Halide ABX3 perovskite particles and their application in controlling photo-flux
Li; Yanan ; et al.
U.S. patent application number 16/033556 was filed with the patent office on 2020-01-16 for halide abx3 perovskite particles and their application in controlling photo-flux. This patent application is currently assigned to 1-Material Inc. The applicant listed for this patent is 1-Material Inc. Invention is credited to Yanan Li, Shuyong Xiao, Dawei Zhang, Shiyong Zhao.
| Application Number | 20200017364 16/033556 |
| Document ID | / |
| Family ID | 69139977 |
| Filed Date | 2020-01-16 |


| United States Patent Application | 20200017364 |
| Kind Code | A1 |
| Li; Yanan ; et al. | January 16, 2020 |
Halide ABX3 perovskite particles and their application in controlling photo-flux
Abstract
The present invention provides a light valve containing ABX.sub.3 perovskite particles; more specifically is related to a light valve containing halide ABX.sub.3 perovskite particles that can control light transmittance. The preferable halide ABX.sub.3 perovskite particles in this invention consist of A being at least one of Cs.sup.+, CH3NH3.sup.+, and Rb.sup.+, B being at least one of Pb.sup.2+, Ge.sup.2+, and Sn.sup.2+, and X being at least one of Cl.sup.-, Br.sup.-, and I.sup.-. This kind of halide ABX.sub.3 perovskite particles were suspended in a liquid suspension to make a light valve with a light transmittance control, which discloses a completely new application for ABX.sub.3 perovskite materials.
| Inventors: | Li; Yanan; (Montreal, CA) ; Zhang; Dawei; (Lachine, CA) ; Zhao; Shiyong; (Longueuil, CA) ; Xiao; Shuyong; (St-Laurent, CA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | 1-Material Inc Dorval CA |
||||||||||
| Family ID: | 69139977 | ||||||||||
| Appl. No.: | 16/033556 | ||||||||||
| Filed: | July 12, 2018 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C01G 19/006 20130101; B82Y 20/00 20130101; C01P 2004/64 20130101; C01G 21/006 20130101; G02F 1/155 20130101; C01P 2004/16 20130101; C01D 17/003 20130101; C01P 2002/34 20130101; B82Y 30/00 20130101; C01G 17/006 20130101; G02F 1/172 20130101 |
| International Class: | C01D 17/00 20060101 C01D017/00; G02F 1/155 20060101 G02F001/155; G02F 1/17 20060101 G02F001/17 |
Claims
1. A light valve, comprising a first layer of a transparent conductive substrate, an active layer containing ABX.sub.3 perovskite particles, and a second layer of transparent conductive substrate, wherein said ABX.sub.3 perovskite particles have a non-spherical morphology and are in a liquid suspension, and wherein A is at least one of Cs.sup.+, CH.sub.3NH.sub.3.sup.+, and Rb.sup.+; B is at least one of Pb.sup.2+, Ge.sup.2+, and Sn.sup.2+; and X is at least one of Cl.sup.-, Br.sup.-, and I.sup.-; wherein said ABX.sub.3 perovskite particles morphology is nanorods having an average length of about 200 nm-500 nm, and an average diameter of 50 nm-100 nm.
2. (canceled)
3. The light valve according to claim 1, wherein A is at least one of Cs.sup.+ and CH.sub.3NH.sub.3.sup.+; B is Pb.sup.2+; X is at least one of Br.sup.- and I.sup.-.
4. (canceled)
5. (canceled)
6. (canceled)
7. The light valve claim 1, wherein said halide ABX.sub.3 perovskite particles are uniformly dispersed in the liquid suspension.
8. (canceled)
9. The light valve according to claim 7, wherein said liquid suspension comprises one or more of a mineral resistive oil, a synthetic resistive oil and a vegetable oil.
10. The light valve according to claim 7, wherein said liquid suspension is sandwiched between two transparent electrodes.
11. A method of controlling light transmittance, comprising using non-spherical ABX.sub.3 perovskite particles suspended in a liquid suspension in a light control device, wherein said ABX.sub.3 perovskite particles are halide ABX.sub.3 perovskite particles, and wherein A is at least one of Cs.sup.+, CH.sub.3NH.sub.3.sup.+, and Rb.sup.+; B is at least one of Pb.sup.2+, Ge.sup.2+, and Sn.sup.2+; and X is at least one of Cl.sup.-, Br.sup.-, and I.sup.-; wherein said ABX.sub.3 perovskite particles morphology is nanorods having an average length of about 200 nm-500 nm, and an average diameter of 50 nm-100 nm.
12. The method according to claim 11, wherein the light control device is a light valve.
Description
TECHNICAL FIELD
[0001] The present invention is related to ABX.sub.3 perovskite particles and a light valve, more specifically is related to the halide ABX.sub.3 perovskite particles and a light control valve that can control the light transmission, and such a device is preferably used for windows, lenses, or a light shutter such as a sunroof. The fascinating multifunctional smart windows exhibit promising features for a wide range of applications in buildings, airplanes, automobiles, etc. The present invention provides a new use for halide ABX.sub.3 perovskite material.
BACKGROUND ART
[0002] This invention presents the method to use halide ABX.sub.3 perovskite particles to control the flux of light in a light control device, or referred as a light valve. Technically, a light valve is a device that can regulate the amount of light passing through a media like a water valve that can control the water flow. Window shade can be viewed as a light valve too. However, in this invention, the light valve is referred a device which can electronically control the light transmittance, and such a device is also scientifically referred as electrochromic device. Depending on science behind an electrochromic device, it can be further classified as polymer dispersed liquid crystal (PDLC) (U.S. patent U.S. Pat. No. 3,585,381), electrochemical device (EC) (U.S. patent U.S. Pat. No. 9,581,877) and suspension particles display (SPD) (U.S. patents U.S. Pat. No. 6,606,185). Specifically, in this invention, the light valve (LV for short hereafter) is referred a device which the light transmittance can be controlled by alternating current (AC). Such a device with controllable light switching and energy-saving advantages can be used as smart windows, rear-view car mirrors, displays, and so on.
[0003] Perovskite, the name of the perovskite, was originated from the Russian geologist Perovski and originally single-pointed the calcium titanate (CaTiO.sub.3) mineral. Later, crystals with similar structures were collectively referred to as perovskites. The cell structure of the ABX.sub.3 perovskite referred to in this patent is shown in the FIG. 3. where `A` and `B` are two cations of very different sizes, and `X` is an anion that bonds to both. Specifically, in this invention, `A` is an alkaline cation or organic ammonium, which has a positive charge, `B` is a transition metal cation or an alkaline earth cation, which has two positive charges; and `X` is a halide anion, which has a negative charge. Among them, `B` cation and 6 `X` anions form octahedral units, and 8 octahedral units occupy the position of the hexahedral apex centered on the `A` cation. This kind of material has a unique structure, giving it excellent optical, electrical, magnetic and thermodynamic properties, and is attracting increasingly studied recently in many applications.
[0004] In 2009, the ABX.sub.3 perovskite material was first reported for solar cells (J. Am. Chem. Soc. 131, 6050-6051, 2009). "Science" rated perovskite solar cells as one of the top 10 scientific breakthroughs in 2013. In January 2018, the Swiss Federal Institute of Technology in Lausanne sets new 23.25% the world record efficiency of perovskite solar cells. In addition, the ABX.sub.3 perovskite material has potential applications in LED (Light Emitting Diodes) (Nature nanotechnology, 9: 687-692, 2014), lasers (Nature Mater., 14: 636-642, 2015), photodetectors (Adv. Materials, 30(8):1704333, 2018), memristors (Advanced Electronic Materials, 2(7): 1600100, 2016).
[0005] However, in the prior art, there is no technology involving making light valves using ABX.sub.3 perovskite materials.
[0006] Therefore, the present invention provides ABX.sub.3 perovskite particles and its application in a light valve, and discloses a new application filed of the ABX.sub.3 perovskite material.
SUMMARY OF THE INVENTION
[0007] This invention presents the method to use ABX.sub.3 perovskite particles to control the flux of light in a light control device, or referred as a light valve. The present invention provides a new use of the ABX.sub.3 perovskite material, and method to make such a material. The present invention further provides a light valve, comprising a liquid suspension having such a material of ABX.sub.3 perovskite material, which can electronically control transmission of light. More specifically, the ABX.sub.3 perovskite particles, A is at least one of Cs.sup.+, CH3NH.sub.3.sup.+, and Rb.sup.+, B is at least one of Pb.sup.2+, Ge.sup.2+, and Sn.sup.2/, and X is at least one of Cl.sup.-, Br.sup.-, and I.sup.-. This halide ABX.sub.3 perovskite is characterized in that have a non-spherical morphology. The feature is that the halide ABX.sub.3 perovskite particles morphology is at least one of nanowires, nanorods (one-dimensional); nanosheets (two-dimensional); cuboids, irregular (three-dimensional) particles.
[0008] According to this invention, the liquid suspension, which is used as a liquid medium to suspend the ABX.sub.3 perovskite particles, comprises one or more a mineral resistive oil, a synthetic resistive oil, and a vegetable oil.
[0009] According to this invention as illustrated in FIG. 1, the said transparent electrode (100) can be made of the same material or different materials, where light can be transmitted through, preferably having a light transmittance equals to or greater than 80%.
BRIEF DESCRIPTION OF THE DRAWINGS
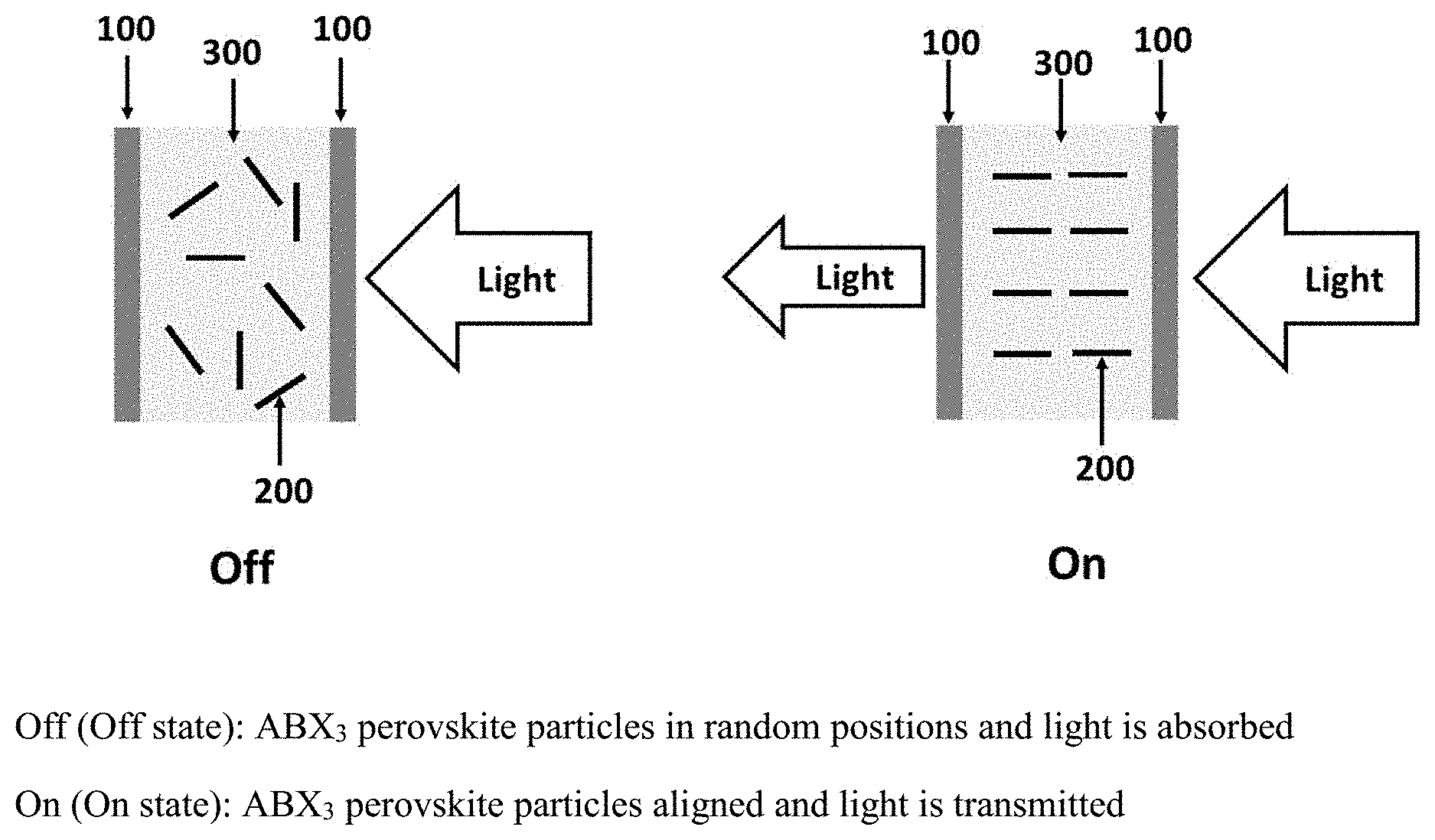
[0010] FIG. 1 presents schematically the light controlling device, wherein, a liquid suspension (300) is sandwiched between two transparent substrates (100) and (100). The halide ABX.sub.3 perovskite particles (200) are suspended in the liquid suspension (300).
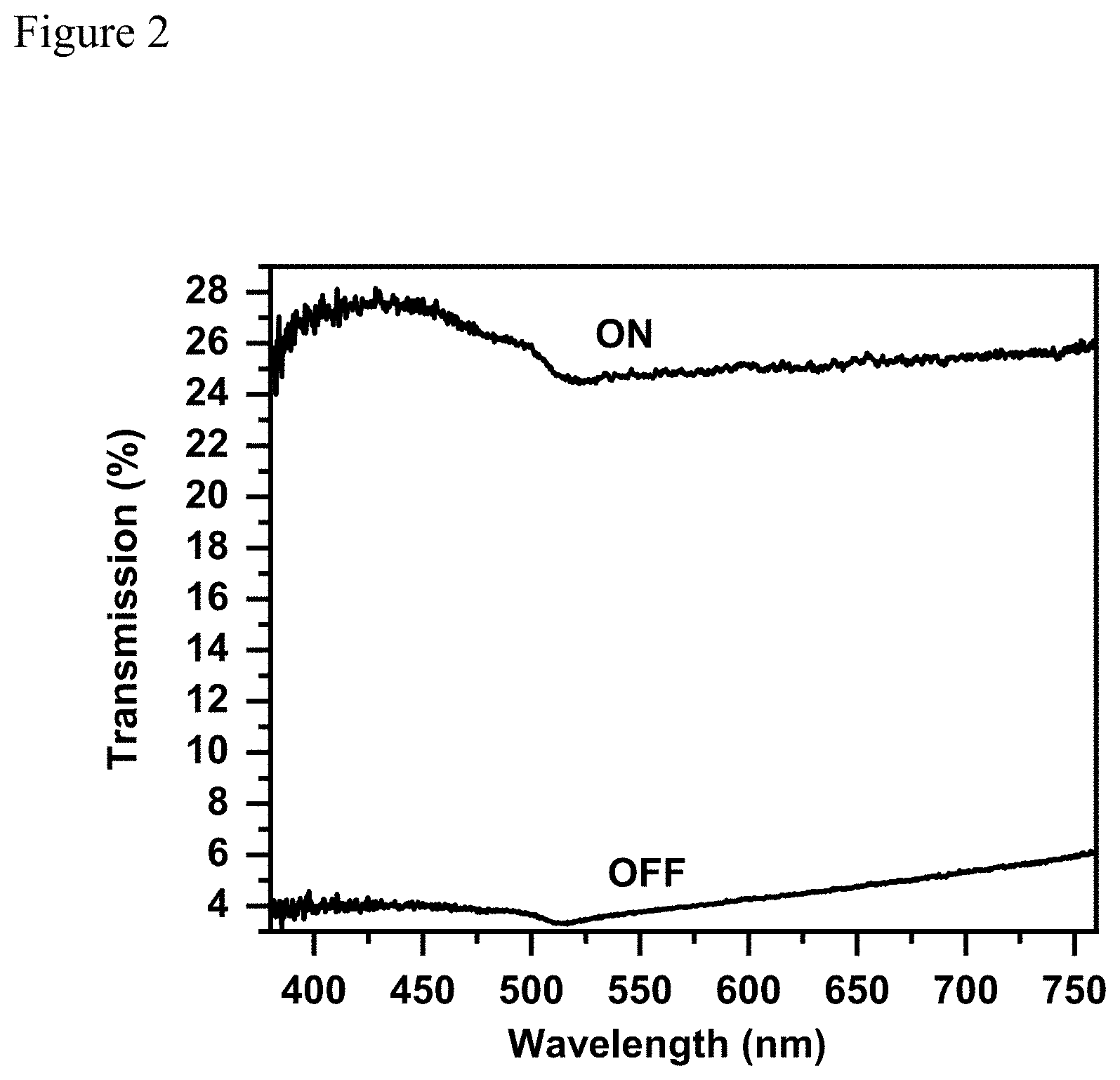
[0011] FIG. 2 presents light transmittance of a light valve (LV) device made according to this invention Example 6 before and after applying an electric voltage of 220V.
[0012] FIG. 3 presents the cell structure of the ABX.sub.3 perovskite.
DETAILED DESCRIPTION OF THE INVENTION
[0013] The present invention provides a new form of halide ABX.sub.3 perovskite particles and the method to use them to control the flux of light in a light control device, or referred as a light valve.
[0014] FIG. 1 presents schematically the light controlling device, wherein, a liquid suspension (300) is sandwiched between two transparent substrates (100) and (100). The halide ABX.sub.3 perovskite particles (200) are suspended in the liquid suspension (300). In the absence of an applied electrical field (OFF state), the halide ABX.sub.3 perovskite particles in the liquid suspension assume random positions due to Brownian movement. Hence, a beam of light passing into the light valve is absorbed/scattered. The light valve is thus relatively dark in the OFF state. When an electric field is applied thereto (ON state), the light control halide ABX.sub.3 perovskite particles are polarized, thereby being arranged in directions parallel to each other in accordance with the electric field, and most of the light can pass through the cell. The light valve is thus relatively transparent in the ON state.
[0015] The present invention provides a new use of the ABX.sub.3 perovskite particles, and method to make such a material. The present invention further provides a light valve, comprising a liquid suspension having such a material of ABX.sub.3 perovskite particles, which can electronically control transmission of light. More specifically, the ABX.sub.3 perovskite particles, A is at least one of Cs.sup.+, CH3NH.sub.3.sup.+, and Rb.sup.+, B is at least one of Pb.sup.2+, Ge.sup.2+, and Sn.sup.2+, and X is at least one of Cl.sup.-, Br.sup.-, and I.sup.-. Sill more preferably, A is at least one of Cs.sup.+ and CH3NH.sub.3.sup.+, B is Pb.sup.2+, X is at least one of Br.sup.- and I.sup.-.
[0016] The halide ABX.sub.3 perovskite particles are characterized in that have a non-spherical morphology. The feature is that the halide ABX.sub.3 perovskite particles morphology is at least one of the nanowires, nanorods (one-dimensional); nanosheets (two-dimensional); cuboids, irregular (three-dimensional) particles.
[0017] As illustrated in FIG. 1, the said ABX.sub.3 perovskite particles (200) which are encapsulated inside the said liquid suspension (300) shall be capable of re-orientating themselves in an electronic field. In terms of geometric dimensions, the ABX.sub.3 perovskite particles are preferably in a form of nanorods having an average length of about 50 nm-2000 nm, more preferably 200 nm-500 nm, and an average diameter of 20 nm-200 nm, more preferably 50 nm-100 nm.
[0018] According to this invention, the liquid suspension (300), which is used as a liquid medium to suspend the ABX.sub.3 perovskite particles, comprises one or more non-aqueous, electrically resistive liquids. Such a liquid or a liquid mixture, referring as the suspension medium, can maintain the suspended ABX.sub.3 perovskite particles in gravitational equilibrium.
[0019] More specifically in this invention, the liquid suspension (300) comprises one or more a mineral resistive oil, a synthetic resistive oil and a vegetable oil. Mineral resistive oils, such as transformer oils; synthetic resistive oils, such as silicone oils, fluorocarbon organic compounds, plasticizers (such as dioctyl phthalate, dibutyl phthalate, diisobutyl phthalate, triisodecyl trimellitate (TDTM), dodecylbenzene, polybutene oil; vegetable oils, such as castor oil, soybean oil, rapeseed oil, are good liquid suspension medium. Technically, the liquid suspension medium used in the light valve of the present invention can be any liquid light valve suspension known in the art and can be formulated according to techniques well known to those skilled in the art.
[0020] According to this invention as illustrated in FIG. 1, the said transparent electrode (100) can be made of the same material or different materials, where light can be transmitted through, preferably having a light transmittance equals to or greater than 80%, more preferably 90%. Either the said transparent electrode (100) can be ITO conductive glass, ITO/PET conductive film, Ag nanowire/PET conductive film, Cu nanowire/PET conductive film. The transparent electrode (100) are preferred to be of the same material for the simplicity of processing and for the same physical properties (such as flexibility and thermal expansion), important for device durability under certain conditions, such as thermal stress.
[0021] As ABX.sub.3 perovskite particles are sensitive to moisture and oxygen, the two transparent electrodes sandwiched by the liquid suspension are sealed with a resistive material, such as epoxy resin, etc., which can be used to seal the sealing material around the two transparent electrodes. The light valve is driven by alternating current to adjust light transmittance, preferably 5-500V alternating current.
[0022] The invention will now be described in more detail with reference to the following examples. However, these examples are given for illustration only and are not intended to limit the scope of the present invention. All chemicals used in the examples are purchased from Sigma-Aldrich Company unless otherwise specified. In all these examples, all parts and percentages are by weight unless otherwise noted. The light transmittance and absorption spectrum of the LV device was measured by an Oceanview spectrometer.
Example 1 Preparation of Cs-Oleate
[0023] Cs.sub.2CO.sub.3 (4.07 g) was loaded into a 250 mL, 3-neck flask along with octadecene (50 mL, ODE) and oleic acid (11.088 g), and the mixture was dried for 1 h at 120.degree. C. and then heated under Ar to 150.degree. C. until all Cs.sub.2CO.sub.3 reacted with oleic acid. Since Cs-Oleate precipitates out of ODE at room temperature, it has to be preheated to make it soluble before usage.
Example 2 Synthesis of CsPbI.sub.3 Nanorods
[0024] N, N-dimethylformamide (100 mL, DMF) and PbI.sub.2 2.306 (5 mmol) were loaded into a 250 mL flask. Acetate acid 4.654 g (77.5 mmol) and dodecylamine 0.797 g (4.3 mmol) were added. After complete solubilization of PbI.sub.2, 5 mL Cs-Oleate solution was added (prepared as described Example 1). Then, the hybrid solution was added into a 5 L flask along with 4200 mL toluene.
[0025] Then, centrifuge the reaction solution at 5000 G for 1.5 hours and discard the supernatant to yield the light control CsPbI.sub.3.
[0026] Then, the CsPbI.sub.3 were further dispersed with 500 mL of toluene, mixed well with shaking and sonication (referring as LCP-Example-2).
Example 3 Synthesis of CsPbBr.sub.3 Nanorods
[0027] In the same manner as in Example 2, only 1.835 g of PbBr.sub.2 was used instead of 2.306 g of PbI.sub.2. A toluene mixture containing CsPbBr.sub.3 is referring as LCP-Example-3.
Example 4 Preparation of LV Suspension Containing CsPbI.sub.3 Nanorods
[0028] In the 250 ml round bottom glass flask was weighted 10 g of TDTM (triisodecyltrimellitate), and the LCP-Example-2 prepared in the Example 2 was added in portions. After thoroughly mixing by shaking, toluene was subsequently removed by a rotary evaporator for 3 hours at 80.degree. C. to yield a LV suspension containing CsPbI.sub.3 referred as LV Suspension Example-4.
Example 5 Preparation of LV Suspension Containing CsPbBr.sub.3 Nanorods
[0029] In the 250 ml round bottom glass flask was weighted 15 g of silicone oil, and the LCP-Example-3 prepared in the Example 3 was added in portions. After thoroughly mixing by shaking, toluene was subsequently removed by a rotary evaporator for 3 hours at 80.degree. C. to yield a LV suspension containing CsPbBr.sub.3 referred as LV Suspension Example-5.
Example 6 LV Devices Made from LV Suspension-Example-4
[0030] In this example, a wet thickness of 200 um of the LV Suspension-Example 4 made in Example 4 was sealed between two transparent electrodes of ITO conductive glass using epoxy resin to produce a light valve referring as LV Device-6. When no electric voltage is applied (OFF State), LV Device-6 exhibits an orange tint and light transmission is measured to be 4.7%. When it was electrically activated using 220 Volts AC at 50 Hz (ON State), the LV Device-6 turns clearer and light transmission is measured to be 25.6%. FIG. 2 presents the absorption spectrum of LV Device-6 at OFF state and ON state respectively.
Example 7 LV Devices Made from LV Suspension-Example-5
[0031] In this example, a wet thickness of 180 um of the LV Suspension-Example 5 made in Example 5 was sealed between two transparent electrodes of ITO conductive glass using epoxy resin to produce a light valve referring as LV Device-7. When no electric voltage is applied (OFF State), LV Device-7 exhibits an orange tint and light transmission is measured to be 6.4%. When it was electrically activated using 220 Volts AC at 50 Hz (ON State), the LV Device-7 turns clearer and light transmission is measured to be 30.2%.
TABLE-US-00001 TABLE 1 Typical performance of LV devices Transmittance % LV Device Off state On state LV Device-6 4.7 25.6 LV Device-7 6.4 30.2
TABLE-US-00002 U.S. PATENT DOCUMENTS 1. U.S. Pat. No. 3,585,381 Theodore L Hodson et al., 1969 2. U.S. Pat. No. 9,581,877 John David Bass et al., 2015 3. U.S. Pat. No. 6,606,185 Robert L. Saxe, 2001 NON U.S. PATENT DOCUMENTS 1. J. Am. Chem. Soc., A. Kojima et al., 2009 131: 6050-6051 2. Nature Nanotechnology, Tan, Zhi-Kuang, et al., 2014 9: 687-692 3. Nature Mater., 14: 636-642 Haiming Zhu, et al., 2015 4. Adv. Mater., 30(8): 1704333 Zhenqian Yang, et al., 2018 5. Advanced Electronic Materials, Zhengguo Xiao, et al., 2016 2: 1600100
* * * * *
D00000

D00001

D00002

D00003

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.