Compositions And Methods Relating To The Treatment Of Diseases
Stimson; William
U.S. patent application number 15/760200 was filed with the patent office on 2019-05-16 for compositions and methods relating to the treatment of diseases. The applicant listed for this patent is Alfacyte Ltd. Invention is credited to William Stimson.
| Application Number | 20190144519 15/760200 |
| Document ID | / |
| Family ID | 57003529 |
| Filed Date | 2019-05-16 |


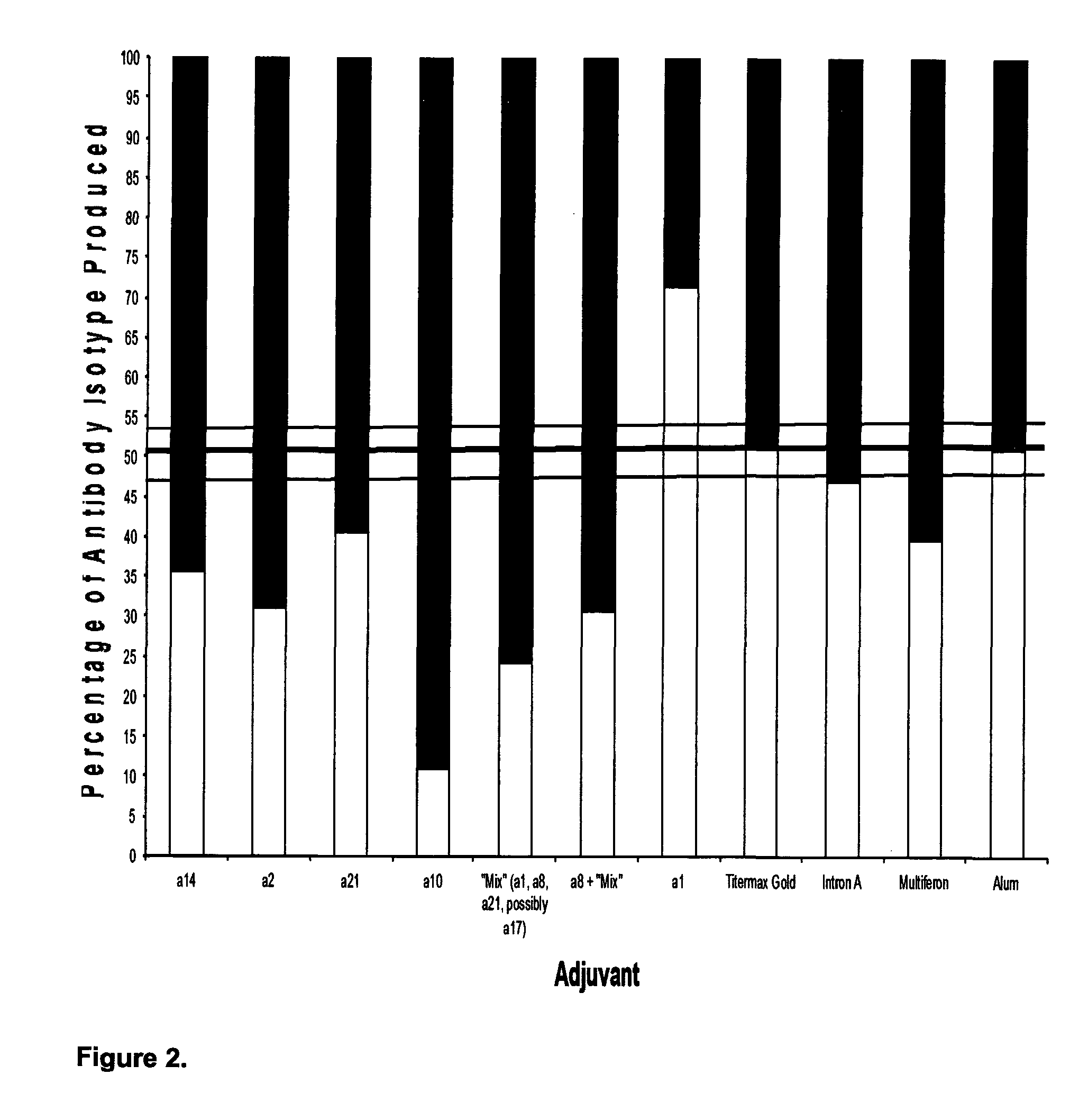

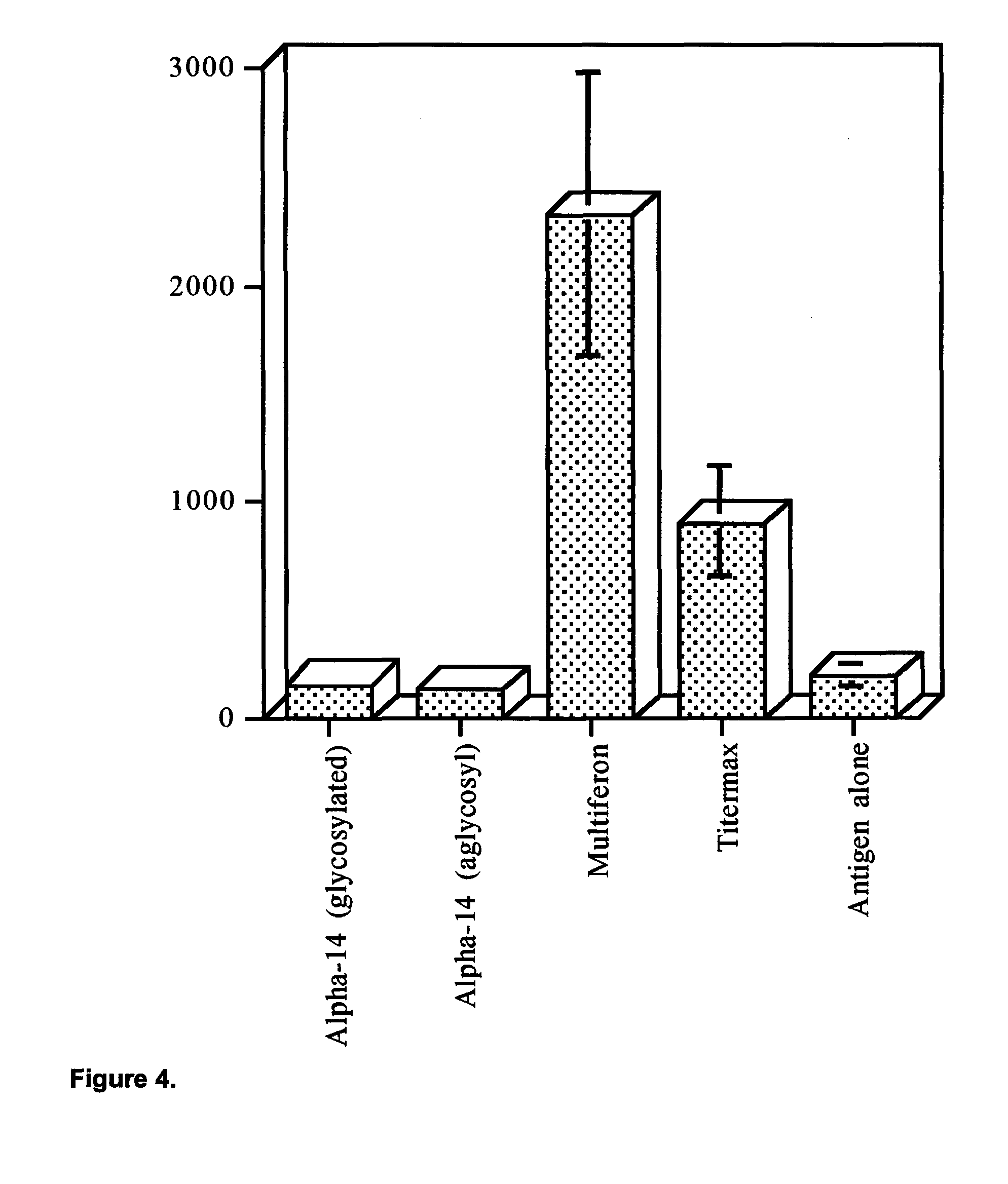
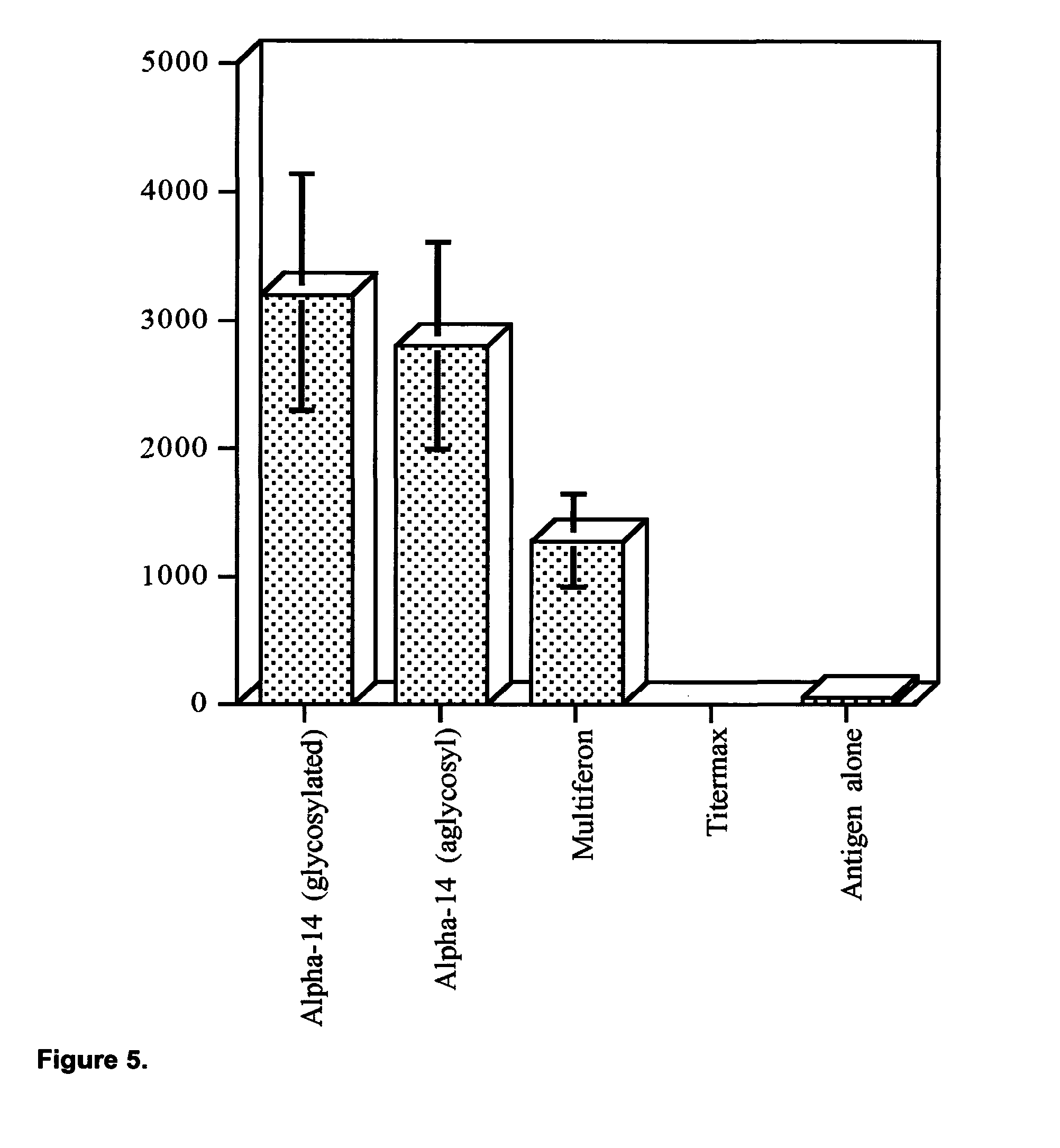
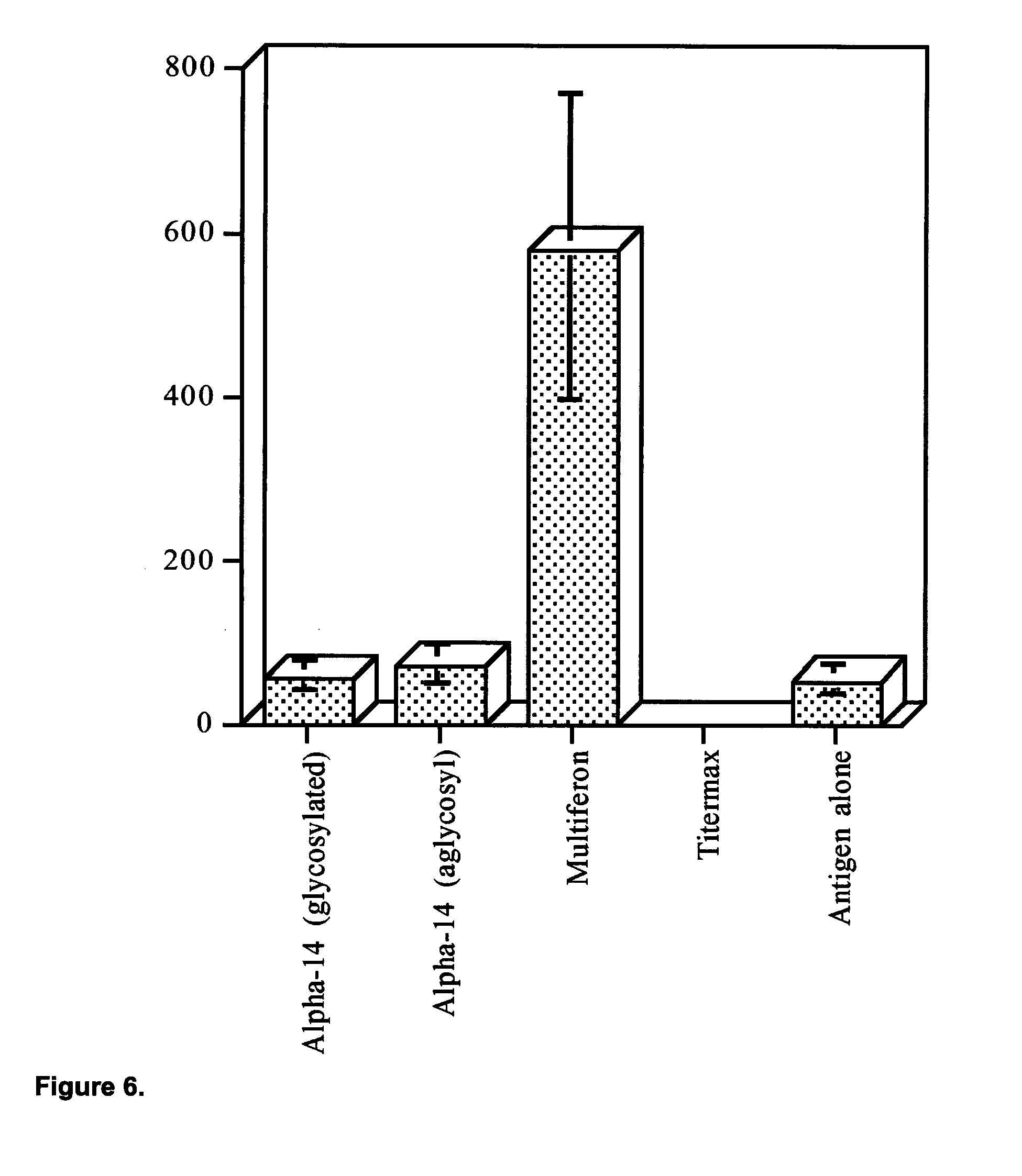




View All Diagrams
| United States Patent Application | 20190144519 |
| Kind Code | A9 |
| Stimson; William | May 16, 2019 |
COMPOSITIONS AND METHODS RELATING TO THE TREATMENT OF DISEASES
Abstract
The present invention relates to compositions and methods for promoting the induction of a cell-mediated immune response (such as that mediated by Th1 cells) and the suppression of a humoral or allergic immune response (such as that mediated by Th2 and Th17 cells). In particular, the invention relates to compositions and methods for preventing or treating allergy, such as food allergy, and associated allergic diseases, and conditions where an exaggerated Th17 response plays a detrimental role, such as inflammatory responses and autoimmune diseases. The invention further extends to the use of the compositions of the invention in the treatment and/or prophylaxis of allergy and associated allergic diseases and also of cancer.
| Inventors: | Stimson; William; (Glasgow, GB) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior Publication: |
|
||||||||||
| Family ID: | 57003529 | ||||||||||
| Appl. No.: | 15/760200 | ||||||||||
| Filed: | September 14, 2016 | ||||||||||
| PCT Filed: | September 14, 2016 | ||||||||||
| PCT NO: | PCT/GB2016/052841 PCKC 00 | ||||||||||
| 371 Date: | March 14, 2018 |
| Current U.S. Class: | 424/85.7 |
| Current CPC Class: | C07K 19/00 20130101; C07K 14/56 20130101; A61P 29/00 20180101; C07K 2319/00 20130101; A61K 38/212 20130101; A61K 39/0011 20130101; A61P 37/08 20180101; A61K 39/35 20130101; A61P 35/00 20180101; A61P 37/00 20180101 |
| International Class: | C07K 14/56 20060101 C07K014/56; A61K 38/21 20060101 A61K038/21; A61K 39/35 20060101 A61K039/35; A61K 39/00 20060101 A61K039/00 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Sep 15, 2015 | GB | 1516303.3 |
| Sep 16, 2015 | GB | 1516437.9 |
Claims
1. A method for the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired, said method comprising the step of: (i) administering to a subject in need thereof a therapeutically effective amount of at least one interferon alpha subtype selected from IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof, wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
2. The method as claimed in claim 1 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is selected from the group consisting of an autoimmune disease, an inflammatory disease, allergy or an associated allergic condition and cancer.
3. The method as claimed in claim 2 wherein the inflammatory disease is inflammatory bowel disease.
4. The method as claimed in claim 3 wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
5. The method as claimed in claim 2 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is allergy or an associated allergic condition suitably food allergy or an associated condition.
6. The method as claimed in claim 2 wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
7. The method as claimed in any one of claims 1 to 6 wherein the method includes a step of administering to the subject a therapeutically effective amount of a vaccine composition for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
8. The method as claimed in claim 7 wherein the vaccine composition comprises at least one allergen capable of mediating a Th2/Th17 immune response.
9. The method as claimed in claim 8 wherein the at least one allergen is a food allergen or a tumour antigen, for example tumour specific antigen or tumour-associated antigen.
10. The method as claimed in any one of claims 7 to 9 wherein the vaccine composition is administered sequentially, separately or simultaneously with the at least one interferon alpha subtype.
11. The method as claimed in any one of claims 1 to 10 wherein the at least one interferon alpha subtype is administered orally.
12. The method as claimed in any one of claims 1 to 11 wherein the at least at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14hybrid wherein the hybrid includes the primary Interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
13. The method as claimed in any one of claims 1 to 12 wherein the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
14. The method as claimed in any one of claims 1 to 13 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
15. The method as claimed in any one of claims 1 to 14 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14 or a hybrid thereof.
16. The method as claimed in any one of claims 1 to 15 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
17. An interferon alpha subtype hybrid comprising the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
18. An interferon alpha subtype comprising the amino acid sequence of SEQ ID NO: 1 or a fragment or variant thereof.
19. At least one interferon alpha subtype selected from the group consisting of IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof wherein the hybrid comprises the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 for use in the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
20. The at least one interferon alpha subtype for use as claimed in claim 17 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is selected from the group consisting of an autoimmune disease, an inflammatory disease, allergy or an associated allergic condition and cancer.
21. The at least one interferon alpha subtype for use as claimed in claim 20 wherein the inflammatory disease is inflammatory bowel disease.
22. The at least one interferon alpha subtype for use as claimed in claim 21 wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
23. The at least one interferon alpha subtype for use as claimed in claim 20 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is allergy or an associated allergic condition preferably wherein the allergy is a food allergy or an associated allergic condition.
24. The at least one interferon alpha subtype for use as claimed in claim 20 wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
25. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 24 wherein the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with a vaccine composition for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
26. The at least one interferon alpha subtype for use as claimed in claim 25 wherein the vaccine composition comprises at least one allergen capable of mediating a Th2/Th17 immune response there against.
27. The at least one interferon alpha subtype for use as claimed in claim 26 wherein the at least one allergen is a food allergen or a tumour antigen, for example a tumour specific antigen or a tumour-associated antigen.
28. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 27 wherein the at least one Interferon alpha subtype is provided for administration orally.
29. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 28 wherein the at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14 hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
30. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 29 wherein wherein the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
31. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 30 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
32. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 31 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14 or a hybrid thereof.
33. The at least one interferon alpha subtype for use as claimed in any one of claims 19 to 32 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
34. Use of at least one interferon alpha subtype selected from the group consisting of IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof including the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 in the preparation of a medicament for the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
35. The use as claimed in claim 34 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is selected from the group consisting of an autoimmune disease, an inflammatory disease, allergy or an associated allergic condition or cancer.
36. The use as claimed in claim 35 wherein the inflammatory disease is inflammatory bowel disease.
37. The use as claimed in claim 36 wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
38. The use as claimed in claim 35 wherein the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired is allergy or an associated allergic condition, preferably wherein the allergy is a food allergy or associated allergic condition.
39. The use as claimed in claim 35 wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
40. The use as claimed in any one of claims 34 to 39 wherein the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with a vaccine composition.
41. The use as claimed in claim 40 wherein the vaccine composition comprises at least one allergen capable of mediating a Th2/Th17 immune response there against.
42. The use as claimed in claim 41 wherein the at least one allergen is a food allergen, or a tumour antigen, for example a tumour specific antigen or a tumour-associated antigen.
43. The use as claimed in any one of claims 35 to 42 wherein the at least one interferon alpha subtype is provided for administration orally.
44. The use as claimed in any one of claims 34 to 43 wherein the at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14 hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
45. The use as claimed in any one of claims 34 to 44 wherein the at least one interferon alpha subtype is a IFN-.alpha.10 IFN-.alpha.14 hybrid and the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
46. The use as claimed in any one of claims 34 to 45 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
47. The use as claimed in any one of claims 34 to 46 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14.
48. The use as claimed in any one of claims 34 to 47 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
49. A composition comprising: (i) a vaccine; and (ii) at least one interferon alpha subtype selected from IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
50. The composition claimed in claim 49 wherein the vaccine comprises at least one allergen capable of mediating a Th2/Th17 immune response there against.
51. The composition as claimed in claim 50 wherein the at least one allergen is a food allergen or a tumour antigen, for example a tumour specific antigen or a tumour-associated antigen.
52. The composition as claimed in any one of claims 49 to 51 wherein the composition is provided for administration orally.
53. The composition as claimed in any one of claims 49 to 52 wherein the at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
54. The composition as claimed in any one of claims 49 to 53 wherein the at least one interferon alpha subtype is a hybrid of IFN-.alpha.10 and IFN-.alpha.14.
55. The composition as claimed in any one of claims 49 to 53 wherein the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
56. The composition as claimed in any one of claims 49 to 55 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
57. The composition as claimed in any one of claims 49 to 56 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14 or a hybrid thereof.
58. The composition as claimed in any one of claims 49 to 57 for use in treating or preventing a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired
59. The composition of claim 58 wherein the condition is selected from the group consisting of autoimmune disease, inflammatory disease, allergy or an associated allergic condition or cancer.
60. The composition of claim 59 wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
61. The use as claimed in any one of claims 49 to 60 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
62. A pharmaceutical composition, wherein the pharmaceutical composition comprises a vaccine and at least one interferon alpha subtype selected from IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14, along with a pharmaceutically acceptable excipient, diluent or carrier.
63. The pharmaceutical composition as claimed in claim 61 wherein the vaccine comprises at least one allergen capable of mediating a Th2 immune response.
64. A method for the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17, said method comprising the step of: (i) administering to a subject in need thereof a therapeutically effective amount of at least one interferon alpha subtype selected from IFN-.alpha.10, IFN-.alpha.14 and a hybrid thereof wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
65. The method as claimed in claim 64 wherein the condition mediated by enhanced expression of IL-17 is inflammatory bowel disease.
66. The method as claimed in claim 65 wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
67. The method as claimed in any one of claims 64 to 66 wherein the at least one interferon alpha subtype is administered orally.
68. The method as claimed in any one of claims 64 to 66 wherein the at least at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
69. The method as claimed in any one of claims 64 to 68 wherein the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
70. The method as claimed in any one of claims 64 to 69 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
71. The method as claimed in any one of claims 64 to 70 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14 or a hybrid thereof.
72. The use as claimed in any one of claims 64 to 70 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
73. At least one interferon alpha subtype selected from IFN-.alpha.10, and IFN-.alpha.14 and a hybrid thereof for use in the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17.
74. The at least one interferon alpha subtype for use as claimed in claim 73 wherein the condition mediated by enhanced expression of IL-17 is inflammatory bowel disease preferably wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
75. The at least one interferon alpha subtype for use as claimed in claim 73 wherein the condition is cancer, preferably wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
76. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 75 wherein the at least one interferon alpha subtype is administered orally.
77. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 76 wherein the at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14 hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
78. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 77 wherein wherein the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
79. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 78 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
80. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 79 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14 or a hybrid thereof.
81. The at least one interferon alpha subtype for use as claimed in any one of claims 73 to 80 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
82. Use of at least one interferon alpha subtype selected from IFN-.alpha.10, IFN-.alpha.14and a hybrid thereof wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 in the preparation of a medicament for the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17.
83. The use as claimed in claim 82 wherein the condition mediated by enhanced expression of IL-17 is inflammatory bowel disease, preferably, wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease.
84. The use as claimed in claim 82 wherein the condition is cancer, preferably wherein the cancer is hepatic cell cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma, genitourinary cancer, prostate cancer, renal cell cancer, or bladder cancer.
85. The use as claimed in any one of claims 82 to 84 wherein the at least one interferon alpha subtype is administered orally.
86. The use as claimed in any one of claims 82 to 85 wherein the at least one interferon alpha subtype is IFN-.alpha.10, IFN-.alpha.14, or an IFN-.alpha.10 IFN-.alpha.14 hybrid wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 and has improved binding to interferon receptor 1 and interferon receptor 2 in comparison to IFN-.alpha.10 or IFN-.alpha.14.
87. The use as claimed in any one of claims 82 to 86 wherein the at least one interferon alpha subtype is a IFN-.alpha.10 IFN-.alpha.14 hybrid and the hybrid comprises the amino acid sequence set forth by SEQ ID NO: 1 or a fragment or variant thereof.
88. The use as claimed in any one of claims 82 to 87 wherein the at least one interferon alpha subtype is a hybrid protein comprising at least part of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes at least 1, at least 2, at least 3, at least 4, at least 5, at least 6 of the amino acids corresponding to primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
89. The use as claimed in any one of claims 82 to 88 wherein the at least one interferon alpha subtype is a recombinant form of IFN-.alpha.10 and/or IFN-.alpha.14.
90. The use as claimed in any one of claims 82 to 88 wherein the hybrid comprises at least one, at least two, at least three, at least four or at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144.
Description
FIELD OF THE INVENTION
[0001] The present invention relates to compositions and methods for promoting the induction of a cell-mediated immune response (such as that mediated by Th1 cells) and the suppression of a humoral or allergic immune response (such as that mediated by Th2 and Th17 cells). In particular, the invention relates to compositions and methods for preventing or treating allergy, such as food allergy, and associated allergic diseases, and conditions where an exaggerated Th17 response plays a detrimental role, such as inflammatory responses and autoimmune diseases. The invention further extends to the use of the compositions of the invention in the treatment and/or prophylaxis of allergy and associated allergic diseases and also of cancer.
BACKGROUND TO THE INVENTION
[0002] It is hypothesised that in certain circumstances, the Th1 response or the Th2/Th17 response can cause disease. An over-reactive Th1 response can generate organ-specific autoimmune disease such as arthritis, multiple sclerosis, or Type I diabetes, while an over-reactive Th2/Th17 response may underlie allergy and atrophy. It is currently believed that Th17 cells play a major role in host defence against pathogens and an exaggerated Th17 response may lead to severe inflammatory responses and autoimmune diseases--inflammatory bowel diseases (IBD), namely, ulcerative colitis (UC) and Crohn's disease (CD), are chronic inflammatory processes of the gastrointestinal tract. In these diseases a disturbed and exaggerated immune response, mainly towards the endogenous microflora, plays a major role. IL-17 expression is increased in both UC and CD. Type I IFNs have been studied in clinical trials in patients with UC and demonstrated efficacy in selected studies. As anti- viral cytokines, it is now known that Type I IFNs can regulate the development of Th17 cells.
[0003] It is known that different pathogens induce different IFN-.alpha. subtypes in vitro and that IFN-.alpha. subtypes have different antiviral, antiproliferative and immunomodulatory activities. Infection via a variety of routes, including orally, has been shown to induce different subtype profiles. IFN-.alpha. subtypes bind to the same receptor, activate common signaling pathways and are expected to have the same biological functions. All IFN-.alpha. subtypes have anti-viral activities, by definition, although their absolute efficacy in this context may vary considerably. In addition, many other biological properties have been described, but with varying potencies, including immunomodulatory and anti-proliferative activities. The pleiotropic effects appear to be due to differential interaction with the receptor chains and signaling through different intracellular pathways to an array of effector molecules. The Type IFN receptor consists of two chains, IFNR1 and IFNR2. There is a range of binding affinities for each of the 12 IFN-.alpha. subtypes with the different receptor chains.
[0004] IFN-.alpha. may have a key role in the regulation of the Th1 response. It has been shown that IFN-.alpha. treatment promotes Th1 cell differentiation indirectly (largely via IFN-.gamma.), but also appears to suppress Th2 cell development through the suppression of IL-4 and IL-13 gene expression. IFN-.alpha. therefore is able to re-establish a Th1/Th2 population balance in diseases and infections that promote a Th2 cell imbalance. In recent years, it became evident that besides its anti-viral effects, several immunomodulatory functions are exerted by IFN-.alpha.. IFN-.alpha. can impact on dendritic cell differentiation and controls the expression of various pro-inflammatory cytokines such as IL-8 or IL-18 and induces several anti-inflammatory mediators such as IL-1 receptor antagonist (IL-1Ra), soluble TNF receptor p55, IL-10 and IL-18 binding protein. However, the mechanisms of actions of IFN-.alpha., and in particular individual IFN-.alpha. subtypes, are still only partly understood.
[0005] In patients with allergy or allergic disease, a Th2-predominant immune response is generated. Th2 cells secrete IL-4 and IL-13 driving B cells to produce Immunoglobulin E (IgE) antibodies specific to an allergen. An allergen is an antigen capable of stimulating a type-I hypersensitivity reaction in atopic individuals mainly through Immunoglobulin E (IgE)-mediated responses. Following that, IgE binds to its high affinity receptor on mast cells, skin cells and mucosal tissues. Upon exposure to the allergen, mast cells release their contents, which include histamine, leukotrienes and prostaglandins. This causes allergic symptoms including, but not limited to, red eyes, itchiness, runny nose, eczema, urticaria, angioedema, shortness of breath, wheezing, coughing an asthma attack, abdominal pain, vomiting diarrhoea or even anaphylaxis.
[0006] Allergic diseases are among the most common form of chronic illness. The World Health Organisation estimates that over 20 percent of the world population is affected and Europe alone has over 80 million sufferers (Global Allergy and Asthma European Network, 2008). An allergic reaction is usually caused by hypersensitivity of the immune system to an allergen, causing a misdirected immune response. Mild allergies, such as hay fever, are very common in the human population. Severe allergies can be caused by dietary allergens, such as food, by environmental allergens, such as the venom of stinging insects, by medication or can be genetically determined.
[0007] Food allergy is a major health concern, which is estimated to affect around 6% of young children and 3-4% of adults in Western societies. Food allergy is hypothesised to result from a breakdown in oral tolerance to ingested antigens or allergens. Food allergies and associated allergic diseases include, but are not limited to, dairy (milk) allergy, including Heiner syndrome, egg allergy, soya allergy, fish (shellfish) allergy, peanut and tree nut allergy, sesame and other seed allergy, gluten (wheat) and grains allergy, fruit and vegetable allergy, caffeine allergy, oral allergy syndrome, alcohol allergy, pollen food allergy syndrome, eosinophilic gastroenteritis, IgE mediated gastrointestinal food allergy and C1 esterase deficiency.
[0008] Management and treatment of allergic disease is usually via three general approaches: (i) avoidance of the allergen; (ii) medications that target disease symptoms and (iii) conventional immunotherapy, known as desensitisation, which aims to enhance the Th1 response in established disease. However, these approaches are far from ideal. Avoidance of allergens is not always possible, medications that target disease symptoms, such as anti-histamines, provide only short-term relief and desensitisation involves the use of the actual allergen, which can result in potentially frequent harmful side-effects. The possibility of anaphylaxis is never completely eliminated in patients suffering from allergic diseases and this causes a great deal of stress to the patient and their families.
[0009] Interferon subtypes IFN-.alpha.10 and IFN-.alpha.14 and hybrids thereof are discussed in PCT Publication Number WO2014/037717 and PCT Application Number PCT/GB2015/050717. In particular IFN-.alpha.10-IFN-.alpha.14 hybrids are disclosed that contain sequences characteristic of the IFN-.alpha.10 and IFN-.alpha.14 subtype binding sites based on a consensus backbone sequence of all 12 alpha-interferons, for example
TABLE-US-00001 (SEQ ID NO: 3) CDLPQTHSLGNRRALILLGQMGRISPFSCLKDRHDFRIPQEEFDGNQFQKAQAISVLHEM ##STR00001## RKYFQRITLYLIERKYSPCAWEVVRAEIMRSLSFSTNLQKRLRRKD.
It would be advantageous to provide alternative hybrids and further compositions and methods that provide alternative immunotherapeutic approaches.
[0010] The present inventor submits that it would be desirable to develop an improved immunotherapeutic approach which involves safer use of an allergen, as lower doses may be employed, and provides longer-term protection against the allergic reaction. Since allergy results from over-reactivity of Th2/Th17 cells and a corresponding lack of activity of the Th1 response, a medication that is able to modify and balance a misdirected Th2/Th17 response would be beneficial in preventing the allergic reaction. Such a medication would further be suitable to treat diseases and conditions where an exaggerated Th17 response plays a role, such as inflammatory bowel diseases. Additionally, the inventor considers the ability to enhance of a Th1-mediated immune response and suppress a Th2/Th17-mediated immune response would be useful in the provision of compositions that mediate immune response in subjects with cancer.
SUMMARY OF THE INVENTION
[0011] The present invention relates to the action of cytokines that promote the induction of a cell-mediated immune response (such as that mediated by Th1 cells) and cytokines that suppress a humoral or allergic immune response (such as that mediated by Th2 and Th17 cells). The present invention relates to hybrids of specific interferon (IFN) subtypes, and in particular to hybrids of IFN-.alpha.10 and IFN-.alpha.14.
[0012] Following extensive experimentation, the present inventor has made the surprising discovery that the administration of a specific interferon alpha (IFN-.alpha.) subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, preferably wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 as part of a composition to modulate the immune system, such as a vaccine, for example comprising an allergen, can result in enhanced activation of the Th1 immune response and suppression of the Th2/Th17 immune response. In particular, the inventor has developed hybrids of IFN-.alpha.10 and IFN-.alpha.14 containing higher affinity binding sites derived from each of IFN-.alpha.10 and IFN-.alpha.14 for the interferon receptors IFNR1 and IFNR2. In particular, the inventor demonstrates that it is advantageous to provide IFN-.alpha.10-IFN-.alpha.14 hybrids with high affinity binding sites derived from IFN-.alpha.10 and IFN-.alpha.14 subtypes that are not based on a consensus sequence of all 12 IFN-.alpha. subtypes which provide SEQ ID NO:3. The hybrids of the present invention are not based on a consensus backbone sequence of all 12 interferon-alphas. Instead, they derive the sequence characteristics of IFN-.alpha.10 and IFN-.alpha.14 subtypes without the sequence characteristics of the other 10 interferon-alpha subtypes.
[0013] In particular, the inventor has discovered that IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprising or consisting of at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, (numbering as used in FIG. 16), in particular 94, 109 or 144 or combinations thereof, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101,102,109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or in particular SEQ ID NO:1 result in higher affinity binding of the interferon receptors IFNR1 and IFNR2. In embodiments, the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence can be SEQ ID NO:3 further comprising the mutation(s) discussed above. These hybrid sequences can be used in all aspects and embodiments of the invention.
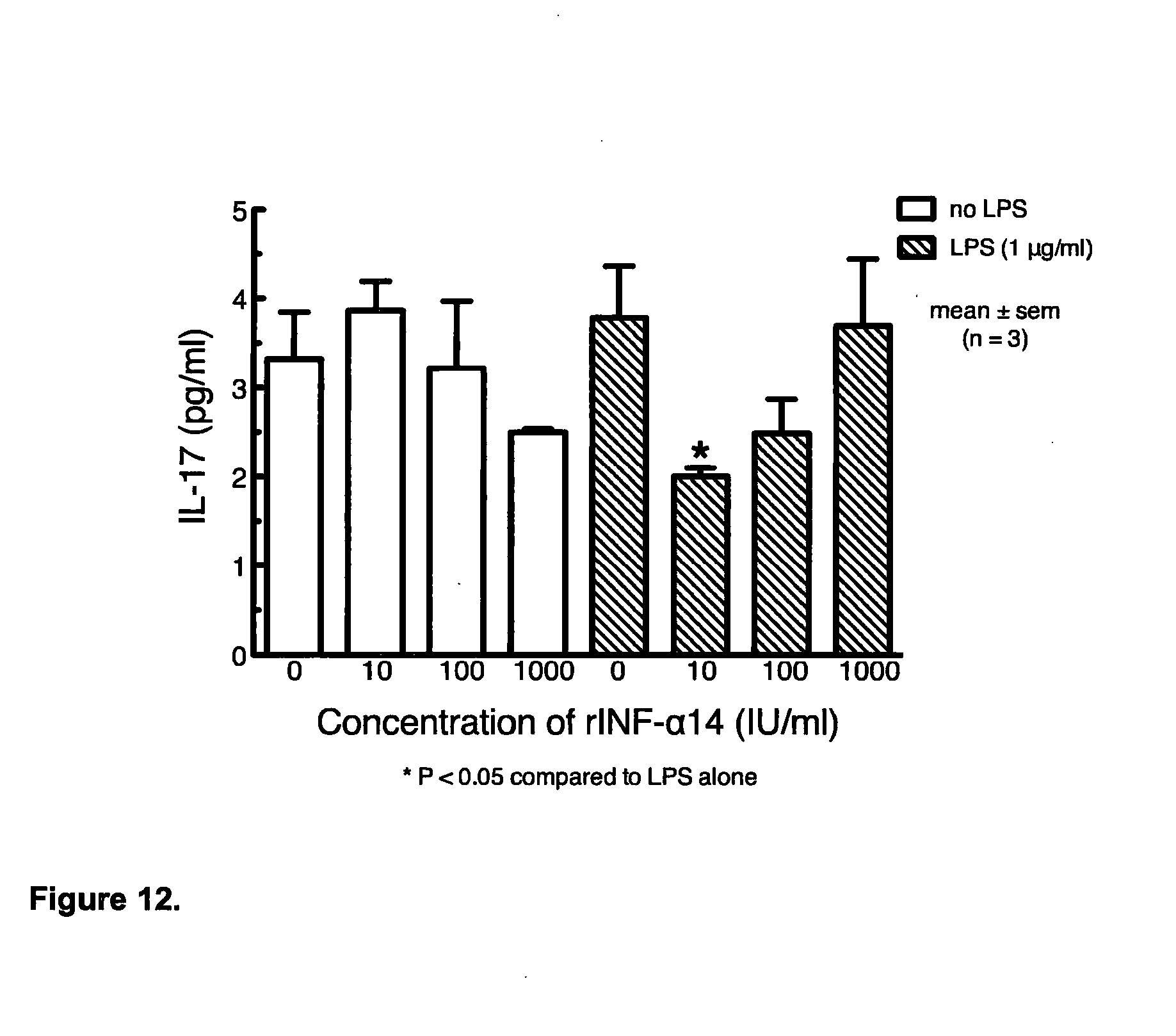
[0014] The inventor has surprisingly discovered that administration of the novel IFN-.alpha.10-IFN-.alpha.14 hybrids result in a greater reduction of IL-17 compared to previous IFN-.alpha.10-IFN-.alpha.14 hybrids. The inventor has discovered that administration of the novel IFN-.alpha.10-IFN-.alpha.14 hybrids result in a 10%, preferably a 20%, preferably a 30%, preferably a 40% and more preferably a 50% greater reduction of IL-17 compared to previous IFN-.alpha.10-IFN-.alpha.14 hybrids.
[0015] This has led to the identification by the inventor of improved therapeutic compositions which have utility in the treatment and/or prophylaxis of allergy and allergic diseases and diseases and conditions where an exaggerated Th17 response plays a role and also to cancer. In particular, the inventor has identified that the administration of at least one food allergen which is capable of mediating a Th2/Th17 immune response with a hybrid of IFN-.alpha.10 and IFN-.alpha.14 preferably wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 can be used in the treatment of food allergy and associated allergic diseases.
[0016] Moreover, the inventor has identified that the administration of a tumour antigen, either a tumour associated or a tumour specific antigen, in combination with a specific interferon alpha (IFN-.alpha.) subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, preferably wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 as part of a composition to modulate the immune system, such as a vaccine, can be used in the treatment of cancer. Suitably, the cancer may be hepatic cancer, lung cancer, in particular non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma or genitourinary cancer.
[0017] Suitably, the tumour associated antigen may be selected from a prostate tumour, a renal cell tumour and a bladder tumour.
[0018] Accordingly a first aspect of the present invention provides a method for the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired, said method comprising the step of: [0019] (i) administering to a subject in need thereof a therapeutically effective amount of at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14.
[0020] Whilst not wishing to be bound by theory, the inventor believes that proteins comprising the amino acid sequence of IFN-.alpha.10 have greater affinity to interferon receptor 2 (IFNR2) and proteins comprising the amino acid sequence of IFN-.alpha.14 have greater affinity to interferon receptor 1 (IFNR1). Thus, substitution of a protein comprising an IFN-.alpha.10 amino acid sequence with amino acids of IFN-.alpha.14 which allow binding to interferon receptor 1 or substitution of a protein comprising an IFN-.alpha.14 amino acid sequence with amino acids of IFN-.alpha.10 which allow binding to interferon receptor 2 is considered to provide a IFN-.alpha.10 IFN-.alpha.14 hybrid protein which should have stronger binding affinity to both interferon receptors 1 and 2 than IFN-.alpha.10 or IFN-.alpha.14 alone. By including the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14 is meant that the hybrid comprises amino acids selected from IFN-.alpha.10 and substituted into an IFN-.alpha.14 amino acid sequence to improve the ability of an IFN-.alpha.14 subtype to bind to an interferon receptor 2 and/or that the hybrid comprises amino acids selected from IFN-.alpha.14 and substituted into an IFN-.alpha.10 amino acid sequence to improve the ability of an IFN-.alpha.10 subtype to bind to an interferon receptor 1.
[0021] Suitably, several amino acid substitutions of protein comprising an IFN-.alpha.10 amino acid sequence with amino acids of IFN-.alpha.14 determined to be involved in binding to interferon receptor 1 may enhance the binding of the protein to interferon receptor 1. Suitably, an amino acid substitution of protein comprising an IFN-.alpha.14 amino acid sequence with amino acids of IFN-.alpha.10 determined to be involved in binding to interferon receptor 2 may enhance the binding of the protein to interferon receptor 2.
[0022] In embodiments the IFN-.alpha.10-IFN-.alpha.14 hybrid can substantially have the amino-acid sequence of IFN-.alpha.10, but be modified in a region between amino residues 80 to 150, or suitably between amino acid residues 84 to 144, or suitably amino acid residues 92 to 115 or suitably between amino acid residues 90 to 110, (utilizing the numbering of the IFN-.alpha.10 sequence providing in FIG. 16) to provide the amino acids provided by the IFN-.alpha.14 sequence. It is considered the amino acid residues in these regions or parts of these regions provide for the binding of IFN-.alpha.14 to interferon receptor 1. In particular, the hybrid sequence may include at least one, at least two, at least three, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10 or at least 11 modifications of the IFN-.alpha.10 sequence to provide the corresponding residues of the IFN-.alpha.14 sequence (suitably substituted residues are noted in bold in FIG. 9) or a conserved mutation thereof. In embodiments, eleven modifications are provided as indicated by the amino acids noted in bold in FIG. 9. In embodiments, the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence may include at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144. In alternative embodiments, IFN-.alpha.14 can be utilised as a backbone structure of the hybrid and the residues which differ between the IFN-.alpha.10 and IFN-.alpha.14 sequences at the N and C terminal regions of the sequences can be provided in the hybrid sequence as those present in the IFN-.alpha.10 sequence. Suitably at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10 or at least 11 substitutions of the IFN-.alpha.14 N-terminal sequence may be made to provide the hybrid sequence to provide residues from IFN-.alpha.10 at those amino acid positions wherein the amino acids are not shared/common between IFN-.alpha.10 and IFN-.alpha.14. Suitably, at least 1, at least 2, or 3 substitutions are provided at the IFN-.alpha.14 C terminal sequence to provide residues from IFN-.alpha.10 to the hybrid sequence at those amino acid positions which are not shared/common between IFN-.alpha.10 and IFN-.alpha.14. In embodiments at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10 or at least 11 substitutions from the N-terminal sequence and at least 1, at least 2, or 3 substitutions from the C-terminal sequence of the IFN-.alpha.14 are made to provide residues from IFN-.alpha.10 to the hybrid at those amino acid positions which have amino acids that are not shared/common between IFN-.alpha.10 and IFN-.alpha.14.
[0023] In embodiments, the hybrid comprises or consists of an amino acid sequence SEQ ID NO: 1 or a functionally active fragment or variant thereof.
[0024] In certain embodiments, the method includes a step of administering to the subject a therapeutically effective amount of a vaccine composition for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired. The vaccine composition may be administered sequentially, separately or simultaneously with the at least one interferon alpha subtype.
[0025] By functionally active is meant an IL-.alpha.10 IL-.alpha.14 hybrid peptide comprising the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14 wherein the administration of peptide to a subject or expression of peptide in a subject promotes enhancement of Th1 mediated immune response and suppression of a Th2/Th17 mediated immune response. Further, functional activity may be indicated by the ability of a hybrid peptide to enhance a Th1 mediated immune response and to suppress a Th2/Th17 mediated response.
[0026] A fragment can comprise at least 50, preferably 100 and more preferably 150 or greater contiguous amino acids from SEQ ID NO: 1 and which is functionally active. Suitably, a fragment may be determined using, for example, C-terminal serial deletion of cDNA such as SEQ ID NO: 2 or SEQ ID NO: 3. Said deletion constructs may then be cloned into suitable plasmids. The activity of these deletion mutants may then be tested for biological activity as described herein.
[0027] By variant is meant an amino acid sequence which is at least 70% homologous to SEQ ID NO: 1, more preferably at least 80% homologous to SEQ ID NO: 1, more preferably at least 90% homologous to SEQ ID NO: 1, even more preferably at least 95% homologous to SEQ ID NO: 1, even more preferably at least 96% homologous to SEQ ID NO: 1, even more preferably at least 97% homologous to SEQ ID NO: 1 and most preferably at least 98% homology with SEQ ID NO: 1. A variant encompasses a polypeptide sequence of SEQ ID NO: 1 which includes substitution of amino acids, especially a substitution(s) which is/are known for having a high probability of not leading to any significant modification of the biological activity or configuration, or folding, of the protein. These substitutions, typically known as conserved substitutions, are known in the art. For example the group of arginine, lysine and histidine are known interchangeable basic amino acids. Suitably, in embodiments amino acids of the same charge, size or hydrophobicity may be substituted with each other. Suitably, any substitution may be selected based on analysis of amino acid sequence alignments of interferon alpha subtypes to provide amino acid substitutions to amino acids which are present in other alpha subtypes at similar or identical positions when the sequences are aligned. Hybrids, and variants and fragments thereof may be generated using suitable molecular biology methods as known in the art.
[0028] In certain embodiments, the vaccine composition comprises at least one antigen. In certain embodiments, the vaccine composition comprises at least one allergen capable of mediating a Th2/Th17 immune response, for example, a food allergen.
[0029] In aspects and embodiments of the invention the antigen can be a tumour antigen, for example a tumour specific antigen or a tumour associated antigen, in particular a tumour antigen can be of a hepatic carcinoma, lung cancer, in particular non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma or of a genitourinary cancer. In particular an antigen of a genitourinary cancer can include an antigen from a prostate cancer, renal cell carcinoma, or bladder cancer. Suitably, an antigen may be a tumour specific antigen or tumour associated antigen provided in an existing cancer vaccine in use or development which would benefit from an adjuvant that enhances T-cell immunity, in particular that enhances a Th1 response or provides an enhancement of a Th1 mediated immune response and suppression of a Th2/Th17-mediated immune response. Suitably a tumour specific or tumour-associated antigen may be obtained from a tumour of a subject to be treated. In embodiments only a tumour-associated antigen can be used.
[0030] In embodiments a tumour antigen, in particular an associated antigen may be an antigen for a prostate cancer antigen, in particular prostate-specific antigen. Suitably a method of providing a prostate specific antigen or a prostate cancer antigen with the interferon-alpha subtypes of the invention maybe used to treat prostate cancer, specifically castration-resistant prostate cancer.
[0031] As will be appreciated by a physician, the subjects who will benefit most from such treatments may be those with minimal disease, as there may be less chance of increasing tumour suppression of the immune system, additionally or alternatively such treatments may benefit subjects with advanced disease who may have significant tumour immune suppression and may benefit more from the use of vaccines in combination with other forms of treatment. Suitably the use of vaccines including tumour antigens, in particular tumour associated antigen may be in combination with other forms of immunotherapy, for example Sunitinib (Sutent by Pfizer) a tyrosine kinase inhibitor.
[0032] In embodiments specific tumour antigens, in particular tumour-associated antigens may be selected from the antigens utilised in the prostate cancer vaccines TroVax and Prostvac.
[0033] In embodiments a tumour antigen, in particular a tumour-associated antigen can be selected from renal cell carcinoma. Suitably a tumour antigen, for example a tumour-associated antigen for renal cell carcinoma may be selected from a heat shock protein or proteins of renal tumour cell lysates, in particular the antigen used in the potential vaccine MVA-5T4.
[0034] Suitably a tumour antigen may be MUC1 from melanoma.
[0035] In embodiments, a tumour antigen, for example a tumour-associated antigen can be selected from bladder cancer. Suitably a tumour-associated antigen may be selected from Bacille Calmette-Guerin (BCG) vaccine, human leukocyte antigen--A*2402 restricted epitope peptides, immucin peptide (a 21mer synthetic vaccine composed of the entire signal peptide of the MUCI protein) human chorionic gonadotropin-colony stimulating factor, or human chorionic gonadotropin-beta.
[0036] In certain embodiments, the method therefore includes a step of administering to the subject a therapeutically effective amount of at least one allergen capable of mediating a Th2/Th17 immune response, for example, a food allergen or tumour antigen, for example a tumour associated antigen. The allergen maybe administered sequentially, separately or simultaneously with the at least one interferon alpha subtype.
[0037] Typically, the subject is a mammal, in particular a human. In certain embodiments, the subject can be suffering from a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
[0038] According to a second aspect of the present invention, there is provided at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a fragment or variant thereof for use in the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
[0039] In certain embodiments, the at least one interferon alpha subtype, in particular a hybrid IFN-.alpha.10 and IFN-.alpha.14 subtype, for example SEQ ID NO: 1, as described herein is provided for simultaneous, separate or sequential administration with a vaccine composition for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired. In certain embodiments, the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with at least one allergen capable of mediating a Th2/Th17 immune response there against, for example, a food allergen, or a tumour antigen, in particular a tumour-associated antigen.
[0040] According to a third aspect of the present invention, there is provided use of at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular wherein the hybrid can be SEQ ID No: 1 or a variant or fragment thereof in the preparation of a medicament for the treatment and/or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired.
[0041] In certain embodiments, the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with a vaccine composition for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired. In certain embodiments, the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with at least one allergen capable of mediating a Th2/Th17 immune response there against, for example, a food allergen, or a tumour antigen, in particular a tumour associated antigen.
[0042] According to a further aspect of the present invention, there is provided a composition comprising: [0043] (i) a vaccine for treatment or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired; and [0044] (ii) at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101,102,109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102,109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular wherein the hybrid can be SEQ ID NO:1 or a variant or fragment, as described herein.
[0045] In certain embodiments, the vaccine comprises at least one allergen capable of mediating a Th2/Th17 immune response, for example, a food allergen or a tumour antigen, in particular a tumour-associated antigen.
[0046] A further aspect of the present invention provides a pharmaceutical composition for enhancement of a Th1 mediated immune response and suppression of a Th2/Th17-mediated immune response, wherein the composition comprises a vaccine for treatment or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired and at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least Five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular wherein the hybrid can be SEQ ID NO:1 or a fragment or variant thereof along with a pharmaceutically acceptable excipient, diluent or carrier.
[0047] In certain embodiments, the vaccine comprises at least one allergen capable of mediating a Th2/Th17 immune response, for example, a food allergen or tumour antigen, in particular a tumour-associated antigen.
[0048] In a further aspect, the present invention extends to improvements in the efficacy of vaccines, for example, anti-allergy or allergic disease vaccines or tumour or cancer vaccines, in particular genitourinary cancer vaccines, for example prostate cancer, renal cancer and or bladder cancer. A composition which comprises a vaccine for treatment or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired, such as at least one allergen capable of mediating a Th2/Th17 immune response, and at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a variant or fragment thereof, has been surprisingly identified by the inventor as providing an unexpectedly efficacious composition for the treatment and/or prophylaxis of diseases, such as allergy or associated allergic diseases.
[0049] Accordingly, a further aspect of the present invention provides a vaccine composition comprising;
(i) a vaccine for treatment or prophylaxis of a condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired; and (ii) at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular can be SEQ ID NO:1 or a variant or fragment thereof.
[0050] In certain embodiments, the vaccine comprises at least one allergen capable of mediating a Th2/Th17 immune response, for example, a food allergen or a tumour antigen, in particular a tumour-associated antigen.
[0051] A further aspect of the present invention provides a vaccine composition for use in the treatment and/or prophylaxis of allergy or cancer, in particular genitourinary cancer, for example prostate cancer, renal cancer or bladder cancer, where an enhancement of a Th1-mediated immune response and the suppression of a Th2/Th17-mediated immune response are desired, said vaccine composition comprising;
(i) at least one allergen capable of mediating a Th2/Th17 immune response; and (ii) at least one interferon alpha subtype which is a hybrid IFN-.alpha.10 and IFN-.alpha.14 subtype, in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102,109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular wherein the hybrid can be SEQ ID NO:1 or a variant or fragment, as described herein.
[0052] A further aspect of the present invention provides for the use of a vaccine composition comprising at least one allergen capable of mediating a Th2/Th17 immune response and at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102,109 or 144, in particular wherein the hybrid can be SEQ ID NO:1 or a variant or fragment thereof, in the preparation of a medicament for the treatment and/or prophylaxis of allergy or associated allergic diseases, or cancer, in particular genitourinary cancer, for example prostate cancer, renal cancer or bladder cancer.
[0053] A further aspect of the present invention provides a method for the treatment and/or prophylaxis of allergy or associated allergic diseases or of cancer, in particular genitourinary cancer for example prostate cancer, renal cancer or bladder cancer the method comprising the step of: [0054] (i) administering a therapeutically effective amount of a vaccine composition or an immunogenic composition which comprises at least one allergen capable of mediating a Th2/Th17 immune response and at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular wherein the hybrid can be SEQ ID NO: 1 or a fragment or variant thereof to a subject in need thereof.
[0055] According to a further aspect of the present invention, there is provided a method for the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17, said method comprising the step of: [0056] (i) administering to a subject in need thereof a therapeutically effective amount of at least one interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a fragment or variant thereof.
[0057] According to a further aspect of the present invention, there is provided at least one interferon alpha subtype comprising or consisting of an IFN-.alpha.10 and IFN-.alpha.14 hybrid in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a variant or fragment thereof for use in the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17.
[0058] Suitably, in aspects and embodiments of the invention, the hybrid may comprise or consist of the amino acid sequence of SEQ ID NO:1.
[0059] According to a further aspect of the present invention, there is provided use of at least one interferon alpha subtype IFN-.alpha.10 and IFN-.alpha.14 hybrid in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least Five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a variant or fragment thereof, in the preparation of a medicament for the treatment and/or prophylaxis of a condition mediated by enhanced expression of IL-17.
[0060] According to a further aspect of the present invention, there is provided a method for modulating an immune response, said method comprising the step of: [0061] (i) administering to a subject in need thereof a therapeutically effective amount of at least one interferon alpha subtype IFN-.alpha.10 and IFN-.alpha.14 hybrid, wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular can be SEQ ID NO:1 or a variant or fragment thereof.
[0062] Suitably, in aspects and embodiments of the invention, the administration or use of at least one interferon alpha subtype comprising or consisting of an IFN-.alpha.10 and IFN-.alpha.14 hybrid in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a variant or fragment thereof results in the full or partial inhibition of IL-17 and/or the full or partial activation of IFN-.gamma..
[0063] According to a further aspect of the present invention, there is provided at least one interferon alpha subtype IFN-.alpha.10 and IFN-.alpha.14 hybrid, wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular can be SEQ ID NO:1 or a variant or fragment thereof for use in modulating an immune response.
[0064] According to a further aspect of the present invention, there is provided use of at least one interferon alpha subtype hybrid IFN-.alpha.10 and IFN-.alpha.14 subtype, wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular SEQ ID NO:1 or a variant or fragment thereof in the preparation of a medicament for modulating an immune response.
[0065] In certain embodiments of the aspects of the invention outlined above, the at least one interferon alpha subtype is provided for simultaneous, separate or sequential administration with a vaccine for treatment or prophylaxis of the condition where an enhancement of a Th1-mediated immune response and suppression of a Th2/Th17-mediated immune response are desired, for example, a vaccine for the treatment or prophylaxis of a condition mediated by enhanced expression of IL-17, e.g. an inflammatory disease or condition or an autoimmune disease, such as inflammatory bowel disease (IBD), ulcerative colitis (UC) or Crohn's disease (CD), cancer, suitably hepatic cancer, lung cancer, in particular non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma or genitourinary cancer, in particular genitourinary cancer, for example prostate cancer, renal cancer or bladder cancer. In certain embodiments, the vaccine composition comprises at least one antigen. In certain embodiments, the vaccine comprises at least one allergen capable of mediating a Th2/Th17 immune response there against, for example, a food allergen.
[0066] In certain embodiments the antigen can be a tumour antigen in particular a tumour specific and/or a tumour-associated antigen.
[0067] In certain embodiments of the aspects of the invention outlined above, the at least one IFN-.alpha. subtype comprises, consists of or is an IFN-.alpha.10 IFN-.alpha.14 hybrid such as a fusion protein, or recombinant protein or the like which includes the primary interferon receptor binding sites of IFN-.alpha.10 and IFN-.alpha.14, and in particular which comprises or consists of the amino acid sequence SEQ ID NO:1 or a variant or fragment thereof. In embodiments the IFN-.alpha.10 IFN-.alpha.14 hybrid can be glycosylated. Suitably the IFN-.alpha.10 IFN-.alpha.14 hybrid can be glycosylated in a similar fashion to IFN-.alpha.14.
[0068] In certain embodiments of the aspects of the invention outlined above, the at least one allergen is at least one food allergen or a tumour specific or tumour-associated tumour allergen, for example a prostate cancer allergen, a renal cancer allergen and or bladder cancer allergen. In certain embodiments, the at least one allergen is a dietary allergen such as food, an environmental allergen such as the venom of stinging insects, or a medication.
[0069] In a further aspect of the invention there is provided a recombinant polypeptide comprising or consisting of SEQ ID NO:1 or a fragment or variant thereof. Nucleic acid sequences derived from the amino acid sequence SEQ ID NO:1 are provided as SEQ ID NO:2. These nucleic acid sequences can form additional aspects to the invention.
[0070] In certain embodiments of the aspects of the invention outlined above, the at least one food allergen is selected from the group consisting of, but not limited to, corn, garlic, oats, coffee, chocolate, pickle, wheat or gluten and their products or derivatives which include durum wheat, spelt (triticum spelta), kamut (triticum poloncium), couscous, bran, wheat bran, wheat germ, wheat gluten, farina, rusk, semolina, durum wheat semolina, flour, wholewheat flour, wheat flour, wheat starch, starch, modified starch, hydrolysed starch, food starch, edible starch, vegetable starch, vegetable gum, vegetable protein, cereal filler, cereal binder, cereal protein; tree nuts (including almonds, cashews, macademia, walnut and brazil nuts); seeds, including sesame, sunflower and poppy seeds; dairy derived antigens, such as milk or milk derivatives, including cheese and yoghurt; fish or shellfish or their derivatives, including from the mollusc phylum (gastropod class: snails and abalone; bivalve class: clam, mussel and oyster; cephalopod class: octopus, squid and scallop), arthropod phylum (crustacean family: crab, lobster, shrimp, prawn and crayfish) or chordate phylum (cartilaginous family: ray and shark; bony fish: cod, salmon and tuna); eggs or egg derivatives; monosodium glutamate (MSG); sulphites or sulphur dioxide; legume allergies to the leguminosae family, which includes peanut, soya (soybean or soya derivatives), bean seeds, peas, green beans, lentils, carob and liquorice; other vegetable allergies such as potato; fruit allergies to the rosaceae family, which includes apple, pear, cherry, peach and plum; fruit allergies to the cucurbitaceae family, which includes cucumber, melon, watermelon, zucchini and pumpkin; and other fruit allergies such as those developed against kiwi, banana, avocado, tomatoes, strawberries and raspberries.
[0071] In certain embodiments, the vaccine or vaccine composition can be a vaccine composition for the treatment or prophylaxis of a condition mediated by enhanced expression of IL-17, e.g. an inflammatory disease or condition or an autoimmune disease, such as inflammatory bowel disease (IBD), ulcerative colitis (UC) or Crohn's disease (CD), or cancer, suitably hepatic cancer, lung cancer, non-small cell lung cancer, ovarian cancer, breast cancer, skin cancer, melanoma or genitourinary cancer, in particular genitourinary cancer, for example prostate cancer, renal cancer or bladder cancer. In certain embodiments, the vaccine or vaccine composition can be a vaccine composition for the treatment or prophylaxis of an inflammatory disease or condition or an autoimmune disease, such as inflammatory bowel disease (IBD), ulcerative colitis (UC) or Crohn's disease (CD).
[0072] In certain embodiments of the aspects of the invention outlined above, the condition where an enhancement of a Th1-mediated immune response and the suppression of a Th2/Th17-mediated immune response arc desired can be a condition mediated by enhanced expression of IL-17, e.g. an inflammatory disease or condition or an autoimmune disease, such as inflammatory bowel disease (IBD), ulcerative colitis (UC) or Crohn's disease (CD).
[0073] In certain embodiments of the aspects of the invention outlined above, the condition where an enhancement of a Th1-mediated immune response and the suppression of a Th2/Th17-mediated immune response are desired can be an inflammatory disease, in particular an inflammatory disease which is mediated by an exaggerated or overactive Th17 immune response. In certain embodiments of the aspects of the invention outlined above, the condition where an enhancement of a Th1-mediated immune response and the suppression of a Th2/Th17-mediated immune response are desired can be an autoimmune disease, in particular an autoimmune disease which is mediated by an exaggerated or overactive Th17 immune response. For example, in certain embodiments the condition can be inflammatory bowel disease (IBD), such as ulcerative colitis (UC) or Crohn's disease (CD). In certain embodiments, the condition can be selected from the group consisting of asthma, allergic rhinitis, atopic dermatitis and food allergy. In certain embodiments, the condition is cancer, in particular a genitourinary cancer, in particular prostate cancer, bladder cancer or renal cancer.
[0074] In certain embodiments of the aspects of the invention outlined above, the condition where an enhancement of a Th1-mediated immune response and the suppression of a Th2/Th17-mediated immune response are desired is an allergy or associated allergic diseases and conditions caused thereby, or cancer wherein an immune response is desired against a tumour-associated antigen, in particular a tumour associated antigen of prostate cancer, renal cancer or bladder caner. In particular, in certain embodiments the condition is a food allergy including food associated or derived allergies and associated allergic diseases and conditions caused thereby.
[0075] In certain embodiments, the food allergy associated allergic diseases or conditions include, but are not limited to, milk/dairy allergy, including Heiner syndrome, egg allergy, soya allergy, fish (shellfish) allergy, peanut and tree nut allergy, sesame and other seed allergy, wheat and grains allergy, fruit and vegetable allergy, caffeine allergy, oral allergy syndrome, alcohol allergy, pollen food allergy syndrome, eosinophilic gastroenteritis, IgE mediated gastrointestinal food allergy and C1 esterase deficiency.
[0076] In certain embodiments of the present invention, the method of administration is oral administration. In certain embodiments, the method of administration is sublingual or buccal administration. In certain embodiments, the method of administration involves placing a lozenge under the patient's tongue. In certain embodiments, the route of administration is ocular or by means of introduction into the nasal cavity, by way of nasal administration. Also it may be introduced by oral administration (swallowing) of a capsule or similar device into the small intestine/duodenum such that the capsule does not dissolve in the stomach, but bypasses same and delivers/releases the interferon alpha subtype only into the small intestine/duodenum.
DETAILED DESCRIPTION OF THE INVENTION
[0077] The inventor of the present invention has surprisingly discovered that administering an IFN-.alpha. subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 subtypes, for example SEQ ID NO:1, as described herein results in the enhancement of a Th1 T cell mediated immune response and the suppression of a Th2/Th17 T cell mediated immune response and can therefore skew the immune response towards a cell-mediated (Th1) path, whilst simultaneously suppressing the allergic (Th2/Th17) response. Surprisingly, this effect is enhanced when the IFN-.alpha. subtype is administered orally.
[0078] In particular, the inventor discovered that IFN-.alpha.10-IFN-.alpha.14 hybrids that contain sequences characteristic of the IFN-.alpha.10 and IFN-.alpha.14 subtype binding sites that are not based on a consensus sequence of all 12 IFN-.alpha. subtypes resulted in a protein with higher affinity binding sites for the two interferon receptors, IFNR1 and IFNR2. This finding can be applied to provide an improved method and improved adjuvant composition for treating and/or preventing conditions where the enhancement of a Th1 T cell mediated immune response and/or the suppression of a Th2/Th17 T cell mediated immune response are desired, for example, inflammatory, autoimmune or allergy conditions, or cancer (including malignant conditions), in particular genitourinary cancers, in particular prostate cancer, renal cancer or bladder cancer. In particular, the hybrid of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular SEQ ID NO:1 or a fragment or variant thereof may be used as an adjuvant in vaccines to boost immune response to antigens and direct the immune response towards a Th1 immune response.
[0079] The inventor has also discovered that a combination of a vaccine composition or a food or tumour specific or tumour-associated antigen allergen which is capable of mediating a Th2/Th17 immune response and an IFN-.alpha. subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14, in particular a hybrid comprising the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular SEQ ID NO:1 or a fragment or variant thereof can result in the activation of a Th1 T cell mediated immune response and the suppression of a Th2/Th17 T cell mediated immune response.
[0080] Tumour progression in normal immunocompetent subjects may reflect a failure of the immune system to recognize the tumour antigens or a subversion of the anti-tumour immune response through induction and activation of regulatory T cells. In subjects with hepatic choriocarcinoma (HCC) studies of IL-17 .alpha. cells have suggested a potential pro-tumour role for IL-17. Increased IL-17 producing cell density within the tumours of HCC patients correlates with both microvessel density and poor prognosis. Further, in subjects with non-small cell lung and ovarian cancer, higher levels of IL-17 within the tumour correlated with higher blood vessel density and shorter survival. Additionally IL-17 has been suggested to have pro-angiogenic roles and this has not been restricted to particular cell populations. Moreover, it has been shown that IL-17A or IL-17A producing cells are elevated in the environment of breast tumours and correlate with poor prognosis.
[0081] Isolation of tumour infiltrating lymphocytes (TILS) from breast cancer biopsies revealed these cells secreted significant amounts of IL-17A, and that recombinant IL-17A recruits the MAPK pathway by upregulating phosphorylated ERK 1/2 in human breast cancer lines thereby promoting proliferation and resistance to conventional chemotherapeutic agents such as Docetaxel. IL-17A has also been indicated to stimulate migration and invasion of breast cancer cells. Importantly IL-17A-neutralizing antibodies abrogated these effects, demonstrating the pathophysiological role of IL-17A as a potential therapeutic target for breast cancer. The inventor has surprisingly discovered that administration of the novel IFN-.alpha.10-IFN-.alpha.14 hybrid result in a greater reduction of IL-17 compared to previous IFN-.alpha.10-IFN-.alpha.14 hybrid. The inventor has discovered that administration of the novel IFN-.alpha.10-IFN-.alpha.14 hybrid results in a 10%, preferably a 20%, preferably a 30%, preferably a 40% and more preferably a 50% greater reduction of IL-17 compared to previous IFN-.alpha.10-IFN-.alpha.14 hybrid. The determination by the inventor or means thus to activate a Th1 T cell mediated immune response and suppress a Th2/Th17 T cell mediated immune response is therefore significant and of utility in cancer. Thus, the present invention may be used for the treatment and prophylaxis of any known cancerous or malignant condition.
[0082] In addition, the inventor has discovered that the administration or use of at least one interferon alpha subtype comprising or consisting of an IFN-.alpha.10 and IFN-.alpha.14 hybrid in particular wherein the hybrid comprises the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, in particular SEQ ID NO:1 or a variant or fragment thereof results in the full or partial inhibition of IL-17 and/or the full or partial activation of IFN-.gamma..
[0083] Moreover, the inventor has surprisingly discovered that orally administering the antigen and IFN-.alpha. subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 in particular a hybrid comprising the primary interferon binding sites of IFN-.alpha.10 and IFN-.alpha.14, in particular wherein the IFN-.alpha.10-IFN-.alpha.14 hybrid sequence comprises at least one mutation selected from amino acids at positions 94, 101, 102, 109 or 144, preferably at least two mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least three mutations selected from amino acids at positions 94, 101, 102, 109 or 144, more preferably at least four mutations selected from amino acids at positions 94, 101, 102, 109 or 144 or more preferably at least five mutations selected from amino acids at positions 94, 101, 102, 109 or 144, and in particular SEQ ID NO:1 or a fragment or variant thereof in combination as discussed herein can result in the activation of a Th1 T cell mediated immune response and the suppression of a Th2/Th17 T cell mediated immune response. A standard flu vaccine was mixed with a low dose of leukocyte-derived interferon alpha (LDA1) and orally administered to mice. The inventor noted that without the interferon, a small anti-flu antibody response was recorded in mice, which was approximately 50 times less than with an injected vaccine. With interferon-alpha, the response from the orally delivered vaccine was exactly the same as the injected vaccine. A series of buccal immunisations using a standard protein antigen and two interferons, LDA1 and an isolated subtype IFN-.alpha.14, surprisingly resulted in oral immunisation of mice to which the composition was administered. However, the inventor surprisingly noted that while the LDA1 gave a balanced response, IFN-.alpha.14 mediated only a significant humoral response. The production of IgG1 is indicative of a Th2 response (humoral immunity) and the production of IgG2a is indicative of a Th1 response (cell-mediated immunity).
[0084] The inventor, whilst not wishing to be bound by theory, has identified that the oral administration of a food allergen capable of mediating a Th2/Th17 immune response and an interferon alpha subtype which is a hybrid of IFN-.alpha.10 and IFN-.alpha.14 can skew the immune response towards a cell-mediated (Th1) path, whilst simultaneously suppressing the allergic (Th2/Th17) response. Accordingly, the inventor has surprisingly shown that the co-administration of an allergen such as a food derived antigen that is causative of allergy or associated allergic diseases in a subject with certain interferon subtypes modulates the resulting immune response and skews it away from the Th2/Th17 response which would have been expected to develop against the allergen or antigen. This surprising finding provides an unexpected approach to treat or prevent allergic responses or diseases which occur in subjects as a result of allergens such as food-derived allergens or tumour associated antigens.
DEFINITIONS
Subject
[0085] As herein defined, a "subject" includes and encompasses mammals such as humans, primates and livestock animals (e.g. sheep, pigs, cattle, horses, donkeys); laboratory test animals such as mice, rabbits, rats and guinea pigs; and companion animals such as dogs and cats.
Treatment/Therapy
[0086] The term "treatment" is used herein to refer to any regimen that can benefit a human or non-human animal. The treatment may be in respect of any existing inflammatory, autoimmune, allergic or allergy-associated condition and the treatment may be prophylactic (preventative treatment). Treatment may include curative or alleviative effects. Reference herein to "therapeutic" and "prophylactic" treatment is to be considered in its broadest context. The term "therapeutic" does not necessarily imply that a subject is treated until total recovery. Similarly, "prophylactic" does not necessarily mean that the subject will not eventually contract a disease condition. Accordingly, therapeutic and/or prophylactic treatment includes amelioration of the symptoms of a particular allergic condition or preventing or otherwise reducing the risk of developing a particular allergic condition. The term "prophylactic" may be considered as reducing the severity or the onset of a particular condition. "Therapeutic" may also reduce the severity of an existing condition.
Administration
[0087] The active ingredients used in the present invention (e.g. vaccine or allergen and IFN-.alpha.10, IFN-.alpha.14 or a hybrid thereof) in particular a hybrid IFN-.alpha.10 and IFN-.alpha.14 subtype, for example SEQ ID NO: 1, as described herein can be administered separately to the same subject, optionally sequentially, or can be co-administered simultaneously as a pharmaceutical, immunogenic or vaccine composition. In certain embodiments, the vaccine or allergen is co-administered with the interferon alpha subtype. The pharmaceutical composition will generally comprise a suitable pharmaceutical excipient, diluent or carrier selected depending on the intended route of administration.
[0088] The active ingredients can be administered to a patient in need of treatment via any suitable route. The precise dose will depend upon a number of factors, as is discussed below in more detail.
[0089] One suitable route of administration is parenterally (including subcutaneous, intramuscular, intravenous, by means of, for example a drip patch). Other suitable routes of administration include (but, are not limited to) oral, ocular, nasal, topical (including buccal and sublingual), infusion, intradermal or administration via oral or nasal inhalation, by means of, for example, a nebuliser or inhaler, or by an implant. Preferable routes of administration include (but, are not limited to) oral, buccal and sublingual. The compositions of the invention may also be administered in such a manner that they are directed to, or released in, specific areas of the gut intestinal tract (such as the small intestine/duodenum). Typically such release will occur after passage through the stomach, this targeted release being achievable through the use of coatings and the like.
[0090] For intravenous injection, the active ingredient will be in the form of a parenterally acceptable aqueous solution which is pyrogen-free and has suitable pH, isotonicity and stability. Those of relevant skill in the art are well able to prepare suitable solutions using, for example, isotonic vehicles such as sodium chloride injection, Ringer's injection, Lactated Ringer's injection. Preservatives, stabilisers, buffers, antioxidants and/or other additives may be included, as required.
[0091] The compositions of the present invention for oral administration may be in tablet, capsule, lozenge, powder or liquid form. Oral administration may involve placing a lozenge under the tongue of the patient A tablet may comprise a solid carrier such as gelatin or an adjuvant. Liquid pharmaceutical compositions generally comprise a liquid carrier such as water, petroleum, animal or vegetable oils, mineral oil or synthetic oil. Physiological saline solution, dextrose or other saccharide solution or glycols such as ethylene glycol, propylene glycol or polyethylene glycol may be included.
[0092] The compositions of the present invention may also be administered via microspheres, liposomes, other microparticulate delivery systems or sustained release formulations placed in certain tissues including blood. Suitable examples of sustained release carriers include semipermeable polymer matrices in the form of shared articles, e.g. suppositories or microcapsules. Examples of the techniques and protocols mentioned above and other techniques and protocols which may be used in accordance with the invention can be found in Remington's Pharmaceutical Sciences, 18th edition, Gennaro, A.R., Lippincott Williams & Wilkins; 20th edition (Dec. 15, 2000) ISBN 0-912734-04-3 and Pharmaceutical Dosage Forms and Drug Delivery Systems; Ansel, H.C. et al. 7.sup.th Edition ISBN 0-683305-72-7, the entire disclosures of which are herein incorporated by reference.
Pharmaceutical Compositions
[0093] As described above, the present invention extends to a pharmaceutical composition for the treatment of inflammatory diseases, autoimmune diseases and allergy such as food allergy and associated allergic diseases and, in particular, for the induction of a Th1 immune response and the suppression or inhibition of a Th2/Th17 immune response.
[0094] Pharmaceutical compositions according to the present invention, and for use in accordance with the present invention, may comprise, in addition to an active ingredient, a pharmaceutically acceptable excipient, carrier, buffer stabiliser or other materials well known to those skilled in the art. Such materials should be non-toxic and should not interfere with the efficacy of the active ingredient. The precise nature of the carrier or other material will depend on the route of administration, which may be, for example, oral, intravenous, intranasal or via oral or nasal inhalation. The formulation may be a liquid, for example, a physiologic salt solution containing non-phosphate buffer at pH 6.8-7.6, or a lyophilised or freeze-dried powder.
Dose
[0095] The composition is preferably administered to an individual in a "therapeutically effective amount" or a "desired amount", this being sufficient to show benefit to the individual. As defined herein, the term an "effective amount" means an amount necessary to at least partly obtain the desired response, or to delay the onset or inhibit progression or halt altogether the onset or progression of a particular condition being treated. The amount varies depending upon the health and physical condition of the subject being treated, the taxonomic group of the subject being treated, the degree of protection desired, the formulation of the composition, the assessment of the medical situation and other relevant factors. It is expected that the amount will fall in a relatively broad range, which may be determined through routine trials. Prescription of treatment, e.g. decisions on dosage etc., is ultimately within the responsibility and at the discretion of general practitioners, physicians or other medical doctors, and typically takes account of the disorder to be treated, the condition of the individual patient, the site of delivery, the method of administration and other factors known to practitioners. The optimal dose can be determined by physicians based on a number of parameters including, for example, age, sex, weight, severity of the condition being treated, the active ingredient being administered and the route of administration. A broad range of doses may be applicable. Considering oral administration to a human patient, for example, from about 10 .mu.g to about 1000 .mu.g of agent may be administered per human dose, optionally for 3 to 4 doses. Dosage regimes may be adjusted to provide the optimum therapeutic response and reduce side effects. For example, several divided doses may be administered daily, weekly, monthly or other suitable time intervals or the dose may be proportionally reduced as indicated by the exigencies of the situation.
[0096] Unless otherwise defined, all technical and scientific terms used herein have the meaning commonly understood by a person who is skilled in the art in the field of the present invention.
Autoimmune Disease
[0097] The term "autoimmune disease" as used herein is understood to mean any disease or condition which is caused by a body's tissues being attacked by its own immune system.
[0098] Throughout the specification, unless the context demands otherwise, the terms "comprise" or "include", or variations such as "comprises" or "comprising", "includes" or "including" will be understood to imply the inclusion of a stated integer or group of integers, but not the exclusion of any other integer or group of integers.
[0099] The present invention will now be exemplified with reference to the following non-limiting Figures and examples which are provided for the purpose of illustration and are not intended to be construed as being limiting on the present invention. Other embodiments of this invention will be apparent to those of ordinary skill in the art in view of this description.
BRIEF DESCRIPTION OF THE FIGURES
[0100] FIG. 1 shows a graph of IgG subtype (IgG1 and IgG2a) production in BALB-c mice immunised with ovalbumin and different subtypes of IFN-.alpha..
[0101] FIG. 2 shows a graph of the percentage of IgG subtype (IgG1 and IgG2a) produced in BALB-c mice immunised with ovalbumin and different subtypes of IFN-.alpha..
[0102] FIG. 3 shows a graph of IgG2a production in BALB-c mice immunised with ovalbumin and MULTIFERON.TM., glycosylated IFN-.alpha.14 and non-glycosylated IFN-.alpha.14 administered via intraperitoneal injection.
[0103] FIG. 4 shows a graph of IgG1 production in BALB-c mice immunised with ovalbumin and MULTIFERON.TM., glycosylated IFN-.alpha.14 and non-glycosylated IFN-.alpha.14 administered via intraperitoneal injection.
[0104] FIG. 5 shows a graph of IgG2a production in BALB-c mice immunised with ovalbumin and MULTIFERON.TM., glycosylated IFN-.alpha.14 and non-glycosylated IFN-.alpha.14 administered orally.
[0105] FIG. 6 shows a graph of IgG1 production in BALB-c mice immunised with ovalbumin and MULTIFERON.TM., glycosylated IFN-.alpha.14 and non-glycosylated IFN-.alpha.14 administered orally.
[0106] FIG. 7 shows inhibition of human PBMC interleukin-17 (IL-17) secretion with lipopolysaccharide (LPS) alone and with LPS and increasing concentrations of IFN-.alpha.2a (black), IFN-.alpha.10 (white) or IFN-14 (grey).
[0107] FIG. 8 shows the inhibition of Interleukin-4 (IL4)-induced CD4+ Th2 cell development using increasing concentrations of IFN-.alpha.2a (black), IFN-.alpha.10 (white) or IFN-14 (grey).
[0108] FIG. 9 shows the IFN-.alpha.10 and IFN-.alpha.14 hybrid amino acid sequence which contains the 2 interferon receptor (IFNaR1 and IFNaR2) binding sites.
[0109] Based on the protein sequence SEQ ID NO:1 using online webservices (for example http://www.ebi.ac.uk/Tools/st/emboss_backtranseq/), a nucleic acid sequence can be obtained. FIG. 10 provides the reverse translation of the protein sequence SEQ ID NO:1 when an E. coli codon usage table is used.
[0110] FIG. 11 shows the alignment of a previously described IFNalpha10 and IFNalpha14 hybrid amino acid sequence with the IFNalpha10 and IFNalpha14 hybrid amino acid sequence as presently claimed.
[0111] FIG. 12 indicates the effect caused by rIFN-.alpha.14 on the production of IL-17 in whole human blood incubated with one microgram E.coli lipopopolysaccharide (LPS) for 48 hours. The .alpha.-14 gave a significant suppression of IL-17 secretion. IL-.alpha.-2 and .alpha.-10 showed no significant suppression.
[0112] FIG. 13 shows the effect caused by rIFN-.alpha.14 on the production of IL-17 from human peripheral blood mononuclear cells incubated with 10 micrograms E. coli lipopolysaccharide (LPS) for 48 hours. The .alpha.-14 caused a significant suppression of IL-17 secretion with and without LPS activation. IL- .alpha.-2 and .alpha.-10 showed no significant changes in the IL-17 concentrations (results not shown).
[0113] FIG. 14 shows the effect caused by rIFN-.alpha.10, rIFN-.alpha.14 and rIFN-.alpha.2 on IL-17 production by whole human blood incubated with PHA for 5 days. The .alpha.-14 is an extremely potent inhibitor (P<0.001 at 1,000 IU/ml) of IL-17 compared with the commonly available .alpha.-2; the .alpha.-10 is more than 20.times. less active in this context
[0114] FIG. 15 shows the effect caused by rIFN-.alpha.10, rIFN-.alpha.14 and rIFN-.alpha.2 on IFN-gamma production by whole human blood incubated with PHA for 5 days. The .alpha.-10 is the most potent interferon--alpha in this context causing enhanced secretion of IFN-gamma--critical for both and innate and adaptive immunity against viruses, intracellular bacterial infections and in the control/elimination of tumours.
[0115] FIG. 16 illustrate a sequence alignment of IFN-alpha10 (SEQ ID NO:4) and IFN-alpha14 (SEQ ID NO:5) amino acid sequences and the hybrid sequence SEQ ID NO: 1 discussed herein.
[0116] FIG. 17 shows the effect caused by rIFN-.alpha.14 and rIFN-.alpha.2 on IL-17 production by whole human blood incubated with PHA for 5 days. The .alpha.-14 is an extremely potent inhibitor of IL-17 compared with the commonly available IFN-.alpha.2 (EC50s for the two subtypes are 100 and 10,000 IU/ml).
[0117] FIG. 18 shows the induction of Interferon-gamma by Interferon-alpha subtypes and in particular demonstrates the effect caused by IFN-.alpha.10, IFN-.alpha.14 and IFN-.alpha.2 on IFN-gamma production by whole human blood incubated with PHA for 5 days. The .alpha.-10 is the most potent interferon--alpha in this context causing enhanced secretion of IFN-gamma.
[0118] FIG. 19 shows the effect of the IFN-.alpha.10-IFN-.alpha.14 hybrid of the present invention (SEQ ID NO:1) on IL-17 production from whole human blood compared to the effect on IL-17 of a previously disclosed IFN-.alpha.10-IFN-.alpha.14 hybrid. The hybrid of the present invention demonstrates an greater reduction in IL-17.
EXAMPLE 1
Identification of Interferon-Alpha Subtypes that are Immunological Adjuvants
[0119] 50 .mu.g ovalbumin and 10.sup.5 IU of interferon subtypes IFN-.alpha.14, IFN-.alpha.2, IFN-.alpha.21, IFN-.alpha.10, an IFN "mix" (including IFN-.alpha.1, IFN-.alpha.8, IFN-.alpha.21 and possibly IFN-.alpha.l7), IFN-.alpha.8, Intron A, MULTIFERON.TM. and IFN-.alpha.1 in 50 .mu.l were administered via intraperitoneal injection three times per week to BALB-c female mice, in groups of 10.
[0120] The serum concentrations of IgG1 mg/ml (Th2 response--humoral immunity to the ovalbumin antigen) and IgG2a mg/ml (Th1 response--cell-mediated immunity to the ovalbumin antigen) were measured by ELISA.
[0121] FIGS. 1 and 2 show the anti-ovalbumin IgG subtype production in BALB-c mice treated with IFN-.alpha.14, IFN-.alpha.2, INF-.alpha.21, IFN-.alpha.10, a "mix" of IFN-.alpha.1, IFN-.alpha.8, IFN-.alpha.21 and possibly IFN-.alpha.17), IFN-.alpha.8, Intron A, MULTIFERON.TM., ovalbumin only, ovalbumin plus human serum albumin (used as a carrier in interferon preparations) and IFN-.alpha.1.
[0122] The inventor demonstrated that IFN-.alpha.10 and IFN-.alpha.14 enhanced the production of IgG2a antibodies significantly which is indicative of an enhanced Th1 immune response. The inventor also demonstrated that IFN-.alpha.10 in particular showed low production of IgG1 antibody which is indicative of suppressing a Th2/Th17 immune response.
EXAMPLE 2
Identification of Antibody Response in BALB-c Mice After Administration of a Composition Comprising a Flu Vaccine and a Low Dose of Leukocyte Derived Interferon-Alpha (LDA1)
[0123] The standard flu vaccine was mixed with a low dose (10.sup.5 IU) of leukocyte derived interferon alpha (LDA1). Without the interferon, a small anti-flu antibody response was recorded in mice, approximately 50 times less than with an injection. With interferon-alpha, the response from the orally delivered vaccine was exactly the same as the injected vaccine. A series of buccal immunisations were carried out using a standard protein antigen (ovalbumin). Two interferons were compared, namely, the LDA1 and an isolated subtype, IFN-.alpha.14. Both produced a remarkable oral immunisation of the mice, but whereas the LDA1 gave a balanced response, the IFN-.alpha.14 gave only a significant humoral response. The production of IgG1 is indicative of a Th2/Th17 response (humoral immunity) and the production of IgG2a is indicative of a Th1 response (cell-mediated immunity).
EXAMPLE 3
The Identification of IFN-Alpha as an Oral Immunological Adjuvant
[0124] 50 .mu.g ovalbumin and 10.sup.5 IU of interferon subtypes, namely MULTIFERON.TM., glycosylated IFN-.alpha.14 and non-glycosylated IFN-.alpha.14, in 50 .mu.l doses were administered three times a week to BALB-c female mice via oral (buccal) and intraperitoneal injection administration.
[0125] The controls used were antigen alone and Titermax--Titermax is a mixture of compounds used in antibody generation and vaccination to stimulate the immune system to recognise an antigen given together with the mixture. Titermax is a recently developed immune adjuvant deemed to be safe in animals.
[0126] Serum concentrations (mg/ml) of IgG1 (indicative of a Th2/Th17 response) and IgG2a (indicative of a Th1 response) anti-ovalbumin antibodies were quantitated by ELISA.
[0127] The production of IgG2a and IgG1 antibodies when MULTIFERON.TM., glycosylated IFN-.alpha.14 and aglycosyl IFN-.alpha.14 (CHO cell-derived) were administered both orally and by injection were compared (see FIGS. 3, 4, 5 and 6).
[0128] The inventor demonstrated that IFN-.alpha.14 showed pronounced immunological adjuvant activity both orally and by injection. No significant difference was seen between the glycosylated and non-glycosylated preparations.
[0129] The inventor also demonstrated that IFN-.alpha.14 only enhanced IgG2a production associated with Th1 responses by the oral route of administration. Hence IFN-.alpha.14 is an activator of cell-mediated immunity when administered orally.
[0130] MULTIFERON.TM. enhanced both IgG1 and IgG2a responses when administered both orally and by injection i.e. it induced both Th1 and Th2 responses significantly.
EXAMPLE 4
In Vitro Determination of the Inhibition of Humoral Immunity (Th2/Th17) by Interferon-Alpha Subtypes--Analysis of Th17 Lymphocytes and Interleukin 17
[0131] A total of 2.times.10.sup.6 human PBMCs were stimulated with lipopolysaccharide (LPS) in the absence or presence of increasing concentrations of recombinant human alpha-IFN. Supernatants were collected after 24 hours and IL-17 concentrations measured by ELISA.
Human Cell Culture
[0132] Human peripheral blood was collected from healthy volunteers and peripheral blood mononuclear cells (PBMCs) were obtained by Lymphoprep gradient centrifugation (Pierce). For PBMC experiments, 2.times.10.sup.6 PBMCs per ml were seeded in 24-well plates and stimulated with lipopolysaccharide (LPS) from Escherichia coli 055:B5 (Sigma) or 2.times.10.sup.6 PBMCs per mL were seeded into 24-well plates and stimulated with 5 mg/mL plate-bound anti-CD3 (clone: UCHT1) and 2.5 mg/ml. anti-CD28 (clone: CD28.2). Naive T cells (CD4+CD45RA) were obtained by magnetically labeling and depletion of non-helper T-cell and memory T-cells performed according to manufacturer's instructions (Miltenyi Biotec). A total of 1.times.10.sup.5 naive T-cells were primed in 96-well flat bottom plates coated with anti-CD3 (clone UCHT1, 2.5 mg/mL) and with anti-CD28 (clone CD28.2, 2.5 mg/mL) antibodies. After 48 h of culture, 20 IU/mL recombinant human IL-2 (Peprotech) was added to the culture.
[0133] For human Th17 differentiation, cells were supplemented with neutralising anti-IL-4 and anti-IFN.gamma. antibodies (both from Peprotech) and with 10 ng/mL recombinant IL-1.beta. and 50 ng/mL recombinant IL-6 (both from Peprotech). Where required, recombinant human IFN.alpha.10/14 was added to the culture. After 5 days of culture, cells were washed, transferred into new plates and expanded until day 12 in the presence of 20 IU/mL recombinant IL-2.
ELISA and Intracellular Cytokine Staining
[0134] The IL-17 producing capacity of primed Th17 cells was assessed by stimulation with 0.1 ng/ml LPS or alternatively can be assessed by the stimulation of human cells with soluble 1 mg/mL anti-CD3 (clone: OKT3) and phorbol-12-13-dibutyrate (PdBu). Concentrations of human IL-17 in cell culture supernatants were determined using commercially available antibody pairs and protein standards (R&D Systems). Absorption was determined using an ELISA reader at 450 nm. For intracellular staining of mouse IFN.gamma. and IL-17, T-cells are stimulated with PMA and ionomycin for 5 hours. Brefeldin A is added for the final 3 h of culture. Intracellular staining can be performed with a BD Cytofix/Cytoperm kit according to the manufacturer's instructions. Cells are incubated with fluorescein isothiocyanate-labeled anti-IFN.gamma. (clone: XMG1.2, BD Pharmingen) and Alexa Fluor 647-labeled anti-mouse mouse IL-17A (clone: eBio17B7, eBioscience). After washing, cells are immediately analysed using Fluorescence-activated cell sorting (FACS).
Results
[0135] As shown by FIG. 7, inhibition of IL-17 was found to occur in the order IFN.alpha.10>IFN.alpha.14>IFN.alpha.2a. P<0.05 (FIG. 7).
EXAMPLE 5
In Vitro Determination of the Inhibition of Humoral Immunity Th2/Th17) by Interferon-Alpha Subtypes--Analysis of Th2 Cells and Associated Cytokines
CRTH2 Background
[0136] CRTH2 (Chemoattractant Receptor-homologous molecule expressed on Th2 cells) is a G-protein coupled receptor expressed by Th2 lymphocytes, eosinophils, and basophils. The receptor mediates the activation and chemotaxis of these cell types in response to prostaglandin D2 (PGD2), the major prostanoid produced by mast cells. PGD2 is released through mast cell degranulation in the initial phase of IgE-mediated reactions. This process is also thought to occur at the site of inflammation, such as the nasal and bronchial mucosa. Through interaction with CRTH2, PGD2 is thought to mediate recruitment and activation of CRTH2-bearing cell types to the site of the allergic reaction, in consequence amplifying and maintaining the allergic inflammation. In the nasal and bronchial mucosa, this pro-inflammatory cascade is thought to start during the so-called late allergic response occurring 3 to 9 hours after allergen challenge. The interaction between PGD2 and CRTH2 would, therefore, contribute to the so-called "Th2 polarisation", with consequent Th2 cytokine production and the typical eosinophilic and basophilic characteristics of the inflammation.
IFN.alpha. Inhibits Human CD4+ Th2 Development
[0137] Purified human CD4+/CD45RA+ cells were activated with plate-bound anti-CD3/anti-CD28 under defined cytokine conditions. Induction of CRTH2 expression was assessed by flow cytometry. All P<0.05, above 100 IU IFN compared with IL-4alone.
Human Subjects
[0138] Peripheral blood was collected from healthy adult donors and cells purified as below.
T Cell Cultures and Analysis
[0139] Peripheral blood was obtained from healthy male adult donors and naive CD4+/CD45RA+ T cells were purified (>92%) from buffy coats by magnetic bead separation (BD Biosciences, USA). CD4+ cells were activated with plate-bound anti-CD3/anti-CD28 and IL-2 (50 U/ml) in complete Iscove's Modified Dulbecco's Medium containing 10% FCS, in the presence of recombinant human recombinant IL-4 (R&D Systems, USA), at a concentration of 20 ng/ml for 7 days. Flow cytometric analysis was performed with hCD294 (chemo-attractant receptor homologous molecule expressed on Th2 cells [CRTH2])-Alexa 647 (BD Biosciences).
Results
[0140] In humans, the PGD2 receptor, CRTH2, is selectively expressed on Th2 cells and is induced by IL-4 during Th2 development. IL-4 promoted the development of cells expressing CRTH2. However, as shown in FIG. 8 all the IFN-alphas markedly blocked IL-4 driven CRTH2 expression, in a dose-dependent manner in the order IFN.alpha.10>IFN.alpha.14>IFN.alpha.2a, thus supporting the concept that these cytokines suppress Th2 (humoral) immunity, but are recognised as potent activators of Th1-associated immunity.
[0141] As shown in FIG. 12, the effect of rIFN-.alpha.14 on the production of IL-17 in human blood incubated with LPS for 48 h was tested.
[0142] Whole human blood was incubated without (open columns) or with 1 .mu.g/ml LPS (cross hatched columns) in the absence and presence of a range of concentrations of rIFN-.alpha.14 (0-1,000 IU/ml) for 48 h at 37.degree. C., in an atmosphere of 5% CO2 in air, in a humidified incubator. Plasma was collected by centrifugation and levels of IL-17 determined by ELISA.
[0143] FIG. 12 indicated a dose response to IFN-.alpha.14 wherein 1 mg=10.sup.-8 IU.
[0144] As shown in FIG. 13 the effect of rIFN-.alpha.14 on the production of IL-17 in human PBMCs incubated with LPS for 48 h was tested.
[0145] Human Peripheral Blood Mononuclear cells (PBMCs), a critical component in the immune system, were isolated from whole human blood by density gradient centrifugation. 2.times.10.sup.6 PBMCs were incubated without (open columns) or with 10 .mu.g/ml LPS (cross hatched columns) in the absence and presence of a range of concentrations of rIFN-.alpha.14 (0-1,000 IU/ml) for 48 h at 37.degree. C., in an atmosphere of 5% CO2 in air, in a humidified incubator. Levels of IL-17 in the supernatant were determined by ELISA.
[0146] As indicated by FIG. 13, increasing concentrations of rIFN-.alpha.14 was found to reduce the IL-17 both in untreated and treated LPS cells.
[0147] As shown in FIG. 14, the effect of rIFN.alpha.10, rIFN.alpha.14 and rIFN2 on IL-17production by whole blood incubated with phytohaemagglutinin (PHA) for 5 days was tested.
[0148] Whole human blood was diluted 1/10 with RPMI 1640 culture medium and incubated without or with 100 .mu.g/ml PHA in the absence and presence of a range of concentrations of rIFN-.alpha.14, rIFN-.alpha.10 and rIFN-.alpha.2 for 5 days at 37.degree. C., in an atmosphere of 5% CO2 in air, in a humidified incubator. At the end of this period, supernatants were aspirated and levels of IL-17 in supernatants measured by ELISA. Values represent the mean .+-. sem, for n=3 incubations. Statistical analysis and IC.sub.50 values were determined using GraphPad Prism 5 (GraphPad Software Inc., California, USA).
[0149] As indicated in FIG. 14 the provision of rIFN-.alpha.14 at higher concentrations (100-1000 IU/ml) caused a greater decrease in IL-17 than the provision of IFN-.alpha.2 or IFN-.alpha.10. rIFN-.alpha.14 is considered to be the most potent interferon tested at reducing IL-17 levels.
[0150] Similar results were achieved as shown in FIG. 17. Here the effect of rIFN.alpha.14 and rIFN2 on IL-17 production by whole blood incubated with phytohaemagglutinin (PHA) for 5 days was tested using the same methodology. As indicated in FIG. 17 the provision of rIFN-.alpha.14 at higher concentrations (100-1000 IU/ml) caused a greater decrease in IL-17 than the provision of IFN-.alpha.2. rIFN-.alpha.14 is considered to be the most potent interferon tested at reducing IL-17 levels.
[0151] As shown in FIG. 15, the effect of rIFN.alpha.10, rIFN.alpha.14 and rIFN.alpha.2 on IFN-gamma production by whole blood incubated with PHA for 5 days.
[0152] Whole human blood was diluted 1/10 with RPMI 1640 culture medium and incubated without or with 100 .mu.g/ml PHA in the absence and presence of a range of concentrations of rIFN-.alpha.10, rIFN-.alpha.14 and rIFN-.alpha.2 for 5 days at 37.degree. C., in an atmosphere of 5% CO.sub.2 in air, in a humidified incubator. At the end of this period supernatants were aspirated and levels of IFN-gamma in supernatants measured by ELISA. Values represent the mean .+-. sem, for n=3 incubations, plasma was collected by centrifugation and levels of IFN-gamma determined by ELISA. Values represent the mean .+-. sem, for n=3 incubations.
[0153] It was determined that rIFN-.alpha.10 was the most effective of the interferons tested at promoting levels of IFN-gamma. IFN-gamma has previously been suggested to be important in providing an anti-cancer effect.
[0154] Similar results were achieved as shown in FIG. 18. Here the effect of rIFN.alpha.14 and rIFN2 on IFN-gamma production by whole blood incubated with phytohaemagglutinin (PHA) for 5 days was tested using the same methodology. As indicated in FIG. 18 the provision of rIFN-.alpha.10 caused a greater increase in IFN-gamma than the provision of IFN-.alpha.2. rIFN-.alpha.10 is considered to be the most potent interferon tested at increasing IFN-gamma levels.
[0155] As shown in FIG. 19, the effect of the IFN-.alpha.10-IFN-.alpha.14 hybrid of the present invention (SEQ ID NO:1) on IL-17 production from whole human blood was compared to the effect on IL-17 of the IFN-.alpha.10-IFN-.alpha.14 hybrid disclosed in PCT/GB2015/050717.
[0156] Whole human blood was diluted 1/10 with RPMI 1640 culture medium and was incubated with PHA (100 .mu.g/ml) in the presence of a range of concentrations of either the IFN-.alpha.10-IFN-.alpha.14 hybrid of the present invention (IFN alpha-hybrid 1) or the IFN-.alpha.10-IFN-.alpha.14 hybrid disclosed in PCT/GB2015/050717 (IFN alpha-hybrid 2) for 5 days at 37.degree. C. in an atmosphere of 5% C02 in air in a humidified incubator. At the end of this period supernatants were collected and levels of IL-17 measured by ELISA. Values represent mean .+-. sem, for n=3 incubations. P<0.05 for all points of the data between the two hybrids except 100 IU/ml, which was non significant The Red diamonds indicate IFN-.alpha.10-IFN-.alpha.14 hybrid 1 and the blue circles indicate IFN-.alpha.10-IFN-.alpha.14 hybrid 2.
[0157] It was determined that the IFN-.alpha.10-IFN-.alpha.14 hybrid of the present invention demonstrates a greater reduction in IL-17.
EXAMPLE 6
Effects of Human Interferon Alpha-14 and Alpha-10 on Unstimulated and Activated Human Mononuclear Leukocytes from Normal Subjects
TABLE-US-00002 [0158] TABLE 1 Synopsis of 400 interleukins, chemokines, and protein markers estimations* following IFN-.alpha.10/14 treatment of human mononuclear cells FOLD NUMBER OF PHA- STIMULATED/PHA- FOLD NUMBER OF UNSTIMULATED/ STIMULATED ALPHA-IFN ANALYTE ALPHA-IFN TREATED CELLS TREATED CELLS CYTOKINES Alpha-14 Alpha-10 Alpha 14 Alpha 10 IL-1a 0 +23 1 +2 IL-1b 0 +70 -2 1 IL-1(F5 to F10) 0 0 0 0 IL-2 0 0 +7 +4 IL-3 0 0 -11 x IL-4 0 0 1 -3 IL-5 0 0 -420 1 IL-6 -19 +1000 1 1 IL-7 0 0 0 0 IL-8 1 +100 1 1 IL-9 0 0 0 0 IL-10 0 +5 +2 +2 IL-11 0 0 0 0 IL-12 p40 0 +350 0 +1 IL-12 p70 0 0 +11 0 IL-13 0 0 -5 1 IL-15 0 0 0 0 IL-16 1 1 1 1 IL-17 0 0 -43 -5 IL-18 0 0 0 0 IL-20 0 0 0 0 IL-21 0 x 0 x IL-23 +4 +3 +6 1 IL-24 0 0 0 0 IL-27 0 1 0 1 IL-28 0 0 0 0 IL-29 0 0 0 0 IL-31 0 0 0 0 IL-33 0 0 0 0 IL-34 0 0 0 0 IFN-gamma 0 0 +600 +3000 G-CSF -1500 +20 1 1 GM-CSF 0 0 0 1 CD MARKERS CD14 +2 +2 +2 +2 CD23 -22 1 -850 -3 CD30 0 0 0 0 CD40 +2 +2 1 1 CD97 -2 1 -5 -5 CD152 (CTLA-4) 1 0 -2 0 CD154 1 x +2 x CD163 -2 1 -2 1 CD200 1 1 -1 1 CD223 (LAG3) 0 0 -3 +3 SELECTED CHEMOKINES AND PROTEINS CXCL1 (GROa) -7600 -12 -3400 1 CXCL5 (ENA-78) -6 +16 -32 -3 CXCL10 (IP10) +460 +10 +1 1 CCL1 (1-309) 0 1 -24 1 CCL7 (MCP-3) -2 -200 -149 1 CCL16 (HCC-4) 0 1 -100 1 CCL20 (MIP-3a) -69 +40 -2 1 MMP-2 +600 +200 +450 +500 (collagenase) MMP-10 -121 1 -2 -2 (proteoglycanase) ACE-2 +12 0 +6 0 PDGF Ralpha -4 -7 -1 -3 Tie-1 -170 -200 -8 -280 ICAM-1 -1 +2 -3 1 TREM-1 +5 -2 +2 -5 E-SELECTIN 1 -7 1 -2 0 = no analyte detected 1 = analyte present but no effect of alpha-10/14 x = not determined The positive effect of alpha-10/14 is denoted by a + The negative effect of alpha-10/14 interferon is denoted by a - *The assay system used was the RayBio Quantibody Human Cytokine Array 9000 (QAH-CAA-9000 provided by Insight Bio Ltd.). This a multiplex ELISA, measuring the concentrations of 400 proteins in a single assay process, including pro- and anti-inflammatory markers, interleukins, cancer markers, chemokines, growth factors and related molecules. Human peripheral blood mononuclear leukocytes (normal blood donors) were treated with 10 ng/ml IFN-.alpha.14/10 for 4 hours prior to assay. Tests were performed on 2 groups of cells - a) unactivated and b) activated with PHA (phytohaemagglutinin) to induce a high level of stimulation.
Effects of Alpha-14 on Activated Immune Cells
[0159] More than 30 interleukins were quantitated but only 6 showed significant changes in the activated cells, indicating the targeted and very specific nature of the interaction of the alpha-14 with the human immune response.
[0160] Interleukin 2 increased by 7-fold, IL-12p70 +11 fold and interferon-.gamma.+600 fold, indicating a strong proliferation of the Th1 (cell-mediated) response while a 6-fold increase in IL-23 is in keeping with its role in cell-mediated immunity and its association with IL-12.
[0161] Very large decreases were observed with IL-3 and IL-5 of 11 and 420-fold respectively. These molecules are associated with the production of myeloid cells and immunoglobulin production (humoral immunity). IL13 also decreased by 5-fold, which is important as this interleukin is implicated in the secretion of IgE, the allergy antibody. Also crucial was the 43-fold decrease in IL-17. This regulatory cytokine is increased in autoimmune diseases, humoral (antibody-mediated) immunity and stimulation of inflammation through attraction of neutrophils.
[0162] CD23 or Fc.epsilon.RII is a receptor for the allergy antibody, IgE, and is displayed widely on different types of leukocytes. CD23 activation controls IgE production and significant increases are seen in patients with allergic disorders. This important marker was decreased by 850-fold, in the activated cells, by alpha-14.
Effects of IFN Alpha-14 on Non-Activated Immune Cells
[0163] IL-6 decreased 19-fold. This cytokine stimulates liver protein synthesis in responses to traumas, causes increases in body temperature and is involved in muscle contraction. However, it is its essential role in antibody-mediated immunity that is important in allergy.
[0164] G-CSF was also decreased by more than 1000-fold. This molecule can stimulate the bone marrow to make Increased numbers of neutrophils that could be involved in inflammation. At the same time the secretion of the chemokine CXCL1 was suppressed by 7,500-fold--this prevents it attracting neutrophils to the site of a response and causing inflammation. Also the concentration of the chemokine, CXCL10 was enhanced by 460-fold--its role is to attract T-lymphocytes to an ongoing immune response.
Effects of IFN Alpha-10 on Activated Immune Cells
[0165] As with alpha-14, alpha-10 only regulated a small number of cytokines out of the numbers assessed. Of particular note were the increases in IL-2 and interferon-.gamma. of 4 and 3000 fold respectively indicating a switch to cell-mediated immunity. IL-17 levels fell by 5 fold, confirming this change in balance.
[0166] The large reduction in CD23 was not evident with alpha-10 and its major effects on chemokines were on Tie-1 (tyrosine kinase crucial in the process of lymphatic remodelling) and TREM-1 (neutrophil activation) where it caused reductions of 280 and 5 fold respectively.
Effects of Alpha-10 on Non-Activated Immune Cells
[0167] Alpha-10 showed significant activity in this context enhancing IL-1.alpha./.beta. by up to 70fold and IL-6,8,10,12 (p40) by 1000,100, 5 and 350 fold in keeping with a strong support for cell-mediated over humoral immunity. G-CSF was also enhanced by 20 fold in total contrast to alpha-14.
[0168] Few changes were recorded with the CD markers but CXCL1 was reduced by 12 fold while CXCL5 and 10 increased by 16 and 10 fold and CCL20 rose by 40 fold. However, CCL7 and Tie-1 fell by 200 fold each. These results are in keeping with a significant movement towards cell-mediated immunity.
RESULT
[0169] The low doses of interferon-alpha 14 and 10 have modified cytokine synthesis in order to enhance cell-mediated immunity at the expense of antibody-mediated immunity. This would be invaluable in enhancing the activities of certain vaccines where a humoral immune response can be detrimental e.g. viral and cancer vaccines.
[0170] In addition the results are totally in keeping with the general understanding that allergy can be alleviated by changing the immune response to an allergen by shifting an antibody response to a cellular response. Such a change would be part of acquired immunity and hence, potentially, a long-term solution by developing tolerance.
[0171] In addition, the alpha-14 significantly suppressed the capacity of leukocytes to make/utilise IgE and hence it inhibited the immediate effects of an allergic reaction, together with reducing inflammatory elements of immunity while enhancing the involvement of more control elements.
[0172] All documents referred to in this specification are herein incorporated by reference.
[0173] Various modifications and variations to the described embodiments of the inventions will be apparent to those skilled in the art without departing from the scope of the invention. Although the invention has been described in connection with specific preferred embodiments, it should be understood that the invention as claimed should not be unduly limited to such specific embodiments. Indeed, various modifications of the described modes of carrying out the invention which are obvious to those skilled in the art are intended to be covered by the present invention.
Sequence CWU 1
1
51166PRTHomo sapiens 1Cys Asp Leu Pro Gln Thr His Ser Leu Gly Asn
Arg Arg Ala Leu Ile 1 5 10 15 Leu Leu Gly Gln Met Gly Arg Ile Ser
Pro Phe Ser Cys Leu Lys Asp 20 25 30 Arg His Asp Phe Arg Ile Pro
Gln Glu Glu Phe Asp Gly Asn Gln Phe 35 40 45 Gln Lys Ala Gln Ala
Ile Ser Val Leu His Glu Met Met Gln Gln Thr 50 55 60 Phe Asn Leu
Phe Ser Thr Lys Asn Ser Ser Ala Ala Trp Asp Glu Thr 65 70 75 80 Leu
Leu Glu Lys Phe Tyr Ile Glu Leu Phe Gln Gln Met Asn Asp Leu 85 90
95 Glu Ala Cys Val Ile Gln Glu Val Gly Val Glu Glu Thr Pro Leu Met
100 105 110 Asn Glu Asp Ser Ile Leu Ala Val Lys Lys Tyr Phe Gln Arg
Ile Thr 115 120 125 Leu Tyr Leu Ile Glu Arg Lys Tyr Ser Pro Cys Ala
Trp Glu Val Val 130 135 140 Arg Ala Glu Ile Met Arg Ser Leu Ser Phe
Ser Thr Asn Leu Gln Lys 145 150 155 160 Arg Leu Arg Arg Lys Asp 165
2507PRTHomo sapiens 2Ala Thr Gly Thr Gly Thr Gly Ala Thr Cys Thr
Gly Cys Cys Gly Cys 1 5 10 15 Ala Gly Ala Cys Cys Cys Ala Thr Ala
Gly Cys Cys Thr Gly Gly Gly 20 25 30 Thr Ala Ala Thr Cys Gly Thr
Cys Gly Thr Gly Cys Ala Cys Thr Gly 35 40 45 Ala Thr Thr Cys Thr
Gly Cys Thr Gly Gly Gly Thr Cys Ala Gly Ala 50 55 60 Thr Gly Gly
Gly Thr Cys Gly Thr Ala Thr Thr Ala Gly Cys Cys Cys 65 70 75 80 Gly
Thr Thr Thr Ala Gly Cys Thr Gly Thr Cys Thr Gly Ala Ala Ala 85 90
95 Gly Ala Thr Cys Gly Thr Cys Ala Thr Gly Ala Thr Thr Thr Thr Cys
100 105 110 Gly Thr Ala Thr Thr Cys Cys Gly Cys Ala Ala Gly Ala Gly
Gly Ala 115 120 125 Ala Thr Thr Thr Gly Ala Thr Gly Gly Cys Ala Ala
Cys Cys Ala Gly 130 135 140 Thr Thr Thr Cys Ala Gly Ala Ala Ala Gly
Cys Ala Cys Ala Gly Gly 145 150 155 160 Cys Ala Ala Thr Thr Ala Gly
Cys Gly Thr Thr Cys Thr Gly Cys Ala 165 170 175 Thr Gly Ala Ala Ala
Thr Gly Ala Thr Gly Cys Ala Gly Cys Ala Gly 180 185 190 Ala Cys Cys
Thr Thr Thr Ala Ala Cys Cys Thr Gly Thr Thr Thr Ala 195 200 205 Gly
Cys Ala Cys Cys Ala Ala Ala Ala Ala Thr Ala Gly Cys Ala Gly 210 215
220 Cys Gly Cys Ala Gly Cys Ala Thr Gly Gly Gly Ala Thr Gly Ala Ala
225 230 235 240 Ala Cys Cys Cys Thr Gly Cys Thr Gly Gly Ala Ala Ala
Ala Ala Thr 245 250 255 Thr Cys Thr Ala Thr Ala Thr Cys Gly Ala Ala
Cys Thr Gly Thr Thr 260 265 270 Thr Cys Ala Gly Cys Ala Gly Ala Thr
Gly Ala Ala Cys Gly Ala Thr 275 280 285 Cys Thr Gly Gly Ala Ala Gly
Cys Ala Thr Gly Thr Gly Thr Thr Ala 290 295 300 Thr Thr Cys Ala Ala
Gly Ala Ala Gly Thr Thr Gly Gly Cys Gly Thr 305 310 315 320 Thr Gly
Ala Ala Gly Ala Ala Ala Cys Ala Cys Cys Gly Cys Thr Gly 325 330 335
Ala Thr Gly Ala Ala Thr Gly Ala Ala Gly Ala Thr Ala Gly Cys Ala 340
345 350 Thr Thr Cys Thr Gly Gly Cys Ala Gly Thr Gly Ala Ala Ala Ala
Ala 355 360 365 Ala Thr Ala Cys Thr Thr Thr Cys Ala Gly Cys Gly Cys
Ala Thr Thr 370 375 380 Ala Cys Cys Cys Thr Gly Thr Ala Thr Cys Thr
Gly Ala Thr Cys Gly 385 390 395 400 Ala Ala Cys Gly Thr Ala Ala Ala
Thr Ala Thr Ala Gly Cys Cys Cys 405 410 415 Gly Thr Gly Thr Gly Cys
Ala Thr Gly Gly Gly Ala Ala Gly Thr Thr 420 425 430 Gly Thr Thr Cys
Gly Thr Gly Cys Ala Gly Ala Ala Ala Thr Thr Ala 435 440 445 Thr Gly
Cys Gly Thr Ala Gly Cys Cys Thr Gly Ala Gly Cys Thr Thr 450 455 460
Thr Ala Gly Cys Ala Cys Cys Ala Ala Thr Cys Thr Gly Cys Ala Ala 465
470 475 480 Ala Ala Ala Cys Gly Thr Cys Thr Gly Cys Gly Thr Cys Gly
Cys Ala 485 490 495 Ala Ala Gly Ala Thr Thr Ala Ala Thr Ala Ala 500
505 3498PRTHomo sapiens 3Thr Gly Tyr Gly Ala Tyr Tyr Thr Asn Cys
Cys Asn Cys Ala Arg Ala 1 5 10 15 Cys Asn Cys Ala Tyr Trp Ser Asn
Tyr Thr Asn Gly Gly Asn Ala Ala 20 25 30 Tyr Met Gly Asn Met Gly
Asn Gly Cys Asn Tyr Thr Asn Ala Thr His 35 40 45 Tyr Thr Asn Tyr
Thr Asn Gly Gly Asn Cys Ala Arg Ala Thr Gly Gly 50 55 60 Gly Asn
Met Gly Asn Ala Thr His Trp Ser Asn Cys Cys Asn Thr Thr 65 70 75 80
Tyr Trp Ser Asn Thr Gly Tyr Tyr Thr Asn Ala Ala Arg Gly Ala Tyr 85
90 95 Met Gly Asn Cys Ala Tyr Gly Ala Tyr Thr Thr Tyr Met Gly Asn
Ala 100 105 110 Thr His Cys Cys Asn Cys Ala Arg Gly Ala Arg Gly Ala
Arg Thr Thr 115 120 125 Tyr Gly Ala Tyr Gly Gly Asn Ala Ala Tyr Cys
Ala Arg Thr Thr Tyr 130 135 140 Cys Ala Arg Ala Ala Arg Gly Cys Asn
Cys Ala Arg Gly Cys Asn Ala 145 150 155 160 Thr His Trp Ser Asn Gly
Thr Asn Tyr Thr Asn Cys Ala Tyr Gly Ala 165 170 175 Arg Ala Thr Gly
Ala Thr Gly Cys Ala Arg Cys Ala Arg Ala Cys Asn 180 185 190 Thr Thr
Tyr Ala Ala Tyr Tyr Thr Asn Thr Thr Tyr Trp Ser Asn Ala 195 200 205
Cys Asn Gly Ala Arg Ala Ala Tyr Trp Ser Asn Trp Ser Asn Gly Cys 210
215 220 Asn Gly Cys Asn Thr Gly Gly Gly Ala Arg Cys Ala Arg Ala Cys
Asn 225 230 235 240 Tyr Thr Asn Tyr Thr Asn Gly Ala Arg Ala Ala Arg
Thr Thr Tyr Trp 245 250 255 Ser Asn Ala Thr His Gly Ala Arg Tyr Thr
Asn Thr Thr Tyr Cys Ala 260 265 270 Arg Cys Ala Arg Ala Thr Gly Ala
Ala Tyr Gly Ala Tyr Tyr Thr Asn 275 280 285 Gly Ala Arg Gly Cys Asn
Thr Gly Tyr Gly Thr Asn Ala Thr His Cys 290 295 300 Ala Arg Gly Ala
Arg Gly Thr Asn Gly Gly Asn Gly Thr Asn Gly Ala 305 310 315 320 Arg
Gly Ala Arg Ala Cys Asn Cys Cys Asn Tyr Thr Asn Ala Thr Gly 325 330
335 Ala Ala Tyr Gly Ala Arg Gly Ala Tyr Trp Ser Asn Ala Thr His Tyr
340 345 350 Thr Asn Gly Cys Asn Gly Thr Asn Met Gly Asn Ala Ala Arg
Thr Ala 355 360 365 Tyr Thr Thr Tyr Cys Ala Arg Met Gly Asn Ala Thr
His Ala Cys Asn 370 375 380 Tyr Thr Asn Thr Ala Tyr Tyr Thr Asn Ala
Thr His Gly Ala Arg Met 385 390 395 400 Gly Asn Ala Ala Arg Thr Ala
Tyr Trp Ser Asn Cys Cys Asn Thr Gly 405 410 415 Tyr Gly Cys Asn Thr
Gly Gly Gly Ala Arg Gly Thr Asn Gly Thr Asn 420 425 430 Met Gly Asn
Gly Cys Asn Gly Ala Arg Ala Thr His Ala Thr Gly Met 435 440 445 Gly
Asn Trp Ser Asn Tyr Thr Asn Trp Ser Asn Thr Thr Tyr Trp Ser 450 455
460 Asn Ala Cys Asn Ala Ala Tyr Tyr Thr Asn Cys Ala Arg Ala Ala Arg
465 470 475 480 Met Gly Asn Tyr Thr Asn Met Gly Asn Met Gly Asn Ala
Ala Arg Gly 485 490 495 Ala Tyr 4189PRTHomo sapiens 4Met Ala Leu
Ser Phe Ser Leu Leu Met Ala Val Leu Val Leu Ser Tyr 1 5 10 15 Lys
Ser Ile Cys Ser Leu Gly Cys Asp Leu Pro Gln Thr His Ser Leu 20 25
30 Gly Asn Arg Arg Ala Leu Ile Leu Leu Gly Gln Met Gly Arg Ile Ser
35 40 45 Pro Phe Ser Cys Leu Lys Asp Arg His Asp Phe Arg Ile Pro
Gln Glu 50 55 60 Glu Phe Asp Gly Asn Gln Phe Gln Lys Ala Gln Ala
Ile Ser Val Leu 65 70 75 80 His Glu Met Ile Gln Gln Thr Phe Asn Leu
Phe Ser Thr Glu Asp Ser 85 90 95 Ser Ala Ala Trp Glu Gln Ser Leu
Leu Glu Lys Phe Ser Thr Glu Leu 100 105 110 Tyr Gln Gln Leu Asn Asp
Leu Glu Ala Cys Val Ile Gln Glu Val Gly 115 120 125 Val Glu Glu Thr
Pro Leu Met Asn Glu Asp Ser Ile Leu Ala Val Arg 130 135 140 Lys Tyr
Phe Gln Arg Ile Thr Leu Tyr Leu Ile Glu Arg Lys Tyr Ser 145 150 155
160 Pro Cys Ala Trp Glu Val Val Arg Ala Glu Ile Met Arg Ser Leu Ser
165 170 175 Phe Ser Thr Asn Leu Gln Lys Arg Leu Arg Arg Lys Asp 180
185 5189PRTHomo sapiens 5Met Ala Leu Pro Phe Ala Leu Met Met Ala
Leu Val Val Leu Ser Cys 1 5 10 15 Lys Ser Ser Cys Ser Leu Gly Cys
Asn Leu Ser Gln Thr His Ser Leu 20 25 30 Asn Asn Arg Arg Thr Leu
Met Leu Met Ala Gln Met Arg Arg Ile Ser 35 40 45 Pro Phe Ser Cys
Leu Lys Asp Arg His Asp Phe Glu Phe Pro Gln Glu 50 55 60 Glu Phe
Asp Gly Asn Gln Phe Gln Lys Ala Gln Ala Ile Ser Val Leu 65 70 75 80
His Glu Met Met Gln Gln Thr Phe Asn Leu Phe Ser Thr Lys Asn Ser 85
90 95 Ser Ala Ala Trp Asp Glu Thr Leu Leu Glu Lys Phe Tyr Ile Glu
Leu 100 105 110 Phe Gln Gln Met Asn Asp Leu Glu Ala Cys Val Ile Gln
Glu Val Gly 115 120 125 Val Glu Glu Thr Pro Leu Met Asn Glu Asp Ser
Ile Leu Ala Val Lys 130 135 140 Lys Tyr Phe Gln Arg Ile Thr Leu Tyr
Leu Met Glu Lys Lys Tyr Ser 145 150 155 160 Pro Cys Ala Trp Glu Val
Val Arg Ala Glu Ile Met Arg Ser Leu Ser 165 170 175 Phe Ser Thr Asn
Leu Gln Lys Arg Leu Arg Arg Lys Asp 180 185
References

D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012
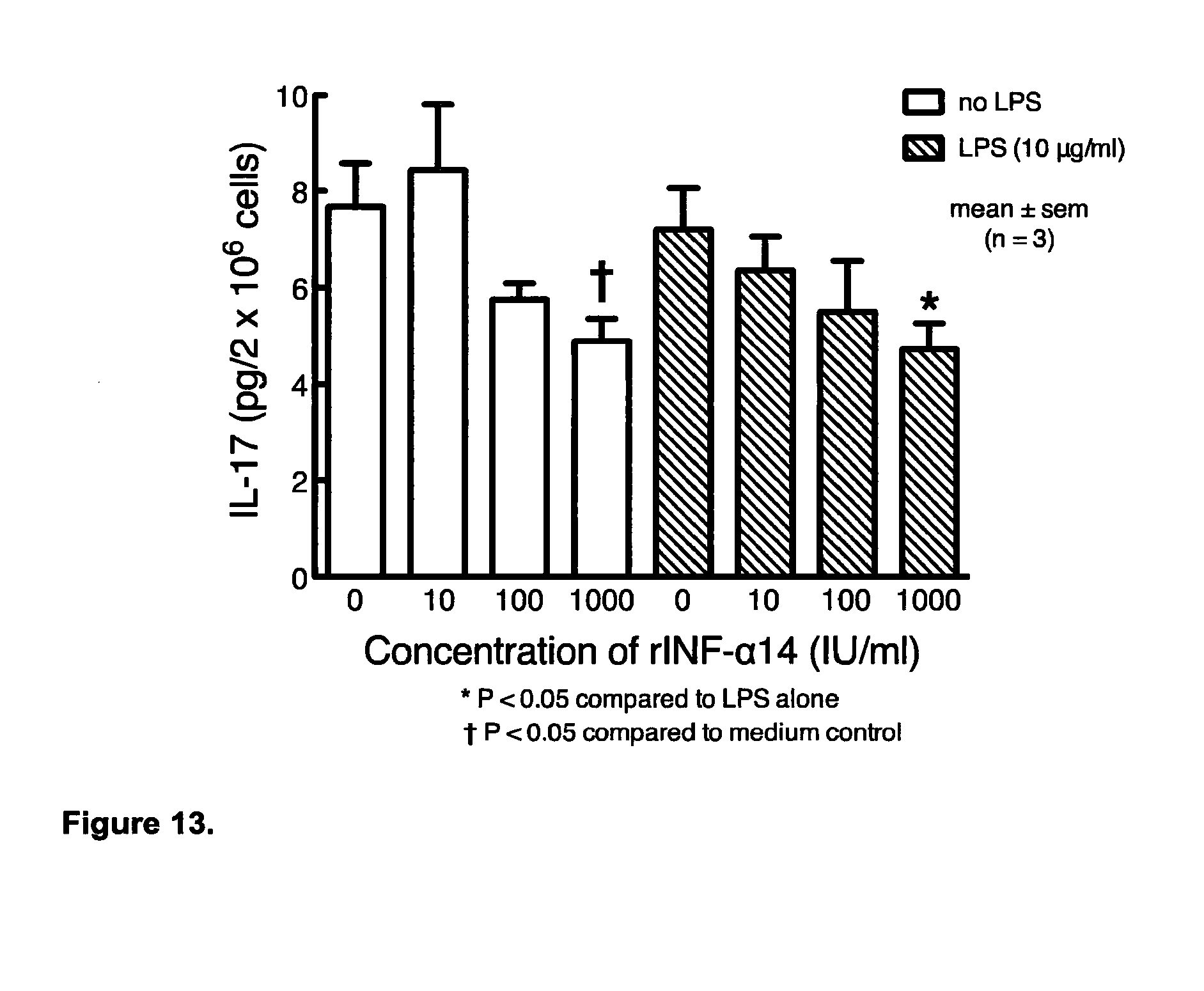
D00013

D00014
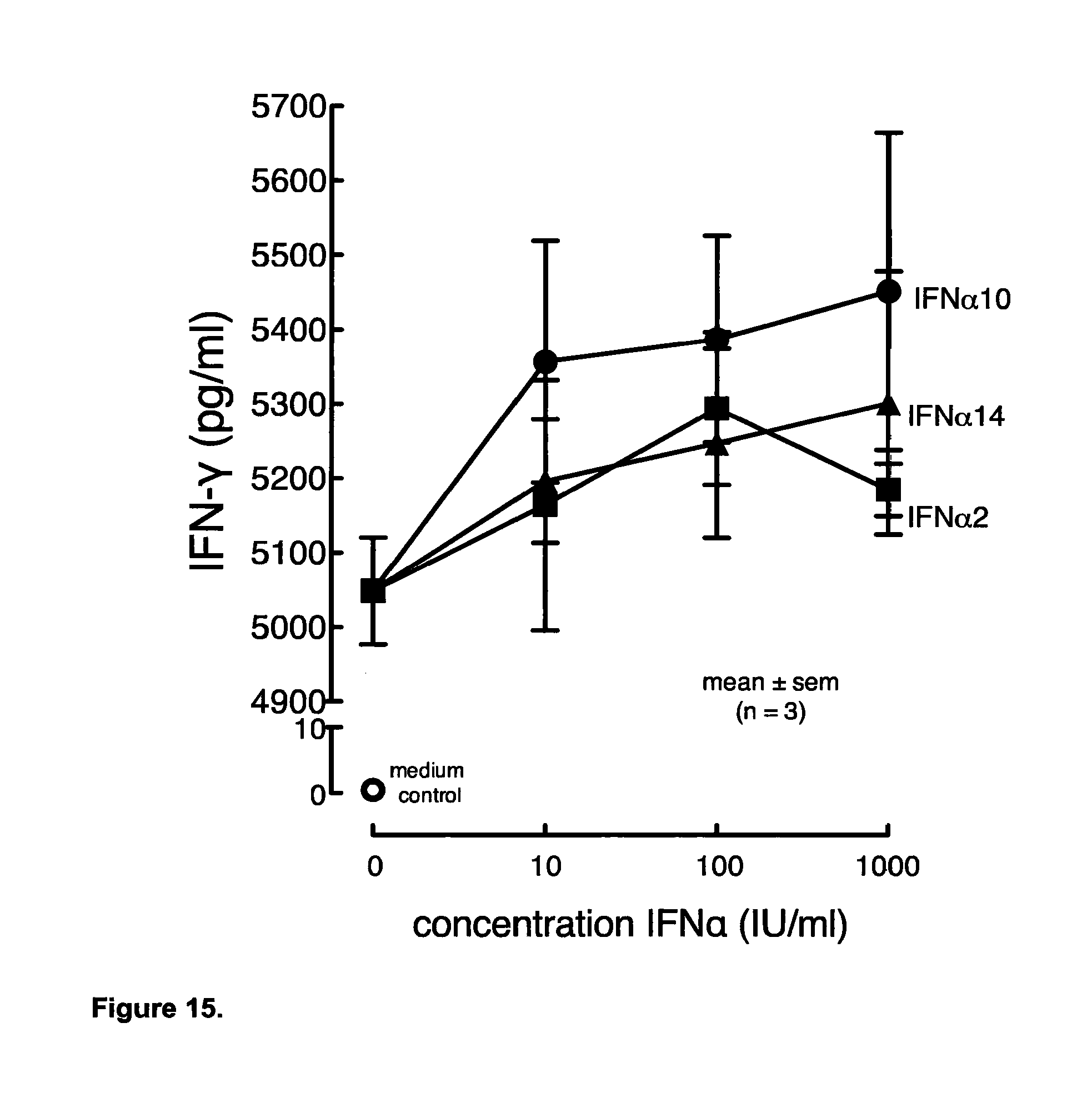
D00015
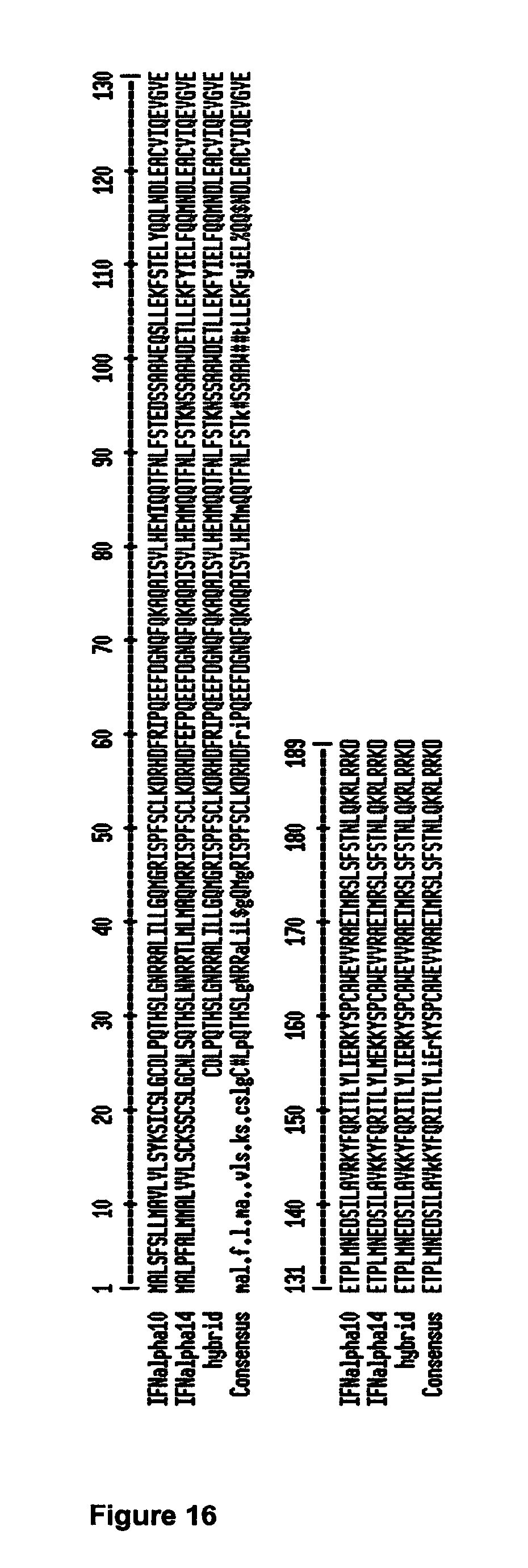
D00016

D00017

D00018

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.