Peptide Inhibitors Of Sodium Channels
Tarran; Robert ; et al.
U.S. patent application number 16/128787 was filed with the patent office on 2019-03-07 for peptide inhibitors of sodium channels. The applicant listed for this patent is Spyryx Biosciences, Inc., The University of North Carolina at Chapel Hill. Invention is credited to Dale J. Christensen, Robert Tarran.
| Application Number | 20190071468 16/128787 |
| Document ID | / |
| Family ID | 55653774 |
| Filed Date | 2019-03-07 |





View All Diagrams
| United States Patent Application | 20190071468 |
| Kind Code | A1 |
| Tarran; Robert ; et al. | March 7, 2019 |
PEPTIDE INHIBITORS OF SODIUM CHANNELS
Abstract
The present invention relates to the ability of specialized non-naturally occurring peptides to bind to sodium channels and inhibit activation of the sodium channels. The invention further relates to methods for regulating of sodium absorption and fluid volume and treating disorders responsive to modulating sodium absorption by modulating the binding of specialized non-naturally occurring peptides to sodium channels.
| Inventors: | Tarran; Robert; (Chapel Hill, NC) ; Christensen; Dale J.; (Cary, NC) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 55653774 | ||||||||||
| Appl. No.: | 16/128787 | ||||||||||
| Filed: | September 12, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15591684 | May 10, 2017 | 10150796 | ||
| 16128787 | ||||
| 14878720 | Oct 8, 2015 | |||
| 15591684 | ||||
| 62061461 | Oct 8, 2014 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 1/00 20180101; C07K 2319/10 20130101; A61P 9/00 20180101; A61P 43/00 20180101; A61P 13/12 20180101; A61P 11/08 20180101; A61P 11/00 20180101; C07K 5/06026 20130101; C07K 7/06 20130101; C07K 7/08 20130101; A61K 9/0073 20130101; C07K 2319/00 20130101; A61P 1/04 20180101; A61P 11/06 20180101; C07K 14/705 20130101; A61P 11/12 20180101; A61K 38/00 20130101 |
| International Class: | C07K 7/08 20060101 C07K007/08; C07K 7/06 20060101 C07K007/06; C07K 5/062 20060101 C07K005/062; A61K 9/00 20060101 A61K009/00; C07K 14/705 20060101 C07K014/705 |
Claims
1. A peptide comprising the sequence: TABLE-US-00005 (SEQ ID NO: 116) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8
wherein: X.sub.1 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.2 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.3 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.4 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.5 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.6 is aspartic acid or a conservative substitution with a natural or non-natural amino acid; X.sub.7 is glutamine or a conservative substitution with a natural or non-natural amino acid; and X.sub.8 is threonine or a conservative substitution with a natural or non-natural amino acid; or a functional fragment thereof.
2. A peptide comprising the sequence: TABLE-US-00006 (SEQ ID NO: 117) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8
wherein: X.sub.1 is leucine, norleucine, or valine; X.sub.2 is proline, 4-hydroxyproline, (2R,5S)-5-phenyl-pyrrolidine-2-carboxylic acid, or 3,4-dehydro-L-proline; X.sub.3 is valine, leucine, norleucine, or N-methylvaline; X.sub.4 is proline, 4-hydroxyproline, (2R,5S)-5-phenyl-pyrrolidine-2-carboxylic acid, or 3,4-dehydro-L-proline; X.sub.5 is leucine, norleucine, or valine; X.sub.6 is aspartic acid or glutamic acid; X.sub.7 is glutamine or asparagine; and X.sub.8 is threonine, serine, or L-.alpha.-methylserine; or a functional fragment thereof.
3. The peptide of claim 1, comprising the sequence of any one of SEQ ID NOS:4-115.
4. A peptide comprising the sequence: TABLE-US-00007 (SEQ ID NO: 118) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8-X.sub.9-X.- sub.10-X.sub.11-X.sub.12-X.sub.13-X.sub.14- X.sub.15
wherein: X.sub.1 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.2 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.3 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.4 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.5 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.6 is aspartic acid or a conservative substitution with a natural or non-natural amino acid; X.sub.7 is glutamine or a conservative substitution with a natural or non-natural amino acid; X.sub.8 is threonine or a conservative substitution with a natural or non-natural amino acid; X.sub.9 is threonine or a conservative substitution with a natural or non-natural amino acid; X.sub.10 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.11 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.12 is asparagine or a conservative substitution with a natural or non-natural amino acid; X.sub.13 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.14 is asparagine or a conservative substitution with a natural or non-natural amino acid; and X.sub.15 is proline or a conservative substitution with a natural or non-natural amino acid; or a functional fragment thereof.
5. The peptide of claim 4, comprising the sequence of SEQ ID NO: 120.
6. A peptide comprising the sequence of SEQ ID NO:127 or SEQ ID NO:128.
7. The peptide of claim 1, wherein the N-terminus is acetylated and/or the C-terminus is amidated.
8. The peptide of claim 1, wherein the peptide comprises at least one non-natural amino acid or at least one terminal modification.
9. The peptide of claim 1, further comprising at least one D-alanine at the N-terminus and/or the C-terminus.
10. A kit comprising the peptide of claim 1.
11. A method of inhibiting the activation of a sodium channel, comprising contacting a sodium channel with the peptide of claim 1.
12. The method of claim 11, wherein the sodium channel is an epithelial sodium channel (ENaC).
13. The method of claim 11, wherein the sodium channel is present in an isolated cell.
14. The method of claim 13, wherein the isolated cell is part of an epithelial cell culture.
15. The method of claim 11, wherein the sodium channel is present in a cell in an animal.
16. The method of claim 15, wherein the animal is a disease model.
17. The method of claim 11, wherein contacting the sodium channel with the peptide comprises delivering the peptide to a cell comprising the sodium channel.
18. The method of claim 11, wherein activation of the sodium channel is inhibited by at least 20%.
19. The method of claim 11, wherein activation of the sodium channel is inhibited by at least 50%.
20. The method of claim 11, wherein activation of the sodium channel is inhibited by at least 90%.
21. A method of inhibiting sodium absorption through a sodium channel, comprising contacting the sodium channel with the peptide of claim 1.
22. The method of claim 21, wherein the sodium channel is ENaC.
23. A method of increasing the volume of fluid lining an epithelial mucosal surface, comprising contacting a sodium channel present on the epithelial mucosal surface with the peptide of claim 1.
24. The method of claim 23, wherein the sodium channel is ENaC.
25. A method of reducing the level of a sodium channel present on the surface of a cell, comprising contacting the sodium channel with the peptide of claim 1.
26. The method of claim 25, wherein the sodium channel is ENaC.
27. A method of treating a disorder responsive to inhibition of sodium absorption across an epithelial mucosal surface in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of the peptide of claim 1.
28. The method of claim 27, wherein the sodium channel is ENaC.
29. The method of claim 27, wherein said disorder is a lung disorder.
30. The method of claim 27, wherein said lung disorder is cystic fibrosis.
31. The method of claim 27, wherein said lung disorder is non-cystic fibrosis bronchiectasis.
32. The method of claim 27, wherein said lung disorder is chronic obstructive pulmonary disease.
33. The method of claim 27, wherein said lung disorder is acute or chronic bronchitis.
34. The method of claim 27, wherein said lung disorder is asthma.
35. The method of claim 27, wherein said disorder is a gastrointestinal disorder.
36. The method of claim 35, wherein said gastrointestinal disorder is inflammatory bowel disease.
37. The method of claim 27, wherein said disorder is a kidney disorder.
38. A method of regulating salt balance, blood volume, and/or blood pressure in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of the peptide of claim 1.
39. The method of claim 27, wherein delivering the peptide reduces the level of a sodium channel present on the surface of a cell in the subject.
40. A method for treating a symptom of a lung disorder, a gastrointestinal disorder, a kidney disorder, or a cardiovascular disorder in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of the peptide of claim 1.
41. The method of claim 40, wherein the lung disorder is cystic fibrosis, non-cystic fibrosis bronchiectasis, chronic obstructive pulmonary disease, acute or chronic bronchitis, or asthma.
42. The method of claim 40, wherein the gastrointestinal disorder is inflammatory bowel disease.
Description
RELATED APPLICATIONS
[0001] This application is a Continuation of U.S. patent application Ser. No. 15/591,684, filed May 10, 2017, which is a Continuation of U.S. patent application Ser. No. 14/878,720, filed Oct. 8, 2015, which claims the benefit of U.S. Provisional Patent Application No. 62/061,461, filed Oct. 8, 2014, the entire contents of which are incorporated by reference herein.
STATEMENT REGARDING ELECTRONIC FILING OF A SEQUENCE LISTING
[0002] A Sequence Listing in ASCII text format, submitted under 37 C.F.R. .sctn. 1.821, entitled 5470-721_ST25.txt, 56,808 bytes in size, generated on Oct. 8, 2015 and filed via EFS-Web, is provided in lieu of a paper copy. The Sequence Listing is incorporated herein by reference into the specification for its disclosures.
FIELD OF THE INVENTION
[0003] The present invention relates to optimized peptides that are specialized non-naturally occurring peptides with improved ability to bind to sodium channels and inhibit activation of the sodium channels. The invention further relates to methods for regulating sodium absorption and fluid volume and treating disorders responsive to modulating sodium absorption by activity of sodium channels.
BACKGROUND OF THE INVENTION
[0004] Epithelial mucosal surfaces are lined with fluids whose volume and composition are precisely controlled. In the airways, a thin film of airway surface liquid helps protect mammalian airways from infection by acting as a lubricant for efficient mucus clearance (Hobbs et al., J. Physiol. 591: 4377 (2013), Knowles et al., J. Clin. Invest. 109:571 (2002)). This layer moves cephalad during mucus clearance and excess liquid that accumulates as two airways converge is eliminated by Na.sup.+-modulated airway surface liquid absorption with Na.sup.+ passing through the epithelial Na.sup.+ channel (ENaC) (Hobbs et al., J. Physiol. 591: 4377 (2013), Knowles et al., J. Clin. Invest. 109:571 (2002)). Critically, the mechanism by which ENaC activity is regulated in the airways is poorly understood. Recently, evidence has been accumulating that molecular regulators in the airway surface liquid can serve as volume sensing signals whose dilution or concentration can alter specific cell surface receptors that control ion transport rates to either absorb or secrete airway surface liquid as needed (Chambers et al., Respir. Physiol. Neurobiol. 159:256 (2007)). As one of the regulated targets, ENaC must be cleaved by intracellular furin-type proteases and/or extracellular channel activating proteases (CAPs) such as prostasin to be active and to conduct Na.sup.+ (Planes et al., Curr. Top. Dev. Biol. 78:23 (2007); Rossier, Proc. Am. Thorac. Soc. 1:4 (2004); Vallet et al., Nature 389:607 (1997); Chraibi et al., J. Gen. Physiol. 111:127 (1998)). ENaC can also be cleaved and activated by exogenous serine proteases such as trypsin, an action that is attenuated by the protease inhibitor aprotinin (Vallet et al., Nature 389:607 (1997)). When human bronchial epithelial cultures are mounted in Ussing chambers where native airway surface liquid is washed away, ENaC is predominantly active, suggesting that cell attached proteases are predominant (Bridges et al., Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L16 (2001); Donaldson et al., J. Biol. Chem. 277:8338 (2002)). In contrast, under thin film conditions, where native airway surface liquid is present, ENaC activity is reduced, suggesting that airway surface liquid contains soluble protease inhibitors (Myerburg et al., J. Biol. Chem. 281:27942 (2006); Tarran et al., J. Gen. Physiol. 127:591 (2006); Gaillard et al., 2010 Pfleugers Arch, 460: 1-17).
[0005] Recently, it has been shown that the Short Palate Lung and Nasal epithelial Clone (SPLUNC1) protein comprises up to 10% of the total protein in the airway surface liquid and can readily be detected in both nasal lavage and tracheal secretions (Bingle, C. D., and Craven, C. J. (2002) PLUNC: a novel family of candidate host defense proteins expressed in the upper airways and nasopharynx Hum Mol Genet 11, 937; Campos, M. A., et al. (2004) Purification and characterization of PLUNC from human tracheobronchial secretions Am J Respir Cell Mol Biol 30, 184; Lindahl, M., Stahlbom, B., and Tagesson, C. (2001); Identification of a new potential airway irritation marker, palate lung nasal epithelial clone protein, in human nasal lavage fluid with two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight Electrophoresis 22, 1795). SPLUNC1 appears to be a volume sensing molecule since it can be secreted onto the mucosal surface of the superficial epithelia where ENaC is expressed (Bartlett et al., J. Leukoc. Biol. 83:1201 (2008); Bingle et al., J. Pathol. 205:491 (2005)). Furthermore, SPLUNC1 has been demonstrated to contain a subdomain that functions as an inhibitor of ENaC through its N-terminal domain.
[0006] The present invention discloses novel specialized non-naturally occurring peptides that mimic the properties of SPLUNC1 in regulation of sodium channels by binding to and inhibiting ion transport to regulate sodium absorption and fluid volume and treat disorders responsive to modulating sodium absorption.
SUMMARY OF THE INVENTION
[0007] The present invention is based, in part, on the design of specialized non-naturally occurring peptides to regulate the activity of sodium channels. Accordingly, in one aspect the invention relates to a method of inhibiting the activation of a sodium channel, comprising contacting a sodium channel with a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the sodium channel is an epithelial sodium channel (ENaC). In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0008] Another aspect of the invention relates to a method of inhibiting sodium absorption through a sodium channel, comprising contacting the sodium channel with a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0009] A further aspect of the invention relates to a method of increasing the volume of fluid lining an epithelial mucosal surface, comprising contacting a sodium channel present on the epithelial mucosal surface with a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0010] Another aspect of the invention relates to a method of reducing the level of a sodium channel present on the surface of a cell, comprising contacting the sodium channel with a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0011] A further aspect of the invention relates to a method of treating a disorder responsive to inhibition of sodium absorption across an epithelial mucosal surface in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0012] Another aspect of the invention relates to a method of regulating salt balance, blood volume, and/or blood pressure in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the specialized non-naturally occurring peptide or a functional fragment thereof binds to the sodium channel.
[0013] Another aspect of the invention relates to a specialized non-naturally occurring peptide or a functional fragment thereof that mimics the sodium channel binding domain of a PLUNC protein and binds to a sodium channel, wherein cleavage of the sodium channel by a protease is inhibited when bound to the peptide.
[0014] Another aspect of the invention relates to a kit comprising the peptide of the invention.
[0015] Another aspect of the invention relates to the use of a specialized non-naturally occurring peptide or a functional fragment thereof for the preparation of a medicament to treat a disorder responsive to inhibition of sodium absorption in a subject in need thereof.
[0016] These and other aspects of the invention are set forth in more detail in the description of the invention below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] FIG. 1 shows the sequence of S18 (SEQ ID NO:1). Highlighted in red are residues essential for ENaC interaction, while highlighted in blue are residues that were found not to contribute.
[0018] FIGS. 2A-2B show the 1.sup.st half (LPVPLDQT (SEQ ID NO: 113) but not the remainder (DQTLPLNVNP (SEQ ID NO: 114) of S18 is required for inhibition of ENaC and preservation of ASL height.
[0019] FIG. 3A shows that S18 (SEQ ID NO:1) and aaLPVPLDQTLPLNVNPaa (SEQ ID NO:2) have equal potency and efficacy, despite SEQ ID NO:2 being flanked by D-alanines. FIG. 3B shows that S18, and aaLPVPLDQTaa (SEQ ID NO:3) have equal potency and efficacy, despite (SEQ ID NO:3) being flanked by D-alanines. FIG. 3A also shows the relative potency of a sample of peptides, including aaLPNlePLDQTaa (SEQ ID NO:5), which displays increased potency, relative to S18 or SEQ ID NO:4.
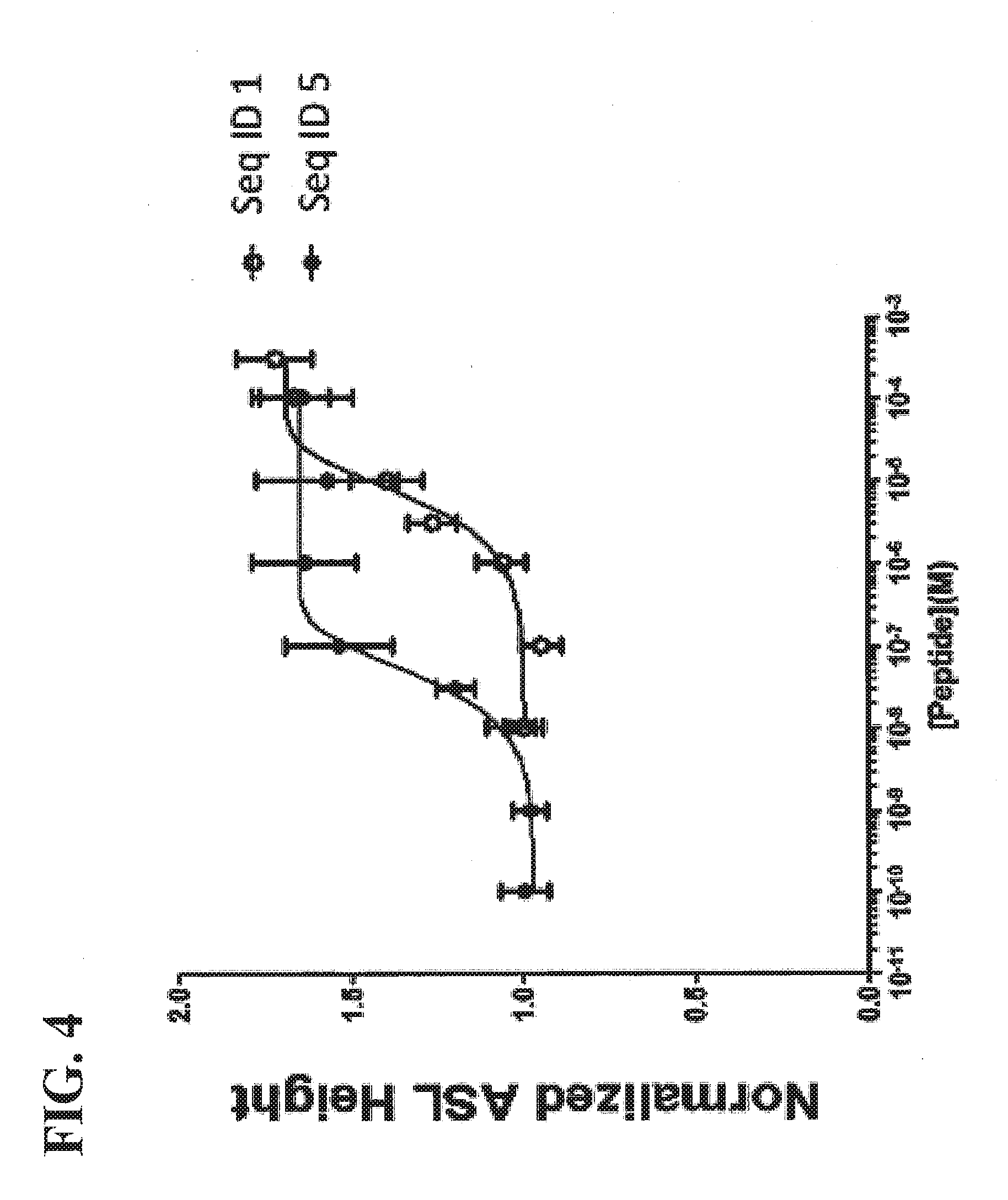
[0020] FIG. 4 shows an additional comparison of S18 and SEQ ID NO:5, and increased potency of SEQ ID NO:5.
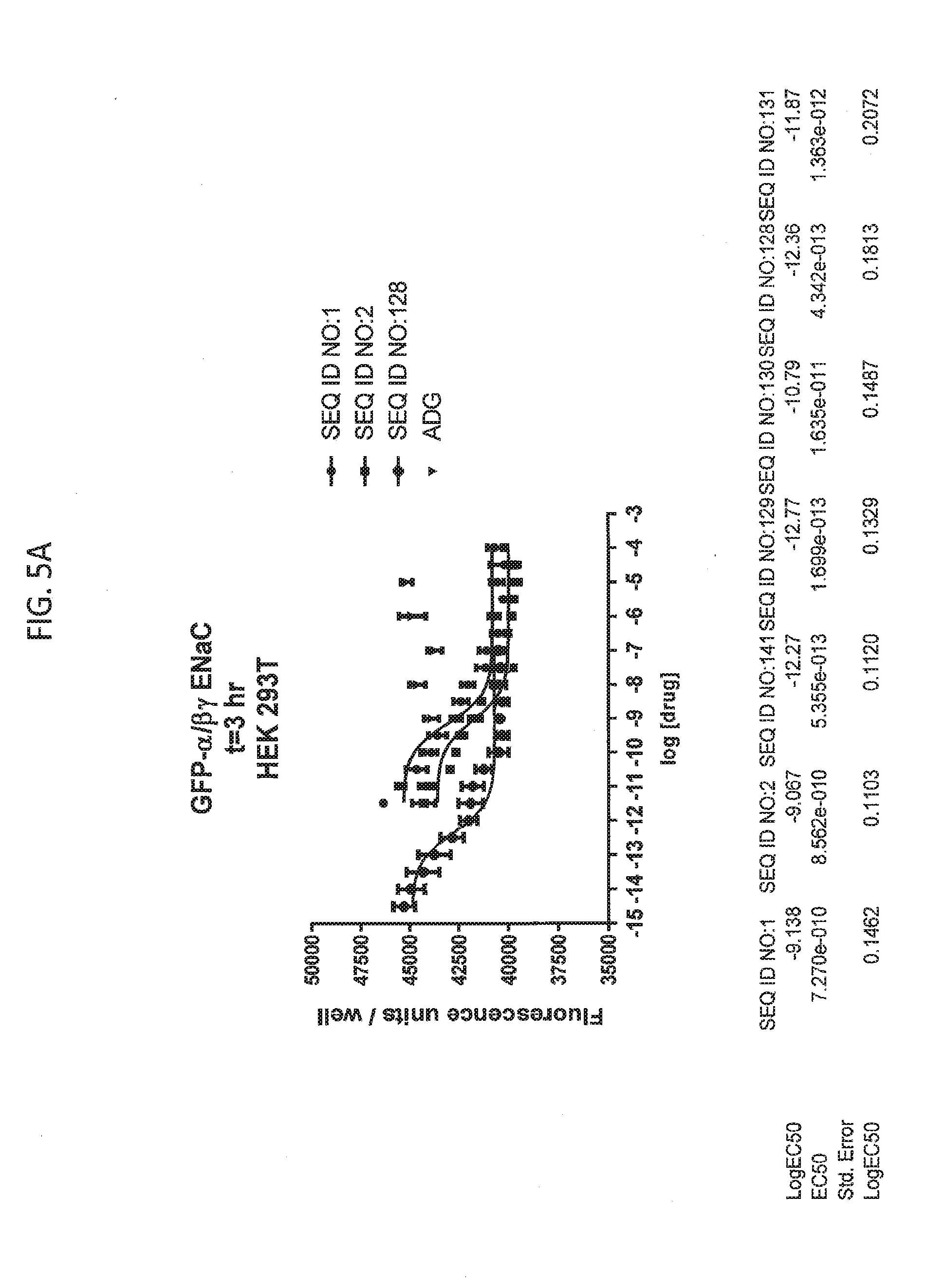
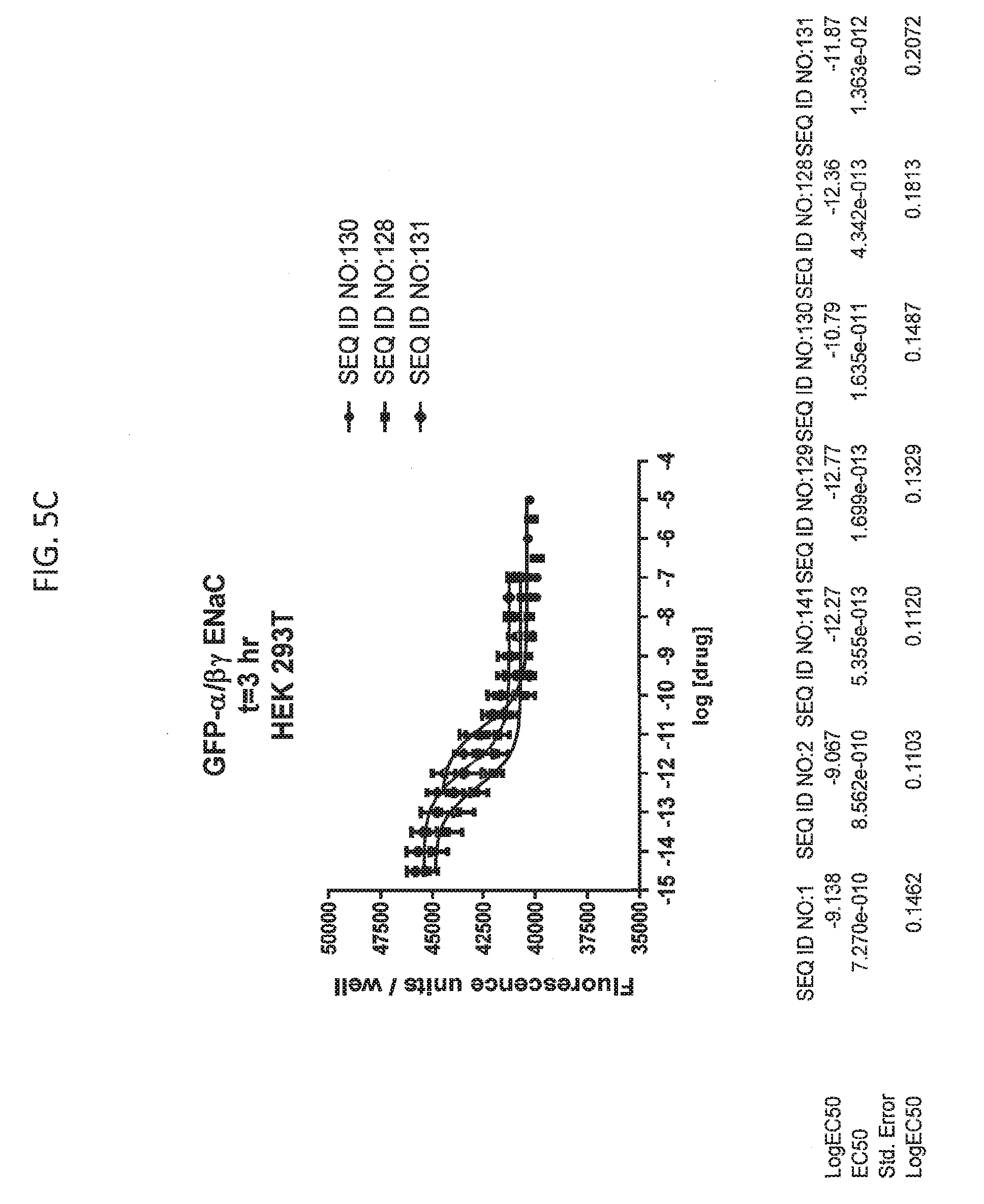
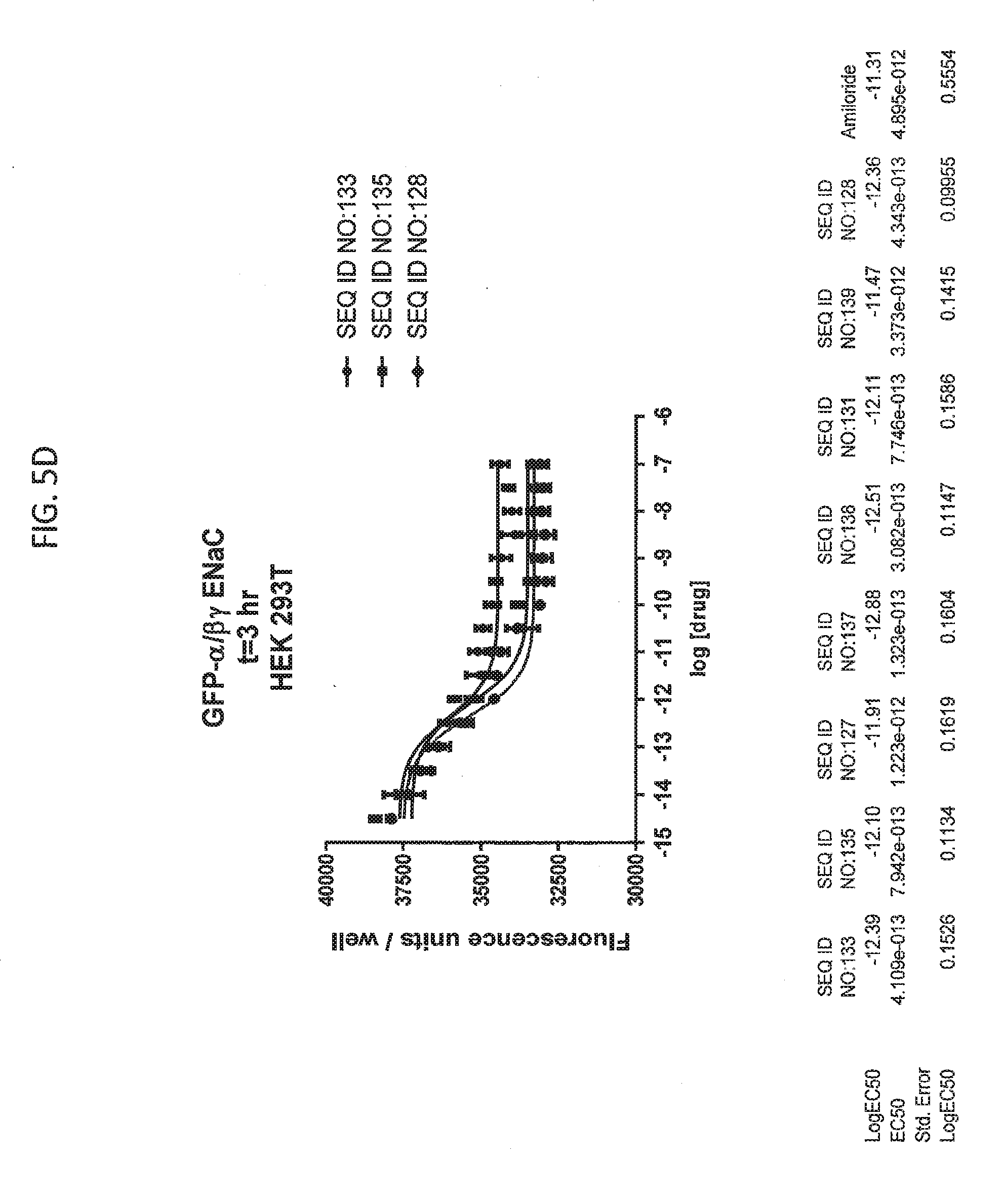
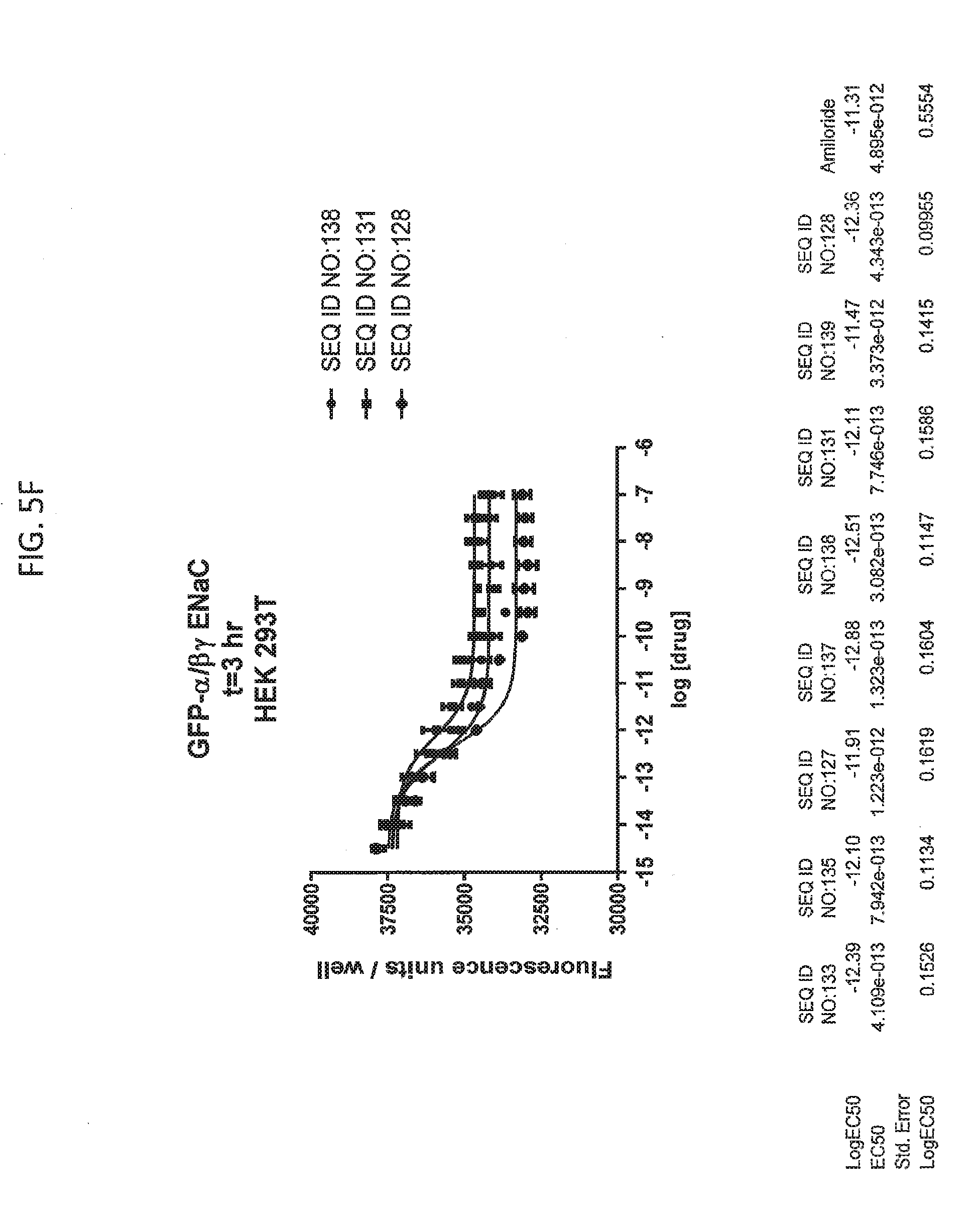
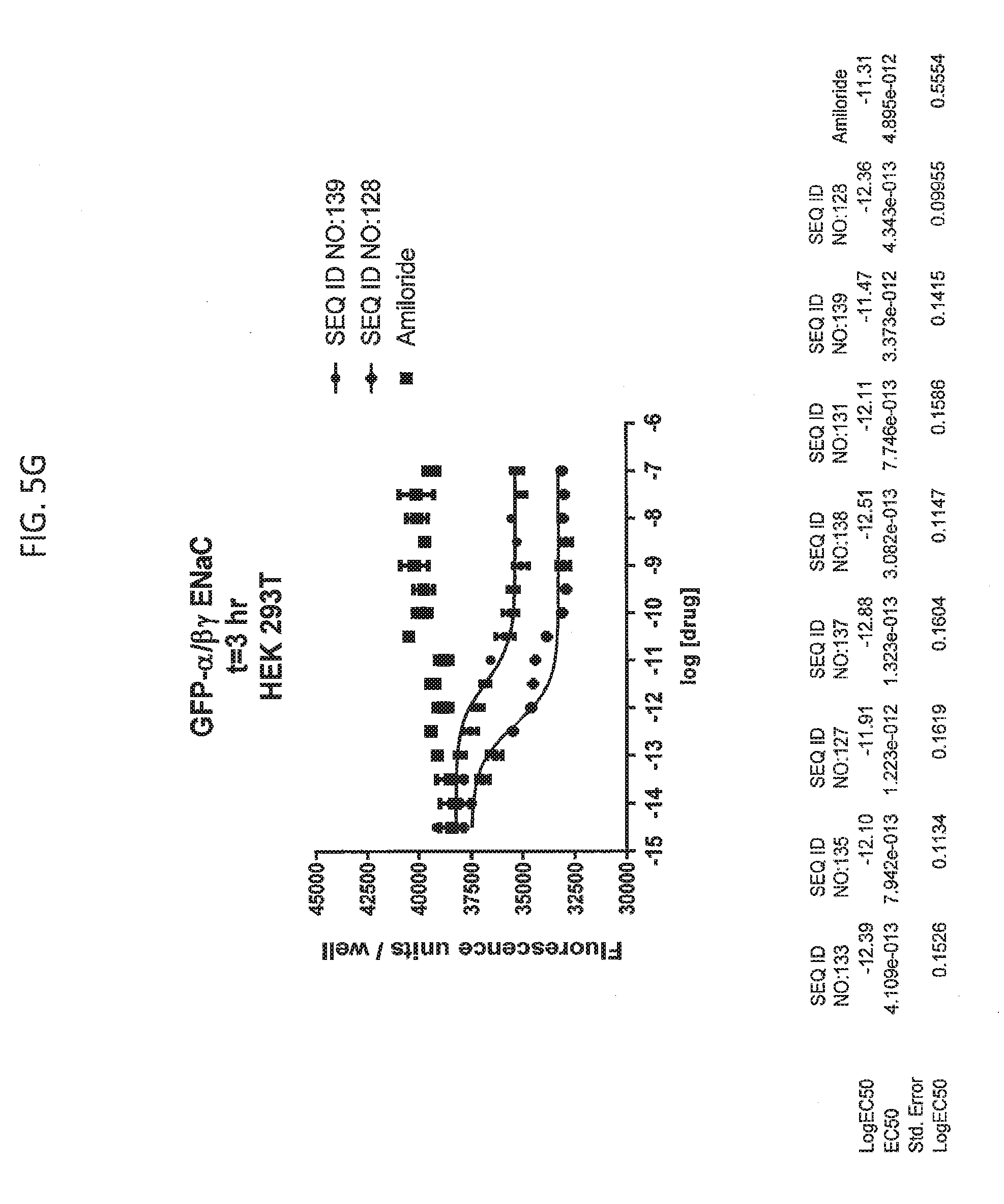
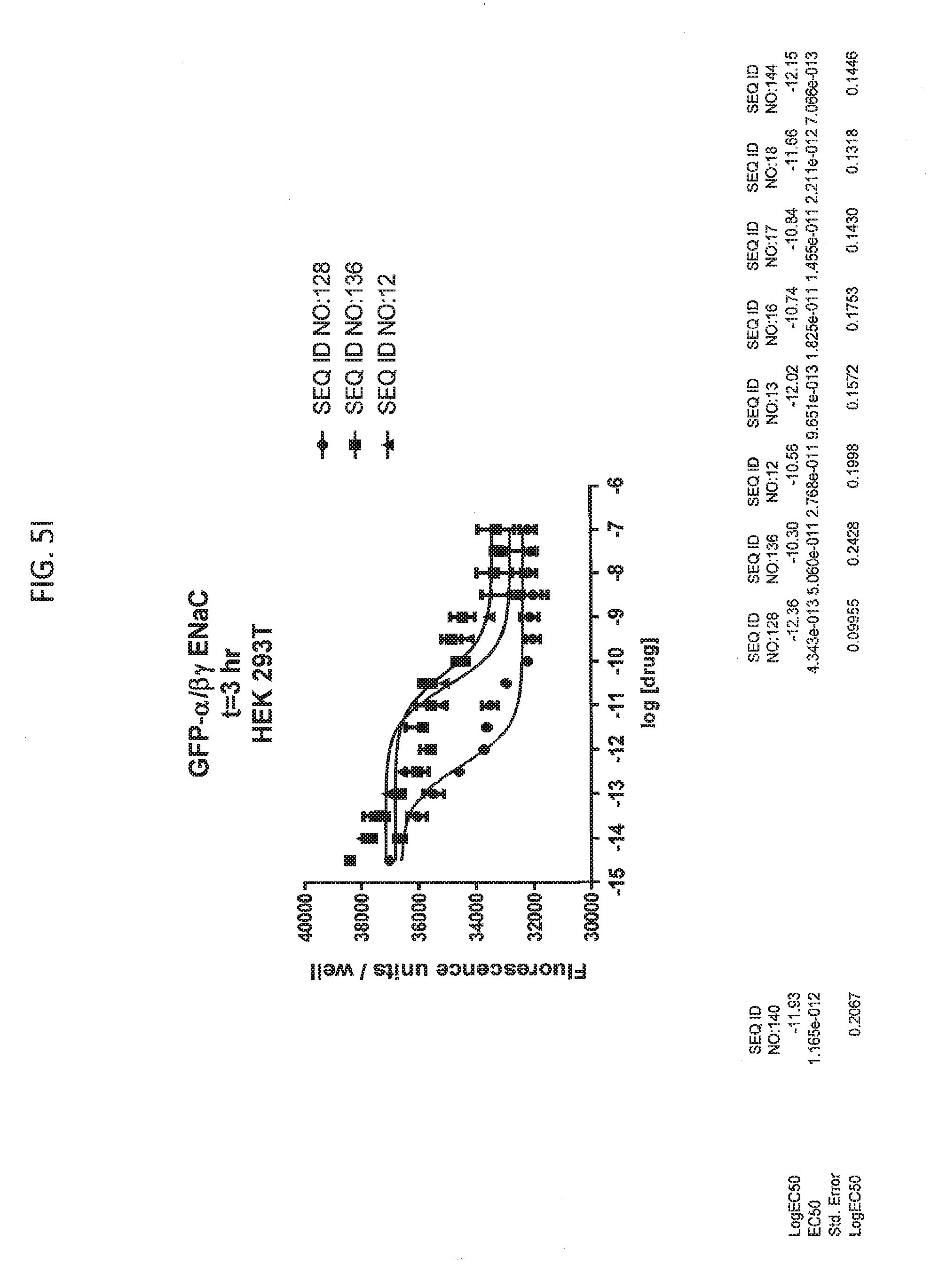
[0021] FIGS. 5A-5U show the results of experiments analyzing the effects of various peptides on internalization of alpha-ENaC in HEK293T cells and effects on cell viability when GFP-tagged alpha-ENaC is co-expressed with beta and gamma ENaC. EC50 values are shown at the bottom of several of the figures. In these figures, ADG is a negative control peptide with the sequence: NH3-ADGGLLLLNNPPPPQTVV-NH2 (SEQ ID NO:143). The peptides used in these experiments were S18 (SEQ ID NO:1), SEQ ID NO:2, SEQ ID NO:141, SEQ ID NO: 129, SEQ ID NO:130, SEQ ID NO:128, SEQ ID NO:131, SEQ ID NO:132, SEQ ID NO:133, SEQ ID NO:134, SEQ ID NO:135, SEQ ID NO:136, SEQ ID NO:137, SEQ ID NO:138, SEQ ID NO:131, SEQ ID NO:139; SEQ ID NO:140, SEQ ID NO:144 and SEQ ID NO:127. Also used in these experiments were peptides of SEQ ID NO:12, SEQ ID NO: 13, SEQ ID NO: 16, SEQ ID NO:17, SEQ ID NO: 18, and SEQ ID NO:136, each with an as cap at both the N- and the C-terminus. Also shown is amiloride, which inhibits ENaC by another mechanism and does not reduce the amount of functional receptor on the surface of cells.
[0022] FIG. 6 shows the results of an experiment analyzing the effects of various peptides on internalization of alpha-ENaC in HEK293T cells when GFP-tagged alpha-ENaC is co-expressed only with gamma ENaC and no beta-ENaC. In this figure, SEQ ID NO:143 (negative control peptide) and water (vehicle) controls are used along with S18 (SEQ ID NO:1) and SEQ ID NO:128. While SEQ ID NO:1 AND SEQ ID NO:128 are effective in reducing alpha-ENaC when beta-ENaC is co-expressed, no effect is observed in this experiment when beta-ENaC is not present.
[0023] FIG. 7A shows the results of an experiment analyzing the effect on percent survival of .beta.ENaC-Tg C57BL:FVB mice after treatment with the S18 peptide (SEQ ID NO:1). S18 (100 mM solution) was administered 3 times per day at 1 .mu.L/g of body weight.
[0024] FIG. 7B shows the results of an experiment analyzing the effect on percent survival of .beta.ENaC-Tg C57BL:FVB mice after treatment with inhaled SEQ ID NO: 142 or SEQ ID NO: 128.
[0025] FIGS. 7C and 7D show the results of an experiment analyzing the effect on percent survival of (.beta.ENaC-Tg C57BL:C3H mice after treatment with SEQ ID NO: 129 or SEQ ID NO: 128.
[0026] FIG. 7E show the results of an experiment analyzing the effect on percent survival (left panel) and weight gain (right panel) of .beta.ENaC-Tg C57BL:C3H mice after treatment with a once daily dose of SEQ ID NO: 128.
[0027] FIG. 7F shows the results of an experiment analyzing the effect on percent survival of .beta.ENaC-Tg C57BL:C3H mice after treatment with SEQ ID NO:127, SEQ ID NO: 134, or SEQ ID NO:136.
DETAILED DESCRIPTION OF THE INVENTION
[0028] The present invention will now be described in more detail with reference to the accompanying drawings, in which preferred embodiments of the invention are shown. This invention may, however, be embodied in different forms and should not be construed as limited to the embodiments set forth herein. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art.
[0029] Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of skill in the art to which this invention belongs. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention. All publications, patent applications, patents, patent publications and other references cited herein are incorporated by reference in their entireties for the teachings relevant to the sentence and/or paragraph in which the reference is presented.
[0030] Amino acids are represented herein in the manner recommended by the IUPAC-IUB Biochemical Nomenclature Commission, or (for amino acids) by either the one-letter code, or the three letter code, both in accordance with 37 C.F.R. .sctn. 1.822 and established usage.
[0031] As used in the description of the invention and the appended claims, the singular forms "a," "an," and "the" are intended to include the plural forms as well, unless the context clearly indicates otherwise.
[0032] Also as used herein, "and/or" refers to and encompasses any and all possible combinations of one or more of the associated listed items, as well as the lack of combinations when interpreted in the alternative ("or").
[0033] The term "consists essentially of" (and grammatical variants), as applied to a peptide sequence of this invention, means a peptide that consists of both the recited sequence (e.g., SEQ ID NO) and a total of ten or less (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10) additional amino acids on the N-terminal and/or C-terminal ends of the recited sequence such that the function of the peptide is not materially altered. The total often or less additional amino acids includes the total number of additional amino acids on both ends added together. The term "materially altered," as applied to peptides of the invention, refers to an increase or decrease in binding activity (e.g., to a sodium channel or specialized non-naturally occurring peptide) of at least about 50% or more as compared to the activity of a peptide consisting of the recited sequence.
[0034] The term "modulate," "modulates," or "modulation" refers to enhancement (e.g., an increase) or inhibition (e.g., a decrease) in the specified level or activity.
[0035] The term "enhance" or "increase" refers to an increase in the specified parameter of at least about 1.25-fold, 1.5-fold, 2-fold, 3-fold, 4-fold, 5-fold, 6-fold, 8-fold, 10-fold, twelve-fold, or even fifteen-fold.
[0036] The term "inhibit" or "reduce" or grammatical variations thereof as used herein refers to a decrease or diminishment in the specified level or activity of at least about 15%, 25%, 35%, 40%, 50%, 60%, 75%, 80%, 90%, 95% or more. In particular embodiments, the inhibition or reduction results in little or essentially no detectible activity (at most, an insignificant amount, e.g., less than about 10% or even 5%).
[0037] The term "contact" or grammatical variations thereof as used with respect to a specialized non-naturally occurring peptide and a sodium channel, refers to bringing the specialized non-naturally occurring peptide and the sodium channel in sufficiently close proximity to each other for one to exert a biological effect on the other. In some embodiments, the term contact means binding of the specialized non-naturally occurring peptide to the sodium channel.
[0038] A "therapeutically effective" amount as used herein is an amount that provides some improvement or benefit to the subject. Alternatively stated, a "therapeutically effective" amount is an amount that will provide some alleviation, mitigation, or decrease in at least one clinical symptom in the subject. Those skilled in the art will appreciate that the therapeutic effects need not be complete or curative, as long as some benefit is provided to the subject.
[0039] By the terms "treat," "treating," or "treatment of," it is intended that the severity of the subject's condition is reduced or at least partially improved or modified and that some alleviation, mitigation or decrease in at least one clinical symptom is achieved.
[0040] The term "fragment," as applied to a peptide, will be understood to mean an amino acid sequence of reduced length relative to a reference peptide or amino acid sequence and comprising, consisting essentially of, and/or consisting of an amino acid sequence of contiguous amino acids identical to the reference peptide or amino acid sequence. Such a peptide fragment according to the invention may be, where appropriate, included in a larger polypeptide of which it is a constituent. In some embodiments, such fragments can comprise, consist essentially of, and/or consist of peptides having a length of at least about 4, 5, 6, 7, 8, 9, 10, or more consecutive amino acids of a peptide or amino acid sequence according to the invention. In other embodiments, such fragments can comprise, consist essentially of, and/or consist of peptides having a length of less than about 10, 9, 8, 7, 6, 5, 4, or less consecutive amino acids of a peptide or amino acid sequence according to the invention.
[0041] As used herein, the terms "protein" and "polypeptide" are used interchangeably and encompass both peptides and proteins, unless indicated otherwise.
[0042] A "fusion protein" is a polypeptide produced when two heterologous nucleotide sequences or fragments thereof coding for two (or more) different polypeptides not found fused together in nature are fused together in the correct translational reading frame. Illustrative fusion polypeptides include fusions of a peptide of the invention (or a fragment thereof) to all or a portion of glutathione-S-transferase, maltose-binding protein, or a reporter protein (e.g., Green Fluorescent Protein, .beta.-glucuronidase, .beta.-galactosidase, luciferase, etc.), hemagglutinin, c-myc, FLAG epitope, etc.
[0043] As used herein, a "functional" peptide or "functional fragment" is one that substantially retains at least one biological activity normally associated with that peptide (e.g., binding to or inhibiting a sodium channel). In particular embodiments, the "functional" peptide or "functional fragment" substantially retains all of the activities possessed by the unmodified peptide. By "substantially retains" biological activity, it is meant that the peptide retains at least about 20%, 30%, 40%, 50%, 60%, 75%, 85%, 90%, 95%, 97%, 98%, 99%, or more, of the biological activity of the native polypeptide (and can even have a higher level of activity than the native peptide). A "non-functional" peptide is one that exhibits little or essentially no detectable biological activity normally associated with the peptide (e.g., at most, only an insignificant amount, e.g., less than about 10% or even 5%). Biological activities such as protein binding and sodium channel inhibitory activity can be measured using assays that are well known in the art and as described herein.
[0044] The term "about," as used herein when referring to a measurable value such as an amount of polypeptide, dose, time, temperature, enzymatic activity or other biological activity and the like, is meant to encompass variations of .+-.20%, .+-.10%, .+-.5%, .+-.1%, .+-.0.5%, or even .+-.0.1% of the specified amount.
[0045] A first aspect of the invention relates to the ability of specialized non-naturally occurring peptides to bind to a sodium channel and prevent activation of the sodium channel, thereby inhibiting the flow of sodium ions. Thus, one aspect of the present invention relates to a method of inhibiting the activation of a sodium channel, comprising contacting (e.g., binding) a sodium channel with a specialized non-naturally occurring peptide or a functional fragment thereof. In one embodiment, the sodium channel is an epithelial sodium channel (ENaC), e.g., human ENaC, or a non-human mammalian ENaC. In another embodiment, the sodium channel is one that is similar in sequence and/or structure to ENaC, such as acid-sensing ion channels (ASIC). The inhibition of sodium channel activation can be measured by any method known in the art or disclosed herein, including, without limitation, measuring sodium flow or change in potential across a membrane, across a cell, or across a natural or artificial lining. The inhibition can be at least about 20%, e.g., at least about 30%, 40%, 50%, 60%, 70%, 80%, 90%, or 100%.
[0046] The method of inhibiting the activation of a sodium channel can be carried out, e.g., on an isolated sodium channel, a sodium channel in an artificial membrane, or a sodium channel in a cell. In one embodiment, the sodium channel is present in an isolated cell, e.g., a cultured primary cell or cell line. In another embodiment, the isolated cell is part of an epithelial cell culture, e.g., a natural or artificial epithelial lining, e.g., a cell culture in a device (such as an Ussing chamber) in which characteristics such as ion flow and/or potential can be measured across lining. In another embodiment, the cell is part of an isolated tissue or a tissue culture. In a further embodiment, the cell can be present in an animal, e.g., an animal that is a disease model or a subject in need of treatment.
[0047] In one embodiment, the step of contacting (e.g., binding) the sodium channel with a specialized non-naturally occurring peptide comprises delivering the specialized non-naturally occurring peptide or a functional fragment or homolog thereof to a cell comprising the sodium channel.
[0048] As used herein, the term "homolog" is used to refer to a polypeptide which differs from a the disclosed specialized non-naturally occurring peptide by modifications to the specialized non-naturally occurring peptide, but which significantly retains a biological activity of the disclosed non-naturally occurring peptide. Minor modifications include, without limitation, changes in one or a few amino acid side chains, changes to one or a few amino acids (including deletions, insertions, and substitutions), changes in stereochemistry of one or a few atoms, and minor derivatizations, including, without limitation, methylation, glycosylation, phosphorylation, acetylation, myristoylation, prenylation, palmitoylation, amidation, and addition of glycosylphosphatidyl inositol. The term "substantially retains," as used herein, refers to a fragment, homolog, or other variant of a peptide that retains at least about 20% of the activity of the naturally occurring peptide (e.g., binding to a sodium channel), e.g., about 30%, 40%, 50% or more. Other biological activities, depending on the peptide, may include enzyme activity, receptor binding, ligand binding, induction of a growth factor, a cell signal transduction event, etc.
[0049] In one embodiment, the method comprises delivering to a cell comprising a sodium channel an isolated specialized non-naturally occurring peptide. In exemplary embodiments, the specialized non-naturally occurring peptide comprises, consists essentially of, or consists of the disclosed specialized non-naturally occurring peptide or a functional fragment thereof. In another embodiment, the isolated specialized non-naturally occurring peptide comprises, consists essentially of, or consists of an amino acid sequence that is at least 70% identical, e.g., at least 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, or 99% identical to the publicly known amino acid sequence or a functional fragment thereof. In some embodiments, the peptides comprise a portion of the natural amino acid sequence of a PLUNC protein with one or more conservative substitutions with natural or non-natural amino acids and/or one or more additions of non-natural amino acids. Conservative substitutions are described below. In some embodiments, the peptides comprise one or more terminal modifications as described below.
[0050] Non-limiting examples of peptides of the invention are disclosed in Table I below. In some embodiments, the peptides of the invention may comprise one or more additional residues at the amino- and/or carboxyl-terminal ends. In some embodiments, the one or more additional residues are D-alanines. For example, a peptide may comprise one or two D-alanines at the amino- and/or carboxyl-terminal ends.
[0051] The specialized non-naturally occurring peptides of the invention also include functional portions or fragments. The length of the fragment is not critical as long as it substantially retains the biological activity of the peptide (e.g., sodium channel binding activity). Illustrative fragments comprise at least about 4, 5, 6, 7, 8, 9, 10, or more contiguous amino acids of a specialized non-naturally occurring peptide. In other embodiments, the fragment comprises no more than about 10, 9, 8, 7, 6, 5, or 4 contiguous amino acids of a specialized non-naturally occurring peptide.
[0052] Likewise, those skilled in the art will appreciate that the present invention also encompasses fusion polypeptides comprising a specialized non-naturally occurring peptides peptide or a functional fragment thereof. As another alternative, the fusion protein can comprise a reporter molecule. In other embodiments, the fusion protein can comprise a polypeptide that provides a function or activity that is the same as or different from the activity of the peptide, e.g., a targeting, binding, or enzymatic activity or function.
[0053] Likewise, it will be understood that the peptides specifically disclosed herein will typically tolerate substitutions in the amino acid sequence and substantially retain biological activity. To identify peptides of the invention other than those specifically disclosed herein, amino acid substitutions may be based on any characteristic known in the art, including the relative similarity or differences of the amino acid side-chain substituents, for example, their hydrophobicity, hydrophilicity, charge, size, and the like.
TABLE-US-00001 L P V P L D Q T L P L N V N P A 2 L P I P L D Q T L P L N V N P A 2 L P V P L D Q T L P L N V N P 2 L P V P L D Q T 2 Nle P V P L D Q T 2 L P Nle P L D Q T 2 L P Nle P L D Q T a a 2 L P Nle P L D Q T a 2 L P V P Nle D Q T 2 L P V P L D N T 2 L P V P L E Q T 2 L P V P L D Q S 2 L P I P L D Q T 2 L P I P L D Q T a a 2 L P I P L D Q T 2 L P V P L D Q T 2 L HP V P L D Q T 2 L HP V P L D Q T 2 L P V HP L D Q T 2 L HP V HP L D Q T 2 L PP V P L D Q T 2 L P V PP L D Q T 2 L YP V P L D Q T 2 L P V YP L D Q T 2 L YP V YP L D Q T 2 L P V P L E Q S 2 L P V P L E Q MS 2 L P V P V D Q T 2 L P V P V E Q S 2 L P V P V D Q S 2 V P V P L D Q T 2 L P L P L D Q S 2 V P L P L D Q S 2 L P L P L D Q T 2 L P L P L D Q T a a 2 V YP L YP V E Q S 2 V P L P V E Q S 2 L P L P L E Q S 2 L P mV P L D Q S 2 L P Nle P L D Q T 2 L P Nle P V E Q S 2 L P Nle P V E Q S 2 L YP Nle YP L E Q S 2 L YP Nle YP V E Q S 2 L P V HP L D Q T 2 L HP V HP L D Q T 2 L PP V P L D Q T 2 L P V PP L D Q T 2 L YP V P L D Q T 2 L P V YP L D Q T 2 L YP V YP L D Q T 2 L P V P L E Q S 2 L P V P L E Q MS 2 L P V P V D Q T 2 L P V P V E Q S 2 L P V P V D Q S 2 V P V P L D Q T 2 L P L P L D Q S 2 V P L P L D Q S 2 L P L P L D Q T 2 V YP L YP V E Q S 2 V P L P V E Q S 2 L P L P L E Q S 2 L P mV P L D Q S 2 L P Nle P L D Q T 2 L P Nle P V E Q S 2 L P Nle P V E Q S 2 L YP Nle YP L E Q S 2 L YP Nle YP V E Q S 2 L HP V P L D Q T 2 L P V HP L D Q T 2 L HP V HP L D Q T 2 L PP V P L D Q T 2 L P V PP L D Q T 2 L YP V P L D Q T 2 L P V YP L D Q T 2 L YP V YP L D Q T 2 L P V P L E Q S 2 L P V P L E Q MS 2 L P V P V D Q T 2 L P V P V E Q S 2 L P V P V D Q S 2 V P V P L D Q T 2 V P V P L D Q S 2 L P L P L D Q S 2 V P L P L D Q S 2 L P L P L D Q T 2 V YP L YP V E Q S 2 V P L P V E Q S 2 L P L P L E Q S 2 L P mV P L D Q S 2 L P Nle P L D Q T 2 L P Nle P V E Q S 2 L P Nle P V E Q S 2 L YP Nle YP L E Q S 2 L YP Nle YP V E Q S 2 L P V HP L D Q T 2 L HP V HP L D Q T 2 L PP V P L D Q T 2 L P V PP L D Q T 2 L YP V P L D Q T 2 L P V YP L D Q T 2 L YP V YP L D Q T 2 L P V P L E Q S 2 L P V P L E Q MS 2 L P V P V D Q T 2 L P V P V E Q S 2 L P V P V D Q S 2 V P V P L D Q T 2 L P L P L D Q S 2 V P L P L D Q S 2 L P L P L D Q T 2 V YP L YP V E Q S 2 V P L P V E Q S 2 L P L P L E Q S 2 L P mV P L D Q S 2 L P Nle P L D Q T 2 L P Nle P V E Q S 2 L P Nle P V E Q S 2 L YP Nle YP L E Q S 2 L YP Nle YP V E Q S 2 L P V P L D Q T 2 D Q T L P L N V N P 2 L P V P L D Q T L P L 2 L P hp P L D Q T 2 L P F P L D Q T 2 L YP Nle P L D Q T a a 2 L P Nle YP L D Q T a a 2 L P Nle P L D Q T L P L N V N P A 2 L YP I P L D Q T 2 L P I P L D Q T L P L N V N P A 2 L P I P L E Q S a 2 Norleucine, HYP = 4-hydroxyproline, DHP = 3,4-dehydro-L-proline, Ahp = aminoheptanoic acid, 2PP = (2R,5S)-5- ylic acid, MS = L-.alpha.-methylserine, and mV = N-methylvaline indicates data missing or illegible when filed
[0054] In identifying amino acid sequences encoding peptides other than those specifically disclosed herein, the hydropathic index of amino acids may be considered. The importance of the hydropathic amino acid index in conferring interactive biologic function on a protein is generally understood in the art (see, Kyte and Doolittle, J. Mol. Biol. 157:105 (1982); incorporated herein by reference in its entirety). It is accepted that the relative hydropathic character of the amino acid contributes to the secondary structure of the resultant protein, which in turn defines the interaction of the protein with other molecules, for example, enzymes, substrates, receptors, DNA, antibodies, antigens, and the like.
[0055] Each amino acid has been assigned a hydropathic index on the basis of its hydrophobicity and charge characteristics (Kyte and Doolittle, id), these are: isoleucine (+4.5); valine (+4.2); leucine (+3.8); phenylalanine (+2.8); cysteine/cystine (+2.5); methionine (+1.9); alanine (+1.8); glycine (-0.4); threonine (-0.7); serine (-0.8); tryptophan (-0.9); tyrosine (-1.3); proline (-1.6); histidine (-3.2); glutamate (-3.5); glutamine (-3.5); aspartate (-3.5); asparagine (-3.5); lysine (-3.9); and arginine (-4.5).
[0056] Accordingly, the hydropathic index of the amino acid (or amino acid sequence) may be considered when modifying the peptides specifically disclosed herein.
[0057] It is also understood in the art that the substitution of amino acids can be made on the basis of hydrophilicity. U.S. Pat. No. 4,554,101 (incorporated herein by reference in its entirety) states that the greatest local average hydrophilicity of a protein, as governed by the hydrophilicity of its adjacent amino acids, correlates with a biological property of the protein.
[0058] As detailed in U.S. Pat. No. 4,554,101, the following hydrophilicity values have been assigned to amino acid residues: arginine (+3.0); lysine (.+-.3.0); aspartate (+3.0.+-.1); glutamate (+3.0.+-.1); serine (+0.3); asparagine (+0.2); glutamine (+0.2); glycine (0); threonine (-0.4); proline (-0.5.+-.I); alanine (-0.5); histidine (-0.5); cysteine (-1.0); methionine (-1.3); valine (-1.5); leucine (-1.8); isoleucine (-1.8); tyrosine (-2.3); phenylalanine (-2.5); tryptophan (-3.4).
[0059] Thus, the hydrophilicity of the amino acid (or amino acid sequence) may be considered when identifying additional peptides beyond those specifically disclosed herein.
[0060] Peptides (and fragments thereof) of the invention include peptides that have at least about 70%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or higher amino acid sequence identity with the peptide sequences disclosed herein.
[0061] As is known in the art, a number of different programs can be used to identify whether a polypeptide has sequence identity or similarity to a known sequence. Sequence identity or similarity may be determined using standard techniques known in the art, including, but not limited to, the local sequence identity algorithm of Smith & Waterman, Adv. Appl. Math. 2:482 (1981), by the sequence identity alignment algorithm of Needleman & Wunsch, J. Mol. Biol. 48:443 (1970), by the search for similarity method of Pearson & Lipman, Proc. Natl. Acad Sci. USA 85:2444 (1988), by computerized implementations of these algorithms (GAP, BESTFIT, FASTA, and TFASTA in the Wisconsin Genetics Software Package, Genetics Computer Group, 575 Science Drive, Madison, Wis.), the Best Fit sequence program described by Devereux et al., Nucl. Acid Res. 12:387 (1984), preferably using the default settings, or by inspection.
[0062] An example of a useful algorithm is PILEUP. PILEUP creates a multiple sequence alignment from a group of related sequences using progressive, pairwise alignments. It can also plot a tree showing the clustering relationships used to create the alignment. PILEUP uses a simplification of the progressive alignment method of Feng & Doolittle, J. Mol. Evol. 35:351 (1987); the method is similar to that described by Higgins & Sharp, CABIOS 5:151 (1989).
[0063] Another example of a useful algorithm is the BLAST algorithm, described in Altschul et al., J. Mol. Biol. 215:403 (1990) and Karlin et al., Proc. Natl. Acad. Sci. USA 90:5873 (1993). A particularly useful BLAST program is the WU-BLAST-2 program which was obtained from Altschul et al., Meth. Enzymol., 266:460 (1996); blast.wustl/edu/blast/README.html. WU-BLAST-2 uses several search parameters, which are preferably set to the default values. The parameters are dynamic values and are established by the program itself depending upon the composition of the particular sequence and composition of the particular database against which the sequence of interest is being searched; however, the values may be adjusted to increase sensitivity.
[0064] An additional useful algorithm is gapped BLAST as reported by Altschul et al., Nucleic Acids Res. 25:3389 (1997).
[0065] A percentage amino acid sequence identity value is determined by the number of matching identical residues divided by the total number of residues of the "longer" sequence in the aligned region. The "longer" sequence is the one having the most actual residues in the aligned region (gaps introduced by WU-Blast-2 to maximize the alignment score are ignored).
[0066] The alignment may include the introduction of gaps in the sequences to be aligned. In addition, for sequences which contain either more or fewer amino acids than the peptides specifically disclosed herein, it is understood that in one embodiment, the percentage of sequence identity will be determined based on the number of identical amino acids in relation to the total number of amino acids. Thus, for example, sequence identity of sequences shorter than a sequence specifically disclosed herein, will be determined using the number of amino acids in the shorter sequence, in one embodiment. In percent identity calculations relative weight is not assigned to various manifestations of sequence variation, such as insertions, deletions, substitutions, etc.
[0067] In one embodiment, only identities are scored positively (+1) and all forms of sequence variation including gaps are assigned a value of "0," which obviates the need for a weighted scale or parameters as described below for sequence similarity calculations. Percent sequence identity can be calculated, for example, by dividing the number of matching identical residues by the total number of residues of the "shorter" sequence in the aligned region and multiplying by 100. The "longer" sequence is the one having the most actual residues in the aligned region.
[0068] Peptides and fragments of the invention can be modified for in vivo use by the addition, at the amino- and/or carboxyl-terminal ends, of a blocking agent to facilitate survival of the relevant polypeptide in vivo. This can be useful in those situations in which the peptide termini tend to be degraded by proteases prior to cellular uptake. Such blocking agents can include, without limitation, additional related or unrelated peptide sequences that can be attached to the amino and/or carboxyl terminal residues of the peptide to be administered. This can be done either chemically during the synthesis of the peptide or by recombinant DNA technology by any suitable methods. For example, one or more non-naturally occurring amino acids, such as D-alanine, can be added to the termini. Alternatively, blocking agents such as pyroglutamic acid or other molecules known in the art can be attached to the amino and/or carboxyl terminal residues, or the amino group at the amino terminus or carboxyl group at the carboxyl terminus can be replaced with a different moiety. Additionally, the peptide terminus can be modified, e.g., by acetylation of the N-terminus and/or amidation of the C-terminus. Likewise, the peptides can be covalently or noncovalently coupled to pharmaceutically acceptable "carrier" proteins prior to administration.
[0069] In one embodiment, the peptides or fragments thereof of the invention are administered directly to a subject. Generally, the compounds of the invention will be suspended in a pharmaceutically-acceptable carrier (e.g., physiological saline) and administered orally or by intravenous infusion, or administered subcutaneously, intramuscularly, intrathecally, intraperitoneally, intrarectally, intravaginally, intranasally, intragastrically, intratracheally, or intrapulmonarily. In another embodiment, the intratracheal or intrapulmonary delivery can be accomplished using a standard nebulizer, jet nebulizer, wire mesh nebulizer, dry powder inhaler, or metered dose inhaler. They can be delivered directly to the site of the disease or disorder, such as lungs, kidney, or intestines. The dosage required depends on the choice of the route of administration; the nature of the formulation; the nature of the patient's illness; the subject's size, weight, surface area, age, and sex; other drugs being administered; and the judgment of the attending physician. Suitable dosages are in the range of 0.01-100.0 .mu.g/kg. Wide variations in the needed dosage are to be expected in view of the variety of peptides, fragments, and homologs available and the differing efficiencies of various routes of administration. For example, oral administration would be expected to require higher dosages than administration by i.v. injection. Variations in these dosage levels can be adjusted using standard empirical routines for optimization as is well understood in the art. Administrations can be single or multiple (e.g., 2-, 3-, 4-, 6-, 8-, 10-; 20-, 50-, 100-, 150-, or more fold). Encapsulation of the peptides, fragments, and homologs in a suitable delivery vehicle (e.g., polymeric microparticles or implantable devices) may increase the efficiency of delivery, particularly for oral delivery.
[0070] According to certain embodiments, the peptides, or fragments thereof can be targeted to specific cells or tissues in vivo. Targeting delivery vehicles, including liposomes and targeted systems are known in the art. For example, a liposome can be directed to a particular target cell or tissue by using a targeting agent, such as an antibody, soluble receptor or ligand, incorporated with the liposome, to target a particular cell or tissue to which the targeting molecule can bind. Targeting liposomes are described, for example, in Ho et al., Biochemistry 25:5500 (1986); Ho et al., J. Biol. Chem. 262:13979 (1987); Ho et al., J. Biol. Chem. 262:13973 (1987); and U.S. Pat. No. 4,957,735 to Huang et al., each of which is incorporated herein by reference in its entirety).
[0071] Another aspect of the invention relates to a method of inhibiting sodium absorption through a sodium channel, comprising contacting (e.g., binding) the sodium channel with a specialized non-naturally occurring peptide or fragment thereof. Inhibition of sodium absorption can be measured by any technique known in the art or disclosed herein.
[0072] Another aspect of the invention relates to a method of increasing the volume of fluid lining an epithelial mucosal surface, comprising contacting (e.g., binding) a sodium channel present on the epithelial mucosal surface with a specialized non-naturally occurring peptide or a functional fragment or homolog thereof. The volume of fluid lining an epithelial mucosal surface can be measured by any technique known in the art or disclosed herein.
[0073] A further aspect of the invention relates to a method of reducing the level of a sodium channel present on the surface of a cell, comprising contacting (e.g., binding) the sodium channel with a specialized non-naturally occurring peptide or a functional fragment or homolog thereof.
[0074] An additional aspect of the invention relates to a method of treating a disorder responsive to inhibition of sodium absorption across an epithelial mucosal surface in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of a specialized non-naturally occurring peptide or a functional fragment or homolog thereof. In one embodiment, the invention encompasses a method for treating a symptom of a disorder responsive to inhibition of sodium absorption across an epithelial mucosal surface in a subject in need thereof, comprising administering a peptide comprising a sequence selected from SEQ ID NOS:2-128 to the subject. The disorder in the methods of the invention can be, in non-limiting examples, a lung disorder (e.g., cystic fibrosis, non-cystic fibrosis bronchiectasis, chronic obstructive pulmonary disease, acute or chronic bronchitis, or asthma), a gastrointestinal disorder (e.g., inflammatory bowel disease), a kidney disorder, or a cardiovascular disorder.
[0075] Another aspect of the invention relates to a method of regulating salt balance, blood volume, and/or blood pressure in a subject in need thereof, comprising delivering to the subject a therapeutically effective amount of a specialized non-naturally occurring peptide or a functional fragment or homolog thereof.
[0076] A third aspect of the invention relates to products that can be used to carry out the methods disclosed herein. Thus, one aspect of the invention relates to a peptide comprising the sequence:
TABLE-US-00002 (SEQ ID NO: 116) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8
wherein: X.sub.1 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.2 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.3 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.4 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.5 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.6 is aspartic acid or a conservative substitution with a natural or non-natural amino acid; X.sub.7 is glutamine or a conservative substitution with a natural or non-natural amino acid; and X.sub.8 is threonine or a conservative substitution with a natural or non-natural amino acid; or a functional fragment thereof.
[0077] Another aspect of the invention relates to a peptide comprising the sequence:
TABLE-US-00003 (SEQ ID NO: 117) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8
wherein: X.sub.1 is leucine, norleucine, or valine; X.sub.2 is proline, 4-hydroxyproline, (2R,5S)-5-phenyl-pyrrolidine-2-carboxylic acid, or 3,4-dehydro-L-proline; X.sub.3 is valine, leucine, norleucine, or N-methylvaline; X.sub.4 is proline, 4-hydroxyproline, (2R,5S)-5-phenyl-pyrrolidine-2-carboxylic acid, or 3,4-dehydro-L-proline; X.sub.5 is leucine, norleucine, or valine; X.sub.6 is aspartic acid or glutamic acid; X.sub.7 is glutamine or asparagine; and X.sub.8 is threonine, serine, or L-.alpha.-methylserine; or a functional fragment thereof.
[0078] A further aspect of the invention relates to a peptide comprising the sequence:
TABLE-US-00004 (SEQ ID NO: 118) X.sub.1-X.sub.2-X.sub.3-X.sub.4-X.sub.5-X.sub.6-X.sub.7-X.sub.8 -X.sub.9-X.sub.10-X.sub.11-X.sub.12-X.sub.13-X.sub.14- X.sub.15
wherein: X.sub.1 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.3 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.3 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.4 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.5 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.6 is aspartic acid or a conservative substitution with a natural or non-natural amino acid; X.sub.7 is glutamine or a conservative substitution with a natural or non-natural amino acid; X.sub.8 is threonine or a conservative substitution with a natural or non-natural amino acid; X.sub.9 is threonine or a conservative substitution with a natural or non-natural amino acid; X.sub.10 is leucine or a conservative substitution with a natural or non-natural amino acid; X.sub.11 is proline or a conservative substitution with a natural or non-natural amino acid; X.sub.12 is asparagine or a conservative substitution with a natural or non-natural amino acid; X.sub.13 is valine or a conservative substitution with a natural or non-natural amino acid; X.sub.14 is asparagine or a conservative substitution with a natural or non-natural amino acid; X.sub.15 is proline or a conservative substitution with a natural or non-natural amino acid; or a functional fragment thereof.
[0079] In some embodiments, the peptide comprises a sequence selected from the group consisting of SEQ ID NOS:4-142 and SEQ ID NO: 144. In one embodiment, the peptide comprises the sequence of SEQ ID NO:5 or SEQ ID NO: 122. In one embodiment, the peptide comprises the sequence of SEQ ID NO:2 (aaLPVPLDQTLPLNVNPaa) or SEQ ID NO:119. In one embodiment, the peptide comprises the sequence of SEQ ID NO: 127 or SEQ ID NO:128 (aaLPIPLDQTaa).
[0080] In certain embodiments, the peptides of the invention comprise at least one modified terminus, e.g., to protect the peptide against degradation. In some embodiments, the N-terminus is acetylated and/or the C-terminus is amidated. In some embodiments, the peptide comprises the sequence of any one of SEQ ID NOS:10-127, 129, 130, 133, 134, or 136-140, further comprising one or two D-alanines at the amino- and/or carboxyl-terminal ends.
[0081] In certain embodiments, the peptides of the invention comprise at least one non-natural amino acid (e.g., 1, 2, 3, or more) or at least one terminal modification (e.g., 1 or 2). In some embodiments, the peptide comprises at least one non-natural amino acid and at least one terminal modification.
[0082] In certain embodiments, the peptide mimics the sodium channel binding domain of a PLUNC protein. The sodium channel binding domain is the minimal fragment of the PLUNC protein required to have substantially the same binding activity to the sodium channel as the full length PLUNC protein. The term "substantially the same binding activity" refers to an activity that is at least about 50% of the binding activity of the full length protein, e.g., at least about 60%, 70%, 80%, or 90% of the binding activity. In some embodiments, the peptide has at least the same binding activity as the full length PLUNC protein. In one embodiment, the sodium channel is ENaC, e.g., human ENaC. In another embodiment, the sodium channel is one that is similar in sequence and/or structure to ENaC, such as acid-sensing ion channels (ASIC).
[0083] The peptides of the present invention can optionally be delivered in conjunction with other therapeutic agents. The additional therapeutic agents can be delivered concurrently with the peptides of the invention. As used herein, the word "concurrently" means sufficiently close in time to produce a combined effect (that is, concurrently can be simultaneously, or it can be two or more events occurring within a short time period before or after each other). In one embodiment of the invention, the specialized non-naturally occurring peptide is delivered to a patient concurrently with a compound that modulates the function of the Cystic fibrosis transmembrane conductance regulator (CFTR) where the combined activity of the specialized non-naturally occurring peptide and the CFTR-targeted agent have superior activity to the CFTR-targeted agent alone. In another embodiment of the invention, the specialized non-naturally occurring peptide is delivered to a patient concurrently with a mucolytic compound where the combined activity of the specialized non-naturally occurring peptide and the mucolytic agent have superior activity to the mucolytic agent alone. In yet another embodiment of the invention, the specialized non-naturally occurring peptide is delivered to a patient concurrently with a long acting B-agonist compound (LABA) where the combined activity of the specialized non-naturally occurring peptide and the LABA agent have superior activity to the LABA alone. In yet another embodiment of the invention, the specialized non-naturally occurring peptide is delivered to a patient concurrently with a glucocorticoid agonist where the combined activity of the specialized non-naturally occurring peptide and the glucocorticoid agent have superior activity to the glucocorticoid alone.
[0084] Another aspect of the invention relates to a kit comprising the peptide of the invention and useful for carrying out the methods of the invention. The kit may further comprise additional reagents for carrying out the methods (e.g., buffers, containers, additional therapeutic agents) as well as instructions.
[0085] As a further aspect, the invention provides pharmaceutical formulations and methods of administering the same to achieve any of the therapeutic effects (e.g., modulation of sodium absorption) discussed above. The pharmaceutical formulation may comprise any of the reagents discussed above in a pharmaceutically acceptable carrier, e.g., a specialized non-naturally occurring peptide or functional fragment thereof.
[0086] By "pharmaceutically acceptable" it is meant a material that is not biologically or otherwise undesirable, i.e., the material can be administered to a subject without causing any undesirable biological effects such as toxicity.
[0087] The formulations of the invention can optionally comprise medicinal agents, pharmaceutical agents, carriers, adjuvants, dispersing agents, diluents, and the like.
[0088] The peptides of the invention can be formulated for administration in a pharmaceutical carrier in accordance with known techniques. See, e.g., Remington, The Science And Practice of Pharmacy (9.sup.th Ed. 1995). In the manufacture of a pharmaceutical formulation according to the invention, the peptide (including the physiologically acceptable salts thereof) is typically admixed with, inter alia, an acceptable carrier. The carrier can be a solid or a liquid, or both, and is preferably formulated with the peptide as a unit-dose formulation, for example, a tablet, which can contain from 0.01 or 0.5% to 95% or 99% by weight of the peptide. One or more peptides can be incorporated in the formulations of the invention, which can be prepared by any of the well-known techniques of pharmacy.
[0089] A further aspect of the invention is a method of treating subjects in vivo, comprising administering to a subject a pharmaceutical composition comprising a peptide of the invention in a pharmaceutically acceptable carrier, wherein the pharmaceutical composition is administered in a therapeutically effective amount. Administration of the peptides of the present invention to a human subject or an animal in need thereof can be by any means known in the art for administering compounds.
[0090] The formulations of the invention include those suitable for oral, rectal, topical, buccal (e.g., sub-lingual), vaginal, parenteral (e.g., subcutaneous, intramuscular including skeletal muscle, cardiac muscle, diaphragm muscle and smooth muscle, intradermal, intravenous, intraperitoneal), topical (i.e., both skin and mucosal surfaces, including airway surfaces), intranasal, transdermal, intraarticular, intrathecal, and inhalation administration, administration to the liver by intraportal delivery, as well as direct organ injection (e.g., into the liver, into the brain for delivery to the central nervous system, into the pancreas, or into a tumor or the tissue surrounding a tumor). The most suitable route in any given case will depend on the nature and severity of the condition being treated and on the nature of the particular peptide which is being used.
[0091] For injection, the carrier will typically be a liquid, such as sterile pyrogen-free water, sterile normal saline, hypertonic saline, pyrogen-free phosphate-buffered saline solution, bacteriostatic water, or Cremophor EL[R] (BASF, Parsippany, N.J.). For other methods of administration, the carrier can be either solid or liquid.
[0092] For oral administration, the peptide can be administered in solid dosage forms, such as capsules, tablets, and powders, or in liquid dosage forms, such as elixirs, syrups, and suspensions. Peptides can be encapsulated in gelatin capsules together with inactive ingredients and powdered carriers, such as glucose, lactose, sucrose, mannitol, starch, cellulose or cellulose derivatives, magnesium stearate, stearic acid, sodium saccharin, talcum, magnesium carbonate and the like. Examples of additional inactive ingredients that can be added to provide desirable color, taste, stability, buffering capacity, dispersion or other known desirable features are red iron oxide, silica gel, sodium lauryl sulfate, titanium dioxide, edible white ink and the like. Similar diluents can be used to make compressed tablets. Both tablets and capsules can be manufactured as sustained release products to provide for continuous release of medication over a period of hours. Compressed tablets can be sugar coated or film coated to mask any unpleasant taste and protect the tablet from the atmosphere, or enteric-coated for selective disintegration in the gastrointestinal tract. Liquid dosage forms for oral administration can contain coloring and flavoring to increase patient acceptance.
[0093] Formulations suitable for buccal (sub-lingual) administration include lozenges comprising the compound in a flavored base, usually sucrose and acacia or tragacanth; and pastilles comprising the compound in an inert base such as gelatin and glycerin or sucrose and acacia.
[0094] Formulations of the present invention suitable for parenteral administration comprise sterile aqueous and non-aqueous injection solutions of the peptide, which preparations are preferably isotonic with the blood of the intended recipient. These preparations can contain anti-oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient. Aqueous and non-aqueous sterile suspensions can include suspending agents and thickening agents. The formulations can be presented in unit/dose or multi-dose containers, for example sealed ampoules and vials, and can be stored in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example, saline or water-for-injection immediately prior to use.
[0095] Extemporaneous injection solutions and suspensions can be prepared from sterile powders, granules and tablets of the kind previously described. For example, in one aspect of the present invention, there is provided an injectable, stable, sterile composition comprising a peptide of the invention, in a unit dosage form in a sealed container. The peptide or salt is provided in the form of a lyophilizate which is capable of being reconstituted with a suitable pharmaceutically acceptable carrier to form a liquid composition suitable for injection thereof into a subject. The unit dosage form typically comprises from about 1 mg to about 10 grams of the peptide or salt. When the peptide or salt is substantially water-insoluble, a sufficient amount of emulsifying agent which is pharmaceutically acceptable can be employed in sufficient quantity to emulsify the peptide or salt in an aqueous carrier. One such useful emulsifying agent is phosphatidyl choline.
[0096] Formulations suitable for rectal administration are preferably presented as unit dose suppositories. These can be prepared by admixing the peptide with one or more conventional solid carriers, for example, cocoa butter, and then shaping the resulting mixture.
[0097] Formulations suitable for topical application to the skin preferably take the form of an ointment, cream, lotion, paste, gel, spray, aerosol, or oil. Carriers which can be used include petroleum jelly, lanoline, polyethylene glycols, alcohols, transdermal enhancers, and combinations of two or more thereof.
[0098] Formulations suitable for transdermal administration can be presented as discrete patches adapted to remain in intimate contact with the epidermis of the recipient for a prolonged period of time. Formulations suitable for transdermal administration can also be delivered by iontophoresis (see, for example, Tyle, Pharm. Res. 3:318 (1986)) and typically take the form of an optionally buffered aqueous solution of the peptides. Suitable formulations comprise citrate or bis/tris buffer (pH 6) or ethanol/water and contain from 0.1 to 0.2M of the compound.
[0099] The peptide can alternatively be formulated for nasal administration or otherwise administered to the lungs of a subject by any suitable means, e.g., administered by an aerosol suspension of respirable particles comprising the peptide, which the subject inhales. The respirable particles can be liquid or solid. The term "aerosol" includes any gas-borne suspended phase, which is capable of being inhaled into the bronchioles or nasal passages. Specifically, aerosol includes a gas-borne suspension of droplets, as can be produced in a metered dose inhaler or nebulizer, or in a mist sprayer. Aerosol also includes a dry powder composition suspended in air or other carrier gas, which can be delivered by insufflation from an inhaler device, for example. See Ganderton & Jones, Drug Delivery to the Respiratory Tract, Ellis Horwood (1987); Gonda (1990) Critical Reviews in Therapeutic Drug Carrier Systems 6:273-313; and Raeburn et al., J. Pharmacol. Toxicol. Meth. 27:143 (1992). Aerosols of liquid particles comprising the peptide can be produced by any suitable means, such as with a pressure-driven aerosol nebulizer or an ultrasonic nebulizer, as is known to those of skill in the art. See, e.g., U.S. Pat. No. 4,501,729. Aerosols of solid particles comprising the peptide can likewise be produced with any solid particulate medicament aerosol generator, by techniques known in the pharmaceutical art.
[0100] Alternatively, one can administer the peptide in a local rather than systemic manner, for example, in a depot or sustained-release formulation.
[0101] Further, the present invention provides liposomal formulations of the peptides disclosed herein and salts thereof. The technology for forming liposomal suspensions is well known in the art. When the peptide or salt thereof is an aqueous-soluble salt, using conventional liposome technology, the same can be incorporated into lipid vesicles. In such an instance, due to the water solubility of the peptide or salt, the peptide or salt will be substantially entrained within the hydrophilic center or core of the liposomes. The lipid layer employed can be of any conventional composition and can either contain cholesterol or can be cholesterol-free. When the peptide or salt of interest is water-insoluble, again employing conventional liposome formation technology, the salt can be substantially entrained within the hydrophobic lipid bilayer which forms the structure of the liposome. In either instance, the liposomes which are produced can be reduced in size, as through the use of standard sonication and homogenization techniques.
[0102] The liposomal formulations containing the peptides disclosed herein or salts thereof, can be lyophilized to produce a lyophilizate which can be reconstituted with a pharmaceutically acceptable carrier, such as water, to regenerate a liposomal suspension.
[0103] In the case of water-insoluble peptides, a pharmaceutical composition can be prepared containing the water-insoluble peptide, such as for example, in an aqueous base emulsion. In such an instance, the composition will contain a sufficient amount of pharmaceutically acceptable emulsifying agent to emulsify the desired amount of the peptide. Particularly useful emulsifying agents include phosphatidyl cholines and lecithin.
[0104] In particular embodiments, the peptide is administered to the subject in a therapeutically effective amount, as that term is defined above. Dosages of pharmaceutically active peptides can be determined by methods known in the art, see, e.g., Remington's Pharmaceutical Sciences (Mack Publishing Co., Easton, Pa.). The therapeutically effective dosage of any specific peptide will vary somewhat from peptide to peptide, and patient to patient, and will depend upon the condition of the patient and the route of delivery. As a general proposition, a dosage from about 0.1 to about 50 mg/kg will have therapeutic efficacy, with all weights being calculated based upon the weight of the peptide, including the cases where a salt is employed. Toxicity concerns at the higher level can restrict intravenous dosages to a lower level such as up to about 10 mg/kg, with all weights being calculated based upon the weight of the peptide, including the cases where a salt is employed. A dosage from about 10 mg/kg to about 50 mg/kg can be employed for oral administration. Typically, a dosage from about 0.5 mg/kg to 5 mg/kg can be employed for intramuscular injection. Particular dosages are about 1 .mu.mol/kg to 50 .mu.mol/kg, and more particularly to about 22 .mu.mol/kg and to 33 .mu.mol/kg of the peptide for intravenous or oral administration, respectively.
[0105] In particular embodiments of the invention, more than one administration (e.g., two, three, four, or more administrations) can be employed over a variety of time intervals (e.g., hourly, daily, weekly, monthly, etc.) to achieve therapeutic effects.
[0106] The present invention finds use in veterinary and medical applications. Suitable subjects include both avians and mammals, with mammals being preferred. The term "avian" as used herein includes, but is not limited to, chickens, ducks, geese, quail, turkeys, and pheasants. The term "mammal" as used herein includes, but is not limited to, humans, bovines, ovines, caprines, equines, felines, canines, lagomorphs, etc. Human subjects include neonates, infants, juveniles, and adults.
[0107] The present invention is more particularly described in the following examples that are intended as illustrative only since numerous modifications and variations therein will be apparent to those skilled in the art.
Example 1
Experimental Methods
[0108] Tissue Procurement and Cell Culture:
[0109] Cells were harvested by enzymatic digestion from human bronchial tissue as previously described under a protocol approved by the UNC School of Medicine IRB (Tarran et al., J. Gen. Physiol. 127:591 (2006)). Human excess donor lungs and excised recipient lungs were obtained at the time of lung transplantation from portions of main stem or lumbar bronchi and cells were harvested by enzymatic digestion. All preparations were maintained at an air-liquid interface in a modified bronchial epithelial medium and used 2-5 weeks after seeding on 12 mm T-Clear inserts (Corning Costar) coated with human placental type VI collagen (Sigma). Phosphate buffered saline (PBS) was used for washing human bronchial epithelial culture mucosal surfaces.
[0110] Confocal Microscopy:
[0111] To label airway surface liquid, Ringer containing Texas Red-dextran (2 mg/ml; Invitrogen) was added to human bronchial epithelial culture mucosal surfaces. Perfluorocarbon was added mucosally to prevent evaporation of the airway surface liquid and the culture placed in a chamber containing 100 .mu.l Ringer on the stage of a Leica SP8 confocal microscope with a 63.times. glycerol immersion objective. 10 points per culture were scanned and an average airway surface liquid height determined. For confocal microscopy human bronchial epithelial cultures were bathed serosally in a modified Ringer solution containing (mM): 116 NaCl, 10 NaHCO.sub.3, 5.1 KCl, 1.2 CaCl.sub.2), 1.2 MgCl.sub.2, 20 TES, 10 glucose, pH 7.4). At all other times, human bronchial epithelial cultures were maintained in a modified BEGM growth medium which contained 24 mM NaHCO.sub.3 gassed with 5% CO.sub.2. Perfluorocarbon (FC-77) was obtained from 3M and had no effect on ASL height as previously reported.
[0112] Internalization of alpha-ENaC: HEK293T cells were transfected with gfp-.alpha.ENaC where gfp was fused at the N-terminus of ENaC and unlabeled .beta.ENaC and .gamma.ENaC using lipofectamine in 384 well plates as per the manufacturer's instructions and as published previously (see, e.g., Hobbs et al. Am J Physiol Lung Cell Mol Physiol. 2013 December; 305(12):L990-L1001. doi: 10.1152/ajplung.00103.2013. Epub 2013 October; Garland et al. Proc Natl Acad Sci USA. 2013 Oct. 1; 110(40):15973-8. doi: 10.1073/pnas.1311999110. Epub 2013 Sep. 16; Tan et al. J Physiol. 2014 Dec. 1; 592(Pt 23):5251-68). Twenty-four hours later, peptide or vehicle control were added at t=0 and fluorescence was read using a Tecan M1000 plate reader 3 h later. Fluorescence was read at 488 and 510 nm.
[0113] FIG. 6 shows the results of an experiment analyzing the effects of various peptides on internalization of alpha-ENaC in HEK293T cells when GFP-tagged alpha-ENaC is co-expressed only with gamma ENaC and no beta-ENaC. In this figure, SEQ ID NO:143 (negative control peptide) and water (vehicle) controls are used along with S18 (SEQ ID NO: 1, and SEQ ID NO:128. While SEQ ID NO:1 AND SEQ ID NO:128 are effective in reducing alpha-ENaC when beta-ENaC is co-expressed, no effect is observed in this experiment when beta-ENaC is not present.
[0114] Efficacy Testing in .beta.ENaC Mice:
[0115] Like CF patients, .beta.ENaC-Tg mice are disease free at birth, but soon develop obstructive lung disease (Zhou, Z., et al. Am J Respir Crit Care Med 178, 1245-1256 (2008)). C57BL:FVB and C57BL:C3H mixed strains have 90% and 50% mortality, respectively, and the reproducibility of the disease on these backgrounds is sufficiently high that reliable data are produced with n of about 8-10 animals/group. Furthermore, the .beta.ENaC-Tg mouse responds to therapeutic interventions in a fashion similar to CF/COPD in humans, and the development of lung disease can be prevented following inhibition of lung disease at birth (Livraghi, A., et al. J Immunol 182, 4357-4367 (2009)). S18-derived peptides or vehicle were dosed one to three times a day by intranasal instillation (1 .mu.l/g body weight) for 14 days in parallel cohorts of mice. Mice were weighed daily and, if a diuretic effect was found, the volume excreted was replaced by sub-cutaneous injections of sterile saline. At the end of the treatment, mice were sacrificed for phenotypic analysis.
[0116] Statistical Analyses:
[0117] All data are presented as the mean.+-.SE for n experiments. Airway cultures derived from three or more separate donors were used for each study and each oocyte study was repeated on three separate occasions. Differences between means were tested for statistical significance using paired or unpaired t tests or their non parametric equivalent as appropriate to the experiment. From such comparisons, differences yielding P.ltoreq.0.05 were judged to be significant. All binding assays were fitted to the Hill equation.
Example 2
Identification of Specialized Non-Naturally Occurring Peptides
[0118] Based on the ability of normal human bronchial epithelial cultures to regulate airway surface liquid height to 7 .mu.m, which was paralleled by a decrease in trypsin-sensitive ENaC activity, we previously demonstrated that SPLUNC1 derived peptides (namely, S18; FIG. 1) can inhibit ENaC with EC.sub.50 in the sub micromolar range for up to 24 h following a single dose (Hobbs et al., 2013). While S18 is resistant to proteolysis and heat-stable, we tested whether we could reduce the size of S1 8, increase its stability and/or increase its potency. Any of these actions would increase its utility as a drug.
[0119] To confirm our alanine-scan data, we made a peptide of subsequence A (LPVPLDQT (SEQ ID NO:113); FIG. 1). As a control, we made an overlapping 2nd peptide, which contained the charged mid-region (DQT), as well as subsequence B (DQTLPLNVNP (SEQ ID NO: 114)). As shown in FIG. 2, LPVPLDQT (SEQ ID NO: 113) retained similar activity to S18, whilst DQTLPLNVNP (SEQ ID NO:114) was inactive. However, additional experiments, where we examined susceptibility to proteases, demonstrated that whilst LPVPLDQT (SEQ ID NO: 113) could inhibit ENaC-led fluid absorption, this peptide was more susceptible to proteolytic degradation. Thus, we generated new peptides where we flanked the C- and N-termini with a pair of D-alanines (shown as lower case `a`). Peptides were added mucosally to HBECs at 30 .mu.M and ASL height measured 3 h later by XZ-confocal microscopy. FIG. 2A shows the comparison of the N-terminal region of S18 (LPVPLDQT) (SEQ ID NO: 113) vs. an overlapping, but C-terminal segment (DQTLPLNVNP) (SEQ ID NO: 114). In FIG. 2B, D-alanines were added to Sub-S18 peptides LPVPLDQT (SEQ ID NO: 113) and LPVPLDQTLPL (SEQ ID NO: 115) to increase the stability of LPVPLDQT (SEQ ID NO: 113). D-alanines are noted as lowercase a. All n=6-9. Data are shown as mean.+-.SEM.
[0120] The 15-mer and 8-mer showed identical activity when compared against S18 (FIGS. 2A-2B). We then generated full dose-responses for S18 vs. the peptide aaLPVPLDQTaa (SEQ ID NO:3). S18 and aaLPVPLDQTaa (SEQ ID NO:3) produced identical dose responses (FIG. 3A), indicating that shortening the peptide and flanking it with unnatural amino acids did not affect its potency or efficacy. Next, we made several new peptides based on the sequence of SEQ ID NO:2. Here, since prolines typically provide kinks in a peptide, they were not altered and instead, we systematically made conservative substitutions of the other amino acids. The dose responses are shown in FIG. 3B. Note, since there are several lines on this graph, the error bars and data symbols were omitted. However, these data were obtained simultaneously using paired airway cultures, so their comparison is valid. Clearly, SEQ ID NO:5 (aaLPNlePLDQTaa) shows a log-fold increase in potency as compared to S18, whilst SEQ ID NO:9 (aaLPVPLDQSaa) and SEQ ID NO:6 (aaLPVPNleDQTaa) show diminished potency and efficacy respectively (FIG. 3B). Other sequences included in FIG. 3B are SEQ ID NO:4 (aaNlePVPLDQTaa), SEQ ID NO:7 (aaLPVPLDNTaa) and SEQ ID NO:8 (aaLPVPLEQTaa).
[0121] Using a fresh batch of airway cells, we performed an additional dose response to SEQ ID NO:5 (FIG. 4). As can be seen, this peptide gave a significant increase in potency, as compared to S18, and the IC.sub.50 was 30 nM.
[0122] The ability of the peptides to modulate internalization of .alpha.ENaC was tested. Results from these experiments are shown in FIGS. 5A-5U. ADG is a negative control peptide with the sequence: NH3-ADGGLLLLNNPPPPQTVV-NH2 (SEQ ID NO: 143). The peptides used in these experiments were S18 (SEQ ID NO:1), SEQ ID NO:2, SEQ ID NO:141, SEQ ID NO:129, SEQ ID NO:130, SEQ ID NO:128, SEQ ID NO:131, SEQ ID NO:132, SEQ ID NO:133, SEQ ID NO:134, SEQ ID NO:135, SEQ ID NO:136, SEQ ID NO:137, SEQ ID NO:138, SEQ ID NO:131, SEQ ID NO:139; SEQ ID NO: 140, SEQ ID NO: 144 and SEQ ID NO:127. Also used in these experiments were peptides of SEQ ID NO:12, SEQ ID NO:13, SEQ ID NO:16, SEQ ID NO: 17, SEQ ID NO: 18, and SEQ ID NO: 136, each with an aa cap at both the N- and the C-terminus. Also shown is amiloride, which inhibits ENaC by another mechanism and does not reduce the amount of functional receptor on the surface of cells. These results demonstrate that the SPLUNC peptides trigger the destruction of the ENaC function to inhibit its activity.
[0123] The efficacy of the peptides was tested in .beta.ENaC mice. Results from these experiments are shown in FIGS. 7A-7F. Fourteen-day treatment with inhaled SEQ ID NO: 128 was more effective than inhaled SEQ ID NO:142 at increasing percent survival of .beta.ENaC-Tg C57BL:FVB mice (FIG. 7B). Fourteen-day treatment with SEQ ID NO:128 also increased percent survival of .beta.ENaC-Tg C57BL:C3H mice (FIGS. 7C and 7D). Once daily dosing with SEQ ID NO:128 increased percent survival of (ENaC-Tg C57BL:C3H mice (FIG. 7E, left panel). The right panel of FIG. 7E shows that SEQ ID NO: 128 did not have a diuretic effect. FIG. 7F shows that treatment with SEQ ID NO:127 increased percent survival of ENaC-Tg C57BL:C3H mice. Sequences of other peptides used in the experiment were SEQ ID NO:129, SEQ ID NO: 134, and SEQ ID NO: 136.
[0124] The foregoing is illustrative of the present invention, and is not to be construed as limiting thereof. The invention is defined by the following claims, with equivalents of the claims to be included therein.
Sequence CWU 1
1
144118PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(18)..(18)AMIDATION 1Gly Gly Leu Pro Val Pro Leu Asp
Gln Thr Leu Pro Leu Asn Val Asn 1 5 10 15 Pro Ala 219PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide sequenceMISC_FEATURE(1)..(2)Xaa is
d-AlaMISC_FEATURE(18)..(19)Xaa is d-AlaMOD_RES(19)..(19)AMIDATION
2Xaa Xaa Leu Pro Val Pro Leu Asp Gln Thr Leu Pro Leu Asn Val Asn 1
5 10 15 Pro Xaa Xaa 312PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(11)..(12)Xaa is D-AlaMOD_RES(12)..(12)AMIDATION
3Xaa Xaa Leu Pro Val Pro Leu Asp Gln Thr Xaa Xaa 1 5 10
412PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(2)Xaa is D-AlaMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(11)..(12)Xaa is
D-AlaMOD_RES(12)..(12)AMIDATION 4Xaa Xaa Xaa Pro Val Pro Leu Asp
Gln Thr Xaa Xaa 1 5 10 512PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(5)..(5)Xaa is NorleucineMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 5Xaa Xaa Leu Pro Xaa Pro Leu Asp
Gln Thr Xaa Xaa 1 5 10 612PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(7)..(7)Xaa is NorleucineMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 6Xaa Xaa Leu Pro Val Pro Xaa Asp
Gln Thr Xaa Xaa 1 5 10 712PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(11)..(12)Xaa is D-AlaMOD_RES(12)..(12)AMIDATION
7Xaa Xaa Leu Pro Val Pro Leu Asp Asn Thr Xaa Xaa 1 5 10
812PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(2)Xaa is D-AlaMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 8Xaa Xaa Leu Pro Val Pro Leu Glu
Gln Thr Xaa Xaa 1 5 10 912PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(11)..(12)Xaa is D-AlaMOD_RES(12)..(12)AMIDATION
9Xaa Xaa Leu Pro Val Pro Leu Asp Gln Ser Xaa Xaa 1 5 10
108PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 10Leu
Pro Val Pro Leu Asp Gln Thr 1 5 118PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 11Leu Xaa Val Pro Leu
Asp Gln Thr 1 5 128PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 12Leu Pro Val Xaa Leu
Asp Gln Thr 1 5 138PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 13Leu Xaa Val Xaa Leu
Asp Gln Thr 1 5 148PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is (2R, 5S)-5-phenyl-pyrrolidine-2-
carboxylic acidMOD_RES(8)..(8)AMIDATION 14Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 158PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is (2R, 5S)-5-phenyl-pyrrolidine-2-
carboxylic acidMOD_RES(8)..(8)AMIDATION 15Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 168PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 16Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 178PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 17Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 188PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 18Leu Xaa Val Xaa Leu Asp
Gln Thr 1 5 198PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 19Leu Pro Val Pro Leu Glu Gln Ser
1 5 208PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(8)..(8)Xaa is
L-alpha-methylserineMOD_RES(8)..(8)AMIDATION 20Leu Pro Val Pro Leu
Glu Gln Xaa 1 5 218PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 21Leu Pro Val Pro Val Asp Gln Thr
1 5 228PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 22Leu Pro Val Pro Val Glu Gln Ser
1 5 238PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 23Leu Pro Val Pro Val Asp Gln Ser
1 5 248PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 24Val Pro Val Pro Leu Asp Gln Thr
1 5 258PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 25Leu Pro Leu Pro Leu Asp Gln Ser
1 5 268PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 26Val Pro Leu Pro Leu Asp Gln Ser
1 5 278PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 27Leu Pro Leu Pro Leu Asp Gln Thr
1 5 288PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 28Val Xaa Leu Xaa Val Glu
Gln Ser 1 5 298PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 29Val Pro Leu Pro Val Glu Gln Ser
1 5 308PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 30Leu Pro Leu Pro Leu Glu Gln Ser
1 5 318PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
N-methylvalineMOD_RES(8)..(8)AMIDATION 31Leu Pro Xaa Pro Leu Asp
Gln Ser 1 5 328PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 32Leu Pro Xaa Pro Leu Asp Gln
Thr 1 5 338PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 33Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 348PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 34Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 358PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 35Leu Xaa Xaa Xaa Leu Glu
Gln Ser 1 5 368PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 36Leu Xaa Xaa Xaa Val Glu
Gln Ser 1 5 378PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 37Leu Pro Val Xaa Leu
Asp Gln Thr 1 5 388PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 38Leu Xaa Val Xaa Leu
Asp Gln Thr 1 5 398PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is (2R,
5S)-5-phenyl-pyrrolidine-2- carboxylic acidMOD_RES(8)..(8)AMIDATION
39Leu Xaa Val Pro Leu Asp Gln Thr 1 5 408PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is (2R,
5S)-5-phenyl-pyrrolidine-2- carboxylic acidMOD_RES(8)..(8)AMIDATION
40Leu Pro Val Xaa Leu Asp Gln Thr 1 5 418PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 41Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 428PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 42Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 438PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 43Leu Xaa Val Xaa Leu Asp
Gln Thr 1 5 448PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 44Leu
Pro Val Pro Leu Glu Gln Ser 1 5 458PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(8)..(8)Xaa is
L-alpha-methylserineMOD_RES(8)..(8)AMIDATION 45Leu Pro Val Pro Leu
Glu Gln Xaa 1 5 468PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 46Leu
Pro Val Pro Val Asp Gln Thr 1 5 478PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 47Leu
Pro Val Pro Val Glu Gln Ser 1 5 488PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 48Leu
Pro Val Pro Val Asp Gln Ser 1 5 498PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 49Val
Pro Val Pro Leu Asp Gln Thr 1 5 508PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 50Leu
Pro Leu Pro Leu Asp Gln Ser 1 5 518PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 51Val
Pro Leu Pro Leu Asp Gln Ser 1 5 528PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 52Leu
Pro Leu Pro Leu Asp Gln Thr 1 5 538PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 53Val Xaa Leu Xaa Val Glu
Gln Ser 1 5 548PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 54Val
Pro Leu Pro Val Glu Gln Ser 1 5 558PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 55Leu
Pro Leu Pro Leu Glu Gln Ser 1 5 568PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
N-methylvalineMOD_RES(8)..(8)AMIDATION 56Leu Pro Xaa Pro Leu Asp
Gln Ser 1 5 578PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 57Leu Pro Xaa Pro Leu Asp Gln
Thr 1 5 588PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 58Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 598PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 59Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 608PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting
peptide sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa
is 4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 60Leu Xaa Xaa Xaa Leu Glu
Gln Ser 1 5 618PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 61Leu Xaa Xaa Xaa Val Glu
Gln Ser 1 5 628PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 62Leu Xaa Val Pro Leu
Asp Gln Thr 1 5 638PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 63Leu Pro Val Xaa Leu
Asp Gln Thr 1 5 648PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 64Leu Xaa Val Xaa Leu
Asp Gln Thr 1 5 658PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is (2R, 5S)-5-phenyl-pyrrolidine-2-
carboxylic acidMOD_RES(8)..(8)AMIDATION 65Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 668PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is (2R, 5S)-5-phenyl-pyrrolidine-2-
carboxylic acidMOD_RES(8)..(8)AMIDATION 66Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 678PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 67Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 688PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 68Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 698PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 69Leu Xaa Val Xaa Leu Asp
Gln Thr 1 5 708PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 70Leu Pro Val Pro Leu Glu Gln Ser
1 5 718PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(8)..(8)Xaa is
L-alpha-methylserineMOD_RES(8)..(8)AMIDATION 71Leu Pro Val Pro Leu
Glu Gln Xaa 1 5 728PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 72Leu Pro Val Pro Val Asp Gln Thr
1 5 738PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 73Leu Pro Val Pro Val Glu Gln Ser
1 5 748PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 74Leu Pro Val Pro Val Asp Gln Ser
1 5 758PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 75Val Pro Val Pro Leu Asp Gln Thr
1 5 768PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 76Leu Pro Leu Pro Leu Asp Gln Ser
1 5 778PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 77Val Pro Leu Pro Leu Asp Gln Ser
1 5 788PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 78Leu Pro Leu Pro Leu Asp Gln Thr
1 5 798PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 79Val Xaa Leu Xaa Val Glu
Gln Ser 1 5 808PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 80Val Pro Leu Pro Val Glu Gln Ser
1 5 818PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 81Leu Pro Leu Pro Leu Glu Gln Ser
1 5 828PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
N-methylvalineMOD_RES(8)..(8)AMIDATION 82Leu Pro Xaa Pro Leu Asp
Gln Ser 1 5 838PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 83Leu Pro Xaa Pro Leu Asp Gln
Thr 1 5 848PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 84Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 858PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 85Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 868PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 86Leu Xaa Xaa Xaa Leu Glu
Gln Ser 1 5 878PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 87Leu Xaa Xaa Xaa Val Glu
Gln Ser 1 5 888PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 88Leu Pro Val Xaa Leu
Asp Gln Thr 1 5 898PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMISC_FEATURE(4)..(4)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 89Leu Xaa Val Xaa Leu
Asp Gln Thr 1 5 908PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is (2R,
5S)-5-phenyl-pyrrolidine-2- carboxylic acidMOD_RES(8)..(8)AMIDATION
90Leu Xaa Val Pro Leu Asp Gln Thr 1 5 918PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is (2R,
5S)-5-phenyl-pyrrolidine-2- carboxylic acidMOD_RES(8)..(8)AMIDATION
91Leu Pro Val Xaa Leu Asp Gln Thr 1 5 928PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 92Leu Xaa Val Pro Leu Asp
Gln Thr 1 5 938PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 93Leu Pro Val Xaa Leu Asp
Gln Thr 1 5 948PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 94Leu Xaa Val Xaa Leu Asp
Gln Thr 1 5 958PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 95Leu
Pro Val Pro Leu Glu Gln Ser 1 5 968PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(8)..(8)Xaa is
L-alpha-methylserineMOD_RES(8)..(8)AMIDATION 96Leu Pro Val Pro Leu
Glu Gln Xaa 1 5 978PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 97Leu
Pro Val Pro Val Asp Gln Thr 1 5 988PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 98Leu
Pro Val Pro Val Glu Gln Ser 1 5 998PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 99Leu
Pro Val Pro Val Asp Gln Ser 1 5 1008PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 100Val
Pro Val Pro Leu Asp Gln Thr 1 5 1018PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 101Leu
Pro Leu Pro Leu Asp Gln Ser 1 5 1028PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 102Val
Pro Leu Pro Leu Asp Gln Ser 1 5 1038PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 103Leu
Pro Leu Pro Leu Asp Gln Thr 1 5 1048PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 104Val Xaa Leu Xaa Val Glu
Gln Ser 1 5 1058PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 105Val
Pro Leu Pro Val Glu Gln Ser 1 5 1068PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 106Leu
Pro Leu Pro Leu Glu Gln Ser 1 5 1078PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
N-methylvalineMOD_RES(8)..(8)AMIDATION 107Leu Pro Xaa Pro Leu Asp
Gln Ser 1 5 1088PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 108Leu Pro Xaa Pro Leu Asp Gln
Thr 1 5 1098PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 109Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 1108PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 110Leu Pro Xaa Pro Val Glu Gln
Ser 1 5 1118PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 111Leu Xaa Xaa Xaa Leu Glu
Gln Ser 1 5 1128PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMISC_FEATURE(3)..(3)Xaa is
NorleucineMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 112Leu Xaa Xaa Xaa Val Glu
Gln Ser 1 5 1138PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 113Leu Pro Val Pro Leu Asp Gln Thr
1 5 11410PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 114Asp Gln Thr Leu Pro Leu Asn Val
Asn Pro 1 5 10 11511PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(11)..(11)AMIDATION 115Leu Pro Val Pro Leu Asp Gln
Thr Leu Pro Leu 1 5 10 1168PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(1)Xaa is L or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(2)..(2)Xaa is P or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(3)..(3)Xaa is
V or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(4)..(4)Xaa is P or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(5)..(5)Xaa is L or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(6)..(6)Xaa is
D or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(7)..(7)Xaa is Q or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(8)..(8)Xaa is T or a conservative substitution
with a natural or non-natural amino acidMOD_RES(8)..(8)AMIDATION
116Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa 1 5 1178PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium
channel
activity inhibiting peptide sequenceMISC_FEATURE(1)..(1)Xaa is L,
Norleucine or VMISC_FEATURE(2)..(2)Xaa is Pro, 4-hydroxyproline,
(2R, 5S)-5-phenyl-pyrrolidine-2-carboxylic acid or
3,4-dehydro-L-prolineMISC_FEATURE(3)..(3)Xaa is V, L, Norleucine or
N-methylvalineMISC_FEATURE(4)..(4)Xaa is Pro, 4-hydroxyproline,
(2R, 5S)-5-phenyl-pyrrolidine-2-carboxylic acid or
3,4-dehydro-L-prolineMISC_FEATURE(5)..(5)Xaa is L, Norleucine or
VMISC_FEATURE(6)..(6)Xaa is D or EMISC_FEATURE(7)..(7)Xaa is Q or
NMISC_FEATURE(8)..(8)Xaa is T, S or
L-alpha-methylserineMOD_RES(8)..(8)AMIDATION 117Xaa Xaa Xaa Xaa Xaa
Xaa Xaa Xaa 1 5 11815PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(1)Xaa is L or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(2)..(2)Xaa is P or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(3)..(3)Xaa is
V or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(4)..(4)Xaa os P or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(5)..(5)Xaa is L or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(6)..(6)Xaa is
D or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(7)..(7)Xaa is Q or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(8)..(8)Xaa is T or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(9)..(9)Xaa is
T or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(10)..(10)Xaa is L or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(11)..(11)Xaa is P or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(12)..(12)Xaa
is N or a conservative substitution with a natural or non-natural
amino acidMISC_FEATURE(13)..(13)Xaa is V or a conservative
substitution with a natural or non-natural amino
acidMISC_FEATURE(14)..(14)Xaa is N or a conservative substitution
with a natural or non-natural amino acidMISC_FEATURE(15)..(15)Xaa
is P or a conservative substitution with a natural or non-natural
amino acidMOD_RES(15)..(15)AMIDATION 118Xaa Xaa Xaa Xaa Xaa Xaa Xaa
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa 1 5 10 15 11915PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide sequenceMOD_RES(15)..(15)AMIDATION
119Leu Pro Val Pro Leu Asp Gln Thr Leu Pro Leu Asn Val Asn Pro 1 5
10 15 1208PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 120Leu Pro Val Pro Leu Asp Gln Thr
1 5 1218PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(1)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 121Xaa Pro Val Pro Leu Asp Gln
Thr 1 5 1228PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(3)..(3)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 122Leu Pro Xaa Pro Leu Asp Gln
Thr 1 5 1238PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(5)..(5)Xaa is
NorleucineMOD_RES(8)..(8)AMIDATION 123Leu Pro Val Pro Xaa Asp Gln
Thr 1 5 1248PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 124Leu Pro Val Pro Leu Asp Asn Thr
1 5 1258PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 125Leu Pro Val Pro Leu Glu Gln Thr
1 5 1268PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 126Leu Pro Val Pro Leu Asp Gln Ser
1 5 1278PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 127Leu Pro Ile Pro Leu Asp Gln Thr
1 5 12812PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(2)Xaa is D-AlaMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 128Xaa Xaa Leu Pro Ile Pro Leu
Asp Gln Thr Xaa Xaa 1 5 10 1298PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(3)..(3)Xaa is
AhpMOD_RES(8)..(8)AMIDATION 129Leu Pro Xaa Pro Leu Asp Gln Thr 1 5
1308PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(8)..(8)AMIDATION 130Leu Pro Phe Pro Leu Asp Gln Thr
1 5 13112PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(2)Xaa is D-AlaMISC_FEATURE(4)..(4)Xaa is
4-hydroxyprolineMISC_FEATURE(5)..(5)Xaa is
NorleucineMISC_FEATURE(11)..(12)Xaa is
D-AlaMOD_RES(12)..(12)AMIDATION 131Xaa Xaa Leu Xaa Xaa Pro Leu Asp
Gln Thr Xaa Xaa 1 5 10 13212PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(5)..(5)Xaa is NorleucineMISC_FEATURE(6)..(6)Xaa
is 4-hydroxyprolineMISC_FEATURE(11)..(12)Xaa is
D-AlaMOD_RES(12)..(12)AMIDATION 132Xaa Xaa Leu Pro Xaa Xaa Leu Asp
Gln Thr Xaa Xaa 1 5 10 13318PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(5)..(5)Xaa is
NorleucineMOD_RES(18)..(18)AMIDATION 133Gly Gly Leu Pro Xaa Pro Leu
Asp Gln Thr Leu Pro Leu Asn Val Asn 1 5 10 15 Pro Ala
1348PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(1)..(1)ACETYLATIONMOD_RES(8)..(8)AMIDATION 134Leu
Pro Ile Pro Leu Asp Gln Thr 1 5 13510PRTArtificial
SequenceDescription of Artificial Sequence Synthetic Sodium channel
activity inhibiting peptide sequenceMISC_FEATURE(1)..(1)Xaa is
D-AlaMISC_FEATURE(4)..(4)Xaa is NorleucineMISC_FEATURE(10)..(10)Xaa
is D-AlaMOD_RES(10)..(10)AMIDATION 135Xaa Leu Pro Xaa Pro Leu Asp
Gln Thr Xaa 1 5 10 1368PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(2)..(2)Xaa is
3,4-dehydro-L-prolineMOD_RES(8)..(8)AMIDATION 136Leu Xaa Val Pro
Leu Asp Gln Thr 1 5 1378PRTArtificial SequenceDescription of
Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMOD_RES(8)..(8)AMIDATION 137Val Pro Val Pro Leu Asp
Gln Ser 1 5 13818PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(18)..(18)AMIDATION 138Gly Gly Leu Pro Ile Pro Leu
Asp Gln Thr Leu Pro Leu Asn Val Asn 1 5 10 15 Pro Ala
1398PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(2)..(2)Xaa is
4-hydroxyprolineMOD_RES(8)..(8)AMIDATION 139Leu Xaa Ile Pro Leu Asp
Gln Thr 1 5 14018PRTArtificial SequenceDescription of Artificial
Sequence Synthetic Sodium channel activity inhibiting peptide
sequenceMOD_RES(18)..(18)AMIDATION 140Gly Gly Leu Pro Ile Pro Leu
Asp Gln Thr Leu Pro Leu Asn Val Asn 1 5 10 15 Pro Ala
14112PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(2)Xaa is D-AlaMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 141Xaa Xaa Leu Pro Leu Pro Leu
Asp Gln Thr Xaa Xaa 1 5 10 14212PRTArtificial SequenceDescription
of Artificial Sequence Synthetic Sodium channel activity inhibiting
peptide sequenceMISC_FEATURE(1)..(2)Xaa is
D-AlaMISC_FEATURE(5)..(5)Xaa is NorleucineMISC_FEATURE(11)..(12)Xaa
is D-AlaMOD_RES(12)..(12)AMIDATION 142Xaa Xaa Leu Pro Xaa Pro Leu
Asp Gln Thr Xaa Xaa 1 5 10 14318PRTArtificial SequenceDescription
of Artificial Sequence Synthetic ADG negative control peptide
sequenceMOD_RES(18)..(18)AMIDATION 143Ala Asp Gly Gly Leu Leu Leu
Leu Asn Asn Pro Pro Pro Pro Gln Thr 1 5 10 15 Val Val
14410PRTArtificial SequenceDescription of Artificial Sequence
Synthetic Sodium channel activity inhibiting peptide
sequenceMISC_FEATURE(1)..(1)Xaa is D-AlaMISC_FEATURE(10)..(10)Xaa
is D-AlaMOD_RES(10)..(10)AMIDATION 144Xaa Leu Pro Ile Pro Leu Glu
Gln Ser Xaa 1 5 10
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

D00012

D00013

D00014

D00015

D00016
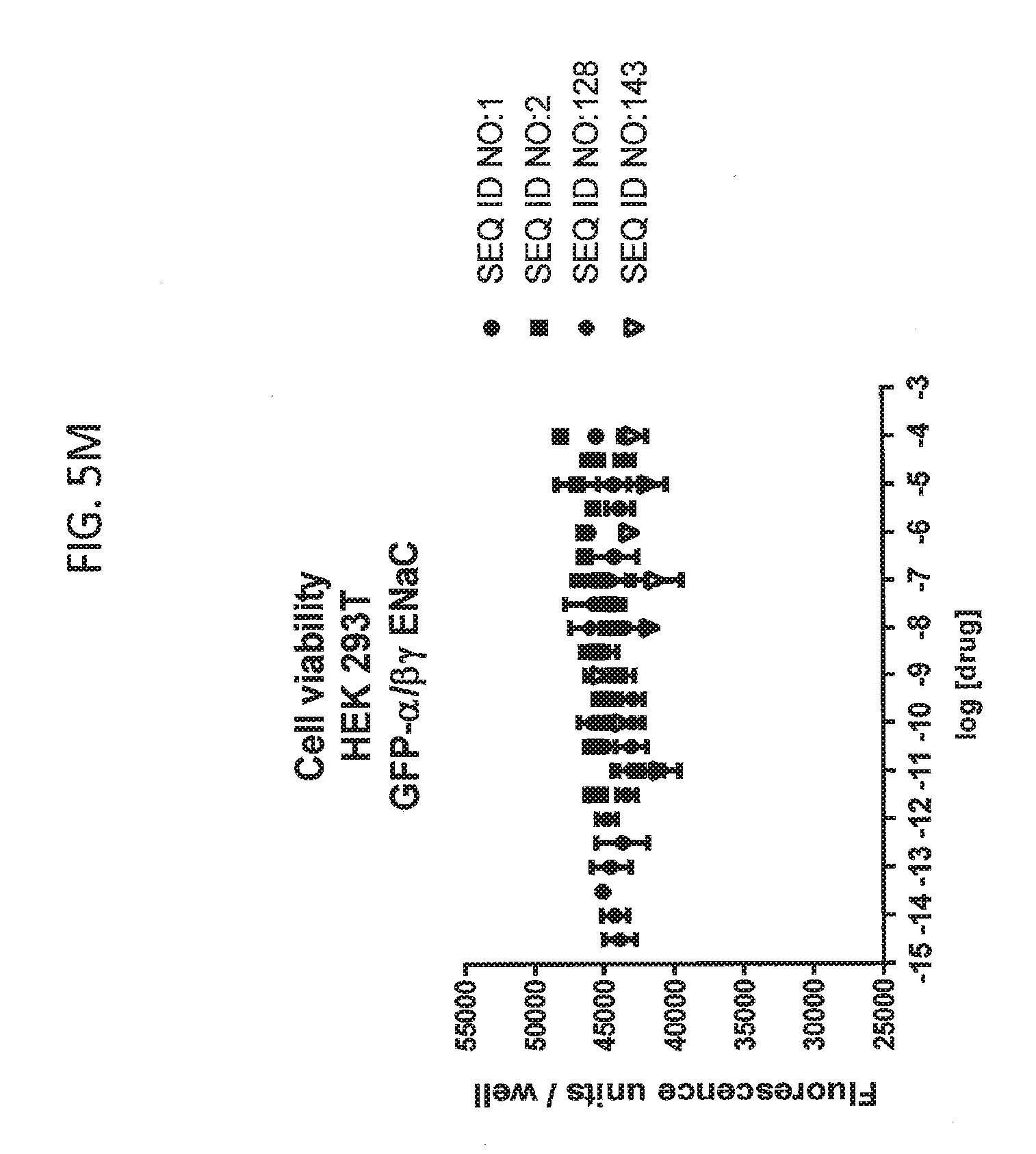
D00017

D00018

D00019
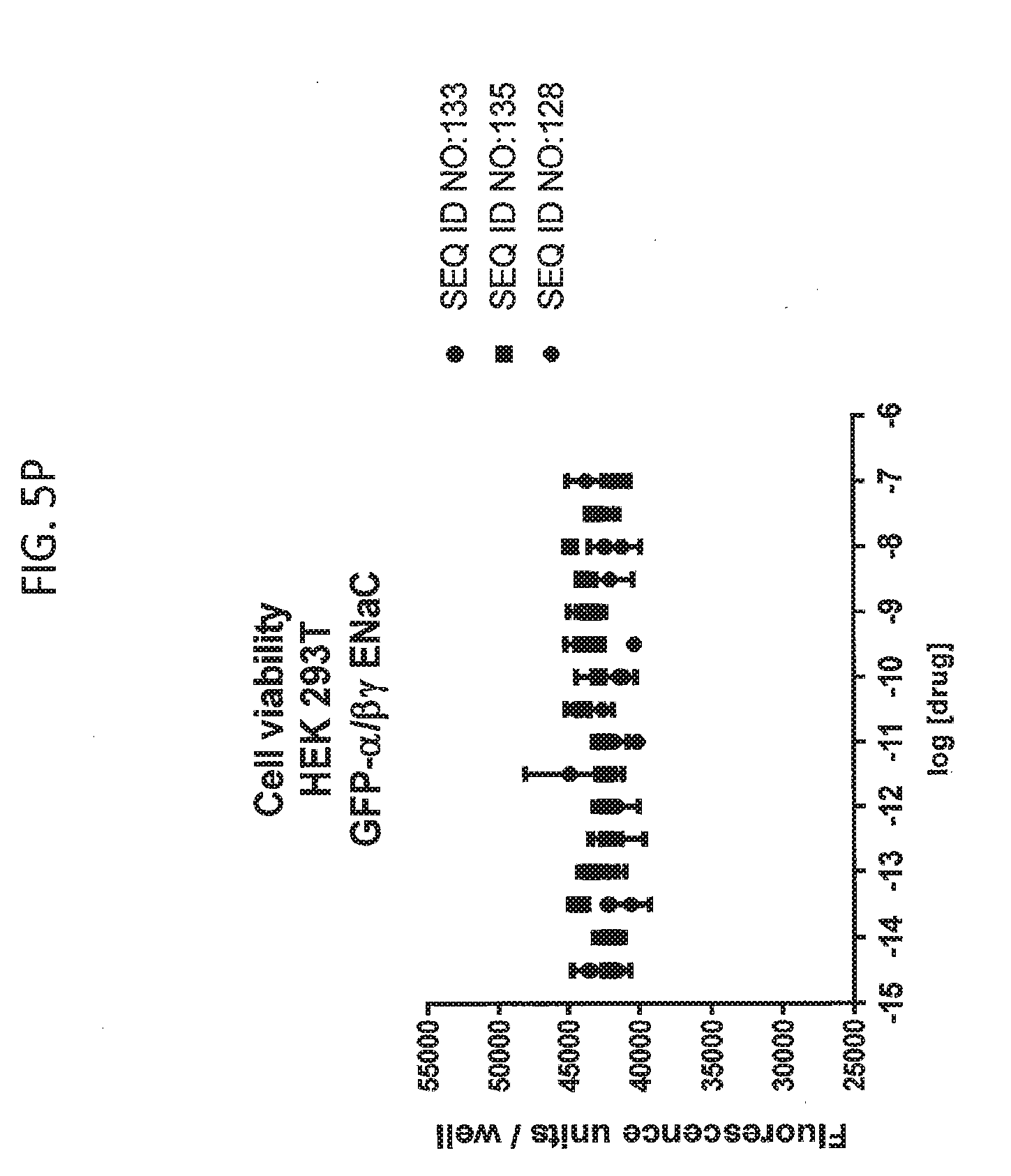
D00020

D00021

D00022
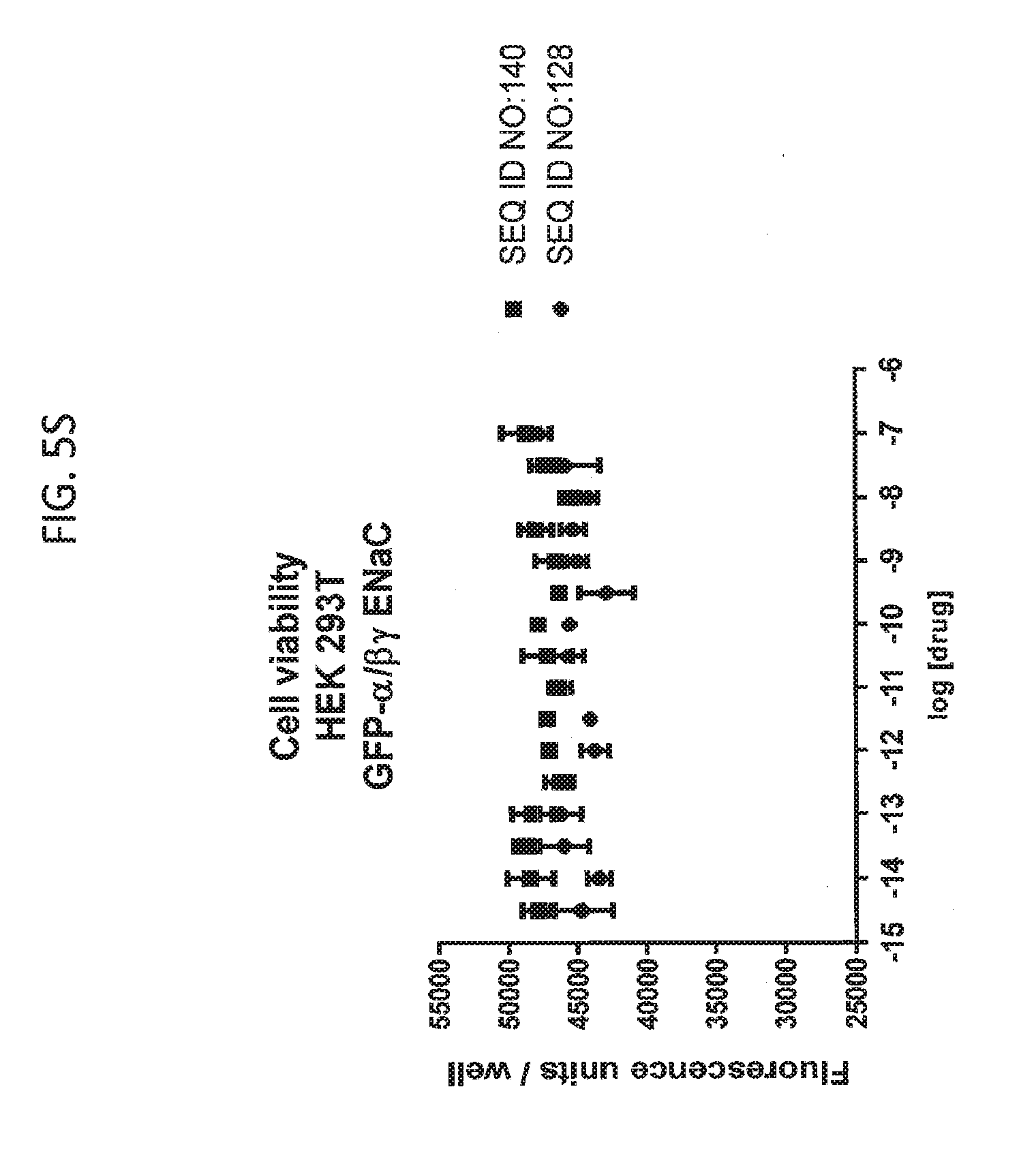
D00023

D00024

D00025
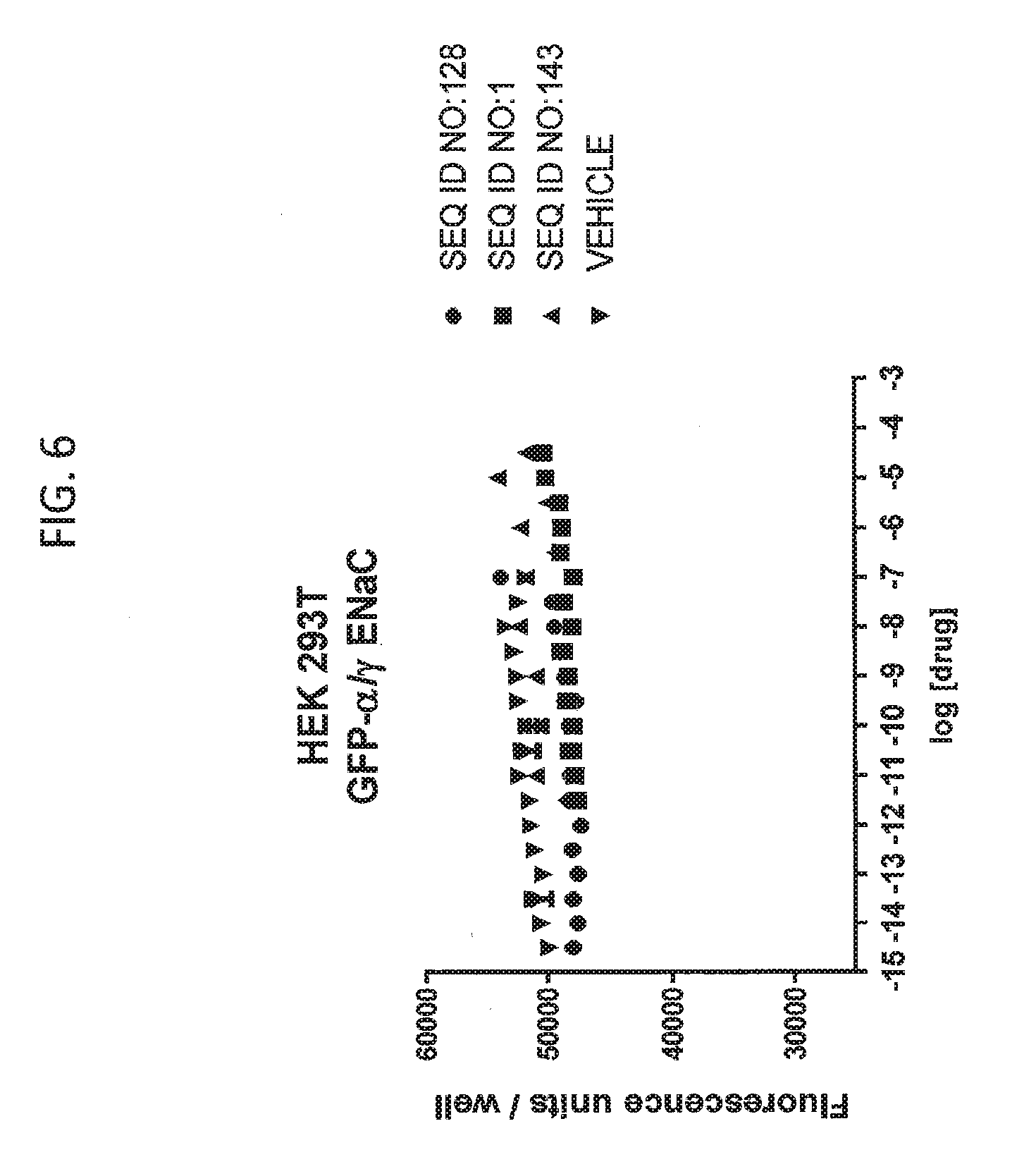
D00026

D00027

D00028
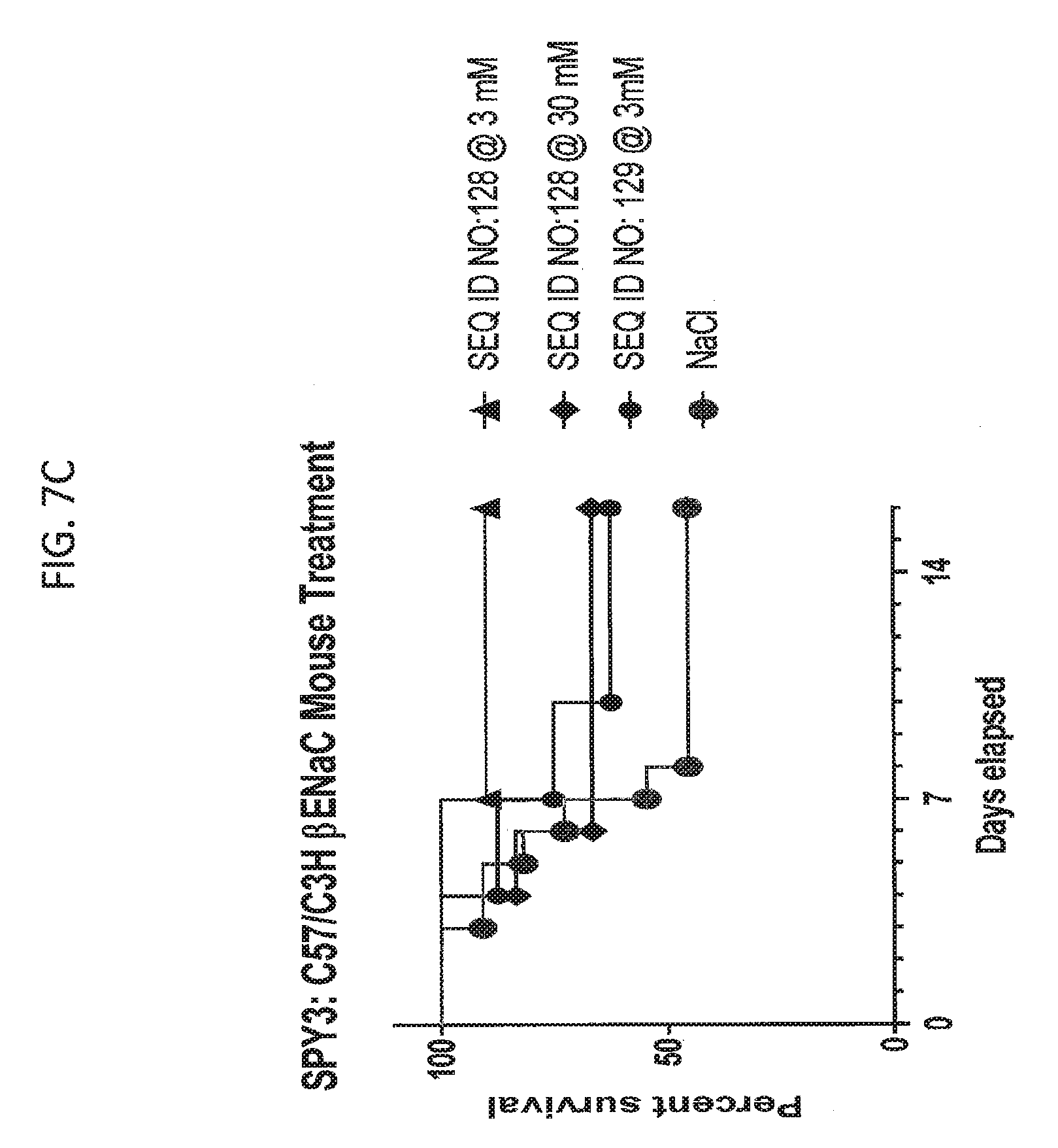
D00029

D00030

D00031

P00899
S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.