Pd-1/tim-3 Bi-specific Antibodies, Compositions Thereof, And Methods Of Making And Using The Same
STAFFORD; Ryan ; et al.
U.S. patent application number 16/487806 was filed with the patent office on 2020-06-18 for pd-1/tim-3 bi-specific antibodies, compositions thereof, and methods of making and using the same. The applicant listed for this patent is SUTRO BIOPHARMA, INC.. Invention is credited to Stephanie ARMSTRONG, Christine CHENG, John LEE, Ryan STAFFORD, Alexander STEINER, Alice YAM, Junhao YANG.
| Application Number | 20200190192 16/487806 |
| Document ID | / |
| Family ID | 68165368 |
| Filed Date | 2020-06-18 |








| United States Patent Application | 20200190192 |
| Kind Code | A1 |
| STAFFORD; Ryan ; et al. | June 18, 2020 |
PD-1/TIM-3 BI-SPECIFIC ANTIBODIES, COMPOSITIONS THEREOF, AND METHODS OF MAKING AND USING THE SAME
Abstract
Provided herein are antibodies that selectively bind to Tim-3 and its isoforms and homologs, and compositions comprising the antibodies. Also provided herein are antibodies that selectively bind to PD-1 and its isoforms and homologs, and compositions comprising the antibodies. In addition, provided herein are bi-specific antibodies and antigen binding constructs that selectively bind to Tim-3 and/or PD-1, their isoforms and homologs, and compositions comprising the antibodies and antigen binding constructs. Also provided are methods of using the antibodies, such as therapeutic and diagnostic methods.
| Inventors: | STAFFORD; Ryan; (Emeryville, CA) ; YAM; Alice; (Tiburon, CA) ; ARMSTRONG; Stephanie; (South San Francisco, CA) ; LEE; John; (San Francisco, CA) ; STEINER; Alexander; (San Francisco, CA) ; YANG; Junhao; (Palo Alto, CA) ; CHENG; Christine; (South San Francisco, CA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 68165368 | ||||||||||
| Appl. No.: | 16/487806 | ||||||||||
| Filed: | February 22, 2018 | ||||||||||
| PCT Filed: | February 22, 2018 | ||||||||||
| PCT NO: | PCT/US18/19248 | ||||||||||
| 371 Date: | August 21, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 62468272 | Mar 7, 2017 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C07K 2317/92 20130101; C07K 2317/76 20130101; H02J 7/025 20130101; C07K 2317/565 20130101; H02J 50/10 20160201; C07K 2317/622 20130101; C07K 16/2818 20130101; C07K 2317/31 20130101; H04B 3/52 20130101 |
| International Class: | C07K 16/28 20060101 C07K016/28 |
Claims
1. A bi-specific antibody or antigen-binding construct, comprising a first binding domain that specifically binds to Tim-3, and a second binding domain that specifically binds to PD-1, wherein: i. the first binding domain comprises: i. a CDR-H3 selected from SEQ ID NOs: 111-118; ii. a Chothia CDR-H2 sequence selected from SEQ ID NOs: 63-80, or a Kabat CDR-H2 sequence selected from SEQ ID NOs: 82-109, or both; iii. a Chothia CDR-H1 sequence selected from SEQ ID NOs: 4-23, or a Kabat CDR-H1 sequence selected from SEQ ID NOs: 39-56, or both and ii. the second binding domain comprises: i. a CDR-H3 sequence selected from SEQ ID NO: 119, or a CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs: 182-220; ii. a Chothia CDR-H2 sequence selected from SEQ ID NO: 81, and a Chothia CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs: 182-220, or a Kabat CDR-H2 sequence selected from SEQ ID NO: 110, and a Kabat CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs: 182-220 or both; iii. a Chothia CDR-H1 sequence selected from SEQ ID NOs: 24-38, and a Chothia CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs: 182-220, or a Kabat CDR-H1 sequence selected from SEQ ID NOs: 57-62, and a Kabat CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs: 182-220 or both.
2.-5. (canceled)
6. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a CDR-L3 sequence selected from SEQ ID NOs: 140-142.
7. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a CDR-L2 sequence selected from SEQ ID NOs: 125-133.
8. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a CDR-L1 sequence selected from SEQ ID NOs: 120-123.
9. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises: i. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 17 and 52; a CDR-H2 comprising one or more of SEQ ID NOs: 77 and 102; and a CDR-H3 comprising SEQ ID NO: 113; ii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 89; and a CDR-H3 comprising SEQ ID NO: 113; iii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 18 and 53; a CDR-H2 comprising one or more of SEQ ID NOs: 77 and 102; and a CDR-H3 comprising SEQ ID NO: 113; iv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 19 and 54; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 102; and a CDR-H3 comprising SEQ ID NO: 113; v. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 22 and 53; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 89; and a CDR-H3 comprising SEQ ID NO: 113; vi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 82; and a CDR-H3 comprising SEQ ID NO: 111; vii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 83; and a CDR-H3 comprising SEQ ID NO: 111; viii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 84; and a CDR-H3 comprising SEQ ID NO: 111; ix. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 85; and a CDR-H3 comprising SEQ ID NO: 111; x. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 86; and a CDR-H3 comprising SEQ ID NO: 111; xi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 4 and 39; a CDR-H2 comprising one or more of SEQ ID NOs: 63 and 87; and a CDR-H3 comprising SEQ ID NO: 111; xii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 5 and 40; a CDR-H2 comprising one or more of SEQ ID NOs: 64 and 88; and a CDR-H3 comprising SEQ ID NO: 112; xiii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 7 and 42; a CDR-H2 comprising one or more of SEQ ID NOs: 66 and 90; and a CDR-H3 comprising SEQ ID NO: 113; xiv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 8 and 43; a CDR-H2 comprising one or more of SEQ ID NOs: 67 and 91; and a CDR-H3 comprising SEQ ID NO: 113; xv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 9 and 44; a CDR-H2 comprising one or more of SEQ ID NOs: 168 and 92; and a CDR-H3 comprising SEQ ID NO: 114; xvi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 10 and 45; a CDR-H2 comprising one or more of SEQ ID NOs: 69 and 93; and a CDR-H3 comprising SEQ ID NO: 114; xvii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 8 and 42; a CDR-H2 comprising one or more of SEQ ID NOs: 70 and 94; and a CDR-H3 comprising SEQ ID NO: 114; xviii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 8 and 42; a CDR-H2 comprising one or more of SEQ ID NOs: 70 and 95; and a CDR-H3 comprising SEQ ID NO: 114; xix. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 11 and 46; a CDR-H2 comprising one or more of SEQ ID NOs: 71 and 96; and a CDR-H3 comprising SEQ ID NO: 115; xx. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 12 and 47; a CDR-H2 comprising one or more of SEQ ID NOs: 72 and 97; and a CDR-H3 comprising SEQ ID NO: 113; xxi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 13 and 48; a CDR-H2 comprising one or more of SEQ ID NOs: 73 and 98; and a CDR-H3 comprising SEQ ID NO: 116; xxii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 14 and 49; a CDR-H2 comprising one or more of SEQ ID NOs: 74 and 99; and a CDR-H3 comprising SEQ ID NO: 117; xxiii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 15 and 50; a CDR-H2 comprising one or more of SEQ ID NOs: 75 and 100; and a CDR-H3 comprising SEQ ID NO: 113; xxiv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 16 and 51; a CDR-H2 comprising one or more of SEQ ID NOs: 76 and 101; and a CDR-H3 comprising SEQ ID NO: 118; xxv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 103; and a CDR-H3 comprising SEQ ID NO: 113; xxvi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 104; and a CDR-H3 comprising SEQ ID NO: 113; xxvii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 78 and 105; and a CDR-H3 comprising SEQ ID NO: 113; xxviii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 79 and 106; and a CDR-H3 comprising SEQ ID NO: 113; xxix. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 80 and 107; and a CDR-H3 comprising SEQ ID NO: 113; xxx. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 108; and a CDR-H3 comprising SEQ ID NO: 113; xxxi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 6 and 41; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 109; and a CDR-H3 comprising SEQ ID NO: 113; xxxii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 20 and 55; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 89; and a CDR-H3 comprising SEQ ID NO: 113; xxxiii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 21 and 56; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 89; and a CDR-H3 comprising SEQ ID NO: 113; or xxxiv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 23 and 53; a CDR-H2 comprising one or more of SEQ ID NOs: 65 and 89; and a CDR-H3 comprising SEQ ID NO: 113.
10. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a V.sub.H is selected from SEQ ID NOs: 144-180.
11. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises: i. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 354; a CDR-L2 comprising SEQ ID NO: 355; and a CDR-L3 comprising SEQ ID NO: 356; ii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 133; and a CDR-L3 comprising SEQ ID NO: 142; iii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 127; and a CDR-L3 comprising SEQ ID NO: 142; iv. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 128; and a CDR-L3 comprising SEQ ID NO: 142; v. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 129; and a CDR-L3 comprising SEQ ID NO: 142; vi. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 130; and a CDR-L3 comprising SEQ ID NO: 142; vii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 131; and a CDR-L3 comprising SEQ ID NO: 142; viii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 123; a CDR-L2 comprising SEQ ID NO: 132; and a CDR-L3 comprising SEQ ID NO: 142; ix. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 120; a CDR-L2 comprising SEQ ID NO: 125; and a CDR-L3 comprising SEQ ID NO: 140; x. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 121; a CDR-L2 comprising SEQ ID NO: 125; and a CDR-L3 comprising SEQ ID NO: 140; or xi. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 122; a CDR-L2 comprising SEQ ID NO: 126; and a CDR-L3 comprising SEQ ID NO: 141.
12. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a V.sub.L sequence selected from SEQ ID NOs: 221-235.
13. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises: i. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; ii. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; iii. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; iv. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; v. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 235, or a variant thereof; vi. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 235, or a variant thereof; vii. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 235, or a variant thereof; viii. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 235, or a variant thereof; ix. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 229, or a variant thereof; x. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 229, or a variant thereof; xi. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 229, or a variant thereof; xii. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 229, or a variant thereof; xiii. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 230, or a variant thereof; xiv. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 230, or a variant thereof; xv. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 230, or a variant thereof; xvi. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 230, or a variant thereof; xvii. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 231, or a variant thereof; xviii. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 231, or a variant thereof; xix. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 231, or a variant thereof; xx. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 231, or a variant thereof; xxi. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 232, or a variant thereof; xxii. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 232, or a variant thereof; xxiii. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 232, or a variant thereof; xxiv. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 232, or a variant thereof; xxv. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 233, or a variant thereof; xxvi. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 233, or a variant thereof; xxvii. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 233, or a variant thereof; xxviii. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 233, or a variant thereof; xxix. the V.sub.H region SEQ ID NO: 165, or a variant thereof, and the V.sub.L region is SEQ ID NO: 234, or a variant thereof; xxx. the V.sub.H region SEQ ID NO: 151, or a variant thereof, and the V.sub.L region is SEQ ID NO: 234, or a variant thereof; xxxi. the V.sub.H region SEQ ID NO: 166, or a variant thereof, and the V.sub.L region is SEQ ID NO: 234, or a variant thereof; xxxii. the V.sub.H region SEQ ID NO: 167, or a variant thereof, and the V.sub.L region is SEQ ID NO: 234, or a variant thereof; xxxiii. the V.sub.H region SEQ ID NO: 179, or a variant thereof, and the V.sub.L region is SEQ ID NO:228, or a variant thereof; xxxiv. the V.sub.H region SEQ ID NO: 179, or a variant thereof, and the V.sub.L region is SEQ ID NO:235, or a variant thereof; xxxv. the V.sub.H region SEQ ID NO: 179, or a variant thereof, and the V.sub.L region is SEQ ID NO:229, or a variant thereof; xxxvi. the V.sub.H region SEQ ID NO: 152, or a variant thereof, and the V.sub.L region is SEQ ID NO:228, or a variant thereof; xxxvii. the V.sub.H region SEQ ID NO: 153, or a variant thereof, and the V.sub.L region is SEQ ID NO:228, or a variant thereof; xxxviii. the V.sub.H region SEQ ID NO: 154, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xxxix. the V.sub.H region SEQ ID NO: 155, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xl. the V.sub.H region SEQ ID NO: 156, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xli. the V.sub.H region SEQ ID NO: 157, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xlii. the V.sub.H region SEQ ID NO: 158, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xliii. the V.sub.H region SEQ ID NO: 159, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; xliv. the V.sub.H region SEQ ID NO: 160, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof; or xlv. the V.sub.H region SEQ ID NO: 161, or a variant thereof, and the V.sub.L region is SEQ ID NO: 228, or a variant thereof.
14.-17. (canceled)
18. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises a CDR-L3 sequence selected from SEQ ID NO: 143 or a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs: 237-269.
19. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises a CDR-L2 sequence selected from SEQ ID NOs: 134-139 or a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs: 237-269.
20. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain comprises a CDR-L1 sequence selected from SEQ ID NO: 124 or a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs: 237-269.
21. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises: i. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 24 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; ii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 38 and 62; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; iii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 37 and 62; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; iv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 36 and 58; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; v. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 35 and 62; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; vi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 34 and 58; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; vii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 33 and 58; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; viii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 32 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; ix. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 31 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; x. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 30 and 59; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 29 and 58; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 28 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xiii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 27 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xiv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 26 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xv. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 25 and 57; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xvi. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 24 and 60; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xvii. a V.sub.H comprising: a CDR-H1 comprising one or more of SEQ ID NOs: 24 and 61; a CDR-H2 comprising one or more of SEQ ID NOs: 81 and 110; and a CDR-H3 comprising SEQ ID NO: 119; xviii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 198; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 198; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 198; xix. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 199; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 199; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 199; xx. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 200; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 200; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 200; xxi. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 201; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 201; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 201; xxii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 202; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 202; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 202; xxiii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 203; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 203; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 203; xxiv. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 204; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 204; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 204; xxv. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 205; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 205; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 205; xxvi. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 206; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 206; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 206; xxvii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 207; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 207; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 207; xxviii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 208; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 208; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 208; xxix. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 209; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 209; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 209; xxx. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 210; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 210; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 210; xxxi. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 211; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 211; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 211; xxxii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 212; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 212; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 212; xxxiii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 213; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 213; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 213; xxxiv. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 214; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 214; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 214; xxxv. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 215; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 215; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 215 xxxvi. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 216; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 216; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 216; xxxvii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 217; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 217; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 217; xxxviii. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 218; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 218; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 218; xxxix. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 219; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 219; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 219; or xl. a V.sub.H comprising: a CDR-H1 comprising a Chothia and/or Kabat CDR-H1 sequence of SEQ ID NO: 220; a CDR-H2 comprising a Chothia and/or Kabat CDR-H2 sequence of SEQ ID NO: 220; and a CDR-H3 comprising a CDR-H3 sequence of SEQ ID NO: 220.
22. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises a V.sub.H is selected from SEQ ID NOs: 181-220.
23. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises: i. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 134; and a CDR-L3 comprising SEQ ID NO: 143; ii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 244; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 244; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 244; iii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 135; and a CDR-L3 comprising SEQ ID NO: 143; iv. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 136; and a CDR-L3 comprising SEQ ID NO: 143; v. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 137; and a CDR-L3 comprising SEQ ID NO: 143; vi. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 138; and a CDR-L3 comprising SEQ ID NO: 143; vii. a V.sub.L comprising: a CDR-L1 comprising SEQ ID NO: 125; a CDR-L2 comprising SEQ ID NO: 139; and a CDR-L3 comprising SEQ ID NO: 143; viii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 242; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 242; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 242; ix. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 243; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 243; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 243; x. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 245; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 245; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 245; xi. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 246; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 246; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 246; xii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 247; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 247; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 247; xiii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 248; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 248; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 248; xiv. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 249; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 249; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 249; xv. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 250; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 250; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 250; xvi. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 251; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 251; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 251; xvii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 252; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 252; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 252; xviii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 253; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 253; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 253; xix. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 254; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 254; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 254; xx. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 255; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 255; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 255; xxi. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 256; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 256; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 256; xxii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 257; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 257; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 257; xxiii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 258; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 258; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 258; xxiv. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 259; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 259; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 259; xxv. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 260; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 260; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 260; xxvi. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 261; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 261; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 261; xxvii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 262; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 262; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 262; xxviii. a V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 263; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 263; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 263; xxix. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 264; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 264; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 264; xxx. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 265; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 265; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 265; xxxi. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 266; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 266; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 266; xxxii. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 267; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 267; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 267; xxxiii. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 268; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 268; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 268; or xxxiv. V.sub.L comprising: a CDR-L1 comprising a CDR-L1 sequence of SEQ ID NO: 269; a CDR-L2 comprising a CDR-L2 sequence of SEQ ID NO: 269; and a CDR-L3 comprising a CDR-L3 sequence of SEQ ID NO: 269.
24. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises a V.sub.L sequence selected from SEQ ID NOs: 236-269.
25. The bi-specific antibody or antigen binding construct of claim 1, wherein the second binding domain comprises: i. the V.sub.H region SEQ ID NO: 181 and the V.sub.L region SEQ ID NO: 244; ii. the V.sub.H region SEQ ID NO: 197 and the V.sub.L region SEQ ID NO: 236; iii. the V.sub.H region SEQ ID NO: 197 and the V.sub.L region SEQ ID NO: 244; iv. the V.sub.H region SEQ ID NO: 181 and the V.sub.L region SEQ ID NO: 236; v. the V.sub.H region SEQ ID NO: 196 and the V.sub.L region SEQ ID NO: 236; vi. the V.sub.H region SEQ ID NO: 195 and the V.sub.L region SEQ ID NO: 236; vii. the V.sub.H region SEQ ID NO: 194 and the V.sub.L region SEQ ID NO: 236; viii. the V.sub.H region SEQ ID NO: 193 and the V.sub.L region SEQ ID NO: 236; ix. the V.sub.H region SEQ ID NO: 192 and the V.sub.L region SEQ ID NO: 236; x. the V.sub.H region SEQ ID NO: 191 and the V.sub.L region SEQ ID NO: 236; xi. the V.sub.H region SEQ ID NO: 190 and the V.sub.L region SEQ ID NO: 236; xii. the V.sub.H region SEQ ID NO: 189 and the V.sub.L region SEQ ID NO: 236; xiii. the V.sub.H region SEQ ID NO: 188 and the V.sub.L region SEQ ID NO: 236; xiv. the V.sub.H region SEQ ID NO: 187 and the V.sub.L region SEQ ID NO: 236; xv. the V.sub.H region SEQ ID NO: 186 and the V.sub.L region SEQ ID NO: 236; xvi. the V.sub.H region SEQ ID NO: 185 and the V.sub.L region SEQ ID NO: 236; xvii. the V.sub.H region SEQ ID NO: 184 and the V.sub.L region SEQ ID NO: 236; xviii. the V.sub.H region SEQ ID NO: 183 and the V.sub.L region SEQ ID NO: 236; xix. the V.sub.H region SEQ ID NO: 182 and the V.sub.L region SEQ ID NO: 236; xx. the V.sub.H region SEQ ID NO: 196 and the V.sub.L region SEQ ID NO: 244; xxi. the V.sub.H region SEQ ID NO: 195 and the V.sub.L region SEQ ID NO: 244; xxii. the V.sub.H region SEQ ID NO: 194 and the V.sub.L region SEQ ID NO: 244; xxiii. the V.sub.H region SEQ ID NO: 193 and the V.sub.L region SEQ ID NO: 244; xxiv. the V.sub.H region SEQ ID NO: 192 and the V.sub.L region SEQ ID NO: 244; xxv. the V.sub.H region SEQ ID NO: 191 and the V.sub.L region SEQ ID NO: 244; xxvi. the V.sub.H region SEQ ID NO: 190 and the V.sub.L region SEQ ID NO: 244; xxvii. the V.sub.H region SEQ ID NO: 189 and the V.sub.L region SEQ ID NO: 244; xxviii. the V.sub.H region SEQ ID NO: 188 and the V.sub.L region SEQ ID NO: 244; xxix. the V.sub.H region SEQ ID NO: 187 and the V.sub.L region SEQ ID NO: 244; xxx. the V.sub.H region SEQ ID NO: 186 and the V.sub.L region SEQ ID NO: 244; xxxi. the V.sub.H region SEQ ID NO: 185 and the V.sub.L region SEQ ID NO: 244; xxxii. the V.sub.H region SEQ ID NO: 184 and the V.sub.L region SEQ ID NO: 244; xxxiii. the V.sub.H region SEQ ID NO: 183 and the V.sub.L region SEQ ID NO: 244; xxxiv. the V.sub.H region SEQ ID NO: 182 and the V.sub.L region SEQ ID NO: 244; or xxxv. the V.sub.H region SEQ ID NO: 181 and the V.sub.L region SEQ ID NO: 244.
26. A bi-specific antibody or antigen-binding construct, comprising a first binding domain that specifically binds to Tim-3, and a second binding domain that specifically binds to PD-1, wherein the first binding domain comprises: i. a V.sub.H region selected from SEQ ID NOs: 144-180, or a variant thereof having 10 or fewer amino acid substitutions; ii. a V.sub.L region selected from SEQ ID NOs: 221-235, or variant thereof of having 10 or fewer amino acid substitutions and the second binding domain comprises: i. a V.sub.H region selected from SEQ ID NOs: 181-220, or a variant thereof having 10 or fewer amino acid substitutions; and ii. a V.sub.L region selected from SEQ ID NOs: 236-269, or variant thereof of having 10 or fewer amino acid substitutions.
27. (canceled)
28. The bi-specific antibody or antigen binding construct of claim 1, wherein the antibody comprises at least one constant region domain.
29. (canceled)
30. The bi-specific antibody or antigen binding construct of claim 1, wherein the bi-specific antibody or antigen binding construct is humanized or human.
31. The bi-specific antibody or antigen binding construct of claim 1, wherein the bi-specific antibody or antigen binding construct is aglycosylated.
32. The bi-specific antibody or antigen binding construct of claim 1, wherein the first binding domain or the second binding domain comprises an antibody fragment.
33.-35. (canceled)
36. The bi-specific antibody or antigen binding construct of claim 32, wherein the antibody fragment is selected from the group consisting of: an Fv fragment, a Fab fragment, a F(ab').sub.2 fragment, a Fab' fragment, an scFv (sFv) fragment, and an scFv-Fc fragment.
37.-48. (canceled)
49. An isolated antibody that specifically binds to human Tim-3, wherein the antibody comprises a CDR-H3 sequence selected from: i. a sequence defined by the consensus sequence .alpha..sub.1-.alpha..sub.2-.alpha..sub.3-Y-R-.alpha..sub.6-.alpha..sub.7- -.alpha..sub.8-.alpha..sub.9-.alpha..sub.10-.alpha..sub.11-.alpha..sub.12-- .alpha..sub.13, where .alpha..sub.1 is Q, S or G; .alpha..sub.2 is G, F, Y, or H; .alpha..sub.3 is G, F, V, or I; .alpha..sub.6 is absent or S; .alpha..sub.7 is Y, S, M, or L; .alpha..sub.8 is D, N, or W; .alpha..sub.9 is D; .alpha..sub.10 is A, W, or S; .alpha..sub.11 is M, Y, F, or L; .alpha..sub.12 is D or V; and .alpha..sub.13 is Y or H; ii. a sequence defined by the consensus sequence G-.beta..sub.2-.beta..sub.3-Y-R-.beta..sub.7-W-D-S-.beta..sub.11-D-.beta.- .sub.13, where .beta..sub.2 is Y or H; .beta..sub.3 is V or I; .beta..sub.7 is M, or L; .beta..sub.11 is Y, F, or L; and .beta..sub.13 is Y or H; and iii. a sequence selected from SEQ ID NOs: 111-118, or a variant thereof having three, two, or one amino acid substitution(s).
50.-116. (canceled)
117. A kit comprising an antibody of claim 1, and instructions for use of the antibody.
118.-119. (canceled)
120. A polynucleotide encoding an antibody of claim 49.
121.-125. (canceled)
126. A pharmaceutical composition comprising the antibody of claim 1 and a pharmaceutically acceptable carrier.
127. A method of treating or preventing a disease or condition in a subject in need thereof, comprising administering to the subject an effective amount of an antibody of claim 1.
128.-161. (canceled)
Description
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application is a national stage of international application no. PCT/US2018/019248, filed Feb. 22, 2018, which claims the benefit of U.S. provisional application No. 62/462,272, filed Feb. 22, 2017, the contents of each of which are hereby incorporated by reference in their entireties.
FIELD
[0002] Provided herein are antibodies with dual binding specificity for T cell immunoglobulin domain- and mucin domain-containing molecule 3 (Tim-3) and for programmed cell death protein (PD-1 or PD1), also referred to as PD-1/Tim-3 bi-specific antibodies. Also provided herein are antibodies with binding specificity for PD-1 or Tim-3. In addition, provided herein are compositions comprising the antibodies, including pharmaceutical compositions, diagnostic compositions, and kits. Also provided are methods of making the bi-specific antibodies, and methods of using the bi-specific antibodies, for example, for therapeutic purposes, diagnostic purposes, and research purposes.
BACKGROUND
[0003] T cell immunoglobulin domain- and mucin domain-containing molecule 3 (Tim-3) is a cell surface protein molecule that belongs to the immunoglobulin superfamily. It is expressed as a transmembrane protein on differentiated type 1 T helper lymphocytes (Thl cells). See Monney et al., Nature 2002, 415:536-541. The Tim-3 protein contains an immunoglobulin variable-like domain and a mucin-like domain. See id. In a mouse model of autoimmune disease, experimental autoimmune encephalomyelitis, antibodies to Tim-3 were shown to increase the number and activation of macrophages, and to enhance clinical and pathologic severity. See id. From this, it has been proposed that Tim-3 is a regulator of immune function. See id. Later studies have shown that Tim-3 is constitutively expressed on cells of the innate immune system and can regulate Thl immunity. Anderson et al., 2007, Science 318:1141-3. Tim-3 has been shown to negatively regulate Thl cells in several studies. See Sabatos et al., Nature Immunol. 4:1102-110; Sanchez-Fueyo et al., 2003, Nature Immunol. 4:1093-1101; Sakuishi et al., 2010, J. Exp. Med. 207:2187-2194. Galectin-9 and CEACAM1 have been proposed as ligands for Tim-3. Zhu et al., 2005, Nature Immunol. 6:1245-1252; Huang et al., 2015, Nature 517:386-390.
[0004] In addition to its roles in immune regulation, autoimmune conditions, and inflammation, Tim-3 has been proposed as a target for cancer therapeutics. See, e.g., Anderson, 2014, Cancer Immunol. Res. 2:393-397. It has been shown that cancer cells can use immune checkpoint regulators such as Tim-3 to suppress the immune response against themselves. See id. Therapeutics that block other checkpoint regulators such as CTLA-4 have proved successful in treating certain cancers. See id. Indeed, target Tim-3 has shown promise for therapies in models of sarcoma, fibrosarcoma, prostate cancer, colon carcinoma, melanoma, and leukemia.
[0005] Programmed cell death protein 1 (PD-1, also referred to as "CD279" and "PD1") is a cell surface protein molecule that belongs to the immunoglobulin superfamily. It is expressed on T and B lymphocytes and macrophages, and plays a role in cell fate and differentiation. See Ishida et al., EMBO 1, 1992, 11:3887-3895, incorporated by reference in its entirety. Activation of PD-1 is thought to negatively regulate the immune response. See Blank et al., Cancer Immunol. Immunother., 2007, 56:739-745; and Freeman et al., J. Exp. Med., 2000, 192:1027-1034, each of which is incorporated by reference in its entirety.
[0006] PD-1 has two known ligands, PD-L1 and PD-L2, which are both members of the B7 family. See Freeman et al., supra; and Latchman et al., Nat. Immunol., 2001, 2:261-268, each of which is incorporated by reference in its entirety. The interaction between PD-1 and these ligands is thought to play a role in a variety of diseases, including cancer (see Ribas and Tumeh, Clin. Cancer Res., 2014, June 26, PMID: 24970841 [Epub ahead of print]), autoimmune disease (see Dai et al., Cell Immunol., 2014, 290:72-79), and infection (see Day et al., Nature, 2006, 443:350-354). Each of the references cited in the preceding sentence is incorporated by reference in its entirety. In particular, the engagement of PD-1 by one of its ligands is thought to inhibit T-cell effector functions in an antigen-specific manner.
[0007] In view of the role of PD-1 and Tim-3 in multiple disease processes, there is a need for methods of modulating the immune regulation and downstream signaling processes activated by both Tim-3 and PD-1. There is also a need for therapeutics that can specifically target cells and tissues that express Tim-3 and/or PD-1.
SUMMARY
[0008] Provided herein are antibodies that selectively bind Tim-3. In some embodiments, the antibodies bind human Tim-3. In some embodiments, the antibodies also bind homologs of human Tim-3. In some aspects, the homologs include a cynomolgus monkey homolog.
[0009] Also provided herein are antibodies that selectively bind PD-1. In some embodiments, the antibodies bind human PD-1. In some embodiments, the antibodies also bind homologs of human PD-1. In some aspects, the homologs include a cynomolgus monkey homolog.
[0010] Also provided herein are bi-specific antibodies or bi-specific antibody constructs that comprise a first binding domain that selectively binds Tim-3, including human Tim-3 or a homolog thereof, and a second binding domain that selectively binds PD-1, including human PD-1 or a homolog thereof.
[0011] In some embodiments, the antibodies comprise at least one CDR sequence defined by a consensus sequence provided in this disclosure. In some embodiments, the antibodies comprise an illustrative CDR, V.sub.H, or V.sub.L sequence provided in this disclosure, or a variant thereof. In some aspects, the variant is a variant with one or more conservative amino acid substitutions.
[0012] Also provided are compositions and kits comprising the antibodies. In some embodiments, the compositions are pharmaceutical compositions. Any suitable pharmaceutical composition may be used. In some embodiments, the pharmaceutical composition is a composition for parenteral administration.
[0013] This disclosure also provides methods of using the anti-Tim-3 antibodies provided herein. In some embodiments, the method is a method of treatment. In some embodiments, the method is a diagnostic method. In some embodiments, the method is an analytical method. In some embodiments, the method is a method of purifying and/or quantifying Tim-3.
[0014] In some embodiments, the antibodies are used to treat a disease or condition. In some aspects, the disease or condition is selected from a cancer, autoimmune disease, and infection.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] FIG. 1 provides a comparison of the Kabat and Chothia numbering systems for CDR-H1. Adapted from Martin A.C.R. (2010). Protein Sequence and Structure Analysis of Antibody Variable Domains. In R. Kontermann & S. Dithel (Eds.), Antibody Engineering vol. 2 (pp. 33-51). Springer-Verlag, Berlin Heidelberg.
[0016] FIG. 2 (A, B) provides alignments of the V.sub.H sequences (SEQ ID NOs: 144-150) and V.sub.L sequences (SEQ ID NOs: 221-227) from the hybridomas provided herein. CDRs according to Chothia are boxed, and CDRs according to Kabat are underlined.
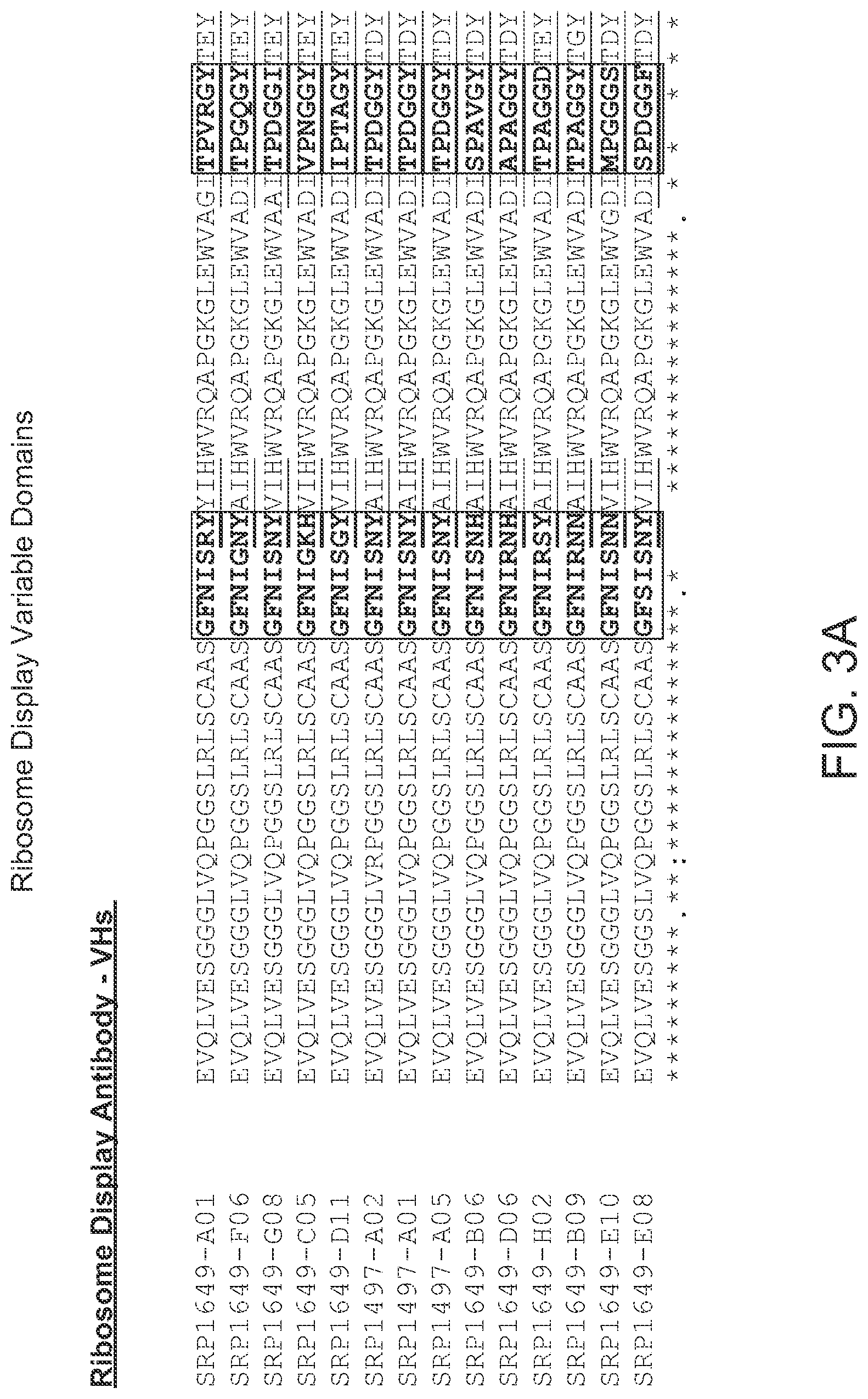
[0017] FIG. 3 (A, B) provides an alignment of the V.sub.H sequences (SEQ ID NOs: 151-164) from the ribosome display selections provided herein. CDRs according to Chothia are boxed, and CDRs according to Kabat are underlined.
[0018] FIG. 4 is a graph that illustrates dose-response activity for IFN-.gamma. in peripheral blood mononuclear cells (PBMCs) isolated from CMV-positive human donors. The activity resulted from exposure of the PBMCs to exemplary bi-specific antibodies disclosed herein.
[0019] FIG. 5 is a graph that illustrates dose-response activity for IL-6 in peripheral blood mononuclear cells (PBMCs) isolated from CMV-positive human donors. The activity resulted from exposure of the PBMCs to exemplary bi-specific antibodies disclosed herein.
[0020] FIG. 6 is a graph that illustrates dose-response activity for TNF-.alpha. in peripheral blood mononuclear cells (PBMCs) isolated from CMV-positive human donors. The activity resulted from exposure of the PBMCs to exemplary bi-specific antibodies disclosed herein.
DETAILED DESCRIPTION
1. Definitions
[0021] Unless otherwise defined, all terms of art, notations and other scientific terminology used herein are intended to have the meanings commonly understood by those of skill in the art to which this invention pertains. In some cases, terms with commonly understood meanings are defined herein for clarity and/or for ready reference, and the inclusion of such definitions herein should not necessarily be construed to represent a difference over what is generally understood in the art. The techniques and procedures described or referenced herein are generally well understood and commonly employed using conventional methodologies by those skilled in the art, such as, for example, the widely utilized molecular cloning methodologies described in Sambrook et al., Molecular Cloning: A Laboratory Manual 2nd ed. (1989) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. As appropriate, procedures involving the use of commercially available kits and reagents are generally carried out in accordance with manufacturer-defined protocols and conditions unless otherwise noted.
[0022] As used herein, the singular forms "a," "an," and "the" include the plural referents unless the context clearly indicates otherwise.
[0023] The term "about" indicates and encompasses an indicated value and a range above and below that value. In certain embodiments, the term "about" indicates the designated value .+-.10%, .+-.5%, or .+-.1%. In certain embodiments, the term "about" indicates the designated value .+-.one standard deviation of that value.
[0024] The terms "first" and "second" are intended to indicate two separate entities, but does not mean that one is before the other in time or space, unless otherwise noted.
[0025] The term "combinations thereof" includes every possible combination of elements to which the term refers to. For example, a sentence stating that "if .alpha..sub.2 is A, then .alpha..sub.3 is not D; .alpha..sub.5 is not S; or .alpha..sub.6 is not S; or combinations thereof" includes the following combinations when .alpha..sub.2 is A: (1) .alpha..sub.3 is not D; (2) .alpha..sub.5 is not S; (3) .alpha..sub.6 is not S; (4) .alpha..sub.3 is not D; .alpha..sub.5 is not S; and .alpha..sub.6 is not S; (5) .alpha..sub.3 is not D and .alpha..sub.5 is not S; (6) .alpha..sub.3 is not D and .alpha..sub.6 is not S; and (7) .alpha..sub.5 is not S and .alpha..sub.6 is not S.
[0026] The terms "Tim-3" and "Tim-3 antigen" are used interchangeably herein. Tim-3 is also known by synonyms, including HAVCR2, T cell immunoglobulin domain- and mucin domain-containing molecule 3, and T cell immunoglobulin and mucin domains-containing molecule 3, among others. Unless specified otherwise, the terms include any variants, isoforms and species homologs of human Tim-3 that are naturally expressed by cells, or that are expressed by cells transfected with a Tim-3 gene. Tim-3 proteins include, for example, human Tim-3 (GI: 20330552; SEQ ID NO: 1). In some embodiments, Tim-3 proteins include cynomolgus monkey Tim-3 (GI: 355750365; SEQ ID NO: 2). In some embodiments, Tim-3 proteins include murine Tim-3 (GI: 17148681; SEQ ID NO: 3).
[0027] The terms "PD-1" and "PD-1 antigen" are used interchangeably herein. Unless specified otherwise, the terms include any variants, isoforms and species homologs of human PD-1 that are naturally expressed by cells, or that are expressed by cells transfected with a PD-1 gene. PD-1 proteins include full-length PD-1 (e.g., human PD-1; GI: 167857792; SEQ ID NO: 292; extracellular domain: Pro21-Gln167), as well as alternative splice variants of PD-1, such as PD-1.DELTA.ex2, PD-1.DELTA.ex3, PD-1.DELTA.ex2,3, and PD-1.DELTA.ex2,3,4. See Nielsen et al., Cellular Immunology, 2005, 235:109-116, incorporated by reference in its entirety. In some embodiments, PD-1 proteins include murine PD-1 (e.g., SEQ ID NO: 293; extracellular domain: Leu25-Gln167). In some embodiments, PD-1 proteins include cynomolgus PD-1 (e.g., SEQ ID NO: 294; extracellular domain: Pro21-Gln167).
[0028] The term "immunoglobulin" refers to a class of structurally related proteins generally comprising two pairs of polypeptide chains: one pair of light (L) chains and one pair of heavy (H) chains. In an "intact immunoglobulin," all four of these chains are interconnected by disulfide bonds. The structure of immunoglobulins has been well characterized. See, e.g., Paul, Fundamental Immunology 7th ed., Ch. 5 (2013) Lippincott Williams & Wilkins, Philadelphia, Pa. Briefly, each heavy chain typically comprises a heavy chain variable region (V.sub.H) and a heavy chain constant region (C.sub.H). The heavy chain constant region typically comprises three domains, abbreviated C.sub.H1, C.sub.H2, and C.sub.H3. Each light chain typically comprises a light chain variable region (V.sub.L) and a light chain constant region. The light chain constant region typically comprises one domain, abbreviated C.sub.L.
[0029] The term "antibody" describes a type of immunoglobulin molecule and is used herein in its broadest sense. An antibody specifically includes intact antibodies (e.g., intact immunoglobulins), and antibody fragments. Antibodies comprise at least one antigen-binding domain. One example of an antigen-binding domain is an antigen binding domain formed by a V.sub.H-V.sub.L dimer.
[0030] A "Tim-3 antibody," "anti-Tim-3 antibody," "Tim-3 Ab," "Tim-3-specific antibody" or "anti-Tim-3 Ab" is an antibody, as described herein, which binds specifically to the antigen Tim-3. In some embodiments, the antibody binds the extracellular domain of Tim-3.
[0031] A "PD-1 antibody," "anti-PD-1 antibody," "PD-1 Ab," "PD-1-specific antibody" or "anti-PD-1 Ab" is an antibody, as described herein, which binds specifically to the antigen PD-1. In some embodiments, the antibody binds the extracellular domain of PD-1.
[0032] The V.sub.H and V.sub.L regions may be further subdivided into regions of hypervariability ("hypervariable regions (HVRs);" also called "complementarity determining regions" (CDRs)) interspersed with regions that are more conserved. The more conserved regions are called framework regions (FRs). Each V.sub.H and V.sub.L generally comprises three CDRs and four FRs, arranged in the following order (from N-terminus to C-terminus): FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4. The CDRs are involved in antigen binding, and influence antigen specificity and binding affinity of the antibody. See Kabat et al., Sequences of Proteins of Immunological Interest 5th ed. (1991) Public Health Service, National Institutes of Health, Bethesda, Md., incorporated by reference in its entirety.
[0033] The light chain from any vertebrate species can be assigned to one of two types, called kappa and lambda, based on the sequence of the constant domain.
[0034] The heavy chain from any vertebrate species can be assigned to one of five different classes (or isotypes): IgA, IgD, IgE, IgG, and IgM. These classes are also designated .alpha., .delta., .epsilon., .gamma., and .mu., respectively. The IgG and IgA classes are further divided into subclasses on the basis of differences in sequence and function. Humans express the following subclasses: IgG1, IgG2, IgG3, IgG4, IgA1, and IgA2.
[0035] The amino acid sequence boundaries of a CDR can be determined by one of skill in the art using any of a number of known numbering schemes, including those described by Kabat et al., supra ("Kabat" numbering scheme); Al-Lazikani et al., 1997, J. Mol. Biol., 273:927-948 ("Chothia" numbering scheme); MacCallum et al., 1996, J. Mol. Biol. 262:732-745 ("Contact" numbering scheme); Lefranc et al., Dev. Comp. Immunol., 2003, 27:55-77 ("IMGT" numbering scheme); and Honegge and Pluckthun, J. Mol. Biol., 2001, 309:657-70 ("AHo" numbering scheme), each of which is incorporated by reference in its entirety.
[0036] Table 1 provides the positions of CDR-L1, CDR-L2, CDR-L3, CDR-H1, CDR-H2, and CDR-H3 as identified by the Kabat and Chothia schemes. For CDR-H1, residue numbering is provided using both the Kabat and Chothia numbering schemes.
[0037] Unless otherwise specified, the numbering scheme used for identification of a particular CDR herein is the Kabat/Chothia numbering scheme. Where the residues encompassed by these two numbering schemes diverge (e.g., CDR-H1 and/or CDR-H2), the numbering scheme is specified as either Kabat or Chothia. For convenience, CDR-H3 is sometimes referred to herein as either Kabat or Chothia. However, this is not intended to imply differences in sequence where they do not exist, and one of skill in the art can readily confirm whether the sequences are the same or different by examining the sequences.
[0038] CDRs may be assigned, for example, using antibody numbering software, such as Abnum, available at http://www.bioinf.org.uk/abs/abnum/, and described in Abhinandan and Martin, Immunology, 2008, 45:3832-3839, incorporated by reference in its entirety.
TABLE-US-00001 TABLE 1 Residues in CDRs according to Kabat and Chothia numbering schemes. CDR Kabat Chothia L1 L24-L34 L24-L34 L2 L50-L56 L50-L56 L3 L89-L97 L89-L97 H1 (Kabat Numbering) H31-H35B H26-H32 or H34* H1 (Chothia Numbering) H31-H35 H26-H32 H2 H50-H65 H52-H56 H3 H95-H102 H95-H102 *The C-terminus of CDR-H1, when numbered using the Kabat numbering convention, varies between H32 and H34, depending on the length of the CDR, as illustrated in FIG. 1.
[0039] The "EU numbering scheme" is generally used when referring to a residue in an antibody heavy chain constant region (e.g., as reported in Kabat et al., supra). Unless stated otherwise, the EU numbering scheme is used to refer to residues in antibody heavy chain constant regions described herein.
[0040] An "antibody fragment" comprises a portion of an intact antibody, such as the antigen binding or variable region of an intact antibody. Antibody fragments include, for example, Fv fragments, Fab fragments, F(ab').sub.2 fragments, Fab' fragments, scFv (sFv) fragments, and scFv-Fc fragments.
[0041] "Fv" fragments comprise a non-covalently-linked dimer of one heavy chain variable domain and one light chain variable domain.
[0042] "Fab" fragments comprise, in addition to the heavy and light chain variable domains, the constant domain of the light chain and the first constant domain (C.sub.H1) of the heavy chain. Fab fragments may be generated, for example, by recombinant methods or by papain digestion of a full-length antibody.
[0043] "F(ab').sub.2" fragments contain two Fab' fragments joined, near the hinge region, by disulfide bonds. F(ab').sub.2 fragments may be generated, for example, by recombinant methods or by pepsin digestion of an intact antibody. The F(ab') fragments can be dissociated, for example, by treatment with .beta.-mercaptoethanol.
[0044] "Single-chain Fv" or "sFv" or "scFv" antibody fragments comprise a V.sub.H domain and a V.sub.L domain in a single polypeptide chain. The V.sub.H and V.sub.L are generally linked by a peptide linker. See Pluckthun A. (1994). In some embodiments, the linker is SEQ ID NO: 168. Antibodies from Escherichia coli. In Rosenberg M. & Moore G. P. (Eds.), The Pharmacology of Monoclonal Antibodies vol. 113 (pp. 269-315). Springer-Verlag, New York, incorporated by reference in its entirety.
[0045] "scFv-Fc" fragments comprise an scFv attached to an Fc domain. For example, an Fc domain may be attached to the C-terminus of the scFv. The Fc domain may follow the V.sub.H or V.sub.L, depending on the orientation of the variable domains in the scFv (i.e., V.sub.H-V.sub.L or V.sub.L-V.sub.H). Any suitable Fc domain known in the art or described herein may be used. In some cases, the Fc domain comprises an IgG1 Fc domain. In some embodiments, the IgG1 Fc domain comprises SEQ ID NO: 270, or a portion thereof, or SEQ ID NO: 276. SEQ ID NO: 270 provides the sequence of C.sub.H1, C.sub.H2, and C.sub.H3 of the human IgG1 constant region. SEQ ID NO: 276 provides the sequence of the constant region used in the illustrative scFv-Fc antibodies provided herein.
[0046] The term "monoclonal antibody" refers to an antibody from a population of substantially homogeneous antibodies. A population of substantially homogeneous antibodies comprises antibodies that are substantially similar and that bind the same epitope(s), except for variants that may normally arise during production of the monoclonal antibody. Such variants are generally present in only minor amounts. A monoclonal antibody is typically obtained by a process that includes the selection of a single antibody from a plurality of antibodies. For example, the selection process can be the selection of a unique clone from a plurality of clones, such as a pool of hybridoma clones, phage clones, yeast clones, bacterial clones, or other recombinant DNA clones. The selected antibody can be further altered, for example, to improve affinity for the target ("affinity maturation"), to humanize the antibody, to improve its production in cell culture, and/or to reduce its immunogenicity in a subject.
[0047] The term "chimeric antibody" refers to an antibody in which a portion of the heavy and/or light chain is derived from a particular source or species, while the remainder of the heavy and/or light chain is derived from a different source or species.
[0048] "Humanized" forms of non-human antibodies are chimeric antibodies that contain minimal sequence derived from the non-human antibody. A humanized antibody is generally a human immunoglobulin (recipient antibody) in which residues from one or more CDRs are replaced by residues from one or more CDRs of a non-human antibody (donor antibody). The donor antibody can be any suitable non-human antibody, such as a mouse, rat, rabbit, chicken, or non-human primate antibody having a desired specificity, affinity, or biological effect. In some instances, selected framework region residues of the recipient antibody are replaced by the corresponding framework region residues from the donor antibody. Humanized antibodies may also comprise residues that are not found in either the recipient antibody or the donor antibody. Such modifications may be made to further refine antibody function. For further details, see Jones et al., Nature, 1986, 321:522-525; Riechmann et al., Nature, 1988, 332:323-329; and Presta, Curr. Op. Struct. Biol., 1992, 2:593-596, each of which is incorporated by reference in its entirety.
[0049] A "human antibody" is one which possesses an amino acid sequence corresponding to that of an antibody produced by a human or a human cell, or derived from a non-human source that utilizes a human antibody repertoire or human antibody-encoding sequences (e.g., obtained from human sources or designed de novo). Human antibodies specifically exclude humanized antibodies.
[0050] An "isolated antibody" is one that has been separated and/or recovered from a component of its natural environment. Components of the natural environment may include enzymes, hormones, and other proteinaceous or nonproteinaceous materials. In some embodiments, an isolated antibody is purified to a degree sufficient to obtain at least 15 residues of N-terminal or internal amino acid sequence, for example by use of a spinning cup sequenator. In some embodiments, an isolated antibody is purified to homogeneity by gel electrophoresis (e.g., SDS-PAGE) under reducing or nonreducing conditions, with detection by Coomassie blue or silver stain. An isolated antibody includes an antibody in situ within recombinant cells, since at least one component of the antibody's natural environment is not present. In some aspects, an isolated antibody is prepared by at least one purification step.
[0051] In some embodiments, an isolated antibody is purified to at least 80%, 85%, 90%, 95%, or 99% by weight. In some embodiments, an isolated antibody is purified to at least 80%, 85%, 90%, 95%, or 99% by volume. In some embodiments, an isolated antibody is provided as a solution comprising at least 85%, 90%, 95%, 98%, 99% to 100% by weight. In some embodiments, an isolated antibody is provided as a solution comprising at least 85%, 90%, 95%, 98%, 99% to 100% by volume.
[0052] "Affinity" refers to the strength of the sum total of non-covalent interactions between a single binding site of a molecule (e.g., an antibody) and its binding partner (e.g., an antigen). Unless indicated otherwise, as used herein, "binding affinity" refers to intrinsic binding affinity, which reflects a 1:1 interaction between members of a binding pair (e.g., antibody and antigen). The affinity of a molecule X for its partner Y can be represented by the dissociation constant (K.sub.D). Affinity can be measured by common methods known in the art, including those described herein. Affinity can be determined, for example, using surface plasmon resonance (SPR) technology, such as a Biacore.RTM. instrument. In some embodiments, the affinity is determined at 25.degree. C.
[0053] With regard to the binding of an antibody to a target molecule, the terms "specific binding," "specifically binds to," "specific for," "selectively binds," and "selective for" a particular antigen (e.g., a polypeptide target) or an epitope on a particular antigen mean binding that is measurably different from a non-specific or non-selective interaction. Specific binding can be measured, for example, by determining binding of a molecule compared to binding of a control molecule. Specific binding can also be determined by competition with a control molecule that mimics the antibody binding site on the target. In that case, specific binding is indicated if the binding of the antibody to the target is competitively inhibited by the control molecule.
[0054] The term "k.sub.d" (sec.sup.-1), as used herein, refers to the dissociation rate constant of a particular antibody-antigen interaction. This value is also referred to as the k.sub.off value.
[0055] The term "k.sub.a" (M.sup.-1.times.sec.sup.-1), as used herein, refers to the association rate constant of a particular antibody-antigen interaction. This value is also referred to as the k.sub.on value.
[0056] The term "K.sub.D" (M), as used herein, refers to the dissociation equilibrium constant of a particular antibody-antigen interaction. K.sub.D=k.sub.d/k.sub.a.
[0057] The term "K.sub.A" (M.sup.-1), as used herein, refers to the association equilibrium constant of a particular antibody-antigen interaction. K.sub.A=k.sub.a/k.sub.d.
[0058] An "affinity matured" antibody is one with one or more alterations in one or more CDRs or FRs that result in an improvement in the affinity of the antibody for its antigen, compared to a parent antibody which does not possess the alteration(s). In one embodiment, an affinity matured antibody has nanomolar or picomolar affinity for the target antigen. Affinity matured antibodies may be produced using a variety of methods known in the art. For example, Marks et al. (Bio/Technology, 1992, 10:779-783, incorporated by reference in its entirety) describes affinity maturation by V.sub.H and V.sub.L domain shuffling. Random mutagenesis of CDR and/or framework residues is described by, for example, Barbas et al. (Proc. Nat. Acad. Sci. U.S.A., 1994, 91:3809-3813); Schier et al., Gene, 1995, 169:147-155; Yelton et al., J. Immunol., 1995, 155:1994-2004; Jackson et al., J. Immunol., 1995, 154:3310-33199; and Hawkins et al, J. Mol. Biol., 1992, 226:889-896, each of which is incorporated by reference in its entirety.
[0059] When used herein in the context of two or more antibodies, the term "competes with" or "cross-competes with" indicates that the two or more antibodies compete for binding to an antigen. In one exemplary assay, an antigen is coated on a plate and allowed to bind a first antibody, after which a second, labeled antibody is added. If the presence of the first antibody reduces binding of the second antibody, then the antibodies compete. In another exemplary assay, a first antibody is coated on a plate and allowed to bind an antigen, and then the second antibody is added. The term "competes with" also includes combinations of antibodies where one antibody reduces binding of another antibody, but where no competition is observed when the antibodies are added in the reverse order. However, in some embodiments, the first and second antibodies inhibit binding of each other, regardless of the order in which they are added. In some embodiments, one antibody reduces binding of another antibody to its antigen by at least 50%, at least 60%, at least 70%, at least 80%, or at least 90%.
[0060] The term "epitope" means a portion of an antigen capable of specific binding to an antibody. Epitopes frequently consist of surface-accessible amino acid residues and/or sugar side chains and may have specific three dimensional structural characteristics, as well as specific charge characteristics. Conformational and non-conformational epitopes are distinguished in that the binding to the former but not the latter is lost in the presence of denaturing solvents. An epitope may comprise amino acid residues that are directly involved in the binding, and other amino acid residues, which are not directly involved in the binding. The epitope to which an antibody binds can be determined using known techniques for epitope determination such as, for example, testing for antibody binding to Tim-3 and/or PD-1 variants with different point-mutations, or to chimeric Tim-3 and/or PD-1 variants as described further in the Examples provided herein.
[0061] Percent "identity" between a polypeptide sequence and a reference sequence, is defined as the percentage of amino acid residues in the polypeptide sequence that are identical to the amino acid residues in the reference sequence, after aligning the sequences and introducing gaps, if necessary, to achieve the maximum percent sequence identity. Alignment for purposes of determining percent amino acid sequence identity can be achieved in various ways that are within the skill in the art, for instance, using publicly available computer software such as BLAST, BLAST-2, ALIGN, MEGALIGN (DNASTAR), CLUSTALW, CLUSTAL OMEGA, or MUSCLE software. Those skilled in the art can determine appropriate parameters for aligning sequences, including any algorithms needed to achieve maximal alignment over the full length of the sequences being compared.
[0062] A "conservative substitution" or a "conservative amino acid substitution," refers to the substitution an amino acid with a chemically or functionally similar amino acid. Conservative substitution tables providing similar amino acids are well known in the art. Polypeptide sequences having such substitutions are known as "conservatively modified variants." By way of example, the groups of amino acids provided in Tables 2-4 are, in some embodiments, considered conservative substitutions for one another.
TABLE-US-00002 TABLE 2 Selected groups of amino acids that are considered conservative substitutions for one another, in certain embodiments. Acidic Residues D and E Basic Residues K, R, and H Hydrophilic Uncharged Residues S, T, N, and Q Aliphatic Uncharged Residues G, A, V, L, and I Non-polar Uncharged Residues C, M, and P Aromatic Residues F, Y, and W
TABLE-US-00003 TABLE 3 Additional selected groups of amino acids that are considered conservative substitutions for one another, in certain embodiments. Group 1 A, S, and T Group 2 D and E Group 3 N and Q Group 4 R and K Group 5 I, L, and M Group 6 F, Y, and W
TABLE-US-00004 TABLE 4 Further selected groups of amino acids that are considered conservative substitutions for one another, in certain embodiments. Group A A and G Group B D and E Group C N and Q Group D R, K, and H Group E I, L, M, V Group F F, Y, and W Group G S and T Group H C and M
[0063] Additional conservative substitutions may be found, for example, in Creighton, Proteins: Structures and Molecular Properties 2nd ed. (1993) W. H. Freeman & Co., New York, N.Y. An antibody generated by making one or more conservative substitutions of amino acid residues in a parent antibody is referred to as a "conservatively modified variant."
[0064] The term "amino acid" refers to the twenty common naturally occurring amino acids. Naturally occurring amino acids include alanine (Ala; A), arginine (Arg; R), asparagine (Asn; N), aspartic acid (Asp; D), cysteine (Cys; C); glutamic acid (Glu; E), glutamine (Gln; Q), Glycine (Gly; G); histidine (His; H), isoleucine (Ile; I), leucine (Leu; L), lysine (Lys; K), methionine (Met; M), phenylalanine (Phe; F), proline (Pro; P), serine (Ser; S), threonine (Thr; T), tryptophan (Trp; W), tyrosine (Tyr; Y), and valine (Val; V).
[0065] "Treating" or "treatment" of any disease or disorder refers, in certain embodiments, to ameliorating a disease or disorder that exists in a subject. In another embodiment, "treating" or "treatment" includes ameliorating at least one physical parameter, which may be indiscernible by the subject. In yet another embodiment, "treating" or "treatment" includes modulating the disease or disorder, either physically (e.g., stabilization of a discernible symptom) or physiologically (e.g., stabilization of a physical parameter) or both. In yet another embodiment, "treating" or "treatment" includes delaying or preventing the onset of the disease or disorder.
[0066] As used herein, the term "therapeutically effective amount" or "effective amount" refers to an amount of an antibody or composition that when administered to a subject is effective to treat a disease or disorder.
[0067] As used herein, the term "subject" means a mammalian subject. Exemplary subjects include, but are not limited to humans, monkeys, dogs, cats, mice, rats, cows, horses, camels, avians, goats, and sheep. In certain embodiments, the subject is a human. In some embodiments, the subject has a cancer that can be treated or diagnosed with an antibody provided herein. In some embodiments, the cancer is a cancer of epithelial origin.
2. Tim-3 Antibodies
[0068] Provided herein are Tim-3 antibodies that selectively bind human Tim-3. In some aspects, the Tim-3 antibody selectively binds to the extracellular domain of human Tim-3.
[0069] In some embodiments, the Tim-3 antibody binds to a homolog of human Tim-3. In some aspects, the Tim-3 antibody binds to a homolog of human Tim-3 from a species selected from monkeys, mice, dogs, cats, rats, cows, horses, goats and sheep. In some aspects, the homolog is a cynomolgus monkey homolog.
[0070] In some embodiments, the Tim-3 antibody has one or more CDRs having particular lengths, in terms of the number of amino acid residues. In some embodiments, the Chothia CDR-H1 of the antibody is 6, 7, or 8 residues in length. In some embodiments, the Kabat CDR-H1 of the antibody is 4, 5, or 6 residues in length. In some embodiments, the Chothia CDR-H2 of the antibody is 5, 6, or 7 residues in length. In some embodiments, the Kabat CDR-H2 of the antibody is 16, 17, or 18 residues in length. In some embodiments, the Kabat/Chothia CDR-H3 of the antibody is 9, 10, 11, 12, or 13 residues in length.
[0071] In some aspects, the Kabat/Chothia CDR-L1 of the antibody is 11, 12, 13, 14, 15, 16, 17, or 18 residues in length. In some aspects, the Kabat/Chothia CDR-L2 of the antibody is 6, 7, or 8 residues in length. In some aspects, the Kabat/Chothia CDR-L3 of the antibody is 8, 9, or 10 residues in length.
[0072] In some embodiments, the Tim-3 antibody comprises a light chain. In some aspects, the light chain is a kappa light chain. In some aspects, the light chain is a lambda light chain.
[0073] In some embodiments, the Tim-3 antibody comprises a heavy chain. In some aspects, the heavy chain is an IgA. In some aspects, the heavy chain is an IgD. In some aspects, the heavy chain is an IgE. In some aspects, the heavy chain is an IgG. In some aspects, the heavy chain is an IgM. In some aspects, the heavy chain is an IgG1. In some aspects, the heavy chain is an IgG2. In some aspects, the heavy chain is an IgG3. In some aspects, the heavy chain is an IgG4. In some aspects, the heavy chain is an IgA1. In some aspects, the heavy chain is an IgA2.
[0074] In some embodiments, the Tim-3 antibody is an antibody fragment. In some aspects, the antibody fragment is an Fv fragment. In some aspects, the antibody fragment is a Fab fragment. In some aspects, the antibody fragment is a F(ab').sub.2 fragment. In some aspects, the antibody fragment is a Fab' fragment. In some aspects, the antibody fragment is an scFv (sFv) fragment. In some aspects, the antibody fragment is an scFv-Fc fragment.
[0075] In some embodiments, the scFv-Fc fragment comprises a constant region wherein the constant region comprises SEQ ID NO: 276. The constant region in SEQ ID NO: 276 differs from the human IgG1 constant region of SEQ ID NO: 270 in several respects. First, the sequence in SEQ ID NO: 276 comprises the linker AAGSDQEPKSS (SEQ ID NO: 282). SEQ ID NO: 276 also does not comprise the CH1 domain of the IgG1 constant region. SEQ ID NO: 276 further comprises a C220S (EU numbering system) mutation, which removes an unpaired cysteine reside that is not needed when the light chain constant region is not present (e.g., in an scFv-Fc format). SEQ ID NO: 276 further comprises two, optional, P to S mutations (P230S and P238S by the EU numbering system). Either or both of these serine residues can be reverted to the naturally occurring proline residues. Finally, SEQ ID NO: 276 comprises an aspartic acid (D) residue at EU position 356 and a leucine (L) residue at EU position 358. In contrast, SEQ ID NO: 270 comprises glutamic acid (E) in EU position 356 and methionine (M) in EU position 358. In some embodiments, the antibodies provided herein comprise constant regions comprising D356/L358, E356/M358, D356/M358, or E356/L358 (EU numbering). However, a skilled person will recognize that the antibodies provide herein may comprise any suitable constant region and that the constant region sequences provided herein are for illustrative purposes.
[0076] In some embodiments, the Tim-3 antibody is a monoclonal antibody. In some embodiments, the Tim-3 antibody is a polyclonal antibody.
[0077] In some embodiments, the Tim-3 antibody is a chimeric antibody. In some embodiments, the Tim-3 antibody is a humanized antibody. In some embodiments, the Tim-3 antibody is a human antibody.
[0078] In some embodiments, the Tim-3 antibody is an affinity matured antibody. In some aspects, the Tim-3 antibody is an affinity matured antibody derived from an illustrative sequence provided in this disclosure.
[0079] In some embodiments, the Tim-3 antibody inhibits the binding of Tim-3 to one or more of its ligands. In some aspects, the Tim-3 antibody inhibits the binding of Tim-3 to a ligand selected from a second Tim-3 molecule, claudin-7, CD44v4-v7, E-cadherin, and CD9.
[0080] In some embodiments, the Tim-3 antibody is provided as a single arm binder. For example, the Tim-3 antibody can be provided as part of a bi-specific antibody or bi-specific antibody construct as disclosed here.
[0081] The Tim-3 antibodies provided herein may be useful for the treatment of a variety of diseases and conditions including cancers. In particular, the Tim-3 antibodies provided herein may be useful for the treatment of cancers of epithelial origin.
[0082] 2.1. Tim-3 CDR-H3 Sequences
[0083] In some embodiments, the Tim-3 antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the CDR-H3 sequence is a CDR-H3 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the CDR-H3 sequence is a CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0084] In some embodiments, the Tim-3 antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 111. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 112. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 113. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 114. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 115. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 116. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 117. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 118.
[0085] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0086] 2.2. Tim-3 V.sub.H Sequences Comprising Illustrative CDRs
[0087] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising one or more CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative CDR-H sequences provided in this disclosure, and variants thereof. In some embodiments, the CDR-H sequences comprise, consist of, or consist essentially of one or more CDR-H sequences provided in a V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0088] 2.2.1. V.sub.H Sequences Comprising Illustrative Kabat CDRs
[0089] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising one or more Kabat CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative Kabat CDR-H sequences provided in this disclosure, and variants thereof.
[0090] 2.2.1.1. Kabat CDR-H3
[0091] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Kabat CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H3 sequence is a Kabat CDR-H3 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Kabat CDR-H3 sequence is a Kabat CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0092] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 111. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 112. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 113. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 114. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 115. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 116. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 117. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 118.
[0093] 2.2.1.2. Kabat CDR-H2
[0094] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Kabat CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H2 sequence is a Kabat CDR-H2 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Kabat CDR-H2 sequence is a Kabat CDR-H2 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0095] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 82. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 83. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 84. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 85. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 86. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 87. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 88. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 89. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 90. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 91. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 92. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 93. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 94. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 95. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 96. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 97. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 98. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 99. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 100. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 101. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 102. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 103. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 104. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 105. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 106. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 107. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 108. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 109.
[0096] 2.2.1.3. Kabat CDR-H1
[0097] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Kabat CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H1 sequence is a Kabat CDR-H1 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Kabat CDR-H1 sequence is a Kabat CDR-H1 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0098] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 39. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 40. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 41. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 42. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 43. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 44. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 45. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 46. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 47. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 48. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 49. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 50. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 51. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 52. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 53. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 54. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 55. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 56.
[0099] 2.2.1.4. Kabat CDR-H3+Kabat CDR-H2
[0100] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118, and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109. In some aspects, the Kabat CDR-H3 sequence and the Kabat CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H3 and Kabat CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0101] 2.2.1.5. Kabat CDR-H3+Kabat CDR-H1
[0102] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118, and a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56. In some aspects, the Kabat CDR-H3 sequence and the Kabat CDR-H1 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H3 and Kabat CDR-H1 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0103] 2.2.1.6. Kabat CDR-H1+Kabat CDR-H2
[0104] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56 and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109. In some aspects, the Kabat CDR-H1 sequence and the Kabat CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H1 and Kabat CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0105] 2.2.1.7. Kabat CDR-H1+Kabat CDR-H2+Kabat CDR-H3
[0106] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56, a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109, and a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118. In some aspects, the Kabat CDR-H1 sequence, Kabat CDR-H2 sequence, and Kabat CDR-H3 sequence are all from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H1, Kabat CDR-H2, and Kabat CDR-H3 are all from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0107] 2.2.1.8. Variants of V.sub.H Sequences Comprising Illustrative Kabat CDRs
[0108] In some embodiments, the V.sub.H sequences provided herein comprise a variant of an illustrative Kabat CDR-H3, CDR-H2, and/or CDR-H1 sequence provided in this disclosure.
[0109] In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H3 sequence provided in this disclosure. In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H3 sequences provided in this disclosure. In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0110] In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H2 sequence provided in this disclosure. In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H2 sequences provided in this disclosure. In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0111] In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H1 sequence provided in this disclosure. In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H1 sequences provided in this disclosure. In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0112] 2.2.2. V.sub.H Sequences Comprising Illustrative Chothia CDRs
[0113] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising one or more Chothia CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative Chothia CDR-H sequences provided in this disclosure, and variants thereof.
[0114] 2.2.2.1. Chothia CDR-H3
[0115] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Chothia CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H3 sequence is a Chothia CDR-H3 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Chothia CDR-H3 sequence is a Chothia CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0116] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 111. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 112. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 113. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 114. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 115. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 116. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 117. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 118.
[0117] 2.2.2.2. Chothia CDR-H2
[0118] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Chothia CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H2 sequence is a Chothia CDR-H2 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Chothia CDR-H2 sequence is a Chothia CDR-H2 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0119] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 63. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 64. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 65. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 66. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 67. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 68. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 69. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 70. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 71. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 72. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 73. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 74. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 75. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 76. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 77. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 78. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 79. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 80.
[0120] 2.2.2.3. Chothia CDR-H1
[0121] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Chothia CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H1 sequence is a Chothia CDR-H1 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the Chothia CDR-H1 sequence is a Chothia CDR-H1 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0122] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 4. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 5. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 6. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 7. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 8. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 9. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 10. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 11. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 12. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 13. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 14. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 15. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 16. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 17. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 18. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 19. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 20. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 21. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 22. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 23.
[0123] 2.2.2.4. Chothia CDR-H3+Chothia CDR-H2
[0124] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118, and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80. In some aspects, the Chothia CDR-H3 sequence and the Chothia CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H3 and Chothia CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0125] 2.2.2.5. Chothia CDR-H3+Chothia CDR-H1
[0126] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118, and a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23. In some aspects, the Chothia CDR-H3 sequence and the Chothia CDR-H1 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H3 and Chothia CDR-H1 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0127] 2.2.2.6. Chothia CDR-H1+Chothia CDR-H2
[0128] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23 and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80. In some aspects, the Chothia CDR-H1 sequence and the Chothia CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H1 and Chothia CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0129] 2.2.2.7. Chothia CDR-H1+Chothia CDR-H2+Chothia CDR-H3
[0130] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23, a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80, and a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118. In some aspects, the Chothia CDR-H1 sequence, Chothia CDR-H2 sequence, and Chothia CDR-H3 sequence are all from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H1, Chothia CDR-H2, and Chothia CDR-H3 are all from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 144-180.
[0131] 2.2.2.8. Variants of V.sub.H Sequences Comprising Illustrative Chothia CDRs
[0132] In some embodiments, the V.sub.H sequences provided herein comprise a variant of an illustrative Chothia CDR-H3, CDR-H2, and/or CDR-H1 sequence provided in this disclosure.
[0133] In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H3 sequence provided in this disclosure. In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H3 sequences provided in this disclosure. In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0134] In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H2 sequence provided in this disclosure. In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H2 sequences provided in this disclosure. In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0135] In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H1 sequence provided in this disclosure. In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H1 sequences provided in this disclosure. In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0136] 2.3. Tim-3 V.sub.H Sequences
[0137] In some embodiments, the Tim-3 antibody comprises, consists of, or consists essentially of a V.sub.H sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some embodiments, the antibody comprises, consists of, or consists essentially of a V.sub.H sequence provided in SEQ ID NOs.: 144-180.
[0138] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 144-180. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 144. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 145. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 146. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 147. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 148. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 149. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 150. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 151. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 152. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 153. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 154. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 155. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 156. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 157. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 158. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 159. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 160. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 161. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 162. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 163. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 164. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 165. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 166. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 167. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 168. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 169. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 170. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 171. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 172. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 173. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 174. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 175. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 176. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 177. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 178. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 179. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 180.
[0139] 2.3.1. Variants of V.sub.H Sequences
[0140] In some embodiments, the V.sub.H sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure.
[0141] In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure. In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.H sequences provided in this disclosure.
[0142] In some embodiments, the V.sub.H sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.H sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0143] 2.4. Tim-3 CDR-L3 Sequences
[0144] In some embodiments, the Tim-3 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 221-235.
[0145] In some embodiments, the Tim-3 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 140. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 141. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 142. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 356.
[0146] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0147] 2.5. Tim-3 V.sub.L Sequences Comprising Illustrative CDRs
[0148] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising one or more CDR-L sequences comprising, consisting of, or consisting essentially of one or more illustrative CDR-L sequences provided in this disclosure, and variants thereof.
[0149] 2.5.1. CDR-L3
[0150] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence, wherein the CDR-L3 sequence comprises, consists of, or consists essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 221-235.
[0151] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 140. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 141. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 142. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 356.
[0152] 2.5.2. CDR-L2
[0153] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence, wherein the CDR-L2 sequence comprises, consists of, or consists essentially of a CDR-L2 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L2 sequence is a CDR-L2 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the CDR-L2 sequence is a CDR-L2 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 221-235.
[0154] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 125. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 126. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 127. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 128. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 129. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 130. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 131. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 132. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 133. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 355.
[0155] 2.5.3. CDR-L1
[0156] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence, wherein the CDR-L1 sequence comprises, consists of, or consists essentially of a CDR-L1 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L1 sequence is a CDR-L1 sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some aspects, the CDR-L1 sequence is a CDR-L1 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 221-235.
[0157] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 120. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 121. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 122. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 123. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 354.
[0158] 2.5.4. CDR-L3+CDR-L2
[0159] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356 and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355. In some aspects, the CDR-L3 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L3 and CDR-L2 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 221-235.
[0160] 2.5.5. CDR-L3+CDR-L1
[0161] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356 and a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354. In some aspects, the CDR-L3 sequence and the CDR-L1 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L3 and CDR-L1 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 221-235.
[0162] 2.5.6. CDR-L1+CDR-L2
[0163] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354 and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355. In some aspects, the CDR-L1 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L1 and CDR-L2 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 221-235.
[0164] 2.5.7. CDR-L1+CDR-L2+CDR-L3
[0165] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from any one of SEQ ID NOs: 120-123 and 354, a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355, and a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356. In some aspects, the CDR-L1 sequence, CDR-L2 sequence, and CDR-L3 sequence are all from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L1, CDR-L2, and CDR-L3 are all from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 221-235.
[0166] 2.5.8. Variants of V.sub.L Sequences Comprising Illustrative CDR-Ls
[0167] In some embodiments, the V.sub.L sequences provided herein comprise a variant of an illustrative CDR-L3, CDR-L2, and/or CDR-L1 sequence provided in this disclosure.
[0168] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0169] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0170] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0171] 2.6. Tim-3 V.sub.L Sequences
[0172] In some embodiments, the Tim-3 antibody comprises, consists of, or consists essentially of a V.sub.L sequence of an scFv-Fc sequence provided in SEQ ID NO: 287. In some embodiments, the antibody comprises, consists of, or consists essentially of a V.sub.L sequence provided in SEQ ID NOs.: 221-235.
[0173] In some embodiments, the Tim-3 antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 221-235. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 221. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 222. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 223. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 224. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 225. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 226. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 227. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 228. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 229. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 230. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 231. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 232. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 233. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 234. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 235.
[0174] 2.6.1. Variants of V.sub.L Sequences
[0175] In some embodiments, the V.sub.L sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure.
[0176] In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure. In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.L sequences provided in this disclosure.
[0177] In some embodiments, the V.sub.L sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.L sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0178] 2.7. Tim-3 Pairs
[0179] 2.7.1. CDR-H3-CDR-L3 Pairs
[0180] In some embodiments, the Tim-3 antibody comprises a CDR-H3 sequence and a CDR-L3 sequence. In some aspects, the CDR-H3 sequence is part of a V.sub.H and the CDR-L3 sequence is part of a V.sub.L.
[0181] In some aspects, the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118, and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356.
[0182] 2.7.1.1. Variants of CDR-H3-CDR-L3 Pairs
[0183] In some embodiments, the CDR-H3-CDR-L3 pairs provided herein comprise a variant of an illustrative CDR-H3 and/or CDR-L1 sequence provided in this disclosure.
[0184] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0185] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0186] 2.7.2. CDR-H1-CDR-L1 Pairs
[0187] In some embodiments, the Tim-3 antibody comprises a CDR-H1 sequence and a CDR-L1 sequence. In some aspects, the CDR-H1 sequence is part of a V.sub.H and the CDR-L1 sequence is part of a V.sub.L.
[0188] In some aspects, the CDR-H1 sequence is a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23, and the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354.
[0189] In some aspects, the CDR-H1 sequence is a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56, and the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354.
[0190] 2.7.2.1. Variants of CDR-H1-CDR-L1 Pairs
[0191] In some embodiments, the CDR-H1-CDR-L1 pairs provided herein comprise a variant of an illustrative CDR-H1 and/or CDR-L1 sequence provided in this disclosure.
[0192] In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H1 sequence provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H1 sequences provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0193] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0194] 2.7.3. CDR-H2-CDR-L2 Pairs
[0195] In some embodiments, the Tim-3 antibody comprises a CDR-H2 sequence and a CDR-L2 sequence. In some aspects, the CDR-H2 sequence is part of a V.sub.H and the CDR-L2 sequence is part of a V.sub.L.
[0196] In some aspects, the CDR-H2 sequence is a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80, and the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355.
[0197] In some aspects, the CDR-H1 sequence is a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109, and the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355.
[0198] 2.7.3.1. Variants of CDR-H2-CDR-L2 Pairs
[0199] In some embodiments, the CDR-H2-CDR-L2 pairs provided herein comprise a variant of an illustrative CDR-H2 and/or CDR-L2 sequence provided in this disclosure.
[0200] In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H2 sequence provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H2 sequences provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0201] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0202] 2.7.4. V.sub.H-V.sub.L Pairs
[0203] In some embodiments, the Tim-3 antibody comprises a V.sub.H sequence and a V.sub.L sequence.
[0204] In some aspects, the V.sub.H sequence is a V.sub.H sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 144-180, and the V.sub.L sequence is a V.sub.L sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 221-235.
[0205] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 221 and SEQ ID NO: 144; SEQ ID NO: 221 and SEQ ID NO: 145; SEQ ID NO: 221 and SEQ ID NO: 146; SEQ ID NO: 221 and SEQ ID NO: 147; SEQ ID NO: 221 and SEQ ID NO: 148; SEQ ID NO: 221 and SEQ ID NO: 149; SEQ ID NO: 221 and SEQ ID NO: 150; SEQ ID NO: 221 and SEQ ID NO: 151; SEQ ID NO: 221 and SEQ ID NO: 152; SEQ ID NO: 221 and SEQ ID NO: 153; SEQ ID NO: 221 and SEQ ID NO: 154; SEQ ID NO: 221 and SEQ ID NO: 155; SEQ ID NO: 221 and SEQ ID NO: 156; SEQ ID NO: 221 and SEQ ID NO: 157; SEQ ID NO: 221 and SEQ ID NO: 158; SEQ ID NO: 221 and SEQ ID NO: 159; SEQ ID NO: 221 and SEQ ID NO: 160; SEQ ID NO: 221 and SEQ ID NO: 161; SEQ ID NO: 221 and SEQ ID NO: 162; SEQ ID NO: 221 and SEQ ID NO: 163; SEQ ID NO: 221 and SEQ ID NO: 164; SEQ ID NO: 221 and SEQ ID NO: 165; SEQ ID NO: 221 and SEQ ID NO: 166; SEQ ID NO: 221 and SEQ ID NO: 167; SEQ ID NO: 221 and SEQ ID NO: 168; SEQ ID NO: 221 and SEQ ID NO: 169; SEQ ID NO: 221 and SEQ ID NO: 170; SEQ ID NO: 221 and SEQ ID NO: 171; SEQ ID NO: 221 and SEQ ID NO: 172; SEQ ID NO: 221 and SEQ ID NO: 173; SEQ ID NO: 221 and SEQ ID NO: 174; SEQ ID NO: 221 and SEQ ID NO: 175; SEQ ID NO: 221 and SEQ ID NO: 176; SEQ ID NO: 221 and SEQ ID NO: 177; SEQ ID NO: 221 and SEQ ID NO: 178; SEQ ID NO: 221 and SEQ ID NO: 179; and SEQ ID NO: 221 and SEQ ID NO: 180.
[0206] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 222 and SEQ ID NO: 144; SEQ ID NO: 222 and SEQ ID NO: 145; SEQ ID NO: 222 and SEQ ID NO: 146; SEQ ID NO: 222 and SEQ ID NO: 147; SEQ ID NO: 222 and SEQ ID NO: 148; SEQ ID NO: 222 and SEQ ID NO: 149; SEQ ID NO: 222 and SEQ ID NO: 150; SEQ ID NO: 222 and SEQ ID NO: 151; SEQ ID NO: 222 and SEQ ID NO: 152; SEQ ID NO: 222 and SEQ ID NO: 153; SEQ ID NO: 222 and SEQ ID NO: 154; SEQ ID NO: 222 and SEQ ID NO: 155; SEQ ID NO: 222 and SEQ ID NO: 156; SEQ ID NO: 222 and SEQ ID NO: 157; SEQ ID NO: 222 and SEQ ID NO: 158; SEQ ID NO: 222 and SEQ ID NO: 159; SEQ ID NO: 222 and SEQ ID NO: 160; SEQ ID NO: 222 and SEQ ID NO: 161; SEQ ID NO: 222 and SEQ ID NO: 162; SEQ ID NO: 222 and SEQ ID NO: 163; SEQ ID NO: 222 and SEQ ID NO: 164; SEQ ID NO: 222 and SEQ ID NO: 165; SEQ ID NO: 222 and SEQ ID NO: 166; SEQ ID NO: 222 and SEQ ID NO: 167; SEQ ID NO: 222 and SEQ ID NO: 168; SEQ ID NO: 222 and SEQ ID NO: 169; SEQ ID NO: 222 and SEQ ID NO: 170; SEQ ID NO: 222 and SEQ ID NO: 171; SEQ ID NO: 222 and SEQ ID NO: 172; SEQ ID NO: 222 and SEQ ID NO: 173; SEQ ID NO: 222 and SEQ ID NO: 174; SEQ ID NO: 222 and SEQ ID NO: 175; SEQ ID NO: 222 and SEQ ID NO: 176; SEQ ID NO: 222 and SEQ ID NO: 177; SEQ ID NO: 222 and SEQ ID NO: 178; SEQ ID NO: 222 and SEQ ID NO: 179; and SEQ ID NO: 222 and SEQ ID NO: 180.
[0207] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 223 and SEQ ID NO: 144; SEQ ID NO: 223 and SEQ ID NO: 145; SEQ ID NO: 223 and SEQ ID NO: 146; SEQ ID NO: 223 and SEQ ID NO: 147; SEQ ID NO: 223 and SEQ ID NO: 148; SEQ ID NO: 223 and SEQ ID NO: 149; SEQ ID NO: 223 and SEQ ID NO: 150; SEQ ID NO: 223 and SEQ ID NO: 151; SEQ ID NO: 223 and SEQ ID NO: 152; SEQ ID NO: 223 and SEQ ID NO: 153; SEQ ID NO: 223 and SEQ ID NO: 154; SEQ ID NO: 223 and SEQ ID NO: 155; SEQ ID NO: 223 and SEQ ID NO: 156; SEQ ID NO: 223 and SEQ ID NO: 157; SEQ ID NO: 223 and SEQ ID NO: 158; SEQ ID NO: 223 and SEQ ID NO: 159; SEQ ID NO: 223 and SEQ ID NO: 160; SEQ ID NO: 223 and SEQ ID NO: 161; SEQ ID NO: 223 and SEQ ID NO: 162; SEQ ID NO: 223 and SEQ ID NO: 163; SEQ ID NO: 223 and SEQ ID NO: 164; SEQ ID NO: 223 and SEQ ID NO: 165; SEQ ID NO: 223 and SEQ ID NO: 166; SEQ ID NO: 223 and SEQ ID NO: 167; SEQ ID NO: 223 and SEQ ID NO: 168; SEQ ID NO: 223 and SEQ ID NO: 169; SEQ ID NO: 223 and SEQ ID NO: 170; SEQ ID NO: 223 and SEQ ID NO: 171; SEQ ID NO: 223 and SEQ ID NO: 172; SEQ ID NO: 223 and SEQ ID NO: 173; SEQ ID NO: 223 and SEQ ID NO: 174; SEQ ID NO: 223 and SEQ ID NO: 175; SEQ ID NO: 223 and SEQ ID NO: 176; SEQ ID NO: 223 and SEQ ID NO: 177; SEQ ID NO: 223 and SEQ ID NO: 178; SEQ ID NO: 223 and SEQ ID NO: 179; and SEQ ID NO: 223 and SEQ ID NO: 180.
[0208] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 224 and SEQ ID NO: 144; SEQ ID NO: 224 and SEQ ID NO: 145; SEQ ID NO: 224 and SEQ ID NO: 146; SEQ ID NO: 224 and SEQ ID NO: 147; SEQ ID NO: 224 and SEQ ID NO: 148; SEQ ID NO: 224 and SEQ ID NO: 149; SEQ ID NO: 224 and SEQ ID NO: 150; SEQ ID NO: 224 and SEQ ID NO: 151; SEQ ID NO: 224 and SEQ ID NO: 152; SEQ ID NO: 224 and SEQ ID NO: 153; SEQ ID NO: 224 and SEQ ID NO: 154; SEQ ID NO: 224 and SEQ ID NO: 155; SEQ ID NO: 224 and SEQ ID NO: 156; SEQ ID NO: 224 and SEQ ID NO: 157; SEQ ID NO: 224 and SEQ ID NO: 158; SEQ ID NO: 224 and SEQ ID NO: 159; SEQ ID NO: 224 and SEQ ID NO: 160; SEQ ID NO: 224 and SEQ ID NO: 161; SEQ ID NO: 224 and SEQ ID NO: 162; SEQ ID NO: 224 and SEQ ID NO: 163; SEQ ID NO: 224 and SEQ ID NO: 164; SEQ ID NO: 224 and SEQ ID NO: 165; SEQ ID NO: 224 and SEQ ID NO: 166; SEQ ID NO: 224 and SEQ ID NO: 167; SEQ ID NO: 224 and SEQ ID NO: 168; SEQ ID NO: 224 and SEQ ID NO: 169; SEQ ID NO: 224 and SEQ ID NO: 170; SEQ ID NO: 224 and SEQ ID NO: 171; SEQ ID NO: 224 and SEQ ID NO: 172; SEQ ID NO: 224 and SEQ ID NO: 173; SEQ ID NO: 224 and SEQ ID NO: 174; SEQ ID NO: 224 and SEQ ID NO: 175; SEQ ID NO: 224 and SEQ ID NO: 176; SEQ ID NO: 224 and SEQ ID NO: 177; SEQ ID NO: 224 and SEQ ID NO: 178; SEQ ID NO: 224 and SEQ ID NO: 179; and SEQ ID NO: 224 and SEQ ID NO: 180.
[0209] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 225 and SEQ ID NO: 144; SEQ ID NO: 225 and SEQ ID NO: 145; SEQ ID NO: 225 and SEQ ID NO: 146; SEQ ID NO: 225 and SEQ ID NO: 147; SEQ ID NO: 225 and SEQ ID NO: 148; SEQ ID NO: 225 and SEQ ID NO: 149; SEQ ID NO: 225 and SEQ ID NO: 150; SEQ ID NO: 225 and SEQ ID NO: 151; SEQ ID NO: 225 and SEQ ID NO: 152; SEQ ID NO: 225 and SEQ ID NO: 153; SEQ ID NO: 225 and SEQ ID NO: 154; SEQ ID NO: 225 and SEQ ID NO: 155; SEQ ID NO: 225 and SEQ ID NO: 156; SEQ ID NO: 225 and SEQ ID NO: 157; SEQ ID NO: 225 and SEQ ID NO: 158; SEQ ID NO: 225 and SEQ ID NO: 159; SEQ ID NO: 225 and SEQ ID NO: 160; SEQ ID NO: 225 and SEQ ID NO: 161; SEQ ID NO: 225 and SEQ ID NO: 162; SEQ ID NO: 225 and SEQ ID NO: 163; SEQ ID NO: 225 and SEQ ID NO: 164; SEQ ID NO: 225 and SEQ ID NO: 165; SEQ ID NO: 225 and SEQ ID NO: 166; SEQ ID NO: 225 and SEQ ID NO: 167; SEQ ID NO: 225 and SEQ ID NO: 168; SEQ ID NO: 225 and SEQ ID NO: 169; SEQ ID NO: 225 and SEQ ID NO: 170; SEQ ID NO: 225 and SEQ ID NO: 171; SEQ ID NO: 225 and SEQ ID NO: 172; SEQ ID NO: 225 and SEQ ID NO: 173; SEQ ID NO: 225 and SEQ ID NO: 174; SEQ ID NO: 225 and SEQ ID NO: 175; SEQ ID NO: 225 and SEQ ID NO: 176; SEQ ID NO: 225 and SEQ ID NO: 177; SEQ ID NO: 225 and SEQ ID NO: 178; SEQ ID NO: 225 and SEQ ID NO: 179; and SEQ ID NO: 225 and SEQ ID NO: 180.
[0210] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 226 and SEQ ID NO: 144; SEQ ID NO: 226 and SEQ ID NO: 145; SEQ ID NO: 226 and SEQ ID NO: 146; SEQ ID NO: 226 and SEQ ID NO: 147; SEQ ID NO: 226 and SEQ ID NO: 148; SEQ ID NO: 226 and SEQ ID NO: 149; SEQ ID NO: 226 and SEQ ID NO: 150; SEQ ID NO: 226 and SEQ ID NO: 151; SEQ ID NO: 226 and SEQ ID NO: 152; SEQ ID NO: 226 and SEQ ID NO: 153; SEQ ID NO: 226 and SEQ ID NO: 154; SEQ ID NO: 226 and SEQ ID NO: 155; SEQ ID NO: 226 and SEQ ID NO: 156; SEQ ID NO: 226 and SEQ ID NO: 157; SEQ ID NO: 226 and SEQ ID NO: 158; SEQ ID NO: 226 and SEQ ID NO: 159; SEQ ID NO: 226 and SEQ ID NO: 160; SEQ ID NO: 226 and SEQ ID NO: 161; SEQ ID NO: 226 and SEQ ID NO: 162; SEQ ID NO: 226 and SEQ ID NO: 163; SEQ ID NO: 226 and SEQ ID NO: 164; SEQ ID NO: 226 and SEQ ID NO: 165; SEQ ID NO: 226 and SEQ ID NO: 166; SEQ ID NO: 226 and SEQ ID NO: 167; SEQ ID NO: 226 and SEQ ID NO: 168; SEQ ID NO: 226 and SEQ ID NO: 169; SEQ ID NO: 226 and SEQ ID NO: 170; SEQ ID NO: 226 and SEQ ID NO: 171; SEQ ID NO: 226 and SEQ ID NO: 172; SEQ ID NO: 226 and SEQ ID NO: 173; SEQ ID NO: 226 and SEQ ID NO: 174; SEQ ID NO: 226 and SEQ ID NO: 175; SEQ ID NO: 226 and SEQ ID NO: 176; SEQ ID NO: 226 and SEQ ID NO: 177; SEQ ID NO: 226 and SEQ ID NO: 178; SEQ ID NO: 226 and SEQ ID NO: 179; and SEQ ID NO: 226 and SEQ ID NO: 180.
[0211] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 227 and SEQ ID NO: 144; SEQ ID NO: 227 and SEQ ID NO: 145; SEQ ID NO: 227 and SEQ ID NO: 146; SEQ ID NO: 227 and SEQ ID NO: 147; SEQ ID NO: 227 and SEQ ID NO: 148; SEQ ID NO: 227 and SEQ ID NO: 149; SEQ ID NO: 227 and SEQ ID NO: 150; SEQ ID NO: 227 and SEQ ID NO: 151; SEQ ID NO: 227 and SEQ ID NO: 152; SEQ ID NO: 227 and SEQ ID NO: 153; SEQ ID NO: 227 and SEQ ID NO: 154; SEQ ID NO: 227 and SEQ ID NO: 155; SEQ ID NO: 227 and SEQ ID NO: 156; SEQ ID NO: 227 and SEQ ID NO: 157; SEQ ID NO: 227 and SEQ ID NO: 158; SEQ ID NO: 227 and SEQ ID NO: 159; SEQ ID NO: 227 and SEQ ID NO: 160; SEQ ID NO: 227 and SEQ ID NO: 161; SEQ ID NO: 227 and SEQ ID NO: 162; SEQ ID NO: 227 and SEQ ID NO: 163; SEQ ID NO: 227 and SEQ ID NO: 164; SEQ ID NO: 227 and SEQ ID NO: 165; SEQ ID NO: 227 and SEQ ID NO: 166; SEQ ID NO: 227 and SEQ ID NO: 167; SEQ ID NO: 227 and SEQ ID NO: 168; SEQ ID NO: 227 and SEQ ID NO: 169; SEQ ID NO: 227 and SEQ ID NO: 170; SEQ ID NO: 227 and SEQ ID NO: 171; SEQ ID NO: 227 and SEQ ID NO: 172; SEQ ID NO: 227 and SEQ ID NO: 173; SEQ ID NO: 227 and SEQ ID NO: 174; SEQ ID NO: 227 and SEQ ID NO: 175; SEQ ID NO: 227 and SEQ ID NO: 176; SEQ ID NO: 227 and SEQ ID NO: 177; SEQ ID NO: 227 and SEQ ID NO: 178; SEQ ID NO: 227 and SEQ ID NO: 179; and SEQ ID NO: 227 and SEQ ID NO: 180.
[0212] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 228 and SEQ ID NO: 144; SEQ ID NO: 228 and SEQ ID NO: 145; SEQ ID NO: 228 and SEQ ID NO: 146; SEQ ID NO: 228 and SEQ ID NO: 147; SEQ ID NO: 228 and SEQ ID NO: 148; SEQ ID NO: 228 and SEQ ID NO: 149; SEQ ID NO: 228 and SEQ ID NO: 150; SEQ ID NO: 228 and SEQ ID NO: 151; SEQ ID NO: 228 and SEQ ID NO: 152; SEQ ID NO: 228 and SEQ ID NO: 153; SEQ ID NO: 228 and SEQ ID NO: 154; SEQ ID NO: 228 and SEQ ID NO: 155; SEQ ID NO: 228 and SEQ ID NO: 156; SEQ ID NO: 228 and SEQ ID NO: 157; SEQ ID NO: 228 and SEQ ID NO: 158; SEQ ID NO: 228 and SEQ ID NO: 159; SEQ ID NO: 228 and SEQ ID NO: 160; SEQ ID NO: 228 and SEQ ID NO: 161; SEQ ID NO: 228 and SEQ ID NO: 162; SEQ ID NO: 228 and SEQ ID NO: 163; SEQ ID NO: 228 and SEQ ID NO: 164; SEQ ID NO: 228 and SEQ ID NO: 165; SEQ ID NO: 228 and SEQ ID NO: 166; SEQ ID NO: 228 and SEQ ID NO: 167; SEQ ID NO: 228 and SEQ ID NO: 168; SEQ ID NO: 228 and SEQ ID NO: 169; SEQ ID NO: 228 and SEQ ID NO: 170; SEQ ID NO: 228 and SEQ ID NO: 171; SEQ ID NO: 228 and SEQ ID NO: 172; SEQ ID NO: 228 and SEQ ID NO: 173; SEQ ID NO: 228 and SEQ ID NO: 174; SEQ ID NO: 228 and SEQ ID NO: 175; SEQ ID NO: 228 and SEQ ID NO: 176; SEQ ID NO: 228 and SEQ ID NO: 177; SEQ ID NO: 228 and SEQ ID NO: 178; SEQ ID NO: 228 and SEQ ID NO: 179; and SEQ ID NO: 228 and SEQ ID NO: 180.
[0213] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 229 and SEQ ID NO: 144; SEQ ID NO: 229 and SEQ ID NO: 145; SEQ ID NO: 229 and SEQ ID NO: 146; SEQ ID NO: 229 and SEQ ID NO: 147; SEQ ID NO: 229 and SEQ ID NO: 148; SEQ ID NO: 229 and SEQ ID NO: 149; SEQ ID NO: 229 and SEQ ID NO: 150; SEQ ID NO: 229 and SEQ ID NO: 151; SEQ ID NO: 229 and SEQ ID NO: 152; SEQ ID NO: 229 and SEQ ID NO: 153; SEQ ID NO: 229 and SEQ ID NO: 154; SEQ ID NO: 229 and SEQ ID NO: 155; SEQ ID NO: 229 and SEQ ID NO: 156; SEQ ID NO: 229 and SEQ ID NO: 157; SEQ ID NO: 229 and SEQ ID NO: 158; SEQ ID NO: 229 and SEQ ID NO: 159; SEQ ID NO: 229 and SEQ ID NO: 160; SEQ ID NO: 229 and SEQ ID NO: 161; SEQ ID NO: 229 and SEQ ID NO: 162; SEQ ID NO: 229 and SEQ ID NO: 163; SEQ ID NO: 229 and SEQ ID NO: 164; SEQ ID NO: 229 and SEQ ID NO: 165; SEQ ID NO: 229 and SEQ ID NO: 166; SEQ ID NO: 229 and SEQ ID NO: 167; SEQ ID NO: 229 and SEQ ID NO: 168; SEQ ID NO: 229 and SEQ ID NO: 169; SEQ ID NO: 229 and SEQ ID NO: 170; SEQ ID NO: 229 and SEQ ID NO: 171; SEQ ID NO: 229 and SEQ ID NO: 172; SEQ ID NO: 229 and SEQ ID NO: 173; SEQ ID NO: 229 and SEQ ID NO: 174; SEQ ID NO: 229 and SEQ ID NO: 175; SEQ ID NO: 229 and SEQ ID NO: 176; SEQ ID NO: 229 and SEQ ID NO: 177; SEQ ID NO: 229 and SEQ ID NO: 178; SEQ ID NO: 229 and SEQ ID NO: 179; and SEQ ID NO: 229 and SEQ ID NO: 180.
[0214] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 230 and SEQ ID NO: 144; SEQ ID NO: 230 and SEQ ID NO: 145; SEQ ID NO: 230 and SEQ ID NO: 146; SEQ ID NO: 230 and SEQ ID NO: 147; SEQ ID NO: 230 and SEQ ID NO: 148; SEQ ID NO: 230 and SEQ ID NO: 149; SEQ ID NO: 230 and SEQ ID NO: 150; SEQ ID NO: 230 and SEQ ID NO: 151; SEQ ID NO: 230 and SEQ ID NO: 152; SEQ ID NO: 230 and SEQ ID NO: 153; SEQ ID NO: 230 and SEQ ID NO: 154; SEQ ID NO: 230 and SEQ ID NO: 155; SEQ ID NO: 230 and SEQ ID NO: 156; SEQ ID NO: 230 and SEQ ID NO: 157; SEQ ID NO: 230 and SEQ ID NO: 158; SEQ ID NO: 230 and SEQ ID NO: 159; SEQ ID NO: 230 and SEQ ID NO: 160; SEQ ID NO: 230 and SEQ ID NO: 161; SEQ ID NO: 230 and SEQ ID NO: 162; SEQ ID NO: 230 and SEQ ID NO: 163; SEQ ID NO: 230 and SEQ ID NO: 164; SEQ ID NO: 230 and SEQ ID NO: 165; SEQ ID NO: 230 and SEQ ID NO: 166; SEQ ID NO: 230 and SEQ ID NO: 167; SEQ ID NO: 230 and SEQ ID NO: 168; SEQ ID NO: 230 and SEQ ID NO: 169; SEQ ID NO: 230 and SEQ ID NO: 170; SEQ ID NO: 230 and SEQ ID NO: 171; SEQ ID NO: 230 and SEQ ID NO: 172; SEQ ID NO: 230 and SEQ ID NO: 173; SEQ ID NO: 230 and SEQ ID NO: 174; SEQ ID NO: 230 and SEQ ID NO: 175; SEQ ID NO: 230 and SEQ ID NO: 176; SEQ ID NO: 230 and SEQ ID NO: 177; SEQ ID NO: 230 and SEQ ID NO: 178; SEQ ID NO: 230 and SEQ ID NO: 179; and SEQ ID NO: 230 and SEQ ID NO: 180.
[0215] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 231 and SEQ ID NO: 144; SEQ ID NO: 231 and SEQ ID NO: 145; SEQ ID NO: 231 and SEQ ID NO: 146; SEQ ID NO: 231 and SEQ ID NO: 147; SEQ ID NO: 231 and SEQ ID NO: 148; SEQ ID NO: 231 and SEQ ID NO: 149; SEQ ID NO: 231 and SEQ ID NO: 150; SEQ ID NO: 231 and SEQ ID NO: 151; SEQ ID NO: 231 and SEQ ID NO: 152; SEQ ID NO: 231 and SEQ ID NO: 153; SEQ ID NO: 231 and SEQ ID NO: 154; SEQ ID NO: 231 and SEQ ID NO: 155; SEQ ID NO: 231 and SEQ ID NO: 156; SEQ ID NO: 231 and SEQ ID NO: 157; SEQ ID NO: 231 and SEQ ID NO: 158; SEQ ID NO: 231 and SEQ ID NO: 159; SEQ ID NO: 231 and SEQ ID NO: 160; SEQ ID NO: 231 and SEQ ID NO: 161; SEQ ID NO: 231 and SEQ ID NO: 162; SEQ ID NO: 231 and SEQ ID NO: 163; SEQ ID NO: 231 and SEQ ID NO: 164; SEQ ID NO: 231 and SEQ ID NO: 165; SEQ ID NO: 231 and SEQ ID NO: 166; SEQ ID NO: 231 and SEQ ID NO: 167; SEQ ID NO: 231 and SEQ ID NO: 168; SEQ ID NO: 231 and SEQ ID NO: 169; SEQ ID NO: 231 and SEQ ID NO: 170; SEQ ID NO: 231 and SEQ ID NO: 171; SEQ ID NO: 231 and SEQ ID NO: 172; SEQ ID NO: 231 and SEQ ID NO: 173; SEQ ID NO: 231 and SEQ ID NO: 174; SEQ ID NO: 231 and SEQ ID NO: 175; SEQ ID NO: 231 and SEQ ID NO: 176; SEQ ID NO: 231 and SEQ ID NO: 177; SEQ ID NO: 231 and SEQ ID NO: 178; SEQ ID NO: 231 and SEQ ID NO: 179; and SEQ ID NO: 231 and SEQ ID NO: 180.
[0216] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 232 and SEQ ID NO: 144; SEQ ID NO: 232 and SEQ ID NO: 145; SEQ ID NO: 232 and SEQ ID NO: 146; SEQ ID NO: 232 and SEQ ID NO: 147; SEQ ID NO: 232 and SEQ ID NO: 148; SEQ ID NO: 232 and SEQ ID NO: 149; SEQ ID NO: 232 and SEQ ID NO: 150; SEQ ID NO: 232 and SEQ ID NO: 151; SEQ ID NO: 232 and SEQ ID NO: 152; SEQ ID NO: 232 and SEQ ID NO: 153; SEQ ID NO: 232 and SEQ ID NO: 154; SEQ ID NO: 232 and SEQ ID NO: 155; SEQ ID NO: 232 and SEQ ID NO: 156; SEQ ID NO: 232 and SEQ ID NO: 157; SEQ ID NO: 232 and SEQ ID NO: 158; SEQ ID NO: 232 and SEQ ID NO: 159; SEQ ID NO: 232 and SEQ ID NO: 160; SEQ ID NO: 232 and SEQ ID NO: 161; SEQ ID NO: 232 and SEQ ID NO: 162; SEQ ID NO: 232 and SEQ ID NO: 163; SEQ ID NO: 232 and SEQ ID NO: 164; SEQ ID NO: 232 and SEQ ID NO: 165; SEQ ID NO: 232 and SEQ ID NO: 166; SEQ ID NO: 232 and SEQ ID NO: 167; SEQ ID NO: 232 and SEQ ID NO: 168; SEQ ID NO: 232 and SEQ ID NO: 169; SEQ ID NO: 232 and SEQ ID NO: 170; SEQ ID NO: 232 and SEQ ID NO: 171; SEQ ID NO: 232 and SEQ ID NO: 172; SEQ ID NO: 232 and SEQ ID NO: 173; SEQ ID NO: 232 and SEQ ID NO: 174; SEQ ID NO: 232 and SEQ ID NO: 175; SEQ ID NO: 232 and SEQ ID NO: 176; SEQ ID NO: 232 and SEQ ID NO: 177; SEQ ID NO: 232 and SEQ ID NO: 178; SEQ ID NO: 232 and SEQ ID NO: 179; and SEQ ID NO: 232 and SEQ ID NO: 180.
[0217] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 233 and SEQ ID NO: 144; SEQ ID NO: 233 and SEQ ID NO: 145; SEQ ID NO: 233 and SEQ ID NO: 146; SEQ ID NO: 233 and SEQ ID NO: 147; SEQ ID NO: 233 and SEQ ID NO: 148; SEQ ID NO: 233 and SEQ ID NO: 149; SEQ ID NO: 233 and SEQ ID NO: 150; SEQ ID NO: 233 and SEQ ID NO: 151; SEQ ID NO: 233 and SEQ ID NO: 152; SEQ ID NO: 233 and SEQ ID NO: 153; SEQ ID NO: 233 and SEQ ID NO: 154; SEQ ID NO: 233 and SEQ ID NO: 155; SEQ ID NO: 233 and SEQ ID NO: 156; SEQ ID NO: 233 and SEQ ID NO: 157; SEQ ID NO: 233 and SEQ ID NO: 158; SEQ ID NO: 233 and SEQ ID NO: 159; SEQ ID NO: 233 and SEQ ID NO: 160; SEQ ID NO: 233 and SEQ ID NO: 161; SEQ ID NO: 233 and SEQ ID NO: 162; SEQ ID NO: 233 and SEQ ID NO: 163; SEQ ID NO: 233 and SEQ ID NO: 164; SEQ ID NO: 233 and SEQ ID NO: 165; SEQ ID NO: 233 and SEQ ID NO: 166; SEQ ID NO: 233 and SEQ ID NO: 167; SEQ ID NO: 233 and SEQ ID NO: 168; SEQ ID NO: 233 and SEQ ID NO: 169; SEQ ID NO: 233 and SEQ ID NO: 170; SEQ ID NO: 233 and SEQ ID NO: 171; SEQ ID NO: 233 and SEQ ID NO: 172; SEQ ID NO: 233 and SEQ ID NO: 173; SEQ ID NO: 233 and SEQ ID NO: 174; SEQ ID NO: 233 and SEQ ID NO: 175; SEQ ID NO: 233 and SEQ ID NO: 176; SEQ ID NO: 233 and SEQ ID NO: 177; SEQ ID NO: 233 and SEQ ID NO: 178; SEQ ID NO: 233 and SEQ ID NO: 179; and SEQ ID NO: 233 and SEQ ID NO: 180.
[0218] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 234 and SEQ ID NO: 144; SEQ ID NO: 234 and SEQ ID NO: 145; SEQ ID NO: 234 and SEQ ID NO: 146; SEQ ID NO: 234 and SEQ ID NO: 147; SEQ ID NO: 234 and SEQ ID NO: 148; SEQ ID NO: 234 and SEQ ID NO: 149; SEQ ID NO: 234 and SEQ ID NO: 150; SEQ ID NO: 234 and SEQ ID NO: 151; SEQ ID NO: 234 and SEQ ID NO: 152; SEQ ID NO: 234 and SEQ ID NO: 153; SEQ ID NO: 234 and SEQ ID NO: 154; SEQ ID NO: 234 and SEQ ID NO: 155; SEQ ID NO: 234 and SEQ ID NO: 156; SEQ ID NO: 234 and SEQ ID NO: 157; SEQ ID NO: 234 and SEQ ID NO: 158; SEQ ID NO: 234 and SEQ ID NO: 159; SEQ ID NO: 234 and SEQ ID NO: 160; SEQ ID NO: 234 and SEQ ID NO: 161; SEQ ID NO: 234 and SEQ ID NO: 162; SEQ ID NO: 234 and SEQ ID NO: 163; SEQ ID NO: 234 and SEQ ID NO: 164; SEQ ID NO: 234 and SEQ ID NO: 165; SEQ ID NO: 234 and SEQ ID NO: 166; SEQ ID NO: 234 and SEQ ID NO: 167; SEQ ID NO: 234 and SEQ ID NO: 168; SEQ ID NO: 234 and SEQ ID NO: 169; SEQ ID NO: 234 and SEQ ID NO: 170; SEQ ID NO: 234 and SEQ ID NO: 171; SEQ ID NO: 234 and SEQ ID NO: 172; SEQ ID NO: 234 and SEQ ID NO: 173; SEQ ID NO: 234 and SEQ ID NO: 174; SEQ ID NO: 234 and SEQ ID NO: 175; SEQ ID NO: 234 and SEQ ID NO: 176; SEQ ID NO: 234 and SEQ ID NO: 177; SEQ ID NO: 234 and SEQ ID NO: 178; SEQ ID NO: 234 and SEQ ID NO: 179; and SEQ ID NO: 234 and SEQ ID NO: 180.
[0219] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 235 and SEQ ID NO: 144; SEQ ID NO: 235 and SEQ ID NO: 145; SEQ ID NO: 235 and SEQ ID NO: 146; SEQ ID NO: 235 and SEQ ID NO: 147; SEQ ID NO: 235 and SEQ ID NO: 148; SEQ ID NO: 235 and SEQ ID NO: 149; SEQ ID NO: 235 and SEQ ID NO: 150; SEQ ID NO: 235 and SEQ ID NO: 151; SEQ ID NO: 235 and SEQ ID NO: 152; SEQ ID NO: 235 and SEQ ID NO: 153; SEQ ID NO: 235 and SEQ ID NO: 154; SEQ ID NO: 235 and SEQ ID NO: 155; SEQ ID NO: 235 and SEQ ID NO: 156; SEQ ID NO: 235 and SEQ ID NO: 157; SEQ ID NO: 235 and SEQ ID NO: 158; SEQ ID NO: 235 and SEQ ID NO: 159; SEQ ID NO: 235 and SEQ ID NO: 160; SEQ ID NO: 235 and SEQ ID NO: 161; SEQ ID NO: 235 and SEQ ID NO: 162; SEQ ID NO: 235 and SEQ ID NO: 163; SEQ ID NO: 235 and SEQ ID NO: 164; SEQ ID NO: 235 and SEQ ID NO: 165; SEQ ID NO: 235 and SEQ ID NO: 166; SEQ ID NO: 235 and SEQ ID NO: 167; SEQ ID NO: 235 and SEQ ID NO: 168; SEQ ID NO: 235 and SEQ ID NO: 169; SEQ ID NO: 235 and SEQ ID NO: 170; SEQ ID NO: 235 and SEQ ID NO: 171; SEQ ID NO: 235 and SEQ ID NO: 172; SEQ ID NO: 235 and SEQ ID NO: 173; SEQ ID NO: 235 and SEQ ID NO: 174; SEQ ID NO: 235 and SEQ ID NO: 175; SEQ ID NO: 235 and SEQ ID NO: 176; SEQ ID NO: 235 and SEQ ID NO: 177; SEQ ID NO: 235 and SEQ ID NO: 178; SEQ ID NO: 235 and SEQ ID NO: 179; and SEQ ID NO: 235 and SEQ ID NO: 180.
[0220] 2.7.4.1. Variants of V.sub.H-V.sub.L Pairs
[0221] In some embodiments, the V.sub.H-V.sub.L pairs provided herein comprise a variant of an illustrative V.sub.H and/or V.sub.L sequence provided in this disclosure.
[0222] In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure. In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.H sequences provided in this disclosure.
[0223] In some embodiments, the V.sub.H sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.H sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0224] In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure. In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.L sequences provided in this disclosure.
[0225] In some embodiments, the V.sub.L sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.L sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0226] 2.8. Tim-3 Antibodies Comprising all Six CDRs
[0227] In some embodiments, the Tim-3 antibody comprises a CDR-H1 sequence, a CDR-H2 sequence, a CDR-H3 sequence, a CDR-L1 sequence, a CDR-L2 sequence, and a CDR-L3 sequence. In some aspects, the CDR sequences are part of a V.sub.H (for CDR-H) or V.sub.L (for CDR-L).
[0228] In some aspects, the CDR-H1 sequence is a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 4-23; the CDR-H2 sequence is a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 63-80; the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118; the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354; the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355; and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356.
[0229] In some aspects, the CDR-H1 sequence is a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 39-56; the CDR-H2 sequence is a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 82-109; the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 111-118; the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 120-123 and 354; the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 125-133 and 355; and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 140-142 and 356.
[0230] 2.8.1. Variants of Antibodies Comprising all Six CDRs
[0231] In some embodiments, the CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, and CDR-L3 provided herein comprise a variant of an illustrative CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, and/or CDR-L3 sequence provided in this disclosure.
[0232] In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia or Kabat CDR-H1 sequence provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia or Kabat CDR-H1 sequences provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia or Kabat CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0233] In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia or Kabat CDR-H2 sequence provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia or Kabat CDR-H2 sequences provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia or Kabat CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0234] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0235] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0236] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0237] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0238] 2.9. Tim-3 Consensus Sequences
[0239] In some embodiments, provided herein are anti-Tim-3 antibodies comprising one or more sequences defined by consensus sequences. Each consensus sequence is based, at least in part, on one or more alignments of two or more useful anti-Tim-3 CDR sequences provided in this disclosure. Based on such alignments, a person of skill in the art would recognize that different amino acid residues may useful in certain positions of the CDRs. Accordingly, each consensus sequence encompasses two or more useful anti-Tim-3 CDR sequences.
[0240] In some embodiments, the Tim-3 antibodies comprise one to six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise two to six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise three to six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise four to six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise five to six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise six of the consensus CDR sequences provided herein. In some embodiments, the antibodies comprise a V.sub.L comprising the CDR-L consensus sequence(s). In some embodiments, the antibodies comprise a V.sub.H comprising the CDR-H consensus sequence(s). In some embodiments, the antibodies comprise a V.sub.H comprising the CDR-H consensus sequence(s) and a V.sub.L comprising the CDR-L consensus sequence(s).
[0241] 2.9.1. CDR-H3 Consensus Sequences
[0242] In some embodiments, the Tim-3 antibody comprises a CDR-H3 sequence defined by the consensus sequence .alpha..sub.1-.alpha..sub.2-.alpha..sub.3-Y-R-.alpha..sub.6-.alpha..sub.7- -.alpha..sub.8-.alpha..sub.9-.alpha..sub.10-.alpha..sub.11-.alpha..sub.12-- .alpha..sub.13, where .alpha..sub.1 is Q, S or G; .alpha..sub.2 is G, F, Y, or H; .alpha..sub.3 is G, F, V, or I; .alpha..sub.6 is absent or S; .alpha..sub.7 is Y, S, M, or L; .alpha..sub.8 is D, N, or W; .alpha..sub.9 is D; .alpha..sub.10 is A, W, or S; .alpha..sub.11 is M, Y, F, or L; .alpha..sub.12 is D or V; and .alpha..sub.13 is Y or H.
[0243] In some embodiments, the Tim-3 antibody comprises a CDR-H3 sequence defined by the consensus sequence G-.beta..sub.2-.beta..sub.3-Y-R-.beta..sub.7-W-D-S-.beta..sub.11-D-.beta.- .sub.13, where .beta..sub.2 is Y or H; .beta..sub.3 is V or I; .beta..sub.7 is M, or L; .beta..sub.11 is Y, F, or L; and .beta..sub.13 is Y or H.
[0244] 2.9.2. Chothia CDR-H1 Consensus Sequences
[0245] In some embodiments, the Tim-3 antibody comprises a Chothia sequence defined by the consensus sequence G-.gamma..sub.2-.gamma..sub.3-.gamma..sub.4-.gamma..sub.5-.gamma..sub.6-.- gamma..sub.7, where .gamma..sub.2 is F or Y; .gamma..sub.3 is S, P or N; .gamma..sub.4 is L, F, or I; y.sub.5 is T, I, S, G, D, K, or R; .gamma..sub.6 is S, G, D, R, N, or K; and .gamma..sub.7 is N, Y or H.
[0246] In some embodiments, the Tim-3 antibody comprises a Chothia sequence defined by the consensus sequence G-F-N-I-.delta..sub.5-.delta..sub.6-.delta..sub.7, where .delta..sub.5 is T, I, S, G, or R; .delta..sub.6 is S, R, N, K, or G; and .delta..sub.7 is N, Y or H.
[0247] 2.9.3. Chothia CDR-H2 Consensus Sequences
[0248] In some embodiments, the Tim-3 antibody comprises a Chothia CDR-H2 sequence defined by the consensus sequence .epsilon..sub.1-.epsilon..sub.2-.epsilon..sub.3-.epsilon..sub.4-G-.epsilo- n..sub.6, where .epsilon..sub.1 is W, N, T, V, I, S, A, or M; .epsilon..sub.2 is S or P; .epsilon..sub.3 is absent, Y, V, G, D, N, T, or A; .epsilon..sub.4 is D, G, N, R, Q, A, or V; and .epsilon..sub.6 is Y, I, D, S, or F.
[0249] In some embodiments, the Tim-3 antibody comprises a Chothia CDR-H2 sequence defined by the consensus sequence .epsilon..sub.1-P-.epsilon..sub.3-.epsilon..sub.4-G-.epsilon..sub.6, where .epsilon..sub.1 is T, V, I, S, A, or M; .epsilon..sub.3 is V, G, D, N, T, or A; and .epsilon..sub.4 is G, R, Q, A, or V; and .epsilon..sub.6 is Y, I, D, S, or F.
[0250] 2.9.4. Kabat CDR-H1 Consensus Sequences
[0251] In some embodiments, the Tim-3 antibody comprises a Kabat CDR-H1 sequence defined by the consensus sequence .xi..sub.1-.xi..sub.2-.xi..sub.3-.xi..sub.4-.xi..sub.5, where .xi..sub.1 is D, S, G, R, N, or K; .xi..sub.2 is Y, H, or N; .xi..sub.3 is G, T, Y, A, or V; .xi..sub.4 is V, M, or I; and .xi..sub.5 is N or H.
[0252] In some embodiments, the Tim-3 antibody comprises a Kabat CDR-H1 sequence defined by the consensus sequence .eta..sub.1-.eta..sub.2-.eta..sub.3-.eta..sub.4-.eta..sub.5, where .eta..sub.1 is G, R, N, K, or S; .eta..sub.2 is Y, H, or N; and .eta..sub.3 is Y, A, or V.
[0253] 2.9.5. Kabat CDR-H2 Consensus Sequences
[0254] In some embodiments, the Tim-3 antibody comprises a Kabat CDR-H2 sequence defined by the consensus sequence .theta..sub.1-I-.theta..sub.3-.theta..sub.4-.theta..sub.5-.theta..sub.6-.- theta..sub.7-.theta..sub.8-T-.theta..sub.10-.theta..sub.11-.theta..sub.12-- .theta..sub.13-.theta..sub.14-.theta..sub.15-.theta..sub.16-.theta..sub.17- , where .theta..sub.1 is V, L, G, D, or A; .theta..sub.3 is W, N, T, V, I, S, A, or M; .theta..sub.4 is S or P; .theta..sub.5 is absent, Y, V, G, D, N, T, or A; .theta..sub.6 is D, N, R, Q, G, A, or V; .theta..sub.7 is G; .theta..sub.8 is I, Y, D, S, or F; .theta..sub.10 is T, E, D, or G; .theta..sub.11 is Y; .theta..sub.12 is N, or A; .theta..sub.13 is P, Q, S, or D; .theta..sub.14 is A, K, or S; .theta..sub.15 is L, F, or V; .theta..sub.16 is Q or K; and .theta..sub.17 is S, G or D.
[0255] In some embodiments, the Tim-3 antibody comprises a Kabat CDR-H2 sequence defined by the consensus sequence V-I-W-S-D-G-S-T-T-Y-N-.theta..sub.13-.theta..sub.14-.theta..sub.15-.theta- ..sub.16, where .theta..sub.13 is P, Q, S, or D; .theta..sub.14 is S, K, or A; .theta..sub.15 is F, L, or V; .theta..sub.16 is Q or K; .theta..sub.17 is G or S.
[0256] In some embodiments, the Tim-3 antibody comprises a Kabat CDR-H2 sequence defined by the consensus sequence .theta..sub.1-I-.theta..sub.3-P-.theta..sub.5-.theta..sub.6-G-.theta..sub- .8-T-.theta..sub.10-Y-A-D-S-V-K-.theta..sub.17, where .theta..sub.1 is D, A, or G; .theta..sub.3 is T, V, I, S, A, or M; .theta..sub.5 is V, G, D, N, T, or A; .theta..sub.6 is R, Q, G, A, or V; .theta..sub.8 is Y, I, D, S, or F; .theta..sub.10 is E, D, or G; and .theta..sub.17 is G or D.
[0257] 2.9.6. CDR-L3 Consensus Sequences
[0258] In some embodiments, the Tim-3 antibody comprises a CDR-L3 sequence defined by the consensus sequence .sub.1-Q-.sub.3-.sub.4-.sub.5-.sub.6-P-.sub.8-T, where .sub.1 is Q or F; .sub.3 S or G; .sub.4 is N or S .sub.5 is E or H; .sub.6 is D or V; and .sub.8 is Y or W.
[0259] In some embodiments, the Tim-3 antibody comprises a CDR-L3 sequence defined by the consensus sequence Q-Q-H-Y-T-T-P-P-T.
[0260] 2.9.7. CDR-L2 Consensus Sequences
[0261] In some embodiments, the Tim-3 antibody comprises a CDR-L2 sequence defined by the consensus sequence .lamda..sub.1-.lamda..sub.2-S-N-.lamda..sub.5-.lamda..sub.6-S, where .lamda..sub.1 is A or K; .lamda..sub.2 is A or V; .lamda..sub.5 is L or R; .lamda..sub.6 is E or F.
[0262] In some embodiments, the Tim-3 antibody comprises a CDR-L2 sequence defined by the consensus sequence .pi..sub.1-.pi..sub.2-.pi..sub.3-.pi..sub.4-L-.pi..sub.6-.pi..sub.7, where .pi..sub.1 is S or D; .pi..sub.2 is A or D; .pi..sub.3 is S or D; .pi..sub.4 is F or D; .pi..sub.6 is Y or D; and .pi..sub.7 is D or S.
[0263] 2.9.8. CDR-L1 Consensus Sequences
[0264] In some embodiments, the Tim-3 antibody comprises a CDR-L1 sequence defined by the consensus sequence .mu..sub.1-.mu..sub.2-S-Q-S-.mu..sub.6-.mu..sub.7-.mu..sub.8-.mu..sub.9-.- mu..sub.10-.mu..sub.11-.mu..sub.12-.mu..sub.13-.mu..sub.14-.mu..sub.15-.mu- ..sub.16, where .mu..sub.1 is K or R; .mu..sub.2 is A or S; .mu..sub.6 is V or I; .mu..sub.7 is D or V; .mu..sub.8 is Y or H; .mu..sub.9 is D or T; .mu..sub.10 is absent or N; .mu..sub.11 is G; .mu..sub.12 is N; .mu..sub.13 is S or T; .mu..sub.14 is Y; .mu..sub.15 is V or L; and .mu..sub.16 is N, A, or E.
[0265] In some embodiments, the Tim-3 antibody comprises a CDR-L1 sequence defined by the consensus sequence K-A-S-S-Q-V-D-Y-D-G-N-S-Y-V-.mu..sub.16, where .mu..sub.16 is N or A.
[0266] In some embodiments, the Tim-3 antibody comprises a CDR-L1 sequence defined by the consensus sequence R-A-S-Q-D-V-N-T-A-V-A.
3. PD-1 Antibodies
[0267] Provided herein are PD-1 antibodies that selectively bind human PD-1. In some aspects, the antibody selectively binds to the extracellular domain of human PD-1. In some aspects, the antibody selectively binds to one or more of full-length human PD-1, PD-1.DELTA.ex2, PD-1.DELTA.ex3, PD-1.DELTA.ex2,3, and PD-1.DELTA.ex2,3,4. See Nielsen et al., Cellular Immunology, 2005, 235:109-116, incorporated by reference in its entirety.
[0268] In some embodiments, the PD-1 antibody binds to a homolog of human PD-1. In some aspects, the antibody binds to a homolog of human Tim-3 from a species selected from monkeys, mice, dogs, cats, rats, cows, horses, goats and sheep. In some aspects, the homolog is a cynomolgus monkey homolog. In some aspects, the homolog is a murine homolog.
[0269] In some embodiments, the PD-1 antibody comprises a light chain. In some aspects, the light chain is a kappa light chain. In some aspects, the light chain is a lambda light chain.
[0270] In some embodiments, the PD-1 antibody comprises a heavy chain. In some aspects, the heavy chain is an IgA. In some aspects, the heavy chain is an IgD. In some aspects, the heavy chain is an IgE. In some aspects, the heavy chain is an IgG. In some aspects, the heavy chain is an IgM. In some aspects, the heavy chain is an IgG1. In some aspects, the heavy chain is an IgG2. In some aspects, the heavy chain is an IgG3. In some aspects, the heavy chain is an IgG4. In some aspects, the heavy chain is an IgA1. In some aspects, the heavy chain is an IgA2.
[0271] In some embodiments, the PD-1 antibody is an antibody fragment. In some aspects, the antibody fragment is an Fv fragment. In some aspects, the antibody fragment is a Fab fragment. In some aspects, the antibody fragment is a F(ab').sub.2 fragment. In some aspects, the antibody fragment is a Fab' fragment. In some aspects, the antibody fragment is an scFv (sFv) fragment. In some aspects, the antibody fragment is an scFv-Fc fragment.
[0272] In some embodiments, the PD-1 antibody is a monoclonal antibody. In some embodiments, the antibody is a polyclonal antibody.
[0273] In some embodiments, the PD-1 antibody is a chimeric antibody. In some embodiments, the antibody is a humanized antibody. In some embodiments, the antibody is a human antibody.
[0274] In some embodiments, the PD-1 antibody is an affinity matured antibody. In some aspects, the antibody is an affinity matured antibody derived from an illustrative sequence provided in this disclosure or in, e.g., WO 2016/077397, which is incorporated herein by reference in its entirety.
[0275] In some embodiments, the PD-1 antibody inhibits the binding of PD-1 to its ligands. In some aspects, the antibody inhibits the binding of PD-1 to PD-L1. In some aspects, the antibody inhibits the binding of PD-1 to PD-L2. In some aspects, the antibody inhibits the binding of PD-1 to PD-L1 and PD-L2.
[0276] In some embodiments, the PD-1 antibody is provided as a single arm binder. For example, the PD-1 antibody can be provided as part of a bi-specific antibody or bi-specific antibody construct as disclosed here.
[0277] The PD-1 antibodies provided herein may be useful for the treatment of a variety of diseases and conditions, including cancers, autoimmune diseases, and infections.
[0278] 3.1 PD-1 CDR-H3 Sequences
[0279] In some embodiments, the PD-1 antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the CDR-H3 sequence is a CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119. In some embodiments, the PD-1 antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0280] In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 220. In some embodiments, the PD-1 antibody comprises a CDR-H3 sequence comprising, consisting of, or consisting essentially of a CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0281] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0282] 3.2 PD-1 V.sub.H Sequences Comprising Illustrative CDRs
[0283] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising one or more CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative CDR-H sequences provided in this disclosure, and variants thereof. In some embodiments, the CDR-H sequences comprise, consist of, or consist essentially of one or more CDR-H sequences provided in a V.sub.H sequence selected from SEQ ID NOs: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising one or more CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative CDR-H sequences provided in WO 2016/077397.
[0284] 3.3.1 V.sub.H Sequences Comprising Illustrative Kabat CDRs
[0285] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising one or more Kabat CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative Kabat CDR-H sequences provided in this disclosure, and variants thereof. In some embodiments, the CDR-H sequences comprise, consist of, or consist essentially of one or more CDR-H sequences provided in a V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0286] 3.2.2.1 Kabat CDR-H3
[0287] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Kabat CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H3 sequence is a Kabat CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Kabat CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0288] In some embodiments, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119.
[0289] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0290] In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0291] 3.2.2.2 Kabat CDR-H2
[0292] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Kabat CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H2 sequence is a Kabat CDR-H2 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Kabat CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0293] In some embodiments, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 110.
[0294] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0295] In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0296] 3.2.2.3 Kabat CDR-H1
[0297] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Kabat CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Kabat CDR-H1 sequence is a Kabat CDR-H1 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Kabat CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0298] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 57-62. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 57. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 58. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 59. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 60. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 61. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 62.
[0299] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0300] In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a Kabat CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0301] 3.2.2.4 Kabat CDR-H3+Kabat CDR-H2
[0302] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119, and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 110. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220, and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220. In some aspects, the Kabat CDR-H3 sequence and the Kabat CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H3 and Kabat CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0303] 3.2.2.5 Kabat CDR-H3+Kabat CDR-H1
[0304] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119, and a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 57-62. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H3 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, and a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220. In some aspects, the Kabat CDR-H3 sequence and the Kabat CDR-H1 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H3 and Kabat CDR-H1 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0305] 3.2.2.6 Kabat CDR-H1+Kabat CDR-H2
[0306] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 57-62, and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 110. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, and a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H2 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220. In some aspects, the Kabat CDR-H1 sequence and the Kabat CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H1 and Kabat CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0307] 3.2.2.7 Kabat CDR-H1+Kabat CDR-H2+Kabat CDR-H3
[0308] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 57-62, a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 110, and a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220, a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220, and a Kabat CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Kabat CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220. In some aspects, the Kabat CDR-H1 sequence, Kabat CDR-H2 sequence, and Kabat CDR-H3 are all from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Kabat CDR-H1, Kabat CDR-H2, and Kabat CDR-H3 are all from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0309] 3.2.2.8 Variants of V.sub.H Sequences Comprising Illustrative Kabat CDRs
[0310] In some embodiments, the V.sub.H sequences provided herein comprise a variant of an illustrative Kabat CDR-H3, CDR-H2, and/or CDR-H1 sequence provided in this disclosure. In some embodiments, the V.sub.H sequences provided herein comprise a variant of an illustrative Kabat CDR-H3, CDR-H2, and/or CDR-H1 sequence provided in WO 2016/077397.
[0311] In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H3 sequence provided in this disclosure. In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H3 sequences provided in this disclosure. In some aspects, the Kabat CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0312] In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H2 sequence provided in this disclosure. In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H2 sequences provided in this disclosure. In some aspects, the Kabat CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0313] In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Kabat CDR-H1 sequence provided in this disclosure. In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Kabat CDR-H1 sequences provided in this disclosure. In some aspects, the Kabat CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Kabat CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0314] 3.3.2 V.sub.H Sequences Comprising Illustrative Chothia CDRs
[0315] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising one or more Chothia CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative Chothia CDR-H sequences provided in this disclosure, and variants thereof. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising one or more Chothia CDR-H sequences comprising, consisting of, or consisting essentially of one or more illustrative Chothia CDR-H sequences provided in WO 2016/077397.
[0316] 3.2.2.1 Chothia CDR-H3
[0317] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Chothia CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H3 sequence is a Chothia CDR-H3 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H3 sequence, wherein the CDR-H3 sequence comprises, consists of, or consists essentially of a Chothia CDR-H3 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0318] In some embodiments, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119.
[0319] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0320] In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H3 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0321] 3.2.2.2 Chothia CDR-H2
[0322] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Chothia CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H2 sequence is a Chothia CDR-H2 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H2 sequence, wherein the CDR-H2 sequence comprises, consists of, or consists essentially of a Chothia CDR-H2 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0323] In some embodiments, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 81.
[0324] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0325] In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H2 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0326] 3.2.2.3 Chothia CDR-H1
[0327] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Chothia CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided herein. In some aspects, the Chothia CDR-H1 sequence is a Chothia CDR-H1 sequence of a V.sub.H sequence provided in SEQ ID NOs.: 181-220. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a CDR-H1 sequence, wherein the CDR-H1 sequence comprises, consists of, or consists essentially of a Chothia CDR-H1 sequence of an illustrative antibody or V.sub.H sequence provided in WO 2016/077397.
[0328] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 24-38. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 24. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 25. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 26. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 27. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 28. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 29. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 30. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 31. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 32. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 33. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 34. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 35. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 36. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 37. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 38.
[0329] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 182-220. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 199.
[0330] In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a Chothia CDR-H1 sequence of a V.sub.H sequence of SEQ ID NO: 220.
[0331] 3.2.2.4 Chothia CDR-H3+Chothia CDR-H2
[0332] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119, and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 81. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220, and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220. In some aspects, the Chothia CDR-H3 sequence and the Chothia CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H3 and Chothia CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0333] 3.2.2.5 Chothia CDR-H3+Chothia CDR-H1
[0334] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119, and a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 24-38. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H3 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, and a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220. In some aspects, the Chothia CDR-H3 sequence and the Chothia CDR-H1 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H3 and Chothia CDR-H1 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0335] 3.2.2.6 Chothia CDR-H1+Chothia CDR-H2
[0336] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 24-38 and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 81. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, and a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H2 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220. In some aspects, the Chothia CDR-H1 sequence and the Chothia CDR-H2 sequence are both from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H1 and Chothia CDR-H2 are both from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0337] 3.2.2.7 Chothia CDR-H1+Chothia CDR-H2+Chothia CDR-H3
[0338] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs:24-38, a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 81, and a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119. In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H2 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220, and a Chothia CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a Chothia CDR-H3 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220. In some aspects, the Chothia CDR-H1 sequence, Chothia CDR-H2 sequence, and Chothia CDR-H3 sequence are all from a single illustrative V.sub.H sequence provided in this disclosure. For example, in some aspects, the Chothia CDR-H1, Chothia CDR-H2, and Chothia CDR-H3 are all from a single illustrative V.sub.H sequence selected from SEQ ID NOs: 181-220.
[0339] 3.2.2.8 Variants of V.sub.H Sequences Comprising Illustrative Chothia CDRs
[0340] In some embodiments, the V.sub.H sequences provided herein comprise a variant of an illustrative Chothia CDR-H3, CDR-H2, and/or CDR-H1 sequence provided in this disclosure.
[0341] In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H3 sequence provided in this disclosure. In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H3 sequences provided in this disclosure. In some aspects, the Chothia CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0342] In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H2 sequence provided in this disclosure. In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H2 sequences provided in this disclosure. In some aspects, the Chothia CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0343] In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia CDR-H1 sequence provided in this disclosure. In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia CDR-H1 sequences provided in this disclosure. In some aspects, the Chothia CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0344] 3.3 PD-1 V.sub.H Sequences
[0345] In some embodiments, the PD-1 antibody comprises, consists of, or consists essentially of a V.sub.H sequence provided in SEQ ID NOs.: 181-220.
[0346] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 181-220. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 181. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 182. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 183. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 184. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 185. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 186. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 187. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 188. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 189. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 190. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 191. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 192. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 193. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 194. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 195. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 196. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 197. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 198. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 199.
[0347] In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 200. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 201. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 202. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 203. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 204. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 205. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 206. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 207. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 208. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 209. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 210. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 211. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 212. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 213. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 214. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 215. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 216. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 217. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 218. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 219. In some aspects, the antibody comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 220.
[0348] 3.3.1 Variants of V.sub.H Sequences
[0349] In some embodiments, the V.sub.H sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure. In some embodiments, the V.sub.H sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.H sequence provided in WO 2016/077397.
[0350] In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure. In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.H sequences provided in this disclosure.
[0351] In some embodiments, the V.sub.H sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.H sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0352] 3.4 PD-1 CDR-L3 Sequences
[0353] In some embodiments, the PD-1 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 236-269. In some embodiments, the PD-1 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided in WO 2016/077397.
[0354] In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143.
[0355] In some embodiments, the PD-1 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 237-269. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 237. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 238. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 239. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 240. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 241. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 242. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 243. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 244. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 245. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 246. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 247. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 248. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 249.
[0356] In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 250. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 251. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 252. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 253. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 254. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 255. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 256. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 257. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 258. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 259. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 260. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 261. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 262. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 263. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 264. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 265. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 266. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 267. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 268. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 269.
[0357] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0358] 3.5 PD-1 V.sub.L Sequences Comprising Illustrative CDRs
[0359] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising one or more CDR-L sequences comprising, consisting of, or consisting essentially of one or more illustrative CDR-L sequences provided in this disclosure, and variants thereof
[0360] 3.5.1 CDR-L3
[0361] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence, wherein the CDR-L3 sequence comprises, consists of, or consists essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L3 sequence is a CDR-L3 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 236-269. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence, wherein the CDR-L3 sequence comprises, consists of, or consists essentially of a CDR-L3 sequence of an illustrative antibody or V.sub.L sequence provided in WO 2016/077397.
[0362] In some embodiments, the antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143.
[0363] In some embodiments, the PD-1 antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 237-269. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 237. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 238. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 239. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 240. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 241. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 242. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 243. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 244. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 245. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 246. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 247. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 248. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 249.
[0364] In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 250. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 251. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 252. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 253. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 254. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 255. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 256. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 257. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 258. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 259. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 260. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 261. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 262. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 263. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 264. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 265. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 266. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 267. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 268. In some aspects, the antibody comprises a CDR-L3 sequence comprising, consisting of, or consisting essentially of a CDR-L3 sequence of a V.sub.L sequence of SEQ ID NO: 269.
[0365] 3.5.2 CDR-L2
[0366] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence, wherein the CDR-L2 sequence comprises, consists of, or consists essentially of a CDR-L2 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L2 sequence is a CDR-L2 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 236-269. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence, wherein the CDR-L2 sequence comprises, consists of, or consists essentially of a CDR-L2 sequence of an illustrative antibody or V.sub.L sequence provided in WO 2016/077397.
[0367] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 134. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 135. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 136. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 137. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 138. In some aspects, the antibody comprises a V.sub.L sequence comprising a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 139.
[0368] In some embodiments, the PD-1 antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 237-269. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 237. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 238. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 239. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 240. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 241. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 242. In some aspects, the antibody comprises a CDR L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 243. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 244. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 245. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 246. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 247. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 248. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 249.
[0369] In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 250. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 251. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 252. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 253. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 254. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 255. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 256. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 257. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 258. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 259. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 260. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 261. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 262. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 263. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 264. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 265. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 266. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 267. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 268. In some aspects, the antibody comprises a CDR-L2 sequence comprising, consisting of, or consisting essentially of a CDR-L2 sequence of a V.sub.L sequence of SEQ ID NO: 269.
[0370] 3.5.3 CDR-L1
[0371] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence, wherein the CDR-L1 sequence comprises, consists of, or consists essentially of a CDR-L1 sequence of an illustrative antibody or V.sub.L sequence provided herein. In some aspects, the CDR-L1 sequence is a CDR-L1 sequence of a V.sub.L sequence provided in SEQ ID NOs.: 236-269. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence, wherein the CDR-L1 sequence comprises, consists of, or consists essentially of a CDR-L1 sequence of an illustrative antibody or V.sub.L sequence provided in WO 2016/077397.
[0372] In some embodiments, the antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124.
[0373] In some embodiments, the PD-1 antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 237-269. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 237. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 238. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 239. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 240. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 241. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 242. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 243. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 244. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 245. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 246. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 247. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 248. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 249.
[0374] In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 250. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 251. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 252. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 253. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 254. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 255. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 256. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 257. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 258. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 259. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 260. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 261. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 262. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 263. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 264. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 265. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 266. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 267. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 268. In some aspects, the antibody comprises a CDR-L1 sequence comprising, consisting of, or consisting essentially of a CDR-L1 sequence of a V.sub.L sequence of SEQ ID NO: 269.
[0375] 3.5.4 CDR-L3+CDR-L2
[0376] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143 and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269, and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269. In some aspects, the CDR-L3 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L3 and CDR-L2 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 236-269. In some aspects, the CDR-L3 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in WO 2016/077397.
[0377] 3.5.5 CDR-L3+CDR-L1
[0378] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143 and a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269, and a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269. In some aspects, the CDR-L3 sequence and the CDR-L1 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L3 and CDR-L1 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 236-269. In some aspects, the CDR-L3 sequence and the CDR-L1 sequence are both from a single illustrative V.sub.L sequence provided in WO 2016/077397.
[0379] 3.5.6 CDR-L1+CDR-L2
[0380] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124 and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269, and a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269. In some aspects, the CDR-L1 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L1 and CDR-L2 are both from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 236-269. In some aspects, the CDR-L1 sequence and the CDR-L2 sequence are both from a single illustrative V.sub.L sequence provided in WO 2016/077397.
[0381] 3.5.7 CDR-L1+CDR-L2+CDR-L3
[0382] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124, a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139, and a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143. In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269, a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269, and a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269. In some aspects, the CDR-L1 sequence, CDR-L2 sequence, and CDR-L3 sequence are all from a single illustrative V.sub.L sequence provided in this disclosure. For example, in some aspects, the CDR-L1, CDR-L2, and CDR-L3 are all from a single illustrative V.sub.L sequence selected from SEQ ID NOs: 236-269. In some aspects, the CDR-L1 sequence, CDR-L2 sequence, and CDR-L3 sequence are all from a single illustrative V.sub.L sequence provided in WO 2016/077397.
[0383] 3.5.8 Variants of V.sub.L Sequences Comprising Illustrative CDR-Ls
[0384] In some embodiments, the V.sub.L sequences provided herein comprise a variant of an illustrative CDR-L3, CDR-L2, and/or CDR-L1 sequence provided in this disclosure.
[0385] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0386] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0387] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0388] 3.6 PD-1 V.sub.L Sequences
[0389] In some embodiments, the PD-1 antibody comprises, consists of, or consists essentially of a V.sub.L sequence provided in SEQ ID NOs.: 236-269.
[0390] In some embodiments, the PD-1 antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 236-269. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 236. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 237. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 238. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 239. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 240. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 241. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 242. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 243. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 244. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 245. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 246. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 247. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 248. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 249.
[0391] In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 250. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 251. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 252. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 253. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 254. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 255. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 256. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 257. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 258. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 259. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 260. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 261. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 262. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 263. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 264. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 265. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 266. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 267. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 268. In some aspects, the antibody comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 269.
[0392] 3.6.1 Variants of V.sub.L Sequences
[0393] In some embodiments, the V.sub.L sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure. In some embodiments, the V.sub.L sequences provided herein comprise, consist of, or consist essentially of a variant of an illustrative V.sub.L sequence provided in WO 2016/077397.
[0394] In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure. In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.L sequences provided in this disclosure.
[0395] In some embodiments, the V.sub.L sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.L sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0396] 3.7 PD-1 Pairs
[0397] 3.7.1 CDR-H3-CDR-L3 Pairs
[0398] In some embodiments, the PD-1 antibody comprises a CDR-H3 sequence and a CDR-L3 sequence. In some aspects, the CDR-H3 sequence is part of a V.sub.H and the CDR-L3 sequence is part of a V.sub.L.
[0399] In some aspects, the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 119 and a CDR-H3 sequence of a a V.sub.H sequence selected from SEQ ID NOs.: 182-220, and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 143 and a CDR-L3 sequence of a a V.sub.L sequence selected from SEQ ID NOs.: 237-269.
[0400] 3.7.1.1 Variants of CDR-H3-CDR-L3 Pairs
[0401] In some embodiments, the CDR-H3-CDR-L3 pairs provided herein comprise a variant of an illustrative CDR-H3 and/or CDR-L1 sequence provided in this disclosure.
[0402] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0403] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0404] 3.7.2 CDR-H1-CDR-L1 Pairs
[0405] In some embodiments, the PD-1 antibody comprises a CDR-H1 sequence and a CDR-L1 sequence. In some aspects, the CDR-H1 sequence is part of a V.sub.H and the CDR-L1 sequence is part of a V.sub.L.
[0406] In some aspects, the CDR-H1 sequence is a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 24-38 and a Chothia CDR-H1 sequence of a a V.sub.H sequence selected from SEQ ID NOs.: 182-220, and the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 124 and a CDR-L1 sequence of a a V.sub.L sequence selected from SEQ ID NOs.: 237-269.
[0407] In some aspects, the CDR-H1 sequence is a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 57-62 and a Kabat CDR-H1 sequence of a a V.sub.H sequence selected from SEQ ID NOs.: 182-220, and the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 124 and a CDR-L1 sequence of a a V.sub.L sequence selected from SEQ ID NOs.: 237-269.
[0408] 3.7.2.1 Variants of CDR-H1-CDR-L1 Pairs
[0409] In some embodiments, the CDR-H1-CDR-L1 pairs provided herein comprise a variant of an illustrative CDR-H1 and/or CDR-L1 sequence provided in this disclosure.
[0410] In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H1 sequence provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H1 sequences provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0411] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0412] 3.7.3 CDR-H2-CDR-L2 Pairs
[0413] In some embodiments, the PD-1 antibody comprises a CDR-H2 sequence and a CDR-L2 sequence. In some aspects, the CDR-H2 sequence is part of a V.sub.H and the CDR-L2 sequence is part of a V.sub.L.
[0414] In some aspects, the CDR-H2 sequence is a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 81 and a Chothia CDR-H2 sequence of a a V.sub.H sequence selected from SEQ ID NOs.: 182-220, and the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139 and a CDR-L2 sequence of a a V.sub.L sequence selected from SEQ ID NOs.: 237-269.
[0415] In some aspects, the CDR-H1 sequence is a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NO: 110 and a Kabat CDR-H2 sequence of a a V.sub.H sequence selected from SEQ ID NOs.: 182-220, and the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of a sequence selected from SEQ ID NOs: 134-139 and a CDR-L2 sequence of a a V.sub.L sequence selected from SEQ ID NOs.: 237-269.
[0416] 3.7.3.1 Variants of CDR-H2-CDR-L2 Pairs
[0417] In some embodiments, the CDR-H2-CDR-L2 pairs provided herein comprise a variant of an illustrative CDR-H2 and/or CDR-L2 sequence provided in this disclosure.
[0418] In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H2 sequence provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H2 sequences provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0419] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0420] 3.7.4 V.sub.H-V.sub.L Pairs
[0421] In some embodiments, the PD-1 antibody comprises a V.sub.H sequence and a V.sub.L sequence.
[0422] In some aspects, the V.sub.H sequence is a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 181-220, and the V.sub.L sequence is a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 236-269.
[0423] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 181 and SEQ ID NO: 236; SEQ ID NO: 181 and SEQ ID NO: 325; SEQ ID NO: 181 and SEQ ID NO: 237; SEQ ID NO: 181 and SEQ ID NO: 238; SEQ ID NO: 181 and SEQ ID NO: 239; SEQ ID NO: 181 and SEQ ID NO: 240; SEQ ID NO: 181 and SEQ ID NO: 241; SEQ ID NO: 181 and SEQ ID NO: 242; SEQ ID NO: 181 and SEQ ID NO: 243; SEQ ID NO: 181 and SEQ ID NO: 244; SEQ ID NO: 181 and SEQ ID NO: 245; SEQ ID NO: 181 and SEQ ID NO: 246; SEQ ID NO: 181 and SEQ ID NO: 247; SEQ ID NO: 181 and SEQ ID NO: 248; SEQ ID NO: 181 and SEQ ID NO: 249; SEQ ID NO: 181 and SEQ ID NO: 250; SEQ ID NO: 181 and SEQ ID NO: 251; SEQ ID NO: 181 and SEQ ID NO: 252; SEQ ID NO: 181 and SEQ ID NO: 253; SEQ ID NO: 181 and SEQ ID NO: 254; SEQ ID NO: 181 and SEQ ID NO: 255; SEQ ID NO: 181 and SEQ ID NO: 256; SEQ ID NO: 181 and SEQ ID NO: 257; SEQ ID NO: 181 and SEQ ID NO: 258; SEQ ID NO: 181 and SEQ ID NO: 259; SEQ ID NO: 181 and SEQ ID NO: 260; SEQ ID NO: 181 and SEQ ID NO: 261; SEQ ID NO: 181 and SEQ ID NO: 262; SEQ ID NO: 181 and SEQ ID NO: 263; SEQ ID NO: 181 and SEQ ID NO: 264; SEQ ID NO: 181 and SEQ ID NO: 265; SEQ ID NO: 181 and SEQ ID NO: 266; SEQ ID NO: 181 and SEQ ID NO: 267; SEQ ID NO: 181 and SEQ ID NO: 268; and SEQ ID NO: 181 and SEQ ID NO: 269.
[0424] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 182 and SEQ ID NO: 236; SEQ ID NO: 182 and SEQ ID NO: 325; SEQ ID NO: 182 and SEQ ID NO: 237; SEQ ID NO: 182 and SEQ ID NO: 238; SEQ ID NO: 182 and SEQ ID NO: 239; SEQ ID NO: 182 and SEQ ID NO: 240; SEQ ID NO: 182 and SEQ ID NO: 241; SEQ ID NO: 182 and SEQ ID NO: 242; SEQ ID NO: 182 and SEQ ID NO: 243; SEQ ID NO: 182 and SEQ ID NO: 244; SEQ ID NO: 182 and SEQ ID NO: 245; SEQ ID NO: 182 and SEQ ID NO: 246; SEQ ID NO: 182 and SEQ ID NO: 247; SEQ ID NO: 182 and SEQ ID NO: 248; SEQ ID NO: 182 and SEQ ID NO: 249; SEQ ID NO: 182 and SEQ ID NO: 250; SEQ ID NO: 182 and SEQ ID NO: 251; SEQ ID NO: 182 and SEQ ID NO: 252; SEQ ID NO: 182 and SEQ ID NO: 253; SEQ ID NO: 182 and SEQ ID NO: 254; SEQ ID NO: 182 and SEQ ID NO: 255; SEQ ID NO: 182 and SEQ ID NO: 256; SEQ ID NO: 182 and SEQ ID NO: 257; SEQ ID NO: 182 and SEQ ID NO: 258; SEQ ID NO: 182 and SEQ ID NO: 259; SEQ ID NO: 182 and SEQ ID NO: 260; SEQ ID NO: 182 and SEQ ID NO: 261; SEQ ID NO: 182 and SEQ ID NO: 262; SEQ ID NO: 182 and SEQ ID NO: 263; SEQ ID NO: 182 and SEQ ID NO: 264; SEQ ID NO: 182 and SEQ ID NO: 265; SEQ ID NO: 182 and SEQ ID NO: 266; SEQ ID NO: 182 and SEQ ID NO: 267; SEQ ID NO: 182 and SEQ ID NO: 268; and SEQ ID NO: 182 and SEQ ID NO: 269.
[0425] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 183 and SEQ ID NO: 236; SEQ ID NO: 183 and SEQ ID NO: 325; SEQ ID NO: 183 and SEQ ID NO: 237; SEQ ID NO: 183 and SEQ ID NO: 238; SEQ ID NO: 183 and SEQ ID NO: 239; SEQ ID NO: 183 and SEQ ID NO: 240; SEQ ID NO: 183 and SEQ ID NO: 241; SEQ ID NO: 183 and SEQ ID NO: 242; SEQ ID NO: 183 and SEQ ID NO: 243; SEQ ID NO: 183 and SEQ ID NO: 244; SEQ ID NO: 183 and SEQ ID NO: 245; SEQ ID NO: 183 and SEQ ID NO: 246; SEQ ID NO: 183 and SEQ ID NO: 247; SEQ ID NO: 183 and SEQ ID NO: 248; SEQ ID NO: 183 and SEQ ID NO: 249; SEQ ID NO: 183 and SEQ ID NO: 250; SEQ ID NO: 183 and SEQ ID NO: 251; SEQ ID NO: 183 and SEQ ID NO: 252; SEQ ID NO: 183 and SEQ ID NO: 253; SEQ ID NO: 183 and SEQ ID NO: 254; SEQ ID NO: 183 and SEQ ID NO: 255; SEQ ID NO: 183 and SEQ ID NO: 256; SEQ ID NO: 183 and SEQ ID NO: 257; SEQ ID NO: 183 and SEQ ID NO: 258; SEQ ID NO: 183 and SEQ ID NO: 259; SEQ ID NO: 183 and SEQ ID NO: 260; SEQ ID NO: 183 and SEQ ID NO: 261; SEQ ID NO: 183 and SEQ ID NO: 262; SEQ ID NO: 183 and SEQ ID NO: 263; SEQ ID NO: 183 and SEQ ID NO: 264; SEQ ID NO: 183 and SEQ ID NO: 265; SEQ ID NO: 183 and SEQ ID NO: 266; SEQ ID NO: 183 and SEQ ID NO: 267; SEQ ID NO: 183 and SEQ ID NO: 268; and SEQ ID NO: 183 and SEQ ID NO: 269.
[0426] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 184 and SEQ ID NO: 236; SEQ ID NO: 184 and SEQ ID NO: 325; SEQ ID NO: 184 and SEQ ID NO: 237; SEQ ID NO: 184 and SEQ ID NO: 238; SEQ ID NO: 184 and SEQ ID NO: 239; SEQ ID NO: 184 and SEQ ID NO: 240; SEQ ID NO: 184 and SEQ ID NO: 241; SEQ ID NO: 184 and SEQ ID NO: 242; SEQ ID NO: 184 and SEQ ID NO: 243; SEQ ID NO: 184 and SEQ ID NO: 244; SEQ ID NO: 184 and SEQ ID NO: 245; SEQ ID NO: 184 and SEQ ID NO: 246; SEQ ID NO: 184 and SEQ ID NO: 247; SEQ ID NO: 184 and SEQ ID NO: 248; SEQ ID NO: 184 and SEQ ID NO: 249; SEQ ID NO: 184 and SEQ ID NO: 250; SEQ ID NO: 184 and SEQ ID NO: 251; SEQ ID NO: 184 and SEQ ID NO: 252; SEQ ID NO: 184 and SEQ ID NO: 253; SEQ ID NO: 184 and SEQ ID NO: 254; SEQ ID NO: 184 and SEQ ID NO: 255; SEQ ID NO: 184 and SEQ ID NO: 256; SEQ ID NO: 184 and SEQ ID NO: 257; SEQ ID NO: 184 and SEQ ID NO: 258; SEQ ID NO: 184 and SEQ ID NO: 259; SEQ ID NO: 184 and SEQ ID NO: 260; SEQ ID NO: 184 and SEQ ID NO: 261; SEQ ID NO: 184 and SEQ ID NO: 262; SEQ ID NO: 184 and SEQ ID NO: 263; SEQ ID NO: 184 and SEQ ID NO: 264; SEQ ID NO: 184 and SEQ ID NO: 265; SEQ ID NO: 184 and SEQ ID NO: 266; SEQ ID NO: 184 and SEQ ID NO: 267; SEQ ID NO: 184 and SEQ ID NO: 268; and SEQ ID NO: 184 and SEQ ID NO: 269.
[0427] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 185 and SEQ ID NO: 236; SEQ ID NO: 185 and SEQ ID NO: 325; SEQ ID NO: 185 and SEQ ID NO: 237; SEQ ID NO: 185 and SEQ ID NO: 238; SEQ ID NO: 185 and SEQ ID NO: 239; SEQ ID NO: 185 and SEQ ID NO: 240; SEQ ID NO: 185 and SEQ ID NO: 241; SEQ ID NO: 185 and SEQ ID NO: 242; SEQ ID NO: 185 and SEQ ID NO: 243; SEQ ID NO: 185 and SEQ ID NO: 244; SEQ ID NO: 185 and SEQ ID NO: 245; SEQ ID NO: 185 and SEQ ID NO: 246; SEQ ID NO: 185 and SEQ ID NO: 247; SEQ ID NO: 185 and SEQ ID NO: 248; SEQ ID NO: 185 and SEQ ID NO: 249; SEQ ID NO: 185 and SEQ ID NO: 250; SEQ ID NO: 185 and SEQ ID NO: 251; SEQ ID NO: 185 and SEQ ID NO: 252; SEQ ID NO: 185 and SEQ ID NO: 253; SEQ ID NO: 185 and SEQ ID NO: 254; SEQ ID NO: 185 and SEQ ID NO: 255; SEQ ID NO: 185 and SEQ ID NO: 256; SEQ ID NO: 185 and SEQ ID NO: 257; SEQ ID NO: 185 and SEQ ID NO: 258; SEQ ID NO: 185 and SEQ ID NO: 259; SEQ ID NO: 185 and SEQ ID NO: 260; SEQ ID NO: 185 and SEQ ID NO: 261; SEQ ID NO: 185 and SEQ ID NO: 262; SEQ ID NO: 185 and SEQ ID NO: 263; SEQ ID NO: 185 and SEQ ID NO: 264; SEQ ID NO: 185 and SEQ ID NO: 265; SEQ ID NO: 185 and SEQ ID NO: 266; SEQ ID NO: 185 and SEQ ID NO: 267; SEQ ID NO: 185 and SEQ ID NO: 268; and SEQ ID NO: 185 and SEQ ID NO: 269.
[0428] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 186 and SEQ ID NO: 236; SEQ ID NO: 186 and SEQ ID NO: 325; SEQ ID NO: 186 and SEQ ID NO: 237; SEQ ID NO: 186 and SEQ ID NO: 238; SEQ ID NO: 186 and SEQ ID NO: 239; SEQ ID NO: 186 and SEQ ID NO: 240; SEQ ID NO: 186 and SEQ ID NO: 241; SEQ ID NO: 186 and SEQ ID NO: 242; SEQ ID NO: 186 and SEQ ID NO: 243; SEQ ID NO: 186 and SEQ ID NO: 244; SEQ ID NO: 186 and SEQ ID NO: 245; SEQ ID NO: 186 and SEQ ID NO: 246; SEQ ID NO: 186 and SEQ ID NO: 247; SEQ ID NO: 186 and SEQ ID NO: 248; SEQ ID NO: 186 and SEQ ID NO: 249; SEQ ID NO: 186 and SEQ ID NO: 250; SEQ ID NO: 186 and SEQ ID NO: 251; SEQ ID NO: 186 and SEQ ID NO: 252; SEQ ID NO: 186 and SEQ ID NO: 253; SEQ ID NO: 186 and SEQ ID NO: 254; SEQ ID NO: 186 and SEQ ID NO: 255; SEQ ID NO: 186 and SEQ ID NO: 256; SEQ ID NO: 186 and SEQ ID NO: 257; SEQ ID NO: 186 and SEQ ID NO: 258; SEQ ID NO: 186 and SEQ ID NO: 259; SEQ ID NO: 186 and SEQ ID NO: 260; SEQ ID NO: 186 and SEQ ID NO: 261; SEQ ID NO: 186 and SEQ ID NO: 262; SEQ ID NO: 186 and SEQ ID NO: 263; SEQ ID NO: 186 and SEQ ID NO: 264; SEQ ID NO: 186 and SEQ ID NO: 265; SEQ ID NO: 186 and SEQ ID NO: 266; SEQ ID NO: 186 and SEQ ID NO: 267; SEQ ID NO: 186 and SEQ ID NO: 268; and SEQ ID NO: 186 and SEQ ID NO: 269.
[0429] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 187 and SEQ ID NO: 236; SEQ ID NO: 187 and SEQ ID NO: 325; SEQ ID NO: 187 and SEQ ID NO: 237; SEQ ID NO: 187 and SEQ ID NO: 238; SEQ ID NO: 187 and SEQ ID NO: 239; SEQ ID NO: 187 and SEQ ID NO: 240; SEQ ID NO: 187 and SEQ ID NO: 241; SEQ ID NO: 187 and SEQ ID NO: 242; SEQ ID NO: 187 and SEQ ID NO: 243; SEQ ID NO: 187 and SEQ ID NO: 244; SEQ ID NO: 187 and SEQ ID NO: 245; SEQ ID NO: 187 and SEQ ID NO: 246; SEQ ID NO: 187 and SEQ ID NO: 247; SEQ ID NO: 187 and SEQ ID NO: 248; SEQ ID NO: 187 and SEQ ID NO: 249; SEQ ID NO: 187 and SEQ ID NO: 250; SEQ ID NO: 187 and SEQ ID NO: 251; SEQ ID NO: 187 and SEQ ID NO: 252; SEQ ID NO: 187 and SEQ ID NO: 253; SEQ ID NO: 187 and SEQ ID NO: 254; SEQ ID NO: 187 and SEQ ID NO: 255; SEQ ID NO: 187 and SEQ ID NO: 256; SEQ ID NO: 187 and SEQ ID NO: 257; SEQ ID NO: 187 and SEQ ID NO: 258; SEQ ID NO: 187 and SEQ ID NO: 259; SEQ ID NO: 187 and SEQ ID NO: 260; SEQ ID NO: 187 and SEQ ID NO: 261; SEQ ID NO: 187 and SEQ ID NO: 262; SEQ ID NO: 187 and SEQ ID NO: 263; SEQ ID NO: 187 and SEQ ID NO: 264; SEQ ID NO: 187 and SEQ ID NO: 265; SEQ ID NO: 187 and SEQ ID NO: 266; SEQ ID NO: 187 and SEQ ID NO: 267; SEQ ID NO: 187 and SEQ ID NO: 268; and SEQ ID NO: 187 and SEQ ID NO: 269.
[0430] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 188 and SEQ ID NO: 236; SEQ ID NO: 188 and SEQ ID NO: 325; SEQ ID NO: 188 and SEQ ID NO: 237; SEQ ID NO: 188 and SEQ ID NO: 238; SEQ ID NO: 188 and SEQ ID NO: 239; SEQ ID NO: 188 and SEQ ID NO: 240; SEQ ID NO: 188 and SEQ ID NO: 241; SEQ ID NO: 188 and SEQ ID NO: 242; SEQ ID NO: 188 and SEQ ID NO: 243; SEQ ID NO: 188 and SEQ ID NO: 244; SEQ ID NO: 188 and SEQ ID NO: 245; SEQ ID NO: 188 and SEQ ID NO: 246; SEQ ID NO: 188 and SEQ ID NO: 247; SEQ ID NO: 188 and SEQ ID NO: 248; SEQ ID NO: 188 and SEQ ID NO: 249; SEQ ID NO: 188 and SEQ ID NO: 250; SEQ ID NO: 188 and SEQ ID NO: 251; SEQ ID NO: 188 and SEQ ID NO: 252; SEQ ID NO: 188 and SEQ ID NO: 253; SEQ ID NO: 188 and SEQ ID NO: 254; SEQ ID NO: 188 and SEQ ID NO: 255; SEQ ID NO: 188 and SEQ ID NO: 256; SEQ ID NO: 188 and SEQ ID NO: 257; SEQ ID NO: 188 and SEQ ID NO: 258; SEQ ID NO: 188 and SEQ ID NO: 259; SEQ ID NO: 188 and SEQ ID NO: 260; SEQ ID NO: 188 and SEQ ID NO: 261; SEQ ID NO: 188 and SEQ ID NO: 262; SEQ ID NO: 188 and SEQ ID NO: 263; SEQ ID NO: 188 and SEQ ID NO: 264; SEQ ID NO: 188 and SEQ ID NO: 265; SEQ ID NO: 188 and SEQ ID NO: 266; SEQ ID NO: 188 and SEQ ID NO: 267; SEQ ID NO: 188 and SEQ ID NO: 268; and SEQ ID NO: 188 and SEQ ID NO: 269.
[0431] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 189 and SEQ ID NO: 236; SEQ ID NO: 189 and SEQ ID NO: 325; SEQ ID NO: 189 and SEQ ID NO: 237; SEQ ID NO: 189 and SEQ ID NO: 238; SEQ ID NO: 189 and SEQ ID NO: 239; SEQ ID NO: 189 and SEQ ID NO: 240; SEQ ID NO: 189 and SEQ ID NO: 241; SEQ ID NO: 189 and SEQ ID NO: 242; SEQ ID NO: 189 and SEQ ID NO: 243; SEQ ID NO: 189 and SEQ ID NO: 244; SEQ ID NO: 189 and SEQ ID NO: 245; SEQ ID NO: 189 and SEQ ID NO: 246; SEQ ID NO: 189 and SEQ ID NO: 247; SEQ ID NO: 189 and SEQ ID NO: 248; SEQ ID NO: 189 and SEQ ID NO: 249; SEQ ID NO: 189 and SEQ ID NO: 250; SEQ ID NO: 189 and SEQ ID NO: 251; SEQ ID NO: 189 and SEQ ID NO: 252; SEQ ID NO: 189 and SEQ ID NO: 253; SEQ ID NO: 189 and SEQ ID NO: 254; SEQ ID NO: 189 and SEQ ID NO: 255; SEQ ID NO: 189 and SEQ ID NO: 256; SEQ ID NO: 189 and SEQ ID NO: 257; SEQ ID NO: 189 and SEQ ID NO: 258; SEQ ID NO: 189 and SEQ ID NO: 259; SEQ ID NO: 189 and SEQ ID NO: 260; SEQ ID NO: 189 and SEQ ID NO: 261; SEQ ID NO: 189 and SEQ ID NO: 262; SEQ ID NO: 189 and SEQ ID NO: 263; SEQ ID NO: 189 and SEQ ID NO: 264; SEQ ID NO: 189 and SEQ ID NO: 265; SEQ ID NO: 189 and SEQ ID NO: 266; SEQ ID NO: 189 and SEQ ID NO: 267; SEQ ID NO: 189 and SEQ ID NO: 268; and SEQ ID NO: 189 and SEQ ID NO: 269.
[0432] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 190 and SEQ ID NO: 236; SEQ ID NO: 190 and SEQ ID NO: 325; SEQ ID NO: 190 and SEQ ID NO: 237; SEQ ID NO: 190 and SEQ ID NO: 238; SEQ ID NO: 190 and SEQ ID NO: 239; SEQ ID NO: 190 and SEQ ID NO: 240; SEQ ID NO: 190 and SEQ ID NO: 241; SEQ ID NO: 190 and SEQ ID NO: 242; SEQ ID NO: 190 and SEQ ID NO: 243; SEQ ID NO: 190 and SEQ ID NO: 244; SEQ ID NO: 190 and SEQ ID NO: 245; SEQ ID NO: 190 and SEQ ID NO: 246; SEQ ID NO: 190 and SEQ ID NO: 247; SEQ ID NO: 190 and SEQ ID NO: 248; SEQ ID NO: 190 and SEQ ID NO: 249; SEQ ID NO: 190 and SEQ ID NO: 250; SEQ ID NO: 190 and SEQ ID NO: 251; SEQ ID NO: 190 and SEQ ID NO: 252; SEQ ID NO: 190 and SEQ ID NO: 253; SEQ ID NO: 190 and SEQ ID NO: 254; SEQ ID NO: 190 and SEQ ID NO: 255; SEQ ID NO: 190 and SEQ ID NO: 256; SEQ ID NO: 190 and SEQ ID NO: 257; SEQ ID NO: 190 and SEQ ID NO: 258; SEQ ID NO: 190 and SEQ ID NO: 259; SEQ ID NO: 190 and SEQ ID NO: 260; SEQ ID NO: 190 and SEQ ID NO: 261; SEQ ID NO: 190 and SEQ ID NO: 262; SEQ ID NO: 190 and SEQ ID NO: 263; SEQ ID NO: 190 and SEQ ID NO: 264; SEQ ID NO: 190 and SEQ ID NO: 265; SEQ ID NO: 190 and SEQ ID NO: 266; SEQ ID NO: 190 and SEQ ID NO: 267; SEQ ID NO: 190 and SEQ ID NO: 268; and SEQ ID NO: 190 and SEQ ID NO: 269.
[0433] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 191 and SEQ ID NO: 236; SEQ ID NO: 191 and SEQ ID NO: 325; SEQ ID NO: 191 and SEQ ID NO: 237; SEQ ID NO: 191 and SEQ ID NO: 238; SEQ ID NO: 191 and SEQ ID NO: 239; SEQ ID NO: 191 and SEQ ID NO: 240; SEQ ID NO: 191 and SEQ ID NO: 241; SEQ ID NO: 191 and SEQ ID NO: 242; SEQ ID NO: 191 and SEQ ID NO: 243; SEQ ID NO: 191 and SEQ ID NO: 244; SEQ ID NO: 191 and SEQ ID NO: 245; SEQ ID NO: 191 and SEQ ID NO: 246; SEQ ID NO: 191 and SEQ ID NO: 247; SEQ ID NO: 191 and SEQ ID NO: 248; SEQ ID NO: 191 and SEQ ID NO: 249; SEQ ID NO: 191 and SEQ ID NO: 250; SEQ ID NO: 191 and SEQ ID NO: 251; SEQ ID NO: 191 and SEQ ID NO: 252; SEQ ID NO: 191 and SEQ ID NO: 253; SEQ ID NO: 191 and SEQ ID NO: 254; SEQ ID NO: 191 and SEQ ID NO: 255; SEQ ID NO: 191 and SEQ ID NO: 256; SEQ ID NO: 191 and SEQ ID NO: 257; SEQ ID NO: 191 and SEQ ID NO: 258; SEQ ID NO: 191 and SEQ ID NO: 259; SEQ ID NO: 191 and SEQ ID NO: 260; SEQ ID NO: 191 and SEQ ID NO: 261; SEQ ID NO: 191 and SEQ ID NO: 262; SEQ ID NO: 191 and SEQ ID NO: 263; SEQ ID NO: 191 and SEQ ID NO: 264; SEQ ID NO: 191 and SEQ ID NO: 265; SEQ ID NO: 191 and SEQ ID NO: 266; SEQ ID NO: 191 and SEQ ID NO: 267; SEQ ID NO: 191 and SEQ ID NO: 268; and SEQ ID NO: 191 and SEQ ID NO: 269.
[0434] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 192 and SEQ ID NO: 236; SEQ ID NO: 192 and SEQ ID NO: 325; SEQ ID NO: 192 and SEQ ID NO: 237; SEQ ID NO: 192 and SEQ ID NO: 238; SEQ ID NO: 192 and SEQ ID NO: 239; SEQ ID NO: 192 and SEQ ID NO: 240; SEQ ID NO: 192 and SEQ ID NO: 241; SEQ ID NO: 192 and SEQ ID NO: 242; SEQ ID NO: 192 and SEQ ID NO: 243; SEQ ID NO: 192 and SEQ ID NO: 244; SEQ ID NO: 192 and SEQ ID NO: 245; SEQ ID NO: 192 and SEQ ID NO: 246; SEQ ID NO: 192 and SEQ ID NO: 247; SEQ ID NO: 192 and SEQ ID NO: 248; SEQ ID NO: 192 and SEQ ID NO: 249; SEQ ID NO: 192 and SEQ ID NO: 250; SEQ ID NO: 192 and SEQ ID NO: 251; SEQ ID NO: 192 and SEQ ID NO: 252; SEQ ID NO: 192 and SEQ ID NO: 253; SEQ ID NO: 192 and SEQ ID NO: 254; SEQ ID NO: 192 and SEQ ID NO: 255; SEQ ID NO: 192 and SEQ ID NO: 256; SEQ ID NO: 192 and SEQ ID NO: 257; SEQ ID NO: 192 and SEQ ID NO: 258; SEQ ID NO: 192 and SEQ ID NO: 259; SEQ ID NO: 192 and SEQ ID NO: 260; SEQ ID NO: 192 and SEQ ID NO: 261; SEQ ID NO: 192 and SEQ ID NO: 262; SEQ ID NO: 192 and SEQ ID NO: 263; SEQ ID NO: 192 and SEQ ID NO: 264; SEQ ID NO: 192 and SEQ ID NO: 265; SEQ ID NO: 192 and SEQ ID NO: 266; SEQ ID NO: 192 and SEQ ID NO: 267; SEQ ID NO: 192 and SEQ ID NO: 268; and SEQ ID NO: 192 and SEQ ID NO: 269.
[0435] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 193 and SEQ ID NO: 236; SEQ ID NO: 193 and SEQ ID NO: 325; SEQ ID NO: 193 and SEQ ID NO: 237; SEQ ID NO: 193 and SEQ ID NO: 238; SEQ ID NO: 193 and SEQ ID NO: 239; SEQ ID NO: 193 and SEQ ID NO: 240; SEQ ID NO: 193 and SEQ ID NO: 241; SEQ ID NO: 193 and SEQ ID NO: 242; SEQ ID NO: 193 and SEQ ID NO: 243; SEQ ID NO: 193 and SEQ ID NO: 244; SEQ ID NO: 193 and SEQ ID NO: 245; SEQ ID NO: 193 and SEQ ID NO: 246; SEQ ID NO: 193 and SEQ ID NO: 247; SEQ ID NO: 193 and SEQ ID NO: 248; SEQ ID NO: 193 and SEQ ID NO: 249; SEQ ID NO: 193 and SEQ ID NO: 250; SEQ ID NO: 193 and SEQ ID NO: 251; SEQ ID NO: 193 and SEQ ID NO: 252; SEQ ID NO: 193 and SEQ ID NO: 253; SEQ ID NO: 193 and SEQ ID NO: 254; SEQ ID NO: 193 and SEQ ID NO: 255; SEQ ID NO: 193 and SEQ ID NO: 256; SEQ ID NO: 193 and SEQ ID NO: 257; SEQ ID NO: 193 and SEQ ID NO: 258; SEQ ID NO: 193 and SEQ ID NO: 259; SEQ ID NO: 193 and SEQ ID NO: 260; SEQ ID NO: 193 and SEQ ID NO: 261; SEQ ID NO: 193 and SEQ ID NO: 262; SEQ ID NO: 193 and SEQ ID NO: 263; SEQ ID NO: 193 and SEQ ID NO: 264; SEQ ID NO: 193 and SEQ ID NO: 265; SEQ ID NO: 193 and SEQ ID NO: 266; SEQ ID NO: 193 and SEQ ID NO: 267; SEQ ID NO: 193 and SEQ ID NO: 268; and SEQ ID NO: 193 and SEQ ID NO: 269.
[0436] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 194 and SEQ ID NO: 236; SEQ ID NO: 194 and SEQ ID NO: 325; SEQ ID NO: 194 and SEQ ID NO: 237; SEQ ID NO: 194 and SEQ ID NO: 238; SEQ ID NO: 194 and SEQ ID NO: 239; SEQ ID NO: 194 and SEQ ID NO: 240; SEQ ID NO: 194 and SEQ ID NO: 241; SEQ ID NO: 194 and SEQ ID NO: 242; SEQ ID NO: 194 and SEQ ID NO: 243; SEQ ID NO: 194 and SEQ ID NO: 244; SEQ ID NO: 194 and SEQ ID NO: 245; SEQ ID NO: 194 and SEQ ID NO: 246; SEQ ID NO: 194 and SEQ ID NO: 247; SEQ ID NO: 194 and SEQ ID NO: 248; SEQ ID NO: 194 and SEQ ID NO: 249; SEQ ID NO: 194 and SEQ ID NO: 250; SEQ ID NO: 194 and SEQ ID NO: 251; SEQ ID NO: 194 and SEQ ID NO: 252; SEQ ID NO: 194 and SEQ ID NO: 253; SEQ ID NO: 194 and SEQ ID NO: 254; SEQ ID NO: 194 and SEQ ID NO: 255; SEQ ID NO: 194 and SEQ ID NO: 256; SEQ ID NO: 194 and SEQ ID NO: 257; SEQ ID NO: 194 and SEQ ID NO: 258; SEQ ID NO: 194 and SEQ ID NO: 259; SEQ ID NO: 194 and SEQ ID NO: 260; SEQ ID NO: 194 and SEQ ID NO: 261; SEQ ID NO: 194 and SEQ ID NO: 262; SEQ ID NO: 194 and SEQ ID NO: 263; SEQ ID NO: 194 and SEQ ID NO: 264; SEQ ID NO: 194 and SEQ ID NO: 265; SEQ ID NO: 194 and SEQ ID NO: 266; SEQ ID NO: 194 and SEQ ID NO: 267; SEQ ID NO: 194 and SEQ ID NO: 268; and SEQ ID NO: 194 and SEQ ID NO: 269.
[0437] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 195 and SEQ ID NO: 236; SEQ ID NO: 195 and SEQ ID NO: 325; SEQ ID NO: 195 and SEQ ID NO: 237; SEQ ID NO: 195 and SEQ ID NO: 238; SEQ ID NO: 195 and SEQ ID NO: 239; SEQ ID NO: 195 and SEQ ID NO: 240; SEQ ID NO: 195 and SEQ ID NO: 241; SEQ ID NO: 195 and SEQ ID NO: 242; SEQ ID NO: 195 and SEQ ID NO: 243; SEQ ID NO: 195 and SEQ ID NO: 244; SEQ ID NO: 195 and SEQ ID NO: 245; SEQ ID NO: 195 and SEQ ID NO: 246; SEQ ID NO: 195 and SEQ ID NO: 247; SEQ ID NO: 195 and SEQ ID NO: 248; SEQ ID NO: 195 and SEQ ID NO: 249; SEQ ID NO: 195 and SEQ ID NO: 250; SEQ ID NO: 195 and SEQ ID NO: 251; SEQ ID NO: 195 and SEQ ID NO: 252; SEQ ID NO: 195 and SEQ ID NO: 253; SEQ ID NO: 195 and SEQ ID NO: 254; SEQ ID NO: 195 and SEQ ID NO: 255; SEQ ID NO: 195 and SEQ ID NO: 256; SEQ ID NO: 195 and SEQ ID NO: 257; SEQ ID NO: 195 and SEQ ID NO: 258; SEQ ID NO: 195 and SEQ ID NO: 259; SEQ ID NO: 195 and SEQ ID NO: 260; SEQ ID NO: 195 and SEQ ID NO: 261; SEQ ID NO: 195 and SEQ ID NO: 262; SEQ ID NO: 195 and SEQ ID NO: 263; SEQ ID NO: 195 and SEQ ID NO: 264; SEQ ID NO: 195 and SEQ ID NO: 265; SEQ ID NO: 195 and SEQ ID NO: 266; SEQ ID NO: 195 and SEQ ID NO: 267; SEQ ID NO: 195 and SEQ ID NO: 268; and SEQ ID NO: 195 and SEQ ID NO: 269.
[0438] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 196 and SEQ ID NO: 236; SEQ ID NO: 196 and SEQ ID NO: 325; SEQ ID NO: 196 and SEQ ID NO: 237; SEQ ID NO: 196 and SEQ ID NO: 238; SEQ ID NO: 196 and SEQ ID NO: 239; SEQ ID NO: 196 and SEQ ID NO: 240; SEQ ID NO: 196 and SEQ ID NO: 241; SEQ ID NO: 196 and SEQ ID NO: 242; SEQ ID NO: 196 and SEQ ID NO: 243; SEQ ID NO: 196 and SEQ ID NO: 244; SEQ ID NO: 196 and SEQ ID NO: 245; SEQ ID NO: 196 and SEQ ID NO: 246; SEQ ID NO: 196 and SEQ ID NO: 247; SEQ ID NO: 196 and SEQ ID NO: 248; SEQ ID NO: 196 and SEQ ID NO: 249; SEQ ID NO: 196 and SEQ ID NO: 250; SEQ ID NO: 196 and SEQ ID NO: 251; SEQ ID NO: 196 and SEQ ID NO: 252; SEQ ID NO: 196 and SEQ ID NO: 253; SEQ ID NO: 196 and SEQ ID NO: 254; SEQ ID NO: 196 and SEQ ID NO: 255; SEQ ID NO: 196 and SEQ ID NO: 256; SEQ ID NO: 196 and SEQ ID NO: 257; SEQ ID NO: 196 and SEQ ID NO: 258; SEQ ID NO: 196 and SEQ ID NO: 259; SEQ ID NO: 196 and SEQ ID NO: 260; SEQ ID NO: 196 and SEQ ID NO: 261; SEQ ID NO: 196 and SEQ ID NO: 262; SEQ ID NO: 196 and SEQ ID NO: 263; SEQ ID NO: 196 and SEQ ID NO: 264; SEQ ID NO: 196 and SEQ ID NO: 265; SEQ ID NO: 196 and SEQ ID NO: 266; SEQ ID NO: 196 and SEQ ID NO: 267; SEQ ID NO: 196 and SEQ ID NO: 268; and SEQ ID NO: 196 and SEQ ID NO: 269.
[0439] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 197 and SEQ ID NO: 236; SEQ ID NO: 197 and SEQ ID NO: 325; SEQ ID NO: 197 and SEQ ID NO: 237; SEQ ID NO: 197 and SEQ ID NO: 238; SEQ ID NO: 197 and SEQ ID NO: 239; SEQ ID NO: 197 and SEQ ID NO: 240; SEQ ID NO: 197 and SEQ ID NO: 241; SEQ ID NO: 197 and SEQ ID NO: 242; SEQ ID NO: 197 and SEQ ID NO: 243; SEQ ID NO: 197 and SEQ ID NO: 244; SEQ ID NO: 197 and SEQ ID NO: 245; SEQ ID NO: 197 and SEQ ID NO: 246; SEQ ID NO: 197 and SEQ ID NO: 247; SEQ ID NO: 197 and SEQ ID NO: 248; SEQ ID NO: 197 and SEQ ID NO: 249; SEQ ID NO: 197 and SEQ ID NO: 250; SEQ ID NO: 197 and SEQ ID NO: 251; SEQ ID NO: 197 and SEQ ID NO: 252; SEQ ID NO: 197 and SEQ ID NO: 253; SEQ ID NO: 197 and SEQ ID NO: 254; SEQ ID NO: 197 and SEQ ID NO: 255; SEQ ID NO: 197 and SEQ ID NO: 256; SEQ ID NO: 197 and SEQ ID NO: 257; SEQ ID NO: 197 and SEQ ID NO: 258; SEQ ID NO: 197 and SEQ ID NO: 259; SEQ ID NO: 197 and SEQ ID NO: 260; SEQ ID NO: 197 and SEQ ID NO: 261; SEQ ID NO: 197 and SEQ ID NO: 262; SEQ ID NO: 197 and SEQ ID NO: 263; SEQ ID NO: 197 and SEQ ID NO: 264; SEQ ID NO: 197 and SEQ ID NO: 265; SEQ ID NO: 197 and SEQ ID NO: 266; SEQ ID NO: 197 and SEQ ID NO: 267; SEQ ID NO: 197 and SEQ ID NO: 268; and SEQ ID NO: 197 and SEQ ID NO: 269.
[0440] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 198 and SEQ ID NO: 236; SEQ ID NO: 198 and SEQ ID NO: 325; SEQ ID NO: 198 and SEQ ID NO: 237; SEQ ID NO: 198 and SEQ ID NO: 238; SEQ ID NO: 198 and SEQ ID NO: 239; SEQ ID NO: 198 and SEQ ID NO: 240; SEQ ID NO: 198 and SEQ ID NO: 241; SEQ ID NO: 198 and SEQ ID NO: 242; SEQ ID NO: 198 and SEQ ID NO: 243; SEQ ID NO: 198 and SEQ ID NO: 244; SEQ ID NO: 198 and SEQ ID NO: 245; SEQ ID NO: 198 and SEQ ID NO: 246; SEQ ID NO: 198 and SEQ ID NO: 247; SEQ ID NO: 198 and SEQ ID NO: 248; SEQ ID NO: 198 and SEQ ID NO: 249; SEQ ID NO: 198 and SEQ ID NO: 250; SEQ ID NO: 198 and SEQ ID NO: 251; SEQ ID NO: 198 and SEQ ID NO: 252; SEQ ID NO: 198 and SEQ ID NO: 253; SEQ ID NO: 198 and SEQ ID NO: 254; SEQ ID NO: 198 and SEQ ID NO: 255; SEQ ID NO: 198 and SEQ ID NO: 256; SEQ ID NO: 198 and SEQ ID NO: 257; SEQ ID NO: 198 and SEQ ID NO: 258; SEQ ID NO: 198 and SEQ ID NO: 259; SEQ ID NO: 198 and SEQ ID NO: 260; SEQ ID NO: 198 and SEQ ID NO: 261; SEQ ID NO: 198 and SEQ ID NO: 262; SEQ ID NO: 198 and SEQ ID NO: 263; SEQ ID NO: 198 and SEQ ID NO: 264; SEQ ID NO: 198 and SEQ ID NO: 265; SEQ ID NO: 198 and SEQ ID NO: 266; SEQ ID NO: 198 and SEQ ID NO: 267; SEQ ID NO: 198 and SEQ ID NO: 268; and SEQ ID NO: 198 and SEQ ID NO: 269.
[0441] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 199 and SEQ ID NO: 236; SEQ ID NO: 199 and SEQ ID NO: 325; SEQ ID NO: 199 and SEQ ID NO: 237; SEQ ID NO: 199 and SEQ ID NO: 238; SEQ ID NO: 199 and SEQ ID NO: 239; SEQ ID NO: 199 and SEQ ID NO: 240; SEQ ID NO: 199 and SEQ ID NO: 241; SEQ ID NO: 199 and SEQ ID NO: 242; SEQ ID NO: 199 and SEQ ID NO: 243; SEQ ID NO: 199 and SEQ ID NO: 244; SEQ ID NO: 199 and SEQ ID NO: 245; SEQ ID NO: 199 and SEQ ID NO: 246; SEQ ID NO: 199 and SEQ ID NO: 247; SEQ ID NO: 199 and SEQ ID NO: 248; SEQ ID NO: 199 and SEQ ID NO: 249; SEQ ID NO: 199 and SEQ ID NO: 250; SEQ ID NO: 199 and SEQ ID NO: 251; SEQ ID NO: 199 and SEQ ID NO: 252; SEQ ID NO: 199 and SEQ ID NO: 253; SEQ ID NO: 199 and SEQ ID NO: 254; SEQ ID NO: 199 and SEQ ID NO: 255; SEQ ID NO: 199 and SEQ ID NO: 256; SEQ ID NO: 199 and SEQ ID NO: 257; SEQ ID NO: 199 and SEQ ID NO: 258; SEQ ID NO: 199 and SEQ ID NO: 259; SEQ ID NO: 199 and SEQ ID NO: 260; SEQ ID NO: 199 and SEQ ID NO: 261; SEQ ID NO: 199 and SEQ ID NO: 262; SEQ ID NO: 199 and SEQ ID NO: 263; SEQ ID NO: 199 and SEQ ID NO: 264; SEQ ID NO: 199 and SEQ ID NO: 265; SEQ ID NO: 199 and SEQ ID NO: 266; SEQ ID NO: 199 and SEQ ID NO: 267; SEQ ID NO: 199 and SEQ ID NO: 268; and SEQ ID NO: 199 and SEQ ID NO: 269.
[0442] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 200 and SEQ ID NO: 236; SEQ ID NO: 200 and SEQ ID NO: 325; SEQ ID NO: 200 and SEQ ID NO: 237; SEQ ID NO: 200 and SEQ ID NO: 238; SEQ ID NO: 200 and SEQ ID NO: 239; SEQ ID NO: 200 and SEQ ID NO: 240; SEQ ID NO: 200 and SEQ ID NO: 241; SEQ ID NO: 200 and SEQ ID NO: 242; SEQ ID NO: 200 and SEQ ID NO: 243; SEQ ID NO: 200 and SEQ ID NO: 244; SEQ ID NO: 200 and SEQ ID NO: 245; SEQ ID NO: 200 and SEQ ID NO: 246; SEQ ID NO: 200 and SEQ ID NO: 247; SEQ ID NO: 200 and SEQ ID NO: 248; SEQ ID NO: 200 and SEQ ID NO: 249; SEQ ID NO: 200 and SEQ ID NO: 250; SEQ ID NO: 200 and SEQ ID NO: 251; SEQ ID NO: 200 and SEQ ID NO: 252; SEQ ID NO: 200 and SEQ ID NO: 253; SEQ ID NO: 200 and SEQ ID NO: 254; SEQ ID NO: 200 and SEQ ID NO: 255; SEQ ID NO: 200 and SEQ ID NO: 256; SEQ ID NO: 200 and SEQ ID NO: 257; SEQ ID NO: 200 and SEQ ID NO: 258; SEQ ID NO: 200 and SEQ ID NO: 259; SEQ ID NO: 200 and SEQ ID NO: 260; SEQ ID NO: 200 and SEQ ID NO: 261; SEQ ID NO: 200 and SEQ ID NO: 262; SEQ ID NO: 200 and SEQ ID NO: 263; SEQ ID NO: 200 and SEQ ID NO: 264; SEQ ID NO: 200 and SEQ ID NO: 265; SEQ ID NO: 200 and SEQ ID NO: 266; SEQ ID NO: 200 and SEQ ID NO: 267; SEQ ID NO: 200 and SEQ ID NO: 268; and SEQ ID NO: 200 and SEQ ID NO: 269.
[0443] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 201 and SEQ ID NO: 236; SEQ ID NO: 201 and SEQ ID NO: 325; SEQ ID NO: 201 and SEQ ID NO: 237; SEQ ID NO: 201 and SEQ ID NO: 238; SEQ ID NO: 201 and SEQ ID NO: 239; SEQ ID NO: 201 and SEQ ID NO: 240; SEQ ID NO: 201 and SEQ ID NO: 241; SEQ ID NO: 201 and SEQ ID NO: 242; SEQ ID NO: 201 and SEQ ID NO: 243; SEQ ID NO: 201 and SEQ ID NO: 244; SEQ ID NO: 201 and SEQ ID NO: 245; SEQ ID NO: 201 and SEQ ID NO: 246; SEQ ID NO: 201 and SEQ ID NO: 247; SEQ ID NO: 201 and SEQ ID NO: 248; SEQ ID NO: 201 and SEQ ID NO: 249; SEQ ID NO: 201 and SEQ ID NO: 250; SEQ ID NO: 201 and SEQ ID NO: 251; SEQ ID NO: 201 and SEQ ID NO: 252; SEQ ID NO: 201 and SEQ ID NO: 253; SEQ ID NO: 201 and SEQ ID NO: 254; SEQ ID NO: 201 and SEQ ID NO: 255; SEQ ID NO: 201 and SEQ ID NO: 256; SEQ ID NO: 201 and SEQ ID NO: 257; SEQ ID NO: 201 and SEQ ID NO: 258; SEQ ID NO: 201 and SEQ ID NO: 259; SEQ ID NO: 201 and SEQ ID NO: 260; SEQ ID NO: 201 and SEQ ID NO: 261; SEQ ID NO: 201 and SEQ ID NO: 262; SEQ ID NO: 201 and SEQ ID NO: 263; SEQ ID NO: 201 and SEQ ID NO: 264; SEQ ID NO: 201 and SEQ ID NO: 265; SEQ ID NO: 201 and SEQ ID NO: 266; SEQ ID NO: 201 and SEQ ID NO: 267; SEQ ID NO: 201 and SEQ ID NO: 268; and SEQ ID NO: 201 and SEQ ID NO: 269.
[0444] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 202 and SEQ ID NO: 236; SEQ ID NO: 202 and SEQ ID NO: 325; SEQ ID NO: 202 and SEQ ID NO: 237; SEQ ID NO: 202 and SEQ ID NO: 238; SEQ ID NO: 202 and SEQ ID NO: 239; SEQ ID NO: 202 and SEQ ID NO: 240; SEQ ID NO: 202 and SEQ ID NO: 241; SEQ ID NO: 202 and SEQ ID NO: 242; SEQ ID NO: 202 and SEQ ID NO: 243; SEQ ID NO: 202 and SEQ ID NO: 244; SEQ ID NO: 202 and SEQ ID NO: 245; SEQ ID NO: 202 and SEQ ID NO: 246; SEQ ID NO: 202 and SEQ ID NO: 247; SEQ ID NO: 202 and SEQ ID NO: 248; SEQ ID NO: 202 and SEQ ID NO: 249; SEQ ID NO: 202 and SEQ ID NO: 250; SEQ ID NO: 202 and SEQ ID NO: 251; SEQ ID NO: 202 and SEQ ID NO: 252; SEQ ID NO: 202 and SEQ ID NO: 253; SEQ ID NO: 202 and SEQ ID NO: 254; SEQ ID NO: 202 and SEQ ID NO: 255; SEQ ID NO: 202 and SEQ ID NO: 256; SEQ ID NO: 202 and SEQ ID NO: 257; SEQ ID NO: 202 and SEQ ID NO: 258; SEQ ID NO: 202 and SEQ ID NO: 259; SEQ ID NO: 202 and SEQ ID NO: 260; SEQ ID NO: 202 and SEQ ID NO: 261; SEQ ID NO: 202 and SEQ ID NO: 262; SEQ ID NO: 202 and SEQ ID NO: 263; SEQ ID NO: 202 and SEQ ID NO: 264; SEQ ID NO: 202 and SEQ ID NO: 265; SEQ ID NO: 202 and SEQ ID NO: 266; SEQ ID NO: 202 and SEQ ID NO: 267; SEQ ID NO: 202 and SEQ ID NO: 268; and SEQ ID NO: 202 and SEQ ID NO: 269.
[0445] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 203 and SEQ ID NO: 236; SEQ ID NO: 203 and SEQ ID NO: 325; SEQ ID NO: 203 and SEQ ID NO: 237; SEQ ID NO: 203 and SEQ ID NO: 238; SEQ ID NO: 203 and SEQ ID NO: 239; SEQ ID NO: 203 and SEQ ID NO: 240; SEQ ID NO: 203 and SEQ ID NO: 241; SEQ ID NO: 203 and SEQ ID NO: 242; SEQ ID NO: 203 and SEQ ID NO: 243; SEQ ID NO: 203 and SEQ ID NO: 244; SEQ ID NO: 203 and SEQ ID NO: 245; SEQ ID NO: 203 and SEQ ID NO: 246; SEQ ID NO: 203 and SEQ ID NO: 247; SEQ ID NO: 203 and SEQ ID NO: 248; SEQ ID NO: 203 and SEQ ID NO: 249; SEQ ID NO: 203 and SEQ ID NO: 250; SEQ ID NO: 203 and SEQ ID NO: 251; SEQ ID NO: 203 and SEQ ID NO: 252; SEQ ID NO: 203 and SEQ ID NO: 253; SEQ ID NO: 203 and SEQ ID NO: 254; SEQ ID NO: 203 and SEQ ID NO: 255; SEQ ID NO: 203 and SEQ ID NO: 256; SEQ ID NO: 203 and SEQ ID NO: 257; SEQ ID NO: 203 and SEQ ID NO: 258; SEQ ID NO: 203 and SEQ ID NO: 259; SEQ ID NO: 203 and SEQ ID NO: 260; SEQ ID NO: 203 and SEQ ID NO: 261; SEQ ID NO: 203 and SEQ ID NO: 262; SEQ ID NO: 203 and SEQ ID NO: 263; SEQ ID NO: 203 and SEQ ID NO: 264; SEQ ID NO: 203 and SEQ ID NO: 265; SEQ ID NO: 203 and SEQ ID NO: 266; SEQ ID NO: 203 and SEQ ID NO: 267; SEQ ID NO: 203 and SEQ ID NO: 268; and SEQ ID NO: 203 and SEQ ID NO: 269.
[0446] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 204 and SEQ ID NO: 236; SEQ ID NO: 204 and SEQ ID NO: 325; SEQ ID NO: 204 and SEQ ID NO: 237; SEQ ID NO: 204 and SEQ ID NO: 238; SEQ ID NO: 204 and SEQ ID NO: 239; SEQ ID NO: 204 and SEQ ID NO: 240; SEQ ID NO: 204 and SEQ ID NO: 241; SEQ ID NO: 204 and SEQ ID NO: 242; SEQ ID NO: 204 and SEQ ID NO: 243; SEQ ID NO: 204 and SEQ ID NO: 244; SEQ ID NO: 204 and SEQ ID NO: 245; SEQ ID NO: 204 and SEQ ID NO: 246; SEQ ID NO: 204 and SEQ ID NO: 247; SEQ ID NO: 204 and SEQ ID NO: 248; SEQ ID NO: 204 and SEQ ID NO: 249; SEQ ID NO: 204 and SEQ ID NO: 250; SEQ ID NO: 204 and SEQ ID NO: 251; SEQ ID NO: 204 and SEQ ID NO: 252; SEQ ID NO: 204 and SEQ ID NO: 253; SEQ ID NO: 204 and SEQ ID NO: 254; SEQ ID NO: 204 and SEQ ID NO: 255; SEQ ID NO: 204 and SEQ ID NO: 256; SEQ ID NO: 204 and SEQ ID NO: 257; SEQ ID NO: 204 and SEQ ID NO: 258; SEQ ID NO: 204 and SEQ ID NO: 259; SEQ ID NO: 204 and SEQ ID NO: 260; SEQ ID NO: 204 and SEQ ID NO: 261; SEQ ID NO: 204 and SEQ ID NO: 262; SEQ ID NO: 204 and SEQ ID NO: 263; SEQ ID NO: 204 and SEQ ID NO: 264; SEQ ID NO: 204 and SEQ ID NO: 265; SEQ ID NO: 204 and SEQ ID NO: 266; SEQ ID NO: 204 and SEQ ID NO: 267; SEQ ID NO: 204 and SEQ ID NO: 268; and SEQ ID NO: 204 and SEQ ID NO: 269.
[0447] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 205 and SEQ ID NO: 236; SEQ ID NO: 205 and SEQ ID NO: 325; SEQ ID NO: 205 and SEQ ID NO: 237; SEQ ID NO: 205 and SEQ ID NO: 238; SEQ ID NO: 205 and SEQ ID NO: 239; SEQ ID NO: 205 and SEQ ID NO: 240; SEQ ID NO: 205 and SEQ ID NO: 241; SEQ ID NO: 205 and SEQ ID NO: 242; SEQ ID NO: 205 and SEQ ID NO: 243; SEQ ID NO: 205 and SEQ ID NO: 244; SEQ ID NO: 205 and SEQ ID NO: 245; SEQ ID NO: 205 and SEQ ID NO: 246; SEQ ID NO: 205 and SEQ ID NO: 247; SEQ ID NO: 205 and SEQ ID NO: 248; SEQ ID NO: 205 and SEQ ID NO: 249; SEQ ID NO: 205 and SEQ ID NO: 250; SEQ ID NO: 205 and SEQ ID NO: 251; SEQ ID NO: 205 and SEQ ID NO: 252; SEQ ID NO: 205 and SEQ ID NO: 253; SEQ ID NO: 205 and SEQ ID NO: 254; SEQ ID NO: 205 and SEQ ID NO: 255; SEQ ID NO: 205 and SEQ ID NO: 256; SEQ ID NO: 205 and SEQ ID NO: 257; SEQ ID NO: 205 and SEQ ID NO: 258; SEQ ID NO: 205 and SEQ ID NO: 259; SEQ ID NO: 205 and SEQ ID NO: 260; SEQ ID NO: 205 and SEQ ID NO: 261; SEQ ID NO: 205 and SEQ ID NO: 262; SEQ ID NO: 205 and SEQ ID NO: 263; SEQ ID NO: 205 and SEQ ID NO: 264; SEQ ID NO: 205 and SEQ ID NO: 265; SEQ ID NO: 205 and SEQ ID NO: 266; SEQ ID NO: 205 and SEQ ID NO: 267; SEQ ID NO: 205 and SEQ ID NO: 268; and SEQ ID NO: 205 and SEQ ID NO: 269.
[0448] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 206 and SEQ ID NO: 236; SEQ ID NO: 206 and SEQ ID NO: 325; SEQ ID NO: 206 and SEQ ID NO: 237; SEQ ID NO: 206 and SEQ ID NO: 238; SEQ ID NO: 206 and SEQ ID NO: 239; SEQ ID NO: 206 and SEQ ID NO: 240; SEQ ID NO: 206 and SEQ ID NO: 241; SEQ ID NO: 206 and SEQ ID NO: 242; SEQ ID NO: 206 and SEQ ID NO: 243; SEQ ID NO: 206 and SEQ ID NO: 244; SEQ ID NO: 206 and SEQ ID NO: 245; SEQ ID NO: 206 and SEQ ID NO: 246; SEQ ID NO: 206 and SEQ ID NO: 247; SEQ ID NO: 206 and SEQ ID NO: 248; SEQ ID NO: 206 and SEQ ID NO: 249; SEQ ID NO: 206 and SEQ ID NO: 250; SEQ ID NO: 206 and SEQ ID NO: 251; SEQ ID NO: 206 and SEQ ID NO: 252; SEQ ID NO: 206 and SEQ ID NO: 253; SEQ ID NO: 206 and SEQ ID NO: 254; SEQ ID NO: 206 and SEQ ID NO: 255; SEQ ID NO: 206 and SEQ ID NO: 256; SEQ ID NO: 206 and SEQ ID NO: 257; SEQ ID NO: 206 and SEQ ID NO: 258; SEQ ID NO: 206 and SEQ ID NO: 259; SEQ ID NO: 206 and SEQ ID NO: 260; SEQ ID NO: 206 and SEQ ID NO: 261; SEQ ID NO: 206 and SEQ ID NO: 262; SEQ ID NO: 206 and SEQ ID NO: 263; SEQ ID NO: 206 and SEQ ID NO: 264; SEQ ID NO: 206 and SEQ ID NO: 265; SEQ ID NO: 206 and SEQ ID NO: 266; SEQ ID NO: 206 and SEQ ID NO: 267; SEQ ID NO: 206 and SEQ ID NO: 268; and SEQ ID NO: 206 and SEQ ID NO: 269.
[0449] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 207 and SEQ ID NO: 236; SEQ ID NO: 207 and SEQ ID NO: 325; SEQ ID NO: 207 and SEQ ID NO: 237; SEQ ID NO: 207 and SEQ ID NO: 238; SEQ ID NO: 207 and SEQ ID NO: 239; SEQ ID NO: 207 and SEQ ID NO: 240; SEQ ID NO: 207 and SEQ ID NO: 241; SEQ ID NO: 207 and SEQ ID NO: 242; SEQ ID NO: 207 and SEQ ID NO: 243; SEQ ID NO: 207 and SEQ ID NO: 244; SEQ ID NO: 207 and SEQ ID NO: 245; SEQ ID NO: 207 and SEQ ID NO: 246; SEQ ID NO: 207 and SEQ ID NO: 247; SEQ ID NO: 207 and SEQ ID NO: 248; SEQ ID NO: 207 and SEQ ID NO: 249; SEQ ID NO: 207 and SEQ ID NO: 250; SEQ ID NO: 207 and SEQ ID NO: 251; SEQ ID NO: 207 and SEQ ID NO: 252; SEQ ID NO: 207 and SEQ ID NO: 253; SEQ ID NO: 207 and SEQ ID NO: 254; SEQ ID NO: 207 and SEQ ID NO: 255; SEQ ID NO: 207 and SEQ ID NO: 256; SEQ ID NO: 207 and SEQ ID NO: 257; SEQ ID NO: 207 and SEQ ID NO: 258; SEQ ID NO: 207 and SEQ ID NO: 259; SEQ ID NO: 207 and SEQ ID NO: 260; SEQ ID NO: 207 and SEQ ID NO: 261; SEQ ID NO: 207 and SEQ ID NO: 262; SEQ ID NO: 207 and SEQ ID NO: 263; SEQ ID NO: 207 and SEQ ID NO: 264; SEQ ID NO: 207 and SEQ ID NO: 265; SEQ ID NO: 207 and SEQ ID NO: 266; SEQ ID NO: 207 and SEQ ID NO: 267; SEQ ID NO: 207 and SEQ ID NO: 268; and SEQ ID NO: 207 and SEQ ID NO: 269.
[0450] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 208 and SEQ ID NO: 236; SEQ ID NO: 208 and SEQ ID NO: 325; SEQ ID NO: 208 and SEQ ID NO: 237; SEQ ID NO: 208 and SEQ ID NO: 238; SEQ ID NO: 208 and SEQ ID NO: 239; SEQ ID NO: 208 and SEQ ID NO: 240; SEQ ID NO: 208 and SEQ ID NO: 241; SEQ ID NO: 208 and SEQ ID NO: 242; SEQ ID NO: 208 and SEQ ID NO: 243; SEQ ID NO: 208 and SEQ ID NO: 244; SEQ ID NO: 208 and SEQ ID NO: 245; SEQ ID NO: 208 and SEQ ID NO: 246; SEQ ID NO: 208 and SEQ ID NO: 247; SEQ ID NO: 208 and SEQ ID NO: 248; SEQ ID NO: 208 and SEQ ID NO: 249; SEQ ID NO: 208 and SEQ ID NO: 250; SEQ ID NO: 208 and SEQ ID NO: 251; SEQ ID NO: 208 and SEQ ID NO: 252; SEQ ID NO: 208 and SEQ ID NO: 253; SEQ ID NO: 208 and SEQ ID NO: 254; SEQ ID NO: 208 and SEQ ID NO: 255; SEQ ID NO: 208 and SEQ ID NO: 256; SEQ ID NO: 208 and SEQ ID NO: 257; SEQ ID NO: 208 and SEQ ID NO: 258; SEQ ID NO: 208 and SEQ ID NO: 259; SEQ ID NO: 208 and SEQ ID NO: 260; SEQ ID NO: 208 and SEQ ID NO: 261; SEQ ID NO: 208 and SEQ ID NO: 262; SEQ ID NO: 208 and SEQ ID NO: 263; SEQ ID NO: 208 and SEQ ID NO: 264; SEQ ID NO: 208 and SEQ ID NO: 265; SEQ ID NO: 208 and SEQ ID NO: 266; SEQ ID NO: 208 and SEQ ID NO: 267; SEQ ID NO: 208 and SEQ ID NO: 268; and SEQ ID NO: 208 and SEQ ID NO: 269.
[0451] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 209 and SEQ ID NO: 236; SEQ ID NO: 209 and SEQ ID NO: 325; SEQ ID NO: 209 and SEQ ID NO: 237; SEQ ID NO: 209 and SEQ ID NO: 238; SEQ ID NO: 209 and SEQ ID NO: 239; SEQ ID NO: 209 and SEQ ID NO: 240; SEQ ID NO: 209 and SEQ ID NO: 241; SEQ ID NO: 209 and SEQ ID NO: 242; SEQ ID NO: 209 and SEQ ID NO: 243; SEQ ID NO: 209 and SEQ ID NO: 244; SEQ ID NO: 209 and SEQ ID NO: 245; SEQ ID NO: 209 and SEQ ID NO: 246; SEQ ID NO: 209 and SEQ ID NO: 247; SEQ ID NO: 209 and SEQ ID NO: 248; SEQ ID NO: 209 and SEQ ID NO: 249; SEQ ID NO: 209 and SEQ ID NO: 250; SEQ ID NO: 209 and SEQ ID NO: 251; SEQ ID NO: 209 and SEQ ID NO: 252; SEQ ID NO: 209 and SEQ ID NO: 253; SEQ ID NO: 209 and SEQ ID NO: 254; SEQ ID NO: 209 and SEQ ID NO: 255; SEQ ID NO: 209 and SEQ ID NO: 256; SEQ ID NO: 209 and SEQ ID NO: 257; SEQ ID NO: 209 and SEQ ID NO: 258; SEQ ID NO: 209 and SEQ ID NO: 259; SEQ ID NO: 209 and SEQ ID NO: 260; SEQ ID NO: 209 and SEQ ID NO: 261; SEQ ID NO: 209 and SEQ ID NO: 262; SEQ ID NO: 209 and SEQ ID NO: 263; SEQ ID NO: 209 and SEQ ID NO: 264; SEQ ID NO: 209 and SEQ ID NO: 265; SEQ ID NO: 209 and SEQ ID NO: 266; SEQ ID NO: 209 and SEQ ID NO: 267; SEQ ID NO: 209 and SEQ ID NO: 268; and SEQ ID NO: 209 and SEQ ID NO: 269.
[0452] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 210 and SEQ ID NO: 236; SEQ ID NO: 210 and SEQ ID NO: 325; SEQ ID NO: 210 and SEQ ID NO: 237; SEQ ID NO: 210 and SEQ ID NO: 238; SEQ ID NO: 210 and SEQ ID NO: 239; SEQ ID NO: 210 and SEQ ID NO: 240; SEQ ID NO: 210 and SEQ ID NO: 241; SEQ ID NO: 210 and SEQ ID NO: 242; SEQ ID NO: 210 and SEQ ID NO: 243; SEQ ID NO: 210 and SEQ ID NO: 244; SEQ ID NO: 210 and SEQ ID NO: 245; SEQ ID NO: 210 and SEQ ID NO: 246; SEQ ID NO: 210 and SEQ ID NO: 247; SEQ ID NO: 210 and SEQ ID NO: 248; SEQ ID NO: 210 and SEQ ID NO: 249; SEQ ID NO: 210 and SEQ ID NO: 250; SEQ ID NO: 210 and SEQ ID NO: 251; SEQ ID NO: 210 and SEQ ID NO: 252; SEQ ID NO: 210 and SEQ ID NO: 253; SEQ ID NO: 210 and SEQ ID NO: 254; SEQ ID NO: 210 and SEQ ID NO: 255; SEQ ID NO: 210 and SEQ ID NO: 256; SEQ ID NO: 210 and SEQ ID NO: 257; SEQ ID NO: 210 and SEQ ID NO: 258; SEQ ID NO: 210 and SEQ ID NO: 259; SEQ ID NO: 210 and SEQ ID NO: 260; SEQ ID NO: 210 and SEQ ID NO: 261; SEQ ID NO: 210 and SEQ ID NO: 262; SEQ ID NO: 210 and SEQ ID NO: 263; SEQ ID NO: 210 and SEQ ID NO: 264; SEQ ID NO: 210 and SEQ ID NO: 265; SEQ ID NO: 210 and SEQ ID NO: 266; SEQ ID NO: 210 and SEQ ID NO: 267; SEQ ID NO: 210 and SEQ ID NO: 268; and SEQ ID NO: 210 and SEQ ID NO: 269.
[0453] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 211 and SEQ ID NO: 236; SEQ ID NO: 211 and SEQ ID NO: 325; SEQ ID NO: 211 and SEQ ID NO: 237; SEQ ID NO: 211 and SEQ ID NO: 238; SEQ ID NO: 211 and SEQ ID NO: 239; SEQ ID NO: 211 and SEQ ID NO: 240; SEQ ID NO: 211 and SEQ ID NO: 241; SEQ ID NO: 211 and SEQ ID NO: 242; SEQ ID NO: 211 and SEQ ID NO: 243; SEQ ID NO: 211 and SEQ ID NO: 244; SEQ ID NO: 211 and SEQ ID NO: 245; SEQ ID NO: 211 and SEQ ID NO: 246; SEQ ID NO: 211 and SEQ ID NO: 247; SEQ ID NO: 211 and SEQ ID NO: 248; SEQ ID NO: 211 and SEQ ID NO: 249; SEQ ID NO: 211 and SEQ ID NO: 250; SEQ ID NO: 211 and SEQ ID NO: 251; SEQ ID NO: 211 and SEQ ID NO: 252; SEQ ID NO: 211 and SEQ ID NO: 253; SEQ ID NO: 211 and SEQ ID NO: 254; SEQ ID NO: 211 and SEQ ID NO: 255; SEQ ID NO: 211 and SEQ ID NO: 256; SEQ ID NO: 211 and SEQ ID NO: 257; SEQ ID NO: 211 and SEQ ID NO: 258; SEQ ID NO: 211 and SEQ ID NO: 259; SEQ ID NO: 211 and SEQ ID NO: 260; SEQ ID NO: 211 and SEQ ID NO: 261; SEQ ID NO: 211 and SEQ ID NO: 262; SEQ ID NO: 211 and SEQ ID NO: 263; SEQ ID NO: 211 and SEQ ID NO: 264; SEQ ID NO: 211 and SEQ ID NO: 265; SEQ ID NO: 211 and SEQ ID NO: 266; SEQ ID NO: 211 and SEQ ID NO: 267; SEQ ID NO: 211 and SEQ ID NO: 268; and SEQ ID NO: 211 and SEQ ID NO: 269.
[0454] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 212 and SEQ ID NO: 236; SEQ ID NO: 212 and SEQ ID NO: 325; SEQ ID NO: 212 and SEQ ID NO: 237; SEQ ID NO: 212 and SEQ ID NO: 238; SEQ ID NO: 212 and SEQ ID NO: 239; SEQ ID NO: 212 and SEQ ID NO: 240; SEQ ID NO: 212 and SEQ ID NO: 241; SEQ ID NO: 212 and SEQ ID NO: 242; SEQ ID NO: 212 and SEQ ID NO: 243; SEQ ID NO: 212 and SEQ ID NO: 244; SEQ ID NO: 212 and SEQ ID NO: 245; SEQ ID NO: 212 and SEQ ID NO: 246; SEQ ID NO: 212 and SEQ ID NO: 247; SEQ ID NO: 212 and SEQ ID NO: 248; SEQ ID NO: 212 and SEQ ID NO: 249; SEQ ID NO: 212 and SEQ ID NO: 250; SEQ ID NO: 212 and SEQ ID NO: 251; SEQ ID NO: 212 and SEQ ID NO: 252; SEQ ID NO: 212 and SEQ ID NO: 253; SEQ ID NO: 212 and SEQ ID NO: 254; SEQ ID NO: 212 and SEQ ID NO: 255; SEQ ID NO: 212 and SEQ ID NO: 256; SEQ ID NO: 212 and SEQ ID NO: 257; SEQ ID NO: 212 and SEQ ID NO: 258; SEQ ID NO: 212 and SEQ ID NO: 259; SEQ ID NO: 212 and SEQ ID NO: 260; SEQ ID NO: 212 and SEQ ID NO: 261; SEQ ID NO: 212 and SEQ ID NO: 262; SEQ ID NO: 212 and SEQ ID NO: 263; SEQ ID NO: 212 and SEQ ID NO: 264; SEQ ID NO: 212 and SEQ ID NO: 265; SEQ ID NO: 212 and SEQ ID NO: 266; SEQ ID NO: 212 and SEQ ID NO: 267; SEQ ID NO: 212 and SEQ ID NO: 268; and SEQ ID NO: 212 and SEQ ID NO: 269.
[0455] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 213 and SEQ ID NO: 236; SEQ ID NO: 213 and SEQ ID NO: 325; SEQ ID NO: 213 and SEQ ID NO: 237; SEQ ID NO: 213 and SEQ ID NO: 238; SEQ ID NO: 213 and SEQ ID NO: 239; SEQ ID NO: 213 and SEQ ID NO: 240; SEQ ID NO: 213 and SEQ ID NO: 241; SEQ ID NO: 213 and SEQ ID NO: 242; SEQ ID NO: 213 and SEQ ID NO: 243; SEQ ID NO: 213 and SEQ ID NO: 244; SEQ ID NO: 213 and SEQ ID NO: 245; SEQ ID NO: 213 and SEQ ID NO: 246; SEQ ID NO: 213 and SEQ ID NO: 247; SEQ ID NO: 213 and SEQ ID NO: 248; SEQ ID NO: 213 and SEQ ID NO: 249; SEQ ID NO: 213 and SEQ ID NO: 250; SEQ ID NO: 213 and SEQ ID NO: 251; SEQ ID NO: 213 and SEQ ID NO: 252; SEQ ID NO: 213 and SEQ ID NO: 253; SEQ ID NO: 213 and SEQ ID NO: 254; SEQ ID NO: 213 and SEQ ID NO: 255; SEQ ID NO: 213 and SEQ ID NO: 256; SEQ ID NO: 213 and SEQ ID NO: 257; SEQ ID NO: 213 and SEQ ID NO: 258; SEQ ID NO: 213 and SEQ ID NO: 259; SEQ ID NO: 213 and SEQ ID NO: 260; SEQ ID NO: 213 and SEQ ID NO: 261; SEQ ID NO: 213 and SEQ ID NO: 262; SEQ ID NO: 213 and SEQ ID NO: 263; SEQ ID NO: 213 and SEQ ID NO: 264; SEQ ID NO: 213 and SEQ ID NO: 265; SEQ ID NO: 213 and SEQ ID NO: 266; SEQ ID NO: 213 and SEQ ID NO: 267; SEQ ID NO: 213 and SEQ ID NO: 268; and SEQ ID NO: 213 and SEQ ID NO: 269.
[0456] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 214 and SEQ ID NO: 236; SEQ ID NO: 214 and SEQ ID NO: 325; SEQ ID NO: 214 and SEQ ID NO: 237; SEQ ID NO: 214 and SEQ ID NO: 238; SEQ ID NO: 214 and SEQ ID NO: 239; SEQ ID NO: 214 and SEQ ID NO: 240; SEQ ID NO: 214 and SEQ ID NO: 241; SEQ ID NO: 214 and SEQ ID NO: 242; SEQ ID NO: 214 and SEQ ID NO: 243; SEQ ID NO: 214 and SEQ ID NO: 244; SEQ ID NO: 214 and SEQ ID NO: 245; SEQ ID NO: 214 and SEQ ID NO: 246; SEQ ID NO: 214 and SEQ ID NO: 247; SEQ ID NO: 214 and SEQ ID NO: 248; SEQ ID NO: 214 and SEQ ID NO: 249; SEQ ID NO: 214 and SEQ ID NO: 250; SEQ ID NO: 214 and SEQ ID NO: 251; SEQ ID NO: 214 and SEQ ID NO: 252; SEQ ID NO: 214 and SEQ ID NO: 253; SEQ ID NO: 214 and SEQ ID NO: 254; SEQ ID NO: 214 and SEQ ID NO: 255; SEQ ID NO: 214 and SEQ ID NO: 256; SEQ ID NO: 214 and SEQ ID NO: 257; SEQ ID NO: 214 and SEQ ID NO: 258; SEQ ID NO: 214 and SEQ ID NO: 259; SEQ ID NO: 214 and SEQ ID NO: 260; SEQ ID NO: 214 and SEQ ID NO: 261; SEQ ID NO: 214 and SEQ ID NO: 262; SEQ ID NO: 214 and SEQ ID NO: 263; SEQ ID NO: 214 and SEQ ID NO: 264; SEQ ID NO: 214 and SEQ ID NO: 265; SEQ ID NO: 214 and SEQ ID NO: 266; SEQ ID NO: 214 and SEQ ID NO: 267; SEQ ID NO: 214 and SEQ ID NO: 268; and SEQ ID NO: 214 and SEQ ID NO: 269.
[0457] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 215 and SEQ ID NO: 236; SEQ ID NO: 215 and SEQ ID NO: 325; SEQ ID NO: 215 and SEQ ID NO: 237; SEQ ID NO: 215 and SEQ ID NO: 238; SEQ ID NO: 215 and SEQ ID NO: 239; SEQ ID NO: 215 and SEQ ID NO: 240; SEQ ID NO: 215 and SEQ ID NO: 241; SEQ ID NO: 215 and SEQ ID NO: 242; SEQ ID NO: 215 and SEQ ID NO: 243; SEQ ID NO: 215 and SEQ ID NO: 244; SEQ ID NO: 215 and SEQ ID NO: 245; SEQ ID NO: 215 and SEQ ID NO: 246; SEQ ID NO: 215 and SEQ ID NO: 247; SEQ ID NO: 215 and SEQ ID NO: 248; SEQ ID NO: 215 and SEQ ID NO: 249; SEQ ID NO: 215 and SEQ ID NO: 250; SEQ ID NO: 215 and SEQ ID NO: 251; SEQ ID NO: 215 and SEQ ID NO: 252; SEQ ID NO: 215 and SEQ ID NO: 253; SEQ ID NO: 215 and SEQ ID NO: 254; SEQ ID NO: 215 and SEQ ID NO: 255; SEQ ID NO: 215 and SEQ ID NO: 256; SEQ ID NO: 215 and SEQ ID NO: 257; SEQ ID NO: 215 and SEQ ID NO: 258; SEQ ID NO: 215 and SEQ ID NO: 259; SEQ ID NO: 215 and SEQ ID NO: 260; SEQ ID NO: 215 and SEQ ID NO: 261; SEQ ID NO: 215 and SEQ ID NO: 262; SEQ ID NO: 215 and SEQ ID NO: 263; SEQ ID NO: 215 and SEQ ID NO: 264; SEQ ID NO: 215 and SEQ ID NO: 265; SEQ ID NO: 215 and SEQ ID NO: 266; SEQ ID NO: 215 and SEQ ID NO: 267; SEQ ID NO: 215 and SEQ ID NO: 268; and SEQ ID NO: 215 and SEQ ID NO: 269.
[0458] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 216 and SEQ ID NO: 236; SEQ ID NO: 216 and SEQ ID NO: 325; SEQ ID NO: 216 and SEQ ID NO: 237; SEQ ID NO: 216 and SEQ ID NO: 238; SEQ ID NO: 216 and SEQ ID NO: 239; SEQ ID NO: 216 and SEQ ID NO: 240; SEQ ID NO: 216 and SEQ ID NO: 241; SEQ ID NO: 216 and SEQ ID NO: 242; SEQ ID NO: 216 and SEQ ID NO: 243; SEQ ID NO: 216 and SEQ ID NO: 244; SEQ ID NO: 216 and SEQ ID NO: 245; SEQ ID NO: 216 and SEQ ID NO: 246; SEQ ID NO: 216 and SEQ ID NO: 247; SEQ ID NO: 216 and SEQ ID NO: 248; SEQ ID NO: 216 and SEQ ID NO: 249; SEQ ID NO: 216 and SEQ ID NO: 250; SEQ ID NO: 216 and SEQ ID NO: 251; SEQ ID NO: 216 and SEQ ID NO: 252; SEQ ID NO: 216 and SEQ ID NO: 253; SEQ ID NO: 216 and SEQ ID NO: 254; SEQ ID NO: 216 and SEQ ID NO: 255; SEQ ID NO: 216 and SEQ ID NO: 256; SEQ ID NO: 216 and SEQ ID NO: 257; SEQ ID NO: 216 and SEQ ID NO: 258; SEQ ID NO: 216 and SEQ ID NO: 259; SEQ ID NO: 216 and SEQ ID NO: 260; SEQ ID NO: 216 and SEQ ID NO: 261; SEQ ID NO: 216 and SEQ ID NO: 262; SEQ ID NO: 216 and SEQ ID NO: 263; SEQ ID NO: 216 and SEQ ID NO: 264; SEQ ID NO: 216 and SEQ ID NO: 265; SEQ ID NO: 216 and SEQ ID NO: 266; SEQ ID NO: 216 and SEQ ID NO: 267; SEQ ID NO: 216 and SEQ ID NO: 268; and SEQ ID NO: 216 and SEQ ID NO: 269.
[0459] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 217 and SEQ ID NO: 236; SEQ ID NO: 217 and SEQ ID NO: 325; SEQ ID NO: 217 and SEQ ID NO: 237; SEQ ID NO: 217 and SEQ ID NO: 238; SEQ ID NO: 217 and SEQ ID NO: 239; SEQ ID NO: 217 and SEQ ID NO: 240; SEQ ID NO: 217 and SEQ ID NO: 241; SEQ ID NO: 217 and SEQ ID NO: 242; SEQ ID NO: 217 and SEQ ID NO: 243; SEQ ID NO: 217 and SEQ ID NO: 244; SEQ ID NO: 217 and SEQ ID NO: 245; SEQ ID NO: 217 and SEQ ID NO: 246; SEQ ID NO: 217 and SEQ ID NO: 247; SEQ ID NO: 217 and SEQ ID NO: 248; SEQ ID NO: 217 and SEQ ID NO: 249; SEQ ID NO: 217 and SEQ ID NO: 250; SEQ ID NO: 217 and SEQ ID NO: 251; SEQ ID NO: 217 and SEQ ID NO: 252; SEQ ID NO: 217 and SEQ ID NO: 253; SEQ ID NO: 217 and SEQ ID NO: 254; SEQ ID NO: 217 and SEQ ID NO: 255; SEQ ID NO: 217 and SEQ ID NO: 256; SEQ ID NO: 217 and SEQ ID NO: 257; SEQ ID NO: 217 and SEQ ID NO: 258; SEQ ID NO: 217 and SEQ ID NO: 259; SEQ ID NO: 217 and SEQ ID NO: 260; SEQ ID NO: 217 and SEQ ID NO: 261; SEQ ID NO: 217 and SEQ ID NO: 262; SEQ ID NO: 217 and SEQ ID NO: 263; SEQ ID NO: 217 and SEQ ID NO: 264; SEQ ID NO: 217 and SEQ ID NO: 265; SEQ ID NO: 217 and SEQ ID NO: 266; SEQ ID NO: 217 and SEQ ID NO: 267; SEQ ID NO: 217 and SEQ ID NO: 268; and SEQ ID NO: 217 and SEQ ID NO: 269.
[0460] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 218 and SEQ ID NO: 236; SEQ ID NO: 218 and SEQ ID NO: 325; SEQ ID NO: 218 and SEQ ID NO: 237; SEQ ID NO: 218 and SEQ ID NO: 238; SEQ ID NO: 218 and SEQ ID NO: 239; SEQ ID NO: 218 and SEQ ID NO: 240; SEQ ID NO: 218 and SEQ ID NO: 241; SEQ ID NO: 218 and SEQ ID NO: 242; SEQ ID NO: 218 and SEQ ID NO: 243; SEQ ID NO: 218 and SEQ ID NO: 244; SEQ ID NO: 218 and SEQ ID NO: 245; SEQ ID NO: 218 and SEQ ID NO: 246; SEQ ID NO: 218 and SEQ ID NO: 247; SEQ ID NO: 218 and SEQ ID NO: 248; SEQ ID NO: 218 and SEQ ID NO: 249; SEQ ID NO: 218 and SEQ ID NO: 250; SEQ ID NO: 218 and SEQ ID NO: 251; SEQ ID NO: 218 and SEQ ID NO: 252; SEQ ID NO: 218 and SEQ ID NO: 253; SEQ ID NO: 218 and SEQ ID NO: 254; SEQ ID NO: 218 and SEQ ID NO: 255; SEQ ID NO: 218 and SEQ ID NO: 256; SEQ ID NO: 218 and SEQ ID NO: 257; SEQ ID NO: 218 and SEQ ID NO: 258; SEQ ID NO: 218 and SEQ ID NO: 259; SEQ ID NO: 218 and SEQ ID NO: 260; SEQ ID NO: 218 and SEQ ID NO: 261; SEQ ID NO: 218 and SEQ ID NO: 262; SEQ ID NO: 218 and SEQ ID NO: 263; SEQ ID NO: 218 and SEQ ID NO: 264; SEQ ID NO: 218 and SEQ ID NO: 265; SEQ ID NO: 218 and SEQ ID NO: 266; SEQ ID NO: 218 and SEQ ID NO: 267; SEQ ID NO: 218 and SEQ ID NO: 268; and SEQ ID NO: 218 and SEQ ID NO: 269.
[0461] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 219 and SEQ ID NO: 236; SEQ ID NO: 219 and SEQ ID NO: 325; SEQ ID NO: 219 and SEQ ID NO: 237; SEQ ID NO: 219 and SEQ ID NO: 238; SEQ ID NO: 219 and SEQ ID NO: 239; SEQ ID NO: 219 and SEQ ID NO: 240; SEQ ID NO: 219 and SEQ ID NO: 241; SEQ ID NO: 219 and SEQ ID NO: 242; SEQ ID NO: 219 and SEQ ID NO: 243; SEQ ID NO: 219 and SEQ ID NO: 244; SEQ ID NO: 219 and SEQ ID NO: 245; SEQ ID NO: 219 and SEQ ID NO: 246; SEQ ID NO: 219 and SEQ ID NO: 247; SEQ ID NO: 219 and SEQ ID NO: 248; SEQ ID NO: 219 and SEQ ID NO: 249; SEQ ID NO: 219 and SEQ ID NO: 250; SEQ ID NO: 219 and SEQ ID NO: 251; SEQ ID NO: 219 and SEQ ID NO: 252; SEQ ID NO: 219 and SEQ ID NO: 253; SEQ ID NO: 219 and SEQ ID NO: 254; SEQ ID NO: 219 and SEQ ID NO: 255; SEQ ID NO: 219 and SEQ ID NO: 256; SEQ ID NO: 219 and SEQ ID NO: 257; SEQ ID NO: 219 and SEQ ID NO: 258; SEQ ID NO: 219 and SEQ ID NO: 259; SEQ ID NO: 219 and SEQ ID NO: 260; SEQ ID NO: 219 and SEQ ID NO: 261; SEQ ID NO: 219 and SEQ ID NO: 262; SEQ ID NO: 219 and SEQ ID NO: 263; SEQ ID NO: 219 and SEQ ID NO: 264; SEQ ID NO: 219 and SEQ ID NO: 265; SEQ ID NO: 219 and SEQ ID NO: 266; SEQ ID NO: 219 and SEQ ID NO: 267; SEQ ID NO: 219 and SEQ ID NO: 268; and SEQ ID NO: 219 and SEQ ID NO: 269.
[0462] In some aspects, the V.sub.H-V.sub.L pairs are selected from SEQ ID NO: 220 and SEQ ID NO: 236; SEQ ID NO: 220 and SEQ ID NO: 325; SEQ ID NO: 220 and SEQ ID NO: 237; SEQ ID NO: 220 and SEQ ID NO: 238; SEQ ID NO: 220 and SEQ ID NO: 239; SEQ ID NO: 220 and SEQ ID NO: 240; SEQ ID NO: 220 and SEQ ID NO: 241; SEQ ID NO: 220 and SEQ ID NO: 242; SEQ ID NO: 220 and SEQ ID NO: 243; SEQ ID NO: 220 and SEQ ID NO: 244; SEQ ID NO: 220 and SEQ ID NO: 245; SEQ ID NO: 220 and SEQ ID NO: 246; SEQ ID NO: 220 and SEQ ID NO: 247; SEQ ID NO: 220 and SEQ ID NO: 248; SEQ ID NO: 220 and SEQ ID NO: 249; SEQ ID NO: 220 and SEQ ID NO: 250; SEQ ID NO: 220 and SEQ ID NO: 251; SEQ ID NO: 220 and SEQ ID NO: 252; SEQ ID NO: 220 and SEQ ID NO: 253; SEQ ID NO: 220 and SEQ ID NO: 254; SEQ ID NO: 220 and SEQ ID NO: 255; SEQ ID NO: 220 and SEQ ID NO: 256; SEQ ID NO: 220 and SEQ ID NO: 257; SEQ ID NO: 220 and SEQ ID NO: 258; SEQ ID NO: 220 and SEQ ID NO: 259; SEQ ID NO: 220 and SEQ ID NO: 260; SEQ ID NO: 220 and SEQ ID NO: 261; SEQ ID NO: 220 and SEQ ID NO: 262; SEQ ID NO: 220 and SEQ ID NO: 263; SEQ ID NO: 220 and SEQ ID NO: 264; SEQ ID NO: 220 and SEQ ID NO: 265; SEQ ID NO: 220 and SEQ ID NO: 266; SEQ ID NO: 220 and SEQ ID NO: 267; SEQ ID NO: 220 and SEQ ID NO: 268; and SEQ ID NO: 220 and SEQ ID NO: 269.
[0463] 3.7.4.1 Variants of V.sub.H-V.sub.L Pairs
[0464] In some embodiments, the V.sub.H-V.sub.L pairs provided herein comprise a variant of an illustrative V.sub.H and/or V.sub.L sequence provided in this disclosure.
[0465] In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.H sequence provided in this disclosure. In some aspects, the V.sub.H sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.1% identity with any of the illustrative V.sub.H sequences provided in this disclosure.
[0466] In some embodiments, the V.sub.H sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.H sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0467] In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a variant of an illustrative V.sub.L sequence provided in this disclosure. In some aspects, the V.sub.L sequence comprises, consists of, or consists essentially of a sequence having at least 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity with any of the illustrative V.sub.L sequences provided in this disclosure.
[0468] In some embodiments, the V.sub.L sequence comprises, consists of, or consists essentially of any of the illustrative V.sub.L sequences provided in this disclosure having 20 or fewer, 19 or fewer, 18 or fewer, 17 or fewer, 16 or fewer, 15 or fewer, 14 or fewer, 13 or fewer, 12 or fewer, 11 or fewer, 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 or fewer amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0469] 3.8 PD-1 Antibodies Comprising all Six CDRs
[0470] In some embodiments, the PD-1 antibody comprises a CDR-H1 sequence, a CDR-H2 sequence, a CDR-H3 sequence, a CDR-L1 sequence, a CDR-L2 sequence, and a CDR-L3 sequence. In some aspects, the CDR sequences are part of a V.sub.H (for CDR-H) or V.sub.L (for CDR-L).
[0471] In some aspects, the CDR-H1 sequence is a Chothia CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 24-38 or a Chothia CDR-H1 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220; the CDR-H2 sequence is a Chothia CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 81 or a Chothia CDR-H2 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220; the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119 or a CDR-H3 sequence of a V.sub.H sequence provided in any one of SEQ ID NOs.: 181-220; the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124 or a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269; the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 134-139 or a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269; and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143 or a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269.
[0472] In some aspects, the CDR-H1 sequence is a Kabat CDR-H1 sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 57-62 or a Kabat CDR-H1 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220; the CDR-H2 sequence is a Kabat CDR-H2 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 110 or a Kabat CDR-H2 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220; the CDR-H3 sequence is a CDR-H3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 119 or a Kabat CDR-H3 sequence of a V.sub.H sequence selected from SEQ ID NOs.: 181-220; the CDR-L1 sequence is a CDR-L1 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 124 or a CDR-L1 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269; the CDR-L2 sequence is a CDR-L2 sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 134-139 or a CDR-L2 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269; and the CDR-L3 sequence is a CDR-L3 sequence comprising, consisting of, or consisting essentially of SEQ ID NO: 143 or or a CDR-L3 sequence of a V.sub.L sequence selected from SEQ ID NOs.: 236-269.
[0473] 3.8.1 Variants of Antibodies Comprising all Six CDRs
[0474] In some embodiments, the CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, and CDR-L3 provided herein comprise a variant of an illustrative CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, and/or CDR-L3 sequence provided in this disclosure.
[0475] In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia or Kabat CDR-H1 sequence provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia or Kabat CDR-H1 sequences provided in this disclosure. In some aspects, the CDR-H1 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia or Kabat CDR-H1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0476] In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a variant of an illustrative Chothia or Kabat CDR-H2 sequence provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative Chothia or Kabat CDR-H2 sequences provided in this disclosure. In some aspects, the CDR-H2 sequence comprises, consists of, or consists essentially of any of the illustrative Chothia or Kabat CDR-H2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0477] In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-H3 sequence provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-H3 sequences provided in this disclosure. In some aspects, the CDR-H3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-H3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0478] In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L1 sequence provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L1 sequences provided in this disclosure. In some aspects, the CDR-L1 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L1 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0479] In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L2 sequence provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L2 sequences provided in this disclosure. In some aspects, the CDR-L2 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L2 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
[0480] In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a variant of an illustrative CDR-L3 sequence provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of a sequence having at least 70%, 75%, 80%, 85%, 90%, or 95% identity with any of the illustrative CDR-L3 sequences provided in this disclosure. In some aspects, the CDR-L3 sequence comprises, consists of, or consists essentially of any of the illustrative CDR-L3 sequences provided in this disclosure, with 1, 2, or 3 amino acid substitutions. In some aspects, the amino acid substitutions are conservative amino acid substitutions.
4. Bi-Specific Antibodies and Antigen-Binding Constructs
[0481] Provided herein are bi-specific antigen-binding constructs, e.g., antibodies, that bind Tim-3 and PD-1. The bi-specific antigen-binding construct includes two antigen-binding polypeptide constructs, e.g., antigen binding domains, that specifically binding to Tim-3 and/or PD-1. In some embodiments, the antigen-binding construct is derived from known antibodies or antigen-binding constructs. In some embodiments, the antigen-binding polypeptide constructs comprise two antigen binding domains that comprise antibody fragments. In some embodiments, the first antigen binding domain and second antigen binding domain each independently comprises an antibody fragment selected from the group of: an scFv, a Fab, and an Fc domain. The antibody fragments may be the same format or different formats from each other. For example, in some embodiments, the antigen-binding polypeptide constructs comprise a first antigen binding domain comprising an scFv and a second antigen binding domain comprising a Fab. In some embodiments, the antigen-binding polypeptide constructs comprise a first antigen binding domain and a second antigen binding domain, wherein both antigen binding domains comprise an scFv. In other embodiments, the first and second antigen binding domains each comprise a Fab. In some embodiments, the first and second antigen binding domains each comprise an Fc domain. Any combination of antibody formats is suitable for the bi-specific antibody constructs disclosed herein.
[0482] In some embodiments, in the bi-specific antibodies disclosed herein, the first and second antigen-binding polypeptide constructs independently comprise different light chains. For example, in some embodiments, the first antigen-binding polypeptide construct comprises a V.sub.L sequence selected from any one of SEQ ID NOs: 221-235, and the second antigen-binding polypeptide construct comprises a V.sub.L sequence selected from any one of SEQ ID NOs: 236-269. In some embodiments, the first antigen-binding polypeptide construct comprises a V.sub.L sequence selected from any one of SEQ ID NOs: 236-269, and the second antigen-binding polypeptide construct comprises a V.sub.L sequence selected from any one of SEQ ID NOs: 221-235. In some embodiments, the first and second antigen-binding polypeptide constructs comprise the same light chain. For example, in some embodiments, the first and second antigen-binding polypeptide constructs comprise a same V.sub.L sequence selected from any one of SEQ ID NOs: 221-269. In some embodiments, the first and second antigen-binding polypeptide constructs further comprise a C.sub.L sequence selected from any one of SEQ ID NOs: 327-338 and 346-347. In some embodiments, the first and second antigen-binding polypeptide constructs comprise the same C.sub.L sequence. In some embodiments, the first and second antigen-binding polypeptide constructs comprise different C.sub.L sequences.
[0483] The term "antigen-binding construct" refers to any agent, e.g., polypeptide or polypeptide complex capable of binding to an antigen. In some aspects an antigen-binding construct is a polypeptide that specifically binds to an antigen of interest. An antigen-binding construct can be a monomer, dimer, multimer, a protein, a peptide, or a protein or peptide complex; an antibody, an antibody fragment, or an antigen-binding fragment thereof; an scFv and the like. An antigen-binding construct can be a polypeptide construct that is monospecific, bi-specific, or multispecific. In some aspects, an antigen-binding construct can include, e.g., one or more antigen-binding components (e.g., Fabs or scFvs) linked to one or more Fc. Further examples of antigen-binding constructs are described below and provided in the Examples.
[0484] The term "bi-specific" includes any agent, e.g., an antigen-binding construct, which has two antigen-binding moieties (e.g. antigen-binding polypeptide constructs), each with a unique binding specificity. For example, a first antigen-binding moiety binds to an epitope on a first antigen, and a second antigen-binding moiety binds to an epitope on a second antigen, where the first antigen is different from the second antigen.
[0485] For example, in some embodiments, a bi-specific agent can bind to, or interact with, (a) a cell surface target molecule and (b) an Fc receptor on the surface of an effector cell. In some embodiments, the agent can bind to, or interact with (a) a first cell surface target molecule and (b) a second cell surface target molecule that is different from the first cell surface target molecule. In some embodiments, the agent can bind to and bridge two cells, i.e. interact with (a) a first cell surface target molecule on a first call and (b) a second cell surface target molecule on a second cell that is different from the first cells surface target molecule on the first cell.
[0486] In contrast, a monospecific antigen-binding construct refers to an antigen-binding construct with a single binding specificity. In other words, both antigen-binding moieties bind to the same epitope on the same antigen. Examples of monospecific antigen-binding constructs include the anti-CD19 antibody HD37 and the anti-CD3 antibody OKT3 for example.
[0487] An antigen-binding construct can be an antibody or antigen-binding portion thereof as disclosed herein.
[0488] Methods of generating bi-specific antibodies and bi-specific antigen-binding constructs are described, for example, in Brinkmann, U., and R. E. Kontermann. 2017. mAbs 9(2):182-212; and in Yang, et al. 2017. Int. J. Mol. Sci. 18(1):48 (21 pages), each of which is incorporated herein by reference in its entirety.
[0489] 4.1 Antigen-Binding Polypeptide Construct-Format
[0490] The bi-specific antigen-binding construct comprises at least two antigen-binding polypeptide constructs, e.g., antigen binding domains. The format of the antigen-binding polypeptide construct determines certain functional characteristics of the bi-specific antigen-binding construct. In some embodiments, the bi-specific antigen-binding construct has an scFv-scFv format, i.e. both antigen-binding polypeptide constructs are scFvs. In some embodiments, the bi-specific antigen-binding construct has a Fab-Fab format, i.e. both antigen-binding polypeptide constructs are Fabs. In some embodiments, the bi-specific antigen-binding construct has an scFv-Fab format, i.e. a first antigen-binding polypeptide construct is an scFv, and a second antigen-binding polypeptide construct is an Fab. The bi-specific antibody or antigen-binding construct can have any form suitable for the antibody or antigen-binding construct, so long as it comprises a first antigen binding domain and a second antigen binding domain that bind to distinct targets.
[0491] In some embodiments, the bi-specific antibody or antigen-binding construct is provided, comprising a first antigen binding domain that specifically binds Tim-3 and a second antigen binding domain that specifically binds PD-1. In some embodiments, a bi-specific antigen construct is provided, comprising a first scFv that specifically binds Tim-3 and a second scFv that specifically binds PD-1. In some embodiments, a bi-specific antigen construct is provided, comprising a first Fab that specifically binds Tim-3 and a second Fab that specifically binds PD-1. In some embodiments, a bi-specific antigen construct is provided, comprising an scFv that specifically binds Tim-3 and a Fab that specifically binds PD-1. In some embodiments, a bi-specific antigen construct is provided, comprising a Fab that specifically binds Tim-3 and an scFv that specifically binds PD-1.
[0492] In some embodiments, the bi-specific antibody or bi-specific antigen-binding construct can be generated as a dual-variable domain antibody. A "dual-variable domain antibody" (also referred to as a DVD-Ig) refers to fusion of an additional V.sub.H domain and V.sub.L domain of a second specificity to a given IgG heavy chain and light chain. Generation of dual-variable domain antibody formats are described, for example, in Wu et al. 2007. Nature Biotechnology 25:1290-1297 and U.S. 2007/0071675, each of which is incorporated herein by reference in its entirety.
[0493] In some embodiments, the bi-specific antibody or bi-specific antigen-binding construct is generated as a cross-over dual-variable domain antibody. A "cross-over dual-variable domain antibody" (also referred to as a CODV-Ig) refers to a format related to the dual-variable domain antibody format wherein the two V.sub.H domains and two V.sub.L domains are linked to allow cross-over pairing of the variable V.sub.H-V.sub.L domains. Generation of cross-over dual-variable domain antibody formats are described, for example, in Steinmetz et al. 2016. mAbs 8:867-878, which is incorporated herein by reference in its entirety.
[0494] Other formats for synthesis and use in bi-specific antibodies or bi-specific antigen-binding constructs are contemplated and described below.
[0495] The format "Single-chain Fv" or "scFv" includes the V.sub.H and V.sub.L domains of an antibody, wherein these domains are present in a single polypeptide chain. In some embodiments, the scFv polypeptide further comprises a polypeptide linker between the V.sub.H and V.sub.L domains. See, e.g., Pluckthun in The Pharmacology of Monoclonal Antibodies, vol. 113, Rosenburg and Moore eds., Springer-Verlag, New York, pp. 269-315 (1994).
[0496] The "Fab fragment" (also referred to as fragment antigen-binding) contains the constant domain (C.sub.L) of the light chain and the first constant domain (C.sub.H1) of the heavy chain along with the variable domains V.sub.L and V.sub.H on the light and heavy chains respectively. The variable domains comprise the complementarity determining loops (CDR, also referred to as hypervariable region) that are involved in antigen-binding. Fab' fragments differ from Fab fragments by the addition of a few residues at the carboxy terminus of the heavy chain CH1 domain including one or more cysteines from the antibody hinge region.
[0497] The "Single domain antibodies" or "sdAb" format is an individual immunoglobulin domain. SdAbs are fairly stable and easy to express as fusion partner with the Fc chain of an antibody (see Harmsen M M, De Haard H J (2007). "Properties, production, and applications of camelid single-domain antibody fragments." Appl. Microbiol Biotechnol. 77(1): 13-22).
[0498] 4.1.1 Format scFv
[0499] Embodiments are directed to bi-specific antigen-binding constructs comprising two antigen-binding polypeptide constructs that are each capable of specific binding to a distinct antigen. In some embodiments, each antigen-binding polypeptide construct is in an scFv format. (i.e., antigen-binding domains composed of a heavy chain variable domain and a light chain variable domain, connected with a polypeptide linker). In some embodiments, the scFv molecules are human. In some embodiments, the scFv molecules are humanized. The scFvs can be optimized for protein expression and yield by the modifications disclosed herein.
[0500] In some embodiments, the scFv is optimized by changing the order of the variable domains V.sub.L and V.sub.H in the scFv. In some embodiments, in the scFv, the C-terminus of the light chain variable region can be linked to the N-terminus of the heavy chain variable region. In some embodiments, in the scFv, the C-terminus of the heavy chain variable region can be linked to the N-terminus of the light chain variable region.
[0501] The variable regions of the scFv can be connected via a linker peptide, or scFv linker, that allows the formation of a functional antigen-binding moiety. In some embodiments, the scFv can be optimized for protein expression and yield by changing composition and/or length of the scFv linker polypeptide. Typical peptide linkers comprise about 2-20 amino acids, and are described herein or known in the art. Suitable, non-immunogenic linker peptides include, for example, (G.sub.4S).sub.n, (SG.sub.4).sub.n, (G.sub.4S).sub.n, G.sub.4(SG.sub.4).sub.n or G.sub.2(SG.sub.2).sub.n linker peptides, wherein n is generally a number between 1 and 10. In some embodiments, n is a number between 4 and 8. In some embodiments, n is a number between 3 and 6. In some embodiments, n is a number between 2 and 4. Other linkers are described, for example, in Bird et al. 1988. Science 242:423-426; Huston et al. 1988. PNAS 85:5879-5883; and McCafferty et al. 1990. Nature 348:552-554.
[0502] The scFv molecule can be optimized for protein expression and yield by including stabilizing disulfide bridges between the heavy and light chain variable domains, for example as described in Reiter et al. (Nat Biotechnol 14, 1239-1245 (1996)). Accordingly, in some embodiments, the bi-specific antigen-binding molecule disclosed herein can comprise an scFv molecule wherein an amino acid in the heavy chain variable domain and an amino acid in the light chain variable domain have been replaced by cysteine so that a disulfide bridge can be formed between the heavy and light chain variable domain.
[0503] ScFvs can also be stabilized by mutation of CDR sequences, as described in the art (Miller et al., Protein Eng Des Sel. 2010 July; 23(7):549-57; Igawa et al., MAbs. 2011 May-June; 3(3):243-5; Perchiacca & Tessier, Annu Rev Chem Biomol Eng. 2012; 3:263-286, each of which is incorporated herein by reference in its entirety.) and as disclosed herein in exemplary embodiments.
[0504] 4.1.2 Fc Domains of Antigen-Binding Constructs
[0505] In some embodiments, the antigen-binding constructs described herein comprise an Fc domain, e.g., a dimeric Fc. The Fc domain is a heterodimeric Fc comprising first and second Fc polypeptides each comprising a modified C.sub.H3 sequence, wherein each modified C.sub.H3 sequence comprises asymmetric amino acid modifications that promote the formation of a heterodimeric Fc and the dimerized C.sub.H3 domains have a melting temperature (Tm) of about 68.degree. C. or higher, and wherein the first Fc polypeptide is linked to the first antigen-binding polypeptide construct, with a first hinge linker, and the second Fc polypeptide is linked to the second antigen-binding polypeptide construct with a second hinge linker.
[0506] The term "Fc domain" or "Fc region" herein refers to a C-terminal region of an immunoglobulin heavy chain that contains at least a portion of the constant region. The term includes native sequence Fc regions and variant Fc regions and is used interchangeably with "Fc." Unless otherwise specified herein, numbering of amino acid residues in the Fc region or constant region is according to the EU numbering system, also called the EU index, as described in Kabat et al, Sequences of Proteins of Immunological Interest, 5th Ed. Public Health Service, National Institutes of Health, Bethesda, Md., 1991. An "Fc polypeptide" of a dimeric Fc as used herein refers to one of the two polypeptides forming the dimeric Fc domain, i.e. a polypeptide comprising C-terminal constant regions of an immunoglobulin heavy chain, capable of stable self-association. For example, an Fc polypeptide of a dimeric IgG Fc comprises an IgG C.sub.H2 and an IgG C.sub.H3 constant domain sequence.
[0507] An Fc domain comprises either a C.sub.H3 domain or a C.sub.H3 and a C.sub.H2 domain. The C.sub.H3 domain comprises two C.sub.H3 sequences, one from each of the two Fc polypeptides of the dimeric Fc. The C.sub.H2 domain comprises two C.sub.H2 sequences, one from each of the two Fc polypeptides of the dimeric Fc.
[0508] In some embodiments, the Fc comprises at least one or two C.sub.H3 sequences. In some aspects, the Fc is coupled, with or without one or more linkers, to a first antigen-binding construct and/or a second antigen-binding construct. In some embodiments, the Fc is a human Fc. In some embodiments, the Fc is a human IgG or IgG1 Fc. In some embodiments, the Fc is a heterodimeric Fc. In some embodiments, the Fc comprises at least one or two C.sub.H2 sequences.
[0509] In some embodiments, the Fc comprises one or more modifications in at least one of the C.sub.H3 sequences. In some embodiments, the Fc comprises one or more modifications in at least one of the C.sub.H2 sequences. For example, the Fc can include one or modifications selected from the group consisting of: V262E, V262D, V262K, V262R, V262S, V264S, V303R, and V305R. In some embodiments, an Fc is a single polypeptide. In some embodiments, an Fc is multiple peptides, e.g., two polypeptides.
[0510] 4.1.3 Modified C.sub.H3 Domains
[0511] In some embodiments, the antigen-binding construct described herein comprises a heterodimeric Fc comprising a modified C.sub.H3 domain that has been asymmetrically modified. The heterodimeric Fc can comprise two heavy chain constant domain polypeptides: a first Fc polypeptide and a second Fc polypeptide, which can be used interchangeably provided that Fc comprises one first Fc polypeptide and one second Fc polypeptide. Generally, the first Fc polypeptide comprises a first C.sub.H3 sequence and the second Fc polypeptide comprises a second C.sub.H3 sequence.
[0512] Two C.sub.H3 sequences that comprise one or more amino acid modifications introduced in an asymmetric fashion generally results in a heterodimeric Fc, rather than a homodimer, when the two C.sub.H3 sequences dimerize. As used herein, "asymmetric amino acid modifications" refers to any modification where an amino acid at a specific position on a first C.sub.H3 sequence is different from the amino acid on a second C.sub.H3 sequence at the same position, and the first and second C.sub.H3 sequence preferentially pair to form a heterodimer, rather than a homodimer. This heterodimerization can be a result of modification of one of the two amino acids at the same respective amino acid position on each sequence; or modification of both amino acids on each sequence at the same respective position on each of the first and second C.sub.H3 sequences. The first and second C.sub.H3 sequence of a heterodimeric Fc can comprise one or more than one asymmetric amino acid modification.
[0513] Typically an Fc can include two contiguous heavy chain sequences (A and B) that are capable of dimerizing. With respect to the antigen binding constructs described herein, in some embodiments the first scFv is linked to chain A of the heterodimeric Fc and the second scFv is linked to chain B of the heterodimeric Fc. In some embodiments the second scFv is linked to chain A of the heterodimeric Fc and the first scFv is linked to chain B of the heterodimeric Fc.
[0514] In some embodiments, one or both sequences of an Fc include one or more mutations or modifications at the following locations: L351, L368, F405, Y407, T366, K392, T394, T350, S400, and/or N390, using EU numbering. In some embodiments, an Fc includes the mutations as disclosed in the art (see Von Kreudenstein et al. 2013. mAbs 5(5):646-654; Von Kreudenstein et al. 2014. Methods 65:77-94; U.S. 2013/0195849; Ridgway et al. 1996. Protein Eng 9(7):617-621; and Brinkmann, U., and R. E. Kontermann. 2017. mAbs 9(2):182-212, each of which is incorporated herein by reference in its entirety.)
[0515] The first and second C.sub.H3 sequences can comprise amino acid mutations as described herein. In some embodiments, the heterodimeric Fc comprises a modified C.sub.H3 domain with a first C.sub.H3 sequence having one or more amino acid modifications selected from L351Y, F405A, and Y407V, and the second C.sub.H3 sequence having one or more amino acid modifications selected from T366L, T366I, K392L, K392M, and T394W. In some embodiments, the heterodimeric Fc comprises a modified C.sub.H3 domain with a first C.sub.H3 sequence having amino acid modifications T350V/T366L/K392L/T394W, and a second C.sub.H3 sequence having amino acid modifications T350V/L351Y/F405A/Y407V.
[0516] Additional modifications within the first and second C.sub.H3, or within the amino acid sequence of human IgG1 Fc, can be found, for example, at U.S. 2016/0326249, which is incorporated herein by reference in its entirety.
[0517] 4.1.4 Modified V.sub.H/V.sub.L and/or C.sub.H1/C.sub.L1 Interactions
[0518] In some embodiments, the bi-specific antibodies or bi-specific antigen-binding constructs disclosed herein comprise one or more light chains. In some embodiments, the bi-specific antibody or bi-specific antigen-binding construct comprises two light chains. In some embodiments, the two light chains are different. In some embodiments, the two light chains are the same.
[0519] In embodiments wherein the two light chains are different, one or more modifications can be introduced into one or both light chains to allow for pairing of a cognate heavy chain and light chain. Accordingly, in some embodiments, the interaction between the variable domain a first light chain and the variable domain of a corresponding first heavy chain (i.e., V.sub.H-V.sub.L interaction) is modified. In some embodiments, the interaction the constant domain of a first light chain and the first constant domain of a corresponding first heavy chain (i.e., C.sub.H1-C.sub.L interaction) is modified. In some embodiments, the modification comprises genetically engineering or genetically modifying residues that are involved in the V.sub.H-V.sub.L and/or the C.sub.H1-C.sub.L interaction. In some embodiments, the modification involves mutating residues to modify electrostatic interactions between the V.sub.H-V.sub.L pairs and/or the C.sub.H1-C.sub.L pairs. The result of modification to the V.sub.H-V.sub.L and/or the C.sub.H1-C.sub.L interactions can result in improved accuracy (or improved "steering") in pairing of cognate heavy and light chains. Exemplary modifications in these domains are described, for example, in Lewis et al. 2014. Nature Biotechnology 32:191-198 and WO 2014/082179, each of which is incorporated herein by reference in its entirety.
[0520] 4.1.5 Hinge Linkers
[0521] In some embodiments, in the bi-specific antigen-binding constructs disclosed herein, the first Fc polypeptide is linked to the first antigen-binding polypeptide construct with a first hinge linker, and the second Fc polypeptide is linked to the second antigen-binding polypeptide construct with a second hinge linker. Examples of hinge linker sequences are well-known to one of skill in the art and can be used in the antigen-binding constructs described herein. Alternatively, modified versions of known hinge linkers can be used.
[0522] The hinge linker polypeptides are selected such that they maintain or optimize the functional activity of the antigen-binding construct. Suitable linker polypeptides include IgG hinge regions such as, for example those from IgG.sub.1, IgG.sub.2, or IgG.sub.4, including the upper hinge sequences and core hinge sequences. The amino acid residues corresponding to the upper and core hinge sequences vary depending on the IgG type, as is known in the art and one of skill in the art would readily be able to identify such sequences for a given IgG type. Modified versions of these exemplary linkers can also be used. For example, modifications to improve the stability of the IgG4 hinge are known in the art (see for example, Labrijn et al. (2009) Nature Biotechnology 27, 767-771). Examples of hinge linker sequences are found, for example, in U.S. 2016/0326249.
[0523] 4.1.6. Bispecific Scaffold arrangements
[0524] The bi-specific antigen-binding construct can have a variety of different arrangements. For example, the bi-specific antigen-binding construct can comprise a 2-chain scFvFc, a 3-chain Fab.times.scFvFc, or a 4-chain IgG-like bispecific construct as described below.
[0525] 4.1.6.1. Generation of a PD1/Tim-3 Bi-Specific Antigen-binding Construct (Two-Chain scFvFc)
[0526] A general scheme for generating embodiments of scFvs for use in a PD-1/Tim3 bi-specific antigen-binding construct is provided below in Tables 5 and 6. In some embodiments, the bi-specific antigen-binding construct comprises a 2-chain scFvFc HC/LC pairing maintained by genetically fusing the V.sub.H to the V.sub.L of both antibodies to form an scFv. In some embodiments, the order of the HC/LC pairing is V.sub.H/V.sub.L. In some embodiments, the order of the HC/LC pairing is V.sub.L/V.sub.H. In any of the foregoing embodiments, the HC/LC pairing can comprise a linker sequence. In some embodiments, the linker sequence comprises a Gly/Ser-rich linker of the sequence (GGGGS)n where n=3, 4, 5, or 6 to provide linkers with lengths of 15, 20, 25, or 30 residues, respectively.
[0527] In some embodiments, the scFv is arranged in a V.sub.H-V.sub.L arrangement. In some embodiments, the scFv comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the scFv comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235.
TABLE-US-00005 TABLE 5 Exemplary V.sub.H-V.sub.L scFv Arrangements Design Name V.sub.H Linker V.sub.L (a) 1353-G10 1353-G10 V.sub.H (GGGGS)3, 1353-G10 V.sub.L V.sub.H-V.sub.L scFv (GGGGS)4, (.lamda.), or 1353- (GGGGS)5, or G10 V.sub.L (.kappa.) (GGGGS)6 (b) 1649-A01 1649-A01 V.sub.H (GGGGS)3, 1649-A01 V.sub.L V.sub.H-V.sub.L scFv (GGGGS)4, (GGGGS)5, or (GGGGS)6
[0528] In some embodiments, the scFv is arranged in a V.sub.L-V.sub.H arrangement. In some embodiments, the scFv comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197. In some embodiments, the scFv comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180.
TABLE-US-00006 TABLE 6 Exemplary V.sub.L-V.sub.H scFv Arrangements Design Name V.sub.L Linker V.sub.H (a) 1353-G10 1353-G10 V.sub.L (GGGGS)3, 1353-G10 V.sub.H V.sub.L-V.sub.H scFv (.lamda.), or 1353- (GGGGS)4, G10 V.sub.L(.kappa.) (GGGGS)5, or (GGGGS)6 (b) 1649-A01 1649-A01 V.sub.L (GGGGS)3, 1649-A01 V.sub.H V.sub.L-V.sub.H scFv (GGGGS)4, (GGGGS)5, or (GGGGS)6
[0529] 4.1.6.2. Generation of a PD-1/Tim-3 Bi-Specific Antigen-Binding Construct (Two-Chain scFvFc with Knob-in-Hole Mutations)
[0530] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a two-chain scFvFc arrangement, wherein the scFvFc pairing comprises knob-in-hole mutations, is provided. In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising an anti-PD-1 scFvFc knob paired with an anti-Tim-3 scFvFc hole. In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising an anti-PD-1 scFvFc hole paired with an anti-Tim-3 scFvFc knob. The scFvFcs include scFvs generated in accordance with Section 4.1.6.1.
[0531] A general scheme for scFvFcs useful in a PD-1/Tim-3 bi-specific antigen-binding construct comprising a two-chain scFv arrangement with knob-in-hole mutations is provided below in Table 7.
TABLE-US-00007 TABLE 7 Exemplary scFvFcs (with Knob-in-hole Mutations) for Two-chain scFvFc Arrangements Design Name scFv Linker C.sub.H2-C.sub.H3 (a) anti-PD-1 1353-G10 V.sub.H-V.sub.L Linker- Fc-Knob, Fc- scFvFc knob scFv, or 1353-G10 hinge Knob V262E, V.sub.L-V.sub.H scFv Fc-Knob - V264S, Fc- knob-D399C, Fc-knob-S354C (b) anti-Tim-3 1649-A01 V.sub.H-V.sub.L Linker- Fc-Knob, Fc- scFvFc knob scFv, or 1649-A01 hinge Knob V262E, V.sub.L-V.sub.H scFv Fc-Knob- V264S, Fc- knob-D399C, Fc-knob-S354C (c) anti-PD-1 1353-G10 V.sub.H-V.sub.L Linker- Fc-Hole, Fc- scFvFc hole scFv, or 1353-G10 hinge hole V262E, Fc- V.sub.L-V.sub.H scFv hole V264S, Fc- hole-Y349C, or Fc-hole-K392C (d) anti-Tim-3 1649-A01 V.sub.H-V.sub.L Linker- Fc-Hole, Fc- scFvFc hole scFv, or 1649-A01 hinge hole V262E, Fc- V.sub.L-V.sub.H scFv hole V264S, or Fc-hole-Y349C, or Fc-hole- K392C
[0532] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a two-chain scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 scFvFc knob (Table 12, design (a)) paired with an anti-Tim-3 scFvFc hole (Table 12, design (d)). In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a two-chain scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 scFvFc hole (Table 12, design (c)) paired with an anti-Tim-3 scFvFc knob (Table 12, design (b)).
[0533] In some embodiments, the anti-PD-1 scFvFc knob is constructed from: (1) an anti-PD-1 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-PD-1 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the anti-PD-1 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 236-245; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 181-197. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO:285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 295-299.
[0534] In some embodiments, the anti-PD-1 scFvFc hole is constructed from: (1) an anti-PD-1 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-PD-1 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the anti-PD-1 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 236-245; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 181-197. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO:285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 300-304.
[0535] In some embodiments, the anti-Tim-3 scFvFc knob is constructed from: (1) an anti-Tim-3 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 144-180. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 295-299.
[0536] In some embodiments, the anti-Tim-3 scFvFc hole is constructed from: (1) an anti-Tim-3 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 144-180. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 300-304.
[0537] 4.1.6.3. Generation of a PD-1/Tim-3 Bi-Specific Antigen-Binding Construct (Three-Chain Fab.times.scFvFc with Knob-in-Hole Mutations)
[0538] In some embodiments, the bi-specific antigen-binding construct comprises a 3-chain Fab.times.scFvFc scaffolds in which an scFv is replaced with a Fab domain. In these embodiments, the asymmetry of the scaffold facilitates correct HC/LC pairing as there is only one HC/LC pairing that can correctly form the Fab domain; the other arm is an scFv.
[0539] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFvFc structure can be prepared using the following arrangements: (1) an anti-PD-1 scFvFc knob paired with an anti-Tim-3 half IgG (HC+LC) hole, (2) an anti-PD-1 scFvFc hole paired with an anti-Tim-3 half IgG (HC+LC) knob, (3) an anti-PD-1 half IgG (HC+LC) knob paired with an anti-Tim-3 scFvFc hole, and (4) an anti-PD-1 half IgG (HC+LC) hole paired with an anti-Tim-3 scFvFc knob. The scFvs included within such scFvFc arrangements can be generated in accordance with Section 4.1.6.1.
[0540] A general scheme for anti-PD-1 half IgGs (HC+LC, knob or hole) and anti-Tim 3 half IgGs (HC+LC, knob or hole) for use in a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFv arrangement with knob-in-hole mutations is provided below in Tables 8 and 9.
TABLE-US-00008 TABLE 8 Exemplary HCs of anti-PD-1 and anti-Tim-3 Half IgGs Design Name V.sub.H C.sub.H1 Linker C.sub.H2-C.sub.H3 (a) anti-PD-1 1353-G10 V.sub.H C.sub.H1-wt Hinge-wt Fc-Knob, Fc-Knob HC knob V262E, Fc-Knob- V264S, Fc-knob- D399C, Fc-knob- S354C (b) anti-Tim-3 1649-A01 V.sub.H C.sub.H1-wt Hinge-wt Fc-Knob, Fc-Knob HC knob V262E, Fc-Knob- V264S, Fc-knob- D399C, Fc-knob- S354C (c) anti-PD-1 1353-G10 V.sub.H C.sub.H1-wt Hinge-wt Fc-Hole, Fc-hole HC hole V262E, Fc-hole V264S, or Fc-hole- Y349C, or Fc-hole- K392C (d) anti-Tim-3 1649-A01 V.sub.H C.sub.H1-wt Hinge-wt Fc-Hole, Fc-hole HC hole V262E, Fc-hole V264S, or Fc-hole- Y349C, or Fc-hole- K392C
TABLE-US-00009 TABLE 9 Exemplary LCs of anti-PD1 and anti-Tim-3 Half IgGs Design Name V.sub.L C.sub.L (a) anti-PD-1 LC (.lamda.) 1353-G10 V.sub.L (.lamda.) C.lamda.-wt (b) anti-PD-1 LC (.kappa.) 1353-G10 V.sub.L (.kappa.) C.kappa.-wt (c) anti-Tim-3 LC 1649-A01 V.sub.L C.kappa.-wt
[0541] In some embodiments, the anti-PD-1 half IgG knob comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a C.sub.H1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a CH.sub.2-CH.sub.3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 295-299, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs:346-347.
[0542] In some embodiments, the anti-PD-1 half IgG hole comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a CH.sub.2-CH.sub.3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 300-304, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 346-347.
[0543] In some embodiments, the anti-Tim-3 half IgG knob comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a CH.sub.2-CH.sub.3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 295-299, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235 and a C.sub.L comprising, consisting of, or consisting essentially of SEQ ID NO: 346.
[0544] In some embodiments, the anti-Tim-3 half IgG hole comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a CH.sub.2-CH.sub.3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 300-304, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235 and a C.sub.L comprising, consisting of, or consisting essentially of SEQ ID NO: 346.
[0545] In some embodiments, the anti-Tim-3 scFvFc hole is constructed from: (1) an anti-Tim-3 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 144-180. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 300-304.
[0546] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 scFvFc knob (Table 7, design (a)) paired with an anti-Tim-3 half IgG hole (HC=Table 8, design (d), LC=Table 9, design (c)).
[0547] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 scFvFc hole (Table 7, design (c)) paired with an anti-Tim-3 half IgG knob (HC=Table 8, design (b), LC=Table 9, design (c)).
[0548] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 half IgG knob (HC=Table 8, design (a), LC=Table 9, design (a) or (b)) paired with an anti-Tim-3 scFvFc hole (Table 7, design (d)).
[0549] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFvFc arrangement is prepared using the following arrangement: an anti-PD-1 half IgG hole (HC=Table 8, design (c), LC=Table 9, design (a) or (b)) hole paired with an anti-Tim-3 scFvFc knob (Table 7, design (b)).
[0550] 4.1.6.4. Generation of a PD-1/Tim-3 Bi-Specific Antigen-Binding Construct (Three-Chain Fab.times.scFvFc with Zw Mutations)
[0551] In some embodiments, the bi-specific antigen-binding construct comprises a three-chain Fab.times.scFvFc scaffold, wherein the Fab and scFvFc structures comprise knob-in-hole mutations. In these embodiments, the asymmetry of the scaffold facilitates correct HC/LC pairing as there is only one HC/LC pairing that can correctly form the Fab domain; the other arm is an scFv.
[0552] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising a three-chain Fab.times.scFvFc structure comprising an anti-PD-1 scFvFc with zwA mutations paired with an anti-Tim-3 half IgG (HC+LC) with zwB mutations. In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising a three-chain Fab.times.scFvFc structure comprising an anti-PD-1 scFvFc with zwB mutations paired with an anti-Tim-3 half IgG (HC+LC) with zwA mutations. In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising a three-chain Fab.times.scFvFc structure comprising an anti-PD-1 half IgG (HC+LC) with zwA mutations paired with an anti-Tim-3 scFvFc with zwB mutations. In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising a three-chain Fab.times.scFvFc structure comprising an anti-PD-1 half IgG (HC+LC) with zwB mutations paired with an anti-Tim-3 scFvFc with zwA mutations. The mutations encompassed by "zwA" mutations include T350V/L351Y/F405A/Y407V in the C.sub.H3 domain. The mutations encompassed by "zwB" mutations include T350V/T366L/K392L/T394W in the C.sub.H3 domain.
[0553] A general scheme for scFvFcs with zw mutations and for anti-PD-1 half IgGs (HC+LC) and anti-Tim-3 half IgGs (HC+LC) with zw mutations for use in a PD-1/Tim-3 bi-specific antigen-binding construct comprising a three-chain Fab.times.scFv arrangement is provided below in Tables 10 and 11. Exemplary LC embodiments for the half IgGs correspond to those in Table 9 above.
TABLE-US-00010 TABLE 10 Exemplary scFvFcs (with zw Mutations) for Three-chain Fab .times. scFvFc Arrangement Design Name scFv Linker CH.sub.2-CH.sub.3 (a) anti-PD-1 1353-G10 V.sub.H- Linker-hinge Fc-zwA, Fc- scFvFc zwA V.sub.L scFv, or zwA V262E, or 1353-G10 V.sub.L- Fc-zwA V264S V.sub.H scFv (b) anti-Tim-3 1649-A01 V.sub.H- Linker-hinge Fc-zwA, Fc- scFvFc zwA V.sub.L scFv, or zwA V262E, or 1649-A01 V.sub.L- Fc-zwA V264S V.sub.H scFv (c) anti-PD-1 1353-G10 V.sub.H- Linker-hinge Fc-zwB, Fc- scFvFc zwB V.sub.L scFv, or zwB V262E, or 1353-G10 V.sub.L- Fc-zwB V264S V.sub.H scFv (d) anti-Tim-3 1649-A01 V.sub.H- Linker-hinge Fc-zwB, Fc- scFvFc zwB V.sub.L scFv, or zwB V262E, or 1649-A01 V.sub.L- Fc-zwB V264S V.sub.H scFv
[0554] In some embodiments, the anti-PD-1 scFvFc zwA is constructed from: (1) an anti-PD-1 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-PD-1 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the anti-PD-1 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 236-245; a linker selected from SEQ ID NOs:278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 181-197. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 305-307.
[0555] In some embodiments, the anti-PD-1 scFvFc zwB is constructed from: (1) an anti-PD-1 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-PD-1 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the anti-PD-1 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 236-245; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 181-197. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 308-310.
[0556] In some embodiments, the anti-Tim-3 scFvFc zwA is constructed from: (1) an anti-Tim-3 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 144-180. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 305-307.
[0557] In some embodiments, the anti-Tim-3 scFvFc zwB is constructed from: (1) an anti-Tim-3 scFv; (2) a linker-hinge; and (3) a CH.sub.2-CH.sub.3 region. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.H-V.sub.L arrangement and comprises a V.sub.H sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a linker selected from SEQ ID NOs: 278-281; and a V.sub.L sequence comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the anti-Tim-3 scFv comprises a V.sub.L-V.sub.H arrangement and comprises a V.sub.L sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 221-235; a linker selected from SEQ ID NOs: 278-281; and a V.sub.H sequence comprising, consisting of, or consisting essentially of SEQ ID NOs: 144-180. In some embodiments, the linker-hinge comprises, consists of, or consists essentially of SEQ ID NO: 285. In some embodiments, the CH.sub.2-CH.sub.3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs.: 308-310.
TABLE-US-00011 TABLE 11 Exemplary HCs of anti-PD-1 and anti-Tim-3 Half IgGs with zw Mutations Design Name V.sub.H C.sub.H1 Linker CH.sub.2-CH.sub.3 (a) anti-PD-1 1353-G10 VH C.sub.H1-wt Hinge-wt Fc-zwA, Fc-zwA HC zwA V262E, or Fc-zwA V264S (b) anti-Tim-3 1649-A01 VH C.sub.H1-wt Hinge-wt Fc-zwA, Fc-zwA HC zwA V262E, or Fc-zwA V264S (c) anti-PD-1 1353-G10 VH C.sub.H1-wt Hinge-wt Fc-zwB, Fc-zwB HC zwB V262E, or Fc-zwB V264S (d) anti-Tim-3 1649-A01 VH C.sub.H1-wt Hinge-wt Fc-zwB, Fc-zwB HC zwB V262E, or Fc-zwB V264S
[0558] In some embodiments, the anti-PD-1 half IgG with zwA mutations comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 305-307, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 346-347.
[0559] In some embodiments, the anti-PD-1 half IgG with zwB mutations comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 181-197; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 308-310, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 236-245 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 346-347.
[0560] In some embodiments, the anti-Tim-3 half IgG with zwA mutations comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 305-307, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 346-347.
[0561] In some embodiments, the anti-Tim-3 half IgG with zwB mutations comprises a heavy chain and a light chain, wherein the heavy chain comprises: a V.sub.H comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 144-180; a CH.sub.1 region comprising, consisting of, or consisting essentially of SEQ ID NO: 345; a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286; and a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of any one of SEQ ID NOs.: 308-310, and wherein the light chain comprises: a V.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 221-235 and a C.sub.L comprising, consisting of, or consisting essentially of any one of SEQ ID NOs: 346-347.
[0562] 4.1.6.5. Generation of a PD-1/Tim-3 Bi-Specific Antigen-Binding Construct (Four-Chain Fab-Fc.times.Fab-Fc (IgG-Like) with Zw Mutations)
[0563] In some embodiments, the bi-specific antigen-binding construct comprises a four-chain IgG-like scaffold comprising a Fab domain fused to the N-termini of a heterodimeric Fc. In such embodiments, this bispecific format comprises four chains: heavy chain 1 (HC1), light chain 1 (LC1), heavy chain 2 (HC2), and light chain 2 (LC2). In some embodiments, the HC and LC sequences are mutated as described herein in sections 4.1.2-4.1.4 to facilitate correct pairing between HCs and LCs. For example, the HC/LC pairing designs listed in Tables 12-14 can be incorporated into the construct to facilitate correct HC/LC pairing. HC1 is designed such that it pairs specifically with LC1 rather than LC2. HC2 is designed such this it pairs specifically with LC2 rather than LC1. Six designs are shown below in Tables 12 and 13 that enforce correct light-chain pairing: (a), (b) (c), (d), (e), and (f). Designs (a) and (b) in Table 12 can be incorporated for an embodiment in which one light chain is a kappa chain (LC1) and the second light chain is a lambda chain (LC2). Designs (c), (d), (e) and (f) in Table 13 can be incorporated for embodiments in which the first and second light chains (LC1, LC2) are kappa chains.
TABLE-US-00012 TABLE 12 Exemplary embodiments of a four-chain bi-specific antibody arrangement (kappa .times. lambda) Bispecific Tim-3 HC1 Tim-3 LC1 PD-1 HC2 PD-1 LC2 (a) HC1(a) LC1(a) HC2(a) LC2(a) (b) HC1(b) LC1(b) HC2(b) LC2(b)
TABLE-US-00013 TABLE 13 Exemplary embodiments of a four-chain bi-specific antibody arrangement (kappa .times. kappa) Bispecific Tim-3 HC1 Tim-3 LC1 PD-1 HC2 PD-1 LC2 (c) HC1(c) LC1(c) HC2(c) LC2(c) (d) HC1(d) LC1(d) HC2(d) LC2(d) (e) HC1(e)(f) LC1(e)(f) HC2(e)(f) LC2(e) (f) HC1(e)(f) LC1(e)(f) HC2(e)(f) LC2(f)
[0564] In some embodiments, the HCs and LCs for a kappa.times.kappa bi-specific construct can be switched around. For example, in some embodiments, the (c), (d), (e), and (f) designs of Table 13 can be swapped such that the Tim-3 HC1+LC1 and PD-1 HC2+LC2 use the opposite light-chain pairing mutations, as illustrated in Table 14.
TABLE-US-00014 TABLE 14 Exemplary embodiments of a four-chain bi-specific antibody arrangement (kappa .times. kappa) Bispecific PD-1 HC1 PD-1 LC1 Tim-3 HC2 Tim-3 LC2 (c) HC1(c) LC1(c) HC2(c) LC2(c) (d) HC1(d) LC1(d) HC2(d) LC2(d) (e) HC1(e)(f) LC1(e)(f) HC2(e)(f) LC2(e) (f) HC1(e)(f) LC1(e)(f) HC2(e)(f) LC2(f)
[0565] In some embodiments, HC1 and HC2 incorporate complementary Zymeworks mutations A (T350V/L351Y/F405A/Y407V; "zwA") and B (T350V/T366L/K392L/T394W; "zwB") to enforce heterodimerization of HC1 with HC2 (see, e.g., Von Kreudenstein et al. 2013. mAbs 5(5):646-654; Von Kreudenstein et al. 2014. Methods 65:77-94; U.S. 2013/0195849, each of which is incorporated herein by reference in its entirety). Accordingly, if HC1 has zwA then HC2 must have zwB; if HC1 has zwB then HC2 must have zwA. Many alternatives to these C.sub.H3 pairing mutations, e.g., knobs-in-holes mutations, can be used instead. Some of these alternatives are described, for example, in Brinkmann, U., and R. E. Kontermann. 2017. mAbs 9(2):182-212; and Yang, et al. 2017. Int. J. Mol. Sci. 18(1):48 (21 pages), each of which is incorporated herein by reference in its entirety. The C.sub.H2 domains can either be wild-type or have stability/solubility/assembly/yield enhancing mutations V262E or V264S.
[0566] 4.1.6.5.1 Tim-3 Heavy Chains (HC1 or HC2)
[0567] A full-length antibody Tim-3 heavy chain typically includes a V.sub.H domain, a C.sub.H1 domain, a linker, and a C.sub.H2-C.sub.H3 region. In some embodiments, the V.sub.H domain comprises, consists, or consists essentially of any one of SEQ ID NOs: 144-180. In some embodiments, the C.sub.H1 domain is selected from SEQ ID NOs: 317-326. In some embodiments, the linker comprises, consists of, or consists essentially of SEQ ID NO: 286. In some embodiments, the C.sub.H2-C.sub.H3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs: 305-310. For example, in an embodiment where the anti-Tim-3 arm is antibody "1" in the bi-specific antibody, an anti-Tim-3 heavy chain with design (a) ("HC1(a)") can be constructed from: (1) a V.sub.H sequence of 1649-A01 (SEQ ID NO: 151); (2) C.sub.H1-(a)1; (3) a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286, and (4) a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of Fc-zwA. Table 15 provides various exemplary components for the V.sub.H domain, C.sub.H1 domain, linker, and C.sub.H2-C.sub.H3 region that are contemplated for generation of a Tim-3 heavy chain sequence.
TABLE-US-00015 TABLE 15 Exemplary Combinations of Components for Tim-3 Heavy Chain (HC1 or HC2) Design V.sub.H C.sub.H1 Linker C.sub.H2-C.sub.H3 (a) 1649-A01; C.sub.H1-(a)1 Hinge-wt Fc-zwA; 1649-A01 S30D; C.sub.H1-(a)2 Fc-zwA V262E; 1649-A01 S30D/R31D; Fc-zwA V264S; 1649-A01 S30D/Y56D; Fc-zwB; 1649-A01 S30D/R31D/Y56D Fc-zwB V262E; Fc-zwB V264S (b) 1649-A01; C.sub.H1-(b)1 Hinge-wt Fc-zwA; 1649-A01 S30D; C.sub.H1-(b)2 Fc-zwA V262E; 1649-A01 S30D/R31D; Fc-zwA V264S; 1649-A01 S30D/Y56D; Fc-zwB; 1649-A01 S30D/R31D/Y56D Fc-zwB V262E; Fc-zwB V264S (c) 1649-A01; C.sub.H1-(c)1 Hinge-wt Fc-zwA; 1649-A01 S30D; C.sub.H1-(c)2 Fc-zwA V262E; 1649-A01 S30D/R31D; Fc-zwA V264S; 1649-A01 S30D/Y56D; Fc-zwB; 1649-A01 S30D/R31D/Y56D Fc-zwB V262E; Fc-zwB V264S (d) 1649-A01; C.sub.H1-(d)1 Hinge-wt Fc-zwA; 1649-A01 S30D; C.sub.H1-(d)2 Fc-zwA V262E; 1649-A01 S30D/R31D; Fc-zwA V264S; 1649-A01 S30D/Y56D; Fc-zwB; 1649-A01 S30D/R31D/Y56D Fc-zwB V262E; Fc-zwB V264S (e)(f) 1649-A01; C.sub.H1-(e)(f)1 Hinge-wt Fc-zwA; 1649-A01 S30D; C.sub.H1-(e)(f)2 Fc-zwA V262E; 1649-A01 S30D/R31D; Fc-zwA V264S; 1649-A01 S30D/Y56D; Fc-zwB; 1649-A01 S30D/R31D/Y56D Fc-zwB V262E; Fc-zwB V264S
[0568] In some embodiments, the V.sub.H sequence can be selected from the group consisting of: the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), and the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165).
[0569] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO:305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0570] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0571] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0572] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0573] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0574] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0575] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0576] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0577] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0578] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 (SEQ ID NO: 151), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0579] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO:305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0580] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0581] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0582] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0583] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0584] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0585] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0586] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0587] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0588] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D (SEQ ID NO: 179), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0589] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO:305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0590] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0591] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0592] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0593] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0594] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0595] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0596] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0597] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0598] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D (SEQ ID NO: 167), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0599] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO:305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0600] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0601] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0602] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0603] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0604] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0605] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0606] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0607] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0608] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/Y56D (SEQ ID NO: 166), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0609] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO:305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0610] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0611] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0612] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0613] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0614] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0615] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0616] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0617] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0618] In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-Tim-3 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1649-A01 S30D/R31D/Y56D (SEQ ID NO: 165), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0619] 4.1.6.5.2 Tim-3 Light Chains (LC1 or LC2)
[0620] A full-length anti-Tim-3 light chain typically includes a V.sub.L domain and a C.sub.L domain. In some embodiments, the V.sub.L domain comprises, consists of, or consists essentially of any one of SEQ ID NOs: 221-235. In some embodiments, the C.sub.L domain comprises, consists of, or consists essentially of any one of SEQ ID NOs: 327-338. For example, in an embodiment where the anti-Tim-3 arm is antibody "1" in the bi-specific antibody, an anti-Tim-3 light chain with design (a) ("LC1(a)") can be constructed from: (1) V.sub.L sequence 1649-A01 (SEQ ID NO: 235); and (2) Ck-(a)1. Table 16 provides various exemplary components for the V.sub.L domain and C.sub.L domain for generation of a Tim-3 light chain sequence.
TABLE-US-00016 TABLE 16 Exemplary Combinations of Components for Tim-3 Light Chain (LC1 or LC2) Design V.sub.L C.sub.L (a) 1649-A01; Ck-(a)1; 1649-A01 T56D Ck-(a)2 (b) 1649-A01; Ck-(b)1; 1649-A01 T56D Ck-(b)2 (c) 1649-A01; Ck-(c)1; 1649-A01 T56D Ck-(c)2 (d) 1649-A01; Ck-(d)1; 1649-A01 T56D Ck-(d)2 (e) 1649-A01; Ck-(e)(f)1; 1649-A01 T56D Ck-(e)2 (f) 1649-A01; Ck-(e)(f)1; 1649-A01 T56D Ck-(f)2
[0621] In some embodiments, the V.sub.L sequence can be selected from the group consisting of: the V.sub.L sequence of 1649-A01 and the V.sub.L sequence of 1649-A01 T56D.
[0622] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(a)1 (SEQ ID NO: 327).
[0623] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(b)1 (SEQ ID NO: 328).
[0624] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(c)1 (SEQ ID NO: 329). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(c)2 (SEQ ID NO: 334).
[0625] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(d)1 (SEQ ID NO: 330). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(d)2 (SEQ ID NO: 335).
[0626] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(e)(f)1 (SEQ ID NO: 331). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(e)2 (SEQ ID NO: 336). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 (SEQ ID NO: 235) and a C.sub.L sequence comprising the sequence of Ck-(f)2 (SEQ ID NO: 337).
[0627] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(a)1 (SEQ ID NO: 327).
[0628] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(b)1 (SEQ ID NO: 328).
[0629] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(c)1 (SEQ ID NO: 329). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(c)2 (SEQ ID NO: 334).
[0630] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(d)1 (SEQ ID NO: 330). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(d)2 (SEQ ID NO: 335).
[0631] In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(e)(f)1 (SEQ ID NO: 331). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(e)2 (SEQ ID NO: 336). In some embodiments, the anti-Tim-3 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1649-A01 T56D (SEQ ID NO: 229) and a C.sub.L sequence comprising the sequence of Ck-(f)2 (SEQ ID NO: 337).
[0632] 4.1.6.5.3 PD-1 Heavy Chains (HC1 or HC2)
[0633] A full-length anti-PD-1 heavy chain typically includes includes a V.sub.H domain, a C.sub.H1 domain, a linker, and a C.sub.H2-C.sub.H3 region. In some embodiments, the V.sub.H domain comprises, consists, or consists essentially of any one of SEQ ID NOs: 181-197. In some embodiments, the C.sub.H1 domain is selected from SEQ ID NOs: 317-326. In some embodiments, the linker comprises, consists of, or consists essentially of SEQ ID NO: 286. In some embodiments, the C.sub.H2-C.sub.H3 region comprises, consists of, or consists essentially of any one of SEQ ID NOs: 305-310. For example, in an embodiment where the anti-PD-1 arm is antibody "2" in the bi-specific antibody, an anti-PD-1 heavy chain with design (a) ("HC2a") can be constructed from: (1) V.sub.H sequence 1353-G10R28T/P30D/H31S (SEQ ID NO: 197); (2) C.sub.H1(a)2; and (3) a linker comprising, consisting of, or consisting essentially of SEQ ID NO: 286, and (4) a C.sub.H2-C.sub.H3 region comprising, consisting of, or consisting essentially of Fc-zwA. Table 17 provides various exemplary components for the V.sub.H domain, C.sub.H1 domain, linker, and C.sub.H2-C.sub.H3 region that are contemplated for generation of a PD-1 heavy chain sequence.
TABLE-US-00017 TABLE 17 Exemplary Combinations of Components for PD-1 Heavy Chain (HC1 or HC2) Design V.sub.H C.sub.H1 Linker C.sub.H2-C.sub.H3 (a) 1353-G10-wt; C.sub.H1-(a)1; Hinge-wt Fc-zwA; 1353-G10-28T/P30D/H31S C.sub.H1-(a)2 Fc-zwA V262E; Fc-zwA V264S; Fc-zwB; Fc-zwB V262E; Fc-zwB V264S (b) 1353-G10-wt; C.sub.H1-(b)1; Hinge-wt Fc-zwA; 1353-G10-28T/P30D/H31S C.sub.H1-(b)2 Fc-zwA V262E; Fc-zwA V264S; Fc-zwB; Fc-zwB V262E; Fc-zwB V264S (c) 1353-G10-wt; C.sub.Hl-(c)1; Hinge-wt Fc-zwA; 1353-G10-28T/P30D/H31S C.sub.H1-(c)2 Fc-zwA V262E; Fc-zwA V264S; Fc-zwB; Fc-zwB V262E; Fc-zwB V264S (d) 1353-G10-wt; C.sub.H1-(d)1; Hinge-wt Fc-zwA; 1353-G10-28T/P30D/H31S C.sub.H1-(d)2 Fc-zwA V262E; Fc-zwA V264S; Fc-zwB; Fc-zwB V262E; Fc-zwB V264S (e)(f) 1353-G10-wt; C.sub.H1-(e)(f)1; Hinge-wt Fc-zwA; 1353-G10-28T/P30D/H31S C.sub.H1-(e)(f)2 Fc-zwA V262E; Fc-zwA V264S; Fc-zwB; Fc-zwB V262E; Fc-zwB V264S
[0634] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0635] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0636] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0637] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0638] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0639] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0640] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0641] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0642] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0643] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0644] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)1 (SEQ ID NO: 317), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0645] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(a)2 (SEQ ID NO: 322), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0646] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0647] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0648] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0649] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0650] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0651] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0652] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0653] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0654] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0655] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0656] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)1 (SEQ ID NO: 318), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0657] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(b)2 (SEQ ID NO: 323), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0658] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0659] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0660] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0661] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0662] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0663] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0664] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0665] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0666] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0667] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0668] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)1 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(01 (SEQ ID NO: 319), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0669] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(c)2 (SEQ ID NO: 324), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0670] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0671] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0672] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0673] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0674] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0675] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0676] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0677] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0678] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0679] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0680] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)1 (SEQ ID NO: 320), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0681] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(d)2 (SEQ ID NO: 325), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0682] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0683] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA (SEQ ID NO: 305).
[0684] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0685] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V262E (SEQ ID NO: 306).
[0686] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0687] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwA V264S (SEQ ID NO: 307).
[0688] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0689] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB (SEQ ID NO: 308).
[0690] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0691] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V262E (SEQ ID NO: 309).
[0692] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)1 (SEQ ID NO: 321), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0693] In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-wt (SEQ ID NO: 181), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310). In some embodiments, the anti-PD-1 heavy chain comprises a V.sub.H sequence comprising the V.sub.H sequence of 1353-G10-R28T/P30D/H31S (SEQ ID NO: 197), a C.sub.H1 sequence comprising the sequence of C.sub.H1-(e)(f)2 (SEQ ID NO: 326), a linker of hinge-wt (SEQ ID NO: 286), and a C.sub.H2-C.sub.H3 region comprising the sequence of Fc-zwB V264S (SEQ ID NO: 310).
[0694] 4.1.6.5.4 PD-1 Light Chains (LC1 or LC2)
[0695] A full-length anti-PD-1 light chain typically includes a V.sub.L domain and a C.sub.L domain. In some embodiments, the V.sub.L domain comprises, consists of, or consists essentially of any one of SEQ ID NOs: 236-245. In some embodiments, the C.sub.L domain comprises, consists of, or consists essentially of any one of SEQ ID NOs: 327-338. For example, in an embodiment where the anti-PD-1 arm is antibody "2" in the bi-specific antibody, an anti-PD-1 light chain with design (a) ("LC2a") can be constructed from: (1) V.sub.L sequence 1353-G10 wt (SEQ ID NO: 236); and (2) Cl-(a)2. Table 18 provides various exemplary components for the V.sub.L domain and C.sub.L domain for generation of a PD-1 light chain sequence.
TABLE-US-00018 TABLE 18 Exemplary Combinations of Components for PD-1 Light Chain (LC1 or LC2) Design V.sub.L C.sub.L (a) 1353-G10 wt (.lamda.) Cl-(a)2 (b) 1353-G10 wt (.lamda.) Cl-(b)2 (c) 1353-G10 Vk1-39 (.kappa.) Ck-(c)1; Ck-(c)2 (d) 1353-G10 Vk1-39 (.kappa.) Ck-(d)1; Ck-(d)2 (e) 1353-G10 Vk1-39 (.kappa.) Ck-(e)(f)1; Ck-(e)(f)2 (f) 1353-G10 Vk1-39 (.kappa.) Ck-(e)(f)1; Ck-(e)(f)2
[0696] In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 wt (SEQ ID NO: 236) and a C.sub.L sequence comprising the sequence of Cl-(a)2 (SEQ ID NO: 332).
[0697] In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 wt (SEQ ID NO: 236) and a C.sub.L sequence comprising the sequence of Cl-(b)2 (SEQ ID NO: 333).
[0698] In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(c)1 (SEQ ID NO: 329). In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(c)2 (SEQ ID NO: 334).
[0699] In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(d)1 (SEQ ID NO: 330). In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(d)2 (SEQ ID NO: 335).
[0700] In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(e)(f)1 (SEQ ID NO: 331). In some embodiments, the anti-PD-1 light chain comprises a V.sub.L sequence comprising the V.sub.L sequence of 1353-G10 Vk1-39 (.kappa.) (also referred to as "1353-G10-kappa", SEQ ID NO: 244) and a C.sub.L sequence comprising the sequence of Ck-(e)(f)2 (SEQ ID NO: 338).
[0701] 4.1.6.5.5 Hybrid Light Chains (LC1 or LC2=V.lamda., +C.kappa.)
[0702] In some embodiments, the anti-Tim-3 or anti-PD-1 light chain comprises a V.sub.L lambda sequence ("V.lamda.") and a C.sub.L kappa sequence ("C.kappa."). In such embodiments, the V.lamda., sequence comprises one or more mutations selected from the group consisting of: E38F, D85T, T105E, V106I, and L106K. In some embodiments, the light chain is an anti-PD-1 light chain comprising, consisting of, or consisting essentially of SEQ ID NOs: 351 or 352.
[0703] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising a hybrid light chain comprising a V.lamda., and a C.kappa. sequence, wherein HC1, LC1, HC2, and LC2 comprise one or more mutations selected from the Table 19:
TABLE-US-00019 TABLE 19 Table of mutations for a bi-specific construct with a hybrid light chain Bispecific HC1 LC1 HC2 LC2 (c) A139W; F116A; Q179K Q124E; L143E; Q124R; L135W; K145T; L135V; Q160E; Q179E T178R T180E (d) Q39E; Q38R; Q39R; Q38E; L143E; Q124R; H172R; Q124E; K145T; Q160K; Q179K Q160E; Q179E T178R T180E
[0704] In some embodiments, HC1 and LC1 indicate PD-1 heavy chain and light chain sequences, respectively, and HC2 and LC2 indicate Tim-3 heavy chain and light chain sequences, respectively. In some embodiments, HC1 and LC1 indicate Tim-3 heavy chain and light chain sequences, respectively, and HC2 and LC2 indicate PD-1 heavy chain and light chain sequences, respectively.
[0705] In some embodiments, the V.sub.H and V.sub.L sequences of HC1, HC2, LC1 and LC2 comprise the following mutations from Table 20:
TABLE-US-00020 TABLE 20 Exemplary mutations for a bi-specific construct with a hybrid light chain V.sub.H1 V.sub.L1 V.sub.H2 V.sub.L2 Q39R Q38E Q39E Q38R
[0706] In some embodiments, HC1 comprises, consists of, or consists essentially of SEQ ID NO:353; LC1 comprises, consists of, or consists essentially of SEQ ID NO: 352; HC2 comprises, consists of, or consists essentially of SEQ ID NO: 151; and LC2 comprises, consists of, or consists essentially of SEQ ID NO: 228.
[0707] The sequences of the various components contemplated for V.sub.H, C.sub.H1, linker, C.sub.H2-C.sub.H3, V.sub.L, and C.sub.L in Section 4.1.6 can be found in Table 30.
[0708] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 181, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 236, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 228.
[0709] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 197, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 236, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 228.
[0710] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 181, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 244, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 228.
[0711] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 197, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 244, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 228.
[0712] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 181, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 236, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 235.
[0713] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 197, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 236, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 235.
[0714] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 181, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 244, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 235.
[0715] In some embodiments, a PD-1/Tim-3 bi-specific antigen-binding construct is provided, comprising: (i) an anti-PD-1 V.sub.H sequence of SEQ ID NO: 197, (ii) an anti-PD-1 V.sub.L sequence of SEQ ID NO: 244, and (iii) an anti-Tim-3 V.sub.H sequence of SEQ ID NO: 179. In some embodiments, the bi-specific antigen-binding construct further comprises a V.sub.L sequence of SEQ ID NO: 235.
[0716] 4.1.7. Additional Mutations
[0717] In some embodiments, an bi-specific antibody or antigen-binding construct as disclosed herein can include additional mutations. In some embodiments, the bi-specific antibody or antigen-binding construct can include a mutation to remove a methionine start residue. In some embodiments, the bi-specific antibody or antigen-binding construct can include a mutation to remove glycosylation (e.g. N297A). In some embodiments, the bi-specific antibody or antigen-binding construct can include a mutation to remove effector function (e.g., AAS mutation, as described in U.S. Patent Publicaiton No. 2016/0075792, which is incorporated herein by reference in its entirety). For example, in some embodiments, one or more of the additional mutations disclosed herein can be used to improve production of bi-specific antibody constructs or bi-specific antibody components in a host cell.
5. Germline
[0718] In some embodiments, an antibody or bi-specific antibody as disclosed herein that specifically binds Tim-3 is an antibody comprising a variable region that is encoded by a particular germline gene, or a variant thereof. The illustrative antibodies provided herein comprise variable regions that are encoded by the heavy chain variable region germline genes V.sub.H3-23 and V.sub.H5-51, or variants thereof and the light chain variable region germline genes V.kappa.3-20 and V.kappa.4-1, or variants thereof.
[0719] One of skill in the art would recognize that the CDR sequences provided herein may also be useful when combined with variable regions encoded by other variable region germline genes, or variants thereof. In particular, the CDR sequences provided herein may be useful when combined with variable regions encoded by variable region germline genes, or variants thereof, that are structurally similar to the variable region germline genes recited above. For example, in some embodiments, a CDR-H sequence provided herein may be combined with a variable region encoded by a variable region germline gene selected from the V.sub.H3 or V.sub.H5 families, or a variant thereof. In some embodiments, a CDR-L sequence provided herein may be combined with a variable region encoded by a variable region germline gene selected from the V.kappa.3 or V.kappa.4 families, or a variant thereof.
6. Affinity
[0720] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for Tim-3 as indicated by K.sub.D, is less than about 10.sup.-5 M, less than about 10.sup.-6M, less than about 10.sup.-7 M, less than about 10.sup.-8 M, less than about 10.sup.-9 M, less than about 10.sup.-10 M, less than about 10.sup.-11 M, or less than about 10.sup.-12 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-11 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-10 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-9 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-8 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-8M and 10.sup.-11M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-8 M and 10.sup.-10 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-9 M and 10.sup.-11 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-10 M and 10.sup.-11 M.
[0721] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for human Tim-3, as determined by surface plasmon resonance at 25.degree. C., and as indicated by K.sub.D, is between about 9.1.times.10.sup.-9 M and about 4.3.times.10.sup.-9 M. In some embodiments, the affinity of the antibody or bi-specific antibody for human Tim-3 is about 9.1.times.10.sup.-9 M, about 8.1.times.10.sup.-9M, about 7.9.times.10.sup.-9M, about 7.6.times.10.sup.-9M, about 7.46.times.10.sup.-9M, about 7.2.times.10.sup.-9M, about 6.8.times.10.sup.-9M, about 6.7.times.10.sup.-9M, about 6.69.times.10.sup.-9M, about 6.2.times.10.sup.-9M, about 6.0.times.10.sup.-9M, about 5.9.times.10.sup.-9 M, about 5.7.times.10.sup.-9 M, about 5.6.times.10.sup.-9 M, about 5.5.times.10.sup.-9 M, about 5.4.times.10.sup.-9 M, about 5.3.times.10.sup.-9 M, about 5.0.times.10.sup.-9 M, about 4.97.times.10.sup.-9 M, about 4.9.times.10.sup.-9 M, about 4.8.times.10.sup.-9 M, or about 4.3.times.10.sup.-9 M.
[0722] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.a of at least about 10.sup.4 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of at least about 10.sup.5 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of at least about 10.sup.6 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of between about 10.sup.4 M.sup.-1.times.sec.sup.-1 and about 10.sup.5 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of between about 10.sup.5 M.sup.-1.times.sec.sup.-1 and about 10.sup.6 M.sup.-1.times.sec.sup.-1.
[0723] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.a when associating with human Tim-3, as determined by surface plasmon resonance at 25.degree. C., of between about 6.71.times.10.sup.4 M.sup.-1.times.sec.sup.-1 and about 2.81.times.10.sup.5 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a when associating with human Tim-3 of about 6.71.times.10.sup.4 M.sup.-1.times.sec.sup.-1, 1.21.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.33.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.35.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.50.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.57.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.85.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.89.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.91.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.97.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 2.02.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 2.27.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 2.75.times.10.sup.5 M.sup.-1.times.sec.sup.-1, or about 2.81.times.10.sup.5 M.sup.-1.times.sec.sup.-1.
[0724] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.d of about 10.sup.-5 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of about 10.sup.-4 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of about 10.sup.-3 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-2 sec.sup.-1 and about 10.sup.-5 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-2 sec.sup.-1 and about 10.sup.-4 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-3 sec.sup.-1 and about 10.sup.-5 sec.sup.-1.
[0725] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.d when dissociating from human Tim-3, as determined by surface plasmon resonance at 25.degree. C., of between about 2.05.times.10.sup.-2 sec.sup.-1 and about 4.25.times.10.sup.-4 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d when dissociating from human Tim-3 of about 2.05.times.10.sup.-2 sec.sup.-1, about 1.40.times.10.sup.-2 sec.sup.-1, about 1.26.times.10.sup.-2 sec.sup.-1, about 3.93.times.10.sup.-3 sec.sup.-1, about 3.74.times.10.sup.-3 sec.sup.-1, about 2.49.times.10.sup.-3 sec.sup.-1, about 2.43.times.10.sup.-3 sec.sup.-1, about 1.82.times.10.sup.-3 sec.sup.-1, about 1.66.times.10.sup.-3 sec.sup.-1, about 1.59.times.10.sup.-3 sec.sup.-1, about 1.17.times.10.sup.-3 sec.sup.-1, about 5.44.times.10.sup.-4 sec.sup.-1, about 4.58.times.10.sup.-4 sec.sup.-1, or about 4.25.times.10.sup.-4 sec.sup.-1.
[0726] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for cynomolgus Tim-3, as determined by surface plasmon resonance at 25.degree. C., and as indicated by K.sub.D, is between about 13.2 nM and about 0.5 nM. In some embodiments, the affinity of the antibody or bi-specific antibody for human Tim-3 is about 13.2 nM, about 12.4 nM, about 9.4 nM, about 9.3 nM, about 7.4 nM, about 6.9 nM, about 5.6 nM, about 5.5 nM, about 5.4 nM, about 5.3 nM, about 4.7 nM, about 4.6 nM, about 4.5 nM, about 4.3 nM, about 4.1 nM, about 3.0 nM, about 2.8 nM, about 2.7 nM, about 2.5 nM, about 2.3 nM, about 2.2 nM, about 2.1 nM, about 1.9 nM, about 1.7 nM, about 1.0 nM, about 0.8 nM, or about 0.5 nM.
[0727] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for PD-1, as indicated by K.sub.D, is less than about 10.sup.-5 M, less than about 10.sup.-6 M, less than about 10.sup.-7M, less than about 10.sup.-8M, less than about 10.sup.-9M, less than about 10.sup.-10 M, less than about 10.sup.-11 M, or less than about 10.sup.-12 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-11 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-10 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-9 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-7 M and 10.sup.-8M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-8M and 10.sup.-11M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-8 M and 10.sup.-10 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-9M and 10.sup.-11 M. In some embodiments, the affinity of the antibody or bi-specific antibody is between about 10.sup.-10 M and 10.sup.-11 M.
[0728] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for human PD-1 is between about 3.85.times.10.sup.-8 M and 2.52.times.10.sup.-10 M. In some embodiment, the affinity of the antibody or bi-specific antibody for human PD-1 is about 2.55.times.10.sup.-8 M, about 1.52.times.10.sup.-8 M, about 9.52.times.10.sup.-9 M, about 1.09.times.10.sup.-8 M, about 4.50.times.10.sup.-9 M, about 1.90.times.10.sup.-9 M, about 4.76.times.10.sup.-9 M, about 4.5.times.10.sup.-9 M, about 1.04.times.10.sup.-8 M, about 9.90.times.10.sup.-9 M, about 9.13.times.10.sup.-10 M, about 2.52.times.10.sup.-10 M, about 2.58.times.10.sup.-9 M, about 3.85.times.10.sup.-8 M, about 3.66.times.10.sup.-9 M, about 3.15.times.10.sup.-9 M, about 5.14.times.10.sup.-9 M, about 2.47.times.10.sup.-9 M, about 2.79.times.10.sup.-9 M, about 1.20.times.10.sup.-9 M, or about 1.28.times.10.sup.-8 M
[0729] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for human PD-1 expressed on the surface of a cell is between about 3.2 and about 0.2 nM. In some embodiment, the affinity of the antibody or bi-specific antibody for human PD-1 expressed on the surface of a cell is about 0.2 nM, about 0.4 nM, about 0.9 nM, about 1 nM, about 0.3 nM, about 0.7 nM, about 0.2 nM, about 0.8 nM, about 3.2 nM, about 2.9 nM, about 1.39 nM, or about 1.34 nM.
[0730] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for murine PD-1 is between about 6.09.times.10.sup.-8 M and 9.08.times.10.sup.-9 M. In some embodiment, the affinity of the antibody or bi-specific antibody for murine PD-1 is about 6.09.times.10.sup.-8 M, about 6.22.times.10.sup.-8 M, or about 9.08.times.10.sup.-9 M.
[0731] In some embodiments, the affinity of an antibody or bi-specific antibody as disclosed herein for cynomolgus PD-1 is between about 2.43.times.10.sup.-8 M and 1.95.times.10.sup.-10 M. In some embodiment, the affinity of the antibody or bi-specific antibody for cynomolgus PD-1 is about 2.43.times.10.sup.-8 M, about 1.55.times.10.sup.-8 M, about 2.22.times.10.sup.-8 M, about 2.56.times.10.sup.-9 M, about 2.54.times.10.sup.-9 M, about 5.61.times.10.sup.-10 M, or about 1.95.times.10.sup.-10 M
[0732] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.a of at least about 10.sup.4 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of at least about 10.sup.5 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of at least about 10.sup.6 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of between about 10.sup.4 M.sup.-1.times.sec.sup.-1 and about 10.sup.5 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a of between about 10.sup.5 M.sup.-1.times.sec.sup.-1 and about 10.sup.6 M.sup.-1.times.sec.sup.-1.
[0733] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.a when associating with human PD-1 of between about 4.74.times.10.sup.4 M.sup.-1.times.sec.sup.-1 and about 1.23.times.10.sup.6 M.sup.-1.times.sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.a when associating with human PD-1 of about 4.88.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.23.times.10.sup.6 M.sup.-1.times.sec.sup.-1, about 7.37.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 6.87.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 5.63.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 5.16.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 2.48.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 7.98.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 1.82.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 4.74.times.10.sup.4 M.sup.-1.times.sec.sup.-1, about 1.85.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 2.00.times.10.sup.5 M.sup.-1.times.sec.sup.-1, about 8.12.times.10.sup.4 M.sup.-1.times.sec.sup.-1, about 1.21.times.10.sup.6 M.sup.-1.times.sec.sup.-1, about 1.16.times.10.sup.6 M.sup.-1.times.sec.sup.-1, about 5.13.times.10.sup.5 M.sup.-1.times.sec.sup.-1, or about 1.86.times.10.sup.5 M.sup.-1.times.sec.sup.-1.
[0734] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.d of about 10.sup.-5 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of about 10.sup.-4 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of about 10.sup.-3 sec.sup.-1 or less. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-2 sec.sup.-1 and about 10.sup.-5 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-2 sec.sup.-1 and about 10.sup.-4 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d of between about 10.sup.-3 sec.sup.-1 and about 10.sup.-5 sec.sup.-1.
[0735] In some embodiments an antibody or bi-specific antibody as disclosed herein has a k.sub.d when dissociating from human PD-1 of between about 1.87.times.10.sup.-2 sec.sup.-1 and about 4.17.times.10.sup.-4 sec.sup.-1. In some embodiments the antibody or bi-specific antibody has a k.sub.d when dissociating from human PD-1 of about 1.24.times.10.sup.-2 sec.sup.-1, about 1.87.times.10.sup.-2 sec.sup.-1, about 7.01.times.10.sup.-3 sec.sup.-1, about 7.74.times.10.sup.-3 sec.sup.-1, about 2.54.times.10.sup.-3 sec.sup.-1, about 9.80.times.10.sup.-4 sec.sup.-1, about 1.18.times.10.sup.-3 sec.sup.-1, about 3.59.times.10.sup.-3 sec.sup.-1, about 4.68.times.10.sup.-4 sec.sup.-1, about 1.82.times.10.sup.-3 sec.sup.-1, about 6.79.times.10.sup.-4 sec.sup.-1, about 6.28.times.10.sup.-4 sec.sup.-1, about 4.17.times.10.sup.-4 sec.sup.-1, about 2.99.times.10.sup.-3 sec.sup.-1, about 3.24.times.10.sup.-3 sec.sup.-1, about 6.17.times.10.sup.-4 sec.sup.-1, or about 2.39.times.10.sup.-3 sec.sup.-1.
[0736] In some aspects, the K.sub.D, k.sub.a, and k.sub.d are determined at 25.degree. C. In some embodiments, the K.sub.D, k.sub.a, and k.sub.d are determined by surface plasmon resonance. In some embodiments, the K.sub.D, k.sub.a, and k.sub.d are determined according to the methods described in the Examples provided herein.
7. Inhibition of PD-L1 and PD-L2 Binding
[0737] In some embodiments, an antibody or bi-specific antibody as disclosed herein inhibits binding of one or more of PD-L1 and PD-L2 to PD-1.
[0738] In some embodiments, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 1 to about 7 nM. In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 1.99, about 2.53, about 5.86, or about 5.96 nM.
[0739] In some embodiments, the antibody or bi-specific antibody inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.01 to about 1 nM. In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.01, about 0.18, about 0.56, or about 0.58 nM.
[0740] In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 5.96 nM, and inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.56 nM. In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 5.86 nM, and inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.58 nM. In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 1.99 nM, and inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.01 nM. In some aspects, the antibody or bi-specific antibody inhibits binding of PD-L1 to PD-1 with an IC.sub.50 of about 2.53 nM, and inhibits binding of PD-L2 to PD-1 with an IC.sub.50 of about 0.18 nM.
8. Epitope Bins
[0741] In some embodiments, an antibody or bi-specific antibody as disclosed herein binds the same epitope as the scFv antibody provided in SEQ ID NO: 287. In some embodiments, the antibody or bi-specific antibody binds to a different epitope from the scFv antibody provided in SEQ ID NO: 287. In some embodiments, the antibody or bi-specific antibody binds the same epitope as antibody encompassing any of SEQ ID NOs: 144-180. In some embodiments, the antibody or bi-specific antibody binds the same epitope as an antibody comprising any of the V.sub.H-V.sub.L pairs, above. In some embodiments, the antibody or bi-specific antibody binds to part of the epitope bound by the scFv antibody provided in SEQ ID NO: 287. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with the scFv antibody provided in SEQ ID NO: 287. In some embodiments, the antibody or bi-specific antibody does not compete for epitope binding with scFv antibody provided in SEQ ID NO: 287. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with an antibody encompassing any of SEQ ID NOs: 144-180. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with an antibody comprising any of the V.sub.H-V.sub.L pairs, above.
[0742] In some embodiments, an antibody or bi-specific antibody as disclosed herein binds the same epitope as the scFv antibody provided in SEQ ID NO: 288. In some embodiments, the antibody or bi-specific antibody binds to a different epitope from the scFv antibody provided in SEQ ID NO: 288. In some embodiments, the antibody or bi-specific antibody binds the same epitope as antibody encompassing any of SEQ ID NOs: 181-220. In some embodiments, the antibody or bi-specific antibody binds the same epitope as an antibody comprising any of the V.sub.H-V.sub.L pairs, above. In some embodiments, the antibody or bi-specific antibody binds to part of the epitope bound by the scFv antibody provided in SEQ ID NO: 288. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with the scFv antibody provided in SEQ ID NO: 288. In some embodiments, the antibody or bi-specific antibody does not compete for epitope binding with scFv antibody provided in SEQ ID NO: 288. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with an antibody encompassing any of SEQ ID NOs: 181-220. In some embodiments, the antibody or bi-specific antibody competes for epitope binding with an antibody comprising any of the V.sub.H-V.sub.L pairs, above.
9. Glycosylation Variants
[0743] In certain embodiments, an antibody or bi-specific antibody as disclosed herein may be altered to increase, decrease or eliminate the extent to which it is glycosylated. Glycosylation of polypeptides is typically either "N-linked" or "O-linked."
[0744] "N-linked" glycosylation refers to the attachment of a carbohydrate moiety to the side chain of an asparagine residue. The tripeptide sequences asparagine-X-serine and asparagine-X-threonine, where X is any amino acid except proline, are the recognition sequences for enzymatic attachment of the carbohydrate moiety to the asparagine side chain. Thus, the presence of either of these tripeptide sequences in a polypeptide creates a potential glycosylation site.
[0745] "O-linked" glycosylation refers to the attachment of one of the sugars N-acetylgalactosamine, galactose, or xylose to a hydroxyamino acid, most commonly serine or threonine, although 5-hydroxyproline or 5-hydroxylysine may also be used.
[0746] Addition or deletion of N-linked glycosylation sites to the antibody or bi-specific antibody may be accomplished by altering the amino acid sequence such that one or more of the above-described tripeptide sequences is created or removed. Addition or deletion of O-linked glycosylation sites may be accomplished by addition, deletion, or substitution of one or more serine or threonine residues in or to (as the case may be) the sequence of an antibody.
10. Fc Variants
[0747] In certain embodiments, amino acid modifications may be introduced into the Fc region of an antibody or bi-specific antibody provided herein to generate an Fc region variant. In certain embodiments, the Fc region variant possesses some, but not all, effector functions. Such antibodies may be useful, for example, in applications in which the half-life of the antibody or bi-specific antibody in vivo is important, yet certain effector functions are unnecessary or deleterious. Examples of effector functions include complement-dependent cytotoxicity (CDC) and antibody-directed complement-mediated cytotoxicity (ADCC). Numerous substitutions or substitutions or deletions with altered effector function are known in the art.
[0748] An alteration in in CDC and/or ADCC activity can be confirmed using in vitro and/or in vivo assays. For example, Fc receptor (FcR) binding assays can be conducted to measure Fc.gamma.R binding. The primary cells for mediating ADCC, NK cells, express Fc.gamma.RIII only, whereas monocytes express Fc.gamma.RI, Fc.gamma.RII and Fc.gamma.RIII FcR expression on hematopoietic cells is summarized in Ravetch and Kinet, Ann. Rev. Immunol., 1991, 9:457-492, incorporated by reference in its entirety.
[0749] Non-limiting examples of in vitro assays to assess ADCC activity of a molecule of interest are provided in U.S. Pat. Nos. 5,500,362 and 5,821,337; Hellstrom et al., Proc. Natl. Acad. Sci. U.S.A., 1986, 83:7059-7063; Hellstrom et al., Proc. Natl. Acad. Sci. U.S.A., 1985, 82:1499-1502; and Bruggemann et al., J. Exp. Med., 1987, 166:1351-1361; each of which is incorporated by reference in its entirety. Useful effector cells for such assays include peripheral blood mononuclear cells (PBMC) and Natural Killer (NK) cells. Alternatively, or additionally, ADCC activity of the molecule of interest may be assessed in vivo, using an animal model such as that disclosed in Clynes et al. Proc. Natl. Acad. Sci. U.S.A., 1998, 95:652-656, incorporated by reference in its entirety.
[0750] C1q binding assays may also be carried out to confirm that the antibody or bi-specific antibody is unable to bind C1q and hence lacks CDC activity. Examples of C1q binding assays include those described in WO 2006/029879 and WO 2005/100402, each of which is incorporated by reference in its entirety.
[0751] Complement activation assays include those described, for example, in Gazzano-Santoro et al., J. Immunol. Methods, 1996, 202:163-171; Cragg et al., Blood, 2003, 101:1045-1052; and Cragg and Glennie, Blood, 2004, 103:2738-2743; each of which is incorporated by reference in its entirety.
[0752] FcRn binding and in vivo clearance (half-life determination) can also be measured, for example, using the methods described in Petkova et al., Intl. Immunol., 2006, 18:1759-1769, incorporated by reference in its entirety.
11. Preparation of Antibodies and Bi-specific Antigen Binding Constructs
[0753] 11.1. Antigen Preparation
[0754] The Tim-3 antigen to be used for isolation of the antibodies and bi-specific antigen binding constructs disclosed herein may be intact Tim-3 or a fragment of Tim-3. The intact Tim-3, or fragment of Tim-3, may be in the form of an isolated protein or protein expressed by a cell. Other forms of Tim-3 useful for generating antibodies will be apparent to those skilled in the art.
[0755] The PD-1 antigen to be used for production of antibodies and bi-specific antigen binding constructs disclosed herein may be intact PD-1 or a fragment of PD-1. The intact PD-1, or fragment of PD-1, may be in the form of an isolated protein or expressed by a cell. Other forms of PD-1 useful for generating antibodies will be apparent to those skilled in the art.
[0756] 11.2. Monoclonal Antibodies
[0757] Monoclonal antibodies may be obtained, for example, using the hybridoma method first described by Kohler et al., Nature, 1975, 256:495-497 (incorporated by reference in its entirety), and/or by recombinant DNA methods (see e.g., U.S. Pat. No. 4,816,567, incorporated by reference in its entirety). Monoclonal antibodies may also be obtained, for example, using phage or yeast-based libraries. See e.g., U.S. Pat. Nos. 8,258,082 and 8,691,730, each of which is incorporated by reference in its entirety.
[0758] In the hybridoma method, a mouse or other appropriate host animal is immunized to elicit lymphocytes that produce or are capable of producing antibodies that will specifically bind to the protein used for immunization. Alternatively, lymphocytes may be immunized in vitro. Lymphocytes are then fused with myeloma cells using a suitable fusing agent, such as polyethylene glycol, to form a hybridoma cell. See Goding J. W., Monoclonal Antibodies: Principles and Practice 3.sup.rd ed. (1986) Academic Press, San Diego, Calif., incorporated by reference in its entirety.
[0759] The hybridoma cells are seeded and grown in a suitable culture medium that contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells. For example, if the parental myeloma cells lack the enzyme hypoxanthine guanine phosphoribosyl transferase (HGPRT or HPRT), the culture medium for the hybridomas typically will include hypoxanthine, aminopterin, and thymidine (HAT medium), which substances prevent the growth of HGPRT-deficient cells.
[0760] Useful myeloma cells are those that fuse efficiently, support stable high-level production of antibody by the selected antibody-producing cells, and are sensitive media conditions, such as the presence or absence of HAT medium. Among these, preferred myeloma cell lines are murine myeloma lines, such as those derived from MOP-21 and MC-11 mouse tumors (available from the Salk Institute Cell Distribution Center, San Diego, Calif.), and SP-2 or X63-Ag8-653 cells (available from the American Type Culture Collection, Rockville, Md.). Human myeloma and mouse-human heteromyeloma cell lines also have been described for the production of human monoclonal antibodies. See e.g., Kozbor, J. Immunol., 1984, 133:3001, incorporated by reference in its entirety.
[0761] After the identification of hybridoma cells that produce antibodies of the desired specificity, affinity, and/or biological activity, selected clones may be subcloned by limiting dilution procedures and grown by standard methods. See Goding, supra. Suitable culture media for this purpose include, for example, D-MEM or RPMI-1640 medium. In addition, the hybridoma cells may be grown in vivo as ascites tumors in an animal.
[0762] DNA encoding the monoclonal antibodies may be readily isolated and sequenced using conventional procedures (e.g., by using oligonucleotide probes that are capable of binding specifically to genes encoding the heavy and light chains of the monoclonal antibodies). Thus, the hybridoma cells can serve as a useful source of DNA encoding antibodies with the desired properties. Once isolated, the DNA may be placed into expression vectors, which are then transfected into host cells such as bacteria (e.g., E. coli), yeast (e.g., Saccharomyces or Pichia sp.), COS cells, Chinese hamster ovary (CHO) cells, or myeloma cells that do not otherwise produce antibody, to produce the monoclonal antibodies.
[0763] 11.3. Humanized Antibodies
[0764] Humanized antibodies may be generated by replacing most, or all, of the structural portions of a non-human monoclonal antibody with corresponding human antibody sequences. Consequently, a hybrid molecule is generated in which only the antigen-specific variable, or CDR, is composed of non-human sequence. Methods to obtain humanized antibodies include those described in, for example, Winter and Milstein, Nature, 1991, 349:293-299; Rader et al., Proc. Nat. Acad. Sci. U.S.A., 1998, 95:8910-8915; Steinberger et al., J. Biol. Chem., 2000, 275:36073-36078; Queen et al., Proc. Natl. Acad. Sci. U.S.A., 1989, 86:10029-10033; and U.S. Pat. Nos. 5,585,089, 5,693,761, 5,693,762, and 6,180,370; each of which is incorporated by reference in its entirety.
[0765] 11.4. Human Antibodies
[0766] Human antibodies can be generated by a variety of techniques known in the art, for example by using transgenic animals (e.g., humanized mice). See, e.g., Jakobovits et al., Proc. Natl. Acad. Sci. U.S.A., 1993, 90:2551; Jakobovits et al., Nature, 1993, 362:255-258; Bruggermann et al., Year in Immuno., 1993, 7:33; and U.S. Pat. Nos. 5,591,669, 5,589,369 and 5,545,807; each of which is incorporated by reference in its entirety. Human antibodies can also be derived from phage-display libraries (see e.g., Hoogenboom et al., J. Mol. Biol., 1991, 227:381-388; Marks et al., J. Mol. Biol., 1991, 222:581-597; and U.S. Pat. Nos. 5,565,332 and 5,573,905; each of which is incorporated by reference in its entirety). Human antibodies may also be generated by in vitro activated B cells (see e.g., U.S. Pat. Nos. 5,567,610 and 5,229,275, each of which is incorporated by reference in its entirety). Human antibodies may also be derived from yeast-based libraries (see e.g., U.S. Pat. No. 8,691,730, incorporated by reference in its entirety). Human antibodies can also be generated and screened by ribosome display (see, e.g., WO 2014/176327 and WO 2014/176439, each of which is incorporated herein by reference in its entirety).
12. Vectors, Host Cells, and Recombinant Methods
[0767] The invention also provides isolated nucleic acids encoding an antibody or bispecific antigen binding construct disclosed herein, vectors and host cells comprising the nucleic acids, and recombinant techniques for the production of the antibodies.
[0768] For recombinant production of the antibody, the nucleic acid(s) encoding it may be isolated and inserted into a replicable vector for further cloning (i.e., amplification of the DNA) or expression. In some aspects, the nucleic acid may be produced by homologous recombination, for example as described in U.S. Pat. No. 5,204,244, incorporated by reference in its entirety.
[0769] Many different vectors are known in the art. The vector components generally include, but are not limited to, one or more of the following: a signal sequence, an origin of replication, one or more marker genes, an enhancer element, a promoter, and a transcription termination sequence, for example as described in U.S. Pat. No. 5,534,615, incorporated by reference in its entirety.
[0770] Illustrative examples of suitable host cells are provided below. These host cells are not meant to be limiting.
[0771] Suitable host cells include any prokaryotic (e.g., bacterial), lower eukaryotic (e.g., yeast), or higher eukaryotic (e.g., mammalian) cells. Suitable prokaryotes include eubacteria, such as Gram-negative or Gram-positive organisms, for example, Enterobacteriaceae such as Escherichia (E. coli), Enterobacter, Erwinia, Klebsiella, Proteus, Salmonella (S. typhimurium), Serratia (S. marcescans), Shigella, Bacilli (B. subtilis and B. licheniformis), Pseudomonas (P. aeruginosa), and Streptomyces. One useful E. coli cloning host is E. coli 294, although other strains such as E. coli B, E. coli X1776, and E. coli W3110 are suitable.
[0772] In addition to prokaryotes, eukaryotic microbes such as filamentous fungi or yeast are also suitable cloning or expression hosts for anti-Tim-3 antibody-encoding vectors. Saccharomyces cerevisiae, or common baker's yeast, is a commonly used lower eukaryotic host microorganism. However, a number of other genera, species, and strains are available and useful, such as Schizosaccharomyces pombe, Kluyveromyces (K. lactis, K. fragilis, K. bulgaricus K. wickeramii, K. waltii, K. drosophilarum, K. thermotolerans, and K. marxianus), Yarrowia, Pichia pastoris, Candida (C. albicans), Trichoderma reesia, Neurospora crassa, Schwanniomyces (S. occidentalis), and filamentous fungi such as, for example Penicillium, Tolypocladium, and Aspergillus (A. nidulans and A. niger).
[0773] Suitable host cells can also include insect cells, such as, for example, Drosophila systems S2, SF9, and SF21 cells and High Five.TM. cells (ThermoFisher Scientific). Drosophila cells can be grown in a suitable medium, such as, for example, Schneider's Drosophila medium or other commercially available media,
[0774] Useful mammalian host cells include COS-7 cells, HEK293 cells; baby hamster kidney (BHK) cells; Chinese hamster ovary (CHO); mouse sertoli cells; African green monkey kidney cells (VERO-76), and the like.
[0775] The host cells used to produce the anti-Tim-3 antibody of this invention may be cultured in a variety of media. Commercially available media such as, for example, Ham's F10, Minimal Essential Medium (MEM), RPMI-1640, and Dulbecco's Modified Eagle's Medium (DMEM) are suitable for culturing the host cells. In addition, any of the media described in Ham et al., Meth. Enz., 1979, 58:44; Barnes et al., Anal. Biochem., 1980, 102:255; and U.S. Pat. Nos. 4,767,704, 4,657,866, 4,927,762, 4,560,655, and 5,122,469, or WO 90/03430 and WO 87/00195 may be used. Each of the foregoing references is incorporated by reference in its entirety.
[0776] Any of these media may be supplemented as necessary with hormones and/or other growth factors (such as insulin, transferrin, or epidermal growth factor), salts (such as sodium chloride, calcium, magnesium, and phosphate), buffers (such as HEPES), nucleotides (such as adenosine and thymidine), antibiotics, trace elements (defined as inorganic compounds usually present at final concentrations in the micromolar range), and glucose or an equivalent energy source. Any other necessary supplements may also be included at appropriate concentrations that would be known to those skilled in the art.
[0777] The culture conditions, such as temperature, pH, and the like, are those previously used with the host cell selected for expression, and will be apparent to the ordinarily skilled artisan.
[0778] When using recombinant techniques, the antibody can be produced intracellularly, in the periplasmic space, or directly secreted into the medium. If the antibody is produced intracellularly, as a first step, the particulate debris, either host cells or lysed fragments, is removed, for example, by centrifugation or ultrafiltration. For example, Carter et al. (Bio/Technology, 1992, 10:163-167) describes a procedure for isolating antibodies which are secreted to the periplasmic space of E. coli. Briefly, cell paste is thawed in the presence of sodium acetate (pH 3.5), EDTA, and phenylmethylsulfonylfluoride (PMSF) over about 30 min. Cell debris can be removed by centrifugation.
[0779] In some embodiments, the antibody is produced in a cell-free system. In some aspects, the cell-free system is an in vitro transcription and translation system as described in Yin et al., mAbs, 2012, 4:217-225, incorporated by reference in its entirety. In some aspects, the cell-free system utilizes a cell-free extract from a eukaryotic cell or from a prokaryotic cell. In some aspects, the prokaryotic cell is E. coli. Cell-free expression of the antibody may be useful, for example, where the antibody accumulates in a cell as an insoluble aggregate, or where yields from periplasmic expression are low.
[0780] Where the antibody is secreted into the medium, supernatants from such expression systems are generally first concentrated using a commercially available protein concentration filter, for example, an Amicon.RTM. or Millipore.RTM. Pellcon.RTM. ultrafiltration unit. A protease inhibitor such as PMSF may be included in any of the foregoing steps to inhibit proteolysis and antibiotics may be included to prevent the growth of adventitious contaminants.
[0781] The antibody composition prepared from the cells can be purified using, for example, hydroxylapatite chromatography, gel electrophoresis, dialysis, and affinity chromatography, with affinity chromatography being a particularly useful purification technique. The suitability of protein A as an affinity ligand depends on the species and isotype of any immunoglobulin Fc domain that is present in the antibody. Protein A can be used to purify antibodies that are based on human .gamma.1, .gamma.2, or .gamma.4 heavy chains (Lindmark et al., J. Immunol. Meth., 1983, 62:1-13, incorporated by reference in its entirety). Protein G is useful for all mouse isotypes and for human .gamma.3 (Guss et al., EMBO 1, 1986, 5:1567-1575, incorporated by reference in its entirety).
[0782] The matrix to which the affinity ligand is attached is most often agarose, but other matrices are available. Mechanically stable matrices such as controlled pore glass or poly(styrenedivinyl)benzene allow for faster flow rates and shorter processing times than can be achieved with agarose. Where the antibody comprises a C.sub.H3 domain, the BakerBond ABX.RTM. resin is useful for purification.
[0783] Other techniques for protein purification, such as fractionation on an ion-exchange column, ethanol precipitation, Reverse Phase HPLC, chromatography on silica, chromatography on heparin Sepharose.RTM., chromatofocusing, SDS-PAGE, and ammonium sulfate precipitation are also available, and can be applied by one of skill in the art. In embodiments where the antibody is a bi-specific antibody or antigen binding construct, separation techniques suitable for such molecules are described, for example, in Xu et al. 2015. mAbs 7:231-242, which is incorporated herein by reference in its entirety.
[0784] Following any preliminary purification step(s), the mixture comprising the antibody of interest and contaminants may be subjected to low pH hydrophobic interaction chromatography using an elution buffer at a pH between about 2.5 to about 4.5, generally performed at low salt concentrations (e.g., from about 0 to about 0.25 M salt).
13. Pharmaceutical Compositions and Methods of Administration
[0785] Any of the antibodies or bi-specific antigen binding constructs provided herein can be provided in any appropriate pharmaceutical composition and be administered by any suitable route of administration. Suitable routes of administration include, but are not limited to, the inhalation, intraarterial, intradermal, intramuscular, intraperitoneal, intravenous, nasal, parenteral, pulmonary, and subcutaneous routes.
[0786] The pharmaceutical composition may comprise one or more pharmaceutical excipients. Any suitable pharmaceutical excipient may be used, and one of ordinary skill in the art is capable of selecting suitable pharmaceutical excipients. Accordingly, the pharmaceutical excipients provided below are intended to be illustrative, and not limiting. Additional pharmaceutical excipients include, for example, those described in the Handbook of Pharmaceutical Excipients, Rowe et al. (Eds.) 6th Ed. (2009), incorporated by reference in its entirety.
[0787] In some embodiments, the pharmaceutical composition comprises an anti-foaming agent. Any suitable anti-foaming agent may be used. In some aspects, the anti-foaming agent is selected from an alcohol, an ether, an oil, a wax, a silicone, a surfactant, and combinations thereof. In some aspects, the anti-foaming agent is selected from a mineral oil, a vegetable oil, ethylene bis stearamide, a paraffin wax, an ester wax, a fatty alcohol wax, a long chain fatty alcohol, a fatty acid soap, a fatty acid ester, a silicon glycol, a fluorosilicone, a polyethylene glycol-polypropylene glycol copolymer, polydimethylsiloxane-silicon dioxide, ether, octyl alcohol, capryl alcohol, sorbitan trioleate, ethyl alcohol, 2-ethyl-hexanol, dimethicone, oleyl alcohol, simethicone, and combinations thereof.
[0788] In some embodiments, the pharmaceutical composition comprises a cosolvent. Illustrative examples of cosolvents include ethanol, poly(ethylene) glycol, butylene glycol, dimethylacetamide, glycerin, and propylene glycol.
[0789] In some embodiments, the pharmaceutical composition comprises a buffer. Illustrative examples of buffers include acetate, borate, carbonate, lactate, malate, phosphate, citrate, hydroxide, diethanolamine, monoethanolamine, glycine, methionine, guar gum, and monosodium glutamate.
[0790] In some embodiments, the pharmaceutical composition comprises a carrier or filler. Illustrative examples of carriers or fillers include lactose, maltodextrin, mannitol, sorbitol, chitosan, stearic acid, xanthan gum, and guar gum.
[0791] In some embodiments, the pharmaceutical composition comprises a surfactant. Illustrative examples of surfactants include d-alpha tocopherol, benzalkonium chloride, benzethonium chloride, cetrimide, cetylpyridinium chloride, docusate sodium, glyceryl behenate, glyceryl monooleate, lauric acid, macrogol 15 hydroxystearate, myristyl alcohol, phospholipids, polyoxyethylene alkyl ethers, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene stearates, polyoxylglycerides, sodium lauryl sulfate, sorbitan esters, and vitamin E polyethylene(glycol) succinate.
[0792] In some embodiments, the pharmaceutical composition comprises an anti-caking agent. Illustrative examples of anti-caking agents include calcium phosphate (tribasic), hydroxymethyl cellulose, hydroxypropyl cellulose, and magnesium oxide.
[0793] Other excipients that may be used with the pharmaceutical compositions include, for example, albumin, antioxidants, antibacterial agents, antifungal agents, bioabsorbable polymers, chelating agents, controlled release agents, diluents, dispersing agents, dissolution enhancers, emulsifying agents, gelling agents, ointment bases, penetration enhancers, preservatives, solubilizing agents, solvents, stabilizing agents, and sugars. Specific examples of each of these agents are described, for example, in the Handbook of Pharmaceutical Excipients, Rowe et al. (Eds.) 6th Ed. (2009), The Pharmaceutical Press, incorporated by reference in its entirety.
[0794] In some embodiments, the pharmaceutical composition comprises a solvent. In some aspects, the solvent is saline solution, such as a sterile isotonic saline solution or dextrose solution. In some aspects, the solvent is water for injection.
[0795] In some embodiments, the pharmaceutical compositions are in a particulate form, such as a microparticle or a nanoparticle. Microparticles and nanoparticles may be formed from any suitable material, such as a polymer or a lipid. In some aspects, the microparticles or nanoparticles are micelles, liposomes, or polymersomes.
[0796] Further provided herein are anhydrous pharmaceutical compositions and dosage forms comprising an antibody, since water can facilitate the degradation of some antibodies.
[0797] Anhydrous pharmaceutical compositions and dosage forms provided herein can be prepared using anhydrous or low moisture containing ingredients and low moisture or low humidity conditions. Pharmaceutical compositions and dosage forms that comprise lactose and at least one active ingredient that comprises a primary or secondary amine can be anhydrous if substantial contact with moisture and/or humidity during manufacturing, packaging, and/or storage is expected.
[0798] An anhydrous pharmaceutical composition should be prepared and stored such that its anhydrous nature is maintained. Accordingly, anhydrous compositions can be packaged using materials known to prevent exposure to water such that they can be included in suitable formulary kits. Examples of suitable packaging include, but are not limited to, hermetically sealed foils, plastics, unit dose containers (e.g., vials), blister packs, and strip packs.
[0799] In certain embodiments, provided are parenteral dosage forms. Parenteral dosage forms can be administered to subjects by various routes including, but not limited to, subcutaneous, intravenous (including bolus injection), intramuscular, and intraarterial. Because their administration typically bypasses subjects' natural defenses against contaminants, parenteral dosage forms are typically, sterile or capable of being sterilized prior to administration to a subject. Examples of parenteral dosage forms include, but are not limited to, solutions ready for injection, dry products ready to be dissolved or suspended in a pharmaceutically acceptable vehicle for injection, suspensions ready for injection, and emulsions.
[0800] Suitable vehicles that can be used to provide parenteral dosage forms are well known to those skilled in the art. Examples include, but are not limited to: Water for Injection USP; aqueous vehicles such as, but not limited to, Sodium Chloride Injection, Ringer's Injection, Dextrose Injection, Dextrose and Sodium Chloride Injection, and Lactated Ringer's Injection; water miscible vehicles such as, but not limited to, ethyl alcohol, polyethylene glycol, and polypropylene glycol; and non-aqueous vehicles such as, but not limited to, corn oil, cottonseed oil, peanut oil, sesame oil, ethyl oleate, isopropyl myristate, and benzyl benzoate.
[0801] Excipients that increase the solubility of one or more of the antibodies disclosed herein can also be incorporated into the parenteral dosage forms.
[0802] 13.1. Dosage and Unit Dosage Forms
[0803] In human therapeutics, the doctor will determine the posology which he considers most appropriate according to a preventive or curative treatment and according to the age, weight, condition and other factors specific to the subject to be treated.
[0804] In certain embodiments, a composition provided herein is a pharmaceutical composition or a single unit dosage form. Pharmaceutical compositions and single unit dosage forms provided herein comprise a prophylactically or therapeutically effective amount of one or more prophylactic or therapeutic antibodies.
[0805] The amount of the antibody or composition which will be effective in the prevention or treatment of a disorder or one or more symptoms thereof will vary with the nature and severity of the disease or condition, and the route by which the antibody is administered. The frequency and dosage will also vary according to factors specific for each subject depending on the specific therapy (e.g., therapeutic or prophylactic agents) administered, the severity of the disorder, disease, or condition, the route of administration, as well as age, body, weight, response, and the past medical history of the subject. Effective doses may be extrapolated from dose-response curves derived from in vitro or animal model test systems.
[0806] In certain embodiments, exemplary doses of a composition include milligram or microgram amounts of the antibody per kilogram of subject or sample weight (e.g., about 10 micrograms per kilogram to about 50 milligrams per kilogram, about 100 micrograms per kilogram to about 25 milligrams per kilogram, or about 100 microgram per kilogram to about 10 milligrams per kilogram). In certain embodiment, the dosage of the antibody provided herein, based on weight of the antibody, administered to prevent, treat, manage, or ameliorate a disorder, or one or more symptoms thereof in a subject is about 0.1 mg/kg, about 1 mg/kg, about 2 mg/kg, about 3 mg/kg, about 4 mg/kg, about 5 mg/kg, about 6 mg/kg, about 10 mg/kg, or about 15 mg/kg or more of a subject's body weight. In another embodiment, the dosage of the composition or a composition provided herein administered to prevent, treat, manage, or ameliorate a disorder, or one or more symptoms thereof in a subject is about 0.1 mg to about 200 mg, about 0.1 mg to about 100 mg, about 0.1 mg to about 50 mg, about 0.1 mg to about 25 mg, about 0.1 mg to about 20 mg, about 0.1 mg to about 15 mg, about 0.1 mg to about 10 mg, about 0.1 mg to about 7.5 mg, about 0.1 mg to about 5 mg, about 0.1 to about 2.5 mg, about 0.25 mg to about 20 mg, about 0.25 to about 15 mg, about 0.25 to about 12 mg, about 0.25 to about 10 mg, about 0.25 mg to about 7.5 mg, about 0.25 mg to about 5 mg, about 0.25 mg to about 2.5 mg, about 0.5 mg to about 20 mg, about 0.5 to about 15 mg, about 0.5 to about 12 mg, about 0.5 to about 10 mg, about 0.5 mg to about 7.5 mg, about 0.5 mg to about 5 mg, about 0.5 mg to about 2.5 mg, about 1 mg to about 20 mg, about 1 mg to about 15 mg, about 1 mg to about 12 mg, about 1 mg to about 10 mg, about 1 mg to about 7.5 mg, about 1 mg to about 5 mg, or about 1 mg to about 2.5 mg.
[0807] The dose can be administered according to a suitable schedule, for example, once, two times, three times, or for times weekly. It may be necessary to use dosages of the antibody outside the ranges disclosed herein in some cases, as will be apparent to those of ordinary skill in the art. Furthermore, it is noted that the clinician or treating physician will know how and when to interrupt, adjust, or terminate therapy in conjunction with subject response.
[0808] Different therapeutically effective amounts may be applicable for different diseases and conditions, as will be readily known by those of ordinary skill in the art. Similarly, amounts sufficient to prevent, manage, treat or ameliorate such disorders, but insufficient to cause, or sufficient to reduce, adverse effects associated with the antibodies provided herein are also encompassed by the herein described dosage amounts and dose frequency schedules. Further, when a subject is administered multiple dosages of a composition provided herein, not all of the dosages need be the same. For example, the dosage administered to the subject may be increased to improve the prophylactic or therapeutic effect of the composition or it may be decreased to reduce one or more side effects that a particular subject is experiencing.
[0809] In certain embodiments, treatment or prevention can be initiated with one or more loading doses of an antibody or composition provided herein followed by one or more maintenance doses.
[0810] In certain embodiments, a dose of an antibody or composition provided herein can be administered to achieve a steady-state concentration of the antibody in blood or serum of the subject. The steady-state concentration can be determined by measurement according to techniques available to those of skill or can be based on the physical characteristics of the subject such as height, weight and age.
[0811] In certain embodiments, administration of the same composition may be repeated and the administrations may be separated by at least aboutl day, about 2 days, about 3 days, about 5 days, about 10 days, about 15 days, about 30 days, about 45 days, about 2 months, about 75 days, about 3 months, or about 6 months. In other embodiments, administration of the same prophylactic or therapeutic agent may be repeated and the administration may be separated by at least about 1 day, about 2 days, about 3 days, about 5 days, about 10 days, about 15 days, about 30 days, about 45 days, about 2 months, about 75 days, about 3 months, or about 6 months.
14. Therapeutic Applications
[0812] For therapeutic applications, the antibodies of the invention are administered to a mammal, generally a human, in a pharmaceutically acceptable dosage form such as those known in the art and those discussed above. For example, the antibodies of the invention may be administered to a human intravenously as a bolus or by continuous infusion over a period of time, by intramuscular, intraperitoneal, intra-cerebrospinal, subcutaneous, intra-articular, intrasynovial, intrathecal, or intratumoral routes. The antibodies also are suitably administered by peritumoral, intralesional, or perilesional routes, to exert local as well as systemic therapeutic effects. The intraperitoneal route may be particularly useful, for example, in the treatment of ovarian tumors.
[0813] The antibodies provided herein may be useful for the treatment of any disease or condition involving Tim-3 and/or PD-1. In some embodiments, the disease or condition is a disease or condition that can be diagnosed by overexpression of Tim-3 and/or PD-1. In some embodiments, the disease or condition is a disease or condition that can benefit from treatment with an anti-Tim-3 antibody and/or anti-PD-1 antibody. In some embodiments, the disease or condition is a cancer. In some embodiments, the disease or condition is an autoimmune disease. In some embodiments, the disease or condition is an infection.
[0814] Any suitable cancer may be treated with the antibodies provided herein. Illustrative suitable cancers include, for example, acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), adrenocortical carcinoma, anal cancer, appendix cancer, astrocytoma, basal cell carcinoma, brain tumor, bile duct cancer, bladder cancer, bone cancer, breast cancer, bronchial tumor, carcinoma of unknown primary origin, cardiac tumor, cervical cancer, chordoma, colon cancer, colorectal cancer, craniopharyngioma, ductal carcinoma, embryonal tumor, endometrial cancer, ependymoma, esophageal cancer, esthesioneuroblastoma, fibrous histiocytoma, Ewing sarcoma, eye cancer, germ cell tumor, gallbladder cancer, gastric cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumor, gestational trophoblastic disease, glioma, head and neck cancer, hepatocellular cancer, histiocytosis, Hodgkin lymphoma, hypopharyngeal cancer, intraocular melanoma, islet cell tumor, Kaposi sarcoma, kidney cancer, Langerhans cell histiocytosis, laryngeal cancer, lip and oral cavity cancer, liver cancer, lobular carcinoma in situ, lung cancer, macroglobulinemia, malignant fibrous histiocytoma, melanoma, Merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer with occult primary, midline tract carcinoma involving NUT gene, mouth cancer, multiple endocrine neoplasia syndrome, multiple myeloma, mycosis fungoides, myelodysplastic syndrome, myelodysplastic/myeloproliferative neoplasm, nasal cavity and par nasal sinus cancer, nasopharyngeal cancer, neuroblastoma, non-small cell lung cancer, oropharyngeal cancer, osteosarcoma, ovarian cancer, pancreatic cancer, papillomatosis, paraganglioma, parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytomas, pituitary tumor, pleuropulmonary blastoma, primary central nervous system lymphoma, prostate cancer, rectal cancer, renal cell cancer, renal pelvis and ureter cancer, retinoblastoma, rhabdoid tumor, salivary gland cancer, Sezary syndrome, skin cancer, small cell lung cancer, small intestine cancer, soft tissue sarcoma, spinal cord tumor, stomach cancer, T-cell lymphoma, teratoid tumor, testicular cancer, throat cancer, thymoma and thymic carcinoma, thyroid cancer, urethral cancer, uterine cancer, vaginal cancer, vulvar cancer, and Wilms tumor.
[0815] In particular embodiments, the cancer is a cancer of epithelial origin. In some aspects, the cancer is a carcinoma. In some aspects, the cancer is selected from an adenocarcinoma, a squamous cell carcinoma, an adenosquamos carcinoma, an anaplastic carcinoma, a large cell carcinoma, small cell carcinoma, and carcinoma of unknown primary origin.
15. Diagnostic Applications
[0816] In some embodiments, the antibodies provided herein are used in diagnostic applications.
[0817] In some embodiments, an antibody or bi-specific antibody as disclosed herein may be useful in assays for Tim-3 protein. In some aspects the antibody or bi-specific antibody can be used to detect the expression of Tim-3 in various cells and tissues. These assays may be useful, for example, in making a diagnosis and/or prognosis for a disease, such as a cancer.
[0818] In some embodiments, an antibody or bi-specific antibody as disclosed herein may be useful in assays for PD-1 protein. In some aspects the antibody or bi-specific antibody can be used to detect the expression of PD-1 in various cells and tissues. These assays may be useful, for example, in making a diagnosis and/or prognosis for a disease, such as a cancer.
[0819] In some diagnostic and prognostic applications, the antibody or bi-specific antibody may be labeled with a detectable moiety. Suitable detectable moieties include, but are not limited to radioisotopes, fluorescent labels, and enzyme-substrate labels. In another embodiment, the antibody or bi-specific antibody need not be labeled, and the presence of the antibody or bi-specific antibody can be detected using a labeled antibody which specifically binds to the anti-Tim-3 antibody.
16. Affinity Purification Reagents
[0820] The antibodies and bi-specific antigen binding constructs disclosed herein may be used as affinity purification agents. In this process, the antibodies or bi-specific antibodies may be immobilized on a solid phase such a resin or filter paper, using methods well known in the art. The immobilized antibody or bi-specific antibody is contacted with a sample containing an antigen protein (or fragment thereof) to be purified, and thereafter the support is washed with a suitable solvent that will remove substantially all the material in the sample except the antigen protein, which is bound to the immobilized antibody. Finally, the support is washed with another suitable solvent, such as glycine buffer, pH 5.0 or glycine buffer, pH 3 to 4, that will release the antigen protein from the antibody. In some embodiments, the antigen protein is Tim-3. In some embodiments, the antigen protein is PD-1.
17. Kits
[0821] In some embodiments, an antibody or bi-specific antigen binding construct provided herein is provided in the form of a kit, i.e., a packaged combination of reagents in predetermined amounts with instructions for performing a procedure. In some embodiments, the procedure is a diagnostic assay. In other embodiments, the procedure is a therapeutic procedure.
[0822] In some embodiments, the kit further comprises a solvent for the reconstitution of the antibody or bispecific antibody. In some embodiments, the antibody or bispecific antibody is provided in the form of a pharmaceutical composition.
EXAMPLES
Example 1: Tim-3 Hybridoma Generation
[0823] Balb/C mice were immunized with the extracellular domain of human Tim-3 fused with human Fc (R&D Systems) using standard immunization methods. The spleens and/or lymph nodes of the mice were harvested and fused with P3X cells to generate the hybridomas (Aragen Biosciences, Morgan Hill, Calif.), similar to published reports. See Chronopoulou et al., 2014, Methods Mol Biol. 1131: 47-70 (2014); Kim et al., 2014, Methods Mol Biol. 1131: 33-45; each incorporated by reference in its entirety. Total RNA was extracted from hybridoma cells using QIAGEN RNeasy Mini Kit (Cat No. 74104) and converted to cDNA using a Clontech SMARTer RACE cDNA Amplification Kit (Cat. No. 634923; Lake Pharma, Belmont, Calif.). Positive clones were identified by gel electrophoresis, cloned using an Invitrogen TOPO kit, and sequenced using standard Sanger methods. Mouse single-chain antibodies were constructed by using total gene synthesis using optimized E. Coli codons and cloned into a standard cell-free expression vector. See Yin et al., supra.
[0824] Mouse hybridoma clone 22E11 showed binding of human Tim-3 expressed on CHO cells and binding of cynomolgus Tim-3 expressed on CHO cells. The k.sub.a for the m22E11 Ab was 2.23.times.10.sup.5 M.sup.-1.times.sec.sup.-1; the k.sub.d was 3.34.times.10.sup.-4 sec.sup.-1, and the K.sub.D was 1.50.times.10.sup.-9 M.
[0825] Mouse hybridoma clone 2D5 showed binding of human Tim-3 expressed on CHO cells, but no binding of cynomolgus Tim-3 expressed on CHO cells. The k.sub.a for the 2D5 Ab was 3.86.times.10.sup.5 M.sup.-1.times.sec.sup.-1; the k.sub.d was 1.66.times.10.sup.-4 sec.sup.-1, and the K.sub.D was 4.31.times.10.sup.-10 M.
[0826] The CDRs for clone m22E11 were grafted onto human antibody frameworks VH1-69, VH3-23, VH4-30-4, VH5-51, VH3-33 (h22E11-5 HC), Vk1-39, Vk2-28, Vk3-11, Vk4-1 and Vk3-20 (h22E11-5) LC by standard methodology to yield humanized antibodies of every HC and LC combination. See Kuramochi et al., 2014, Methods in Molecular Biology 1060: 123-137. In the results below, "h22E11" indicates a humanized variant of m22E11. The heavy chains are indicated by framework designation (e.g., "VH1-69") and the light chains are indicated by framework designation (e.g., "Vk1-39").
TABLE-US-00021 TABLE 21 Antibodies From 2D5 and 22E11 Antibody V.sub.H V.sub.L 250.180.2.22E11 22E11-VH 22E11-VL 421.51.2.2D5.5E9 2D5-VH 2D5-VL h22E11-VK1-39 .times. h22E11-VH1-69 h22E11-VH1-69-VH h22E11-Vk1-39-VL h22E11-VK1-39 .times. h22E11-VH3-23 h22E11-VH3-23-VH h22E11-Vk1-39-VL h22E11-VK1-39 .times. h22E11-VH4-30-4 h22E11-VH4-30-4-VH h22E11-Vk1-39-VL h22E11-VK1-39 .times. h22E11-VH5-51 h22E11-VH5-51-VH h22E11-Vk1-39-VL h22E11-VK1-39 .times. h22E11-5-IgG-HC h22E11-5-VH h22E11-Vk1-39-VL h22E11-VK2-28 .times. h22E11-VH1-69 h22E11-VH1-69-VH h22E11-Vk2-28-VL h22E11-VK2-28 .times. h22E11-VH3-23 h22E11-VH3-23-VH h22E11-Vk2-28-VL h22E11-VK2-28 .times. h22E11-VH4-30-4 h22E11-VH4-30-4-VH h22E11-Vk2-28-VL h22E11-VK2-28 .times. h22E11-VH5-51 h22E11-VH5-51-VH h22E11-Vk2-28-VL h22E11-VK2-28 .times. h22E11-5-IgG-HC h22E11-5-VH h22E11-Vk2-28-VL h22E11-VK3-11 .times. h22E11-VH1-69 h22E11-VH1-69-VH h22E11-Vk3-11-VL h22E11-VK3-11 .times. h22E11-VH3-23 h22E11-VH3-23-VH h22E11-Vk3-11-VL h22E11-VK3-11 .times. h22E11-VH4-30-4 h22E11-VH4-30-4-VH h22E11-Vk3-11-VL h22E11-VK3-11 .times. h22E11-VH5-51 h22E11-VH5-51-VH h22E11-Vk3-11-VL h22E11-VK3-11 .times. h22E11-5-IgG-HC h22E11-5-VH h22E11-Vk3-11-VL h22E11-VK4-1 .times. h22E11-VH1-69 h22E11-VH1-69-VH h22E11-Vk4-1-VL h22E11-VK4-1 .times. h22E11-VH3-23 h22E11-VH3-23-VH h22E11-Vk4-1-VL h22E11-VK4-1 .times. h22E11-VH4-30-4 h22E11-VH4-30-4-VH h22E11-Vk4-1-VL h22E11-VK4-1 .times. h22E11-VH5-51 h22E11-VH5-51-VH h22E11-Vk4-1-VL h22E11-VK4-1 .times. h22E11-5-IgG-HC h22E11-5-VH h22E11-Vk4-1-VL h22E11-5-IgG-LC .times. h22E11-VH1-69 h22E11-VH1-69-VH h22E11-5-VL h22E11-5-IgG-LC .times. h22E11-VH3-23 h22E11-VH3-23-VH h22E11-5-VL h22E11-5-IgG-LC .times. h22E11-VH4-30-4 h22E11-VH4-30-4-VH h22E11-5-VL h22E11-5-IgG-LC .times. h22E11-VH5-51 h22E11-VH5-51-VH h22E11-5-VL h22E11-5-IgG-LC .times. h22E11-5-IgG-HC h22E11-5-VH h22E11-5-VL h22E11-5 IgG h22E11-5-VH h22E11-5-VL
Example 2: Generation and Primary Screening of Anti-Tim-3 Antibodies
[0827] Antibody Fab libraries were constructed using a standard overlap extension PCR protocol with mutagenic primers targeting complementary determining regions (CDRs). See Heckman and Pease, Nat. Protoc., 2007, 2:924-932; Stafford et al., 2014, Protein Eng. Des. Sel. 27:97-109, both incorporated by reference in their entireties.
[0828] Initial antibody leads from ribosome display (e.g. SRP1497 antibodies) were derived from a naive human library which was constructed by overlapping PCR using trastuzumab HC as the base template. CDRs H1 and H2 were randomized with the same design as described by Lee et al., 2004, J. Mol. Biol. 2004, 340:1073-1093 using oligonucleotides purchased from Integrated DNA Technologies. In this design, CDRs H1 and H2 closely match the observed amino acid distributions of natural human antibodies. CDR H3 was diversified using oligonucleotides incorporating trimer phosphoramidite mixtures (TRIMs) for amino acid randomization. The TRIM oligos were synthesized as described by Yagodkin A et al., Nucleosides Nucleotides Nucleic Acids 2007, 26:473-97. Specifically, six separate oligonucleotides containing TRIMs were used to make 6 separate H3 loop-lengths (13-18; as defined by Zemlin et al.) to match the most common loop lengths observed in the human repertoire. Together these loop lengths comprise approximately 54.5% of the naturally-occurring loop length variation in human IgGs as reported by Zemlin et al., J. Mol. Biol. 2003, 334:733-749. The frequency distribution of each amino acid was designed to closely match the observed distribution of amino acids in CDR H3 of human IgGs as reported by Zemlin et al. Altogether, the library closely matches natural human antibody variation which is known in the field to improve antibody stability and folding of antibodies as described by Zhai et al., J Mol Biol. 2011, 412:55-71. The HC library was paired with a constant, unmodified trastuzumab LC throughout the selection process as described by Stafford et al., Protein Eng Des Sel 2014, 27:97-109.
[0829] Affinity maturated antibody leads (e.g. SRP1649 antibodies) were derived from a focused library, biased towards the lead (1497-A05) which was constructed by overlapping PCR using "soft-randomized" oligonucleotides purchased from Eurofins MWG Operon. Soft-randomization is a process in which a biased distribution of nucleotides is used for each soft-randomized codon such that the parent amino acid sequence is coded more frequently than other amino acids .about.30% of the time. Other amino acids are coded at each position but at a lower percentage. At each soft-randomized position, 70% of the parent nucleotide is mixed with 10% of the other three nucleotides. For the library, CDRs H1, H2, and H3 were soft-randomized simultaneously and selected by standard ribosome display protocols. As with the selection of initial leads, the affinity matured antibodies were paired with a constant, unmodified trastuzumab LC throughout the selection process as described by Stafford et al., Protein Eng Des Sel 2014, 27:97-109. For the affinity maturation, the selection was run against human and cyno TIM3 to yield human and cyno cross-reactive antibodies.
[0830] Selections for novel antibodies were performed using standard ribosome display protocols. See Dreier and PlUckthun, Methods Mol. Biol., 2003, 687:283-306, Clifton, N.J., incorporated by reference in its entirety. Fab ribosome display selections were performed according to published protocols. See Stafford et al., 2014, Protein Eng. Des. Sel. 27:97-109, incorporated by reference in its entirety. After multiple rounds of selection, the DNA from RT-PCR output was cloned into an optimized vector for cell-free expression using standard molecular biology techniques. See Yin et al., mAbs, 2012, 4:217-225, incorporated by reference in its entirety. All constructs were HIS- and FLAG-tagged to streamline purification and testing during screening.
[0831] Libraries of antibody variants generated by selection workflow were transformed into E. coli and grown on agar plates with antibiotic (kanamycin). Individual colonies were grown in liquid broth (TB+kanamycin), and used as a template for DNA amplification via rolling circle amplification (RCA). The Fab-HC variants, together with about 2.5 .mu.g/mL of a trastuzumab LC, were then expressed in cell-free protein synthesis reactions as described in Zawada et al., Biotechnol. Bioeng., 2011, 108:1570-1578, incorporated by reference in its entirety.
[0832] Briefly, cell-free extracts were treated with 50 .mu.M iodoacetamide for 30 min at room temperature (20.degree. C.) and added to a premix containing cell-free components (see Groff et al., mAbs, 2014, 6:671-678, incorporated by reference in its entirety) and 10% (v/v) RCA DNA template (approximately 10 .mu.g/mL DNA) for HC variants, in addition to 2.5 ug/mL Trastuzumab LC which is present for antibody assembly but does not contribute to the binding to the target antigen. Sixty microliters of cell-free reactions were incubated at 30.degree. C. for 12 hr on a shaker at 650 rpm in 96-well plates. Four hundred to one-thousand-five-hundred colonies were screened, depending on the predicted diversity of different selection campaigns.
[0833] Following synthesis, each reaction was diluted 1:50 into PBST (PBS at pH 7.4 with 0.2% Tween-20+0.2% BSA) and expressed variants were tested for functional activity via ELISA-based binding to recombinant human Tim-3 extracellular domain (ECD) (Acro Biosystems, TM3-H5229, Accession # Q8TDQ0; R&D Systems, 2365-TM, Accession # Q8TDQ0). Standard ELISA-based methods were employed. Specifically, 384-well plates were coated with 2 .mu.g/mL recombinant Tim-3 diluted in bicarbonate buffer, and then blocked with bovine serum albumin (BSA). Antibody variants of interest were allowed to bind to the Tim-3-coated plates, and detected with secondary antibodies (e.g., HRP-conjugated anti-human Fc or anti-FLAG) and then detected with chemiluminescent substrate (Pierce ELISA SuperSignal.TM. Substrate). Chemiluminescence was quantified on a Molecular Devices SpectraMax.RTM. M5 plate reader. Top hits were selected based on ELISA signal or signal/noise ratio and their nucleotides were sequenced. Based on functional activity and sequence analysis, a subset of variants was selected for further scale-up and characterization. The resulting antibodies are reported in Table 22, below, beginning with the designation SRP1497.
[0834] The top leads from ELISA-based screening were cultured and plasmid minipreps were performed using a QiAprep.RTM. 96 Turbo miniprep kit (Qiagen) according to the manufacturer's instructions. 10 .mu.g/mL miniprepped DNA was added to 4 mL cell-free reactions and incubated overnight for 12 hr at 30.degree. C., at 650 rpm. In the case of IgG variants with a common Trastuzumab LC, 7.5 ug/mL of the HC variant DNA and 2.5 ug/mL of the common Trastuzumab LC were added to the reaction.
[0835] Expressed variants from clarified cell-free reactions were purified via immobilized metal ion affinity chromatography (IMAC) purification using a semi-automated high throughput batch purification method. Briefly, purifications were performed in a 96-well plate format where 50 .mu.L/well of IMAC resin (Ni Sepharose High Performance, GE Healthcare) was equilibrated in IMAC binding buffer (50 mM Tris pH 8.0, 300 mM NaCl, 10 mM imidazole), incubated with 1 mL cell-free reaction for 15 minutes followed by two washes in IMAC binding buffer. His-tagged antibody variants were then eluted using 200 .mu.L IMAC elution buffer (50 mM Tris pH 8.0, 300 mM NaCl, 500 mM imidazole) and buffer exchanged into PBS using a 96-well Zeba plate (7 kD MWCO, Thermo Fisher). Purified antibodies were quantified via high throughput capillary electrophoresis using the LabChip GXII (Perkin Elmer) against a Herceptin standard curve, according to the manufacturer's instructions.
TABLE-US-00022 TABLE 22 Naive (1497) and Affinity Matured (1649) Antibodies Antibody V.sub.H V.sub.L SRP1497-A01 SRP1497-A01 trastuzumab VL SRP1497-A02 SRP1497-A02 trastuzumab VL SRP1497-A05 SRP1497-A05 trastuzumab VL SRP1649-A01 SRP1649-A01 trastuzumab VL SRP1649-B06 SRP1649-B06 trastuzumab VL SRP1649-B09 SRP1649-B09 trastuzumab VL SRP1649-C05 SRP1649-C05 trastuzumab VL SRP1649-D06 SRP1649-D06 trastuzumab VL SRP1649-D11 SRP1649-D11 trastuzumab VL SRP1649-E08 SRP1649-E08 trastuzumab VL SRP1649-E10 SRP1649-E10 trastuzumab VL SRP1649-F06 SRP1649-F06 trastuzumab VL SRP1649-G08 SRP1649-G08 trastuzumab VL SRP1649-H02 SRP1649-H02 trastuzumab VL
Example 3: Affinity and Kinetic Binding Analyses of Anti-Tim-3 Antibodies
[0836] Monoclonal Anti-FLAG M2 IgG (Sigma-Aldrich # F9291) was immobilized onto a CM5 chip (GE Life Sciences) using amine coupling chemistry (from Amine Coupling Kit, GE Life Sciences). The immobilization steps were carried out at a flow rate of 25 .mu.L/min in 1.times.HBS-EP+buffer (GE Life Sciences; 10.times. Stock diluted before use). The sensor surfaces were activated for 7 min with a mixture of NHS (0.05 M) and EDC (0.2 M). The Anti-Flag M2 IgG was injected over all 4 flow cells at a concentration of 25 .mu.g/mL in 10 mM sodium acetate, pH 4.5, for 7 min. Ethanolamine (1 M, pH 8.5) was injected for 7 min to block any remaining activated groups. An average of 12,000 response units (RU) of capture antibody was immobilized on each flow cell.
[0837] Off-rate and kinetic binding experiments were performed at 25.degree. C. using 1.times. HBS-EP+buffer. Test and control antibodies were injected over the Anti-FLAG surface at concentrations of 5-10 .mu.g/mL for 12 seconds at a flow rate of 10 .mu.L/min on flow cells 2, 3 and 4, followed by a buffer wash for 30 seconds at the same flow rate. Kinetic characterization of antibody samples was carried out with a single concentration of antigen (for off-rate ranking) or a dilution series of antigen (for kinetic characterization) and 1 injection of 0 nM antigen. After capturing ligand (antibody) on the anti-FLAG surface, the analyte (human TIM-3-Fc or TIM-3-HIS) was bound at 50, 25, 12.5, 6.25 and 0 nM for 180 seconds, followed by a 600 second dissociation phase at a flow rate of 50 .mu.l/min. Between each ligand capture and analyte binding cycle, regeneration was carried out using 2 injections of 10 mM glycine pH 2.0 for 30 seconds at 30 .mu.L/min, followed by a 30 second buffer wash step.
[0838] The data were fit with the Biacore T200 Evaluation software, using a 1:1 Langmuir binding model. K.sub.D (affinity, nM) was determined as a ratio of the kinetic rate constants calculated from the fits of the association and dissociation phases.
Example 4: Tim-3/Galectin9 Competition ELISA
[0839] Anti-Tim-3 variants were tested for their ability to block a Tim-3/Galectin9 interaction. Galectin-9 (R&D Systems) was adsorbed on Nunc 384-well white Maxisorp plates at 2 .mu.g/mL in sodium bicarbonate buffer (pH 8.9) and incubated at 30.degree. C. for 1 hour or overnight at 4.degree. C. The plate was washed 3 times with PBS pH 7.4 with 0.05% Tween20 and blocked with 2% bovine serum albumin (BSA) in PBS pH 7.4+0.1% Tween20 for 1 hour at 30.degree. C. The blocking solution was aspirated, and a dilution series of antibody was mixed with 10 nM biotinylated Tim-3-Fc (R&D Systems) in 0.2% BSA in PBS pH 7.4+0.1% Tween20 (diluent buffer) and incubated at 30.degree. C. for 1 hour. The plate was washed, and streptavidin-HRP (Pierce) in diluent buffer was added to all wells. After 1 hour incubation at 30.degree. C., the plate was washed, followed by detection with SuperSignal Pico Chemiluminescent Substrate (Thermo Pierce). Luminescence was detected on a SpectraMax.RTM. M5 plate reader (Molecular Devices).
Example 5: Tim-3 ELISA
[0840] Anti-Tim-3 variants were tested for their ability to bind human or cynomolgous Tim-3. Recombinant Tim-3 protein (R&D Systems, huTim-3-Fc, 2365-TM, Accession # Q8TDQ0; cyTim-3-Fc, 7914-TM, Accession # EHH54703) was adsorbed on Nunc 384-well white Maxisorp plates at 2 .mu.g/mL in sodium bicarbonate buffer (pH 8.9) and incubated at 30.degree. C. for 1 hour or overnight at 4.degree. C. The plate was washed 3 times with PBS pH 7.4 with 0.05% Tween20 and blocked with 2% bovine serum albumin (BSA) in PBS pH 7.4+0.1% Tween20 for 1 hour at 30.degree. C. The blocking solution was aspirated, and a dilution series of anti-Tim-3 antibody in 0.2% BSA in PBS pH 7.4+0.1% Tween20 (diluent buffer) was pipetted to the ELISA plate and incubated at 30.degree. C. for 1 hour. The plate was washed, and anti-Flag-HRP (Sigma-Aldrich, A8592) in diluent buffer was added to all wells. After 1 hour incubation at 30.degree. C., the plate was washed, followed by detection with SuperSignal Pico Chemiluminescent Substrate (Thermo Pierce). Luminescence was detected on a SpectraMax.RTM. M5 plate reader (Molecular Devices).
Example 6: Flow Cytometry-Based Cell Binding Assay of Anti-Tim-3 Antibodies
[0841] CHO-k cells were transfected to stably express Tim-3 on the cell surface. CHO parental and stably transfected CHO-Tim-3 cells (human or cynomolgus Tim-3) were cultured in RPMI w/10% fetal calf serum (FCS), Penicillin/Streptomycin (or Pen/Strep) and glutamine (or Gln). On day of assay, cells were washed with DPBS, detached with Accutase.TM. (BD Biosciences; San Jose, Calif.), and resuspended in RPMI media.
[0842] A mixture of fluoresecent-labeled parental CHO cells and unlabeled CHO-Tim-3 cells were prepared as follows. Parental CHO cells in RPMI media were incubated with 1 uM CellTrace.TM. Oregon Green488.RTM. (Life Technologies) at 37.degree. C. for 15 to 30 minutes. Cells were then washed 3.times. with RPMI media. Labeled parental CHO and unlabeled CHO-Tim-3 cells were combined at 1:1 ratio, washed 1.times. in ice-cold FACS buffer (DPBS buffer supplemented with 0.5% bovine serum albumin) and seeded at 100 .mu.l per well containing a total of 200,000 cells in 96 well polypropylene plates. Cells were spun down at 1.5K rpm and resuspended with test antibodies diluted in FACS buffer and incubated on ice for 60 mins. Cells were washed twice with FACS buffer and incubated on ice for 30 mins with R-phycoerythrin AffiniPure F(ab')2 fragment, goat anti-Human IgG, Fc.gamma. fragment specific secondary detection antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.) diluted at 1:200 with FACS buffer. Cells were washed twice with FACS buffer, fixed in 4% paraformaldehyde in PBS (Santa Cruz Biotechnology; Dallas, Tex.) for 20 mins on ice in the dark, washed twice with FACS buffer and analyzed using the BD LSR II Flow Cytometer (BD Biosciences; San Jose, Calif.). Data were analyzed using FlowJo (FlowJo, LLC; Ashland, Oreg.) to determine mean fluorescence intensities. Binding constants were calculated using the statistical software, GraphPad Prism (GraphPad Software; La Jolla, Calif.) using the nonlinear regression equation, one site--specific binding with Hill slope. Secondary antibody alone was used as a control, in addition to measuring non-specific antibody binding to CHO parental cells.
Example 7: CMV Recall Assay of Anti-Tim-3 Antibodies
[0843] CD14+ monocytes and CD3+ T cells were obtained from peripheral blood mononuclear (PBMC) isolated from CMV+ human donors (AllCells, Alameda, Calif.) using MACS Cell Separation kits (Miltenyi Biotec). CD14+ monocytes were differentiated into immature dendritic cells (DC) by culturing cells at 10.sup.6 cells/ml for 7 days in presence of GM-CSF and IL-4 (Peprotech) in X-Vivo 15 media (Lonza) containing 2% human AB serum (Sigma-Aldrich), penicillin-streptomycin (Corning Mediatech) and GlutaMAX (Life Technologies). Following differentiation, DCs were matured by culturing in X-Vivo 15+2% human AB serum media at 10.sup.6 cells/ml for 2 days in the presence of GM-C SF, IL-4, TNF-a, IL-1b, IL-6 (Peprotech) and prostaglandin E2 (Sigma-Aldrich). To set-up the CMV recall assay, mature DCs were collected, washed and 10,000 DCs and 100,000 pan CD3+ T cells were plated per well in a 96-well U-bottom plate in a total volume of 100 .mu.l media containing peptide pools for the CMV IE-1 and CMV pp65 protein (Miltenyi Biotec). IgG antibodies (50 ul) were added starting at a final concentration of 133 nM with 5-fold serial dilutions. Cells were co-cultured with peptides and antibodies for 5-6 days. Conditioned media was collected and tested for human IFN-g levels by ELISA (BD Biosciences).
Example 8: DC/CD4+ T Cell Mixed Lymphocyte Reaction (MLR)) of Anti-Tim-3 Antibodies
[0844] CD14+ monocytes and CD4+ T cells were obtained from PBMC isolated from human donors using MACS Cell Separation kits. CD14+ monocytes were differentiated into immature DC by culturing cells at 10.sup.6 cells/ml cell density for 7 days in presence of GM-CSF and IL-4 in RPMI media containing 10% fetal bovine serum, penicillin-streptomycin and GlutaMAX. Following differentiation, DCs were matured by culturing in RPMI+10% FBS media at 10.sup.6 cells/ml cell density for 2 days in the presence of GM-CSF, IL-4, TNF-a, IL-1b, IL-6 and prostaglandin E2. To set-up the DC/CD4+ T cell MLR, mature DCs were collected, washed and 10,000 DCs and 100,000 CD4+ T cells were plated per well in a 96-well U-bottom plate in a total volume of 100 .mu.l media. IgG antibodies (50 ul, final volume of 150 .mu.l per well) were added starting at a final concentration of 133 nM with 5-fold serial dilutions. Cells were co-cultured with peptides and antibodies for 5-6 days. Conditioned media was collected and tested for human IFN-g levels by ELISA.
Example 9: Cell Binding of Anti-Tim-3 Antibodies on Activated Primary Human T Cells
[0845] CD4+ T cells were obtained from PBMC isolated from human donors using MACS Cell Separation kit. CD4+ T cells (2e6 cells/ml) were activated with CD3/CD28 Human T-Activator Dynabeads (Life Technologies) in RPMI+10% FBS media containing 100 U/ml human IL-2 (Peprotech) for 2-3 days. Activated CD4+ T cells expressing Tim-3 were used test anti-Tim-3 antibodies for FACS cell binding.
Example 10: Characteristics of Illustrative Anti-Tim-3 Antibodies
[0846] Tables 23-24 show results obtained using the illustrative antibodies described herein.
[0847] Table 23 shows results from humanized variants of mouse hybridoma clone m22E11 with various human frameworks.
TABLE-US-00023 TABLE 23 Humanized h22E11 Antibody Characterization Human Tim-3 Fc Human Human Cyno Gal9 MLR (Biacore) Tim-3 Tim-3 Tim-3 ELISA activity Humanized K.sub.D (nM) (CHO) (T-Cell) (CHO) comp IFNg Antibody k.sub.d (1/s) estimated K.sub.D (nM) K.sub.D (nM) K.sub.D (nM) IC.sub.50 (nM) release h22E11-VK1-39 .times. 9.40E-04 6 0.6 2.6 1.7 Not Not h22E11-VH1-69 tested tested h22E11-VK1-39 .times. 9.40E-04 5.6 0.5 1.1 4.5 Not Not h22E11-VH3-23 tested tested h22E11-VK1-39 .times. 1.26E-03 9.1 0.7 poor 5.5 Not Not h22E11-VH4-30-4 tested tested h22E11-VK1-39 .times. 9.47E-04 5.3 0.4 0.9 2.1 Not Not h22E11-VH5-51 tested tested h22E11-VK1-39 .times. 1.02E-03 6.2 0.6 2.4 4.7 Not Not h22E11-5-IgG-HC tested tested h22E11-VK2-28 .times. 8.63E-04 5.4 0.7 2.3 1.7 Not Not h22E11-VH1-69 tested tested h22E11-VK2-28 .times. 9.11E-04 5.9 0.8 1.7 5.4 Not Not h22E11-VH3-23 tested tested h22E11-VK2-28 .times. 1.09E-03 7.9 0.8 9.2 4.3 Not Not h22E11-VH4-30-4 tested tested h22E11-VK2-28 .times. 9.03E-04 4.8 0.6 0.7 2.3 Not Not h22E11-VH5-51 tested tested h22E11-VK2-28 .times. 1.01E-03 6.7 0.8 3.3 4.1 Not Not h22E11-5-IgG-HC tested tested h22E11-VK3-11 .times. 8.67E-04 4.9 1 4 2.8 Not Not h22E11-VH1-69 tested tested h22E11-VK3-11 .times. 9.34E-04 5.3 0.7 0.9 6.9 Not positive h22E11-VH3-23 tested h22E11-VK3-11 .times. 1.07E-03 7.2 1 7.1 4.5 Not Not h22E11-VH4-30-4 tested tested h22E11-VK3-11 .times. 8.75E-04 4.3 0.8 0.6 2.7 Not positive h22E11-VH5-51 tested h22E11-VK3-11 .times. 1.04E-03 5.9 0.8 1.1 5.3 0.64 Not h22E11-5-IgG-HC tested h22E11-VK4-1 .times. 8.63E-04 5.5 1 6.3 2.5 Not Not h22E11-VH1-69 tested tested h22E11-VK4-1 .times. 8.75E-04 5 0.9 0.9 4.6 0.67 Not h22E11-VH3-23 tested h22E11-VK4-1 .times. 1.19E-03 8.1 1.1 5.7 5 Not Not h22E11-VH4-30-4 tested tested h22E11-VK4-1 .times. 8.81E-04 4.8 0.8 0.6 1.9 Not Not h22E11-VH5-51 tested tested h22E11-VK4-1 .times. 9.73E-04 5.7 0.8 1.4 5.3 Not Not h22E11-5-IgG-HC tested tested h22E11-5-IgG-LC .times. 9.91E-04 4.9 0.9 1.6 3 Not Not h22E11-VH1-69 tested tested h22E11-5-IgG-LC .times. 8.95E-04 5.3 0.8 1.2 13.2 Not Not h22E11-VH3-23 tested tested h22E11-5-IgG-LC .times. 1.17E-03 7.6 3.7 poor poor Not Not h22E11-VH4-30-4 tested tested h22E11-5-IgG-LC .times. 1.10E-03 5 poor poor poor Not Not h22E11-VH5-51 tested tested h22E11-5-IgG-LC .times. 1.10E-03 6.8 poor poor poor Not Not h22E11-5-IgG-HC tested tested h22E11-5 IgG (lot 2) Not Not 1.0 Not poor 0.77 Not tested tested tested tested
[0848] Table 24 shows results obtained from antibodies isolated from a naive Fab TRIM ribosome display library, constructed on a Trastuzumab HC framework.
[0849] The "EC.sub.50" value is the concentration of the antibody at which half-maximum signal is achieved in an ELISA assay where Tim-3 protein is adsorbed onto a plate and then bound by the respective antibody provided herein. The anti-Tim-3 antibody is detected with horseradish peroxidase (HRP)-conjugated anti-human Fc antibody.
TABLE-US-00024 TABLE 24 Results obtained from antibodies isolated from a first affinity matured library, based on the SRP1464-B04 antibody provided in Table 21. Human Cyno Human Tim-3-Fc Cell Tim-3-Fc Tim-3-Fc Gal9- IgG (Biacore) Binding ELISA ELISA blockade Antibody k.sub.a (1/Ms) k.sub.d (1/s) K.sub.D (M) K.sub.D (nM) EC.sub.50 (nM) EC.sub.50 (nM) IC.sub.50 (nM) SRP1497-A01 1.97E+05 4.25E-04 2.15E-09 1.17 0.30 22.56 0.8 SRP1497-A02 1.85E+05 5.44E-04 2.95E-09 1.54 0.34 13.74 1.0 SRP1497-A05 2.02E+05 4.58E-04 2.27E-09 5.6 0.24 6.193 1.1
[0850] Table 25 shows results obtained from antibodies isolated from a second affinity matured library constructed by performing soft randomization on the SRP1497-A05 antibody.
TABLE-US-00025 TABLE 25 Results obtained from antibodies isolated from a second affinity matured library. Human Cyno Human Tim-3 Tim-3 Tim-3 Gal9- IgG (Biacore) (CHO) (CHO) blockade Antibody k.sub.a (1/Ms) k.sub.d (1/s) K.sub.D (M) K.sub.D (nM) K.sub.D (nM) IC.sub.50 (nM) SRP1497-A05 3.38E+05 2.19E-02 6.47E-08 0.5 +/- Not tested SRP1649-A01 6.71E+04 1.17E-03 1.74E-08 0.4 0.5 0.26 SRP1649-B06 1.89E+05 1.26E-02 6.69E-08 0.2 1.9 Not tested SRP1649-B09 1.33E+05 2.49E-03 1.88E-08 0.2 9.4 Not tested SRP1649-C05 1.21E+05 1.66E-03 1.37E-08 0.2 0.8 Not tested SRP1649-D06 2.75E+05 2.05E-02 7.46E-08 0.1 1.0 Not tested SRP1649-D11 1.50E+05 1.59E-03 1.06E-08 0.1 2.2 Not tested SRP1649-E08 1.35E+05 1.82E-03 1.35E-08 0.2 12.4 Not tested SRP1649-E10 2.81E+05 1.40E-02 4.97E-08 0.2 7.4 Not tested SRP1649-F06 2.27E+05 3.93E-03 1.73E-08 0.2 5.6 0.63 SRP1649-G08 1.91E+05 2.43E-03 1.28E-08 0.2 9.3 Not tested SRP1649-H02 1.57E+05 3.74E-03 2.39E-08 0.2 2.2 Not tested
Example 11: Preparation of Tim-3 scFvs
[0851] A single-chain antibody is made in either the V.sub.HV.sub.L or V.sub.LV.sub.H orientation with a linker sequence between the V.sub.H and V.sub.L domains. Typically, scFv linkers are composed of (GGGGS).sub.n repeats where n=3, 4, 5, or 6 for linkers of 15, 20, 25, or 30 residues respectively. For cell-free expression, an N-terminal Met is added, but for mammalian expression a leader peptide is added. On the C-terminal end of the scFv, an Fc sequence can be added to extend in vivo half-life or the scFv can be used directly. An optional linker sequence can be incorporated between the scFv and the Fc. An exemplary scFv-Fc linker sequence is AAGSDQEPKSS (SEQ ID NO: 282). C-terminal affinity tags can optionally be added to facilitate purification and assay development. An exemplary affinity tag is a C-terminal FlagHis tag GSGDYKDDDDKGSGHHHHHH (SEQ ID NO: 277). A stop codon is typically inserted at the end of the sequence. An exemplary scFv of the present disclosure is SEQ ID NO: 287, with an N-terminal Met residue, a V.sub.H domain, a GGGGSGGGGSGGGGS (SEQ ID NO: 278) linker, a V.sub.L domain, an AAGSDQEPKSS (SEQ ID NO: 282) linker, an Fc domain, a FlagHis tag, and a stop codon.
Example 12: Mutation Design in Anti-Tim-3 and Anti-PD1
[0852] To improve solubility and reduce aggregation propensity, aspartic acid mutations were initially introduced into the heavy chain of affinity matured anti-Tim-3 antibody 1649-A01 around and in CDR H1 (S30D, R31D) and around and in light chain CDR L2 (S50D, A51D, S52D, F53D, Y55D, S56D). This general approach of targeting H1 and L2 to reduce aggregation propensity has been described previously (Dudgeon et al. 2012. PNAS 109(27):10879-10884, incorporated herein by reference in its entirety). Additional aspartic acid mutations were also designed around CDR H2 (G50D, V53D, Y56D, E58D) to determine if these mutations also reduce aggregation propensity by a similar mechanism. Trastuzumab (also known as Herceptin or 4D5) is known to be a highly stable antibody with desirable biophysical properties, including folding and low aggregation propensity (Jung and Pluckthun. 1997. Protein Eng 10(8):959-966, incorporated herein by reference in its entirety). Thus, the 1649-A01 heavy chain sequence was also aligned to the 4D5 sequence using MAFFT (Katoh et al. 2013. Mol Biol Evol 30:772-780, incorporated herein by reference in its entirety) to identify additional mutations (S30K, Y32T, G50R, V53T, R54N, E58R, D65G) that could improve stability and/or reduce aggregation propensity. Following screening of the mutations as described below, combination mutants were designed to further reduce aggregation propensity (S30D/R31D, S30D/Y56D, S30D/R31D/Y56D). Only combination mutants without NxT/S glycosylation motifs were chosen to prevent glycosylation during expression in CHO.
[0853] To improve solubility and reduce aggregation propensity, an aspartic acid mutation was also made to PD-1 antibody 1353-G10 in CDR H1 (P30D). See, e.g., WO 2016/060033, incorporated herein by reference in its entirety. The mutation was introduced using the general approach described above and in the literature (Dudgeon et al. 2012. PNAS 109(27):10879-10884). IgBLAST (Ye et al. 2013. Nucleic Acids Res 41 (Web Server issue):W34-W40, incorporated herein by reference in its entirety) was used to analyze the 1353-G10 heavy chain. The analysis showed that R28T and H31S mutations would render the sequence closer to the native VH1-18 human germline sequence, which could reduce immunogenicity in humans and improve biophysical behavior, e.g., reduction of aggregationpropensity, transition melting temperature, etc. Lastly, to improve the stability of the light chain of 1353-G10, the CDRs were grafted onto a kappa framework Vk1-39 in a similar manner as described previously (Lehmann et al. 2015. mAbs 7(6):1058-1071, incorporated herein by reference in its entirety). The stabilizing mutations and kappa-grafted light chain were also made in the context of an scFv framework, both alone and in combination with each other. The sequences of these scFv are presented as SEQ ID NO: 339 (stabilizing mutations and kappa grafted light chain), SEQ ID NO: 340 (stabilizing mutations), and SEQ ID NO: 341 (kappa grafted light chain), respectively.
[0854] Affinity and kinetic binding analysis was carried out on bi-specific antibody candidates as described in Example 3. The analytes used for analyte binding (for kinetic characterization of the antibodies) were human Tim-3-Fc or cynomolgus Tim-3-Fc, R&D Systems, Minneapolis, Minn.). Additional characterization of bi-specific antibody candidates are described in Example 13 to Example 18.
Example 13: Differential Scanning Fluorimetry
[0855] A protein thermal shift assay was carried out by mixing the protein to be assayed with an environmentally sensitive dye (SYPRO.RTM. Orange, Life Technologies Cat. # S-6650) in a phosphate buffered solution (PBS), and monitoring the fluorescence of the mixture in real time as it underwent controlled thermal denaturation. Protein solutions between 0.2-2 mg/mL were mixed at a 1:1 volumetric ratio with a 1:500 PBS-diluted solution of SYPRO.RTM. Orange (SYPRO.RTM. Orange stock dye is 5000.times. in DMSO). Aliquots of 10 .mu.L of the protein-dye mixture were dispensed in quadruplicate in a 384-well microplate (Bio-Rad Cat # MSP-3852), and the plate was sealed with an optically clear sealing film (Bio-Rad Cat # MSB-1001) and placed in a 384-well plate real-time thermocycler (Bio-Rad CFX384 Real Time System). The protein-dye mixture was heated from 25.degree. C. to 95.degree. C., at increments of 0.1.degree. C. per cycle (.about.1.5.degree. C. per minute), allowing 3 seconds of equilibration at each temperature before taking a fluorescence measurement. At the end of the experiment, the first and second transition melting temperatures (Tm1 and Tm2, respectively) were determined using the Bio-Rad CFX manager software.
Example 14: Turbidity Assay
[0856] Affinity-matured anti-Tim-3 1649-A01 antibody and its derivatives were tested for their aggregation propensity with a turbidity assay. Antibody variants were formulated in PBS with 0.3 M sodium chloride and ranged from 0.5-1 mg/mL in concentration. Samples were subsequently aliquoted to a clear microtiter plate and incubated at 55.degree. C. for 8 hours, during which absorbance at 280 nm and 600 nm were measured at periodic time intervals using a Spectramax plate reader. Antibody samples were given a qualitative score from "-" to "++++", where "-" reflected little to no change in A600 absorbance and "++++" reflected a significant increase in A600 absorbance indicative of aggregation. Antibody variants with reduced aggregation propensity (i.e., little to no absorbance at A600) were selected for further characterization and development.
Example 15: PD-1 Competition Assay
[0857] The ability of an anti-PD-1 antibody or an anti-PD-1 bi-specific antibody construct to block PD-1 binding to PD-L1 was measured as by PD-1 competition assay. CHO cells expressing human PD-L1 were seeded at 100,000 cells per 96-well in FACS buffer (1.times. PBS, 0.5% BSA, 0.1% sodium azide). All steps were performed on ice. Antibody titrations were prepared in FACS buffer and added to cells, followed by addition of 4 ug/ml of biotinylated recombinant human PD-1 (AcroBiosystems). Cells were incubated on ice for 1 hour and washed twice. Biotinylated PD-1 binding on CHO-human PD-L1 cells was detected with streptavidin-PE (1:100 dilution, Ebioscience) for 30 minutes, and cells were subsequently washed twice. Cells were fixed with 2% paraformaldehyde in PBS for 30 minutes. Fixed cells were washed with FACS buffer before flow cytometry analysis. FlowJo X was used to generate median MFI values for each sample and Prism 6 software was used to create one site, specific binding with Hill slope curves (Log transform) to determine IC.sub.50 values.
Example 16: Bridging Assay
[0858] To validate that the generated bi-specific antibodies could bind to both protein targets simultaneously, bridging assays were developed. A homogeneous bead-based assay based on Perkin Elmer's AlphaLISA.RTM. platform (Perkin Elmer, Waltham, Mass.) was utilized. Briefly, recombinant human Tim-3-Fc protein and human PD1 protein were conjugated to AlphaLISA.RTM. acceptor and donor beads, respectively, per the manufacturer's instructions in a reductive amination reaction utilizing sodium borohydride. When Tim-3-acceptor beads and PD1-donor beads are brought into close proximity by binding to a PD-1.times.Tim-3 bi-specific antibody, excitation of the donor bead (680 nm) triggers an emission from the acceptor bead (615 nm). Thus, PD-1.times.Tim-3 bi-specific bridging was measured over a dilution series of each antibody and the result was reported as an EC50 and maximal signal (B max) of each curve.
Example 17: PD-1.times.Tim-3 Cell-based Bridging Assay
[0859] A cell-based bridging assay was developed using the PathHunter.RTM. platform (DiscoverX, Fremont, Calif.) to detect simultaneous binding of PD-1 and Tim-3 on the cell surface. Briefly, a split beta-galactosidase reporter enzyme was utilized such that enzyme activity could only be detected when the enzyme acceptor (EA) and ProLink (PK) fragments are allowed to assemble. The EA-fragment was C-terminally fused to PD-1 (residues 1-199) and the PK fragment was C-terminally fused to Tim-3 (residues 1-223). Both fusion constructs were coexpressed in U20S cells, and stable cell pools were generated. To assess PD-1.times.Tim-3 bi-specific bridging, 10,000 cells per well were added to 96-well plates. The bi-specific antibodies were serially diluted (3-fold) starting at 10 ug/mL and incubated with the cells for 16 hours at 37.degree. C. Binding was subsequently detected by measuring beta-galactosidase activity using the Beta-Glo.RTM. Assay System (Promega Cat. # TM239) and the plates were read on an Envision luminometer (integration time of 0.5 sec/well). Prism 6 software was used to create agonist vs. response curves with variable slope to determine EC.sub.50 values.
Example 18: CMV Recall Assay for Bi-specific Antibodies
[0860] Peripheral blood mononuclear cells (PBMC) were initially isolated from CMV-positive human donors (Stanford Blood Center) by differential gradient centrifugation using NycoPrep.TM. 1.077 (Axis-Shield). PBMC were cryopreserved with Recovery.TM. Cell Culture Freezing Medium (Life Technologies) in liquid nitrogen. For the assay, PBMC (0.2.times.10.sup.6 cells/well) were cultured in serum-free media containing 2% human AB serum and CMV peptide pools for the CMV IE-1 and CMV pp65 proteins (Miltenyi Biotec) in a total volume of 100 ul per well. Anti-PD-1/Tim-3 bi-specific antibody candidates (50 ul/well) were added as 3x stock with 5-fold serial dilution titration. Cells were cultured for 5-6 days. Conditioned media was collected and tested for human IFN-.gamma., IL-6 and TNF-.alpha. levels by ELISA (BD Biosciences).
[0861] Representative data for a CMV-peptide responsive human donor is shown in FIGS. 4-6. Anti-PD-1/Tim-3 bi-specific antibody (1353-G10-scFv.times.1649-A01-Fab, "BsAb A") mediates greater dose-response activity for IFN-.gamma., IL-6 and TNF-.alpha. release from PBMC in response to antigen-peptide stimulation alone (media alone) and PD-1.times. stump bi-specific ("BsAb stump"), indicating that the anti-PD1 arm alone, 1353-G10, and more so in combination with the anti-Tim-3 arm, 1649-A01, as a bi-specific antibody shows potent activity. Furthermore, anti-PD-1/Tim-3 bi-specific (1353-G10-scFv.times.1649-A01-Fab, "BsAb A") mediates equivalent or greater dose-response activity compared to a competitor anti-PD-1 IgG (nivolumab, also known as Opdivo) alone or as a mixture with anti-Tim-3 ABTIM3 (Novartis) IgG.
Example 19: Characteristics of Illustrative PD-1/Tim-3 Bi-Specific Antibodies
[0862] Table 26 shows the results obtained using the illustrative bi-specific antibodies described herein. The bi-specific antibody of protein sample A contains the Fab of PD-1 antibody 1353-G10 and the scFv of Tim-3 humanized antibody h22E11. The bi-specific antibody of protein sample B contains the scFv of PD-1 antibody 1353-G10 and the Fab of Tim-3 humanized antibody h22E11. The bi-specific antibody of protein sample C contains the scFv of PD-1 antibody 1353-G10 and the Fab of Tim-3 humanized antibody 1649-A01. The bi-specific antibody of protein sample D contains the Fab of PD-1 antibody 1353-G10 and stump, where the stump refers to the Fc domain (hinge+C.sub.H1+C.sub.H2 regions). The results indicate that the bi-specific antibodies of samples A to C exhibited specific binding to human and cynomolgus PD-1 and to human and cynomolgus Tim-3 expressed on the cell surface of CHO cells. In addition, the bi-specific antibodies illustrated (1) the ability to effectively compete for and block the interaction between PD-1 and PDL-1 (see, e.g., the PDL-1 competition IC.sub.50 values); (2) simultaneous binding to PD-1 and TIM-3 antigens (see, e.g., AlphaLISA bridging results); and (3) better capability for bringing a higher proportion of PD-1 and TIM-3 conjugated beads into close proximity relative to the antibody construct without the TIM-3 binding arm (see, e.g., B max values of the AlphaLISA bridging assay, Samples A-C versus Sample D).
[0863] Table 27 shows additional results obtained from illustrative bi-specific antibodies described herein. The bi-specific antibodies exemplified in the table comprise an scFvFc PD-1 antibody 1353-G10 with mutations T350V/T366L/K392L/T394W ("zwB") in the C.sub.H3 domain and Tim-3 antibody 1649-A01 with mutations T350V/L351Y/F405A/Y407V ("zwA") in the C.sub.H3 domain, or its derivatives (Von Kreudenstein et al. 2013. mAbs 5(5):646-654; Von Kreudenstein et al. 2014. Methods 65:77-94; and U.S. 2013/0195849, each of which is incorporated herein by reference in its entirety). Several point mutations to an aspartate residue were introduced in various combinations throughout the anti-TIM-3 antibody (heavy chain: 530D, R31D, Y56D; light chain: S56D). All bi-specific antibodies showed similar ability to block recombinant human PD-1 binding to CHO cells expressing human PD-L1. In addition, all bi-specific antibodies illustrated the ability to bind simultaneously to human PD-1 and human TIM-3 that are expressed on the same cells. Overall, the mutations did not have an impact on binding affinity to recombinant CHO cell lines overexpressing PD-1 or TIM-3, nor was there a significant difference in kinetic affinity to TIM-3 antigen measured by SPR. As there were observed improvements in aggregation propensity by introduction of the mutations (Example 14), but no significant differences in the thermostability of the anti-TIM-3 Fab arm of the bispecific antibody (Tm2), this suggested that some of these point mutations may be beneficial for antibody stability without impacting functional activity.
[0864] Tables 28 and 29 provide assay results and kinetics of antigen binding to PD-1 and Tim-3 using the illustrative bi-specific antibodies. The PD-1 antibody 1353-G10 was produced either as an IgG or as a bi-specific antibody with Tim-3 antibody 1649-A01. In the bi-specific antibody format, the PD-1 antibody was provided as an scFv, the Tim-3 antibody was provided as a Fab fragment, and a mutation was introduced at V262E of the heavy chain to improve biophysical properties of the bi-specific format. In addition, the PD-1 antibody was provided as wild-type 1353-G10 IgG (SEQ ID NO: 181; Samples E, I), as a triple mutant derivative (SEQ ID NO: 197; Samples F, J), as a kappa graft derivative (SEQ ID NO: 244; Samples G, K), or as both a triple mutant and kappa graft derivative (SEQ ID NO: 197, SEQ ID NO: 244; Samples H, L). In the context of the bi-specific antibody, the 1353-G10 scFv triple mutant derivative had the impact of improved thermostability at the expense of slightly reducing affinity. The kappa-grafted 1353-G10 further improved thermostability without significantly impacting PD-1 affinity. In combination, the triple mutation and the kappa graft further enhanced thermostability, which was more pronounced in the context of the bispecific antibody when 1353-G10 was in a scFv conformation as opposed to the IgG conformation.
TABLE-US-00026 TABLE 26 Characteristics of Bi-specific Antibodies AlphaLISA CHO-Tim-3 bridging CHO-PD-1 cell binding cell binding EC50 hPD-1, hPD-1, cPD-1, cPD-1, hTim-3, Sample Description (nM) Bmax Kd (nM) Bmax Kd (nM) Bmax Kd (nM) A 1353-G10-Fab .times. 0.48 49743 1.7 17256 3.1 10314 7.8 h22E11 scFv 51.39 B 1353-G10-scFv .times. 0.16 31379 1.1 11642 5.2 7794 11 h22E11 Fab 51.39 C 1353-G10-scFv .times. 0.05 455697 1.3 11234 2.7 7901 4.4 1649-A01-Fab D 1353-G10-Fab .times. 263 0.8 19122 1.2 10287 -- Stump CHO-Tim-3 cell binding PDL1 Activated Activated hTim-3, cTim-3, cTim-3, competition CD4 T cell CD8 Tcell Sample Bmax Kd (nM) Bmax IC50 (nM) Kd (nM) Bmax Kd (nM) Bmax A 5803 8.9 5228 23 0.4 1703 0.7 754 B 5991 14 4222 23 1.4 1496 1.5 712 C 12113 29 5468 11 0.4 1586 0.9 832 D -- -- -- 12 0.3 1642 0.3 746
TABLE-US-00027 TABLE 27 Characteristics of Bi-specific Antibodies PD1 .times. Tim-3 bi-specifics CHO- CHO- CHO- CHO- PDL1- PD-1-Tim-3 (1353-G10 scFvFc-zwB .times. hPD-1, hPD-1, hTim-3, hTim-3, competition, cell bridging, Tm2 Tim-3, Tim-3, Tim-3, 1649 derivatives) Kd (nM) Bmax Kd (nM) Bmax IC50 (nM) EC50 (nM) (.degree. C.) ka (1/Ms) kd (1/s) KD (M) 1649-A01 Fab-zwA (parent) 1.4 13319 3.9 14691 191 0.1 79 5.2E+04 6.0E-04 1.2E-08 1649-A01 Hc zwA S30D/ 1.3 12548 3.2 11699 128 0.08 79.2 5.8E+04 4.1E-04 7.1E-09 TrstmbLC S56D 1649-A01 Hc zwA R31D/ 2.7 14022 3.5 11258 117 0.1 80.6 5.6E+04 4.9E-04 8.6E-09 TrstmbLC S56D 1649-A01 Hc zwA Y56D/ 2.3 13324 4.5 11211 82 0.11 79.1 5.7E+04 6.1E-04 1.1E-08 TrstmbLC S56D 1649-A01 Hc zwA S30D/ 1.1 9864 3.7 10465 138 0.1 79.6 5.9E+04 5.1E-04 8.7E-09 R31D/TrstmbLC S56D 1649-A01 Hc zwA S30D/ 1.2 9654 3.6 10000 108 0.06 78.7 6.7E+04 6.5E-04 9.7E-09 Y56D/TrsimbLC S56D 1649-A01 Hc zwA R31D/ 1.6 12635 3.3 11380 148 0.09 80.4 6.4E+04 5.6E-04 8.8E-09 Y56D/TrstmbLC S56D 1649-A01 Hc zwA S30D/ 2.1 12998 4.3 10880 147 0.1 78.7 5.8E+04 6.5E-04 1.1E-08 R31D/Y56D/TrstmbLC S56D
TABLE-US-00028 TABLE 28 Characteristics of PD-1 antibodies in both bi-specific format and IgG format Thermostability PD-1 kinetics Tim-3 kinetics (.degree. C.) Sample Description Scaffold ka (1/Ms) kd (1/s) KD (M) ka (1/Ms) kd (1/s) KD (M) Tm1 Tm2 E 1353-G10 wt fab .times. scFv 4.5E+05 8.6E-04 1.9E-09 3.9E+04 5.4E-04 1.4E-08 50.8 78.1 BsAb V262E F 1353-G10 H1 VH fab .times. scFv 8.4E+05 3.1E-03 3.7E-09 4.7E+04 5.8E-04 1.2E-08 52.5 78.1 BsAb V262E G 1353-G10 vk1-39 fab .times. scFv 3.1E+05 7.3E-04 2.4E-09 4.6E+04 5.8E-04 1.3E-08 54.5 78 BsAb V262E H 1353-G10 H1/vk1-39 fab .times. scFv 5.0E+05 2.8E-03 5.7E-09 5.3E+04 7.3E-04 1.4E-08 55.2 77.9 BsAb V262E I 1353-G10 wt IgG 6.2E+05 5.9E-04 9.6E-10 62.2 not detected J 1353-G10 H1 HC IgG 1.1E+06 2.6E-03 2.3E-09 62.3 not detected K 1353-G10 vk1-39 LC IgG 5.8E+05 4.9E-04 8.5E-10 64.1 70.7 L 1353-G10 H1/vk1-39 IgG 1.0E+06 2.8E-03 2.6E-09 63.2 70.5
TABLE-US-00029 TABLE 29 Characteristics of PD-1 antibodies in bi-specific format and IgG format hPD-1 cPD-1 hTim-3 cTim-3 Sample Description Scaffold Concentration Binding Kd (nM) Bmax Binding Kd (nM) Bmax Binding Binding E 1353-G10 wt fab .times. scFv 10 nM + + + + BsAb V262E F 1353-G10 H1 VH fab .times. scFv 10 nM + + + + BsAb V262E G 1353-G10 vk1-39 fab .times. scFv 10 nM + + + + BsAb V262E H 1353-G10 H1/vk1-39 fab .times. scFv 10 nM + + + + BsAb V262E I 1353-G10 wt IgG 0.1 2985 0.01 2772 J 1353-G10 H1 HC IgG 0.5 3412 0.3 2959 K 1353-G10 vk1-39 LC IgG 0.8 3631 0.1 3973 L 1353-G10 H1/vk1-39 IgG 12.9 4938 2.3 2800
Example 20: Sequences
[0865] Table 30 provides sequences referred to herein.
TABLE-US-00030 TABLE 30 Sequences SEQ ID NO: Molecule Region Scheme Sequence 1 Human Tim-3 MFSHLPFDCVLLLLLLLLTRSSEVEYRAE VGQNAYLPCFYIPAAPGNLVPVCWGKGAC PVFECGNVVLRTDERDVNYWTSRYWLNGD FRKGDVSLTIENVILADSGIYCCRIQIPG IMNDEKFNLKLVIKPAKVTPAPTLQRDFT AAFPRMLTTRGHGPAETQTLGSLPDINLT QISTLANELRDSRLANDLRDSGATIRIGI YIGAGICAGLALALIFGALIFKWYSHSKE KIQNLSLISLANLPPSGLANAVAEGIRSE ENIYTIEENVYEVEEPNEYYCYVSSRQQP SQPLGCRFAMP 2 Cynomolgus Tim-3 MFSHLPFDCVLLLLLLLLTRSSEVEYIAE VGQNAYLPCSYTPAPPGNLVPVCWGKGAC PVFDCSNVVLRTDNRDVNDRTSGRYWLKG DFHKGDVSLTIENVTLADSGVYCCRIQIP GIMNDEKHNVKLVVIKPAKVTPAPTLQRD LTSAFPRMLTTGEHGPAETQTPGSLPDVN LTVSNFFCELQIFTLTNELRDSGATIRTA IYIAAGISAGLALALIFGALIFKWYSHSK EKTQNLSISLANIPPSGLANAVAEGIRSE ENIYTIEEDVYEVEEPNEYYCYVSSGQQP SQPLGCRVAMP 3 Mouse Tim-3 MFSGLTLNCVLLLLQLLLARSLENAYVFE VGKNAYLPCSYTLSTPGALVPMCWGKGFC PWSQCTNELLRTDERNVTYQKSSRYQLKG DLNKGDVSLIIKNVTLDDHGTYCCRIQFP GLMNDKKLELKLDIKAAKVTPAQTAHGDS TTASPRTLTTERNGSETQTLVTLHNNNGT KISTWADEIKDSGETIRTAIHIGVGVSAG LTLALIIGVLILKWYSCKKKKLSSLSLIT LANLPPGGLANAGAVRIRSEENIYTIEEN VYEVENSNEYYCYVNSQQPS 4 h22E11-VH5-51-VH CDR-H1 Chothia GFSLTSY 5 2D5-VH CDR-H1 Chothia GYPFIGY 6 SRP1649-A01 CDR-H1 Chothia GFNISRY 7 SRP1649-F06 CDR-H1 Chothia GFNIGNY 8 SRP1649-G08 CDR-H1 Chothia GFNISNY 9 SRP1649-C05 CDR-H1 Chothia GFNIGKH 10 SRP1649-D11 CDR-H1 Chothia GFNISGY 11 SRP1649-B06 CDR-H1 Chothia GFNISNH 12 SRP1649-D06 CDR-H1 Chothia GFNIRNH 13 SRP1649-H02 CDR-H1 Chothia GFNIRSY 14 SRP1649-B09 CDR-H1 Chothia GFNIRNN 15 SRP1649-E10 CDR-H1 Chothia GFNISNN 16 SRP1649-E08 CDR-H1 Chothia GFSISNY 17 1649-A01 CDR-H1 Chothia GFNIDDY S30D/R31D/Y56D 18 1649-A01 CDR-H1 Chothia GFNIDRY S30D/Y56D 19 1649-A01 CDR-H1 Chothia GFNIDDY S30D/R31D 20 1649-A01 Y32T CDR-H1 Chothia GFNISRT 21 1649-A01 R31D CDR-H1 Chothia GFNISDY 22 1649-A01 S30D CDR-H1 Chothia GFNIDRY 23 1649-A01 S30K CDR-H1 Chothia GFNIKRY 24 1353-G10-wt CDR-H1 Chothia GYRFPHY 25 1353-G10 Y27D CDR-H1 Chothia GDRFPHY 26 1353-G10 R28D CDR-H1 Chothia GYDFPHY 27 1353-G10 F29D CDR-H1 Chothia GYRDPHY 28 1353-G10 P30D CDR-H1 Chothia GYRFDHY 29 1353-G10 H31D CDR-H1 Chothia GYRFPDY 30 1353-G10 Y32D CDR-H1 Chothia GYRFPHD 31 1353-G10 R28T/P30D CDR-H1 Chothia GYTFDHY 32 1353-G10 R28D/P30D CDR-H1 Chothia GYDFDHY 33 1353-G10 CDR-H1 Chothia GYDFPDY R28D/H31D 34 1353-G10 R28T/H31D CDR-H1 Chothia GYTFPDY 35 1353-G10 CDR-H1 Chothia GYDFTSY R28D/P30T/H31S 36 1353-G10 CDR-H1 Chothia GYTFTDY R28T/P30T/H31D 37 1353-G10 CDR-H1 Chothia GYTFTSY R28T/P30T/H31S 38 1353-G10- CDR-H1 Chothia GYTFDSY R28T/P30D/H31S 39 h22E11-VHS-51-VH CDR-H1 Kabat SYGVH 40 2D5-VH CDR-H1 Kabat GYTMN 41 SRP1649-A01 CDR-H1 Kabat RYYIH 42 SRP1649-F06 CDR-H1 Kabat NYAIH 43 SRP1649-G08 CDR-H1 Kabat NYVIH 44 SRP1649-C05 CDR-H1 Kabat KHVIH 45 SRP1649-D11 CDR-H1 Kabat GYVIH 46 SRP1649-B06 CDR-H1 Kabat NHAIH 47 SRP1649-D06 CDR-H1 Kabat NHAIH 48 SRP1649-H02 CDR-H1 Kabat SYAIH 49 SRP1649-B09 CDR-H1 Kabat NNAIH 50 SRP1649-E10 CDR-H1 Kabat NNVIH 51 SRP1649-E08 CDR-H1 Kabat NYVIH 52 1649-A01 CDR-H1 Kabat DYYIH S30D/R31D/Y56D 53 1649-A01 CDR-H1 Kabat RYYIH S30D/Y56D 54 1649-A01 CDR-H1 Kabat DYYIH S30D/R31D 55 1649-A01 Y32T CDR-H1 Kabat RTYIH 56 1649-A01 R31D CDR-H1 Kabat DYYIH 57 1353-G10-wt CDR-H1 Kabat HYGIS 58 1353-G10 H31D CDR-H1 Kabat DYGIS 59 1353-G10 Y32D CDR-H1 Kabat HDGIS 60 1353-G10 G33D CDR-H1 Kabat HYDIS 61 1353-G10 S35D CDR-H1 Kabat HYGID 62 1353-G10- CDR-H1 Kabat SYGIS R28T/P30D/H31S 63 h22E11-VH5-51-VH CDR-H2 Chothia WS-DGS 64 2D5-VH CDR-H2 Chothia NPYNGI 65 SRP1649-A01 CDR-H2 Chothia TPVRGY 66 SRP1649-F06 CDR-H2 Chothia TPGQGY 67 SRP1649-G08 CDR-H2 Chothia TPDGGI 68 SRP1649-C05 CDR-H2 Chothia VPNGGY 69 SRP1649-D11 CDR-H2 Chothia IPTAGY 70 SRP1497-A02 CDR-H2 Chothia TPDGGY 71 SRP1649-B06 CDR-H2 Chothia SPAVGY 72 SRP1649-D06 CDR-H2 Chothia APAGGY 73 SRP1649-H02 CDR-H2 Chothia TPAGGD 74 SRP1649-B09 CDR-H2 Chothia TPAGGY 75 SRP1649-E10 CDR-H2 Chothia MPGGGS 76 SRP1649-E08 CDR-H2 Chothia SPDGGF 77 1649-A01 CDR-H2 Chothia TPVRGD S30D/R31D/Y56D 78 1649-A01 R54N CDR-H2 Chothia TPVNGY 79 1649-A01 V53D CDR-H2 Chothia TPDRGY 80 1649-A01 V53T CDR-H2 Chothia TPTRGY 81 1353-G10-wt CDR-H2 Chothia SAYNGN 82 h22E11-VH5-51-VH CDR-H2 Kabat VIWS-DGSTTYNPSFQG 83 h22E11-VH1-69-VH CDR-H2 Kabat VIWS-DGSTTYNQKFQG 84 22E11-VH CDR-H2 Kabat VIWS-DGSTTYNSALKS 85 h22E11-VH4-30-4-VH CDR-H2 Kabat VIWS-DGSTTYNPSLKS 86 h22E11-VH3-23-VH CDR-H2 Kabat VIWS-DGSTTYNDSVKG 87 h22E11-5-VH CDR-H2 Kabat VIWS-DGSTTYNSALKS 88 2D5-VH CDR-H2 Kabat LINPYNGITTYNQKFKG 89 SRP1649-A01 CDR-H2 Kabat GITPVRGYTEYADSVKD 90 SRP1649-F06 CDR-H2 Kabat DITPGQGYTEYADSVKD 91 SRP1649-G08 CDR-H2 Kabat AITPDGGITEYADSVKG 92 SRP1649-C05 CDR-H2 Kabat DIVPNGGYTEYADSVKD 93 SRP1649-D11 CDR-H2 Kabat DIIPTAGYTEYADSVKG 94 SRP1497-A02 CDR-H2 Kabat DITPDGGYTDYADSVKG 95 SRP1497-A05 CDR-H2 Kabat DITPDGGYTDYADSVKD 96 SRP1649-B06 CDR-H2 Kabat DISPAVGYTDYADSVKD 97 SRP1649-D06 CDR-H2 Kabat DIAPAGGYTDYADSVKD 98 SRP1649-H02 CDR-H2 Kabat DITPAGGDTEYADSVKG 99 SRP1649-B09 CDR-H2 Kabat DITPAGGYTGYADSVKD 100 SRP1649-E10 CDR-H2 Kabat DIMPGGGSTDYADSVKD 101 SRP1649-E08 CDR-H2 Kabat DISPDGGFTDYADSVKD
102 1649-A01 CDR-H2 Kabat GITPVRGDTEYADSVKD S30D/R31D/Y56D 103 1649-A01 E58D CDR-H2 Kabat GITPVRGYTDYADSVKD 104 1649-A01 E58R CDR-H2 Kabat GITPVRGYTRYADSVKD 105 1649-A01 R54N CDR-H2 Kabat GITPVNGYTEYADSVKD 106 1649-A01 V53D CDR-H2 Kabat GITPDRGYTEYADSVKD 107 1649-A01 V53T CDR-H2 Kabat GITPTRGYTEYADSVKD 108 1649-A01 G50D CDR-H2 Kabat DITPVRGYTEYADSVKD 109 1649-A01 G50R CDR-H2 Kabat RITPVRGYTEYADSVKD 110 1353-G10-wt CDR-H2 Kabat WISAYNGNTNYAQKLQG 111 h22E11-VH5-51-VH CDR-H3 QGGYR-YDDAMDY 112 2D5-VH CDR-H3 SFFYGSSNDWLVY 113 SRP1649-A01 CDR-H3 GYVYR-MWDSYDY 114 SRP1649-C05 CDR-H3 GYVYR-MWDSFDY 115 SRP1649-B06 CDR-H3 GYVYR-MWDSFDH 116 SRP1649-H02 CDR-H3 GYIYR-MWDSYDY 117 SRP1649-B09 CDR-H3 GYIYR-MWDSLDY 118 SRP1649-E08 CDR-H3 GHVYR-LWDSFDY 119 1353-G10-wt CDR-H3 DVDYG-T-GS-GY 120 h22E11-Vk3-11-VL CDR-L1 KASQSVDYD-GNSYVN 121 h22E11-5-VL CDR-L1 KASQSVDYD-GNSYVA 122 2D5-VL CDR-L1 RSSQSIVHTNGNTYLE 123 1649-A01 CDR-L1 RASQDVNTAVA 124 1353-G10-wt CDR-L1 SGDALPKQYAY 125 h22E11-Vk3-11-VL CDR-L2 AASNLES 126 2D5-VL CDR-L2 KVSNRFS 127 1649-A01 T56D CDR-L2 SASFLYD 128 1649-A01 Y55D CDR-L2 SASFLDS 129 1649-A01 F53D CDR-L2 SASDLYS 130 1649-A01 S52D CDR-L2 SADFLYS 131 1649-A01 A51D CDR-L2 SDSFLYS 132 1649-A01 S50D CDR-L2 DASFLYS 133 1649-A01 CDR-L2 SASFLYS 134 1353-G10-wt CDR-L2 KDTERPS 135 1353-G10 K50D CDR-L2 DDTERPS 136 1353-G10 T52D CDR-L2 KDDERPS 137 1353-G10 E53D CDR-L2 KDTDRPS 138 1353-G10 P55D CDR-L2 KDTERDS 139 1353-G10 S56D CDR-L2 KDTERPD 140 h22E11-Vk3-11-VL CDR-L3 QQSNEDPYT 141 2D5-VL CDR-L3 FQGSHVPWT 142 1649-A01 CDR-L3 QQHYTTPPT 143 1353-G10-wt CDR-L3 QSADNSITYRV 144 2D5-VH VH EVQLQQSGPELVKPGTSMKISCRASGYPF IGYTMNWVKQSHGGNLEWIGLINPYNGIT TYNQKFKGRATLSVDTSSTIAYMELLSLT SDDSAEYYCARSFFYGSSNDWLVYWGQGT LVTVSA 145 22E11-VH VH QVQLKESGPDLVAPSQSLSITCTVSGFSL TSYGVHWVRQPPGKGLEWLVVIWSDGSTT YNSALKSRLTISKDNSKSQVFLKMNSLQT DDTAMYYCARQGGYRYDDAMDYWGQGTSV AVSS 146 h22E11-VH5-51-VH VH EVQLVQSGAEVKKPGESLKISCKVSGFSL TSYGVHWVRQMPGKGLEWLVVIWSDGSTT YNPSFQGQVTISKDKSISTVYLQWSSLKA SDTAMYYCARQGGYRYDDAMDYWGQGTLV TVSS 147 h22E11-VH4-30-4-H VH QVQLQESGPGLVKPSQTLSLTCTVSGFSL TSYGVHWIRQPPGKGLEWLVVIWSDGSTT YNPSLKSRVTISKDTSKNQVSLKLSSVTA ADTAVYYCARQGGYRYDDAMDYWGQGTLV TVSS 148 h22E11-VH3-23-VH VH EVQLLESGGGLVQPGGSLRLSCAVSGFSL TSYGVHWVRQAPGKGLEWLVVIWSDGSTT YNDSVKGRFTISKDNSKNTVYLQMNSLRA EDTAVYYCARQGGYRYDDAMDYWGQGTLV TVSS 149 h22E11-VH1-69-VH VH QVQLVQSGAEVKKPGSSVKVSCKVSGFSL TSYGVHWVRQAPGQGLEWLVVIWSDGSTT YNQKFQGRVTITKDESTSTVYMELSSLRS EDTAVYYCARQGGYRYDDAMDYWGQGTLV TVSS 150 h22E11-5-VH VH EVQLVESGGGLVQPGGSLRLSCAVSGFSL TSYGVHWVRQAPGKGLEWLVVIWSDGSTT YNSALKSRFTISKDNAKNSVYLQMNSLRA EDTAVYYCARQGGYRYDDAMDYWGQGTLV TVSS 151 SRP1649-A01 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 152 SRP1649-B06 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SNHAIHWVRQAPGKGLEWVADISPAVGYT DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDHWGQGTL VTVSS 153 SRP1649-C05 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI GKHVIHWVRQAPGKGLEWVADIVPNGGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDYWGQGTL VTVSS 154 SRP1649-D06 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI RNHAIHWVRQAPGKGLEWVADIAPAGGYT DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 155 SRP1649-D11 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SGYVIHWVRQAPGKGLEWVADIIPTAGYT EYADSVKGRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDYWGQGTL VTVSS 156 SRP1649-E08 VH EVQLVESGGSLVQPGGSLRLSCAASGFSI SNYVIHWVRQAPGKGLEWVADISPDGGFT DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGHVYRLWDSFDYWGRGTL VTVSS 157 SRP1649-E10 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SNNVIHWVRQAPGKGLEWVGDIMPGGGST DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 158 SRP1649-F06 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI GNYAIHWVRQAPGKGLEWVADITPGQGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 159 SRP1649-G08 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SNYVIHWVRQAPGKGLEWVAAITPDGGIT EYADSVKGRFAISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 160 SRP1649-H02 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI RSYAIHWVRQAPGKGLEWVADITPAGGDT EYADSVKGRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYIYRMWDSYDYWGQGTL VTVSS 161 SRP1649-B09 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI RNNAIHWVRQAPGKGLEWVADITPAGGYT GYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYIYRMWDSLDYWGQGTL VTVSS 162 SRP1497-A05 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SNYAIHWVRQAPGKGLEWVADITPDGGYT DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDYWGQGTL VTVSS 163 SRP1497-A02 VH EVQLVESGGGLVRPGGSLRLSCAASGFNI SNYAIHWVRQAPGKGLEWVADITPDGGYT DYADSVKGRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDYWGQGTL VTVSS 164 SRP1497-A01 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SNYAIHWVRQAPGKGLEWVADITPDGGYT DYADSVKGRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSFDYWGQGTL VTVSS 165 1649-A01 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI S30D/R31D/Y56D DDYYIHWVRQAPGKGLEWVAGITPVRGDT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 166 1649-A01 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI S30D/Y56D DRYYIHWVRQAPGKGLEWVAGITPVRGDT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 167 1649-A01 VH EVQLVESGGGLVQPGGSLRLSCAASGFNI S30D/R31D DDYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 168 1649-A01 D65G VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKGRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 169 1649-A01 E58D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVRGYT DYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 170 1649-A01 E58R VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVRGYT RYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 171 1649-A01 Y56D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVRGDT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL
VTVSS 172 1649-A01 R54N VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPVNGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 173 1649-A01 V53D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPDRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 174 1649-A01 V53T VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVAGITPTRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 175 1649-A01 G50D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVADITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 176 1649-A01 G50R VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRYYIHWVRQAPGKGLEWVARITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 177 1649-A01 Y32T VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SRTYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 178 1649-A01 R31D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI SDYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 179 1649-A01 S30D VH EVQLVESGGGLVQPGGSLRLSCAASGFNI DRYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 180 1649-A01 S30K VH EVQLVESGGGLVQPGGSLRLSCAASGFNI KRYYIHWVRQAPGKGLEWVAGITPVRGYT EYADSVKDRFTISADTSKNTAYLQMNSLR AEDTAVYYCARGYVYRMWDSYDYWGQGTL VTVSS 181 1353-G10-wt VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 182 1353-G10 Y27D VH EVQLVQSGAEVKKPGASVKVSCKASGDRF PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 183 1353-G10 R28D VH EVQLVQSGAEVKKPGASVKVSCKASGYDF PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 184 1353-G10 F29D VH EVQLVQSGAEVKKPGASVKVSCKASGYRD PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 185 1353-G10 P30D VH EVQLVQSGAEVKKPGASVKVSCKASGYRF DHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 186 1353-G10 H31D VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PDYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 187 1353-G10 Y32D VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PHDGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 188 1353-G10 G33D VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PHYDISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 189 1353-G10 S35D VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PHYGIDWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 190 1353-G10 R28T/P30D VH EVQLVQSGAEVKKPGASVKVSCKASGYTF DHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 191 1353-G10 R28D/P30D VH EVQLVQSGAEVKKPGASVKVSCKASGYDF DHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 192 1353-G10 VH EVQLVQSGAEVKKPGASVKVSCKASGYDF R28D/H31D PDYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 193 1353-G10 R28T/H31D VH EVQLVQSGAEVKKPGASVKVSCKASGYTF PDYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 194 1353-G10 VH EVQLVQSGAEVKKPGASVKVSCKASGYDF R28D/P30T/H31S TSYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 195 1353-G10 VH EVQLVQSGAEVKKPGASVKVSCKASGYTF R28T/P30T/H31D TDYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 196 1353-G10 VH EVQLVQSGAEVKKPGASVKVSCKASGYTF R28T/P30T/H31S TSYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 197 1353-G10- VH EVQLVQSGAEVKKPGASVKVSCKASGYTF R28T/P30D/H31S DSYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSS 198 1084 VH QVQLQQSGAELMKPGASVKMSCKTTGYIF SSYWIGWVKQRPGHGLEWIGKIFPGSGSA DYNENFKGKATFTVDTSSNTAYMQLSSLT SEDSAVYYCARGYGNYLYFDVWGAGTTVT VSS 199 1353-A09 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF TWYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDSEYSSGSGYWGQGTLVT VSS 200 1353-C07 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF STFGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYSSGSGYWGQGTLVT VSS 201 1353-E07 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF ETYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDAEYSLGSGYWGQGTLVT VSS 202 1353-F09 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF RQYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDAEYGSGSGYWGQGTLVT VSS 203 1353-G08 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF TRYGISWVRQAPGQGLEWMGWVSAHNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDADYGSGSGYWGQGTLVT VSS 204 1353-H08 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF TRQGISWVRQAPGQGLEWMGWISAYNGNT KYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGSGSGYWGQGTLVT VSS 205 1353-H09 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDAEYGSGSGYWGQGTLVT VSS 206 1B10 VH DVQLQESGPGLVKPSQSLSLTCTVTGHSI TSDYAWNWIRQFPGNKLEWMGYISYSGRT SYNPSLTSRISITRDTSKNQFFLQLNSVT TEDTATYYCARGYALDYWGQGTSVTVSS 207 1E9 VH EVKLVESGGGLVSPGGSLKLSCAASGFTF STFGMSWVRQTPEKRLEWVATISGGGSDT YYPDSVQGRFIISRYNAKNNLYLQMNSLR PEDTALYYCARQGYDVYSWFAYWGQGTLV TVSA 208 4B10 VH EVKLVESGGGLVKPGGSLKLSCAASGFTF STYGMSWVRQTPEKRLQWVATISGGGSNT YYSDSVKGRFTISRDNAKNNLYLQMSSLR SEDTALYYCARQRDSAWFASWGQGTLVTV SA 209 h1E9-1 VH EVQLVESGGGLVKPGGSLRLSCAASGFTF STFGMSWVRQAPGKGLEWVSTISGGGSDT YYPDSVQGRFTISRDNAKNSLYLQMNSLR AEDTAVYYCARQGYDVYSWFAYWGQGTLV TVSS 210 h1E9-2 VH EVQLVESGGGLVKPGGSLRLSCAASGFTF STFGMSWVRQAPGKGLEWVSTISGGGSDT YYPDSVQGRFTISRDNAKNSLYLQMNSLR AEDTAVYYCARQGYDVYSWFAYWGQGTLV TVSS 211 h1E9-4 VH EVQLVESGGGLVQPGGSLRLSCAASGFTF STFGMSWVRQAPGKGLEWVATISGGGSDT YYPDSVQGRFTISRDNAKNSLYLQMNSLR AEDTAVYYCARQGYDVYSWFAYWGQGTLV TVSS 212 h1E9-5 VH EVQLVESGGGLVQPGGSLRLSCAASGFTF STFGMSWVRQAPGKGLEWVATISGGGSDT YYPDSVQGRFTISRDNAKNSLYLQMNSLR AEDTAVYYCARQGYDVYSWFAYWGQGTLV TVSS 213 h4B10-1 VH EVQLVESGGGLVKPGGSLRLSCAASGFTF STYGMSWVRQAPGKGLEWVATISGGGSNT YYSDSVKGRFTISRDDSKNTLYLQMNSLK
TEDTAVYYCARQRDSAWFASWGQGTLVTV SS 214 h4B10-2 VH EVQLVESGGGLVKPGGSLRLSCAASGFTF STYGMSWVRQAPGKGLEWVATISGGGSNT YYSDSVKGRFTISRDDSKNTLYLQMNSLK TEDTAVYYCARQRDSAWFASWGQGTLVTV SS 215 h4B10-3 VH EVQLVESGGGLVKPGGSLRLSCAASGFTF STYGMSWVRQAPGKGLEWVATISGGGSNT YYSDSVKGRFTISRDDSKNTLYLQMNSLK TEDTAVYYCARQRDSAWFASWGQGTLVTV SS 216 PD1-17 VH QVQLQESGPGVVKPSGTLSLTCAISGGSI GSGGSIRSTRWWSWVRQSPGKGLEWIGEI YHSGSTNYNPSLKSRVTISLDKSRNHFSL RLNSVTAADTAVYYCARQDYGDSGDWYFD LWGKGTMVTVSS 217 PD1-28 VH EVQLVQSGAEVKKPGASVKVSCKASGYRF TSYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDADYSSGSGYWGQGTLVT VSS 218 PD1-33 VH QVQLVQSGAEVKKPGASVRVSCKASGYTL TSYYIHWVRQAPGQGLEWMGIINPRGATI SYAQKFQGRVTMTRDTSTSTVYMELRNLK SEDTALYYCATAGIYGFDFDYWGRGTLVT VSS 219 PD1-35 VH QVQLQESGPGLVKPSQTLSLTCTVSGGSI SSGAYYWSWIRQHPGKGLEWIGYIYYNGN TYYNPSLRSLVTISVDASKNQFSLKLSSV TAADTAVYYCARASDYVWGGYRYMDAFDI WGRGTLITVSS 220 PD1-F2 VH EVQLVQSGGGVVQPGRSLRLSCAASGFTF SSYWCDRMSWVRQAPGKGLEWVSAISG SGGSTYYADSVKGRFTISRDNSKNTLYLQ MNSLRAEDTAVYYCAKENWGSYFDLWGQG TTVTVSS 221 2D5-VL VL DVLMTQTPLSLPVSLGDQASISCRSSQSI VHTNGNTYLEWYLQKPGQSPKLLIYKVSN RFSGVPDRFSGSGSGTDFTLKISRVEAED LGVYYCFQGSHVPWTFGGGTELEIK 222 22E11-VL VL DIVLTQSPASLAVSLGQRATISCKASQSV DYDGNSYVNWYQQKPGQPPKLLIYAASNL ESGIPARFSGSGSGTDFTLNIHPVEEEDA ATYYCQQSNEDPYTFGGGTKLEIK 223 h22E11-Vk4-1-VL VL DIVLTQSPDSLAVSLGERATINCKASQSV DYDGNSYVNWYQQKPGQPPKLLIYAASNL ESGVPDRFSGSGSGTDFTLTISSLQAEDV AVYYCQQSNEDPYTFGQGTKVEIK 224 h22E11-Vk3-11-VL VL EIVLTQSPATLSLSPGERATLSCKASQSV DYDGNSYVNWYQQKPGQAPRLLIYAASNL ESGIPARFSGSGSGTDFTLTISSLEPEDF AVYYCQQSNEDPYTFGQGTKVEIK 225 h22E11-Vk2-28-VL VL DIVLTQSPLSLPVTPGEPASISCKASQSV DYDGNSYVNWYLQKPGQSPQLLIYAASNL ESGVPDRFSGSGSGTDFTLKISRVEAEDV GVYYCQQSNEDPYTFGQGTKVEIK 226 h22E11-Vk1-39-VL VL DIQLTQSPSSLSASVGDRVTITCKASQSV DYDGNSYVNWYQQKPGKAPKLLIYAASNL ESGVPSRFSGSGSGTDFTLTISSLQPEDF ATYYCQQSNEDPYTFGQGTKVEIK 227 h22E11-5-VL VL EIVLTQSPGTLSLSPGERATLSCKASQSV DYDGNSYVAWYQQKPGQAPRLLIYAASNL ESGIPDRFSGSGSGTDFTLTISRLEPEDF AVYYCQQSNEDPYTFGQGTKVEIK 228 trastuzumab VL VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSASFLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 229 1649-A01 T56D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSASFLYDGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 230 1649-A01 Y55D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSASFLDSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 231 1649-A01 F53D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSASDLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 232 1649-A01 S52D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSADFLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 233 1649-A01 A51D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSDSFLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 234 1649-A01 S50D VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYDASFLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 235 1649-A01 VL DIQMTQSPSSLSASVGDRVTITCRASQDV NTAVAWYQQKPGKAPKLLIYSASFLYSGV PSRFSGSRSGTDFTLTISSLQPEDFATYY CQQHYTTPPTFGQGTKVEIK 236 1353-G10-wt VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 237 1353-G10 K50D(.lamda.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYDDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 238 1353-G10 T52D(.lamda.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDDERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 239 1353-G10 E53D(.lamda.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTDRPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 240 1353-G10 P55D(.lamda.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERDSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 241 1353-G10 S56D(.lamda.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPDGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 242 1353-G10 VL1c (.kappa.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDFATYYC QSADNSITYRVFGGGTKVEIK 243 1353-G10 VL1d (.kappa.) VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQRKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDFATYYC QSADNSITYRVFGGGTKVEIK 244 1353-G10-kappa VL DIQLTQSPSSLSASVGDRVTITCSGDALP KQYAYWYQQKPGKAPKLLIYKDTERPSGV PSRFSGSSSGTKVTLTISSLQPEDFATYY CQSADNSITYRVFGGGTKVEIK 245 1353-G10 Vk3-20 (.kappa.) VL EIVLTQSPGTLSLSPGERATLSCSGDALP KQYAYWYQQKPGQAPRLLIYKDTERPSGI PDRFSGSSSGTKVTLTISRLEPEDFAVYY CQSADNSITYRVFGGGTKVEIK 246 10B4 VL NIVMTQTPKFLLVSAGDRITITCKASQSV SDDVAWYQQKPGQSPKLLISYAFKRYIGV PDRFTGSGYGTDFTFTISTVQAEDLAVYF CQQNYNSPYTFGGGTKLELKR 247 1353-A09 VL SYELTQPPSVSVSPGQTARITCSGDALTT QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 248 1353-C07 VL SYELTQPPSVSVSPGQTARITCSGDALSE QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 249 1353-E07 VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 250 1353-F09 VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVLYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 251 1353-G08 VL SYELTQPPSVSVSPGQTARITCSGDALPM QYGYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 252 1353-H08 VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 253 1353-H09 VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 254 1B10 VL QIVLSQSPAILSASPGEKVTMTCRTSSSV NYMHWFQQKPGSSPKPWIYATSKLASGVP ARFSGSGSGTSYSLTISRVEAEDAATYFC QQWISDPWTFGGGTKLEIK 255 1E9 VL DIILTQSPASLAVSLGQRAAISCRASESV DNSGISFMSWFQQKPGQPPKLLIYTASNQ GSGVPARFSGSGSGTEFSLNIHPMEEDDT ANYFCQQSKEVPWTFGGGTKLEIR 256 4B10 VL DIVLTQSPASLAVSLGQRATISCRASENV DDYGVSFMNWFQQKPGQPPKLLIYPASNQ GSGVPARFSGSGSGTDFSLNIHPMEEDDT ANYFCQQSKEVPWTFGGGTKLEIK 257 h1E9-1 VL DIQLTQSPSFLSASVGDRVTITCRASESV DNSGISFMSWYQQKPGKAPKLLIYTASNQ GSGVPSRFSGSGSGTEFTLTISSLQPEDF ATYYCQQSKEVPWTFGQGTKVEIK 258 h1E9-2 VL EIVLTQSPATLSLSPGERATLSCRASESV DNSGISFMSWYQQKPGQAPRLLIYTASNQ GSGIPARFSGSGSGTDFTLTISSLEPEDF AVYYCQQSKEVPWTFGQGTKVEIK 259 h1E9-4 VL DIQLTQSPSFLSASVGDRVTITCRASESV DNSGISFMSWYQQKPGKAPKLLIYTASNQ GSGVPSRFSGSGSGTEFTLTISSLQPEDF ATYYCQQSKEVPWTFGQGTKVEIK 260 h1E9-5 VL EIVLTQSPATLSLSPGERATLSCRASESV DNSGISFMSWYQQKPGQAPRLLIYTASNQ GSGIPARFSGSGSGTDFTLTISSLEPEDF AVYYCQQSKEVPWTFGQGTKVEIK 261 h4B10-1 VL EIVLTQSPATLSLSPGERATLSCRASENV DDYGVSFMNWYQQKPGQAPRLLIYPASNQ GSGIPARFSGSGSGTDFTLTISSLEPEDF AVYYCQQSKEVPWTFGQGTKVEIK 262 h4B10-2 VL EIVLTQSPGTLSLSPGERATLSCRASENV
DDYGVSFMNWYQQKPGQAPRLLIYPASNQ GSGIPDRFSGSGSGTDFTLTISRLEPEDF AVYYCQQSKEVPWTFGQGTKVEIK 263 h4B10-3 VL DIVMTQSPDSLAVSLGERATINCRASENV DDYGVSFMNWYQQKPGQPPKLLIYPASNQ GSGVPDRFSGSGSGTDFTLTISSLQAEDV AVYYCQQSKEVPWTFGGGTKLEIK 264 PD1-17 VL NFMLTQPHSVSESPGKTVTISCTRSSGSI ASNSVQWYQQRPGSSPTTVIYEDNQRPSG VPDRFSGSIDSSSNSASLTVSGLKTEDEA DYYCQSSDSSAVVFGSGTKLTVL 265 PD1-28 VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDEADYYC QSADNSITYRVFGGGTKVTVL 266 PD1-33 VL QSALTQPASVSGSPGQSITISCTGTSNDV GGYNYVSWYQHHPGKAPKLIIYDVTNRPS GVSDRFSGSKSGNTASLTISGLLAEDEGD YYCSSYTIVTNFEVLFGGGTKLTV 267 PD1-35 VL QSVLTQPPSASGTPGQRVTISCSGSNSNI GSNSVNWYQQLPGTAPKLLIYGNNQRPSG VPDRFSGSKSGTSASLAISGLQSENEADY YCAAWDDSLNGPVFGRGTKVTVL 268 PD1-F2 VL DIVMTQSPSTLSASVGDRVTITCRASQGI SSWLAWYQQKPGRAPKVLIYKASTLES GVPSRFSGSGSGTDFTLTISSLQPEDFAT YYCQQSYSTPWTFGQGTKLEIK 269 1353-G10-wt-c VL SYELTQPPSVSVSPGQTARITCSGDALPK QYAYWYQQKPGQAPVMVIYKDTERPSGIP ERFSGSSSGTKVTLTISGVQAEDFATYYC QSADNSITYRVFGGGTKVEIK 270 Human IgG1 HC ASTKGPSVFPLAPSSKSTSGGTAALGCLV Constant KDYFPEPVTVSWNSGALTSGVHTFPAVLQ SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSCDKTHTCPPCPAPE LLGGPSVFLFPPKPKDTLMISRIPEVTCV VVDVSHEDPEVKFNWYVDGVEVHNAKTKP REEQYNSTYRVVSVLTVLHQDWLNGKEYK CKVSNKALPAPIEKTISKAKGQPREPQVY TLPPSREEMTKNQVSLTCLVKGFYPSDIA VEWESNGQPENNYKTTPPVLDSDGSFFLY SKLTVDKSRWQQGNVFSCSVMHEALHNHY TQKSLSLSPGK 271 Human IgG LC RTVAAPSVFIFPPSDEQLKSGTASVVCLL Constant Ckappa NNFYPREAKVQWKVDNALQSGNSQESVTE QDSKDSTYSLSSTLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 272 Mouse IgG1 HC AKTTPPSVYPLAPGSAAQTNSMVTLGCLV Constant KGYFPEPVTVTWNSGSLSSGVHTFPAVLQ SDLYTLSSSVTVPSSTWPSETVTCNVAHP ASSTKVDKKIVPRDCGCKPCICTVPEVSS VFIFPPKPKDVLTITLTPKVTCVVVDISK DDPEVQFSWFVDDVEVHTAQTQPREEQFN STFRSVSELPIMHQDWLNGKEFKCRVNSA AFPAPIEKTISKTKGRPKAPQVYTIPPPK EQMAKDKVSLTCMITDFFPEDITVEWQWN GQPAENYKNTQPIMDTDGSYFVYSKLNVQ KSNWEAGNTFTCSVLHEGLHNHHTEKSLS HSPG 273 Mouse IgG LC RADAAPTVSIFPPSSEQLTSGGASVVCFL Constant Ckappa NNFYPKDINVKWKIDGSERQNGVLNSWTD QDSKDSTYSMSSTLTLTKDEYERHNSYTC EATHKTSTSPIVKSFNRNEC 274 Kappa LC HMTVAAPSVFIFPPSDEQLKSGTASVVCL LNNFYPREAKVQWKVDNALQSGNSQESVT EQDSKDSTYSLSSTLTLSKADYEKHKVYA CEVTHQGLSSPVTKSFNRGEC 275 Lambda LD GQPKAAPSVTLFPPSSEELQANKATLVCL ISDFYPGAVTVAWKADSSPVKAGVETTTP SKQSNNKYAASSYLSLTPEQWKSHRSYSC QVTHEGSTVEKTVAPTECS 276 IgG1 Fc from scFv-Fc AAGSDQEPKSSDKTHTCPPCSAPELLGGS SVFLFPPKPKDTLMISRTPEVTCVVVDVS HEDPEVKFNWYVDGVEVHNAKTKPREEQY NSTYRVVSVLTVLHQDWLNGKEYKCKVSN KALPAPIEKTISKAKGQPREPQVYTLPPS RDELTKNQVSLTCLVKGFYPSDIAVEWES NGQPENNYKTTPPVLDSDGSFFLYSKLTV DKSRWQQGNVFSCSVMHEALHNHYTQKSL SLSPGKGSGDYKDDDDKGSG 277 FlagHis Tag GSGDYKDDDDKGSGHHHHHH 278 Linker (GGGGS).sub.3 GGGGSGGGGSGGGGS 279 Linker (GGGGS).sub.4 GGGGSGGGGSGGGGSGGGGS 280 Linker (GGGGS).sub.5 GGGGSGGGGSGGGGSGGGGSGGGGS 281 Linker (GGGGS).sub.6 GGGGSGGGGSGGGGSGGGGSGGGGSGGGG S 282 Linker AAGSDQEPKSS 283 Linker AAGSDQ 284 Linker APGPSAPSHRSLPSRAFG 285 Linker-hinge AAGSDQEPKSSDKTHTCPPCP 286 Hinge - wt DKTHTCPPCP 287 Tim-3 scFv-Fc MEVQLVQSGAEVKKPGESLKISCKVSGFS LTSYGVHWVRQMPGKGLEWLVVIWSDGST TYNPSFQGQVTISKDKSISTVYLQWSSLK ASDTAMYYCARQGGYRYDDAMDYWGQGTL VTVSSGGGGSGGGGSGGGGSDIQLTQSPS SLSASVGDRVTITCKASQSVDYDGNSYVN WYQQKPGKAPKLLIYAASNLESGVPSRFS GSGSGTDFTLTISSLQPEDFATYYCQQSN EDPYTFGQGTKVEIKAAGSDQEPKSSDKT HTCPPCPAPELLGGPSVFLFPPKPKDTLM ISRTPEVTCVVVDVSHEDPEVKFNWYVDG VEVHNAKTKPREEQYNSTYRVVSVLTVLH QDWLNGKEYKCKVSNKALPAPIEKTISKA KGQPREPQVYTLPPSREEMTKNQVSLTCL VKGFYPSDIAVEWESNGQPENNYKTTPPV LDSDGSFFLYSKLTVDKSRWQQGNVFSCS VMHEALHNHYTQKSLSLSPGKGSGDYKDD DDKGSGHHHHHH 288 1353-G10 scFv-Fc MEVQLVQSGAEVKKPGASVKVSCKASGYR FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSGGGGSGGGGSGGGGSSYELTQPPSV SVSPGQTARITCSGDALPKQYAYWYQQKP GQAPVMVIYKDTERPSGIPERFSGSSSGT KVTLTISGVQAEDEADYYCQSADNSITYR VFGGGTKVTVLAAGSDQEPKKLAAGSDQE PKSSDKTHTCPPCSAPELLGGSSVFLFPP KPKDTLMISRTPEVTCVVVDVSHEDPEVK FNWYVDGVEVHNAKTKPREEQYNSTYRVV SVLTVLHQDWLNGKEYKCKVSNKALPAPI EKTISKAKGQPREPQVYTLPPSRDELTKN QVSLTCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGKGSGDYKDDDDKGSGH HHHHH 289 1353-G10 scFv-Fc scFv-Fc MEVQLVQSGAEVKKPGASVKVSCKASGYR hole FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSGGGGSGGGGSGGGGSSYELTQPPSV SVSPGQTARITCSGDALPKQYAYWYQQKP GQAPVMVIYKDTERPSGIPERFSGSSSGT KVTLTISGVQAEDEADYYCQSADNSITYR VFGGGTKVTVLAAGSDQEPKSSDKTHTCP PCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWL NGKEYKCKVSNKALPAPIEKTISKAKGQP REPQVYTLPPSRDELTKNQVSLSCAVKGF YPSDIAVEWESNGQPENNYKTTPPVLDSD GSFFLVSKLTVDKSRWQQGNVFSCSVMHE ALHNHYTQKSLSLSPGK 290 1353-G10 scFv-Fc scFv-Fc MEVQLVQSGAEVKKPGASVKVSCKASGYR knob FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSGGGGSGGGGSGGGGSSYELTQPPSV SVSPGQTARITCSGDALPKQYAYWYQQKP GQAPVMVIYKDTERPSGIPERFSGSSSGT KVTLTISGVQAEDEADYYCQSADNSITYR VFGGGTKVTVLAAGSDQEPKSSDKTHTCP PCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWL NGKEYKCKVSNKALPAPIEKTISKAKGQP REPQVYTLPPSRDELTKNQVSLWCLVKGF YPSDIAVEWESNGQPENNYKTTPPVLDSD GSFFLYSKLTVDKSRWQQGNVFSCSVMHE ALHNHYTQKSLSLSPGK 291 1353-G10 scFv-Fc scFv-Fc MEVQLVQSGAEVKKPGASVKVSCKASGYR zwA FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSGGGGSGGGGSGGGGSSYELTQPPSV SVSPGQTARITCSGDALPKQYAYWYQQKP GQAPVMVIYKDTERPSGIPERFSGSSSGT KVTLTISGVQAEDEADYYCQSADNSITYR VFGGGTKVTVLAAGSDQEPKSSDKTHTCP PCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWL NGKEYKCKVSNKALPAPIEKTISKAKGQP REPQVYVYPPSREEMTKNQVSLTCLVKGF YPSDIAVEWESNGQPENNYKTTPPVLDSD GSFALVSKLTVDKSRWQQGNVFSCSVMHE ALHNHYTQKSLSLSPGK 292 hPD-1 MQIPQAPWPVVWAVLQLGWRPGWFLDSPD RPWNPPTFSPALLVVTEGDNATFTCSFSN TSESFVLNWYRMSPSNQTDKLAAFPEDRS QPGQDCRERVTQLPNGRDFHMSVVRARRN DSGTYLCGAISLAPKAQIKESLRAELRVT ERRAEVPTAHPSPSPRPAGQFQTLVVGVV GGLLGSLVLLVWVLAVICSRAARGTIGAR RTGQPLKEDPSAVPVFSVDYGELDFQWRE KTPEPPVPCVPEQTEYATIVFPSGMGTSS PARRGSADGPRSAQPLRPEDGHCSWPL 293 murine PD-1 MWVRQVPWSFTWAVLQLSWQSGWLLEVPN GPWRSLTFYPAWLTVSEGANATFTCSLSN WSEDLMLNWNRLSPSNQTEKQAAFCNGLS QPVQDARFQIIQLPNRHDFHMNILDTRRN DSGIYLCGAISLHPKAKIEESPGAELVVT ERILETSTRYPSPSPKPEGRFQGMVIGIM SALVGIPVLLLLAWALAVFCSTSMSEARG AGSKDDTLKEEPSAAPVPSVAYEELDFQG REKTPELPTACVHTEYATIVFTEGLGASA MGRRGSADGLQGPRPPRHEDGHCSWPL 294 cyno PD-1 MWVRQVPWSFTWAVLQLSWQSGWLLEVPN GPWRSLTFYPAWLTVSEGANATFTCSLSN WSEDLMLNWNRLSPSNQTEKQAAFCNGLS QPVQDARFQIIQLPNRHDFHMNILDTRRN DSGIYLCGAISLHPKAKIEESPGAELVVT ERILETSTRYPSPSPKPEGRFQGMVIGIM SALVGIPVLLLLAWALAVFCSTSMSEARG AGSKDDTLKEEPSAAPVPSVAYEELDFQG REKTPELPTACVHTEYATIVFTEGLGASA MGRRGSADGLQGPRPPRHEDGHCSWPL 295 CH.sub.2-CH.sub.3, Fc-knob APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLWCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK
296 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-knob-V262E TCEVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLWCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 297 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-knob-V264S TCVVSDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLWCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 298 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-knob-D399C TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSRDELTKNQVSLWCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLCSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 299 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-knob-S354C TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPCRDELTKNQVSLWCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 300 CH.sub.2-CH.sub.3, Fc-hole APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLSCAVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 301 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-hole-V262E TCEVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLSCAVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 302 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-hole-V264S TCVVSDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSREEMTKNQVSLSCAVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 303 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-hole-Y349C TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVCTLPPSRDELTKNQVSLSCAVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF FLVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 304 CH.sub.2-CH.sub.3, APELLGGPSVFLFPPKPKDTLMISRTPEV Fc-hole-K392C TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSRDELTKNQVSLSCAVKGFYPS DIAVEWESNGQPENNYCTTPPVLDSDGSF FLVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 305 Fc-zwA APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVYPPSREEMTKNQVSLTCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF ALVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 306 Fc-zwA-V262E APELLGGPSVFLFPPKPKDTLMISRTPEV TCEVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVYPPSREEMTKNQVSLTCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF ALVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 307 Fc-zwA-V264S APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVSDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVYPPSREEMTKNQVSLTCLVKGFYPS DIAVEWESNGQPENNYKTTPPVLDSDGSF ALVSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 308 Fc-zwB APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVLPPSREEMTKNQVSLLCLVKGFYPS DIAVEWESNGQPENNYLTWPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 309 Fc-zwB-V262E APELLGGPSVFLFPPKPKDTLMISRTPEV TCEVVDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVLPPSREEMTKNQVSLLCLVKGFYPS DIAVEWESNGQPENNYLTWPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 310 Fc-zwB-V264S APELLGGPSVFLFPPKPKDTLMISRTPEV TCVVSDVSHEDPEVKFNWYVDGVEVHNAK TKPREEQYNSTYRVVSVLTVLHQDWLNGK EYKCKVSNKALPAPIEKTISKAKGQPREP QVYVLPPSREEMTKNQVSLLCLVKGFYPS DIAVEWESNGQPENNYLTWPPVLDSDGSF FLYSKLTVDKSRWQQGNVFSCSVMHEALH NHYTQKSLSLSPGK 311 hinge-C.sub.H2-C.sub.H3-zwA DKTHTCPPCPAPELLGGPSVFLFPPKPKD TLMISRTPEVTCVVVDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVYPPSREEMTKNQVSL TCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFALVSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 312 hinge-C.sub.H2-C.sub.H3-zwA DKTHTCPPCPAPELLGGPSVFLFPPKPKD V262E TLMISRTPEVTCEVVDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVYPPSREEMTKNQVSL TCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFALVSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 313 hinge-C.sub.H2-C.sub.H3-zwA DKTHTCPPCPAPELLGGPSVFLFPPKPKD V264S TLMISRTPEVTCVVSDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVYPPSREEMTKNQVSL TCLVKGFYPSDIAVEWESNGQPENNYKTT PPVLDSDGSFALVSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 314 hinge-C.sub.H2-C.sub.H3-zwB DKTHTCPPCPAPELLGGPSVFLFPPKPKD TLMISRTPEVTCVVVDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVLPPSREEMTKNQVSL LCLVKGFYPSDIAVEWESNGQPENNYLTW PPVLDSDGSFFLYSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 315 hinge-C.sub.H2-C.sub.H3-zwB DKTHTCPPCPAPELLGGPSVFLFPPKPKD V262E TLMISRTPEVTCEVVDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVLPPSREEMTKNQVSL LCLVKGFYPSDIAVEWESNGQPENNYLTW PPVLDSDGSFFLYSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 316 hinge-C.sub.H2-C.sub.H3-zwB DKTHTCPPCPAPELLGGPSVFLFPPKPKD V264S TLMISRTPEVTCVVSDVSHEDPEVKFNWY VDGVEVHNAKTKPREEQYNSTYRVVSVLT VLHQDWLNGKEYKCKVSNKALPAPIEKTI SKAKGQPREPQVYVLPPSREEMTKNQVSL LCLVKGFYPSDIAVEWESNGQPENNYLTW PPVLDSDGSFFLYSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK 317 C.sub.H1-(a)1 ASTKGPSVFPEAPSSKSTSGGTAALGCLV TDYFPEPVTVSWNSGALTSGVHTFPAVLE SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 318 C.sub.H1-(b)1 ASTKGPSVFPRAPSSKSTSGGTAALGCLV KDYFPEPVTVSWNSGALTSGVHTFPAVLQ SSGLYKLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 319 C.sub.H1-(c)1 ASTKGPSVFPLAPSSKSTSGGTAWLGCEV TDYFPEPVTVSWNSGALTSGVHTFPAVLE SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 320 C.sub.H1-(d)1 ASTKGPSVFPLAPSSKSTSGGTAALGCLV KDYFPEPVTVSWNSGALTSGVRTFPAVLK SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 321 C.sub.H1-(e)(f)1 ASTKGPSVFPLAPSSKSTSGGTAALGCLV KGYFPEPVTVSWNSGALTSGVHTFPAVLK SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 322 C.sub.H1-(a)2 ASTKGPSVFPLAPSSKSTSGGTAALGCLV KDYFPEPVTVSWNSGALTSGVHTFPAVLQ SSGLYSLKSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 323 C.sub.H1-(b)2 ASTKGPSVFPLAPSSKSTSGGTAALGCEV TDYFPEPVTVSWNSGALTSGVHTFPAVLE SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 324 C.sub.H1-(c)2 ASTKGPSVFPLAPSSKSTSGGTAALGCLV KDYFPEPVTVSWNSGALTSGVHTFPAVLK SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 325 C.sub.H1-(d)2 ASTKGPSVFPLAPSSKSTSGGTAALGCEV TDYFPEPVTVSWNSGALTSGVHTFPAVLE SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 326 C.sub.H1-(e)(f)2 ASTKGPSVFPLAPSSKSTSGGTAALGCEV TDYFPEPVTVSWNSGALTSGVHTFPAVLQ SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 327 Ck-(a)1 RTVAAPSVFIFPPSDEQLKSGTARVGCLL NNFYPREAKVQWKVDNALQSGNSQESVTE QDSKDSTYSLRSTLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 328 Ck-(b)1 RTVAAPSVFIFPPSDEQLKSGTASVGCLL NNFYPREAKVQWKVDNALQSGNSQESVTE QDSKDSTYSLDSELTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 329 Ck-(c)1 RTVAAPSVAIFPPSDERLKSGTASVVCVL NNFYPREAKVQWKVDNALQSGNSQESVTE
QDSKDSTYSLSSRLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 330 Ck-(d)1 RTVAAPSVFIFPPSDEELKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSEESVTE QDSKDSTYSLSSTLELSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 331 Ck-(e)(f)1 RTVAAPSVFIFPPSDEELKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSEESVTE QDSKDSTYSLSSTLELSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 332 Cl-(a)2 GQPKAAPSVTLFPPSSEELQANKATLVCL ISDFYPGAVTVAWKADSSPVKAGVETTTP SKQSNNKYAAESELSLTPEQWKSHRSYSC QVTHEGSTVEKTVAPTECS 333 Cl-(b)2 GQPKAAPSVTLFPPSSEQLQANKARLVCL ISDFYPGAVTVAWKADSSPVKAGVETTTP SKQSNNKYAASSYLSLTPEQWKSHRSYSC QVTHEGSTVEKTVAPTECS 334 Ck-(c)2 RTVAAPSVFIFPPSDEELKSGTASVVCWL NNFYPREAKVQWKVDNALQSGNSEESVTE QDSKDSTYSLSSTLELSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 335 Ck-(d)2 RTVAAPSVFIFPPSDERLKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSKESVTE QDSKDSTYSLSSRLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 336 Ck-(e)2 RTVAAPSVFIFPPSDERLKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSKESVTE QDSKDSTYSLSSRLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 337 Ck-(f)2 RTVAAPSVFIFPPSDERLKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSQESVTE QDSKDSTYSLSSTLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 338 Ck-(e)(f)2 RTVAAPSVFIFPPSDEELKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSEESVTE QDSKDSTYSLSSTLELSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 339 aPD-1-1353- scFv EVQLVQSGAEVKKPGASVKVSCKASGYTF G10_R28T/P30D/H3 DSYGISWVRQAPGQGLEWMGWISAYNGNT 1S_Vk1-39_LC NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSSGGGGSGGGGSGGGGSDIQLTQSPSSL SASVGDRVTITCSGDALPKQYAYWYQQKP GKAPKLLIYKDTERPSGVPSRFSGSSSGT KVTLTISSLQPEDFATYYCQSADNSITYR VFGGGTKVEIK 340 anti-PD-1 1353-G10 scFv EVQLVQSGAEVKKPGASVKVSCKASGYTF scFvFc zwB DSYGISWVRQAPGQGLEWMGWISAYNGNT R28T/P30D/H31S NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSSGGGGSGGGGSGGGGSSYELTQPPSVS VSPGQTARITCSGDALPKQYAYWYQQKPG QAPVMVIYKDTERPSGIPERFSGSSSGTK VTLTISGVQAEDEADYYCQSADNSITYRV FGGGTKVTVL 341 anti-PD-1 1353-G10 scFv EVQLVQSGAEVKKPGASVKVSCKASGYRF Vk1-39 PHYGISWVRQAPGQGLEWMGWISAYNGNT NYAQKLQGRVTMTTDTSTNTAYMELRSLR SDDTAVYYCARDVDYGTGSGYWGQGTLVT VSSGGGGSGGGGSGGGGSDIQLTQSPSSL SASVGDRVTITCSGDALPKQYAYWYQQKP GKAPKLLIYKDTERPSGVPSRFSGSSSGT KVTLTISSLQPEDFATYYCQSADNSITYR VFGGGTKVEIK 342 Anti-PD1 1353-G10 HC MEVQLVQSGAEVKKPGASVKVSCKASGYR HC knob FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSASTKGPSVFPLAPSSKSTSGGTAAL GCLVKDYFPEPVTVSWNSGALTSGVHTFP AVLQSSGLYSLSSVVTVPSSSLGTQTYIC NVNHKPSNTKVDKKVEPKSCDKTHTCPPC PAPELLGGPSVFLFPPKPKDTLMISRTPE VTCVVVDVSHEDPEVKFNWYVDGVEVHNA KTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKVSNKALPAPIEKTISKAKGQPRE PQVYTLPPSREEMTKNQVSLWCLVKGFYP SDIAVEWESNGQPENNYKTTPPVLDSDGS FFLYSKLTVDKSRWQQGNVFSCSVMHEAL HNHYTQKSLSLSPGK 343 Anti-PD1 1353-G10 HC MEVQLVQSGAEVKKPGASVKVSCKASGYR HC hole FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSASTKGPSVFPLAPSSKSTSGGTAAL GCLVKDYFPEPVTVSWNSGALTSGVHTFP AVLQSSGLYSLSSVVTVPSSSLGTQTYIC NVNHKPSNTKVDKKVEPKSCDKTHTCPPC PAPELLGGPSVFLFPPKPKDTLMISRTPE VTCVVVDVSHEDPEVKFNWYVDGVEVHNA KTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKVSNKALPAPIEKTISKAKGQPRE PQVYTLPPSREEMTKNQVSLSCAVKGFYP SDIAVEWESNGQPENNYKTTPPVLDSDGS FFLVSKLTVDKSRWQQGNVFSCSVMHEAL HNHYTQKSLSLSPGK 344 Anti-PD1 1353-G10 LC MSYELTQPPSVSVSPGQTARITCSGDALP LC KQYAYWYQQKPGQAPVMVIYKDTERPSGI PERFSGSSSGTKVTLTISGVQAEDEADYY CQSADNSITYRVFGGGTKVTVLGQPKAAP SVTLFPPSSEELQANKATLVCLISDFYPG AVTVAWKADSSPVKAGVETTTPSKQSNNK YAASSYLSLTPEQWKSHRSYSCQVTHEGS TVEKTVAPTECS 345 C.sub.H1-Wt ASTKGPSVFPLAPSSKSTSGGTAALGCLV KDYFPEPVTVSWNSGALTSGVHTFPAVLQ SSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKKVEPKSC 346 C.kappa.-wt RTVAAPSVFIFPPSDEQLKSGTASVVCLL NNFYPREAKVQWKVDNALQSGNSQESVTE QDSKDSTYSLSSTLTLSKADYEKHKVYAC EVTHQGLSSPVTKSFNRGEC 347 C.lamda.-wt GQPKAAPSVTLFPPSSEELQANKATLVCL ISDFYPGAVTVAWKADSSPVKAGVETTTP SKQSNNKYAASSYLSLTPEQWKSHRSYSC QVTHEGSTVEKTVAPTECS 348 1353-G10 scFv-Fc scFv-Fc MEVQLVQSGAEVKKPGASVKVSCKASGYR zwB FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSGGGGSGGGGSGGGGSSYELTQPPSV SVSPGQTARITCSGDALPKQYAYWYQQKP GQAPVMVIYKDTERPSGIPERFSGSSSGT KVTLTISGVQAEDEADYYCQSADNSITYR VFGGGTKVTVLAAGSDQEPKSSDKTHTCP PCPAPELLGGPSVFLFPPKPKDTLMISRT PEVTCVVVDVSHEDPEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWL NGKEYKCKVSNKALPAPIEKTISKAKGQP REPQVYVLPPSREEMTKNQVSLLCLVKGF YPSDIAVEWESNGQPENNYLTWPPVLDSD GSFFLYSKLTVDKSRWQQGNVFSCSVMHE ALHNHYTQKSLSLSPGK 349 1353-G10 HC zwA HC MEVQLVQSGAEVKKPGASVKVSCKASGYR FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSASTKGPSVFPLAPSSKSTSGGTAAL GCLVKDYFPEPVTVSWNSGALTSGVHTFP AVLQSSGLYSLSSVVTVPSSSLGTQTYIC NVNHKPSNTKVDKKVEPKSCDKTHTCPPC PAPELLGGPSVFLFPPKPKDTLMISRTPE VTCVVVDVSHEDPEVKFNWYVDGVEVHNA KTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKVSNKALPAPIEKTISKAKGQPRE PQVYVYPPSREEMTKNQVSLTCLVKGFYP SDIAVEWESNGQPENNYKTTPPVLDSDGS FALVSKLTVDKSRWQQGNVFSCSVMHEAL HNHYTQKSLSLSPGK 350 1353-G10 HC zwB HC MEVQLVQSGAEVKKPGASVKVSCKASGYR FPHYGISWVRQAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSASTKGPSVFPLAPSSKSTSGGTAAL GCLVKDYFPEPVTVSWNSGALTSGVHTFP AVLQSSGLYSLSSVVTVPSSSLGTQTYIC NVNHKPSNTKVDKKVEPKSCDKTHTCPPC PAPELLGGPSVFLFPPKPKDTLMISRTPE VTCVVVDVSHEDPEVKFNWYVDGVEVHNA KTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKVSNKALPAPIEKTISKAKGQPRE PQVYVLPPSREEMTKNQVSLLCLVKGFYP SDIAVEWESNGQPENNYLTWPPVLDSDGS FFLYSKLTVDKSRWQQGNVFSCSVMHEAL HNHYTQKSLSLSPGK 351 1353-G10 LC1c LC MSYELTQPPSVSVSPGQTARITCSGDALP KQYAYWYQQKPGQAPVMVIYKDTERPSGI PERFSGSSSGTKVTLTISGVQAEDFATYY CQSADNSITYRVFGGGTKVEIKRTVAAPS VAIFPPSDERLKSGTASVVCVLNNFYPRE AKVQWKVDNALQSGNSQESVTEQDSKDST YSLSSRLTLSKADYEKHKVYACEVTHQGL SSPVTKSFNRGEC 352 1353-G10 LC1d LC MSYELTQPPSVSVSPGQTARITCSGDALP KQYAYWYQRKPGQAPVMVIYKDTERPSGI PERFSGSSSGTKVTLTISGVQAEDFATYY CQSADNSITYRVFGGGTKVEIKRTVAAPS VFIFPPSDERLKSGTASVVCLLNNFYPRE AKVQWKVDNALQSGNSKESVTEQDSKDST YSLSSRLTLSKADYEKHKVYACEVTHQGL SSPVTKSFNRGEC 353 1353-G10 HC1d zwB HC MEVQLVQSGAEVKKPGASVKVSCKASGYR FPHYGISWVREAPGQGLEWMGWISAYNGN TNYAQKLQGRVTMTTDTSTNTAYMELRSL RSDDTAVYYCARDVDYGTGSGYWGQGTLV TVSSASTKGPSVFPLAPSSKSTSGGTAAL GCEVTDYFPEPVTVSWNSGALTSGVHTFP AVLESSGLYSLSSVVTVPSSSLGTQTYIC NVNHKPSNTKVDKKVEPKSCDKTHTCPPC PAPELLGGPSVFLFPPKPKDTLMISRTPE VTCVVVDVSHEDPEVKFNWYVDGVEVHNA KTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKVSNKALPAPIEKTISKAKGQPRE PQVYVLPPSREEMTKNQVSLLCLVKGFYP SDIAVEWESNGQPENNYLTWPPVLDSDGS FFLYSKLTVDKSRWQQGNVFSCSVMHEAL HNHYTQKSLSLSPGK 354 trastuzumab CDR-L1 RASQDVNTA-VA 355 trastuzumab CDR-L2 SASFLYS 356 trastuzumab CDR-L3 QQHYTTPPT
EQUIVALENTS
[0866] The disclosure set forth above may encompass multiple distinct inventions with independent utility. Although each of these inventions has been disclosed in its preferred form(s), the specific embodiments thereof as disclosed and illustrated herein are not to be considered in a limiting sense, because numerous variations are possible. The subject matter of the inventions includes all novel and nonobvious combinations and subcombinations of the various elements, features, functions, and/or properties disclosed herein. The following claims particularly point out certain combinations and subcombinations regarded as novel and nonobvious. Inventions embodied in other combinations and subcombinations of features, functions, elements, and/or properties may be claimed in this application, in applications claiming priority from this application, or in related applications. Such claims, whether directed to a different invention or to the same invention, and whether broader, narrower, equal, or different in scope in comparison to the original claims, also are regarded as included within the subject matter of the inventions of the present disclosure.
Sequence CWU 1
1
3561301PRTHomo sapiensmisc_featureHuman Tim 3 1Met Phe Ser His Leu
Pro Phe Asp Cys Val Leu Leu Leu Leu Leu Leu1 5 10 15Leu Leu Thr Arg
Ser Ser Glu Val Glu Tyr Arg Ala Glu Val Gly Gln 20 25 30Asn Ala Tyr
Leu Pro Cys Phe Tyr Thr Pro Ala Ala Pro Gly Asn Leu 35 40 45Val Pro
Val Cys Trp Gly Lys Gly Ala Cys Pro Val Phe Glu Cys Gly 50 55 60Asn
Val Val Leu Arg Thr Asp Glu Arg Asp Val Asn Tyr Trp Thr Ser65 70 75
80Arg Tyr Trp Leu Asn Gly Asp Phe Arg Lys Gly Asp Val Ser Leu Thr
85 90 95Ile Glu Asn Val Ile Leu Ala Asp Ser Gly Ile Tyr Cys Cys Arg
Ile 100 105 110Gln Ile Pro Gly Ile Met Asn Asp Glu Lys Phe Asn Leu
Lys Leu Val 115 120 125Ile Lys Pro Ala Lys Val Thr Pro Ala Pro Thr
Leu Gln Arg Asp Phe 130 135 140Thr Ala Ala Phe Pro Arg Met Leu Thr
Thr Arg Gly His Gly Pro Ala145 150 155 160Glu Thr Gln Thr Leu Gly
Ser Leu Pro Asp Ile Asn Leu Thr Gln Ile 165 170 175Ser Thr Leu Ala
Asn Glu Leu Arg Asp Ser Arg Leu Ala Asn Asp Leu 180 185 190Arg Asp
Ser Gly Ala Thr Ile Arg Ile Gly Ile Tyr Ile Gly Ala Gly 195 200
205Ile Cys Ala Gly Leu Ala Leu Ala Leu Ile Phe Gly Ala Leu Ile Phe
210 215 220Lys Trp Tyr Ser His Ser Lys Glu Lys Ile Gln Asn Leu Ser
Leu Ile225 230 235 240Ser Leu Ala Asn Leu Pro Pro Ser Gly Leu Ala
Asn Ala Val Ala Glu 245 250 255Gly Ile Arg Ser Glu Glu Asn Ile Tyr
Thr Ile Glu Glu Asn Val Tyr 260 265 270Glu Val Glu Glu Pro Asn Glu
Tyr Tyr Cys Tyr Val Ser Ser Arg Gln 275 280 285Gln Pro Ser Gln Pro
Leu Gly Cys Arg Phe Ala Met Pro 290 295 3002301PRTMacaca
fascicularismisc_featureCynomolgus Tim 3 2Met Phe Ser His Leu Pro
Phe Asp Cys Val Leu Leu Leu Leu Leu Leu1 5 10 15Leu Leu Thr Arg Ser
Ser Glu Val Glu Tyr Ile Ala Glu Val Gly Gln 20 25 30Asn Ala Tyr Leu
Pro Cys Ser Tyr Thr Pro Ala Pro Pro Gly Asn Leu 35 40 45Val Pro Val
Cys Trp Gly Lys Gly Ala Cys Pro Val Phe Asp Cys Ser 50 55 60Asn Val
Val Leu Arg Thr Asp Asn Arg Asp Val Asn Asp Arg Thr Ser65 70 75
80Gly Arg Tyr Trp Leu Lys Gly Asp Phe His Lys Gly Asp Val Ser Leu
85 90 95Thr Ile Glu Asn Val Thr Leu Ala Asp Ser Gly Val Tyr Cys Cys
Arg 100 105 110Ile Gln Ile Pro Gly Ile Met Asn Asp Glu Lys His Asn
Val Lys Leu 115 120 125Val Val Ile Lys Pro Ala Lys Val Thr Pro Ala
Pro Thr Leu Gln Arg 130 135 140Asp Leu Thr Ser Ala Phe Pro Arg Met
Leu Thr Thr Gly Glu His Gly145 150 155 160Pro Ala Glu Thr Gln Thr
Pro Gly Ser Leu Pro Asp Val Asn Leu Thr 165 170 175Val Ser Asn Phe
Phe Cys Glu Leu Gln Ile Phe Thr Leu Thr Asn Glu 180 185 190Leu Arg
Asp Ser Gly Ala Thr Ile Arg Thr Ala Ile Tyr Ile Ala Ala 195 200
205Gly Ile Ser Ala Gly Leu Ala Leu Ala Leu Ile Phe Gly Ala Leu Ile
210 215 220Phe Lys Trp Tyr Ser His Ser Lys Glu Lys Thr Gln Asn Leu
Ser Ile225 230 235 240Ser Leu Ala Asn Ile Pro Pro Ser Gly Leu Ala
Asn Ala Val Ala Glu 245 250 255Gly Ile Arg Ser Glu Glu Asn Ile Tyr
Thr Ile Glu Glu Asp Val Tyr 260 265 270Glu Val Glu Glu Pro Asn Glu
Tyr Tyr Cys Tyr Val Ser Ser Gly Gln 275 280 285Gln Pro Ser Gln Pro
Leu Gly Cys Arg Val Ala Met Pro 290 295 3003281PRTMus
musculusmisc_featureMouse Tim 3 3Met Phe Ser Gly Leu Thr Leu Asn
Cys Val Leu Leu Leu Leu Gln Leu1 5 10 15Leu Leu Ala Arg Ser Leu Glu
Asn Ala Tyr Val Phe Glu Val Gly Lys 20 25 30Asn Ala Tyr Leu Pro Cys
Ser Tyr Thr Leu Ser Thr Pro Gly Ala Leu 35 40 45Val Pro Met Cys Trp
Gly Lys Gly Phe Cys Pro Trp Ser Gln Cys Thr 50 55 60Asn Glu Leu Leu
Arg Thr Asp Glu Arg Asn Val Thr Tyr Gln Lys Ser65 70 75 80Ser Arg
Tyr Gln Leu Lys Gly Asp Leu Asn Lys Gly Asp Val Ser Leu 85 90 95Ile
Ile Lys Asn Val Thr Leu Asp Asp His Gly Thr Tyr Cys Cys Arg 100 105
110Ile Gln Phe Pro Gly Leu Met Asn Asp Lys Lys Leu Glu Leu Lys Leu
115 120 125Asp Ile Lys Ala Ala Lys Val Thr Pro Ala Gln Thr Ala His
Gly Asp 130 135 140Ser Thr Thr Ala Ser Pro Arg Thr Leu Thr Thr Glu
Arg Asn Gly Ser145 150 155 160Glu Thr Gln Thr Leu Val Thr Leu His
Asn Asn Asn Gly Thr Lys Ile 165 170 175Ser Thr Trp Ala Asp Glu Ile
Lys Asp Ser Gly Glu Thr Ile Arg Thr 180 185 190Ala Ile His Ile Gly
Val Gly Val Ser Ala Gly Leu Thr Leu Ala Leu 195 200 205Ile Ile Gly
Val Leu Ile Leu Lys Trp Tyr Ser Cys Lys Lys Lys Lys 210 215 220Leu
Ser Ser Leu Ser Leu Ile Thr Leu Ala Asn Leu Pro Pro Gly Gly225 230
235 240Leu Ala Asn Ala Gly Ala Val Arg Ile Arg Ser Glu Glu Asn Ile
Tyr 245 250 255Thr Ile Glu Glu Asn Val Tyr Glu Val Glu Asn Ser Asn
Glu Tyr Tyr 260 265 270Cys Tyr Val Asn Ser Gln Gln Pro Ser 275
28047PRTArtificial SequenceSynthetic h22E11 VH5 51 VH, CDR H1 4Gly
Phe Ser Leu Thr Ser Tyr1 557PRTArtificial SequenceSynthetic 2D5 VH,
CDR H1 5Gly Tyr Pro Phe Ile Gly Tyr1 567PRTArtificial
SequenceSynthetic SRP1649 A01, CDR H1 6Gly Phe Asn Ile Ser Arg Tyr1
577PRTArtificial SequenceSynthetic SRP1649 F06, CDR H1 7Gly Phe Asn
Ile Gly Asn Tyr1 587PRTArtificial SequenceSynthetic SRP1649 G08,
CDR H1 8Gly Phe Asn Ile Ser Asn Tyr1 597PRTArtificial
SequenceSynthetic SRP1649 C05, CDR H1 9Gly Phe Asn Ile Gly Lys His1
5107PRTArtificial SequenceSynthetic SRP1649 D11, CDR H1 10Gly Phe
Asn Ile Ser Gly Tyr1 5117PRTArtificial SequenceSynthetic SRP1649
B06, CDR H1 11Gly Phe Asn Ile Ser Asn His1 5127PRTArtificial
SequenceSynthetic SRP1649 D06, CDR H1 12Gly Phe Asn Ile Arg Asn
His1 5137PRTArtificial SequenceSynthetic SRP1649 H02, CDR H1 13Gly
Phe Asn Ile Arg Ser Tyr1 5147PRTArtificial SequenceSynthetic
SRP1649 B09, CDR H1 14Gly Phe Asn Ile Arg Asn Asn1
5157PRTArtificial SequenceSynthetic SRP1649 E10, CDR H1 15Gly Phe
Asn Ile Ser Asn Asn1 5167PRTArtificial SequenceSynthetic SRP1649
E08, CDR H1 16Gly Phe Ser Ile Ser Asn Tyr1 5177PRTArtificial
SequenceSynthetic 1649-A01 S30D/R31D/Y56D, CDR-H1 17Gly Phe Asn Ile
Asp Asp Tyr1 5187PRTArtificial SequenceSynthetic 1649-A01
S30D/Y56D, CDR-H1 18Gly Phe Asn Ile Asp Arg Tyr1 5197PRTArtificial
SequenceSynthetic 1649-A01 S30D/R31D, CDR-H1 19Gly Phe Asn Ile Asp
Asp Tyr1 5207PRTArtificial SequenceSynthetic 1649-A01 Y32T, CDR-H1
20Gly Phe Asn Ile Ser Arg Thr1 5217PRTArtificial SequenceSynthetic
1649-A01 R31D, CDR-H1 21Gly Phe Asn Ile Ser Asp Tyr1
5227PRTArtificial SequenceSynthetic 1649-A01 S30D, CDR-H1 22Gly Phe
Asn Ile Asp Arg Tyr1 5237PRTArtificial SequenceSynthetic 1649-A01
S30K, CDR-H1 23Gly Phe Asn Ile Lys Arg Tyr1 5247PRTArtificial
SequenceSynthetic 1353-G10-wt, CDR-H1 24Gly Tyr Arg Phe Pro His
Tyr1 5257PRTArtificial SequenceSynthetic 1353-G10 Y27D, CDR-H1
25Gly Asp Arg Phe Pro His Tyr1 5267PRTArtificial SequenceSynthetic
1353-G10 R28D, CDR-H1 26Gly Tyr Asp Phe Pro His Tyr1
5277PRTArtificial SequenceSynthetic 1353-G10 F29D, CDR-H1 27Gly Tyr
Arg Asp Pro His Tyr1 5287PRTArtificial SequenceSynthetic 1353-G10
P30D, CDR-H1 28Gly Tyr Arg Phe Asp His Tyr1 5297PRTArtificial
SequenceSynthetic 1353-G10 H31D, CDR-H1 29Gly Tyr Arg Phe Pro Asp
Tyr1 5307PRTArtificial SequenceSynthetic 1353-G10 Y32D, CDR-H1
30Gly Tyr Arg Phe Pro His Asp1 5317PRTArtificial SequenceSynthetic
1353-G10 R28T/P30D, CDR-H1 31Gly Tyr Thr Phe Asp His Tyr1
5327PRTArtificial SequenceSynthetic 1353-G10 R28D/P30D, CDR-H1
32Gly Tyr Asp Phe Asp His Tyr1 5337PRTArtificial SequenceSynthetic
1353-G10 R28D/H31D, CDR-H1 33Gly Tyr Asp Phe Pro Asp Tyr1
5347PRTArtificial SequenceSynthetic 1353-G10 R28T/H31D, CDR-H1
34Gly Tyr Thr Phe Pro Asp Tyr1 5357PRTArtificial SequenceSynthetic
1353-G10 R28D/P30T/H31S, CDR-H1 35Gly Tyr Asp Phe Thr Ser Tyr1
5367PRTArtificial SequenceSynthetic 1353-G10 R28T/P30T/H31D, CDR-H1
36Gly Tyr Thr Phe Thr Asp Tyr1 5377PRTArtificial SequenceSynthetic
1353-G10 R28T/P30T/H31S, CDR-H1 37Gly Tyr Thr Phe Thr Ser Tyr1
5387PRTArtificial SequenceSynthetic 1353-G10-R28T/P30D/H31S, CDR-H1
38Gly Tyr Thr Phe Asp Ser Tyr1 5395PRTArtificial SequenceSynthetic
h22E11 VH5 51 VH, CDR H1 39Ser Tyr Gly Val His1 5405PRTArtificial
SequenceSynthetic 2D5 VH, CDR H1 40Gly Tyr Thr Met Asn1
5415PRTArtificial SequenceSynthetic SRP1649 A01, CDR H1 41Arg Tyr
Tyr Ile His1 5425PRTArtificial SequenceSynthetic SRP1649 F06, CDR
H1 42Asn Tyr Ala Ile His1 5435PRTArtificial SequenceSynthetic
SRP1649 G08, CDR H1 43Asn Tyr Val Ile His1 5445PRTArtificial
SequenceSynthetic SRP1649 C05, CDR H1 44Lys His Val Ile His1
5455PRTArtificial SequenceSynthetic SRP1649 D11, CDR H1 45Gly Tyr
Val Ile His1 5465PRTArtificial SequenceSynthetic SRP1649 B06, CDR
H1 46Asn His Ala Ile His1 5475PRTArtificial SequenceSynthetic
SRP1649 D06, CDR H1 47Asn His Ala Ile His1 5485PRTArtificial
SequenceSynthetic SRP1649 H02, CDR H1 48Ser Tyr Ala Ile His1
5495PRTArtificial SequenceSynthetic SRP1649 B09, CDR H1 49Asn Asn
Ala Ile His1 5505PRTArtificial SequenceSynthetic SRP1649 E10, CDR
H1 50Asn Asn Val Ile His1 5515PRTArtificial SequenceSynthetic
SRP1649 E08, CDR H1 51Asn Tyr Val Ile His1 5525PRTArtificial
SequenceSynthetic 1649-A01 S30D/R31D/Y56D, CDR-H1 52Asp Tyr Tyr Ile
His1 5535PRTArtificial SequenceSynthetic 1649-A01 S30D/Y56D, CDR-H1
53Arg Tyr Tyr Ile His1 5545PRTArtificial SequenceSynthetic 1649-A01
S30D/R31D, CDR-H1 54Asp Tyr Tyr Ile His1 5555PRTArtificial
SequenceSynthetic 1649-A01 Y32T, CDR-H1 55Arg Thr Tyr Ile His1
5565PRTArtificial SequenceSynthetic 1649-A01 R31D, CDR-H1 56Asp Tyr
Tyr Ile His1 5575PRTArtificial SequenceSynthetic 1353-G10-wt,
CDR-H1 57His Tyr Gly Ile Ser1 5585PRTArtificial SequenceSynthetic
1353-G10 H31D, CDR-H1 58Asp Tyr Gly Ile Ser1 5595PRTArtificial
SequenceSynthetic 1353-G10 Y32D, CDR-H1 59His Asp Gly Ile Ser1
5605PRTArtificial SequenceSynthetic 1353-G10 G33D, CDR-H1 60His Tyr
Asp Ile Ser1 5615PRTArtificial SequenceSynthetic 1353-G10 S35D,
CDR-H1 61His Tyr Gly Ile Asp1 5625PRTArtificial SequenceSynthetic
1353-G10-R28T/P30D/H31S, CDR-H1 62Ser Tyr Gly Ile Ser1
5635PRTArtificial SequenceSynthetic h22E11 VH5 51 VH, CDR H2 63Trp
Ser Asp Gly Ser1 5646PRTArtificial SequenceSynthetic 2D5 VH, CDR H2
64Asn Pro Tyr Asn Gly Ile1 5656PRTArtificial SequenceSynthetic
SRP1649 A01, CDR H2 65Thr Pro Val Arg Gly Tyr1 5666PRTArtificial
SequenceSynthetic SRP1649 F06, CDR H2 66Thr Pro Gly Gln Gly Tyr1
5676PRTArtificial SequenceSynthetic SRP1649 G08, CDR H2 67Thr Pro
Asp Gly Gly Ile1 5686PRTArtificial SequenceSynthetic SRP1649 C05,
CDR H2 68Val Pro Asn Gly Gly Tyr1 5696PRTArtificial
SequenceSynthetic SRP1649 D11, CDR H2 69Ile Pro Thr Ala Gly Tyr1
5706PRTArtificial SequenceSynthetic SRP1497 A02, CDR H2 70Thr Pro
Asp Gly Gly Tyr1 5716PRTArtificial SequenceSynthetic SRP1649 B06,
CDR H2 71Ser Pro Ala Val Gly Tyr1 5726PRTArtificial
SequenceSynthetic SRP1649 D06, CDR H2 72Ala Pro Ala Gly Gly Tyr1
5736PRTArtificial SequenceSynthetic SRP1649 H02, CDR H2 73Thr Pro
Ala Gly Gly Asp1 5746PRTArtificial SequenceSynthetic SRP1649 B09,
CDR H2 74Thr Pro Ala Gly Gly Tyr1 5756PRTArtificial
SequenceSynthetic SRP1649 E10, CDR H2 75Met Pro Gly Gly Gly Ser1
5766PRTArtificial SequenceSynthetic SRP1649 E08, CDR H2 76Ser Pro
Asp Gly Gly Phe1 5776PRTArtificial SequenceSynthetic 1649-A01
S30D/R31D/Y56D, CDR H2 77Thr Pro Val Arg Gly Asp1 5786PRTArtificial
SequenceSynthetic 1649-A01 R54N, CDR H2 78Thr Pro Val Asn Gly Tyr1
5796PRTArtificial SequenceSynthetic 1649-A01 V53D, CDR H2 79Thr Pro
Asp Arg Gly Tyr1 5806PRTArtificial SequenceSynthetic 1649-A01 V53T,
CDR H2 80Thr Pro Thr Arg Gly Tyr1 5816PRTArtificial
SequenceSynthetic 1353-G10-wt, CDR H2 81Ser Ala Tyr Asn Gly Asn1
58216PRTArtificial SequenceSynthetic h22E11 VH5 51 VH, CDR H2 82Val
Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Pro Ser Phe Gln Gly1 5 10
158316PRTArtificial SequenceSynthetic h22E11 VH1 69 VH, CDR H2
83Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Gln Lys Phe Gln Gly1
5 10 158416PRTArtificial SequenceSynthetic 22E11 VH, CDR H2 84Val
Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Ser Ala Leu Lys Ser1 5 10
158516PRTArtificial SequenceSynthetic h22E11 VH4 30 4 VH, CDR H2
85Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Pro Ser Leu Lys Ser1
5 10 158616PRTArtificial SequenceSynthetic h22E11 VH3 23 VH, CDR H2
86Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Asp Ser Val Lys Gly1
5 10 158716PRTArtificial SequenceSynthetic h22E11 5 VH, CDR H2
87Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Ser Ala Leu Lys Ser1
5 10 158817PRTArtificial SequenceSynthetic 2D5 VH, CDR H2 88Leu Ile
Asn Pro Tyr Asn Gly Ile Thr Thr Tyr Asn Gln Lys Phe Lys1 5 10
15Gly8917PRTArtificial SequenceSynthetic SRP1649 A01, CDR H2 89Gly
Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Asp9017PRTArtificial SequenceSynthetic SRP1649 F06, CDR H2 90Asp
Ile Thr Pro Gly Gln Gly Tyr Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Asp9117PRTArtificial SequenceSynthetic SRP1649 G08, CDR H2 91Ala
Ile Thr Pro Asp Gly Gly Ile Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Gly9217PRTArtificial SequenceSynthetic SRP1649 C05, CDR H2 92Asp
Ile Val Pro Asn Gly Gly Tyr Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Asp9317PRTArtificial SequenceSynthetic SRP1649 D11, CDR H2 93Asp
Ile Ile Pro Thr Ala Gly Tyr Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Gly9417PRTArtificial SequenceSynthetic SRP1497 A02, CDR H2 94Asp
Ile Thr Pro Asp Gly Gly Tyr Thr Asp Tyr Ala Asp Ser Val Lys1 5 10
15Gly9517PRTArtificial SequenceSynthetic SRP1497 A05, CDR H2 95Asp
Ile Thr Pro Asp Gly Gly Tyr Thr Asp Tyr Ala Asp Ser Val Lys1 5 10
15Asp9617PRTArtificial SequenceSynthetic SRP1649 B06, CDR H2 96Asp
Ile Ser Pro Ala Val Gly Tyr Thr Asp Tyr Ala Asp Ser Val Lys1 5 10
15Asp9717PRTArtificial SequenceSynthetic SRP1649 D06, CDR H2 97Asp
Ile Ala Pro Ala Gly Gly Tyr Thr Asp Tyr Ala Asp Ser Val Lys1 5 10
15Asp9817PRTArtificial SequenceSynthetic SRP1649 H02, CDR H2 98Asp
Ile Thr Pro Ala Gly Gly Asp Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Gly9917PRTArtificial SequenceSynthetic SRP1649 B09, CDR H2 99Asp
Ile Thr Pro Ala Gly Gly Tyr Thr Gly Tyr Ala Asp Ser Val Lys1 5 10
15Asp10017PRTArtificial SequenceSynthetic SRP1649 E10, CDR H2
100Asp Ile Met Pro Gly Gly Gly Ser Thr Asp Tyr Ala Asp Ser Val Lys1
5 10
15Asp10117PRTArtificial SequenceSynthetic SRP1649 E08, CDR H2
101Asp Ile Ser Pro Asp Gly Gly Phe Thr Asp Tyr Ala Asp Ser Val Lys1
5 10 15Asp10217PRTArtificial SequenceSynthetic 1649-A01
S30D/R31D/Y56D, CDR H2 102Gly Ile Thr Pro Val Arg Gly Asp Thr Glu
Tyr Ala Asp Ser Val Lys1 5 10 15Asp10317PRTArtificial
SequenceSynthetic 1649-A01 E58D, CDR H2 103Gly Ile Thr Pro Val Arg
Gly Tyr Thr Asp Tyr Ala Asp Ser Val Lys1 5 10
15Asp10417PRTArtificial SequenceSynthetic 1649-A01 E58R, CDR H2
104Gly Ile Thr Pro Val Arg Gly Tyr Thr Arg Tyr Ala Asp Ser Val Lys1
5 10 15Asp10517PRTArtificial SequenceSynthetic 1649-A01 R54N, CDR
H2 105Gly Ile Thr Pro Val Asn Gly Tyr Thr Glu Tyr Ala Asp Ser Val
Lys1 5 10 15Asp10617PRTArtificial SequenceSynthetic 1649-A01 V53D,
CDR H2 106Gly Ile Thr Pro Asp Arg Gly Tyr Thr Glu Tyr Ala Asp Ser
Val Lys1 5 10 15Asp10717PRTArtificial SequenceSynthetic 1649-A01
V53T, CDR H2 107Gly Ile Thr Pro Thr Arg Gly Tyr Thr Glu Tyr Ala Asp
Ser Val Lys1 5 10 15Asp10817PRTArtificial SequenceSynthetic
1649-A01 G50D, CDR H2 108Asp Ile Thr Pro Val Arg Gly Tyr Thr Glu
Tyr Ala Asp Ser Val Lys1 5 10 15Asp10917PRTArtificial
SequenceSynthetic 1649-A01 G50R, CDR H2 109Arg Ile Thr Pro Val Arg
Gly Tyr Thr Glu Tyr Ala Asp Ser Val Lys1 5 10
15Asp11017PRTArtificial SequenceSynthetic 1353-G10-wt, CDR H2
110Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu Gln1
5 10 15Gly11112PRTArtificial SequenceSynthetic h22E11 VH5 51 VH,
CDR H3 111Gln Gly Gly Tyr Arg Tyr Asp Asp Ala Met Asp Tyr1 5
1011213PRTArtificial SequenceSynthetic 2D5 VH, CDR H3 112Ser Phe
Phe Tyr Gly Ser Ser Asn Asp Trp Leu Val Tyr1 5 1011312PRTArtificial
SequenceSynthetic SRP1649 A01, CDR H3 113Gly Tyr Val Tyr Arg Met
Trp Asp Ser Tyr Asp Tyr1 5 1011412PRTArtificial SequenceSynthetic
SRP1649 C05, CDR H3 114Gly Tyr Val Tyr Arg Met Trp Asp Ser Phe Asp
Tyr1 5 1011512PRTArtificial SequenceSynthetic SRP1649 B06, CDR H3
115Gly Tyr Val Tyr Arg Met Trp Asp Ser Phe Asp His1 5
1011612PRTArtificial SequenceSynthetic SRP1649 H02, CDR H3 116Gly
Tyr Ile Tyr Arg Met Trp Asp Ser Tyr Asp Tyr1 5 1011712PRTArtificial
SequenceSynthetic SRP1649 B09, CDR H3 117Gly Tyr Ile Tyr Arg Met
Trp Asp Ser Leu Asp Tyr1 5 1011812PRTArtificial SequenceSynthetic
SRP1649 E08, CDR H3 118Gly His Val Tyr Arg Leu Trp Asp Ser Phe Asp
Tyr1 5 1011910PRTArtificial SequenceSynthetic 1353-G10-wt, CDR H3
119Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr1 5 1012015PRTArtificial
SequenceSynthetic h22E11 Vk3 11 VL, CDR L1 120Lys Ala Ser Gln Ser
Val Asp Tyr Asp Gly Asn Ser Tyr Val Asn1 5 10 1512115PRTArtificial
SequenceSynthetic h22E11 5 VL, CDR L1 121Lys Ala Ser Gln Ser Val
Asp Tyr Asp Gly Asn Ser Tyr Val Ala1 5 10 1512216PRTArtificial
SequenceSynthetic 2D5 VL, CDR L1 122Arg Ser Ser Gln Ser Ile Val His
Thr Asn Gly Asn Thr Tyr Leu Glu1 5 10 1512311PRTArtificial
SequenceSynthetic 1649-A01, CDR L1 123Arg Ala Ser Gln Asp Val Asn
Thr Ala Val Ala1 5 1012411PRTArtificial SequenceSynthetic
1353-G10-wt, CDR L1 124Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala Tyr1
5 101257PRTArtificial SequenceSynthetic h22E11 Vk3 11 VL, CDR L2
125Ala Ala Ser Asn Leu Glu Ser1 51267PRTArtificial
SequenceSynthetic 2D5 VL, CDR L2 126Lys Val Ser Asn Arg Phe Ser1
51277PRTArtificial SequenceSynthetic 1649-A01 T56D, CDR L2 127Ser
Ala Ser Phe Leu Tyr Asp1 51287PRTArtificial SequenceSynthetic
1649-A01 Y55D, CDR L2 128Ser Ala Ser Phe Leu Asp Ser1
51297PRTArtificial SequenceSynthetic 1649-A01 F53D, CDR L2 129Ser
Ala Ser Asp Leu Tyr Ser1 51307PRTArtificial SequenceSynthetic
1649-A01 S52D, CDR L2 130Ser Ala Asp Phe Leu Tyr Ser1
51317PRTArtificial SequenceSynthetic 1649-A01 A51D, CDR L2 131Ser
Asp Ser Phe Leu Tyr Ser1 51327PRTArtificial SequenceSynthetic
1649-A01 S50D, CDR L2 132Asp Ala Ser Phe Leu Tyr Ser1
51337PRTArtificial SequenceSynthetic 1649-A01, CDR L2 133Ser Ala
Ser Phe Leu Tyr Ser1 51347PRTArtificial SequenceSynthetic
1353-G10-wt, CDR L2 134Lys Asp Thr Glu Arg Pro Ser1
51357PRTArtificial SequenceSynthetic 1353-G10 K50D, CDR L2 135Asp
Asp Thr Glu Arg Pro Ser1 51367PRTArtificial SequenceSynthetic
1353-G10 T52D, CDR L2 136Lys Asp Asp Glu Arg Pro Ser1
51377PRTArtificial SequenceSynthetic 1353-G10 E53D, CDR L2 137Lys
Asp Thr Asp Arg Pro Ser1 51387PRTArtificial SequenceSynthetic
1353-G10 P55D, CDR L2 138Lys Asp Thr Glu Arg Asp Ser1
51397PRTArtificial SequenceSynthetic 1353-G10 S56D, CDR L2 139Lys
Asp Thr Glu Arg Pro Asp1 51409PRTArtificial SequenceSynthetic
h22E11 Vk3 11 VL, CDR L3 140Gln Gln Ser Asn Glu Asp Pro Tyr Thr1
51419PRTArtificial SequenceSynthetic 2D5 VL, CDR L3 141Phe Gln Gly
Ser His Val Pro Trp Thr1 51429PRTArtificial SequenceSynthetic
1649-A01, CDR L3 142Gln Gln His Tyr Thr Thr Pro Pro Thr1
514311PRTArtificial SequenceSynthetic 1353-G10-wt, CDR L3 143Gln
Ser Ala Asp Asn Ser Ile Thr Tyr Arg Val1 5 10144122PRTArtificial
SequenceSynthetic 2D5 VH, VH 144Glu Val Gln Leu Gln Gln Ser Gly Pro
Glu Leu Val Lys Pro Gly Thr1 5 10 15Ser Met Lys Ile Ser Cys Arg Ala
Ser Gly Tyr Pro Phe Ile Gly Tyr 20 25 30Thr Met Asn Trp Val Lys Gln
Ser His Gly Gly Asn Leu Glu Trp Ile 35 40 45Gly Leu Ile Asn Pro Tyr
Asn Gly Ile Thr Thr Tyr Asn Gln Lys Phe 50 55 60Lys Gly Arg Ala Thr
Leu Ser Val Asp Thr Ser Ser Thr Ile Ala Tyr65 70 75 80Met Glu Leu
Leu Ser Leu Thr Ser Asp Asp Ser Ala Glu Tyr Tyr Cys 85 90 95Ala Arg
Ser Phe Phe Tyr Gly Ser Ser Asn Asp Trp Leu Val Tyr Trp 100 105
110Gly Gln Gly Thr Leu Val Thr Val Ser Ala 115
120145120PRTArtificial SequenceSynthetic 22E11 VH, VH 145Gln Val
Gln Leu Lys Glu Ser Gly Pro Asp Leu Val Ala Pro Ser Gln1 5 10 15Ser
Leu Ser Ile Thr Cys Thr Val Ser Gly Phe Ser Leu Thr Ser Tyr 20 25
30Gly Val His Trp Val Arg Gln Pro Pro Gly Lys Gly Leu Glu Trp Leu
35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Ser Ala Leu
Lys 50 55 60Ser Arg Leu Thr Ile Ser Lys Asp Asn Ser Lys Ser Gln Val
Phe Leu65 70 75 80Lys Met Asn Ser Leu Gln Thr Asp Asp Thr Ala Met
Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg Tyr Asp Asp Ala Met
Asp Tyr Trp Gly Gln 100 105 110Gly Thr Ser Val Ala Val Ser Ser 115
120146120PRTArtificial SequenceSynthetic h22E11 VH5 51 VH, VH
146Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Glu1
5 10 15Ser Leu Lys Ile Ser Cys Lys Val Ser Gly Phe Ser Leu Thr Ser
Tyr 20 25 30Gly Val His Trp Val Arg Gln Met Pro Gly Lys Gly Leu Glu
Trp Leu 35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn Pro
Ser Phe Gln 50 55 60Gly Gln Val Thr Ile Ser Lys Asp Lys Ser Ile Ser
Thr Val Tyr Leu65 70 75 80Gln Trp Ser Ser Leu Lys Ala Ser Asp Thr
Ala Met Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg Tyr Asp Asp
Ala Met Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser
Ser 115 120147120PRTArtificial SequenceSynthetic h22E11 VH4 30 4 H,
VH 147Gln Val Gln Leu Gln Glu Ser Gly Pro Gly Leu Val Lys Pro Ser
Gln1 5 10 15Thr Leu Ser Leu Thr Cys Thr Val Ser Gly Phe Ser Leu Thr
Ser Tyr 20 25 30Gly Val His Trp Ile Arg Gln Pro Pro Gly Lys Gly Leu
Glu Trp Leu 35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr Asn
Pro Ser Leu Lys 50 55 60Ser Arg Val Thr Ile Ser Lys Asp Thr Ser Lys
Asn Gln Val Ser Leu65 70 75 80Lys Leu Ser Ser Val Thr Ala Ala Asp
Thr Ala Val Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg Tyr Asp
Asp Ala Met Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val
Ser Ser 115 120148120PRTArtificial SequenceSynthetic h22E11 VH3 23
VH, VH 148Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Val Ser Gly Phe Ser Leu
Thr Ser Tyr 20 25 30Gly Val His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Leu 35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr
Asn Asp Ser Val Lys 50 55 60Gly Arg Phe Thr Ile Ser Lys Asp Asn Ser
Lys Asn Thr Val Tyr Leu65 70 75 80Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg Tyr
Asp Asp Ala Met Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr
Val Ser Ser 115 120149120PRTArtificial SequenceSynthetic h22E11 VH1
69 VH, VH 149Gln Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys
Pro Gly Ser1 5 10 15Ser Val Lys Val Ser Cys Lys Val Ser Gly Phe Ser
Leu Thr Ser Tyr 20 25 30Gly Val His Trp Val Arg Gln Ala Pro Gly Gln
Gly Leu Glu Trp Leu 35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr
Tyr Asn Gln Lys Phe Gln 50 55 60Gly Arg Val Thr Ile Thr Lys Asp Glu
Ser Thr Ser Thr Val Tyr Met65 70 75 80Glu Leu Ser Ser Leu Arg Ser
Glu Asp Thr Ala Val Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg
Tyr Asp Asp Ala Met Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val
Thr Val Ser Ser 115 120150120PRTArtificial SequenceSynthetic h22E11
5 VH, VH 150Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Val Ser Gly Phe Ser Leu
Thr Ser Tyr 20 25 30Gly Val His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Leu 35 40 45Val Val Ile Trp Ser Asp Gly Ser Thr Thr Tyr
Asn Ser Ala Leu Lys 50 55 60Ser Arg Phe Thr Ile Ser Lys Asp Asn Ala
Lys Asn Ser Val Tyr Leu65 70 75 80Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys Ala 85 90 95Arg Gln Gly Gly Tyr Arg Tyr
Asp Asp Ala Met Asp Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr
Val Ser Ser 115 120151121PRTArtificial SequenceSynthetic SRP1649
A01, VH 151Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile
Ser Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Glu
Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr
Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala
Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg
Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val
Thr Val Ser Ser 115 120152121PRTArtificial SequenceSynthetic
SRP1649 B06, VH 152Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val
Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe
Asn Ile Ser Asn His 20 25 30Ala Ile His Trp Val Arg Gln Ala Pro Gly
Lys Gly Leu Glu Trp Val 35 40 45Ala Asp Ile Ser Pro Ala Val Gly Tyr
Thr Asp Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala
Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu
Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val
Tyr Arg Met Trp Asp Ser Phe Asp His Trp Gly 100 105 110Gln Gly Thr
Leu Val Thr Val Ser Ser 115 120153121PRTArtificial
SequenceSynthetic SRP1649 C05, VH 153Glu Val Gln Leu Val Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys
Ala Ala Ser Gly Phe Asn Ile Gly Lys His 20 25 30Val Ile His Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Asp Ile Val
Pro Asn Gly Gly Tyr Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg
Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu
Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser Phe Asp Tyr Trp Gly
100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser 115
120154121PRTArtificial SequenceSynthetic SRP1649 D06, VH 154Glu Val
Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser
Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Arg Asn His 20 25
30Ala Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45Ala Asp Ile Ala Pro Ala Gly Gly Tyr Thr Asp Tyr Ala Asp Ser
Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr
Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser
Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser
115 120155121PRTArtificial SequenceSynthetic SRP1649 D11, VH 155Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10
15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Gly Tyr
20 25 30Val Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45Ala Asp Ile Ile Pro Thr Ala Gly Tyr Thr Glu Tyr Ala Asp
Ser Val 50 55 60Lys Gly Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn
Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp
Ser Phe Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser
Ser 115 120156121PRTArtificial SequenceSynthetic SRP1649 E08, VH
156Glu Val Gln Leu Val Glu Ser Gly Gly Ser Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Ser Ile Ser Asn
Tyr 20 25 30Val Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Ala Asp Ile Ser Pro Asp Gly Gly Phe Thr Asp Tyr Ala
Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys
Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn
Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly
His Val Tyr Arg Leu Trp Asp Ser Phe Asp Tyr Trp Gly 100 105 110Arg
Gly Thr Leu Val Thr Val Ser Ser 115 120157121PRTArtificial
SequenceSynthetic SRP1649 E10, VH 157Glu Val Gln Leu Val Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys
Ala Ala Ser Gly Phe Asn Ile Ser Asn Asn 20 25 30Val Ile His Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Gly Asp Ile Met
Pro Gly Gly Gly Ser Thr Asp Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg
Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu
Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp Gly
100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser 115
120158121PRTArtificial SequenceSynthetic SRP1649 F06, VH 158Glu Val
Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser
Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Gly Asn Tyr 20 25
30Ala Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45Ala Asp Ile Thr Pro Gly Gln Gly Tyr Thr Glu Tyr Ala Asp Ser
Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr
Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser
Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser
115 120159121PRTArtificial SequenceSynthetic SRP1649 G08, VH 159Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10
15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Asn Tyr
20 25 30Val Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45Ala Ala Ile Thr Pro Asp Gly Gly Ile Thr Glu Tyr Ala Asp
Ser Val 50 55 60Lys Gly Arg Phe Ala Ile Ser Ala Asp Thr Ser Lys Asn
Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp
Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser
Ser 115 120160121PRTArtificial SequenceSynthetic SRP1649 H02, VH
160Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Arg Ser
Tyr 20 25 30Ala Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Ala Asp Ile Thr Pro Ala Gly Gly Asp Thr Glu Tyr Ala
Asp Ser Val 50 55 60Lys Gly Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys
Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Ile Tyr Arg Met Trp
Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val
Ser Ser 115 120161121PRTArtificial SequenceSynthetic SRP1649 B09,
VH 161Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly
Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Arg
Asn Asn 20 25 30Ala Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45Ala Asp Ile Thr Pro Ala Gly Gly Tyr Thr Gly Tyr
Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser
Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Ile Tyr Arg Met
Trp Asp Ser Leu Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr
Val Ser Ser 115 120162121PRTArtificial SequenceSynthetic SRP1497
A05, VH 162Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile
Ser Asn Tyr 20 25 30Ala Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Val 35 40 45Ala Asp Ile Thr Pro Asp Gly Gly Tyr Thr Asp
Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr
Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala
Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg
Met Trp Asp Ser Phe Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val
Thr Val Ser Ser 115 120163121PRTArtificial SequenceSynthetic
SRP1497 A02, VH 163Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val
Arg Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe
Asn Ile Ser Asn Tyr 20 25 30Ala Ile His Trp Val Arg Gln Ala Pro Gly
Lys Gly Leu Glu Trp Val 35 40 45Ala Asp Ile Thr Pro Asp Gly Gly Tyr
Thr Asp Tyr Ala Asp Ser Val 50 55 60Lys Gly Arg Phe Thr Ile Ser Ala
Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu
Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val
Tyr Arg Met Trp Asp Ser Phe Asp Tyr Trp Gly 100 105 110Gln Gly Thr
Leu Val Thr Val Ser Ser 115 120164121PRTArtificial
SequenceSynthetic SRP1497 A01, VH 164Glu Val Gln Leu Val Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys
Ala Ala Ser Gly Phe Asn Ile Ser Asn Tyr 20 25 30Ala Ile His Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Asp Ile Thr
Pro Asp Gly Gly Tyr Thr Asp Tyr Ala Asp Ser Val 50 55 60Lys Gly Arg
Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu
Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser Phe Asp Tyr Trp Gly
100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser 115
120165121PRTArtificial SequenceSynthetic 1649-A01 S30D/R31D/Y56D,
VH 165Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly
Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Asp
Asp Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Asp Thr Glu Tyr
Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser
Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met
Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr
Val Ser Ser 115 120166121PRTArtificial SequenceSynthetic 1649-A01
S30D/Y56D, VH 166Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val
Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe
Asn Ile Asp Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly
Lys Gly Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Asp
Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala
Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu
Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val
Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr
Leu Val Thr Val Ser Ser 115 120167121PRTArtificial
SequenceSynthetic 1649-A01 S30D/R31D, VH 167Glu Val Gln Leu Val Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser
Cys Ala Ala Ser Gly Phe Asn Ile Asp Asp Tyr 20 25 30Tyr Ile His Trp
Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Gly Ile
Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp
Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75
80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp
Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser 115
120168121PRTArtificial SequenceSynthetic 1649-A01 D65G, VH 168Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10
15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg Tyr
20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala Asp
Ser Val 50 55 60Lys Gly Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn
Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp
Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser
Ser 115 120169121PRTArtificial SequenceSynthetic 1649-A01 E58D, VH
169Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg
Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Asp Tyr Ala
Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys
Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp
Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val
Ser Ser 115 120170121PRTArtificial SequenceSynthetic 1649-A01 E58R,
VH 170Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly
Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser
Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Arg Tyr
Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser
Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met
Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr
Val Ser Ser 115 120171121PRTArtificial SequenceSynthetic 1649-A01
Y56D, VH 171Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile
Ser Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Asp Thr Glu
Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr
Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala
Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg
Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val
Thr Val Ser Ser 115 120172121PRTArtificial SequenceSynthetic
1649-A01 R54N, VH 172Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Phe Asn Ile Ser Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Asn Gly
Tyr Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser
Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr
Val Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly
Thr Leu Val Thr Val Ser Ser 115 120173121PRTArtificial
SequenceSynthetic 1649-A01 V53D, VH 173Glu Val Gln Leu Val Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys
Ala Ala Ser Gly Phe Asn Ile Ser Arg Tyr 20 25 30Tyr Ile His Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Gly Ile Thr
Pro Asp Arg Gly Tyr Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg
Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu
Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp Gly
100 105 110Gln Gly Thr Leu Val Thr Val Ser Ser 115
120174121PRTArtificial SequenceSynthetic 1649-A01 V53T, VH 174Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10
15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg Tyr
20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45Ala Gly Ile Thr Pro Thr Arg Gly Tyr Thr Glu Tyr Ala Asp
Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn
Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp
Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser
Ser 115 120175121PRTArtificial SequenceSynthetic 1649-A01 G50D, VH
175Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg
Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Ala Asp Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala
Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys
Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp
Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val
Ser Ser 115
120176121PRTArtificial SequenceSynthetic 1649-A01 G50R, VH 176Glu
Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10
15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg Tyr
20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45Ala Arg Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala Asp
Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys Asn
Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp Asp
Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val Ser
Ser 115 120177121PRTArtificial SequenceSynthetic 1649-A01 Y32T, VH
177Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1
5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser Arg
Thr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu
Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr Ala
Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser Lys
Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met Trp
Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr Val
Ser Ser 115 120178121PRTArtificial SequenceSynthetic 1649-A01 R31D,
VH 178Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly
Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile Ser
Asp Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Glu Tyr
Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr Ser
Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg Met
Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val Thr
Val Ser Ser 115 120179121PRTArtificial SequenceSynthetic 1649-A01
S30D, VH 179Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro
Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Ile
Asp Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro Gly Lys Gly
Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly Tyr Thr Glu
Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser Ala Asp Thr
Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala
Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr Val Tyr Arg
Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly Thr Leu Val
Thr Val Ser Ser 115 120180121PRTArtificial SequenceSynthetic
1649-A01 S30K, VH 180Glu Val Gln Leu Val Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly
Phe Asn Ile Lys Arg Tyr 20 25 30Tyr Ile His Trp Val Arg Gln Ala Pro
Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Gly Ile Thr Pro Val Arg Gly
Tyr Thr Glu Tyr Ala Asp Ser Val 50 55 60Lys Asp Arg Phe Thr Ile Ser
Ala Asp Thr Ser Lys Asn Thr Ala Tyr65 70 75 80Leu Gln Met Asn Ser
Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Gly Tyr
Val Tyr Arg Met Trp Asp Ser Tyr Asp Tyr Trp Gly 100 105 110Gln Gly
Thr Leu Val Thr Val Ser Ser 115 120181119PRTArtificial
SequenceSynthetic 1353-G10-wt, VH 181Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Pro His Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115182119PRTArtificial
SequenceSynthetic 1353-G10 Y27D, VH 182Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Asp Arg Phe Pro His Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115183119PRTArtificial
SequenceSynthetic 1353-G10 R28D, VH 183Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Asp Phe Pro His Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115184119PRTArtificial
SequenceSynthetic 1353-G10 F29D, VH 184Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Asp Pro His Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115185119PRTArtificial
SequenceSynthetic 1353-G10 P30D, VH 185Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Asp His Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115186119PRTArtificial
SequenceSynthetic 1353-G10 H31D, VH 186Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Pro Asp Tyr 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115187119PRTArtificial
SequenceSynthetic 1353-G10 Y32D, VH 187Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Pro His Asp 20 25 30Gly Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115188119PRTArtificial
SequenceSynthetic 1353-G10 G33D, VH 188Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Pro His Tyr 20 25 30Asp Ile Ser Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115189119PRTArtificial
SequenceSynthetic 1353-G10 S35D, VH 189Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys
Lys Ala Ser Gly Tyr Arg Phe Pro His Tyr 20 25 30Gly Ile Asp Trp Val
Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser
Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg
Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met
Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90
95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly
100 105 110Thr Leu Val Thr Val Ser Ser 115190119PRTArtificial
SequenceSynthetic 1353-G10 R28T/P30D, VH 190Glu Val Gln Leu Val Gln
Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser
Cys Lys Ala Ser Gly Tyr Thr Phe Asp His Tyr 20 25 30Gly Ile Ser Trp
Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile
Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly
Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75
80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln
Gly 100 105 110Thr Leu Val Thr Val Ser Ser 115191119PRTArtificial
SequenceSynthetic 1353-G10 R28D/P30D, VH 191Glu Val Gln Leu Val Gln
Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser
Cys Lys Ala Ser Gly Tyr Asp Phe Asp His Tyr 20 25 30Gly Ile Ser Trp
Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile
Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly
Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75
80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln
Gly 100 105 110Thr Leu Val Thr Val Ser Ser 115192119PRTArtificial
SequenceSynthetic 1353-G10 R28D/H31D, VH 192Glu Val Gln Leu Val Gln
Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser
Cys Lys Ala Ser Gly Tyr Asp Phe Pro Asp Tyr 20 25 30Gly Ile Ser Trp
Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile
Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly
Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75
80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln
Gly 100 105 110Thr Leu Val Thr Val Ser Ser 115193119PRTArtificial
SequenceSynthetic 1353-G10 R28T/H31D, VH 193Glu Val Gln Leu Val Gln
Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser
Cys Lys Ala Ser Gly Tyr Thr Phe Pro Asp Tyr 20 25 30Gly Ile Ser Trp
Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile
Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly
Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75
80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln
Gly 100 105 110Thr Leu Val Thr Val Ser Ser 115194119PRTArtificial
SequenceSynthetic 1353-G10 R28D/P30T/H31S, VH 194Glu Val Gln Leu
Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys
Val Ser Cys Lys Ala Ser Gly Tyr Asp Phe Thr Ser Tyr 20 25 30Gly Ile
Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly
Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55
60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65
70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr
Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly
Gln Gly 100 105 110Thr Leu Val Thr Val Ser Ser
115195119PRTArtificial SequenceSynthetic 1353-G10 R28T/P30T/H31D,
VH 195Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly
Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr
Asp Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu
Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr
Ala Gln Lys Leu 50
55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala
Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr
Trp Gly Gln Gly 100 105 110Thr Leu Val Thr Val Ser Ser
115196119PRTArtificial SequenceSynthetic 1353-G10 R28T/P30T/H31S,
VH 196Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly
Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr
Ser Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu
Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr
Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser
Thr Asn Thr Ala Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr
Gly Ser Gly Tyr Trp Gly Gln Gly 100 105 110Thr Leu Val Thr Val Ser
Ser 115197119PRTArtificial SequenceSynthetic
1353-G10-R28T/P30D/H31S, VH 197Glu Val Gln Leu Val Gln Ser Gly Ala
Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala
Ser Gly Tyr Thr Phe Asp Ser Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln
Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr
Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr
Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu Leu
Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg
Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115198119PRTArtificial
SequenceSynthetic 10B4, VH 198Gln Val Gln Leu Gln Gln Ser Gly Ala
Glu Leu Met Lys Pro Gly Ala1 5 10 15Ser Val Lys Met Ser Cys Lys Thr
Thr Gly Tyr Ile Phe Ser Ser Tyr 20 25 30Trp Ile Gly Trp Val Lys Gln
Arg Pro Gly His Gly Leu Glu Trp Ile 35 40 45Gly Lys Ile Phe Pro Gly
Ser Gly Ser Ala Asp Tyr Asn Glu Asn Phe 50 55 60Lys Gly Lys Ala Thr
Phe Thr Val Asp Thr Ser Ser Asn Thr Ala Tyr65 70 75 80Met Gln Leu
Ser Ser Leu Thr Ser Glu Asp Ser Ala Val Tyr Tyr Cys 85 90 95Ala Arg
Gly Tyr Gly Asn Tyr Leu Tyr Phe Asp Val Trp Gly Ala Gly 100 105
110Thr Thr Val Thr Val Ser Ser 115199119PRTArtificial
SequenceSynthetic 1353-A09, VH 199Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Thr Trp Tyr 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Ser Glu Tyr Ser Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115200119PRTArtificial
SequenceSynthetic 1353-C07, VH 200Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Ser Thr Phe 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Val Asp Tyr Ser Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115201119PRTArtificial
SequenceSynthetic 1353-E07, VH 201Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Glu Thr Tyr 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Ala Glu Tyr Ser Leu Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115202119PRTArtificial
SequenceSynthetic 1353-F09, VH 202Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Arg Gln Tyr 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Ala Glu Tyr Gly Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115203119PRTArtificial
SequenceSynthetic 1353-G08, VH 203Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Thr Arg Tyr 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Val Ser Ala
His Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Ala Asp Tyr Gly Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115204119PRTArtificial
SequenceSynthetic 1353-H08, VH 204Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Thr Arg Gln 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Lys Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Val Asp Tyr Gly Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115205119PRTArtificial
SequenceSynthetic 1353-H09, VH 205Glu Val Gln Leu Val Gln Ser Gly
Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys Val Ser Cys Lys
Ala Ser Gly Tyr Arg Phe Pro His Tyr 20 25 30Gly Ile Ser Trp Val Arg
Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly Trp Ile Ser Ala
Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55 60Gln Gly Arg Val
Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65 70 75 80Met Glu
Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Asp Ala Glu Tyr Gly Ser Gly Ser Gly Tyr Trp Gly Gln Gly 100 105
110Thr Leu Val Thr Val Ser Ser 115206115PRTArtificial
SequenceSynthetic 1B10, VH 206Asp Val Gln Leu Gln Glu Ser Gly Pro
Gly Leu Val Lys Pro Ser Gln1 5 10 15Ser Leu Ser Leu Thr Cys Thr Val
Thr Gly His Ser Ile Thr Ser Asp 20 25 30Tyr Ala Trp Asn Trp Ile Arg
Gln Phe Pro Gly Asn Lys Leu Glu Trp 35 40 45Met Gly Tyr Ile Ser Tyr
Ser Gly Arg Thr Ser Tyr Asn Pro Ser Leu 50 55 60Thr Ser Arg Ile Ser
Ile Thr Arg Asp Thr Ser Lys Asn Gln Phe Phe65 70 75 80Leu Gln Leu
Asn Ser Val Thr Thr Glu Asp Thr Ala Thr Tyr Tyr Cys 85 90 95Ala Arg
Gly Tyr Ala Leu Asp Tyr Trp Gly Gln Gly Thr Ser Val Thr 100 105
110Val Ser Ser 115207120PRTArtificial SequenceSynthetic 1E9, VH
207Glu Val Lys Leu Val Glu Ser Gly Gly Gly Leu Val Ser Pro Gly Gly1
5 10 15Ser Leu Lys Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr
Phe 20 25 30Gly Met Ser Trp Val Arg Gln Thr Pro Glu Lys Arg Leu Glu
Trp Val 35 40 45Ala Thr Ile Ser Gly Gly Gly Ser Asp Thr Tyr Tyr Pro
Asp Ser Val 50 55 60Gln Gly Arg Phe Ile Ile Ser Arg Tyr Asn Ala Lys
Asn Asn Leu Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Pro Glu Asp
Thr Ala Leu Tyr Tyr Cys 85 90 95Ala Arg Gln Gly Tyr Asp Val Tyr Ser
Trp Phe Ala Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser
Ala 115 120208118PRTArtificial SequenceSynthetic 4B10, VH 208Glu
Val Lys Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10
15Ser Leu Lys Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Tyr
20 25 30Gly Met Ser Trp Val Arg Gln Thr Pro Glu Lys Arg Leu Gln Trp
Val 35 40 45Ala Thr Ile Ser Gly Gly Gly Ser Asn Thr Tyr Tyr Ser Asp
Ser Val 50 55 60Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn
Asn Leu Tyr65 70 75 80Leu Gln Met Ser Ser Leu Arg Ser Glu Asp Thr
Ala Leu Tyr Tyr Cys 85 90 95Ala Arg Gln Arg Asp Ser Ala Trp Phe Ala
Ser Trp Gly Gln Gly Thr 100 105 110Leu Val Thr Val Ser Ala
115209120PRTArtificial SequenceSynthetic h1E9-1, VH 209Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Phe 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ser Thr Ile Ser Gly Gly Gly Ser Asp Thr Tyr Tyr Pro Asp Ser Val
50 55 60Gln Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Gly Tyr Asp Val Tyr Ser Trp Phe Ala
Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser 115
120210120PRTArtificial SequenceSynthetic h1E9-2, VH 210Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Phe 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ser Thr Ile Ser Gly Gly Gly Ser Asp Thr Tyr Tyr Pro Asp Ser Val
50 55 60Gln Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Gly Tyr Asp Val Tyr Ser Trp Phe Ala
Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser 115
120211120PRTArtificial SequenceSynthetic h1E9-4, VH 211Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Phe 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ala Thr Ile Ser Gly Gly Gly Ser Asp Thr Tyr Tyr Pro Asp Ser Val
50 55 60Gln Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Gly Tyr Asp Val Tyr Ser Trp Phe Ala
Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser 115
120212120PRTArtificial SequenceSynthetic h1E9-5, VH 212Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Phe 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ala Thr Ile Ser Gly Gly Gly Ser Asp Thr Tyr Tyr Pro Asp Ser Val
50 55 60Gln Gly Arg Phe Thr Ile Ser Arg Asp Asn Ala Lys Asn Ser Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Gly Tyr Asp Val Tyr Ser Trp Phe Ala
Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser 115
120213118PRTArtificial SequenceSynthetic h4B10-1, VH 213Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Tyr 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ala Thr Ile Ser Gly Gly Gly Ser Asn Thr Tyr Tyr Ser Asp Ser Val
50 55 60Lys Gly Arg Phe Thr Ile Ser Arg Asp Asp Ser Lys Asn Thr Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Lys Thr Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Arg Asp Ser Ala Trp Phe Ala Ser Trp
Gly Gln Gly Thr 100 105 110Leu Val Thr Val Ser Ser
115214118PRTArtificial SequenceSynthetic h4B10-2, VH 214Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Thr Tyr 20 25 30Gly
Met Ser Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45Ala Thr Ile Ser Gly Gly Gly Ser Asn Thr Tyr Tyr Ser Asp Ser Val
50 55 60Lys Gly Arg Phe Thr Ile Ser Arg Asp Asp Ser Lys Asn Thr Leu
Tyr65 70 75 80Leu Gln Met Asn Ser Leu Lys Thr Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Gln Arg Asp Ser Ala Trp Phe Ala Ser Trp
Gly Gln Gly Thr 100 105 110Leu Val Thr Val Ser Ser
115215118PRTArtificial SequenceSynthetic h4B10-3, VH 215Glu Val Gln
Leu Val Glu Ser Gly Gly Gly Leu Val Lys Pro Gly Gly1 5 10 15Ser Leu
Arg Leu Ser Cys Ala
Ala Ser Gly Phe Thr Phe Ser Thr Tyr 20 25 30Gly Met Ser Trp Val Arg
Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala Thr Ile Ser Gly
Gly Gly Ser Asn Thr Tyr Tyr Ser Asp Ser Val 50 55 60Lys Gly Arg Phe
Thr Ile Ser Arg Asp Asp Ser Lys Asn Thr Leu Tyr65 70 75 80Leu Gln
Met Asn Ser Leu Lys Thr Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala
Arg Gln Arg Asp Ser Ala Trp Phe Ala Ser Trp Gly Gln Gly Thr 100 105
110Leu Val Thr Val Ser Ser 115216128PRTArtificial SequenceSynthetic
PD1-17, VH 216Gln Val Gln Leu Gln Glu Ser Gly Pro Gly Val Val Lys
Pro Ser Gly1 5 10 15Thr Leu Ser Leu Thr Cys Ala Ile Ser Gly Gly Ser
Ile Gly Ser Gly 20 25 30Gly Ser Ile Arg Ser Thr Arg Trp Trp Ser Trp
Val Arg Gln Ser Pro 35 40 45Gly Lys Gly Leu Glu Trp Ile Gly Glu Ile
Tyr His Ser Gly Ser Thr 50 55 60Asn Tyr Asn Pro Ser Leu Lys Ser Arg
Val Thr Ile Ser Leu Asp Lys65 70 75 80Ser Arg Asn His Phe Ser Leu
Arg Leu Asn Ser Val Thr Ala Ala Asp 85 90 95Thr Ala Val Tyr Tyr Cys
Ala Arg Gln Asp Tyr Gly Asp Ser Gly Asp 100 105 110Trp Tyr Phe Asp
Leu Trp Gly Lys Gly Thr Met Val Thr Val Ser Ser 115 120
125217119PRTArtificial SequenceSynthetic PD1-28, VH 217Glu Val Gln
Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val
Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe Thr Ser Tyr 20 25 30Gly
Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40
45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu
50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala
Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val
Tyr Tyr Cys 85 90 95Ala Arg Asp Ala Asp Tyr Ser Ser Gly Ser Gly Tyr
Trp Gly Gln Gly 100 105 110Thr Leu Val Thr Val Ser Ser
115218119PRTArtificial SequenceSynthetic PD1-33, VH 218Gln Val Gln
Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val
Arg Val Ser Cys Lys Ala Ser Gly Tyr Thr Leu Thr Ser Tyr 20 25 30Tyr
Ile His Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40
45Gly Ile Ile Asn Pro Arg Gly Ala Thr Ile Ser Tyr Ala Gln Lys Phe
50 55 60Gln Gly Arg Val Thr Met Thr Arg Asp Thr Ser Thr Ser Thr Val
Tyr65 70 75 80Met Glu Leu Arg Asn Leu Lys Ser Glu Asp Thr Ala Leu
Tyr Tyr Cys 85 90 95Ala Thr Ala Gly Ile Tyr Gly Phe Asp Phe Asp Tyr
Trp Gly Arg Gly 100 105 110Thr Leu Val Thr Val Ser Ser
115219127PRTArtificial SequenceSynthetic PD1-35, VH 219Gln Val Gln
Leu Gln Glu Ser Gly Pro Gly Leu Val Lys Pro Ser Gln1 5 10 15Thr Leu
Ser Leu Thr Cys Thr Val Ser Gly Gly Ser Ile Ser Ser Gly 20 25 30Ala
Tyr Tyr Trp Ser Trp Ile Arg Gln His Pro Gly Lys Gly Leu Glu 35 40
45Trp Ile Gly Tyr Ile Tyr Tyr Asn Gly Asn Thr Tyr Tyr Asn Pro Ser
50 55 60Leu Arg Ser Leu Val Thr Ile Ser Val Asp Ala Ser Lys Asn Gln
Phe65 70 75 80Ser Leu Lys Leu Ser Ser Val Thr Ala Ala Asp Thr Ala
Val Tyr Tyr 85 90 95Cys Ala Arg Ala Ser Asp Tyr Val Trp Gly Gly Tyr
Arg Tyr Met Asp 100 105 110Ala Phe Asp Ile Trp Gly Arg Gly Thr Leu
Ile Thr Val Ser Ser 115 120 125220121PRTArtificial
SequenceSynthetic PD1-F2, VH 220Glu Val Gln Leu Val Gln Ser Gly Gly
Gly Val Val Gln Pro Gly Arg1 5 10 15Ser Leu Arg Leu Ser Cys Ala Ala
Ser Gly Phe Thr Phe Ser Ser Tyr 20 25 30Trp Cys Asp Arg Met Ser Trp
Val Arg Gln Ala Pro Gly Lys Gly Leu 35 40 45Glu Trp Val Ser Ala Ile
Ser Gly Ser Gly Gly Ser Thr Tyr Tyr Ala 50 55 60Asp Ser Val Lys Gly
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn65 70 75 80Thr Leu Tyr
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val 85 90 95Tyr Tyr
Cys Ala Lys Glu Asn Trp Gly Ser Tyr Phe Asp Leu Trp Gly 100 105
110Gln Gly Thr Thr Val Thr Val Ser Ser 115 120221112PRTArtificial
SequenceSynthetic 2D5 VL, VL 221Asp Val Leu Met Thr Gln Thr Pro Leu
Ser Leu Pro Val Ser Leu Gly1 5 10 15Asp Gln Ala Ser Ile Ser Cys Arg
Ser Ser Gln Ser Ile Val His Thr 20 25 30Asn Gly Asn Thr Tyr Leu Glu
Trp Tyr Leu Gln Lys Pro Gly Gln Ser 35 40 45Pro Lys Leu Leu Ile Tyr
Lys Val Ser Asn Arg Phe Ser Gly Val Pro 50 55 60Asp Arg Phe Ser Gly
Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile65 70 75 80Ser Arg Val
Glu Ala Glu Asp Leu Gly Val Tyr Tyr Cys Phe Gln Gly 85 90 95Ser His
Val Pro Trp Thr Phe Gly Gly Gly Thr Glu Leu Glu Ile Lys 100 105
110222111PRTArtificial SequenceSynthetic 22E11 VL, VL 222Asp Ile
Val Leu Thr Gln Ser Pro Ala Ser Leu Ala Val Ser Leu Gly1 5 10 15Gln
Arg Ala Thr Ile Ser Cys Lys Ala Ser Gln Ser Val Asp Tyr Asp 20 25
30Gly Asn Ser Tyr Val Asn Trp Tyr Gln Gln Lys Pro Gly Gln Pro Pro
35 40 45Lys Leu Leu Ile Tyr Ala Ala Ser Asn Leu Glu Ser Gly Ile Pro
Ala 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Asn
Ile His65 70 75 80Pro Val Glu Glu Glu Asp Ala Ala Thr Tyr Tyr Cys
Gln Gln Ser Asn 85 90 95Glu Asp Pro Tyr Thr Phe Gly Gly Gly Thr Lys
Leu Glu Ile Lys 100 105 110223111PRTArtificial SequenceSynthetic
h22E11 Vk4 1 VL, VL 223Asp Ile Val Leu Thr Gln Ser Pro Asp Ser Leu
Ala Val Ser Leu Gly1 5 10 15Glu Arg Ala Thr Ile Asn Cys Lys Ala Ser
Gln Ser Val Asp Tyr Asp 20 25 30Gly Asn Ser Tyr Val Asn Trp Tyr Gln
Gln Lys Pro Gly Gln Pro Pro 35 40 45Lys Leu Leu Ile Tyr Ala Ala Ser
Asn Leu Glu Ser Gly Val Pro Asp 50 55 60Arg Phe Ser Gly Ser Gly Ser
Gly Thr Asp Phe Thr Leu Thr Ile Ser65 70 75 80Ser Leu Gln Ala Glu
Asp Val Ala Val Tyr Tyr Cys Gln Gln Ser Asn 85 90 95Glu Asp Pro Tyr
Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys 100 105
110224111PRTArtificial SequenceSynthetic h22E11 Vk3 11 VL, VL
224Glu Ile Val Leu Thr Gln Ser Pro Ala Thr Leu Ser Leu Ser Pro Gly1
5 10 15Glu Arg Ala Thr Leu Ser Cys Lys Ala Ser Gln Ser Val Asp Tyr
Asp 20 25 30Gly Asn Ser Tyr Val Asn Trp Tyr Gln Gln Lys Pro Gly Gln
Ala Pro 35 40 45Arg Leu Leu Ile Tyr Ala Ala Ser Asn Leu Glu Ser Gly
Ile Pro Ala 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr
Leu Thr Ile Ser65 70 75 80Ser Leu Glu Pro Glu Asp Phe Ala Val Tyr
Tyr Cys Gln Gln Ser Asn 85 90 95Glu Asp Pro Tyr Thr Phe Gly Gln Gly
Thr Lys Val Glu Ile Lys 100 105 110225111PRTArtificial
SequenceSynthetic h22E11 Vk2 28 VL, VL 225Asp Ile Val Leu Thr Gln
Ser Pro Leu Ser Leu Pro Val Thr Pro Gly1 5 10 15Glu Pro Ala Ser Ile
Ser Cys Lys Ala Ser Gln Ser Val Asp Tyr Asp 20 25 30Gly Asn Ser Tyr
Val Asn Trp Tyr Leu Gln Lys Pro Gly Gln Ser Pro 35 40 45Gln Leu Leu
Ile Tyr Ala Ala Ser Asn Leu Glu Ser Gly Val Pro Asp 50 55 60Arg Phe
Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Lys Ile Ser65 70 75
80Arg Val Glu Ala Glu Asp Val Gly Val Tyr Tyr Cys Gln Gln Ser Asn
85 90 95Glu Asp Pro Tyr Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys
100 105 110226111PRTArtificial SequenceSynthetic h22E11 Vk1 39 VL,
VL 226Asp Ile Gln Leu Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val
Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Lys Ala Ser Gln Ser Val Asp
Tyr Asp 20 25 30Gly Asn Ser Tyr Val Asn Trp Tyr Gln Gln Lys Pro Gly
Lys Ala Pro 35 40 45Lys Leu Leu Ile Tyr Ala Ala Ser Asn Leu Glu Ser
Gly Val Pro Ser 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe
Thr Leu Thr Ile Ser65 70 75 80Ser Leu Gln Pro Glu Asp Phe Ala Thr
Tyr Tyr Cys Gln Gln Ser Asn 85 90 95Glu Asp Pro Tyr Thr Phe Gly Gln
Gly Thr Lys Val Glu Ile Lys 100 105 110227111PRTArtificial
SequenceSynthetic h22E11 5 VL, VL 227Glu Ile Val Leu Thr Gln Ser
Pro Gly Thr Leu Ser Leu Ser Pro Gly1 5 10 15Glu Arg Ala Thr Leu Ser
Cys Lys Ala Ser Gln Ser Val Asp Tyr Asp 20 25 30Gly Asn Ser Tyr Val
Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro 35 40 45Arg Leu Leu Ile
Tyr Ala Ala Ser Asn Leu Glu Ser Gly Ile Pro Asp 50 55 60Arg Phe Ser
Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser65 70 75 80Arg
Leu Glu Pro Glu Asp Phe Ala Val Tyr Tyr Cys Gln Gln Ser Asn 85 90
95Glu Asp Pro Tyr Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys 100
105 110228107PRTArtificial SequenceSynthetic trastuzumab VL, VL
228Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1
5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val Asn Thr
Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu
Leu Ile 35 40 45Tyr Ser Ala Ser Phe Leu Tyr Ser Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln
His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln Gly Thr Lys Val Glu
Ile Lys 100 105229107PRTArtificial SequenceSynthetic 1649-A01 T56D,
VL 229Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val
Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val Asn
Thr Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys
Leu Leu Ile 35 40 45Tyr Ser Ala Ser Phe Leu Tyr Asp Gly Val Pro Ser
Arg Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr Ile
Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln
Gln His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln Gly Thr Lys Val
Glu Ile Lys 100 105230107PRTArtificial SequenceSynthetic 1649-A01
Y55D, VL 230Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser
Val Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val
Asn Thr Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro
Lys Leu Leu Ile 35 40 45Tyr Ser Ala Ser Phe Leu Asp Ser Gly Val Pro
Ser Arg Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr
Ile Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr Tyr Tyr Cys
Gln Gln His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln Gly Thr Lys
Val Glu Ile Lys 100 105231107PRTArtificial SequenceSynthetic
1649-A01 F53D, VL 231Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu
Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser
Gln Asp Val Asn Thr Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly
Lys Ala Pro Lys Leu Leu Ile 35 40 45Tyr Ser Ala Ser Asp Leu Tyr Ser
Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe
Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr
Tyr Tyr Cys Gln Gln His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln
Gly Thr Lys Val Glu Ile Lys 100 105232107PRTArtificial
SequenceSynthetic 1649-A01 S52D, VL 232Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile Thr
Cys Arg Ala Ser Gln Asp Val Asn Thr Ala 20 25 30Val Ala Trp Tyr Gln
Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45Tyr Ser Ala Asp
Phe Leu Tyr Ser Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Arg Ser
Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75 80Glu
Asp Phe Ala Thr Tyr Tyr Cys Gln Gln His Tyr Thr Thr Pro Pro 85 90
95Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys 100
105233107PRTArtificial SequenceSynthetic 1649-A01 A51D, VL 233Asp
Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1 5 10
15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val Asn Thr Ala
20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu
Ile 35 40 45Tyr Ser Asp Ser Phe Leu Tyr Ser Gly Val Pro Ser Arg Phe
Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser
Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln His
Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln Gly Thr Lys Val Glu Ile
Lys 100 105234107PRTArtificial SequenceSynthetic 1649-A01 S50D, VL
234Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1
5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val Asn Thr
Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu
Leu Ile 35 40 45Tyr Asp Ala Ser Phe Leu Tyr Ser Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln
His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln Gly Thr Lys Val Glu
Ile Lys 100 105235107PRTArtificial SequenceSynthetic 1649-A01, VL
235Asp Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly1
5 10 15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Asp Val Asn Thr
Ala 20 25 30Val Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu
Leu Ile 35 40 45Tyr Ser Ala Ser Phe Leu Tyr Ser Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60Ser Arg Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr
Tyr Tyr Cys Gln Gln His Tyr Thr Thr Pro Pro 85 90 95Thr Phe Gly Gln
Gly Thr Lys Val Glu Ile Lys 100 105236108PRTArtificial
SequenceSynthetic 1353-G10-wt, VL 236Ser Tyr Glu Leu Thr Gln Pro
Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys
Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln
Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40 45Lys Asp Thr Glu
Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly
Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp
Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90
95Arg Val Phe Gly Gly Gly Thr Lys Val Thr Val Leu 100
105237108PRTArtificial SequenceSynthetic 1353-G10 K50D (?), VL
237Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1
5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr
Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val
Ile Tyr 35 40 45Asp Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe
Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly
Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala
Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val
Thr Val Leu 100 105238108PRTArtificial SequenceSynthetic 1353-G10
T52D (?), VL 238Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser
Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro
Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro
Val Met Val Ile Tyr 35 40 45Lys Asp Asp Glu Arg Pro Ser Gly Ile Pro
Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr
Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys
Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly
Thr Lys Val Thr Val Leu 100 105239108PRTArtificial
SequenceSynthetic 1353-G10 E53D (?), VL 239Ser Tyr Glu Leu Thr Gln
Pro Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr
Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln
Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40 45Lys Asp Thr
Asp Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser
Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala Glu65 70 75
80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr Tyr
85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val Thr Val Leu 100
105240108PRTArtificial SequenceSynthetic 1353-G10 P55D (?), VL
240Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1
5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr
Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val
Ile Tyr 35 40 45Lys Asp Thr Glu Arg Asp Ser Gly Ile Pro Glu Arg Phe
Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly
Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala
Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val
Thr Val Leu 100 105241108PRTArtificial SequenceSynthetic 1353-G10
S56D (?), VL 241Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser
Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro
Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro
Val Met Val Ile Tyr 35 40 45Lys Asp Thr Glu Arg Pro Asp Gly Ile Pro
Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr
Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys
Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly
Thr Lys Val Thr Val Leu 100 105242108PRTArtificial
SequenceSynthetic 1353-G10 VL1c (?), VL 242Ser Tyr Glu Leu Thr Gln
Pro Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr
Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln
Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40 45Lys Asp Thr
Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser
Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala Glu65 70 75
80Asp Phe Ala Thr Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr Tyr
85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105243108PRTArtificial SequenceSynthetic 1353-G10 VL1d (?), VL
243Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1
5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr
Ala 20 25 30Tyr Trp Tyr Gln Arg Lys Pro Gly Gln Ala Pro Val Met Val
Ile Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe
Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly
Val Gln Ala Glu65 70 75 80Asp Phe Ala Thr Tyr Tyr Cys Gln Ser Ala
Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val
Glu Ile Lys 100 105244109PRTArtificial SequenceSynthetic
1353-G10-kappa, VL 244Asp Ile Gln Leu Thr Gln Ser Pro Ser Ser Leu
Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Ser Gly Asp
Ala Leu Pro Lys Gln Tyr 20 25 30Ala Tyr Trp Tyr Gln Gln Lys Pro Gly
Lys Ala Pro Lys Leu Leu Ile 35 40 45Tyr Lys Asp Thr Glu Arg Pro Ser
Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Ser Ser Gly Thr Lys Val
Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe Ala Thr
Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr 85 90 95Tyr Arg Val Phe
Gly Gly Gly Thr Lys Val Glu Ile Lys 100 105245109PRTArtificial
SequenceSynthetic 1353-G10 Vk3-20 (?), VL 245Glu Ile Val Leu Thr
Gln Ser Pro Gly Thr Leu Ser Leu Ser Pro Gly1 5 10 15Glu Arg Ala Thr
Leu Ser Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr 20 25 30Ala Tyr Trp
Tyr Gln Gln Lys Pro Gly Gln Ala Pro Arg Leu Leu Ile 35 40 45Tyr Lys
Asp Thr Glu Arg Pro Ser Gly Ile Pro Asp Arg Phe Ser Gly 50 55 60Ser
Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Arg Leu Glu Pro65 70 75
80Glu Asp Phe Ala Val Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr
85 90 95Tyr Arg Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105246108PRTArtificial SequenceSynthetic 10B4, VL 246Asn Ile Val
Met Thr Gln Thr Pro Lys Phe Leu Leu Val Ser Ala Gly1 5 10 15Asp Arg
Ile Thr Ile Thr Cys Lys Ala Ser Gln Ser Val Ser Asp Asp 20 25 30Val
Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Lys Leu Leu Ile 35 40
45Ser Tyr Ala Phe Lys Arg Tyr Ile Gly Val Pro Asp Arg Phe Thr Gly
50 55 60Ser Gly Tyr Gly Thr Asp Phe Thr Phe Thr Ile Ser Thr Val Gln
Ala65 70 75 80Glu Asp Leu Ala Val Tyr Phe Cys Gln Gln Asn Tyr Asn
Ser Pro Tyr 85 90 95Thr Phe Gly Gly Gly Thr Lys Leu Glu Leu Lys Arg
100 105247108PRTArtificial SequenceSynthetic 1353-A09, VL 247Ser
Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10
15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Thr Thr Gln Tyr Ala
20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile
Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser
Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val
Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp
Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val Thr
Val Leu 100 105248108PRTArtificial SequenceSynthetic 1353-C07, VL
248Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1
5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Ser Glu Gln Tyr
Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val
Ile Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe
Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly
Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala
Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val
Thr Val Leu 100 105249108PRTArtificial SequenceSynthetic 1353-E07,
VL 249Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly
Gln1 5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln
Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met
Val Ile Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg
Phe Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser
Gly Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser
Ala Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys
Val Thr Val Leu 100 105250108PRTArtificial SequenceSynthetic
1353-F09, VL 250Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser
Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro
Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro
Val Met Val Leu Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro
Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr
Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys
Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly
Thr Lys Val Thr Val Leu 100 105251108PRTArtificial
SequenceSynthetic 1353-G08, VL 251Ser Tyr Glu Leu Thr Gln Pro Pro
Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys Ser
Gly Asp Ala Leu Pro Met Gln Tyr Gly 20 25 30Tyr Trp Tyr Gln Gln Lys
Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40 45Lys Asp Thr Glu Arg
Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly Thr
Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp Glu
Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90 95Arg
Val Phe Gly Gly Gly Thr Lys Val Thr Val Leu 100
105252108PRTArtificial SequenceSynthetic 1353-H08, VL 252Ser Tyr
Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr
Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25
30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr
35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly
Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln
Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn
Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val Thr Val
Leu 100 105253108PRTArtificial SequenceSynthetic 1353-H09, VL
253Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1
5 10 15Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr
Ala 20 25 30Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val
Ile Tyr 35 40 45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe
Ser Gly Ser 50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly
Val Gln Ala Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala
Asp Asn Ser Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val
Thr Val Leu 100 105254106PRTArtificial SequenceSynthetic 1B10, VL
254Gln Ile Val Leu Ser Gln Ser Pro Ala Ile Leu Ser Ala Ser Pro Gly1
5 10 15Glu Lys Val Thr Met Thr Cys Arg Thr Ser Ser Ser Val Asn Tyr
Met 20 25 30His Trp Phe Gln Gln Lys Pro Gly Ser Ser Pro Lys Pro Trp
Ile Tyr 35 40 45Ala Thr Ser Lys Leu Ala Ser Gly Val Pro Ala Arg Phe
Ser Gly Ser 50 55 60Gly Ser Gly Thr Ser Tyr Ser Leu Thr Ile Ser Arg
Val Glu Ala Glu65 70 75 80Asp Ala Ala Thr Tyr Phe Cys Gln Gln Trp
Ile Ser Asp Pro Trp Thr 85 90 95Phe Gly Gly Gly Thr Lys Leu Glu Ile
Lys 100 105255111PRTArtificial SequenceSynthetic 1E9, VL 255Asp Ile
Ile Leu Thr Gln Ser Pro Ala Ser Leu Ala Val Ser Leu Gly1 5 10 15Gln
Arg Ala Ala Ile Ser Cys Arg Ala Ser Glu Ser Val Asp Asn Ser 20 25
30Gly Ile Ser Phe Met Ser Trp Phe Gln Gln Lys Pro Gly Gln Pro Pro
35 40 45Lys Leu Leu Ile Tyr Thr Ala Ser Asn Gln Gly Ser Gly Val Pro
Ala 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Glu Phe Ser Leu Asn
Ile His65 70 75 80Pro Met Glu Glu Asp Asp Thr Ala Met Tyr Phe Cys
Gln Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe Gly Gly Gly Thr Lys
Leu Glu Ile Arg 100 105 110256111PRTArtificial SequenceSynthetic
4B10, VL 256Asp Ile Val Leu Thr Gln Ser Pro Ala Ser Leu Ala Val Ser
Leu Gly1 5 10 15Gln Arg Ala Thr Ile Ser Cys Arg Ala Ser Glu Asn Val
Asp Asp Tyr 20 25 30Gly Val Ser Phe Met Asn Trp Phe Gln Gln Lys Pro
Gly Gln Pro Pro 35 40 45Lys Leu Leu Ile Tyr Pro Ala Ser Asn Gln Gly
Ser Gly Val Pro Ala 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp
Phe Ser Leu Asn Ile His65 70 75 80Pro Met Glu Glu Asp Asp Thr Ala
Met Tyr Phe Cys Gln Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe Gly
Gly Gly Thr Lys Leu Glu Ile Lys 100 105 110257111PRTArtificial
SequenceSynthetic h1E9-1, VL 257Asp Ile Gln Leu Thr Gln Ser Pro Ser
Phe Leu Ser Ala Ser Val Gly1 5 10
15Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Glu Ser Val Asp Asn Ser
20 25 30Gly Ile Ser Phe Met Ser Trp Tyr Gln Gln Lys Pro Gly Lys Ala
Pro 35 40 45Lys Leu Leu Ile Tyr Thr Ala Ser Asn Gln Gly Ser Gly Val
Pro Ser 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Glu Phe Thr Leu
Thr Ile Ser65 70 75 80Ser Leu Gln Pro Glu Asp Phe Ala Thr Tyr Tyr
Cys Gln Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe Gly Gln Gly Thr
Lys Val Glu Ile Lys 100 105 110258111PRTArtificial
SequenceSynthetic h1E9-2, VL 258Glu Ile Val Leu Thr Gln Ser Pro Ala
Thr Leu Ser Leu Ser Pro Gly1 5 10 15Glu Arg Ala Thr Leu Ser Cys Arg
Ala Ser Glu Ser Val Asp Asn Ser 20 25 30Gly Ile Ser Phe Met Ser Trp
Tyr Gln Gln Lys Pro Gly Gln Ala Pro 35 40 45Arg Leu Leu Ile Tyr Thr
Ala Ser Asn Gln Gly Ser Gly Ile Pro Ala 50 55 60Arg Phe Ser Gly Ser
Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser65 70 75 80Ser Leu Glu
Pro Glu Asp Phe Ala Val Tyr Tyr Cys Gln Gln Ser Lys 85 90 95Glu Val
Pro Trp Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys 100 105
110259111PRTArtificial SequenceSynthetic h1E9-4, VL 259Asp Ile Gln
Leu Thr Gln Ser Pro Ser Phe Leu Ser Ala Ser Val Gly1 5 10 15Asp Arg
Val Thr Ile Thr Cys Arg Ala Ser Glu Ser Val Asp Asn Ser 20 25 30Gly
Ile Ser Phe Met Ser Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro 35 40
45Lys Leu Leu Ile Tyr Thr Ala Ser Asn Gln Gly Ser Gly Val Pro Ser
50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Glu Phe Thr Leu Thr Ile
Ser65 70 75 80Ser Leu Gln Pro Glu Asp Phe Ala Thr Tyr Tyr Cys Gln
Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe Gly Gln Gly Thr Lys Val
Glu Ile Lys 100 105 110260111PRTArtificial SequenceSynthetic
h1E9-5, VL 260Glu Ile Val Leu Thr Gln Ser Pro Ala Thr Leu Ser Leu
Ser Pro Gly1 5 10 15Glu Arg Ala Thr Leu Ser Cys Arg Ala Ser Glu Ser
Val Asp Asn Ser 20 25 30Gly Ile Ser Phe Met Ser Trp Tyr Gln Gln Lys
Pro Gly Gln Ala Pro 35 40 45Arg Leu Leu Ile Tyr Thr Ala Ser Asn Gln
Gly Ser Gly Ile Pro Ala 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr
Asp Phe Thr Leu Thr Ile Ser65 70 75 80Ser Leu Glu Pro Glu Asp Phe
Ala Val Tyr Tyr Cys Gln Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe
Gly Gln Gly Thr Lys Val Glu Ile Lys 100 105 110261111PRTArtificial
SequenceSynthetic h4B10-1, VL 261Glu Ile Val Leu Thr Gln Ser Pro
Ala Thr Leu Ser Leu Ser Pro Gly1 5 10 15Glu Arg Ala Thr Leu Ser Cys
Arg Ala Ser Glu Asn Val Asp Asp Tyr 20 25 30Gly Val Ser Phe Met Asn
Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro 35 40 45Arg Leu Leu Ile Tyr
Pro Ala Ser Asn Gln Gly Ser Gly Ile Pro Ala 50 55 60Arg Phe Ser Gly
Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser65 70 75 80Ser Leu
Glu Pro Glu Asp Phe Ala Val Tyr Tyr Cys Gln Gln Ser Lys 85 90 95Glu
Val Pro Trp Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys 100 105
110262111PRTArtificial SequenceSynthetic h4B10-2, VL 262Glu Ile Val
Leu Thr Gln Ser Pro Gly Thr Leu Ser Leu Ser Pro Gly1 5 10 15Glu Arg
Ala Thr Leu Ser Cys Arg Ala Ser Glu Asn Val Asp Asp Tyr 20 25 30Gly
Val Ser Phe Met Asn Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro 35 40
45Arg Leu Leu Ile Tyr Pro Ala Ser Asn Gln Gly Ser Gly Ile Pro Asp
50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile
Ser65 70 75 80Arg Leu Glu Pro Glu Asp Phe Ala Val Tyr Tyr Cys Gln
Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe Gly Gln Gly Thr Lys Val
Glu Ile Lys 100 105 110263111PRTArtificial SequenceSynthetic
h4B10-3, VL 263Asp Ile Val Met Thr Gln Ser Pro Asp Ser Leu Ala Val
Ser Leu Gly1 5 10 15Glu Arg Ala Thr Ile Asn Cys Arg Ala Ser Glu Asn
Val Asp Asp Tyr 20 25 30Gly Val Ser Phe Met Asn Trp Tyr Gln Gln Lys
Pro Gly Gln Pro Pro 35 40 45Lys Leu Leu Ile Tyr Pro Ala Ser Asn Gln
Gly Ser Gly Val Pro Asp 50 55 60Arg Phe Ser Gly Ser Gly Ser Gly Thr
Asp Phe Thr Leu Thr Ile Ser65 70 75 80Ser Leu Gln Ala Glu Asp Val
Ala Val Tyr Tyr Cys Gln Gln Ser Lys 85 90 95Glu Val Pro Trp Thr Phe
Gly Gly Gly Thr Lys Leu Glu Ile Lys 100 105 110264110PRTArtificial
SequenceSynthetic PD1-17, VL 264Asn Phe Met Leu Thr Gln Pro His Ser
Val Ser Glu Ser Pro Gly Lys1 5 10 15Thr Val Thr Ile Ser Cys Thr Arg
Ser Ser Gly Ser Ile Ala Ser Asn 20 25 30Ser Val Gln Trp Tyr Gln Gln
Arg Pro Gly Ser Ser Pro Thr Thr Val 35 40 45Ile Tyr Glu Asp Asn Gln
Arg Pro Ser Gly Val Pro Asp Arg Phe Ser 50 55 60Gly Ser Ile Asp Ser
Ser Ser Asn Ser Ala Ser Leu Thr Val Ser Gly65 70 75 80Leu Lys Thr
Glu Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ser Asp Ser 85 90 95Ser Ala
Val Val Phe Gly Ser Gly Thr Lys Leu Thr Val Leu 100 105
110265108PRTArtificial SequenceSynthetic PD1-28, VL 265Ser Tyr Glu
Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala
Arg Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25 30Tyr
Trp Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40
45Lys Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser
50 55 60Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala
Glu65 70 75 80Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn Ser
Ile Thr Tyr 85 90 95Arg Val Phe Gly Gly Gly Thr Lys Val Thr Val Leu
100 105266111PRTArtificial SequenceSynthetic PD1-33, VL 266Gln Ser
Ala Leu Thr Gln Pro Ala Ser Val Ser Gly Ser Pro Gly Gln1 5 10 15Ser
Ile Thr Ile Ser Cys Thr Gly Thr Ser Asn Asp Val Gly Gly Tyr 20 25
30Asn Tyr Val Ser Trp Tyr Gln His His Pro Gly Lys Ala Pro Lys Leu
35 40 45Ile Ile Tyr Asp Val Thr Asn Arg Pro Ser Gly Val Ser Asp Arg
Phe 50 55 60Ser Gly Ser Lys Ser Gly Asn Thr Ala Ser Leu Thr Ile Ser
Gly Leu65 70 75 80Leu Ala Glu Asp Glu Gly Asp Tyr Tyr Cys Ser Ser
Tyr Thr Ile Val 85 90 95Thr Asn Phe Glu Val Leu Phe Gly Gly Gly Thr
Lys Leu Thr Val 100 105 110267110PRTArtificial SequenceSynthetic
PD1-35, VL 267Gln Ser Val Leu Thr Gln Pro Pro Ser Ala Ser Gly Thr
Pro Gly Gln1 5 10 15Arg Val Thr Ile Ser Cys Ser Gly Ser Asn Ser Asn
Ile Gly Ser Asn 20 25 30Ser Val Asn Trp Tyr Gln Gln Leu Pro Gly Thr
Ala Pro Lys Leu Leu 35 40 45Ile Tyr Gly Asn Asn Gln Arg Pro Ser Gly
Val Pro Asp Arg Phe Ser 50 55 60Gly Ser Lys Ser Gly Thr Ser Ala Ser
Leu Ala Ile Ser Gly Leu Gln65 70 75 80Ser Glu Asn Glu Ala Asp Tyr
Tyr Cys Ala Ala Trp Asp Asp Ser Leu 85 90 95Asn Gly Pro Val Phe Gly
Arg Gly Thr Lys Val Thr Val Leu 100 105 110268107PRTArtificial
SequenceSynthetic PD1-F2, VL 268Asp Ile Val Met Thr Gln Ser Pro Ser
Thr Leu Ser Ala Ser Val Gly1 5 10 15Asp Arg Val Thr Ile Thr Cys Arg
Ala Ser Gln Gly Ile Ser Ser Trp 20 25 30Leu Ala Trp Tyr Gln Gln Lys
Pro Gly Arg Ala Pro Lys Val Leu Ile 35 40 45Tyr Lys Ala Ser Thr Leu
Glu Ser Gly Val Pro Ser Arg Phe Ser Gly 50 55 60Ser Gly Ser Gly Thr
Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro65 70 75 80Glu Asp Phe
Ala Thr Tyr Tyr Cys Gln Gln Ser Tyr Ser Thr Pro Trp 85 90 95Thr Phe
Gly Gln Gly Thr Lys Leu Glu Ile Lys 100 105269108PRTArtificial
SequenceSynthetic 1353-G10-wt-c, VL 269Ser Tyr Glu Leu Thr Gln Pro
Pro Ser Val Ser Val Ser Pro Gly Gln1 5 10 15Thr Ala Arg Ile Thr Cys
Ser Gly Asp Ala Leu Pro Lys Gln Tyr Ala 20 25 30Tyr Trp Tyr Gln Gln
Lys Pro Gly Gln Ala Pro Val Met Val Ile Tyr 35 40 45Lys Asp Thr Glu
Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser 50 55 60Ser Ser Gly
Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala Glu65 70 75 80Asp
Phe Ala Thr Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr Tyr 85 90
95Arg Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105270330PRTArtificial SequenceSynthetic Human IgG1 HC Constant
270Ala Ser Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1
5 10 15Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp
Tyr 20 25 30Phe Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu
Thr Ser 35 40 45Gly Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly
Leu Tyr Ser 50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu
Gly Thr Gln Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser
Asn Thr Lys Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys Asp Lys
Thr His Thr Cys Pro Pro Cys 100 105 110Pro Ala Pro Glu Leu Leu Gly
Gly Pro Ser Val Phe Leu Phe Pro Pro 115 120 125Lys Pro Lys Asp Thr
Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys 130 135 140Val Val Val
Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp145 150 155
160Tyr Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu
165 170 175Glu Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr
Val Leu 180 185 190His Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys
Lys Val Ser Asn 195 200 205Lys Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly 210 215 220Gln Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Glu Glu225 230 235 240Met Thr Lys Asn Gln
Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr 245 250 255Pro Ser Asp
Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn 260 265 270Asn
Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe 275 280
285Leu Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn
290 295 300Val Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His
Tyr Thr305 310 315 320Gln Lys Ser Leu Ser Leu Ser Pro Gly Lys 325
330271107PRTArtificial SequenceSynthetic Human IgG LC Constant
Ckappa 271Arg Thr Val Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser
Asp Glu1 5 10 15Gln Leu Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu
Asn Asn Phe 20 25 30Tyr Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp
Asn Ala Leu Gln 35 40 45Ser Gly Asn Ser Gln Glu Ser Val Thr Glu Gln
Asp Ser Lys Asp Ser 50 55 60Thr Tyr Ser Leu Ser Ser Thr Leu Thr Leu
Ser Lys Ala Asp Tyr Glu65 70 75 80Lys His Lys Val Tyr Ala Cys Glu
Val Thr His Gln Gly Leu Ser Ser 85 90 95Pro Val Thr Lys Ser Phe Asn
Arg Gly Glu Cys 100 105272323PRTArtificial SequenceSynthetic Mouse
IgG1 HC Constant 272Ala Lys Thr Thr Pro Pro Ser Val Tyr Pro Leu Ala
Pro Gly Ser Ala1 5 10 15Ala Gln Thr Asn Ser Met Val Thr Leu Gly Cys
Leu Val Lys Gly Tyr 20 25 30Phe Pro Glu Pro Val Thr Val Thr Trp Asn
Ser Gly Ser Leu Ser Ser 35 40 45Gly Val His Thr Phe Pro Ala Val Leu
Gln Ser Asp Leu Tyr Thr Leu 50 55 60Ser Ser Ser Val Thr Val Pro Ser
Ser Thr Trp Pro Ser Glu Thr Val65 70 75 80Thr Cys Asn Val Ala His
Pro Ala Ser Ser Thr Lys Val Asp Lys Lys 85 90 95Ile Val Pro Arg Asp
Cys Gly Cys Lys Pro Cys Ile Cys Thr Val Pro 100 105 110Glu Val Ser
Ser Val Phe Ile Phe Pro Pro Lys Pro Lys Asp Val Leu 115 120 125Thr
Ile Thr Leu Thr Pro Lys Val Thr Cys Val Val Val Asp Ile Ser 130 135
140Lys Asp Asp Pro Glu Val Gln Phe Ser Trp Phe Val Asp Asp Val
Glu145 150 155 160Val His Thr Ala Gln Thr Gln Pro Arg Glu Glu Gln
Phe Asn Ser Thr 165 170 175Phe Arg Ser Val Ser Glu Leu Pro Ile Met
His Gln Asp Trp Leu Asn 180 185 190Gly Lys Glu Phe Lys Cys Arg Val
Asn Ser Ala Ala Phe Pro Ala Pro 195 200 205Ile Glu Lys Thr Ile Ser
Lys Thr Lys Gly Arg Pro Lys Ala Pro Gln 210 215 220Val Tyr Thr Ile
Pro Pro Pro Lys Glu Gln Met Ala Lys Asp Lys Val225 230 235 240Ser
Leu Thr Cys Met Ile Thr Asp Phe Phe Pro Glu Asp Ile Thr Val 245 250
255Glu Trp Gln Trp Asn Gly Gln Pro Ala Glu Asn Tyr Lys Asn Thr Gln
260 265 270Pro Ile Met Asp Thr Asp Gly Ser Tyr Phe Val Tyr Ser Lys
Leu Asn 275 280 285Val Gln Lys Ser Asn Trp Glu Ala Gly Asn Thr Phe
Thr Cys Ser Val 290 295 300Leu His Glu Gly Leu His Asn His His Thr
Glu Lys Ser Leu Ser His305 310 315 320Ser Pro
Gly273107PRTArtificial SequenceSynthetic Mouse IgG LC Constant
Ckappa 273Arg Ala Asp Ala Ala Pro Thr Val Ser Ile Phe Pro Pro Ser
Ser Glu1 5 10 15Gln Leu Thr Ser Gly Gly Ala Ser Val Val Cys Phe Leu
Asn Asn Phe 20 25 30Tyr Pro Lys Asp Ile Asn Val Lys Trp Lys Ile Asp
Gly Ser Glu Arg 35 40 45Gln Asn Gly Val Leu Asn Ser Trp Thr Asp Gln
Asp Ser Lys Asp Ser 50 55 60Thr Tyr Ser Met Ser Ser Thr Leu Thr Leu
Thr Lys Asp Glu Tyr Glu65 70 75 80Arg His Asn Ser Tyr Thr Cys Glu
Ala Thr His Lys Thr Ser Thr Ser 85 90 95Pro Ile Val Lys Ser Phe Asn
Arg Asn Glu Cys 100 105274108PRTArtificial SequenceSynthetic Kappa
LC 274His Met Thr Val Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser
Asp1 5 10 15Glu Gln Leu Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu
Asn Asn 20 25 30Phe Tyr Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp
Asn Ala Leu 35 40 45Gln Ser Gly Asn Ser Gln Glu Ser Val Thr Glu Gln
Asp Ser Lys Asp 50 55 60Ser Thr Tyr Ser Leu Ser
Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr65 70 75 80Glu Lys His Lys
Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser 85 90 95Ser Pro Val
Thr Lys Ser Phe Asn Arg Gly Glu Cys 100 105275106PRTArtificial
SequenceSynthetic Lambda LD 275Gly Gln Pro Lys Ala Ala Pro Ser Val
Thr Leu Phe Pro Pro Ser Ser1 5 10 15Glu Glu Leu Gln Ala Asn Lys Ala
Thr Leu Val Cys Leu Ile Ser Asp 20 25 30Phe Tyr Pro Gly Ala Val Thr
Val Ala Trp Lys Ala Asp Ser Ser Pro 35 40 45Val Lys Ala Gly Val Glu
Thr Thr Thr Pro Ser Lys Gln Ser Asn Asn 50 55 60Lys Tyr Ala Ala Ser
Ser Tyr Leu Ser Leu Thr Pro Glu Gln Trp Lys65 70 75 80Ser His Arg
Ser Tyr Ser Cys Gln Val Thr His Glu Gly Ser Thr Val 85 90 95Glu Lys
Thr Val Ala Pro Thr Glu Cys Ser 100 105276252PRTArtificial
SequenceSynthetic IgG1 Fc from scFv Fc 276Ala Ala Gly Ser Asp Gln
Glu Pro Lys Ser Ser Asp Lys Thr His Thr1 5 10 15Cys Pro Pro Cys Ser
Ala Pro Glu Leu Leu Gly Gly Ser Ser Val Phe 20 25 30Leu Phe Pro Pro
Lys Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro 35 40 45Glu Val Thr
Cys Val Val Val Asp Val Ser His Glu Asp Pro Glu Val 50 55 60Lys Phe
Asn Trp Tyr Val Asp Gly Val Glu Val His Asn Ala Lys Thr65 70 75
80Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val
85 90 95Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys 100 105 110Lys Val Ser Asn Lys Ala Leu Pro Ala Pro Ile Glu Lys
Thr Ile Ser 115 120 125Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val
Tyr Thr Leu Pro Pro 130 135 140Ser Arg Asp Glu Leu Thr Lys Asn Gln
Val Ser Leu Thr Cys Leu Val145 150 155 160Lys Gly Phe Tyr Pro Ser
Asp Ile Ala Val Glu Trp Glu Ser Asn Gly 165 170 175Gln Pro Glu Asn
Asn Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp 180 185 190Gly Ser
Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp 195 200
205Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met His Glu Ala Leu His
210 215 220Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro Gly Lys
Gly Ser225 230 235 240Gly Asp Tyr Lys Asp Asp Asp Asp Lys Gly Ser
Gly 245 25027720PRTArtificial SequenceSynthetic FlagHis Tag 277Gly
Ser Gly Asp Tyr Lys Asp Asp Asp Asp Lys Gly Ser Gly His His1 5 10
15His His His His 2027815PRTArtificial SequenceSynthetic Linker
(GGGGS)3 278Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly
Ser1 5 10 1527920PRTArtificial SequenceSynthetic Linker (GGGGS)4
279Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly1
5 10 15Gly Gly Gly Ser 2028025PRTArtificial SequenceSynthetic
Linker (GGGGS)5 280Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly
Gly Gly Ser Gly1 5 10 15Gly Gly Gly Ser Gly Gly Gly Gly Ser 20
2528130PRTArtificial SequenceSynthetic Linker (GGGGS)6 281Gly Gly
Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly1 5 10 15Gly
Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser 20 25
3028211PRTArtificial SequenceSynthetic Linker 282Ala Ala Gly Ser
Asp Gln Glu Pro Lys Ser Ser1 5 102836PRTArtificial
SequenceSynthetic Linker 283Ala Ala Gly Ser Asp Gln1
528418PRTArtificial SequenceSynthetic Linker 284Ala Pro Gly Pro Ser
Ala Pro Ser His Arg Ser Leu Pro Ser Arg Ala1 5 10 15Phe
Gly28521PRTArtificial SequenceSynthetic Linker-hinge 285Ala Ala Gly
Ser Asp Gln Glu Pro Lys Ser Ser Asp Lys Thr His Thr1 5 10 15Cys Pro
Pro Cys Pro 2028610PRTArtificial SequenceSynthetic Hinge - wt
286Asp Lys Thr His Thr Cys Pro Pro Cys Pro1 5 10287505PRTArtificial
SequenceSynthetic Tim-3 scFv Fc 287Met Glu Val Gln Leu Val Gln Ser
Gly Ala Glu Val Lys Lys Pro Gly1 5 10 15Glu Ser Leu Lys Ile Ser Cys
Lys Val Ser Gly Phe Ser Leu Thr Ser 20 25 30Tyr Gly Val His Trp Val
Arg Gln Met Pro Gly Lys Gly Leu Glu Trp 35 40 45Leu Val Val Ile Trp
Ser Asp Gly Ser Thr Thr Tyr Asn Pro Ser Phe 50 55 60Gln Gly Gln Val
Thr Ile Ser Lys Asp Lys Ser Ile Ser Thr Val Tyr65 70 75 80Leu Gln
Trp Ser Ser Leu Lys Ala Ser Asp Thr Ala Met Tyr Tyr Cys 85 90 95Ala
Arg Gln Gly Gly Tyr Arg Tyr Asp Asp Ala Met Asp Tyr Trp Gly 100 105
110Gln Gly Thr Leu Val Thr Val Ser Ser Gly Gly Gly Gly Ser Gly Gly
115 120 125Gly Gly Ser Gly Gly Gly Gly Ser Asp Ile Gln Leu Thr Gln
Ser Pro 130 135 140Ser Ser Leu Ser Ala Ser Val Gly Asp Arg Val Thr
Ile Thr Cys Lys145 150 155 160Ala Ser Gln Ser Val Asp Tyr Asp Gly
Asn Ser Tyr Val Asn Trp Tyr 165 170 175Gln Gln Lys Pro Gly Lys Ala
Pro Lys Leu Leu Ile Tyr Ala Ala Ser 180 185 190Asn Leu Glu Ser Gly
Val Pro Ser Arg Phe Ser Gly Ser Gly Ser Gly 195 200 205Thr Asp Phe
Thr Leu Thr Ile Ser Ser Leu Gln Pro Glu Asp Phe Ala 210 215 220Thr
Tyr Tyr Cys Gln Gln Ser Asn Glu Asp Pro Tyr Thr Phe Gly Gln225 230
235 240Gly Thr Lys Val Glu Ile Lys Ala Ala Gly Ser Asp Gln Glu Pro
Lys 245 250 255Ser Ser Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala
Pro Glu Leu 260 265 270Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro
Lys Pro Lys Asp Thr 275 280 285Leu Met Ile Ser Arg Thr Pro Glu Val
Thr Cys Val Val Val Asp Val 290 295 300Ser His Glu Asp Pro Glu Val
Lys Phe Asn Trp Tyr Val Asp Gly Val305 310 315 320Glu Val His Asn
Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser 325 330 335Thr Tyr
Arg Val Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu 340 345
350Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala
355 360 365Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg
Glu Pro 370 375 380Gln Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met
Thr Lys Asn Gln385 390 395 400Val Ser Leu Thr Cys Leu Val Lys Gly
Phe Tyr Pro Ser Asp Ile Ala 405 410 415Val Glu Trp Glu Ser Asn Gly
Gln Pro Glu Asn Asn Tyr Lys Thr Thr 420 425 430Pro Pro Val Leu Asp
Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu 435 440 445Thr Val Asp
Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser 450 455 460Val
Met His Glu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser465 470
475 480Leu Ser Pro Gly Lys Gly Ser Gly Asp Tyr Lys Asp Asp Asp Asp
Lys 485 490 495Gly Ser Gly His His His His His His 500
505288512PRTArtificial SequenceSynthetic 1353-G10, scFv-Fc 288Met
Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly1 5 10
15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro His
20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu
Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala
Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr
Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg Ser Asp Asp
Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr Gly Thr Gly
Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser
Gly Gly Gly Gly Ser Gly Gly Gly 115 120 125Gly Ser Gly Gly Gly Gly
Ser Ser Tyr Glu Leu Thr Gln Pro Pro Ser 130 135 140Val Ser Val Ser
Pro Gly Gln Thr Ala Arg Ile Thr Cys Ser Gly Asp145 150 155 160Ala
Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly Gln 165 170
175Ala Pro Val Met Val Ile Tyr Lys Asp Thr Glu Arg Pro Ser Gly Ile
180 185 190Pro Glu Arg Phe Ser Gly Ser Ser Ser Gly Thr Lys Val Thr
Leu Thr 195 200 205Ile Ser Gly Val Gln Ala Glu Asp Glu Ala Asp Tyr
Tyr Cys Gln Ser 210 215 220Ala Asp Asn Ser Ile Thr Tyr Arg Val Phe
Gly Gly Gly Thr Lys Val225 230 235 240Thr Val Leu Ala Ala Gly Ser
Asp Gln Glu Pro Lys Lys Leu Ala Ala 245 250 255Gly Ser Asp Gln Glu
Pro Lys Ser Ser Asp Lys Thr His Thr Cys Pro 260 265 270Pro Cys Ser
Ala Pro Glu Leu Leu Gly Gly Ser Ser Val Phe Leu Phe 275 280 285Pro
Pro Lys Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val 290 295
300Thr Cys Val Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys
Phe305 310 315 320Asn Trp Tyr Val Asp Gly Val Glu Val His Asn Ala
Lys Thr Lys Pro 325 330 335Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg
Val Val Ser Val Leu Thr 340 345 350Val Leu His Gln Asp Trp Leu Asn
Gly Lys Glu Tyr Lys Cys Lys Val 355 360 365Ser Asn Lys Ala Leu Pro
Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala 370 375 380Lys Gly Gln Pro
Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser Arg385 390 395 400Asp
Glu Leu Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly 405 410
415Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro
420 425 430Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp
Gly Ser 435 440 445Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp Lys Ser
Arg Trp Gln Gln 450 455 460Gly Asn Val Phe Ser Cys Ser Val Met His
Glu Ala Leu His Asn His465 470 475 480Tyr Thr Gln Lys Ser Leu Ser
Leu Ser Pro Gly Lys Gly Ser Gly Asp 485 490 495Tyr Lys Asp Asp Asp
Asp Lys Gly Ser Gly His His His His His His 500 505
510289481PRTArtificial SequenceSynthetic 1353-G10 scFv-Fc hole,
scFv-Fc 289Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys
Pro Gly1 5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg
Phe Pro His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln
Gly Leu Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr
Asn Tyr Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp
Thr Ser Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg
Ser Asp Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr
Gly Thr Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr
Val Ser Ser Gly Gly Gly Gly Ser Gly Gly Gly 115 120 125Gly Ser Gly
Gly Gly Gly Ser Ser Tyr Glu Leu Thr Gln Pro Pro Ser 130 135 140Val
Ser Val Ser Pro Gly Gln Thr Ala Arg Ile Thr Cys Ser Gly Asp145 150
155 160Ala Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly
Gln 165 170 175Ala Pro Val Met Val Ile Tyr Lys Asp Thr Glu Arg Pro
Ser Gly Ile 180 185 190Pro Glu Arg Phe Ser Gly Ser Ser Ser Gly Thr
Lys Val Thr Leu Thr 195 200 205Ile Ser Gly Val Gln Ala Glu Asp Glu
Ala Asp Tyr Tyr Cys Gln Ser 210 215 220Ala Asp Asn Ser Ile Thr Tyr
Arg Val Phe Gly Gly Gly Thr Lys Val225 230 235 240Thr Val Leu Ala
Ala Gly Ser Asp Gln Glu Pro Lys Ser Ser Asp Lys 245 250 255Thr His
Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly Pro 260 265
270Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met Ile Ser
275 280 285Arg Thr Pro Glu Val Thr Cys Val Val Val Asp Val Ser His
Glu Asp 290 295 300Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val
Glu Val His Asn305 310 315 320Ala Lys Thr Lys Pro Arg Glu Glu Gln
Tyr Asn Ser Thr Tyr Arg Val 325 330 335Val Ser Val Leu Thr Val Leu
His Gln Asp Trp Leu Asn Gly Lys Glu 340 345 350Tyr Lys Cys Lys Val
Ser Asn Lys Ala Leu Pro Ala Pro Ile Glu Lys 355 360 365Thr Ile Ser
Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val Tyr Thr 370 375 380Leu
Pro Pro Ser Arg Asp Glu Leu Thr Lys Asn Gln Val Ser Leu Ser385 390
395 400Cys Ala Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp
Glu 405 410 415Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro
Pro Val Leu 420 425 430Asp Ser Asp Gly Ser Phe Phe Leu Val Ser Lys
Leu Thr Val Asp Lys 435 440 445Ser Arg Trp Gln Gln Gly Asn Val Phe
Ser Cys Ser Val Met His Glu 450 455 460Ala Leu His Asn His Tyr Thr
Gln Lys Ser Leu Ser Leu Ser Pro Gly465 470 475
480Lys290481PRTArtificial SequenceSynthetic 1353-G10 scFv-Fc knob,
scFv-Fc 290Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys
Pro Gly1 5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg
Phe Pro His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln
Gly Leu Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr
Asn Tyr Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp
Thr Ser Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg
Ser Asp Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr
Gly Thr Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr
Val Ser Ser Gly Gly Gly Gly Ser Gly Gly Gly 115 120 125Gly Ser Gly
Gly Gly Gly Ser Ser Tyr Glu Leu Thr Gln Pro Pro Ser 130 135 140Val
Ser Val Ser Pro Gly Gln Thr Ala Arg Ile Thr Cys Ser Gly Asp145 150
155 160Ala Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly
Gln 165 170 175Ala Pro Val Met Val Ile Tyr Lys Asp Thr Glu Arg Pro
Ser Gly Ile 180 185 190Pro Glu Arg Phe Ser Gly Ser Ser Ser Gly Thr
Lys Val Thr Leu Thr 195 200 205Ile Ser Gly Val Gln Ala Glu Asp Glu
Ala Asp Tyr Tyr Cys Gln Ser 210 215 220Ala Asp Asn Ser Ile Thr Tyr
Arg Val Phe Gly Gly Gly Thr Lys Val225 230 235 240Thr Val Leu Ala
Ala Gly Ser Asp Gln Glu Pro Lys Ser Ser Asp Lys 245 250 255Thr His
Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly Pro 260 265
270Ser Val Phe Leu Phe
Pro Pro Lys Pro Lys Asp Thr Leu Met Ile Ser 275 280 285Arg Thr Pro
Glu Val Thr Cys Val Val Val Asp Val Ser His Glu Asp 290 295 300Pro
Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His Asn305 310
315 320Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg
Val 325 330 335Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn
Gly Lys Glu 340 345 350Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro
Ala Pro Ile Glu Lys 355 360 365Thr Ile Ser Lys Ala Lys Gly Gln Pro
Arg Glu Pro Gln Val Tyr Thr 370 375 380Leu Pro Pro Ser Arg Asp Glu
Leu Thr Lys Asn Gln Val Ser Leu Trp385 390 395 400Cys Leu Val Lys
Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu 405 410 415Ser Asn
Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val Leu 420 425
430Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp Lys
435 440 445Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met
His Glu 450 455 460Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser
Leu Ser Pro Gly465 470 475 480Lys291481PRTArtificial
SequenceSynthetic 1353-G10 scFv-Fc zwA, scFv-Fc 291Met Glu Val Gln
Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly1 5 10 15Ala Ser Val
Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro His 20 25 30Tyr Gly
Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp 35 40 45Met
Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys 50 55
60Leu Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala65
70 75 80Tyr Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr
Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp
Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser Gly Gly Gly Gly
Ser Gly Gly Gly 115 120 125Gly Ser Gly Gly Gly Gly Ser Ser Tyr Glu
Leu Thr Gln Pro Pro Ser 130 135 140Val Ser Val Ser Pro Gly Gln Thr
Ala Arg Ile Thr Cys Ser Gly Asp145 150 155 160Ala Leu Pro Lys Gln
Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly Gln 165 170 175Ala Pro Val
Met Val Ile Tyr Lys Asp Thr Glu Arg Pro Ser Gly Ile 180 185 190Pro
Glu Arg Phe Ser Gly Ser Ser Ser Gly Thr Lys Val Thr Leu Thr 195 200
205Ile Ser Gly Val Gln Ala Glu Asp Glu Ala Asp Tyr Tyr Cys Gln Ser
210 215 220Ala Asp Asn Ser Ile Thr Tyr Arg Val Phe Gly Gly Gly Thr
Lys Val225 230 235 240Thr Val Leu Ala Ala Gly Ser Asp Gln Glu Pro
Lys Ser Ser Asp Lys 245 250 255Thr His Thr Cys Pro Pro Cys Pro Ala
Pro Glu Leu Leu Gly Gly Pro 260 265 270Ser Val Phe Leu Phe Pro Pro
Lys Pro Lys Asp Thr Leu Met Ile Ser 275 280 285Arg Thr Pro Glu Val
Thr Cys Val Val Val Asp Val Ser His Glu Asp 290 295 300Pro Glu Val
Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His Asn305 310 315
320Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg Val
325 330 335Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly
Lys Glu 340 345 350Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala
Pro Ile Glu Lys 355 360 365Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg
Glu Pro Gln Val Tyr Val 370 375 380Tyr Pro Pro Ser Arg Glu Glu Met
Thr Lys Asn Gln Val Ser Leu Thr385 390 395 400Cys Leu Val Lys Gly
Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu 405 410 415Ser Asn Gly
Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val Leu 420 425 430Asp
Ser Asp Gly Ser Phe Ala Leu Val Ser Lys Leu Thr Val Asp Lys 435 440
445Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met His Glu
450 455 460Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser
Pro Gly465 470 475 480Lys292288PRTArtificial SequenceSynthetic
hPD-1 292Met Gln Ile Pro Gln Ala Pro Trp Pro Val Val Trp Ala Val
Leu Gln1 5 10 15Leu Gly Trp Arg Pro Gly Trp Phe Leu Asp Ser Pro Asp
Arg Pro Trp 20 25 30Asn Pro Pro Thr Phe Ser Pro Ala Leu Leu Val Val
Thr Glu Gly Asp 35 40 45Asn Ala Thr Phe Thr Cys Ser Phe Ser Asn Thr
Ser Glu Ser Phe Val 50 55 60Leu Asn Trp Tyr Arg Met Ser Pro Ser Asn
Gln Thr Asp Lys Leu Ala65 70 75 80Ala Phe Pro Glu Asp Arg Ser Gln
Pro Gly Gln Asp Cys Arg Phe Arg 85 90 95Val Thr Gln Leu Pro Asn Gly
Arg Asp Phe His Met Ser Val Val Arg 100 105 110Ala Arg Arg Asn Asp
Ser Gly Thr Tyr Leu Cys Gly Ala Ile Ser Leu 115 120 125Ala Pro Lys
Ala Gln Ile Lys Glu Ser Leu Arg Ala Glu Leu Arg Val 130 135 140Thr
Glu Arg Arg Ala Glu Val Pro Thr Ala His Pro Ser Pro Ser Pro145 150
155 160Arg Pro Ala Gly Gln Phe Gln Thr Leu Val Val Gly Val Val Gly
Gly 165 170 175Leu Leu Gly Ser Leu Val Leu Leu Val Trp Val Leu Ala
Val Ile Cys 180 185 190Ser Arg Ala Ala Arg Gly Thr Ile Gly Ala Arg
Arg Thr Gly Gln Pro 195 200 205Leu Lys Glu Asp Pro Ser Ala Val Pro
Val Phe Ser Val Asp Tyr Gly 210 215 220Glu Leu Asp Phe Gln Trp Arg
Glu Lys Thr Pro Glu Pro Pro Val Pro225 230 235 240Cys Val Pro Glu
Gln Thr Glu Tyr Ala Thr Ile Val Phe Pro Ser Gly 245 250 255Met Gly
Thr Ser Ser Pro Ala Arg Arg Gly Ser Ala Asp Gly Pro Arg 260 265
270Ser Ala Gln Pro Leu Arg Pro Glu Asp Gly His Cys Ser Trp Pro Leu
275 280 285293288PRTArtificial SequenceSynthetic murine PD-1 293Met
Trp Val Arg Gln Val Pro Trp Ser Phe Thr Trp Ala Val Leu Gln1 5 10
15Leu Ser Trp Gln Ser Gly Trp Leu Leu Glu Val Pro Asn Gly Pro Trp
20 25 30Arg Ser Leu Thr Phe Tyr Pro Ala Trp Leu Thr Val Ser Glu Gly
Ala 35 40 45Asn Ala Thr Phe Thr Cys Ser Leu Ser Asn Trp Ser Glu Asp
Leu Met 50 55 60Leu Asn Trp Asn Arg Leu Ser Pro Ser Asn Gln Thr Glu
Lys Gln Ala65 70 75 80Ala Phe Cys Asn Gly Leu Ser Gln Pro Val Gln
Asp Ala Arg Phe Gln 85 90 95Ile Ile Gln Leu Pro Asn Arg His Asp Phe
His Met Asn Ile Leu Asp 100 105 110Thr Arg Arg Asn Asp Ser Gly Ile
Tyr Leu Cys Gly Ala Ile Ser Leu 115 120 125His Pro Lys Ala Lys Ile
Glu Glu Ser Pro Gly Ala Glu Leu Val Val 130 135 140Thr Glu Arg Ile
Leu Glu Thr Ser Thr Arg Tyr Pro Ser Pro Ser Pro145 150 155 160Lys
Pro Glu Gly Arg Phe Gln Gly Met Val Ile Gly Ile Met Ser Ala 165 170
175Leu Val Gly Ile Pro Val Leu Leu Leu Leu Ala Trp Ala Leu Ala Val
180 185 190Phe Cys Ser Thr Ser Met Ser Glu Ala Arg Gly Ala Gly Ser
Lys Asp 195 200 205Asp Thr Leu Lys Glu Glu Pro Ser Ala Ala Pro Val
Pro Ser Val Ala 210 215 220Tyr Glu Glu Leu Asp Phe Gln Gly Arg Glu
Lys Thr Pro Glu Leu Pro225 230 235 240Thr Ala Cys Val His Thr Glu
Tyr Ala Thr Ile Val Phe Thr Glu Gly 245 250 255Leu Gly Ala Ser Ala
Met Gly Arg Arg Gly Ser Ala Asp Gly Leu Gln 260 265 270Gly Pro Arg
Pro Pro Arg His Glu Asp Gly His Cys Ser Trp Pro Leu 275 280
285294288PRTArtificial SequenceSynthetic cyno PD-1 294Met Trp Val
Arg Gln Val Pro Trp Ser Phe Thr Trp Ala Val Leu Gln1 5 10 15Leu Ser
Trp Gln Ser Gly Trp Leu Leu Glu Val Pro Asn Gly Pro Trp 20 25 30Arg
Ser Leu Thr Phe Tyr Pro Ala Trp Leu Thr Val Ser Glu Gly Ala 35 40
45Asn Ala Thr Phe Thr Cys Ser Leu Ser Asn Trp Ser Glu Asp Leu Met
50 55 60Leu Asn Trp Asn Arg Leu Ser Pro Ser Asn Gln Thr Glu Lys Gln
Ala65 70 75 80Ala Phe Cys Asn Gly Leu Ser Gln Pro Val Gln Asp Ala
Arg Phe Gln 85 90 95Ile Ile Gln Leu Pro Asn Arg His Asp Phe His Met
Asn Ile Leu Asp 100 105 110Thr Arg Arg Asn Asp Ser Gly Ile Tyr Leu
Cys Gly Ala Ile Ser Leu 115 120 125His Pro Lys Ala Lys Ile Glu Glu
Ser Pro Gly Ala Glu Leu Val Val 130 135 140Thr Glu Arg Ile Leu Glu
Thr Ser Thr Arg Tyr Pro Ser Pro Ser Pro145 150 155 160Lys Pro Glu
Gly Arg Phe Gln Gly Met Val Ile Gly Ile Met Ser Ala 165 170 175Leu
Val Gly Ile Pro Val Leu Leu Leu Leu Ala Trp Ala Leu Ala Val 180 185
190Phe Cys Ser Thr Ser Met Ser Glu Ala Arg Gly Ala Gly Ser Lys Asp
195 200 205Asp Thr Leu Lys Glu Glu Pro Ser Ala Ala Pro Val Pro Ser
Val Ala 210 215 220Tyr Glu Glu Leu Asp Phe Gln Gly Arg Glu Lys Thr
Pro Glu Leu Pro225 230 235 240Thr Ala Cys Val His Thr Glu Tyr Ala
Thr Ile Val Phe Thr Glu Gly 245 250 255Leu Gly Ala Ser Ala Met Gly
Arg Arg Gly Ser Ala Asp Gly Leu Gln 260 265 270Gly Pro Arg Pro Pro
Arg His Glu Asp Gly His Cys Ser Trp Pro Leu 275 280
285295217PRTArtificial SequenceSynthetic CH2-CH3, Fc-knob 295Ala
Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1 5 10
15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val
20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp
Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg
Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr
Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys
Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile
Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr Thr
Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val Ser
Leu Trp Cys Leu Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile Ala
Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155 160Tyr
Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu 165 170
175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn Val
180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr
Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215296217PRTArtificial SequenceSynthetic CH2-CH3, Fc-knob-V262E
296Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Glu 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val
Ser Leu Trp Cys Leu Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215297217PRTArtificial SequenceSynthetic CH2-CH3, Fc-knob-V264S
297Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Ser Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val
Ser Leu Trp Cys Leu Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215298217PRTArtificial SequenceSynthetic CH2-CH3, Fc-knob-D399C
298Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Asp Glu Leu 115 120 125Thr Lys Asn Gln Val
Ser Leu Trp Cys Leu Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Cys Ser Asp Gly Ser Phe Phe Leu
165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys
210
215299217PRTArtificial SequenceSynthetic CH2-CH3, Fc-knob-S354C
299Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Cys Arg Asp Glu Leu 115 120 125Thr Lys Asn Gln Val
Ser Leu Trp Cys Leu Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215300217PRTArtificial SequenceSynthetic CH2-CH3, Fc-hole 300Ala
Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1 5 10
15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val
20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp
Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg
Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr
Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys
Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile
Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr Thr
Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val Ser
Leu Ser Cys Ala Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile Ala
Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155 160Tyr
Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu 165 170
175Val Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn Val
180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr
Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215301217PRTArtificial SequenceSynthetic CH2-CH3, Fc-hole-V262E
301Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Glu 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val
Ser Leu Ser Cys Ala Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Val Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215302217PRTArtificial SequenceSynthetic CH2-CH3, Fc-hole-V264S
302Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Ser Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val
Ser Leu Ser Cys Ala Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Val Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215303217PRTArtificial SequenceSynthetic CH2-CH3, Fc-hole-Y349C
303Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Cys
Thr Leu Pro Pro Ser Arg Asp Glu Leu 115 120 125Thr Lys Asn Gln Val
Ser Leu Ser Cys Ala Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Lys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Val Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215304217PRTArtificial SequenceSynthetic CH2-CH3, Fc-hole-K392C
304Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1
5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys
Val 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu
Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr
Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr
Thr Leu Pro Pro Ser Arg Asp Glu Leu 115 120 125Thr Lys Asn Gln Val
Ser Leu Ser Cys Ala Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile
Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn145 150 155
160Tyr Cys Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu
165 170 175Val Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly
Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu Ala Leu His Asn
His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210
215305217PRTArtificial SequenceSynthetic Fc-zwA 305Ala Pro Glu Leu
Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys1 5 10 15Pro Lys Asp
Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val 20 25 30Val Val
Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr 35 40 45Val
Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu 50 55
60Gln Tyr Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His65
70 75 80Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys 85 90 95Ala Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys
Gly Gln 100 105 110Pro Arg Glu Pro Gln Val Tyr Val Tyr Pro Pro Ser
Arg Glu Glu Met 115 120 125Thr Lys Asn Gln Val Ser Leu Thr Cys Leu
Val Lys Gly Phe Tyr Pro 130 135 140Ser Asp Ile Ala Val Glu Trp Glu
Ser Asn Gly Gln Pro Glu Asn Asn145 150 155 160Tyr Lys Thr Thr Pro
Pro Val Leu Asp Ser Asp Gly Ser Phe Ala Leu 165 170 175Val Ser Lys
Leu Thr Val Asp Lys Ser Arg Trp Gln Gln Gly Asn Val 180 185 190Phe
Ser Cys Ser Val Met His Glu Ala Leu His Asn His Tyr Thr Gln 195 200
205Lys Ser Leu Ser Leu Ser Pro Gly Lys 210 215306217PRTArtificial
SequenceSynthetic Fc-zwA-V262E 306Ala Pro Glu Leu Leu Gly Gly Pro
Ser Val Phe Leu Phe Pro Pro Lys1 5 10 15Pro Lys Asp Thr Leu Met Ile
Ser Arg Thr Pro Glu Val Thr Cys Glu 20 25 30Val Val Asp Val Ser His
Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr 35 40 45Val Asp Gly Val Glu
Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu 50 55 60Gln Tyr Asn Ser
Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His65 70 75 80Gln Asp
Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys 85 90 95Ala
Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln 100 105
110Pro Arg Glu Pro Gln Val Tyr Val Tyr Pro Pro Ser Arg Glu Glu Met
115 120 125Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe
Tyr Pro 130 135 140Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln
Pro Glu Asn Asn145 150 155 160Tyr Lys Thr Thr Pro Pro Val Leu Asp
Ser Asp Gly Ser Phe Ala Leu 165 170 175Val Ser Lys Leu Thr Val Asp
Lys Ser Arg Trp Gln Gln Gly Asn Val 180 185 190Phe Ser Cys Ser Val
Met His Glu Ala Leu His Asn His Tyr Thr Gln 195 200 205Lys Ser Leu
Ser Leu Ser Pro Gly Lys 210 215307217PRTArtificial
SequenceSynthetic Fc-zwA-V264S 307Ala Pro Glu Leu Leu Gly Gly Pro
Ser Val Phe Leu Phe Pro Pro Lys1 5 10 15Pro Lys Asp Thr Leu Met Ile
Ser Arg Thr Pro Glu Val Thr Cys Val 20 25 30Val Ser Asp Val Ser His
Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr 35 40 45Val Asp Gly Val Glu
Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu 50 55 60Gln Tyr Asn Ser
Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His65 70 75 80Gln Asp
Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys 85 90 95Ala
Leu Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln 100 105
110Pro Arg Glu Pro Gln Val Tyr Val Tyr Pro Pro Ser Arg Glu Glu Met
115 120 125Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe
Tyr Pro 130 135 140Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln
Pro Glu Asn Asn145 150 155 160Tyr Lys Thr Thr Pro Pro Val Leu Asp
Ser Asp Gly Ser Phe Ala Leu 165 170 175Val Ser Lys Leu Thr Val Asp
Lys Ser Arg Trp Gln Gln Gly Asn Val 180 185 190Phe Ser Cys Ser Val
Met His Glu Ala Leu His Asn His Tyr Thr Gln 195 200 205Lys Ser Leu
Ser Leu Ser Pro Gly Lys 210 215308217PRTArtificial
SequenceSynthetic Fc-zwB 308Ala Pro Glu Leu Leu Gly Gly Pro Ser Val
Phe Leu Phe Pro Pro Lys1 5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg
Thr Pro Glu Val Thr Cys Val 20 25 30Val Val Asp Val Ser His Glu Asp
Pro Glu Val Lys Phe Asn Trp Tyr 35 40 45Val Asp Gly Val Glu Val His
Asn Ala Lys Thr Lys Pro Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr
Arg Val Val Ser Val Leu Thr Val Leu His65 70 75 80Gln Asp Trp Leu
Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro
Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro
Arg Glu Pro Gln Val Tyr Val Leu Pro Pro Ser Arg Glu Glu Met 115 120
125Thr Lys Asn Gln Val Ser Leu Leu Cys Leu Val Lys Gly Phe Tyr Pro
130 135 140Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu
Asn Asn145 150 155 160Tyr Leu Thr Trp Pro Pro Val Leu Asp Ser Asp
Gly Ser Phe Phe Leu 165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser
Arg Trp Gln Gln Gly Asn Val 180 185 190Phe Ser Cys Ser Val Met His
Glu Ala Leu His Asn His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu
Ser Pro Gly Lys 210 215309217PRTArtificial SequenceSynthetic
Fc-zwB-V262E 309Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe
Pro Pro Lys1 5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu
Val Thr Cys Glu 20 25 30Val Val Asp Val Ser His Glu Asp Pro Glu Val
Lys Phe Asn Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys
Thr Lys Pro Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val
Ser Val Leu Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys
Glu Tyr Lys Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile
Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro
Gln Val Tyr Val Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys
Asn Gln Val Ser Leu Leu Cys Leu Val Lys Gly Phe Tyr Pro 130 135
140Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn
Asn145 150 155 160Tyr Leu Thr Trp Pro Pro Val Leu Asp Ser Asp Gly
Ser Phe Phe Leu 165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg
Trp Gln Gln Gly Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu
Ala Leu His Asn His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser
Pro Gly Lys 210 215310217PRTArtificial SequenceSynthetic
Fc-zwB-V264S 310Ala Pro Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe
Pro Pro Lys1 5 10 15Pro Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu
Val Thr Cys Val 20 25 30Val Ser Asp Val Ser His Glu Asp Pro Glu Val
Lys Phe Asn Trp Tyr 35 40 45Val Asp Gly Val Glu Val His Asn Ala Lys
Thr Lys Pro Arg Glu Glu 50 55 60Gln Tyr Asn Ser Thr Tyr Arg Val Val
Ser Val Leu Thr Val Leu His65 70 75 80Gln Asp Trp Leu Asn Gly Lys
Glu Tyr Lys Cys Lys Val Ser Asn Lys 85 90 95Ala Leu Pro Ala Pro Ile
Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln 100 105 110Pro Arg Glu Pro
Gln Val Tyr Val Leu Pro Pro Ser Arg Glu Glu Met 115 120 125Thr Lys
Asn Gln Val Ser Leu Leu Cys Leu Val Lys Gly Phe Tyr Pro 130 135
140Ser Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn
Asn145 150 155 160Tyr Leu Thr Trp Pro Pro Val Leu Asp Ser Asp Gly
Ser Phe Phe Leu 165 170 175Tyr Ser Lys Leu Thr Val Asp Lys Ser Arg
Trp Gln Gln Gly Asn Val 180 185 190Phe Ser Cys Ser Val Met His Glu
Ala Leu His Asn His Tyr Thr Gln 195 200 205Lys Ser Leu Ser Leu Ser
Pro Gly Lys 210 215311227PRTArtificial SequenceSynthetic
hinge-CH2-CH3-zwA 311Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala
Pro Glu Leu Leu Gly1 5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys
Pro Lys Asp Thr Leu Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys
Val Val Val Asp Val Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn
Trp Tyr Val Asp Gly Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro
Arg Glu Glu Gln Tyr Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val
Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys
Cys Lys Val Ser Asn Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys
Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120
125Tyr Val Tyr Pro Pro Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser
130 135 140Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala
Val Glu145 150 155 160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr
Lys Thr Thr Pro Pro 165 170 175Val Leu Asp Ser Asp Gly Ser Phe Ala
Leu Val Ser Lys Leu Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln
Gly Asn Val Phe Ser Cys Ser Val Met 195 200 205His Glu Ala Leu His
Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225312227PRTArtificial SequenceSynthetic hinge-CH2-CH3-zwA V262E
312Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly1
5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu
Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys Glu Val Val Asp Val
Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr
Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val Leu Thr Val Leu His
Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys Thr Ile Ser Lys Ala
Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120 125Tyr Val Tyr Pro Pro
Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser 130 135 140Leu Thr Cys
Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu145 150 155
160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro
165 170 175Val Leu Asp Ser Asp Gly Ser Phe Ala Leu Val Ser Lys Leu
Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser
Cys Ser Val Met 195 200 205His Glu Ala Leu His Asn His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225313227PRTArtificial SequenceSynthetic hinge-CH2-CH3-zwA V264S
313Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly1
5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu
Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Ser Asp Val
Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr
Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val Leu Thr Val Leu His
Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys Thr Ile Ser Lys Ala
Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120 125Tyr Val Tyr Pro Pro
Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser 130 135 140Leu Thr Cys
Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu145 150 155
160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro
165 170 175Val Leu Asp Ser Asp Gly Ser Phe Ala Leu Val Ser Lys Leu
Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser
Cys Ser Val Met 195 200 205His Glu Ala Leu His Asn His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225314227PRTArtificial SequenceSynthetic hinge-CH2-CH3-zwB
314Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly1
5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu
Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Val Asp Val
Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr
Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val Leu Thr Val Leu His
Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys Thr Ile Ser Lys Ala
Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120 125Tyr Val Leu Pro Pro
Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser 130 135 140Leu Leu Cys
Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu145 150 155
160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Leu Thr Trp Pro Pro
165 170 175Val Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu
Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser
Cys Ser Val Met 195 200 205His Glu Ala Leu His Asn His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225315227PRTArtificial SequenceSynthetic hinge-CH2-CH3-zwB V262E
315Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly1
5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu
Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys Glu Val Val Asp Val
Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr
Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val Leu Thr Val Leu His
Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys Thr Ile Ser Lys Ala
Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120 125Tyr Val Leu Pro Pro
Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser 130 135 140Leu Leu Cys
Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu145 150 155
160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Leu Thr Trp Pro Pro
165 170 175Val Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu
Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser
Cys Ser Val Met 195 200 205His Glu Ala Leu His Asn His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225316227PRTArtificial SequenceSynthetic hinge-CH2-CH3-zwB V264S
316Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly1
5 10 15Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu
Met 20 25 30Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Ser Asp Val
Ser His 35 40 45Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val 50 55 60His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr
Asn Ser Thr Tyr65 70 75 80Arg Val Val Ser Val Leu Thr Val Leu His
Gln Asp Trp Leu Asn Gly 85 90 95Lys Glu Tyr Lys Cys Lys Val Ser Asn
Lys Ala Leu Pro Ala Pro Ile 100 105 110Glu Lys Thr Ile Ser Lys Ala
Lys Gly Gln Pro Arg Glu Pro Gln Val 115 120 125Tyr Val Leu Pro Pro
Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser 130 135 140Leu Leu Cys
Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu145 150 155
160Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Leu Thr Trp Pro Pro
165 170 175Val Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu
Thr Val 180 185 190Asp Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser
Cys Ser Val Met 195 200 205His Glu Ala Leu His Asn His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser 210 215 220Pro Gly
Lys225317103PRTArtificial SequenceSynthetic CH1-(a)1 317Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Glu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Thr Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Glu Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100318103PRTArtificial SequenceSynthetic CH1-(b)1 318Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Arg Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Lys
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100319103PRTArtificial SequenceSynthetic CH1-(c)1 319Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Trp Leu Gly Cys Glu Val Thr Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Glu Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100320103PRTArtificial SequenceSynthetic CH1-(d)1 320Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val Arg Thr Phe Pro Ala Val Leu Lys Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100321103PRTArtificial SequenceSynthetic CH1-(e)(f)1 321Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Gly Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Lys Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100322103PRTArtificial SequenceSynthetic CH1 (a)2 322Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Gln Ser Ser Gly Leu Tyr Ser
50 55 60Leu Lys Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100323103PRTArtificial SequenceSynthetic CH1 (b)2 323Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Glu Val Thr Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Glu Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser Cys
100324103PRTArtificial SequenceSynthetic CH1 (c)2 324Ala Ser Thr
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr
Ser Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr 20 25 30Phe
Pro Glu Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40
45Gly Val His Thr Phe Pro Ala Val Leu Lys Ser Ser Gly Leu Tyr Ser
50 55 60Leu Ser Ser Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gln
Thr65 70 75 80Tyr Ile Cys Asn Val Asn His Lys Pro Ser Asn Thr Lys
Val Asp Lys 85
90 95Lys Val Glu Pro Lys Ser Cys 100325103PRTArtificial
SequenceSynthetic CH1 (d)2 325Ala Ser Thr Lys Gly Pro Ser Val Phe
Pro Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr Ser Gly Gly Thr Ala Ala
Leu Gly Cys Glu Val Thr Asp Tyr 20 25 30Phe Pro Glu Pro Val Thr Val
Ser Trp Asn Ser Gly Ala Leu Thr Ser 35 40 45Gly Val His Thr Phe Pro
Ala Val Leu Glu Ser Ser Gly Leu Tyr Ser 50 55 60Leu Ser Ser Val Val
Thr Val Pro Ser Ser Ser Leu Gly Thr Gln Thr65 70 75 80Tyr Ile Cys
Asn Val Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys 85 90 95Lys Val
Glu Pro Lys Ser Cys 100326103PRTArtificial SequenceSynthetic
CH1-(e)(f)2 326Ala Ser Thr Lys Gly Pro Ser Val Phe Pro Leu Ala Pro
Ser Ser Lys1 5 10 15Ser Thr Ser Gly Gly Thr Ala Ala Leu Gly Cys Glu
Val Thr Asp Tyr 20 25 30Phe Pro Glu Pro Val Thr Val Ser Trp Asn Ser
Gly Ala Leu Thr Ser 35 40 45Gly Val His Thr Phe Pro Ala Val Leu Gln
Ser Ser Gly Leu Tyr Ser 50 55 60Leu Ser Ser Val Val Thr Val Pro Ser
Ser Ser Leu Gly Thr Gln Thr65 70 75 80Tyr Ile Cys Asn Val Asn His
Lys Pro Ser Asn Thr Lys Val Asp Lys 85 90 95Lys Val Glu Pro Lys Ser
Cys 100327107PRTArtificial SequenceSynthetic Ck-(a)1 327Arg Thr Val
Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Gln Leu
Lys Ser Gly Thr Ala Arg Val Gly Cys Leu Leu Asn Asn Phe 20 25 30Tyr
Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40
45Ser Gly Asn Ser Gln Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser
50 55 60Thr Tyr Ser Leu Arg Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr
Glu65 70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly
Leu Ser Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105328107PRTArtificial SequenceSynthetic Ck-(b)1 328Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Gln Leu Lys
Ser Gly Thr Ala Ser Val Gly Cys Leu Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Gln Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Asp Ser Glu Leu Thr Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105329107PRTArtificial SequenceSynthetic Ck-(c)1 329Arg Thr Val Ala
Ala Pro Ser Val Ala Ile Phe Pro Pro Ser Asp Glu1 5 10 15Arg Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Val Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Gln Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Arg Leu Thr Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105330107PRTArtificial SequenceSynthetic Ck-(d)1 330Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Glu Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Glu Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Thr Leu Glu Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105331107PRTArtificial SequenceSynthetic Ck-(e)(f)1 331Arg Thr Val
Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Glu Leu
Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr
Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40
45Ser Gly Asn Ser Glu Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser
50 55 60Thr Tyr Ser Leu Ser Ser Thr Leu Glu Leu Ser Lys Ala Asp Tyr
Glu65 70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly
Leu Ser Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105332106PRTArtificial SequenceSynthetic Cl-(a)2 332Gly Gln Pro Lys
Ala Ala Pro Ser Val Thr Leu Phe Pro Pro Ser Ser1 5 10 15Glu Glu Leu
Gln Ala Asn Lys Ala Thr Leu Val Cys Leu Ile Ser Asp 20 25 30Phe Tyr
Pro Gly Ala Val Thr Val Ala Trp Lys Ala Asp Ser Ser Pro 35 40 45Val
Lys Ala Gly Val Glu Thr Thr Thr Pro Ser Lys Gln Ser Asn Asn 50 55
60Lys Tyr Ala Ala Glu Ser Glu Leu Ser Leu Thr Pro Glu Gln Trp Lys65
70 75 80Ser His Arg Ser Tyr Ser Cys Gln Val Thr His Glu Gly Ser Thr
Val 85 90 95Glu Lys Thr Val Ala Pro Thr Glu Cys Ser 100
105333106PRTArtificial SequenceSynthetic Cl-(b)2 333Gly Gln Pro Lys
Ala Ala Pro Ser Val Thr Leu Phe Pro Pro Ser Ser1 5 10 15Glu Gln Leu
Gln Ala Asn Lys Ala Arg Leu Val Cys Leu Ile Ser Asp 20 25 30Phe Tyr
Pro Gly Ala Val Thr Val Ala Trp Lys Ala Asp Ser Ser Pro 35 40 45Val
Lys Ala Gly Val Glu Thr Thr Thr Pro Ser Lys Gln Ser Asn Asn 50 55
60Lys Tyr Ala Ala Ser Ser Tyr Leu Ser Leu Thr Pro Glu Gln Trp Lys65
70 75 80Ser His Arg Ser Tyr Ser Cys Gln Val Thr His Glu Gly Ser Thr
Val 85 90 95Glu Lys Thr Val Ala Pro Thr Glu Cys Ser 100
105334107PRTArtificial SequenceSynthetic Ck-(c)2 334Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Glu Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Trp Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Glu Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Thr Leu Glu Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105335107PRTArtificial SequenceSynthetic Ck-(d)2 335Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Arg Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Lys Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Arg Leu Thr Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105336107PRTArtificial SequenceSynthetic Ck-(e)2 336Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Arg Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Lys Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Arg Leu Thr Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105337107PRTArtificial SequenceSynthetic Ck-(f)2 337Arg Thr Val Ala
Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Arg Leu Lys
Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr Pro
Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40 45Ser
Gly Asn Ser Gln Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser 50 55
60Thr Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu65
70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly Leu Ser
Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105338107PRTArtificial SequenceSynthetic Ck-(e)(f)2 338Arg Thr Val
Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1 5 10 15Glu Leu
Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe 20 25 30Tyr
Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln 35 40
45Ser Gly Asn Ser Glu Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser
50 55 60Thr Tyr Ser Leu Ser Ser Thr Leu Glu Leu Ser Lys Ala Asp Tyr
Glu65 70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr His Gln Gly
Leu Ser Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly Glu Cys 100
105339243PRTArtificial SequenceSynthetic
aPD-1-1353-G10_R28T/P30D/H31S_Vk1- 39_LC, scFv 339Glu Val Gln Leu
Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly Ala1 5 10 15Ser Val Lys
Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Asp Ser Tyr 20 25 30Gly Ile
Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp Met 35 40 45Gly
Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys Leu 50 55
60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala Tyr65
70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr
Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly
Gln Gly 100 105 110Thr Leu Val Thr Val Ser Ser Gly Gly Gly Gly Ser
Gly Gly Gly Gly 115 120 125Ser Gly Gly Gly Gly Ser Asp Ile Gln Leu
Thr Gln Ser Pro Ser Ser 130 135 140Leu Ser Ala Ser Val Gly Asp Arg
Val Thr Ile Thr Cys Ser Gly Asp145 150 155 160Ala Leu Pro Lys Gln
Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly Lys 165 170 175Ala Pro Lys
Leu Leu Ile Tyr Lys Asp Thr Glu Arg Pro Ser Gly Val 180 185 190Pro
Ser Arg Phe Ser Gly Ser Ser Ser Gly Thr Lys Val Thr Leu Thr 195 200
205Ile Ser Ser Leu Gln Pro Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Ser
210 215 220Ala Asp Asn Ser Ile Thr Tyr Arg Val Phe Gly Gly Gly Thr
Lys Val225 230 235 240Glu Ile Lys340242PRTArtificial
SequenceSynthetic anti-PD-1 1353-G10 scFvFc zwB R28T/P30D/H31S,
scFv 340Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly
Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Asp
Ser Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu
Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr
Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser
Thr Asn Thr Ala Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr
Gly Ser Gly Tyr Trp Gly Gln Gly 100 105 110Thr Leu Val Thr Val Ser
Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly 115 120 125Ser Gly Gly Gly
Gly Ser Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val 130 135 140Ser Val
Ser Pro Gly Gln Thr Ala Arg Ile Thr Cys Ser Gly Asp Ala145 150 155
160Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly Gln Ala
165 170 175Pro Val Met Val Ile Tyr Lys Asp Thr Glu Arg Pro Ser Gly
Ile Pro 180 185 190Glu Arg Phe Ser Gly Ser Ser Ser Gly Thr Lys Val
Thr Leu Thr Ile 195 200 205Ser Gly Val Gln Ala Glu Asp Glu Ala Asp
Tyr Tyr Cys Gln Ser Ala 210 215 220Asp Asn Ser Ile Thr Tyr Arg Val
Phe Gly Gly Gly Thr Lys Val Thr225 230 235 240Val
Leu341243PRTArtificial SequenceSynthetic anti-PD-1 1353-G10 Vk1-39,
scFv 341Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly
Ala1 5 10 15Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro
His Tyr 20 25 30Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu
Glu Trp Met 35 40 45Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr
Ala Gln Lys Leu 50 55 60Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser
Thr Asn Thr Ala Tyr65 70 75 80Met Glu Leu Arg Ser Leu Arg Ser Asp
Asp Thr Ala Val Tyr Tyr Cys 85 90 95Ala Arg Asp Val Asp Tyr Gly Thr
Gly Ser Gly Tyr Trp Gly Gln Gly 100 105 110Thr Leu Val Thr Val Ser
Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly 115 120 125Ser Gly Gly Gly
Gly Ser Asp Ile Gln Leu Thr Gln Ser Pro Ser Ser 130 135 140Leu Ser
Ala Ser Val Gly Asp Arg Val Thr Ile Thr Cys Ser Gly Asp145 150 155
160Ala Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln Lys Pro Gly Lys
165 170 175Ala Pro Lys Leu Leu Ile Tyr Lys Asp Thr Glu Arg Pro Ser
Gly Val 180 185 190Pro Ser Arg Phe Ser Gly Ser Ser Ser Gly Thr Lys
Val Thr Leu Thr 195 200 205Ile Ser Ser Leu Gln Pro Glu Asp Phe Ala
Thr Tyr Tyr Cys Gln Ser 210 215 220Ala Asp Asn Ser Ile Thr Tyr Arg
Val Phe Gly Gly Gly Thr Lys Val225 230 235 240Glu Ile
Lys342450PRTArtificial SequenceSynthetic Anti-PD1 1353-G10 HC knob,
HC 342Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro
Gly1 5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe
Pro His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly
Leu Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn
Tyr Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp Thr
Ser Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg Ser
Asp Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr Gly
Thr Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val
Ser Ser Ala Ser Thr Lys Gly Pro Ser Val 115 120
125Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala
130 135 140Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr
Val Ser145 150 155 160Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His
Thr Phe Pro Ala Val 165 170 175Leu Gln Ser Ser Gly Leu Tyr Ser Leu
Ser Ser Val Val Thr Val Pro 180 185 190Ser Ser Ser Leu Gly Thr Gln
Thr Tyr Ile Cys Asn Val Asn His Lys 195 200 205Pro Ser Asn Thr Lys
Val Asp Lys Lys Val Glu Pro Lys Ser Cys Asp 210 215 220Lys Thr His
Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly225 230 235
240Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met Ile
245 250 255Ser Arg Thr Pro Glu Val Thr Cys Val Val Val Asp Val Ser
His Glu 260 265 270Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly
Val Glu Val His 275 280 285Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln
Tyr Asn Ser Thr Tyr Arg 290 295 300Val Val Ser Val Leu Thr Val Leu
His Gln Asp Trp Leu Asn Gly Lys305 310 315 320Glu Tyr Lys Cys Lys
Val Ser Asn Lys Ala Leu Pro Ala Pro Ile Glu 325 330 335Lys Thr Ile
Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val Tyr 340 345 350Thr
Leu Pro Pro Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser Leu 355 360
365Trp Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp
370 375 380Glu Ser Asn Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro
Pro Val385 390 395 400Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser
Lys Leu Thr Val Asp 405 410 415Lys Ser Arg Trp Gln Gln Gly Asn Val
Phe Ser Cys Ser Val Met His 420 425 430Glu Ala Leu His Asn His Tyr
Thr Gln Lys Ser Leu Ser Leu Ser Pro 435 440 445Gly Lys
450343450PRTArtificial SequenceSynthetic Anti-PD1 1353-G10 HC hole,
HC 343Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro
Gly1 5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe
Pro His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly
Leu Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn
Tyr Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp Thr
Ser Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg Ser
Asp Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr Gly
Thr Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val
Ser Ser Ala Ser Thr Lys Gly Pro Ser Val 115 120 125Phe Pro Leu Ala
Pro Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala 130 135 140Leu Gly
Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser145 150 155
160Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val
165 170 175Leu Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr
Val Pro 180 185 190Ser Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn
Val Asn His Lys 195 200 205Pro Ser Asn Thr Lys Val Asp Lys Lys Val
Glu Pro Lys Ser Cys Asp 210 215 220Lys Thr His Thr Cys Pro Pro Cys
Pro Ala Pro Glu Leu Leu Gly Gly225 230 235 240Pro Ser Val Phe Leu
Phe Pro Pro Lys Pro Lys Asp Thr Leu Met Ile 245 250 255Ser Arg Thr
Pro Glu Val Thr Cys Val Val Val Asp Val Ser His Glu 260 265 270Asp
Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His 275 280
285Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg
290 295 300Val Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn
Gly Lys305 310 315 320Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu
Pro Ala Pro Ile Glu 325 330 335Lys Thr Ile Ser Lys Ala Lys Gly Gln
Pro Arg Glu Pro Gln Val Tyr 340 345 350Thr Leu Pro Pro Ser Arg Glu
Glu Met Thr Lys Asn Gln Val Ser Leu 355 360 365Ser Cys Ala Val Lys
Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp 370 375 380Glu Ser Asn
Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val385 390 395
400Leu Asp Ser Asp Gly Ser Phe Phe Leu Val Ser Lys Leu Thr Val Asp
405 410 415Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val
Met His 420 425 430Glu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu
Ser Leu Ser Pro 435 440 445Gly Lys 450344215PRTArtificial
SequenceSynthetic Anti-PD1 1353-G10 LC, LC 344Met Ser Tyr Glu Leu
Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly1 5 10 15Gln Thr Ala Arg
Ile Thr Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr 20 25 30Ala Tyr Trp
Tyr Gln Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile 35 40 45Tyr Lys
Asp Thr Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly 50 55 60Ser
Ser Ser Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala65 70 75
80Glu Asp Glu Ala Asp Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr
85 90 95Tyr Arg Val Phe Gly Gly Gly Thr Lys Val Thr Val Leu Gly Gln
Pro 100 105 110Lys Ala Ala Pro Ser Val Thr Leu Phe Pro Pro Ser Ser
Glu Glu Leu 115 120 125Gln Ala Asn Lys Ala Thr Leu Val Cys Leu Ile
Ser Asp Phe Tyr Pro 130 135 140Gly Ala Val Thr Val Ala Trp Lys Ala
Asp Ser Ser Pro Val Lys Ala145 150 155 160Gly Val Glu Thr Thr Thr
Pro Ser Lys Gln Ser Asn Asn Lys Tyr Ala 165 170 175Ala Ser Ser Tyr
Leu Ser Leu Thr Pro Glu Gln Trp Lys Ser His Arg 180 185 190Ser Tyr
Ser Cys Gln Val Thr His Glu Gly Ser Thr Val Glu Lys Thr 195 200
205Val Ala Pro Thr Glu Cys Ser 210 215345103PRTArtificial
SequenceSynthetic CH1-wt 345Ala Ser Thr Lys Gly Pro Ser Val Phe Pro
Leu Ala Pro Ser Ser Lys1 5 10 15Ser Thr Ser Gly Gly Thr Ala Ala Leu
Gly Cys Leu Val Lys Asp Tyr 20 25 30Phe Pro Glu Pro Val Thr Val Ser
Trp Asn Ser Gly Ala Leu Thr Ser 35 40 45Gly Val His Thr Phe Pro Ala
Val Leu Gln Ser Ser Gly Leu Tyr Ser 50 55 60Leu Ser Ser Val Val Thr
Val Pro Ser Ser Ser Leu Gly Thr Gln Thr65 70 75 80Tyr Ile Cys Asn
Val Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys 85 90 95Lys Val Glu
Pro Lys Ser Cys 100346107PRTArtificial SequenceSynthetic C-kappa-wt
346Arg Thr Val Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu1
5 10 15Gln Leu Lys Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn
Phe 20 25 30Tyr Pro Arg Glu Ala Lys Val Gln Trp Lys Val Asp Asn Ala
Leu Gln 35 40 45Ser Gly Asn Ser Gln Glu Ser Val Thr Glu Gln Asp Ser
Lys Asp Ser 50 55 60Thr Tyr Ser Leu Ser Ser Thr Leu Thr Leu Ser Lys
Ala Asp Tyr Glu65 70 75 80Lys His Lys Val Tyr Ala Cys Glu Val Thr
His Gln Gly Leu Ser Ser 85 90 95Pro Val Thr Lys Ser Phe Asn Arg Gly
Glu Cys 100 105347106PRTArtificial SequenceSynthetic C-lambda-wt
347Gly Gln Pro Lys Ala Ala Pro Ser Val Thr Leu Phe Pro Pro Ser Ser1
5 10 15Glu Glu Leu Gln Ala Asn Lys Ala Thr Leu Val Cys Leu Ile Ser
Asp 20 25 30Phe Tyr Pro Gly Ala Val Thr Val Ala Trp Lys Ala Asp Ser
Ser Pro 35 40 45Val Lys Ala Gly Val Glu Thr Thr Thr Pro Ser Lys Gln
Ser Asn Asn 50 55 60Lys Tyr Ala Ala Ser Ser Tyr Leu Ser Leu Thr Pro
Glu Gln Trp Lys65 70 75 80Ser His Arg Ser Tyr Ser Cys Gln Val Thr
His Glu Gly Ser Thr Val 85 90 95Glu Lys Thr Val Ala Pro Thr Glu Cys
Ser 100 105348481PRTArtificial SequenceSynthetic 1353-G10 scFv-Fc
zwB, scFv-Fc 348Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys
Lys Pro Gly1 5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr
Arg Phe Pro His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly
Gln Gly Leu Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn
Thr Asn Tyr Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr
Asp Thr Ser Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu
Arg Ser Asp Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp
Tyr Gly Thr Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val
Thr Val Ser Ser Gly Gly Gly Gly Ser Gly Gly Gly 115 120 125Gly Ser
Gly Gly Gly Gly Ser Ser Tyr Glu Leu Thr Gln Pro Pro Ser 130 135
140Val Ser Val Ser Pro Gly Gln Thr Ala Arg Ile Thr Cys Ser Gly
Asp145 150 155 160Ala Leu Pro Lys Gln Tyr Ala Tyr Trp Tyr Gln Gln
Lys Pro Gly Gln 165 170 175Ala Pro Val Met Val Ile Tyr Lys Asp Thr
Glu Arg Pro Ser Gly Ile 180 185 190Pro Glu Arg Phe Ser Gly Ser Ser
Ser Gly Thr Lys Val Thr Leu Thr 195 200 205Ile Ser Gly Val Gln Ala
Glu Asp Glu Ala Asp Tyr Tyr Cys Gln Ser 210 215 220Ala Asp Asn Ser
Ile Thr Tyr Arg Val Phe Gly Gly Gly Thr Lys Val225 230 235 240Thr
Val Leu Ala Ala Gly Ser Asp Gln Glu Pro Lys Ser Ser Asp Lys 245 250
255Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu Gly Gly Pro
260 265 270Ser Val Phe Leu Phe Pro Pro Lys Pro Lys Asp Thr Leu Met
Ile Ser 275 280 285Arg Thr Pro Glu Val Thr Cys Val Val Val Asp Val
Ser His Glu Asp 290 295 300Pro Glu Val Lys Phe Asn Trp Tyr Val Asp
Gly Val Glu Val His Asn305 310 315 320Ala Lys Thr Lys Pro Arg Glu
Glu Gln Tyr Asn Ser Thr Tyr Arg Val 325 330 335Val Ser Val Leu Thr
Val Leu His Gln Asp Trp Leu Asn Gly Lys Glu 340 345 350Tyr Lys Cys
Lys Val Ser Asn Lys Ala Leu Pro Ala Pro Ile Glu Lys 355 360 365Thr
Ile Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln Val Tyr Val 370 375
380Leu Pro Pro Ser Arg Glu Glu Met Thr Lys Asn Gln Val Ser Leu
Leu385 390 395 400Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp Ile Ala
Val Glu Trp Glu 405 410 415Ser Asn Gly Gln Pro Glu Asn Asn Tyr Leu
Thr Trp Pro Pro Val Leu 420 425 430Asp Ser Asp Gly Ser Phe Phe Leu
Tyr Ser Lys Leu Thr Val Asp Lys 435 440 445Ser Arg Trp Gln Gln Gly
Asn Val Phe Ser Cys Ser Val Met His Glu 450 455 460Ala Leu His Asn
His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro Gly465 470 475
480Lys349450PRTArtificial SequenceSynthetic 1353-G10 HC zwA, HC
349Met Glu Val Gln Leu Val Gln Ser Gly Ala Glu Val Lys Lys Pro Gly1
5 10 15Ala Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro
His 20 25 30Tyr Gly Ile Ser Trp Val Arg Gln Ala Pro Gly Gln Gly Leu
Glu Trp 35 40 45Met Gly Trp Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr
Ala Gln Lys 50 55 60Leu Gln Gly Arg Val Thr Met Thr Thr Asp Thr Ser
Thr Asn Thr Ala65 70 75 80Tyr Met Glu Leu Arg Ser Leu Arg Ser Asp
Asp Thr Ala Val Tyr Tyr 85 90 95Cys Ala Arg Asp Val Asp Tyr Gly Thr
Gly Ser Gly Tyr Trp Gly Gln 100 105 110Gly Thr Leu Val Thr Val Ser
Ser Ala Ser Thr Lys Gly Pro Ser Val 115 120 125Phe Pro Leu Ala Pro
Ser Ser Lys Ser Thr Ser Gly Gly Thr Ala Ala 130 135 140Leu Gly Cys
Leu Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser145 150 155
160Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val
165 170 175Leu Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr
Val Pro 180 185 190Ser Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn
Val Asn His Lys 195 200 205Pro Ser Asn Thr Lys Val Asp Lys Lys Val
Glu Pro Lys Ser Cys Asp 210 215 220Lys Thr His Thr Cys Pro Pro Cys
Pro Ala Pro Glu Leu Leu Gly Gly225 230 235 240Pro Ser Val Phe Leu
Phe Pro Pro Lys Pro Lys Asp Thr Leu Met Ile 245 250 255Ser Arg Thr
Pro Glu Val Thr Cys Val Val Val Asp Val Ser His Glu 260 265 270Asp
Pro Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His 275 280
285Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg
290 295 300Val Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn
Gly Lys305 310 315 320Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu
Pro Ala Pro Ile Glu 325 330 335Lys Thr Ile Ser Lys Ala Lys Gly Gln
Pro Arg Glu Pro Gln Val Tyr 340 345 350Val Tyr Pro Pro Ser Arg Glu
Glu Met Thr Lys Asn Gln Val Ser Leu 355 360 365Thr Cys Leu Val Lys
Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp 370 375 380Glu Ser Asn
Gly Gln Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val385 390 395
400Leu Asp Ser Asp Gly Ser Phe Ala Leu Val Ser Lys Leu Thr Val Asp
405 410 415Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val
Met His 420 425 430Glu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu
Ser Leu Ser Pro 435 440 445Gly Lys 450350450PRTArtificial
SequenceSynthetic 1353-G10 HC zwB, HC 350Met Glu Val Gln Leu Val
Gln Ser Gly Ala Glu Val Lys Lys Pro Gly1 5 10 15Ala Ser Val Lys Val
Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro His 20 25 30Tyr Gly Ile Ser
Trp Val Arg Gln Ala Pro Gly Gln Gly Leu Glu Trp 35 40 45Met Gly Trp
Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys 50 55 60Leu Gln
Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala65 70 75
80Tyr Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr
85 90 95Cys Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly
Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly
Pro Ser Val 115 120 125Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser
Gly Gly Thr Ala Ala 130 135 140Leu Gly Cys Leu Val Lys Asp Tyr Phe
Pro Glu Pro Val Thr Val Ser145 150 155 160Trp Asn Ser Gly Ala Leu
Thr Ser Gly Val His Thr Phe Pro Ala Val 165
170 175Leu Gln Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val
Pro 180 185 190Ser Ser Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val
Asn His Lys 195 200 205Pro Ser Asn Thr Lys Val Asp Lys Lys Val Glu
Pro Lys Ser Cys Asp 210 215 220Lys Thr His Thr Cys Pro Pro Cys Pro
Ala Pro Glu Leu Leu Gly Gly225 230 235 240Pro Ser Val Phe Leu Phe
Pro Pro Lys Pro Lys Asp Thr Leu Met Ile 245 250 255Ser Arg Thr Pro
Glu Val Thr Cys Val Val Val Asp Val Ser His Glu 260 265 270Asp Pro
Glu Val Lys Phe Asn Trp Tyr Val Asp Gly Val Glu Val His 275 280
285Asn Ala Lys Thr Lys Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg
290 295 300Val Val Ser Val Leu Thr Val Leu His Gln Asp Trp Leu Asn
Gly Lys305 310 315 320Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu
Pro Ala Pro Ile Glu 325 330 335Lys Thr Ile Ser Lys Ala Lys Gly Gln
Pro Arg Glu Pro Gln Val Tyr 340 345 350Val Leu Pro Pro Ser Arg Glu
Glu Met Thr Lys Asn Gln Val Ser Leu 355 360 365Leu Cys Leu Val Lys
Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp 370 375 380Glu Ser Asn
Gly Gln Pro Glu Asn Asn Tyr Leu Thr Trp Pro Pro Val385 390 395
400Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp
405 410 415Lys Ser Arg Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val
Met His 420 425 430Glu Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu
Ser Leu Ser Pro 435 440 445Gly Lys 450351216PRTArtificial
SequenceSynthetic 1353-G10 LC1c, LC 351Met Ser Tyr Glu Leu Thr Gln
Pro Pro Ser Val Ser Val Ser Pro Gly1 5 10 15Gln Thr Ala Arg Ile Thr
Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr 20 25 30Ala Tyr Trp Tyr Gln
Gln Lys Pro Gly Gln Ala Pro Val Met Val Ile 35 40 45Tyr Lys Asp Thr
Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly 50 55 60Ser Ser Ser
Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala65 70 75 80Glu
Asp Phe Ala Thr Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr 85 90
95Tyr Arg Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys Arg Thr Val
100 105 110Ala Ala Pro Ser Val Ala Ile Phe Pro Pro Ser Asp Glu Arg
Leu Lys 115 120 125Ser Gly Thr Ala Ser Val Val Cys Val Leu Asn Asn
Phe Tyr Pro Arg 130 135 140Glu Ala Lys Val Gln Trp Lys Val Asp Asn
Ala Leu Gln Ser Gly Asn145 150 155 160Ser Gln Glu Ser Val Thr Glu
Gln Asp Ser Lys Asp Ser Thr Tyr Ser 165 170 175Leu Ser Ser Arg Leu
Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys 180 185 190Val Tyr Ala
Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr 195 200 205Lys
Ser Phe Asn Arg Gly Glu Cys 210 215352216PRTArtificial
SequenceSynthetic 1353-G10 LC1d, LC 352Met Ser Tyr Glu Leu Thr Gln
Pro Pro Ser Val Ser Val Ser Pro Gly1 5 10 15Gln Thr Ala Arg Ile Thr
Cys Ser Gly Asp Ala Leu Pro Lys Gln Tyr 20 25 30Ala Tyr Trp Tyr Gln
Arg Lys Pro Gly Gln Ala Pro Val Met Val Ile 35 40 45Tyr Lys Asp Thr
Glu Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly 50 55 60Ser Ser Ser
Gly Thr Lys Val Thr Leu Thr Ile Ser Gly Val Gln Ala65 70 75 80Glu
Asp Phe Ala Thr Tyr Tyr Cys Gln Ser Ala Asp Asn Ser Ile Thr 85 90
95Tyr Arg Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys Arg Thr Val
100 105 110Ala Ala Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Arg
Leu Lys 115 120 125Ser Gly Thr Ala Ser Val Val Cys Leu Leu Asn Asn
Phe Tyr Pro Arg 130 135 140Glu Ala Lys Val Gln Trp Lys Val Asp Asn
Ala Leu Gln Ser Gly Asn145 150 155 160Ser Lys Glu Ser Val Thr Glu
Gln Asp Ser Lys Asp Ser Thr Tyr Ser 165 170 175Leu Ser Ser Arg Leu
Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys 180 185 190Val Tyr Ala
Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr 195 200 205Lys
Ser Phe Asn Arg Gly Glu Cys 210 215353450PRTArtificial
SequenceSynthetic 1353-G10 HC1d zwB, HC 353Met Glu Val Gln Leu Val
Gln Ser Gly Ala Glu Val Lys Lys Pro Gly1 5 10 15Ala Ser Val Lys Val
Ser Cys Lys Ala Ser Gly Tyr Arg Phe Pro His 20 25 30Tyr Gly Ile Ser
Trp Val Arg Glu Ala Pro Gly Gln Gly Leu Glu Trp 35 40 45Met Gly Trp
Ile Ser Ala Tyr Asn Gly Asn Thr Asn Tyr Ala Gln Lys 50 55 60Leu Gln
Gly Arg Val Thr Met Thr Thr Asp Thr Ser Thr Asn Thr Ala65 70 75
80Tyr Met Glu Leu Arg Ser Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr
85 90 95Cys Ala Arg Asp Val Asp Tyr Gly Thr Gly Ser Gly Tyr Trp Gly
Gln 100 105 110Gly Thr Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly
Pro Ser Val 115 120 125Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser
Gly Gly Thr Ala Ala 130 135 140Leu Gly Cys Glu Val Thr Asp Tyr Phe
Pro Glu Pro Val Thr Val Ser145 150 155 160Trp Asn Ser Gly Ala Leu
Thr Ser Gly Val His Thr Phe Pro Ala Val 165 170 175Leu Glu Ser Ser
Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val Pro 180 185 190Ser Ser
Ser Leu Gly Thr Gln Thr Tyr Ile Cys Asn Val Asn His Lys 195 200
205Pro Ser Asn Thr Lys Val Asp Lys Lys Val Glu Pro Lys Ser Cys Asp
210 215 220Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro Glu Leu Leu
Gly Gly225 230 235 240Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys
Asp Thr Leu Met Ile 245 250 255Ser Arg Thr Pro Glu Val Thr Cys Val
Val Val Asp Val Ser His Glu 260 265 270Asp Pro Glu Val Lys Phe Asn
Trp Tyr Val Asp Gly Val Glu Val His 275 280 285Asn Ala Lys Thr Lys
Pro Arg Glu Glu Gln Tyr Asn Ser Thr Tyr Arg 290 295 300Val Val Ser
Val Leu Thr Val Leu His Gln Asp Trp Leu Asn Gly Lys305 310 315
320Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu Pro Ala Pro Ile Glu
325 330 335Lys Thr Ile Ser Lys Ala Lys Gly Gln Pro Arg Glu Pro Gln
Val Tyr 340 345 350Val Leu Pro Pro Ser Arg Glu Glu Met Thr Lys Asn
Gln Val Ser Leu 355 360 365Leu Cys Leu Val Lys Gly Phe Tyr Pro Ser
Asp Ile Ala Val Glu Trp 370 375 380Glu Ser Asn Gly Gln Pro Glu Asn
Asn Tyr Leu Thr Trp Pro Pro Val385 390 395 400Leu Asp Ser Asp Gly
Ser Phe Phe Leu Tyr Ser Lys Leu Thr Val Asp 405 410 415Lys Ser Arg
Trp Gln Gln Gly Asn Val Phe Ser Cys Ser Val Met His 420 425 430Glu
Ala Leu His Asn His Tyr Thr Gln Lys Ser Leu Ser Leu Ser Pro 435 440
445Gly Lys 45035411PRTArtificial SequenceSynthetic trastuzumab,
CDR-L1 354Arg Ala Ser Gln Asp Val Asn Thr Ala Val Ala1 5
103557PRTArtificial SequenceSynthetic trastuzumab, CDR-L2 355Ser
Ala Ser Phe Leu Tyr Ser1 53569PRTArtificial SequenceSynthetic
trastuzumab, CDR-L3 356Gln Gln His Tyr Thr Thr Pro Pro Thr1 5
References
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.