Ketorolac Tromethamine Compositions For Treating Or Preventing Ocular Pain
Farnes; Eldon Quinn ; et al.
U.S. patent application number 16/806694 was filed with the patent office on 2020-06-25 for ketorolac tromethamine compositions for treating or preventing ocular pain. The applicant listed for this patent is Allergan, Inc.. Invention is credited to Mayssa Attar, Ching-Ming Chang, Eldon Quinn Farnes, Richard S. Graham, Rhett M. Schiffman, Devin F. Welty.
| Application Number | 20200197336 16/806694 |
| Document ID | / |
| Family ID | 40852460 |
| Filed Date | 2020-06-25 |

| United States Patent Application | 20200197336 |
| Kind Code | A1 |
| Farnes; Eldon Quinn ; et al. | June 25, 2020 |
KETOROLAC TROMETHAMINE COMPOSITIONS FOR TREATING OR PREVENTING OCULAR PAIN
Abstract
The present invention provides an aqueous ophthalmic solution comprising an effective amount of ketorolac which comprises carboxymethyl cellulose in an aqueous solution wherein said concentration of carboxymethyl cellulose is selected to provide an increased absorption of ketorolac in the eye of a patient that is at least 130% greater than the absorption of a comparative aqueous ketorolac ophthalmic solution having the same concentration of ketorolac.
| Inventors: | Farnes; Eldon Quinn; (Laguna Beach, CA) ; Attar; Mayssa; (Placentia, CA) ; Schiffman; Rhett M.; (Laguna Beach, CA) ; Chang; Ching-Ming; (Tustin, CA) ; Graham; Richard S.; (Irvine, CA) ; Welty; Devin F.; (Foothill Ranch, CA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 40852460 | ||||||||||
| Appl. No.: | 16/806694 | ||||||||||
| Filed: | March 2, 2020 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 13446870 | Apr 13, 2012 | |||
| 16806694 | ||||
| 12953622 | Nov 24, 2010 | |||
| 13446870 | ||||
| 12396131 | Mar 2, 2009 | 7842714 | ||
| 12953622 | ||||
| 61111919 | Nov 6, 2008 | |||
| 61096096 | Sep 11, 2008 | |||
| 61067925 | Mar 3, 2008 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 27/02 20180101; A61K 47/38 20130101; A61K 47/10 20130101; A61K 47/02 20130101; A61P 29/00 20180101; A61K 47/12 20130101; A61K 31/14 20130101; A61K 9/0048 20130101; A61K 47/186 20130101; A61K 31/407 20130101 |
| International Class: | A61K 31/14 20060101 A61K031/14; A61K 9/00 20060101 A61K009/00; A61K 47/18 20060101 A61K047/18; A61K 47/12 20060101 A61K047/12; A61K 47/38 20060101 A61K047/38; A61K 47/02 20060101 A61K047/02; A61K 31/407 20060101 A61K031/407; A61K 47/10 20060101 A61K047/10 |
Claims
1. A method for treating ocular pain and inflammation in a patient comprising administering a formulation to the patient comprising about 0.45% w/v ketorolac tromethamine and about 0.5% w/v carboxymethyl cellulose.
2. The method of claim 1 wherein the twice daily topical administration of the first composition to an eye increases the bioavailability of ketorolac in the eye relative to the twice daily topical administration of a second composition to an eye comprising about 0.5% (w/v) ketorolac tromethamine and no carboxymethyl cellulose.
3. The method of claim 1 wherein the first composition comprises 0.45% w/v ketorolac tromethamine.
4. The method of claim 1 wherein the first composition comprises at least one ingredient selected from the group consisting of sodium chloride, sodium citrate dihydrate, sodium hydroxide, hydrochloric acid and water.
5. The method of claim 4 further comprising 0.5% w/v carboxymethyl cellulose comprised of 0.325% w/v medium viscosity carboxymethyl cellulose and 0.175% w/v high viscosity carboxymethyl cellulose.
6. The method of claim 2 wherein the first composition increases bioavailability of the ketorolac in the eye relative to a second composition by up to about 78%.
7. The method of claim 1 wherein the first composition reduces ocular pain and inflammation following cataract surgery.
8. The method of claim 2 wherein the first composition reduces ocular pain and inflammation following cataract surgery to a greater extent than the second composition.
9. A method for treating ocular pain and inflammation in a patient comprising administering a formulation to the patient comprising about 0.45% w/v ketorolac tromethamine and about 0.5% w/v carboxymethyl cellulose, wherein the twice daily topical administration of the first composition to the eye increases bioavailability of ketorolac in the eye relative to the twice daily topical administration of a second composition comprising about 0.4% w/v ketorolac tromethamine and no carboxymethyl cellulose.
10. The method of claim 9 wherein the first composition increases bioavailability of ketorolac in the eye relative to the second composition by up to about 185%.
11. The method of claim 9 wherein the first composition comprises 0.45% w/v ketorolac tromethamine.
12. The method of claim 9 wherein the first composition comprises 0.5% w/v carboxymethyl cellulose.
13. The method of claim 11 wherein the first composition is used for treating pain and inflammation following cataract surgery.
14. The method of claim 12 wherein the 0.5% w/v carboxymethyl cellulose is comprised of 0.325% w/v medium viscosity carboxymethyl cellulose and 0.175% w/v carboxymethyl cellulose.
15. The method of claim 9 wherein the first composition further comprises sodium citrate dihydrate.
16. The method of claim 11 wherein the first composition reduces ocular pain and inflammation following cataract surgery to a greater extent than the second composition.
17. The method of claim 9 wherein the first composition reduces ocular pain and inflammation and results in less hyperemia than the second composition despite having a higher concentration of ketorolac.
18. The method of claim 9 wherein the first composition reduces ocular pain and inflammation and results in less ocular burning and stinging than the second composition.
19. A method for treating ocular pain and inflammation in a patient suffering therefrom comprising 0.45% w/v ketorolac tromethamine and 0.5% w/v carboxymethyl cellulose, wherein the twice daily topical administration of the first composition to an eye increases the bioavailability of the ketorolac in the eye relative to the four times daily topical administration of a second composition to an eye comprising about 0.4% (w/v) ketorolac tromethamine and no carboxymethyl cellulose.
20. The method of claim 19 wherein administering the first composition twice a day to an eye of a patient increases the bioavailability of ketorolac in the eye by up to about two times greater relative to the second composition administered to an eye of a patient four times a day.
21. The method of claim 19, wherein the first composition reduces ocular pain and inflammation with less ocular irritation as compared to the second composition.
22. The method of claim 19, wherein the first composition reduces ocular pain and inflammation and results in less hyperemia than the second composition.
23. The method of claim 19, wherein the first composition reduces ocular pain and inflammation and results in less ocular burning and stinging than the second composition.
24. The method of claim 19 wherein the 0.5% w/v carboxymethyl cellulose is comprised of 0.325% w/v medium viscosity carboxymethyl cellulose and 0.175% w/v carboxymethyl cellulose.
25. A method for treating ocular pain and inflammation in a patient suffering therefrom comprising 0.45% w/v ketorolac tromethamine, 0.5% w/v carboxy methyl cellulose, 0.7% w/v sodium chloride, 0.2% sodium citrate dihydrate and water.
26. The method of claim 25 wherein the pH of the composition is about 6.8.
27. The method of claim 25 wherein the 0.5% carboxymethyl cellulose is comprised of 0.325% w/v medium viscosity carboxymethyl cellulose and 0.175% w/v high viscosity carboxymethyl cellulose.
28. The method of claim 25 comprising sodium hydroxide and hydrochloric acid.
29. The method of claim 25 wherein the composition is used for treating ocular pain and inflammation following cataract surgery.
Description
RELATED APPLICATION
[0001] This application is a divisional of U.S. patent application Ser. No. 13/446,870, filed Apr. 13, 2012, which is a continuation of U.S. patent application Ser. No. 12/953,622, filed Nov. 24, 2010, now abandoned, which is a continuation of U.S. patent application Ser. No. 12/396,131, filed Mar. 2, 2009, now U.S. Pat. No. 7,842,714, issued Nov. 30, 2010, and to which claims the benefit of U.S. Provisional Application Ser. No. 61/067,925, filed Mar. 3, 2008; Ser. No. 61/096,096 filed Sep. 11, 2008; and Ser. No. 61/111,919 filed Nov. 6, 2008, the disclosures of which are hereby incorporated in their entirety herein by reference.
FIELD OF THE INVENTION
[0002] This invention relates to pharmaceutical compositions. More particularly, this invention relates to topical ophthalmic solutions comprising 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, otherwise known as ketorolac, and the use of ketorolac for treating or preventing ocular pain.
DESCRIPTION OF THE RELATED ART
[0003] Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are used to control pain and postoperative inflammation. All drugs are associated with some adverse effects. With the use of NSAIDS in topical ophthalmic treatment of patients, surface toxicity has been a concern, and incidents of keratitis, corneal subepithelial infiltrates, ulceration, and corneal melts have been reported (Guidera et al, Ophthalmology, 2001, 108 (5), pp. 936-944; Solomon et al, J Cataract Refract Surg, 2001, 27 (8), pp. 1232-1237; Teal et al, J Cataract Refract Surg, 1995, 21(5), pp. 516-518). Further, patients often report burning or stinging on instillation (Jaanus et al, Antiinflammatory Drugs. Clinical Ocular Pharmacology, Bartlet, J. D. and Jaanus, S. D., Ed., Boston: Heineman, 2001, pp. 265-298). The burning or stinging could be related to the concentration of the active component of the formulation.
[0004] Ketorolac tromethamine 0.5% (w/v) ophthalmic solution, available from Allergan, Inc., under the tradeneme ACULAR.RTM., is a safe and effective NSAID with proven analgesic and anti-inflammatory activity. The most common adverse event associated with the use of the 0.5% ketorolac formulation is ocular irritation, primarily burning and stinging upon instillation. Ketorolac tromethamine 0.4% (w/v) ophthalmic solution, under the trade name ACULAR LS.RTM., has shown improved bioavailability and less stinging on instillation than ACULAR.RTM., but there remains a need for an improved ketorolac tromethamine formulation with greater bioavailability and greater tolerability, minimized ocular surface toxicity, improved patient comfort, increased retention time of the active ingredient and improved wound healing capabilities during use.
[0005] It is one object of this invention to provide a ketorolac formulation for instillation in the eye to eliminate or reduce ocular irritation, to improve tolerability, compliance, duration and effect of ketorolac, to allow for dosing from four times daily to twice daily, and to increase the effectiveness of treatment by being free of benzalkonium chloride or other preservatives.
[0006] It is another object of the invention to improve bioavailability and increase the ocular absorption of ketorolac yet provide an aqueous solution having an optimized concentration of ketorolac.
[0007] It is another object of the invention to extend the effects of ketorolac and allow for a decrease in required daily dosage.
[0008] It is another object of the invention to provide reduction of inflammation associated with cataract surgery and reduction of pain associated with cataract surgery in comparison to other ketorolac formulations.
[0009] It is another object of the invention to create a ketorolac formulation with improved wound healing capabilities.
[0010] Other objects of this invention will become apparent from a reading of the present specification.
BRIEF DESCRIPTION OF DRAWINGS
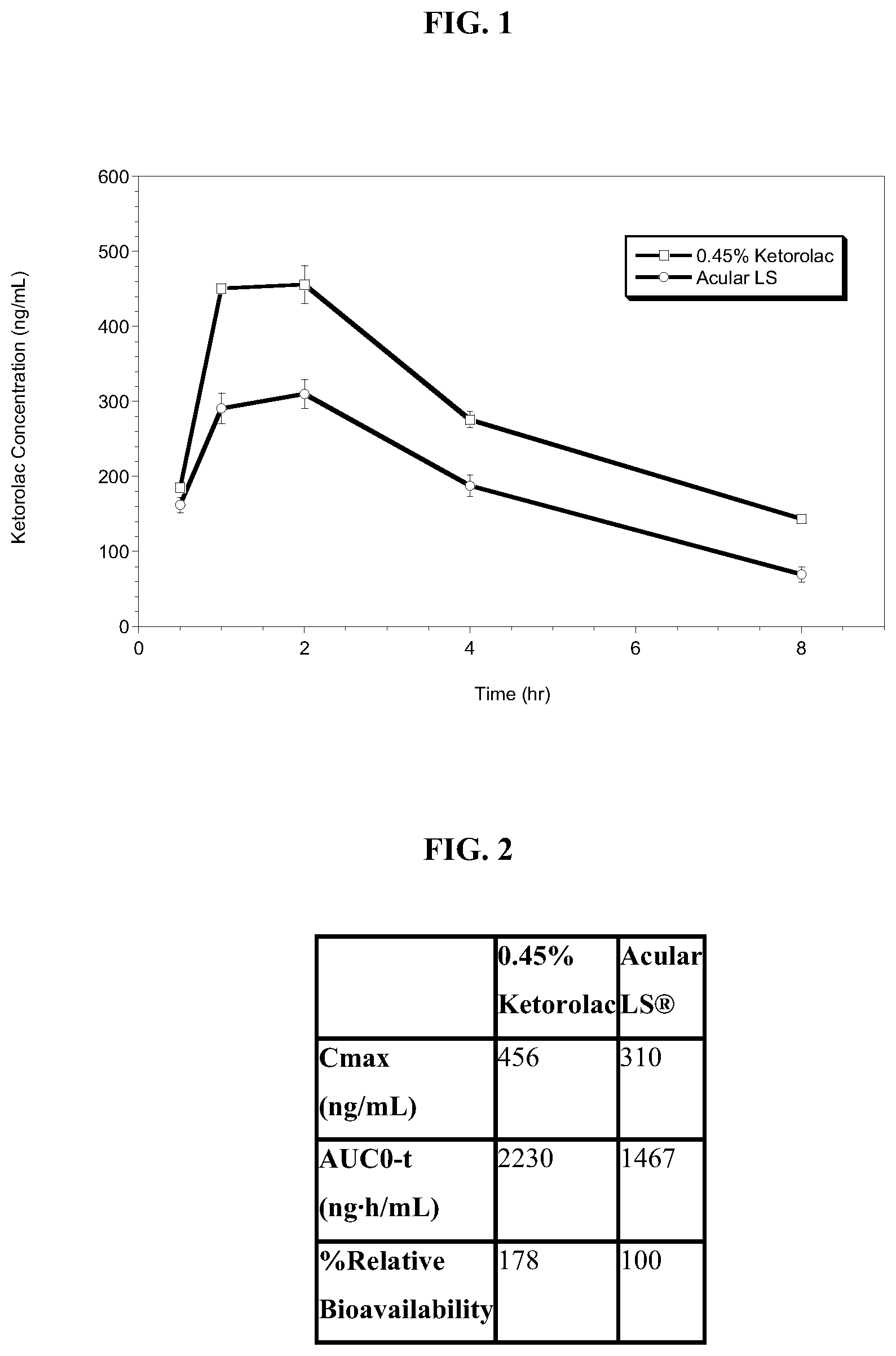
[0011] FIG. 1 shows the ocular pharmacokinetics of the results in Example 7 of the increased and prolonged ketorolac exposure in the aqueous humor of the 0.45% w/v ketorolac solution in comparison to ACULAR LS.RTM.;
[0012] FIG. 2 shows the results of FIG. 1 in table form of Cmax, AUC and percent relative bioavailability of the ocular pharmacokinetics in Example 7 of the aqueous humor relative bioavailability of 0.45% w/v ketorolac solution in comparison to ACULAR LS.RTM.;
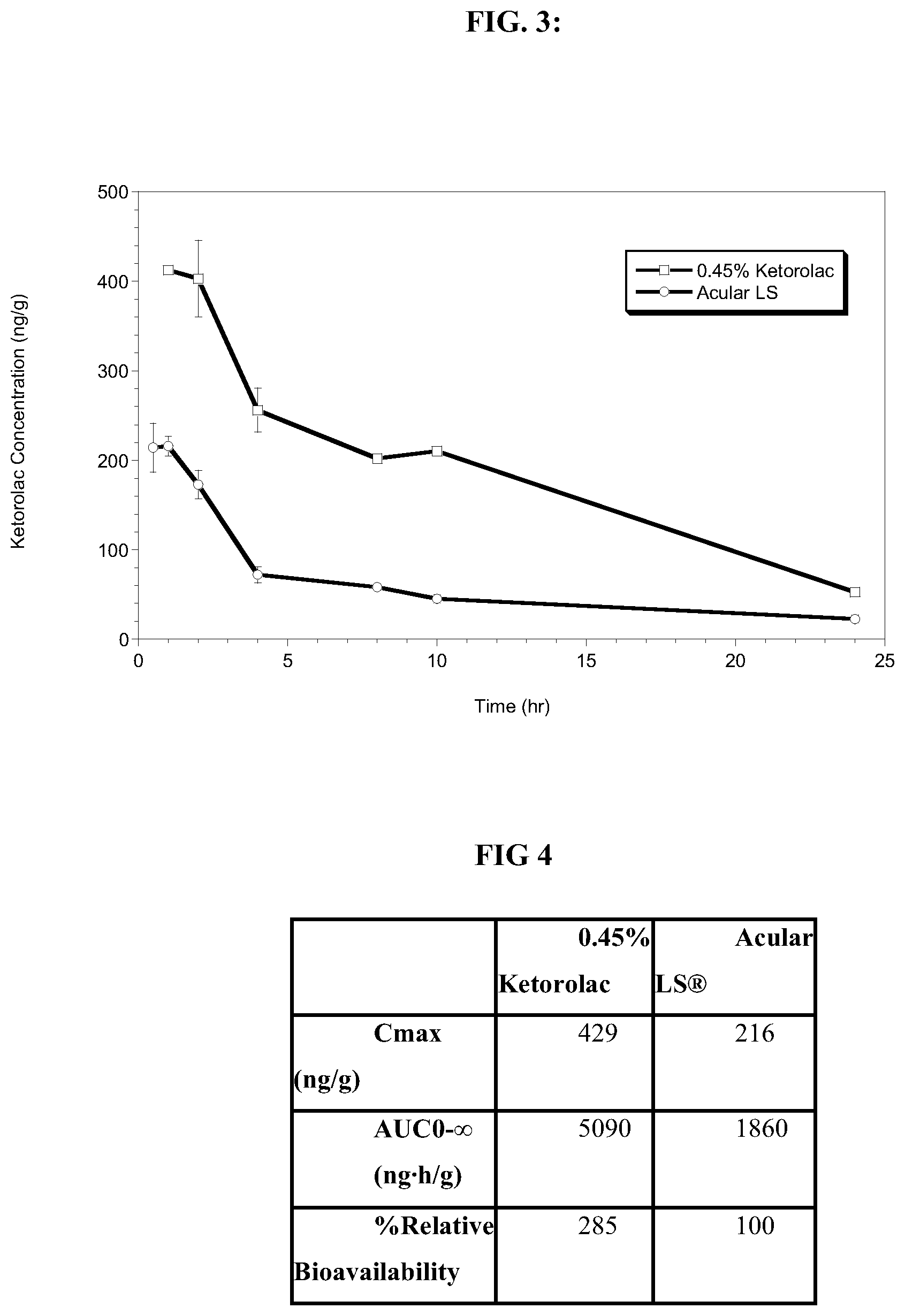
[0013] FIG. 3 shows the ocular pharmacokinetics of the results in Example 7 of the increased and prolonged ketorolac exposure in the iris-ciliary body of 0.45% w/v ketorolac solution in comparison to ACULAR LS.RTM.;
[0014] FIG. 4 shows the results of FIG. 3 in table form of Cmax, AUC and percent relative bioavailability of the increased and prolonged exposure in the iris ciliary body of 0.45% w/v ketorolac solution in comparison to ACULAR LS.RTM.;
[0015] FIG. 5 shows a multiple dose simulation of Example 7 of 0.45% ketorolac BID in comparison to ACULAR LS.RTM. QID in the iris ciliary body; and,
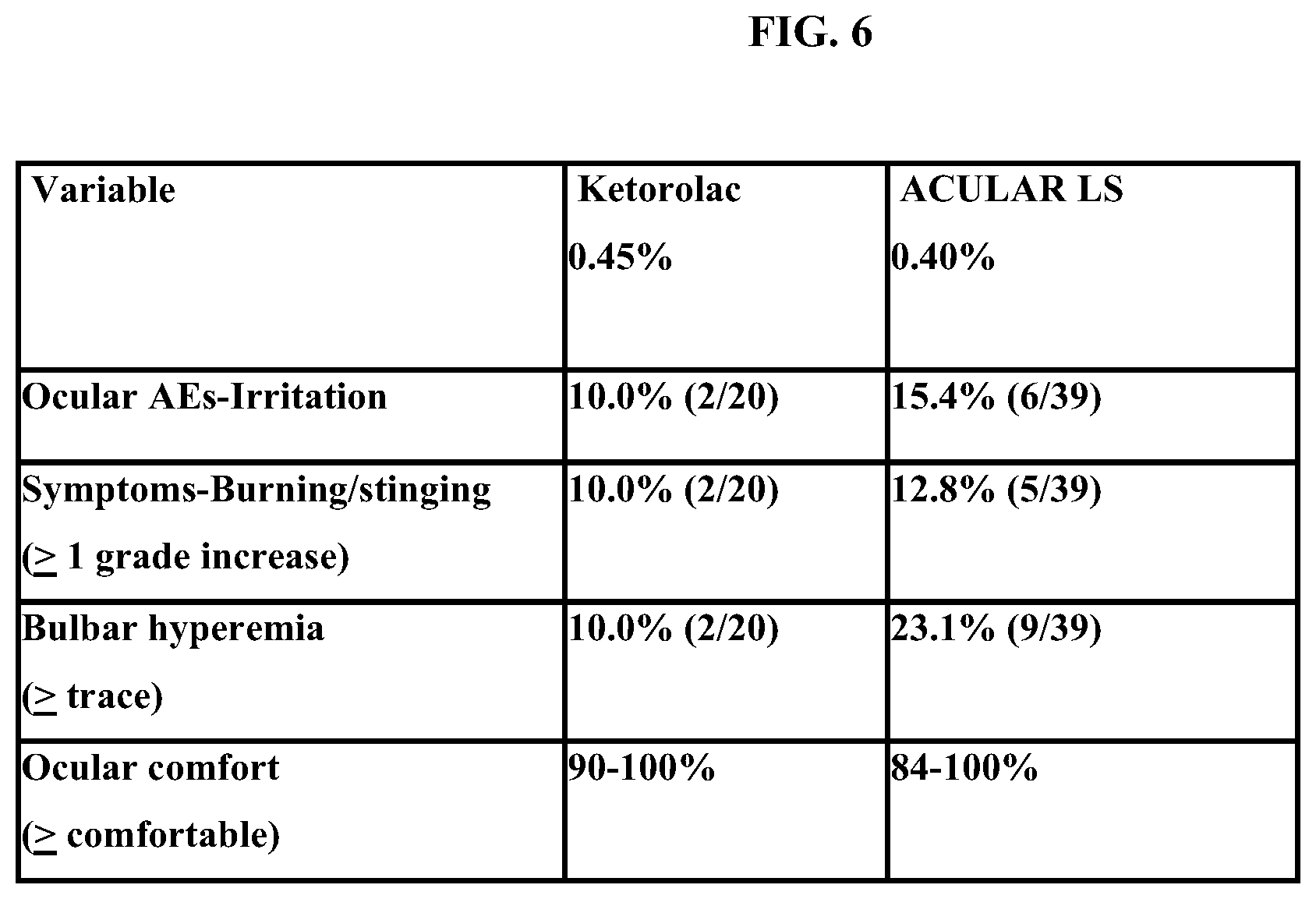
[0016] FIG. 6 shows safety and tolerability results in human clinical trials of 0.45% w/v ketorolac solution vs. ACULAR LS.
SUMMARY OF THE INVENTION
[0017] The present invention provides an aqueous ophthalmic formulation comprising an effective amount of ketorolac but having an optimized concentration of ketorolac in comparison other commercially available ketorolac products. The aqueous ophthalmic solution of the present invention comprises carboxymethyl cellulose, e.g. sodium carboxymethyl cellulose, having a pH within the range of from about 6.8 to 7.4, which is comfortable when topically applied to the eye of a patient, wherein the concentration of carboxymethyl cellulose and, preferably, the pH, is selected to provide an increased absorption of ketorolac in the eye of a patient as compared to a comparative ketorolac solution that differs only in not including the carboxymethyl cellulose. That is, the absorption of ketorolac may be 130% or greater than the absorption of a comparative aqueous ketorolac ophthalmic solution having the same or higher concentration of ketorolac.
[0018] More preferably, the aqueous ophthalmic solution of this invention has a pH within the range of from 6.8 to 7.4, particularly 6.8.
[0019] More preferably, the aqueous ophthalmic solution of the present invention has a concentration of carboxymethyl cellulose of from about 0.2 to about 2 percent, by weight, even more preferably from about 0.5 to 1.5 percent, by weight, and most preferably about 0.5% w/v.
[0020] Even more preferably, the aqueous ophthalmic solution of the present invention comprises a mixture of medium viscosity and high viscosity sodium carboxymethyl cellulose.
[0021] More preferably, the aqueous ophthalmic solution of the invention comprises an effective amount of ketorolac of from 0.25 to 0.50 percent, by weight, or about 0.45 percent, by weight.
[0022] More preferably, the aqueous ophthalmic solution of the invention has a viscosity of from 5 to 50 cps, preferably from 10 to 30 cps.
[0023] It has been surprisingly discovered that optimizing the concentration of ketorolac tromethamine reduces the occurrence of adverse events while maintaining clinical efficacy. Additionally, it has been discovered that the optimized concentration of ketorolac tromethamine in combination with carboxymethyl cellulose offers surprising and clear benefits in terms of formulation in that no preservative, chelating agent, and surfactant are required for formulation. Thus, finding a way to increase the absorption of ketorolac benefits the patient who can use a solution having an optimized concentration of ketorolac and obtain similar results in terms of efficiency as compared to a ketorolac solution having a higher concentration of ketorolac.
[0024] Thus, this invention relates to an aqueous topical ophthalmic composition comprising 0.25 to 0.50 percent by weight, more preferably from 0.35% to 0.45% by weight and most preferably about 0.45% ketorolac tromethamine by weight/volume. The present invention also contains from 0.2 to 2 percent by weight, more preferably from 0.5 to 1.5 percent by weight and most preferably about 0.5% w/v percent of medium and high molecular weight sodium carboxymethyl cellulose. Another aspect of this invention relates to a method of treating or preventing ocular pain in a person comprising topically administering to said patient a sterile composition comprising from 0.25 to 0.50 percent, by weight, more particularly from 0.35% to 0.45% by weight, or about 0.45% w/v ketorolac tromethamine in combination with from 0.2 to 2 percent, by weight, preferably from 0.5 to 1.5 percent by weight, and most preferably 0.5% percent by weight/volume, sodium carboxymethyl cellulose and mixtures thereof.
[0025] While not intending to limit the scope of this invention in any way, of particular interest in relationship to this invention is the use of aqueous topical ophthalmic compositions of 0.45% (w/v) ketorolac tromethamine for the treatment of ocular pain, especially for the treatment of ocular pain in postoperative photorefractive keratectomy (PRK) surgery patients which improves healing. It is surprising that the lower concentration of ketorolac as compared to the Acular.RTM. product, discussed herein, would reduce the incidence of adverse events and enhance comfort while maintaining clinical efficacy. Two drops (0.1 mL) of 0.5% ketorolac tromethamine ophthalmic solution instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved measurable levels in 8 of 9 patients' eyes (mean ketorolac concentration 95 ng/mL aqueous humor, range 40 to 170 ng/mL). Ocular administration of ketorolac tromethamine reduces prostaglandin E.sub.2 (PGE.sub.2) levels in aqueous humor. The mean concentration of PGE.sub.2 was 80 pg/mL in the aqueous humor of eyes receiving vehicle and 28 pg/mL in the eyes receiving 0.5% ketorolac tromethamine ophthalmic solution.
[0026] Ocular administration of 0.45% w/v ketorolac tromethamine ophthalmic solution increases relative bioavailability of ketorolac in the aqueous humor of rabbits to greater than 200% and in the iris-ciliary body to nearly 300%, compared with 0.5% ketorolac tromethamine ophthalmic solution. This enhanced ketorolac bioavailability allows for a reduction in dosing frequency from QID with 0.5% ketorolac tromethamine ophthalmic solution to BID with 0.45% ketorolac solution. Preclinical data indicate systemic ketorolac exposure levels achieved following ocular administration of 0.45% ketorolac solution are comparable to levels achieved with 0.5% ketorolac tromethamine ophthalmic solution.
DETAILED DESCRIPTION OF THE INVENTION
[0027] During the reformulation of Allergan's marketed Acular LS.RTM. product (0.40% w/v ketorolac), it was surprisingly found that a test formulation containing 0.45% ketorolac tromethamine and sodium carboxymethyl cellulose (NaCMC) exhibited significantly better ocular absorption in rabbits than did the currently marketed product, i.e. Acular LS.RTM..
[0028] Since the viscosities of the two test solutions were virtually identical, the mechanism for achieving increased ocular penetration compared to the control formulation cannot be accounted for only by the viscosity of the test solutions. In fact, a comparison of two identical carboxymethyl cellulose-containing solutions which differ only in having viscosity of 11 and 22 cps shows similar absorption of ketorolac into the aqueous humor. While not wishing to be bound by theory, it is believed that there is a functional relationship between the sodium carboxymethyl cellulose and either [,] the ketorolac or some component of the ocular surface that facilitates absorption of ketorolac.
[0029] All of the aqueous topical ophthalmic solutions of this invention are contemplated for use in treating or preventing ocular pain. Preferably, all of the solutions of this invention are contemplated for use when said ocular pain is a result of photorefractive keratectomy surgery (PRK).
[0030] One important aspect of this invention is that the solutions of the present invention have a concentration of ketorolac tromethamine which is optimized to reduce side effects, while maintaining clinical efficacy in treating ocular pain. As such, the concentration of ketorolac tromethamine in compositions related to this invention is preferably from 0.35% to 0.45%, most preferably the concentration of ketorolac tromethamine in the aqueous topical ophthalmic solution of this invention is 0.45% ketorolac tromethamine, by weight.
[0031] Carboxymethyl cellulose (CMC) is a carboxymethyl derivative of cellulose formed by the reaction of cellulose with alkali and chloroacetic acid. As a result of said reaction, carboxymethyl groups are bound to some of the hydroxyl groups of the glucopyranose units that make up the backbone of cellulose. The degree of substitution of carboxymethyl varies from about 0.6 to 0.95 per glucopyranose unit. CMC is used in aqueous solutions usually as the sodium salt to increase viscosity.
[0032] Carboxymethyl cellulose is available in various molecular weights. Low molecular weight carboxymethyl cellulose has a Mw of about 90,000 and a 2% solution thereof will have a viscosity of about 1.1 cP at 25.degree. C. Medium weight carboxymethyl cellulose has a Mw of about 250,000. High molecular weight carboxymethyl cellulose has a Mw of about 700,000 and a 2% solution will have a viscosity of about 12 cP at 25.degree. C.
[0033] For the purpose of the present invention, it is desirable to use a mixture of medium and high molecular weight sodium carboxymethyl cellulose. For example, from 25/75 to 75/25 carboxymethyl cellulose, preferably from 30/70 to 70/30 and most preferably about 35/65 medium/high molecular weight sodium carboxymethyl cellulose.
[0034] The fact that the concentration of ketorolac tromethamine in compositions related to this invention achieves greater or equal absorption of ketorolac into the aqueous humor of the eye and includes carboxymethyl cellulose, allows the solutions of the present invention to be prepared with no preservative, surfactant and chelating agent. This is a significant advantage over prior art ketorolac formulations as preservatives, surfactants and chelating agents can cause irritation to the eye resulting in less patient compliance and less effectiveness of prior art ketorolac formulations.
[0035] The term preservative has the meaning commonly understood in the ophthalmic art. Preservatives are used to prevent bacterial contamination in multiple-use ophthalmic preparations, and, while not intending to be limiting, examples include benzalkonium chloride, stabilized oxychloro complexes (otherwise known as Purite.RTM.), phenylmercuric acetate, chlorobutanol, benzyl alcohol, parabens, and thimerosal. Preferably, the ketorolac solution of the present invention is preservative free.
[0036] The term surfactant used in this invention has the meaning commonly understood in the art. Surfactants are used to help solubilize the therapeutically active agent or other insoluble components of the composition. Anionic, cationic, amphoteric, zwitterionic, and nonionic surfactants may all be used in this invention. If a surfactant is included in the solutions of this invention, preferably, a nonionic surfactant is used. While not intending to limit the scope of the invention, some examples of useful nonionic surfactants are polysorbates, poloxamers, alcohol ethoxylates, ethylene glycol-propylene glycol block copolymers, fatty acid amides, and alkylphenol ethoxylates, and phospholipids. Most preferably, the surfactant is an octylphenol ethoxylate with an average of 40 ethoxylate groups. This type of surfactant, also known as octoxynol-40 or Igepal CA-897.RTM., can be purchased under the Igepal CA-897.RTM. trade name from Rhone-Poulenc. Preferably, the ketorolac solution of the present invention is surfactant free.
[0037] The term chelating agent refers to a compound that is capable of complexing a metal, as understood by those of ordinary skill in the chemical art. Chelating agents are used in ophthalmic compositions to enhance preservative effectiveness. While not intending to be limiting, some useful chelating agents for the purposes of this invention are edetate salts like edetate disodium, edetate calcium disodium, edetate sodium, edetate trisodium, and edetate dipotassium. Preferably, the ketorolac solution of the present invention is chelator free.
[0038] In addition to surfactants, preservatives, and chelating agents, tonicity agents and other excipients are often used in ophthalmic compositions. Tonicity agents are often used in ophthalmic compositions to adjust the concentration of dissolved material to the desired isotonic range. Tonicity agents are known to those skilled in the ophthalmic art, and, while not intending to be limiting, some examples include glycerin, mannitol, sorbitol, sodium chloride, and other electrolytes. Preferably, the tonicity agent is sodium chloride.
[0039] One preferred embodiment of this invention relates to an aqueous topical ophthalmic composition comprising 0.4% ketorolac tromethamine, from 0.2 to 2.0%, by weight, sodium carboxymethyl cellulose.
[0040] The most preferred embodiment of this invention relates to an aqueous topical ophthalmic composition consisting of 0.45% (w/v) of ketorolac tromethamine, 0.5% w/v of carboxymethyl cellulose sodium, e.g. a mixture of medium and high viscosity sodium carboxymethyl cellulose, sodium chloride, sodium citrate dehydrate, sodium hydroxide, hydrochloric acid and purified water.
Example 1
[0041] Unless otherwise specified, all steps in this procedure were carried out at room temperature. The following procedure was followed in accordance with the amounts listed in Table 1 below. Purified water was charged into the main batch vessel. Mixing was initiated to produce a vortex sufficient to disperse and/or dissolve all product ingredients without excessive aeration or foam formation. The following components were added directly into the vortex in order, allowing each to dissolve before adding the next: sodium chloride, calcium chloride, dihydrate magnesium chloride, hexahydrate, boric acid, sodium borate, sodium carboxymethyl cellulose as a an percent aqueous solution comprising including a mixture of 65% medium molecular weight and 35% high molecular weight carboxymethyl cellulose. The solution was mixed for no longer than 15 minutes. A specified amount of 1N sodium hydroxide, was then added. The pH was checked and, if needed, was adjusted to 7.3 with 1N sodium hydroxide or 1N hydrochloric acid. Ketorolac tromethamine was then added based on "as is" assay and mixed until completely dissolved based on visual inspection. When dissolved, the solution pH was again checked and if needed adjusted to pH 7.3-7.5 (final target pH is 7.4) with 1N sodium hydroxide or 1N hydrochloric acid. Purified water was then added to bring the bulk solution to final volume and allowed to mix for at least 15 minutes to ensure uniformity. The solution was then sterile filtered for use.
TABLE-US-00001 TABLE 1 0.4% Ketorolac Tromethamine Ophthalmic Solution of the Invention Ketorolac Tromethamine 0.4% CMC, Med Visc. 0.65% CMC Low Visc. 0.35% Potassium chloride 0.14% Calcium chloride, dihydrate 0.060% Magnesium chloride, hexahydrate 0.060% Boric acid .060% Sodium borate .1225%
Example 2
[0042] Unless otherwise specified, all steps in this procedure were carried out at room temperature. The following procedure was followed in accordance with the amounts listed in Table 2 below. Purified water at 90% of batch size was charged into the main batch vessel. Mixing was initiated to produce a vortex sufficient to disperse and/or dissolve all product ingredients without excessive aeration or foam formation. The following components were added directly into the vortex in order, allowing each to dissolve before adding the next: sodium chloride, edetate disodium, octoxynol-40 (as a 70% stock solution) and benzalkonium chloride (as a 10% stock solution). The amount of benzalkonium chloride added took into account the assay of the stock solution used. The solution was mixed for no longer than 15 minutes. A specified amount of 1N sodium hydroxide, 1.85 mL per liter of final bulk product, was then added. The pH was checked and if needed was adjusted to 10.7-11.0 with 1N sodium hydroxide or 1N hydrochloric acid. Ketorolac tromethamine was then added based on "as is" assay and mixed until completely dissolved based on visual inspection. When dissolved, the solution pH was again checked and if needed adjusted to pH 7.3-7.5 (final target pH is 7.4) with 1N sodium hydroxide or 1N hydrochloric acid. Purified water was then added to bring the bulk solution to final volume and allowed to mix for at least 15 minutes to ensure uniformity. The solution was then sterile filtered for use.
TABLE-US-00002 TABLE 2 0.4% Ketorolac Tromethamine Ophthalmic Solution (Comparative) Ketorolac Tromethamine 0.4% Edetate Disodium 0.015% NaCl 0.79% Benzalkonium Chloride 0.006% Octoxynol-40 0.003% Ph 7.4
Example 3
[0043] This example was prepared according to the procedure of Example 1, except that hydroxypropyl cellulose was used in place of the sodium carboxymethyl cellulose in an amount sufficient to provide a viscosity equivalent to the viscosity of the composition of Example 1.
Example 4
[0044] The following composition was manufactured on a volume basis at ambient temperates from two principal parts. Each part is manufactured separately and then combined under controlled sequences to form a sterile bulk product: the first part (Part 1) involves the dissolution of carboxymethyl cellulose sodium in water followed by bulk heat sterilization, and the second part (Part 2) involves dissolution of ketorolac tromethamine and salts in water sterile filtration throng a 0.2 micron membrane into a sterile pressure vessel. The sterile bulk solution is then clarity filtered through a 20 micron polypropylene membrane filter into the filling machine reservoir.
[0045] The sterile post-clarity filtered solution is then filled by a UD filling machine via blow-fill-seal process into UD vials using virgin LDPE resin without colorant. The filling is done in an ISO Class 5 environment. The nominal fill is 0.4 mL into 0.9 mL capacity vials.
TABLE-US-00003 TABLE 3 0.45% w/v Ketorolac Tromethamine Ophthalmic Solution Concentration Ingredient Function (% w/v) Ketorolac tromethamine Active 0.45% Carboxymethyl cellulose Thickening Agent 0.325% Sodium (Med. Viscosity) Carboxymethyl cellulose Thickening Agent 0.175% Sodium (High Viscosity) NaCl Tonicity Agent 0.7% Sodium Citrate Buffer 0.2% Dihydrate Sodium Hydroxide (1N) pH adjustment Adjust to pH 6.8 Hydrochloric Acid (1N) pH adjustment Adjust to pH 6.8 Purified Water Vehicle Q.S.
Example 5
[0046] Comparison of Aqueous Humor Ketorolac Pharmacokinetics Following a Single Ocular Instillation of 0.45% Ketorolac Tromethamine Formulations with Varying pH to Acular LS.RTM. in New Zealand White Rabbits.
[0047] Study Objectives: [0048] 1) To compare aqueous humor ketorolac pharmacokinetics following a single ocular instillation of 0.45% ketorolac tromethamine formulations with varying pH and Acular LS.RTM. to New Zealand White rabbits; [0049] 2) This Example was designed to determine whether decreasing the pH of the composition would increase the absorption of ketorolac into the eye; and, [0050] 3) In addition, one arm of this trial was designed to test the effect of decreasing viscosity of the composition from 22 cps to 11 cps.
[0051] The specifics of this study are as follows:
Rabbit Aqueous Humor Ketorolac Concentrations following Administration of Three 0.45% Ketorolac Tromethamine Formulations and Acular LS
TABLE-US-00004 TABLE 4 Treatment Groups 0.45% Ketorolac Tromethamine 22 cps pH = 7.4 0.45% Ketorolac Tromethamine 22 cps pH = 7.2 0.45% Ketorolac Tromethamine 22 cps pH = 7.0 0.45% Ketorolac Tromethamine 11 cps pH = 7.0 0.45% Ketorolac Tromethamine 22 cps pH = 6.8 Acular LS pH = 7.4 Dosing Route: Topical ocular Animal Gender: NZW Rabbits/Female Dosing Regimen Single dose, bilateral Time points: 1, 2 and 4 hrs post-dose # Rabbits: 3 rabbits/time point + 1 rabbit blank Total = 39 rabbits Tissues/Matrices: Aqueous Humor Bioanalysis: LC-MS/MS Data analysis: AUC.sub.0-t, C.sub.max
[0052] The results of the study are reported in Table 5, below.
TABLE-US-00005 TABLE 5 PK Parameters AUC.sub.0-4 .+-. SE C.sub.max .+-. SD Relative Formulation (ng h/ml) (ng/ml) % F* 0.45% CMC 22 cps 627 .+-. 51 265 .+-. 71 135 pH 7.4 w.o "outlier" 0.45% CMC 22 cps 713 .+-. 96 322 .+-. 153 153 pH 7.4 0.45% CMC 22 cps 620 .+-. 50 240 .+-. 84 133 pH 7.2 0.45% CMC 22 cps 658 .+-. 73 268 .+-. 125 142 pH 7.0 0.45% CMC 22 cps 939 .+-. 163 389 .+-. 258 202 pH 6.8 0.45% CMC 11 cps 649 .+-. 74 347 .+-. 218 139 pH 7.0 Acular LS .RTM. 465 .+-. 65 211 .+-. 106 100
Summary of the Results
[0053] The sodium carboxymethyl cellulose-containing formulations perform better than Acular LS.RTM. with a relative bioavailability ranging from 133% (0.45% Keto 22 cps pH 7.2) to 202% (0.45% Keto 22 cps pH 6.8). However, there is not a clear pH effect-because the 0.45% Keto 22 cps pH 7.4 has a relative bioavailability of 153%, although one anomalous result may be driving this observation. Nevertheless, the solution having a pH of 6.8 shows the best bioavailability.
Example 6
[0054] A multicenter, randomized, double-masked, parallel-group study is carried out using the 0.4% ketorolac tromethamine formulations of Examples 2 and 3. The study subjects consisted of 157 patients (78-79/group) undergoing unilateral PRK surgery. The key inclusion criteria for the study is that each subject a) is a candidate for unilateral photorefractive keratectomy surgery (PRK) within 7 days after the initial visit, b) have best-corrected ETDRS visual acuity of 20/100 or better, and c) is capable of wearing a soft bandage contact lens. Key exclusion criteria are a history of refractive ocular surgery and sensitivity to study medication or its vehicle, Tylenol #3.RTM., or Ocuflox.RTM.. The patient demographics are shown in Table 6. A total of 157 patients are enrolled with an age range of 20-66 years. There are no significant demographic differences between treatment groups.
TABLE-US-00006 TABLE 6 Patient Demographics n % Gender Female 91 58 Male 66 42 Age, mean .+-. SD 39 .+-. 10 Race Caucasian 148 94 Black 5 3 Hispanic 2 1 Asian 1 1 Other 1 1
[0055] Each subject receives the Ocuflox.RTM. 5 min prior to study medication. The study subjects then receive ketorolac tromethamine 0.4% ophthalmic solution of Example 2 or Example 3, 1 drop QID for up to 4 days. Then all subjects are then instructed to take Tylenol #3.RTM. as needed for intolerable pain (escape medication). Patients use electronic diaries with date and time stamp to record the ocular pain they experience as one of the following: no pain; mild; moderate; severe; and intolerable.
[0056] The pain intensity is less for the subjects who receive the solution of Example 2 during the first 12 hours post-PRK compared to those who receive the solution of Example 3. In particular, during the first 12 hours post-PRK, the group that receives the solution of Example 2 had fewer patients with severe or intolerable pain compared with the group which receives the solution of Example 3. In particular, the median pain intensity reported by the group which receives the solution of Example 2 was 1 grade less than with the group which receives the solution of Example 3 (moderate vs. severe on a 5-point scale of 0=no pain to 4=intolerable pain). Additionally, pain intensity is also less for the group which receives Example 2 compared with the group which receives the solution of Example 3.
[0057] This clinical study shows that the solution of invention provides a greater degree of absorption of ketorolac as compared to the solution without sodium carboxymethylcellulose despite the fact that the solutions have the same concentration of ketorolac and are at the same viscosity.
[0058] In summary, the 0.4% ketorolac formulation is clinically effective in treating post PRK ocular pain. In patients treated with 0.4 ketorolac tromethamine--the patients treated with the solution comprising sodium carboxymethyl cellulose experienced significantly greater and faster pain relief, and used less escape medication compared to the patients treated with the solution comprising hydroxypropyl cellulose.
Example 7
[0059] Rabbit Ocular Pharmacokinetic Evaluation of Ketorolac Tromethamine 0.45%
[0060] NZW Rabbits/Female
[0061] Dosing Regimen: Single ocular dose, bilateral
[0062] Time points: 0.5, 1, 2, 4, 8, 10 and 24 hrs post-dose
[0063] Tissues/Matrices: Aqueous Humor and Iris-ciliary body
[0064] Bioanalysis: LC-MS/MS
[0065] Data Analysis: Pharmacokinetic analyses and simulation
[0066] Conclusions:
[0067] As shown in FIGS. 1-5, Example 7 shows there is an:
1) Increase in relative bioavailability of ketorolac as compared to Acular LS.RTM.; 2) Increased ketorolac concentrations are maintained longer post-dose; and 3) Together these data support a reduction in dosing frequency from 4.times./day to 2.times./day.
CONCLUSION
[0068] As shown in FIG. 6, Acular 0.45% is safe and well-tolerated among human patients when given 5 times over a half-day and compares very favorably to ACULAR LS.
[0069] The present invention is not to be limited in scope by the exemplified embodiments, which are only intended as illustrations of specific aspects of the invention. Various modifications of the invention, in addition to those disclosed herein, will be apparent to those skilled in the art by a careful reading of the specification, including the claims, as originally filed. It is intended that all such modifications will fall within the scope of the appended claims.
* * * * *
D00001

D00002

D00003

D00004

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.