Method For Treating Cancer
Cho; Der-Yang ; et al.
U.S. patent application number 16/390308 was filed with the patent office on 2020-03-19 for method for treating cancer. The applicant listed for this patent is China Medical University Hospital. Invention is credited to Shao-Chih Chiu, Der-Yang Cho, Shi-Wei Huang, Chia-Ing Jan, Chih-Ming Pan.
| Application Number | 20200085868 16/390308 |
| Document ID | / |
| Family ID | 67770364 |
| Filed Date | 2020-03-19 |











View All Diagrams
| United States Patent Application | 20200085868 |
| Kind Code | A1 |
| Cho; Der-Yang ; et al. | March 19, 2020 |
Method For Treating Cancer
Abstract
The present disclosure relates to a method for treating cancer including steps as follows. A chemotherapy drug is administered to a subject in need for a treatment of cancer. Then a composition containing a plurality of chimeric antigen receptor expressing cells is administered to the subject, wherein the chimeric antigen receptor expressing cells expresses a chimeric antigen receptor specific to human leukocyte antigen G (HLA-G).
| Inventors: | Cho; Der-Yang; (Taichung City, TW) ; Chiu; Shao-Chih; (Taichung City, TW) ; Jan; Chia-Ing; (Taichung City, TW) ; Pan; Chih-Ming; (Taichung City, TW) ; Huang; Shi-Wei; (Taichung City, TW) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 67770364 | ||||||||||
| Appl. No.: | 16/390308 | ||||||||||
| Filed: | April 22, 2019 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C07K 2317/565 20130101; C07K 2319/00 20130101; A61K 31/711 20130101; A61K 2039/505 20130101; C07K 14/70539 20130101; C07K 16/2833 20130101; C12N 2800/80 20130101; A61K 35/17 20130101; A61K 31/495 20130101; A61K 31/7068 20130101; A61K 31/555 20130101; C12N 5/0646 20130101; C12N 9/22 20130101; C07K 2319/03 20130101; A61K 45/06 20130101; A61K 31/4188 20130101; C07K 2319/33 20130101; C12N 2510/00 20130101; A61K 31/704 20130101; A61K 31/282 20130101; C07K 2317/622 20130101; A61P 35/00 20180101; A61K 35/17 20130101; A61K 2300/00 20130101; A61K 31/704 20130101; A61K 2300/00 20130101; A61K 31/495 20130101; A61K 2300/00 20130101; A61K 31/7068 20130101; A61K 2300/00 20130101; A61K 31/555 20130101; A61K 2300/00 20130101; A61K 31/711 20130101; A61K 2300/00 20130101 |
| International Class: | A61K 35/17 20060101 A61K035/17; A61P 35/00 20060101 A61P035/00; A61K 31/7068 20060101 A61K031/7068; A61K 31/282 20060101 A61K031/282; A61K 31/4188 20060101 A61K031/4188; A61K 31/704 20060101 A61K031/704 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Sep 17, 2018 | TW | 107132664 |
Claims
1. A method for treating a cancer, comprising: administering a chemotherapy drug to a subject in need for a treatment of cancer; and administering a composition containing a plurality of chimeric antigen receptor expressing cells to the subject, wherein the chimeric antigen receptor expressing cells expresses a chimeric antigen receptor specific to human leukocyte antigen G (HLA-G).
2. The method of claim 1, wherein the cancer comprises a breast cancer, a polymorphic glioblastoma, a pancreatic cancer and an ovarian cancer.
3. The method of claim 2, wherein the chemotherapy drug comprises doxorubicin, temozolomide, gemcitabine and carboplatin.
4. The method of claim 1, wherein the chimeric antigen receptor expressing cell comprises an immune cell and a chimeric antigen receptor expression plasmid expresses the chimeric antigen receptor specific to HLA-G.
5. The method of claim 4, wherein the immune cell is a T lymphocyte.
6. The method of claim 4, wherein the immune cell is a natural killer (NK) cell.
7. The method of claim 6, wherein the NK cell is a NK-92 cell line or a primary NK cell.
8. The method of claim 1, wherein the chimeric antigen receptor comprises, in order from an N-terminus to a C-terminus: an anti-HLA-G antibody comprising an amino acid sequence of SEQ ID NO: 1; an HLA-G receptor comprising an amino acid sequence of SEQ ID NO: 2; and a costimulatory domain comprising an amino acid sequence of SEQ ID NO: 3.
9. The method of claim 8, wherein the chimeric antigen receptor further comprises a suicide protein comprising an amino acid sequence of SEQ ID NO: 4, and the suicide protein is linked to the C-terminus of the costimulatory domain.
10. The method of claim 1, wherein the chimeric antigen receptor further comprises a 2A peptide, wherein the 2A peptide links the HLA-G receptor and the costimulatory domain.
Description
RELATED APPLICATIONS
[0001] This application claims priority to Taiwan Application Serial Number 107132664, filed Sep. 17, 2018, which is herein incorporated by reference.
SEQUENCE LISTING
[0002] The sequence listing submitted via EFS, in compliance with 37 CFR .sctn. 1.52(e)(5), is incorporated herein by reference. The sequence listing text file submitted via EFS contains the file "CP-4343-US_SequenceListing", created on Mar. 19, 2019, which is 15,771 bytes in size.
BACKGROUND
Technical Field
[0003] The present disclosure relates to a method for treating cancer. More particularly, the present disclosure relates to a method for treating cancer with a chemotherapy drug and chimeric antigen receptor expressing cells.
Description of Related Art
[0004] Cancer, also known as malignancy, is a state of abnormal proliferation of cells, and these proliferating cells may invade other parts of the body as a disease caused by a malfunction in the control of cell division and proliferation.
[0005] The number of people suffering from cancer worldwide has a growing trend. Cancer is one of the top ten causes of death for the Chinese people and has been the top ten causes of death for twenty-seven consecutive years.
[0006] Conventional cancer treatments include surgery, radiation therapy, chemotherapy, and target therapy. Cancer immunotherapy is another method for treating cancer except the above methods. The immune system of the patient is activated in the cancer immunotherapy by using tumor cells or tumor antigens to induce specific cellular and humoral immune responses for enhancing the anti-cancer ability of the patient, preventing the growth, spread, and recurrence of tumors, and achieving the purpose of removing or controlling tumors.
[0007] There are three main directions for the cancer immunotherapy: the tumor vaccine, the cell therapy and the immune checkpoint inhibitor. The chimeric antigen receptor immune cell technology is one of the cell therapy developing very rapidly in recent years. In conventional technology, the chimeric antigen receptor immune cell transfecting a chimeric protein, which couples the antigen binding portion having capable of recognizing a certain tumor antigen of the antibody to the intracellular portion of the CD3-.delta. chain or Fc.epsilon.Rl.gamma. in vitro, into the immune cell by a transduction method to express the chimeric antigen receptor. The chimeric antigen receptor immune cell technology has a significant therapeutic effect in the treatment of acute leukemia and non-Hodgkin's lymphoma, and it is considered to be one of the most promising treatment for cancer. However, the cell therapy of the chimeric antigen receptor immune cell currently has the following disadvantages: lack of unique tumor-associated antigens, low efficiency of homing of immune cells to tumor sites, and inability to overcome the immunosuppressive microenvironment of solid tumors. Accordingly, the efficacy of the chimeric antigen receptor immune cell in solid tumors is greatly limited.
SUMMARY
[0008] According to one aspect of the present disclosure, a method for treating a cancer includes steps as follows. A chemotherapy drug is administered to a subject in need for a treatment of cancer. Then a composition containing a plurality of chimeric antigen receptor expressing cells is administered to the subject, wherein the chimeric antigen receptor expressing cells expresses a chimeric antigen receptor specific to human leukocyte antigen G (HLA-G).
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The patent or application file contains at least one drawing executed in color. Copies of this patent or patent application publication with color drawing(s) will be provided by Office upon request and payment of the necessary fee. The present disclosure can be more fully understood by reading the following detailed description of the embodiment, with reference made to the accompanying drawings as follows:
[0010] FIGS. 1A, 1B, 1C, 1D, 1E, 1F, 1G and 1H are analytical results of immunofluorescence staining assay showing a HLA-G expression level of tumor cells treated with a chemotherapy drug.
[0011] FIGS. 2A, 2B, 2C, 2D and 2E are analytical results of flow cytometry showing a HLA-G expression of tumor cells treated with a chemotherapy drug.
[0012] FIG. 3 is a graph showing an expression level of chimeric antigen receptors in a chimeric antigen receptor expressing cell according to Example 1 of the present disclosure.
[0013] FIGS. 4A, 4B, 4C, 4D, 4E, 4F, 4G, 4H and 4I show analytical results of tumor cell death induced by chimeric antigen receptor expressing cells according to Example 1 of the present disclosure.
[0014] FIG. 5 is a graph showing an expression level of chimeric antigen receptors in a chimeric antigen receptor expressing cell according to Example 2 of the present disclosure.
[0015] FIGS. 6A, 6B, 6C, 6D, 6E, 6F, 6G, 6H and 6I show analytical results of tumor cell death induced by chimeric antigen receptor expressing cells according to Example 2 of the present disclosure.
[0016] FIG. 7 is a graph showing an expression level of a chimeric antigen receptor in a chimeric antigen receptor expressing cell according to Example 3 of the present disclosure.
[0017] FIGS. 8A, 8B, 8C, 8D, 8E, 8F, 8G, 8H and 8I show analytical results of tumor cell death induced by chimeric antigen receptor expressing cells according to Example 3 of the present disclosure.
[0018] FIG. 9 is a schematic view showing the theoretical structure and mechanism of a chimeric antigen receptor in the plasma membrane of a chimeric antigen receptor expressing cell of the present disclosure.
DETAILED DESCRIPTION
[0019] A method for treating cancer is provided. The method includes administering a chemotherapy drug to a subject in need for a treatment of cancer, and administering a composition containing a plurality of chimeric antigen receptor expressing cells to the subject, wherein the chimeric antigen receptor expressing cells expresses a chimeric antigen receptor specific to human leukocyte antigen G (HLA-G). The chemotherapeutic drug can induce the plasma membrane of the tumor cells to express a large amount of the human leukocyte antigen G, thereby enhancing a toxicity of the chimeric antigen receptor cell to the tumor cell.
[0020] The term "human leukocyte antigen G (HLA-G)" is a protein that in humans is encoded by the HLA-G gene. The HLA-G belongs to nonclassical class I major histocompatibility complex (MHC) with a heavy chain of approximately 45 kDa. HLA-G is expressed on fetal derived placental cells, and is active in the negative regulation of immune response. HLA-G may play a role in immune tolerance in pregnancy.
[0021] According to the method for treating cancer of the present disclosure, the cancer can include breast cancer, polymorphic glioblastoma, pancreatic cancer and ovarian cancer, and the chemotherapy drug can include doxorubicin (Dox), temozolomide (TMZ), gemcitabine (Gem) and carboplatin (CB).
[0022] According to the method for treating cancer of the present disclosure, the chimeric antigen receptor can include, in order from an N-terminus to a C-terminus, an anti-HLA-G antibody including an amino acid sequence of SEQ ID NO: 1, an HLA-G receptor including an amino acid sequence of SEQ ID NO: 2, and a costimulatory domain including an amino acid sequence of SEQ ID NO: 3. Preferably, a suicide protein including an amino acid sequence of SEQ ID NO: 4 is linked to the C-terminus of the costimulatory domain, and a 2A peptide including an amino acid sequence of SEQ ID NO: 10 links the HLA-G receptor and the costimulatory domain. In detail, the anti-HLA-G antibody including the amino acid sequence of SEQ ID NO: 1 includes a heavy chain (HC) immunoglobulin variable domain sequence and a light chain (LC) immunoglobulin variable domain sequence. The HC immunoglobulin variable domain sequence includes a CDRH1 including an amino acid sequence of SEQ ID NO: 5, a CDRH2 including an amino acid sequence of SEQ ID NO: 6, and a CDRH3 including an amino acid sequence of SEQ ID NO: 7. The LC immunoglobulin variable domain sequence includes a CDRL2 including an amino acid sequence of SEQ ID NO: 8, and a CDRL3 including an amino acid sequence of SEQ ID NO: 9. The costimulatory domain including an amino acid sequence of SEQ ID NO: 3 is DNAX activating protein 12 (DAP12). The suicide protein including an amino acid sequence of SEQ ID NO: 4 is iCas9 protein.
[0023] According to the method for treating cancer of the present disclosure, the chimeric antigen receptor expression plasmid can include a promoter and a nucleic acid encoding the chimeric antigen receptor. The nucleic acid encoding the chimeric antigen receptor can include, in order from a 5' end to a 3' end, an anti-HLA-G antibody coding fragment, an HLA-G receptor coding fragment, and a costimulatory domain coding fragment. Preferably, a suicide gene is linked to the 3' end of the costimulatory domain coding fragment, and a 2A peptide coding fragment links the HLA-G receptor coding fragment and the costimulatory domain coding fragment.
[0024] In detail, according to one example of this embodiment, the chimeric antigen receptor expression plasmid is Lenti-EF1a-CAR-100517-S1A plasmid, wherein the insert thereof includes a promoter, an anti-HLA-G antibody coding fragment, an HLA-G receptor coding fragment, and a costimulatory domain coding fragment. The promoter is the EF-1 alpha promoter including a nucleic acid sequence of SEQ ID NO: 16. The anti-HLA-G antibody coding fragment includes the nucleic acid sequence of SEQ ID NO: 11. The HLA-G receptor coding fragment includes the nucleic acid sequence of SEQ ID NO: 12. The costimulatory domain coding fragment includes the nucleic acid sequence of SEQ ID NO: 13. In addition, the insert of the Lenti-EF1a-CAR-100517-S1A plasmid further includes a signal peptide coding fragment including a nucleic acid sequence of SEQ ID NO: 17, the suicide gene including the nucleic acid sequence of SEQ ID NO: 14, and the 2A peptide coding fragment including the nucleic acid sequence of SEQ ID NO: 15. The signal peptide coding fragment is linked to the 5' end of the anti-HLA-G antibody coding fragment, the suicide gene is linked to the 3' end of the costimulatory domain coding fragment, and the 2A peptide coding fragment links the HLA-G receptor coding fragment and the costimulatory domain coding fragment. Then, the insert is constructed on Creative Biolabs vector (Creative Biolabs, N.Y., USA) to obtain the Lenti-EF1a-CAR-100517-S1A plasmid. The Creative Biolabs vector is a lentivirus vector system, so that the constructed chimeric antigen receptor expression plasmid can be transfected into expression cells to produce lentiviruses, and the chimeric antigen receptor can be subsequently transduced into the immune cells using lentiviruses.
[0025] According to the method for treating cancer of the present disclosure, the chimeric antigen receptor expressing cell can include an immune cell and a chimeric antigen receptor expression plasmid expresses the chimeric antigen receptor specific to HLA-G. The chimeric antigen receptor expressing cell of the present disclosure is obtained by transducing the chimeric antigen receptor of the present disclosure into the immune cell using lentiviruses. Preferably, the immune cell can be a T lymphocyte or a natural killer (NK) cell. More preferably, the NK cell can be a NK-92 cell line or a primary NK cell. In detail, the constructed Lenti-EF1a-CAR-100517-S1A plasmid is transfected into a 293T cell line using lipofectamine 3000 (Invitrogen) to prepare the lentiviruses carrying the chimeric antigen receptor of the present disclosure. For obtaining one example of the chimeric antigen receptor expressing cell, the supernatant containing the prepared lentiviruses and Opti-MEM (Invitrogen) containing 8 .mu.g/ml of polybrene (Sigma-Aldrich) are used to culture the primary T lymphocytes for 3 days to transduce the chimeric antigen receptor of the present disclosure into the primary T lymphocytes. For obtaining another example of the chimeric antigen receptor expressing cell, the supernatant containing the prepared lentiviruses and the Opti-MEM (Invitrogen) containing 50 .mu.g/ml of protamine sulfate (Sigma-Aldrich) are used to culture the primary NK cells or the NK-92 cell line for 7 days to transduce the chimeric antigen receptor of the present disclosure into the primary NK cell or the NK-92 cell line. The obtained chimeric antigen receptor expressing cell has an effect of inducing tumor cell death in mammals, so that the chimeric antigen receptor expressing cell can be used for inhibiting a proliferation of tumor cells in a subject in need for a treatment of a tumor. Preferably, the tumor cell can be a breast cancer cell, a polymorphic glioblastoma cell, a pancreatic cancer cell or an ovarian cancer cell.
[0026] Reference will now be made in detail to the present embodiments of the present disclosure, examples of which are illustrated in the accompanying drawings.
EXAMPLES
I. Treatment of Chemotherapy Drug Increases the HLA-G Expression Level on the Plasma Membrane of Tumor Cells
[0027] To investigate effect of the treatment of the chemotherapy drug on the tumor cells, the tumor cells are treated with the chemotherapy drug, and then detecting the HLA-G expression level of the tumor cells.
[0028] The tumor cells used are human breast cancer cell line MDA-MB-231, human malignant brain tumor cell line DBTRG-05MG (hereinafter referred to as DBTRG), human pancreatic cancer cell line AsPC1, and human ovarian cancer cell line SKOV3. The tumor cell lines used are all purchased from the American Type Culture Collection (ATCC). The human breast cancer cell line MDA-MB-231 is a triple-negative breast cancer cell line, that is, the hormone receptor (ER, PR) and HER-2 receptor thereof are negative, and the human breast cancer cell line MDA-MB-231 is cultured in RPMI culture medium containing 10% fetal bovine serum (FBS). The human malignant brain tumor cell line DBTRG is cultured in DMEM culture medium containing 10% FBS. The human pancreatic cancer cell line AsPC1 is cultured in RPMI culture medium containing 10% FBS. The human ovarian cancer cell line SKOV3 is cultured in McCoy's 5A culture medium containing 10% FBS.
[0029] First, the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3 are seeded in a 6-well plate at a density of 2.times.10.sup.5 cells/well. The cells are subsequently incubated for 24 hours. Each type of the tumor cells is divided into two groups. In a control, the tumor cells are untreated. In an experiment group, the tumor cells are treated with the chemotherapy drug for 48 hours. The chemotherapy drug used for treating the human breast cancer cell line MDA-MB-231 is doxorubicin (200 nM), the chemotherapy drug used for treating the human malignant brain tumor cell line DBTRG is temozolomide (80 .mu.g/mL), the chemotherapy drug used for treating the human pancreatic cancer cell line AsPC1 is gemcitabine (20 .mu.M), the chemotherapy drug used for treating the human ovarian cancer cell line SKOV3 is carboplatin (20 .mu.M). Then, the HLA-G expression level of the tumor cells are detected by immunofluorescence staining assay and flow cytometry.
[0030] Please refer to FIGS. 1A, 1B, 10, 1D, 1E, 1F, 1G and 1H, which are analytical results of immunofluorescence staining assay showing the HLA-G expression level of tumor cells treated with the chemotherapy drug. FIG. 1A is analytical result of immunofluorescence staining assay showing the HLA-G expression level in the control group of the human breast cancer cell line MDA-MB-231, and FIG. 1B is analytical result of immunofluorescence staining assay showing the HLA-G expression level of the human breast cancer cell line MDA-MB-231 treated with doxorubicin (Dox). FIG. 1C is analytical result of immunofluorescence staining assay showing the HLA-G expression level in the control group of the human malignant brain tumor cell line DBTRG, and FIG. 1D is analytical result of immunofluorescence staining assay showing the HLA-G expression level of the human malignant brain tumor cell line DBTRG treated with temozolomide (TMZ). FIG. 1E is analytical result of immunofluorescence staining assay showing the HLA-G expression level in the control group of the human pancreatic cancer cell line AsPC1, and FIG. 1F is analytical result of immunofluorescence staining assay showing the HLA-G expression level of the human pancreatic cancer cell line AsPC1 treated with gemcitabine (Gem). FIG. 1G is analytical result of immunofluorescence staining assay showing the HLA-G expression level in the control group of the human ovarian cancer cell line SKOV3, and FIG. 1H is analytical result of immunofluorescence staining assay showing the HLA-G expression level of the human ovarian cancer cell line SKOV3 treated with carboplatin (CB).
[0031] In FIGS. 1A and 1B, treatment of doxorubicin can increase the HLA-G expression level on the plasma membrane of the human breast cancer cell line MDA-MB-231. In FIGS. 1C and 1D, treatment of temozolomide can increase the HLA-G expression level on the plasma membrane of the human malignant brain tumor cell line DBTRG. In FIGS. 1E and 1F, treatment of gemcitabine can increase the HLA-G expression level on the plasma membrane of the human pancreatic cancer cell line AsPC1. In FIGS. 1G and 1H, treatment of carboplatin can increase the HLA-G expression level on the plasma membrane of the human ovarian cancer cell line SKOV3.
[0032] Please refer to FIGS. 2A, 2B, 2C, 2D and 2E, which are analytical results of flow cytometry showing a HLA-G expression of tumor cells treated with a chemotherapy drug. FIG. 2A is analytical result of flow cytometry of the human breast cancer cell line MDA-MB-231. FIG. 2B is analytical result of flow cytometry of the human malignant brain tumor cell line DBTRG. FIG. 2C is analytical result of flow cytometry of the human pancreatic cancer cell line AsPC1. FIG. 2D is analytical result of flow cytometry of the human ovarian cancer cell line SKOV3. FIG. 2E is a statistical chart of FIGS. 2A, 2B, 2C and 2D.
[0033] In FIG. 2A, the mean fluorescence intensity (MFI) of the control group of the human breast cancer cell line MDA-MB-231 is only 12.27%, while the MFI of the experiment group of the human breast cancer cell line MDA-MB-231 can reach 64.45%, which is statistically significant (p<0.001). In FIG. 2B, the MFI of the control group of the human malignant brain tumor cell line DBTRG is only 14.01%, while the MFI of the experiment group of the human malignant brain tumor cell line DBTRG can reach 22.33%, which is statistically significant (p<0.001). In FIG. 2C, the MFI of the control group of the human pancreatic cancer cell line AsPC1 is only 13.18%, while the MFI of the experiment group of the human pancreatic cancer cell line AsPC1 can reach 41.44%, which is statistically significant (p<0.01). In FIG. 2D, the MFI of the control group of the human ovarian cancer cell line SKOV3 is only 14.69%, while the MFI of the experiment group of the human ovarian cancer cell line SKOV3 can reach 38.58%, which is statistically significant (p 21 0.01).
[0034] The results in FIGS. 1A to 2E indicate that the treatment of the chemotherapy drug can increase the HLA-G expression level on the plasma membrane of the tumor cells. Therefore, the method for treating cancer of the present disclosure further administers a composition containing a plurality of chimeric antigen receptor expressing cells to the subject in need for a treatment of cancer, in which the chimeric antigen receptor expressing cells expresses the chimeric antigen receptor specific to HLA-G, in order to enhance the effect of killing tumor cells. The treatment of the chemotherapy drug and the composition containing the chimeric antigen receptor expressing cells can be in a sequence or simultaneous.
II. Method for Treating Cancer of the Present Disclosure 2.1. Example 1
[0035] In the following, an Example 1, an Example 2 and an Example 3 will be further provided to illustrate the accompanied efficacies of the method for treating cancer of the present disclosure on inducing tumor cell death. However, the present disclosure is not limited thereto.
[0036] The Lenti-EF1a-CAR-100517-S1A plasmid is transduced into the NK-92 cell line to obtain the chimeric antigen receptor expressing cell of Example 1 of the present disclosure, and the expression level of the chimeric antigen receptor of the obtained chimeric antigen receptor expressing cell of Example 1 is analyzed by flow cytometry. Please refer to FIG. 3, which is a graph showing the expression level of chimeric antigen receptors in the chimeric antigen receptor expressing cell according to Example 1 of the present disclosure. FIG. 3 shows the expression level of the chimeric antigen receptor of the parental NK-92 cell line without transducing the chimeric antigen receptor of the present disclosure, and the expression level of the chimeric antigen receptor of the chimeric antigen receptor expressing cell of Example 1 on day 3 and day 7 after transduction the chimeric antigen receptor. In FIG. 3, the MFI of the parental NK-92 cell line is only 9.98%, while the MFI of the chimeric antigen receptor expressing cell of Example 1 on day 3 and day 7 after transduction can reach 20.11% and 65.07%, respectively. The results indicate that the chimeric antigen receptor expressing cell of Example 1 can stably express the chimeric antigen receptor of the present disclosure.
[0037] The effects of the method for treating cancer of the present disclosure by using the chimeric antigen receptor expressing cell of Example 1 of the present disclosure on inducing the death of the breast cancer cells, the glioblastoma multiforme cells, the pancreatic cancer cells, and the ovarian cancer cells are further demonstrated in following experiments.
[0038] First, the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3 are seeded in a 12-well plate at a density of 1.times.10.sup.5 cells/well. The cells are subsequently incubated for 24 hours. Each type of the tumor cells is divided into six groups. In a control, the tumor cells are untreated. In a group 1, the tumor cells are treated with the chemotherapy drug. In a group 2, the tumor cells are treated with the parental NK-92 cell line. In a group 3, the tumor cells are treated with the parental NK-92 cell line and the chemotherapy drug. In the groups 2 and 3, the number of the parental NK-92 cell line treated is 1.times.10.sup.5 cells. In a group 4, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 1. In a group 5, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 1 and the chemotherapy drug. In the groups 4 and 5, the number of the chimeric antigen receptor expressing cell of Example 1 treated is 1.times.10.sup.5 cells. The chemotherapy drug used for treating the human breast cancer cell line MDA-MB-231 is doxorubicin (200 nM), the chemotherapy drug used for treating the human malignant brain tumor cell line DBTRG is temozolomide (80 .mu.g/mL), the chemotherapy drug used for treating the human pancreatic cancer cell line AsPC1 is gemcitabine (20 .mu.M), the chemotherapy drug used for treating the human ovarian cancer cell line SKOV3 is carboplatin (20 .mu.M). The treated cells are stained with Annexin V-FITC and propidium iodide (PI), and the apoptosis and the death of the tumor cells are detected by the flow cytometry. The sum of the percentage of cells stained with Annexin V-FITC and/or PI (that is the percentage of cells in the first quadrant, the second quadrant, and the fourth quadrant of the bivariate flow cytometry scatter plot) are calculated to obtain the cytotoxicity. The results of the cytotoxicity are counted after the three independent trials in each group.
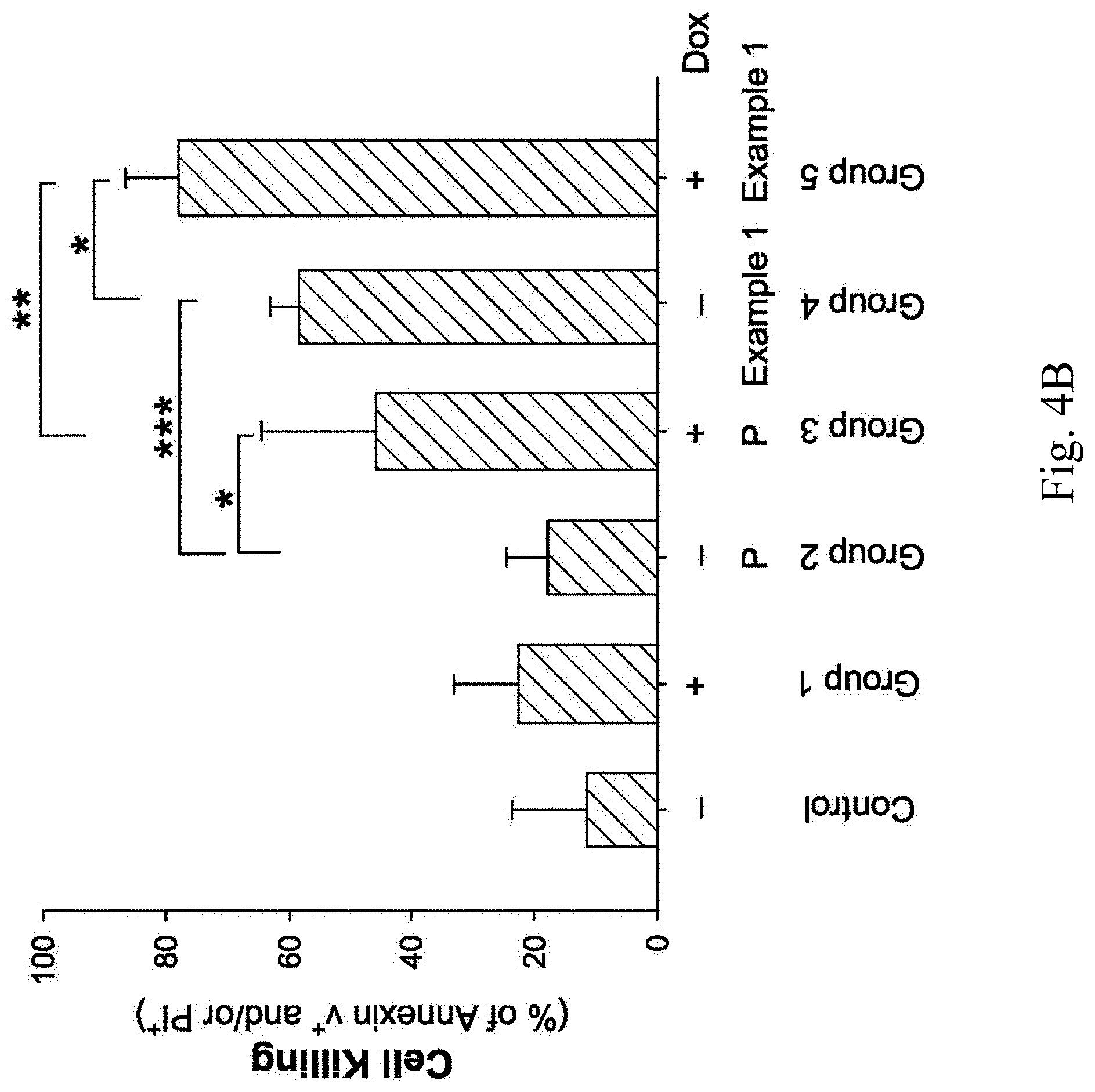
[0039] FIGS. 4A, 4B, 4C, 4D, 4E, 4F, 4G, 4H and 4I show analytical results of tumor cell death induced by the chimeric antigen receptor expressing cells according to Example 1 of the present disclosure. FIG. 4A is a graph showing the analytical results of the death of the human breast cancer cell line MDA-MB-231 induced by the chimeric antigen receptor expressing cell of Example 1, and FIG. 4B is a statistical chart of FIG. 4A after the three independent trials. FIG. 4C is a graph showing the analytical results of the death of the human malignant brain tumor cell line DBTRG induced by the chimeric antigen receptor expressing cell of Example 1, and FIG. 4D is a statistical chart of FIG. 4C after the three independent trials. FIG. 4E is a graph showing the analytical results of the death of the human pancreatic cancer cell line AsPC1 induced by the chimeric antigen receptor expressing cell of Example 1, and FIG. 4F is a statistical chart of FIG. 4E after the three independent trials. FIG. 4G is a graph showing the analytical results of the death of the human ovarian cancer cell line SKOV3 induced by the chimeric antigen receptor expressing cell of Example 1, and FIG. 4H is a statistical chart of FIG. 4G after the three independent trials. FIG. 4I is a statistical chart of FIGS. 4A, 4C, 4E and 4G after the three independent trials, wherein P represents the parental NK-92 cell line, H represents the chimeric antigen receptor expressing cell of Example 1, D represents doxorubicin, T represents temozolomide, G represents gemcitabine, and C represents carboplatin.
[0040] Please refer to FIGS. 4A and 4B. In the control, the death rate of the human breast cancer cell line MDA-MB-231 is only about 10%. In the group 1 treated with the doxorubicin and the group 2 treated with the parental NK-92 cell line, the death rate of the human breast cancer cell line MDA-MB-231 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the doxorubicin and the parental NK-92 cell line, the death rate of the human breast cancer cell line MDA-MB-231 can increase to 40%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 1, the death rate of the human breast cancer cell line MDA-MB-231 is about 60%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the doxorubicin and the chimeric antigen receptor expressing cell of Example 1, the death rate of the human breast cancer cell line MDA-MB-231 can reach 80%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.01) compared to the group 3, respectively.
[0041] Please refer to FIGS. 4C and 4D. In the control, the death rate of the human malignant brain tumor cell line DBTRG is less than 10%. In the group 1 treated with the temozolomide and the group 2 treated with the parental NK-92 cell line, the death rate of the human malignant brain tumor cell line DBTRG is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the temozolomide and the parental NK-92 cell line, the death rate of the human malignant brain tumor cell line DBTRG can increase to 40%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 1, the death rate of the human malignant brain tumor cell line DBTRG is more than 60%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the temozolomide and the chimeric antigen receptor expressing cell of Example 1, the death rate of the human malignant brain tumor cell line DBTRG can reach more than 80%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.001) compared to the group 3, respectively.
[0042] Please refer to FIGS. 4E and 4F. In the control, the death rate of the human pancreatic cancer cell line AsPC1 is less than 10%. In the group 1 treated with the gemcitabine and the group 2 treated with the parental NK-92 cell line, the death rate of the human pancreatic cancer cell line AsPC1 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the gemcitabine and the parental NK-92 cell line, the death rate of the human pancreatic cancer cell line AsPC1 can increase to 30%, but there is no statistically significant difference compared to the control. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 1, the death rate of the human pancreatic cancer cell line AsPC1 is approximately 40%, and there is a statistically significant difference (p<0.01) compared to the group 2. Furthermore, in the group 5 treated with the gemcitabine and the chimeric antigen receptor expressing cell of Example 1, the death rate of the human pancreatic cancer cell line AsPC1 can reach 60%, and there is a statistically significant difference (p<0.001) compared to the group 4 and a statistically significant difference (p<0.001) compared to the group 3, respectively.
[0043] Please refer to FIGS. 4G and 4H. In the control, the death rate of the human ovarian cancer cell line SKOV3 is less than 10%. In the group 1 treated with the carboplatin and the group 2 treated with the parental NK-92 cell line, the death rate of the human ovarian cancer cell line SKOV3 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the carboplatin and the parental NK-92 cell line, the death rate of the human ovarian cancer cell line SKOV3 can increase to 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 1, the death rate of the human ovarian cancer cell line SKOV3 is approximately 40%, and there is a statistically significant difference (p<0.01) compared to the group 2. Furthermore, in the group 5 treated with the carboplatin and the chimeric antigen receptor expressing cell of Example 1, the death rate of the human ovarian cancer cell line SKOV3 can reach 60%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.01) compared to the group 3, respectively.
[0044] Please refer to FIG. 4I, the results indicate that the chimeric antigen receptor expressing cell of Example 1 can be used to treat with the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3 for excellent cell killing. Therefore, the chimeric antigen receptor expressing cell of the present disclosure can be used for inhibiting the proliferation of the tumor cells in the subject in need for the treatment of the tumor. Preferably, the tumor cell can be the breast cancer cell, the polymorphic glioblastoma cell, the pancreatic cancer cell or the ovarian cancer cell. Further, the simultaneous treatment of the chemotherapy drug and the chimeric antigen receptor expressing cell of Example 1 can significantly increase the toxic effect on inducing death of the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3. The results indicate that the method for treating cancer of the present disclosure can effectively inhibit the growth of the tumor cells and treat cancer.
2.2. Example 2
[0045] The Lenti-EF1a-CAR-100517-S1A plasmid is transduced into the primary NK cell to obtain the chimeric antigen receptor expressing cell of Example 2 of the present disclosure, and the expression level of the chimeric antigen receptor of the obtained chimeric antigen receptor expressing cell of Example 2 is analyzed by the flow cytometry. Please refer to FIG. 5, which is a graph showing an expression level of chimeric antigen receptors in a chimeric antigen receptor expressing cell according to Example 2 of the present disclosure. FIG. 5 shows the expression level of the chimeric antigen receptor of the parental primary NK cell without transducing the chimeric antigen receptor of the present disclosure, and the expression level of the chimeric antigen receptor of the chimeric antigen receptor expressing cell of Example 2 on day 3 and day 7 after transduction the chimeric antigen receptor. In FIG. 5, the MFI of the parental primary NK cell is 22.09%, while the MFI of the chimeric antigen receptor expressing cell of Example 2 on day 3 and day 7 after transduction can reach 29.02% and 50.21%, respectively. The results indicate that the chimeric antigen receptor expressing cell of Example 2 can stably express the chimeric antigen receptor of the present disclosure.
[0046] The effects of the method for treating cancer of the present disclosure by using the chimeric antigen receptor expressing cell of Example 2 of the present disclosure on inducing the death of the breast cancer cells, the glioblastoma multiforme cells, the pancreatic cancer cells, and the ovarian cancer cells are further demonstrated in following experiments.
[0047] First, the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3 are seeded in a 12-well plate at a density of 1.times.10.sup.5 cells/well. The cells are subsequently incubated for 24 hours. Each type of the tumor cells is divided into six groups. In a control, the tumor cells are untreated. In a group 1, the tumor cells are treated with the chemotherapy drug. In a group 2, the tumor cells are treated with the parental primary NK cell. In a group 3, the tumor cells are treated with the parental primary NK cell and the chemotherapy drug. In the groups 2 and 3, the number of the parental primary NK cell treated is 1.times.10.sup.5 cells. In a group 4, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 2. In a group 5, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 2 and the chemotherapy drug. In the groups 4 and 5, the number of the chimeric antigen receptor expressing cell of Example 2 treated is 1.times.10.sup.5 cells. The chemotherapy drug used for treating the human breast cancer cell line MDA-MB-231 is doxorubicin (200 nM), the chemotherapy drug used for treating the human malignant brain tumor cell line DBTRG is temozolomide (80 .mu.g/mL), the chemotherapy drug used for treating the human pancreatic cancer cell line AsPC1 is gemcitabine (20 .mu.M), the chemotherapy drug used for treating the human ovarian cancer cell line SKOV3 is carboplatin (20 .mu.M). The treated cells are stained with Annexin V-FITC and propidium iodide (PI), and the apoptosis and the death of the tumor cells are detected by the flow cytometry. The sum of the percentage of cells stained with Annexin V-FITC and/or PI (that is the percentage of cells in the first quadrant, the second quadrant, and the fourth quadrant of the bivariate flow cytometry scatter plot) are calculated to obtain the cytotoxicity. The results of the cytotoxicity are counted after the three independent trials in each group.
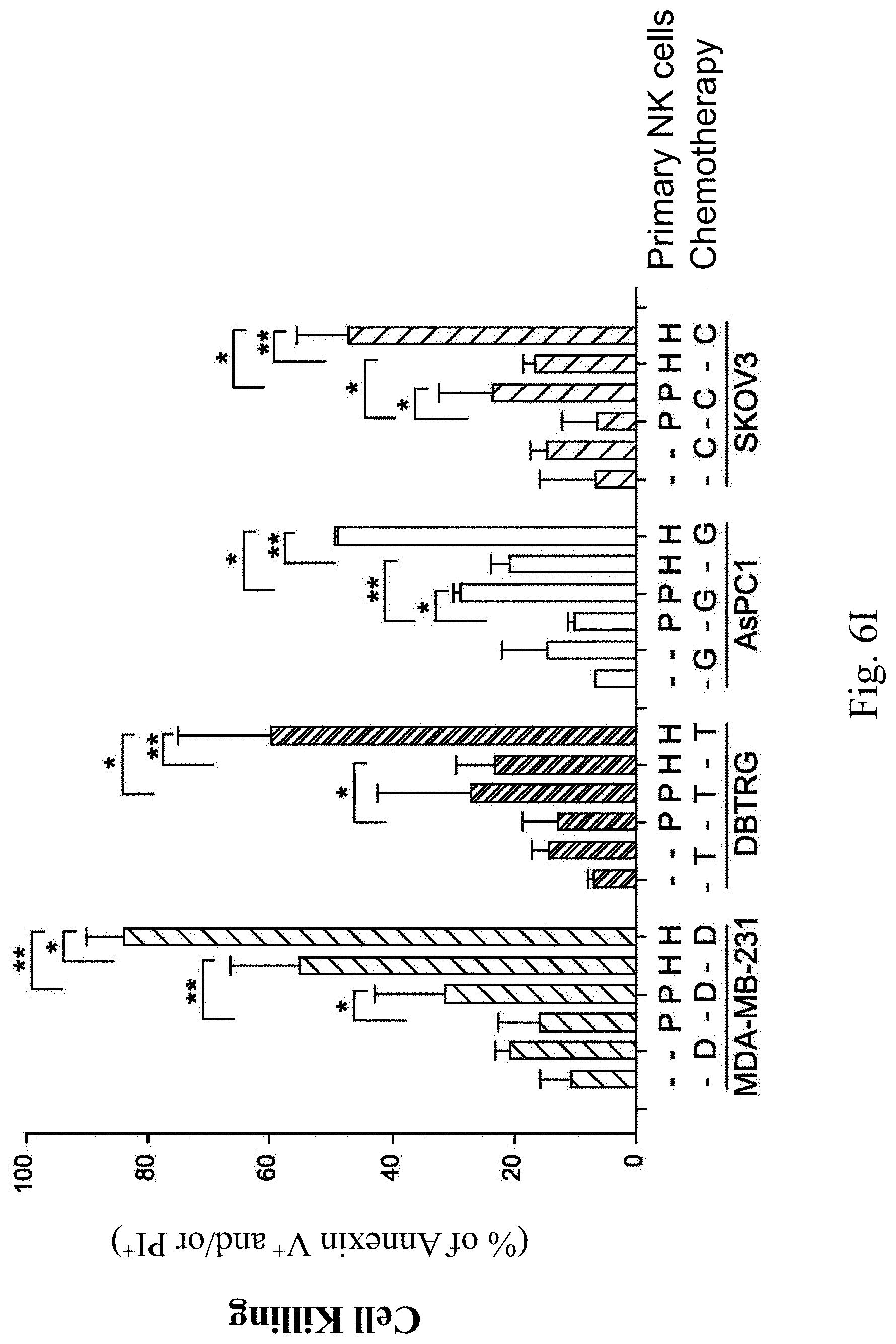
[0048] FIGS. 6A, 6B, 6C, 6D, 6E, 6F, 6G, 6H and 6I show analytical results of tumor cell death induced by the chimeric antigen receptor expressing cells according to Example 2 of the present disclosure. FIG. 6A is a graph showing the analytical results of the death of the human breast cancer cell line MDA-MB-231 induced by the chimeric antigen receptor expressing cell of Example 2, and FIG. 6B is a statistical chart of FIG. 6A after the three independent trials. FIG. 6C is a graph showing the analytical results of the death of the human malignant brain tumor cell line DBTRG induced by the chimeric antigen receptor expressing cell of Example 2, and FIG. 6D is a statistical chart of FIG. 6C after the three independent trials. FIG. 6E is a graph showing the analytical results of the death of the human pancreatic cancer cell line AsPC1 induced by the chimeric antigen receptor expressing cell of Example 2, and FIG. 6F is a statistical chart of FIG. 6E after the three independent trials. FIG. 6G is a graph showing the analytical results of the death of the human ovarian cancer cell line SKOV3 induced by the chimeric antigen receptor expressing cell of Example 2, FIG. 6H is a statistical chart of FIG. 6G after the three independent trials. FIG. 6I is a statistical chart of FIGS. 6A, 6C, 6E and 6G after the three independent trials, wherein P represents the parental primary NK cell, H represents the chimeric antigen receptor expressing cell of Example 2, D represents doxorubicin, T represents temozolomide, G represents gemcitabine, and C represents carboplatin.
[0049] Please refer to FIGS. 6A and 6B. In the control, the death rate of the human breast cancer cell line MDA-MB-231 is only about 10%. In the group 1 treated with the doxorubicin and the group 2 treated with the parental primary NK cell, the death rate of the human breast cancer cell line MDA-MB-231 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the doxorubicin and the parental primary NK cell, the death rate of the human breast cancer cell line MDA-MB-231 can increase to 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 2, the death rate of the human breast cancer cell line MDA-MB-231 is more than 50%, and there is a statistically significant difference (p<0.01) compared to the group 2. Furthermore, in the group 5 treated with the doxorubicin and the chimeric antigen receptor expressing cell of Example 2, the death rate of the human breast cancer cell line MDA-MB-231 can reach 80%, and there is a statistically significant difference (p <0.05) compared to the group 4 and a statistically significant difference (p<0.01) compared to the group 3, respectively.
[0050] Please refer to FIGS. 6C and 6D. In the control, the death rate of the human malignant brain tumor cell line DBTRG is less than 10%. In the group 1 treated with the temozolomide and the group 2 treated with the parental primary NK cell, the death rate of the human malignant brain tumor cell line DBTRG is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the temozolomide and the parental primary NK cell, the death rate of the human malignant brain tumor cell line DBTRG can increase to about 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 2, the death rate of the human malignant brain tumor cell line DBTRG is more than 20%, and there is a statistically significant difference (p<0.05) compared to the group 2. Furthermore, in the group 5 treated with the temozolomide and the chimeric antigen receptor expressing cell of Example 2, the death rate of the human malignant brain tumor cell line DBTRG can reach about 60%, and there is a statistically significant difference (p<0.01) compared to the group 4 and a statistically significant difference (p<0.05) compared to the group 3, respectively.
[0051] Please refer to FIGS. 6E and 6F. In the control, the death rate of the human pancreatic cancer cell line AsPC1 is less than 10%. In the group 1 treated with the gemcitabine and the group 2 treated with the parental primary NK cell, the death rate of the human pancreatic cancer cell line AsPC1 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the gemcitabine and the parental primary NK cell, the death rate of the human pancreatic cancer cell line AsPC1 can increase to 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 2, the death rate of the human pancreatic cancer cell line AsPC1 is approximately 20%, and there is a statistically significant difference (p<0.01) compared to the group 2. Furthermore, in the group 5 treated with the gemcitabine and the chimeric antigen receptor expressing cell of Example 2, the death rate of the human pancreatic cancer cell line AsPC1 can reach 50%, and there is a statistically significant difference (p<0.01) compared to the group 4 and a statistically significant difference (p<0.05) compared to the group 3, respectively.
[0052] Please refer to FIGS. 6G and 6H. In the control, the death rate of the human ovarian cancer cell line SKOV3 is less than 10%. In the group 1 treated with the carboplatin and the group 2 treated with the parental primary NK cell, the death rate of the human ovarian cancer cell line SKOV3 is comparable to that of the control. In the group 3 treated with the carboplatin and the parental primary NK cell, the death rate of the human ovarian cancer cell line SKOV3 can increase to more than 20%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 2, the death rate of the human ovarian cancer cell line SKOV3 is approximately 20%, and there is a statistically significant difference (p<0.05) compared to the group 2. Furthermore, in the group 5 treated with the carboplatin and the chimeric antigen receptor expressing cell of Example 2, the death rate of the human ovarian cancer cell line SKOV3 can reach 50%, and there is a statistically significant difference (p 21 0.01) compared to the group 4 and a statistically significant difference (p<0.05) compared to the group 3, respectively.
[0053] Please refer to FIG. 6I, the results indicate that the chimeric antigen receptor expressing cell of Example 2 can be used to treat with the breast cancer cell, the polymorphic glioblastoma cell, the pancreatic cancer cell or the ovarian cancer cell for excellent cell killing. Therefore, the chimeric antigen receptor expressing cell of the present disclosure can be used for inhibiting the proliferation of the tumor cells in the subject in need for the treatment of the tumor. Further, the simultaneous treatment of the chemotherapy drug and the chimeric antigen receptor expressing cell of Example 2 can significantly increase the toxic effect on inducing death of the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3. The results indicate that the method for treating cancer of the present disclosure can effectively inhibit the growth of the tumor cells and treat cancer.
2.3. Example 3
[0054] The chimeric antigen receptor of the present disclosure is transduced into the primary T lymphocyte to obtain the chimeric antigen receptor expressing cell of Example 3 of the present disclosure, and the expression level of the chimeric antigen receptor of the obtained chimeric antigen receptor expressing cell of Example 3 is analyzed by the flow cytometry. Please refer to FIG. 7, which is a graph showing an expression level of a chimeric antigen receptor in a chimeric antigen receptor expressing cell according to Example 3 of the present disclosure. FIG. 7 shows the expression level of the chimeric antigen receptor of the parental primary T lymphocyte without transducing the chimeric antigen receptor of the present disclosure, and the expression level of the chimeric antigen receptor of the chimeric antigen receptor expressing cell of Example 3 on day 3 and day 7 after transduction the chimeric antigen receptor. In FIG. 7, the MFI of the parental primary T lymphocyte only is 9.36%, while the MFI of the chimeric antigen receptor expressing cell of Example 3 on day 3 and day 7 after transduction can reach 34.1% and 88.64%, respectively. The results indicate that the chimeric antigen receptor expressing cell of Example 3 can stably express the chimeric antigen receptor of the present disclosure.
[0055] The effects of the chimeric antigen receptor expressing cell of Example 3 of the present disclosure on inducing the death of the breast cancer cells, the glioblastoma multiforme cells, the pancreatic cancer cells, and the ovarian cancer cells are further demonstrated in following experiments.
[0056] First, the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3 are seeded in a 12-well plate at a density of 1.times.10.sup.5 cells/well. The cells are subsequently incubated for 24 hours. Each type of the tumor cells is divided into six groups. In a control, the tumor cells are untreated. In a group 1, the tumor cells are treated with the chemotherapy drug. In a group 2, the tumor cells are treated with the parental primary T lymphocyte. In a group 3, the tumor cells are treated with the parental T lymphocyte and the chemotherapy drug. In the groups 2 and 3, the number of the parental primary T lymphocyte treated is 1.times.10.sup.5 cells. In a group 4, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 3. In a group 5, the tumor cells are treated with the chimeric antigen receptor expressing cell of Example 3 and the chemotherapy drug. In the groups 4 and 5, the number of the chimeric antigen receptor expressing cell of Example 3 treated is 1.times.10.sup.5 cells. The chemotherapy drug used for treating the human breast cancer cell line MDA-MB-231 is doxorubicin (200 nM), the chemotherapy drug used for treating the human malignant brain tumor cell line DBTRG is temozolomide (80 .mu.g/mL), the chemotherapy drug used for treating the human pancreatic cancer cell line AsPC1 is gemcitabine (20 .mu.M), the chemotherapy drug used for treating the human ovarian cancer cell line SKOV3 is carboplatin (20 .mu.M). The treated cells are stained with Annexin V-FITC and propidium iodide (PI), and the apoptosis and the death of the tumor cells are detected by the flow cytometry. The sum of the percentage of cells stained with Annexin V-FITC and/or PI (that is the percentage of cells in the first quadrant, the second quadrant, and the fourth quadrant of the bivariate flow cytometry scatter plot) are calculated to obtain the cytotoxicity. The results of the cytotoxicity are counted after the three independent trials in each group.
[0057] FIGS. 8A, 8B, 8C, 8D, 8E, 8F, 8G, 8H and 8I show analytical results of tumor cell death induced by the chimeric antigen receptor expressing cells according to Example 3 of the present disclosure. FIG. 8A is a graph showing the analytical results of the death of the human breast cancer cell line MDA-MB-231 induced by the chimeric antigen receptor expressing cell of Example 3, and FIG. 8B is a statistical chart of FIG. 8A after the three independent trials. FIG. 8C is a graph showing the analytical results of the death of the human malignant brain tumor cell line DBTRG induced by the chimeric antigen receptor expressing cell of Example 3, and FIG. 8D is a statistical chart of FIG. 8C after the three independent trials. FIG. 8E is a graph showing the analytical results of the death of the human pancreatic cancer cell line AsPC1 induced by the chimeric antigen receptor expressing cell of Example 3, and FIG. 8F is a statistical chart of FIG. 8E after the three independent trials. FIG. 8G is a graph showing the analytical results of the death of the human ovarian cancer cell line SKOV3 induced by the chimeric antigen receptor expressing cell of Example 3, and FIG. 8H is a statistical chart of FIG. 8G after the three independent trials. FIG. 8I is a statistical chart of FIGS. 8A, 8C, 8E and 8G after the three independent trials, wherein P represents the parental primary T lymphocyte, H represents the chimeric antigen receptor expressing cell of Example 3, D represents doxorubicin, T represents temozolomide, G represents gemcitabine, and C represents carboplatin.
[0058] Please refer to FIGS. 8A and 8B. In the control, the death rate of the human breast cancer cell line MDA-MB-231 is only about 10%. In the group 1 treated with the doxorubicin and the group 2 treated with the parental primary T lymphocyte, the death rate of the human breast cancer cell line MDA-MB-231 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the doxorubicin and the parental primary T lymphocyte, the death rate of the human breast cancer cell line MDA-MB-231 can increase to 20%, and there is a statistically significant difference (p<0.01) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 3, the death rate of the human breast cancer cell line MDA-MB-231 is more than 30%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the doxorubicin and the chimeric antigen receptor expressing cell of Example 3, the death rate of the human breast cancer cell line MDA-MB-231 can reach about 50%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.001) compared to the group 3, respectively.
[0059] Please refer to FIGS. 8C and 8D. In the control, the death rate of the human malignant brain tumor cell line DBTRG is less than 20%. In the group 1 treated with the temozolomide and the group 2 treated with the parental primary T lymphocyte, the death rate of the human malignant brain tumor cell line DBTRG is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the temozolomide and the parental primary T lymphocyte, the death rate of the human malignant brain tumor cell line DBTRG can increase to about 30%, there is no statistically significant difference compared to the control. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 3, the death rate of the human malignant brain tumor cell line DBTRG is more than 50%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the temozolomide and the chimeric antigen receptor expressing cell of Example 3, the death rate of the human malignant brain tumor cell line DBTRG can reach about 80%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.001) compared to the group 3, respectively.
[0060] Please refer to FIGS. 8E and 8F. In the control, the death rate of the human pancreatic cancer cell line AsPC1 is less than 20%. In the group 1 treated with the gemcitabine and the group 2 treated with the parental primary T lymphocyte, the death rate of the human pancreatic cancer cell line AsPC1 is comparable to that of the control. In the group 3 treated with the gemcitabine and the parental primary T lymphocyte, the death rate of the human pancreatic cancer cell line AsPC1 can increase to 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 3, the death rate of the human pancreatic cancer cell line AsPC1 is increased to more than 50%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the gemcitabine and the chimeric antigen receptor expressing cell of Example 3, the death rate of the human pancreatic cancer cell line AsPC1 can reach 60%, and there is a statistically significant difference (p<0.01) compared to the group 4 and a statistically significant difference (p<0.01) compared to the group 3, respectively.
[0061] Please refer to FIGS. 8G and 8H. In the control, the death rate of the human ovarian cancer cell line SKOV3 is less than 10%. In the group 1 treated with the parental primary T lymphocyte, the death rate of the human ovarian cancer cell line SKOV3 is increased, but there is no statistically significant difference compared to the control. In the group 3 treated with the carboplatin and the parental primary T lymphocyte, the death rate of the human ovarian cancer cell line SKOV3 can increase to about 30%, and there is a statistically significant difference (p<0.05) compared to the group 2. In the group 4 treated with the chimeric antigen receptor expressing cell of Example 3, the death rate of the human ovarian cancer cell line SKOV3 is approximately 60%, and there is a statistically significant difference (p<0.001) compared to the group 2. Furthermore, in the group 5 treated with the carboplatin and the chimeric antigen receptor expressing cell of Example 3, the death rate of the human ovarian cancer cell line SKOV3 can reach more than 60%, and there is a statistically significant difference (p<0.05) compared to the group 4 and a statistically significant difference (p<0.001) compared to the group 3, respectively.
[0062] Please refer to FIG. 8I, the results indicate that the chimeric antigen receptor expressing cell of Example 3 can be used to treat with the breast cancer cell, the polymorphic glioblastoma cell, the pancreatic cancer cell or the ovarian cancer cell for excellent cell killing. Therefore, the chimeric antigen receptor expressing cell of the present disclosure can be used for inhibiting the proliferation of the tumor cells in the subject in need for the treatment of the tumor. Further, the simultaneous treatment of the chemotherapy drug and the chimeric antigen receptor expressing cell of Example 3 can significantly increase the toxic effect on inducing death of the human breast cancer cell line MDA-MB-231, the human malignant brain tumor cell line DBTRG, the human pancreatic cancer cell line AsPC1 and the human ovarian cancer cell line SKOV3. The results indicate that the method for treating cancer of the present disclosure can effectively inhibit the growth of the tumor cells and treat cancer.
[0063] FIG. 9 is a schematic view showing the theoretical structure and mechanism of the chimeric antigen receptor in the plasma membrane of the chimeric antigen receptor expressing cell of the present disclosure. The chimeric antigen receptor expressing cell of the present disclosure is a genetically engineered NK cell or T cell which expresses the chimeric antigen receptor of the present disclosure, and the chimeric antigen receptor of the present disclosure is a tumor-targeting receptor complex included the anti-HLA-G antibody (scFv), the HLA-G receptor (KIR) and the costimulatory domain (DAP12). Preferably, the chimeric antigen receptor of the present disclosure can further include the suicide protein iCas9. The chimeric antigen receptor expressing cell of the present disclosure can specifically recognize the HLA-G on the tumor plasma membrane. When the tumor cells are treated with the chemotherapy drug, the HLA-G expression on the plasma membrane of the tumor cell can be positively regulated. Accordingly, the chimeric antigen receptor expressing cell of the present disclosure binds to the HLA-G, which is specifically recognized on the surface of the tumor cell, signal transduction is triggered, and a signal cascade is generated to cause activation and proliferation of the chimeric antigen receptor expressing cell of the present disclosure. In turn, it also triggers exocytosis of lytic granules and killing of the target tumor cells.
[0064] To sum up, the treatment of the chemotherapy drug can increase the HLA-G expression level on the plasma membrane of tumor cells. The chimeric antigen receptor expressed by the chimeric antigen receptor expressing cell of the present disclosure has excellent specific binding ability to the tumor cells, especially specific binding to HLA-G expressed on the plasma membrane of tumor cells, and can specifically target the tumor cells to avoid the off-target effect, thereby effectively killing the tumor cells. Accordingly, the method for treating cancer of the present disclosure can effectively inhibit the proliferation of the tumor cells in the subject in need for the treatment of the tumor and thereby treat cancer.
[0065] Although the present disclosure has been described in considerable detail with reference to certain embodiments thereof, other embodiments are possible. Therefore, the spirit and scope of the appended claims should not be limited to the description of the embodiments contained herein.
[0066] It will be apparent to those skilled in the art that various modifications and variations can be made to the structure of the present disclosure without departing from the scope or spirit of the disclosure. In view of the foregoing, it is intended that the present disclosure cover modifications and variations of this disclosure provided they fall within the scope of the following claims.
Sequence CWU 1
1
171246PRTArtificial Sequenceanti-HLA-G antibody 1Glu Val Gln Leu
Gln Glu Ser Gly Gly Gly Leu Val Gln Pro Lys Gly1 5 10 15Ser Leu Lys
Leu Ser Cys Ala Ala Phe Gly Phe Thr Phe Asn Thr Tyr 20 25 30Ala Met
His Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45Ala
Arg Ile Arg Ser Lys Ser Asn Asn Tyr Ala Thr Tyr Tyr Ala Asp 50 55
60Ser Val Lys Asp Arg Phe Thr Ile Ser Arg Asp Asp Ser Gln Ser Met65
70 75 80Leu Ser Leu Gln Met Asn Asn Leu Lys Thr Glu Asp Thr Ala Ile
Tyr 85 90 95Tyr Cys Val Arg Gly Gly Tyr Trp Ser Phe Asp Val Trp Gly
Ala Gly 100 105 110Thr Thr Val Thr Val Ser Ser Gly Gly Gly Gly Ser
Gly Gly Gly Gly 115 120 125Ser Gly Gly Gly Gly Ser Asp Ile Val Ile
Thr Gln Thr Thr Pro Ser 130 135 140Val Pro Val Thr Pro Gly Glu Ser
Val Ser Ile Ser Cys Arg Ser Ser145 150 155 160Lys Ser Leu Leu His
Ser Asn Gly Asn Thr Tyr Leu Tyr Trp Phe Leu 165 170 175Gln Arg Pro
Gly Gln Ser Pro Gln Leu Leu Ile Ser Arg Met Ser Ser 180 185 190Leu
Ala Ser Gly Val Pro Asp Arg Phe Ser Gly Ser Gly Ser Gly Thr 195 200
205Ala Phe Thr Leu Arg Ile Ser Arg Val Glu Ala Glu Asp Val Gly Val
210 215 220Tyr Tyr Cys Met Gln His Leu Glu Tyr Pro Tyr Thr Phe Gly
Gly Gly225 230 235 240Thr Lys Leu Glu Ile Lys 245275PRTArtificial
SequenceHLA-G receptor 2Ser Pro Thr Glu Pro Ser Ser Lys Thr Gly Asn
Pro Arg His Leu His1 5 10 15Val Leu Ile Gly Thr Ser Val Val Lys Ile
Pro Phe Thr Ile Leu Leu 20 25 30Phe Phe Leu Leu His Arg Trp Cys Ser
Asn Lys Lys Asn Ala Ala Val 35 40 45Met Asp Gln Glu Pro Ala Gly Asn
Arg Thr Val Asn Ser Glu Asp Ser 50 55 60Asp Glu Gln Asp His Gln Glu
Val Ser Tyr Ala65 70 753113PRTArtificial Sequencecostimulatory
domain 3Met Gly Gly Leu Glu Pro Cys Ser Arg Leu Leu Leu Leu Pro Leu
Leu1 5 10 15Leu Ala Val Ser Gly Leu Arg Pro Val Gln Ala Gln Ala Gln
Ser Asp 20 25 30Cys Ser Cys Ser Thr Val Ser Pro Gly Val Leu Ala Gly
Ile Val Met 35 40 45Gly Asp Leu Val Leu Thr Val Leu Ile Ala Leu Ala
Val Tyr Phe Leu 50 55 60Gly Arg Leu Val Pro Arg Gly Arg Gly Ala Ala
Glu Ala Ala Thr Arg65 70 75 80Lys Gln Arg Ile Thr Glu Thr Glu Ser
Pro Tyr Gln Glu Leu Gln Gly 85 90 95Gln Arg Ser Asp Val Tyr Ser Asp
Leu Asn Thr Gln Arg Pro Tyr Tyr 100 105 110Lys4439PRTArtificial
Sequencesuicide protein 4Met Gly Val Gln Val Glu Thr Ile Ser Pro
Gly Asp Gly Arg Thr Phe1 5 10 15Pro Lys Arg Gly Gln Thr Cys Val Val
His Tyr Thr Gly Met Leu Glu 20 25 30Asp Gly Lys Lys Val Asp Ser Ser
Arg Asp Arg Asn Lys Pro Phe Lys 35 40 45Phe Met Leu Gly Lys Gln Glu
Val Ile Arg Gly Trp Glu Glu Gly Val 50 55 60Ala Gln Met Ser Val Gly
Gln Arg Ala Lys Leu Thr Ile Ser Pro Asp65 70 75 80Tyr Ala Tyr Gly
Ala Thr Gly His Pro Gly Ile Ile Pro Pro His Ala 85 90 95Thr Leu Val
Phe Asp Val Glu Leu Leu Lys Leu Glu Ser Gly Gly Gly 100 105 110Ser
Thr Asn Arg Gln Ala Ala Lys Leu Ser Lys Pro Thr Leu Glu Asn 115 120
125Leu Thr Pro Val Val Leu Arg Pro Glu Ile Arg Lys Pro Glu Val Leu
130 135 140Arg Pro Glu Thr Pro Arg Pro Val Asp Ile Gly Ser Gly Gly
Phe Gly145 150 155 160Asp Val Gly Ala Leu Glu Ser Leu Arg Gly Asn
Ala Asp Leu Ala Tyr 165 170 175Ile Leu Ser Met Glu Pro Cys Gly His
Cys Leu Ile Ile Asn Asn Val 180 185 190Asn Phe Cys Arg Glu Ser Gly
Leu Arg Thr Arg Thr Gly Ser Asn Ile 195 200 205Asp Cys Glu Lys Leu
Arg Arg Arg Phe Ser Ser Leu His Phe Met Val 210 215 220Glu Val Lys
Gly Asp Leu Thr Ala Lys Lys Met Val Leu Ala Leu Leu225 230 235
240Glu Leu Ala Gln Gln Asp His Gly Ala Leu Asp Cys Cys Val Val Val
245 250 255Ile Leu Ser His Gly Cys Gln Ala Ser His Leu Gln Phe Pro
Gly Ala 260 265 270Val Tyr Gly Thr Asp Gly Cys Pro Val Ser Val Glu
Lys Ile Val Asn 275 280 285Ile Phe Asn Gly Thr Ser Cys Pro Ser Leu
Gly Gly Lys Pro Lys Leu 290 295 300Phe Phe Ile Gln Ala Cys Gly Gly
Glu Gln Lys Asp His Gly Phe Glu305 310 315 320Val Ala Ser Thr Ser
Pro Glu Asp Glu Ser Pro Gly Ser Asn Pro Glu 325 330 335Pro Asp Ala
Thr Pro Phe Gln Glu Gly Leu Arg Thr Phe Asp Gln Leu 340 345 350Asp
Ala Ile Ser Ser Leu Pro Thr Pro Ser Asp Ile Phe Val Ser Tyr 355 360
365Ser Thr Phe Pro Gly Phe Val Ser Trp Arg Asp Pro Lys Ser Gly Ser
370 375 380Trp Tyr Val Glu Thr Leu Asp Asp Ile Phe Glu Gln Trp Ala
His Ser385 390 395 400Glu Asp Leu Gln Ser Leu Leu Leu Arg Val Ala
Asn Ala Val Ser Val 405 410 415Lys Gly Ile Tyr Lys Gln Met Pro Gly
Cys Phe Asn Phe Leu Arg Lys 420 425 430Lys Leu Phe Phe Lys Thr Ser
43558PRTArtificial SequenceCDRH1 5Gly Phe Thr Phe Asn Thr Tyr Ala1
5610PRTArtificial SequenceCDRH2 6Ile Arg Ser Lys Ser Asn Asn Tyr
Ala Thr1 5 10710PRTArtificial SequenceCDRH3 7Val Arg Gly Gly Tyr
Trp Ser Phe Asp Val1 5 10811PRTArtificial SequenceCDRL2 8Lys Ser
Leu Leu His Ser Asn Gly Asn Thr Tyr1 5 1099PRTArtificial
SequenceCDRL3 9Met Gln His Leu Glu Tyr Pro Tyr Thr1
51022PRTArtificial SequenceP2A 10Gly Ser Gly Ala Thr Asn Phe Ser
Leu Leu Lys Gln Ala Gly Asp Val1 5 10 15Glu Glu Asn Pro Gly Pro
2011738DNAArtificial Sequenceanti-HLA-G antibody coding fragment
11gaggttcagc tgcaagagtc tggcggagga ctggtgcagc ctaagggaag cctgaagctg
60agctgtgccg ccttcggctt caccttcaac acctacgcca tgcactgggt ccgacaggcc
120cctggaaaag gccttgaatg ggtcgcccgg atcagaagca agagcaacaa
ttacgccacc 180tactacgccg acagcgtgaa ggacagattc accatcagcc
gggacgacag ccagagcatg 240ctgagcctgc agatgaacaa cctgaaaacc
gaggacaccg ccatctacta ctgcgtcaga 300ggcggctact ggtccttcga
tgtttgggga gccggcacca ccgtgacagt ttctagcgga 360ggcggtggat
ctggcggcgg aggaagtggt ggcggaggtt ctgatatcgt gatcacccag
420accacaccta gcgtgccagt gacacctggc gagagcgtgt ccatcagctg
cagaagcagc 480aagagcctgc tgcacagcaa cggcaatacc tacctgtact
ggttcctgca gaggcccgga 540cagtctcctc agctgctgat ctccagaatg
agcagcctgg ctagcggcgt gcccgataga 600ttttctggca gcggctctgg
caccgccttc acactgagaa tcagcagagt ggaagccgag 660gacgtgggcg
tgtactactg tatgcagcac ctggaatacc cctacacctt cggcggaggc
720accaagctgg aaatcaag 73812225DNAArtificial SequenceHLA-G receptor
coding fragment 12tcacccactg aaccaagctc caaaaccggt aaccccagac
acctgcatgt tctgattggg 60acctcagtgg tcaaaatccc tttcaccatc ctcctcttct
ttctccttca tcgctggtgc 120tccaacaaaa aaaatgctgc tgtaatggac
caagagcctg cagggaacag aacagtgaac 180agcgaggatt ctgatgaaca
agaccatcag gaggtgtcat acgca 22513339DNAArtificial
Sequencecostimulatory domain coding fragment 13atggggggac
ttgaaccctg cagcaggctc ctgctcctgc ctctcctgct ggctgtaagt 60ggtctccgtc
ctgtccaggc ccaggcccag agcgattgca gttgctctac ggtgagcccg
120ggcgtgctgg cagggatcgt gatgggagac ctggtgctga cagtgctcat
tgccctggcc 180gtgtacttcc tgggccggct ggtccctcgg gggcgagggg
ctgcggaggc agcgacccgg 240aaacagcgta tcactgagac cgagtcgcct
tatcaggagc tccagggtca gaggtcggat 300gtctacagcg acctcaacac
acagaggccg tattacaaa 339141317DNAArtificial Sequencesuicide gene
14atgggagtgc aggtggaaac catctcccca ggagacgggc gcaccttccc caagcgcggc
60cagacctgcg tggtgcacta caccgggatg cttgaagatg gaaagaaagt ggattcctcc
120cgggacagaa acaagccctt taagtttatg ctaggcaagc aggaggtgat
ccgaggctgg 180gaagaagggg ttgcccagat gagtgtgggt cagagagcca
aactgactat atctccagat 240tatgcctatg gtgccactgg gcacccaggc
atcatcccac cacatgccac tctcgtcttc 300gatgtggagc ttctaaaact
ggaatctgga ggaggttcta ctaacaggca agcagcaaag 360ttgtcgaagc
caaccctaga aaaccttacc ccagtggtgc tcagaccaga gattcgcaaa
420ccagaggttc tcagaccgga aacacccaga ccagtggaca ttggttctgg
aggatttggt 480gatgtcggtg ctcttgagag tttgagggga aatgcagatt
tggcttacat cctgagcatg 540gagccctgtg gccactgcct cattatcaac
aatgtgaact tctgccgtga gtccgggctc 600cgcacccgca ctggctccaa
catcgactgt gagaagttgc ggcgtcgctt ctcctcgctg 660catttcatgg
tggaggtgaa gggcgacctg actgccaaga aaatggtgct ggctttgctg
720gagctggcgc agcaggacca cggtgctctg gactgctgcg tggtggtcat
tctctctcac 780ggctgtcagg ccagccacct gcagttccca ggggctgtct
acggcacaga tggatgccct 840gtgtcggtcg agaagattgt gaacatcttc
aatgggacca gctgccccag cctgggaggg 900aagcccaagc tctttttcat
ccaggcctgt ggtggggagc agaaagacca tgggtttgag 960gtggcctcca
cttcccctga agacgagtcc cctggcagta accccgagcc agatgccacc
1020ccgttccagg aaggtttgag gaccttcgac cagctggacg ccatatctag
tttgcccaca 1080cccagtgaca tctttgtgtc ctactctact ttcccaggtt
ttgtttcctg gagggacccc 1140aagagtggct cctggtacgt tgagaccctg
gacgacatct ttgagcagtg ggctcactct 1200gaagacctgc agtccctcct
gcttagggtc gctaatgctg tttcggtgaa agggatttat 1260aaacagatgc
ctggttgctt taatttcctc cggaaaaaac ttttctttaa aacatca
13171566DNAArtificial Sequence2A peptide coding fragment
15ggatctggcg ccaccaactt cagcctgctg aagcaggcag gcgacgtgga agagaaccct
60ggccct 66161335DNAArtificial Sequencepromoter 16gagtaattca
tacaaaagga ctcgcccctg ccttggggaa tcccagggac cgtcgttaaa 60ctcccactaa
cgtagaaccc agagatcgct gcgttcccgc cccctcaccc gcccgctctc
120gtcatcactg aggtggagaa gagcatgcgt gaggctccgg tgcccgtcag
tgggcagagc 180gcacatcgcc cacagtcccc gagaagttgg ggggaggggt
cggcaattga accggtgcct 240agagaaggtg gcgcggggta aactgggaaa
gtgatgtcgt gtactggctc cgcctttttc 300ccgagggtgg gggagaaccg
tatataagtg cagtagtcgc cgtgaacgtt ctttttcgca 360acgggtttgc
cgccagaaca caggtaagtg ccgtgtgtgg ttcccgcggg cctggcctct
420ttacgggtta tggcccttgc gtgccttgaa ttacttccac gcccctggct
gcagtacgtg 480attcttgatc ccgagcttcg ggttggaagt gggtgggaga
gttcgaggcc ttgcgcttaa 540ggagcccctt cgcctcgtgc ttgagttgag
gcctggcttg ggcgctgggg ccgccgcgtg 600cgaatctggt ggcaccttcg
cgcctgtctc gctgctttcg ataagtctct agccatttaa 660aatttttgat
gacctgctgc gacgcttttt ttctggcaag atagtcttgt aaatgcgggc
720caagatctgc acactggtat ttcggttttt ggggccgcgg gcggcgacgg
ggcccgtgcg 780tcccagcgca catgttcggc gaggcggggc ctgcgagcgc
ggccaccgag aatcggacgg 840gggtagtctc aagctggccg gcctgctctg
gtgcctggcc tcgcgccgcc gtgtatcgcc 900ccgccctggg cggcaaggct
ggcccggtcg gcaccagttg cgtgagcgga aagatggccg 960cttcccggcc
ctgctgcagg gagctcaaaa tggaggacgc ggcgctcggg agagcgggcg
1020ggtgagtcac ccacacaaag gaaaagggcc tttccgtcct cagccgtcgc
ttcatgtgac 1080tccacggagt accgggcgcc gtccaggcac ctcgattagt
tctcgagctt ttggagtacg 1140tcgtctttag gttgggggga ggggttttat
gcgatggagt ttccccacac tgagtgggtg 1200gagactgaag ttaggccagc
ttggcacttg atgtaattct ccttggaatt tgcccttttt 1260gagtttggat
cttggttcat tctcaagcct cagacagtgg ttcaaagttt ttttcttcca
1320tttcaggtgt cgtga 13351763DNAArtificial Sequencesignal peptide
17atggccctcc ctgtcaccgc cctgctgctt ccgctggctc ttctgctcca cgccgctcgg
60ccc 63
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

D00013

D00014

D00015

D00016

D00017

D00018

D00019

D00020

D00021

D00022

D00023

D00024

D00025

D00026

D00027

D00028

D00029
D00030

D00031

D00032

D00033

D00034

D00035

D00036

D00037

D00038

D00039

D00040

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.