gly Phe Leu Pro Asn Asp Ala Glu Glu Leu
Phe Ile Phe Leu Thr1 5 10 15Glu Ile Thr Glu Ile Thr Ile Pro Cys Arg
Val Thr Asp Pro Gln Leu 20 25 30Val Val Thr Leu His Glu Lys Lys Gly
Asp Val Ala Leu Pro Val Pro 35 40 45Tyr Asp His Gln Arg Gly Phe Ser
Gly Ile Phe Glu Asp Arg Ser Tyr 50 55 60Ile Cys Lys Thr Thr Ile Gly
Asp Arg Glu Val Asp Ser Asp Ala Tyr65 70 75 80Tyr Val Tyr Arg Leu
Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala 85 90 95Val Gln Thr Val
Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile 100 105 110Val Ile
Gly Asn Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys 115 120
125Glu Ser Gly Arg Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met
130 135 140Pro Tyr His Ile Arg Ser Ile Leu His Ile Pro Ser Ala Glu
Leu Glu145 150 155 160Asp Ser Gly Thr Tyr Thr Cys Asn Val Thr Glu
Ser Val Asn Asp His 165 170 175Gln Asp Glu Lys Ala Ile Asn Ile Thr
Val Val Glu Ser Gly Glu Val 180 185 190Gln Leu Val Glu Ser Gly Gly
Gly Leu Val Gln Pro Gly Gly Ser Leu 195 200 205Arg Leu Ser Cys Ala
Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Gly Met 210 215 220Asn Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Gly Trp225 230 235
240Ile Asn Thr Tyr Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe Lys Arg
245 250 255Arg Phe Thr Phe Ser Leu Asp Thr Ser Lys Ser Thr Ala Tyr
Leu Gln 260 265 270Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr
Tyr Cys Ala Lys 275 280 285Tyr Pro His Tyr Tyr Gly Ser Ser His Trp
Tyr Phe Asp Val Trp Gly 290 295 300Gln Gly Thr Leu Val Thr Val Ser
Ser Ala Ser Thr Lys Gly Pro Ser305 310 315 320Val Phe Pro Leu Ala
Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala 325 330 335Ala Leu Gly
Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val 340 345 350Ser
Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala 355 360
365Val Leu Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val
370 375 380Pro Ser Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val
Asn His385 390 395 400Lys Pro Ser Asn Thr Lys Val Asp Lys Lys Val
Glu Pro Lys Ser Cys 405 410 415Asp Lys Thr His Thr
42029450PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 29Val Gly Phe Leu Pro Asn Asp Ala Glu Glu Leu
Phe Ile Phe Leu Thr1 5 10 15Glu Ile Thr Glu Ile Thr Ile Pro Cys Arg
Val Thr Asp Pro Gln Leu 20 25 30Val Val Thr Leu His Glu Lys Lys Gly
Asp Val Ala Leu Pro Val Pro 35 40 45Tyr Asp His Gln Arg Gly Phe Ser
Gly Ile Phe Glu Asp Arg Ser Tyr 50 55 60Ile Cys Lys Thr Thr Ile Gly
Asp Arg Glu Val Asp Ser Asp Ala Tyr65 70 75 80Tyr Val Tyr Arg Leu
Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala 85 90 95Val Gln Thr Val
Val Arg Gln Gly Glu Asn Ile Thr Leu Met Cys Ile 100 105 110Val Ile
Gly Asn Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys 115 120
125Glu Ser Gly Arg Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met
130 135 140Pro Tyr His Ile Arg Ser Ile Leu His Ile Pro Ser Ala Glu
Leu Glu145 150 155 160Asp Ser Gly Thr Tyr Thr Cys Asn Val Thr Glu
Ser Val Asn Asp His 165 170 175Gln Asp Glu Lys Ala Ile Asn Ile Thr
Val Val Glu Ser Gly Gly Gly 180 185 190Gly Ser Gly Gly Gly Gly Ser
Gly Gly Gly Gly Ser Gly Gly Gly Gly 195 200 205Ser Gly Gly Gly
Gly Ser Gly Gly Gly Gly Ser Glu Val Gln Leu Val 210 215 220Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly Ser Leu Arg Leu Ser225 230 235
240Cys Ala Ala Ser Gly Tyr Thr Phe Thr Asn Tyr Gly Met Asn Trp Val
245 250 255Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Gly Trp Ile
Asn Thr 260 265 270Tyr Thr Gly Glu Pro Thr Tyr Ala Ala Asp Phe Lys
Arg Arg Phe Thr 275 280 285Phe Ser Leu Asp Thr Ser Lys Ser Thr Ala
Tyr Leu Gln Met Asn Ser 290 295 300Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys Ala Lys Tyr Pro His305 310 315 320Tyr Tyr Gly Ser Ser
His Trp Tyr Phe Asp Val Trp Gly Gln Gly Thr 325 330 335Leu Val Thr
Val Ser Ser Ala Ser Thr Lys Gly Pro Ser Val Phe Pro 340 345 350Leu
Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly 355 360
365Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser Trp Asn
370 375 380Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val
Leu Gln385 390 395 400Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val
Thr Val Pro Ser Ser 405 410 415Ser Leu Gly Thr Gln Thr Tyr Ile Cys
Asn Val Asn His Lys Pro Ser 420 425 430Asn Thr Lys Val Asp Lys Lys
Val Glu Pro Lys Ser Cys Asp Lys Thr 435 440 445His Thr
45030450PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 30Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Tyr Thr Phe Thr Asn Tyr 20 25 30Gly Met Asn Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Trp Ile Asn Thr Tyr Thr Gly
Glu Pro Thr Tyr Ala Ala Asp Phe 50 55 60Lys Arg Arg Phe Thr Phe Ser
Leu Asp Thr Ser Lys Ser Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Lys Tyr Pro
His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val 100 105 110Trp Gly
Gln Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly 115 120
125Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly
130 135 140Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu
Pro Val145 150 155 160Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser
Gly Val His Thr Phe 165 170 175Pro Ala Val Leu Gln Ser Ser Gly Leu
Tyr Ser Leu Ser Ser Val Val 180 185 190Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr Tyr Ile Cys Asn Val 195 200 205Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys 210 215 220Ser Cys Asp
Lys Thr His Thr Gly Gly Gly Ser Gly Gly Gly Gly Ser225 230 235
240Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly
245 250 255Gly Gly Gly Ser Val Gly Phe Leu Pro Asn Asp Ala Glu Glu
Leu Phe 260 265 270Ile Phe Leu Thr Glu Ile Thr Glu Ile Thr Ile Pro
Cys Arg Val Thr 275 280 285Asp Pro Gln Leu Val Val Thr Leu His Glu
Lys Lys Gly Asp Val Ala 290 295 300Leu Pro Val Pro Tyr Asp His Gln
Arg Gly Phe Ser Gly Ile Phe Glu305 310 315 320Asp Arg Ser Tyr Ile
Cys Lys Thr Thr Ile Gly Asp Arg Glu Val Asp 325 330 335Ser Asp Ala
Tyr Tyr Val Tyr Arg Leu Gln Val Ser Ser Ile Asn Val 340 345 350Ser
Val Asn Ala Val Gln Thr Val Val Arg Gln Gly Glu Asn Ile Thr 355 360
365Leu Met Cys Ile Val Ile Gly Asn Glu Val Val Asn Phe Glu Trp Thr
370 375 380Tyr Pro Arg Lys Glu Ser Gly Arg Leu Val Glu Pro Val Thr
Asp Phe385 390 395 400Leu Leu Asp Met Pro Tyr His Ile Arg Ser Ile
Leu His Ile Pro Ser 405 410 415Ala Glu Leu Glu Asp Ser Gly Thr Tyr
Thr Cys Asn Val Thr Glu Ser 420 425 430Val Asn Asp His Gln Asp Glu
Lys Ala Ile Asn Ile Thr Val Val Glu 435 440 445Ser Gly
450317719DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 31gacggatcgg gagatctccc gatcccctat
ggtgcactct cagtacaatc tgctctgatg 60ccgcatagtt aagccagtat ctgctccctg
cttgtgtgtt ggaggtcgct gagtagtgcg 120cgagcaaaat ttaagctaca
acaaggcaag gcttgaccga caattgcatg aagaatctgc 180ttagggttag
gcgttttgcg ctgcttcgcg atgtacgggc cagatatacg cgttgacatt
240gattattgac tagttattaa tagtaatcaa ttacggggtc attagttcat
agcccatata 300tggagttccg cgttacataa cttacggtaa atggcccgcc
tggctgaccg cccaacgacc 360cccgcccatt gacgtcaata atgacgtatg
ttcccatagt aacgccaata gggactttcc 420attgacgtca atgggtggag
tatttacggt aaactgccca cttggcagta catcaagtgt 480atcatatgcc
aagtacgccc cctattgacg tcaatgacgg taaatggccc gcctggcatt
540atgcccagta catgacctta tgggactttc ctacttggca gtacatctac
gtattagtca 600tcgctattac catggtgatg cggttttggc agtacatcaa
tgggcgtgga tagcggtttg 660actcacgggg atttccaagt ctccacccca
ttgacgtcaa tgggagtttg ttttggcacc 720aaaatcaacg ggactttcca
aaatgtcgta acaactccgc cccattgacg caaatgggcg 780gtaggcgtgt
acggtgggag gtctatataa gcagagctct ctggctaact agagaaccca
840ctgcttactg gcttatcgaa attaatacga ctcactatag ggagacccaa
gctggctagc 900gtttaaactt aagcttggta ccgagctcgg atccactagt
ccagtgtggt ggaattctgc 960agatatccag cacagtggcg gccgccatga
aagctgtggt gctggccgtg gctctggtct 1020tcctgacagg gagccaggct
ctggtcgtca cacccccggg gccagagctt gtcctcaatg 1080tctccagcac
cttcgttctg acctgctcgg gttcagctcc ggtggtgtgg gaacggatgt
1140cccaggagcc cccacaggaa atggccaagg cccaggatgg caccttctcc
agcgtgctca 1200cactgaccaa cctcactggg ctagacacgg gagaatactt
ttgcacccac aatgactccc 1260gtggactgga gaccgatgag cggaaacggc
tctacatctt tgtgccagat cccaccgtgg 1320gcttcctccc taatgatgcc
gaggaactat tcatctttct cacggaaata actgagatca 1380ccattccatg
ccgagtaaca gacccacagc tggtggtgac actgcacgag aagaaagggg
1440acgttgcact gcctgtcccc tatgatcacc aacgtggctt ttctggtatc
tttgaggaca 1500gaagctacat ctgcaaaacc accattgggg acagggaggt
ggattctgat gcctactatg 1560tctacagact ccaggtgtca tccatcaacg
tctctgtgaa cgcagtgcag actgtggtcc 1620gccagggtga gaacatcacc
ctcatgtgca ttgtgatcgg gaatgaggtg gtcaacttcg 1680agtggacata
cccccgcaaa gaaagtgggc ggctggtgga gccggtgact gacttcctct
1740tggatatgcc ttaccacatc cgctccatcc tgcacatccc cagtgccgag
ttagaagact 1800cggggaccta cacctgcaat gtgacggaga gtgtgaatga
ccatcaggat gaaaaggcca 1860tcaacatcac cgtggttgag agcggcggtg
gtggcggctc cggtggaggc ggaagcgagg 1920tgcagctggt ggaatccggc
ggaggcctgg tccagcctgg cggatccctg agactgtcct 1980gtgccgcctc
cggctacgac ttcacccatt acggcatgaa ctgggtccga caggcccctg
2040gcaagggcct ggaatgggtc ggatggatca acacctacac cggcgagccc
acctacgccg 2100ccgacttcaa gcggcggttc accttctccc tggacacctc
caagtccacc gcctacctgc 2160agatgaactc cctgcgggcc gaggacaccg
ccgtgtacta ctgcgccaag tacccctact 2220actacggcac ctcccactgg
tacttcgacg tgtggggcca gggcaccctg gtcaccgtgt 2280cctccgcctc
taccaagggc ccctccgtgt tccctctggc cccctccagc aagtccacct
2340ctggcggcac cgccgctctg ggctgcctgg tcaaggacta cttccccgag
cccgtgaccg 2400tgtcctggaa ctctggcgcc ctgacctccg gcgtgcacac
ctttccagcc gtgctgcagt 2460cctccggcct gtactccctg tcctccgtcg
tgaccgtgcc ctccagctct ctgggcaccc 2520agacctacat ctgcaacgtg
aaccacaagc cctccaacac caaggtggac aagaaggtgg 2580aacccaagtc
ctgcgacaag acccacacct gtcccccctg ccctgcccct gaagcagccg
2640gtgcacccag cgtgttcctg ttccccccaa agcccaagga caccctgatg
atctcccgga 2700cccccgaagt gacctgcgtg gtggtggacg tgtcccacga
ggaccctgaa gtgaagttca 2760attggtacgt ggacggcgtg gaagtgcaca
atgccaagac caagcccaga gaggaacagt 2820acaactccac ctaccgggtg
gtgtccgtgc tgaccgtgct gcatcaggac tggctgaacg 2880gcaaagagta
caagtgcaag gtctccaaca aggccctgcc tgcccccatc gaaaagacca
2940tctccaaggc caagggccag ccccgcgagc ctcaggtgta cacactgcca
cccagccggg 3000aagagatgac caagaaccag gtctccctga cctgtctggt
caagggcttc tacccctccg 3060atatcgccgt cgaatgggag tccaacggcc
agcccgagaa caactacaag accacccccc 3120ctgtgctgga ctccgacggc
tcattcttcc tgtactccaa gctgaccgtg gacaagtccc 3180ggtggcagca
gggcaacgtg ttctcctgct ccgtgatgca cgaggccctg cacaaccact
3240acacccagaa gtccctgtcc tgcagccccg gcaagtgata atctagaggg
cccgtttaaa 3300cccgctgatc agcctcgact gtgccttcta gttgccagcc
atctgttgtt tgcccctccc 3360ccgtgccttc cttgaccctg gaaggtgcca
ctcccactgt cctttcctaa taaaatgagg 3420aaattgcatc gcattgtctg
agtaggtgtc attctattct ggggggtggg gtggggcagg 3480acagcaaggg
ggaggattgg gaagacaata gcaggcatgc tggggatgcg gtgggctcta
3540tggcttctga ggcggaaaga accagctggg gctctagggg gtatccccac
gcgccctgta 3600gcggcgcatt aagcgcggcg ggtgtggtgg ttacgcgcag
cgtgaccgct acacttgcca 3660gcgccctagc gcccgctcct ttcgctttct
tcccttcctt tctcgccacg ttcgccggct 3720ttccccgtca agctctaaat
cgggggctcc ctttagggtt ccgatttagt gctttacggc 3780acctcgaccc
caaaaaactt gattagggtg atggttcacg tagtgggcca tcgccctgat
3840agacggtttt tcgccctttg acgttggagt ccacgttctt taatagtgga
ctcttgttcc 3900aaactggaac aacactcaac cctatctcgg tctattcttt
tgatttataa gggattttgc 3960cgatttcggc ctattggtta aaaaatgagc
tgatttaaca aaaatttaac gcgaattaat 4020tctgtggaat gtgtgtcagt
tagggtgtgg aaagtcccca ggctccccag caggcagaag 4080tatgcaaagc
atgcatctca attagtcagc aaccaggtgt ggaaagtccc caggctcccc
4140agcaggcaga agtatgcaaa gcatgcatct caattagtca gcaaccatag
tcccgcccct 4200aactccgccc atcccgcccc taactccgcc cagttccgcc
cattctccgc cccatggctg 4260actaattttt tttatttatg cagaggccga
ggccgcctct gcctctgagc tattccagaa 4320gtagtgagga ggcttttttg
gaggcctagg cttttgcaaa aagctcccgg gagcttgtat 4380atccattttc
ggatctgatc aagagacagg atgaggatcg tttcgcatga ttgaacaaga
4440tggattgcac gcaggttctc cggccgcttg ggtggagagg ctattcggct
atgactgggc 4500acaacagaca atcggctgct ctgatgccgc cgtgttccgg
ctgtcagcgc aggggcgccc 4560ggttcttttt gtcaagaccg acctgtccgg
tgccctgaat gaactgcagg acgaggcagc 4620gcggctatcg tggctggcca
cgacgggcgt tccttgcgca gctgtgctcg acgttgtcac 4680tgaagcggga
agggactggc tgctattggg cgaagtgccg gggcaggatc tcctgtcatc
4740tcaccttgct cctgccgaga aagtatccat catggctgat gcaatgcggc
ggctgcatac 4800gcttgatccg gctacctgcc cattcgacca ccaagcgaaa
catcgcatcg agcgagcacg 4860tactcggatg gaagccggtc ttgtcgatca
ggatgatctg gacgaagagc atcaggggct 4920cgcgccagcc gaactgttcg
ccaggctcaa ggcgcgcatg cccgacggcg aggatctcgt 4980cgtgacccat
ggcgatgcct gcttgccgaa tatcatggtg gaaaatggcc gcttttctgg
5040attcatcgac tgtggccggc tgggtgtggc ggaccgctat caggacatag
cgttggctac 5100ccgtgatatt gctgaagagc ttggcggcga atgggctgac
cgcttcctcg tgctttacgg 5160tatcgccgct cccgattcgc agcgcatcgc
cttctatcgc cttcttgacg agttcttctg 5220agcgggactc tggggttcga
aatgaccgac caagcgacgc ccaacctgcc atcacgagat 5280ttcgattcca
ccgccgcctt ctatgaaagg ttgggcttcg gaatcgtttt ccgggacgcc
5340ggctggatga tcctccagcg cggggatctc atgctggagt tcttcgccca
ccccaacttg 5400tttattgcag cttataatgg ttacaaataa agcaatagca
tcacaaattt cacaaataaa 5460gcattttttt cactgcattc tagttgtggt
ttgtccaaac tcatcaatgt atcttatcat 5520gtctgtatac cgtcgacctc
tagctagagc ttggcgtaat catggtcata gctgtttcct 5580gtgtgaaatt
gttatccgct cacaattcca cacaacatac gagccggaag cataaagtgt
5640aaagcctggg gtgcctaatg agtgagctaa ctcacattaa ttgcgttgcg
ctcactgccc 5700gctttccagt cgggaaacct gtcgtgccag ctgcattaat
gaatcggcca acgcgcgggg 5760agaggcggtt tgcgtattgg gcgctcttcc
gcttcctcgc tcactgactc gctgcgctcg 5820gtcgttcggc tgcggcgagc
ggtatcagct cactcaaagg cggtaatacg gttatccaca 5880gaatcagggg
ataacgcagg aaagaacatg tgagcaaaag gccagcaaaa ggccaggaac
5940cgtaaaaagg ccgcgttgct ggcgtttttc cataggctcc gcccccctga
cgagcatcac 6000aaaaatcgac gctcaagtca gaggtggcga aacccgacag
gactataaag ataccaggcg 6060tttccccctg gaagctccct cgtgcgctct
cctgttccga ccctgccgct taccggatac 6120ctgtccgcct ttctcccttc
gggaagcgtg gcgctttctc atagctcacg ctgtaggtat 6180ctcagttcgg
tgtaggtcgt tcgctccaag ctgggctgtg tgcacgaacc ccccgttcag
6240cccgaccgct gcgccttatc cggtaactat cgtcttgagt ccaacccggt
aagacacgac 6300ttatcgccac tggcagcagc cactggtaac aggattagca
gagcgaggta tgtaggcggt 6360gctacagagt tcttgaagtg gtggcctaac
tacggctaca ctagaagaac agtatttggt 6420atctgcgctc tgctgaagcc
agttaccttc ggaaaaagag ttggtagctc ttgatccggc 6480aaacaaacca
ccgctggtag cggttttttt gtttgcaagc agcagattac gcgcagaaaa
6540aaaggatctc aagaagatcc tttgatcttt tctacggggt ctgacgctca
gtggaacgaa 6600aactcacgtt aagggatttt ggtcatgaga ttatcaaaaa
ggatcttcac ctagatcctt 6660ttaaattaaa aatgaagttt taaatcaatc
taaagtatat atgagtaaac ttggtctgac 6720agttaccaat gcttaatcag
tgaggcacct atctcagcga tctgtctatt tcgttcatcc 6780atagttgcct
gactccccgt cgtgtagata actacgatac gggagggctt accatctggc
6840cccagtgctg caatgatacc gcgagaccca cgctcaccgg ctccagattt
atcagcaata 6900aaccagccag ccggaagggc cgagcgcaga agtggtcctg
caactttatc cgcctccatc 6960cagtctatta attgttgccg ggaagctaga
gtaagtagtt cgccagttaa tagtttgcgc 7020aacgttgttg ccattgctac
aggcatcgtg gtgtcacgct cgtcgtttgg tatggcttca 7080ttcagctccg
gttcccaacg atcaaggcga gttacatgat cccccatgtt gtgcaaaaaa
7140gcggttagct ccttcggtcc tccgatcgtt gtcagaagta agttggccgc
agtgttatca 7200ctcatggtta tggcagcact gcataattct cttactgtca
tgccatccgt aagatgcttt 7260tctgtgactg gtgagtactc aaccaagtca
ttctgagaat agtgtatgcg gcgaccgagt 7320tgctcttgcc cggcgtcaat
acgggataat accgcgccac atagcagaac tttaaaagtg 7380ctcatcattg
gaaaacgttc ttcggggcga aaactctcaa ggatcttacc gctgttgaga
7440tccagttcga tgtaacccac tcgtgcaccc aactgatctt cagcatcttt
tactttcacc 7500agcgtttctg ggtgagcaaa aacaggaagg caaaatgccg
caaaaaaggg aataagggcg 7560acacggaaat gttgaatact catactcttc
ctttttcaat attattgaag catttatcag 7620ggttattgtc tcatgagcgg
atacatattt gaatgtattt agaaaaataa acaaataggg 7680gttccgcgca
catttccccg aaaagtgcca cctgacgtc 7719326129DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
32gacggatcgg gagatctccc gatcccctat ggtgcactct cagtacaatc tgctctgatg
60ccgcatagtt aagccagtat ctgctccctg cttgtgtgtt ggaggtcgct gagtagtgcg
120cgagcaaaat ttaagctaca acaaggcaag gcttgaccga caattgcatg
aagaatctgc 180ttagggttag gcgttttgcg ctgcttcgcg atgtacgggc
cagatatacg cgttgacatt 240gattattgac tagttattaa tagtaatcaa
ttacggggtc attagttcat agcccatata 300tggagttccg cgttacataa
cttacggtaa atggcccgcc tggctgaccg cccaacgacc 360cccgcccatt
gacgtcaata atgacgtatg ttcccatagt aacgccaata gggactttcc
420attgacgtca atgggtggag tatttacggt aaactgccca cttggcagta
catcaagtgt 480atcatatgcc aagtacgccc cctattgacg tcaatgacgg
taaatggccc gcctggcatt 540atgcccagta catgacctta tgggactttc
ctacttggca gtacatctac gtattagtca 600tcgctattac catggtgatg
cggttttggc agtacatcaa tgggcgtgga tagcggtttg 660actcacgggg
atttccaagt ctccacccca ttgacgtcaa tgggagtttg ttttggcacc
720aaaatcaacg ggactttcca aaatgtcgta acaactccgc cccattgacg
caaatgggcg 780gtaggcgtgt acggtgggag gtctatataa gcagagctct
ctggctaact agagaaccca 840ctgcttactg gcttatcgaa attaatacga
ctcactatag ggagacccaa gctggctagc 900gtttaaactt aagcttggta
ccgagctcgg atccactagt ccagtgtggt ggaattctgc 960agatatccag
cacagtggcg gccgccatgg gatggagctg tatcatcctc ttcttggtgg
1020caacagctac aggcgtgcac tccgacatcc agctgaccca gtccccctcc
agcctgtccg 1080cctctgtggg cgacagagtg accatcacct gttccgccag
ccaggacatc tccaactacc 1140tgaactggta tcagcagaag cccggcaagg
cccccaaggt gctgatctac ttcacctcct 1200ccctgcactc cggcgtgccc
tccagattct ccggctctgg ctccggcacc gactttaccc 1260tgaccatctc
cagcctgcag cccgaggact tcgccaccta ctactgccag cagtactcca
1320ccgtgccctg gaccttcggc cagggcacca aggtggaaat caagcggacc
gtggccgctc 1380cctccgtgtt catcttccca ccctccgacg agcagctgaa
gtccggaacc gcctccgtcg 1440tgtgcctgct gaacaacttc tacccccgcg
aggccaaggt gcagtggaag gtggacaacg 1500ccctgcagag cggcaactcc
caggaatccg tcaccgagca ggactccaag gacagcacct 1560actccctgtc
cagcaccctg accctgtcca aggccgacta cgagaagcac aaggtgtacg
1620cctgcgaagt gacccaccag ggcctcagct ccccagtgac caagtccttc
aaccggggcg 1680agtgctagta atctagaggg cccgtttaaa cccgctgatc
agcctcgact gtgccttcta 1740gttgccagcc atctgttgtt tgcccctccc
ccgtgccttc cttgaccctg gaaggtgcca 1800ctcccactgt cctttcctaa
taaaatgagg aaattgcatc gcattgtctg agtaggtgtc 1860attctattct
ggggggtggg gtggggcagg acagcaaggg ggaggattgg gaagacaata
1920gcaggcatgc tggggatgcg gtgggctcta tggcttctga ggcggaaaga
accagctggg 1980gctctagggg gtatccccac gcgccctgta gcggcgcatt
aagcgcggcg ggtgtggtgg 2040ttacgcgcag cgtgaccgct acacttgcca
gcgccctagc gcccgctcct ttcgctttct 2100tcccttcctt tctcgccacg
ttcgccggct ttccccgtca agctctaaat cgggggctcc 2160ctttagggtt
ccgatttagt gctttacggc acctcgaccc caaaaaactt gattagggtg
2220atggttcacg tagtgggcca tcgccctgat agacggtttt tcgccctttg
acgttggagt 2280ccacgttctt taatagtgga ctcttgttcc aaactggaac
aacactcaac cctatctcgg 2340tctattcttt tgatttataa gggattttgc
cgatttcggc ctattggtta aaaaatgagc 2400tgatttaaca aaaatttaac
gcgaattaat tctgtggaat gtgtgtcagt tagggtgtgg 2460aaagtcccca
ggctccccag caggcagaag tatgcaaagc atgcatctca attagtcagc
2520aaccaggtgt ggaaagtccc caggctcccc agcaggcaga agtatgcaaa
gcatgcatct 2580caattagtca gcaaccatag tcccgcccct aactccgccc
atcccgcccc taactccgcc 2640cagttccgcc cattctccgc cccatggctg
actaattttt tttatttatg cagaggccga 2700ggccgcctct gcctctgagc
tattccagaa gtagtgagga ggcttttttg gaggcctagg 2760cttttgcaaa
aagctcccgg gagcttgtat atccattttc ggatctgatc aagagacagg
2820atgaggatcg tttcgcatga ttgaacaaga tggattgcac gcaggttctc
cggccgcttg
2880ggtggagagg ctattcggct atgactgggc acaacagaca atcggctgct
ctgatgccgc 2940cgtgttccgg ctgtcagcgc aggggcgccc ggttcttttt
gtcaagaccg acctgtccgg 3000tgccctgaat gaactgcagg acgaggcagc
gcggctatcg tggctggcca cgacgggcgt 3060tccttgcgca gctgtgctcg
acgttgtcac tgaagcggga agggactggc tgctattggg 3120cgaagtgccg
gggcaggatc tcctgtcatc tcaccttgct cctgccgaga aagtatccat
3180catggctgat gcaatgcggc ggctgcatac gcttgatccg gctacctgcc
cattcgacca 3240ccaagcgaaa catcgcatcg agcgagcacg tactcggatg
gaagccggtc ttgtcgatca 3300ggatgatctg gacgaagagc atcaggggct
cgcgccagcc gaactgttcg ccaggctcaa 3360ggcgcgcatg cccgacggcg
aggatctcgt cgtgacccat ggcgatgcct gcttgccgaa 3420tatcatggtg
gaaaatggcc gcttttctgg attcatcgac tgtggccggc tgggtgtggc
3480ggaccgctat caggacatag cgttggctac ccgtgatatt gctgaagagc
ttggcggcga 3540atgggctgac cgcttcctcg tgctttacgg tatcgccgct
cccgattcgc agcgcatcgc 3600cttctatcgc cttcttgacg agttcttctg
agcgggactc tggggttcga aatgaccgac 3660caagcgacgc ccaacctgcc
atcacgagat ttcgattcca ccgccgcctt ctatgaaagg 3720ttgggcttcg
gaatcgtttt ccgggacgcc ggctggatga tcctccagcg cggggatctc
3780atgctggagt tcttcgccca ccccaacttg tttattgcag cttataatgg
ttacaaataa 3840agcaatagca tcacaaattt cacaaataaa gcattttttt
cactgcattc tagttgtggt 3900ttgtccaaac tcatcaatgt atcttatcat
gtctgtatac cgtcgacctc tagctagagc 3960ttggcgtaat catggtcata
gctgtttcct gtgtgaaatt gttatccgct cacaattcca 4020cacaacatac
gagccggaag cataaagtgt aaagcctggg gtgcctaatg agtgagctaa
4080ctcacattaa ttgcgttgcg ctcactgccc gctttccagt cgggaaacct
gtcgtgccag 4140ctgcattaat gaatcggcca acgcgcgggg agaggcggtt
tgcgtattgg gcgctcttcc 4200gcttcctcgc tcactgactc gctgcgctcg
gtcgttcggc tgcggcgagc ggtatcagct 4260cactcaaagg cggtaatacg
gttatccaca gaatcagggg ataacgcagg aaagaacatg 4320tgagcaaaag
gccagcaaaa ggccaggaac cgtaaaaagg ccgcgttgct ggcgtttttc
4380cataggctcc gcccccctga cgagcatcac aaaaatcgac gctcaagtca
gaggtggcga 4440aacccgacag gactataaag ataccaggcg tttccccctg
gaagctccct cgtgcgctct 4500cctgttccga ccctgccgct taccggatac
ctgtccgcct ttctcccttc gggaagcgtg 4560gcgctttctc atagctcacg
ctgtaggtat ctcagttcgg tgtaggtcgt tcgctccaag 4620ctgggctgtg
tgcacgaacc ccccgttcag cccgaccgct gcgccttatc cggtaactat
4680cgtcttgagt ccaacccggt aagacacgac ttatcgccac tggcagcagc
cactggtaac 4740aggattagca gagcgaggta tgtaggcggt gctacagagt
tcttgaagtg gtggcctaac 4800tacggctaca ctagaagaac agtatttggt
atctgcgctc tgctgaagcc agttaccttc 4860ggaaaaagag ttggtagctc
ttgatccggc aaacaaacca ccgctggtag cggttttttt 4920gtttgcaagc
agcagattac gcgcagaaaa aaaggatctc aagaagatcc tttgatcttt
4980tctacggggt ctgacgctca gtggaacgaa aactcacgtt aagggatttt
ggtcatgaga 5040ttatcaaaaa ggatcttcac ctagatcctt ttaaattaaa
aatgaagttt taaatcaatc 5100taaagtatat atgagtaaac ttggtctgac
agttaccaat gcttaatcag tgaggcacct 5160atctcagcga tctgtctatt
tcgttcatcc atagttgcct gactccccgt cgtgtagata 5220actacgatac
gggagggctt accatctggc cccagtgctg caatgatacc gcgagaccca
5280cgctcaccgg ctccagattt atcagcaata aaccagccag ccggaagggc
cgagcgcaga 5340agtggtcctg caactttatc cgcctccatc cagtctatta
attgttgccg ggaagctaga 5400gtaagtagtt cgccagttaa tagtttgcgc
aacgttgttg ccattgctac aggcatcgtg 5460gtgtcacgct cgtcgtttgg
tatggcttca ttcagctccg gttcccaacg atcaaggcga 5520gttacatgat
cccccatgtt gtgcaaaaaa gcggttagct ccttcggtcc tccgatcgtt
5580gtcagaagta agttggccgc agtgttatca ctcatggtta tggcagcact
gcataattct 5640cttactgtca tgccatccgt aagatgcttt tctgtgactg
gtgagtactc aaccaagtca 5700ttctgagaat agtgtatgcg gcgaccgagt
tgctcttgcc cggcgtcaat acgggataat 5760accgcgccac atagcagaac
tttaaaagtg ctcatcattg gaaaacgttc ttcggggcga 5820aaactctcaa
ggatcttacc gctgttgaga tccagttcga tgtaacccac tcgtgcaccc
5880aactgatctt cagcatcttt tactttcacc agcgtttctg ggtgagcaaa
aacaggaagg 5940caaaatgccg caaaaaaggg aataagggcg acacggaaat
gttgaatact catactcttc 6000ctttttcaat attattgaag catttatcag
ggttattgtc tcatgagcgg atacatattt 6060gaatgtattt agaaaaataa
acaaataggg gttccgcgca catttccccg aaaagtgcca 6120cctgacgtc
6129331106PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 33Met Arg Leu Pro Gly Ala Met Pro Ala Leu Ala
Leu Lys Gly Glu Leu1 5 10 15Leu Leu Leu Ser Leu Leu Leu Leu Leu Glu
Pro Gln Ile Ser Gln Gly 20 25 30Leu Val Val Thr Pro Pro Gly Pro Glu
Leu Val Leu Asn Val Ser Ser 35 40 45Thr Phe Val Leu Thr Cys Ser Gly
Ser Ala Pro Val Val Trp Glu Arg 50 55 60Met Ser Gln Glu Pro Pro Gln
Glu Met Ala Lys Ala Gln Asp Gly Thr65 70 75 80Phe Ser Ser Val Leu
Thr Leu Thr Asn Leu Thr Gly Leu Asp Thr Gly 85 90 95Glu Tyr Phe Cys
Thr His Asn Asp Ser Arg Gly Leu Glu Thr Asp Glu 100 105 110Arg Lys
Arg Leu Tyr Ile Phe Val Pro Asp Pro Thr Val Gly Phe Leu 115 120
125Pro Asn Asp Ala Glu Glu Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu
130 135 140Ile Thr Ile Pro Cys Arg Val Thr Asp Pro Gln Leu Val Val
Thr Leu145 150 155 160His Glu Lys Lys Gly Asp Val Ala Leu Pro Val
Pro Tyr Asp His Gln 165 170 175Arg Gly Phe Ser Gly Ile Phe Glu Asp
Arg Ser Tyr Ile Cys Lys Thr 180 185 190Thr Ile Gly Asp Arg Glu Val
Asp Ser Asp Ala Tyr Tyr Val Tyr Arg 195 200 205Leu Gln Val Ser Ser
Ile Asn Val Ser Val Asn Ala Val Gln Thr Val 210 215 220Val Arg Gln
Gly Glu Asn Ile Thr Leu Met Cys Ile Val Ile Gly Asn225 230 235
240Glu Val Val Asn Phe Glu Trp Thr Tyr Pro Arg Lys Glu Ser Gly Arg
245 250 255Leu Val Glu Pro Val Thr Asp Phe Leu Leu Asp Met Pro Tyr
His Ile 260 265 270Arg Ser Ile Leu His Ile Pro Ser Ala Glu Leu Glu
Asp Ser Gly Thr 275 280 285Tyr Thr Cys Asn Val Thr Glu Ser Val Asn
Asp His Gln Asp Glu Lys 290 295 300Ala Ile Asn Ile Thr Val Val Glu
Ser Gly Tyr Val Arg Leu Leu Gly305 310 315 320Glu Val Gly Thr Leu
Gln Phe Ala Glu Leu His Arg Ser Arg Thr Leu 325 330 335Gln Val Val
Phe Glu Ala Tyr Pro Pro Pro Thr Val Leu Trp Phe Lys 340 345 350Asp
Asn Arg Thr Leu Gly Asp Ser Ser Ala Gly Glu Ile Ala Leu Ser 355 360
365Thr Arg Asn Val Ser Glu Thr Arg Tyr Val Ser Glu Leu Thr Leu Val
370 375 380Arg Val Lys Val Ala Glu Ala Gly His Tyr Thr Met Arg Ala
Phe His385 390 395 400Glu Asp Ala Glu Val Gln Leu Ser Phe Gln Leu
Gln Ile Asn Val Pro 405 410 415Val Arg Val Leu Glu Leu Ser Glu Ser
His Pro Asp Ser Gly Glu Gln 420 425 430Thr Val Arg Cys Arg Gly Arg
Gly Met Pro Gln Pro Asn Ile Ile Trp 435 440 445Ser Ala Cys Arg Asp
Leu Lys Arg Cys Pro Arg Glu Leu Pro Pro Thr 450 455 460Leu Leu Gly
Asn Ser Ser Glu Glu Glu Ser Gln Leu Glu Thr Asn Val465 470 475
480Thr Tyr Trp Glu Glu Glu Gln Glu Phe Glu Val Val Ser Thr Leu Arg
485 490 495Leu Gln His Val Asp Arg Pro Leu Ser Val Arg Cys Thr Leu
Arg Asn 500 505 510Ala Val Gly Gln Asp Thr Gln Glu Val Ile Val Val
Pro His Ser Leu 515 520 525Pro Phe Lys Val Val Val Ile Ser Ala Ile
Leu Ala Leu Val Val Leu 530 535 540Thr Ile Ile Ser Leu Ile Ile Leu
Ile Met Leu Trp Gln Lys Lys Pro545 550 555 560Arg Tyr Glu Ile Arg
Trp Lys Val Ile Glu Ser Val Ser Ser Asp Gly 565 570 575His Glu Tyr
Ile Tyr Val Asp Pro Met Gln Leu Pro Tyr Asp Ser Thr 580 585 590Trp
Glu Leu Pro Arg Asp Gln Leu Val Leu Gly Arg Thr Leu Gly Ser 595 600
605Gly Ala Phe Gly Gln Val Val Glu Ala Thr Ala His Gly Leu Ser His
610 615 620Ser Gln Ala Thr Met Lys Val Ala Val Lys Met Leu Lys Ser
Thr Ala625 630 635 640Arg Ser Ser Glu Lys Gln Ala Leu Met Ser Glu
Leu Lys Ile Met Ser 645 650 655His Leu Gly Pro His Leu Asn Val Val
Asn Leu Leu Gly Ala Cys Thr 660 665 670Lys Gly Gly Pro Ile Tyr Ile
Ile Thr Glu Tyr Cys Arg Tyr Gly Asp 675 680 685Leu Val Asp Tyr Leu
His Arg Asn Lys His Thr Phe Leu Gln His His 690 695 700Ser Asp Lys
Arg Arg Pro Pro Ser Ala Glu Leu Tyr Ser Asn Ala Leu705 710 715
720Pro Val Gly Leu Pro Leu Pro Ser His Val Ser Leu Thr Gly Glu Ser
725 730 735Asp Gly Gly Tyr Met Asp Met Ser Lys Asp Glu Ser Val Asp
Tyr Val 740 745 750Pro Met Leu Asp Met Lys Gly Asp Val Lys Tyr Ala
Asp Ile Glu Ser 755 760 765Ser Asn Tyr Met Ala Pro Tyr Asp Asn Tyr
Val Pro Ser Ala Pro Glu 770 775 780Arg Thr Cys Arg Ala Thr Leu Ile
Asn Glu Ser Pro Val Leu Ser Tyr785 790 795 800Met Asp Leu Val Gly
Phe Ser Tyr Gln Val Ala Asn Gly Met Glu Phe 805 810 815Leu Ala Ser
Lys Asn Cys Val His Arg Asp Leu Ala Ala Arg Asn Val 820 825 830Leu
Ile Cys Glu Gly Lys Leu Val Lys Ile Cys Asp Phe Gly Leu Ala 835 840
845Arg Asp Ile Met Arg Asp Ser Asn Tyr Ile Ser Lys Gly Ser Thr Phe
850 855 860Leu Pro Leu Lys Trp Met Ala Pro Glu Ser Ile Phe Asn Ser
Leu Tyr865 870 875 880Thr Thr Leu Ser Asp Val Trp Ser Phe Gly Ile
Leu Leu Trp Glu Ile 885 890 895Phe Thr Leu Gly Gly Thr Pro Tyr Pro
Glu Leu Pro Met Asn Glu Gln 900 905 910Phe Tyr Asn Ala Ile Lys Arg
Gly Tyr Arg Met Ala Gln Pro Ala His 915 920 925Ala Ser Asp Glu Ile
Tyr Glu Ile Met Gln Lys Cys Trp Glu Glu Lys 930 935 940Phe Glu Ile
Arg Pro Pro Phe Ser Gln Leu Val Leu Leu Leu Glu Arg945 950 955
960Leu Leu Gly Glu Gly Tyr Lys Lys Lys Tyr Gln Gln Val Asp Glu Glu
965 970 975Phe Leu Arg Ser Asp His Pro Ala Ile Leu Arg Ser Gln Ala
Arg Leu 980 985 990Pro Gly Phe His Gly Leu Arg Ser Pro Leu Asp Thr
Ser Ser Val Leu 995 1000 1005Tyr Thr Ala Val Gln Pro Asn Glu Gly
Asp Asn Asp Tyr Ile Ile 1010 1015 1020Pro Leu Pro Asp Pro Lys Pro
Glu Val Ala Asp Glu Gly Pro Leu 1025 1030 1035Glu Gly Ser Pro Ser
Leu Ala Ser Ser Thr Leu Asn Glu Val Asn 1040 1045 1050Thr Ser Ser
Thr Ile Ser Cys Asp Ser Pro Leu Glu Pro Gln Asp 1055 1060 1065Glu
Pro Glu Pro Glu Pro Gln Leu Glu Leu Gln Val Glu Pro Glu 1070 1075
1080Pro Glu Leu Glu Gln Leu Pro Asp Ser Gly Cys Pro Ala Pro Arg
1085 1090 1095Ala Glu Ala Glu Asp Ser Phe Leu 1100
1105341338PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 34Met Val Ser Tyr Trp Asp Thr Gly Val Leu Leu
Cys Ala Leu Leu Ser1 5 10 15Cys Leu Leu Leu Thr Gly Ser Ser Ser Gly
Ser Lys Leu Lys Asp Pro 20 25 30Glu Leu Ser Leu Lys Gly Thr Gln His
Ile Met Gln Ala Gly Gln Thr 35 40 45Leu His Leu Gln Cys Arg Gly Glu
Ala Ala His Lys Trp Ser Leu Pro 50 55 60Glu Met Val Ser Lys Glu Ser
Glu Arg Leu Ser Ile Thr Lys Ser Ala65 70 75 80Cys Gly Arg Asn Gly
Lys Gln Phe Cys Ser Thr Leu Thr Leu Asn Thr 85 90 95Ala Gln Ala Asn
His Thr Gly Phe Tyr Ser Cys Lys Tyr Leu Ala Val 100 105 110Pro Thr
Ser Lys Lys Lys Glu Thr Glu Ser Ala Ile Tyr Ile Phe Ile 115 120
125Ser Asp Thr Gly Arg Pro Phe Val Glu Met Tyr Ser Glu Ile Pro Glu
130 135 140Ile Ile His Met Thr Glu Gly Arg Glu Leu Val Ile Pro Cys
Arg Val145 150 155 160Thr Ser Pro Asn Ile Thr Val Thr Leu Lys Lys
Phe Pro Leu Asp Thr 165 170 175Leu Ile Pro Asp Gly Lys Arg Ile Ile
Trp Asp Ser Arg Lys Gly Phe 180 185 190Ile Ile Ser Asn Ala Thr Tyr
Lys Glu Ile Gly Leu Leu Thr Cys Glu 195 200 205Ala Thr Val Asn Gly
His Leu Tyr Lys Thr Asn Tyr Leu Thr His Arg 210 215 220Gln Thr Asn
Thr Ile Ile Asp Val Gln Ile Ser Thr Pro Arg Pro Val225 230 235
240Lys Leu Leu Arg Gly His Thr Leu Val Leu Asn Cys Thr Ala Thr Thr
245 250 255Pro Leu Asn Thr Arg Val Gln Met Thr Trp Ser Tyr Pro Asp
Glu Lys 260 265 270Asn Lys Arg Ala Ser Val Arg Arg Arg Ile Asp Gln
Ser Asn Ser His 275 280 285Ala Asn Ile Phe Tyr Ser Val Leu Thr Ile
Asp Lys Met Gln Asn Lys 290 295 300Asp Lys Gly Leu Tyr Thr Cys Arg
Val Arg Ser Gly Pro Ser Phe Lys305 310 315 320Ser Val Asn Thr Ser
Val His Ile Tyr Asp Lys Ala Phe Ile Thr Val 325 330 335Lys His Arg
Lys Gln Gln Val Leu Glu Thr Val Ala Gly Lys Arg Ser 340 345 350Tyr
Arg Leu Ser Met Lys Val Lys Ala Phe Pro Ser Pro Glu Val Val 355 360
365Trp Leu Lys Asp Gly Leu Pro Ala Thr Glu Lys Ser Ala Arg Tyr Leu
370 375 380Thr Arg Gly Tyr Ser Leu Ile Ile Lys Asp Val Thr Glu Glu
Asp Ala385 390 395 400Gly Asn Tyr Thr Ile Leu Leu Ser Ile Lys Gln
Ser Asn Val Phe Lys 405 410 415Asn Leu Thr Ala Thr Leu Ile Val Asn
Val Lys Pro Gln Ile Tyr Glu 420 425 430Lys Ala Val Ser Ser Phe Pro
Asp Pro Ala Leu Tyr Pro Leu Gly Ser 435 440 445Arg Gln Ile Leu Thr
Cys Thr Ala Tyr Gly Ile Pro Gln Pro Thr Ile 450 455 460Lys Trp Phe
Trp His Pro Cys Asn His Asn His Ser Glu Ala Arg Cys465 470 475
480Asp Phe Cys Ser Asn Asn Glu Glu Ser Phe Ile Leu Asp Ala Asp Ser
485 490 495Asn Met Gly Asn Arg Ile Glu Ser Ile Thr Gln Arg Met Ala
Ile Ile 500 505 510Glu Gly Lys Asn Lys Met Ala Ser Thr Leu Val Val
Ala Asp Ser Arg 515 520 525Ile Ser Gly Ile Tyr Ile Cys Ile Ala Ser
Asn Lys Val Gly Thr Val 530 535 540Gly Arg Asn Ile Ser Phe Tyr Ile
Thr Asp Val Pro Asn Gly Phe His545 550 555 560Val Asn Leu Glu Lys
Met Pro Thr Glu Gly Glu Asp Leu Lys Leu Ser 565 570 575Cys Thr Val
Asn Lys Phe Leu Tyr Arg Asp Val Thr Trp Ile Leu Leu 580 585 590Arg
Thr Val Asn Asn Arg Thr Met His Tyr Ser Ile Ser Lys Gln Lys 595 600
605Met Ala Ile Thr Lys Glu His Ser Ile Thr Leu Asn Leu Thr Ile Met
610 615 620Asn Val Ser Leu Gln Asp Ser Gly Thr Tyr Ala Cys Arg Ala
Arg Asn625 630 635 640Val Tyr Thr Gly Glu Glu Ile Leu Gln Lys Lys
Glu Ile Thr Ile Arg 645 650 655Asp Gln Glu Ala Pro Tyr Leu Leu Arg
Asn Leu Ser Asp His Thr Val 660 665 670Ala Ile Ser Ser Ser Thr Thr
Leu Asp Cys His Ala Asn Gly Val Pro 675 680 685Glu Pro Gln Ile Thr
Trp Phe Lys Asn Asn His Lys Ile Gln Gln Glu 690 695 700Pro Gly Ile
Ile Leu Gly Pro Gly Ser Ser Thr Leu Phe Ile Glu Arg705 710 715
720Val Thr Glu Glu Asp Glu Gly Val Tyr His Cys Lys Ala Thr Asn Gln
725 730 735Lys Gly Ser Val Glu Ser Ser Ala Tyr Leu Thr Val Gln Gly
Thr Ser 740 745 750Asp Lys Ser Asn Leu Glu Leu Ile Thr Leu Thr Cys
Thr Cys Val Ala 755 760 765Ala Thr Leu Phe Trp Leu Leu Leu Thr Leu
Phe Ile Arg Lys Met Lys 770 775 780Arg Ser Ser Ser Glu Ile Lys Thr
Asp Tyr Leu Ser Ile Ile Met Asp785 790 795
800Pro Asp Glu Val Pro Leu Asp Glu Gln Cys Glu Arg Leu Pro Tyr Asp
805 810 815Ala Ser Lys Trp Glu Phe Ala Arg Glu Arg Leu Lys Leu Gly
Lys Ser 820 825 830Leu Gly Arg Gly Ala Phe Gly Lys Val Val Gln Ala
Ser Ala Phe Gly 835 840 845Ile Lys Lys Ser Pro Thr Cys Arg Thr Val
Ala Val Lys Met Leu Lys 850 855 860Glu Gly Ala Thr Ala Ser Glu Tyr
Lys Ala Leu Met Thr Glu Leu Lys865 870 875 880Ile Leu Thr His Ile
Gly His His Leu Asn Val Val Asn Leu Leu Gly 885 890 895Ala Cys Thr
Lys Gln Gly Gly Pro Leu Met Val Ile Val Glu Tyr Cys 900 905 910Lys
Tyr Gly Asn Leu Ser Asn Tyr Leu Lys Ser Lys Arg Asp Leu Phe 915 920
925Phe Leu Asn Lys Asp Ala Ala Leu His Met Glu Pro Lys Lys Glu Lys
930 935 940Met Glu Pro Gly Leu Glu Gln Gly Lys Lys Pro Arg Leu Asp
Ser Val945 950 955 960Thr Ser Ser Glu Ser Phe Ala Ser Ser Gly Phe
Gln Glu Asp Lys Ser 965 970 975Leu Ser Asp Val Glu Glu Glu Glu Asp
Ser Asp Gly Phe Tyr Lys Glu 980 985 990Pro Ile Thr Met Glu Asp Leu
Ile Ser Tyr Ser Phe Gln Val Ala Arg 995 1000 1005Gly Met Glu Phe
Leu Ser Ser Arg Lys Cys Ile His Arg Asp Leu 1010 1015 1020Ala Ala
Arg Asn Ile Leu Leu Ser Glu Asn Asn Val Val Lys Ile 1025 1030
1035Cys Asp Phe Gly Leu Ala Arg Asp Ile Tyr Lys Asn Pro Asp Tyr
1040 1045 1050Val Arg Lys Gly Asp Thr Arg Leu Pro Leu Lys Trp Met
Ala Pro 1055 1060 1065Glu Ser Ile Phe Asp Lys Ile Tyr Ser Thr Lys
Ser Asp Val Trp 1070 1075 1080Ser Tyr Gly Val Leu Leu Trp Glu Ile
Phe Ser Leu Gly Gly Ser 1085 1090 1095Pro Tyr Pro Gly Val Gln Met
Asp Glu Asp Phe Cys Ser Arg Leu 1100 1105 1110Arg Glu Gly Met Arg
Met Arg Ala Pro Glu Tyr Ser Thr Pro Glu 1115 1120 1125Ile Tyr Gln
Ile Met Leu Asp Cys Trp His Arg Asp Pro Lys Glu 1130 1135 1140Arg
Pro Arg Phe Ala Glu Leu Val Glu Lys Leu Gly Asp Leu Leu 1145 1150
1155Gln Ala Asn Val Gln Gln Asp Gly Lys Asp Tyr Ile Pro Ile Asn
1160 1165 1170Ala Ile Leu Thr Gly Asn Ser Gly Phe Thr Tyr Ser Thr
Pro Ala 1175 1180 1185Phe Ser Glu Asp Phe Phe Lys Glu Ser Ile Ser
Ala Pro Lys Phe 1190 1195 1200Asn Ser Gly Ser Ser Asp Asp Val Arg
Tyr Val Asn Ala Phe Lys 1205 1210 1215Phe Met Ser Leu Glu Arg Ile
Lys Thr Phe Glu Glu Leu Leu Pro 1220 1225 1230Asn Ala Thr Ser Met
Phe Asp Asp Tyr Gln Gly Asp Ser Ser Thr 1235 1240 1245Leu Leu Ala
Ser Pro Met Leu Lys Arg Phe Thr Trp Thr Asp Ser 1250 1255 1260Lys
Pro Lys Ala Ser Leu Lys Ile Asp Leu Arg Val Thr Ser Lys 1265 1270
1275Ser Lys Glu Ser Gly Leu Ser Asp Val Ser Arg Pro Ser Phe Cys
1280 1285 1290His Ser Ser Cys Gly His Val Ser Glu Gly Lys Arg Arg
Phe Thr 1295 1300 1305Tyr Asp His Ala Glu Leu Glu Arg Lys Ile Ala
Cys Cys Ser Pro 1310 1315 1320Pro Pro Asp Tyr Asn Ser Val Val Leu
Tyr Ser Thr Pro Pro Ile 1325 1330 1335351356PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
35Met Gln Ser Lys Val Leu Leu Ala Val Ala Leu Trp Leu Cys Val Glu1
5 10 15Thr Arg Ala Ala Ser Val Gly Leu Pro Ser Val Ser Leu Asp Leu
Pro 20 25 30Arg Leu Ser Ile Gln Lys Asp Ile Leu Thr Ile Lys Ala Asn
Thr Thr 35 40 45Leu Gln Ile Thr Cys Arg Gly Gln Arg Asp Leu Asp Trp
Leu Trp Pro 50 55 60Asn Asn Gln Ser Gly Ser Glu Gln Arg Val Glu Val
Thr Glu Cys Ser65 70 75 80Asp Gly Leu Phe Cys Lys Thr Leu Thr Ile
Pro Lys Val Ile Gly Asn 85 90 95Asp Thr Gly Ala Tyr Lys Cys Phe Tyr
Arg Glu Thr Asp Leu Ala Ser 100 105 110Val Ile Tyr Val Tyr Val Gln
Asp Tyr Arg Ser Pro Phe Ile Ala Ser 115 120 125Val Ser Asp Gln His
Gly Val Val Tyr Ile Thr Glu Asn Lys Asn Lys 130 135 140Thr Val Val
Ile Pro Cys Leu Gly Ser Ile Ser Asn Leu Asn Val Ser145 150 155
160Leu Cys Ala Arg Tyr Pro Glu Lys Arg Phe Val Pro Asp Gly Asn Arg
165 170 175Ile Ser Trp Asp Ser Lys Lys Gly Phe Thr Ile Pro Ser Tyr
Met Ile 180 185 190Ser Tyr Ala Gly Met Val Phe Cys Glu Ala Lys Ile
Asn Asp Glu Ser 195 200 205Tyr Gln Ser Ile Met Tyr Ile Val Val Val
Val Gly Tyr Arg Ile Tyr 210 215 220Asp Val Val Leu Ser Pro Ser His
Gly Ile Glu Leu Ser Val Gly Glu225 230 235 240Lys Leu Val Leu Asn
Cys Thr Ala Arg Thr Glu Leu Asn Val Gly Ile 245 250 255Asp Phe Asn
Trp Glu Tyr Pro Ser Ser Lys His Gln His Lys Lys Leu 260 265 270Val
Asn Arg Asp Leu Lys Thr Gln Ser Gly Ser Glu Met Lys Lys Phe 275 280
285Leu Ser Thr Leu Thr Ile Asp Gly Val Thr Arg Ser Asp Gln Gly Leu
290 295 300Tyr Thr Cys Ala Ala Ser Ser Gly Leu Met Thr Lys Lys Asn
Ser Thr305 310 315 320Phe Val Arg Val His Glu Lys Pro Phe Val Ala
Phe Gly Ser Gly Met 325 330 335Glu Ser Leu Val Glu Ala Thr Val Gly
Glu Arg Val Arg Ile Pro Ala 340 345 350Lys Tyr Leu Gly Tyr Pro Pro
Pro Glu Ile Lys Trp Tyr Lys Asn Gly 355 360 365Ile Pro Leu Glu Ser
Asn His Thr Ile Lys Ala Gly His Val Leu Thr 370 375 380Ile Met Glu
Val Ser Glu Arg Asp Thr Gly Asn Tyr Thr Val Ile Leu385 390 395
400Thr Asn Pro Ile Ser Lys Glu Lys Gln Ser His Val Val Ser Leu Val
405 410 415Val Tyr Val Pro Pro Gln Ile Gly Glu Lys Ser Leu Ile Ser
Pro Val 420 425 430Asp Ser Tyr Gln Tyr Gly Thr Thr Gln Thr Leu Thr
Cys Thr Val Tyr 435 440 445Ala Ile Pro Pro Pro His His Ile His Trp
Tyr Trp Gln Leu Glu Glu 450 455 460Glu Cys Ala Asn Glu Pro Ser Gln
Ala Val Ser Val Thr Asn Pro Tyr465 470 475 480Pro Cys Glu Glu Trp
Arg Ser Val Glu Asp Phe Gln Gly Gly Asn Lys 485 490 495Ile Glu Val
Asn Lys Asn Gln Phe Ala Leu Ile Glu Gly Lys Asn Lys 500 505 510Thr
Val Ser Thr Leu Val Ile Gln Ala Ala Asn Val Ser Ala Leu Tyr 515 520
525Lys Cys Glu Ala Val Asn Lys Val Gly Arg Gly Glu Arg Val Ile Ser
530 535 540Phe His Val Thr Arg Gly Pro Glu Ile Thr Leu Gln Pro Asp
Met Gln545 550 555 560Pro Thr Glu Gln Glu Ser Val Ser Leu Trp Cys
Thr Ala Asp Arg Ser 565 570 575Thr Phe Glu Asn Leu Thr Trp Tyr Lys
Leu Gly Pro Gln Pro Leu Pro 580 585 590Ile His Val Gly Glu Leu Pro
Thr Pro Val Cys Lys Asn Leu Asp Thr 595 600 605Leu Trp Lys Leu Asn
Ala Thr Met Phe Ser Asn Ser Thr Asn Asp Ile 610 615 620Leu Ile Met
Glu Leu Lys Asn Ala Ser Leu Gln Asp Gln Gly Asp Tyr625 630 635
640Val Cys Leu Ala Gln Asp Arg Lys Thr Lys Lys Arg His Cys Val Val
645 650 655Arg Gln Leu Thr Val Leu Glu Arg Val Ala Pro Thr Ile Thr
Gly Asn 660 665 670Leu Glu Asn Gln Thr Thr Ser Ile Gly Glu Ser Ile
Glu Val Ser Cys 675 680 685Thr Ala Ser Gly Asn Pro Pro Pro Gln Ile
Met Trp Phe Lys Asp Asn 690 695 700Glu Thr Leu Val Glu Asp Ser Gly
Ile Val Leu Lys Asp Gly Asn Arg705 710 715 720Asn Leu Thr Ile Arg
Arg Val Arg Lys Glu Asp Glu Gly Leu Tyr Thr 725 730 735Cys Gln Ala
Cys Ser Val Leu Gly Cys Ala Lys Val Glu Ala Phe Phe 740 745 750Ile
Ile Glu Gly Ala Gln Glu Lys Thr Asn Leu Glu Ile Ile Ile Leu 755 760
765Val Gly Thr Ala Val Ile Ala Met Phe Phe Trp Leu Leu Leu Val Ile
770 775 780Ile Leu Arg Thr Val Lys Arg Ala Asn Gly Gly Glu Leu Lys
Thr Gly785 790 795 800Tyr Leu Ser Ile Val Met Asp Pro Asp Glu Leu
Pro Leu Asp Glu His 805 810 815Cys Glu Arg Leu Pro Tyr Asp Ala Ser
Lys Trp Glu Phe Pro Arg Asp 820 825 830Arg Leu Lys Leu Gly Lys Pro
Leu Gly Arg Gly Ala Phe Gly Gln Val 835 840 845Ile Glu Ala Asp Ala
Phe Gly Ile Asp Lys Thr Ala Thr Cys Arg Thr 850 855 860Val Ala Val
Lys Met Leu Lys Glu Gly Ala Thr His Ser Glu His Arg865 870 875
880Ala Leu Met Ser Glu Leu Lys Ile Leu Ile His Ile Gly His His Leu
885 890 895Asn Val Val Asn Leu Leu Gly Ala Cys Thr Lys Pro Gly Gly
Pro Leu 900 905 910Met Val Ile Val Glu Phe Cys Lys Phe Gly Asn Leu
Ser Thr Tyr Leu 915 920 925Arg Ser Lys Arg Asn Glu Phe Val Pro Tyr
Lys Thr Lys Gly Ala Arg 930 935 940Phe Arg Gln Gly Lys Asp Tyr Val
Gly Ala Ile Pro Val Asp Leu Lys945 950 955 960Arg Arg Leu Asp Ser
Ile Thr Ser Ser Gln Ser Ser Ala Ser Ser Gly 965 970 975Phe Val Glu
Glu Lys Ser Leu Ser Asp Val Glu Glu Glu Glu Ala Pro 980 985 990Glu
Asp Leu Tyr Lys Asp Phe Leu Thr Leu Glu His Leu Ile Cys Tyr 995
1000 1005Ser Phe Gln Val Ala Lys Gly Met Glu Phe Leu Ala Ser Arg
Lys 1010 1015 1020Cys Ile His Arg Asp Leu Ala Ala Arg Asn Ile Leu
Leu Ser Glu 1025 1030 1035Lys Asn Val Val Lys Ile Cys Asp Phe Gly
Leu Ala Arg Asp Ile 1040 1045 1050Tyr Lys Asp Pro Asp Tyr Val Arg
Lys Gly Asp Ala Arg Leu Pro 1055 1060 1065Leu Lys Trp Met Ala Pro
Glu Thr Ile Phe Asp Arg Val Tyr Thr 1070 1075 1080Ile Gln Ser Asp
Val Trp Ser Phe Gly Val Leu Leu Trp Glu Ile 1085 1090 1095Phe Ser
Leu Gly Ala Ser Pro Tyr Pro Gly Val Lys Ile Asp Glu 1100 1105
1110Glu Phe Cys Arg Arg Leu Lys Glu Gly Thr Arg Met Arg Ala Pro
1115 1120 1125Asp Tyr Thr Thr Pro Glu Met Tyr Gln Thr Met Leu Asp
Cys Trp 1130 1135 1140His Gly Glu Pro Ser Gln Arg Pro Thr Phe Ser
Glu Leu Val Glu 1145 1150 1155His Leu Gly Asn Leu Leu Gln Ala Asn
Ala Gln Gln Asp Gly Lys 1160 1165 1170Asp Tyr Ile Val Leu Pro Ile
Ser Glu Thr Leu Ser Met Glu Glu 1175 1180 1185Asp Ser Gly Leu Ser
Leu Pro Thr Ser Pro Val Ser Cys Met Glu 1190 1195 1200Glu Glu Glu
Val Cys Asp Pro Lys Phe His Tyr Asp Asn Thr Ala 1205 1210 1215Gly
Ile Ser Gln Tyr Leu Gln Asn Ser Lys Arg Lys Ser Arg Pro 1220 1225
1230Val Ser Val Lys Thr Phe Glu Asp Ile Pro Leu Glu Glu Pro Glu
1235 1240 1245Val Lys Val Ile Pro Asp Asp Asn Gln Thr Asp Ser Gly
Met Val 1250 1255 1260Leu Ala Ser Glu Glu Leu Lys Thr Leu Glu Asp
Arg Thr Lys Leu 1265 1270 1275Ser Pro Ser Phe Gly Gly Met Val Pro
Ser Lys Ser Arg Glu Ser 1280 1285 1290Val Ala Ser Glu Gly Ser Asn
Gln Thr Ser Gly Tyr Gln Ser Gly 1295 1300 1305Tyr His Ser Asp Asp
Thr Asp Thr Thr Val Tyr Ser Ser Glu Glu 1310 1315 1320Ala Glu Leu
Leu Lys Leu Ile Glu Ile Gly Val Gln Thr Gly Ser 1325 1330 1335Thr
Ala Gln Ile Leu Gln Pro Asp Ser Gly Thr Thr Leu Ser Ser 1340 1345
1350Pro Pro Val 1355361363PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 36Met Gln Arg Gly Ala Ala
Leu Cys Leu Arg Leu Trp Leu Cys Leu Gly1 5 10 15Leu Leu Asp Gly Leu
Val Ser Gly Tyr Ser Met Thr Pro Pro Thr Leu 20 25 30Asn Ile Thr Glu
Glu Ser His Val Ile Asp Thr Gly Asp Ser Leu Ser 35 40 45Ile Ser Cys
Arg Gly Gln His Pro Leu Glu Trp Ala Trp Pro Gly Ala 50 55 60Gln Glu
Ala Pro Ala Thr Gly Asp Lys Asp Ser Glu Asp Thr Gly Val65 70 75
80Val Arg Asp Cys Glu Gly Thr Asp Ala Arg Pro Tyr Cys Lys Val Leu
85 90 95Leu Leu His Glu Val His Ala Asn Asp Thr Gly Ser Tyr Val Cys
Tyr 100 105 110Tyr Lys Tyr Ile Lys Ala Arg Ile Glu Gly Thr Thr Ala
Ala Ser Ser 115 120 125Tyr Val Phe Val Arg Asp Phe Glu Gln Pro Phe
Ile Asn Lys Pro Asp 130 135 140Thr Leu Leu Val Asn Arg Lys Asp Ala
Met Trp Val Pro Cys Leu Val145 150 155 160Ser Ile Pro Gly Leu Asn
Val Thr Leu Arg Ser Gln Ser Ser Val Leu 165 170 175Trp Pro Asp Gly
Gln Glu Val Val Trp Asp Asp Arg Arg Gly Met Leu 180 185 190Val Ser
Thr Pro Leu Leu His Asp Ala Leu Tyr Leu Gln Cys Glu Thr 195 200
205Thr Trp Gly Asp Gln Asp Phe Leu Ser Asn Pro Phe Leu Val His Ile
210 215 220Thr Gly Asn Glu Leu Tyr Asp Ile Gln Leu Leu Pro Arg Lys
Ser Leu225 230 235 240Glu Leu Leu Val Gly Glu Lys Leu Val Leu Asn
Cys Thr Val Trp Ala 245 250 255Glu Phe Asn Ser Gly Val Thr Phe Asp
Trp Asp Tyr Pro Gly Lys Gln 260 265 270Ala Glu Arg Gly Lys Trp Val
Pro Glu Arg Arg Ser Gln Gln Thr His 275 280 285Thr Glu Leu Ser Ser
Ile Leu Thr Ile His Asn Val Ser Gln His Asp 290 295 300Leu Gly Ser
Tyr Val Cys Lys Ala Asn Asn Gly Ile Gln Arg Phe Arg305 310 315
320Glu Ser Thr Glu Val Ile Val His Glu Asn Pro Phe Ile Ser Val Glu
325 330 335Trp Leu Lys Gly Pro Ile Leu Glu Ala Thr Ala Gly Asp Glu
Leu Val 340 345 350Lys Leu Pro Val Lys Leu Ala Ala Tyr Pro Pro Pro
Glu Phe Gln Trp 355 360 365Tyr Lys Asp Gly Lys Ala Leu Ser Gly Arg
His Ser Pro His Ala Leu 370 375 380Val Leu Lys Glu Val Thr Glu Ala
Ser Thr Gly Thr Tyr Thr Leu Ala385 390 395 400Leu Trp Asn Ser Ala
Ala Gly Leu Arg Arg Asn Ile Ser Leu Glu Leu 405 410 415Val Val Asn
Val Pro Pro Gln Ile His Glu Lys Glu Ala Ser Ser Pro 420 425 430Ser
Ile Tyr Ser Arg His Ser Arg Gln Ala Leu Thr Cys Thr Ala Tyr 435 440
445Gly Val Pro Leu Pro Leu Ser Ile Gln Trp His Trp Arg Pro Trp Thr
450 455 460Pro Cys Lys Met Phe Ala Gln Arg Ser Leu Arg Arg Arg Gln
Gln Gln465 470 475 480Asp Leu Met Pro Gln Cys Arg Asp Trp Arg Ala
Val Thr Thr Gln Asp 485 490 495Ala Val Asn Pro Ile Glu Ser Leu Asp
Thr Trp Thr Glu Phe Val Glu 500 505 510Gly Lys Asn Lys Thr Val Ser
Lys Leu Val Ile Gln Asn Ala Asn Val 515 520 525Ser Ala Met Tyr Lys
Cys Val Val Ser Asn Lys Val Gly Gln Asp Glu 530 535
540Arg Leu Ile Tyr Phe Tyr Val Thr Thr Ile Pro Asp Gly Phe Thr
Ile545 550 555 560Glu Ser Lys Pro Ser Glu Glu Leu Leu Glu Gly Gln
Pro Val Leu Leu 565 570 575Ser Cys Gln Ala Asp Ser Tyr Lys Tyr Glu
His Leu Arg Trp Tyr Arg 580 585 590Leu Asn Leu Ser Thr Leu His Asp
Ala His Gly Asn Pro Leu Leu Leu 595 600 605Asp Cys Lys Asn Val His
Leu Phe Ala Thr Pro Leu Ala Ala Ser Leu 610 615 620Glu Glu Val Ala
Pro Gly Ala Arg His Ala Thr Leu Ser Leu Ser Ile625 630 635 640Pro
Arg Val Ala Pro Glu His Glu Gly His Tyr Val Cys Glu Val Gln 645 650
655Asp Arg Arg Ser His Asp Lys His Cys His Lys Lys Tyr Leu Ser Val
660 665 670Gln Ala Leu Glu Ala Pro Arg Leu Thr Gln Asn Leu Thr Asp
Leu Leu 675 680 685Val Asn Val Ser Asp Ser Leu Glu Met Gln Cys Leu
Val Ala Gly Ala 690 695 700His Ala Pro Ser Ile Val Trp Tyr Lys Asp
Glu Arg Leu Leu Glu Glu705 710 715 720Lys Ser Gly Val Asp Leu Ala
Asp Ser Asn Gln Lys Leu Ser Ile Gln 725 730 735Arg Val Arg Glu Glu
Asp Ala Gly Arg Tyr Leu Cys Ser Val Cys Asn 740 745 750Ala Lys Gly
Cys Val Asn Ser Ser Ala Ser Val Ala Val Glu Gly Ser 755 760 765Glu
Asp Lys Gly Ser Met Glu Ile Val Ile Leu Val Gly Thr Gly Val 770 775
780Ile Ala Val Phe Phe Trp Val Leu Leu Leu Leu Ile Phe Cys Asn
Met785 790 795 800Arg Arg Pro Ala His Ala Asp Ile Lys Thr Gly Tyr
Leu Ser Ile Ile 805 810 815Met Asp Pro Gly Glu Val Pro Leu Glu Glu
Gln Cys Glu Tyr Leu Ser 820 825 830Tyr Asp Ala Ser Gln Trp Glu Phe
Pro Arg Glu Arg Leu His Leu Gly 835 840 845Arg Val Leu Gly Tyr Gly
Ala Phe Gly Lys Val Val Glu Ala Ser Ala 850 855 860Phe Gly Ile His
Lys Gly Ser Ser Cys Asp Thr Val Ala Val Lys Met865 870 875 880Leu
Lys Glu Gly Ala Thr Ala Ser Glu His Arg Ala Leu Met Ser Glu 885 890
895Leu Lys Ile Leu Ile His Ile Gly Asn His Leu Asn Val Val Asn Leu
900 905 910Leu Gly Ala Cys Thr Lys Pro Gln Gly Pro Leu Met Val Ile
Val Glu 915 920 925Phe Cys Lys Tyr Gly Asn Leu Ser Asn Phe Leu Arg
Ala Lys Arg Asp 930 935 940Ala Phe Ser Pro Cys Ala Glu Lys Ser Pro
Glu Gln Arg Gly Arg Phe945 950 955 960Arg Ala Met Val Glu Leu Ala
Arg Leu Asp Arg Arg Arg Pro Gly Ser 965 970 975Ser Asp Arg Val Leu
Phe Ala Arg Phe Ser Lys Thr Glu Gly Gly Ala 980 985 990Arg Arg Ala
Ser Pro Asp Gln Glu Ala Glu Asp Leu Trp Leu Ser Pro 995 1000
1005Leu Thr Met Glu Asp Leu Val Cys Tyr Ser Phe Gln Val Ala Arg
1010 1015 1020Gly Met Glu Phe Leu Ala Ser Arg Lys Cys Ile His Arg
Asp Leu 1025 1030 1035Ala Ala Arg Asn Ile Leu Leu Ser Glu Ser Asp
Val Val Lys Ile 1040 1045 1050Cys Asp Phe Gly Leu Ala Arg Asp Ile
Tyr Lys Asp Pro Asp Tyr 1055 1060 1065Val Arg Lys Gly Ser Ala Arg
Leu Pro Leu Lys Trp Met Ala Pro 1070 1075 1080Glu Ser Ile Phe Asp
Lys Val Tyr Thr Thr Gln Ser Asp Val Trp 1085 1090 1095Ser Phe Gly
Val Leu Leu Trp Glu Ile Phe Ser Leu Gly Ala Ser 1100 1105 1110Pro
Tyr Pro Gly Val Gln Ile Asn Glu Glu Phe Cys Gln Arg Leu 1115 1120
1125Arg Asp Gly Thr Arg Met Arg Ala Pro Glu Leu Ala Thr Pro Ala
1130 1135 1140Ile Arg Arg Ile Met Leu Asn Cys Trp Ser Gly Asp Pro
Lys Ala 1145 1150 1155Arg Pro Ala Phe Ser Glu Leu Val Glu Ile Leu
Gly Asp Leu Leu 1160 1165 1170Gln Gly Arg Gly Leu Gln Glu Glu Glu
Glu Val Cys Met Ala Pro 1175 1180 1185Arg Ser Ser Gln Ser Ser Glu
Glu Gly Ser Phe Ser Gln Val Ser 1190 1195 1200Thr Met Ala Leu His
Ile Ala Gln Ala Asp Ala Glu Asp Ser Pro 1205 1210 1215Pro Ser Leu
Gln Arg His Ser Leu Ala Ala Arg Tyr Tyr Asn Trp 1220 1225 1230Val
Ser Phe Pro Gly Cys Leu Ala Arg Gly Ala Glu Thr Arg Gly 1235 1240
1245Ser Ser Arg Met Lys Thr Phe Glu Glu Phe Pro Met Thr Pro Thr
1250 1255 1260Thr Tyr Lys Gly Ser Val Asp Asn Gln Thr Asp Ser Gly
Met Val 1265 1270 1275Leu Ala Ser Glu Glu Phe Glu Gln Ile Glu Ser
Arg His Arg Gln 1280 1285 1290Glu Ser Gly Phe Ser Cys Lys Gly Pro
Gly Gln Asn Val Ala Val 1295 1300 1305Thr Arg Ala His Pro Asp Ser
Gln Gly Arg Arg Arg Arg Pro Glu 1310 1315 1320Arg Gly Ala Arg Gly
Gly Gln Val Phe Tyr Asn Ser Glu Tyr Gly 1325 1330 1335Glu Leu Ser
Glu Pro Ser Glu Glu Asp His Cys Ser Pro Ser Ala 1340 1345 1350Arg
Val Thr Phe Phe Thr Asp Asn Ser Tyr 1355 1360375PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 37Gly
Gly Gly Gly Ser1 5384PRTArtificial SequenceDescription of
Artificial Sequence Synthetic peptide 38Gly Gly Gly
Ser1395PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptide 39Gly Gly Gly Glu Ser1 54010PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 40Gly
Gly Gly Gly Ser Gly Gly Gly Gly Ser1 5 104121PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 41Gly
Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly1 5 10
15Gly Gly Gly Ser Gly 204210PRTArtificial SequenceDescription of
Artificial Sequence Synthetic peptide 42Gly Tyr Asp Phe Thr His Tyr
Gly Met Asn1 5 104317PRTArtificial SequenceDescription of
Artificial Sequence Synthetic peptide 43Trp Ile Asn Thr Tyr Thr Gly
Glu Pro Thr Tyr Ala Ala Asp Phe Lys1 5 10 15Arg4414PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 44Tyr
Pro Tyr Tyr Tyr Gly Thr Ser His Trp Tyr Phe Asp Val1 5
104511PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptide 45Ser Ala Ser Gln Asp Ile Ser Asn Tyr Leu Asn1 5
10467PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptide 46Phe Thr Ser Ser Leu His Ser1 5479PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 47Gln
Gln Tyr Ser Thr Val Pro Trp Thr1 54810PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 48Gly
Tyr Thr Phe Thr Asn Tyr Gly Met Asn1 5 104914PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 49Tyr
Pro His Tyr Tyr Gly Ser Ser His Trp Tyr Phe Asp Val1 5
105020PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptidemisc_feature(1)..(20)This sequence may encompass
1-4 'Gly-Gly-Gly- Gly-Ser' repeating units 50Gly Gly Gly Gly Ser
Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly1 5 10 15Gly Gly Gly Ser
20514PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptidemisc_feature(1)..(4)This sequence may encompass
1-4 'Gly' residues 51Gly Gly Gly Gly1528PRTArtificial
SequenceDescription of Artificial Sequence Synthetic
peptidemisc_feature(1)..(8)This sequence may encompass 1-4
'Gly-Gly' repeating units 52Gly Gly Gly Gly Gly Gly Gly Gly1
55316PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptidemisc_feature(1)..(16)This sequence may encompass
1-4 'Gly-Gly-Gly- Ser' repeating units 53Gly Gly Gly Ser Gly Gly
Gly Ser Gly Gly Gly Ser Gly Gly Gly Ser1 5 10 155420PRTArtificial
SequenceDescription of Artificial Sequence Synthetic
peptidemisc_feature(1)..(20)This sequence may encompass 1-4
'Gly-Gly-Gly- Glu-Ser' repeating units 54Gly Gly Gly Glu Ser Gly
Gly Gly Glu Ser Gly Gly Gly Glu Ser Gly1 5 10 15Gly Gly Glu Ser
20555PRTArtificial SequenceDescription of Artificial Sequence
Synthetic peptide 55Ser Gly Gly Gly Cys1 55629PRTArtificial
SequenceDescription of Artificial Sequence Synthetic peptide 56Gly
Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly1 5 10
15Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser 20 25












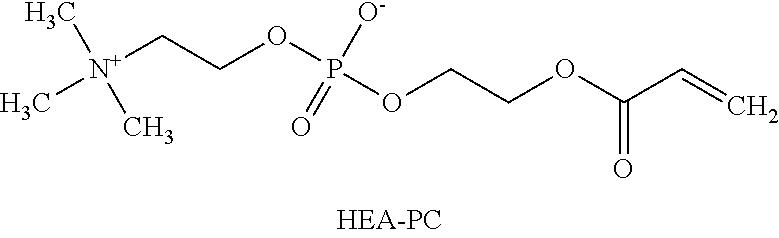
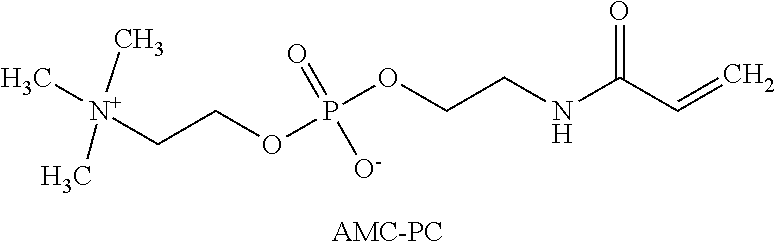

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008
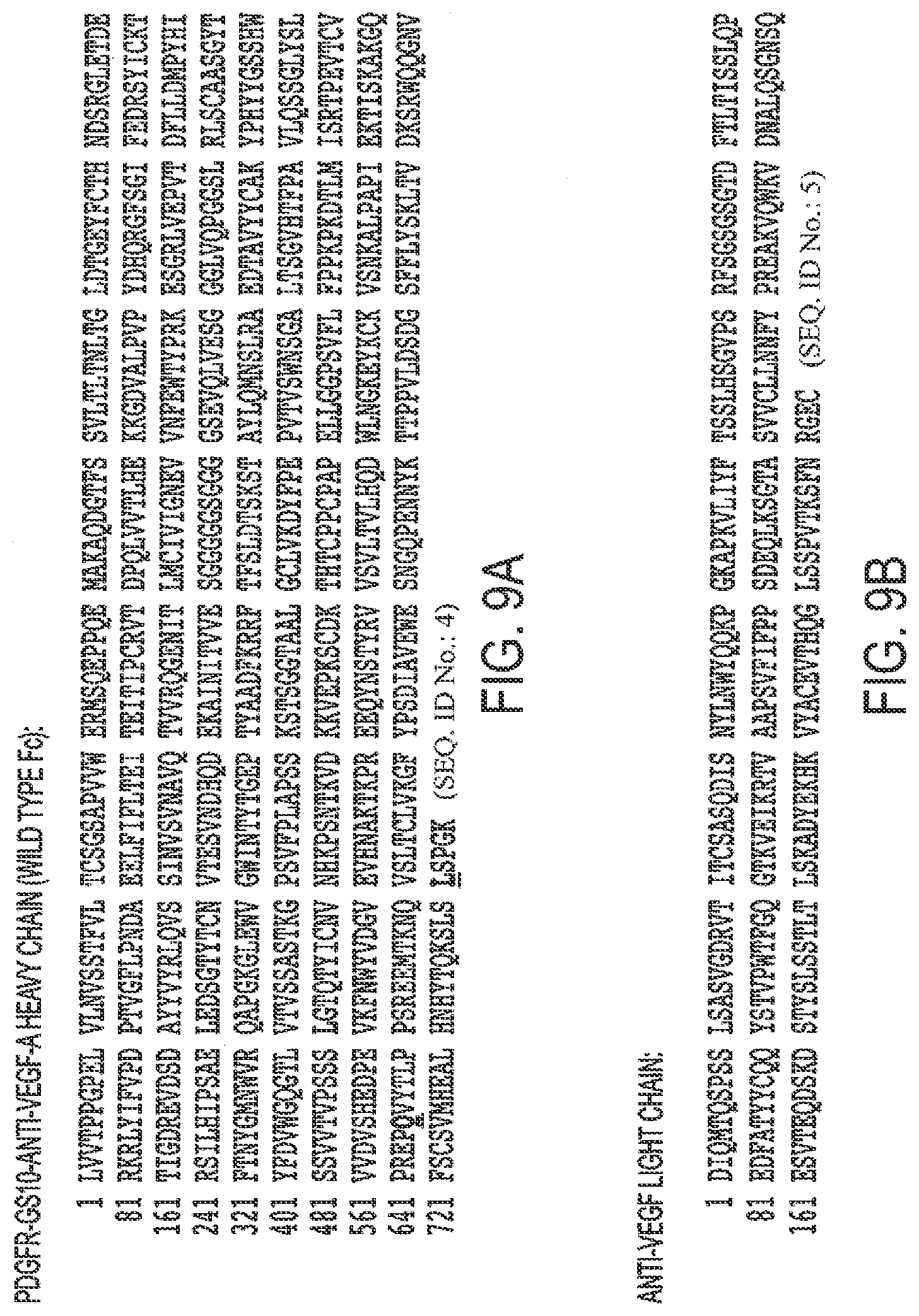
D00009

D00010

D00011
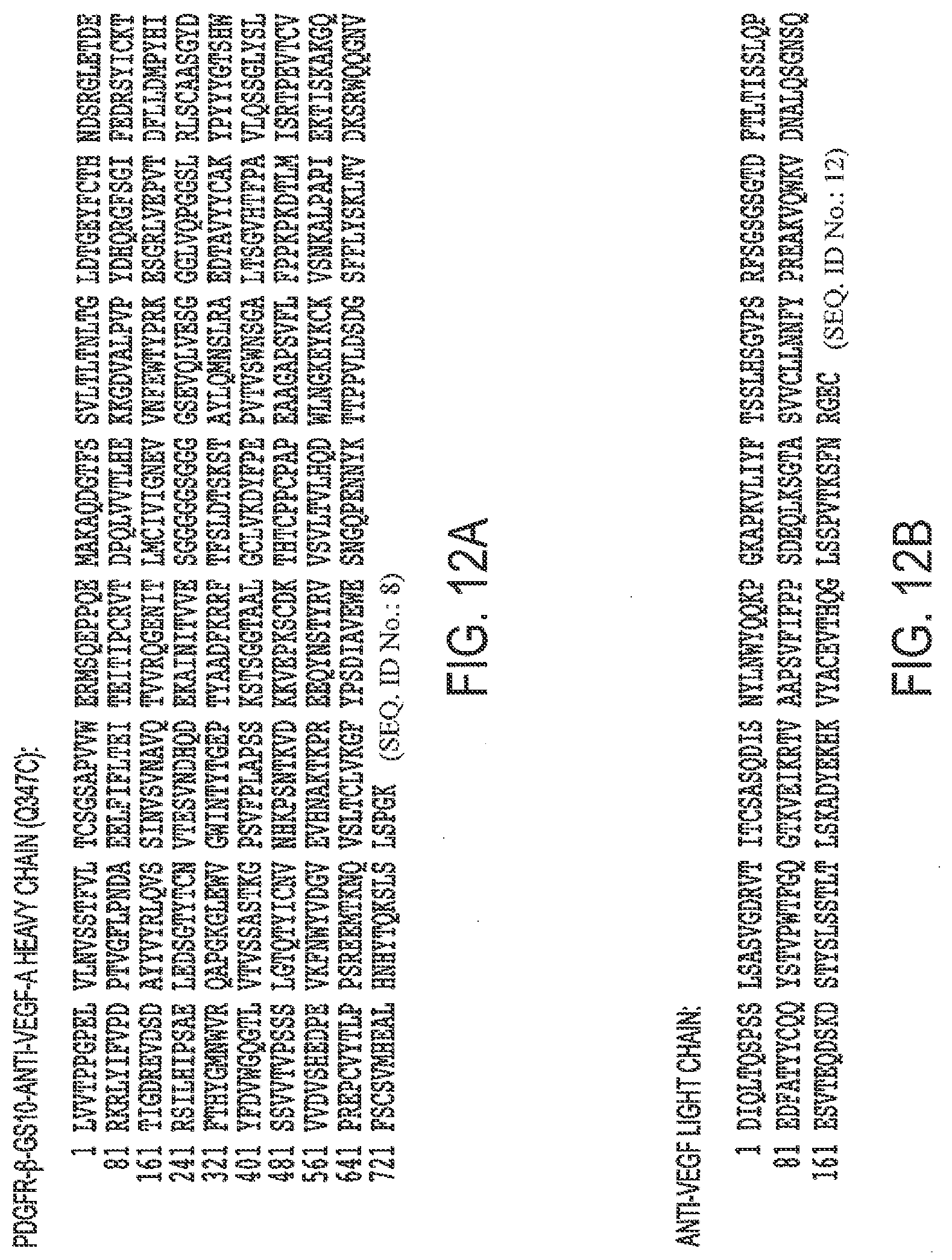
D00012
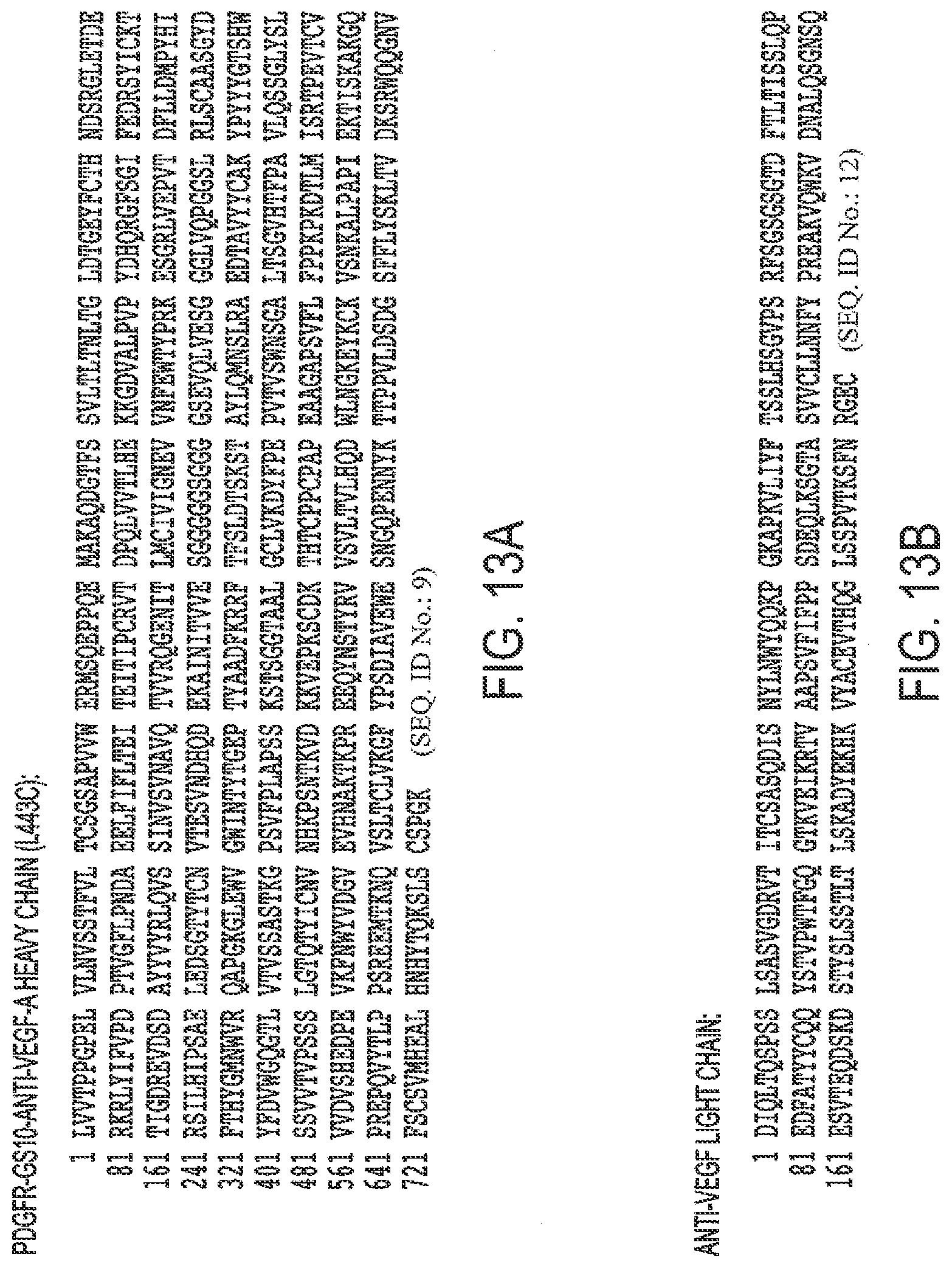
D00013

D00014

D00015

D00016

D00017

D00018

D00019

D00020
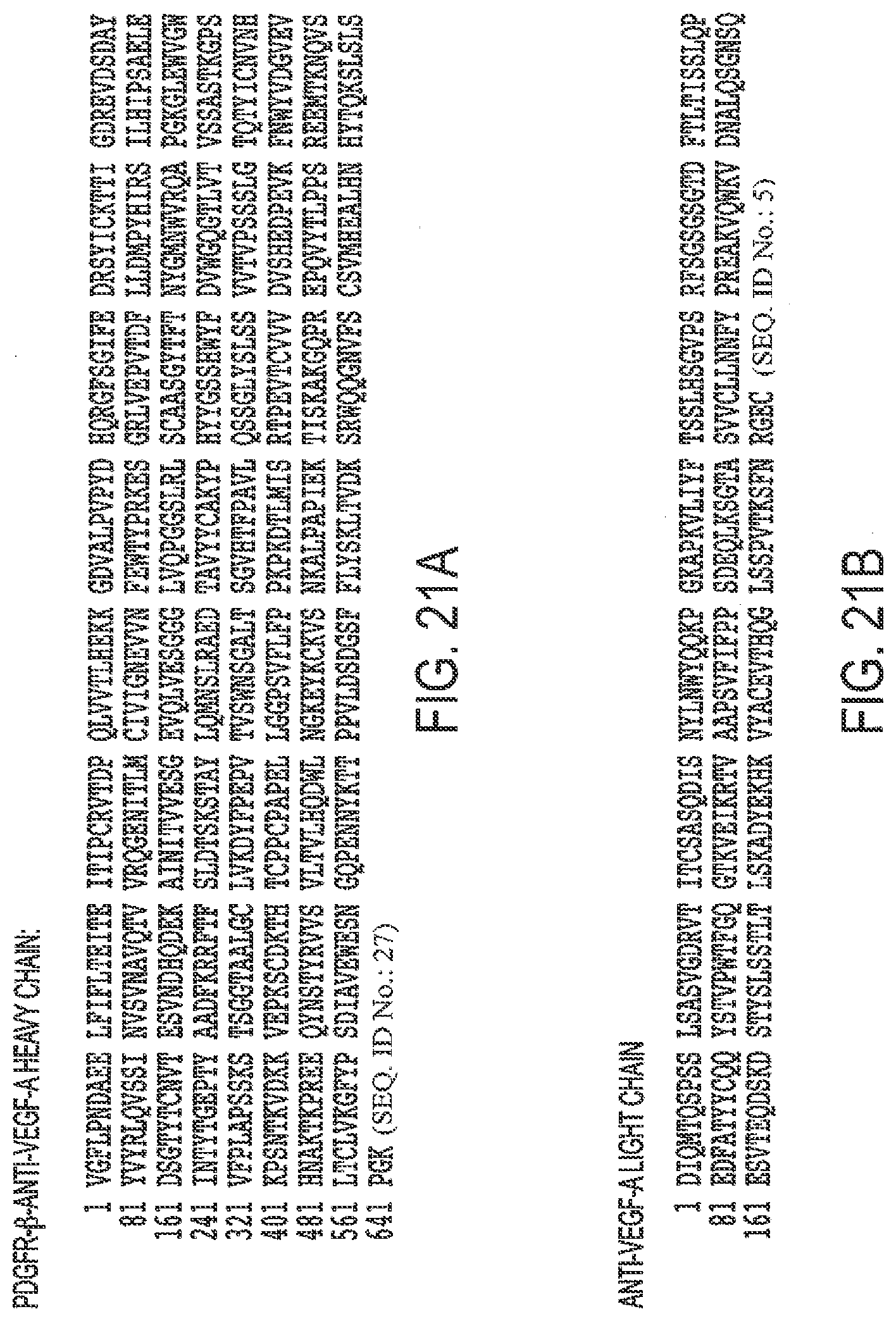
D00021
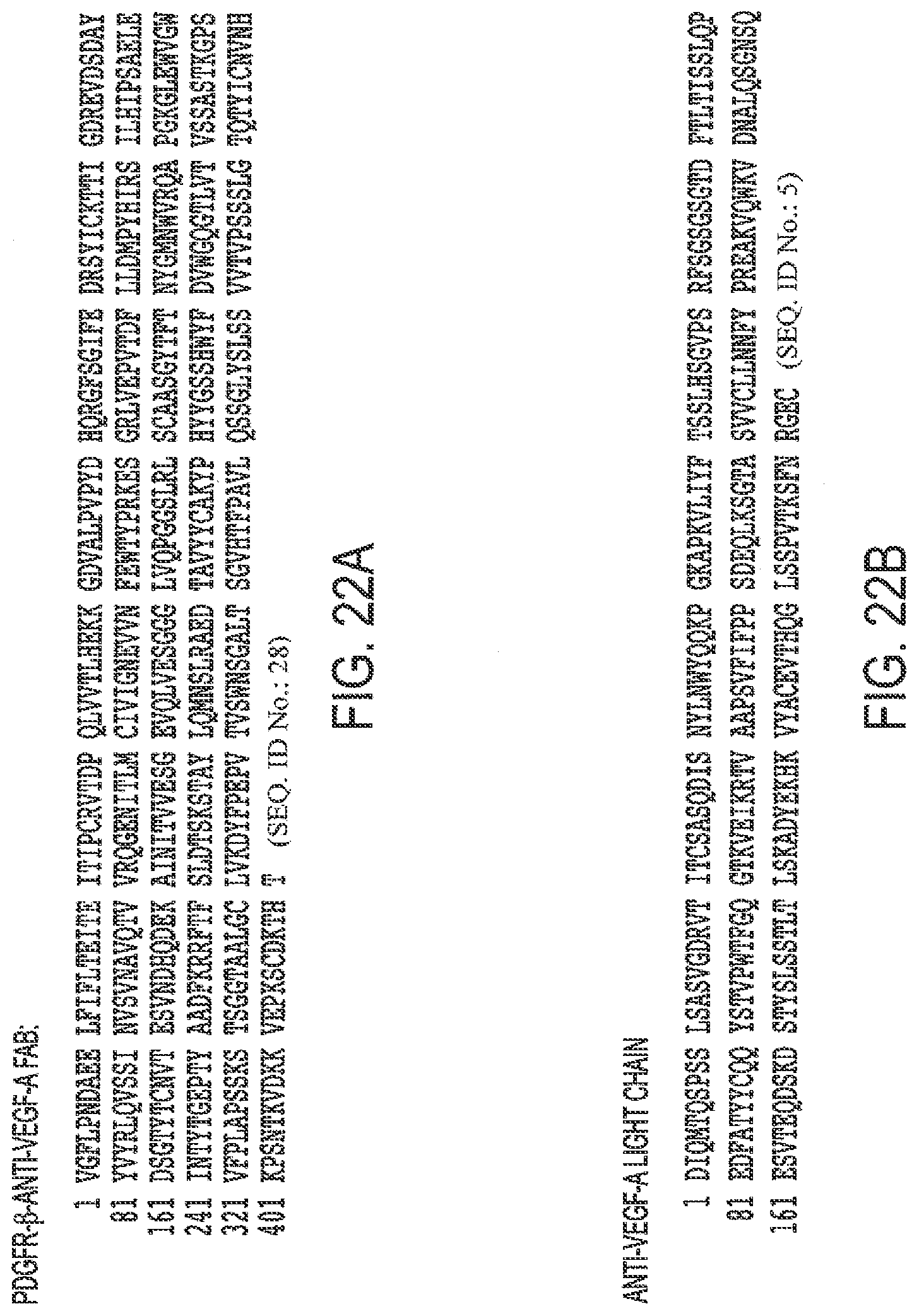
D00022

D00023

D00024

D00025

D00026

D00027
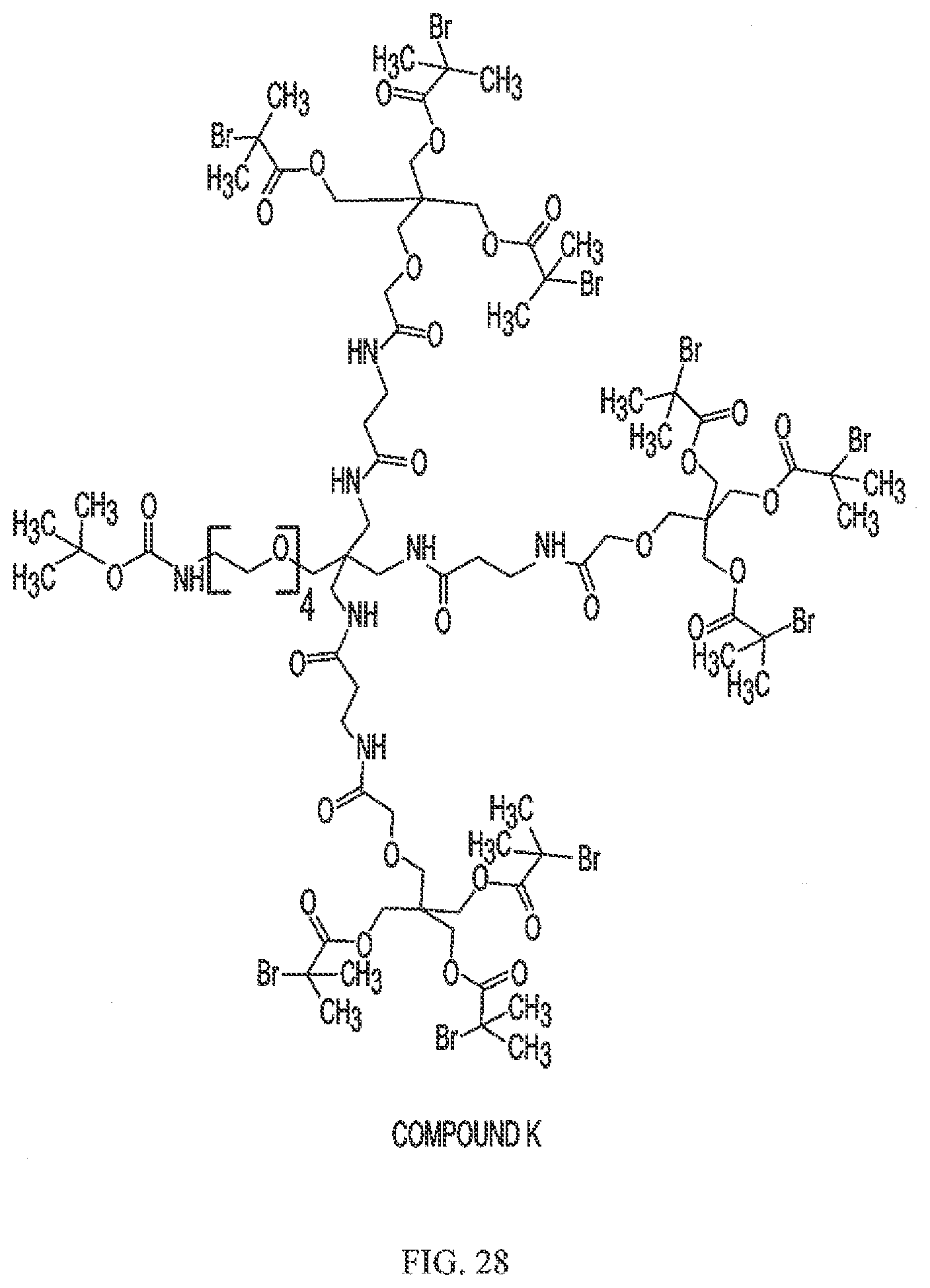
D00028

D00029

D00030
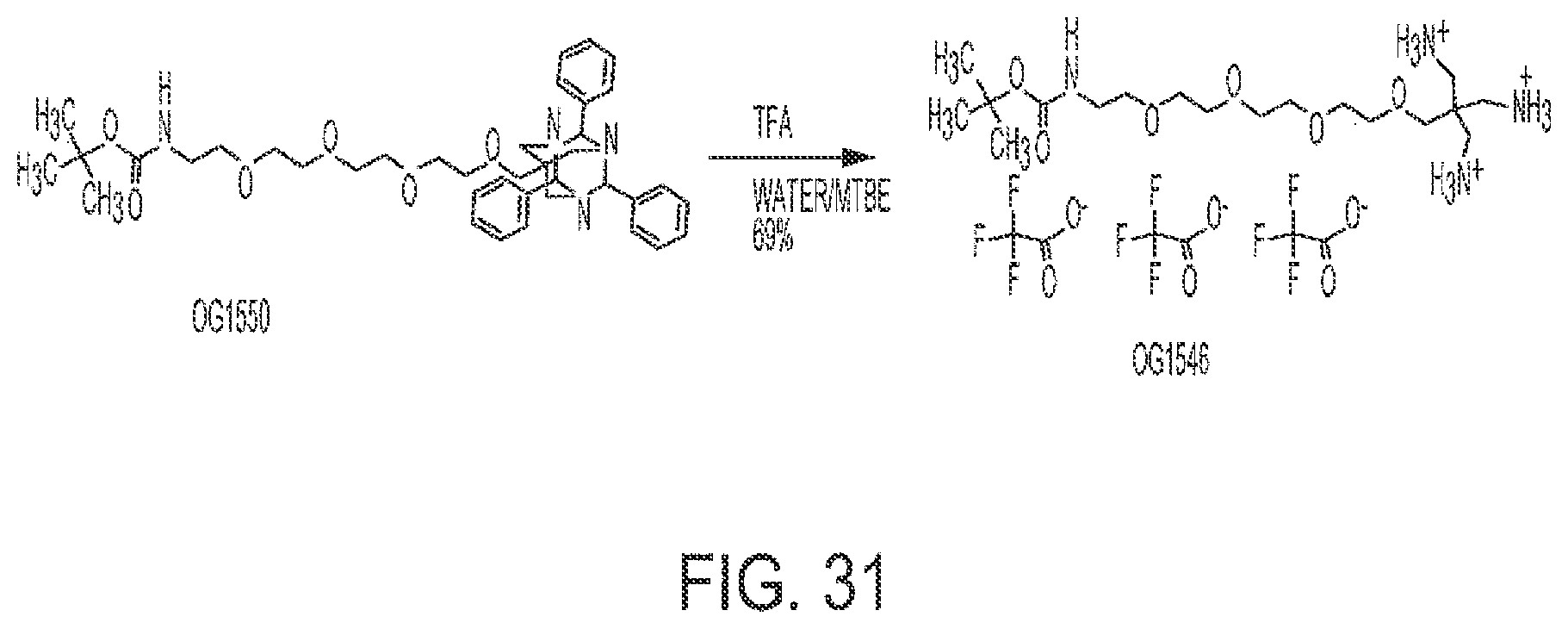
D00031

D00032
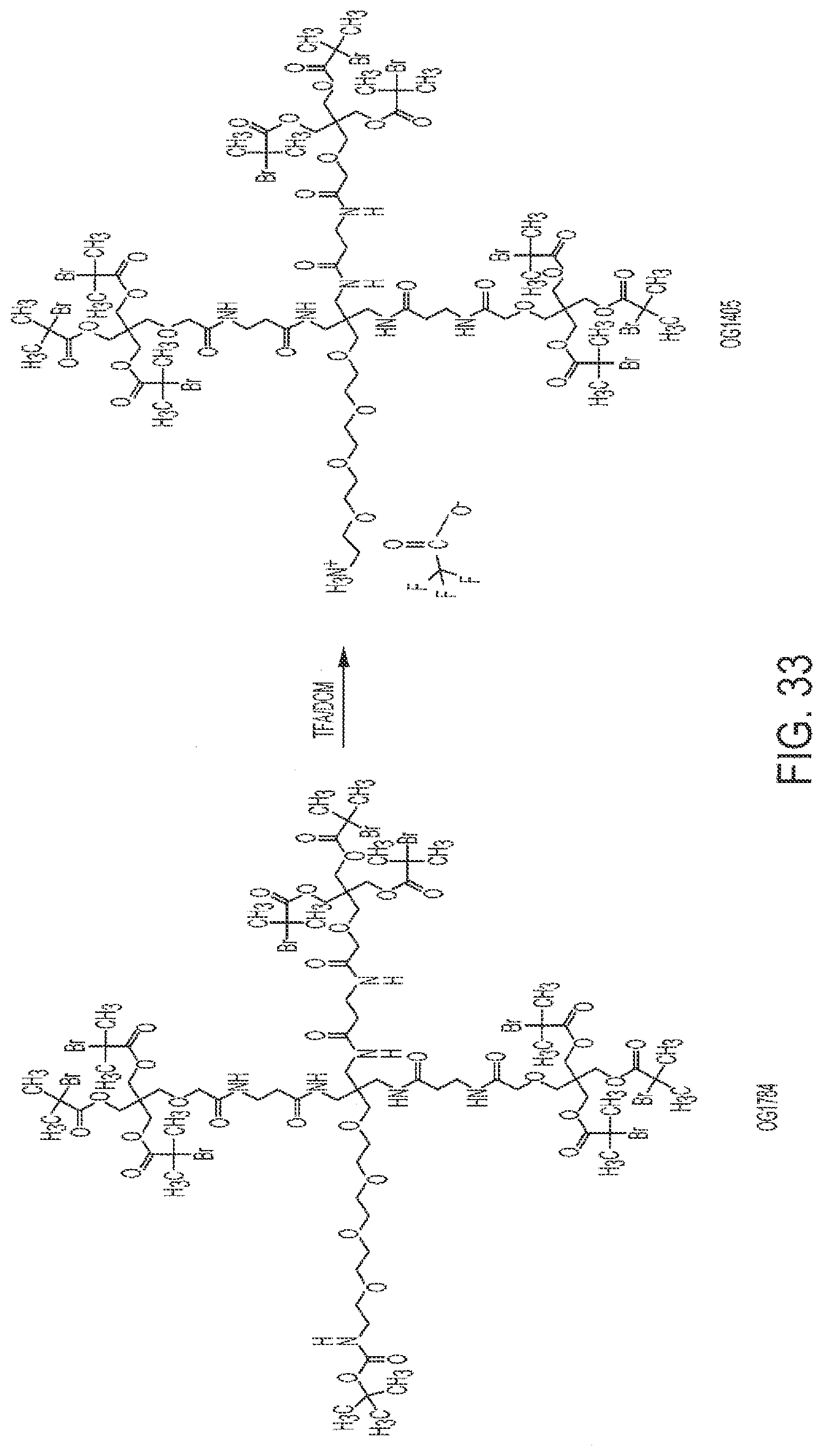
D00033
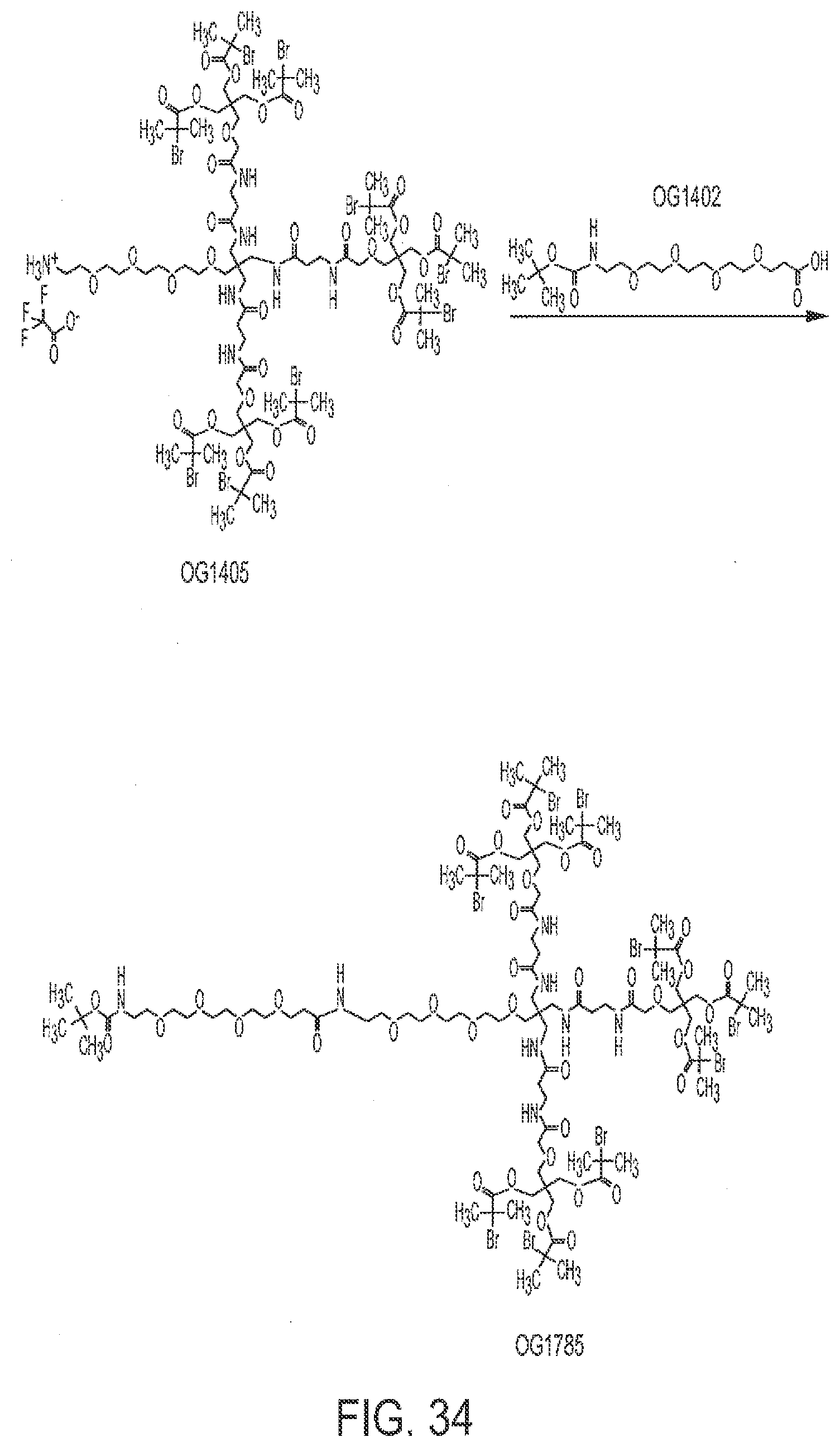
D00034

D00035
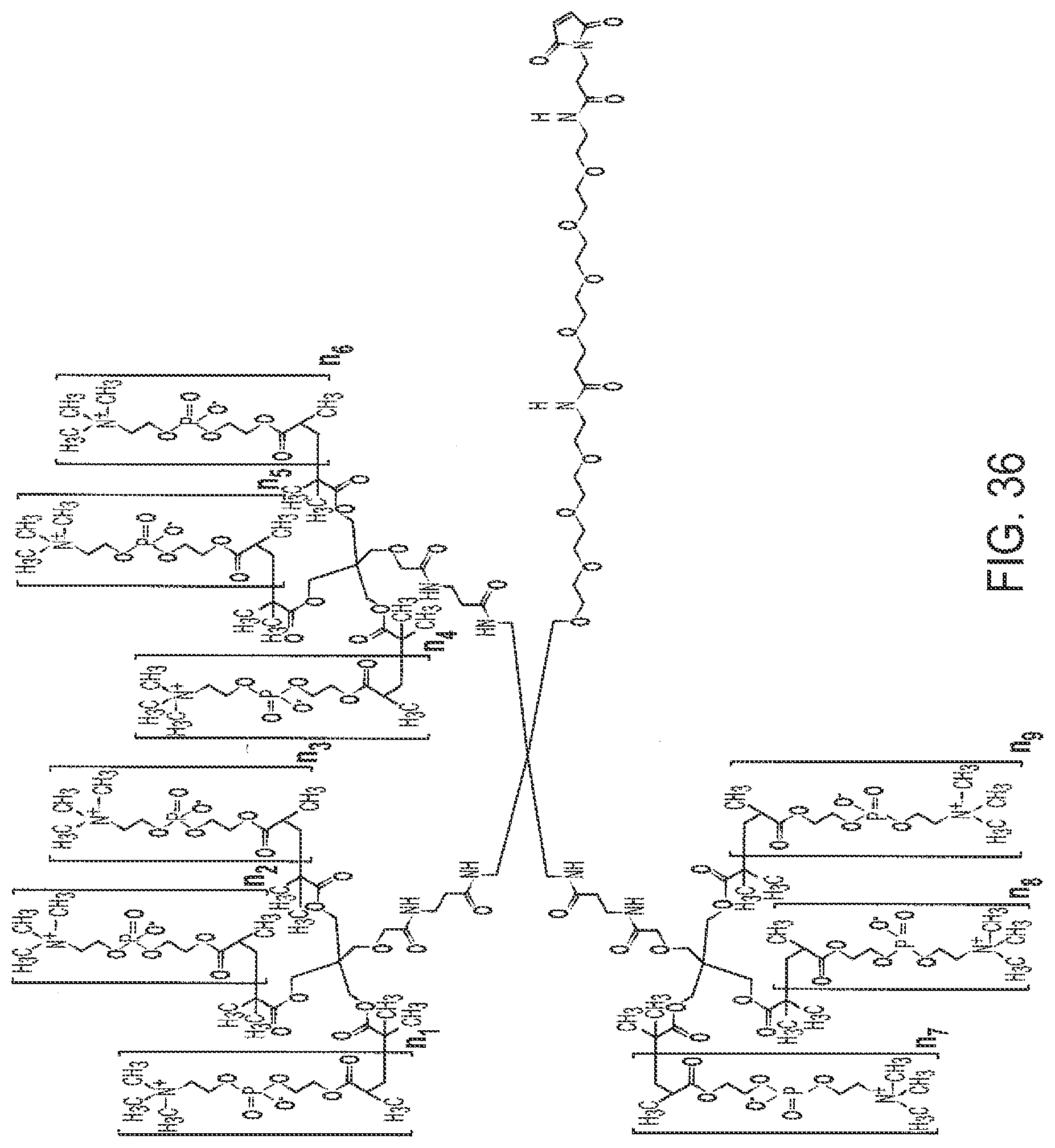
D00036

D00037

D00038

D00039

D00040

D00041
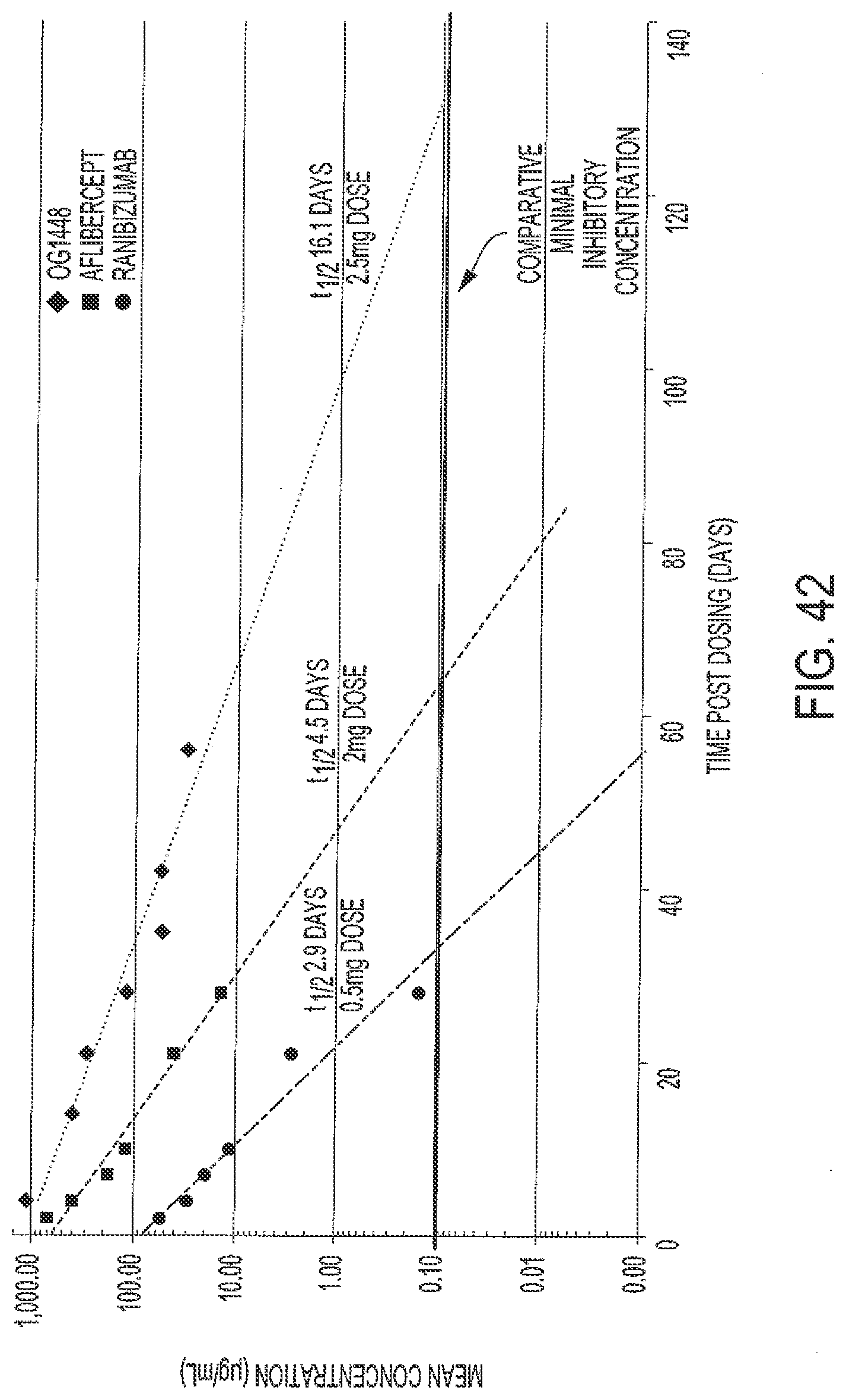
P00001

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.