Biofilm Disrupting Composition
Kumar; Theerthankar Das Ashish ; et al.
U.S. patent application number 16/470887 was filed with the patent office on 2020-01-16 for biofilm disrupting composition. The applicant listed for this patent is The University of Sydney, Whiteley Corporation Pty Ltd. Invention is credited to Trevor Owen Glasbey, Theerthankar Das Ashish Kumar, Jim Manos, Gregory Stuart Whiteley.
| Application Number | 20200016231 16/470887 |
| Document ID | / |
| Family ID | 62624084 |
| Filed Date | 2020-01-16 |











View All Diagrams
| United States Patent Application | 20200016231 |
| Kind Code | A1 |
| Kumar; Theerthankar Das Ashish ; et al. | January 16, 2020 |
BIOFILM DISRUPTING COMPOSITION
Abstract
A biofilm disrupting composition for use in treating biofilm-mediated infections due to non-Pseudomonas micro-organisms in the Cystic Fibrosis patient. One embodiment of the composition of the invention comprises at least one biologically acceptable thiol based antioxidant and at least one antibiotic. Another embodiment of the composition of the invention comprises at least one biologically acceptable thiol based antioxidant, at least one enzyme and at least one antibiotic. The invention is also directed to the process of preparing the composition of the invention, the use of the composition for the manufacture of a medicament for disrupting biofilms formed by non-Pseudomonad micro-organisms, and a method of disrupting biofilm formed by non-Pseudomonad micro-organisms in a patient, comprising administering to the patient the composition of the invention.
| Inventors: | Kumar; Theerthankar Das Ashish; (Kensington, AU) ; Manos; Jim; (Oatley, AU) ; Whiteley; Gregory Stuart; (Queenscliff, AU) ; Glasbey; Trevor Owen; (Tanilba Bay, AU) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 62624084 | ||||||||||
| Appl. No.: | 16/470887 | ||||||||||
| Filed: | December 8, 2017 | ||||||||||
| PCT Filed: | December 8, 2017 | ||||||||||
| PCT NO: | PCT/AU2017/051349 | ||||||||||
| 371 Date: | June 18, 2019 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61K 38/063 20130101; A61K 9/08 20130101; A61K 38/465 20130101; A61K 9/06 20130101; A61K 45/06 20130101; A61K 31/7036 20130101; A61K 33/30 20130101; A61P 31/04 20180101; A61K 31/14 20130101; A61K 9/0014 20130101; A61K 31/496 20130101; C12Y 301/21001 20130101; A61P 27/02 20180101; A61K 33/18 20130101; A61K 47/38 20130101; A61P 31/00 20180101; A61K 38/063 20130101; A61K 2300/00 20130101; A61K 31/496 20130101; A61K 2300/00 20130101; A61K 33/30 20130101; A61K 2300/00 20130101; A61K 31/14 20130101; A61K 2300/00 20130101; A61K 33/18 20130101; A61K 2300/00 20130101; A61K 38/465 20130101; A61K 2300/00 20130101 |
| International Class: | A61K 38/06 20060101 A61K038/06; A61K 31/496 20060101 A61K031/496; A61K 31/7036 20060101 A61K031/7036; A61K 38/46 20060101 A61K038/46; A61P 31/04 20060101 A61P031/04 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Dec 22, 2016 | AU | 2016905326 |
Claims
1-20. (canceled)
21. A biofilm disrupting composition when used for disrupting biofilms formed by non-Pseudomonad micro-organisms comprising: (a) at least one biologically acceptable thiol based antioxidant, and (b) at least one antibiotic.
22. The composition according to claim 21 wherein the biologically acceptable thiol based antioxidant is selected from the group consisting of mercaptoethanol, N-acetyl cysteine, glutathione, thiamphenicol glycinate, acetylcysteinate, sodium mercaptoethane sulfonate, lipoic acid and erdosteine.
23. The composition according to claim 22 wherein the biologically acceptable thiol based antioxidant is glutathione.
24. The composition according to claim 21 wherein the antibiotic is selected from the group consisting of antibiotic classes Penicillins, Tetracyclines, Cephalosporins, Quinolones, Lincomycins, Macrolides, Sulfonamides, Glycopeptides, Aminoglycosides and Carbapenems and mixtures thereof.
25. The composition according to claim 24 wherein the antibiotic is selected from the group consisting of ciprofloxacin, dexamethasone, amoxicillin/clavulanate, cefixime, cefaclor, clarithromycin, levofloxacin, moxifloxacin and telithromycin.
26. A biofilm disrupting composition when used for disrupting biofilms formed by non-Pseudomonad micro-organisms comprising: (a) at least one biologically acceptable thiol based antioxidant, (b) at least one enzyme and (c) at least one antibiotic.
27. The composition according to claim 26 wherein the biologically acceptable thiol based antioxidant is selected from the group consisting of mercaptoethanol, N-acetyl cysteine, glutathione, thiamphenicol glycinate, acetylcysteinate, sodium mercaptoethane sulfonate, lipoic acid and erdosteine.
28. The composition according to claim 27 wherein the biologically acceptable thiol based antioxidant is glutathione.
29. The composition according to claim 26 wherein the antibiotic is selected from the group consisting of antibiotic classes Penicillins, Tetracyclines, Cephalosporins, Quinolones, Lincomycins, Macrolides, Sulfonamides, Glycopeptides, Aminoglycosides and Carbapenems and mixtures thereof.
30. The composition according to claim 29 wherein the antibiotic is selected from the group consisting of ciprofloxacin, dexamethasone, amoxicillin/clavulanate, cefixime, cefaclor, clarithromycin, levofloxacin, moxifloxacin and telithromycin.
31. The composition according to claim 26 wherein the enzyme is selected from the group consisting of DNase, amylase, cellulase, and proteinase.
32. The composition according to claim 31 wherein the enzyme is DNase.
33. A process of preparing a biofilm disrupting composition according to claim 21, which process comprises combining at least one biologically acceptable thiol based antioxidant and at least one antibiotic, to form said composition.
34. A process of preparing a biofilm disrupting composition according to claim 26, which process comprises combining at least one biologically acceptable thiol based antioxidant, at least one antibiotic and at least one enzyme, to form said composition.
35. The use of a composition comprising: (a) at least one biologically acceptable thiol based antioxidant, and (b) at least one antibiotic, for the manufacture of a medicament for disrupting biofilms formed by non-Pseudomonad micro-organisms.
36. The use according to claim 35 wherein the biologically acceptable thiol based antioxidant is selected from the group consisting of mercaptoethanol, N-acetyl cysteine, glutathione, thiamphenicol glycinate, acetylcysteinate, sodium mercaptoethane sulfonate, lipoic acid and erdosteine.
37. The use according to claim 36 wherein the biologically acceptable thiol based antioxidant is glutathione.
38. The use according to claim 35 wherein the antibiotic is selected from the group consisting of antibiotic classes Penicillins, Tetracyclines, Cephalosporins, Quinolones, Lincomycins, Macrolides, Sulfonamides, Glycopeptides, Aminoglycosides and Carbapenems and mixtures thereof.
39. The use according to claim 38 wherein the antibiotic is selected from the group consisting of ciprofloxacin, dexamethasone, amoxicillin/clavulanate, cefixime, cefaclor, clarithromycin, levofloxacin, moxifloxacin and telithromycin.
40. The use according to claim 39 wherein the enzyme is selected from the group consisting of DNase, amylase, cellulase, and proteinase.
41. The use of a composition comprising: (a) at least one biologically acceptable thiol based antioxidant, (b) at least one enzyme and (c) at least one antibiotic, for the manufacture of a medicament for disrupting biofilms formed by non-Pseudomonad micro-organisms.
42. The use according to claim 41 wherein the biologically acceptable thiol based antioxidant is selected from the group consisting of mercaptoethanol, N-acetyl cysteine, glutathione, thiamphenicol glycinate, acetylcysteinate, sodium mercaptoethane sulfonate, lipoic acid and erdosteine.
43. The use according to claim 42 wherein the biologically acceptable thiol based antioxidant is glutathione.
44. The use according to claim 41 wherein the antibiotic is selected from the group consisting of antibiotic classes Penicillins, Tetracyclines, Cephalosporins, Quinolones, Lincomycins, Macrolides, Sulfonamides, Glycopeptides, Aminoglycosides and Carbapenems and mixtures thereof.
45. The use according to claim 44 wherein the antibiotic is selected from the group consisting of ciprofloxacin, dexamethasone, amoxicillin/clavulanate, cefixime, cefaclor, clarithromycin, levofloxacin, moxifloxacin and telithromycin.
46. The use according to claim 45 wherein the enzyme is selected from the group consisting of DNase, amylase, cellulase, and proteinase.
47. A method of disrupting biofilm formed by non-Pseudomonad micro-organisms in a patient, which method comprises administering to said patient a composition according to claim 21 in an amount which effectively disrupts said biofilm.
48. A method of disrupting biofilm formed by non-Pseudomonad micro-organisms in a patient, which method comprises administering to said patient a composition according to claim 26 in an amount which effectively disrupts said biofilm.
Description
FIELD OF THE INVENTION
[0001] The invention relates to a biofilm disrupting composition for use in treating biofilm-mediated infections due to non-Pseudomonas micro-organisms in the Cystic Fibrosis patient.
BACKGROUND OF INVENTION
[0002] A biofilm is any group of microorganisms in which cells stick to each other and often these cells adhere to a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). The biofilm EPS is typically comprised of a polymeric conglomeration generally composed of extracellular DNA (eDNA), proteins, and polysaccharides. Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial and hospital settings. The sessile microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single-cells that may float or swim in a liquid medium.
[0003] Microbes form a biofilm in response to many factors, which may include cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or in some cases, by exposure of planktonic cells to sub-inhibitory concentrations of antibiotics. When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated.
[0004] Within a biofilm structure, microorganisms demonstrate significantly greater resistance to both biocides and antibiotics. This resistance feature of sessile microbes is a dual function of the enhanced genetic expression and also shielding by the surrounding polymeric materials of the biofilm. Traditional antimicrobial therapies have been ineffective in the treatment of bacterial infections when the bacteria are located within a biofilm which is within, adherent to, or above tissue or located within a void such as the lungs or bladder, or in nasal passages or on the surface of a wound or burn. The additional risk of a multi-drug-resistant-organism increases the likelihood of significant morbidity or even death.
[0005] Inhaled glutathione (GSH) therapy has been used to reduce oxidative stress in cystic fibrosis patients and inhibit proliferation of Pseudomonas infections in cystic fibrosis patients, including increasing susceptibility of the Pseudomonas to antibiotics (Zhang Y and Duan K, "Glutathione exhibits antibacterial activity and increases tetracycline efficacy against Pseudomonas aeruginosa"; Sci. China Ser. C, (2009) 52:501-505.
[0006] It has also been found that GSH and DNase I can be combined for treating chronic Pseudomonas infections in individuals with cystic fibrosis (Klare et al., Canberra ASM meeting, July 2015). Further, GSH and DNase I have been found to be useful in the disruption of Pseudomonas biofilms in cystic fibrosis-like media and increasing susceptibility of the Pseudomonas aeruginosa to antibiotics (Klare et al., 2016 Antimicrobial Agents Chemotherapy 60 (8) 4539-4551), particularly when incorporated with an antibiotic such as Ciprofloxacin.
[0007] The effectiveness of the combination of Glutathione and DNase I can be ascribed to the nature of the biofilms formed by Pseudomonas spp. The biofilm matrix consists predominantly of polysaccharides, proteins, and nucleic acids. Despite macromolecule heterogeneity, most research has focused on the role of bacterially produced exopolysaccharides (EPSs) in biofilm establishment and maturation.
[0008] The integral role of extracellular DNA in biofilm formation was first identified in P. aeruginosa by Whitchurch et al (Whitchurch C B, Tolker-Nielsen T, Ragas P C, Mattick J S. "Extracellular DNA required for bacterial biofilm formation". Science (2002) 295:1487. doi:10.1126/science.295.5559.1487), but eDNA has since been shown to be a ubiquitous biofilm matrix polymer across most Gram-positive and Gram-negative bacterial species. In fact, within P. aeruginosa biofilms, eDNA is the most abundant matrix polymer (see Matsukawa M, Greenberg E P. "Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development". J Bacteriol (2004) 186:4449-4456. doi:10.1128/JB.186.14.4449-4456.2004. and Okshevsky M, Meyer R L. "The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms". Crit Rev Microbiol (2013) 41:341-352). The eDNA also serves as a structural component of the EPS and contributes to its viscosity.
[0009] Pseudomonas aeruginosa is also known to release exoproducts into the EPS. One of these exoproducts, the blue, redox-active phenazine derivative called pyocyanin also contributes to the viscosity of the EPS by intercalating directly with the EPS. Pyocyanin has also been demonstrated to promote the release of eDNA from P. aeruginosa (see Das T, and Manefield M, "Pyocyanin Promotes Extracellular DNA Release in Pseudomonas aeruginosa"; Plos One (2012), 7, e46718).
[0010] Pyocyanin also contributes to the disease processes in Cystic Fibrosis. In vitro studies have shown that pyocyanin has multiple deleterious effects on mammalian cells, such as inhibition of cell respiration, ciliary function, epidermal cell growth and prostacyclin release, disruption of calcium homeostasis, and inactivation of catalase. Pyocyanin also induces apoptosis in neutrophils and modulates the glutathione redox cycle in lung epithelial and endothelial cells. It also inactivates al protease inhibitor and contributes to the imbalance of protease-antiprotease activity, which is readily detected in the airways of patients with CF lung disease. More recently, it was shown that pyocyanin inactivates the vacuolar ATPase of lung epithelial cells (See Lau G W et al., "Pseudomonas aeruginosa Pyocyanin Is Critical for Lung Infection in Mice". Infect. Immun. (2004) 72 4275-4278 and references therein)
[0011] The most commonly identified organisms in respiratory specimens taken from Cystic Fibrosis patients are various species and forms of Pseudomonas. It can be seen that 48.5 percent of patients tested produced positive Pseudomonas aeruginosa cultures, with the mucoid form showing in 32.0 percent. Its prevalence is greater in adult patients, with 60.9 percent of tested adult CF patients producing samples indicating the mucoid form of Pseudomonas aeruginosa, three times the corresponding proportion for adolescents and much higher than that for children.
[0012] While prevalence of Pseudomonas organisms is lower in children than in adults, although increasing with rising age, young children are more likely than adult patients to produce cultures showing presence of Staphylococcus aureus (see table 1). Half of all child patients and adolescent patients aged 6 to 17 years had this bacterial infection. Haemophilus influenza is also evident in relatively high proportions of child patients, highest in children aged from 2 to 5 years, where this organism was cultured for almost one third of children. The youngest age groups also had the highest proportions with positive cultures of the bacteria Escherichia coli; 12 percent, for those in the age (source: "CYSTIC FIBROSIS IN AUSTRALIA 2014", 17th Annual Report from the Australian Cystic Fibrosis Data Registry, Cystic Fibrosis Australia (2016), North Ryde, NSW, Australia).
TABLE-US-00001 TABLE 1 Percentages of other respiratory cultures by age group (other than Pseudomonas) Age Range 0-1 2-5 6-11 12-17 18-29 30+ Total Bacteria: Staphylococcus aureus 39.0 42.6 47.6 50.3 38.6 30.7 41.8 Haemophilus influenzae 28.0 32.3 23.7 11.3 7.1 2.3 14.5 Burkholderia cepacia 0.0 0.0 1.6 2.1 4.2 2.8 2.3 Stenotrophomonas maltophilia 5.0 3.8 10.2 14.4 7.7 6.5 8.7 Escherichia coli 12.0 8.9 6.5 3.1 1.0 0.6 4.0 MRSA (Methicillin Resistant 2.0 1.7 1.9 3.4 2.5 3.4 2.6 Staphylococcus aureus) Alcaligenes xylosoxidans 0.0 0.4 3.0 2.6 5.0 5.6 3.5 Serratia marcescens 2.0 1.7 0.9 1.3 0.8 0.0 0.9 Klebsiella (any species) 9.0 3.0 0.7 1.6 0.6 1.1 1.6 Non-tuberculous mycobacterium 0.0 0.0 0.0 3.1 4.6 4.2 2.5 Fungi: Candida 18.0 22.1 24.6 34.8 27.8 29.3 27.6 Aspergillus (any species) 8.0 10.2 22.0 33.5 29.2 22.3 24.0 Scediosporium (any species) 0.0 0.4 2.6 6.3 5.0 3.4 3.7 Other organisms not listed 28.0 33.2 30.4 29.1 20.8 19.7 26.0 above Normal flora only 63.0 79.1 82.8 77.5 38.0 28.5 59.4 No growth/sterile culture 10.0 8.1 7.0 4.5 4.2 3.4 5.4 Patients tested 100 235 431 382 518 355 2021
[0013] It is evident therefore that whilst the bulk of respiratory infections in Cystic Fibrosis sufferers are due to Pseudomonas, a significant number of patients show infections due to other biofilm generating organisms such as Staphylococcus aureus and Haemophilus influenzae, neither of which release pyocyanin.
DESCRIPTION OF FIGURES
[0014] FIG. 1 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Pseudomonas aeruginosa both individually and in combination (two component combination), according to comparative Example 2.
[0015] FIG. 2 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Staphylococcus aureus (MRSA) both individually and in combination (two component combination), according to Example 3.
[0016] FIG. 3 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Staphylococcus aureus MSSA both individually and in combination (two component combination), according to Example 4.
[0017] FIG. 4 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Streptococcus agalactiae both individually and in combination (two component combination), according to Example 5.
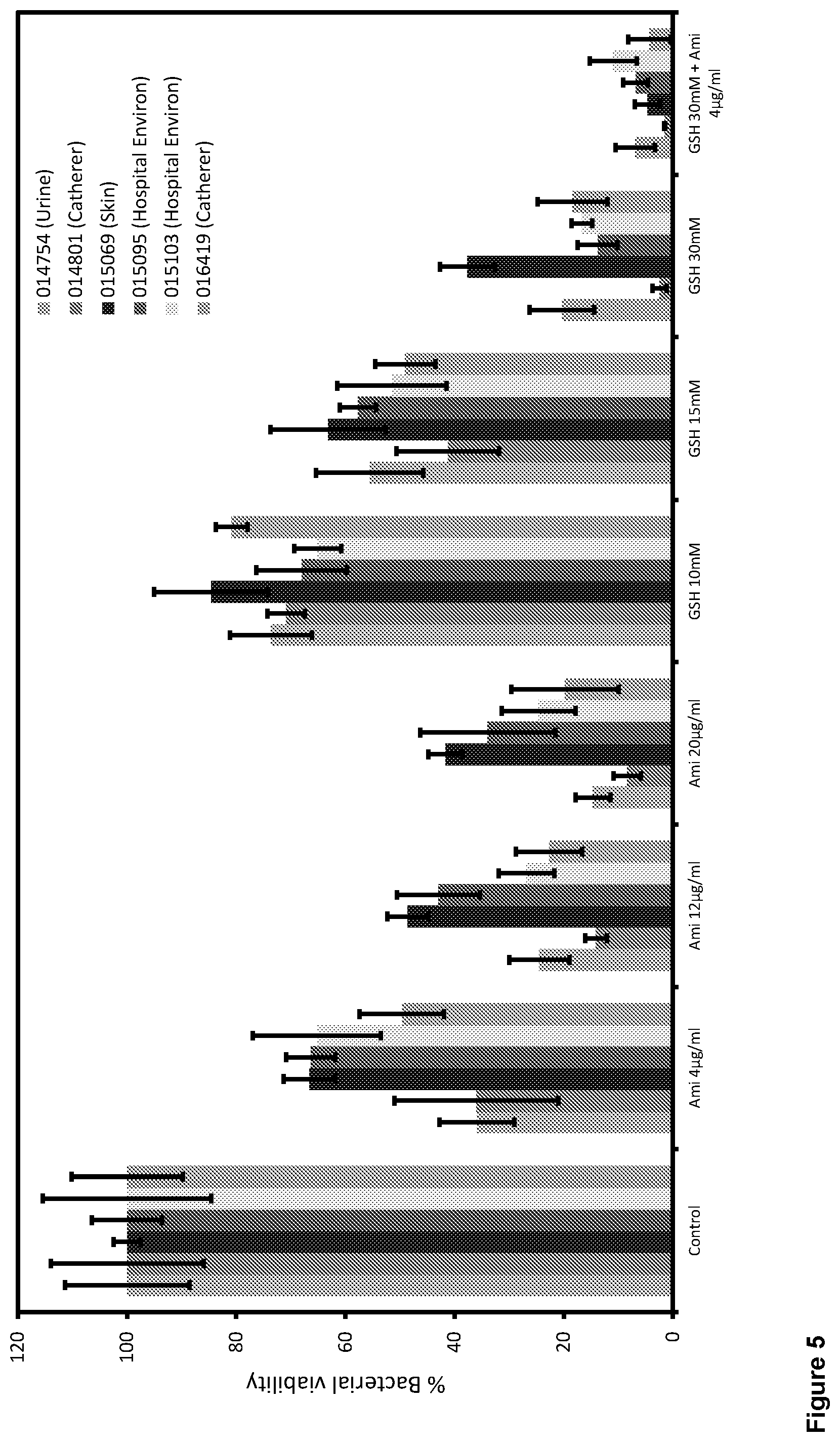
[0018] FIG. 5 shows the effects of amikacin and glutathione on the biofilm viability of Acinetobacter baumaunii multi drug resistant (MRAB) both individually and in combination (two component combination), according to Example 6.
[0019] FIG. 6 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Klebsiella pneumoniae both individually and in combination (two component combination), according to Example 7.
[0020] FIG. 7 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Enterobacter species both individually and in combination (two component combination), according to Example 8.
[0021] FIG. 8 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Escherichia coli both individually and in combination (two component combination), according to Example 9.
[0022] FIG. 9 shows the effects of ciprofloxacin and glutathione on the biofilm viability of Streptococcus pyogenes both individually and in combination (two component combination), according to Example 10.
[0023] FIG. 10 shows the effects of ciprofloxacin, glutathione and DNase I on the biofilm viability of Staphylococcus aureus (MRSA) both individually, and in both dual and triple combination (three component combination), according to Example 11.
[0024] FIG. 11 shows the effects of ciprofloxacin, glutathione and DNase I on the biofilm viability of Staphylococcus aureus (MSSA) both individually, and in both dual and triple combination (three component combination), according to Example 12.
[0025] FIG. 12 shows the effects of ciprofloxacin, glutathione and DNase I on the biofilm viability of Streptococcus agalactiae both individually, and in both dual and triple combination (three component combination), according to Example 13.
[0026] FIG. 13 shows the effects of amikacin, glutathione and DNase I on the biofilm viability of Acinetobacter baumannii (MRAB) both individually, and in both dual and triple combination (three component combination), according to Example 14.
SUMMARY OF THE INVENTION
[0027] Whilst it may be expected that the combination of DNase I and an antibiotic will show activity against biofilms, it has been surprisingly found that a biologically acceptable thiol based antioxidant can also serve to disrupt biofilms formed from organisms other than the pyocyanin-producing Pseudomonas.
[0028] It has also been unexpectedly found that the combination of a biologically acceptable thiol based antioxidant, an enzyme and an antibiotic also demonstrate a synergistic effect against biofilms formed by organisms other than Pseudomonas (ie non-Pseudomonad organisms).
[0029] According to a first embodiment of the invention, there is provided a biofilm disrupting composition comprising: [0030] (a) at least one biologically acceptable thiol based antioxidant, and [0031] (b) at least one antibiotic, [0032] wherein said composition is capable of disrupting biofilms formed by non-Pseudomonad micro-organisms.
[0033] According to a second embodiment of the invention, there is provided a biofilm disrupting composition when used for disrupting biofilms formed by non-Pseudomonad micro-organisms comprising: [0034] (a) at least one biologically acceptable thiol based antioxidant, and [0035] (b) at least one antibiotic.
[0036] According to a third embodiment of the invention, there is provided a biofilm disrupting composition comprising: [0037] (a) at least one biologically acceptable thiol based antioxidant, [0038] (b) at least one enzyme and [0039] (c) at least one antibiotic, wherein said composition is capable of disrupting biofilms formed by non-Pseudomonad micro-organisms.
[0040] According to a fourth embodiment of the invention, there is provided a biofilm disrupting composition when used for disrupting biofilms formed by non-Pseudomonad micro-organisms comprising: [0041] (a) at least one biologically acceptable thiol based antioxidant, [0042] (b) at least one enzyme and [0043] (d) at least one antibiotic.
[0044] According to a fifth embodiment of the invention, there is provided a process of preparing a biofilm disrupting composition according to the first and second embodiments, which process comprises combining at least one biologically acceptable thiol based antioxidant and at least one antibiotic, to form said composition.
[0045] According to a sixth embodiment of the invention, there is provided a process of preparing a biofilm disrupting composition according to the third and fourth embodiments, which process comprises combining at least one biologically acceptable thiol based antioxidant, at least one antibiotic and at least one enzyme, to form said composition.
[0046] According to a seventh embodiment of the invention, there is provided the use of a composition comprising: [0047] (a) at least one biologically acceptable thiol based antioxidant, and [0048] (b) at least one antibiotic, for the manufacture of a medicament for disrupting biofilms formed by non-Pseudomonad micro-organisms.
[0049] According to an eighth embodiment of the invention, there is provided the use of a composition comprising: [0050] (a) at least one biologically acceptable thiol based antioxidant, [0051] (b) at least one enzyme and [0052] (c) at least one antibiotic, for the manufacture of a medicament for disrupting biofilms formed by non-Pseudomonad micro-organisms.
[0053] According to a ninth embodiment of the invention, there is provided a method of disrupting biofilm formed by non-Pseudomonad micro-organisms in a patient, which method comprises administering to said patient a composition according to any one of the first, second, third or fourth embodiments in an amount which effectively disrupts said biofilm.
[0054] By biologically acceptable thiol based antioxidant it is understood that this is a substance that is tolerated without ill effect by a living body, and contains a sulfhydryl moiety, and where said substance can inhibit the oxidation of other molecules.
[0055] Throughout the description and claims of the specification, the word "comprise" and variations of the word, such as "comprising" and "comprises", is not intended to exclude other additives, components, integers or steps.
[0056] The ingredients of the composition of the invention act synergistically providing superior biofilm disruption.
DETAILED DESCRIPTION OF THE INVENTION
[0057] Biologically Acceptable Thiol Based Antioxidant
[0058] The composition of the invention comprises at least one biologically acceptable thiol based antioxidant. This is a biologically and pharmaceutically acceptable compound capable of reducing disulfide bonds formed within cytoplasmic proteins to cysteines by serving as an electron donor.
[0059] Examples of suitable biologically acceptable thiol based antioxidants include mercaptoethanol, N-acetyl cysteine (NAC), glutathione (GSH), thiamphenicol glycinate acetylcysteinate (TGA), sodium mercaptoethane sulfonate, dithiothreitol (DTT), dithiobutylamine and other similar compounds. Other examples of suitable biologically acceptable thiol based antioxidants are compounds such as lipoic acid or erdosteine, which are capable of generating free thiol groups in vivo following first pass metabolism. In a preferred embodiment the biologically acceptable thiol based antioxidant is glutathione (GSH).
[0060] Antibiotic
[0061] The composition of the invention comprises at least one antibiotic capable of killing either or both Gram Positive or Gram Negative organisms. The antibiotic may be selected from the non-limiting group of antibiotic classes consisting of Penicillins, Tetracyclines, Cephalosporins, Quinolones, Lincomycins, Macrolides, Sulfonamides, Glycopeptides, Aminoglycosides and Carbapenems. Specific examples of useful antibiotics include ciprofloxacin, amoxicillin/clavulanate, cefixime, cefalosporin cefaclor, clarithromycin, levofloxacin, moxifloxacin, gentamycin, vancomycin and telithromycin.
[0062] Enzymes
[0063] The composition of the invention comprises one or more enzymes capable of degrading one or more of the biopolymers that make up the biofilm.
[0064] The enzymes may be selected from the (non-limiting) group consisting of protease, amylase, cellulase, and DNase. In a preferred embodiment, the composition of the invention will contain two or more of these enzyme types. Preferably at least one of the enzymes is DNase.
[0065] Optional Ancillary Agents
[0066] The biofilm disrupting composition may optionally contain other ingredients such as tonicity modifiers, pH buffers, colourants, preservatives and perfumes.
[0067] Tonicity Modifying Agent
[0068] The composition of the invention may also contain tonicity modifying ingredients. These may comprise inorganic salts, for example sodium bromide, potassium bromide, sodium chloride, potassium chloride, sodium acetate, potassium acetate, sodium citrate, potassium citrate, sodium phosphate, potassium phosphate, or may comprise organic tonicity modifiers such as propylene glycol, glycerol, mannitol, arabitol, glucose, fructose etc. The composition of the invention may be isotonic (i.e. 250-350 mOsmal/Kg) or hypotonic (i.e. <250 mOsmal/Kg).
[0069] Colouring Agent
[0070] The composition of the invention may also comprise colouring agents. The colouring agents may be added to provide a function to the composition, such as the staining of components found within the bacterial biofilm, or may just be added to provide an aesthetically pleasing solution. When the colouring agent is added to stain components of the biofilm, the resultant staining may provide a visual cue as to the presence of the biofilm, thus also provide a means of monitoring its removal. Suitable colouring agents capable of staining biofilm components (for example protein, polysaccharide or bacterial cell walls) will include Coomassie Brilliant Blue, Crystal Violet, erythrosine and tartrazine.
[0071] Processing Aids
[0072] The biofilm disrupting composition may be in solid form, or the composition may be a solution. In the case of a solid mixture of ingredients, the mixture may comprise one or more processing aids such as mannitol, starch, glucose, sucrose etc. in order to allow the composition to be processed into micronized particles, preferably with a mean particle size of less than 500 microns. In a more preferred embodiment, the micronized composition will have a mean particle size of less than 100 microns, and in a particularly preferred embodiment, the micronized composition will have a mean particle size of less than 40 microns. The micronized composition of this particularly preferred embodiment is suitable for inhalation and useful for the disruption and removal of bacterial biofilms found in the lungs in conditions such as cystic fibrosis, bronchitis, chronic obstructive pulmonary disease (COPD), and other airway infections in which biofilms due to non-Pseudomonad micro-organisms are implicated, such as recurrent rhinosinusitis or pharyngotonsillitis.
[0073] In the case of a liquid composition, the mixture may contain one or more processing aids such as wetting agents, defoaming agents, antioxidants, viscosity modifiers etc.
[0074] Observed Results
[0075] Glutathione (GSH) especially at 30 milli Molar (3 times higher than biological intracellular concentration which is 2-10 millimolar) shows significantly higher effect than antibiotics in killing/disrupting biofilms, even against non-Pseudomonad organisms (see FIGS. 1-13).
[0076] Combining GSH with low concentration of antibiotics (FIGS. 2-13) enhances biofilms disruptions and killing.
[0077] DNase I by itself has no effect on Staphylococcus aureus and Streptococcus agalactiae biofilms disruption (see FIGS. 10 and 11), but Dnase I by itself has some effect on MRAB biofilms disruption (see FIG. 13).
[0078] Consequently 3 component combination therapy (3CT) (GSH+DNase I+Ciprofloxacin) used for Staphylococcus aureus and Streptococcus agalactiae has similar effect as GSH+ciprofloxacin (2 component combination therapy (2CT). 3CT use for MRAB (GSH+DNase I+Amikacin) showed significantly better disruption/killing.
EXAMPLES
[0079] In the following examples, all clinical isolates were taken from various hospitals in the Sydney, NSW area. Streptococcus agalactiae from Cow mastitis, isolated on a NSW farm.
[0080] All strains sensitive to Ciprofloxacin (except for Acinetobacter baumannii). Many strains are resistant to different antibiotics including: penicillin, gentamicin, amoxycillin/Clavulanate, Cefazolin, am ikacin.
Example 1: Experimental Protocol
[0081] Bacterial isolates were grown in Tryptone Soya Broth (TSB) medium for 24 hours at 37.degree. C., in a shaking incubator set at 150 rpm. After this time the organisms were harvested by centrifugation (5000.times.g, 5 min at 10.degree. C.). After centrifugation, the supernatant liquid was removed and the bacterial pellet was suspended in 1.times.Phosphate Buffered Saline (PBS). To initiate biofilm growth, the bacterial suspension from PBS was immediately re-suspended in TSB and 250 .mu.L of bacterial cell suspension (OD.sub.600=0.5.+-.0.05) were added into the wells of 96-well plates (Corning Corp. USA) and incubated at 37.degree. C. for 48 h at 150 r.p.m.
[0082] After 48 h, biofilm were washed once with 1.times.PBS followed by treatment (for 24 h, 37.degree. C., 150 rpm under different conditions: either with Ciprofloxacin or Amikacin, DNase I (40 U solution, Sigma Aldrich) or GSH (different concentration 10, 15 and 30 milliMolar solutions in PBS) individually or combination: DNase I+ciprofloxacin or Amikacin, GSH+DNase I, GSH+ciprofloxacin or Amikacin and GSH+DNase I+Ciprofloxacin or Amikacin. The composition of the treatments used are given in the subsequent examples
[0083] After 24 h, treated biofilms supernatant were replaced with 200 .mu.L of 1.times.PBS, followed by addition of 15 .mu.L of a 0.05% w/v solution of resazurin (Sigma-Aldrich), and incubated further at 37.degree. C., 150 r.p.m.
[0084] After a further 24 h, the fluorescent intensity of the biofilm was determined at Ex544 nm and Em590 nm (Tecan infinite M1000 pro microplate reader). Results are plotted as percentage (%) of bacterial vaiability calculated based on fluorescent intensity. Control or untreated bacterial biofilm fluorescent intensity always considered as 100% bacterial viability and % viability under rest of treatment conditions were calculated with reference to control.
[0085] It should be noted that the Resazurin assay works by recording fluorescence intensity and depends upon two factors (total number of bacteria and total number of viable bacteria in a given biofilm sample).
Example 2: Pseudomonas aeruginosa (Clinical Isolates)
[0086] Example 2 is a comparative, prior art, example demonstrating the use of the combination of glutathione with an antibiotic, ciprofloxacin. As previously discussed, it is widely believed that the role of the glutathione is to deactivate the pyocyanin released by Pseudomonas aeruginosa. It is noted that the treatments shown in column 1 of Table 2 were tested against three clinical isolates of Pseudomonas aeruginosa. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 1).
TABLE-US-00002 TABLE 2 Pseudomonas aeruginosa isolate and source PA0053 365707 Left 364077 Scalp Treatment DFU ankle wound wound Control 100.0 100.0 100.0 CIP 0.5 .mu.g/ml 12.2 22.0 9.7 CIP 1 .mu.g/ml 8.7 21.3 6.8 CIP2 .mu.g/ml 5.8 25.9 6.5 GSH 10 mM 89.8 103.5 95.6 GSH 15 mM 74.5 100.9 93.0 GSH 30 mM 7.3 11.2 30.3 2 part CT 3.2 2.3 5.2 GSH 30 mM CIP 0.5 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
[0087] The following examples illustrate the efficacy of the combination of a biologically acceptable thiol based antioxidant, with an antibiotic against biofilms formed by organisms other than Pseudomonas (i.e. non-Pseudomonad organisms).
Example 3: Methicillin Resistant Staphylococcus aureus (MRSA: Clinical Isolates)
[0088] The treatments shown in column 1 of Table 3 were tested against three strains of methicillin-resistant Staphylococcus aureus. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 2).
TABLE-US-00003 TABLE 3 MRSA 27060 MRSA 30616 MRSA - 0799 Treatment Left leg Chin vesicle DFU Control 100.00 100.00 100.00 CIP 10 .mu.g/ml 42.93 40.01 61.08 CIP 20 .mu.g/ml 25.74 31.38 54.21 CIP30 .mu.g/ml 19.53 24.56 51.60 GSH 10 mM 88.83 73.12 86.73 GSH 15 mM 65.24 58.82 75.03 GSH 30 mM 1.02 4.54 33.12 2 part CT -0.30 0.61 21.83 GSH 30 mM CIP 10 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 4: Methicillin Sensitive Staphylococcus aureus (MSSA: Clinical Isolate)
[0089] The treatments shown in column 1 of Table 4 were tested against three strains of methicillin-sensitive Staphylococcus aureus. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 3).
TABLE-US-00004 TABLE 4 MSSA - 34397 MSSA - 34654 MSSA - 0800 Treatment Left elbow Right toe DFU Control 100.00 100.00 100.00 CIP 10 .mu.g/ml 49.93 37.08 41.69 CIP 20 .mu.g/ml 36.78 32.05 32.03 CIP 30 .mu.g/ml 29.81 32.44 21.58 GSH 10 mM 88.67 86.11 82.44 GSH 15 mM 61.34 70.52 61.70 GSH 30 mM 1.06 15.33 20.09 2 part CT 0.77 5.85 9.68 GSH 30 mM CIP10 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 5: Staphylococcus agalactiae (Ex Cow Mastitis)
[0090] The treatments shown in column 1 of Table 5 were tested against four strains of Staphylococcus agalactiae, (veterinary strains ex Cow mastitis). The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 4).
TABLE-US-00005 TABLE 5 S. S. S. S. S. agalactiae agalactiae agalactiae agalactiae agalactiae Treatment # 30 # 44 # 70 # 88 # 106 Control 100.00 100.00 100.00 100.00 100.00 CIP 4 .mu.g/ml 74.20 63.40 74.10 71.60 72.50 CIP 8 .mu.g/ml 66.38 66.52 65.26 69.79 70.13 CIP 12 69.29 63.28 58.93 63.77 65.90 .mu.g/ml GSH 10 mM 103.78 86.99 89.17 87.95 102.63 GSH 15 mM 80.69 73.92 79.41 81.62 83.17 GSH 30 mM 35.30 29.16 38.30 25.00 29.20 2 part CT (1) 22.30 25.90 24.80 17.80 25.30 GSH 30 mM CIP 4 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 6: Multi Drug Resistant Acinetobacter baumannii
[0091] The treatments shown in column 1 of Table 6 were tested against six clinical isolates of Multidrug resistant Acinetobacter Baumannii, (MRAB). The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 5). As these MRAB strains were resistant to ciprofloxacin, Amikacin was used instead.
TABLE-US-00006 TABLE 6 MRAB 1 MRAB 2 MRAB 3 MRAB 4 MRAB 5 MRAB 12 Strain ID 014754 014801 015069 015095 015103 016419 Source Urine Catheter Skin Hospital Hospital Catheter Environ Environ Control 100.00 100.00 100.00 100.00 100.00 100.00 AMI 4 .mu.g/ml 35.91 35.99 66.60 66.36 65.23 49.66 AMI12 .mu.g/ml 24.47 14.11 48.60 42.91 26.85 22.70 AMI20 .mu.g/ml 14.67 8.36 41.70 33.92 24.60 19.80 GSH 10 mM 73.61 70.84 84.56 68.00 65.08 80.83 GSH 15 mM 55.58 41.23 63.18 57.70 51.49 49.05 GSH 30 mM 20.35 2.45 37.70 13.77 16.71 18.41 2 part CT (2) 6.90 1.53 4.69 6.85 10.96 4.30 GSH 30 mM AMI 4 .mu.g/ml Control = no treatment AMI = Amikacin GHS = glutathione
Example 7: Klebsiella pneumoniae (Clinical Isolates)
[0092] The treatments shown in column 1 of table 7 were tested against three clinical isolates of Klebsiella pneumoniae. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 6).
TABLE-US-00007 TABLE 7 Klebsiella pneumoniae isolate and source 374450 Catheter 377951 Left 385261 Neck Treatment urine hip wound wound Control 100.00 100.00 100.00 CIP 10 .mu.g/ml 37.55 54.58 29.48 CIP 20 .mu.g/ml 22.13 40.39 21.00 CIP30 .mu.g/ml 21.29 25.39 21.97 GSH 10 mM 85.95 81.60 78.75 GSH 15 mM 72.81 70.81 62.46 GSH 30 mM 16.76 9.13 14.12 2 part CT 10.32 1.80 4.15 GSH 30 mM CIP 10 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 8 Enterobacter Species (Clinical Isolates)
[0093] The treatments shown in column 1 of Table 8 were tested against three clinical isolates of Enterobacter species. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 7).
TABLE-US-00008 TABLE 8 Enterobacter species and source E. cloacae E aerogenes E. cloacae 359315 Left 359475 Treatment 357768 Ear foot wound Sternum Control 100.0 100.0 100.0 CIP 0.5 .mu.g/ml 39.2 64.0 14.6 CIP 1 .mu.g/ml 23.4 53.8 6.3 CIP2 .mu.g/ml 25.0 64.1 5.9 GSH 10 mM 62.8 84.7 79.0 GSH 15 mM 38.9 74.0 55.1 GSH 30 mM 0.0 19.5 14.6 2 part CT 0.0 8.5 2.3 GSH 30 mM CIP 0.5 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 9 Escherichia coli (Clinical Isolates)
[0094] The treatments shown in column 1 of Table 9 were tested against three clinical isolates of Enterobacter species. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 8).
TABLE-US-00009 TABLE 9 Escherichia coli and source 362805 365714 366290 Drain Exit Wound fluid Control 100.0 100.0 100.0 CIP 0.5 .mu.g/ml 57.9 40.7 29.9 CIP 1 .mu.g/ml 48.1 36.4 29.4 CIP2 .mu.g/ml 48.7 31.3 30.8 GSH 10 mM 75.7 107.5 69.8 GSH 15 mM 71.8 93.4 68.7 GSH 30 mM 0.7 12.3 3.9 2 part CT 1.1 6.0 0.7 GSH 30 mM CIP 0.5 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 10: Streptococcus pyogenes (Clinical Isolates)
[0095] The treatments shown in column 1 of Table 10 were tested against three clinical isolates of Enterobacter species. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 9).
TABLE-US-00010 TABLE 10 Streptococcus pyogenes and source 361194 Left 386596 Head 371982 Skin leg boil Wound Wound Control 100.0 100.0 100.0 CIP 4 .mu.g/ml 18.3 49.6 54.3 CIP 8 .mu.g/ml 10.1 46.8 56.5 CIP 12 .mu.g/ml 6.4 39.3 56.1 GSH 10 mM 55.6 67.7 99.8 GSH 15 mM 35.8 42.7 90.2 GSH 30 mM 4.3 22.3 15.4 2 part CT 0.1 11.8 4.8 GSH 30 mM CIP 4 ug/ml Control = no treatment CIP = Ciprofloxacin GHS = glutathione
[0096] The following examples illustrate the efficacy of the triple combination of a biologically acceptable thiol based antioxidant, an antibiotic, along with an enzyme against biofilms formed by organisms other than Pseudomonas (ie non-Pseudomonad organisms). These represent the three part combination therapy (3 part CT).
Example 11: Methicillin Resistant Staphylococcus aureus (Clinical Isolates)
[0097] The treatments shown in column 1 of Table 11 were tested against three strains of methicillin-resistant Staphylococcus aureus. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 10).
TABLE-US-00011 TABLE 11 MRSA 27060 MRSA 30616 MRSA - 0799 Treatment Left leg Chin vesicle DFU Control 100.00 100.00 100.00 CIP 10 .mu.g/ml 42.93 40.01 61.08 CIP 20 .mu.g/ml 25.74 31.38 54.21 CIP30 .mu.g/ml 19.53 24.56 51.60 GSH 10 mM 88.83 73.12 86.73 GSH 15 mM 65.24 58.82 75.03 GSH 30 mM 1.02 4.54 33.12 DNase I 40 U 101.74 108.30 86.56 2 part CT -0.30 0.61 21.83 GSH 30 mM CIP 10 ug/ml 3 part CT 0.00 1.70 17.11 GSH 30 mM CIP 10 ug/ml DNase I Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 12: Methicillin Sensitive Staphylococcus aureus (Clinical Isolate)
[0098] The treatments shown in column 1 of Table 12 were tested against three strains of methicillin-sensitive Staphylococcus aureus. The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 11).
TABLE-US-00012 TABLE 12 MSSA - 34397 MSSA - 34654 MSSA - 0800 Treatment Left elbow Right toe DFU Control 100.00 100.00 100.00 CIP 10 .mu.g/ml 49.93 37.08 41.69 CIP 20 .mu.g/ml 36.78 32.05 32.03 CIP 30 .mu.g/ml 29.81 32.44 21.58 GSH 10 mM 88.67 86.11 82.44 GSH 15 mM 61.34 70.52 61.70 GSH 30 mM 1.06 15.33 20.09 DNase I 40 U 116.55 125.77 83.01 2 part CT 0.77 5.85 9.68 GSH 30 mM CIP10 ug/ml 3 part CT 0.96 3.55 8.24 GSH 30 mM CIP 10 ug/ml DNase I Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 13: Staphylococcus agalactiae (Ex Cow Mastitis)
[0099] The treatments shown in column 1 of Table 13 were tested against four strains of Staphylococcus agalactiae, (veterinary strains ex Cow mastitis). The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 12).
TABLE-US-00013 TABLE 13 S. S. S. S. S. agalactiae agalactiae agalactiae agalactiae agalactiae Treatment # 30 # 44 # 70 # 88 #106 Control 100.00 100.00 100.00 100.00 100.00 CIP 4 .mu.g/ml 74.20 63.40 74.10 71.60 72.50 CIP 8 .mu.g/ml 66.38 66.52 65.26 69.79 70.13 CIP 12 69.29 63.28 58.93 63.77 65.90 .mu.g/ml GSH 10 mM 103.78 86.99 89.17 87.95 102.63 GSH 15 mM 80.69 73.92 79.41 81.62 83.17 GSH 30 mM 35.30 29.16 38.30 25.00 29.20 DNase I 71.50 58.50 70.60 69.60 69.30 40 U 2 part CT (1) 22.30 25.90 24.80 17.80 25.30 GSH 30 mM CIP 4 ug/ml 2 part CT (2) 36.90 28.90 34.20 21.00 28.50 GSH 30 mM DNase 1 40 U 2 part CT (3) 62.87 57.90 62.60 66.60 60.30 CIP 30 mM DNase 1 40 U 3 part CT (1) GSH 10 mM 53.10 47.90 53.10 35.00 44.80 Cip 4 ug/ml DNase 1 40 U 3 part CT (2) GSH 30 mM 20.60 17.60 27.50 16.30 24.00 CIP 10 ug/ml DNase 1 40 U Control = no treatment CIP = Ciprofloxacin GHS = glutathione
Example 14: Multi Drug Resistant Acinetobacter baumannii
[0100] The treatments shown in column 1 of Table 14 were tested against six clinical isolates of Multidrug resistant Acinetobacter Baumannii, (MRAB). The treatments used are given in column 1, and the % bacterial viability for each strain tested are given in the subsequent columns (see also FIG. 13). As these MRAB strains were resistant to ciprofloxacin, Amikacin was used instead.
TABLE-US-00014 TABLE 14 MRAB 1 MRAB 2 MRAB 3 MRAB 4 MRAB 5 MRAB 12 Strain ID 014754 014801 015069 015095 015103 016419 Source Urine Catheter Skin Hospital Hospital Catheter Environ Environ Control 100.00 100.00 100.00 100.00 100.00 100.00 AMI 4 .mu.g/ml 35.91 35.99 66.60 66.36 65.23 49.66 AMI12 .mu.g/ml 24.47 14.11 48.60 42.91 26.85 22.70 AMI20 .mu.g/ml 14.67 8.36 41.70 33.92 24.60 19.80 GSH 10 mM 73.61 70.84 84.56 68.00 65.08 80.83 GSH 15 mM 55.58 41.23 63.18 57.70 51.49 49.05 GSH 30 mM 20.35 2.45 37.70 13.77 16.71 18.41 DNaseI 46.43 43.91 71.60 83.09 89.54 77.12 2 part CT (2) 6.90 1.53 4.69 6.85 10.96 4.30 GSH 30 mM AMI 4 .mu.g/ml 2 part CT (2) 18.61 27.60 29.50 62.75 46.11 39.81 DNase1 40 U AMI 4 .mu.g/ml 2 part CT (3) 9.96 2.51 36.40 5.02 9.30 15.12 GSH 30 mM DNase1 40 U 3 part CT (1) 15.67 12.06 24.20 39.98 49.98 22.24 GSH 10 mM AMI 4 ug/ml DNase1 40 U 3 part CT (2) 3.80 0.17 0.11 4.13 5.20 2.28 GSH 30 mM AMI 10 ug/ml DNase1 40 U Control = no treatment AMI = Amikacin GHS = glutathione
* * * * *
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

D00013

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.