Steel Sheet Suitable For Enamelling And Method For Producing Such A Sheet
Van Steenberge; Nele ; et al.
U.S. patent application number 16/230485 was filed with the patent office on 2019-04-18 for steel sheet suitable for enamelling and method for producing such a sheet. This patent application is currently assigned to Arcelormittal Investigacion Y Desarrollo SL. The applicant listed for this patent is Lode Duprez, Philippe Gousselot, Marc Leveaux, Nele Van Steenberge. Invention is credited to Lode Duprez, Philippe Gousselot, Marc Leveaux, Nele Van Steenberge.
| Application Number | 20190112684 16/230485 |
| Document ID | / |
| Family ID | 44509976 |
| Filed Date | 2019-04-18 |

| United States Patent Application | 20190112684 |
| Kind Code | A1 |
| Van Steenberge; Nele ; et al. | April 18, 2019 |
STEEL SHEET SUITABLE FOR ENAMELLING AND METHOD FOR PRODUCING SUCH A SHEET
Abstract
A rolled steel sheet suitable for enameling, having a carbon profile, defined by a gradient in the C-level from a level C.sub.surface at at least one surface of the sheet, to a level C.sub.bulk in the bulk of the sheet, C.sub.bulk being higher than C.sub.surface, and with C.sub.bulk higher than 0 and lower than 0.08 wt %, C.sub.surface between 0 and 0.015 wt %, Al between 0.012 wt % and 0.07 wt %, Mn between 0.12 wt % and 0.45 wt % and O lower than 0.01 wt % and wherein the depth where the C-level reaches (C.sub.bulk+C.sub.surface)/2, is higher than 75 .mu.m.
| Inventors: | Van Steenberge; Nele; (Gent, BE) ; Leveaux; Marc; (Marcq-en-Baroeul, FR) ; Duprez; Lode; (Lokeren, BE) ; Gousselot; Philippe; (Metz, FR) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | Arcelormittal Investigacion Y
Desarrollo SL Sestao ES |
||||||||||
| Family ID: | 44509976 | ||||||||||
| Appl. No.: | 16/230485 | ||||||||||
| Filed: | December 21, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 13502566 | Nov 29, 2012 | |||
| PCT/EP11/55477 | Apr 8, 2011 | |||
| 16230485 | ||||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C21D 9/46 20130101; C21D 8/0257 20130101; C22C 38/002 20130101; C21D 3/04 20130101; C21D 9/56 20130101; C22C 38/04 20130101; C22C 38/06 20130101; C21D 8/0205 20130101; C22C 38/16 20130101 |
| International Class: | C21D 8/02 20060101 C21D008/02; C22C 38/16 20060101 C22C038/16; C22C 38/06 20060101 C22C038/06; C22C 38/04 20060101 C22C038/04; C21D 3/04 20060101 C21D003/04; C21D 9/56 20060101 C21D009/56; C21D 9/46 20060101 C21D009/46; C22C 38/00 20060101 C22C038/00 |
Claims
1. A rolled steel sheet suitable for enameling, said sheet having a carbon profile, defined by a gradient in the C-level from a level C.sub.surface at at least one surface of the sheet, to a level C.sub.bulk in the bulk of the sheet, C.sub.bulk being higher than C.sub.surface, and comprising: C.sub.bulk higher than 0 and lower than or equal to 0.08 wt %, C.sub.surface between 0 and 0.015 wt %, Al between 0.012 wt % and 0.07 wt %, Mn between 0.12 wt % and 0.45 wt %, O lower than 0.01 wt % and optionally: Cu between 0.025 wt % and 0.1 wt %, S between 0.008 wt % and 0.04 wt %, Ca between 0.0005 wt % and 0.005 wt %, the balance being Fe and incidental impurities, wherein a particular depth where the C-level reaches a midpoint C-level of (C.sub.bulk+C.sub.surface)/2 is higher than 75 micron, the midpoint C-level being a fixed value greater than 0 that is a single point on the gradient.
2. Steel sheet according to claim 1, having an r.sub.m value between 1.8 and 2.1.
3. Steel sheet according to claim 1, wherein C.sub.surface is between 0.005 wt % and 0.015 wt %.
4. Steel sheet according to claim 1, wherein C.sub.surface is between 0 and 0.005 wt %.
5. Steel sheet according to claim 1, wherein C.sub.bulk is between 0.02 wt % and 0.08 wt %.
6. Steel sheet according to claim 5, wherein C.sub.bulk is between 0.025 wt % and 0.08 wt %.
7. Steel sheet according to claim 5, wherein C.sub.bulk is between 0.025 wt % and 0.06 wt %.
8. Steel sheet according to claim 1, wherein the Al-level is between 0.02 wt % and 0.06 wt %.
9. Steel sheet according to claim 1, wherein said depth is between 130 .mu.m and 200 .mu.m.
10. Enameled steel sheet consisting of a steel sheet according to claim 1, provided with an enamel layer.
11. Steel product produced from a sheet according to claim 1.
12. Enameled steel product consisting of a product according to claim 11, provided with an enamel layer.
Description
CROSS-REFERENCE TO RELATED PATENT APPLICATIONS
[0001] This patent application is a divisional of co-pending U.S. patent application Ser. No. 13/502,566, filed Nov. 29, 2012, which is a National Stage Entry of PCT/EP11/55477, filed Apr. 8, 2011, the entire teachings and disclosure of which are incorporated herein by reference thereto.
FIELD OF THE INVENTION
[0002] The present invention is related to a steel sheet suitable for enameling, and to a method for the superficial decarburization of a steel sheet, as a preparation for enameling the steel.
BACKGROUND OF THE INVENTION
[0003] The carbon level of a steel sheet has an important influence on the results in terms of surface quality of an enamel layer applied on the surface of the sheet. A high carbon level at the steel surface may give rise to CO-gas bubble formation, which shows up as black spots and craters in the enamel surface. On the other hand, when a sufficiently high carbon level is present initially in the bulk, this carbon forms coarse cementite during hot rolling which cracks upon cold rolling. These cracks are capable of capturing hydrogen which enters the steel during the enameling process. When hydrogen is insufficiently captured, pressure will rise at the steel/enamel surface which gives rise to the so-called `fish-scale` deformation of the enamel.
[0004] It is therefore advantageous to decarburize the steel only in a layer at the surface of the steel, i.e. to conduct a superficial decarburization. Document JP-A-2282421 describes such a method, wherein a continuously cast and annealed non-aging steel sheet for enameling is produced, characterized in that a continuous-cast steel slab containing C between 0.0025 and 0.0050 wt %, Si max. 0.03 wt %, Mn between 0.1 and 0.6 wt %, P between 0.005 and 0.03 wt %, S between 0.005 and 0.03 wt %, Al max. 0.01 wt %, N max. 0.004 wt %, Cu between 0.01 and 0.06 wt %, 0 between 0.02 and 0.06 wt %, V between 0.01 and 0.06 wt %, the balance Fe and inevitable impurities, is hot-rolled with a finishing temperature higher than or equal to 800.degree. C., and a coiling temperature of 600-800.degree. C., cold-rolled with a reduction ratio higher than or equal to 60% and subjected to a decarburization annealing at 700-900.degree. C. for 30 sec-3 min, carried out in a continuous annealing furnace having a decarburizing atmosphere composed of 1-20% water vapour, gaseous hydrogen in an amount higher than or equal to twice the water vapour amount, and the balance being mainly gaseous nitrogen, so as to reduce the C-level to be less than or equal to 0.002 wt %. JP-A-6116634 describes a similar method, but wherein the starting material has no vanadium and the initial C level is up to 0.015 wt % and B is added instead of V for H-trapping.
[0005] Both prior art methods result in fully or superficially decarburized steel sheets which are suitable for enameling by Direct White Enameling (DWE), wherein one white enamel coating is applied on the surface, followed by one firing step. As the initial carbon level is quite low, fish-scale resistance due to cementite formation is not achieved. To compensate for this, a steel with a high oxygen level and a limited Al-level is used in the prior art together with specific alloying elements such as B, V. Thanks to the limited Al-content, oxides of Si and Mn and nitrides of B and V are formed within the bulk of the steel which are beneficial against fish-scaling. However, this alloying involves extra costs and leads to some technical difficulties, e.g. involving casting with V. Furthermore, the prior art decarburized sheets have rather low formability as testified by the values of the Lankford coefficient (r.sub.m). These values do not exceed 1.8 which is a concern when deep-drawing is foreseen. In particular, FIG. 1 of JP6116634 shows that r.sub.m values between 1.6 and 1.8 are only achieved for a very narrow range of carbon level before decarburizing annealing. Below 0.0050 wt % C and above 0.0150 wt % C, the r.sub.m value is deteriorated.
[0006] The present invention aims to provide a partially decarburized steel sheet suitable for enameling which do not suffer from the drawbacks of the above cited prior art.
BRIEF SUMMARY OF THE INVENTION
[0007] The invention is related to steel sheets and products and to a production method as disclosed in the appended claims. The invention is thus related to a rolled steel sheet suitable for enameling, said sheet having a carbon profile, defined by a gradient in the carbon level (C-level) from a level C.sub.surface at at least one surface of the sheet, to a level C.sub.bulk in the bulk of the sheet, C.sub.bulk being higher than C.sub.surface, and with: [0008] C.sub.bulk higher than 0 and lower than or equal to 0.08 wt %, [0009] C.sub.surface between 0 and 0.015 wt %, [0010] Al between 0.012 wt % and 0.07 wt %, [0011] Mn between 0.12 wt % and 0.45 wt %, [0012] O lower than 0.01 wt %
[0013] and optionally: [0014] Cu between 0.025 wt % and 0.1 wt %, [0015] S between 0.008 wt % and 0.04 wt %, [0016] Ca between 0.0005 wt % and 0.005 wt % the balance being Fe and incidental impurities, and wherein the depth where the C-level reaches (C.sub.bulk+C.sub.surface)/2, is higher than 75 mm.
[0017] According to a preferred embodiment, the steel sheet of the invention has an r.sub.m value between 1.8 and 2.1.
[0018] According to specific embodiments, C.sub.surface is between 0.005 wt % and 0.015 wt %, or between 0 and 0.005 wt %.
[0019] According to other specific embodiments, C.sub.bulk is between 0.02 wt % and 0.08 wt %, or between 0.025 wt % and 0.08 wt % or between 0.025 wt % and 0.06 wt %.
[0020] According to another embodiment, the Al-level is between 0.02 wt % and 0.06 wt %.
[0021] According to a further embodiment, said depth is between 130 mm and 200 mm.
[0022] The invention is equally related to an enameled steel sheet consisting of a steel sheet according to any of the above paragraphs, provided with an enamel layer.
[0023] The invention is further related to a steel product produced from a sheet according to the invention, and to an enameled steel product consisting of a such a product, provided with an enamel layer.
[0024] The invention is further related to a steel product produced from a sheet according to the invention, and to an enameled steel product consisting of a such a product, provided with an enamel layer. The invention is also related to a method for producing a rolled steel sheet for enameling, comprising the steps of: [0025] subjecting a steel slab to hot rolling followed by coiling, and cold rolling, so as to obtain a cold-rolled steel sheet, said slab comprising the following initial composition: [0026] C between 0.02 wt % and 0.08 wt %, [0027] Al between 0.012 wt % and 0.07 wt %, [0028] Mn between 0.12 wt % and 0.45 wt %, [0029] O lower than 0.01 wt % [0030] and optionally: [0031] Cu between 0.025 wt % and 0.1 wt %, [0032] S between 0.008 wt % and 0.04 wt %, [0033] Ca between 0.0005 wt % and 0.005 wt %, [0034] the balance being Fe and incidental impurities, [0035] subjecting said cold-rolled sheet to continuous annealing step, wherein said sheet is exposed during a decarburizing time to a decarburizing atmosphere comprising water vapour and hydrogen gas, wherein the H.sub.2 content is between 1 vol % and 95 vol %, the H.sub.2O content between 0.04 vol % and 33 vol %, the remainder being mainly nitrogen gas, the ratio pH.sub.2O/pH.sub.2 being between 0.04 and 0.5.
[0036] According to a preferred embodiment, said continuous annealing takes place at an anneal temperature between 760.degree. C. and 850.degree. C., and during a decarburizing time between 45 s and 300 s.
[0037] According to a specific embodiment, the anneal temperature is between 800.degree. C. and 850.degree. C.
[0038] According to specific embodiments of the method of the invention, the initial C-level is between 0.025 wt % and 0.08 wt % or between 0.025 wt % and 0.06 wt %.
[0039] According to a further embodiment, the initial Al-level is between 0.02 wt % and 0.06 wt %.
[0040] According to a specific embodiment, the ratio pH.sub.2O/pH.sub.2 is between 0.04 and 0.25.
[0041] The method of the invention may further comprise an over-ageing step at a temperature between 350.degree. C. and 450.degree. C. during a timespan between 100 s and 500 s. The method may further comprise a skinpass step with a reduction of between 0.3% and 1.5%.
BRIEF DESCRIPTION OF THE DRAWINGS
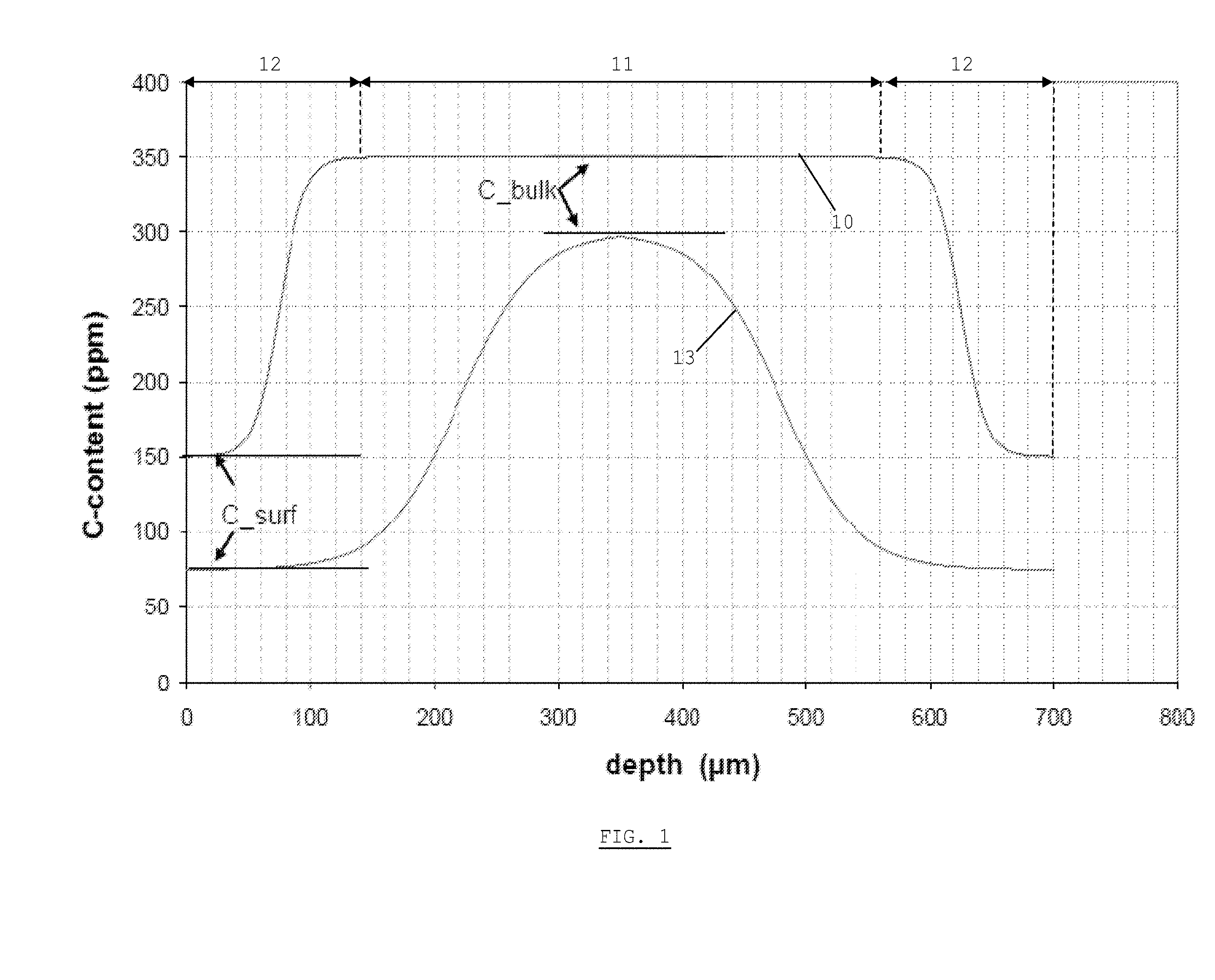
[0042] FIG. 1 illustrates the carbon profile in a steel sheet according to the invention.
[0043] FIG. 2 illustrates an example of an annealing step usable in the method of the invention.
DETAILED DESCRIPTION OF THE INVENTION
[0044] The steel sheet of the invention has a C-profile, defined by a gradient in the C-level from a lower value C.sub.surface at the surface to a higher value C.sub.bulk in the bulk. The sheet is obtainable by a method which includes a continuous decarburization step, as will be described further in this text. FIG. 1 illustrates the carbon-distribution across the thickness of two sheets according to the invention, with a thickness of 0.7 mm. Curve 10 illustrates a sheet which comprises a bulk portion 11, where the C-level C.sub.bulk is substantially constant, and two surface portions 12 (one on each side of the sheet), each surface portion exhibiting the C-profile. The surface level is defined as the minimum value of the C-profile, measured by a suitable measurement technique (e.g. Glow Discharge Optical Emission Spectroscopy (GD-OES), which allows composition measurement as depth analysis). In a steel sheet according to the invention, the C-level at the surface is maximum 0.015 wt %, whereas C.sub.bulk is higher than zero and lower than or equal to 0.08 wt %. At the same time, C.sub.bulk is higher than C.sub.surface. According to an embodiment, C.sub.surface is between 0.005 wt % and 0.015 wt %. According to another embodiment, C.sub.surface is between 0 and 0.005 wt %.
[0045] Curve 10 is an example of a sheet where the decarburization has not taken place over the entire thickness of the sheet. This means that the level C.sub.bulk is equal to the initial C-level applied in the production method (described further in more detail). According to embodiments which correspond to embodiments of the method of the invention (see further), C.sub.bulk is then between 0.02 wt % and 0.08 wt %, or between 0.025 wt % and 0.08 wt, or between 0.025 wt % and 0.06 wt % or between 0.025 wt % and 0.05 wt %. Curve 13 illustrates the case where decarburization has continued until the middle plane of the sheet. In this case, C.sub.bulk is smaller than the initial C-level of the method, and the C-profile extends over each half-width of the sheet.
[0046] The decarburized sheet according to the invention further comprises Al, Mn and possibly S, Cu and Ca. Contrary to the prior art references, the oxygen level is to be kept lower than 0.01 wt %. According to a preferred embodiment of the steel sheet of the invention, oxygen is not added deliberately to the composition, but is allowed only at impurity levels. Fish scaling resistance is ensured by the higher initial C-level, so no oxide formation is required for this purpose. This means that no special alloying elements such as V are included. Also, N is kept as low as possible.
[0047] The Al-level in the sheet of the invention is between 0.012 wt % and 0.07 wt %, which is higher than the allowed Al-level in the prior art references cited above. In the cited prior art documents Al needs to be limited to avoid deoxidation, so as to ensure the formation of the oxides that will work against fish-scaling. In the method of the invention (see further), Al is mandatory for deoxidation and binding of free N to avoid the ageing of the mechanical properties. When the Al-level is lower than 0.012 wt %, insufficient deoxidation takes place, and binding of N is required through other means. Adding Al at levels higher than 0.07 wt % means an increase in cost of the process, and a deterioration of the enameling quality. A more preferred range for the Al-level, related to more optimized conditions in terms of deoxidation and cost/enameling quality is between 0.02 wt % and 0.06 wt %.
[0048] Mn is present between 0.12 wt % and 0.45 wt %. This element is added to control the strength properties of the steel and to avoid the formation of free sulphur.
[0049] Copper, Sulphur and Calcium may optionally be added above the impurity level, more precisely in the ranges 0.025 wt % to 0.1 wt %, 0.008 wt % to 0.04 wt % and 0.0005 wt % to 0.005 wt % respectively. These elements improve the enameling quality.
[0050] The balance of the composition of the steel sheet according to the invention consists of Fe and incidental impurities. The following elements may be present as impurities at levels which are preferably lower than the values (in wt %) given in table 1:
TABLE-US-00001 TABLE 1 impurity levels Si <0.1 P <0.03 Ti <0.01 Cr <0.2 Ni <0.2 As <0.02 Sn <0.02 Nb <0.01 V <0.01 Sb <0.02 Mo <0.03 B <0.0005 N <0.007
[0051] In a steel sheet of the invention, the depth of the C-profile, being defined as the depth where the C-level reaches (C.sub.bulk+C.sub.surface)/2, is higher than 75 mm, to ensure good enameling capability. According to an embodiment, said depth is between 130 mm and 200 mm.
[0052] Steel sheets according to the invention, i.e. with a C-level at the surface between 0 and 0.015 wt % are suitable for 2C/1F enameling, i.e. enameling by applying a ground coat enamel, followed by an outer enamel coating, both coatings being subjected to one firing step, and for 1C/1F enameling, i.e. enameling by applying one enamel layer subjected to one firing step. Steel sheets with low C-levels (i.e. 0.005 wt % and less) at the surface may be suitable also for Direct White Enameling (DWE).
[0053] According to a preferred embodiment, the r.sub.m value of a steel sheet according to the invention is between 1.8 and 2.1. This means that the steel sheet has better formability than the prior art steel sheets referred to above. In the present description, the `r` value refers to the plastic strain ratio (also known as the anisotropy factor), being the ratio of the true strain in the width direction to the true strain in the thickness direction when a sheet material is pulled in uniaxial tension beyond its elastic limit. The `r.sub.m` value is defined as 1/4(r.sub.90+2*r.sub.45+r.sub.0), with r.sub.90, r.sub.45 and r.sub.0 the r-values as defined above, measured on samples oriented respectively at 90.degree., 45.degree. and 0.degree. with respect to the rolling direction. In a steel sheet according to the invention, fish scaling resistance is ensured by the higher initial C-level applied in the method (see further).
[0054] The steel sheet of the invention can be produced by subjecting a steel slab with a specific initial steel composition to hot rolling, coiling and cold rolling, and by subjecting the cold-rolled sheet to continuous superficial decarburization. The initial composition is mainly characterized by a higher C-level compared to the prior art, and by a higher Al-level and a lower oxygen level. No deliberate addition of elements like V, Nb or B is done, while still allowing to produce enameled steel sheets with a high fish scale resistance and good enamel surface quality. The initial C-level is between 0.02 wt % and 0.08 wt %, more preferably between 0.025 wt % and 0.08 wt %. This is higher than the initial C-levels disclosed in the prior art references referred to above. Despite such higher initial C-levels, the method of the invention allows to obtain steel sheets with improved formability characteristics compared to the prior art. Whereas JP6116634 indicates that above 0.015 wt % of initial carbon, it is not possible to obtain acceptable decarburization and good formability, the starting composition of the invention does not encounter these problems. Decarburization is possible down to an acceptable level, while formability is excellent. When the initial C-level is lower than 0.02 wt %, insufficient cementite formation occurs which deteriorates fish scale resistance. C-levels above 0.08 wt % lead to too high strength levels and thus reduced formability. Specific ranges for the initial C-level, related to more optimized characteristics in terms of fish scale resistance and strength/formability are between 0.025 wt % and 0.06 wt % and between 0.025 wt % and 0.05 wt %.
[0055] The initial steel composition according to the method of the invention further comprises Al, Mn and possibly O, S, Cu and Ca in the same ranges as the decarburized sheet described above, the balance being Fe and the incidental impurities listed in Table 1. A more preferred range for the initial Al-level, related to more optimized conditions in terms of deoxidation and cost/enameling quality is between 0.02 wt % and 0.06 wt %. According to a preferred embodiment of the method of the invention, oxygen is not added deliberately to the composition, but is allowed only at impurity levels.
[0056] The method of the invention comprises standard steps of hot rolling and cold rolling a steel slab of the above composition. According to the preferred embodiment, the slab is heated or reheated at a temperature above 1050.degree. C., subjected to hot rolling with a finishing temperature between 850.degree. C. and 950.degree. C., and coiling at coiling temperature between 620.degree. C. and 770.degree. C. Still according to the preferred embodiment, cold rolling is performed with a reduction of minimum 50%. The final thickness of the cold rolled sheet is preferably between 0.2 and 2 mm.
[0057] The decarburization anneal is done in an annealing furnace for continuous annealing (i.e. annealing while the cold-rolled sheet moves through the furnace at a given speed, said speed determining the anneal time, i.e. the time spent at the annealing temperature) as known in the art, possibly provided with a vapour injection device for applying a given annealing atmosphere.
[0058] FIG. 2 shows an example of a lay-out of an annealing furnace usable in the method of the invention, starting with heating phase 1 wherein the temperature rises to the annealing temperature. Phase 2 represents the actual annealing (soaking) phase. Phase 3 is an overageing step. Phase 2 can consist of one or more periods with a different (constant or average) annealing temperature and a different annealing atmosphere in each period. Practically speaking, the different periods at different conditions can be obtained by dividing the annealing zone in subsections and by injecting H.sub.2O vapour into an atmosphere comprising H.sub.2, at various points along the annealing line (see example further in this description).
[0059] FIG. 2 shows an example of a lay-out of an annealing furnace usable in the method of the invention, starting with heating phase 1 wherein the temperature rises to the annealing temperature. Phase 2 represents the actual annealing (soaking) phase. Phase 3 is an overageing step. Phase 2 can consist of one or more periods with a different (constant or average) annealing temperature and a different annealing atmosphere in each period. Practically speaking, the different periods at different conditions can be obtained by dividing the annealing zone in subsections and by injecting H.sub.2O vapour into an atmosphere comprising H.sub.2, at various points along the annealing line (see example further in this description).
[0060] According to one embodiment, the decarburizing atmosphere can be prepared with a mixture of H.sub.2 and N.sub.2 with between 1.5 and 5% H.sub.2 in which H.sub.2O vapour is injected so that pH.sub.2O/pH.sub.2 is between 0.04 and 0.5. The minimum value of this ratio ensures that sufficient H.sub.2O is present to obtain decarburization according to the formula C+H.sub.2O.fwdarw.CO+H.sub.2. The maximum of said range ensures that oxidation of Fe and of the furnace is avoided. A more preferred range for pH.sub.2O/pH.sub.2, related to more optimized conditions in terms of sufficient decarburization and avoiding the occurrence of Fe-oxidation is between 0.04 and 0.25.
[0061] In the method of the invention, the decarburizing atmosphere is applied during at least one of said periods with a different (constant or average) annealing temperature and a different annealing atmosphere in each period, preferably during the totality of phase 2. In the following, the `decarburizing time` refers to the time spent under the conditions of the decarburizing atmosphere.
[0062] The decarburizing time and the anneal temperature are chosen so as to obtain a steel sheet according to the invention. It is within the skilled person's knowledge to find suitable combinations of decarburizing time and anneal temperature based on the examples given further in this description. According to a preferred embodiment, the decarburizing time is between 45 s and 300 s and the anneal temperature between 760.degree. C. and 850.degree. C. When the ratio pH.sub.2O/pH.sub.2 is lower than about 0.1, the decarburizing time is preferably higher than 70 s. A more preferred range of the anneal temperature, applicable in combination with any decarburizing time between 45 s and 300 s is between 800.degree. C. and 850.degree. C. The temperature is not necessarily constant during the decarburizing time. Fluctuations of the temperature may occur due to variations in the line speed for example. An over-ageing step may be applied at a temperature between 350.degree. C. and 450.degree. C. during a timespan between 100 s and 500 s. A skinpass may further be applied with a reduction of between 0.3% and 1.5%.
EXAMPLES
[0063] Results from industrial trials performed by the applicant will be described hereafter, as well as a number of laboratory trials. All tested samples were produced from starting compositions according to the invention. The coiling temperature was 725.degree. C. Two industrial trials were conducted. The thickness of the cold rolled sheet subjected to decarburization annealing in industrial trial 1 was 0.6 mm; in the second industrial trial the thickness was 1 mm.
[0064] The continuous annealing line in which the industrial trials were conducted consists of a heating section, two soaking areas, a cooling and an overaging part. The annealing atmosphere consisted mainly of a mixture of H.sub.2 and N.sub.2, with H.sub.2O vapour being injected in the first and/or the second soaking area. In the first trial, H.sub.2O vapour was injected only in the second soaking area. In the second trial, H.sub.2O was injected in the first and the second soaking area. Overageing was performed in both trials at 400.degree. C. The overageing time depended on the line speed, e.g. at 180 m/min line speed, the overageing time was 232 s. Table 2 shows the annealing conditions for both trials (numbered trial 1 and trial 2). Table 3 shows the composition besides C, for a number of the samples shown in table 1.
[0065] In industrial trial 1, the pH.sub.2O/pH.sub.2 ratio is below the range of 0.04-0.5 in the first soaking area (due to the fact that no H.sub.2O injection is done). The decarburizing time in this trial is the time spent in the second soaking area, where the pH.sub.2O/pH.sub.2 is within said range. This is an example therefore of a process wherein phase 2 as shown in FIG. 1 comprises a first period wherein the conditions of the present invention are not met, and a second period wherein these conditions are met. Such a process falls within the scope of the present invention.
[0066] In industrial trial 2, H.sub.2O-injection was performed in both soaking areas. The decarburizing time indicated here is the time spent in soaking areas 1 and 2. The anneal temperature is the average of the temperatures in soaking areas 1 and 2. The pH.sub.2O/pH.sub.2 values indicated in table 1 are the average values in soaking area 1 in which more H.sub.2O was injected. However, the pH.sub.2O/pH.sub.2 in soaking area 2 is estimated to be also within the range of 0.04-0.5. In industrial trial 2, longer decarburizing times could be achieved as compared to the first trial, for similar line velocities, leading to stronger decarburization.
[0067] The laboratory trials (marked `trial 3` in table 2), were conducted on samples which were subjected to a simulation of the continuous annealing step, at the conditions shown in table 2. These trials were conducted in an atmosphere of HN.sub.x with 5% H.sub.2, with H.sub.2O added to obtain pH.sub.2O/pH.sub.2 in the range between 0.04 and 0.5.
[0068] Samples from all three trials were subjected to an enameling process wherein a ground coat enamel is deposited, this enamel being designed especially to determine the role of C in the enamel characteristics. It was found that the adhesion of the enameling layer was good for all tested samples. The enameling aspect was good for C-levels at the surface of maximum 0.015 wt %, and for profile depths of 75 mm up to 250 mm, as shown in table 1. There is no reason to conclude from the results however that the enameling quality deteriorates at higher depth values than 250 mm. Such higher depth values are therefore not excluded from the scope of the invention. All tested samples showed a good fish scale resistance.
[0069] Table 2 summarizes the results after decarburization in terms of the C-level at the surface (i.e. minimum level of the C-profile, measured by GD-OES), the depth of the C-profile, and the quality of an enamel layer produced on the surface of the samples. Samples 25 to 35 yielded a bad enameling aspect, which can be ascribed to either an insufficient depth of the C-profile (as determined by the depth where the C-level reaches (C.sub.surface+C.sub.bulk/2), and/or a C level at the surface which is too high. The reason for these negative results can be ascribed to the test conditions, either the anneal temperature which is too low, the decarburizing time too short, or the pH.sub.2O/pH.sub.2 ratio too low, or a combination of these factors.
[0070] Table 4 shows the mechanical properties of a number of samples taken from the sheets of the industrial trials 1 and 2. Importantly, the formability in terms of the r.sub.m value is excellent, despite the initial C-level which is higher than in the prior art: r.sub.m is between 1.8 and 2.1. These results prove that the method of the invention allows to produce steel sheets suitable for enameling, starting from an initial C-level higher than 0.02 wt %, the resulting sheets allowing good enameling quality and fish scale resistance, and having very good formability characteristics.
TABLE-US-00002 TABLE 2 Overview of experimental conditions and results decarbu- anneal Sample Trial initial C rizing time temp. pH.sub.2O/pH.sub.2 C_surf depth enameling Acc. to Nro nr (ppm.sup.1) (s) (.degree. C.) (--) (ppm) (.mu.m) aspect invention? 1 1 362 45 807 0.12 126 90 good y 2 3 330 70 770 0.05 123 92 good y 3 3 330 70 770 0.12 108 100 good y 4 3 330 70 770 0.24 47 94* good y 5 1 367 90 782 0.16 132 123 good y 6 1 367 90 785 0.16 133 130 good y 7 2 330 90 810 0.13 87 130* good y 8 2 346 90 817 0.09 89 116* good y 9 2 386 105 801 0.12 106 130 good y 10 2 348 105 809 0.11 88 145* good y 11 2 316 105 817 0.12 78 180 good y 12 2 346 105 824 0.09 81 142* good y 13 2 360 126 799 0.09 106 138* good y 14 2 348 126 814 0.09 78 124* good y 15 2 375 126 824 0.09 76 140* good y 16 3 330 155 840 0.05 51 94* good y 17 3 330 155 840 0.12 61 94* good y 18 3 330 155 840 0.24 35 94* good y 22 1 500 45 795 0.08 211 40 bad n 23 1 491 45 796 0.09 164 75 bad n 24 1 490 45 800 0.09 177 49 bad n 25 1 360 45 793 0.09 150 70 bad n 26 1 360 45 794 0.09 167 75 bad n 27 1 360 45 795 0.09 176 68 bad n 28 3 330 70 770 0.01 198 57 bad n 29 2 363 105 743 0.17 187 135 bad n 30 2 370 105 752 0.16 165 120 bad n 31 3 330 155 840 0.01 192 89 bad n .sup.11 wt % = 10.sup.4 ppm
[0071] The depth-values given in table 1 are values of the depth where the C-level reaches (C.sub.surface+C.sub.bulk)/2. The depth measurements indicated with `*` show the maximum depth which could be measured with the applied equipment. The real value is thus higher than this value.
TABLE-US-00003 TABLE 3 composition of samples (C-level in table 1, remaining elements are beneath impurity level, the remainder is Fe) Sample Nr.degree. Al (wt %) Mn (wt %) Cu (wt %) S (wt %) 1 0.024 0.18 0.022 0.0049 5 0.03 0.18 0.028 0.0046 6 0.028 0.19 0.041 0.0052 9 0.035 0.18 0.029 0.0088 11 0.034 0.17 0.028 0.0077
TABLE-US-00004 TABLE 4 mechanical properties initial Anneal Anneal Sample C-level Temp time pH2O/pH2 Rp0.2 Rm Thickness Nro (ppm) (.degree. C.) (s) (--) (MPa) (MPa) r_m (mm) 23 490 796 209 0.09 189 318 1.8 0.6 1 362 807 126 0.12 182 318 1.9 0.6 5 367 782 105 0.16 189 319 1.8 0.6 19 391 821 105 0.08 185 320 1.8 1 15 375 824 45 0.09 187 331 2.0 1 11 316 817 45 0.12 188 329 1.8 1 9 386 801 90 0.12 183 319 1.9 1
* * * * *
D00001

D00002

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.