Fgfr/pd-1 Combination Therapy For The Treatment Of Cancer
KARKERA; Jayaprakash ; et al.
U.S. patent application number 16/661671 was filed with the patent office on 2020-04-09 for fgfr/pd-1 combination therapy for the treatment of cancer. This patent application is currently assigned to ASTEX THERAPEUTICS LTD. The applicant listed for this patent is ASTEX THERAPEUTICS LTD. Invention is credited to Jayaprakash KARKERA, Matthew V. LORENZI, Suso Jesus PLATERO, Raluca VERONA.
| Application Number | 20200108141 16/661671 |
| Document ID | / |
| Family ID | 56134542 |
| Filed Date | 2020-04-09 |




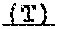
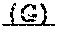
| United States Patent Application | 20200108141 |
| Kind Code | A1 |
| KARKERA; Jayaprakash ; et al. | April 9, 2020 |
FGFR/PD-1 COMBINATION THERAPY FOR THE TREATMENT OF CANCER
Abstract
Provided herein are combination therapies for the treatment of cancer. In particular, the disclosed methods are directed to treatment of cancer in a patient comprising administering an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient.
| Inventors: | KARKERA; Jayaprakash; (Germantown, MD) ; PLATERO; Suso Jesus; (Washington Crossing, PA) ; VERONA; Raluca; (Swarthmore, PA) ; LORENZI; Matthew V.; (Philadelphia, PA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | ASTEX THERAPEUTICS LTD CAMBRIDGE GB |
||||||||||
| Family ID: | 56134542 | ||||||||||
| Appl. No.: | 16/661671 | ||||||||||
| Filed: | October 23, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15079136 | Mar 24, 2016 | 10478494 | ||
| 16661671 | ||||
| 62142569 | Apr 3, 2015 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 35/00 20180101; C12Q 1/6886 20130101; C07K 16/2818 20130101; A61K 39/3955 20130101; C12Q 2600/158 20130101; A61K 31/498 20130101; G01N 33/57492 20130101; A61P 43/00 20180101; A61K 45/06 20130101; G01N 2333/70596 20130101; G01N 2800/52 20130101; A61K 31/498 20130101; A61K 2300/00 20130101; A61K 39/3955 20130101; A61K 2300/00 20130101 |
| International Class: | A61K 39/395 20060101 A61K039/395; A61K 31/498 20060101 A61K031/498; G01N 33/574 20060101 G01N033/574; C12Q 1/6886 20060101 C12Q001/6886; C07K 16/28 20060101 C07K016/28; A61K 45/06 20060101 A61K045/06 |
Claims
1. A method of treating cancer in a patient, comprising: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient.
2. The method of claim 1, further comprising evaluating the presence of one or more FGFR variants in the biological sample before the administering step.
3. The method of claim 1, further comprising evaluating PD-L1 expression in a biological sample.
4. The method of claim 3, wherein the biological sample for the one or more FGFR variants and the PD-L1 is the same biological sample.
5. The method of claim 3, wherein the biological sample for the one or more FGFR variants is different from the biological sample for PD-L1 expression.
6. The method of claim 1, wherein the biological sample is blood, lymph fluid, bone marrow, a solid tumor sample, or any combination thereof.
7. The method of claim 1, wherein the administering step is performed if PD-L1 expression is low in the biological sample.
8. The method of claim 1, wherein the cancer is lung cancer, bladder cancer, gastric cancer, breast cancer, ovarian cancer, head and neck cancer, esophageal cancer, glioblastoma, or any combination thereof.
9. The method of claim 8, wherein the lung cancer is non-small cell lung cancer (NSCLC) adenocarcinoma, NSCLC squamous cell carcinoma, small cell lung cancer, or any combination thereof.
10. The method of claim 1, wherein the one or more FGFR variants comprise an FGFR mutation, an FGFR amplification, an FGFR fusion gene, or a combination thereof.
11. The method of claim 10, wherein the FGFR fusion gene is FGFR2:AFF3; FGFR2:BICC1; FGFR2:CASP7; FGFR2:CCDC6; FGFR2:OFD1; FGFR3:BAIAP2L1; FGFR3:TACC3-Intron; FGFR3:TACC3V1; FGFR3:TACC3V3; or a combination thereof.
12. The method of claim 1, wherein the antibody that blocks the interaction between PD-1 and PD-L1 is an anti-PD-1 antibody, an anti-PD-L1 antibody, or a combination thereof.
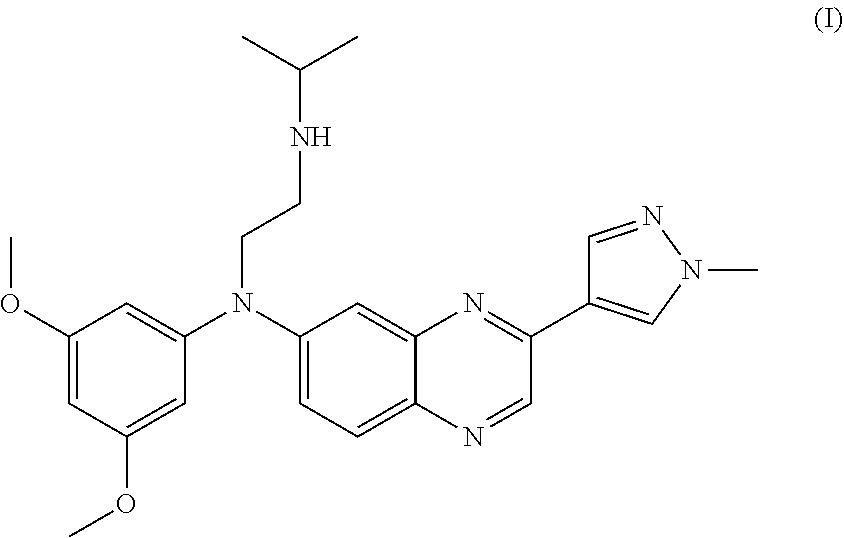
13. The method of claim 1, wherein the FGFR inhibitor is the compound of formula (I): ##STR00003## or a pharmaceutically acceptable salt thereof.
14. A method of treating cancer in a patient comprising: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1; monitoring the efficacy of the antibody; and if the antibody is not efficacious, evaluating a biological sample from the patient for a presence of one or more FGFR variants; and administering to the patient a pharmaceutically effective amount of an FGFR inhibitor if the one or more FGFR variants are present in the sample.
15. The method of claim 14, wherein the evaluating step further comprises measuring an expression level of PD-L1 in a biological sample and wherein the second administering step comprises administering the FGFR inhibitor if the expression level of PD-L1 is low.
16. The method of claim 15, wherein the biological sample for the one or more FGFR variants and the PD-L1 is the same biological sample.
17. The method of claim 15, wherein the biological sample for the one or more FGFR variants is different from the biological sample for PD-L1 expression.
18. (canceled)
19. The method of claim 16, wherein the cancer is lung cancer, bladder cancer, gastric cancer, breast cancer, ovarian cancer, head and neck cancer, esophageal cancer, glioblastoma, or any combination thereof.
20. The method of claim 19, wherein the lung cancer is non-small cell lung cancer (NSCLC) adenocarcinoma, NSCLC squamous cell carcinoma, small cell lung cancer, or any combination thereof.
21-23. (canceled)
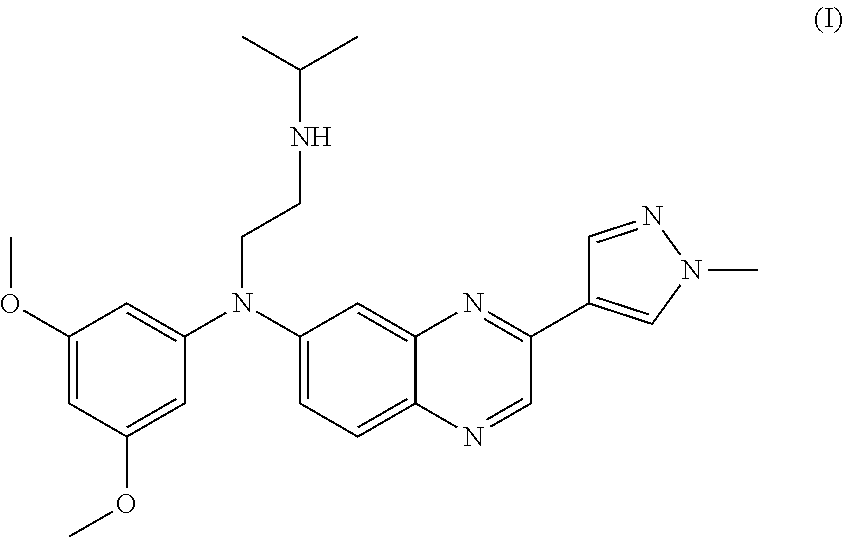
24. The method of claim 16, wherein the FGFR inhibitor is the compound of formula (I): ##STR00004## or a pharmaceutically acceptable salt thereof.
Description
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation of U.S. patent application Ser. No. 15/079,136, which was filed on Mar. 24, 2016 and claims priority to U.S. Provisional Application No. 62/142,569, filed on Apr. 3, 2015. The entire contents of the prior applications are hereby incorporated by reference herein.
SEQUENCE LISTING
[0002] The instant application contains a Sequence Listing which has been submitted electronically in ASCII format and is hereby incorporated by reference in its entirety. Said ASCII copy, created on Mar. 22, 2016, is named PRD3366USNP_SL.txt (H1594748) and is 53,086 bytes in size.
TECHNICAL FIELD
[0003] Provided herein are combination therapies for the treatment of cancer. In particular, the disclosed methods are directed to treatment of cancer in a patient comprising administering an antibody that blocks the interaction between PD-1 and PD-L1 and a fibroblast growth factor receptor (FGFR) inhibitor.
BACKGROUND
[0004] For cancer patients failing the main therapeutic option (front-line therapy) for that cancer type, there is often no accepted standard of care for second and subsequent-line therapy, unless a particular genetic abnormality is identified and a specific therapy is available. Fibroblast growth factor receptors (FGFRs) are a family of receptor tyrosine kinases involved in regulating cell survival, proliferation, migration and differentiation. FGFR alterations have been observed in some cancers. To date, there are no approved therapies that are efficacious in patients with FGFR alterations.
SUMMARY
[0005] Disclosed herein are methods of using a combination therapy that comprises an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor to treat cancer in a patient. In some embodiments, the methods comprise administering to a patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient.
[0006] In other embodiments, the methods of treating cancer in a patient comprise: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1; monitoring the efficacy of the antibody; and, if the antibody is not efficacious, evaluating a biological sample from the patient for a presence of one or more FGFR variants and administering to the patient a pharmaceutically effective amount of an FGFR inhibitor if the one or more FGFR variants are present in the sample.
[0007] Also, disclosed herein are uses of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for the manufacture of a medicament for the treatment of cancer, in particular for the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient. In some embodiments, the medicament contains a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the medicament is used in a patient wherein one or more FGFR variants are present in a biological sample from the patient.
[0008] Also disclosed herein are combinations of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for use in the treatment of cancer, in particular for use in the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient. In some embodiments, the combination comprises a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor for use in the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient. In other embodiments, the combination for treating cancer comprises administration of a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1; monitoring the efficacy of the antibody; and, if the antibody is not efficacious, evaluating a biological sample from the patient for a presence of one or more FGFR variants, followed by administration to the patient a pharmaceutically effective amount of an FGFR inhibitor if the one or more FGFR variants are present in the sample.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The summary, as well as the following detailed description, is further understood when read in conjunction with the appended drawings. For the purpose of illustrating the disclosed methods, there are shown in the drawings exemplary embodiments of the methods; however, the methods are not limited to the specific embodiments disclosed. In the drawings:
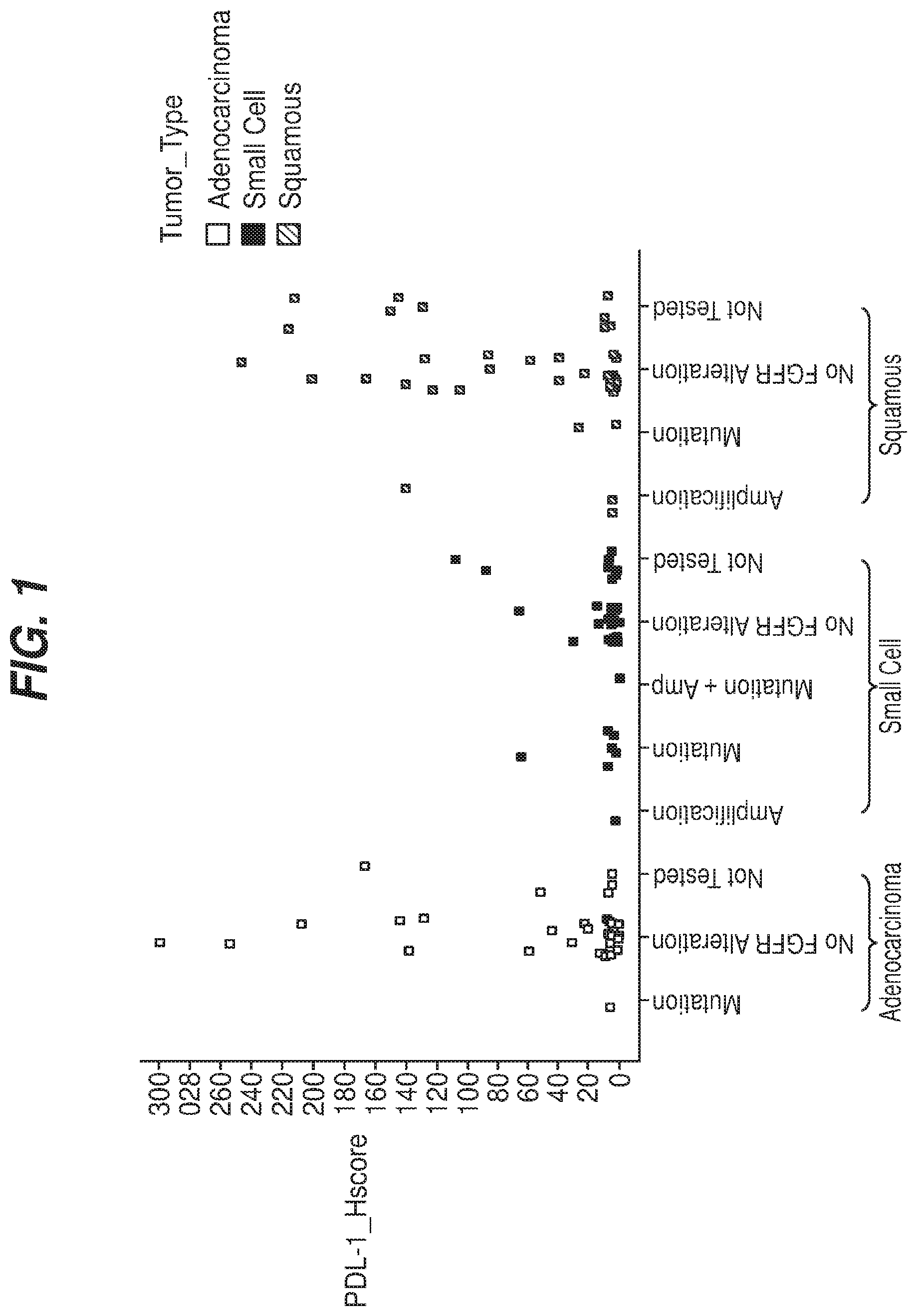
[0010] FIG. 1 illustrates PD-L1 expression in a 120 lung cancer samples set by histology and FGFR mutant and amplification status. PD-L1 H-scores (Y-axis) were plotted for NSCLC adenocarcinoma (left), small cell lung cancer (middle), and NSCLC squamous (right). The FGFR mutation and/or amplification status versus the PD-L1 staining for each of the 120 samples is shown. Mutation--an FGFR mutation was identified, but no amplification or fusion was detected; No FGFR Alteration--no mutation, amplification, or fusion was detected; Amplification--an FGFR gene amplification was identified, but no FGFR mutation or fusion was detected; Mutation+Amp--samples were positive for both FGFR mutation and gene amplification, but no fusion was detected; Not Tested--IHC for PD-L1 was performed, but sample was not tested on Foundation Medicine panel.
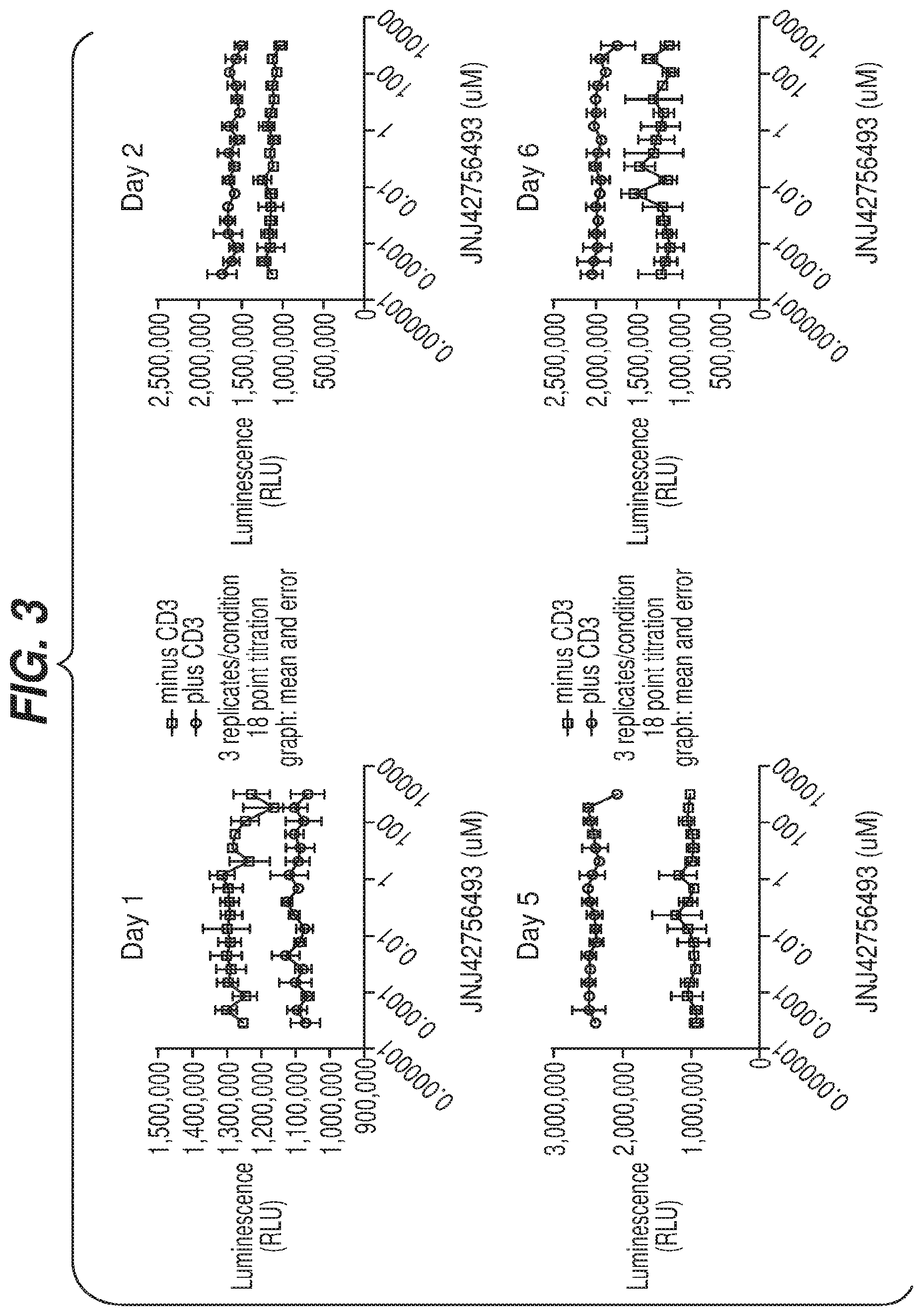
[0011] FIG. 2 illustrates PD-L1 expression in an 80 non-small-cell lung carcinoma (NSCLC) sample set by FGFR fusion status by NSCLC histology. PD-L1 H-scores (Y-axis) were plotted for NSCLC adenocarcinoma (left), and NSCLC squamous (right). The FGFR fusion status versus the PD-L1 staining for each of the 80 samples is shown. Fusion Positive--an FGFR fusion was detected; Fusion Wild-Type--no FGFR fusion was detected; Not Tested--insufficient sample for testing or quality control (QC) failure.
[0012] FIG. 3 illustrates the effect of JNJ42756493 on immune cell viability. Normal donor peripheral blood mononuclear cells (PBMCs), either unstimulated or stimulated with anti-CD3 antibodies, were treated with increasing concentrations of JNJ42756493 (0.0000077, 0.000023, 0.000070, 0.00021, 0.00063, 0.00188, 0.00565, 0.01694, 0.051, 0.152, 0.457, 1.372, 4.115, 12.346, 37.037, 111.111, 333.333, and 1000 nM). On days 1, 2, 5 and 6 after plating, cell viability was assessed by CellTiter-Glo (Promega).
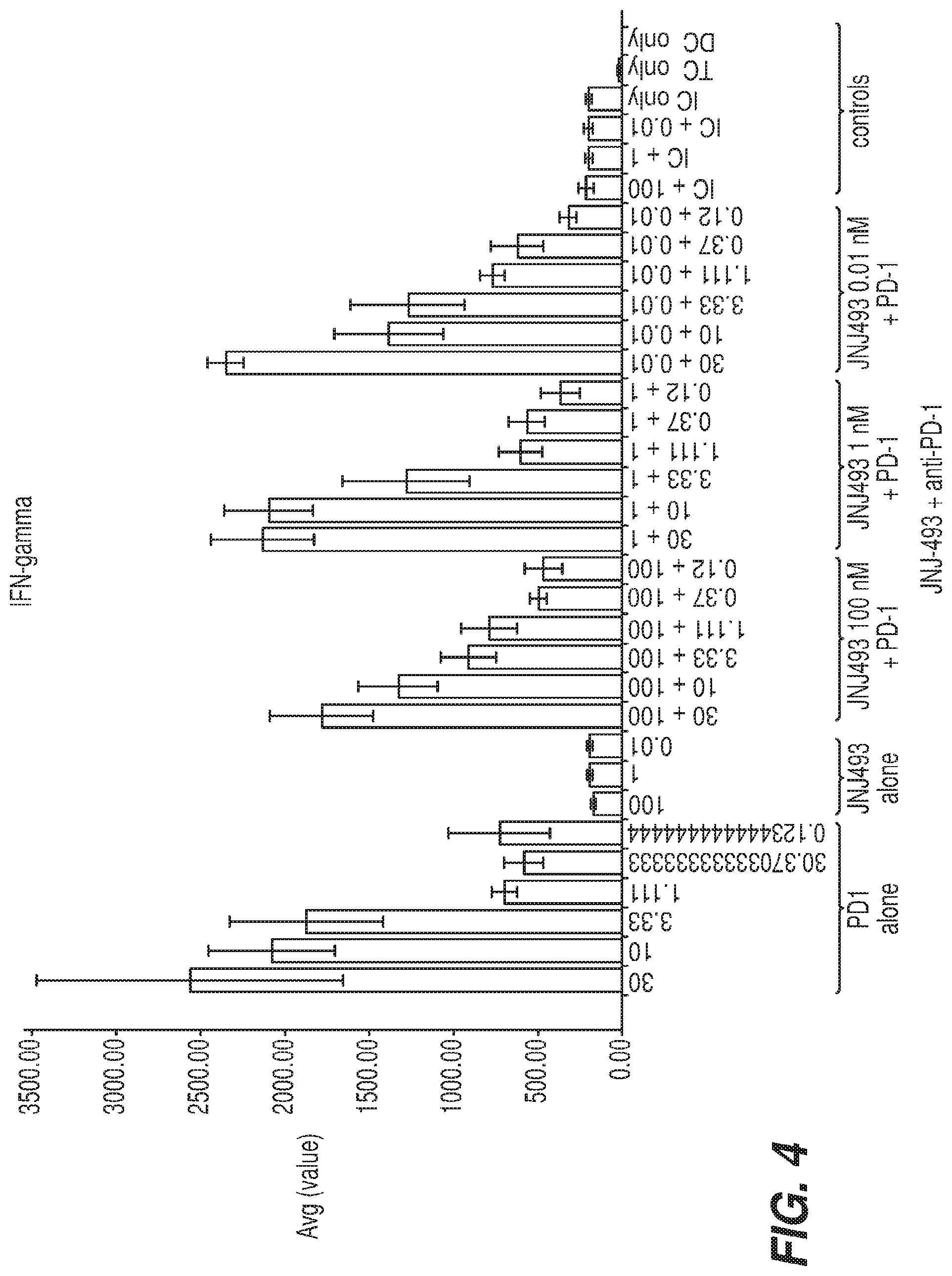
[0013] FIG. 4 illustrates the effect of JNJ42756493 on IFN-.gamma. levels induced by anti-PD-1 antibodies in a Mixed Lymphocyte Reaction (MLR) Assay. Cultures of CD4.sup.+ T and allogeneic dendritic cells were treated with anti-PD-1 antibodies (concentrations left to right--30, 10, 3.33, 1.11, 0.37, 0.12 nM). JNJ42756493 was added at 100, 1, or 0.01 nM alone (concentrations left to right), together with anti-PD-1 antibodies (100, 1, or 0.01 nM JNJ42756493 together with 30, 10, 3.33, 1.11, 0.37, or 0.12 nM of anti-PD-1 antibody), or in the presence of isotype control (IC). 5 days after treatment, IFN-.gamma. levels in the supernatant were measured by Meso Scale Discovery (MSD).
[0014] FIG. 5 illustrates the effect of JNJ42756493 on IFN-.gamma. levels induced by anti-PD-1 antibodies in a Cytomegalovirus antigen assay (CMV) Assay. Peripheral blood mononuclear cells (PMBCs) were stimulated with CMV antigen and treated with anti-PD-1 antibodies (concentration left to right--30, 10, 3.33, 1.11, 0.37, 0.12 nM) as indicated. JNJ42756493 was added at 100, 1, or 0.01 nM alone (concentrations left to right), together with anti-PD-1 antibodies (100, 1, or 0.01 nM JNJ42756493 together with 30, 10, 3.33, 1.11, 0.37, or 0.12 nM of anti-PD-1 antibody), or in the presence of isotype control (IC). 6 days after treatment, IFN-.gamma. levels in the supernatant were measured by MSD.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0015] The disclosed methods may be understood more readily by reference to the following detailed description taken in connection with the accompanying figures, which form a part of this disclosure. It is to be understood that the disclosed methods are not limited to the specific methods described and/or shown herein, and that the terminology used herein is for the purpose of describing particular embodiments by way of example only and is not intended to be limiting of the claimed methods.
[0016] Unless specifically stated otherwise, any description as to a possible mechanism or mode of action or reason for improvement is meant to be illustrative only, and the disclosed methods are not to be constrained by the correctness or incorrectness of any such suggested mechanism or mode of action or reason for improvement.
[0017] Reference to a particular numerical value includes at least that particular value, unless the context clearly dictates otherwise. When a range of values is expressed, another embodiment includes from the one particular value and/or to the other particular value. Further, reference to values stated in ranges include each and every value within that range. All ranges are inclusive and combinable.
[0018] When values are expressed as approximations, by use of the antecedent "about," it will be understood that the particular value forms another embodiment.
[0019] The term "about" when used in reference to numerical ranges, cutoffs, or specific values is used to indicate that the recited values may vary by up to as much as 10% from the listed value. Thus, the term "about" is used to encompass variations of +10% or less, variations of .+-.5% or less, variations of .+-.1% or less, variations of .+-.0.5% or less, or variations of .+-.0.1% or less from the specified value.
[0020] It is to be appreciated that certain features of the disclosed methods which are, for clarity, described herein in the context of separate embodiments, may also be provided in combination in a single embodiment. Conversely, various features of the disclosed methods that are, for brevity, described in the context of a single embodiment, may also be provided separately or in any subcombination.
[0021] As used herein, the singular forms "a," "an," and "the" include the plural.
[0022] The following abbreviations are used throughout the disclosure: FFPE (formalin-fixed, paraffin-embedded); NSCLC (non-small-cell lung carcinoma); SCLC (small-cell lung cancer); FGFR (fibroblast growth factor receptor); PD-1 (programmed cell death 1); PD-L1 (programmed death-ligand 1); FGFR3:TACC3 (fusion between genes encoding FGFR3 and transforming acidic coiled-coil containing protein 3); FGFR3:BAIAP2L1 (fusion between genes encoding FGFR3 and brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 1); FGFR2:AFF3 (fusion between genes encoding FGFR2 and AF4/FMR2 family, member 3); FGFR2:BICC1 (fusion between genes encoding FGFR2 and bicaudal C homolog 1); FGFR2: CASP7 (fusion between genes encoding FGFR2 and caspase 7); FGFR2:CCDC6 (fusion between genes encoding FGFR2 and coiled-coil domain containing 6); FGFR2:OFD1 (fusion between genes encoding FGFR2 and oral-facial-digital syndrome 1).
[0023] The term "antibody" refers to (a) immunoglobulin polypeptides, i.e., polypeptides of the immunoglobulin family that contain an antigen binding site that specifically binds to a specific antigen (e.g., PD-1 or PD-L1), including all immunoglobulin isotypes (IgG, IgA, IgE, IgM, IgD, and IgY), classes (e.g. IgG1, IgG2, IgG3, IgG4, IgA1, IgA2), subclasses, and various monomeric and polymeric forms of each isotype, unless otherwise specified, and (b) conservatively substituted variants of such immunoglobulin polypeptides that immunospecifically bind to the antigen (e.g., PD-1 or PD-L1). Antibodies are generally described in, for example, Harlow & Lane, Antibodies: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1988). Unless otherwise apparent from the context, reference to an antibody also includes antibody derivatives as described in more detail below.
[0024] "Antibody fragments" comprise a portion of a full length antibody, generally the antigen-binding or variable region thereof, such as Fab, Fab', F(ab').sub.2, and Fv fragments; diabodies; linear antibodies; single-chain antibody molecules; and multispecific antibodies formed from antibody fragments. Various techniques have been developed for the production of antibody fragments, including proteolytic digestion of antibodies and recombinant production in host cells; however, other techniques for the production of antibody fragments will be apparent to the skilled practitioner. In some embodiments, the antibody fragment of choice is a single chain Fv fragment (scFv). "Single-chain Fv" or "scFv" antibody fragments comprise the V.sub.H and V.sub.L domains of antibody, wherein these domains are present in a single polypeptide chain. Generally, the Fv polypeptide further comprises a polypeptide linker between the V.sub.H and V.sub.L domains which enables the scFv to form the desired structure for antigen binding. For a review of scFv and other antibody fragments, see James D. Marks, Antibody Engineering, Chapter 2, Oxford University Press (1995) (Carl K. Borrebaeck, Ed.).
[0025] An "antibody derivative" means an antibody, as defined above, that is modified by covalent attachment of a heterologous molecule such as, e.g., by attachment of a heterologous polypeptide (e.g., a cytotoxin) or therapeutic agent (e.g., a chemotherapeutic agent), or by glycosylation, deglycosylation, acetylation or phosphorylation not normally associated with the antibody, and the like.
[0026] The term "monoclonal antibody" refers to an antibody that is derived from a single cell clone, including any eukaryotic or prokaryotic cell clone, or a phage clone, and not the method by which it is produced. Thus, the term "monoclonal antibody" is not limited to antibodies produced through hybridoma technology.
[0027] "Biological sample" refers to any sample from a patient in which cancerous cells can be obtained and protein expression can be evaluated and/or RNA can be isolated. Suitable biological samples include, but are not limited to, blood, lymph fluid, bone marrow, sputum, a solid tumor sample, or any combination thereof. In some embodiments, the biological sample can be formalin-fixed paraffin-embedded tissue (FFPET).
[0028] As used here, "block(s) the interaction" refers to the ability of an anti-PD-1 antibody or an anti-PD-L1 antibody to inhibit or reduce binding of PD-L1 to PD-1, such that signaling/functioning through PD-1 is abolished or diminished.
[0029] As used herein, "FGFR variant" refers to an alteration in the wild type FGFR gene, including, but not limited to, FGFR fusion genes, FGFR mutations, FGFR amplifications, or any combination thereof. The terms "variant" and "alteration" are used interchangeably herein. "FGFR fusion" or "FGFR fusion gene" refers to a gene encoding a portion of FGFR (e.g., FGRF2 or FGFR3) and one of the herein disclosed fusion partners created by a translocation between the two genes.
[0030] As used herein, "patient" is intended to mean any animal, in particular, mammals. Thus, the methods are applicable to human and nonhuman animals, although most preferably with humans. "Patient" and "subject" may be used interchangeably herein.
[0031] "Pharmaceutically effective amount" refers to an amount of an antibody that blocks the interaction between PD-1 and PD-L1 and an amount of an FGFR inhibitor that treats the patient.
[0032] As used herein, "pharmaceutically acceptable salt" embraces salts with inorganic and organic acids, such as hydrochloric acid, nitric acid, sulfuric acid, phosphoric acid, and the like. Examples of pharmaceutically acceptable salts are discussed in Berge, et al. (1977) "Pharmaceuticall Acceptable Salts," J. Pharm. Sci., Vol. 66, pp. 1-19.
[0033] As used herein, "treating" and like terms refer to reducing the severity and/or frequency of cancer symptoms, eliminating cancer symptoms and/or the underlying cause of said symptoms, reducing the frequency or likelihood of cancer symptoms and/or their underlying cause, and improving or remediating damage caused, directly or indirectly, by cancer.
[0034] Disclosed herein are methods of treating cancer in a patient comprising: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient.
[0035] PD-1 is a cell surface receptor expressed on the surface of CD4+ and CD8+ T cells, B cells, and myeloid cells. The ligands of PD-1, PD-L1 and PD-L2, are expressed on immune cells; in addition, PD-L1 is also expressed on cancer cells. When engaged by its ligands, PD-1 downregulates the immune response by reducing T cell proliferation, cytokine production and effector function. Antibodies against PD-1 (anti-PD-1 antibodies) and/or its ligands (anti-PD-L1 antibodies, for example) can block the interaction between PD-1 and PD-L1, thereby inhibiting the downregulation of the immune response. The disclosed methods comprise administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1. In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-1 antibody. In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-L1 antibody. In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-1 antibody and an anti-PD-L1 antibody.
[0036] Exemplary anti-PD-1 antibodies include, but are not limited to, OPDIVO.RTM. (nivolumab) (Bristol-Myers Squibb) and KEYTRUDA.RTM. (pembrolizumab) (Merck). Exemplary anti-PD-L1 antibodies include, but are not limited to, MPDL3208A (Roche) and MEDI4736 (AstraZeneca).
[0037] Exemplary FGFR inhibitors are described in U.S. Publ. No. 2013/0072457 A1 (incorporated herein by reference) and include N-(3,5-dimethoxyphenyl)-N'-(1-methylethyl)-N-[3-(1-methyl-1H-pyrazol-4-yl- )quinoxalin-6-yl]ethane-1,2-diamine (referred to herein "JNJ-42756493"), including any tautomeric or stereochemically isomeric forms thereof, N-oxides thereof, pharmaceutically acceptable salts thereof, or solvates thereof (suitable R groups are also disclosed in U.S. Publ. No. 2013/0072457 A1). Thus, in some embodiments, the FGFR inhibitor can be the compound of formula (I):
##STR00001##
or a pharmaceutically acceptable salt thereof. In some aspects, the pharmaceutically acceptable salt is a HCl salt.
[0038] The antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor can be administered as a single therapeutic agent or can be co-administered as individual agents. When administered as individual agents, the antibody and FGFR inhibitor can be administered contemporaneously or sequentially in either order. In some embodiments, the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor can be administered contemporaneously. In some embodiments, the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor can be administered sequentially. In some aspects, for example, the antibody that blocks the interaction between PD-1 and PD-L1 can be administered first, followed by administration of the FGFR inhibitor. In other aspects, the FGFR inhibitor can be administered first, followed by administration of the antibody that blocks the interaction between PD-1 and PD-L1. When administered sequentially, the antibody and FGFR inhibitor can be administered within seconds, minutes, hours, days, or weeks of each other.
[0039] The pharmaceutically effective amount of the antibody that blocks the interaction between PD-1 and PD-L1 and of the FGFR inhibitor will be dependent on several factors including, but not limited to, stage and severity of the cancer, as well as other factors relating to the health of the patient. Those skilled in the art would know how to determine the pharmaceutically effective amount.
[0040] The disclosed methods are suitable for treating cancer in a patient if one or more FGFR variants are present in a biological sample from the patient. In some embodiments, the FGFR variant can be one or more FGFR fusion genes. In some embodiments, the FGFR variant can be one or more FGFR mutations. In some embodiments, the FGFR variant can be one or more FGFR amplifications. In some embodiments, a combination of the one or more FGFR variants can be present in the biological sample from the patient. For example, in some embodiments, the FGFR variants can be one or more FGFR fusion genes and one or more FGFR mutations. In some embodiments, the FGFR variants can be one or more FGFR fusion genes and one or more FGFR amplifications. In some embodiments, the FGFR variants can be one or more FGFR mutations and one or more FGFR amplifications. In yet other embodiments, the FGFR variants can be one or more FGFR fusion genes, mutations, and amplifications.
[0041] Exemplary FGFR fusion genes are provided in Table 1 and include, but are not limited to: FGFR2:AFF3; FGFR2:BICC1; FGFR2:CASP7; FGFR2:CCDC6; FGFR2:OFD1; FGFR3:BAIAP2L1; FGFR3:TACC3-Intron; FGFR3:TACC3V1; FGFR3:TACC3V3; or a combination thereof. The sequences of the FGFR fusion genes are disclosed in Table 7.
TABLE-US-00001 TABLE 1 Exemplary FGFR fusion genes Fusion Gene FGFR Exon Partner Exon FGFR2 FGFR2:AFF3 19 8 FGFR2:BICC1 19 3 FGFR2:CASP7 19 4 FGFR2:CCDC6 19 2 FGFR2:OFD1 19 3 FGFR3 FGFR3:BAIAP2L1 18 2 FGFR3:TACC3 Intron 18 4 FGFR3:TACC3 v1 18 11 FCFR3:TACC3 v3 18 10
[0042] FGFR mutations include FGFR single nucleotide polymorphism (SNP). "FGFR single nucleotide polymorphism" (SNP) refers to a FGFR2 or FGFR3 gene in which a single nucleotide differs among individuals. In particular, FGFR single nucleotide polymorphism" (SNP) refers to a FGFR3 gene in which a single nucleotide differs among individuals. The presence of one or more of the following FGFR SNPs in a biological sample from a patient can be determined by methods known to those of ordinary skill in the art or methods disclosed in U.S. Provisional Patent App. No. 62/056,159, U.S. Patent Publication No. US2016-0090633, and WO 2016/048833, FGFR3 R248C, FGFR3 S249C, FGFR3 G370C, FGFR3 Y373C, or any combination thereof. The sequences of the FGFR SNPs are provided in Table 2.
TABLE-US-00002 TABLE 2 FGFR3 mutant Sequence FGFR3 R248C TCGGACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAGCATCCGGCAGACGT ACACGCTGGACGTGCTGGAG GCTCCCCGCACCGGCCCATCCTGCAGGCGGGGCTGCCA GGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGGAGTTCCACTGCAAGGTGTACAGTGCG CACAGCCCCACATCCAGTGGCTCAAGCACGTGGAGGTGAATGGCAGCAAGGTGGGCCCGGA CGGCACACCCTACGTTACCGTGCTCA (SEQ ID NO: 1) FGFR3 S249C GACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAGCATCCGGCAGACGTACA CGCTGGACGTGCTGGGTGAGGGCCCTGGGGCGGCGCGGGGGTGGGGGCGGCAGTGGCGGTG GTGGTGAGGGAGGGGGTGGCCCCTGAGCGTCATCTGCCCCCACAGAGCGCT CCCGCAC CGGCCCATCCTGCAGGCGGGGCTGCCGGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGG AGTTCCACTGCAAGGTGTACAGTGACGCACAGCCCCACATCCAGTGGCTCAAGCACGTGGA GGTGAATGGCAGCAAGGTGGGCCCGGACGGCACACCCTACGTTACCGTGCTCAAGGTGGGC CACCGTGTGCACGT (SEQ ID NO: 2) FGFR3 G370C GCGGGCAATTCTATTGGGTTTTCTCATCACTCTGCGTGGCTGGTGGTGCTGCCAGCCGAGG AGGAGCTGGTGGAGGCTGACGAGGCG GCAGTGTGTATGCAGGCATCCTCAGCTACGGG GTGGGCTTCTTCCTGTTCATCCTGGTGGTGGCGGCTGTGACGCTCTGCCGCCTGCGCAGCC CCCCCAAGAAAGGCCTGGGCTCCCCCACCGTGCACAAGATCTCCCGCTTCCCG (SEQ ID NO: 3) FGFR3 Y373C* CTAGAGGTTCTCTCCTTGCACAACGTCACCTTTGAGGACGCCGGGGAGTACACCTGCCTGG CGGGCAATTCTATTGGGTTTTCTCATCACTCTGCGTGGCTGGTGGTGCTGCCAGCCGAGGA GGAGCTGGTGGAGGCTGACGAGGCGGGCAGTGTGT TGCAGGCATCCTCAGCTACGGGG TGGGCTTCTTCCTGTTCATCCTGGTGGTGGCGGCTGTGACGCTCTGCCGCCTGCGCAGCCC CCCCAAGAAAGGCCTGGGCTCCCCCACCGTGCACAAGATCTCCCGCTTCCCGCTCAAGC (SEQ ID NO: 4) Sequences correspond to nucleotides 920-1510 of FGFR3 (Genebank ID # NM_000142.4). Nucleotides in bold underline represent the SNP. *Sometimes mistakenly referred to as Y375C in the literature.
[0043] The methods can further comprise evaluating the presence of one or more FGFR variants in the biological sample before the administering step. Suitable methods for evaluating a biological sample for the presence of one or more FGFR variants are disclosed elsewhere herein.
[0044] The disclosed methods can be dependent upon PD-L1 expression in the cancer or can be carried out irrespectively of PD-L1 expression in the cancer. In some embodiments, for example, the methods can comprise administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient and PD-L1 expression in the biological sample from the patient is at a specified level or within a specified range. In some aspects, for example, the methods can be carried out if the PD-L1 expression is high in the biological sample. Accordingly, in some embodiments the methods can comprise administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if PD-L1 expression is high and one or more FGFR variants are present in a biological sample from the patient. Alternatively, the methods can be carried out if the PD-L1 expression is low in the biological sample. Accordingly, the methods can comprise administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if PD-L1 expression is low and one or more FGFR variants are present in a biological sample from the patient. The methods can be carried out if the PD-L1 expression is moderate. Accordingly, the methods can comprise administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if PD-L1 expression is moderate and one or more FGFR variants are present in a biological sample from the patient. As discussed elsewhere herein, PD-L1 expression levels can be based upon a numerical H-score (low includes an H-score of about 0 to about 99; moderate includes an H-score of about 100 to about 199; and high includes an H-score of about 200 to about 300) or can be based upon a comparison to a reference value.
[0045] In other embodiments, the methods can be carried out irrespectively of PD-L1 expression in the biological sample from the patient and can be based on the presence of one or more FGFR variants without factoring in PD-L1 expression.
[0046] The methods can further comprise evaluating PD-L1 expression in the biological sample from the patient. Exemplary methods of evaluating PD-L1 expression are disclosed elsewhere herein. PD-L1 expression can be evaluated before, during, or after the administering step.
[0047] In some embodiments, the methods can comprise evaluating the presence of one or more FGFR variants and PD-L1 expression in the biological sample from the patient before the administering step.
[0048] Suitable biological samples for evaluating PD-L1 expression, evaluating the presence of one or more FGFR variants, or for evaluating both PD-L1 expression and the presence of one or more FGFR variants include, but are not limited to, blood, lymph fluid, bone marrow, a solid tumor sample, or any combination thereof.
[0049] The disclosed methods can be used to treat a variety of cancer types including, but not limited to, lung cancer, bladder cancer, gastric cancer, breast cancer, ovarian cancer, head and neck cancer, esophageal cancer, glioblastoma, or any combination thereof. In some embodiments, the methods can be used to treat lung cancer. The lung cancer can be non-small cell lung cancer (NSCLC) adenocarcinoma, NSCLC squamous cell carcinoma, small cell lung cancer, or any combination thereof. Thus, in some aspects, the methods can be used to treat NSCLC adenocarcinoma. In other aspects, the methods can be used to treat NSCLC squamous cell carcinoma. In yet other aspects, the methods can be used to treat small cell lung cancer. In some embodiments, the methods can be used to treat bladder cancer. In some embodiments, the methods can be used to treat gastric cancer. In some embodiments, the methods can be used to treat breast cancer. In some embodiments, the methods can be used to treat ovarian cancer. In some embodiments, the methods can be used to treat head and neck cancer. In some embodiments, the methods can be used to treat esophageal cancer. In some embodiments, the methods can be used to treat glioblastoma. In some embodiments, the methods can be used to treat any combination of the above cancers.
[0050] Also disclosed are methods of treating cancer in a patient comprising: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1; monitoring the efficacy of the antibody; and if the antibody is not efficacious, evaluating a biological sample from the patient for a presence of one or more FGFR variants and administering to the patient a pharmaceutically effective amount of an FGFR inhibitor if the one or more FGFR variants are present in the sample.
[0051] The efficacy of the antibody can be monitored by, for example, evaluating the patient's symptoms for progression of the cancer, evaluating the severity of the cancer symptoms, evaluating the frequency of the cancer symptoms, measuring tumor size, or any combination thereof. Without intent to be limiting, progression or failure to reduce the progression of the cancer, increased severity or no change in severity of the cancer symptoms, increased frequency or no change in the frequency of the cancer symptoms, increased size or no change in size of the tumor, or any combination thereof, can be indications that the antibody is not efficacious.
[0052] In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-1 antibody. In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-L1 antibody. In some embodiments, the methods can comprise administering to the patient a pharmaceutically effective amount of an anti-PD-1 antibody and an anti-PD-L1 antibody. Exemplary anti-PD-1 antibodies include, but are not limited to, OPDIVO.RTM. (nivolumab) (Bristol-Myers Squibb) and KEYTRUDA.RTM. (pembrolizumab) (Merck). Exemplary anti-PD-L1 antibodies include, but are not limited to, MPDL3208A (Roche) and MEDI4736 (AstraZeneca).
[0053] Exemplary FGFR inhibitors include those disclosed above, including N-(3,5-dimethoxyphenyl)-N'-(1-methylethyl)-N-[3-(1-methyl-1H-pyrazol-4-yl- )quinoxalin-6-yl]ethane-1,2-diamine (referred to herein "JNJ-42756493"), including any tautomeric or stereochemically isomeric forms thereof, N-oxides thereof, pharmaceutically acceptable salts thereof, or solvates thereof (suitable R groups are also disclosed in U.S. Publ. No. 2013/0072457 A1). In some embodiments, the FGFR inhibitor can be the compound of formula (I):
##STR00002##
or a pharmaceutically acceptable salt thereof. In some aspects, the pharmaceutically acceptable salt is a HCl salt.
[0054] The pharmaceutically effective amount of the antibody and FGFR inhibitor will be dependent on several factors including, but not limited to, stage and severity of the cancer, as well as other factors relating to the health of the patient. Those skilled in the art would know how to determine the pharmaceutically effective amount.
[0055] The disclosed methods are suitable for treating cancer in a patient if one or more FGFR variants are present in a biological sample from the patient. In some embodiments, the FGFR variant can be one or more FGFR fusion genes. In some embodiments, the FGFR variant can be one or more FGFR mutations. In some embodiments, the FGFR variant can be one or more FGFR amplifications. In some embodiments, a combination of the one or more FGFR variants can be present in the biological sample from the patient. For example, in some embodiments, the FGFR variants can be one or more FGFR fusion genes and one or more FGFR mutations. In some embodiments, the FGFR variants can be one or more FGFR fusion genes and one or more FGFR amplifications. In some embodiments, the FGFR variants can be one or more FGFR mutations and one or more FGFR amplifications. In yet other embodiments, the FGFR variants can be one or more FGFR fusion genes, mutations, and amplifications. Exemplary FGFR fusion genes are provided in Table 1 and include, but are not limited to: FGFR2:AFF3; FGFR2:BICC1; FGFR2:CASP7; FGFR2:CCDC6; FGFR2:OFD1; FGFR3:BAIAP2L1; FGFR3:TACC3-Intron; FGFR3:TACC3V1; FGFR3:TACC3V3; or a combination thereof.
[0056] Suitable methods for evaluating a biological sample for the presence of one or more FGFR variants are disclosed elsewhere herein.
[0057] The disclosed methods can be dependent upon PD-L1 expression in the biological sample or can be carried out irrespectively of PD-L1 expression in the cancer. In some aspects, for example, if the antibody is not efficacious, the methods can comprise measuring an expression level of PD-L1 in the biological sample and administering to the patient a pharmaceutically effective amount of an FGFR inhibitor if the PD-L1 expression is at a specified level or within a specified range. Methods of evaluating PD-L1 expression are disclosed elsewhere herein. The methods can be carried out if the PD-1 expression in the biological sample is low. In some embodiments, for example, the evaluating step can further comprise measuring an expression level of PD-L1 in the biological sample and the second administering step can comprise administering the FGFR inhibitor if the expression level of PD-L1 is low. In some aspects, methods of treating cancer in a patient comprise: administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1; monitoring the efficacy of the antibody; and if the antibody is not efficacious, evaluating a biological sample from the patient for a presence of one or more FGFR variants and measuring an expression level of PD-L1 in the biological sample, and administering to the patient a pharmaceutically effective amount of an FGFR inhibitor if the one or more FGFR variants are present and if the expression level of PD-L1 is low in the sample.
[0058] The methods can be carried out if the PD-1 expression in the biological sample is moderate. Thus, the evaluating step can further comprise measuring an expression level of PD-L1 in the biological sample and the second administering step can comprise administering the FGFR inhibitor if the expression level of PD-L1 is moderate. The methods can be carried out if the PD-1 expression in the biological sample is high. For example, the evaluating step can further comprise measuring an expression level of PD-L1 in the biological sample and the second administering step can comprise administering the FGFR inhibitor if the expression level of PD-L1 is high.
[0059] As discussed elsewhere herein, PD-L1 expression levels can be based upon a numerical H-score (low includes an H-score of about 0 to about 99; moderate includes an H-score of about 100 to about 199; and high includes an H-score of about 200 to about 300) or can be based upon a comparison to a reference value.
[0060] In other embodiments, the methods can be carried out irrespectively of PD-L1 expression in the cancer and can be based on the presence of one or more FGFR variants in the biological sample without factoring in PD-L1 expression.
[0061] Suitable biological samples include, but are not limited to, blood, lymph fluid, bone marrow, a solid tumor sample, or any combination thereof.
[0062] The disclosed methods can be used to treat a variety of cancer types including, but not limited to, lung cancer, bladder cancer, gastric cancer, breast cancer, ovarian cancer, head and neck cancer, esophageal cancer, glioblastoma, or any combination thereof. In some embodiments, the methods can be used to treat lung cancer. The lung cancer can be non-small cell lung cancer (NSCLC) adenocarcinoma, NSCLC squamous cell carcinoma, small cell lung cancer, or any combination thereof. Thus, in some aspects, the methods can be used to treat NSCLC adenocarcinoma. In other aspects, the methods can be used to treat NSCLC squamous cell carcinoma. In yet other aspects, the methods can be used to treat small cell lung cancer. In some embodiments, the methods can be used to treat bladder cancer. In some embodiments, the methods can be used to treat gastric cancer. In some embodiments, the methods can be used to treat breast cancer. In some embodiments, the methods can be used to treat ovarian cancer. In some embodiments, the methods can be used to treat head and neck cancer. In some embodiments, the methods can be used to treat esophageal cancer. In some embodiments, the methods can be used to treat glioblastoma. In some embodiments, the methods can be used to treat any combination of the above cancers.
[0063] Also, disclosed herein are uses of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for the manufacture of a medicament for the treatment of cancer, in particular for the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient. Each of the above embodiments described in terms of a method of treating cancer can be employed in the use of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for the manufacture of a medicament for the treatment of cancer, in particular for the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient.
[0064] Also disclosed herein are combinations of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for use in the treatment of cancer, in particular for the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient. Each of the above embodiments described in terms of a method of treating cancer can be employed with a combination of an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor for use in the treatment of cancer, in particular for the treatment of cancer in a patient wherein one or more FGFR variants are present in a biological sample from the patient.
Evaluating a Sample for the Presence of One or More FGFR Variants
[0065] The following methods for evaluating a biological sample for the presence of one or more FGFR variants apply equally to any of the above disclosed methods of treatment.
[0066] Suitable methods for evaluating a biological sample for the presence of one or more FGFR variants are described in the methods section herein and in U.S. Provisional Patent App. No. 62/056,159, U.S. Patent Publication No. US2016-0090633, and WO 2016/048833, which are incorporated herein in their entirety. For example, and without intent to be limiting, evaluating a biological sample for the presence of one or more FGFR variants can comprise any combination of the following steps: isolating RNA from the biological sample; synthesizing cDNA from the RNA; and amplifying the cDNA (preamplified or non-preamplified). In some embodiments, evaluating a biological sample for the presence of one or more FGFR variants can comprise: amplifying cDNA from the patient with a pair of primers that bind to and amplify one or more FGFR variants; and determining whether the one or more FGFR variants are present in the sample. In some aspects, the cDNA can be pre-amplified. In some aspects, the evaluating step can comprise isolating RNA from the sample, synthesizing cDNA from the isolated RNA, and pre-amplifying the cDNA.
[0067] Suitable primer pairs for performing an amplification step include, but are not limited to, those disclosed in U.S. Provisional Patent App. No. 62/056,159, U.S. Patent Publication No. US2016-0090633, and WO 2016/048833, as exemplified below:
TABLE-US-00003 FGFR3TACC3 V1 (SEQ ID NO: 1) Forward: GACCTGGACCGTGTCCTTACC (SEQ ID NO: 2) Reverse: CTTCCCCAGTTCCAGGTTCTT FGFR3TACC3 V3 (SEQ ID NO: 3) Forward: AGGACCTGGACCGTGTCCTT (SEQ ID NO: 4) Reverse: TATAGGTCCGGTGGACAGGG FGFR3TACC3 Intron (SEQ ID NO: 5) Forward: GGCCATCCTGCCCCC (SEQ ID NO: 6) Reverse: GAGCAGTCCAGGTCAGCCAG FGFR3BAIAP2L1 (SEQ ID NO: 7) Forward: CTGGACCGTGTCCTTACCGT (SEQ ID NO: 8) Reverse: GCAGCCCAGGATTGAACTGT FGFR2BICC1 (SEQ ID NO: 9) Forward: TGGATCGAATTCTCACTCTCACA (SEQ ID NO: 10) Reverse: GCCAAGCAATCTGCGTATTTG FGFR2AFF3 (SEQ ID NO: 11) Forward: TGGTAGAAGACTTGGATCGAATTCT (SEQ ID NO: 12) Reverse: TCTCCCGGATTATTTCTTCAACA FGFR2CASP7 (SEQ ID NO: 13) Forward: GCTCTTCAATACAGCCCTGATCA (SEQ ID NO: 14) Reverse: ACTTGGATCGAATTCTCACTCTCA FGFR2CCDC6 (SEQ ID NO: 15) Forward: TGGATCGAATTCTCACTCTCACA (SEQ ID NO: 16) Reverse: GCAAAGCCTGAATTTTCTTGAATAA FGFR2OFD1 (SEQ ID NO: 17) Forward: AGGGTGCATCAACTCATGAATTAG (SEQ ID NO: 18) Reverse: ACTTGGATCGAATTCTCACTCTCA
[0068] The presence of one or more FGFR variants can be evaluated at any suitable time point including upon diagnosis, following tumor resection, following first-line therapy, during clinical treatment, or any combination thereof.
Evaluating PD-L1 Expression in the Cancer
[0069] The following methods for evaluating PD-L1 expression in a biological sample apply equally to any of the above disclosed methods of treatment.
[0070] In some embodiments, the disclosed methods can be dependent upon PD-L1 expression in the biological sample from the patient. Thus, administering to the patient a pharmaceutically effective amount of an antibody that blocks the interaction between PD-1 and PD-L1 and a pharmaceutically effective amount of an FGFR inhibitor may be based upon PD-L1 expression and the presence of one or more FGFR variants in the biological sample from the patient. The methods can comprise evaluating PD-L1 expression in a biological sample from the patient. The biological sample from which PD-L1 expression is evaluated can be the same biological sample from which the presence of one or more FGFR variants are evaluated, or the biological samples from which PD-L1 expression is evaluated can be a different biological sample from which the presence of one or more FGFR variants are evaluated. "Same biological sample" refers to a single sample from which both PD-L1 expression and FGFR variants are evaluated. "Different biological sample" includes the same source of sample (blood, lymph fluid, bone marrow, a solid tumor sample, etc.) taken at different time points or different sources of sample. For example, a blood sample can be obtained from the patient, evaluated for PD-L1 expression or the presence of one or more FGFR variants, and at a later time point, another blood sample can be obtained from the patient and evaluated for the presence of one or more FGFR variants or PD-L1 expression. Conversely, a blood sample can be obtained from the patient and evaluated for PD-L1 expression and/or the presence of one or more FGFR variants and a solid tumor sample can be obtained from the patient and evaluated for the presence of one or more FGFR variants and/or PD-L1 expression.
[0071] In some embodiments, the level of PD-L1 expression can be converted into a numerical H-score (as described in the methods section herein). The level of PD-L1 expression can be converted into a numerical H-score of: low PD-L1 expression, which includes an H-score of about 0 to about 99; moderate PD-L1 expression, which includes an H-score of about 100 to about 199; or high PD-L1 expression, which includes an H-score of about 200 to about 300. Treating the patient can be based upon these H-scores. For example, if the treatment methods are carried out on a patient with a low H-score, that patient would have PD-L1 expression corresponding to an H-score of about 0 to about 99. If the treatment methods are carried out on a patient with a moderate H-score, that patient would have PD-L1 expression corresponding to an H-score of about 100 to about 199. If the treatment methods are carried out on a patient with a high H-score, that patient would have PD-L1 expression corresponding to an H-score of about 200 to about 300.
[0072] In other embodiments, the level of PD-L1 expression can be compared to a reference PD-L1 expression level. In a preferred embodiment, the reference PD-L1 expression level can be predetermined. For example, a reference data set may be established using samples from unrelated patients with low, moderate and high PD-L1 expression levels. This data set can represent a standard by which relative PD-L1 expression levels are compared among patients and/or quantified using the H-Score method. In some embodiments, the reference PD-L1 expression level can be determined by comparing a patient population that is administered the antibody that blocks the interaction between PD-1 and PD-L1 to a patient population that is administered placebo. The PD-L1 expression level for each patient in the respective populations can be determined in accordance with the methods described herein. Clinical outcomes (e.g., progression-free survival or overall survival) for the patient populations can be monitored. Clinical outcomes for the patient populations relative to PD-L1 expression levels can then be compared. The reference PD-L1 expression level can correspond to the PD-L1 expression level above which the patient population that is administered the antibody that blocks the interaction between PD-1 and PD-L1 demonstrates a statistically significant improvement in at least one clinical outcome relative to the patient population that is administered placebo. A patient PD-L1 expression level that is less than the reference PD-L1 expression level, particularly when combined with the presence of one or more FGFR variants in a patient sample, can be indicative that the patient will benefit from treatment with the antibody that blocks the interaction between PD-1 and PD-L1 in combination with an FGFR inhibitor. For example, in some embodiments, the methods can comprise administering an antibody that blocks the interaction between PD-1 and PD-L1 and an FGFR inhibitor, wherein the antibody that blocks the interaction between PD-1 and PD-L1 and the FGFR inhibitor are administered if one or more FGFR variants are present in a biological sample from the patient and the PD-L1 expression in the biological sample is less than a reference PD-L1 expression level, wherein the reference PD-L1 expression level corresponds to a PD-L1 expression level above which treatment with the antibody that blocks the interaction between PD-1 and PD-L1 alone is likely to be efficacious.
[0073] Methods for determining PD-L1 expression include, but are not limited to, immunohistochemistry (IHC), Western Blotting, microscopy, immunoprecipitation, BCA assays, spectrophotometry, or any combination thereof. Exemplary methods for evaluating PD-L1 expression are described in the methods section herein.
[0074] PD-L1 expression can be evaluated at any suitable time point including upon diagnosis, following tumor resection, following first-line therapy, during clinical treatment, or any combination thereof.
[0075] The following examples are provided to further describe some of the embodiments disclosed herein. The examples are intended to illustrate, not to limit, the disclosed embodiments.
EXAMPLES
Methods
PD-L1 Immunohistochemistry
[0076] PD-L1 immunohistochemistry (IHC) was performed at a CRO (QualTek, Newtown, Pa.). Samples were stained using a CD274 PD-L1 (RUO) assay. Slides stained with a CD274 PD-L1 (RUO) assay were examined in random order and/or in blinded fashion by a board-certified clinical pathologist, the Medical Director of QualTek Clinical Laboratories (CAP/CLIA facility). The entire tissue section was evaluated for CD274 PD-L1. Only viable tissue was evaluated; areas of necrosis or obviously poorly fixed areas of tissue were not evaluated.
[0077] The tumor H-Score was calculated from the intensity of CD274 PD-L1 membrane reactivity on a four-point semi-quantitative scale (0: null, negative or non-specific staining of cell membranes; 1+: low or weak intensity staining of cell membranes; 2+: medium or moderate intensity staining of cell membranes; and 3+: high or strong intensity staining of cell membranes) and the estimated percentage of CD274 PD-L1 positive tumor cells (0-100%) for each discrete intensity value.
[0078] Tumor CD274 PD-L1 membrane reactivity was captured by a standard H-Score--the tumor H-Score minimum of 0 and the tumor H-Score maximum of 300: Tumor H-Score=([% positive cells at 1+]*1)+([% positive cells at 2+]*2)+([% positive cells at 3+]*3)
Next-Generation Sequencing (NGS)
[0079] NGS for FGFR mutations and gene amplification was performed by Foundation Medicine, Cambridge, Mass. using the FoundationOne panel (http://www.foundationmedicine.com).
FGFR Fusions
[0080] FGFR fusions were determined using a proprietary qRT-PCR assay developed by Janssen Oncology Translational Research as described in U.S. Provisional Application No. 62/056,159, U.S. Patent Publication No. US2016-0090633, and WO 2016/048833.
Results
[0081] PD-L1 Expression in Tumors with FGFR Fusions and Mutations
[0082] To determine the overlap of PD-L1 expression with FGFR alterations, immunohistochemistry (IHC) for PD-L1 was performed on human tumor tissue samples which were subsequently assessed for FGFR alterations. FGFR amplifications and mutations were identified using next-generation sequencing (Foundation Medicine panel, FMI). FGFR fusions were screened for using a Janssen-developed qRT-PCR assay.
Correlation of FGFR Mutations and Amplification with PD-L1
[0083] PD-L1 expression was first assessed in a set of 120 commercially sourced lung FFPE tumor tissues comprised of forty of each of the following lung tumor histologies; non-small-cell lung carcinoma (NSCLC) adenocarcinoma; NSCLC squamous cell carcinoma; and small-cell lung cancer (SCLC). FGFR mutations and gene amplification were detected using the Foundation Medicine panel. PD-L1 staining versus FGFR status was plotted for each tumor type (FIG. 1). PD-L1 expression was largely reserved to tumors without FGFR mutations or amplifications. Out of nine samples with FGFR mutations, no PD-L1 staining was observed in seven samples (78%). Two of the nine samples showed very low PD-L1 staining with H-scores of 20 and 70, respectively. Of four samples with FGFR gene amplification, one sample showed moderate-high PD-L1 staining (H-score=140), with three having almost no staining (H-score=4, n=1). No staining was observed in the one tumor sample harboring both an FGFR mutation and FGFR gene amplification. FGFR mutation and amplification status was unknown for 24 tumor samples, of which nine exhibited PD-L1 staining with H-scores ranging from 55 to 220.
FGFR Fusions and PD-L1 Expression in Bladder and NSCLC
[0084] The set of 120 lung FFPE tumor tissues was subsequently screened for FGFR fusions using a Janssen-developed qRT-PCR assay (as described in U.S. Provisional Application No. 62/056,159, U.S. Patent Publication No. US2016-0090633, and WO 2016/048833) detecting nine fusions (Table 1). Results for PD-L1 expression by FGFR fusion status for the NSCLC tumor samples (n=80) are shown in FIG. 2. Twenty-three percent (7/31) of NSCLC adenocarcinoma samples, and 52% (13/25) of NSCLC squamous cell carcinoma tumor samples were positive for FGFR fusions. All fusion-positive adenocarcinoma samples exhibited no or low PD-L1 expression, 6/7 (86%) or 1/7 (14%), respectively (Table 3). Fusion-negative adenocarcinoma samples showed a range of PD-L1 from no expression (12/31, 39%), low (12/31, 39%), moderate (4/31, 13%), to high PD-L1 (3/31, 10%) (Table 3). Fusion-positive squamous cell carcinoma sample PD-L1 H-scores were equally distributed across the no expression, low, moderate, or high expression categories (4/31, 31% each respectively) (Table 4). Fusion-negative squamous samples also showed a range of H-scores from no expression (6/25, 24%), low (11/25, 44%), moderate (5/25, 20%), and high expression (3/25, 12%) (Table 4).
TABLE-US-00004 TABLE 3 NSCLC Adenocarcinoma-PD-L1 H-Scores by FGFR fusion status NSCLC H-Score Range Adenocarcinoma 0 1-25 26-50 51-99 100-199 200-300 Category: No Low Mod. High Fusion 6 -- -- 1 -- -- Positive (86%) (14%) Fusion 12 9 2 1 4 3 Negative (39%) (29%) (6%) (3%) (13%) (10%)
TABLE-US-00005 TABLE 4 NSCLC Squamous Cell Carcinoma-PD-L1 H-Scores by FGFR fusion status NSCLC H-Score Range Squamous 0 1-25 26-50 51-99 100-199 200-300 Category: No Low Mod. High Fusion 4 2 1 1 4 1 Positive (31%) (15%) (8%) (8%) (31%) (8%) Fusion 6 8 2 1 5 3 Negative (24%) (32%) (8%) (4%) (20%) (12%)
[0085] Forty-five commercially sourced bladder tumors were sequenced for mutations by the Foundation Medicine panel (FMI), stained for PD-L1 expression, and screened for FGFR gene fusions using the Janssen qRT-PCR assay. Forty-two of 45 samples (93%) were positive for FGFR fusions. Five samples (11%) were positive for an FGFR mutation (FGFR3-R248C or FGFR3-S249C), all of which were also positive for FGFR fusions. PD-L1 staining H-scores for samples with FGFR alterations are summarized in Table 5, and listed in Table 6. For FGFR fusion positive samples, 22/37 (59%) were negative for PD-L1 staining. Ten FGFR fusion-positive samples (27%) expressed low levels of PD-L1, and five samples (14%) showed high PD-L1 expression. All samples with both FGFR mutations and FGFR fusions in the same tumor sample (n=5) were negative for PD-L1 staining. Overall, PD-L1 staining was absent in 64% (27/42) of bladder samples with FGFR alterations, keeping in mind that almost all of the tumors in this sample set were positive for FGFR fusions.
[0086] FGFR mutation and PD-L1 expression data were available for seven commercially sourced metastatic NSCLC samples with FGFR fusions (Janssen). No PD-L1 staining was observed in 4/7 (57%) of samples. Two samples exhibited very low PD-L1 staining, H-scores of 4 and 15. One sample showed moderate PD-L1 with an H-score of 160. Interestingly, the FGFR fusion-positive sample with moderate PD-L1 staining harbored an FGFR4 V550I mutation--an FGFR gatekeeper residue mutation with potential to confer resistance to tyrosine kinase inhibitors.
[0087] Overall these data show that the majority of commercially available tumor samples harboring FGFR alterations have very little expression or do not express PD-L1.
TABLE-US-00006 TABLE 5 PD-L1 staining in FGFR fusion positive bladder samples H-Score Range n = 42 0 1-25 26-50 51-99 100-199 200-300 Category: No Low Mod. High Fusion Positive 22 8 -- 2 -- 5 Fusion + 5 -- -- -- -- -- Mutation %, of Total 64% 19% 0% 5% 0% 12% FGFR+ Samples Expressing per Category
TABLE-US-00007 TABLE 6 PD-L1 expression, FGFR fusion and mutation status in commercial bladder and NSCLC tumor samples Janssen Tumor FGFR H-Score Sample ID Type FGFR Fusion Gene(s) Mutation (0-300) 2329 Bladder None None 300 2425 Bladder FGFR3:BAIA/FGFR2:CASP7/ None 300 FGFR2:OFD1 F26993.C3a Bladder FGFR3:BAIA/FGFR2:AFF/ None 300 FGFR2:CASP7/FGFR2:CCDC6 F5244.E22b Bladder FGFR2:CASP7 None 300 F28052.E14a Bladder FGFR2:BICC1/FGFR2:AFF3/ None 280 FGFR2:CCDC6 F27999.D25 Bladder FGFR3:BAIA/FGFR2:CCDC6 None 250 F7799.H25b Bladder FGFR3:BAIAP2L/FGFR2:CASP7/ None 70 FGFR2:OFD F28057.D1a Bladder FGFR3:BAIA None 60 F15377.A2 Bladder FGFR2:AFF3 None 21 F28137.G3b Bladder FGFR3:TACC3v3/FGFR2:AFF3 None 20 F7538.A1b Bladder FGFR3:BAIAP2L/FGFR2:BICC1/ None 20 FGFR:AFF3/FGFR2:CASP7 F26375.A2 Bladder FGFR3:BAIA/FGFR2:AFF/ None 18 FGFR2:CASP7 F7830.G3ba Bladder FGFR2:CASP7 None 10 F7860.B2b Bladder FGFR2:AFF3FGFR2:CASP7 None 10 F27338.C4a Bladder FGFR3:BAIA/FGFR2:CASP7 None 6 F5242.G10ba Bladder FGFR2:CASP7 None 3 2319 Bladder FGFR2:CASP7 None 0 2321 Bladder None None 0 2346 Bladder FGFR3:BAIA/FGFR2:CASP7/ None 0 FGFR2:OFD1 2347 Bladder FGFR3:BAIAP2L1/ FGFR3-S249C 0 FGFR2:CCDC6 2362 Bladder FGFR3:TACC3v1/FGFR3:TACC3v3/ FGFR3-S249C 0 FGFR3:BAIA/FGFR2:BICC1/FGFR2: AFF3/FGFR2:CASP7/FGFR2: CCDC6 2376 Bladder FGFR3:TACC3,v1/FGFR2: None 0 BICC1/FGFR2:CASP7 2381 Bladder FGFR3:BAIA/FGFR2:AFF3/ FGFR3-R248C 0 FGFR2:CASP7 FGFR3-S249C 2430 Bladder FGFR3:BAIA/FGFR2:CASP7 None 0 2434 Bladder FGFR3:BAIA None 0 2458 Bladder FGFR3:BAIA/FGFR2:AFF3/ FGFR3-R248C 0 FGFR2:CASP7 2455 Bladder None None 0 2473 Bladder FGFR2:AFF3/FGFR2:OFD1 None 0 2480 Bladder FGFR2:OFD1 None 0 2518 Bladder FGFR3:BAIA/FGFR2:AFF3/ None 0 FGFR2:CASP7/FGFGFR2:OFD1 2533 Bladder FGFR2:OFD1 None 0 2541 Bladder FGFR2:CASP7/FGFR2:OFD1 None 0 2561 Bladder FGFR3:BAIA/FGFR2:BICC1/ None 0 FGFR2:AFF3/FGFR2:CASP7 2563 Bladder FGFR2:OFD1 None 0 4916 Bladder FGFR2:OFD1 None 0 F27064.CFS Bladder FGFR3:BAIA/FGFR2:AFF/ None 0 FGFR2:CASP7 F28132.Ba Bladder FGFR3:TACC3v1/FGFR3:BAIAP2L/ None 0 FGFR2:BICC1/FGFR2:CCDC6 F7269.C2 Bladder FGFR3:BAIAP2L/FGFR2:CASP7 None 0 F7271.AFSb Bladder FGFR2:AFF3/FGFR2:CASP7 None 0 F7467.D1bb Bladder FGFR2:AFF3/FGFR2:CASP7/ None 0 FGFR2:CCDC6 F7484.BFSc Bladder FGFR2:AFF3 None 0 F7502.D1b Bladder FGFR2:AFF3/FGFR2:CASP7 FGFR3-S249C 0 F7789.DFSb Bladder FGFR3:BAIAP2L/FGFR2:CASP7 FGFR2-M537I 0 F7876.D1bb Bladder FGFR3:BAIAP2L/FGFR2:OFD1 None 0 I-7290.E13a Bladder FGFR2:CASP7 None 0 CNT06GK NSCLC FGFR3:TACC3intron FGFR4-V550I 160 CNT0RHX NSCLC FGFR3:BAIAP2L None 15 CNT0RFD NSCLC FGFR2:BICC1 None 4 CNT06FI NSCLC FGFR2:AFF3 None 0 CNT06FJ NSCLC FGFR2:CCDC6 None 0 CNT06G5 NSCLC FGFR3:TACC3v1/FGFR3:TACC3intron/ None 0 FGFR2:AFF3 CNT0RFX NSCLC FGFR3:BAIAP2L/FGFR2:CASP7 None 0
FGFR In Vitro Experiments
[0088] To determine the effects of JNJ427564493 on immune cell viability in vitro, peripheral blood mononuclear cells (PBMCs) from normal donors were stimulated with anti-CD3 antibodies to activate T cells, in the presence of increasing concentrations of JNJ42756493. Unstimulated PBMCs were also included to determine if JNJ42756493 affected unactivated immune populations. Cell viability was assessed at four different time points, over 6 days. FIG. 3 shows the luminescence signal, as a measurement of cell viability, in the presence of increasing concentrations of JNJ42756493 (up to 1 .mu.M) at days 1, 2, 5 and 6 post-treatment. For both the stimulated and unstimulated groups, at all time points tested, cell viability remained constant with increasing concentrations of compound. These data suggest that the addition of JNJ42756493 does not impair immune cell viability.
[0089] JNJ42756493 was next tested to analyze the impact on the activity of anti-PD-1 antibodies in two in vitro functional assays: Mixed Lymphocyte Reaction (MLR); and Cytomegalovirus antigen assay (CMV). For the MLR assay, CD4.sup.+ T cells are stimulated with allogeneic dendritic cells, leading to T cell activation and IFN-.gamma. secretion. In this assay, anti-PD-1 antibodies caused dose-dependent increases in IFN-.gamma. levels (FIG. 4, PD-1 alone). When T cells and DCs were treated with 0.01, 1 or 100 nM of JNJ42756493, IFN-.gamma. levels were similar to those observed in the untreated samples (FIG. 4, JNJ-493 alone vs controls), suggesting that FGFR inhibition does not affect T cell activation. Furthermore, combinations of JNJ42756493 with anti-PD-1 antibodies caused similar IFN-.gamma. secretion as observed with anti-PD-1 treatment alone (FIG. 4, JNJ-493+ anti-PD-1 compared to PD-1 alone). These results suggest that JNJ42756493 does not impair the functional activity of anti-PD-1 antibodies in the MLR assay.
[0090] In the CMV assay, PBMCs from CMV-reactive donors were stimulated by the addition of CMV antigen. CMV-reactive T cells are active, expand and secrete pro-inflammatory cytokines such as IFN-.gamma.. In the presence of anti-PD-1 antibodies, significantly higher levels of IFN-.gamma. were secreted upon CMV stimulation (FIG. 5, PD-1 alone). In contrast, JNJ42756493 alone had no impact on cytokine levels (FIG. 5, JNJ-493 alone). Similarly, JNJ42756493 combinations with anti-PD-1 antibodies led to similar increases of IFN-.gamma. as seen with anti-PD-1 alone (FIG. 5, JNJ42756493+anti-PD-1 compared to PD-1 alone). These data show that JNJ42756493 does not affect the activity of anti-PD-1 antibodies in the CMV assay.
[0091] Those skilled in the art will appreciate that numerous changes and modifications can be made to the preferred embodiments and that such changes and modifications can be made without departing from the spirit of the invention. It is, therefore, intended that the appended claims cover all such equivalent variations as fall within the true spirit and scope of the invention.
[0092] The disclosures of each patent, patent application, and publication cited or described in this document are hereby incorporated herein by reference, in its entirety.
Nucleotide Sequence of FGFR Fusion Genes
[0093] The nucleotide sequences for the FGFR fusion cDNA are provided in Table 7. The underlined sequences correspond to either FGFR3 or FGFR2, the sequences in black represent the fusion partners and the sequence in italic fonts represent the intron sequence of the FGFR3 gene.
TABLE-US-00008 TABLE 7 FGFR3: TACC3 v1 >ATGGGCGCCCCTGCCTGCGCCCTCGCGCTCTGCGTGGCCGTGGCCATCGTGGCCGGCG (2850 base pairs) CCTCCTCGGAGTCCTTGGGGACGGAGCAGCGCGTCGTGGGGCGAGCGGCAGAAGTCCCG (SEQ ID NO: 19) GGCCCAGAGCCCGGCCAGCAGGAGCAGTTGGTCTTCGGCAGCGGGGATGCTGTGGAGCT GAGCTGTCCCCCGCCCGGGGGTGGTCCCATGGGGCCCACTGTCTGGGTCAAGGATGGCA CAGGGCTGGTGCCCTCGGAGCGTGTCCTGGTGGGGCCCCAGCGGCTGCAGGTGCTGAAT GCCTCCCACGAGGACTCCGGGGCCTACAGCTGCCGGCAGCGGCTCACGCAGCGCGTACT GTGCCACTTCAGTGTGCGGGTGACAGACGCTCCATCCTCGGGAGATGACGAAGACGGGG AGGACGAGGCTGAGGACACAGGTGTGGACACAGGGGCCCCTTACTGGACACGGCCCGAG CGGATGGACAAGAAGCTGCTGGCCGTGCCGGCCGCCAACACCGTCCGCTTCCGCTGCCC AGCCGCTGGCAACCCCACTCCCTCCATCTCCTGGCTGAAGAACGGCAGGGAGTTCCGCG GCGAGCACCGCATTGGAGGCATCAAGCTGCGGCATCAGCAGTGGAGCCTGGTCATGGAA AGCGTGGTGCCCTCGGACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAG CATCCGGCAGACGTACACGCTGGACGTGCTGGAGCGCTCCCCGCACCGGCCCATCCTGC AGGCGGGGCTGCCGGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGGAGTTCCACTGC AAGGTGTACAGTGACGCACAGCCCCACATCCAGTGGCTCAAGCACGTGGAGGTGAATGG CAGCAAGGTGGGCCCGGACGGCACACCCTACGTTACCGTGCTCAAGACGGCGGGCGCTA ACACCACCGACAAGGAGCTAGAGGTTCTCTCCTTGCACAACGTCACCTTTGAGGACGCC GGGGAGTACACCTGCCTGGCGGGCAATTCTATTGGGTTTTCTCATCACTCTGCGTGGCT GGTGGTGCTGCCAGCCGAGGAGGAGCTGGTGGAGGCTGACGAGGCGGGCAGTGTGTATG CAGGCATCCTCAGCTACGGGGTGGGCTTCTTCCTGTTCATCCTGGTGGTGGCGGCTGTG ACGCTCTGCCGCCTGCGCAGCCCCCCCAAGAAAGGCCTGGGCTCCCCCACCGTGCACAA GATCTCCCGCTTCCCGCTCAAGCGACAGGTGTCCCTGGAGTCCAACGCGTCCATGAGCT CCAACACACCACTGGTGCGCATCGCAAGGCTGTCCTCAGGGGAGGGCCCCACGCTGGCC AATGTCTCCGAGCTCGAGCTGCCTGCCGACCCCAAATGGGAGCTGTCTCGGGCCCGGCT GACCCTGGGCAAGCCCCTTGGGGAGGGCTGCTTCGGCCAGGTGGTCATGGCGGAGGCCA TCGGCATTGACAAGGACCGGGCCGCCAAGCCTGTCACCGTAGCCGTGAAGATGCTGAAA GACGATGCCACTGACAAGGACCTGTCGGACCTGGTGTCTGAGATGGAGATGATGAAGAT GATCGGGAAACACAAAAACATCATCAACCTGCTGGGCGCCTGCACGCAGGGCGGGCCCC TGTACGTGCTGGTGGAGTACGCGGCCAAGGGTAACCTGCGGGAGTTTCTGCGGGCGCGG CGGCCCCCGGGCCTGGACTACTCCTTCGACACCTGCAAGCCGCCCGAGGAGCAGCTCAC CTTCAAGGACCTGGTGTCCTGTGCCTACCAGGTGGCCCGGGGCATGGAGTACTTGGCCT CCCAGAAGTGCATCCACAGGGACCTGGCTGCCCGCAATGTGCTGGTGACCGAGGACAAC GTGATGAAGATCGCAGACTTCGGGCTGGCCCGGGACGTGCACAACCTCGACTACTACAA GAAGACGACCAACGGCCGGCTGCCCGTGAAGTGGATGGCGCCTGAGGCCTTGTTTGACC GAGTCTACACTCACCAGAGTGACGTCTGGTCCTTTGGGGTCCTGCTCTGGGAGATCTTC ACGCTGGGGGGCTCCCCGTACCCCGGCATCCCTGTGGAGGAGCTCTTCAAGCTGCTGAA GGAGGGCCACCGCATGGACAAGCCCGCCAACTGCACACACGACCTGTACATGATCATGC GGGAGTGCTGGCATGCCGCGCCCTCCCAGAGGCCCACCTTCAAGCAGCTGGTGGAGGAC CTGGACCGTGTCCTTACCGTGACGTCCACCGACGTAAAGGCGACACAGGAGGAGAACCG GGAGCTGAGGAGCAGGTGTGAGGAGCTCCACGGGAAGAACCTGGAACTGGGGAAGATCA TGGACAGGTTCGAAGAGGTTGTGTACCAGGCCATGGAGGAAGTTCAGAAGCAGAAGGAA CTTTCCAAAGCTGAAATCCAGAAAGTTCTAAAAGAAAAAGACCAACTTACCACAGATCT GAACTCCATGGAGAAGTCCTTCTCCGACCTCTTCAAGCGTTTTGAGAAACAGAAAGAGG TGATCGAGGGCTACCGCAAGAACGAAGAGTCACTGAAGAAGTGCGTGGAGGATTACCTG GCAAGGATCACCCAGGAGGGCCAGAGGTACCAAGCCCTGAAGGCCCACGCGGAGGAGAA GCTGCAGCTGGCAAACGAGGAGATCGCCCAGGTCCGGAGCAAGGCCCAGGCGGAAGCGT TGGCCCTCCAGGCCAGCCTGAGGAAGGAGCAGATGCGCATCCAGTCGCTGGAGAAGACA GTGGAGCAGAAGACTAAAGAGAACGAGGAGCTGACCAGGATCTGCGACGACCTCATCTC CAAGATGGAGAAGATCTGA FGFR3: TACC3 v3 >ATGGGCGCCCCTGCCTGCGCCCTCGCGCTCTGCGTGGCCGTGGCCATCGTGGCCGGCG (2955 base pairs) CCTCCTCGGAGTCCTTGGGGACGGAGCAGCGCGTCGTGGGGCGAGCGGCAGAAGTCCCG (SEQ ID NO: 20) GGCCCAGAGCCCGGCCAGCAGGAGCAGTTGGTCTTCGGCAGCGGGGATGCTGTGGAGCT GAGCTGTCCCCCGCCCGGGGGTGGTCCCATGGGGCCCACTGTCTGGGTCAAGGATGGCA CAGGGCTGGTGCCCTCGGAGCGTGTCCTGGTGGGGCCCCAGCGGCTGCAGGTGCTGAAT GCCTCCCACGAGGACTCCGGGGCCTACAGCTGCCGGCAGCGGCTCACGCAGCGCGTACT GTGCCACTTCAGTGTGCGGGTGACAGACGCTCCATCCTCGGGAGATGACGAAGACGGGG AGGACGAGGCTGAGGACACAGGTGTGGACACAGGGGCCCCTTACTGGACACGGCCCGAG CGGATGGACAAGAAGCTGCTGGCCGTGCCGGCCGCCAACACCGTCCGCTTCCGCTGCCC AGCCGCTGGCAACCCCACTCCCTCCATCTCCTGGCTGAAGAACGGCAGGGAGTTCCGCG GCGAGCACCGCATTGGAGGCATCAAGCTGCGGCATCAGCAGTGGAGCCTGGTCATGGAA AGCGTGGTGCCCTCGGACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAG CATCCGGCAGACGTACACGCTGGACGTGCTGGAGCGCTCCCCGCACCGGCCCATCCTGC AGGCGGGGCTGCCGGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGGAGTTCCACTGC AAGGTGTACAGTGACGCACAGCCCCACATCCAGTGGCTCAAGCACGTGGAGGTGAATGG CAGCAAGGTGGGCCCGGACGGCACACCCTACGTTACCGTGCTCAAGACGGCGGGCGCTA ACACCACCGACAAGGAGCTAGAGGTTCTCTCCTTGCACAACGTCACCTTTGAGGACGCC GGGGAGTACACCTGCCTGGCGGGCAATTCTATTGGGTTTTCTCATCACTCTGCGTGGCT GGTGGTGCTGCCAGCCGAGGAGGAGCTGGTGGAGGCTGACGAGGCGGGCAGTGTGTATG CAGGCATCCTCAGCTACGGGGTGGGCTTCTTCCTGTTCATCCTGGTGGTGGCGGCTGTG ACGCTCTGCCGCCTGCGCAGCCCCCCCAAGAAAGGCCTGGGCTCCCCCACCGTGCACAA GATCTCCCGCTTCCCGCTCAAGCGACAGGTGTCCCTGGAGTCCAACGCGTCCATGAGCT CCAACACACCACTGGTGCGCATCGCAAGGCTGTCCTCAGGGGAGGGCCCCACGCTGGCC AATGTCTCCGAGCTCGAGCTGCCTGCCGACCCCAAATGGGAGCTGTCTCGGGCCCGGCT GACCCTGGGCAAGCCCCTTGGGGAGGGCTGCTTCGGCCAGGTGGTCATGGCGGAGGCCA TCGGCATTGACAAGGACCGGGCCGCCAAGCCTGTCACCGTAGCCGTGAAGATGCTGAAA GACGATGCCACTGACAAGGACCTGTCGGACCTGGTGTCTGAGATGGAGATGATGAAGAT GATCGGGAAACACAAAAACATCATCAACCTGCTGGGCGCCTGCACGCAGGGCGGGCCCC TGTACGTGCTGGTGGAGTACGCGGCCAAGGGTAACCTGCGGGAGTTTCTGCGGGCGCGG CGGCCCCCGGGCCTGGACTACTCCTTCGACACCTGCAAGCCGCCCGAGGAGCAGCTCAC CTTCAAGGACCTGGTGTCCTGTGCCTACCAGGTGGCCCGGGGCATGGAGTACTTGGCCT CCCAGAAGTGCATCCACAGGGACCTGGCTGCCCGCAATGTGCTGGTGACCGAGGACAAC GTGATGAAGATCGCAGACTTCGGGCTGGCCCGGGACGTGCACAACCTCGACTACTACAA GAAGACGACCAACGGCCGGCTGCCCGTGAAGTGGATGGCGCCTGAGGCCTTGTTTGACC GAGTCTACACTCACCAGAGTGACGTCTGGTCCTTTGGGGTCCTGCTCTGGGAGATCTTC ACGCTGGGGGGCTCCCCGTACCCCGGCATCCCTGTGGAGGAGCTCTTCAAGCTGCTGAA GGAGGGCCACCGCATGGACAAGCCCGCCAACTGCACACACGACCTGTACATGATCATGC GGGAGTGCTGGCATGCCGCGCCCTCCCAGAGGCCCACCTTCAAGCAGCTGGTGGAGGAC CTGGACCGTGTCCTTACCGTGACGTCCACCGACGTGCCAGGCCCACCCCCAGGTGTTCC CGCGCCTGGGGGCCCACCCCTGTCCACCGGACCTATAGTGGACCTGCTCCAGTACAGCC AGAAGGACCTGGATGCAGTGGTAAAGGCGACACAGGAGGAGAACCGGGAGCTGAGGAGC AGGTGTGAGGAGCTCCACGGGAAGAACCTGGAACTGGGGAAGATCATGGACAGGTTCGA AGAGGTTGTGTACCAGGCCATGGAGGAAGTTCAGAAGCAGAAGGAACTTTCCAAAGCTG AAATCCAGAAAGTTCTAAAAGAAAAAGACCAACTTACCACAGATCTGAACTCCATGGAG AAGTCCTTCTCCGACCTCTTCAAGCGTTTTGAGAAACAGAAAGAGGTGATCGAGGGCTA CCGCAAGAACGAAGAGTCACTGAAGAAGTGCGTGGAGGATTACCTGGCAAGGATCACCC AGGAGGGCCAGAGGTACCAAGCCCTGAAGGCCCACGCGGAGGAGAAGCTGCAGCTGGCA AACGAGGAGATCGCCCAGGTCCGGAGCAAGGCCCAGGCGGAAGCGTTGGCCCTCCAGGC CAGCCTGAGGAAGGAGCAGATGCGCATCCAGTCGCTGGAGAAGACAGTGGAGCAGAAGA CTAAAGAGAACGAGGAGCTGACCAGGATCTGCGACGACCTCATCTCCAAGATGGAGAAG ATCTGA FGFR3 >ATGGGCGCCCCTGCCTGCGCCCTCGCGCTCTGCGTGGCCGTGGCCATCGTGGCCGGCG Intron: TACC3 CCTCCTCGGAGTCCTTGGGGACGGAGCAGCGCGTCGTGGGGCGAGCGGCAGAAGTCCCG (4462 base pairs) GGCCCAGAGCCCGGCCAGCAGGAGCAGTTGGTCTTCGGCAGCGGGGATGCTGTGGAGCT (SEQ ID NO: 21) GAGCTGTCCCCCGCCCGGGGGTGGTCCCATGGGGCCCACTGTCTGGGTCAAGGATGGCA CAGGGCTGGTGCCCTCGGAGCGTGTCCTGGTGGGGCCCCAGCGGCTGCAGGTGCTGAAT GCCTCCCACGAGGACTCCGGGGCCTACAGCTGCCGGCAGCGGCTCACGCAGCGCGTACT GTGCCACTTCAGTGTGCGGGTGACAGACGCTCCATCCTCGGGAGATGACGAAGACGGGG AGGACGAGGCTGAGGACACAGGTGTGGACACAGGGGCCCCTTACTGGACACGGCCCGAG CGGATGGACAAGAAGCTGCTGGCCGTGCCGGCCGCCAACACCGTCCGCTTCCGCTGCCC AGCCGCTGGCAACCCCACTCCCTCCATCTCCTGGCTGAAGAACGGCAGGGAGTTCCGCG GCGAGCACCGCATTGGAGGCATCAAGCTGCGGCATCAGCAGTGGAGCCTGGTCATGGAA AGCGTGGTGCCCTCGGACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAG CATCCGGCAGACGTACACGCTGGACGTGCTGGAGCGCTCCCCGCACCGGCCCATCCTGC AGGCGGGGCTGCCGGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGGAGTTCCACTGC AAGGTGTACAGTGACGCACAGCCCCACATCCAGTGGCTCAAGCACGTGGAGGTGAATGG CAGCAAGGTGGGCCCGGACGGCACACCCTACGTTACCGTGCTCAAGACGGCGGGCGCTA ACACCACCGACAAGGAGCTAGAGGTTCTCTCCTTGCACAACGTCACCTTTGAGGACGCC GGGGAGTACACCTGCCTGGCGGGCAATTCTATTGGGTTTTCTCATCACTCTGCGTGGCT GGTGGTGCTGCCAGCCGAGGAGGAGCTGGTGGAGGCTGACGAGGCGGGCAGTGTGTATG CAGGCATCCTCAGCTACGGGGTGGGCTTCTTCCTGTTCATCCTGGTGGTGGCGGCTGTG ACGCTCTGCCGCCTGCGCAGCCCCCCCAAGAAAGGCCTGGGCTCCCCCACCGTGCACAA GATCTCCCGCTTCCCGCTCAAGCGACAGGTGTCCCTGGAGTCCAACGCGTCCATGAGCT CCAACACACCACTGGTGCGCATCGCAAGGCTGTCCTCAGGGGAGGGCCCCACGCTGGCC AATGTCTCCGAGCTCGAGCTGCCTGCCGACCCCAAATGGGAGCTGTCTCGGGCCCGGCT GACCCTGGGCAAGCCCCTTGGGGAGGGCTGCTTCGGCCAGGTGGTCATGGCGGAGGCCA TCGGCATTGACAAGGACCGGGCCGCCAAGCCTGTCACCGTAGCCGTGAAGATGCTGAAA GACGATGCCACTGACAAGGACCTGTCGGACCTGGTGTCTGAGATGGAGATGATGAAGAT GATCGGGAAACACAAAAACATCATCAACCTGCTGGGCGCCTGCACGCAGGGCGGGCCCC TGTACGTGCTGGTGGAGTACGCGGCCAAGGGTAACCTGCGGGAGTTTCTGCGGGCGCGG CGGCCCCCGGGCCTGGACTACTCCTTCGACACCTGCAAGCCGCCCGAGGAGCAGCTCAC CTTCAAGGACCTGGTGTCCTGTGCCTACCAGGTGGCCCGGGGCATGGAGTACTTGGCCT CCCAGAAGTGCATCCACAGGGACCTGGCTGCCCGCAATGTGCTGGTGACCGAGGACAAC GTGATGAAGATCGCAGACTTCGGGCTGGCCCGGGACGTGCACAACCTCGACTACTACAA GAAGACGACCAACGGCCGGCTGCCCGTGAAGTGGATGGCGCCTGAGGCCTTGTTTGACC GAGTCTACACTCACCAGAGTGACGTCTGGTCCTTTGGGGTCCTGCTCTGGGAGATCTTC ACGCTGGGGGGCTCCCCGTACCCCGGCATCCCTGTGGAGGAGCTCTTCAAGCTGCTGAA GGAGGGCCACCGCATGGACAAGCCCGCCAACTGCACACACGACCTGTACATGATCATGC GGGAGTGCTGGCATGCCGCGCCCTCCCAGAGGCCCACCTTCAAGCAGCTGGTGGAGGAC CTGGACCGTGTCCTTACCGTGACGTCCACCGACgtgagtgctggctctggcctggtgcc acccgcctatgcccctccccctgccgtccccggccatcctgccccccagagtgctgagg tgtggggcgggccttTCTGGCCCAGGTGCCCTGGCTGACCTGGACTGCTCAAGCTCTTC CCAGAGCCCAGGAAGTTCTGAGAACCAAATGGTGTCTCCAGGAAAAGTGTCTGGCAGCC CTGAGCAAGCCGTGGAGGAAAACCTTAGTTCCTATTCCTTAGACAGAAGAGTGACACCC GCCTCTGAGACCCTAGAAGACCCTTGCAGGACAGAGTCCCAGCACAAAGCGGAGACTCC GCACGGAGCCGAGGAAGAATGCAAAGCGGAGACTCCGCACGGAGCCGAGGAGGAATGCC GGCACGGTGGGGTCTGTGCTCCCGCAGCAGTGGCCACTTCGCCTCCTGGTGCAATCCCT AAGGAAGCCTGCGGAGGAGCACCCCTGCAGGGTCTGCCTGGCGAAGCCCTGGGCTGCCC TGCGGGTGTGGGCACCCCCGTGCCAGCAGATGGCACTCAGACCCTTACCTGTGCACACA CCTCTGCTCCTGAGAGCACAGCCCCAACCAACCACCTGGTGGCTGGCAGGGCCATGACC CTGAGTCCTCAGGAAGAAGTGGCTGCAGGCCAAATGGCCAGCTCCTCGAGGAGCGGACC TGTAAAACTAGAATTTGATGTATCTGATGGCGCCACCAGCAAAAGGGCACCCCCACCAA GGAGACTGGGAGAGAGGTCCGGCCTCAAGCCTCCCTTGAGGAAAGCAGCAGTGAGGCAG CAAAAGGCCCCGCAGGAGGTGGAGGAGGACGACGGTAGGAGCGGAGCAGGAGAGGACCC CCCCATGCCAGCTTCTCGGGGCTCTTACCACCTCGACTGGGACAAAATGGATGACCCAA ACTTCATCCCGTTCGGAGGTGACACCAAGTCTGGTTGCAGTGAGGCCCAGCCCCCAGAA AGCCCTGAGACCAGGCTGGGCCAGCCAGCGGCTGAACAGTTGCATGCTGGGCCTGCCAC GGAGGAGCCAGGTCCCTGTCTGAGCCAGCAGCTGCATTCAGCCTCAGCGGAGGACACGC CTGTGGTGCAGTTGGCAGCCGAGACCCCAACAGCAGAGAGCAAGGAGAGAGCCTTGAAC TCTGCCAGCACCTCGCTTCCCACAAGCTGTCCAGGCAGTGAGCCAGTGCCCACCCATCA GCAGGGGCAGCCTGCCTTGGAGCTGAAAGAGGAGAGCTTCAGAGACCCCGCTGAGGTTC TAGGCACGGGCGCGGAGGTGGATTACCTGGAGCAGTTTGGAACTTCCTCGTTTAAGGAG TCGGCCTTGAGGAAGCAGTCCTTATACCTCAAGTTCGACCCCCTCCTGAGGGACAGTCC TGGTAGACCAGTGCCCGTGGCCACCGAGACCAGCAGCATGCACGGTGCAAATGAGACTC CCTCAGGACGTCCGCGGGAAGCCAAGCTTGTGGAGTTCGATTTCTTGGGAGCACTGGAC ATTCCTGTGCCAGGCCCACCCCCAGGTGTTCCCGCGCCTGGGGGCCCACCCCTGTCCAC CGGACCTATAGTGGACCTGCTCCAGTACAGCCAGAAGGACCTGGATGCAGTGGTAAAGG CGACACAGGAGGAGAACCGGGAGCTGAGGAGCAGGTGTGAGGAGCTCCACGGGAAGAAC CTGGAACTGGGGAAGATCATGGACAGGTTCGAAGAGGTTGTGTACCAGGCCATGGAGGA AGTTCAGAAGCAGAAGGAACTTTCCAAAGCTGAAATCCAGAAAGTTCTAAAAGAAAAAG ACCAACTTACCACAGATCTGAACTCCATGGAGAAGTCCTTCTCCGACCTCTTCAAGCGT TTTGAGAAACAGAAAGAGGTGATCGAGGGCTACCGCAAGAACGAAGAGTCACTGAAGAA GTGCGTGGAGGATTACCTGGCAAGGATCACCCAGGAGGGCCAGAGGTACCAAGCCCTGA AGGCCCACGCGGAGGAGAAGCTGCAGCTGGCAAACGAGGAGATCGCCCAGGTCCGGAGC AAGGCCCAGGCGGAAGCGTTGGCCCTCCAGGCCAGCCTGAGGAAGGAGCAGATGCGCAT CCAGTCGCTGGAGAAGACAGTGGAGCAGAAGACTAAAGAGAACGAGGAGCTGACCAGGA TCTGCGACGACCTCATCTCCAAGATGGAGAAGATCTGA FGFR3: BAIAP2L1 >ATGGGCGCCCCTGCCTGCGCCCTCGCGCTCTGCGTGGCCGTGGCCATCGTGGCCGGCG (3765 base pairs) CCTCCTCGGAGTCCTTGGGGACGGAGCAGCGCGTCGTGGGGCGAGCGGCAGAAGTCCCG (SEQ ID NO: 22) GGCCCAGAGCCCGGCCAGCAGGAGCAGTTGGTCTTCGGCAGCGGGGATGCTGTGGAGCT GAGCTGTCCCCCGCCCGGGGGTGGTCCCATGGGGCCCACTGTCTGGGTCAAGGATGGCA CAGGGCTGGTGCCCTCGGAGCGTGTCCTGGTGGGGCCCCAGCGGCTGCAGGTGCTGAAT GCCTCCCACGAGGACTCCGGGGCCTACAGCTGCCGGCAGCGGCTCACGCAGCGCGTACT GTGCCACTTCAGTGTGCGGGTGACAGACGCTCCATCCTCGGGAGATGACGAAGACGGGG AGGACGAGGCTGAGGACACAGGTGTGGACACAGGGGCCCCTTACTGGACACGGCCCGAG CGGATGGACAAGAAGCTGCTGGCCGTGCCGGCCGCCAACACCGTCCGCTTCCGCTGCCC AGCCGCTGGCAACCCCACTCCCTCCATCTCCTGGCTGAAGAACGGCAGGGAGTTCCGCG GCGAGCACCGCATTGGAGGCATCAAGCTGCGGCATCAGCAGTGGAGCCTGGTCATGGAA AGCGTGGTGCCCTCGGACCGCGGCAACTACACCTGCGTCGTGGAGAACAAGTTTGGCAG GCAGCATCCGGCAGACGTACACGCTGGACGTGCTGGAGCGCTCCCCGCACCGGCCCATC CTGCAGGCGGGGCTGCCGGCCAACCAGACGGCGGTGCTGGGCAGCGACGTGGAGTTCCA CTGCAAGGTGTACAGTGACGCACAGCCCCACATCCAGTGGCTCAAGCACGTGGAGGTGA ATGGCAGCAAGGTGGGCCCGGACGGCACACCCTACGTTACCGTGCTCAAGTCCTGGATC AGTGAGAGTGTGGAGGCCGACGTGCGCCTCCGCCTGGCCAATGTGTCGGAGCGGGACGG GGGCGAGTACCTCTGTCGAGCCACCAATTTCATAGGCGTGGCCGAGAAGGCCTTTTGGC TGAGCGTTCACGGGCCCCGAGCAGCCGAGGAGGAGCTGGTGGAGGCTGACGAGGCGGGC AGTGTGTATGCAGGCATCCTCAGCTACGGGGTGGGCTTCTTCCTGTTCATCCTGGTGGT GGCGGCTGTGACGCTCTGCCGCCTGCGCAGCCCCCCCAAGAAAGGCCTGGGCTCCCCCA CCGTGCACAAGATCTCCCGCTTCCCGCTCAAGCGACAGGTGTCCCTGGAGTCCAACGCG TCCATGAGCTCCAACACACCACTGGTGCGCATCGCAAGGCTGTCCTCAGGGGAGGGCCC CACGCTGGCCAATGTCTCCGAGCTCGAGCTGCCTGCCGACCCCAAATGGGAGCTGTCTC GGGCCCGGCTGACCCTGGGCAAGCCCCTTGGGGAGGGCTGCTTCGGCCAGGTGGTCATG GCGGAGGCCATCGGCATTGACAAGGACCGGGCCGCCAAGCCTGTCACCGTAGCCGTGAA GATGCTGAAAGACGATGCCACTGACAAGGACCTGTCGGACCTGGTGTCTGAGATGGAGA TGATGAAGATGATCGGGAAACACAAAAACATCATCAACCTGCTGGGCGCCTGCACGCAG GGCGGGCCCCTGTACGTGCTGGTGGAGTACGCGGCCAAGGGTAACCTGCGGGAGTTTCT GCGGGCGCGGCGGCCCCCGGGCCTGGACTACTCCTTCGACACCTGCAAGCCGCCCGAGG AGCAGCTCACCTTCAAGGACCTGGTGTCCTGTGCCTACCAGGTGGCCCGGGGCATGGAG TACTTGGCCTCCCAGAAGTGCATCCACAGGGACCTGGCTGCCCGCAATGTGCTGGTGAC CGAGGACAACGTGATGAAGATCGCAGACTTCGGGCTGGCCCGGGACGTGCACAACCTCG ACTACTACAAGAAGACGACCAACGGCCGGCTGCCCGTGAAGTGGATGGCGCCTGAGGCC TTGTTTGACCGAGTCTACACTCACCAGAGTGACGTCTGGTCCTTTGGGGTCCTGCTCTG GGAGATCTTCACGCTGGGGGGCTCCCCGTACCCCGGCATCCCTGTGGAGGAGCTCTTCA AGCTGCTGAAGGAGGGCCACCGCATGGACAAGCCCGCCAACTGCACACACGACCTGTAC ATGATCATGCGGGAGTGCTGGCATGCCGCGCCCTCCCAGAGGCCCACCTTCAAGCAGCT GGTGGAGGACCTGGACCGTGTCCTTACCGTGACGTCCACCGACAATGTTATGGAACAGT TCAATCCTGGGCTGCGAAATTTAATAAACCTGGGGAAAAATTATGAGAAAGCTGTAAAC GCTATGATCCTGGCAGGAAAAGCCTACTACGATGGAGTGGCCAAGATCGGTGAGATTGC CACTGGGTCCCCCGTGTCAACTGAACTGGGACATGTCCTCATAGAGATTTCAAGTACCC ACAAGAAACTCAACGAGAGTCTTGATGAAAATTTTAAAAAATTCCACAAAGAGATTATC CATGAGCTGGAGAAGAAGATAGAACTTGACGTGAAATATATGAACGCAACTCTAAAAAG ATACCAAACAGAACACAAGAATAAATTAGAGTCTTTGGAGAAATCCCAAGCTGAGTTGA AGAAGATCAGAAGGAAAAGCCAAGGAAGCCGAAACGCACTCAAATATGAACACAAAGAA ATTGAGTATGTGGAGACCGTTACTTCTCGTCAGAGTGAAATCCAGAAATTCATTGCAGA TGGTTGCAAAGAGGCTCTGCTTGAAGAGAAGAGGCGCTTCTGCTTTCTGGTTGATAAGC ACTGTGGCTTTGCAAACCACATACATTATTATCACTTACAGTCTGCAGAACTACTGAAT TCCAAGCTGCCTCGGTGGCAGGAGACCTGTGTTGATGCCATCAAAGTGCCAGAGAAAAT CATGAATATGATCGAAGAAATAAAGACCCCAGCCTCTACCCCCGTGTCTGGAACTCCTC AGGCTTCACCCATGATCGAGAGAAGCAATGTGGTTAGGAAAGATTACGACACCCTTTCT AAATGCTCACCAAAGATGCCCCCCGCTCCTTCAGGCAGAGCATATACCAGTCCCTTGAT CGATATGTTTAATAACCCAGCCACGGCTGCCCCGAATTCACAAAGGGTAAATAATTCAA CAGGTACTTCCGAAGATCCCAGTTTACAGCGATCAGTTTCGGTTGCAACGGGACTGAAC ATGATGAAGAAGCAGAAAGTGAAGACCATCTTCCCGCACACTGCGGGCTCCAACAAGAC CTTACTCAGCTTTGCACAGGGAGATGTCATCACGCTGCTCATCCCCGAGGAGAAGGATG GCTGGCTCTATGGAGAACACGACGTGTCCAAGGCGAGGGGTTGGTTCCCGTCGTCGTAC
ACGAAGTTGCTGGAAGAAAATGAGACAGAAGCAGTGACCGTGCCCACGCCAAGCCCCAC ACCAGTGAGAAGCATCAGCACCGTGAACTTGTCTGAGAATAGCAGTGTTGTCATCCCCC CACCCGACTACTTGGAATGCTTGTCCATGGGGGCAGCTGCCGACAGGAGAGCAGATTCG GCCAGGACGACATCCACCTTTAAGGCCCCAGCGTCCAAGCCCGAGACCGCGGCTCCTAA CGATGCCAACGGGACTGCAAAGCCGCCTTTTCTCAGCGGAGAAAACCCCTTTGCCACTG TGAAACTCCGCCCGACTGTGACGAATGATCGCTCGGCACCCATCATTCGATGA FGFR2: BICC1 >ATGGTCAGCTGGGGTCGTTTCATCTGCCTGGTCGTGGTCACCATGGCAACCTTGTCCC (4989 base pairs) TGGCCCGGCCCTCCTTCAGTTTAGTTGAGGATACCACATTAGAGCCAGAAGAGCCACCA (SEQ ID NO: 23) ACCAAATACCAAATCTCTCAACCAGAAGTGTACGTGGCTGCGCCAGGGGAGTCGCTAGA GGTGCGCTGCCTGTTGAAAGATGCCGCCGTGATCAGTTGGACTAAGGATGGGGTGCACT TGGGGCCCAACAATAGGACAGTGCTTATTGGGGAGTACTTGCAGATAAAGGGCGCCACG CCTAGAGACTCCGGCCTCTATGCTTGTACTGCCAGTAGGACTGTAGACAGTGAAACTTG GTACTTCATGGTGAATGTCACAGATGCCATCTCATCCGGAGATGATGAGGATGACACCG ATGGTGCGGAAGATTTTGTCAGTGAGAACAGTAACAACAAGAGAGCACCATACTGGACC AACACAGAAAAGATGGAAAAGCGGCTCCATGCTGTGCCTGCGGCCAACACTGTCAAGTT TCGCTGCCCAGCCGGGGGGAACCCAATGCCAACCATGCGGTGGCTGAAAAACGGGAAGG AGTTTAAGCAGGAGCATCGCATTGGAGGCTACAAGGTACGAAACCAGCACTGGAGCCTC ATTATGGAAAGTGTGGTCCCATCTGACAAGGGAAATTATACCTGTGTAGTGGAGAATGA ATACGGGTCCATCAATCACACGTACCACCTGGATGTTGTGGAGCGATCGCCTCACCGGC CCATCCTCCAAGCCGGACTGCCGGCAAATGCCTCCACAGTGGTCGGAGGAGACGTAGAG TTTGTCTGCAAGGTTTACAGTGATGCCCAGCCCCACATCCAGTGGATCAAGCACGTGGA AAAGAACGGCAGTAAATACGGGCCCGACGGGCTGCCCTACCTCAAGGTTCTCAAGGCCG CCGGTGTTAACACCACGGACAAAGAGATTGAGGTTCTCTATATTCGGAATGTAACTTTT GAGGACGCTGGGGAATATACGTGCTTGGCGGGTAATTCTATTGGGATATCCTTTCACTC TGCATGGTTGACAGTTCTGCCAGCGCCTGGAAGAGAAAAGGAGATTACAGCTTCCCCAG ACTACCTGGAGATAGCCATTTACTGCATAGGGGTCTTCTTAATCGCCTGTATGGTGGTA ACAGTCATCCTGTGCCGAATGAAGAACACGACCAAGAAGCCAGACTTCAGCAGCCAGCC GGCTGTGCACAAGCTGACCAAACGTATCCCCCTGCGGAGACAGGTAACAGTTTCGGCTG AGTCCAGCTCCTCCATGAACTCCAACACCCCGCTGGTGAGGATAACAACACGCCTCTCT TCAACGGCAGACACCCCCATGCTGGCAGGGGTCTCCGAGTATGAACTTCCAGAGGACCC AAAATGGGAGTTTCCAAGAGATAAGCTGACACTGGGCAAGCCCCTGGGAGAAGGTTGCT TTGGGCAAGTGGTCATGGCGGAAGCAGTGGGAATTGACAAAGACAAGCCCAAGGAGGCG GTCACCGTGGCCGTGAAGATGTTGAAAGATGATGCCACAGAGAAAGACCTTTCTGATCT GGTGTCAGAGATGGAGATGATGAAGATGATTGGGAAACACAAGAATATCATAAATCTTC TTGGAGCCTGCACACAGGATGGGCCTCTCTATGTCATAGTTGAGTATGCCTCTAAAGGC AACCTCCGAGAATACCTCCGAGCCCGGAGGCCACCCGGGATGGAGTACTCCTATGACAT TAACCGTGTTCCTGAGGAGCAGATGACCTTCAAGGACTTGGTGTCATGCACCTACCAGC TGGCCAGAGGCATGGAGTACTTGGCTTCCCAAAAATGTATTCATCGAGATTTAGCAGCC AGAAATGTTTTGGTAACAGAAAACAATGTGATGAAAATAGCAGACTTTGGACTCGCCAG AGATATCAACAATATAGACTATTACAAAAAGACCACCAATGGGCGGCTTCCAGTCAAGT GGATGGCTCCAGAAGCCCTGTTTGATAGAGTATACACTCATCAGAGTGATGTCTGGTCC TTCGGGGTGTTAATGTGGGAGATCTTCACTTTAGGGGGCTCGCCCTACCCAGGGATTCC CGTGGAGGAACTTTTTAAGCTGCTGAAGGAAGGACACAGAATGGATAAGCCAGCCAACT GCACCAACGAACTGTACATGATGATGAGGGACTGTTGGCATGCAGTGCCCTCCCAGAGA CCAACGTTCAAGCAGTTGGTAGAAGACTTGGATCGAATTCTCACTCTCACAACCAATGA GATCATGGAGGAAACAAATACGCAGATTGCTTGGCCATCAAAACTGAAGATCGGAGCCA AATCCAAGAAAGATCCCCATATTAAGGTTTCTGGAAAGAAAGAAGATGTTAAAGAAGCC AAGGAAATGATCATGTCTGTCTTAGACACAAAAAGCAATCGAGTCACACTGAAGATGGA TGTTTCACATACAGAACATTCACATGTAATCGGCAAAGGTGGCAACAATATTAAAAAAG TGATGGAAGAAACCGGATGCCATATCCACTTTCCAGATTCCAACAGGAATAACCAAGCA GAAAAAAGCAACCAGGTATCTATAGCGGGACAACCAGCAGGAGTAGAATCTGCCCGAGT TAGAATTCGGGAGCTGCTTCCTTTGGTGCTGATGTTTGAGCTACCAATTGCTGGAATTC TTCAACCGGTTCCTGATCCTAATTCCCCCTCTATTCAGCATATATCACAAACGTACAAT ATTTCAGTATCATTTAAACAGCGTTCCCGAATGTATGGTGCTACTGTCATAGTACGAGG GTCTCAGAATAACACTAGTGCTGTGAAGGAAGGAACTGCCATGCTGTTAGAACATCTTG CTGGGAGCTTAGCATCAGCTATTCCTGTGAGCACACAACTAGATATTGCAGCTCAACAT CATCTCTTTATGATGGGTCGAAATGGGAGCAACATCAAACATATCATGCAGAGAACAGG TGCTCAGATCCACTTTCCTGATCCCAGTAATCCACAAAAGAAATCTACCGTCTACCTCC AGGGCACCATTGAGTCTGTCTGTCTTGCAAGGCAATATCTCATGGGTTGTCTTCCTCTT GTGTTGATGTTTGATATGAAGGAAGAAATTGAAGTAGATCCACAATTCATTGCGCAGTT GATGGAACAGCTTGATGTCTTCATCAGTATTAAACCAAAGCCCAAACAGCCAAGCAAGT CTGTGATTGTGAAAAGTGTTGAGCGAAATGCCTTAAATATGTATGAAGCAAGGAAATGT CTCCTCGGACTTGAAAGCAGTGGGGTTACCATAGCAACCAGTCCATCCCCAGCATCCTG CCCTGCCGGCCTGGCATGTCCCAGCCTGGATATCTTAGCTTCAGCAGGCCTTGGACTCA CTGGACTAGGTCTTTTGGGACCCACCACCTTATCTCTGAACACTTCAACAACCCCAAAC TCACTCTTGAATGCTCTTAATAGCTCAGTCAGTCCTTTGCAAAGTCCAAGTTCTGGTAC ACCCAGCCCCACATTATGGGCACCCCCACTTGCTAATACTTCAAGTGCCACAGGTTTTT CTGCTATACCACACCTTATGATTCCATCTACTGCCCAAGCCACATTAACTAATATTTTG TTGTCTGGAGTGCCCACCTATGGGCACACAGCTCCATCTCCCCCTCCTGGCTTGACTCC TGTTGATGTCCATATCAACAGTATGCAGACCGAAGGCAAAAAAATCTCTGCTGCTTTAA ATGGACATGCACAGTCTCCAGATATAAAATATGGTGCAATATCCACTTCATCACTTGGA GAAAAAGTGCTGAGTGCAAATCACGGGGATCCGTCCATCCAGACAAGTGGGTCTGAGCA GACATCTCCCAAATCAAGCCCCACTGAAGGTTGTAATGATGCTTTTGTTGAAGTAGGCA TGCCTCGAAGTCCTTCCCATTCTGGGAATGCTGGTGACTTGAAACAGATGATGTGTCCC TCCAAGGTTTCCTGTGCCAAAAGGCAGACAGTGGAACTATTGCAAGGCACGAAAAACTC ACACTTACACAGCACTGACAGGTTGCTCTCAGACCCTGAACTGAGTGCTACCGAAAGCC CTTTGGCTGACAAGAAGGCTCCAGGGAGTGAGCGCGCTGCAGAGAGGGCAGCAGCTGCC CAGCAAAACTCCGAAAGGGCCCACCTTGCTCCACGGTCATCATATGTCAACATGCAGGC ATTTGACTATGAACAGAAGAAGCTATTAGCCACCAAAGCTATGTTAAAGAAACCAGTGG TGACGGAGGTCAGAACGCCCACAAATACCTGGAGTGGCCTGGGTTTTTCTAAATCCATG CCAGCTGAAACTATCAAGGAGTTGAGAAGGGCCAATCATGTGTCCTATAAGCCCACAAT GACAACCACTTATGAGGGCTCATCCATGTCCCTTTCACGGTCCAACAGTCGTGAGCACT TGGGAGGTGGAAGCGAATCTGATAACTGGAGAGACCGAAATGGAATTGGACCTGGAAGT CATAGTGAATTTGCAGCTTCTATTGGCAGCCCTAAGCGTAAACAAAACAAATCAACGGA ACACTATCTCAGCAGTAGCAATTACATGGACTGCATTTCCTCGCTGACAGGAAGCAATG GCTGTAACTTAAATAGCTCTTTCAAAGGTTCTGACCTCCCTGAGCTCTTCAGCAAACTG GGCCTGGGCAAATACACAGATGTTTTCCAGCAACAAGAGATCGATCTTCAGACATTCCT CACTCTCACAGATCAGGATCTGAAGGAGCTGGGAATAACTACTTTTGGTGCCAGGAGGA AAATGCTGCTTGCAATTTCAGAACTAAATAAAAACCGAAGAAAGCTTTTTGAATCGCCA AATGCACGCACCTCTTTCCTGGAAGGTGGAGCGAGTGGAAGGCTACCCCGTCAGTATCA CTCAGACATTGCTAGTGTCAGTGGCCGCTGGTAG FGFR2: AFF3 >ATGGTCAGCTGGGGTCGTTTCATCTGCCTGGTCGTGGTCACCATGGCAACCTTGTCCC (5109 base pairs) TGGCCCGGCCCTCCTTCAGTTTAGTTGAGGATACCACATTAGAGCCAGAAGAGCCACCA (SEQ ID NO: 24) ACCAAATACCAAATCTCTCAACCAGAAGTGTACGTGGCTGCGCCAGGGGAGTCGCTAGA GGTGCGCTGCCTGTTGAAAGATGCCGCCGTGATCAGTTGGACTAAGGATGGGGTGCACT TGGGGCCCAACAATAGGACAGTGCTTATTGGGGAGTACTTGCAGATAAAGGGCGCCACG CCTAGAGACTCCGGCCTCTATGCTTGTACTGCCAGTAGGACTGTAGACAGTGAAACTTG GTACTTCATGGTGAATGTCACAGATGCCATCTCATCCGGAGATGATGAGGATGACACCG ATGGTGCGGAAGATTTTGTCAGTGAGAACAGTAACAACAAGAGAGCACCATACTGGACC AACACAGAAAAGATGGAAAAGCGGCTCCATGCTGTGCCTGCGGCCAACACTGTCAAGTT TCGCTGCCCAGCCGGGGGGAACCCAATGCCAACCATGCGGTGGCTGAAAAACGGGAAGG AGTTTAAGCAGGAGCATCGCATTGGAGGCTACAAGGTACGAAACCAGCACTGGAGCCTC ATTATGGAAAGTGTGGTCCCATCTGACAAGGGAAATTATACCTGTGTAGTGGAGAATGA ATACGGGTCCATCAATCACACGTACCACCTGGATGTTGTGGAGCGATCGCCTCACCGGC CCATCCTCCAAGCCGGACTGCCGGCAAATGCCTCCACAGTGGTCGGAGGAGACGTAGAG TTTGTCTGCAAGGTTTACAGTGATGCCCAGCCCCACATCCAGTGGATCAAGCACGTGGA AAAGAACGGCAGTAAATACGGGCCCGACGGGCTGCCCTACCTCAAGGTTCTCAAGGCCG CCGGTGTTAACACCACGGACAAAGAGATTGAGGTTCTCTATATTCGGAATGTAACTTTT GAGGACGCTGGGGAATATACGTGCTTGGCGGGTAATTCTATTGGGATATCCTTTCACTC TGCATGGTTGACAGTTCTGCCAGCGCCTGGAAGAGAAAAGGAGATTACAGCTTCCCCAG ACTACCTGGAGATAGCCATTTACTGCATAGGGGTCTTCTTAATCGCCTGTATGGTGGTA ACAGTCATCCTGTGCCGAATGAAGAACACGACCAAGAAGCCAGACTTCAGCAGCCAGCC GGCTGTGCACAAGCTGACCAAACGTATCCCCCTGCGGAGACAGGTAACAGTTTCGGCTG AGTCCAGCTCCTCCATGAACTCCAACACCCCGCTGGTGAGGATAACAACACGCCTCTCT TCAACGGCAGACACCCCCATGCTGGCAGGGGTCTCCGAGTATGAACTTCCAGAGGACCC AAAATGGGAGTTTCCAAGAGATAAGCTGACACTGGGCAAGCCCCTGGGAGAAGGTTGCT TTGGGCAAGTGGTCATGGCGGAAGCAGTGGGAATTGACAAAGACAAGCCCAAGGAGGCG GTCACCGTGGCCGTGAAGATGTTGAAAGATGATGCCACAGAGAAAGACCTTTCTGATCT GGTGTCAGAGATGGAGATGATGAAGATGATTGGGAAACACAAGAATATCATAAATCTTC TTGGAGCCTGCACACAGGATGGGCCTCTCTATGTCATAGTTGAGTATGCCTCTAAAGGC AACCTCCGAGAATACCTCCGAGCCCGGAGGCCACCCGGGATGGAGTACTCCTATGACAT TAACCGTGTTCCTGAGGAGCAGATGACCTTCAAGGACTTGGTGTCATGCACCTACCAGC TGGCCAGAGGCATGGAGTACTTGGCTTCCCAAAAATGTATTCATCGAGATTTAGCAGCC AGAAATGTTTTGGTAACAGAAAACAATGTGATGAAAATAGCAGACTTTGGACTCGCCAG AGATATCAACAATATAGACTATTACAAAAAGACCACCAATGGGCGGCTTCCAGTCAAGT GGATGGCTCCAGAAGCCCTGTTTGATAGAGTATACACTCATCAGAGTGATGTCTGGTCC TTCGGGGTGTTAATGTGGGAGATCTTCACTTTAGGGGGCTCGCCCTACCCAGGGATTCC CGTGGAGGAACTTTTTAAGCTGCTGAAGGAAGGACACAGAATGGATAAGCCAGCCAACT GCACCAACGAACTGTACATGATGATGAGGGACTGTTGGCATGCAGTGCCCTCCCAGAGA CCAACGTTCAAGCAGTTGGTAGAAGACTTGGATCGAATTCTCACTCTCACAACCAATGA GGAGAGTAGATCTGGAGAAACCAACAGCTGTGTTGAAGAAATAATCCGGGAGATGACCT GGCTTCCACCACTTTCTGCTATTCAAGCACCTGGCAAAGTGGAACCAACCAAATTTCCA TTTCCAAATAAGGACTCTCAGCTTGTATCCTCTGGACACAATAATCCAAAGAAAGGTGA TGCAGAGCCAGAGAGTCCAGACAGTGGCACATCGAATACATCAATGCTGGAAGATGACC TTAAGCTAAGCAGTGATGAAGAGGAGAATGAACAGCAGGCAGCTCAGAGAACGGCTCTC CGCGCTCTCTCTGACAGCGCCGTGGTCCAGCAGCCCAACTGCAGAACCTCGGTGCCTTC CAGCAAGGGCAGCAGCAGCAGCAGCAGCAGCGGCAGCAGCAGCTCCTCCAGCGACTCAG AGAGCAGCTCCGGATCTGACTCGGAGACCGAGAGCAGCTCCAGCGAGAGTGAGGGCAGC AAGCCCCCCCACTTCTCCAGCCCCGAGGCTGAACCGGCATCCTCTAACAAGTGGCAGCT GGATAAATGGCTAAACAAAGTTAATCCCCACAAGCCTCCTATTCTGATCCAAAATGAAA GCCACGGGTCAGAGAGCAATCAGTACTACAACCCGGTGAAAGAGGACGTCCAGGACTGT GGGAAAGTCCCCGACGTTTGCCAGCCCAGCCTGAGAGAGAAGGAGATCAAGAGCACTTG CAAGGAGGAGCAAAGGCCAAGGACAGCCAACAAGGCCCCTGGGAGTAAAGGCGTGAAGC AGAAGTCCCCGCCCGCGGCCGTGGCCGTGGCGGTGAGCGCAGCCGCCCCGCCACCCGCA GTGCCCTGTGCGCCCGCGGAGAACGCGCCCGCGCCTGCCCGGAGGTCCGCGGGCAAGAA GCCCACCAGGCGCACCGAGAGGACCTCAGCCGGGGACGGCGCCAACTGCCACCGGCCCG AGGAGCCCGCGGCCGCGGACGCGCTGGGGACGAGCGTGGTGGTCCCCCCGGAGCCCACC AAAACCAGGCCCTGTGGCAACAACAGAGCGAGCCACCGCAAGGAGCTGCGCTCCTCCGT GACCTGCGAGAAGCGCCGCACGCGGGGGCTAAGCAGGATCGTCCCCAAATCCAAGGAGT TCATTGAGACAGAGTCGTCATCTTCATCCTCCTCCTCGGACTCCGACCTGGAGTCCGAG CAGGAGGAGTACCCTCTGTCCAAAGCACAGACCGTGGCTGCCTCTGCCTCCTCCGGGAA TGATCAGAGGCTGAAGGAGGCCGCTGCCAACGGGGGCAGTGGTCCTAGGGCCCCTGTAG GCTCCATCAACGCCAGGACCACCAGTGACATCGCCAAGGAGCTGGAGGAGCAGTTCTAC ACACTGGTCCCCTTTGGCCGGAACGAACTTCTCTCCCCTCTAAAGGACAGTGATGAGAT CAGGTCTCTCTGGGTCAAAATCGACCTGACCCTCCTGTCCAGGATCCCAGAACACCTGC CCCAGGAGCCAGGGGTATTGAGCGCCCCTGCCACCAAGGACTCTGAGAGCGCACCGCCC AGCCACACCTCGGACACACCTGCAGAAAAGGCTTTGCCAAAATCCAAGAGGAAACGCAA GTGTGACAACGAAGACGACTACAGGGAGATCAAGAAGTCCCAGGGAGAGAAAGACAGCT CTTCAAGACTGGCCACCTCCACCAGTAATACTTTGTCTGCAAACCACTGCAACATGAAC ATCAACAGTGTGGCAATACCAATAAATAAAAATGAAAAAATGCTTCGGTCGCCCATCTC ACCCCTCTCTGATGCATCTAAACACAAATACACCAGCGAGGACTTAACTTCTTCCAGCC GACCTAATGGCAACAGTTTGTTTACTTCAGCCTCTTCCAGCAAAAAGCCTAAGGCCGAC AGCCAGCTGCAGCCTCACGGCGGAGACCTCACGAAAGCAGCTCACAACAATTCTGAAAA CATTCCCCTCCACAAGTCACGGCCGCAGACGAAGCCGTGGTCTCCAGGCTCCAACGGCC ACAGGGACTGCAAGAGGCAGAAACTTGTCTTCGATGATATGCCTCGCAGTGCCGATTAT TTTATGCAAGAAGCTAAACGAATGAAGCATAAAGCAGATGCAATGGTGGAAAAGTTTGG AAAGGCTTTGAACTATGCTGAAGCAGCATTGTCGTTTATCGAGTGTGGAAATGCAATGG AACAAGGCCCCATGGAATCCAAATCTCCTTATACGATGTATTCAGAAACAGTAGAGCTC ATCAGGTATGCTATGAGACTAAAAACCCACTCAGGCCCCAATGCCACACCAGAAGACAA ACAACTGGCTGCATTATGTTACCGATGCCTGGCCCTCCTGTACTGGCGGATGTTTCGAC TCAAAAGGGACCACGCTGTAAAGTATTCAAAAGCACTAATCGACTATTTCAAGAACTCA TCTAAAGCCGCCCAAGCCCCATCTCCGTGGGGGGCCAGTGGAAAGAGCACTGGAACCCC ATCCCCCATGTCTCCCAACCCCTCTCCCGCCAGCTCCGTGGGGTCTCAGGGCAGCCTCT CCAACGCCAGCGCCCTGTCCCCGTCGACCATCGTCAGCATCCCACAGCGCATCCACCAG ATGGCGGCCAACCACGTCAGCATCACCAACAGCATCCTGCACAGCTACGACTACTGGGA GATGGCCGACAACCTGGCCAAGGAAAACCGAGAATTCTTCAACGACCTGGATCTGCTCA TGGGGCCGGTCACCCTGCACAGCAGCATGGAGCACCTGGTCCAGTACTCCCAACAGGGC CTGCACTGGCTGCGGAACAGCGCCCACCTGTCATAG FGFR2: CASP7 >ATGGTCAGCTGGGGTCGTTTCATCTGCCTGGTCGTGGTCACCATGGCAACCTTGTCCC (3213 base pairs) TGGCCCGGCCCTCCTTCAGTTTAGTTGAGGATACCACATTAGAGCCAGAAGAGCCACCA (SEQ ID NO: 25) ACCAAATACCAAATCTCTCAACCAGAAGTGTACGTGGCTGCGCCAGGGGAGTCGCTAGA GGTGCGCTGCCTGTTGAAAGATGCCGCCGTGATCAGTTGGACTAAGGATGGGGTGCACT TGGGGCCCAACAATAGGACAGTGCTTATTGGGGAGTACTTGCAGATAAAGGGCGCCACG CCTAGAGACTCCGGCCTCTATGCTTGTACTGCCAGTAGGACTGTAGACAGTGAAACTTG GTACTTCATGGTGAATGTCACAGATGCCATCTCATCCGGAGATGATGAGGATGACACCG ATGGTGCGGAAGATTTTGTCAGTGAGAACAGTAACAACAAGAGAGCACCATACTGGACC AACACAGAAAAGATGGAAAAGCGGCTCCATGCTGTGCCTGCGGCCAACACTGTCAAGTT TCGCTGCCCAGCCGGGGGGAACCCAATGCCAACCATGCGGTGGCTGAAAAACGGGAAGG AGTTTAAGCAGGAGCATCGCATTGGAGGCTACAAGGTACGAAACCAGCACTGGAGCCTC ATTATGGAAAGTGTGGTCCCATCTGACAAGGGAAATTATACCTGTGTAGTGGAGAATGA ATACGGGTCCATCAATCACACGTACCACCTGGATGTTGTGGAGCGATCGCCTCACCGGC CCATCCTCCAAGCCGGACTGCCGGCAAATGCCTCCACAGTGGTCGGAGGAGACGTAGAG TTTGTCTGCAAGGTTTACAGTGATGCCCAGCCCCACATCCAGTGGATCAAGCACGTGGA AAAGAACGGCAGTAAATACGGGCCCGACGGGCTGCCCTACCTCAAGGTTCTCAAGGCCG CCGGTGTTAACACCACGGACAAAGAGATTGAGGTTCTCTATATTCGGAATGTAACTTTT GAGGACGCTGGGGAATATACGTGCTTGGCGGGTAATTCTATTGGGATATCCTTTCACTC TGCATGGTTGACAGTTCTGCCAGCGCCTGGAAGAGAAAAGGAGATTACAGCTTCCCCAG ACTACCTGGAGATAGCCATTTACTGCATAGGGGTCTTCTTAATCGCCTGTATGGTGGTA ACAGTCATCCTGTGCCGAATGAAGAACACGACCAAGAAGCCAGACTTCAGCAGCCAGCC GGCTGTGCACAAGCTGACCAAACGTATCCCCCTGCGGAGACAGGTAACAGTTTCGGCTG AGTCCAGCTCCTCCATGAACTCCAACACCCCGCTGGTGAGGATAACAACACGCCTCTCT TCAACGGCAGACACCCCCATGCTGGCAGGGGTCTCCGAGTATGAACTTCCAGAGGACCC AAAATGGGAGTTTCCAAGAGATAAGCTGACACTGGGCAAGCCCCTGGGAGAAGGTTGCT TTGGGCAAGTGGTCATGGCGGAAGCAGTGGGAATTGACAAAGACAAGCCCAAGGAGGCG GTCACCGTGGCCGTGAAGATGTTGAAAGATGATGCCACAGAGAAAGACCTTTCTGATCT GGTGTCAGAGATGGAGATGATGAAGATGATTGGGAAACACAAGAATATCATAAATCTTC TTGGAGCCTGCACACAGGATGGGCCTCTCTATGTCATAGTTGAGTATGCCTCTAAAGGC AACCTCCGAGAATACCTCCGAGCCCGGAGGCCACCCGGGATGGAGTACTCCTATGACAT TAACCGTGTTCCTGAGGAGCAGATGACCTTCAAGGACTTGGTGTCATGCACCTACCAGC TGGCCAGAGGCATGGAGTACTTGGCTTCCCAAAAATGTATTCATCGAGATTTAGCAGCC AGAAATGTTTTGGTAACAGAAAACAATGTGATGAAAATAGCAGACTTTGGACTCGCCAG AGATATCAACAATATAGACTATTACAAAAAGACCACCAATGGGCGGCTTCCAGTCAAGT GGATGGCTCCAGAAGCCCTGTTTGATAGAGTATACACTCATCAGAGTGATGTCTGGTCC TTCGGGGTGTTAATGTGGGAGATCTTCACTTTAGGGGGCTCGCCCTACCCAGGGATTCC CGTGGAGGAACTTTTTAAGCTGCTGAAGGAAGGACACAGAATGGATAAGCCAGCCAACT GCACCAACGAACTGTACATGATGATGAGGGACTGTTGGCATGCAGTGCCCTCCCAGAGA CCAACGTTCAAGCAGTTGGTAGAAGACTTGGATCGAATTCTCACTCTCACAACCAATGA GATGGCAGATGATCAGGGCTGTATTGAAGAGCAGGGGGTTGAGGATTCAGCAAATGAAG ATTCAGTGGATGCTAAGCCAGACCGGTCCTCGTTTGTACCGTCCCTCTTCAGTAAGAAG AAGAAAAATGTCACCATGCGATCCATCAAGACCACCCGGGACCGAGTGCCTACATATCA GTACAACATGAATTTTGAAAAGCTGGGCAAATGCATCATAATAAACAACAAGAACTTTG ATAAAGTGACAGGTATGGGCGTTCGAAACGGAACAGACAAAGATGCCGAGGCGCTCTTC AAGTGCTTCCGAAGCCTGGGTTTTGACGTGATTGTCTATAATGACTGCTCTTGTGCCAA GATGCAAGATCTGCTTAAAAAAGCTTCTGAAGAGGACCATACAAATGCCGCCTGCTTCG CCTGCATCCTCTTAAGCCATGGAGAAGAAAATGTAATTTATGGGAAAGATGGTGTCACA CCAATAAAGGATTTGACAGCCCACTTTAGGGGGGATAGATGCAAAACCCTTTTAGAGAA ACCCAAACTCTTCTTCATTCAGGCTTGCCGAGGGACCGAGCTTGATGATGGCATCCAGG CCGACTCGGGGCCCATCAATGACACAGATGCTAATCCTCGATACAAGATCCCAGTGGAA GCTGACTTCCTCTTCGCCTATTCCACGGTTCCAGGCTATTACTCGTGGAGGAGCCCAGG AAGAGGCTCCTGGTTTGTGCAAGCCCTCTGCTCCATCCTGGAGGAGCACGGAAAAGACC TGGAAATCATGCAGATCCTCACCAGGGTGAATGACAGAGTTGCCAGGCACTTTGAGTCT CAGTCTGATGACCCACACTTCCATGAGAAGAAGCAGATCCCCTGTGTGGTCTCCATGCT CACCAAGGAACTCTACTTCAGTCAATAG FGFR2: CCDC6 >ATGGTCAGCTGGGGTCGTTTCATCTGCCTGGTCGTGGTCACCATGGCAACCTTGTCCC (3423 base pairs) TGGCCCGGCCCTCCTTCAGTTTAGTTGAGGATACCACATTAGAGCCAGAAGAGCCACCA (SEQ ID NO: 26)
ACCAAATACCAAATCTCTCAACCAGAAGTGTACGTGGCTGCGCCAGGGGAGTCGCTAGA GGTGCGCTGCCTGTTGAAAGATGCCGCCGTGATCAGTTGGACTAAGGATGGGGTGCACT TGGGGCCCAACAATAGGACAGTGCTTATTGGGGAGTACTTGCAGATAAAGGGCGCCACG CCTAGAGACTCCGGCCTCTATGCTTGTACTGCCAGTAGGACTGTAGACAGTGAAACTTG GTACTTCATGGTGAATGTCACAGATGCCATCTCATCCGGAGATGATGAGGATGACACCG ATGGTGCGGAAGATTTTGTCAGTGAGAACAGTAACAACAAGAGAGCACCATACTGGACC AACACAGAAAAGATGGAAAAGCGGCTCCATGCTGTGCCTGCGGCCAACACTGTCAAGTT TCGCTGCCCAGCCGGGGGGAACCCAATGCCAACCATGCGGTGGCTGAAAAACGGGAAGG AGTTTAAGCAGGAGCATCGCATTGGAGGCTACAAGGTACGAAACCAGCACTGGAGCCTC ATTATGGAAAGTGTGGTCCCATCTGACAAGGGAAATTATACCTGTGTAGTGGAGAATGA ATACGGGTCCATCAATCACACGTACCACCTGGATGTTGTGGAGCGATCGCCTCACCGGC CCATCCTCCAAGCCGGACTGCCGGCAAATGCCTCCACAGTGGTCGGAGGAGACGTAGAG TTTGTCTGCAAGGTTTACAGTGATGCCCAGCCCCACATCCAGTGGATCAAGCACGTGGA AAAGAACGGCAGTAAATACGGGCCCGACGGGCTGCCCTACCTCAAGGTTCTCAAGGCCG CCGGTGTTAACACCACGGACAAAGAGATTGAGGTTCTCTATATTCGGAATGTAACTTTT GAGGACGCTGGGGAATATACGTGCTTGGCGGGTAATTCTATTGGGATATCCTTTCACTC TGCATGGTTGACAGTTCTGCCAGCGCCTGGAAGAGAAAAGGAGATTACAGCTTCCCCAG ACTACCTGGAGATAGCCATTTACTGCATAGGGGTCTTCTTAATCGCCTGTATGGTGGTA ACAGTCATCCTGTGCCGAATGAAGAACACGACCAAGAAGCCAGACTTCAGCAGCCAGCC GGCTGTGCACAAGCTGACCAAACGTATCCCCCTGCGGAGACAGGTAACAGTTTCGGCTG AGTCCAGCTCCTCCATGAACTCCAACACCCCGCTGGTGAGGATAACAACACGCCTCTCT TCAACGGCAGACACCCCCATGCTGGCAGGGGTCTCCGAGTATGAACTTCCAGAGGACCC AAAATGGGAGTTTCCAAGAGATAAGCTGACACTGGGCAAGCCCCTGGGAGAAGGTTGCT TTGGGCAAGTGGTCATGGCGGAAGCAGTGGGAATTGACAAAGACAAGCCCAAGGAGGCG GTCACCGTGGCCGTGAAGATGTTGAAAGATGATGCCACAGAGAAAGACCTTTCTGATCT GGTGTCAGAGATGGAGATGATGAAGATGATTGGGAAACACAAGAATATCATAAATCTTC TTGGAGCCTGCACACAGGATGGGCCTCTCTATGTCATAGTTGAGTATGCCTCTAAAGGC AACCTCCGAGAATACCTCCGAGCCCGGAGGCCACCCGGGATGGAGTACTCCTATGACAT TAACCGTGTTCCTGAGGAGCAGATGACCTTCAAGGACTTGGTGTCATGCACCTACCAGC TGGCCAGAGGCATGGAGTACTTGGCTTCCCAAAAATGTATTCATCGAGATTTAGCAGCC AGAAATGTTTTGGTAACAGAAAACAATGTGATGAAAATAGCAGACTTTGGACTCGCCAG AGATATCAACAATATAGACTATTACAAAAAGACCACCAATGGGCGGCTTCCAGTCAAGT GGATGGCTCCAGAAGCCCTGTTTGATAGAGTATACACTCATCAGAGTGATGTCTGGTCC TTCGGGGTGTTAATGTGGGAGATCTTCACTTTAGGGGGCTCGCCCTACCCAGGGATTCC CGTGGAGGAACTTTTTAAGCTGCTGAAGGAAGGACACAGAATGGATAAGCCAGCCAACT GCACCAACGAACTGTACATGATGATGAGGGACTGTTGGCATGCAGTGCCCTCCCAGAGA CCAACGTTCAAGCAGTTGGTAGAAGACTTGGATCGAATTCTCACTCTCACAACCAATGA GCAAGCCAGGGCTGAGCAGGAAGAAGAATTCATTAGTAACACTTTATTCAAGAAAATTC AGGCTTTGCAGAAGGAGAAAGAAACCCTTGCTGTAAATTATGAGAAAGAAGAAGAATTC CTCACTAATGAGCTCTCCAGAAAATTGATGCAGTTGCAGCATGAGAAAGCCGAACTAGA ACAGCATCTTGAACAAGAGCAGGAATTTCAGGTCAACAAACTGATGAAGAAAATTAAAA AACTGGAGAATGACACCATTTCTAAGCAACTTACATTAGAACAGTTGAGACGGGAGAAG ATTGACCTTGAAAATACATTGGAACAAGAACAAGAAGCACTAGTTAATCGCCTCTGGAA AAGGATGGATAAGCTTGAAGCTGAAAAGCGAATCCTGCAGGAAAAATTAGACCAGCCCG TCTCTGCTCCACCATCGCCTAGAGATATCTCCATGGAGATTGATTCTCCAGAAAATATG ATGCGTCACATCAGGTTTTTAAAGAATGAAGTGGAACGGCTGAAGAAGCAACTGAGAGC TGCTCAGTTACAGCATTCAGAGAAAATGGCACAGTATCTGGAGGAGGAACGTCACATGA GAGAAGAGAACTTGAGGCTCCAGAGGAAGCTGCAGAGGGAGATGGAGAGAAGAGAAGCC CTCTGTCGACAGCTCTCCGAGAGTGAGTCCAGCTTAGAAATGGACGACGAAAGGTATTT TAATGAGATGTCTGCACAAGGATTAAGACCTCGCACTGTGTCCAGCCCGATCCCTTACA CACCTTCTCCGAGTTCAAGCAGGCCTATATCACCTGGTCTATCATATGCAAGTCACACG GTTGGTTTCACGCCACCAACTTCACTGACTAGAGCTGGAATGTCTTATTACAATTCCCC GGGTCTTCACGTGCAGCACATGGGAACATCCCATGGTATCACAAGGCCTTCACCACGGA GAAGCAACAGTCCTGACAAATTCAAACGGCCCACGCCGCCTCCATCTCCCAACACACAG ACCCCAGTCCAGCCACCTCCGCCTCCACCTCCGCCACCCATGCAGCCCACGGTCCCCTC AGCAGCCACCTCGCAGCCTACTCCTTCGCAACATTCGGCGCACCCCTCCTCCCAGCCTT AA FGFR2: OFD1 >ATGGTCAGCTGGGGTCGTTTCATCTGCCTGGTCGTGGTCACCATGGCAACCTTGTCCC (5229 base pairs) TGGCCCGGCCCTCCTTCAGTTTAGTTGAGGATACCACATTAGAGCCAGAAGAGCCACCA (SEQ ID NO: 27) ACCAAATACCAAATCTCTCAACCAGAAGTGTACGTGGCTGCGCCAGGGGAGTCGCTAGA GGTGCGCTGCCTGTTGAAAGATGCCGCCGTGATCAGTTGGACTAAGGATGGGGTGCACT TGGGGCCCAACAATAGGACAGTGCTTATTGGGGAGTACTTGCAGATAAAGGGCGCCACG CCTAGAGACTCCGGCCTCTATGCTTGTACTGCCAGTAGGACTGTAGACAGTGAAACTTG GTACTTCATGGTGAATGTCACAGATGCCATCTCATCCGGAGATGATGAGGATGACACCG ATGGTGCGGAAGATTTTGTCAGTGAGAACAGTAACAACAAGAGAGCACCATACTGGACC AACACAGAAAAGATGGAAAAGCGGCTCCATGCTGTGCCTGCGGCCAACACTGTCAAGTT TCGCTGCCCAGCCGGGGGGAACCCAATGCCAACCATGCGGTGGCTGAAAAACGGGAAGG AGTTTAAGCAGGAGCATCGCATTGGAGGCTACAAGGTACGAAACCAGCACTGGAGCCTC ATTATGGAAAGTGTGGTCCCATCTGACAAGGGAAATTATACCTGTGTAGTGGAGAATGA ATACGGGTCCATCAATCACACGTACCACCTGGATGTTGTGGAGCGATCGCCTCACCGGC CCATCCTCCAAGCCGGACTGCCGGCAAATGCCTCCACAGTGGTCGGAGGAGACGTAGAG TTTGTCTGCAAGGTTTACAGTGATGCCCAGCCCCACATCCAGTGGATCAAGCACGTGGA AAAGAACGGCAGTAAATACGGGCCCGACGGGCTGCCCTACCTCAAGGTTCTCAAGGCCG CCGGTGTTAACACCACGGACAAAGAGATTGAGGTTCTCTATATTCGGAATGTAACTTTT GAGGACGCTGGGGAATATACGTGCTTGGCGGGTAATTCTATTGGGATATCCTTTCACTC TGCATGGTTGACAGTTCTGCCAGCGCCTGGAAGAGAAAAGGAGATTACAGCTTCCCCAG ACTACCTGGAGATAGCCATTTACTGCATAGGGGTCTTCTTAATCGCCTGTATGGTGGTA ACAGTCATCCTGTGCCGAATGAAGAACACGACCAAGAAGCCAGACTTCAGCAGCCAGCC GGCTGTGCACAAGCTGACCAAACGTATCCCCCTGCGGAGACAGGTAACAGTTTCGGCTG AGTCCAGCTCCTCCATGAACTCCAACACCCCGCTGGTGAGGATAACAACACGCCTCTCT TCAACGGCAGACACCCCCATGCTGGCAGGGGTCTCCGAGTATGAACTTCCAGAGGACCC AAAATGGGAGTTTCCAAGAGATAAGCTGACACTGGGCAAGCCCCTGGGAGAAGGTTGCT TTGGGCAAGTGGTCATGGCGGAAGCAGTGGGAATTGACAAAGACAAGCCCAAGGAGGCG GTCACCGTGGCCGTGAAGATGTTGAAAGATGATGCCACAGAGAAAGACCTTTCTGATCT GGTGTCAGAGATGGAGATGATGAAGATGATTGGGAAACACAAGAATATCATAAATCTTC TTGGAGCCTGCACACAGGATGGGCCTCTCTATGTCATAGTTGAGTATGCCTCTAAAGGC AACCTCCGAGAATACCTCCGAGCCCGGAGGCCACCCGGGATGGAGTACTCCTATGACAT TAACCGTGTTCCTGAGGAGCAGATGACCTTCAAGGACTTGGTGTCATGCACCTACCAGC TGGCCAGAGGCATGGAGTACTTGGCTTCCCAAAAATGTATTCATCGAGATTTAGCAGCC AGAAATGTTTTGGTAACAGAAAACAATGTGATGAAAATAGCAGACTTTGGACTCGCCAG AGATATCAACAATATAGACTATTACAAAAAGACCACCAATGGGCGGCTTCCAGTCAAGT GGATGGCTCCAGAAGCCCTGTTTGATAGAGTATACACTCATCAGAGTGATGTCTGGTCC TTCGGGGTGTTAATGTGGGAGATCTTCACTTTAGGGGGCTCGCCCTACCCAGGGATTCC CGTGGAGGAACTTTTTAAGCTGCTGAAGGAAGGACACAGAATGGATAAGCCAGCCAACT GCACCAACGAACTGTACATGATGATGAGGGACTGTTGGCATGCAGTGCCCTCCCAGAGA CCAACGTTCAAGCAGTTGGTAGAAGACTTGGATCGAATTCTCACTCTCACAACCAATGA GACACAACTTCGAAACCAGCTAATTCATGAGTTGATGCACCCTGTATTGAGTGGAGAAC TGCAGCCTCGGTCCATTTCAGTAGAAGGGAGCTCCCTCTTAATAGGCGCCTCTAACTCT TTAGTGGCAGATCACTTACAAAGATGTGGCTATGAATATTCACTTTCTGTTTTCTTTCC AGAAAGTGGTTTGGCAAAAGAAAAGGTATTTACTATGCAGGATCTATTACAACTCATTA AAATCAACCCTACTTCCAGTCTCTACAAATCACTGGTTTCAGGATCTGATAAAGAAAAT CAAAAAGGTTTTCTTATGCATTTTTTAAAAGAATTGGCAGAATATCATCAAGCTAAAGA GAGTTGTAATATGGAAACTCAGACAAGTTCGACATTTAACAGAGATTCTCTGGCTGAGA AGCTTCAGCTTATTGATGATCAGTTTGCAGATGCTTACCCTCAGCGTATCAAGTTCGAA TCTTTAGAAATAAAGCTAAATGAGTATAAGAGAGAAATAGAAGAGCAACTTCGGGCAGA AATGTGTCAAAAGTTGAAGTTTTTTAAAGATACCGAGATAGCAAAAATTAAAATGGAAG CAAAAAAAAAGTATGAAAAGGAGTTAACCATGTTCCAGAATGATTTTGAAAAAGCTTGT CAAGCAAAATCTGAAGCTCTCGTTCTTCGGGAAAAGAGTACCCTTGAAAGAATTCACAA GCACCAAGAGATTGAAACAAAAGAAATTTATGCTCAAAGGCAACTTTTACTAAAAGATA TGGATTTGCTAAGAGGAAGAGAAGCAGAGCTGAAGCAAAGAGTTGAAGCTTTTGAATTG AACCAGAAGCTCCAGGAAGAAAAACATAAAAGCATAACTGAGGCACTTAGGAGACAGGA GCAGAATATAAAGAGTTTTGAGGAGACCTATGACCGAAAGCTCAAGAATGAACTTCTAA AGTATCAACTTGAACTGAAGGATGACTACATCATTAGAACTAATCGACTGATTGAAGAT GAAAGGAAGAATAAAGAAAAAGCTGTTCATTTGCAAGAGGAGCTCATAGCTATTAATTC AAAAAAGGAGGAACTCAATCAATCTGTAAATCGTGTGAAAGAACTTGAGCTTGAATTAG AGTCTGTCAAAGCCCAGTCTTTGGCAATAACAAAACAAAACCATATGCTGAATGAAAAG GTTAAAGAGATGAGTGATTATTCACTACTAAAAGAAGAGAAACTGGAGCTTCTGGCACA AAATAAATTACTTAAACAACAACTGGAAGAGAGTAGAAATGAAAACCTGCGTCTCCTAA ACCGCCTAGCTCAGCCGGCTCCTGAACTTGCAGTCTTTCAGAAAGAACTACGGAAAGCC GAAAAGGCTATAGTGGTTGAGCATGAGGAGTTCGAAAGCTGCAGGCAAGCTCTGCACAA ACAACTGCAAGACGAAATTGAGCATTCTGCACAGCTGAAGGCCCAGATTCTAGGTTACA AAGCTTCTGTAAAGAGTTTAACTACTCAGGTTGCCGATTTAAAATTGCAACTGAAGCAA ACTCAGACAGCCCTAGAGAATGAAGTGTACTGCAATCCAAAGCAGTCTGTGATCGATCG TTCTGTCAATGGATTAATAAATGGCAATGTGGTGCCTTGCAATGGTGAGATAAGTGGGG ATTTCTTGAACAATCCTTTTAAACAGGAAAACGTTCTAGCACGTATGGTTGCATCAAGG ATCACAAATTATCCAACTGCATGGGTGGAGGGTAGTTCCCCTGATTCTGACCTTGAGTT TGTAGCCAATACTAAGGCAAGGGTCAAAGAGCTTCAGCAAGAGGCCGAACGCTTGGAAA AGGCTTTCAGAAGTTACCATCGGAGAGTCATTAAAAACTCTGCCAAAAGCCCACTAGCA GCAAAGAGCCCACCATCTCTGCACTTGCTGGAAGCCTTCAAAAACATTACTTCCAGTTC CCCGGAAAGACATATTTTTGGAGAGGACAGAGTTGTCTCTGAGCAGCCTCAAGTGGGCA CACTTGAAGAAAGGAATGACGTCGTGGAAGCACTGACAGGCAGTGCAGCCTCGAGGCTC CGCGGGGGCACTTCCTCCAGACGCCTCTCTTCCACACCCCTTCCAAAAGCAAAAAGAAG CCTCGAAAGTGAAATGTATCTGGAAGGTCTGGGCAGATCACACATTGCTTCCCCCAGTC CTTGTCCTGACAGAATGCCCCTACCATCACCCACTGAGTCTAGGCACAGCCTCTCCATC CCTCCTGTCTCCAGCCCTCCGGAGCAGAAAGTGGGTCTTTATCGAAGACAAACTGAACT TCAAGACAAAAGTGAATTTTCAGATGTGGACAAGCTAGCTTTTAAGGATAATGAGGAGT TTGAATCATCTTTTGAATCTGCAGGGAACATGCCAAGGCAGTTGGAAATGGGCGGGCTT TCTCCTGCCGGGGATATGTCTCATGTGGACGCTGCTGCAGCTGCTGTGCCCCTCTCATA TCAGCACCCAAGTGTAGATCAGAAACAAATTGAAGAACAAAAGGAAGAAGAAAAAATAC GGGAACAGCAAGTGAAAGAACGAAGGCAGAGAGAAGAAAGAAGGCAGAGTAACCTACAA GAAGTTTTAGAAAGGGAACGAAGAGAACTAGAAAAACTGTATCAGGAAAGGAAGATGAT TGAAGAATCACTGAAGATTAAAATAAAAAAGGAATTAGAAATGGAAAATGAATTAGAAA TGAGTAATCAAGAAATAAAAGACAAATCTGCTCACAGTGAAAATCCTTTAGAGAAATAC ATGAAAATCATCCAGCAGGAGCAAGACCAGGAGTCGGCAGATAAGAGCTCAAAAAAGAT GGTCCAAGAAGGCTCCCTAGTGGACACGCTGCAATCTAGTGACAAAGTCGAAAGTTTAA CAGGCTTTTCTCATGAAGAACTAGACGACTCTTGGTAA
Sequence CWU 1
1
27121DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 1gacctggacc gtgtccttac c 21221DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
2cttccccagt tccaggttct t 21320DNAArtificial SequenceDescription of
Artificial Sequence Synthetic primer 3aggacctgga ccgtgtcctt
20420DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 4tataggtccg gtggacaggg 20515DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
5ggccatcctg ccccc 15620DNAArtificial SequenceDescription of
Artificial Sequence Synthetic primer 6gagcagtcca ggtcagccag
20720DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 7ctggaccgtg tccttaccgt 20820DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
8gcagcccagg attgaactgt 20923DNAArtificial SequenceDescription of
Artificial Sequence Synthetic primer 9tggatcgaat tctcactctc aca
231021DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 10gccaagcaat ctgcgtattt g 211125DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
11tggtagaaga cttggatcga attct 251223DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
12tctcccggat tatttcttca aca 231323DNAArtificial SequenceDescription
of Artificial Sequence Synthetic primer 13gctcttcaat acagccctga tca
231424DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 14acttggatcg aattctcact ctca 241523DNAArtificial
SequenceDescription of Artificial Sequence Synthetic primer
15tggatcgaat tctcactctc aca 231625DNAArtificial SequenceDescription
of Artificial Sequence Synthetic primer 16gcaaagcctg aattttcttg
aataa 251724DNAArtificial SequenceDescription of Artificial
Sequence Synthetic primer 17agggtgcatc aactcatgaa ttag
241824DNAArtificial SequenceDescription of Artificial Sequence
Synthetic primer 18acttggatcg aattctcact ctca 24192850DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
19atgggcgccc ctgcctgcgc cctcgcgctc tgcgtggccg tggccatcgt ggccggcgcc
60tcctcggagt ccttggggac ggagcagcgc gtcgtggggc gagcggcaga agtcccgggc
120ccagagcccg gccagcagga gcagttggtc ttcggcagcg gggatgctgt
ggagctgagc 180tgtcccccgc ccgggggtgg tcccatgggg cccactgtct
gggtcaagga tggcacaggg 240ctggtgccct cggagcgtgt cctggtgggg
ccccagcggc tgcaggtgct gaatgcctcc 300cacgaggact ccggggccta
cagctgccgg cagcggctca cgcagcgcgt actgtgccac 360ttcagtgtgc
gggtgacaga cgctccatcc tcgggagatg acgaagacgg ggaggacgag
420gctgaggaca caggtgtgga cacaggggcc ccttactgga cacggcccga
gcggatggac 480aagaagctgc tggccgtgcc ggccgccaac accgtccgct
tccgctgccc agccgctggc 540aaccccactc cctccatctc ctggctgaag
aacggcaggg agttccgcgg cgagcaccgc 600attggaggca tcaagctgcg
gcatcagcag tggagcctgg tcatggaaag cgtggtgccc 660tcggaccgcg
gcaactacac ctgcgtcgtg gagaacaagt ttggcagcat ccggcagacg
720tacacgctgg acgtgctgga gcgctccccg caccggccca tcctgcaggc
ggggctgccg 780gccaaccaga cggcggtgct gggcagcgac gtggagttcc
actgcaaggt gtacagtgac 840gcacagcccc acatccagtg gctcaagcac
gtggaggtga atggcagcaa ggtgggcccg 900gacggcacac cctacgttac
cgtgctcaag acggcgggcg ctaacaccac cgacaaggag 960ctagaggttc
tctccttgca caacgtcacc tttgaggacg ccggggagta cacctgcctg
1020gcgggcaatt ctattgggtt ttctcatcac tctgcgtggc tggtggtgct
gccagccgag 1080gaggagctgg tggaggctga cgaggcgggc agtgtgtatg
caggcatcct cagctacggg 1140gtgggcttct tcctgttcat cctggtggtg
gcggctgtga cgctctgccg cctgcgcagc 1200ccccccaaga aaggcctggg
ctcccccacc gtgcacaaga tctcccgctt cccgctcaag 1260cgacaggtgt
ccctggagtc caacgcgtcc atgagctcca acacaccact ggtgcgcatc
1320gcaaggctgt cctcagggga gggccccacg ctggccaatg tctccgagct
cgagctgcct 1380gccgacccca aatgggagct gtctcgggcc cggctgaccc
tgggcaagcc ccttggggag 1440ggctgcttcg gccaggtggt catggcggag
gccatcggca ttgacaagga ccgggccgcc 1500aagcctgtca ccgtagccgt
gaagatgctg aaagacgatg ccactgacaa ggacctgtcg 1560gacctggtgt
ctgagatgga gatgatgaag atgatcggga aacacaaaaa catcatcaac
1620ctgctgggcg cctgcacgca gggcgggccc ctgtacgtgc tggtggagta
cgcggccaag 1680ggtaacctgc gggagtttct gcgggcgcgg cggcccccgg
gcctggacta ctccttcgac 1740acctgcaagc cgcccgagga gcagctcacc
ttcaaggacc tggtgtcctg tgcctaccag 1800gtggcccggg gcatggagta
cttggcctcc cagaagtgca tccacaggga cctggctgcc 1860cgcaatgtgc
tggtgaccga ggacaacgtg atgaagatcg cagacttcgg gctggcccgg
1920gacgtgcaca acctcgacta ctacaagaag acgaccaacg gccggctgcc
cgtgaagtgg 1980atggcgcctg aggccttgtt tgaccgagtc tacactcacc
agagtgacgt ctggtccttt 2040ggggtcctgc tctgggagat cttcacgctg
gggggctccc cgtaccccgg catccctgtg 2100gaggagctct tcaagctgct
gaaggagggc caccgcatgg acaagcccgc caactgcaca 2160cacgacctgt
acatgatcat gcgggagtgc tggcatgccg cgccctccca gaggcccacc
2220ttcaagcagc tggtggagga cctggaccgt gtccttaccg tgacgtccac
cgacgtaaag 2280gcgacacagg aggagaaccg ggagctgagg agcaggtgtg
aggagctcca cgggaagaac 2340ctggaactgg ggaagatcat ggacaggttc
gaagaggttg tgtaccaggc catggaggaa 2400gttcagaagc agaaggaact
ttccaaagct gaaatccaga aagttctaaa agaaaaagac 2460caacttacca
cagatctgaa ctccatggag aagtccttct ccgacctctt caagcgtttt
2520gagaaacaga aagaggtgat cgagggctac cgcaagaacg aagagtcact
gaagaagtgc 2580gtggaggatt acctggcaag gatcacccag gagggccaga
ggtaccaagc cctgaaggcc 2640cacgcggagg agaagctgca gctggcaaac
gaggagatcg cccaggtccg gagcaaggcc 2700caggcggaag cgttggccct
ccaggccagc ctgaggaagg agcagatgcg catccagtcg 2760ctggagaaga
cagtggagca gaagactaaa gagaacgagg agctgaccag gatctgcgac
2820gacctcatct ccaagatgga gaagatctga 2850202955DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
20atgggcgccc ctgcctgcgc cctcgcgctc tgcgtggccg tggccatcgt ggccggcgcc
60tcctcggagt ccttggggac ggagcagcgc gtcgtggggc gagcggcaga agtcccgggc
120ccagagcccg gccagcagga gcagttggtc ttcggcagcg gggatgctgt
ggagctgagc 180tgtcccccgc ccgggggtgg tcccatgggg cccactgtct
gggtcaagga tggcacaggg 240ctggtgccct cggagcgtgt cctggtgggg
ccccagcggc tgcaggtgct gaatgcctcc 300cacgaggact ccggggccta
cagctgccgg cagcggctca cgcagcgcgt actgtgccac 360ttcagtgtgc
gggtgacaga cgctccatcc tcgggagatg acgaagacgg ggaggacgag
420gctgaggaca caggtgtgga cacaggggcc ccttactgga cacggcccga
gcggatggac 480aagaagctgc tggccgtgcc ggccgccaac accgtccgct
tccgctgccc agccgctggc 540aaccccactc cctccatctc ctggctgaag
aacggcaggg agttccgcgg cgagcaccgc 600attggaggca tcaagctgcg
gcatcagcag tggagcctgg tcatggaaag cgtggtgccc 660tcggaccgcg
gcaactacac ctgcgtcgtg gagaacaagt ttggcagcat ccggcagacg
720tacacgctgg acgtgctgga gcgctccccg caccggccca tcctgcaggc
ggggctgccg 780gccaaccaga cggcggtgct gggcagcgac gtggagttcc
actgcaaggt gtacagtgac 840gcacagcccc acatccagtg gctcaagcac
gtggaggtga atggcagcaa ggtgggcccg 900gacggcacac cctacgttac
cgtgctcaag acggcgggcg ctaacaccac cgacaaggag 960ctagaggttc
tctccttgca caacgtcacc tttgaggacg ccggggagta cacctgcctg
1020gcgggcaatt ctattgggtt ttctcatcac tctgcgtggc tggtggtgct
gccagccgag 1080gaggagctgg tggaggctga cgaggcgggc agtgtgtatg
caggcatcct cagctacggg 1140gtgggcttct tcctgttcat cctggtggtg
gcggctgtga cgctctgccg cctgcgcagc 1200ccccccaaga aaggcctggg
ctcccccacc gtgcacaaga tctcccgctt cccgctcaag 1260cgacaggtgt
ccctggagtc caacgcgtcc atgagctcca acacaccact ggtgcgcatc
1320gcaaggctgt cctcagggga gggccccacg ctggccaatg tctccgagct
cgagctgcct 1380gccgacccca aatgggagct gtctcgggcc cggctgaccc
tgggcaagcc ccttggggag 1440ggctgcttcg gccaggtggt catggcggag
gccatcggca ttgacaagga ccgggccgcc 1500aagcctgtca ccgtagccgt
gaagatgctg aaagacgatg ccactgacaa ggacctgtcg 1560gacctggtgt
ctgagatgga gatgatgaag atgatcggga aacacaaaaa catcatcaac
1620ctgctgggcg cctgcacgca gggcgggccc ctgtacgtgc tggtggagta
cgcggccaag 1680ggtaacctgc gggagtttct gcgggcgcgg cggcccccgg
gcctggacta ctccttcgac 1740acctgcaagc cgcccgagga gcagctcacc
ttcaaggacc tggtgtcctg tgcctaccag 1800gtggcccggg gcatggagta
cttggcctcc cagaagtgca tccacaggga cctggctgcc 1860cgcaatgtgc
tggtgaccga ggacaacgtg atgaagatcg cagacttcgg gctggcccgg
1920gacgtgcaca acctcgacta ctacaagaag acgaccaacg gccggctgcc
cgtgaagtgg 1980atggcgcctg aggccttgtt tgaccgagtc tacactcacc
agagtgacgt ctggtccttt 2040ggggtcctgc tctgggagat cttcacgctg
gggggctccc cgtaccccgg catccctgtg 2100gaggagctct tcaagctgct
gaaggagggc caccgcatgg acaagcccgc caactgcaca 2160cacgacctgt
acatgatcat gcgggagtgc tggcatgccg cgccctccca gaggcccacc
2220ttcaagcagc tggtggagga cctggaccgt gtccttaccg tgacgtccac
cgacgtgcca 2280ggcccacccc caggtgttcc cgcgcctggg ggcccacccc
tgtccaccgg acctatagtg 2340gacctgctcc agtacagcca gaaggacctg
gatgcagtgg taaaggcgac acaggaggag 2400aaccgggagc tgaggagcag
gtgtgaggag ctccacggga agaacctgga actggggaag 2460atcatggaca
ggttcgaaga ggttgtgtac caggccatgg aggaagttca gaagcagaag
2520gaactttcca aagctgaaat ccagaaagtt ctaaaagaaa aagaccaact
taccacagat 2580ctgaactcca tggagaagtc cttctccgac ctcttcaagc
gttttgagaa acagaaagag 2640gtgatcgagg gctaccgcaa gaacgaagag
tcactgaaga agtgcgtgga ggattacctg 2700gcaaggatca cccaggaggg
ccagaggtac caagccctga aggcccacgc ggaggagaag 2760ctgcagctgg
caaacgagga gatcgcccag gtccggagca aggcccaggc ggaagcgttg
2820gccctccagg ccagcctgag gaaggagcag atgcgcatcc agtcgctgga
gaagacagtg 2880gagcagaaga ctaaagagaa cgaggagctg accaggatct
gcgacgacct catctccaag 2940atggagaaga tctga 2955214462DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
21atgggcgccc ctgcctgcgc cctcgcgctc tgcgtggccg tggccatcgt ggccggcgcc
60tcctcggagt ccttggggac ggagcagcgc gtcgtggggc gagcggcaga agtcccgggc
120ccagagcccg gccagcagga gcagttggtc ttcggcagcg gggatgctgt
ggagctgagc 180tgtcccccgc ccgggggtgg tcccatgggg cccactgtct
gggtcaagga tggcacaggg 240ctggtgccct cggagcgtgt cctggtgggg
ccccagcggc tgcaggtgct gaatgcctcc 300cacgaggact ccggggccta
cagctgccgg cagcggctca cgcagcgcgt actgtgccac 360ttcagtgtgc
gggtgacaga cgctccatcc tcgggagatg acgaagacgg ggaggacgag
420gctgaggaca caggtgtgga cacaggggcc ccttactgga cacggcccga
gcggatggac 480aagaagctgc tggccgtgcc ggccgccaac accgtccgct
tccgctgccc agccgctggc 540aaccccactc cctccatctc ctggctgaag
aacggcaggg agttccgcgg cgagcaccgc 600attggaggca tcaagctgcg
gcatcagcag tggagcctgg tcatggaaag cgtggtgccc 660tcggaccgcg
gcaactacac ctgcgtcgtg gagaacaagt ttggcagcat ccggcagacg
720tacacgctgg acgtgctgga gcgctccccg caccggccca tcctgcaggc
ggggctgccg 780gccaaccaga cggcggtgct gggcagcgac gtggagttcc
actgcaaggt gtacagtgac 840gcacagcccc acatccagtg gctcaagcac
gtggaggtga atggcagcaa ggtgggcccg 900gacggcacac cctacgttac
cgtgctcaag acggcgggcg ctaacaccac cgacaaggag 960ctagaggttc
tctccttgca caacgtcacc tttgaggacg ccggggagta cacctgcctg
1020gcgggcaatt ctattgggtt ttctcatcac tctgcgtggc tggtggtgct
gccagccgag 1080gaggagctgg tggaggctga cgaggcgggc agtgtgtatg
caggcatcct cagctacggg 1140gtgggcttct tcctgttcat cctggtggtg
gcggctgtga cgctctgccg cctgcgcagc 1200ccccccaaga aaggcctggg
ctcccccacc gtgcacaaga tctcccgctt cccgctcaag 1260cgacaggtgt
ccctggagtc caacgcgtcc atgagctcca acacaccact ggtgcgcatc
1320gcaaggctgt cctcagggga gggccccacg ctggccaatg tctccgagct
cgagctgcct 1380gccgacccca aatgggagct gtctcgggcc cggctgaccc
tgggcaagcc ccttggggag 1440ggctgcttcg gccaggtggt catggcggag
gccatcggca ttgacaagga ccgggccgcc 1500aagcctgtca ccgtagccgt
gaagatgctg aaagacgatg ccactgacaa ggacctgtcg 1560gacctggtgt
ctgagatgga gatgatgaag atgatcggga aacacaaaaa catcatcaac
1620ctgctgggcg cctgcacgca gggcgggccc ctgtacgtgc tggtggagta
cgcggccaag 1680ggtaacctgc gggagtttct gcgggcgcgg cggcccccgg
gcctggacta ctccttcgac 1740acctgcaagc cgcccgagga gcagctcacc
ttcaaggacc tggtgtcctg tgcctaccag 1800gtggcccggg gcatggagta
cttggcctcc cagaagtgca tccacaggga cctggctgcc 1860cgcaatgtgc
tggtgaccga ggacaacgtg atgaagatcg cagacttcgg gctggcccgg
1920gacgtgcaca acctcgacta ctacaagaag acgaccaacg gccggctgcc
cgtgaagtgg 1980atggcgcctg aggccttgtt tgaccgagtc tacactcacc
agagtgacgt ctggtccttt 2040ggggtcctgc tctgggagat cttcacgctg
gggggctccc cgtaccccgg catccctgtg 2100gaggagctct tcaagctgct
gaaggagggc caccgcatgg acaagcccgc caactgcaca 2160cacgacctgt
acatgatcat gcgggagtgc tggcatgccg cgccctccca gaggcccacc
2220ttcaagcagc tggtggagga cctggaccgt gtccttaccg tgacgtccac
cgacgtgagt 2280gctggctctg gcctggtgcc acccgcctat gcccctcccc
ctgccgtccc cggccatcct 2340gccccccaga gtgctgaggt gtggggcggg
cctttctggc ccaggtgccc tggctgacct 2400ggactgctca agctcttccc
agagcccagg aagttctgag aaccaaatgg tgtctccagg 2460aaaagtgtct
ggcagccctg agcaagccgt ggaggaaaac cttagttcct attccttaga
2520cagaagagtg acacccgcct ctgagaccct agaagaccct tgcaggacag
agtcccagca 2580caaagcggag actccgcacg gagccgagga agaatgcaaa
gcggagactc cgcacggagc 2640cgaggaggaa tgccggcacg gtggggtctg
tgctcccgca gcagtggcca cttcgcctcc 2700tggtgcaatc cctaaggaag
cctgcggagg agcacccctg cagggtctgc ctggcgaagc 2760cctgggctgc
cctgcgggtg tgggcacccc cgtgccagca gatggcactc agacccttac
2820ctgtgcacac acctctgctc ctgagagcac agccccaacc aaccacctgg
tggctggcag 2880ggccatgacc ctgagtcctc aggaagaagt ggctgcaggc
caaatggcca gctcctcgag 2940gagcggacct gtaaaactag aatttgatgt
atctgatggc gccaccagca aaagggcacc 3000cccaccaagg agactgggag
agaggtccgg cctcaagcct cccttgagga aagcagcagt 3060gaggcagcaa
aaggccccgc aggaggtgga ggaggacgac ggtaggagcg gagcaggaga
3120ggaccccccc atgccagctt ctcggggctc ttaccacctc gactgggaca
aaatggatga 3180cccaaacttc atcccgttcg gaggtgacac caagtctggt
tgcagtgagg cccagccccc 3240agaaagccct gagaccaggc tgggccagcc
agcggctgaa cagttgcatg ctgggcctgc 3300cacggaggag ccaggtccct
gtctgagcca gcagctgcat tcagcctcag cggaggacac 3360gcctgtggtg
cagttggcag ccgagacccc aacagcagag agcaaggaga gagccttgaa
3420ctctgccagc acctcgcttc ccacaagctg tccaggcagt gagccagtgc
ccacccatca 3480gcaggggcag cctgccttgg agctgaaaga ggagagcttc
agagaccccg ctgaggttct 3540aggcacgggc gcggaggtgg attacctgga
gcagtttgga acttcctcgt ttaaggagtc 3600ggccttgagg aagcagtcct
tatacctcaa gttcgacccc ctcctgaggg acagtcctgg 3660tagaccagtg
cccgtggcca ccgagaccag cagcatgcac ggtgcaaatg agactccctc
3720aggacgtccg cgggaagcca agcttgtgga gttcgatttc ttgggagcac
tggacattcc 3780tgtgccaggc ccacccccag gtgttcccgc gcctgggggc
ccacccctgt ccaccggacc 3840tatagtggac ctgctccagt acagccagaa
ggacctggat gcagtggtaa aggcgacaca 3900ggaggagaac cgggagctga
ggagcaggtg tgaggagctc cacgggaaga acctggaact 3960ggggaagatc
atggacaggt tcgaagaggt tgtgtaccag gccatggagg aagttcagaa
4020gcagaaggaa ctttccaaag ctgaaatcca gaaagttcta aaagaaaaag
accaacttac 4080cacagatctg aactccatgg agaagtcctt ctccgacctc
ttcaagcgtt ttgagaaaca 4140gaaagaggtg atcgagggct accgcaagaa
cgaagagtca ctgaagaagt gcgtggagga 4200ttacctggca aggatcaccc
aggagggcca gaggtaccaa gccctgaagg cccacgcgga 4260ggagaagctg
cagctggcaa acgaggagat cgcccaggtc cggagcaagg cccaggcgga
4320agcgttggcc ctccaggcca gcctgaggaa ggagcagatg cgcatccagt
cgctggagaa 4380gacagtggag cagaagacta aagagaacga ggagctgacc
aggatctgcg acgacctcat 4440ctccaagatg gagaagatct ga
4462223765DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 22atgggcgccc ctgcctgcgc cctcgcgctc
tgcgtggccg tggccatcgt ggccggcgcc 60tcctcggagt ccttggggac ggagcagcgc
gtcgtggggc gagcggcaga agtcccgggc 120ccagagcccg gccagcagga
gcagttggtc ttcggcagcg gggatgctgt ggagctgagc 180tgtcccccgc
ccgggggtgg tcccatgggg cccactgtct gggtcaagga tggcacaggg
240ctggtgccct cggagcgtgt cctggtgggg ccccagcggc tgcaggtgct
gaatgcctcc 300cacgaggact ccggggccta cagctgccgg cagcggctca
cgcagcgcgt actgtgccac 360ttcagtgtgc gggtgacaga cgctccatcc
tcgggagatg acgaagacgg ggaggacgag 420gctgaggaca caggtgtgga
cacaggggcc ccttactgga cacggcccga gcggatggac 480aagaagctgc
tggccgtgcc ggccgccaac accgtccgct tccgctgccc agccgctggc
540aaccccactc cctccatctc ctggctgaag aacggcaggg agttccgcgg
cgagcaccgc 600attggaggca tcaagctgcg gcatcagcag tggagcctgg
tcatggaaag cgtggtgccc 660tcggaccgcg gcaactacac ctgcgtcgtg
gagaacaagt ttggcagcat ccggcagacg 720tacacgctgg acgtgctgga
gcgctccccg caccggccca tcctgcaggc ggggctgccg 780gccaaccaga
cggcggtgct gggcagcgac gtggagttcc actgcaaggt gtacagtgac
840gcacagcccc acatccagtg gctcaagcac gtggaggtga atggcagcaa
ggtgggcccg 900gacggcacac cctacgttac cgtgctcaag tcctggatca
gtgagagtgt ggaggccgac 960gtgcgcctcc gcctggccaa tgtgtcggag
cgggacgggg gcgagtacct ctgtcgagcc 1020accaatttca taggcgtggc
cgagaaggcc ttttggctga gcgttcacgg gccccgagca 1080gccgaggagg
agctggtgga ggctgacgag gcgggcagtg tgtatgcagg catcctcagc
1140tacggggtgg gcttcttcct gttcatcctg gtggtggcgg ctgtgacgct
ctgccgcctg 1200cgcagccccc ccaagaaagg cctgggctcc cccaccgtgc
acaagatctc ccgcttcccg 1260ctcaagcgac aggtgtccct ggagtccaac
gcgtccatga gctccaacac accactggtg 1320cgcatcgcaa ggctgtcctc
aggggagggc cccacgctgg ccaatgtctc cgagctcgag 1380ctgcctgccg
accccaaatg ggagctgtct cgggcccggc tgaccctggg caagcccctt
1440ggggagggct gcttcggcca ggtggtcatg gcggaggcca tcggcattga
caaggaccgg 1500gccgccaagc ctgtcaccgt agccgtgaag atgctgaaag
acgatgccac tgacaaggac 1560ctgtcggacc tggtgtctga gatggagatg
atgaagatga tcgggaaaca caaaaacatc 1620atcaacctgc tgggcgcctg
cacgcagggc gggcccctgt acgtgctggt ggagtacgcg 1680gccaagggta
acctgcggga gtttctgcgg gcgcggcggc ccccgggcct ggactactcc
1740ttcgacacct gcaagccgcc cgaggagcag ctcaccttca aggacctggt
gtcctgtgcc 1800taccaggtgg cccggggcat ggagtacttg gcctcccaga
agtgcatcca cagggacctg 1860gctgcccgca atgtgctggt gaccgaggac
aacgtgatga agatcgcaga cttcgggctg 1920gcccgggacg tgcacaacct
cgactactac aagaagacga ccaacggccg gctgcccgtg
1980aagtggatgg cgcctgaggc cttgtttgac cgagtctaca ctcaccagag
tgacgtctgg 2040tcctttgggg tcctgctctg ggagatcttc acgctggggg
gctccccgta ccccggcatc 2100cctgtggagg agctcttcaa gctgctgaag
gagggccacc gcatggacaa gcccgccaac 2160tgcacacacg acctgtacat
gatcatgcgg gagtgctggc atgccgcgcc ctcccagagg 2220cccaccttca
agcagctggt ggaggacctg gaccgtgtcc ttaccgtgac gtccaccgac
2280aatgttatgg aacagttcaa tcctgggctg cgaaatttaa taaacctggg
gaaaaattat 2340gagaaagctg taaacgctat gatcctggca ggaaaagcct
actacgatgg agtggccaag 2400atcggtgaga ttgccactgg gtcccccgtg
tcaactgaac tgggacatgt cctcatagag 2460atttcaagta cccacaagaa
actcaacgag agtcttgatg aaaattttaa aaaattccac 2520aaagagatta
tccatgagct ggagaagaag atagaacttg acgtgaaata tatgaacgca
2580actctaaaaa gataccaaac agaacacaag aataaattag agtctttgga
gaaatcccaa 2640gctgagttga agaagatcag aaggaaaagc caaggaagcc
gaaacgcact caaatatgaa 2700cacaaagaaa ttgagtatgt ggagaccgtt
acttctcgtc agagtgaaat ccagaaattc 2760attgcagatg gttgcaaaga
ggctctgctt gaagagaaga ggcgcttctg ctttctggtt 2820gataagcact
gtggctttgc aaaccacata cattattatc acttacagtc tgcagaacta
2880ctgaattcca agctgcctcg gtggcaggag acctgtgttg atgccatcaa
agtgccagag 2940aaaatcatga atatgatcga agaaataaag accccagcct
ctacccccgt gtctggaact 3000cctcaggctt cacccatgat cgagagaagc
aatgtggtta ggaaagatta cgacaccctt 3060tctaaatgct caccaaagat
gccccccgct ccttcaggca gagcatatac cagtcccttg 3120atcgatatgt
ttaataaccc agccacggct gccccgaatt cacaaagggt aaataattca
3180acaggtactt ccgaagatcc cagtttacag cgatcagttt cggttgcaac
gggactgaac 3240atgatgaaga agcagaaagt gaagaccatc ttcccgcaca
ctgcgggctc caacaagacc 3300ttactcagct ttgcacaggg agatgtcatc
acgctgctca tccccgagga gaaggatggc 3360tggctctatg gagaacacga
cgtgtccaag gcgaggggtt ggttcccgtc gtcgtacacg 3420aagttgctgg
aagaaaatga gacagaagca gtgaccgtgc ccacgccaag ccccacacca
3480gtgagaagca tcagcaccgt gaacttgtct gagaatagca gtgttgtcat
ccccccaccc 3540gactacttgg aatgcttgtc catgggggca gctgccgaca
ggagagcaga ttcggccagg 3600acgacatcca cctttaaggc cccagcgtcc
aagcccgaga ccgcggctcc taacgatgcc 3660aacgggactg caaagccgcc
ttttctcagc ggagaaaacc cctttgccac tgtgaaactc 3720cgcccgactg
tgacgaatga tcgctcggca cccatcattc gatga 3765234989DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
23atggtcagct ggggtcgttt catctgcctg gtcgtggtca ccatggcaac cttgtccctg
60gcccggccct ccttcagttt agttgaggat accacattag agccagaaga gccaccaacc
120aaataccaaa tctctcaacc agaagtgtac gtggctgcgc caggggagtc
gctagaggtg 180cgctgcctgt tgaaagatgc cgccgtgatc agttggacta
aggatggggt gcacttgggg 240cccaacaata ggacagtgct tattggggag
tacttgcaga taaagggcgc cacgcctaga 300gactccggcc tctatgcttg
tactgccagt aggactgtag acagtgaaac ttggtacttc 360atggtgaatg
tcacagatgc catctcatcc ggagatgatg aggatgacac cgatggtgcg
420gaagattttg tcagtgagaa cagtaacaac aagagagcac catactggac
caacacagaa 480aagatggaaa agcggctcca tgctgtgcct gcggccaaca
ctgtcaagtt tcgctgccca 540gccgggggga acccaatgcc aaccatgcgg
tggctgaaaa acgggaagga gtttaagcag 600gagcatcgca ttggaggcta
caaggtacga aaccagcact ggagcctcat tatggaaagt 660gtggtcccat
ctgacaaggg aaattatacc tgtgtagtgg agaatgaata cgggtccatc
720aatcacacgt accacctgga tgttgtggag cgatcgcctc accggcccat
cctccaagcc 780ggactgccgg caaatgcctc cacagtggtc ggaggagacg
tagagtttgt ctgcaaggtt 840tacagtgatg cccagcccca catccagtgg
atcaagcacg tggaaaagaa cggcagtaaa 900tacgggcccg acgggctgcc
ctacctcaag gttctcaagg ccgccggtgt taacaccacg 960gacaaagaga
ttgaggttct ctatattcgg aatgtaactt ttgaggacgc tggggaatat
1020acgtgcttgg cgggtaattc tattgggata tcctttcact ctgcatggtt
gacagttctg 1080ccagcgcctg gaagagaaaa ggagattaca gcttccccag
actacctgga gatagccatt 1140tactgcatag gggtcttctt aatcgcctgt
atggtggtaa cagtcatcct gtgccgaatg 1200aagaacacga ccaagaagcc
agacttcagc agccagccgg ctgtgcacaa gctgaccaaa 1260cgtatccccc
tgcggagaca ggtaacagtt tcggctgagt ccagctcctc catgaactcc
1320aacaccccgc tggtgaggat aacaacacgc ctctcttcaa cggcagacac
ccccatgctg 1380gcaggggtct ccgagtatga acttccagag gacccaaaat
gggagtttcc aagagataag 1440ctgacactgg gcaagcccct gggagaaggt
tgctttgggc aagtggtcat ggcggaagca 1500gtgggaattg acaaagacaa
gcccaaggag gcggtcaccg tggccgtgaa gatgttgaaa 1560gatgatgcca
cagagaaaga cctttctgat ctggtgtcag agatggagat gatgaagatg
1620attgggaaac acaagaatat cataaatctt cttggagcct gcacacagga
tgggcctctc 1680tatgtcatag ttgagtatgc ctctaaaggc aacctccgag
aatacctccg agcccggagg 1740ccacccggga tggagtactc ctatgacatt
aaccgtgttc ctgaggagca gatgaccttc 1800aaggacttgg tgtcatgcac
ctaccagctg gccagaggca tggagtactt ggcttcccaa 1860aaatgtattc
atcgagattt agcagccaga aatgttttgg taacagaaaa caatgtgatg
1920aaaatagcag actttggact cgccagagat atcaacaata tagactatta
caaaaagacc 1980accaatgggc ggcttccagt caagtggatg gctccagaag
ccctgtttga tagagtatac 2040actcatcaga gtgatgtctg gtccttcggg
gtgttaatgt gggagatctt cactttaggg 2100ggctcgccct acccagggat
tcccgtggag gaacttttta agctgctgaa ggaaggacac 2160agaatggata
agccagccaa ctgcaccaac gaactgtaca tgatgatgag ggactgttgg
2220catgcagtgc cctcccagag accaacgttc aagcagttgg tagaagactt
ggatcgaatt 2280ctcactctca caaccaatga gatcatggag gaaacaaata
cgcagattgc ttggccatca 2340aaactgaaga tcggagccaa atccaagaaa
gatccccata ttaaggtttc tggaaagaaa 2400gaagatgtta aagaagccaa
ggaaatgatc atgtctgtct tagacacaaa aagcaatcga 2460gtcacactga
agatggatgt ttcacataca gaacattcac atgtaatcgg caaaggtggc
2520aacaatatta aaaaagtgat ggaagaaacc ggatgccata tccactttcc
agattccaac 2580aggaataacc aagcagaaaa aagcaaccag gtatctatag
cgggacaacc agcaggagta 2640gaatctgccc gagttagaat tcgggagctg
cttcctttgg tgctgatgtt tgagctacca 2700attgctggaa ttcttcaacc
ggttcctgat cctaattccc cctctattca gcatatatca 2760caaacgtaca
atatttcagt atcatttaaa cagcgttccc gaatgtatgg tgctactgtc
2820atagtacgag ggtctcagaa taacactagt gctgtgaagg aaggaactgc
catgctgtta 2880gaacatcttg ctgggagctt agcatcagct attcctgtga
gcacacaact agatattgca 2940gctcaacatc atctctttat gatgggtcga
aatgggagca acatcaaaca tatcatgcag 3000agaacaggtg ctcagatcca
ctttcctgat cccagtaatc cacaaaagaa atctaccgtc 3060tacctccagg
gcaccattga gtctgtctgt cttgcaaggc aatatctcat gggttgtctt
3120cctcttgtgt tgatgtttga tatgaaggaa gaaattgaag tagatccaca
attcattgcg 3180cagttgatgg aacagcttga tgtcttcatc agtattaaac
caaagcccaa acagccaagc 3240aagtctgtga ttgtgaaaag tgttgagcga
aatgccttaa atatgtatga agcaaggaaa 3300tgtctcctcg gacttgaaag
cagtggggtt accatagcaa ccagtccatc cccagcatcc 3360tgccctgccg
gcctggcatg tcccagcctg gatatcttag cttcagcagg ccttggactc
3420actggactag gtcttttggg acccaccacc ttatctctga acacttcaac
aaccccaaac 3480tcactcttga atgctcttaa tagctcagtc agtcctttgc
aaagtccaag ttctggtaca 3540cccagcccca cattatgggc acccccactt
gctaatactt caagtgccac aggtttttct 3600gctataccac accttatgat
tccatctact gcccaagcca cattaactaa tattttgttg 3660tctggagtgc
ccacctatgg gcacacagct ccatctcccc ctcctggctt gactcctgtt
3720gatgtccata tcaacagtat gcagaccgaa ggcaaaaaaa tctctgctgc
tttaaatgga 3780catgcacagt ctccagatat aaaatatggt gcaatatcca
cttcatcact tggagaaaaa 3840gtgctgagtg caaatcacgg ggatccgtcc
atccagacaa gtgggtctga gcagacatct 3900cccaaatcaa gccccactga
aggttgtaat gatgcttttg ttgaagtagg catgcctcga 3960agtccttccc
attctgggaa tgctggtgac ttgaaacaga tgatgtgtcc ctccaaggtt
4020tcctgtgcca aaaggcagac agtggaacta ttgcaaggca cgaaaaactc
acacttacac 4080agcactgaca ggttgctctc agaccctgaa ctgagtgcta
ccgaaagccc tttggctgac 4140aagaaggctc cagggagtga gcgcgctgca
gagagggcag cagctgccca gcaaaactcc 4200gaaagggccc accttgctcc
acggtcatca tatgtcaaca tgcaggcatt tgactatgaa 4260cagaagaagc
tattagccac caaagctatg ttaaagaaac cagtggtgac ggaggtcaga
4320acgcccacaa atacctggag tggcctgggt ttttctaaat ccatgccagc
tgaaactatc 4380aaggagttga gaagggccaa tcatgtgtcc tataagccca
caatgacaac cacttatgag 4440ggctcatcca tgtccctttc acggtccaac
agtcgtgagc acttgggagg tggaagcgaa 4500tctgataact ggagagaccg
aaatggaatt ggacctggaa gtcatagtga atttgcagct 4560tctattggca
gccctaagcg taaacaaaac aaatcaacgg aacactatct cagcagtagc
4620aattacatgg actgcatttc ctcgctgaca ggaagcaatg gctgtaactt
aaatagctct 4680ttcaaaggtt ctgacctccc tgagctcttc agcaaactgg
gcctgggcaa atacacagat 4740gttttccagc aacaagagat cgatcttcag
acattcctca ctctcacaga tcaggatctg 4800aaggagctgg gaataactac
ttttggtgcc aggaggaaaa tgctgcttgc aatttcagaa 4860ctaaataaaa
accgaagaaa gctttttgaa tcgccaaatg cacgcacctc tttcctggaa
4920ggtggagcga gtggaaggct accccgtcag tatcactcag acattgctag
tgtcagtggc 4980cgctggtag 4989245109DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
24atggtcagct ggggtcgttt catctgcctg gtcgtggtca ccatggcaac cttgtccctg
60gcccggccct ccttcagttt agttgaggat accacattag agccagaaga gccaccaacc
120aaataccaaa tctctcaacc agaagtgtac gtggctgcgc caggggagtc
gctagaggtg 180cgctgcctgt tgaaagatgc cgccgtgatc agttggacta
aggatggggt gcacttgggg 240cccaacaata ggacagtgct tattggggag
tacttgcaga taaagggcgc cacgcctaga 300gactccggcc tctatgcttg
tactgccagt aggactgtag acagtgaaac ttggtacttc 360atggtgaatg
tcacagatgc catctcatcc ggagatgatg aggatgacac cgatggtgcg
420gaagattttg tcagtgagaa cagtaacaac aagagagcac catactggac
caacacagaa 480aagatggaaa agcggctcca tgctgtgcct gcggccaaca
ctgtcaagtt tcgctgccca 540gccgggggga acccaatgcc aaccatgcgg
tggctgaaaa acgggaagga gtttaagcag 600gagcatcgca ttggaggcta
caaggtacga aaccagcact ggagcctcat tatggaaagt 660gtggtcccat
ctgacaaggg aaattatacc tgtgtagtgg agaatgaata cgggtccatc
720aatcacacgt accacctgga tgttgtggag cgatcgcctc accggcccat
cctccaagcc 780ggactgccgg caaatgcctc cacagtggtc ggaggagacg
tagagtttgt ctgcaaggtt 840tacagtgatg cccagcccca catccagtgg
atcaagcacg tggaaaagaa cggcagtaaa 900tacgggcccg acgggctgcc
ctacctcaag gttctcaagg ccgccggtgt taacaccacg 960gacaaagaga
ttgaggttct ctatattcgg aatgtaactt ttgaggacgc tggggaatat
1020acgtgcttgg cgggtaattc tattgggata tcctttcact ctgcatggtt
gacagttctg 1080ccagcgcctg gaagagaaaa ggagattaca gcttccccag
actacctgga gatagccatt 1140tactgcatag gggtcttctt aatcgcctgt
atggtggtaa cagtcatcct gtgccgaatg 1200aagaacacga ccaagaagcc
agacttcagc agccagccgg ctgtgcacaa gctgaccaaa 1260cgtatccccc
tgcggagaca ggtaacagtt tcggctgagt ccagctcctc catgaactcc
1320aacaccccgc tggtgaggat aacaacacgc ctctcttcaa cggcagacac
ccccatgctg 1380gcaggggtct ccgagtatga acttccagag gacccaaaat
gggagtttcc aagagataag 1440ctgacactgg gcaagcccct gggagaaggt
tgctttgggc aagtggtcat ggcggaagca 1500gtgggaattg acaaagacaa
gcccaaggag gcggtcaccg tggccgtgaa gatgttgaaa 1560gatgatgcca
cagagaaaga cctttctgat ctggtgtcag agatggagat gatgaagatg
1620attgggaaac acaagaatat cataaatctt cttggagcct gcacacagga
tgggcctctc 1680tatgtcatag ttgagtatgc ctctaaaggc aacctccgag
aatacctccg agcccggagg 1740ccacccggga tggagtactc ctatgacatt
aaccgtgttc ctgaggagca gatgaccttc 1800aaggacttgg tgtcatgcac
ctaccagctg gccagaggca tggagtactt ggcttcccaa 1860aaatgtattc
atcgagattt agcagccaga aatgttttgg taacagaaaa caatgtgatg
1920aaaatagcag actttggact cgccagagat atcaacaata tagactatta
caaaaagacc 1980accaatgggc ggcttccagt caagtggatg gctccagaag
ccctgtttga tagagtatac 2040actcatcaga gtgatgtctg gtccttcggg
gtgttaatgt gggagatctt cactttaggg 2100ggctcgccct acccagggat
tcccgtggag gaacttttta agctgctgaa ggaaggacac 2160agaatggata
agccagccaa ctgcaccaac gaactgtaca tgatgatgag ggactgttgg
2220catgcagtgc cctcccagag accaacgttc aagcagttgg tagaagactt
ggatcgaatt 2280ctcactctca caaccaatga ggagagtaga tctggagaaa
ccaacagctg tgttgaagaa 2340ataatccggg agatgacctg gcttccacca
ctttctgcta ttcaagcacc tggcaaagtg 2400gaaccaacca aatttccatt
tccaaataag gactctcagc ttgtatcctc tggacacaat 2460aatccaaaga
aaggtgatgc agagccagag agtccagaca gtggcacatc gaatacatca
2520atgctggaag atgaccttaa gctaagcagt gatgaagagg agaatgaaca
gcaggcagct 2580cagagaacgg ctctccgcgc tctctctgac agcgccgtgg
tccagcagcc caactgcaga 2640acctcggtgc cttccagcaa gggcagcagc
agcagcagca gcagcggcag cagcagctcc 2700tccagcgact cagagagcag
ctccggatct gactcggaga ccgagagcag ctccagcgag 2760agtgagggca
gcaagccccc ccacttctcc agccccgagg ctgaaccggc atcctctaac
2820aagtggcagc tggataaatg gctaaacaaa gttaatcccc acaagcctcc
tattctgatc 2880caaaatgaaa gccacgggtc agagagcaat cagtactaca
acccggtgaa agaggacgtc 2940caggactgtg ggaaagtccc cgacgtttgc
cagcccagcc tgagagagaa ggagatcaag 3000agcacttgca aggaggagca
aaggccaagg acagccaaca aggcccctgg gagtaaaggc 3060gtgaagcaga
agtccccgcc cgcggccgtg gccgtggcgg tgagcgcagc cgccccgcca
3120cccgcagtgc cctgtgcgcc cgcggagaac gcgcccgcgc ctgcccggag
gtccgcgggc 3180aagaagccca ccaggcgcac cgagaggacc tcagccgggg
acggcgccaa ctgccaccgg 3240cccgaggagc ccgcggccgc ggacgcgctg
gggacgagcg tggtggtccc cccggagccc 3300accaaaacca ggccctgtgg
caacaacaga gcgagccacc gcaaggagct gcgctcctcc 3360gtgacctgcg
agaagcgccg cacgcggggg ctaagcagga tcgtccccaa atccaaggag
3420ttcattgaga cagagtcgtc atcttcatcc tcctcctcgg actccgacct
ggagtccgag 3480caggaggagt accctctgtc caaagcacag accgtggctg
cctctgcctc ctccgggaat 3540gatcagaggc tgaaggaggc cgctgccaac
gggggcagtg gtcctagggc ccctgtaggc 3600tccatcaacg ccaggaccac
cagtgacatc gccaaggagc tggaggagca gttctacaca 3660ctggtcccct
ttggccggaa cgaacttctc tcccctctaa aggacagtga tgagatcagg
3720tctctctggg tcaaaatcga cctgaccctc ctgtccagga tcccagaaca
cctgccccag 3780gagccagggg tattgagcgc ccctgccacc aaggactctg
agagcgcacc gcccagccac 3840acctcggaca cacctgcaga aaaggctttg
ccaaaatcca agaggaaacg caagtgtgac 3900aacgaagacg actacaggga
gatcaagaag tcccagggag agaaagacag ctcttcaaga 3960ctggccacct
ccaccagtaa tactttgtct gcaaaccact gcaacatgaa catcaacagt
4020gtggcaatac caataaataa aaatgaaaaa atgcttcggt cgcccatctc
acccctctct 4080gatgcatcta aacacaaata caccagcgag gacttaactt
cttccagccg acctaatggc 4140aacagtttgt ttacttcagc ctcttccagc
aaaaagccta aggccgacag ccagctgcag 4200cctcacggcg gagacctcac
gaaagcagct cacaacaatt ctgaaaacat tcccctccac 4260aagtcacggc
cgcagacgaa gccgtggtct ccaggctcca acggccacag ggactgcaag
4320aggcagaaac ttgtcttcga tgatatgcct cgcagtgccg attattttat
gcaagaagct 4380aaacgaatga agcataaagc agatgcaatg gtggaaaagt
ttggaaaggc tttgaactat 4440gctgaagcag cattgtcgtt tatcgagtgt
ggaaatgcaa tggaacaagg ccccatggaa 4500tccaaatctc cttatacgat
gtattcagaa acagtagagc tcatcaggta tgctatgaga 4560ctaaaaaccc
actcaggccc caatgccaca ccagaagaca aacaactggc tgcattatgt
4620taccgatgcc tggccctcct gtactggcgg atgtttcgac tcaaaaggga
ccacgctgta 4680aagtattcaa aagcactaat cgactatttc aagaactcat
ctaaagccgc ccaagcccca 4740tctccgtggg gggccagtgg aaagagcact
ggaaccccat cccccatgtc tcccaacccc 4800tctcccgcca gctccgtggg
gtctcagggc agcctctcca acgccagcgc cctgtccccg 4860tcgaccatcg
tcagcatccc acagcgcatc caccagatgg cggccaacca cgtcagcatc
4920accaacagca tcctgcacag ctacgactac tgggagatgg ccgacaacct
ggccaaggaa 4980aaccgagaat tcttcaacga cctggatctg ctcatggggc
cggtcaccct gcacagcagc 5040atggagcacc tggtccagta ctcccaacag
ggcctgcact ggctgcggaa cagcgcccac 5100ctgtcatag
5109253213DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 25atggtcagct ggggtcgttt catctgcctg
gtcgtggtca ccatggcaac cttgtccctg 60gcccggccct ccttcagttt agttgaggat
accacattag agccagaaga gccaccaacc 120aaataccaaa tctctcaacc
agaagtgtac gtggctgcgc caggggagtc gctagaggtg 180cgctgcctgt
tgaaagatgc cgccgtgatc agttggacta aggatggggt gcacttgggg
240cccaacaata ggacagtgct tattggggag tacttgcaga taaagggcgc
cacgcctaga 300gactccggcc tctatgcttg tactgccagt aggactgtag
acagtgaaac ttggtacttc 360atggtgaatg tcacagatgc catctcatcc
ggagatgatg aggatgacac cgatggtgcg 420gaagattttg tcagtgagaa
cagtaacaac aagagagcac catactggac caacacagaa 480aagatggaaa
agcggctcca tgctgtgcct gcggccaaca ctgtcaagtt tcgctgccca
540gccgggggga acccaatgcc aaccatgcgg tggctgaaaa acgggaagga
gtttaagcag 600gagcatcgca ttggaggcta caaggtacga aaccagcac
References




D00001

D00002

D00003

D00004

D00005

P00001
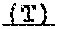
P00002
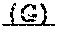
S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.