CpG-OLIGODEOXYNUCLEOTIDE, IMMUNOGENIC COMPOSITION INCLUDING THE SAME, AND METHOD OF INDUCING IMMUNE RESPONSE BY THE SAME
CHUANG; Tsung-Hsien ; et al.
U.S. patent application number 15/722949 was filed with the patent office on 2019-04-04 for cpg-oligodeoxynucleotide, immunogenic composition including the same, and method of inducing immune response by the same. The applicant listed for this patent is National Health Research Institutes. Invention is credited to Tsung-Hsien CHUANG, Chao-Yang LAI, Yi-Ling LIU, Chih-Hao LU, Da-Wei YEH.
| Application Number | 20190100756 15/722949 |
| Document ID | / |
| Family ID | 65895934 |
| Filed Date | 2019-04-04 |











View All Diagrams
| United States Patent Application | 20190100756 |
| Kind Code | A1 |
| CHUANG; Tsung-Hsien ; et al. | April 4, 2019 |
CpG-OLIGODEOXYNUCLEOTIDE, IMMUNOGENIC COMPOSITION INCLUDING THE SAME, AND METHOD OF INDUCING IMMUNE RESPONSE BY THE SAME
Abstract
A CpG-oligodeoxynucleotide (CpG-ODN) for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host is provided. The CpG-ODN includes one or more copies of the sequences of GTCGTT, one or more copies of the sequences of GTT and one or more copies of the sequences of TTTT, wherein at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT. Further, an immunogenic composition including the CpG-ODN and a method of inducing immune response by the same are also provided.
| Inventors: | CHUANG; Tsung-Hsien; (Miaoli County, TW) ; YEH; Da-Wei; (Miaoli County, TW) ; LAI; Chao-Yang; (Miaoli County, TW) ; LIU; Yi-Ling; (Miaoli County, TW) ; LU; Chih-Hao; (Miaoli County, TW) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 65895934 | ||||||||||
| Appl. No.: | 15/722949 | ||||||||||
| Filed: | October 2, 2017 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C12N 15/117 20130101; C12N 2310/17 20130101; A61K 39/39 20130101; A61K 31/711 20130101; A61K 2039/55561 20130101; A61K 31/7088 20130101 |
| International Class: | C12N 15/117 20060101 C12N015/117; A61K 39/39 20060101 A61K039/39 |
Claims
1. A CpG-oligodeoxynucleotide (CpG-ODN) for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host, comprising: one or more copies of the sequence of GTCGTT; one or more copies of the sequence of GTT; and one or more copies of the sequence of TTTT; wherein at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT, and the CpG-ODN comprises the sequence of SEQ ID NO: 1.
2. (canceled)
3. A CpG-oligodeoxynucleotide (CpG-ODN) for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host, comprising: one or more copies of the sequence of GTCGTT; one or more copies of the sequence of GTT; and one or more copies of the sequence of TTTT; wherein at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT, and the CpG-ODN has a length of 19 nucleotides.
4. A CpG-oligodeoxynucleotide (CpG-ODN) for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host, comprising: one or more copies of the sequence of GTCGTT; one or more copies of the sequence of GTT; and one or more copies of the sequence of TTTT; wherein at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT, and the CpG-ODN comprises the sequence of SEQ ID NO: 2 or SEQ ID NO: 3.
5. (canceled)
6. The CpG-ODN as in claim 1, wherein the host is selected from the group consisting of human, aves, rodent and pisces.
7. An immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, comprising: the CpG-ODN as in claim 1; and a vehicle, an excipient or a combination thereof.
8. The immunogenic composition as in claim 7, further comprising an antigen selected from the group consisting of: virus, bacterium, protozoa, and tumor.
9. A method of inducing an immune response of a host for treating or preventing a disorder, comprising: preparing an immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, including an effective dose of a CpG-ODN, wherein the CpG-ODN is as in claim 1; and administrating the immunogenic composition to the host.
10. The method as in claim 9, wherein the host is selected from the group consisting of human, aves, rodent and pisces.
11. The method as in claim 9, wherein the effective dose is 0.01 mg/kg body weight to 20 mg/kg body weight.
12. The method as in claim 9, wherein the disorder is selected from the group consisting of breast cancer, prostate cancer, melanoma, lymphoma, non-small-cell lung cancer, basal cell carcinoma, glioblastoma, ovarian cancer, and an infectious disease induced by hepatitis B virus, B. anthrax, malaria, S. pneumoniae, herpes simplex virus, influenza virus or a combination thereof.
13. The method as in claim 9, wherein the administrating comprises administrating orally or by means of injection.
14. An immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, comprising: the CpG-ODN as in claim 3; and a vehicle, an excipient or a combination thereof.
15. An immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, comprising: the CpG-ODN as in claim 4; and a vehicle, an excipient or a combination thereof.
16. A method of inducing an immune response of a host for treating or preventing a disorder, comprising: preparing an immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, including an effective dose of a CpG-ODN, wherein the CpG-ODN is as in claim 3; and administrating the immunogenic composition to the host.
17. A method of inducing an immune response of a host for treating or preventing a disorder, comprising: preparing an immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, including an effective dose of a CpG-ODN, wherein the CpG-ODN is as in claim 4; and administrating the immunogenic composition to the host.
Description
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001] The present invention relates to a CpG-oligodeoxynucleotide (CpG-ODN), an immunogenic composition including the same and methods of inducing immune response by the same, in particular, to a CpG-ODN comprising a GTCGTT motif for inducing a TLR9 activated immune response and/or a TLR21 activated immune response, an immunogenic composition including the same and a method of activating such immune responses.
2. Description of the Related Art
[0002] CpG-oligodeoxynucleotides (CpG-ODNs) are known as potent immune modulators which induce the production of inflammatory cytokines and a T helper 1 (Th1) polarized immune response, resulting in the expression of costimulatory molecules in antigen-presenting cells and increased activation of B cells, T cells, NK cells and other immunocytes.
[0003] Generally, various CpG-ODNs have species-specific activities based on the compositions and lengths thereof. For instance, CpG-1826 (SEQ ID NO: 4) containing two copies of the GACGTT motif and having a length of 20 nucleotides is more potent in activating murine cells and less effective in activating human cells than CpG-2007 (SEQ ID NO: 11) containing three copies of the GTCGTT motif and having 22 nucleotides. Hence, various kinds of CpG-ODN have been designed and artificially synthesized for inducing the immune responses of a specific receptor in a specific species.
[0004] On the other hand, the choices of the target receptor which is activated by the CpG-ODNs may be also a key point to cost down and popularize the CpG-ODNs based treatment. A large number of researchers have found that using Toll-like receptors (TLRs) as a target induced by the CpG-ODNs in a host may be a better choice because of its key role in the innate immune system. For instance, TLR21 in zebrafish (Danio rerio), pufferfish (Takifugu rubripes) and chicken (Gallus gallus) have shown being able to be activated by CpG-ODNs (Brownlie R. et al. Molecular immunology. 2009; 46(15):3163-70).
[0005] However, different types of TLRs are expressed in different species, it may cause unnecessary cost on the developments of various types of CpG-ODN appropriating to corresponding species. Hence, developing a single type of CpG-ODN that is able to induce a plurality of types of TLRs, that is, is able to induce the immune responses in different kinds of species and has better activation effects will be a substantial solution of the problem.
SUMMARY OF THE INVENTION
[0006] In the present invention, cells of groupers expressing both TLR21 and TLR9, mammals expressing TLR9 are used in the test of activation effects by the CpG-ODNs of the present invention to show that the CpG-ODN is potent to induce the immune responses in different species by activating different types of TLRs.
[0007] In the present invention, a CpG-oligodeoxynucleotide (CpG-ODN) for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host is provided. The CpG-ODN comprises one or more copies of the sequence of GTCGTT, one or more copies of the sequence of GTT and one or more copies of the sequence of TTTT, wherein at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT.
[0008] Preferably, the CpG-ODN comprises the sequence of SEQ ID NO: 1.
[0009] Preferably, the CpG-ODN has a length of 19 nucleotides.
[0010] Preferably, the CpG-ODN comprises the sequence of SEQ ID NO: 2.
[0011] Preferably, the CpG-ODN comprises the sequence of SEQ ID NO: 3.
[0012] Preferably, the host is selected from a group consisting of human, aves, rodent and pisces.
[0013] An immunogenic composition for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof, comprising: the CpG-ODNs mentioned above, and a vehicle, an excipient or a combination thereof.
[0014] Preferably, the immunogenic composition further comprises an antigen selected from the group consisting of: virus, bacterium, protozoa, and tumor.
[0015] Additionally, a method of inducing an immune response of a host for treating or preventing a disorder is provided, comprising: preparing an immunogenic composition mentioned above which includes an effective dose of CpG-ODN of the present invention; and administrating the immunogenic composition to the host.
[0016] Preferably, the host is selected from the group consisting of human, aves, rodent and pisces.
[0017] Preferably, the disorder is selected from the group consisting of breast cancer, prostate cancer, melanoma, lymphoma, non-small-cell lung cancer, basal cell carcinoma, glioblastoma, ovarian cancer, and an infectious disease induced by hepatitis B virus, B. anthrax, malaria, S. pneumoniae, herpes simplex virus, influenza virus or a combination thereof.
[0018] Preferably, the effective dose is 0.01 mg/kg body weight to 20 mg/kg body weight.
[0019] Preferably, the administrating comprises administrating orally or by means of injection.
[0020] Preferably, the CpG-ODN of present invention is able to activate both TLR21 and/or TLR9 in a host, that is, is able inducing immune responses in species of human, aves, rodent, pisces, and other species expressing TLR21 and/or TLR9.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The patent or application file contains at least one drawing executed in color. Copies of this patent or patent application publication with color drawing(s) will be provided by the Office upon request and payment of the necessary fee.
[0022] In the histograms of appended drawings, the statistics information is as follows: Data represent means.+-.SD (n=3 independent experiments).*P<0.05, **P<0.01 indicates in comparison with a control group.
[0023] Further, the sequences of the CpG-ODNs labeled in the drawings are shown in Table 1.
[0024] FIG. 1A shows the computational modeling of the ectodomain protein structures of orange-spotted grouper (osg, E. coioides) and giant grouper (gg, E. lanceolatus) TLR21s. FIGS. 1B-1F show the protein sequence alignment of osgTLR21A, osgTLR21B, and ggTLR21 Toll/interleukin receptor (TIR) domains.
[0025] FIGS. 2A and 2B show the protein identity of the protein sequences between ggTLR21 and various TLRs referring to the information obtained from the GenBank database.
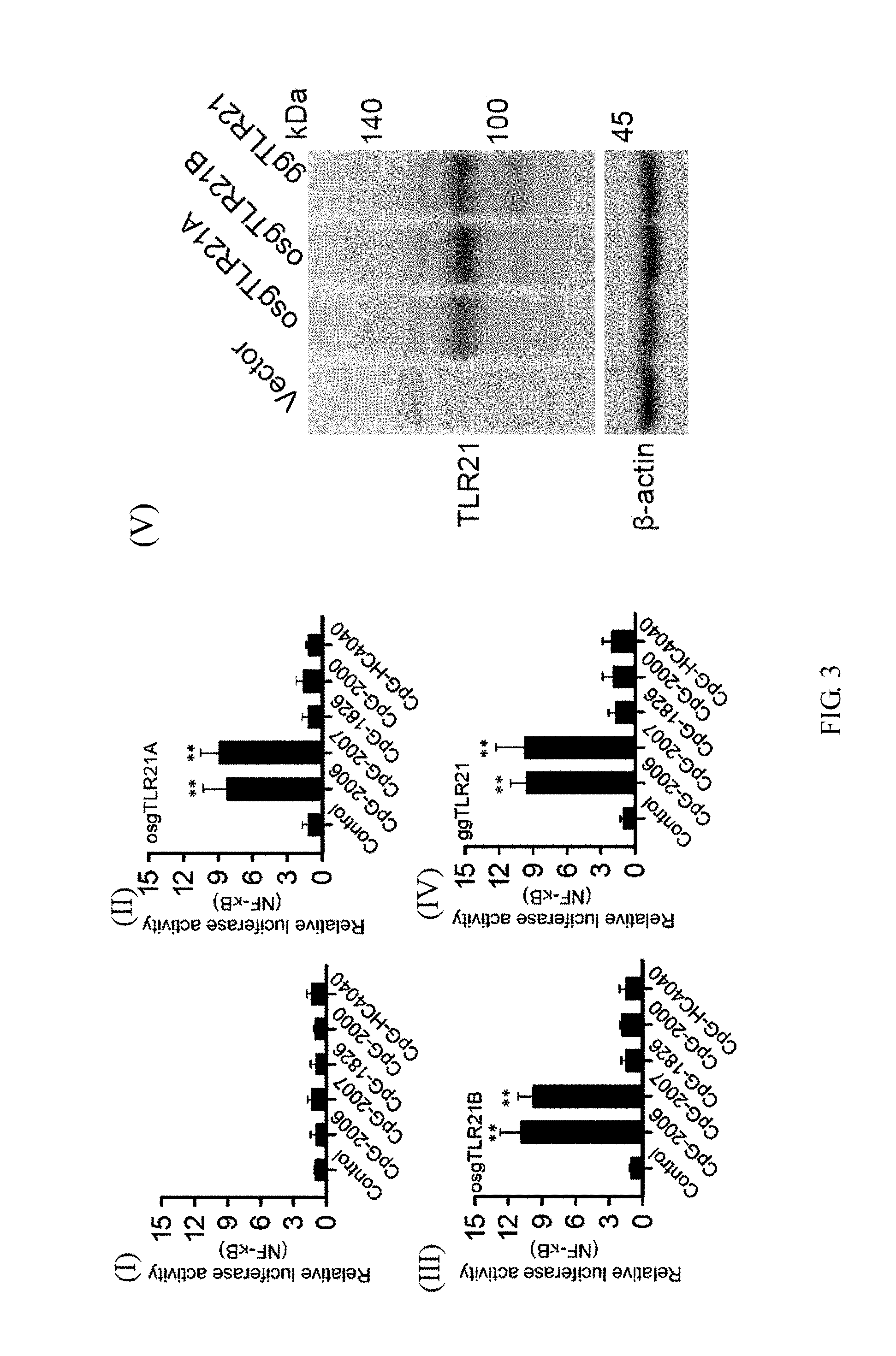
[0026] FIG. 3 shows activation of grouper (Epinephelus spp.) TLR21 s induced by different types of CpG-ODNs, wherein part (V) of FIG. 3 shows immunoblot analysis results of the expression of the grouper TLR21s using .beta.-actin as a loading control.
[0027] FIG. 4 shows histograms illustrating activation of grouper (Epinephelus spp.) TLR21s induced by different types of CpG-ODNs and the trimmed derivatives thereof.
[0028] FIG. 5 shows histograms illustrating activation of grouper (Epinephelus spp.) TLR21s induced by different types of CpG-ODNs and the trimmed derivatives of the CpG-ODNs in FIG. 3 having higher activities.
[0029] FIG. 6 shows histograms illustrating activation of grouper (Epinephelus spp.) TLR21s induced by different types of the trimmed derivatives of the CpG-ODNs in FIG. 3 having higher activities.
[0030] FIG. 7 shows histograms illustrating activation of grouper (Epinephelus spp.) TLR21s induced by different types of the trimmed derivatives of the CpG-ODNs in FIG. 3 having higher activities.
[0031] FIG. 8 shows histograms illustrating activation of grouper (Epinephelus spp.) TLR21s, human TLR9 and mouse TLR9 induced by different types of CpG-ODNs and the trimmed derivatives thereof.
[0032] FIG. 9 shows histograms illustrating induction of different kinds of cytokine expression in orange-spotted grouper (Epinephelus coioides) head kidney cells by different types of CpG-ODNs and the trimmed derivatives thereof.
[0033] FIG. 10 shows histograms illustrating induction of different kinds of cytokine expression in orange-spotted grouper (Epinephelus coioides) splenocytes by different types of CpG-ODNs and the trimmed derivatives thereof.
[0034] FIG. 11 shows histograms illustrating induction of different kinds of cytokine production in human peripheral blood mononuclear cells (PBMCs) by different types of CpG-ODNs and the trimmed derivatives thereof.
[0035] FIG. 12 shows histograms illustrating induction of different kinds of cytokine production in mouse splenocytes by different types of CpG-ODNs and the trimmed derivatives thereof.
[0036] FIG. 13 shows histograms illustrating induction of different kinds of cytokine production in mouse bone marrow-derived macrophages (BMDMs) by different types of CpG-ODNs and the trimmed derivatives thereof.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0037] For the ease of realizing the technical features, contents, advantages and effects of the present invention by examiners, a detailed description of the embodiments, the appended drawings and tables of the present invention will be stated hereinafter. The drawings are merely used to illustrate and support the content of the specification and are not intended to be limiting in any way regarding the implementation of the present invention that must be explained first.
[0038] Methods and procedures of preparing and obtaining materials used in the implementation and used for prove the substantial effects of the present invention will be described below.
[0039] In the embodiments of the present invention, head kidney cells and splenocytes of groupers were isolated according to the procedures as follows. Orange-spotted groupers and giant groupers are anesthetized in water containing 0.2 g/L Tricaine, and the head kidneys and spleens thereof are aseptically removed. The organs were then gently minced and passed through a 70-.mu.m strainer with homogenization buffer (standard Hank's balanced salt solution (sHBSS) supplemented with 15 mM HEPES, 10% fetal bovine serum (FBS), 1.times. Antibiotic-Antimycotic, and 50 U/ml heparin). Subsequently, 1 ml of the homogenized tissue suspension is placed into 8 ml of distilled water and 1 ml of 10.times. phosphate-buffered saline (PBS). Following the removal of gross debris, the cell suspension was centrifuged and washed with 1.times.PBS twice. The cell pellet was then resuspended in Leibovitz's L-15 cell culture medium (Gibco, Carlsbad, Calif., USA).
[0040] Bone marrow-derived macrophages (BMDMs) and splenocytes of C57BL/6 mice were collected and cultured according to the procedures as follows. In terms of BMDMs, mouse bone marrow cells from mouse tibias and femurs were cultured in complete Dulbecco's Modified Eagle Medium (DMEM) with 30% L929 conditioned medium for 5 days, followed by DMEM medium with 10% FBS. In terms of splenocytes, they were cultured in RPMI1640 medium with 10% FBS after obtained. Furthermore, human peripheral blood mononuclear cells (PBMCs) were cultured in RPMI1640 medium with 10% FBS.
[0041] TLR21 cDNA of giant groupers (ggTLR21 cDNA) were molecular cloned by procedures as follows. Total RNAs were purified from grouper splenocytes using TRIzol, and first-strand cDNA libraries were then prepared using the SuperScript.RTM. III First-Strand Synthesis System (Invitrogen, Carlsbad, Calif., USA), which is known in prior arts (Yeh D. W. et al. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110(51):20711-6). To clone ggTLR21 cDNA, a pair of forward and reverse primers (5'-GAACAGATTCCTGTACCATGTTCATC-3' (SEQ ID NO: 51) and 5'-GCTTGTATGAATTGTCACACTGCAC-3' (SEQ ID NO: 52) was designed based on the sequences of the 5'- and 3'-untranslated regions of TLR 21 of orange-spotted groupers (osgTLR21) (GenBank: GU198366.2). A ggTLR21 cDNA containing both of the 5'- and 3'-untranslated regions and the complete coding region was cloned from the prepared giant grouper spleen first-strand cDNA library using polymerase chain reaction (PCR) amplification. Further, the cloned ggTLR21 cDNA sequence is submitted to the GenBank database (accession number: KM024068).
[0042] Multiple alignments of the amino acid sequences of the TLR21s were performed using ClustalW2. The structural model of the TLR21 protein was predicted with SWISS MODEL, using TLR13 as a template.
[0043] The expression constructs for osgTLR21s and ggTLR21 were generated through PCR amplification of the corresponding protein-coding regions from the generated first-strand cDNA libraries derived from the orange spotted and giant grouper spleens. The forward and reverse primers based on the 5'- and 3'-end cDNA sequences for the coding region of osgTLR21 (GU198366.2) and ggTLR21 (KM024068) are designed and subcloned the amplified DNA fragments into a PEF6 vector in frame with a FLAG tag at their C-terminal ends. The expression vectors for human and mouse TLR9 were generated following previously described methods (Liu J. Comparative immunology, microbiology and infectious diseases. 2012; 35(5):443-51).
[0044] In the embodiments of present invention, TLR21 and TLR9 activation assays are performed according to the procedures as follows. Human embryonic kidney (HEK) 293 cells were grown in DMEM supplemented with 10% FBS. Then, the procedures stated in previous art (Yeh D. W. et al. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110(51):20711-6) were performed. Relative luciferase activities were calculated as the fold change compared with an unstimulated control.
[0045] Reverse transcription-quantitative PCR (RT-qPCR) analysis of gene expression was performed according to the procedures as follows. Cells were treated with different CpG-ODNs for 4 h. Then, the total RNA is purified using TRIzol and the transcription is performed using, for instance, the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, Calif., USA). Further, RT-qPCR was carried out using appropriate detection methods or kits, for instance, an ABI PRISM 790 reverse 0HT Sequence Detection System and KAPA SYBR.RTM. fast qPCR kit (KK4605) for gene expression analysis. The expression of mRNA was normalized to .beta.-actin. as below. In Table 1, the sequences of comparative examples are used for explaining the preparation and for comparing with the CpG-ODN of the examples (SEQ ID NOs: 2-3) of the present invention so as to show difference on their effects. Further, the primer sequences used in RT-qPCR are listed in Tables 2 and 3.
TABLE-US-00001 TABLE 1 CpG-ODN SEQUENCE Comparative examples CpG-1826 TCCATGACGTTCCTGACGTT (SEQ ID NO: 4) CpG-2000 TCCATGACGTTCCTGCAGTTCCTGACGTT (SEQ ID NO: 5) CpG-HC4040 TGACTGTGAACGTTCGAGATGA (SEQ ID NO: 6) CpG-2006 TCGTCGTTTTGTCGTTTTGTCGTT (SEQ ID NO: 7) CpG-261 TCGTCGTTTTGTCGTT (SEQ ID NO: 8) CpG-262 GTTTTGTCGTTTTGTCGTT (SEQ ID NO: 9) CpG-263 TCGTTTTGTCGTTTTG (SEQ ID NO: 10) CpG-2007 TCGTCGTTGTCGTTTTGTCGTT (SEQ ID NO: 11) CpG-271 TCGTCGTTGTCGTTTTGT (SEQ ID NO: 12) CpG-272 GTTGTCGTTTTGTCGTT (SEQ ID NO: 13) CpG-273 TCGTTGTCGTTTTGT (SEQ ID NO: 14) CpG-2721 GTTGTCGTTGTCGTT (SEQ ID NO: 15) CpG-2723 GTGTCGTTTTGTCGTT (SEQ ID NO: 16) CpG-2724 GTTGTCGTTTTGTC (SEQ ID NO: 17) CpG-2725 GTTGTCGTTTCC (SEQ ID NO: 18) CpG-2726 GTTGTGCTTTTTTGTCGTT (SEQ ID NO: 19) CpG-2728 GTTGTGCTTTTTTGTGCTT (SEQ ID NO: 20) CpG-2729 (SEQ ID NO: 21) GTCGTTTTTTGTCGTT CpG-2730 (SEQ ID NO: 22) GTTGTCGTTTTTT Examples of embodiments CpG-2731 GTCGTTTTTT (SEQ ID NO: 1) CpG-2722 GTTGTCGTTTTTTGTCGTT (SEQ ID NO: 2) CpG-2727 GTTGTCGTTTTTTGTGCTT (SEQ ID NO: 3)
TABLE-US-00002 TABLE 2 Grouper primers used in RT-qPCR .beta.-actin forward 5'-GACATGGTGCGGTTTCTCTT-3' (SEQ ID NO: 23) reverse 5'-GCCTCTGCTGTGCTGATGTA-3' (SEQ ID NO: 24) TNF-.alpha. forward 5'-GGATCTGGCGCTACTCAGAC-3' (SEQ ID NO: 25) reverse 5'-TCCGATAGCTGGTTGGTTTC-3' (SEQ ID NO: 26) IL-1.beta. forward 5'-GACATGGTGCGGTTTCTCTT-3' (SEQ ID NO: 27) reverse 5'-GCCTCTGCTGTGCTGATGTA-3' (SEQ ID NO: 28) IL-6 forward 5'-CCTGAAGGACCTCGACAATC-3' (SEQ ID NO: 29) reverse 5'-TCCTGACAGCCAGACTTCCT-3' (SEQ ID NO: 30) IL-8 forward 5'-GAGCTGCACTGTCGCTGTAT-3' (SEQ ID NO: 31) reverse 5'-TGTTGGCCATGATCCTGTTA-3' (SEQ ID NO: 32) Mx forward 5'-CCATCTGACGCAACTGAGAA-3' (SEQ ID NO: 33) reverse 5'-TCCACCTCGCAAACTCTCTT-3' (SEQ ID NO: 34) IFN1 forward 5'-CTGTGTCCTTCCCGAATCAT-3' (SEQ ID NO: 35) reverse 5'-TGCACAGTACAGGAGCGAAG-3' (SEQ ID NO: 36) IFN forward 5'-GACCACCAAGATGGAGGCTA-3' gamma (SEQ ID NO: 37) reverse 5'-TACCGGTGTTTCCTCAGGTC-3' (SEQ ID NO: 38) CCL4 forward 5'-GTGGTACTGGCCCAAAGAAA-3' (SEQ ID NO: 39) reverse 5'-GGCTGAAGGTCTGACACACA-3' (SEQ ID NO: 40)
TABLE-US-00003 TABLE 3 Mouse primers used in RT-qPCR gapdh forward 5'-ACCCAGAAGACTGTGGATGG-3' (SEQ ID NO: 41) reverse 5'-CACATTGGGGGTAGGAACAC-3' (SEQ ID NO: 42) tnf-.alpha. forward 5'-GGATCTGGCGCTACTCAGAC-3' (SEQ ID NO: 43) reverse 5'-TCCGATAGCTGGTTGGTTTC-3' (SEQ ID NO: 44) il-1.beta. forward 5'-CAGGCAGGCAGTATCACTCA-3' (SEQ ID NO: 45) reverse 5'-AGCTCATATGGGTCCGACAG-3' (SEQ ID NO: 46) il-6 forward 5'-AGTTGCCTTCTTGGGACTGA-3' (SEQ ID NO: 47) reverse 5'-TCCACGATTTCCCAGAGAAC-3' (SEQ ID NO: 48) cxcl1 forward 5'-GCTGGGATTCACCTCAAGAA-3' (SEQ ID NO: 49) reverse 5'-CTTGGGGACACCTTTTAGCA-3' (SEQ ID NO: 50)
[0046] Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis are performed according to the procedures as follows. Cells were lysed with lysis buffer (100 mM NaCl, 50 mM Tris-Cl (pH 7.5), 0.5 mM ethylenediaminetetraacetic acid (EDTA), 1% octylphenoxypolyethoxyethanol (NP-40)) containing complete protease inhibitor cocktail (Roche Life Science, Indianapolis, Ind., USA). Then, cell lysates were separated by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. Subsequently, membranes were incubated with the indicated antibody and then with horseradish peroxidase (HRP)-conjugated secondary antibody of which the immunoreactive bands were visualized using chemiluminescent HRP substrate (Immobilon Western; Millipore, Temecula, Calif., USA) and the UVP BioSpectrum Imaging System.
[0047] Human cytokine production results were measured by the procedures as follows. PBMCs were treated with different CpG-ODNs for 24 h, and then the culture supernatant is collected for cytokine measurement. The production of tumor necrosis factor (TNF)-.alpha., interleukin (IL)-1.beta., IL-12p70, and interferon (IFN)-.gamma. were measured using ELISA Kits.
[0048] Furthermore, all of the results shown in the present disclosure are expressed as the mean.+-.SD of three independent experiments. Statistical analyses were performed using Student's t-test with a significance level of P<0.05.
[0049] Here, several embodiments will be described with the results and the appended drawings for of the present invention for ease of realizing the effects of the CpG-ODNs provided by the present invention.
[0050] In an embodiment of the present invention, ggTLR21 may be cloned according to the methods mentioned above. The cDNA from osgTLR21A and osgTLR21B may be cloned according to the previous publication (Li Y. W. et al. Cryptocaryon irritans infection. Fish & shellfish immunology. 2012; 32(3):476-81). The main difference between their encoded protein sequences is the absence of four amino acid residues at the C-terminal end of osgTLR21B, as shown in FIGS. 1B-1F. Based on the expected high identity between nucleotide sequences of the same gene in giant grouper and orange-spotted grouper, the inventor designs two primers based on the 5'- and 3'-untranslated regions of osgTLR21 to clone ggTLR21. This results in a full-length ggTLR21 cDNA which is homologous to osgTLR21A but not osgTLR21B.
[0051] Then, the characteristics of the grouper TLR21s used in the present invention may be analyzed. To compare the grouper TLR21s with other TLRs, the inventor aligns their protein sequences with those of different zebrafish TLRs using ClustalW2 and constructed an evolution tree as shown in FIG. 2A, wherein the proteins sequences and labels thereof are obtained from the GenBank database. As results, the protein identity was 98.26% between ggTLR21 and osgTLR21A, 98.15% between ggTLR21 and osgTLR21B, 54.44% between zebrafish (zeb) TLR21 and ggTLR21, and 28.30% between zebTLR22 and ggTLR21A. TLR22 was closest to TLR21 in the evolution tree, with TLR13 and TLR20f the next closest.
[0052] Further, as shown in FIG. 2B, phylogenetic analysis using the protein sequences of TLR21s from different fish and avian species show that the grouper TLR21s were most closely related to Oplegnathus fasciatus TLR21, followed by Paralichthys olivaceus and Scophthalmus maximus TLR21s, and were more distantly related to chicken (Gullas gullas), duck (Anas platyrhynchos), and goose (Anser cygnoides) TLR21s.
[0053] On the other hand, the sequences and structures of TLR21s between difference species of groupers may be also analyzed. As results, ggTLR21 contains 979 amino acid residues, while osgTLR21A and osgTLR21B contain 979 and 975, respectively. Alignment of these three protein sequences shows that they contain an extracellular domain (ectodomain), a transmembrane domain, and a Toll/IL-1 (TIR) cytosolic domain. They also have 23 copies of leucine-rich repeats (LRRs) and a C-terminal leucine-rich repeat (LRR-CT) in their ectodomain. Only 14 of 741 amino acid residues are differed between the ectodomain of osgTLR21 and ggTLR21. Several three-dimensional structures of different TLR ectodomains have been resolved in prior arts, of which TLR13 is phylogenetically closest to TLR21. Therefore, the inventor predicts the three-dimensional structures of the osgTLR21 and ggTLR21 ectodomains with SWISS MODEL software using TLR13 as a template to further examine their difference. This shows that their ectodomains are relatively similar to horseshoe-shaped solenoid three-dimensional structures, except for the extrusion of a small helix structure at the 315-330 amino acid regions in osgTLR21, as shown in FIG. 1A in which from left to right is the result of predicted ectodomain structure of osgTLR21 (black), ggTLR21 (white), and superimposition (black: osgTLR21; white: ggTLR21) of these two ectodomains. (N: N-terminal end, C: C-terminal end of the ectodomain). Referring to FIGS. 1B-1F, the three motifs boxes 1-3 that are required for signal transduction of mammalian TLRs are conserved in the TIR domains of all three grouper TLR21s. However, osgTLR21B differs from osgTLR21A and ggTLR21 in that it lacks a four amino acid residue region at the C-terminal end after box 3 (Asterisk, identical residues; single dot, conservative substitutions; two dots, highly conservative substitutions).
[0054] Subsequently, to investigate whether the structural differences between these grouper TLR21s translates into differences in their activity in response to different types of CpG-ODNs stimulation, the inventor co-transfects HEK293 cells with the expression vector for these TLR21s and the nuclear factor (NF)-.kappa.B controlled luciferase reporter gene to establish a cell-based activation assay. Then, the inventor treats these cells with CpG-ODNs with different hexamer motifs and sequences, as shown in Table 1, and measures the induced luciferase reporter activities. As shown in parts (I) to (IV) of FIG. 3, it was found that osgTLR21A, osgTLR21B, and ggTLR21 experience the same level of activation by CpG-ODNs containing GTCGTT hexamer motifs (i.e., CpG-2006 [SEQ ID NO: 7] and CpG-2007), but do not respond to CpG-ODNs containing GACGTT and AACGTT hexamer motifs (i.e., CpG-1826, CpG-2000 [SEQ ID NO: 5], and CpG-HC4040 [SEQ ID NO: 6]). The results suggest that all three grouper TLRs are functional and these minor structural differences do not significantly affect their response to CpG-ODN stimulation.
[0055] According to the above results, in an attempt to develop CpG-ODNs for strong activation of grouper TLR21s, the inventor trims the length of CpG-2006 and CpG-2007 to retain only the left or right two of the three GTCGTT hexamer motifs to generate CpG-261 (SEQ ID NO: 8) and CpG-271 (SEQ ID NO: 12), and CpG-262 (SEQ ID NO: 9) and CpG-272 (SEQ ID NO: 13), respectively. Further, the inventor also trims their length to retain only the middle copy (Table 1). Then, the activities of these CpG-ODNs are determined by an osgTLR21 and ggTLR21 cell-based activation assay and being compared with CpG-2006 and CpG-2007. As results shown in parts (I) to (III) of FIG. 4, it was found that CpG-272 has the best activities toward the osgTLR21s and ggTLR21 of the hexamer motif to generate CpG-263 (SEQ ID NO: 10) and CpG-273 (SEQ ID NO: 14). Therefore, inventor further hexamer motifs to generate CpG-2721 (SEQ ID NO: 15), CpG-2722 (SEQ ID NO: 2), CpG-2723 (SEQ ID NOs: 16), CpG-2724 (SEQ ID NO: 17) and CpG-2725 (SEQ ID NO: 18) (Table 1). A further cell-based assay was performed and the results indicate that CpG-2722 has the best activity toward the osgTLR21s and ggTLR21, as shown in part (I) to (III) of FIG. 5.
[0056] Since the CpG-hexamer motifs activating the grouper TLR21s were found in above results, the inventor further investigates whether the number of CpG-hexamer motifs and structure of the CpG-2722 influence the activation of grouper TLR21s. In the above results, CpG-2722 which contains two copies of the GTCGTT hexamer motif in a length of nineteen nucleotides has the best activity. To determine whether both copies are required for its activity, the inventor reverses the CpG-dideoxynucleotides in its 5'- and 3'-CpG-hexamer motif to generate CpG-2726 (SEQ ID NO: 19), CpG-2727 (SEQ ID NO: 3) and CpG-2728 (SEQ ID NO: 20) (Table 1). As the results shown in part (I) to (III) of FIG. 6 activation assays of from osgTLR21 and ggTLR21 indicated that the activity of CpG-2727 is as good as that of CpG-2722, whereas CpG-2726 and CpG-2728 were unable to activate the osgTLR21s and ggTLR21 grouper TLR21s. These findings suggest that only one copy of the 5'-CpG-hexamer motif is required for CpG-2722 activity. The CpG-2722 was further trimmed to generate CpG-2729 (SEQ ID NO: 21) by removing three nucleotides from 5'-end, CpG-2730 (SEQ ID NO: 22) by removing the 3'-CpG-hexamer motif, and CpG-2731 (SEQ ID NO: 1) by removing both of the three nucleotides from 5'-end and the 3'-CpG-hexamer motif (Table 1).
[0057] Furthermore, the activities of these CpG-ODNs were tested and the results are shown as in parts (I) to (III) of FIG. 7. The results showed that the three 5'-end nucleotides were required for the CpG-2722 to strongly active both of the osgTLR21 and ggTLR21, and although the 3'-CpG-hexamer motif is not required for the activity of CpG-2722, these nucleotides are required to maintain the nineteen nucleotide length of CpG-2722 for its activity.
[0058] Additionally, as shown in parts (I) to (IV) of FIG. 8, the inventor tests the activities of CpG-2722 and CpG-2727 on human (h) and mouse (m) TLR9s to determine whether they are specific to grouper TLR21s. Interestingly, in the hTLR9 activation assay, according to the results, it was found that the activities of CpG-2722 and CpG-2727 were as good as those of CpG-2006 and CpG-2007, which have been optimized for the activation of human cells. Furthermore, in the mTLR9 activation assay, although the activities of CpG-2722 and CpG-2727 were not as good as that of CpG-1826, which has been optimized for the activation of mouse cells, both had better activities toward mTLR9 than CpG-2006 and CpG-2007.
[0059] Further, the inventor investigates the effects of CpG-2722 and CpG-2727 on the induction of cytokine productions in grouper, human, and mouse cells to compare their activities on these cells with that showed on the cell-based activation assays in parts (I) to (IV) of FIG. 8. Head kidney cells and splenocytes are purified from orange-spotted groupers, treated with different CpG-ODNs for determining the induction of the cytokines IL-1.alpha., IL-6, IL-8, and IFN.gamma.. In consistent with results obtained from the cell based assays as show in parts (I) to (IV) of FIG. 9 and parts (I) to (IV) of FIG. 10, it was found that CpG-2722 and CpG-2727 have greater effects on the activation of cytokine production in orange-spotted grouper cells than CpG-1826, CpG-2006, and CpG-2007. In addition, an ELISA analysis is also performed to show that CpG-2722 and CpG-2727 are as potent as CpG-2006 and CpG-2007 in inducing cytokine production in human PBMCs, whereas CpG-1826 had weak activity on these cells, as shown in parts (I) to (IV) of FIG. 11. Furthermore, CpG-2722 and CpG-2727 were found more potent than the CpG-2006 and 2007 in inducing cytokine expression in mouse splenocytes and BMDMs as the results shown in parts (I) to (IV) of FIG. 12 and parts (I) to (IV) of FIG. 13. These findings suggest that CpG-2722 and CpG-2727, which were developed for grouper TLR21s, also effectively activate immune responses in human and mouse cells.
[0060] According to the embodiments and results stated above, more details of the present invention may be described and explained in following paragraphs.
[0061] Generally, TLR9 and TLR21 are the cellular receptors for CpG-ODNs. TLR9 is expressed in both mammalian and fish species, and has been better studied in terms of the structural requirements for CpG-ODN to strongly and species-specifically activate this TLR. CpG-ODNs that have been optimized for humans are currently being investigated for their application as vaccine adjuvants, as well as immunotherapies for allergies, infectious diseases, and cancers. Similarly, CpG-ODNs have also been shown to exert potent immunostimulatory activities in chicken, duck, and fish, which contain TLR21 in previous arts, suggesting potential veterinary uses of CpG-ODNs as immune modulators and vaccine adjuvants. However, few studies have investigated the structural requirements for CpG-ODN to activate TLR21, and it remains unclear whether there is a common structure that will activate both TLR9 and TLR21. Therefore, in the present invention, the inventor develops CpG-ODNs for strong activation of TLR21s in grouper, which are considered an important aquaculture species in Asia due to their rapid growth and good price, and investigated the interaction between CpG-ODNs, grouper TLR21s, and human and mouse TLR9s.
[0062] The inventor found that CpG-ODNs that contained the GTCGTT motif, such as CpG-2006 and CpG-2007, may activates grouper TLR21s, whereas those that contained the GACGTT and AACGTT motifs, such as CpG-1826, CpG-2000, and HC4040, does not. Similarly, it has previously been shown that zebTLR9s broadly recognize CpG-ODNs with different CpG-hexamer motifs, with those containing the GACGTT or AACGTT motifs having better activity toward TLR9, and with the GTCGTT motif having better activity toward zebTLR21 (Yeh D. W. et al. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110(51):20711-6). Thus, the grouper TLR21s have a similar CpG-hexamer recognition profile to zebTLR21.
[0063] In the present invention, two CpG-ODNs that have better activities toward grouper TLR21s than CpG-2006 and CpG-2007 are provided. CpG-2722 has a length of 19 deoxynucleotides and contains two GTCGTT motifs with four spacing nucleotides between them, while CpG-2727 is a modification of CpG-2722 in which the CpG-dideoxynucleotides in the GTCGTT motif has been reversed. Therefore, the good activity of CpG-2722 suggests that one copy of the GTCGTT motif is sufficient for strong activation of grouper TLR21s. In a previous study that developed CpG-ODNs that strongly activate rabbit TLR9, it was found that the length of a CpG-ODN also affects its activity (Chuang T H, et al. PloS one. 2014; 9(9):e108808). Thus, the fact that CpG-2722 and CpG-2727 contain the same type of CpG-hexamer as CpG-2006 and CpG-2007, but have different lengths and numbers of the CpG hexamer suggests that the key structural elements for designing CpG-ODNs to strongly activate TLR21 are the same as those for TLR9.
[0064] In the embodiments and results stated above, the activities of CpG-2722 and CpG-2727 in cells isolated from the head kidneys and spleens of orange-spotted groupers, which should contain both TLR9 and TLR21, are also investigated. This shows that although the activation profile is not entirely the same as for grouper TLR21s, CpG-2722 and CpG-2727 exhibits a greater effect on cytokine production in these cells than CpG-2006, CpG-2007, and CpG-1826. Thus, the CpG-2722 and CpG-2727 may also have good activity toward grouper TLR9, and it appears that both TLR9 and TLR21 cooperatively mediate the immunostimulatory activities of CpG-ODNs in groupers, as previously demonstrated for zebTLR9 and zebTLR21 by CpG-ODNs with the GTCGTT motif (Yeh D. W. et al. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110(51):20711-6). Alternatively, this may suggest that TLR21 is the major receptor for CpG-ODNs in grouper cells.
[0065] On other hand, human and mouse TLR9s have been shown to have species-specific ligand recognition properties, with human TLR9 being strongly activated by CpG-2006 and CpG-2007, but weakly activated by CpG-1826, and mouse TLR9 being preferentially activated by CpG-1826. Here, the inventor found that the activities of CpG-2722 and CpG-2727 were as strong as those of CpG-2006 and CpG-2007 in terms of the activation of human TLR9 and cytokine production in human PBMCs. Furthermore, these two CpG-ODNs also have better effects than CpG-2006 and CpG-2007 on the activation of mouse TLR9 and cytokine production in mouse cells. This suggests that the structures of these two developed CpG-ODNs can better override the specific-specific ligand recognition properties of human and mouse TLR9s, and also share a common structure for the activation of TLR9s and TLR21s from different species. Thus, these two CpG-ODNs may have potential for use as immunomodulatory or vaccine adjuvants in a range of species.
[0066] In summary, the present invention provides CpG-ODNs for inducing a TLR9 activated immune response, a TLR21 activated immune response or a combination thereof in a host, wherein the CpG-ODNs comprises one or more copies of the sequence of GTCGTT, one or more copies of the sequence of GTT and one or more copies of the sequence of TTTT. Further, at least one copy of the sequence of GTCGTT is encoded between the sequence of GTT and the sequence of TTTT.
[0067] Preferably, the sequence of TTTT may be encoded right after the GTCGTT, that is, may be the sequence of GTCGTTTTTT (SEQ ID NO: 1).
[0068] Preferably, the CpG-ODNs having better activities toward TLR9 and TLR21 are CpG-2722 (SEQ ID NO: 2) and CpG-2727 (SEQ ID NO: 3) which show better efficiencies in the results described above.
[0069] The host of which the immune responses is being to be activated by the CpG-ODNs provided by the present invention may be species expressing TLR9 and/or TLR21 such as human, aves, rodent and pisces or any other appropriate species.
[0070] Also, the CpG-ODNs of the present invention may be combined with a vehicle, and/or an excipient or any other pharmaceutically acceptable additives as an immunogenic composition to improve the practicality or biocompatibility thereof.
[0071] Preferably, the immunogenic composition may also be combined with an antigen to achieve the improvement of the treatment, specific targeting, or any other additional functions. Preferably, the antigen may be HBsAg, listeriolysin O, apical membrane antigen 1, MART1, and leishmanial.
[0072] The CpG-ODNs and the immunogenic compositions of the present invention may also be used in a method of activating an immune response of a host. The method may comprise steps of preparing an effective dose of the CpG-ODN or an immunogenic compositions containing effective dose of the CpG-ODN and administrating the prepared products into a host.
[0073] In the method of activating an immune response of a host of the present invention, the effective dose may preferably be 0.01 mg/kg to 20 mg/kg, more preferably 0.1 mg/kg to 20 mg/kg, 0.2 mg/kg to 10 mg/kg, 0.5 mg/kg to 5 mg/kg.
[0074] Preferably, the host of the present invention may be a living subject under a demand of a treatment or prevention of a disorder comprising a cancer, such as breast, prostate, melanoma, lymphoma, non-small-cell lung cancer, basal cell carcinoma, glioblastoma, and ovarian cancer and an infectious disease such as induced by hepatitis B virus, B. anthrax, malaria, S. pneumoniae, herpes simplex virus, and influenza virus.
[0075] Further, in the method of the present invention, the administrating may be administrating orally, by means of an injection or any other general administration methods.
[0076] While the means of specific embodiments in present invention has been described by reference drawings, numerous modifications and variations could be made thereto by those skilled in the art without departing from the scope and spirit of the invention set forth in the claims. The modifications and variations should in a range limited by the specification of the present invention.
Sequence CWU 1
1
52110DNAArtificial SequenceAn aspect of the CpG-ODN of the present
invention. 1gtcgtttttt 10219DNAArtificial SequenceAn aspect of the
CpG-ODN of the present invention. 2gttgtcgttt tttgtcgtt
19319DNAArtificial SequenceAn aspect of the CpG-ODN of the present
invention 3gttgtcgttt tttgtgctt 19420DNAArtificial
SequenceCpG-1826, a comparative example of the present invention.
4tccatgacgt tcctgacgtt 20529DNAArtificial SequenceCpG-2000, a
comparative example of the present invention. 5tccatgacgt
tcctgcagtt cctgacgtt 29622DNAArtificial SequenceCpG-HC4040, a
comparative example of the present invention. 6tgactgtgaa
cgttcgagat ga 22724DNAArtificial SequenceCpG-2006, a comparative
example of the present invention. 7tcgtcgtttt gtcgttttgt cgtt
24816DNAArtificial SequenceCpG-261, a comparative example of the
present invention. 8tcgtcgtttt gtcgtt 16919DNAArtificial
SequenceCpG-262, a comparative example of the present invention.
9gttttgtcgt tttgtcgtt 191016DNAArtificial SequenceCpG-263, a
comparative example of the present invention. 10tcgttttgtc gttttg
161122DNAArtificial SequenceCpG-2007, a comparative example of the
present invention. 11tcgtcgttgt cgttttgtcg tt 221218DNAArtificial
SequenceCpG-271, a comparative example of the present invention.
12tcgtcgttgt cgttttgt 181317DNAArtificial SequenceCpG-272, a
comparative example of the present invention. 13gttgtcgttt tgtcgtt
171415DNAArtificial SequenceCpG-273, a comparative example of the
present invention. 14tcgttgtcgt tttgt 151515DNAArtificial
SequenceCpG-2721, a comparative example of the present invention.
15gttgtcgttg tcgtt 151616DNAArtificial SequenceCpG-2723, a
comparative example of the present invention. 16gtgtcgtttt gtcgtt
161714DNAArtificial SequenceCpG-2724, a comparative example of the
present invention. 17gttgtcgttt tgtc 141812DNAArtificial
SequenceCpG-2725, a comparative example of the present invention.
18gttgtcgttt cc 121919DNAArtificial SequenceCpG-2726, a comparative
example of the present invention. 19gttgtgcttt tttgtcgtt
192019DNAArtificial SequenceCpG-2728, a comparative example of the
present invention. 20gttgtgcttt tttgtgctt 192116DNAArtificial
SequenceCpG-2729, a comparative example of the present invention.
21gtcgtttttt gtcgtt 162213DNAArtificial SequenceCpG-2730, a
comparative example of the present invention. 22gttgtcgttt ttt
132320DNAArtificial SequenceA forward primer of grouper ]-actin
used in qRT-PCR. 23gacatggtgc ggtttctctt 202420DNAArtificial
SequenceA reverse primer of grouper ]-actin used in qRT-PCR.
24gcctctgctg tgctgatgta 202520DNAArtificial SequenceA forward
primer of grouper TNF-\ used in qRT-PCR. 25ggatctggcg ctactcagac
202620DNAArtificial SequenceA reverse primer of grouper TNF-\ used
in qRT-PCR. 26tccgatagct ggttggtttc 202720DNAArtificial SequenceA
forward primer of grouper IL-1] used in qRT-PCR. 27gacatggtgc
ggtttctctt 202820DNAArtificial SequenceA reverse primer of grouper
IL-1] used in qRT-PCR. 28gcctctgctg tgctgatgta 202920DNAArtificial
SequenceA forward primer of grouper IL-6 used in qRT-PCR.
29cctgaaggac ctcgacaatc 203020DNAArtificial SequenceA reverse
primer of grouper IL-6 used in qRT-PCR. 30tcctgacagc cagacttcct
203120DNAArtificial SequenceA forward primer of grouper IL-8 used
in qRT-PCR. 31gagctgcact gtcgctgtat 203220DNAArtificial SequenceA
reverse primer of grouper IL-8 used in qRT-PCR. 32tgttggccat
gatcctgtta 203320DNAArtificial SequenceA forward primer of grouper
Mx used in qRT-PCR. 33ccatctgacg caactgagaa 203420DNAArtificial
SequenceA reverse primer of grouper Mx used in qRT-PCR.
34tccacctcgc aaactctctt 203520DNAArtificial SequenceA forward
primer of grouper IFN1 used in qRT-PCR. 35ctgtgtcctt cccgaatcat
203620DNAArtificial SequenceA reverse primer of grouper IFN1 used
in qRT-PCR. 36tgcacagtac aggagcgaag 203720DNAArtificial SequenceA
forward primer of grouper IFN gamma used in qRT-PCR. 37gaccaccaag
atggaggcta 203820DNAArtificial SequenceA reverse primer of grouper
IFN gamma used in qRT-PCR. 38taccggtgtt tcctcaggtc
203920DNAArtificial SequenceA forward primer of grouper CCL4 used
in qRT-PCR. 39gtggtactgg cccaaagaaa 204020DNAArtificial SequenceA
reverse primer of grouper CCL4 used in qRT-PCR. 40ggctgaaggt
ctgacacaca 204120DNAArtificial SequenceA forward primer of mouse
gapdh used in qRT-PCR. 41acccagaaga ctgtggatgg 204220DNAArtificial
SequenceA reverse primer of mouse gapdh used in qRT-PCR.
42cacattgggg gtaggaacac 204320DNAArtificial SequenceA forward
primer of mouse tnf-\ used in qRT-PCR. 43ggatctggcg ctactcagac
204420DNAArtificial SequenceA reverse primer of mouse tnf-\ used in
qRT-PCR. 44tccgatagct ggttggtttc 204520DNAArtificial SequenceA
forward primer of mouse il-1] used in qRT-PCR. 45caggcaggca
gtatcactca 204620DNAArtificial SequenceA reverse primer of mouse
il-1] used in qRT-PCR. 46agctcatatg ggtccgacag 204720DNAArtificial
SequenceA forward primer of mouse il-6 used in qRT-PCR.
47agttgccttc ttgggactga 204820DNAArtificial SequenceA reverse
primer of mouse il-6 used in qRT-PCR. 48tccacgattt cccagagaac
204920DNAArtificial SequenceA forward primer of mouse cxcl1 used in
qRT-PCR. 49gctgggattc acctcaagaa 205020DNAArtificial SequenceA
reverse primer of mouse cxcl1 used in qRT-PCR. 50cttggggaca
ccttttagca 205126DNAArtificial SequenceA forward primer for cloning
ggTLR21 cDNA. 51gaacagattc ctgtaccatg ttcatc 265225DNAArtificial
SequenceA reverse primer for cloning ggTLR21 cDNA. 52gcttgtatga
attgtcacac tgcac 25
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

D00013

D00014

D00015

D00016

D00017

D00018

D00019

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.