Methods For Treating Or Preventing Ophthalmological Conditions
Patel; Samir ; et al.
U.S. patent application number 16/434018 was filed with the patent office on 2019-12-19 for methods for treating or preventing ophthalmological conditions. This patent application is currently assigned to IVERIC bio, Inc.. The applicant listed for this patent is IVERIC bio, Inc.. Invention is credited to Douglas Brooks, Richard Everett, Samir Patel, Shane Xinxin Tian.
| Application Number | 20190381087 16/434018 |
| Document ID | / |
| Family ID | 52277261 |
| Filed Date | 2019-12-19 |


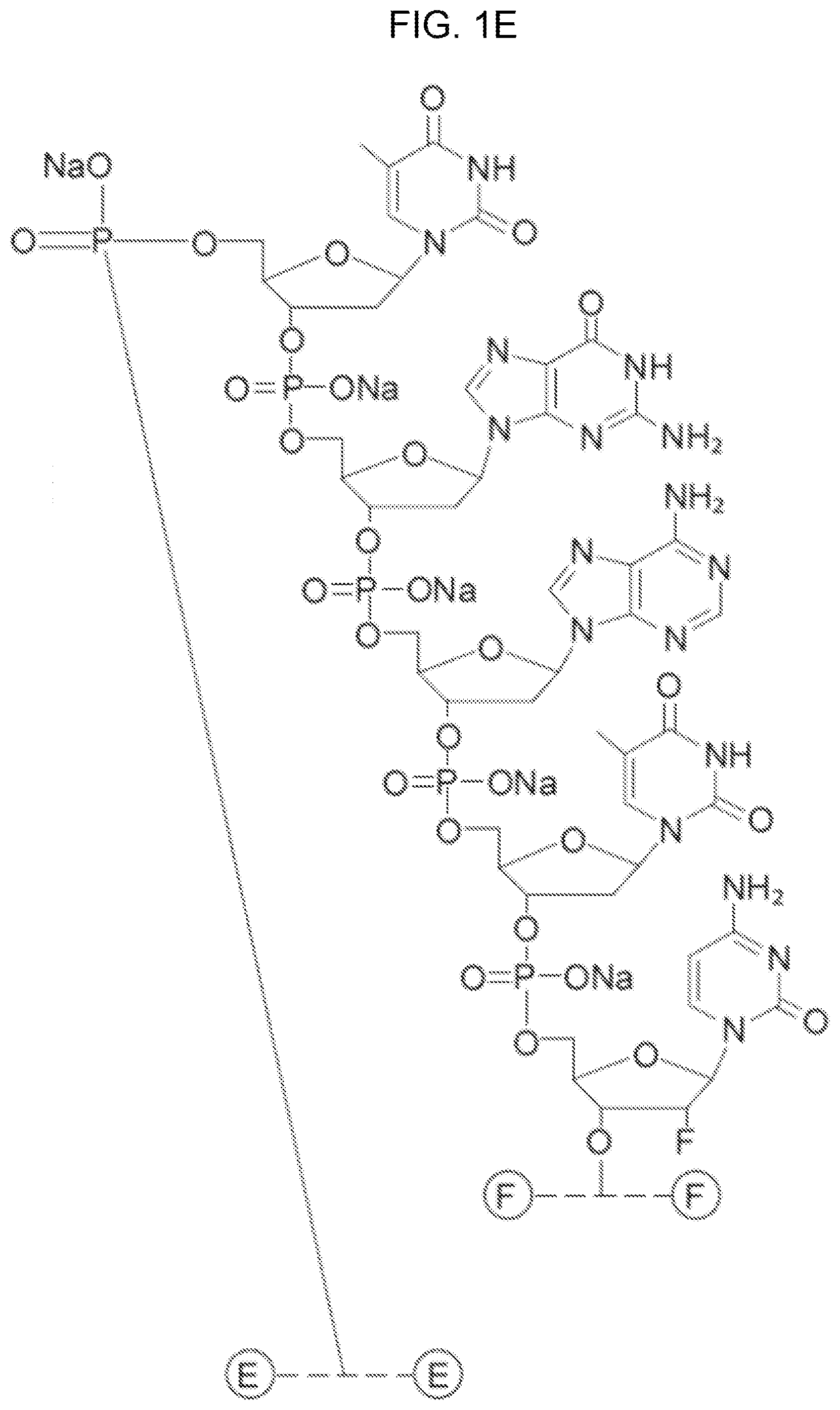

View All Diagrams
| United States Patent Application | 20190381087 |
| Kind Code | A1 |
| Patel; Samir ; et al. | December 19, 2019 |
METHODS FOR TREATING OR PREVENTING OPHTHALMOLOGICAL CONDITIONS
Abstract
The present invention relates to methods for treating and preventing ophthalmological disease and disorders, comprising administering Antagonist A or another pharmaceutically acceptable salt thereof, optionally in combination with another treatment, to a subject in need thereof. The present invention also relates to methods for treating and preventing ophthalmological disease and disorders, comprising administering an anti-C5 agent (e.g., ARC1905), optionally in combination with another treatment, to a subject in need thereof.
| Inventors: | Patel; Samir; (Princeton, NJ) ; Everett; Richard; (Randolph, NJ) ; Brooks; Douglas; (Durham, NC) ; Tian; Shane Xinxin; (Oakland, NJ) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | IVERIC bio, Inc. New York NY |
||||||||||
| Family ID: | 52277261 | ||||||||||
| Appl. No.: | 16/434018 | ||||||||||
| Filed: | June 6, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15144429 | May 2, 2016 | |||
| 16434018 | ||||
| 14329702 | Jul 11, 2014 | |||
| 15144429 | ||||
| 61931125 | Jan 24, 2014 | |||
| 61931116 | Jan 24, 2014 | |||
| 61931135 | Jan 24, 2014 | |||
| 61926848 | Jan 13, 2014 | |||
| 61926825 | Jan 13, 2014 | |||
| 61926812 | Jan 13, 2014 | |||
| 61911894 | Dec 4, 2013 | |||
| 61911854 | Dec 4, 2013 | |||
| 61911860 | Dec 4, 2013 | |||
| 61866503 | Aug 15, 2013 | |||
| 61866502 | Aug 15, 2013 | |||
| 61866507 | Aug 15, 2013 | |||
| 61845938 | Jul 12, 2013 | |||
| 61845936 | Jul 12, 2013 | |||
| 61845935 | Jul 12, 2013 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 25/00 20180101; C07K 2317/76 20130101; C12N 2310/322 20130101; C07K 16/22 20130101; A61P 9/10 20180101; A61K 31/7088 20130101; A61P 9/00 20180101; A61K 9/0048 20130101; C12N 2320/31 20130101; A61P 27/02 20180101; C12N 2310/321 20130101; C12N 2310/317 20130101; A61P 35/00 20180101; C12N 2310/16 20130101; A61K 39/3955 20130101; C07K 2317/55 20130101; C12N 2320/30 20130101; C12N 15/115 20130101; A61K 45/06 20130101; A61P 43/00 20180101; C12N 2310/314 20130101; A61K 31/713 20130101; C12N 2310/351 20130101; A61K 9/143 20130101; C07K 2317/24 20130101; A61K 39/3955 20130101; A61K 2300/00 20130101; C12N 2310/322 20130101; C12N 2310/3533 20130101; C12N 2310/321 20130101; C12N 2310/3521 20130101; A61K 31/713 20130101; A61K 2300/00 20130101 |
| International Class: | A61K 31/7088 20060101 A61K031/7088; A61K 31/713 20060101 A61K031/713; A61K 39/395 20060101 A61K039/395; C12N 15/115 20060101 C12N015/115; A61K 45/06 20060101 A61K045/06; A61K 9/00 20060101 A61K009/00; C07K 16/22 20060101 C07K016/22; A61K 9/14 20060101 A61K009/14 |
Claims
1. A method for treating or preventing wet age-related macular degeneration (wet AMD), comprising administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein (a) and (b) are administered in an amount that is effective for treating or preventing wet AMD, and wherein the administering occurs once every month, .+-.about seven days, for a first administration period of at least 3 consecutive months, followed by administering (a) and (b) for a second administration period at a frequency of at least every other month .+-.about seven days beginning at two months .+-.about seven days after the day of the last month of the first administration period on which (a) and (b) are administered.
2. The method of claim 1, wherein (a) and (b) are administered within about 60 minutes of each other.
3. The method of claim 1, wherein the VEGF antagonist is ranibizumab, bevacizumab, pegaptanib sodium, ESBA 1008 or aflibercept.
4. The method of claim 1, wherein the VEGF antagonist is ranibizumab or bevacizumab, wherein (a) and (b) are administered at a frequency of once every month .+-.about seven days during the second administration period and wherein the second administration period is at least about nine months.
5. The method of claim 4, further comprising measuring the subject's visual acuity.
6. The method of claim 5, further comprising administering to the subject (a) and (b) in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity on the last two of any three consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity on the first of the three consecutive months.
7. The method of claim 5, further comprising administering to the subject (a) and (b) every other month in an amount that is effective for treating or preventing wet AMD, wherein the subject's visual acuity on the last two of any three consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity on the first of the three consecutive months.
8. The method of claim 7, further comprising administering to the subject (a) and (b) every month in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity on the last two of any three consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity on the first of the three consecutive months.
9. The method of claim 1, wherein the VEGF antagonist is aflibercept.
10. The method of claim 1, wherein the total number of months does not exceed 24.
11. The method of claim 4, wherein the subject has intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid at one month, .+-.about seven days, immediately following the second administration period.
12. The method of claim 11, further comprising: administering to the subject on each month .+-.about seven days, beginning on the month that immediately follows the second administration period (a) and (b) in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity on the last two of any three consecutive months that follow the 12 consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity on the first of the three consecutive months.
13. The method of claim 12, wherein the total number of months does not exceed 24.
14. The method of claim 1, wherein Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally in an amount of about 1.5 mg/eye.
15. The method of claim 4, wherein the VEGF antagonist is bevacizumab and is administered intravitreally in an amount of about 1.25 mg/eye.
16. The method of claim 9, wherein the VEGF antagonist is administered intravitreally in an amount of about 2 mg/eye.
17. The method of claim 4, wherein the VEGF antagonist is ranibizumab and is administered intravitreally in an amount of about 0.5 mg/eye.
18. The method of claim 1, further comprising administering an anti-C5 agent.
19. The method of claim 1, further comprising administering (a) and (b) on a month in which the subject has intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid.
20. A method for treating or preventing sub-retinal fibrosis, comprising administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof in an amount that is effective for treating or preventing sub-retinal fibrosis.
21. The method of claim 20, further comprising administering to the subject (b) a VEGF antagonist, wherein (a) and (b) are administered in an amount that is effective for treating or preventing sub-retinal fibrosis.
22. The method of claim 20, wherein the subject has wet age-related macular degeneration (wet AMD).
23. The method of claim 22, wherein the sub-retinal fibrosis is associated with the wet AMD.
24. The method of claim 20, wherein administering Antagonist A or another pharmaceutically acceptable salt thereof results in a decrease in the size of sub-retinal hyper-reflective material (SHRM) as evidenced by spectral domain optical coherence tomography (SD-OCT) or results in stabilization of the subject's vision.
25. The method of claim 20, wherein Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally in an amount of about 1.5 mg/eye.
26. The method of claim 21, wherein the VEGF antagonist is bevacizumab, ranibizumab, aflibercept, pegaptanib sodium or ESBA1008.
27. The method of claim 26, wherein the VEGF antagonist is bevacizumab and is administered intravitreally in an amount of about 1.25 mg/eye.
28. The method of claim 26, wherein the VEGF antagonist is aflibercept and is administered intravitreally in an amount of about 2 mg/eye.
29. The method of claim 26, wherein the VEGF antagonist is ranibizumab and is administered intravitreally in an amount of about 0.5 mg/eye.
30. The method of claim 21, further comprising administering an anti-C5 agent.
31. A method for treating or preventing von Hippel-Lindau (VHL) disease, comprising administering to a subject in need thereof Antagonist A or another pharmaceutically acceptable salt thereof in an amount that is effective for treating or preventing VHL disease.
32. The method of claim 31, further comprising administering a VEGF antagonist.
33. The method of claim 31, wherein Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally in an amount of about 1.5 mg/eye.
34. The method of claim 32, wherein the VEGF antagonist is bevacizumab and is administered intravitreally in an amount of about 1.25 mg/eye.
35. The method of claim 32, wherein the VEGF antagonist is aflibercept and is administered intravitreally in an amount of about 2 mg/eye.
36. The method of claim 32, wherein the VEGF antagonist is ranibizumab and is administered intravitreally in an amount of about 0.5 mg/eye.
37. The method of claim 32, further comprising administering an anti-C5 agent.
Description
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation of U.S. application Ser. No. 14/329,702, filed Jul. 11, 20114, which claims the benefit of U.S. provisional application nos. 61/845,938, filed Jul. 12, 2013, 61/845,935, filed Jul. 12, 2013, 61/845,936, filed Jul. 12, 2013, 61/866,502, filed Aug. 15, 2013, 61/866,503, filed Aug. 15, 2013, 61/866,507, filed Aug. 15, 2013, 61/911,854, filed Dec. 4, 2013, 61/911,860, filed Dec. 4, 2013, 61/911,894, filed Dec. 4, 2013, 61/926,812, filed Jan. 13, 2014, 61/926,825, filed Jan. 13, 2014, 61/926,848, filed Jan. 13, 2014, 61/931,116, filed Jan. 24, 2014, 61/931,125, filed Jan. 24, 2014, and 61/931,135, filed Jan. 24, 2014, each of which is incorporated by reference herein in its entirety.
SEQUENCE LISTING
[0002] The Sequence Listing associated with this application is provided in text format in lieu of a paper copy, and is hereby incorporated by reference into the specification. The name of the text file containing the Sequence Listing is OPHT_012_06US_SeqList_ST25.txt. The text file is about 372 KB, was created on Jul. 10, 2014, and is being submitted electronically via EFS-Web.
FIELD OF THE INVENTION
[0003] This invention relates to methods and compositions useful for the treatment or prevention of an ophthalmological disease or disorder, comprising administration of an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof.
BACKGROUND OF THE INVENTION
[0004] Various disorders of the eye are characterized, caused by, or result in choroidal, retinal or iris neovascularization or retinal edema. One of these disorders is macular degeneration. Age-related macular degeneration (AMD) is a disease that affects approximately one in ten Americans over the age of 65. One type of AMD, "wet-AMD," accounts only for approximately 10% of age-related macular degeneration cases but results in approximately 90% of cases of legal blindness from macular degeneration in the elderly. Another disorder of the eye is diabetic retinopathy. Diabetic retinopathy can affect up to 80% of all patients having diabetes for 10 years or more and is the third leading cause of adult blindness, accounting for almost 7% of blindness in the USA. Other disorders include hypertensive retinopathy, central serous chorioretinopathy, cystoid macular edema, Coats disease and ocular or adnexal neoplasms such as choroidal hemangioma, retinal pigment epithelial carcinoma, retinal vein occlusions and intraocular lymphoma.
[0005] Therefore, although advances in the understanding of the molecular events accompanying neovascularization have been made, there exists a need to utilize this understanding to develop improved methods for treating or preventing neovascular diseases disorders, including ocular neovascular diseases and disorders such as the neovascularization that occurs with AMD, diabetic retinopathy, and retinal vein occlusions.
SUMMARY OF THE INVENTION
[0006] The present invention relates to methods and compositions useful for the treatment or prevention of an ophthalmological disease or disorder.
[0007] The present invention provides a method for treating or preventing wet age-related macular degeneration (wet AMD), comprising administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein (a) and (b) are administered in an amount that is effective for treating or preventing wet AMD, and wherein the administering occurs once every month, .+-.about seven days, for a first administration period of at least 3 consecutive months, followed by administering (a) and (b) for a second administration period at a frequency of at least every other month .+-.about seven days beginning at two months .+-.about seven days after the day of the last month of the first administration period on which (a) and (b) are administered.
[0008] Also provided herein is a method for treating or preventing sub-retinal fibrosis, comprising administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof in an amount that is effective for treating or preventing sub-retinal fibrosis.
[0009] A method for treating or preventing von Hippel-Lindau (VHL) disease, comprising administering to a subject in need thereof Antagonist A or another pharmaceutically acceptable salt thereof in an amount that is effective for treating or preventing VHL disease is also provided herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Reference is made to the following detailed description, which sets forth illustrative embodiments and the accompanying drawings of which:
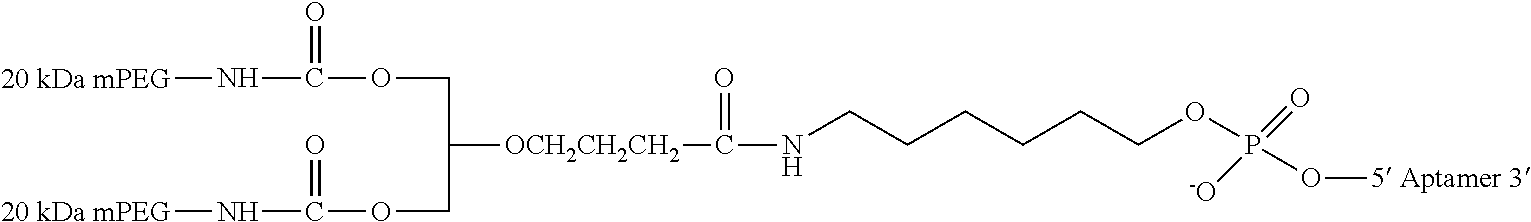
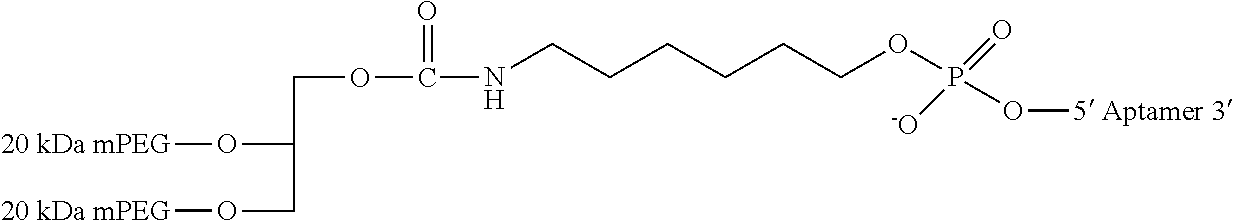
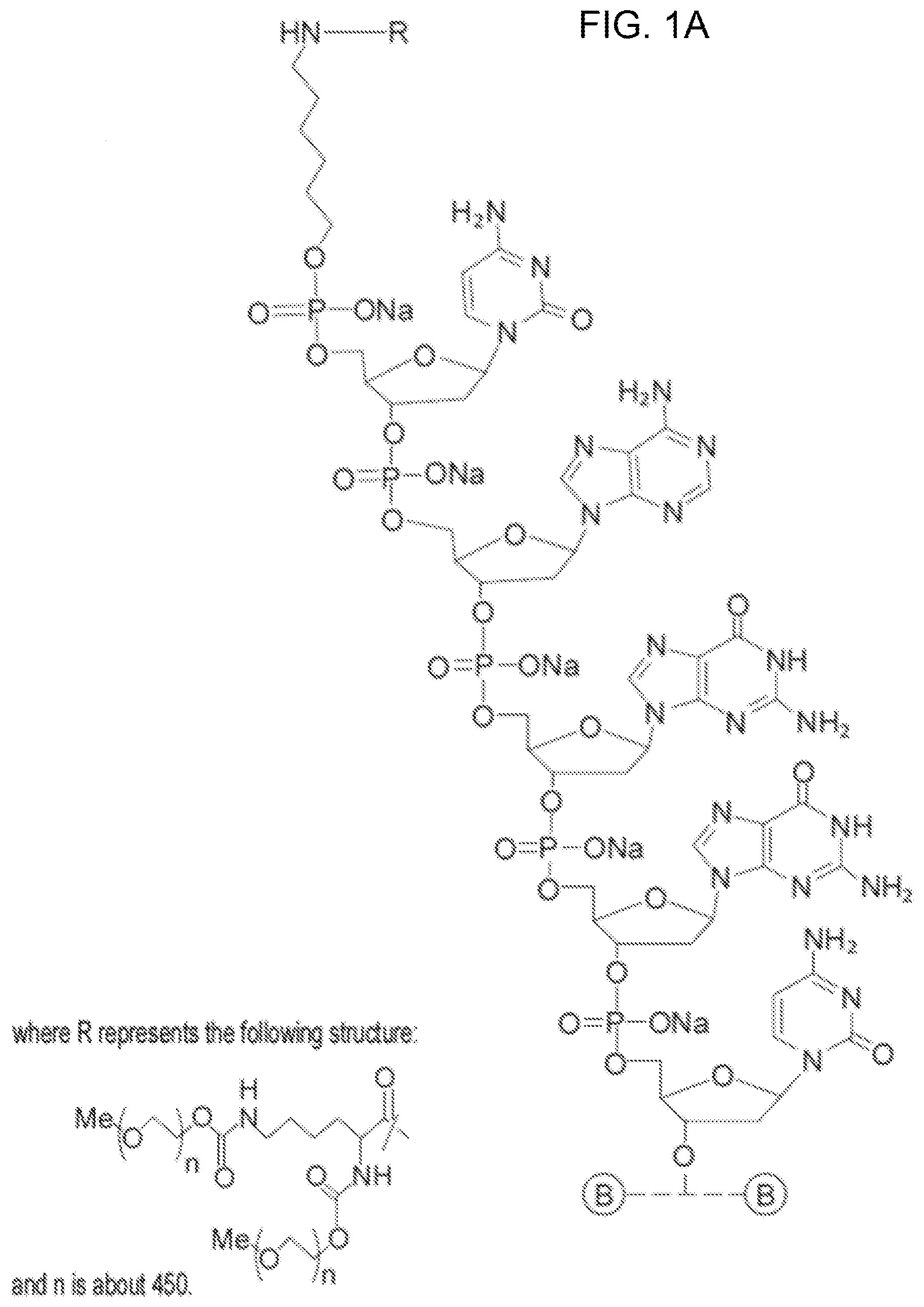
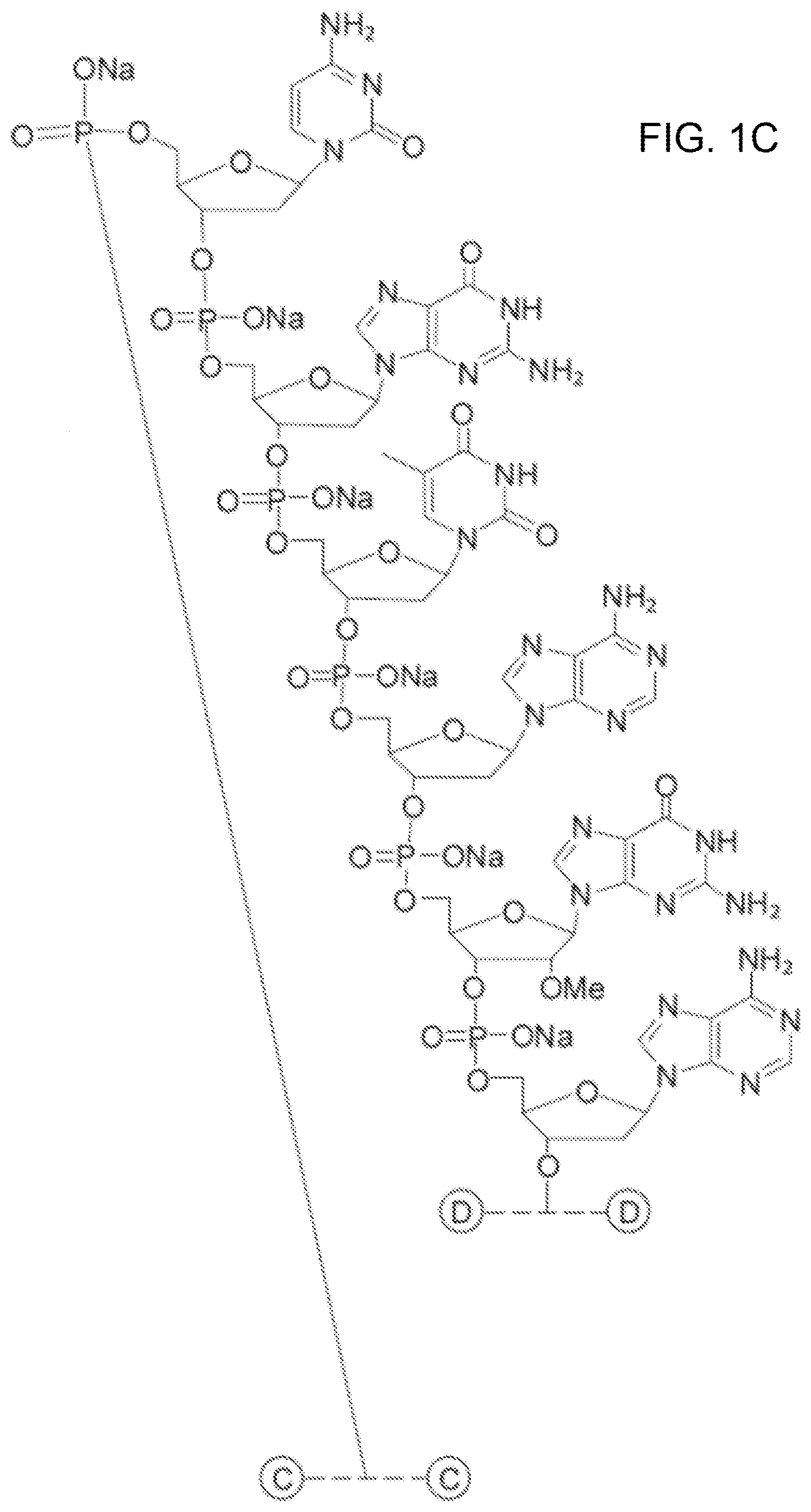
[0011] FIGS. 1A-F show the chemical structure of Antagonist A, wherein the 5' end of its aptamer (SEQ ID NO: 1) is modified with Me(OCH.sub.2CH.sub.2).sub.nOC(O)NH(CH.sub.2).sub.4CH(NHC(O)O(CH.sub.2CH.s- ub.2O).sub.nMe)C(O)NH(CH.sub.2).sub.6--, where n is about 450. The designations {circle around (B)}-{circle around (F)} indicate a continuation from a previous panel.
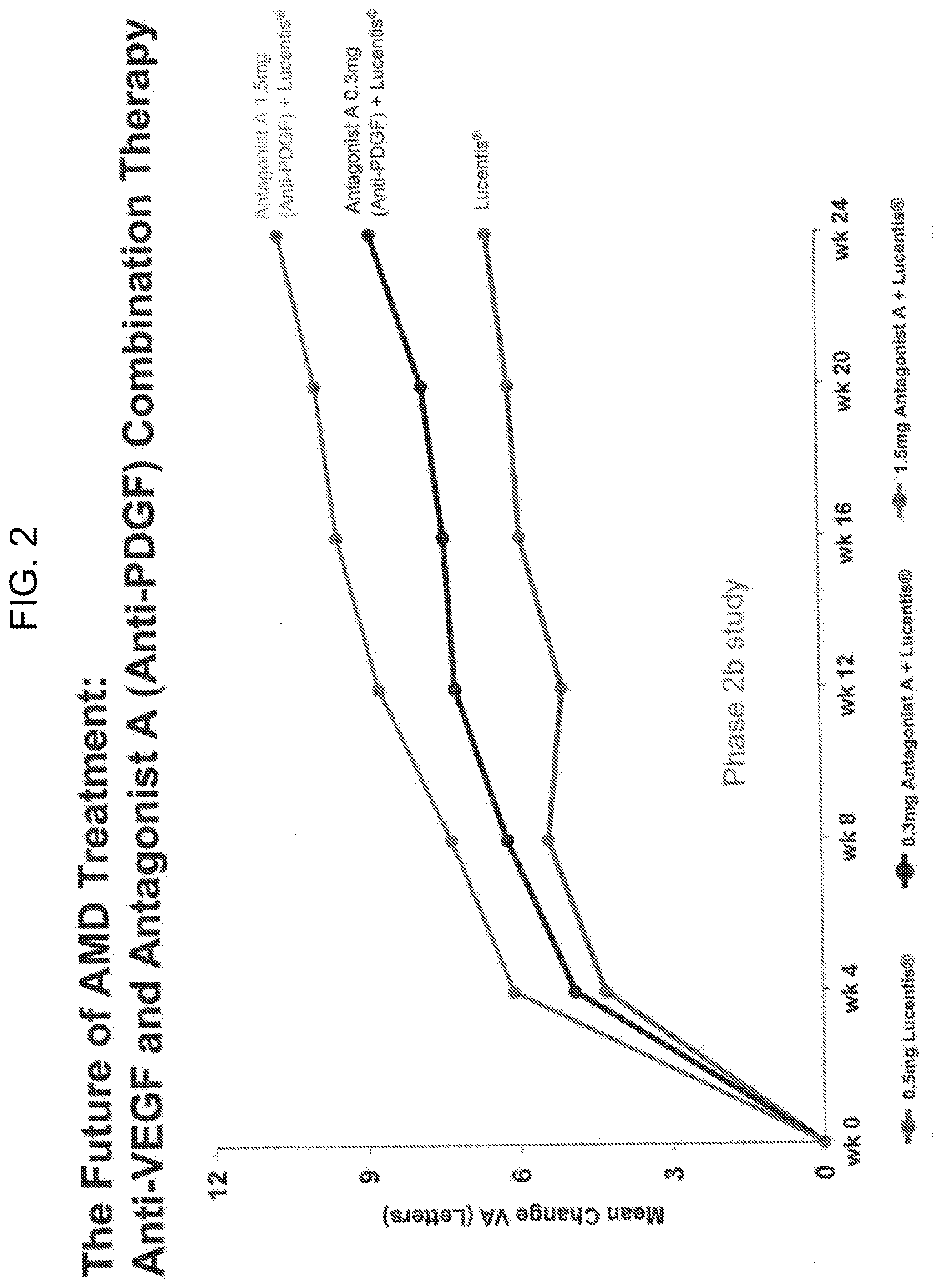
[0012] FIG. 2 shows a graph depicting the mean change in visual acuity in wet AMD patients in a phase 2b clinical trial, who were treated with 0.5 mg of Lucentis.RTM. alone or with 0.5 mg of Lucentis.RTM. and either 1.5 mg of Antagonist A or 0.3 mg of Antagonist A.
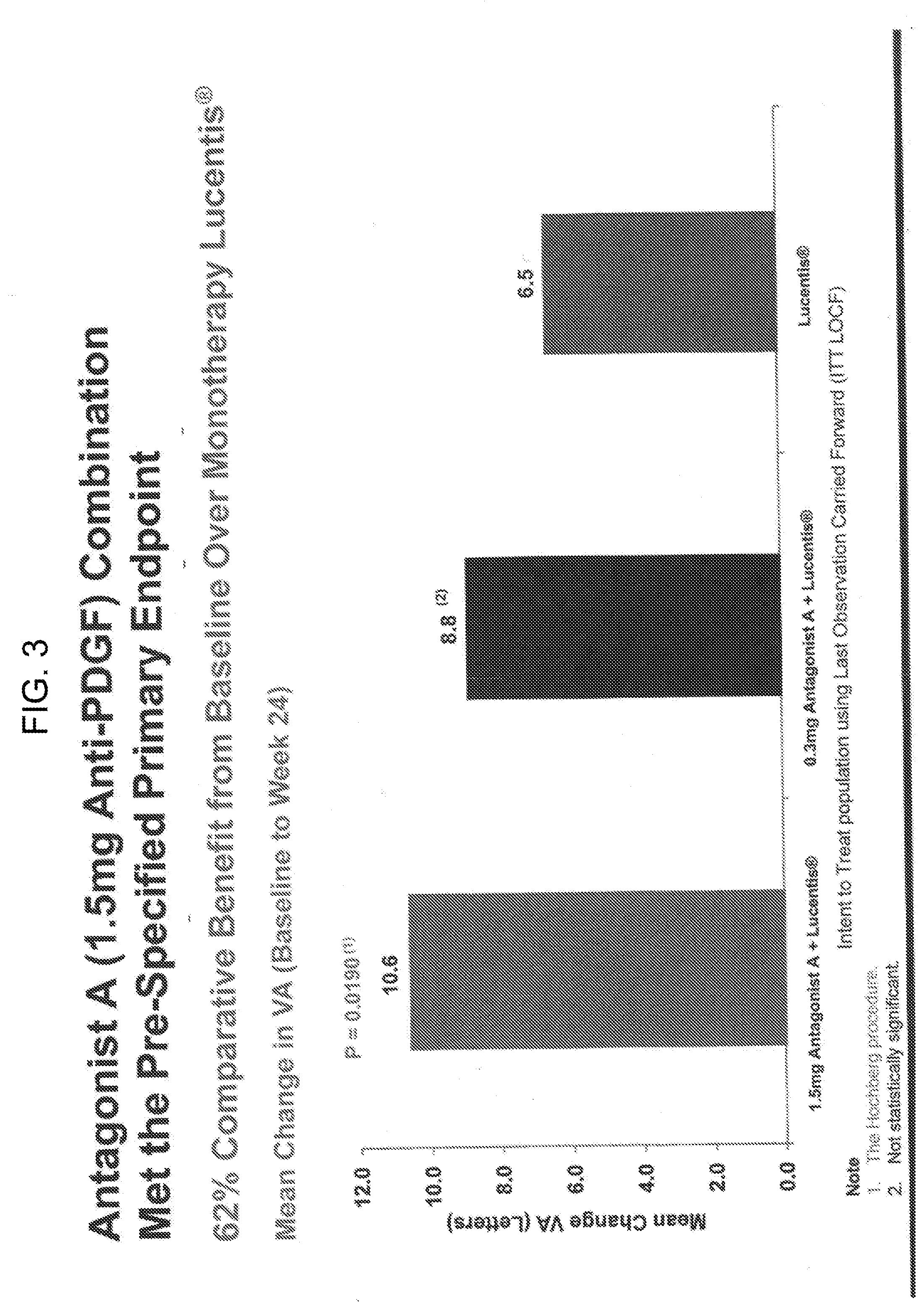
[0013] FIG. 3 shows a bar graph showing comparative visual-acuity benefit in wet AMD patients with treatment with 0.5 mg of Lucentis.RTM. and either 1.5 mg or 0.3 mg of Antagonist A as compared to treatment with Lucentis.RTM. monotherapy (0.5 mg).
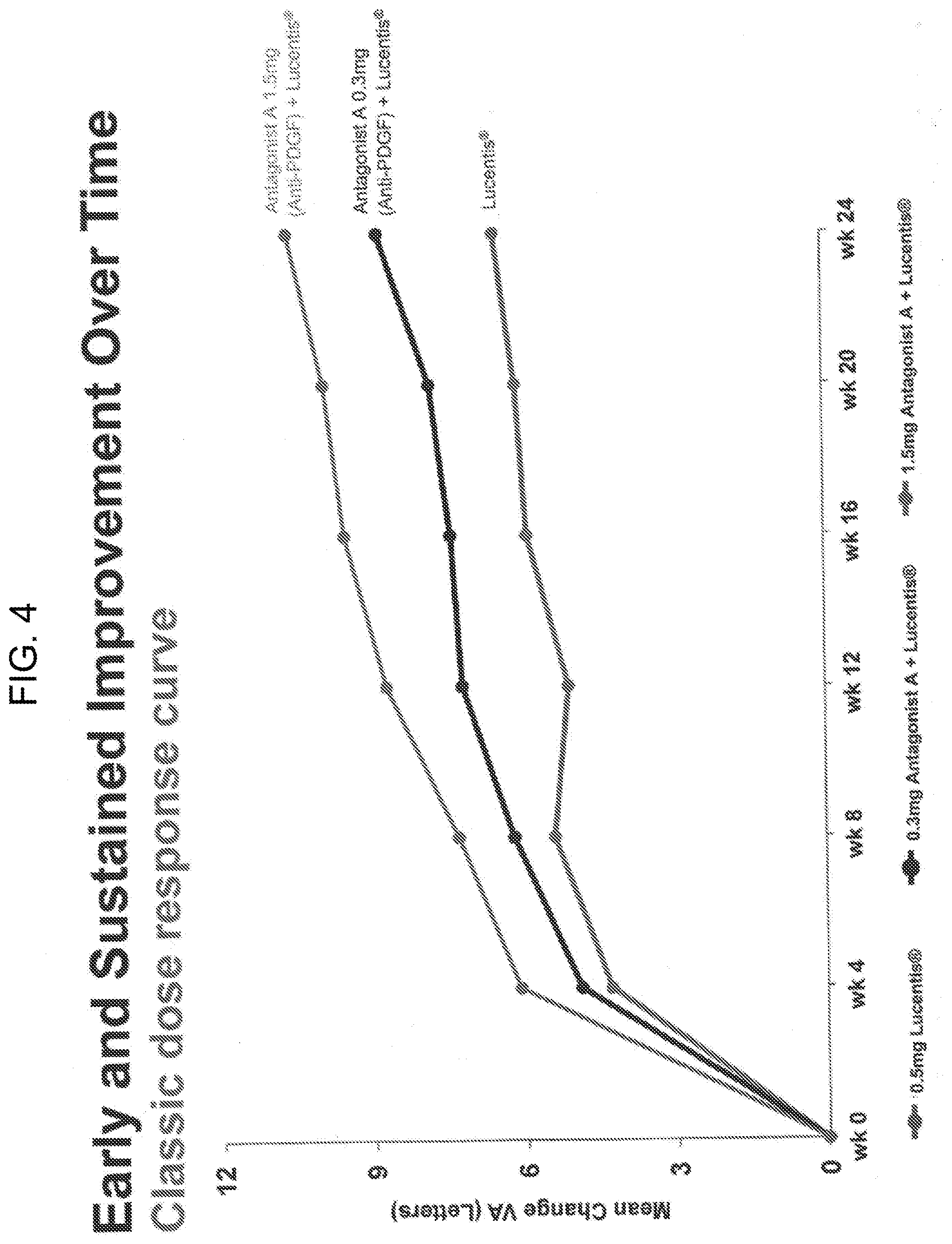
[0014] FIG. 4 shows a graph depicting the early and sustained visual-acuity improvement over time in wet AMD patients treated with Lucentis.RTM. monotherapy (0.5 mg) or with 0.5 mg of Lucentis.RTM. and either 1.5 mg of Antagonist or 0.3 mg of Antagonist A.
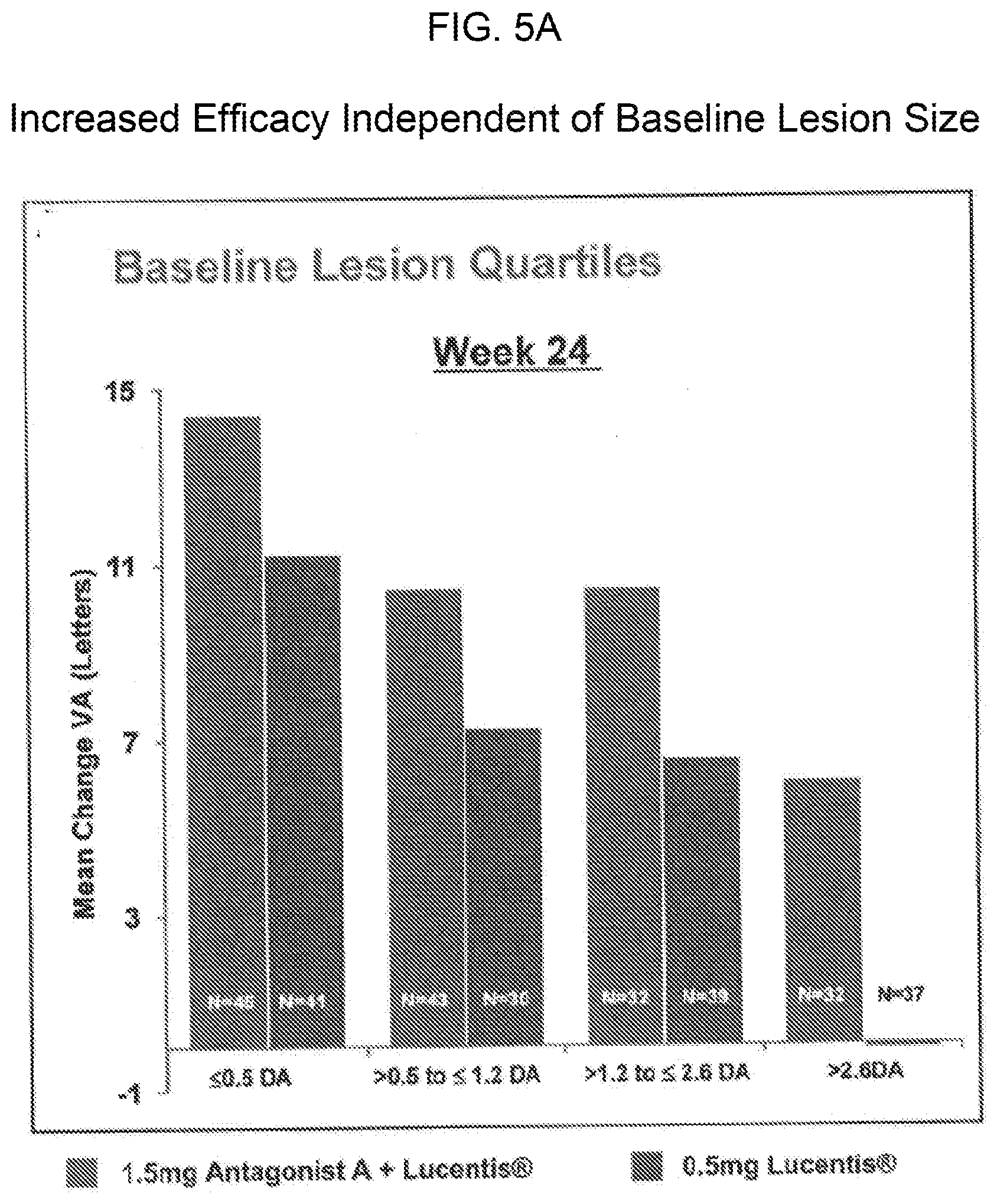
[0015] FIGS. 5A and 5A provide bar graphs showing that the increased efficacy of treatment with 0.5 mg of Lucentis.RTM. and either 1.5 mg or 0.3 mg of Antagonist A as compared to treatment with Lucentis.RTM. monotherapy (0.5 mg) in patients with wet AMD is independent of baseline lesion size or baseline vision. FIG. 5A shows the mean change in visual acuity for patients in each of the indicated baseline lesion quartiles, and FIG. 5B shows the mean change in visual acuity for patients with the indicated baseline vision.
[0016] FIGS. 6A and 6B provide bar graphs showing that the cohort of patients treated with a combination of 0.5 mg of Lucentis.RTM. and 1.5 mg of Antagonist A included a greater proportion of patients with significant visual gain (FIG. 6A) and fewer patients with visual loss (FIG. 6B) as compared to the cohort of patients with treated Lucentis.RTM. monotherapy (0.5 mg).
[0017] FIGS. 7A-C provide bar graphs showing that patients treated with 0.5 mg of Lucentis.RTM. and 1.5 mg of Antagonist A exhibited a greater mean improvement in final visual acuity as compared to patients treated with Lucentis.RTM. monotherapy (0.5 mg). FIG. 7A shows the percentage of patients who demonstrated a visual acuity of 20/40 or better; FIG. 7B shows the percentage of patients who demonstrated a visual acuity of 20/25 or better; and FIG. 7C shows the percentage of patients who demonstrated a visual acuity of 20/200 or worse.
[0018] FIGS. 8A and 8B provide bar graphs showing increased reduction in choroidal neovascularization (CNV) lesion size in small and large baseline CNV lesions in wet AMD patients treated with both 0.5 mg of Lucentis.RTM. and 1.5 mg of Antagonist A as compared to patients treated with Lucentis.RTM. monotherapy (0.5 mg). FIG. 8A shows the results in all patients, and FIG. 8B shows the results in patients with a visual outcome >3-lines.
[0019] FIG. 9 shows a graph depicting the mean change in geographic atrophy (GA) lesion area in dry AMD patients measured at 24 weeks in patients treated with either a 0.3 mg or 1 mg dose of ARC1905 monthly from weeks 0 to 24 in a phase 2a trial.
[0020] FIG. 10 shows a graph depicting the mean change in GA lesion area in dry AMD patients measured at 24 weeks and 48 weeks in patients treated with either a 0.3 mg or 1 mg dose of ARC1905 monthly from weeks 0 to 48 in a phase 2a trial.
[0021] FIG. 11 shows Early Treatment for Diabetic Retinopathy Study ("ETDRS") Chart 1.
[0022] FIG. 12 shows Early Treatment for Diabetic Retinopathy Study ("ETDRS") Chart 2.
[0023] FIG. 13 shows Early Treatment for Diabetic Retinopathy Study ("ETDRS") Chart R.
DETAILED DESCRIPTION OF THE INVENTION
[0024] In certain aspects, the present invention provides new and improved methods and compositions for treating and preventing ophthalmological diseases and disorders, including, e.g., new uses, combination therapies, treatment and dosing regimens, and coformulations.
[0025] In one aspect, the invention provides methods for treating or preventing an ophthalmological disease or disorder, comprising administering to a subject in need thereof an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof. In particular embodiments, the subject is administered Antagonist A or another pharmaceutically acceptable salt thereof and not administered an anti-C5 agent. In some embodiments, the subject is administered Antagonist A or another pharmaceutically acceptable salt thereof and not administered a VEGF antagonist.
[0026] In particular embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof is administered in combination with a VEGF antagonist. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered in combination with ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008.
[0027] In particular embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof is administered in combination with a VEGF antagonist and an anti-C5 agent. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered in combination with a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008), and ARC1905.
[0028] The invention also provides treatment regimens, including treatment and dosing regimens, related to the coadministration of Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, optionally also in combination with an anti-C5 agent.
[0029] In further embodiments, another agent (e.g., an agent that is not Antagonist A, VEGF antagonist or an anti-C5 agent) that is useful for treating or preventing an ophthalmological disease or disorder is administered. In some embodiments, the methods comprise administering one or more (e.g., two) VEGF antagonists and/or one or more (e.g., two) anti-C5 agents to the subject in need thereof.
[0030] In another aspect, the invention provides methods for treating or preventing an ophthalmological disease or disorder, comprising administering to a subject in need thereof an effective amount of an anti-C5 agent (e.g., ARC1905). In particular embodiments, the subject is not administered Antagonist A or another pharmaceutically acceptable salt thereof. In some embodiments, the subject is not administered a VEGF antagonist.
[0031] In addition, the invention provides coformulations that comprise Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist. In certain embodiments, the coformulations further comprise an anti-C5 agent. In certain embodiments, the coformulations are pharmaceutically compositions comprising an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist, and a pharmaceutically acceptable carrier or vehicle. In certain embodiments, the coformulations are pharmaceutically compositions comprising an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist, and anti-C5 agent, and a pharmaceutically acceptable carrier or vehicle.
[0032] In one embodiment, the present invention provides methods for treating or preventing an ophthalmological disease or disorder, comprising administering to a subject in need thereof Antagonist A or another pharmaceutically acceptable salt thereof and optionally a VEGF antagonist, wherein the methods further comprise performing a surgery to treat the ophthalmological disease or disorder and/or administration of an anti-C5 agent.
Definitions and Abbreviations
[0033] As used herein, the following terms and phrases shall have the meanings set forth below. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood to one of skill in the art to which this invention belongs.
[0034] The term "about" when used in connection with a referenced numeric indication means the referenced numeric indication plus or minus up to 10% of that referenced numeric indication. For example, "about 100" means from 90 to 110 and "about six" means from 5.4 to 6.6.
[0035] The term "antagonist" refers to an agent that inhibits, either partially or fully, the activity or production of a target molecule. In particular, the term "antagonist," as applied selectively herein, means an agent capable of decreasing levels of gene expression, mRNA levels, protein levels or protein activity of the target molecule. Illustrative forms of antagonists include, for example, proteins, polypeptides, peptides (such as cyclic peptides), antibodies or antibody fragments, peptide mimetics, nucleic acid molecules, antisense molecules, ribozymes, aptamers, RNAi molecules, and small organic molecules. Illustrative non-limiting mechanisms of antagonist inhibition include repression of ligand synthesis and/or stability (e.g., using, antisense, ribozymes or RNAi compositions targeting the ligand gene/nucleic acid), blocking of binding of the ligand to its cognate receptor (e.g., using anti-ligand aptamers, antibodies or a soluble, decoy cognate receptor), repression of receptor synthesis and/or stability (e.g., using, antisense, ribozymes or RNAi compositions targeting the ligand receptor gene/nucleic acid), blocking of the binding of the receptor to its cognate receptor (e.g., using receptor antibodies) and blocking of the activation of the receptor by its cognate ligand (e.g., using receptor tyrosine kinase inhibitors). In addition, the antagonist may directly or indirectly inhibit the target molecule.
[0036] The term "antibody fragment" includes a portion of an antibody that is an antigen binding fragment or single chains thereof. An antibody fragment can be a synthetically or genetically engineered polypeptide. Examples of binding fragments encompassed within the term "antigen-binding portion" of an antibody include (i) a Fab fragment, a monovalent fragment consisting of the V.sub.L, V.sub.H, C.sub.L and C.sub.H1 domains; (ii) a F(ab').sub.2 fragment, a bivalent fragment comprising two Fab fragments linked by a disulfide bridge at the hinge region; (iii) a Fd fragment consisting of the V.sub.H and C.sub.H1 domains; (iv) a Fv fragment consisting of the V.sub.L and V.sub.H domains of a single arm of an antibody, (v) a dAb fragment (Ward et al., (1989) Nature 341:544-546), which consists of a V.sub.H domain; and (vi) an isolated complementarity determining region (CDR). Furthermore, although the two domains of the Fv fragment, V.sub.L and V.sub.H, are coded for by separate genes, they can be joined, using recombinant methods, by a synthetic linker that enables them to be made as a single protein chain in which the V.sub.L and V.sub.H regions pair to form monovalent molecules (known as single chain Fv (scFv); see e.g., Bird et al. (1988) Science 242:423-426; and Huston et al. (1988) Proc. Natl. Acad. Sci. USA 85:5879-5883). Such single chain antibodies are also intended to be encompassed within the term "antigen-binding fragment" of an antibody. These antibody fragments are obtained using conventional techniques known to those in the art, and the fragments can be screened for utility in the same manner as whole antibodies.
[0037] The term "aptamer" refers to a peptide or nucleic acid that has an inhibitory effect on a target. Inhibition of the target by the aptamer can occur by binding of the target, by catalytically altering the target, by reacting with the target in a way which modifies the target or the functional activity of the target, by ionically or covalently attaching to the target as in a suicide inhibitor or by facilitating the reaction between the target and another molecule. Aptamers can be peptides, ribonucleotides, deoxyribonucleotides, other nucleic acids or a mixture of the different types of nucleic acids. Aptamers can comprise one or more modified amino acid, bases, sugars, polyethylene glycol spacers or phosphate backbone units as described in further detail herein.
[0038] A nucleotide sequence is "complementary" to another nucleotide sequence if each of the bases of the two sequences matches, i.e., are capable of forming Watson Crick base pairs. The complement of a nucleic acid strand can be the complement of a coding strand or the complement of a non-coding strand.
[0039] The phrase "conserved residue" refers to an amino acid of a group of amino acids having particular common properties. A functional way to define common properties among individual amino acids is to analyze the normalized frequencies of amino acid changes among corresponding proteins of homologous organisms. According to such analyses, groups of amino acids may be characterized where amino acids within a group exchange preferentially with each other, and therefore resemble each other most in their impact on the overall protein structure (Schulz, G. E. and R. H. Schirmer, Principles of Protein Structure, Springer-Verlag). Examples of amino acid groups defined in this manner include:
[0040] (i) a charged group, consisting of Glu and Asp, Lys, Arg and His,
[0041] (ii) a positively-charged group, consisting of Lys, Arg and His,
[0042] (iii) a negatively-charged group, consisting of Glu and Asp,
[0043] (iv) an aromatic group, consisting of Phe, Tyr and Trp,
[0044] (v) a nitrogen ring group, consisting of His and Trp,
[0045] (vi) a large aliphatic nonpolar group, consisting of Val, Leu and Ile,
[0046] (vii) a slightly-polar group, consisting of Met and Cys,
[0047] (viii) a small-residue group, consisting of Ser, Thr, Asp, Asn, Gly, Ala, Glu, Gln and Pro,
[0048] (ix) an aliphatic group consisting of Val, Leu, Ile, Met and Cys, and
[0049] (x) a small hydroxyl group consisting of Ser and Thr.
[0050] Members of each of the above groups are conserved residues.
[0051] The term "label" includes, but is not limited to, a radioactive isotope, a fluorophore, a chemiluminescent moiety, an enzyme, an enzyme substrate, an enzyme cofactor, an enzyme inhibitor, a dye, a metal ion, a ligand (e.g., biotin or a hapten) and the like. Examples of fluorophore labels include fluorescein, rhodamine, dansyl, umbelliferone, Texas red, luminol, NADPH, alpha-beta-galactosidase and horseradish peroxidase.
[0052] The term "nucleic acid" refers to a polynucleotide such as deoxyribonucleic acid (DNA) or ribonucleic acid (RNA). The term also includes analogs of RNA or DNA made from nucleotide analogs, and, as applicable to the embodiment being described, single (sense or antisense) and double-stranded polynucleotides, ESTs, chromosomes, cDNAs, mRNAs, and rRNAs.
[0053] The terms "RNA interference," "RNAi," "miRNA," and "siRNA" refer to any method by which expression of a gene or gene product is decreased by introducing into a target cell one or more double-stranded RNAs, which are homologous to a gene of interest (particularly to the messenger RNA of the gene of interest, e.g., PDGF or VEGF).
[0054] The term "neovascularization" refers to new blood vessel formation in abnormal tissue or in abnormal positions.
[0055] The term "angiogenesis" refers to formation of new blood vessels in normal or in abnormal tissue or positions.
[0056] The term "ophthalmological disease" includes diseases of the eye and the ocular adnexa.
[0057] The term "ocular neovascular disorder" refers to an ocular disorder characterized by neovascularization. In one embodiment, the ocular neovascular disorder is a disorder other than cancer. Examples of ocular neovascular disorders include diabetic retinopathy and age-related macular degeneration.
[0058] The term "mammal" includes a human, monkey, cow, hog, sheep, horse, dog, cat, rabbit, rat and mouse. In certain embodiments, a subject is a mammal.
[0059] The term "PDGF" refers to a platelet-derived growth factor that regulates cell growth or division. As used herein, the term "PDGF" includes the various subtypes of PDGF including PDGF-B (see SEQ ID NOS: 2 (nucleic acid) and 3 (polypeptide)), PDGF-A (see SEQ ID NOS: 4 (nucleic acid) and 5 (polypeptide), PDGF-C (see SEQ ID NOS: 6 (nucleic acid) and 7 (polypeptide)), PDGF-D, variants 1 (see SEQ ID NOS: 8 (nucleic acid) and 9 (polypeptide)) and 2 (see SEQ ID NOS: 10 (nucleic acid) and 11 (polypeptide)), and dimerized forms thereof, including PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD. Platelet derived growth factors includes homo- or heterodimers of A-chain (PDGF-A) and B-chain (PDGF-B) that exert their action via binding to and dimerization of two related receptor tyrosine kinase platelet-derived growth factor cell surface receptors (i.e., PDGFRs), PDGFR-.alpha. (see SEQ ID NOS: 12 (nucleic acid) and 13 (polypeptide)) and PDGFR-.beta. (see SEQ ID NOS: 14 (nucleic acid) and 15 (polypeptide)). In addition, PDGF-C and PDGF-D, two additional protease-activated ligands for the PDGFR complexes, have been identified (Li et al., (2000) Nat. Cell. Biol. 2: 302-9; Bergsten et al., (2001) Nat. Cell. Biol. 3: 512-6; and Uutele et al., (2001) Circulation 103: 2242-47). Due to the different ligand binding specificities of the PDGFRs, it is known that PDGFR-.alpha./.alpha. binds PDGF-AA, PDGF-BB, PDGF-AB, and PDGF-CC; PDGFR-.beta./.beta. binds PDGF-BB and PDGF-DD; whereas PDGFR-.alpha./.beta. binds PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD (Betsholtz et al., (2001) BioEssays 23: 494-507). As used herein, the term "PDGF" also refers to those members of the class of growth factors that induce DNA synthesis and mitogenesis through the binding and activation of a PDGFR on a responsive cell type. PDGFs can effect, for example: directed cell migration (chemotaxis) and cell activation; phospholipase activation; increased phosphatidylinositol turnover and prostaglandin metabolism; stimulation of both collagen and collagenase synthesis by responsive cells; alteration of cellular metabolic activities, including matrix synthesis, cytokine production, and lipoprotein uptake; induction, indirectly, of a proliferative response in cells lacking PDGF receptors; and potent vasoconstrictor activity. The term "PDGF" can be used to refer to a "PDGF" polypeptide, a "PDGF" encoding gene or nucleic acid, or a dimerized form thereof.
[0060] The term "PDGF-A" refers to an A chain polypeptide of PDGF or its corresponding encoding gene or nucleic acid.
[0061] The term "PDGF-B" refers to a B chain polypeptide of PDGF or its corresponding encoding gene or nucleic acid.
[0062] The term "PDGF-C" refers to a C chain polypeptide of PDGF or its corresponding encoding gene or nucleic acid.
[0063] The term "PDGF-D" refers to a D chain polypeptide of PDGF or its corresponding encoding gene or nucleic acid, including variants 1 and 2 of the D chain polypeptide of PDGF.
[0064] The term "PDGF-AA" refers to a dimer having two PDGF-A chain polypeptides.
[0065] The term "PDGF-AB" refers to a dimer having one PDGF-A chain polypeptide and one PDGF-B chain polypeptide.
[0066] The term "PDGF-BB" refers to a dimer having two PDGF-B chain polypeptides.
[0067] The term "PDGF-CC" refers to a dimer having two PDGF-C chain polypeptides.
[0068] The term "PDGF-DD" refers to a dimer having two PDGF-D chain polypeptides.
[0069] The term "VEGF" refers to a vascular endothelial growth factor that induces angiogenesis or an angiogenic process. As used herein, the term "VEGF" includes the various subtypes of VEGF (also known as vascular permeability factor (VPF) and VEGF-A) (see SEQ ID NOS: 16 (nucleic acid) and 17 (polypeptide)) that arise by, e.g., alternative splicing of the VEGF-A/VPF gene including VEGF.sub.121, VEGF.sub.165 and VEGF.sub.189. Further, as used herein, the term "VEGF" includes VEGF-related angiogenic factors such as PIGF (placenta growth factor), VEGF-B, VEGF-C, VEGF-D and VEGF-E, which act through a cognate VEFG receptor (i.e., VEGFR) to induce angiogenesis or an angiogenic process. The term "VEGF" includes any member of the class of growth factors that binds to a VEGF receptor such as VEGFR-1 (Flt-1) (see SEQ ID NOS: 18 (nucleic acid) and 19 (polypeptide)), VEGFR-2 (KDR/Flk-1) (see SEQ ID NOS: 20 (nucleic acid) and 21 (polypeptide)), or VEGFR-3 (FLT-4). The term "VEGF" can be used to refer to a "VEGF" polypeptide or a "VEGF" encoding gene or nucleic acid.
[0070] The term "PDGF antagonist" refers to an agent that reduces, or inhibits, either partially or fully, the activity or production of a PDGF. In certain embodiments, the PDGF antagonist inhibits one or more of PDGF-A, PDGF-B, PDGF-C and PDGF-D. In certain embodiments, the PDGF antagonist inhibits one or more of PDGF-A, PDGF-B, and PDGF-C. In some embodiments, the PDGF antagonist inhibits a dimerized form of PDGF, such as PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD. In certain embodiments, the PDGF antagonist inhibits PDGF-BB. In other embodiments, the PDGF antagonist inhibits PDGF-AB. A PDGF antagonist can directly or indirectly reduce or inhibit the activity or production of a specific PDGF such as PDGF-B. Furthermore, "PDGF antagonists" consistent with the above definition of "antagonist," include agents that act on a PDGF ligand or its cognate receptor so as to reduce or inhibit a PDGF-associated receptor signal. Examples of "PDGF antagonists" include antisense molecules, ribozymes or RNAi that target a PDGF nucleic acid; anti-PDGF aptamers, anti-PDGF antibodies to PDGF itself or its receptor, or soluble PDGF receptor decoys that prevent binding of a PDGF to its cognate receptor; antisense molecules, ribozymes or RNAi that target a cognate PDGF receptor (PDGFR) nucleic acid; anti-PDGFR aptamers or anti-PDGFR antibodies that bind to a cognate PDGFR receptor; and PDGFR tyrosine kinase inhibitors.
[0071] The term "VEGF antagonist" refers to an agent that reduces, or inhibits, either partially or fully, the activity or production of a VEGF. In certain embodiments, the VEGF antagonist inhibits one or more of VEGF-A, VEGF-B, VEGF-C and VEGF-D. A VEGF antagonist can directly or indirectly reduce or inhibit the activity or production of a specific VEGF such as VEGF.sub.165. Furthermore, "VEGF antagonists" consistent with the above definition of "antagonist," include agents that act on either a VEGF ligand or its cognate receptor so as to reduce or inhibit a VEGF-associated receptor signal. Examples of "VEGF antagonists" include antisense molecules, ribozymes or RNAi that target a VEGF nucleic acid; anti-VEGF aptamers, anti-VEGF antibodies to VEGF itself or its receptor, or soluble VEGF receptor decoys that prevent binding of a VEGF to its cognate receptor; antisense molecules, ribozymes, or RNAi that target a cognate VEGF receptor (VEGFR) nucleic acid; anti-VEGFR aptamers or anti-VEGFR antibodies that bind to a cognate VEGFR receptor; and VEGFR tyrosine kinase inhibitors. In certain embodiments, the VEGF antagonist is a peptide, e.g., a peptide comprising three or more amino acid residues. In certain embodiments, the VEGF antagonist is a bicyclic peptide.
[0072] The term "effective amount" when used in connection with an active agent, refers to an amount of the active agent, e.g., a PDGF antagonist, a VEGF antagonist or an anti-C5 agent, alone or in combination with another active agent, that is useful to treat or prevent an ophthalmological disease or disorder. The "effective amount" can vary depending upon the mode of administration, specific locus of the ophthalmological disease or disorder, the age, body weight, and general health of the subject. The effective amount of two or more active agents is the combined amount of the active agents that is useful for treating or preventing an ophthalmological disease or disorder, even if the amount of one of the agents, in the absence of one or more of the other agents, is ineffective to treat or prevent the ophthalmological disease or disorder.
[0073] A "variant" of polypeptide X refers to a polypeptide having the amino acid sequence of polypeptide X in which is altered in one or more amino acid residues. The variant can have "conservative" changes, wherein a substituted amino acid has similar structural or chemical properties (e.g., replacement of leucine with isoleucine). More rarely, a variant can have "nonconservative" changes (e.g., replacement of glycine with tryptophan). Analogous minor variations may also include amino acid deletions or insertions, or both. Guidance in determining which amino acid residues may be substituted, inserted, or deleted without eliminating biological or immunological activity can be determined using computer programs well known in the art, for example, LASERGENE software (DNASTAR).
[0074] The term "variant," when used in the context of a polynucleotide sequence, can encompass a polynucleotide sequence related to that of gene or the coding sequence thereof. This definition also includes, for example, "allelic," "splice," "species," or "polymorphic" variants. A splice variant can have significant identity to a reference molecule, but will generally have a greater or lesser number of polynucleotides due to alternative splicing of exons during mRNA processing. The corresponding polypeptide can possess additional functional domains or an absence of domains. Species variants are polynucleotide sequences that vary from one species to another. The resulting polypeptides generally will have significant amino acid identity relative to each other. A polymorphic variant is a variation in the polynucleotide sequence of a particular gene between individuals of a given species.
[0075] The term "anti-C5 agent" refers to an agent that reduces, or inhibits, either partially or fully, the activity or production of a C5 complement protein or a variant thereof. An anti-C5 agent can directly or indirectly reduce or inhibit the activity or production of a C5 complement protein or variant thereof. An anti-C5 agent can reduce or inhibit the conversion of C5 complement protein into its component polypeptides C5a and C5b. Anti-C5 agents can also reduce or inhibit the activity or production of C5a and/or C5b. Examples of "anti-C5 agents" include antisense molecules, ribozymes or RNAi that target a C5 nucleic acid; anti-C5 aptamers including anti-C5a and anti-C5b aptamers, anti-C5 antibodies directed against C5, C5a, C5b, or C5b-9, or soluble C5 receptor decoys that prevent binding of a C5 complement protein or variant or fragment thereof (e.g., C5a or C5b) to a binding partner or receptor.
[0076] Agents Useful for Treatment or Prevention of an Opthalmological Disease or Disorder
[0077] Antagonist A
[0078] Antagonist A is a PEGylated, anti-PDGF aptamer having the sequence CAGGCUACGC GTAGAGCAUC ATGATCCUGT (SEQ ID NO: 1) (see Example 3 of US Patent Application Publication No. 20050096257, incorporated herein by reference in its entirety) having 2'-fluoro-2'-deoxyuridine at positions 6, 19 and 28; 2'-fluoro-2'-deoxycytidine at positions 8, 20, 26, and 27; 2'-O-Methyl-2'-deoxyguanosine at positions 9, 14, 16, and 29; 2'-O-Methyl-2'-deoxyadenosine at position 21; an inverted orientation T (i.e., 3'-3'-linked) at position 30; and two heaxethylene-glycol phosphoramidite linkages that join together the 9.sup.th and 10.sup.th nucleotides and 21.sup.st and 22.sup.nd nucleotides via phosphodiester linkages between the linker and the respective nucleotides.
[0079] The chemical name of Antagonist A is [(monomethoxy 20K polyethylene glycol carbamoyl-N2-) (monomethoxy 20K polyethylene glycol carbamoyl-N6-)]-lysine-amido-6-hexandilyl-(1-5)-2'-deoxycytidylyl-(3'-5')- -2'-deoxyadenylyl-(3'-5')-2'-deoxyguanylyl-(3'-5')-2'-deoxyguanylyl-(3'-5)- -2'-deoxycytidylyl-(3'-5)-2'-deoxy-2'-fluorouridylyl-(3'-5')-2'-deoxyadeny- lyl-(3'-5)-2'-deoxy-2'-fluorocytidylyl-(3'-5')-2'-deoxy-2'-methoxyguanylyl- -(3'-1)-PO.sub.3-hexa(ethyloxy)-(18-5)-2'-deoxycytidylyl-(3'-5')-2'-deoxyg- uanylyl-(3'-5')-thymidylyl-(3'-5)-2'-deoxyadenylyl-(3'-5')-2'-deoxy-2'-met- hoxyguanylyl-(3'-5)-2'-deoxyadenylyl-(3'-5)-2'-deoxy-2'-methoxyguanylyl-(3- '-5)-2'-deoxycytidylyl-(3'-5)-2'-deoxyadenylyl-(3'-5)-2'-deoxy-2'-fluorour- idylyl-(3'-5)-2'-deoxy-2'-fluorocytidylyl-(3'-5)-2'-deoxy-2'-methoxyadenyl- yl-(3'-1)-PO.sub.3-hexa(ethyloxy)-(18-5)-thymidylyl-(3'-5)-2'-deoxyguanyly- l-(3'-5)-2'-deoxyadenylyl-(3'-5)-thymidylyl-(3'-5)-2'-deoxy-2'-fluorocytid- ylyl-(3'-5)-2'-deoxy-2'-fluorocytidylyl-(3'-5)-2'-deoxy-2'-fluorouridylyl-- (3'-5)-2'-deoxy-2'-methoxyguanylyl-(3'-3)-thymidine.
[0080] The structure of Antagonist A is shown in FIG. 1.
[0081] The sequence of Antagonist A is:
[0082] 5'-[mPEG2 40 kD]-[HN--(CH.sub.2).sub.6O] CAGGCU.sub.fAC.sub.fG.sub.m [PO.sub.3(CH.sub.2CH.sub.2O).sub.6] CGTAG.sub.mAG.sub.mCAU.sub.fC.sub.fA.sub.m [PO.sub.3(CH.sub.2CH.sub.2O).sub.6]TGATC.sub.fC.sub.fU.sub.fG.sub.m-[3T]-- 3', whose aptamer sequence is set forth in (SEQ ID NO: 1),
[0083] where [3T] refers to an inverted thymidine nucleotide that is attached to the 3' end of the oligonucleotide at the 3' position on the ribose sugar, and [mPEG2 40 kD] represents two 20 kD polyethylene glycol (PEG) polymer chains, in one embodiment two about 20 kD PEG polymer chains, that are covalently attached to the two amino groups of a lysine residue via carbamate linkages. This moiety is in turn linked with the oligonucleotide via the amino linker described below.
[0084] [HN--(CH.sub.2).sub.6O] represents a bifunctional .alpha.-hydroxy-.omega.-amino linker that is covalently attached to the PEG polymer via an amide bond. The linker is attached to the oligonucleotide at the 5'-end of Antagonist A by a phosphodiester linkage.
[0085] [PO.sub.3(CH.sub.2CH.sub.2O).sub.6] represents the hexaethylene glycol (HEX) moieties that join segments of the oligonucleotide via phosphodiester linkages. Antagonist A has two HEX linkages that join together the 9.sup.th and 10.sup.th nucleotides and 21.sup.st and 22.sup.nd nucleotides via phosphodiester linkages between the linker and the respective nucleotides.
[0086] C, A, G, and T represent the single letter code for the 2'-deoxy derivatives of cytosine, adenosine, guanosine, and thymidine nucleic acids, respectively. Antagonist A has four 2'-deoxyribocytosine, six 2'-deoxyriboadenosine, four 2'-deoxyriboguanosine, and four 2'-deoxyribothymidine.
[0087] G.sub.m and A.sub.m represent 2'-methoxy substituted forms of guanosine and adenosine, respectively. Antagonist A has four 2'-methoxyguanosines and one 2'-methoxyadenosine. C.sub.f and U.sub.f represent the 2'-fluoro substituted forms of cytosine and uridine, respectively. Antagonist A has four 2'-fluorocytosines and three 2'-fluorouridines.
[0088] The phosphodiester linkages in the oligonucleotide, with the exception of the 3'-terminus, connect the 5'- and 3'-oxygens of the ribose ring with standard nucleoside phosphodiester linkages. The phosphodiester linkage between the 3'-terminal thymidine and the penultimate G.sub.m links their respective 3'-oxygens, which is referred to as the 3',3'-cap.
[0089] Antagonist A has a molecular weight from 40,000 to 60,000 Daltons, in one embodiment from about 40,000 to about 60,000 Daltons, and can be colorless to slightly yellow in solution. Antagonist A can be present in a solution of monobasic sodium phosphate monohydrate and dibasic sodium phosphate heptahydrate as buffering agents and sodium chloride as a tonicity adjuster. Antagonist A is a hydrophilic polymer. The Antagonist A is soluble in water and in phosphate-buffered saline (PBS), as assessed by visual inspection, to at least 50 mg (based on oligonucleotide weight)/mL solution.
[0090] Antagonist A can be synthesized using an iterative chemical synthesis procedure to produce the oligonucleotide portion, which is then covalently bonded to a pegylation reagent, as further described in Example 4 of US Patent Publication NO. 2012/0100136.
[0091] Antagonist A is a persodium salt. Other pharmaceutically acceptable salts, however, of Antagonist are useful in the compositions and methods disclosed herein.
[0092] VEGF Antagonists
[0093] In some embodiments, the VEGF antagonist is ranibizumab (commercially available under the trademark Lucentis.RTM. (Genentech, San Francisco, Calif.); see FIG. 1 of U.S. Pat. No. 7,060,269 for the heavy chain and light chain variable region sequences), bevacizumab (commercially available under the trademark Avastin.RTM. (Genentech, San Francisco, Calif.); see FIG. 1 of U.S. Pat. No. 6,054,297 for the heavy chain and light chain variable region sequences), aflibercept (commercially available under the trademarkEylea.RTM. (Regeneron, Tarrytown, N.Y.), KH902 VEGF receptor-Fc fusion protein (see Zhang et al. (2008) Mol Vis. 14:37-49), 2C3 antibody (see U.S. Pat. No. 6,342,221, Column 8, lines 48-67, Column 9, lines 1-21), ORA102 (available from Ora Bio, Ltd.), pegaptanib (e.g., pegaptanib sodium; commercially available under the trademark Macugen.RTM. (Valeant Pharmaceuticals, Bridgewater, N.J.; see FIG. 1 of U.S. Pat. No. 6,051,698)), bevasiranib (see Dejneka et al. (2008) Mol Vis. 14:997-1005), SIRNA-027 (Shen et al. (2006) Gene Ther. 13:225-34), decursin (see U.S. Pat. No. 6,525,089 (Column 3, lines 5-16)), decursinol (see Ahn et al. (1997) Planta Med. 63:360-1), picropodophyllin (see Economou (2008) Investigative Ophthalmology & Visual Science. 49:2620-6), guggulsterone (see Kim et al. (2008) Oncol. Rep. 20:1321-7), PLG101 (see Ahmadi and Lim (2008) Expert Opin Pharmacother. 9:3045-52), PLG201 (see Ahmadi and Lim (2008)), eicosanoid LXA4 (see Baker et al (2009) J Immun. 182:3819-26), PTK787 (commercially available under the trademark Vitalanib.TM.; see Barakat and Kaiser (2009) Expert Opin Investig Drugs 18:637-46), pazopanib (see Takahashi et al. (2009) Arch Ophthalmol. 127:494-9), axitinib (see Hu-Lowe et al. (2008) Clin Cancer Res. 14:7272-83), CDDO-Me (see Sogno et al. (2009) Recent Results Cancer Res. 181:209-12), CDDO-Imm (see Sogno et al. (2009)), shikonin (see Hisa et al. (1998) Anticancer Res. 18:783-90), beta-hydroxyisovalerylshikonin (see Hisa et al. (1998)), ganglioside GM3 (Chung et al. (2009) Glycobio. 19:229-39), DC101 antibody (see U.S. Pat. No. 6,448,077, Column 2, lines 61-65), Mab25 antibody (see U.S. Pat. No. 6,448,077, Column 2, lines 61-65), Mab73 antibody (see U.S. Pat. No. 6,448,077, Column 2, lines 61-65), 4A5 antibody (see U.S. Pat. No. 6,383,484, Column 12, lines 50-54), 4E10 antibody (see U.S. Pat. No. 6,383,484, Column 10, lines 66-67, Column 11, lines 1-2), 5F12 antibody (see U.S. Pat. No. 6,383,484, Column 10, lines 62-65), VA01 antibody (see U.S. Pat. No. 5,730,977, Column 6, lines 26-30), BL2 antibody (U.S. Pat. No. 5,730,977, Column 6, lines 30-32), VEGF-related protein (see U.S. Pat. No. 6,451,764, FIG. 1), sFLT01 (see Pechan et al. (2009) Gene Ther. 16:10-6), sFLT02 (see Pechan et al. (2009)), Peptide B3 (see Lacal et al. (2008) Eur J Cancer 44:1914-21), TG100801 (see Palanki et al. (2008) J Med Chem. 51:1546-59), sorafenib (commercially available under the trademark Nexavar.TM.; see Kernt et al. (2008) Acta Ophthalmol. 86:456-8), G6-31 antibody (see Crawford et al. (2009) Cancer Cell 15:21-34), ESBA1008 (see U.S. Pat. No. 8,349,322), tivozanib (see U.S. Pat. No. 6,821,987, incorporated by reference in its entirety; Campas et al. (2009) Drugs Fut 2009, 34(10): 793), or a pharmaceutically acceptable salt thereof.
[0094] In another embodiment, the VEGF antagonist is an antibody or an antibody fragment which binds to an epitope VEGF-A (SEQ ID NO: 22) or VEGF-B (SEQ ID NO: 23), or any portion of the epitopes. In one embodiment, the VEGF antagonist is an antibody or antibody fragment that binds to one or more of an epitope of VEGF (e.g., SEQ ID NOS: 22 and 23). In another embodiment, the VEGF antagonist is an antibody or an antibody fragment which binds to an epitope of VEGF, such as an epitope of VEGF-A, VEGF-B, VEGF-C, VEGF-D, or VEGF-E. In some embodiments, the VEGF antagonist binds to an epitope of VEGF such that binding of VEGF and VEGFR are inhibited. In one embodiment, the epitope encompasses a component of the three dimensional structure of VEGF that is displayed, such that the epitope is exposed on the surface of the folded VEGF molecule. In one embodiment, the epitope is a linear amino acid sequence from VEGF.
[0095] In some embodiments, an inhibitory antibody directed against VEGF is known in the art, e.g., those described in U.S. Pat. Nos. 6,524,583, 6,451,764 (VRP antibodies), U.S. Pat. Nos. 6,448,077, 6,416,758, 6,403,088 (to VEGF-C), U.S. Pat. No. 6,383,484 (to VEGF-D), U.S. Pat. No. 6,342,221 (anti-VEGF antibodies), U.S. Pat. Nos. 6,342,219 6,331,301 (VEGF-B antibodies), and U.S. Pat. No. 5,730,977, and PCT publications WO96/30046, WO 97/44453, and WO 98/45331, the contents of which are incorporated by reference in their entirety.
[0096] Other non-antibody VEGF antagonists include antibody mimetics (e.g., Affibody.RTM. molecules, affilins, affitins, anticalins, avimers, Kunitz domain peptides, and monobodies) with VEGF antagonist activity. This includes recombinant binding proteins comprising an ankyrin repeat domain that binds VEGF-A and prevents it from binding to VEGFR-2. One example is MP0112, also known as AGN 150998 (DARPin.RTM.). The ankyrin binding domain may have an amino acid sequence of SEQ ID NO: 97.
[0097] Recombinant binding proteins comprising an ankyrin repeat domain that binds VEGF-A and prevents it from binding to VEGFR-2 are described in more detail in WO2010/060748 and WO2011/135067.
[0098] Further specific antibody mimetics with VEGF antagonist activity are the 40 kD pegylated anticalin PRS-050 and the monobody angiocept (CT-322).
[0099] The aforementioned non-antibody VEGF antagonist may be modified to further improve their pharmacokinetic properties or bioavailability. For example, a non-antibody VEGF antagonist may be chemically modified (e.g., pegylated) to extend its in vivo half-life. Alternatively or in addition, it may be modified by glycosylation or the addition of further glycosylation sites not present in the protein sequence of the natural protein from which the VEGF antagonist was derived.
[0100] Other non-antibody VEGF antagonist immunoadhesin currently in pre-clinical development is a recombinant human soluble VEGF receptor fusion protein similar to VEGF-trap containing extracellular ligand-binding domains 3 and 4 from VEGFR2/KDR, and domain 2 from VEGFR1/Flt-1; these domains are fused to a human IgG Fc protein fragment (Li et al., 2011 Molecular Vision 17:797-803). This antagonist binds to isoforms VEGF-A. VEGF-B and VEGF-C. The molecule is prepared using two different production processes resulting in different glycosylation patterns on the final proteins. The two glycoforms are referred to as KH902 (conbercept) and KH906. The fusion protein can have the amino acid sequence of SEQ ID NO: 98 and, like VEGF-trap, can be present as a dimer. This fusion protein and related molecules are further characterized in EP1767546.
[0101] Anti-C5 Agents
[0102] In certain embodiments, the anti-C5 agent modulates a function of a C5 complement protein or a variant thereof. In some embodiments, the anti-C5 agent inhibits a function of C5 complement protein or a variant thereof. In one embodiment, the function inhibited by the anti-C5 agent is C5 complement protein cleavage.
[0103] A C5 complement protein variant as used herein encompasses a variant that performs substantially the same function as a C5 complement protein function. A C5 complement protein variant in some embodiments comprises substantially the same structure and in some embodiments comprises at least 80% sequence identity, in some embodiments at least 90% sequence identity, and in some embodiments at least 95% sequence identity to the amino acid sequence of the C5 complement protein comprising the amino acid sequence SEQ ID NO: 24.
[0104] In some embodiments, the anti-C5 agent is selected from a nucleic acid molecule, an aptamer, an antisense molecule, an RNAi molecule, a protein, a peptide, a cyclic peptide, an antibody or antibody fragment, a sugar, a polymer, or a small molecule. In certain embodiments, the anti-C5 agent is an anti-C5 agent described in PCT Patent Application Publication No. WO 2007/103549.
[0105] In particular embodiments, the anti-C5 agent is an anti-C5 aptamer. Aptamers are nucleic acid molecules having specific binding affinity to molecules through interactions other than classic Watson-Crick base pairing. Aptamers, like peptides generated by phage display or monoclonal antibodies ("mAbs"), are capable of specifically binding to selected targets and modulating the target's activity, e.g., through binding aptamers may block their target's ability to function. The aptamers may be unpegylated or pegylated. In particular embodiments, the aptamers may contain one or more 2' sugar modifications, such as 2'-O-alkyl (e.g., 2'-O-methyl or 2'-O-methoxyethyl) or 2'-fluoro modifications.
[0106] Illustrative C5 specific aptamers include the aptamers disclosed in PCT Publication No. WO 2007/103549, which is incorporated by reference in its entirety. Illustrative C5 specific aptamers include the aptamers ARC185 (SEQ ID NO: 25), ARC186 (SEQ ID NO: 26), ARC188 (SEQ ID NO: 27), ARC189 (SEQ ID NO: 28), ARC243 (SEQ ID NO: 29), ARC244 (SEQ ID NO: 30), ARC250 (SEQ ID NO: 31), ARC296 (SEQ ID NO: 32), ARC297 (SEQ ID NO: 33), ARC330 (SEQ ID NO: 34), ARC331 (SEQ ID NO: 35), ARC332 (SEQ ID NO: 36), ARC333 (SEQ ID NO: 37), ARC334 (SEQ ID NO: 38), ARC411 (SEQ ID NO: 39), ARC412 (SEQ ID NO: 40), ARC413 (SEQ ID NO: 41), ARC414 (SEQ ID NO: 42), ARC415 (SEQ ID NO: 43), ARC416 (SEQ ID NO: 44), ARC417 (SEQ ID NO: 45), ARC418 (SEQ ID NO: 46), ARC419 (SEQ ID NO: 47), ARC420 (SEQ ID NO: 48), ARC421 (SEQ ID NO: 49), ARC422 (SEQ ID NO: 50), ARC423 (SEQ ID NO: 51), ARC424 (SEQ ID NO: 52), ARC425 (SEQ ID NO: 53), ARC426 (SEQ ID NO: 54), ARC427 (SEQ ID NO: 55), ARC428 (SEQ ID NO: 56), ARC429 (SEQ ID NO: 57), ARC430 (SEQ ID NO: 58), ARC431 (SEQ ID NO: 59), ARC432 (SEQ ID NO: 60), ARC433 (SEQ ID NO: 61), ARC434 (SEQ ID NO: 62), ARC435 (SEQ ID NO: 63), ARC436 (SEQ ID NO: 64), ARC437 (SEQ ID NO: 65), ARC438 (SEQ ID NO: 66), ARC439 (SEQ ID NO: 67), ARC440 (SEQ ID NO: 68), ARC457 (SEQ ID NO: 69), ARC458 (SEQ ID NO: 70), ARC459 (SEQ ID NO: 71), ARC473 (SEQ ID NO: 72), ARC522 (SEQ ID NO: 73), ARC523 (SEQ ID NO: 74), ARC524 (SEQ ID NO: 75), ARC525 (SEQ ID NO: 76), ARC532 (SEQ ID NO: 77), ARC543 (SEQ ID NO: 78), ARC544 (SEQ ID NO: 79), ARC550 (SEQ ID NO: 80), ARC551 (SEQ ID NO: 81), ARC552 (SEQ ID NO: 82), ARC553 (SEQ ID NO: 83), ARC554 (SEQ ID NO: 84), ARC657 (SEQ ID NO: 85), ARC658 (SEQ ID NO: 86), ARC672 (SEQ ID NO: 87), ARC706 (SEQ ID NO: 88), ARC913 (SEQ ID NO: 89), ARC874 (SEQ ID NO: 90), ARC954 (SEQ ID NO: 91), ARC1537 (SEQ ID NO: 92), ARC1730 (SEQ ID NO: 93), or a pharmaceutically acceptable salt thereof.
[0107] In some embodiments, the anti-C5 agent is an aptamer with SEQ ID NO: 94, 95, or 96.
[0108] In a particular embodiment, the anti-C5 agent is a C5 specific aptamer comprising the nucleotide sequence of SEQ ID NO: 26 conjugated to a polyethylene glycol moiety via a linker. In some embodiments, the polyethylene glycol moiety has a molecular weight greater than about 10 kDa, particularly a molecular weight of about 20 kDa, more particularly about 30 kDa and more particulary about 40 kDa. In some embodiments, the polyethylene glycol moiety is conjugated via a linker to the 5' end of the aptamer. In some embodiments, the PEG conjugated to the 5' end of is a PEG of about 40 kDa molecular weight. In particular embodiments the about 40 kDa PEG is a branched PEG. In some embodiments the branched about 40 kDa PEG is 1,3-bis(mPEG-[about 20 kDa])-propyl-2-(4'-butamide). In other embodiments the branched about 40 kDa PEG is 2,3-bis(mPEG-[about 20 kDa])-propyl-1-carbamoyl.
[0109] In a particular embodiment, the C5 specific aptamer is a compound, ARC187, having the structure set forth below:
##STR00001##
[0110] or a pharmaceutically acceptable salt thereof, where Aptamer=fCmGfCfCGfCmGmGfUfCfUfCmAmGmGfCGfCfUmGmAmGfUfCfUmGmAmGf UfUfUAfCf CfUmGfCmG-3T (SEQ ID NO: 26)
[0111] wherein fC and fU=2'-fluoro nucleotides, and mG and mA=2'-OMe nucleotides and all other nucleotides are 2'-OH and where 3T indicates an inverted deoxy thymidine. In some embodiments, each 20 kDa mPEG of the above structure has a molecular weight of about 20 kDa.
[0112] In another particular embodiment, the C5 specific aptamer is a compound, ARC1905, having the structure set forth below:
##STR00002##
[0113] or a pharmaceutically acceptable salt thereof, where Aptamer=fCmGfCfCGfCmGmGfUfCfUfCmAmGmGfCGfCfUmGmAmGfUfCfUmGmAmGfUfUfUAfCf CfUmGfCmG-3T (SEQ ID NO: 26)
[0114] wherein fC and fU=2'-fluoro nucleotides, and mG and mA=2'-OMe nucleotides and all other nucleotides are 2'-OH and where 3T indicates and inverted deoxy thymidine. In some embodiments, each 20 kDa mPEG of the above structure has a molecular weight of about 20 kDa.
[0115] In other embodiments, the anti-C5 agent is an antisense oligonucleotide or ribozyme targeted to C5 that effects C5 inhibition by inhibiting protein translation from the messenger RNA or by targeting degradation of the corresponding C5 mRNA.
[0116] In still other embodiments, the anti-C5 agent is an anti-C5 RNA interference (RNAi) construct. Certain double stranded oligonucleotides useful to effect RNAi against C5 complement protein are less than 30 base pairs in length and may comprise about 25, 24, 23, 22, 21, 20, 19, 18 or 17 base pairs of ribonucleic acid and comprise a sequence with substantial sequence identity to the mRNA sequence of complement C5 protein, particularly human complement C5 protein. Optionally, the dsRNA oligonucleotides may include 3' overhang ends. Non-limiting illustrative 2-nucleotide 3' overhangs are composed of ribonucleotide residues of any type and may even be composed of 2'-deoxythymidine resides, which lowers the cost of RNA synthesis and may enhance nuclease resistance of siRNAs in the cell culture medium and within transfected cells (see Elbashi et al., (2001) Nature, 411: 494-8).
[0117] Other Agents for Treatment or Prevention of an Ophthalmological Disease or Disorder
[0118] In another embodiment, another agent useful for treating or preventing an ophthalmological disease or disorder is volociximab or a pharmaceutically acceptable salt thereof (Ramakrishnan et al. (2008) J Exp Ther Oncol. 5:273-86, which is hereby incorporated by reference in its entirety).
[0119] In some embodiments, a plurality of aptamers can be associated with a single Non-Immunogenic, High Molecular Weight Compound, such as Polyalkylene Glycol or PEG, or a Lipophilic Compound, such as a glycerolipid. The aptamers can all be to one target or to different targets. In embodiments where a compound comprises more than one PDGF aptamer, there can be an increase in avidity due to multiple binding interactions with a target, such as PDGF or VEGF. In yet further embodiments, a plurality of Polyalkylene Glycol, PEG, glycerol lipid molecules can be attached to each other. In these embodiments, one or more aptamers can be associated with each Polyalkylene Glycol, PEG, or glycerol lipid. This can result in an increase in avidity of each aptamer to its target. In addition, in embodiments where there are aptamers to PDGF or aptamers to PDGF and different Targets associated with Polyalkylene Glycol, PEG, or glycerol lipid, a drug can also be associated with, e.g., covalently bonded to, Polyalkylene Glycol, PEG, or glycerol lipid. Thus the compound would provide targeted delivery of the drug, with Polyalkylene Glycol, PEG, or glycerol lipid serving as a Linker, optionally, with one or more additional linkers.
[0120] Aptamers can be 5'-capped and/or 3'-capped with a 5'-5' inverted nucleoside cap structure at the 5' end and/or a 3'-3' inverted nucleoside cap structure at the 3' end. In several embodiments, Antagonist A, Antagonist B, Antagonist C, Antagonist D, pegaptanib, bevasiranib and Sirna-027 are 5' or 3' end-capped.
[0121] Methods for Treating or Preventing an Ophthalmological Disease or Disorder
[0122] The invention provides methods and compositions useful for treating or preventing ophthalmological diseases and disorders, including but not limited to any of the ophthalmological diseases and disorders described herein.
[0123] In some embodiments, the methods for treating or preventing an ophthalmological disease or disorder disclosed herein improve retinal attachment success, improve visual acuity, or stabilize vision. In some embodiments, the methods disclosed herein prevent or retard the rate of further vision loss in a subject.
[0124] In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof in combination with a VEGF antagonist or pharmaceutically acceptable salt thereof and/or an anti-C5 agent improves retinal attachment success, improves visual acuity, or stabilizes vision to a degree that is greater than administration of Antagonist A or another pharmaceutically acceptable salt thereof alone, the VEGF antagonist or pharmaceutically acceptable salt thereof alone, or the anti-C5 agent alone. In some embodiments, the administration of Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist or pharmaceutically acceptable salt thereof, and optionally, an anti-C5 agent, has a synergistic effect in treating or preventing an ophthalmological disease or disorder. For example, the administration of both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist or pharmaceutically acceptable salt thereof can improve retinal attachment success, improve visual acuity, or stabilize vision to a degree that is greater than an additive effect of administering both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist or pharmaceutically acceptable salt thereof. In some embodiments, administration of Antagonist A, alone or in combination with a VEGF antagonist and/or an anti-C5 agent, according to the methods described herein, e.g., treatment or dosing regimens, improves retinal attachment success, improves visual acuity, or stabilizes vision to a degree that is greater than administration of Antagonist A, alone or in combination with a VEGF antagonist and/or an anti-C5 agent, according to previously described methods.
[0125] In particular embodiments, any of the methods and compositions of the present invention are used to treat or prevent an ophthalmological disease or disorder in particular subjects. For example, in certain embodiments, subjects treated according to a method described herein are defined or identified based on their previous treatments for the disease or disorder, specific manifestations of their disease or disorder being treated, and/or other characteristics. In one embodiment, the subject has a defined phenotype or medical history.
[0126] Accordingly, any of the methods described herein may further comprise identifying the subject to be treated, such as by determining whether the subject was previously administered a VEGF antagonist for treating or preventing the disease or disorder or whether the subject had previously failed monotherapy with a VEGF antagonist, e.g., by inquiring of the subject or his health care provider, or by reviewing the subject's medical records.
[0127] In one embodiment, the subject was previously treated with a VEGF antagonist or anti-VEGF monotherapy for any ocular disease or disorder for which a VEGF antagonist is used, or for any of the ocular diseases or disorders described herein (e.g., wet-type AMD).
[0128] In particular embodiments, the methods and compositions described herein are useful for treating or preventing an ophthalmological disease or disorder in a subject who is anti-VEGF resistant, was previously administered or treated with anti-VEGF monotherapy, does not respond or had not responded favorably or adequately to anti-VEGF monotherapy, and/or failed monotherapy with a VEGF antagonist. In some embodiments, a subject who failed monotherapy is anti-VEGF resistant, has complement-mediated inflammation, and/or did not respond adequately to anti-VEGF monotherapy. In one embodiment, the subject who failed monotherapy with a VEGF antagonist is a subject who experienced a poor visual or anatomic outcome after treatment or administration with a VEGF antagonist. In one embodiment, the subject did not exhibit improved vision or exhibited reduced vision following anti-VEGF monotherapy.
[0129] In certain embodiments, the subject does not respond or had not responded favorably or adequately to anti-VEGF monotherapy, as determined by the subject's vision loss or by the subject's lack of significant vision gain following anti-VEGF monotherapy. In one embodiment, the subject's lack of significant vision gain following anti-VEGF monotherapy is determined by the subject's loss of ability to read one or more, in some embodiments three or more, and in some embodiments fifteen or more, letters of a standardized chart of vision testing, e.g., the Early Treatment for Diabetic Retinopathy Study Chart ("ETDRS chart"). In some embodiments, the vision testing is as described in Early Treatment Diabetic Retinopathy Study Research Group (ETDRS), Manual of Operations, Baltimore: ETDRS Coordinating Center, University of Maryland. Available from: National Technical Information Service, 5285 Port Royal Road, Springfield, Va. 22161; Accession No. PB85 223006/AS; Ferris et al., Am J Ophthalmol 94:91-96, 1982; or Example 4, as described herein. In some embodiments, the vision testing uses one or more charts available from http://www.nei.nih.gov/photo/keyword.asp?conditions=Eye+Charts&match=all, e.g., ETDRS visual acuity Chart 1, 2 and/or R.
[0130] In another embodiment, the subject's vision loss following anti-VEGF monotherapy is determined by the subject's loss of ability to read one or more, in some embodiments three or more, letters or lines of a standardized chart of vision testing, e.g., the ETDRS chart, from baseline. In one embodiment, the subject's lack of significant vision gain following anti-VEGF monotherapy is determined by the subject's inability to read an additional one or more, in some embodiment three or more, and in some embodiments fifteen or more, letters of a standardized chart of vision testing, e.g., the ETDRS chart, from baseline. In another embodiment, the subject's lack of significant vision gain following anti-VEGF monotherapy is determined by the subject's inability to read an additional one or more, in some embodiments three or more, lines of a standardized chart of visual testing, e.g., the ETDRS chart, from baseline. In some embodiments, a subject's vision loss or lack of significant vision gain is determined by the subject's visual loss or anatomic signs of poor treatment response, for example, persistent leakage, increased hemorrhage, persistent or increased retinal pigment epithelium (RPE) detachment, signs of neovascular activity, or growth of neovascularization or increased deposition of abnormal matrix or fibrosis. In particular embodiments, a subject's vision loss or lack of significant vision gain is determined at 12 weeks or at 24 weeks following the initiation of treatment.
[0131] In certain embodiments, the subject is anti-VEGF-resistant to a VEGF antagonist, e.g., anti-VEGF monotherapy. In one embodiment, a subject is anti-VEGF resistant if the subject was previously administered with a VEGF antagonist, e.g., anti-VEGF monotherapy, that did not result in the treatment or prevention of the ophthalmological disease or disorder; resulted in only a temporary treatment or prevention of the ophthalmological disease or disorder and rendered the subject in further need of treatment or prevention of the ophthalmological disease or disorder; or that resulted in the subject's visual decline and rendered the subject in further need of treatment or prevention of the ophthalmological disease or disorder.
[0132] In another embodiment, a subject is anti-VEGF resistant if the subject was previously treated or administered with an anti-VEGF treatment, e.g., anti-VEGF monotherapy, and failed to achieve any visual gain or experienced visual decline. In some embodiments, the subject did not respond adequately to anti-VEGF treatment. In one embodiment, the subject was administered the anti-VEGF treatment for one year or longer. In some such embodiments, the subject is in need of treatment for wet AMD.
[0133] Accordingly, the present invention provides methods for treating, preventing, or stabilizing wet AMD in a subject, such as a subject who has failed monotherapy with a VEGF antagonist (e.g., is anti-VEGF resistant, has complement-mediated inflammation, and/or did not respond adequately to anti-VEGF monotherapy). In particular embodiments, the methods comprise determining whether the subject was previously administered or treated with anti-VEGF monotherapy. In certain embodiments, anti-VEGF monotherapy means administration of only one or more VEGF antagonists. In certain embodiments, anti-VEGF monotherapy includes the optional administration of other drugs that are not agents specifically adapted for treating an ophthalmological disease or disorder, e.g, wet AMD.
[0134] In certain embodiments, the methods and compositions described herein are useful for treating or preventing an ophthalmological disease or disorder in a subject that is treatment-naive. In some embodiments, the subject is treatment-naive if the subject was not previously treated for the ophthalmological disease or disorder. In some embodiments, the subject is treatment-naive if the subject was not previously administered or treated with a VEGF antagonist or anti-VEGF monotherapy ("anti-VEGF-treatment-naive"). In particular embodiments, the methods further comprise determining whether the subject was previously treated for the ophthalmological disease or disorder or administered a VEGF antagonist or anti-VEGF monotherapy, e.g., by inquiring of the subject or his or her health care provider, or by reviewing the subject's medical records. In certain embodiments, anti-VEGF monotherapy means administration of only one or more VEGF antagonists. In certain embodiments, anti-VEGF monotherapy includes the optional administration of other drugs that are not agents specifically adapted for treating an ophthalmological disease or disorder, e.g, wet AMD. In some embodiments, the subject is treatment-naive if the subject was not previously treated for AMD (e.g., wet AMD). In some embodiments, the subject is treatment-naive if the subject was not previously treated, or has underwent no previous treatment for AMD (e.g., wet AMD) in either eye. In yet other embodiments, the subject is treatment-naive if the subject was not previously treated, or has underwent no previous treatment, for AMD (e.g., wet AMD; e.g., in either eye) except for one or more oral supplements of vitamins and minerals. In some embodiments, the subject is treatment-naive if the subject was not previously administered a therapeutic agent used for the treatment of AMD (e.g., wet AMD).
[0135] In certain embodiments, the subject has complement-mediated inflammation. In certain embodiments, the subject is anti-VEGF resistant and has complement-mediated inflammation. In certain embodiments, the complement-mediated inflammation is present in an eye of the subject. In certain embodiments, the complement-mediated inflammation results from previous administration with anti-VEGF monotherapy. In other embodiments, the subject has or has been diagnosed with complement-mediated inflammation. In still other embodiments, the subject did not respond adequately to anti-VEGF monotherapy and has or has been diagnosed with complement-mediated inflammation. In certain embodiments, complement-mediated inflammation is diagnosed in the subject using a genetic screening method. Such genetic screening methods are known to those of skill in the art and include, but are not limited to, screening for mutations in complement genes, such as complement factor H (CFH), CFI, CFHR5, and MCP, BF, and C2 genes.
[0136] In certain embodiments, the methods and compositions described herein are useful for treating or preventing an ophthalmological disease or disorder in a subject who is newly diagnosed with the ophthalmological disease or disorder. In some embodiments, the subject is newly diagnosed if the subject was not previously diagnosed for the ophthalmological disease or disorder. In some embodiments, the subject is newly diagnosed with age-related macular degeneration. In some embodiments, the subject is newly diagnosed with dry age-related macular degeneration. In some embodiments, the subject is newly diagnosed with wet-type AMD. In particular embodiments, the methods further comprise determining whether the subject was previously diagnosed for the ophthalmological disease or disorder, e.g., by inquiring of the subject or his or her health care provider, or by reviewing the subject's medical records.
[0137] In some embodiments of the invention, the methods and compositions described herein are useful for treating or preventing an ophthalmological disease or disorder that is a neovascular disorder. In other embodiments of the invention, the ophthalmological disease or disorder results in retinal edema. Illustrative ophthalmological diseases or disorders that can be treated or prevented are described herein.
[0138] Treatment or Prevention of Age-Related Macular Degeneration
[0139] In one embodiment, the ophthalmological disease or disorder treated or prevented by any of the methods or compositions described herein is age-related macular degeneration. Vision changes that can be associated with macular degeneration include distortions and/or blind spots (scotoma) detected using an Amsler grid, changes in dark adaptation (diagnostic of rod cell health), changes in color interpretation (diagnostic of cone cell health), or a decrease in visual acuity. Examples of age-related macular degeneration are nonneovascular (also known as "dry") and neovascular (also known as "wet" or "exudative") macular degeneration.
[0140] In one embodiment, the dry age-related macular degeneration is associated with the formation of drusen. In one embodiment, treating or preventing dry macular degeneration encompasses treating or preventing an abnormality of the retinal pigment epithelium and/or underlying vasculature, known as choriocapilaries. Examples of abnormalities of the retinal pigment epithelium include geographic atrophy, non-geographic atrophy, focal hypopigmentation, and focal hyperpigmentation. In another embodiment, treating or preventing wet age-related macular degeneration encompasses treating or preventing choroidal neovascularization or pigment epithelial detachment.
[0141] In one embodiment, the invention provides methods for treating or preventing wet age-related macular degeneration. Another aspect of the present invention is methods for treating, preventing, or inhibiting a choroidal neovascular complex in a subject, e.g., inhibiting the formation or growth of a choroidal neovascular complex.
[0142] In another aspect of the invention, the invention provides methods for treating or preventing choroidal neovascularization in a subject. In some embodiments, the choroidal neovascularization is subfoveal choroidal neovascularization. In some embodiments, the subfoveal choroidal neovascularization is due to age-related macular degeneration. In one embodiment, the subfoveal choroidal neovascularization is secondary to exudative type AMD. In other embodiments, the subfoveal choroidal neovascularization is present in subjects who have exudative type AMD, and in other embodiments, subfoveal choroidal neovascularization is present in subjects who do not have exudative type AMD. In some embodiments, the subfoveal choroidal neovascularization is secondary to inflammatory, traumatic, myopic, idiopathic or neoplastic afflictions of the macula.
[0143] In some embodiments, wet age-related macular degeneration is classified according to the appearance of its choroidal neovascularization (CNV), into classic, occult or mixed (classic and occult) CNV types, as determined by an angiography, known as fluorescence angiography. Classic, occult or mixed (classic and occult) CNV classification can be based on the time, intensity and level of definition of dye appearance, and leakage from the CNV, as assessed by the fluorescein angiography. In some embodiments, the subject has classic CNV (e.g., pure classic) or mixed CNV (predominantly or minimally classic CNV). In some embodiments, the subject has occult CNV (e.g., pure occult CNV).
[0144] The administration of Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist and/or anti-C5 agent can have a synergistic effect in treating or preventing classic CNV or occult CNV. For example, administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can improve visual acuity or stabilize vision to a degree that is greater than an additive effect of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist. In another example, administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can reduce CNV or inhibit the growth of CNV to a greater degree than administration of Antagonist A or another pharmaceutically acceptable salt thereof or the VEGF antagonist. In some embodiments, administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can reduce CNV in a shorter timeframe or with a lower dosage amount or frequency, as compared to the timeframe or dosage amount with administration of Antagonist A or another pharmaceutically acceptable salt thereof or the VEGF antagonist. In some embodiments, administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can reduce CNV or inhibit the growth of CNV to a greater degree than an additive effect of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist. In some embodiments, administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can reduce CNV in a shorter timeframe or with a lower dosage amount or frequency, as compared to an additive timeframe, dosage amount or frequency with administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist.
[0145] In one embodiment, the present invention provides methods for treating, preventing, or stabilizing non-exudative type ("dry type") AMD. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof, an anti-C5 agent, the combination of Antagonist A or another pharmaceutically acceptable salt thereof and an anti-C5 agent, or the combination of an anti-C5 agent and a VEGF antagonist is administered in an amount effective to maintain about the same level of drusen or reduce the level of drusen (e.g., amount, size, number, area and/or morphology) (e.g., size, number, area and/or morphology) as compared to the subject's drusen level prior to administration of Antagonist A or another pharmaceutically acceptable salt thereof, the anti-C5 agent, the combination of Antagonist A or another pharmaceutically acceptable salt thereof and the anti-C5 agent, or the combination of an anti-C5 agent and a VEGF antagonist, respectively. In a particular embodiment, the level of drusen is reduced by at least or about 5%, at least or about 10%, at least or about 20%, at least or about 30%, at least or about 40%, or at least or about 50%.
[0146] In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, an anti-C5 agent, the combination of Antagonist A or another pharmaceutically acceptable salt thereof and the anti-C5 agent, or the combination of the anti-C5 agent and a VEGF antagonist is administered in an amount effective to inhibit, slow, or prevent the progression of non-exudative type AMD to geographic atrophy (GA). GA is an advanced form of non-exudative type AMD. In other embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof and/or the anti-C5 agent or a pharmaceutically acceptable salt thereof is administered in an amount effective to reduce the growth or area of a GA lesion over time as compared to that in a subject not receiving Antagonist A or another pharmaceutically acceptable salt thereof and/or the anti-C5 agent. In other embodiments, the anti-C5 agent or a pharmaceutically acceptable salt thereof and a VEGF antagonist is administered in an amount effective to reduce the growth or area of a GA lesion over time as compared to that in a subject not receiving the anti-C5 agent and/or the VEGF antagonist. In a particular embodiment, the change in area or growth of the geographic atrophy lesion over time is reduced by at least or about 5%, at least or about 10%, at least or about 20%, at least or about 30%, at least or about 40%, or at least or about 50%. Methods of identifying and assessing the size of geographic lesions are known to those of skill in the art and include autofluorescence imaging and optical coherence tomography.
[0147] In particular embodiments, a subject in whom non-exudative AMD converts to exudative AMD, e.g., when new blood vessels invade the overlying retina, is treated. The present invention further provides methods for treating, preventing, or stabilizing drusen retinopathy secondary to complement-mediated immune disorders, including drusen retinopathy secondary to membranoproliferative glomerulonephritis type II disease. In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and/or an anti-C5 agent and/or a VEGF antagonist is administered in an amount effective to reduce retinal drusen in subjects having or having been diagnosed with membranoproliferative glomerulonephritis type II disease or exudative-type AMD as compared to the level of retinal drusen prior to administration of Antagonist A or another pharmaceutically acceptable salt thereof and/or an anti-C5 agent and/or a VEGF antagonist. In certain embodiments, the level of drusen is reduced by at least or about 5%, at least or about 10%, at least or about 20%, at least or about 30%, at least or about 40%, or at least or about 50%.
[0148] In one embodiment, the ophthalmological disease or disorder is polypoidal choroidal vasculopathy (PCV), a variant of wet AMD.
[0149] Treatment or Prevention of a Condition Associated with Choroidal Neovascularization
[0150] In one embodiment, the ophthalmological disease or disorder is a condition associated with choroidal neovascularization. Examples of conditions associated with choroidal neovascularization include a degenerative, inflammatory, traumatic or idiopathic condition. Treating or preventing a degenerative disorder associated with choroidal neovascularization also encompasses treating or preventing a heredodegerative disorder. Examples of heredodegenerative disorders include vitelliform macular dystrophy, fundus flavimaculatus and optic nerve head drusen. Examples of degenerative conditions associated with choroidal neovascularization include myopic degeneration or angioid streaks. In some embodiments, treating or preventing an inflammatory disorder associated with choroidal neovascularization encompasses treating or preventing ocular histoplasmosis syndrome, multifocal choroiditis, serpininous choroiditis, toxoplasmosis, toxocariasis, rubella, Vogt-Koyanagi-Harada syndrome, Behcet syndrome or sympathetic ophthalmia. In some embodiments, treating or preventing a traumatic disorder associated with choroidal neovascularization encompasses treating or preventing choroidal rupture or a traumatic condition caused by intense photocoagulation.
[0151] Treatment or Prevention of Proliferative Retinopathy
[0152] One particular aspect of the invention provides methods and compositions for treating or preventing proliferative vitreoretinopathy (PVR). In some embodiments, the PVR is a moderate form. In other embodiments, the PVR is a severe form. In some embodiments, the PVR is a recurrent form. In one embodiment, the subject with PVR also has or had retinal detachment, or the subject has PVR associated with retinal detachment, or PVR related scarring (e.g., scarring resulting from PVR, e.g., retinal scarring). In some embodiments, the PVR is characterized based on the configuration of the retina and the location of the scar tissue, such as in shown in Table 2 (See Lean J, et al. Classification of proliferative vitreoretinopathy used in the silicone study. The Silicone study group. Ophthalmology 1989; 96:765-771). Any of these categories or types of PVR can be treated or prevented according to the present invention.
TABLE-US-00001 TABLE 2 Classification of PVR Type Type of Location no. contraction of PVR Summary of Clinical Signs 1 Focal Posterior Starfold 2 Diffuse Posterior Confluent irregular retinal folds in posterior retina; remainder of retina drawn posteriorly; optic disc may not be visible 3 Sub-retinal Posterior "Napkin ring" around disc or "clothesline" elevation of retina 4 Circumferential Anterior Irregular retinal folds in the anterior retina; series of radial folds more posteriorly; peripheral retina within vitreous base stretched inward 5 Perpendicular Anterior Smooth circumferential fold of retina at insertion of posterior hyaloid 6 Anterior Anterior Circumferential fold of retina at insertion of posterior hyaloid pulled forward; trough of peripheral retina anteriorly; ciliary processes stretched with possible hypotony; iris retracted
[0153] The present methods for treating PVR can further comprise administering another agent useful for treating PVR, such as a corticosteriod; antineoplastic drug, such as 5-fluorouracil; colchicine; retinoid; heparin; epidermal growth factor receptor (EGFR) inhibitor, such as gefitinib or erlotinib.
[0154] Another aspect of the invention is methods for treating or preventing a proliferative retinopathy, such as one related to PVR (e.g., treating or preventing an ocular manifestation of a proliferative retinopathy), such as proliferative diabetic retinopathy, sickle cell retinopathy, post traumatic retinopathy, hyperviscosity syndromes, Aortic arch syndromes, ocular ischemic syndromes, carotid-cavernous fistula, multiple sclerosis, retinal vasculitis, systemic lupus erythematosus, arteriolitis with SS-A autoantibody, acute multifocal hemorrhagic vasculitis, vasculitis resulting from infection, vasculitis resulting from Behcet's disease, sarcoidosis, coagulopathies, sickling hemoglobinopathies, AC and C- thalassemia, small vessel hyalinosis, incontinentia pigmenti, Eales' disease, branch retinal artery or vein occlusion, frosted branch angiitis, idiopathic retinal vasculitis, aneurysms, neuroretinitis, retinal embolization, retinopathy of prematurity, Uveitis, pars planitis, acute retinal necrosis, birdshot retinochoroidopathy, long-standing retinal detachment, choroidal melanoma, radiation retinopathy, familial exudative vitreoretinopathy, inherited retinal venous beading, retinoschisis, retinitis pigmentosa, or autosomal dominant vitreoretinochoroidopathy.
[0155] Another aspect of the invention is methods for treating or preventing a disease or condition that is a cause that results in proliferative retinopathy or PVR. In one embodiment, post-retinal detachment (e.g., that causes or results in PVR) is treated or prevented. In another embodiment, proliferative diabetic retinopathy (e.g., that causes or results in PVR) or sickle-cell retinopathy (e.g., that causes or results in PVR), as well as scarring caused by one or more of these disorders is treated or prevented.
[0156] Treatment or Prevention of Glaucoma
[0157] In one embodiment, the opthalmological disease or disorder is glaucoma. In one embodiment the glaucoma is open angle glaucoma, primary open angle glaucoma, secondary open angle glaucoma, closed angle glaucoma, glaucoma that is associated with diabetes, glaucoma that is associated with diabetic retinopathy, angle closure glaucoma, narrow angle glaucoma or acute glaucoma.
[0158] Treatment or Prevention of a Neoplasm
[0159] In one embodiment, the ophthalmological disease or disorder is a neoplasm. Examples of neoplams include an eyelid tumor, a conjunctival tumor, a choroidal tumor, an iris tumor, an optic nerve tumor, a retinal tumor, an infiltrative intraocular tumor or an orbital tumor. Examples of an eyelid tumor include basal cell carcinoma, squamous carcinoma, sebaceous carcinoma, malignant melanoma, capillary hemangioma, hydrocystoma, nevus or seborrheic keratosis. Examples of a conjunctival tumor include conjunctival Kaposi's sarcoma, squamous carcinoma, intraepithelial neoplasia of the conjunctiva, epibular dermoid, lymphoma of the conjunctiva, melanoma, pingueculum, or pterygium. Examples of a choroidal tumor include choroidal nevus, choroidal hemangioma, metastatic choroidal tumor, choroidal osteoma, choroidal melanoma, ciliary body melanoma or nevus of Ota. Examples of an iris tumor include anterior uveal metastasis, iris cyst, iris melanocytoma, iris melanoma, or pearl cyst of the iris. Examples of an optic nerve tumor include optic nerve melanocytoma, optic nerve sheath meningioma, choroidal melanoma affecting the optic nerve, or circumpapillary metastasis with optic neuropathy. Examples of a retinal tumor include retinal pigment epithelial (RPE) hypertrophy, RPE adenoma, RPE carcinoma, retinoblastoma, or hamartoma of the RPE. In some embodiments, the present invention provides methods for inhibiting retinal pigment epithelium (RPE) or glial cells, such as inhibiting the migration of RPE or glial cells. Examples of an infiltrative intraocular tumor include chronic lymphocytic leukemia, infiltrative choroidopathy, or intraocular lymphoma. Examples of an orbital tumor include adenoid cystic carcinoma of the lacrimal gland, cavernous hemangioma of the orbit, lymphangioma of the orbit, orbital mucocele, orbital pseudotumor, orbital rhabdomyosarcoma, periocular hemangioma of childhood, or sclerosing orbital psuedotumor.
[0160] Another aspect of the invention is methods for treating or preventing von Hippel-Lindau (VHL) disease (e.g., treating or preventing visual loss associated VHL disease). In some embodiments, VHL disease is characterized by tumors. The tumors may be malignant or benign. In another embodiment, a benign or malignant tumor in the eye (e.g., ocular tumor) or a cyst (e.g., an ocular cyst), associated with VHL is treated or prevented. In some embodiments, the tumors are hemangioblastomas. In some embodiments, the tumors are von Hippel angioma or retinal capillary hemangiomas (e.g., juxtapapillary hemangioma).
[0161] In some embodiments, the subject with VHL disease has a deficiency of the protein "pvHL."
[0162] In some embodiments, the VHL disease is severe (e.g., a subject with severe VHL disease has a lesion that cannot be effectively treated with a non-pharmacologic modality (e.g., laser or cryotherapy), for example, as the lesion resides over or adjacent to a significant neural structure (e.g., optic nerve, macula, papillomacular bundle) that can be damaged with laser or cryotherapy).
[0163] In some embodiments, the methods for treating or preventing VHL disease comprise treating an ocular or non-ocular manifestation (e.g., benign or malignant neoplasm or cyst of the kidney, adrenal gland, pancreas, brain, spinal cord, inner ear, epididymis, or broad ligament) of VHL.
[0164] In some embodiments, the subjected being treated has a family history of VHL disease or one or more of retinal capillary hemangioma (RCH), spinal or cerebellar hemangioblastoma, pheochromocytoma, multiple pancreatic cysts, epididymal or broad ligament cystadenoma, multiple renal cysts, and renal cell carcinoma. In some embodiments, the subject has one or more RCH, spinal and cerebellar hemangioblastoma, pheochromocytoma, multiple pancreatic cysts, epididymal or broad ligament cystadenomas, multiple renal cysts, or renal cell carcinoma before the age of 60 years. In some embodiments, the subject has two or more hemangioblastomas of the retina or brain or a single hemangioblastoma in association with a visceral manifestation, such as kidney or pancreatic cysts; renal cell carcinoma; adrenal or extra-adrenal pheochromocytomas; endolymphatic sac tumors; papillary cystadenomas of the epididymis or broad ligament; or neuroendocrine tumors of the pancreas. In some embodiments, the subject has a disease-causing germline mutation in the VHL gene.
[0165] In some embodiments, the subject has RCH that exhibit activity, such as associated intra- or sub-retinal exudation or lipid deposition (which may reflect ongoing vascular incompetence and is not reflective of residual changes following previous treatment or secondary to coexistent retinal traction); increased size of the tumor compared to a previous time point as assessed by fundus photography or fluorescein angiography (FA); associated intra-, sub-, or pre-retinal hemorrhage not secondary to previous treatment, as assessed by fundus photography or FA; appearance of new feeder vessels or greater dilation or tortuosity of existing feeder vessels compared to a previous time point; and/or vitreous cell or haze indicative of vitreous exudation, in the absence of other ocular features potentially responsible for such findings. In some embodiments, the subject has RCH that is not readily treatable using cryotherapy or thermal laser because of its size, posterior location, poor previous response to conventional therapy, or other factors.
[0166] In some embodiments, methods or compositions of the invention are used to treat or prevent a complication of VHL, visual dysfunction (e.g., from VHL), or a fibrous complication of VHL (e.g., fibrous meningioma). In certain embodiments, the methods or compositions of the present invention are used to treat a manifestation of VHL as vascular proliferation that comprises fine, superficial, juxtapapillary vessels that are often associated with fibrovascular proliferation and epiretinal membrane formation.
[0167] Treatment or Prevention of Scarring or Fibrosis
[0168] Another aspect the invention provides methods for treating, inhibiting or preventing scarring or fibrosis (e.g., scarring or fibrosis is under the macular region of the retina). In some embodiments, the scarring is a fibrovascular scar (e.g., in the retina). In some embodiments, the fibrosis is hepatic, pulmonary or renal fibrosis. In some embodiments, the fibrosis is ocular fibrosis. In some embodiments, the fibrosis is sub-retinal fibrosis (e.g., associated with neovascular AMD). In some embodiments, the sub-retinal fibrosis is not associated with neovascular AMD. In some embodiments, the fibrosis is subfoveal fibrosis. In some embodiments, the subfoveal fibrosis is with retinal atrophy. In some embodiments, subfoveal fibrosis or sub-retinal fibrosis develops after administration of a VEGF antagonist, e.g., anti-VEGF monotherapy.
[0169] In some embodiments, the scarring results from glaucoma surgery, or follows glaucoma surgery, such as trabeculectomy, filtering surgery (such as partial thickness filtering surgery), glaucoma filtering procedures, minimally invasive glaucoma surgery, glaucoma valve implant surgery, glaucoma seton surgery, glaucoma tube shunt placement, glaucoma stent placement, or combined cataract and glaucoma surgery. In some embodiments, the methods of the present invention are useful to treat or prevent scarring relating to or resulting from glaucoma surgery (e.g., that can result in scar related proliferation). In some embodiments, the scarring is sub-retinal scarring. In some embodiments, the scarring is sub-retinal scarring that occurs following choroidal neovascular regression.
[0170] In particular embodiments, methods for treating, inhibiting or preventing sub-retinal fibrosis (e.g., reducing the formation of sub-retinal fibrosis) comprise administering to a subject in need thereof an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist. In some embodiments, the subject has or is diagnosed with AMD (e.g., wet AMD). In some embodiments, the subject has or is diagnosed with advanced wet AMD.
[0171] Treatment or Prevention of Other Ophthalmological Diseases and Disorders
[0172] In certain embodiments, the ophthalmological disease or disorder is a cataract (e.g., age-related cataract), diabetic macula edema, macular telangiectasia (e.g., type 1 or 2 macular telangiectasia), atrophic macular degeneration, chorioretinopathy (e.g., central serous chorioretinopathy), retinal inflammatory vasculopathy, pathological retinal angiogenesis, age-related maculopathy, retinoblastoma, Pseudoxanthoma elasticum, a vitreoretinal disease, choroidal sub-retinal neovascularization, central serous chorioretinopathy, ischemic retinopathy, hypertensive retinopathy or diabetic retinopathy (e.g., nonproliferative or proliferative diabetic retinopathy, such as macular edema or macular ischemia), retinopathy of prematurity (e.g., associated with abnormal growth of blood vessels in the vascular bed supporting the developing retina), venous occlusive disease (e.g., a retinal vein occlusion, branch retinal vein occlusion or central retinal vein occlusion), arterial occlusive disease (e.g., branch retinal artery occlusion (BRAO), central retinal artery occlusion or ocular ischemic syndrome), central serous chorioretinopathy (CSC), cystoid macular edema (CME) (e.g., affecting the central retina or macula, or after cataract surgery), retinal telangiectasia (e.g., characterized by dilation and tortuosity of retinal vessels and formation of multiple aneurysms, idiopathic JXT, Leber's miliary aneurysms, or Coats' disease), arterial macroaneurysm, retinal angiomatosis, radiation-induced retinopathy (RIRP), or rubeosis iridis (e.g., associated with the formation of neovascular glaucoma, diabetic retinopathy, central retinal vein occlusion, ocular ischemic syndrome, or chronic retinal detachment).
[0173] In other embodiments, the ophthalmological disease or disorder is sickle cell disease (SCD), anemia, or sickle cell retinopathy (e.g., non-neovascular or non-proliferative ocular manifestations). In some embodiments, vaso-occlusive phenomena or hemolysis associated with SCD is treated or prevented. In some embodiments, ocular manifestations of SCD include vascular occlusions in the conjunctiva, iris, retina, or choroid. Non-neovascular or non-proliferative ocular manifestations can include conjunctival vascular occlusions which transform smooth vessels into comma-shaped fragments, iris atrophy, retinal "salmon patch" hemorrhages, retinal pigmentary changes and other abnormalities of the retinal vasculature, macula, choroid, and optic disc. In some embodiments, neovascularization or the proliferative ocular manifestation involves the growth of abnormal vascular fronds which can lead to vitreous hemorrhage, retinal detachment, epiretinal membranes, resulting in vision loss. In some embodiments, the methods further comprise performing another treatment, such as diathermy, cryotherapy, laser photocoagulation or surgery (e.g., vitrectomy).
[0174] In one embodiment, the ophthalmological disease or disorder is a condition associated with peripheral retinal neovascularization. Examples of conditions associated with peripheral retinal neovascularization include ischemic vascular disease, inflammatory disease with possible ischemia, incontinentia pigmenti, retinitis pigmentosa, retinoschisis or chronic retinal detachment.
[0175] Examples of ischemic vascular disease include proliferative diabetic retinopathy, branch retinal vein occlusion, branch retinal arteriolar occlusion, carotid cavernous fistula, sickling hemoglobinopathy, non-sickling hemoglobinopathy, IRVAN syndrome (retinal vasculitic disorder characterized by idiopathic retinal vasculitis, an aneurysm, and neuroretinitis), retinal embolization, retinopathy of prematurity, familial exudative vitreoretinopathy, hyperviscosity syndrome, aortic arch syndrome or Eales disease. Examples of sickling hemoglobinopathy include SS hemoglobinopathy and SC hemoglobinopathy. Examples of non-sickling hemoglobinopathy include AC hemoglobinopathy and AS hemoglobinopathy. Examples of hyperviscosity syndrome include leukemia, Waldenstrom macroglobulinemia, multiple myeloma, polycythemia or myeloproliferative disorder.
[0176] In some embodiments, treating or preventing an inflammatory disease with possible ischemia encompasses treating or preventing retinal vasculitis associated with systemic disease, retinal vasculitis associated with an infectious agent, uveitis or birdshot retinopathy. Examples of systemic diseases include systemic lupus erythematosis, Behcet's disease, inflammatory bowel disease, sarcoidosis, multiple sclerosis, Wegener's granulomatosis and polyarteritis nodosa. Examples of infectious agents include a bacterial agent that is the causative agent for syphilis, tuberculosis, Lyme disease or cat-scratch disease, a virus such as herpesvirus, or a parasite such as Toxocara canis or Toxoplasma gondii. Examples of uveitis include pars planitis or Fuchs uveitis syndrome.
[0177] Compositions for Therapeutic or Prophylactic Administration
[0178] Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonists, or anti-C5 agents can be administered as a component of a composition that further comprises a pharmaceutically acceptable carrier or vehicle, e.g., a pharmaceutical composition. In certain embodiments, each therapeutic agent is administered to the subject in a separate composition. However, in other embodiments, two or more therapeutic agents may be administered to the subject in the same composition. In one embodiment, a composition of the invention comprises an effective amount of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist, and/or an anti-C5 agent and a pharmaceutically acceptable carrier or vehicle. In another embodiment, a composition comprising Antagonist A or another pharmaceutically acceptable salt thereof and another composition comprising a VEGF antagonist are administered. In some embodiments, another composition comprising an anti-C5 agent is administered. In some embodiments, a composition comprising Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist is administered. In some embodiments, another composition comprising an anti-C5 agent is also administered.
[0179] Administration of each antagonist may be by any suitable means that results in an amount of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist, and/or anti-C5 agent that is effective for the treatment or prevention of an ophthalmological disease or disorder. Each antagonist, for example, can be admixed with a suitable carrier substance, and is generally present in an amount of 1-95% by weight of the total weight of the composition. The composition may be provided in a dosage form that is suitable for ophthalmic, oral, parenteral (e.g., intravenous, intramuscular, subcutaneous), rectal, transdermal, nasal, or inhalant administration. In one embodiment, the composition is in a form that is suitable for injection directly in the eye. The composition may be in form of, e.g., tablets, capsules, pills, powders, granulates, suspensions, emulsions, solutions, gels including hydrogels, pastes, ointments, creams, plasters, delivery devices, suppositories, enemas, injectables, implants, sprays, drops or aerosols. The compositions comprising one or more antagonists can be formulated according to conventional pharmaceutical practice (see, e.g., Remington: The Science and Practice of Pharmacy, (20th ed.) ed. A. R. Gennaro, 2000, Lippincott Williams & Wilkins, Philadelphia, Pa. and Encyclopedia of Pharmaceutical Technology, eds., J. Swarbrick and J. C. Boylan, 1988-2002, Marcel Dekker, New York).
[0180] The compositions are, in one useful aspect, administered parenterally (e.g., by intramuscular, intraperitoneal, intravenous, intraocular, intravitreal, retro-bulbar, subconjunctival, subtenon or subcutaneous injection or implant) or systemically. Formulations for parenteral or systemic administration include sterile aqueous or non-aqueous solutions, suspensions, or emulsions. A variety of aqueous carriers can be used, e.g., water, buffered water, saline, and the like. Examples of other suitable vehicles include polypropylene glycol, polyethylene glycol, vegetable oils, gelatin, hydrogels, hydrogenated naphalenes, and injectable organic esters, such as ethyl oleate. Such formulations may also contain auxiliary substances, such as preserving, wetting, buffering, emulsifying, and/or dispersing agents. Biocompatible, biodegradable lactide polymer, lactide/glycolide copolymer, or polyoxyethylene-polyoxypropylene copolymers may be used to control the release of the active ingredients.
[0181] Alternatively, the compositions can be administered by oral ingestion. Compositions intended for oral use can be prepared in solid or liquid forms, according to any method known to the art for the manufacture of pharmaceutical compositions.
[0182] Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules. Generally, these pharmaceutical preparations contain active ingredients admixed with non-toxic pharmaceutically acceptable excipients. These include, for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, sucrose, glucose, mannitol, cellulose, starch, calcium phosphate, sodium phosphate, kaolin and the like. Binding agents, buffering agents, and/or lubricating agents (e.g., magnesium stearate) may also be used. Tablets and pills can additionally be prepared with enteric coatings. The compositions may optionally contain sweetening, flavoring, coloring, perfuming, and preserving agents in order to provide a more palatable preparation.
[0183] Compositions useful for ophthalmic use include tablets comprising one or more antagonists in admixture with a pharmaceutically acceptable excipient. These excipients may be, for example, inert diluents or fillers (e.g., sucrose and sorbitol), lubricating agents, glidants, and antiadhesives (e.g., magnesium stearate, zinc stearate, stearic acid, silicas, hydrogenated vegetable oils, or talc).
[0184] The antagonists of the present invention may be admixed in a tablet or other vehicle, or may be partitioned. In one example, one antagonist is contained on the inside of the tablet, and the other antagonist is on the outside, such that a substantial portion of the other antagonist is released prior to the release of the contained antagonist. If desired, antagonists in a tablet form may be administered using a drug delivery device (see below).
[0185] For example, compositions of the present invention may be administered intraocularly by intravitreal injection into the eye as well as by subconjunctival and subtenon injections. Other routes of administration include transcleral, retrobulbar, intraperitoneal, intramuscular, and intravenous. Alternatively, compositions can be administered using a drug delivery device or an intraocular implant (see below).
[0186] In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof or VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008) is administered intravitreally with a 30-gauge or 27-gauge needle. In some embodiments, a 0.5 inch needle is used. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally with a 30-gauge 0.5 inch needle and a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008) is administered intravitreally with a 27-gauge needle. In some embodiments, 50 .mu.L (1.5 mg in 0.05 mL) of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally with a 30-gauge 0.5 inch needle and 504, of a VEGF antagonist (e.g., 0.5 mg of ranibizumab, 1.25 mg of bevacizuamb, or 2.0 mg of aflibercept) is administered intravitreally with a 27-gauge needle.
[0187] Liquid dosage forms for oral administration can include pharmaceutically acceptable emulsions, solutions, suspensions, syrups, and soft gelatin capsules. These forms can contain inert diluents commonly used in the art, such as water or an oil medium, and can also include adjuvants, such as wetting agents, emulsifying agents, and suspending agents.
[0188] In some instances, the compositions can also be administered topically, for example, by patch or by direct application to a region, such as the epidermis or the eye, susceptible to or affected by a neovascular disorder, or by iontophoresis.
[0189] In one embodiment, the compositions can comprise one or more pharmaceutically acceptable excipients. In one embodiment, excipients for compositions that comprise an antagonist include, but are not limited to, buffering agents, nonionic surfactants, preservatives, tonicity agents, sugars, amino acids, and pH-adjusting agents. Suitable buffering agents include, but are not limited to, monobasic sodium phosphate, dibasic sodium phosphate, and sodium acetate. Suitable nonionic surfactants include, but are not limited to, polyoxyethylene sorbitan fatty acid esters such as polysorbate 20 and polysorbate 80. Suitable preservatives include, but are not limited to, benzyl alcohol. Suitable tonicity agents include, but are not limited to sodium chloride, mannitol, and sorbitol. Suitable sugars include, but are not limited to, .alpha.,.alpha.-trehalose. Suitable amino acids include, but are not limited to glycine and histidine. Suitable pH-adjusting agents include, but are not limited to, hydrochloric acid, acetic acid, and sodium hydroxide. In one embodiment, the pH-adjusting agent or agents are present in an amount effective to provide a pH of about 3 to about 8, about 4 to about 7, about 5 to about 6, about 6 to about 7, or about 7 to about 7.5. In one embodiment, the compositions do not comprise a preservative. In another embodiment, the composition does not comprise an antimicrobial agent. In another embodiment, the composition does not comprise a bacteriostat. Suitable excipients for a VEGF antagonist also include those described in U.S. Pat. No. 7,365,166, the contents of which are herein incorporated by reference in their entirety.
[0190] In one embodiment, the composition is in the form of an aqueous solution that is suitable for injection. In one embodiment, a composition is in the form of an aqueous solution that is suitable for injection. In one embodiment, a composition comprises Antagonist A or another pharmaceutically acceptable salt thereof, a buffering agent, a pH-adjusting agent, and water for injection. In another embodiment, a composition comprises Antagonist A or another pharmaceutically acceptable salt thereof, monobasic sodium phosphate, dibasic sodium phosphate, sodium chloride, hydrochloride acid, and sodium hydroxide.
[0191] In one embodiment, the composition comprises a VEGF antagonist, a buffering agent, a sugar, a nonionic surfactant, and water for injection. In another embodiment, the composition comprises a VEGF antagonist, monobasic sodium phosphate, dibasic sodium phosphate, .alpha.,.alpha.-trehalose dehydrate, and polysorbate 20. In one embodiment, the composition comprises a VEGF antagonist, a buffering agent, a pH-adjusting agent, a tonicity agent, and water that is suitable for injection. In another embodiment, the composition comprises a VEGF antagonist, monobasic sodium phosphate, dibasic sodium phosphate, sodium chloride, hydrochloric acid, and sodium hydroxide. In one embodiment, the VEGF antagonist is a pegylated anti-VEGF aptamer, e.g., pegaptanib sodium
[0192] In another embodiment, the VEGF antagonist is ranibizumab, bevacizumab, aflibercept or ESBA1008. This invention provides the pharmaceutically acceptable salts of the antagonists. An antagonist of the present invention can possess a sufficiently basic functional group, which can react with any of a number of inorganic and organic acids, to form a pharmaceutically acceptable salt. A pharmaceutically-acceptable acid addition salt is formed from a pharmaceutically-acceptable acid, as is well known in the art. Such salts include the pharmaceutically acceptable salts listed in Journal of Pharmaceutical Science, 66, 2-19 (1977) and The Handbook of Pharmaceutical Salts; Properties, Selection, and Use. P. H. Stahl and C. G. Wermuth (ED.s), Verlag, Zurich (Switzerland) 2002, which are hereby incorporated by reference in their entirety.
[0193] Examples of a pharmaceutically acceptable salts include sulfate, citrate, acetate, oxalate, chloride, bromide, iodide, nitrate, bisulfate, phosphate, acid phosphate, isonicotinate, lactate, salicylate, acid citrate, tartrate, oleate, tannate, pantothenate, bitartrate, ascorbate, succinate, maleate, gentisinate, fumarate, gluconate, glucaronate, saccharate, formate, benzoate, glutamate, methanesulfonate, ethanesulfonate, benzenesulfonate, p-toluenesulfonate, camphorsulfonate, pamoate, phenylacetate, trifluoroacetate, acrylate, chlorobenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, methylbenzoate, o-acetoxybenzoate, naphthalene-2-benzoate, isobutyrate, phenylbutyrate, .alpha.-hydroxybutyrate, butyne-1,4-dicarboxylate, hexyne-1,4-dicarboxylate, caprate, caprylate, cinnamate, glycollate, heptanoate, hippurate, malate, hydroxymaleate, malonate, mandelate, mesylate, nicotinate, phthalate, teraphthalate, propiolate, propionate, phenylpropionate, sebacate, suberate, p-bromobenzenesulfonate, chlorobenzenesulfonate, ethylsulfonate, 2-hydroxyethylsulfonate, methylsulfonate, naphthalene-1-sulfonate, naphthalene-2-sulfonate, naphthalene-1,5-sulfonate, xylenesulfonate, and tartarate salts. The term "pharmaceutically acceptable salt" includes a hydrate of a compound of the invention and also refers to a salt of an antagonist of the present invention having an acidic functional group, such as a carboxylic acid functional group or a hydrogen phosphate functional group, and a base. Suitable bases include, but are not limited to, hydroxides of alkali metals such as sodium, potassium, and lithium; hydroxides of alkaline earth metal such as calcium and magnesium; hydroxides of other metals, such as aluminum and zinc; ammonia, and organic amines, such as unsubstituted or hydroxy-substituted mono-, di-, or tri-alkylamines, dicyclohexylamine; tributyl amine; pyridine; N-methyl, N-ethylamine; diethylamine; triethylamine; mono-, bis-, or tris-(2-OH-lower alkylamines), such as mono-; bis-, or tris-(2-hydroxyethyl)amine, 2-hydroxy-tert-butylamine, or tris-(hydroxymethyl)methylamine, N,N-di-lower alkyl-N-(hydroxyl-lower alkyl)-amines, such as N,N-dimethyl-N-(2-hydroxyethyl)amine or tri-(2-hydroxyethyl)amine; N-methyl-D-glucamine; and amino acids such as arginine, lysine, and the like. In one embodiment, the pharmaceutically acceptable salt is a sodium salt. In another embodiment, the pharmaceutically acceptable salt is a persodium salt.
[0194] The present invention further provides comprising Antagonist A or another pharmaceutically acceptable salt thereof. In one embodiment, the present compositions comprise about 30.0 mg of Antagonist A or another pharmaceutically acceptable salt thereof, about 0.3 mg of monobasic sodium phosphate monohydrate, about 2.1 mg of dibasic sodium phosphate heptahydrate and about 9.0 mg of sodium chloride per about 1 mL. In some embodiments, hydrochloric acid and/or sodium hydroxide are present as needed to adjust the pH of the composition. In some embodiments, the pH is about pH 5.5 to about pH 7.5 or about pH 6.0.
[0195] In some embodiments, the compositions comprise about 3% (w/v) of Antagonist A or another pharmaceutically acceptable salt thereof, about 0.03% (w/v) of monobasic sodium phosphate monohydrate, about 0.2% (w/v) of dibasic sodium phosphate heptahydrate, about 0.9% (w/v) of sodium chloride and about 95.9% (w/v) of water. In some embodiments, hydrochloric acid and/or sodium hydroxide are present as needed to adjust the pH of the composition. In some embodiments, the pH is about pH 5.5 to about pH 7.5 or about pH 6.0.
[0196] In certain embodiments, the concentration of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and/or an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof) in a composition is about 0.002 mg/mL to about 50 mg/mL. In some embodiments, the concentration of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and/or an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof) in a composition is less than or about 100 mg/mL, less than about 50 mg/mL, less than about 40 mg/mL, less than about 30 mg/mL, less than about 25 mg/mL, less than about 20 mg/mL, less than about 15 mg/mL, less than about 10 mg/mL, or less than about 5 mg/mL. In certain embodiments, the concentration of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and/or an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof) in a composition is about 0.3 mg/mL to about 100 mg/mL, about 0.3 mg/mL to about 50 mg/mL, about 0.3 mg/mL to about 40 mg/mL, about 0.3 mg/mL to about 30 mg/mL, about 0.3 to about 25 mg/mL, about 0.3 mg/mL to about 20 mg/mL, about 0.3 mg/mL to about 15 mg/mL, about 0.3 mg/mL to about 10 mg/mL, about 1 mg/mL to about 100 mg/mL, about 1 mg/mL to about 50 mg/mL, about 1 mg/mL to about 40 mg/mL, about 1 mg/mL to about 30 mg/mL, about 1 mg/mL to about 25 mg/mL, about 1 mg/mL to about 20 mg/mL, about 1 mg/mL to about 15 mg/mL, about 1 mg/mL to about 10 mg/mL, about 1 mg/mL to about 5 mg/mL, about 5 mg/mL to about 100 mg/mL, or about 5 mg/mL to about 50 mg/mL.
[0197] In certain embodiments, methods of the invention comprise administering Antagonist A and optionally one or both of a VEGF antagonist and an anti-C5 agent as a component of a pharmaceutical composition. In one embodiment, the present invention provides compositions comprising an effective amount of: (a) Antagonist A or another pharmaceutically acceptable salt thereof; and (b) a VEGF antagonist or a pharmaceutically acceptable salt thereof. In certain embodiments, the compositions further comprise an effective amount of an anti-C5 agent or a pharmaceutically acceptable salt thereof. In some embodiments, the compositions stabilize one or more of the Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist, and the anti-C5 agent. In certain embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist and/or the anti-C5 agent does not adversely affect the activity of the other active agent(s) present in the composition. In particular embodiments, at least about 90% of one or more of the active agents in the composition, e.g., Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist, or anti-C5 agent, is chemically stable when the composition is stored at a temperature of from about 2.0.degree. C. to about 8.0.degree. C. for at least about twelve weeks.
[0198] In particular embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist or the anti-C5 agent is chemically stable when it shows no sign of decomposition or modification resulting in formation of a new chemical entity. In particular embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist or the anti-C5 agent is chemically stable when at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, a least about 95%, or at least about 99% of Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist or the anti-C5 agent shows no sign of decomposition or modification resulting in formation of a new chemical entity, e.g., when stored at a temperature of from about 2.0.degree. C. to about 8.0.degree. C. for at least about twelve weeks.
[0199] In certain embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof does not adversely affect the activity of the VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008) or the ARC1905 or a pharmaceutically acceptable salt thereof. In certain embodiments, the VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008) does not adversely affect the activity of the Antagonist A or another pharmaceutically acceptable salt thereof, or ARC1905 or a pharmaceutically acceptable salt thereof. In certain embodiments, ARC1905 or a pharmaceutically acceptable salt thereof does not adversely affect the activity of the Antagonist A or another pharmaceutically acceptable salt thereof, or the VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008).
[0200] In particular embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof and ranibizumab, bevacizumab, aflibercept, pegaptanib sodium or ESBA1008, or a pharmaceutically acceptable salt thereof, and the compositions are physically or chemically stable with respect to both active agents at a particular pH or suitable for parenteral administration. In particular embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof; ranibizumab, bevacizumab, aflibercept, pegaptanib sodium or ESBA1008 or a pharmaceutically acceptable salt thereof and ARC1905 or a pharmaceutically acceptable salt thereof, and the compositions are physically or chemically stable with respect to all active agents at a particular pH or suitable for parenteral administration. In particular embodiments, a composition is physically stable if at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, or at least about 99% of all active agents, i.e., the Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist, and the anti-C5 agent (when present) present in the composition show no sign of aggregation, precipitation or denaturation upon visual examination of color or clarity, or as measured by UV light scattering or by size exclusion chromatography (SEC) or differential scanning calorimetry (DSC).
[0201] In particular embodiments, the compositions of the invention are considered physically stable if after storage the average number of particles detected does not exceed about 50 particles/mL, where the particles have a diameter >about 10 .mu.m and does not exceed 5 particles/mL, where the particles have a diameter >25 .mu.m, as measured by the Light Obscuration Particle Count Test described in (788) Particulate Matter in Injections, Revised Bulletin, Official Oct. 1, 2011, The United States Pharmacopeial Convention.
[0202] In particular embodiments, the compositions are considered physically stable if after storage the average number of particles detected does not exceed 50 particles/mL, where the particles have a diameter >10 .mu.m; does not exceed 5 particles/mL, where the particles have a diameter >25 .mu.m; and does not exceed 2 particles/mL, where the particles have a diameter >50 .mu.M, as measured by the microscopic method particle count test described in (788) Particulate Matter in Injections, Revised Bulletin, Official Oct. 1, 2011, The United States Pharmacopeial Convention.
[0203] In particular embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium) and, optionally, an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof) and are chemically stable for at least eight weeks or at least twelve weeks at 25.degree. C. or for at least twelve weeks or at least sixteen weeks or at least 24 weeks at 4.degree. C. In particular embodiments, at least 80% of each of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist, and anti-C5 agent (if present) show no sign of decomposition or modification resulting in formation of a new chemical entity under at least one of these conditions.
[0204] In particular embodiments, compositions comprise the following: (1) Antagonist A or another pharmaceutically acceptable salt thereof; (2) a VEGF antagonist; optionally, (3) an anti-C5 agent; (4) a buffer; optionally, (5) a tonicity modifier; and, optionally, (6) a surfactant. In specific embodiments of such compositions, the buffer is an acetate, phosphate, Tris or histidine buffer, or a mixture thereof; the tonicity modifier is sodium chloride, mannitol, sorbitol, or trehalose, or a mixture thereof; and the surfactant is polysorbate 20. In various embodiments, Antagonist A or another pharmaceutically acceptable salt thereof is present in compositions of the invention at a concentration of about 0.1 mg/mL to about 200 mg/mL; and the VEGF antagonist is present at a concentration of about 0.1 mg/mL to about 200 mg/mL. When present, the anti-C5 agent is present at a concentration of about 0.1 mg/mL to about 200 mg/mL. The buffer is present at a concentration of about 1 mM to about 200 mM; the tonicity modifier is present at a concentration of about 10 mM to about 200 mM (sodium chloride), about 1% to about 10% (w/v) (sorbitol), or about 1% to about 20% (w/v) (trehalose); and the surfactant, when present, is present at a concentration of about 0.005% to about 0.05% or a concentration of about 0.001% to about 0.05%.
[0205] In particular embodiments, the ratio of the concentration (mass of Antagonist A or another pharmaceutically acceptable salt thereof less that of its --R group/volume of composition) of Antagonist A or another pharmaceutically acceptable salt thereof to the concentration (mass/volume of composition) of the VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008), ARC1905, or a pharmaceutically acceptable salt thereof, present in the composition is less than, or less than or equal to, 25.0, less than, or less than or equal to, 10.0, less than, or less than or equal to, 9.0, less than, or less than or equal to, 8.0, less than, or less than or equal to, 7.0, less than, or less than or equal to, 6.0, less than, or less than or equal to, 5.0, less than, or less than or equal to, 4.0, less than, or less than or equal to, 3.0, less than, or less than or equal to, 2.0 or less than, or less than or equal to, 1.0. Antagonist A's --R group is depicted in FIG. 1. In particular embodiments, the ratio of the concentration (mass of Antagonist A or another pharmaceutically acceptable salt thereof less that of its --R group/volume of composition) of Antagonist A or another pharmaceutically acceptable salt thereof to the concentration (mass/volume of composition) of the VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008), ARC1905, or a pharmaceutically acceptable salt thereof, present in the composition is in the range of about 1 to about 10, about 2 to about 5, about 3 about 4, or about 5. In certain embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, pegaptanib sodium, or ESBA1008), and ARC1905 or a pharmaceutically acceptable salt thereof.
[0206] In one particular embodiment, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008, or pegaptanib sodium), and, optionally, an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof), wherein the ratio of the concentration of PDGF antagonist to the concentration of VEGF antagonist (and/or anti-C5 agent) is less than 2; and the compositions further comprise sodium chloride at a concentration of about 10 mM to about 200 mM, histidine at a concentration of about 1 mM to about 100 mM, and polysorbate (e.g., polysorbate 20) at a concentration of about 0.005% to about 0.05%, where the pH of the composition is about 5.5 to about 7.0.
[0207] In certain embodiments, the compositions comprise one or more of a tonicity modifier, a surfactant, and a buffer suitable to achieve or maintain the particular pH or be suitable for parenteral administration. Appropriate buffers include those described herein as well as others known in the art, such as, e.g., Good's buffers, e.g., MES.
[0208] In certain embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and a tonicity modifier that is sorbitol or sodium chloride, or mixtures thereof. In certain embodiments, the compositions further comprise an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In particular embodiments, the tonicity modifier is sorbitol, and the pH of the composition is about 5.0 to about 8.0, about 5.0 to about 7.0, about 6.0 or about 7.0. In particular embodiments, the tonicity modifier is sodium chloride, and the pH of the composition is about 5.0 to about 8.0, about 5.0 to about 7.0, about 5.5 to about 7.5, about 6.0 to about 8.0, about 8.0, about 7.0, or about 6.0. In certain embodiments, the tonicity modifier is sorbitol at about 1% to about 10% (w/v), or about 1% (w/v), about 2% (w/v), about 3% (w/v), about 4% (w/v), about 5% (w/v), about 6% (w/v), about 7% (w/v), about 8% (w/v), about 9% (w/v), or about 10% (w/v). In particular embodiments, the tonicity modifier is sodium chloride at a concentration of about 10 mM to about 200 mM, about 50 mM to 200 mM, about 75 mM to about 200 mM, about 50 mM to about 150 mM, about 100 mM, about 110 mM, about 120 mM, about 130 mM about 140 mM or about 150 mM. In one embodiment, the tonicity modifier is sodium chloride at a concentration of about 130 mM. In other embodiments, the tonicity modifier is sodium chloride at a concentration of about 75 mM or about 120 mM. With respect to tonicity modifier concentration, "mM" refers to milimoles of the tonicity modifier per liter of composition.
[0209] In certain embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and a buffer capable of achieving or maintaining the pH of the composition within a desired range. In certain embodiments, the compositions further comprise an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In certain embodiments, the compositions comprise histidine (e.g., L-histidine or a pharmaceutically acceptable salt thereof) or phosphate as a buffer, e.g., sodium phosphate, potassium phosphate, or both. In certain embodiments, the buffer is present at a concentration of about 1 mM to about 200 mM, about 1 mM to about 150 mM, about 1 mM to about 20 mM, about 1 mM to about 10 mM, about 2 mM to about 100 mM, about 2 mM to about 20 mM, about 5 mM to about 20 mM, or about 10 mM. In particular embodiments, the pH of the buffered composition is about 5.0 to about 8.0, about 5.0 to about 7.0, about 5.5 to about 7.5, about 5.5 to about 7.0, or about 6.0. In one embodiment, the buffered composition has a pH of about 5.5 to about 7.0. In certain embodiments, the buffer comprises histidine at a concentration of about 1 mM to about 200 mM, about 1 mM to about 150 mM, about 2 mM to about 100 mM, about 5 mM to about 20 mM, or about 10 mM, and the buffered composition has a pH of about 5.5 to about 7.0, or about 6.0. In one particular embodiment, the buffer comprises histidine at a concentration of about 10 mM and the pH of the histidine-buffered composition is about 6.0. With respect to buffer concentration, "mM" refers to millimoles of buffer (e.g., histidine) per liter of composition.
[0210] In certain embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and a buffer that comprises phosphate, alone or in combination with histidine. In certain embodiments, the compositions further comprise an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). The phosphate buffer may be, e.g., a sodium phosphate or potassium phosphate buffer. In certain embodiments, the buffer comprises phosphate at a concentration of about 1 mM to about 200 mM, about 1 mM to about 50 mM, about 2 mM to about 200 mM, about 2 mM to about 50 mM, about 5 mM to about 200 mM, about 5 mM to about 100 mM, about 5 mM to about 50 mM, about 10 mM to about 150 mM, about 10 mM to about 100 mM, about 5 mM, about 10 mM, about 25 mM, or about 50 mM. In particular embodiments, the pH of the buffered composition is about 5.0 to about 8.0, about 6.0 to about 8.0, about 5.5 to about 7.5, about 5.5 to about 7.0, about 6.0, about 7.0, or about 8.0. In one embodiment, the buffer comprises phosphate, and the buffered composition has a pH of about 6.0 to about 8.0. In certain embodiments, the buffer comprises phosphate at a concentration of about 5 mM to about 200 mM, about 5 mM to about 150 mM, about 5 mM to about 100 mM, about 5 mM, about 8 mM, about 10 mM, about 25 mM, or about 50 mM, and the buffered composition has a pH of about 5.5 to about 7.5, about 5.5 to about 7.0, or about 6.0. In one particular embodiment, the buffer comprises phosphate at a concentration of about 10 mM, and the buffered composition has a pH of about 6.2.
[0211] In certain embodiments, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof), a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), and a surfactant. In certain embodiments, the compositions further comprise an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In particular embodiments, the surfactant is polysorbate 20 at a concentration of about 0.001% (w/v) to about 0.05% (w/v), about 0.002% (w/v) to about 0.05% (w/v), about 0.005% (w/v) to about 0.05% (w/v), about 0.01% (w/v) to about 0.05% (w/v), or about 0.02% (w/v).
[0212] In one embodiment, the compositions comprise Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium), histidine, and NaCl. In certain embodiments, the compositions further comprise an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). The composition may further comprise polysorbate.
[0213] In certain embodiments, the compositions comprise an effective amount of: (a) about 0.3 mg/mL to about 30 mg/mL of Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 0.5 mg/mL to about 20 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium); and one or both of: (c) a buffer capable of achieving or maintaining the pH of the compositions at about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, the compositions further comprise (e) about 0.3 mg/mL to about 30 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In certain embodiments, the buffer is about 1 mM to about 20 mM L-histidine or about 1 mM to about 20 mM sodium phosphate, and the tonicity modifier is about 10 mM to about 200 mM NaCl, about 1% to about 20% (w/v) sorbitol, or about 1% to about 20% (w/v) trehalose. In particular embodiments, the compositions further comprise: (f) about 0.001% (w/v) to about 0.05% (w/v) surfactant.
[0214] In certain embodiments, the compositions comprise: (a) about 0.3 mg/mL to about 30 mg/mL of Antagonist A or another pharmaceutically acceptable salt thereof; and (b) about 0.5 mg/mL to about 20 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium). In certain embodiments, the compositions further comprise (c) about 0.3 mg/mL to about 30 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In certain embodiments, any of these the compositions further comprise one or both of: (d) about 1 mM to about 20 mM L-histidine; and (e) about 10 mM to about 200 mM NaCl. In further embodiments, the compositions further comprise: (f) about 0.001% (w/v) to about 0.05% (w/v) surfactant, which is optionally polysorbate. In a particular embodiment, the compositions comprise: (a) about 0.3 mg/mL to about 30 mg/mL of Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 0.5 mg/mL to about 20 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium); (c) about 1 mM to about 20 mM L-histidine; and (d) about 10 mM to about 200 mM NaCl, wherein the pH of the compositions is about pH 5.0 to about pH 7.0. In certain embodiments, the compositions further comprise (e) about 0.3 mg/mL to about 30 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In certain embodiments, the compositions further comprise: (f) about 0.01% (w/v) polysorbate 20.
[0215] In certain embodiments, compositions comprise: (a) about 1.0 mg/mL to about 100 mg/mL, or about 5.0 mg/mL to about 50 mg/mL of Antagonist A or another pharmaceutically acceptable salt thereof); and (b) about 1.0 mg/mL to about 50 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium). In certain embodiments, the compositions further comprise (c) about 1.0 mg/mL to about 100 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In other embodiments, any of the compositions further comprise one or both of (d) about 1 mM to about 20 mM L-histidine; and (e) about 10 mM to about 200 mM NaCl. In further embodiments, any of the compositions further comprise: (f) about 0.001% (w/v) to about 0.05% (w/v) surfactant, which is optionally polysorbate.
[0216] In certain embodiments, compositions comprise: (a) about 0.3 mg/mL to about 30 mg/mL of Antagonist A or another pharmaceutically acceptable salt thereof); (b) about 0.5 mg/mL to about 20 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium); and one or both of (c) a buffer capable of achieving or maintaining the pH of the composition to about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, the compositions further comprise about 0.3 mg/mL to about 30 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In particular embodiments, the buffer, where present, is about 1 mM to about 20 mM L-histidine or about 1 mM to about 20 mM sodium phosphate; and the tonicity modifier, where present, is about 10 mM to about 200 mM NaCl, about 1% to about 20% (w/v) sorbitol, or about 1% to about 20% (w/v) trehalose. In certain embodiments, the buffer is about 1 mM to about 20 mM L-histidine; and the tonicity modifier is about 10 mM to about 200 mM NaCl, wherein the pH of the compositions is about pH 5.0 to about pH 7.0.
[0217] Any of the compositions can also comprise a surfactant, e.g., about 0.001% (w/v) to about 0.05% (w/v) surfactant.
[0218] In certain embodiments the compositions comprise: (a) about 3 mg/mL to about 90 mg/mL Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 1.0 mg/mL to about 30 mg/mL of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium); and one or both of (c) a buffer capable of achieving or maintaining the pH of the compositions to about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, any of the compositions further comprises (e) about 3 mg/mL to about 90 mg/mL of an anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof). In particular embodiments, the buffer, where present, comprises about 1 mM to about 100 mM sodium phosphate or about 1.0 mM to about 10 mM histidine.HCl; and the tonicity modifier, where present, is about 0.5% (w/v) to about 10% (w/v) trehalose.
[0219] In certain embodiments, a composition of the invention comprises: (a) about 0.3 mg/mL to about 30 mg/mL Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 0.5 mg/mL to about 20 mg/mL ranibizumab or a pharmaceutically acceptable salt thereof; and one or both of: (c) a buffer capable of achieving or maintaining the pH of the composition at about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, the buffer is about 1 mM to about 20 mM L-histidine or about 1 mM to about 20 mM sodium phosphate, and the tonicity modifier is about 10 mM to about 200 mM NaCl, about 1% to about 20% (w/v) sorbitol, or about 1% to about 20% (w/v) trehalose. In particular embodiments, the composition of the invention further comprises: (e) about 0.001% (w/v) to about 0.05% (w/v) surfactant. In particular embodiments, the composition further comprises: (f) an anti-C5 agent, another PDGF antagonist, or another VEGF antagonist. In particular embodiments, the anti-C5 agent is ARC 186, ARC 187, or ARC1905, and the other VEGF antagonist is bevacizumab or aflibercept.
[0220] In certain embodiments, a composition of the invention comprises: (a) about 0.3 mg/mL to about 30 mg/mL Antagonist A or another pharmaceutically acceptable salt thereof; and (b) about 0.5 mg/mL to about 25 mg/mL bevacizumab or a pharmaceutically acceptable salt thereof; and one or both of: (c) a buffer capable of achieving or maintaining the pH of the composition at about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, the buffer is about 5 mM to about 200 mM sodium phosphate or about 5 mM to about 200 mM Tris.HCl, and the tonicity modifier is about 10 mM to about 200 mM NaCl, about 1% to about 20% (w/v) sorbitol, or about 1% to about 20% (w/v) trehalose. In particular embodiments, the composition of the invention further comprises: (e) about 0.001% (w/v) to about 0.05% (w/v) surfactant. In particular embodiments, the composition further comprises: (f) an anti-C5 agent, another PDGF antagonist, and/or another VEGF antagonist. In particular embodiments, the anti-C5 agent is ARC186, ARC187, or ARC1905, and the other VEGF antagonist is ranibizumab or aflibercept.
[0221] In certain embodiments, a composition of the invention comprises: (a) about 0.3 mg/mL to about 30 mg/mL Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 5 mg/mL to about 40 mg/mL aflibercept or a pharmaceutically acceptable salt thereof; and one or more of: (c) a buffer capable of achieving or maintaining the pH of the composition at about pH 5.0 to about pH 8.0; (d) a tonicity modifier; and (e) 0 to about 10% (w/v) sucrose. In certain embodiments, the buffer is about 5 mM to about 50 mM phosphate, and the tonicity modifier is about 10 mM to about 200 mM NaCl. In particular embodiments, the composition of the invention further comprises: (f) about 0.001% (w/v) to about 0.05% (w/v) surfactant. In particular embodiments, the composition further comprises: (g) an anti-C5 agent, another PDGF antagonist, and/or another VEGF antagonist. In particular embodiments, the anti-C5 agent is ARC186, ARC187, or ARC1905, and the other VEGF antagonist is ranibizumab or bevacizumab.
[0222] In certain embodiments, a composition of the invention comprises: (a) about 3 mg/mL to about 90 mg/mL Antagonist A or another pharmaceutically acceptable salt thereof; (b) about 1.0 mg/mL to about 30 mg/mL ranibizumab or a pharmaceutically acceptable salt thereof; and one or both of: (c) a buffer capable of achieving or maintaining the pH of the composition at about pH 5.0 to about pH 8.0; and (d) a tonicity modifier. In certain embodiments, the buffer comprises about 1 mM to about 100 mM sodium phosphate or about 1.0 mM to about 10 mM histidine.HCl, and the tonicity modifier is about 0.5% (w/v) to about 10% (w/v) trehalose. In particular embodiments, the composition further comprises: (e) an anti-C5 agent, another PDGF antagonist, and/or another VEGF antagonist. In particular embodiments, the anti-C5 agent is ARC186, ARC187, or ARC1905, and the other VEGF antagonist is bevacizumab or aflibercept.
[0223] Illustrative compositions include F1-F31, as described in Tables 3 and 4. Illustrative compositions are also described in PCT Application Publication No. WO2013/181495. Any of these compositions may further comprise an anti-C5 agent, such as ARC1905 or a pharmaceutically acceptable salt thereof.
TABLE-US-00002 TABLE 3 Composition Matrix for Illustrative Antagonist A: Ranibizumab Compositions [Ant. A] [ran.] Polysorbate Comp. Buffer pH Tonicity Modifier (mg/mL) (mg/mL) 20 (% w/v) F1 10 mM Sodium Phosphate 7.3 150 mM NaCl 3 0 0% F2 10 mM Sodium Acetate 5.0 5% (w/v) Sorbitol 3 5 0.01% F3 10 mM Sodium Acetate 5.0 130 mM NaCl 3 5 0.01% F4 10 mM Histidine.cndot.HCl 5.5 10% (w/v) Trehalose 0 5 0.01% F5 10 mM Histidine.cndot.HCl 6.0 5% (w/v) Sorbitol 3 5 0.01% F6 10 mM Histidine.cndot.HCl 6.0 130 mM NaCl 3 5 0.01% F7 10 mM Sodium Phosphate 7.0 5% (w/v) Sorbitol 3 5 0.01% F8 10 mM Sodium Phosphate 7.0 130 mM NaCl 3 5 0.01% F9 10 mM Tris.cndot.HCl 8.0 5% (w/v) Sorbitol 3 5 0.01% F10 10 mM Tris.cndot.HCl 8.0 130 mM NaCl 3 5 0.01% F11 5 mM Sodium Phosphate + 6.5 75 mM NaCl + 3 5 0.005% 5 mM Histidine 5% (w/v) Trehalose F27 10 mM Sodium Phosphate 7.3 150 mM NaCl 30 0 0% F28 10 mM Histidine.cndot.HCl 5.5 10% (w/v) Trehalose 0 10 0.01% F29 10 mM Histidine.cndot.HCl 5.5 10% (w/v) Trehalose 0 40 0.01% F30 5 mM Sodium Phosphate + 75 mM NaCl + 5% 15 5 0.005% 5 mM Histidine.cndot.HCl (w/v) Trehalose F31 8 mM Sodium Phosphate + 120 mM NaCl + 2% 24 8 0.002% 2 mM Histidine.cndot.HCl (w/v) Trehalose "Ant. A" is Antagonist A; "ran." is ranibizumab
TABLE-US-00003 TABLE 4 Composition Matrix for Illustrative Antagonist A: Bevacizumab Compositions Antagonist A Concentration Bevacizumab (mg/mL, oligo Concentration Comp. Buffer pH Tonicity Modifier wt.) (mg/mL) Surfactant F12 10 mM 7.3 150 mM Sodium 30 0.0 0% Phosphate Chloride F13 50 mM 4 5% (w/v) Sorbitol 3 12.5 0.02% Acetate Polysorbate 20 F14 50 mM 4 130 mM Sodium 3 12.5 0.02% Acetate Chloride Polysorbate 20 F15 50 mM 5 5% (w/v) Sorbitol 3 12.5 0.02% Acetate Polysorbate 20 F16 50 mM 5 130 mM Sodium 3 12.5 0.02% Acetate Chloride Polysorbate 20 F17 50 mM 6 5% (w/v) Sorbitol 3 12.5 0.02% Phosphate Polysorbate 20 F18 50 mM 6.2 6% (w/v) Trehalose 0 12.5 0.02% Phosphate Polysorbate 20 F19 50 mM 6 130 mM Sodium 3 12.5 0.02% Phosphate Chloride Polysorbate 20 F20 50 mM 7 5% (w/v) Sorbitol 3 12.5 0.02% Phosphate Polysorbate 20 F21 50 mM 7 130 mM Sodium 3 12.5 0.02% Phosphate Chloride Polysorbate 20 F22 50 mM 8 5% (w/v) Sorbitol 3 12.5 0.02% Tris Polysorbate 20 F23 50 mM 8 130 mM Sodium 3 12.5 0.02% Tris Chloride Polysorbate 20 F24 30 mM 6.3 75 mM sodium 15 12.5 0.02% Phosphate Chloride + 3% (w/v) Polysorbate 20 Trehalose F25 10 mM 7.3 150 mM Sodium 3 0.0 0% Phosphate Chloride F26 30 mM 6.3 75 mM sodium 3 12.5 0.02% Phosphate Chloride + 3% (w/v) Polysorbate 20 Trehalose
[0224] Administration and Dosage
[0225] The methods or compositions according to the invention can be administered alone or in conjunction with another therapy and can be provided at home, a doctor's office, a clinic, a hospital's outpatient department, or a hospital. Treatment can begin at a hospital so that the doctor can observe the therapy's effects closely and make any adjustments that are needed. The duration of the administration can depend on the type of ophthalmological disease or disorder being treated or prevented, the age and condition of the subject, the stage and type of the subject's disease or disorder, and how the subject responds to the treatment. Additionally, a subject having a greater risk of developing an ophthalmological disease or disorder (e.g., a diabetic patient) can receive treatment to inhibit or delay the onset of symptoms. In one embodiment, the present methods or compositions allow for the administration of a relatively lower dose of each antagonist.
[0226] The dosage and frequency of administration of each antagonist can be controlled independently. For example, one antagonist can be administered three times per day, while the other antagonist can be administered once per day. Administration can be performed in on-and-off cycles that include rest periods so that the subject's body has a chance to recover from a side effect, if any. The antagonists can also be present in the same composition.
[0227] In other embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and optionally, a VEGF antagonist and/or anti-C5 agent are administered prior to, during, and/or after another treatment. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist and/or anti-C5 agent are administered concurrently, such as in a co-formulation, prior to, during, and/or after the other treatment. In other embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist are administered sequentially, prior to, during, and/or after the other treatment. In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof is administered prior to the administration of the VEGF antagonist. In other embodiments, Antagonist A or another pharmaceutically acceptable salt thereof is administered subsequent to the administration of the VEGF antagonist. In some embodiments, the other treatment is performing surgery. Examples of other treatment include pneumatic retinopexy, laser retinopexy, scleral buckling, and pars plana vitrectomy (PPV), laser photocoagulation, or cryotherapy.
[0228] Administration of a composition disclosed herein with performing another treatment can improve retinal attachment success, improve visual acuity, reduce choroidal neovascularization or stabilize vision to a degree that is greater than performing the other treatment alone. For example, in some embodiments, the administration of both Antagonist A or another pharmaceutically acceptable salt thereof with performing another treatment can improve retinal attachment success, improve visual acuity, or stabilize vision to a degree that is greater than an additive effect of both Antagonist A or another pharmaceutically acceptable salt thereof with performing the other treatment. In some embodiments, the synergistic effect is in reducing the size or growth of a tumor (e.g., in treating or preventing VHL disease, retinal capillary hemangioma, or von Hippel angioma). In some embodiments, the synergistic effect is reducing or inhibiting scarring or fibrosis (e.g., ocular scarring of fibrosis, such as subretinal fibrosis).
[0229] Administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can improve retinal attachment success, improve visual acuity, or stabilize vision to a degree that is greater than administration of Antagonist A or another pharmaceutically acceptable salt thereof or the VEGF antagonist. In some embodiments, the administration of Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can have a synergistic effect in treating or preventing an ophthalmological disease or disorder. For example, the administration of both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can improve retinal attachment success, improve visual acuity, or stabilize vision to a degree that is greater than an additive effect of administering both Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist. In some embodiments, the synergistic effect is in reducing the size or growth of a tumor (e.g., in treating or preventing VHL disease, retinal capillary hemangioma, or von Hippel angioma). In some embodiments, the synergistic effect is reducing or inhibiting scarring or fibrosis (e.g., ocular scarring of fibrosis, such as subretinal fibrosis).
[0230] In some embodiments, the methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent, in which two or more of Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist and the anti-C5 agent are present in the same composition. In certain embodiments, the PDGF antagonist and the VEGF antagonist are present in the same composition; in certain embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and the anti-C5 agent are present in the same composition; and in certain embodiments, the VEGF antagonist and the anti-C5 agent are present in the same composition. In some embodiments, all three of Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist and the anti-C5 agent are present in the same composition.
[0231] In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist and the anti-C5 agent are administered sequentially. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered prior to the VEGF antagonist or the anti-C5 agent. In one embodiment, the VEGF antagonist is administered prior to Antagonist A or another pharmaceutically acceptable salt thereof or the anti-C5 agent. In one embodiment, the anti-C5 agent is administered prior to the VEGF antagonist or Antagonist A or another pharmaceutically acceptable salt thereof. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered prior to the VEGF antagonist and anti-C5 agent. In one embodiment, the VEGF antagonist is administered prior to Antagonist A or another pharmaceutically acceptable salt thereof and he-C5 agent. In one embodiment, the anti-C5 agent is administered prior to the VEGF antagonist and PDGF antagonist.
[0232] In certain embodiments, the subject is administered two or more active agents (e.g., Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist) in a staggered dosing regimen, wherein one or more of the two or more active agents is administered before another one or more of the two or more active agents is administered to the subject.
[0233] In certain embodiments, the one or more active agent(s) is administered at least one day before the other one or more active agent(s). Accordingly, in some embodiments the present methods comprise administering on one or more days Antagonist A or another pharmaceutically acceptable salt thereof, one or more VEGF antagonists or one or more anti-C5 agents.
[0234] In one embodiment, the order of administration is: Antagonist A or another pharmaceutically acceptable salt thereof, followed by VEGF antagonist, followed by anti-C5 agent. In another embodiment, the order of administration is: Antagonist A or another pharmaceutically acceptable salt thereof, followed by anti-C5 agent, followed by VEGF antagonist. In another embodiment, the order of administration is: VEGF antagonist, followed by anti-C5 agent, followed by Antagonist A or another pharmaceutically acceptable salt thereof. In another embodiment, the order of administration is: VEGF antagonist, followed by Antagonist A or another pharmaceutically acceptable salt thereof, followed by anti-C5 agent. In yet another embodiment the order of administration is: anti-C5 agent, followed by Antagonist A or another pharmaceutically acceptable salt thereof, followed by VEGF antagonist. In another embodiment the order of administration is: anti-C5 agent, followed by VEGF antagonist, followed by PDGF antagonist.
[0235] In some embodiments, the Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist are administered concurrently, and the anti-C5 agent is administered prior to or subsequent to administration of the PDGF antagonist and VEGF antagonist. In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and the anti-C5 agent are administered concurrently, and the VEGF antagonist is administered prior to or subsequent to administration of Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist. In some embodiments, the VEGF antagonist and anti-C5 agent are administered concurrently, and Antagonist A or another pharmaceutically acceptable salt thereof is administered prior to or subsequent to administration of the anti-C5 agent and VEGF antagonist.
[0236] In other embodiments, the order of administration is: Antagonist A or another pharmaceutically acceptable salt thereof, followed by VEGF antagonist and anti-C5 agent, wherein the VEGF antagonist and anti-C5 agent are present in the same composition. In another embodiment, the order of administration is: VEGF antagonist, followed by anti-C5 agent and Antagonist A or another pharmaceutically acceptable salt thereof, wherein the anti-C5 agent and PDGF antagonist are present in the same composition. In yet another embodiment the order of administration is: anti-C5 agent, followed by Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist, wherein the PDGF antagonist and VEGF antagonist are present in the same composition.
[0237] In still other embodiments, the order of administration is: Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist, wherein Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist are present in the same composition, followed by anti-C5 agent. In another embodiment, the order of administration is: Antagonist A or another pharmaceutically acceptable salt thereof and anti-C5 agent, wherein Antagonist A or another pharmaceutically acceptable salt thereof and the anti-C5 agent are present in the same composition, followed by VEGF antagonist. In another embodiment, the order of administration is: VEGF antagonist and anti-C5 agent, wherein the VEGF antagonist and anti-C5 agent are present in the same composition, followed by Antagonist A or another pharmaceutically acceptable salt thereof.
[0238] For example, Antagonist A or another pharmaceutically acceptable salt thereof can be administered prior to or subsequent to administration of a VEGF antagonist and/or an anti-C5 agent; a VEGF antagonist can be administered prior to or subsequent to administration of Antagonist A or another pharmaceutically acceptable salt thereof and/or anti-C5 agent; or an anti-C5 agent can be administered prior to or subsequent to administration of Antagonist A or another pharmaceutically acceptable salt thereof and/or a VEGF antagonist.
[0239] In some embodiments, the present methods comprise administering a first agent prior to administering a second agent. In some embodiments, the present methods comprise administering a first agent prior to administering a second agent and administering the second agent prior to administering a third agent.
[0240] In some embodiments, the present methods comprise concurrently administering a first agent and a second agent. In some embodiments, the present methods comprise concurrently administering a first agent and a second agent prior to administering a third agent.
[0241] In some embodiments, the present methods comprise administering a first agent prior to concurrently administering a second agent and third agent.
[0242] In some embodiments, the present methods comprise concurrently administering a first agent, a second agent and a third agent.
[0243] Illustrative groups of first agent, second agent and third agent are set forth below in Tables 5 and 6.
TABLE-US-00004 TABLE 5 Group First Agent Second Agent Third Agent A Antagonist A or another VEGF antagonist Anti-C5 Agent pharmaceutically acceptable salt thereof B Antagonist A or another Anti-C5 Agent VEGF antagonist pharmaceutically acceptable salt thereof C VEGF antagonist Antagonist A or another Anti-C5 Agent pharmaceutically acceptable salt thereof D VEGF antagonist Anti-C5 Agent Antagonist A or another pharmaceutically acceptable salt thereof E Anti-C5 Agent Antagonist A or another VEGF antagonist pharmaceutically acceptable salt thereof F Anti-C5 Agent VEGF antagonist Antagonist A or another pharmaceutically acceptable salt thereof
TABLE-US-00005 TABLE 6 Group First Agent Second Agent Third Agent A Antagonist A ranibizumab ARC1905 B Antagonist A bevacizumab ARC1905 C Antagonist A aflibercept ARC1905 D Antagonist A pegaptanib sodium ARC1905 E Antagonist A ESBA1008 ARC1905 F Antagonist A ARC1905 ranibizumab G Antagonist A ARC1905 bevacizumab H Antagonist A ARC1905 aflibercept I Antagonist A ARC1905 pegaptanib sodium J Antagonist A ARC1905 ESBA1008 K ranibizumab Antagonist A ARC1905 L bevacizumab Antagonist A ARC1905 M aflibercept Antagonist A ARC1905 N pegaptanib sodium Antagonist A ARC1905 O ESBA1008 Antagonist A ARC1905 P ranibizumab ARC1905 Antagonist A Q bevacizumab ARC1905 Antagonist A R aflibercept ARC1905 Antagonist A S pegaptanib sodium ARC1905 Antagonist A T ESBA1008 ARC1905 Antagonist A U ARC1905 Antagonist A ranibizumab V ARC1905 Antagonist A bevacizumab W ARC1905 Antagonist A aflibercept X ARC1905 Antagonist A pegaptanib sodium Y ARC1905 Antagonist A ESBA1008 Z ARC1905 ranibizumab Antagonist A AA ARC1905 bevacizumab Antagonist A AB ARC1905 aflibercept Antagonist A AC ARC1905 pegaptanib sodium Antagonist A AD ARC1905 ESBA1008 Antagonist A
[0244] In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof and two or more VEGF antagonists. In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof and two or more anti-C5 agents. In some embodiments, the present methods comprise administering a VEGF antagonist and two or more anti-C5 agents.
[0245] In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to administering two or more VEGF antagonists. In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to administering a first VEGF antagonist and administering the first VEGF antagonist prior to administering a second VEGF antagonist.
[0246] In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist. In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof and a first VEGF antagonist prior to administering a second VEGF antagonist.
[0247] In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to concurrently administering a first VEGF antagonist and a second VEGF antagonist.
[0248] In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof, a first VEGF antagonist and a second VEGF antagonist.
[0249] In some embodiments, the present methods comprise administering a VEGF antagonist prior to administering two PDGF antagonists (e.g., Antagonist A or another pharmaceutically acceptable salt thereof and another PDGF antagonist). In some embodiments, the present methods comprise administering a VEGF antagonist prior to administering a first PDGF antagonist and administering the first PDGF antagonist prior to administering a second PDGF antagonist.
[0250] In some embodiments, the present methods comprise concurrently administering a VEGF antagonist and Antagonist A or another pharmaceutically acceptable salt thereof. In some embodiments, the present methods comprise concurrently administering a VEGF antagonist and a first PDGF antagonist prior to administering a second PDGF antagonist.
[0251] In some embodiments, the present methods comprise administering a VEGF antagonist prior to concurrently administering a first PDGF antagonist and a second PDGF antagonist.
[0252] In some embodiments, the present methods comprise concurrently administering a VEGF antagonist, a first PDGF antagonist and a second PDGF antagonist.
[0253] In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to administering two or more anti-C5 agents. In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to administering a first anti-C5 agent and administering the first anti-C5 agent prior to administering a second anti-C5 agent.
[0254] In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof and an anti-C5 agent. In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof and a first anti-C5 agent prior to administering a second anti-C5 agent.
[0255] In some embodiments, the present methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof prior to concurrently administering a first anti-C5 agent and a second anti-C5 agent.
[0256] In some embodiments, the present methods comprise concurrently administering Antagonist A or another pharmaceutically acceptable salt thereof, a first anti-C5 agent and a second anti-C5 agent.
[0257] In some embodiments, the present methods comprise administering an anti-C5 agent prior to administering two or more PDGF antagonists. In some embodiments, the present methods comprise administering an anti-C5 agent prior to administering a first PDGF antagonist and administering the first PDGF antagonist prior to administering a second PDGF antagonist.
[0258] In some embodiments, the present methods comprise concurrently administering an anti-C5 agent and Antagonist A or another pharmaceutically acceptable salt thereof. In some embodiments, the present methods comprise concurrently administering an anti-C5 agent and a first PDGF antagonist prior to administering a second PDGF antagonist.
[0259] In some embodiments, the present methods comprise administering an anti-C5 agent prior to concurrently administering a first PDGF antagonist and a second PDGF antagonist.
[0260] In some embodiments, the present methods comprise concurrently administering an anti-C5 agent, a first PDGF antagonist and a second PDGF antagonist.
[0261] In some embodiments, the present methods comprise administering a VEGF antagonist prior to administering two or more anti-C5 agents. In some embodiments, the present methods comprise administering a VEGF antagonist prior to administering a first anti-C5 agent and administering the first anti-C5 agent prior to administering a second anti-C5 agent.
[0262] In some embodiments, the present methods comprise concurrently administering a VEGF antagonist and an anti-C5 agent. In some embodiments, the present methods comprise concurrently administering a VEGF antagonist and a first anti-C5 agent prior to administering a second anti-C5 agent.
[0263] In some embodiments, the present methods comprise administering a VEGF antagonist prior to concurrently administering a first anti-C5 agent and a second anti-C5 agent.
[0264] In some embodiments, the present methods comprise concurrently administering a VEGF antagonist, a first anti-C5 agent and a second anti-C5 agent.
[0265] In some embodiments, the present methods comprise administering an anti-C5 agent prior to administering two or more VEGF antagonists. In some embodiments, the present methods comprise administering an anti-C5 agent prior to administering a first VEGF antagonist and administering the first VEGF antagonist prior to administering a second VEGF antagonist.
[0266] In some embodiments, the present methods comprise concurrently administering an anti-C5 agent and a VEGF antagonist. In some embodiments, the present methods comprise concurrently administering an anti-C5 agent and a first VEGF antagonist prior to administering a second VEGF antagonist.
[0267] In some embodiments, the present methods comprise administering an anti-C5 agent prior to concurrently administering a first VEGF antagonist and a second VEGF antagonist.
[0268] In some embodiments, the present methods comprise concurrently administering an anti-C5 agent, a first VEGF antagonist and a second VEGF antagonist.
[0269] In some embodiments, the first agent and second agent are PDGF antagonists, which can be the same or different. In some embodiment, the first agent and second agent are VEGF antagonists, which can be the same or different. In some embodiments, the first agent and second agent are anti-C5 agents, which can be the same or different.
[0270] In some embodiments, the first agent and third agent are PDGF antagonists, which can be the same or different. In some embodiment, the first agent and third agent are VEGF antagonists, which can be the same or different. In some embodiments, the first agent and third agent are anti-C5 agents, which can be the same or different.
[0271] In some embodiments, the second agent and third agent are PDGF antagonists, which can be the same or different. In some embodiment, the second agent and third agent are VEGF antagonists, which can be the same or different. In some embodiments, the second agent and third agent are anti-C5 agents, which can be the same or different.
[0272] Illustrative groups of first agent, second agent and third agent are set forth below in Tables 7, 8, 9 and 10.
TABLE-US-00006 TABLE 7 Group First Agent Second Agent Third Agent A PDGF Antagonist VEGF antagonist VEGF antagonist B VEGF antagonist PDGF Antagonist VEGF antagonist C VEGF antagonist VEGF antagonist PDGF Antagonist D PDGF Antagonist Anti-C5 Agent Anti-C5 Agent E Anti-C5 Agent PDGF Antagonist Anti-C5 Agent F Anti-C5 Agent Anti-C5 Agent PDGF Antagonist G PDGF Antagonist PDGF Antagonist VEGF antagonist H PDGF Antagonist VEGF antagonist PDGF Antagonist I VEGF antagonist PDGF Antagonist PDGF Antagonist J PDGF Antagonist PDGF Antagonist Anti-C5 Agent K PDGF Antagonist Anti-C5 Agent PDGF Antagonist L Anti-C5 Agent PDGF Antagonist PDGF Antagonist
TABLE-US-00007 TABLE 8 Group First Agent Second Agent Third Agent A PDGF Antagonist First VEGF antagonist Second VEGF antagonist B First VEGF antagonist PDGF Antagonist Second VEGF antagonist C First VEGF antagonist Second VEGF antagonist PDGF Antagonist D PDGF Antagonist First Anti-C5 Agent Second Anti-C5 Agent E First Anti-C5 Agent PDGF Antagonist Second Anti-C5 Agent F First Anti-C5 Agent Second Anti-C5 Agent PDGF Antagonist G First PDGF Antagonist Second PDGF Antagonist VEGF antagonist H First PDGF Antagonist VEGF antagonist Second PDGF Antagonist I VEGF antagonist First PDGF Antagonist Second PDGF Antagonist J First PDGF Antagonist Second PDGF Antagonist Anti-C5 Agent K First PDGF Antagonist Anti-C5 Agent Second PDGF Antagonist L Anti-C5 Agent First PDGF Antagonist Second PDGF Antagonist
TABLE-US-00008 TABLE 9 Group First Agent Second Agent Third Agent A Antagonist A ranibizumab Antagonist A B Antagonist A ranibizumab ranibizumab C Antagonist A bevacizumab Antagonist A D Antagonist A bevacizumab bevacizumab E Antagonist A aflibercept Antagonist A F Antagonist A aflibercept aflibercept G Antagonist A pegaptanib sodium Antagonist A H Antagonist A pegaptanib sodium pegaptanib sodium I Antagonist A ESBA1008 Antagonist A J Antagonist A ESBA1008 ESBA1008 K Antagonist A ARC1905 Antagonist A L Antagonist A ARC1905 ARC1905 M ranibizumab Antagonist A ranibizumab N ranibizumab Antagonist A Antagonist A O bevacizumab Antagonist A bevacizumab P bevacizumab Antagonist A Antagonist A Q aflibercept Antagonist A aflibercept R aflibercept Antagonist A Antagonist A S pegaptanib sodium Antagonist A pegaptanib sodium T pegaptanib sodium Antagonist A Antagonist A U ESBA1008 Antagonist A ESBA1008 V ESBA1008 Antagonist A Antagonist A W ARC1905 Antagonist A ARC1905 X ARC1905 Antagonist A Antagonist A Y ranibizumab ranibizumab Antagonist A Z bevacizumab bevacizumab Antagonist A AA aflibercept aflibercept Antagonist A AB pegaptanib sodium pegaptanib sodium Antagonist A AC ESBA1008 ESBA1008 Antagonist A AD ARC1905 ARC1905 Antagonist A AE ranibizumab ranibizumab bevacizumab AF ranibizumab bevacizumab ranibizumab AG ranibizumab ranibizumab aflibercept AH ranibizumab aflibercept ranibizumab AI ranibizumab ranibizumab pegaptanib sodium AJ ranibizumab pegaptanib sodium ranibizumab AK ranibizumab ranibizumab ESBA1008 AL ranibizumab ESBA1008 ranibizumab AM ranibizumab ranibizumab ARC1905 AN ranibizumab ARC1905 ranibizumab AO bevacizumab bevacizumab ranibizumab AP bevacizumab ranibizumab bevacizumab AQ bevacizumab bevacizumab aflibercept AR bevacizumab aflibercept bevacizumab AS bevacizumab bevacizumab pegaptanib sodium AT bevacizumab pegaptanib sodium bevacizumab AU bevacizumab bevacizumab ESBA1008 AV bevacizumab ESBA1008 bevacizumab AW bevacizumab bevacizumab ARC1905 AX bevacizumab ARC1905 bevacizumab AY aflibercept aflibercept ranibizumab AZ aflibercept ranibizumab aflibercept BA aflibercept aflibercept bevacizumab BB aflibercept bevacizumab aflibercept BC aflibercept aflibercept pegaptanib sodium BD aflibercept pegaptanib sodium aflibercept BE aflibercept aflibercept ESBA1008 BF aflibercept ESBA1008 aflibercept BG aflibercept aflibercept ARC1905 BH aflibercept ARC1905 aflibercept BI pegaptanib sodium pegaptanib sodium ranibizumab BJ pegaptanib sodium ranibizumab pegaptanib sodium BK pegaptanib sodium pegaptanib sodium bevacizumab BL pegaptanib sodium bevacizumab pegaptanib sodium BM pegaptanib sodium pegaptanib sodium aflibercept BN pegaptanib sodium aflibercept pegaptanib sodium BO pegaptanib sodium pegaptanib sodium ESBA1008 BP pegaptanib sodium ESBA1008 pegaptanib sodium BQ pegaptanib sodium pegaptanib sodium ARC1905 BR pegaptanib sodium ARC1905 pegaptanib sodium BS ESBA1008 ESBA1008 ranibizumab BT ESBA1008 ranibizumab ESBA1008 BU ESBA1008 ESBA1008 bevacizumab BV ESBA1008 bevacizumab ESBA1008 BW ESBA1008 ESBA1008 aflibercept BX ESBA1008 aflibercept ESBA1008 BY ESBA1008 ESBA1008 pegaptanib sodium BZ ESBA1008 pegaptanib sodium ESBA1008 CA ESBA1008 ESBA1008 ARC1905 CB ESBA1008 ARC1905 ESBA1008 CC ARC1905 ARC1905 ranibizumab CD ARC1905 ranibizumab ARC1905 CE ARC1905 ARC1905 bevacizumab CF ARC1905 bevacizumab ARC1905 CO ARC1905 ARC1905 aflibercept CH ARC1905 aflibercept ARC1905 CI ARC1905 ARC1905 pegaptanib sodium CJ ARC1905 pegaptanib sodium ARC1905 CK ARC1905 ARC1905 ESBA1008 CL ARC1905 ESBA1008 ESBA1008
TABLE-US-00009 TABLE 10 Group First Agent Second Agent Third Agent A Antagonist A ranibizumab bevacizumab B Antagonist A ranibizumab aflibercept C Antagonist A ranibizumab pegaptanib sodium D Antagonist A bevacizumab aflibercept E Antagonist A bevacizumab pegaptanib sodium F Antagonist A aflibercept pegaptanib sodium G ranibizumab bevacizumab Antagonist A H ranibizumab aflibercept Antagonist A I ranibizumab pegaptanib sodium Antagonist A J bevacizumab aflibercept Antagonist A K bevacizumab pegaptanib sodium Antagonist A L aflibercept pegaptanib sodium Antagonist A M ranibizumab Antagonist A bevacizumab N ranibizumab Antagonist A aflibercept O ranibizumab Antagonist A pegaptanib sodium P bevacizumab Antagonist A aflibercept Q bevacizumab Antagonist A pegaptanib sodium R aflibercept Antagonist A pegaptanib sodium S bevacizumab ranibizumab Antagonist A T aflibercept ranibizumab Antagonist A U pegaptanib sodium ranibizumab Antagonist A V aflibercept bevacizumab Antagonist A W pegaptanib sodium bevacizumab Antagonist A X pegaptanib sodium aflibercept Antagonist A Y bevacizumab Antagonist A ranibizumab Z aflibercept Antagonist A ranibizumab AA pegaptanib sodium Antagonist A ranibizumab AB aflibercept Antagonist A bevacizumab AC pegaptanib sodium Antagonist A bevacizumab AD pegaptanib sodium Antagonist A aflibercept AE Antagonist A ARC187 ARC1905 AF Antagonist A ARC1905 ARC187 AG ARC187 ARC1905 Antagonist A AH ARC1905 ARC187 Antagonist A AI ARC187 Antagonist A ARC1905 AJ ARC1905 Antagonist A ARC187
[0273] In one embodiment, two or more agents are administered concurrently. In one embodiment, the two or more agents administered concurrently are present in the same composition. In another embodiment, the two or more agents administered concurrently are each present in a separate composition.
[0274] In certain embodiments, the time period from administration of a first agent to administration of a second agent is at least 1 min, at least 5 min, at least 10 min, at least 15 min, at least 30 min, or at least one hour. In certain embodiments, the time period from administration of a first agent to administration of a second agent is between 1 min and 2 hours, between 5 min and 2 hours, between 10 min and 2 hours, between 15 min and 2 hours, between 30 min and 2 hours, between 45 min and 2 hours, between 1 hour and 2 hours, or between 30 min and 1 hour. In certain embodiments, the time period from administration of a first agent to administration of a second agent is about 1 min, about 2 min, about 3 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 35 min, about 40 min, about 45 min, about 50 min, about 55 min, about 60 min, about 90 min, or about 120 min. In certain embodiments, a second agent is administered within 90 days, 30 days, 10 days, 5 days, 2 days, 1 day, 24 hours, 1 hour, 30 minutes, 10 minutes, 5 minutes or one minute after administration of a second agent.
[0275] In certain embodiments, the time period from administration of a second agent to administration of a third agent is at least 1 min, at least 5 min, at least 10 min, at least 15 min, at least 30 min, or at least one hour. In certain embodiments, the time period between administration of a second agent and administration of a third agent is between 1 min and 2 hours, between 5 min and 2 hours, between 10 min and 2 hours, between 15 min and 2 hours, between 30 min and 2 hours, between 45 min and 2 hours, between 1 hour and 2 hours, or between 30 min and 1 hour. In certain embodiments, the time period between administration of a second agent and administration of a third agent is about 1 min, about 2 min, about 3 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 35 min, about 40 min, about 45 min, about 50 min, about 55 min, about 60 min, about 90 min, or about 120 min. In certain embodiments, a third agent is administered within 90 days, 30 days, 10 days, 5 days, 2 days, 1 day, 24 hours, 1 hour, 30 minutes, 10 minutes, 5 minutes or one minute after administration of a second agent.
[0276] In certain embodiments, the time period between concurrent administration of a first agent and a second agent and administration of a third agent is at least 1 min, at least 5 min, at least 10 min, at least 15 min, at least 30 min, or at least one hour. In certain embodiments, the time period between concurrent administration of a first agent and a second agent and administration of a third agent is between 1 min and 2 hours, between 5 min and 2 hours, between 10 min and 2 hours, between 15 min and 2 hours, between 30 min and 2 hours, between 45 min and 2 hours, between 1 hour and 2 hours, or between 30 min and 1 hour. In certain embodiments, the time period from concurrent administration of a first agent and a second agent to administration of a third agent is about 1 min, about 2 min, about 3 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 35 min, about 40 min, about 45 min, about 50 min, about 55 min, about 60 min, about 90 min, or about 120 min. In certain embodiments, administration of a third agent is within 90 days, 30 days, 10 days, 5 days, 2 days, 1 day, 24 hours, 1 hour, 30 minutes, 10 minutes, 5 minutes or one minute of concurrent administration of a first agent and a second agent.
[0277] In certain embodiments, the time period from administration of a first agent to concurrent administration a second agent and a third agent is at least 1 min, at least 5 min, at least 10 min, at least 15 min, at least 30 min, or at least one hour. In certain embodiments, the time period from administration of a first agent to concurrent administration of a second agent and a third agent is between 1 min and 2 hours, between 5 min and 2 hours, between 10 min and 2 hours, between 15 min and 2 hours, between 30 min and 2 hours, between 45 min and 2 hours, between 1 hour and 2 hours, or between 30 min and 1 hour. In certain embodiments, the time period from administration of a first agent to concurrent administration of a second agent and a third agent is about 1 min, about 2 min, about 3 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 35 min, about 40 min, about 45 min, about 50 min, about 55 min, about 60 min, about 90 min, or about 120 min. In certain embodiments, concurrent administration of a second agent and a third agent is within 90 days, 30 days, 10 days, 5 days, 2 days, 1 day, 24 hours, 1 hour, 30 minutes, 10 minutes, 5 minutes or one minute of administration of a first agent.
[0278] The administration of two or more, such as three or more, active agents (e.g., Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist and an anti-C5 agent) can have a synergistic effect in treating or preventing a disease or disorder, e.g., an ophthalmological disease or disorder. For example, administration of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent (or any two of these active agents) can improve retinal attachment success, improve visual acuity, reduce choroidal neovascularization or stabilize vision to a degree that is greater than an additive effect of the active agents.
[0279] In certain embodiments, the invention provides methods for treating or preventing an ophthalmological disease or disorder, comprising administering to a subject in need thereof one or more, in some embodiments two or more or three or more, active agents via an apparatus. In other embodiments, the methods further comprise performing surgery on the subject. In other embodiments, the methods further comprise administering another active agent, such as an antineoplastic drug, including but not limited to any of those described herein. In particular embodiments, the methods further comprise administering another active agent and performing surgery on the subject.
[0280] In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent to a subject results in improved vision, such as increased visual acuity. In some embodiments, the subject experienced moderate vision loss, defined as losing 15 letters or more from baseline on ETDRS visual acuity testing, measured at week 24, prior to treatment with Antagonist A or another pharmaceutically acceptable salt thereof.
[0281] In some embodiments, visual acuity testing is as described in Early Treatment Diabetic Retinopathy Study Research Group (ETDRS), Manual of Operations, Baltimore: ETDRS Coordinating Center, University of Maryland. Available from: National Technical Information Service, 5285 Port Royal Road, Springfield, Va. 22161; Accession No. PB85 223006/AS; Ferris et al., Am J Ophthalmol 94:91-96, 1982; or Example 4, as described herein. In some embodiments, the visual acuity testing uses one or more charts available from http://www.nei.nih.gov/photo/keyword.asp?conditions=Eye+Charts&match=all, e.g., ETDRS visual acuity Chart 1, 2 and/or R.
[0282] In other embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist results in fewer ocular adverse events, a decrease in size of RCH (e.g., measured by fundus photography and FA), a decrease in exudation (measured by fundus photography, OCT, and FA), or a decrease in epiretinal proliferation or retinal traction (assessed by fundus photography), compared to those experienced by a subject who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof. In some embodiments, the subject does not require, and the methods do not comprise, ablative treatment of RCH or ocular surgery.
[0283] In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in improved vision independent of baseline lesion size or baseline vision, compared to vision of a subject who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof, or compared to a subject administered anti-VEGF monotherapy. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in the subject having a visual acuity of 20/40 or better, or 20/25 or better vision. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent to a subject results in an increased reduction in CNV size in the subject, compared to CNV size in a patient who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof, or compared to a subject administered anti-VEGF monotherapy. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in a reduction in CNV size (e.g., reduction in disc area (DA) size). In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent to a subject result in an increased reduction in DA in the subject, compared to DA in a patient who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof, or compared to a subject administered anti-VEGF monotherapy. In some embodiments, the increased reduction in CNV size is in subjects with small baseline CNV, e.g., less than or equal to 1.62 DA (disc area). In some embodiments, the increased reduction in CNV size (e.g., in disc area) is in subjects with large baseline CNV, e.g., greater than 1.62 DA. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in neovascular regression. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in reduced neovascular growth, compared to that occurring in a subject who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof, or compared to a subject administered anti-VEGF monotherapy. In some embodiments, the reduced neovascular growth is anti-fibrosis. In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in a decrease in or absence of hyper-reflective material, e.g., sub-retinal hyper-reflective material, such as a decrease in the size of sub-retinal hyper-reflective material (SHRM) as evidenced by spectral domain optical coherence tomography (SD-OCT). In some embodiments, administration of Antagonist A or another pharmaceutically acceptable salt thereof, and optionally a VEGF antagonist and/or an anti-C5 agent, to a subject results in an increase in resolution of hyper-reflective material, e.g., sub-retinal hyper-reflective material, such as compared to a subject who was not administered with Antagonist A or another pharmaceutically acceptable salt thereof, or compared to a subject administered a VEGF antagonist, anti-VEGF monotherapy, and/or an anti-C5 agent.
[0284] In some embodiments, a subject with improved vision has a greater than 3-line, 4-line or 5-line gain in visual acuity. In one embodiment, a subject's visual acuity is determined using a protocol such as the Early Treatment for Diabetic Retinopathy Study ("ETDRS") or the Age-Related Eye Disease Study ("AREDS") protocol. In some embodiments, visual acuity is measured using a modified ETDRS and/or AREDS protocol, such as the measurement of visual acuity described in Ferris et al., Am J Ophthalmol 94:91-96, 1982. In some embodiments, visual acuity is measured as described in Early Treatment Diabetic Retinopathy Study Research Group (ETDRS), Manual of Operations, Baltimore: ETDRS Coordinating Center, University of Maryland. Available from: National Technical Information Service, 5285 Port Royal Road, Springfield, Va. 22161; Accession No. PB85 223006/AS. In other embodiments, visual acuity testing is measured as described in Example 4 below. In some embodiments, the visual acuity testing uses one or more charts available from http://www.nei.nih.gov/photo/keyword.asp?conditions=Eye+Charts&match=all, e.g., ETDRS visual acuity Chart 1, 2 and/or R.
[0285] In one embodiment, a subject's visual acuity is determined by one or more of the following procedures: (1) measurement of best-corrected visual acuity (BCVA) with required manifest refraction; (2) measurement of corrected visual acuity with conditional manifest refraction; or (3) measurement of corrected visual acuity without manifest refraction.
[0286] In one embodiment, each of the PDGF and VEGF antagonists is administered in an amount effective to treat or prevent an ophthalmological disease or disorder. The amount of antagonist that is admixed with the carrier materials to produce a single dosage can vary depending upon the subject being treated and the particular mode of administration.
[0287] The dosage of each antagonist can depend on several factors including the severity of the condition, whether the condition is to be treated or prevented, and the age, weight, and health of the person to be treated. Additionally, pharmacogenomic (the effect of genotype on the pharmacokinetic, pharmacodynamic or efficacy profile of a therapeutic) information about a particular patient may affect dosage used. Furthermore, the exact individual dosages can be adjusted somewhat depending on a variety of factors, including the specific combination of antagonists being administered, the time of administration, the route of administration, the nature of the formulation, the rate of excretion, the particular ophthalmological disease or disorder being treated, the severity of the disorder, and the anatomical location of the neovascular disorder. Some variations in the dosage can be expected.
[0288] Generally, when orally administered to a subject, the dosage of an antagonist of the present invention is normally 0.001 mg/kg/day to 100 mg/kg/day, 0.01 mg/kg/day to 50 mg/kg/day, or 0.1 mg/kg/day to 10 mg/kg/day. Generally, when orally administered to a human, the dosage of an antagonist of the present invention is normally 0.001 mg to 300 mg per day, 1 mg to 200 mg per day, or 5 mg to 50 mg per day. Dosages up to 200 mg per day may be necessary. For administration of an antagonist of the present invention by parenteral injection, the dosage is normally 0.1 mg to 250 mg per day, 1 mg to 20 mg per day, or 3 mg to 5 mg per day. Injections may be given up to four times daily. In some embodiments, the dosage of a PDGF or VEGF antagonist for use in the present invention is normally 0.1 mg to 1500 mg per day, or 0.5 mg to 10 mg per day, or 0.5 mg to 5 mg per day. A dosage of up to 3000 mg per day can be administered.
[0289] In some embodiments, for administration by parenteral injection of a three active agents (e.g., Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and an anti-C5 agent or other combination disclosed herein), the dosage of each of the PDGF antagonist, VEGF antagonist and anti-C5 agent, is typically 0.1 mg to 250 mg per day, 1 mg to 20 mg per day, or 3 mg to 5 mg per day. Injections may be given up to four times daily. Generally, when parenterally administered, the dosage of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist, or anti-C5 agent is typically 0.1 mg to 1500 mg per day, or 0.5 mg to 10 mg per day, or 0.5 mg to 5 mg per day. A dosage of at least up to 3000 mg per day can be administered.
[0290] In some embodiments, in which Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and/or anti-C5 agent are ophthalmologically administered to a human, for example intravitreally, the dosage of each of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent is typically 0.003 mg to 5.0 mg per eye per administration, or 0.03 mg to 3.0 mg per eye per administration, or 0.1 mg to 1.0 mg per eye per administration. In one embodiment, the dosage of each of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent is about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.25 mg, about 1.5 mg, about 2.0 mg or about 3.0 mg per eye. In one embodiment, the dosage Antagonist A or another pharmaceutically acceptable salt thereof is about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.25 mg, about 1.5 mg, about 2.0 mg, about 3.0 mg, or about 4.0 mg per eye. In another embodiment, the dosage of a VEGF antagonist (e.g., ranibizumab, bevacizumab, aflibercept, ESBA1008 or pegaptanib sodium) is about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.25 mg, about 1.5 mg, about 1.65 mg, about 2.0 mg, about 3.0 mg, or about 4.0 mg per eye. In another embodiment, the dosage of the anti-C5 agent (e.g., ARC1905 or a pharmaceutically acceptable salt thereof) is about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.25 mg, about 1.5 mg, about 1.65 mg, about 2.0 mg, about 3.0 mg, or about 4.0 per eye.
[0291] In certain embodiments where a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, and optionally an anti-C5 agent, the dosage of Antagonist A or another pharmaceutically acceptable salt thereof) is about 1.5 mg, and the dosage of the VEGF antagonist (e.g., ranibizumab) is about 0.5 mg. In certain embodiments where a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 3.0 mg, and the dosage of the VEGF antagonist (e.g., ranibizumab) is about 0.5 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof) is about 1.5 mg, and the dosage of the VEGF antagonist (e.g., bevacizumab) is about 1.25 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 3.0 mg, and the dosage of the VEGF antagonist (e.g., bevacizumab) is about 1.25 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 1.5 mg, and the dosage of the VEGF antagonist (e.g., aflibercept) is about 2.0 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 3.0 mg, and the dosage of the VEGF antagonist (e.g., aflibercept) is about 2.0 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 1.5 mg, and the dosage of the VEGF antagonist, e.g., pegaptanib sodium, is about 1.65 mg. In certain embodiments, a subject is administered both Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist, wherein the dosage of Antagonist A or another pharmaceutically acceptable salt thereof is about 3.0 mg, and the dosage of the VEGF antagonist, e.g., pegaptanib sodium, is about 1.65 mg.
[0292] The dosage can range from about 0.01 mL to about 0.2 mL administered per eye, or about 0.03 mL to about 0.15 mL administered per eye, or about 0.05 mL to about 0.10 mL administered per eye.
[0293] Antagonist A or a pharmaceutically acceptable salt thereof can be delivered intravitreally at up to about 30 mg/ml with injection volumes up to 100 .mu.L.
[0294] Illustrative Antagonist A/VEGF antagonist combination pairs and their dosages are set forth in Table 11:
TABLE-US-00010 TABLE 11 Combination No. PDGF Antagonist VEGF Antagonist 1 Antagonist A (about 1.5 mg) ranibizumab (about 0.5 mg) 2 Antagonist A (about 3.0 mg) ranibizumab (about 0.5 mg) 3 Antagonist A (about 1.5 mg) bevacizumab (about 1.25 mg) 4 Antagonist A (about 3.0 mg) bevacizumab (about 1.25 mg) 5 Antagonist A (about 1.5 mg) aflibercept (about 2.0 mg) 6 Antagonist A (about 3.0 mg) aflibercept (about 2.0 mg) 7 Antagonist A (about 3.0 mg) pegaptanib sodium (about 1.65 mg) 8 Antagonist A (about 3.0 mg) pegaptanib sodium (about 1.65 mg)
[0295] In particular embodiments wherein the subject is administered an anti-C5 agent in combination with Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist, the anti-C5 agent may be administered at a dosage of about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.25 mg, about 1.5 mg, about 2.0 mg or about 3.0 mg per eye.
[0296] In certain embodiments, ocular dosages of compositions comprising anti-C5 aptamers, such as ARC1905 and ARC187, or a pharmaceutically acceptable salt thereof, can range from about 0.01 mg to about 5 mg/eye or from about 0.1 mg to about 3 mg/eye. For instance, ocular dosages of compositions comprising ARC1905, ARC187, or a pharmaceutically acceptable salt thereof may be about 0.01 mg, about 0.03 mg, about 0.05 mg, about 0.1 mg, about 0.3 mg, about 0.5 mg, about 1 mg, about 1.5 mg, about 2 mg, about 2.5 mg, about 3 mg, about 3.5 mg, about 4 mg, about 4.5 mg, or about 5 mg. Such dosages may be administered ocularly, for example by intravitreal injection, weekly, biweekly, monthly, or quarterly, optionally by a sustained release device or formulation. In some embodiments, the anti-C5 aptamers (e.g., ARC1905, ARC187, or a pharmaceutically acceptable salt thereof) can be administered in multiple injections (e.g., intravitreal injections) over a period of months separated by varying time intervals. In certain such embodiments, initial injections received early in the treatment regimen are separated by a shorter interval than injections received later in the treatment regimen. For instance, one dosage regimen, particularly useful in methods for treating, preventing, or stabilizing AMD (e.g., non-exudative type AMD or geographic atrophy), comprises administering initial injections at the start of treatment (e.g., first two, three, four, or five injections) of anti-C5 aptamer (e.g., ARC1905, ARC187, or a pharmaceutically acceptable salt thereof) on a monthly basis and administering subsequent injections at longer intervals (e.g., every three, four, five, or six months). By way of example, the first three injections of anti-C5 aptamer are administered to a subject every month, whereas the fourth and fifth injections are administered three or four months after the previous injection. Intervals between injections of anti-C5 aptamer may be adjusted based on the subject's response to treatment as measured, for example, by change in geographic atrophy lesion size or improvement or stabilization of visual acuity.
[0297] In some embodiments, an anti-C5 aptamer is administered to a subject with a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 0.03 mg, and the dosage of the VEGF antagonist, e.g., ranibizumab, is about 0.5 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 1.0 mg, and the dosage of the VEGF antagonist, e.g., ranibizumab, is about 0.5 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 2.0 mg, and the dosage of the VEGF antagonist, e.g., ranibizumab, is about 0.5 mg.
[0298] In some embodiments, an anti-C5 aptamer is administered to a subject with a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 0.03 mg, and the dosage of the VEGF antagonist, e.g., bevacizumab, is about 1.25 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 1.0 mg, and the dosage of the VEGF antagonist, e.g., bevacizumab, is about 1.25 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 2.0 mg, and the dosage of the VEGF antagonist, e.g., bevacizumab, is about 1.25 mg.
[0299] In some embodiments, an anti-C5 aptamer is administered to a subject with a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 0.03 mg, and the dosage of the VEGF antagonist, e.g., aflibercept, is about 2.0 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 1.0 mg, and the dosage of the VEGF antagonist, e.g., aflibercept, is about 2.0 mg. In certain embodiments, a subject is administered both an anti-C5 aptamer and a VEGF antagonist, wherein the dosage of the anti-C5 aptamer is about 2.0 mg, and the dosage of the VEGF antagonist, e.g., aflibercept, is about 2.0 mg.
[0300] Administration of each antagonist can, independently, be one to four times daily or one to four times per month or one to six times per year or once every two, three, four or five years. Administration can be for the duration of one day or one month, two months, three months, six months, one year, two years, three years, and may even be for the life of the patient. In one embodiment, the administration is performed once a month for three months. Chronic, long-term administration will be indicated in many cases. The dosage may be administered as a single dose or divided into multiple doses. In general, the desired dosage should be administered at set intervals for a prolonged period, usually at least over several weeks or months, although longer periods of administration of several months or years or more may be needed.
[0301] In addition to treating pre-existing ophthalmological diseases and disorders, the compositions can be administered prophylactically in order to prevent or slow the onset of these disease and disorders. The term "prevent" encompasses inhibiting or delaying the onset or progression of a disease or disorder. In prophylactic applications, the composition can be administered to a patient susceptible to or otherwise at risk of a particular ophthalmological disease or disorder.
[0302] In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist are administered to a subject in need of treatment therewith, typically in the form of an injectable pharmaceutical composition. Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist can be administered either in separate compositions or in a pharmaceutical composition comprising both the PDGF antagonist and VEGF antagonist. The administration can be by injection, for example by intraocular injection, or by using a drug delivery device. Parenteral, systemic, or transdermal administration is also within the scope of the invention. The administration of Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist can be sequential in time or concurrent. When administered sequentially, the administration of each can be by the same or different route. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered within 90 days, 30 days, 10 days, 5 days, 24 hours, 1 hour, 30 minutes, 10 minutes, 5 minutes or one minute of administration of a VEGF antagonist. Where Antagonist A or another pharmaceutically acceptable salt thereof is administered prior to the VEGF antagonist, the VEGF antagonist is administered within a time and in an amount such that the total amount of Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist is effective to treat or prevent an ophthalmological disease or disorder. Where the VEGF antagonist is administered prior to Antagonist A or another pharmaceutically acceptable salt thereof, Antagonist A or another pharmaceutically acceptable salt thereof is administered within a time and in an amount such that the total amount of Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist is effective to treat or prevent an ophthalmological disease or disorder.
[0303] In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof or VEGF antagonist (e.g., ranibizumab, bevacizumab, pegaptanib sodium, ESBA1008 or aflibercept) is administered intravitreally with a 30-gauge or 27-gauge needle. In some embodiments, a 0.5 inch needle is used. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally with a 30-gauge 0.5 inch needle and a VEGF antagonist (e.g., ranibizumab, bevacizumab, pegaptanib sodium, ESBA1008 or aflibercept) is administered intravitreally with a 27-gauge needle. In some embodiments, 50 .mu.L (1.5 mg in 0.05 mL) of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally with a 30-gauge 0.5 inch needle and 50 .mu.L (0.5 mg in 0.05 mL) of a VEGF antagonist (e.g., ranibizumab, bevacizumab, pegaptanib sodium or aflibercept) is administered intravitreally with a 27-gauge needle.
[0304] In certain embodiments where Antagonist A or another pharmaceutically acceptable salt thereof such as Antagonist A or another pharmaceutically acceptable salt thereof is used in combination with a VEGF antagonist, such as ranibizumab, bevacizumab, ESBA1008, pegaptanib sodium or aflibercept, one of these two agents is first administered to the subject, and then the other agent is administered to the subject. In particular embodiments, the two agents are both administered to the same eye of the subject. In particular embodiments, the two agents are both administered to both eyes of the subject. The two agents may be administered to an eye in either order, i.e., Antagonist A or another pharmaceutically acceptable salt thereof may be administered first, and then the VEGF antagonist administered, or the VEGF antagonist may be administered first, and then Antagonist A or another pharmaceutically acceptable salt thereof administered. The agent administered second may be administered immediately following administration of the agent administered first, or the agent administered second may be administered after a time period following administration of the agent administered first.
[0305] In certain embodiments, the time period from administration of the first agent to administration of the second agent is at least 1 min, at least 5 min, at least 10 min, at least 15 min, at least 30 min, or at least one hour. In certain embodiments, the time period from administration of the first agent to administration of the second agent is between 1 min and 2 hours, between 5 min and 2 hours, between 10 min and 2 hours, between 15 min and 2 hours, between 30 min and 2 hours, between 45 min and 2 hours, between 1 hour and 2 hours, or between 30 min and 1 hour. In certain embodiments, the time period from administration of the first agent to administration of the second agent is about 1 min, about 2 min, about 3 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 35 min, about 40 min, about 45 min, about 50 min, about 55 min, about 60 min, about 90 min, or about 120 min.
[0306] In certain embodiments, the present invention provides methods for treating or preventing any of the ophthalmological diseases described herein, comprising providing to a subject in need thereof Antagonist A or another pharmaceutically acceptable salt thereof at a first time point, and providing to the subject a VEGF antagonist, e.g., aflibercept, bevacizumab, ranibizumab, ESBA1008, or pegaptanib sodium, at a second time point, wherein the amount of time between the first time point and the second time point is about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days.
[0307] In certain embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and the VEGF antagonist are administered intravitreally. In certain embodiments, about 1.5 mg or 3.0 mg of Antagonist A or another pharmaceutically acceptable salt thereof to an eye, and about 0.5 mg, about 1.25 mg, about 1.65 mg, or about 2.0 mg of the VEGF antagonist is administered to an eye. In some embodiments, the VEGF antagonist is administered intravitreally about 30 minutes after Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally. In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally about 30 minutes after the VEGF antagonist is administered intravitreally.
[0308] In one embodiment, a VEGF antagonist is administered to at least one eye of the subject, about 1 hour is allowed to elapse following administration of the VEGF antagonist, and then Antagonist A or another pharmaceutically acceptable salt thereof is administered to the same eye. In one embodiment, Antagonist A or another pharmaceutically acceptable salt thereof is administered to at least one eye of the subject, about 1 hour is allowed to lapse following administration of the PDGF antagonist, and then a VEGF antagonist is administered to the same eye.
[0309] In certain embodiments, the PDGF antagonist and the VEGF antagonist are administered to each eye in a total combined volume of less than or about 50 .mu.L, less than or about 60 .mu.L, less than or about 70 .mu.L, less than or about 80 .mu.L, less than or about 90 .mu.L, less than or about 100 .mu.L, less than or about 120 .mu.L, less than or about 150 .mu.L, or less than or about 200 .mu.L.
[0310] In certain embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are administered intraocularly, e.g., intravitreally. In particular embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are administered to the mammal via a single injection, e.g., a single intraocular or intravitreal injection. In particular embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are administered sequentially. In certain embodiments, two or more of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist and an anti-C5 agent are administered at the same time, e.g., in the same composition. In particular embodiments, one of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist and an anti-C5 agent is administered, and within about 30 seconds, one or two of others are subsequently administered. In particular embodiments, all three of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist and an anti-C5 agent are administered within about 30 seconds or one minute of each other. In other embodiments, one of Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist and an anti-C5 agent is administered, and one or both of the others are administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later. In other embodiments, one or two of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are administered, and the other is administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later. In certain embodiments, one of the PDGF antagonist, VEGF antagonist and anti-C5 agent is administered; and another is administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later; and the remaining one is administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later. In certain embodiments wherein two of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are present in the same composition, the composition is administered and the PDGF antagonist, VEGF antagonist or anti-C5 agent that is not present in the composition is administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later. In other embodiments wherein two of Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist and anti-C5 agent are present in the same composition, Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist or anti-C5 agent that is not present in the composition is administered, and the composition is administered about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours, about 24 hours, about 36 hours, about 48 hours, about three days, about four days, about five days, about six days, or about seven days later.
[0311] In certain embodiments, Antagonist A or another pharmaceutically acceptable salt thereof, e.g., Antagonist A or another pharmaceutically acceptable salt thereof, is administered about every 24 hours for two or more, three or more, four or more, five or more, six or more, or seven or more days, and a VEGF antagonist, e.g., aflibercept, bevacizumab, ESBA1008, pegaptanib sodium or ranimizumab, is administered about 48 hours following the first administration of Antagonist A or another pharmaceutically acceptable salt thereof. In certain embodiments, Antagonist A or another pharmaceutically acceptable salt thereof is administered on each of four successive days, i.e., day 1, day 2, day 3 and day 4, and the VEGF antagonist (e.g., bevacizumab, ranicizumab, ESBA1008, pegaptanib sodium or aflibercept) is administered on the third day, i.e., day 3. In particular embodiments, a composition comprising Antagonist A or another pharmaceutically acceptable salt thereof, e.g., Antagonist A or another pharmaceutically acceptable salt thereof, is administered to a subject, and a composition comprising a VEGF antagonist is administered to the subject about forty-eight hours later.
[0312] In one embodiment, about 50 mg/kg of Antagonist A or another pharmaceutically acceptable salt thereof (e.g., Antagonist A or another pharmaceutically acceptable salt thereof) is administered, e.g., intraperitoneally, on day 1, day 2, day 3 and day 4, and about 1 mg/kg of a VEGF antagonist (e.g., bevacizumab, ranibizumab, ESBA1008, pegaptanib sodium, or aflibercept) is administered on day 3. In one embodiment, about 50 mg/kg of Antagonist A or another pharmaceutically acceptable salt thereof (e.g., Antagonist A or another pharmaceutically acceptable salt thereof) is administered on day 1, day 2, day 3 and day 4, and about 5 mg/kg of a VEGF antagonist (e.g., bevacizumab, ranibizumab, ESBA1008, pegaptanib sodium, or aflibercept) is administered on day 3.
[0313] In one embodiment, about 50 mg/kg of Antagonist A or another pharmaceutically acceptable salt thereof is administered on day 1, day 2, day 3 and day 4, and about 1 mg/kg of aflibercept is administered on day 3. In one embodiment, about 50 mg/kg of Antagonist A or another pharmaceutically acceptable salt thereof is administered on day 1, day 2, day 3 and day 4, and about 5 mg/kg of aflibercept is administered on day 3.
[0314] In one embodiment, about 0.03 mg, about 0.3 mg, about 0.5 mg, about 1.0 mg, about 1.5 mg or about 3.0 mg of Antagonist A or another pharmaceutically acceptable salt thereof (e.g., Antagonist A or another pharmaceutically acceptable salt thereof) is administered intravitreally on day 1, day 2, day 3 and day 4, and about 0.5 mg, about 1.0 mg, about 1.5 mg, about 1.65 mg, about 3.0 mg, or about 4.0 mg of a VEGF antagonist (e.g., bevacizumab, ranibizumab, ESBA1008, pegaptanib sodium, or aflibercept) is administered intravitreally on day 3. In one embodiment, about 0.3 mg or about 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally on day 1, day 2, day 3 and day 4, and about 0.5 mg of ranibizumab is administered intravitreally on day 3. In one embodiment, about 0.3 mg or about 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally on day 1, day 2, day 3 and day 4, and about 1.25 mg of bevacizumab is administered intravitreally on day 3. In one embodiment, about 0.3 mg or about 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally on day 1, day 2, day 3 and day 4, and about 2.0 mg of aflibercept is administered intravitreally on day 3. In one embodiment, about 0.3 mg or about 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof is administered intravitreally on day 1, day 2, day 3 and day 4, and about 1.65 mg of pegaptanib sodium is administered intravitreally on day 3.
[0315] In some embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and VEGF antagonist are administered every four weeks or every 30 days, for six treatments. In some embodiments, the VEGF antagonist is ranibizumab. In some embodiments, 0.3 mg of Antagonist A or another pharmaceutically acceptable salt thereof and 0.5 mg of ranibizumab are administered every four weeks or every 30 days, for six treatments. In some embodiments, 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof and 0.5 mg of ranibizumab are administered every four weeks or every 30 days, for six treatments.
[0316] In some embodiments, 0.3 mg of Antagonist A or another pharmaceutically acceptable salt thereof and 1.25 mg of bevacizumab, 2.0 mg of aflibercept, or 1.65 mg of pegaptanib sodium are administered every four weeks or every 30 days, for six treatments. In some embodiments, 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof and 1.25 mg of bevacizumab, 2.0 mg of aflibercept, or 1.65 mg of pegaptanib sodium are administered every four weeks or every 30 days, for six treatments.
[0317] In some embodiments, the methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof, bevacizumab and aflibercept. In some embodiments, the methods comprise administering Antagonist A or another pharmaceutically acceptable salt thereof, bevacizumab and aflibercept every four weeks or every 30 days, for six treatments. In some embodiments, the methods comprise administering 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof, 1.25 mg of bevacizumab, and 2 mg of aflibercept. In some embodiments, the methods comprise administering 1.5 mg of Antagonist A or another pharmaceutically acceptable salt thereof, 1.25 mg of bevacizumab, and 2 mg of aflibercept every four weeks or every 30 days, for six treatments.
[0318] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein (a) and (b) are administered in an amount that is effective for treating or preventing an ocular condition (e.g., wet AMD), and wherein the administering occurs once every month, .+-.about seven days, for 12 consecutive months.
[0319] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein: (a) and (b) are administered in an amount that is effective for treating or preventing an ocular condition (e.g., wet AMD); and the administering occurs once every month, .+-.about seven days, for a first 12 consecutive months, and immediately thereafter once every two months, .+-.about seven days, for a second 12 consecutive months, commencing on the second month of the second 12 consecutive months.
[0320] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein: (a) and (b) are administered in an amount that is effective for treating or preventing an ocular condition (e.g., wet AMD); and the administering occurs once every month, .+-.about seven days, for 24 consecutive months is also provided herein.
[0321] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein: (a) and (b) are administered in an amount that is effective for treating or preventing an ocular condition (e.g., wet AMD); and the administering occurs once every month, .+-.about seven days, for three consecutive months, and immediately thereafter once every two months, .+-.about seven days, for 12 consecutive months, commencing on the second month of the 12 consecutive months.
[0322] In some embodiments, the methods comprise continuous treatment, continuous and discontinuous treatments, and/or retreatments, e.g., for the treatment or preventing of wet-type AMD or subfoveal neovascular AMD. In some embodiments, continuous treatment comprises administering to Antagonist A or another pharmaceutically acceptable salt thereof and an anti-VEGF agent monthly (.+-.7 days) for at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 consecutive months. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered within about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours of administration of the VEGF antagonist. In some embodiments, the VEGF antagonist is administered prior to administration of Antagonist A or a pharmaceutically acceptable salt thereof. In other embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered prior to administration of the VEGF antagonist. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist are administered as a co-formulation. In some embodiments, the amount of Antagonist A or a pharmaceutically acceptable salt thereof administered is about 1.5 mg/eye and the amount of VEGF antagonist administered is about 0.5 mg/eye (e.g., ranibizumab), about 1.25 mg/eye (e.g., bevacizumab), about 1.65 mg/eye (e.g., pegaptanib sodium), or about 2.0 mg/eye (e.g., aflibercept).
[0323] In some embodiments, the methods further comprise measuring the subject's visual acuity. In some embodiments, the subject's visual acuity is measured once every month, .+-.about seven days. In some embodiments, visual acuity is stable when it is stable for three consecutive months. In some embodiments, visual acuity is stable when at each of the last two of the three consecutive months, visual acuity is within 5 ETDRS letters (better or worse) of the subject's visual acuity at the first of the three consecutive months (i.e., the month immediately preceding the first of the two consecutive following months).
[0324] In some embodiments, a subject is administered in accordance with the present methods until the subject's visual acuity is stable. In some embodiments, a subject is administered in accordance with the present methods until the subject's visual acuity is stable for three consecutive months. In some embodiments, a subject is administered in accordance with the present methods until the subject's visual acuity at each of the last two of the three consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity of the first of the three consecutive months. In some embodiments, a subject is administered in accordance with the present methods until the subject experiences no new or significant intraretinal or sub-retinal hemorrhage, or no increase of .gtoreq.50 .mu.m in foveal intraretinal fluid. In some embodiments, a subject is administered in accordance with the present methods until the subject's visual acuity measured at each of the last two of the three consecutive months is .ltoreq.a five-ETDRS-letter difference from the subject's visual acuity of the first of the three consecutive months, and the subject experiences no new or significant intraretinal or sub-retinal hemorrhage, and no increase of .gtoreq.50 .mu.m in foveal intraretinal fluid.
[0325] In some embodiments, discontinuous treatment is administered after continuous treatment, in which discontinuous treatment is based on a physician's discretion, and the subject has stabilized vision as determined by .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity after continuous and discontinuous treatment.
[0326] In some embodiments, subjects with a loss of visual acuity of >5 ETDRS letters from the previous monthly assessment, new and significant intraretinal or sub-retinal hemorrhage, and/or an increase of .gtoreq.50 .mu.m in foveal intraretinal fluid are retreated.
[0327] In some embodiments, the continuous method comprises administering Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist in an amount that is effective for treating or preventing wet AMD, wherein the administering occurs once every month, .+-.about seven days, for 12 consecutive months. In some embodiments, the methods further comprise measuring the subject's visual acuity at one month, .+-.about seven days, immediately following the 12 consecutive months, wherein the subject's visual acuity measured on the twelfth of the 12 consecutive months and the one month immediately following the 12 consecutive months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on the eleventh of the 12 consecutive months.
[0328] In some embodiments, the methods further comprise measuring the subject's visual acuity once every month, .+-.about seven days, on each of an additional 11 consecutive months. In some embodiments, the subject's visual acuity measured on any two consecutive months of the additional 11 consecutive months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the two consecutive months.
[0329] In some embodiments, the subject's visual acuity measured on the twelfth of the 12 consecutive months and the one month immediately following the 12 consecutive months is not .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on the eleventh of the 12 consecutive months and the subject is retreated. In some embodiments, retreatment comprises administering to the patient on the one month immediately following the 12 consecutive months Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist in an amount that is effective for treating or preventing wet AMD, measuring the patient's visual acuity on a month, .+-.about seven days, immediately following the one month immediately following the 12 consecutive months, and administering to the subject on each immediately following month Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity on any two consecutive following months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive following months. In some embodiments, the total number of months does not exceed 24.
[0330] In some embodiments, wherein the subject's visual acuity measured on the one month immediately following the 12 consecutive months is not .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on the twelfth of the 12 consecutive months and is not solely attributable to newly diagnosed foveal atrophy or worsening ocular media opacity, the method further comprises administering to the subject on the one month immediately following the 12 consecutive months Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist in an amount that is effective for treating or preventing wet AMD; and administering to the subject on each immediately following month (a) and (b) in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity measured on any two consecutive following months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive following months. In some embodiments, the total number of months does not exceed 24.
[0331] In some embodiments, wherein the subject presents intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid at one month, .+-.about seven days, immediately following the 12 consecutive months, the method further comprises administering to the subject on the one month immediately following the 12 consecutive months Antagonist A or another pharmaceutically acceptable salt thereof an a VEGF antagonist in an amount that is effective for treating or preventing wet AMD; and administering to the subject on each immediately following month (a) and (b) in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity measured on any two consecutive following months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive following months. In some embodiments, the total number of months does not exceed 24.
[0332] Also provided herein is a method comprising administering Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist intravitreally once every month, .+-.about seven days, for a first 12 consecutive months, and immediately thereafter once every two months, .+-.about seven days, for a second 12 consecutive months, commencing on the second month of the second 12 consecutive months. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered within about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours of administration of the VEGF antagonist. In some embodiments, the VEGF antagonist is administered prior to administration of Antagonist A or a pharmaceutically acceptable salt thereof. In other embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered prior to administration of the VEGF antagonist. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist are administered as a co-formulation. In some embodiments, the amount of Antagonist A or a pharmaceutically acceptable salt thereof administered is about 1.5 mg/eye and the amount of VEGF antagonist administered is about 0.5 mg/eye (e.g., ranibizumab), about 1.25 mg/eye (e.g., bevacizumab), about 1.65 mg/eye (e.g., pegaptanib sodium), or about 2.0 mg/eye (e.g., aflibercept).
[0333] In some embodiments, the method further comprises measuring the subject's visual acuity once every month, .+-.about seven days, during the first 12 consecutive months and second 12 consecutive months. In some embodiments, the subject's visual acuity measured on any one of the first, third, fifth, seven, ninth and eleventh months of the second consecutive 12 months decreased at least five ETDRS letters relative to the patient's visual acuity measured on the month immediately preceding the first, third, fifth, seven, ninth or eleventh month of the second consecutive 12 months.
[0334] In some embodiments, the methods further comprises administering to the subject an amount of Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist effective for treating or preventing wet AMD on the month in which the subject's visual acuity measured the decrease of at least five ETDRS letters relative to the patient's visual acuity measured on the immediately preceding month.
[0335] In some embodiments, the method further comprises administering Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist on any one of the first, third, fifth, seven, ninth and eleventh months of the second consecutive 12 months.
[0336] In some embodiments, the decrease in visual acuity is attributed to solely newly diagnosed foveal atrophy or opacified ocular media.
[0337] In some embodiments, the subject presents intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid on any one of the first, third, fifth, seven, ninth and eleventh months of the second consecutive 12 months.
[0338] In some embodiments, the method further comprises administering Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist on month in which the subject presents intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid.
[0339] Also provided herein is a method comprising administering Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist intravitreally once every month, .+-.about seven days, for 24 consecutive months. In other embodiments, Antagonist A or another pharmaceutically acceptable salt thereof and a VEGF antagonist are administered intravitreally once a month for three months and then every other month for the next 21 months. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered within about 1 min, about 2 min, about 5 min, about 10 min, about 15 min, about 20 min, about 25 min, about 30 min, about 40 min, about 50 min, about 60 min, about 90 min, about 2 hours, about 4 hours, about 6 hours, about 8 hours, about 12 hours of administration of the VEGF antagonist. In some embodiments, the VEGF antagonist is administered prior to administration of Antagonist A or a pharmaceutically acceptable salt thereof. In other embodiments, Antagonist A or a pharmaceutically acceptable salt thereof is administered prior to administration of the VEGF antagonist. In some embodiments, Antagonist A or a pharmaceutically acceptable salt thereof and a VEGF antagonist are administered as a co-formulation. In some embodiments, the amount of Antagonist A or a pharmaceutically acceptable salt thereof administered is about 1.5 mg/eye and the amount of VEGF antagonist administered is about 0.5 mg/eye (e.g., ranibizumab), about 1.25 mg/eye (e.g., bevacizumab), about 1.65 mg/eye (e.g., pegaptanib sodium), or about 2.0 mg/eye (e.g., aflibercept).
[0340] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) an VEGF antagonist, wherein (a) and (b) are administered in an amount that is effective for treating or preventing an ophthalmological disease or disorder (e.g., wet AMD), and wherein the administering occurs once every month, .+-.about seven days, for a first administration period of at least 3 consecutive months, followed by administering (a) and (b) for a second administration period at a frequency of at least every other month .+-.about seven days beginning at two months .+-.about seven days after the day of the last month of the first administration period on which (a) and (b) are administered. In some embodiments, the first administration period is for at least 6 consecutive months. In some embodiments, the VEGF antagonist is ranibizumab or bevacizumab, wherein (a) and (b) are administered at a frequency of once every month .+-.about seven days during the second administration period and wherein the second administration period is at least about nine months.
[0341] In some embodiments, the methods further comprise measuring the subject's visual acuity on a day that is prior to and within about one month of administration of (a) and (b). In some embodiments, the methods further comprise administering to the subject (a) and (b) in an amount that is effective for treating or preventing an ophthalmological disease or disorder (e.g., wet AMD), until the subject's visual acuity on any two consecutive following months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive following months.
[0342] In some embodiments, the method further comprise administering to the subject (a) and (b) every other month in an amount that is effective for treating or preventing an ophthalmological disease or disorder (e.g., wet AMD), until the subject's visual acuity on any two consecutive visual acuity assessments is not .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a visual acuity assessment immediately preceding the first of the two consecutive visual acuity assessments.
[0343] In other embodiments, the methods further comprise administering to the subject (a) and (b) every month in an amount that is effective for treating or preventing an ophthalmological disease or disorder (e.g., wet AMD), until the subject's visual acuity on any two consecutive following months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive following months.
[0344] In some embodiments, the methods comprise administering to a subject in need thereof (a) Antagonist A or another pharmaceutically acceptable salt thereof and (b) aflibercept, wherein (a) and (b) are administered in an amount that is effective for treating or preventing an ophthalmological disease or disorder (e.g., wet AMD), and wherein the administering occurs once every month, .+-.about seven days, for a first administration period of at least 3 consecutive months, followed by administering (a) and (b) for a second administration period at a frequency of at least every other month .+-.about seven days beginning at two months .+-.about seven days after the day of the last month of the first administration period on which (a) and (b) are administered.
[0345] In some embodiments, the subject has intraretinal or sub-retinal hemorrhage or a .gtoreq.50 .mu.m increase in foveal intraretinal fluid at one month, .+-.about seven days, immediately following the second administration period. In some embodiments, the methods further comprise administering to the subject on each month .+-.about seven days, beginning on the month that immediately follows the second administration period (a) and (b) in an amount that is effective for treating or preventing wet AMD, until the subject's visual acuity measured on any two consecutive months that follow the 12 consecutive months is .ltoreq.a five-ETDRS-letter difference in the subject's visual acuity measured on a month immediately preceding the first of the two consecutive months.
[0346] In some embodiments, the total number of months of treatment does not exceed 24.
[0347] Pharmaceutical compositions according to the invention may be formulated to release Antagonist A or another pharmaceutically acceptable salt thereof, a VEGF antagonist, or an anti-C5 agent, substantially immediately upon administration or at any predetermined time period after administration, using controlled release formulations. For example, a pharmaceutical composition can be provided in sustained-release form. The use of immediate or sustained release compositions depends on the nature of the condition being treated. If the condition consists of an acute disorder, treatment with an immediate release form can be utilized over a prolonged release composition. For certain preventative or long-term treatments, a sustained released composition can also be appropriate.
[0348] Administration of one or both of the antagonists of, or an anti-C5 agent, in controlled release formulations can be useful where the antagonist, either alone or in combination, has (i) a narrow therapeutic index (e.g., the difference between the plasma concentration leading to harmful side effects or toxic reactions and the plasma concentration leading to a therapeutic effect is small; generally, the therapeutic index, TI, is defined as the ratio of median lethal dose (LD.sub.50) to median effective dose (ED.sub.50)); (ii) a narrow absorption window in the gastro-intestinal tract; or (iii) a short biological half-life, so that frequent dosing during a day is required in order to sustain the plasma level at a therapeutic level.
[0349] Many strategies can be pursued to obtain controlled release in which the rate of release outweighs the rate of degradation or metabolism of the therapeutic antagonist. For example, controlled release can be obtained by the appropriate selection of formulation parameters and ingredients, including, e.g., appropriate controlled release compositions and coatings. Examples include single or multiple unit tablet or capsule compositions, oil solutions, suspensions, emulsions, microcapsules, microspheres, nanoparticles, patches, and liposomes. Methods for preparing such sustained or controlled release formulations are well known in the art.
[0350] Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist, or the anti-C5 agent can also be delivered using a drug-delivery device such as an implant. Such implants can be biodegradable and/or biocompatible, or can be non-biodegradable. The implants can be permeable to Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist, or the anti-C5 agent. Ophthalmic drug delivery devices can be inserted into a chamber of the eye, such as the anterior or posterior chamber or can be implanted in or on the sclera, choroidal space, or an a vascularized region exterior to the vitreous. In one embodiment, the implant can be positioned over an avascular region, such as on the sclera, so as to allow for transcleral diffusion of Antagonist A or another pharmaceutically acceptable salt thereof, the VEGF antagonist, or the anti-C5 agent to the desired site of treatment, e.g., the intraocular space and macula of the eye. Furthermore, the site of transcleral diffusion can be proximal to a site of neovascularization such as a site proximal to the macula. Suitable drug delivery devices are described, for example, in U.S. Publication Nos. 2008/0286334; 2008/0145406; 2007/0184089; 2006/0233860; 2005/0244500; 2005/0244471; and 2005/0244462, and U.S. Pat. Nos. 6,808,719 and 5,322,691, the contents of each of which is herein incorporated by reference in its entirety.
[0351] In one embodiment, the implant comprises Antagonist A or another pharmaceutically acceptable salt thereof and/or VEGF antagonist dispersed in a biodegradable polymer matrix. The matrix can comprise PLGA (polylactic acid-polyglycolic acid copolymer), an ester-end capped polymer, an acid end-capped polymer, or a mixture thereof. In another embodiment, the implant comprises Antagonist A or another pharmaceutically acceptable salt thereof and/or a VEGF antagonist, a surfactant, and lipophilic compound. The lipophilic compound can be present in an amount of about 80-99% by weight of the implant. Suitable lipophilic compounds include, but are not limited to, glyceryl palmitostearate, diethylene glycol monostearate, propylene glycol monostearate, glyceryl monostearate, glyceryl monolinoleate, glyceryl monooleate, glyceryl monopalmitate, glyceryl monolaurate, glyceryl dilaurate, glyceryl monomyristate, glyceryl dimyristate, glyceryl monopalmitate, glyceryl dipalmitate, glyceryl monostearate, glyceryl distearate, glyceryl monooleate, glyceryl dioleate, glyceryl monolinoleate, glyceryl dilinoleate, glyceryl monoarachidate, glyceryl diarachidate, glyceryl monobehenate, glyceryl dibehenate, and mixtures thereof. In another embodiment, the implant comprises Antagonist A or another pharmaceutically acceptable salt thereof and/or a VEGF antagonist housed within a hollow sleeve. The PDGF antagonist or VEGF antagonist, or both, are delivered to the eye by inserting the sleeve into the eye, releasing the implant from the sleeve into the eye, and then removing the sleeve from the eye. An example of this delivery device is described in U.S. Publication No. 2005/0244462, which is hereby incorporated by reference in its entirety.
[0352] In one embodiment, the implant is a flexible ocular insert device adapted for the controlled sustained release of Antagonist A or another pharmaceutically acceptable salt thereof and/or a VEGF antagonist into the eye. In one embodiment, the device includes an elongated body of a polymeric material in the form of a rod or tube containing Antagonist A or another pharmaceutically acceptable salt thereof, VEGF antagonist or both, and with at least two anchoring protrusions extending radially outwardly from the body. The device may have a length of at least 8 mm and the diameter of its body portion including the protrusions does not exceed 1.9 mm. The sustained release mechanism can, for example, be by diffusion or by osmosis or bioerosion. The insert device can be inserted into the upper or lower formix of the eye so as to be independent of movement of the eye by virtue of the formix anatomy. The protrusions can be of various shapes such as, for example, ribs, screw threads, dimples or bumps, truncated cone-shaped segments or winding braid segments. In a further embodiment, the polymeric material for the body is selected as one which swells in a liquid environment. Thus a device of smaller initial size can be employed. The insert device can be of a size and configuration such that, upon insertion into the upper or lower formix, the device remains out of the field of vision so as to be well retained in place and imperceptible by a recipient over a prolonged period of use. The device can be retained in the upper or lower formix for 7 to 14 days or longer. An example of this device is described in U.S. Pat. No. 5,322,691, which is hereby incorporated by reference in its entirety.
[0353] Kits
[0354] The invention relates to kits comprising one or more pharmaceutical compositions and instructions for use. At least two antagonists can be formulated together or in separate compositions and in individual dosage amounts. The antagonists are also useful when formulated as pharmaceutically acceptable salts. In one embodiment, the kits comprise a composition comprising Antagonist A or another pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier or vehicle and another composition comprising a VEGF antagonist and a pharmaceutically acceptable carrier or vehicle. In another embodiment, the kits comprise a composition comprising a VEGF antagonist, Antagonist A or another pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier or vehicle. Each of the kits' compositions can be contained in a container. In some embodiments, the kits comprise an anti-C5 agent.
[0355] The kits can comprise (1) an amount of Antagonist A or another pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier, vehicle, or diluent in a first unit dosage form; (2) an amount of a VEGF antagonist and a pharmaceutically acceptable carrier, vehicle, or diluent in a second unit dosage form; and (3) a container. The container can be used to separate components and include, for example, a divided bottle or a divided foil packet. The separate antagonist compositions may also, if desired, be contained within a single, undivided container. In some embodiments, the kits comprise an anti-C5 agent.
[0356] The kits can also comprise directions for the administration of the antagonists. The kits are particularly advantageous when the separate components are administered in different dosage forms, are administered at different dosage levels, or when titration of the individual antagonists is desired.
EXAMPLES
Example 1: Antagonist a and Ranibizumab Combination Therapy for Treating Subfoveal Neovascular Lesions Secondary to Neovascular Age Related Macular Degeneration (NVAMD)
[0357] In this study, 449 subjects with subfoveal neovascular lesions secondary to NVAMD received six monthly intravitreous injections of Antagonist A given in combination with ranibizumab (administered as Lucentis.RTM., commercially available from Genentech, South San Francisco, Calif.). Antagonist A was injected as the formulation shown in Table 12. The primary efficacy endpoint in the study was the mean change in visual acuity from baseline at the week 24 visit. As pre-specified in the analysis plan, the Hochberg procedure (Hochberg, Y. (1988). A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 75, 800-802) was employed to account for multiple dose comparisons.
[0358] The subjects were randomized in a 1:1:1 ratio to the groups shown in Table 13.
TABLE-US-00011 TABLE 12 Antagonist A Formulation 30 mg/mL Reference to Solution Percent Name of Ingredient Standards Function Composition (w/v) Antagonist A In-house standard Drug substance 30.0 mg 3% Monobasic Sodium USP/Ph. Eur pH buffering agent 0.3 mg 0.03% Phosphate Monohydrate Dibasic Sodium Phosphate USP/Ph. Eur pH buffering agent 2.1 mg 0.2% Heptahydrate Sodium Chloride USP/Ph. Eur Tonicity adjuster 9.0 mg 0.9% Hydrochloric Acid NF/Ph. Eur pH adjuster As needed Sodium Hydroxide NF/Ph. Eur pH adjuster As needed Water for Injection USP/Ph. Eur Diluent q.s. 95.9% Nitrogen NF/Ph. Eur Inert gas overlay -- -- Total Volume 1 ml Volume in Final Drug 230 microliters Product Presentation
TABLE-US-00012 TABLE 13 Antagonist A and Ranibizumab Combination Therapy for Subfoveal Neovascular Lesions Secondary to NVAMD Treatment Groups Group No. Group Name Treatment Regimen 1 Combination Subjects were administered 0.3 mg/eye of Therapy (0.3 mg) Antagonist A and 0.5 mg/ eye of Lucentis .RTM. 2 Combination Subjects were administered 1.5 mg/eye of Therapy (1.5 mg) Antagonist A and 0.5 mg/ eye of Lucentis .RTM. 3 Ranibizumab Subjects were administered Antagonist A Monotherapy Sham and 0.5 mg/eye of Lucentis .RTM.
[0359] Combination therapy proved superior in terms of mean visual gain when compared to eyes that were treated with anti-VEGF monotherapy. Subjects treated with Lucentis.RTM. and either 1.5 mg/eye or 0.3 mg/eye Antagonist A showed an increase in visual acuity compared with those treated with Lucentis.RTM. alone (FIG. 2). The combination of 1.5 mg/eye of Antagonist A and 0.5 mg of Lucentis.RTM. met the pre-specified, alpha protected primary endpoint of superiority in mean change of visual acuity gain compared to ranibizumab monotherapy from baseline to 24 weeks (10.6 ETDRS letters at week 24, compared to 6.5 letters, p=0.019, representing a 62% additional benefit). (FIG. 3) Subjects treated with Lucentis.RTM. and either 1.5 mg or 0.3 mg Antagonist A showed a 62% comparative benefit from baseline compared to treatment with Lucentis.RTM. alone.
[0360] In addition, the mean change in vision over time demonstrated the benefit of combination therapy at each measured time point over 24 weeks. (FIG. 4) That benefit was sustained during the study and demonstrated increasing differentiation of the curves at study closure.
[0361] Treatment with 0.5 mg of Lucentis.RTM. and either 1.5 mg or 0.3 mg Antagonist A in wet AMD patients also had increased efficacy as compared to patients treated with Lucentis.RTM. alone, independent of baseline lesion size or vision. (FIGS. 5A and 5B)
[0362] A greater percentage of subjects in the Combination Therapy (1.5 mg) group achieved enhanced visual outcomes compared to those in the Ranibizumab Monotherapy group with respect to multiple treatment endpoints at week 24, as shown in FIG. 6A, and Table 14.
TABLE-US-00013 TABLE 14 Percentage of Subjects in the Combination Therapy (1.5 mg) Group and Ranibizumab Monotherapy Group with Visual Acuity Improvement Percentage of Patients Combination Ranibizumab Treatment Endpoint Therapy (1.5 mg) Monotherapy >3-lines of visual acuity 36.4% 28.6% improvement >4-lines of visual acuity 19.9% 11.6% improvement >5-lines of visual acuity 11.9% 4.1% improvement .gtoreq.20/40 vision after treatment 37.0% 31.9% .gtoreq.20/25 vision after treatment 12.3% 5.6%
[0363] Moreover, fewer subjects in the Combination Therapy (1.5 mg) group demonstrated a loss of visual acuity as compared to the number of subjects in the Ranibizumab Monotherapy group at week 24, as shown in FIG. 6B and Table 15.
TABLE-US-00014 TABLE 15 Percentage of Subjects in the Combination Therapy (1.5 mg) Group and Ranibizumab Monotherapy Group with Visual Acuity Loss Percentage of Patients Combination Ranibizumab Treatment Endpoint Therapy (1.5 mg) Monotherapy .gtoreq.1-lines of visual acuity loss 8.3% 21.5% .gtoreq.2-lines of visual acuity loss 3.4% 12.5% .ltoreq.20/125 vision after treatment 19.2% 27.8% .ltoreq.20/200 vision after treatment 10.3% 13.9%
[0364] Subjects treated with Lucentis.RTM. and 1.5 mg Antagonist A showed improved final visual acuity compared to patients treated with Lucentis.RTM. monotherapy. (FIG. 7) Subjects in the Combination Therapy (1.5 mg) group also showed increased reduction in CNV size in small and large baseline CNV as compared to subjects in the Ranibizumab Monotherapy group (FIGS. 8A and 8B).
[0365] Combination therapy was well tolerated. There were no events of endophthalmitis, retinal detachment, retinal tear or iatrogenic traumatic cataract after a total of 4431 intravitreal injections (1776 administrations of Antagonist A and 2655 administrations of Lucentis.RTM.). As expected, mean intraocular pressure (TOP) increased after each intravitreal injection consistent with a volume effect. However, mean TOP in all arms returned to pre-injection levels at the next visit, including at the end of the study. The systemic safety profile of combination therapy was similar to that of ranibizumab monotherapy.
[0366] The results of the trial show statistically significant superior efficacy of the combination treatment with Antagonist A and ranibizumab over Lucentis.RTM. (ranibizumab) monotherapy for the treatment of wet AMD.
Example 2: ARC1905 for the Treatment of Wet AMD
[0367] Forty-three patients with subfoveal neovascular AMD received six monthly administrations of ARC1905 (0.3 mg/eye, 1 mg/eye or 2 mg/eye) in combination with Lucentis. The mean change in visual acuity at week 24 was an increase of +13.6, +11.7 and +15.3 letters at the doses of 0.3 mg, 1 mg and 2 mg, respectively. Furthermore, 46%, 47% and 60% of patients gained 3 or more lines of visual acuity at the doses of 0.3 mg, 1 mg, and 2 mg, respectively.
Example 3: ARC1905 for the Treatment and Prevention of Dry AMD
[0368] Forty-seven patients with dry AMD were enrolled to receive five intravitreal injections of either 0.3 mg/eye or 1.0 mg/eye of ARC1905 over a 36-week treatment period. FIG. 9 shows the mean change in geographic atrophy (GA) lesion area in dry AMD patients measured at week 24 in patients treated with either 0.3 mg or 1.0 mg doses of ARC1905 at weeks 0, 4, and 8. FIG. 10 shows the mean change in GA lesion in dry AMD patients measured at week 24 and week 48 in patients treated with either 0.3 mg or 1.0 mg doses of ARC1905 at weeks 0, 4, 8, 24, and 36. The results show a dose-dependent reduction in growth of the GA lesion, indicating ARC1905 can slow the progression of GA in non-exudative type AMD patients
Example 4: Visual Acuity Testing Using ETDRS Chart
[0369] Best-corrected visual acuity is measured using standard charts, lighting, and procedures. Best correction is determined by careful refraction at that visit.
[0370] Chart 1 (FIG. 11) is used for testing the visual acuity of the right eye. Chart 2 (FIG. 12) is used for testing the left eye. Chart R (FIG. 13) is used for testing refraction. Subjects do not see any of the charts before the examination.
[0371] A distance of 4 meters is between the subject's eyes and the visual acuity chart. With the box light off, not more than 15 foot-candles of light (161.4 Lux) fall on the center of the chart. To measure the amount of light, the room is set up for visual acuity testing, but with the box light off. The light meter is placed at the fourth line from the top of the chart, with its back against the chart and the reading is taken. If more than one lane is available for testing visual acuity, the visual acuity of an individual subject should be measured in the same lane at each visit. If different lanes are used to test visual acuity, they each meet the same standards.
[0372] Retroilluminated ETDRS charts are used. The illuminator box is either wall-mounted or mounted on a stand (available from Lighthouse Low Vision Services). The light box is mounted at a height such that the top of the third row letter is 49+2 inches from the floor.
[0373] The visual acuity light box is equipped with two 20-watt fluorescent tubes (available from General Electric Cool Daylight) and a ballast which partially covers the tubes. Because the illumination of fluorescent tubes generally diminishes by 5 percent during the first 100 hours and by another 5 percent during the next 2000 hours, new tubes are kept on for 4 days (96 hours) continuously, and replaced once a year.
[0374] A sticker is placed on the back of the light box, indicating the date on which the present tubes were installed. A spare set of burned in bulbs is available.
[0375] Each tube is partly covered by a 14-inch fenestrated sleeve, which is open in the back. This serves as a baffle to reduce illumination. Each sleeve is centered on the tube with the opening towards the back.
[0376] All eyes are tested at 4 meters first, even if the refraction was performed at 1 meter. The subject is seated comfortably directly in front of the chart so that the eyes remain at the 4 meter distance. Testing begins with the right eye. The subject's left eye is occluded. A folded tissue or eye pad lightly taped over the eye behind the trial frame serves as an effective occluder that allows eccentric fixation without inadvertent use of the covered eye. After testing the right eye, occlusion of the right eye is done before Chart 2 is put up for testing the left eye.
[0377] The lens correction from the subjective refraction is in the trial frame worn by the subject.
[0378] The subject is asked to read the letters slowly, approximately one letter per second. The subject is told that only one chance is given to read each letter on the chart. If the subject is unsure about the identity of the letter, then the subject is encouraged to guess.
[0379] The subject begins by reading the top line of the chart and continue reading every letter on each smaller line, from left to right on each line. The examiner circles every correct letter read and totals each line and the whole column (0 if no letters are correct) on the data collection form. An X is put through letters read incorrectly. Letters, for which no guess was attempted, are not circled. When a subject reaches a level where he/she cannot guess, the examiner may stop the test provided that the subject has made errors on previous guesses, which is a clear indication that the best visual acuity has been obtained.
[0380] When a subject cannot read at least 20 letters on the chart at 4.0 meters, the subject is tested at 1.0 meter. The distance from the subject to the chart should be measured again using the rigid one meter stick. The distance is measured from the outer canthus to the center of the fourth letter (right eye) or the second letter (left eye) of the third line of the chart. The spherical correction in the trial frame should be changed by adding +0.75 to correct for the closer test distance. The subject may fixate eccentrically or turn or shake his/her head to improve visual acuity. If this is done, the examiner ensures that the fellow eye remains occluded both centrally and peripherally and that the subject does not move forward in the chair. Particular care should be taken to ensure the subject does not move forward when testing at 1 meter. The subject is reminded to blink.
[0381] The examiner does not tell the subject if a letter was identified correctly. The subject may be encouraged by neutral comments, such as "good", "next", and "OK".
[0382] The examiner does not stand close to the chart during testing. The examiner's attention is focused on the subject and the data collection form. If the subject has difficulty locating the next line to read, the examiner may go up to the chart and point to the next line to be read, and then moves away from the chart.
[0383] When it is possible to measure the visual acuity of the eye at 4.0 meters (i.e., 20 or more letters read at 4 meters), the visual acuity score for that eye is recorded as the number of letters correct plus 30. The subject gets credit for the 30 1M letters even though they did not have to read them. Otherwise, the visual acuity score is the number of letters read correctly at 1.0 meter plus the number, if any, read at 4M. If no letters are read correctly at either 4.0 meters or 1 meter, then the visual acuity score is recorded as 0.
INCORPORATION BY REFERENCE
[0384] All publications and patent applications disclosed in this specification are herein incorporated by reference to the same extent as if each individual publication or patent application was specifically and individually indicated to be incorporated by reference.
Sequence CWU 1
1
98130DNAArtificial SequenceSynthetic anti-PDGF
aptamermisc_feature(1)..(1)May be modified with two 20 kD
polyethylene glycol polymer chains that are covalently attached to
the two amino groups of a lysine residue via carbamate
linkagesmisc_feature(1)..(1)May be modified with a bifunctional
alpha- hydroxy-omega-amino linker covalently attached to the
polyethylene glycol polymer chains via an amide
bondmisc_feature(6)..(6)May be
2'-fluoro-2'-deoxyuridinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(10)May be linked via
hexaethylene glycol moieties via phosphodiester
linkagesmisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(21)..(22)May be linked
via hexaethylene glycol moieties via phosphodiester
linkagesmisc_feature(26)..(27)May be
2'-fluoro-2'-deoxycytidinemisc_feature(28)..(28)May be
2'-fluoro-2'-deoxyuridinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(30)..(30)May be an
inverted orientation T (3'-3'-linked) 1caggcuacgc gtagagcauc
atgatccugt 3022137DNAHomo sapiens 2ccctgcctgc ctccctgcgc acccgcagcc
tcccccgctg cctccctagg gctcccctcc 60ggccgccagc gcccattttt cattccctag
atagagatac tttgcgcgca cacacataca 120tacgcgcgca aaaaggaaaa
aaaaaaaaaa aagcccaccc tccagcctcg ctgcaaagag 180aaaaccggag
cagccgcagc tcgcagctcg cagcccgcag cccgcagagg acgcccagag
240cggcgagcgg gcgggcagac ggaccgacgg actcgcgccg cgtccacctg
tcggccgggc 300ccagccgagc gcgcagcggg cacgccgcgc gcgcggagca
gccgtgcccg ccgcccgggc 360ccgccgccag ggcgcacacg ctcccgcccc
cctacccggc ccgggcggga gtttgcacct 420ctccctgccc gggtgctcga
gctgccgttg caaagccaac tttggaaaaa gttttttggg 480ggagacttgg
gccttgaggt gcccagctcc gcgctttccg attttggggg cctttccaga
540aaatgttgca aaaaagctaa gccggcgggc agaggaaaac gcctgtagcc
ggcgagtgaa 600gacgaaccat cgactgccgt gttccttttc ctcttggagg
ttggagtccc ctgggcgccc 660ccacacggct agacgcctcg gctggttcgc
gacgcagccc cccggccgtg gatgctgcac 720tcgggctcgg gatccgccca
ggtagcggcc tcggacccag gtcctgcgcc caggtcctcc 780cctgcccccc
agcgacggag ccggggccgg gggcggcggc gccgggggca tgcgggtgag
840ccgcggctgc agaggcctga gcgcctgatc gccgcggacc cgagccgagc
ccacccccct 900ccccagcccc ccaccctggc cgcgggggcg gcgcgctcga
tctacgcgtt cggggccccg 960cggggccggg cccggagtcg gcatgaatcg
ctgctgggcg ctcttcctgt ctctctgctg 1020ctacctgcgt ctggtcagcg
ccgaggggga ccccattccc gaggagcttt atgagatgct 1080gagtgaccac
tcgatccgct cctttgatga tctccaacgc ctgctgcacg gagaccccgg
1140agaggaagat ggggccgagt tggacctgaa catgacccgc tcccactctg
gaggcgagct 1200ggagagcttg gctcgtggaa gaaggagcct gggttccctg
accattgctg agccggccat 1260gatcgccgag tgcaagacgc gcaccgaggt
gttcgagatc tcccggcgcc tcatagaccg 1320caccaacgcc aacttcctgg
tgtggccgcc ctgtgtggag gtgcagcgct gctccggctg 1380ctgcaacaac
cgcaacgtgc agtgccgccc cacccaggtg cagctgcgac ctgtccaggt
1440gagaaagatc gagattgtgc ggaagaagcc aatctttaag aaggccacgg
tgacgctgga 1500agaccacctg gcatgcaagt gtgagacagt ggcagctgca
cggcctgtga cccgaagccc 1560ggggggttcc caggagcagc gagccaaaac
gccccaaact cgggtgacca ttcggacggt 1620gcgagtccgc cggcccccca
agggcaagca ccggaaattc aagcacacgc atgacaagac 1680ggcactgaag
gagacccttg gagcctaggg gcatcggcag gagagtgtgt gggcagggtt
1740atttaatatg gtatttgctg tattgccccc atggggcctt ggagtagata
atattgtttc 1800cctcgtccgt ctgtctcgat gcctgattcg gacggccaat
ggtgcctccc ccacccctcc 1860acgtgtccgt ccacccttcc atcagcgggt
ctcctcccag cggcctccgg ctcttgccca 1920gcagctcaag aagaaaaaga
aggactgaac tccatcgcca tcttcttccc ttaactccaa 1980gaacttggga
taagagtgtg agagagactg atggggtcgc tctttggggg aaacgggttc
2040cttcccctgc acctggcctg ggccacacct gagcgctgtg gactgtcctg
aggagccctg 2100aggacctctc agcatagcct gcctgatccc tgaaccc
21373241PRTHomo sapiens 3Met Asn Arg Cys Trp Ala Leu Phe Leu Ser
Leu Cys Cys Tyr Leu Arg1 5 10 15Leu Val Ser Ala Glu Gly Asp Pro Ile
Pro Glu Glu Leu Tyr Glu Met 20 25 30Leu Ser Asp His Ser Ile Arg Ser
Phe Asp Asp Leu Gln Arg Leu Leu 35 40 45His Gly Asp Pro Gly Glu Glu
Asp Gly Ala Glu Leu Asp Leu Asn Met 50 55 60Thr Arg Ser His Ser Gly
Gly Glu Leu Glu Ser Leu Ala Arg Gly Arg65 70 75 80Arg Ser Leu Gly
Ser Leu Thr Ile Ala Glu Pro Ala Met Ile Ala Glu 85 90 95Cys Lys Thr
Arg Thr Glu Val Phe Glu Ile Ser Arg Arg Leu Ile Asp 100 105 110Arg
Thr Asn Ala Asn Phe Leu Val Trp Pro Pro Cys Val Glu Val Gln 115 120
125Arg Cys Ser Gly Cys Cys Asn Asn Arg Asn Val Gln Cys Arg Pro Thr
130 135 140Gln Val Gln Leu Arg Pro Val Gln Val Arg Lys Ile Glu Ile
Val Arg145 150 155 160Lys Lys Pro Ile Phe Lys Lys Ala Thr Val Thr
Leu Glu Asp His Leu 165 170 175Ala Cys Lys Cys Glu Thr Val Ala Ala
Ala Arg Pro Val Thr Arg Ser 180 185 190Pro Gly Gly Ser Gln Glu Gln
Arg Ala Lys Thr Pro Gln Thr Arg Val 195 200 205Thr Ile Arg Thr Val
Arg Val Arg Arg Pro Pro Lys Gly Lys His Arg 210 215 220Lys Phe Lys
His Thr His Asp Lys Thr Ala Leu Lys Glu Thr Leu Gly225 230 235
240Ala42305DNAHomo sapiens 4ttcttggggc tgatgtccgc aaatatgcag
aattaccggc cgggtcgctc ctgaagccag 60cgcggggagc gagcgcggcg gcggccagca
ccgggaacgc accgaggaag aagcccagcc 120cccgccctcc gccccttccg
tccccacccc ctacccggcg gcccaggagg ctccccggct 180gcggcgcgca
ctccctgttt ctcctcctcc tggctggcgc tgcctgcctc tccgcactca
240ctgctcgccg ggcgccgtcc gccagctccg tgctccccgc gccaccctcc
tccgggccgc 300gctccctaag ggatggtact gaatttcgcc gccacaggag
accggctgga gcgcccgccc 360cgcgcctcgc ctctcctccg agcagccagc
gcctcgggac gcgatgagga ccttggcttg 420cctgctgctc ctcggctgcg
gatacctcgc ccatgttctg gccgaggaag ccgagatccc 480ccgcgaggtg
atcgagaggc tggcccgcag tcagatccac agcatccggg acctccagcg
540actcctggag atagactccg tagggagtga ggattctttg gacaccagcc
tgagagctca 600cggggtccac gccactaagc atgtgcccga gaagcggccc
ctgcccattc ggaggaagag 660aagcatcgag gaagctgtcc ccgctgtctg
caagaccagg acggtcattt acgagattcc 720tcggagtcag gtcgacccca
cgtccgccaa cttcctgatc tggcccccgt gcgtggaggt 780gaaacgctgc
accggctgct gcaacacgag cagtgtcaag tgccagccct cccgcgtcca
840ccaccgcagc gtcaaggtgg ccaaggtgga atacgtcagg aagaagccaa
aattaaaaga 900agtccaggtg aggttagagg agcatttgga gtgcgcctgc
gcgaccacaa gcctgaatcc 960ggattatcgg gaagaggaca cggatgtgag
gtgaggatga gccgcagccc tttcctggga 1020catggatgta catggcgtgt
tacattcctg aacctactat gtacggtgct ttattgccag 1080tgtgcggtct
ttgttctcct ccgtgaaaaa ctgtgtccga gaacactcgg gagaacaaag
1140agacagtgca catttgttta atgtgacatc aaagcaagta ttgtagcact
cggtgaagca 1200gtaagaagct tccttgtcaa aaagagagag agagagagag
agagagaaaa caaaaccaca 1260aatgacaaaa acaaaacgga ctcacaaaaa
tatctaaact cgatgagatg gagggtcgcc 1320ccgtgggatg gaagtgcaga
ggtctcagca gactggattt ctgtccgggt ggtcacaggt 1380gcttttttgc
cgaggatgca gagcctgctt tgggaacgac tccagagggg tgctggtggg
1440ctctgcaggg cccgcaggaa gcaggaatgt cttggaaacc gccacgcgaa
ctttagaaac 1500cacacctcct cgctgtagta tttaagccca tacagaaacc
ttcctgagag ccttaagtgg 1560tttttttttt tgtttttgtt ttgttttttt
tttttttgtt tttttttttt tttttttttt 1620ttacaccata aagtgattat
taagcttcct tttactcttt ggctagcttt tttttttttt 1680tttttttttt
ttttttttaa ttatctcttg gatgacattt acaccgataa cacacaggct
1740gctgtaactg tcaggacagt gcgacggtat ttttcctagc aagatgcaaa
ctaatgagat 1800gtattaaaat aaacatggta tacctaccta tgcatcattt
cctaaatgtt tctggctttg 1860tgtttctccc ttaccctgct ttatttgtta
atttaagcca ttttgaaaga actatgcgtc 1920aaccaatcgt acgccgtccc
tgcggcacct gccccagagc ccgtttgtgg ctgagtgaca 1980acttgttccc
cgcagtgcac acctagaatg ctgtgttccc acgcggcacg tgagatgcat
2040tgccgcttct gtctgtgttg ttggtgtgcc ctggtgccgt ggtggcggtc
actccctctg 2100ctgccagtgt ttggacagaa cccaaattct ttatttttgg
taagatattg tgctttacct 2160gtattaacag aaatgtgtgt gtgtggtttg
tttttttgta aaggtgaagt ttgtatgttt 2220acctaatatt acctgttttg
tatacctgag agcctgctat gttcttcttt tgttgatcca 2280aaattaaaaa
aaaaatacca ccaac 23055196PRTHomo sapiens 5Met Arg Thr Leu Ala Cys
Leu Leu Leu Leu Gly Cys Gly Tyr Leu Ala1 5 10 15His Val Leu Ala Glu
Glu Ala Glu Ile Pro Arg Glu Val Ile Glu Arg 20 25 30Leu Ala Arg Ser
Gln Ile His Ser Ile Arg Asp Leu Gln Arg Leu Leu 35 40 45Glu Ile Asp
Ser Val Gly Ser Glu Asp Ser Leu Asp Thr Ser Leu Arg 50 55 60Ala His
Gly Val His Ala Thr Lys His Val Pro Glu Lys Arg Pro Leu65 70 75
80Pro Ile Arg Arg Lys Arg Ser Ile Glu Glu Ala Val Pro Ala Val Cys
85 90 95Lys Thr Arg Thr Val Ile Tyr Glu Ile Pro Arg Ser Gln Val Asp
Pro 100 105 110Thr Ser Ala Asn Phe Leu Ile Trp Pro Pro Cys Val Glu
Val Lys Arg 115 120 125Cys Thr Gly Cys Cys Asn Thr Ser Ser Val Lys
Cys Gln Pro Ser Arg 130 135 140Val His His Arg Ser Val Lys Val Ala
Lys Val Glu Tyr Val Arg Lys145 150 155 160Lys Pro Lys Leu Lys Glu
Val Gln Val Arg Leu Glu Glu His Leu Glu 165 170 175Cys Ala Cys Ala
Thr Thr Ser Leu Asn Pro Asp Tyr Arg Glu Glu Asp 180 185 190Thr Asp
Val Arg 19563018DNAHomo sapiens 6gcccggagag ccgcatctat tggcagcttt
gttattgatc agaaactgct cgccgccgac 60ttggcttcca gtctggctgc gggcaaccct
tgagttttcg cctctgtcct gtcccccgaa 120ctgacaggtg ctcccagcaa
cttgctgggg acttctcgcc gctcccccgc gtccccaccc 180cctcattcct
ccctcgcctt cacccccacc cccaccactt cgccacagct caggatttgt
240ttaaaccttg ggaaactggt tcaggtccag gttttgcttt gatccttttc
aaaaactgga 300gacacagaag agggctctag gaaaaagttt tggatgggat
tatgtggaaa ctaccctgcg 360attctctgct gccagagcag gctcggcgct
tccaccccag tgcagccttc ccctggcggt 420ggtgaaagag actcgggagt
cgctgcttcc aaagtgcccg ccgtgagtga gctctcaccc 480cagtcagcca
aatgagcctc ttcgggcttc tcctgctgac atctgccctg gccggccaga
540gacaggggac tcaggcggaa tccaacctga gtagtaaatt ccagttttcc
agcaacaagg 600aacagaacgg agtacaagat cctcagcatg agagaattat
tactgtgtct actaatggaa 660gtattcacag cccaaggttt cctcatactt
atccaagaaa tacggtcttg gtatggagat 720tagtagcagt agaggaaaat
gtatggatac aacttacgtt tgatgaaaga tttgggcttg 780aagacccaga
agatgacata tgcaagtatg attttgtaga agttgaggaa cccagtgatg
840gaactatatt agggcgctgg tgtggttctg gtactgtacc aggaaaacag
atttctaaag 900gaaatcaaat taggataaga tttgtatctg atgaatattt
tccttctgaa ccagggttct 960gcatccacta caacattgtc atgccacaat
tcacagaagc tgtgagtcct tcagtgctac 1020ccccttcagc tttgccactg
gacctgctta ataatgctat aactgccttt agtaccttgg 1080aagaccttat
tcgatatctt gaaccagaga gatggcagtt ggacttagaa gatctatata
1140ggccaacttg gcaacttctt ggcaaggctt ttgtttttgg aagaaaatcc
agagtggtgg 1200atctgaacct tctaacagag gaggtaagat tatacagctg
cacacctcgt aacttctcag 1260tgtccataag ggaagaacta aagagaaccg
ataccatttt ctggccaggt tgtctcctgg 1320ttaaacgctg tggtgggaac
tgtgcctgtt gtctccacaa ttgcaatgaa tgtcaatgtg 1380tcccaagcaa
agttactaaa aaataccacg aggtccttca gttgagacca aagaccggtg
1440tcaggggatt gcacaaatca ctcaccgacg tggccctgga gcaccatgag
gagtgtgact 1500gtgtgtgcag agggagcaca ggaggatagc cgcatcacca
ccagcagctc ttgcccagag 1560ctgtgcagtg cagtggctga ttctattaga
gaacgtatgc gttatctcca tccttaatct 1620cagttgtttg cttcaaggac
ctttcatctt caggatttac agtgcattct gaaagaggag 1680acatcaaaca
gaattaggag ttgtgcaaca gctcttttga gaggaggcct aaaggacagg
1740agaaaaggtc ttcaatcgtg gaaagaaaat taaatgttgt attaaataga
tcaccagcta 1800gtttcagagt taccatgtac gtattccact agctgggttc
tgtatttcag ttctttcgat 1860acggcttagg gtaatgtcag tacaggaaaa
aaactgtgca agtgagcacc tgattccgtt 1920gccttgctta actctaaagc
tccatgtcct gggcctaaaa tcgtataaaa tctggatttt 1980tttttttttt
tttgctcata ttcacatatg taaaccagaa cattctatgt actacaaacc
2040tggtttttaa aaaggaacta tgttgctatg aattaaactt gtgtcgtgct
gataggacag 2100actggatttt tcatatttct tattaaaatt tctgccattt
agaagaagag aactacattc 2160atggtttgga agagataaac ctgaaaagaa
gagtggcctt atcttcactt tatcgataag 2220tcagtttatt tgtttcattg
tgtacatttt tatattctcc ttttgacatt ataactgttg 2280gcttttctaa
tcttgttaaa tatatctatt tttaccaaag gtatttaata ttctttttta
2340tgacaactta gatcaactat ttttagcttg gtaaattttt ctaaacacaa
ttgttatagc 2400cagaggaaca aagatgatat aaaatattgt tgctctgaca
aaaatacatg tatttcattc 2460tcgtatggtg ctagagttag attaatctgc
attttaaaaa actgaattgg aatagaattg 2520gtaagttgca aagacttttt
gaaaataatt aaattatcat atcttccatt cctgttattg 2580gagatgaaaa
taaaaagcaa cttatgaaag tagacattca gatccagcca ttactaacct
2640attccttttt tggggaaatc tgagcctagc tcagaaaaac ataaagcacc
ttgaaaaaga 2700cttggcagct tcctgataaa gcgtgctgtg ctgtgcagta
ggaacacatc ctatttattg 2760tgatgttgtg gttttattat cttaaactct
gttccataca cttgtataaa tacatggata 2820tttttatgta cagaagtatg
tctcttaacc agttcactta ttgtactctg gcaatttaaa 2880agaaaatcag
taaaatattt tgcttgtaaa atgcttaata tcgtgcctag gttatgtggt
2940gactatttga atcaaaaatg tattgaatca tcaaataaaa gaatgtggct
attttgggga 3000gaaaattaaa aaaaaaaa 30187345PRTHomo sapiens 7Met Ser
Leu Phe Gly Leu Leu Leu Leu Thr Ser Ala Leu Ala Gly Gln1 5 10 15Arg
Gln Gly Thr Gln Ala Glu Ser Asn Leu Ser Ser Lys Phe Gln Phe 20 25
30Ser Ser Asn Lys Glu Gln Asn Gly Val Gln Asp Pro Gln His Glu Arg
35 40 45Ile Ile Thr Val Ser Thr Asn Gly Ser Ile His Ser Pro Arg Phe
Pro 50 55 60His Thr Tyr Pro Arg Asn Thr Val Leu Val Trp Arg Leu Val
Ala Val65 70 75 80Glu Glu Asn Val Trp Ile Gln Leu Thr Phe Asp Glu
Arg Phe Gly Leu 85 90 95Glu Asp Pro Glu Asp Asp Ile Cys Lys Tyr Asp
Phe Val Glu Val Glu 100 105 110Glu Pro Ser Asp Gly Thr Ile Leu Gly
Arg Trp Cys Gly Ser Gly Thr 115 120 125Val Pro Gly Lys Gln Ile Ser
Lys Gly Asn Gln Ile Arg Ile Arg Phe 130 135 140Val Ser Asp Glu Tyr
Phe Pro Ser Glu Pro Gly Phe Cys Ile His Tyr145 150 155 160Asn Ile
Val Met Pro Gln Phe Thr Glu Ala Val Ser Pro Ser Val Leu 165 170
175Pro Pro Ser Ala Leu Pro Leu Asp Leu Leu Asn Asn Ala Ile Thr Ala
180 185 190Phe Ser Thr Leu Glu Asp Leu Ile Arg Tyr Leu Glu Pro Glu
Arg Trp 195 200 205Gln Leu Asp Leu Glu Asp Leu Tyr Arg Pro Thr Trp
Gln Leu Leu Gly 210 215 220Lys Ala Phe Val Phe Gly Arg Lys Ser Arg
Val Val Asp Leu Asn Leu225 230 235 240Leu Thr Glu Glu Val Arg Leu
Tyr Ser Cys Thr Pro Arg Asn Phe Ser 245 250 255Val Ser Ile Arg Glu
Glu Leu Lys Arg Thr Asp Thr Ile Phe Trp Pro 260 265 270Gly Cys Leu
Leu Val Lys Arg Cys Gly Gly Asn Cys Ala Cys Cys Leu 275 280 285His
Asn Cys Asn Glu Cys Gln Cys Val Pro Ser Lys Val Thr Lys Lys 290 295
300Tyr His Glu Val Leu Gln Leu Arg Pro Lys Thr Gly Val Arg Gly
Leu305 310 315 320His Lys Ser Leu Thr Asp Val Ala Leu Glu His His
Glu Glu Cys Asp 325 330 335Cys Val Cys Arg Gly Ser Thr Gly Gly 340
34583997DNAHomo sapiens 8tctcaggggc cgcggccggg gctggagaac
gctgctgctc cgctcgcctg ccccgctaga 60ttcggcgctg cccgccccct gcagcctgtg
ctgcagctgc cggccaccgg agggggcgaa 120caaacaaacg tcaacctgtt
gtttgtcccg tcaccattta tcagctcagc accacaagga 180agtgcggcac
ccacacgcgc tcggaaagtt cagcatgcag gaagtttggg gagagctcgg
240cgattagcac agcgacccgg gccagcgcag ggcgagcgca ggcggcgaga
gcgcagggcg 300gcgcggcgtc ggtcccggga gcagaacccg gctttttctt
ggagcgacgc tgtctctagt 360cgctgatccc aaatgcaccg gctcatcttt
gtctacactc taatctgcgc aaacttttgc 420agctgtcggg acacttctgc
aaccccgcag agcgcatcca tcaaagcttt gcgcaacgcc 480aacctcaggc
gagatgagag caatcacctc acagacttgt accgaagaga tgagaccatc
540caggtgaaag gaaacggcta cgtgcagagt cctagattcc cgaacagcta
ccccaggaac 600ctgctcctga catggcggct tcactctcag gagaatacac
ggatacagct agtgtttgac 660aatcagtttg gattagagga agcagaaaat
gatatctgta ggtatgattt tgtggaagtt 720gaagatatat ccgaaaccag
taccattatt agaggacgat ggtgtggaca caaggaagtt 780cctccaagga
taaaatcaag aacgaaccaa attaaaatca cattcaagtc cgatgactac
840tttgtggcta aacctggatt caagatttat tattctttgc tggaagattt
ccaacccgca 900gcagcttcag agaccaactg ggaatctgtc acaagctcta
tttcaggggt atcctataac 960tctccatcag taacggatcc cactctgatt
gcggatgctc tggacaaaaa aattgcagaa 1020tttgatacag tggaagatct
gctcaagtac ttcaatccag agtcatggca agaagatctt 1080gagaatatgt
atctggacac ccctcggtat cgaggcaggt cataccatga ccggaagtca
1140aaagttgacc tggataggct caatgatgat gccaagcgtt acagttgcac
tcccaggaat 1200tactcggtca atataagaga
agagctgaag ttggccaatg tggtcttctt tccacgttgc 1260ctcctcgtgc
agcgctgtgg aggaaattgt ggctgtggaa ctgtcaactg gaggtcctgc
1320acatgcaatt cagggaaaac cgtgaaaaag tatcatgagg tattacagtt
tgagcctggc 1380cacatcaaga ggaggggtag agctaagacc atggctctag
ttgacatcca gttggatcac 1440catgaacgat gtgattgtat ctgcagctca
agaccacctc gataagagaa tgtgcacatc 1500cttacattaa gcctgaaaga
acctttagtt taaggagggt gagataagag acccttttcc 1560taccagcaac
caaacttact actagcctgc aatgcaatga acacaagtgg ttgctgagtc
1620tcagccttgc tttgttaatg ccatggcaag tagaaaggta tatcatcaac
ttctatacct 1680aagaatatag gattgcattt aataatagtg tttgaggtta
tatatgcaca aacacacaca 1740gaaatatatt catgtctatg tgtatataga
tcaaatgttt tttttggtat atataaccag 1800gtacaccaga gcttacatat
gtttgagtta gactcttaaa atcctttgcc aaaataaggg 1860atggtcaaat
atatgaaaca tgtctttaga aaatttagga gataaattta tttttaaatt
1920ttgaaacaca aaacaatttt gaatcttgct ctcttaaaga aagcatcttg
tatattaaaa 1980atcaaaagat gaggctttct tacatataca tcttagttga
ttattaaaaa aggaaaaata 2040tggtttccag agaaaaggcc aatacctaag
cattttttcc atgagaagca ctgcatactt 2100acctatgtgg actataataa
cctgtctcca aaaccatgcc ataataatat aagtgcttta 2160gaaattaaat
cattgtgttt tttatgcatt ttgctgaggc atgcttattc atttaacacc
2220tatctcaaaa acttacttag aaggtttttt attatagtcc tacaaaagac
aatgtataag 2280ctgtaacaga attttgaatt gtttttcttt gcaaaacccc
tccacaaaag caaatccttt 2340caagaatggc atgggcattc tgtatgaacc
tttccagatg gtgttcagtg aaagatgtgg 2400gtagttgaga acttaaaaag
tgaacattga aacatcgacg taactggaaa ttaggtggga 2460tatttgatag
gatccatatc taataatgga ttcgaactct ccaaactaca ccaattaatt
2520taatgtatct tgcttttgtg ttcccgtctt tttgaaatat agacatggat
ttataatggc 2580attttatatt tggcaggcca tcatagatta tttacaacct
aaaagctttt gtgtatcaaa 2640aaaatcacat tttattaatg taaatttcta
atcgtatact tgctcactgt tctgatttcc 2700tgtttctgaa ccaagtaaaa
tcagtcctag aggctatggt tcttaatcta tggagcttgc 2760tttaagaagc
cagttgtcaa ttgtggtaac acaagtttgg ccctgctgtc ctactgttta
2820atagaaaact gttttacatt ggttaatggt atttagagta attttttctc
tctgcctcct 2880ttgtgtctgt tttaaaggag actaactcca ggagtaggaa
atgattcatc atcctccaaa 2940gcaagaggct taagagagaa acaccgaaat
tcagatagct cagggactgc taacagagaa 3000ctacattttt cttattgcct
tgaaagttaa aaggaaagca gatttcttca gtgactttgt 3060ggtcctacta
actacaacca gtttgggtga cagggctggt aaagtcccag tgttagatga
3120gtgacctaaa tatacttaga tttctaagta tggtgctctc aggtccaagt
tcaactattc 3180ttaagcagtg caattcttcc cagttatttg agatgaaaga
tctctgctta ttgaagatgt 3240accttctaaa actttcctaa aagtgtctga
tgtttttact caagagggga gtggtaaaat 3300taaatactct attgttcaat
tctctaaaat cccagaacac aatcagaaat agctcaggca 3360gacactaata
attaagaacg ctcttcctct tcataactgc tttgcaagtt tcctgtgaaa
3420acatcagttt cctgtaccaa agtcaaaatg aacgttacat cactctaacc
tgaacagctc 3480acaatgtagc tgtaaatata aaaaatgaga gtgttctacc
cagttttcaa taaaccttcc 3540aggctgcaat aaccagcaag gttttcagtt
aaagccctat ctgcactttt tatttattag 3600ctgaaatgta agcaggcata
ttcactcact tttctttgcc tttcctgaga gttttattaa 3660aacttctccc
ttggttacct gttatctttt gcacttctaa catgtagcca ataaatctat
3720ttgatagcca tcaaaggaat aaaaagctgg ccgtacaaat tacatttcaa
aacaaaccct 3780aataaatcca catttccgca tggctcattc acctggaata
atgcctttta ttgaatatgt 3840tcttataggg caaaacactt tcataagtag
agttttttat gttttttgtc atatcggtaa 3900catgcagctt tttcctctca
tagcattttc tatagcgaat gtaatatgcc tcttatcttc 3960atgaaaaata
aatattgctt ttgaacaaaa ctaaaaa 39979370PRTHomo sapiens 9Met His Arg
Leu Ile Phe Val Tyr Thr Leu Ile Cys Ala Asn Phe Cys1 5 10 15Ser Cys
Arg Asp Thr Ser Ala Thr Pro Gln Ser Ala Ser Ile Lys Ala 20 25 30Leu
Arg Asn Ala Asn Leu Arg Arg Asp Glu Ser Asn His Leu Thr Asp 35 40
45Leu Tyr Arg Arg Asp Glu Thr Ile Gln Val Lys Gly Asn Gly Tyr Val
50 55 60Gln Ser Pro Arg Phe Pro Asn Ser Tyr Pro Arg Asn Leu Leu Leu
Thr65 70 75 80Trp Arg Leu His Ser Gln Glu Asn Thr Arg Ile Gln Leu
Val Phe Asp 85 90 95Asn Gln Phe Gly Leu Glu Glu Ala Glu Asn Asp Ile
Cys Arg Tyr Asp 100 105 110Phe Val Glu Val Glu Asp Ile Ser Glu Thr
Ser Thr Ile Ile Arg Gly 115 120 125Arg Trp Cys Gly His Lys Glu Val
Pro Pro Arg Ile Lys Ser Arg Thr 130 135 140Asn Gln Ile Lys Ile Thr
Phe Lys Ser Asp Asp Tyr Phe Val Ala Lys145 150 155 160Pro Gly Phe
Lys Ile Tyr Tyr Ser Leu Leu Glu Asp Phe Gln Pro Ala 165 170 175Ala
Ala Ser Glu Thr Asn Trp Glu Ser Val Thr Ser Ser Ile Ser Gly 180 185
190Val Ser Tyr Asn Ser Pro Ser Val Thr Asp Pro Thr Leu Ile Ala Asp
195 200 205Ala Leu Asp Lys Lys Ile Ala Glu Phe Asp Thr Val Glu Asp
Leu Leu 210 215 220Lys Tyr Phe Asn Pro Glu Ser Trp Gln Glu Asp Leu
Glu Asn Met Tyr225 230 235 240Leu Asp Thr Pro Arg Tyr Arg Gly Arg
Ser Tyr His Asp Arg Lys Ser 245 250 255Lys Val Asp Leu Asp Arg Leu
Asn Asp Asp Ala Lys Arg Tyr Ser Cys 260 265 270Thr Pro Arg Asn Tyr
Ser Val Asn Ile Arg Glu Glu Leu Lys Leu Ala 275 280 285Asn Val Val
Phe Phe Pro Arg Cys Leu Leu Val Gln Arg Cys Gly Gly 290 295 300Asn
Cys Gly Cys Gly Thr Val Asn Trp Arg Ser Cys Thr Cys Asn Ser305 310
315 320Gly Lys Thr Val Lys Lys Tyr His Glu Val Leu Gln Phe Glu Pro
Gly 325 330 335His Ile Lys Arg Arg Gly Arg Ala Lys Thr Met Ala Leu
Val Asp Ile 340 345 350Gln Leu Asp His His Glu Arg Cys Asp Cys Ile
Cys Ser Ser Arg Pro 355 360 365Pro Arg 370103979DNAHomo sapiens
10tctcaggggc cgcggccggg gctggagaac gctgctgctc cgctcgcctg ccccgctaga
60ttcggcgctg cccgccccct gcagcctgtg ctgcagctgc cggccaccgg agggggcgaa
120caaacaaacg tcaacctgtt gtttgtcccg tcaccattta tcagctcagc
accacaagga 180agtgcggcac ccacacgcgc tcggaaagtt cagcatgcag
gaagtttggg gagagctcgg 240cgattagcac agcgacccgg gccagcgcag
ggcgagcgca ggcggcgaga gcgcagggcg 300gcgcggcgtc ggtcccggga
gcagaacccg gctttttctt ggagcgacgc tgtctctagt 360cgctgatccc
aaatgcaccg gctcatcttt gtctacactc taatctgcgc aaacttttgc
420agctgtcggg acacttctgc aaccccgcag agcgcatcca tcaaagcttt
gcgcaacgcc 480aacctcaggc gagatgactt gtaccgaaga gatgagacca
tccaggtgaa aggaaacggc 540tacgtgcaga gtcctagatt cccgaacagc
taccccagga acctgctcct gacatggcgg 600cttcactctc aggagaatac
acggatacag ctagtgtttg acaatcagtt tggattagag 660gaagcagaaa
atgatatctg taggtatgat tttgtggaag ttgaagatat atccgaaacc
720agtaccatta ttagaggacg atggtgtgga cacaaggaag ttcctccaag
gataaaatca 780agaacgaacc aaattaaaat cacattcaag tccgatgact
actttgtggc taaacctgga 840ttcaagattt attattcttt gctggaagat
ttccaacccg cagcagcttc agagaccaac 900tgggaatctg tcacaagctc
tatttcaggg gtatcctata actctccatc agtaacggat 960cccactctga
ttgcggatgc tctggacaaa aaaattgcag aatttgatac agtggaagat
1020ctgctcaagt acttcaatcc agagtcatgg caagaagatc ttgagaatat
gtatctggac 1080acccctcggt atcgaggcag gtcataccat gaccggaagt
caaaagttga cctggatagg 1140ctcaatgatg atgccaagcg ttacagttgc
actcccagga attactcggt caatataaga 1200gaagagctga agttggccaa
tgtggtcttc tttccacgtt gcctcctcgt gcagcgctgt 1260ggaggaaatt
gtggctgtgg aactgtcaac tggaggtcct gcacatgcaa ttcagggaaa
1320accgtgaaaa agtatcatga ggtattacag tttgagcctg gccacatcaa
gaggaggggt 1380agagctaaga ccatggctct agttgacatc cagttggatc
accatgaacg atgtgattgt 1440atctgcagct caagaccacc tcgataagag
aatgtgcaca tccttacatt aagcctgaaa 1500gaacctttag tttaaggagg
gtgagataag agaccctttt cctaccagca accaaactta 1560ctactagcct
gcaatgcaat gaacacaagt ggttgctgag tctcagcctt gctttgttaa
1620tgccatggca agtagaaagg tatatcatca acttctatac ctaagaatat
aggattgcat 1680ttaataatag tgtttgaggt tatatatgca caaacacaca
cagaaatata ttcatgtcta 1740tgtgtatata gatcaaatgt tttttttggt
atatataacc aggtacacca gagcttacat 1800atgtttgagt tagactctta
aaatcctttg ccaaaataag ggatggtcaa atatatgaaa 1860catgtcttta
gaaaatttag gagataaatt tatttttaaa ttttgaaaca caaaacaatt
1920ttgaatcttg ctctcttaaa gaaagcatct tgtatattaa aaatcaaaag
atgaggcttt 1980cttacatata catcttagtt gattattaaa aaaggaaaaa
tatggtttcc agagaaaagg 2040ccaataccta agcatttttt ccatgagaag
cactgcatac ttacctatgt ggactataat 2100aacctgtctc caaaaccatg
ccataataat ataagtgctt tagaaattaa atcattgtgt 2160tttttatgca
ttttgctgag gcatgcttat tcatttaaca cctatctcaa aaacttactt
2220agaaggtttt ttattatagt cctacaaaag acaatgtata agctgtaaca
gaattttgaa 2280ttgtttttct ttgcaaaacc cctccacaaa agcaaatcct
ttcaagaatg gcatgggcat 2340tctgtatgaa cctttccaga tggtgttcag
tgaaagatgt gggtagttga gaacttaaaa 2400agtgaacatt gaaacatcga
cgtaactgga aattaggtgg gatatttgat aggatccata 2460tctaataatg
gattcgaact ctccaaacta caccaattaa tttaatgtat cttgcttttg
2520tgttcccgtc tttttgaaat atagacatgg atttataatg gcattttata
tttggcaggc 2580catcatagat tatttacaac ctaaaagctt ttgtgtatca
aaaaaatcac attttattaa 2640tgtaaatttc taatcgtata cttgctcact
gttctgattt cctgtttctg aaccaagtaa 2700aatcagtcct agaggctatg
gttcttaatc tatggagctt gctttaagaa gccagttgtc 2760aattgtggta
acacaagttt ggccctgctg tcctactgtt taatagaaaa ctgttttaca
2820ttggttaatg gtatttagag taattttttc tctctgcctc ctttgtgtct
gttttaaagg 2880agactaactc caggagtagg aaatgattca tcatcctcca
aagcaagagg cttaagagag 2940aaacaccgaa attcagatag ctcagggact
gctaacagag aactacattt ttcttattgc 3000cttgaaagtt aaaaggaaag
cagatttctt cagtgacttt gtggtcctac taactacaac 3060cagtttgggt
gacagggctg gtaaagtccc agtgttagat gagtgaccta aatatactta
3120gatttctaag tatggtgctc tcaggtccaa gttcaactat tcttaagcag
tgcaattctt 3180cccagttatt tgagatgaaa gatctctgct tattgaagat
gtaccttcta aaactttcct 3240aaaagtgtct gatgttttta ctcaagaggg
gagtggtaaa attaaatact ctattgttca 3300attctctaaa atcccagaac
acaatcagaa atagctcagg cagacactaa taattaagaa 3360cgctcttcct
cttcataact gctttgcaag tttcctgtga aaacatcagt ttcctgtacc
3420aaagtcaaaa tgaacgttac atcactctaa cctgaacagc tcacaatgta
gctgtaaata 3480taaaaaatga gagtgttcta cccagttttc aataaacctt
ccaggctgca ataaccagca 3540aggttttcag ttaaagccct atctgcactt
tttatttatt agctgaaatg taagcaggca 3600tattcactca cttttctttg
cctttcctga gagttttatt aaaacttctc ccttggttac 3660ctgttatctt
ttgcacttct aacatgtagc caataaatct atttgatagc catcaaagga
3720ataaaaagct ggccgtacaa attacatttc aaaacaaacc ctaataaatc
cacatttccg 3780catggctcat tcacctggaa taatgccttt tattgaatat
gttcttatag ggcaaaacac 3840tttcataagt agagtttttt atgttttttg
tcatatcggt aacatgcagc tttttcctct 3900catagcattt tctatagcga
atgtaatatg cctcttatct tcatgaaaaa taaatattgc 3960ttttgaacaa
aactaaaaa 397911364PRTHomo sapiens 11Met His Arg Leu Ile Phe Val
Tyr Thr Leu Ile Cys Ala Asn Phe Cys1 5 10 15Ser Cys Arg Asp Thr Ser
Ala Thr Pro Gln Ser Ala Ser Ile Lys Ala 20 25 30Leu Arg Asn Ala Asn
Leu Arg Arg Asp Asp Leu Tyr Arg Arg Asp Glu 35 40 45Thr Ile Gln Val
Lys Gly Asn Gly Tyr Val Gln Ser Pro Arg Phe Pro 50 55 60Asn Ser Tyr
Pro Arg Asn Leu Leu Leu Thr Trp Arg Leu His Ser Gln65 70 75 80Glu
Asn Thr Arg Ile Gln Leu Val Phe Asp Asn Gln Phe Gly Leu Glu 85 90
95Glu Ala Glu Asn Asp Ile Cys Arg Tyr Asp Phe Val Glu Val Glu Asp
100 105 110Ile Ser Glu Thr Ser Thr Ile Ile Arg Gly Arg Trp Cys Gly
His Lys 115 120 125Glu Val Pro Pro Arg Ile Lys Ser Arg Thr Asn Gln
Ile Lys Ile Thr 130 135 140Phe Lys Ser Asp Asp Tyr Phe Val Ala Lys
Pro Gly Phe Lys Ile Tyr145 150 155 160Tyr Ser Leu Leu Glu Asp Phe
Gln Pro Ala Ala Ala Ser Glu Thr Asn 165 170 175Trp Glu Ser Val Thr
Ser Ser Ile Ser Gly Val Ser Tyr Asn Ser Pro 180 185 190Ser Val Thr
Asp Pro Thr Leu Ile Ala Asp Ala Leu Asp Lys Lys Ile 195 200 205Ala
Glu Phe Asp Thr Val Glu Asp Leu Leu Lys Tyr Phe Asn Pro Glu 210 215
220Ser Trp Gln Glu Asp Leu Glu Asn Met Tyr Leu Asp Thr Pro Arg
Tyr225 230 235 240Arg Gly Arg Ser Tyr His Asp Arg Lys Ser Lys Val
Asp Leu Asp Arg 245 250 255Leu Asn Asp Asp Ala Lys Arg Tyr Ser Cys
Thr Pro Arg Asn Tyr Ser 260 265 270Val Asn Ile Arg Glu Glu Leu Lys
Leu Ala Asn Val Val Phe Phe Pro 275 280 285Arg Cys Leu Leu Val Gln
Arg Cys Gly Gly Asn Cys Gly Cys Gly Thr 290 295 300Val Asn Trp Arg
Ser Cys Thr Cys Asn Ser Gly Lys Thr Val Lys Lys305 310 315 320Tyr
His Glu Val Leu Gln Phe Glu Pro Gly His Ile Lys Arg Arg Gly 325 330
335Arg Ala Lys Thr Met Ala Leu Val Asp Ile Gln Leu Asp His His Glu
340 345 350Arg Cys Asp Cys Ile Cys Ser Ser Arg Pro Pro Arg 355
360126574DNAHomo sapiens 12aagagcaaaa agcgaaggcg caatctggac
actgggagat tcggagcgca gggagtttga 60gagaaacttt tattttgaag agaccaaggt
tgaggggggg cttatttcct gacagctatt 120tacttagagc aaatgattag
ttttagaagg atggactata acattgaatc aattacaaaa 180cgcggttttt
gagcccatta ctgttggagc tacagggaga gaaacagagg aggagactgc
240aagagatcat tggaggccgt gggcacgctc tttactccat gtgtgggaca
ttcattgcgg 300aataacatcg gaggagaagt ttcccagagc tatggggact
tcccatccgg cgttcctggt 360cttaggctgt cttctcacag ggctgagcct
aatcctctgc cagctttcat taccctctat 420ccttccaaat gaaaatgaaa
aggttgtgca gctgaattca tccttttctc tgagatgctt 480tggggagagt
gaagtgagct ggcagtaccc catgtctgaa gaagagagct ccgatgtgga
540aatcagaaat gaagaaaaca acagcggcct ttttgtgacg gtcttggaag
tgagcagtgc 600ctcggcggcc cacacagggt tgtacacttg ctattacaac
cacactcaga cagaagagaa 660tgagcttgaa ggcaggcaca tttacatcta
tgtgccagac ccagatgtag cctttgtacc 720tctaggaatg acggattatt
tagtcatcgt ggaggatgat gattctgcca ttataccttg 780tcgcacaact
gatcccgaga ctcctgtaac cttacacaac agtgaggggg tggtacctgc
840ctcctacgac agcagacagg gctttaatgg gaccttcact gtagggccct
atatctgtga 900ggccaccgtc aaaggaaaga agttccagac catcccattt
aatgtttatg ctttaaaagc 960aacatcagag ctggatctag aaatggaagc
tcttaaaacc gtgtataagt caggggaaac 1020gattgtggtc acctgtgctg
tttttaacaa tgaggtggtt gaccttcaat ggacttaccc 1080tggagaagtg
aaaggcaaag gcatcacaat gctggaagaa atcaaagtcc catccatcaa
1140attggtgtac actttgacgg tccccgaggc cacggtgaaa gacagtggag
attacgaatg 1200tgctgcccgc caggctacca gggaggtcaa agaaatgaag
aaagtcacta tttctgtcca 1260tgagaaaggt ttcattgaaa tcaaacccac
cttcagccag ttggaagctg tcaacctgca 1320tgaagtcaaa cattttgttg
tagaggtgcg ggcctaccca cctcccagga tatcctggct 1380gaaaaacaat
ctgactctga ttgaaaatct cactgagatc accactgatg tggaaaagat
1440tcaggaaata aggtatcgaa gcaaattaaa gctgatccgt gctaaggaag
aagacagtgg 1500ccattatact attgtagctc aaaatgaaga tgctgtgaag
agctatactt ttgaactgtt 1560aactcaagtt ccttcatcca ttctggactt
ggtcgatgat caccatggct caactggggg 1620acagacggtg aggtgcacag
ctgaaggcac gccgcttcct gatattgagt ggatgatatg 1680caaagatatt
aagaaatgta ataatgaaac ttcctggact attttggcca acaatgtctc
1740aaacatcatc acggagatcc actcccgaga caggagtacc gtggagggcc
gtgtgacttt 1800cgccaaagtg gaggagacca tcgccgtgcg atgcctggct
aagaatctcc ttggagctga 1860gaaccgagag ctgaagctgg tggctcccac
cctgcgttct gaactcacgg tggctgctgc 1920agtcctggtg ctgttggtga
ttgtgatcat ctcacttatt gtcctggttg tcatttggaa 1980acagaaaccg
aggtatgaaa ttcgctggag ggtcattgaa tcaatcagcc cagatggaca
2040tgaatatatt tatgtggacc cgatgcagct gccttatgac tcaagatggg
agtttccaag 2100agatggacta gtgcttggtc gggtcttggg gtctggagcg
tttgggaagg tggttgaagg 2160aacagcctat ggattaagcc ggtcccaacc
tgtcatgaaa gttgcagtga agatgctaaa 2220acccacggcc agatccagtg
aaaaacaagc tctcatgtct gaactgaaga taatgactca 2280cctggggcca
catttgaaca ttgtaaactt gctgggagcc tgcaccaagt caggccccat
2340ttacatcatc acagagtatt gcttctatgg agatttggtc aactatttgc
ataagaatag 2400ggatagcttc ctgagccacc acccagagaa gccaaagaaa
gagctggata tctttggatt 2460gaaccctgct gatgaaagca cacggagcta
tgttatttta tcttttgaaa acaatggtga 2520ctacatggac atgaagcagg
ctgatactac acagtatgtc cccatgctag aaaggaaaga 2580ggtttctaaa
tattccgaca tccagagatc actctatgat cgtccagcct catataagaa
2640gaaatctatg ttagactcag aagtcaaaaa cctcctttca gatgataact
cagaaggcct 2700tactttattg gatttgttga gcttcaccta tcaagttgcc
cgaggaatgg agtttttggc 2760ttcaaaaaat tgtgtccacc gtgatctggc
tgctcgcaac gtcctcctgg cacaaggaaa 2820aattgtgaag atctgtgact
ttggcctggc cagagacatc atgcatgatt cgaactatgt 2880gtcgaaaggc
agtacctttc tgcccgtgaa gtggatggct cctgagagca tctttgacaa
2940cctctacacc acactgagtg atgtctggtc ttatggcatt ctgctctggg
agatcttttc 3000ccttggtggc accccttacc ccggcatgat ggtggattct
actttctaca ataagatcaa 3060gagtgggtac cggatggcca agcctgacca
cgctaccagt gaagtctacg agatcatggt 3120gaaatgctgg aacagtgagc
cggagaagag accctccttt taccacctga gtgagattgt 3180ggagaatctg
ctgcctggac aatataaaaa gagttatgaa aaaattcacc tggacttcct
3240gaagagtgac catcctgctg tggcacgcat gcgtgtggac tcagacaatg
catacattgg 3300tgtcacctac aaaaacgagg aagacaagct gaaggactgg
gagggtggtc tggatgagca 3360gagactgagc gctgacagtg gctacatcat
tcctctgcct gacattgacc ctgtccctga 3420ggaggaggac ctgggcaaga
ggaacagaca cagctcgcag acctctgaag agagtgccat 3480tgagacgggt
tccagcagtt ccaccttcat caagagagag gacgagacca ttgaagacat
3540cgacatgatg gatgacatcg gcatagactc ttcagacctg gtggaagaca
gcttcctgta 3600actggcggat tcgaggggtt ccttccactt ctggggccac
ctctggatcc cgttcagaaa 3660accactttat tgcaatgcag aggttgagag
gaggacttgg
ttgatgttta aagagaagtt 3720cccagccaag ggcctcgggg agcgttctaa
atatgaatga atgggatatt ttgaaatgaa 3780ctttgtcagt gttgcctctt
gcaatgcctc agtagcatct cagtggtgtg tgaagtttgg 3840agatagatgg
ataagggaat aataggccac agaaggtgaa ctttgtgctt caaggacatt
3900ggtgagagtc caacagacac aatttatact gcgacagaac ttcagcattg
taattatgta 3960aataactcta accaaggctg tgtttagatt gtattaacta
tcttctttgg acttctgaag 4020agaccactca atccatccat gtacttccct
cttgaaacct gatgtcagct gctgttgaac 4080tttttaaaga agtgcatgaa
aaaccatttt tgaaccttaa aaggtactgg tactatagca 4140ttttgctatc
ttttttagtg ttaaagagat aaagaataat aattaaccaa ccttgtttaa
4200tagatttggg tcatttagaa gcctgacaac tcattttcat attgtaatct
atgtttataa 4260tactactact gttatcagta atgctaaatg tgtaataatg
taacatgatt tccctccaga 4320gaaagcacaa tttaaaacaa tccttactaa
gtaggtgatg agtttgacag tttttgacat 4380ttatattaaa taacatgttt
ctctataaag tatggtaata gctttagtga attaaattta 4440gttgagcata
gagaacaaag taaaagtagt gttgtccagg aagtcagaat ttttaactgt
4500actgaatagg ttccccaatc catcgtatta aaaaacaatt aactgccctc
tgaaataatg 4560ggattagaaa caaacaaaac tcttaagtcc taaaagttct
caatgtagag gcataaacct 4620gtgctgaaca taacttctca tgtatattac
ccaatggaaa atataatgat cagcaaaaag 4680actggatttg cagaagtttt
tttttttttt ttcttcatgc ctgatgaaag ctttggcgac 4740cccaatatat
gtattttttg aatctatgaa cctgaaaagg gtcagaagga tgcccagaca
4800tcagcctcct tctttcaccc cttaccccaa agagaaagag tttgaaactc
gagaccataa 4860agatattctt tagtggaggc tggatgtgca ttagcctgga
tcctcagttc tcaaatgtgt 4920gtggcagcca ggatgactag atcctgggtt
tccatccttg agattctgaa gtatgaagtc 4980tgagggaaac cagagtctgt
atttttctaa actccctggc tgttctgatc ggccagtttt 5040cggaaacact
gacttaggtt tcaggaagtt gccatgggaa acaaataatt tgaactttgg
5100aacagggttg gcattcaacc acgcaggaag cctactattt aaatccttgg
cttcaggtta 5160gtgacattta atgccatcta gctagcaatt gcgaccttaa
tttaactttc cagtcttagc 5220tgaggctgag aaagctaaag tttggttttg
acaggttttc caaaagtaaa gatgctactt 5280cccactgtat gggggagatt
gaactttccc cgtctcccgt cttctgcctc ccactccata 5340ccccgccaag
gaaaggcatg tacaaaaatt atgcaattca gtgttccaag tctctgtgta
5400accagctcag tgttttggtg gaaaaaacat tttaagtttt actgataatt
tgaggttaga 5460tgggaggatg aattgtcaca tctatccaca ctgtcaaaca
ggttggtgtg ggttcattgg 5520cattctttgc aatactgctt aattgctgat
accatatgaa tgaaacatgg gctgtgatta 5580ctgcaatcac tgtgctatcg
gcagatgatg ctttggaaga tgcagaagca ataataaagt 5640acttgactac
ctactggtgt aatctcaatg caagccccaa ctttcttatc caactttttc
5700atagtaagtg cgaagactga gccagattgg ccaattaaaa acgaaaacct
gactaggttc 5760tgtagagcca attagacttg aaatacgttt gtgtttctag
aatcacagct caagcattct 5820gtttatcgct cactctccct tgtacagcct
tattttgttg gtgctttgca ttttgatatt 5880gctgtgagcc ttgcatgaca
tcatgaggcc ggatgaaact tctcagtcca gcagtttcca 5940gtcctaacaa
atgctcccac ctgaatttgt atatgactgc atttgtgtgt gtgtgtgtgt
6000tttcagcaaa ttccagattt gtttcctttt ggcctcctgc aaagtctcca
gaagaaaatt 6060tgccaatctt tcctactttc tatttttatg atgacaatca
aagccggcct gagaaacact 6120atttgtgact ttttaaacga ttagtgatgt
ccttaaaatg tggtctgcca atctgtacaa 6180aatggtccta tttttgtgaa
gagggacata agataaaatg atgttataca tcaatatgta 6240tatatgtatt
tctatataga cttggagaat actgccaaaa catttatgac aagctgtatc
6300actgccttcg tttatatttt tttaactgtg ataatcccca caggcacatt
aactgttgca 6360cttttgaatg tccaaaattt atattttaga aataataaaa
agaaagatac ttacatgttc 6420ccaaaacaat ggtgtggtga atgtgtgaga
aaaactaact tgatagggtc taccaataca 6480aaatgtatta cgaatgcccc
tgttcatgtt tttgttttaa aacgtgtaaa tgaagatctt 6540tatatttcaa
taaatgatat ataatttaaa gtta 6574131089PRTHomo sapiens 13Met Gly Thr
Ser His Pro Ala Phe Leu Val Leu Gly Cys Leu Leu Thr1 5 10 15Gly Leu
Ser Leu Ile Leu Cys Gln Leu Ser Leu Pro Ser Ile Leu Pro 20 25 30Asn
Glu Asn Glu Lys Val Val Gln Leu Asn Ser Ser Phe Ser Leu Arg 35 40
45Cys Phe Gly Glu Ser Glu Val Ser Trp Gln Tyr Pro Met Ser Glu Glu
50 55 60Glu Ser Ser Asp Val Glu Ile Arg Asn Glu Glu Asn Asn Ser Gly
Leu65 70 75 80Phe Val Thr Val Leu Glu Val Ser Ser Ala Ser Ala Ala
His Thr Gly 85 90 95Leu Tyr Thr Cys Tyr Tyr Asn His Thr Gln Thr Glu
Glu Asn Glu Leu 100 105 110Glu Gly Arg His Ile Tyr Ile Tyr Val Pro
Asp Pro Asp Val Ala Phe 115 120 125Val Pro Leu Gly Met Thr Asp Tyr
Leu Val Ile Val Glu Asp Asp Asp 130 135 140Ser Ala Ile Ile Pro Cys
Arg Thr Thr Asp Pro Glu Thr Pro Val Thr145 150 155 160Leu His Asn
Ser Glu Gly Val Val Pro Ala Ser Tyr Asp Ser Arg Gln 165 170 175Gly
Phe Asn Gly Thr Phe Thr Val Gly Pro Tyr Ile Cys Glu Ala Thr 180 185
190Val Lys Gly Lys Lys Phe Gln Thr Ile Pro Phe Asn Val Tyr Ala Leu
195 200 205Lys Ala Thr Ser Glu Leu Asp Leu Glu Met Glu Ala Leu Lys
Thr Val 210 215 220Tyr Lys Ser Gly Glu Thr Ile Val Val Thr Cys Ala
Val Phe Asn Asn225 230 235 240Glu Val Val Asp Leu Gln Trp Thr Tyr
Pro Gly Glu Val Lys Gly Lys 245 250 255Gly Ile Thr Met Leu Glu Glu
Ile Lys Val Pro Ser Ile Lys Leu Val 260 265 270Tyr Thr Leu Thr Val
Pro Glu Ala Thr Val Lys Asp Ser Gly Asp Tyr 275 280 285Glu Cys Ala
Ala Arg Gln Ala Thr Arg Glu Val Lys Glu Met Lys Lys 290 295 300Val
Thr Ile Ser Val His Glu Lys Gly Phe Ile Glu Ile Lys Pro Thr305 310
315 320Phe Ser Gln Leu Glu Ala Val Asn Leu His Glu Val Lys His Phe
Val 325 330 335Val Glu Val Arg Ala Tyr Pro Pro Pro Arg Ile Ser Trp
Leu Lys Asn 340 345 350Asn Leu Thr Leu Ile Glu Asn Leu Thr Glu Ile
Thr Thr Asp Val Glu 355 360 365Lys Ile Gln Glu Ile Arg Tyr Arg Ser
Lys Leu Lys Leu Ile Arg Ala 370 375 380Lys Glu Glu Asp Ser Gly His
Tyr Thr Ile Val Ala Gln Asn Glu Asp385 390 395 400Ala Val Lys Ser
Tyr Thr Phe Glu Leu Leu Thr Gln Val Pro Ser Ser 405 410 415Ile Leu
Asp Leu Val Asp Asp His His Gly Ser Thr Gly Gly Gln Thr 420 425
430Val Arg Cys Thr Ala Glu Gly Thr Pro Leu Pro Asp Ile Glu Trp Met
435 440 445Ile Cys Lys Asp Ile Lys Lys Cys Asn Asn Glu Thr Ser Trp
Thr Ile 450 455 460Leu Ala Asn Asn Val Ser Asn Ile Ile Thr Glu Ile
His Ser Arg Asp465 470 475 480Arg Ser Thr Val Glu Gly Arg Val Thr
Phe Ala Lys Val Glu Glu Thr 485 490 495Ile Ala Val Arg Cys Leu Ala
Lys Asn Leu Leu Gly Ala Glu Asn Arg 500 505 510Glu Leu Lys Leu Val
Ala Pro Thr Leu Arg Ser Glu Leu Thr Val Ala 515 520 525Ala Ala Val
Leu Val Leu Leu Val Ile Val Ile Ile Ser Leu Ile Val 530 535 540Leu
Val Val Ile Trp Lys Gln Lys Pro Arg Tyr Glu Ile Arg Trp Arg545 550
555 560Val Ile Glu Ser Ile Ser Pro Asp Gly His Glu Tyr Ile Tyr Val
Asp 565 570 575Pro Met Gln Leu Pro Tyr Asp Ser Arg Trp Glu Phe Pro
Arg Asp Gly 580 585 590Leu Val Leu Gly Arg Val Leu Gly Ser Gly Ala
Phe Gly Lys Val Val 595 600 605Glu Gly Thr Ala Tyr Gly Leu Ser Arg
Ser Gln Pro Val Met Lys Val 610 615 620Ala Val Lys Met Leu Lys Pro
Thr Ala Arg Ser Ser Glu Lys Gln Ala625 630 635 640Leu Met Ser Glu
Leu Lys Ile Met Thr His Leu Gly Pro His Leu Asn 645 650 655Ile Val
Asn Leu Leu Gly Ala Cys Thr Lys Ser Gly Pro Ile Tyr Ile 660 665
670Ile Thr Glu Tyr Cys Phe Tyr Gly Asp Leu Val Asn Tyr Leu His Lys
675 680 685Asn Arg Asp Ser Phe Leu Ser His His Pro Glu Lys Pro Lys
Lys Glu 690 695 700Leu Asp Ile Phe Gly Leu Asn Pro Ala Asp Glu Ser
Thr Arg Ser Tyr705 710 715 720Val Ile Leu Ser Phe Glu Asn Asn Gly
Asp Tyr Met Asp Met Lys Gln 725 730 735Ala Asp Thr Thr Gln Tyr Val
Pro Met Leu Glu Arg Lys Glu Val Ser 740 745 750Lys Tyr Ser Asp Ile
Gln Arg Ser Leu Tyr Asp Arg Pro Ala Ser Tyr 755 760 765Lys Lys Lys
Ser Met Leu Asp Ser Glu Val Lys Asn Leu Leu Ser Asp 770 775 780Asp
Asn Ser Glu Gly Leu Thr Leu Leu Asp Leu Leu Ser Phe Thr Tyr785 790
795 800Gln Val Ala Arg Gly Met Glu Phe Leu Ala Ser Lys Asn Cys Val
His 805 810 815Arg Asp Leu Ala Ala Arg Asn Val Leu Leu Ala Gln Gly
Lys Ile Val 820 825 830Lys Ile Cys Asp Phe Gly Leu Ala Arg Asp Ile
Met His Asp Ser Asn 835 840 845Tyr Val Ser Lys Gly Ser Thr Phe Leu
Pro Val Lys Trp Met Ala Pro 850 855 860Glu Ser Ile Phe Asp Asn Leu
Tyr Thr Thr Leu Ser Asp Val Trp Ser865 870 875 880Tyr Gly Ile Leu
Leu Trp Glu Ile Phe Ser Leu Gly Gly Thr Pro Tyr 885 890 895Pro Gly
Met Met Val Asp Ser Thr Phe Tyr Asn Lys Ile Lys Ser Gly 900 905
910Tyr Arg Met Ala Lys Pro Asp His Ala Thr Ser Glu Val Tyr Glu Ile
915 920 925Met Val Lys Cys Trp Asn Ser Glu Pro Glu Lys Arg Pro Ser
Phe Tyr 930 935 940His Leu Ser Glu Ile Val Glu Asn Leu Leu Pro Gly
Gln Tyr Lys Lys945 950 955 960Ser Tyr Glu Lys Ile His Leu Asp Phe
Leu Lys Ser Asp His Pro Ala 965 970 975Val Ala Arg Met Arg Val Asp
Ser Asp Asn Ala Tyr Ile Gly Val Thr 980 985 990Tyr Lys Asn Glu Glu
Asp Lys Leu Lys Asp Trp Glu Gly Gly Leu Asp 995 1000 1005Glu Gln
Arg Leu Ser Ala Asp Ser Gly Tyr Ile Ile Pro Leu Pro 1010 1015
1020Asp Ile Asp Pro Val Pro Glu Glu Glu Asp Leu Gly Lys Arg Asn
1025 1030 1035Arg His Ser Ser Gln Thr Ser Glu Glu Ser Ala Ile Glu
Thr Gly 1040 1045 1050Ser Ser Ser Ser Thr Phe Ile Lys Arg Glu Asp
Glu Thr Ile Glu 1055 1060 1065Asp Ile Asp Met Met Asp Asp Ile Gly
Ile Asp Ser Ser Asp Leu 1070 1075 1080Val Glu Asp Ser Phe Leu
1085145718DNAHomo sapiens 14ctcctgaggc tgccagcagc cagcagtgac
tgcccgccct atctgggacc caggatcgct 60ctgtgagcaa cttggagcca gagaggagat
caacaaggag gaggagagag ccggcccctc 120agccctgctg cccagcagca
gcctgtgctc gccctgccca acgcagacag ccagacccag 180ggcggcccct
ctggcggctc tgctcctccc gaaggatgct tggggagtga ggcgaagctg
240ggccgctcct ctcccctaca gcagccccct tcctccatcc ctctgttctc
ctgagccttc 300aggagcctgc accagtcctg cctgtccttc tactcagctg
ttacccactc tgggaccagc 360agtctttctg ataactggga gagggcagta
aggaggactt cctggagggg gtgactgtcc 420agagcctgga actgtgccca
caccagaagc catcagcagc aaggacacca tgcggcttcc 480gggtgcgatg
ccagctctgg ccctcaaagg cgagctgctg ttgctgtctc tcctgttact
540tctggaacca cagatctctc agggcctggt cgtcacaccc ccggggccag
agcttgtcct 600caatgtctcc agcaccttcg ttctgacctg ctcgggttca
gctccggtgg tgtgggaacg 660gatgtcccag gagcccccac aggaaatggc
caaggcccag gatggcacct tctccagcgt 720gctcacactg accaacctca
ctgggctaga cacgggagaa tacttttgca cccacaatga 780ctcccgtgga
ctggagaccg atgagcggaa acggctctac atctttgtgc cagatcccac
840cgtgggcttc ctccctaatg atgccgagga actattcatc tttctcacgg
aaataactga 900gatcaccatt ccatgccgag taacagaccc acagctggtg
gtgacactgc acgagaagaa 960aggggacgtt gcactgcctg tcccctatga
tcaccaacgt ggcttttctg gtatctttga 1020ggacagaagc tacatctgca
aaaccaccat tggggacagg gaggtggatt ctgatgccta 1080ctatgtctac
agactccagg tgtcatccat caacgtctct gtgaacgcag tgcagactgt
1140ggtccgccag ggtgagaaca tcaccctcat gtgcattgtg atcgggaatg
aggtggtcaa 1200cttcgagtgg acataccccc gcaaagaaag tgggcggctg
gtggagccgg tgactgactt 1260cctcttggat atgccttacc acatccgctc
catcctgcac atccccagtg ccgagttaga 1320agactcgggg acctacacct
gcaatgtgac ggagagtgtg aatgaccatc aggatgaaaa 1380ggccatcaac
atcaccgtgg ttgagagcgg ctacgtgcgg ctcctgggag aggtgggcac
1440actacaattt gctgagctgc atcggagccg gacactgcag gtagtgttcg
aggcctaccc 1500accgcccact gtcctgtggt tcaaagacaa ccgcaccctg
ggcgactcca gcgctggcga 1560aatcgccctg tccacgcgca acgtgtcgga
gacccggtat gtgtcagagc tgacactggt 1620tcgcgtgaag gtggcagagg
ctggccacta caccatgcgg gccttccatg aggatgctga 1680ggtccagctc
tccttccagc tacagatcaa tgtccctgtc cgagtgctgg agctaagtga
1740gagccaccct gacagtgggg aacagacagt ccgctgtcgt ggccggggca
tgccccagcc 1800gaacatcatc tggtctgcct gcagagacct caaaaggtgt
ccacgtgagc tgccgcccac 1860gctgctgggg aacagttccg aagaggagag
ccagctggag actaacgtga cgtactggga 1920ggaggagcag gagtttgagg
tggtgagcac actgcgtctg cagcacgtgg atcggccact 1980gtcggtgcgc
tgcacgctgc gcaacgctgt gggccaggac acgcaggagg tcatcgtggt
2040gccacactcc ttgcccttta aggtggtggt gatctcagcc atcctggccc
tggtggtgct 2100caccatcatc tcccttatca tcctcatcat gctttggcag
aagaagccac gttacgagat 2160ccgatggaag gtgattgagt ctgtgagctc
tgacggccat gagtacatct acgtggaccc 2220catgcagctg ccctatgact
ccacgtggga gctgccgcgg gaccagcttg tgctgggacg 2280caccctcggc
tctggggcct ttgggcaggt ggtggaggcc acggctcatg gcctgagcca
2340ttctcaggcc acgatgaaag tggccgtcaa gatgcttaaa tccacagccc
gcagcagtga 2400gaagcaagcc cttatgtcgg agctgaagat catgagtcac
cttgggcccc acctgaacgt 2460ggtcaacctg ttgggggcct gcaccaaagg
aggacccatc tatatcatca ctgagtactg 2520ccgctacgga gacctggtgg
actacctgca ccgcaacaaa cacaccttcc tgcagcacca 2580ctccgacaag
cgccgcccgc ccagcgcgga gctctacagc aatgctctgc ccgttgggct
2640ccccctgccc agccatgtgt ccttgaccgg ggagagcgac ggtggctaca
tggacatgag 2700caaggacgag tcggtggact atgtgcccat gctggacatg
aaaggagacg tcaaatatgc 2760agacatcgag tcctccaact acatggcccc
ttacgataac tacgttccct ctgcccctga 2820gaggacctgc cgagcaactt
tgatcaacga gtctccagtg ctaagctaca tggacctcgt 2880gggcttcagc
taccaggtgg ccaatggcat ggagtttctg gcctccaaga actgcgtcca
2940cagagacctg gcggctagga acgtgctcat ctgtgaaggc aagctggtca
agatctgtga 3000ctttggcctg gctcgagaca tcatgcggga ctcgaattac
atctccaaag gcagcacctt 3060tttgccttta aagtggatgg ctccggagag
catcttcaac agcctctaca ccaccctgag 3120cgacgtgtgg tccttcggga
tcctgctctg ggagatcttc accttgggtg gcacccctta 3180cccagagctg
cccatgaacg agcagttcta caatgccatc aaacggggtt accgcatggc
3240ccagcctgcc catgcctccg acgagatcta tgagatcatg cagaagtgct
gggaagagaa 3300gtttgagatt cggcccccct tctcccagct ggtgctgctt
ctcgagagac tgttgggcga 3360aggttacaaa aagaagtacc agcaggtgga
tgaggagttt ctgaggagtg accacccagc 3420catccttcgg tcccaggccc
gcttgcctgg gttccatggc ctccgatctc ccctggacac 3480cagctccgtc
ctctatactg ccgtgcagcc caatgagggt gacaacgact atatcatccc
3540cctgcctgac cccaaacccg aggttgctga cgagggccca ctggagggtt
cccccagcct 3600agccagctcc accctgaatg aagtcaacac ctcctcaacc
atctcctgtg acagccccct 3660ggagccccag gacgaaccag agccagagcc
ccagcttgag ctccaggtgg agccggagcc 3720agagctggaa cagttgccgg
attcggggtg ccctgcgcct cgggcggaag cagaggatag 3780cttcctgtag
ggggctggcc cctaccctgc cctgcctgaa gctccccccc tgccagcacc
3840cagcatctcc tggcctggcc tgaccgggct tcctgtcagc caggctgccc
ttatcagctg 3900tccccttctg gaagctttct gctcctgacg tgttgtgccc
caaaccctgg ggctggctta 3960ggaggcaaga aaactgcagg ggccgtgacc
agccctctgc ctccagggag gccaactgac 4020tctgagccag ggttccccca
gggaactcag ttttcccata tgtaagatgg gaaagttagg 4080cttgatgacc
cagaatctag gattctctcc ctggctgaca ggtggggaga ccgaatccct
4140ccctgggaag attcttggag ttactgaggt ggtaaattaa cttttttctg
ttcagccagc 4200tacccctcaa ggaatcatag ctctctcctc gcacttttat
ccacccagga gctagggaag 4260agaccctagc ctccctggct gctggctgag
ctagggccta gccttgagca gtgttgcctc 4320atccagaaga aagccagtct
cctccctatg atgccagtcc ctgcgttccc tggcccgagc 4380tggtctgggg
ccattaggca gcctaattaa tgctggaggc tgagccaagt acaggacacc
4440cccagcctgc agcccttgcc cagggcactt ggagcacacg cagccatagc
aagtgcctgt 4500gtccctgtcc ttcaggccca tcagtcctgg ggctttttct
ttatcaccct cagtcttaat 4560ccatccacca gagtctagaa ggccagacgg
gccccgcatc tgtgatgaga atgtaaatgt 4620gccagtgtgg agtggccacg
tgtgtgtgcc agtatatggc cctggctctg cattggacct 4680gctatgaggc
tttggaggaa tccctcaccc tctctgggcc tcagtttccc cttcaaaaaa
4740tgaataagtc ggacttatta actctgagtg ccttgccagc actaacattc
tagagtattc 4800caggtggttg cacatttgtc cagatgaagc aaggccatat
accctaaact tccatcctgg 4860gggtcagctg ggctcctggg agattccaga
tcacacatca cactctgggg actcaggaac 4920catgcccctt ccccaggccc
ccagcaagtc tcaagaacac agctgcacag gccttgactt 4980agagtgacag
ccggtgtcct ggaaagcccc cagcagctgc cccagggaca tgggaagacc
5040acgggacctc tttcactacc cacgatgacc tccgggggta tcctgggcaa
aagggacaaa 5100gagggcaaat gagatcacct cctgcagccc accactccag
cacctgtgcc gaggtctgcg 5160tcgaagacag aatggacagt gaggacagtt
atgtcttgta aaagacaaga agcttcagat 5220gggtacccca agaaggatgt
gagaggtggg cgctttggag gtttgcccct cacccaccag 5280ctgccccatc
cctgaggcag cgctccatgg gggtatggtt ttgtcactgc ccagacctag
5340cagtgacatc tcattgtccc cagcccagtg ggcattggag gtgccagggg
agtcagggtt 5400gtagccaaga cgcccccgca cggggagggt tgggaagggg
gtgcaggaag ctcaacccct 5460ctgggcacca accctgcatt gcaggttggc
accttacttc cctgggatcc ccagagttgg 5520tccaaggagg gagagtgggt
tctcaatacg gtaccaaaga tataatcacc taggtttaca 5580aatattttta
ggactcacgt taactcacat ttatacagca gaaatgctat tttgtatgct
5640gttaagtttt tctatctgtg tacttttttt taagggaaag attttaatat
taaacctggt 5700gcttctcact cacaaaaa 5718151106PRTHomo sapiens 15Met
Arg Leu Pro Gly Ala Met Pro Ala Leu Ala Leu Lys Gly Glu Leu1 5 10
15Leu Leu Leu Ser Leu Leu Leu Leu Leu Glu Pro Gln Ile Ser Gln Gly
20 25 30Leu Val Val Thr Pro Pro Gly Pro Glu Leu Val Leu Asn Val Ser
Ser 35 40 45Thr Phe Val Leu Thr Cys Ser Gly Ser Ala Pro Val Val Trp
Glu Arg 50 55 60Met Ser Gln Glu Pro Pro Gln Glu Met Ala Lys Ala Gln
Asp Gly Thr65 70 75 80Phe Ser Ser Val Leu Thr Leu Thr Asn Leu Thr
Gly Leu Asp Thr Gly 85 90 95Glu Tyr Phe Cys Thr His Asn Asp Ser Arg
Gly Leu Glu Thr Asp Glu 100 105 110Arg Lys Arg Leu Tyr Ile Phe Val
Pro Asp Pro Thr Val Gly Phe Leu 115 120 125Pro Asn Asp Ala Glu Glu
Leu Phe Ile Phe Leu Thr Glu Ile Thr Glu 130 135 140Ile Thr Ile Pro
Cys Arg Val Thr Asp Pro Gln Leu Val Val Thr Leu145 150 155 160His
Glu Lys Lys Gly Asp Val Ala Leu Pro Val Pro Tyr Asp His Gln 165 170
175Arg Gly Phe Ser Gly Ile Phe Glu Asp Arg Ser Tyr Ile Cys Lys Thr
180 185 190Thr Ile Gly Asp Arg Glu Val Asp Ser Asp Ala Tyr Tyr Val
Tyr Arg 195 200 205Leu Gln Val Ser Ser Ile Asn Val Ser Val Asn Ala
Val Gln Thr Val 210 215 220Val Arg Gln Gly Glu Asn Ile Thr Leu Met
Cys Ile Val Ile Gly Asn225 230 235 240Glu Val Val Asn Phe Glu Trp
Thr Tyr Pro Arg Lys Glu Ser Gly Arg 245 250 255Leu Val Glu Pro Val
Thr Asp Phe Leu Leu Asp Met Pro Tyr His Ile 260 265 270Arg Ser Ile
Leu His Ile Pro Ser Ala Glu Leu Glu Asp Ser Gly Thr 275 280 285Tyr
Thr Cys Asn Val Thr Glu Ser Val Asn Asp His Gln Asp Glu Lys 290 295
300Ala Ile Asn Ile Thr Val Val Glu Ser Gly Tyr Val Arg Leu Leu
Gly305 310 315 320Glu Val Gly Thr Leu Gln Phe Ala Glu Leu His Arg
Ser Arg Thr Leu 325 330 335Gln Val Val Phe Glu Ala Tyr Pro Pro Pro
Thr Val Leu Trp Phe Lys 340 345 350Asp Asn Arg Thr Leu Gly Asp Ser
Ser Ala Gly Glu Ile Ala Leu Ser 355 360 365Thr Arg Asn Val Ser Glu
Thr Arg Tyr Val Ser Glu Leu Thr Leu Val 370 375 380Arg Val Lys Val
Ala Glu Ala Gly His Tyr Thr Met Arg Ala Phe His385 390 395 400Glu
Asp Ala Glu Val Gln Leu Ser Phe Gln Leu Gln Ile Asn Val Pro 405 410
415Val Arg Val Leu Glu Leu Ser Glu Ser His Pro Asp Ser Gly Glu Gln
420 425 430Thr Val Arg Cys Arg Gly Arg Gly Met Pro Gln Pro Asn Ile
Ile Trp 435 440 445Ser Ala Cys Arg Asp Leu Lys Arg Cys Pro Arg Glu
Leu Pro Pro Thr 450 455 460Leu Leu Gly Asn Ser Ser Glu Glu Glu Ser
Gln Leu Glu Thr Asn Val465 470 475 480Thr Tyr Trp Glu Glu Glu Gln
Glu Phe Glu Val Val Ser Thr Leu Arg 485 490 495Leu Gln His Val Asp
Arg Pro Leu Ser Val Arg Cys Thr Leu Arg Asn 500 505 510Ala Val Gly
Gln Asp Thr Gln Glu Val Ile Val Val Pro His Ser Leu 515 520 525Pro
Phe Lys Val Val Val Ile Ser Ala Ile Leu Ala Leu Val Val Leu 530 535
540Thr Ile Ile Ser Leu Ile Ile Leu Ile Met Leu Trp Gln Lys Lys
Pro545 550 555 560Arg Tyr Glu Ile Arg Trp Lys Val Ile Glu Ser Val
Ser Ser Asp Gly 565 570 575His Glu Tyr Ile Tyr Val Asp Pro Met Gln
Leu Pro Tyr Asp Ser Thr 580 585 590Trp Glu Leu Pro Arg Asp Gln Leu
Val Leu Gly Arg Thr Leu Gly Ser 595 600 605Gly Ala Phe Gly Gln Val
Val Glu Ala Thr Ala His Gly Leu Ser His 610 615 620Ser Gln Ala Thr
Met Lys Val Ala Val Lys Met Leu Lys Ser Thr Ala625 630 635 640Arg
Ser Ser Glu Lys Gln Ala Leu Met Ser Glu Leu Lys Ile Met Ser 645 650
655His Leu Gly Pro His Leu Asn Val Val Asn Leu Leu Gly Ala Cys Thr
660 665 670Lys Gly Gly Pro Ile Tyr Ile Ile Thr Glu Tyr Cys Arg Tyr
Gly Asp 675 680 685Leu Val Asp Tyr Leu His Arg Asn Lys His Thr Phe
Leu Gln His His 690 695 700Ser Asp Lys Arg Arg Pro Pro Ser Ala Glu
Leu Tyr Ser Asn Ala Leu705 710 715 720Pro Val Gly Leu Pro Leu Pro
Ser His Val Ser Leu Thr Gly Glu Ser 725 730 735Asp Gly Gly Tyr Met
Asp Met Ser Lys Asp Glu Ser Val Asp Tyr Val 740 745 750Pro Met Leu
Asp Met Lys Gly Asp Val Lys Tyr Ala Asp Ile Glu Ser 755 760 765Ser
Asn Tyr Met Ala Pro Tyr Asp Asn Tyr Val Pro Ser Ala Pro Glu 770 775
780Arg Thr Cys Arg Ala Thr Leu Ile Asn Glu Ser Pro Val Leu Ser
Tyr785 790 795 800Met Asp Leu Val Gly Phe Ser Tyr Gln Val Ala Asn
Gly Met Glu Phe 805 810 815Leu Ala Ser Lys Asn Cys Val His Arg Asp
Leu Ala Ala Arg Asn Val 820 825 830Leu Ile Cys Glu Gly Lys Leu Val
Lys Ile Cys Asp Phe Gly Leu Ala 835 840 845Arg Asp Ile Met Arg Asp
Ser Asn Tyr Ile Ser Lys Gly Ser Thr Phe 850 855 860Leu Pro Leu Lys
Trp Met Ala Pro Glu Ser Ile Phe Asn Ser Leu Tyr865 870 875 880Thr
Thr Leu Ser Asp Val Trp Ser Phe Gly Ile Leu Leu Trp Glu Ile 885 890
895Phe Thr Leu Gly Gly Thr Pro Tyr Pro Glu Leu Pro Met Asn Glu Gln
900 905 910Phe Tyr Asn Ala Ile Lys Arg Gly Tyr Arg Met Ala Gln Pro
Ala His 915 920 925Ala Ser Asp Glu Ile Tyr Glu Ile Met Gln Lys Cys
Trp Glu Glu Lys 930 935 940Phe Glu Ile Arg Pro Pro Phe Ser Gln Leu
Val Leu Leu Leu Glu Arg945 950 955 960Leu Leu Gly Glu Gly Tyr Lys
Lys Lys Tyr Gln Gln Val Asp Glu Glu 965 970 975Phe Leu Arg Ser Asp
His Pro Ala Ile Leu Arg Ser Gln Ala Arg Leu 980 985 990Pro Gly Phe
His Gly Leu Arg Ser Pro Leu Asp Thr Ser Ser Val Leu 995 1000
1005Tyr Thr Ala Val Gln Pro Asn Glu Gly Asp Asn Asp Tyr Ile Ile
1010 1015 1020Pro Leu Pro Asp Pro Lys Pro Glu Val Ala Asp Glu Gly
Pro Leu 1025 1030 1035Glu Gly Ser Pro Ser Leu Ala Ser Ser Thr Leu
Asn Glu Val Asn 1040 1045 1050Thr Ser Ser Thr Ile Ser Cys Asp Ser
Pro Leu Glu Pro Gln Asp 1055 1060 1065Glu Pro Glu Pro Glu Pro Gln
Leu Glu Leu Gln Val Glu Pro Glu 1070 1075 1080Pro Glu Leu Glu Gln
Leu Pro Asp Ser Gly Cys Pro Ala Pro Arg 1085 1090 1095Ala Glu Ala
Glu Asp Ser Phe Leu 1100 1105163626DNAHomo sapiens 16tcgcggaggc
ttggggcagc cgggtagctc ggaggtcgtg gcgctggggg ctagcaccag 60cgctctgtcg
ggaggcgcag cggttaggtg gaccggtcag cggactcacc ggccagggcg
120ctcggtgctg gaatttgata ttcattgatc cgggttttat ccctcttctt
ttttcttaaa 180catttttttt taaaactgta ttgtttctcg ttttaattta
tttttgcttg ccattcccca 240cttgaatcgg gccgacggct tggggagatt
gctctacttc cccaaatcac tgtggatttt 300ggaaaccagc agaaagagga
aagaggtagc aagagctcca gagagaagtc gaggaagaga 360gagacggggt
cagagagagc gcgcgggcgt gcgagcagcg aaagcgacag gggcaaagtg
420agtgacctgc ttttgggggt gaccgccgga gcgcggcgtg agccctcccc
cttgggatcc 480cgcagctgac cagtcgcgct gacggacaga cagacagaca
ccgcccccag ccccagctac 540cacctcctcc ccggccggcg gcggacagtg
gacgcggcgg cgagccgcgg gcaggggccg 600gagcccgcgc ccggaggcgg
ggtggagggg gtcggggctc gcggcgtcgc actgaaactt 660ttcgtccaac
ttctgggctg ttctcgcttc ggaggagccg tggtccgcgc gggggaagcc
720gagccgagcg gagccgcgag aagtgctagc tcgggccggg aggagccgca
gccggaggag 780ggggaggagg aagaagagaa ggaagaggag agggggccgc
agtggcgact cggcgctcgg 840aagccgggct catggacggg tgaggcggcg
gtgtgcgcag acagtgctcc agccgcgcgc 900gctccccagg ccctggcccg
ggcctcgggc cggggaggaa gagtagctcg ccgaggcgcc 960gaggagagcg
ggccgcccca cagcccgagc cggagaggga gcgcgagccg cgccggcccc
1020ggtcgggcct ccgaaaccat gaactttctg ctgtcttggg tgcattggag
ccttgccttg 1080ctgctctacc tccaccatgc caagtggtcc caggctgcac
ccatggcaga aggaggaggg 1140cagaatcatc acgaagtggt gaagttcatg
gatgtctatc agcgcagcta ctgccatcca 1200atcgagaccc tggtggacat
cttccaggag taccctgatg agatcgagta catcttcaag 1260ccatcctgtg
tgcccctgat gcgatgcggg ggctgctgca atgacgaggg cctggagtgt
1320gtgcccactg aggagtccaa catcaccatg cagattatgc ggatcaaacc
tcaccaaggc 1380cagcacatag gagagatgag cttcctacag cacaacaaat
gtgaatgcag accaaagaaa 1440gatagagcaa gacaagaaaa aaaatcagtt
cgaggaaagg gaaaggggca aaaacgaaag 1500cgcaagaaat cccggtataa
gtcctggagc gttccctgtg ggccttgctc agagcggaga 1560aagcatttgt
ttgtacaaga tccgcagacg tgtaaatgtt cctgcaaaaa cacagactcg
1620cgttgcaagg cgaggcagct tgagttaaac gaacgtactt gcagatgtga
caagccgagg 1680cggtgagccg ggcaggagga aggagcctcc ctcagggttt
cgggaaccag atctctcacc 1740aggaaagact gatacagaac gatcgataca
gaaaccacgc tgccgccacc acaccatcac 1800catcgacaga acagtcctta
atccagaaac ctgaaatgaa ggaagaggag actctgcgca 1860gagcactttg
ggtccggagg gcgagactcc ggcggaagca ttcccgggcg ggtgacccag
1920cacggtccct cttggaattg gattcgccat tttatttttc ttgctgctaa
atcaccgagc 1980ccggaagatt agagagtttt atttctggga ttcctgtaga
cacacccacc cacatacata 2040catttatata tatatatatt atatatatat
aaaaataaat atctctattt tatatatata 2100aaatatatat attctttttt
taaattaaca gtgctaatgt tattggtgtc ttcactggat 2160gtatttgact
gctgtggact tgagttggga ggggaatgtt cccactcaga tcctgacagg
2220gaagaggagg agatgagaga ctctggcatg atcttttttt tgtcccactt
ggtggggcca 2280gggtcctctc ccctgcccag gaatgtgcaa ggccagggca
tgggggcaaa tatgacccag 2340ttttgggaac accgacaaac ccagccctgg
cgctgagcct ctctacccca ggtcagacgg 2400acagaaagac agatcacagg
tacagggatg aggacaccgg ctctgaccag gagtttgggg 2460agcttcagga
cattgctgtg ctttggggat tccctccaca tgctgcacgc gcatctcgcc
2520cccaggggca ctgcctggaa gattcaggag cctgggcggc cttcgcttac
tctcacctgc 2580ttctgagttg cccaggagac cactggcaga tgtcccggcg
aagagaagag acacattgtt 2640ggaagaagca gcccatgaca gctccccttc
ctgggactcg ccctcatcct cttcctgctc 2700cccttcctgg ggtgcagcct
aaaaggacct atgtcctcac accattgaaa ccactagttc 2760tgtcccccca
ggagacctgg ttgtgtgtgt gtgagtggtt gaccttcctc catcccctgg
2820tccttccctt cccttcccga ggcacagaga gacagggcag gatccacgtg
cccattgtgg 2880aggcagagaa aagagaaagt gttttatata cggtacttat
ttaatatccc tttttaatta 2940gaaattaaaa cagttaattt aattaaagag
tagggttttt tttcagtatt cttggttaat 3000atttaatttc aactatttat
gagatgtatc ttttgctctc tcttgctctc ttatttgtac 3060cggtttttgt
atataaaatt catgtttcca atctctctct ccctgatcgg tgacagtcac
3120tagcttatct tgaacagata tttaattttg ctaacactca gctctgccct
ccccgatccc 3180ctggctcccc agcacacatt cctttgaaat aaggtttcaa
tatacatcta catactatat 3240atatatttgg caacttgtat ttgtgtgtat
atatatatat atatgtttat gtatatatgt 3300gattctgata aaatagacat
tgctattctg ttttttatat gtaaaaacaa aacaagaaaa 3360aatagagaat
tctacatact aaatctctct ccttttttaa ttttaatatt tgttatcatt
3420tatttattgg tgctactgtt tatccgtaat aattgtgggg aaaagatatt
aacatcacgt 3480ctttgtctct agtgcagttt ttcgagatat tccgtagtac
atatttattt ttaaacaacg 3540acaaagaaat acagatatat cttaaaaaaa
aaaaagcatt ttgtattaaa gaatttaatt 3600ctgatctcaa aaaaaaaaaa aaaaaa
362617395PRTHomo sapiens 17Met Thr Asp Arg Gln Thr Asp Thr Ala Pro
Ser Pro Ser Tyr His Leu1 5 10 15Leu Pro Gly Arg Arg Arg Thr Val Asp
Ala Ala Ala Ser Arg Gly Gln 20 25 30Gly Pro Glu Pro Ala Pro Gly Gly
Gly Val Glu Gly Val Gly Ala Arg 35 40 45Gly Val Ala Leu Lys Leu Phe
Val Gln Leu Leu Gly Cys Ser Arg Phe 50 55 60Gly Gly Ala Val Val Arg
Ala Gly Glu Ala Glu Pro Ser Gly Ala Ala65 70 75 80Arg Ser Ala Ser
Ser Gly Arg Glu Glu Pro Gln Pro Glu Glu Gly Glu 85 90 95Glu Glu Glu
Glu Lys Glu Glu Glu Arg Gly Pro Gln Trp Arg Leu Gly 100 105 110Ala
Arg Lys Pro Gly Ser Trp Thr Gly Glu Ala Ala Val Cys Ala Asp 115 120
125Ser Ala Pro Ala Ala Arg Ala Pro Gln Ala Leu Ala Arg Ala Ser Gly
130 135 140Arg Gly Gly Arg Val Ala Arg Arg Gly Ala Glu Glu Ser Gly
Pro Pro145 150 155 160His Ser Pro Ser Arg Arg Gly Ser Ala Ser Arg
Ala Gly Pro Gly Arg 165 170 175Ala Ser Glu Thr Met Asn Phe Leu Leu
Ser Trp Val His Trp Ser Leu 180 185 190Ala Leu Leu Leu Tyr Leu His
His Ala Lys Trp Ser Gln Ala Ala Pro 195 200 205Met Ala Glu Gly Gly
Gly Gln Asn His His Glu Val Val Lys Phe Met 210 215 220Asp Val Tyr
Gln Arg Ser Tyr Cys His Pro Ile Glu Thr Leu Val Asp225 230 235
240Ile Phe Gln Glu Tyr Pro Asp Glu Ile Glu Tyr Ile Phe Lys Pro Ser
245 250 255Cys Val Pro Leu Met Arg Cys Gly Gly Cys Cys Asn Asp Glu
Gly Leu 260 265 270Glu Cys Val Pro Thr Glu Glu Ser Asn Ile Thr Met
Gln Ile Met Arg 275 280 285Ile Lys Pro His Gln Gly Gln His Ile Gly
Glu Met Ser Phe Leu Gln 290 295 300His Asn Lys Cys Glu Cys Arg Pro
Lys Lys Asp Arg Ala Arg Gln Glu305 310 315 320Lys Lys Ser Val Arg
Gly Lys Gly Lys Gly Gln Lys Arg Lys Arg Lys 325 330 335Lys Ser Arg
Tyr Lys Ser Trp Ser Val Pro Cys Gly Pro Cys Ser Glu 340 345 350Arg
Arg Lys His Leu Phe Val Gln Asp Pro Gln Thr Cys Lys Cys Ser 355 360
365Cys Lys Asn Thr Asp Ser Arg Cys Lys Ala Arg Gln Leu Glu Leu Asn
370 375 380Glu Arg Thr Cys Arg Cys Asp Lys Pro Arg Arg385 390
395184017DNAHomo sapiens 18atggtcagct actgggacac cggggtcctg
ctgtgcgcgc tgctcagctg tctgcttctc 60acaggatcta gttcaggttc aaaattaaaa
gatcctgaac tgagtttaaa aggcacccag 120cacatcatgc aagcaggcca
gacactgcat ctccaatgca ggggggaagc agcccataaa 180tggtctttgc
ctgaaatggt gagtaaggaa agcgaaaggc tgagcataac taaatctgcc
240tgtggaagaa atggcaaaca attctgcagt actttaacct tgaacacagc
tcaagcaaac 300cacactggct tctacagctg caaatatcta gctgtaccta
cttcaaagaa gaaggaaaca 360gaatctgcaa tctatatatt tattagtgat
acaggtagac ctttcgtaga gatgtacagt 420gaaatccccg aaattataca
catgactgaa ggaagggagc tcgtcattcc ctgccgggtt 480acgtcaccta
acatcactgt tactttaaaa aagtttccac ttgacacttt gatccctgat
540ggaaaacgca taatctggga cagtagaaag ggcttcatca tatcaaatgc
aacgtacaaa 600gaaatagggc ttctgacctg tgaagcaaca gtcaatgggc
atttgtataa gacaaactat 660ctcacacatc gacaaaccaa tacaatcata
gatgtccaaa taagcacacc acgcccagtc 720aaattactta gaggccatac
tcttgtcctc aattgtactg ctaccactcc cttgaacacg 780agagttcaaa
tgacctggag ttaccctgat gaaaaaaata agagagcttc cgtaaggcga
840cgaattgacc aaagcaattc ccatgccaac atattctaca gtgttcttac
tattgacaaa 900atgcagaaca aagacaaagg actttatact tgtcgtgtaa
ggagtggacc atcattcaaa 960tctgttaaca cctcagtgca tatatatgat
aaagcattca tcactgtgaa acatcgaaaa 1020cagcaggtgc ttgaaaccgt
agctggcaag cggtcttacc ggctctctat gaaagtgaag 1080gcatttccct
cgccggaagt tgtatggtta aaagatgggt tacctgcgac tgagaaatct
1140gctcgctatt tgactcgtgg ctactcgtta attatcaagg acgtaactga
agaggatgca 1200gggaattata caatcttgct gagcataaaa cagtcaaatg
tgtttaaaaa cctcactgcc 1260actctaattg tcaatgtgaa accccagatt
tacgaaaagg ccgtgtcatc gtttccagac 1320ccggctctct acccactggg
cagcagacaa atcctgactt gtaccgcata tggtatccct 1380caacctacaa
tcaagtggtt ctggcacccc tgtaaccata atcattccga agcaaggtgt
1440gacttttgtt ccaataatga agagtcctct atcctggatg ctgacagcaa
catgggaaac 1500agaattgaga gcatcactca gcgcatggca ataatagaag
gaaagaataa gatggctagc 1560accttggttg tggctgactc tagaatttct
ggaatctaca tttgcatagc ttccaataaa 1620gttgggactg tgggaagaaa
cataagcttt tatatcacag atgtgccaaa tgggtttcat 1680gttaacttgg
aaaaaatgcc gacggaagga gaggacctga aactgtcttg cacagttaac
1740aagttcttat acagagacgt tacttggatt ttactgcgga cagttaataa
cagaacaatg 1800cactacagta ttagcaagca aaaaatggcc atcactaagg
agcactccat cactcttaat 1860cttaccatca tgaatgtttc cctgcaagat
tcaggcacct atgcctgcag agccaggaat
1920gtatacacag gggaagaaat cctccagaag aaagaaatta caatcagaga
tcaggaagca 1980ccatacctcc tgcgaaacct cagtgatcac acagtggcca
tcagcagttc caccacttta 2040gactgtcatg ctaatggtgt ccccgagcct
cagatcactt ggtttaaaaa caaccacaaa 2100atacaacaag agcctggaat
tattttagga ccaggaagca gcacgctgtt tattgaaaga 2160gtcacagaag
aggatgaagg tgtctatcac tgcaaagcca ccaaccagaa gggctctgtg
2220gaaagttcag catacctcac tgttcaagga acctcggaca agtctaatct
ggagctgatc 2280actctaacat gcacctgtgt ggctgcgact ctcttctggc
tcctattaac cctctttatc 2340cgaaaaatga aaaggtcttc ttctgaaata
aagactgact acctatcaat tataatggac 2400ccagatgaag ttcctttgga
tgagcagtgt gagcggctcc cttatgatgc cagcaagtgg 2460gagtttgccc
gggagagact taaactgggc aaatcacttg gaagaggggc ttttggaaaa
2520gtggttcaag catcagcatt tggcattaag aaatcaccta cgtgccggac
tgtggctgtg 2580aaaatgctga aagagggggc cacggccagc gagtacaaag
ctctgatgac tgagctaaaa 2640atcttgaccc acattggcca ccatctgaac
gtggttaacc tgctgggagc ctgcaccaag 2700caaggagggc ctctgatggt
gattgttgaa tactgcaaat atggaaatct ctccaactac 2760ctcaagagca
aacgtgactt attttttctc aacaaggatg cagcactaca catggagcct
2820aagaaagaaa aaatggagcc aggcctggaa caaggcaaga aaccaagact
agatagcgtc 2880accagcagcg aaagctttgc gagctccggc tttcaggaag
ataaaagtct gagtgatgtt 2940gaggaagagg aggattctga cggtttctac
aaggagccca tcactatgga agatctgatt 3000tcttacagtt ttcaagtggc
cagaggcatg gagttcctgt cttccagaaa gtgcattcat 3060cgggacctgg
cagcgagaaa cattctttta tctgagaaca acgtggtgaa gatttgtgat
3120tttggccttg cccgggatat ttataagaac cccgattatg tgagaaaagg
agatactcga 3180cttcctctga aatggatggc tcctgaatct atctttgaca
aaatctacag caccaagagc 3240gacgtgtggt cttacggagt attgctgtgg
gaaatcttct ccttaggtgg gtctccatac 3300ccaggagtac aaatggatga
ggacttttgc agtcgcctga gggaaggcat gaggatgaga 3360gctcctgagt
actctactcc tgaaatctat cagatcatgc tggactgctg gcacagagac
3420ccaaaagaaa ggccaagatt tgcagaactt gtggaaaaac taggtgattt
gcttcaagca 3480aatgtacaac aggatggtaa agactacatc ccaatcaatg
ccatactgac aggaaatagt 3540gggtttacat actcaactcc tgccttctct
gaggacttct tcaaggaaag tatttcagct 3600ccgaagttta attcaggaag
ctctgatgat gtcagatatg taaatgcttt caagttcatg 3660agcctggaaa
gaatcaaaac ctttgaagaa cttttaccga atgccacctc catgtttgat
3720gactaccagg gcgacagcag cactctgttg gcctctccca tgctgaagcg
cttcacctgg 3780actgacagca aacccaaggc ctcgctcaag attgacttga
gagtaaccag taaaagtaag 3840gagtcggggc tgtctgatgt cagcaggccc
agtttctgcc attccagctg tgggcacgtc 3900agcgaaggca agcgcaggtt
cacctacgac cacgctgagc tggaaaggaa aatcgcgtgc 3960tgctccccgc
ccccagacta caactcggtg gtcctgtact ccaccccacc catctag
4017191338PRTHomo sapiens 19Met Val Ser Tyr Trp Asp Thr Gly Val Leu
Leu Cys Ala Leu Leu Ser1 5 10 15Cys Leu Leu Leu Thr Gly Ser Ser Ser
Gly Ser Lys Leu Lys Asp Pro 20 25 30Glu Leu Ser Leu Lys Gly Thr Gln
His Ile Met Gln Ala Gly Gln Thr 35 40 45Leu His Leu Gln Cys Arg Gly
Glu Ala Ala His Lys Trp Ser Leu Pro 50 55 60Glu Met Val Ser Lys Glu
Ser Glu Arg Leu Ser Ile Thr Lys Ser Ala65 70 75 80Cys Gly Arg Asn
Gly Lys Gln Phe Cys Ser Thr Leu Thr Leu Asn Thr 85 90 95Ala Gln Ala
Asn His Thr Gly Phe Tyr Ser Cys Lys Tyr Leu Ala Val 100 105 110Pro
Thr Ser Lys Lys Lys Glu Thr Glu Ser Ala Ile Tyr Ile Phe Ile 115 120
125Ser Asp Thr Gly Arg Pro Phe Val Glu Met Tyr Ser Glu Ile Pro Glu
130 135 140Ile Ile His Met Thr Glu Gly Arg Glu Leu Val Ile Pro Cys
Arg Val145 150 155 160Thr Ser Pro Asn Ile Thr Val Thr Leu Lys Lys
Phe Pro Leu Asp Thr 165 170 175Leu Ile Pro Asp Gly Lys Arg Ile Ile
Trp Asp Ser Arg Lys Gly Phe 180 185 190Ile Ile Ser Asn Ala Thr Tyr
Lys Glu Ile Gly Leu Leu Thr Cys Glu 195 200 205Ala Thr Val Asn Gly
His Leu Tyr Lys Thr Asn Tyr Leu Thr His Arg 210 215 220Gln Thr Asn
Thr Ile Ile Asp Val Gln Ile Ser Thr Pro Arg Pro Val225 230 235
240Lys Leu Leu Arg Gly His Thr Leu Val Leu Asn Cys Thr Ala Thr Thr
245 250 255Pro Leu Asn Thr Arg Val Gln Met Thr Trp Ser Tyr Pro Asp
Glu Lys 260 265 270Asn Lys Arg Ala Ser Val Arg Arg Arg Ile Asp Gln
Ser Asn Ser His 275 280 285Ala Asn Ile Phe Tyr Ser Val Leu Thr Ile
Asp Lys Met Gln Asn Lys 290 295 300Asp Lys Gly Leu Tyr Thr Cys Arg
Val Arg Ser Gly Pro Ser Phe Lys305 310 315 320Ser Val Asn Thr Ser
Val His Ile Tyr Asp Lys Ala Phe Ile Thr Val 325 330 335Lys His Arg
Lys Gln Gln Val Leu Glu Thr Val Ala Gly Lys Arg Ser 340 345 350Tyr
Arg Leu Ser Met Lys Val Lys Ala Phe Pro Ser Pro Glu Val Val 355 360
365Trp Leu Lys Asp Gly Leu Pro Ala Thr Glu Lys Ser Ala Arg Tyr Leu
370 375 380Thr Arg Gly Tyr Ser Leu Ile Ile Lys Asp Val Thr Glu Glu
Asp Ala385 390 395 400Gly Asn Tyr Thr Ile Leu Leu Ser Ile Lys Gln
Ser Asn Val Phe Lys 405 410 415Asn Leu Thr Ala Thr Leu Ile Val Asn
Val Lys Pro Gln Ile Tyr Glu 420 425 430Lys Ala Val Ser Ser Phe Pro
Asp Pro Ala Leu Tyr Pro Leu Gly Ser 435 440 445Arg Gln Ile Leu Thr
Cys Thr Ala Tyr Gly Ile Pro Gln Pro Thr Ile 450 455 460Lys Trp Phe
Trp His Pro Cys Asn His Asn His Ser Glu Ala Arg Cys465 470 475
480Asp Phe Cys Ser Asn Asn Glu Glu Ser Ser Ile Leu Asp Ala Asp Ser
485 490 495Asn Met Gly Asn Arg Ile Glu Ser Ile Thr Gln Arg Met Ala
Ile Ile 500 505 510Glu Gly Lys Asn Lys Met Ala Ser Thr Leu Val Val
Ala Asp Ser Arg 515 520 525Ile Ser Gly Ile Tyr Ile Cys Ile Ala Ser
Asn Lys Val Gly Thr Val 530 535 540Gly Arg Asn Ile Ser Phe Tyr Ile
Thr Asp Val Pro Asn Gly Phe His545 550 555 560Val Asn Leu Glu Lys
Met Pro Thr Glu Gly Glu Asp Leu Lys Leu Ser 565 570 575Cys Thr Val
Asn Lys Phe Leu Tyr Arg Asp Val Thr Trp Ile Leu Leu 580 585 590Arg
Thr Val Asn Asn Arg Thr Met His Tyr Ser Ile Ser Lys Gln Lys 595 600
605Met Ala Ile Thr Lys Glu His Ser Ile Thr Leu Asn Leu Thr Ile Met
610 615 620Asn Val Ser Leu Gln Asp Ser Gly Thr Tyr Ala Cys Arg Ala
Arg Asn625 630 635 640Val Tyr Thr Gly Glu Glu Ile Leu Gln Lys Lys
Glu Ile Thr Ile Arg 645 650 655Asp Gln Glu Ala Pro Tyr Leu Leu Arg
Asn Leu Ser Asp His Thr Val 660 665 670Ala Ile Ser Ser Ser Thr Thr
Leu Asp Cys His Ala Asn Gly Val Pro 675 680 685Glu Pro Gln Ile Thr
Trp Phe Lys Asn Asn His Lys Ile Gln Gln Glu 690 695 700Pro Gly Ile
Ile Leu Gly Pro Gly Ser Ser Thr Leu Phe Ile Glu Arg705 710 715
720Val Thr Glu Glu Asp Glu Gly Val Tyr His Cys Lys Ala Thr Asn Gln
725 730 735Lys Gly Ser Val Glu Ser Ser Ala Tyr Leu Thr Val Gln Gly
Thr Ser 740 745 750Asp Lys Ser Asn Leu Glu Leu Ile Thr Leu Thr Cys
Thr Cys Val Ala 755 760 765Ala Thr Leu Phe Trp Leu Leu Leu Thr Leu
Phe Ile Arg Lys Met Lys 770 775 780Arg Ser Ser Ser Glu Ile Lys Thr
Asp Tyr Leu Ser Ile Ile Met Asp785 790 795 800Pro Asp Glu Val Pro
Leu Asp Glu Gln Cys Glu Arg Leu Pro Tyr Asp 805 810 815Ala Ser Lys
Trp Glu Phe Ala Arg Glu Arg Leu Lys Leu Gly Lys Ser 820 825 830Leu
Gly Arg Gly Ala Phe Gly Lys Val Val Gln Ala Ser Ala Phe Gly 835 840
845Ile Lys Lys Ser Pro Thr Cys Arg Thr Val Ala Val Lys Met Leu Lys
850 855 860Glu Gly Ala Thr Ala Ser Glu Tyr Lys Ala Leu Met Thr Glu
Leu Lys865 870 875 880Ile Leu Thr His Ile Gly His His Leu Asn Val
Val Asn Leu Leu Gly 885 890 895Ala Cys Thr Lys Gln Gly Gly Pro Leu
Met Val Ile Val Glu Tyr Cys 900 905 910Lys Tyr Gly Asn Leu Ser Asn
Tyr Leu Lys Ser Lys Arg Asp Leu Phe 915 920 925Phe Leu Asn Lys Asp
Ala Ala Leu His Met Glu Pro Lys Lys Glu Lys 930 935 940Met Glu Pro
Gly Leu Glu Gln Gly Lys Lys Pro Arg Leu Asp Ser Val945 950 955
960Thr Ser Ser Glu Ser Phe Ala Ser Ser Gly Phe Gln Glu Asp Lys Ser
965 970 975Leu Ser Asp Val Glu Glu Glu Glu Asp Ser Asp Gly Phe Tyr
Lys Glu 980 985 990Pro Ile Thr Met Glu Asp Leu Ile Ser Tyr Ser Phe
Gln Val Ala Arg 995 1000 1005Gly Met Glu Phe Leu Ser Ser Arg Lys
Cys Ile His Arg Asp Leu 1010 1015 1020Ala Ala Arg Asn Ile Leu Leu
Ser Glu Asn Asn Val Val Lys Ile 1025 1030 1035Cys Asp Phe Gly Leu
Ala Arg Asp Ile Tyr Lys Asn Pro Asp Tyr 1040 1045 1050Val Arg Lys
Gly Asp Thr Arg Leu Pro Leu Lys Trp Met Ala Pro 1055 1060 1065Glu
Ser Ile Phe Asp Lys Ile Tyr Ser Thr Lys Ser Asp Val Trp 1070 1075
1080Ser Tyr Gly Val Leu Leu Trp Glu Ile Phe Ser Leu Gly Gly Ser
1085 1090 1095Pro Tyr Pro Gly Val Gln Met Asp Glu Asp Phe Cys Ser
Arg Leu 1100 1105 1110Arg Glu Gly Met Arg Met Arg Ala Pro Glu Tyr
Ser Thr Pro Glu 1115 1120 1125Ile Tyr Gln Ile Met Leu Asp Cys Trp
His Arg Asp Pro Lys Glu 1130 1135 1140Arg Pro Arg Phe Ala Glu Leu
Val Glu Lys Leu Gly Asp Leu Leu 1145 1150 1155Gln Ala Asn Val Gln
Gln Asp Gly Lys Asp Tyr Ile Pro Ile Asn 1160 1165 1170Ala Ile Leu
Thr Gly Asn Ser Gly Phe Thr Tyr Ser Thr Pro Ala 1175 1180 1185Phe
Ser Glu Asp Phe Phe Lys Glu Ser Ile Ser Ala Pro Lys Phe 1190 1195
1200Asn Ser Gly Ser Ser Asp Asp Val Arg Tyr Val Asn Ala Phe Lys
1205 1210 1215Phe Met Ser Leu Glu Arg Ile Lys Thr Phe Glu Glu Leu
Leu Pro 1220 1225 1230Asn Ala Thr Ser Met Phe Asp Asp Tyr Gln Gly
Asp Ser Ser Thr 1235 1240 1245Leu Leu Ala Ser Pro Met Leu Lys Arg
Phe Thr Trp Thr Asp Ser 1250 1255 1260Lys Pro Lys Ala Ser Leu Lys
Ile Asp Leu Arg Val Thr Ser Lys 1265 1270 1275Ser Lys Glu Ser Gly
Leu Ser Asp Val Ser Arg Pro Ser Phe Cys 1280 1285 1290His Ser Ser
Cys Gly His Val Ser Glu Gly Lys Arg Arg Phe Thr 1295 1300 1305Tyr
Asp His Ala Glu Leu Glu Arg Lys Ile Ala Cys Cys Ser Pro 1310 1315
1320Pro Pro Asp Tyr Asn Ser Val Val Leu Tyr Ser Thr Pro Pro Ile
1325 1330 1335205830DNAHomo sapiens 20actgagtccc gggaccccgg
gagagcggtc agtgtgtggt cgctgcgttt cctctgcctg 60cgccgggcat cacttgcgcg
ccgcagaaag tccgtctggc agcctggata tcctctccta 120ccggcacccg
cagacgcccc tgcagccgcc ggtcggcgcc cgggctccct agccctgtgc
180gctcaactgt cctgcgctgc ggggtgccgc gagttccacc tccgcgcctc
cttctctaga 240caggcgctgg gagaaagaac cggctcccga gttctgggca
tttcgcccgg ctcgaggtgc 300aggatgcaga gcaaggtgct gctggccgtc
gccctgtggc tctgcgtgga gacccgggcc 360gcctctgtgg gtttgcctag
tgtttctctt gatctgccca ggctcagcat acaaaaagac 420atacttacaa
ttaaggctaa tacaactctt caaattactt gcaggggaca gagggacttg
480gactggcttt ggcccaataa tcagagtggc agtgagcaaa gggtggaggt
gactgagtgc 540agcgatggcc tcttctgtaa gacactcaca attccaaaag
tgatcggaaa tgacactgga 600gcctacaagt gcttctaccg ggaaactgac
ttggcctcgg tcatttatgt ctatgttcaa 660gattacagat ctccatttat
tgcttctgtt agtgaccaac atggagtcgt gtacattact 720gagaacaaaa
acaaaactgt ggtgattcca tgtctcgggt ccatttcaaa tctcaacgtg
780tcactttgtg caagataccc agaaaagaga tttgttcctg atggtaacag
aatttcctgg 840gacagcaaga agggctttac tattcccagc tacatgatca
gctatgctgg catggtcttc 900tgtgaagcaa aaattaatga tgaaagttac
cagtctatta tgtacatagt tgtcgttgta 960gggtatagga tttatgatgt
ggttctgagt ccgtctcatg gaattgaact atctgttgga 1020gaaaagcttg
tcttaaattg tacagcaaga actgaactaa atgtggggat tgacttcaac
1080tgggaatacc cttcttcgaa gcatcagcat aagaaacttg taaaccgaga
cctaaaaacc 1140cagtctggga gtgagatgaa gaaatttttg agcaccttaa
ctatagatgg tgtaacccgg 1200agtgaccaag gattgtacac ctgtgcagca
tccagtgggc tgatgaccaa gaagaacagc 1260acatttgtca gggtccatga
aaaacctttt gttgcttttg gaagtggcat ggaatctctg 1320gtggaagcca
cggtggggga gcgtgtcaga atccctgcga agtaccttgg ttacccaccc
1380ccagaaataa aatggtataa aaatggaata ccccttgagt ccaatcacac
aattaaagcg 1440gggcatgtac tgacgattat ggaagtgagt gaaagagaca
caggaaatta cactgtcatc 1500cttaccaatc ccatttcaaa ggagaagcag
agccatgtgg tctctctggt tgtgtatgtc 1560ccaccccaga ttggtgagaa
atctctaatc tctcctgtgg attcctacca gtacggcacc 1620actcaaacgc
tgacatgtac ggtctatgcc attcctcccc cgcatcacat ccactggtat
1680tggcagttgg aggaagagtg cgccaacgag cccagccaag ctgtctcagt
gacaaaccca 1740tacccttgtg aagaatggag aagtgtggag gacttccagg
gaggaaataa aattgaagtt 1800aataaaaatc aatttgctct aattgaagga
aaaaacaaaa ctgtaagtac ccttgttatc 1860caagcggcaa atgtgtcagc
tttgtacaaa tgtgaagcgg tcaacaaagt cgggagagga 1920gagagggtga
tctccttcca cgtgaccagg ggtcctgaaa ttactttgca acctgacatg
1980cagcccactg agcaggagag cgtgtctttg tggtgcactg cagacagatc
tacgtttgag 2040aacctcacat ggtacaagct tggcccacag cctctgccaa
tccatgtggg agagttgccc 2100acacctgttt gcaagaactt ggatactctt
tggaaattga atgccaccat gttctctaat 2160agcacaaatg acattttgat
catggagctt aagaatgcat ccttgcagga ccaaggagac 2220tatgtctgcc
ttgctcaaga caggaagacc aagaaaagac attgcgtggt caggcagctc
2280acagtcctag agcgtgtggc acccacgatc acaggaaacc tggagaatca
gacgacaagt 2340attggggaaa gcatcgaagt ctcatgcacg gcatctggga
atccccctcc acagatcatg 2400tggtttaaag ataatgagac ccttgtagaa
gactcaggca ttgtattgaa ggatgggaac 2460cggaacctca ctatccgcag
agtgaggaag gaggacgaag gcctctacac ctgccaggca 2520tgcagtgttc
ttggctgtgc aaaagtggag gcatttttca taatagaagg tgcccaggaa
2580aagacgaact tggaaatcat tattctagta ggcacggcgg tgattgccat
gttcttctgg 2640ctacttcttg tcatcatcct acggaccgtt aagcgggcca
atggagggga actgaagaca 2700ggctacttgt ccatcgtcat ggatccagat
gaactcccat tggatgaaca ttgtgaacga 2760ctgccttatg atgccagcaa
atgggaattc cccagagacc ggctgaagct aggtaagcct 2820cttggccgtg
gtgcctttgg ccaagtgatt gaagcagatg cctttggaat tgacaagaca
2880gcaacttgca ggacagtagc agtcaaaatg ttgaaagaag gagcaacaca
cagtgagcat 2940cgagctctca tgtctgaact caagatcctc attcatattg
gtcaccatct caatgtggtc 3000aaccttctag gtgcctgtac caagccagga
gggccactca tggtgattgt ggaattctgc 3060aaatttggaa acctgtccac
ttacctgagg agcaagagaa atgaatttgt cccctacaag 3120accaaagggg
cacgattccg tcaagggaaa gactacgttg gagcaatccc tgtggatctg
3180aaacggcgct tggacagcat caccagtagc cagagctcag ccagctctgg
atttgtggag 3240gagaagtccc tcagtgatgt agaagaagag gaagctcctg
aagatctgta taaggacttc 3300ctgaccttgg agcatctcat ctgttacagc
ttccaagtgg ctaagggcat ggagttcttg 3360gcatcgcgaa agtgtatcca
cagggacctg gcggcacgaa atatcctctt atcggagaag 3420aacgtggtta
aaatctgtga ctttggcttg gcccgggata tttataaaga tccagattat
3480gtcagaaaag gagatgctcg cctccctttg aaatggatgg ccccagaaac
aatttttgac 3540agagtgtaca caatccagag tgacgtctgg tcttttggtg
ttttgctgtg ggaaatattt 3600tccttaggtg cttctccata tcctggggta
aagattgatg aagaattttg taggcgattg 3660aaagaaggaa ctagaatgag
ggcccctgat tatactacac cagaaatgta ccagaccatg 3720ctggactgct
ggcacgggga gcccagtcag agacccacgt tttcagagtt ggtggaacat
3780ttgggaaatc tcttgcaagc taatgctcag caggatggca aagactacat
tgttcttccg 3840atatcagaga ctttgagcat ggaagaggat tctggactct
ctctgcctac ctcacctgtt 3900tcctgtatgg aggaggagga agtatgtgac
cccaaattcc attatgacaa cacagcagga 3960atcagtcagt atctgcagaa
cagtaagcga aagagccggc ctgtgagtgt aaaaacattt 4020gaagatatcc
cgttagaaga accagaagta aaagtaatcc cagatgacaa ccagacggac
4080agtggtatgg ttcttgcctc agaagagctg aaaactttgg aagacagaac
caaattatct 4140ccatcttttg gtggaatggt gcccagcaaa agcagggagt
ctgtggcatc tgaaggctca 4200aaccagacaa gcggctacca gtccggatat
cactccgatg acacagacac caccgtgtac 4260tccagtgagg aagcagaact
tttaaagctg atagagattg gagtgcaaac cggtagcaca 4320gcccagattc
tccagcctga ctcggggacc acactgagct ctcctcctgt ttaaaaggaa
4380gcatccacac cccaactccc ggacatcaca tgagaggtct gctcagattt
tgaagtgttg 4440ttctttccac cagcaggaag tagccgcatt tgattttcat
ttcgacaaca gaaaaaggac 4500ctcggactgc agggagccag tcttctaggc
atatcctgga agaggcttgt gacccaagaa 4560tgtgtctgtg tcttctccca
gtgttgacct gatcctcttt tttcattcat ttaaaaagca 4620ttatcatgcc
cctgctgcgg gtctcaccat gggtttagaa caaagagctt caagcaatgg
4680ccccatcctc aaagaagtag cagtacctgg ggagctgaca cttctgtaaa
actagaagat
4740aaaccaggca acgtaagtgt tcgaggtgtt gaagatggga aggatttgca
gggctgagtc 4800tatccaagag gctttgttta ggacgtgggt cccaagccaa
gccttaagtg tggaattcgg 4860attgatagaa aggaagacta acgttacctt
gctttggaga gtactggagc ctgcaaatgc 4920attgtgtttg ctctggtgga
ggtgggcatg gggtctgttc tgaaatgtaa agggttcaga 4980cggggtttct
ggttttagaa ggttgcgtgt tcttcgagtt gggctaaagt agagttcgtt
5040gtgctgtttc tgactcctaa tgagagttcc ttccagaccg ttagctgtct
ccttgccaag 5100ccccaggaag aaaatgatgc agctctggct ccttgtctcc
caggctgatc ctttattcag 5160aataccacaa agaaaggaca ttcagctcaa
ggctccctgc cgtgttgaag agttctgact 5220gcacaaacca gcttctggtt
tcttctggaa tgaataccct catatctgtc ctgatgtgat 5280atgtctgaga
ctgaatgcgg gaggttcaat gtgaagctgt gtgtggtgtc aaagtttcag
5340gaaggatttt acccttttgt tcttccccct gtccccaacc cactctcacc
ccgcaaccca 5400tcagtatttt agttatttgg cctctactcc agtaaacctg
attgggtttg ttcactctct 5460gaatgattat tagccagact tcaaaattat
tttatagccc aaattataac atctattgta 5520ttatttagac ttttaacata
tagagctatt tctactgatt tttgcccttg ttctgtcctt 5580tttttcaaaa
aagaaaatgt gttttttgtt tggtaccata gtgtgaaatg ctgggaacaa
5640tgactataag acatgctatg gcacatatat ttatagtctg tttatgtaga
aacaaatgta 5700atatattaaa gccttatata taatgaactt tgtactattc
acattttgta tcagtattat 5760gtagcataac aaaggtcata atgctttcag
caattgatgt cattttatta aagaacattg 5820aaaaacttga 5830211356PRTHomo
sapiens 21Met Gln Ser Lys Val Leu Leu Ala Val Ala Leu Trp Leu Cys
Val Glu1 5 10 15Thr Arg Ala Ala Ser Val Gly Leu Pro Ser Val Ser Leu
Asp Leu Pro 20 25 30Arg Leu Ser Ile Gln Lys Asp Ile Leu Thr Ile Lys
Ala Asn Thr Thr 35 40 45Leu Gln Ile Thr Cys Arg Gly Gln Arg Asp Leu
Asp Trp Leu Trp Pro 50 55 60Asn Asn Gln Ser Gly Ser Glu Gln Arg Val
Glu Val Thr Glu Cys Ser65 70 75 80Asp Gly Leu Phe Cys Lys Thr Leu
Thr Ile Pro Lys Val Ile Gly Asn 85 90 95Asp Thr Gly Ala Tyr Lys Cys
Phe Tyr Arg Glu Thr Asp Leu Ala Ser 100 105 110Val Ile Tyr Val Tyr
Val Gln Asp Tyr Arg Ser Pro Phe Ile Ala Ser 115 120 125Val Ser Asp
Gln His Gly Val Val Tyr Ile Thr Glu Asn Lys Asn Lys 130 135 140Thr
Val Val Ile Pro Cys Leu Gly Ser Ile Ser Asn Leu Asn Val Ser145 150
155 160Leu Cys Ala Arg Tyr Pro Glu Lys Arg Phe Val Pro Asp Gly Asn
Arg 165 170 175Ile Ser Trp Asp Ser Lys Lys Gly Phe Thr Ile Pro Ser
Tyr Met Ile 180 185 190Ser Tyr Ala Gly Met Val Phe Cys Glu Ala Lys
Ile Asn Asp Glu Ser 195 200 205Tyr Gln Ser Ile Met Tyr Ile Val Val
Val Val Gly Tyr Arg Ile Tyr 210 215 220Asp Val Val Leu Ser Pro Ser
His Gly Ile Glu Leu Ser Val Gly Glu225 230 235 240Lys Leu Val Leu
Asn Cys Thr Ala Arg Thr Glu Leu Asn Val Gly Ile 245 250 255Asp Phe
Asn Trp Glu Tyr Pro Ser Ser Lys His Gln His Lys Lys Leu 260 265
270Val Asn Arg Asp Leu Lys Thr Gln Ser Gly Ser Glu Met Lys Lys Phe
275 280 285Leu Ser Thr Leu Thr Ile Asp Gly Val Thr Arg Ser Asp Gln
Gly Leu 290 295 300Tyr Thr Cys Ala Ala Ser Ser Gly Leu Met Thr Lys
Lys Asn Ser Thr305 310 315 320Phe Val Arg Val His Glu Lys Pro Phe
Val Ala Phe Gly Ser Gly Met 325 330 335Glu Ser Leu Val Glu Ala Thr
Val Gly Glu Arg Val Arg Ile Pro Ala 340 345 350Lys Tyr Leu Gly Tyr
Pro Pro Pro Glu Ile Lys Trp Tyr Lys Asn Gly 355 360 365Ile Pro Leu
Glu Ser Asn His Thr Ile Lys Ala Gly His Val Leu Thr 370 375 380Ile
Met Glu Val Ser Glu Arg Asp Thr Gly Asn Tyr Thr Val Ile Leu385 390
395 400Thr Asn Pro Ile Ser Lys Glu Lys Gln Ser His Val Val Ser Leu
Val 405 410 415Val Tyr Val Pro Pro Gln Ile Gly Glu Lys Ser Leu Ile
Ser Pro Val 420 425 430Asp Ser Tyr Gln Tyr Gly Thr Thr Gln Thr Leu
Thr Cys Thr Val Tyr 435 440 445Ala Ile Pro Pro Pro His His Ile His
Trp Tyr Trp Gln Leu Glu Glu 450 455 460Glu Cys Ala Asn Glu Pro Ser
Gln Ala Val Ser Val Thr Asn Pro Tyr465 470 475 480Pro Cys Glu Glu
Trp Arg Ser Val Glu Asp Phe Gln Gly Gly Asn Lys 485 490 495Ile Glu
Val Asn Lys Asn Gln Phe Ala Leu Ile Glu Gly Lys Asn Lys 500 505
510Thr Val Ser Thr Leu Val Ile Gln Ala Ala Asn Val Ser Ala Leu Tyr
515 520 525Lys Cys Glu Ala Val Asn Lys Val Gly Arg Gly Glu Arg Val
Ile Ser 530 535 540Phe His Val Thr Arg Gly Pro Glu Ile Thr Leu Gln
Pro Asp Met Gln545 550 555 560Pro Thr Glu Gln Glu Ser Val Ser Leu
Trp Cys Thr Ala Asp Arg Ser 565 570 575Thr Phe Glu Asn Leu Thr Trp
Tyr Lys Leu Gly Pro Gln Pro Leu Pro 580 585 590Ile His Val Gly Glu
Leu Pro Thr Pro Val Cys Lys Asn Leu Asp Thr 595 600 605Leu Trp Lys
Leu Asn Ala Thr Met Phe Ser Asn Ser Thr Asn Asp Ile 610 615 620Leu
Ile Met Glu Leu Lys Asn Ala Ser Leu Gln Asp Gln Gly Asp Tyr625 630
635 640Val Cys Leu Ala Gln Asp Arg Lys Thr Lys Lys Arg His Cys Val
Val 645 650 655Arg Gln Leu Thr Val Leu Glu Arg Val Ala Pro Thr Ile
Thr Gly Asn 660 665 670Leu Glu Asn Gln Thr Thr Ser Ile Gly Glu Ser
Ile Glu Val Ser Cys 675 680 685Thr Ala Ser Gly Asn Pro Pro Pro Gln
Ile Met Trp Phe Lys Asp Asn 690 695 700Glu Thr Leu Val Glu Asp Ser
Gly Ile Val Leu Lys Asp Gly Asn Arg705 710 715 720Asn Leu Thr Ile
Arg Arg Val Arg Lys Glu Asp Glu Gly Leu Tyr Thr 725 730 735Cys Gln
Ala Cys Ser Val Leu Gly Cys Ala Lys Val Glu Ala Phe Phe 740 745
750Ile Ile Glu Gly Ala Gln Glu Lys Thr Asn Leu Glu Ile Ile Ile Leu
755 760 765Val Gly Thr Ala Val Ile Ala Met Phe Phe Trp Leu Leu Leu
Val Ile 770 775 780Ile Leu Arg Thr Val Lys Arg Ala Asn Gly Gly Glu
Leu Lys Thr Gly785 790 795 800Tyr Leu Ser Ile Val Met Asp Pro Asp
Glu Leu Pro Leu Asp Glu His 805 810 815Cys Glu Arg Leu Pro Tyr Asp
Ala Ser Lys Trp Glu Phe Pro Arg Asp 820 825 830Arg Leu Lys Leu Gly
Lys Pro Leu Gly Arg Gly Ala Phe Gly Gln Val 835 840 845Ile Glu Ala
Asp Ala Phe Gly Ile Asp Lys Thr Ala Thr Cys Arg Thr 850 855 860Val
Ala Val Lys Met Leu Lys Glu Gly Ala Thr His Ser Glu His Arg865 870
875 880Ala Leu Met Ser Glu Leu Lys Ile Leu Ile His Ile Gly His His
Leu 885 890 895Asn Val Val Asn Leu Leu Gly Ala Cys Thr Lys Pro Gly
Gly Pro Leu 900 905 910Met Val Ile Val Glu Phe Cys Lys Phe Gly Asn
Leu Ser Thr Tyr Leu 915 920 925Arg Ser Lys Arg Asn Glu Phe Val Pro
Tyr Lys Thr Lys Gly Ala Arg 930 935 940Phe Arg Gln Gly Lys Asp Tyr
Val Gly Ala Ile Pro Val Asp Leu Lys945 950 955 960Arg Arg Leu Asp
Ser Ile Thr Ser Ser Gln Ser Ser Ala Ser Ser Gly 965 970 975Phe Val
Glu Glu Lys Ser Leu Ser Asp Val Glu Glu Glu Glu Ala Pro 980 985
990Glu Asp Leu Tyr Lys Asp Phe Leu Thr Leu Glu His Leu Ile Cys Tyr
995 1000 1005Ser Phe Gln Val Ala Lys Gly Met Glu Phe Leu Ala Ser
Arg Lys 1010 1015 1020Cys Ile His Arg Asp Leu Ala Ala Arg Asn Ile
Leu Leu Ser Glu 1025 1030 1035Lys Asn Val Val Lys Ile Cys Asp Phe
Gly Leu Ala Arg Asp Ile 1040 1045 1050Tyr Lys Asp Pro Asp Tyr Val
Arg Lys Gly Asp Ala Arg Leu Pro 1055 1060 1065Leu Lys Trp Met Ala
Pro Glu Thr Ile Phe Asp Arg Val Tyr Thr 1070 1075 1080Ile Gln Ser
Asp Val Trp Ser Phe Gly Val Leu Leu Trp Glu Ile 1085 1090 1095Phe
Ser Leu Gly Ala Ser Pro Tyr Pro Gly Val Lys Ile Asp Glu 1100 1105
1110Glu Phe Cys Arg Arg Leu Lys Glu Gly Thr Arg Met Arg Ala Pro
1115 1120 1125Asp Tyr Thr Thr Pro Glu Met Tyr Gln Thr Met Leu Asp
Cys Trp 1130 1135 1140His Gly Glu Pro Ser Gln Arg Pro Thr Phe Ser
Glu Leu Val Glu 1145 1150 1155His Leu Gly Asn Leu Leu Gln Ala Asn
Ala Gln Gln Asp Gly Lys 1160 1165 1170Asp Tyr Ile Val Leu Pro Ile
Ser Glu Thr Leu Ser Met Glu Glu 1175 1180 1185Asp Ser Gly Leu Ser
Leu Pro Thr Ser Pro Val Ser Cys Met Glu 1190 1195 1200Glu Glu Glu
Val Cys Asp Pro Lys Phe His Tyr Asp Asn Thr Ala 1205 1210 1215Gly
Ile Ser Gln Tyr Leu Gln Asn Ser Lys Arg Lys Ser Arg Pro 1220 1225
1230Val Ser Val Lys Thr Phe Glu Asp Ile Pro Leu Glu Glu Pro Glu
1235 1240 1245Val Lys Val Ile Pro Asp Asp Asn Gln Thr Asp Ser Gly
Met Val 1250 1255 1260Leu Ala Ser Glu Glu Leu Lys Thr Leu Glu Asp
Arg Thr Lys Leu 1265 1270 1275Ser Pro Ser Phe Gly Gly Met Val Pro
Ser Lys Ser Arg Glu Ser 1280 1285 1290Val Ala Ser Glu Gly Ser Asn
Gln Thr Ser Gly Tyr Gln Ser Gly 1295 1300 1305Tyr His Ser Asp Asp
Thr Asp Thr Thr Val Tyr Ser Ser Glu Glu 1310 1315 1320Ala Glu Leu
Leu Lys Leu Ile Glu Ile Gly Val Gln Thr Gly Ser 1325 1330 1335Thr
Ala Gln Ile Leu Gln Pro Asp Ser Gly Thr Thr Leu Ser Ser 1340 1345
1350Pro Pro Val 13552216PRTHomo sapiens 22Cys Asn Asp Glu Gly Leu
Glu Cys Val Pro Thr Glu Glu Ser Asn Ile1 5 10 152316PRTHomo sapiens
23Cys Pro Asp Asp Gly Leu Glu Cys Val Pro Thr Gly Gln His Gln Val1
5 10 15241676PRTHomo sapiens 24Met Gly Leu Leu Gly Ile Leu Cys Phe
Leu Ile Phe Leu Gly Lys Thr1 5 10 15Trp Gly Gln Glu Gln Thr Tyr Val
Ile Ser Ala Pro Lys Ile Phe Arg 20 25 30Val Gly Ala Ser Glu Asn Ile
Val Ile Gln Val Tyr Gly Tyr Thr Glu 35 40 45Ala Phe Asp Ala Thr Ile
Ser Ile Lys Ser Tyr Pro Asp Lys Lys Phe 50 55 60Ser Tyr Ser Ser Gly
His Val His Leu Ser Ser Glu Asn Lys Phe Gln65 70 75 80Asn Ser Ala
Ile Leu Thr Ile Gln Pro Lys Gln Leu Pro Gly Gly Gln 85 90 95Asn Pro
Val Ser Tyr Val Tyr Leu Glu Val Val Ser Lys His Phe Ser 100 105
110Lys Ser Lys Arg Met Pro Ile Thr Tyr Asp Asn Gly Phe Leu Phe Ile
115 120 125His Thr Asp Lys Pro Val Tyr Thr Pro Asp Gln Ser Val Lys
Val Arg 130 135 140Val Tyr Ser Leu Asn Asp Asp Leu Lys Pro Ala Lys
Arg Glu Thr Val145 150 155 160Leu Thr Phe Ile Asp Pro Glu Gly Ser
Glu Val Asp Met Val Glu Glu 165 170 175Ile Asp His Ile Gly Ile Ile
Ser Phe Pro Asp Phe Lys Ile Pro Ser 180 185 190Asn Pro Arg Tyr Gly
Met Trp Thr Ile Lys Ala Lys Tyr Lys Glu Asp 195 200 205Phe Ser Thr
Thr Gly Thr Ala Tyr Phe Glu Val Lys Glu Tyr Val Leu 210 215 220Pro
His Phe Ser Val Ser Ile Glu Pro Glu Tyr Asn Phe Ile Gly Tyr225 230
235 240Lys Asn Phe Lys Asn Phe Glu Ile Thr Ile Lys Ala Arg Tyr Phe
Tyr 245 250 255Asn Lys Val Val Thr Glu Ala Asp Val Tyr Ile Thr Phe
Gly Ile Arg 260 265 270Glu Asp Leu Lys Asp Asp Gln Lys Glu Met Met
Gln Thr Ala Met Gln 275 280 285Asn Thr Met Leu Ile Asn Gly Ile Ala
Gln Val Thr Phe Asp Ser Glu 290 295 300Thr Ala Val Lys Glu Leu Ser
Tyr Tyr Ser Leu Glu Asp Leu Asn Asn305 310 315 320Lys Tyr Leu Tyr
Ile Ala Val Thr Val Ile Glu Ser Thr Gly Gly Phe 325 330 335Ser Glu
Glu Ala Glu Ile Pro Gly Ile Lys Tyr Val Leu Ser Pro Tyr 340 345
350Lys Leu Asn Leu Val Ala Thr Pro Leu Phe Leu Lys Pro Gly Ile Pro
355 360 365Tyr Pro Ile Lys Val Gln Val Lys Asp Ser Leu Asp Gln Leu
Val Gly 370 375 380Gly Val Pro Val Thr Leu Asn Ala Gln Thr Ile Asp
Val Asn Gln Glu385 390 395 400Thr Ser Asp Leu Asp Pro Ser Lys Ser
Val Thr Arg Val Asp Asp Gly 405 410 415Val Ala Ser Phe Val Leu Asn
Leu Pro Ser Gly Val Thr Val Leu Glu 420 425 430Phe Asn Val Lys Thr
Asp Ala Pro Asp Leu Pro Glu Glu Asn Gln Ala 435 440 445Arg Glu Gly
Tyr Arg Ala Ile Ala Tyr Ser Ser Leu Ser Gln Ser Tyr 450 455 460Leu
Tyr Ile Asp Trp Thr Asp Asn His Lys Ala Leu Leu Val Gly Glu465 470
475 480His Leu Asn Ile Ile Val Thr Pro Lys Ser Pro Tyr Ile Asp Lys
Ile 485 490 495Thr His Tyr Asn Tyr Leu Ile Leu Ser Lys Gly Lys Ile
Ile His Phe 500 505 510Gly Thr Arg Glu Lys Phe Ser Asp Ala Ser Tyr
Gln Ser Ile Asn Ile 515 520 525Pro Val Thr Gln Asn Met Val Pro Ser
Ser Arg Leu Leu Val Tyr Tyr 530 535 540Ile Val Thr Gly Glu Gln Thr
Ala Glu Leu Val Ser Asp Ser Val Trp545 550 555 560Leu Asn Ile Glu
Glu Lys Cys Gly Asn Gln Leu Gln Val His Leu Ser 565 570 575Pro Asp
Ala Asp Ala Tyr Ser Pro Gly Gln Thr Val Ser Leu Asn Met 580 585
590Ala Thr Gly Met Asp Ser Trp Val Ala Leu Ala Ala Val Asp Ser Ala
595 600 605Val Tyr Gly Val Gln Arg Gly Ala Lys Lys Pro Leu Glu Arg
Val Phe 610 615 620Gln Phe Leu Glu Lys Ser Asp Leu Gly Cys Gly Ala
Gly Gly Gly Leu625 630 635 640Asn Asn Ala Asn Val Phe His Leu Ala
Gly Leu Thr Phe Leu Thr Asn 645 650 655Ala Asn Ala Asp Asp Ser Gln
Glu Asn Asp Glu Pro Cys Lys Glu Ile 660 665 670Leu Arg Pro Arg Arg
Thr Leu Gln Lys Lys Ile Glu Glu Ile Ala Ala 675 680 685Lys Tyr Lys
His Ser Val Val Lys Lys Cys Cys Tyr Asp Gly Ala Cys 690 695 700Val
Asn Asn Asp Glu Thr Cys Glu Gln Arg Ala Ala Arg Ile Ser Leu705 710
715 720Gly Pro Arg Cys Ile Lys Ala Phe Thr Glu Cys Cys Val Val Ala
Ser 725 730 735Gln Leu Arg Ala Asn Ile Ser His Lys Asp Met Gln Leu
Gly Arg Leu 740 745 750His Met Lys Thr Leu Leu Pro Val Ser Lys Pro
Glu Ile Arg Ser Tyr 755 760 765Phe Pro Glu Ser Trp Leu Trp Glu Val
His Leu Val Pro Arg Arg Lys 770 775 780Gln Leu Gln Phe Ala Leu Pro
Asp Ser Leu Thr Thr Trp Glu Ile Gln785 790 795 800Gly Val Gly Ile
Ser Asn Thr Gly Ile Cys Val Ala Asp Thr Val Lys 805 810 815Ala Lys
Val Phe Lys Asp Val Phe Leu Glu Met Asn Ile Pro Tyr Ser 820 825
830Val Val Arg Gly Glu Gln Ile Gln Leu Lys Gly Thr Val Tyr Asn Tyr
835 840 845Arg Thr Ser Gly Met Gln Phe Cys Val Lys Met Ser Ala Val
Glu Gly 850 855 860Ile Cys Thr Ser Glu Ser Pro Val Ile Asp His Gln
Gly Thr Lys Ser865 870 875
880Ser Lys Cys Val Arg Gln Lys Val Glu Gly Ser Ser Ser His Leu Val
885 890 895Thr Phe Thr Val Leu Pro Leu Glu Ile Gly Leu His Asn Ile
Asn Phe 900 905 910Ser Leu Glu Thr Trp Phe Gly Lys Glu Ile Leu Val
Lys Thr Leu Arg 915 920 925Val Val Pro Glu Gly Val Lys Arg Glu Ser
Tyr Ser Gly Val Thr Leu 930 935 940Asp Pro Arg Gly Ile Tyr Gly Thr
Ile Ser Arg Arg Lys Glu Phe Pro945 950 955 960Tyr Arg Ile Pro Leu
Asp Leu Val Pro Lys Thr Glu Ile Lys Arg Ile 965 970 975Leu Ser Val
Lys Gly Leu Leu Val Gly Glu Ile Leu Ser Ala Val Leu 980 985 990Ser
Gln Glu Gly Ile Asn Ile Leu Thr His Leu Pro Lys Gly Ser Ala 995
1000 1005Glu Ala Glu Leu Met Ser Val Val Pro Val Phe Tyr Val Phe
His 1010 1015 1020Tyr Leu Glu Thr Gly Asn His Trp Asn Ile Phe His
Ser Asp Pro 1025 1030 1035Leu Ile Glu Lys Gln Lys Leu Lys Lys Lys
Leu Lys Glu Gly Met 1040 1045 1050Leu Ser Ile Met Ser Tyr Arg Asn
Ala Asp Tyr Ser Tyr Ser Val 1055 1060 1065Trp Lys Gly Gly Ser Ala
Ser Thr Trp Leu Thr Ala Phe Ala Leu 1070 1075 1080Arg Val Leu Gly
Gln Val Asn Lys Tyr Val Glu Gln Asn Gln Asn 1085 1090 1095Ser Ile
Cys Asn Ser Leu Leu Trp Leu Val Glu Asn Tyr Gln Leu 1100 1105
1110Asp Asn Gly Ser Phe Lys Glu Asn Ser Gln Tyr Gln Pro Ile Lys
1115 1120 1125Leu Gln Gly Thr Leu Pro Val Glu Ala Arg Glu Asn Ser
Leu Tyr 1130 1135 1140Leu Thr Ala Phe Thr Val Ile Gly Ile Arg Lys
Ala Phe Asp Ile 1145 1150 1155Cys Pro Leu Val Lys Ile Asp Thr Ala
Leu Ile Lys Ala Asp Asn 1160 1165 1170Phe Leu Leu Glu Asn Thr Leu
Pro Ala Gln Ser Thr Phe Thr Leu 1175 1180 1185Ala Ile Ser Ala Tyr
Ala Leu Ser Leu Gly Asp Lys Thr His Pro 1190 1195 1200Gln Phe Arg
Ser Ile Val Ser Ala Leu Lys Arg Glu Ala Leu Val 1205 1210 1215Lys
Gly Asn Pro Pro Ile Tyr Arg Phe Trp Lys Asp Asn Leu Gln 1220 1225
1230His Lys Asp Ser Ser Val Pro Asn Thr Gly Thr Ala Arg Met Val
1235 1240 1245Glu Thr Thr Ala Tyr Ala Leu Leu Thr Ser Leu Asn Leu
Lys Asp 1250 1255 1260Ile Asn Tyr Val Asn Pro Val Ile Lys Trp Leu
Ser Glu Glu Gln 1265 1270 1275Arg Tyr Gly Gly Gly Phe Tyr Ser Thr
Gln Asp Thr Ile Asn Ala 1280 1285 1290Ile Glu Gly Leu Thr Glu Tyr
Ser Leu Leu Val Lys Gln Leu Arg 1295 1300 1305Leu Ser Met Asp Ile
Asp Val Ser Tyr Lys His Lys Gly Ala Leu 1310 1315 1320His Asn Tyr
Lys Met Thr Asp Lys Asn Phe Leu Gly Arg Pro Val 1325 1330 1335Glu
Val Leu Leu Asn Asp Asp Leu Ile Val Ser Thr Gly Phe Gly 1340 1345
1350Ser Gly Leu Ala Thr Val His Val Thr Thr Val Val His Lys Thr
1355 1360 1365Ser Thr Ser Glu Glu Val Cys Ser Phe Tyr Leu Lys Ile
Asp Thr 1370 1375 1380Gln Asp Ile Glu Ala Ser His Tyr Arg Gly Tyr
Gly Asn Ser Asp 1385 1390 1395Tyr Lys Arg Ile Val Ala Cys Ala Ser
Tyr Lys Pro Ser Arg Glu 1400 1405 1410Glu Ser Ser Ser Gly Ser Ser
His Ala Val Met Asp Ile Ser Leu 1415 1420 1425Pro Thr Gly Ile Ser
Ala Asn Glu Glu Asp Leu Lys Ala Leu Val 1430 1435 1440Glu Gly Val
Asp Gln Leu Phe Thr Asp Tyr Gln Ile Lys Asp Gly 1445 1450 1455His
Val Ile Leu Gln Leu Asn Ser Ile Pro Ser Ser Asp Phe Leu 1460 1465
1470Cys Val Arg Phe Arg Ile Phe Glu Leu Phe Glu Val Gly Phe Leu
1475 1480 1485Ser Pro Ala Thr Phe Thr Val Tyr Glu Tyr His Arg Pro
Asp Lys 1490 1495 1500Gln Cys Thr Met Phe Tyr Ser Thr Ser Asn Ile
Lys Ile Gln Lys 1505 1510 1515Val Cys Glu Gly Ala Ala Cys Lys Cys
Val Glu Ala Asp Cys Gly 1520 1525 1530Gln Met Gln Glu Glu Leu Asp
Leu Thr Ile Ser Ala Glu Thr Arg 1535 1540 1545Lys Gln Thr Ala Cys
Lys Pro Glu Ile Ala Tyr Ala Tyr Lys Val 1550 1555 1560Ser Ile Thr
Ser Ile Thr Val Glu Asn Val Phe Val Lys Tyr Lys 1565 1570 1575Ala
Thr Leu Leu Asp Ile Tyr Lys Thr Gly Glu Ala Val Ala Glu 1580 1585
1590Lys Asp Ser Glu Ile Thr Phe Ile Lys Lys Val Thr Cys Thr Asn
1595 1600 1605Ala Glu Leu Val Lys Gly Arg Gln Tyr Leu Ile Met Gly
Lys Glu 1610 1615 1620Ala Leu Gln Ile Lys Tyr Asn Phe Ser Phe Arg
Tyr Ile Tyr Pro 1625 1630 1635Leu Asp Ser Leu Thr Trp Ile Glu Tyr
Trp Pro Arg Asp Thr Thr 1640 1645 1650Cys Ser Ser Cys Gln Ala Phe
Leu Ala Asn Leu Asp Glu Phe Ala 1655 1660 1665Glu Asp Ile Phe Leu
Asn Gly Cys 1670 16752542RNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'OH-guanosinemisc_feature(2)..(2)May be
2'OH-adenosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'OH-guanosinemisc_feature(5)..(5)May be
2'OH-adenosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxyuridinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'OH-guanosinemisc_feature(10)..(10)May be
2'OH-guanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxycytidinemisc_feature(15)..(15)May be
2'OH-adenosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxyuridinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxyuridinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxycytidinemisc_feature(22)..(22)May be
2'OH-guanosinemisc_feature(23)..(23)May be
2'OH-adenosinemisc_feature(24)..(24)May be
2'OH-guanosinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'OH-guanosinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxyuridinemisc_feature(28)..(28)May be
2'OH-guanosinemisc_feature(29)..(29)May be
2'OH-adenosinemisc_feature(30)..(30)May be
2'OH-guanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxyuridinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'OH-guanosinemisc_feature(41)..(41)May be
2'-fluoro-2'-deoxyuridinemisc_feature(42)..(42)May be
2'-fluoro-2'-deoxycytidine 25gacgaugcgg ucucaugcgu cgagugugag
uuuaccuucg uc 422638DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'OH-adenosinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxycytidinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxyuridinemisc_feature(35)..(35)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(38)..(38)May be an
inverted orientation T (3'-3'-linked) 26cgccgcgguc ucaggcgcug
agucugaguu accugcgt 382744RNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'OH-adenosinemisc_feature(2)..(2)May be
2'OH-guanosinemisc_feature(3)..(3)May be
2'OH-guanosinemisc_feature(4)..(4)May be
2'OH-adenosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'OH-guanosinemisc_feature(7)..(7)May be
2'OH-adenosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxyuridinemisc_feature(9)..(9)May be
2'OH-guanosinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'OH-guanosinemisc_feature(12)..(12)May be
2'OH-guanosinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxycytidinemisc_feature(15)..(15)May be
2'-fluoro-2'-deoxyuridinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-adenosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxyuridinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'OH-guanosinemisc_feature(22)..(22)May be
2'-fluoro-2'-deoxyuridinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxycytidinemisc_feature(24)..(24)May be
2'OH-guanosinemisc_feature(25)..(25)May be
2'OH-adenosinemisc_feature(26)..(26)May be
2'OH-guanosinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxyuridinemisc_feature(28)..(28)May be
2'OH-guanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'OH-guanosinemisc_feature(31)..(31)May be
2'OH-adenosinemisc_feature(32)..(32)May be
2'OH-guanosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxyuridinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'OH-adenosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxycytidinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxyuridinemisc_feature(40)..(40)May be
2'-fluoro-2'-deoxyuridinemisc_feature(41)..(41)May be
2'-fluoro-2'-deoxycytidinemisc_feature(42)..(42)May be
2'OH-guanosinemisc_feature(43)..(43)May be
2'-fluoro-2'-deoxyuridinemisc_feature(44)..(44)May be
2'-fluoro-2'-deoxycytidine 27aggacgaugc ggucucaugc gucgagugug
aguuuaccuu cguc 442840RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'OH-adenosinemisc_feature(2)..(2)May be
2'OH-guanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxycytidinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxyuridinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosine 28agcgccgcgg ucucaggcgc ugagucugag
uuuaccugcg 402946RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'OH-guanosinemisc_feature(2)..(2)May be
2'OH-guanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'OH-guanosinemisc_feature(5)..(5)May be
2'OH-adenosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxyuridinemisc_feature(7)..(7)May be
2'-fluoro-2'-deoxyuridinemisc_feature(8)..(8)May be
2'OH-adenosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxycytidinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxyuridinemisc_feature(11)..(11)May be
2'OH-guanosinemisc_feature(12)..(12)May be
2'OH-guanosinemisc_feature(13)..(13)May be
2'OH-guanosinemisc_feature(14)..(14)May be
2'OH-adenosinemisc_feature(15)..(15)May be
2'-fluoro-2'-deoxycytidinemisc_feature(16)..(16)May be
2'OH-guanosinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'OH-adenosinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxycytidinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxyuridinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxycytidinemisc_feature(22)..(22)May be
2'OH-guanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxycytidinemisc_feature(24)..(24)May be
2'OH-guanosinemisc_feature(25)..(25)May be
2'OH-adenosinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxyuridinemisc_feature(27)..(27)May be
2'OH-guanosinemisc_feature(28)..(28)May be
2'-fluoro-2'-deoxyuridinemisc_feature(29)..(29)May be
2'OH-guanosinemisc_feature(30)..(30)May be
2'OH-adenosinemisc_feature(31)..(31)May be
2'OH-guanosinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxycytidinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'OH-adenosinemisc_feature(36)..(36)May be
2'OH-guanosinemisc_feature(37)..(37)May be
2'OH-adenosinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxycytidinemisc_feature(39)..(39)May be
2'OH-guanosinemisc_feature(40)..(40)May be
2'OH-adenosinemisc_feature(41)..(41)May be
2'-fluoro-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-fluoro-2'-deoxyuridinemisc_feature(43)..(43)May be
2'-fluoro-2'-deoxycytidinemisc_feature(44)..(44)May be
2'OH-guanosinemisc_feature(45)..(45)May be
2'-fluoro-2'-deoxycytidinemisc_feature(46)..(46)May be
2'-fluoro-2'-deoxycytidine 29ggcgauuacu gggacggacu cgcgauguga
gcccagacga cucgcc 463040RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'OH-guanosinemisc_feature(2)..(2)May be
2'OH-guanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxyuridinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxyuridinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-fluoro-2'-deoxyuridinemisc_feature(8)..(8)May be
2'OH-guanosinemisc_feature(9)..(9)May be
2'OH-adenosinemisc_feature(10)..(10)May be
2'OH-adenosinemisc_feature(11)..(11)May be
2'OH-guanosinemisc_feature(12)..(12)May be
2'OH-adenosinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxyuridinemisc_feature(15)..(15)May be
2'OH-adenosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxyuridinemisc_feature(17)..(17)May be
2'-fluoro-2'-deoxyuridinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxyuridinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxycytidinemisc_feature(20)..(20)May be
2'OH-guanosinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxycytidinemisc_feature(22)..(22)May be
2'OH-guanosinemisc_feature(23)..(23)May be
2'OH-adenosinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxyuridinemisc_feature(25)..(25)May be
2'OH-guanosinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxyuridinemisc_feature(27)..(27)May be
2'OH-guanosinemisc_feature(28)..(28)May be
2'OH-adenosinemisc_feature(29)..(29)May be
2'OH-adenosinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxycytidinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxycytidinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'OH-guanosinemisc_feature(36)..(36)May be
2'OH-adenosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxycytidinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-fluoro-2'-deoxycytidine 30ggcuucugaa gauuauuucg cgaugugaac
uccagacccc 403140RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'OH-guanosinemisc_feature(2)..(2)May be
2'OH-guanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'OH-guanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'OH-guanosinemisc_feature(10)..(10)May be
2'OH-guanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxycytidinemisc_feature(15)..(15)May be
2'OH-adenosinemisc_feature(16)..(16)May be
2'OH-guanosinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxyuridinemisc_feature(22)..(22)May be
2'OH-guanosinemisc_feature(23)..(23)May be
2'OH-adenosinemisc_feature(24)..(24)May be
2'OH-guanosinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxycytidinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxyuridinemisc_feature(28)..(28)May be
2'OH-guanosinemisc_feature(29)..(29)May be
2'OH-adenosinemisc_feature(30)..(30)May be
2'OH-guanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'OH-guanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'OH-guanosine 31ggcgccgcgg ucucaggcgc ugagucugag uuuaccugcg
403239DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
deoxycytidinemisc_feature(34)..(34)May be
deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 32cgccgcgguc tcaggcgcug
agtctgaguu uaccugcgt 393339DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
deoxycytidinemisc_feature(34)..(34)May be
deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 33cgccgcgguc tcaggcgcug
agtctgaguu uaccugcgt 393438DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'OH-cytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 34cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 383538DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 35cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 383638DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 36cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 383738DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 37cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 383838DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 38cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 383938DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 39cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384037DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxyuridinemisc_feature(35)..(35)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyguanosine 40cgccgcgguc tcaggcgcug agtctgaguu
uacugcg 374138DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 41cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384237DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxyuridinemisc_feature(35)..(35)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyguanosine 42cgccgcgguc tcaggcgcug agtctgaguu
uacugcg 374338DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'OH-cytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 43cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384438DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 44cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384538DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 45cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384638DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 46cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 384738DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-thymidinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 47cgccgcgguc tcaggcgctg agtctgaguu
uaccugcg 384838DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-thymidinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 48cgccgcgguc tcaggcgctg agtctgaguu
uaccugcg 384938DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'OH-thymidinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 49cgccgcgguc tcaggcgcug agtctgagtu
uaccugcg 385038DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'OH-thymidinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 50cgccgcgguc tcaggcgcug agtctgagut
uaccugcg 385138DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 51cgccgcgguc tcaggcgcug agtctgaguu
taccugcg 385238DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'OH-thymidinemisc_feature(30)..(30)May be
2'OH-thymidinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 52cgccgcgguc tcaggcgcug agtctgagtt
taccugcg 385338DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'OH-thymidinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 53cgccgcgguc tcaggcgcug agtctgaguu
uacctgcg 385438DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
deoxycytidinemisc_feature(34)..(34)May be
deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 54cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 385538DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 55cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 385638DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 56cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 385738DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 57cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 385837DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'OH-guanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxyuridinemisc_feature(9)..(9)May be
deoxycytidinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxyuridinemisc_feature(11)..(11)May be
deoxycytidinemisc_feature(12)..(12)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
deoxycytidinemisc_feature(16)..(16)May be
2'OH-guanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxyuridinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(22)..(22)May be
2'-fluoro-2'-deoxyuridinemisc_feature(23)..(23)May be
deoxycytidinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxyuridinemisc_feature(25)..(25)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(28)..(28)May be
2'-fluoro-2'-deoxyuridinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'OH-adenosinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxycytidinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxyuridinemisc_feature(35)..(35)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyguanosine 58cgcgcggucu caggcgcuga gucugaguuu
accugcg 375938DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 59cgccgcgguc ucaggcgcug agucugaguu
uaccugcg 386038DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 60cgccgcgguc ucaggcgcug agucugaguu
uaccugcg 386138DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 61cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386238DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 62cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386338DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 63cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386438DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 64cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386538DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 65cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386638DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
deoxyguanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 66cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386738DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
deoxyguanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'OH-cytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 67cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386838DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
deoxyadenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosine 68cgccgcgguc tcaggcgcug agtctgaguu
uaccugcg 386940DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-fluoro-2'-deoxycytidinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxyuridinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'OH-guanosinemisc_feature(7)..(7)May be
2'-fluoro-2'-deoxycytidinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxyuridinemisc_feature(11)..(11)May be
deoxycytidinemisc_feature(12)..(12)May be
2'OH-thymidinemisc_feature(13)..(13)May be
deoxycytidinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
deoxycytidinemisc_feature(18)..(18)May be
2'OH-guanosinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxycytidinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxyuridinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(24)..(24)May be
2'OH-thymidinemisc_feature(25)..(25)May be
deoxycytidinemisc_feature(26)..(26)May be
2'OH-thymidinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'OH-adenosinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxyuridinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxycytidinemisc_feature(39)..(39)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxycytidine 69gcgucgcggu ctcaggcgcu gagtctgagu
uuaccuacgc 407038DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
deoxycytidinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'OH-thymidinemisc_feature(24)..(24)May be
deoxycytidinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxycytidine 70gggcgcgguc tcaggcgcug agtctgaguu
uaccuccc 387139DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-fluoro-2'-deoxycytidinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'OH-guanosinemisc_feature(7)..(7)May be
2'-fluoro-2'-deoxycytidinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxyuridinemisc_feature(11)..(11)May be
deoxycytidinemisc_feature(12)..(12)May be
2'OH-thymidinemisc_feature(13)..(13)May be
deoxycytidinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
deoxycytidinemisc_feature(18)..(18)May be
2'OH-guanosinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxycytidinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxyuridinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(24)..(24)May be
2'OH-thymidinemisc_feature(25)..(25)May be
deoxycytidinemisc_feature(26)..(26)May be
2'OH-thymidinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'OH-adenosinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-O-Methyl-2'-deoxycytidine 71gcgccgcggu ctcaggcgcu gagtctgagu
uuacugcgc 397243DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-fluoro-2'-deoxycytidinemisc_feature(8)..(8)May be
2'OH-guanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxycytidinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxyuridinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxycytidinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxyuridinemisc_feature(15)..(15)May be
2'-fluoro-2'-deoxycytidinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxycytidinemisc_feature(20)..(20)May be
2'OH-guanosinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxycytidinemisc_feature(22)..(22)May be
2'-fluoro-2'-deoxyuridinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(25)..(25)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxyuridinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxycytidinemisc_feature(28)..(28)May be
2'-fluoro-2'-deoxyuridinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxyuridinemisc_feature(37)..(37)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(38)..(38)May be
2'-fluoro-2'-deoxycytidinemisc_feature(39)..(39)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(40)..(40)May be
2'-fluoro-2'-deoxyuridinemisc_feature(41)..(41)May be
2'-fluoro-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-fluoro-2'-deoxyuridinemisc_feature(43)..(43)May be an inverted
orientation T (3'-3'-linked) 72ggacgccgcg gucucaggcg cugagucugg
uuuacugcgu cut 437342DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'OH-thymidinemisc_feature(14)..(14)May be
deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
deoxycytidinemisc_feature(27)..(27)May be
2'OH-thymidinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
deoxycytidinemisc_feature(36)..(36)May be
deoxycytidinemisc_feature(37)..(37)May be
2'OH-thymidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(41)..(41)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-O-Methyl-2'-deoxycytidine 73ggcgccgcgg uctcaggcgc ugagtctgag
tuuacctgcg cc 427440DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'OH-thymidinemisc_feature(14)..(14)May be
deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
deoxycytidinemisc_feature(27)..(27)May be
2'OH-thymidinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxythymidinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
deoxycytidinemisc_feature(34)..(34)May be
deoxycytidinemisc_feature(35)..(35)May be
2'OH-thymidinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxycytidine 74ggcgccgcgg uctcaggcgc ugagtctgat
tacctgcgcc 407542DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
deoxycytidinemisc_feature(6)..(6)May be
deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'OH-thymidinemisc_feature(14)..(14)May be
deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
deoxycytidinemisc_feature(27)..(27)May be
2'OH-thymidinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'OH-thymidinemisc_feature(33)..(33)May be
2'OH-thymidinemisc_feature(34)..(34)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(35)..(35)May be
deoxycytidinemisc_feature(36)..(36)May be
deoxycytidinemisc_feature(37)..(37)May be
2'OH-thymidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(41)..(41)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-O-Methyl-2'-deoxycytidine 75ggcgccgcgg tctcaggcgc ugagtctgag
tttacctgcg cc 427642DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
deoxycytidinemisc_feature(6)..(6)May be
deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'OH-thymidinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'OH-thymidinemisc_feature(32)..(32)May be
2'OH-thymidinemisc_feature(33)..(33)May be
2'OH-thymidinemisc_feature(34)..(34)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(35)..(35)May be
deoxycytidinemisc_feature(36)..(36)May be
deoxycytidinemisc_feature(37)..(37)May be
2'OH-thymidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(41)..(41)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-O-Methyl-2'-deoxycytidine 76ggcgccgcgg tcucaggcgc ugagucugag
tttacctgcg cc 427740RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May have biotin conjugated to the 5'
endmisc_feature(1)..(1)May be 2'OH-adenosinemisc_feature(2)..(2)May
be 2'OH-guanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-fluoro-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-fluoro-2'-deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-fluoro-2'-deoxycytidinemisc_feature(27)..(27)May be
2'-fluoro-2'-deoxyuridinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosine 77agcgccgcgg ucucaggcgc ugagucugag
uuuaccugcg 407842DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
deoxycytidinemisc_feature(13)..(13)May be
2'OH-thymidinemisc_feature(14)..(14)May be
deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'OH-thymidinemisc_feature(26)..(26)May be
deoxycytidinemisc_feature(27)..(27)May be
2'OH-thymidinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(41)..(41)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-O-Methyl-2'-deoxycytidine 78ggcgccgcgg uctcaggcgc ugagtctgag
uuuaccugcg cc 427942RNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(5)..(5)May be
2'-fluoro-2'-deoxycytidinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'OH-guanosinemisc_feature(8)..(8)May be
2'-fluoro-2'-deoxycytidinemisc_feature(9)..(9)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(10)..(10)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(16)..(16)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(17)..(17)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(19)..(19)May be
2'OH-guanosinemisc_feature(20)..(20)May be
2'-fluoro-2'-deoxycytidinemisc_feature(21)..(21)May be
2'-fluoro-2'-deoxyuridinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(24)..(24)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(25)..(25)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(30)..(30)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-fluoro-2'-deoxyuridinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxyuridinemisc_feature(34)..(34)May be
2'OH-adenosinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxycytidinemisc_feature(36)..(36)May be
2'-fluoro-2'-deoxycytidinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxyuridinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be
2'-fluoro-2'-deoxycytidinemisc_feature(40)..(40)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(41)..(41)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(42)..(42)May be
2'-O-Methyl-2'-deoxycytidine 79ggcgccgcgg ucucaggcgc ugagucugag
uuuaccugcg cc 428039DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 80cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398139DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 81cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398239DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'OH-thymidinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 82cgccgcgguc ucaggcgcug
agucugagtu uaccugcgt 398339DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 83cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398439DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-O-Methyl-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-O-Methyl-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'OH-thymidinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 84cgccgcgguc ucaggcgcug
agucugagtu uaccugcgt 398539DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May have a 20 kDa polyethylene
glycol group attached via a hexylamine
linkermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 85cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398639DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May have a 30 kDa polyethylene
glycol group attached via a hexylamine
linkermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 86cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398739DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May have a hexylamine terminal
groupmisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 87cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398839DNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May have a 10 kDa polyethylene
glycol group attached via a hexylamine
linkermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 88cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 398975RNAArtificial SequenceSynthetic C5
specific aptamer 89gggagaggag agaacguucu accuugguuu ggcacaggca
uacauacgca ggggucgauc 60gaucgaucau cgaug 759032RNAArtificial
SequenceSynthetic C5 specific aptamer 90ccuugguuug gcacaggcau
acauacgcag gg 329147RNAArtificial SequenceSynthetic C5 specific
aptamer 91cguucuaccu ugguuuggca caggcauaca uacgcagggg ucgaucg
479239DNAArtificial SequenceSynthetic C5 specific
aptamermisc_feature(1)..(1)May have a 40 kDa polyethylene glycol
group attached via a hexylamine linkermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(39)..(39)May be an
inverted orientation T (3'-3'-linked) 92cgccgcgguc ucaggcgcug
agucugaguu uaccugcgt 399338RNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(1)..(1)May have a 20 kDa polyethylene
glycol group attached via a hexylamine
linkermisc_feature(1)..(1)May be
2'-fluoro-2'-deoxycytidinemisc_feature(2)..(2)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(3)..(3)May be
2'-fluoro-2'-deoxycytidinemisc_feature(4)..(4)May be
2'-fluoro-2'-deoxycytidinemisc_feature(5)..(5)May be
2'OH-guanosinemisc_feature(6)..(6)May be
2'-fluoro-2'-deoxycytidinemisc_feature(7)..(7)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(8)..(8)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(9)..(9)May be
2'-fluoro-2'-deoxyuridinemisc_feature(10)..(10)May be
2'-fluoro-2'-deoxycytidinemisc_feature(11)..(11)May be
2'-fluoro-2'-deoxyuridinemisc_feature(12)..(12)May be
2'-fluoro-2'-deoxycytidinemisc_feature(13)..(13)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(14)..(14)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(15)..(15)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(16)..(16)May be
2'-fluoro-2'-deoxycytidinemisc_feature(17)..(17)May be
2'OH-guanosinemisc_feature(18)..(18)May be
2'-fluoro-2'-deoxycytidinemisc_feature(19)..(19)May be
2'-fluoro-2'-deoxyuridinemisc_feature(20)..(20)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(21)..(21)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(22)..(22)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(23)..(23)May be
2'-fluoro-2'-deoxyuridinemisc_feature(24)..(24)May be
2'-fluoro-2'-deoxycytidinemisc_feature(25)..(25)May be
2'-fluoro-2'-deoxyuridinemisc_feature(26)..(26)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(27)..(27)May be
2'-O-Methyl-2'-deoxyadenosinemisc_feature(28)..(28)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(29)..(29)May be
2'-fluoro-2'-deoxyuridinemisc_feature(30)..(30)May be
2'-fluoro-2'-deoxyuridinemisc_feature(31)..(31)May be
2'-fluoro-2'-deoxyuridinemisc_feature(32)..(32)May be
2'OH-adenosinemisc_feature(33)..(33)May be
2'-fluoro-2'-deoxycytidinemisc_feature(34)..(34)May be
2'-fluoro-2'-deoxycytidinemisc_feature(35)..(35)May be
2'-fluoro-2'-deoxyuridinemisc_feature(36)..(36)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(37)..(37)May be
2'-fluoro-2'-deoxycytidinemisc_feature(38)..(38)May be
2'-O-Methyl-2'-deoxyguanosinemisc_feature(38)..(38)May have a 20
kDa polyethylene glycol group attached via a hexylamine linker
93cgccgcgguc ucaggcgcug agucugaguu uaccugcg 389480RNAArtificial
SequenceSynthetic C5 specific aptamer 94gggagaggag agaacguucu
accuugguuu ggcccaggca uauauacgca gggauugauc 60cguuacgacu agcaucgaug
809579RNAArtificial SequenceSynthetic C5 specific aptamer
95gggagaggag agaacguucu accuuagguu cgcacuguca uacauacaca cgggcaaucg
60guuacgacua gcaucgaug 799675RNAArtificial SequenceSynthetic C5
specific aptamermisc_feature(34)..(34)n is a, c, g, or
umisc_feature(43)..(43)n is a, c, g, or u 96gggagaggag agaacguucu
accuugguuu ggcncaggca uanauacgca cgggucgauc 60gguuacgacu agcau
7597126PRTUnknownankyrin binding domain 97Gly Ser Asp Leu Gly Lys
Lys Leu Leu Glu Ala Ala Arg Ala Gly Gln1 5 10 15Asp Asp Glu Val Arg
Ile Leu Met Ala Asn Gly Ala Asp Val Asn Thr 20 25 30Ala Asp Ser Thr
Gly Trp Thr Pro Leu His Leu Ala Val Pro Trp Gly 35 40 45His Leu Glu
Ile Val Glu Val Leu Leu Lys Tyr Gly Ala Asp Val Asn 50 55 60Ala Lys
Asp Phe Gln Gly Trp Thr Pro Leu His Leu Ala Ala Ala Ile65 70 75
80Gly His Gln Glu Ile Val Glu Val Leu Leu Lys Asn Gly Ala Asp Val
85 90 95Asn Ala Gln Asp Lys Phe Gly Lys Thr Ala Phe Asp Ile Ser Ile
Asp 100 105 110Asn Gly Asn Glu Asp Leu Ala Glu Ile Leu Gln Lys Ala
Ala 115 120 12598552PRTArtificial Sequencerecombinant human soluble
VEGF receptor fusion protein 98Met Val Ser Tyr Trp Asp Thr Gly Val
Leu Leu Cys Ala Leu Leu Ser1 5 10 15Cys Leu Leu Leu Thr Gly Ser Ser
Ser Gly Gly Arg Pro Phe Val Glu 20 25 30Met Tyr Ser Glu Ile Pro Glu
Ile Ile His Met Thr Glu Gly Arg Glu 35 40 45Leu Val Ile Pro Cys Arg
Val Thr Ser Pro Asn Ile Thr Val Thr Leu 50 55 60Lys Lys Phe Pro Leu
Asp Thr Leu Ile Pro Asp Gly Lys Arg Ile Ile65 70 75 80Trp Asp Ser
Arg Lys Gly Phe Ile Ile Ser Asn Ala Thr Tyr Lys Glu 85 90 95Ile Gly
Leu Leu Thr Cys Glu Ala Thr Val Asn Gly His Leu Tyr Lys 100 105
110Thr Asn Tyr Leu Thr His Arg Gln Thr Asn Thr Ile Ile Asp Val Val
115 120 125Leu Ser Pro Ser His Gly Ile Glu Leu Ser Val Gly Glu Lys
Leu Val 130 135 140Leu Asn Cys Thr Ala Arg Thr Glu Leu Asn Val Gly
Ile Asp Phe Asn145 150 155 160Trp Glu Tyr Pro Ser Ser Lys His Gln
His Lys Lys Leu Val Asn Arg 165 170 175Asp Leu Lys Thr Gln Ser Gly
Ser Glu Met Lys Lys Phe Leu Ser Thr 180 185 190Leu Thr Ile Asp Gly
Val Thr Arg Ser Asp Gln Gly Leu Tyr Thr Cys 195 200 205Ala Ala Ser
Ser Gly Leu Met Thr Lys Lys Asn Ser Thr Phe Val Arg 210 215 220Val
His Glu Lys Pro Phe Val Ala Phe Gly Ser Gly Met Glu Ser Leu225 230
235 240Val Glu Ala Thr Val Gly Glu Arg Val Arg Leu Pro Ala Lys Tyr
Leu 245 250 255Gly Tyr Pro Pro Pro Glu Ile Lys Trp Tyr Lys Asn Gly
Ile Pro Leu 260 265 270Glu Ser Asn His Thr Ile Lys Ala Gly His Val
Leu Thr Ile Met Glu 275 280 285Val Ser Glu Arg Asp Thr Gly Asn Tyr
Thr Val Ile Leu Thr Asn Pro 290 295 300Ile Ser Lys Glu Lys Gln Ser
His Val Val Ser Leu Val Val Tyr Val305 310 315 320Pro Pro Gly Pro
Gly Asp Lys Thr His Thr Cys Pro Leu Cys Pro Ala 325 330 335Pro Glu
Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro 340 345
350Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val
355 360 365Val Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp
Tyr Val 370 375 380Asp Gly Val Glu Val His Asn Ala Lys Thr Lys Pro
Arg Glu Glu Gln385 390 395 400Tyr Asn Ser Thr Tyr Arg Val Val Ser
Val Leu Thr Val Leu His Gln 405 410 415Asp Trp Leu Asn Gly Lys Glu
Tyr Lys Cys Lys Val Ser Asn Lys Ala 420 425 430Leu Pro Ala Pro Ile
Glu Lys Thr Ile Ser Lys Ala Lys Gly Gln Pro 435 440 445Arg Glu Pro
Gln Val Tyr Thr Leu Pro Pro Ser Arg Asp Glu Leu Thr 450 455 460Lys
Asn Gln Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro Ser465 470
475 480Asp Ile Ala Val Glu Trp Glu Ser Asn Gly Gln Pro Glu Asn Asn
Tyr 485 490 495Lys Ala Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe
Phe Leu Tyr 500 505 510Ser Lys Leu Thr Val Asp Lys Ser Arg Trp Gln
Gln Gly Asn Val Phe 515 520 525Ser Cys Ser Val Met His Glu Ala Leu
His Asn His Tyr Thr Gln Lys 530 535 540Ser Leu Ser Leu Ser Pro Gly
Lys545 550
References


D00001

D00002

D00003

D00004

D00005
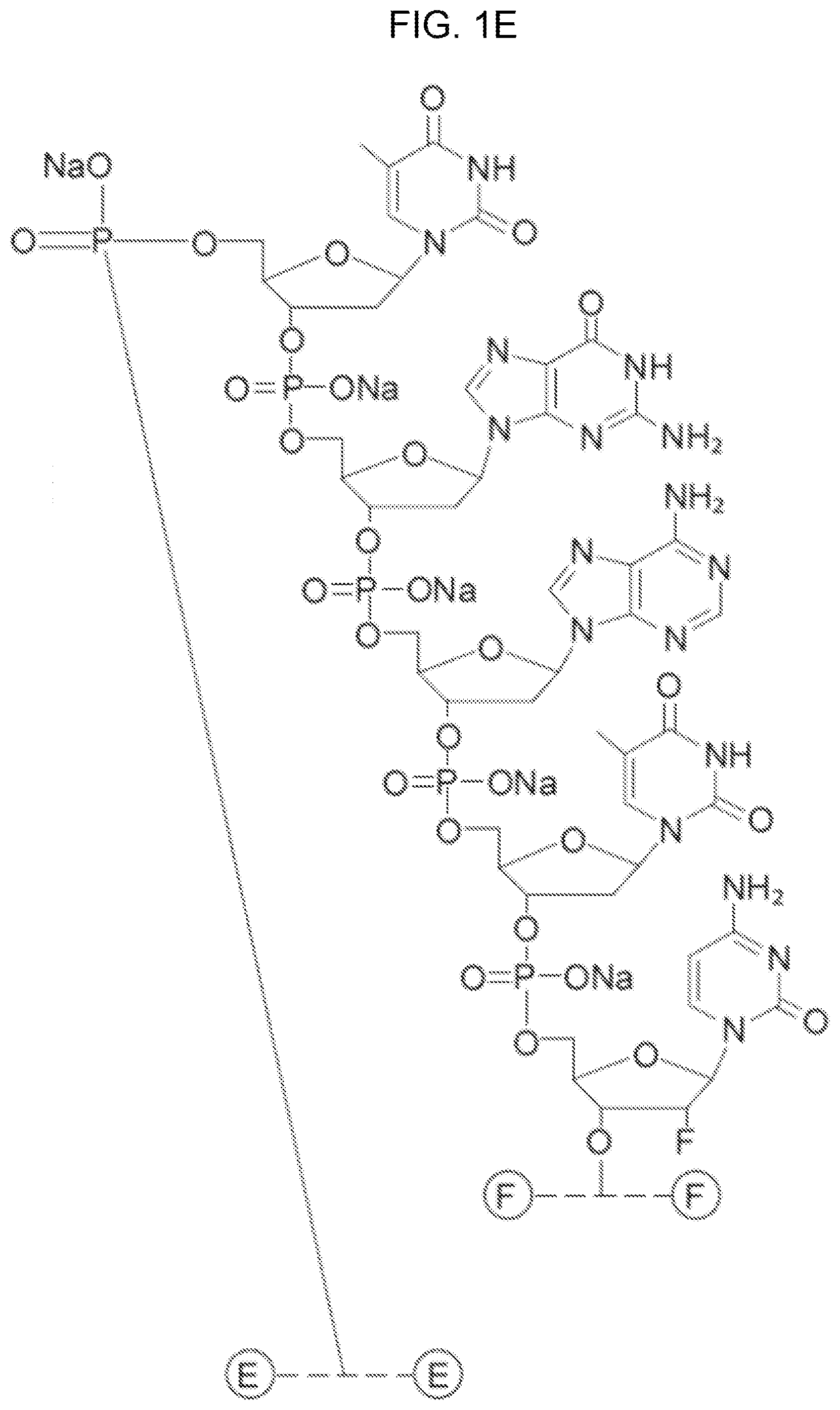
D00006

D00007

D00008

D00009

D00010

D00011
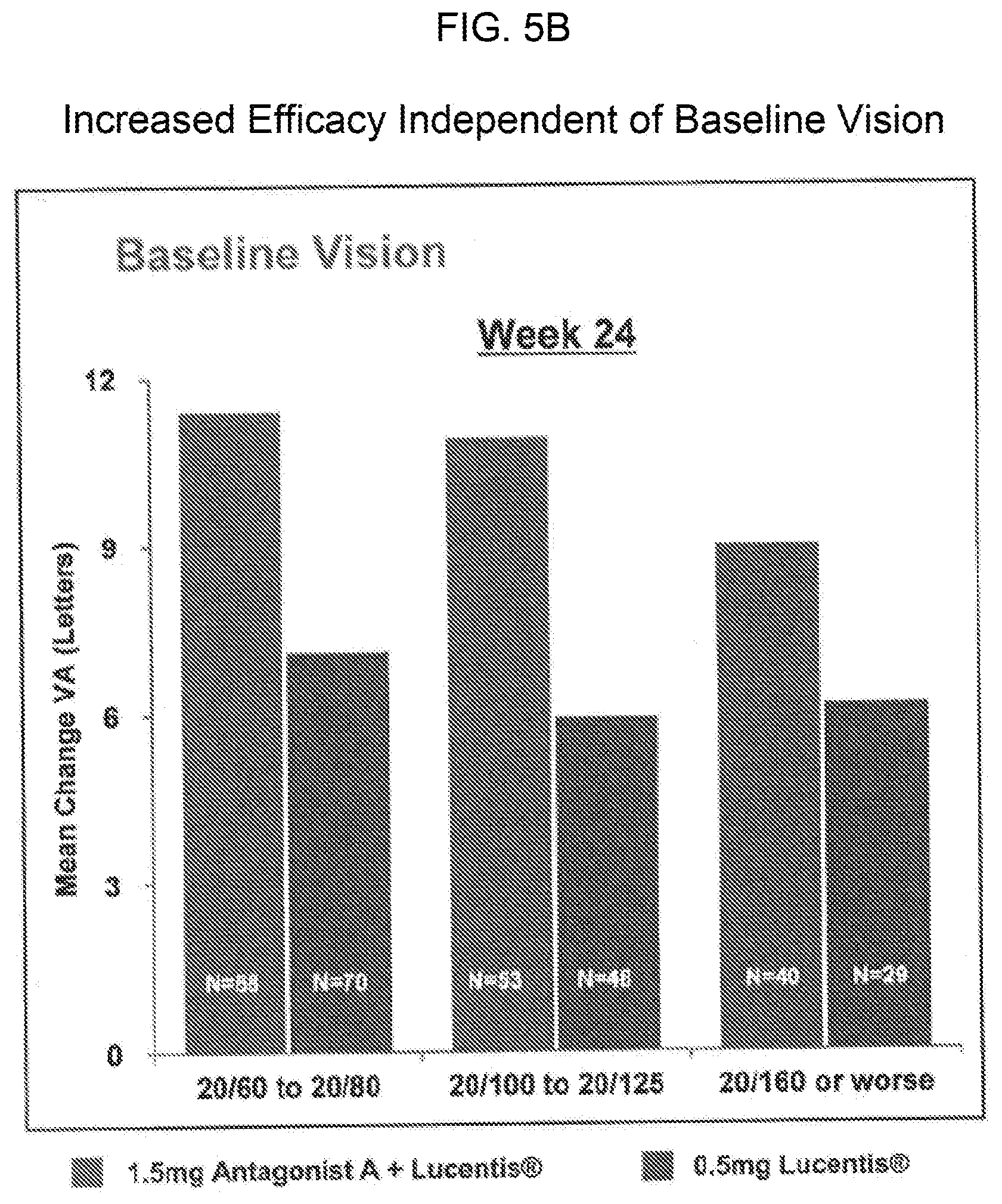
D00012
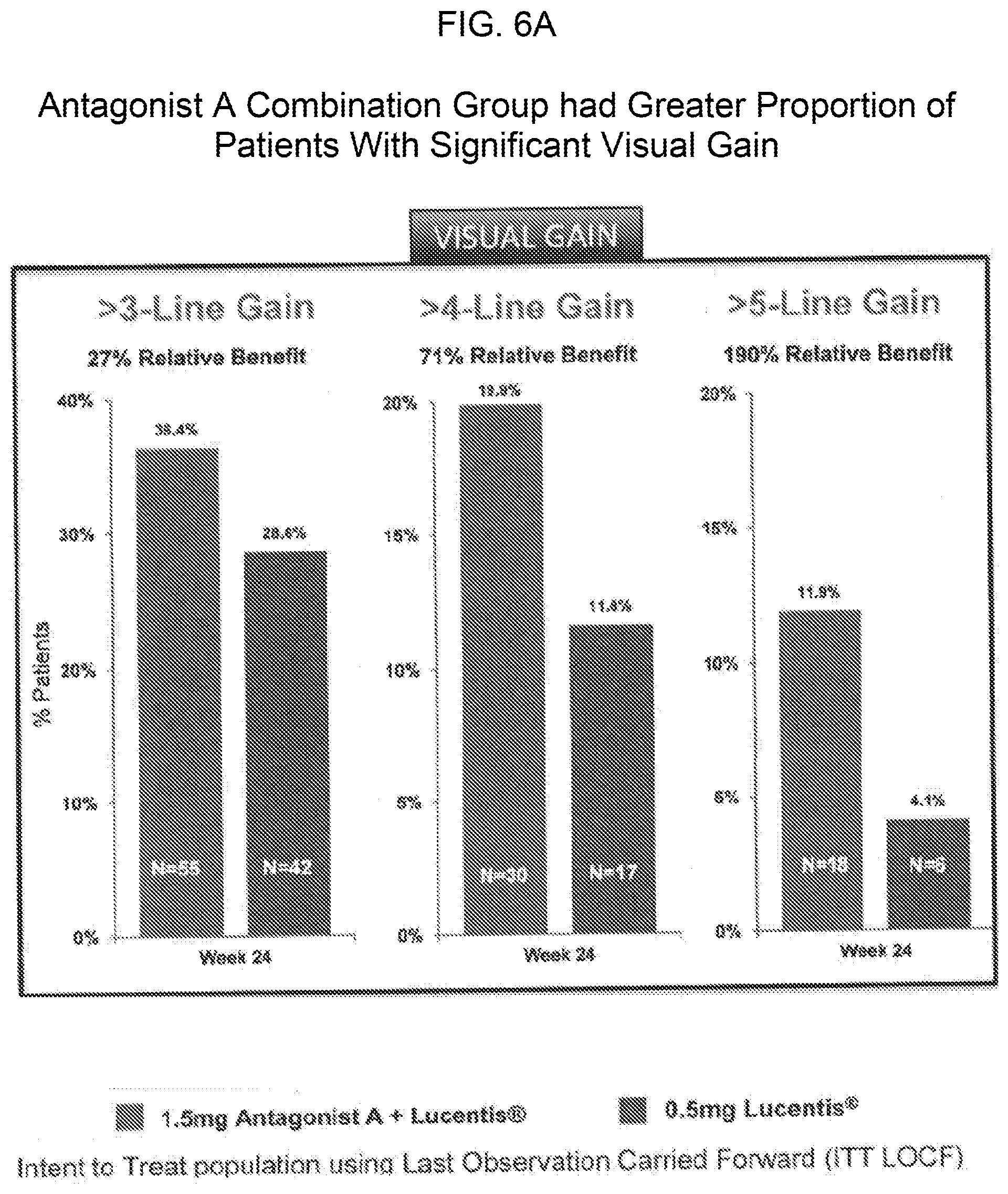
D00013
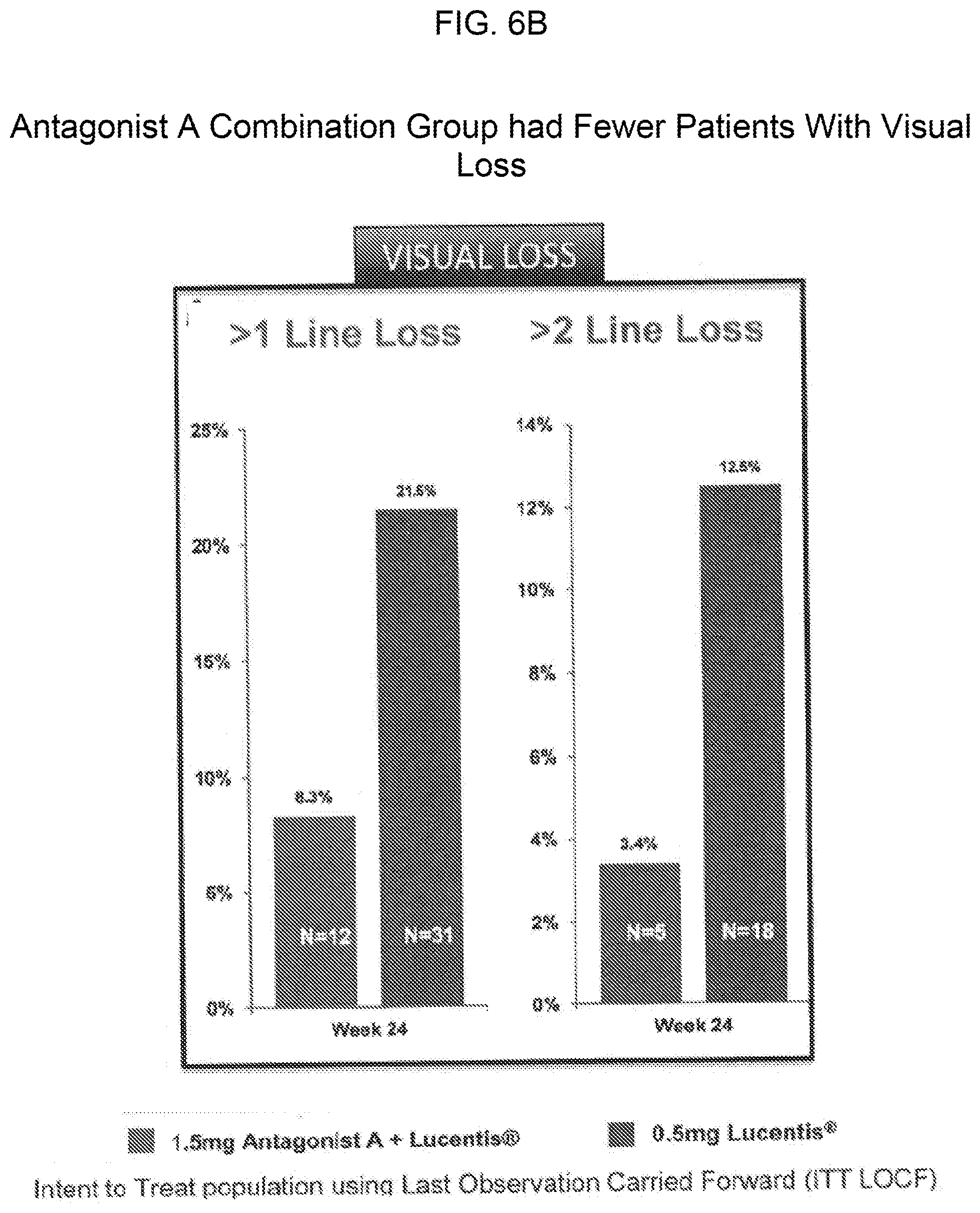
D00014

D00015

D00016

D00017

D00018

D00019

D00020
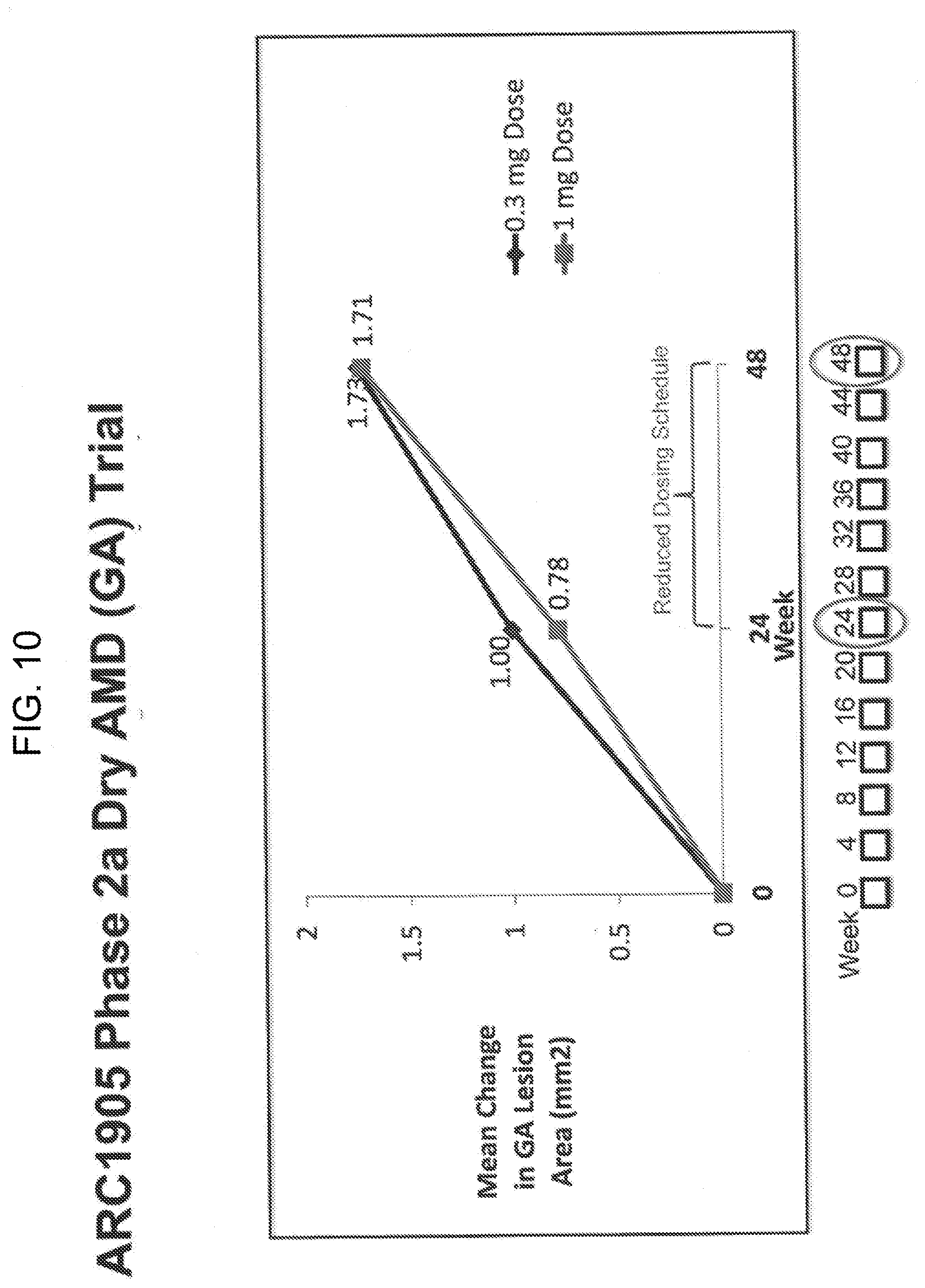
D00021

D00022
D00023

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.