Moisture Wicking And Cooling Capsules Having An Outer Shell Comprising A Siloxane And Methods For Making Same
Ryan; Kayla L. M. ; et al.
U.S. patent application number 16/747126 was filed with the patent office on 2020-05-14 for moisture wicking and cooling capsules having an outer shell comprising a siloxane and methods for making same. This patent application is currently assigned to MICROTEK LABORATORIES, INC.. The applicant listed for this patent is MICROTEK LABORATORIES, INC.. Invention is credited to Carl M. Lentz, Kayla L. M. Ryan.
| Application Number | 20200146378 16/747126 |
| Document ID | / |
| Family ID | 63245484 |
| Filed Date | 2020-05-14 |







| United States Patent Application | 20200146378 |
| Kind Code | A1 |
| Ryan; Kayla L. M. ; et al. | May 14, 2020 |
MOISTURE WICKING AND COOLING CAPSULES HAVING AN OUTER SHELL COMPRISING A SILOXANE AND METHODS FOR MAKING SAME
Abstract
Microcapsules or macrocapsules have a core composition that includes a phase change material (PCM) encapsulated within a polymer wall with an outer shell having a siloxane tethered to an exterior surface of the polymer wall by a surfactant. The siloxane may form a crystalline or a sol-gel outer shell. Methods of making such capsules and textile fabrics and clothing incorporating such capsules include treating pre-formed capsules with a surfactant solution followed by treating with a compound containing a siloxane functional group. The surfactant connects or tethers the siloxane to the exterior surface of the polymer wall and the siloxane forms an outer shell of the capsules.
| Inventors: | Ryan; Kayla L. M.; (Alamogordo, NM) ; Lentz; Carl M.; (Waynesville, OH) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | MICROTEK LABORATORIES, INC. Dayton OH |
||||||||||
| Family ID: | 63245484 | ||||||||||
| Appl. No.: | 16/747126 | ||||||||||
| Filed: | January 20, 2020 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15906015 | Feb 27, 2018 | 10561182 | ||
| 16747126 | ||||
| 62464733 | Feb 28, 2017 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A42B 3/125 20130101; A63B 21/4037 20151001; B01J 20/265 20130101; C09K 5/066 20130101; B01J 20/3217 20130101; A41D 2600/10 20130101; A41D 27/02 20130101; A63B 2209/00 20130101; B01J 20/28004 20130101; B01J 20/3246 20130101; B01J 20/3293 20130101; B01J 20/3208 20130101; A41D 31/02 20130101; A42B 3/066 20130101; B01J 20/28019 20130101 |
| International Class: | A41D 31/02 20060101 A41D031/02; C09K 5/06 20060101 C09K005/06; A42B 3/12 20060101 A42B003/12; A41D 27/02 20060101 A41D027/02; B01J 20/32 20060101 B01J020/32; B01J 20/28 20060101 B01J020/28; B01J 20/26 20060101 B01J020/26 |
Claims
1. A capsule comprising: a core composition comprising a phase change material (PCM); a polymer wall encapsulating the core composition; and an outer shell comprising a reaction product of a silicate and a metal-oxygen containing compound or a reaction product of a metal-oxygen containing compound and a compound containing a siloxane functional group chemically bonded to an exterior surface of the polymer wall by a surfactant; wherein the capsules wick moisture.
2. The capsule of claim 1, wherein the surfactant comprises one or more of a cationic surfactant, an anionic surfactant, and a non-ionic surfactant.
3. The capsule of claim 1, wherein the PCM comprises a C.sub.10-C.sub.40 hydrocarbyl.
4. The capsule of claim 1, wherein the metal complex containing oxygen comprises NaAlSi.sub.2O.sub.6.
5. The capsule of claim 1, wherein the reaction product forms a crystalline outer shell and the outer shell is a discontinuous shell.
6. The capsule of claim 1, wherein the surfactant solution is in an organic solvent and the siloxane forms a sol-gel outer shell.
7. The capsule claim 1, wherein the capsule is a microcapsule or a macrocapsule.
8. The capsule of claim 1, wherein the capsule is a microcapsule having a diameter of about 15 .mu.m to about 25 .mu.m.
9. The capsule of claim 1, wherein the polymer wall comprises one or more of a melamine formaldehyde, a crosslinked melamine formaldehyde, a resorcinol urea formaldehyde, a poly-urea formaldehyde, an acrylic polymer, and a gelatin.
10. The capsule of claim 9, wherein the polymer wall comprises a crosslinked melamine formaldehyde and the cross-linked melamine comprises melamine formaldehyde polymerized with a crosslinking agent comprising: (a) a reaction product of a cyclic urea (U) and a multifunctional aldehyde (A), and (b) at least one crosslinker selected from the group consisting of (b1) reaction products of an aminotriazine and at least one aldehyde selected from the group consisting of aliphatic monoaldehydes and multifunctional aliphatic aldehydes having the structure Y(CHO).sub.n, where Y is an n-functional aliphatic residue, and n is greater than 1, where U is not dihydroxyethylene urea if the crosslinker (b) is (b1), (b2) reaction products of urea and/or cyclic ureas and formaldehyde, (b3) alkoxycarbonylaminotriazines, (b4) multifunctional isocyanates which may be partially or completely blocked, (b5) reaction products of phenols and aliphatic monoaldehydes, (b6) multifunctional epoxides, (b7) multifunctional aziridines, (b8) multifunctional carbodiimides, wherein any of the crosslinkers (a) and (b) which have hydroxyl groups may be etherified with one or more linear, branched, or cyclic aliphatic alcohols.
11. The capsule of claim 10, wherein the capsule has a free formaldehyde level of less than 100 pm.
12. The capsule of claim 1, wherein the capsule has an enthalpy of greater than 75 J/g as measured by differential scanning calorimetry.
13. A textile fabric comprising a plurality of capsules according to claim 1.
14. The textile fabric of claim 13, wherein the textile fabric is an article of clothing.
15. The textile fabric of claim 13, wherein each capsule of the plurality of capsules has an enthalpy of greater than 75 J/g as measured by differential scanning calorimetry.
16. The textile fabric of claim 13, wherein the plurality of capsules forms a layer having a thickness of about 1 mm to about 50 mm on a surface of the textile fabric.
Description
RELATED APPLICATIONS
[0001] This application is a continuation of U.S. application Ser. No. 15/906,015, filed Feb. 27, 2018, which claims the benefit of U.S. Provisional Application No. 62/464,733, filed Feb. 28, 2017, which is incorporated herein in its entirety.
TECHNICAL FIELD
[0002] The present disclosure relates to capsules having a polymer wall encapsulating a core composition including a phase change material (PCM) and an outer shell comprising a siloxane tethered to the polymer wall by a surfactant.
BACKGROUND
[0003] Articles made from cotton fabric and other natural material fabrics (such as linen, wool, etc.) are generally absorbent, and may feel comfortable under conditions of very light perspiration. However, under conditions of heavier perspiration, these fabrics feel wet, heavy and clingy, restricting movement and becoming uncomfortable to wear.
[0004] Wicking technology for apparel has been developed to address this problem. Generally, wicking aims to pull moisture away from the skin of the wearer and make the wearer more comfortable while being active. In the early to mid-nineties, patent applications were filed for moisture-managing clothing, where a fabric would pull moisture away from the skin via a dual-layered fabric, where the layer closest to the skin would be hydrophobic, and the outer layer would be hydrophilic. In some cases, these two components were woven into the same layer of clothing, in order to make the material thinner. The hydrophobic layer would wick moisture, so as to pull it away from the wearer, then force the moisture into contact with the hydrophilic layer, which would then absorb the moisture and spread it out along the surface of an article of clothing. The thinning of the moisture would allow evaporation from the clothing at a quicker rate than normal fabric. The hydrophobic layer would typically consist of a polyester or cotton fiber, whereas the hydrophilic layer would typically consist of a nylon or polypropylene fiber. One main issue with this is that the wicking and evaporating of the moisture is not noticeable to the wearer.
[0005] Accordingly, there is a need for better wicking and overall moisture control in clothing articles.
SUMMARY
[0006] In one aspect, capsules, such as microcapsules or macrocapsules, are disclosed that have a core composition comprising a phase changing material encapsulated in a polymer wall and having an outer shell formed of a siloxane chemically bonded to an exterior surface of the polymer wall by a surfactant. The surfactant is one or more of a cationic surfactant, an anionic surfactant, and a non-ionic surfactant, and the polymer wall comprises one or more of a melamine formaldehyde, a crosslinked melamine formaldehyde, a resorcinol urea formaldehyde, a poly-urea formaldehyde, an acrylic polymer, and a gelatin. The siloxane can form a crystalline outer shell or a sol-gel outer shell. When the capsules are microcapsules, the microcapsules may have a diameter of about 15 .mu.m to about 25 .mu.m. The capsules have an enthalpy of greater than 75 J/g as measured by differential scanning calorimetry.
[0007] In all embodiments, the PCM can be a C.sub.10-C.sub.40 hydrocarbyl and in some embodiments, the siloxane is NaAlSi.sub.2O.sub.6. The siloxane can form a crystalline outer shell or a sol-gel outer shell.
[0008] In one embodiment, the polymer wall is a crosslinked melamine formaldehyde and the cross-linked melamine comprises melamine formaldehyde polymerized with a crosslinking agent comprising:
[0009] (a) a reaction product of a cyclic urea (U) and a multifunctional aldehyde (A), and
[0010] (b) at least one crosslinker selected from the group consisting of [0011] (b1) reaction products of an aminotriazine and at least one aldehyde selected from the group consisting of aliphatic monoaldehydes and multifunctional aliphatic aldehydes having the structure Y(CHO).sub.n, where Y is an n-functional aliphatic residue, and n is greater than 1, where U is not dihydroxyethylene urea if the crosslinker (b) is (b1), [0012] (b2) reaction products of urea and/or cyclic ureas and formaldehyde, [0013] (b3) alkoxycarbonylaminotriazines, [0014] (b4) multifunctional isocyanates which may be partially or completely blocked, [0015] (b5) reaction products of phenols and aliphatic monoaldehydes, [0016] (b6) multifunctional epoxides, [0017] (b7) multifunctional aziridines, [0018] (b8) multifunctional carbodiimides, wherein any of the crosslinkers (a) and (b) which have hydroxyl groups may be etherified with one or more linear, branched, or cyclic aliphatic alcohols. The crosslinked melamine formaldehyde capsules have a free formaldehyde level of less than 100 pm.
[0019] In another aspect, textile fabrics are disclosed that have a plurality of the capsules disclosed herein incorporated therein, which may be present as a layer on a surface of the textile fabric at a thickness of about 1 mm to about 50 mm. The textile fabric may be bedding or an article of clothing. The plurality of capsules has an enthalpy that is greater than 75 J/g as measured by differential scanning calorimetry.
[0020] In another aspect, methods for producing an outer shell of a capsule, such as a microcapsule or macrocapsule, are disclosed. The method includes the provision of pre-formed capsules that have a core composition comprising a phase changing material encapsulated by a polymer wall, treating pre-formed capsules with a surfactant solution to form a surfactant-coated capsule (the surfactant is tethered to the exterior surface of the capsule), and treating the surfactant-coated capsule with a compound containing a siloxane functional group. The surfactant connects the compound containing the siloxane functional group to the exterior surface of the polymer wall and the compound containing the siloxane functional group forms an outer shell of the capsule. The compound containing a siloxane functional group may be one or more of sodium silicate, tetramethyl orthosilicate, and tetraethyl orthosilicate. In one embodiment, the surfactant solution is in an organic solvent and the outer shell is a sol-gel reaction product.
[0021] The surfactant solution is an aqueous solution, and the method further comprises, subsequent to treating with the compound containing the siloxane functional group, treating the surfactant-coated capsules with a metal-oxygen containing compound to form a crystalline outer shell. The metal-oxygen containing compound comprises one or more of sodium, aluminum, calcium, potassium, iron, manganese, or magnesium as an acetate, phosphate, sulfate, ethoxide, silicate, hydroxide, oxide hydroxide, nitrate, thiocyanate, chlorate, and/or nitrite.
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] FIG. 1 is an SEM image, 0.10 mm scale, of capsules having a melamine-formaldehyde polymer wall encapsulating a phase change material before addition of a sodium aluminum silicate crystalline shell.
[0023] FIG. 2 is an SEM image, 10 .mu.m scale, of the capsules of FIG. 1 after the addition of a sodium aluminum silicate (NaAlSi.sub.2O.sub.6) crystalline shell, which is tethered to the melamine-formaldehyde polymer wall.
[0024] FIG. 3 is a flow diagram of addition of the NaAlSi.sub.2O.sub.6 shell to pre-formed capsules, represented as a cross-sectional view.
[0025] FIG. 4 is a flow diagram of an alternate embodiment of addition of a crystalline silicate compound as an outer shell, represented as a cross-sectional view, to pre-formed capsules.
[0026] FIG. 5A is a FTIR spectra of the pre-formed PCM microcapsules (no tethered outer shell).
[0027] FIG. 5B is a FTIR spectra of NaAlSi.sub.2O.sub.6 coated PCM microcapsules.
[0028] FIG. 6 is an FTIR spectra of siloxane coated microcapsules.
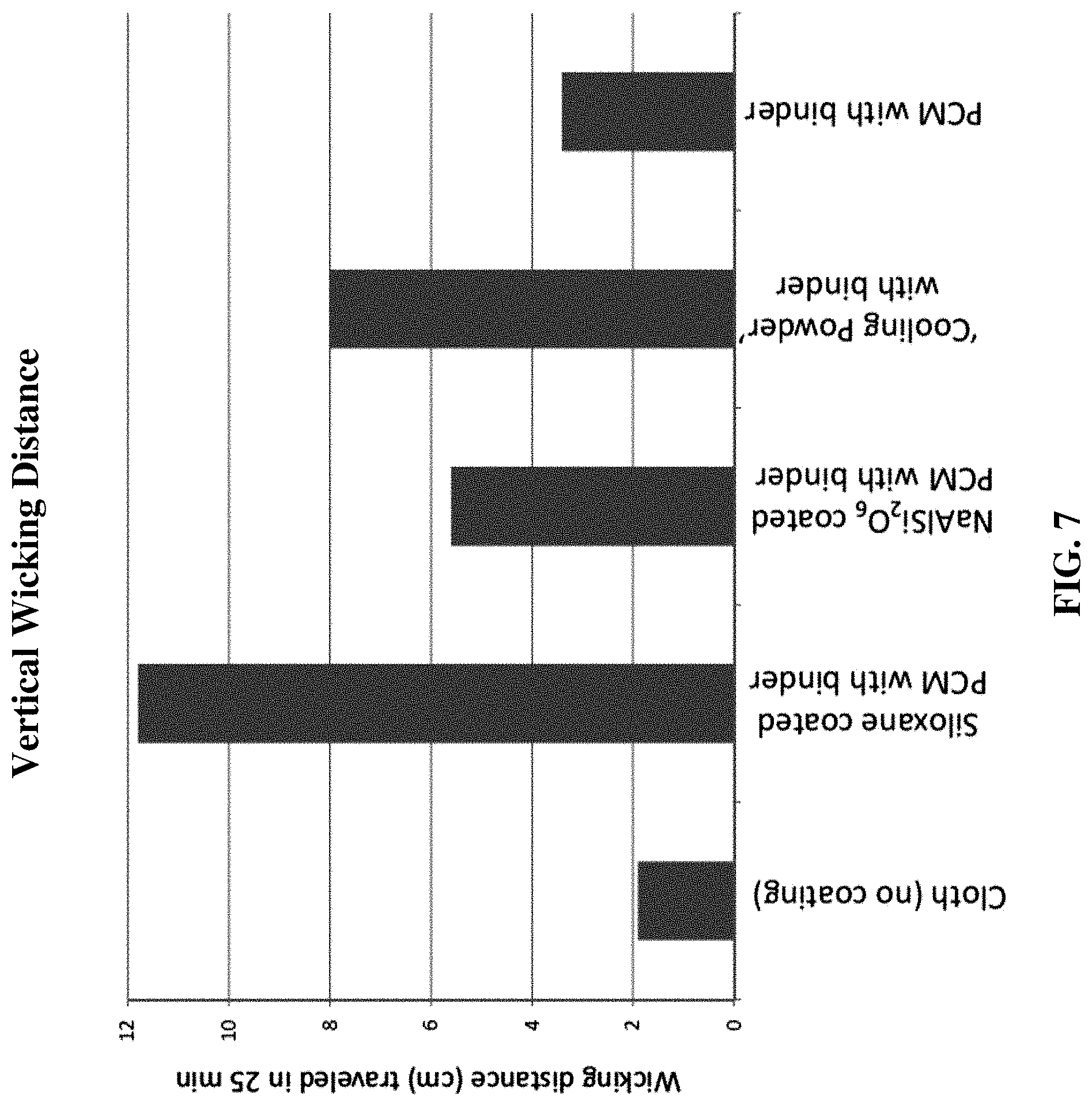
[0029] FIG. 7 is a bar graph comparing the vertical wicking distance of a cloth comprising inventive examples compared to existing wicking "cooling powder."
[0030] FIG. 8 is a bar graph comparing the heat absorbance (J/g) of inventive examples compared to existing wicking "cooling powder."
DETAILED DESCRIPTION
[0031] The following detailed description will illustrate the general principles of the invention, examples of which are additionally illustrated in the accompanying drawings. In the drawings, like reference numbers indicate identical or functionally similar elements.
[0032] Provided in this disclosure are capsules including a core encapsulated by a polymer wall, and an outer shell having a siloxane tethered to the exterior surface of the polymer wall, which renders the capsule capable of wicking moisture, such as water and/or sweat. The core of such capsules comprises a phase changing material, which enables the capsules to have a high heat of absorption. As such, the capsules are suitable for inclusion in a textile material or fabric to cool a wearer of an article of clothing made therefrom and to pull moisture away from the wearer's body. To attach the siloxane to the exterior of the pre-formed capsules, a surfactant acts as a tether to connect the crystalline silicate compound to the exterior surface of the polymer wall, typically through electrostatic interactions, i.e., a chemical bond. For example, a hydrophobic portion of a surfactant can interact with the polymer wall via hydrophobic association.
[0033] As used herein, the term "about" allows a degree of variability in a value or range, for example, within 10% of a stated value or of a stated limit of a range for all embodiments, but within 5% of a stated value or of a stated limit of a range in more preferred embodiments.
[0034] As used herein, the term "hydrocarbon" refers to a functional group or molecule that includes carbon and hydrogen atoms. The term can also refer to a functional group or molecule that normally includes both carbon and hydrogen atoms but wherein some or all the hydrogen atoms are substituted with other functional groups.
[0035] As used herein, the term "substituted" refers to an organic group as defined in which one or more hydrogen atoms contained therein are replaced by one or more substituents. The term "functional group" or "substituent" as used herein refers to a group that can be or is substituted onto a molecule or onto an organic group. Examples of substituents or functional groups include, but are not limited to, a halogen (e.g., F, Cl, Br, and I); an oxygen atom in groups such as hydroxy groups, alkoxy groups, aryloxy groups, aralkyloxy groups, oxo(carbonyl) groups, carboxyl groups including carboxylic acids, carboxylates, and carboxylate esters; a sulfur atom in groups such as thiol groups, alkyl and aryl sulfide groups, sulfoxide groups, sulfone groups, sulfonyl groups, and sulfonamide groups; a nitrogen atom in groups such as amines, hydroxyamines, nitriles, nitro groups, N-oxides, hydrazides, azides, and enamines; and other heteroatoms in various other groups.
[0036] Referring to FIGS. 1 and 2, SEM image of capsules are shown. FIG. 1 comprises capsules having a melamine-formaldehyde polymer wall encapsulating a phase change material before addition of a sodium aluminum silicate (NaAlSi.sub.2O.sub.6) crystalline shell. FIG. 2 comprises the same capsules after addition of the NaAlSi.sub.2O.sub.6 crystalline shell.
[0037] Referring to FIG. 3, capsule 200c has an outer NaAlSi.sub.2O.sub.6 shell 206 surrounding a polymer wall 204 encapsulating a PCM 202 as the core. The NaAlSi.sub.2O.sub.6 shell 206 is typically an outermost shell or coating, but in some embodiments another coating can be applied thereto. For example, an adhesive coating (e.g., a discontinuous adhesive coating) can be applied to allow the capsules to adhere to a surface. The capsule 200c having the NaAlSi.sub.2O.sub.6 shell 206 is formed from a preformed capsule 200 that has one or more PCMs 202 as the core encapsulated within the polymer wall 204. Polymer wall 204 is used as a scaffold on which a surfactant 208 is tethered, in FIG. 3 a cationic surfactant, and the surfactant 208 tethers the NaAlSi.sub.2O.sub.6 shell 206 to the exterior surface 205 of the polymer wall 204.
[0038] As shown in FIG. 3, capsule 200 is treated with a cationic surfactant to form surfactant-coated capsule 200a. The surfactant-coated capsule 200a is then treated with a sodium silicate 213 to form sodium silicate-coated capsule 200b. The silicate-coated capsule 200b is then treated with an aluminum complex 214 containing oxygen, specifically nano-boehmite in this example, to form a NaAlSi.sub.2O.sub.6 shell 206. The nano-boehmite is dissolved in water, such as deionized water, and the NaAlSi.sub.2O.sub.6 shell 206 formed may be a continuous (i.e., a full, endless coating) or a discontinuous (partial) shell. The polymer wall 204, PCM 202, and surfactant 208 can be any polymer wall, PCM, or cationic surfactant described herein.
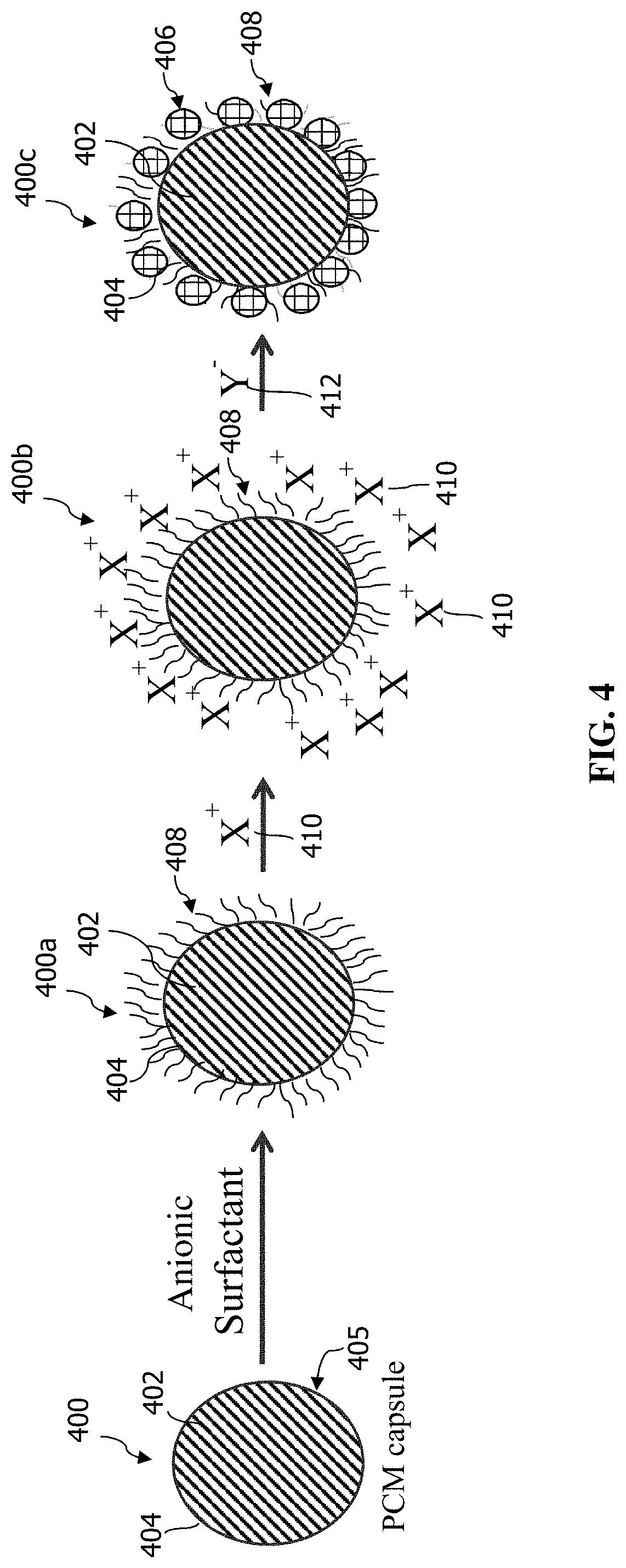
[0039] Referring to FIG. 4, in another embodiment, the outer shell, here 406, may be formed on the exterior surface 405 of the polymer wall 404 of a pre-formed capsule 400 containing a core composition 402 by introducing an anionic surfactant 408 to form the surfactant-containing intermediate capsule 400a. The surfactant 408 is introduced as an aqueous solution. The pre-formed capsule 400 can be added to the surfactant solution (or vice versa) with stirring and heating (if desired) for sufficient time to allow the surfactant 408 to tether to the polymer wall 404 thereof. After the surfactant 408 is applied to the polymer wall 404, the surfactant-containing intermediate capsule 400a is treated with a metal-oxygen containing compound to form a secondary intermediate capsule 400b. For example, the metal-oxygen containing compound (such as boehmite) can be added to an aqueous solution of surfactant-containing intermediate capsule 400a and stirred for a sufficient time to allow the compound to chemically bond to the surfactant 408. The secondary intermediate capsule 400b is then treated with a compound containing a siloxane functional group 412 to form a crystalline, silicate outer shell 406. The compound containing a siloxane functional group 412 is dissolved in water, such as deionized water, and the crystalline outer shell 406 formed may be a continuous (i.e., a full, endless coating) or a discontinuous (partial) shell.
[0040] While FIGS. 3 and 4 are described as starting with pre-formed capsules, the methods herein may further include forming the capsules. The capsules can be formed by encapsulating a PCM in a polymer shell using known methods of encapsulations such as coacervation, polymer-polymer phase separation, interfacial and dispersion polymerization, in-situ polymerization, solvent phase separation, desolvation, solvent evaporation, spray drying, spray chilling, matrix encapsulation, various types of fluid bed, extrusion, and various hybrid systems. The PCM and polymer shell can be any PCM and polymer shell described herein.
[0041] As discussed above with respect to the example set forth in FIG. 3, the outer shell comprising the siloxane may form a crystalline outer shell; see resultant capsules 200c in FIG. 3 and the SEM image in FIG. 2. When the method of making the capsules includes aqueous solutions for the surfactant and the compound containing a siloxane functional group and a metal-oxygen containing compound is used to precipitate the siloxane as a silicate (or vice versa), the outer shell will be crystalline. In other embodiments, as demonstrated in working example 5, the outer shell comprising the siloxane may form a sol-gel product, which is not crystalline. Here, the solutions have organic solvents and, after the surfactant has been tethered to the exterior surface of the polymer wall, a silicate is added, and adequate time is provided for the silicate to be chemically bonded to the surfactant. Then, a strong base is added to catalyze the reaction of the silicate to form the outer shell.
[0042] Each PCM in the core can include a substituted or unsubstituted, saturated or unsaturated C.sub.10-C.sub.40 hydrocarbon, particularly a C.sub.10-C.sub.40 hydrocarbon having a melting point of about -30.degree. C. to about 70.degree. C., and mixtures thereof. In some embodiments, the PCM can be an ester, an alcohol, a carboxylic acid, a salt hydrate, an ether, or a mixture thereof. The PCM of the core can be selected for the purpose having a melting point that would be comfortable to the wearer. Some preferred embodiments include a C.sub.15-C.sub.25 hydrocarbon or mixtures thereof, since these hydrocarbons have melting points in a range that is comfortable to a wearer of an article of clothing having the capsules disclosed herein incorporated therein.
[0043] Examples of saturated or unsaturated C.sub.10-C.sub.40 hydrocarbons, which are branched or linear, include, but are not limited to, n-tetradecane, n-pentadecane, n-hexadecane, n-heptadecane, n-octadecane, n-nonadecane, n-eicosane, n-heneicosane, n-docosane, n-tricosane, n-tetracosane, n-pentacosane, n-hexacosane, n-heptacosane, and n-octacosane. Examples of cyclic hydrocarbons include, but are not limited to, cyclohexane, cyclooctane, and cyclodecane. Examples of aromatic hydrocarbyl compounds include, but are not limited to, benzene, naphthalene, biphenyl, and o- or n-terphenyl. Examples of C.sub.10-C.sub.40-alkyl-substituted aromatic hydrocarbons include, but are not limited to, dodecylbenzene, tetradecylbenzene, hexadecylbenzene, hexylnaphthalene or decyinaphthalene. Examples of saturated or unsaturated C.sub.10-C.sub.30-fatty acids include, but are not limited to, lauric, stearic, oleic or behenic acid, and eutectic mixtures of decanoic acid with myristic, palmitic or lauric acid. Examples of fatty alcohols include, but are not limited to, lauryl, stearyl, oleyl, myristyl, cetyl alcohol, mixtures such as coconut fatty alcohol, and the so-called oxo alcohols which are obtained by hydroformylation of .alpha.-olefins and further reactions. Examples of C.sub.a-alkyl esters include, but are not limited to, C.sub.1-C.sub.10-alkyl esters of fatty acids, such as propyl palmitate, methyl stearate or methyl palmitate, and their eutectic mixtures or methyl cinnamate. Examples of natural and synthetic waxes include, but are not limited to, montan acid waxes, montan ester waxes, polyethylene wax, oxidized waxes, polyvinyl ether wax, and ethylene vinyl acetate wax. In some embodiments, the PCM is a paraffin wax.
[0044] In all embodiments, the PCM can include one or more of docosane, docosene, eicosane, heneicosane, heptadecane, hexadecane, nonadecane, octadecane, tetracosane, and tricosane. In some embodiments, the core composition includes octadecane or is octadecane.
[0045] The core composition can have a melting point of about -30.degree. C. to about 70.degree. C. For textile materials suitable for articles of clothing a melting point in a range that is comfortable to the wearer is preferred. Accordingly, a core composition with a melting point of 15.degree. C. to about 40.degree. C. or more particularly about 25.degree. C. to about 35.degree. C. is useful. If the capsules are intended to be incorporated in a material or fabric to be used in cold environments, a PCM, or mixture of PCMs, with a lower melting point can be selected, for example about -20.degree. C. to about 5.degree. C. If the capsules are intended to be incorporated in a fabric to be used in hot environments, a PCM, or mixture of PCMs, with a higher melting point can be selected, for example about 25.degree. C. to about 45.degree. C.
[0046] In all embodiments, the capsules typically have a relatively high payload of a core composition relative to the amount of material forming the polymer wall and the outer shell. The core in the capsules can be about 10% to about 90% by weight of the capsule. In some embodiments, the PCM is about 70% to about 80% by weight, more particularly about 75% to about 85%, and even more particularly about 77% to about 81% by weight of the capsule. In some embodiments, the PCM is at least 50% by weight of capsule, more particularly at least 70%, and even more particularly at least 80% by weight of the capsule.
[0047] The polymer wall can include a melamine formaldehyde, a crosslinked melamine formaldehyde, a resorcinol urea formaldehyde, a poly-urea formaldehyde, an acrylic polymer, a gelatin, or a mixture thereof, or another known wall material made using known methods such as in-situ polymerization, interfacial polycondensation, interfacial cross-linking, or any other known method. In some embodiments, the polymer wall includes crosslinked melamine formaldehyde.
[0048] The polymer wall and capsule can have low levels of formaldehyde. For example, melamine formaldehyde walled microcapsules can have levels of free formaldehyde around 200 ppm, however, a crosslinked melamine formaldehyde walled microcapsule (as discussed in detail below) can have free formaldehyde levels of less than about 100 pm, less than about 50 ppm, less than about 20 ppm, or less than about 10 ppm.
[0049] The in-situ polymerization process of polycondensation can prepare capsules having a melamine formaldehyde polymer wall and a PCM core. The process can include a melamine formaldehyde prepolymer, soluble in a continuous water phase, and a hydrophobic PCM, as dispersed core droplets. As the polymerization reaction starts in the aqueous solution, the formed oligomers start to collapse on the surface of the core droplets. On the surface, polymerization continues, and crosslinking occurs that results in the formation of a solid melamine formaldehyde wall.
[0050] Capsules having a gelatin wall encapsulating a core material are known, as taught in Onder et al. Encapsulation of Phase Change Materials by Complex Coacervation to Improve Thermal Performances of Woven Fabrics, Thermochimica Acta. 2008, 467, 63-72, and in Patrick et al. Optimization Process by Complex Coacervation of Fish Oil Using Gelatin/SDS/NaCMC and Secondary Coating Application with Sodium Polyphosphate, IJSBAR. 2014, 17, 74-94.
[0051] For a crosslinked melamine microcapsule, reference is made to co-pending U.S. Provisional Application No. 62/206,367 for methods of making the microcapsule, which is incorporated herein by reference. These microcapsules are made from a melamine formaldehyde prepolymer comprising a crosslinking agent, the crosslinking agent being a mixture of:
[0052] (a) a reaction product of a cyclic urea (U) and a multifunctional aldehyde (A), and
[0053] (b) at least one crosslinker selected from the group consisting of [0054] (b1) reaction products of an aminotriazine and at least one aldehyde selected from the group consisting of aliphatic monoaldehydes and multifunctional aliphatic aldehydes having the structure Y(CHO).sub.n, where Y is an n-functional aliphatic residue, and n is greater than 1, where U is not dihydroxyethylene urea if the crosslinker (b) is (b1), [0055] (b2) reaction products of urea and/or cyclic ureas and formaldehyde, [0056] (b3) alkoxycarbonylaminotriazines, [0057] (b4) multifunctional isocyanates which may be partially or completely blocked, [0058] (b5) reaction products of phenols and aliphatic monoaldehydes, [0059] (b6) multifunctional epoxides, [0060] (b7) multifunctional aziridines, [0061] (b8) multifunctional carbodiimides, wherein any of the crosslinkers (a) and (b) which have hydroxyl groups may be etherified with one or more linear, branched, or cyclic aliphatic alcohols, polymerized by adjusting the pH and/or addition of urea. The crosslinking agent (b) is preferably at least one crosslinker selected from the group consisting of (b1), (b2), (b3), and (b5). These cross-linked melamine microcapsules have MF prepolymer present in a ratio by weight percent to the crosslinking agent of 1:1 to 4:1, more preferably 1.5:1 to 3.75:1. These capsules have an initial free formaldehyde level of less than 100 ppm, more preferably less than 80 ppm, less than 60 ppm, and even more preferably less than 40 ppm. Such a crosslinking agent is available from Allnex USA Inc.
[0062] In one embodiment, the crosslinking agent has the reaction product of a cyclic urea U and a multifunctional aliphatic aldehyde A, portion (a), in a mixture with one or more of (b1), (b2), (b3) and (b5). Mixtures of the reaction product of a cyclic urea (U) and a multifunctional aldehyde (A) and one or more of the crosslinkers (b) have a ratio of the mass of the reaction product to the mass of the crosslinker (b) (or to the sum of the masses of all crosslinkers (b)) from 1/99 to 99/1, preferably from 10/90 to 90/10, and more preferably from 30/70 to 70/30.
[0063] The multifunctional aldehyde A has the formula OHC--R'--CHO where R' may be a direct bond or a divalent radical which may preferably be a linear, branched or cyclic aliphatic radical and may have from one to twenty carbon atoms, both these options for R' leading to a divalent aldehyde having exactly two --CHO groups, or an aliphatic divalent radical which may be linear, branched or cyclic and may have from one to twenty carbon atoms, which radical carries at least one additional aldehyde group --CHO, which latter option leads to trivalent or polyvalent aldehydes having at least three aldehyde groups. Preferred aldehydes are divalent aliphatic aldehydes, particularly glyoxal, malonic dialdehyde, succinic dialdehyde, and glutaric dialdehyde. Especially preferred is glyoxal in an aqueous solution, as anhydrous solid which has to be cooled as its melting temperature is 15.degree. C., or in the form of its dimer or trimer, optionally in solid hydrated form as dihydrates, or in the form of its addition products with sulphites or hydrogen sulphites which decompose under acidic conditions.
[0064] The cyclic ureas U which may be used according to the present invention have at least one unsubstituted amidic --NH group. These cyclic ureas are cycloaliphatic or bicycloaliphatic compounds having an element of the structure --NH--CO--NH-- within a ring structure, the total number of ring atoms preferably being from 5 to 7 (ethylene urea, 1,2-propylene urea, 1,3-propylene urea, 1,4-butylene urea or tetramethylene urea). Particularly preferred is ethylene urea or a mixture comprising ethylene urea, especially a mixture comprising at least a mass fraction of 50% of ethylene urea. In the case of a bicyclic compound, the simplest structure is glycoluril or acetylene diurea. Hydroxy functional ureas are not useful for the present invention. The cyclic ureas may be substituted, preferably by alkyl groups on the N-- or C-atoms, or both, the alkyl residues preferably having from one to four carbon atoms. At least one of the nitrogen atoms must remain unsubstituted to enable reaction with the aldehyde functional molecule. Preferably, at least one cyclic urea is selected from the group consis
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.