Non-toxic Vehicle To Solubilize, Deliver, And Obtain Biological Activity Of Steroid Hormones At Cell, Tissue, And Organ Targets,
Shaak; Thomas ; et al.
U.S. patent application number 16/557132 was filed with the patent office on 2019-12-26 for non-toxic vehicle to solubilize, deliver, and obtain biological activity of steroid hormones at cell, tissue, and organ targets,. This patent application is currently assigned to Government of the United States as Represented by the Secretary of the Air Force. The applicant listed for this patent is Government of the United States as Represented by the Secretary of the Air Force. Invention is credited to Thomas Shaak, Suizhao Wang.
| Application Number | 20190388349 16/557132 |
| Document ID | / |
| Family ID | 62905385 |
| Filed Date | 2019-12-26 |








View All Diagrams
| United States Patent Application | 20190388349 |
| Kind Code | A1 |
| Shaak; Thomas ; et al. | December 26, 2019 |
NON-TOXIC VEHICLE TO SOLUBILIZE, DELIVER, AND OBTAIN BIOLOGICAL ACTIVITY OF STEROID HORMONES AT CELL, TISSUE, AND ORGAN TARGETS, IN VITRO AND IN VIVO
Abstract
A non-toxic vehicle for hormone therapy. The non-toxic vehicle includes a cationic lipid or a neutral lipid and polyethylene glycol.
| Inventors: | Shaak; Thomas; (Ocean Springs, MS) ; Wang; Suizhao; (Biloxi, MS) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | Government of the United States as
Represented by the Secretary of the Air Force Wright-Patterson AFB OH |
||||||||||
| Family ID: | 62905385 | ||||||||||
| Appl. No.: | 16/557132 | ||||||||||
| Filed: | August 30, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15875637 | Jan 19, 2018 | |||
| 16557132 | ||||
| 62449205 | Jan 23, 2017 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61K 31/568 20130101; A61B 2017/00707 20130101; A61K 2300/00 20130101; G09B 23/34 20130101; A61B 17/32053 20130101; A61M 1/0088 20130101; G09B 23/306 20130101; A61K 31/565 20130101; A61B 10/02 20130101; A61K 47/10 20130101; A61K 47/24 20130101; A61K 31/573 20130101; A61K 9/0019 20130101; A61P 35/00 20180101; A61K 9/1271 20130101; A61K 39/39 20130101 |
| International Class: | A61K 9/127 20060101 A61K009/127; A61K 31/573 20060101 A61K031/573; A61K 31/565 20060101 A61K031/565; A61K 9/00 20060101 A61K009/00; A61P 35/00 20060101 A61P035/00; A61K 31/568 20060101 A61K031/568; A61K 47/10 20060101 A61K047/10; A61K 47/24 20060101 A61K047/24; A61K 39/39 20060101 A61K039/39; A61M 1/00 20060101 A61M001/00; G09B 23/30 20060101 G09B023/30; G09B 23/34 20060101 G09B023/34 |
Goverment Interests
RIGHTS OF THE GOVERNMENT
[0002] The invention described herein may be manufactured and used by or for the Government of the United States for all governmental purposes without the payment of any royalty.
Claims
1. A method of preparing a hormone treatment with a non-toxic vehicle, the method comprising: preparing a first solution comprising an aqueous solution of a hormone and polyethylene glycol 300; preparing a second solution comprising a cationic lipid and polyethylene glycol 300, wherein a concentration of the cationic lipid in the polyethylene glycol 300 is 5%; combining the first solution with the second solution.
2. The method of claim 1, wherein the cationic lipid is selected from the group consisting of N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride; 2,3-dioleyloxy-N[2-(spermine-carboxaindo)ethyl]-N,N-dimethyl-1-- propanaminium; 5-carboxyspermylglycinedioctadecylaminde; N,N-dimethyl-N-ethylcarboxamidochloesterol; and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine.
3. The method of claim 1, wherein the hormone is one or more of estradiol, testosterone, dihydrotestosterone ("DHT"), and a glucocorticoid.
4. The method of claim 1, further comprising: introducing a neutral lipid to the second solution before combining the first and second solutions.
5. The method of claim 4, wherein the neutral lipid is selected from the group consisting of dioleoylphosphatidylethanolamine; palmitoyl-snglycero-phosphoethanolamine; and 1,2-dimyristoyl-snglycero-3-phospho-ethanolamine.
Description
[0001] This application is a continuation of U.S. application Ser. No. 15/875,637, which claims the benefit of and priority to prior filed Provisional Application Ser. No. 62/449,205, filed Jan. 23, 2017. The contents of each application is expressly incorporated herein by reference in its entirety, each in its entirety.
FIELD OF THE INVENTION
[0003] The present invention relates generally to animal models and, more particularly, to animal models for educational and investigational uses.
BACKGROUND OF THE INVENTION
[0004] Steroid hormone therapy is generally considered the treatment with any steroid hormone, such as estrogen, progesterone, androgens, and the like. Such treatments have been found useful in combating the symptoms of menopause, supplementing cancer treatments, and hormone replacement, to name a few. However, the use and application of steroid hormones in hormone therapy has been hampered in conventional methodologies by two difficulties: 1) an inability to dissolve sufficient amounts of steroid hormones in a solvent and 2) a lack of delivery vehicle for which a measurable dose of hormone may be delivered to a target tissue that produces a biological effect or systemic response. While some conventional mechanisms have utilized polyethylene glycol ("PEG") for transmitting the hormone to the target tissue, there are disadvantages to using PEG alone, such as solubility and toxicity.
[0005] As a result, there remains a need for non-toxic vehicles that facilitate the solubility and delivery of small doses of hormone while effectuating a systemic response.
SUMMARY OF THE INVENTION
[0006] The present invention overcomes the foregoing problems and other shortcomings, drawbacks, and challenges of conventional hormone therapy delivery. While the invention will be described in connection with certain embodiments, it will be understood that the invention is not limited to these embodiments. To the contrary, this invention includes all alternatives, modifications, and equivalents as may be included within the spirit and scope of the present invention.
[0007] According to embodiments of the present invention, a non-toxic vehicle for hormone therapy includes a cationic lipid or a neutral lipid and polyethylene glycol.
[0008] Other embodiments of the present invention are directed to a method of preparing a hormone treatment with non-toxic vehicle. The method includes preparing a first solution and a second solution. The first solution includes an aqueous solution of a hormone and polyethylene glycol. The second solution includes a cationic lipid, a neutral lipid, or both, and polyethylene glycol. The prepared first and second solutions are then combined.
[0009] Additional objects, advantages, and novel features of the invention will be set forth in part in the description which follows, and in part will become apparent to those skilled in the art upon examination of the following or may be learned by practice of the invention. The objects and advantages of the invention may be realized and attained by means of the instrumentalities and combinations particularly pointed out in the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The accompanying drawings, which are incorporated in and constitute a part of this specification, illustrate embodiments of the present invention and, together with a general description of the invention given above, and the detailed description of the embodiments given below, serve to explain the principles of the present invention.
[0011] FIGS. 1-3 are a flowcharts illustrating a method of preparing and using an immune system model according to embodiments of the present invention.
[0012] FIG. 4 is an exemplary photograph of 293T cells of normal, distinct phenotype.
[0013] FIG. 5 is an exemplary photograph of 293T cells treated with PEG 300 only.
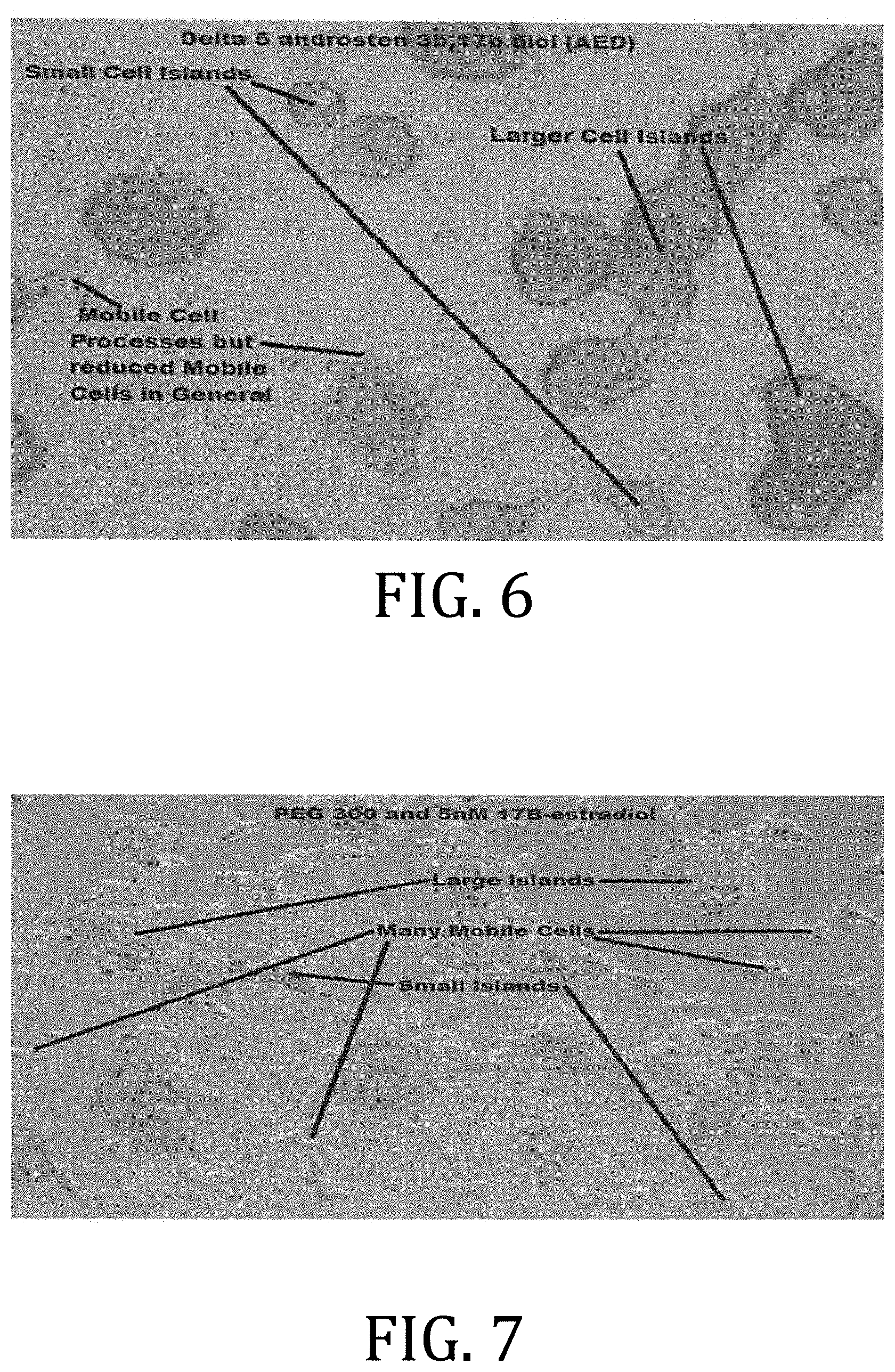
[0014] FIG. 6 is an exemplary photograph of 293T cells treated with PEG 300 and .beta.AED
[0015] FIG. 7 is an exemplary photograph of 293T cells treated with PEG 300 and 17.beta.-estradiol.
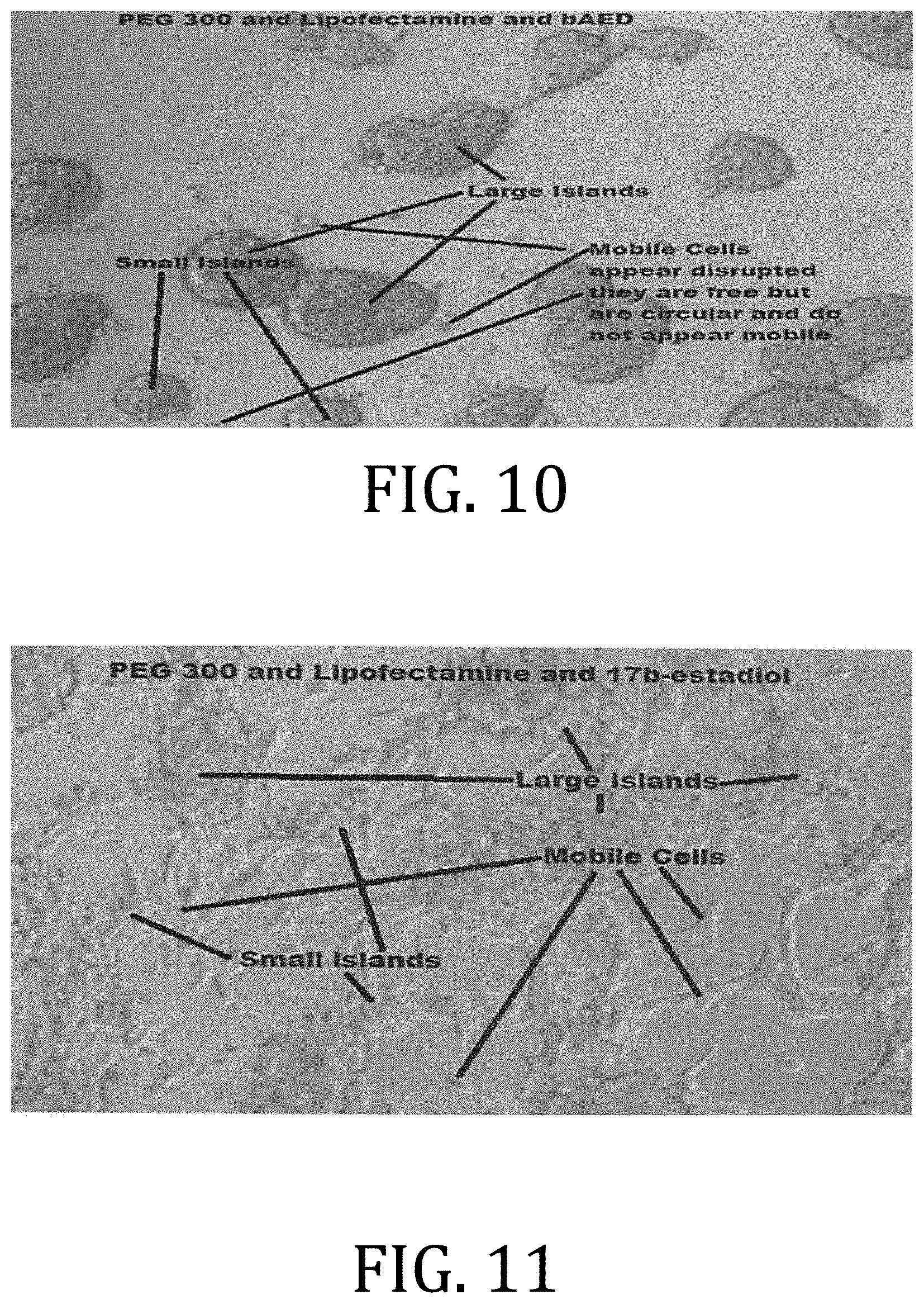
[0016] FIG. 8 is an exemplary photograph of 293T cells treated with PEG 300, .beta.AED, and 17.beta.-estradiol.
[0017] FIG. 9 is an exemplary photograph of 293T cells treated with PEG 300 and LIPOFECTAMINE.
[0018] FIG. 10 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, and .beta.AED
[0019] FIG. 11 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, and 17.beta.-estradiol.
[0020] FIG. 12 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, .beta.AED, and 17.beta.-estradiol.
[0021] FIG. 13 is a graphical representation of a number of mobile cells per field of view of in vitro androstene hormone treatments in 293T cells.
[0022] FIG. 14 is an exemplary photograph of MCF-7 cells treated with a PEG 300 and LIPOFECTAMINE solution, diluted 1:1000.
[0023] FIG. 15 is an exemplary photograph of MCF-7 cells treated with a .beta.-AED, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000.
[0024] FIG. 16 is an exemplary photograph of MCF-7 cells treated with a .beta.-AET, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000.
[0025] FIG. 17 is an exemplary photograph of MCF-7 cells treated with an E2, PEG 300, and LIPOFECTAMINE solution, diluted 1:5000.
[0026] FIG. 18 is an exemplary photograph of MCF-7 cells treated with a .beta.-AED, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000, and a solution of E2, PEG 300, and LIPOFECTAMINE, diluted 1:5000.
[0027] FIG. 19 is an exemplary photograph of MCF-7 cells treated with a .beta.-AET, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000, and a solution of E2, PEG 300, and LIPOFECTAMINE, diluted 1:5000.
[0028] FIG. 20 is an exemplary photograph of MCF-7 cells treated with a .beta.-AET, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000, and a solution of .beta.-AET, PEG 300, and LIPOFECTAMINE, diluted 1:1000.
[0029] FIG. 21 is an exemplary photograph of MCF-7 cells treated with a .beta.-AED, PEG 300, and LIPOFECTAMINE solution, diluted 1:1000, a solution of .beta.-AET, PEG 300, and LIPOFECTAMINE, diluted 1:1000, and a solution of E2, PEG 300, and LIPOFECTAMINE, diluted 1:5000.
[0030] It should be understood that the appended drawings are not necessarily to scale, presenting a somewhat simplified representation of various features illustrative of the basic principles of the invention. The specific design features of the sequence of operations as disclosed herein, including, for example, specific dimensions, orientations, locations, and shapes of various illustrated components, will be determined in part by the particular intended application and use environment. Certain features of the illustrated embodiments have been enlarged or distorted relative to others to facilitate visualization and clear understanding. In particular, thin features may be thickened, for example, for clarity or illustration.
DETAILED DESCRIPTION OF THE INVENTION
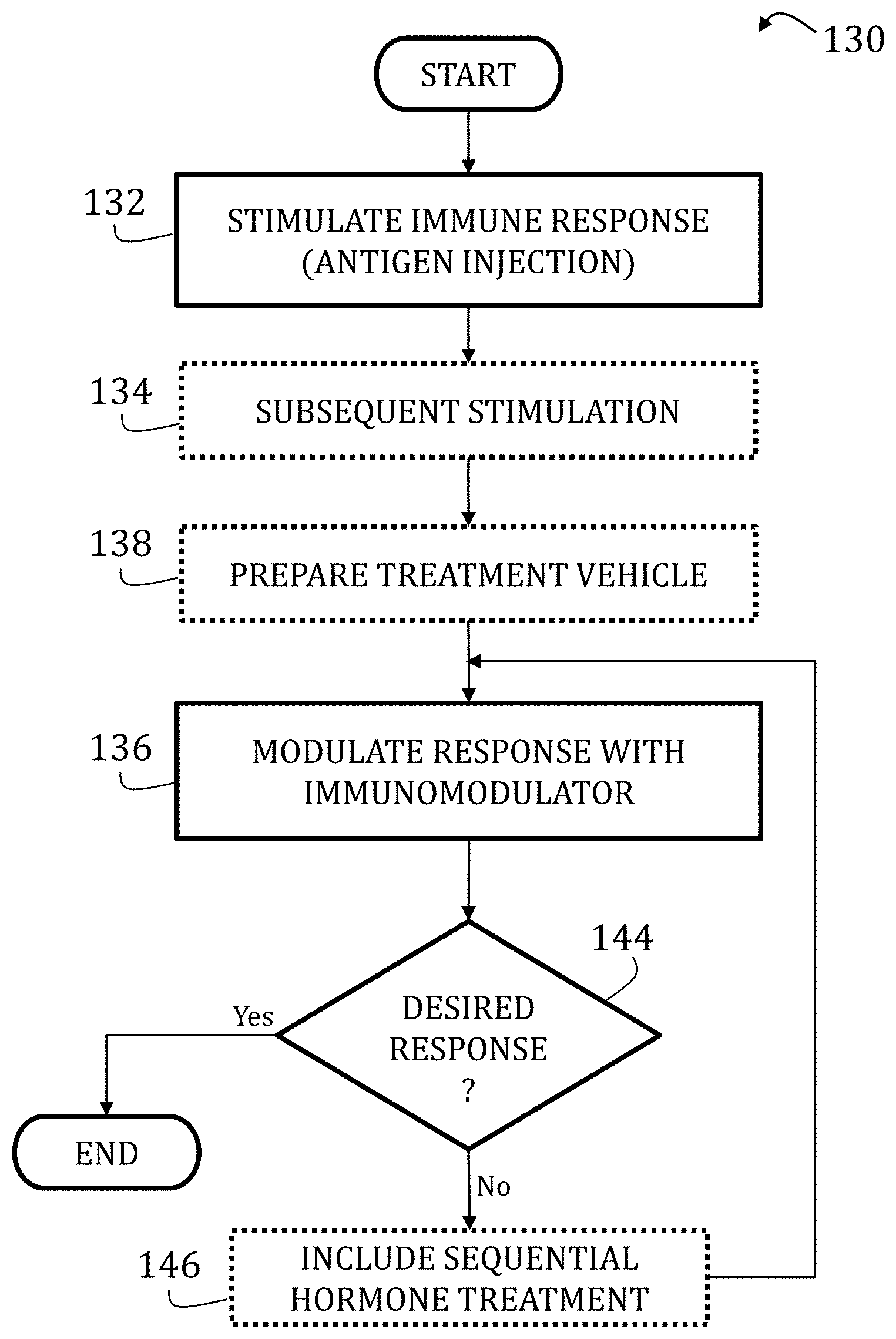
[0031] Turning now to the figures, and in particular to FIG. 1, a flow chart 130 illustrating a method of preparing an immune system model according to an embodiment of the present invention is shown. Again, while not necessary, the illustrated embodiments include S. scrofa; however, other appropriate mammals may be used in the alternative as the specimen.
[0032] At start, an immune response is stimulated (Block 132) by injecting a particular antigen into the specimen. Antigens may include commercially-available bacterial, fungal, or viral specific antigens configured to stimulate the formation of protective antibodies (i.e., a thymic dependent response), such as those found in a vaccine.
[0033] Chronic inflammation conditions include a prolonged and extended period of inflammation. The chronic inflammatory response to would related antigens is marked by increased granulocytes, Th1 T cells, M1 macrophages, and a decreased amount of dendritic cells. More particularly, the thymic immune response includes responses to T cell dependent antigenic proteins, which may include animal, bacterial, viral, or fungal proteins. Non-thymic immune responses include non-specific antigens (non-protein, less specific particles) that may illicit a non-specific immune reaction, such as an allergic response or asthma.
[0034] Systemic baseline conditions before and after stimulating the immune response may extend to the brain, skin, thymus, spleen, liver, adrenals, gonads, lymph nodes, small intestine, small intestine mesentery, and circulatory system.
[0035] Continued stimulation generally depends on the system investigated. Generally, 30 days (or roughly four weeks) are necessary for a primary and immune response to generate antibodies from an antigen exposure. A secondary response may be obtained by reinjecting the antigen after the 30 day primary response. Accordingly, the desired response may be achieved by a first stimulation in the first 30 days and, optionally depending on the phenome to be investigated, at least one subsequent stimulation every 30 days (or approximately every four weeks) (Block 134).
[0036] With desired response stimulated, modulation of the immune response may be initiated (Block 136). Immunomodulators include those chemical agents that modify the immune response by stimulating antibody formation or inhibiting white blood cell activity. According to embodiments of the present invention, immunomodulators may include one or more of delta 5-androsten-3B,17B-diol (.DELTA..sup.5-diol) and delta 5-androsten-3B, 7B,17B-triol (.DELTA..sup.5-triol), and further optionally with one or more of estradiol, testosterone, and dihydrotestosterone ("DHT").
[0037] If desired, delivery of the immunomodulator may be assisted, according to one embodiment of the present invention, by preparing a non-toxic vehicle (Block 138). Briefly, embodiments of the present invention overcome previous difficulties of steroid hormone solubility and delivery of measureable doses to produce biological effects. Accordingly, and as illustrated in the flow chart 138 of FIG. 2, the non-toxic vehicle includes preparation of a composition comprising a cationic or neutral lipid and polyethylene glycol ("PEG") (Block 140). Suitable cationic and neutral lipids may include, for example, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride ("DOTMA"); dioleoylphosphatidylethanolamine ("DOPE"); 2,3-dioleyloxy-N-[2-(spermine-carboxaindo)ethyl]-N,N-dimethyl-1-propanami- nium ("DOSPA"); 5-carboxyspermylglycinedioctadecylaminde ("DOGS"); N,N-dimethyl-N-ethylcarboxamidochloesterol ("DC-Chol"); 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine ("DPPE"); palmitoyl-sn-glycero-phosphoethanolamine ("PPE"); and 1,2-dimyristoyl-snglycero-3-phospho-ethanolamine ("DMPE").
[0038] Cellular membranes generally comprise a phospholipid bilayer such that outer surfaces of the cellular membrane comprise the hydrophilic head groups of the phospholipids with the hydrophobic tails directed centrally between the outer surfaces. Cationic and neutral lipids may assist in trafficking hormone to and across the cellular membrane. Suitable cationic and neutral lipid solutions may include, for example, the commercially-available LIPOFECTAMINE (ThermoFisher Scientific Inc., Waltham, Mass.) or any appropriate PEG formulation in any weight may be used, including but not limited to, PEG 200, PEG 300, PEG 1500, and so forth.
[0039] The liposomal formulation may be prepared in an aqueous solution comprising two solutions. In a first solution, the immunomodulator (.beta.-AED (Steraloids, Inc., Newport, R.I.) or (.beta.-AET (Steraloids, Inc.)) is dissolved in a polyester (for example, PEG), the latter of which may be pre-warmed. In a second solution, a dilution of the cationic and neutral lipid, for example, a 3:1 mixture of cationic to neutral lipid (DOSPA:DOPE) as in LIPOFECTAMINE, may be introduced into a polyester, mixed, and incubated (incubation may be at 42.degree. C. for 30 min). The first and second solutions may then be combined.
[0040] A solution comprising the immunomodulator may then be introduced to the liposomal formulation (Block 142). Evidence of undissolved hormone may include the presence of crystals in in vitro cultures. If crystals are present, then the solution may be warned to above 42.degree. C.
[0041] While not wishing to be bound by theory, it is thought that the cationic liposome formulation provides a solvation and delivery base. While PEG has the ability to, alone, dissolve and deliver hormones, when cationic liposome formulations are used, the PEG solution is diluted, improving solubility, and decreasing toxicity.
[0042] Returning to FIG. 1, the immunomodulator, with or without the treatment vehicle of Block 138, may administered directly or indirectly through the mandibular gland, the thoracic duct, or through the lymphatic drainage system. Alternatively, the immunomodulator may be subcutaneously injected in, or delivered by a transdermal patch applied to, an anterior portion of the patient where lymph vessels of the thoracic limb drain to various lymph nodes (mandibular, parotid, accessory mandibular, lateral retropharyngical, ventral superficial cervical, dorsal superficial cervical, and axillares primae costae (costo-axillary)). Alternatively still, the immunomodulator may be subcutaneously injected in, or delivered by a transdermal patch applied to, a posterior portion of the patient where lymph vessels of the abdominal wall, pelvic wall, and pelvic limbs drain to various lymph nodes (subiliac, superficial inguinal, accessory superficial inguinal, superficial popliteal, deep popliteal, external sacral, anterior sacral, medial iliacs, and lateral iliac).
[0043] Generally, only one application (whether subcutaneous injection or transdermal patch) is required for immunomodulation ("Yes" branch of Decision Block 144). However, it would be appreciated by those having ordinary skill in the art and the benefit of the disclosure made herein that other embodiment of the invention of immunomodulatory schemes or other combinations of immunomodulators may be induced by one subcutaneous injection or transdermal application.
[0044] The initial dosage of immunomodulatory (Block 136), or any subsequent dosage ("No" branch of Decision Block 144) may require sequential hormone treatments (Block 146) with a repeat dose of the immunomodulatory. For example, hydrocortisone followed by an application of 37 ng/kg or higher of androst-5-ene-3.beta.,17.beta.-diol (".beta.-AED") or 5-androstene-3.beta.,7.beta.,17.beta.-triol (".beta.-AET"). Still other combinations with additional hormones, such as testosterone or estrogen, may be used to achieve a desired method of immunomodulation.
[0045] Referring now to FIG. 3, a flowchart illustrating a method 150 for causing cellular death of triple-negative breast cancer cells by hormone modulation is described according to an embodiment of the present invention is shown. Triple-negative breast cancer comprises a heterogeneous subset of breast cancer-types. While accounting for 15% to 25% of all breast cancer cases, the triple negative cancers are not supported by estrogen and progesterone because the cells are ER and PR receptor negative and have an increased number of HER2 receptors.
[0046] At start, a combination of adrenal hormones, including .DELTA.5-androsten-3.beta.,17.beta.-diol and .DELTA.5-androsten-3.beta.,7.beta.,17.beta.-triol (both at a concentration of about 25 .mu.M) with or without estradiol ("E2") is prepared (at a concentration of about 5 nM) (Block 152). The adrenal hormone solutions may be prepared and administered separately or together.
[0047] Optionally, the preparation may include a suitable vehicle (Block 154), such as PEG, ethanol, dimethylsulfoxide ("DMSO"), or the PEG-LIPOFECTAMINE vehicle according to other embodiments of the present invention, for example as described with reference to FIG. 2. In such embodiments, the respective adrenal hormone solution may be mixed with the cationic or neutral lipid and polyethyleneglycol.
[0048] In vitro treatment may include applying the adrenal hormones with vehicle to a cell growth medium. In vivo treatment may be injected directly into a tumor, applied to an area from which a tumor was surgically removed, or injected subcutaneously.
[0049] Only one application (whether subcutaneous injection or transdermal patch) is required for immunomodulation ("Yes" branch of Decision Block 156). However, it would be appreciated by those having ordinary skill in the art and the benefit of the disclosure made herein that other embodiment of the invention of immunomodulatory schemes or other combinations of immunomodulators may be induced by one subcutaneous injection or transdermal application.
[0050] The initial dosage of immunomodulatory (Block 154), or any subsequent dosage ("No" branch of Decision Block 156) may require sequential hormone treatments (Block 158) with a repeat dose of the immunomodulatory. For example, hydrocortisone, testosterone, or estrogen, may be used to achieve a desired method of immunomodulation.
[0051] Although not specifically illustrated herein, it would be understood that the various embodiments of the treatment vehicle described herein need not be limited to delivery of an immunomodulator or in the modeling of an immune response. Accordingly, the treatment vehicle may be used, when appropriate or desired, to achieve delivery of hormone, steroid, or the like to achieve a desired physiological effect that would otherwise difficult to achieve.
[0052] Preparation and delivery of hormone in according to embodiments as describe herein provide the benefit in that the treated cultures are not morphologically or phenotypically changed as compared to control cells. Full dissolution of the hormone reduces toxicity and reduces interference with physiological effects of the hormones. Moreover, use of the non-toxic vehicle reduces component residue at the point of application, allowing for more precise measurement of dosage administration.
[0053] The following examples illustrate particular properties and advantages of some of the embodiments of the present invention. Furthermore, these are examples of reduction to practice of the present invention and confirmation that the principles described in the present invention are therefore valid but should not be construed as in any way limiting the scope of the invention.
EXAMPLE 1
[0054] A non-toxic vehicle for administration of androstene hormones according to embodiments of the present invention was prepared from a first solution and a second solution. The first solution, comprising liposomal formulation, was placed in PEG 300 to a concentration of 5% and heated in a sonicating water bath to about 45.degree. C. The second solution, comprising a selected hormone (one or more of 5.DELTA.-androsten-3.beta., 17.alpha.-diol, 5.DELTA.-androsten-3.beta., 17.beta.-diol, or 5.DELTA.-androsten-3.beta.,7.beta.,17.beta.-triol), was placed in PEG 300 and heated in a sonicating water bath to about 45.degree. C. The first and second solutions were then combine and stirred until dissolved.
[0055] Biological function was tested in 293T cells, which is a human embryonic cell line transformed with Large T antigen or SV40. This hypotriploid (polyploid) cell line is particularly useful in such transformation studies and has very distinct phenotypes when grown. All androstene hormone treatment applications were at concentrations of 25 .mu.M.
[0056] FIG. 4 is an exemplary photograph of 293T cells of normal, distinct phenotype. There are small cell islands, large cell islands, and mobile cells/cell processes.
[0057] FIG. 5 is an exemplary photograph of 293T cells treated with PEG 300 only. The cells demonstrate the same characteristics as the normal cells of FIG. 3.
[0058] FIG. 6 is an exemplary photograph of 293T cells treated with PEG 300 and .beta.AED. Resultant cell islands are larger and there is a significant reduction in mobile cells.
[0059] FIG. 7 is an exemplary photograph of 293T cells treated with PEG 300 and 17.beta.-estradiol. The culture includes many more cell processes as compared to estradiol alone.
[0060] FIG. 8 is an exemplary photograph of 293T cells treated with PEG 300, .beta.AED, and 17.beta.-estradiol. There is a visible decrease in mobile cells over the treatment with .beta.AED alone.
[0061] FIG. 9 is an exemplary photograph of 293T cells treated with PEG 300 and LIPOFECTAMINE, the latter of which increased granularity. Otherwise, cells were similar to the Blanks, despite presence of PEG 300.
[0062] FIG. 10 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, and .beta.AED. The treatment combination demonstrated a drastic decrease in, or morphological change, in mobile elements.
[0063] FIG. 11 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, and 17.beta.-estradiol. The combination produced many large islands with mobile processes, small islands with mobile process, and mobile cells forming interconnections with other elements.
[0064] FIG. 12 is an exemplary photograph of 293T cells treated with PEG 300, LIPOFECTAMINE, .beta.AED, and 17.beta.-estradiol. As compared to cells of FIG. 11, mobile cells and processes are largely non-existent.
[0065] FIG. 13 is a graphical representation of a number of mobile cells per field of view of in vitro androstene hormone treatments in 293T cells, described above, wherein column A is a blank, column B is PEG 300 with LIPOFECTAMINE, column C is PEG 300 with LIPOFECTAMINE with 25 .mu.M .beta.-AED, column D is PEG 300 with LIPOFECTAMINE with 5 nM E2, column E is PEG 300 with LIPOFECTAMINE with 25 .mu.M .beta.-AED and 5 nM E2, column F is PEG 300 with LIPOFECTAMINE, column G is PEG 300 with LIPOFECTAMINE and 5 nM E2, column H is PEG 300 with LIPOFECTAMINE and 25 .mu.M .beta.-AED, and column I is PEG 300 with LIPOFECTAMINE, 25 .mu.M .beta.-AED, and 5 nM E2.
EXAMPLE 2
[0066] Hormone dependent, noninvasive, epithelial phenotype (ER/PR positive MCF-7 breast cancer cells) and hormone independent, invasive, mesenchymal phenotype (ER/PR negative MDA-MB-231 breast cancer cell) cell suspensions were acquired and prepared in complete medium (DMEM/F12, 10% FBS, 1% penicillin/streptomycin (Gibco)). Cellular concentration was adjusted to 2.times.10.sup.5 cells/mL.
[0067] Androstene hormone solutions according to embodiments of the present invention were prepared from a first solution and a second solution. For the first solution, 3.0 mg of .beta.-AED or .beta.-AET was added to warm PEG 300 to prepare 50 mM stock. For the second solution, 10 .mu.L of LIPOFECTAMINE (3:1 DOSPA: DOPE) was introduced to 100 .mu.L of PEG 300, mixed, and incubated in a water bath at 42.degree. C. for 30 min. The first and second solutions were then mixed in a sonicator water bath at 42.degree. C. for 1 hr. The final concentration was 50 mM .beta.-AED (or AET, as used).
[0068] A .beta.-estradiol solution was also prepared by adding 2.7 mg of .beta.-estradiol to 200 .mu.L warmed PEG 300 with LIPOFECTAMINE and then further diluted 1:1000 with PEG-300. The final concentration was 50 .mu.M.
[0069] A diluted .beta.-AED (or .beta.-AET) solution was prepared with complete medium at a final concentration of 50 .mu.M (1:1000 dilute of 50 mM). Likewise, a diluted .beta.-E2 solution was prepared with complete medium at a final concentration of 10 nM (1:5000 dilute of 50 .mu.M).
[0070] Using a 24-well plate, mixtures of 0.5 mL of cells per well with 0.5 mL of medium diluted hormone vehicle. Cell cultures were maintained at 90% humidity, 7% CO.sub.2, and 37.degree. C.
[0071] Results are provided in tabular format below:
TABLE-US-00001 TABLE 1 MDA-MB-231 MCF7 Cell Death Cell Death Additional Treatment Amount Amount Observation .beta.-AED Not observed Not observed Cell particles observed .beta.-AED + E2 70% Not observed Cell particles observed .beta.-AED + .beta.-AET 80% Not observed Cell particles observed .beta.-AED + .beta.-AET + E2 90% Not observed Cell particles observed .beta.-AET Not observed Not observed N/A E2 Not observed Not observed N/A
[0072] The MDA-MB-231 cell cultures treated with (1) 25 .mu.M .beta.-AED and 5 nM E2, (2) 25 .mu.M .beta.-AED and 25 .mu.M AET, or (3) 25 .mu.M .beta.-AED, 25 .mu.M AET and 5 nM E2 yielded cell death rates of 70%, 80%, and 90%, respectively. Cell death was not observed in MCF7 cell cultures treated with the same doses.
[0073] FIG. 14 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000.
[0074] FIG. 15 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M. As compared to FIG. 14, the cell density of FIG. 15 is not as heath and there are areas of decreased density. FIG. 15 also presents small particles (identified by arrows) that were produced by cells in response to the treatment with .beta.-AED
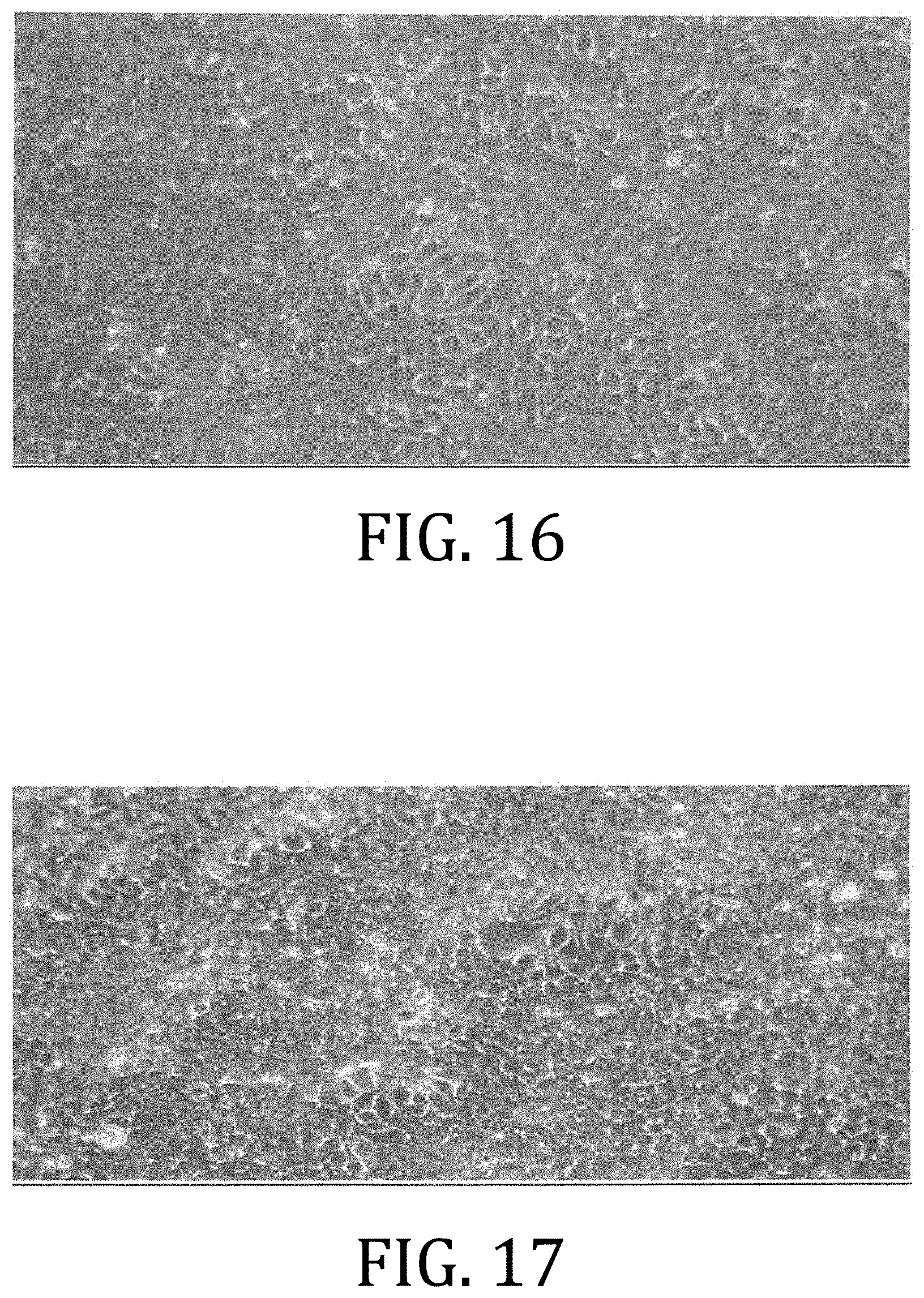
[0075] FIG. 16 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AET in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M. The cell growth is thick and similar to the cell growth of FIG. 14.
[0076] FIG. 17 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 .mu.M solution of E2 in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:5000 to a concentration of 5 nM. The cell growth is thick and similar to the cell growth of FIGS. 14 and 16.
[0077] FIG. 18 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M and 25 .mu.M stock solution of E2 in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:5000 to a concentration of 5 nM. The cell growth is thick and similar to the cell growth of FIGS. 14, 16, and 17.
[0078] FIG. 19 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AET in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M and 25 .mu.M stock solution of E2 in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:5000 to a concentration of 5 nM. The cell growth is thick and similar to the cell growth of FIGS. 14 and 16-18.
[0079] FIG. 20 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M and 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M. The cell growth is thick and similar to the cell growth of FIGS. 14 and 16-19.
[0080] FIG. 21 is a microscopic photograph of MCF-7 cells grown for 5 days in a cell medium comprising 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M, 25 mM stock solution of .beta.-AED in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:1000 to a concentration of 25 .mu.M, and 25 .mu.M stock solution of E2 in PEG 300 with 5% LIPOFECTAMINE solution diluted 1:5000 to a concentration of 5 nM. While the figure presents a full growth of cells, the cell layer is not as thick as was presented in FIGS. 14 and 16-19. The treatment caused the cells to produce small cell bodies.
[0081] While the present invention has been illustrated by a description of one or more embodiments thereof and while these embodiments have been described in considerable detail, they are not intended to restrict or in any way limit the scope of the appended claims to such detail. Additional advantages and modifications will readily appear to those skilled in the art. The invention in its broader aspects is therefore not limited to the specific details, representative apparatus and method, and illustrative examples shown and described. Accordingly, departures may be made from such details without departing from the scope of the general inventive concept.
* * * * *
D00000

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.