Zoonotic Disease Rna Vaccines
Ciaramella; Giuseppe ; et al.
U.S. patent application number 16/494988 was filed with the patent office on 2020-01-30 for zoonotic disease rna vaccines. This patent application is currently assigned to ModernaTX, Inc.. The applicant listed for this patent is ModernaTX, Inc.. Invention is credited to Kerry Benenato, Giuseppe Ciaramella, Sunny Himansu, Ellalahewage Sathyajith Kumarasinghe, Vladimir Presnyak.
| Application Number | 20200030432 16/494988 |
| Document ID | / |
| Family ID | 63522661 |
| Filed Date | 2020-01-30 |








View All Diagrams
| United States Patent Application | 20200030432 |
| Kind Code | A1 |
| Ciaramella; Giuseppe ; et al. | January 30, 2020 |
ZOONOTIC DISEASE RNA VACCINES
Abstract
The disclosure relates to Lassa virus, Nipah virus, and betacoronavirus ribonucleic acid vaccines as well as methods of using the vaccines and compositions comprising the vaccines.
| Inventors: | Ciaramella; Giuseppe; (Sudbury, MA) ; Himansu; Sunny; (Winchester, MA) ; Presnyak; Vladimir; (Manchester, NH) ; Benenato; Kerry; (Sudbury, MA) ; Kumarasinghe; Ellalahewage Sathyajith; (Harvard, MA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | ModernaTX, Inc. Cambridge MA |
||||||||||
| Family ID: | 63522661 | ||||||||||
| Appl. No.: | 16/494988 | ||||||||||
| Filed: | March 16, 2018 | ||||||||||
| PCT Filed: | March 16, 2018 | ||||||||||
| PCT NO: | PCT/US2018/022777 | ||||||||||
| 371 Date: | September 17, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 62473174 | Mar 17, 2017 | |||
| 62473202 | Mar 17, 2017 | |||
| 62473219 | Mar 17, 2017 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 31/14 20180101; A61K 39/12 20130101; C12N 2760/18634 20130101; C12N 2770/20034 20130101; C12N 15/86 20130101; A61K 31/7105 20130101; A61K 2039/545 20130101; A61P 37/04 20180101; C12N 2760/14134 20130101; A61K 2039/53 20130101; A61K 31/7115 20130101; A61P 31/16 20180101; A61K 31/7105 20130101; A61K 2300/00 20130101; A61K 31/7115 20130101; A61K 2300/00 20130101 |
| International Class: | A61K 39/12 20060101 A61K039/12; A61K 31/7115 20060101 A61K031/7115; A61P 31/14 20060101 A61P031/14; C12N 15/86 20060101 C12N015/86 |
Claims
1. A zoonotic disease vaccine, comprising a ribonucleic acid (RNA) comprising an open reading frame (ORF) encoding an antigen selected from Lassa virus antigens, Nipah virus antigens, and betacoronavirus antigens, wherein intramuscular (IM) administration of a therapeutically effective amount of the vaccine to a subject induces an immune response in the subject.
2. The zoonotic disease vaccine of claim 1, wherein the ORF encodes a Lassa virus antigen.
3. The zoonotic disease vaccine of claim 2, wherein the Lassa virus antigen comprises a glycoprotein.
4. The zoonotic disease vaccine of claim 3, wherein the Lassa virus antigen comprises a Lassa virus glycoprotein precursor (GPC), a structurally stabilized Lassa virus GPC, an ectodomain of Lassa virus glycoprotein 1 (GP1), or a Lassa virus glycoprotein 2 (GP2).
5. The zoonotic disease vaccine of claim 4, wherein the Lassa virus antigen comprises amino acid residues 59-259 of a Lassa virus GPC.
6. The zoonotic disease vaccine of claim 2, wherein the Lassa virus antigen comprises a nucleocapsid protein (NP).
7. The zoonotic disease vaccine of claim 2, wherein the Lassa virus antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 1-3, but does not include wild-type protein sequence.
8. The zoonotic disease vaccine of claim 2, wherein the Lassa virus antigen has an amino acid sequence of any one of SEQ ID NO: 1-3.
9. The zoonotic disease vaccine of claim 2, wherein the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by any one of SEQ ID NO: 6, 7 or 9, but does not include wild-type protein sequence.
10. The zoonotic disease vaccine of claim 2, wherein the RNA comprising an ORF sequence comprises a nucleic acid sequence of any one of SEQ ID NO: 6, 7 or 9.
11. The zoonotic disease vaccine of claim 1, wherein the ORF encodes a Nipah virus antigen and/or a Hendra virus antigen.
12. The zoonotic disease vaccine of claim 11, wherein the Nipah virus antigen and/or a Hendra virus antigen comprises a hemagglutinin-neuraminidase protein (HN), a hemagglutinin protein (H), or a glycoprotein (G).
13. The zoonotic disease vaccine of claim 12, wherein the Nipah virus antigen and/or a Hendra virus antigen comprises an attachment glycoprotein, optionally a type II membrane protein.
14. The zoonotic disease vaccine of claim 12, wherein the Nipah virus antigen and/or a Hendra virus antigen comprises a fusion (F) glycoprotein.
15. The zoonotic disease vaccine of claim 14, wherein the F glycoprotein comprises a trimeric class I fusogenic envelope glycoprotein containing two heptad repeat (HR) regions and a hydrophobic fusion peptide.
16. The zoonotic disease vaccine of any one of claims 11-15, wherein the Nipah virus antigen and/or a Hendra virus antigen is a Nipah virus antigen.
17. The zoonotic disease vaccine of any one of claims 11-15, wherein the Nipah virus antigen and/or a Hendra virus antigen is a Hendra virus antigen.
18. The zoonotic disease vaccine of claim 11, wherein the Nipah virus antigen and/or a Hendra virus antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 10-13 but does not include wild-type protein sequence.
19. The zoonotic disease vaccine of claim 11, wherein the Nipah virus antigen and/or a Hendra virus antigen has an amino acid sequence of any one of SEQ ID NO: 10-13.
20. The zoonotic disease vaccine of claim 11, wherein the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by SEQ ID NO: 16 or 17, but does not include wild-type protein sequence.
21. The zoonotic disease vaccine of claim 11, wherein the RNA comprising an ORF sequence comprises a nucleic acid sequence of SEQ ID NO: 16 or 17.
22. The zoonotic disease vaccine of claim 1, wherein the ORF encodes a middle east respiratory syndrome coronavirus (MERS-CoV) antigen and/or a severe acute respiratory syndrome-like coronavirus WIV1 (SL-CoV-WIV1) antigen.
23. The zoonotic disease vaccine of claim 22, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen comprises a betacoronavirus structural protein.
24. The zoonotic disease vaccine of claim 23, wherein the betacoronavirus structural protein is spike protein, envelope protein, nucleocapsid protein, or membrane protein.
25. The zoonotic disease vaccine of claim 24, wherein the betacoronavirus structural protein is spike protein.
26. The zoonotic disease vaccine of claim 25, wherein the betacoronavirus structural protein a S1 subunit of the spike protein or a S2 subunit of the spike protein.
27. The zoonotic disease vaccine of any one of claims 22-26, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen is a MERS-CoV antigen.
28. The zoonotic disease vaccine of any one of claims 22-26, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen is a SL-CoV-WIV1 antigen.
29. The zoonotic disease vaccine of claim 22, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified SEQ ID NO: 18 but does not include wild-type protein sequence.
30. The zoonotic disease vaccine of claim 22, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen has an amino acid sequence of SEQ ID NO: 18.
31. The zoonotic disease vaccine of claim 22, wherein the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by SEQ ID NO: 18, but does not include wild-type protein sequence.
32. The zoonotic disease vaccine of claim 22, wherein the RNA comprising an ORF sequence comprises a nucleic acid sequence of SEQ ID NO: 18.
33. The zoonotic disease vaccine of any one of claims 1-32, wherein IM administration of a therapeutically effective amount of the vaccine to a subject induces a neutralizing antibody titer in the subject.
34. The zoonotic disease vaccine of claim 33, wherein the neutralizing antibody titer is at least 100 neutralizing units per milliliter (NU/mL), at least 500 NU/mL, or at least 1000 NU/mL.
35. The zoonotic disease vaccine of claim 33 or 34, wherein the neutralizing antibody titer is sufficient to reduce viral infection of B cells by at least 50% relative to a neutralizing antibody titer of an unvaccinated control subject or relative to a neutralizing antibody titer of a subject vaccinated with a live attenuated viral vaccine, an inactivated viral vaccine, or a protein subunit viral vaccine.
36. The zoonotic disease vaccine of any one of claims 33-35, wherein the neutralizing antibody titer is induced in the subject following fewer than three doses of the vaccine.
37. The zoonotic disease vaccine of any one of claims 1-36, wherein a single dose is of 10 .mu.g-100 .mu.g.
38. The zoonotic disease vaccine of any one of claims 33-37, wherein the neutralizing antibody titer and/or a T cell immune response is sufficient to reduce the rate of asymptomatic viral infection relative to the neutralizing antibody titer of unvaccinated control subjects.
39. The zoonotic disease vaccine of any one of claims 33-38, wherein the neutralizing antibody titer and/or a T cell immune response is sufficient to prevent viral latency the subject.
40. The zoonotic disease vaccine of any one of claims 33-39, wherein the neutralizing antibody titer is sufficient to block fusion of virus with epithelial cells and/or B cells of the subject.
41. The zoonotic disease vaccine of any one of claims 33-40, wherein the neutralizing antibody titer is induced within 20 days following a single 10-100 .mu.g of the vaccine, or within 40 days following a second 10-100 .mu.g dose of the vaccine.
42. The zoonotic disease vaccine of any one of claims 33-40, wherein IM administration of a therapeutically effective amount of the vaccine to a subject induces a T cell immune response in the subject.
43. The zoonotic disease vaccine of claim 42, wherein the T cell immune response comprises a CD4.sup.+ T cell immune response and/or a CD8.sup.+ T cell immune response.
44. The zoonotic disease vaccine of any one of claims 1-43, wherein the antigen is expressed on the surface of cells of the subject.
45. The zoonotic disease vaccine of any one of claims 1-44, wherein the vaccine comprises (a) a ribonucleic acid (RNA) having an open reading frame (ORF) encoding two antigens, or (b) two RNAs, each having an ORF encoding an antigen.
46. The zoonotic disease vaccine of any one of claims 1-45, wherein the vaccine comprises a RNA having an ORF encoding two antigens formulated in a lipid nanoparticle.
47. The zoonotic disease vaccine of any one of claims 1-46, wherein the vaccine comprises two RNAs, each having an ORF encoding an antigen, wherein the two RNAs are formulated in a single lipid nanoparticle or wherein the each RNAs is formulated in a single lipid nanoparticle.
48. The zoonotic disease vaccine of any one of claims 1-47, further comprising at least one additional RNA having an ORF encoding at least one additional antigen.
49. The zoonotic disease vaccine of any one of claims 46-48, wherein the lipid nanoparticle comprises a molar ratio of 20-60% ionizable cationic lipid, 5-25% non-cationic lipid, 25-55% sterol, and 0.5-15% PEG-modified lipid
50. The zoonotic disease vaccine of any one of claims 1-49, wherein the antigen is fused to a signal peptide.
51. The zoonotic disease vaccine of any one of claims 1-50, wherein the antigen is fused to a scaffold moiety.
52. The zoonotic disease vaccine of claim 51, wherein the scaffold moiety is selected from the group consisting of: ferritin, encapsulin, lumazine synthase, hepatitis B surface antigen, and hepatitis B core antigen.
53. The zoonotic disease vaccine of any one of claims 1-52, wherein the RNA comprises messenger RNA (mRNA).
54. The zoonotic disease vaccine of any one of claims 1-53, wherein the RNA further comprises a 5'UTR and/or a 3'UTR.
55. The zoonotic disease vaccine of any one of claims 1-54, wherein the RNA is unmodified.
56. The zoonotic disease vaccine of any one of claims 1-54, wherein the RNA comprise a modified nucleotide.
57. The zoonotic disease vaccine of claim 56, wherein at least 80% of the uracil in the ORF comprise 1-methyl-pseudouridine modification.
58. A method comprising administering to a subject the zoonotic disease vaccine of any one of claims 1-57 in a therapeutically effective amount to induce an immune response in the subject.
59. The method of claim 58, wherein the therapeutically effective amount induces a neutralizing antibody titer and/or a T cell immune response in the subject.
60. The method of claim 59, wherein efficacy of the vaccine is at least 80% relative to unvaccinated control subjects.
61. The method of any one of claims 58-60, wherein detectable levels of the antigen are produced in the serum of the subject at 1-72 hours post administration of the vaccine.
62. The method of any one of claims 59-61, wherein a neutralizing antibody titer of at least 100 NU/ml, at least 500 NU/ml, or at least 1000 NU/ml is produced in the serum of the subject at 1-72 hours post administration of the vaccine.
63. The method of any one of claims 58-62, wherein the therapeutically effective amount is a total dose of 20 .mu.g-200 .mu.g or a total dose of 50 .mu.g-100 .mu.g.
Description
RELATED APPLICATIONS
[0001] This application claims the benefit under 35 U.S.C. .sctn. 119(e) of U.S. provisional application No. 62/473,174, filed Mar. 17, 2017, U.S. provisional application No. 62/473,202, filed Mar. 17, 2017, and U.S. provisional application No. 62/473,219, filed Mar. 17, 2017, each of which is incorporated by reference herein in its entirety.
BACKGROUND
[0002] Zoonotic diseases are infectious diseases that are naturally transmitted from vertebrate animals to humans and vice versa. They are caused by all types of pathogenic agents, including bacteria, parasites, fungi, viruses and prions. In regions densely populated with both people and livestock, zoonotic diseases can spread very quickly. With changes in the environment, human behavior and habitat, increasingly these infections are emerging from wildlife species. Specific examples of zoonotic viruses include Lassa virus, Nipah virus, and betacoronaviruses.
[0003] Lassa Virus.
[0004] Lassa virus (LASV), a segmented negative-sense RNA virus that belongs to the family Arenaviridae, is endemic to West Africa. Transmission typically occurs through contact with infected rodents or virus-contaminated rodent excreta, and person-to-person transmission. The LASV expresses just one protein on its surface, termed GPC, which mediates both attachment to and entry of host cells. GPC is a class I viral fusion protein that forms trimers on the viral surface. Each monomer in the trimer is assembled by distinct GP1 and GP2 subunits that mediate receptor binding and membrane fusion, respectively. Notably, on the viral surface, GP2 is coiled about the base of GP1 in a structure that is only metastable. The complex is prone to rapid disassembly of GP1 from GP2 and rearrangement of the GP2 into a much more stable six-helix bundle. The release of energy achieved by collapsing of the metastable viral-surface conformation to the much more stable six-helix bundle conformation drives fusion of viral and host membranes during infection. Because of its metastability, it is difficult to maintain GPC on its trimeric pre-fusion configuration when expressed recombinantly or even when expressed on some particle surfaces. Antibodies against the resulting separated subunits are not potently neutralizing. As a result, prior vaccine approaches that included natural GPC failed to elicit an effective antibody response, leading vaccine manufacturers to instead focus on induction of cell-mediated immunity as the most likely correlate of protection. Further, in the absence of knowledge about how to create or purify stabilized Lassa virus GPC trimeric, vaccine makers did not have the necessary reagents to evaluate the most ideal antibody responses.
[0005] The structure of the viral surface GP trimer remained unknown for Lassa and all other arenaviruses until this year. After a ten-year effort in engineering LASV GPC, using the GOC to evaluate human antibody responses from survivors, several high-resolution three-dimensional structures of the Lassa virus GPC in complex with these antibodies have been identified.
[0006] Nipah Virus.
[0007] Nipah virus (NiV), of the genus henipahvirus (which includes Hendra virus) is part of the paramyxovirus family (see FIG. 7). Nipah first emerged in Malaysia in 1998, initially in domestic pigs and subsequently causing severe disease in humans, eventually killing over 1000 people. New outbreaks have occurred every year since, with fatality rates ranging from 40-70%. Nipah virus is classified as a BSL-4 agent and as a Category C priority pathogen by the CDC and NIAID. The primary reservoir is Pteropus bats; however, the virus is able to infect and replicate in many mammals (Luby et al 2013; Angeletti et al 2016).
[0008] There are no vaccines currently available against Nipah virus. Considering that the population of people that live in the same regions as pteropus bats is approximately 2 billion, the unmet need for a protective vaccine is high.
[0009] Coronavirus.
[0010] Human Coronaviruses are highly contagious enveloped, positive single stranded RNA viruses of the Coronaviridae family. They are the common etiological agents of mild to moderate upper respiratory tract infections. However, novel coronaviruses such as Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) can result in severe lower respiratory tract infections and high mortality. MERS-CoV was first identified in 2012 within the Arabian Peninsula and since its initial outbreak, Sporadic MERS-CoV infections continue to appear within the Arabian Peninsula. The epidemiology of MERS-CoV infection in humans remains unclear and convoluted with Bats and Dromedary Camels being the major reservoirs for the virus. As of June 2016, the World Health Organization has reported a total of 1,769 MERS-CoV infections with a mortality rate of 36% and an ongoing risk of human to human transmission. The absence of a vaccine for MERS-CoV poses a severe global health threat due to its pandemic potential.
SUMMARY
[0011] Some aspects of the present disclosure provide zoonotic disease vaccines, comprising a ribonucleic acid (RNA) comprising an open reading frame (ORF) encoding an antigen selected from Lassa virus antigens, Nipah virus antigens, and betacoronavirus antigens, wherein intramuscular (IM) administration of a therapeutically effective amount of the vaccine to a subject induces an immune response in the subject.
[0012] In some embodiments, the ORF encodes a Lassa virus antigen.
[0013] In some embodiments, the Lassa virus antigen comprises a glycoprotein.
[0014] In some embodiments, the Lassa virus antigen comprises a Lassa virus glycoprotein precursor (GPC), a structurally stabilized Lassa virus GPC, an ectodomain of Lassa virus glycoprotein 1 (GP1), or a Lassa virus glycoprotein 2 (GP2).
[0015] In some embodiments, the Lassa virus antigen comprises amino acid residues 59-259 of a Lassa virus GPC.
[0016] In some embodiments, the Lassa virus antigen comprises a nucleocapsid protein (NP).
[0017] In some embodiments, the Lassa virus antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 1-3, but does not include wild-type protein sequence.
[0018] In some embodiments, the Lassa virus antigen has an amino acid sequence of any one of SEQ ID NO: 1-3.
[0019] In some embodiments, the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by any one of SEQ ID NO: 6, 7 or 9, but does not include wild-type protein sequence.
[0020] In some embodiments, the RNA comprising an ORF sequence comprises a nucleic acid sequence of any one of SEQ ID NO: 6, 7 or 9.
[0021] In some embodiments, the ORF encodes a Nipah virus antigen and/or a Hendra virus antigen.
[0022] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen comprises a hemagglutinin-neuraminidase protein (HN), a hemagglutinin protein (H), or a glycoprotein (G).
[0023] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen comprises an attachment glycoprotein, optionally a type II membrane protein.
[0024] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen comprises a fusion (F) glycoprotein.
[0025] In some embodiments, the F glycoprotein comprises a trimeric class I fusogenic envelope glycoprotein containing two heptad repeat (HR) regions and a hydrophobic fusion peptide.
[0026] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen is a Nipah virus antigen.
[0027] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen is a Hendra virus antigen.
[0028] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 10-13 but does not include wild-type protein sequence.
[0029] In some embodiments, the Nipah virus antigen and/or a Hendra virus antigen has an amino acid sequence of any one of SEQ ID NO: 10-13.
[0030] In some embodiments, the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by SEQ ID NO: 16 or 17, but does not include wild-type protein sequence.
[0031] In some embodiments, the RNA comprising an ORF sequence comprises a nucleic acid sequence of SEQ ID NO: 16 or 17.
[0032] In some embodiments, the ORF encodes a middle east respiratory syndrome coronavirus (MERS-CoV) antigen and/or a severe acute respiratory syndrome-like coronavirus WIV1 (SL-CoV-WIV1) antigen.
[0033] In some embodiments, the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen comprises a betacoronavirus structural protein.
[0034] In some embodiments, the betacoronavirus structural protein is spike protein, envelope protein, nucleocapsid protein, or membrane protein.
[0035] In some embodiments, rein the betacoronavirus structural protein is spike protein.
[0036] In some embodiments, the betacoronavirus structural protein a S1 subunit of the spike protein or a S2 subunit of the spike protein.
[0037] In some embodiments, the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen is a MERS-CoV antigen.
[0038] In some embodiments, the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen is a SL-CoV-WIV1 antigen.
[0039] In some embodiments, wherein the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen has an amino acid sequence that has at least 90%, at least 95%, or at least 99% identity to an amino acid sequence identified SEQ ID NO: 18 but does not include wild-type protein sequence.
[0040] In some embodiments, the MERS-CoV antigen and/or a SL-CoV-WIV1 antigen has an amino acid sequence of SEQ ID NO: 18.
[0041] In some embodiments, the RNA comprising an ORF sequence has at least 90%, at least 95%, or at least 99% identity to a nucleic acid sequence identified by SEQ ID NO: 18, but does not include wild-type protein sequence.
[0042] In some embodiments, the RNA comprising an ORF sequence comprises a nucleic acid sequence of SEQ ID NO: 18.
[0043] In some embodiments, IM administration of a therapeutically effective amount of the vaccine to a subject induces a neutralizing antibody titer in the subject.
[0044] In some embodiments, the neutralizing antibody titer is at least 100 neutralizing units per milliliter (NU/mL), at least 500 NU/mL, or at least 1000 NU/mL.
[0045] In some embodiments, the neutralizing antibody titer is sufficient to reduce viral infection of B cells by at least 50% relative to a neutralizing antibody titer of an unvaccinated control subject or relative to a neutralizing antibody titer of a subject vaccinated with a live attenuated viral vaccine, an inactivated viral vaccine, or a protein subunit viral vaccine.
[0046] In some embodiments, the neutralizing antibody titer is induced in the subject following fewer than three doses of the vaccine.
[0047] In some embodiments, a single dose is of 10 .mu.g-100 .mu.g.
[0048] In some embodiments, the neutralizing antibody titer and/or a T cell immune response is sufficient to reduce the rate of asymptomatic viral infection relative to the neutralizing antibody titer of unvaccinated control subjects.
[0049] In some embodiments, the neutralizing antibody titer and/or a T cell immune response is sufficient to prevent viral latency the subject.
[0050] In some embodiments, the neutralizing antibody titer is sufficient to block fusion of virus with epithelial cells and/or B cells of the subject.
[0051] In some embodiments, the neutralizing antibody titer is induced within 20 days following a single 10-100 .mu.g of the vaccine, or within 40 days following a second 10-100 .mu.g dose of the vaccine.
[0052] In some embodiments, IM administration of a therapeutically effective amount of the vaccine to a subject induces a T cell immune response in the subject.
[0053] In some embodiments, the T cell immune response comprises a CD4.sup.+ T cell immune response and/or a CD8.sup.+ T cell immune response.
[0054] In some embodiments, the antigen is expressed on the surface of cells of the subject.
[0055] In some embodiments, the vaccine comprises
(a) a ribonucleic acid (RNA) having an open reading frame (ORF) encoding two antigens, or (b) two RNAs, each having an ORF encoding an antigen.
[0056] In some embodiments, the vaccine comprises a RNA having an ORF encoding two antigens formulated in a lipid nanoparticle.
[0057] In some embodiments, the vaccine comprises two RNAs, each having an ORF encoding an antigen, wherein the two RNAs are formulated in a single lipid nanoparticle or wherein the each RNAs is formulated in a single lipid nanoparticle.
[0058] In some embodiments, the vaccine further comprises at least one additional RNA having an ORF encoding at least one additional antigen.
[0059] In some embodiments, the lipid nanoparticle comprises a molar ratio of 20-60% ionizable cationic lipid, 5-25% non-cationic lipid, 25-55% sterol, and 0.5-15% PEG-modified lipid In some embodiments, the antigen is fused to a signal peptide.
[0060] In some embodiments, the antigen is fused to a scaffold moiety.
[0061] In some embodiments, the scaffold moiety is selected from the group consisting of: ferritin, encapsulin, lumazine synthase, hepatitis B surface antigen, and hepatitis B core antigen.
[0062] In some embodiments, the RNA comprises messenger RNA (mRNA).
[0063] In some embodiments, the RNA further comprises a 5'UTR and/or a 3'UTR.
[0064] In some embodiments, the RNA is unmodified.
[0065] In some embodiments, the RNA comprise a modified nucleotide.
[0066] In some embodiments, at least 80% of the uracil in the ORF comprise 1-methyl-pseudouridine modification.
[0067] Some aspects of the present disclosure provide methods comprising administering to a subject the zoonotic disease vaccine in a therapeutically effective amount to induce an immune response in the subject.
[0068] In some embodiments, the therapeutically effective amount induces a neutralizing antibody titer and/or a T cell immune response in the subject.
[0069] In some embodiments, the vaccine is at least 80% relative to unvaccinated control subjects.
[0070] In some embodiments, detectable levels of the antigen are produced in the serum of the subject at 1-72 hours post administration of the vaccine.
[0071] In some embodiments, a neutralizing antibody titer of at least 100 NU/ml, at least 500 NU/ml, or at least 1000 NU/ml is produced in the serum of the subject at 1-72 hours post administration of the vaccine.
[0072] In some embodiments, the therapeutically effective amount is a total dose of 20 .mu.g-200 .mu.g or a total dose of 50 .mu.g-100 .mu.g.
[0073] Each of the limitations of the invention can encompass various embodiments of the invention. It is, therefore, anticipated that each of the limitations of the invention involving any one element or combinations of elements can be included in each aspect of the invention. This invention is not limited in its application to the details of construction and the arrangement of components set forth in the following description or illustrated in the drawings. The invention is capable of other embodiments and of being practiced or of being carried out in various ways.
BRIEF DESCRIPTION OF THE DRAWINGS
[0074] The accompanying drawings are not intended to be drawn to scale. In the drawings, each identical or nearly identical component that is illustrated in various figures is represented by a like numeral. For purposes of clarity, not every component may be labeled in every drawing. In the drawings:
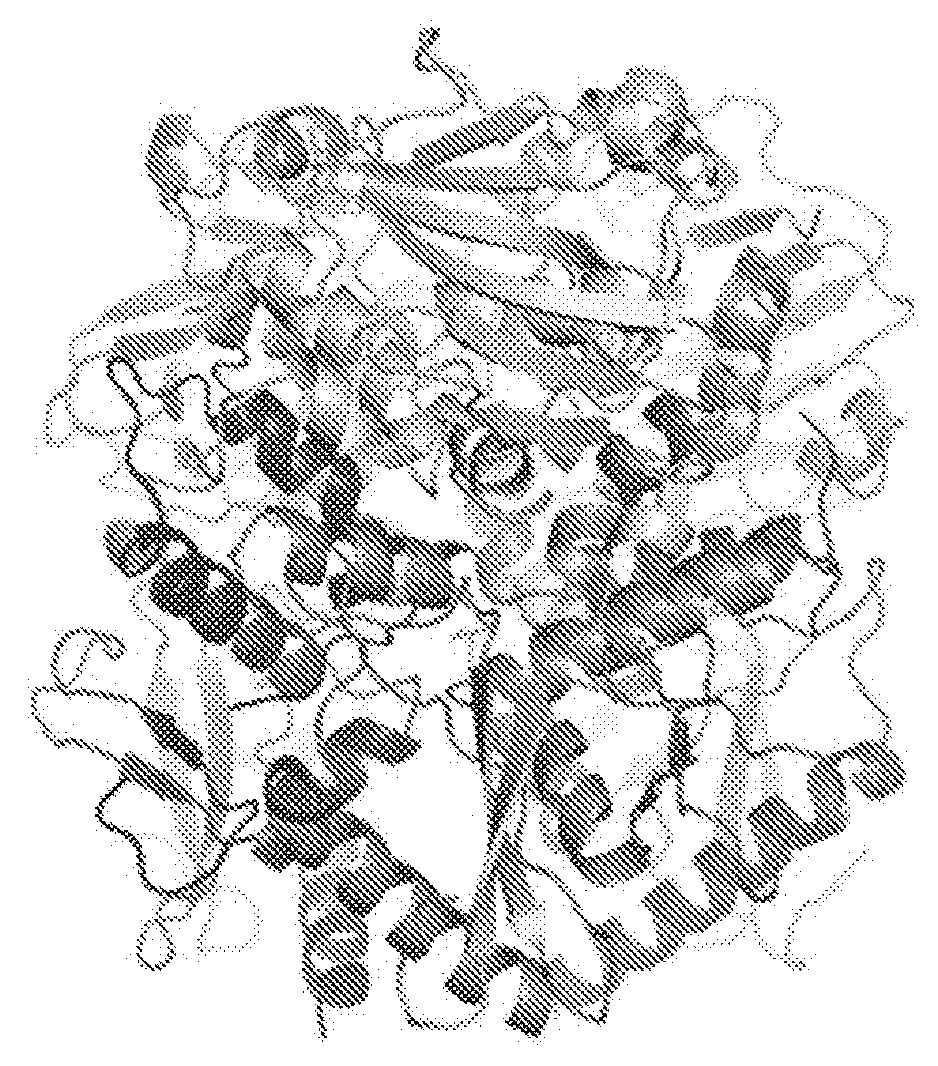
[0075] FIG. 1 shows the crystal structure of Lassa virus GPC in its trimeric, pre-fusion viral surface conformation. The three monomers are colored purple, orange and green, respectively, with the GP1 subunits in a light shade and GP2 subunits in a darker shade of each color. These structures illustrate the assembly surfaces of the trimer and quaternary epitopes at the base and apex that are formed only when the subunits assemble together in the trimer.
[0076] FIG. 2 shows anti-Ebola virus glycoprotein mouse IgG titers on 7 and 19 days post dose 2.
[0077] FIG. 3 shows the Ebola lethal challenge model study design. AG1 represents the designated Ebola GP mRNA vaccine, and AG2 represents the mRNA vaccine expressing wild type GP.
[0078] FIG. 4 shows mortality analysis of Guinea pigs in the Ebola challenge model.
[0079] FIG. 5 shows the average group weight loss post Ebola challenge.
[0080] FIG. 6 shows morbidity scores for individual animals.
[0081] FIG. 7 shows the paramyxovirus family.
[0082] FIG. 8 shows experimental design for the cotton rat challenge study.
[0083] FIG. 9 shows viral titers (top panel) and serum PIV3 neutralizing antibody titers (bottom panel) in cotton rats.
[0084] FIG. 10 shows viral titers (top panel) and serum PIV3 neutralizing antibody titers (bottom panel) in African green monkeys.
[0085] FIG. 11 shows VN titers in Balb/C mice after 2-dose immunization with MERS-CoV spike protein mRNA vaccine.
[0086] FIG. 12 shows VN titers against MERS-CoV after prime only (left), prime-boost (middle) or placebo (right) treatment. Individual values are shown as well as the geometric mean titer.
[0087] FIG. 13 shows MERS-CoV PCR and titration levels in nose swabs after challenge in prime only (left), prime-boost (middle) or placebo (right) treated animals. Panels A-C: Individual PCR values are shown as well as the lower limit of detection (1.2 log 10 CDU/mL). Samples below the lower limit of detection are plotted as 1.1 log 10 CDU/mL. Panels D-F: Individual viral titration values are shown as well as the lower limit of detection (0.8 log 10 TCID50/mL). Samples below the lower limit of detection are plotted as 0.7 log 10 TCID50/mL.
[0088] FIG. 14 shows MERS-CoV PCR and titration levels in throat swabs after challenge in prime only (left), prime-boost (middle) or placebo (right) treated animals. Panels A-C: Individual PCR values are shown as well as the lower limit of detection (1.2 log 10 CDU/mL). Samples below the lower limit of detection are plotted as 1.1 log 10 CDU/mL. Panels D-F: Individual titration values are shown as well as the lower limit of detection (0.8 log 10 TCID50/mL). Samples below the lower limit of detection are plotted as 0.7 log 10 TCID50/mL.
[0089] FIG. 15 shows MERS-CoV PCR (left panel) and titration (right panel) results in pooled lung samples after challenge in prime only (1a), prime-boost (1b) or placebo (2) treated groups. Individual values are shown as well as the (range of the) lower limit of detection of PCR (2.8 log 10 CDU/g) and virus titration (1.2-1.4 log 10 TCID50/g).
DETAILED DESCRIPTION
Lassa Virus Vaccines
[0090] LASV (LASV) is an arenavirus (negative ssRNA) that represents a significant unmet global health care need. LASV expresses just one protein on its surface, termed GPC, which mediates both attachment to and entry of host cells. GPC is a class I viral fusion protein that forms trimers on the viral surface. Each monomer in the trimer is assembled by distinct GP1 and GP2 subunits that mediate receptor binding and membrane fusion, respectively. Notably, on the viral surface, GP2 is coiled about the base of GP1 in structure that is only metastable. The complex is prone to rapid disassembly of GP1 from GP2 and rearrangement of the GP2 into a much more stable six-helix bundle. The release of energy achieved by collapsing of the metastable viral-surface conformation to the much more stable six-helix bundle conformation drives fusion of viral and host membranes during infection. However, because of its metastability, it is difficult to maintain GPC on its trimeric pre-fusion configuration when expressed recombinantly or even when expressed on some particle surfaces. Antibodies against the resulting separated subunits are not potently neutralizing. As a result, prior vaccine approaches that included natural GPC failed to elicit an effective antibody response, leading vaccine manufacturers to instead focus on induction of cell-mediated immunity as the most likely correlate of protection. Further, in the absence of knowledge about how to create or purify stabilized LASV GPC trimeric, vaccine makers did not have the necessary reagents to evaluate the most ideal antibody responses.
[0091] The mRNA vaccines of the disclosure have been designed to express viral membrane bound proteins (B cell antigens) as well as intracellular proteins (T cell antigens). Arenaviruses including LASV are pleomorphic enveloped viruses with membrane GP glycoprotein as the major surface antigen. In some respects the Lassa glycoprotein is a potent vaccine antigen with structural similarities to Ebola glycoproteins. The disclosure in some aspects includes, a mRNA vaccine expressing full length-membrane bound Lassa glycoprotein precursor GPC. The GPC precursor mRNA once translated will be matured through a natural process by the cellular proteases into the fully matured GP glycoprotein. The membrane anchored version of this protein will form trimers on cell surfaces and recognized by the immune system to generate humoral and cellular responses.
[0092] The most effective anti LASV antibodies are directed against a quaternary epitopes on GPC (those only formed when both GP1 and GP2 are intertwined, and three GP1-GP2 monomers form the proper trimer). Engineering and stabilization of GPC to firmly remain in this assembly allows recognition by the most potent human antibodies, and that the potent antibodies themselves are sufficient to provide post-exposure protection, even late in the disease course. The properly stabilized GPC trimer displays key quaternary epitopes that lead to broadly reactive, potent, and protective antibodies. The mRNA vaccines of the disclosure in some embodiments are designed to produce these unique stabilized GPCs in order to provoke production of the type and quality of neutralizing antibody necessary for eliminating the virus in the host.
Nipah Virus Vaccines
[0093] Nipah virus (NiV) and Hendra virus (HeV) are part of the paramyxovirus family. Virus-cell fusion by the paramyxoviruses is mediated by both an attachment protein (which can vary by genus) and a fusion (F) protein, which is well conserved throughout the family. There are currently no commercially available vaccines available against Nipah virus.
[0094] Parainfluenza virus 3 (PIV3, genus respirovirus), is closely related to Nipah virus. A mRNA vaccine against PIV3 encoding the PIV3 F protein, which exists functionally as a membrane bound trimer of two disulfide-linked subunits has been developed. Applicants have demonstrated that this PIV3 mRNA vaccine drives the efficient expression of this protein in its biologically relevant conformation, thus generating a robust neutralizing response.
[0095] Paramyxoviruses such as HeV and NiV possess two major membrane-anchored glycoproteins in the envelope of the viral particle. One glycoprotein is required for virion attachment to receptors on host cells and is designated as either hemagglutinin-neuraminidase protein (HN) or hemagglutinin protein (H), and the other is glycoprotein (G), which has neither hemagglutination nor neuraminidase activities. The attachment glycoproteins are type II membrane proteins, where the molecule's amino (N) terminus is oriented toward the cytoplasm and the protein's carboxy (C) terminus is extracellular. The other major glycoprotein is the fusion (F) glycoprotein, which is a trimeric class I fusogenic envelope glycoprotein containing two heptad repeat (HR) regions and a hydrophobic fusion peptide. HeV and NiV infect cells though a pH-independent membrane fusion process into receptive host cells through the concerted action of their attachment G glycoprotein and F glycoprotein following receptor binding. The primary function of the HeV and NiV attachment G glycoprotein is to engage appropriate receptors on the surfaces of host cells, which for the majority of well-characterized paramyxoviruses are sialic acid moieties. The HeV and NiV G glycoproteins utilize the host cell protein receptors ephrin B2 and/or ephrin B3 and antibodies have been developed which block viral attachment by the G glycoprotein.
[0096] According to the disclosure, mRNA vaccines based on Nipah and Hendra F proteins have been developed. Additionally, soluble Nipah glycoprotein (G) vaccines and Hendra glycoprotein (G) vaccines are encompassed by the disclosure. In some aspects the vaccines may include F and G alone and/or in combination at different ratios.
[0097] The fusion glycoprotein (F) of Nipah virus mediates membrane fusion and is required for viral entry. Nipah F, like RSV F, is a class I fusion protein and they have similar structures and functions. The vaccines of the disclosure include stabilizing mutations to maintain the prefusion structure of Nipah F. Ideally stabilized mutants will maintain biophysical properties including structure and antigenicity.
Betacoronavirus Vaccines
[0098] Embodiments of the present disclosure provide RNA (e.g., mRNA) vaccines that include polynucleotide encoding a Middle East respiratory syndrome coronavirus (MERS-CoV) antigen and/or Bat SARS-like coronavirus WIV1, (SL-CoV-WIV1).
[0099] MERS-CoV is a positive-sense, single-stranded RNA virus of the genus Betacoronavirus. The genomes are phylogenetically classified into two clades, clade A and clade B. It has a strong tropism for non-ciliated bronchial epithelial cells, evades the innate immune response and antagonizes interferon (IFN) production in infected cells. Dipeptyl peptidase 4 (DDP4, also known as CD26) has been identified as a functional cellular receptor for MERS-CoV. Its enzymatic activity is not required for infection, although its amino acid sequence is highly conserved across species and is expressed in the human bronchial epithelium and kidneys. Most infected individuals develop severe acute respiratory illnesses, including fever, cough, and shortness of breath, and the virus can be fatal. The disease may be transmitted among humans, generally among those in close contact.
[0100] Bat SARS-like coronavirus WIV1, (SL-CoV-WIV1) or SARS-like coronavirus WIV1 (WIV1), was isolated recently from Chinese rufous horseshoe bats. It is a single-stranded, enveloped, positive-sense RNA betacoronavirus. It has been demonstrated by phylogenetic analysis direct transmission of SARS from bats to humans may occur without intermediary Chinese civets.
[0101] The genome of MERS-CoV encodes at least four unique accessory proteins, such as 3, 4a, 4b and 5, two replicase proteins (open reading frame 1a and 1b), and four major structural proteins, including spike (S), envelope (E), nucleocapsid (N), and membrane (M) proteins (Almazan F et al. MBio 2013; 4(5):e00650-13). The accessory proteins play nonessential roles in MERS-CoV replication, but they are likely structural proteins or interferon antagonists, modulating in vivo replication efficiency and/or pathogenesis, as in the case of SARS-CoV (Almazan F et al. MBio 2013; 4(5):e00650-13; Totura A L et al. Curr Opin Virol 2012; 2(3):264-75; Scobey T et al. Proc Natl Acad Sci USA 2013; 110(40):16157-62). The other proteins of MERS-CoV maintain different functions in virus replication. The E protein, for example, involves in virulence, and deleting the E-coding gene results in replication-competent and propagation-defective viruses or attenuated viruses (Almazan F et al. MBio 2013; 4(5):e00650-13). The S protein is particularly essential in mediating virus binding to cells expressing receptor dipeptidyl peptidase-4 (DPP4) through receptor-binding domain (RBD) in the S1 subunit, whereas the S2 subunit subsequently mediates virus entry via fusion of the virus and target cell membranes (Li F. J Virol 2015; 89(4):1954-64; Raj V S et al. Nature 2013; 495(7440):251-4).
[0102] In some aspects of the disclosure, the vaccine encodes the major antigenic component for MERS-CoV or SL-CoV-WIV1, the spike (S) glycoprotein. Spike protein is a typical type I viral fusion protein that exists as trimer on the viral surface with each monomer consisting of a Head (S1) and stem (S2) domain similar to influenza Hemagglutinin (HA). The S1 domain of the spike glycoprotein includes the receptor binding domain (RBD) that engages with the dipeptidyl peptidase-4 (DPP4) receptor and mediates viral fusion into the host cell, an N-terminal domain that may make initial contact with target cells, and 2 subdomains, all of which are susceptible to neutralizing antibodies. S2 domain consists of a six helix bundle fusion core involved in membrane fusion with the host endosomal membrane and is also a target for neutralization.
[0103] Spike protein for betacoronaviruses has been shown to be an effective target for vaccines as antibodies against this protein are generated during natural infection and are protective in a passive transfer animal model (REF). It has been demonstrated that mRNA vaccine for MERS-CoV elicits high levels of neutralizing antibodies and significantly reduces viral load in infected animals (see Examples).
[0104] The data demonstrate that expressing a stable trimeric Spike protein in its prefusion conformation (pre-S) (pre-S trimer) increases the magnitude and breadth of neutralizing activity against diverse strains of MERS CoV.
[0105] The zoonotic disease RNA vaccines described herein are superior to current vaccines in several ways. For example, the lipid nanoparticle (LNP) delivery system used herein increases the efficacy of RNA vaccines in comparison to other formulations, including a protamine-based approach described in the literature. The use of this LNP delivery system enables the effective delivery of chemically-modified RNA vaccines or unmodified RNA vaccines, without requiring additional adjuvant to produce a therapeutic result (e.g., production neutralizing antibody titer and/or a T cell response). In some embodiments, the zoonotic disease RNA vaccines disclosed herein are superior to conventional vaccines by a factor of at least 10 fold, 20, fold, 40, fold, 50 fold, 100 fold, 500 fold, or 1,000 fold when administered intramuscularly (IM) or intradermally (ID). These results can be achieved even when significantly lower doses of the RNA (e.g., mRNA) are administered in comparison with RNA doses used in other classes of lipid based formulations.
[0106] The LNP used in the studies described herein has been used previously to deliver siRNA in various animal models as well as in humans. In view of the observations made in association with the siRNA delivery of LNP formulations, the fact that LNP is useful in vaccines is quite surprising, particularly when immunity to an antigen has been hard to generate. It has been observed that therapeutic delivery of siRNA formulated in LNP causes an undesirable inflammatory response associated with a transient IgM response, typically leading to a reduction in antigen production and a compromised immune response. In contrast to the findings observed with siRNA, the LNP-mRNA formulations of the present disclosure are demonstrated herein to generate enhanced IgG levels, sufficient for prophylactic and therapeutic methods rather than transient IgM responses.
Exemplary Zoonotic Disease Antigens
[0107] Antigens are proteins capable of inducing an immune response (e.g., causing an immune system to produce antibodies against the antigens). Herein, use of the term antigen encompasses immunogenic proteins and immunogenic fragments (an immunogenic fragment that induces (or is capable of inducing) an immune response to a zoonotic disease antigen), unless otherwise stated. It should be understood that the term "protein` encompasses peptides and the term "antigen" encompasses antigenic fragments.
[0108] A number of different antigens are associated with zoonotic diseases such as Lassa virus, Nipah virus, and betacoronavirus. Zoonotic disease vaccines, as provided herein, comprise at least one (one or more) ribonucleic acid (RNA, e.g., mRNA) having an open reading frame encoding at least one Lassa virus, Nipah virus, or betacoronavirus antigen. Non-limiting examples of zoonotic disease antigens are provided below.
[0109] Exemplary zoonotic disease antigens are provided in the Sequence Listing elsewhere herein. For example, the antigens may be encoded by (thus the RNA may comprise or consist of) any one of sequences set forth in SEQ ID NO: 6, 7, 9, 16, 17, or 20. In some embodiments, the antigens comprise a sequence set forth in SEQ ID NO: 1, 2, 3, 10, 11, 12, 13, or 18. In some embodiments, the aforementioned sequences may further comprise a 5' cap (e.g., 7mG(5')ppp(5')NlmpNp), a polyA tail, or a 5' cap and a polyA tail.
[0110] It should be understood that the zoonotic disease vaccines of the present disclosure may comprise any of the RNA open reading frames (ORFs), or encode any of the protein ORFs, described herein, with or without a signal sequence. It should also be understood that the zoonotic disease vaccines of the present disclosure may include any 5' untranslated region (UTR) and/or any 3' UTR. Any UTR sequence (e.g., of the prior art) may be used or exchanged for any of the UTR sequences described herein. UTRs may also be omitted from the vaccine constructs provided herein.
Nucleic Acids
[0111] The zoonotic disease vaccines of the present disclosure comprise at least one (one or more) ribonucleic acid (RNA) having an open reading frame encoding at least one zoonotic disease antigen. In some embodiments, the zoonotic disease antigen is a Lassa virus antigen. In some embodiments, the zoonotic disease antigen is a Nipah virus antigen. In some embodiments, the zoonotic disease antigen is a betcoronavirus antigen. In some embodiments, the RNA is a messenger RNA (mRNA) having an open reading frame encoding at least one zoonotic disease antigen. In some embodiments, the RNA (e.g., mRNA) further comprises a (at least one) 5'UTR, 3'UTR, a polyA tail and/or a 5' cap.
[0112] Nucleic acids comprise a polymer of nucleotides (nucleotide monomers), also referred to as polynucleotides. Nucleic acids may be or may include, for example, deoxyribonucleic acids (DNAs), ribonucleic acids (RNAs), threose nucleic acids (TNAs), glycol nucleic acids (GNAs), peptide nucleic acids (PNAs), locked nucleic acids (LNAs, including LNA having a .beta.-D-ribo configuration, .alpha.-LNA having an .alpha.-L-ribo configuration (a diastereomer of LNA), 2'-amino-LNA having a 2'-amino functionalization, and 2'-amino-.alpha.-LNA having a 2'-amino functionalization), ethylene nucleic acids (ENA), cyclohexenyl nucleic acids (CeNA) and/or chimeras and/or combinations thereof.
[0113] Messenger RNA (mRNA) is any ribonucleic acid that encodes a (at least one) protein (a naturally-occurring, non-naturally-occurring, or modified polymer of amino acids) and can be translated to produce the encoded protein in vitro, in vivo, in situ or ex vivo. The skilled artisan will appreciate that, except where otherwise noted, nucleic acid sequences set forth in the instant application may recite "T"s in a representative DNA sequence but where the sequence represents RNA (e.g., mRNA), the "T"s would be substituted for "U"s. Thus, any of the DNAs disclosed and identified by a particular sequence identification number herein also disclose the corresponding RNA (e.g., mRNA) sequence complementary to the DNA, where each "T" of the DNA sequence is substituted with "U."
[0114] It should be understood that the mRNA polynucleotides of the vaccines as provided herein are synthetic molecules, i.e., they are not naturally-occurring molecules. That is, the mRNA polynucleotides of the present disclosure are isolated mRNA polynucleotides. As is known in the art, "isolated polynucleotides" refer to polynucleotides that are substantially physically separated from other cellular material (e.g., separated from cells and/or systems that produce the polynucleotides) or from other material that hinders their use in the vaccines of the present disclosure. Isolated polynucleotides are substantially pure in that they have been substantially separated from the substances with which they may be associated in living or viral systems. Thus, mRNA polynucleotide vaccines are not associated with living or viral systems, such as cells or viruses. The mRNA polynucleotide vaccines do not include viral components (e.g., viral capsids, viral enzymes, or other viral proteins, for example, those needed for viral-based replication), and the mRNA polynucleotide vaccines are not packaged within, encapsulated within, linked to, or otherwise associated with a virus or viral particle. In some embodiments, the mRNA vaccines comprise a lipid nanoparticle that consists of, or consists essentially of, one or more mRNA polynucleotides (e.g., mRNA polynucleotides encoding one or more zoonotic viral antigen(s)).
[0115] An open reading frame (ORF) is a continuous stretch of DNA or RNA beginning with a start codon (e.g., methionine (ATG or AUG)) and ending with a stop codon (e.g., TAA, TAG or TGA, or UAA, UAG or UGA). An ORF typically encodes a protein. It will be understood that the sequences disclosed herein may further comprise additional elements, e.g., 5' and 3' UTRs, but that those elements, unlike the ORF, need not necessarily be present in a vaccine of the present disclosure.
Variants
[0116] In some embodiments, an RNA of the present disclosure encodes a zoonotic disease antigen variant. Antigen or other polypeptide variants refers to molecules that differ in their amino acid sequence from a wild-type, native or reference sequence. The antigen/polypeptide variants may possess substitutions, deletions, and/or insertions at certain positions within the amino acid sequence, as compared to a native or reference sequence. Ordinarily, variants possess at least 50% identity to a wild-type, native or reference sequence. In some embodiments, variants share at least 80%, or at least 90% identity with a wild-type, native or reference sequence.
[0117] Variant antigens/polypeptides encoded by nucleic acids of the disclosure may contain amino acid changes that confer any of a number of desirable properties, e.g., that enhance their immunogenicity, enhance their expression, and/or improve their stability or PK/PD properties in a subject. Variant antigens/polypeptides can be made using routine mutagenesis techniques and assayed as appropriate to determine whether they possess the desired property. Assays to determine expression levels and immunogenicity are well known in the art and exemplary such assays are set forth in the Examples section. Similarly, PK/PD properties of a protein variant can be measured using art recognized techniques, e.g., by determining expression of antigens in a vaccinated subject over time and/or by looking at the durability of the induced immune response. The stability of protein(s) encoded by a variant nucleic acid may be measured by assaying thermal stability or stability upon urea denaturation or may be measured using in silico prediction. Methods for such experiments and in silico determinations are known in the art.
[0118] In some embodiments, a zoonotic disease vaccine comprises an mRNA ORF having a nucleotide sequence identified by any one of the sequences provided herein (see e.g., Sequence Listing), or having a nucleotide sequence at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, or at least 99% identical to a nucleotide sequence identified by any one of the sequence provided herein.
[0119] The term "identity" refers to a relationship between the sequences of two or more polypeptides (e.g. antigens) or polynucleotides (nucleic acids), as determined by comparing the sequences. Identity also refers to the degree of sequence relatedness between or among sequences as determined by the number of matches between strings of two or more amino acid residues or nucleic acid residues. Identity measures the percent of identical matches between the smaller of two or more sequences with gap alignments (if any) addressed by a particular mathematical model or computer program (e.g., "algorithms"). Identity of related antigens or nucleic acids can be readily calculated by known methods. "Percent (%) identity" as it applies to polypeptide or polynucleotide sequences is defined as the percentage of residues (amino acid residues or nucleic acid residues) in the candidate amino acid or nucleic acid sequence that are identical with the residues in the amino acid sequence or nucleic acid sequence of a second sequence after aligning the sequences and introducing gaps, if necessary, to achieve the maximum percent identity. Methods and computer programs for the alignment are well known in the art. It is understood that identity depends on a calculation of percent identity but may differ in value due to gaps and penalties introduced in the calculation. Generally, variants of a particular polynucleotide or polypeptide (e.g., antigen) have at least 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% but less than 100% sequence identity to that particular reference polynucleotide or polypeptide as determined by sequence alignment programs and parameters described herein and known to those skilled in the art. Such tools for alignment include those of the BLAST suite (Stephen F. Altschul, et al (1997), "Gapped BLAST and PSI-BLAST: a new generation of protein database search programs", Nucleic Acids Res. 25:3389-3402). Another popular local alignment technique is based on the Smith-Waterman algorithm (Smith, T. F. & Waterman, M. S. (1981) "Identification of common molecular subsequences." J. Mol. Biol. 147:195-197). A general global alignment technique based on dynamic programming is the Needleman-Wunsch algorithm (Needleman, S. B. & Wunsch, C. D. (1970) "A general method applicable to the search for similarities in the amino acid sequences of two proteins." J. Mol. Biol. 48:443-453). More recently a Fast Optimal Global Sequence Alignment Algorithm (FOGSAA) has been developed that purportedly produces global alignment of nucleotide and protein sequences faster than other optimal global alignment methods, including the Needleman-Wunsch algorithm.
[0120] As such, polynucleotides encoding peptides or polypeptides containing substitutions, insertions and/or additions, deletions and covalent modifications with respect to reference sequences, in particular the polypeptide (e.g., antigen) sequences disclosed herein, are included within the scope of this disclosure. For example, sequence tags or amino acids, such as one or more lysines, can be added to peptide sequences (e.g., at the N-terminal or C-terminal ends). Sequence tags can be used for peptide detection, purification or localization. Lysines can be used to increase peptide solubility or to allow for biotinylation. Alternatively, amino acid residues located at the carboxy and amino terminal regions of the amino acid sequence of a peptide or protein may optionally be deleted providing for truncated sequences. Certain amino acids (e.g., C-terminal or N-terminal residues) may alternatively be deleted depending on the use of the sequence, as for example, expression of the sequence as part of a larger sequence which is soluble, or linked to a solid support. In some embodiments, sequences for (or encoding) signal sequences, termination sequences, transmembrane domains, linkers, multimerization domains (such as, e.g., foldon regions) and the like may be substituted with alternative sequences that achieve the same or a similar function. In some embodiments, cavities in the core of proteins can be filled to improve stability, e.g., by introducing larger amino acids. In other embodiments, buried hydrogen bond networks may be replaced with hydrophobic resides to improve stability. In yet other embodiments, glycosylation sites may be removed and replaced with appropriate residues. Such sequences are readily identifiable to one of skill in the art. It should also be understood that some of the sequences provided herein contain sequence tags or terminal peptide sequences (e.g., at the N-terminal or C-terminal ends) that may be deleted, for example, prior to use in the preparation of an RNA (e.g., mRNA) vaccine.
[0121] As recognized by those skilled in the art, protein fragments, functional protein domains, and homologous proteins are also considered to be within the scope of zoonotic disease antigens of interest. For example, provided herein is any protein fragment (meaning a polypeptide sequence at least one amino acid residue shorter than a reference antigen sequence but otherwise identical) of a reference protein, provided that the fragment is immunogenic and confers a protective immune response to the zoonotic disease pathogen. In addition to variants that are identical to the reference protein but are truncated, in some embodiments, an antigen includes 2, 3, 4, 5, 6, 7, 8, 9, 10, or more mutations, as shown in any of the sequences provided or referenced herein. Antigens/antigenic polypeptides can range in length from about 4, 6, or 8 amino acids to full length proteins.
Stabilizing Elements
[0122] Naturally-occurring eukaryotic mRNA molecules can contain stabilizing elements, including, but not limited to untranslated regions (UTR) at their 5'-end (5' UTR) and/or at their 3'-end (3' UTR), in addition to other structural features, such as a 5'-cap structure or a 3'-poly(A) tail. Both the 5' UTR and the 3' UTR are typically transcribed from the genomic DNA and are elements of the premature mRNA. Characteristic structural features of mature mRNA, such as the 5'-cap and the 3'-poly(A) tail are usually added to the transcribed (premature) mRNA during mRNA processing.
[0123] In some embodiments, a vaccine includes at least one RNA polynucleotide having an open reading frame encoding at least one antigenic polypeptide having at least one modification, at least one 5' terminal cap, and is formulated within a lipid nanoparticle. 5'-capping of polynucleotides may be completed concomitantly during the in vitro-transcription reaction using the following chemical RNA cap analogs to generate the 5'-guanosine cap structure according to manufacturer protocols: 3'-O-Me-m7G(5')ppp(5') G [the ARCA cap]; G(5')ppp(5')A; G(5')ppp(5')G; m7G(5')ppp(5')A; m7G(5')ppp(5')G (New England BioLabs, Ipswich, Mass.). 5'-capping of modified RNA may be completed post-transcriptionally using a Vaccinia Virus Capping Enzyme to generate the "Cap 0" structure: m7G(5')ppp(5')G (New England BioLabs, Ipswich, Mass.). Cap 1 structure may be generated using both Vaccinia Virus Capping Enzyme and a 2'-O methyl-transferase to generate: m7G(5')ppp(5')G-2'-O-methyl. Cap 2 structure may be generated from the Cap 1 structure followed by the 2'-O-methylation of the 5'-antepenultimate nucleotide using a 2'-O methyl-transferase. Cap 3 structure may be generated from the Cap 2 structure followed by the 2'-O-methylation of the 5'-preantepenultimate nucleotide using a 2'-O methyl-transferase. Enzymes may be derived from a recombinant source.
[0124] The 3'-poly(A) tail is typically a stretch of adenine nucleotides added to the 3'-end of the transcribed mRNA. It can, in some instances, comprise up to about 400 adenine nucleotides. In some embodiments, the length of the 3'-poly(A) tail may be an essential element with respect to the stability of the individual mRNA.
[0125] In some embodiments, zoonotic disease RNA vaccines may include one or more stabilizing elements. Stabilizing elements may include for instance a histone stem-loop. A stem-loop binding protein (SLBP), a 32 kDa protein has been identified. It is associated with the histone stem-loop at the 3'-end of the histone messages in both the nucleus and the cytoplasm. Its expression level is regulated by the cell cycle; it peaks during the S-phase, when histone mRNA levels are also elevated. The protein has been shown to be essential for efficient 3'-end processing of histone pre-mRNA by the U7 snRNP. SLBP continues to be associated with the stem-loop after processing, and then stimulates the translation of mature histone mRNAs into histone proteins in the cytoplasm. The RNA binding domain of SLBP is conserved through metazoa and protozoa; its binding to the histone stem-loop depends on the structure of the loop. The minimum binding site includes at least three nucleotides 5' and two nucleotides 3' relative to the stem-loop.
[0126] In some embodiments, zoonotic disease RNA vaccines include a coding region, at least one histone stem-loop, and optionally, a poly(A) sequence or polyadenylation signal. The poly(A) sequence or polyadenylation signal generally should enhance the expression level of the encoded protein. The encoded protein, in some embodiments, is not a histone protein, a reporter protein (e.g. Luciferase, GFP, EGFP, .beta.-Galactosidase, EGFP), or a marker or selection protein (e.g. alpha-Globin, Galactokinase and Xanthine:guanine phosphoribosyl transferase (GPT)).
[0127] In some embodiments, the combination of a poly(A) sequence or polyadenylation signal and at least one histone stem-loop, even though both represent alternative mechanisms in nature, acts synergistically to increase the protein expression beyond the level observed with either of the individual elements. The synergistic effect of the combination of poly(A) and at least one histone stem-loop does not depend on the order of the elements or the length of the poly(A) sequence.
[0128] In some embodiments, zoonotic disease RNA vaccines do not comprise a histone downstream element (HDE). "Histone downstream element" (HDE) includes a purine-rich polynucleotide stretch of approximately 15 to 20 nucleotides 3' of naturally occurring stem-loops, representing the binding site for the U7 snRNA, which is involved in processing of histone pre-mRNA into mature histone mRNA. In some embodiments, the nucleic acid does not include an intron.
[0129] In some embodiments, zoonotic disease RNA vaccines may or may not contain an enhancer and/or promoter sequence, which may be modified or unmodified or which may be activated or inactivated. In some embodiments, the histone stem-loop is generally derived from histone genes, and includes an intramolecular base pairing of two neighbored partially or entirely reverse complementary sequences separated by a spacer, consisting of a short sequence, which forms the loop of the structure. The unpaired loop region is typically unable to base pair with either of the stem loop elements. It occurs more often in RNA, as is a key component of many RNA secondary structures, but may be present in single-stranded DNA as well. Stability of the stem-loop structure generally depends on the length, number of mismatches or bulges, and base composition of the paired region. In some embodiments, wobble base pairing (non-Watson-Crick base pairing) may result. In some embodiments, the at least one histone stem-loop sequence comprises a length of 15 to 45 nucleotides.
[0130] In some embodiments, zoonotic disease RNA vaccines may have one or more AU-rich sequences removed. These sequences, sometimes referred to as AURES are destabilizing sequences found in the 3'UTR. The AURES may be removed from the RNA vaccines. Alternatively the AURES may remain in the RNA vaccine.
Signal Peptides
[0131] In some embodiments, a zoonotic disease vaccine comprises a RNA having an ORF that encodes a signal peptide fused to the zoonotic disease antigen. Signal peptides, comprising the N-terminal 15-60 amino acids of proteins, are typically needed for the translocation across the membrane on the secretory pathway and, thus, universally control the entry of most proteins both in eukaryotes and prokaryotes to the secretory pathway. In eukaryotes, the signal peptide of a nascent precursor protein (pre-protein) directs the ribosome to the rough endoplasmic reticulum (ER) membrane and initiates the transport of the growing peptide chain across it for processing. ER processing produces mature proteins, wherein the signal peptide is cleaved from precursor proteins, typically by a ER-resident signal peptidase of the host cell, or they remain uncleaved and function as a membrane anchor. A signal peptide may also facilitate the targeting of the protein to the cell membrane.
[0132] A signal peptide may have a length of 15-60 amino acids. For example, a signal peptide may have a length of 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, or 60 amino acids. In some embodiments, a signal peptide has a length of 20-60, 25-60, 30-60, 35-60, 40-60, 45-60, 50-60, 55-60, 15-55, 20-55, 25-55, 30-55, 35-55, 40-55, 45-55, 50-55, 15-50, 20-50, 25-50, 30-50, 35-50, 40-50, 45-50, 15-45, 20-45, 25-45, 30-45, 35-45, 40-45, 15-40, 20-40, 25-40, 30-40, 35-40, 15-35, 20-35, 25-35, 30-35, 15-30, 20-30, 25-30, 15-25, 20-25, or 15-20 amino acids.
[0133] Signal peptides from heterologous genes (which regulate expression of genes other than zoonotic disease antigens in nature) are known in the art and can be tested for desired properties and then incorporated into a nucleic acid of the disclosure. In some embodiments, the signal peptide may comprise one of the following sequences:
TABLE-US-00001 (SEQ ID NO: 21) MDSKGSSQKGSRLLLLLVVSNLLLPQGVVG, (SEQ ID NO: 22) MDWTWILFLVAAATRVHS; (SEQ ID NO: 23) METPAQLLFLLLLWLPDTTG; (SEQ ID NO: 24) MLGSNSGQRVVFTILLLLVAPAYS; (SEQ ID NO: 25) MKCLLYLAFLFIGVNCA; (SEQ ID NO: 26) MWLVSLAIVTACAGA.
Fusion Proteins
[0134] In some embodiments, a zoonotic disease RNA vaccine of the present disclosure includes an RNA encoding an antigenic fusion protein. Thus, the encoded antigen or antigens may include two or more proteins (e.g., protein and/or protein fragment) joined together.
[0135] Alternatively, the protein to which a protein antigen is fused does not promote a strong immune response to itself, but rather to the zoonotic disease antigen. Antigenic fusion proteins, in some embodiments, retain the functional property from each original protein.
Scaffold Moieties
[0136] The RNA (e.g., mRNA) vaccines as provided herein, in some embodiments, encode fusion proteins which comprise zoonotic disease antigens linked to scaffold moieties. In some embodiments, such scaffold moieties impart desired properties to an antigen encoded by a nucleic acid of the disclosure. For example scaffold proteins may improve the immunogenicity of an antigen, e.g., by altering the structure of the antigen, altering the uptake and processing of the antigen, and/or causing the antigen to bind to a binding partner.
[0137] In some embodiments, the scaffold moiety is protein that can self-assemble into protein nanoparticles that are highly symmetric, stable, and structurally organized, with diameters of 10-150 nm, a highly suitable size range for optimal interactions with various cells of the immune system. In some embodiments, viral proteins or virus-like particles can be used to form stable nanoparticle structures. Examples of such viral proteins are known in the art. For example, in some embodiments, the scaffold moiety is a hepatitis B surface antigen (HBsAg). HBsAg forms spherical particles with an average diameter of -22 nm and which lacked nucleic acid and hence are non-infectious (Lopez-Sagaseta, J. et al. Computational and Structural Biotechnology Journal 14 (2016) 58-68). In some embodiments, the scaffold moiety is a hepatitis B core antigen (HBcAg) self-assembles into particles of 24-31 nm diameter, which resembled the viral cores obtained from HBV-infected human liver. HBcAg produced in self-assembles into two classes of differently sized nanoparticles of 300 .ANG. and 360 .ANG. diameter, corresponding to 180 or 240 protomers. In some embodiments a zoonotic disease antigen is fused to HBsAG or HBcAG to facilitate self-assembly of nanoparticles displaying the zoonotic disease antigen.
[0138] In another embodiment, bacterial protein platforms may be used. Non-limiting examples of these self-assembling proteins include ferritin, lumazine and encapsulin.
[0139] Ferritin is a protein whose main function is intracellular iron storage. Ferritin is made of 24 subunits, each composed of a four-alpha-helix bundle, that self-assemble in a quaternary structure with octahedral symmetry (Cho K. J. et al. J Mol Biol. 2009; 390:83-98). Several high-resolution structures of ferritin have been determined, confirming that Helicobacter pylori ferritin is made of 24 identical protomers, whereas in animals, there are ferritin light and heavy chains that can assemble alone or combine with different ratios into particles of 24 subunits (Granier T. et al. J Biol Inorg Chem. 2003; 8:105-111; Lawson D. M. et al. Nature. 1991; 349:541-544). Ferritin self-assembles into nanoparticles with robust thermal and chemical stability. Thus, the ferritin nanoparticle is well-suited to carry and expose antigens.
[0140] Lumazine synthase (LS) is also well-suited as a nanoparticle platform for antigen display. LS, which is responsible for the penultimate catalytic step in the biosynthesis of riboflavin, is an enzyme present in a broad variety of organisms, including archaea, bacteria, fungi, plants, and eubacteria (Weber S. E. Flavins andFlavoproteins. Methods and Protocols, Series: Methods in Molecular Biology. 2014). The LS monomer is 150 amino acids long, and consists of beta-sheets along with tandem alpha-helices flanking its sides. A number of different quaternary structures have been reported for LS, illustrating its morphological versatility: from homopentamers up to symmetrical assemblies of 12 pentamers forming capsids of 150 .ANG. diameter. Even LS cages of more than 100 subunits have been described (Zhang X. et al. J Mol Biol. 2006; 362:753-770).
[0141] Encapsulin, a novel protein cage nanoparticle isolated from thermophile Thermotoga maritima, may also be used as a platform to present antigens on the surface of self-assembling nanoparticles. Encapsulin is assembled from 60 copies of identical 31 kDa monomers having a thin and icosahedral T=1 symmetric cage structure with interior and exterior diameters of 20 and 24 nm, respectively (Sutter M. et al. Nat Struct Mol Biol. 2008, 15: 939-947). Although the exact function of encapsulin in T. maritima is not clearly understood yet, its crystal structure has been recently solved and its function was postulated as a cellular compartment that encapsulates proteins such as DyP (Dye decolorizing peroxidase) and Flp (Ferritin like protein), which are involved in oxidative stress responses (Rahmanpour R. et al. FEBS J 2013, 280: 2097-2104).
Linkers and Cleavable Peptides
[0142] In some embodiments, the mRNAs of the disclosure encode more than one polypeptide, referred to herein as fusion proteins. In some embodiments, the mRNA further encodes a linker located between at least one or each domain of the fusion protein. The linker can be, for example, a cleavable linker or protease-sensitive linker. In some embodiments, the linker is selected from the group consisting of F2A linker, P2A linker, T2A linker, E2A linker, and combinations thereof. This family of self-cleaving peptide linkers, referred to as 2A peptides, has been described in the art (see for example, Kim, J. H. et al. (2011) PLoS ONE 6:e18556). In some embodiments, the linker is an F2A linker. In some embodiments, the linker is a GGGS linker. In some embodiments, the fusion protein contains three domains with intervening linkers, having the structure: domain-linker-domain-linker-domain.
[0143] Cleavable linkers known in the art may be used in connection with the disclosure. Exemplary such linkers include: F2A linkers, T2A linkers, P2A linkers, E2A linkers (See, e.g., WO2017/127750). The skilled artisan will appreciate that other art-recognized linkers may be suitable for use in the constructs of the disclosure (e.g., encoded by the nucleic acids of the disclosure). The skilled artisan will likewise appreciate that other polycistronic constructs (mRNA encoding more than one antigen/polypeptide separately within the same molecule) may be suitable for use as provided herein.
Sequence Optimization
[0144] In some embodiments, an ORF encoding an antigen of the disclosure is codon optimized. Codon optimization methods are known in the art. For example, an ORF of any one or more of the sequences provided herein may be codon optimized. Codon optimization, in some embodiments, may be used to match codon frequencies in target and host organisms to ensure proper folding; bias GC content to increase mRNA stability or reduce secondary structures; minimize tandem repeat codons or base runs that may impair gene construction or expression; customize transcriptional and translational control regions; insert or remove protein trafficking sequences; remove/add post translation modification sites in encoded protein (e.g., glycosylation sites); add, remove or shuffle protein domains; insert or delete restriction sites; modify ribosome binding sites and mRNA degradation sites; adjust translational rates to allow the various domains of the protein to fold properly; or reduce or eliminate problem secondary structures within the polynucleotide. Codon optimization tools, algorithms and services are known in the art--non-limiting examples include services from GeneArt (Life Technologies), DNA2.0 (Menlo Park Calif.) and/or proprietary methods. In some embodiments, the open reading frame (ORF) sequence is optimized using optimization algorithms.
[0145] In some embodiments, a codon optimized sequence shares less than 95% sequence identity to a naturally-occurring or wild-type sequence ORF (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen). In some embodiments, a codon optimized sequence shares less than 90% sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen). In some embodiments, a codon optimized sequence shares less than 85% sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen). In some embodiments, a codon optimized sequence shares less than 80% sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen). In some embodiments, a codon optimized sequence shares less than 75% sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen).
[0146] In some embodiments, a codon optimized sequence shares between 65% and 85% (e.g., between about 67% and about 85% or between about 67% and about 80%) sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen). In some embodiments, a codon optimized sequence shares between 65% and 75% or about 80% sequence identity to a naturally-occurring or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a zoonotic disease antigen).
[0147] In some embodiments, a codon-optimized sequence encodes an antigen that is as immunogenic as, or more immunogenic than (e.g., at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 100%, or at least 200% more), than a zoonotic disease antigen encoded by a non-codon-optimized sequence. In some embodiments, a codon-optimized sequence shares between 65% and 85% (e.g., between about 67% and about 85%, or between about 67% and about 80%) sequence identity to a naturally-occurring sequence or a wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a polypeptide or protein of interest (e.g., an antigenic protein or polypeptide)). In some embodiments, a codon-optimized sequence shares between 65% and 75%, or about 80% sequence identity to a naturally-occurring sequence or wild-type sequence (e.g., a naturally-occurring or wild-type mRNA sequence encoding a polypeptide or protein of interest (e.g., an antigenic protein or polypeptide)).
[0148] When transfected into mammalian host cells, the modified mRNAs have a stability of between 12-18 hours, or greater than 18 hours, e.g., 24, 36, 48, 60, 72, or greater than 72 hours and are capable of being expressed by the mammalian host cells.
[0149] In some embodiments, a codon optimized RNA may be one in which the levels of G/C are enhanced. The G/C-content of nucleic acid molecules (e.g., mRNA) may influence the stability of the RNA. RNA having an increased amount of guanine (G) and/or cytosine (C) residues may be functionally more stable than RNA containing a large amount of adenine (A) and thymine (T) or uracil (U) nucleotides. As an example, WO02/098443 discloses a pharmaceutical composition containing an mRNA stabilized by sequence modifications in the translated region. Due to the degeneracy of the genetic code, the modifications work by substituting existing codons for those that promote greater RNA stability without changing the resulting amino acid. The approach is limited to coding regions of the RNA.
Chemically Unmodified Nucleotides
[0150] In some embodiments, at least one RNA (e.g., mRNA) of a zoonotic disease vaccines of the present disclosure is not chemically modified and comprises the standard ribonucleotides consisting of adenosine, guanosine, cytosine and uridine. In some embodiments, nucleotides and nucleosides of the present disclosure comprise standard nucleoside residues such as those present in transcribed RNA (e.g. A, G, C, or U). In some embodiments, nucleotides and nucleosides of the present disclosure comprise standard deoxyribonucleosides such as those present in DNA (e.g. dA, dG, dC, or dT).
Chemical Modifications
[0151] Zoonotic disease RNA vaccines of the present disclosure comprise, in some embodiments, at least one nucleic acid (e.g., RNA) having an open reading frame encoding at least one zoonotic disease antigen, wherein the nucleic acid comprises nucleotides and/or nucleosides that can be standard (unmodified) or modified as is known in the art. In some embodiments, nucleotides and nucleosides of the present disclosure comprise modified nucleotides or nucleosides. Such modified nucleotides and nucleosides can be naturally-occurring modified nucleotides and nucleosides or non-naturally occurring modified nucleotides and nucleosides. Such modifications can include those at the sugar, backbone, or nucleobase portion of the nucleotide and/or nucleoside as are recognized in the art.
[0152] In some embodiments, a naturally-occurring modified nucleotide or nucleotide of the disclosure is one as is generally known or recognized in the art. Non-limiting examples of such naturally occurring modified nucleotides and nucleotides can be found, inter alia, in the widely recognized MODOMICS database.
[0153] In some embodiments, a non-naturally occurring modified nucleotide or nucleoside of the disclosure is one as is generally known or recognized in the art. Non-limiting examples of such non-naturally occurring modified nucleotides and nucleosides can be found, inter alia, in published US application Nos. PCT/US2012/058519; PCT/US2013/075177; PCT/US2014/058897; PCT/US2014/058891; PCT/US2014/070413; PCT/US2015/36773; PCT/US2015/36759; PCT/US2015/36771; or PCT/IB2017/051367 all of which are incorporated by reference herein.
[0154] Hence, nucleic acids of the disclosure (e.g., DNA nucleic acids and RNA nucleic acids, such as mRNA nucleic acids) can comprise standard nucleotides and nucleosides, naturally-occurring nucleotides and nucleosides, non-naturally-occurring nucleotides and nucleosides, or any combination thereof.
[0155] Nucleic acids of the disclosure (e.g., DNA nucleic acids and RNA nucleic acids, such as mRNA nucleic acids), in some embodiments, comprise various (more than one) different types of standard and/or modified nucleotides and nucleosides. In some embodiments, a particular region of a nucleic acid contains one, two or more (optionally different) types of standard and/or modified nucleotides and nucleosides.
[0156] In some embodiments, a modified RNA nucleic acid (e.g., a modified mRNA nucleic acid), introduced to a cell or organism, exhibits reduced degradation in the cell or organism, respectively, relative to an unmodified nucleic acid comprising standard nucleotides and nucleosides.
[0157] In some embodiments, a modified RNA nucleic acid (e.g., a modified mRNA nucleic acid), introduced into a cell or organism, may exhibit reduced immunogenicity in the cell or organism, respectively (e.g., a reduced innate response) relative to an unmodified nucleic acid comprising standard nucleotides and nucleosides.
[0158] Nucleic acids (e.g., RNA nucleic acids, such as mRNA nucleic acids), in some embodiments, comprise non-natural modified nucleotides that are introduced during synthesis or post-synthesis of the nucleic acids to achieve desired functions or properties. The modifications may be present on internucleotide linkages, purine or pyrimidine bases, or sugars. The modification may be introduced with chemical synthesis or with a polymerase enzyme at the terminal of a chain or anywhere else in the chain. Any of the regions of a nucleic acid may be chemically modified.
[0159] The present disclosure provides for modified nucleosides and nucleotides of a nucleic acid (e.g., RNA nucleic acids, such as mRNA nucleic acids). A "nucleoside" refers to a compound containing a sugar molecule (e.g., a pentose or ribose) or a derivative thereof in combination with an organic base (e.g., a purine or pyrimidine) or a derivative thereof (also referred to herein as "nucleobase"). A "nucleotide" refers to a nucleoside, including a phosphate group. Modified nucleotides may by synthesized by any useful method, such as, for example, chemically, enzymatically, or recombinantly, to include one or more modified or non-natural nucleosides. Nucleic acids can comprise a region or regions of linked nucleosides. Such regions may have variable backbone linkages. The linkages can be standard phosphodiester linkages, in which case the nucleic acids would comprise regions of nucleotides.
[0160] Modified nucleotide base pairing encompasses not only the standard adenosine-thymine, adenosine-uracil, or guanosine-cytosine base pairs, but also base pairs formed between nucleotides and/or modified nucleotides comprising non-standard or modified bases, wherein the arrangement of hydrogen bond donors and hydrogen bond acceptors permits hydrogen bonding between a non-standard base and a standard base or between two complementary non-standard base structures, such as, for example, in those nucleic acids having at least one chemical modification. One example of such non-standard base pairing is the base pairing between the modified nucleotide inosine and adenine, cytosine or uracil. Any combination of base/sugar or linker may be incorporated into nucleic acids of the present disclosure.
[0161] In some embodiments, modified nucleobases in nucleic acids (e.g., RNA nucleic acids, such as mRNA nucleic acids) comprise 1-methyl-pseudouridine (m1.psi.), 1-ethyl-pseudouridine (e1.psi.), 5-methoxy-uridine (mo5U), 5-methyl-cytidine (m5C), and/or pseudouridine (v). In some embodiments, modified nucleobases in nucleic acids (e.g., RNA nucleic acids, such as mRNA nucleic acids) comprise 5-methoxymethyl uridine, 5-methylthio uridine, 1-methoxymethyl pseudouridine, 5-methyl cytidine, and/or 5-methoxy cytidine. In some embodiments, the polyribonucleotide includes a combination of at least two (e.g., 2, 3, 4 or more) of any of the aforementioned modified nucleobases, including but not limited to chemical modifications.
[0162] In some embodiments, a RNA nucleic acid of the disclosure comprises 1-methyl-pseudouridine (m1.psi.) substitutions at one or more or all uridine positions of the nucleic acid.
[0163] In some embodiments, a RNA nucleic acid of the disclosure comprises 1-methyl-pseudouridine (m1.psi.) substitutions at one or more or all uridine positions of the nucleic acid and 5-methyl cytidine substitutions at one or more or all cytidine positions of the nucleic acid.
[0164] In some embodiments, a RNA nucleic acid of the disclosure comprises pseudouridine (.psi.) substitutions at one or more or all uridine positions of the nucleic acid.
[0165] In some embodiments, a RNA nucleic acid of the disclosure comprises pseudouridine (.psi.) substitutions at one or more or all uridine positions of the nucleic acid and 5-methyl cytidine substitutions at one or more or all cytidine positions of the nucleic acid.
[0166] In some embodiments, a RNA nucleic acid of the disclosure comprises uridine at one or more or all uridine positions of the nucleic acid.
[0167] In some embodiments, nucleic acids (e.g., RNA nucleic acids, such as mRNA nucleic acids) are uniformly modified (e.g., fully modified, modified throughout the entire sequence) for a particular modification. For example, a nucleic acid can be uniformly modified with 1-methyl-pseudouridine, meaning that all uridine residues in the mRNA sequence are replaced with 1-methyl-pseudouridine. Similarly, a nucleic acid can be uniformly modified for any type of nucleoside residue present in the sequence by replacement with a modified residue such as those set forth above.
[0168] The nucleic acids of the present disclosure may be partially or fully modified along the entire length of the molecule. For example, one or more or all or a given type of nucleotide (e.g., purine or pyrimidine, or any one or more or all of A, G, U, C) may be uniformly modified in a nucleic acid of the disclosure, or in a predetermined sequence region thereof (e.g., in the mRNA including or excluding the polyA tail). In some embodiments, all nucleotides X in a nucleic acid of the present disclosure (or in a sequence region thereof) are modified nucleotides, wherein X may be any one of nucleotides A, G, U, C, or any one of the combinations A+G, A+U, A+C, G+U, G+C, U+C, A+G+U, A+G+C, G+U+C or A+G+C.
[0169] The nucleic acid may contain from about 1% to about 100% modified nucleotides (either in relation to overall nucleotide content, or in relation to one or more types of nucleotide, i.e., any one or more of A, G, U or C) or any intervening percentage (e.g., from 1% to 20%, from 1% to 25%, from 1% to 50%, from 1% to 60%, from 1% to 70%, from 1% to 80%, from 1% to 90%, from 1% to 95%, from 10% to 20%, from 10% to 25%, from 10% to 50%, from 10% to 60%, from 10% to 70%, from 10% to 80%, from 10% to 90%, from 10% to 95%, from 10% to 100%, from 20% to 25%, from 20% to 50%, from 20% to 60%, from 20% to 70%, from 20% to 80%, from 20% to 90%, from 20% to 95%, from 20% to 100%, from 50% to 60%, from 50% to 70%, from 50% to 80%, from 50% to 90%, from 50% to 95%, from 50% to 100%, from 70% to 80%, from 70% to 90%, from 70% to 95%, from 70% to 100%, from 80% to 90%, from 80% to 95%, from 80% to 100%, from 90% to 95%, from 90% to 100%, and from 95% to 100%). It will be understood that any remaining percentage is accounted for by the presence of unmodified A, G, U, or C.
[0170] The nucleic acids may contain at a minimum 1% and at maximum 100% modified nucleotides, or any intervening percentage, such as at least 5% modified nucleotides, at least 10% modified nucleotides, at least 25% modified nucleotides, at least 50% modified nucleotides, at least 80% modified nucleotides, or at least 90% modified nucleotides. For example, the nucleic acids may contain a modified pyrimidine such as a modified uracil or cytosine. In some embodiments, at least 5%, at least 10%, at least 25%, at least 50%, at least 80%, at least 90% or 100% of the uracil in the nucleic acid is replaced with a modified uracil (e.g., a 5-substituted uracil). The modified uracil can be replaced by a compound having a single unique structure, or can be replaced by a plurality of compounds having different structures (e.g., 2, 3, 4 or more unique structures). In some embodiments, at least 5%, at least 10%, at least 25%, at least 50%, at least 80%, at least 90% or 100% of the cytosine in the nucleic acid is replaced with a modified cytosine (e.g., a 5-substituted cytosine). The modified cytosine can be replaced by a compound having a single unique structure, or can be replaced by a plurality of compounds having different structures (e.g., 2, 3, 4 or more unique structures).
Untranslated Regions (UTRs)
[0171] The nucleic acids of the present disclosure may comprise one or more regions or parts which act or function as an untranslated region. Where nucleic acids are designed to encode at least one antigen of interest, the nucleic may comprise one or more of these untranslated regions (UTRs). Wild-type untranslated regions of a nucleic acid are transcribed but not translated. In mRNA, the 5' UTR starts at the transcription start site and continues to the start codon but does not include the start codon; whereas, the 3' UTR starts immediately following the stop codon and continues until the transcriptional termination signal. There is growing body of evidence about the regulatory roles played by the UTRs in terms of stability of the nucleic acid molecule and translation. The regulatory features of a UTR can be incorporated into the polynucleotides of the present disclosure to, among other things, enhance the stability of the molecule. The specific features can also be incorporated to ensure controlled down-regulation of the transcript in case they are misdirected to undesired organs sites. A variety of 5'UTR and 3'UTR sequences are known and available in the art.
[0172] A 5' UTR is region of an mRNA that is directly upstream (5') from the start codon (the first codon of an mRNA transcript translated by a ribosome). A 5' UTR does not encode a protein (is non-coding). Natural 5'UTRs have features that play roles in translation initiation. They harbor signatures like Kozak sequences which are commonly known to be involved in the process by which the ribosome initiates translation of many genes. Kozak sequences have the consensus CCR(A/G)CCAUGG (SEQ ID NO: 27), where R is a purine (adenine or guanine) three bases upstream of the start codon (AUG), which is followed by another `G`0.5'UTR also have been known to form secondary structures which are involved in elongation factor binding.
[0173] In some embodiments of the disclosure, a 5' UTR is a heterologous UTR, i.e., is a UTR found in nature associated with a different ORF. In another embodiment, a 5' UTR is a synthetic UTR, i.e., does not occur in nature. Synthetic UTRs include UTRs that have been mutated to improve their properties, e.g., which increase gene expression as well as those which are completely synthetic. Exemplary 5' UTRs include Xenopus or human derived a-globin or b-globin (U.S. Pat. Nos. 8,278,063; 9,012,219), human cytochrome b-245 a polypeptide, and hydroxysteroid (17b) dehydrogenase, and Tobacco etch virus (U.S. Pat. Nos. 8,278,063, 9,012,219). CMV immediate-early 1 (IE1) gene (US2014/0206753, WO2013/185069), the sequence GGGAUCCUACC (SEQ ID NO: 28) (WO2014/144196) may also be used. In another embodiment, 5' UTR of a TOP gene is a 5' UTR of a TOP gene lacking the 5' TOP motif (the oligopyrimidine tract) (e.g., WO2015/101414, WO2015/101415, WO2015/062738, WO2015/024667, WO2015/024667; 5' UTR element derived from ribosomal protein Large 32 (L32) gene (WO2015/101414, WO2015/101415, WO2015/062738), 5' UTR element derived from the 5'UTR of an hydroxysteroid (17-.beta.) dehydrogenase 4 gene (HSD17B4) (WO2015/024667), or a 5' UTR element derived from the 5' UTR of ATP5A1 (WO2015/024667) can be used. In some embodiments, an internal ribosome entry site (IRES) is used instead of a 5' UTR.
[0174] A 3' UTR is region of an mRNA that is directly downstream (3') from the stop codon (the codon of an mRNA transcript that signals a termination of translation). A 3' UTR does not encode a protein (is non-coding). Natural or wild type 3' UTRs are known to have stretches of adenosines and uridines embedded in them. These AU rich signatures are particularly prevalent in genes with high rates of turnover. Based on their sequence features and functional properties, the AU rich elements (AREs) can be separated into three classes (Chen et al, 1995): Class I AREs contain several dispersed copies of an AUUUA motif within U-rich regions. C-Myc and MyoD contain class I AREs. Class II AREs possess two or more overlapping UUAUUUA(U/A)(U/A) (SEQ ID NO: 29) nonamers. Molecules containing this type of AREs include GM-CSF and TNF-a. Class III ARES are less well defined. These U rich regions do not contain an AUUUA motif. c-Jun and Myogenin are two well-studied examples of this class. Most proteins binding to the AREs are known to destabilize the messenger, whereas members of the ELAV family, most notably HuR, have been documented to increase the stability of mRNA. HuR binds to AREs of all the three classes. Engineering the HuR specific binding sites into the 3' UTR of nucleic acid molecules will lead to HuR binding and thus, stabilization of the message in vivo.
[0175] Introduction, removal or modification of 3' UTR AU rich elements (AREs) can be used to modulate the stability of nucleic acids (e.g., RNA) of the disclosure. When engineering specific nucleic acids, one or more copies of an ARE can be introduced to make nucleic acids of the disclosure less stable and thereby curtail translation and decrease production of the resultant protein. Likewise, AREs can be identified and removed or mutated to increase the intracellular stability and thus increase translation and production of the resultant protein. Transfection experiments can be conducted in relevant cell lines, using nucleic acids of the disclosure and protein production can be assayed at various time points post-transfection. For example, cells can be transfected with different ARE-engineering molecules and by using an ELISA kit to the relevant protein and assaying protein produced at 6 hour, 12 hour, 24 hour, 48 hour, and 7 days post-transfection.
[0176] 3' UTRs may be heterologous or synthetic. With respect to 3' UTRs, globin UTRs, including Xenopus .beta.-globin UTRs and human .beta.-globin UTRs are known in the art (U.S. Pat. Nos. 8,278,063, 9,012,219, US2011/0086907). A modified .beta.-globin construct with enhanced stability in some cell types by cloning two sequential human .beta.-globin 3'UTRs head to tail has been developed and is well known in the art (US2012/0195936, WO2014/071963). In addition a2-globin, al-globin, UTRs and mutants thereof are also known in the art (WO2015/101415, WO2015/024667). Other 3' UTRs described in the mRNA constructs in the non-patent literature include CYBA (Ferizi et al., 2015) and albumin (Thess et al., 2015). Other exemplary 3' UTRs include that of bovine or human growth hormone (wild type or modified) (WO2013/185069, US2014/0206753, WO2014/152774), rabbit .beta. globin and hepatitis B virus (HBV), .alpha.-globin 3' UTR and Viral VEEV 3' UTR sequences are also known in the art. In some embodiments, the sequence UUUGAAUU (WO2014/144196) is used. In some embodiments, 3' UTRs of human and mouse ribosomal protein are used. Other examples include rps9 3'UTR (WO2015/101414), FIG. 4 (WO2015/101415), and human albumin 7 (WO2015/101415).
[0177] Those of ordinary skill in the art will understand that 5'UTRs that are heterologous or synthetic may be used with any desired 3' UTR sequence. For example, a heterologous 5'UTR may be used with a synthetic 3'UTR with a heterologous 3'' UTR.
[0178] Non-UTR sequences may also be used as regions or subregions within a nucleic acid. For example, introns or portions of introns sequences may be incorporated into regions of nucleic acid of the disclosure. Incorporation of intronic sequences may increase protein production as well as nucleic acid levels.
[0179] Combinations of features may be included in flanking regions and may be contained within other features. For example, the ORF may be flanked by a 5' UTR which may contain a strong Kozak translational initiation signal and/or a 3' UTR which may include an oligo(dT) sequence for templated addition of a poly-A tail. 5' UTR may comprise a first polynucleotide fragment and a second polynucleotide fragment from the same and/or different genes such as the 5' UTRs described in US Patent Application Publication No. 2010/0293625 and PCT/US2014/069155, herein incorporated by reference in its entirety.
[0180] It should be understood that any UTR from any gene may be incorporated into the regions of a nucleic acid. Furthermore, multiple wild-type UTRs of any known gene may be utilized. It is also within the scope of the present disclosure to provide artificial UTRs which are not variants of wild type regions. These UTRs or portions thereof may be placed in the same orientation as in the transcript from which they were selected or may be altered in orientation or location. Hence a 5' or 3' UTR may be inverted, shortened, lengthened, made with one or more other 5' UTRs or 3' UTRs. As used herein, the term "altered" as it relates to a UTR sequence, means that the UTR has been changed in some way in relation to a reference sequence. For example, a 3' UTR or 5' UTR may be altered relative to a wild-type or native UTR by the change in orientation or location as taught above or may be altered by the inclusion of additional nucleotides, deletion of nucleotides, swapping or transposition of nucleotides. Any of these changes producing an "altered" UTR (whether 3' or 5') comprise a variant UTR.
[0181] In some embodiments, a double, triple or quadruple UTR such as a 5' UTR or 3' UTR may be used. As used herein, a "double" UTR is one in which two copies of the same UTR are encoded either in series or substantially in series. For example, a double beta-globin 3' UTR may be used as described in US Patent publication 20100129877, the contents of which are incorporated herein by reference in its entirety.
[0182] It is also within the scope of the present disclosure to have patterned UTRs. As used herein "patterned UTRs" are those UTRs which reflect a repeating or alternating pattern, such as ABABAB or AABBAABBAABB or ABCABCABC or variants thereof repeated once, twice, or more than 3 times. In these patterns, each letter, A, B, or C represent a different UTR at the nucleotide level.
[0183] In some embodiments, flanking regions are selected from a family of transcripts whose proteins share a common function, structure, feature or property. For example, polypeptides of interest may belong to a family of proteins which are expressed in a particular cell, tissue or at some time during development. The UTRs from any of these genes may be swapped for any other UTR of the same or different family of proteins to create a new polynucleotide. As used herein, a "family of proteins" is used in the broadest sense to refer to a group of two or more polypeptides of interest which share at least one function, structure, feature, localization, origin, or expression pattern.
[0184] The untranslated region may also include translation enhancer elements (TEE). As a non-limiting example, the TEE may include those described in US Application No. 2009/0226470, herein incorporated by reference in its entirety, and those known in the art.
In Vitro Transcription of RNA
[0185] cDNA encoding the polynucleotides described herein may be transcribed using an in vitro transcription (IVT) system. In vitro transcription of RNA is known in the art and is described in International Publication WO2014/152027, which is incorporated by reference herein in its entirety.
[0186] In some embodiments, the RNA transcript is generated using a non-amplified, linearized DNA template in an in vitro transcription reaction to generate the RNA transcript. In some embodiments, the template DNA is isolated DNA. In some embodiments, the template DNA is cDNA. In some embodiments, the cDNA is formed by reverse transcription of a RNA polynucleotide, for example, but not limited to Lassa virus, Nipah virus, or betacoronavirus RNA, e.g. mRNA. In some embodiments, cells, e.g., bacterial cells, e.g., E. coli, e.g., DH-1 cells are transfected with the plasmid DNA template. In some embodiments, the transfected cells are cultured to replicate the plasmid DNA which is then isolated and purified. In some embodiments, the DNA template includes a RNA polymerase promoter, e.g., a T7 promoter located 5' to and operably linked to the gene of interest.
[0187] In some embodiments, an in vitro transcription template encodes a 5' untranslated (UTR) region, contains an open reading frame, and encodes a 3' UTR and a polyA tail. The particular nucleic acid sequence composition and length of an in vitro transcription template will depend on the mRNA encoded by the template.
[0188] A "5' untranslated region" (UTR) refers to a region of an mRNA that is directly upstream (i.e., 5') from the start codon (i.e., the first codon of an mRNA transcript translated by a ribosome) that does not encode a polypeptide. When RNA transcripts are being generated, the 5' UTR may comprise a promoter sequence. Such promoter sequences are known in the art. It should be understood that such promoter sequences will not be present in a vaccine of the disclosure.
[0189] A "3' untranslated region" (UTR) refers to a region of an mRNA that is directly downstream (i.e., 3') from the stop codon (i.e., the codon of an mRNA transcript that signals a termination of translation) that does not encode a polypeptide.
[0190] An "open reading frame" is a continuous stretch of DNA beginning with a start codon (e.g., methionine (ATG)), and ending with a stop codon (e.g., TAA, TAG or TGA) and encodes a polypeptide.
[0191] A "polyA tail" is a region of mRNA that is downstream, e.g., directly downstream (i.e., 3'), from the 3' UTR that contains multiple, consecutive adenosine monophosphates. A polyA tail may contain 10 to 300 adenosine monophosphates. For example, a polyA tail may contain 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210, 220, 230, 240, 250, 260, 270, 280, 290 or 300 adenosine monophosphates. In some embodiments, a polyA tail contains 50 to 250 adenosine monophosphates. In a relevant biological setting (e.g., in cells, in vivo) the poly(A) tail functions to protect mRNA from enzymatic degradation, e.g., in the cytoplasm, and aids in transcription termination, and/or export of the mRNA from the nucleus and translation.
[0192] In some embodiments, a nucleic acid includes 200 to 3,000 nucleotides. For example, a nucleic acid may include 200 to 500, 200 to 1000, 200 to 1500, 200 to 3000, 500 to 1000, 500 to 1500, 500 to 2000, 500 to 3000, 1000 to 1500, 1000 to 2000, 1000 to 3000, 1500 to 3000, or 2000 to 3000 nucleotides).
[0193] An in vitro transcription system typically comprises a transcription buffer, nucleotide triphosphates (NTPs), an RNase inhibitor and a polymerase.
[0194] The NTPs may be manufactured in house, may be selected from a supplier, or may be synthesized as described herein. The NTPs may be selected from, but are not limited to, those described herein including natural and unnatural (modified) NTPs.
[0195] Any number of RNA polymerases or variants may be used in the method of the present disclosure. The polymerase may be selected from, but is not limited to, a phage RNA polymerase, e.g., a T7 RNA polymerase, a T3 RNA polymerase, a SP6 RNA polymerase, and/or mutant polymerases such as, but not limited to, polymerases able to incorporate modified nucleic acids and/or modified nucleotides, including chemically modified nucleic acids and/or nucleotides. Some embodiments exclude the use of DNase.
[0196] In some embodiments, the RNA transcript is capped via enzymatic capping. In some embodiments, the RNA comprises 5' terminal cap, for example, 7mG(5')ppp(5')NlmpNp.
Chemical Synthesis
[0197] Solid-Phase Chemical Synthesis.
[0198] Nucleic acids the present disclosure may be manufactured in whole or in part using solid phase techniques. Solid-phase chemical synthesis of nucleic acids is an automated method wherein molecules are immobilized on a solid support and synthesized step by step in a reactant solution. Solid-phase synthesis is useful in site-specific introduction of chemical modifications in the nucleic acid sequences.
[0199] Liquid Phase Chemical Synthesis.
[0200] The synthesis of nucleic acids of the present disclosure by the sequential addition of monomer building blocks may be carried out in a liquid phase.
[0201] Combination of Synthetic Methods.
[0202] The synthetic methods discussed above each has its own advantages and limitations. Attempts have been conducted to combine these methods to overcome the limitations. Such combinations of methods are within the scope of the present disclosure. The use of solid-phase or liquid-phase chemical synthesis in combination with enzymatic ligation provides an efficient way to generate long chain nucleic acids that cannot be obtained by chemical synthesis alone.
Ligation of Nucleic Acid Regions or Subregions
[0203] Assembling nucleic acids by a ligase may also be used. DNA or RNA ligases promote intermolecular ligation of the 5' and 3' ends of polynucleotide chains through the formation of a phosphodiester bond. Nucleic acids such as chimeric polynucleotides and/or circular nucleic acids may be prepared by ligation of one or more regions or subregions. DNA fragments can be joined by a ligase catalyzed reaction to create recombinant DNA with different functions. Two oligodeoxynucleotides, one with a 5' phosphoryl group and another with a free 3' hydroxyl group, serve as substrates for a DNA ligase.
Purification
[0204] Purification of the nucleic acids described herein may include, but is not limited to, nucleic acid clean-up, quality assurance and quality control. Clean-up may be performed by methods known in the arts such as, but not limited to, AGENCOURT.RTM. beads (Beckman Coulter Genomics, Danvers, Mass.), poly-T beads, LNATM oligo-T capture probes (EXIQON.RTM. Inc, Vedbaek, Denmark) or HPLC based purification methods such as, but not limited to, strong anion exchange HPLC, weak anion exchange HPLC, reverse phase HPLC (RP-HPLC), and hydrophobic interaction HPLC (HIC-HPLC). The term "purified" when used in relation to a nucleic acid such as a "purified nucleic acid" refers to one that is separated from at least one contaminant. A "contaminant" is any substance that makes another unfit, impure or inferior. Thus, a purified nucleic acid (e.g., DNA and RNA) is present in a form or setting different from that in which it is found in nature, or a form or setting different from that which existed prior to subjecting it to a treatment or purification method.
[0205] A quality assurance and/or quality control check may be conducted using methods such as, but not limited to, gel electrophoresis, UV absorbance, or analytical HPLC.
[0206] In some embodiments, the nucleic acids may be sequenced by methods including, but not limited to reverse-transcriptase-PCR.
Quantification
[0207] In some embodiments, the nucleic acids of the present disclosure may be quantified in exosomes or when derived from one or more bodily fluid. Bodily fluids include peripheral blood, serum, plasma, ascites, urine, cerebrospinal fluid (CSF), sputum, saliva, bone marrow, synovial fluid, aqueous humor, amniotic fluid, cerumen, breast milk, broncheoalveolar lavage fluid, semen, prostatic fluid, cowper's fluid or pre-ejaculatory fluid, sweat, fecal matter, hair, tears, cyst fluid, pleural and peritoneal fluid, pericardial fluid, lymph, chyme, chyle, bile, interstitial fluid, menses, pus, sebum, vomit, vaginal secretions, mucosal secretion, stool water, pancreatic juice, lavage fluids from sinus cavities, bronchopulmonary aspirates, blastocyl cavity fluid, and umbilical cord blood. Alternatively, exosomes may be retrieved from an organ selected from the group consisting of lung, heart, pancreas, stomach, intestine, bladder, kidney, ovary, testis, skin, colon, breast, prostate, brain, esophagus, liver, and placenta.
[0208] Assays may be performed using construct specific probes, cytometry, qRT-PCR, real-time PCR, PCR, flow cytometry, electrophoresis, mass spectrometry, or combinations thereof while the exosomes may be isolated using immunohistochemical methods such as enzyme linked immunosorbent assay (ELISA) methods. Exosomes may also be isolated by size exclusion chromatography, density gradient centrifugation, differential centrifugation, nanomembrane ultrafiltration, immunoabsorbent capture, affinity purification, microfluidic separation, or combinations thereof.
[0209] These methods afford the investigator the ability to monitor, in real time, the level of nucleic acids remaining or delivered. This is possible because the nucleic acids of the present disclosure, in some embodiments, differ from the endogenous forms due to the structural or chemical modifications.
[0210] In some embodiments, the nucleic acid may be quantified using methods such as, but not limited to, ultraviolet visible spectroscopy (UV/Vis). A non-limiting example of a UV/Vis spectrometer is a NANODROP.RTM. spectrometer (ThermoFisher, Waltham, Mass.). The quantified nucleic acid may be analyzed in order to determine if the nucleic acid may be of proper size, check that no degradation of the nucleic acid has occurred. Degradation of the nucleic acid may be checked by methods such as, but not limited to, agarose gel electrophoresis, HPLC based purification methods such as, but not limited to, strong anion exchange HPLC, weak anion exchange HPLC, reverse phase HPLC (RP-HPLC), and hydrophobic interaction HPLC (HIC-HPLC), liquid chromatography-mass spectrometry (LCMS), capillary electrophoresis (CE) and capillary gel electrophoresis (CGE).
Lipid Nanoparticles (LNPs)
[0211] In some embodiments, zoonotic disease RNA (e.g., mRNA) vaccines of the disclosure are formulated in a lipid nanoparticle (LNP). Lipid nanoparticles typically comprise ionizable cationic lipid, non-cationic lipid, sterol and PEG lipid components along with the nucleic acid cargo of interest. The lipid nanoparticles of the disclosure can be generated using components, compositions, and methods as are generally known in the art, see for example PCT/US2016/052352; PCT/US2016/068300; PCT/US2017/037551; PCT/US2015/027400; PCT/US2016/047406; PCT/US2016000129; PCT/US2016/014280; PCT/US2016/014280; PCT/US2017/038426; PCT/US2014/027077; PCT/US2014/055394; PCT/US2016/52117; PCT/US2012/069610; PCT/US2017/027492; PCT/US2016/059575 and PCT/US2016/069491, all of which are incorporated by reference herein in their entirety.
[0212] Vaccines of the present disclosure are typically formulated in lipid nanoparticle. In some embodiments, the lipid nanoparticle comprises at least one ionizable cationic lipid, at least one non-cationic lipid, at least one sterol, and/or at least one polyethylene glycol (PEG)-modified lipid.
[0213] In some embodiments, the lipid nanoparticle comprises a molar ratio of 20-60% ionizable cationic lipid. For example, the lipid nanoparticle may comprise a molar ratio of 20-50%, 20-40%, 20-30%, 30-60%, 30-50%, 30-40%, 40-60%, 40-50%, or 50-60% ionizable cationic lipid. In some embodiments, the lipid nanoparticle comprises a molar ratio of 20%, 30%, 40%, 50, or 60% ionizable cationic lipid.
[0214] In some embodiments, the lipid nanoparticle comprises a molar ratio of 5-25% non-cationic lipid. For example, the lipid nanoparticle may comprise a molar ratio of 5-20%, 5-15%, 5-10%, 10-25%, 10-20%, 10-25%, 15-25%, 15-20%, or 20-25% non-cationic lipid. In some embodiments, the lipid nanoparticle comprises a molar ratio of 5%, 10%, 15%, 20%, or 25% non-cationic lipid.
[0215] In some embodiments, the lipid nanoparticle comprises a molar ratio of 25-55% sterol. For example, the lipid nanoparticle may comprise a molar ratio of 25-50%, 25-45%, 25-40%, 25-35%, 25-30%, 30-55%, 30-50%, 30-45%, 30-40%, 30-35%, 35-55%, 35-50%, 35-45%, 35-40%, 40-55%, 40-50%, 40-45%, 45-55%, 45-50%, or 50-55% sterol. In some embodiments, the lipid nanoparticle comprises a molar ratio of 25%, 30%, 35%, 40%, 45%, 50%, or 55% sterol.
[0216] In some embodiments, the lipid nanoparticle comprises a molar ratio of 0.5-15% PEG-modified lipid. For example, the lipid nanoparticle may comprise a molar ratio of 0.5-10%, 0.5-5%, 1-15%, 1-10%, 1-5%, 2-15%, 2-10%, 2-5%, 5-15%, 5-10%, or 10-15%. In some embodiments, the lipid nanoparticle comprises a molar ratio of 0.5%, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, or 15% PEG-modified lipid.
[0217] In some embodiments, the lipid nanoparticle comprises a molar ratio of 20-60% ionizable cationic lipid, 5-25% non-cationic lipid, 25-55% sterol, and 0.5-15% PEG-modified lipid.
[0218] In some embodiments, an ionizable cationic lipid of the disclosure comprises a compound of Formula (I):
##STR00001##
[0219] or a salt or isomer thereof, wherein:
[0220] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0221] R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0222] R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a carbocycle, heterocycle, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --N(R).sub.2, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and --C(R)N(R).sub.2C(O)OR, and each n is independently selected from 1, 2, 3, 4, and 5;
[0223] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0224] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0225] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0226] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0227] R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle;
[0228] R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle;
[0229] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0230] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0231] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0232] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl;
[0233] each Y is independently a C.sub.3-6 carbocycle;
[0234] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0235] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13.
[0236] In some embodiments, a subset of compounds of Formula (I) includes those in which when R.sub.4 is --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, or --CQ(R).sub.2, then (i) Q is not --N(R).sub.2 when n is 1, 2, 3, 4 or 5, or (ii) Q is not 5, 6, or 7-membered heterocycloalkyl when n is 1 or 2.
[0237] In some embodiments, another subset of compounds of Formula (I) includes those in which
[0238] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0239] R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0240] R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a C.sub.3-6 carbocycle, a 5- to 14-membered heteroaryl having one or more heteroatoms selected from N, O, and S, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --CRN(R).sub.2C(O)OR, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and a 5- to 14-membered heterocycloalkyl having one or more heteroatoms selected from N, O, and S which is substituted with one or more substituents selected from oxo (.dbd.O), OH, amino, mono- or di-alkylamino, and C.sub.1-3 alkyl, and each n is independently selected from 1, 2, 3, 4, and 5;
[0241] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0242] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0243] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0244] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0245] R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle;
[0246] R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle;
[0247] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0248] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0249] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0250] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl;
[0251] each Y is independently a C.sub.3-6 carbocycle;
[0252] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0253] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
[0254] or salts or isomers thereof.
[0255] In some embodiments, another subset of compounds of Formula (I) includes those in which
[0256] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0257] R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0258] R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a C.sub.3-6 carbocycle, a 5- to 14-membered heterocycle having one or more heteroatoms selected from N, O, and S, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --CRN(R).sub.2C(O)OR, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and --C(.dbd.NR.sub.9)N(R).sub.2, and each n is independently selected from 1, 2, 3, 4, and 5; and when Q is a 5- to 14-membered heterocycle and (i) R.sub.4 is --(CH.sub.2).sub.nQ in which n is 1 or 2, or (ii) R.sub.4 is --(CH.sub.2).sub.nCHQR in which n is 1, or (iii) R.sub.4 is --CHQR, and --CQ(R).sub.2, then Q is either a 5- to 14-membered heteroaryl or 8- to 14-membered heterocycloalkyl;
[0259] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0260] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0261] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0262] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0263] R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle;
[0264] R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle;
[0265] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0266] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0267] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0268] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl;
[0269] each Y is independently a C.sub.3-6 carbocycle;
[0270] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0271] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
[0272] or salts or isomers thereof.
[0273] In some embodiments, another subset of compounds of Formula (I) includes those in which
[0274] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0275] R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0276] R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a C.sub.3-6 carbocycle, a 5- to 14-membered heteroaryl having one or more heteroatoms selected from N, O, and S, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --CRN(R).sub.2C(O)OR, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and --C(.dbd.NR.sub.9)N(R).sub.2, and each n is independently selected from 1, 2, 3, 4, and 5;
[0277] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0278] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0279] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0280] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0281] R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle;
[0282] R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle;
[0283] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0284] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0285] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0286] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl;
[0287] each Y is independently a C.sub.3-6 carbocycle;
[0288] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0289] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
[0290] or salts or isomers thereof.
[0291] In some embodiments, another subset of compounds of Formula (I) includes those in which
[0292] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0293] R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.2-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0294] R.sub.4 is --(CH.sub.2).sub.nQ or --(CH.sub.2).sub.nCHQR, where Q is --N(R).sub.2, and n is selected from 3, 4, and 5;
[0295] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0296] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0297] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0298] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0299] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0300] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0301] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0302] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.1-12 alkenyl;
[0303] each Y is independently a C.sub.3-6 carbocycle;
[0304] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0305] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
[0306] or salts or isomers thereof.
[0307] In some embodiments, another subset of compounds of Formula (I) includes those in which
[0308] R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R';
[0309] R.sub.2 and R.sub.3 are independently selected from the group consisting of C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle;
[0310] R.sub.4 is selected from the group consisting of --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, and --CQ(R).sub.2, where Q is --N(R).sub.2, and n is selected from 1, 2, 3, 4, and 5;
[0311] each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0312] each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0313] M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group;
[0314] R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0315] each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H;
[0316] each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H;
[0317] each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl;
[0318] each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.1-12 alkenyl;
[0319] each Y is independently a C.sub.3-6 carbocycle;
[0320] each X is independently selected from the group consisting of F, Cl, Br, and I; and
[0321] m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
[0322] or salts or isomers thereof.
[0323] In some embodiments, a subset of compounds of Formula (I) includes those of Formula (IA):
##STR00002##
[0324] or a salt or isomer thereof, wherein 1 is selected from 1, 2, 3, 4, and 5; m is selected from 5, 6, 7, 8, and 9; M.sub.1 is a bond or M'; R.sub.4 is unsubstituted C.sub.1-3 alkyl, or --(CH.sub.2).sub.nQ, in which Q is OH, --NHC(S)N(R).sub.2, --NHC(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)R.sub.8, --NHC(.dbd.NR.sub.9)N(R).sub.2, --NHC(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, heteroaryl or heterocycloalkyl; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --P(O)(OR')O--, --S--S--, an aryl group, and a heteroaryl group; and R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, and C.sub.2-14 alkenyl.
[0325] In some embodiments, a subset of compounds of Formula (I) includes those of Formula (II):
##STR00003##
or a salt or isomer thereof, wherein 1 is selected from 1, 2, 3, 4, and 5; M.sub.1 is a bond or M'; R.sub.4 is unsubstituted C.sub.1-3 alkyl, or --(CH.sub.2).sub.nQ, in which n is 2, 3, or 4, and Q is OH, --NHC(S)N(R).sub.2, --NHC(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)R.sub.8, --NHC(.dbd.NR.sub.9)N(R).sub.2, --NHC(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, heteroaryl or heterocycloalkyl; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --P(O)(OR')O--, --S--S--, an aryl group, and a heteroaryl group; and R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, and C.sub.2-14 alkenyl.
[0326] In some embodiments, a subset of compounds of Formula (I) includes those of Formula (IIa), (IIb), (IIc), or (lie):
##STR00004##
[0327] or a salt or isomer thereof, wherein R.sub.4 is as described herein.
[0328] In some embodiments, a subset of compounds of Formula (I) includes those of Formula (IId):
##STR00005##
[0329] or a salt or isomer thereof, wherein n is 2, 3, or 4; and m, R', R'', and R.sub.2 through R.sub.6 are as described herein. For example, each of R.sub.2 and R.sub.3 may be independently selected from the group consisting of C.sub.5-14 alkyl and C.sub.5-14 alkenyl.
[0330] In some embodiments, an ionizable cationic lipid of the disclosure comprises a compound having structure:
##STR00006##
[0331] In some embodiments, an ionizable cationic lipid of the disclosure comprises a compound having structure:
##STR00007##
[0332] In some embodiments, a non-cationic lipid of the disclosure comprises 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLPC), 1,2-dimyristoyl-sn-gly cero-phosphocholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-diundecanoyl-sn-glycero-phosphocholine (DUPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-di-O-octadecenyl-sn-glycero-3-phosphocholine (18:0 Diether PC), 1-oleoyl-2 cholesterylhemisuccinoyl-sn-glycero-3-phosphocholine (OChemsPC), 1-hexadecyl-sn-glycero-3-phosphocholine (C16 Lyso PC), 1,2-dilinolenoyl-sn-glycero-3-phosphocholine, 1,2-diarachidonoyl-sn-glycero-3-phosphocholine, 1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine (ME 16.0 PE), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine, 1,2-dilinoleoyl-sn-glycero-3-phosphoethanolamine, 1,2-dilinolenoyl-sn-glycero-3-phosphoethanolamine, 1,2-diarachidonoyl-sn-glycero-3-phosphoethanolamine, 1,2-didocosahexaenoyl-sn-glycero-3-phosphoethanolamine, 1,2-dioleoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt (DOPG), sphingomyelin, and mixtures thereof.
[0333] In some embodiments, a PEG modified lipid of the disclosure comprises a PEG-modified phosphatidylethanolamine, a PEG-modified phosphatidic acid, a PEG-modified ceramide, a PEG-modified dialkylamine, a PEG-modified diacylglycerol, a PEG-modified dialkylglycerol, and mixtures thereof. In some embodiments, the PEG-modified lipid is PEG-DMG, PEG-c-DOMG (also referred to as PEG-DOMG), PEG-DSG and/or PEG-DPG.
[0334] In some embodiments, a sterol of the disclosure comprises cholesterol, fecosterol, sitosterol, ergosterol, campesterol, stigmasterol, brassicasterol, tomatidine, ursolic acid, alpha-tocopherol, and mixtures thereof.
[0335] In some embodiments, a LNP of the disclosure comprises an ionizable cationic lipid of Compound 1, wherein the non-cationic lipid is DSPC, the structural lipid that is cholesterol, and the PEG lipid is PEG-DMG.
[0336] In some embodiments, a LNP of the disclosure comprises an N:P ratio of from about 2:1 to about 30:1.
[0337] In some embodiments, a LNP of the disclosure comprises an N:P ratio of about 6:1.
[0338] In some embodiments, a LNP of the disclosure comprises an N:P ratio of about 3:1.
[0339] In some embodiments, a LNP of the disclosure comprises a wt/wt ratio of the ionizable cationic lipid component to the RNA of from about 10:1 to about 100:1.
[0340] In some embodiments, a LNP of the disclosure comprises a wt/wt ratio of the ionizable cationic lipid component to the RNA of about 20:1.
[0341] In some embodiments, a LNP of the disclosure comprises a wt/wt ratio of the ionizable cationic lipid component to the RNA of about 10:1.
[0342] In some embodiments, a LNP of the disclosure has a mean diameter from about 50 nm to about 150 nm.
[0343] In some embodiments, a LNP of the disclosure has a mean diameter from about 70 nm to about 120 nm.
Multivalent Vaccines
[0344] The zoonotic disease vaccines, as provided herein, may include an RNA (e.g. mRNA) or multiple RNAs encoding two or more antigens of the same or different zoonotic disease species. In some embodiments, a zoonotic disease vaccine includes an RNA or multiple RNAs encoding two or more antigens. In some embodiments, the RNA (at least one RNA) of a zoonotic disease vaccine may encode 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, or more antigens.
[0345] In some embodiments, two or more different RNA (e.g., mRNA) encoding antigens may be formulated in the same lipid nanoparticle. In other embodiments, two or more different RNA encoding antigens may be formulated in separate lipid nanoparticles (each RNA formulated in a single lipid nanoparticle). The lipid nanoparticles may then be combined and administered as a single vaccine composition (e.g., comprising multiple RNA encoding multiple antigens) or may be administered separately.
Broad Spectrum RNA (e.g., mRNA) Vaccines
[0346] There may be situations where persons are at risk for infection with more than one strain of LASV. RNA (e.g., mRNA) therapeutic vaccines are particularly amenable to combination vaccination approaches due to a number of factors including, but not limited to, speed of manufacture, ability to rapidly tailor vaccines to accommodate perceived geographical threat, and the like. Moreover, because the vaccines utilize the human body to produce the antigenic protein, the vaccines are amenable to the production of larger, more complex antigenic proteins, allowing for proper folding, surface expression, antigen presentation, etc. in the human subject. To protect against more than one strain of a viral infection, a combination vaccine can be administered that includes RNA (e.g., mRNA) encoding at least one antigenic polypeptide protein (or antigenic portion thereof) of a first virus and further includes RNA encoding at least one antigenic polypeptide protein (or antigenic portion thereof) of a second virus. RNA (e.g., mRNA) can be co-formulated, for example, in a single lipid nanoparticle (LNP) or can be formulated in separate LNPs for co-administration.
Combination Vaccines
[0347] The zoonotic disease vaccines, as provided herein, may include an RNA or multiple RNAs encoding two or more antigens of the same or different viral strains. Also provided herein are combination vaccines that include RNA encoding one or more zoonotic disease antigen(s) and one or more antigen(s) of a different organisms (e.g., bacterial and/or viral organism). Thus, the vaccines of the present disclosure may be combination vaccines that target one or more antigens of the same strain/species, or one or more antigens of different strains/species, e.g., antigens which induce immunity to organisms which are found in the same geographic areas where the risk of Lassa virus, Nipah virus, or betacoronavirus infection is high or organisms to which an individual is likely to be exposed to when exposed to the virus.
Flagellin Adjuvants
[0348] Flagellin is an approximately 500 amino acid monomeric protein that polymerizes to form the flagella associated with bacterial motion. Flagellin is expressed by a variety of flagellated bacteria (Salmonella typhimurium for example) as well as non-flagellated bacteria (such as Escherichia coli). Sensing of flagellin by cells of the innate immune system (dendritic cells, macrophages, etc.) is mediated by the Toll-like receptor 5 (TLR5) as well as by Nod-like receptors (NLRs) Ipaf and Naip5. TLRs and NLRs have been identified as playing a role in the activation of innate immune response and adaptive immune response. As such, flagellin provides an adjuvant effect in a vaccine.
[0349] The nucleotide and amino acid sequences encoding known flagellin polypeptides are publicly available in the NCBI GenBank database. The flagellin sequences from S. Typhimurium, H. Pylori, V. Cholera, S. marcesens, S. flexneri, T. Pallidum, L. pneumophila, B. burgdorferei, C. difficile, R. meliloti, A. tumefaciens, R. lupini, B. clarridgeiae, P. Mirabilis, B. subtilus, L. monocytogenes, P. aeruginosa, and E. coli, among others are known.
[0350] A flagellin polypeptide, as used herein, refers to a full length flagellin protein, immunogenic fragments thereof, and peptides having at least 50% sequence identify to a flagellin protein or immunogenic fragments thereof. Exemplary flagellin proteins include flagellin from Salmonella typhi (UniPro Entry number: Q56086), Salmonella typhimurium (AOAOC9DG09), Salmonella enteritidis (AOAOC9BAB7), and Salmonella choleraesuis (Q6V2X8). In some embodiments, the flagellin polypeptide has at least 60%, 70%, 75%, 80%, 90%, 95%, 97%, 98%, or 99% sequence identify to a flagellin protein or immunogenic fragments thereof.
[0351] In some embodiments, the flagellin polypeptide is an immunogenic fragment. An immunogenic fragment is a portion of a flagellin protein that provokes an immune response. In some embodiments, the immune response is a TLR5 immune response. An example of an immunogenic fragment is a flagellin protein in which all or a portion of a hinge region has been deleted or replaced with other amino acids. For example, an antigenic polypeptide may be inserted in the hinge region. Hinge regions are the hypervariable regions of a flagellin. Hinge regions of a flagellin are also referred to as "D3 domain or region, "propeller domain or region," "hypervariable domain or region" and "variable domain or region." "At least a portion of a hinge region," as used herein, refers to any part of the hinge region of the flagellin, or the entirety of the hinge region. In other embodiments an immunogenic fragment of flagellin is a 20, 25, 30, 35, or 40 amino acid C-terminal fragment of flagellin.
[0352] The flagellin monomer is formed by domains D0 through D3. D0 and D1, which form the stem, are composed of tandem long alpha helices and are highly conserved among different bacteria. The D1 domain includes several stretches of amino acids that are useful for TLR5 activation. The entire D1 domain or one or more of the active regions within the domain are immunogenic fragments of flagellin. Examples of immunogenic regions within the D1 domain include residues 88-114 and residues 411-431 (in Salmonella typhimurium FliC flagellin). Within the 13 amino acids in the 88-100 region, at least 6 substitutions are permitted between Salmonella flagellin and other flagellins that still preserve TLR5 activation. Thus, immunogenic fragments of flagellin include flagellin like sequences that activate TLR5 and contain a 13 amino acid motif that is 53% or more identical to the Salmonella sequence in 88-100 of FliC (LQRVRELAVQSAN; SEQ ID NO: 31).
Pharmaceutical Formulations
[0353] Provided herein are compositions (e.g., pharmaceutical compositions), methods, kits and reagents for prevention or treatment of zoonotic disease in humans and other mammals, for example. zoonotic disease RNA (e.g., mRNA) vaccines can be used as therapeutic or prophylactic agents. They may be used in medicine to prevent and/or treat infectious disease.
[0354] In some embodiments, a zoonotic disease vaccine containing RNA polynucleotides as described herein can be administered to a subject (e.g., a mammalian subject, such as a human subject), and the RNA polynucleotides are translated in vivo to produce an antigenic polypeptide (antigen).
[0355] An "effective amount" of a zoonotic disease vaccine is based, at least in part, on the target tissue, target cell type, means of administration, physical characteristics of the RNA (e.g., length, nucleotide composition, and/or extent of modified nucleosides), other components of the vaccine, and other determinants, such as age, body weight, height, sex and general health of the subject. Typically, an effective amount of a zoonotic disease vaccine provides an induced or boosted immune response as a function of antigen production in the cells of the subject. In some embodiments, an effective amount of the zoonotic disease RNA vaccine containing RNA polynucleotides having at least one chemical modifications are more efficient than a composition containing a corresponding unmodified polynucleotide encoding the same antigen or a peptide antigen. Increased antigen production may be demonstrated by increased cell transfection (the percentage of cells transfected with the RNA vaccine), increased protein translation and/or expression from the polynucleotide, decreased nucleic acid degradation (as demonstrated, for example, by increased duration of protein translation from a modified polynucleotide), or altered antigen specific immune response of the host cell.
[0356] The term "pharmaceutical composition" refers to the combination of an active agent with a carrier, inert or active, making the composition especially suitable for diagnostic or therapeutic use in vivo or ex vivo. A "pharmaceutically acceptable carrier," after administered to or upon a subject, does not cause undesirable physiological effects. The carrier in the pharmaceutical composition must be "acceptable" also in the sense that it is compatible with the active ingredient and can be capable of stabilizing it. One or more solubilizing agents can be utilized as pharmaceutical carriers for delivery of an active agent. Examples of a pharmaceutically acceptable carrier include, but are not limited to, biocompatible vehicles, adjuvants, additives, and diluents to achieve a composition usable as a dosage form. Examples of other carriers include colloidal silicon oxide, magnesium stearate, cellulose, and sodium lauryl sulfate. Additional suitable pharmaceutical carriers and diluents, as well as pharmaceutical necessities for their use, are described in Remington's Pharmaceutical Sciences.
[0357] In some embodiments, RNA vaccines (including polynucleotides and their encoded polypeptides) in accordance with the present disclosure may be used for treatment or prevention of zoonotic disease. Zoonotic disease RNA vaccines may be administered prophylactically or therapeutically as part of an active immunization scheme to healthy individuals or early in infection during the incubation phase or during active infection after onset of symptoms. In some embodiments, the amount of RNA vaccines of the present disclosure provided to a cell, a tissue or a subject may be an amount effective for immune prophylaxis.
[0358] Zoonotic disease RNA (e.g., mRNA) vaccines may be administered with other prophylactic or therapeutic compounds. As a non-limiting example, a prophylactic or therapeutic compound may be an adjuvant or a booster. As used herein, when referring to a prophylactic composition, such as a vaccine, the term "booster" refers to an extra administration of the prophylactic (vaccine) composition. A booster (or booster vaccine) may be given after an earlier administration of the prophylactic composition. The time of administration between the initial administration of the prophylactic composition and the booster may be, but is not limited to, 1 minute, 2 minutes, 3 minutes, 4 minutes, 5 minutes, 6 minutes, 7 minutes, 8 minutes, 9 minutes, 10 minutes, 15 minutes, 20 minutes 35 minutes, 40 minutes, 45 minutes, 50 minutes, 55 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 11 hours, 12 hours, 13 hours, 14 hours, 15 hours, 16 hours, 17 hours, 18 hours, 19 hours, 20 hours, 21 hours, 22 hours, 23 hours, 1 day, 36 hours, 2 days, 3 days, 4 days, 5 days, 6 days, 1 week, 10 days, 2 weeks, 3 weeks, 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months, 1 year, 18 months, 2 years, 3 years, 4 years, 5 years, 6 years, 7 years, 8 years, 9 years, 10 years, 11 years, 12 years, 13 years, 14 years, 15 years, 16 years, 17 years, 18 years, 19 years, 20 years, 25 years, 30 years, 35 years, 40 years, 45 years, 50 years, 55 years, 60 years, 65 years, 70 years, 75 years, 80 years, 85 years, 90 years, 95 years or more than 99 years. In exemplary embodiments, the time of administration between the initial administration of the prophylactic composition and the booster may be, but is not limited to, 1 week, 2 weeks, 3 weeks, 1 month, 2 months, 3 months, 6 months or 1 year.
[0359] In some embodiments, zoonotic disease RNA vaccines may be administered intramuscularly, intranasally or intradermally, similarly to the administration of inactivated vaccines known in the art.
[0360] The zoonotic disease RNA vaccines may be utilized in various settings depending on the prevalence of the infection or the degree or level of unmet medical need. As a non-limiting example, the RNA vaccines may be utilized to treat and/or prevent a variety of infectious disease. RNA vaccines have superior properties in that they produce much larger antibody titers, better neutralizing immunity, produce more durable immune responses, and/or produce responses earlier than commercially available vaccines.
[0361] Provided herein are pharmaceutical compositions including zoonotic disease RNA vaccines and RNA vaccine compositions and/or complexes optionally in combination with one or more pharmaceutically acceptable excipients.
[0362] Zoonotic disease RNA (e.g., mRNA) vaccines may be formulated or administered alone or in conjunction with one or more other components. For instance, zoonotic disease RNA vaccines (vaccine compositions) may comprise other components including, but not limited to, adjuvants.
[0363] In some embodiments, zoonotic disease RNA vaccines do not include an adjuvant (they are adjuvant free).
[0364] Zoonotic disease RNA (e.g., mRNA) vaccines may be formulated or administered in combination with one or more pharmaceutically-acceptable excipients. In some embodiments, vaccine compositions comprise at least one additional active substances, such as, for example, a therapeutically-active substance, a prophylactically-active substance, or a combination of both.
[0365] Vaccine compositions may be sterile, pyrogen-free or both sterile and pyrogen-free. General considerations in the formulation and/or manufacture of pharmaceutical agents, such as vaccine compositions, may be found, for example, in Remington: The Science and Practice of Pharmacy 21st ed., Lippincott Williams & Wilkins, 2005 (incorporated herein by reference in its entirety).
[0366] In some embodiments, zoonotic disease RNA vaccines are administered to humans, human patients or subjects. For the purposes of the present disclosure, the phrase "active ingredient" generally refers to the RNA vaccines or the polynucleotides contained therein, for example, RNA polynucleotides (e.g., mRNA polynucleotides) encoding antigens.
[0367] Formulations of the vaccine compositions described herein may be prepared by any method known or hereafter developed in the art of pharmacology. In general, such preparatory methods include the step of bringing the active ingredient (e.g., mRNA polynucleotide) into association with an excipient and/or one or more other accessory ingredients, and then, if necessary and/or desirable, dividing, shaping and/or packaging the product into a desired single- or multi-dose unit.
[0368] Relative amounts of the active ingredient, the pharmaceutically acceptable excipient, and/or any additional ingredients in a pharmaceutical composition in accordance with the disclosure will vary, depending upon the identity, size, and/or condition of the subject treated and further depending upon the route by which the composition is to be administered. By way of example, the composition may comprise between 0.1% and 100%, e.g., between 0.5 and 50%, between 1-30%, between 5-80%, at least 80% (w/w) active ingredient.
[0369] In some embodiments, zoonotic disease RNA vaccines are formulated using one or more excipients to: (1) increase stability; (2) increase cell transfection; (3) permit the sustained or delayed release (e.g., from a depot formulation); (4) alter the biodistribution (e.g., target to specific tissues or cell types); (5) increase the translation of encoded protein in vivo; and/or (6) alter the release profile of encoded protein (antigen) in vivo. In addition to traditional excipients such as any and all solvents, dispersion media, diluents, or other liquid vehicles, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, excipients can include, without limitation, lipidoids, liposomes, lipid nanoparticles, polymers, lipoplexes, core-shell nanoparticles, peptides, proteins, cells transfected with zoonotic disease RNA vaccines (e.g., for transplantation into a subject), hyaluronidase, nanoparticle mimics and combinations thereof.
Dosing/Administration
[0370] Provided herein are compositions (e.g., pharmaceutical compositions), methods, kits and reagents for prevention and/or treatment of zoonotic disease in humans and other mammals. zoonotic disease RNA vaccines can be used as therapeutic or prophylactic agents. In some aspects, the RNA vaccines of the disclosure are used to provide prophylactic protection from zoonotic disease. In some aspects, the RNA vaccines of the disclosure are used to treat a zoonotic disease infection. In some embodiments, the zoonotic disease vaccines of the present disclosure are used in the priming of immune effector cells, for example, to activate peripheral blood mononuclear cells (PBMCs) ex vivo, which are then infused (re-infused) into a subject.
[0371] A subject may be any mammal, including non-human primate and human subjects. Typically, a subject is a human subject.
[0372] In some embodiments, the zoonotic disease vaccines are administered to a subject (e.g., a mammalian subject, such as a human subject) in an effective amount to induce an antigen-specific immune response. The RNA encoding the zoonotic disease antigen is expressed and translated in vivo to produce the antigen, which then stimulates an immune response in the subject.
[0373] Prophylactic protection from zoonotic disease can be achieved following administration of a zoonotic disease RNA vaccine of the present disclosure. Vaccines can be administered once, twice, three times, four times or more but it is likely sufficient to administer the vaccine once (optionally followed by a single booster). It is possible, although less desirable, to administer the vaccine to an infected individual to achieve a therapeutic response. Dosing may need to be adjusted accordingly.
[0374] A method of eliciting an immune response in a subject against zoonotic disease is provided in aspects of the present disclosure. The method involves administering to the subject a zoonotic disease RNA vaccine comprising at least one RNA (e.g., mRNA) having an open reading frame encoding at least one zoonotic disease antigen, thereby inducing in the subject an immune response specific to zoonotic disease antigen, wherein anti-antigen antibody titer in the subject is increased following vaccination relative to anti-antigen antibody titer in a subject vaccinated with a prophylactically effective dose of a traditional vaccine against the zoonotic disease. An "anti-antigen antibody" is a serum antibody the binds specifically to the antigen.
[0375] A prophylactically effective dose is an effective dose that prevents infection with the virus at a clinically acceptable level. In some embodiments, the effective dose is a dose listed in a package insert for the vaccine. A traditional vaccine, as used herein, refers to a vaccine other than the mRNA vaccines of the present disclosure. For instance, a traditional vaccine includes, but is not limited, to live microorganism vaccines, killed microorganism vaccines, subunit vaccines, protein antigen vaccines, DNA vaccines, virus like particle (VLP) vaccines, etc. In exemplary embodiments, a traditional vaccine is a vaccine that has achieved regulatory approval and/or is registered by a national drug regulatory body, for example the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA).
[0376] In some embodiments, the anti-antigen antibody titer in the subject is increased 1 log to 10 log following vaccination relative to anti-antigen antibody titer in a subject vaccinated with a prophylactically effective dose of a traditional vaccine against the zoonotic disease or an unvaccinated subject. In some embodiments, the anti-antigen antibody titer in the subject is increased 1 log, 2 log, 3 log, 4 log, 5 log, or 10 log following vaccination relative to anti-antigen antibody titer in a subject vaccinated with a prophylactically effective dose of a traditional vaccine against the zoonotic disease or an unvaccinated subject.
[0377] A method of eliciting an immune response in a subject against a zoonotic disease is provided in other aspects of the disclosure. The method involves administering to the subject a zoonotic disease RNA vaccine comprising at least one RNA polynucleotide having an open reading frame encoding at least one zoonotic disease antigen, thereby inducing in the subject an immune response specific to zoonotic disease antigen, wherein the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine against the zoonotic disease at 2 times to 100 times the dosage level relative to the RNA vaccine.
[0378] In some embodiments, the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine at twice the dosage level relative to the zoonotic disease RNA vaccine. In some embodiments, the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine at three times the dosage level relative to the zoonotic disease RNA vaccine. In some embodiments, the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine at 4 times, 5 times, 10 times, 50 times, or 100 times the dosage level relative to the zoonotic disease RNA vaccine. In some embodiments, the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine at 10 times to 1000 times the dosage level relative to the zoonotic disease RNA vaccine. In some embodiments, the immune response in the subject is equivalent to an immune response in a subject vaccinated with a traditional vaccine at 100 times to 1000 times the dosage level relative to the zoonotic disease RNA vaccine.
[0379] In other embodiments, the immune response is assessed by determining [protein] antibody titer in the subject. In other embodiments, the ability of serum or antibody from an immunized subject is tested for its ability to neutralize viral uptake or reduce zoonotic disease transformation of human B lymphocytes. In other embodiments, the ability to promote a robust T cell response(s) is measured using art recognized techniques.
[0380] Other aspects the disclosure provide methods of eliciting an immune response in a subject against a zoonotic disease by administering to the subject a zoonotic disease RNA vaccine comprising at least one RNA polynucleotide having an open reading frame encoding at least one zoonotic disease antigen, thereby inducing in the subject an immune response specific to zoonotic disease antigen, wherein the immune response in the subject is induced 2 days to 10 weeks earlier relative to an immune response induced in a subject vaccinated with a prophylactically effective dose of a traditional vaccine against the zoonotic disease. In some embodiments, the immune response in the subject is induced in a subject vaccinated with a prophylactically effective dose of a traditional vaccine at 2 times to 100 times the dosage level relative to the RNA vaccine.
[0381] In some embodiments, the immune response in the subject is induced 2 days, 3 days, 1 week, 2 weeks, 3 weeks, 5 weeks, or 10 weeks earlier relative to an immune response induced in a subject vaccinated with a prophylactically effective dose of a traditional vaccine.
[0382] Also provided herein are methods of eliciting an immune response in a subject against a zoonotic disease by administering to the subject a zoonotic disease RNA vaccine having an open reading frame encoding a first antigen, wherein the RNA polynucleotide does not include a stabilization element, and wherein an adjuvant is not co-formulated or co-administered with the vaccine.
[0383] Zoonotic disease RNA (e.g., mRNA) vaccines may be administered by any route which results in a therapeutically effective outcome. These include, but are not limited, to intradermal, intramuscular, intranasal, and/or subcutaneous administration. The present disclosure provides methods comprising administering RNA vaccines to a subject in need thereof. The exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the disease, the particular composition, its mode of administration, its mode of activity, and the like. zoonotic disease RNA (e.g., mRNA) vaccines compositions are typically formulated in dosage unit form for ease of administration and uniformity of dosage. It will be understood, however, that the total daily usage of zoonotic disease RNA (e.g., mRNA) vaccines compositions may be decided by the attending physician within the scope of sound medical judgment. The specific therapeutically effective, prophylactically effective, or appropriate imaging dose level for any particular patient will depend upon a variety of factors including the disorder being treated and the severity of the disorder; the activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed; and like factors well known in the medical arts.
[0384] The effective amount of a zoonotic disease vaccine, as provided herein, may be as low as 20 rig, administered for example as a single dose or as two 10 .mu.g doses. In some embodiments, the effective amount is a total dose of 20 .mu.g-200 .mu.g. For example, the effective amount may be a total dose of 20 .mu.g, 25 .mu.g, 30 .mu.g, 35 .mu.g, 40 .mu.g, 45 .mu.g, 50 .mu.g, 55 .mu.g, 60 .mu.g, 65 .mu.g, 70 .mu.g, 75 .mu.g, 80 .mu.g, 85 .mu.g, 90 .mu.g, 95 .mu.g, 100 .mu.g, 110 .mu.g, 120 .mu.g, 130 .mu.g, 140 .mu.g, 150 .mu.g, 160 .mu.g, 170 .mu.g, 180 .mu.g, 190 .mu.g or 200 .mu.g. In some embodiments, the effective amount is a total dose of 25 .mu.g-200 .mu.g. In some embodiments, the effective amount is a total dose of 50 .mu.g-200 .mu.g.
[0385] In some embodiments, zoonotic disease RNA (e.g., mRNA) vaccines compositions may be administered at dosage levels sufficient to deliver 0.0001 mg/kg to 100 mg/kg, 0.001 mg/kg to 0.05 mg/kg, 0.005 mg/kg to 0.05 mg/kg, 0.001 mg/kg to 0.005 mg/kg, 0.05 mg/kg to 0.5 mg/kg, 0.01 mg/kg to 50 mg/kg, 0.1 mg/kg to 40 mg/kg, 0.5 mg/kg to 30 mg/kg, 0.01 mg/kg to 10 mg/kg, 0.1 mg/kg to 10 mg/kg, or 1 mg/kg to 25 mg/kg, of subject body weight per day, one or more times a day, per week, per month, etc. to obtain the desired therapeutic, diagnostic, prophylactic, or imaging effect (see e.g., the range of unit doses described in International Publication No. WO2013/078199, herein incorporated by reference in its entirety). The desired dosage may be delivered three times a day, two times a day, once a day, every other day, every third day, every week, every two weeks, every three weeks, every four weeks, every 2 months, every three months, every 6 months, etc. In certain embodiments, the desired dosage may be delivered using multiple administrations (e.g., two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, or more administrations). When multiple administrations are employed, split dosing regimens such as those described herein may be used. In exemplary embodiments, zoonotic disease RNA (e.g., mRNA) vaccines compositions may be administered at dosage levels sufficient to deliver 0.0005 mg/kg to 0.01 mg/kg, e.g., about 0.0005 mg/kg to about 0.0075 mg/kg, e.g., about 0.0005 mg/kg, about 0.001 mg/kg, about 0.002 mg/kg, about 0.003 mg/kg, about 0.004 mg/kg or about 0.005 mg/kg.
[0386] In some embodiments, zoonotic disease RNA (e.g., mRNA) vaccine compositions may be administered once or twice (or more) at dosage levels sufficient to deliver 0.025 mg/kg to 0.250 mg/kg, 0.025 mg/kg to 0.500 mg/kg, 0.025 mg/kg to 0.750 mg/kg, or 0.025 mg/kg to 1.0 mg/kg.
[0387] In some embodiments, zoonotic disease RNA (e.g., mRNA) vaccine compositions may be administered twice (e.g., Day 0 and Day 7, Day 0 and Day 14, Day 0 and Day 21, Day 0 and Day 28, Day 0 and Day 60, Day 0 and Day 90, Day 0 and Day 120, Day 0 and Day 150, Day 0 and Day 180, Day 0 and 3 months later, Day 0 and 6 months later, Day 0 and 9 months later, Day 0 and 12 months later, Day 0 and 18 months later, Day 0 and 2 years later, Day 0 and 5 years later, or Day 0 and 10 years later) at a total dose of or at dosage levels sufficient to deliver a total dose of 0.0100 mg, 0.025 mg, 0.050 mg, 0.075 mg, 0.100 mg, 0.125 mg, 0.150 mg, 0.175 mg, 0.200 mg, 0.225 mg, 0.250 mg, 0.275 mg, 0.300 mg, 0.325 mg, 0.350 mg, 0.375 mg, 0.400 mg, 0.425 mg, 0.450 mg, 0.475 mg, 0.500 mg, 0.525 mg, 0.550 mg, 0.575 mg, 0.600 mg, 0.625 mg, 0.650 mg, 0.675 mg, 0.700 mg, 0.725 mg, 0.750 mg, 0.775 mg, 0.800 mg, 0.825 mg, 0.850 mg, 0.875 mg, 0.900 mg, 0.925 mg, 0.950 mg, 0.975 mg, or 1.0 mg. Higher and lower dosages and frequency of administration are encompassed by the present disclosure. For example, a zoonotic disease RNA (e.g., mRNA) vaccine composition may be administered three or four times.
[0388] In some embodiments, zoonotic disease RNA (e.g., mRNA) vaccine compositions may be administered twice (e.g., Day 0 and Day 7, Day 0 and Day 14, Day 0 and Day 21, Day 0 and Day 28, Day 0 and Day 60, Day 0 and Day 90, Day 0 and Day 120, Day 0 and Day 150, Day 0 and Day 180, Day 0 and 3 months later, Day 0 and 6 months later, Day 0 and 9 months later, Day 0 and 12 months later, Day 0 and 18 months later, Day 0 and 2 years later, Day 0 and 5 years later, or Day 0 and 10 years later) at a total dose of or at dosage levels sufficient to deliver a total dose of 0.010 mg, 0.025 mg, 0.100 mg or 0.400 mg.
[0389] In some embodiments, the zoonotic disease RNA (e.g., mRNA) vaccine for use in a method of vaccinating a subject is administered the subject a single dosage of between 10 .mu.g/kg and 400 .mu.g/kg of the nucleic acid vaccine in an effective amount to vaccinate the subject. In some embodiments, the RNA vaccine for use in a method of vaccinating a subject is administered the subject a single dosage of between 10 .mu.g and 400 .mu.g of the nucleic acid vaccine in an effective amount to vaccinate the subject. In some embodiments, a zoonotic disease RNA (e.g., mRNA) vaccine for use in a method of vaccinating a subject is administered to the subject as a single dosage of 25-1000 .mu.g (e.g., a single dosage of mRNA encoding a zoonotic disease antigen). In some embodiments, a zoonotic disease RNA vaccine is administered to the subject as a single dosage of 25, 50, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, 800, 850, 900, 950 or 1000 .mu.g. For example, a zoonotic disease RNA vaccine may be administered to a subject as a single dose of 25-100, 25-500, 50-100, 50-500, 50-1000, 100-500, 100-1000, 250-500, 250-1000, or 500-1000 .mu.g. In some embodiments, a zoonotic disease RNA (e.g., mRNA) vaccine for use in a method of vaccinating a subject is administered to the subject as two dosages, the combination of which equals 25-1000 .mu.g of the zoonotic disease RNA (e.g., mRNA) vaccine.
[0390] AN zoonotic disease RNA (e.g., mRNA) vaccine pharmaceutical composition described herein can be formulated into a dosage form described herein, such as an intranasal, intratracheal, or injectable (e.g., intravenous, intraocular, intravitreal, intramuscular, intradermal, intracardiac, intraperitoneal, and subcutaneous).
Vaccine Efficacy
[0391] Some aspects of the present disclosure provide formulations of the zoonotic disease RNA (e.g., mRNA) vaccine, wherein the zoonotic disease RNA vaccine is formulated in an effective amount to produce an antigen specific immune response in a subject (e.g., production of antibodies specific to an anti-zoonotic disease antigen). "An effective amount" is a dose of an zoonotic disease RNA (e.g., mRNA) vaccine effective to produce an antigen-specific immune response. Also provided herein are methods of inducing an antigen-specific immune response in a subject.
[0392] As used herein, an immune response to a vaccine or LNP of the present disclosure is the development in a subject of a humoral and/or a cellular immune response to a (one or more) zoonotic disease protein(s) present in the vaccine. For purposes of the present disclosure, a "humoral" immune response refers to an immune response mediated by antibody molecules, including, e.g., secretory (IgA) or IgG molecules, while a "cellular" immune response is one mediated by T-lymphocytes (e.g., CD4+ helper and/or CD8+ T cells (e.g., CTLs) and/or other white blood cells. One important aspect of cellular immunity involves an antigen-specific response by cytolytic T-cells (CTLs). CTLs have specificity for peptide antigens that are presented in association with proteins encoded by the major histocompatibility complex (MHC) and expressed on the surfaces of cells. CTLs help induce and promote the destruction of intracellular microbes or the lysis of cells infected with such microbes. Another aspect of cellular immunity involves and antigen-specific response by helper T-cells. Helper T-cells act to help stimulate the function, and focus the activity nonspecific effector cells against cells displaying peptide antigens in association with MHC molecules on their surface. A cellular immune response also leads to the production of cytokines, chemokines, and other such molecules produced by activated T-cells and/or other white blood cells including those derived from CD4+ and CD8+ T-cells.
[0393] In some embodiments, the antigen-specific immune response is characterized by measuring an anti-zoonotic disease antigen antibody titer produced in a subject administered an zoonotic disease RNA (e.g., mRNA) vaccine as provided herein. An antibody titer is a measurement of the amount of antibodies within a subject, for example, antibodies that are specific to a particular antigen (e.g., an anti-zoonotic disease antigen) or epitope of an antigen. Antibody titer is typically expressed as the inverse of the greatest dilution that provides a positive result. Enzyme-linked immunosorbent assay (ELISA) is a common assay for determining antibody titers, for example.
[0394] In some embodiments, an antibody titer is used to assess whether a subject has had an infection or to determine whether immunizations are required. In some embodiments, an antibody titer is used to determine the strength of an autoimmune response, to determine whether a booster immunization is needed, to determine whether a previous vaccine was effective, and to identify any recent or prior infections. In accordance with the present disclosure, an antibody titer may be used to determine the strength of an immune response induced in a subject by the zoonotic disease RNA (e.g., mRNA) vaccine.
[0395] In some embodiments, an anti-zoonotic disease antigen antibody titer produced in a subject is increased by at least 1 log relative to a control. For example, anti-zoonotic disease antigen antibody titer produced in a subject may be increased by at least 1.5, at least 2, at least 2.5, or at least 3 log relative to a control. In some embodiments, the anti-zoonotic disease antigen antibody titer produced in the subject is increased by 1, 1.5, 2, 2.5 or 3 log relative to a control. In some embodiments, the anti-zoonotic disease antigen antibody titer produced in the subject is increased by 1-3 log relative to a control. For example, the anti-zoonotic disease antigen antibody titer produced in a subject may be increased by 1-1.5, 1-2, 1-2.5, 1-3, 1.5-2, 1.5-2.5, 1.5-3, 2-2.5, 2-3, or 2.5-3 log relative to a control.
[0396] In some embodiments, the anti-zoonotic disease antigen antibody titer produced in a subject is increased at least 2 times relative to a control. For example, the anti-zoonotic disease antigen antibody titer produced in a subject may be increased at least 3 times, at least 4 times, at least 5 times, at least 6 times, at least 7 times, at least 8 times, at least 9 times, or at least 10 times relative to a control. In some embodiments, the anti-zoonotic disease antigen antibody titer produced in the subject is increased 2, 3, 4, 5, 6, 7, 8, 9, or 10 times relative to a control. In some embodiments, the anti-zoonotic disease antigen antibody titer produced in a subject is increased 2-10 times relative to a control. For example, the anti-zoonotic disease antigen antibody titer produced in a subject may be increased 2-10, 2-9, 2-8, 2-7, 2-6, 2-5, 2-4, 2-3, 3-10, 3-9, 3-8, 3-7, 3-6, 3-5, 3-4, 4-10, 4-9, 4-8, 4-7, 4-6, 4-5, 5-10, 5-9, 5-8, 5-7, 5-6, 6-10, 6-9, 6-8, 6-7, 7-10, 7-9, 7-8, 8-10, 8-9, or 9-10 times relative to a control.
[0397] A control, in some embodiments, is the anti-zoonotic disease antigen antibody titer produced in a subject who has not been administered an zoonotic disease RNA (e.g., mRNA) vaccine. In some embodiments, a control is an anti-zoonotic disease antigen antibody titer produced in a subject administered a recombinant or purified zoonotic disease protein vaccine. Recombinant protein vaccines typically include protein antigens that either have been produced in a heterologous expression system (e.g., bacteria or yeast) or purified from large amounts of the pathogenic organism.
[0398] In some embodiments, the ability of an zoonotic disease vaccine to be effective is measured in a murine model. For example, the zoonotic disease vaccines may be administered to a murine model and the murine model assayed for induction of neutralizing antibody titers. Viral challenge studies may also be used to assess the efficacy of a vaccine of the present disclosure. For example, the zoonotic disease vaccines may be administered to a murine model, the murine model challenged with zoonotic disease antigen, and the murine model assayed for survival and/or immune response (e.g., neutralizing antibody response, T cell response (e.g., cytokine response)).
[0399] In some embodiments, an effective amount of an zoonotic disease RNA (e.g., mRNA) vaccine is a dose that is reduced compared to the standard of care dose of a recombinant zoonotic disease protein vaccine. A "standard of care," as provided herein, refers to a medical or psychological treatment guideline and can be general or specific. "Standard of care" specifies appropriate treatment based on scientific evidence and collaboration between medical professionals involved in the treatment of a given condition. It is the diagnostic and treatment process that a physician/clinician should follow for a certain type of patient, illness or clinical circumstance. A "standard of care dose," as provided herein, refers to the dose of a recombinant or purified zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or an zoonotic disease VLP vaccine, that a physician/clinician or other medical professional would administer to a subject to treat or prevent a zoonotic disease, or a zoonotic disease-related condition, while following the standard of care guideline for treating or preventing a zoonotic disease, or a zoonotic disease related condition.
[0400] In some embodiments, the anti-zoonotic disease antigen antibody titer produced in a subject administered an effective amount of a zoonotic disease RNA vaccine is equivalent to an anti-zoonotic disease antigen antibody titer produced in a control subject administered a standard of care dose of a recombinant or purified zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or a zoonotic disease VLP vaccine.
[0401] In some embodiments, an effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a dose equivalent to an at least 2-fold reduction in a standard of care dose of a recombinant or purified zoonotic disease protein vaccine. For example, an effective amount of a zoonotic disease RNA vaccine may be a dose equivalent to an at least 3-fold, at least 4-fold, at least 5-fold, at least 6-fold, at least 7-fold, at least 8-fold, at least 9-fold, or at least 10-fold reduction in a standard of care dose of a recombinant or purified zoonotic disease protein vaccine. In some embodiments, an effective amount of a zoonotic disease RNA vaccine is a dose equivalent to an at least at least 100-fold, at least 500-fold, or at least 1000-fold reduction in a standard of care dose of a recombinant or purified zoonotic disease protein vaccine. In some embodiments, an effective amount of a zoonotic disease RNA vaccine is a dose equivalent to a 2-, 3-, 4-, 5-, 6-, 7-, 8-, 9-, 10-, 20-, 50-, 100-, 250-, 500-, or 1000-fold reduction in a standard of care dose of a recombinant or purified zoonotic disease protein vaccine. In some embodiments, the anti-zoonotic disease antigen antibody titer produced in a subject administered an effective amount of a zoonotic disease RNA vaccine is equivalent to an anti-zoonotic disease antigen antibody titer produced in a control subject administered the standard of care dose of a recombinant or protein zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or a zoonotic disease VLP vaccine. In some embodiments, an effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a dose equivalent to a 2-fold to 1000-fold (e.g., 2-fold to 100-fold, 10-fold to 1000-fold) reduction in the standard of care dose of a recombinant or purified zoonotic disease protein vaccine, wherein the anti-zoonotic disease antigen antibody titer produced in the subject is equivalent to an anti-zoonotic disease antigen antibody titer produced in a control subject administered the standard of care dose of a recombinant or purified zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or a zoonotic disease VLP vaccine.
[0402] In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a dose equivalent to a 2 to 1000-, 2 to 900-, 2 to 800-, 2 to 700-, 2 to 600-, 2 to 500-, 2 to 400-, 2 to 300-, 2 to 200-, 2 to 100-, 2 to 90-, 2 to 80-, 2 to 70-, 2 to 60-, 2 to 50-, 2 to 40-, 2 to 30-, 2 to 20-, 2 to 10-, 2 to 9-, 2 to 8-, 2 to 7-, 2 to 6-, 2 to 5-, 2 to 4-, 2 to 3-, 3 to 1000-, 3 to 900-, 3 to 800-, 3 to 700-, 3 to 600-, 3 to 500-, 3 to 400-, 3 to 3 to 00-, 3 to 200-, 3 to 100-, 3 to 90-, 3 to 80-, 3 to 70-, 3 to 60-, 3 to 50-, 3 to 40-, 3 to 30-, 3 to 20-, 3 to 10-, 3 to 9-, 3 to 8-, 3 to 7-, 3 to 6-, 3 to 5-, 3 to 4-, 4 to 1000-, 4 to 900-, 4 to 800-, 4 to 700-, 4 to 600-, 4 to 500-, 4 to 400-, 4 to 300-, 4 to 200-, 4 to 100-, 4 to 90-, 4 to 80-, 4 to 70-, 4 to 60-, 4 to 50, 4 to 40-, 4 to 30-, 4 to 20-, 4 to 10-, 4 to 9-, 4 to 8-, 4 to 7-, 4 to 6-, 4 to 5-, 4 to 4-, 5 to 1000-, 5 to 900-, 5 to 800-, 5 to 700-, 5 to 600-, 5 to 500-, 5 to 400-, 5 to 300-, 5 to 200-, 5 to 100-, 5 to 90-, 5 to 80-, 5 to 70-, 5 to 60-, 5 to 50-, 5 to 40-, 5 to 30-, 5 to 20-, 5 to 10-, 5 to 9-, 5 to 8, 5 to 7-, 5 to 6-, 6 to 1000-, 6 to 900-, 6 to 800-, 6 to 700-, 6 to 600-, 6 to 500-, 6 to 400-, 6 to 300-, 6 to 200-, 6 to 100-, 6 to 90-, 6 to 80-, 6 to 70-, 6 to 60-, 6 to 50-, 6 to 40-, 6 to 30-, 6 to 20-, 6 to 10-, 6 to 9- , 6 to 8-, 6 to 7-, 7 to 1000-, 7 to 900-, 7 to 800-, 7 to 700-, 7 to 600-, 7 to 500-, 7 to 400-, 7 to 300-, 7 to 200-, 7 to 100-, 7 to 90-, 7 to 80-, 7 to 70-, 7 to 60-, 7 to 50-, 7 to 40-, 7 to 30-, 7 to 20-, 7 to 10-, 7 to 9-, 7 to 8-, 8 to 1000-, 8 to 900-, 8 to 800-, 8 to 700-, 8 to 600-, 8 to 500-, 8 to 400-, 8 to 300-, 8 to 200-, 8 to 100-, 8 to 90-, 8 to 80-, 8 to 70-, 8 to 60-, 8 to 50-, 8 to 40-, 8 to 30-, 8 to 20-, 8 to 10-, 8 to 9-, 9 to 1000-, 9 to 900-, 9 to 800-, 9 to 700-, 9 to 600-, 9 to 500-, 9 to 400-, 9 to 300-, 9 to 200-, 9 to 100-, 9 to 90-, 9 to 80-, 9 to 70-, 9 to 60-, 9 to 50-, 9 to 40-, 9 to 30-, 9 to 20-, 9 to 10-, 10 to 1000-, 10 to 900-, 10 to 800-, 10 to 700-, 10 to 600-, 10 to 500-, 10 to 400-, 10 to 300-, 10 to 200-, 10 to 100-, 10 to 90-, 10 to 80-, 10 to 70-, 10 to 60-, 10 to 50-, 10 to 40-, 10 to 30-, 10 to 20-, 20 to 1000-, 20 to 900-, 20 to 800-, 20 to 700-, 20 to 600-, 20 to 500-, 20 to 400-, 20 to 300-, 20 to 200-, 20 to 100-, 20 to 90-, 20 to 80-, 20 to 70-, 20 to 60-, 20 to 50-, 20 to 40-, 20 to 30-, 30 to 1000-, 30 to 900-, 30 to 800-, 30 to 700-, 30 to 600-, 30 to 500-, 30 to 400-, 30 to 300-, 30 to 200-, 30 to 100-, 30 to 90-, 30 to 80-, 30 to 70-, 30 to 60-, 30 to 50-, 30 to 40-, 40 to 1000-, 40 to 900-, 40 to 800-, 40 to 700-, 40 to 600-, 40 to 500-, 40 to 400-, 40 to 300-, 40 to 200-, 40 to 100-, 40 to 90-, 40 to 80-, 40 to 70-, 40 to 60-, 40 to 50-, 50 to 1000-, 50 to 900-, 50 to 800-, 50 to 700-, 50 to 600-, 50 to 500-, 50 to 400-, 50 to 300-, 50 to 200-, 50 to 100-, 50 to 90-, 50 to 80-, 50 to 70-, 50 to 60-, 60 to 1000-, 60 to 900-, 60 to 800-, 60 to 700-, 60 to 600-, 60 to 500-, 60 to 400-, 60 to 300-, 60 to 200-, 60 to 100-, 60 to 90-, 60 to 80-, 60 to 70-, 70 to 1000-, 70 to 900-, 70 to 800-, 70 to 700-, 70 to 600-, 70 to 500-, 70 to 400-, 70 to 300-, 70 to 200-, 70 to 100-, 70 to 90-, 70 to 80-, 80 to 1000-, 80 to 900-, 80 to 800-, 80 to 700-, 80 to 600-, 80 to 500-, 80 to 400-, 80 to 300-, 80 to 200-, 80 to 100-, 80 to 90-, 90 to 1000-, 90 to 900-, 90 to 800-, 90 to 700-, 90 to 600-, 90 to 500-, 90 to 400-, 90 to 300-, 90 to 200-, 90 to 100-, 100 to 1000-, 100 to 900-, 100 to 800-, 100 to 700-, 100 to 600-, 100 to 500-, 100 to 400-, 100 to 300-, 100 to 200-, 200 to 1000-, 200 to 900-, 200 to 800-, 200 to 700-, 200 to 600-, 200 to 500-, 200 to 400-, 200 to 300-, 300 to 1000-, 300 to 900-, 300 to 800-, 300 to 700-, 300 to 600-, 300 to 500-, 300 to 400-, 400 to 1000-, 400 to 900-, 400 to 800-, 400 to 700-, 400 to 600-, 400 to 500-, 500 to 1000-, 500 to 900-, 500 to 800-, 500 to 700-, 500 to 600-, 600 to 1000-, 600 to 900-, 600 to 800-, 600 to 700-, 700 to 1000-, 700 to 900-, 700 to 800-, 800 to 1000-, 800 to 900-, or 900 to 1000-fold reduction in the standard of care dose of a recombinant zoonotic disease protein vaccine. In some embodiments, such as the foregoing, the anti-zoonotic disease antigen antibody titer produced in the subject is equivalent to an anti-zoonotic disease antigen antibody titer produced in a control subject administered the standard of care dose of a recombinant or purified zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or a zoonotic disease VLP vaccine. In some embodiments, the effective amount is a dose equivalent to (or equivalent to an at least) 2-, 3-, 4-, 5-, 6-, 7-, 8-, 9-, 10-, 20-, 30-, 40-, 50-, 60-, 70-, 80-, 90-, 100-, 110-, 120-, 130-, 140-, 150-, 160-, 170-, 1280-, 190-, 200-, 210-, 220-, 230-, 240-, 250-, 260-, 270-, 280-, 290-, 300-, 310-, 320-, 330-, 340-, 350-, 360-, 370-, 380-, 390-, 400-, 410-, 420-, 430-, 440-, 450-, 4360-, 470-, 480-, 490-, 500-, 510-, 520-, 530-, 540-, 550-, 560-, 5760-, 580-, 590-, 600-, 610-, 620-, 630-, 640-, 650-, 660-, 670-, 680-, 690-, 700-, 710-, 720-, 730-, 740-, 750-, 760-, 770-, 780-, 790-, 800-, 810-, 820-, 830-, 840-, 850-, 860-, 870-, 880-, 890-, 900-, 910-, 920-, 930-, 940-, 950-, 960-, 970-, 980-, 990-, or 1000-fold reduction in the standard of care dose of a recombinant zoonotic disease protein vaccine. In some embodiments, such as the foregoing, an anti-zoonotic disease antigen antibody titer produced in the subject is equivalent to an anti-zoonotic disease antigen antibody titer produced in a control subject administered the standard of care dose of a recombinant or purified zoonotic disease protein vaccine, or a live attenuated or inactivated zoonotic disease vaccine, or a zoonotic disease VLP vaccine.
[0403] In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a total dose of 50-1000 .mu.g. In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a total dose of 50-1000, 50-900, 50-800, 50-700, 50-600, 50-500, 50-400, 50-300, 50-200, 50-100, 50-90, 50-80, 50-70, 50-60, 60-1000, 60-900, 60-800, 60-700, 60-600, 60-500, 60-400, 60-300, 60-200, 60-100, 60-90, 60-80, 60-70, 70-1000, 70-900, 70-800, 70-700, 70-600, 70-500, 70-400, 70-300, 70-200, 70-100, 70-90, 70-80, 80-1000, 80-900, 80-800, 80-700, 80-600, 80-500, 80-400, 80-300, 80-200, 80-100, 80-90, 90-1000, 90-900, 90-800, 90-700, 90-600, 90-500, 90-400, 90-300, 90-200, 90-100, 100-1000, 100-900, 100-800, 100-700, 100-600, 100-500, 100-400, 100-300, 100-200, 200-1000, 200-900, 200-800, 200-700, 200-600, 200-500, 200-400, 200-300, 300-1000, 300-900, 300-800, 300-700, 300-600, 300-500, 300-400, 400-1000, 400-900, 400-800, 400-700, 400-600, 400-500, 500-1000, 500-900, 500-800, 500-700, 500-600, 600-1000, 600-900, 600-900, 600-700, 700-1000, 700-900, 700-800, 800-1000, 800-900, or 900-1000 .mu.g. In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a total dose of 50, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750, 800, 850, 900, 950 or 1000 .mu.g. In some embodiments, the effective amount is a dose of 25-500 .mu.g administered to the subject a total of two times. In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a dose of 25-500, 25-400, 25-300, 25-200, 25-100, 25-50, 50-500, 50-400, 50-300, 50-200, 50-100, 100-500, 100-400, 100-300, 100-200, 150-500, 150-400, 150-300, 150-200, 200-500, 200-400, 200-300, 250-500, 250-400, 250-300, 300-500, 300-400, 350-500, 350-400, 400-500 or 450-500 .mu.g administered to the subject a total of two times. In some embodiments, the effective amount of a zoonotic disease RNA (e.g., mRNA) vaccine is a total dose of 25, 50, 100, 150, 200, 250, 300, 350, 400, 450, or 500 .mu.g administered to the subject a total of two times.
[0404] Vaccine efficacy may be assessed using standard analyses (see, e.g., Weinberg et al., J Infect Dis. 2010 Jun. 1; 201(11):1607-10). For example, vaccine efficacy may be measured by double-blind, randomized, clinical controlled trials. Vaccine efficacy may be expressed as a proportionate reduction in disease attack rate (AR) between the unvaccinated (ARU) and vaccinated (ARV) study cohorts and can be calculated from the relative risk (RR) of disease among the vaccinated group with use of the following formulas:
Efficacy=(ARU-ARV)/ARU.times.100; and
Efficacy=(1-RR).times.100.
[0405] Likewise, vaccine effectiveness may be assessed using standard analyses (see, e.g., Weinberg et al., J Infect Dis. 2010 Jun. 1; 201(11):1607-10). Vaccine effectiveness is an assessment of how a vaccine (which may have already proven to have high vaccine efficacy) reduces disease in a population. This measure can assess the net balance of benefits and adverse effects of a vaccination program, not just the vaccine itself, under natural field conditions rather than in a controlled clinical trial. Vaccine effectiveness is proportional to vaccine efficacy (potency) but is also affected by how well target groups in the population are immunized, as well as by other non-vaccine-related factors that influence the `real-world` outcomes of hospitalizations, ambulatory visits, or costs. For example, a retrospective case control analysis may be used, in which the rates of vaccination among a set of infected cases and appropriate controls are compared. Vaccine effectiveness may be expressed as a rate difference, with use of the odds ratio (OR) for developing infection despite vaccination:
Effectiveness=(1-OR).times.100.
[0406] In some embodiments, efficacy of the zoonotic disease vaccine is at least 60% relative to unvaccinated control subjects. For example, efficacy of the zoonotic disease vaccine may be at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least 95%, at least 98%, or 100% relative to unvaccinated control subjects.
[0407] Sterilizing Immunity.
[0408] Sterilizing immunity refers to a unique immune status that prevents effective pathogen infection into the host. In some embodiments, the effective amount of a zoonotic disease vaccine of the present disclosure is sufficient to provide sterilizing immunity in the subject for at least 1 year. For example, the effective amount of a zoonotic disease vaccine of the present disclosure is sufficient to provide sterilizing immunity in the subject for at least 2 years, at least 3 years, at least 4 years, or at least 5 years. In some embodiments, the effective amount of a zoonotic disease vaccine of the present disclosure is sufficient to provide sterilizing immunity in the subject at an at least 5-fold lower dose relative to control. For example, the effective amount may be sufficient to provide sterilizing immunity in the subject at an at least 10-fold lower, 15-fold, or 20-fold lower dose relative to a control.
[0409] Detectable Antigen.
[0410] In some embodiments, the effective amount of a zoonotic disease vaccine of the present disclosure is sufficient to produce detectable levels of zoonotic disease antigen as measured in serum of the subject at 1-72 hours post administration.
[0411] Titer.
[0412] An antibody titer is a measurement of the amount of antibodies within a subject, for example, antibodies that are specific to a particular antigen (e.g., an anti-zoonotic disease antigen). Antibody titer is typically expressed as the inverse of the greatest dilution that provides a positive result. Enzyme-linked immunosorbent assay (ELISA) is a common assay for determining antibody titers, for example.
[0413] In some embodiments, the effective amount of a zoonotic disease vaccine of the present disclosure is sufficient to produce a 1,000-10,000 neutralizing antibody titer produced by neutralizing antibody against the zoonotic disease antigen as measured in serum of the subject at 1-72 hours post administration. In some embodiments, the effective amount is sufficient to produce a 1,000-5,000 neutralizing antibody titer produced by neutralizing antibody against the zoonotic disease antigen as measured in serum of the subject at 1-72 hours post administration. In some embodiments, the effective amount is sufficient to produce a 5,000-10,000 neutralizing antibody titer produced by neutralizing antibody against the zoonotic disease antigen as measured in serum of the subject at 1-72 hours post administration.
[0414] In some embodiments, the neutralizing antibody titer is at least 100 NT.sub.50. For example, the neutralizing antibody titer may be at least 200, 300, 400, 500, 600, 700, 800, 900 or 1000 NT.sub.50. In some embodiments, the neutralizing antibody titer is at least 10,000 NT.sub.50.
[0415] In some embodiments, the neutralizing antibody titer is at least 100 neutralizing units per milliliter (NU/mL). For example, the neutralizing antibody titer may be at least 200, 300, 400, 500, 600, 700, 800, 900 or 1000 NU/mL. In some embodiments, the neutralizing antibody titer is at least 10,000 NU/mL.
[0416] In some embodiments, an anti-zoonotic disease antigen antibody titer produced in the subject is increased by at least 1 log relative to a control. For example, an anti-zoonotic disease antigen antibody titer produced in the subject may be increased by at least 2, 3, 4, 5, 6, 7, 8, 9 or 10 log relative to a control.
[0417] In some embodiments, an anti-zoonotic disease antigen antibody titer produced in the subject is increased at least 2 times relative to a control. For example, an anti-zoonotic disease antigen antibody titer produced in the subject is increased by at least 3, 4, 5, 6, 7, 8, 9 or 10 times relative to a control.
[0418] In some embodiments, a geometric mean, which is the nth root of the product of n numbers, is generally used to describe proportional growth. Geometric mean, in some embodiments, is used to characterize antibody titer produced in a subject.
[0419] A control may be, for example, an unvaccinated subject, or a subject administered a live attenuated zoonotic disease vaccine, an inactivated zoonotic disease vaccine, or a protein subunit zoonotic disease vaccine.
Additional Embodiments
[0420] One aspect of the disclosure is a Lassa virus (LASV) vaccine, comprising at least one RNA polynucleotide having an open reading frame encoding at least one LASV antigenic polypeptide. In some embodiments, the LASV antigenic polypeptide is a Lassa glycoprotein precursor GPC. In some embodiments, the LASV antigenic polypeptide is a structurally stabilized GPC. In some embodiments, the LASV antigenic polypeptide is a ectodomain of LASV glycoprotein 1 (GP1). In some embodiments, the LASV antigenic polypeptide is a glycoprotein. In some embodiments, the glycoprotein comprises amino acid residues 59-259 of the LASV glycoprotein precursor (GPC). In some embodiments, the LASV antigenic polypeptide is glycoprotein 2 (GP2). In some embodiments, the LASV antigenic polypeptide is a nucleocapsid protein (NP). In some embodiments, the LASV antigenic polypeptide is fused to a signal peptide.
[0421] In some embodiments, the LASV antigenic has an amino acid sequence that has at least 90% identity to an amino acid sequence identified by any one of SEQ ID NO: 1-3, but does not include wild-type protein sequence. In some embodiments, the LASV antigenic has an amino acid sequence that has at least 95% identity to an amino acid sequence identified by any one of SEQ ID NO: 1-3, but does not include wild-type protein sequence. In some embodiments, the LASV antigenic has an amino acid sequence that has at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 1-3, but does not include wild-type protein sequence. In some embodiments, the LASV antigenic polypeptide has an amino acid sequence of any one of SEQ ID NO: 1-3.
[0422] In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 80% identity to any one of SEQ ID NO: 6, 7, or 9, but does not include wild-type mRNA sequence. In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 85% identity to any one of SEQ ID NO: 6, 7, or 9, but does not include wild-type mRNA sequence. In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 90% identity to any one of SEQ ID NO: 6, 7, or 9, but does not include wild-type mRNA sequence. In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 95% identity to any one of SEQ ID NO: 6, 7, or 9, but does not include wild-type mRNA sequence.
[0423] In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 98% identity to any one of SEQ ID NO: 4-9, but does not include wild-type mRNA sequence. In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence of any one of SEQ ID NO: 6, 7, or 9. In some embodiments, the LASV antigenic polypeptide has membrane fusion activity, attaches to cell receptors, causes fusion of viral and cellular membranes, and/or is responsible for binding of the virus to a cell being infected. In some embodiments, the at least one RNA polynucleotide having an open reading frame encoding at least one LASV antigenic polypeptide comprises at least one chemical modification. In some embodiments, the chemical modification is selected from pseudouridine, N1-methylpseudouridine, N1-ethylpseudouridine, 2-thiouridine, 4'-thiouridine, 5-methylcytosine, 5-methyluridine, 2-thio-1-methyl-1-deaza-pseudouridine, 2-thio-1-methyl-pseudouridine, 2-thio-5-aza-uridine, 2-thio-dihydropseudouridine, 2-thio-dihydrouridine, 2-thio-pseudouridine, 4-methoxy-2-thio-pseudouridine, 4-methoxy-pseudouridine, 4-thio-1-methyl-pseudouridine, 4-thio-pseudouridine, 5-aza-uridine, dihydropseudouridine, 5-methoxyuridine and 2'-O-methyl uridine. In some embodiments, the chemical modification is in the carbon 5-position of the uracil. In some embodiments, the chemical modification is a N1-methylpseudouridine or N1-ethylpseudouridine. In some embodiments, at least 80% of the uracil in the open reading frame have a chemical modification. In some embodiments, at least 90% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame is modified to include N1-methyl pseudouridine at the 5-position of the uracil. In some embodiments, at least one RNA polynucleotide having an open reading frame encoding at least one LASV antigenic polypeptide further encodes at least one 5' terminal cap. In some embodiments, the 5' terminal cap is 7mG(5')ppp(5')NlmpNp.
[0424] In some embodiments, the RNA polynucleotide having an open reading frame encoding at least one LASV antigenic polypeptide is formulated in a cationic lipid nanoparticle. In some embodiments, the cationic lipid nanoparticle has a mean diameter of 50-200 nm. In some embodiments, the cationic lipid nanoparticle comprises a cationic lipid, a PEG-modified lipid, a sterol and a non-cationic lipid. In some embodiments, the cationic lipid nanoparticle comprises a molar ratio of about 20-60% cationic lipid, 0.5-15% PEG-modified lipid, 25-55% sterol, and 5-25% non-cationic lipid. In some embodiments, the cationic lipid is an ionizable cationic lipid and the non-cationic lipid is a neutral lipid, and the sterol is a cholesterol. In some embodiments, the cationic lipid is selected from 2,2-dilinoleyl-4-dimethylaminoethyl-[1,3]-dioxolane (DLin-KC2-DMA), dilinoleyl-methyl-4-dimethylaminobutyrate (DLin-MC3-DMA), and di((Z)-non-2-en-1-yl) 9-((4-(dimethylamino)butanoyl)oxy)heptadecanedioate (L319). In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (I), optionally Compound 3, 18, 20, 25, 26, 29, 30, 60, 108-112, or 122. In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (II). In some embodiments, the cationic lipid nanoparticle has a polydispersity value of less than 0.4. In some embodiments, the cationic lipid nanoparticle has a net neutral charge at a neutral pH value. In some embodiments, further comprising an adjuvant.
[0425] In some embodiments, the open reading frame encoding at least one LASV antigenic polypeptide is codon-optimized. In some embodiments, the LASV vaccine is multivalent. In some embodiments, the LASV vaccine is formulated in an effective amount to produce an antigen-specific immune response. In some embodiments, the LASV vaccine is for use in a method of inducing an antigen specific immune response in a subject, the method comprising administering to the subject the LASV vaccine in an amount effective to produce an antigen specific immune response in the subject.
[0426] One aspect of the disclosure is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the LASV vaccine as described herein, wherein the effective dose is sufficient to produce detectable levels of antigen as measured in serum of the subject at 1-72 hours post administration. In some embodiments, the cut off index of the antigen is 1-2.
[0427] One aspect of the disclosure is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the LASV vaccine as described herein, wherein the effective dose is sufficient to produce a 1,000-10,000 neutralization titer produced by neutralizing antibody against said antigen as measured in serum of the subject at 1-72 hours post administration.
[0428] One aspect of the disclosure is a composition comprising the LASV vaccine as described herein formulated in a lipid nanoparticle comprising compounds of Formula (I), (IA) and/or Formula (II), discussed below.
[0429] One aspect of the disclosure is a method of inducing an immune response in a subject, the method comprising administering to the subject the LASV vaccine as described herein in an amount effective to produce an antigen-specific immune response in the subject. In some embodiments, the antigen specific immune response comprises a T cell response or a B cell response. In some embodiments, the subject is administered a single dose of the vaccine. In some embodiments, the subject is administered a booster dose of the vaccine. In some embodiments, the vaccine is administered to the subject by intradermal injection or intramuscular injection. In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by at least 1 log relative to a control. In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by 1-3 log relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased at least 2 times relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased 2-10 times relative to a control. In some embodiments, the control is an anti-antigenic polypeptide antibody titer produced in a subject who has not been administered a vaccine against the virus.
[0430] One aspect of the disclosure is a paramyxovirus vaccine, comprising: at least one RNA polynucleotide having an open reading frame encoding at least one Nipah virus (NiV) and/or Hendra virus (HeV) antigenic polypeptide. In some embodiments, the NiV and/or HeV antigenic polypeptide is a hemagglutinin-neuraminidase protein (HN) or hemagglutinin protein (H). In some embodiments, the NiV and/or HeV antigenic polypeptide is a glycoprotein (G). In some embodiments, the NiV and/or HeV antigenic polypeptide is an attachment glycoproteins which is a type II membrane protein. In some embodiments, the NiV and/or HeV antigenic polypeptide is a fusion (F) glycoprotein. In some embodiments, the F glycoprotein comprises a trimeric class I fusogenic envelope glycoprotein containing two heptad repeat (HR) regions and a hydrophobic fusion peptide.
[0431] In some embodiments, the NiV and/or HeV antigenic polypeptide is NiV antigenic polypeptide. In some embodiments, the NiV and/or HeV antigenic polypeptide is HeV antigenic polypeptide. In some embodiments, the NiV and/or HeV antigenic polypeptide is fused to a signal peptide. In some embodiments, the NiV and/or HeV antigenic has an amino acid sequence that has at least 90% identity to an amino acid sequence identified by any one of SEQ ID NO: 10-13, but does not include wild-type protein sequence. In some embodiments, the NiV and/or HeV antigenic has an amino acid sequence that has at least 95% identity to an amino acid sequence identified by any one of SEQ ID NO: 10-13, but does not include wild-type protein sequence. In some embodiments, the NiV and/or HeV antigenic has an amino acid sequence that has at least 99% identity to an amino acid sequence identified by any one of SEQ ID NO: 10-13, but does not include wild-type protein sequence. In some embodiments, the NiV and/or HeV antigenic polypeptide has an amino acid sequence of any one of SEQ ID NO: 10-13.
[0432] In some embodiments, the at least one RNA polynucleotide has a nucleic acid sequence that has at least 80% identity to any one of SEQ ID NO: 16 or 17, but does not include wild-type mRNA sequence.
[0433] In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 85% identity to SEQ ID NO: 16 or 17, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 90% identity to SEQ ID NO: 16 or 17, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 95% identity to SEQ ID NO: 16 or 17, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 98% identity to SEQ ID NO: 16 or 17, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence of SEQ ID NO: 16 or 17.
[0434] In some embodiments, the antigenic polypeptide has membrane fusion activity, attaches to cell receptors, causes fusion of viral and cellular membranes, and/or is responsible for binding of the virus to a cell being infected. In some embodiments, at least one RNA polynucleotide comprises at least one chemical modification. In some embodiments, the chemical modification is selected from pseudouridine, N1-methylpseudouridine, N1-ethylpseudouridine, 2-thiouridine, 4'-thiouridine, 5-methylcytosine, 5-methyluridine, 2-thio-1-methyl-1-deaza-pseudouridine, 2-thio-1-methyl-pseudouridine, 2-thio-5-aza-uridine, 2-thio-dihydropseudouridine, 2-thio-dihydrouridine, 2-thio-pseudouridine, 4-methoxy-2-thio-pseudouridine, 4-methoxy-pseudouridine, 4-thio-1-methyl-pseudouridine, 4-thio-pseudouridine, 5-aza-uridine, dihydropseudouridine, 5-methoxyuridine and 2'-O-methyl uridine. In some embodiments, the chemical modification is in the 5-position of the uracil.
[0435] In some embodiments, the chemical modification is a N1-methylpseudouridine or N1-ethylpseudouridine. In some embodiments, at least 80% of the uracil in the open reading frame have a chemical modification. In some embodiments, at least 90% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame is modified to include N1-methyl pseudouridine at the 5-position of the uracil. In some embodiments, at least one RNA polynucleotide further encodes at least one 5' terminal cap. In some embodiments, the 5' terminal cap is 7mG(5')ppp(5')NlmpNp. In some embodiments, the RNA polynucleotide is formulated in a cationic lipid nanoparticle. In some embodiments, the cationic lipid nanoparticle has a mean diameter of 50-200 nm. In some embodiments, the cationic lipid nanoparticle comprises a cationic lipid, a PEG-modified lipid, a sterol and a non-cationic lipid. In some embodiments, the cationic lipid nanoparticle comprises a molar ratio of about 20-60% cationic lipid, 0.5-15% PEG-modified lipid, 25-55% sterol, and 5-25% non-cationic lipid. In some embodiments, the cationic lipid is an ionizable cationic lipid and the non-cationic lipid is a neutral lipid, and the sterol is a cholesterol.
[0436] In some embodiments, the cationic lipid is selected from 2,2-dilinoleyl-4-dimethylaminoethyl-[1,3]-dioxolane (DLin-KC2-DMA), dilinoleyl-methyl-4-dimethylaminobutyrate (DLin-MC3-DMA), and di((Z)-non-2-en-1-yl) 9-((4-(dimethylamino)butanoyl)oxy)heptadecanedioate (L319). In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (I), optionally Compound 3, 18, 20, 25, 26, 29, 30, 60, 108-112, or 122. In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (II). In some embodiments, the cationic lipid nanoparticle has a polydispersity value of less than 0.4. In some embodiments, the cationic lipid nanoparticle has a net neutral charge at a neutral pH value. Some embodiments further comprise an adjuvant. In some embodiments, the open reading frame is codon-optimized. In some embodiments, the vaccine is multivalent. Some embodiments are formulated in an effective amount to produce an antigen-specific immune response. Some embodiments are for use in a method of inducing an antigen specific immune response in a subject, the method comprising administering to the subject the vaccine in an amount effective to produce an antigen specific immune response in the subject.
[0437] One aspect of the disclosure is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the paramyxovirus vaccine as described herein, wherein the effective dose is sufficient to produce detectable levels of antigen as measured in serum of the subject at 1-72 hours post administration. In some embodiments, the cut off index of the antigen is 1-2.
[0438] One aspect of the disclosure is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the paramyxovirus vaccine as described herein, wherein the effective dose is sufficient to produce a 1,000-10,000 neutralization titer produced by neutralizing antibody against said antigen as measured in serum of the subject at 1-72 hours post administration.
[0439] One aspect of the disclosure is a composition comprising the paramyxovirus vaccine as described herein formulated in a lipid nanoparticle comprising compounds of Formula (I), (IA), and/or Formula (II), discussed below.
[0440] One aspect of the disclosure is a method of inducing an immune response in a subject, the method comprising administering to the subject the paramyxovirus vaccine as described herein in an amount effective to produce an antigen-specific immune response in the subject. In some embodiments, the antigen specific immune response comprises a T cell response or a B cell response. In some embodiments, the subject is administered a single dose of the vaccine. In some embodiments, the subject is administered a booster dose of the vaccine. In some embodiments, the vaccine is administered to the subject by intradermal injection or intramuscular injection. In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by at least 1 log relative to a control. In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by 1-3 log relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased at least 2 times relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased 2-10 times relative to a control. In some embodiments, the control is an anti-antigenic polypeptide antibody titer produced in a subject who has not been administered a vaccine against the virus.
[0441] One aspect of the invention is a betacoronavirus vaccine, comprising: at least one ribonucleic acid (RNA) polynucleotide having an open reading frame encoding at least one MERS-CoV or SARS-like coronavirus WIV1 (SL-CoV-WIV1) antigenic polypeptide. In some embodiments, the antigenic polypeptide is a betacoronavirus structural protein. In some embodiments, the betacoronavirus structural protein is spike protein (S), envelope protein (E), nucleocapsid protein (N) or membrane protein (M). In some embodiments, the betacoronavirus structural protein is spike protein (S). In some embodiments, the antigenic polypeptide is a S1 subunit of the spike protein (S). In some embodiments, the antigenic polypeptide is a S2 subunit of the spike protein (S). In some embodiments, the antigenic polypeptide is an SL-CoV-WIV1 antigenic polypeptide. In some embodiments, the antigenic polypeptide is a MERS-CoV antigenic polypeptide. In some embodiments, the open reading from is codon-optimized. In some embodiments, the vaccine is multivalent. In some embodiments, at least one RNA polynucleotide encodes at least 2 antigenic polypeptides. In some embodiments, at least one RNA polynucleotide encodes at least 10 antigenic polypeptides. In some embodiments, at least one RNA polynucleotide encodes at least 100 antigenic polypeptides. In some embodiments, at least one RNA polynucleotide encodes 2-100 antigenic polypeptides.
[0442] In some embodiments, the MERS-CoV or SL-CoV-WIV1 antigenic polypeptide has an amino acid sequence that has at least 90% identity to an amino acid sequence identified by SEQ ID NO: 18, but does not include wild-type protein sequence. In some embodiments, the MERS-CoV or SL-CoV-WIV1 antigenic polypeptide has an amino acid sequence that has at least 95% identity to an amino acid sequence identified by SEQ ID NO: 18, but does not include wild-type protein sequence. In some embodiments, the MERS-CoV or SL-CoV-WIV1 antigenic polypeptide has an amino acid sequence of SEQ ID NO: 18.
[0443] In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 80% identity to SEQ ID NO: 19 or 20, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence that has at least 90% identity to SEQ ID NO: 19 or 20, but does not include wild-type mRNA sequence. In some embodiments, at least one RNA polynucleotide has a nucleic acid sequence of SEQ ID NO: 19 or 20.
[0444] In some embodiments, at least one RNA polynucleotide comprises at least one chemical modification. In some embodiments, the chemical modification is selected from pseudouridine, N1-methylpseudouridine, N1-ethylpseudouridine, 2-thiouridine, 4'-thiouridine, 5-methylcytosine, 5-methyluridine, 2-thio-1-methyl-1-deaza-pseudouridine, 2-thio-1-methyl-pseudouridine, 2-thio-5-aza-uridine, 2-thio-dihydropseudouridine, 2-thio-dihydrouridine, 2-thio-pseudouridine, 4-methoxy-2-thio-pseudouridine, 4-methoxy-pseudouridine, 4-thio-1-methyl-pseudouridine, 4-thio-pseudouridine, 5-aza-uridine, dihydropseudouridine, 5-methoxyuridine and 2'-O-methyl uridine. In some embodiments, the chemical modification is in the 5-position of the uracil. In some embodiments, the chemical modification is a N1-methylpseudouridine or N1-ethylpseudouridine. In some embodiments, at least 80% of the uracil in the open reading frame have a chemical modification. In some embodiments, at least 90% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame have a chemical modification. In some embodiments, 100% of the uracil in the open reading frame is modified to include N1-methyl pseudouridine at the 5-position of the uracil. In some embodiments, at least one RNA polynucleotide further encodes at least one 5' terminal cap. In some embodiments, the 5' terminal cap is 7mG(5')ppp(5')NlmpNp.
[0445] In some embodiments, the RNA polynucleotide is formulated in a cationic lipid nanoparticle. In some embodiments, the cationic lipid nanoparticle has a mean diameter of 50-200 nm. In some embodiments, the cationic lipid nanoparticle comprises a cationic lipid, a PEG-modified lipid, a sterol and a non-cationic lipid. In some embodiments, the cationic lipid nanoparticle comprises a molar ratio of about 20-60% cationic lipid, 0.5-15% PEG-modified lipid, 25-55% sterol, and 5-25% non-cationic lipid. In some embodiments, the cationic lipid is an ionizable cationic lipid and the non-cationic lipid is a neutral lipid, and the sterol is a cholesterol. In some embodiments, the cationic lipid is selected from 2,2-dilinoleyl-4-dimethylaminoethyl-[1,3]-dioxolane (DLin-KC2-DMA), dilinoleyl-methyl-4-dimethylaminobutyrate (DLin-MC3-DMA), and di((Z)-non-2-en-1-yl) 9-((4-(dimethylamino)butanoyl)oxy)heptadecanedioate (L319). In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (I), optionally Compound 3, 18, 20, 25, 26, 29, 30, 60, 108-112, or 122. In some embodiments, the cationic lipid nanoparticle comprises a compound of Formula (II). In some embodiments, the cationic lipid nanoparticle has a polydispersity value of less than 0.4. In some embodiments, the cationic lipid nanoparticle has a net neutral charge at a neutral pH value. Some embodiments further comprise an adjuvant. In some embodiments, the open reading frame is codon-optimized. In some embodiments, the vaccine is multivalent. Some embodiments are formulated in an effective amount to produce an antigen-specific immune response. Some embodiments are for use in a method of inducing an antigen specific immune response in a subject, the method comprising administering to the subject the vaccine in an amount effective to produce an antigen specific immune response in the subject.
[0446] One aspect of the invention is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the betacoronavirus vaccine as described herein, wherein the effective dose is sufficient to produce detectable levels of antigen as measured in serum of the subject at 1-72 hours post administration. In some embodiments, the cut off index of the antigen is 1-2.
[0447] One aspect of the invention is a pharmaceutical composition for use in vaccination of a subject comprising an effective dose of the betacoronavirus vaccine as described herein, wherein the effective dose is sufficient to produce a 1,000-10,000 neutralization titer produced by neutralizing antibody against said antigen as measured in serum of the subject at 1-72 hours post administration.
[0448] One aspect of the invention is a composition comprising the betacoronavirus vaccine as described herein formulated in a lipid nanoparticle comprising compounds of Formula (I):
##STR00008##
or a salt or isomer thereof, wherein: R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R'; R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle; R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a carbocycle, heterocycle, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --N(R).sub.2, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and --C(R)N(R).sub.2C(O)OR, and each n is independently selected from 1, 2, 3, 4, and 5; each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group; R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle; R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle; each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H; each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl; each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl; each Y is independently a C.sub.3-6 carbocycle; each X is independently selected from the group consisting of F, Cl, Br, and I; and m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13.
[0449] In some embodiments, a subset of compounds of Formula (I) includes those in which when R.sub.4 is --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, or --CQ(R).sub.2, then (i) Q is not --N(R).sub.2 when n is 1, 2, 3, 4 or 5, or (ii) Q is not 5, 6, or 7-membered heterocycloalkyl when n is 1 or 2. In some embodiments, a subset of compounds of Formula (I) includes those in which R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R'; R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle; R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a C.sub.3-6 carbocycle, a 5- to 14-membered heteroaryl having one or more heteroatoms selected from N, O, and S, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --CRN(R).sub.2C(O)OR, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and a 5- to 14-membered heterocycloalkyl having one or more heteroatoms selected from N, O, and S which is substituted with one or more substituents selected from oxo (.dbd.O), OH, amino, mono- or di-alkylamino, and C.sub.1-3 alkyl, and each n is independently selected from 1, 2, 3, 4, and 5; each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group; R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle; R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle; each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H; each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl; each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl; each Y is independently a C.sub.3-6 carbocycle; each X is independently selected from the group consisting of F, Cl, Br, and I; and m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13,
or salts or isomers thereof.
[0450] In some embodiments, a subset of compounds of Formula (I) includes those in which R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R'; R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle; R.sub.4 is selected from the group consisting of a C.sub.3-6 carbocycle, --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, --CQ(R).sub.2, and unsubstituted C.sub.1-6 alkyl, where Q is selected from a C.sub.3-6 carbocycle, a 5- to 14-membered heterocycle having one or more heteroatoms selected from N, O, and S, --OR, --O(CH.sub.2).sub.nN(R).sub.2, --C(O)OR, --OC(O)R, --CX.sub.3, --CX.sub.2H, --CXH.sub.2, --CN, --C(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)C(O)N(R).sub.2, --N(R)C(S)N(R).sub.2, --CRN(R).sub.2C(O)OR, --N(R)R.sub.8, --O(CH.sub.2).sub.nOR, --N(R)C(.dbd.NR.sub.9)N(R).sub.2, --N(R)C(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, --N(OR)C(O)R, --N(OR)S(O).sub.2R, --N(OR)C(O)OR, --N(OR)C(O)N(R).sub.2, --N(OR)C(S)N(R).sub.2, --N(OR)C(.dbd.NR.sub.9)N(R).sub.2, --N(OR)C(.dbd.CHR.sub.9)N(R).sub.2, --C(.dbd.NR.sub.9)R, --C(O)N(R)OR, and --C(.dbd.NR.sub.9)N(R).sub.2, and each n is independently selected from 1, 2, 3, 4, and 5; and when Q is a 5- to 14-membered heterocycle and (i) R.sub.4 is --(CH.sub.2).sub.nQ in which n is 1 or 2, or (ii) R.sub.4 is --(CH.sub.2).sub.nCHQR in which n is 1, or (iii) R.sub.4 is --CHQR, and --CQ(R).sub.2, then Q is either a 5- to 14-membered heteroaryl or 8- to 14-membered heterocycloalkyl; each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group; R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; R.sub.8 is selected from the group consisting of C.sub.3-6 carbocycle and heterocycle; R.sub.9 is selected from the group consisting of H, CN, NO.sub.2, C.sub.1-6 alkyl, --OR, --S(O).sub.2R, --S(O).sub.2N(R).sub.2, C.sub.2-6 alkenyl, C.sub.3-6 carbocycle and heterocycle; each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H; each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl; each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.2-12 alkenyl; each Y is independently a C.sub.3-6 carbocycle; each X is independently selected from the group consisting of F, Cl, Br, and I; and m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13, or salts or isomers thereof.
[0451] In some embodiments, the subset of compounds of Formula (I) includes those in which R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R'; R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.2-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle; R.sub.4 is --(CH.sub.2).sub.nQ or --(CH.sub.2).sub.nCHQR, where Q is --N(R).sub.2, and n is selected from 3, 4, and 5; each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group; R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H; each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl; each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.1-12 alkenyl; each Y is independently a C.sub.3-6 carbocycle; each X is independently selected from the group consisting of F, Cl, Br, and I; and m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13, or salts or isomers thereof.
[0452] In some embodiments, a subset of compounds of Formula (I) includes those in which R.sub.1 is selected from the group consisting of C.sub.5-30 alkyl, C.sub.5-20 alkenyl, --R*YR'', --YR'', and --R''M'R'; R.sub.2 and R.sub.3 are independently selected from the group consisting of C.sub.1-14 alkyl, C.sub.2-14 alkenyl, --R*YR'', --YR'', and --R*OR'', or R.sub.2 and R.sub.3, together with the atom to which they are attached, form a heterocycle or carbocycle; R.sub.4 is selected from the group consisting of --(CH.sub.2).sub.nQ, --(CH.sub.2).sub.nCHQR, --CHQR, and --CQ(R).sub.2, where Q is --N(R).sub.2, and n is selected from 1, 2, 3, 4, and 5; each R.sub.5 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R.sub.6 is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --N(R')C(O)--, --C(O)--, --C(S)--, --C(S)S--, --SC(S)--, --CH(OH)--, --P(O)(OR')O--, --S(O).sub.2--, --S--S--, an aryl group, and a heteroaryl group; R.sub.7 is selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R is independently selected from the group consisting of C.sub.1-3 alkyl, C.sub.2-3 alkenyl, and H; each R' is independently selected from the group consisting of C.sub.1-18 alkyl, C.sub.2-18 alkenyl, --R*YR'', --YR'', and H; each R'' is independently selected from the group consisting of C.sub.3-14 alkyl and C.sub.3-14 alkenyl; each R* is independently selected from the group consisting of C.sub.1-12 alkyl and C.sub.1-12 alkenyl; each Y is independently a C.sub.3-6 carbocycle; each X is independently selected from the group consisting of F, Cl, Br, and I; and m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13, or salts or isomers thereof.
[0453] In some embodiments, a subset of compounds of Formula (I) includes those of Formula (IA): (IA), or a salt or isomer thereof, wherein 1 is selected from 1, 2, 3, 4, and 5; m is selected from 5, 6, 7, 8, and 9; M.sub.1 is a bond or M'; R.sub.4 is unsubstituted C.sub.1-3 alkyl, or --(CH.sub.2).sub.nQ, in which Q is OH, --NHC(S)N(R).sub.2, --NHC(O)N(R).sub.2, --N(R)C(O)R, --N(R)S(O).sub.2R, --N(R)R.sub.8, --NHC(.dbd.NR.sub.9)N(R).sub.2, --NHC(.dbd.CHR.sub.9)N(R).sub.2, --OC(O)N(R).sub.2, --N(R)C(O)OR, heteroaryl or heterocycloalkyl; M and M' are independently selected from --C(O)O--, --OC(O)--, --C(O)N(R')--, --P(O)(OR')O--, --S--S--, an aryl group, and a heteroaryl group; and R.sub.2 and R.sub.3 are independently selected from the group consisting of H, C.sub.1-14 alkyl, and C.sub.2-14 alkenyl.
[0454] One aspect of the invention is a method of inducing an immune response in a subject, the method comprising administering to the subject the betacoronavirus vaccine as described herein in an amount effective to produce an antigen-specific immune response in the subject. In some embodiments, the antigen specific immune response comprises a T cell response or a B cell response. In some embodiments, the subject is administered a single dose of the vaccine. In some embodiments, the subject is administered a booster dose of the vaccine. In some embodiments, the vaccine is administered to the subject by intradermal injection or intramuscular injection.
[0455] In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by at least 1 log relative to a control. In some embodiments, an anti-antigenic polypeptide antibody titer produced in the subject is increased by 1-3 log relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased at least 2 times relative to a control. In some embodiments, the anti-antigenic polypeptide antibody titer produced in the subject is increased 2-10 times relative to a control. In some embodiments, the control is an anti-antigenic polypeptide antibody titer produced in a subject who has not been administered a vaccine against the virus.
EXAMPLES
Example 1: Ebola Vaccine Immunogenicity Study
[0456] 8-10 week old female Balb/c mice were immunized intramuscularly with 10 .mu.g of Ebola mRNA vaccines or recombinant Zaire ebolavirus Glycoprotein on day 0 and 14. Serum samples were collected on day 21, 33, 52 and 77 to measure antibody response.
[0457] Animals receiving 2 doses of the Ebola mRNA vaccine antigens had high levels of GP specific IgG titers 1 week after 2nd dose (FIG. 2). The results from this study were used to select a lead vaccine candidate to be tested in a Guinea pig challenge model.
Example 2: Guinea Pig Challenge Model
[0458] Guinea pigs (n=5) were immunized intramuscularly with 20 .mu.g of select mRNA vaccine constructs (AG1 and AG2) on Day 0 and 21. Three weeks after the second dose, animals were challenged with 1000 pfu (XLD50) through Intraperitoneal injection of Guinea pig adapted Zaire Ebola virus strain Mayinga-76. Serial bleeds were collected 3, 6, 9 and 12 days after challenge to measure viremia. Animals were monitored for morbidity and mortality for 4 weeks after challenge (FIG. 3).
[0459] All animals receiving the mRNA vaccine (AG1 and AG2) were completely protected in the lethal challenge model while all placebo treated animals succumbed to infection by day 10. Furthermore, animals receiving Ebola GP mRNA vaccines did not demonstrate any significant morbidity or weight loss after challenge.
Example 3: Product Development Strategy
[0460] Two Phase 1/2 clinical trials are planned and will be a safety, immunogenicity and dose-selection studies in non-endemic and endemic settings. The first clinical study (FIH) will be initiated in the US and will include approximately 90 subjects. Three dose levels of investigational vaccine will be tested compared to placebo in a staggered manner. To mitigate risk of different immunogenicity in subjects from endemic and non-endemic setting, the second clinical study in endemic setting will be initiated in collaboration with a local clinical study site. Following evaluation of immunogenicity and safety data from both clinical studies (at 1 month post-vaccination), a dose of vaccine for further development will be selected.
Example 4: PIV3 mRNA Vaccine as a Demonstration
[0461] An mRNA vaccine was designed based on the PIV3 fusion protein and tested in two animal models, cotton rat and African green monkey, for immunogenicity and protection from viral challenge.
[0462] First, cotton rats were dosed 10 .mu.g, or 25 .mu.g of the mRNA PIV3 vaccine, placebo, or formalin inactivated (FI) PIV3 vaccine at days 0 and 28. Blood was collected pre-dose and on days 27 and 56 (28 days post dose 2) for immunogenicity testing by viral neutralization assay. On day 57 the animals were challenged with PIV3 and viral titer measured 5 days post challenge on lung and nose samples.
[0463] As shown in FIG. 9, both the 10 .mu.g and 25 .mu.g doses of mRNA vaccine completely protected cotton rats from a challenge that results in viral loads of 4 to 5 logs in lung and nose respectively, while FI vaccine showed no significant protection. The right panel shows that this protection was the result of neutralizing titers in the range of 7 to 9 logs.
[0464] The second model used to assess our mRNA PIV3 vaccine was African green monkey, which were screened as PIV3 seronegative before the experiment. The design was similar to the cotton rat study, but with animals dosed at 5, 25, or 50 ug of the vaccine. As shown in FIG. 10, absolute neutralizing titers in serum were lower than in the cotton rat model, however the 25 and 50 .mu.g doses still conferred complete protection from detectable viral load. The 5 ug dose resulted in a reduction in viral load at 5 days post challenge of approximately 1.5 to 2 logs in nose and lung, respectively, relative to placebo.
[0465] While the results above suggest a high probability of success in generating an mRNA vaccine based on Nipah F protein, soluble Nipah glycoprotein (G) vaccines have also been shown to be protective in vivo. Leveraging the flexibility of this mRNA platform we will design and test constructs of the Nipah and Hendra glycoprotein G protein as well, ultimately testing the efficacy of F and G alone and in combination at different ratios. This flexible mRNA technology allows multiple constructs to be combined and administered as one vaccine. It also enables selection of the ideal ratio of antigens to elicit the optimal immune response.
Example 5: MERS-CoV Spike Protein mRNA Vaccine
Mouse Immunogenicity
[0466] To determine the immunogenicity of MERS-CoV spike protein mRNA vaccine, female balb/c mice were immunized intramuscularly with 10 .mu.g of the vaccine on Day 0 and 28. Virus neutralizing (VN) antibody titers in the mouse sera in response to MERS spike protein mRNA vaccine measured on Day 0, 21, 42 and 56 using an in vitro neutralization assay. All animals were confirmed to be seronegative at the beginning of the study.
[0467] As shown in FIG. 11, a single dose of the mRNA vaccine induced neutralizing antibodies with an average serum titer of 1:320 on day 21. After the second dose on day 21, the VN antibody titers were boosted to 1:3000 by day 42 and further boosted up to 1:4800 by day 56. In contrast, placebo treated mice had no detectable VN antibody titer throughout the study.
Rabbit Challenge
[0468] Oryctolagus cuniculus (Rabbit) has been recently identified as a suitable animal model for MERS-CoV infection. The sequence homology for the receptor gene for MERS-CoV, DPP4(dipeptidyl peptidase 4), between humans and rabbits is such that it allows proficient infection of rabbits with MERS-CoV (Ra et al., J Virol 2014; Haagmans et al., J Virol, 2015). Nevertheless, replication of MERS-CoV in rabbits require a very high viral inoculum administered through the intra-nasal and intra-tracheal route.
[0469] In order to assess the efficacy of MERS-CoV spike protein mRNA vaccine, 6 month old New Zealand white rabbits were challenged 6 weeks after prime with EMC/2012 MERS-CoV. The vaccine was tested in a one or two dose regimen, with the boost spaced 3 weeks apart on day 21 for group 2, and each dose was 20 .mu.g. Nasal and Throat swabs were collected from one day prior to challenge; to the end of study, 4 days post challenge. Serum from animals was collected on Day 0, 21, 35, 42 and 47 for measuring virus neutralizing antibody titers.
[0470] In the Single dose group (prime only), all animals became VN positive two weeks after the vaccination and remained equally high until one week before challenge. At the time of challenge (day 0) a minor decrease in VN antibodies was observed, which was boosted upon challenge virus MERS-CoV (see FIG. 12). Similarly, all animals receiving 2 doses (prime-boost) of the vaccine became VN positive two weeks after the first vaccination and responses were boosted after the second vaccination on day -21. VN antibody responses remained high until the time of challenge and were not further boosted upon challenge on day 0. No VN antibody responses could be detected in any of the placebo treated animals during the vaccination and challenge phase of the study (FIG. 12).
Analysis of PCR and Virus Titration in Rabbit Nose Swabs
[0471] In the prime only group (1a), virus could be detected by PCR on day 1 after challenge in all animals. Three animals remained PCR positive until the end of follow up, while 3 animals became PCR negative in within 2 to 4 days post challenge (FIG. 13, Panel A). None of the PCR positive signals detected after challenge could be confirmed by virus titration (FIG. 13, Panel D).
[0472] In the prime-boost group (1b), virus could be detected by PCR on day 1 after challenge in three out of six animals, which remained positive on day 2 after challenge and were PCR negative by day 3 post challenge (FIG. 13, Panel B). None of the PCR positive signals detected after challenge could be confirmed by virus titration (FIG. 13, Panel E).
Analysis of PCR and Virus Titration in Rabbit Throat Swabs
[0473] In the prime only group (1a), virus could be detected by PCR on day 1 after challenge in all animals. One animal remained PCR positive until day 3 after challenge, however all animals were PCR negative day 4 post challenge (FIG. 14, Panel A). None of the PCR positive signals detected after challenge could be confirmed by virus titration (FIG. 14, Panel D).
[0474] In the prime-boost group (1b), virus could be detected by PCR day 1 after challenge in three out of six animals and all were PCR negative the following day. Additionally, two of these animals were PCR positive on the last day of follow up (FIG. 14, Panel B). PCR signals could not be detected in any of the other three animals. None of the PCR positive signals detected after challenge could be confirmed by virus titration (FIG. 14, Panel E).
[0475] In all placebo animals (group 2) virus could be detected by PCR on day 1 after challenge and remained PCR positive until the last sample that was analyzed. Only three PCR positive signals could be confirmed by virus titration (FIG. 14, Panels C and F).
[0476] Viral loads were also measured in the right nasal turbinates post mortem at the day of scheduled euthanasia (4 dpi). Levels of viral RNA were measured using a MERS-CoV-specific TaqMan PCR and levels of infectious (replication competent) virus using Vero cell culture.
[0477] Of the prime only group, samples from 2 out of 6 animals were positive by PCR, but all were undetectable by virus titration. The remaining four animals of group 1a were negative in PCR and virus titration. In the prime-boost group, 1 of 6 animals was positive by PCR, which again could not be detected by virus titration. The remaining five animals of group 1b were negative in PCR and virus titration. Finally all placebo animals were positive by PCR and in four animals the PCR positive signal could be confirmed by virus titration.
Analysis of Viral Load in Rabbit Lungs
[0478] Rabbit lungs were dissected into 9 separated regions post mortem for individual for assessment of viral load by region of the lung. For determining the viral load in the total lung the different sections of the lungs were pooled (equal amount of material for each section) and these samples were tested by both PCR and titration (FIG. 15). Results by PCR showed that only one animal in the prime-boost group was PCR negative in the lungs. In contrast, while PCR positive signals could be detected in almost all animals, virus titration on the total lung samples resulted in only two positive animals, both in the placebo group.
Summary
[0479] The patterns of viral load observed by PCR and by titration observed in each of the sample types in the rabbit challenge model are suggestive of a high level of protection from viral replication. The lack of any replicating virus in most of the vaccinated animal samples indicates that any PCR signal found in those same samples is likely due to the detection of residual nucleic acid sequences from input virus during the challenge itself.
[0480] The body of the immunogenicity and viral challenge data indicate that the vaccines of the invention generate robust immunologic responses with high neutralizing titers that are protective from viral replication upon challenge.
EQUIVALENTS
[0481] All references, patents and patent applications disclosed herein are incorporated by reference with respect to the subject matter for which each is cited, which in some cases may encompass the entirety of the document.
[0482] The indefinite articles "a" and "an," as used herein in the specification and in the claims, unless clearly indicated to the contrary, should be understood to mean "at least one." It should also be understood that, unless clearly indicated to the contrary, in any methods claimed herein that include more than one step or act, the order of the steps or acts of the method is not necessarily limited to the order in which the steps or acts of the method are recited.
[0483] In the claims, as well as in the specification above, all transitional phrases such as "comprising," "including," "carrying," "having," "containing," "involving," "holding," "composed of," and the like are to be understood to be open-ended, i.e., to mean including but not limited to. Only the transitional phrases "consisting of" and "consisting essentially of" shall be closed or semi-closed transitional phrases, respectively, as set forth in the United States Patent Office Manual of Patent Examining Procedures, Section 2111.03.
[0484] The terms "about" and "substantially" preceding a numerical value mean .+-.10% of the recited numerical value.
[0485] Where a range of values is provided, each value between the upper and lower ends of the range are specifically contemplated and described herein.
[0486] The entire contents of International Application Nos. PCT/US2015/027400, PCT/US2016/043348, PCT/US2016/043332, PCT/US2016/058327, PCT/US2016/058324, PCT/US2016/058314, PCT/US2016/058310, PCT/US2016/058321, PCT/US2016/058297, PCT/US2016/058319, and PCT/US2016/058314 are incorporated herein by reference.
SEQUENCES
[0487] It should be understood that any of the mRNA sequences described herein may include a 5' UTR and/or a 3' UTR. The UTR sequences may be selected from the following sequences, or other known UTR sequences may be used. It should also be understood that any of the mRNA constructs described herein may further comprise a polyA tail and/or cap (e.g., 7mG(5')ppp(5')NlmpNp). Further, while many of the mRNAs and encoded antigen sequences described herein include a signal peptide and/or a peptide tag (e.g., C-terminal His tag), it should be understood that the indicated signal peptide and/or peptide tag may be substituted for a different signal peptide and/or peptide tag, or the signal peptide and/or peptide tag may be omitted.
[0488] Exemplary Sequences: Human IgG kappa signal sequence included in protein and nucleic acid sequences are underlined
TABLE-US-00002 Lassa_GPC protein (SEQ ID NO: 1) MGQIVTFFQEVPHVIEEVMNIVLIALSLLAILKGIYNVATCGLFGLVSFLLLCGRSCSTTYKGVYELQTLELD MASLNMTMPLSCTKNNSHHYIMVGNETGLELTLTNTSIINHKFCNLSDAHKKDLYDHALMSIISTFHLSIPN FNQYEAMSCDFNGGKISVQYNLSHTYAVDAANHCGTIANGVLQTFMRMAWGGSYIALDSGKGSWDCIM TSYQYLIIQNTTWEDHCQFSRPSPIGYLGLLSQRTRDIYISRRLLGTFTWTLSDSEGNETPGGYCLTRWMLIE AELKCFGNTAVAKCNEKHDEEFCDMLRLFDFNKQAIMRLKTEAQMSIQLINKAVNALINDQLIMKNHLRD IMGIPYCNYSKYWYLNHTVTGKTSLPRCWLVSNGSYLNETRFSDDIEQQADNMITEMLQKEYLDRQGKTP LGLVDLFVFSTSFYLISIFLHLVKIPTHRHIIGKPCPKPHRLNHMGICSCGLYKHPGVPVKWKR Lassa_GPC nucleic acid (SEQ ID NO: 4) ATGGGCCAGATCGTGACATTCTTCCAAGAGGTGCCCCACGTGATCGAGGAAGTGATGAACATCGTCCT GATCGCCCTGAGCCTGCTGGCCATCCTGAAGGGCATCTACAACGTGGCCACCTGTGGCCTGTTTGGCC TGGTGTCATTCCTGCTGCTGTGCGGCAGAAGCTGCAGCACCACATACAAGGGCGTGTACGAGCTGCAG ACCCTGGAACTGGATATGGCCAGCCTGAACATGACCATGCCTCTGAGCTGCACCAAGAACAACAGCC ACCACTACATCATGGTCGGAAACGAGACAGGACTGGAACTGACCCTGACCAACACCAGCATCATCAA CCACAAGTTCTGCAACCTGAGCGACGCCCACAAGAAGGACCTGTACGATCACGCCCTGATGAGCATCA TCTCCACCTTCCACCTGAGCATCCCCAACTTCAACCAGTACGAGGCCATGAGCTGCGACTTCAACGGC GGCAAGATCAGCGTGCAGTACAATCTGAGCCACACCTACGCCGTGGACGCCGCCAATCACTGTGGCAC AATTGCCAATGGCGTGCTGCAGACATTCATGCGGATGGCCTGGGGCGGCTCTTATATCGCCCTGGATT CTGGCAAAGGCAGCTGGGACTGCATCATGACCAGCTACCAGTACCTGATCATCCAGAACACCACCTGG GAAGATCACTGCCAGTTCAGCAGACCCTCTCCTATCGGCTATCTGGGCCTGCTGAGCCAGAGAACCCG GGACATCTACATCAGCAGAAGGCTGCTGGGCACCTTCACCTGGACACTGTCTGACAGCGAGGGCAAC GAAACACCTGGCGGCTACTGCCTGACCAGATGGATGCTGATTGAGGCCGAGCTGAAGTGCTTCGGCAA TACCGCCGTGGCCAAGTGCAACGAGAAGCACGACGAGGAATTCTGCGACATGCTGCGGCTGTTCGATT TCAACAAGCAGGCCATCATGCGGCTCAAGACCGAGGCTCAGATGTCCATCCAGCTGATCAACAAGGC CGTGAATGCCCTGATCAACGATCAGCTCATCATGAAGAACCACCTCCGGGATATCATGGGCATCCCTT ACTGCAACTACAGCAAGTACTGGTATCTCAACCACACCGTGACCGGCAAGACCAGCCTGCCTAGATGT TGGCTGGTGTCCAACGGCAGCTACCTGAACGAGACACGGTTCAGCGACGACATCGAGCAGCAGGCCG ACAACATGATCACCGAGATGCTGCAGAAAGAGTACCTGGACCGGCAGGGCAAGACACCTCTGGGACT CGTGGATCTGTTCGTGTTCAGCACCAGCTTCTACCTGATCTCTATCTTCCTGCACCTGGTCAAGATCCC CACACACCGGCACATCATCGGCAAGCCCTGTCCTAAGCCTCACCGGCTGAACCACATGGGAATCTGTA GCTGCGGCCTGTACAAGCACCCTGGCGTGCCAGTGAAGTGGAAGAGA Lassa_GPC mRNA (SEQ ID NO: 6) AUGGGCCAGAUCGUGACAUUCUUCCAAGAGGUGCCCCACGUGAUCGAGGAAGUGAUGAACAUCGU CCUGAUCGCCCUGAGCCUGCUGGCCAUCCUGAAGGGCAUCUACAACGUGGCCACCUGUGGCCUGUU UGGCCUGGUGUCAUUCCUGCUGCUGUGCGGCAGAAGCUGCAGCACCACAUACAAGGGCGUGUACGA GCUGCAGACCCUGGAACUGGAUAUGGCCAGCCUGAACAUGACCAUGCCUCUGAGCUGCACCAAGAA CAACAGCCACCACUACAUCAUGGUCGGAAACGAGACAGGACUGGAACUGACCCUGACCAACACCAG CAUCAUCAACCACAAGUUCUGCAACCUGAGCGACGCCCACAAGAAGGACCUGUACGAUCACGCCCU GAUGAGCAUCAUCUCCACCUUCCACCUGAGCAUCCCCAACUUCAACCAGUACGAGGCCAUGAGCUG CGACUUCAACGGCGGCAAGAUCAGCGUGCAGUACAAUCUGAGCCACACCUACGCCGUGGACGCCGC CAAUCACUGUGGCACAAUUGCCAAUGGCGUGCUGCAGACAUUCAUGCGGAUGGCCUGGGGCGGCUC UUAUAUCGCCCUGGAUUCUGGCAAAGGCAGCUGGGACUGCAUCAUGACCAGCUACCAGUACCUGAU CAUCCAGAACACCACCUGGGAAGAUCACUGCCAGUUCAGCAGACCCUCUCCUAUCGGCUAUCUGGG CCUGCUGAGCCAGAGAACCCGGGACAUCUACAUCAGCAGAAGGCUGCUGGGCACCUUCACCUGGAC ACUGUCUGACAGCGAGGGCAACGAAACACCUGGCGGCUACUGCCUGACCAGAUGGAUGCUGAUUGA GGCCGAGCUGAAGUGCUUCGGCAAUACCGCCGUGGCCAAGUGCAACGAGAAGCACGACGAGGAAUU CUGCGACAUGCUGCGGCUGUUCGAUUUCAACAAGCAGGCCAUCAUGCGGCUCAAGACCGAGGCUCA GAUGUCCAUCCAGCUGAUCAACAAGGCCGUGAAUGCCCUGAUCAACGAUCAGCUCAUCAUGAAGAA CCACCUCCGGGAUAUCAUGGGCAUCCCUUACUGCAACUACAGCAAGUACUGGUAUCUCAACCACAC CGUGACCGGCAAGACCAGCCUGCCUAGAUGUUGGCUGGUGUCCAACGGCAGCUACCUGAACGAGAC ACGGUUCAGCGACGACAUCGAGCAGCAGGCCGACAACAUGAUCACCGAGAUGCUGCAGAAAGAGUA CCUGGACCGGCAGGGCAAGACACCUCUGGGACUCGUGGAUCUGUUCGUGUUCAGCACCAGCUUCUA CCUGAUCUCUAUCUUCCUGCACCUGGUCAAGAUCCCCACACACCGGCACAUCAUCGGCAAGCCCUG UCCUAAGCCUCACCGGCUGAACCACAUGGGAAUCUGUAGCUGCGGCCUGUACAAGCACCCUGGCGU GCCAGUGAAGUGGAAGAGA Lassa_Nucleoprotein with or without signal sequence-protein (SEQ ID NO: 2) MSASKEVKSFLWTQSLRRELSGYCSNIKLQVVKDAQALLHGLDFSEVSNVQRLMRKQKRDDGDLKRLRD LNQAVNNLVELKSTQQKSVLRVGTLSSDDLLVLAADLEKLKSKVVRTERPLSSGIYMGNLSSQQLDQRKA LLNMIGMTGGNGGRNTTSDGIVRVWDVKNAELLNNQFGTMPSLTLACLTKQGQVDLNDAVQALTDLGLI YTAKYPNSSDLDRLAQSHPILNMIDTKKSSLNISGYNFSLGAAVKAGACMLDGGNMLETIKVSPQTMDGIL KSILKVKRSLGMFISDTPGERNPYENILYKICLSGDGWPYIASRTSIVGRAWENTVVDLESDNKPQKTGNGG SNKSLQSAGFAAGLTYSQLMTLKDSMLQLDPNAKTWMDIEGRPEDPVEIALYQPSSGCYIHFFREPTDLKQ FKQDAKYSHGIDVTDLFAAQPGLTSAVIEALPRNMVITCQGSEDIRKLLESQGRRDIKLIDISLSKVDSRKFE NAVWDQFKDLCHMHTGIVVEKKKRGGKEEITPHCALMDCIMFDAAVSGGVDAKVLRAVLPRDMVFRTS TPKVVL (SEQ ID NO: 3) METPAQLLFLLLLWLPDTTGMSASKEVKSFLWTQSLRRELSGYCSNIKLQVVKDAQALLHGLDFSEVSNV QRLMRKQKRDDGDLKRLRDLNQAVNNLVELKSTQQKSVLRVGTLSSDDLLVLAADLEKLKSKVVRTERP LSSGIYMGNLSSQQLDQRKALLNMIGMTGGNGGRNTTSDGIVRVWDVKNAELLNNQFGTMPSLTLACLT KQGQVDLNDAVQALTDLGLIYTAKYPNSSDLDRLAQSHPILNMIDTKKSSLNISGYNFSLGAAVKAGACM LDGGNMLETIKVSPQTMDGILKSILKVKRSLGMFISDTPGERNPYENILYKICLSGDGWPYIASRTSIVGRAW ENTVVDLESDNKPQKTGNGGSNKSLQSAGFAAGLTYSQLMTLKDSMLQLDPNAKTWMDIEGRPEDPVEIA LYQPSSGCYIHFFREPTDLKQFKQDAKYSHGIDVTDLFAAQPGLTSAVIEALPRNMVITCQGSEDIRKLLESQ GRRDIKLIDISLSKVDSRKFENAVWDQFKDLCHMHTGIVVEKKKRGGKEEITPHCALMDCIMFDAAVSGG VDAKVLRAVLPRDMVFRTSTPKVVL Lassa_Nucleoprotein with or without signal sequence-nucleic acid (SEQ ID NO: 5) ATGGAGACTCCTGCCCAGCTCTTGTTCCTTTTGCTATTGTGGCTTCCCGACACCACCGGCATGAGCGCC AGCAAGGAGGTCAAGAGCTTCCTCTGGACCCAGAGCCTAAGAAGAGAGCTTAGCGGCTACTGCAGCA ACATCAAGCTTCAGGTGGTGAAGGACGCCCAGGCCCTGCTGCACGGCCTGGACTTCAGCGAGGTGAG CAACGTGCAGAGACTGATGAGAAAGCAGAAGCGAGACGACGGCGACCTGAAGCGTCTGCGGGACCTG AACCAGGCCGTGAACAACCTGGTGGAGCTTAAGAGCACCCAGCAGAAGTCTGTGCTGAGAGTGGGCA CCCTGAGCAGCGACGACCTGCTGGTGCTGGCCGCCGACCTGGAGAAGCTGAAGTCTAAGGTCGTCAG AACCGAGCGGCCATTGAGCTCAGGCATCTACATGGGCAACCTTAGCAGTCAGCAGCTGGACCAGAGA AAGGCCTTGCTGAACATGATCGGCATGACCGGCGGCAACGGCGGCAGAAACACCACCAGCGACGGCA TCGTGAGAGTGTGGGACGTGAAGAACGCCGAGCTACTCAACAACCAGTTCGGCACCATGCCCAGCCT GACCCTGGCCTGCCTGACCAAGCAGGGCCAGGTGGACCTCAATGACGCCGTGCAGGCACTAACCGAC CTTGGCCTGATCTACACCGCCAAGTACCCCAACTCTTCAGACCTGGACAGACTGGCGCAGTCCCACCC CATCTTAAATATGATTGACACCAAGAAGTCATCCCTTAACATCAGTGGCTACAACTTCAGCCTGGGCG CCGCCGTGAAGGCCGGCGCCTGCATGCTGGACGGCGGAAATATGCTGGAAACTATCAAGGTGAGCCC TCAGACCATGGACGGTATCCTGAAGTCCATTTTGAAGGTTAAGAGATCCCTGGGTATGTTCATCAGCG ACACCCCAGGCGAGAGAAACCCCTACGAGAACATCCTGTACAAGATCTGCCTGAGTGGCGACGGCTG GCCCTACATCGCGAGCAGAACCAGCATCGTGGGAAGGGCCTGGGAGAACACCGTGGTGGATCTTGAG AGCGACAACAAGCCCCAGAAGACCGGAAATGGCGGTTCAAACAAGAGCCTGCAGAGCGCCGGCTTCG CCGCCGGCCTGACCTACAGCCAGCTGATGACCCTGAAGGACAGCATGCTACAATTGGATCCCAACGCC AAGACTTGGATGGACATCGAGGGCAGACCCGAGGACCCCGTGGAGATCGCCCTGTACCAGCCCTCAT CCGGCTGCTACATCCACTTCTTCAGAGAGCCCACAGATCTGAAGCAGTTCAAGCAGGACGCGAAGTAT AGCCATGGCATAGACGTCACCGATTTATTCGCGGCCCAGCCGGGCCTTACGAGCGCCGTGATCGAGGC GCTGCCCAGAAACATGGTGATCACCTGCCAGGGCAGCGAGGACATCAGAAAGCTCCTTGAATCTCAA GGCCGGAGAGATATTAAGCTGATAGATATCAGCTTATCTAAGGTTGACAGCAGAAAGTTCGAGAACG CTGTATGGGACCAATTCAAGGACCTGTGCCACATGCATACGGGCATAGTGGTAGAGAAGAAGAAGCG TGGCGGAAAGGAGGAGATCACACCTCACTGCGCCCTGATGGACTGCATCATGTTCGACGCGGCAGTCT CCGGCGGCGTCGACGCAAAGGTCCTCCGGGCCGTGCTGCCAAGGGACATGGTGTTCCGGACAAGCAC CCCTAAGGTAGTGCTG Lassa_Nucleoprotein with or without signal sequence-mRNA (SEQ ID NO: 7) AUGGAGACUCCUGCCCAGCUCUUGUUCCUUUUGCUAUUGUGGCUUCCCGACACCACCGGCAUGAGC GCCAGCAAGGAGGUCAAGAGCUUCCUCUGGACCCAGAGCCUAAGAAGAGAGCUUAGCGGCUACUGC AGCAACAUCAAGCUUCAGGUGGUGAAGGACGCCCAGGCCCUGCUGCACGGCCUGGACUUCAGCGAG GUGAGCAACGUGCAGAGACUGAUGAGAAAGCAGAAGCGAGACGACGGCGACCUGAAGCGUCUGCG GGACCUGAACCAGGCCGUGAACAACCUGGUGGAGCUUAAGAGCACCCAGCAGAAGUCUGUGCUGAG AGUGGGCACCCUGAGCAGCGACGACCUGCUGGUGCUGGCCGCCGACCUGGAGAAGCUGAAGUCUAA GGUCGUCAGAACCGAGCGGCCAUUGAGCUCAGGCAUCUACAUGGGCAACCUUAGCAGUCAGCAGCU GGACCAGAGAAAGGCCUUGCUGAACAUGAUCGGCAUGACCGGCGGCAACGGCGGCAGAAACACCAC CAGCGACGGCAUCGUGAGAGUGUGGGACGUGAAGAACGCCGAGCUACUCAACAACCAGUUCGGCAC CAUGCCCAGCCUGACCCUGGCCUGCCUGACCAAGCAGGGCCAGGUGGACCUCAAUGACGCCGUGCA GGCACUAACCGACCUUGGCCUGAUCUACACCGCCAAGUACCCCAACUCUUCAGACCUGGACAGACU GGCGCAGUCCCACCCCAUCUUAAAUAUGAUUGACACCAAGAAGUCAUCCCUUAACAUCAGUGGCUA CAACUUCAGCCUGGGCGCCGCCGUGAAGGCCGGCGCCUGCAUGCUGGACGGCGGAAAUAUGCUGGA AACUAUCAAGGUGAGCCCUCAGACCAUGGACGGUAUCCUGAAGUCCAUUUUGAAGGUUAAGAGAU CCCUGGGUAUGUUCAUCAGCGACACCCCAGGCGAGAGAAACCCCUACGAGAACAUCCUGUACAAGA UCUGCCUGAGUGGCGACGGCUGGCCCUACAUCGCGAGCAGAACCAGCAUCGUGGGAAGGGCCUGGG AGAACACCGUGGUGGAUCUUGAGAGCGACAACAAGCCCCAGAAGACCGGAAAUGGCGGUUCAAAC AAGAGCCUGCAGAGCGCCGGCUUCGCCGCCGGCCUGACCUACAGCCAGCUGAUGACCCUGAAGGAC AGCAUGCUACAAUUGGAUCCCAACGCCAAGACUUGGAUGGACAUCGAGGGCAGACCCGAGGACCCC GUGGAGAUCGCCCUGUACCAGCCCUCAUCCGGCUGCUACAUCCACUUCUUCAGAGAGCCCACAGAU CUGAAGCAGUUCAAGCAGGACGCGAAGUAUAGCCAUGGCAUAGACGUCACCGAUUUAUUCGCGGCC
CAGCCGGGCCUUACGAGCGCCGUGAUCGAGGCGCUGCCCAGAAACAUGGUGAUCACCUGCCAGGGC AGCGAGGACAUCAGAAAGCUCCUUGAAUCUCAAGGCCGGAGAGAUAUUAAGCUGAUAGAUAUCAG CUUAUCUAAGGUUGACAGCAGAAAGUUCGAGAACGCUGUAUGGGACCAAUUCAAGGACCUGUGCC ACAUGCAUACGGGCAUAGUGGUAGAGAAGAAGAAGCGUGGCGGAAAGGAGGAGAUCACACCUCAC UGCGCCCUGAUGGACUGCAUCAUGUUCGACGCGGCAGUCUCCGGCGGCGUCGACGCAAAGGUCCUC CGGGCCGUGCUGCCAAGGGACAUGGUGUUCCGGACAAGCACCCCUAAGGUAGUGCUG (SEQ ID NO: 8) ATGAGCGCCAGCAAGGAGGTCAAGAGCTTCCTCTGGACCCAGAGCCTAAGAAGAGAGCTTAGCGGCT ACTGCAGCAACATCAAGCTTCAGGTGGTGAAGGACGCCCAGGCCCTGCTGCACGGCCTGGACTTCAGC GAGGTGAGCAACGTGCAGAGACTGATGAGAAAGCAGAAGCGAGACGACGGCGACCTGAAGCGTCTG CGGGACCTGAACCAGGCCGTGAACAACCTGGTGGAGCTTAAGAGCACCCAGCAGAAGTCTGTGCTGA GAGTGGGCACCCTGAGCAGCGACGACCTGCTGGTGCTGGCCGCCGACCTGGAGAAGCTGAAGTCTAA GGTCGTCAGAACCGAGCGGCCATTGAGCTCAGGCATCTACATGGGCAACCTTAGCAGTCAGCAGCTGG ACCAGAGAAAGGCCTTGCTGAACATGATCGGCATGACCGGCGGCAACGGCGGCAGAAACACCACCAG CGACGGCATCGTGAGAGTGTGGGACGTGAAGAACGCCGAGCTACTCAACAACCAGTTCGGCACCATG CCCAGCCTGACCCTGGCCTGCCTGACCAAGCAGGGCCAGGTGGACCTCAATGACGCCGTGCAGGCACT AACCGACCTTGGCCTGATCTACACCGCCAAGTACCCCAACTCTTCAGACCTGGACAGACTGGCGCAGT CCCACCCCATCTTAAATATGATTGACACCAAGAAGTCATCCCTTAACATCAGTGGCTACAACTTCAGC CTGGGCGCCGCCGTGAAGGCCGGCGCCTGCATGCTGGACGGCGGAAATATGCTGGAAACTATCAAGG TGAGCCCTCAGACCATGGACGGTATCCTGAAGTCCATTTTGAAGGTTAAGAGATCCCTGGGTATGTTC ATCAGCGACACCCCAGGCGAGAGAAACCCCTACGAGAACATCCTGTACAAGATCTGCCTGAGTGGCG ACGGCTGGCCCTACATCGCGAGCAGAACCAGCATCGTGGGAAGGGCCTGGGAGAACACCGTGGTGGA TCTTGAGAGCGACAACAAGCCCCAGAAGACCGGAAATGGCGGTTCAAACAAGAGCCTGCAGAGCGCC GGCTTCGCCGCCGGCCTGACCTACAGCCAGCTGATGACCCTGAAGGACAGCATGCTACAATTGGATCC CAACGCCAAGACTTGGATGGACATCGAGGGCAGACCCGAGGACCCCGTGGAGATCGCCCTGTACCAG CCCTCATCCGGCTGCTACATCCACTTCTTCAGAGAGCCCACAGATCTGAAGCAGTTCAAGCAGGACGC GAAGTATAGCCATGGCATAGACGTCACCGATTTATTCGCGGCCCAGCCGGGCCTTACGAGCGCCGTGA TCGAGGCGCTGCCCAGAAACATGGTGATCACCTGCCAGGGCAGCGAGGACATCAGAAAGCTCCTTGA ATCTCAAGGCCGGAGAGATATTAAGCTGATAGATATCAGCTTATCTAAGGTTGACAGCAGAAAGTTCG AGAACGCTGTATGGGACCAATTCAAGGACCTGTGCCACATGCATACGGGCATAGTGGTAGAGAAGAA GAAGCGTGGCGGAAAGGAGGAGATCACACCTCACTGCGCCCTGATGGACTGCATCATGTTCGACGCG GCAGTCTCCGGCGGCGTCGACGCAAAGGTCCTCCGGGCCGTGCTGCCAAGGGACATGGTGTTCCGGAC AAGCACCCCTAAGGTAGTGCTG mRNA (SEQ ID NO: 9) AUGAGCGCCAGCAAGGAGGUCAAGAGCUUCCUCUGGACCCAGAGCCUAAGAAGAGAGCUUAGCGGC UACUGCAGCAACAUCAAGCUUCAGGUGGUGAAGGACGCCCAGGCCCUGCUGCACGGCCUGGACUUC AGCGAGGUGAGCAACGUGCAGAGACUGAUGAGAAAGCAGAAGCGAGACGACGGCGACCUGAAGCG UCUGCGGGACCUGAACCAGGCCGUGAACAACCUGGUGGAGCUUAAGAGCACCCAGCAGAAGUCUGU GCUGAGAGUGGGCACCCUGAGCAGCGACGACCUGCUGGUGCUGGCCGCCGACCUGGAGAAGCUGAA GUCUAAGGUCGUCAGAACCGAGCGGCCAUUGAGCUCAGGCAUCUACAUGGGCAACCUUAGCAGUCA GCAGCUGGACCAGAGAAAGGCCUUGCUGAACAUGAUCGGCAUGACCGGCGGCAACGGCGGCAGAAA CACCACCAGCGACGGCAUCGUGAGAGUGUGGGACGUGAAGAACGCCGAGCUACUCAACAACCAGUU CGGCACCAUGCCCAGCCUGACCCUGGCCUGCCUGACCAAGCAGGGCCAGGUGGACCUCAAUGACGC CGUGCAGGCACUAACCGACCUUGGCCUGAUCUACACCGCCAAGUACCCCAACUCUUCAGACCUGGA CAGACUGGCGCAGUCCCACCCCAUCUUAAAUAUGAUUGACACCAAGAAGUCAUCCCUUAACAUCAG UGGCUACAACUUCAGCCUGGGCGCCGCCGUGAAGGCCGGCGCCUGCAUGCUGGACGGCGGAAAUAU GCUGGAAACUAUCAAGGUGAGCCCUCAGACCAUGGACGGUAUCCUGAAGUCCAUUUUGAAGGUUA AGAGAUCCCUGGGUAUGUUCAUCAGCGACACCCCAGGCGAGAGAAACCCCUACGAGAACAUCCUGU ACAAGAUCUGCCUGAGUGGCGACGGCUGGCCCUACAUCGCGAGCAGAACCAGCAUCGUGGGAAGGG CCUGGGAGAACACCGUGGUGGAUCUUGAGAGCGACAACAAGCCCCAGAAGACCGGAAAUGGCGGU UCAAACAAGAGCCUGCAGAGCGCCGGCUUCGCCGCCGGCCUGACCUACAGCCAGCUGAUGACCCUG AAGGACAGCAUGCUACAAUUGGAUCCCAACGCCAAGACUUGGAUGGACAUCGAGGGCAGACCCGAG GACCCCGUGGAGAUCGCCCUGUACCAGCCCUCAUCCGGCUGCUACAUCCACUUCUUCAGAGAGCCC ACAGAUCUGAAGCAGUUCAAGCAGGACGCGAAGUAUAGCCAUGGCAUAGACGUCACCGAUUUAUU CGCGGCCCAGCCGGGCCUUACGAGCGCCGUGAUCGAGGCGCUGCCCAGAAACAUGGUGAUCACCUG CCAGGGCAGCGAGGACAUCAGAAAGCUCCUUGAAUCUCAAGGCCGGAGAGAUAUUAAGCUGAUAG AUAUCAGCUUAUCUAAGGUUGACAGCAGAAAGUUCGAGAACGCUGUAUGGGACCAAUUCAAGGAC CUGUGCCACAUGCAUACGGGCAUAGUGGUAGAGAAGAAGAAGCGUGGCGGAAAGGAGGAGAUCAC ACCUCACUGCGCCCUGAUGGACUGCAUCAUGUUCGACGCGGCAGUCUCCGGCGGCGUCGACGCAAA GGUCCUCCGGGCCGUGCUGCCAAGGGACAUGGUGUUCCGGACAAGCACCCCUAAGGUAGUGCUG Nipah_G (SEQ ID NO: 10) METPAQLLFLLLLWLPDTTGMPAENKKVRFENTTSDKGKNPSKVIKSYYGTMDIKKINEGLLDSKILSAFN TVIALLGSIVIIVMNIMIIQNYTRSTDNQAVIKDALQGIQQQIKGLADKIGTEIGPKVSLIDTSSTITIPANIG- LL GSKISQSTASINENVNEKCKFTLPPLKIHECNISCPNPLPFREYRPQTEGVSNLVGLPDNICLQKTSNQILKPK LISYTLPVVGQSGTCITDPLLAMDEGYFAYSHLERIGSCSRGVSKQRIIGVGEVLDRGDEVPSLFMTNVWTP PNPNTVYHCSAVYNNEFYYVLCAVSTVGDPILNSTYWSGSLMMTRLAVKPKSNGGGYNQHQLALRSIEK GRYDKVMPYGPSGIKQGDTLYFPAVGFLVRTEFKYNDSNCPITKCQYSKPENCRLSMGIRPNSHYILRSGLL KYNLSDGENPKIVFIEISDQRLSIGSPSKVYDSLGQPVFYQASFSWDTMIKFGDVQTVNPLVVNWRDNTVIS RPGQSQCPRFNTCPEICWEGVYNDAFLIDRINWISAGVFLDSNQTAENPVFTVFKDNEILYRAQLASEDTNA QKTITNCFLLKNKIWCISLVEIYDTGDNVIRPKLFAVKIPEQCT (SEQ ID NO: 11) MPAENKKVRFENTTSDKGKNPSKVIKSYYGTMDIKKINEGLLDSKILSAFNTVIALLGSIVIIVMNIMIIQNYT RSTDNQAVIKDALQGIQQQIKGLADKIGTEIGPKVSLIDTSSTITIPANIGLLGSKISQSTASINENVNEKCKF- T LPPLKIHECNISCPNPLPFREYRPQTEGVSNLVGLPDNICLQKTSNQILKPKLISYTLPVVGQSGTCITDPLLA MDEGYFAYSHLERIGSCSRGVSKQRIIGVGEVLDRGDEVPSLFMTNVWTPPNPNTVYHCSAVYNNEFYYV LCAVSTVGDPILNSTYWSGSLMMTRLAVKPKSNGGGYNQHQLALRSIEKGRYDKVMPYGPSGIKQGDTLY FPAVGFLVRTEFKYNDSNCPITKCQYSKPENCRLSMGIRPNSHYILRSGLLKYNLSDGENPKIVFIEISDQRLS IGSPSKVYDSLGQPVFYQASFSWDTMIKFGDVQTVNPLVVNWRDNTVISRPGQSQCPRFNTCPEICWEGVY NDAFLIDRINWISAGVFLDSNQTAENPVFTVFKDNEILYRAQLASEDTNAQKTITNCFLLKNKIWCISLVEIY DTGDNVIRPKLFAVKIPEQCT (SEQ ID NO: 14) ATGGAAACCCCTGCTCAGCTGCTGTTCCTGCTGCTGCTGTGGCTGCCTGATACAACAGGCATGCCCGCC GAGAACAAGAAAGTTCGCTTCGAGAACACCACCAGCGACAAGGGCAAGAACCCCAGCAAAGTGATCA AGAGCTACTACGGCACCATGGACATCAAGAAGATCAACGAGGGCCTGCTGGACAGCAAGATCCTGAG CGCCTTCAACACCGTGATTGCCCTGCTGGGCTCTATCGTGATCATCGTGATGAACATCATGATCATCCA GAACTACACCCGGTCCACCGACAACCAGGCCGTGATTAAGGATGCTCTGCAGGGAATCCAGCAGCAG ATCAAAGGCCTGGCCGACAAGATCGGCACAGAGATCGGCCCTAAGGTGTCCCTGATCGACACCAGCA GCACCATCACAATCCCCGCCAATATCGGACTGCTGGGATCCAAGATCAGCCAGAGCACCGCCAGCATC AACGAGAACGTGAACGAGAAGTGCAAGTTCACCCTGCCTCCACTGAAGATCCACGAGTGCAACATCA GCTGCCCCAATCCTCTGCCATTCAGAGAGTACAGACCCCAGACAGAGGGCGTGTCCAATCTCGTGGGC CTGCCTGACAATATCTGCCTGCAGAAGACCAGCAACCAGATCCTGAAGCCTAAGCTGATCTCCTACAC ACTGCCCGTCGTGGGCCAGAGCGGCACCTGTATTACAGATCCTCTGCTGGCCATGGACGAGGGCTACT TTGCCTACAGCCACCTGGAAAGAATCGGCAGCTGTAGCCGGGGAGTGTCCAAGCAGAGAATCATCGG CGTGGGCGAAGTGCTGGATAGAGGCGACGAAGTGCCCAGCCTGTTCATGACCAATGTGTGGACCCCTC CTAATCCTAACACCGTGTACCACTGCAGCGCCGTGTACAACAACGAGTTCTACTACGTGCTGTGCGCC GTGTCCACAGTGGGCGACCCTATCCTGAACAGCACCTATTGGAGCGGCAGCCTGATGATGACCAGACT GGCCGTGAAGCCCAAGAGCAATGGCGGCGGATACAACCAGCATCAGCTGGCCCTGCGGTCCATCGAG AAGGGCAGATACGACAAAGTGATGCCTTACGGCCCCAGCGGCATCAAGCAAGGCGATACCCTGTACT TTCCCGCCGTGGGATTTCTCGTGCGGACCGAGTTCAAGTACAACGACAGCAACTGCCCCATCACCAAG TGCCAGTACAGCAAGCCCGAGAACTGCAGACTGAGCATGGGCATCAGACCCAACAGCCACTACATCC TGAGAAGCGGCCTGCTGAAGTACAACCTGAGCGACGGCGAGAACCCCAAGATCGTGTTCATCGAGAT CAGCGACCAGCGGCTGTCTATCGGCAGCCCTAGCAAGGTGTACGACTCTCTGGGACAGCCAGTGTTCT ACCAGGCCTCCTTCAGCTGGGACACCATGATCAAGTTCGGCGACGTGCAGACCGTGAATCCCCTGGTG GTCAACTGGCGGGACAATACCGTGATCAGCAGACCTGGCCAGTCTCAGTGCCCCAGATTCAACACATG CCCCGAGATCTGTTGGGAAGGCGTGTACAATGACGCCTTCCTGATCGATCGGATCAACTGGATCTCTG CCGGCGTGTTCCTGGACTCCAATCAGACAGCCGAGAATCCTGTGTTCACCGTGTTCAAGGACAATGAG ATCCTGTATCGGGCCCAGCTGGCCTCCGAGGATACAAATGCCCAGAAGACAATCACCAACTGCTTTCT GCTCAAGAACAAGATCTGGTGCATCAGCCTGGTGGAAATCTACGACACCGGCGACAACGTGATCAGG CCCAAGCTGTTCGCCGTGAAGATCCCTGAGCAGTGCACA mRNA (SEQ ID NO: 16) AUGGAAACCCCUGCUCAGCUGCUGUUCCUGCUGCUGCUGUGGCUGCCUGAUACAACAGGCAUGCCC GCCGAGAACAAGAAAGUUCGCUUCGAGAACACCACCAGCGACAAGGGCAAGAACCCCAGCAAAGUG AUCAAGAGCUACUACGGCACCAUGGACAUCAAGAAGAUCAACGAGGGCCUGCUGGACAGCAAGAUC CUGAGCGCCUUCAACACCGUGAUUGCCCUGCUGGGCUCUAUCGUGAUCAUCGUGAUGAACAUCAUG AUCAUCCAGAACUACACCCGGUCCACCGACAACCAGGCCGUGAUUAAGGAUGCUCUGCAGGGAAUC CAGCAGCAGAUCAAAGGCCUGGCCGACAAGAUCGGCACAGAGAUCGGCCCUAAGGUGUCCCUGAUC GACACCAGCAGCACCAUCACAAUCCCCGCCAAUAUCGGACUGCUGGGAUCCAAGAUCAGCCAGAGC ACCGCCAGCAUCAACGAGAACGUGAACGAGAAGUGCAAGUUCACCCUGCCUCCACUGAAGAUCCAC GAGUGCAACAUCAGCUGCCCCAAUCCUCUGCCAUUCAGAGAGUACAGACCCCAGACAGAGGGCGUG UCCAAUCUCGUGGGCCUGCCUGACAAUAUCUGCCUGCAGAAGACCAGCAACCAGAUCCUGAAGCCU AAGCUGAUCUCCUACACACUGCCCGUCGUGGGCCAGAGCGGCACCUGUAUUACAGAUCCUCUGCUG GCCAUGGACGAGGGCUACUUUGCCUACAGCCACCUGGAAAGAAUCGGCAGCUGUAGCCGGGGAGUG UCCAAGCAGAGAAUCAUCGGCGUGGGCGAAGUGCUGGAUAGAGGCGACGAAGUGCCCAGCCUGUU CAUGACCAAUGUGUGGACCCCUCCUAAUCCUAACACCGUGUACCACUGCAGCGCCGUGUACAACAA
CGAGUUCUACUACGUGCUGUGCGCCGUGUCCACAGUGGGCGACCCUAUCCUGAACAGCACCUAUUG GAGCGGCAGCCUGAUGAUGACCAGACUGGCCGUGAAGCCCAAGAGCAAUGGCGGCGGAUACAACCA GCAUCAGCUGGCCCUGCGGUCCAUCGAGAAGGGCAGAUACGACAAAGUGAUGCCUUACGGCCCCAG CGGCAUCAAGCAAGGCGAUACCCUGUACUUUCCCGCCGUGGGAUUUCUCGUGCGGACCGAGUUCAA GUACAACGACAGCAACUGCCCCAUCACCAAGUGCCAGUACAGCAAGCCCGAGAACUGCAGACUGAG CAUGGGCAUCAGACCCAACAGCCACUACAUCCUGAGAAGCGGCCUGCUGAAGUACAACCUGAGCGA CGGCGAGAACCCCAAGAUCGUGUUCAUCGAGAUCAGCGACCAGCGGCUGUCUAUCGGCAGCCCUAG CAAGGUGUACGACUCUCUGGGACAGCCAGUGUUCUACCAGGCCUCCUUCAGCUGGGACACCAUGAU CAAGUUCGGCGACGUGCAGACCGUGAAUCCCCUGGUGGUCAACUGGCGGGACAAUACCGUGAUCAG CAGACCUGGCCAGUCUCAGUGCCCCAGAUUCAACACAUGCCCCGAGAUCUGUUGGGAAGGCGUGUA CAAUGACGCCUUCCUGAUCGAUCGGAUCAACUGGAUCUCUGCCGGCGUGUUCCUGGACUCCAAUCA GACAGCCGAGAAUCCUGUGUUCACCGUGUUCAAGGACAAUGAGAUCCUGUAUCGGGCCCAGCUGGC CUCCGAGGAUACAAAUGCCCAGAAGACAAUCACCAACUGCUUUCUGCUCAAGAACAAGAUCUGGUG CAUCAGCCUGGUGGAAAUCUACGACACCGGCGACAACGUGAUCAGGCCCAAGCUGUUCGCCGUGAA GAUCCCUGAGCAGUGCACA Nipah_F (SEQ ID NO: 12) METPAQLLFULLWLPDTTGILHYEKLSKIGLVKGITRKYKIKSNPLTKDIVIKIVIIPNVSNIVISQCTGSVME- N YKTRLNGILTPIKGALEIYKNNTHDLVGDVRLAGVIMAGVAIGIATAAQITAGVALYEAMKNADNINKLKS SIESTNEAVVKLQETAEKTVYVLTALQDYINTNLVPTIDKISCKQTELSLDLALSKYLSDLLFVFGPNLQDPV SNSMTIQAISQAFGGNYETLLRTLGYATEDFDDLLESDSITGQIIYVDLSGYYIIVRVYFPILTEIQQAYIQEL- L PVSFNNDNSEWISIVPNFILVRNTLISNIEIGFCLITKRSVICNQDYATPMTNNMRECLTGSTEKCPRELVVSS HVPRFALSNGVLFANCISVTCQCQTTGRAISQSGEQTLLMIDNTTCPTAVLGNVIISLGKYLGSVNYNSEGIA IGPPVFTDKVDISSQISSMNQSLQQSKDYIKEAQRLLDTVNPSLISMLSMIILYVLSIASLCIGLITFISFIIV- EKK RNTYSRLEDRRVRPTSSGDLYYIGT (SEQ ID NO: 13) ILHYEKLSKIGLVKGITRKYKIKSNPLTKDIVIKMIPNVSNMSQCTGSVMENYKTRLNGILTPIKGALEIYKN NTHDLVGDVRLAGVIMAGVAIGIATAAQITAGVALYEAMKNADNINKLKSSIESTNEAVVKLQETAEKTV YVLTALQDYINTNLVPTIDKISCKQTELSLDLALSKYLSDLLFVFGPNLQDPVSNSMTIQAISQAFGGNYETL LRTLGYATEDFDDLLESDSITGQIIYVDLSGYYIIVRVYFPILTEIQQAYIQELLPVSFNNDNSEWISIVPNFI- LV RNTLISNIEIGFCLITKRSVICNQDYATPMTNNMRECLTGSTEKCPRELVVSSHVPRFALSNGVLFANCISVT CQCQTTGRAISQSGEQTLLMIDNTTCPTAVLGNVIISLGKYLGSVNYNSEGIAIGPPVFTDKVDISSQISSMNQ SLQQSKDYIKEAQRLLDTVNPSLISMLSMIILYVLSIASLCIGLITFISFIIVEKKRNTYSRLEDRRVRPTSSG- DL YYIGT (SEQ ID NO: 15) ATGGAAACCCCTGCTCAGCTGCTGTTCCTGCTGCTGCTGTGGCTGCCTGATACAACAGGCATGCCCGCC GAGAACAAGAAAGTTCGCTTCGAGAACACCACCAGCGACAAGGGCAAGAACCCCAGCAAAGTGATCA AGAGCTACTACGGCACCATGGACATCAAGAAGATCAACGAGGGCCTGCTGGACAGCAAGATCCTGAG CGCCTTCAACACCGTGATTGCCCTGCTGGGCTCTATCGTGATCATCGTGATGAACATCATGATCATCCA GAACTACACCCGGTCCACCGACAACCAGGCCGTGATTAAGGATGCTCTGCAGGGAATCCAGCAGCAG ATCAAAGGCCTGGCCGACAAGATCGGCACAGAGATCGGCCCTAAGGTGTCCCTGATCGACACCAGCA GCACCATCACAATCCCCGCCAATATCGGACTGCTGGGATCCAAGATCAGCCAGAGCACCGCCAGCATC AACGAGAACGTGAACGAGAAGTGCAAGTTCACCCTGCCTCCACTGAAGATCCACGAGTGCAACATCA GCTGCCCCAATCCTCTGCCATTCAGAGAGTACAGACCCCAGACAGAGGGCGTGTCCAATCTCGTGGGC CTGCCTGACAATATCTGCCTGCAGAAGACCAGCAACCAGATCCTGAAGCCTAAGCTGATCTCCTACAC ACTGCCCGTCGTGGGCCAGAGCGGCACCTGTATTACAGATCCTCTGCTGGCCATGGACGAGGGCTACT TTGCCTACAGCCACCTGGAAAGAATCGGCAGCTGTAGCCGGGGAGTGTCCAAGCAGAGAATCATCGG CGTGGGCGAAGTGCTGGATAGAGGCGACGAAGTGCCCAGCCTGTTCATGACCAATGTGTGGACCCCTC CTAATCCTAACACCGTGTACCACTGCAGCGCCGTGTACAACAACGAGTTCTACTACGTGCTGTGCGCC GTGTCCACAGTGGGCGACCCTATCCTGAACAGCACCTATTGGAGCGGCAGCCTGATGATGACCAGACT GGCCGTGAAGCCCAAGAGCAATGGCGGCGGATACAACCAGCATCAGCTGGCCCTGCGGTCCATCGAG AAGGGCAGATACGACAAAGTGATGCCTTACGGCCCCAGCGGCATCAAGCAAGGCGATACCCTGTACT TTCCCGCCGTGGGATTTCTCGTGCGGACCGAGTTCAAGTACAACGACAGCAACTGCCCCATCACCAAG TGCCAGTACAGCAAGCCCGAGAACTGCAGACTGAGCATGGGCATCAGACCCAACAGCCACTACATCC TGAGAAGCGGCCTGCTGAAGTACAACCTGAGCGACGGCGAGAACCCCAAGATCGTGTTCATCGAGAT CAGCGACCAGCGGCTGTCTATCGGCAGCCCTAGCAAGGTGTACGACTCTCTGGGACAGCCAGTGTTCT ACCAGGCCTCCTTCAGCTGGGACACCATGATCAAGTTCGGCGACGTGCAGACCGTGAATCCCCTGGTG GTCAACTGGCGGGACAATACCGTGATCAGCAGACCTGGCCAGTCTCAGTGCCCCAGATTCAACACATG CCCCGAGATCTGTTGGGAAGGCGTGTACAATGACGCCTTCCTGATCGATCGGATCAACTGGATCTCTG CCGGCGTGTTCCTGGACTCCAATCAGACAGCCGAGAATCCTGTGTTCACCGTGTTCAAGGACAATGAG ATCCTGTATCGGGCCCAGCTGGCCTCCGAGGATACAAATGCCCAGAAGACAATCACCAACTGCTTTCT GCTCAAGAACAAGATCTGGTGCATCAGCCTGGTGGAAATCTACGACACCGGCGACAACGTGATCAGG CCCAAGCTGTTCGCCGTGAAGATCCCTGAGCAGTGCACA mRNA (SEQ ID NO: 17) AUGGAAACCCCUGCUCAGCUGCUGUUCCUGCUGCUGCUGUGGCUGCCUGAUACAACAGGCAUGCCC GCCGAGAACAAGAAAGUUCGCUUCGAGAACACCACCAGCGACAAGGGCAAGAACCCCAGCAAAGUG AUCAAGAGCUACUACGGCACCAUGGACAUCAAGAAGAUCAACGAGGGCCUGCUGGACAGCAAGAUC CUGAGCGCCUUCAACACCGUGAUUGCCCUGCUGGGCUCUAUCGUGAUCAUCGUGAUGAACAUCAUG AUCAUCCAGAACUACACCCGGUCCACCGACAACCAGGCCGUGAUUAAGGAUGCUCUGCAGGGAAUC CAGCAGCAGAUCAAAGGCCUGGCCGACAAGAUCGGCACAGAGAUCGGCCCUAAGGUGUCCCUGAUC GACACCAGCAGCACCAUCACAAUCCCCGCCAAUAUCGGACUGCUGGGAUCCAAGAUCAGCCAGAGC ACCGCCAGCAUCAACGAGAACGUGAACGAGAAGUGCAAGUUCACCCUGCCUCCACUGAAGAUCCAC GAGUGCAACAUCAGCUGCCCCAAUCCUCUGCCAUUCAGAGAGUACAGACCCCAGACAGAGGGCGUG UCCAAUCUCGUGGGCCUGCCUGACAAUAUCUGCCUGCAGAAGACCAGCAACCAGAUCCUGAAGCCU AAGCUGAUCUCCUACACACUGCCCGUCGUGGGCCAGAGCGGCACCUGUAUUACAGAUCCUCUGCUG GCCAUGGACGAGGGCUACUUUGCCUACAGCCACCUGGAAAGAAUCGGCAGCUGUAGCCGGGGAGUG UCCAAGCAGAGAAUCAUCGGCGUGGGCGAAGUGCUGGAUAGAGGCGACGAAGUGCCCAGCCUGUU CAUGACCAAUGUGUGGACCCCUCCUAAUCCUAACACCGUGUACCACUGCAGCGCCGUGUACAACAA CGAGUUCUACUACGUGCUGUGCGCCGUGUCCACAGUGGGCGACCCUAUCCUGAACAGCACCUAUUG GAGCGGCAGCCUGAUGAUGACCAGACUGGCCGUGAAGCCCAAGAGCAAUGGCGGCGGAUACAACCA GCAUCAGCUGGCCCUGCGGUCCAUCGAGAAGGGCAGAUACGACAAAGUGAUGCCUUACGGCCCCAG CGGCAUCAAGCAAGGCGAUACCCUGUACUUUCCCGCCGUGGGAUUUCUCGUGCGGACCGAGUUCAA GUACAACGACAGCAACUGCCCCAUCACCAAGUGCCAGUACAGCAAGCCCGAGAACUGCAGACUGAG CAUGGGCAUCAGACCCAACAGCCACUACAUCCUGAGAAGCGGCCUGCUGAAGUACAACCUGAGCGA CGGCGAGAACCCCAAGAUCGUGUUCAUCGAGAUCAGCGACCAGCGGCUGUCUAUCGGCAGCCCUAG CAAGGUGUACGACUCUCUGGGACAGCCAGUGUUCUACCAGGCCUCCUUCAGCUGGGACACCAUGAU CAAGUUCGGCGACGUGCAGACCGUGAAUCCCCUGGUGGUCAACUGGCGGGACAAUACCGUGAUCAG CAGACCUGGCCAGUCUCAGUGCCCCAGAUUCAACACAUGCCCCGAGAUCUGUUGGGAAGGCGUGUA CAAUGACGCCUUCCUGAUCGAUCGGAUCAACUGGAUCUCUGCCGGCGUGUUCCUGGACUCCAAUCA GACAGCCGAGAAUCCUGUGUUCACCGUGUUCAAGGACAAUGAGAUCCUGUAUCGGGCCCAGCUGGC CUCCGAGGAUACAAAUGCCCAGAAGACAAUCACCAACUGCUUUCUGCUCAAGAACAAGAUCUGGUG CAUCAGCCUGGUGGAAAUCUACGACACCGGCGACAACGUGAUCAGGCCCAAGCUGUUCGCCGUGAA GAUCCCUGAGCAGUGCACA >gi|940378825|gb|ALK02457.1| spikeprotein [SARS-likecoronavirusWIV16] (SEQ ID NO: 18) MFIFLFFLTLTSGSDLESCTTFDDVQAPNYPQHSSSRRGVYYPDEIFRSDTLYLTQDLFLPFYSNVTGFHTINH RFDNPVIPFKDGVYFAATEKSNVVRGWVFGSTMNNKSQSVIIINNSTNVVIRACNFELCDNPFFAVSKPTGT QTHTMIFDNAFNCTFEYISDSFSLDVAEKSGNFKHLREFVFKNKDGFLYVYKGYQPIDVVRDLPSGFNILKPI FKLPLGINITNFRAILTAFLPAQDTWGTSAAAYFVGYLKPATFMLKYDENGTITDAVDCSQNPLAELKCSV KSFEIDKGIYQTSNFRVAPSKEVVRFPNITNLCPFGEVFNATTFPSVYAWERKRISNCVADYSVLYNSTSFST FKCYGVSATKLNDLCFSNVYADSFVVKGDDVRQIAPGQTGVIADYNYKLPDDFTGCVLAWNTRNIDATQT GNYNYKYRSLRHGKLRPFERDISNVPFSPDGKPCTPPAFNCYWPLNDYGFYITNGIGYQPYRVVVLSFELLN APATVCGPKLSTDLIKNQCVNFNFNGLTGTGVLTPSSKRFQPFQQFGRDVLDFTDSVRDPKTSEILDISPCSF GGVSVITPGTNTSSEVAVLYQDVNCTDVPVAIHADQLTPSWRVYSTGNNVFQTQAGCLIGAEHVDTSYEC DIPIGAGICASYHTVSSLRSTSQKSIVAYTMSLGADSSIAYSNNTIAIPTNFSISITTEVMPVSMAKTSVDCNM YICGDSTECANLLLQYGSFCTQLNRALSGIAVEQDRNTREVFAQVKQMYKTPTLKDFGGFNFSQILPDPLKP TKRSFIEDLLFNKVTLADAGFMKQYGECLGDINARDLICAQKFNGLTVLPPLLTDDMIAAYTAALVSGTAT AGWTFGAGAALQIPFAMQMAYRFNGIGVTQNVLYENQKQIANQFNKAISQIQESLTTTSTALGKLQDVVN QNAQALNTLVKQLSSNFGAISSVLNDILSRLDKVEAEVQIDRLITGRLQSLQTYVTQQLIRAAEIRASANLA ATKMSECVLGQSKRVDFCGKGYHLMSFPQAAPHGVVFLHVTYVPSQERNFTTAPAICHEGKAYFPREGVF VFNGTSWFITQRNFFSPQIITTDNTFVSGSCDVVIGIINNTVYDPLQPELDSFKEELDKYFKNHTSPDVDLGDI SGINASVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIKWPWYVWLGFIAGLIAIVMVTILLCCMTSCC SCLKGACSCGSCCKFDEDDSEPVLKGVKLHYT >WIV16_FL_CO_Spike (SEQ ID NO: 19) ATGTTTATCTTCCTGTTCTTCCTGACCCTGACCAGCGGCAGCGACCTGGAAAGCTGCACCACCTTCGAC GACGTGCAGGCCCCCAACTACCCTCAGCACAGCTCTAGCAGACGGGGCGTGTACTACCCCGACGAGAT CTTCAGAAGCGACACCCTGTACCTGACCCAGGACCTGTTCCTGCCCTTCTACAGCAACGTGACCGGCTT CCACACCATCAACCACAGATTCGACAACCCCGTGATCCCCTTCAAGGACGGGGTGTACTTTGCCGCCA CCGAGAAGTCCAATGTCGTGCGGGGATGGGTGTTCGGCAGCACCATGAACAACAAGAGCCAGAGCGT GATCATCATCAACAACAGCACCAACGTCGTGATCCGGGCCTGCAACTTCGAGCTGTGCGACAACCCAT TCTTCGCCGTGTCCAAGCCCACCGGCACCCAGACCCACACCATGATCTTCGACAACGCCTTCAACTGC ACCTTCGAGTACATCAGCGACAGCTTCAGCCTGGACGTGGCCGAGAAAAGCGGCAACTTCAAGCACCT GAGAGAATTCGTGTTCAAGAACAAGGACGGCTTCCTGTACGTGTACAAGGGCTACCAGCCCATCGACG
TCGTGCGCGATCTGCCCAGCGGCTTCAACATCCTGAAGCCCATCTTCAAGCTGCCCCTGGGCATCAAC ATCACCAACTTCCGGGCTATCCTGACCGCCTTCCTGCCCGCCCAGGATACCTGGGGAACAAGCGCCGC TGCCTACTTCGTGGGCTACCTGAAGCCTGCCACCTTCATGCTGAAGTACGACGAGAACGGCACCATCA CCGACGCCGTGGACTGCAGCCAGAATCCTCTGGCCGAGCTGAAGTGCAGCGTGAAGTCCTTCGAGATC GACAAGGGCATCTACCAGACCAGCAACTTCAGAGTGGCCCCCAGCAAAGAAGTCGTGCGGTTCCCCA ATATCACCAACCTGTGCCCCTTCGGCGAGGTGTTCAACGCCACCACCTTTCCCAGCGTGTACGCCTGGG AGCGGAAGCGGATCAGCAACTGCGTGGCCGACTACAGCGTGCTGTACAACTCCACCAGCTTCTCCACC TTCAAGTGCTACGGCGTGTCCGCCACCAAGCTGAACGACCTGTGCTTCAGCAATGTGTACGCCGACTC CTTCGTCGTGAAGGGCGACGATGTGCGCCAGATCGCCCCTGGACAGACAGGCGTGATCGCCGATTACA ACTACAAGCTGCCTGACGACTTCACCGGCTGCGTGCTGGCCTGGAACACCAGAAACATCGACGCCACC CAGACAGGCAACTACAATTACAAGTACAGAAGCCTGCGGCACGGCAAGCTGCGGCCCTTCGAGAGGG ACATCTCCAACGTGCCCTTCAGCCCCGACGGCAAGCCTTGTACCCCCCCTGCCTTTAACTGCTACTGGC CCCTGAACGACTACGGCTTCTACATCACAAACGGCATCGGCTATCAGCCCTACCGGGTGGTGGTGCTG TCCTTTGAGCTGCTGAATGCCCCTGCCACCGTGTGCGGCCCTAAGCTGAGCACCGACCTGATCAAGAA CCAGTGCGTGAACTTCAACTTCAACGGCCTGACCGGCACCGGCGTGCTGACACCTAGCAGCAAGAGAT TCCAGCCCTTCCAGCAGTTCGGCCGGGACGTGCTGGATTTCACCGACAGCGTGCGGGACCCCAAGACC AGCGAGATCCTGGACATCAGCCCCTGCAGCTTCGGCGGAGTGTCCGTGATCACCCCCGGCACCAATAC CAGCTCTGAGGTGGCCGTGCTGTATCAGGACGTGAACTGCACCGATGTGCCCGTGGCCATCCACGCCG ATCAGCTGACCCCATCTTGGCGGGTGTACTCCACCGGCAACAACGTGTTCCAGACACAAGCCGGCTGC CTGATCGGAGCCGAGCACGTGGACACCAGCTACGAGTGCGACATCCCTATCGGCGCTGGCATCTGCGC CAGCTACCACACCGTGTCCAGCCTGAGAAGCACCAGCCAGAAATCTATCGTGGCCTACACCATGAGCC TGGGCGCCGACAGCTCTATCGCCTACTCCAACAACACAATCGCCATCCCCACCAATTTCAGCATCTCC ATCACCACCGAAGTGATGCCCGTGTCCATGGCCAAGACCTCCGTGGATTGCAACATGTACATCTGCGG CGACAGCACCGAGTGCGCCAACCTGCTGCTGCAGTACGGCAGCTTCTGCACCCAGCTGAACAGAGCCC TGAGCGGAATCGCCGTGGAACAGGACAGAAACACCCGGGAAGTGTTCGCCCAAGTGAAGCAGATGTA TAAGACCCCCACCCTGAAGGATTTCGGCGGCTTTAACTTCAGCCAGATCCTGCCCGACCCTCTGAAGC CTACCAAGCGGAGCTTCATCGAGGACCTGCTGTTCAACAAAGTGACCCTGGCCGACGCCGGCTTTATG AAGCAGTATGGCGAGTGCCTGGGCGACATCAACGCCCGGGATCTGATCTGCGCCCAGAAGTTTAACG GACTGACCGTGCTGCCCCCTCTGCTGACCGACGATATGATCGCCGCCTACACAGCCGCCCTGGTGTCT GGCACAGCTACCGCCGGATGGACATTTGGAGCTGGCGCCGCTCTGCAGATCCCCTTTGCCATGCAGAT GGCCTACCGGTTCAATGGCATCGGCGTGACCCAGAATGTGCTGTACGAGAACCAGAAGCAGATCGCC AACCAGTTCAACAAGGCCATTAGCCAGATTCAGGAAAGCCTGACCACCACCAGCACCGCCCTGGGCA AACTGCAGGACGTCGTGAACCAGAACGCCCAGGCCCTGAACACCCTCGTGAAGCAGCTGAGCAGCAA TTTCGGCGCCATCAGCTCCGTGCTGAACGATATCCTGAGCAGACTGGACAAGGTGGAAGCAGAGGTGC AGATCGACCGGCTGATCACCGGCAGACTGCAGAGCCTGCAGACCTACGTGACACAGCAGCTGATTAG AGCCGCCGAGATCAGGGCCAGCGCCAATCTGGCCGCCACAAAGATGAGCGAGTGTGTGCTGGGCCAG AGCAAGCGGGTGGACTTCTGCGGCAAGGGCTATCACCTGATGAGCTTCCCCCAGGCCGCTCCTCACGG CGTGGTGTTTCTGCACGTGACATACGTGCCCAGCCAGGAACGGAACTTCACCACCGCCCCAGCCATCT GCCACGAGGGCAAGGCCTACTTCCCCCGGGAAGGCGTGTTCGTGTTTAACGGCACCTCCTGGTTTATC ACCCAGCGGAATTTCTTCAGTCCGCAGATCATCACCACAGACAACACCTTCGTGTCCGGCAGCTGCGA CGTCGTGATTGGCATCATTAACAACACCGTGTACGACCCCCTGCAGCCCGAGCTGGACAGCTTCAAAG AGGAACTGGACAAGTACTTCAAGAACCACACCTCCCCCGACGTGGACCTGGGCGATATCTCCGGCATC AATGCCAGCGTCGTGAATATCCAGAAAGAGATCGATCGCCTGAACGAGGTGGCCAAGAACCTGAATG AGAGCCTGATCGACCTGCAGGAACTGGGGAAGTACGAGCAGTACATCAAGTGGCCTTGGTACGTGTG GCTGGGCTTTATCGCCGGCCTGATCGCCATCGTGATGGTCACCATCCTGCTGTGCTGCATGACCAGCTG TTGCAGCTGTCTGAAGGGCGCCTGCAGCTGTGGCTCCTGCTGCAAGTTCGATGAGGACGACAGCGAGC CTGTGCTGAAAGGCGTGAAGCTGCACTACACC (SEQ ID NO: 20) AUGUUUAUCUUCCUGUUCUUCCUGACCCUGACCAGCGGCAGCGACCUGGAAAGCUGCACCACCUUC GACGACGUGCAGGCCCCCAACUACCCUCAGCACAGCUCUAGCAGACGGGGCGUGUACUACCCCGAC GAGAUCUUCAGAAGCGACACCCUGUACCUGACCCAGGACCUGUUCCUGCCCUUCUACAGCAACGUG ACCGGCUUCCACACCAUCAACCACAGAUUCGACAACCCCGUGAUCCCCUUCAAGGACGGGGUGUAC UUUGCCGCCACCGAGAAGUCCAAUGUCGUGCGGGGAUGGGUGUUCGGCAGCACCAUGAACAACAAG AGCCAGAGCGUGAUCAUCAUCAACAACAGCACCAACGUCGUGAUCCGGGCCUGCAACUUCGAGCUG UGCGACAACCCAUUCUUCGCCGUGUCCAAGCCCACCGGCACCCAGACCCACACCAUGAUCUUCGAC AACGCCUUCAACUGCACCUUCGAGUACAUCAGCGACAGCUUCAGCCUGGACGUGGCCGAGAAAAGC GGCAACUUCAAGCACCUGAGAGAAUUCGUGUUCAAGAACAAGGACGGCUUCCUGUACGUGUACAA GGGCUACCAGCCCAUCGACGUCGUGCGCGAUCUGCCCAGCGGCUUCAACAUCCUGAAGCCCAUCUU CAAGCUGCCCCUGGGCAUCAACAUCACCAACUUCCGGGCUAUCCUGACCGCCUUCCUGCCCGCCCAG GAUACCUGGGGAACAAGCGCCGCUGCCUACUUCGUGGGCUACCUGAAGCCUGCCACCUUCAUGCUG AAGUACGACGAGAACGGCACCAUCACCGACGCCGUGGACUGCAGCCAGAAUCCUCUGGCCGAGCUG AAGUGCAGCGUGAAGUCCUUCGAGAUCGACAAGGGCAUCUACCAGACCAGCAACUUCAGAGUGGCC CCCAGCAAAGAAGUCGUGCGGUUCCCCAAUAUCACCAACCUGUGCCCCUUCGGCGAGGUGUUCAAC GCCACCACCUUUCCCAGCGUGUACGCCUGGGAGCGGAAGCGGAUCAGCAACUGCGUGGCCGACUAC AGCGUGCUGUACAACUCCACCAGCUUCUCCACCUUCAAGUGCUACGGCGUGUCCGCCACCAAGCUG AACGACCUGUGCUUCAGCAAUGUGUACGCCGACUCCUUCGUCGUGAAGGGCGACGAUGUGCGCCAG AUCGCCCCUGGACAGACAGGCGUGAUCGCCGAUUACAACUACAAGCUGCCUGACGACUUCACCGGC UGCGUGCUGGCCUGGAACACCAGAAACAUCGACGCCACCCAGACAGGCAACUACAAUUACAAGUAC AGAAGCCUGCGGCACGGCAAGCUGCGGCCCUUCGAGAGGGACAUCUCCAACGUGCCCUUCAGCCCC GACGGCAAGCCUUGUACCCCCCCUGCCUUUAACUGCUACUGGCCCCUGAACGACUACGGCUUCUAC AUCACAAACGGCAUCGGCUAUCAGCCCUACCGGGUGGUGGUGCUGUCCUUUGAGCUGCUGAAUGCC CCUGCCACCGUGUGCGGCCCUAAGCUGAGCACCGACCUGAUCAAGAACCAGUGCGUGAACUUCAAC UUCAACGGCCUGACCGGCACCGGCGUGCUGACACCUAGCAGCAAGAGAUUCCAGCCCUUCCAGCAG UUCGGCCGGGACGUGCUGGAUUUCACCGACAGCGUGCGGGACCCCAAGACCAGCGAGAUCCUGGAC AUCAGCCCCUGCAGCUUCGGCGGAGUGUCCGUGAUCACCCCCGGCACCAAUACCAGCUCUGAGGUG GCCGUGCUGUAUCAGGACGUGAACUGCACCGAUGUGCCCGUGGCCAUCCACGCCGAUCAGCUGACC CCAUCUUGGCGGGUGUACUCCACCGGCAACAACGUGUUCCAGACACAAGCCGGCUGCCUGAUCGGA GCCGAGCACGUGGACACCAGCUACGAGUGCGACAUCCCUAUCGGCGCUGGCAUCUGCGCCAGCUAC CACACCGUGUCCAGCCUGAGAAGCACCAGCCAGAAAUCUAUCGUGGCCUACACCAUGAGCCUGGGC GCCGACAGCUCUAUCGCCUACUCCAACAACACAAUCGCCAUCCCCACCAAUUUCAGCAUCUCCAUC ACCACCGAAGUGAUGCCCGUGUCCAUGGCCAAGACCUCCGUGGAUUGCAACAUGUACAUCUGCGGC GACAGCACCGAGUGCGCCAACCUGCUGCUGCAGUACGGCAGCUUCUGCACCCAGCUGAACAGAGCC CUGAGCGGAAUCGCCGUGGAACAGGACAGAAACACCCGGGAAGUGUUCGCCCAAGUGAAGCAGAU GUAUAAGACCCCCACCCUGAAGGAUUUCGGCGGCUUUAACUUCAGCCAGAUCCUGCCCGACCCUCU GAAGCCUACCAAGCGGAGCUUCAUCGAGGACCUGCUGUUCAACAAAGUGACCCUGGCCGACGCCGG CUUUAUGAAGCAGUAUGGCGAGUGCCUGGGCGACAUCAACGCCCGGGAUCUGAUCUGCGCCCAGAA GUUUAACGGACUGACCGUGCUGCCCCCUCUGCUGACCGACGAUAUGAUCGCCGCCUACACAGCCGC CCUGGUGUCUGGCACAGCUACCGCCGGAUGGACAUUUGGAGCUGGCGCCGCUCUGCAGAUCCCCUU UGCCAUGCAGAUGGCCUACCGGUUCAAUGGCAUCGGCGUGACCCAGAAUGUGCUGUACGAGAACCA GAAGCAGAUCGCCAACCAGUUCAACAAGGCCAUUAGCCAGAUUCAGGAAAGCCUGACCACCACCAG CACCGCCCUGGGCAAACUGCAGGACGUCGUGAACCAGAACGCCCAGGCCCUGAACACCCUCGUGAA GCAGCUGAGCAGCAAUUUCGGCGCCAUCAGCUCCGUGCUGAACGAUAUCCUGAGCAGACUGGACAA GGUGGAAGCAGAGGUGCAGAUCGACCGGCUGAUCACCGGCAGACUGCAGAGCCUGCAGACCUACGU GACACAGCAGCUGAUUAGAGCCGCCGAGAUCAGGGCCAGCGCCAAUCUGGCCGCCACAAAGAUGAG CGAGUGUGUGCUGGGCCAGAGCAAGCGGGUGGACUUCUGCGGCAAGGGCUAUCACCUGAUGAGCU UCCCCCAGGCCGCUCCUCACGGCGUGGUGUUUCUGCACGUGACAUACGUGCCCAGCCAGGAACGGA ACUUCACCACCGCCCCAGCCAUCUGCCACGAGGGCAAGGCCUACUUCCCCCGGGAAGGCGUGUUCG UGUUUAACGGCACCUCCUGGUUUAUCACCCAGCGGAAUUUCUUCAGUCCGCAGAUCAUCACCACAG ACAACACCUUCGUGUCCGGCAGCUGCGACGUCGUGAUUGGCAUCAUUAACAACACCGUGUACGACC CCCUGCAGCCCGAGCUGGACAGCUUCAAAGAGGAACUGGACAAGUACUUCAAGAACCACACCUCCC CCGACGUGGACCUGGGCGAUAUCUCCGGCAUCAAUGCCAGCGUCGUGAAUAUCCAGAAAGAGAUCG AUCGCCUGAACGAGGUGGCCAAGAACCUGAAUGAGAGCCUGAUCGACCUGCAGGAACUGGGGAAG UACGAGCAGUACAUCAAGUGGCCUUGGUACGUGUGGCUGGGCUUUAUCGCCGGCCUGAUCGCCAUC GUGAUGGUCACCAUCCUGCUGUGCUGCAUGACCAGCUGUUGCAGCUGUCUGAAGGGCGCCUGCAGC UGUGGCUCCUGCUGCAAGUUCGAUGAGGACGACAGCGAGCCUGUGCUGAAAGGCGUGAAGCUGCA CUACACC
Sequence CWU 1 SEQUENCE LISTING <160> NUMBER OF SEQ ID
NOS: 31 <210> SEQ ID NO 1 <211> LENGTH: 490 <212>
TYPE: PRT <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polypeptide
<400> SEQUENCE: 1 Met Gly Gln Ile Val Thr Phe Phe Gln Glu Val
Pro His Val Ile Glu 1 5 10 15 Glu Val Met Asn Ile Val Leu Ile Ala
Leu Ser Leu Leu Ala Ile Leu 20 25 30 Lys Gly Ile Tyr Asn Val Ala
Thr Cys Gly Leu Phe Gly Leu Val Ser 35 40 45 Phe Leu Leu Leu Cys
Gly Arg Ser Cys Ser Thr Thr Tyr Lys Gly Val 50 55 60 Tyr Glu Leu
Gln Thr Leu Glu Leu Asp Met Ala Ser Leu Asn Met Thr 65 70 75 80 Met
Pro Leu Ser Cys Thr Lys Asn Asn Ser His His Tyr Ile Met Val 85 90
95 Gly Asn Glu Thr Gly Leu Glu Leu Thr Leu Thr Asn Thr Ser Ile Ile
100 105 110 Asn His Lys Phe Cys Asn Leu Ser Asp Ala His Lys Lys Asp
Leu Tyr 115 120 125 Asp His Ala Leu Met Ser Ile Ile Ser Thr Phe His
Leu Ser Ile Pro 130 135 140 Asn Phe Asn Gln Tyr Glu Ala Met Ser Cys
Asp Phe Asn Gly Gly Lys 145 150 155 160 Ile Ser Val Gln Tyr Asn Leu
Ser His Thr Tyr Ala Val Asp Ala Ala 165 170 175 Asn His Cys Gly Thr
Ile Ala Asn Gly Val Leu Gln Thr Phe Met Arg 180 185 190 Met Ala Trp
Gly Gly Ser Tyr Ile Ala Leu Asp Ser Gly Lys Gly Ser 195 200 205 Trp
Asp Cys Ile Met Thr Ser Tyr Gln Tyr Leu Ile Ile Gln Asn Thr 210 215
220 Thr Trp Glu Asp His Cys Gln Phe Ser Arg Pro Ser Pro Ile Gly Tyr
225 230 235 240 Leu Gly Leu Leu Ser Gln Arg Thr Arg Asp Ile Tyr Ile
Ser Arg Arg 245 250 255 Leu Leu Gly Thr Phe Thr Trp Thr Leu Ser Asp
Ser Glu Gly Asn Glu 260 265 270 Thr Pro Gly Gly Tyr Cys Leu Thr Arg
Trp Met Leu Ile Glu Ala Glu 275 280 285 Leu Lys Cys Phe Gly Asn Thr
Ala Val Ala Lys Cys Asn Glu Lys His 290 295 300 Asp Glu Glu Phe Cys
Asp Met Leu Arg Leu Phe Asp Phe Asn Lys Gln 305 310 315 320 Ala Ile
Met Arg Leu Lys Thr Glu Ala Gln Met Ser Ile Gln Leu Ile 325 330 335
Asn Lys Ala Val Asn Ala Leu Ile Asn Asp Gln Leu Ile Met Lys Asn 340
345 350 His Leu Arg Asp Ile Met Gly Ile Pro Tyr Cys Asn Tyr Ser Lys
Tyr 355 360 365 Trp Tyr Leu Asn His Thr Val Thr Gly Lys Thr Ser Leu
Pro Arg Cys 370 375 380 Trp Leu Val Ser Asn Gly Ser Tyr Leu Asn Glu
Thr Arg Phe Ser Asp 385 390 395 400 Asp Ile Glu Gln Gln Ala Asp Asn
Met Ile Thr Glu Met Leu Gln Lys 405 410 415 Glu Tyr Leu Asp Arg Gln
Gly Lys Thr Pro Leu Gly Leu Val Asp Leu 420 425 430 Phe Val Phe Ser
Thr Ser Phe Tyr Leu Ile Ser Ile Phe Leu His Leu 435 440 445 Val Lys
Ile Pro Thr His Arg His Ile Ile Gly Lys Pro Cys Pro Lys 450 455 460
Pro His Arg Leu Asn His Met Gly Ile Cys Ser Cys Gly Leu Tyr Lys 465
470 475 480 His Pro Gly Val Pro Val Lys Trp Lys Arg 485 490
<210> SEQ ID NO 2 <211> LENGTH: 569 <212> TYPE:
PRT <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 2 Met Ser Ala Ser Lys Glu Val Lys Ser Phe Leu Trp Thr Gln
Ser Leu 1 5 10 15 Arg Arg Glu Leu Ser Gly Tyr Cys Ser Asn Ile Lys
Leu Gln Val Val 20 25 30 Lys Asp Ala Gln Ala Leu Leu His Gly Leu
Asp Phe Ser Glu Val Ser 35 40 45 Asn Val Gln Arg Leu Met Arg Lys
Gln Lys Arg Asp Asp Gly Asp Leu 50 55 60 Lys Arg Leu Arg Asp Leu
Asn Gln Ala Val Asn Asn Leu Val Glu Leu 65 70 75 80 Lys Ser Thr Gln
Gln Lys Ser Val Leu Arg Val Gly Thr Leu Ser Ser 85 90 95 Asp Asp
Leu Leu Val Leu Ala Ala Asp Leu Glu Lys Leu Lys Ser Lys 100 105 110
Val Val Arg Thr Glu Arg Pro Leu Ser Ser Gly Ile Tyr Met Gly Asn 115
120 125 Leu Ser Ser Gln Gln Leu Asp Gln Arg Lys Ala Leu Leu Asn Met
Ile 130 135 140 Gly Met Thr Gly Gly Asn Gly Gly Arg Asn Thr Thr Ser
Asp Gly Ile 145 150 155 160 Val Arg Val Trp Asp Val Lys Asn Ala Glu
Leu Leu Asn Asn Gln Phe 165 170 175 Gly Thr Met Pro Ser Leu Thr Leu
Ala Cys Leu Thr Lys Gln Gly Gln 180 185 190 Val Asp Leu Asn Asp Ala
Val Gln Ala Leu Thr Asp Leu Gly Leu Ile 195 200 205 Tyr Thr Ala Lys
Tyr Pro Asn Ser Ser Asp Leu Asp Arg Leu Ala Gln 210 215 220 Ser His
Pro Ile Leu Asn Met Ile Asp Thr Lys Lys Ser Ser Leu Asn 225 230 235
240 Ile Ser Gly Tyr Asn Phe Ser Leu Gly Ala Ala Val Lys Ala Gly Ala
245 250 255 Cys Met Leu Asp Gly Gly Asn Met Leu Glu Thr Ile Lys Val
Ser Pro 260 265 270 Gln Thr Met Asp Gly Ile Leu Lys Ser Ile Leu Lys
Val Lys Arg Ser 275 280 285 Leu Gly Met Phe Ile Ser Asp Thr Pro Gly
Glu Arg Asn Pro Tyr Glu 290 295 300 Asn Ile Leu Tyr Lys Ile Cys Leu
Ser Gly Asp Gly Trp Pro Tyr Ile 305 310 315 320 Ala Ser Arg Thr Ser
Ile Val Gly Arg Ala Trp Glu Asn Thr Val Val 325 330 335 Asp Leu Glu
Ser Asp Asn Lys Pro Gln Lys Thr Gly Asn Gly Gly Ser 340 345 350 Asn
Lys Ser Leu Gln Ser Ala Gly Phe Ala Ala Gly Leu Thr Tyr Ser 355 360
365 Gln Leu Met Thr Leu Lys Asp Ser Met Leu Gln Leu Asp Pro Asn Ala
370 375 380 Lys Thr Trp Met Asp Ile Glu Gly Arg Pro Glu Asp Pro Val
Glu Ile 385 390 395 400 Ala Leu Tyr Gln Pro Ser Ser Gly Cys Tyr Ile
His Phe Phe Arg Glu 405 410 415 Pro Thr Asp Leu Lys Gln Phe Lys Gln
Asp Ala Lys Tyr Ser His Gly 420 425 430 Ile Asp Val Thr Asp Leu Phe
Ala Ala Gln Pro Gly Leu Thr Ser Ala 435 440 445 Val Ile Glu Ala Leu
Pro Arg Asn Met Val Ile Thr Cys Gln Gly Ser 450 455 460 Glu Asp Ile
Arg Lys Leu Leu Glu Ser Gln Gly Arg Arg Asp Ile Lys 465 470 475 480
Leu Ile Asp Ile Ser Leu Ser Lys Val Asp Ser Arg Lys Phe Glu Asn 485
490 495 Ala Val Trp Asp Gln Phe Lys Asp Leu Cys His Met His Thr Gly
Ile 500 505 510 Val Val Glu Lys Lys Lys Arg Gly Gly Lys Glu Glu Ile
Thr Pro His 515 520 525 Cys Ala Leu Met Asp Cys Ile Met Phe Asp Ala
Ala Val Ser Gly Gly 530 535 540 Val Asp Ala Lys Val Leu Arg Ala Val
Leu Pro Arg Asp Met Val Phe 545 550 555 560 Arg Thr Ser Thr Pro Lys
Val Val Leu 565 <210> SEQ ID NO 3 <211> LENGTH: 589
<212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 3 Met Glu Thr Pro Ala Gln Leu Leu
Phe Leu Leu Leu Leu Trp Leu Pro 1 5 10 15 Asp Thr Thr Gly Met Ser
Ala Ser Lys Glu Val Lys Ser Phe Leu Trp 20 25 30 Thr Gln Ser Leu
Arg Arg Glu Leu Ser Gly Tyr Cys Ser Asn Ile Lys 35 40 45 Leu Gln
Val Val Lys Asp Ala Gln Ala Leu Leu His Gly Leu Asp Phe 50 55 60
Ser Glu Val Ser Asn Val Gln Arg Leu Met Arg Lys Gln Lys Arg Asp 65
70 75 80 Asp Gly Asp Leu Lys Arg Leu Arg Asp Leu Asn Gln Ala Val
Asn Asn 85 90 95 Leu Val Glu Leu Lys Ser Thr Gln Gln Lys Ser Val
Leu Arg Val Gly 100 105 110 Thr Leu Ser Ser Asp Asp Leu Leu Val Leu
Ala Ala Asp Leu Glu Lys 115 120 125 Leu Lys Ser Lys Val Val Arg Thr
Glu Arg Pro Leu Ser Ser Gly Ile 130 135 140 Tyr Met Gly Asn Leu Ser
Ser Gln Gln Leu Asp Gln Arg Lys Ala Leu 145 150 155 160 Leu Asn Met
Ile Gly Met Thr Gly Gly Asn Gly Gly Arg Asn Thr Thr 165 170 175 Ser
Asp Gly Ile Val Arg Val Trp Asp Val Lys Asn Ala Glu Leu Leu 180 185
190 Asn Asn Gln Phe Gly Thr Met Pro Ser Leu Thr Leu Ala Cys Leu Thr
195 200 205 Lys Gln Gly Gln Val Asp Leu Asn Asp Ala Val Gln Ala Leu
Thr Asp 210 215 220 Leu Gly Leu Ile Tyr Thr Ala Lys Tyr Pro Asn Ser
Ser Asp Leu Asp 225 230 235 240 Arg Leu Ala Gln Ser His Pro Ile Leu
Asn Met Ile Asp Thr Lys Lys 245 250 255 Ser Ser Leu Asn Ile Ser Gly
Tyr Asn Phe Ser Leu Gly Ala Ala Val 260 265 270 Lys Ala Gly Ala Cys
Met Leu Asp Gly Gly Asn Met Leu Glu Thr Ile 275 280 285 Lys Val Ser
Pro Gln Thr Met Asp Gly Ile Leu Lys Ser Ile Leu Lys 290 295 300 Val
Lys Arg Ser Leu Gly Met Phe Ile Ser Asp Thr Pro Gly Glu Arg 305 310
315 320 Asn Pro Tyr Glu Asn Ile Leu Tyr Lys Ile Cys Leu Ser Gly Asp
Gly 325 330 335 Trp Pro Tyr Ile Ala Ser Arg Thr Ser Ile Val Gly Arg
Ala Trp Glu 340 345 350 Asn Thr Val Val Asp Leu Glu Ser Asp Asn Lys
Pro Gln Lys Thr Gly 355 360 365 Asn Gly Gly Ser Asn Lys Ser Leu Gln
Ser Ala Gly Phe Ala Ala Gly 370 375 380 Leu Thr Tyr Ser Gln Leu Met
Thr Leu Lys Asp Ser Met Leu Gln Leu 385 390 395 400 Asp Pro Asn Ala
Lys Thr Trp Met Asp Ile Glu Gly Arg Pro Glu Asp 405 410 415 Pro Val
Glu Ile Ala Leu Tyr Gln Pro Ser Ser Gly Cys Tyr Ile His 420 425 430
Phe Phe Arg Glu Pro Thr Asp Leu Lys Gln Phe Lys Gln Asp Ala Lys 435
440 445 Tyr Ser His Gly Ile Asp Val Thr Asp Leu Phe Ala Ala Gln Pro
Gly 450 455 460 Leu Thr Ser Ala Val Ile Glu Ala Leu Pro Arg Asn Met
Val Ile Thr 465 470 475 480 Cys Gln Gly Ser Glu Asp Ile Arg Lys Leu
Leu Glu Ser Gln Gly Arg 485 490 495 Arg Asp Ile Lys Leu Ile Asp Ile
Ser Leu Ser Lys Val Asp Ser Arg 500 505 510 Lys Phe Glu Asn Ala Val
Trp Asp Gln Phe Lys Asp Leu Cys His Met 515 520 525 His Thr Gly Ile
Val Val Glu Lys Lys Lys Arg Gly Gly Lys Glu Glu 530 535 540 Ile Thr
Pro His Cys Ala Leu Met Asp Cys Ile Met Phe Asp Ala Ala 545 550 555
560 Val Ser Gly Gly Val Asp Ala Lys Val Leu Arg Ala Val Leu Pro Arg
565 570 575 Asp Met Val Phe Arg Thr Ser Thr Pro Lys Val Val Leu 580
585 <210> SEQ ID NO 4 <211> LENGTH: 1470 <212>
TYPE: DNA <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polynucleotide
<400> SEQUENCE: 4 atgggccaga tcgtgacatt cttccaagag gtgccccacg
tgatcgagga agtgatgaac 60 atcgtcctga tcgccctgag cctgctggcc
atcctgaagg gcatctacaa cgtggccacc 120 tgtggcctgt ttggcctggt
gtcattcctg ctgctgtgcg gcagaagctg cagcaccaca 180 tacaagggcg
tgtacgagct gcagaccctg gaactggata tggccagcct gaacatgacc 240
atgcctctga gctgcaccaa gaacaacagc caccactaca tcatggtcgg aaacgagaca
300 ggactggaac tgaccctgac caacaccagc atcatcaacc acaagttctg
caacctgagc 360 gacgcccaca agaaggacct gtacgatcac gccctgatga
gcatcatctc caccttccac 420 ctgagcatcc ccaacttcaa ccagtacgag
gccatgagct gcgacttcaa cggcggcaag 480 atcagcgtgc agtacaatct
gagccacacc tacgccgtgg acgccgccaa tcactgtggc 540 acaattgcca
atggcgtgct gcagacattc atgcggatgg cctggggcgg ctcttatatc 600
gccctggatt ctggcaaagg cagctgggac tgcatcatga ccagctacca gtacctgatc
660 atccagaaca ccacctggga agatcactgc cagttcagca gaccctctcc
tatcggctat 720 ctgggcctgc tgagccagag aacccgggac atctacatca
gcagaaggct gctgggcacc 780 ttcacctgga cactgtctga cagcgagggc
aacgaaacac ctggcggcta ctgcctgacc 840 agatggatgc tgattgaggc
cgagctgaag tgcttcggca ataccgccgt ggccaagtgc 900 aacgagaagc
acgacgagga attctgcgac atgctgcggc tgttcgattt caacaagcag 960
gccatcatgc ggctcaagac cgaggctcag atgtccatcc agctgatcaa caaggccgtg
1020 aatgccctga tcaacgatca gctcatcatg aagaaccacc tccgggatat
catgggcatc 1080 ccttactgca actacagcaa gtactggtat ctcaaccaca
ccgtgaccgg caagaccagc 1140 ctgcctagat gttggctggt gtccaacggc
agctacctga acgagacacg gttcagcgac 1200 gacatcgagc agcaggccga
caacatgatc accgagatgc tgcagaaaga gtacctggac 1260 cggcagggca
agacacctct gggactcgtg gatctgttcg tgttcagcac cagcttctac 1320
ctgatctcta tcttcctgca cctggtcaag atccccacac accggcacat catcggcaag
1380 ccctgtccta agcctcaccg gctgaaccac atgggaatct gtagctgcgg
cctgtacaag 1440 caccctggcg tgccagtgaa gtggaagaga 1470 <210>
SEQ ID NO 5 <211> LENGTH: 1767 <212> TYPE: DNA
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 5 atggagactc ctgcccagct cttgttcctt ttgctattgt ggcttcccga
caccaccggc 60 atgagcgcca gcaaggaggt caagagcttc ctctggaccc
agagcctaag aagagagctt 120 agcggctact gcagcaacat caagcttcag
gtggtgaagg acgcccaggc cctgctgcac 180 ggcctggact tcagcgaggt
gagcaacgtg cagagactga tgagaaagca gaagcgagac 240 gacggcgacc
tgaagcgtct gcgggacctg aaccaggccg tgaacaacct ggtggagctt 300
aagagcaccc agcagaagtc tgtgctgaga gtgggcaccc tgagcagcga cgacctgctg
360 gtgctggccg ccgacctgga gaagctgaag tctaaggtcg tcagaaccga
gcggccattg 420 agctcaggca tctacatggg caaccttagc agtcagcagc
tggaccagag aaaggccttg 480 ctgaacatga tcggcatgac cggcggcaac
ggcggcagaa acaccaccag cgacggcatc 540 gtgagagtgt gggacgtgaa
gaacgccgag ctactcaaca accagttcgg caccatgccc 600 agcctgaccc
tggcctgcct gaccaagcag ggccaggtgg acctcaatga cgccgtgcag 660
gcactaaccg accttggcct gatctacacc gccaagtacc ccaactcttc agacctggac
720 agactggcgc agtcccaccc catcttaaat atgattgaca ccaagaagtc
atcccttaac 780 atcagtggct acaacttcag cctgggcgcc gccgtgaagg
ccggcgcctg catgctggac 840 ggcggaaata tgctggaaac tatcaaggtg
agccctcaga ccatggacgg tatcctgaag 900 tccattttga aggttaagag
atccctgggt atgttcatca gcgacacccc aggcgagaga 960 aacccctacg
agaacatcct gtacaagatc tgcctgagtg gcgacggctg gccctacatc 1020
gcgagcagaa ccagcatcgt gggaagggcc tgggagaaca ccgtggtgga tcttgagagc
1080 gacaacaagc cccagaagac cggaaatggc ggttcaaaca agagcctgca
gagcgccggc 1140 ttcgccgccg gcctgaccta cagccagctg atgaccctga
aggacagcat gctacaattg 1200 gatcccaacg ccaagacttg gatggacatc
gagggcagac ccgaggaccc cgtggagatc 1260 gccctgtacc agccctcatc
cggctgctac atccacttct tcagagagcc cacagatctg 1320 aagcagttca
agcaggacgc gaagtatagc catggcatag acgtcaccga tttattcgcg 1380
gcccagccgg gccttacgag cgccgtgatc gaggcgctgc ccagaaacat ggtgatcacc
1440 tgccagggca gcgaggacat cagaaagctc cttgaatctc aaggccggag
agatattaag 1500 ctgatagata tcagcttatc taaggttgac agcagaaagt
tcgagaacgc tgtatgggac 1560 caattcaagg acctgtgcca catgcatacg
ggcatagtgg tagagaagaa gaagcgtggc 1620 ggaaaggagg agatcacacc
tcactgcgcc ctgatggact gcatcatgtt cgacgcggca 1680 gtctccggcg
gcgtcgacgc aaaggtcctc cgggccgtgc tgccaaggga catggtgttc 1740
cggacaagca cccctaaggt agtgctg 1767 <210> SEQ ID NO 6
<211> LENGTH: 1470 <212> TYPE: RNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE: 6
augggccaga ucgugacauu cuuccaagag gugccccacg ugaucgagga agugaugaac
60 aucguccuga ucgcccugag ccugcuggcc auccugaagg gcaucuacaa
cguggccacc 120 uguggccugu uuggccuggu gucauuccug cugcugugcg
gcagaagcug cagcaccaca 180 uacaagggcg uguacgagcu gcagacccug
gaacuggaua uggccagccu gaacaugacc 240 augccucuga gcugcaccaa
gaacaacagc caccacuaca ucauggucgg aaacgagaca 300 ggacuggaac
ugacccugac caacaccagc aucaucaacc acaaguucug caaccugagc 360
gacgcccaca agaaggaccu guacgaucac gcccugauga gcaucaucuc caccuuccac
420 cugagcaucc ccaacuucaa ccaguacgag gccaugagcu gcgacuucaa
cggcggcaag 480 aucagcgugc aguacaaucu gagccacacc uacgccgugg
acgccgccaa ucacuguggc 540 acaauugcca auggcgugcu gcagacauuc
augcggaugg ccuggggcgg cucuuauauc 600 gcccuggauu cuggcaaagg
cagcugggac ugcaucauga ccagcuacca guaccugauc 660 auccagaaca
ccaccuggga agaucacugc caguucagca gacccucucc uaucggcuau 720
cugggccugc ugagccagag aacccgggac aucuacauca gcagaaggcu gcugggcacc
780 uucaccugga cacugucuga cagcgagggc aacgaaacac cuggcggcua
cugccugacc 840 agauggaugc ugauugaggc cgagcugaag ugcuucggca
auaccgccgu ggccaagugc 900 aacgagaagc acgacgagga auucugcgac
augcugcggc uguucgauuu caacaagcag 960 gccaucaugc ggcucaagac
cgaggcucag auguccaucc agcugaucaa caaggccgug 1020 aaugcccuga
ucaacgauca gcucaucaug aagaaccacc uccgggauau caugggcauc 1080
ccuuacugca acuacagcaa guacugguau cucaaccaca ccgugaccgg caagaccagc
1140 cugccuagau guuggcuggu guccaacggc agcuaccuga acgagacacg
guucagcgac 1200 gacaucgagc agcaggccga caacaugauc accgagaugc
ugcagaaaga guaccuggac 1260 cggcagggca agacaccucu gggacucgug
gaucuguucg uguucagcac cagcuucuac 1320 cugaucucua ucuuccugca
ccuggucaag auccccacac accggcacau caucggcaag 1380 cccuguccua
agccucaccg gcugaaccac augggaaucu guagcugcgg ccuguacaag 1440
cacccuggcg ugccagugaa guggaagaga 1470 <210> SEQ ID NO 7
<211> LENGTH: 1767 <212> TYPE: RNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE: 7
auggagacuc cugcccagcu cuuguuccuu uugcuauugu ggcuucccga caccaccggc
60 augagcgcca gcaaggaggu caagagcuuc cucuggaccc agagccuaag
aagagagcuu 120 agcggcuacu gcagcaacau caagcuucag guggugaagg
acgcccaggc ccugcugcac 180 ggccuggacu ucagcgaggu gagcaacgug
cagagacuga ugagaaagca gaagcgagac 240 gacggcgacc ugaagcgucu
gcgggaccug aaccaggccg ugaacaaccu gguggagcuu 300 aagagcaccc
agcagaaguc ugugcugaga gugggcaccc ugagcagcga cgaccugcug 360
gugcuggccg ccgaccugga gaagcugaag ucuaaggucg ucagaaccga gcggccauug
420 agcucaggca ucuacauggg caaccuuagc agucagcagc uggaccagag
aaaggccuug 480 cugaacauga ucggcaugac cggcggcaac ggcggcagaa
acaccaccag cgacggcauc 540 gugagagugu gggacgugaa gaacgccgag
cuacucaaca accaguucgg caccaugccc 600 agccugaccc uggccugccu
gaccaagcag ggccaggugg accucaauga cgccgugcag 660 gcacuaaccg
accuuggccu gaucuacacc gccaaguacc ccaacucuuc agaccuggac 720
agacuggcgc agucccaccc caucuuaaau augauugaca ccaagaaguc aucccuuaac
780 aucaguggcu acaacuucag ccugggcgcc gccgugaagg ccggcgccug
caugcuggac 840 ggcggaaaua ugcuggaaac uaucaaggug agcccucaga
ccauggacgg uauccugaag 900 uccauuuuga agguuaagag aucccugggu
auguucauca gcgacacccc aggcgagaga 960 aaccccuacg agaacauccu
guacaagauc ugccugagug gcgacggcug gcccuacauc 1020 gcgagcagaa
ccagcaucgu gggaagggcc ugggagaaca ccguggugga ucuugagagc 1080
gacaacaagc cccagaagac cggaaauggc gguucaaaca agagccugca gagcgccggc
1140 uucgccgccg gccugaccua cagccagcug augacccuga aggacagcau
gcuacaauug 1200 gaucccaacg ccaagacuug gauggacauc gagggcagac
ccgaggaccc cguggagauc 1260 gcccuguacc agcccucauc cggcugcuac
auccacuucu ucagagagcc cacagaucug 1320 aagcaguuca agcaggacgc
gaaguauagc cauggcauag acgucaccga uuuauucgcg 1380 gcccagccgg
gccuuacgag cgccgugauc gaggcgcugc ccagaaacau ggugaucacc 1440
ugccagggca gcgaggacau cagaaagcuc cuugaaucuc aaggccggag agauauuaag
1500 cugauagaua ucagcuuauc uaagguugac agcagaaagu ucgagaacgc
uguaugggac 1560 caauucaagg accugugcca caugcauacg ggcauagugg
uagagaagaa gaagcguggc 1620 ggaaaggagg agaucacacc ucacugcgcc
cugauggacu gcaucauguu cgacgcggca 1680 gucuccggcg gcgucgacgc
aaagguccuc cgggccgugc ugccaaggga caugguguuc 1740 cggacaagca
ccccuaaggu agugcug 1767 <210> SEQ ID NO 8 <211> LENGTH:
1707 <212> TYPE: DNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 8 atgagcgcca
gcaaggaggt caagagcttc ctctggaccc agagcctaag aagagagctt 60
agcggctact gcagcaacat caagcttcag gtggtgaagg acgcccaggc cctgctgcac
120 ggcctggact tcagcgaggt gagcaacgtg cagagactga tgagaaagca
gaagcgagac 180 gacggcgacc tgaagcgtct gcgggacctg aaccaggccg
tgaacaacct ggtggagctt 240 aagagcaccc agcagaagtc tgtgctgaga
gtgggcaccc tgagcagcga cgacctgctg 300 gtgctggccg ccgacctgga
gaagctgaag tctaaggtcg tcagaaccga gcggccattg 360 agctcaggca
tctacatggg caaccttagc agtcagcagc tggaccagag aaaggccttg 420
ctgaacatga tcggcatgac cggcggcaac ggcggcagaa acaccaccag cgacggcatc
480 gtgagagtgt gggacgtgaa gaacgccgag ctactcaaca accagttcgg
caccatgccc 540 agcctgaccc tggcctgcct gaccaagcag ggccaggtgg
acctcaatga cgccgtgcag 600 gcactaaccg accttggcct gatctacacc
gccaagtacc ccaactcttc agacctggac 660 agactggcgc agtcccaccc
catcttaaat atgattgaca ccaagaagtc atcccttaac 720 atcagtggct
acaacttcag cctgggcgcc gccgtgaagg ccggcgcctg catgctggac 780
ggcggaaata tgctggaaac tatcaaggtg agccctcaga ccatggacgg tatcctgaag
840 tccattttga aggttaagag atccctgggt atgttcatca gcgacacccc
aggcgagaga 900 aacccctacg agaacatcct gtacaagatc tgcctgagtg
gcgacggctg gccctacatc 960 gcgagcagaa ccagcatcgt gggaagggcc
tgggagaaca ccgtggtgga tcttgagagc 1020 gacaacaagc cccagaagac
cggaaatggc ggttcaaaca agagcctgca gagcgccggc 1080 ttcgccgccg
gcctgaccta cagccagctg atgaccctga aggacagcat gctacaattg 1140
gatcccaacg ccaagacttg gatggacatc gagggcagac ccgaggaccc cgtggagatc
1200 gccctgtacc agccctcatc cggctgctac atccacttct tcagagagcc
cacagatctg 1260 aagcagttca agcaggacgc gaagtatagc catggcatag
acgtcaccga tttattcgcg 1320 gcccagccgg gccttacgag cgccgtgatc
gaggcgctgc ccagaaacat ggtgatcacc 1380 tgccagggca gcgaggacat
cagaaagctc cttgaatctc aaggccggag agatattaag 1440 ctgatagata
tcagcttatc taaggttgac agcagaaagt tcgagaacgc tgtatgggac 1500
caattcaagg acctgtgcca catgcatacg ggcatagtgg tagagaagaa gaagcgtggc
1560 ggaaaggagg agatcacacc tcactgcgcc ctgatggact gcatcatgtt
cgacgcggca 1620 gtctccggcg gcgtcgacgc aaaggtcctc cgggccgtgc
tgccaaggga catggtgttc 1680 cggacaagca cccctaaggt agtgctg 1707
<210> SEQ ID NO 9 <211> LENGTH: 1707 <212> TYPE:
RNA <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 9 augagcgcca gcaaggaggu caagagcuuc cucuggaccc agagccuaag
aagagagcuu 60 agcggcuacu gcagcaacau caagcuucag guggugaagg
acgcccaggc ccugcugcac 120 ggccuggacu ucagcgaggu gagcaacgug
cagagacuga ugagaaagca gaagcgagac 180 gacggcgacc ugaagcgucu
gcgggaccug aaccaggccg ugaacaaccu gguggagcuu 240 aagagcaccc
agcagaaguc ugugcugaga gugggcaccc ugagcagcga cgaccugcug 300
gugcuggccg ccgaccugga gaagcugaag ucuaaggucg ucagaaccga gcggccauug
360 agcucaggca ucuacauggg caaccuuagc agucagcagc uggaccagag
aaaggccuug 420 cugaacauga ucggcaugac cggcggcaac ggcggcagaa
acaccaccag cgacggcauc 480 gugagagugu gggacgugaa gaacgccgag
cuacucaaca accaguucgg caccaugccc 540 agccugaccc uggccugccu
gaccaagcag ggccaggugg accucaauga cgccgugcag 600 gcacuaaccg
accuuggccu gaucuacacc gccaaguacc ccaacucuuc agaccuggac 660
agacuggcgc agucccaccc caucuuaaau augauugaca ccaagaaguc aucccuuaac
720 aucaguggcu acaacuucag ccugggcgcc gccgugaagg ccggcgccug
caugcuggac 780 ggcggaaaua ugcuggaaac uaucaaggug agcccucaga
ccauggacgg uauccugaag 840 uccauuuuga agguuaagag aucccugggu
auguucauca gcgacacccc aggcgagaga 900 aaccccuacg agaacauccu
guacaagauc ugccugagug gcgacggcug gcccuacauc 960 gcgagcagaa
ccagcaucgu gggaagggcc ugggagaaca ccguggugga ucuugagagc 1020
gacaacaagc cccagaagac cggaaauggc gguucaaaca agagccugca gagcgccggc
1080 uucgccgccg gccugaccua cagccagcug augacccuga aggacagcau
gcuacaauug 1140 gaucccaacg ccaagacuug gauggacauc gagggcagac
ccgaggaccc cguggagauc 1200 gcccuguacc agcccucauc cggcugcuac
auccacuucu ucagagagcc cacagaucug 1260 aagcaguuca agcaggacgc
gaaguauagc cauggcauag acgucaccga uuuauucgcg 1320 gcccagccgg
gccuuacgag cgccgugauc gaggcgcugc ccagaaacau ggugaucacc 1380
ugccagggca gcgaggacau cagaaagcuc cuugaaucuc aaggccggag agauauuaag
1440 cugauagaua ucagcuuauc uaagguugac agcagaaagu ucgagaacgc
uguaugggac 1500 caauucaagg accugugcca caugcauacg ggcauagugg
uagagaagaa gaagcguggc 1560 ggaaaggagg agaucacacc ucacugcgcc
cugauggacu gcaucauguu cgacgcggca 1620 gucuccggcg gcgucgacgc
aaagguccuc cgggccgugc ugccaaggga caugguguuc 1680 cggacaagca
ccccuaaggu agugcug 1707 <210> SEQ ID NO 10 <211>
LENGTH: 622 <212> TYPE: PRT <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polypeptide <400> SEQUENCE: 10 Met Glu Thr Pro Ala
Gln Leu Leu Phe Leu Leu Leu Leu Trp Leu Pro 1 5 10 15 Asp Thr Thr
Gly Met Pro Ala Glu Asn Lys Lys Val Arg Phe Glu Asn 20 25 30 Thr
Thr Ser Asp Lys Gly Lys Asn Pro Ser Lys Val Ile Lys Ser Tyr 35 40
45 Tyr Gly Thr Met Asp Ile Lys Lys Ile Asn Glu Gly Leu Leu Asp Ser
50 55 60 Lys Ile Leu Ser Ala Phe Asn Thr Val Ile Ala Leu Leu Gly
Ser Ile 65 70 75 80 Val Ile Ile Val Met Asn Ile Met Ile Ile Gln Asn
Tyr Thr Arg Ser 85 90 95 Thr Asp Asn Gln Ala Val Ile Lys Asp Ala
Leu Gln Gly Ile Gln Gln 100 105 110 Gln Ile Lys Gly Leu Ala Asp Lys
Ile Gly Thr Glu Ile Gly Pro Lys 115 120 125 Val Ser Leu Ile Asp Thr
Ser Ser Thr Ile Thr Ile Pro Ala Asn Ile 130 135 140 Gly Leu Leu Gly
Ser Lys Ile Ser Gln Ser Thr Ala Ser Ile Asn Glu 145 150 155 160 Asn
Val Asn Glu Lys Cys Lys Phe Thr Leu Pro Pro Leu Lys Ile His 165 170
175 Glu Cys Asn Ile Ser Cys Pro Asn Pro Leu Pro Phe Arg Glu Tyr Arg
180 185 190 Pro Gln Thr Glu Gly Val Ser Asn Leu Val Gly Leu Pro Asp
Asn Ile 195 200 205 Cys Leu Gln Lys Thr Ser Asn Gln Ile Leu Lys Pro
Lys Leu Ile Ser 210 215 220 Tyr Thr Leu Pro Val Val Gly Gln Ser Gly
Thr Cys Ile Thr Asp Pro 225 230 235 240 Leu Leu Ala Met Asp Glu Gly
Tyr Phe Ala Tyr Ser His Leu Glu Arg 245 250 255 Ile Gly Ser Cys Ser
Arg Gly Val Ser Lys Gln Arg Ile Ile Gly Val 260 265 270 Gly Glu Val
Leu Asp Arg Gly Asp Glu Val Pro Ser Leu Phe Met Thr 275 280 285 Asn
Val Trp Thr Pro Pro Asn Pro Asn Thr Val Tyr His Cys Ser Ala 290 295
300 Val Tyr Asn Asn Glu Phe Tyr Tyr Val Leu Cys Ala Val Ser Thr Val
305 310 315 320 Gly Asp Pro Ile Leu Asn Ser Thr Tyr Trp Ser Gly Ser
Leu Met Met 325 330 335 Thr Arg Leu Ala Val Lys Pro Lys Ser Asn Gly
Gly Gly Tyr Asn Gln 340 345 350 His Gln Leu Ala Leu Arg Ser Ile Glu
Lys Gly Arg Tyr Asp Lys Val 355 360 365 Met Pro Tyr Gly Pro Ser Gly
Ile Lys Gln Gly Asp Thr Leu Tyr Phe 370 375 380 Pro Ala Val Gly Phe
Leu Val Arg Thr Glu Phe Lys Tyr Asn Asp Ser 385 390 395 400 Asn Cys
Pro Ile Thr Lys Cys Gln Tyr Ser Lys Pro Glu Asn Cys Arg 405 410 415
Leu Ser Met Gly Ile Arg Pro Asn Ser His Tyr Ile Leu Arg Ser Gly 420
425 430 Leu Leu Lys Tyr Asn Leu Ser Asp Gly Glu Asn Pro Lys Ile Val
Phe 435 440 445 Ile Glu Ile Ser Asp Gln Arg Leu Ser Ile Gly Ser Pro
Ser Lys Val 450 455 460 Tyr Asp Ser Leu Gly Gln Pro Val Phe Tyr Gln
Ala Ser Phe Ser Trp 465 470 475 480 Asp Thr Met Ile Lys Phe Gly Asp
Val Gln Thr Val Asn Pro Leu Val 485 490 495 Val Asn Trp Arg Asp Asn
Thr Val Ile Ser Arg Pro Gly Gln Ser Gln 500 505 510 Cys Pro Arg Phe
Asn Thr Cys Pro Glu Ile Cys Trp Glu Gly Val Tyr 515 520 525 Asn Asp
Ala Phe Leu Ile Asp Arg Ile Asn Trp Ile Ser Ala Gly Val 530 535 540
Phe Leu Asp Ser Asn Gln Thr Ala Glu Asn Pro Val Phe Thr Val Phe 545
550 555 560 Lys Asp Asn Glu Ile Leu Tyr Arg Ala Gln Leu Ala Ser Glu
Asp Thr 565 570 575 Asn Ala Gln Lys Thr Ile Thr Asn Cys Phe Leu Leu
Lys Asn Lys Ile 580 585 590 Trp Cys Ile Ser Leu Val Glu Ile Tyr Asp
Thr Gly Asp Asn Val Ile 595 600 605 Arg Pro Lys Leu Phe Ala Val Lys
Ile Pro Glu Gln Cys Thr 610 615 620 <210> SEQ ID NO 11
<211> LENGTH: 602 <212> TYPE: PRT <213> ORGANISM:
Artificial sequence <220> FEATURE: <223> OTHER
INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 11 Met Pro
Ala Glu Asn Lys Lys Val Arg Phe Glu Asn Thr Thr Ser Asp 1 5 10 15
Lys Gly Lys Asn Pro Ser Lys Val Ile Lys Ser Tyr Tyr Gly Thr Met 20
25 30 Asp Ile Lys Lys Ile Asn Glu Gly Leu Leu Asp Ser Lys Ile Leu
Ser 35 40 45 Ala Phe Asn Thr Val Ile Ala Leu Leu Gly Ser Ile Val
Ile Ile Val 50 55 60 Met Asn Ile Met Ile Ile Gln Asn Tyr Thr Arg
Ser Thr Asp Asn Gln 65 70 75 80 Ala Val Ile Lys Asp Ala Leu Gln Gly
Ile Gln Gln Gln Ile Lys Gly 85 90 95 Leu Ala Asp Lys Ile Gly Thr
Glu Ile Gly Pro Lys Val Ser Leu Ile 100 105 110 Asp Thr Ser Ser Thr
Ile Thr Ile Pro Ala Asn Ile Gly Leu Leu Gly 115 120 125 Ser Lys Ile
Ser Gln Ser Thr Ala Ser Ile Asn Glu Asn Val Asn Glu 130 135 140 Lys
Cys Lys Phe Thr Leu Pro Pro Leu Lys Ile His Glu Cys Asn Ile 145 150
155 160 Ser Cys Pro Asn Pro Leu Pro Phe Arg Glu Tyr Arg Pro Gln Thr
Glu 165 170 175 Gly Val Ser Asn Leu Val Gly Leu Pro Asp Asn Ile Cys
Leu Gln Lys 180 185 190 Thr Ser Asn Gln Ile Leu Lys Pro Lys Leu Ile
Ser Tyr Thr Leu Pro 195 200 205 Val Val Gly Gln Ser Gly Thr Cys Ile
Thr Asp Pro Leu Leu Ala Met 210 215 220 Asp Glu Gly Tyr Phe Ala Tyr
Ser His Leu Glu Arg Ile Gly Ser Cys 225 230 235 240 Ser Arg Gly Val
Ser Lys Gln Arg Ile Ile Gly Val Gly Glu Val Leu 245 250 255 Asp Arg
Gly Asp Glu Val Pro Ser Leu Phe Met Thr Asn Val Trp Thr 260 265 270
Pro Pro Asn Pro Asn Thr Val Tyr His Cys Ser Ala Val Tyr Asn Asn 275
280 285 Glu Phe Tyr Tyr Val Leu Cys Ala Val Ser Thr Val Gly Asp Pro
Ile 290 295 300 Leu Asn Ser Thr Tyr Trp Ser Gly Ser Leu Met Met Thr
Arg Leu Ala 305 310 315 320 Val Lys Pro Lys Ser Asn Gly Gly Gly Tyr
Asn Gln His Gln Leu Ala 325 330 335 Leu Arg Ser Ile Glu Lys Gly Arg
Tyr Asp Lys Val Met Pro Tyr Gly 340 345 350 Pro Ser Gly Ile Lys Gln
Gly Asp Thr Leu Tyr Phe Pro Ala Val Gly 355 360 365 Phe Leu Val Arg
Thr Glu Phe Lys Tyr Asn Asp Ser Asn Cys Pro Ile 370 375 380 Thr Lys
Cys Gln Tyr Ser Lys Pro Glu Asn Cys Arg Leu Ser Met Gly 385 390 395
400 Ile Arg Pro Asn Ser His Tyr Ile Leu Arg Ser Gly Leu Leu Lys Tyr
405 410 415 Asn Leu Ser Asp Gly Glu Asn Pro Lys Ile Val Phe Ile Glu
Ile Ser 420 425 430 Asp Gln Arg Leu Ser Ile Gly Ser Pro Ser Lys Val
Tyr Asp Ser Leu 435 440 445 Gly Gln Pro Val Phe Tyr Gln Ala Ser Phe
Ser Trp Asp Thr Met Ile 450 455 460 Lys Phe Gly Asp Val Gln Thr Val
Asn Pro Leu Val Val Asn Trp Arg 465 470 475 480 Asp Asn Thr Val Ile
Ser Arg Pro Gly Gln Ser Gln Cys Pro Arg Phe 485 490 495 Asn Thr Cys
Pro Glu Ile Cys Trp Glu Gly Val Tyr Asn Asp Ala Phe 500 505 510 Leu
Ile Asp Arg Ile Asn Trp Ile Ser Ala Gly Val Phe Leu Asp Ser 515 520
525 Asn Gln Thr Ala Glu Asn Pro Val Phe Thr Val Phe Lys Asp Asn Glu
530 535 540 Ile Leu Tyr Arg Ala Gln Leu Ala Ser Glu Asp Thr Asn Ala
Gln Lys 545 550 555 560 Thr Ile Thr Asn Cys Phe Leu Leu Lys Asn Lys
Ile Trp Cys Ile Ser 565 570 575 Leu Val Glu Ile Tyr Asp Thr Gly Asp
Asn Val Ile Arg Pro Lys Leu 580 585 590 Phe Ala Val Lys Ile Pro Glu
Gln Cys Thr 595 600 <210> SEQ ID NO 12 <211> LENGTH:
540 <212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 12 Met Glu Thr Pro Ala Gln Leu
Leu Phe Leu Leu Leu Leu Trp Leu Pro 1 5 10 15 Asp Thr Thr Gly Ile
Leu His Tyr Glu Lys Leu Ser Lys Ile Gly Leu 20 25 30 Val Lys Gly
Ile Thr Arg Lys Tyr Lys Ile Lys Ser Asn Pro Leu Thr 35 40 45 Lys
Asp Ile Val Ile Lys Met Ile Pro Asn Val Ser Asn Met Ser Gln 50 55
60 Cys Thr Gly Ser Val Met Glu Asn Tyr Lys Thr Arg Leu Asn Gly Ile
65 70 75 80 Leu Thr Pro Ile Lys Gly Ala Leu Glu Ile Tyr Lys Asn Asn
Thr His 85 90 95 Asp Leu Val Gly Asp Val Arg Leu Ala Gly Val Ile
Met Ala Gly Val 100 105 110 Ala Ile Gly Ile Ala Thr Ala Ala Gln Ile
Thr Ala Gly Val Ala Leu 115 120 125 Tyr Glu Ala Met Lys Asn Ala Asp
Asn Ile Asn Lys Leu Lys Ser Ser 130 135 140 Ile Glu Ser Thr Asn Glu
Ala Val Val Lys Leu Gln Glu Thr Ala Glu 145 150 155 160 Lys Thr Val
Tyr Val Leu Thr Ala Leu Gln Asp Tyr Ile Asn Thr Asn 165 170 175 Leu
Val Pro Thr Ile Asp Lys Ile Ser Cys Lys Gln Thr Glu Leu Ser 180 185
190 Leu Asp Leu Ala Leu Ser Lys Tyr Leu Ser Asp Leu Leu Phe Val Phe
195 200 205 Gly Pro Asn Leu Gln Asp Pro Val Ser Asn Ser Met Thr Ile
Gln Ala 210 215 220 Ile Ser Gln Ala Phe Gly Gly Asn Tyr Glu Thr Leu
Leu Arg Thr Leu 225 230 235 240 Gly Tyr Ala Thr Glu Asp Phe Asp Asp
Leu Leu Glu Ser Asp Ser Ile 245 250 255 Thr Gly Gln Ile Ile Tyr Val
Asp Leu Ser Gly Tyr Tyr Ile Ile Val 260 265 270 Arg Val Tyr Phe Pro
Ile Leu Thr Glu Ile Gln Gln Ala Tyr Ile Gln 275 280 285 Glu Leu Leu
Pro Val Ser Phe Asn Asn Asp Asn Ser Glu Trp Ile Ser 290 295 300 Ile
Val Pro Asn Phe Ile Leu Val Arg Asn Thr Leu Ile Ser Asn Ile 305 310
315 320 Glu Ile Gly Phe Cys Leu Ile Thr Lys Arg Ser Val Ile Cys Asn
Gln 325 330 335 Asp Tyr Ala Thr Pro Met Thr Asn Asn Met Arg Glu Cys
Leu Thr Gly 340 345 350 Ser Thr Glu Lys Cys Pro Arg Glu Leu Val Val
Ser Ser His Val Pro 355 360 365 Arg Phe Ala Leu Ser Asn Gly Val Leu
Phe Ala Asn Cys Ile Ser Val 370 375 380 Thr Cys Gln Cys Gln Thr Thr
Gly Arg Ala Ile Ser Gln Ser Gly Glu 385 390 395 400 Gln Thr Leu Leu
Met Ile Asp Asn Thr Thr Cys Pro Thr Ala Val Leu 405 410 415 Gly Asn
Val Ile Ile Ser Leu Gly Lys Tyr Leu Gly Ser Val Asn Tyr 420 425 430
Asn Ser Glu Gly Ile Ala Ile Gly Pro Pro Val Phe Thr Asp Lys Val 435
440 445 Asp Ile Ser Ser Gln Ile Ser Ser Met Asn Gln Ser Leu Gln Gln
Ser 450 455 460 Lys Asp Tyr Ile Lys Glu Ala Gln Arg Leu Leu Asp Thr
Val Asn Pro 465 470 475 480 Ser Leu Ile Ser Met Leu Ser Met Ile Ile
Leu Tyr Val Leu Ser Ile 485 490 495 Ala Ser Leu Cys Ile Gly Leu Ile
Thr Phe Ile Ser Phe Ile Ile Val 500 505 510 Glu Lys Lys Arg Asn Thr
Tyr Ser Arg Leu Glu Asp Arg Arg Val Arg 515 520 525 Pro Thr Ser Ser
Gly Asp Leu Tyr Tyr Ile Gly Thr 530 535 540 <210> SEQ ID NO
13 <211> LENGTH: 520 <212> TYPE: PRT <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 13
Ile Leu His Tyr Glu Lys Leu Ser Lys Ile Gly Leu Val Lys Gly Ile 1 5
10 15 Thr Arg Lys Tyr Lys Ile Lys Ser Asn Pro Leu Thr Lys Asp Ile
Val 20 25 30 Ile Lys Met Ile Pro Asn Val Ser Asn Met Ser Gln Cys
Thr Gly Ser 35 40 45 Val Met Glu Asn Tyr Lys Thr Arg Leu Asn Gly
Ile Leu Thr Pro Ile 50 55 60 Lys Gly Ala Leu Glu Ile Tyr Lys Asn
Asn Thr His Asp Leu Val Gly 65 70 75 80 Asp Val Arg Leu Ala Gly Val
Ile Met Ala Gly Val Ala Ile Gly Ile 85 90 95 Ala Thr Ala Ala Gln
Ile Thr Ala Gly Val Ala Leu Tyr Glu Ala Met 100 105 110 Lys Asn Ala
Asp Asn Ile Asn Lys Leu Lys Ser Ser Ile Glu Ser Thr 115 120 125 Asn
Glu Ala Val Val Lys Leu Gln Glu Thr Ala Glu Lys Thr Val Tyr 130 135
140 Val Leu Thr Ala Leu Gln Asp Tyr Ile Asn Thr Asn Leu Val Pro Thr
145 150 155 160 Ile Asp Lys Ile Ser Cys Lys Gln Thr Glu Leu Ser Leu
Asp Leu Ala 165 170 175 Leu Ser Lys Tyr Leu Ser Asp Leu Leu Phe Val
Phe Gly Pro Asn Leu 180 185 190 Gln Asp Pro Val Ser Asn Ser Met Thr
Ile Gln Ala Ile Ser Gln Ala 195 200 205 Phe Gly Gly Asn Tyr Glu Thr
Leu Leu Arg Thr Leu Gly Tyr Ala Thr 210 215 220 Glu Asp Phe Asp Asp
Leu Leu Glu Ser Asp Ser Ile Thr Gly Gln Ile 225 230 235 240 Ile Tyr
Val Asp Leu Ser Gly Tyr Tyr Ile Ile Val Arg Val Tyr Phe 245 250 255
Pro Ile Leu Thr Glu Ile Gln Gln Ala Tyr Ile Gln Glu Leu Leu Pro 260
265 270 Val Ser Phe Asn Asn Asp Asn Ser Glu Trp Ile Ser Ile Val Pro
Asn 275 280 285 Phe Ile Leu Val Arg Asn Thr Leu Ile Ser Asn Ile Glu
Ile Gly Phe 290 295 300 Cys Leu Ile Thr Lys Arg Ser Val Ile Cys Asn
Gln Asp Tyr Ala Thr 305 310 315 320 Pro Met Thr Asn Asn Met Arg Glu
Cys Leu Thr Gly Ser Thr Glu Lys 325 330 335 Cys Pro Arg Glu Leu Val
Val Ser Ser His Val Pro Arg Phe Ala Leu 340 345 350 Ser Asn Gly Val
Leu Phe Ala Asn Cys Ile Ser Val Thr Cys Gln Cys 355 360 365 Gln Thr
Thr Gly Arg Ala Ile Ser Gln Ser Gly Glu Gln Thr Leu Leu 370 375 380
Met Ile Asp Asn Thr Thr Cys Pro Thr Ala Val Leu Gly Asn Val Ile 385
390 395 400 Ile Ser Leu Gly Lys Tyr Leu Gly Ser Val Asn Tyr Asn Ser
Glu Gly 405 410 415 Ile Ala Ile Gly Pro Pro Val Phe Thr Asp Lys Val
Asp Ile Ser Ser 420 425 430 Gln Ile Ser Ser Met Asn Gln Ser Leu Gln
Gln Ser Lys Asp Tyr Ile 435 440 445 Lys Glu Ala Gln Arg Leu Leu Asp
Thr Val Asn Pro Ser Leu Ile Ser 450 455 460 Met Leu Ser Met Ile Ile
Leu Tyr Val Leu Ser Ile Ala Ser Leu Cys 465 470 475 480 Ile Gly Leu
Ile Thr Phe Ile Ser Phe Ile Ile Val Glu Lys Lys Arg 485 490 495 Asn
Thr Tyr Ser Arg Leu Glu Asp Arg Arg Val Arg Pro Thr Ser Ser 500 505
510 Gly Asp Leu Tyr Tyr Ile Gly Thr 515 520 <210> SEQ ID NO
14 <211> LENGTH: 1866 <212> TYPE: DNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE:
14 atggaaaccc ctgctcagct gctgttcctg ctgctgctgt ggctgcctga
tacaacaggc 60 atgcccgccg agaacaagaa agttcgcttc gagaacacca
ccagcgacaa gggcaagaac 120 cccagcaaag tgatcaagag ctactacggc
accatggaca tcaagaagat caacgagggc 180 ctgctggaca gcaagatcct
gagcgccttc aacaccgtga ttgccctgct gggctctatc 240 gtgatcatcg
tgatgaacat catgatcatc cagaactaca cccggtccac cgacaaccag 300
gccgtgatta aggatgctct gcagggaatc cagcagcaga tcaaaggcct ggccgacaag
360 atcggcacag agatcggccc taaggtgtcc ctgatcgaca ccagcagcac
catcacaatc 420 cccgccaata tcggactgct gggatccaag atcagccaga
gcaccgccag catcaacgag 480 aacgtgaacg agaagtgcaa gttcaccctg
cctccactga agatccacga gtgcaacatc 540 agctgcccca atcctctgcc
attcagagag tacagacccc agacagaggg cgtgtccaat 600 ctcgtgggcc
tgcctgacaa tatctgcctg cagaagacca gcaaccagat cctgaagcct 660
aagctgatct cctacacact gcccgtcgtg ggccagagcg gcacctgtat tacagatcct
720 ctgctggcca tggacgaggg ctactttgcc tacagccacc tggaaagaat
cggcagctgt 780 agccggggag tgtccaagca gagaatcatc ggcgtgggcg
aagtgctgga tagaggcgac 840 gaagtgccca gcctgttcat gaccaatgtg
tggacccctc ctaatcctaa caccgtgtac 900 cactgcagcg ccgtgtacaa
caacgagttc tactacgtgc tgtgcgccgt gtccacagtg 960 ggcgacccta
tcctgaacag cacctattgg agcggcagcc tgatgatgac cagactggcc 1020
gtgaagccca agagcaatgg cggcggatac aaccagcatc agctggccct gcggtccatc
1080 gagaagggca gatacgacaa agtgatgcct tacggcccca gcggcatcaa
gcaaggcgat 1140 accctgtact ttcccgccgt gggatttctc gtgcggaccg
agttcaagta caacgacagc 1200 aactgcccca tcaccaagtg ccagtacagc
aagcccgaga actgcagact gagcatgggc 1260 atcagaccca acagccacta
catcctgaga agcggcctgc tgaagtacaa cctgagcgac 1320 ggcgagaacc
ccaagatcgt gttcatcgag atcagcgacc agcggctgtc tatcggcagc 1380
cctagcaagg tgtacgactc tctgggacag ccagtgttct accaggcctc cttcagctgg
1440 gacaccatga tcaagttcgg cgacgtgcag accgtgaatc ccctggtggt
caactggcgg 1500 gacaataccg tgatcagcag acctggccag tctcagtgcc
ccagattcaa cacatgcccc 1560 gagatctgtt gggaaggcgt gtacaatgac
gccttcctga tcgatcggat caactggatc 1620 tctgccggcg tgttcctgga
ctccaatcag acagccgaga atcctgtgtt caccgtgttc 1680 aaggacaatg
agatcctgta tcgggcccag ctggcctccg aggatacaaa tgcccagaag 1740
acaatcacca actgctttct gctcaagaac aagatctggt gcatcagcct ggtggaaatc
1800 tacgacaccg gcgacaacgt gatcaggccc aagctgttcg ccgtgaagat
ccctgagcag 1860 tgcaca 1866 <210> SEQ ID NO 15 <211>
LENGTH: 1866 <212> TYPE: DNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 15 atggaaaccc
ctgctcagct gctgttcctg ctgctgctgt ggctgcctga tacaacaggc 60
atgcccgccg agaacaagaa agttcgcttc gagaacacca ccagcgacaa gggcaagaac
120 cccagcaaag tgatcaagag ctactacggc accatggaca tcaagaagat
caacgagggc 180 ctgctggaca gcaagatcct gagcgccttc aacaccgtga
ttgccctgct gggctctatc 240 gtgatcatcg tgatgaacat catgatcatc
cagaactaca cccggtccac cgacaaccag 300 gccgtgatta aggatgctct
gcagggaatc cagcagcaga tcaaaggcct ggccgacaag 360 atcggcacag
agatcggccc taaggtgtcc ctgatcgaca ccagcagcac catcacaatc 420
cccgccaata tcggactgct gggatccaag atcagccaga gcaccgccag catcaacgag
480 aacgtgaacg agaagtgcaa gttcaccctg cctccactga agatccacga
gtgcaacatc 540 agctgcccca atcctctgcc attcagagag tacagacccc
agacagaggg cgtgtccaat 600 ctcgtgggcc tgcctgacaa tatctgcctg
cagaagacca gcaaccagat cctgaagcct 660 aagctgatct cctacacact
gcccgtcgtg ggccagagcg gcacctgtat tacagatcct 720 ctgctggcca
tggacgaggg ctactttgcc tacagccacc tggaaagaat cggcagctgt 780
agccggggag tgtccaagca gagaatcatc ggcgtgggcg aagtgctgga tagaggcgac
840 gaagtgccca gcctgttcat gaccaatgtg tggacccctc ctaatcctaa
caccgtgtac 900 cactgcagcg ccgtgtacaa caacgagttc tactacgtgc
tgtgcgccgt gtccacagtg 960 ggcgacccta tcctgaacag cacctattgg
agcggcagcc tgatgatgac cagactggcc 1020 gtgaagccca agagcaatgg
cggcggatac aaccagcatc agctggccct gcggtccatc 1080 gagaagggca
gatacgacaa agtgatgcct tacggcccca gcggcatcaa gcaaggcgat 1140
accctgtact ttcccgccgt gggatttctc gtgcggaccg agttcaagta caacgacagc
1200 aactgcccca tcaccaagtg ccagtacagc aagcccgaga actgcagact
gagcatgggc 1260 atcagaccca acagccacta catcctgaga agcggcctgc
tgaagtacaa cctgagcgac 1320 ggcgagaacc ccaagatcgt gttcatcgag
atcagcgacc agcggctgtc tatcggcagc 1380 cctagcaagg tgtacgactc
tctgggacag ccagtgttct accaggcctc cttcagctgg 1440 gacaccatga
tcaagttcgg cgacgtgcag accgtgaatc ccctggtggt caactggcgg 1500
gacaataccg tgatcagcag acctggccag tctcagtgcc ccagattcaa cacatgcccc
1560 gagatctgtt gggaaggcgt gtacaatgac gccttcctga tcgatcggat
caactggatc 1620 tctgccggcg tgttcctgga ctccaatcag acagccgaga
atcctgtgtt caccgtgttc 1680 aaggacaatg agatcctgta tcgggcccag
ctggcctccg aggatacaaa tgcccagaag 1740 acaatcacca actgctttct
gctcaagaac aagatctggt gcatcagcct ggtggaaatc 1800 tacgacaccg
gcgacaacgt gatcaggccc aagctgttcg ccgtgaagat ccctgagcag 1860 tgcaca
1866 <210> SEQ ID NO 16 <211> LENGTH: 1866 <212>
TYPE: RNA <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polynucleotide
<400> SEQUENCE: 16 auggaaaccc cugcucagcu gcuguuccug
cugcugcugu ggcugccuga uacaacaggc 60 augcccgccg agaacaagaa
aguucgcuuc gagaacacca ccagcgacaa gggcaagaac 120 cccagcaaag
ugaucaagag cuacuacggc accauggaca ucaagaagau caacgagggc 180
cugcuggaca gcaagauccu gagcgccuuc aacaccguga uugcccugcu gggcucuauc
240 gugaucaucg ugaugaacau caugaucauc cagaacuaca cccgguccac
cgacaaccag 300 gccgugauua aggaugcucu gcagggaauc cagcagcaga
ucaaaggccu ggccgacaag 360 aucggcacag agaucggccc uaaggugucc
cugaucgaca ccagcagcac caucacaauc 420 cccgccaaua ucggacugcu
gggauccaag aucagccaga gcaccgccag caucaacgag 480 aacgugaacg
agaagugcaa guucacccug ccuccacuga agauccacga gugcaacauc 540
agcugcccca auccucugcc auucagagag uacagacccc agacagaggg cguguccaau
600 cucgugggcc ugccugacaa uaucugccug cagaagacca gcaaccagau
ccugaagccu 660 aagcugaucu ccuacacacu gcccgucgug ggccagagcg
gcaccuguau uacagauccu 720 cugcuggcca uggacgaggg cuacuuugcc
uacagccacc uggaaagaau cggcagcugu 780 agccggggag uguccaagca
gagaaucauc ggcgugggcg aagugcugga uagaggcgac 840 gaagugccca
gccuguucau gaccaaugug uggaccccuc cuaauccuaa caccguguac 900
cacugcagcg ccguguacaa caacgaguuc uacuacgugc ugugcgccgu guccacagug
960 ggcgacccua uccugaacag caccuauugg agcggcagcc ugaugaugac
cagacuggcc 1020 gugaagccca agagcaaugg cggcggauac aaccagcauc
agcuggcccu gcgguccauc 1080 gagaagggca gauacgacaa agugaugccu
uacggcccca gcggcaucaa gcaaggcgau 1140 acccuguacu uucccgccgu
gggauuucuc gugcggaccg aguucaagua caacgacagc 1200 aacugcccca
ucaccaagug ccaguacagc aagcccgaga acugcagacu gagcaugggc 1260
aucagaccca acagccacua cauccugaga agcggccugc ugaaguacaa ccugagcgac
1320 ggcgagaacc ccaagaucgu guucaucgag aucagcgacc agcggcuguc
uaucggcagc 1380 ccuagcaagg uguacgacuc ucugggacag ccaguguucu
accaggccuc cuucagcugg 1440 gacaccauga ucaaguucgg cgacgugcag
accgugaauc cccugguggu caacuggcgg 1500 gacaauaccg ugaucagcag
accuggccag ucucagugcc ccagauucaa cacaugcccc 1560 gagaucuguu
gggaaggcgu guacaaugac gccuuccuga ucgaucggau caacuggauc 1620
ucugccggcg uguuccugga cuccaaucag acagccgaga auccuguguu caccguguuc
1680 aaggacaaug agauccugua ucgggcccag cuggccuccg aggauacaaa
ugcccagaag 1740 acaaucacca acugcuuucu gcucaagaac aagaucuggu
gcaucagccu gguggaaauc 1800 uacgacaccg gcgacaacgu gaucaggccc
aagcuguucg ccgugaagau cccugagcag 1860 ugcaca 1866 <210> SEQ
ID NO 17 <211> LENGTH: 1866 <212> TYPE: RNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE:
17 auggaaaccc cugcucagcu gcuguuccug cugcugcugu ggcugccuga
uacaacaggc 60 augcccgccg agaacaagaa aguucgcuuc gagaacacca
ccagcgacaa gggcaagaac 120 cccagcaaag ugaucaagag cuacuacggc
accauggaca ucaagaagau caacgagggc 180 cugcuggaca gcaagauccu
gagcgccuuc aacaccguga uugcccugcu gggcucuauc 240 gugaucaucg
ugaugaacau caugaucauc cagaacuaca cccgguccac cgacaaccag 300
gccgugauua aggaugcucu gcagggaauc cagcagcaga ucaaaggccu ggccgacaag
360 aucggcacag agaucggccc uaaggugucc cugaucgaca ccagcagcac
caucacaauc 420 cccgccaaua ucggacugcu gggauccaag aucagccaga
gcaccgccag caucaacgag 480 aacgugaacg agaagugcaa guucacccug
ccuccacuga agauccacga gugcaacauc 540 agcugcccca auccucugcc
auucagagag uacagacccc agacagaggg cguguccaau 600 cucgugggcc
ugccugacaa uaucugccug cagaagacca gcaaccagau ccugaagccu 660
aagcugaucu ccuacacacu gcccgucgug ggccagagcg gcaccuguau uacagauccu
720 cugcuggcca uggacgaggg cuacuuugcc uacagccacc uggaaagaau
cggcagcugu 780 agccggggag uguccaagca gagaaucauc ggcgugggcg
aagugcugga uagaggcgac 840 gaagugccca gccuguucau gaccaaugug
uggaccccuc cuaauccuaa caccguguac 900 cacugcagcg ccguguacaa
caacgaguuc uacuacgugc ugugcgccgu guccacagug 960 ggcgacccua
uccugaacag caccuauugg agcggcagcc ugaugaugac cagacuggcc 1020
gugaagccca agagcaaugg cggcggauac aaccagcauc agcuggcccu gcgguccauc
1080 gagaagggca gauacgacaa agugaugccu uacggcccca gcggcaucaa
gcaaggcgau 1140 acccuguacu uucccgccgu gggauuucuc gugcggaccg
aguucaagua caacgacagc 1200 aacugcccca ucaccaagug ccaguacagc
aagcccgaga acugcagacu gagcaugggc 1260 aucagaccca acagccacua
cauccugaga agcggccugc ugaaguacaa ccugagcgac 1320 ggcgagaacc
ccaagaucgu guucaucgag aucagcgacc agcggcuguc uaucggcagc 1380
ccuagcaagg uguacgacuc ucugggacag ccaguguucu accaggccuc cuucagcugg
1440 gacaccauga ucaaguucgg cgacgugcag accgugaauc cccugguggu
caacuggcgg 1500 gacaauaccg ugaucagcag accuggccag ucucagugcc
ccagauucaa cacaugcccc 1560 gagaucuguu gggaaggcgu guacaaugac
gccuuccuga ucgaucggau caacuggauc 1620 ucugccggcg uguuccugga
cuccaaucag acagccgaga auccuguguu caccguguuc 1680 aaggacaaug
agauccugua ucgggcccag cuggccuccg aggauacaaa ugcccagaag 1740
acaaucacca acugcuuucu gcucaagaac aagaucuggu gcaucagccu gguggaaauc
1800 uacgacaccg gcgacaacgu gaucaggccc aagcuguucg ccgugaagau
cccugagcag 1860 ugcaca 1866 <210> SEQ ID NO 18 <211>
LENGTH: 1255 <212> TYPE: PRT <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polypeptide <400> SEQUENCE: 18 Met Phe Ile Phe Leu
Phe Phe Leu Thr Leu Thr Ser Gly Ser Asp Leu 1 5 10 15 Glu Ser Cys
Thr Thr Phe Asp Asp Val Gln Ala Pro Asn Tyr Pro Gln 20 25 30 His
Ser Ser Ser Arg Arg Gly Val Tyr Tyr Pro Asp Glu Ile Phe Arg 35 40
45 Ser Asp Thr Leu Tyr Leu Thr Gln Asp Leu Phe Leu Pro Phe Tyr Ser
50 55 60 Asn Val Thr Gly Phe His Thr Ile Asn His Arg Phe Asp Asn
Pro Val 65 70 75 80 Ile Pro Phe Lys Asp Gly Val Tyr Phe Ala Ala Thr
Glu Lys Ser Asn 85 90 95 Val Val Arg Gly Trp Val Phe Gly Ser Thr
Met Asn Asn Lys Ser Gln 100 105 110 Ser Val Ile Ile Ile Asn Asn Ser
Thr Asn Val Val Ile Arg Ala Cys 115 120 125 Asn Phe Glu Leu Cys Asp
Asn Pro Phe Phe Ala Val Ser Lys Pro Thr 130 135 140 Gly Thr Gln Thr
His Thr Met Ile Phe Asp Asn Ala Phe Asn Cys Thr 145 150 155 160 Phe
Glu Tyr Ile Ser Asp Ser Phe Ser Leu Asp Val Ala Glu Lys Ser 165 170
175 Gly Asn Phe Lys His Leu Arg Glu Phe Val Phe Lys Asn Lys Asp Gly
180 185 190 Phe Leu Tyr Val Tyr Lys Gly Tyr Gln Pro Ile Asp Val Val
Arg Asp 195 200 205 Leu Pro Ser Gly Phe Asn Ile Leu Lys Pro Ile Phe
Lys Leu Pro Leu 210 215 220 Gly Ile Asn Ile Thr Asn Phe Arg Ala Ile
Leu Thr Ala Phe Leu Pro 225 230 235 240 Ala Gln Asp Thr Trp Gly Thr
Ser Ala Ala Ala Tyr Phe Val Gly Tyr 245 250 255 Leu Lys Pro Ala Thr
Phe Met Leu Lys Tyr Asp Glu Asn Gly Thr Ile 260 265 270 Thr Asp Ala
Val Asp Cys Ser Gln Asn Pro Leu Ala Glu Leu Lys Cys 275 280 285 Ser
Val Lys Ser Phe Glu Ile Asp Lys Gly Ile Tyr Gln Thr Ser Asn 290 295
300 Phe Arg Val Ala Pro Ser Lys Glu Val Val Arg Phe Pro Asn Ile Thr
305 310 315 320 Asn Leu Cys Pro Phe Gly Glu Val Phe Asn Ala Thr Thr
Phe Pro Ser 325 330 335 Val Tyr Ala Trp Glu Arg Lys Arg Ile Ser Asn
Cys Val Ala Asp Tyr 340 345 350 Ser Val Leu Tyr Asn Ser Thr Ser Phe
Ser Thr Phe Lys Cys Tyr Gly 355 360 365 Val Ser Ala Thr Lys Leu Asn
Asp Leu Cys Phe Ser Asn Val Tyr Ala 370 375 380 Asp Ser Phe Val Val
Lys Gly Asp Asp Val Arg Gln Ile Ala Pro Gly 385 390 395 400 Gln Thr
Gly Val Ile Ala Asp Tyr Asn Tyr Lys Leu Pro Asp Asp Phe 405 410 415
Thr Gly Cys Val Leu Ala Trp Asn Thr Arg Asn Ile Asp Ala Thr Gln 420
425 430 Thr Gly Asn Tyr Asn Tyr Lys Tyr Arg Ser Leu Arg His Gly Lys
Leu 435 440 445 Arg Pro Phe Glu Arg Asp Ile Ser Asn Val Pro Phe Ser
Pro Asp Gly 450 455 460 Lys Pro Cys Thr Pro Pro Ala Phe Asn Cys Tyr
Trp Pro Leu Asn Asp 465 470 475 480 Tyr Gly Phe Tyr Ile Thr Asn Gly
Ile Gly Tyr Gln Pro Tyr Arg Val 485 490 495 Val Val Leu Ser Phe Glu
Leu Leu Asn Ala Pro Ala Thr Val Cys Gly 500 505 510 Pro Lys Leu Ser
Thr Asp Leu Ile Lys Asn Gln Cys Val Asn Phe Asn 515 520 525 Phe Asn
Gly Leu Thr Gly Thr Gly Val Leu Thr Pro Ser Ser Lys Arg 530 535 540
Phe Gln Pro Phe Gln Gln Phe Gly Arg Asp Val Leu Asp Phe Thr Asp 545
550 555 560 Ser Val Arg Asp Pro Lys Thr Ser Glu Ile Leu Asp Ile Ser
Pro Cys 565 570 575 Ser Phe Gly Gly Val Ser Val Ile Thr Pro Gly Thr
Asn Thr Ser Ser 580 585 590 Glu Val Ala Val Leu Tyr Gln Asp Val Asn
Cys Thr Asp Val Pro Val 595 600 605 Ala Ile His Ala Asp Gln Leu Thr
Pro Ser Trp Arg Val Tyr Ser Thr 610 615 620 Gly Asn Asn Val Phe Gln
Thr Gln Ala Gly Cys Leu Ile Gly Ala Glu 625 630 635 640 His Val Asp
Thr Ser Tyr Glu Cys Asp Ile Pro Ile Gly Ala Gly Ile 645 650 655 Cys
Ala Ser Tyr His Thr Val Ser Ser Leu Arg Ser Thr Ser Gln Lys 660 665
670 Ser Ile Val Ala Tyr Thr Met Ser Leu Gly Ala Asp Ser Ser Ile Ala
675 680 685 Tyr Ser Asn Asn Thr Ile Ala Ile Pro Thr Asn Phe Ser Ile
Ser Ile 690 695 700 Thr Thr Glu Val Met Pro Val Ser Met Ala Lys Thr
Ser Val Asp Cys 705 710 715 720 Asn Met Tyr Ile Cys Gly Asp Ser Thr
Glu Cys Ala Asn Leu Leu Leu 725 730 735 Gln Tyr Gly Ser Phe Cys Thr
Gln Leu Asn Arg Ala Leu Ser Gly Ile 740 745 750 Ala Val Glu Gln Asp
Arg Asn Thr Arg Glu Val Phe Ala Gln Val Lys 755 760 765 Gln Met Tyr
Lys Thr Pro Thr Leu Lys Asp Phe Gly Gly Phe Asn Phe 770 775 780 Ser
Gln Ile Leu Pro Asp Pro Leu Lys Pro Thr Lys Arg Ser Phe Ile 785 790
795 800 Glu Asp Leu Leu Phe Asn Lys Val Thr Leu Ala Asp Ala Gly Phe
Met 805 810 815 Lys Gln Tyr Gly Glu Cys Leu Gly Asp Ile Asn Ala Arg
Asp Leu Ile 820 825 830 Cys Ala Gln Lys Phe Asn Gly Leu Thr Val Leu
Pro Pro Leu Leu Thr 835 840 845 Asp Asp Met Ile Ala Ala Tyr Thr Ala
Ala Leu Val Ser Gly Thr Ala 850 855 860 Thr Ala Gly Trp Thr Phe Gly
Ala Gly Ala Ala Leu Gln Ile Pro Phe 865 870 875 880 Ala Met Gln Met
Ala Tyr Arg Phe Asn Gly Ile Gly Val Thr Gln Asn 885 890 895 Val Leu
Tyr Glu Asn Gln Lys Gln Ile Ala Asn Gln Phe Asn Lys Ala 900 905 910
Ile Ser Gln Ile Gln Glu Ser Leu Thr Thr Thr Ser Thr Ala Leu Gly 915
920 925 Lys Leu Gln Asp Val Val Asn Gln Asn Ala Gln Ala Leu Asn Thr
Leu 930 935 940 Val Lys Gln Leu Ser Ser Asn Phe Gly Ala Ile Ser Ser
Val Leu Asn 945 950 955 960 Asp Ile Leu Ser Arg Leu Asp Lys Val Glu
Ala Glu Val Gln Ile Asp 965 970 975 Arg Leu Ile Thr Gly Arg Leu Gln
Ser Leu Gln Thr Tyr Val Thr Gln 980 985 990 Gln Leu Ile Arg Ala Ala
Glu Ile Arg Ala Ser Ala Asn Leu Ala Ala 995 1000 1005 Thr Lys Met
Ser Glu Cys Val Leu Gly Gln Ser Lys Arg Val Asp 1010 1015 1020 Phe
Cys Gly Lys Gly Tyr His Leu Met Ser Phe Pro Gln Ala Ala 1025 1030
1035 Pro His Gly Val Val Phe Leu His Val Thr Tyr Val Pro Ser Gln
1040 1045 1050 Glu Arg Asn Phe Thr Thr Ala Pro Ala Ile Cys His Glu
Gly Lys 1055 1060 1065 Ala Tyr Phe Pro Arg Glu Gly Val Phe Val Phe
Asn Gly Thr Ser 1070 1075 1080 Trp Phe Ile Thr Gln Arg Asn Phe Phe
Ser Pro Gln Ile Ile Thr 1085 1090 1095 Thr Asp Asn Thr Phe Val Ser
Gly Ser Cys Asp Val Val Ile Gly 1100 1105 1110 Ile Ile Asn Asn Thr
Val Tyr Asp Pro Leu Gln Pro Glu Leu Asp 1115 1120 1125 Ser Phe Lys
Glu Glu Leu Asp Lys Tyr Phe Lys Asn His Thr Ser 1130 1135 1140 Pro
Asp Val Asp Leu Gly Asp Ile Ser Gly Ile Asn Ala Ser Val 1145 1150
1155 Val Asn Ile Gln Lys Glu Ile Asp Arg Leu Asn Glu Val Ala Lys
1160 1165 1170 Asn Leu Asn Glu Ser Leu Ile Asp Leu Gln Glu Leu Gly
Lys Tyr 1175 1180 1185 Glu Gln Tyr Ile Lys Trp Pro Trp Tyr Val Trp
Leu Gly Phe Ile 1190 1195 1200 Ala Gly Leu Ile Ala Ile Val Met Val
Thr Ile Leu Leu Cys Cys 1205 1210 1215 Met Thr Ser Cys Cys Ser Cys
Leu Lys Gly Ala Cys Ser Cys Gly 1220 1225 1230 Ser Cys Cys Lys Phe
Asp Glu Asp Asp Ser Glu Pro Val Leu Lys 1235 1240 1245 Gly Val Lys
Leu His Tyr Thr 1250 1255 <210> SEQ ID NO 19 <211>
LENGTH: 3765 <212> TYPE: DNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 19 atgtttatct
tcctgttctt cctgaccctg accagcggca gcgacctgga aagctgcacc 60
accttcgacg acgtgcaggc ccccaactac cctcagcaca gctctagcag acggggcgtg
120 tactaccccg acgagatctt cagaagcgac accctgtacc tgacccagga
cctgttcctg 180 cccttctaca gcaacgtgac cggcttccac accatcaacc
acagattcga caaccccgtg 240 atccccttca aggacggggt gtactttgcc
gccaccgaga agtccaatgt cgtgcgggga 300 tgggtgttcg gcagcaccat
gaacaacaag agccagagcg tgatcatcat caacaacagc 360 accaacgtcg
tgatccgggc ctgcaacttc gagctgtgcg acaacccatt cttcgccgtg 420
tccaagccca ccggcaccca gacccacacc atgatcttcg acaacgcctt caactgcacc
480 ttcgagtaca tcagcgacag cttcagcctg gacgtggccg agaaaagcgg
caacttcaag 540 cacctgagag aattcgtgtt caagaacaag gacggcttcc
tgtacgtgta caagggctac 600 cagcccatcg acgtcgtgcg cgatctgccc
agcggcttca acatcctgaa gcccatcttc 660 aagctgcccc tgggcatcaa
catcaccaac ttccgggcta tcctgaccgc cttcctgccc 720 gcccaggata
cctggggaac aagcgccgct gcctacttcg tgggctacct gaagcctgcc 780
accttcatgc tgaagtacga cgagaacggc accatcaccg acgccgtgga ctgcagccag
840 aatcctctgg ccgagctgaa gtgcagcgtg aagtccttcg agatcgacaa
gggcatctac 900 cagaccagca acttcagagt ggcccccagc aaagaagtcg
tgcggttccc caatatcacc 960 aacctgtgcc ccttcggcga ggtgttcaac
gccaccacct ttcccagcgt gtacgcctgg 1020 gagcggaagc ggatcagcaa
ctgcgtggcc gactacagcg tgctgtacaa ctccaccagc 1080 ttctccacct
tcaagtgcta cggcgtgtcc gccaccaagc tgaacgacct gtgcttcagc 1140
aatgtgtacg ccgactcctt cgtcgtgaag ggcgacgatg tgcgccagat cgcccctgga
1200 cagacaggcg tgatcgccga ttacaactac aagctgcctg acgacttcac
cggctgcgtg 1260 ctggcctgga acaccagaaa catcgacgcc acccagacag
gcaactacaa ttacaagtac 1320 agaagcctgc ggcacggcaa gctgcggccc
ttcgagaggg acatctccaa cgtgcccttc 1380 agccccgacg gcaagccttg
taccccccct gcctttaact gctactggcc cctgaacgac 1440 tacggcttct
acatcacaaa cggcatcggc tatcagccct accgggtggt ggtgctgtcc 1500
tttgagctgc tgaatgcccc tgccaccgtg tgcggcccta agctgagcac cgacctgatc
1560 aagaaccagt gcgtgaactt caacttcaac ggcctgaccg gcaccggcgt
gctgacacct 1620 agcagcaaga gattccagcc cttccagcag ttcggccggg
acgtgctgga tttcaccgac 1680 agcgtgcggg accccaagac cagcgagatc
ctggacatca gcccctgcag cttcggcgga 1740 gtgtccgtga tcacccccgg
caccaatacc agctctgagg tggccgtgct gtatcaggac 1800 gtgaactgca
ccgatgtgcc cgtggccatc cacgccgatc agctgacccc atcttggcgg 1860
gtgtactcca ccggcaacaa cgtgttccag acacaagccg gctgcctgat cggagccgag
1920 cacgtggaca ccagctacga gtgcgacatc cctatcggcg ctggcatctg
cgccagctac 1980 cacaccgtgt ccagcctgag aagcaccagc cagaaatcta
tcgtggccta caccatgagc 2040 ctgggcgccg acagctctat cgcctactcc
aacaacacaa tcgccatccc caccaatttc 2100 agcatctcca tcaccaccga
agtgatgccc gtgtccatgg ccaagacctc cgtggattgc 2160 aacatgtaca
tctgcggcga cagcaccgag tgcgccaacc tgctgctgca gtacggcagc 2220
ttctgcaccc agctgaacag agccctgagc ggaatcgccg tggaacagga cagaaacacc
2280 cgggaagtgt tcgcccaagt gaagcagatg tataagaccc ccaccctgaa
ggatttcggc 2340 ggctttaact tcagccagat cctgcccgac cctctgaagc
ctaccaagcg gagcttcatc 2400 gaggacctgc tgttcaacaa agtgaccctg
gccgacgccg gctttatgaa gcagtatggc 2460 gagtgcctgg gcgacatcaa
cgcccgggat ctgatctgcg cccagaagtt taacggactg 2520 accgtgctgc
cccctctgct gaccgacgat atgatcgccg cctacacagc cgccctggtg 2580
tctggcacag ctaccgccgg atggacattt ggagctggcg ccgctctgca gatccccttt
2640 gccatgcaga tggcctaccg gttcaatggc atcggcgtga cccagaatgt
gctgtacgag 2700 aaccagaagc agatcgccaa ccagttcaac aaggccatta
gccagattca ggaaagcctg 2760 accaccacca gcaccgccct gggcaaactg
caggacgtcg tgaaccagaa cgcccaggcc 2820 ctgaacaccc tcgtgaagca
gctgagcagc aatttcggcg ccatcagctc cgtgctgaac 2880 gatatcctga
gcagactgga caaggtggaa gcagaggtgc agatcgaccg gctgatcacc 2940
ggcagactgc agagcctgca gacctacgtg acacagcagc tgattagagc cgccgagatc
3000 agggccagcg ccaatctggc cgccacaaag atgagcgagt gtgtgctggg
ccagagcaag 3060 cgggtggact tctgcggcaa gggctatcac ctgatgagct
tcccccaggc cgctcctcac 3120 ggcgtggtgt ttctgcacgt gacatacgtg
cccagccagg aacggaactt caccaccgcc 3180 ccagccatct gccacgaggg
caaggcctac ttcccccggg aaggcgtgtt cgtgtttaac 3240 ggcacctcct
ggtttatcac ccagcggaat ttcttcagtc cgcagatcat caccacagac 3300
aacaccttcg tgtccggcag ctgcgacgtc gtgattggca tcattaacaa caccgtgtac
3360 gaccccctgc agcccgagct ggacagcttc aaagaggaac tggacaagta
cttcaagaac 3420 cacacctccc ccgacgtgga cctgggcgat atctccggca
tcaatgccag cgtcgtgaat 3480 atccagaaag agatcgatcg cctgaacgag
gtggccaaga acctgaatga gagcctgatc 3540 gacctgcagg aactggggaa
gtacgagcag tacatcaagt ggccttggta cgtgtggctg 3600 ggctttatcg
ccggcctgat cgccatcgtg atggtcacca tcctgctgtg ctgcatgacc 3660
agctgttgca gctgtctgaa gggcgcctgc agctgtggct cctgctgcaa gttcgatgag
3720 gacgacagcg agcctgtgct gaaaggcgtg aagctgcact acacc 3765
<210> SEQ ID NO 20 <211> LENGTH: 3765 <212> TYPE:
RNA <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 20 auguuuaucu uccuguucuu ccugacccug accagcggca gcgaccugga
aagcugcacc 60 accuucgacg acgugcaggc ccccaacuac ccucagcaca
gcucuagcag acggggcgug 120 uacuaccccg acgagaucuu cagaagcgac
acccuguacc ugacccagga ccuguuccug 180 cccuucuaca gcaacgugac
cggcuuccac accaucaacc acagauucga caaccccgug 240 auccccuuca
aggacggggu guacuuugcc gccaccgaga aguccaaugu cgugcgggga 300
uggguguucg gcagcaccau gaacaacaag agccagagcg ugaucaucau caacaacagc
360 accaacgucg ugauccgggc cugcaacuuc gagcugugcg acaacccauu
cuucgccgug 420 uccaagccca ccggcaccca gacccacacc augaucuucg
acaacgccuu caacugcacc 480 uucgaguaca ucagcgacag cuucagccug
gacguggccg agaaaagcgg caacuucaag 540 caccugagag aauucguguu
caagaacaag gacggcuucc uguacgugua caagggcuac 600 cagcccaucg
acgucgugcg cgaucugccc agcggcuuca acauccugaa gcccaucuuc 660
aagcugcccc ugggcaucaa caucaccaac uuccgggcua uccugaccgc cuuccugccc
720 gcccaggaua ccuggggaac aagcgccgcu gccuacuucg ugggcuaccu
gaagccugcc 780 accuucaugc ugaaguacga cgagaacggc accaucaccg
acgccgugga cugcagccag 840 aauccucugg ccgagcugaa gugcagcgug
aaguccuucg agaucgacaa gggcaucuac 900 cagaccagca acuucagagu
ggcccccagc aaagaagucg ugcgguuccc caauaucacc 960 aaccugugcc
ccuucggcga gguguucaac gccaccaccu uucccagcgu guacgccugg 1020
gagcggaagc ggaucagcaa cugcguggcc gacuacagcg ugcuguacaa cuccaccagc
1080 uucuccaccu ucaagugcua cggcgugucc gccaccaagc ugaacgaccu
gugcuucagc 1140 aauguguacg ccgacuccuu cgucgugaag ggcgacgaug
ugcgccagau cgccccugga 1200 cagacaggcg ugaucgccga uuacaacuac
aagcugccug acgacuucac cggcugcgug 1260 cuggccugga acaccagaaa
caucgacgcc acccagacag gcaacuacaa uuacaaguac 1320 agaagccugc
ggcacggcaa gcugcggccc uucgagaggg acaucuccaa cgugcccuuc 1380
agccccgacg gcaagccuug uacccccccu gccuuuaacu gcuacuggcc ccugaacgac
1440 uacggcuucu acaucacaaa cggcaucggc uaucagcccu accggguggu
ggugcugucc 1500 uuugagcugc ugaaugcccc ugccaccgug ugcggcccua
agcugagcac cgaccugauc 1560 aagaaccagu gcgugaacuu caacuucaac
ggccugaccg gcaccggcgu gcugacaccu 1620 agcagcaaga gauuccagcc
cuuccagcag uucggccggg acgugcugga uuucaccgac 1680 agcgugcggg
accccaagac cagcgagauc cuggacauca gccccugcag cuucggcgga 1740
guguccguga ucacccccgg caccaauacc agcucugagg uggccgugcu guaucaggac
1800 gugaacugca ccgaugugcc cguggccauc cacgccgauc agcugacccc
aucuuggcgg 1860 guguacucca ccggcaacaa cguguuccag acacaagccg
gcugccugau cggagccgag 1920 cacguggaca ccagcuacga gugcgacauc
ccuaucggcg cuggcaucug cgccagcuac 1980 cacaccgugu ccagccugag
aagcaccagc cagaaaucua ucguggccua caccaugagc 2040 cugggcgccg
acagcucuau cgccuacucc aacaacacaa ucgccauccc caccaauuuc 2100
agcaucucca ucaccaccga agugaugccc guguccaugg ccaagaccuc cguggauugc
2160 aacauguaca ucugcggcga cagcaccgag ugcgccaacc ugcugcugca
guacggcagc 2220 uucugcaccc agcugaacag agcccugagc ggaaucgccg
uggaacagga cagaaacacc 2280 cgggaagugu ucgcccaagu gaagcagaug
uauaagaccc ccacccugaa ggauuucggc 2340 ggcuuuaacu ucagccagau
ccugcccgac ccucugaagc cuaccaagcg gagcuucauc 2400 gaggaccugc
uguucaacaa agugacccug gccgacgccg gcuuuaugaa gcaguauggc 2460
gagugccugg gcgacaucaa cgcccgggau cugaucugcg cccagaaguu uaacggacug
2520 accgugcugc ccccucugcu gaccgacgau augaucgccg ccuacacagc
cgcccuggug 2580 ucuggcacag cuaccgccgg auggacauuu ggagcuggcg
ccgcucugca gauccccuuu 2640 gccaugcaga uggccuaccg guucaauggc
aucggcguga cccagaaugu gcuguacgag 2700 aaccagaagc agaucgccaa
ccaguucaac aaggccauua gccagauuca ggaaagccug 2760 accaccacca
gcaccgcccu gggcaaacug caggacgucg ugaaccagaa cgcccaggcc 2820
cugaacaccc ucgugaagca gcugagcagc aauuucggcg ccaucagcuc cgugcugaac
2880 gauauccuga gcagacugga caagguggaa gcagaggugc agaucgaccg
gcugaucacc 2940 ggcagacugc agagccugca gaccuacgug acacagcagc
ugauuagagc cgccgagauc 3000 agggccagcg ccaaucuggc cgccacaaag
augagcgagu gugugcuggg ccagagcaag 3060 cggguggacu ucugcggcaa
gggcuaucac cugaugagcu ucccccaggc cgcuccucac 3120 ggcguggugu
uucugcacgu gacauacgug cccagccagg aacggaacuu caccaccgcc 3180
ccagccaucu gccacgaggg caaggccuac uucccccggg aaggcguguu cguguuuaac
3240 ggcaccuccu gguuuaucac ccagcggaau uucuucaguc cgcagaucau
caccacagac 3300 aacaccuucg uguccggcag cugcgacguc gugauuggca
ucauuaacaa caccguguac 3360 gacccccugc agcccgagcu ggacagcuuc
aaagaggaac uggacaagua cuucaagaac 3420 cacaccuccc ccgacgugga
ccugggcgau aucuccggca ucaaugccag cgucgugaau 3480 auccagaaag
agaucgaucg ccugaacgag guggccaaga accugaauga gagccugauc 3540
gaccugcagg aacuggggaa guacgagcag uacaucaagu ggccuuggua cguguggcug
3600 ggcuuuaucg ccggccugau cgccaucgug auggucacca uccugcugug
cugcaugacc 3660 agcuguugca gcugucugaa gggcgccugc agcuguggcu
ccugcugcaa guucgaugag 3720 gacgacagcg agccugugcu gaaaggcgug
aagcugcacu acacc 3765 <210> SEQ ID NO 21 <211> LENGTH:
30 <212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 21 Met Asp Ser Lys Gly Ser Ser
Gln Lys Gly Ser Arg Leu Leu Leu Leu 1 5 10 15 Leu Val Val Ser Asn
Leu Leu Leu Pro Gln Gly Val Val Gly 20 25 30 <210> SEQ ID NO
22 <211> LENGTH: 18 <212> TYPE: PRT <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 22
Met Asp Trp Thr Trp Ile Leu Phe Leu Val Ala Ala Ala Thr Arg Val 1 5
10 15 His Ser <210> SEQ ID NO 23 <211> LENGTH: 20
<212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 23 Met Glu Thr Pro Ala Gln Leu
Leu Phe Leu Leu Leu Leu Trp Leu Pro 1 5 10 15 Asp Thr Thr Gly 20
<210> SEQ ID NO 24 <211> LENGTH: 24 <212> TYPE:
PRT <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 24 Met Leu Gly Ser Asn Ser Gly Gln Arg Val Val Phe Thr
Ile Leu Leu 1 5 10 15 Leu Leu Val Ala Pro Ala Tyr Ser 20
<210> SEQ ID NO 25 <211> LENGTH: 17 <212> TYPE:
PRT <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 25 Met Lys Cys Leu Leu Tyr Leu Ala Phe Leu Phe Ile Gly
Val Asn Cys 1 5 10 15 Ala <210> SEQ ID NO 26 <211>
LENGTH: 15 <212> TYPE: PRT <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polypeptide <400> SEQUENCE: 26 Met Trp Leu Val Ser
Leu Ala Ile Val Thr Ala Cys Ala Gly Ala 1 5 10 15 <210> SEQ
ID NO 27 <211> LENGTH: 9 <212> TYPE: RNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE:
27 ccrccaugg 9 <210> SEQ ID NO 28 <211> LENGTH: 11
<212> TYPE: RNA <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polynucleotide <400> SEQUENCE: 28 gggauccuac c 11 <210>
SEQ ID NO 29 <211> LENGTH: 9 <212> TYPE: RNA
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <220>
FEATURE: <221> NAME/KEY: misc_feature <222> LOCATION:
(8)..(9) <223> OTHER INFORMATION: n can be u or a <400>
SEQUENCE: 29 uuauuuann 9 <210> SEQ ID NO 30 <400>
SEQUENCE: 30 000 <210> SEQ ID NO 31 <211> LENGTH: 13
<212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 31 Leu Gln Arg Val Arg Glu Leu
Ala Val Gln Ser Ala Asn 1 5 10
1 SEQUENCE LISTING <160> NUMBER OF SEQ ID NOS: 31 <210>
SEQ ID NO 1 <211> LENGTH: 490 <212> TYPE: PRT
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 1 Met Gly Gln Ile Val Thr Phe Phe Gln Glu Val Pro His Val
Ile Glu 1 5 10 15 Glu Val Met Asn Ile Val Leu Ile Ala Leu Ser Leu
Leu Ala Ile Leu 20 25 30 Lys Gly Ile Tyr Asn Val Ala Thr Cys Gly
Leu Phe Gly Leu Val Ser 35 40 45 Phe Leu Leu Leu Cys Gly Arg Ser
Cys Ser Thr Thr Tyr Lys Gly Val 50 55 60 Tyr Glu Leu Gln Thr Leu
Glu Leu Asp Met Ala Ser Leu Asn Met Thr 65 70 75 80 Met Pro Leu Ser
Cys Thr Lys Asn Asn Ser His His Tyr Ile Met Val 85 90 95 Gly Asn
Glu Thr Gly Leu Glu Leu Thr Leu Thr Asn Thr Ser Ile Ile 100 105 110
Asn His Lys Phe Cys Asn Leu Ser Asp Ala His Lys Lys Asp Leu Tyr 115
120 125 Asp His Ala Leu Met Ser Ile Ile Ser Thr Phe His Leu Ser Ile
Pro 130 135 140 Asn Phe Asn Gln Tyr Glu Ala Met Ser Cys Asp Phe Asn
Gly Gly Lys 145 150 155 160 Ile Ser Val Gln Tyr Asn Leu Ser His Thr
Tyr Ala Val Asp Ala Ala 165 170 175 Asn His Cys Gly Thr Ile Ala Asn
Gly Val Leu Gln Thr Phe Met Arg 180 185 190 Met Ala Trp Gly Gly Ser
Tyr Ile Ala Leu Asp Ser Gly Lys Gly Ser 195 200 205 Trp Asp Cys Ile
Met Thr Ser Tyr Gln Tyr Leu Ile Ile Gln Asn Thr 210 215 220 Thr Trp
Glu Asp His Cys Gln Phe Ser Arg Pro Ser Pro Ile Gly Tyr 225 230 235
240 Leu Gly Leu Leu Ser Gln Arg Thr Arg Asp Ile Tyr Ile Ser Arg Arg
245 250 255 Leu Leu Gly Thr Phe Thr Trp Thr Leu Ser Asp Ser Glu Gly
Asn Glu 260 265 270 Thr Pro Gly Gly Tyr Cys Leu Thr Arg Trp Met Leu
Ile Glu Ala Glu 275 280 285 Leu Lys Cys Phe Gly Asn Thr Ala Val Ala
Lys Cys Asn Glu Lys His 290 295 300 Asp Glu Glu Phe Cys Asp Met Leu
Arg Leu Phe Asp Phe Asn Lys Gln 305 310 315 320 Ala Ile Met Arg Leu
Lys Thr Glu Ala Gln Met Ser Ile Gln Leu Ile 325 330 335 Asn Lys Ala
Val Asn Ala Leu Ile Asn Asp Gln Leu Ile Met Lys Asn 340 345 350 His
Leu Arg Asp Ile Met Gly Ile Pro Tyr Cys Asn Tyr Ser Lys Tyr 355 360
365 Trp Tyr Leu Asn His Thr Val Thr Gly Lys Thr Ser Leu Pro Arg Cys
370 375 380 Trp Leu Val Ser Asn Gly Ser Tyr Leu Asn Glu Thr Arg Phe
Ser Asp 385 390 395 400 Asp Ile Glu Gln Gln Ala Asp Asn Met Ile Thr
Glu Met Leu Gln Lys 405 410 415 Glu Tyr Leu Asp Arg Gln Gly Lys Thr
Pro Leu Gly Leu Val Asp Leu 420 425 430 Phe Val Phe Ser Thr Ser Phe
Tyr Leu Ile Ser Ile Phe Leu His Leu 435 440 445 Val Lys Ile Pro Thr
His Arg His Ile Ile Gly Lys Pro Cys Pro Lys 450 455 460 Pro His Arg
Leu Asn His Met Gly Ile Cys Ser Cys Gly Leu Tyr Lys 465 470 475 480
His Pro Gly Val Pro Val Lys Trp Lys Arg 485 490 <210> SEQ ID
NO 2 <211> LENGTH: 569 <212> TYPE: PRT <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 2
Met Ser Ala Ser Lys Glu Val Lys Ser Phe Leu Trp Thr Gln Ser Leu 1 5
10 15 Arg Arg Glu Leu Ser Gly Tyr Cys Ser Asn Ile Lys Leu Gln Val
Val 20 25 30 Lys Asp Ala Gln Ala Leu Leu His Gly Leu Asp Phe Ser
Glu Val Ser 35 40 45 Asn Val Gln Arg Leu Met Arg Lys Gln Lys Arg
Asp Asp Gly Asp Leu 50 55 60 Lys Arg Leu Arg Asp Leu Asn Gln Ala
Val Asn Asn Leu Val Glu Leu 65 70 75 80 Lys Ser Thr Gln Gln Lys Ser
Val Leu Arg Val Gly Thr Leu Ser Ser 85 90 95 Asp Asp Leu Leu Val
Leu Ala Ala Asp Leu Glu Lys Leu Lys Ser Lys 100 105 110 Val Val Arg
Thr Glu Arg Pro Leu Ser Ser Gly Ile Tyr Met Gly Asn 115 120 125 Leu
Ser Ser Gln Gln Leu Asp Gln Arg Lys Ala Leu Leu Asn Met Ile 130 135
140 Gly Met Thr Gly Gly Asn Gly Gly Arg Asn Thr Thr Ser Asp Gly Ile
145 150 155 160 Val Arg Val Trp Asp Val Lys Asn Ala Glu Leu Leu Asn
Asn Gln Phe 165 170 175 Gly Thr Met Pro Ser Leu Thr Leu Ala Cys Leu
Thr Lys Gln Gly Gln 180 185 190 Val Asp Leu Asn Asp Ala Val Gln Ala
Leu Thr Asp Leu Gly Leu Ile 195 200 205 Tyr Thr Ala Lys Tyr Pro Asn
Ser Ser Asp Leu Asp Arg Leu Ala Gln 210 215 220 Ser His Pro Ile Leu
Asn Met Ile Asp Thr Lys Lys Ser Ser Leu Asn 225 230 235 240 Ile Ser
Gly Tyr Asn Phe Ser Leu Gly Ala Ala Val Lys Ala Gly Ala 245 250 255
Cys Met Leu Asp Gly Gly Asn Met Leu Glu Thr Ile Lys Val Ser Pro 260
265 270 Gln Thr Met Asp Gly Ile Leu Lys Ser Ile Leu Lys Val Lys Arg
Ser 275 280 285 Leu Gly Met Phe Ile Ser Asp Thr Pro Gly Glu Arg Asn
Pro Tyr Glu 290 295 300 Asn Ile Leu Tyr Lys Ile Cys Leu Ser Gly Asp
Gly Trp Pro Tyr Ile 305 310 315 320 Ala Ser Arg Thr Ser Ile Val Gly
Arg Ala Trp Glu Asn Thr Val Val 325 330 335 Asp Leu Glu Ser Asp Asn
Lys Pro Gln Lys Thr Gly Asn Gly Gly Ser 340 345 350 Asn Lys Ser Leu
Gln Ser Ala Gly Phe Ala Ala Gly Leu Thr Tyr Ser 355 360 365 Gln Leu
Met Thr Leu Lys Asp Ser Met Leu Gln Leu Asp Pro Asn Ala 370 375 380
Lys Thr Trp Met Asp Ile Glu Gly Arg Pro Glu Asp Pro Val Glu Ile 385
390 395 400 Ala Leu Tyr Gln Pro Ser Ser Gly Cys Tyr Ile His Phe Phe
Arg Glu 405 410 415 Pro Thr Asp Leu Lys Gln Phe Lys Gln Asp Ala Lys
Tyr Ser His Gly 420 425 430 Ile Asp Val Thr Asp Leu Phe Ala Ala Gln
Pro Gly Leu Thr Ser Ala 435 440 445 Val Ile Glu Ala Leu Pro Arg Asn
Met Val Ile Thr Cys Gln Gly Ser 450 455 460 Glu Asp Ile Arg Lys Leu
Leu Glu Ser Gln Gly Arg Arg Asp Ile Lys 465 470 475 480 Leu Ile Asp
Ile Ser Leu Ser Lys Val Asp Ser Arg Lys Phe Glu Asn 485 490 495 Ala
Val Trp Asp Gln Phe Lys Asp Leu Cys His Met His Thr Gly Ile 500 505
510 Val Val Glu Lys Lys Lys Arg Gly Gly Lys Glu Glu Ile Thr Pro His
515 520 525 Cys Ala Leu Met Asp Cys Ile Met Phe Asp Ala Ala Val Ser
Gly Gly 530 535 540 Val Asp Ala Lys Val Leu Arg Ala Val Leu Pro Arg
Asp Met Val Phe 545 550 555 560 Arg Thr Ser Thr Pro Lys Val Val Leu
565 <210> SEQ ID NO 3 <211> LENGTH: 589 <212>
TYPE: PRT <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polypeptide
<400> SEQUENCE: 3 Met Glu Thr Pro Ala Gln Leu Leu Phe Leu Leu
Leu Leu Trp Leu Pro 1 5 10 15 Asp Thr Thr Gly Met Ser Ala Ser Lys
Glu Val Lys Ser Phe Leu Trp 20 25 30 Thr Gln Ser Leu Arg Arg Glu
Leu Ser Gly Tyr Cys Ser Asn Ile Lys 35 40 45 Leu Gln Val Val Lys
Asp Ala Gln Ala Leu Leu His Gly Leu Asp Phe 50 55 60 Ser Glu Val
Ser Asn Val Gln Arg Leu Met Arg Lys Gln Lys Arg Asp 65 70 75 80
Asp Gly Asp Leu Lys Arg Leu Arg Asp Leu Asn Gln Ala Val Asn Asn 85
90 95 Leu Val Glu Leu Lys Ser Thr Gln Gln Lys Ser Val Leu Arg Val
Gly 100 105 110 Thr Leu Ser Ser Asp Asp Leu Leu Val Leu Ala Ala Asp
Leu Glu Lys 115 120 125 Leu Lys Ser Lys Val Val Arg Thr Glu Arg Pro
Leu Ser Ser Gly Ile 130 135 140 Tyr Met Gly Asn Leu Ser Ser Gln Gln
Leu Asp Gln Arg Lys Ala Leu 145 150 155 160 Leu Asn Met Ile Gly Met
Thr Gly Gly Asn Gly Gly Arg Asn Thr Thr 165 170 175 Ser Asp Gly Ile
Val Arg Val Trp Asp Val Lys Asn Ala Glu Leu Leu 180 185 190 Asn Asn
Gln Phe Gly Thr Met Pro Ser Leu Thr Leu Ala Cys Leu Thr 195 200 205
Lys Gln Gly Gln Val Asp Leu Asn Asp Ala Val Gln Ala Leu Thr Asp 210
215 220 Leu Gly Leu Ile Tyr Thr Ala Lys Tyr Pro Asn Ser Ser Asp Leu
Asp 225 230 235 240 Arg Leu Ala Gln Ser His Pro Ile Leu Asn Met Ile
Asp Thr Lys Lys 245 250 255 Ser Ser Leu Asn Ile Ser Gly Tyr Asn Phe
Ser Leu Gly Ala Ala Val 260 265 270 Lys Ala Gly Ala Cys Met Leu Asp
Gly Gly Asn Met Leu Glu Thr Ile 275 280 285 Lys Val Ser Pro Gln Thr
Met Asp Gly Ile Leu Lys Ser Ile Leu Lys 290 295 300 Val Lys Arg Ser
Leu Gly Met Phe Ile Ser Asp Thr Pro Gly Glu Arg 305 310 315 320 Asn
Pro Tyr Glu Asn Ile Leu Tyr Lys Ile Cys Leu Ser Gly Asp Gly 325 330
335 Trp Pro Tyr Ile Ala Ser Arg Thr Ser Ile Val Gly Arg Ala Trp Glu
340 345 350 Asn Thr Val Val Asp Leu Glu Ser Asp Asn Lys Pro Gln Lys
Thr Gly 355 360 365 Asn Gly Gly Ser Asn Lys Ser Leu Gln Ser Ala Gly
Phe Ala Ala Gly 370 375 380 Leu Thr Tyr Ser Gln Leu Met Thr Leu Lys
Asp Ser Met Leu Gln Leu 385 390 395 400 Asp Pro Asn Ala Lys Thr Trp
Met Asp Ile Glu Gly Arg Pro Glu Asp 405 410 415 Pro Val Glu Ile Ala
Leu Tyr Gln Pro Ser Ser Gly Cys Tyr Ile His 420 425 430 Phe Phe Arg
Glu Pro Thr Asp Leu Lys Gln Phe Lys Gln Asp Ala Lys 435 440 445 Tyr
Ser His Gly Ile Asp Val Thr Asp Leu Phe Ala Ala Gln Pro Gly 450 455
460 Leu Thr Ser Ala Val Ile Glu Ala Leu Pro Arg Asn Met Val Ile Thr
465 470 475 480 Cys Gln Gly Ser Glu Asp Ile Arg Lys Leu Leu Glu Ser
Gln Gly Arg 485 490 495 Arg Asp Ile Lys Leu Ile Asp Ile Ser Leu Ser
Lys Val Asp Ser Arg 500 505 510 Lys Phe Glu Asn Ala Val Trp Asp Gln
Phe Lys Asp Leu Cys His Met 515 520 525 His Thr Gly Ile Val Val Glu
Lys Lys Lys Arg Gly Gly Lys Glu Glu 530 535 540 Ile Thr Pro His Cys
Ala Leu Met Asp Cys Ile Met Phe Asp Ala Ala 545 550 555 560 Val Ser
Gly Gly Val Asp Ala Lys Val Leu Arg Ala Val Leu Pro Arg 565 570 575
Asp Met Val Phe Arg Thr Ser Thr Pro Lys Val Val Leu 580 585
<210> SEQ ID NO 4 <211> LENGTH: 1470 <212> TYPE:
DNA <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 4 atgggccaga tcgtgacatt cttccaagag gtgccccacg tgatcgagga
agtgatgaac 60 atcgtcctga tcgccctgag cctgctggcc atcctgaagg
gcatctacaa cgtggccacc 120 tgtggcctgt ttggcctggt gtcattcctg
ctgctgtgcg gcagaagctg cagcaccaca 180 tacaagggcg tgtacgagct
gcagaccctg gaactggata tggccagcct gaacatgacc 240 atgcctctga
gctgcaccaa gaacaacagc caccactaca tcatggtcgg aaacgagaca 300
ggactggaac tgaccctgac caacaccagc atcatcaacc acaagttctg caacctgagc
360 gacgcccaca agaaggacct gtacgatcac gccctgatga gcatcatctc
caccttccac 420 ctgagcatcc ccaacttcaa ccagtacgag gccatgagct
gcgacttcaa cggcggcaag 480 atcagcgtgc agtacaatct gagccacacc
tacgccgtgg acgccgccaa tcactgtggc 540 acaattgcca atggcgtgct
gcagacattc atgcggatgg cctggggcgg ctcttatatc 600 gccctggatt
ctggcaaagg cagctgggac tgcatcatga ccagctacca gtacctgatc 660
atccagaaca ccacctggga agatcactgc cagttcagca gaccctctcc tatcggctat
720 ctgggcctgc tgagccagag aacccgggac atctacatca gcagaaggct
gctgggcacc 780 ttcacctgga cactgtctga cagcgagggc aacgaaacac
ctggcggcta ctgcctgacc 840 agatggatgc tgattgaggc cgagctgaag
tgcttcggca ataccgccgt ggccaagtgc 900 aacgagaagc acgacgagga
attctgcgac atgctgcggc tgttcgattt caacaagcag 960 gccatcatgc
ggctcaagac cgaggctcag atgtccatcc agctgatcaa caaggccgtg 1020
aatgccctga tcaacgatca gctcatcatg aagaaccacc tccgggatat catgggcatc
1080 ccttactgca actacagcaa gtactggtat ctcaaccaca ccgtgaccgg
caagaccagc 1140 ctgcctagat gttggctggt gtccaacggc agctacctga
acgagacacg gttcagcgac 1200 gacatcgagc agcaggccga caacatgatc
accgagatgc tgcagaaaga gtacctggac 1260 cggcagggca agacacctct
gggactcgtg gatctgttcg tgttcagcac cagcttctac 1320 ctgatctcta
tcttcctgca cctggtcaag atccccacac accggcacat catcggcaag 1380
ccctgtccta agcctcaccg gctgaaccac atgggaatct gtagctgcgg cctgtacaag
1440 caccctggcg tgccagtgaa gtggaagaga 1470 <210> SEQ ID NO 5
<211> LENGTH: 1767 <212> TYPE: DNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE: 5
atggagactc ctgcccagct cttgttcctt ttgctattgt ggcttcccga caccaccggc
60 atgagcgcca gcaaggaggt caagagcttc ctctggaccc agagcctaag
aagagagctt 120 agcggctact gcagcaacat caagcttcag gtggtgaagg
acgcccaggc cctgctgcac 180 ggcctggact tcagcgaggt gagcaacgtg
cagagactga tgagaaagca gaagcgagac 240 gacggcgacc tgaagcgtct
gcgggacctg aaccaggccg tgaacaacct ggtggagctt 300 aagagcaccc
agcagaagtc tgtgctgaga gtgggcaccc tgagcagcga cgacctgctg 360
gtgctggccg ccgacctgga gaagctgaag tctaaggtcg tcagaaccga gcggccattg
420 agctcaggca tctacatggg caaccttagc agtcagcagc tggaccagag
aaaggccttg 480 ctgaacatga tcggcatgac cggcggcaac ggcggcagaa
acaccaccag cgacggcatc 540 gtgagagtgt gggacgtgaa gaacgccgag
ctactcaaca accagttcgg caccatgccc 600 agcctgaccc tggcctgcct
gaccaagcag ggccaggtgg acctcaatga cgccgtgcag 660 gcactaaccg
accttggcct gatctacacc gccaagtacc ccaactcttc agacctggac 720
agactggcgc agtcccaccc catcttaaat atgattgaca ccaagaagtc atcccttaac
780 atcagtggct acaacttcag cctgggcgcc gccgtgaagg ccggcgcctg
catgctggac 840 ggcggaaata tgctggaaac tatcaaggtg agccctcaga
ccatggacgg tatcctgaag 900 tccattttga aggttaagag atccctgggt
atgttcatca gcgacacccc aggcgagaga 960 aacccctacg agaacatcct
gtacaagatc tgcctgagtg gcgacggctg gccctacatc 1020 gcgagcagaa
ccagcatcgt gggaagggcc tgggagaaca ccgtggtgga tcttgagagc 1080
gacaacaagc cccagaagac cggaaatggc ggttcaaaca agagcctgca gagcgccggc
1140 ttcgccgccg gcctgaccta cagccagctg atgaccctga aggacagcat
gctacaattg 1200 gatcccaacg ccaagacttg gatggacatc gagggcagac
ccgaggaccc cgtggagatc 1260 gccctgtacc agccctcatc cggctgctac
atccacttct tcagagagcc cacagatctg 1320 aagcagttca agcaggacgc
gaagtatagc catggcatag acgtcaccga tttattcgcg 1380 gcccagccgg
gccttacgag cgccgtgatc gaggcgctgc ccagaaacat ggtgatcacc 1440
tgccagggca gcgaggacat cagaaagctc cttgaatctc aaggccggag agatattaag
1500 ctgatagata tcagcttatc taaggttgac agcagaaagt tcgagaacgc
tgtatgggac 1560 caattcaagg acctgtgcca catgcatacg ggcatagtgg
tagagaagaa gaagcgtggc 1620 ggaaaggagg agatcacacc tcactgcgcc
ctgatggact gcatcatgtt cgacgcggca 1680 gtctccggcg gcgtcgacgc
aaaggtcctc cgggccgtgc tgccaaggga catggtgttc 1740 cggacaagca
cccctaaggt agtgctg 1767 <210> SEQ ID NO 6 <211> LENGTH:
1470 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 6 augggccaga
ucgugacauu cuuccaagag gugccccacg ugaucgagga agugaugaac 60
aucguccuga ucgcccugag ccugcuggcc auccugaagg gcaucuacaa cguggccacc
120 uguggccugu uuggccuggu gucauuccug cugcugugcg gcagaagcug
cagcaccaca 180 uacaagggcg uguacgagcu gcagacccug gaacuggaua
uggccagccu gaacaugacc 240 augccucuga gcugcaccaa gaacaacagc
caccacuaca ucauggucgg aaacgagaca 300 ggacuggaac ugacccugac
caacaccagc aucaucaacc acaaguucug caaccugagc 360 gacgcccaca
agaaggaccu guacgaucac gcccugauga gcaucaucuc caccuuccac 420
cugagcaucc ccaacuucaa ccaguacgag gccaugagcu gcgacuucaa cggcggcaag
480 aucagcgugc aguacaaucu gagccacacc uacgccgugg acgccgccaa
ucacuguggc 540 acaauugcca auggcgugcu gcagacauuc augcggaugg
ccuggggcgg cucuuauauc 600 gcccuggauu cuggcaaagg cagcugggac
ugcaucauga ccagcuacca guaccugauc 660 auccagaaca ccaccuggga
agaucacugc caguucagca gacccucucc uaucggcuau 720 cugggccugc
ugagccagag aacccgggac aucuacauca gcagaaggcu gcugggcacc 780
uucaccugga cacugucuga cagcgagggc aacgaaacac cuggcggcua cugccugacc
840 agauggaugc ugauugaggc cgagcugaag ugcuucggca auaccgccgu
ggccaagugc 900 aacgagaagc acgacgagga auucugcgac augcugcggc
uguucgauuu caacaagcag 960 gccaucaugc ggcucaagac cgaggcucag
auguccaucc agcugaucaa caaggccgug 1020 aaugcccuga ucaacgauca
gcucaucaug aagaaccacc uccgggauau caugggcauc 1080 ccuuacugca
acuacagcaa guacugguau cucaaccaca ccgugaccgg caagaccagc 1140
cugccuagau guuggcuggu guccaacggc agcuaccuga acgagacacg guucagcgac
1200 gacaucgagc agcaggccga caacaugauc accgagaugc ugcagaaaga
guaccuggac 1260 cggcagggca agacaccucu gggacucgug gaucuguucg
uguucagcac cagcuucuac 1320 cugaucucua ucuuccugca ccuggucaag
auccccacac accggcacau caucggcaag 1380 cccuguccua agccucaccg
gcugaaccac augggaaucu guagcugcgg ccuguacaag 1440 cacccuggcg
ugccagugaa guggaagaga 1470 <210> SEQ ID NO 7 <211>
LENGTH: 1767 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 7 auggagacuc
cugcccagcu cuuguuccuu uugcuauugu ggcuucccga caccaccggc 60
augagcgcca gcaaggaggu caagagcuuc cucuggaccc agagccuaag aagagagcuu
120 agcggcuacu gcagcaacau caagcuucag guggugaagg acgcccaggc
ccugcugcac 180 ggccuggacu ucagcgaggu gagcaacgug cagagacuga
ugagaaagca gaagcgagac 240 gacggcgacc ugaagcgucu gcgggaccug
aaccaggccg ugaacaaccu gguggagcuu 300 aagagcaccc agcagaaguc
ugugcugaga gugggcaccc ugagcagcga cgaccugcug 360 gugcuggccg
ccgaccugga gaagcugaag ucuaaggucg ucagaaccga gcggccauug 420
agcucaggca ucuacauggg caaccuuagc agucagcagc uggaccagag aaaggccuug
480 cugaacauga ucggcaugac cggcggcaac ggcggcagaa acaccaccag
cgacggcauc 540 gugagagugu gggacgugaa gaacgccgag cuacucaaca
accaguucgg caccaugccc 600 agccugaccc uggccugccu gaccaagcag
ggccaggugg accucaauga cgccgugcag 660 gcacuaaccg accuuggccu
gaucuacacc gccaaguacc ccaacucuuc agaccuggac 720 agacuggcgc
agucccaccc caucuuaaau augauugaca ccaagaaguc aucccuuaac 780
aucaguggcu acaacuucag ccugggcgcc gccgugaagg ccggcgccug caugcuggac
840 ggcggaaaua ugcuggaaac uaucaaggug agcccucaga ccauggacgg
uauccugaag 900 uccauuuuga agguuaagag aucccugggu auguucauca
gcgacacccc aggcgagaga 960 aaccccuacg agaacauccu guacaagauc
ugccugagug gcgacggcug gcccuacauc 1020 gcgagcagaa ccagcaucgu
gggaagggcc ugggagaaca ccguggugga ucuugagagc 1080 gacaacaagc
cccagaagac cggaaauggc gguucaaaca agagccugca gagcgccggc 1140
uucgccgccg gccugaccua cagccagcug augacccuga aggacagcau gcuacaauug
1200 gaucccaacg ccaagacuug gauggacauc gagggcagac ccgaggaccc
cguggagauc 1260 gcccuguacc agcccucauc cggcugcuac auccacuucu
ucagagagcc cacagaucug 1320 aagcaguuca agcaggacgc gaaguauagc
cauggcauag acgucaccga uuuauucgcg 1380 gcccagccgg gccuuacgag
cgccgugauc gaggcgcugc ccagaaacau ggugaucacc 1440 ugccagggca
gcgaggacau cagaaagcuc cuugaaucuc aaggccggag agauauuaag 1500
cugauagaua ucagcuuauc uaagguugac agcagaaagu ucgagaacgc uguaugggac
1560 caauucaagg accugugcca caugcauacg ggcauagugg uagagaagaa
gaagcguggc 1620 ggaaaggagg agaucacacc ucacugcgcc cugauggacu
gcaucauguu cgacgcggca 1680 gucuccggcg gcgucgacgc aaagguccuc
cgggccgugc ugccaaggga caugguguuc 1740 cggacaagca ccccuaaggu agugcug
1767 <210> SEQ ID NO 8 <211> LENGTH: 1707 <212>
TYPE: DNA <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polynucleotide
<400> SEQUENCE: 8 atgagcgcca gcaaggaggt caagagcttc ctctggaccc
agagcctaag aagagagctt 60 agcggctact gcagcaacat caagcttcag
gtggtgaagg acgcccaggc cctgctgcac 120 ggcctggact tcagcgaggt
gagcaacgtg cagagactga tgagaaagca gaagcgagac 180 gacggcgacc
tgaagcgtct gcgggacctg aaccaggccg tgaacaacct ggtggagctt 240
aagagcaccc agcagaagtc tgtgctgaga gtgggcaccc tgagcagcga cgacctgctg
300 gtgctggccg ccgacctgga gaagctgaag tctaaggtcg tcagaaccga
gcggccattg 360 agctcaggca tctacatggg caaccttagc agtcagcagc
tggaccagag aaaggccttg 420 ctgaacatga tcggcatgac cggcggcaac
ggcggcagaa acaccaccag cgacggcatc 480 gtgagagtgt gggacgtgaa
gaacgccgag ctactcaaca accagttcgg caccatgccc 540 agcctgaccc
tggcctgcct gaccaagcag ggccaggtgg acctcaatga cgccgtgcag 600
gcactaaccg accttggcct gatctacacc gccaagtacc ccaactcttc agacctggac
660 agactggcgc agtcccaccc catcttaaat atgattgaca ccaagaagtc
atcccttaac 720 atcagtggct acaacttcag cctgggcgcc gccgtgaagg
ccggcgcctg catgctggac 780 ggcggaaata tgctggaaac tatcaaggtg
agccctcaga ccatggacgg tatcctgaag 840 tccattttga aggttaagag
atccctgggt atgttcatca gcgacacccc aggcgagaga 900 aacccctacg
agaacatcct gtacaagatc tgcctgagtg gcgacggctg gccctacatc 960
gcgagcagaa ccagcatcgt gggaagggcc tgggagaaca ccgtggtgga tcttgagagc
1020 gacaacaagc cccagaagac cggaaatggc ggttcaaaca agagcctgca
gagcgccggc 1080 ttcgccgccg gcctgaccta cagccagctg atgaccctga
aggacagcat gctacaattg 1140 gatcccaacg ccaagacttg gatggacatc
gagggcagac ccgaggaccc cgtggagatc 1200 gccctgtacc agccctcatc
cggctgctac atccacttct tcagagagcc cacagatctg 1260 aagcagttca
agcaggacgc gaagtatagc catggcatag acgtcaccga tttattcgcg 1320
gcccagccgg gccttacgag cgccgtgatc gaggcgctgc ccagaaacat ggtgatcacc
1380 tgccagggca gcgaggacat cagaaagctc cttgaatctc aaggccggag
agatattaag 1440 ctgatagata tcagcttatc taaggttgac agcagaaagt
tcgagaacgc tgtatgggac 1500 caattcaagg acctgtgcca catgcatacg
ggcatagtgg tagagaagaa gaagcgtggc 1560 ggaaaggagg agatcacacc
tcactgcgcc ctgatggact gcatcatgtt cgacgcggca 1620 gtctccggcg
gcgtcgacgc aaaggtcctc cgggccgtgc tgccaaggga catggtgttc 1680
cggacaagca cccctaaggt agtgctg 1707 <210> SEQ ID NO 9
<211> LENGTH: 1707 <212> TYPE: RNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE: 9
augagcgcca gcaaggaggu caagagcuuc cucuggaccc agagccuaag aagagagcuu
60 agcggcuacu gcagcaacau caagcuucag guggugaagg acgcccaggc
ccugcugcac 120 ggccuggacu ucagcgaggu gagcaacgug cagagacuga
ugagaaagca gaagcgagac 180 gacggcgacc ugaagcgucu gcgggaccug
aaccaggccg ugaacaaccu gguggagcuu 240 aagagcaccc agcagaaguc
ugugcugaga gugggcaccc ugagcagcga cgaccugcug 300 gugcuggccg
ccgaccugga gaagcugaag ucuaaggucg ucagaaccga gcggccauug 360
agcucaggca ucuacauggg caaccuuagc agucagcagc uggaccagag aaaggccuug
420 cugaacauga ucggcaugac cggcggcaac ggcggcagaa acaccaccag
cgacggcauc 480 gugagagugu gggacgugaa gaacgccgag cuacucaaca
accaguucgg caccaugccc 540 agccugaccc uggccugccu gaccaagcag
ggccaggugg accucaauga cgccgugcag 600 gcacuaaccg accuuggccu
gaucuacacc gccaaguacc ccaacucuuc agaccuggac 660 agacuggcgc
agucccaccc caucuuaaau augauugaca ccaagaaguc aucccuuaac 720
aucaguggcu acaacuucag ccugggcgcc gccgugaagg ccggcgccug caugcuggac
780 ggcggaaaua ugcuggaaac uaucaaggug agcccucaga ccauggacgg
uauccugaag 840 uccauuuuga agguuaagag aucccugggu auguucauca
gcgacacccc aggcgagaga 900 aaccccuacg agaacauccu guacaagauc
ugccugagug gcgacggcug gcccuacauc 960 gcgagcagaa ccagcaucgu
gggaagggcc ugggagaaca ccguggugga ucuugagagc 1020 gacaacaagc
cccagaagac cggaaauggc gguucaaaca agagccugca gagcgccggc 1080
uucgccgccg gccugaccua cagccagcug augacccuga aggacagcau gcuacaauug
1140 gaucccaacg ccaagacuug gauggacauc gagggcagac ccgaggaccc
cguggagauc 1200 gcccuguacc agcccucauc cggcugcuac auccacuucu
ucagagagcc cacagaucug 1260 aagcaguuca agcaggacgc gaaguauagc
cauggcauag acgucaccga uuuauucgcg 1320 gcccagccgg gccuuacgag
cgccgugauc gaggcgcugc ccagaaacau ggugaucacc 1380 ugccagggca
gcgaggacau cagaaagcuc cuugaaucuc aaggccggag agauauuaag 1440
cugauagaua ucagcuuauc uaagguugac agcagaaagu ucgagaacgc uguaugggac
1500 caauucaagg accugugcca caugcauacg ggcauagugg uagagaagaa
gaagcguggc 1560 ggaaaggagg agaucacacc ucacugcgcc cugauggacu
gcaucauguu cgacgcggca 1620 gucuccggcg gcgucgacgc aaagguccuc
cgggccgugc ugccaaggga caugguguuc 1680 cggacaagca ccccuaaggu agugcug
1707 <210> SEQ ID NO 10 <211> LENGTH: 622 <212>
TYPE: PRT <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polypeptide
<400> SEQUENCE: 10
Met Glu Thr Pro Ala Gln Leu Leu Phe Leu Leu Leu Leu Trp Leu Pro 1 5
10 15 Asp Thr Thr Gly Met Pro Ala Glu Asn Lys Lys Val Arg Phe Glu
Asn 20 25 30 Thr Thr Ser Asp Lys Gly Lys Asn Pro Ser Lys Val Ile
Lys Ser Tyr 35 40 45 Tyr Gly Thr Met Asp Ile Lys Lys Ile Asn Glu
Gly Leu Leu Asp Ser 50 55 60 Lys Ile Leu Ser Ala Phe Asn Thr Val
Ile Ala Leu Leu Gly Ser Ile 65 70 75 80 Val Ile Ile Val Met Asn Ile
Met Ile Ile Gln Asn Tyr Thr Arg Ser 85 90 95 Thr Asp Asn Gln Ala
Val Ile Lys Asp Ala Leu Gln Gly Ile Gln Gln 100 105 110 Gln Ile Lys
Gly Leu Ala Asp Lys Ile Gly Thr Glu Ile Gly Pro Lys 115 120 125 Val
Ser Leu Ile Asp Thr Ser Ser Thr Ile Thr Ile Pro Ala Asn Ile 130 135
140 Gly Leu Leu Gly Ser Lys Ile Ser Gln Ser Thr Ala Ser Ile Asn Glu
145 150 155 160 Asn Val Asn Glu Lys Cys Lys Phe Thr Leu Pro Pro Leu
Lys Ile His 165 170 175 Glu Cys Asn Ile Ser Cys Pro Asn Pro Leu Pro
Phe Arg Glu Tyr Arg 180 185 190 Pro Gln Thr Glu Gly Val Ser Asn Leu
Val Gly Leu Pro Asp Asn Ile 195 200 205 Cys Leu Gln Lys Thr Ser Asn
Gln Ile Leu Lys Pro Lys Leu Ile Ser 210 215 220 Tyr Thr Leu Pro Val
Val Gly Gln Ser Gly Thr Cys Ile Thr Asp Pro 225 230 235 240 Leu Leu
Ala Met Asp Glu Gly Tyr Phe Ala Tyr Ser His Leu Glu Arg 245 250 255
Ile Gly Ser Cys Ser Arg Gly Val Ser Lys Gln Arg Ile Ile Gly Val 260
265 270 Gly Glu Val Leu Asp Arg Gly Asp Glu Val Pro Ser Leu Phe Met
Thr 275 280 285 Asn Val Trp Thr Pro Pro Asn Pro Asn Thr Val Tyr His
Cys Ser Ala 290 295 300 Val Tyr Asn Asn Glu Phe Tyr Tyr Val Leu Cys
Ala Val Ser Thr Val 305 310 315 320 Gly Asp Pro Ile Leu Asn Ser Thr
Tyr Trp Ser Gly Ser Leu Met Met 325 330 335 Thr Arg Leu Ala Val Lys
Pro Lys Ser Asn Gly Gly Gly Tyr Asn Gln 340 345 350 His Gln Leu Ala
Leu Arg Ser Ile Glu Lys Gly Arg Tyr Asp Lys Val 355 360 365 Met Pro
Tyr Gly Pro Ser Gly Ile Lys Gln Gly Asp Thr Leu Tyr Phe 370 375 380
Pro Ala Val Gly Phe Leu Val Arg Thr Glu Phe Lys Tyr Asn Asp Ser 385
390 395 400 Asn Cys Pro Ile Thr Lys Cys Gln Tyr Ser Lys Pro Glu Asn
Cys Arg 405 410 415 Leu Ser Met Gly Ile Arg Pro Asn Ser His Tyr Ile
Leu Arg Ser Gly 420 425 430 Leu Leu Lys Tyr Asn Leu Ser Asp Gly Glu
Asn Pro Lys Ile Val Phe 435 440 445 Ile Glu Ile Ser Asp Gln Arg Leu
Ser Ile Gly Ser Pro Ser Lys Val 450 455 460 Tyr Asp Ser Leu Gly Gln
Pro Val Phe Tyr Gln Ala Ser Phe Ser Trp 465 470 475 480 Asp Thr Met
Ile Lys Phe Gly Asp Val Gln Thr Val Asn Pro Leu Val 485 490 495 Val
Asn Trp Arg Asp Asn Thr Val Ile Ser Arg Pro Gly Gln Ser Gln 500 505
510 Cys Pro Arg Phe Asn Thr Cys Pro Glu Ile Cys Trp Glu Gly Val Tyr
515 520 525 Asn Asp Ala Phe Leu Ile Asp Arg Ile Asn Trp Ile Ser Ala
Gly Val 530 535 540 Phe Leu Asp Ser Asn Gln Thr Ala Glu Asn Pro Val
Phe Thr Val Phe 545 550 555 560 Lys Asp Asn Glu Ile Leu Tyr Arg Ala
Gln Leu Ala Ser Glu Asp Thr 565 570 575 Asn Ala Gln Lys Thr Ile Thr
Asn Cys Phe Leu Leu Lys Asn Lys Ile 580 585 590 Trp Cys Ile Ser Leu
Val Glu Ile Tyr Asp Thr Gly Asp Asn Val Ile 595 600 605 Arg Pro Lys
Leu Phe Ala Val Lys Ile Pro Glu Gln Cys Thr 610 615 620 <210>
SEQ ID NO 11 <211> LENGTH: 602 <212> TYPE: PRT
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 11 Met Pro Ala Glu Asn Lys Lys Val Arg Phe Glu Asn Thr
Thr Ser Asp 1 5 10 15 Lys Gly Lys Asn Pro Ser Lys Val Ile Lys Ser
Tyr Tyr Gly Thr Met 20 25 30 Asp Ile Lys Lys Ile Asn Glu Gly Leu
Leu Asp Ser Lys Ile Leu Ser 35 40 45 Ala Phe Asn Thr Val Ile Ala
Leu Leu Gly Ser Ile Val Ile Ile Val 50 55 60 Met Asn Ile Met Ile
Ile Gln Asn Tyr Thr Arg Ser Thr Asp Asn Gln 65 70 75 80 Ala Val Ile
Lys Asp Ala Leu Gln Gly Ile Gln Gln Gln Ile Lys Gly 85 90 95 Leu
Ala Asp Lys Ile Gly Thr Glu Ile Gly Pro Lys Val Ser Leu Ile 100 105
110 Asp Thr Ser Ser Thr Ile Thr Ile Pro Ala Asn Ile Gly Leu Leu Gly
115 120 125 Ser Lys Ile Ser Gln Ser Thr Ala Ser Ile Asn Glu Asn Val
Asn Glu 130 135 140 Lys Cys Lys Phe Thr Leu Pro Pro Leu Lys Ile His
Glu Cys Asn Ile 145 150 155 160 Ser Cys Pro Asn Pro Leu Pro Phe Arg
Glu Tyr Arg Pro Gln Thr Glu 165 170 175 Gly Val Ser Asn Leu Val Gly
Leu Pro Asp Asn Ile Cys Leu Gln Lys 180 185 190 Thr Ser Asn Gln Ile
Leu Lys Pro Lys Leu Ile Ser Tyr Thr Leu Pro 195 200 205 Val Val Gly
Gln Ser Gly Thr Cys Ile Thr Asp Pro Leu Leu Ala Met 210 215 220 Asp
Glu Gly Tyr Phe Ala Tyr Ser His Leu Glu Arg Ile Gly Ser Cys 225 230
235 240 Ser Arg Gly Val Ser Lys Gln Arg Ile Ile Gly Val Gly Glu Val
Leu 245 250 255 Asp Arg Gly Asp Glu Val Pro Ser Leu Phe Met Thr Asn
Val Trp Thr 260 265 270 Pro Pro Asn Pro Asn Thr Val Tyr His Cys Ser
Ala Val Tyr Asn Asn 275 280 285 Glu Phe Tyr Tyr Val Leu Cys Ala Val
Ser Thr Val Gly Asp Pro Ile 290 295 300 Leu Asn Ser Thr Tyr Trp Ser
Gly Ser Leu Met Met Thr Arg Leu Ala 305 310 315 320 Val Lys Pro Lys
Ser Asn Gly Gly Gly Tyr Asn Gln His Gln Leu Ala 325 330 335 Leu Arg
Ser Ile Glu Lys Gly Arg Tyr Asp Lys Val Met Pro Tyr Gly 340 345 350
Pro Ser Gly Ile Lys Gln Gly Asp Thr Leu Tyr Phe Pro Ala Val Gly 355
360 365 Phe Leu Val Arg Thr Glu Phe Lys Tyr Asn Asp Ser Asn Cys Pro
Ile 370 375 380 Thr Lys Cys Gln Tyr Ser Lys Pro Glu Asn Cys Arg Leu
Ser Met Gly 385 390 395 400 Ile Arg Pro Asn Ser His Tyr Ile Leu Arg
Ser Gly Leu Leu Lys Tyr 405 410 415 Asn Leu Ser Asp Gly Glu Asn Pro
Lys Ile Val Phe Ile Glu Ile Ser 420 425 430 Asp Gln Arg Leu Ser Ile
Gly Ser Pro Ser Lys Val Tyr Asp Ser Leu 435 440 445 Gly Gln Pro Val
Phe Tyr Gln Ala Ser Phe Ser Trp Asp Thr Met Ile 450 455 460 Lys Phe
Gly Asp Val Gln Thr Val Asn Pro Leu Val Val Asn Trp Arg 465 470 475
480 Asp Asn Thr Val Ile Ser Arg Pro Gly Gln Ser Gln Cys Pro Arg Phe
485 490 495 Asn Thr Cys Pro Glu Ile Cys Trp Glu Gly Val Tyr Asn Asp
Ala Phe 500 505 510 Leu Ile Asp Arg Ile Asn Trp Ile Ser Ala Gly Val
Phe Leu Asp Ser 515 520 525 Asn Gln Thr Ala Glu Asn Pro Val Phe Thr
Val Phe Lys Asp Asn Glu 530 535 540 Ile Leu Tyr Arg Ala Gln Leu Ala
Ser Glu Asp Thr Asn Ala Gln Lys 545 550 555 560 Thr Ile Thr Asn Cys
Phe Leu Leu Lys Asn Lys Ile Trp Cys Ile Ser 565 570 575 Leu Val Glu
Ile Tyr Asp Thr Gly Asp Asn Val Ile Arg Pro Lys Leu 580 585 590 Phe
Ala Val Lys Ile Pro Glu Gln Cys Thr 595 600 <210> SEQ ID NO
12 <211> LENGTH: 540 <212> TYPE: PRT <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polypeptide <400> SEQUENCE:
12
Met Glu Thr Pro Ala Gln Leu Leu Phe Leu Leu Leu Leu Trp Leu Pro 1 5
10 15 Asp Thr Thr Gly Ile Leu His Tyr Glu Lys Leu Ser Lys Ile Gly
Leu 20 25 30 Val Lys Gly Ile Thr Arg Lys Tyr Lys Ile Lys Ser Asn
Pro Leu Thr 35 40 45 Lys Asp Ile Val Ile Lys Met Ile Pro Asn Val
Ser Asn Met Ser Gln 50 55 60 Cys Thr Gly Ser Val Met Glu Asn Tyr
Lys Thr Arg Leu Asn Gly Ile 65 70 75 80 Leu Thr Pro Ile Lys Gly Ala
Leu Glu Ile Tyr Lys Asn Asn Thr His 85 90 95 Asp Leu Val Gly Asp
Val Arg Leu Ala Gly Val Ile Met Ala Gly Val 100 105 110 Ala Ile Gly
Ile Ala Thr Ala Ala Gln Ile Thr Ala Gly Val Ala Leu 115 120 125 Tyr
Glu Ala Met Lys Asn Ala Asp Asn Ile Asn Lys Leu Lys Ser Ser 130 135
140 Ile Glu Ser Thr Asn Glu Ala Val Val Lys Leu Gln Glu Thr Ala Glu
145 150 155 160 Lys Thr Val Tyr Val Leu Thr Ala Leu Gln Asp Tyr Ile
Asn Thr Asn 165 170 175 Leu Val Pro Thr Ile Asp Lys Ile Ser Cys Lys
Gln Thr Glu Leu Ser 180 185 190 Leu Asp Leu Ala Leu Ser Lys Tyr Leu
Ser Asp Leu Leu Phe Val Phe 195 200 205 Gly Pro Asn Leu Gln Asp Pro
Val Ser Asn Ser Met Thr Ile Gln Ala 210 215 220 Ile Ser Gln Ala Phe
Gly Gly Asn Tyr Glu Thr Leu Leu Arg Thr Leu 225 230 235 240 Gly Tyr
Ala Thr Glu Asp Phe Asp Asp Leu Leu Glu Ser Asp Ser Ile 245 250 255
Thr Gly Gln Ile Ile Tyr Val Asp Leu Ser Gly Tyr Tyr Ile Ile Val 260
265 270 Arg Val Tyr Phe Pro Ile Leu Thr Glu Ile Gln Gln Ala Tyr Ile
Gln 275 280 285 Glu Leu Leu Pro Val Ser Phe Asn Asn Asp Asn Ser Glu
Trp Ile Ser 290 295 300 Ile Val Pro Asn Phe Ile Leu Val Arg Asn Thr
Leu Ile Ser Asn Ile 305 310 315 320 Glu Ile Gly Phe Cys Leu Ile Thr
Lys Arg Ser Val Ile Cys Asn Gln 325 330 335 Asp Tyr Ala Thr Pro Met
Thr Asn Asn Met Arg Glu Cys Leu Thr Gly 340 345 350 Ser Thr Glu Lys
Cys Pro Arg Glu Leu Val Val Ser Ser His Val Pro 355 360 365 Arg Phe
Ala Leu Ser Asn Gly Val Leu Phe Ala Asn Cys Ile Ser Val 370 375 380
Thr Cys Gln Cys Gln Thr Thr Gly Arg Ala Ile Ser Gln Ser Gly Glu 385
390 395 400 Gln Thr Leu Leu Met Ile Asp Asn Thr Thr Cys Pro Thr Ala
Val Leu 405 410 415 Gly Asn Val Ile Ile Ser Leu Gly Lys Tyr Leu Gly
Ser Val Asn Tyr 420 425 430 Asn Ser Glu Gly Ile Ala Ile Gly Pro Pro
Val Phe Thr Asp Lys Val 435 440 445 Asp Ile Ser Ser Gln Ile Ser Ser
Met Asn Gln Ser Leu Gln Gln Ser 450 455 460 Lys Asp Tyr Ile Lys Glu
Ala Gln Arg Leu Leu Asp Thr Val Asn Pro 465 470 475 480 Ser Leu Ile
Ser Met Leu Ser Met Ile Ile Leu Tyr Val Leu Ser Ile 485 490 495 Ala
Ser Leu Cys Ile Gly Leu Ile Thr Phe Ile Ser Phe Ile Ile Val 500 505
510 Glu Lys Lys Arg Asn Thr Tyr Ser Arg Leu Glu Asp Arg Arg Val Arg
515 520 525 Pro Thr Ser Ser Gly Asp Leu Tyr Tyr Ile Gly Thr 530 535
540 <210> SEQ ID NO 13 <211> LENGTH: 520 <212>
TYPE: PRT <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polypeptide
<400> SEQUENCE: 13 Ile Leu His Tyr Glu Lys Leu Ser Lys Ile
Gly Leu Val Lys Gly Ile 1 5 10 15 Thr Arg Lys Tyr Lys Ile Lys Ser
Asn Pro Leu Thr Lys Asp Ile Val 20 25 30 Ile Lys Met Ile Pro Asn
Val Ser Asn Met Ser Gln Cys Thr Gly Ser 35 40 45 Val Met Glu Asn
Tyr Lys Thr Arg Leu Asn Gly Ile Leu Thr Pro Ile 50 55 60 Lys Gly
Ala Leu Glu Ile Tyr Lys Asn Asn Thr His Asp Leu Val Gly 65 70 75 80
Asp Val Arg Leu Ala Gly Val Ile Met Ala Gly Val Ala Ile Gly Ile 85
90 95 Ala Thr Ala Ala Gln Ile Thr Ala Gly Val Ala Leu Tyr Glu Ala
Met 100 105 110 Lys Asn Ala Asp Asn Ile Asn Lys Leu Lys Ser Ser Ile
Glu Ser Thr 115 120 125 Asn Glu Ala Val Val Lys Leu Gln Glu Thr Ala
Glu Lys Thr Val Tyr 130 135 140 Val Leu Thr Ala Leu Gln Asp Tyr Ile
Asn Thr Asn Leu Val Pro Thr 145 150 155 160 Ile Asp Lys Ile Ser Cys
Lys Gln Thr Glu Leu Ser Leu Asp Leu Ala 165 170 175 Leu Ser Lys Tyr
Leu Ser Asp Leu Leu Phe Val Phe Gly Pro Asn Leu 180 185 190 Gln Asp
Pro Val Ser Asn Ser Met Thr Ile Gln Ala Ile Ser Gln Ala 195 200 205
Phe Gly Gly Asn Tyr Glu Thr Leu Leu Arg Thr Leu Gly Tyr Ala Thr 210
215 220 Glu Asp Phe Asp Asp Leu Leu Glu Ser Asp Ser Ile Thr Gly Gln
Ile 225 230 235 240 Ile Tyr Val Asp Leu Ser Gly Tyr Tyr Ile Ile Val
Arg Val Tyr Phe 245 250 255 Pro Ile Leu Thr Glu Ile Gln Gln Ala Tyr
Ile Gln Glu Leu Leu Pro 260 265 270 Val Ser Phe Asn Asn Asp Asn Ser
Glu Trp Ile Ser Ile Val Pro Asn 275 280 285 Phe Ile Leu Val Arg Asn
Thr Leu Ile Ser Asn Ile Glu Ile Gly Phe 290 295 300 Cys Leu Ile Thr
Lys Arg Ser Val Ile Cys Asn Gln Asp Tyr Ala Thr 305 310 315 320 Pro
Met Thr Asn Asn Met Arg Glu Cys Leu Thr Gly Ser Thr Glu Lys 325 330
335 Cys Pro Arg Glu Leu Val Val Ser Ser His Val Pro Arg Phe Ala Leu
340 345 350 Ser Asn Gly Val Leu Phe Ala Asn Cys Ile Ser Val Thr Cys
Gln Cys 355 360 365 Gln Thr Thr Gly Arg Ala Ile Ser Gln Ser Gly Glu
Gln Thr Leu Leu 370 375 380 Met Ile Asp Asn Thr Thr Cys Pro Thr Ala
Val Leu Gly Asn Val Ile 385 390 395 400 Ile Ser Leu Gly Lys Tyr Leu
Gly Ser Val Asn Tyr Asn Ser Glu Gly 405 410 415 Ile Ala Ile Gly Pro
Pro Val Phe Thr Asp Lys Val Asp Ile Ser Ser 420 425 430 Gln Ile Ser
Ser Met Asn Gln Ser Leu Gln Gln Ser Lys Asp Tyr Ile 435 440 445 Lys
Glu Ala Gln Arg Leu Leu Asp Thr Val Asn Pro Ser Leu Ile Ser 450 455
460 Met Leu Ser Met Ile Ile Leu Tyr Val Leu Ser Ile Ala Ser Leu Cys
465 470 475 480 Ile Gly Leu Ile Thr Phe Ile Ser Phe Ile Ile Val Glu
Lys Lys Arg 485 490 495 Asn Thr Tyr Ser Arg Leu Glu Asp Arg Arg Val
Arg Pro Thr Ser Ser 500 505 510 Gly Asp Leu Tyr Tyr Ile Gly Thr 515
520 <210> SEQ ID NO 14 <211> LENGTH: 1866 <212>
TYPE: DNA <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polynucleotide
<400> SEQUENCE: 14 atggaaaccc ctgctcagct gctgttcctg
ctgctgctgt ggctgcctga tacaacaggc 60 atgcccgccg agaacaagaa
agttcgcttc gagaacacca ccagcgacaa gggcaagaac 120 cccagcaaag
tgatcaagag ctactacggc accatggaca tcaagaagat caacgagggc 180
ctgctggaca gcaagatcct gagcgccttc aacaccgtga ttgccctgct gggctctatc
240 gtgatcatcg tgatgaacat catgatcatc cagaactaca cccggtccac
cgacaaccag 300 gccgtgatta aggatgctct gcagggaatc cagcagcaga
tcaaaggcct ggccgacaag 360 atcggcacag agatcggccc taaggtgtcc
ctgatcgaca ccagcagcac catcacaatc 420 cccgccaata tcggactgct
gggatccaag atcagccaga gcaccgccag catcaacgag 480 aacgtgaacg
agaagtgcaa gttcaccctg cctccactga agatccacga gtgcaacatc 540
agctgcccca atcctctgcc attcagagag tacagacccc agacagaggg cgtgtccaat
600 ctcgtgggcc tgcctgacaa tatctgcctg cagaagacca gcaaccagat
cctgaagcct 660 aagctgatct cctacacact gcccgtcgtg ggccagagcg
gcacctgtat tacagatcct 720 ctgctggcca tggacgaggg ctactttgcc
tacagccacc tggaaagaat cggcagctgt 780 agccggggag tgtccaagca
gagaatcatc ggcgtgggcg aagtgctgga tagaggcgac 840 gaagtgccca
gcctgttcat gaccaatgtg tggacccctc ctaatcctaa caccgtgtac 900
cactgcagcg ccgtgtacaa caacgagttc tactacgtgc tgtgcgccgt gtccacagtg
960 ggcgacccta tcctgaacag cacctattgg agcggcagcc tgatgatgac
cagactggcc 1020 gtgaagccca agagcaatgg cggcggatac aaccagcatc
agctggccct gcggtccatc 1080 gagaagggca gatacgacaa agtgatgcct
tacggcccca gcggcatcaa gcaaggcgat 1140 accctgtact ttcccgccgt
gggatttctc gtgcggaccg agttcaagta caacgacagc 1200 aactgcccca
tcaccaagtg ccagtacagc aagcccgaga actgcagact gagcatgggc 1260
atcagaccca acagccacta catcctgaga agcggcctgc tgaagtacaa cctgagcgac
1320 ggcgagaacc ccaagatcgt gttcatcgag atcagcgacc agcggctgtc
tatcggcagc 1380 cctagcaagg tgtacgactc tctgggacag ccagtgttct
accaggcctc cttcagctgg 1440 gacaccatga tcaagttcgg cgacgtgcag
accgtgaatc ccctggtggt caactggcgg 1500 gacaataccg tgatcagcag
acctggccag tctcagtgcc ccagattcaa cacatgcccc 1560 gagatctgtt
gggaaggcgt gtacaatgac gccttcctga tcgatcggat caactggatc 1620
tctgccggcg tgttcctgga ctccaatcag acagccgaga atcctgtgtt caccgtgttc
1680 aaggacaatg agatcctgta tcgggcccag ctggcctccg aggatacaaa
tgcccagaag 1740 acaatcacca actgctttct gctcaagaac aagatctggt
gcatcagcct ggtggaaatc 1800 tacgacaccg gcgacaacgt gatcaggccc
aagctgttcg ccgtgaagat ccctgagcag 1860 tgcaca 1866 <210> SEQ
ID NO 15 <211> LENGTH: 1866 <212> TYPE: DNA <213>
ORGANISM: Artificial sequence <220> FEATURE: <223>
OTHER INFORMATION: Synthetic Polynucleotide <400> SEQUENCE:
15 atggaaaccc ctgctcagct gctgttcctg ctgctgctgt ggctgcctga
tacaacaggc 60 atgcccgccg agaacaagaa agttcgcttc gagaacacca
ccagcgacaa gggcaagaac 120 cccagcaaag tgatcaagag ctactacggc
accatggaca tcaagaagat caacgagggc 180 ctgctggaca gcaagatcct
gagcgccttc aacaccgtga ttgccctgct gggctctatc 240 gtgatcatcg
tgatgaacat catgatcatc cagaactaca cccggtccac cgacaaccag 300
gccgtgatta aggatgctct gcagggaatc cagcagcaga tcaaaggcct ggccgacaag
360 atcggcacag agatcggccc taaggtgtcc ctgatcgaca ccagcagcac
catcacaatc 420 cccgccaata tcggactgct gggatccaag atcagccaga
gcaccgccag catcaacgag 480 aacgtgaacg agaagtgcaa gttcaccctg
cctccactga agatccacga gtgcaacatc 540 agctgcccca atcctctgcc
attcagagag tacagacccc agacagaggg cgtgtccaat 600 ctcgtgggcc
tgcctgacaa tatctgcctg cagaagacca gcaaccagat cctgaagcct 660
aagctgatct cctacacact gcccgtcgtg ggccagagcg gcacctgtat tacagatcct
720 ctgctggcca tggacgaggg ctactttgcc tacagccacc tggaaagaat
cggcagctgt 780 agccggggag tgtccaagca gagaatcatc ggcgtgggcg
aagtgctgga tagaggcgac 840 gaagtgccca gcctgttcat gaccaatgtg
tggacccctc ctaatcctaa caccgtgtac 900 cactgcagcg ccgtgtacaa
caacgagttc tactacgtgc tgtgcgccgt gtccacagtg 960 ggcgacccta
tcctgaacag cacctattgg agcggcagcc tgatgatgac cagactggcc 1020
gtgaagccca agagcaatgg cggcggatac aaccagcatc agctggccct gcggtccatc
1080 gagaagggca gatacgacaa agtgatgcct tacggcccca gcggcatcaa
gcaaggcgat 1140 accctgtact ttcccgccgt gggatttctc gtgcggaccg
agttcaagta caacgacagc 1200 aactgcccca tcaccaagtg ccagtacagc
aagcccgaga actgcagact gagcatgggc 1260 atcagaccca acagccacta
catcctgaga agcggcctgc tgaagtacaa cctgagcgac 1320 ggcgagaacc
ccaagatcgt gttcatcgag atcagcgacc agcggctgtc tatcggcagc 1380
cctagcaagg tgtacgactc tctgggacag ccagtgttct accaggcctc cttcagctgg
1440 gacaccatga tcaagttcgg cgacgtgcag accgtgaatc ccctggtggt
caactggcgg 1500 gacaataccg tgatcagcag acctggccag tctcagtgcc
ccagattcaa cacatgcccc 1560 gagatctgtt gggaaggcgt gtacaatgac
gccttcctga tcgatcggat caactggatc 1620 tctgccggcg tgttcctgga
ctccaatcag acagccgaga atcctgtgtt caccgtgttc 1680 aaggacaatg
agatcctgta tcgggcccag ctggcctccg aggatacaaa tgcccagaag 1740
acaatcacca actgctttct gctcaagaac aagatctggt gcatcagcct ggtggaaatc
1800 tacgacaccg gcgacaacgt gatcaggccc aagctgttcg ccgtgaagat
ccctgagcag 1860 tgcaca 1866 <210> SEQ ID NO 16 <211>
LENGTH: 1866 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 16 auggaaaccc
cugcucagcu gcuguuccug cugcugcugu ggcugccuga uacaacaggc 60
augcccgccg agaacaagaa aguucgcuuc gagaacacca ccagcgacaa gggcaagaac
120 cccagcaaag ugaucaagag cuacuacggc accauggaca ucaagaagau
caacgagggc 180 cugcuggaca gcaagauccu gagcgccuuc aacaccguga
uugcccugcu gggcucuauc 240 gugaucaucg ugaugaacau caugaucauc
cagaacuaca cccgguccac cgacaaccag 300 gccgugauua aggaugcucu
gcagggaauc cagcagcaga ucaaaggccu ggccgacaag 360 aucggcacag
agaucggccc uaaggugucc cugaucgaca ccagcagcac caucacaauc 420
cccgccaaua ucggacugcu gggauccaag aucagccaga gcaccgccag caucaacgag
480 aacgugaacg agaagugcaa guucacccug ccuccacuga agauccacga
gugcaacauc 540 agcugcccca auccucugcc auucagagag uacagacccc
agacagaggg cguguccaau 600 cucgugggcc ugccugacaa uaucugccug
cagaagacca gcaaccagau ccugaagccu 660 aagcugaucu ccuacacacu
gcccgucgug ggccagagcg gcaccuguau uacagauccu 720 cugcuggcca
uggacgaggg cuacuuugcc uacagccacc uggaaagaau cggcagcugu 780
agccggggag uguccaagca gagaaucauc ggcgugggcg aagugcugga uagaggcgac
840 gaagugccca gccuguucau gaccaaugug uggaccccuc cuaauccuaa
caccguguac 900 cacugcagcg ccguguacaa caacgaguuc uacuacgugc
ugugcgccgu guccacagug 960 ggcgacccua uccugaacag caccuauugg
agcggcagcc ugaugaugac cagacuggcc 1020 gugaagccca agagcaaugg
cggcggauac aaccagcauc agcuggcccu gcgguccauc 1080 gagaagggca
gauacgacaa agugaugccu uacggcccca gcggcaucaa gcaaggcgau 1140
acccuguacu uucccgccgu gggauuucuc gugcggaccg aguucaagua caacgacagc
1200 aacugcccca ucaccaagug ccaguacagc aagcccgaga acugcagacu
gagcaugggc 1260 aucagaccca acagccacua cauccugaga agcggccugc
ugaaguacaa ccugagcgac 1320 ggcgagaacc ccaagaucgu guucaucgag
aucagcgacc agcggcuguc uaucggcagc 1380 ccuagcaagg uguacgacuc
ucugggacag ccaguguucu accaggccuc cuucagcugg 1440 gacaccauga
ucaaguucgg cgacgugcag accgugaauc cccugguggu caacuggcgg 1500
gacaauaccg ugaucagcag accuggccag ucucagugcc ccagauucaa cacaugcccc
1560 gagaucuguu gggaaggcgu guacaaugac gccuuccuga ucgaucggau
caacuggauc 1620 ucugccggcg uguuccugga cuccaaucag acagccgaga
auccuguguu caccguguuc 1680 aaggacaaug agauccugua ucgggcccag
cuggccuccg aggauacaaa ugcccagaag 1740 acaaucacca acugcuuucu
gcucaagaac aagaucuggu gcaucagccu gguggaaauc 1800 uacgacaccg
gcgacaacgu gaucaggccc aagcuguucg ccgugaagau cccugagcag 1860 ugcaca
1866 <210> SEQ ID NO 17 <211> LENGTH: 1866 <212>
TYPE: RNA <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polynucleotide
<400> SEQUENCE: 17 auggaaaccc cugcucagcu gcuguuccug
cugcugcugu ggcugccuga uacaacaggc 60 augcccgccg agaacaagaa
aguucgcuuc gagaacacca ccagcgacaa gggcaagaac 120 cccagcaaag
ugaucaagag cuacuacggc accauggaca ucaagaagau caacgagggc 180
cugcuggaca gcaagauccu gagcgccuuc aacaccguga uugcccugcu gggcucuauc
240 gugaucaucg ugaugaacau caugaucauc cagaacuaca cccgguccac
cgacaaccag 300 gccgugauua aggaugcucu gcagggaauc cagcagcaga
ucaaaggccu ggccgacaag 360 aucggcacag agaucggccc uaaggugucc
cugaucgaca ccagcagcac caucacaauc 420 cccgccaaua ucggacugcu
gggauccaag aucagccaga gcaccgccag caucaacgag 480 aacgugaacg
agaagugcaa guucacccug ccuccacuga agauccacga gugcaacauc 540
agcugcccca auccucugcc auucagagag uacagacccc agacagaggg cguguccaau
600 cucgugggcc ugccugacaa uaucugccug cagaagacca gcaaccagau
ccugaagccu 660 aagcugaucu ccuacacacu gcccgucgug ggccagagcg
gcaccuguau uacagauccu 720 cugcuggcca uggacgaggg cuacuuugcc
uacagccacc uggaaagaau cggcagcugu 780 agccggggag uguccaagca
gagaaucauc ggcgugggcg aagugcugga uagaggcgac 840 gaagugccca
gccuguucau gaccaaugug uggaccccuc cuaauccuaa caccguguac 900
cacugcagcg ccguguacaa caacgaguuc uacuacgugc ugugcgccgu guccacagug
960 ggcgacccua uccugaacag caccuauugg agcggcagcc ugaugaugac
cagacuggcc 1020 gugaagccca agagcaaugg cggcggauac aaccagcauc
agcuggcccu gcgguccauc 1080 gagaagggca gauacgacaa agugaugccu
uacggcccca gcggcaucaa gcaaggcgau 1140 acccuguacu uucccgccgu
gggauuucuc gugcggaccg aguucaagua caacgacagc 1200 aacugcccca
ucaccaagug ccaguacagc aagcccgaga acugcagacu gagcaugggc 1260
aucagaccca acagccacua cauccugaga agcggccugc ugaaguacaa ccugagcgac
1320 ggcgagaacc ccaagaucgu guucaucgag aucagcgacc agcggcuguc
uaucggcagc 1380 ccuagcaagg uguacgacuc ucugggacag ccaguguucu
accaggccuc cuucagcugg 1440 gacaccauga ucaaguucgg cgacgugcag
accgugaauc cccugguggu caacuggcgg 1500 gacaauaccg ugaucagcag
accuggccag ucucagugcc ccagauucaa cacaugcccc 1560 gagaucuguu
gggaaggcgu guacaaugac gccuuccuga ucgaucggau caacuggauc 1620
ucugccggcg uguuccugga cuccaaucag acagccgaga auccuguguu caccguguuc
1680 aaggacaaug agauccugua ucgggcccag cuggccuccg aggauacaaa
ugcccagaag 1740
acaaucacca acugcuuucu gcucaagaac aagaucuggu gcaucagccu gguggaaauc
1800 uacgacaccg gcgacaacgu gaucaggccc aagcuguucg ccgugaagau
cccugagcag 1860 ugcaca 1866 <210> SEQ ID NO 18 <211>
LENGTH: 1255 <212> TYPE: PRT <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polypeptide <400> SEQUENCE: 18 Met Phe Ile Phe Leu
Phe Phe Leu Thr Leu Thr Ser Gly Ser Asp Leu 1 5 10 15 Glu Ser Cys
Thr Thr Phe Asp Asp Val Gln Ala Pro Asn Tyr Pro Gln 20 25 30 His
Ser Ser Ser Arg Arg Gly Val Tyr Tyr Pro Asp Glu Ile Phe Arg 35 40
45 Ser Asp Thr Leu Tyr Leu Thr Gln Asp Leu Phe Leu Pro Phe Tyr Ser
50 55 60 Asn Val Thr Gly Phe His Thr Ile Asn His Arg Phe Asp Asn
Pro Val 65 70 75 80 Ile Pro Phe Lys Asp Gly Val Tyr Phe Ala Ala Thr
Glu Lys Ser Asn 85 90 95 Val Val Arg Gly Trp Val Phe Gly Ser Thr
Met Asn Asn Lys Ser Gln 100 105 110 Ser Val Ile Ile Ile Asn Asn Ser
Thr Asn Val Val Ile Arg Ala Cys 115 120 125 Asn Phe Glu Leu Cys Asp
Asn Pro Phe Phe Ala Val Ser Lys Pro Thr 130 135 140 Gly Thr Gln Thr
His Thr Met Ile Phe Asp Asn Ala Phe Asn Cys Thr 145 150 155 160 Phe
Glu Tyr Ile Ser Asp Ser Phe Ser Leu Asp Val Ala Glu Lys Ser 165 170
175 Gly Asn Phe Lys His Leu Arg Glu Phe Val Phe Lys Asn Lys Asp Gly
180 185 190 Phe Leu Tyr Val Tyr Lys Gly Tyr Gln Pro Ile Asp Val Val
Arg Asp 195 200 205 Leu Pro Ser Gly Phe Asn Ile Leu Lys Pro Ile Phe
Lys Leu Pro Leu 210 215 220 Gly Ile Asn Ile Thr Asn Phe Arg Ala Ile
Leu Thr Ala Phe Leu Pro 225 230 235 240 Ala Gln Asp Thr Trp Gly Thr
Ser Ala Ala Ala Tyr Phe Val Gly Tyr 245 250 255 Leu Lys Pro Ala Thr
Phe Met Leu Lys Tyr Asp Glu Asn Gly Thr Ile 260 265 270 Thr Asp Ala
Val Asp Cys Ser Gln Asn Pro Leu Ala Glu Leu Lys Cys 275 280 285 Ser
Val Lys Ser Phe Glu Ile Asp Lys Gly Ile Tyr Gln Thr Ser Asn 290 295
300 Phe Arg Val Ala Pro Ser Lys Glu Val Val Arg Phe Pro Asn Ile Thr
305 310 315 320 Asn Leu Cys Pro Phe Gly Glu Val Phe Asn Ala Thr Thr
Phe Pro Ser 325 330 335 Val Tyr Ala Trp Glu Arg Lys Arg Ile Ser Asn
Cys Val Ala Asp Tyr 340 345 350 Ser Val Leu Tyr Asn Ser Thr Ser Phe
Ser Thr Phe Lys Cys Tyr Gly 355 360 365 Val Ser Ala Thr Lys Leu Asn
Asp Leu Cys Phe Ser Asn Val Tyr Ala 370 375 380 Asp Ser Phe Val Val
Lys Gly Asp Asp Val Arg Gln Ile Ala Pro Gly 385 390 395 400 Gln Thr
Gly Val Ile Ala Asp Tyr Asn Tyr Lys Leu Pro Asp Asp Phe 405 410 415
Thr Gly Cys Val Leu Ala Trp Asn Thr Arg Asn Ile Asp Ala Thr Gln 420
425 430 Thr Gly Asn Tyr Asn Tyr Lys Tyr Arg Ser Leu Arg His Gly Lys
Leu 435 440 445 Arg Pro Phe Glu Arg Asp Ile Ser Asn Val Pro Phe Ser
Pro Asp Gly 450 455 460 Lys Pro Cys Thr Pro Pro Ala Phe Asn Cys Tyr
Trp Pro Leu Asn Asp 465 470 475 480 Tyr Gly Phe Tyr Ile Thr Asn Gly
Ile Gly Tyr Gln Pro Tyr Arg Val 485 490 495 Val Val Leu Ser Phe Glu
Leu Leu Asn Ala Pro Ala Thr Val Cys Gly 500 505 510 Pro Lys Leu Ser
Thr Asp Leu Ile Lys Asn Gln Cys Val Asn Phe Asn 515 520 525 Phe Asn
Gly Leu Thr Gly Thr Gly Val Leu Thr Pro Ser Ser Lys Arg 530 535 540
Phe Gln Pro Phe Gln Gln Phe Gly Arg Asp Val Leu Asp Phe Thr Asp 545
550 555 560 Ser Val Arg Asp Pro Lys Thr Ser Glu Ile Leu Asp Ile Ser
Pro Cys 565 570 575 Ser Phe Gly Gly Val Ser Val Ile Thr Pro Gly Thr
Asn Thr Ser Ser 580 585 590 Glu Val Ala Val Leu Tyr Gln Asp Val Asn
Cys Thr Asp Val Pro Val 595 600 605 Ala Ile His Ala Asp Gln Leu Thr
Pro Ser Trp Arg Val Tyr Ser Thr 610 615 620 Gly Asn Asn Val Phe Gln
Thr Gln Ala Gly Cys Leu Ile Gly Ala Glu 625 630 635 640 His Val Asp
Thr Ser Tyr Glu Cys Asp Ile Pro Ile Gly Ala Gly Ile 645 650 655 Cys
Ala Ser Tyr His Thr Val Ser Ser Leu Arg Ser Thr Ser Gln Lys 660 665
670 Ser Ile Val Ala Tyr Thr Met Ser Leu Gly Ala Asp Ser Ser Ile Ala
675 680 685 Tyr Ser Asn Asn Thr Ile Ala Ile Pro Thr Asn Phe Ser Ile
Ser Ile 690 695 700 Thr Thr Glu Val Met Pro Val Ser Met Ala Lys Thr
Ser Val Asp Cys 705 710 715 720 Asn Met Tyr Ile Cys Gly Asp Ser Thr
Glu Cys Ala Asn Leu Leu Leu 725 730 735 Gln Tyr Gly Ser Phe Cys Thr
Gln Leu Asn Arg Ala Leu Ser Gly Ile 740 745 750 Ala Val Glu Gln Asp
Arg Asn Thr Arg Glu Val Phe Ala Gln Val Lys 755 760 765 Gln Met Tyr
Lys Thr Pro Thr Leu Lys Asp Phe Gly Gly Phe Asn Phe 770 775 780 Ser
Gln Ile Leu Pro Asp Pro Leu Lys Pro Thr Lys Arg Ser Phe Ile 785 790
795 800 Glu Asp Leu Leu Phe Asn Lys Val Thr Leu Ala Asp Ala Gly Phe
Met 805 810 815 Lys Gln Tyr Gly Glu Cys Leu Gly Asp Ile Asn Ala Arg
Asp Leu Ile 820 825 830 Cys Ala Gln Lys Phe Asn Gly Leu Thr Val Leu
Pro Pro Leu Leu Thr 835 840 845 Asp Asp Met Ile Ala Ala Tyr Thr Ala
Ala Leu Val Ser Gly Thr Ala 850 855 860 Thr Ala Gly Trp Thr Phe Gly
Ala Gly Ala Ala Leu Gln Ile Pro Phe 865 870 875 880 Ala Met Gln Met
Ala Tyr Arg Phe Asn Gly Ile Gly Val Thr Gln Asn 885 890 895 Val Leu
Tyr Glu Asn Gln Lys Gln Ile Ala Asn Gln Phe Asn Lys Ala 900 905 910
Ile Ser Gln Ile Gln Glu Ser Leu Thr Thr Thr Ser Thr Ala Leu Gly 915
920 925 Lys Leu Gln Asp Val Val Asn Gln Asn Ala Gln Ala Leu Asn Thr
Leu 930 935 940 Val Lys Gln Leu Ser Ser Asn Phe Gly Ala Ile Ser Ser
Val Leu Asn 945 950 955 960 Asp Ile Leu Ser Arg Leu Asp Lys Val Glu
Ala Glu Val Gln Ile Asp 965 970 975 Arg Leu Ile Thr Gly Arg Leu Gln
Ser Leu Gln Thr Tyr Val Thr Gln 980 985 990 Gln Leu Ile Arg Ala Ala
Glu Ile Arg Ala Ser Ala Asn Leu Ala Ala 995 1000 1005 Thr Lys Met
Ser Glu Cys Val Leu Gly Gln Ser Lys Arg Val Asp 1010 1015 1020 Phe
Cys Gly Lys Gly Tyr His Leu Met Ser Phe Pro Gln Ala Ala 1025 1030
1035 Pro His Gly Val Val Phe Leu His Val Thr Tyr Val Pro Ser Gln
1040 1045 1050 Glu Arg Asn Phe Thr Thr Ala Pro Ala Ile Cys His Glu
Gly Lys 1055 1060 1065 Ala Tyr Phe Pro Arg Glu Gly Val Phe Val Phe
Asn Gly Thr Ser 1070 1075 1080 Trp Phe Ile Thr Gln Arg Asn Phe Phe
Ser Pro Gln Ile Ile Thr 1085 1090 1095 Thr Asp Asn Thr Phe Val Ser
Gly Ser Cys Asp Val Val Ile Gly 1100 1105 1110 Ile Ile Asn Asn Thr
Val Tyr Asp Pro Leu Gln Pro Glu Leu Asp 1115 1120 1125 Ser Phe Lys
Glu Glu Leu Asp Lys Tyr Phe Lys Asn His Thr Ser 1130 1135 1140 Pro
Asp Val Asp Leu Gly Asp Ile Ser Gly Ile Asn Ala Ser Val 1145 1150
1155 Val Asn Ile Gln Lys Glu Ile Asp Arg Leu Asn Glu Val Ala Lys
1160 1165 1170 Asn Leu Asn Glu Ser Leu Ile Asp Leu Gln Glu Leu Gly
Lys Tyr 1175 1180 1185 Glu Gln Tyr Ile Lys Trp Pro Trp Tyr Val Trp
Leu Gly Phe Ile 1190 1195 1200 Ala Gly Leu Ile Ala Ile Val Met Val
Thr Ile Leu Leu Cys Cys 1205 1210 1215 Met Thr Ser Cys Cys Ser Cys
Leu Lys Gly Ala Cys Ser Cys Gly 1220 1225 1230 Ser Cys Cys Lys Phe
Asp Glu Asp Asp Ser Glu Pro Val Leu Lys
1235 1240 1245 Gly Val Lys Leu His Tyr Thr 1250 1255 <210>
SEQ ID NO 19 <211> LENGTH: 3765 <212> TYPE: DNA
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 19 atgtttatct tcctgttctt cctgaccctg accagcggca gcgacctgga
aagctgcacc 60 accttcgacg acgtgcaggc ccccaactac cctcagcaca
gctctagcag acggggcgtg 120 tactaccccg acgagatctt cagaagcgac
accctgtacc tgacccagga cctgttcctg 180 cccttctaca gcaacgtgac
cggcttccac accatcaacc acagattcga caaccccgtg 240 atccccttca
aggacggggt gtactttgcc gccaccgaga agtccaatgt cgtgcgggga 300
tgggtgttcg gcagcaccat gaacaacaag agccagagcg tgatcatcat caacaacagc
360 accaacgtcg tgatccgggc ctgcaacttc gagctgtgcg acaacccatt
cttcgccgtg 420 tccaagccca ccggcaccca gacccacacc atgatcttcg
acaacgcctt caactgcacc 480 ttcgagtaca tcagcgacag cttcagcctg
gacgtggccg agaaaagcgg caacttcaag 540 cacctgagag aattcgtgtt
caagaacaag gacggcttcc tgtacgtgta caagggctac 600 cagcccatcg
acgtcgtgcg cgatctgccc agcggcttca acatcctgaa gcccatcttc 660
aagctgcccc tgggcatcaa catcaccaac ttccgggcta tcctgaccgc cttcctgccc
720 gcccaggata cctggggaac aagcgccgct gcctacttcg tgggctacct
gaagcctgcc 780 accttcatgc tgaagtacga cgagaacggc accatcaccg
acgccgtgga ctgcagccag 840 aatcctctgg ccgagctgaa gtgcagcgtg
aagtccttcg agatcgacaa gggcatctac 900 cagaccagca acttcagagt
ggcccccagc aaagaagtcg tgcggttccc caatatcacc 960 aacctgtgcc
ccttcggcga ggtgttcaac gccaccacct ttcccagcgt gtacgcctgg 1020
gagcggaagc ggatcagcaa ctgcgtggcc gactacagcg tgctgtacaa ctccaccagc
1080 ttctccacct tcaagtgcta cggcgtgtcc gccaccaagc tgaacgacct
gtgcttcagc 1140 aatgtgtacg ccgactcctt cgtcgtgaag ggcgacgatg
tgcgccagat cgcccctgga 1200 cagacaggcg tgatcgccga ttacaactac
aagctgcctg acgacttcac cggctgcgtg 1260 ctggcctgga acaccagaaa
catcgacgcc acccagacag gcaactacaa ttacaagtac 1320 agaagcctgc
ggcacggcaa gctgcggccc ttcgagaggg acatctccaa cgtgcccttc 1380
agccccgacg gcaagccttg taccccccct gcctttaact gctactggcc cctgaacgac
1440 tacggcttct acatcacaaa cggcatcggc tatcagccct accgggtggt
ggtgctgtcc 1500 tttgagctgc tgaatgcccc tgccaccgtg tgcggcccta
agctgagcac cgacctgatc 1560 aagaaccagt gcgtgaactt caacttcaac
ggcctgaccg gcaccggcgt gctgacacct 1620 agcagcaaga gattccagcc
cttccagcag ttcggccggg acgtgctgga tttcaccgac 1680 agcgtgcggg
accccaagac cagcgagatc ctggacatca gcccctgcag cttcggcgga 1740
gtgtccgtga tcacccccgg caccaatacc agctctgagg tggccgtgct gtatcaggac
1800 gtgaactgca ccgatgtgcc cgtggccatc cacgccgatc agctgacccc
atcttggcgg 1860 gtgtactcca ccggcaacaa cgtgttccag acacaagccg
gctgcctgat cggagccgag 1920 cacgtggaca ccagctacga gtgcgacatc
cctatcggcg ctggcatctg cgccagctac 1980 cacaccgtgt ccagcctgag
aagcaccagc cagaaatcta tcgtggccta caccatgagc 2040 ctgggcgccg
acagctctat cgcctactcc aacaacacaa tcgccatccc caccaatttc 2100
agcatctcca tcaccaccga agtgatgccc gtgtccatgg ccaagacctc cgtggattgc
2160 aacatgtaca tctgcggcga cagcaccgag tgcgccaacc tgctgctgca
gtacggcagc 2220 ttctgcaccc agctgaacag agccctgagc ggaatcgccg
tggaacagga cagaaacacc 2280 cgggaagtgt tcgcccaagt gaagcagatg
tataagaccc ccaccctgaa ggatttcggc 2340 ggctttaact tcagccagat
cctgcccgac cctctgaagc ctaccaagcg gagcttcatc 2400 gaggacctgc
tgttcaacaa agtgaccctg gccgacgccg gctttatgaa gcagtatggc 2460
gagtgcctgg gcgacatcaa cgcccgggat ctgatctgcg cccagaagtt taacggactg
2520 accgtgctgc cccctctgct gaccgacgat atgatcgccg cctacacagc
cgccctggtg 2580 tctggcacag ctaccgccgg atggacattt ggagctggcg
ccgctctgca gatccccttt 2640 gccatgcaga tggcctaccg gttcaatggc
atcggcgtga cccagaatgt gctgtacgag 2700 aaccagaagc agatcgccaa
ccagttcaac aaggccatta gccagattca ggaaagcctg 2760 accaccacca
gcaccgccct gggcaaactg caggacgtcg tgaaccagaa cgcccaggcc 2820
ctgaacaccc tcgtgaagca gctgagcagc aatttcggcg ccatcagctc cgtgctgaac
2880 gatatcctga gcagactgga caaggtggaa gcagaggtgc agatcgaccg
gctgatcacc 2940 ggcagactgc agagcctgca gacctacgtg acacagcagc
tgattagagc cgccgagatc 3000 agggccagcg ccaatctggc cgccacaaag
atgagcgagt gtgtgctggg ccagagcaag 3060 cgggtggact tctgcggcaa
gggctatcac ctgatgagct tcccccaggc cgctcctcac 3120 ggcgtggtgt
ttctgcacgt gacatacgtg cccagccagg aacggaactt caccaccgcc 3180
ccagccatct gccacgaggg caaggcctac ttcccccggg aaggcgtgtt cgtgtttaac
3240 ggcacctcct ggtttatcac ccagcggaat ttcttcagtc cgcagatcat
caccacagac 3300 aacaccttcg tgtccggcag ctgcgacgtc gtgattggca
tcattaacaa caccgtgtac 3360 gaccccctgc agcccgagct ggacagcttc
aaagaggaac tggacaagta cttcaagaac 3420 cacacctccc ccgacgtgga
cctgggcgat atctccggca tcaatgccag cgtcgtgaat 3480 atccagaaag
agatcgatcg cctgaacgag gtggccaaga acctgaatga gagcctgatc 3540
gacctgcagg aactggggaa gtacgagcag tacatcaagt ggccttggta cgtgtggctg
3600 ggctttatcg ccggcctgat cgccatcgtg atggtcacca tcctgctgtg
ctgcatgacc 3660 agctgttgca gctgtctgaa gggcgcctgc agctgtggct
cctgctgcaa gttcgatgag 3720 gacgacagcg agcctgtgct gaaaggcgtg
aagctgcact acacc 3765 <210> SEQ ID NO 20 <211> LENGTH:
3765 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 20 auguuuaucu
uccuguucuu ccugacccug accagcggca gcgaccugga aagcugcacc 60
accuucgacg acgugcaggc ccccaacuac ccucagcaca gcucuagcag acggggcgug
120 uacuaccccg acgagaucuu cagaagcgac acccuguacc ugacccagga
ccuguuccug 180 cccuucuaca gcaacgugac cggcuuccac accaucaacc
acagauucga caaccccgug 240 auccccuuca aggacggggu guacuuugcc
gccaccgaga aguccaaugu cgugcgggga 300 uggguguucg gcagcaccau
gaacaacaag agccagagcg ugaucaucau caacaacagc 360 accaacgucg
ugauccgggc cugcaacuuc gagcugugcg acaacccauu cuucgccgug 420
uccaagccca ccggcaccca gacccacacc augaucuucg acaacgccuu caacugcacc
480 uucgaguaca ucagcgacag cuucagccug gacguggccg agaaaagcgg
caacuucaag 540 caccugagag aauucguguu caagaacaag gacggcuucc
uguacgugua caagggcuac 600 cagcccaucg acgucgugcg cgaucugccc
agcggcuuca acauccugaa gcccaucuuc 660 aagcugcccc ugggcaucaa
caucaccaac uuccgggcua uccugaccgc cuuccugccc 720 gcccaggaua
ccuggggaac aagcgccgcu gccuacuucg ugggcuaccu gaagccugcc 780
accuucaugc ugaaguacga cgagaacggc accaucaccg acgccgugga cugcagccag
840 aauccucugg ccgagcugaa gugcagcgug aaguccuucg agaucgacaa
gggcaucuac 900 cagaccagca acuucagagu ggcccccagc aaagaagucg
ugcgguuccc caauaucacc 960 aaccugugcc ccuucggcga gguguucaac
gccaccaccu uucccagcgu guacgccugg 1020 gagcggaagc ggaucagcaa
cugcguggcc gacuacagcg ugcuguacaa cuccaccagc 1080 uucuccaccu
ucaagugcua cggcgugucc gccaccaagc ugaacgaccu gugcuucagc 1140
aauguguacg ccgacuccuu cgucgugaag ggcgacgaug ugcgccagau cgccccugga
1200 cagacaggcg ugaucgccga uuacaacuac aagcugccug acgacuucac
cggcugcgug 1260 cuggccugga acaccagaaa caucgacgcc acccagacag
gcaacuacaa uuacaaguac 1320 agaagccugc ggcacggcaa gcugcggccc
uucgagaggg acaucuccaa cgugcccuuc 1380 agccccgacg gcaagccuug
uacccccccu gccuuuaacu gcuacuggcc ccugaacgac 1440 uacggcuucu
acaucacaaa cggcaucggc uaucagcccu accggguggu ggugcugucc 1500
uuugagcugc ugaaugcccc ugccaccgug ugcggcccua agcugagcac cgaccugauc
1560 aagaaccagu gcgugaacuu caacuucaac ggccugaccg gcaccggcgu
gcugacaccu 1620 agcagcaaga gauuccagcc cuuccagcag uucggccggg
acgugcugga uuucaccgac 1680 agcgugcggg accccaagac cagcgagauc
cuggacauca gccccugcag cuucggcgga 1740 guguccguga ucacccccgg
caccaauacc agcucugagg uggccgugcu guaucaggac 1800 gugaacugca
ccgaugugcc cguggccauc cacgccgauc agcugacccc aucuuggcgg 1860
guguacucca ccggcaacaa cguguuccag acacaagccg gcugccugau cggagccgag
1920 cacguggaca ccagcuacga gugcgacauc ccuaucggcg cuggcaucug
cgccagcuac 1980 cacaccgugu ccagccugag aagcaccagc cagaaaucua
ucguggccua caccaugagc 2040 cugggcgccg acagcucuau cgccuacucc
aacaacacaa ucgccauccc caccaauuuc 2100 agcaucucca ucaccaccga
agugaugccc guguccaugg ccaagaccuc cguggauugc 2160 aacauguaca
ucugcggcga cagcaccgag ugcgccaacc ugcugcugca guacggcagc 2220
uucugcaccc agcugaacag agcccugagc ggaaucgccg uggaacagga cagaaacacc
2280 cgggaagugu ucgcccaagu gaagcagaug uauaagaccc ccacccugaa
ggauuucggc 2340 ggcuuuaacu ucagccagau ccugcccgac ccucugaagc
cuaccaagcg gagcuucauc 2400 gaggaccugc uguucaacaa agugacccug
gccgacgccg gcuuuaugaa gcaguauggc 2460 gagugccugg gcgacaucaa
cgcccgggau cugaucugcg cccagaaguu uaacggacug 2520 accgugcugc
ccccucugcu gaccgacgau augaucgccg ccuacacagc cgcccuggug 2580
ucuggcacag cuaccgccgg auggacauuu ggagcuggcg ccgcucugca gauccccuuu
2640 gccaugcaga uggccuaccg guucaauggc aucggcguga cccagaaugu
gcuguacgag 2700 aaccagaagc agaucgccaa ccaguucaac aaggccauua
gccagauuca ggaaagccug 2760 accaccacca gcaccgcccu gggcaaacug
caggacgucg ugaaccagaa cgcccaggcc 2820 cugaacaccc ucgugaagca
gcugagcagc aauuucggcg ccaucagcuc cgugcugaac 2880 gauauccuga
gcagacugga caagguggaa gcagaggugc agaucgaccg gcugaucacc 2940
ggcagacugc agagccugca gaccuacgug acacagcagc ugauuagagc cgccgagauc
3000
agggccagcg ccaaucuggc cgccacaaag augagcgagu gugugcuggg ccagagcaag
3060 cggguggacu ucugcggcaa gggcuaucac cugaugagcu ucccccaggc
cgcuccucac 3120 ggcguggugu uucugcacgu gacauacgug cccagccagg
aacggaacuu caccaccgcc 3180 ccagccaucu gccacgaggg caaggccuac
uucccccggg aaggcguguu cguguuuaac 3240 ggcaccuccu gguuuaucac
ccagcggaau uucuucaguc cgcagaucau caccacagac 3300 aacaccuucg
uguccggcag cugcgacguc gugauuggca ucauuaacaa caccguguac 3360
gacccccugc agcccgagcu ggacagcuuc aaagaggaac uggacaagua cuucaagaac
3420 cacaccuccc ccgacgugga ccugggcgau aucuccggca ucaaugccag
cgucgugaau 3480 auccagaaag agaucgaucg ccugaacgag guggccaaga
accugaauga gagccugauc 3540 gaccugcagg aacuggggaa guacgagcag
uacaucaagu ggccuuggua cguguggcug 3600 ggcuuuaucg ccggccugau
cgccaucgug auggucacca uccugcugug cugcaugacc 3660 agcuguugca
gcugucugaa gggcgccugc agcuguggcu ccugcugcaa guucgaugag 3720
gacgacagcg agccugugcu gaaaggcgug aagcugcacu acacc 3765 <210>
SEQ ID NO 21 <211> LENGTH: 30 <212> TYPE: PRT
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 21 Met Asp Ser Lys Gly Ser Ser Gln Lys Gly Ser Arg Leu
Leu Leu Leu 1 5 10 15 Leu Val Val Ser Asn Leu Leu Leu Pro Gln Gly
Val Val Gly 20 25 30 <210> SEQ ID NO 22 <211> LENGTH:
18 <212> TYPE: PRT <213> ORGANISM: Artificial sequence
<220> FEATURE: <223> OTHER INFORMATION: Synthetic
Polypeptide <400> SEQUENCE: 22 Met Asp Trp Thr Trp Ile Leu
Phe Leu Val Ala Ala Ala Thr Arg Val 1 5 10 15 His Ser <210>
SEQ ID NO 23 <211> LENGTH: 20 <212> TYPE: PRT
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 23 Met Glu Thr Pro Ala Gln Leu Leu Phe Leu Leu Leu Leu
Trp Leu Pro 1 5 10 15 Asp Thr Thr Gly 20 <210> SEQ ID NO 24
<211> LENGTH: 24 <212> TYPE: PRT <213> ORGANISM:
Artificial sequence <220> FEATURE: <223> OTHER
INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 24 Met Leu
Gly Ser Asn Ser Gly Gln Arg Val Val Phe Thr Ile Leu Leu 1 5 10 15
Leu Leu Val Ala Pro Ala Tyr Ser 20 <210> SEQ ID NO 25
<211> LENGTH: 17 <212> TYPE: PRT <213> ORGANISM:
Artificial sequence <220> FEATURE: <223> OTHER
INFORMATION: Synthetic Polypeptide <400> SEQUENCE: 25 Met Lys
Cys Leu Leu Tyr Leu Ala Phe Leu Phe Ile Gly Val Asn Cys 1 5 10 15
Ala <210> SEQ ID NO 26 <211> LENGTH: 15 <212>
TYPE: PRT <213> ORGANISM: Artificial sequence <220>
FEATURE: <223> OTHER INFORMATION: Synthetic Polypeptide
<400> SEQUENCE: 26 Met Trp Leu Val Ser Leu Ala Ile Val Thr
Ala Cys Ala Gly Ala 1 5 10 15 <210> SEQ ID NO 27 <211>
LENGTH: 9 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <400> SEQUENCE: 27 ccrccaugg 9
<210> SEQ ID NO 28 <211> LENGTH: 11 <212> TYPE:
RNA <213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polynucleotide <400>
SEQUENCE: 28 gggauccuac c 11 <210> SEQ ID NO 29 <211>
LENGTH: 9 <212> TYPE: RNA <213> ORGANISM: Artificial
sequence <220> FEATURE: <223> OTHER INFORMATION:
Synthetic Polynucleotide <220> FEATURE: <221> NAME/KEY:
misc_feature <222> LOCATION: (8)..(9) <223> OTHER
INFORMATION: n can be u or a <400> SEQUENCE: 29 uuauuuann 9
<210> SEQ ID NO 30 <400> SEQUENCE: 30 000 <210>
SEQ ID NO 31 <211> LENGTH: 13 <212> TYPE: PRT
<213> ORGANISM: Artificial sequence <220> FEATURE:
<223> OTHER INFORMATION: Synthetic Polypeptide <400>
SEQUENCE: 31 Leu Gln Arg Val Arg Glu Leu Ala Val Gln Ser Ala Asn 1
5 10

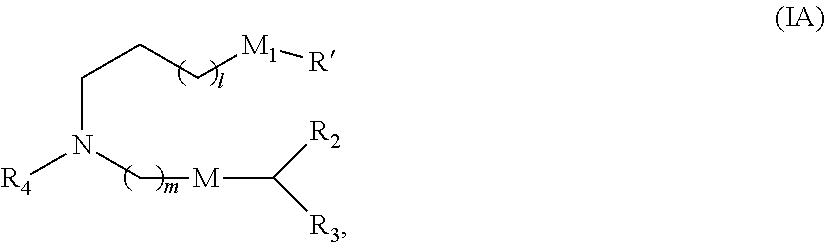






D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.