Artificial Forisome Bodies With Seo-f Fusion Proteins, Plant Or Yeast Cells With Vectors For Encoding These Proteins And Vectors
Mueller; Boje ; et al.
U.S. patent application number 16/377019 was filed with the patent office on 2019-10-03 for artificial forisome bodies with seo-f fusion proteins, plant or yeast cells with vectors for encoding these proteins and vectors. The applicant listed for this patent is Fraunhofer-Gesellschaft zur Foerderung der angewandten Forschung e.V.. Invention is credited to Rainer Fischer, Boje Mueller, Dirk Pruefer.
| Application Number | 20190300586 16/377019 |
| Document ID | / |
| Family ID | 48446274 |
| Filed Date | 2019-10-03 |





| United States Patent Application | 20190300586 |
| Kind Code | A1 |
| Mueller; Boje ; et al. | October 3, 2019 |
ARTIFICIAL FORISOME BODIES WITH SEO-F FUSION PROTEINS, PLANT OR YEAST CELLS WITH VECTORS FOR ENCODING THESE PROTEINS AND VECTORS FOR ENCODING SEO-F FUSION PROTEINS
Abstract
Artificial forisome bodies include a fusion protein of at least one SEO-F protein or an at least 50-amino acid portion of an SEO-F protein, and at least one additional protein or peptide, with the exception of GFP and the Venus protein. The additional protein or peptide has a mass of at most 30 kDa, or the forisome body further includes an unfused SEO-F protein or a form of the protein having C-terminal deletions of up to 45 amino acids and/or N-terminal deletions of up to 13 amino acids, in which the unfused SEO-F protein is a protein capable of forming homomeric forisome bodies in the absence of additional SEO-F proteins.
| Inventors: | Mueller; Boje; (Muenster, DE) ; Pruefer; Dirk; (Muenster, DE) ; Fischer; Rainer; (Aachen, DE) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 48446274 | ||||||||||
| Appl. No.: | 16/377019 | ||||||||||
| Filed: | April 5, 2019 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 14399472 | Nov 6, 2014 | |||
| PCT/EP2013/059190 | May 2, 2013 | |||
| 16377019 | ||||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C12N 9/1205 20130101; C12Y 101/01049 20130101; C07K 14/445 20130101; C07K 2319/00 20130101; C12Y 207/01001 20130101; C12N 9/92 20130101; C12Y 503/01009 20130101; C12N 9/0006 20130101; C07K 14/415 20130101; C12N 15/8242 20130101 |
| International Class: | C07K 14/415 20060101 C07K014/415; C12N 9/04 20060101 C12N009/04; C12N 9/92 20060101 C12N009/92; C12N 9/12 20060101 C12N009/12; C07K 14/445 20060101 C07K014/445 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| May 9, 2012 | EP | 12167377.6 |
Claims
1. An artificial forisome body comprising a fusion protein of (A) a SEO-F (Sieve Element Occlusion by Forisome) protein having an amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, or a fragment of the amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, wherein said fragment results from deleting up to 45 amino acids from the C-terminus and/or up to 13 amino acids from the N-terminus of the amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, and (B) at least one additional protein, wherein the at least one additional protein is not a fluorescent protein, (a) wherein the additional protein has a mass of at most 30 kDa, and wherein the artificial forisome body does not contain an unfused SEO-F protein or an unfused SEO-F protein having C-terminal deletions of up to 45 amino acids and/or N-terminal deletions of up to 13 amino acids, or (b) wherein the forisome body further comprises an unfused SEO-F protein, wherein said unfused SEO-F protein is (i) a protein having the amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, or (ii) a protein comprising a fragment of the amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, wherein said fragment results from deleting up to 45 amino acids from the C-terminus and/or up to 13 amino acids from the N-terminus of the amino acid sequence of SEQ ID NO: 4, 5, 6, 7 or 8, and wherein the unfused SEO-F protein has ability to form homomeric forisome bodies in the absence of additional SEO-F proteins.
2. The artificial forisome body according to claim 1, with the exception of forisome bodies that are comprised entirely or partly of a fusion protein that contains an artificial fluorescent variant or a portion of a fluorescent portion of a green fluorescent protein (GFP) or Venus protein.
3. The artificial forisome body according to claim 1, wherein the artificial forisome body comprises a fusion protein of (A) a SEO-F (Sieve Element Occlusion by Forisome) protein having the amino acid sequence of SEQ ID NO: 4, or a fragment of the amino acid sequence of SEQ ID NO: 4, wherein said fragment results from deleting up to 45 amino acids from the C-terminus and/or up to 13 amino acids from the N-terminus of the amino acid sequence of SEQ ID NO: 4, and (B) the at least one additional protein.
4. The artificial forisome body according to claim 1, wherein the additional protein is selected from the group consisting of proteins that contribute to metabolism, proteins capable of triggering an immune response and/or proteins having a therapeutic benefit, and proteins that are useful for biotechnological applications.
5. The artificial forisome body according to claim 1, wherein the additional protein is selected from the group consisting of enzymes, antibodies, and antigens, wherein the additional protein can be immobilized on a substrate due to their affinity reaction with a substrate-bound biological or biochemically produced material.
6. The artificial forisome body according to claim 1, wherein the fusion protein contains an enzyme fused to the N-terminal end of the SEO-F protein or a portion thereof, or wherein the fusion protein comprises a protein that has a therapeutic benefit or is useful for biotechnological applications, and which is fused to the C-terminal end of the SEO-F protein or portion thereof.
7. The artificial forisome body according to claim 1, comprising at least two fusion proteins, each comprising an enzyme, such that a product of a reaction of one substrate with a first of said enzymes can serve as a substrate for a second of said enzymes.
8. A plant cell or yeast cell comprising: a first vector encoding a fusion protein of at least one SEO-F protein or a portion thereof comprising at least 50 amino acids and at least one additional protein, with the exception of a green fluorescent protein (GFP) and Venus protein and artificial fluorescent variants or fluorescent portions of the GFP protein or the Venus protein, and optionally a second vector encoding a SEO-F protein or a form of said protein having C-terminal deletions of up to 45 amino acids and/or N-terminal deletions of up to 13 amino acids, wherein the SEO-F protein is capable of forming forisome bodies in the absence of further homomeric SEO-F proteins, with the proviso that optionally any number of the four cysteines that are located in the native SEO-F proteins in the C-terminal region between aa 600 and aa 670, may be replaced by amino acids that are not capable for forming an SS-bond.
9. The plant cell or yeast cell according to claim 8, wherein the additional protein has a mass of at most 30 kDa.
10. The plant cell or yeast cell according to claim 8, wherein the additional protein is a portion of a second SEO-F protein, with the proviso that one of the two SEO-F proteins in unfused form is capable of forming homomeric forisome bodies and the fusion protein is comprised of an N-terminal SEO-F protein portion and a C-terminal SEO-F protein portion, wherein the portions represent an SEO-F protein that is complete or C-terminally deleted by up to approximately 50 amino acids and/or N-terminally by up to 13 amino acids.
11. The plant cell or yeast cell according to claim 8, wherein the additional protein is selected from the group consisting of proteins that contribute to metabolism, proteins capable of triggering an immune response and/or proteins having a therapeutic benefit, and proteins that are useful for biotechnological applications.
12. The plant cell or yeast cell according to claim 8, wherein the additional protein is selected from the group consisting of enzymes, antibodies, and antigens, wherein the additional protein can be immobilized on a substrate due to their affinity reaction with a substrate-bound biological or biochemically produced material.
13. The plant cell or yeast cell according to claim 8, wherein the fusion protein comprises an enzyme which is fused to the N-terminal end of the SEO-F protein or a portion thereof, or wherein the fusion protein comprises a protein that has a therapeutic benefit or is useful for biotechnological applications, and is fused to the C-terminal end of the SEO-F protein or portion thereof.
14. The plant cell or yeast cell according to claim 8, wherein the first vector encodes a fusion protein comprising an amino acid sequence of a first enzyme, characterized in that the cell contains at least one further vector encoding a fusion protein comprising the amino acid sequence of a second enzyme, wherein a reaction product of a substrate with the first enzyme is suitable as a substrate for the second enzyme.
15. A vector capable of being amplified in the yeast cell according to claim 8, comprising a region encoding a fusion protein comprised of at least one SEO-F protein or a portion thereof comprising at least 50 amino acids and at least one further protein or peptide, with the exception of the GFP and the Venus protein.
16. The vector according to claim 15, wherein the additional protein is selected from the group consisting of proteins that contribute to metabolism, proteins capable of triggering an immune response and/or proteins having a therapeutic benefit, and proteins that are useful for biotechnological applications.
17. The vector according to claim 15, wherein the additional protein or peptide is selected from the group consisting of enzymes, antibodies, and antigens, wherein the additional protein can be immobilized on a substrate due to their affinity reaction with a substrate-bound biological or biochemically produced material.
18. The vector according to claim 15, wherein the fusion protein comprises an enzyme fused to the N-terminal end of the SEO-F protein or a portion thereof, or wherein the fusion protein comprises a protein that has a therapeutic benefit or can be used for biotechnological applications, and is fused to the C-terminal end of the SEO-F protein or the portion thereof.
19. A method for producing an artificial forisome body comprising a fusion protein of at least one SEO-F (Sieve Element Occlusion by Forisome) protein or an at least 50-amino acid portion thereof, and at least one additional protein, with the exception of a green fluorescent protein (GFP) and Venus protein, wherein (a) the additional protein or peptide has a mass of at most 30 kDa, and the artificial forisome body does not contain an unfused SEO-F protein or a form of said protein having C-terminal deletions of up to 45 amino acids and/or N-terminal deletions of up to 13 amino acids, or (b) the forisome body further comprises an unfused SEO-F protein or a form of said protein having C-terminal deletions of up to 45 amino acids and/or N-terminal deletions of up to 13 amino acids, wherein the unfused SEO-F protein is selected from proteins having the capability of forming homomeric forisome bodies in the absence of additional SEO-F proteins, or (c) the further protein or peptide is a portion of a second SEO-F protein, with the proviso that one of the two SEO-F proteins in its unfused form is capable of forming homomeric forisome bodies, and the fusion protein is comprised of an N-terminal SEO-F protein portion and a C-terminal SEO-F protein portion, wherein the portions represent an SEO-F protein that is complete or C-terminally deleted by up to 50 amino acids and/or N-terminally deleted by up to 13 amino acids, with the proviso that optionally any number of the four cysteines located in the C-terminal portion between aa 600 and aa 670 of the native SEO-F proteins, may be replaced by amino acids that are not capable of forming an SS-bond.
20. The method according to claim 19, wherein the fusion protein does not contain any artificial fluorescent variant and no fluorescent portion of the GFP protein or the Venus fluorescent protein.
21. The artificial forisome body according to claim 2, with the exception of forisome bodies that are composed exclusively or partly of a fluorescent fusion protein.
22. The method according to claim 20, wherein the fusion protein does not comprise fluorescent protein portions.
Description
INCORPORATION BY REFERENCE TO ANY PRIORITY APPLICATIONS
[0001] Any and all applications for which a foreign or domestic priority claim is identified in the Application Data Sheet as filed with the present application are hereby incorporated by reference under 37 CFR 1.57.
SEQUENCE LISTING IN ELECTRONIC FORMAT
[0002] The present application is being filed along with an Electronic Sequence Listing as an ASCII text file via EFS-Web. The Electronic Sequence Listing is provided as a file entitled 2019-04-02_SEQ-SWKC001001C1.txt created and last saved on Apr. 5, 2019, which is approximately 57 kilobytes in size. The information in the Electronic Sequence Listing is incorporated herein by reference in its entirety in accordance with 35 U.S.C. .sctn. 1.52(e).
BACKGROUND OF THE INVENTION
Field of the Invention
[0003] The present invention relates to artificial forisome bodies having properties useful protein-chemistry, plant cells and yeast cells with a combination of vectors that enable formation and isolation of said forisome bodies in the cell, and novel vectors encoding SEO-F fusion proteins.
Description of the Related Art
[0004] Forisomes are plant protein bodies (mechanoproteins), which are found exclusively in the phloem of plants of the Fabaceae family (legumes). They are located in the sieve plates of the phloem system. When the phloem is wounded, forisomes undergo a calcium-dependent conformational change that converts them from a condensed state to a thickened, dispersed state that allows them to plug the sieve elements and prevent the loss of valuable sugar molecules. Forisomes exist as fibrillar substructures packed into large, compact bundles. In vitro, divalent cations, pH changes, or electrical stimuli can trigger forisomes to undergo numerous ATP-independent repeatable cycles of contractions and alternating expansions.
[0005] A forisome is comprised of several million subunits. These subunits are homologous proteins that, according to their function, are named "Sieve Element Occlusion by Forisomes" (SEO-F). The thesis by Gundula Noll (2005) describes expression of several genes that code for these proteins using bacterial expression vectors. It was determined that in Medicago trunculata at least four subunits (SEO-F1 to SEO-F4) exist (G. Noll et al., Plant Mol. Biol. 65:285-294 (2007), HC Pelissier et al., Plant Cell Physiol. 49:1699-1710 (2008)). All four subunits have meanwhile been sequenced; their sequences are shown as SEQ-ID NO: 1-4 in Sequence Listing. The sequences of SEO-F1 proteins of the species Dipteryx panamensis, Lotus japonicus, Pisum sativum and Vicia faba are shown as SEQ-ID NO: 5-8 in Sequence Listing. In plants, the different SEO-F proteins assemble to forisome protein bodies. Expression of the corresponding genes in foreign organisms (tobacco plants, yeast) has meanwhile demonstrated that in Medicago trunculata each of the two sub-units, namely SEO-F1 and SEO-F4, assemble into homomeric artificial forisomes in the absence of other subunits, see G. Noll et al., Bioengineered Bugs 2:2, 1-4 (2011), 2011 Landes Bioscience. The SEO-F2 subunit, in contrast, cannot assemble into homomeric forisomes, but can co-assemble both with the SEO-F1 subunit as well as with the SEO-F4 subunit.
[0006] Some SEO-F fusion proteins have previously been generated for analytical purposes. Accordingly, G. Noll performed forisome gene-enzyme coupling in the context of her dissertation (2005) for the purpose of producing antibodies in E. coli. However, formation of forisome bodies was hereby not detectable. H. C. Pelissier et al. describe loc. cit. a fusion protein consisting of a forisome subunit and the green fluorescent protein (GFP) that allowed them to demonstrate the assembly of this subunit to a forisome body in transgenic plants in which the fusion protein was stored. In Appl. Microbiol. Biotechnol. (2010) 88:689-698 (2010) B. Muller et al. describe the preparation of four fusion protein vectors that encode one of the MtSEO1 to MtSE04 genes of Medicago truncatula and the Venus yellow fluorescent protein gene. The fusion protein was successfully expressed in epidermal cells of N. benthamiana; when the respective MtSEO gene was co-expressed with MtSEO-F1 or MtSEO-F4, protein complexes were formed that resembled a forisome body but had a different phenotypes. Using the same experimental approach, in the case of MtSEO-F2 and MtSEO-F3 protein was detectable that was localized in the cytoplasm only. In addition, MtSEO-F1/MtSEO-F1venus and MtSEO-F4/MtSEO-F4venus were coexpressed in yeast to demonstrate the possibility of producing such artificial forisome bodies in larger quantities. Furthermore, large quantities of artificial forisomes can be produced by single expression of MtSEO-F1 or MtSEO-F4.
[0007] In the past decades, great strides have been made in protein biochemistry, however the purification of recombinant proteins often still presents a substantial challenge, for example for membrane-associated or toxic proteins. In particular with enzymes, it is often observed that the quantity of the enzyme and/or its activity is not within a desirable range making the cost of the assay or the like unreasonably high because of the amount of enzyme required. The expression of recombinant proteins itself may in turn be problematic; some of these proteins may not be folded correctly in the expression organism, or deposited in an inactive form as inclusion bodies within the cell. A further requirement for production is the re-usability of enzymes, which is often accomplished by immobilization on support materials (agarose, nylon). This immobilization often results in strongly reduced enzyme activities, leading to disproportionately high costs of the subsequent assays. Purification of polyclonal antibodies in particular, which is usually performed by chromatographic methods, also remains to be improved. The inventors have therefore set themselves the task to remedy this situation by providing proteins that, on the one hand, can be produced with reasonable effort and, on the other hand, have a structure or form that facilitates the use of these proteins for the afore-mentioned purposes, and/or improves the results obtained with their use compared to results obtained with known proteins or other materials previously used for this purpose.
SUMMARY OF THE INVENTION
[0008] To solve this object, the invention proposes to provide modified forisomes. They can improve and simplify many areas of protein chemistry by the biochemically active structures that are contained in the form of fusion proteins therein. When the fusion introduces enzymatic functions to the forisomes, the forisomes can serve as carrier proteins to which the enzymes are immovably coupled, thus circumventing attachment to an external matrix. The forisome may also provide a protective function to the foreign coupled protein in the context of recombinant protein production, e.g., by simplifying their purification: The foreign protein can be easily isolated in the form of forisomes and, if needed, subsequently excised by means of appropriate protease cleavage sites and corresponding proteolytic enzymes. When antigenic structures are introduced into the forisome by fusion, these structures can be employed for purification of antibodies. In addition, by selectively varying their binding properties or by changing their conformation, the bodies according to the invention may be used for micromechanical purposes.
[0009] From the above-cited work in combination with the analysis of the SEO-F genes and proteins, it is known that a fusion protein consisting of a SEO-F1 or SEO-F4 protein, a fluorescent tag, and a corresponding native protein are capable of forming forisome bodies. However, the inventors of the present invention found that the assembly of forisomes from, or with, fusion proteins containing any SEO-F unit fused to any protein is not possible. They were nevertheless able to produce artificial forisome bodies containing foreign proteins that were suitable for the purpose of the invention. These forisome bodies can be expressed in yeast, thus allowing large production of forisomes. The authors were successful because it was shown that SEO-F proteins and/or fragments thereof may be combined with either the C-terminus or the N-terminus of a variety of proteins and, optionally, of peptides, whereby forisomes are formed, provided one of the following conditions is met.
[0010] The object of the invention is accordingly achieved by providing artificial forisome bodies comprising a fusion protein of at least one SEO-F protein or an at least 50-amino acid portion thereof, and at least one additional protein or peptide, wherein [0011] (a) the additional protein or peptide has a mass of at most 30 kDa, preferably of at most 25 kDa, and/or [0012] (b) the forisome body further comprises an unfused, often native SEO-F protein or a form of said protein having C-terminal deletions of up to approximately 50, in particular of up to 45, and preferably of up to 43 amino acids and/or N-terminal deletions of up to 13 amino acids, wherein the unfused SEO-F protein has the property of forming homomeric forisome bodies in the absence of additional SEO-F proteins, or [0013] (c) the additional protein or peptide is a portion of a second SEO-F protein, with the proviso that one of the two SEO-F proteins in its unfused form is capable of forming homomeric forisome bodies, and the fusion protein is comprised of an N-terminal SEO-F protein portion and a C-terminal SEO-F protein portion, wherein the fusion is within a region that is identical or approximately identical in both SEO-F proteins and is located within an identical or substantially identical region of the proteins relative to a region that is relevant for their function, so that the fusion protein represents a complete SEO-F protein, wherein however up to approximately 50 amino acids, in particular up to 45 amino acids, and preferably up to 43 amino acids of the C-terminus and/or 13 amino acids of the N-terminus may be deleted.
[0014] Of course, the present invention also encompasses forisome bodies that fulfill more than one of the conditions (a), (b) and (c).
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] FIG. 1 shows the schematic illustration of motif M1. Motif M1 is characteristic for proteins of the SEO family and located near their C-terminus in the SEO-CTD. Of special interest are the four highly conserved cysteines of this motif. In the forisome subunit MtSEO-F1, these cysteines are at the positions 615, 620, 633 and 634 of the amino acid sequence.
[0016] FIG. 2 shows an activity assay for the purified MtSEO-F1/MtSEO-F2-G6PDH forizymes and MtSEO-F1 control forisomes based on measuring the formation of NADPH by monitoring the absorbance at 340 nm in a G6PDH enzyme assay.
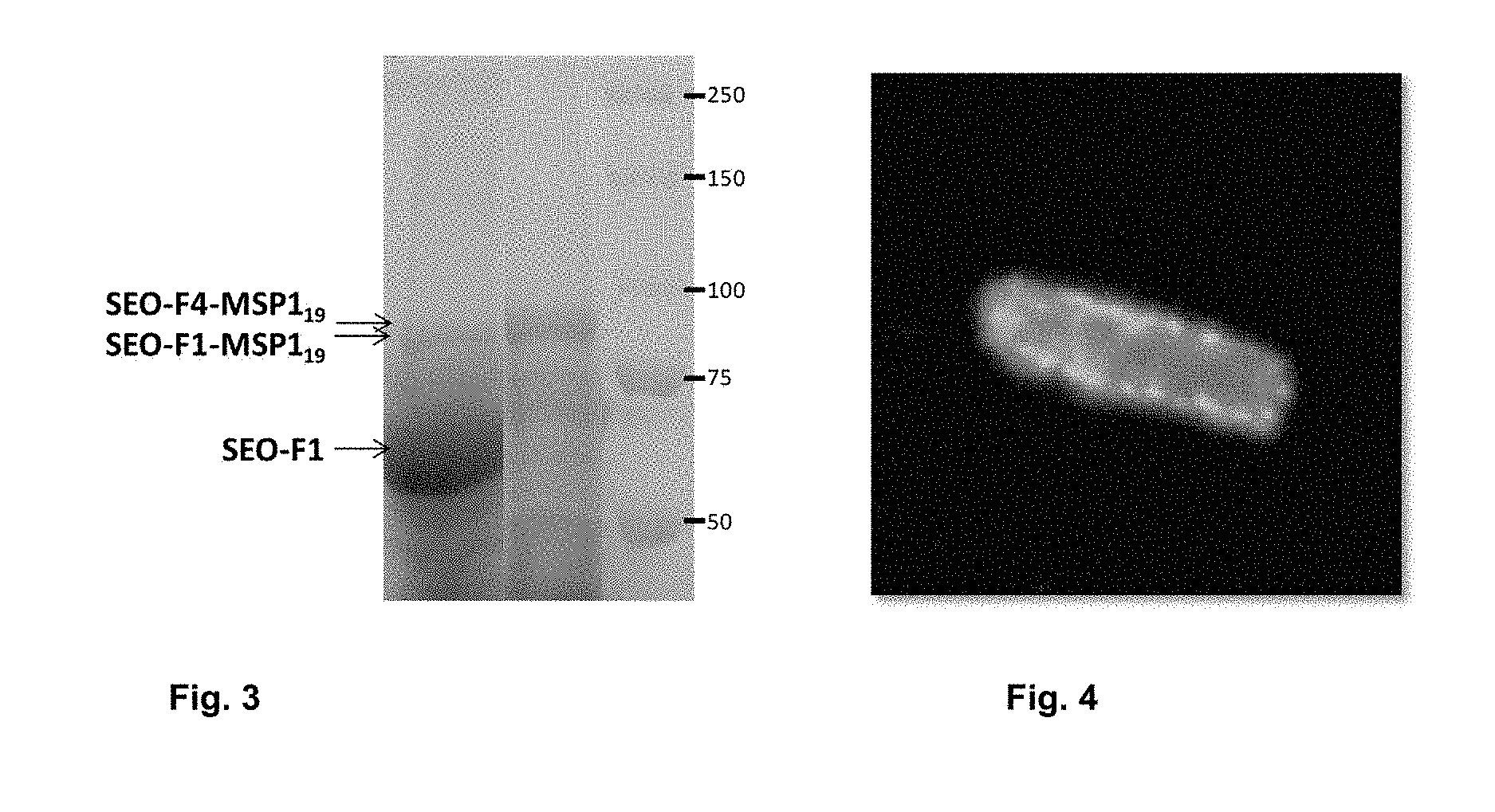
[0017] FIG. 3 shows the recombinant protein purification of MSP.sub.19, using protein bodies consisting of either one or two forisome subunits.
[0018] FIG. 4 shows utilizing the interaction of the B-domain and IgG antibodies for immobilizing artificial forisomes. For visualization, the B-domain is fused to the SEO-F1 subunit of the forisome body and detected by a fluorescence-coupled IgG antibody.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
[0019] As already mentioned above, forisome bodies assembled from the combination of fusion proteins MtSEO-F1venus and MtSEO-F4venus with corresponding native proteins such as MtSEO-F1/MtSEO-F1venus and MtSEO-F4/MtSEO-F4venus are known from the prior art. These shall be excluded from the scope of the patent. They were produced for the purpose of forisome detection, while the present invention is in no way aimed at detecting forisomes, but at solving specific problems that occur in protein chemistry. Therefore, all such forisome bodies shall fall outside the scope of the patent that were generated from or with fusion proteins having a SEO-F protein or SEO-F peptide fused to a GFP protein (see SEQ ID NO:9 in Sequence Listing) or to a Venus protein (see SEQ ID NO:10 in Sequence Listing) or a portion thereof, or fused to a (artificial) variant thereof, provided the fusion protein is fluorescent, optionally by use of excitation light (such as blue or ultraviolet light). Those forisome bodies shall also not fall within the scope of the patent that are constructed to contain fusion proteins fused to other fluorescent or otherwise visually detectable peptides and proteins, e.g., chemiluminescent proteins, provided said fusion proteins are not biochemically active or activatable in the sense hereinafter defined. In the broadest sense, this may optionally apply to any forisome body assembled from or comprising fusion proteins containing protein components of non-SEO-F molecules that serve no other purpose, or are generally not intended to serve another purpose than detecting the presence of the desired fusion. The exceptions named of course extend to all products of this invention that contain the above-mentioned forisome bodies or with which said forisome bodies and/or corresponding fusion proteins can be produced.
[0020] The invention is aimed at the production of forisome fusion proteins that confer artificial biochemical activity or activatability or altered mechanical properties to the forisomes. Therefore, the additional peptide or protein is selected from the group of biochemically active or activatable proteins or peptides, and portions of a second SEO-F protein.
[0021] The term "biochemically active or activatable proteins or peptides" according to the invention includes, among others, any protein involved in metabolism such as enzymes-due to their biocatalytic effects-, any protein capable of eliciting an immune reaction, or proteins that are therapeutically beneficial such as in particular antibodies and antigens, all peptides or proteins having binding sites for foreign proteins or peptides, and other biotechnologically useful proteins and peptides. The term "biotechnologically useful" according to the invention includes for example any protein and peptide whose synthesis may be of significance for medical applications or diagnostic methods. Several proteins can be immobilized due to their affinity reaction with substrate-bound biological or biochemically-produced materials in order to enable their re-usability. Such proteins or peptides are also included in the term "biotechnologically useful." Not covered by the term on the other hand are proteins or peptides that are (exclusively) designed to detect the fusion protein formation such as optically detectable, in particular fluorescent proteins, especially when said proteins or peptides do not possess biocatalytic activity or any other of the above-mentioned properties.
[0022] The inventors have found that forisome bodies can generally always be assembled in yeast when the fusion proteins of the invention are co-expressed with an unfused, for example native SEO-F protein, provided said SEO-F proteins has the property of forming homomeric forisome bodies in the absence of other SEO-F proteins, see condition (b). This is likely due to the fact that because of the presence of homomer-forming SEO-F molecule, the number and characteristics of the structures relevant for assembly is relatively high.
[0023] Surprisingly, however, the inventors have found that the above-defined fusion proteins assemble to forisome bodies even in the absence of said unfused SEO-F proteins in yeast when the proportion of foreign protein does not exceed a certain size. The inventors have found that this occurs when the non-SEO-F-portion has a mass of at most 30 kDa. It is more advantageous to limit the size to approximately 25 kDa (condition (a)). The forisome bodies thus obtainable are somewhat thinner and more fibrous, but can still be purified.
[0024] In regards to the definition of artificial forisomes in provision (c) it must be mentioned that in the context of the invention it was determined that SEO-F fusion products having all required properties of a SEO-F protein can be produced artificially. This requires that at least a portion of the fusion protein is derived from a SEO-F protein that is capable of forming homomeric forisomes. It is believed that in these proteins the structures that are required for assembly and thus contribute to the formation of forisomes are more pronounced. The aforementioned possibility that a certain deletion, which can be more extensive in the C-terminal region than in the N-terminal region, is, according to inventor's preliminary opinion without being absolutely bound thereto, due to the fact that the structures relevant for assembly are not located within these regions.
[0025] The inventor's discovery that according to conditions (c) artificial SEO-F proteins can be obtained that have the ability of assembling to homomeric forisomes, i.e., without additional, for example unfused protein, enables the preparation of forisomes having assembly properties that can be appropriately controlled, e.g. increased. In this way the mechanical properties of such forisomes can be adjusted to the desired applications. For example, the conditions (Ca.sup.2+ concentration and/or pH and/or electrical stimuli) required for conformational changes can be varied so that the forisomes can also be technically used under conditions that are not able capable of inducing conformational changes in native forisomes.
[0026] According to the invention, the fusion protein may contain the additional protein C-terminally i.e. based on the cloning vector and the DNA reading frame, "upstream", or N-terminally, i.e. based on the cloning vector and the DNA reading frame "downstream."
[0027] Particular advantages of using the present invention can be achieved in the following areas: [0028] a) Enzyme immobilization is used for industrial enzymes as it offers the advantage of re-using enzymes and minimizing contaminations in the enzyme product. However, the carrier material generally reduces the stability and activity of enzymes compared to their soluble forms. To date, enzyme immobilization is conducted mainly by adsorption, entrapment, cross-linking, or covalent binding of the enzyme to substrate materials. Disadvantages of immobilization methods include for example insufficient binding of the enzyme following adsorption and inclusion, the use of toxic chemicals for cross-linking, and blockage of essential functional amino acids groups when covalent bonds are introduced. The support materials used to date are synthetic polymers such as acrylic resins, hydrogels and silica, smart polymers such as PNIPAM, or biopolymers such as agarose, cellulose, starch, and chitosan. For example, glucose-6-phosphate dehydrogenase immobilized to agarose beads with an activity of 1000-1750 Units/gram agarose is commercially available. The purification of the enzyme, and the subsequent coupling of the enzyme to the carrier material thereby represent two separate steps, wherein the enzyme activity after immobilization is greatly reduced. [0029] b) Depending on the properties of the protein, expression of recombinant proteins may be problematic. For example toxic proteins affect the vitality of the expression organism and reduce the amount of recombinant protein produced. Other proteins are not properly folded or deposited as inclusion bodies in inactive forms within the cell. Other problems may occur during purification of the recombinant protein. For example, the isolation of membrane proteins is complicated by their interaction with membrane components, or proteins may be degraded during the purification process. Furthermore, the process of protein purification is usually very expensive and often requires the use of large amounts of environmentally harmful chemicals. In practice, even in industrial manufacturing process, the purification consists of multiple steps. The steps involved include precipitation, filtration, or chromatographic methods. The most important criteria of these methods are the purification efficiency, cost efficiency, and biological sustainability. For example, precipitation is very cost-effective, but delivers a low degree of purity and requires use of large amounts of chemicals, while filtration or chromatographic methods are often very expensive. For this reason, the development of new purification methods that increase the purity of the product, reduce costs, and minimize the use of chemicals are of great interest to the industry. [0030] c) Polyclonal antibodies are generated by injecting animals with respective antigens (proteins or peptides). Several weeks later, the polyclonal serum may be harvested from the blood of the animal. For the generation of monoclonal antibodies, plasma cells from spleen or lymph nodes of immunized animals are isolated, fused with tumor cells, and grown in sterile culture. After several rounds of selection, hybridoma cultures can be obtained that originate from a single cell and secrete the desired monoclonal antibody. In particular with polyclonal antibodies, more rarely with monoclonal antibodies, the serum contains not only the desired antibodies but also undesired antibodies (e.g., keratin antibodies) and/or substances that interfere with detection (e.g., proteins that are similar to the antigen used, or proteins that aggregate and interfere with detection methods.) These substances must be removed from the desired antibody. To date, this has been accomplished by chromatographic methods wherein the antigen is bound to a column matrix. The matrix is subsequently incubated with the "impure" antibody solution, allowing the specific antibodies to bind to the antigen, and thus to the matrix. After the matrix is washed, the antibodies are eluted from the column (e.g., by a solution with an acidic pH.) A simplification of this laborious method and increased efficiency is extremely desirable. [0031] d) The advantages of the invention, however, are not limited only to the manufacture and the properties of foreign proteins; they can be advantageously used in the field of forisomes itself: As mentioned above, forisomes are plant mechanoproteins that can be employed e.g., as control modules in microfluidic systems due to their calcium or pH-inducible conformational changes. These properties allowed A. Q. Shen et al. in Smart Struct. Syst. 2, 225-235 (2006) and K. Uhlig et al. in J. Microelektromech. Sys. 17, 1322-1328 (2008) to demonstrate that the flux of fluorescent particles in microchannels could be controlled using forisomes integrated therein. However, targeted, permanent attachment of forisomes can only be achieved to date manually with the help of micromanipulation techniques that require a very large amount of time and effort. Thus, Shen et al. and Uhlig et al. (loc. cit.) took advantage of forisome's natural adhesion to glass. Forisomes thereby adhere to surfaces when pressed against them. However, the adhesion does not enable permanent attachment of the forisomes in a fluid stream. In addition, the strength of the forisome reaction is reduced upon their adhesion to surfaces (G. A. Noll et al., Bioeng. Bugs 2, 111-114 (2011)).
[0032] The provision of forisomes with conformational properties that can be altered by known stimuli (e.g., upon conversion from a condensed to dispersed state and vice versa at a different pH or with a different Ca.sup.2+ concentration) is also desirable.
[0033] The inventors of the present invention succeeded in providing a material that has advantages in all four of the mentioned areas. It was thereby found that expression of fusion proteins is often possible when forisome proteins that can form homomers independently of the presence of other forisome subunits are co-expressed in the same cell. In contrast, the expression of the fusion protein alone yields usable product only when small foreign proteins are used, while in other cases forisome bodies are not formed and instead the protein is present in soluble form or deposited in the cell as "inclusion body."
[0034] When a fusion protein is co-expressed with a homomeric forisome body forming SEO-F subunit and/or a when a fusion protein of a relatively small foreign protein component is expressed, stable forisome bodies can be expressed in plants and in yeast having substantially the form of native forisomes, despite the presence of a foreign protein or peptide. Thus, the invention offers the possibility of producing individually modulatable functionalized artificial forisomes. This was surprising in itself, but also in particular the finding that the assembly of the forisome bodies did not impede the functional activity of the foreign protein. Using the example of enzymes fused to SEO-F units it was shown that the forisome bodies reduced the activity of the foreign proteins to a lesser extent than commercially applied immobilization matrices; it may be assumed that this applies to all fusion proteins, despite not having being demonstrated for a number of other proteins due to lack of quantitative comparisons.
[0035] The use of MtSEO-F1 and MtSEO-F4 is particularly preferred; however, SEO-F subunits from other sources may be used equally well.
[0036] It has been found that it is not necessary for the entire amino acid chain of a respective native SEO-F subunit to be present in the fusion protein. Instead, even a relatively small amount thereof suffices, for example, a region of approximately 60 to 250 amino acids in length, as the inventors were able to determine via fusion with fluorescent proteins. This also corresponds to the finding that the presence of homomer-forming proteins such as SEO-F1 and/or SEO-F4 determines whether forisome are formed when the foreign protein exceeds a certain size.
[0037] The SEO-F component of the fusion protein can be derived from any SEO-F subunit; preferably, it is derived from the subunits SEO-F1, SEO-F2 and SEO-F4, especially from MtSEO-F1, MtSEO-F2 and MtSEO-F4.
[0038] The coexpressed, unfused SEO F protein, if present, should be substantially or at least in large part complete in order to ensure forisome formation. The inventors have found, however, that it is not required for the entire chain of respective subunits to be present. An N-terminal deletion of at least up to 13 amino acids and/or C-terminal deletions of at least up to 43 amino acids, optionally of up to 45 and possibly up to 50 amino acids are acceptable without the forisome bodies of the present invention being adversely affected.
[0039] The forisome bodies of the present invention may be comprised of any number of subunits; generally, a species of a non-fused SEO-F subunit in combination with a species of a fusion protein is sufficient, or a species of the fusion protein alone, provided the foreign protein component does not exceed the mentioned size. The forisome bodies generally consist of from approximately 10.sup.6-10.sup.7 individual protein chains, wherein optionally the ratio of the number of unfused SEO-F subunits to the number of fusion proteins is approximately between 4:1-1:1, depending on the type and size of the foreign protein.
[0040] Individual forisome bodies of the invention are generally comprised of only one type of fusion protein; however, they may also contain several different fusion proteins. A specific, particularly advantageous example thereof is illustrated below in point 1).
[0041] The origin of the native source of the respective forisome subunits is not significant for the invention. It was possible to produce forisome bodies according to the invention with SEO-F genes, for example, from the organisms Dipteryx panamensis, Pisum sativum, Vicia faba, Canavalia gladiata and Lotus japonicus. This suggests that it is possible to employ corresponding genes of any plants of the Fabacea family in the invention. In addition, genetically modified or synthetic SEO-F genes and/or forisomes subunits may be employed provided all of the conserved regions of genes of this plant family are preserved and/or present.
[0042] It has been suspected for some time that a sequence of four cysteines in the amino acid sequence of the various forisomes subunits greatly affects their structure and stability. These cysteines are located in the C-terminal portion of the amino acid sequence (following position 600) of all three forisomes subunits SEO-F1, F2, and SEO-SEO-F4, in each case within a highly conserved motif CPNPXCGRVMEVXSXXYKCC (where X denotes a variable amino acid). This motif is highly conserved in all SEO genes (i.e., also in those of other plant families). The corresponding sequence motif is shown in FIG. 1. However, the inventors have shown that the presence of this region is not essential for forisomes formation: As mentioned above, it is possible to use a SEO-F-protein in form of a coexpressed unfused SEO-F protein or a fusion protein comprising two SEO-F components, having a C-terminal deletion of up to 43, possibly even up to 45 or even 50 amino acids without the inventive feature of protein chain aggregation being lost. However, when the complete sequence of a SEO-F1 or SEO-F4 is used, or at least a sequence in which at least a part or all of the said conserved motif is present, the above cysteines obviously have a significant role: It has been shown that when the mentioned cysteines are partially or completely replaced, for example by "site-directed mutagenesis," by amino acids which do not allow disulfide bond formation, e.g., glycine or alanine, the conformational states of the forisome bodies changes as follows: If the last two of said cysteines (cysteines C21 and C22 in the sequence motif) are mutated, the protein fibrils no longer assemble in all cases to forisome bodies, but may form a random fiber network. Without being bound by theory, it can therefore be assumed that the disulfide bonds between said cysteines of two SEO-F subunits are responsible for the ordered assembly of the protein fibrils. If, in contrast, at least one of the first two said cysteines (cysteines C3 and C8 in the sequence motif) is mutated, a typical forisome body is assembled upon its expression which, however, completely dissolves when calcium ions and NaHSO.sub.3 are added. Calcium thereby triggers the protein fibrils to repel, while the addition of NaHSO.sub.3 disrupts remaining disulfide bonds. It can therefore be assumed that the C3 and C8 cysteines are involved in the association of individual subunits to form fibers, which allows the protein to adopt its soluble form upon mutagenesis.
[0043] The fibrous bodies may have advantageous properties and are encompassed by the invention. The term "artificial forisome," as used in the present invention, is therefore intended to also encompass the fiber network in at least one embodiment of the invention.
[0044] The production of soluble forisomes-bodies as described above is particularly advantageous, as it may facilitate the purification of proteins, as shown in the examples below.
[0045] As mentioned above, the preparation of forisome bodies is preferably performed in cells of plants or yeast, with the use of yeast cells being particularly beneficial because they enable the production of large amounts of artificial forisome bodies. The invention is therefore also directed to the corresponding transformed cells. Finally, the invention also comprises novel vector constructs by means of which forisome bodies according to the invention can be produced.
[0046] The invention shall be detailed with reference to several examples that demonstrate the breadth of application of the invention on the one hand and on the other specify the individual measures that enable the expert to carry out the invention. It should therefore be clear that the above examples are not meant to be limiting.
[0047] 1) Forisome Bodies with Enzyme-Linked Fusion Proteins
[0048] The linking of enzymes to SEO-F proteins allows the artificial forisomes to be functionalized in such a manner that they can serve as substrates for enzymes. Enzymes may thus be immobilized. Enzyme-linked forisomes are constructed as follows: They consist of a first, optionally shortened, SEO-F subunit that is fused to an enzyme, and optionally a second SEO-F unit selected from SEO-F1 and SEO-F4, which may be deleted as described above if necessary or if desired. The enzyme may be fused to the C- or N-terminus of the fusion protein. Fusions proteins can be generated by coexpression in organisms suitable for expression such as yeast (e.g., Saccharomyces cerevisiae), bacteria (e.g., Escherichia coli) or plants (e.g., tobacco). The (co-) expression in yeast is particularly preferred. The resulting enzyme-linked forisomes are characterized by high stability. They are isolated from the expression organism (e.g., by disruption of yeast cells) and are separated from cell components, e.g., by centrifugation/density gradient centrifugation. Appropriate enzyme activity assays are used to verify the activity of the coupled enzyme. Using glucose-6-phosphate dehydrogenase as an enzyme fused to the N-terminus of a forisome subunit, a significantly higher enzymatic activity was measured in comparison to the commercially available immobilized enzyme (2700 Units/gram forisome compared to 1000-1750 U/g agarose, see SEQ ID NO:4 in Sequence Listing). The enzyme was isolated directly in an immobilized form from the production organism, thereby omitting the step of substrate coupling in the enzyme production. This not only facilitates the procedure, but obviously and surprising causes an extreme increase in activity. Fusion proteins containing hexokinase and phosphoglucoisomerase that were prepared in a similar manner yielded similar results.
[0049] When not only one, but two or even more fusion proteins are coexpressed, wherein the fusion partners are selected so that the reaction product of the first enzyme is a substrate for the second enzyme and its reaction product is optionally a substrate for a third protein, etc., reaction complexes can be generated that allow certain reaction pathways to take place.
[0050] In the fusion protein, the enzyme can also be bound to the C-terminus of the SEO-F protein.
[0051] 2) Purification of Recombinant Proteins
[0052] As mentioned above, the artificial forisome bodies of the invention can also be used as purification systems for recombinant proteins. Said proteins are thereby fused to a SEO-F subunit and the fusion protein is optionally co-expressed with a second SEO-F subunit that is able to form homomers in the absence of other subunits, as described above, for example, in yeast or plant cells. The recombinant protein may be present at the C-terminus or N-terminus of the fusion protein, and optionally contain a protease restriction site that enables the foreign protein to be cleaved from the forisome body following purification. The isolation and purification of the obtained artificial forisomes is performed by cell disruption and e.g., centrifugation/density gradient centrifugation. Alternatively, the protein polymer can be converted from the solid state polymer to a soluble state, in particular following mutation of one or more of the above-described C-terminal conserved cysteines by means of high Ca.sup.2+ concentration (<2 mM), or by a combination of high Ca.sup.2+ concentration (<2 mM) and reducing conditions (>18.5 .mu.M NaHSO.sub.3). Thus, forisome technology presents an entirely new purification system that completely omits traditional methods such as precipitation, filtration, and chromatography, and instead is based on centrifugation and the conformational state of the protein. Based on this technology, purification of a variety of proteins can be simplified and the cost reduced. In addition, the purification system offers the advantage that toxic effects or interactions of the proteins to be purified with the membrane can be minimized or prevented by fusion to forisomes. Using this approach, the malaria antigen MSP1.sub.19 for example was successfully purified by the present invention; this is extremely difficult by other means due to the strong interaction of MSP1.sub.19 with the membrane. The purification is illustrated by immunological detection of the antigen, which is shown in FIG. 3.
[0053] 3) Purification of Antibodies
[0054] The invention enables purification of polyclonal or monoclonal antibodies to be performed using artificial forisomes, thereby avoiding previous chromatographic separation steps. For this purpose, the antigen is cloned upstream or downstream to a forisome gene (MtSEO-F1 or MtSEO-F2 or MtSEO-F4 and/or a portion thereof, as defined above) by the methods described previously. The antigen-MtSEO-F-fusion product is subsequently expressed in yeast, optionally together with MtSEO-F1 and MtSEO-F4 having C-terminal and/or N-terminal deletions of up to 13 amino acids. This procedure yields artificial forisomes that contain the antigen in the yeast cells.
[0055] The yeast cells are grown, pelleted by centrifugation, and the cells disrupted. The artificial forisomes carrying the antigen are now free in solution and can be used to purify the polyclonal or monoclonal antibodies as follows.
[0056] The antigen-containing artificial forisomes are incubated with antibody serum, whereby the specific antibodies bind to the artificial forisomes. The forisome are pelleted by centrifugation, washed, and the antibodies subsequently eluted via a pH change. The antibody solution is then neutralized and can now be used for various applications (Western blot, immunoprecipitation, ELISA, antibody therapy, etc.).
[0057] 4) Modification of Forisomes Properties
[0058] With the help of the invention, forisomes can be modified artificially to acquire new, technologically useful properties. For example, the binding of forisomes to surfaces can be improved by including SEO-F subunits fused to protein or (protein or peptide) tags in said forisomes. This approach enables their positioning and immobilization in microchannels. Examples include the fusion with the B-domain of the Staphylococcus aureus protein A, with glutathione S-transferase or with biotin, which allows selective surface functionalization of the artificial forisome produced in the organism and subsequent isolation in a manner that enables their covalent binding to surfaces coated with IgG, glutathione, or streptavidin. As before, stable forisome can be obtained when a SEO-F subunit capable of forming homomers in the absence of other subunits is coexpressed with the fusion protein, or the foreign protein component in the expression product is not too large. This circumvents problems associated with accurate positioning of forisomes employed as mechanoproteins to surfaces or to micro channels. If a fusion is performed with a further SEO-F protein instead of with a foreign protein, a mechanoprotein body is obtained having conformational change properties that can be modified by the Ca.sup.2+ concentration and pH.
[0059] The following examples of specific embodiments are intended to deepen the understanding of the invention.
Example 1--Enzyme Immobilization Using Artificial Forisomes (Enzyme Coupling)
[0060] I. The forisome genes MtSEO-F1 and MtSEO-F2 and MtSEO-F4 with and without translational stop codon were amplified from M. truncatula cDNA using the following oligonucleotides (the restriction sites are underlined):
TABLE-US-00001 MtSEO-F1 fw Ncol: 5'-AGA ACC ATG GGA TCA TTG TCC AAT GGA ACT AAA C-3' MtSEO-F1 rev Xhol with stop: 5'-AGA CTC GAG TCA TAT CTT GCC ATT CTG TGG AGC-3' MtSEO-F1 rev Xhol without stop: 5'-AGA CTC GAG CAT ATC TTG CCA TTC TGT GGA GC-3' MtSEO-F2 fw Ncol: 5'-AGA ACC ATG GGA TCC ACT GCA TTG TCC TAT AAT G-3' MtSEO-F2 rev Xhol with stop: 5'-AGA CTC GAG TCA AAT GCA ACT ATC TGG-3' MtSEO-F2 rev Xhol without stop: 5'-AGA CTC GAG ATG CAG CAA CTA TCT GGA-3' MtSEO-F4 fw Ncol: 5'-AGA ACC ATG GGA TCC CTT TCC AAC TTA GGA AG-3' MtSEO-F4 rev Xhol with stop: 5'-AGA CTC GAG TCA AAC ACC AAG ATT GTT TGG-3' MtSEO-F4 rev Xhol without stop: 5'- AGA CTC GAG ACA CCA AGA TTG TTT GGT TC-3'
[0061] The amplicons were digested with the restriction enzymes NcoI/XhoI and cloned into the corresponding restriction sites of the pENTR4.TM. vector (Invitrogen, Germany). In this way, pENTR4-MtSEO-F vectors with and without stop codons were generated.
[0062] II. The genes of the enzymes hexokinase 2 (HXK2), phosphoglucoisomerase (PGI) and glucose-6-phosphate dehydrogenase (G6PDH) from Saccharomyces cerevisiae were amplified as cDNA using the following oligonucleotides (the restriction sites are underlined).
TABLE-US-00002 G6PDH fw Xhol: 5'-AGA CTC GAG AAT GAG TGA AGG CCC CGT C-3' G6PDH rev Xhol: 5'-AGA CTC GAG CTA ATT ATC CTT CGT ATC TTC-3' HXK2 fw Xhol: 5'-AGA CTC GAG AAT GGT TCA TTT AGG TCC AAA-3' HXK2 rev Xhol: 5'-AG ACT CGA GTT AAG CAC CGA TGA TAC CA-3' PGI Xhol fw: 5'-AGA CTC GAG AAT GTC CAA TAA CTC ATT CAC-3' PGI Xhol rev: 5'-AGA CTC GAG ATC ACA TCC ATT CCT TGA ATT G-3' Invertase Xhol fw: 5'-AGA CTC GAG AGC ATC AAT GAC AAA CGA AAC-3' Invertase Xhol rev: 5'-AGA CTC GAG CTA TTT TAC TTC CCT TAC TTG G-3'
[0063] The amplicons were digested with XhoI and cloned into the corresponding restriction site of the pENTR4-MtSEO-F vectors without stop (see a) I). In this way, the following vectors were obtained: pENTR4-MtSEO-F1-G6PDH, pENTR4-MtSEO-F2-G6PDH, pENTR4-MtSEO-F4-G6PDH, pENTR4-MtSEO-F1-HXK2, pENTR4-MtSEO-F2-HXK2, pENTR4-MtSEO-F4-HXK2, pENTR4-MtSEO-F1-PGI, pENTR4-MtSEO-F2-PGI and pENTR4-MtSEO-F4-PGI.
[0064] III. The vectors pENTR4-MtSEO-F1 with stop and pENTR4-MtSEO-F4 with stop were recombined with the yeast vectors 425GPD-ccdB (Addgene, USA). The resulting expression constructs 425GPD-MtSEO-F1 and 425GPD-MtSEO-F4 were transformed into the yeast strain InvSc1 (Invitrogen, Germany). For selection, the correction of the yeast strain leucine auxotrophy was used. The resulting yeast cells produce artificial forisomes of MtSEO-F1 or MtSEO-F4 that were used as the basis for enzyme coupling.
[0065] IV. The above-mentioned pENTR4 vectors with MtSEO-F-enzyme fusions (see 1.II.) were recombined with the yeast vector 424GPD-ccdB (Addgene, USA). The resulting vectors (424GPD-MtSEO-F1-G6PDH, 424GPD-MtSEO-F2-G6PDH, 424GPD-MtSEO-F4-G6PDH, 424GPD-MtSEO-F1-HXK2, 424GPD-MtSEO-F2-HXK2, 424GPD-MtSEO-F4-HXK2, 424GPD-MtSEO-F1-PGI, 424GPD-MtSEO-F2-PGI, 424GPD-MtSEO-F4-PGI) were each transformed into yeast that already contained a plasmid (425GPD-MtSEO-F1 or 425GPD-MtSEO-F4) to generate artificial forisomes of MtSEO-F1 or MtSEO-F4 (see a) III.) The resulting double mutants (e.g., 425GPD-MtSEO-F1/424GPD-MtSEO-F2-G6PDH) are therefore corrected for their leucine as well as tryptophan auxotrophy.
[0066] V. Expression yeasts producing enzyme-coupled forisomes (see a) I.-IV.) were grown in a volume of 50 ml until the OD.sub.600 nm was between 5-7 and harvested by centrifugation (1000.times.g, 10 min). The yeast pellet was washed with 50 ml of V-medium (10 mM Tris, 10 mM EDTA, 100 mM KCl, pH 7.4), centrifuged again (1000.times.g, 10 min) and frozen at -20.degree. C. The frozen cell pellet was resuspended in 1 ml V-medium, and approximately 500 mg glass beads (425-600 .mu.m) were added. The cells were disrupted in 1.5 ml tubes at 30 Hz in the Mixer Mill MM400 (Retsch, Germany). The artificial forisome with the insoluble cell components were subsequently pelleted by centrifugation and resuspended in 0.5 ml V-medium. The solution was loaded on a sucrose or Nycodenz density gradient in which the sucrose or Nycodenz concentration increased from 40% to 70%. The gradient was centrifuged in a Beckman ultracentrifuge at 163,000.times.g at 4.degree. C. for 3 h.
[0067] The forisome-containing phase was subsequently removed from the gradient with a pipette, diluted 1:2 with V-medium and divided into 2 equal aliquots. The aliquots were centrifuged for 10 minutes at 100.times.g and the supernatant removed. The forisomes of the first aliquot were then taken up in 50 .mu.l V-medium and used to determine the molecular mass and concentration of the enzyme-coupled artificial forisomes by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The second aliquot was taken up in 50 .mu.l enzyme buffer (for G6PDH-coupled forisomes: 250 mM glycylglycine buffer, pH 7.4; HXK2-coupled forisomes: 0.05 M Tris-HCl buffer with 13.3 mM MgCl.sub.2, pH 8; PGI-coupled forisomes: 250 mM glycylglycine buffer, pH 7.4). This aliquot was used to determine the activity of the forisomen-coupled enzymes using specific enzyme assays.
[0068] VI. The molecular mass and concentration of enzyme-coupled, artificial forisomes (see a) IV) were determined by SDS-PAGE analysis. The different forisome proteins comprising the enzyme-linked, artificial forisomes (e.g., MtSEO-F1 and MtSEO-F2 enzyme fusion protein) are thereby separated. The presence of the individual proteins was determined by comparing the mass predicted by bioinformatics (e.g., MtSEO-F2-G6PDH=124.7 kilodaltons) with the actual mass of the protein in the gel (MtSEO-F2-G6PDH=approx. 130 kDa). The protein concentration was determined using a standard series of defined protein amounts that was loaded simultaneously and/or by using the protein marker Precision Plus Protein Standards unstained (Bio-Rad). We were able to obtain a total amount of protein (single MtSEO-F protein+MtSEO-F-enzyme fusion) between 56-124 .mu.g of artificial, enzyme-linked forisomes, depending on the selected forisome protein and enzyme fusion, from a 50 ml yeast expression culture. The proportion of MtSEO-F-enzyme fusion relative to the total protein content is between 10%-50% depending on the fusion partner. We obtained the largest quantities, both of total protein (124 .mu.g/50 ml culture) and enzyme fusion protein (37 .mu.g/50 ml culture) when PGI-coupled enzyme forisomes were generated (MtSEO-F1/MtSEO-F2-PGI).
[0069] The activity of the forisome-immobilized enzymes was determined by specific spectrophotometric enzyme assays. For glucose-6-phosphate dehydrogenase, the protocol recommended by Sigma-Aldrich (Germany) was used. The assay is based on the G6PDH-catalyzed conversion of glucose-6-phosphate into 6-phosphogluconolactone. In this reaction, nicotinamide adenine dinucleotide phosphate (NADP.sup.+) is reduced to NADPH:
##STR00001##
[0070] The absorbance of NADPH in the wavelength range of 340 nm can be measured photometrically and used to calculate enzyme activity. For this assay, the purified enzyme forisomes from the second aliquot (see a) V.) were used. Using the determined concentration of the enzyme-linked forisomes (see a)VI.) the measured enzyme activities per gram of artificial forisome was calculated. Depending on the construct (see a) III.) activities between 2000-2700 Units per gram of artificial forisome were obtained for forisome-immobilized glucose-6-phosphate dehydrogenase. In comparison, glucose-6-phosphate dehydrogenase immobilized to agarose beads that is commercially available from Sigma-Aldrich (Germany) has only between 1000 to 1750 Units per gram of agarose. Thus, the forisome-immobilized glucose-6-phosphate dehydrogenase of the present invention exhibits a markedly higher specific enzyme activity (enzyme activity based on the amount of carrier material). FIG. 2 shows an enzyme activity assay of glucose-6-phosphate dehydrogenase that is coupled to the forisome bodies SEO-F1 and SEO-F2 of the invention.
[0071] The activity of forisome-immobilized hexokinase 2 and phosphoglucoisomerase was determined using a similar assay principle. In this case, only two successive enzyme reactions were used to measure the enzyme activity based on the increase of NADPH absorbance at 340 nm. For hexokinase 2, the protocol recommended by Worthington (Lakewood, N.J., USA) was used, which is based on the following reaction:
##STR00002##
[0072] Glucose-6-phosphate dehydrogenase required for the second reaction was added to the assay in the form of commercially available soluble enzyme with a defined activity. Depending on the construct (see a) III.) activities between 6000-8000 Units per gram of artificial forisome were obtained for forisome-immobilized hexokinase 2. In contrast, agarose-immobilized hexokinase available from Sigma-Aldrich has an activity of only 50-75 U.
[0073] For phosphoglucoisomerase, the protocol recommended by Sigma-Aldrich (Germany) was used which is based on the following reaction:
##STR00003##
[0074] Depending on the construct (see a) III.), activities between 6000-8000 Units per gram of artificial forisome were obtained for forisome-immobilized phosphoglucoisomerase. In contrast, agarose-immobilized phosphoglucoisomerase available from Sigma-Aldrich has an activity of only 300-600 U.
Example 2--Purification of Proteins
[0075] 2.1 Purification of Recombinant Proteins Using Unmutated Forisome Genes or in Absence of Unmutated Forisome Genes
[0076] I. The coding sequence of a fragment of the malaria surface antigen MSP (MSP1.sub.19) was amplified from a sequence within a vector using the following oligonucleotides (restriction sites are underlined).
TABLE-US-00003 MSP1.sub.19 Ncol fw: 5'-AGACCATGGACCTGCGTATTTCTCAG-3' MSP1.sub.19 Ncol FaXa rev: 5'-AGACCATGGTACGACCTTCGATCC TGCATATAGAAATGCC-3' MSP1.sub.19 Xhol FaXa fw: 5'-AGACTCGAGAATCGAAGGTCGTGAC CTGCGTATTTCTCAG-3' MSP1.sub.19 Xbal rev: 5'-AGATCTAGATCACCTGCATATAGAAAT G-3'
[0077] The primers MSP1.sub.19 NcoI FaXa rev and MSP1.sub.19 XhoI FaXa fw contain the coding sequence of the recognition site for the protease Factor Xa (shown in italics) in addition to the gene-specific sequences. The first amplicon was treated with the restriction enzyme NcoI and cloned into the NcoI site of the vectors pENTR4-MtSEO-F1 with stop codon, pENTR4-MtSEO-F2 with stop codon and pENTR4-MtSEO-F4 with stop codon (see a)I.) to generate the vectors pENTR4-MSP1.sub.19-MtSEO-F1, pENTR4-MSP1.sub.19-MtSEO-F2 and pENTR4-MSP1.sub.19-MtSEO-F4. The second amplicon was treated with the restriction enzymes XhoI and XbaI, and cloned into the XhoI/XbaI-restriction sites of the vectors pENTR4-MtSEO-F1 without stop codon, pENTR4-MtSEO-F2 without stop codon and pENTR4-MtSEO-F4 without stop codon (see a)I.) to generate the vectors pENTR4-MtSEO-F1-MSP1.sub.19, pENTR4-MtSEO-F2-MSP1.sub.19 and pENTR4-MtSEO-F4-MSP1.sub.19. For preparation of the expression vectors 424GPD-MSP1.sub.19-MtSEO-F1, 424GPD-MSP1.sub.19-MtSEO-F2, 424GPD-MSP1.sub.19-MtSEO-F4, 424GPD-MtSEO-F1-MSP1.sub.19, 424GPD-MtSEO-F2-MSP1.sub.19 and 424GPD-MtSEO-F4-MSP1.sub.19 the generated vectors were recombined with the yeast vector 424GPD-ccdB (Addgene, USA).
[0078] II. The vectors 424GPD-MSP1.sub.19-MtSEO-F4 and 424GPD-MtSEO-F4-MSP.sub.19 were transformed into the yeast strain InvSc1 (Invitrogen, Germany) using the correction of tryptophan auxotrophy of the yeast strain for selection. The fusion proteins comprised of MSP1.sub.19 and MtSEO-F4 form forisomes without additional expression of an additional MtSEO-F protein.
[0079] The vectors 424GPD-MSP1.sub.19-MtSEO-F1, 424GPD-MSP1.sub.19-MtSEO-F2, 424GPD-MtSEO-F1-MSP1.sub.19 and 424GPD-MtSEO-F2-MSP1.sub.19 were transformed into yeast that already contained a plasmid (425GPD-MtSEO-F1) to generate artificial forisomes of MtSEO-F1 (see a) III.). The resulting yeast (e.g., 425GPD-MtSEO-F1/424GPD-MSP1.sub.19-MtSEO-F1) are corrected for their leucine and tryptophane auxotrophy and provide artificial forisomes fused to the MSP1.sub.19 protein.
[0080] III. The artificial forisomes fused to MSP1.sub.19 were purified as described in 1.V and detected and quantified by SDS-PAGE and Western blotting. All constructs were suitable for purification. However, the inventors obtained the highest purification yield of 0.42 mg MSP1.sub.19 protein per liter of cell culture with the 424GPD-MPS1.sub.19-Mt.SEO-F4 construct. Future optimization by modifying culture and purification conditions will lead to higher yields of protein available for purification. Furthermore, the MSP1.sub.19 protein can be cleaved from the artificial protein by incubation with Factor Xa protease. In addition, the inventors have observed that certain reducing and calcium-containing buffer conditions (4 mM CaCl.sub.2), 200 .mu.M NaHSO.sub.3, 10 mM TRIS, 100 mM KCl, pH 7.2) can lead to disassembly of artificial forisomes (especially when the cysteines in position 615 and 620 of the MtSEO-F1 protein are mutated). This conversion from the insoluble form to the soluble form may also be used for protein isolation and purification. FIG. 3 shows the purification of MSP1.sub.19 using forisome bodies of SEO-F1 or SEO-F4. The immunological detection of MPS1.sub.19 is shown.
[0081] 2.1b Purification of Recombinant Proteins Using Forisome Genes Containing Mutated Cysteines
[0082] The cysteines located at positions 3 and 8 in the sequence motif (FIG. 1) of the MtSEO-F1gene were mutated to serines using the QuikChange II Site-Directed Mutagenesis Kit from Agilent Technologies (CA, USA) according to manufacturer's instructions. The vector pENTR4-MtSEO-F1 with and without stop codons (Example 1) served as a substrate. The cysteines at position 3 and position 8 correspond to amino acids 615 and 620 of the MtSEO-F1 protein. The resulting mutated MtSEO-F1 gene is therefore hereinafter named MtSEO-F1 (C615S/C620S).
[0083] The coding sequence of a fragment of the malaria surface antigen MSP (MSP1.sub.19) was cloned into the vector pENTR4.TM. (Invitrogen, Germany) upstream and downstream of MtSEO-F1 (C615S/C620S) as described in Example 2.1a.
[0084] By recombination of the vector pENTR4-MtSEO-F1 (C615S/C620S) with the yeast vectors 425GPD-ccdB (Addgene, USA) and recombination of the vectors pENTR4-MSP1.sub.19-MtSEO-F1 (C615S/C620S) and pENTR4-MtSEO-F1 (C615S/C620S)-MSP1.sub.19 with the yeast vectors 424GPD-ccdB (Addgene, USA), the expression vectors 425GPD-MtSEO-F1 (C615S/C620S), 424GPD-MSP1.sub.19-MtSEO-F1 (C615S/C620S), 424GPD-MtSEO-F1 (C615S/C620S)-MSP1.sub.19 [were generated].
[0085] The following combinations of yeast vectors were transformed into the yeast strain InvSc1 (Invitrogen, Germany)
425GPD-MtSEO-F1(C615S/C620S)+424GPD-MSP1.sub.19-MtSEO-F1(C615S/C620S)
and
425GPD-MtSEO-F1(C615S/C620S)+424GPD-MtSEO-F1(C615S/C620S)-MSP1.sub.19
[0086] The correction of the leucine and tryptophan auxotrophy of the yeast strain was used for selection. The resulting yeasts produce artificial forisomes comprised of MtSEO-F1 (C615S/C620S) that contain MSP1.sub.19 protein.
[0087] Almost 100% of the resulting artificial forisomes can be converted into the soluble form with reducing buffer containing calcium ions (4 mM CaCl.sub.2), 200 .mu.M NaHSO.sub.3, 10 mM TRIS, 100 mM KCl, pH 7.2), while only a small proportion of the non-mutated version converts to the soluble form.
[0088] The purification process can thereby be abbreviated. After cultivation, the yeast cells containing artificial forisomes with MSP1.sub.19 protein can be disrupted, the artificial forisome and yeast components separated from soluble components by centrifugation, and the protein-forisome-fusions products then brought into solution.
[0089] 2.2 Purification of Antibodies Using Artificial Forisomes
[0090] I. The coding sequence of the Small Rubber Particle Protein 3 (SRPP3) was amplified from sequences within a vector with the following oligonucleotides (restriction sites are underlined).
TABLE-US-00004 SRPP3 Xhol fw: 5'-AGA CTCGAG A ATGACCGACGCTGCTT C-3' SRPP 3 Xhol rev: 5'-AGA CTCGAG TCATGTTTCCTCCACAAT C-3'
[0091] The amplicon was treated with the restriction enzyme XhoI and cloned into the XhoI site of the vector pENTR4-MtSEO-F1 without stop codon (see a)I.) to generate the vector pENTR4-MtSEO-F1-SRPP3. To generate the expression vector 424GPD-MtSEO-F1-SRPP3 the resulting vector was recombined with the yeast vector 424GPD-ccdB (Addgene, USA).
[0092] II. The vector 424GPD-MtSEO-F1-SRPP3 was transformed into yeast cells that already contained a plasmid (425GPD-MtSEO-F1) to produce artificial forisomes of MtSEO-F1 (see 1.III.). The resulting yeasts (e.g., 425GPD-MtSEO-F1/424GPD-MtSEO-F1-SRPP3) are corrected for their leucine and tryptophan auxotrophy and present artificial forisomes fused to the SRPP3 protein. The yeasts were grown in a volume of 50 ml to OD.sub.600, centrifuged and resuspended in 1 ml V-medium (10 mM Tris, 10 mM EDTA, 100 mM KCl, pH 7.4), and disrupted by means of a ball mill. The artificial forisomes carrying the antigen were then free in solution and could be used in the following for purification of polyclonal or monoclonal antibodies.
[0093] III. The artificial forisomes containing antigen were incubated for 30 minutes with 500 .mu.l of a polyclonal anti-SRPP3 serum that was produced in rabbit. The specific antibodies thereby bound to the artificial forisomes. The forisomes were pelleted by centrifugation (4000.times.g, 4 min), and washed three times with 1 ml PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na.sub.2HPO.sub.4, 2 mM KH.sub.2PO.sub.4, pH 7.4). Then the antibodies were eluted with 450 .mu.l 0.1 M glycine-HCl solution (pH 2.7) for 5 min. Subsequently, the antibody solution was neutralized with 50 .mu.l 1 M Tris-HCl solution (pH 8.5). Subsequent blots demonstrated the high specificity of the purified antibody was detectable (without serum contamination). The antibodies purified by means of the forisome technology were used for various purposes (Western Blot, immunoprecipitation, ELISA, antibody therapy, etc.). The principle of this purification is shown in FIG. 3; FIG. 4 shows the use of the interaction between the B-domain and the IgG antibody for immobilization of artificial forisomes. An artificial forisome consisting of SEO-F1 subunits coupled to the B-domain binds fluorescent IgG antibodies.
Example 3 Immobilization of Artificial Forisomes to Technical Surfaces (Surface Coupling)
[0094] I. The coding sequence of glutathione-S-transferase (GST) was amplified from sequences within the pGex-3X vector (GE Healthcare, USA) using the following oligonucleotides
[0095] (restriction sites are underlined).
TABLE-US-00005 GST Ncol Xhol fw: 5'-AGA CCA TGG GAC TCG AGA ATG TCC CCT ATA CTA GGT TA-3' GST Sall rev: 5'-AGA GTC GAC TTA ACG ACC TTC GAT CAG ATC-3'
[0096] The fragment was treated with the restriction enzymes NcoI/SalI and cloned into the NcoI/XhoI-digested pENTR4.TM. cloning vector, resulting in the vector pENTR4-GST. Subsequently, the amplicon containing the MtSEO-F1 gene with stop (see SEQ ID NO:1 in Sequence Listing) was cloned into the NcoI/XhoI-sites of the resulting vector to generate the vector pENTR4-GST-MtSEO-F1. To generate the expression vector 424GPD-GST-MtSEO-F1, the vector pENTR4-GST-MtSEO-F1 was recombined with the yeast vector 424GPD-ccdB (Addgene, USA). The expression vector was transformed into yeasts cells that already contained a plasmid (425GPD-MtSEO-F1) to produce artificial forisomes of MtSEO-F1 (see a) III.). The resulting yeast (425GPD-MtSEO-F1/424GPD-GST-MtSEO-F1) are corrected for their leucine and tryptophan auxotrophy and present artificial forisomes with a GST-tag. They were purified as described in a)V. and the presence of the respective proteins (MtSEO-F1 and GST-MtSEO-F1) was detectable by SDS-PAGE. It was further shown that the resulting artificial GST-coupled forisomes bound to a glutathione-coupled matrix (Glutathione Sepharose 4B, Amersham Bioscience, USA).
[0097] II. The coding sequence of the B domain of Staphylococcus aureus protein A was amplified from sequences within the vector 424GPD-ccdB-TAP (Addgene, USA) using the following oligonucleotides (restriction sites are underlined).
TABLE-US-00006 B domain Ncol fw: 5'-AGACCATGGCGGATAACAAATTCAAC A-3' B domain Ncol rev: 5'-AGACCATGGCTTTTGGTGCTTGAGCA TC-3' B domain Xhol fw: 5'-AGACTCGAGAGCGGATAACAAATTCAA C-3' B domain Xhol rev: 5'-AGACTCGAGTCATTTTGGTGCTTGAGC ATC-3'
[0098] The first amplicon was treated with the restriction enzyme NcoI and cloned into the NcoI restriction site of pENTR4-MtSEO-F1 with stop codon and pENTR4-MtSEO-F4 with stop codon (see a) I) to generate the vectors pENTR4-B-domain-MtSEO-F1 and pENTR4-B-domain-MtSEO-F4. The second amplicon was treated with the restriction enzyme XhoI and cloned into the XhoI restriction site of the vector pENTR4-MtSEO-F1 without stop codon and pENTR4-MtSEO-F4 without stop codon (see a)I.) to generate the vectors pENTR4-MtSEO-F1-B-domain and pENTR4-MtSEO-F4-B-domain. To produce the expression vectors 424GPD-B-domain-MtSEO-F1, 424GPD-B-domain-MtSEO-F4, 424GPD-MtSEO-F1-B-domain, 424GPD-MtSEO-F4-B domain, the vectors produced were recombined with the yeast vector 424GPD-ccdB (Addgene, USA).
[0099] The vectors 424GPD-B-domain-MtSEO-F4 and 424GPD-MtSEO-F4-B-domain were transformed into the yeast strain InvSc1 (Invitrogen, Germany). For selection, the correction of tryptophan auxotrophy of the yeast strain was used. The fusion proteins of the B domain and MtSEO-F4 assembled to forisome-like structures without additional expression of another MtSEO-F protein.
[0100] The vectors 424GPD-B-domain-MtSEO-F1 and 424GPD-MtSEO-F1-B-domain were transformed into yeast that already contained a plasmid (425GPD-MtSEO-F1) to produce artificial forisomes of MtSEO-F1 (see 1.III.). The resulting yeasts (e.g., 425GPD-MtSEO-F1/424GPD-B-domain-MtSEO-F1) are corrected for their leucine and tryptoph anauxotrophy and present artificial forisomes fused to a B-domain. All of the artificial forisomes generated that contained B-domains bound to IgG-coupled Sepharose (GE Healthcare, USA).
[0101] 2.3 Preparation and Purification of Artificial SEO-F Forisomes Containing Two Different SEO-F Proteins
[0102] 2.3.1: Fusion of Amino Acids 1-96 of MtSEO-F4 and Amino Acids 73-648 of MtSEO-F1.
[0103] The N-terminal MtSEO-F4-fragment and the C-terminal MtSEO-F1 fragment were amplified with the oligonucleotides
TABLE-US-00007 MtSEO-F4 MSLSN Ncol fw 5'-AGACCATGGGATCCCTTTCCAAC TTAGGAAGTG-3' MtSEO-F4 LISCQ Ncol rev 5'-AGACCATGGCCTGACAAGAAAT CAGCTT-3' MtSEO-F1 MITTR Ncol fw 5'-AGACCATGGGAATGATAACCACC CCTC-3' MtSEO-F1 QNGKI Xhol rev 5'-AGACTCGAGGTCATATCTTGCC ATTCTGTGGAG-3'
[0104] and cloned into the NcoI/XhoI-digestion pENTR4 vector. The resulting vector pENTR4-MtSEO-F4(1-288 bp)/MtSEO-F1 (219-1944 bp) was subsequently recombined with the plant expression vector pBatTL and the yeast expression vector 425GPD-ccdB. The resulting pBatTL-MtSEO-F4(1-288 bp)/MtSEO-F1 (219-1944 bp) was transformed into Agrobacterium, which was used to infiltrate N. benthamiana plants (see Muller et al., 2010). The resulting vector 425GPD-MtSEO-F4(1-288 bp)/MtSEO-F1(219-1944 bp) was transformed into the yeast strain InvSc1. In both systems, the development of artificial forisomes was observed microscopically. The purification was carried out as described above in Example 1 V.
[0105] 2.3.2: Fusion of Amino Acids 1-583 of MtSEO-F1 with Amino Acids 620-670 of MtSEO-F2.
[0106] The N-terminal MtSEO-F1 fragment and the C-terminal MtSEO-F2 fragment were amplified using the oligonucleotides
TABLE-US-00008 MtSEO-F1 MSLNS Ncol fw 5'-AGACCATGGGATCATTGTCCAAT GGAACTA-3' MtSEO-F1 FKEYY Xhol rev 5'-AGACTCGAGTGATAGTATTCTT TGAATGCAAT-3' MtSEO-F2 DTKLS Xhol fw 5'-AGACTCGAGTGATACTAAGCTTT CAGAGAT-3' MtSEO-F2 DSCCI Xhol 5'-rev AAACTCGAGTCAAATGCAGCAA CTATCTGGATCATC-3'
[0107] and cloned into the NcoI/XhoI-digested vector pENTR4. The resulting vector pENTR4-MtSEO-F1 (1-1749 bp)/MtSEO-F2(1860-2010 bp) was recombined with the plant expression vector pBatTL and the yeast expression vector 425GPD-ccdB. The generated pBatTL-MtSEO-F1 (1-1749 bp)/MtSEO-F2 (1860-2010 bp) was transformed into Agrobacterium, which was used to infiltrate N. benthamiana plants (see Muller et al., 2010). The resulting vector 425GPD-MtSEO-F1 (1-1749 bp)/MtSEO-F2(1860-2010 bp) was transformed into the yeast strain InvSc1. In both systems, the formation of artificial forisome could be observed microscopically. The purification was carried out as described in Example 1 V above.
Sequence CWU 1
1
471647PRTMedicago truncatula 1Met Ser Leu Ser Asn Gly Thr Lys Leu
Pro Asn Pro Phe Asp Leu Asp1 5 10 15Glu Ser Gln Ile Leu Asp Lys Val
Tyr Leu Thr His Leu His Asp Asp 20 25 30Asp Lys Cys Asp Lys Asp Val
Leu Phe His Ile Leu Ser Asn Val Ile 35 40 45Leu Arg Thr Arg Leu Ala
Glu Ser Arg Ala Glu Phe Glu Pro Glu Phe 50 55 60Arg Thr Leu Lys Leu
Ile Ser Cys Gln Met Ile Thr Thr Pro Arg Gly65 70 75 80Glu Arg Tyr
Val His Gln Thr Thr Met Trp Ile Leu Gln Gln Leu Lys 85 90 95Thr Tyr
Ser Trp Asp Ala Lys Ala Leu Ile Ala Leu Ala Ala Phe Thr 100 105
110Leu Glu Tyr Gly Asn Leu Leu Tyr Leu Thr Glu Thr Ser Thr Ser Ser
115 120 125Asp Gln Leu Val Asn Ser Leu Lys Ile Leu Asn Gln Ile Gln
Asn Arg 130 135 140Lys Val Thr Val Pro Ala Thr Asp Leu Val Glu Leu
Ile Met Asp Val145 150 155 160Leu Leu His Ile His Glu Trp Ala Thr
Arg Ser Gly Val Gly Tyr Asn 165 170 175Thr Leu Asp Val Pro Ser Leu
Ser Asp Ala Leu Gln Asp Ile Pro Val 180 185 190Ala Val Tyr Trp Ile
Ile Ala Ser Thr Val Ala Ala Thr Gly Asn Ile 195 200 205Ile Gly Val
Ser Asp Tyr Thr Leu Ser Asp Phe Lys Glu Lys Leu Asn 210 215 220Phe
Val Asp Ser Lys Leu Lys Glu His Leu Lys Leu Ser Lys Trp Gln225 230
235 240Ile Asp Ser Val Glu Glu Tyr Leu Lys Arg Lys Lys Ala Ile Ser
Asn 245 250 255Pro Lys Asp Ile Ile Asp Phe Leu Lys Leu Leu Ile Gln
Arg Asn Gly 260 265 270Asp Asn Leu Leu Ile Tyr Asp Gly Thr Thr Lys
Asn Lys Thr Asp Ile 275 280 285Glu Val Phe Lys Asp Lys Tyr Val Leu
Leu Phe Ile Ser Ser Leu Asn 290 295 300Lys Val Asp Asp Glu Ile Leu
Leu Leu Asn Ser Ile His Asp Arg Leu305 310 315 320Gln Asp Asn Pro
Gln Val Ile Lys Gly Tyr Lys Lys Glu Asp Phe Lys 325 330 335Ile Leu
Trp Ile Pro Ile Trp Asp Val Asp Asp Gln Lys Ile Lys Phe 340 345
350Asp Ser Leu Lys Asn Lys Ile Arg Phe Tyr Ala Val Asp Tyr Phe Ser
355 360 365Glu Leu Pro Gly Ile Arg Leu Ile Arg Glu His Leu Asn Tyr
Ser Asp 370 375 380Lys Pro Ile Val Pro Val Leu Ser Pro Leu Gly Glu
Lys Met Asn Asp385 390 395 400Asp Ala Met Asp Leu Ile Phe Gln Trp
Gly Ile Asp Ala Leu Pro Phe 405 410 415Arg Lys Gln Asp Gly Tyr Asp
Leu Thr Gln Lys Trp Lys Trp Phe Trp 420 425 430Asp Val Thr Lys Arg
Val Asn Leu Gly Ile Gln Val Lys Gly Asp Arg 435 440 445Tyr Ile Phe
Ile Tyr Gly Gly Ser Asp Lys Lys Trp Ile Gln Asp Phe 450 455 460Thr
Leu Ala Leu Glu Lys Thr Lys Arg His Glu Thr Ile Leu Arg Ala465 470
475 480Asp Ala Ile Ile Glu His Tyr His Leu Gly Lys Asp Asp Pro Lys
Ile 485 490 495Val Pro Arg Phe Trp Ile Glu Ile Glu Ser Lys Arg Leu
Lys Lys His 500 505 510Gln Asp Gly Ile Asp Cys Glu Ile Gln Asp Ile
Val Lys Ser Leu Leu 515 520 525Cys Leu Lys Gln Asp Pro Gln Gly Trp
Val Ile Leu Thr Lys Gly Tyr 530 535 540Asn Val Lys Leu Leu Gly His
Gly Glu Pro Met Tyr Gln Thr Leu Ala545 550 555 560Asp Phe Asp Ile
Trp Lys Asp Arg Val Leu Gln Lys Glu Gly Phe Asp 565 570 575Ile Ala
Phe Lys Glu Tyr Tyr Asp Thr Lys Val Lys Asp Thr Tyr Val 580 585
590Lys Gln Pro Cys Glu Ile Ile Asn Val Asp Asn Asn Ile Asn Gly Asn
595 600 605Val Ile Ala Thr Ile Ser Cys Pro Asn Pro Thr Cys Gly Arg
Val Met 610 615 620Glu Val Ser Ser Val Asn Tyr Lys Cys Cys His Arg
Asp Asp Ala Ala625 630 635 640Ala Pro Gln Asn Gly Lys Ile
6452675PRTMedicago truncatula 2Met Ser Thr Ala Leu Ser Tyr Asn Val
Pro Ile Ser Gly Thr Thr Thr1 5 10 15Gln Lys Asn Asp Thr Ser Gln Gln
Gln Lys Ser Gln Leu Pro Asn Pro 20 25 30Phe Lys Leu Glu Asp Ile Glu
Ile Leu Asn Lys Val Tyr Leu Thr His 35 40 45Val Asn Asp Asn Met Lys
Tyr Asp Arg Asp Thr Leu Phe Asn Leu Val 50 55 60Ser Asn Ile Ile Ser
Ala Ser Thr Gln Thr Ser Gly Thr Asn Ser Gly65 70 75 80Leu Asn Thr
Gln Ile Ser Phe Lys Pro Asp Phe Ser Val Leu Lys Arg 85 90 95Ile Ser
Cys Gln Met Ile Thr Thr Arg Gly Thr Ala Glu Cys Ala His 100 105
110Gln Thr Thr Met Trp Val Leu His His Leu Arg Gly Phe Ser Trp Glu
115 120 125Ala Lys Ala Leu Ile Thr Leu Ala Ala Phe Ser Leu Glu Tyr
Gly Ala 130 135 140Ile Met His Leu His Arg Ile Gln Ser Ser Asp Thr
Leu Gly Asn Ser145 150 155 160Leu Lys Gln Leu Ser Gln Val Gln Phe
Arg Lys Val Pro Ala Asp Ile 165 170 175Thr Glu Leu Val Thr Phe Leu
Leu Gln Val Leu Gln Asp Ile Lys Thr 180 185 190Trp Ala Ala Trp Ser
Ala Phe Gly Tyr Asp Leu Asp Asp Val Asn Ser 195 200 205Leu Pro Asp
Ala Met Gln Trp Ile Pro Leu Val Val Tyr Trp Thr Val 210 215 220Ala
Thr Ile Val Ala Cys Thr Gly Asn Leu Val Gly Ile Ser Glu His225 230
235 240Lys Leu Ser Asp Tyr Val Lys Ser Leu Ser Asp Val Val Lys Glu
Leu 245 250 255Arg Arg His Leu Lys Ser Cys Glu Leu Glu Ile Gly Lys
Ile His Glu 260 265 270Asn Glu Asn Leu Leu Lys Asp Ser Asp Asn Ile
Lys Asp Val Val Ala 275 280 285Phe Leu Arg Leu Leu Ile Lys Gly Asn
Gly Thr Asp Gln Ile Pro Pro 290 295 300Ile Phe Ile Gly Asn Asp Gln
Val Lys Thr Gly Ile Glu Val Phe Lys305 310 315 320Lys Lys His Val
Leu Leu Phe Val Ser Gly Leu Asp Thr Leu Arg Asp 325 330 335Glu Ile
Leu Leu Leu Asn Ser Ile Tyr Lys Arg Leu Gln Asp Lys Pro 340 345
350Gln Glu Val Leu Lys Gly Ser Phe Lys Lys Glu Asp Phe Lys Ile Leu
355 360 365Trp Ile Pro Ile Val Asn Lys Trp Asp Glu Asp Arg Lys Lys
Glu Phe 370 375 380Lys Asn Leu Lys Glu Ser Met Lys Trp Tyr Val Leu
Glu His Phe Ser385 390 395 400Glu Leu Pro Gly Arg Gly Ile Ile Lys
Lys Lys Leu Asn Tyr Asp Ile 405 410 415Gly Tyr Pro Pro Ile Leu Ala
Val Ile Asn Pro Gln Gly Asp Ile Ile 420 425 430Asn Lys Asp Ala Met
Glu Ile Ile Phe Gln Trp Gly Ile Asp Ala Phe 435 440 445Pro Phe Arg
Ile Ser Asp Ala Glu Asp Ile Phe Lys Lys Trp Glu Trp 450 455 460Phe
Trp Lys Leu Met Lys Lys Val Asp Val Asn Ile Glu Lys Met Ser465 470
475 480Trp Asp Arg Tyr Ile Phe Ile Tyr Gly Gly Asn Asp Pro Lys Trp
Ile 485 490 495Gln Asp Phe Thr Arg Ala Ile Gly Ser Ile Lys Lys His
Gln Thr Ile 500 505 510Gln Asn Val Asp Val Asn Ile Asp Tyr His Gln
Leu Gly Lys Asn Asn 515 520 525Pro Thr Glu Ile Pro Tyr Phe Trp Met
Gly Ile Asp Gly Arg Lys Gln 530 535 540Gln Asn Lys Thr Cys Lys Asp
Ser Val Asp Cys Glu Ile Gln Thr Ala545 550 555 560Val Lys Lys Leu
Leu Cys Leu Lys Gln Asp Pro Leu Gly Trp Val Leu 565 570 575Leu Ser
Arg Gly Arg His Val Thr Val Phe Gly His Gly Glu Pro Met 580 585
590Tyr Gln Thr Val Ala Asp Phe Asp Lys Trp Lys Asn Asn Val Val Glu
595 600 605Lys Glu Ser Phe Asp Glu Ala Phe Lys Glu Tyr Tyr Asp Thr
Lys Leu 610 615 620Ser Glu Ile Ser Ser Ser Ala Ser Cys Ala Val Asn
Ser Ser Asp Val625 630 635 640Leu Ala Thr Ile Thr Cys Pro Asn Pro
Phe Cys Gly Arg Val Met Glu 645 650 655Val Thr Ser Val Asn Tyr Lys
Cys Cys His Arg Asp Asp Pro Asp Ser 660 665 670Cys Cys Ile
6753701PRTMedicago truncatula 3Met Ser Ser Ser Met Ala Pro Ser Ser
Leu Val Ser Asn Val Ser Ala1 5 10 15Tyr Ser Gln Gln Ala Arg Thr Ser
Asn Pro Leu Ala Trp Ser Asp Asp 20 25 30Lys Ile Leu Glu Thr Val Tyr
Leu Thr His Val His Thr Gly Glu Arg 35 40 45Tyr Asp Val Glu Ser Leu
Phe Asn Leu Thr Ser Asn Ile Leu Lys Arg 50 55 60Ser Thr Ala Val Ala
Asp Ser Val Ala Ser Lys Thr Gly Thr Pro Val65 70 75 80Gly Leu Val
Glu Asp Arg Leu Pro Leu Ser Gly Tyr Glu Pro Pro Ile 85 90 95Arg Lys
Leu Lys His Ile Ser Ala Gln Met Met Ser Thr Leu Pro Gly 100 105
110Glu His His Ala His Met Thr Thr Met Ser Ile Leu Asp Gln Leu Lys
115 120 125Ser His Thr Trp Asp Gly Lys Ala Ile Phe Ala Leu Ala Ala
Phe Ser 130 135 140Leu Glu Tyr Gly Asn Phe Trp His Leu Val Gln Thr
Pro Ser Gly Asp145 150 155 160Thr Leu Gly Arg Ser Leu Ala Thr Met
Asn Arg Val Gln Ser Val Asp 165 170 175Lys Asn Arg Gln Ala Ile Ala
Asp Tyr Asn Ser Leu Val Lys Asn Leu 180 185 190Leu Phe Ala Val Glu
Cys Ile Thr Glu Leu Glu Lys Leu Ser Thr Lys 195 200 205Gly Tyr Glu
His Lys Asp Val Pro Ala Leu Ser Glu Ala Met Gln Glu 210 215 220Ile
Pro Val Ala Val Tyr Trp Ala Ile Ile Thr Ala Ile Ile Cys Ala225 230
235 240Asn His Leu Asp Leu Leu Phe Gly Asp Ser Asp Asp Arg Tyr Glu
Leu 245 250 255Ser Ser Tyr Asp Val Lys Leu Ala Ser Ile Val Ser Lys
Leu Lys Ala 260 265 270His Leu Thr Arg Ser Arg Lys His Ile Gly Glu
Leu Glu Asp Tyr Trp 275 280 285Arg Arg Lys Arg Val Leu Gln Thr Pro
Thr Glu Ile Val Glu Val Leu 290 295 300Lys Val Leu Val Phe His Asn
Glu Ile Gln Asp Pro Leu Val Phe Asp305 310 315 320Gly Leu Asn Arg
Gln Met Val Ser Ile Glu Val Phe Arg Lys Lys His 325 330 335Val Leu
Val Phe Ile Ser Gly Leu Asp Ser Ile Arg Asp Glu Ile Arg 340 345
350Leu Leu Gln Ser Ile Tyr Val Gly Leu Gln Glu Glu Pro Arg Glu Leu
355 360 365Lys Gly Tyr Arg Lys Glu Asp Phe Lys Ile Leu Trp Ile Pro
Ile Val 370 375 380Asp Asp Trp Thr Leu Leu His Lys Ala Glu Phe Asp
Asn Leu Lys Leu385 390 395 400Glu Met Pro Trp Tyr Val Val Glu Tyr
Phe Tyr Pro Leu Ala Gly Ile 405 410 415Arg Leu Ile Arg Glu Asp Leu
Ser Tyr Lys Asn Lys Pro Ile Leu Pro 420 425 430Val Leu Asn Pro Leu
Gly Arg Ile Val Asn His Asn Ala Met His Met 435 440 445Ile Phe Val
Trp Gly Ile Asp Ala Phe Pro Phe Arg Pro Thr Asp Asp 450 455 460Glu
Ser Leu Thr Gln Lys Trp Asn Trp Phe Trp Ala Glu Met Lys Lys465 470
475 480Val Tyr Pro Arg Leu Gln Asp Leu Ile Lys Gly Asp Thr Phe Ile
Phe 485 490 495Ile Tyr Gly Gly Thr Asp Pro Lys Trp Thr Gln Asp Phe
Ala Leu Ala 500 505 510Ile Glu Lys Ile Lys Arg His Glu Ile Thr Arg
Lys Ala Asp Ala Val 515 520 525Ile Glu His Phe His Phe Gly Lys Glu
Asp Lys Arg Ile Val Pro Arg 530 535 540Phe Trp Ile Gly Ile Glu Ser
Leu Phe Ala Asn Met Ile Gln Lys Lys545 550 555 560His Lys Asp Pro
Thr Ile Asp Glu Ile Lys Ser Leu Leu Cys Leu Lys 565 570 575Gln Asp
Gln Pro Gly Trp Val Leu Leu Ser Lys Gly Pro Asn Val Lys 580 585
590Leu Leu Gly Arg Gly Asp Gln Met Tyr Ala Thr Ala Val Asp Phe Glu
595 600 605Ile Trp Lys Glu Lys Val Leu Glu Lys Ala Gly Phe Asp Val
Ala Phe 610 615 620Lys Glu Tyr Tyr Glu Arg Lys Arg Arg Glu Tyr Pro
Val Ala Cys Ala625 630 635 640Asn Met Gln Leu Ala Asn Tyr Pro Ser
Asp Ile Leu Asp Pro Ile Tyr 645 650 655Cys Pro Asp Ser Asn Cys Gly
Arg Ser Met Glu Ile Ala Ser Val Ser 660 665 670Tyr Lys Cys Cys His
Gly His Thr His Glu Asn Ala Glu Val Ala Pro 675 680 685Ala Glu Ser
Gly Gly Phe Val Gln Ile Glu Lys Arg Ser 690 695 7004671PRTMedicago
truncatula 4Met Ser Leu Ser Asn Leu Gly Ser Ala Thr Ala Thr Asn Ser
Ser Leu1 5 10 15Asn Gln Lys Asn Ala Thr Asn Ser Leu Gln Asn Lys Ala
Asn Phe Leu 20 25 30Pro Asn Pro Phe Asp Leu His Asp Pro Gln Ile Leu
Asp Arg Val Tyr 35 40 45Leu Thr His Val Thr Asp Asp Glu Phe Cys Asp
Thr Asn Ile Ile Phe 50 55 60Glu Leu Val Ser Ser Val Val Leu Gln Thr
Ile Pro Lys Ile Ser Val65 70 75 80Thr Ser Phe Lys Pro Glu Phe Pro
Thr Leu Lys Leu Ile Ser Cys Gln 85 90 95Met Ile Thr Thr Arg Asn Asp
Pro His Cys Val His Gln Thr Thr Leu 100 105 110Trp Ile Leu Gln Asn
Leu Arg Ser Tyr Ser Trp Asp Ala Lys Ala Leu 115 120 125Ile Thr Leu
Ala Ala Phe Thr Leu Glu Tyr Gly Asn Tyr Leu Gln Leu 130 135 140Asn
Arg Val Thr Thr Thr Asp Thr Leu Gly Asn Ser Leu Arg Val Leu145 150
155 160Asn Gln Val Gln Thr Arg Lys Ile Ser Asn Asp Val Thr Glu Leu
Val 165 170 175Lys Tyr Ile Val Asp Met Leu Ile His Leu Asn Val Trp
Ala Thr Trp 180 185 190Ser Ala Asp Gly Tyr Asp Pro Val Asp Val Pro
Ala Leu Thr Asp Ala 195 200 205Leu Gln Glu Ile Pro Val Phe Val Tyr
Trp Thr Ile Ala Ser Ile Val 210 215 220Ala Ser Thr Gly Asn Leu Val
Gly Val Ser Asp Tyr Lys Leu Ser Ala225 230 235 240Tyr Lys Glu Arg
Leu Ser Arg Val Val Glu Glu Leu Val Lys His Leu 245 250 255Ala Thr
Cys Glu Arg Gln Ile Arg Asn Val Asp Asp Leu Thr Ser Arg 260 265
270Thr Asn Asn Tyr Arg Lys Pro Lys Asp Ile Val Asp Cys Leu Lys Ala
275 280 285Leu Ile His Arg Asn Gly Thr Asp Ile Pro Gln Ile Tyr Gln
Gly Asn 290 295 300Val Gln Val Lys Ser Gly Leu Asp Ile Phe Lys Gln
Lys His Val Leu305 310 315 320Leu Phe Ile Ser Ser Leu Asp Arg Ile
Gln Asp Glu Ile Thr Leu Leu 325 330 335Asn Ser Ile Tyr Glu Arg Leu
Gln Glu Asn Pro Lys Glu Ser Lys Gly 340 345 350Phe Met Lys Glu Asp
Phe Lys Ile Leu Trp Ile Pro Ile Val Lys Lys 355 360 365Trp Asp Asp
Ile Gln Ile Glu Asn Phe Lys Ala Leu Lys Ser Gly Ile 370 375 380Lys
Trp Tyr Val Val Glu Tyr Phe Ser Glu Leu Pro Gly Leu Lys Ile385 390
395 400Ile Lys Asp Pro Glu Leu Ile Gly Tyr Ile Asp Asn Pro Ile Ile
Pro 405 410 415Val Phe Asn Pro Lys Gly Ile Ile Thr Asn Glu Asp Ala
Met Asp Leu 420 425 430Ile Phe Gln Trp Gly Ile Asp Ala Phe Pro Phe
Arg Lys Ser Asp Gly 435
440 445Asn Asp Leu Lys Leu Lys Trp Asn Trp Leu Trp Asp Val Ile Lys
Lys 450 455 460Ala Thr Pro Gly Leu Leu Val Lys Val Asp Arg Tyr Ile
Phe Ile Tyr465 470 475 480Gly Gly Thr Asn Lys Lys Trp Ile Gln Asp
Phe Thr Leu Glu Leu Glu 485 490 495Lys Ile Lys Arg His Glu Thr Ile
Lys Arg Ala Asp Val Ile Ile Glu 500 505 510Asn Tyr Gln Val Gly Lys
Asp Asp Pro Asn Arg Val Pro Ser Phe Trp 515 520 525Met Gly Ile Glu
Arg Lys Lys Gln Asn Lys Lys His Gln Glu Thr Val 530 535 540Asp Cys
Lys Ile Gln Glu Ile Val Lys Asp Leu Phe Cys Leu Arg Arg545 550 555
560Asp Pro Gln Gly Trp Ile Ile Leu Ser Lys Gly His Ser Ile Lys Leu
565 570 575Leu Gly His Gly Glu Pro Ala Tyr Gln Thr Leu Val Glu Phe
Gln Asn 580 585 590Trp Lys Asp Lys Val Leu Glu Lys Glu Gly Phe Asp
Ile Ala Phe Lys 595 600 605Glu Tyr Tyr Gln Met Lys Ala Lys Glu Ile
Ser Gly Arg Glu Pro Cys 610 615 620Glu Val Leu Asn Val Asp Thr Tyr
Ser Ser Asn Val Ile Gly Thr Ile625 630 635 640Ser Cys Pro Asn Pro
Met Cys Gly Arg Val Met Glu Val Ser Ser Ile 645 650 655His Tyr Lys
Cys Cys His Arg Asp Glu Pro Asn Asn Leu Gly Val 660 665
6705651PRTDipteryx panamensis 5Met Ser Leu Ser Asn Gly Ala Ser Ser
Thr Thr Leu Ser Gln Gln Lys1 5 10 15Thr Gln Leu Pro Asn Pro Phe Asp
Leu Thr Asp Ser Gln Ile Leu Asp 20 25 30Lys Val Tyr Leu Ser His Ala
His Asp Asp Glu Glu Cys Asp Arg Asp 35 40 45Thr Leu Leu Asp Leu Val
Ser Ile Ile Ile Leu Lys Ser Gln Arg Pro 50 55 60Ile Pro Leu Ala Lys
Tyr Lys Pro Glu Phe Pro Thr Leu Lys Leu Ile65 70 75 80Ser Cys Gln
Met Ile Thr Thr Arg Gly Val Val His Cys Ala His Gln 85 90 95Thr Thr
Met Trp Ile Leu Gln His Leu Arg Ser Phe Ser Trp Asp Ala 100 105
110Lys Ala Leu Ile Thr Val Ala Ala Phe Ser Leu Glu Tyr Gly Asn Phe
115 120 125Arg His Leu Gln Ile Pro Thr Ser Asp Gln Leu Gly Asn Ala
Leu Lys 130 135 140Gln Leu Asn Gln Val Asn Asn Gly Lys Leu Ser Asp
Asp Ile Thr Glu145 150 155 160Leu Ala Thr Val Thr Val Arg Val Leu
Gln His Leu Lys Glu Trp Ala 165 170 175Ala Trp Ser Ala Ala Gly Tyr
Asp Thr Glu Asp Val Pro Ala Leu Ser 180 185 190Asp Ala Leu Gln Val
Ile Pro Phe Val Val Tyr Trp Thr Ile Ala Ser 195 200 205Ile Val Ala
Ser Thr Gly Asn Leu Ile Gly Val Ser Asp Tyr Lys Leu 210 215 220Ser
Asp Phe Lys Asp Lys Leu Asp Arg Val Val Lys Thr Leu Asn Asp225 230
235 240His Leu Asp Glu Cys Lys Lys Gln Ile Asp Val Ile Asp Asn Tyr
Asn 245 250 255Trp Arg Arg Lys Ala Phe Glu Asn Pro Lys Asp Ile Val
Asp Leu Leu 260 265 270Lys Leu Leu Ile His Ser Lys Gly Ser Pro Ile
Pro Gln Ile Tyr Asp 275 280 285Gly Arg Thr Thr Thr Lys Thr Asp Ile
Glu Val Phe Lys Gln Lys Tyr 290 295 300Val Leu Leu Phe Ile Ser Ser
Leu Asp Ser Ile Asp Asp Glu Ile Arg305 310 315 320Leu Leu Asn Ser
Ile Tyr Asp Arg Leu Lys Glu Asp Pro Lys Glu Val 325 330 335Lys Gly
Phe Asn Lys Glu Asp Phe Lys Ile Leu Trp Ile Pro Ile Val 340 345
350Asp Ser Trp Asp Lys Asp Ser Val Glu Lys Tyr Lys Thr Leu Lys Thr
355 360 365Lys Ile Lys Trp Tyr Ala Val Glu Phe Leu Ser Leu Val Pro
Gly Ile 370 375 380Arg Leu Val Arg Glu Val Leu Lys Phe Glu Thr Lys
Pro Ile Ile Pro385 390 395 400Val Ile Ser Pro Gln Gly Lys Arg Ile
Asn Asp Asn Ala Met Asp Ile 405 410 415Ile Phe Glu Trp Gly Val Asp
Ala Phe Pro Phe Arg Lys Glu Asp Gly 420 425 430Asp Gln Leu Thr Gln
Lys Trp Lys Trp Phe Trp Asp Val Ile Lys Lys 435 440 445Val Asn Pro
Ala Ile Gln Val Glu Pro Glu Ser Tyr Ile Phe Ile Tyr 450 455 460Gly
Gly Thr Asp Asn Lys Trp Ile Gln Asp Phe Thr Leu Ala Val Asp465 470
475 480Lys Val Lys Arg His Asp Thr Ile Lys Arg Ala Asp Ala Ile Ile
Glu 485 490 495His His Gln Leu Ala Lys Asp Asp Ser Ile Val Pro Arg
Phe Trp Ile 500 505 510Gly Ile Glu Ser Lys Thr His Lys Lys His Gln
Glu Ala Val Asp Cys 515 520 525Gln Ile Gln Thr Ile Val Lys Ser Leu
Leu Cys Leu Lys Arg Asp Pro 530 535 540Gln Gly Trp Ala Ile Leu Ser
Lys Gly Asn Asn Val Lys Ile Leu Gly545 550 555 560His Gly Glu Pro
Met Leu Gln Thr Leu Thr Gln Phe Glu Ser Trp Lys 565 570 575Asp Lys
Val Leu Glu Lys Glu Gly Phe Asp Ile Ala Leu Lys Glu Phe 580 585
590Tyr Asp Gly Lys Val Glu Ser Leu Ser Tyr Arg Gln Pro Cys Glu Tyr
595 600 605Leu Asn Ile Asp Ser Gln Ser Ser Ser Val Ile Ala Thr Ile
Thr Cys 610 615 620Pro Asn Pro Thr Cys Gly Arg Val Met Glu Val Thr
Ser Val Asn Tyr625 630 635 640Arg Cys Cys His Arg Asp Gly Gln Lys
Ile Cys 645 6506668PRTLotus japonicus 6Met Ser His Val Pro Lys Ala
Ala Ser Asn Gly Ala Leu Ile Gln His1 5 10 15Ser Gly Thr Ser Pro Asn
Gln Lys Ala Tyr Leu Pro Ser Pro Phe Glu 20 25 30Leu Lys Asp Pro Gln
Ile Leu Asp Arg Val Tyr Leu Thr His Val Asn 35 40 45Asp Asp Glu Ile
Cys Asp Thr Lys Ile Leu Phe Asp Leu Val Ser Thr 50 55 60Val Val Leu
Gln Ser Val Ser Gln Ile Pro Ala Thr Ser Phe Lys Pro65 70 75 80Glu
Phe Ser Thr Leu Lys Leu Ile Ser Cys Gln Met Ile Thr Thr Arg 85 90
95Asn Ala Asp His Cys Val His Gln Thr Thr Met Trp Ile Leu Gln Asn
100 105 110Leu Arg Ser Tyr Ser Trp Asp Ala Lys Ala Ile Ile Thr Leu
Ala Ala 115 120 125Phe Thr Leu Glu Tyr Gly Asn Tyr Leu His Leu Ser
Arg Ala Ala Val 130 135 140Ala Asp Thr Leu Gly Ser Ser Leu Arg Gln
Leu Asn Gln Val His Thr145 150 155 160Arg Lys Val Pro Ala Asp Ile
Thr Lys Leu Val Thr Phe Ile Val His 165 170 175Ala Phe Gln His Leu
Lys Glu Trp Ala Thr Trp Ala Asp Glu Gly Tyr 180 185 190Glu Pro Glu
Glu Val Pro Ser Leu Thr Glu Ala Leu Gln His Val Pro 195 200 205Val
Ala Val Tyr Trp Thr Ile Ala Ala Ile Val Ala Ser Thr Gly Asn 210 215
220Leu Val Gly Val Ser Thr Tyr Asn Leu Gln Gly Tyr Ile Asp Arg
Leu225 230 235 240Asp Glu His Val Thr Lys Leu Ala Glu Gln Leu Asn
Ser Cys Lys Leu 245 250 255Gln Ile Gly His Val Asp Asp Tyr Phe Asn
Arg Arg Lys Ile Phe Asp 260 265 270Lys Pro Lys Asp Ile Val Asp Leu
Leu Lys Ala Leu Ile His Arg Asn 275 280 285Gly Ala Gln Gly Pro Gln
Ile Phe Glu Gly Gly Val Ile Val Lys Gln 290 295 300Gly Leu Glu Val
Phe Arg Gln Lys His Val Leu Leu Phe Ile Ser Gly305 310 315 320Leu
Asn Ser Ile Val Asp Glu Ile Leu Leu Leu Asn Ser Ile Tyr Asn 325 330
335Arg Leu Gln Asp Asn Pro Thr Glu Val Ile Lys Gly Phe Lys Lys Glu
340 345 350Asp Phe Lys Ile Leu Trp Val Pro Met Val Asp Arg Trp Asp
Glu Ala 355 360 365Ser Arg Glu Gln Tyr Leu Asn Thr Trp Lys Arg Gly
Ile Lys Trp Tyr 370 375 380Ile Val Glu Tyr Phe Phe Glu Leu Pro Gly
Arg Arg Ile Ile Thr Asp385 390 395 400Pro Glu Arg Leu Gly Tyr Glu
Gly Asn Pro Ile Ile Pro Val Phe Asn 405 410 415Pro Gln Gly Met Leu
Thr Asn Asp Asn Ala Met Asp Leu Ile Phe Gln 420 425 430Trp Gly Ile
Asp Ala Phe Pro Phe Arg Lys Ser Asp Gly Ile Asp Leu 435 440 445Thr
Leu Lys Trp Lys Trp Leu Trp Asp Ile Ile Lys Lys Ala Thr Pro 450 455
460Gly Leu Gln Val Lys Val Asp Arg Tyr Ile Phe Ile Phe Gly Ser
Thr465 470 475 480Asn Asn Lys Trp Ile Gln Asp Phe Thr Ile Glu Leu
Asp Lys Leu Lys 485 490 495Arg Asn Glu Thr Val Lys Arg Ala Asp Val
Ile Ile Glu Gln Tyr Gln 500 505 510Leu Gly Lys Asp Asp Pro Asn Arg
Val Pro Ser Phe Trp Met Gly Val 515 520 525Glu Arg Lys Lys Gln Asn
Lys Lys His Gln Glu Ala Val Asp Cys Glu 530 535 540Ile Gln Gly Ile
Val Lys Ser Leu Phe Cys Leu Lys Arg Asp Pro Gln545 550 555 560Gly
Trp Val Ile Leu Ser Lys Gly His Asn Ile Lys Leu Leu Gly His 565 570
575Gly Glu Ala Val Tyr Gln Thr Val Val Glu Phe Pro Asn Trp Lys Glu
580 585 590Lys Val Leu Glu Arg Glu Gly Phe Asp Ile Ala Phe Lys Glu
Tyr Tyr 595 600 605Asp Ile Lys Ala Lys Glu Ile Ser Ala Arg Gln Pro
Cys Glu Ile Ile 610 615 620Asn Val Asp Ser Tyr Ser Ala Asn Val Ile
Ala Thr Ile Thr Cys Pro625 630 635 640Asn Pro Met Cys Gly Arg Val
Met Glu Val Thr Ser Val Asn Tyr Lys 645 650 655Cys Cys His Ser Asp
Ala Pro Asn Gly Phe Gly Ile 660 6657685PRTPisum sativum 7Met Ser
Phe Ser Asn Ser Ala Ala Ala Ala Thr Gly Thr Leu Val Gln1 5 10 15His
Gly Gly Asn Ala Thr Asn Asn Asn Ser Leu Ile Gln Lys Asn Ala 20 25
30Thr Ser Pro His Ser His His Lys Ala Asn Asn Tyr Leu Pro Asn Pro
35 40 45Phe Glu Leu His Asp Ser Gln Ile Leu Asp Lys Val Tyr Leu Thr
His 50 55 60Val Thr Asp Asp Gln Phe Cys Asp Thr Asp Ile Ile Phe Asp
Leu Val65 70 75 80Ser Thr Leu Val Leu Gln Thr Asn Thr Gln Ile Pro
Val Thr Gly Phe 85 90 95Lys Pro Asp Phe Pro Thr Leu Lys Leu Ile Ser
Cys Gln Met Ile Thr 100 105 110Thr Arg Ser Ala Ala His Cys Val His
Gln Thr Thr Leu Trp Ile Leu 115 120 125Gln Asn Leu Arg Ser Tyr Ser
Trp Asp Ala Lys Ala Leu Ile Thr Leu 130 135 140Ala Ala Phe Thr Leu
Glu Tyr Gly Asn Tyr Leu His Leu Thr Arg Val145 150 155 160Thr Ala
Thr Asp Pro Ile Gly Asn Ser Leu Arg Gln Leu Asn Gln Ile 165 170
175Gln Thr Arg Asn Ile Ser Thr Asp Ile Thr Glu Leu Val Ser Phe Ile
180 185 190Val His Gln Leu Leu His Leu Lys Glu Trp Ala Thr Trp Ser
Ala Glu 195 200 205Gly Tyr Asp Pro Glu Asp Val Pro Ala Leu Thr Glu
Ala Leu Gln Glu 210 215 220Ile Pro Val Phe Val Tyr Trp Thr Ile Ala
Ser Ile Val Ala Ser Thr225 230 235 240Gly Asn Leu Val Gly Val Ser
Asp Tyr Lys Leu Ser Glu Tyr Arg Glu 245 250 255Arg Leu Ser Gly Ile
Val Gln Lys Leu Val Val His Leu Asn Asn Cys 260 265 270Lys Leu Gln
Ile Ser Tyr Ile Asp Asp Leu Phe Asn Arg Lys Lys Ile 275 280 285Phe
Asp Lys Pro Lys Asp Ile Val Asp Cys Leu Lys Ala Leu Ile His 290 295
300Arg Asn Gly Thr Asp Ser Pro Gln Ile Tyr Glu Gly Ala Ile His
Val305 310 315 320Lys Thr Gly Leu Glu Val Phe Arg Asn Lys His Val
Leu Val Phe Ile 325 330 335Ser Ser Leu Asp Ser Ile Glu Asp Glu Ile
Ser Leu Leu Asn Ser Ile 340 345 350Tyr Glu Arg Leu Gln Glu Asn Ser
Lys Glu Ser Ile Lys Gly Phe Lys 355 360 365Lys Glu Asp Phe Lys Ile
Leu Trp Ile Pro Ile Val Asn Asn Trp Asp 370 375 380Asp Ile Arg Lys
Glu Arg Phe Arg Ala Leu Lys Ser Gly Ile Lys Trp385 390 395 400Tyr
Ala Val Glu Tyr Phe Tyr Glu Leu Pro Gly His Arg Ile Ile Thr 405 410
415Asp Pro Glu Arg Ile Gly Tyr Ile Gly Asn Pro Ile Ile Pro Val Phe
420 425 430Asn Pro Gln Gly Tyr Ile Thr Asn Ile Asp Ala Met Asp Leu
Ile Phe 435 440 445Gln Trp Gly Ile Asp Ala Phe Pro Phe Arg Lys Ser
Asp Gly Ile Asp 450 455 460Leu Thr Leu Lys Trp Lys Trp Leu Trp Asp
Val Ile Lys Lys Ala Thr465 470 475 480Pro Gly Leu Gln Val Lys Gly
Asp Arg Tyr Ile Phe Ile Tyr Gly Gly 485 490 495Thr Asn Asn Lys Trp
Ile Gln Asp Phe Thr Leu Glu Leu Glu Lys Ile 500 505 510Lys Arg His
Glu Ile Leu Lys Arg Ala Asp Val Ile Ile Glu Asn Tyr 515 520 525Gln
Leu Gly Lys Glu Asp Pro Asn Arg Val Pro Ser Phe Trp Ile Gly 530 535
540Val Glu Arg Lys Lys Gln Asn Lys Lys His Gln Glu Ala Leu Asp
Cys545 550 555 560Glu Ile Gln Asp Ile Val Lys Ser Leu Phe Cys Leu
Lys Arg Asp Pro 565 570 575Gln Gly Trp Ile Ile Leu Ser Lys Gly Gln
Asn Ile Lys Leu Leu Gly 580 585 590His Gly Glu Pro Ala Tyr Gln Thr
Leu Ala Glu Phe Gln Asn Trp Lys 595 600 605Asp Arg Val Leu Glu Lys
Glu Gly Phe Asp Ile Ala Phe Lys Glu Tyr 610 615 620Tyr Glu Met Lys
Ala Lys Glu Leu Ser Gly Arg Gln Pro Cys Glu Val625 630 635 640Val
Asn Val Asp Thr Tyr Ser Ser Asn Val Ile Ala Thr Ile Ala Cys 645 650
655Pro Asn Pro Met Cys Gly Arg Val Met Glu Val Ser Ser Ala His Tyr
660 665 670Lys Cys Cys His Arg Asp Glu Pro Asn Asn Phe Gly Val 675
680 6858684PRTVicia faba 8Met Ser Phe Ser Asn Ser Pro Ala Ala Ala
Thr Gly Thr Leu Val Gln1 5 10 15His Gly Gly Asn Gly Thr Asn Asn Ser
Leu Ile Gln Lys Thr Ala Thr 20 25 30Ser Ser His Pro His His Lys Ala
Asn Asn Tyr Leu Pro Asn Pro Phe 35 40 45Glu Leu His Asp Ser His Ile
Leu Asp Lys Val Tyr Leu Thr His Val 50 55 60Thr Asp Asp Glu Phe Cys
Asp Thr Asp Ile Ile Phe Asp Leu Val Ser65 70 75 80Thr Leu Ile Leu
Gln Ser Asn Thr Gln Ile Pro Val Thr Gly Phe Lys 85 90 95Pro Asp Phe
Pro Thr Leu Lys Leu Ile Ser Cys Gln Met Ile Thr Thr 100 105 110Arg
Ser Val Ala His Cys Val His Gln Thr Thr Leu Trp Ile Leu Gln 115 120
125Asn Leu Arg Ser Tyr Ser Trp Asp Ala Lys Ala Leu Ile Thr Leu Ala
130 135 140Ala Phe Thr Leu Glu Tyr Gly Asn Tyr Leu Gln Leu Asn Arg
Val Thr145 150 155 160Ala Thr Asp Pro Ile Gly Asn Ser Leu Arg Gln
Leu Asn Gln Ile Gln 165 170 175Thr Arg Lys Ile Ser Thr Asp Ile Pro
Glu Leu Val Asn Phe Ile Val 180 185 190His Lys Leu Leu His Leu Lys
Glu Trp Ala Ala Trp Ser Ala Glu Gly 195 200 205Tyr Asp Pro Glu Asp
Val Pro Ala Leu Thr Glu Ala Leu Gln Glu Ile 210 215 220Pro Val Phe
Val Tyr Trp Thr Ile Ala Ser Ile Val Ala Ser Thr Gly225
230 235 240Asn Leu Val Gly Val Ser Asp Tyr Asn Leu Ser Glu Tyr Arg
Glu Arg 245 250 255Leu Ser Gly Ile Val Gln Lys Leu Val Val His Leu
Asn Asn Cys Lys 260 265 270Leu Gln Ile Ser Tyr Ile Asp Asp Leu Phe
Asn Arg Arg Lys Ile Phe 275 280 285Asp Lys Pro Lys Asp Ile Val Asp
Cys Leu Lys Ala Leu Ile His His 290 295 300Asn Gly Ala Asp Ser Pro
Gln Ile Tyr Glu Gly Ala Ile His Val Lys305 310 315 320Thr Gly Leu
Glu Val Phe Arg His Lys His Val Leu Met Phe Ile Ser 325 330 335Ser
Leu Asp Ser Ile Glu Asp Glu Ile Ser Leu Leu Asn Ser Ile Tyr 340 345
350Glu Arg Leu Gln Glu Asn Ser Lys Glu Ser Ile Lys Gly Phe Lys Lys
355 360 365Glu Asp Phe Lys Ile Leu Trp Ile Pro Ile Val Asn Asn Trp
Asp Asp 370 375 380Ile Arg Lys Glu Arg Phe Arg Ala Leu Lys Ser Gly
Ile Lys Trp Tyr385 390 395 400Ala Val Glu Tyr Phe Tyr Glu Leu Pro
Gly His Arg Ile Ile Thr Asp 405 410 415Pro Glu Arg Ile Gly Tyr Ile
Gly Asn Pro Ile Ile Pro Val Phe Asn 420 425 430Pro His Gly Tyr Ile
Thr Asn Ile Asp Ala Met Asp Leu Ile Phe Gln 435 440 445Trp Gly Ile
Asp Ala Phe Pro Phe Arg Lys Ser Asp Gly Ile Asp Leu 450 455 460Thr
Phe Lys Trp Lys Trp Leu Trp Asp Val Ile Lys Lys Ala Thr Pro465 470
475 480Gly Leu Gln Val Lys Gly Asp Arg Tyr Ile Phe Ile Tyr Gly Gly
Thr 485 490 495Asn Asn Lys Trp Ile Gln Asp Phe Thr Leu Glu Leu Glu
Lys Ile Lys 500 505 510Arg His Glu Thr Leu Lys Arg Ala Asp Val Ile
Ile Asp Asn Tyr Gln 515 520 525Leu Gly Lys Asp Asp Pro Asn Arg Val
Pro Ser Phe Trp Ile Gly Val 530 535 540Glu Arg Lys Lys Gln Asn Lys
Lys His Gln Glu Ala Val Asp Cys Glu545 550 555 560Ile Gln Asp Ile
Val Lys Ser Leu Phe Cys Leu Lys Arg Asp Pro Gln 565 570 575Gly Trp
Val Ile Leu Ser Lys Gly Gln Asn Ile Lys Leu Leu Gly His 580 585
590Gly Glu Pro Ala Tyr Gln Thr Leu Ala Glu Phe Gln Asn Trp Lys Asp
595 600 605Arg Val Leu Glu Lys Glu Gly Phe Asp Ile Ala Phe Lys Glu
Tyr Tyr 610 615 620Glu Met Lys Ala Lys Glu Leu Ser Gly Arg Glu Pro
Cys Glu Val Val625 630 635 640Asn Val Asp Thr Tyr Ser Ser Asn Val
Ile Ala Thr Ile Ala Cys Pro 645 650 655Asn Pro Met Cys Gly Arg Val
Met Glu Val Ser Ser Val His Tyr Lys 660 665 670Cys Cys His Arg Asp
Glu Pro Asn Asn Phe Gly Val 675 6809238PRTAequorea victoria 9Met
Ser Lys Gly Glu Glu Leu Phe Thr Gly Val Val Pro Ile Leu Val1 5 10
15Glu Leu Asp Gly Asp Val Asn Gly His Lys Phe Ser Val Ser Gly Glu
20 25 30Gly Glu Gly Asp Ala Thr Tyr Gly Lys Leu Thr Leu Lys Phe Ile
Cys 35 40 45Thr Thr Gly Lys Leu Pro Val Pro Trp Pro Thr Leu Val Thr
Thr Leu 50 55 60Thr Tyr Gly Val Gln Cys Phe Ser Arg Tyr Pro Asp His
Met Lys Gln65 70 75 80His Asp Phe Phe Lys Ser Ala Met Pro Glu Gly
Tyr Val Gln Glu Arg 85 90 95Thr Ile Phe Phe Lys Asp Asp Gly Asn Tyr
Lys Thr Arg Ala Glu Val 100 105 110Lys Phe Glu Gly Asp Thr Leu Val
Asn Arg Ile Glu Leu Lys Gly Ile 115 120 125Asp Phe Lys Glu Asp Gly
Asn Ile Leu Gly His Lys Leu Glu Tyr Asn 130 135 140Tyr Asn Ser His
Asn Val Tyr Ile Met Ala Asp Lys Gln Lys Asn Gly145 150 155 160Ile
Lys Val Asn Phe Lys Ile Arg His Asn Ile Glu Asp Gly Ser Val 165 170
175Gln Leu Ala Asp His Tyr Gln Gln Asn Thr Pro Ile Gly Asp Gly Pro
180 185 190Val Leu Leu Pro Asp Asn His Tyr Leu Ser Thr Gln Ser Ala
Leu Ser 195 200 205Lys Asp Pro Asn Glu Lys Arg Asp His Met Val Leu
Leu Glu Phe Val 210 215 220Thr Ala Ala Gly Ile Thr His Gly Met Asp
Glu Leu Tyr Lys225 230 23510239PRTAequorea victoria 10Met Val Ser
Lys Gly Glu Glu Leu Phe Thr Gly Val Val Pro Ile Leu1 5 10 15Val Glu
Leu Asp Gly Asp Val Asn Gly His Lys Phe Ser Val Ser Gly 20 25 30Glu
Gly Glu Gly Asp Ala Thr Tyr Gly Lys Leu Thr Leu Lys Leu Ile 35 40
45Cys Thr Thr Gly Lys Leu Pro Val Pro Trp Pro Thr Leu Val Thr Thr
50 55 60Phe Gly Tyr Gly Leu Gln Cys Phe Ala Arg Tyr Pro Asp His Met
Lys65 70 75 80Gln His Asp Phe Phe Lys Ser Ala Met Pro Glu Gly Tyr
Val Gln Glu 85 90 95Arg Thr Ile Phe Phe Lys Asp Asp Gly Asn Tyr Lys
Thr Arg Ala Glu 100 105 110Val Lys Phe Glu Gly Asp Thr Leu Val Asn
Arg Ile Glu Leu Lys Gly 115 120 125Ile Asp Phe Lys Glu Asp Gly Asn
Ile Leu Gly His Lys Leu Glu Tyr 130 135 140Asn Tyr Asn Ser His Asn
Val Tyr Ile Thr Ala Asp Lys Gln Lys Asn145 150 155 160Gly Ile Lys
Ala Asn Phe Lys Ile Arg His Asn Ile Glu Asp Gly Gly 165 170 175Val
Gln Leu Ala Asp His Tyr Gln Gln Asn Thr Pro Ile Gly Asp Gly 180 185
190Pro Val Leu Leu Pro Asp Asn His Tyr Leu Ser Tyr Gln Ser Ala Leu
195 200 205Ser Lys Asp Pro Asn Glu Lys Arg Asp His Met Val Leu Leu
Glu Phe 210 215 220Val Thr Ala Ala Gly Ile Thr Leu Gly Met Asp Glu
Leu Tyr Lys225 230 2351134DNAArtificial sequenceoligonucleotide
11agaaccatgg gatcattgtc caatggaact aaac 341233DNAArtificial
sequenceoligonucleotide 12agactcgagt catatcttgc cattctgtgg agc
331332DNAArtificial sequenceoligonucleotide 13agactcgagc atatcttgcc
attctgtgga gc 321434DNAArtificial sequenceoligonucleotide
14agaaccatgg gatccactgc attgtcctat aatg 341530DNAArtificial
sequenceoligonucleotide 15agactcgagt caaatgcagc aactatctgg
301627DNAArtificial sequenceoligonucleotide 16agactcgaga tgcagcaact
atctgga 271732DNAArtificial sequenceoligonucleotide 17agaaccatgg
gatccctttc caacttagga ag 321830DNAArtificial
sequenceoligonucleotide 18agactcgagt caaacaccaa gattgtttgg
301929DNAArtificial sequenceoligonucleotide 19agactcgaga caccaagatt
gtttggttc 292028DNAArtificial sequenceoligonucleotide 20agactcgaga
atgagtgaag gccccgtc 282130DNAArtificial sequenceoligonucleotide
21agactcgagc taattatcct tcgtatcttc 302230DNAArtificial
sequenceoligonucleotide 22agactcgaga atggttcatt taggtccaaa
302328DNAArtificial sequenceoligonucleotide 23agactcgagt taagcaccga
tgatacca 282430DNAArtificial sequenceoligonucleotide 24agactcgaga
atgtccaata actcattcac 302531DNAArtificial sequenceoligonucleotide
25agactcgaga tcacatccat tccttgaatt g 312630DNAArtificial
sequenceoligonucleotide 26agactcgaga gcatcaatga caaacgaaac
302731DNAArtificial sequenceoligonucleotide 27agactcgagc tattttactt
cccttacttg g 312826DNAArtificial sequenceoligonucleotide
28agaccatgga cctgcgtatt tctcag 262940DNAArtificial
sequenceoligonucleotide 29agaccatggt acgaccttcg atcctgcata
tagaaatgcc 403040DNAArtificial sequenceoligonucleotide 30agactcgaga
atcgaaggtc gtgacctgcg tatttctcag 403128DNAArtificial
sequenceoligonucleotide 31agatctagat cacctgcata tagaaatg
283227DNAArtificial sequenceoligonucleotide 32agactcgaga atgaccgacg
ctgcttc 273328DNAArtificial sequenceoligonucleotide 33agactcgagt
catgtttcct ccacaatc 283438DNAArtificial sequenceoligonucleotide
34agaccatggg actcgagaat gtcccctata ctaggtta 383530DNAArtificial
sequenceoligonucleotide 35agagtcgact taacgacctt cgatcagatc
303627DNAArtificial sequenceoligonucleotide 36agaccatggc ggataacaaa
ttcaaca 273728DNAArtificial sequenceoligonucleotide 37agaccatggc
ttttggtgct tgagcatc 283828DNAArtificial sequenceoligonucleotide
38agactcgaga gcggataaca aattcaac 283930DNAArtificial
sequenceoligonucleotide 39agactcgagt cattttggtg cttgagcatc
304033DNAArtificial sequenceoligonucleotide 40agaccatggg atccctttcc
aacttaggaa gtg 334128DNAArtificial sequenceoligonucleotide
41agaccatggc ctgacaagaa atcagctt 284227DNAArtificial
sequenceoligonucleotide 42agaccatggg aatgataacc acccctc
274333DNAArtificial sequenceoligonucleotide 43agactcgagg tcatatcttg
ccattctgtg gag 334430DNAArtificial sequenceoligonucleotide
44agaccatggg atcattgtcc aatggaacta 304532DNAArtificial
sequenceoligonucleotide 45agactcgagt gatagtattc tttgaatgca at
324630DNAArtificial sequenceoligonucleotide 46agactcgagt gatactaagc
tttcagagat 304736DNAArtificial sequenceoligonucleotide 47aaactcgagt
caaatgcagc aactatctgg atcatc 36
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.