Method For Producing Noble Metal Nanocomposites
ALSHATWI; ALI A. ; et al.
U.S. patent application number 16/714436 was filed with the patent office on 2020-04-16 for method for producing noble metal nanocomposites. The applicant listed for this patent is KING SAUD UNIVERSITY. Invention is credited to ALI A. ALSHATWI, Jegan Athinarayanan, Vaiyapuri Subbarayan Periasamy.
| Application Number | 20200115802 16/714436 |
| Document ID | / |
| Family ID | 60039451 |
| Filed Date | 2020-04-16 |









View All Diagrams
| United States Patent Application | 20200115802 |
| Kind Code | A1 |
| ALSHATWI; ALI A. ; et al. | April 16, 2020 |
METHOD FOR PRODUCING NOBLE METAL NANOCOMPOSITES
Abstract
The method for producing noble metal nanocomposites involves reducing noble metal ions (Ag, Au and Pt) on graphene oxide (GO) or carbon nanotubes (CNT) by using Artocarpus integer leaves extract as a reducing agent. As synthesized MNPs/GO and MNPs/CNT composites have been characterized using X-ray diffraction (XRD), transmission electron microscope (TEM) imaging, and energy dispersive X-ray spectroscopy (EDX). The TEM images of prepared materials showed that the nanocomposites were 1-30 nm in size with spherical nanoparticles embedded on the surface of GO and CNT. This synthetic route is easy and rapid for preparing a variety of nanocomposites. The method avoids use of toxic chemicals, and the prepared nanocomposites can be used for biosensor, fuel cell, and biomedical applications.
| Inventors: | ALSHATWI; ALI A.; (Riyadh, SA) ; Athinarayanan; Jegan; (Riyadh, SA) ; Periasamy; Vaiyapuri Subbarayan; (Riyadh, SA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 60039451 | ||||||||||
| Appl. No.: | 16/714436 | ||||||||||
| Filed: | December 13, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 16001112 | Jun 6, 2018 | |||
| 16714436 | ||||
| 15474760 | Mar 30, 2017 | 10106895 | ||
| 16001112 | ||||
| 14666307 | Mar 24, 2015 | |||
| 15474760 | ||||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C23C 18/1639 20130101; B82Y 30/00 20130101; C01B 32/174 20170801; C23C 18/1635 20130101; Y10S 977/892 20130101; C23C 18/44 20130101; C23C 18/31 20130101; Y10S 977/847 20130101; Y10S 977/81 20130101; Y10S 977/948 20130101; B82Y 15/00 20130101; C23C 18/1662 20130101; Y10S 977/748 20130101; B82Y 40/00 20130101; C23C 18/1658 20130101; H01B 1/02 20130101; Y10S 977/92 20130101; B82Y 5/00 20130101; C23C 18/1882 20130101; Y10S 977/904 20130101 |
| International Class: | C23C 18/44 20060101 C23C018/44; C01B 32/174 20060101 C01B032/174; C23C 18/16 20060101 C23C018/16; C23C 18/31 20060101 C23C018/31; C23C 18/18 20060101 C23C018/18 |
Claims
1-17. (canceled)
18. A noble metal nanocomposite, comprising: a composite of nanoparticles of noble metal embedded in a graphene oxide, wherein the nanoparticles have a particle size between 1 nm and 30 nm and the noble metal is selected from the group consisting of platinum and gold.
19. The noble metal nanocomposite according to claim 18, wherein the noble metal was reduced on the graphene oxide by using an extract of Artocarpus integrifolia leaves as a reducing agent.
20. The noble metal nanocomposite according to claim 18, wherein the noble metal is platinum and the nanoparticles have a particle size between 1 nm and 3 nm.
21. The noble metal nanocomposite according to claim 18, wherein the noble metal is gold and the nanoparticles have a particle size between 10 nm and 20 nm.
22. The noble metal nanocomposite according to claim 18, wherein the nanoparticles are substantially spherical.
Description
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application is a division of U.S. application Ser. No. 16/001,112, filed Jun. 6, 2018, pending, which is division of U.S. application Ser. No. 15/474,760, filed Mar. 30, 2017, now U.S. Pat. No. 10,106,895, issued Oct. 23, 2018, which is a continuation-in-part of U.S. application Ser. No. 14/666,307 filed Mar. 24, 2015, abandoned.
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0002] The present invention relates to composites, and particularly to a method for producing noble metal nanocomposites that have noble metal nanoparticles (MNP) embedded in a carbon-based substrate of graphene oxide (GO) or carbon nanotubes (CNT).
2. Description of the Related Art
[0003] Noble metal nanoparticles have gained remarkable attention due to their excellent physical, chemical and biological properties. On the other hand, carbon-based nano-materials, including graphene oxide (GO) sheets and carbon nanotubes (CNT), are promising supporting materials for noble metal nanoparticles to produce new nanocomposites that can be used in a wide variety of applications because of their distinctive electronic, thermal, and mechanical properties. Currently, the search for synthetic routes for embedding metal nanoparticles on carbon-based materials is a rapidly growing research area in nanoscience and nanotechnology. So far, there have been a number of attempts to carry out a synthesis of noble metal nanoparticles embedded graphene oxide and carbon nanotubes, including chemical reduction, electrochemical, thermal decomposition, ultraviolet and microwave irradiation. However, these methods use hazardous chemicals, high pressure, energy, and temperatures that lead to environmental pollution. Green synthesis is a most promising method for metal nanoparticles (MNPs) synthesis that is considered cost effective, simple, rapid, and eco-friendly, since it does not require toxic chemicals. We have developed a new synthetic route for synthesis of MNPs on GO/CNT. These synthesized samples can be used for biosensors, fuel cells, and biomedical applications.
[0004] Thus, a method for producing noble metal nanocomposites solving the aforementioned problems is desired.
SUMMARY OF THE INVENTION
[0005] The method for producing noble metal nanocomposites involves reducing noble metal ions (Ag, Au and Pt) on graphene oxide (GO) or carbon nanotubes (CNT) by using Artocarpus integer (champedak) leaves extract as a reducing agent. As synthesized MNPs/GO and MNPs/CNT composites have been characterized using X-ray diffraction (XRD), transmission electron microscope (TEM) imaging, and energy dispersive X-ray spectroscopy (EDX). The TEM images of prepared materials showed that the nanocomposites were 1-30 nm in size with spherical nanoparticles embedded on the surface of GO and CNT. This synthetic route is easy and rapid for preparing a variety of nanocomposites. The method avoids use of toxic chemicals, and the prepared nanocomposites can be used for biosensor, fuel cell, and biomedical applications.
[0006] These and other features of the present invention will become readily apparent upon further review of the following specification and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] FIG. 1A is a TEM image of a Pt-CNT nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0008] FIG. 1B is a TEM image of the same Pt-CNT nanocomposite sample shown in FIG. 1A, but at higher magnification.
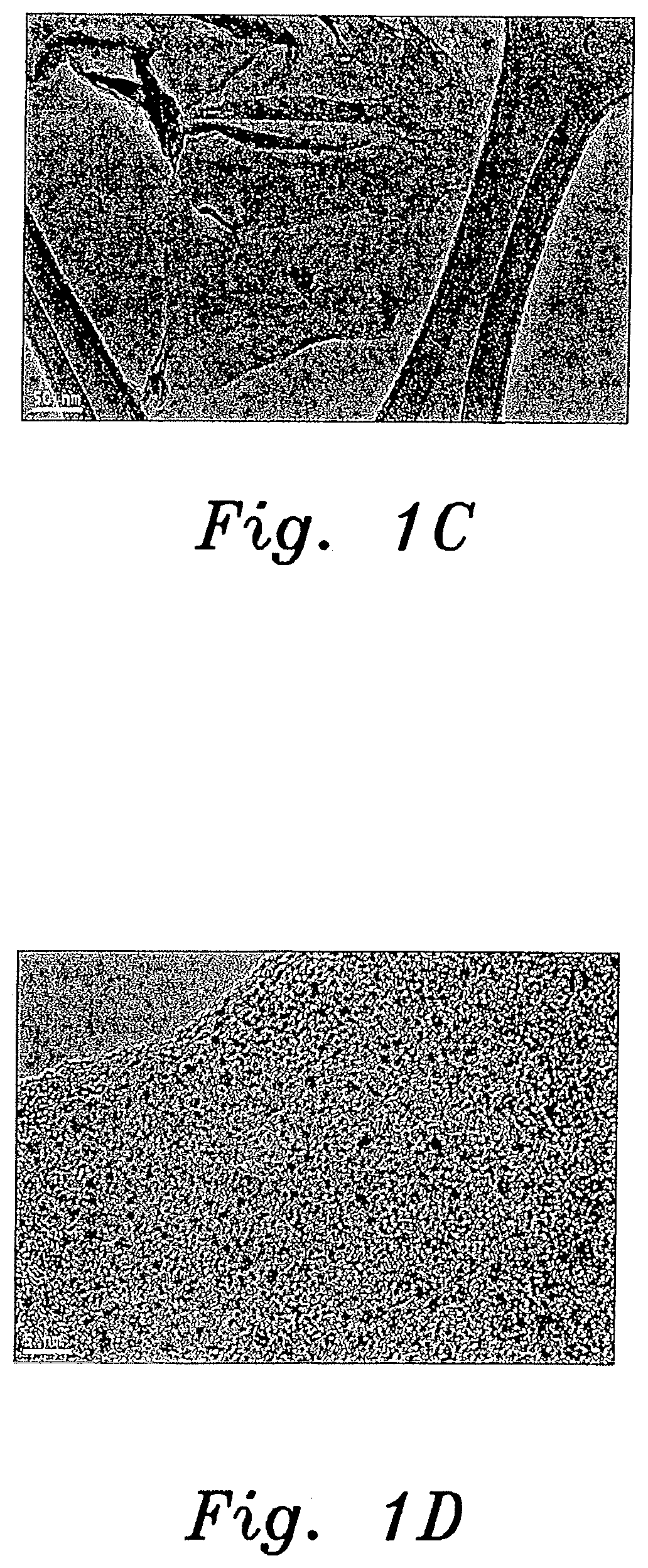
[0009] FIG. 1C is a TEM image of a Pt-GO nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0010] FIG. 1D is a TEM image of the same Pt-GO nanocomposite sample shown in FIG. 1C, but at higher magnification.
[0011] FIG. 2A is an EDX spectrum of the Pt-CNT nanocomposite sample shown in FIGS. 1A and 1B.
[0012] FIG. 2B is an EDX spectrum of the Pt-GO nanocomposite sample shown in FIGS. 1C and 1D.
[0013] FIG. 3A is a TEM image of an Au-CNT nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0014] FIG. 3B is a TEM image of the same Au-CNT nanocomposite sample shown in FIG. 3A, but at higher magnification.
[0015] FIG. 3C is a TEM image of an Au-GO nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0016] FIG. 3D is a TEM image of the same Au-GO nanocomposite sample shown in FIG. 3C, but at higher magnification.
[0017] FIG. 4A is an EDX spectrum of the Au-CNT nanocomposite sample shown in FIGS. 3A and 3B.
[0018] FIG. 4B is an EDX spectrum of the Au-GO nanocomposite sample shown in FIGS. 3C and 3D.
[0019] FIG. 5A is a TEM image of an Ag-CNT nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0020] FIG. 5B is a TEM image of the same Ag-CNT nanocomposite sample shown in FIG. 5A, but at higher magnification.
[0021] FIG. 5C is a TEM image of an Ag-GO nanocomposite sample prepared by a method for producing noble metal nanocomposites according to the present invention.
[0022] FIG. 5D is a TEM image of the same Ag-GO nanocomposite sample shown in FIG. 5C, but at higher magnification.
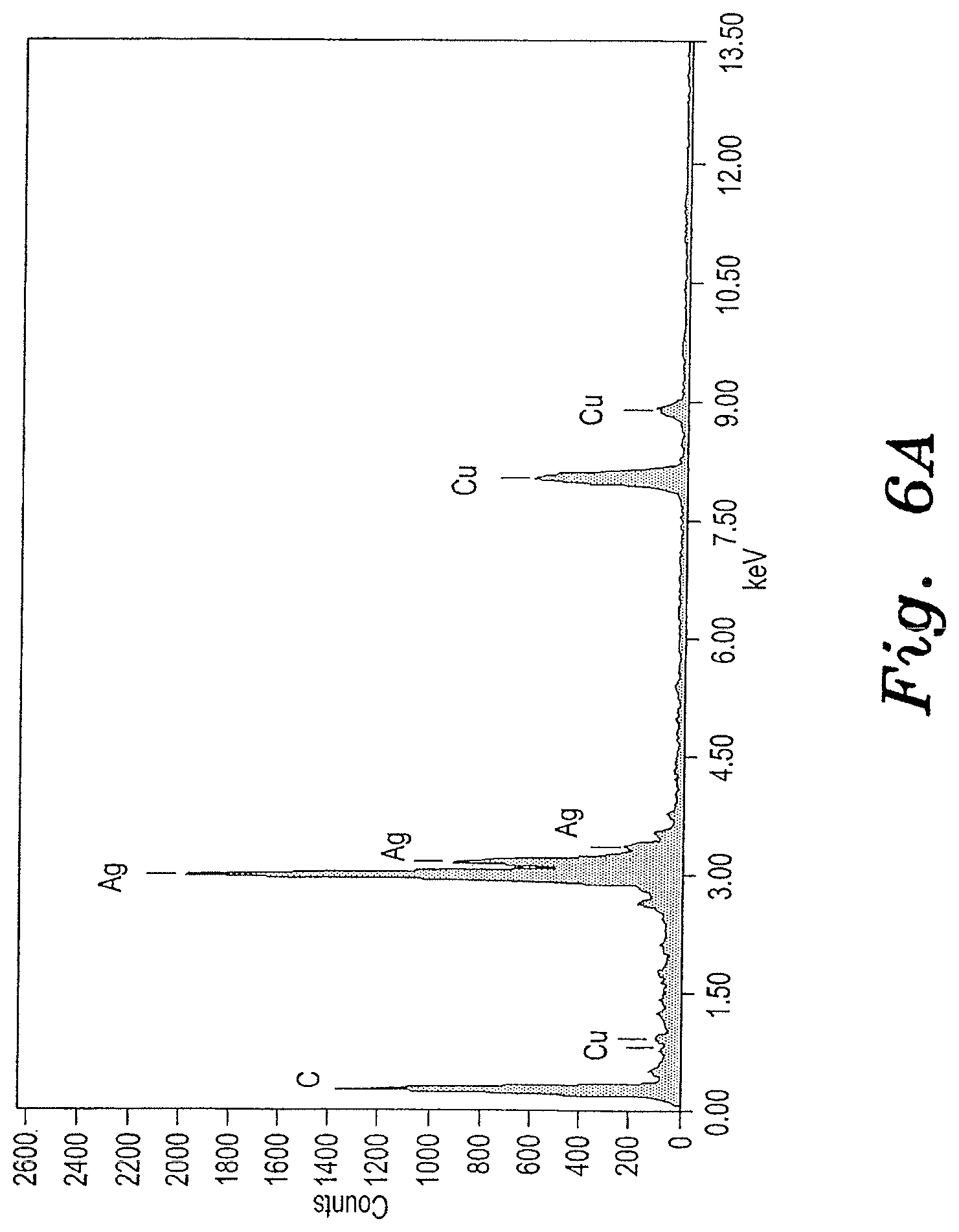
[0023] FIG. 6A is an EDX spectrum of the Ag-CNT nanocomposite sample shown in FIGS. 5A and 5B.
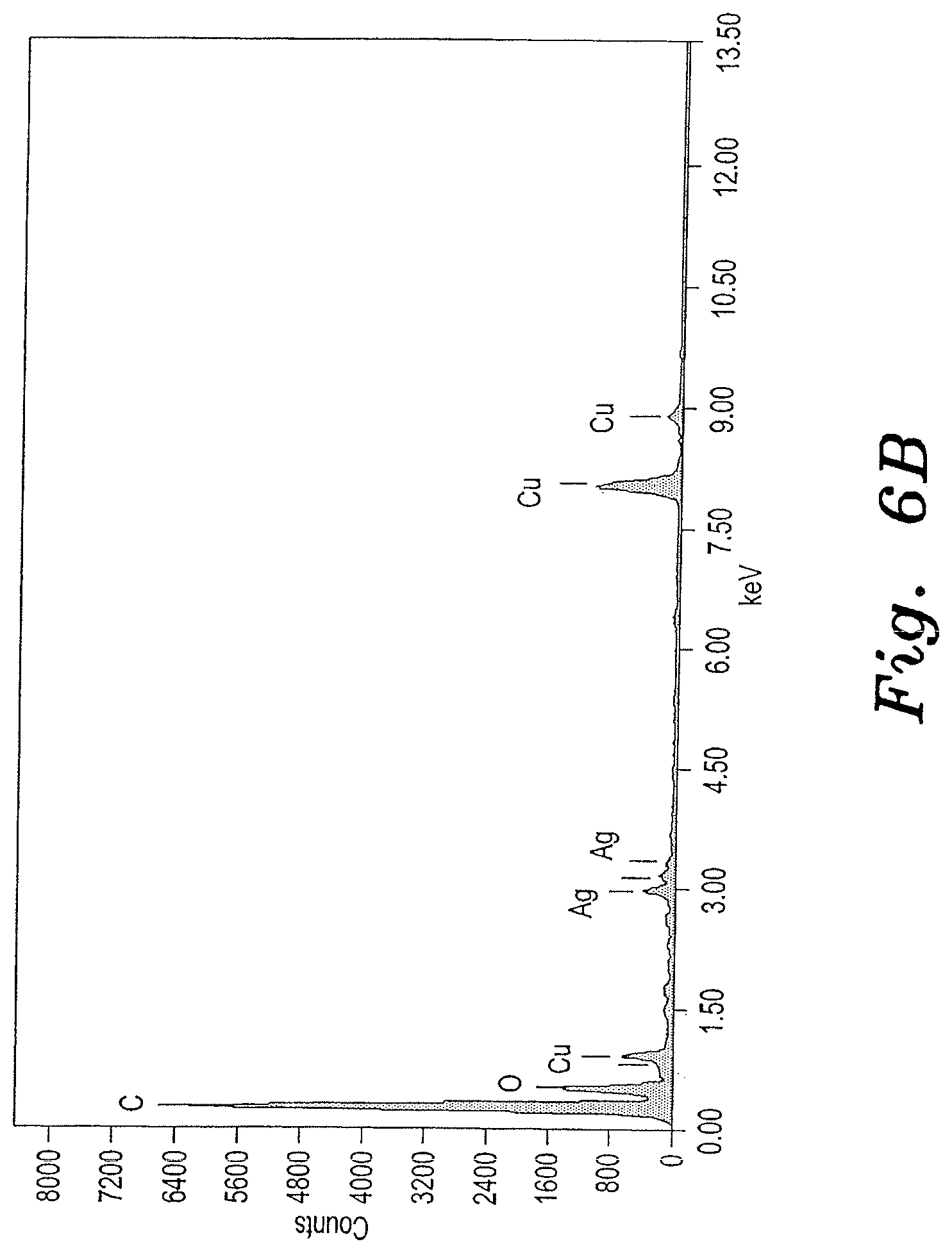
[0024] FIG. 6B is an EDX spectrum of the Ag-GO nanocomposite sample shown in FIGS. 5C and 5D.
[0025] Similar reference characters denote corresponding features consistently throughout the attached drawings.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0026] The method for producing noble metal nanocomposites involves reducing noble metal ions (Ag, Au and Pt) on graphene oxide (GO) or carbon nanotubes (CNT) by using Artocarpus integer (champedak) leaves extract as a reducing agent. As synthesized MNPs/GO and MNPs/CNT composites have been characterized using X-ray diffraction (XRD), transmission electron microscope (TEM) imaging, and energy dispersive X-ray spectroscopy (EDX). The TEM images of prepared materials showed that the nanocomposites were 2-20 nm in size with spherical nanoparticles embedded on the surface of GO and CNT. This synthetic route is easy and rapid for preparing a variety of nanocomposites. The method avoids use of toxic chemicals, and the prepared nanocomposites can be used for biosensor, fuel cell, and biomedical applications.
[0027] In the following examples, leaves of Artocarpus integer were collected from the Kanyakumari Dist., Tamil Nadu (India). Graphite and CNT were purchased from S.D.Fine, Inida and Sigma, USA respectively. Milli Q water was used throughout the experiments.
[0028] Freshly harvested A. integer leaves were washed several times with deionized water. About 10 g of leaves were finely chopped and stirred in 200 ml of double-distilled water at 95.degree. C. for 5 min and filtered using a Whatman #1 filter paper to obtain the leaf extract. The filtrate was used as the reducing agent.
[0029] Graphene oxide was synthesized from graphite by modified Hummers method. Briefly, 1.0 g graphite powder was dispersed in 24 mL concentrated H.sub.2SO.sub.4 under stirring at 0.degree. C. Subsequently, 3.0 g of KMnO.sub.4 was added gradually to the mixture and kept in an ice bath. The mixture was stirred for 30 min. The mixture was diluted gradually with 45 mL Milli-Q water. The mixture was re-diluted with 140 mL Milli-Q water and treated with drop-wise addition of 3% hydrogen peroxide. The color of the mixture changed to yellow-brown during the drop-wise addition of H.sub.2O.sub.2. The mixture was filtered and washed with HCl solution (5%) and then repeatedly washed with water. Finally, the dark brown graphene oxide (GO) powder was obtained through drying at 50.degree. C. in a vacuum oven.
[0030] Functionalized MWCNT (multi-wall carbon nanotubes) were prepared by brutal oxidation using an H.sub.2SO.sub.4--HNO.sub.3 mixture (3:1 v/v ratio). About 1 g of MWCNT was refluxed with 100 ml of the acid mixture at 120.degree. C. for 6 h. After cooling, the reaction mixture was diluted with 500 ml of Milli-Q water and filtered through vacuum filtration. The obtained product was washed several times with Milli-Q water until the acid was removed. The functionalized MWCNT were used for further experiments.
[0031] To obtain platinum nanocomposites, about 20 mg of either GO or the functionalized MWCNT was dispersed in 20 ml Milli-Q water under sonication for 30 minutes. About 5 ml of 1.times.10.sup.-2 M H.sub.2PtCl.sub.6 solution was added drop-wise in GO or the functionalized MWCNT separately under stirring. Following that, the mixture was kept at room temperature for aging and GO-Pt.sup.+ or MWCNT-Pt.sup.+ complex formation. Excess metal ions of GO-Pt.sup.+ or MWCNT-Pt.sup.+ mixture were removed by centrifugation. Then 5 ml of the leaves broth (extract) was added to the obtained GO-metal complex or MWCNT-metal complex and mixed well. After 15 minutes incubation, the samples were used for further physico-chemical characterization.
[0032] To obtain gold nanocomposites, about 20 mg GO or the functionalized MWCNT was dispersed in 20 ml Milli-Q water under sonication for 30 minutes. About 5 ml of 1.times.10.sup.-2 M HAuCl.sub.4 solution was added drop-wise in GO or the functionalized MWCNT separately under stirring. Following that, the mixture was kept at room temperature for aging and GO-Au.sup.+ or MWCNT-Au.sup.+ complex formation. Excess metal ions of the GO-Au.sup.+ or the MWCNT-Au.sup.+ mixture was removed by centrifugation. Then, about 5 ml of the leaves broth (extract) was added to the obtained GO-metal complex or the MWCNT-metal complex and mixed well. After 15 minutes incubation, the samples were used for further physico-chemical characterization.
[0033] To obtain silver nanocomposite, about 20 mg GO or the functionalized MWCNT was dispersed in 20 ml Milli-Q water under sonication for 30 minutes. About 5 ml of 1.times.10.sup.-2 M AgNO.sub.3 solution was added drop-wise in the GO or the functionalized MWCNT separately under stirring. Following that, the mixture was kept at room temperature for aging and GO-Ag.sup.+ or MWCNT-Ag.sup.+ complex formation. Excess metal ions of the GO-Ag.sup.+ or the MWCNT-Ag.sup.+ mixture were removed by centrifugation. Then, 5 ml of the leaves broth (extract) was added to the obtained GO-metal complex or the MWCNT-metal complex and mixed well. After 15 minutes incubation, the samples were used for further physico-chemical characterization.
[0034] Chemical compositions of prepared noble metal nanocomposites were characterized by using Energy Dispersive X-ray analysis (EDAX or EDX). See FIGS. 2A, 2B, 4A, 4B, 6A and 6B. The crystalline nature of the prepared samples was analyzed using X-ray diffraction (XRD). The surface morphology, particle size and diameter of the prepared materials were characterized by using Transmission electron microscope (TEM) (JEOL, JEM2100, Japan). See FIGS. 1A, 1B, 1C, 1D, 3A, 3B, 3C, 3D, 5A, 5B, 5C and 5D.
[0035] The crystalline nature of the platinum, gold, and silver nanocomposites was confirmed by the X-ray diffraction analysis. The typical XRD patterns of the prepared samples could be indexed to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes of face-centered cubic bulk metallic counterparts.
[0036] The morphology and particle size of the prepared platinum nanocomposites were analyzed using transmission electron microscopy. FIGS. 1A-1D show TEM images of the platinum nanocomposites. The platinum nanoparticle size varied between 1-3 nm. The TEM images suggested well dispersed platinum particles on the graphene oxide and carbon nanotube substrates, respectively. The elemental profiles of the prepared nanocomposites were analyzed using TEM with an energy dispersive spectroscopy (EDX) setup. The EDX spectrum (FIG. 2A) for the Pt-CNT nanocomposite showed Pt, C, and Cu peaks, which suggested the presence of platinum nanoparticles on the CNT. FIG. 2B exhibited the Pt, C, O, and Cu peaks, which indicates the presence of platinum nanoparticles on graphene oxide. The Cu peaks correspond to the copper grid used for TEM analysis.
[0037] The morphology and particle size of the prepared gold nanocomposites were analyzed using transmission electron microscopy. FIGS. 3A-3D show TEM images of the gold nanocomposites. Our results suggested that 10-20 nm spherically shaped gold nanoparticles are uniformly formed on GO and CNT. The elemental profiles of the prepared nanocomposites were analyzed using TEM with an energy dispersive spectroscopy (EDX) setup. The EDX spectrum (FIG. 4A) for the Au-CNT nanocomposite showed Au, C, and Cu peaks, which suggested the presence of gold nanoparticles on the CNT. FIG. 4B exhibited the Au, C, O, and Cu peaks, which indicates the presence of gold nanoparticles (Au) on graphene oxide (C, O). The Cu peaks correspond to the copper grid used for TEM analysis.
[0038] The morphology and particle size of the prepared silver nanocomposites were analyzed using transmission electron microscopy. FIGS. 5A-5D show TEM images of the silver nanocomposites. The TEM results confirmed that 20-30 nm silver nanoparticles are present on the GO and CNT substrates. The elemental profiles of the prepared nanocomposites were analyzed using TEM with an energy dispersive spectroscopy (EDX) setup. The EDX spectrum (FIG. 6A) for the Pt-GO nanocomposite showed Ag, C, and Cu peaks, which suggested the presence of silver nanoparticles on the CNT. FIG. 6B exhibited the Ag, C, O, and Cu peaks, which indicates the presence of silver nanoparticles on graphene oxide. The Cu peaks correspond to the copper grid used for TEM analysis.
[0039] It is to be understood that the present invention is not limited to the embodiments described above, but encompasses any and all embodiments within the scope of the following claims.
* * * * *
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.