Tricyclic Quinoline And Quinoxaline Derivatives
Koolman; Hannes ; et al.
U.S. patent application number 16/179050 was filed with the patent office on 2019-03-07 for tricyclic quinoline and quinoxaline derivatives. The applicant listed for this patent is AbbVie Deutschland GmbH & Co. KG, AbbVie Inc.. Invention is credited to Margaretha Henrica Maria Bakker, Wilfried Braje, Karla Drescher, Andreas Haupt, Carolin Hoft, Hannes Koolman, Viktor Lakics, Helmut Mack, Ana Lucia Relo, Ruxu Xu, Xiaona Zhao.
| Application Number | 20190071443 16/179050 |
| Document ID | / |
| Family ID | 50275085 |
| Filed Date | 2019-03-07 |





View All Diagrams
| United States Patent Application | 20190071443 |
| Kind Code | A1 |
| Koolman; Hannes ; et al. | March 7, 2019 |
TRICYCLIC QUINOLINE AND QUINOXALINE DERIVATIVES
Abstract
The present invention relates to tricyclic quinoline and quinoxaline derivatives, to a pharmaceutical composition containing such compounds, to their use as modulators, especially agonists or partial agonists, of the 5-HT.sub.2C receptor, their use for preparing a medicament for the prevention or treatment of conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor, and to a method for preventing or treating conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor.
| Inventors: | Koolman; Hannes; (North Chicago, IL) ; Braje; Wilfried; (Ludwigshafen, DE) ; Mack; Helmut; (Ludwigshafen, DE) ; Haupt; Andreas; (Ludwigshafen, DE) ; Relo; Ana Lucia; (Ludwigshafen, DE) ; Drescher; Karla; (Ludwigshafen, DE) ; Bakker; Margaretha Henrica Maria; (Ludwigshafen, DE) ; Lakics; Viktor; (Ludwigshafen, DE) ; Hoft; Carolin; (Ludwigshafen, DE) ; Xu; Ruxu; (Tianjin, CN) ; Zhao; Xiaona; (Tianjin, CN) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 50275085 | ||||||||||
| Appl. No.: | 16/179050 | ||||||||||
| Filed: | November 2, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 14026354 | Sep 13, 2013 | 10118926 | ||
| 16179050 | ||||
| 61793033 | Mar 15, 2013 | |||
| 61701514 | Sep 14, 2012 | |||
| 61701531 | Sep 14, 2012 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 25/24 20180101; A61P 15/00 20180101; A61P 27/02 20180101; A61P 15/10 20180101; A61P 25/00 20180101; A61P 17/02 20180101; A61P 25/28 20180101; A61P 25/20 20180101; A61P 25/22 20180101; C07D 487/06 20130101; A61P 17/06 20180101; A61P 25/16 20180101; A61P 13/10 20180101; A61P 25/18 20180101; A61P 3/04 20180101; A61P 15/12 20180101; A61P 25/06 20180101; C07D 471/06 20130101; A61P 7/12 20180101; C07D 487/16 20130101; A61P 17/00 20180101; A61P 9/00 20180101; A61P 25/08 20180101; A61P 25/30 20180101; A61P 25/36 20180101; A61P 29/00 20180101; A61P 43/00 20180101; A61P 3/10 20180101; A61P 1/00 20180101; A61P 25/32 20180101; A61P 25/34 20180101 |
| International Class: | C07D 471/06 20060101 C07D471/06; C07D 487/16 20060101 C07D487/16; C07D 487/06 20060101 C07D487/06 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Sep 11, 2013 | CN | 201310410951.1 |
Claims
1. A compound of the formula I ##STR00025## wherein G is (CR.sup.3aR.sup.3b).sup.n; X is NR.sup.6 or CR.sup.7R.sup.8; R.sup.1 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; each R.sup.2 is independently selected from the group consisting of cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1--C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; R.sup.3a and R.sup.3b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; R.sup.4a and R.sup.4b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or R.sup.4a and R.sup.4b form together a group .dbd.O or .dbd.S; R.sup.5a and R.sup.5b, independently of each other, are selected from the group consisting of hydrogen, deuterium, halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; where R.sup.5a and R.sup.5b are not simultaneously hydroxy; or R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; R.sup.6 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, --SO.sub.2R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of deuterium, halogen, cyano, nitro, hydroxyl, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; where R.sup.7 and R.sup.8 are not simultaneously hydroxyl; and where R.sup.7 is not hydroxyl if R.sup.8 is C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, phenoxy or benzyloxy; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; each R.sup.9 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkenyl, fluorinated C.sub.3-C.sub.8-cycloalkenyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1--C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or two radicals R.sup.9 bound on neighboring carbon atoms, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; each R.sup.10 is independently selected from the group consisting of hydrogen, cyano, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the five last-mentioned radicals may be substituted with one or more substituents R.sup.11; each R.sup.11 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --COOH, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, C
.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy and fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy; or two radicals R.sup.11, together with the atom(s) they are bound to, form a saturated, partially unsaturated or maximally unsaturated 3-, 4-, 5-, 6- or 7-membered carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members; R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, phenyl and benzyl, where the phenyl moieties in the two last-mentioned radicals may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; or, if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; a is 0, 1 or 2; b is 0, 1, 2 or 3; and n is 1 or 2; and the N-oxides, tautomeric forms, stereoisomers and pharmaceutically acceptable salts thereof, and the compound of the general formula I, wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope.
2. The compound as claimed in claim 1, wherein at least one hydrogen atom has been replaced by a deuterium atom.
3. The compound as claimed in any of the preceding claims, where R.sup.1 is selected from hydrogen and C.sub.1-C.sub.6-alkyl and is preferably hydrogen.
4. The compound as claimed in any of the preceding claims, where R.sup.2 is selected from cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, in particular from C.sub.1-C.sub.6-alkyl and especially from methyl.
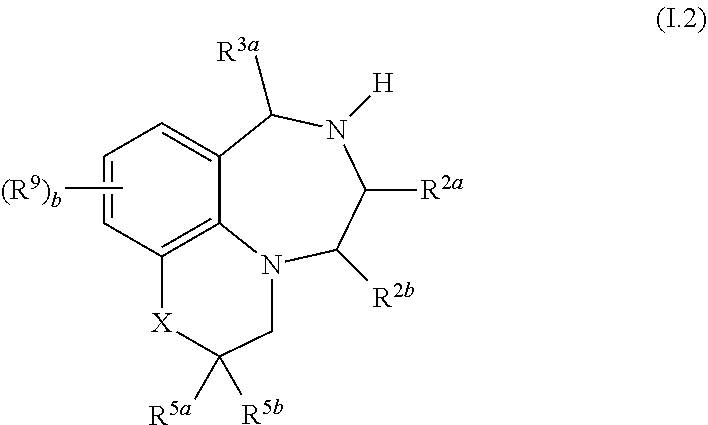
5. The compound as claimed in any of the preceding claims, where R.sup.3a and R.sup.3b independently of each other, are selected from hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy.
6. The compound as claimed in claim 5, where R.sup.3a is selected from hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, and R.sup.3b is hydrogen.
7. The compound as claimed in claim 6, where R.sup.3a is selected from hydrogen and methyl, and R.sup.3b is hydrogen.
8. The compound as claimed in claim 7, where R.sup.3a and R.sup.3b are hydrogen.
9. The compound as claimed in any of the preceding claims, where R.sup.4a and R.sup.4b independently of each other, are selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl or form together a group .dbd.O, and are in particular hydrogen or methyl or form together a group .dbd.O, and are especially hydrogen.
10. The compound as claimed in any of the preceding claims, where R.sup.5a is selected from hydrogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; and in case that X is CR.sup.7R.sup.8 is further selected from halogen; or R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; and R.sup.5b is selected from hydrogen and deuterium.
11. The compound as claimed in claim 10, where R.sup.5a is selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl; and in case that X is CR.sup.7R.sup.8 is further selected from halogen; and is in particular hydrogen; or R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5; or R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5; and R.sup.5b is selected from hydrogen and deuterium.
12. The compound as claimed in claim 10, where R.sup.5a is selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl; and in case that X is CR.sup.7R.sup.8 is further selected from halogen; and is in particular hydrogen; or R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5, where two hydrogen atoms bound to adjacent CH.sub.2 groups may be replaced by two radicals R.sup.11, where the two radicals R.sup.11 form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5; or R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5; and R.sup.5b is selected from hydrogen and deuterium.
13. The compound as claimed in any of claims 1 to 9, where R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; and form preferably a 5- or 6-membered saturated or partially unsaturated carbocyclic ring, and where the ring may be substituted with one or more substituents R.sup.11.
14. The compound as claimed in any of the preceding claims, where X is NR.sup.6, where R.sup.6 is as defined in claim 1.
15. The compound as claimed in claim 14, where X is NR.sup.6, where R.sup.6 is --SO.sub.2R.sup.10, where R.sup.10 is as defined in claim 1.
16. The compound as claimed in claim 15, where R.sup.10 is selected from C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl, where phenyl may be substituted with 1, 2, 3, 4 or 5 radicals R.sup.11.
17. The compound as claimed in claim 14, where X is NR.sup.6, where R.sup.6 is selected from hydrogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy, --C(.dbd.O)R.sup.10, where R.sup.10 is selected from C.sub.1-C.sub.6-alkyl and C.sub.3-C.sub.6-cycloalkyl; phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11.
18. The compound as claimed in claim 17, where R.sup.6 is selected from hydrogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, C.sub.3-C.sub.6-cycloalkylcarbonyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members; and is in particular selected from C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members.
19. The compound as claimed in claim 18, where R.sup.6 is selected from C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and oxetanyl.
20. The compound as claimed in claim 14, where X is NR.sup.6, where R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11.
21. The compound as claimed in claim 20, where R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5 and in particular 3, 4 or 5.
22. The compound as claimed in claim 20, where R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5 and in particular 3, 4 or 5, where two hydrogen atoms bound to adjacent CH.sub.2 groups may be replaced by two radicals R.sup.11, where the two radicals R.sup.11 form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5 and in particular 1, 2 or 3.
23. The compound as claimed in any of claims 1 to 13, where X is CR.sup.7R.sup.8, where R.sup.7 and R.sup.8 are as defined in claim 1.
24. The compound as claimed in claim 23, where R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of halogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12R.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; in which case R.sup.8 has one of the above meanings or is deuterium.
25. The compound as claimed in claim 22, where R.sup.7 and R.sup.8, independently of each other, are selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, benzyloxy and a 3-, 4-, 5- or 6-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the four last-mentioned radicals may be substituted with one or more substituents R.sup.11; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; in which case R.sup.8 has one of the above meanings or is deuterium; and R.sup.5b is selected from hydrogen and deuterium.
26. The compound as claimed in claim 23, where R.sup.7 and R.sup.8, independently of each other, are selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl, or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5; in which case R.sup.8 has one of the above meanings or is deuterium; and R.sup.5b is selected from hydrogen and deuterium.
27. The compound as claimed in claim 24, where R.sup.8 is selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl; and R.sup.7 is selected from C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl, preferably from methyl and CF.sub.3 and is in particular methyl; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3 or 4; in which case R.sup.8 has one of the above meanings or is deuterium; and R.sup.5b is selected from hydrogen and deuterium.
28. The compound as claimed in claim 22, where R.sup.7 is C.sub.1-C.sub.6-alkyl and is in particular methyl; R.sup.8 is selected from hydroxyl, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy and phenyl and in particular from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3 or 4 and where R.sup.8 is methyl; and R.sup.5b is hydrogen.
29. The compound as claimed in any of claims 20 to 26, wherein the ring formed by R.sup.7 and R.sup.8 together with the carbon atom they are bound is a carbocyclic ring, in particular a saturated carbocyclic ring.
30. The compound as claimed in claim 27, where R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3, 4-, 5- or 6-membered saturated or partially unsaturated carbocyclic ring, preferably a 3, 4- or 5-membered saturated carbocyclic ring, in particular a 4-membered saturated carbocyclic ring, where the ring may be substituted with one or more substituents R.sup.11.
31. The compound as claimed in any of claims 20 to 26, wherein the ring formed by R.sup.7 and R.sup.8 together with the carbon atom they are bound is a heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO and SO.sub.2 as ring members preferably containing 1 or 2 heteroatoms selected from O, S and N as ring members.
32. The compound as claimed in claim 29, wherein the heterocyclic ring is a saturated 3-, 4- or 5-membered heterocyclic ring containing one heteroatom selected from O, S and N as ring member, where the ring may be substituted with one or more substituents R.sup.11, and is in particular oxetanyl which may be substituted with one or more substituents R.sup.11.
33. The compound as claimed in any of the preceding claims, where each R.sup.9 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members, where the heterocyclic ring may be substituted with one or more substituents R.sup.11, in particular from halogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl and C.sub.1-C.sub.6-alkoxy, and is especially halogen.
34. The compound as claimed in any of the preceding claims, where each R.sup.11 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, and is in particular halogen or C.sub.1-C.sub.4-alkyl.
35. The compound as claimed in any of the preceding claims, where a is 0 or 1, in particular 0.
36. The compound as claimed in claim 33, where a is 1 and R.sup.2 is bound in 3-position to the nitrogen ring atom carrying R.sup.1.
37. The compound as claimed in any of the preceding claims, where b is 0 or 1.
38. The compound as claimed in any of the preceding claims, where n is 1.
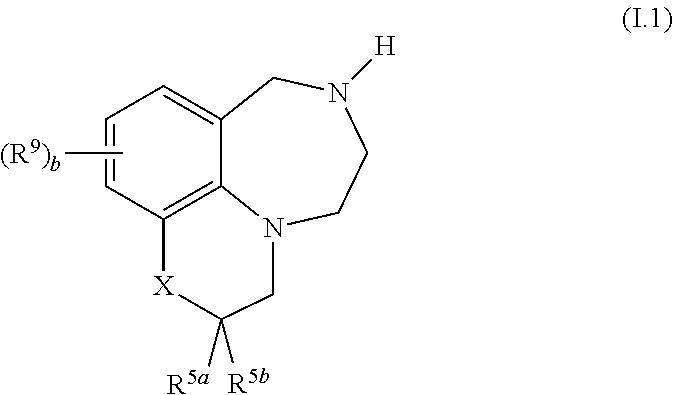
39. The compound as claimed in any of the preceding claims, of formula I.1 ##STR00026## wherein X, R.sup.5a, R.sup.5b, R.sup.9 and b are as defined in any of claims 1 and 10 to 34.
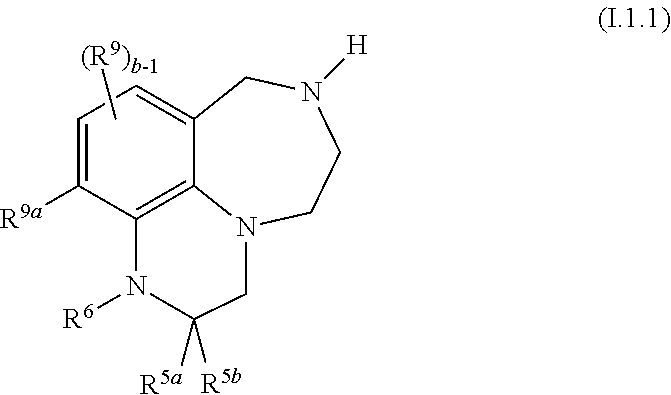
40. The compound as claimed in claim 37, of formula I.1.1 ##STR00027## wherein R.sup.9a is selected from H, halogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and fluorinated C.sub.3-C.sub.6-cycloalkyl; and R.sup.5a, R.sup.5b, R.sup.6, R.sup.9 and b are as defined in any of claims 1 and 10 to 35.
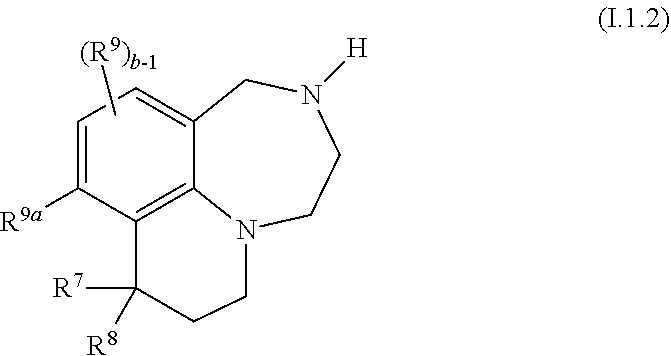
41. The compound as claimed in claim 37, of formula I.1.2 ##STR00028## wherein R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, in particular from methyl and CF.sub.3, and is especially methyl; R.sup.8 is selected from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; and in particular from methyl and CF.sub.3; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered, in particular a 3- or 4-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; R.sup.9a is H, Cl, F or methyl; and R.sup.9 and b are as defined in any of claims 1, 31, 32, 34 and 35.
42. The compound as claimed in claim 37, of formula I.1.3 ##STR00029## wherein R.sup.8 is selected from deuterium, F, Cl, CN and CH.sub.3.
43. The compound as claimed in any of claims 1 to 36, of formula I.2 ##STR00030## wherein X, R.sup.5a, R.sup.5b, R.sup.9 and b are as defined in any of claims 1 and 10 to 35.
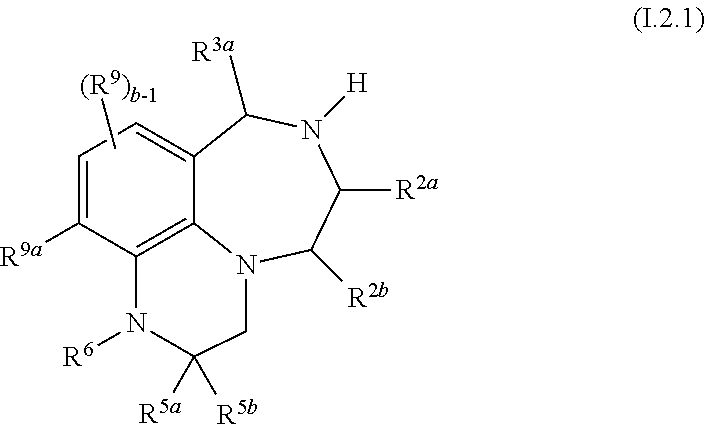
44. The compound as claimed in claim 41 of formula I.2.1 ##STR00031## wherein R.sup.2a, R.sup.2b and R.sup.3a, independently of each other, are selected from hydrogen and methyl; and where in particular at most one of R.sup.2a, R.sup.2b and R.sup.3a is methyl; R.sup.9a is selected from H, halogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and fluorinated C.sub.3-C.sub.6-cycloalkyl; and R.sup.5a, R.sup.5b, R.sup.6, R.sup.9 and b are as defined in any of claims 1 and 10 to 19, 30, 31 and 32.
45. The compound as claimed in claim 42, where R.sup.2b is methyl and R.sup.2a and R.sup.3a are hydrogen.
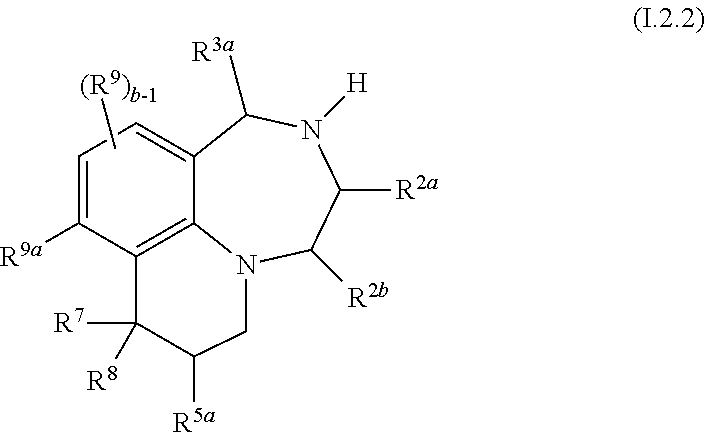
46. The compound as claimed in claim 41, of formula I.2.2 ##STR00032## wherein R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a, independently of each other, are selected from hydrogen, methyl and ethyl, in particular from hydrogen and methyl; R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, in particular from methyl and CF.sub.3, and is especially methyl; R.sup.8 is selected from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; and in particular from methyl and CF.sub.3; or R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered, in particular a 3- or 4-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; R.sup.9a is H, Cl, F or methyl; and R.sup.9 and b are as defined in any of claims 1, 31, 32 and 34.
47. The compound as claimed in claim 44, wherein R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a are selected from hydrogen and methyl, with the proviso that at most 2, preferably at most 1, of R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a are methyl.
48. The compound as claimed in claim 45, where R.sup.2b is methyl and R.sup.2a, R.sup.3a and R.sup.5a are hydrogen.
49. The compound as claimed in any of claims 44 to 46, where R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3, 4-, 5- or 6-membered saturated or partially unsaturated carbocyclic ring, preferably a 3, 4- or 5-membered saturated carbocyclic ring, in particular a 4-membered saturated carbocyclic ring, where the ring may be substituted with one or more substituents R.sup.11.
50. A compound of formula I as claimed in any of the preceding claims, selected from 8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolone; 1,2,3,4-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopent[- 2]en]-6(7H)-one; 8-methyl-8-phenyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-- 6(2H)-one; 8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quin- olin-6(2H)-one; 1,2,3,4-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopenta- n]-6(7H)-one; 1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclope- ntane]; 12a-methyl-4,5,6,7,10,11,12,12a-octahydrocyclopenta[c][1,4]diazepi- no[6,7,1-ij]quinolin-9(9aH)-one; 8-methyl-8-(trifluoromethyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,- 1-ij]quinolone; 12a-methyl-4,5,6,7,10,11,12,12a-octahydrocyclopenta[c][1,4]diazepino[6,7,- 1-ij]quinolin-9(9aH)-one; 12a-methyl-4,5,6,7,9,9a, 10,11,12,12a-decahydrocyclopenta[c][1,4]diazepino-[6,7,1-ij]quinoline; 8-methyl-8-(trifluoromethyl)-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-i- j]quinolin-6(2H)-one; 12a-methyl-4,5,6,7,9,9a, 10,11,12,12a-decahydrocyclopenta[c][1,4]diazepino-[6,7,1-ij]quinoline; 8-methyl-8-phenyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinol- ine; (R)-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[- 1,2-a]quinoxaline; (S)-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 9-chloro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qu- inoline; 10-chloro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,- 7,1-ij]quinoline; 9-chloro-8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quino- lin-6(2H)-one; 10-chloro-8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quin- olin-6(2H)-one; 9-fluoro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qu- inoline; 10-fluoro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,- 7,1-ij]quinoline; 1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclohe- xane]; 1',2',3',4',6',7'-hexahydrospiro[cyclobutane-1,8'-]1,4]diazepino[6,- 7,1-ij]quinoline; 9'-fluoro-1',2',3',4',6',7'-hexahydrospiro[cyclobutane-1,8'-[1,4]diazepin- o[6,7,1-ij]quinoline]; 7,8,8-trimethyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinolin- e]; 9-fluoro-1-methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]q- uinoline-8,1'-cyclobutane]; 3-methyl-1',2',3',4',6',7'-hexahydrospiro[cyclobutane-1,8'-[1,4]diazepino- [6,7,1-ij]quinoline]; 3-benzyl-2',3',7',8',9',10'-hexahydro-1H-3',8',10a'-triaza-cyclohepta[de]- naphthalene; 2,3,5,6,7,8-Hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline; 1-Cyclobutyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline- ; 1-Methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline; 1-(Oxetan-3-yl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxal- ine; 1-(Cyclopropylmethyl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-d- e]quinoxaline; 1-(Cyclopentylmethyl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline; Cyclopropyl(2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxalin-1-yl)methanone; Cyclopentyl(2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxalin-1- -yl)methanone; 1-Cyclopropyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxalin- e; 1-Cyclopentyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxal- ine; 1-Cyclopropyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-- de]quinoxaline; 6,6-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline; 5,6,7,9,9a, 10,11,12,13,14-decahydro-4H-azepino[1,2-a][1,4]diazepino[1,7,6-de]quinoxa- line; 7-Methyl-5,6,7,9,9.sup.a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 1-Fluoro-7-methyl-5,6,7,9,9.sup.a,10,11,12-octahydro-4H-[1,4]diazepino[1,- 7,6-de]pyrrolo[1,2-a]quinoxaline; 1,2,3,4,6,7-Hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopr- opane]; 4-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinol- ine-8,1'-cyclopropane]; 11-Fluoro-8,8-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]q- uinoline; 10-Methoxy-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[- 6,7,1-ij]quinoline; 4,8,8-Trimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin- e; 4-Ethyl-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]q- uinoline; 6,8,8-Trimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij- ]quinoline; 8-ethyl-8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoli- ne; (R)-8-ethyl-8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]- quinoline; (S)-8-ethyl-8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,- 7,1-ij]quinoline; 4-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane]; 1-Fluoro-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 1-Bromo-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 1-Methyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 1-Cyclopropyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 1-Cyclobutyl-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyr- rolo[1,2-a]quinoxaline; 1-Cyclopentyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline; 8-Methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol; 8-Methoxy-8-methyl-2,3,4,67,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinol- ine; 2,8-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoli- n-8-ol; 11-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quino- line-8,1'-cyclobutane]; 9-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane]; 4,5,6,7,9,9a,9b,10,10a, 11-Decahydrocyclopropa[3,4]pyrrolo[1,2-a][1,4]diazepino[1,7,6-de]quinoxal- ine; 1-Ethyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline; 1-Propyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline; 1-Cyclobutyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline; 5-Methyl-1-(oxetan-3-yl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepin- o[1,7,6-de]quinoxaline; 1-Ethyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxa- line; 5-Methyl-1-propyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]q- uinoxaline; 9-Chloro-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane]; 10-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,- 1'-cyclobutane]; 10-Chloro-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,- 1'-cyclobutane]; and the stereoisomers and pharmaceutically acceptable salts thereof.
51. A pharmaceutical composition comprising a therapeutically effective amount of at least one compound as claimed in any of the preceding claims or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof, or comprising at least one compound as defined in any of the preceding claims wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope, preferably wherein at least one hydrogen atom has been replaced by a deuterium atom, in combination with at least one pharmaceutically acceptable carrier and/or auxiliary substance.
52. The compound as claimed in any of claims 1 to 50 or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for use as a medicament.
53. The compound as claimed in any of claims 1 to 50 or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the treatment of disorders which respond to the modulation of the 5-HT.sub.2C receptor.
54. The use of a compound as claimed in any of claims 1 to 50 or of an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the manufacture of a medicament for the treatment of disorders which respond to the modulation of the 5-HT.sub.2C receptor.
55. A method for treating disorders which respond to the modulation of the 5-HT.sub.2C receptor, which method comprises administering to a subject in need thereof at least one compound as defined in any of claims 1 to 50 or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
56. The compound as claimed in claim 53 or the use as claimed in claim 54 or the method as claimed in claim 55, where the disorders are selected from the group consisting of damage of the central nervous system, disorders of the central nervous system, eating disorders, ocular hypertension, cardiovascular disorders, gastrointestinal disorders and diabetes.
57. The compound or the use or the method as claimed in claim 56, where the disorders are selected from the group consisting of bipolar disorder, depression, atypical depression, mood episodes, adjustment disorders, anxiety, panic disorders, post-traumatic syndrome, psychoses, schizophrenia, cognitive deficits of schizophrenia, memory loss, dementia of aging, Alzheimer's disease, behavioral disorders associated with dementia, social phobia, mental disorders in childhood, attention deficit hyperactivity disorder, organic mental disorders, autism, mutism, disruptive behavior disorder, impulse control disorder, borderline personality disorder, obsessive compulsive disorder, migraine and other conditions associated with cephalic pain or other pain, raised intracranial pressure, seizure disorders, epilepsy, substance use disorders, alcohol abuse, cocaine abuse, tobacco abuse, smoking cessation, sexual dysfunction/erectile dysfunction in males, sexual dysfunction in females, premenstrual syndrome, late luteal phase syndrome, chronic fatigue syndrome, sleep disorders, sleep apnoea, chronic fatigue syndrome, psoriasis, Parkinson's disease, spinal cord injury, trauma, stroke, pain, bladder dysfunction/urinary incontinence, encephalitis, meningitis, eating disorders, obesity, bulimia, weight loss, anorexia nervosa, ocular hypertension, cardiovascular disorders, gastrointestinal disorders, diabetes insipidus, diabetes mellitus, type I diabetes, type II diabetes, type III diabetes, diabetes secondary to pancreatic diseases, diabetes related to steroid use, diabetes complications, hyperglycemia and insulin resistance.
58. The compound or the use or the method as claimed in claim 57, where the disorders are selected from schizophrenia, depression, bipolar disorders, obesity and substance use disorders.
Description
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This is a non-provisional of U.S. Patent Application No. 61/701,514, filed on Sep. 14, 2012, U.S. Patent Application No. 61/701,531, filed on Sep. 14, 2012, U.S. Patent Application No. 61/793,033, filed on Mar. 15, 2013, and claims priority to Chinese Patent Application No. CN 201310410951.1, filed on Sep. 11, 2013, the contents of all of which are fully incorporated herein by reference.
FIELD OF THE INVENTION
[0002] The present invention relates to tricyclic quinoline and quinoxaline derivatives, to a pharmaceutical composition containing such compounds, to their use as modulators, especially agonists or partial agonists, of the 5-HT.sub.2C receptor, their use for preparing a medicament for the prevention or treatment of conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor, to a method for preventing or treating conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor, and processes for preparing such compounds and compositions.
BACKGROUND OF THE INVENTION
[0003] Diseases, disorders and conditions where 5-HT.sub.2C modulation is desired are for example depression, anxiety, schizophrenia, bipolar disorder, obsessive compulsive disorder, migraine, pain, epilepsy, substance abuse, eating disorders, obesity, diabetes, erectile dysfunction and others.
[0004] Serotonin (5-hydroxytryptamine, 5-HT), a monoamine neurotransmitter and local hormone, is formed by the hydroxylation and decarboxylation of tryptophan. The greatest concentration is found in the enterochromaffin cells of the gastrointestinal tract, the remainder being predominantly present in platelets and in the Central Nervous System (CNS). 5-HT is implicated in a vast array of physiological and pathophysiological pathways. In the periphery, it contracts a number of smooth muscles and induces endothelium-dependent vasodilation. In the CNS, it is believed to be involved in a wide range of functions, including the control of appetite, mood, anxiety, hallucinations, sleep, vomiting and pain perception.
[0005] Neurons that secrete 5-HT are termed serotonergic. The function of 5-HT is exerted upon its interaction with specific (serotonergic) neurons. Seven types of 5-HT receptors have been identified: 5-HT.sub.1 (with subtypes 5-HT.sub.1A, 5-HT.sub.1B, 5-HT.sub.1D, 5-HT.sub.1E and 5-HT.sub.1F), 5-HT.sub.2 (with subtypes 5-HT.sub.2A, 5-HT.sub.2B and 5-HT.sub.2C), 5-HT.sub.3, 5-HT.sub.4, 5-HT.sub.5 (with subtypes 5-HT.sub.5A and 5-HT.sub.5B), 5-HT.sub.6 and 5-HT.sub.7. Most of these receptors are coupled to G-proteins that affect the activities of adenylate cyclase or phospholipase C.gamma..
[0006] Alterations in the activity of multiple neurotransmitter receptor systems (dopamine, serotonin, glutamate, GABA, acetylcholine) have been implicated in the manifestation of the symptoms of schizophrenia. The most widely accepted "Dopamine Hypothesis of Schizophrenia" in its simplest form states that the positive symptoms of this pathology relate to a functional hyperactivity of the mesolimbic dopaminergic system, while the negative and cognitive aspects can be traced to a functional hypoactivity of the mesocortical dopaminergic projections. Atypical antipsychotics block the mesolimbic dopaminergic neurotransmission, thereby controlling positive symptoms, with little or no effect on the nigrostriatal system, leading to less induction of extrapyramidal side effects (EPS).
[0007] Primary negative and cognitive symptoms of schizophrenia reflect a dysfunction of the frontal cortex ("hypofrontality"), which is thought to be induced by a decreased tone in the mesocortical dopaminergic projection field [Davis K L, Kahn R S, Ko G and Davidson M (1991). Dopamine in schizophrenia: a review and re-conceptualization. Am J Psychiatry 148: 1474-86. Weinberger D R and Berman K F (1996). Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci 351: 1495-503]. Agents that selectively enhance dopamine levels in the cortex have the potential to address the negative symptoms of this disorder. Atypical antipsychotics lack robust efficacy against negative and cognitive components of the schizophrenic syndrome.
[0008] The schizophrenic symptomatology is further complicated by the occurrence of drug-induced so-called secondary negative symptoms and cognitive impairment, which are difficult to distinguish from primary negative and cognitive symptoms [Remington G and Kapur S (2000). Atypical antipsychotics: are some more atypical than others?Psychopharmacol 148: 3-15]. The occurrence of secondary negative symptoms not only limits therapeutic efficacy but also, together with these side effects, negatively affects patient compliance.
[0009] It may thus be hypothesized that a novel mechanistic approach that blocks dopaminergic neurotransmission in the limbic system but does not affect the striatal and pituitary projection fields, and stimulates frontocortical projection fields, would provide an efficacious treatment for all parts of the schizophrenic pathology, including its positive, negative and cognitive symptoms. Moreover, a selective compound that is substantially free of the ancillary pharmacology that characterizes current agents would be expected to avoid a variety of off-target side effects that plague current treatments such as extrapyramidal side effects (EPS) and weight gain.
[0010] The 5-HT.sub.2C receptor, previously named 5-HT1C, is a G-protein-coupled receptor, which couples to multiple cellular effector systems including the phospholipase C, A and D pathways. It is found primarily in the brain and its distribution is particularly high in the plexus choroideus, where it is assumed to control cerebrospinal fluid production [Kaufman M J, Hirata F (1996) Cyclic GMP inhibits phosphoinositide turnover in choroid plexus: evidence for interactions between second messengers concurrently triggered by 5-HT.sub.2C receptors. Neurosci Lett 206:153-156]. Very high levels were also found in the retrosplenial, piriform and entorhinal cortex, anterior olfactory nucleus, lateral septal nucleus, subthalamic nucleus, amygdala, subiculum and ventral part of CA3, lateral habenula, substantia nigra pars compacta, several brainstem nuclei and the whole grey matter of the spinal cord [Pompeiano M, Palacios J M, Mengod G (1994). Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT.sub.2A and 5-HT.sub.2C receptors. Brain Res Mol Brain Res 23:163-178]. A comparison of the distribution of 5-HT.sub.2C mRNA with that of 5-HT.sub.2C protein in monkey and human brains has revealed both pre- and postsynaptic localization [Lopez-Gimenez J F, Mengod G, Palacios J M, Vilaro M T (2001) Regional distribution and cellular localization of 5-HT.sub.2C receptor mRNA in monkey brain: comparison with [.sup.3H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse 42:12-26].
[0011] It is anticipated that modulation of the 5-HT.sub.2C receptor will improve disorders such as depression, anxiety, schizophrenia, cognitive deficits of schizophrenia, obsessive compulsive disorder, bipolar disorder, migraine, epilepsy, substance abuse, eating disorders, obesity, diabetes, sexual dysfunction/erectile dysfunction, sleep disorders, psoriasis, Parkinson's disease, pain conditions and disorders, and spinal cord injury, smoking cessation, ocular hypertension and Alzheimer's disease. Modulators of the 5-HT.sub.2C receptor are also shown to be useful in the modulation of bladder function, including the prevention or treatment of urinary incontinence.
[0012] There is an ongoing need for providing compounds having high affinity and selectivity for the 5-HT.sub.2C receptor. In particular the compounds should have low affinity to adrenergic receptors, such as the .alpha..sub.1-adrenergic receptor, histamine receptors, such as the H.sub.1-receptor, and dopaminergic receptors, such as the D.sub.2-receptor, in order to avoid or reduce side effects associated with modulation of these receptors, such as postural hypotension, reflex tachycardia, potentiation of the antihypertensive effect of prazosin, terazosin, doxazosin and labetalol or dizziness associated with the blockade of the al-adrenergic receptor, weight gain, sedation, drowsiness or potentiation of central depressant drugs associated with the blockade of the H.sub.1-receptor, or extrapyramidal movement disorder, such as dystonia, parkinsonism, akathisia, tardive dyskinesia or rabbit syndrome, or endocrine effects, such as prolactin elevation (galactorrhea, gynecomastia, mentstrual changes, sexual dysfunction in males), associated with the blockade of the D.sub.2-receptor.
[0013] The present invention provides compounds which have an affinity for the 5-HT.sub.2C, thus allowing the treatment of disorders related to or affected by the 5-HT.sub.2C receptor.
SUMMARY OF THE INVENTION
[0014] The invention is directed to tricyclic quinoline and quinoxaline derivatives, compositions comprising such compounds, their use as modulators, especially agonists or partial agonists, of the 5-HT.sub.2C receptor, their use for preparing a medicament for the prevention or treatment of conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor, to a method for preventing or treating conditions and disorders which respond to the modulation of 5-HT.sub.2C receptor, and processes for preparing such compounds and compositions.
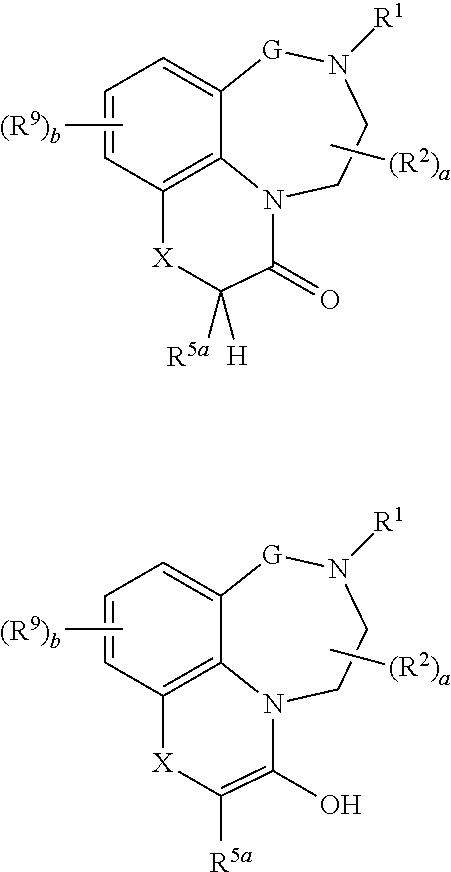
[0015] In one aspect, the present invention relates to compounds of the formula (I):
##STR00001## [0016] wherein [0017] G is (CR.sup.3aR.sup.3b).sub.n; [0018] X is NR.sup.6 or CR.sup.7R.sup.8; [0019] R.sup.1 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0020] each R.sup.2 is independently selected from the group consisting of cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0021] NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0022] R.sup.3a and R.sup.3b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0023] --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0024] R.sup.4a and R.sup.4b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, [0025] NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0026] R.sup.4a and R.sup.4b form together a group .dbd.O or .dbd.S; [0027] R.sup.5a and R.sup.5b, independently of each other, are selected from the group consisting of hydrogen, deuterium, halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; where R.sup.5a and R.sup.5b are not simultaneously hydroxy; or [0028] R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring (i.e. a spiro ring), where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0029] R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0030] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0031] R.sup.6 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, --SO.sub.2R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0032] R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of deuterium, halogen, cyano, nitro, hydroxyl, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; where R.sup.7 and R.sup.8 are not simultaneously hydroxyl; and where R.sup.7 is not hydroxyl if R.sup.8 is C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, phenoxy or benzyloxy; or [0033] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring (i.e. a spiro ring), where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0034] each R.sup.9 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkenyl, fluorinated C.sub.3-C.sub.8-cycloalkenyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b. --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0035] two radicals R.sup.9 bound on neighbouring carbon atoms, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0036] each R.sup.10 is independently selected from the group consisting of hydrogen, cyano, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the five last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0037] each R.sup.11 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C
.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --COOH, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy and fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy; [0038] or two radicals R.sup.11, together with the atom(s) they are bound to, form a saturated, partially unsaturated or maximally unsaturated 3-, 4-, 5-, 6- or 7-membered carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members; [0039] R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, phenyl and benzyl, where the phenyl moieties in the two last-mentioned radicals may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; or, [0040] if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; [0041] a is 0, 1 or 2; [0042] b is 0, 1, 2 or 3; and [0043] n is 1 or 2; and the N-oxides, tautomeric forms, stereoisomers and pharmaceutically acceptable salts thereof, and the compound of the general formula I, wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope.
[0044] In another aspect, the invention relates to compounds of formula I as defined above, [0045] wherein [0046] G is (CR.sup.3aR.sup.3b).sub.n; [0047] X is NR.sup.6 or CR.sup.7R.sup.8; [0048] R.sup.1 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0049] each R.sup.2 is independently selected from the group consisting of cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0050] NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0051] R.sup.3a and R.sup.3b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1--C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0052] NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0053] R.sup.4a and R.sup.4b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, [0054] --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or R.sup.4a and R.sup.4b form together a group .dbd.O or .dbd.S; [0055] R.sup.5a and R.sup.5b, independently of each other, are selected from the group consisting of hydrogen, deuterium, halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0056] R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0057] R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0058] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0059] R.sup.6 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, --SO.sub.2R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0060] R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of deuterium, halogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0061] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0062] each R.sup.9 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkenyl, fluorinated C.sub.3-C.sub.8-cycloalkenyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0063] two radicals R.sup.9 bound on neighbouring carbon atoms, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0064] each R.sup.10 is independently selected from the group consisting of hydrogen, cyano, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the five last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0065] each R.sup.11 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --COOH, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6
-alkoxycarbonyl, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy and fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy; [0066] R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, phenyl and benzyl, where the phenyl moieties in the two last-mentioned radicals may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; or, [0067] if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; [0068] a is 0, 1 or 2; [0069] b is 0, 1, 2 or 3; and [0070] n is 1 or 2; [0071] and the N-oxides, tautomeric forms, stereoisomers and pharmaceutically acceptable salts thereof, and the compound of the general formula I, wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope.
[0072] Preferably, R.sup.5a and R.sup.5b are not simultaneously hydroxyl.
[0073] In another aspect, the invention relates to compounds of formula I
##STR00002## [0074] wherein [0075] G is (CR.sup.3aR.sup.3b).sub.n; [0076] X is NR.sup.6 or CR.sup.7R.sup.8; [0077] R.sup.1 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0078] each R.sup.2 is independently selected from the group consisting of cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0079] NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0080] R.sup.3a and R.sup.3b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0081] NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0082] R.sup.4a and R.sup.4b, independently of each other, are selected from the group consisting of hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, [0083] --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0084] R.sup.4a and R.sup.4b form together a group .dbd.O or .dbd.S; R.sup.5a and R.sup.5b, independently of each other, are selected from the group consisting of hydrogen, deuterium, halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0085] R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0086] R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0087] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0088] R.sup.6 is selected from the group consisting of hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --C(.dbd.O)R.sup.10, --SO.sub.2R.sup.10, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0089] R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of deuterium, halogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0090] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0091] each R.sup.9 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.3-C.sub.8-cycloalkenyl, fluorinated C.sub.3-C.sub.8-cycloalkenyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0092] two radicals R.sup.9 bound on neighbouring carbon atoms, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0093] each R.sup.10 is independently selected from the group consisting of hydrogen, cyano, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the five last-mentioned radicals may be substituted with one or more substituents R.sup.11; [0094] each R.sup.11 is independently selected from the group consisting of halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sup.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --COOH, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy and fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy; [0095] R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, cyano, C
.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, phenyl and benzyl, where the phenyl moieties in the two last-mentioned radicals may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; or, [0096] if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; [0097] a is 0, 1 or 2; [0098] b is 0, 1, 2 or 3; and [0099] n is 1 or 2; [0100] and the N-oxides, tautomeric forms, stereoisomers and pharmaceutically acceptable salts thereof, and the compound of the general formula I, wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope.
[0101] Preferably, R.sup.5a and R.sup.5b are not simultaneously hydroxyl.
[0102] In another aspect, the invention relates to a pharmaceutical composition comprising a therapeutically effective amount of at least one compound of formula I or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof, or comprising at least one compound as defined above or below wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope, preferably wherein at least one hydrogen atom has been replaced by a deuterium atom, in combination with at least one pharmaceutically acceptable carrier and/or auxiliary substance.
[0103] In yet another aspect, the invention relates to a compound of formula I or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for use as a medicament.
[0104] In yet another aspect, the invention relates to a compound of formula I or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the treatment of disorders which responds to the modulation of the 5-HT.sub.2C receptor.
[0105] In yet another aspect, the invention relates to the use of a compound of formula I or of an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the manufacture of a medicament for the treatment of disorders which respond to the modulation of the 5-HT.sub.2C receptor.
[0106] In yet another aspect, the invention relates to the use of a compound of formula I or of an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the manufacture of a medicament for the treatment of disorders selected from the group consisting of damage of the central nervous system, disorders of the central nervous system, eating disorders, ocular hypertension, cardiovascular disorders, gastrointestinal disorders and diabetes, and especially from the group consisting of bipolar disorder, depression, atypical depression, mood episodes, adjustment disorders, anxiety, panic disorders, post-traumatic syndrome, psychoses, schizophrenia, cognitive deficits of schizophrenia, memory loss, dementia of aging, Alzheimer's disease, behavioral disorders associated with dementia, social phobia, mental disorders in childhood, attention deficit hyperactivity disorder, organic mental disorders, autism, mutism, disruptive behavior disorder, impulse control disorder, borderline personality disorder, obsessive compulsive disorder, migraine and other conditions associated with cephalic pain or other pain, raised intracranial pressure, seizure disorders, epilepsy, substance use disorders, alcohol abuse, cocaine abuse, tobacco abuse, smoking cessation, sexual dysfunction/erectile dysfunction in males, sexual dysfunction in females, premenstrual syndrome, late luteal phase syndrome, chronic fatigue syndrome, sleep disorders, sleep apnoea, chronic fatigue syndrome, psoriasis, Parkinson's disease, spinal cord injury, trauma, stroke, pain, bladder dysfunction/urinary incontinence, encephalitis, meningitis, eating disorders, obesity, bulimia, weight loss, anorexia nervosa, ocular hypertension, cardiovascular disorders, gastrointestinal disorders, diabetes insipidus, diabetes mellitus, type I diabetes, type II diabetes, type III diabetes, diabetes secondary to pancreatic diseases, diabetes related to steroid use, diabetes complications, hyperglycemia and insulin resistance.
[0107] In yet another aspect, the invention relates to a method for treating disorders which respond to the modulation of the 5-HT.sub.2C receptor, which method comprises administering to a subject in need thereof at least one compound of formula I or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
[0108] In yet another aspect, the invention relates to a method for treating disorders selected from the group consisting of damage of the central nervous system, disorders of the central nervous system, eating disorders, ocular hypertension, cardiovascular disorders, gastrointestinal disorders and diabetes, and especially from the group consisting of bipolar disorder, depression, atypical depression, mood episodes, adjustment disorders, anxiety, panic disorders, post-traumatic syndrome, psychoses, schizophrenia, cognitive deficits of schizophrenia, memory loss, dementia of aging, Alzheimer's disease, behavioral disorders associated with dementia, social phobia, mental disorders in childhood, attention deficit hyperactivity disorder, organic mental disorders, autism, mutism, disruptive behavior disorder, impulse control disorder, borderline personality disorder, obsessive compulsive disorder, migraine and other conditions associated with cephalic pain or other pain, raised intracranial pressure, seizure disorders, epilepsy, substance use disorders, alcohol abuse, cocaine abuse, tobacco abuse, smoking cessation, sexual dysfunction/erectile dysfunction in males, sexual dysfunction in females, premenstrual syndrome, late luteal phase syndrome, chronic fatigue syndrome, sleep disorders, sleep apnoea, chronic fatigue syndrome, psoriasis, Parkinson's disease, spinal cord injury, trauma, stroke, pain, bladder dysfunction/urinary incontinence, encephalitis, meningitis, eating disorders, obesity, bulimia, weight loss, anorexia nervosa, ocular hypertension, cardiovascular disorders, gastrointestinal disorders, diabetes insipidus, diabetes mellitus, type I diabetes, type II diabetes, type III diabetes, diabetes secondary to pancreatic diseases, diabetes related to steroid use, diabetes complications, hyperglycemia and insulin resistance, which method comprises administering to a subject in need thereof at least one compound of formula I or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
DETAILED DESCRIPTION
[0109] The compounds of the formula I may exist in different spatial arrangements. For example, if the compounds possess one or more centers of asymmetry, polysubstituted rings or double bonds, or as different tautomers, the present invention contemplates the possible use of enantiomeric mixtures, in particular racemates, diastereomeric mixtures and tautomeric mixtures, such as the respective essentially pure enantiomers, diastereomers and tautomers of the compounds of formula I and/or their salts.
[0110] It is likewise possible to use physiologically tolerated salts of the compounds of the formula I, especially acid addition salts with physiologically tolerated acids. Examples of suitable physiologically tolerated organic and inorganic acids are hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid, acetic acid, trifluoroacetic acid, C.sub.1-C.sub.4-alkylsulfonic acids, such as methanesulfonic acid, aromatic sulfonic acids, such as benzenesulfonic acid and toluenesulfonic acid, oxalic acid, maleic acid, fumaric acid, lactic acid, tartaric acid, adipic acid and benzoic acid. Other utilizable acids are described in Fortschritte der Arzneimittelforschung [Advances in drug research], Volume 10, pages 224 et seq., Birkhauser Verlag, Basel and Stuttgart, 1966.
[0111] The compounds of formula I may also be present in the form of tautomers. In one aspect, tautomery may be present in compounds I wherein R.sup.4a and R.sup.4b form together a group .dbd.O and R.sup.5a or R.sup.5b is H. For example, the compounds of formula I may have the following tautomeric formulae:
##STR00003##
[0112] In another aspect, tautomery may be present in compounds I containing rings which have one or more C.dbd.O groups as ring members which are neighboured to a CH.sub.2 group.
[0113] The organic moieties mentioned in the above definitions of the variables are, like the term halogen, collective terms for individual listings of the individual group members. The prefix C.sub.n-C.sub.m indicates in each case the possible number of carbon atoms in the group.
[0114] The term "halogen" denotes in each case fluorine, bromine, chlorine or iodine. In one aspect, the halogen may be fluorine, chlorine or bromine.
[0115] The term "alkyl" as used herein and in the alkyl moieties of alkoxy, alkylthio, alkylsulfinyl, alkylsulfonyl, alkylcarbonyl, alkoxycarbonyl and the like refers to saturated straight-chain or branched hydrocarbon radicals having 1 to 2 ("C.sub.1-C.sub.2-alkyl"), 1 to 3 ("C.sub.1-C.sub.3-alkyl"), 1 to 4 ("C.sub.1-C.sub.4-alkyl") or 1 to 6 ("C.sub.1-C.sub.6-alkyl") carbon atoms. C.sub.1-C.sub.2-Alkyl is methyl or ethyl. C.sub.1-C.sub.3-Alkyl is additionally propyl and isopropyl. C.sub.1-C.sub.4-Alkyl is additionally butyl, 1-methylpropyl (sec-butyl), 2-methylpropyl (isobutyl) or 1,1-dimethylethyl (tert-butyl). C.sub.1-C.sub.6-Alkyl is additionally also, for example, pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, hexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl, or 1-ethyl-2-methylpropyl.
[0116] The term "fluorinated alkyl" as used herein refers to straight-chain or branched alkyl groups having 1 to 2 ("fluorinated C.sub.1-C.sub.2-alkyl"), 1 to 3 ("fluorinated C.sub.1-C.sub.3-alkyl"), 1 to 4 ("fluorinated C.sub.1-C.sub.4-alkyl") or 1 to 6 ("fluorinated C.sub.1-C.sub.6-alkyl") carbon atoms (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by fluorine atoms. Fluorinated C.sub.1-C.sub.2-alkyl is an alkyl group having 1 or 2 carbon atoms (as mentioned above), where at least one of the hydrogen atoms, e.g. 1, 2, 3, 4 or 5 hydrogen atoms in these groups are replaced by fluorine atoms, such as difluoromethyl, trifluoromethyl, 1-fluoroethyl, (R)-1-fluoroethyl, (S)-1-fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, or pentafluoroethyl. Fluorinated C.sub.1-C.sub.4-alkyl is a straight-chain or branched alkyl group having 1 to 4 carbon atoms (as mentioned above), where at least one of the hydrogen atoms, e.g. 1, 2, 3, 4 or 5 hydrogen atoms in these groups are replaced by fluorine atoms. Examples are, apart those listed above for C.sub.1-C.sub.2-fluoroalkyl, 1-fluoropropyl, (R)-1-fluoropropyl, (S)-1-fluoropropyl, 2-fluoropropyl, (R)-2-fluoropropyl, (S)-2-fluoropropyl, 3-fluoropropyl, 1,1-difluoropropyl, 2,2-difluoropropyl, 1,2-difluoropropyl, 2,3-difluoropropyl, 1,3-difluoropropyl, 3,3-difluoropropyl, 1,1,2-trifluoropropyl, 1,2,2-trifluoropropyl, 1,2,3-trifluoropropyl, 2,2,3-trifluoropropyl, 3,3,3-trifluoropropyl, 1,1,1-trifluoroprop-2-yl, 2-fluoro-1-methylethyl, (R)-2-fluoro-1-methylethyl, (S)-2-fluoro-1-methylethyl, 2,2-difluoro-1-methylethyl, (R)-2,2-difluoro-1-methylethyl, (S)-2,2-difluoro-1-methylethyl, 1,2-difluoro-1-methylethyl, (R)-1,2-difluoro-1-methylethyl, (S)-1,2-difluoro-1-methylethyl, 2,2,2-trifluoro-1-methylethyl, (R)-2,2,2-trifluoro-1-methylethyl, (S)-2,2,2-trifluoro-1-methylethyl, 2-fluoro-1-(fluoromethyl)ethyl, 1-(difluoromethyl)-2,2-difluoroethyl, 1-(trifluoromethyl)-2,2,2-trifluoroethyl, 1-(trifluoromethyl)-1,2,2,2-tetrafluoroethyl, 1-fluorobutyl, (R)-1-fluorobutyl, (S)-1-fluorobutyl, 2-fluorobutyl, (R)-2-fluorobutyl, (S)-2-fluorobutyl, 3-fluorobutyl, (R)-3-fluorobutyl, (S)-3-fluorobutyl, 4-fluorobutyl, 1,1-difluorobutyl, 2,2-difluorobutyl, 3,3-difluorobutyl, 4,4-difluorobutyl, 4,4,4-trifluorobutyl and the like. Fluorinated C.sub.1-C.sub.6-alkyl is a straight-chain or branched alkyl group having 1 to 6 carbon atoms (as mentioned above), where at least one of the hydrogen atoms, e.g. 1, 2, 3, 4 or 5 hydrogen atoms in these groups are replaced by fluorine atoms. Additionally examples include for C.sub.1-C.sub.4-fluoroalkyl, 1-fluoropentyl, (R)-1-fluoropentyl, (S)-1-fluoropentyl, 2-fluoropentyl, (R)-2-fluoropentyl, (S)-2-fluoropentyl, 3-fluoropentyl, (R)-3-fluoropentyl, (S)-3-fluoropentyl, 4-fluoropentyl, (R)-4-fluoropentyl, (S)-4-fluoropentyl, 5-fluoropentyl, (R)-5-fluoropentyl, (S)-5-fluoropentyl, 1-fluorohexyl, (R)-1-fluorohexyl, (S)-1-fluorohexyl, 2-fluorohexyl, (R)-2-fluorohexyl, (S)-2-fluorohexyl, 3-fluorohexyl, (R)-3-fluorohexyl, (S)-3-fluorohexyl, 4-fluorohexyl, (R)-4-fluorohexyl, (S)-4-fluorohexyl, 5-fluorohexyl, (R)-5-fluorohexyl, (S)-5-fluorohexyl, 6-fluorohexyl, (R)-6-fluorohexyl, (S)-6-fluorohexyl, and the like.
[0117] The term "alkenyl" as used herein refers to monounsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("C.sub.2-C.sub.3-alkenyl"), 2 to 4 ("C.sub.2-C.sub.4-alkenyl") or 2 to 6 ("C.sub.2-C.sub.6-alkenyl") carbon atoms and a double bond in any position, such as C.sub.2-C.sub.3-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl or 1-methylethenyl; C.sub.2-C.sub.4-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl or 2-methyl-2-propenyl; and C.sub.2-C.sub.6-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl, 2-methyl-2-propenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 1-methyl-1-butenyl, 2-methyl-1-butenyl, 3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl, 3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-3-butenyl, 3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl, 1,2-dimethyl-1-propenyl, 1,2-dimethyl-2-propenyl, 1-ethyl-1-propenyl, 1-ethyl-2-propenyl, 1-hexenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-1-pentenyl, 2-methyl-1-pentenyl, 3-methyl-1-pentenyl, 4-methyl-1-pentenyl, 1-methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2-pentenyl, 4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl, 3-methyl-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-pentenyl, 2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl, 1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl, 1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl, 1,2-dimethyl-3-butenyl, 1,3-dimethyl-1-butenyl, 1,3-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl, 2,2-dimethyl-3-butenyl, 2,3-dimethyl-1-butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl, 3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl, 1-ethyl-1-butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl, 2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl, 1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl, 1-ethyl-2-methyl-1-propenyl, 1-ethyl-2-methyl-2-propenyl and the like.
[0118] The term "fluorinated alkenyl" as used herein refers to unsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("fluorinated C.sub.2-C.sub.3-alkenyl"), 2 to 4 ("fluorinated C.sub.2-C.sub.4-alkenyl") or 2 to 6 ("fluorinated C.sub.2-C.sub.6-alkenyl") carbon atoms and a double bond in any position (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by fluorine atoms, such as, fluorovinyl, fluoroallyl and the like.
[0119] The term "alkynyl" as used herein refers to straight-chain or branched hydrocarbon groups having 2 to 3 ("C.sub.2-C.sub.3-alkynyl"), 2 to 4 ("C.sub.2-C.sub.4-alkynyl") or 2 to 6 ("C.sub.2-C.sub.6-alkynyl") carbon atoms and one or two triple bonds in any position, such as C.sub.2-C.sub.3-alkynyl, such as ethynyl, 1-propynyl or 2-propynyl; C.sub.2-C.sub.4-alkynyl, such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl and the like, and C.sub.2-C.sub.6-alkynyl, such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl, 3-methyl-1-butynyl, 1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl, 1-methyl-3-pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl, 2-methyl-4-pentynyl, 3-methyl-1-pentynyl, 3-methyl-4-pentynyl, 4-methyl-1-pentynyl, 4-methyl-2-pentynyl, 1,1-dimethyl-2-butynyl, 1,1-dimethyl-3-butynyl, 1,2-dimethyl-3-butynyl, 2,2-dimethyl-3-butynyl, 3,3-dimethyl-1-butynyl, 1-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl, 1-ethyl-1-methyl-2-propynyl and the like.
[0120] The term "fluorinated alkynyl" as used herein refers to unsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("fluorinated C.sub.2-C.sub.3-alkynyl"), 3 to 4 ("fluorinated C.sub.3-C.sub.4-alkynyl") or 2 to 6 ("fluorinated C.sub.2-C.sub.6-alkynyl") carbon atoms and one or two triple bonds in any position (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by fluorine atoms.
[0121] The term "cycloalkyl" as used herein refers to mono- or bicyclic saturated hydrocarbon radicals having 3 to 8 ("C.sub.3-C.sub.8-cycloalkyl"), in particular 3 to 6 carbon atoms ("C.sub.3-C.sub.6-cycloalkyl") or 3 to 5 carbon atoms ("C.sub.3-C.sub.5-cycloalkyl") or 3 or 4 carbon atoms ("C.sub.3-C.sub.4-cycloalkyl"). In one aspect, C.sub.3-C.sub.4-cycloalkyl, C.sub.3-C.sub.5-cycloalkyl and C.sub.3-C.sub.6-cycloalkyl are monocyclic. Examples for C.sub.3-C.sub.4-cycloalkyl are cyclopropyl and cyclobutyl. Examples of monocyclic radicals having 3 to 5 carbon atoms are cyclopropyl, cyclobutyl and cyclopentyl. Examples of monocyclic radicals having 3 to 6 carbon atoms are cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. Examples of monocyclic radicals having 3 to 8 carbon atoms are cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl. Examples of bicyclic radicals having 7 or 8 carbon atoms comprise bicyclo[2.2.1]heptyl, bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl and bicyclo[3.2.1]octyl. In one aspect, the term cycloalkyl denotes a monocyclic saturated hydrocarbon radical.
[0122] The term "fluorinated cycloalkyl" as used herein refers to mono- or bicyclic saturated hydrocarbon groups having 3 to 8 ("C.sub.3-C.sub.8-halocycloalkyl") or preferably 3 to 6 ("C.sub.3-C.sub.6-halocycloalkyl") or 3 to 5 ("C.sub.3-C.sub.8-halocycloalkyl") carbon ring members (as mentioned above) in which some or all of the hydrogen atoms are replaced by fluorine atoms. Examples include 1-fluorocyclopropyl, 2-fluorocyclopropyl, (S)- and (R)-2,2-difluorocyclopropyl, 1,2-difluorocyclopropyl, 2,3-difluorocyclopropyl, pentafluorocyclopropyl, 1-fluorocyclobutyl, 2-fluorocyclobutyl, 3-fluorocyclobutyl, 2,2-difluorocyclobutyl, 3,3-difluorocyclobutyl, 1,2-difluorocyclobutyl, 1,3-difluorocyclobutyl, 2,3-difluorocyclobutyl, 2,4-difluorocyclobutyl, 1,2,2-trifluorocyclobutyl, 1-fluorocycloheptyl, 2-fluorocycloheptyl, 3-fluorocycloheptyl, 4-fluorocycloheptyl, 1,2-difluorocycloheptyl, 1,3-difluorocycloheptyl, 1,4-difluorocycloheptyl, 2,2-difluorocycloheptyl, 2,3-difluorocycloheptyl, 2,4-difluorocycloheptyl, 2,5-difluorocycloheptyl, 2,6-difluorocycloheptyl, 2,7-difluorocycloheptyl, 3,3-difluorocycloheptyl, 3,4-difluorocycloheptyl, 3,5-difluorocycloheptyl, 3,6-difluorocycloheptyl, 4,4-difluorocycloheptyl, 4,5-difluorocycloheptyl, and the like.
[0123] The term "cycloalkenyl" as used herein refers to monocyclic partially unsaturated, non-aromatic hydrocarbon radicals having 3 to 8 ("C.sub.3-C.sub.8-cycloalkenyl"), in particular 5 to 7 carbon atoms ("C.sub.5-C.sub.7-cycloalkenyl") or 5 or 6 carbon atoms ("C.sub.5-C.sub.6-cycloalkenyl") and one or more non-cumulative, preferably one, C--C double bonds in the ring. Examples for C.sub.5-C.sub.6-cycloalkenyl are cyclopent-1-en-1-yl, cyclopent-1-en-3-yl, cyclopent-1-en-4-yl, cyclopenta-1,3-dien-1-yl, cyclopenta-1,3-dien-2-yl, cyclopenta-1,3-dien-5-yl, cyclohex-1-en-1-yl, cyclohex-1-en-3-yl, cyclohex-1-en-4-yl, cyclohexa-1,3-dien-1-yl, cyclohexa-1,3-dien-2-yl, cyclohexa-1,3-dien-5-yl, cyclohexa-1,4-dien-1-yl and cyclohexa-1,4-dien-3-yl. Examples of C.sub.5-C.sub.7-cycloalkenyl are, apart those mentioned above, include for C.sub.5-C.sub.6-cycloalkenyl, cyclohept-1-en-1-yl, cyclohept-1-en-3-yl, cyclohept-1-en-4-yl, cyclohept-1-en-5-yl, cyclohepta-1,3-dien-1-yl, cyclohepta-1,3-dien-2-yl, cyclohepta-1,3-dien-5-yl, cyclohepta-1,3-dien-6-yl, cyclohepta-1,4-dien-1-yl, cyclohepta-1,4-dien-2-yl, cyclohepta-1,4-dien-3-yl and cyclohepta-1,4-dien-6-yl. Examples of C.sub.3-C.sub.8-cycloalkenyl are, apart those mentioned above for C.sub.5-C.sub.6-cycloalkenyl, cycloprop-1-en-1-yl, cycloprop-1-en-3-yl, cyclobut-1-en-1-yl, cyclobut-1-en-3-yl, cyclooct-1-en-1-yl, cyclooct-1-en-3-yl, cyclooct-1-en-4-yl, cyclooct-1-en-5-yl, cycloocta-1,3-dien-1-yl, cycloocta-1,3-dien-2-yl, cycloocta-1,3-dien-5-yl, cycloocta-1,3-dien-6-yl, cycloocta-1,4-dien-1-yl, cycloocta-1,4-dien-2-yl, cycloocta-1,4-dien-3-yl, cycloocta-1,4-dien-6-yl, cycloocta-1,4-dien-7-yl, cycloocta-1,5-dien-1-yl, and cycloocta-1,5-dien-3-yl.
[0124] The term "fluorinated cycloalkenyl" as used herein refers to monocyclic partially unsaturated, non-aromatic hydrocarbon radicals having 3 to 8 ("fluorinated C.sub.3-C.sub.8-cycloalkenyl"), in particular 5 to 7 carbon atoms ("fluorinated C.sub.5-C.sub.7-cycloalkenyl") or 5 or 6 carbon atoms ("fluorinated C.sub.5-C.sub.6-cycloalkenyl") and one or more non-cumulative, preferably one, C--C double bonds in the ring and in which some or all of the hydrogen atoms are replaced by fluorine atoms.
[0125] The term "cycloalkyl-C.sub.1-C.sub.4-alkyl" refers to a C.sub.3-C.sub.8-cycloalkyl group ("C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl"), preferably a C.sub.3-C.sub.6-cycloalkyl group ("C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl") as defined above which is bound to the remainder of the molecule via a C.sub.1-C.sub.4-alkyl group, as defined above. The term "cycloalkyl-C.sub.1-C.sub.2-alkyl" refers to a C.sub.3-C.sub.8-cycloalkyl group ("C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.2-alkyl"), preferably a C.sub.3-C.sub.6-cycloalkyl group ("C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl") as defined above which is bound to the remainder of the molecule via a C.sub.1-C.sub.2-alkyl group, as defined above. Examples for C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl are cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, cyclobutylethyl, cyclopentylmethyl, cyclopentylethyl, cyclohexylmethyl and cyclohexylethyl. Examples for C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl, apart those mentioned for C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, are cyclopropylpropyl, cyclopropylbutyl, cyclobutylpropyl, cyclobutylbutyl, cyclopentylpropyl, cyclopentylbutyl, cyclohexylpropyl and cyclohexylbutyl. Examples for C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.2-alkyl, apart those mentioned for C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, are cycloheptylmethyl, cycloheptylethyl, cyclooctylmethyl and cyclooctylethyl. Examples for C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl, apart those mentioned for C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.2-alkyl, are cycloheptylpropyl, cycloheptylbutyl, cyclooctylpropyland cyclooctylbutyl.
[0126] The term "fluorinated cycloalkyl-C.sub.1-C.sub.4-alkyl" refers to a fluorinated C.sub.3-C.sub.8-cycloalkyl group ("fluorinated C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.4-alkyl"), preferably a fluorinated C.sub.3-C.sub.6-cycloalkyl group ("fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl") as defined above which is bound to the remainder of the molecule via a C.sub.1-C.sub.4-alkyl group, as defined above. The term "fluorinated cycloalkyl-C.sub.1-C.sub.2-alkyl" refers to a fluorinated C.sub.3-C.sub.8-cycloalkyl group ("fluorinated C.sub.3-C.sub.8-cycloalkyl-C.sub.1-C.sub.2-alkyl"), preferably a fluorinated C.sub.3-C.sub.6-cycloalkyl group ("fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl") as defined above which is bound to the remainder of the molecule via a C.sub.1-C.sub.2-alkyl group, as defined above.
[0127] The term "C.sub.1-C.sub.2-alkoxy" is a C.sub.1-C.sub.2-alkyl group, as defined above, attached via an oxygen atom. The term "C.sub.1-C.sub.3-alkoxy" is a C.sub.1-C.sub.3-alkyl group, as defined above, attached via an oxygen atom. The term "C.sub.1-C.sub.4-alkoxy" is a C.sub.1-C.sub.4-alkyl group, as defined above, attached via an oxygen atom. The term "C.sub.1-C.sub.6-alkoxy" is a C.sub.1-C.sub.6-alkyl group, as defined above, attached via an oxygen atom. C.sub.1-C.sub.2-Alkoxy is methoxy or ethoxy. C.sub.1-C.sub.3-Alkoxy is additionally, for example, n-propoxy and 1-methylethoxy (isopropoxy). C.sub.1-C.sub.4-Alkoxy is additionally, for example, butoxy, 1-methylpropoxy (sec-butoxy), 2-methylpropoxy (isobutoxy) or 1,1-dimethylethoxy (tert-butoxy). C.sub.1-C.sub.6-Alkoxy is additionally, for example, pentoxy, 1-methylbutoxy, 2-methylbutoxy, 3-methylbutoxy, 1,1-dimethylpropoxy, 1,2-dimethylpropoxy, 2,2-dimethylpropoxy, 1-ethylpropoxy, hexoxy, 1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy, 4-methylpentoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy, 1,3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy, 1-ethyl-1-methylpropoxy or 1-ethyl-2-methylpropoxy. C.sub.1-C.sub.8-Alkoxy is additionally, for example, heptyloxy, octyloxy, 2-ethylhexyloxy and positional isomers thereof.
[0128] The term "fluorinated C.sub.1-C.sub.2-alkoxy" is a fluorinated C.sub.1-C.sub.2-alkyl group, as defined above, attached via an oxygen atom. The term "fluorinated C.sub.1-C.sub.3-alkoxy" is a fluorinated C.sub.1-C.sub.3-alkyl group, as defined above, attached via an oxygen atom. The term "fluorinated C.sub.1-C.sub.6-haloalkoxy" is a fluorinated C.sub.1-C.sub.6-alkyl group, as defined above, attached via an oxygen atom. Fluorinated C.sub.1-C.sub.2-alkoxy is, for example, OCH.sub.2F, OCHF.sub.2, OCF.sub.3, 1-fluoroethoxy, (R)-1-fluoroethoxy, (S)-1-fluoroethoxy, 2-fluoroethoxy, 1,1-difluoroethoxy, 1,2-difluoroethoxy, 2,2-difluoroethoxy, 1,1,2-trifluoroethoxy, 1,2,2-trifluoroethoxy, 2,2,2-trifluoroethoxy, 1,1,2,2-tetrafluoroethoxy or OC.sub.2F.sub.5. Fluorinated C.sub.1-C.sub.3-alkoxy is additionally, for example, 1-fluoropropoxy, (R)-1-fluoropropoxy, (S)-1-fluoropropoxy, 2-fluoropropoxy, (R)-2-fluoropropoxy, (S)-2-fluoropropoxy, 3-fluoropropoxy, 1,1-difluoropropoxy, 2,2-difluoropropoxy, 2,3-difluoropropoxy, 3,3-difluoropropoxy, 3,3,3-trifluoropropoxy, (R)-2-fluoro-1-methylethoxy, (S)-2-fluoro-1-methylethoxy, (R)-2,2-difluoro-1-methylethoxy, (S)-2,2-difluoro-1-methylethoxy, (R)-1,2-difluoro-1-methylethoxy, (S)-1,2-difluoro-1-methylethoxy, (R)-2,2,2-trifluoro-1-methylethoxy, (S)-2,2,2-trifluoro-1-methylethoxy, 2-fluoro-1-(fluoromethyl)ethoxy, 1-(difluoromethyl)-2,2-difluoroethoxy, OCH.sub.2--C.sub.2F.sub.5, OCF.sub.2--C.sub.2F.sub.5 or 1-(CH.sub.2F)-2-fluoroethoxy. Fluorinated C.sub.1-C.sub.4-alkoxy is additionally, for example, 1-fluorobutoxy, (R)-1-fluorobutoxy, (S)-1-fluorobutoxy, 2-fluorobutoxy, 3-fluorobutoxy, 4-fluorobutoxy, 1,1-difluorobutoxy, 2,2-difluorobutoxy, 3,3-difluorobutoxy, 4,4-difluorobutoxy, 4,4,4-trifluorobutoxy or nonafluorobutoxy. Fluorinated C.sub.1-C.sub.6-alkoxy is additionally, for example, 5-fluoropentoxy, undecafluoropentoxy, 6-fluorohexoxy or tridecafluorohexoxy.
[0129] The term "C.sub.1-C.sub.4-alkoxy-C.sub.1-C.sub.4-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 4 carbon atoms, as defined above, where one hydrogen atom is replaced by a C.sub.1-C.sub.4-alkoxy group, as defined above. The term "C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 4 carbon atoms, as defined above, where one hydrogen atom is replaced by a C.sub.1-C.sub.6-alkoxy group, as defined above. Examples are methoxymethyl, ethoxymethyl, propoxymethyl, isopropoxymethyl, n-butoxymethyl, sec-butoxymethyl, isobutoxymethyl, tert-butoxymethyl, 1-methoxyethyl, 1-ethoxyethyl, 1-propoxyethyl, 1-isopropoxyethyl, 1-n-butoxyethyl, 1-sec-butoxyethyl, 1-isobutoxyethyl, 1-tert-butoxyethyl, 2-methoxyethyl, 2-ethoxyethyl, 2-propoxyethyl, 2-isopropoxyethyl, 2-n-butoxyethyl, 2-sec-butoxyethyl, 2-isobutoxyethyl, 2-tert-butoxyethyl, 1-methoxypropyl, 1-ethoxypropyl, 1-propoxypropyl, 1-isopropoxypropyl, 1-n-butoxypropyl, 1-sec-butoxypropyl, 1-isobutoxypropyl, 1-tert-butoxypropyl, 2-methoxypropyl, 2-ethoxypropyl, 2-propoxypropyl, 2-isopropoxypropyl, 2-n-butoxypropyl, 2-sec-butoxypropyl, 2-isobutoxypropyl, 2-tert-butoxypropyl, 3-methoxypropyl, 3-ethoxypropyl, 3-propoxypropyl, 3-isopropoxypropyl, 3-n-butoxypropyl, 3-sec-butoxypropyl, 3-isobutoxypropyl, 3-tert-butoxypropyl and the like.
[0130] The term "hydroxy-C.sub.1-C.sub.4-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 4 carbon atoms, as defined above, where one hydrogen atom is replaced by a hydroxy group. The term "hydroxy-C.sub.1-C.sub.6-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 6 carbon atoms, as defined above, where one hydrogen atom is replaced by a hydroxy group. Examples for hydroxy-C.sub.1-C.sub.4-alkyl include hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl, 1-hydroxypropyl, 2-hydroxypropyl, 3-hydroxypropyl, 1-hydroxyprop-2-yl, 2-hydroxyprop-2-yl, 1-hydroxybutyl, 2-hydroxybutyl, 3-hydroxybutyl, 4-hydroxybutyl, 1-hydroxybut-2-yl, 2-hydroxybut-2-yl, 3-hydroxybut-2-yl, 4-hydroxybut-2-yl, hydroxy-tert-butyl and the like. Examples for hydroxy-C.sub.1-C.sub.6-alkyl are, apart those mentioned for hydroxy-C.sub.1-C.sub.4-alkyl, include, 1-hydroxypentyl, 2-hydroxypentyl, 3-hydroxypentyl, 4-hydroxypentyl, 5-hydroxypentyl, 1-hydroxyhexyl, 2-hydroxyhexyl, 3-hydroxyhexyl, 4-hydroxyhexyl, 5-hydroxyhexyl, 6-hydroxyhexyl and the like.
[0131] The term "hydroxy-C.sub.1-C.sub.4-alkoxy" as used herein, refers to a C.sub.1-C.sub.4-alkoxy group, as defined above, where one hydrogen atom is replaced by a hydroxy group. The term "hydroxy-C.sub.1-C.sub.6-alkoxy" as used herein, refers to a C.sub.1-C.sub.6-alkoxy group, as defined above, where one hydrogen atom is replaced by a hydroxy group. Examples for hydroxy-C.sub.1-C.sub.4-alkoxy include hydroxymethoxy, 1-hydroxyethoxy, 2-hydroxyethoxy, 1-hydroxypropoxy, 2-hydroxypropoxy, 3-hydroxypropoxy, 1-hydroxy-2-propoxy, 2-hydroxy-2-propoxy, 1-hydroxybutoxy, 2-hydroxybutoxy, 3-hydroxybutoxy, 4-hydroxybutoxy, 1-hydroxy-2-butoxy, 2-hydroxy-2-butoxy, 3-hydroxy-2-butoxy, 4-hydroxy-2-butoxy, hydroxy-tert-butoxy and the like. Examples for hydroxy-C.sub.1-C.sub.6-alkoxy include, apart those mentioned for hydroxy-C.sub.1-C.sub.4-alkoxy, 1-hydroxypentoxy, 2-hydroxypentoxy, 3-hydroxypentoxy, 4-hydroxypentoxy, 5-hydroxypentoxy, 1-hydroxyhexoxy, 2-hydroxyhexoxy, 3-hydroxyhexoxy, 4-hydroxyhexoxy, 5-hydroxyhexoxy, 6-hydroxyhexoxy and the like.
[0132] The term "C.sub.1-C.sub.4-alkoxy-C.sub.1-C.sub.4-alkoxy" as used herein, refers to a C.sub.1-C.sub.4-alkoxy group, as defined above, where one hydrogen atom is replaced by a C.sub.1-C.sub.4-alkoxy group, as defined above. The term "C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy" as used herein, refers to a C.sub.1-C.sub.4-alkoxy group, as defined above, where one hydrogen atom is replaced by a C.sub.1-C.sub.4-alkoxy group, as defined above. Examples are methoxymethoxy, ethoxymethoxy, propoxymethoxy, isopropoxymethoxy, butoxymethoxy, sec-butoxymethoxy, isobutoxymethoxy, tert-butoxymethoxy, 1-methoxyethoxy, 1-ethoxyethoxy, 1-propoxyethoxy, 1-isopropoxyethoxy, 1-butoxyethoxy, 1-sec-butoxyethoxy, 1-isobutoxyethoxy, 1-tert-butoxyethoxy, 2-methoxyethoxy, 2-ethoxyethoxy, 2-propoxyethoxy, 2-isopropoxyethoxy, 2-butoxyethoxy, 2-sec-butoxyethoxy, 2-isobutoxyethoxy, 2-tert-butoxyethoxy, 1-methoxypropoxy, 1-ethoxypropoxy, 1-propoxypropoxy, 1-isopropoxypropoxy, 1-butoxypropoxy, 1-sec-butoxypropoxy, 1-isobutoxypropoxy, 1-tert-butoxypropoxy, 2-methoxypropoxy, 2-ethoxypropoxy, 2-propoxypropoxy, 2-isopropoxypropoxy, 2-butoxypropoxy, 2-sec-butoxypropoxy, 2-isobutoxypropoxy, 2-tert-butoxypropoxy, 3-methoxypropoxy, 3-ethoxypropoxy, 3-propoxypropoxy, 3-isopropoxypropoxy, 3-butoxypropoxy, 3-sec-butoxypropoxy, 3-isobutoxypropoxy, 3-tert-butoxypropoxy and the like.
[0133] The term "C.sub.1-C.sub.2-alkylthio" is a C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfur atom. The term "C.sub.1-C.sub.3-alkylthio" refers to a C.sub.1-C.sub.3-alkyl group, as defined above, attached via a sulfur atom. The term "C.sub.1-C.sub.4-alkylthio" is a C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfur atom. The term "C.sub.1-C.sub.6-alkylthio" refers to a C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfur atom. The term "C.sub.1-C.sub.10-alkylthio" refers to a C.sub.1-C.sub.10-alkyl group, as defined above, attached via a sulfur atom. C.sub.1-C.sub.2-Alkylthio is methylthio or ethylthio. C.sub.1-C.sub.3-Alkylthio is additionally, for example, n-propylthio or 1-methylethylthio (isopropylthio). C.sub.1-C.sub.4-Alkylthio is additionally, for example, butylthio, 1-methylpropylthio (sec-butylthio), 2-methylpropylthio (isobutylthio) or 1,1-dimethylethylthio (tert-butylthio). C.sub.1-C.sub.6-Alkylthio is additionally, for example, pentylthio, 1-methylbutylthio, 2-methylbutylthio, 3-methylbutylthio, 1,1-dimethylpropylthio, 1,2-dimethylpropylthio, 2,2-dimethylpropylthio, 1-ethylpropylthio, hexylthio, 1-methylpentylthio, 2-methylpentylthio, 3-methylpentylthio, 4-methylpentylthio, 1,1-dimethylbutylthio, 1,2-dimethylbutylthio, 1,3-dimethylbutylthio, 2,2-dimethylbutylthio, 2,3-dimethylbutylthio, 3,3-dimethylbutylthio, 1-ethylbutylthio, 2-ethylbutylthio, 1,1,2-trimethylpropylthio, 1,2,2-trimethylpropylthio, 1-ethyl-1-methylpropylthio or 1-ethyl-2-methylpropylthio.
[0134] The term "fluorinated C.sub.1-C.sub.2-alkylthio" refers to a fluorinated C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfur atom. The term "fluorinated C.sub.1-C.sub.3-alkylthio" refers to a fluorinated C.sub.1-C.sub.3-alkyl group, as defined above, attached via a sulfur atom. The term "fluorinated C.sub.1-C.sub.4-alkylthio" refers to a fluorinated C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfur atom. The term "fluorinated C.sub.1-C.sub.6-alkylthio" refers to a fluorinated C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfur atom. Fluorinated C.sub.1-C.sub.2-alkylthio refers to, for example, SCH.sub.2F, SCHF.sub.2, SCF.sub.3, 2-fluoroethylthio, 2,2-difluoroethylthio, 2,2,2-trifluoroethylthio, or SC.sub.2F.sub.5. Fluorinated C.sub.1-C.sub.3-alkylthio may additionally, for example, include 2-fluoropropylthio, 3-fluoropropylthio, 2,2-difluoropropylthio, 2,3-difluoropropylthio, 3,3,3-trifluoropropylthio, SCH.sub.2--C.sub.2F.sub.5, SCF.sub.2--C.sub.2F.sub.5 or 1-(CH.sub.2F)-2-fluoroethylthio. Fluorinated C.sub.1-C.sub.4-alkylthio may additionally, for example, include 4-fluorobutylthio or nonafluorobutylthio. Fluorinated C.sub.1-C.sub.6-alkylthio is additionally, for example, 5-fluoropentylthio, undecafluoropentylthio, 6-fluorohexylthio or dodecafluorohexylthio.
[0135] The term "C.sub.1-C.sub.2-alkylsulfinyl" refers to a C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C.sub.1-C.sub.4-alkylsulfinyl" is a C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C.sub.1-C.sub.6-alkylsulfinyl" is a C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. C.sub.1-C.sub.2-Alkylsulfinyl is methylsulfinyl or ethylsulfinyl. C.sub.1-C.sub.4-Alkylsulfinyl is additionally, for example, n-propylsulfinyl, 1-methylethylsulfinyl (isopropylsulfinyl), butylsulfinyl, 1-methylpropylsulfinyl (sec-butylsulfinyl), 2-methylpropylsulfinyl (isobutylsulfinyl) or 1,1-dimethylethylsulfinyl (tert-butylsulfinyl). C.sub.1-C.sub.6-Alkylsulfinyl is additionally, for example, pentylsulfinyl, 1-methylbutylsulfinyl, 2-methylbutylsulfinyl, 3-methylbutylsulfinyl, 1,1-dimethylpropylsulfinyl, 1,2-dimethylpropylsulfinyl, 2,2-dimethylpropylsulfinyl, 1-ethylpropylsulfinyl, hexylsulfinyl, 1-methylpentylsulfinyl, 2-methylpentylsulfinyl, 3-methylpentylsulfinyl, 4-methylpentylsulfinyl, 1,1-dimethylbutylsulfinyl, 1,2-dimethylbutylsulfinyl, 1,3-dimethylbutylsulfinyl, 2,2-dimethylbutylsulfinyl, 2,3-dimethylbutylsulfinyl, 3,3-dimethylbutylsulfinyl, 1-ethylbutylsulfinyl, 2-ethylbutylsulfinyl, 1,1,2-trimethylpropylsulfinyl, 1,2,2-trimethylpropylsulfinyl, 1-ethyl-1-methylpropylsulfinyl or 1-ethyl-2-methylpropylsulfinyl.
[0136] The term "fluorinated C.sub.1-C.sub.2-alkylsulfinyl" refers to a fluorinated C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "fluorinated C.sub.1-C.sub.3-alkylsulfinyl" refers to a fluorinated C.sub.1-C.sub.3-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "fluorinated C.sub.1-C.sub.4-alkylsulfinyl" refers to a fluorinated C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "fluorinated C.sub.1-C.sub.6-alkylsulfinyl" refers to a fluorinated C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. Fluorinated C.sub.1-C.sub.2-alkylsulfinyl is, for example, S(O)CH.sub.2F, S(O)CHF.sub.2, S(O)CF.sub.3, 2-fluoroethylsulfinyl, 2,2-difluoroethylsulfinyl, 2,2,2-trifluoroethylsulfinyl, or S(O)C.sub.2F.sub.5. Fluorinated C.sub.1-C.sub.3-alkylsulfinyl may additionally, for example, include 2-fluoropropylsulfinyl, 3-fluoropropylsulfinyl, 2,2-difluoropropylsulfinyl, 2,3-difluoropropylsulfinyl, 3,3,3-trifluoropropylsulfinyl, S(O)CH.sub.2--C.sub.2F.sub.5, S(O)CF.sub.2--C.sub.2F.sub.5 or 1-(CH.sub.2F)-2-fluoroethylsulfinyl. Fluorinated C.sub.1-C.sub.4-alkylsulfinyl may additionally, for example, include 4-fluorobutylsulfinyl or nonafluorobutylsulfinyl. Fluorinated C.sub.1-C.sub.6-alkylsulfinyl may additionally, for example, include 5-fluoropentylsulfinyl, undecafluoropentylsulfinyl, 6-fluorohexylsulfinyl or dodecafluorohexylsulfinyl.
[0137] The term "C.sub.1-C.sub.2-alkylsulfonyl" refers to a C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. The term "C.sub.1-C.sub.4-alkylsulfonyl" refers to a C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. The term "C.sub.1-C.sub.6-alkylsulfonyl" is a C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. C.sub.1-C.sub.2-Alkylsulfonyl refers to a methylsulfonyl or ethylsulfonyl. C.sub.1-C.sub.4-Alkylsulfonyl is additionally, for example, n-propylsulfonyl, 1-methylethylsulfonyl (isopropylsulfonyl), butylsulfonyl, 1-methylpropylsulfonyl (sec-butylsulfonyl), 2-methylpropylsulfonyl (isobutylsulfonyl) or 1,1-dimethylethylsulfonyl (tert-butylsulfonyl). C.sub.1-C.sub.6-Alkylsulfonyl is additionally, for example, pentylsulfonyl, 1-methylbutylsulfonyl, 2-methylbutylsulfonyl, 3-methylbutylsulfonyl, 1,1-dimethylpropylsulfonyl, 1,2-dimethylpropylsulfonyl, 2,2-dimethylpropylsulfonyl, 1-ethylpropylsulfonyl, hexylsulfonyl, 1-methylpentylsulfonyl, 2-methylpentylsulfonyl, 3-methylpentylsulfonyl, 4-methylpentylsulfonyl, 1,1-dimethylbutylsulfonyl, 1,2-dimethylbutylsulfonyl, 1,3-dimethylbutylsulfonyl, 2,2-dimethylbutylsulfonyl, 2,3-dimethylbutylsulfonyl, 3,3-dimethylbutylsulfonyl, 1-ethylbutylsulfonyl, 2-ethylbutylsulfonyl, 1,1,2-trimethylpropylsulfonyl, 1,2,2-trimethylpropylsulfonyl, 1-ethyl-1-methylpropylsulfonyl or 1-ethyl-2-methylpropylsulfonyl.
[0138] The term "fluorinated C.sub.1-C.sub.2-alkylsulfonyl" refers to a fluorinated C.sub.1-C.sub.2-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. The term "fluorinated C.sub.1-C.sub.3-alkylsulfonyl" refers to a fluorinated C.sub.1-C.sub.3-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. The term "fluorinated C.sub.1-C.sub.4-alkylsulfonyl" refers to a fluorinated C.sub.1-C.sub.4-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. The term "fluorinated C.sub.1-C.sub.6-alkylsulfonyl" refers to a fluorinated C.sub.1-C.sub.6-alkyl group, as defined above, attached via a sulfonyl [S(O).sub.2] group. Fluorinated C.sub.1-C.sub.2-alkylsulfonyl is, for example, S(O).sub.2CH.sub.2F, S(O).sub.2CHF.sub.2, S(O).sub.2CF.sub.3, 2-fluoroethylsulfonyl, 2,2-difluoroethylsulfonyl, 2,2,2-trifluoroethylsulfonyl, or S(O).sub.2C.sub.2F.sub.5. Fluorinated C.sub.1-C.sub.3-alkylsulfonyl is additionally, for example, 2-fluoropropylsulfonyl, 3-fluoropropylsulfonyl, 2,2-difluoropropylsulfonyl, 2,3-difluoropropylsulfonyl, 3,3,3-trifluoropropylsulfonyl, S(O).sub.2CH.sub.2--C.sub.2F.sub.5, S(O).sub.2CF.sub.2--C.sub.2F.sub.5 or 1-(CH.sub.2F)-2-fluoroethylsulfonyl. Fluorinated C.sub.1-C.sub.4-alkylsulfonyl is additionally, for example, 4-fluorobutylsulfonyl or nonafluorobutylsulfonyl. Fluorinated C.sub.1-C.sub.6-alkylsulfonyl is additionally, for example, 5-fluoropentylsulfonyl, undecafluoropentylsulfonyl, 6-fluorohexylsulfonyl or dodecafluorohexylsulfonyl.
[0139] C.sub.1-C.sub.4-Alkylcarbonyl refers to a straight-chain or branched alkyl group having from 1 to 4 carbon atoms), which is bound to the remainder of the molecule via a carbonyl group (CO), such as in acetyl, propionyl, isopropylcarbonyl, butylcarbonyl, sec-butylcarbonyl, isobutylcarbonyl, and tert-butylcarbonyl. C.sub.1-C.sub.6-Alkylcarbonyl is a straight-chain or branched alkyl group having from 1 to 6 carbon atoms, which is bound to the remainder of the molecule via a carbonyl group (CO). Examples include, apart those listed above for C.sub.1-C.sub.4-alkylcarbonylpentylcarbonyl, hexylcarbonyl and the constitutional isomers thereof.
[0140] Fluorinated C.sub.1-C.sub.4-alkylcarbonyl refers to a straight-chain or branched fluorinated alkyl group having from 1 to 4 carbon atoms as defined above, which is bound to the remainder of the molecule via a carbonyl group (CO). Fluorinated C.sub.1-C.sub.6-alkylcarbonyl is a straight-chain or branched fluorinated alkyl group having from 1 to 6 carbon atoms as defined above, which is bound to the remainder of the molecule via a carbonyl group (CO). Examples include trifluoromethylcarbonyl, 2,2,2-trifluoroethylcarbonyl and the like.
[0141] C.sub.3-C.sub.6-cycloalkylcarbonyl relates to a C.sub.3-C.sub.6-cycloalkyl group as defined above which is bound to the remainder of the molecule via a carbonyl group (CO), such as in cyclopropylcarbonyl, cyclobutylcarbonyl, cyclopentylcarbonyl and cyclohexylcarbonyl.
[0142] C.sub.1-C.sub.6-Alkoxycarbonyl refers to a straight-chain or branched alkoxy group having from 1 to 6, especially 1 to 4 carbon atoms (.dbd.C.sub.1-C.sub.4-alkoxycarbonyl), in particular 1 to 3 carbon atoms (.dbd.C.sub.1-C.sub.3-alkoxycarbonyl), which is bound to the remainder of the molecule via a carbonyl group (CO), such as in methoxycarbonyl, ethoxycarbonyl, propyloxycarbonyl, and isopropyloxycarbonyl.
[0143] Fluorinated C.sub.1-C.sub.6-alkoxycarbonyl refers to a straight-chain or branched fluorinated alkoxy group having from 1 to 6, especially 1 to 4 carbon atoms (=fluorinated C.sub.1-C.sub.4-alkoxycarbonyl), in particular 1 to 3 carbon atoms (=fluorinated C.sub.1-C.sub.3-alkoxycarbonyl) as defined above, which is bound to the remainder of the molecule via a carbonyl group (CO). Examples include trifluoromethoxycarbonyl, 2,2,2-trifluoroethoxycarbonyl and the like.
[0144] C.sub.1-C.sub.4-Alkylcarbonyloxy refers to a straight-chain or branched alkyl group having from 1 to 4 carbon atoms, which is bound to the remainder of the molecule via a carbonyloxy group [C(O)--O--], such as in acet(yl)oxy, propionyloxy, isopropylcarbonyloxy, butylcarbonyloxy, sec-butylcarbonyloxy, isobutylcarbonyloxy, and tert-butylcarbonyloxy. C.sub.1-C.sub.6-Alkylcarbonyloxy is a straight-chain or branched alkyl group having from 1 to 6 carbon atoms, which is bound to the remainder of the molecule via a carbonyloxy group [C(O--O--]. Examples include, apart those listed above for C.sub.1-C.sub.4-alkylcarbonyloxy, pentylcarbonyloxy, hexylcarbonyloxy and the constitutional isomers thereof.
[0145] Fluorinated C.sub.1-C.sub.4-alkylcarbonyloxy refers to a straight-chain or branched fluorinated alkyl group having from 1 to 4 carbon atoms as defined above, which is bound to the remainder of the molecule via a carbonyloxy group [C(O)--O--]. Fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy is a straight-chain or branched fluorinated alkyl group having from 1 to 6 carbon atoms as defined above, which is bound to the remainder of the molecule via a carbonyloxy group [C(O)--O--]. Examples include trifluoromethylcarbonyloxy, 2,2,2-trifluoroethylcarbonyloxy and the like.
[0146] Phenyl-C.sub.1-C.sub.2-alkyl is a phenyl group bound to the remainder of the molecule via a C.sub.1-C.sub.2-alkyl group. Examples are benzyl, 1-phenylethyl and 2-phenylethyl (phenethyl).
[0147] The term "3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members" denotes a 3-, 4-, 5-, 6-, 7- or 8-membered, preferably a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximum unsaturated heteromonocyclic ring containing 1, 2, 3 or 4 (preferably 1, 2 or 3) heteroatoms or heteroatom groups selected from N, O, S, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members.
[0148] Unsaturated rings contain at least one C--C and/or C--N and/or N--N double bond(s). Maximally unsaturated rings contain as many conjugated C--C and/or C--N and/or N--N double bonds as allowed by the ring size. Maximally unsaturated 5- or 6-membered heterocyclic rings are aromatic. 7- and 8-membered rings cannot be aromatic. They are homoaromatic (7-membered ring, 3 double bonds) or have 4 double bonds (8-membered ring). Partially unsaturated rings contain less than the maximum number of C--C and/or C--N and/or N--N double bond(s) allowed by the ring size. The heterocyclic ring may be attached to the remainder of the molecule via a carbon ring member or via a nitrogen ring member. As a matter of course, the heterocyclic ring contains at least one carbon ring atom. If the ring contains more than one O ring atom, these are not adjacent.
[0149] Examples of a 3-, 4-, 5-, 6- or 7-membered saturated heterocyclic ring include: Oxiranyl, thiiranyl, aziridinyl, oxetanyl, thietanyl, azetidinyl, tetrahydrofuran-2-yl, tetrahydrofuran-3-yl, tetrahydrofuran-2-on-3-yl, tetrahydrofuran-2-on-4-yl, tetrahydrofuran-2-on-5-yl, tetrahydrofuran-2-thion-3-yl, tetrahydrofuran-2-thion-4-yl, tetrahydrofuran-2-thion-5-yl, tetrahydrothien-2-yl, tetrahydrothien-3-yl, tetrahydrothien-2-on-3-yl, tetrahydrothien-2-on-4-yl, tetrahydrothien-2-on-5-yl, tetrahydrothien-2-thion-3-yl, tetrahydrothien-2-thion-4-yl, tetrahydrothien-2-thion-5-yl, pyrrolidin-1-yl, pyrrolidine-2-on-1-yl, pyrrolidine-2,5-dion-1-yl, pyrrolidine-2-thion-1-yl, pyrrolidin-2-yl, pyrrolidin-3-yl, pyrrolidine-2-on-3-yl, pyrrolidine-2-on-4-yl, pyrrolidine-2-on-5-yl, pyrrolidine-2,5-dion-3-yl, pyrrolidine-2-thion-3-yl, pyrrolidine-2-thion-4-yl, pyrrolidine-2-thion-5-yl, pyrazolidin-1-yl, pyrazolidin-3-yl, pyrazolidin-4-yl, pyrazolidin-5-yl, imidazolidin-1-yl, imidazolidin-2-on-1-yl, imidazolidin-2-thion-1-yl, imidazolidin-2-yl, imidazolidin-4-yl, imidazolidin-2-on-4-yl, imidazolidin-2-thion-4-yl, oxazolidin-2-yl, oxazolidin-3-yl, oxazolidin-4-yl, oxazolidin-5-yl, isoxazolidin-2-yl, isoxazolidin-3-yl, isoxazolidin-4-yl, isoxazolidin-5-yl, thiazolidin-2-yl, thiazolidin-3-yl, thiazolidin-4-yl, thiazolidin-5-yl, isothiazolidin-2-yl, isothiazolidin-3-yl, isothiazolidin-4-yl, isothiazolidin-5-yl, 1,2,4-oxadiazolidin-3-yl, 1,2,4-oxadiazolidin-5-yl, 1,2,4-thiadiazolidin-3-yl, 1,2,4-thiadiazolidin-5-yl, 1,2,4-triazolidin-3-yl, 1,3,4-oxadiazolidin-2-yl, 1,3,4-thiadiazolidin-2-yl, 1,3,4-triazolidin-1-yl, 1,3,4-triazolidin-2-yl, 2-tetrahydropyranyl, 4-tetrahydropyranyl, 1,3-dioxan-5-yl, 1,4-dioxan-2-yl, piperidin-1-yl, piperidin-2-on-1-yl, piperidin-2,5-dion-1-yl, piperidine-2-thion-1-yl, piperidin-2-yl, piperidin-3-yl, piperidin-2-on-3-yl, piperidin-2,5-dion-3-yl, piperidin-2-thion-3-yl, piperidin-4-yl, hexahydropyridazin-3-yl, hexahydropyridazin-4-yl, hexahydropyrimidin-2-yl, hexahydropyrimidin-4-yl, hexahydropyrimidin-5-yl, piperazin-1-yl, piperazin-2-yl, 1,3,5-hexahydrotriazin-1-yl, 1,3,5-hexahydrotriazin-2-yl and 1,2,4-hexahydrotriazin-3-yl, morpholin-2-yl, morpholin-3-yl, morpholin-4-yl, thiomorpholin-2-yl, thiomorpholin-3-yl, thiomorpholin-4-yl, 1-oxothiomorpholin-2-yl, 1-oxothiomorpholin-3-yl, 1-oxothiomorpholin-4-yl, 1,1-dioxothiomorpholin-2-yl, 1,1-dioxothiomorpholin-3-yl, 1,1-dioxothiomorpholin-4-yl, azepan-1-, -2-, -3- or -4-yl, oxepan-2-, -3-, -4- or -5-yl, hexahydro-1,3-diazepinyl, hexahydro-1,4-diazepinyl, hexahydro-1,3-oxazepinyl, hexahydro-1,4-oxazepinyl, hexahydro-1,3-dioxepinyl, hexahydro-1,4-dioxepinyl and the like.
[0150] Examples of a 3-, 4-, 5-, 6-, 7- or 8-membered saturated heterocyclic ring further include oxocane, thiocane, azocane, [1,3]diazocane, [1,4]diazocane, [1,5]diazocane, [1,5]oxazocane and the like.
[0151] Examples of a 3-, 4-, 5-, 6- or 7-membered partially unsaturated heterocyclic ring include: 2,3-dihydrofur-2-yl, 2,3-dihydrofur-3-yl, 2,4-dihydrofur-2-yl, 2,4-dihydrofur-3-yl, 2,3-dihydrothien-2-yl, 2,3-dihydrothien-3-yl, 2,4-dihydrothien-2-yl, 2,4-dihydrothien-3-yl, 2-pyrrolin-2-yl, 2-pyrrolin-3-yl, 3-pyrrolin-2-yl, 3-pyrrolin-3-yl, 2-isoxazolin-3-yl, 3-isoxazolin-3-yl, 4-isoxazolin-3-yl, 2-isoxazolin-4-yl, 3-isoxazolin-4-yl, 4-isoxazolin-4-yl, 2-isoxazolin-5-yl, 3-isoxazolin-5-yl, 4-isoxazolin-5-yl, 2-isothiazolin-3-yl, 3-isothiazolin-3-yl, 4-isothiazolin-3-yl, 2-isothiazolin-4-yl, 3-isothiazolin-4-yl, 4-isothiazolin-4-yl, 2-isothiazolin-5-yl, 3-isothiazolin-5-yl, 4-isothiazolin-5-yl, 2,3-dihydropyrazol-1-yl, 2,3-dihydropyrazol-2-yl, 2,3-dihydropyrazol-3-yl, 2,3-dihydropyrazol-4-yl, 2,3-dihydropyrazol-5-yl, 3,4-dihydropyrazol-1-yl, 3,4-dihydropyrazol-3-yl, 3,4-dihydropyrazol-4-yl, 3,4-dihydropyrazol-5-yl, 4,5-dihydropyrazol-1-yl, 4,5-dihydropyrazol-3-yl, 4,5-dihydropyrazol-4-yl, 4,5-dihydropyrazol-5-yl, 2,3-dihydrooxazol-2-yl, 2,3-dihydrooxazol-3-yl, 2,3-dihydrooxazol-4-yl, 2,3-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2-yl, 3,4-dihydrooxazol-3-yl, 3,4-dihydrooxazol-4-yl, 3,4-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2-yl, 3,4-dihydrooxazol-3-yl, 3,4-dihydrooxazol-4-yl, 2-, 3-, 4-, 5- or 6-di- or tetrahydropyridinyl, 3-di- or tetrahydropyridazinyl, 4-di- or tetrahydropyridazinyl, 2-di- or tetrahydropyrimidinyl, 4-di- or tetrahydropyrimidinyl, 5-di- or tetrahydropyrimidinyl, di- or tetrahydropyrazinyl, 1,3,5-di- or tetrahydrotriazin-2-yl, 1,2,4-di- or tetrahydrotriazin-3-yl, 2,3,4,5-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl, 3,4,5,6-tetrahydro[2H]azepin-2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,4,7-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,6,7-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl, tetrahydrooxepinyl, such as 2,3,4,5-tetrahydro[1H]oxepin-2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,4,7-tetrahydro[1H]oxepin-2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,6,7-tetrahydro[1H]oxepin-2-, -3-,-4-, -5-, -6- or -7-yl, tetrahydro-1,3-diazepinyl, tetrahydro-1,4-diazepinyl, tetrahydro-1,3-oxazepinyl, tetrahydro-1,4-oxazepinyl, tetrahydro-1,3-dioxepinyl and tetrahydro-1,4-dioxepinyl.
[0152] Examples of a 3-, 4-, 5-, 6-, 7- or 8-membered partially unsaturated heterocyclic ring further include 1,2,3,4,5,6-hexahydroazocine, 2,3,4,5,6,7-hexahydroazocine, 1,2,3,4,5,8-hexahydroazocine, 1,2,3,4,7,8-hexahydroazocine, 1,2,3,4,5,6-hexahydro-[1,5]diazocine,1,2,3,4,7,8-hexahydro-[1,5]diazocine and the like.
[0153] Examples of a 3-, 4-, 5-, 6- or 7-membered maximally unsaturated (including aromatic) heterocyclic ring include 5- or 6-membered heteroaromatic rings, such as 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 1-pyrazolyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 1-imidazolyl, 2-imidazolyl, 4-imidazolyl, 1,3,4-triazol-1-yl, 1,3,4-triazol-2-yl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 1-oxopyridin-2-yl, 1-oxopyridin-3-yl, 1-oxopyridin-4-yl, 3-pyridazinyl, 4-pyridazinyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl and 2-pyrazinyl, and also homoaromatic radicals, such as 1H-azepine, 1H-[1,3]-diazepine and 1H-[1,4]-diazepine.
[0154] Examples of a 3-, 4-, 5-, 6-, 7- or 8-membered maximally unsaturated heterocyclic ring further include [1,3]diazocine, [1,5]diazocine and [1,5]diazocine.
[0155] A 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members is either saturated, partially unsaturated and carbocyclic (if it contains only C.dbd.O and/or C.dbd.S as heteroatom group and no further heteroatoms or heteroatom groups) or saturated, partially unsaturated or maximally unsaturated heterocyclic. Examples are, in addition to the heterocyclic rings mentioned above, carbocyclic rings, such as cyclopropanonyl, cyclobutanonyl, cyclopentanonyl, cyclohexanonyl, cyclohexandionyl, cycloheptanonyl, cyclooctanonyl, cyclopropanthionyl, cyclobutanthionyl, cyclopentanthionyl, cyclohexanthionyl, cyclohexandithionyl, cycloheptanthionyl, cyclooctanthionyl, cyclopropenonyl, cyclopentenonyl, cyclohexenonyl and the like.
[0156] The remarks made above and in the following with respect to preferred aspects of the invention, e.g. to preferred meanings of the variables R.sup.1, R.sup.2, R.sup.3a, R.sup.3b, R.sup.4a, R.sup.4b, R.sup.5a, R.sup.5b, R.sup.6, R.sup.7, R.sup.8, R.sup.9, a, b and n of compounds I, to preferred compounds I and to preferred embodiments of the method or the use according to the invention, apply in each case on their own or in particular to combinations thereof.
[0157] In a preferred embodiment, R.sup.1 is selected from hydrogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, --C(.dbd.O)R.sup.10, phenyl and benzyl, where the phenyl moiety in the two last-mentioned radicals may carry 1, 2 or 3 radicals R.sup.11, where R.sup.10 and R.sup.11 have one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0158] In the above group --C(.dbd.O)R.sup.10 as a meaning for R.sup.1, R.sup.10 is preferably selected from C.sub.1-C.sub.4-alkyl and C.sub.1-C.sub.4-alkoxy and more preferably from C.sub.1-C.sub.2-alkyl and tert-butoxy.
[0159] More preferably, R.sup.1 is selected from hydrogen and C.sub.1-C.sub.6-alkyl, in particular from hydrogen and methyl, and is specifically hydrogen.
[0160] In a preferred embodiment, R.sup.2 is selected from cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, more preferably from C.sub.1-C.sub.6-alkyl, in particular from methyl, ethyl, propyl and isopropyl, more particularly from methyl and ethyl, and is specifically methyl.
[0161] In a preferred embodiment, R.sup.3a and R.sup.3b, independently of each other, are selected from hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy. More preferably, R.sup.3a is selected from hydrogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, and R.sup.3b is hydrogen. Even more preferably, R.sup.3a is selected from hydrogen and methyl and R.sup.3b is hydrogen. In particular, both R.sup.3a and R.sup.3b are hydrogen.
[0162] In a preferred embodiment, R.sup.4a and R.sup.4b, independently of each other, are selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl or form together a group .dbd.O. More preferably, they are hydrogen or form together a group .dbd.O. In another embodiment, one of R.sup.4a and R.sup.4b is hydrogen and the other is methyl; especially if X is CR.sup.7R.sup.8. In particular, both R.sup.4a and R.sup.4b are hydrogen.
[0163] In a preferred embodiment, [0164] R.sup.5a is selected from hydrogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; and in case that X is CR.sup.7R.sup.8 is further selected from halogen; or [0165] R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0166] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; and [0167] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen; [0168] where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0169] More preferably, [0170] R.sup.5a is selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl; and in case that X is CR.sup.7R.sup.8 is further selected from halogen; and is preferably selected from hydrogen and methyl, or [0171] R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5, preferably 2, 3 or 4; or [0172] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5, preferably 2, 3 or 4; and [0173] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen.
[0174] In an alternative more preferred embodiment, [0175] R.sup.5a is selected from hydrogen, C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl; and in case that [0176] X is CR.sup.7R.sup.8 is further selected from halogen; and is preferably selected from hydrogen and methyl, or [0177] R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5, where two hydrogen atoms bound to adjacent CH.sub.2 groups may be replaced by two radicals R.sup.11, where the two radicals R.sup.11 form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5, preferably 1, 2 or 3; or [0178] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5, preferably 2, 3 or 4; and [0179] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen.
[0180] In particular, [0181] R.sup.5a is hydrogen; and in case that X is CR.sup.7R.sup.8, is selected from hydrogen and methyl and is in particular hydrogen; or [0182] R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 3, 4 or 5, where two hydrogen atoms bound to adjacent CH.sub.2 groups may be replaced by two radicals R.sup.11, where the two radicals R.sup.11 form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5, preferably 1, 2 or 3 and specifically 1; or [0183] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3 or 4, in particular 3; and [0184] R.sup.5b is hydrogen.
[0185] In an alternatively preferred embodiment, R.sup.5a and R.sup.5b, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; and form in particular a 5- or 6-membered saturated or partially unsaturated carbocyclic ring, and where the ring may be substituted with one or more substituents R.sup.11.
[0186] In one embodiment, X is NR.sup.6, where R.sup.6 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0187] Preferably, [0188] R.sup.6 is selected from the group consisting of hydrogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, --C(.dbd.O)R.sup.10, --SO.sub.2R.sup.10, phenyl and benzyl, where the phenyl moiety in the two last-mentioned radicals may carry 1, 2 or 3 radicals R.sup.11; or [0189] R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0190] where R.sup.10 and R.sup.11 have one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0191] In the above group --C(.dbd.O)R.sup.10, R.sup.6, R.sup.10 is preferably selected from C.sub.1-C.sub.4-alkyl and C.sub.1-C.sub.4-alkoxy and more preferably from C.sub.1-C.sub.2-alkyl and tert-butoxy.
[0192] In the above group --SO.sub.2R.sup.10, R.sup.6, R.sup.10 is preferably selected from C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl, where phenyl may be substituted with 1, 2, 3, 4 or 5, preferably 1, 2 or 3, in particular 1, radicals R.sup.11, where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0193] In one more preferred embodiment, R.sup.6 is --SO.sub.2R.sup.10, where R.sup.10 has one of the general meanings given above or is preferably selected from C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl, where phenyl may be substituted with 1, 2, 3, 4 or 5, preferably 1, 2 or 3, in particular 1, radicals R.sup.11, where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0194] In another preferred embodiment, R.sup.6 is selected from hydrogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy, --C(.dbd.O)R.sup.10, where R.sup.10 is selected from C.sub.1-C.sub.6-alkyl and C.sub.3-C.sub.6-cycloalkyl; phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11.
[0195] In another preferred embodiment, R.sup.6 is selected from C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, phenyl, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11.
[0196] In a more preferred embodiment, R.sup.6 is phenyl-C.sub.1-C.sub.2-alkyl, and in particular benzyl.
[0197] In another more preferred embodiment, R.sup.6 is selected from C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl and fluorinated C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, and in particular from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-methyl and fluorinated C.sub.3-C.sub.6-cycloalkyl-methyl.
[0198] In another more preferred embodiment, R.sup.6 is a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 and optionally also 1 or 2 C.dbd.O and/or C.dbd.S groups as ring members, where the cyclic moieties in the three last-mentioned radicals may be substituted with one or more substituents R.sup.11. Even more preferably, R.sup.6 is a 3-, 4- or 5-membered saturated heterocyclic ring containing 1 or 2 heteroatoms independently selected from N, O and S, where the heterocyclic ring may be substituted with one or two substituents selected from halogen and C.sub.1-C.sub.4-alkyl, and is in particular oxetanyl which may carry one or two substituents selected from halogen and C.sub.1-C.sub.4-alkyl and especially from F.
[0199] In another more preferred embodiment, R.sup.6 is selected from hydrogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, C.sub.3-C.sub.6-cycloalkylcarbonyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy, phenyl-C.sub.1-C.sub.2-alkyl and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members; and is in particular selected from C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl, C.sub.3-C.sub.6-cycloalkyl-C.sub.1-C.sub.2-alkyl, and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members, where the saturated heterocyclic ring preferably contains 1 heteroatom selected from O and S as ring member. Specifically, R.sup.6 is selected from C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and oxetanyl and very specifically from ethyl, n-propyl, cyclopropyl, cyclobutyl and 3-oxetanyl.
[0200] In another more preferred embodiment, R.sup.5a and R.sup.6, together with the atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0201] where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0202] In particular, R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5, preferably 2, 3 or 4 or, in particular, 3, 4 or 5. Alternatively, R.sup.5a and R.sup.6 form together a group (CH.sub.2).sub.r, where r is 2, 3, 4 or 5 and in particular 3, 4 or 5, where two hydrogen atoms bound to adjacent CH.sub.2 groups may be replaced by two radicals R.sup.11, where the two radicals R.sup.11 form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5, in particular 1, 2 or 3 and specifically 1.
[0203] In an alternative embodiment, X is CR.sup.7R.sup.8, where R.sup.7 and R.sup.8 have one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0204] In case that R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a ring, this ring is spiro-bound to the carbon atom carrying R.sup.7 and R.sup.8.
[0205] Preferably, [0206] R.sup.7 and R.sup.8, independently of each other, are selected from the group consisting of halogen, cyano, nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.2-C.sub.6-alkenyl, fluorinated C.sub.2-C.sub.6-alkenyl, C.sub.2-C.sub.6-alkynyl, fluorinated C.sub.2-C.sub.6-alkynyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.6-hydroxyalkoxy, C.sub.1-C.sub.6-alkoxy-C.sub.1-C.sub.4-alkoxy, C.sub.1-C.sub.6-alkylthio, fluorinated C.sub.1-C.sub.6-alkylthio, C.sub.1-C.sub.6-alkylsulfinyl, fluorinated C.sub.1-C.sub.6-alkylsulfinyl, C.sub.1-C.sub.6-alkylsulfonyl, fluorinated C.sub.1-C.sub.6-alkylsulfonyl, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b, [0207] --NR.sup.12aC(O)R.sup.10, --C(.dbd.O)R.sup.10, SO.sub.2NR.sup.12aR.sup.12b, C.sub.1-C.sub.6-alkylcarbonyloxy, fluorinated C.sub.1-C.sub.6-alkylcarbonyloxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, phenylsulfonyl, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the six last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0208] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0209] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0210] in which case R.sup.8 has one of the above meanings or is deuterium; [0211] where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0212] More preferably, [0213] R.sup.7 and R.sup.8, independently of each other, are selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, phenyl, phenyl-C.sub.1-C.sub.2-alkyl, benzyloxy and a 3-, 4-, 5- or 6-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the four last-mentioned radicals may be substituted with one or more substituents R.sup.11; or [0214] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; or [0215] R.sup.5a and R.sup.7, together with the carbon atoms they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may contain 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents R.sup.11; [0216] in which case R.sup.8 has one of the above meanings or is deuterium; and [0217] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen; where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0218] Even more preferably, [0219] R.sup.7 and R.sup.8, independently of each other, are selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl, or [0220] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0221] R.sup.5 and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5, preferably 2, 3 or 4; in which case R.sup.8 has one of the above meanings or is deuterium; and [0222] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen; where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0223] In particular, [0224] R.sup.8 is selected from halogen, cyano, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl and phenyl; and [0225] R.sup.7 is selected from C.sub.1-C.sub.6-alkyl and fluorinated C.sub.1-C.sub.6-alkyl, preferably from methyl and CF.sub.3 and is in particular methyl; or [0226] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0227] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3, 4 or 5, preferably 2, 3 or 4; in which case R.sup.8 has one of the above meanings or is deuterium; and [0228] R.sup.5b is selected from hydrogen and deuterium and is preferably hydrogen; [0229] where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0230] In an alternative particular embodiment, [0231] R.sup.7 is C.sub.1-C.sub.6-alkyl and is in particular methyl; [0232] R.sup.8 is selected from hydroxyl, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy and phenyl and in particular from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; or [0233] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0234] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3 or 4 and where R.sup.8 is methyl; and [0235] R.sup.5b is hydrogen.
[0236] Specifically, [0237] R.sup.8 is selected from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.2-alkyl and phenyl and specifically from methyl, CF.sub.3 and phenyl; and [0238] R.sup.7 is methyl; or [0239] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5- or 6-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0240] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 3 or 4, specifically 3; in which case R.sup.8 is selected from methyl and deuterium; and [0241] R.sup.5b is selected from hydrogen and deuterium and is specifically hydrogen; where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0242] In an alternative specific embodiment, [0243] R.sup.7 is methyl; [0244] R.sup.8 is selected from hydroxyl, methyl, ethyl, CF.sub.3, methoxy and phenyl; or [0245] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5- or 6-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0246] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 2, 3 or 4 and in particular and where R.sup.8 is methyl; and [0247] R.sup.5b is hydrogen.
[0248] Very specifically, [0249] R.sup.8 is selected from methyl, CF.sub.3 and phenyl; and [0250] R.sup.7 is methyl; or [0251] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 4, 5- or 6-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or form a 3-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; or [0252] R.sup.5a and R.sup.7 form together a group (CH.sub.2).sub.s, where s is 3 or 4, specifically 3; in which case R.sup.8 is selected from methyl and deuterium and in particular methyl; and [0253] R.sup.5b is selected from hydrogen and deuterium and is specifically hydrogen; where R.sup.11 has one of the general meanings given above, or, in particular, one of the preferred meanings given below.
[0254] In one particular embodiment, the ring formed by R.sup.7 and R.sup.8 together with the carbon atom they are bound to is a carbocyclic ring, preferably a saturated or partially unsaturated carbocyclic ring, in particular a saturated carbocyclic ring.
[0255] If R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a carbocyclic ring, this is particularly preferably a 4- or 5-membered saturated carbocyclic ring (i.e. R.sup.7 and R.sup.8 form together a group --(CH.sub.2).sub.3-- or --(CH.sub.2).sub.4--) and in particular a 4-membered saturated carbocyclic ring (i.e. R.sup.7 and R.sup.8 form together a group --(CH.sub.2).sub.3-), where the ring may be substituted with one or more substituents R.sup.11. Specifically, the ring is not substituted. Alternatively, the carbocyclic ring is 3-membered (i.e. R.sup.7 and R.sup.8 form together a group --(CH.sub.2).sub.2--)--), where the ring may be substituted with one or more substituents R.sup.11. Specifically, the ring is not substituted.
[0256] In an alternative particular embodiment, the ring formed by R.sup.7 and R.sup.8 together with the carbon atom they are bound to is a heterocyclic ring containing 1, 2, 3 or 4 heteroatoms or heteroatom-containing groups selected from O, S, N, SO and SO.sub.2 as ring members, preferably containing 1 or 2 heteroatoms or heteroatom-containing groups selected from O, S, N, SO and SO.sub.2 as ring members. Preferably, the heterocyclic ring is 3-, 4-, 5- or 6-membered and saturated and may carry one or more substituents R.sup.11. In particular, the heterocyclic ring is oxetanyl which may carry one or more substituents R.sup.11.
[0257] Specifically, R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3- or 4-membered saturated carbocyclic ring (i.e. R.sup.7 and R.sup.8 form together a group --(CH.sub.2).sub.2-- or --(CH.sub.2).sub.3--) and in particular a 4-membered saturated carbocyclic ring (i.e. R.sup.7 and R.sup.8 form together a group --(CH.sub.2).sub.3--); the ring not being substituted.
[0258] In a preferred embodiment, each R.sup.9 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy and a 3-, 4-, 5- or 6-membered saturated heterocyclic ring containing 1 or 2 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO and SO.sub.2 as ring members, where the heterocyclic ring may be substituted with one or more substituents R.sup.11. The heterocyclic ring is specifically oxetanyl.
[0259] More preferably, each R.sup.9 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, even more preferably from halogen, cyano, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy and fluorinated C.sub.1-C.sub.4-alkoxy, and is in particular selected from halogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl and C.sub.1-C.sub.6-alkoxy, more particularly from halogen, C.sub.1-C.sub.6-alkyl and C.sub.1-C.sub.6-alkoxy, and is specifically halogen, especially F or Cl, and more specifically F.
[0260] In case that X is NR.sup.6, in alternatively more preferred embodiment, each R.sup.9 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.3-C.sub.6-cycloalkyl, fluorinated C.sub.3-C.sub.6-cycloalkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, even more preferably from halogen, C.sub.1-C.sub.6-alkyl and C.sub.3-C.sub.6-cycloalkyl, and in particular from halogen and C.sub.1-C.sub.6-alkyl.
[0261] In a preferred embodiment, R.sup.10 is selected from hydrogen, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-hydroxyalkyl, C.sub.3-C.sub.8-cycloalkyl, fluorinated C.sub.3-C.sub.8-cycloalkyl, C.sub.1-C.sub.6-alkoxy, fluorinated C.sub.1-C.sub.6-alkoxy, --NR.sup.12aR.sup.12b, --CH.sub.2NR.sup.12aR.sup.12b phenyl, phenyl-C.sub.1-C.sub.2-alkyl, phenoxy, benzyloxy and a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partially unsaturated or maximally unsaturated ring containing 1, 2, 3 or 4 heteroatoms or heteroatom groups independently selected from N, O, S, NO, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, where the cyclic moieties in the five last-mentioned radicals may be substituted with one or more substituents R.sup.11, where R.sup.11, R.sup.12a and R.sup.12b have one of the general meanings given above, or, in particular, one of the preferred meanings given below. More preferably, R.sup.10 is selected from hydrogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, and even more preferably from C.sub.1-C.sub.4-alkyl and C.sub.1-C.sub.4-alkoxy. In particular, R.sup.10 is selected from C.sub.1-C.sub.2-alkyl and tert-butoxy.
[0262] In a preferred embodiment, each R.sup.11 is independently selected from halogen, cyano, nitro, hydroxy, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy, and is in particular halogen or C.sub.1-C.sub.4-alkyl. Alternatively, two radicals R.sup.11 bound on adjacent ring atoms form together a group (CH.sub.2).sub.t, where t is 1, 2, 3, 4 or 5 and in particular 1, 2 or 3.
[0263] In a preferred embodiment, R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from hydrogen, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkylcarbonyl, fluorinated C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl, fluorinated C.sub.1-C.sub.6-alkoxycarbonyl, phenyl and benzyl, where the phenyl moieties in the two last-mentioned radicals may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy; or,
if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic ring, where the ring may further contain 1 or 2 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2, C.dbd.O and C.dbd.S as ring members, and where the ring may be substituted with one or more substituents selected from halogen, cyano nitro, C.sub.1-C.sub.6-alkyl, fluorinated C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkoxy and fluorinated C.sub.1-C.sub.6-alkoxy.
[0264] More preferably, R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from hydrogen, C.sub.1-C.sub.6-alkyl, C.sub.1-C.sub.6-alkylcarbonyl, C.sub.1-C.sub.6-alkoxycarbonyl and benzyl, where the phenyl moiety in the last-mentioned radical may carry 1, 2 or 3 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy and fluorinated C.sub.1-C.sub.4-alkoxy; or, if R.sup.12a and R.sup.12b are bound to the same nitrogen atom, together with this nitrogen atom may form a 5- or 6-membered saturated or aromatic heterocyclic ring, where the ring may further contain 1 or 2 heteroatoms or heteroatom-containing groups selected from O, S, N, SO, SO.sub.2 and C.dbd.O as ring members, and where the ring may be substituted with 1 or 2 substituents selected from halogen, cyano nitro, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy and fluorinated C.sub.1-C.sub.4-alkoxy.
[0265] In particular, R.sup.12a and R.sup.12b, independently of each other and independently of each occurrence, are selected from hydrogen and C.sub.1-C.sub.6-alkyl.
[0266] In a preferred embodiment, a is 0 or 1 and in particular 0.
[0267] In a particular embodiment, a is 1 and R.sup.2 is bound in 3-position to the nitrogen ring atom carrying R.sup.1 (this is for the example the position of R.sup.2b in below formula I.2).
[0268] In a preferred embodiment, b is 0, 1 or 2 and in particular 0 or 1.
[0269] In a preferred embodiment, n is 1.
[0270] In a particular embodiment, the compound of formula I is a compound of formula I.1
##STR00004##
wherein X, R.sup.5a, R.sup.5b, R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0271] In a specific embodiment, the compound of formula I is a compound of formula I.1.1
##STR00005## [0272] wherein [0273] R.sup.9a is selected from H, halogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and fluorinated C.sub.3-C.sub.6-cycloalkyl, especially from H or F; and [0274] R.sup.5a, R.sup.5b, R.sup.6, R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0275] In another specific embodiment, the compound of formula I is a compound of formula I.1.2
##STR00006## [0276] wherein [0277] R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl; [0278] R.sup.8 is selected from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; or [0279] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered, in particular a 3- or 4-membered saturated or partially unsaturated ring, especially a saturated carbocyclic ring, where the ring may be substituted with one or more substituents R.sup.11, and form especially a 3- or 4-membered saturated carbocyclic ring; [0280] R.sup.9a is H, Cl, F or methyl, especially H, Cl or F; and [0281] R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0282] In an alternative embodiment, in compounds I.1.2, [0283] R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, preferably from methyl and CF.sub.3, and is in particular methyl; [0284] R.sup.8 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, and preferably from methyl and CF.sub.3; or [0285] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11, and form preferably a 5- or 6-membered saturated or partially unsaturated ring; [0286] R.sup.9a is H or F; and [0287] R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0288] In another specific embodiment, the compound of formula I is a compound of formula I.1.3
##STR00007##
wherein R.sup.8 is selected from deuterium, F, Cl, CN and CH.sub.3, in particular from deuterium, F, Cl and CN.
[0289] Among these, preference is given to the trans compounds, i.e. of formula I.1.3-trans:
##STR00008## [0290] wherein R.sup.8 is selected from deuterium, F, Cl, CN and CH.sub.3, in particular from deuterium, F, Cl and CN.
[0291] In another particular embodiment, the compound of formula I is a compound of formula I.2
##STR00009##
wherein X, R.sup.5a, R.sup.5b, R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0292] In another particular embodiment, the compound of formula I is a compound of formula I.2.1
##STR00010## [0293] wherein [0294] R.sup.2a, R.sup.2b and R.sup.3a, independently of each other, are selected from hydrogen and methyl; and where in particular at most one of R.sup.2a, R.sup.2b and R.sup.3a is methyl; [0295] R.sup.9a is selected from H, halogen, C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.3-C.sub.6-cycloalkyl and fluorinated C.sub.3-C.sub.6-cycloalkyl; and [0296] R.sup.5a, R.sup.5b, R.sup.6, R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0297] In particular, in compounds I.2.1, [0298] R.sup.9a is H or F; and [0299] R.sup.5a, R.sup.5b, R.sup.6, R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0300] In particular, in compounds I.2.1, R.sup.2b is methyl and R.sup.2a and R.sup.3a are hydrogen.
[0301] In another particular embodiment, the compound of formula I is a compound of formula I.2.2
##STR00011## [0302] wherein [0303] R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a, independently of each other, are selected from hydrogen, methyl and ethyl, in particular from hydrogen and methyl; [0304] R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, in particular from methyl and CF.sub.3, and is especially methyl; [0305] R.sup.8 is selected from C.sub.1-C.sub.4-alkyl, fluorinated C.sub.1-C.sub.4-alkyl, C.sub.1-C.sub.4-alkoxy, fluorinated C.sub.1-C.sub.4-alkoxy and phenyl; and in particular from methyl and CF.sub.3; or [0306] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered, in particular a 3- or 4-membered saturated or partially unsaturated, especially a saturated carbocyclic ring, ring, where the ring may be substituted with one or more substituents R.sup.11, and form especially a 3- or 4-membered saturated carbocyclic ring; [0307] R.sup.9a is H, Cl, F or methyl, especially H, Cl or F; and [0308] R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0309] In particular, in compound I.2.2, [0310] R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a, independently of each other, are selected from hydrogen and methyl; [0311] R.sup.7 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, preferably from methyl and CF.sub.3, and is in particular methyl; [0312] R.sup.8 is selected from C.sub.1-C.sub.4-alkyl and fluorinated C.sub.1-C.sub.4-alkyl, and preferably from methyl and CF.sub.3; or [0313] R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated or partially unsaturated ring, where the ring may be substituted with one or more substituents R.sup.11; [0314] R.sup.9a is H or F; and [0315] R.sup.9 and b have one of the above general, or, in particular, one of the above preferred meanings.
[0316] In particular, in compound I.2.2, R.sup.2b is methyl and R.sup.2a, R.sup.3a and R.sup.5a are hydrogen.
[0317] In the above formulae I.1.1, I.1.2, I.2.1 and I.2.2, (b-1) is preferably 0.
[0318] Preferably, in the above formulae I.2, I.2.1 and I.2.2, R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a are selected from hydrogen and methyl, with the proviso that at most two, preferably at most one, of the substituents R.sup.2a, R.sup.2b, R.sup.3a and R.sup.5a are methyl. In particular, R.sup.2b is hydrogen and R.sup.2a, R.sup.3a and R.sup.5a are selected from hydrogen and methyl, with the proviso that at most two, preferably at most one, of the substituents R.sup.2a, R.sup.3a and R.sup.5a are methyl. However, more particularly, R.sup.2b is hydrogen or methyl, especially methyl, and R.sup.2a, R.sup.3a and R.sup.5a are hydrogen (if the latter does not form a ring together with R.sup.6 or R.sup.7 and the atoms they are bond to).
[0319] Preferably, in the above formulae I.2, I.2.1 and I1.2.2, R.sup.7 and R.sup.8, together with the carbon atom they are bound to, form a 3, 4-, 5- or 6-membered saturated or partially unsaturated carbocyclic ring, preferably a 3, 4- or 5-membered saturated carbocyclic ring, in particular a 4-membered saturated carbocyclic ring, where the ring may be substituted with one or more substituents R.sup.11.
[0320] Examples of preferred compounds are compounds of the following formulae I.a to I.ii, where the variables have one of the general or preferred meanings given above.
[0321] Examples of preferred compounds are the individual compounds compiled in the tables 1 to 36 below. Moreover, the meanings mentioned below for the individual variables in the tables are per se, independently of the combination in which they are mentioned, a particularly preferred embodiment of the substituents in question.
##STR00012## ##STR00013## ##STR00014## ##STR00015## ##STR00016## ##STR00017##
[0322] Table 1
[0323] Compounds of the formula I.a in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0324] Table 2
[0325] Compounds of the formula I.b in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0326] Table 3
[0327] Compounds of the formula I.c in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0328] Table 4
[0329] Compounds of the formula I.d in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0330] Table 5
[0331] Compounds of the formula I.e in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0332] Table 6
[0333] Compounds of the formula I.f in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0334] Table 7
[0335] Compounds of the formula I.g in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0336] Table 8
[0337] Compounds of the formula I.h in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0338] Table 9
[0339] Compounds of the formula I.i in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0340] Table 10
[0341] Compounds of the formula I.j in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0342] Table 11
[0343] Compounds of the formula I.k in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0344] Table 12
[0345] Compounds of the formula I.1 in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0346] Table 13
[0347] Compounds of the formula I.m in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0348] Table 14
[0349] Compounds of the formula I.n in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0350] Table 15
[0351] Compounds of the formula I.o in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0352] Table 16
[0353] Compounds of the formula I.p in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0354] Table 17
[0355] Compounds of the formula I.q in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0356] Table 18
[0357] Compounds of the formula I.r in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0358] Table 19
[0359] Compounds of the formula I.s in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0360] Table 20
[0361] Compounds of the formula I.t in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0362] Table 21
[0363] Compounds of the formula I.u in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0364] Table 22
[0365] Compounds of the formula I.v in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0366] Table 23
[0367] Compounds of the formula I.w in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0368] Table 24
[0369] Compounds of the formula I.x in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0370] Table 25
[0371] Compounds of the formula I.y in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0372] Table 26
[0373] Compounds of the formula I.z in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0374] Table 27
[0375] Compounds of the formula I.zz in which the combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a for a compound corresponds in each case to one row of Table B.
[0376] Table 28
[0377] Compounds of the formula I.aa in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0378] Table 29
[0379] Compounds of the formula I.bb in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0380] Table 30
[0381] Compounds of the formula I.cc in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0382] Table 31
[0383] Compounds of the formula I.dd in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0384] Table 32
[0385] Compounds of the formula I.ee in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0386] Table 33
[0387] Compounds of the formula I.ff in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0388] Table 34
[0389] Compounds of the formula I.gg in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0390] Table 35
[0391] Compounds of the formula I.hh in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
[0392] Table 36
[0393] Compounds of the formula I.ii in which the combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a for a compound corresponds in each case to one row of Table A.
TABLE-US-00001 TABLE A No. R.sup.9a R.sup.5b R.sup.5a R.sup.6 A-1 H H H --SO.sub.2--CH.sub.3 A-2 F H H --SO.sub.2--CH.sub.3 A-3 Cl H H --SO.sub.2--CH.sub.3 A-4 Br H H --SO.sub.2--CH.sub.3 A-5 CH.sub.3 H H --SO.sub.2--CH.sub.3 A-6 CF.sub.3 H H --SO.sub.2--CH.sub.3 A-7 OCH.sub.3 H H --SO.sub.2--CH.sub.3 A-8 OCF.sub.3 H H --SO.sub.2--CH.sub.3 A-9 cyclopropyl H H --SO.sub.2--CH.sub.3 A-10 cyclobutyl H H --SO.sub.2--CH.sub.3 A-11 cyclopentyl H H --SO.sub.2--CH.sub.3 A-12 cyclohexyl H H --SO.sub.2--CH.sub.3 A-13 H CH.sub.3 H --SO.sub.2--CH.sub.3 A-14 F CH.sub.3 H --SO.sub.2--CH.sub.3 A-15 Cl CH.sub.3 H --SO.sub.2--CH.sub.3 A-16 Br CH.sub.3 H --SO.sub.2--CH.sub.3 A-17 CH.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.3 A-18 CF.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.3 A-19 OCH.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.3 A-20 OCF.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.3 A-21 cyclopropyl CH.sub.3 H --SO.sub.2--CH.sub.3 A-22 cyclobutyl CH.sub.3 H --SO.sub.2--CH.sub.3 A-23 cyclopentyl CH.sub.3 H --SO.sub.2--CH.sub.3 A-24 cyclohexyl CH.sub.3 H --SO.sub.2--CH.sub.3 A-25 H CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-26 F CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-27 Cl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-28 Br CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-29 CH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-30 CF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-31 OCH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-32 OCF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-33 cyclopropyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-34 cyclobutyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-35 cyclopentyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-36 cyclohexyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.3 A-37 H H H --SO.sub.2--CH.sub.2CH.sub.3 A-38 F H H --SO.sub.2--CH.sub.2CH.sub.3 A-39 Cl H H --SO.sub.2--CH.sub.2CH.sub.3 A-40 Br H H --SO.sub.2--CH.sub.2CH.sub.3 A-41 CH.sub.3 H H --SO.sub.2--CH.sub.2CH.sub.3 A-42 CF.sub.3 H H --SO.sub.2--CH.sub.2CH.sub.3 A-43 OCH.sub.3 H H --SO.sub.2--CH.sub.2CH.sub.3 A-44 OCF.sub.3 H H --SO.sub.2--CH.sub.2CH.sub.3 A-45 cyclopropyl H H --SO.sub.2--CH.sub.2CH.sub.3 A-46 cyclobutyl H H --SO.sub.2--CH.sub.2CH.sub.3 A-47 cyclopentyl H H --SO.sub.2--CH.sub.2CH.sub.3 A-48 cyclohexyl H H --SO.sub.2--CH.sub.2CH.sub.3 A-49 H CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-50 F CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-51 Cl CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-52 Br CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-53 CH.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-54 CF.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-55 OCH.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-56 OCF.sub.3 CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-57 cyclopropyl CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-58 cyclobutyl CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-59 cyclopentyl CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-60 cyclohexyl CH.sub.3 H --SO.sub.2--CH.sub.2CH.sub.3 A-61 H CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-62 F CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-63 Cl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-64 Br CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-65 CH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-66 CF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-67 OCH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-68 OCF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-69 cyclopropyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-70 cyclobutyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-71 cyclopentyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-72 cyclohexyl CH.sub.3 CH.sub.3 --SO.sub.2--CH.sub.2CH.sub.3 A-73 H H H --SO.sub.2--CF.sub.3 A-74 F H H --SO.sub.2--CF.sub.3 A-75 Cl H H --SO.sub.2--CF.sub.3 A-76 Br H H --SO.sub.2--CF.sub.3 A-77 CH.sub.3 H H --SO.sub.2--CF.sub.3 A-78 CF.sub.3 H H --SO.sub.2--CF.sub.3 A-79 OCH.sub.3 H H --SO.sub.2--CF.sub.3 A-80 OCF.sub.3 H H --SO.sub.2--CF.sub.3 A-81 cyclopropyl H H --SO.sub.2--CF.sub.3 A-82 cyclobutyl H H --SO.sub.2--CF.sub.3 A-83 cyclopentyl H H --SO.sub.2--CF.sub.3 A-84 cyclohexyl H H --SO.sub.2--CF.sub.3 A-85 H CH.sub.3 H --SO.sub.2--CF.sub.3 A-86 F CH.sub.3 H --SO.sub.2--CF.sub.3 A-87 Cl CH.sub.3 H --SO.sub.2--CF.sub.3 A-88 Br CH.sub.3 H --SO.sub.2--CF.sub.3 A-89 CH.sub.3 CH.sub.3 H --SO.sub.2--CF.sub.3 A-90 CF.sub.3 CH.sub.3 H --SO.sub.2--CF.sub.3 A-91 OCH.sub.3 CH.sub.3 H --SO.sub.2--CF.sub.3 A-92 OCF.sub.3 CH.sub.3 H --SO.sub.2--CF.sub.3 A-93 cyclopropyl CH.sub.3 H --SO.sub.2--CF.sub.3 A-94 cyclobutyl CH.sub.3 H --SO.sub.2--CF.sub.3 A-95 cyclopentyl CH.sub.3 H --SO.sub.2--CF.sub.3 A-96 cyclohexyl CH.sub.3 H --SO.sub.2--CF.sub.3 A-97 H CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-98 F CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-99 Cl CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-100 Br CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-101 CH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-102 CF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-103 OCH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-104 OCF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-105 cyclopropyl CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-106 cyclobutyl CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-107 cyclopentyl CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-108 cyclohexyl CH.sub.3 CH.sub.3 --SO.sub.2--CF.sub.3 A-109 H H H --SO.sub.2--C.sub.6H.sub.5* A-110 F H H --SO.sub.2--C.sub.6H.sub.5 A-111 Cl H H --SO.sub.2--C.sub.6H.sub.5 A-112 Br H H --SO.sub.2--C.sub.6H.sub.5 A-113 CH.sub.3 H H --SO.sub.2--C.sub.6H.sub.5 A-114 CF.sub.3 H H --SO.sub.2--C.sub.6H.sub.5 A-115 OCH.sub.3 H H --SO.sub.2--C.sub.6H.sub.5 A-116 OCF.sub.3 H H --SO.sub.2--C.sub.6H.sub.5 A-117 cyclopropyl H H --SO.sub.2--C.sub.6H.sub.5 A-118 cyclobutyl H H --SO.sub.2--C.sub.6H.sub.5 A-119 cyclopentyl H H --SO.sub.2--C.sub.6H.sub.5 A-120 cyclohexyl H H --SO.sub.2--C.sub.6H.sub.5 A-121 H CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-122 F CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-123 Cl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-124 Br CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-125 CH.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-126 CF.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-127 OCH.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-128 OCF.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-129 cyclopropyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-130 cyclobutyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-131 cyclopentyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-132 cyclohexyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.5 A-133 H CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-134 F CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-135 Cl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-136 Br CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-137 CH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-138 CF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-139 OCH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-140 OCF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-141 cyclopropyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-142 cyclobutyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-143 cyclopentyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-144 cyclohexyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.5 A-145 H H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3** A-146 F H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-147 Cl H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-148 Br H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-149 CH.sub.3 H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-150 CF.sub.3 H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-151 OCH.sub.3 H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-152 OCF.sub.3 H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-153 cyclopropyl H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-154 cyclobutyl H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-155 cyclopentyl H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-156 cyclohexyl H H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-157 H CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-158 F CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-159 Cl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-160 Br CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-161 CH.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-162 CF.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-163 OCH.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-164 OCF.sub.3 CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-165 cyclopropyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-166 cyclobutyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-167 cyclopentyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-168 cyclohexyl CH.sub.3 H --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-169 H CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-170 F CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-171 Cl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-172 Br CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-173 CH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-174 CF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-175 OCH.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-176 OCF.sub.3 CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-177 cyclopropyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-178 cyclobutyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-179 cyclopentyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-180 cyclohexyl CH.sub.3 CH.sub.3 --SO.sub.2--C.sub.6H.sub.4CH.sub.3 A-181 H H H benzyl A-182 F H H benzyl A-183 Cl H H benzyl A-184 Br H H benzyl A-185 CH.sub.3 H H benzyl A-186 CF.sub.3 H H benzyl A-187 OCH.sub.3 H H benzyl A-188 OCF.sub.3 H H benzyl A-189 cyclopropyl H H benzyl A-190 cyclobutyl H H benzyl A-191 cyclopentyl H H benzyl A-192 cyclohexyl H H benzyl A-193 H CH.sub.3 H benzyl A-194 F CH.sub.3 H benzyl A-195 Cl CH.sub.3 H benzyl A-196 Br CH.sub.3 H benzyl A-197 CH.sub.3 CH.sub.3 H benzyl A-198 CF.sub.3 CH.sub.3 H benzyl A-199 OCH.sub.3 CH.sub.3 H benzyl A-200 OCF.sub.3 CH.sub.3 H benzyl A-201 cyclopropyl CH.sub.3 H benzyl A-202 cyclobutyl CH.sub.3 H benzyl A-203 cyclopentyl CH.sub.3 H benzyl A-204 cyclohexyl CH.sub.3 H benzyl A-205 H CH.sub.3 CH.sub.3 benzyl A-206 F CH.sub.3 CH.sub.3 benzyl A-207 Cl CH.sub.3 CH.sub.3 benzyl A-208 Br CH.sub.3 CH.sub.3 benzyl A-209 CH.sub.3 CH.sub.3 CH.sub.3 benzyl A-210 CF.sub.3 CH.sub.3 CH.sub.3 benzyl A-211 OCH.sub.3 CH.sub.3 CH.sub.3 benzyl A-212 OCF.sub.3 CH.sub.3 CH.sub.3 benzyl A-213 cyclopropyl CH.sub.3 CH.sub.3 benzyl A-214 cyclobutyl CH.sub.3 CH.sub.3 benzyl A-215 cyclopentyl CH.sub.3 CH.sub.3 benzyl A-216 cyclohexyl CH.sub.3 CH.sub.3 benzyl A-217 H H H H A-218 F H H H A-219 Cl H H H A-220 Br H H H A-221 CH.sub.3 H H H A-222 CF.sub.3 H H H A-223 OCH.sub.3 H H H A-224 OCF.sub.3 H H H A-225 cyclopropyl H H H A-226 cyclobutyl H H H A-227 cyclopentyl H H H A-228 cyclohexyl H H H A-229 H CH.sub.3 H H A-230 F CH.sub.3 H H A-231 Cl CH.sub.3 H H A-232 Br CH.sub.3 H H A-233 CH.sub.3 CH.sub.3 H H A-234 CF.sub.3 CH.sub.3 H H A-235 OCH.sub.3 CH.sub.3 H H A-236 OCF.sub.3 CH.sub.3 H H A-237 cyclopropyl CH.sub.3 H H A-238 cyclobutyl CH.sub.3 H H A-239 cyclopentyl CH.sub.3 H H A-240 cyclohexyl CH.sub.3 H H A-241 H CH.sub.3 CH.sub.3 H A-242 F CH.sub.3 CH.sub.3 H A-243 Cl CH.sub.3 CH.sub.3 H A-244 Br CH.sub.3 CH.sub.3 H A-245 CH.sub.3 CH.sub.3 CH.sub.3 H A-246 CF.sub.3 CH.sub.3 CH.sub.3 H
A-247 OCH.sub.3 CH.sub.3 CH.sub.3 H A-248 OCF.sub.3 CH.sub.3 CH.sub.3 H A-249 cyclopropyl CH.sub.3 CH.sub.3 H A-250 cyclobutyl CH.sub.3 CH.sub.3 H A-251 cyclopentyl CH.sub.3 CH.sub.3 H A-252 cyclohexyl CH.sub.3 CH.sub.3 H A-253 H H H CH.sub.3 A-254 F H H CH.sub.3 A-255 Cl H H CH.sub.3 A-256 Br H H CH.sub.3 A-257 CH.sub.3 H H CH.sub.3 A-258 CF.sub.3 H H CH.sub.3 A-259 OCH.sub.3 H H CH.sub.3 A-260 OCF.sub.3 H H CH.sub.3 A-261 cyclopropyl H H CH.sub.3 A-262 cyclobutyl H H CH.sub.3 A-263 cyclopentyl H H CH.sub.3 A-264 cyclohexyl H H CH.sub.3 A-265 H CH.sub.3 H CH.sub.3 A-266 F CH.sub.3 H CH.sub.3 A-267 Cl CH.sub.3 H CH.sub.3 A-268 Br CH.sub.3 H CH.sub.3 A-269 CH.sub.3 CH.sub.3 H CH.sub.3 A-270 CF.sub.3 CH.sub.3 H CH.sub.3 A-271 OCH.sub.3 CH.sub.3 H CH.sub.3 A-272 OCF.sub.3 CH.sub.3 H CH.sub.3 A-273 cyclopropyl CH.sub.3 H CH.sub.3 A-274 cyclobutyl CH.sub.3 H CH.sub.3 A-275 cyclopentyl CH.sub.3 CH.sub.3 H A-276 cyclohexyl CH.sub.3 H CH.sub.3 A-277 H CH.sub.3 CH.sub.3 CH.sub.3 A-278 F CH.sub.3 CH.sub.3 CH.sub.3 A-279 Cl CH.sub.3 CH.sub.3 CH.sub.3 A-280 Br CH.sub.3 CH.sub.3 CH.sub.3 A-281 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 A-282 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 A-283 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 A-284 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 A-285 cyclopropyl CH.sub.3 CH.sub.3 CH.sub.3 A-286 cyclobutyl CH.sub.3 CH.sub.3 CH.sub.3 A-287 cyclopentyl CH.sub.3 CH.sub.3 CH.sub.3 A-288 cyclohexyl CH.sub.3 CH.sub.3 CH.sub.3 A-289 H H H C.sub.2H.sub.5 A-290 F H H C.sub.2H.sub.5 A-291 Cl H H C.sub.2H.sub.5 A-292 Br H H C.sub.2H.sub.5 A-293 CH.sub.3 H H C.sub.2H.sub.5 A-294 CF.sub.3 H H C.sub.2H.sub.5 A-295 OCH.sub.3 H H C.sub.2H.sub.5 A-296 OCF.sub.3 H H C.sub.2H.sub.5 A-297 cyclopropyl H H C.sub.2H.sub.5 A-298 cyclobutyl H H C.sub.2H.sub.5 A-299 cyclopentyl H H C.sub.2H.sub.5 A-300 cyclohexyl H H C.sub.2H.sub.5 A-301 H CH.sub.3 H C.sub.2H.sub.5 A-302 F CH.sub.3 H C.sub.2H.sub.5 A-303 Cl CH.sub.3 H C.sub.2H.sub.5 A-304 Br CH.sub.3 H C.sub.2H.sub.5 A-305 CH.sub.3 CH.sub.3 H C.sub.2H.sub.5 A-306 CF.sub.3 CH.sub.3 H C.sub.2H.sub.5 A-307 OCH.sub.3 CH.sub.3 H C.sub.2H.sub.5 A-308 OCF.sub.3 CH.sub.3 H C.sub.2H.sub.5 A-309 cyclopropyl CH.sub.3 H C.sub.2H.sub.5 A-310 cyclobutyl CH.sub.3 H C.sub.2H.sub.5 A-311 cyclopentyl CH.sub.3 H C.sub.2H.sub.5 A-312 cyclohexyl CH.sub.3 H C.sub.2H.sub.5 A-313 H CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-314 F CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-315 Cl CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-316 Br CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-317 CH.sub.3 CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-318 CF.sub.3 CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-319 OCH.sub.3 CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-320 OCF.sub.3 CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-321 cyclopropyl CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-322 cyclobutyl CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-323 cyclopentyl CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-324 cyclohexyl CH.sub.3 CH.sub.3 C.sub.2H.sub.5 A-325 H H H n-propyl A-326 F H H n-propyl A-327 Cl H H n-propyl A-328 Br H H n-propyl A-329 CH.sub.3 H H n-propyl A-330 CF.sub.3 H H n-propyl A-331 OCH.sub.3 H H n-propyl A-332 OCF.sub.3 H H n-propyl A-333 cyclopropyl H H n-propyl A-334 cyclobutyl H H n-propyl A-335 cyclopentyl H H n-propyl A-336 cyclohexyl H H n-propyl A-337 H CH.sub.3 H n-propyl A-338 F CH.sub.3 H n-propyl A-339 Cl CH.sub.3 H n-propyl A-340 Br CH.sub.3 H n-propyl A-341 CH.sub.3 CH.sub.3 H n-propyl A-342 CF.sub.3 CH.sub.3 H n-propyl A-343 OCH.sub.3 CH.sub.3 H n-propyl A-344 OCF.sub.3 CH.sub.3 H n-propyl A-345 cyclopropyl CH.sub.3 H n-propyl A-346 cyclobutyl CH.sub.3 H n-propyl A-347 cyclopentyl CH.sub.3 H n-propyl A-348 cyclohexyl CH.sub.3 H n-propyl A-349 H CH.sub.3 CH.sub.3 n-propyl A-350 F CH.sub.3 CH.sub.3 n-propyl A-351 Cl CH.sub.3 CH.sub.3 n-propyl A-352 Br CH.sub.3 CH.sub.3 n-propyl A-353 CH.sub.3 CH.sub.3 CH.sub.3 n-propyl A-354 CF.sub.3 CH.sub.3 CH.sub.3 n-propyl A-355 OCH.sub.3 CH.sub.3 CH.sub.3 n-propyl A-356 OCF.sub.3 CH.sub.3 CH.sub.3 n-propyl A-357 cyclopropyl CH.sub.3 CH.sub.3 n-propyl A-358 cyclobutyl CH.sub.3 CH.sub.3 n-propyl A-359 cyclopentyl CH.sub.3 CH.sub.3 n-propyl A-360 cyclohexyl CH.sub.3 CH.sub.3 n-propyl A-361 H H H isopropyl A-362 F H H isopropyl A-363 Cl H H isopropyl A-364 Br H H isopropyl A-365 CH.sub.3 H H isopropyl A-366 CF.sub.3 H H isopropyl A-367 OCH.sub.3 H H isopropyl A-368 OCF.sub.3 H H isopropyl A-369 cyclopropyl H H isopropyl A-370 cyclobutyl H H isopropyl A-371 cyclopentyl H H isopropyl A-372 cyclohexyl H H isopropyl A-373 H CH.sub.3 H isopropyl A-374 F CH.sub.3 H isopropyl A-375 Cl CH.sub.3 H isopropyl A-376 Br CH.sub.3 H isopropyl A-377 CH.sub.3 CH.sub.3 H isopropyl A-378 CF.sub.3 CH.sub.3 H isopropyl A-379 OCH.sub.3 CH.sub.3 H isopropyl A-380 OCF.sub.3 CH.sub.3 H isopropyl A-381 cyclopropyl CH.sub.3 H isopropyl A-382 cyclobutyl CH.sub.3 H isopropyl A-383 cyclopentyl CH.sub.3 H isopropyl A-384 cyclohexyl CH.sub.3 H isopropyl A-385 H CH.sub.3 CH.sub.3 isopropyl A-386 F CH.sub.3 CH.sub.3 isopropyl A-387 Cl CH.sub.3 CH.sub.3 isopropyl A-388 Br CH.sub.3 CH.sub.3 isopropyl A-389 CH.sub.3 CH.sub.3 CH.sub.3 isopropyl A-390 CF.sub.3 CH.sub.3 CH.sub.3 isopropyl A-391 OCH.sub.3 CH.sub.3 CH.sub.3 isopropyl A-392 OCF.sub.3 CH.sub.3 CH.sub.3 isopropyl A-393 cyclopropyl CH.sub.3 CH.sub.3 isopropyl A-394 cyclobutyl CH.sub.3 CH.sub.3 isopropyl A-395 cyclopentyl CH.sub.3 CH.sub.3 isopropyl A-396 cyclohexyl CH.sub.3 CH.sub.3 isopropyl A-397 H H H n-butyl A-398 F H H n-butyl A-399 Cl H H n-butyl A-400 Br H H n-butyl A-401 CH.sub.3 H H n-butyl A-402 CF.sub.3 H H n-butyl A-403 OCH.sub.3 H H n-butyl A-404 OCF.sub.3 H H n-butyl A-405 cyclopropyl H H n-butyl A-406 cyclobutyl H H n-butyl A-407 cyclopentyl H H n-butyl A-408 cyclohexyl H H n-butyl A-409 H CH.sub.3 H n-butyl A-410 F CH.sub.3 H n-butyl A-411 Cl CH.sub.3 H n-butyl A-412 Br CH.sub.3 H n-butyl A-413 CH.sub.3 CH.sub.3 H n-butyl A-414 CF.sub.3 CH.sub.3 H n-butyl A-415 OCH.sub.3 CH.sub.3 H n-butyl A-416 OCF.sub.3 CH.sub.3 H n-butyl A-417 cyclopropyl CH.sub.3 H n-butyl A-418 cyclobutyl CH.sub.3 H n-butyl A-419 cyclopentyl CH.sub.3 H n-butyl A-420 cyclohexyl CH.sub.3 H n-butyl A-421 H CH.sub.3 CH.sub.3 n-butyl A-422 F CH.sub.3 CH.sub.3 n-butyl A-423 Cl CH.sub.3 CH.sub.3 n-butyl A-424 Br CH.sub.3 CH.sub.3 n-butyl A-425 CH.sub.3 CH.sub.3 CH.sub.3 n-butyl A-426 CF.sub.3 CH.sub.3 CH.sub.3 n-butyl A-427 OCH.sub.3 CH.sub.3 CH.sub.3 n-butyl A-428 OCF.sub.3 CH.sub.3 CH.sub.3 n-butyl A-429 cyclopropyl CH.sub.3 CH.sub.3 n-butyl A-430 cyclobutyl CH.sub.3 CH.sub.3 n-butyl A-431 cyclopentyl CH.sub.3 CH.sub.3 n-butyl A-432 cyclohexyl CH.sub.3 CH.sub.3 n-butyl A-433 H H H cyclopropyl A-434 F H H cyclopropyl A-435 Cl H H cyclopropyl A-436 Br H H cyclopropyl A-437 CH.sub.3 H H cyclopropyl A-438 CF.sub.3 H H cyclopropyl A-439 OCH.sub.3 H H cyclopropyl A-440 OCF.sub.3 H H cyclopropyl A-441 cyclopropyl H H cyclopropyl A-442 cyclobutyl H H cyclopropyl A-443 cyclopentyl H H cyclopropyl A-444 cyclohexyl H H cyclopropyl A-445 H CH.sub.3 H cyclopropyl A-446 F CH.sub.3 H cyclopropyl A-447 Cl CH.sub.3 H cyclopropyl A-448 Br CH.sub.3 H cyclopropyl A-449 CH.sub.3 CH.sub.3 H cyclopropyl A-450 CF.sub.3 CH.sub.3 H cyclopropyl A-451 OCH.sub.3 CH.sub.3 H cyclopropyl A-452 OCF.sub.3 CH.sub.3 H cyclopropyl A-453 cyclopropyl CH.sub.3 H cyclopropyl A-454 cyclobutyl CH.sub.3 H cyclopropyl A-455 cyclopentyl CH.sub.3 H cyclopropyl A-456 cyclohexyl CH.sub.3 H cyclopropyl A-457 H CH.sub.3 CH.sub.3 cyclopropyl A-458 F CH.sub.3 CH.sub.3 cyclopropyl A-459 Cl CH.sub.3 CH.sub.3 cyclopropyl A-460 Br CH.sub.3 CH.sub.3 cyclopropyl A-461 CH.sub.3 CH.sub.3 CH.sub.3 cyclopropyl A-462 CF.sub.3 CH.sub.3 CH.sub.3 cyclopropyl A-463 OCH.sub.3 CH.sub.3 CH.sub.3 cyclopropyl A-464 OCF.sub.3 CH.sub.3 CH.sub.3 cyclopropyl A-465 cyclopropyl CH.sub.3 CH.sub.3 cyclopropyl A-466 cyclobutyl CH.sub.3 CH.sub.3 cyclopropyl A-467 cyclopentyl CH.sub.3 CH.sub.3 cyclopropyl A-468 cyclohexyl CH.sub.3 CH.sub.3 cyclopropyl A-469 H H H cyclobutyl A-470 F H H cyclobutyl A-471 Cl H H cyclobutyl A-472 Br H H cyclobutyl A-473 CH.sub.3 H H cyclobutyl A-474 CF.sub.3 H H cyclobutyl A-475 OCH.sub.3 H H cyclobutyl A-476 OCF.sub.3 H H cyclobutyl A-477 cyclopropyl H H cyclobutyl A-478 cyclobutyl H H cyclobutyl A-479 cyclopentyl H H cyclobutyl A-480 cyclohexyl H H cyclobutyl A-481 H CH.sub.3 H cyclobutyl A-482 F CH.sub.3 H cyclobutyl A-483 Cl CH.sub.3 H cyclobutyl A-484 Br CH.sub.3 H cyclobutyl A-485 CH.sub.3 CH.sub.3 H cyclobutyl A-486 CF.sub.3 CH.sub.3 H cyclobutyl A-487 OCH.sub.3 CH.sub.3 H cyclobutyl A-488 OCF.sub.3 CH.sub.3 H cyclobutyl A-489 cyclopropyl CH.sub.3 H cyclobutyl A-490 cyclobutyl CH.sub.3 H cyclobutyl A-491 cyclopentyl CH.sub.3 H cyclobutyl A-492 cyclohexyl CH.sub.3 H cyclobutyl A-493 H CH.sub.3 CH.sub.3 cyclobutyl A-494 F CH.sub.3 CH.sub.3 cyclobutyl A-495 Cl CH.sub.3 CH.sub.3 cyclobutyl A-496 Br CH.sub.3 CH.sub.3 cyclobutyl A-497 CH.sub.3 CH.sub.3 CH.sub.3 cyclobutyl
A-498 CF.sub.3 CH.sub.3 CH.sub.3 cyclobutyl A-499 OCH.sub.3 CH.sub.3 CH.sub.3 cyclobutyl A-500 OCF.sub.3 CH.sub.3 CH.sub.3 cyclobutyl A-501 cyclopropyl CH.sub.3 CH.sub.3 cyclobutyl A-502 cyclobutyl CH.sub.3 CH.sub.3 cyclobutyl A-503 cyclopentyl CH.sub.3 CH.sub.3 cyclobutyl A-504 cyclohexyl CH.sub.3 CH.sub.3 cyclobutyl A-505 H H H cyclopentyl A-506 F H H cyclopentyl A-507 Cl H H cyclopentyl A-508 Br H H cyclopentyl A-509 CH.sub.3 H H cyclopentyl A-510 CF.sub.3 H H cyclopentyl A-511 OCH.sub.3 H H cyclopentyl A-512 OCF.sub.3 H H cyclopentyl A-513 cyclopropyl H H cyclopentyl A-514 cyclobutyl H H cyclopentyl A-515 cyclopentyl H H cyclopentyl A-516 cyclohexyl H H cyclopentyl A-517 H CH.sub.3 H cyclopentyl A-518 F CH.sub.3 H cyclopentyl A-519 Cl CH.sub.3 H cyclopentyl A-520 Br CH.sub.3 H cyclopentyl A-521 CH.sub.3 CH.sub.3 H cyclopentyl A-522 CF.sub.3 CH.sub.3 H cyclopentyl A-523 OCH.sub.3 CH.sub.3 H cyclopentyl A-524 OCF.sub.3 CH.sub.3 H cyclopentyl A-525 cyclopropyl CH.sub.3 H cyclopentyl A-526 cyclobutyl CH.sub.3 H cyclopentyl A-527 cyclopentyl CH.sub.3 H cyclopentyl A-528 cyclohexyl CH.sub.3 H cyclopentyl A-529 H CH.sub.3 CH.sub.3 cyclopentyl A-530 F CH.sub.3 CH.sub.3 cyclopentyl A-531 Cl CH.sub.3 CH.sub.3 cyclopentyl A-532 Br CH.sub.3 CH.sub.3 cyclopentyl A-533 CH.sub.3 CH.sub.3 CH.sub.3 cyclopentyl A-534 CF.sub.3 CH.sub.3 CH.sub.3 cyclopentyl A-535 OCH.sub.3 CH.sub.3 CH.sub.3 cyclopentyl A-536 OCF.sub.3 CH.sub.3 CH.sub.3 cyclopentyl A-537 cyclopropyl CH.sub.3 CH.sub.3 cyclopentyl A-538 cyclobutyl CH.sub.3 CH.sub.3 cyclopentyl A-539 cyclopentyl CH.sub.3 CH.sub.3 cyclopentyl A-540 cyclohexyl CH.sub.3 CH.sub.3 cyclopentyl A-541 H H H cyclohexyl A-542 F H H cyclohexyl A-543 Cl H H cyclohexyl A-544 Br H H cyclohexyl A-545 CH.sub.3 H H cyclohexyl A-546 CF.sub.3 H H cyclohexyl A-547 OCH.sub.3 H H cyclohexyl A-548 OCF.sub.3 H H cyclohexyl A-549 cyclopropyl H H cyclohexyl A-550 cyclobutyl H H cyclohexyl A-551 cyclopentyl H H cyclohexyl A-552 cyclohexyl H H cyclohexyl A-553 H CH.sub.3 H cyclohexyl A-554 F CH.sub.3 H cyclohexyl A-555 Cl CH.sub.3 H cyclohexyl A-556 Br CH.sub.3 H cyclohexyl A-557 CH.sub.3 CH.sub.3 H cyclohexyl A-558 CF.sub.3 CH.sub.3 H cyclohexyl A-559 OCH.sub.3 CH.sub.3 H cyclohexyl A-560 OCF.sub.3 CH.sub.3 H cyclohexyl A-561 cyclopropyl CH.sub.3 H cyclohexyl A-562 cyclobutyl CH.sub.3 H cyclohexyl A-563 cyclopentyl CH.sub.3 H cyclohexyl A-564 cyclohexyl CH.sub.3 H cyclohexyl A-565 H CH.sub.3 CH.sub.3 cyclohexyl A-566 F CH.sub.3 CH.sub.3 cyclohexyl A-567 Cl CH.sub.3 CH.sub.3 cyclohexyl A-568 Br CH.sub.3 CH.sub.3 cyclohexyl A-569 CH.sub.3 CH.sub.3 CH.sub.3 cyclohexyl A-570 CF.sub.3 CH.sub.3 CH.sub.3 cyclohexyl A-571 OCH.sub.3 CH.sub.3 CH.sub.3 cyclohexyl A-572 OCF.sub.3 CH.sub.3 CH.sub.3 cyclohexyl A-573 cyclopropyl CH.sub.3 CH.sub.3 cyclohexyl A-574 cyclobutyl CH.sub.3 CH.sub.3 cyclohexyl A-575 cyclopentyl CH.sub.3 CH.sub.3 cyclohexyl A-576 cyclohexyl CH.sub.3 CH.sub.3 cyclohexyl A-577 H H H oxetan-.sub.3-yl A-578 F H H oxetan-.sub.3-yl A-579 Cl H H oxetan-.sub.3-yl A-580 Br H H oxetan-.sub.3-yl A-581 CH.sub.3 H H oxetan-.sub.3-yl A-582 CF.sub.3 H H oxetan-.sub.3-yl A-583 OCH.sub.3 H H oxetan-.sub.3-yl A-584 OCF.sub.3 H H oxetan-.sub.3-yl A-585 cyclopropyl H H oxetan-.sub.3-yl A-586 cyclobutyl H H oxetan-.sub.3-yl A-587 cyclopentyl H H oxetan-.sub.3-yl A-588 cyclohexyl H H oxetan-.sub.3-yl A-589 H CH.sub.3 H oxetan-.sub.3-yl A-590 F CH.sub.3 H oxetan-.sub.3-yl A-591 Cl CH.sub.3 H oxetan-.sub.3-yl A-592 Br CH.sub.3 H oxetan-.sub.3-yl A-593 CH.sub.3 CH.sub.3 H oxetan-.sub.3-yl A-594 CF.sub.3 CH.sub.3 H oxetan-.sub.3-yl A-595 OCH.sub.3 CH.sub.3 H oxetan-.sub.3-yl A-596 OCF.sub.3 CH.sub.3 H oxetan-.sub.3-yl A-597 cyclopropyl CH.sub.3 H oxetan-.sub.3-yl A-598 cyclobutyl CH.sub.3 H oxetan-.sub.3-yl A-599 cyclopentyl CH.sub.3 H oxetan-.sub.3-yl A-600 cyclohexyl CH.sub.3 H oxetan-.sub.3-yl A-601 H CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-602 F CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-603 Cl CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-604 Br CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-605 CH.sub.3 CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-606 CF.sub.3 CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-607 OCH.sub.3 CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-608 OCF.sub.3 CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-609 cyclopropyl CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-610 cyclobutyl CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-611 cyclopentyl CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-612 cyclohexyl CH.sub.3 CH.sub.3 oxetan-.sub.3-yl A-613 H H H --CH.sub.2-cyclopropyl A-614 F H H --CH.sub.2-cyclopropyl A-615 Cl H H --CH.sub.2-cyclopropyl A-616 Br H H --CH.sub.2-cyclopropyl A-617 CH.sub.3 H H --CH.sub.2-cyclopropyl A-618 CF.sub.3 H H --CH.sub.2-cyclopropyl A-619 OCH.sub.3 H H --CH.sub.2-cyclopropyl A-620 OCF.sub.3 H H --CH.sub.2-cyclopropyl A-621 cyclopropyl H H --CH.sub.2-cyclopropyl A-622 cyclobutyl H H --CH.sub.2-cyclopropyl A-623 cyclopentyl H H --CH.sub.2-cyclopropyl A-624 cyclohexyl H H --CH.sub.2-cyclopropyl A-625 H CH.sub.3 H --CH.sub.2-cyclopropyl A-626 F CH.sub.3 H --CH.sub.2-cyclopropyl A-627 Cl CH.sub.3 H --CH.sub.2-cyclopropyl A-628 Br CH.sub.3 H --CH.sub.2-cyclopropyl A-629 CH.sub.3 CH.sub.3 H --CH.sub.2-cyclopropyl A-630 CF.sub.3 CH.sub.3 H --CH.sub.2-cyclopropyl A-631 OCH.sub.3 CH.sub.3 H --CH.sub.2-cyclopropyl A-632 OCF.sub.3 CH.sub.3 H --CH.sub.2-cyclopropyl A-633 cyclopropyl CH.sub.3 H --CH.sub.2-cyclopropyl A-634 cyclobutyl CH.sub.3 H --CH.sub.2-cyclopropyl A-635 cyclopentyl CH.sub.3 H --CH.sub.2-cyclopropyl A-636 cyclohexyl CH.sub.3 H --CH.sub.2-cyclopropyl A-637 H CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-638 F CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-639 Cl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-640 Br CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-641 CH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-642 CF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-643 OCH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-644 OCF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-645 cyclopropyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-646 cyclobutyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-647 cyclopentyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-648 cyclohexyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopropyl A-649 H H H --CH.sub.2-cyclobutyl A-650 F H H --CH.sub.2-cyclobutyl A-651 Cl H H --CH.sub.2-cyclobutyl A-652 Br H H --CH.sub.2-cyclobutyl A-653 CH.sub.3 H H --CH.sub.2-cyclobutyl A-654 CF.sub.3 H H --CH.sub.2-cyclobutyl A-655 OCH.sub.3 H H --CH.sub.2-cyclobutyl A-656 OCF.sub.3 H H --CH.sub.2-cyclobutyl A-657 cyclopropyl H H --CH.sub.2-cyclobutyl A-658 cyclobutyl H H --CH.sub.2-cyclobutyl A-659 cyclopentyl H H --CH.sub.2-cyclobutyl A-660 cyclohexyl H H --CH.sub.2-cyclobutyl A-661 H CH.sub.3 H --CH.sub.2-cyclobutyl A-662 F CH.sub.3 H --CH.sub.2-cyclobutyl A-663 Cl CH.sub.3 H --CH.sub.2-cyclobutyl A-664 Br CH.sub.3 H --CH.sub.2-cyclobutyl A-665 CH.sub.3 CH.sub.3 H --CH.sub.2-cyclobutyl A-666 CF.sub.3 CH.sub.3 H --CH.sub.2-cyclobutyl A-667 OCH.sub.3 CH.sub.3 H --CH.sub.2-cyclobutyl A-668 OCF.sub.3 CH.sub.3 H --CH.sub.2-cyclobutyl A-669 cyclopropyl CH.sub.3 H --CH.sub.2-cyclobutyl A-670 cyclobutyl CH.sub.3 H --CH.sub.2-cyclobutyl A-671 cyclopentyl CH.sub.3 H --CH.sub.2-cyclobutyl A-672 cyclohexyl CH.sub.3 H --CH.sub.2-cyclobutyl A-673 H CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-674 F CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-675 Cl CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-676 Br CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-677 CH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-678 CF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-679 OCH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-680 OCF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-681 cyclopropyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-682 cyclobutyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-683 cyclopentyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-684 cyclohexyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclobutyl A-685 H H H --CH.sub.2-cyclopentyl A-686 F H H --CH.sub.2-cyclopentyl A-687 Cl H H --CH.sub.2-cyclopentyl A-688 Br H H --CH.sub.2-cyclopentyl A-689 CH.sub.3 H H --CH.sub.2-cyclopentyl A-690 CF.sub.3 H H --CH.sub.2-cyclopentyl A-691 OCH.sub.3 H H --CH.sub.2-cyclopentyl A-692 OCF.sub.3 H H --CH.sub.2-cyclopentyl A-693 cyclopropyl H H --CH.sub.2-cyclopentyl A-694 cyclobutyl H H --CH.sub.2-cyclopentyl A-695 cyclopentyl H H --CH.sub.2-cyclopentyl A-696 cyclohexyl H H --CH.sub.2-cyclopentyl A-697 H CH.sub.3 H --CH.sub.2-cyclopentyl A-698 F CH.sub.3 H --CH.sub.2-cyclopentyl A-699 Cl CH.sub.3 H --CH.sub.2-cyclopentyl A-700 Br CH.sub.3 H --CH.sub.2-cyclopentyl A-701 CH.sub.3 CH.sub.3 H --CH.sub.2-cyclopentyl A-702 CF.sub.3 CH.sub.3 H --CH.sub.2-cyclopentyl A-703 OCH.sub.3 CH.sub.3 H --CH.sub.2-cyclopentyl A-704 OCF.sub.3 CH.sub.3 H --CH.sub.2-cyclopentyl A-705 cyclopropyl CH.sub.3 H --CH.sub.2-cyclopentyl A-706 cyclobutyl CH.sub.3 H --CH.sub.2-cyclopentyl A-707 cyclopentyl CH.sub.3 H --CH.sub.2-cyclopentyl A-708 cyclohexyl CH.sub.3 H --CH.sub.2-cyclopentyl A-709 H CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-710 F CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-711 Cl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-712 Br CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-713 CH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-714 CF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-715 OCH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-716 OCF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-717 cyclopropyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-718 cyclobutyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-719 cyclopentyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-720 cyclohexyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclopentyl A-721 H H H --CH.sub.2-cyclohexyl A-722 F H H --CH.sub.2-cyclohexyl A-723 Cl H H --CH.sub.2-cyclohexyl A-724 Br H H --CH.sub.2-cyclohexyl A-725 CH.sub.3 H H --CH.sub.2-cyclohexyl A-726 CF.sub.3 H H --CH.sub.2-cyclohexyl A-727 OCH.sub.3 H H --CH.sub.2-cyclohexyl A-728 OCF.sub.3 H H --CH.sub.2-cyclohexyl A-729 cyclopropyl H H --CH.sub.2-cyclohexyl A-730 cyclobutyl H H --CH.sub.2-cyclohexyl A-731 cyclopentyl H H --CH.sub.2-cyclohexyl A-732 cyclohexyl H H --CH.sub.2-cyclohexyl A-733 H CH.sub.3 H --CH.sub.2-cyclohexyl A-734 F CH.sub.3 H --CH.sub.2-cyclohexyl A-735 Cl CH.sub.3 H --CH.sub.2-cyclohexyl A-736 Br CH.sub.3 H --CH.sub.2-cyclohexyl A-737 CH.sub.3 CH.sub.3 H --CH.sub.2-cyclohexyl A-738 CF.sub.3 CH.sub.3 H --CH.sub.2-cyclohexyl A-739 OCH.sub.3 CH.sub.3 H --CH.sub.2-cyclohexyl A-740 OCF.sub.3 CH.sub.3 H --CH.sub.2-cyclohexyl A-741 cyclopropyl CH.sub.3 H --CH.sub.2-cyclohexyl A-742 cyclobutyl CH.sub.3 H --CH.sub.2-cyclohexyl A-743 cyclopentyl CH.sub.3 H --CH.sub.2-cyclohexyl A-744 cyclohexyl CH.sub.3 H --CH.sub.2-cyclohexyl A-745 H CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-746 F CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-747 Cl CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-748 Br CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl
A-749 CH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-750 CF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-751 OCH.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-752 OCF.sub.3 CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-753 cyclopropyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-754 cyclobutyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-755 cyclopentyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-756 cyclohexyl CH.sub.3 CH.sub.3 --CH.sub.2-cyclohexyl A-757 H H H --C(O)-cyclopropyl A-758 F H H --C(O)-cyclopropyl A-759 Cl H H --C(O)-cyclopropyl A-760 Br H H --C(O)-cyclopropyl A-761 CH.sub.3 H H --C(O)-cyclopropyl A-762 CF.sub.3 H H --C(O)-cyclopropyl A-763 OCH.sub.3 H H --C(O)-cyclopropyl A-764 OCF.sub.3 H H --C(O)-cyclopropyl A-765 cyclopropyl H H --C(O)-cyclopropyl A-766 cyclobutyl H H --C(O)-cyclopropyl A-767 cyclopentyl H H --C(O)-cyclopropyl A-768 cyclohexyl H H --C(O)-cyclopropyl A-769 H CH.sub.3 H --C(O)-cyclopropyl A-770 F CH.sub.3 H --C(O)-cyclopropyl A-771 Cl CH.sub.3 H --C(O)-cyclopropyl A-772 Br CH.sub.3 H --C(O)-cyclopropyl A-773 CH.sub.3 CH.sub.3 H --C(O)-cyclopropyl A-774 CF.sub.3 CH.sub.3 H --C(O)-cyclopropyl A-775 OCH.sub.3 CH.sub.3 H --C(O)-cyclopropyl A-776 OCF.sub.3 CH.sub.3 H --C(O)-cyclopropyl A-777 cyclopropyl CH.sub.3 H --C(O)-cyclopropyl A-778 cyclobutyl CH.sub.3 H --C(O)-cyclopropyl A-779 cyclopentyl CH.sub.3 H --C(O)-cyclopropyl A-780 cyclohexyl CH.sub.3 H --C(O)-cyclopropyl A-781 H CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-782 F CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-783 Cl CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-784 Br CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-785 CH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-786 CF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-787 OCH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-788 OCF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-789 cyclopropyl CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-790 cyclobutyl CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-791 cyclopentyl CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-792 cyclohexyl CH.sub.3 CH.sub.3 --C(O)-cyclopropyl A-793 H H H --C(O)-cyclobutyl A-794 F H H --C(O)-cyclobutyl A-795 Cl H H --C(O)-cyclobutyl A-796 Br H H --C(O)-cyclobutyl A-797 CH.sub.3 H H --C(O)-cyclobutyl A-798 CF.sub.3 H H --C(O)-cyclobutyl A-799 OCH.sub.3 H H --C(O)-cyclobutyl A-800 OCF.sub.3 H H --C(O)-cyclobutyl A-801 cyclopropyl H H --C(O)-cyclobutyl A-802 cyclobutyl H H --C(O)-cyclobutyl A-803 cyclopentyl H H --C(O)-cyclobutyl A-804 cyclohexyl H H --C(O)-cyclobutyl A-805 H CH.sub.3 H --C(O)-cyclobutyl A-806 F CH.sub.3 H --C(O)-cyclobutyl A-807 Cl CH.sub.3 H --C(O)-cyclobutyl A-808 Br CH.sub.3 H --C(O)-cyclobutyl A-809 CH.sub.3 CH.sub.3 H --C(O)-cyclobutyl A-810 CF.sub.3 CH.sub.3 H --C(O)-cyclobutyl A-811 OCH.sub.3 CH.sub.3 H --C(O)-cyclobutyl A-812 OCF.sub.3 CH.sub.3 H --C(O)-cyclobutyl A-813 cyclopropyl CH.sub.3 H --C(O)-cyclobutyl A-814 cyclobutyl CH.sub.3 H --C(O)-cyclobutyl A-815 cyclopentyl CH.sub.3 H --C(O)-cyclobutyl A-816 cyclohexyl CH.sub.3 H --C(O)-cyclobutyl A-817 H CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-818 F CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-819 Cl CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-820 Br CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-821 CH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-822 CF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-823 OCH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-824 OCF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-825 cyclopropyl CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-826 cyclobutyl CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-827 cyclopentyl CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-828 cyclohexyl CH.sub.3 CH.sub.3 --C(O)-cyclobutyl A-829 H H H --C(O)-cyclopentyl A-830 F H H --C(O)-cyclopentyl A-831 Cl H H --C(O)-cyclopentyl A-832 Br H H --C(O)-cyclopentyl A-833 CH.sub.3 H H --C(O)-cyclopentyl A-834 CF.sub.3 H H --C(O)-cyclopentyl A-835 OCH.sub.3 H H --C(O)-cyclopentyl A-836 OCF.sub.3 H H --C(O)-cyclopentyl A-837 cyclopropyl H H --C(O)-cyclopentyl A-838 cyclobutyl H H --C(O)-cyclopentyl A-839 cyclopentyl H H --C(O)-cyclopentyl A-840 cyclohexyl H H --C(O)-cyclopentyl A-841 H CH.sub.3 H --C(O)-cyclopentyl A-842 F CH.sub.3 H --C(O)-cyclopentyl A-843 Cl CH.sub.3 H --C(O)-cyclopentyl A-844 Br CH.sub.3 H --C(O)-cyclopentyl A-845 CH.sub.3 CH.sub.3 H --C(O)-cyclopentyl A-846 CF.sub.3 CH.sub.3 H --C(O)-cyclopentyl A-847 OCH.sub.3 CH.sub.3 H --C(O)-cyclopentyl A-848 OCF.sub.3 CH.sub.3 H --C(O)-cyclopentyl A-849 cyclopropyl CH.sub.3 H --C(O)-cyclopentyl A-850 cyclobutyl CH.sub.3 H --C(O)-cyclopentyl A-851 cyclopentyl CH.sub.3 H --C(O)-cyclopentyl A-852 cyclohexyl CH.sub.3 H --C(O)-cyclopentyl A-853 H CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-854 F CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-855 Cl CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-856 Br CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-857 CH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-858 CF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-859 OCH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-860 OCF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-861 cyclopropyl CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-862 cyclobutyl CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-863 cyclopentyl CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-864 cyclohexyl CH.sub.3 CH.sub.3 --C(O)-cyclopentyl A-865 H H H --C(O)-cyclohexyl A-866 F H H --C(O)-cyclohexyl A-867 Cl H H --C(O)-cyclohexyl A-868 Br H H --C(O)-cyclohexyl A-869 CH.sub.3 H H --C(O)-cyclohexyl A-870 CF.sub.3 H H --C(O)-cyclohexyl A-871 OCH.sub.3 H H --C(O)-cyclohexyl A-872 OCF.sub.3 H H --C(O)-cyclohexyl A-873 cyclopropyl H H --C(O)-cyclohexyl A-874 cyclobutyl H H --C(O)-cyclohexyl A-875 cyclopentyl H H --C(O)-cyclohexyl A-876 cyclohexyl H H --C(O)-cyclohexyl A-877 H CH.sub.3 H --C(O)-cyclohexyl A-878 F CH.sub.3 H --C(O)-cyclohexyl A-879 Cl CH.sub.3 H --C(O)-cyclohexyl A-880 Br CH.sub.3 H --C(O)-cyclohexyl A-881 CH.sub.3 CH.sub.3 H --C(O)-cyclohexyl A-882 CF.sub.3 CH.sub.3 H --C(O)-cyclohexyl A-883 OCH.sub.3 CH.sub.3 H --C(O)-cyclohexyl A-884 OCF.sub.3 CH.sub.3 H --C(O)-cyclohexyl A-885 cyclopropyl CH.sub.3 H --C(O)-cyclohexyl A-886 cyclobutyl CH.sub.3 H --C(O)-cyclohexyl A-887 cyclopentyl CH.sub.3 H --C(O)-cyclohexyl A-888 cyclohexyl CH.sub.3 H --C(O)-cyclohexyl A-889 H CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-890 F CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-891 Cl CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-892 Br CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-893 CH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-894 CF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-895 OCH.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-896 OCF.sub.3 CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-897 cyclopropyl CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-898 cyclobutyl CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-899 cyclopentyl CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-900 cyclohexyl CH.sub.3 CH.sub.3 --C(O)-cyclohexyl A-901 H H --(CH.sub.2).sub.3-- A-902 F H --(CH.sub.2).sub.3-- A-903 Cl H --(CH.sub.2).sub.3-- A-904 Br H --(CH.sub.2).sub.3-- A-905 CH.sub.3 H --(CH.sub.2).sub.3-- A-906 CF.sub.3 H --(CH.sub.2).sub.3-- A-907 OCH.sub.3 H --(CH.sub.2).sub.3-- A-908 OCF.sub.3 H --(CH.sub.2).sub.3-- A-909 cyclopropyl H --(CH.sub.2).sub.3-- A-910 cyclobutyl H --(CH.sub.2).sub.3-- A-911 cyclopentyl H --(CH.sub.2).sub.3-- A-912 cyclohexyl H --(CH.sub.2).sub.3-- A-913 H CH.sub.3 --(CH.sub.2).sub.3-- A-914 F CH.sub.3 --(CH.sub.2).sub.3-- A-915 Cl CH.sub.3 --(CH.sub.2).sub.3-- A-916 Br CH.sub.3 --(CH.sub.2).sub.3-- A-917 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- A-918 CF.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- A-919 OCH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- A-920 OCF.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- A-921 cyclopropyl CH.sub.3 --(CH.sub.2).sub.3-- A-922 cyclobutyl CH.sub.3 --(CH.sub.2).sub.3-- A-923 cyclopentyl CH.sub.3 --(CH.sub.2).sub.3-- A-924 cyclohexyl CH.sub.3 --(CH.sub.2).sub.3-- A-925 H D --(CH.sub.2).sub.3-- A-926 F D --(CH.sub.2).sub.3-- A-927 Cl D --(CH.sub.2).sub.3-- A-928 Br D --(CH.sub.2).sub.3-- A-929 CH.sub.3 D --(CH.sub.2).sub.3-- A-930 CF.sub.3 D --(CH.sub.2).sub.3-- A-931 OCH.sub.3 D --(CH.sub.2).sub.3-- A-932 OCF.sub.3 D --(CH.sub.2).sub.3-- A-933 cyclopropyl D --(CH.sub.2).sub.3-- A-934 cyclobutyl D --(CH.sub.2).sub.3-- A-935 cyclopentyl D --(CH.sub.2).sub.3-- A-936 cyclohexyl D --(CH.sub.2).sub.3-- A-937 H H --(CH.sub.2).sub.4-- A-938 F H --(CH.sub.2).sub.4-- A-939 Cl H --(CH.sub.2).sub.4-- A-940 Br H --(CH.sub.2).sub.4-- A-941 CH.sub.3 H --(CH.sub.2).sub.4-- A-942 CF.sub.3 H --(CH.sub.2).sub.4-- A-943 OCH.sub.3 H --(CH.sub.2).sub.4-- A-944 OCF.sub.3 H --(CH.sub.2).sub.4-- A-945 cyclopropyl H --(CH.sub.2).sub.4-- A-946 cyclobutyl H --(CH.sub.2).sub.4-- A-947 cyclopentyl H --(CH.sub.2).sub.4-- A-948 cyclohexyl H --(CH.sub.2).sub.4-- A-949 H CH.sub.3 --(CH.sub.2).sub.4-- A-950 F CH.sub.3 --(CH.sub.2).sub.4-- A-951 Cl CH.sub.3 --(CH.sub.2).sub.4-- A-952 Br CH.sub.3 --(CH.sub.2).sub.4-- A-953 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- A-954 CF.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- A-955 OCH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- A-956 OCF.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- A-957 cyclopropyl CH.sub.3 --(CH.sub.2).sub.4-- A-958 cyclobutyl CH.sub.3 --(CH.sub.2).sub.4-- A-959 cyclopentyl CH.sub.3 --(CH.sub.2).sub.4-- A-960 cyclohexyl CH.sub.3 --(CH.sub.2).sub.4-- A-961 H D --(CH.sub.2).sub.4-- A-962 F D --(CH.sub.2).sub.4-- A-963 Cl D --(CH.sub.2).sub.4-- A-964 Br D --(CH.sub.2).sub.4-- A-965 CH.sub.3 D --(CH.sub.2).sub.4-- A-966 CF.sub.3 D --(CH.sub.2).sub.4-- A-967 OCH.sub.3 D --(CH.sub.2).sub.4-- A-968 OCF.sub.3 D --(CH.sub.2).sub.4-- A-969 cyclopropyl D --(CH.sub.2).sub.4-- A-970 cyclobutyl D --(CH.sub.2).sub.4-- A-971 cyclopentyl D --(CH.sub.2).sub.4-- A-972 cyclohexyl D --(CH.sub.2).sub.4-- A-973 H H --(CH.sub.2).sub.5-- A-974 F H --(CH.sub.2).sub.5-- A-975 Cl H --(CH.sub.2).sub.5-- A-976 Br H --(CH.sub.2).sub.5-- A-977 CH.sub.3 H --(CH.sub.2).sub.5-- A-978 CF.sub.3 H --(CH.sub.2).sub.5-- A-979 OCH.sub.3 H --(CH.sub.2).sub.5-- A-980 OCF.sub.3 H --(CH.sub.2).sub.5-- A-981 cyclopropyl H --(CH.sub.2).sub.5-- A-982 cyclobutyl H --(CH.sub.2).sub.5-- A-983 cyclopentyl H --(CH.sub.2).sub.5-- A-984 cyclohexyl H --(CH.sub.2).sub.5-- A-985 H CH.sub.3 --(CH.sub.2).sub.5-- A-986 F CH.sub.3 --(CH.sub.2).sub.5-- A-987 Cl CH.sub.3 --(CH.sub.2).sub.5-- A-988 Br CH.sub.3 --(CH.sub.2).sub.5-- A-989 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- A-990 CF.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- A-991 OCH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- A-992 OCF.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- A-993 cyclopropyl CH.sub.3 --(CH.sub.2).sub.5-- A-994 cyclobutyl CH.sub.3 --(CH.sub.2).sub.5-- A-995 cyclopentyl CH.sub.3 --(CH.sub.2).sub.5-- A-996 cyclohexyl CH.sub.3 --(CH.sub.2).sub.5-- A-997 H D --(CH.sub.2).sub.5-- A-998 F D --(CH.sub.2).sub.5-- A-999 Cl D --(CH.sub.2).sub.5--
A-1000 Br D --(CH.sub.2).sub.5-- A-1001 CH.sub.3 D --(CH.sub.2).sub.5-- A-1002 CF.sub.3 D --(CH.sub.2).sub.5-- A-1003 OCH.sub.3 D --(CH.sub.2).sub.5-- A-1004 OCF.sub.3 D --(CH.sub.2).sub.5-- A-1005 cyclopropyl D --(CH.sub.2).sub.5-- A-1006 cyclobutyl D --(CH.sub.2).sub.5-- A-1007 cyclopentyl D --(CH.sub.2).sub.5-- A-1008 cyclohexyl D --(CH.sub.2).sub.5-- A-1009 F H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)--*** A-1010 Cl H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1011 Br H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1012 CH.sub.3 H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1013 CF.sub.3 H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1014 OCH.sub.3 H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1015 OCF.sub.3 H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1016 cyclopropyl H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1017 cyclobutyl H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1018 cyclopentyl H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1019 cyclohexyl H --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1020 H CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1021 F CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1022 Cl CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1023 Br CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1024 CH.sub.3 CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1025 CF.sub.3 CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1026 OCH.sub.3 CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1027 OCF.sub.3 CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1028 cyclopropyl CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1029 cyclobutyl CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1030 cyclopentyl CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1031 cyclohexyl CH.sub.3 --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1032 H D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1033 F D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1034 Cl D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1035 Br D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1036 CH.sub.3 D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1037 CF.sub.3 D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1038 OCH.sub.3 D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1039 OCF.sub.3 D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1040 cyclopropyl D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1041 cyclobutyl D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1042 cyclopentyl D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- A-1043 cyclohexyl D --(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- *C.sub.6H.sub.5 = phenyl **C.sub.6H.sub.4CH.sub.3 = 4-methylphenyl (p-tolyl) ***--(CH.sub.2--CH--(.mu.-CH.sub.2)--CH)-- = ##STR00018##
TABLE-US-00002 TABLE B No. R.sup.9a R.sup.5b R.sup.5a R.sup.7 R.sup.8 B-1 H H H CH.sub.3 CH.sub.3 B-2 F H H CH.sub.3 CH.sub.3 B-3 Cl H H CH.sub.3 CH.sub.3 B-4 Br H H CH.sub.3 CH.sub.3 B-5 CH.sub.3 H H CH.sub.3 CH.sub.3 B-6 CF.sub.3 H H CH.sub.3 CH.sub.3 B-7 OCH.sub.3 H H CH.sub.3 CH.sub.3 B-8 OCF.sub.3 H H CH.sub.3 CH.sub.3 B-9 H F H CH.sub.3 CH.sub.3 B-10 F F H CH.sub.3 CH.sub.3 B-11 Cl F H CH.sub.3 CH.sub.3 B-12 Br F H CH.sub.3 CH.sub.3 B-13 CH.sub.3 F H CH.sub.3 CH.sub.3 B-14 CF.sub.3 F H CH.sub.3 CH.sub.3 B-15 OCH.sub.3 F H CH.sub.3 CH.sub.3 B-16 OCF.sub.3 F H CH.sub.3 CH.sub.3 B-17 H Cl H CH.sub.3 CH.sub.3 B-18 F Cl H CH.sub.3 CH.sub.3 B-19 Cl Cl H CH.sub.3 CH.sub.3 B-20 Br Cl H CH.sub.3 CH.sub.3 B-21 CH.sub.3 Cl H CH.sub.3 CH.sub.3 B-22 CF.sub.3 Cl H CH.sub.3 CH.sub.3 B-23 OCH.sub.3 Cl H CH.sub.3 CH.sub.3 B-24 OCF.sub.3 Cl H CH.sub.3 CH.sub.3 B-25 H CH.sub.3 H CH.sub.3 CH.sub.3 B-26 F CH.sub.3 H CH.sub.3 CH.sub.3 B-27 Cl CH.sub.3 H CH.sub.3 CH.sub.3 B-28 Br CH.sub.3 H CH.sub.3 CH.sub.3 B-29 CH.sub.3 CH.sub.3 H CH.sub.3 CH.sub.3 B-30 CF.sub.3 CH.sub.3 H CH.sub.3 CH.sub.3 B-31 OCH.sub.3 CH.sub.3 H CH.sub.3 CH.sub.3 B-32 OCF.sub.3 CH.sub.3 H CH.sub.3 CH.sub.3 B-33 H CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-34 F CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-35 Cl CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-36 Br CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-37 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-38 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-39 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-40 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 B-41 H H H CH.sub.3 CH.sub.2CH.sub.3 B-42 F H H CH.sub.3 CH.sub.2CH.sub.3 B-43 Cl H H CH.sub.3 CH.sub.2CH.sub.3 B-44 Br H H CH.sub.3 CH.sub.2CH.sub.3 B-45 CH.sub.3 H H CH.sub.3 CH.sub.2CH.sub.3 B-46 CF.sub.3 H H CH.sub.3 CH.sub.2CH.sub.3 B-47 OCH.sub.3 H H CH.sub.3 CH.sub.2CH.sub.3 B-48 OCF.sub.3 H H CH.sub.3 CH.sub.2CH.sub.3 B-49 H F H CH.sub.3 CH.sub.2CH.sub.3 B-50 F F H CH.sub.3 CH.sub.2CH.sub.3 B-51 Cl F H CH.sub.3 CH.sub.2CH.sub.3 B-52 Br F H CH.sub.3 CH.sub.2CH.sub.3 B-53 CH.sub.3 F H CH.sub.3 CH.sub.2CH.sub.3 B-54 CF.sub.3 F H CH.sub.3 CH.sub.2CH.sub.3 B-55 OCH.sub.3 F H CH.sub.3 CH.sub.2CH.sub.3 B-56 OCF.sub.3 F H CH.sub.3 CH.sub.2CH.sub.3 B-57 H Cl H CH.sub.3 CH.sub.2CH.sub.3 B-58 F Cl H CH.sub.3 CH.sub.2CH.sub.3 B-59 Cl Cl H CH.sub.3 CH.sub.2CH.sub.3 B-60 Br Cl H CH.sub.3 CH.sub.2CH.sub.3 B-61 CH.sub.3 Cl H CH.sub.3 CH.sub.2CH.sub.3 B-62 CF.sub.3 Cl H CH.sub.3 CH.sub.2CH.sub.3 B-63 OCH.sub.3 Cl H CH.sub.3 CH.sub.2CH.sub.3 B-64 OCF.sub.3 Cl H CH.sub.3 CH.sub.2CH.sub.3 B-65 H CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-66 F CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-67 Cl CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-68 Br CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-69 CH.sub.3 CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-70 CF.sub.3 CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-71 OCH.sub.3 CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-72 OCF.sub.3 CH.sub.3 H CH.sub.3 CH.sub.2CH.sub.3 B-73 H CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-74 F CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-75 Cl CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-76 Br CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-77 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-78 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-79 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-80 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.2CH.sub.3 B-81 H H H CH.sub.3 CF.sub.3 B-82 F H H CH.sub.3 CF.sub.3 B-83 Cl H H CH.sub.3 CF.sub.3 B-84 Br H H CH.sub.3 CF.sub.3 B-85 CH.sub.3 H H CH.sub.3 CF.sub.3 B-86 CF.sub.3 H H CH.sub.3 CF.sub.3 B-87 OCH.sub.3 H H CH.sub.3 CF.sub.3 B-88 OCF.sub.3 H H CH.sub.3 CF.sub.3 B-89 H F H CH.sub.3 CF.sub.3 B-90 F F H CH.sub.3 CF.sub.3 B-91 Cl F H CH.sub.3 CF.sub.3 B-92 Br F H CH.sub.3 CF.sub.3 B-93 CH.sub.3 F H CH.sub.3 CF.sub.3 B-94 CF.sub.3 F H CH.sub.3 CF.sub.3 B-95 OCH.sub.3 F H CH.sub.3 CF.sub.3 B-96 OCF.sub.3 F H CH.sub.3 CF.sub.3 B-97 H Cl H CH.sub.3 CF.sub.3 B-98 F Cl H CH.sub.3 CF.sub.3 B-99 Cl Cl H CH.sub.3 CF.sub.3 B-100 Br Cl H CH.sub.3 CF.sub.3 B-101 CH.sub.3 Cl H CH.sub.3 CF.sub.3 B-102 CF.sub.3 Cl H CH.sub.3 CF.sub.3 B-103 OCH.sub.3 Cl H CH.sub.3 CF.sub.3 B-104 OCF.sub.3 Cl H CH.sub.3 CF.sub.3 B-105 H CH.sub.3 H CH.sub.3 CF.sub.3 B-106 F CH.sub.3 H CH.sub.3 CF.sub.3 B-107 Cl CH.sub.3 H CH.sub.3 CF.sub.3 B-108 Br CH.sub.3 H CH.sub.3 CF.sub.3 B-109 CH.sub.3 CH.sub.3 H CH.sub.3 CF.sub.3 B-110 CF.sub.3 CH.sub.3 H CH.sub.3 CF.sub.3 B-111 OCH.sub.3 CH.sub.3 H CH.sub.3 CF.sub.3 B-112 OCF.sub.3 CH.sub.3 H CH.sub.3 CF.sub.3 B-113 H CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-114 F CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-115 Cl CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-116 Br CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-117 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-118 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-119 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-120 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 B-121 H H H CF.sub.3 CF.sub.3 B-122 F H H CF.sub.3 CF.sub.3 B-123 Cl H H CF.sub.3 CF.sub.3 B-124 Br H H CF.sub.3 CF.sub.3 B-125 CH.sub.3 H H CF.sub.3 CF.sub.3 B-126 CF.sub.3 H H CF.sub.3 CF.sub.3 B-127 OCH.sub.3 H H CF.sub.3 CF.sub.3 B-128 OCF.sub.3 H H CF.sub.3 CF.sub.3 B-129 H F H CF.sub.3 CF.sub.3 B-130 F F H CF.sub.3 CF.sub.3 B-131 Cl F H CF.sub.3 CF.sub.3 B-132 Br F H CF.sub.3 CF.sub.3 B-133 CH.sub.3 F H CF.sub.3 CF.sub.3 B-134 CF.sub.3 F H CF.sub.3 CF.sub.3 B-135 OCH.sub.3 F H CF.sub.3 CF.sub.3 B-136 OCF.sub.3 F H CF.sub.3 CF.sub.3 B-137 H Cl H CF.sub.3 CF.sub.3 B-138 F Cl H CF.sub.3 CF.sub.3 B-139 Cl Cl H CF.sub.3 CF.sub.3 B-140 Br Cl H CF.sub.3 CF.sub.3 B-141 CH.sub.3 Cl H CF.sub.3 CF.sub.3 B-142 CF.sub.3 Cl H CF.sub.3 CF.sub.3 B-143 OCH.sub.3 Cl H CF.sub.3 CF.sub.3 B-144 OCF.sub.3 Cl H CF.sub.3 CF.sub.3 B-145 H CH.sub.3 H CF.sub.3 CF.sub.3 B-146 F CH.sub.3 H CF.sub.3 CF.sub.3 B-147 Cl CH.sub.3 H CF.sub.3 CF.sub.3 B-148 Br CH.sub.3 H CF.sub.3 CF.sub.3 B-149 CH.sub.3 CH.sub.3 H CF.sub.3 CF.sub.3 B-150 CF.sub.3 CH.sub.3 H CF.sub.3 CF.sub.3 B-151 OCH.sub.3 CH.sub.3 H CF.sub.3 CF.sub.3 B-152 OCF.sub.3 CH.sub.3 H CF.sub.3 CF.sub.3 B-153 H CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-154 F CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-155 Cl CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-156 Br CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-157 CH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-158 CF.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-159 OCH.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-160 OCF.sub.3 CH.sub.3 CH.sub.3 CF.sub.3 CF.sub.3 B-161 H H H CH.sub.3 C.sub.6H.sub.5* B-162 F H H CH.sub.3 C.sub.6H.sub.5 B-163 Cl H H CH.sub.3 C.sub.6H.sub.5 B-164 Br H H CH.sub.3 C.sub.6H.sub.5 B-165 CH.sub.3 H H CH.sub.3 C.sub.6H.sub.5 B-166 CF.sub.3 H H CH.sub.3 C.sub.6H.sub.5 B-167 OCH.sub.3 H H CH.sub.3 C.sub.6H.sub.5 B-168 OCF.sub.3 H H CH.sub.3 C.sub.6H.sub.5 B-169 H F H CH.sub.3 C.sub.6H.sub.5 B-170 F F H CH.sub.3 C.sub.6H.sub.5 B-171 Cl F H CH.sub.3 C.sub.6H.sub.5 B-172 Br F H CH.sub.3 C.sub.6H.sub.5 B-173 CH.sub.3 F H CH.sub.3 C.sub.6H.sub.5 B-174 CF.sub.3 F H CH.sub.3 C.sub.6H.sub.5 B-175 OCH.sub.3 F H CH.sub.3 C.sub.6H.sub.5 B-176 OCF.sub.3 F H CH.sub.3 C.sub.6H.sub.5 B-177 H Cl H CH.sub.3 C.sub.6H.sub.5 B-178 F Cl H CH.sub.3 C.sub.6H.sub.5 B-179 Cl Cl H CH.sub.3 C.sub.6H.sub.5 B-180 Br Cl H CH.sub.3 C.sub.6H.sub.5 B-181 CH.sub.3 Cl H CH.sub.3 C.sub.6H.sub.5 B-182 CF.sub.3 Cl H CH.sub.3 C.sub.6H.sub.5 B-183 OCH.sub.3 Cl H CH.sub.3 C.sub.6H.sub.5 B-184 OCF.sub.3 Cl H CH.sub.3 C.sub.6H.sub.5 B-185 H CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-186 F CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-187 Cl CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-188 Br CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-189 CH.sub.3 CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-190 CF.sub.3 CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-191 OCH.sub.3 CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-192 OCF.sub.3 CH.sub.3 H CH.sub.3 C.sub.6H.sub.5 B-193 H CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-194 F CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-195 Cl CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-196 Br CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-197 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-198 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-199 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-200 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 C.sub.6H.sub.5 B-201 H H H CH.sub.3 OCH.sub.3 B-202 F H H CH.sub.3 OCH.sub.3 B-203 Cl H H CH.sub.3 OCH.sub.3 B-204 Br H H CH.sub.3 OCH.sub.3 B-205 CH.sub.3 H H CH.sub.3 OCH.sub.3 B-206 CF.sub.3 H H CH.sub.3 OCH.sub.3 B-207 OCH.sub.3 H H CH.sub.3 OCH.sub.3 B-208 OCF.sub.3 H H CH.sub.3 OCH.sub.3 B-209 H F H CH.sub.3 OCH.sub.3 B-210 F F H CH.sub.3 OCH.sub.3 B-211 Cl F H CH.sub.3 OCH.sub.3 B-212 Br F H CH.sub.3 OCH.sub.3 B-213 CH.sub.3 F H CH.sub.3 OCH.sub.3 B-214 CF.sub.3 F H CH.sub.3 OCH.sub.3 B-215 OCH.sub.3 F H CH.sub.3 OCH.sub.3 B-216 OCF.sub.3 F H CH.sub.3 OCH.sub.3 B-217 H Cl H CH.sub.3 OCH.sub.3 B-218 F Cl H CH.sub.3 OCH.sub.3 B-219 Cl Cl H CH.sub.3 OCH.sub.3 B-220 Br Cl H CH.sub.3 OCH.sub.3 B-221 CH.sub.3 Cl H CH.sub.3 OCH.sub.3 B-222 CF.sub.3 Cl H CH.sub.3 OCH.sub.3 B-223 OCH.sub.3 Cl H CH.sub.3 OCH.sub.3 B-224 OCF.sub.3 Cl H CH.sub.3 OCH.sub.3 B-225 H CH.sub.3 H CH.sub.3 OCH.sub.3 B-226 F CH.sub.3 H CH.sub.3 OCH.sub.3 B-227 Cl CH.sub.3 H CH.sub.3 OCH.sub.3 B-228 Br CH.sub.3 H CH.sub.3 OCH.sub.3 B-229 CH.sub.3 CH.sub.3 H CH.sub.3 OCH.sub.3 B-230 CF.sub.3 CH.sub.3 H CH.sub.3 OCH.sub.3 B-231 OCH.sub.3 CH.sub.3 H CH.sub.3 OCH.sub.3 B-232 OCF.sub.3 CH.sub.3 H CH.sub.3 OCH.sub.3 B-233 H CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-234 F CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-235 Cl CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-236 Br CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-237 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-238 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-239 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-240 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OCH.sub.3 B-241 H H H CH.sub.3 OH B-242 F H H CH.sub.3 OH B-243 Cl H H CH.sub.3 OH B-244 Br H H CH.sub.3 OH B-245 CH.sub.3 H H CH.sub.3 OH B-246 CF.sub.3 H H CH.sub.3 OH
B-247 OCH.sub.3 H H CH.sub.3 OH B-248 OCF.sub.3 H H CH.sub.3 OH B-249 H F H CH.sub.3 OH B-250 F F H CH.sub.3 OH B-251 Cl F H CH.sub.3 OH B-252 Br F H CH.sub.3 OH B-253 CH.sub.3 F H CH.sub.3 OH B-254 CF.sub.3 F H CH.sub.3 OH B-255 OCH.sub.3 F H CH.sub.3 OH B-256 OCF.sub.3 F H CH.sub.3 OH B-257 H Cl H CH.sub.3 OH B-258 F Cl H CH.sub.3 OH B-259 Cl Cl H CH.sub.3 OH B-260 Br Cl H CH.sub.3 OH B-261 CH.sub.3 Cl H CH.sub.3 OH B-262 CF.sub.3 Cl H CH.sub.3 OH B-263 OCH.sub.3 Cl H CH.sub.3 OH B-264 OCF.sub.3 Cl H CH.sub.3 OH B-265 H CH.sub.3 H CH.sub.3 OH B-266 F CH.sub.3 H CH.sub.3 OH B-267 Cl CH.sub.3 H CH.sub.3 OH B-268 Br CH.sub.3 H CH.sub.3 OH B-269 CH.sub.3 CH.sub.3 H CH.sub.3 OH B-270 CF.sub.3 CH.sub.3 H CH.sub.3 OH B-271 OCH.sub.3 CH.sub.3 H CH.sub.3 OH B-272 OCF.sub.3 CH.sub.3 H CH.sub.3 OH B-273 H CH.sub.3 CH.sub.3 CH.sub.3 OH B-274 F CH.sub.3 CH.sub.3 CH.sub.3 OH B-275 Cl CH.sub.3 CH.sub.3 CH.sub.3 OH B-276 Br CH.sub.3 CH.sub.3 CH.sub.3 OH B-277 CH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OH B-278 CF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OH B-279 OCH.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OH B-280 OCF.sub.3 CH.sub.3 CH.sub.3 CH.sub.3 OH B-281 H H H --(CH.sub.2).sub.2-- B-282 F H H --(CH.sub.2).sub.2-- B-283 Cl H H --(CH.sub.2).sub.2-- B-284 Br H H --(CH.sub.2).sub.2-- B-285 CH.sub.3 H H --(CH.sub.2).sub.2-- B-286 CF.sub.3 H H --(CH.sub.2).sub.2-- B-287 OCH.sub.3 H H --(CH.sub.2).sub.2-- B-288 OCF.sub.3 H H --(CH.sub.2).sub.2-- B-289 H F H --(CH.sub.2).sub.2-- B-290 F F H --(CH.sub.2).sub.2-- B-291 Cl F H --(CH.sub.2).sub.2-- B-292 Br F H --(CH.sub.2).sub.2-- B-293 CH.sub.3 F H --(CH.sub.2).sub.2-- B-294 CF.sub.3 F H --(CH.sub.2).sub.2-- B-295 OCH.sub.3 F H --(CH.sub.2).sub.2-- B-296 OCF.sub.3 F H --(CH.sub.2).sub.2-- B-297 H Cl H --(CH.sub.2).sub.2-- B-298 F Cl H --(CH.sub.2).sub.2-- B-299 Cl Cl H --(CH.sub.2).sub.2-- B-300 Br Cl H --(CH.sub.2).sub.2-- B-301 CH.sub.3 Cl H --(CH.sub.2).sub.2-- B-302 CF.sub.3 Cl H --(CH.sub.2).sub.2-- B-303 OCH.sub.3 Cl H --(CH.sub.2).sub.2-- B-304 OCF.sub.3 Cl H --(CH.sub.2).sub.2-- B-305 H CH.sub.3 H --(CH.sub.2).sub.2-- B-306 F CH.sub.3 H --(CH.sub.2).sub.2-- B-307 Cl CH.sub.3 H --(CH.sub.2).sub.2-- B-308 Br CH.sub.3 H --(CH.sub.2).sub.2-- B-309 CH.sub.3 CH.sub.3 H --(CH.sub.2).sub.2-- B-310 CF.sub.3 CH.sub.3 H --(CH.sub.2).sub.2-- B-311 OCH.sub.3 CH.sub.3 H --(CH.sub.2).sub.2-- B-312 OCF.sub.3 CH.sub.3 H --(CH.sub.2).sub.2-- B-313 H CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-314 F CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-315 Cl CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-316 Br CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-317 CH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-318 CF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-319 OCH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-320 OCF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.2-- B-321 H H H --(CH.sub.2).sub.3-- B-322 F H H --(CH.sub.2).sub.3-- B-323 Cl H H --(CH.sub.2).sub.3-- B-324 Br H H --(CH.sub.2).sub.3-- B-325 CH.sub.3 H H --(CH.sub.2).sub.3-- B-326 CF.sub.3 H H --(CH.sub.2).sub.3-- B-327 OCH.sub.3 H H --(CH.sub.2).sub.3-- B-328 OCF.sub.3 H H --(CH.sub.2).sub.3-- B-329 H F H --(CH.sub.2).sub.3-- B-330 F F H --(CH.sub.2).sub.3-- B-331 Cl F H --(CH.sub.2).sub.3-- B-332 Br F H --(CH.sub.2).sub.3-- B-333 CH.sub.3 F H --(CH.sub.2).sub.3-- B-334 CF.sub.3 F H --(CH.sub.2).sub.3-- B-335 OCH.sub.3 F H --(CH.sub.2).sub.3-- B-336 OCF.sub.3 F H --(CH.sub.2).sub.3-- B-337 H Cl H --(CH.sub.2).sub.3-- B-338 F Cl H --(CH.sub.2).sub.3-- B-339 Cl Cl H --(CH.sub.2).sub.3-- B-340 Br Cl H --(CH.sub.2).sub.3-- B-341 CH.sub.3 Cl H --(CH.sub.2).sub.3-- B-342 CF.sub.3 Cl H --(CH.sub.2).sub.3-- B-343 OCH.sub.3 Cl H --(CH.sub.2).sub.3-- B-344 OCF.sub.3 Cl H --(CH.sub.2).sub.3-- B-345 H CH.sub.3 H --(CH.sub.2).sub.3-- B-346 F CH.sub.3 H --(CH.sub.2).sub.3-- B-347 Cl CH.sub.3 H --(CH.sub.2).sub.3-- B-348 Br CH.sub.3 H --(CH.sub.2).sub.3-- B-349 CH.sub.3 CH.sub.3 H --(CH.sub.2).sub.3-- B-350 CF.sub.3 CH.sub.3 H --(CH.sub.2).sub.3-- B-351 OCH.sub.3 CH.sub.3 H --(CH.sub.2).sub.3-- B-352 OCF.sub.3 CH.sub.3 H --(CH.sub.2).sub.3-- B-353 H CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-354 F CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-355 Cl CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-356 Br CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-357 CH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-358 CF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-359 OCH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-360 OCF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.3-- B-361 H H H --(CH.sub.2).sub.4-- B-362 F H H --(CH.sub.2).sub.4-- B-363 Cl H H --(CH.sub.2).sub.4-- B-364 Br H H --(CH.sub.2).sub.4-- B-365 CH.sub.3 H H --(CH.sub.2).sub.4-- B-366 CF.sub.3 H H --(CH.sub.2).sub.4-- B-367 OCH.sub.3 H H --(CH.sub.2).sub.4-- B-368 OCF.sub.3 H H --(CH.sub.2).sub.4-- B-369 H F H --(CH.sub.2).sub.4-- B-370 F F H --(CH.sub.2).sub.4-- B-371 Cl F H --(CH.sub.2).sub.4-- B-372 Br F H --(CH.sub.2).sub.4-- B-373 CH.sub.3 F H --(CH.sub.2).sub.4-- B-374 CF.sub.3 F H --(CH.sub.2).sub.4-- B-375 OCH.sub.3 F H --(CH.sub.2).sub.4-- B-376 OCF.sub.3 F H --(CH.sub.2).sub.4-- B-377 H Cl H --(CH.sub.2).sub.4-- B-378 F Cl H --(CH.sub.2).sub.4-- B-379 Cl Cl H --(CH.sub.2).sub.4-- B-380 Br Cl H --(CH.sub.2).sub.4-- B-381 CH.sub.3 Cl H --(CH.sub.2).sub.4-- B-382 CF.sub.3 Cl H --(CH.sub.2).sub.4-- B-383 OCH.sub.3 Cl H --(CH.sub.2).sub.4-- B-384 OCF.sub.3 Cl H --(CH.sub.2).sub.4-- B-385 H CH.sub.3 H --(CH.sub.2).sub.4-- B-386 F CH.sub.3 H --(CH.sub.2).sub.4-- B-387 Cl CH.sub.3 H --(CH.sub.2).sub.4-- B-388 Br CH.sub.3 H --(CH.sub.2).sub.4-- B-389 CH.sub.3 CH.sub.3 H --(CH.sub.2).sub.4-- B-390 CF.sub.3 CH.sub.3 H --(CH.sub.2).sub.4-- B-391 OCH.sub.3 CH.sub.3 H --(CH.sub.2).sub.4-- B-392 OCF.sub.3 CH.sub.3 H --(CH.sub.2).sub.4-- B-393 H CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-394 F CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-395 Cl CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-396 Br CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-397 CH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-398 CF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-399 OCH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-400 OCF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.4-- B-401 H H H --(CH.sub.2).sub.5-- B-402 F H H --(CH.sub.2).sub.5-- B-403 Cl H H --(CH.sub.2).sub.5-- B-404 Br H H --(CH.sub.2).sub.5-- B-405 CH.sub.3 H H --(CH.sub.2).sub.5-- B-406 CF.sub.3 H H --(CH.sub.2).sub.5-- B-407 OCH.sub.3 H H --(CH.sub.2).sub.5-- B-408 OCF.sub.3 H H --(CH.sub.2).sub.5-- B-409 H F H --(CH.sub.2).sub.5-- B-410 F F H --(CH.sub.2).sub.5-- B-411 Cl F H --(CH.sub.2).sub.5-- B-412 Br F H --(CH.sub.2).sub.5-- B-413 CH.sub.3 F H --(CH.sub.2).sub.5-- B-414 CF.sub.3 F H --(CH.sub.2).sub.5-- B-415 OCH.sub.3 F H --(CH.sub.2).sub.5-- B-416 OCF.sub.3 F H --(CH.sub.2).sub.5-- B-417 H Cl H --(CH.sub.2).sub.5-- B-418 F Cl H --(CH.sub.2).sub.5-- B-419 Cl Cl H --(CH.sub.2).sub.5-- B-420 Br Cl H --(CH.sub.2).sub.5-- B-421 CH.sub.3 Cl H --(CH.sub.2).sub.5-- B-422 CF.sub.3 Cl H --(CH.sub.2).sub.5-- B-423 OCH.sub.3 Cl H --(CH.sub.2).sub.5-- B-424 OCF.sub.3 Cl H --(CH.sub.2).sub.5-- B-425 H CH.sub.3 H --(CH.sub.2).sub.5-- B-426 F CH.sub.3 H --(CH.sub.2).sub.5-- B-427 Cl CH.sub.3 H --(CH.sub.2).sub.5-- B-428 Br CH.sub.3 H --(CH.sub.2).sub.5-- B-429 CH.sub.3 CH.sub.3 H --(CH.sub.2).sub.5-- B-430 CF.sub.3 CH.sub.3 H --(CH.sub.2).sub.5-- B-431 OCH.sub.3 CH.sub.3 H --(CH.sub.2).sub.5-- B-432 OCF.sub.3 CH.sub.3 H --(CH.sub.2).sub.5-- B-433 H CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-434 F CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-435 Cl CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-436 Br CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-437 CH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-438 CF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-439 OCH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-440 OCF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.5-- B-441 H H H --(CH.sub.2).sub.6-- B-442 F H H --(CH.sub.2).sub.6-- B-443 Cl H H --(CH.sub.2).sub.6-- B-444 Br H H --(CH.sub.2).sub.6-- B-445 CH.sub.3 H H --(CH.sub.2).sub.6-- B-446 CF.sub.3 H H --(CH.sub.2).sub.6-- B-447 OCH.sub.3 H H --(CH.sub.2).sub.6-- B-448 OCF.sub.3 H H --(CH.sub.2).sub.6-- B-449 H F H --(CH.sub.2).sub.6-- B-450 F F H --(CH.sub.2).sub.6-- B-451 Cl F H --(CH.sub.2).sub.6-- B-452 Br F H --(CH.sub.2).sub.6-- B-453 CH.sub.3 F H --(CH.sub.2).sub.6-- B-454 CF.sub.3 F H --(CH.sub.2).sub.6-- B-455 OCH.sub.3 F H --(CH.sub.2).sub.6-- B-456 OCF.sub.3 F H --(CH.sub.2).sub.6-- B-457 H Cl H --(CH.sub.2).sub.6-- B-458 F Cl H --(CH.sub.2).sub.6-- B-459 Cl Cl H --(CH.sub.2).sub.6-- B-460 Br Cl H --(CH.sub.2).sub.6-- B-461 CH.sub.3 Cl H --(CH.sub.2).sub.6-- B-462 CF.sub.3 Cl H --(CH.sub.2).sub.6-- B-463 OCH.sub.3 Cl H --(CH.sub.2).sub.6-- B-464 OCF.sub.3 Cl H --(CH.sub.2).sub.6-- B-465 H CH.sub.3 H --(CH.sub.2).sub.6-- B-466 F CH.sub.3 H --(CH.sub.2).sub.6-- B-467 Cl CH.sub.3 H --(CH.sub.2).sub.6-- B-468 Br CH.sub.3 H --(CH.sub.2).sub.6-- B-469 CH.sub.3 CH.sub.3 H --(CH.sub.2).sub.6-- B-470 CF.sub.3 CH.sub.3 H --(CH.sub.2).sub.6-- B-471 OCH.sub.3 CH.sub.3 H --(CH.sub.2).sub.6-- B-472 OCF.sub.3 CH.sub.3 H --(CH.sub.2).sub.6-- B-473 H CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-474 F CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-475 Cl CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-476 Br CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-477 CH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-478 CF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-479 OCH.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-480 OCF.sub.3 CH.sub.3 CH.sub.3 --(CH.sub.2).sub.6-- B-481 H H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-482 F H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-483 Cl H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-484 Br H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-485 CH.sub.3 H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-486 CF.sub.3 H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-487 OCH.sub.3 H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-488 OCF.sub.3 H H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-489 H F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-490 F F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-491 Cl F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-492 Br F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-493 CH.sub.3 F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-494 CF.sub.3 F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-495 OCH.sub.3 F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-496 OCF.sub.3 F H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-497 H Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2--
B-498 F Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-499 Cl Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-500 Br Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-501 CH.sub.3 Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-502 CF.sub.3 Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-503 OCH.sub.3 Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-504 OCF.sub.3 Cl H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-505 H CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-506 F CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-507 Cl CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-508 Br CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-509 CH.sub.3 CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-510 CF.sub.3 CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-511 OCH.sub.3 CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-512 OCF.sub.3 CH.sub.3 H --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-513 H CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-514 F CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-515 Cl CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-516 Br CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-517 CH.sub.3 CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-518 CF.sub.3 CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-519 OCH.sub.3 CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-520 OCF.sub.3 CH.sub.3 CH.sub.3 --CH.dbd.CH--CH.sub.2--CH.sub.2-- B-521 H H --(CH.sub.2).sub.3-- CH.sub.3 B-522 F H --(CH.sub.2).sub.3-- CH.sub.3 B-523 Cl H --(CH.sub.2).sub.3-- CH.sub.3 B-524 Br H --(CH.sub.2).sub.3-- CH.sub.3 B-525 CH.sub.3 H --(CH.sub.2).sub.3-- CH.sub.3 B-526 CF.sub.3 H --(CH.sub.2).sub.3-- CH.sub.3 B-527 OCH.sub.3 H --(CH.sub.2).sub.3-- CH.sub.3 B-528 OCF.sub.3 H --(CH.sub.2).sub.3-- CH.sub.3 B-529 H D --(CH.sub.2).sub.3-- CH.sub.3 B-530 F D --(CH.sub.2).sub.3-- CH.sub.3 B-531 Cl D --(CH.sub.2).sub.3-- CH.sub.3 B-532 Br D --(CH.sub.2).sub.3-- CH.sub.3 B-533 CH.sub.3 D --(CH.sub.2).sub.3-- CH.sub.3 B-534 CF.sub.3 D --(CH.sub.2).sub.3-- CH.sub.3 B-535 OCH.sub.3 D --(CH.sub.2).sub.3-- CH.sub.3 B-536 OCF.sub.3 D --(CH.sub.2).sub.3-- CH.sub.3 B-537 H H --(CH.sub.2).sub.3-- D B-538 F H --(CH.sub.2).sub.3-- D B-539 Cl H --(CH.sub.2).sub.3-- D B-540 Br H --(CH.sub.2).sub.3-- D B-541 CH.sub.3 H --(CH.sub.2).sub.3-- D B-542 CF.sub.3 H --(CH.sub.2).sub.3-- D B-543 OCH.sub.3 H --(CH.sub.2).sub.3-- D B-544 OCF.sub.3 H --(CH.sub.2).sub.3-- D B-545 H D --(CH.sub.2).sub.3-- D B-546 F D --(CH.sub.2).sub.3-- D B-547 Cl D --(CH.sub.2).sub.3-- D B-548 Br D --(CH.sub.2).sub.3-- D B-549 CH.sub.3 D --(CH.sub.2).sub.3-- D B-550 CF.sub.3 D --(CH.sub.2).sub.3-- D B-551 OCH.sub.3 D --(CH.sub.2).sub.3-- D B-552 OCF.sub.3 D --(CH.sub.2).sub.3-- D B-553 H H --(CH.sub.2).sub.4-- CH.sub.3 B-554 F H --(CH.sub.2).sub.4-- CH.sub.3 B-555 Cl H --(CH.sub.2).sub.4-- CH.sub.3 B-556 Br H --(CH.sub.2).sub.4-- CH.sub.3 B-557 CH.sub.3 H --(CH.sub.2).sub.4-- CH.sub.3 B-558 CF.sub.3 H --(CH.sub.2).sub.4-- CH.sub.3 B-559 OCH.sub.3 H --(CH.sub.2).sub.4-- CH.sub.3 B-560 OCF.sub.3 H --(CH.sub.2).sub.4-- CH.sub.3 B-561 H D --(CH.sub.2).sub.4-- CH.sub.3 B-562 F D --(CH.sub.2).sub.4-- CH.sub.3 B-563 Cl D --(CH.sub.2).sub.4-- CH.sub.3 B-564 Br D --(CH.sub.2).sub.4-- CH.sub.3 B-565 CH.sub.3 D --(CH.sub.2).sub.4-- CH.sub.3 B-566 CF.sub.3 D --(CH.sub.2).sub.4-- CH.sub.3 B-567 OCH.sub.3 D --(CH.sub.2).sub.4-- CH.sub.3 B-568 OCF.sub.3 D --(CH.sub.2).sub.4-- CH.sub.3 B-569 H H --(CH.sub.2).sub.4-- D B-570 F H --(CH.sub.2).sub.4-- D B-571 Cl H --(CH.sub.2).sub.4-- D B-572 Br H --(CH.sub.2).sub.4-- D B-573 CH.sub.3 H --(CH.sub.2).sub.4-- D B-574 CF.sub.3 H --(CH.sub.2).sub.4-- D B-575 OCH.sub.3 H --(CH.sub.2).sub.4-- D B-576 OCF.sub.3 H --(CH.sub.2).sub.4-- D B-577 H D --(CH.sub.2).sub.4-- D B-578 F D --(CH.sub.2).sub.4-- D B-579 Cl D --(CH.sub.2).sub.4-- D B-580 Br D --(CH.sub.2).sub.4-- D B-581 CH.sub.3 D --(CH.sub.2).sub.4-- D B-582 CF.sub.3 D --(CH.sub.2).sub.4-- D B-583 OCH.sub.3 D --(CH.sub.2).sub.4-- D B-584 OCF.sub.3 D --(CH.sub.2).sub.4-- D *C.sub.6H.sub.5 = phenyl
[0394] In a specific embodiment, the invention relates to compounds I selected from the compounds of the examples, either in form of free bases or of any pharmaceutically acceptable salt thereof or a steroisomer thereof.
[0395] The compounds of the present invention can be prepared by using routine techniques familiar to a skilled person. In particular, the compounds of the formula I can be prepared according to the following schemes, wherein the variables, if not stated otherwise, are as defined above.
[0396] Compounds of formula I wherein X is CR.sup.7R.sup.8 and R.sup.5b is H (=compounds I') can be synthesized as described in scheme 1 below. The protected tetrahydrobenzodiazepine or tetrahydroquinoxaline 1, wherein Z is a hydrogen or a halogen atom, such as Cl, Br or I and PG is a common protective group, such as a carbamate, especially boc, is acylated with the acrylic acid derivative 2, wherein LG is an appropriate leaving group, such as Cl or an anhydride or a chloroformate, in the presence of a base, such as triethylamine or Hunig's base, in an organic solvent, such as ether or methylene chloride. Reaction of 3 with a Lewis acid or a Bronstedt acid HA or irradiation with a suitable wavelength commonly derived from a mercury lamp in an adequate solvent, such as acetone or toluene, in a common photoreactor yields cyclization to 4. Reduction of the carbonyl group with common reduction agents like borohydrides such as sodium borohydride or borane-tetrahydrofurane-complex yields 5, which is deprotected using suitable reagents such as strong bases or acids to I', wherein R.sup.4a and R.sup.4b and R.sup.5b are H. Compounds I wherein R.sup.4a and R.sup.4b form together .dbd.O can be obtained by skipping the reduction step to 5 and deprotecting 4.
##STR00019##
[0397] If desired, substituents R.sup.1 different from hydrogen can be introduced for example via alkylation under typical conditions such as stirring in an appropriate solvent in the presence of an alkylhalide and a base or via other common substitution reactions.
[0398] Compounds I wherein R.sup.5b is different from H can be prepared, for example, by reacting compound 4 with a compound LG-R.sup.5b in the presence of a base, wherein LG is an appropriate leaving group, such as Cl or Br.
[0399] Compounds I wherein R.sup.5b is different from H and R.sup.8 is CH.sub.3 (=compounds I'') can be prepared, for example, as out lined in scheme 2 below. Acylation of 1 with the allylic acid derivative 6, wherein LG is an appropriate leaving group, such as Cl or an anhydride or a chloroformate, in the presence of a base, such as triethylamine or Hunig's base, in an organic solvent, such as ether or methylene chloride, yields 7, which is reacted in a Heck-type reaction, e.g. employing palladium acetate in the presence of a base, such as potassium carbonate, to yield the cyclization product 8. Reduction of the carbonyl group with common reduction agents like borohydrides such as sodium borohydride or borane-tetrahydrofurane-complex yields 9, which is deprotected using suitable reagents such as strong bases or acids to I'', wherein R.sup.4a and R.sup.4b are H. Compounds I wherein R.sup.4a and R.sup.4b form together .dbd.O can be obtained by skipping the reduction step to 9 and deprotecting 8. If desired, substituents R.sup.1 different from hydrogen can be introduced for example via alkylation under typical conditions such as stirring in an appropriate solvent in the presence of an alkylhalide and a base or via other common substitution reactions.
##STR00020##
[0400] Compounds I wherein X is NR.sup.6 (=compounds I''') can be prepared as outlined in scheme 3 below. Acylation of 1 with the .beta.-amino acid derivative 10, wherein PG' is a protection group different from PG and LG is an appropriate leaving group, such as Cl or an anhydride or a chloroformate, in the presence of a base, such as triethylamine or Hunig's base, in an organic solvent, such as ether or methylene chloride, yields 11. This is first selectively deprotected at the amino group NR.sup.6PG'. The partially deprotected compound is then reacted in a cyclization reaction to 12 under Buchwald-Hartwig reaction conditions such as the use of a Pd catalyst, e.g. tetrakis(triphenylphosphine) palladium in the presence of as base, such as sodium tert-butylate and potassium carbonate. Reduction of the carbonyl group of 12 with common reduction agents like borohydrides such as sodium borohydride or borane-tetrahydrofurane-complex yields 13, which is deprotected using suitable reagents such as strong bases or acids to I''', wherein R.sup.4a and R.sup.4b are H. Compounds I wherein R.sup.4a and R.sup.4b form together .dbd.O can be obtained by skipping the reduction step to 13 and deprotecting 12. If desired, substituents R.sup.1 different from hydrogen can be introduced for example via alkylation under typical conditions such as stirring in an appropriate solvent in the presence of an alkylhalide and a base or via other common substitution reactions.
##STR00021##
[0401] Alternatively to the method depicted in scheme 1, compounds I wherein X is CR.sup.7R.sup.8 and R.sup.5b is H (=compounds I') can be synthesized as described in scheme 4 below. Readily available anilines 14 are derivatized with carbonyl moieties 15 by acylation procedures employing appropriate leaving groups LG, such as chlorides or anhydrides, in the presence of a base such as triethylamine or Hunig's base, in an organic solvent, such as diethyl ether or methylene chloride to yield 16. Cyclization products 17 are received by irradiation with a suitable wavelength commonly derived from a mercury lamp in an adequate solvent such as acetone or toluene in a common photoreactor known to those skilled in the art. Reduction with common reduction agents like borohydrides such as sodium borohydride or borane-tetrahydrofurane-complex yield suitable intermediates 18 that are alkylated with alkylamides 19 employing suitable leaving groups LG such as chlorides or bromides. After reduction to the corresponding amines 20 cyclization is performed in a suitable solvent like acetonitrile or methanol at room temperature or higher temperature by addition of an acid and substituted ketones or aldehydes to obtain compounds I'.
##STR00022##
[0402] Alternatively to the method depicted in scheme 3, compounds I wherein X is NR.sup.6 (=compounds I''') can be prepared as outlined in scheme 5 below.
[0403] Readily available starting materials 22 are alkylated with alkylamides 19 employing suitable leaving groups LG such as chlorides or bromides to give 23. After reduction to the corresponding amines 24 cyclization is performed in a suitable solvent like acetonitrile or methanol at room temperature or higher temperature by addition of an acid and substituted ketones or aldehydes 21 to obtain compounds I'''.
##STR00023##
[0404] Compounds I wherein R.sup.4a and R.sup.4b are not H or do not form together a group .dbd.O can be prepared by standard derivatization methods of compounds wherein R.sup.4a and R.sup.4b form together a group .dbd.O. For instance, compounds wherein R.sup.4a and R.sup.4b form together a group .dbd.S may be prepared by reaction with a sulfurization agent, such as Lawesson's reagent or P.sub.2S.sub.5. Alkyl and related groups as radicals R.sup.4a and R.sup.4b may be introduced via Grignard reduction. Amino and related groups may be introduced via reductive amination. Hydroxyl group R.sup.4a or R.sup.4b may be introduced by reducing the carbonyl group. This may be alkylated to yield alkoxy and related groups R.sup.4a and R.sup.4b or substituted by diverse groups.
[0405] If not otherwise indicated, the above-described reactions are generally carried out in a solvent at temperatures between room temperature and the boiling temperature of the solvent employed. Alternatively, the activation energy which is required for the reaction can be introduced into the reaction mixture using microwaves, something which has proved to be of value, in particular, in the case of the reactions catalyzed by transition metals (with regard to reactions using microwaves, see Tetrahedron 2001, 57, p. 9199 ff. p. 9225 ff. and also, in a general manner, "Microwaves in Organic Synthesis", Andre Loupy (Ed.), Wiley-VCH 2002.
[0406] The acid addition salts of compounds I are prepared in a customary manner by mixing the free base with a corresponding acid, where appropriate in solution in an organic solvent, for example a lower alcohol, such as methanol, ethanol or propanol, an ether, such as methyl tert-butyl ether or diisopropyl ether, a ketone, such as acetone or methyl ethyl ketone, or an ester, such as ethyl acetate.
[0407] Routine experimentations, including appropriate manipulation of the reaction conditions, reagents and sequence of the synthetic route, protection of any chemical functionality that may not be compatible with the reaction conditions, and deprotection at a suitable point in the reaction sequence of the preparation methods are within routine techniques.
[0408] Suitable protecting groups and the methods for protecting and deprotecting different substituents using such suitable protecting groups are well known to those skilled in the art; examples of which may be found in T. Greene and P. Wuts, Protective Groups in Organic Synthesis (3.sup.rd ed.), John Wiley & Sons, NY (1999), which is herein incorporated by reference in its entirety. Synthesis of the compounds of the invention may be accomplished by methods analogous to those described in the synthetic schemes described hereinabove and in specific examples.
[0409] Starting materials, if not commercially available, may be prepared by procedures selected from standard organic chemical techniques, techniques that are analogous to the synthesis of known, structurally similar compounds, or techniques that are analogous to the above described schemes or the procedures described in the synthetic examples section.
[0410] When an optically active form of a compound of the invention is required, it may be obtained by carrying out one of the procedures described herein using an optically active starting material (prepared, for example, by asymmetric induction of a suitable reaction step), or by resolution of a mixture of the stereoisomers of the compound or intermediates using a standard procedure (such as chromatographic separation, recrystallization or enzymatic resolution).
[0411] Similarly, when a pure geometric isomer of a compound of the invention is required, it may be obtained by carrying out one of the above procedures using a pure geometric isomer as a starting material, or by resolution of a mixture of the geometric isomers of the compound or intermediates using a standard procedure such as chromatographic separation.
[0412] Moreover, the present invention relates to compounds of formula I as defined above, wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope (e.g., hydrogen by deuterium, .sup.12C by .sup.13C, .sup.14N by .sup.15N, .sup.16O by .sup.18O) and preferably wherein at least one hydrogen atom has been replaced by a deuterium atom.
[0413] Additionally, the compounds according to the invention include more of the respective isotope than occurs naturally occurs and which is present in compounds I.
[0414] Stable isotopes (e.g., deuterium, .sup.13C, .sup.15N, .sup.18O) are nonradioactive isotopes which contain one additional neutron than the normally abundant isotope of the respective atom. Deuterated compounds have been used in pharmaceutical research to investigate the in vivo metabolic fate of the compounds by evaluation of the mechanism of action and metabolic pathway of the non deuterated parent compound (Blake et al. J. Pharm. Sci. 64, 3, 367-391 (1975)). Such metabolic studies are important in the design of safe, effective therapeutic drugs, either because the in vivo active compound administered to the patient or because the metabolites produced from the parent compound prove to be toxic or carcinogenic (Foster et al., Advances in Drug Research Vol. 14, pp. 2-36, Academic press, London, 1985; Kato et al., J. Labelled Comp. Radiopharmaceut., 36(10):927-932 (1995); Kushner et al., Can. J. Physiol. Pharmacol., 77, 79-88 (1999)).
[0415] Incorporation of a heavy atom, particularly substitution of deuterium for hydrogen, can give rise to an isotope effect that could alter the pharmacokinetics of the drug.
[0416] Stable isotope labeling of a drug can alter its physico-chemical properties such as pK.sub.a and lipid solubility. These changes may influence the fate of the drug at different steps along its passage through the body. Absorption, distribution, metabolism or excretion can be changed. Absorption and distribution are processes that depend primarily on the molecular size and the lipophilicity of the substance. These effects and alterations can affect the pharmacodynamic response of the drug molecule if the isotopic substitution affects a region involved in a ligand-receptor interaction.
[0417] Drug metabolism can give rise to large isotopic effect if the breaking of a chemical bond to a deuterium atom is the rate limiting step in the process. While some of the physical properties of a stable isotope-labeled molecule are different from those of the unlabeled one, the chemical and biological properties are the same, with one important exception: because of the increased mass of the heavy isotope, any bond involving the heavy isotope and another atom will be stronger than the same bond between the light isotope and that atom. In any reaction in which the breaking of this bond is the rate limiting step, the reaction will proceed slower for the molecule with the heavy isotope due to "kinetic isotope effect". A reaction involving breaking a C--D bond can be up to 700 percent slower than a similar reaction involving breaking a C--H bond. If the C--D bond is not involved in any of the steps leading to the metabolite, there may not be any effect to alter the behavior of the drug. If a deuterium is placed at a site involved in the metabolism of a drug, an isotope effect will be observed only if breaking of the C--D bond is the rate limiting step. There is evidence to suggest that whenever cleavage of an aliphatic C--H bond occurs, usually by oxidation catalyzed by a mixed-function oxidase, replacement of the hydrogen by deuterium will lead to observable isotope effect. It is also important to understand that the incorporation of deuterium at the site of metabolism slows its rate to the point where another metabolite produced by attack at a carbon atom not substituted by deuterium becomes the major pathway a process called "metabolic switching".
[0418] Deuterium tracers, such as deuterium-labeled drugs and doses, in some cases repeatedly, of thousands of milligrams of deuterated water, are also used in healthy humans of all ages, including neonates and pregnant women, without reported incident (e.g. Pons G and Rey E, Pediatrics 1999 104: 633; Coward W A et al., Lancet 1979 7: 13; Schwarcz H P, Control. Clin. Trials 1984 5(4 Suppl): 573; Rodewald L E et al., J. Pediatr. 1989 114: 885; Butte N F et al. Br. J. Nutr. 1991 65: 3; MacLennan A H et al. Am. J. Obstet Gynecol. 1981 139: 948). Thus, it is clear that any deuterium released, for instance, during the metabolism of compounds of this invention poses no health risk.
[0419] The weight percentage of hydrogen in a mammal (approximately 9%) and natural abundance of deuterium (approximately 0.015%) indicates that a 70 kg human normally contains nearly a gram of deuterium. Furthermore, replacement of up to about 15% of normal hydrogen with deuterium has been effected and maintained for a period of days to weeks in mammals, including rodents and dogs, with minimal observed adverse effects (Czajka D M and Finkel A J, Ann. N.Y. Acad. Sci. 1960 84: 770; Thomson J F, Ann. New York Acad. Sci 1960 84: 736; Czakja D M et al., Am. J. Physiol. 1961 201: 357). Higher deuterium concentrations, usually in excess of 20%, can be toxic in animals. However, acute replacement of as high as 15%-23% of the hydrogen in humans' fluids with deuterium was found not to cause toxicity (Blagojevic N et al. in "Dosimetry & Treatment Planning for Neutron Capture Therapy", Zamenhof R, Solares G and Harling O Eds. 1994. Advanced Medical Publishing, Madison Wis. pp. 125-134; Diabetes Metab. 23: 251 (1997)).
[0420] Increasing the amount of deuterium present in a compound above its natural abundance is called enrichment or deuterium-enrichment. Examples of the amount of enrichment include from about 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 16, 21, 25, 29, 33, 37, 42, 46, 50, 54, 58, 63, 67, 71, 75, 79, 84, 88, 92, 96, to about 100 mol %.
[0421] The hydrogens present on a particular organic compound have different capacities for exchange with deuterium. Certain hydrogen atoms are easily exchangeable under physiological conditions and, if replaced by deuterium atoms, it is expected that they will readily exchange for protons after administration to a patient. Certain hydrogen atoms may be exchanged for deuterium atoms by the action of a deuteric acid such as D.sub.2SO.sub.4/D.sub.2O. Alternatively, deuterium atoms may be incorporated in various combinations during the synthesis of compounds of the invention. Certain hydrogen atoms are not easily exchangeable for deuterium atoms. However, deuterium atoms at the remaining positions may be incorporated by the use of deuterated starting materials or intermediates during the construction of compounds of the invention.
[0422] Deuterated and deuterium-enriched compounds of the invention can be prepared by using known methods described in the literature. Such methods can be carried out utilizing corresponding deuterated and optionally, other isotope-containing reagents and/or intermediates to synthesize the compounds delineated herein, or invoking standard synthetic protocols known in the art for introducing isotopic atoms to a chemical structure. Relevant procedures and intermediates are disclosed, for instance in Lizondo, J et al., Drugs Fut, 21(11), 1116 (1996); Brickner, S J et al., J Med Chem, 39(3), 673 (1996); Mallesham, B et al., Org Lett, 5(7), 963 (2003); PCT publications WO1997010223, WO2005099353, WO1995007271, WO2006008754; U.S. Pat. Nos. 7,538,189; 7,534,814; 7,531,685; 7,528,131; 7,521,421; 7,514,068; 7,511,013; and US Patent Application Publication Nos. 20090137457; 20090131485; 20090131363; 20090118238; 20090111840; 20090105338; 20090105307; 20090105147; 20090093422; 20090088416; 20090082471, the methods are hereby incorporated by reference.
[0423] The present invention further relates to a pharmaceutical composition comprising a therapeutically effective amount of at least one compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof, or comprising at least one compound as defined in any of the preceding claims wherein at least one of the atoms has been replaced by its stable, non-radioactive isotope, preferably wherein at least one hydrogen atom has been replaced by a deuterium atom, in combination with at least one pharmaceutically acceptable carrier and/or auxiliary substance.
[0424] The present invention further relates to a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for use as a medicament.
[0425] The present invention also relates to a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the treatment of disorders which respond to the modulation of the 5-HT.sub.2C receptor.
[0426] The present invention also relates to the use of a compound I as defined above or of an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the manufacture of a medicament for the treatment of disorders which respond to the modulation of the 5-HT.sub.2C receptor, and to a method for treating disorders which respond to the modulation of the 5-HT.sub.2C receptor, which method comprises administering to a subject in need thereof at least one compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
[0427] The compounds of the present invention are modulators of the 5-HT.sub.2C receptor. Specifically, the compounds of formula I are agonists or partial agonists of the 5-HT.sub.2C receptor. Thus, in a specific embodiment, the invention relates to a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the treatment of disorders which respond to 5-HT.sub.2C receptor agonists, further to the use of a compound I as defined above or of an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for the manufacture of a medicament for the treatment of disorders which respond to 5-HT.sub.2C receptor agonists, and to a method for treating disorders which respond to 5-HT.sub.2C receptor agonists, which method comprises administering to a subject in need thereof at least one compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
[0428] Within the meaning of the invention, the term "disorder" denotes disturbances and/or anomalies which are as a rule regarded as being pathological conditions or functions and which can manifest themselves in the form of particular signs, symptoms and/or malfunctions. While the treatment according to the invention can be directed toward individual disorders, i.e. anomalies or pathological conditions, it is also possible for several anomalies, which may be causatively linked to each other, to be combined into patterns, i.e. syndromes, which can be treated in accordance with the invention.
[0429] In one aspect of the invention, the diseases to be treated are disorders are damage of the central nervous system, disorders of the central nervous system, eating disorders, ocular hypertension, cardiovascular disorders, gastrointestinal disorders and diabetes.
[0430] Disorders or diseases of the central nervous system are understood as meaning disorders which affect the spinal cord and, in particular, the brain. These are, for example, cognitive dysfunction, attention deficit disorder/hyperactivity syndrome and cognitive deficits related with schizophrenia, attention deficit/hyperactivity syndrome, personality disorders, affective disorders, motion or motor disorders, pain, migraine, sleep disorders (including disturbances of the Circadian rhythm), feeding disorders, diseases associated with neurodegeneration, addiction diseases, obesity or psoriasis.
[0431] Examples of cognitive dysfunction are deficits in memory, cognition, and learning, Alzheimer's disease, age-related cognitive decline, and mild cognitive impairment, or any combinations thereof. Examples of personality disorders are schizophrenia and cognitive deficits related to schizophrenia. Examples of affective disorders are depression, anxiety, bipolar disorder and obsessive compulsive disorders, or any combination thereof. Examples of motion or motor disorders are Parkinson's disease and epilepsy. Examples of feeding disorders are obesity, bulimia, weight loss and anorexia, especially anorexia nervosa. Examples of diseases associated with neurodegeneration are stroke, spinal or head trauma, and head injuries, such as hydrocephalus.
[0432] Pain condition includes nociceptive pain, neuropathic pain or a combination thereof. Such pain conditions or disorders can include, but are not limited to, post-operative pain, osteoarthritis pain, pain due to inflammation, rheumatoid arthritis pain, musculoskeletal pain, burn pain (including sunburn), ocular pain, the pain associated with dental conditions (such as dental caries and gingivitis), post-partum pain, bone fracture, herpes, HIV, traumatic nerve injury, stroke, post-ischemia, fibromyalgia, reflex sympathetic dystrophy, complex regional pain syndrome, spinal cord injury, sciatica, phantom limb pain, diabetic neuropathy, hyperalgesia and cancer.
[0433] In certain other embodiments, the disease condition is bladder dysfunction, including urinary incontinence.
[0434] Diabetes includes diabetes insipidus, diabetes mellitus, type I diabetes, type II diabetes, type III diabetes, diabetes secondary to pancreatic diseases, diabetes related to steroid use, diabetes complications, hyperglycemia and insulin resistance.
[0435] The addiction diseases include psychiatric disorders and behavioral disturbances which are caused by the abuse of psychotropic substances, such as pharmaceuticals or narcotics, and also other addiction diseases, such as addiction to gaming (impulse control disorders not elsewhere classified). Examples of addictive substances are: opioids (e.g. morphine, heroin and codeine), cocaine; nicotine; alcohol; substances which interact with the GABA chloride channel complex, sedatives, hypnotics and tranquilizers, for example benzodiazepines; LSD; cannabinoids; psychomotor stimulants, such as 3,4-methylenedioxy-N-methylamphetamine (ecstasy); amphetamine and amphetamine-like substances such as methylphenidate, other stimulants including caffeine and nicotine. Addictive substances which come particularly into consideration are opioids, cocaine, amphetamine or amphetamine-like substances, nicotine and alcohol. Especially, addiction disorders include alcohol abuse, cocaine abuse, tobacco abuse and smoking cessation.
[0436] With regard to the treatment of addiction diseases, particular preference is given to those compounds according to the invention of the formula (I) which themselves do not possess any psychotropic effect. This can also be observed in a test using rats, which, after having been administered compounds which can be used in accordance with the invention, reduce their self administration of psychotropic substances, for example cocaine.
[0437] Examples of gastrointestinal disorders are irritable bowel syndrome.
[0438] Preferably, the disorders are selected from the group consisting of bipolar disorder, depression, atypical depression, mood episodes, adjustment disorders, anxiety, panic disorders, post-traumatic syndrome, psychoses, schizophrenia, cognitive deficits of schizophrenia, memory loss, dementia of aging, Alzheimer's disease, behavioral disorders associated with dementia, social phobia, mental disorders in childhood, attention deficit hyperactivity disorder, organic mental disorders, autism, mutism, disruptive behavior disorder, impulse control disorder, borderline personality disorder, obsessive compulsive disorder, migraine and other conditions associated with cephalic pain or other pain, raised intracranial pressure, seizure disorders, epilepsy, substance use disorders, alcohol abuse, cocaine abuse, tobacco abuse, smoking cessation, sexual dysfunction/erectile dysfunction in males, sexual dysfunction in females, premenstrual syndrome, late luteal phase syndrome, chronic fatigue syndrome, sleep disorders, sleep apnoea, chronic fatigue syndrome, psoriasis, Parkinson's disease, spinal cord injury, trauma, stroke, pain, bladder dysfunction/urinary incontinence, encephalitis, meningitis, eating disorders, obesity, bulimia, weight loss, anorexia nervosa, ocular hypertension, cardiovascular disorders, gastrointestinal disorders, diabetes insipidus, diabetes mellitus, type I diabetes, type II diabetes, type III diabetes, diabetes secondary to pancreatic diseases, diabetes related to steroid use, diabetes complications, hyperglycemia and insulin resistance, and are specifically schizophrenia, depression, bipolar disorders, obesity or substance use disorders.
[0439] The compounds of the invention may be used for a preventive treatment (prophylaxis), in particular as relapse prophylaxis or phase prophylaxis, but are preferably used for a treatment in its proper sense, i.e. for the treatment of acute or chronic signs, symptoms and/or malfunctions. The treatment can be orientated symptomatically, for example as the suppression of symptoms. It can be effected over a short period, be orientated over the medium term or can be a long-term treatment, for example within the context of a maintenance therapy.
[0440] In another embodiment, the present invention relates to the use of a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for preparing a medicament for preventing (the development of) a disease condition as described above and to a method for preventing (the development of) a disease condition as described above comprises administering to the subject in need of treatment thereof (e.g., a mammal, such as a human) a therapeutically effective amount of a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof. As used herein, the term "prevent" a disease condition by administration of any of the compounds described herein means that the detectable physical characteristics or symptoms of the disease or condition do not develop following the administration of the compound described herein. Alternatively, the method comprises administering to the subject a therapeutically effective amount of a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof, in combination with a therapeutically effective amount of at least one cognitive enhancing drug.
[0441] In yet another embodiment, the present invention relates to the use a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof for preparing a medicament for preventing the progression (e.g., worsening) of a disease condition and to a method for preventing the progression (e.g., worsening) of a disease condition, which method comprises administering to the subject in need of treatment thereof (e.g., a mammal, such as a human) a therapeutically effective amount of a compound I as defined above or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof.
[0442] There are several lines of evidence suggesting that 5-HT.sub.2C agonists or partial agonists would have therapeutic use in a variety of diseases, disorders and conditions.
[0443] Knockout mice models lacking the 5-HT.sub.2C receptor exhibit hyperphagia, obesity and are more prone to seizures and sudden death [Tecott L H, Sun L M, Akana S F, Strack A M, Lowenstein D H, Dallman M F, Julius D (1995) Eating disorder and epilepsy in mice lacking 5-HT.sub.2C serotonin receptors. Nature 374:542-546]. They also exhibit compulsive-like behavior [Chou-Green J M, Holscher T D, Dallman M F, Akana S F (2003). Compulsive behavior in the 5-HT.sub.2C receptor knockout mouse. Phys. Behav. 78:641-649], hyperresponsiveness to repeated stress [Chou-Green J M, Holscher T D, Dallman M F, Akana S F (2003). Repeated stress in young and old 5-HT.sub.2C receptor knockout mouse. Phys. Behav. 79:217-226], wakefulness [Frank M G, Stryker M P, Tecott L H (2002). Sleep and sleep homeostasis in mice lacking the 5-HT.sub.2C receptor. Neuropsychopharmacology 27:869-873], hyperactivity and drug dependence [Rocha BA, Goulding E H, O'Dell L E, Mead A N, Coufal N G, Parsons L H, Tecott L H (2002). Enhanced locomotor, reinforcing and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J. Neurosci. 22:10039-10045].
[0444] 5-HT.sub.2C is unique among other G-protein-coupled receptors (GPCRs) in that its pre-mRNA is a substrate for base modification via hydrolytic deamination of adenosines to yield inosines. Five adenosines, located within a sequence encoding the putative second intracellular domain can be converted to inosines. This editing can alter the coding potential of the triplet codons and allows for the generation of multiple different receptor isoforms. The edited receptor isoforms were shown to have reduced ability to interact with G-proteins in the absence of agonist stimulation [Werry, TD, Loiacono R, Sexton P A, Christopoulos A (2008). RNA editing of the serotonin 5-HT.sub.2C receptor and its effects on cell signaling, pharmacology and brain function. Pharmac. Therap. 119:7-23].
[0445] Edited 5-HT.sub.2C isoforms with reduced function are significantly expressed in the brains of depressed suicide victims [Schmauss C (2003) Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist 9:237-242. Iwamoto K, Kato T (2003). RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neurosci. Lett. 346:169-172] and in the learned helplessness rats (a well established animal model of depression) [Iwamotoa K, Nakatanib N, Bundoa M, Yoshikawab T, Katoa T (2005). Altered RNA editing of serotonin 2C receptor in a rat model of depression. Neurosci. Res. 53: 69-76] suggesting a link between 5-HT.sub.2C function and depression. There are also implications of edited 5-HT.sub.2C isoforms and spatial memory [Du Y, Stasko M, Costa A C, Davissone M T, Gardiner K J (2007). Editing of the serotonin 2C receptor pre-mRNA Effects of the Morris Water Maze. Gene 391:186-197]. In addition, fully edited isoforms of the human 5-HT.sub.2C receptor display a striking reduction in sensitivity to lysergic acid diethylamide (LSD) and to atypical antipsychotic drugs clozapine and loxapine, suggesting a possible role of the receptor in the etiology and pharmacology of schizophrenia [Niswender C M, Herrick-Davis K,. Dilley G E, Meltzer H Y, Overholser J C, Stockmeier C A, Emeson R B, Sanders-Bush E (2001). RNA Editing of the Human Serotonin 5-HT.sub.2C Receptor: Alterations in Suicide and Implications for Serotonergic Pharmacotherapy. Neuropsychopharm. 24:478-491].
[0446] Recently, the availability of potent and selective 5-HT.sub.2C receptor agonists made it possible to directly investigate the effects of 5-HT.sub.2C agonists and their therapeutic potential. Thus recent studies demonstrated that selective 5-HT.sub.2C agonists resulted in decreased food intake and body weight gain in normal and obese rats [Smith B M, et al. (2008). Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT.sub.2C receptor agonist for the treatment of obesity. J Med Chem 51:305-313. Thomsen W J, Grottick A J, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shama H, Smith B, Chalmers D, Behan D (2008) Lorcaserin, A Novel Selective Human 5-HT.sub.2C Agonist: In Vitro and In Vivo Pharmacological Characterization. J Pharmacol Exp Ther. 325:577-587. Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison B L, Sabb A, Sabalski J, Stack G, Welmaker G, Barrett J E, Dunlop J (2006) Antiobesity-like effects of the 5-HT.sub.2C receptor agonist WAY-161503. Brain Res. 1073-1074:240-251. Dunlop J, Sabb A L, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel R L, Stack G, Schechter L, Harrison B L, Rosenzweig-Lipson S (2005). WAY-163909 [97bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,l- hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther. 313:862-869.].
[0447] Furthermore, selective 5-HT.sub.2C receptor agonists produce antidepressant effects in animal models of depression comparable to those of SSRIs but with a much faster onset of action and a therapeutic window that avoids antidepressant-induced sexual dysfunction. These agonists were also effective in animal models of compulsive behavior such as scheduled induced polydipsia and they also exhibited decreased hyperactivity and aggression in rodents [Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg J E, Grauer S, Brennan J, Cryan J F, Sukoff Rizzo S J, Dunlop J, Barrett J E, Marquis K L (2007) Antidepressant-like effects of the novel, selective, 5-HT.sub.2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berlin) 192:159-170. Rosenzweig-Lipson S, Dunlop J, Marquis K L (2007) 5-HT.sub.2C receptor agonists as an innovative approach for psychiatric disorders. Drug news Perspect, 20: 565-571. Cryan, J F, Lucki I (2000). Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine 2C receptors. J. Pharm. Exp. Ther. 295:1120-1126.].
[0448] Acute or chronic administration of 5-HT.sub.2C agonists decreases the firing rate of ventral tegmental area dopamine neurons but not that of substantia nigra. In addition 5-HT.sub.2c agonists reduce dopamine levels in the nucleus accumbens but not in the striatum (the region of the brain mostly associated with extrapyramidal side effects) [Di Matteo, V., Di Giovanni, G., Di Mascio, M., & Esposito, E. (1999). SB 242084, a selective serotonin 2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38, 1195-1205. Di Giovanni, G., Di Matteo, V., Di Mascio, M., & Esposito, E. (2000). Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 35, 53-61. Marquis K L, Sabb A L, Logue S F, Brennan J A, Piesla M J, Comery T A, Grauer S M, Ashby C R, Jr., Nguyen H Q, Dawson L A, Barrett J E, Stack G, Meltzer H Y, Harrison B L, Rosenzweig-Lipson S (2007) WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino- [6,7,lhi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486-496.]. Therefore it is expected that 5-HT.sub.2C receptor agonists will selectively decrease mesolimibic dopamine levels without affecting the nigrostriatal pathway thus avoiding the EPS side effects of typical antipsychotics. Several 5-HT.sub.2C receptor agonists have shown antipsychotic activity in animal models of schizophrenia without EPS based on the lack of effect in catalepsy [Marquis K L, Sabb A L, Logue S F, Brennan J A, Piesla M J, Comery T A, Grauer S M, Ashby C R, Jr., Nguyen H Q, Dawson L A, Barrett J E, Stack G, Meltzer H Y, Harrison B L, Rosenzweig-Lipson S (2007) WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino- [6,7,lhi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486-496. Siuciak J A, Chapin D S, McCarthy S A, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour P A, Swick A, Iredale P A (2007) CP-809,101, a selective 5-HT.sub.2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52:279-290]. The antipsychotic activity of 5-HT.sub.2C receptor agonists without EPS coupled with their beneficial effects in mood disorders and cognition and their antiobesity like effects render 5-HT.sub.2C receptor agonists as unique agents to treat schizophrenia [Rosenzweig-Lipson S, Dunlop J, Marquis K L (2007) 5-HT.sub.2C receptor agonists as an innovative approach for psychiatric disorders. Drug news Perspect, 20: 565-571. Dunlop J, Marquis K L, Lim H K, Leung L, Kao J, Cheesman C, Rosenzweig-Lipson S (2006). Pharmacological profile of the 5-HT.sub.2C receptor agonist WAY-163909; therapeutic potential in multiple indications. CNS Dug Rev. 12:167-177.].
[0449] In addition 5-HT.sub.2C modulation has been implicated in epilepsy [Isaac M (2005). Serotonergic 5-HT.sub.2C receptors as a potential therapeutic target for the antiepileptic drugs. Curr. Topics fed. Chem. 5:59:67], psoriasis [Thorslund K, Nordlind K (2007). Serotonergic drugs--a possible role in the treatment of psoriasis? Drug News Perspect 20:521-525], Parkinson's disease and related motor disorders [Esposito E, Di Matteo V, Pierucci M, Benigno A, Di Giavanni, G (2007). Role of central 5-HT.sub.2C receptor in the control of basal ganglia functions. The Basal Ganglia Pathophysiology: Recent Advances 97-127], behavioral deficits [Barr A M, Lahmann-Masten V, Paulus M, Gainetdinov R P, Caron M G, Geyer M A (2004). The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology 29:221-228], anxiety [Dekeyne A, Mannoury la Cour C, Gobert A, Brocco M, Lejuene F, Serres F, Sharp T, Daszuta A, Soumier A, Papp M, Rivet J M, Flik G, Crerners T I, Muller O, Lavielle G, Millan M J (2208). S32006, a novel 5-HT.sub.2C receptor antagonists displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology 199:549-568. Nunes-de-Souza V, Nunes-de-Souza R L, Rodgers R J, Canto-de-Souza A (2008). 5-HT2 receptor activation in the midbrain periaqueductal grey (PAG) reduces anxiety-like behavior in mice. Behav. Brain Res. 187:72-79.], migraine [Leone M, Rigamonti A, D'Amico D, Grazzi L, Usai S, Bussone G (2001). The serotonergic system in migraine. Journal of Headache and Pain 2(Suppl. 1):S43-S46], Alzheimer's disease [Arjona A A, Pooler A M, Lee R K, Wurtman R J (2002). Effect of a 5-HT.sub.2C serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain Res. 951:135-140], pain and spinal cord injury [Nakae A, Nakai K, Tanaka T, Hagihira S, Shibata M, Ueda K, Masimo T (2008). The role of RNA editing of the serotonin 2C receptor in a rat model of oro-facial neuropathic pain. The European Journal of Neuroscience 27:2373-2379. Nakae A, Nakai K, Tanaka T, Takashina M, Hagihira S, Shibata M, Ueda K, Mashimo T (2008). Serotonin 2C receptor mRNA editing in neuropathic pain model. Neurosci. Res. 60:228-231. Kao T, Shumsky J S, Jacob-Vadakot S, Timothy H B, Murray M, Moxon, K A (2006). Role of the 5-HT.sub.2C receptor in improving weight-supported stepping in adult rats spinalized as neonates. Brain Res. 1112:159-168.], sexual dysfunction [Motofei I G (2008). A dual physiological character for sexual function: the role of serotonergic receptors. BJU international 101:531-534. Shimada I, Maeno K, Kondoh Y, Kaku H, Sugasawa K, Kimura Y, Hatanaka K; Naitou Y, Wanibuchi F, Sakamoto S; Tsukamoto S (2008). Synthesis and structure-activity relationships of a series of benzazepine derivatives as 5-HT.sub.2C receptor agonists. Bioorg. Med. Chem. 16:3309-3320.], smoking cessation [Fletcher P J, Le A D, Higgins G A (2008). Serotonin receptors as potential targets for modulation of nicotine use and dependence. Progress Brain Res. 172:361-83], substance dependence [Bubar M J, Cunningham K A (2008). Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Progress Brain Res. 172:319-46], and ocular hypertension [Sharif N A, McLaughlin M A, Kelly C R (2006). AL-34662: a potent, selective, and efficacious ocular hypotensive serotonin-2 receptor agonist. J Ocul Pharmacol Ther. 23:1-13].
[0450] Further, 5HT modulation can be useful in the treatment of pain, both neuropathic and nociceptive pain, see for example U.S. Patent application publication US2007/0225277. Obata, Hideaki; Ito, Naomi; Sasaki, Masayuki; Saito, Shigeru; Goto, Fumio. Possible involvement of spinal noradrenergic mechanisms in the antiallodynic effect of intrathecally administered 5-HT2C receptor agonists in the rats with peripheral nerve injury. European Journal of Pharmacology (2007), 567(1-2), 89-94. Serotonin2C receptor mRNA editing in neuropathic pain model. Nakae, Aya; Nakai, Kunihiro; Tanaka, Tatsuya; Takashina, Masaki; Hagihira, Satoshi; Shibata, Masahiko; Ueda, Koichi; Mashimo, Takashi. Department of Anesthesiology & Intensive Care Medicine, Graduate School of Medicine, Osaka University, Neuroscience Research (Amsterdam, Netherlands) (2008), 60(2), 228-231. Antiallodynic effects of intrathecally administered 5-HT2C receptor agonists in rats with nerve injury. Obata, Hideaki; Saito, Shigeru; Sakurazawa, Shinobu; Sasaki, Masayuki; Usui, Tadashi; Goto, Fumio. Department of Anesthesiology, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan. Pain (2004), 108(1-2), 163-169. Influence of 5,7-dihydroxytryptamine (5,7-DHT) on the antinociceptive effect of serotonin (5-HT) 5-HT2C receptor agonist in male and female rats. Brus, Ryszard; Kasperska, Alicja; Oswiecimska, Joanna; Szkilnik, Ryszard. Department of Pharmacology, Silesian Medical University, Zabrze, Pol. Medical Science Monitor (1997), 3(5), 654-656.
[0451] Modulation of 5HT2 receptors may be beneficial in the treatment of conditions related to bladder function, in particular, urinary incontinence. [Discovery of a novel azepine series of potent and selective 5-HT2C agonists as potential treatments for urinary incontinence. Brennan, Paul E.; Whitlock, Gavin A.; Ho, Danny K. H.; Conlon, Kelly; McMurray, Gordon. Bioorganic & Medicinal Chemistry Letters (2009), 19(17), 4999-5003. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anesthetized female rat. Mbaki, Y.; Ramage, A. G. Department of Pharmacology, University College London, London, UK. British Journal of Pharmacology (2008), 155(3), 343-356.] In particular, compounds with agonist activity at 5-HT.sub.2C have been shown to be useful in treating urinary incontinence, see for example U.S. Patent application publications US2008/0146583 and US 2007/0225274.
[0452] In the use and the method of the invention, an effective quantity of one or more compounds, as a rule formulated in accordance with pharmaceutical and veterinary practice, is administered to the individual to be treated, preferably a mammal, in particular a human being, productive animal or domestic animal. Whether such a treatment is indicated, and in which form it is to take place, depends on the individual case and is subject to medical assessment (diagnosis) which takes into consideration signs, symptoms and/or malfunctions which are present, the risks of developing particular signs, symptoms and/or malfunctions, and other factors.
[0453] Actual dosage levels of active ingredients in the pharmaceutical compositions of the present invention can be varied so as to obtain an amount of the active compound(s) that is effective to achieve the desired therapeutic response for a particular subject (e.g., a mammal, preferably, a human (patient)), compositions and mode of administration. The selected dosage level will depend upon the activity of the particular compound, the route of administration, the severity of the condition being treated and the condition and prior medical history of the patient being treated. However, it is within the skill of the art to start doses of the compound at levels lower than required to achieve the desired therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
[0454] Compounds of the present invention can also be administered to a subject as a pharmaceutical composition comprising the compounds of interest in combination with at least one pharmaceutically acceptable carriers. The phrase "therapeutically effective amount" of the compound of the present invention means a sufficient amount of the compound to treat disorders, at a reasonable benefit/risk ratio applicable to any medical treatment. It will be understood, however, that the total daily usage of the compounds and compositions of the present invention will be decided by the attending physician within the scope of sound medical judgment. The specific therapeutically effective dose level for any particular patient will depend upon a variety of factors including the disorder being treated and the severity of the disorder; activity of the specific compound employed; the specific composition employed; the age, body weight, general health, sex and diet of the patient; the time of administration, route of administration, and rate of excretion of the specific compound employed; the duration of the treatment; drugs used in combination or coincidental with the specific compound employed; and like factors well-known in the medical arts. For example, it is well within the skill of the art to start doses of the compound at levels lower than required to achieve the desired therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
[0455] The total daily dose of the compounds of this invention administered to a subject (namely, a mammal, such as a human) ranges from about 0.01 mg/kg body weight to about 100 mg/kg body weight. More preferable doses can be in the range of from about 0.01 mg/kg body weight to about 30 mg/kg body weight. If desired, the effective daily dose can be divided into multiple doses for purposes of administration. Consequently, single dose compositions may contain such amounts or submultiples thereof to make up the daily dose.
[0456] In one aspect, the present invention provides pharmaceutical compositions. The pharmaceutical compositions of the present invention comprise the compounds of the present invention or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt or solvate thereof. The pharmaceutical compositions of the present invention comprise compounds of the present invention that can be formulated together with at least one non-toxic pharmaceutically acceptable carrier.
[0457] In yet another embodiment, the present invention provides a pharmaceutical composition comprising compounds of the present invention or an N-oxide, a tautomeric form, a stereoisomer or a pharmaceutically acceptable salt thereof, and one or more pharmaceutically acceptable carriers, alone or in combination with one or more compounds that are not the compounds of the present invention. Examples of one or more compounds that can be combined with the compounds of the present invention in pharmaceutical compositions, include, but are not limited to, one or more cognitive enhancing drugs.
[0458] The pharmaceutical compositions of this present invention can be administered to a subject (e.g., a mammal, such as a human) orally, rectally, parenterally, intracisternally, intravaginally, intraperitoneally, topically (as by powders, ointments or drops), bucally or as an oral or nasal spray. The term "parenterally" as used herein, refers to modes of administration which include intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular injection and infusion.
[0459] The term "pharmaceutically acceptable carrier" as used herein, means a non-toxic, inert solid, semi-solid or liquid filler, diluent, encapsulating material or formulation auxiliary of any type. Some examples of materials which can serve as pharmaceutically acceptable carriers are sugars such as, but not limited to, lactose, glucose and sucrose; starches such as, but not limited to, corn starch and potato starch; cellulose and its derivatives such as, but not limited to, sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients such as, but not limited to, cocoa butter and suppository waxes; oils such as, but not limited to, peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; glycols; such a propylene glycol; esters such as, but not limited to, ethyl oleate and ethyl laurate; agar; buffering agents such as, but not limited to, magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as well as other non-toxic compatible lubricants such as, but not limited to, sodium lauryl sulfate and magnesium stearate, as well as coloring agents, releasing agents, coating agents, sweetening, flavoring and perfuming agents, preservatives and antioxidants can also be present in the composition, according to the judgment of the formulator.
[0460] Pharmaceutical compositions of the present invention for parenteral injection comprise pharmaceutically acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions or emulsions as well as sterile powders for reconstitution into sterile injectable solutions or dispersions just prior to use. Examples of suitable aqueous and nonaqueous carriers, diluents, solvents or vehicles include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol and the like), vegetable oils (such as olive oil), injectable organic esters (such as ethyl oleate) and suitable mixtures thereof. Proper fluidity can be maintained, for example, by the use of coating materials such as lecithin, by the maintenance of the required particle size in the case of dispersions and by the use of surfactants.
[0461] These compositions may also contain adjuvants such as preservatives, wetting agents, emulsifying agents and dispersing agents. Prevention of the action of microorganisms can be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol, phenol sorbic acid and the like. It may also be desirable to include isotonic agents such as sugars, sodium chloride and the like. Prolonged absorption of the injectable pharmaceutical form can be brought about by the inclusion of agents which delay absorption such as aluminum monostearate and gelatin.
[0462] In some cases, in order to prolong the effect of the drug, it is desirable to slow the absorption of the drug from subcutaneous or intramuscular injection. This can be accomplished by the use of a liquid suspension of crystalline or amorphous material with poor water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle.
[0463] Injectable depot forms are made by forming microencapsule matrices of the drug in biodegradable polymers such as polylactide-polyglycolide. Depending upon the ratio of drug to polymer and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include poly(orthoesters) and poly(anhydrides). Depot injectable formulations are also prepared by entrapping the drug in liposomes or microemulsions which are compatible with body tissues.
[0464] The injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium just prior to use.
[0465] Solid dosage forms for oral administration include capsules, tablets, pills, powders and granules. In such solid dosage forms, the active compound may be mixed with at least one inert, pharmaceutically acceptable excipient or carrier, such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol and silicic acid; b) binders such as carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidone, sucrose and acacia; c) humectants such as glycerol; d) disintegrating agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates and sodium carbonate; e) solution retarding agents such as paraffin; f) absorption accelerators such as quaternary ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol monostearate; h) absorbents such as kaolin and bentonite clay and i) lubricants such as talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate and mixtures thereof. In the case of capsules, tablets and pills, the dosage form may also comprise buffering agents.
[0466] Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such carriers as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
[0467] The solid dosage forms of tablets, dragees, capsules, pills and granules can be prepared with coatings and shells such as enteric coatings and other coatings well-known in the pharmaceutical formulating art. They may optionally contain opacifying agents and may also be of a composition such that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions which can be used include polymeric substances and waxes.
[0468] The active compounds can also be in micro-encapsulated form, if appropriate, with one or more of the above-mentioned carriers.
[0469] Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, solutions, suspensions, syrups and elixirs. In addition to the active compounds, the liquid dosage forms may contain inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethyl formamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan and mixtures thereof.
[0470] Besides inert diluents, the oral compositions may also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring and perfuming agents.
[0471] Suspensions, in addition to the active compounds, may contain suspending agents as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar, tragacanth and mixtures thereof.
[0472] Compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable non-irritating carriers or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at room temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
[0473] Compounds of the present invention can also be administered in the form of liposomes. As is known in the art, liposomes are generally derived from phospholipids or other lipid substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid crystals which are dispersed in an aqueous medium. Any non-toxic, physiologically acceptable and metabolizable lipid capable of forming liposomes can be used. The present compositions in liposome form can contain, in addition to a compound of the present invention, stabilizers, preservatives, excipients and the like. The preferred lipids are natural and synthetic phospholipids and phosphatidyl cholines (lecithins) used separately or together.
[0474] Methods to form liposomes are known in the art. See, for example, Prescott, Ed., Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p. 33 et seq.
[0475] Dosage forms for topical administration of a compound of the present invention include powders, sprays, ointments and inhalants. The active compound may be mixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives, buffers or propellants which may be required. Ophthalmic formulations, eye ointments, powders and solutions are also contemplated as being within the scope of this invention.
[0476] The compounds of the present invention can be used in the form of pharmaceutically acceptable salts derived from inorganic or organic acids. The phrase "pharmaceutically acceptable salt" means those salts which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like and are commensurate with a reasonable benefit/risk ratio.
[0477] Pharmaceutically acceptable salts are well known in the art. For example, S. M. Berge et al. describe pharmaceutically acceptable salts in detail in (J. Pharmaceutical Sciences, 1977, 66: 1 et seq.). The salts can be prepared in situ during the final isolation and purification of the compounds of the invention or separately by reacting a free base function with a suitable organic acid. Representative acid addition salts include, but are not limited to acetate, adipate, alginate, citrate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate, camphorate, camphorsulfonate, digluconate, glycerophosphate, hemisulfate, heptanoate, hexanoate, fumarate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethansulfonate (isothionate), lactate, malate, maleate, methanesulfonate, nicotinate, 2-naphthalenesulfonate, oxalate, palmitoate, pectinate, persulfate, 3-phenylpropionate, picrate, pivalate, propionate, succinate, tartrate, thiocyanate, phosphate, glutamate, bicarbonate, p-toluenesulfonate and undecanoate. Also, the basic nitrogen-containing groups can be quaternized with such agents as lower alkyl halides such as, but not limited to, methyl, ethyl, propyl, and butyl chlorides, bromides and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl and diamyl sulfates; long chain halides such as, but not limited to, decyl, lauryl, myristyl and stearyl chlorides, bromides and iodides; arylalkyl halides like benzyl and phenethyl bromides and others. Water or oil-soluble or dispersible products are thereby obtained. Examples of acids which can be employed to form pharmaceutically acceptable acid addition salts include such inorganic acids as hydrochloric acid, hydrobromic acid, sulfuric acid, and phosphoric acid and such organic acids as acetic acid, fumaric acid, maleic acid, 4-methylbenzenesulfonic acid, succinic acid and citric acid.
[0478] Basic addition salts can be prepared in situ during the final isolation and purification of compounds of this invention by reacting a carboxylic acid-containing moiety with a suitable base such as, but not limited to, the hydroxide, carbonate or bicarbonate of a pharmaceutically acceptable metal cation or with ammonia or an organic primary, secondary or tertiary amine. Pharmaceutically acceptable salts include, but are not limited to, cations based on alkali metals or alkaline earth metals such as, but not limited to, lithium, sodium, potassium, calcium, magnesium and aluminum salts and the like and nontoxic quaternary ammonia and amine cations including ammonium, tetramethylammonium, tetraethylammonium, methylammonium, dimethylammonium, trimethylammonium, triethylammonium, diethylammonium, ethylammonium and the like. Other representative organic amines useful for the formation of base addition salts include ethylenediamine, ethanolamine, diethanolamine, piperidine, piperazine and the like.
[0479] The compounds of the present invention can exist in unsolvated as well as solvated forms, including hydrated forms, such as hemi-hydrates. In general, the solvated forms, with pharmaceutically acceptable solvents such as water and ethanol among others are equivalent to the unsolvated forms for the purposes of the invention.
[0480] The following examples serve to explain the invention without limiting it.
EXAMPLES
[0481] The compounds were either characterized via proton-NMR in d.sub.6-dimethylsulfoxide or d-chloroform or d.sub.4-methanol on a 400 MHz or 500 MHz NMR instrument (Bruker AVANCE), or by mass spectrometry, generally recorded via HPLC-MS in a fast gradient on C18-material (electrospray-ionisation (ESI) mode).
[0482] The magnetic nuclear resonance spectral properties (NMR) refer to the chemical shifts (.delta.) expressed in parts per million (ppm). The relative area of the shifts in the .sup.1H-NMR spectrum corresponds to the number of hydrogen atoms for a particular functional type in the molecule. The nature of the shift, as regards multiplicity, is indicated as singlet (s), broad singlet (s. br.), doublet (d), broad doublet (d br.), triplet (t), broad triplet (t br.), quartet (q), quintet (quint.) and multiplet (m).
[0483] Enantiomers were separated/purified either by chiral supercritical fluid chromatography (SFC) (method A) or by chiral HPLC (method B).
Method a--Chiral Analytical SFC
[0484] Analytical SFC was performed on an Aurora A5 SFC Fusion and Agilent 1100 system running under Agilent Chemstation software control. The SFC system included a 10-way column switcher, CO2 pump, modifier pump, oven, and backpressure regulator. The mobile phase comprised of supercritical CO2 supplied by a beverage-grade CO2 cylinder with a modifier mixture of methanol at a flow rate of 3 mL/min. Oven temperature was at 35.degree. C. and the outlet pressure at 150 bar. The UV detector was set to collect at wavelengths of 220 nm and 254 nm. The mobile phase gradient started with 5% modifier and held it for 0.1 minutes at a flow rate of 1 mL/min, then the flow rate was ramped up to 3 mL/min and held for 0.4 min. The modifier was ramped from 5% to 15% over the next 8 minutes at 3 mL/min then held for 1 minute at 15% modifier (3 mL/min). The gradient was ramped down from 15% to 5% modifier over 0.5 min (3 mL/min). The instrument was fitted with a Chiralpak AS-H column with dimensions of 4.6 mm i.d..times.150 mm length with 5 .mu.m particles.
Method B--Chiral HPLC
[0485] System: KNAUER preparative HPLC Pump: Preparative pump 1800 Detector: Smartline UV detector 2600 257 nm Sample pump: Knauer HPLC-Pump K-120 Fractional collector: Smartline Valves Drive S6
Software: ChromGate.RTM. V3.1.7, KNAUER Instrument Control
Column: Whelk O 4.6 mm ID.times.250 mm
[0486] Column temperature: 25.degree. C. Mobile phase: hexane/isopropylamine 95/5 Flow rate: 1 ml/min
I. PREPARATION EXAMPLES
Example 1
8,8-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-1 of Table B)
1.1 Preparation of tert-butyl 1-(3-methylbut-2-enoyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carbo- xylate
[0487] 1 g (4.04 mmol) of tert-butyl 2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate was dissolved in 20 mL of dichloromethane and treated with 1.54 mL (8.86 mL) of triethylamine followed by 0.53 mL (4.83 mmol) of 3-methylbut-2-enoyl chloride. The reaction mixture was stirred over night at room temperature, poured onto water (50 mL) and extracted three times with 50 mL of dichloromethane each. The organic phases were combined, washed with a saturated solution of sodium chloride, dried over magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 5-10% methanol in dichloromethane) to yield 1.27 g of the title compound as a white solid.
[0488] ESI-MS: m/z (%): 275.10 (100, [M-C.sub.4H.sub.9+H].sup.+).
1.2 Preparation of tert-butyl 8,8-dimethyl-6-oxo-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin- e-2(6H)-carboxylate
[0489] A solution of 0.33 g (1.0 mmol) of tert-butyl 1-(3-methylbut-2-enoyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carbo- xylate of step 1.1 was irradiated with a 150 W mercury lamp in an immersion well reactor with a pyrex filter in 20 mL of acetone until completion of the reaction monitored by liquid chromatography. The solution was concentrated in vacuo and the residue was purified by column chromatography on silica (eluent: 10-30% ethyl acetate in heptane) to yield 202 mg of the title compound as a beige solid.
[0490] ESI-MS: m/z (%): 275.10 (100, [M-C.sub.4H.sub.9+H].sup.+).
1.3 Preparation of tert-butyl 8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-2(6H- )-carboxylate
[0491] A solution of 38 mg (0.115 mmol) tert-butyl 8,8-dimethyl-6-oxo-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin- e-2(6H)-carboxylate of step 1.2 in 1 mL of tetrahydrofuran was treated with 0.46 mL of 1 molar solution of borohydride-tetrahydrofuran complex in tetrahydrofuran. The mixture was stirred over night, and then quenched with water and diluted hydrochloric acid. The pH was adjusted to pH 9 by addition of aqueous sodium hydroxide solution and the mixture extracted three times with 10 mL of dichloromethane each. The solution was dried over magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 20-30% ethyl acetate in heptane) to yield 21 mg of the title compound as a clear oil.
[0492] ESI-MS: m/z (%): 317.20 (100, [M+H].sup.+).
1.4 Preparation of 8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline
[0493] 287 mg (0.9 mmol) of tert-butyl 8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-2(6H- )-carboxylate of step 1.3 were dissolved in 5 mL of dichloromethane and treated with 2.5 mL of trifluoroacetic acid. The mixture was stirred over night at room temperature and then extracted once with water (10 mL). The organic phase was then extracted twice with a diluted solution of sodium hydroxide (10 mL each), dried over magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 10-30% methanol in dichloromethane) to yield 141 mg of the title compound as a yellow oil.
[0494] ESI-MS: m/z (%): 217.15 (100, [M+H].sup.+).
[0495] .sup.1H-NMR (500 MHz, CDCl.sub.3): .delta.=7.15 (d, 1H), 6.85 (d, 1H), 6.75 (m, 1H), 3.80 (s, 2H), 3.15 (m, 2H), 3.00 (s, 4H), 1.65 (m, 2H), 1.20 (s, 6H).
Example 2
1,2,3,4-Tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopent[2- ]en]-6(7H)-one 2,2,2-trifluoroacetate (Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-481 of Table B)
2.1 Preparation of tert-butyl 6-oxo-3,4,6,7-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cycl- opent[2]ene]-2(1H)-carboxylate (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-401 of Table B, Wherein However R.sup.1 is not H, but Boc)
[0496] A solution of 50 mg (0.128 mmol) tert-butyl 9-chloro-1-(2-cyclopentenylacetyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-- 4(5H)-carboxylate was dissolved in 1 mL of degassed dry acetonitrile under argon, treated with 1.4 mg (0.006 mmol) of palladium acetate, 6.10 mg (0.013 mmol) dicyclohexyl(2',4',6'-triisopropylbiphenyl-2-yl)phosphine and 53 mg (0.384 mmol) potassium carbonate. The mixture was stirred for 10 hours at 120.degree. C. in a synthesis microwave system. After cooling to room temperature the mixture was filtered over celite, diluted with dichloromethane and then extracted twice with a diluted solution of sodium chloride (10 mL each), dried over magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 10-30% ethyl acetate in heptane) to yield 40 mg of the title compound as a yellow oil.
[0497] ESI-MS: m/z (%): 299.10 (100, [M-C.sub.4H.sub.9+H].sup.+).
2.2 Preparation of 1,2,3,4-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopent[- 2]en]-6(7H)-one 2,2,2-trifluoroacetate
[0498] Boc-deprotection of the compound obtained in step 2.1 analogously to example 1.4 yielded the title compound.
[0499] ESI-MS: m/z (%): 255 (100, [M+H].sup.+).
Example 3
8-Methyl-8-phenyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-6- (2H)-one, hydrochloride (Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-161 of Table B)
[0500] 392 mg (1 mmol) of tert-butyl 1-(3-phenylbut-2-enoyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carbo- xylate were treated with 1 g of polyphosphoric acid. The mixture was heated to 90.degree. C. for 2 hours and then quenched with a saturated solution of potassium carbonate at 0.degree. C. The mixture was extracted three times with ethyl acetate (20 mL each), the combined organic fractions dried over magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 10-30% methanol in dichloromethane) to yield 86 mg of the title compound as a yellow foam.
[0501] ESI-MS: m/z (%): 293.1 (100, [M+H].sup.+).
[0502] 1H-NMR (500 MHz, DMSO-d6): .delta.=10.05 (bs, 1H), 9.85 (bs, 1H), 7.40 (d, 1H), 7.35 (d, 1H), 7.25 (m, 2H), 7.20 (m, 2H), 7.10 (m, 2H), 4.55 (m, 1H), 4.35 (m, 1H), 4.15 (m, 1H), 3.55 (m, 1H), 3.25 (m, 2H), 3.10 (m, 1H), 2.70 (m, 1H), 1.60 (s, 3H).
[0503] The following example was prepared analogously to example 1.
Example 4
8,8-Dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-6(2H)-- one (Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-1 of Table B)
[0504] ESI-MS: m/z (%): 231 (100, [M+H].sup.+).
[0505] 1H-NMR (500 MHz, CDCl.sub.3): .delta.=7.20 (d, 1H), 7.00 (m, 2H), 4.10 (s, 4H), 3.20 (m, 2H), 2.50 (s, 2H), 1.30 (s, 6H).
[0506] The following examples 5 and 6 were prepared analogously to example 2.
Example 5
1,2,3,4-Tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopentan- ]-6(7H)-one (Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-361 of Table B)
[0507] ESI-MS: m/z (%): 257 (100, [M+H].sup.+).
Example 6
1,2,3,4,6,7-Hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopen- tane](Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-361 of Table B)
[0508] ESI-MS: m/z (%): 243 (100, [M+H].sup.+).
[0509] .sup.1H-NMR (500 MHz, CDCl.sub.3): .delta.=7.15 (d, 1H), 6.95 (d, 1H), 6.80 (m, 1H), 3.90 (s, 2H), 3.20 (m, 2H), 3.05 (m, 4H), 1.95-1.65 (m, 10H).
[0510] The following examples 7 to 13 were prepared analogously to example 1.
Example 7
12a-Methyl-4,5,6,7,10,11,12,12a-octahydrocyclopenta[c][1,4]diazepino[6,7,1- -ij]quinolin-9(9aH)-one (Racemic Cis Diastereomer of the Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-521 of Table B)
[0511] ESI-MS: m/z (%): 257 (100, [M+H].sup.+).
Example 8
8-Methyl-8-(trifluoromethyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1- -ij]quinoline (Single Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-81 of Table B)
[0512] ESI-MS: m/z (%): 271 (100, [M+H].sup.+).
[0513] The retention time according to method A is 1.396 min.
Example 9
8-Methyl-8-(trifluoromethyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1- -ij]quinoline (Single Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-81 of Table B)
[0514] ESI-MS: m/z (%): 271 (100, [M+H].sup.+).
[0515] The retention time according to method A is 1.543 min.
Example 10
[0516] 12a-Methyl-4,5,6,7,10,11,12,12a-octahydrocyclopenta[c][1,4]diazepin- o[6,7,1-ij]quinolin-9(9aH)-one (Racemic Trans Diastereomer of the Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-521 of Table B)
[0517] ESI-MS: m/z (%): 257 (100, [M+H].sup.+).
Example 11
12a-Methyl-4,5,6,7,9,9a, 10,11,12,12a-decahydrocyclopenta[c][1,4]diazepino[6,7,1-ij]quinoline (Racemic Cis Diastereomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-521 of Table B)
[0518] ESI-MS: m/z (%): 243 (100, [M+H].sup.+).
[0519] .sup.1H-NMR (500 MHz, CDCl.sub.3): .delta.=7.15 (d, 1H), 6.90 (d, 1H), 6.80 (m, 1H), 3.90 (m, 2H), 3.15 (m, 1H), 3.05 (m, 3H), 2.95 (m, 1H), 2.80 (m, 1H), 1.95 (m, 1H), 1.90 (m, 1H), 1.80 (m, 2H), 1.60 (m, 1H), 1.55 (m, 1H), 1.30 (m, 1H), 1.20 (s, 3H).
Example 12
8-Methyl-8-(trifluoromethyl)-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij- ]quinolin-6(2H)-one (Racemic Mixture of Compound of the Formula I.j, I.k or 1.1 in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-81 of Table B)
[0520] ESI-MS: m/z (%): 285 (100, [M+H]f).
Example 13
12a-Methyl-4,5,6,7,9,9a,10,11,12,12a-decahydrocyclopenta[c][1,4]diazepino-- [6,7,1-ij]quinoline Hydrochloride (Racemic Trans Diastereomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-521 of Table B)
[0521] ESI-MS: m/z (%): 243 (100, [M+H].sup.+).
[0522] .sup.1H-NMR (500 MHz, CDCl.sub.3): .delta.=9.45 (bs, 1H), 9.05 (bs, 1H), 7.05 (d, 1H), 7.00 (d, 1H), 6.70 (m, 1H), 4.30 (m, 1H), 3.95 (m, 1H), 3.55 (m, 2H), 3.35 (m, 1H), 3.25 (m, 2H), 2.95 (m, 1H), 1.95 (m, 1H), 1.85 (m, 1H), 1.80 (m, 1H), 1.70 (m, 1H), 1.55 (m, 1H), 1.40 (m, 1H), 1.25 (m, 1H), 0.90 (s, 3H).
[0523] The following examples 14 and 15 were prepared analogously to example 3.
Example 14
8-Methyl-8-phenyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoli- ne Hydrochloride (Racemic Mixture in Form of the Salt of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-161 of Table B)
[0524] ESI-MS: m/z (%): 279 (100, [M+H].sup.+)
[0525] .sup.1H-NMR (500 MHz, CDCl.sub.3): .delta.=7.22-7.27 (m, 2H), 7.11-7.19 (m, 3H), 6.95-7.04 (m, 2H), 6.78 (dd, J=8.0, 7.5 Hz, 1H), 3.99 (d, J=14.4 Hz, 1H), 3.87 99 (d, J=14.5 Hz, 1H), 3.20-3.26 (m, 1H), 3.01-3.08 (m, 4H), 2.87-2.94 (m, 1H), 2.09-2.15 (m, 1H), 1.92-1.99 (m, 1H), 1.73 (s, 3H) ppm.
Example 15
8-Methyl-8-phenyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoli- ne (Racemic Mixture of Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R and R.sup.9a is as in Row B-161 of Table B)
[0526] ESI-MS: m/z (%): 279 (100, [M+H].sup.+).
[0527] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 7.25 (m, 2H), 7.15 (m, 3H), 7.05 (d, 1H), 6.95 (d, 1H), 6.80 (m, 1H), 4.00 (m, 1H), 3.90 (m, 1H), 3.25 (m, 1H), 3.05 (m, 4H), 2.90 (m, 1H), 2.10 (m, 1H), 1.95 (m, 1H), 1.75 (s, 3H).
Example 16
(R)-5,6,7,9,9a, 10,11,12-Octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (2,2,2-trifluoroacetate) (R-Enantiomer of the Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row A-865 of Table A)
16.1 Preparation of (R)-tert-butyl 9-oxo-6,7,9a, 10,11,12-hexahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline-5- (9H)-carboxylate
[0528] 65 mg of (R)-tert-butyl 9-chloro-1-(pyrrolidine-2-carbonyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine- -4(5H)-carboxylate was stirred together with 1.8 mg of 2'-(di-tert-butylphosphino)-N,N-dimethylbiphenyl-2-amine, 2.5 mg Pd.sub.2(dba).sub.3 and 19 mg sodium 2-methylpropan-2-olate at 100.degree. C. over 10 hours in toluene. The reaction mixture was then cooled to room temperature and evaporated. The residue was purified by column chromatography on silica (eluent: 10-25% ethyl acetate in heptane) to yield 20 mg of the title compound as a beige solid.
[0529] ESI-MS: m/z (%): 344.20 (100, [M+H].sup.+).
16.2 Preparation of (R)-tert-butyl 6,7,9a, 10,11,12-hexahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline-5- (9H)-carboxylate (R-Enantiomer of the Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row A-121 of Table a, Wherein However R.sup.1 is not H, but Boc)
[0530] Reduction of the compound obtained in step 16.1 analogously to example 1.3 yielded the title compound.
[0531] ESI-MS: m/z (%): 330.20 (100, [M+H].sup.+).
16.3 Preparation of (R)-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-- a]quinoxaline bis(2,2,2-trifluoroacetate) (R-Enantiomer of the Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row A-121 of Table A)
[0532] Boc-deprotection of the compound obtained in step 16.2 analogously to example 1.4 yielded the title compound.
[0533] ESI-MS: m/z (%): 230.15 (100, [M+H].sup.+).
[0534] Example 17 was prepared analogously to example 16 employing (S)-tert-butyl 9-chloro-1-(pyrrolidine-2-carbonyl)-2,3-dihydro-1H-benzo[e][1,4]diazepine- -4(5H)-carboxylate as starting material which was derived from (R)-1-(((9H-fluoren-9-yl)methoxy)carbonyl)pyrrolidine-2-carboxylic acid.
Example 17
(S)-5,6,7,9,9a, 10,11,12-Octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (2,2,2-trifluoroacetate) (S-Enantiomer of the Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row A-865 of Table A)
[0535] ESI-MS: m/z (%): 230 (100, [M+H].sup.+).
[0536] The following examples 18 to 23 were prepared analogously to example 1.
Example 18
9-Chloro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qui- noline, (2,2,2-trifluoroacetate) (Compound of the Formula I.g in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R and R.sup.9a is as in Row B-3 of Table B)
[0537] ESI-MS: m/z (%): 251 (100, [M+H].sup.+).
[0538] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.95 (bs, 2H), 7.35 (d, 1H), 7.25 (d, 1H), 4.15 (s, 2H), 3.30-3.20 (m, 6H), 1.65 (m, 2H), 1.25 (s, 6H).
Example 19
10-Chloro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qu- inoline (2,2,2-trifluoroacetate) (Compound of the Formula I.h in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-3 of Table B)
[0539] ESI-MS: m/z (%): 251 (100, [M+H].sup.+).
[0540] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 9.10 (bs, 2H), 7.35 (m, 1H), 7.20 (m, 1H), 4.15 (s, 2H), 3.30-3.20 (m, 6H), 1.65 (m, 2H), 1.25 (s, 6H).
Example 20
9-Chloro-8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinol- in-6(2H)-one 2,2,2-trifluoroacetate (Compound of the Formula I.j in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-3 of Table B)
[0541] ESI-MS: m/z (%): 265 (100, [M+H].sup.+).
Example 21
10-Chloro-8,8-dimethyl-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quino- lin-6(2H)-one 2,2,2-trifluoroacetate (Compound of the Formula I.k in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-3 of Table B)
[0542] ESI-MS: m/z (%): 265 (100, [M+H].sup.+).
Example 22
9-Fluoro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qui- noline (Compound of the Formula I.g in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-2 of Table B)
[0543] ESI-MS: m/z (%): 235 (100, [M+H].sup.+).
[0544] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.95 (m, 1H), 6.55 (m, 1H), 3.95 (s, 2H), 3.20-3.10 (m, 6H), 1.70 (m, 2H), 1.40 (s, 6H).
Example 23
10-Fluoro-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qu- inoline (2,2,2-trifluoroacetate) (Compound of the Formula I.h in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-2 of Table B)
[0545] ESI-MS: m/z (%): 235 (100, [M+H].sup.+).
[0546] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 9.05 (bs, 2H), 7.05 (d, 1H), 6.80 (m, 1H), 4.20 (s, 2H), 3.35 (m, 2H), 3.30 (m, 3.25), 3.25 (m, 2H), 1.70 (m, 2H), 1.30 (s, 6H).
[0547] The following examples 24 to 26 were prepared analogously to example 2.
Example 24
1,2,3,4,6,7-Hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclohex- ane] (2,2,2-trifluoroacetate) (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-401 of Table B)
[0548] ESI-MS: m/z (%): 257.2 (100, [M+H].sup.+).
[0549] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 8.85 (bs, 2H), 7.45 (d, 1H), 7.15 (d, 1H), 6.90 (m, 1H), 4.15 (s, 2H), 3.25 (m, 4H), 3.15 (m, 2H), 1.80 (m, 2H), 1.70 (m, 4H), 1.55 (m, 4H), 1.30 (m, 2H).
Example 25
1',2',3',4',6',7'-Hexahydrospiro[cyclobutane-1,8'-[1,4]diazepino[6,7,1-ij]- quinoline], Hydrochloride (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-321 of Table B)
[0550] ESI-MS [M+H+]=229.20
[0551] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 8.80 (bs, 2H), 7.50 (d, 1H), 6.95 (d, 1H), 6.90 (m, 1H), 4.10 (s, 2H), 3.25 (m, 2H), 3.20 (m, 2H), 3.10 (m, 2H), 2.35 (m, 2H), 2.00 (m, 2H), 1.90 (m, 4H).
Example 26
9'-Fluoro-1',2',3',4',6',7'-hexahydrospiro[cyclobutane-1,8'-[1,4]diazepino- [6,7,1-ij]quinoline], Hydrochloride (Compound of the Formula I.g in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-322 of Table B)
[0552] ESI-MS [M+H+]=247.15
[0553] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): [ppm]: 9.00 (bs, 2H), 7.25 (m, 1H), 6.80 (m, 1H), 4.10 (s, 2H), 3.25 (m, 2H), 3.20 (m, 2H), 3.10 (m, 2H), 2.65 (m, 2H), 2.00 (m, 4H), 1.85 (m, 2H).
[0554] The following examples 27 and 28 were prepared analogously to example 1.
Example 27
Enantiomer of 7,8,8-trimethyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinolin- e](Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-25 of Table B)
[0555] ESI-MS [M+H+]=231.20
[0556] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 7.25 (d, 1H), 7.00 (d, 1H), 6.85 (dd, 1H), 4.00 (m, 1H), 3.90 (m, 1H), 3.15 (m, 4H), 3.05 (m, 2H), 1.85 (m, 1H), 1.30 (s, 3H), 1.10 (s, 3H), 0.95 (d, 3H).
[0557] The retention time of the Boc-protected precursor according to method B is 10.363.
Example 28
Enantiomer of 7,8,8-trimethyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinolin- e](Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-25 of Table B)
[0558] ESI-MS [M+H+]=231.20
[0559] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 7.25 (d, 1H), 7.00 (d, 1H), 6.85 (dd, 1H), 4.00 (m, 1H), 3.90 (m, 1H), 3.15 (m, 4H), 3.05 (m, 2H), 1.85 (m, 1H), 1.30 (s, 3H), 1.10 (s, 3H), 0.95 (d, 3H).
[0560] The retention time of the Boc-protected precursor according to method B is 11.410 min.
[0561] The following example 29 was prepared analogously to example 1.
Example 29
3-Methyl-1',2',3',4',6',7'-hexahydrospiro[cyclobutane-1,8'-[1,4]diazepino[- 6,7,1-ij]quinoline], Hydrochloride (Compound of the Formula I.p, I.q or I.r in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-321 of Table B)
[0562] ESI-MS [M+H+]=243.20
[0563] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): [ppm]: 9.30 (bs, 1H), 8.55 (bs, 1H), 7.60 (d, 1H), 7.20 (d, 1H), 6.95 (dd, 1H), 4.20 (m, 1H), 4.05 (m, 1H), 3.35 (m, 1H), 3.20 (m, 2H), 2.90 (m, 1H), 2.40 (m, 1H), 2.25 (m, 1H), 2.05 (m, 1H), 1.95 (m, 6H), 1.2 (d, 3H).
Example 30
3-Benzyl-2',3',7',8',9',10'-hexahydro-1H-3',8',10a'-triaza-cyclohepta[de]n- aphthalene (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row A-181 of Table A)
30.1 Preparation of 2-(4-benzyl-3,4-dihydro-2H-quinoxalin-1-yl)-acetamide
[0564] A solution of 1 g (4.46 mmol) 1-benzyl-1,2,3,4-tetrahydroquinoxaline in 10 ml of dimethylformamide was treated with 3.14 ml (18.15 mmol) of N-Ethyl-N-isopropylpropan-2-amine, and subsequently 863 mg (9.23 mmol) 2-chloroacetamide was added. The reaction mixture was heated in a microwave system at 100.degree. C. for four hours. The resulting mixture was quenched with water and extracted once with ethylacetate. The aqueous phase was set to pH 10 with sodium hydroxide solution and extracted 3.times. with ethylacetate. The combined organic extracts were dried with magnesium sulfate, evaporated till dryness and directly purified by column chromatography on silica (eluent: starting with heptane and then up to 100% ethylacetate) to yield 1.224 g of the title compound.
[0565] ESI-MS: m/z (%): 282.10 (100, [M+H].sup.+).
30.2 Preparation of 2-(4-Benzyl-3,4-dihydro-2H-quinoxalin-1-yl)-ethylamine
[0566] To 1.224 g (4.35 mmol) of 2-(4-benzyl-3,4-dihydro-2H-quinoxalin-1-yl)-acetamide were added 10.88 ml (21.75 mmol) borane dimethylsulfide THF solution and subsequently heated to 60.degree. C. for six hours in a microwave system. The mixture was quenched with 1 molar hydrochloric acid and methanol and heated for 15 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethylacetate and extracted 3.times. with 1 molar hydrochlorid acid. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and evaporated to yield 1.089 g of the title compound as an oil.
[0567] ESI-MS: m/z (%): 268.15 (100, [M+H].sup.+).
30.3 Preparation of 3-benzyl-2,3,7,8,9,10-hexahydro-1H-3,8,10a-triaza-cyclohepta[de]-naphthal- ene
[0568] A solution of 1.089 g (4.07 mmol) of 2-(4-benzyl-3,4-dihydro-2H-quinoxalin-1-yl)-ethylamine and 20 ml ethanol was treated with 122 mg (4.07 mmol) of formaldehyde and 511 mg (4.48 mmol) of trifluoroacetic acid and stirred over night at room temperature. The solvent was evaporated and dichloromethane was added to the crude mixture. The organic phase was washed with 1 molar sodium hydroxide solution. The organic phase was dried and purified by column chromatography on silica (eluent: starting with dichloromethane and then up to 100% methanol) to yield 448 mg of the title compound.
[0569] ESI-MS [M+H+]=280.10 (100, [M+H].sup.+).
[0570] .sup.1H NMR (CDCl.sub.3, 500 MHz): [ppm]: 7.35 (m, 3H), 7.30 (m, 2H), 6.80 (dd, 1H), 6.65 (d, 1H), 6.60 (d, 1H), 4.55 (s, 2H), 4.10 (s, 2H), 3.30 (m, 4H), 3.25 (m, 2H), 3.10 (m, 2H).
Example 31
9-Fluoro-1-methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quino- line-8,1'-cyclobutane], Trifluoroacetic Acid Salt (Compound of the Formula I.m in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-322 of Table B)
31.1 Preparation of 2-cyclobutylidene-N-(3-fluorophenyl)-acetamide
[0571] 1.5 g (13.50 mmol) of 3-fluoroaniline were dissolved in 20 ml of dichloromethane and treated with 5.19 g (29.7 mmol) of N-ethyl-N-isopropylpropan-2-amine. Within 10 min 12.82 g (16.20 mmol) of 2-cyclobutylideneacetyl chloride were added at 0.degree. C. and the solution was stirred overnight at room temperature. The mixture was poured on ice water and extracted 2.times. with dichloromethane. The combined organic phases were washed once with saturated sodium chloride solution, dried with MgSO.sub.4 and evaporated in vacuo. The crude oil was treated with heptane and decanted twice, redissolved in ether-dichloromethane and treated with heptane till crystallization, yielding 2.2 g (77%) of a beige solid.
[0572] ESI-MS: m/z (%): 206 (100, [M+H].sup.+).
31.2 Preparation of 5'-fluoro-1'H-spiro[cyclobutane-1,4'-quinolin]-2'(3'H)-one
[0573] A solution of 400 mg (1.94 mmol) of 2-cyclobutylidene-N-(3-fluorophenyl)-acetamide obtained in step 31.1 was dissolved in 19.5 ml of toluene and irradiated with a Hg middle pressure lamp in a suitable device till completion of the reaction indicated by liquid chromatography. The crude solution was concentrated in vacuo and the residue was purified by column chromatography on silica (eluent: 10-30% ethyl acetate in heptane) to yield 55 mg (14%) of the title compound as a beige solid.
[0574] ESI-MS: m/z (%): 206 (100, [M+H].sup.+).
31.3 Preparation of 5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinoline]
[0575] A solution of 50 mg (0.244 mmol) of 5'-fluoro-1'H-spiro[cyclobutane-1,4'-quinolin]-2'(3'H)-one obtained in step 31.2 in 1 mL of tetrahydrofuran was treated with 0.73 mL of 1 M solution of borohydride-tetrahydrofuran complex in tetrahydrofuran. The mixture was stirred at reflux for 2 h, quenched with water and evaporated till dryness. The crude compound was used in the subsequent step without purification.
[0576] ESI-MS: m/z (%): 192 (100, [M+H].sup.+).
31.4 Preparation of 2-(5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)-ac- etamide
[0577] A solution of 50 mg (0.26 mmol) of 5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinoline] obtained in step 31.3 in 1 ml of dimethylformamide was treated with 6.2 mg (0.26 mmol) of sodium hydride followed by 72 mg (0.52 mmol) 2-bromoacetamide and heated in a microwave system at 100.degree. C. for one hour. The resulting mixture was quenched with water, evaporated till dryness and directly purified by column chromatography on silica (eluent: 10% methanol in dichloromethane) to yield 70 mg of the title compound with about 80% purity determined by LCMS.
[0578] ESI-MS: m/z (%): 249 (100, [M+H].sup.+).
31.5 Preparation of 2-(5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)eth- anamine
[0579] A solution of 40 mg (0.16 mmol) of 2-(5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)-ac- etamide obtained in step 31.4 in 1 ml of tetrahydrofurane was treated with 38 .mu.l (0.38 mmol) of borane dimethylsulfide and heated to 70.degree. C. for two hours. The mixture was quenched with water and hydrochloric acid, diluted with dichloromethane and extracted twice with water. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and evaporated to yield 10 mg of the title compound as an oil.
[0580] ESI-MS: m/z (%): 235 (100, [M+H].sup.+).
31.6 Preparation of 9-fluoro-1-methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quin- oline-8,1'-cyclobutane], Trifluoroacetic Acid Salt
[0581] A solution of 10 mg (0.043 mmol) of 2-(5'-fluoro-2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)eth- an-amine obtained in step 31.5 in 0.4 ml of acetonitrile/methanol was treated with 3.8 mg (0.085 mmol) of acetaldehyde and 6.5 l (0.085 mmol) of trifluoroacetic acid and stirred over night at room temperature. The solvent was evaporated and the crude mixture purified with reversed phase liquid chromatography to yield 1.5 mg of the title compound as beige solid.
[0582] ESI-MS: m/z (%): 261 (100, [M+H].sup.+).
[0583] .sup.1H-NMR (500 MHz, pyridine-d5): .delta.=7.05 (dd, 1H), 6.80 (dd, 1H), 4.20 (q, 1H), 3.05 (m, 2H), 3.00 (m, 2H), 2.85 (m, 2H), 2.75 (m, 2H), 2.05 (m, 2H), 1.90-1.70 (m, 4H), 1.50 (d, 3H) ppm.
Example 32
2,3,5,6,7,8-Hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-217 of Table A)
32.1 Preparation of tert-butyl 1-benzyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-- carboxylate
[0584] To a solution of 388 mg (1.39 mmol) of 1-benzyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (compound of example 30) in 15 ml of dichloromethane was treated with 0.49 ml (2.78 mmol) of N,N-diisopropylethylamine. 333 mg (1.53 mmol) of di-tert-butyl dicarbonate were dissolved in 5 ml of dichloromethane, slowly added to the reaction mixture and the solution was then stirred overnight at room temperature. The solution was diluted with dichloromethane and treated twice with 10% aqueous solution of citric acid. The organic phase was washed once with saturated sodium chloride solution, dried with MgSO.sub.4 and concentrated in vacuo, yielding 559 mg of the crude product. The crude compound was used in the subsequent step without purification.
[0585] ESI-MS [M+H.sup.+]=380.20
32.2 Preparation of tert-butyl 2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]-quinoxaline-7(8H)-carboxyl- ate
[0586] 559 mg (1.47 mmol) of tert-butyl 1-benzyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-- carboxylate (559 mg, 1,473 mmol) (from step 32.1) were dissolved in 10 ml of methanol. The solution was hydrogenated with a 10% Pd/C cartridge using the H-Cube apparatus (from Thales Nano) at 80 bar and 70.degree. C. with a flow rate of 0.5 ml/min for 6 hours. The solution was concentrated in vacuo yielding 320 mg of the crude product. The crude compound was used in the subsequent step without purification.
[0587] ESI-MS [M+H.sup.+]=290.20
32.3 Preparation of 2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline
[0588] 30 mg (0.104 mmol) of tert-butyl 2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-carboxyla- te (from step 32.2) were dissolved in 2 ml of dichloromethane and treated with 0.20 ml (2.59 mmol) of trifluoroacetic acid at 0.degree. C. The solution was allowed to warm up to room temperature and stirred for 2 h. The reaction mixture was diluted with dichloromethane and extracted once with a 1 molar aqueous solution of sodium hydroxide. The aqueous phase was extracted 3.times. with ethyl acetate. The combined organic phases were dried with magnesium sulfate, and concentrated in vacuo yielding 14 mg (0.076 mmol) of the title compound.
[0589] ESI-MS [M+H.sup.+]=190.10
[0590] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.75 (m, 1H), 6.55 (d, 1H), 6.50 (d, 1H), 3.90 (s, 2H), 3.25 (m, 4H), 3.10 (m, 2H), 2.95 (m, 2H).
Example 33
1-Cyclobutyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline 2,2,2-trifluoroacetate (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-433 of Table A)
33.1 Preparation of tert-butyl 1-cyclobutyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(- 8H)-carboxylate
[0591] A solution of 79 mg (0.237 mmol) of tert-butyl 2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-carboxyla- te (from step 32.2) in 2 ml THF was treated with 38.3 mg (0.546 mmol) of cyclobutanone and molecular sieves (4 .ANG.). The reaction mixture was stirred at room temperature overnight. 231 mg (1.09 mmol) of sodium triacetoxyborohydride was added and the reaction mixture was stirred overnight at room temperature. The reaction mixture was diluted with ethyl acetate and extracted once with a 1 molar aqueous solution of sodium hydroxide. The organic phase was dried with magnesium sulfate, concentrated in vacuo and the residue was purified by column chromatography on silica (eluent: 0-10% methanol in dichloromethane) yielding 78 mg (0.205 mmol) of the title compound.
[0592] ESI-MS [M+H.sup.+]=344.20
33.2 Preparation of 1-cyclobutyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline 2,2,2-trifluoroacetate
[0593] 78.4 mg (0.228 mmol) of tert-butyl 1-cyclobutyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(- 8H)-carboxylate (from step 33.1) were dissolved in 1 ml of dichloromethane and treated with 0.53 ml (6.85 mmol) of trifluoroacetic acid at 0.degree. C. The solution was stirred for 2 h and then concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-30% methanol in dichloromethane) to yield 69 mg of crude product, which was purified again via preparative HPLC to yield 46 mg of the title compound.
[0594] ESI-MS [M+H.sup.+]=244.20
[0595] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.70 (bs, 2H), 6.80 (m, 1H), 6.75 (d, 1H), 6.70 (d, 1H), 4.10 (m, 2H), 4.05 (m, 1H), 3.25 (m, 2H), 3.20 (m, 2H), 3.10 (m, 4H), 2.20 (m, 2H), 2.10 (m, 2H), 1.65 (m, 2H).
Example 34
1-Methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-253 of Table A)
[0596] The title compound was prepared in analogy to example 33, using however formaldehyde instead of cyclobutanone.
[0597] ESI-MS [M+H.sup.+]=204.10
[0598] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.85 (m, 1H), 6.65 (d, 1H), 6.55 (d, 1H), 3.90 (s, 2H), 3.35 (m, 2H), 3.15 (m, 2H), 3.10 (m, 2H), 2.95 (m, 5H).
Example 35
1-(Oxetan-3-yl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxali- ne 2,2,2-trifluoroacetate (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-541 of Table A)
[0599] The title compound was prepared in analogy to example 33, using however oxetan-3-one instead of cyclobutanone.
[0600] ESI-MS [M+H.sup.+]=246.15
[0601] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.80 (m, 2H), 6.75 (m, 2H), 6.30 (d, 1H), 4.80 (m, 2H), 4.65 (m, 3H), 4.10 (s, 2H), 3.35 (m, 2H), 3.20 (m, 4H), 3.10 (m, 2H).
Example 36
1-(Cyclopropylmethyl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qui- noxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-577 of Table A)
[0602] The title compound was prepared in analogy to example 33, using however cyclopropanecarboxaldehyde instead of cyclobutanone.
[0603] ESI-MS [M+H.sup.+]=244.20
[0604] 1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.85 (m, 1H), 6.75 (m, 1H), 6.55 (m, 1H), 4.00 (m, 2H), 3.35-3.15 (m, 8H), 3.00 (m, 2H), 1.05 (m, 1H), 0.50 (m, 2H), 0.20 (m, 2H).
Example 37
1-(Cyclopentylmethyl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qui- noxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-649 of Table A)
[0605] The title compound was prepared in analogy to example 33, using however cyclopentanecarboxaldehyde instead of cyclobutanone.
[0606] ESI-MS [M+H.sup.+]=272.20
[0607] .sup.1H NMR (DMSO-d.sup.6, 500 MHz): .delta. [ppm]: 6.80 (m, 1H), 6.65 (d, 1H), 6.50 (d, 1H), 3.95 (m, 2H), 3.30-3.20 (m, 6H), 3.15 (m, 2H), 2.95 (m, 2H), 2.30 (m, 1H), 1.90-1.55 (m, 6H), 1.25 (m, 2H).
Example 38
Cyclopropyl(2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxalin-1-- yl)methanone (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-721 of Table A)
38.1 Preparation of tert-butyl 1-(cyclopropanecarbonyl)-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline-7(8H)-carboxylate
[0608] To 50 mg (0.173 mmol) of tert-butyl 2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-carboxyla- te dissolved in 1 ml of dichloromethane were added 0.77 ml (4.43 mmol) of N,N-diisopropylethylamine in an inert atmosphere at 0.degree. C. 19 .mu.l (0.21 mmol) of cyclopropanecarbonyl chloride was dissolved in 1 ml of dichloromethane and slowly added to the reaction mixture. The reaction mixture was stirred at room temperature for 5 h. The solution was diluted with dichloromethane and treated twice with 10% aqueous solution of citric acid. The organic phase was washed once with saturated sodium chloride solution, dried with MgSO.sub.4 and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-10% methanol in dichloromethane) yielding 35 mg (0.098 mmol) of the title compound.
[0609] ESI-MS [M-tert-butyl+H.sup.+]=302.10
38.2 Preparation of cyclopropyl(2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]-quinoxalin-- 1-yl)methanone
[0610] 35 mg (0.098 mmol) of tert-butyl 1-(cyclopropanecarbonyl)-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline-7(8H)-carboxylate were dissolved in 2 ml of dichloromethane and treated with 150 .mu.l (1.96 mmol) of trifluoroacetic acid at 0.degree. C. The solution was stirred overnight at room temperature. 100 .mu.l (1.31 mmol) of trifluoroacetic acid were added and the reaction mixture was stirred for 1 h. The reaction mixture was treated 3.times. with acidified water. The combined aqueous phase was basified with 1 N sodium hydroxide solution and extracted 5.times. with dichloromethane. The combined organic phase was dried with MgSO.sub.4 and concentrated in vacuo to yield 23 mg of the title compound.
[0611] ESI-MS [M+H.sup.+]=258.20
[0612] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.30 (m, 1H), 7.05 (m, 1H), 6.80 (m, 1H), 4.10 (s, 2H), 3.90 (m, 2H), 3.45 (m, 4H), 3.30 (m, 2H), 2.10 (m, 1H), 1.15 (m, 2H), 0.85 (m, 2H).
Example 39
Cyclopentyl(2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxalin-1-- yl)methanone (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-793 of Table A)
[0613] The title compound was prepared in analogy to example 38 using cyclopentanecarbonyl chloride.
[0614] ESI-MS [M+H.sup.+]=286.20
[0615] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.00 (m, 2H), 6.80 (m, 1H), 4.10 (s, 2H), 3.90 (m, 2H), 3.45-3.20 (m, 7H), 1.80 (m, 6H), 1.55 (m, 2H).
Example 40
1-Cyclopropyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-397 of Table A)
40.1 Preparation of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)acetamide
[0616] A solution of 500 mg (2.87 mmol) of 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline in 5 ml dimethylformamide was treated with 2.0 ml (11.68 mmol) of N-ethyl-N-isopropylpropan-2-amine, and subsequently 555 mg (5.94 mmol) 2-chloroacetamide were added. The reaction mixture was heated in a microwave system at 100.degree. C. for 12 hours. The reaction mixture was quenched with water and basified with 1 N sodium hydroxide solution. The aqueous phase was extracted 3.times. with dichloromethane. The combined organic extracts were dried with magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 620 mg of the title compound.
[0617] ESI-MS [M+H+]=232.20
40.2 Preparation of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)ethanamine
[0618] To 620 mg (2.68 mmol) of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1 (2H)-yl)acetamide in 2 ml of THF was added 5.4 ml (10.80 mmol) of 2 molar borane dimethylsulfide THF solution and subsequently heated for 7 hours at 60.degree. C. in a microwave system. An additional 2.7 ml (5.40 mmol) of 2 molar borane dimethylsulfide THF solution was added to the reaction mixture and heated for 10 hours at 60.degree. C. in a microwave system. To the reaction mixture were added 2 ml of methanol, acidified with 2 molar hydrochloric acid and heated for 15 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethyl acetate and extracted twice with 1 molar hydrochloric acid. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-15% methanol in dichloromethane) to yield 347 mg of the title compound.
[0619] ESI-MS [M+H.sup.+]=218.20
40.3 Preparation of 1-Cyclopropyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]-quinoxali- ne
[0620] A solution of 347 mg (1.6 mmol) of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)ethanamine in 5 ml of ethanol were treated with 119 l (1.6 mmol) of 37% aqueous formaldehyde solution and 135 l (1.76 mmol) of trifluoroacetic acid. The reaction mixture was stirred for 36 h at room temperature. Water was added and the reaction mixture was basified using 2 N aqueous sodium hydroxide solution. The aqueous phase was extracted three times with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 177 mg of the title compound.
[0621] ESI-MS [M+H.sup.+]=230.20
[0622] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.10 (d, 1H), 6.80 (m, 1H), 6.60 (d, 1H), 3.85 (s, 2H), 3.20 (m, 2H), 3.10 (m, 2H), 3.00 (m, 4H), 2.25 (m, 1H), 0.80 (m, 2H), 0.55 (m, 2H).
Example 41
1-Cyclopentyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-469 of Table A)
[0623] The title compound was prepared in analogy to example 40 using 1-cyclopentyl-1,2,3,4-tetrahydroquinoxaline as starting material.
[0624] ESI-MS [M+H.sup.+]=258.20
[0625] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.80 (d, 1H), 6.70 (m, 1H), 6.45 (d, 1H), 4.25 (m, 1H), 3.75 (s, 2H), 3.15 (m, 2H), 3.05 (m, 2H), 2.90 (m, 2H), 2.85 (m, 2H), 1.80 (m, 2H), 1.65 (m, 2H), 1.55 (m, 4H).
Example 42
1-Cyclopropyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-397 of Table A)
42.1 Preparation of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1 (2H)-yl)propanamide
[0626] A solution of 300 mg (1.72 mmol) of 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline in 3 ml dimethylformamide was treated with 1.2 ml (7.0 mmol) of N-ethyl-N-isopropylpropan-2-amine, 387 mg (2.58 mmol) of sodium iodide and subsequently 523 mg (3.44 mmol) of 2-bromopropanamide were added. The reaction mixture was heated in a microwave system at 100.degree. C. for 8 hours. The reaction mixture was quenched with water and basified with 1 N sodium hydroxide solution. The aqueous phase was extracted 3.times. with dichloromethane. The combined organic extracts were dried with magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 453 mg of the title compound.
[0627] ESI-MS [M+H.sup.+]=246.20
42.2 Preparation of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)propan-1-amine
[0628] To 453 mg (1.85 mmol) of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)propanamide in 2 ml THF were added 4.3 ml (8.60 mmol) of 2 molar borane dimethylsulfide THF solution and subsequently heated for 9 hours at 60.degree. C. and 2 hours at 70.degree. C. in a microwave system. To the reaction mixture was added 2 ml of methanol, acidified with 2 molar hydrochloric acid and heated for 15 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethyl acetate and extracted twice with 1 molar hydrochloric acid. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-15% methanol in dichloromethane) to yield 209 mg of the title compound.
[0629] ESI-MS [M+H.sup.+]=232.20
42.3 Preparation of 1-cyclopropyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino-[1,7,6-de]- quinoxaline
[0630] A solution of 209 mg (0.9 mmol) of 2-(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)propan-1-amine in 3 ml of ethanol were treated with 67 l (0.9 mmol) of 37% aqueous formaldehyde solution and 76 l (0.99 mmol) of trifluoroacetic acid. The reaction mixture was stirred for 36 h at room temperature. Water was added and the reaction mixture was basified using 2 N aqueous sodium hydroxide solution. The aqueous phase was extracted three times with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 136 mg of the title compound.
[0631] ESI-MS [M+H.sup.+]=244.20
[0632] 1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.00 (d, 1H), 6.65 (m, 1H), 6.50 (d, 1H), 3.75 (d, 1H), 3.45 (d, 1H), 3.30-3.15 (m, 4H), 3.05 (m, 1H), 2.80 (d, 1H), 2.65 (d, 1H), 2.20 (m, 1H), 0.90 (d, 3H), 0.80 (m, 2H), 0.50 (m, 2H).
Example 43
1-Cyclopentyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qu- inoxaline (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-469 of Table A)
[0633] The title compound was prepared in analogy to example using however 1-cyclopentyl-1,2,3,4-tetrahydroquinoxaline instead of 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline.
[0634] ESI-MS [M+H.sup.+]=272.20
[0635] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.70 (d, 1H), 6.65 (m, 1H), 6.40 (d, 1H), 4.20 (m, 1H), 3.75 (d, 1H), 3.50 (d, 1H), 3.25-3.10 (m, 3H), 3.00 (m, 2H), 2.85 (d, 1H), 2.70 (d, 1H), 1.80 (m, 2H), 1.70-1.55 (m, 5H), 1.50 (m, 1H), 0.90 (d, 3H).
Example 44
5,6,7,9,9a, 10,11,12,13,14-decahydro-4H-azepino[1,2-a][1,4]diazepino[1,7,6-de]quinoxa- line (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-937 of Table A)
44.1 Preparation of ethyl 1-(2-nitrophenyl)azepane-2-carboxylate
[0636] A solution of 450 mg (3.19 mmol) of 1-fluoro-2-nitrobenzene, 546 mg (3.19 mmol) of ethyl azepane-2-carboxylate and 0.89 ml (6.38 mmol) of triethylamine in 10 ml acetonitrile was stirred overnight at reflux. The reaction mixture was concentrated in vacuo. To the residue was added aqueous sodium bicarbonate solution and extracted twice with ethyl acetate. The combined organic extracts were dried with sodium sulfate and concentrated in vacuo to yield 780 mg of the crude title compound. The crude product was used without further purification in the next step.
[0637] ESI-MS [M+H.sup.+]=293.10
44.2 Preparation of 6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-6(5H)-one
[0638] 780 mg (2.67 mmol) of ethyl 1-(2-nitrophenyl)azepane-2-carboxylate were dissolved in 30 ml methanol and treated with 284 mg of 10% Pd/C in a hydrogen atmosphere. The solution was stirred at room temperature for 1 h. The catalyst was filtered off and the solution was concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-50% ethyl acetate in heptane) to yield 159 mg of the title compound.
[0639] ESI-MS [M+H.sup.+]=217.10
44.3 Preparation of 5,6,6a,7,8,9,10,11-octahydroazepino[1,2-a]quinoxaline
[0640] To 105 mg (0.485 mmol) 6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-6(5H)-one in 4 ml of THF (tetrahydrofuran) were added 0.97 ml (0.97 mmol) of 1 molar borane dimethylsulfide THF solution and subsequently heated for 1 h at 80.degree. C. in a microwave system. The reaction mixture was poured on ice water, acidified with hydrochloric acid solution to pH=5 and extracted twice with ethyl acetate. The combined organic phases were dried and concentrated in vacuo. The residue was acidified with hydrochloric acid solution and extracted once with ether. The aqueous phase was basified with aqueous sodium bicarbonate solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo to yield 85 mg of the crude title compound.
[0641] ESI-MS [M+H.sup.+]=203.20
44.4 Preparation of 2-(6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-5(6H)-yl)-acetamide
[0642] A solution of 85 mg (0.40 mmol) of 5,6,6a,7,8,9,10,11-octahydroazepino[1,2-a]quinoxaline in 2 ml of dimethylformamide was treated with 16 mg (0.40 mmol) of sodium hydride (60% in mineral oil) and stirred for 15 min at room temperature. 112 mg (1.2 mmol) of 2-chloroacetamide were added and the reaction mixture was heated in a microwave system at 100.degree. C. for 4 hours. 209 .mu.l (1.2 mmol) of N-ethyl-N-isopropylpropan-2-amine were added to the reaction mixture and heated at 100.degree. C. for 2 hours. 110 mg (0.8 mmol) of 2-bromoacetamide were added and the reaction mixture was heated in a microwave system at 100.degree. C. for 2 hours. The reaction mixture was basified with sodium bicarbonate solution and extracted 2.times. with ethyl acetate. The combined organic extracts were dried with magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-50% ethyl acetate in heptane) to yield 42 mg of the title compound.
[0643] ESI-MS [M+H.sup.+]=260.20
44.5 Preparation of 2-(6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-5(6H)-yl)-ethanamine
[0644] To 42 mg (0.146 mmol) of 2-(6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-5(6H)-yl)acetamide in 1 ml of THF were added 292 .mu.l (8.60 mmol) of 2 molar borane dimethylsulfide THF solution and subsequently heated for 6 hours at 60.degree. C. in a microwave system. Additional 292 .mu.l (8.60 mmol) of 2 molar borane dimethylsulfide THF solution were added to the reaction mixture and heated for 4 hours at 60.degree. C. in a microwave system. To the reaction mixture was added 2 ml of methanol, acidified with 2 molar aqueous hydrochloric acid solution and heated for 15 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethyl acetate and extracted twice with 1 molar hydrochloric acid. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo to yield 36 mg of the title compound.
[0645] ESI-MS [M+H.sup.+]=246.20
44.6 Preparation of 5,6,7,9,9a,10,11,12,13,14-decahydro-4H-azepino[1,2-a][1,4]-diazepino[1,7,- 6-de]quinoxaline
[0646] A solution of 36 mg (0.147 mmol) of 2-(6a,7,8,9,10,11-hexahydroazepino[1,2-a]quinoxalin-5(6H)-yl)ethanamine in 2 ml of ethanol were treated with 11 .mu.l (0.147 mmol) of 37% aqueous formaldehyde solution and 12 .mu.l (0.161 mmol) of trifluoroacetic acid. The reaction mixture was stirred overnight at room temperature. The reaction mixture was concentrated in vacuo, treated with aqueous sodium bicarbonate solution and extracted twice with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 12.7 mg of the title compound.
[0647] ESI-MS [M+H.sup.+]=258.20
[0648] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.65 (m, 1H), 6.50 (d, 1H), 6.35 (d, 1H), 3.70 (m, 1H), 3.60 (m, 1H), 3.20 (m, 2H), 3.10 (m, 1H), 3.00 (m, 3H), 2.95 (m, 1H), 2.85 (m, 1H), 2.80 (m, 1H), 1.80 (m, 2H), 1.65-1.50 (m, 4H), 1.40 (m, 2H).
Example 45
7-Methyl-5,6,7,9,9.sup.a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-865 of Table A)
[0649] The title compound was prepared in analogy to example 42, using however 1,2,3,3a,4,5-hexahydropyrrolo[1,2-a]quinoxaline and 2-bromopropanamide.
[0650] ESI-MS [M+H.sup.+]=244.20
[0651] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.80 (m, 1H), 6.50 (d, 1H), 6.45 (d, 1H), 3.95 (d, 1H), 3.70 (d, 1H), 3.50 (m, 2H), 3.15 (m, 2H), 2.85 (m, 2H), 2.75 (m, 1H), 2.20 (m, 1H), 2.10 (m, 1H), 2.00 (m, 1H), 1.90 (m, 1H), 1.35 (m, 1H), 1.20 (d, 3H).
Example 46
1-Fluoro-7-methyl-5,6,7,9,9.sup.a,10,11,12-octahydro-4H-[1,4]diazepino[1,7- ,6-de]pyrrolo[1,2-a]quinoxaline (Compound of the Formula I.Gg in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-866 of Table A)
[0652] The title compound was prepared in analogy to example 42, using however 9-fluoro-1,2,3,3a,4,5-hexahydropyrrolo[1,2-a]quinoxaline and 2-bromopropanamide.
[0653] ESI-MS [M+H.sup.+]=262.20
[0654] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.60 (m, 1H), 6.55 (m, 1H), 3.90 (d, 1H), 3.80 (m, 1H), 3.55 (d, 1H), 3.20 (d, 1H), 3.00 (m, 2H), 2.80 (m, 2H), 2.55 (m, 1H), 2.15 (m, 2H), 1.80 (m, 2H), 1.50 (m, 1H), 1.25 (d, 3H).
Example 47
1,2,3,4,6,7-Hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopro- pane](Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-281 of Table B)
47.1 Preparation of 2-(2',3'-dihydro-1 .sup.1H-spiro[cyclopropane-1,4'-quinolin]-1'-yl)-acetamide
[0655] A solution of 500 mg (3.14 mmol) of 2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-quinoline] in 5 ml of dimethylformamide was treated with 2.2 ml (12.8 mmol) of N-ethyl-N-isopropylpropan-2-amine and 706 mg (4.71 mmol) of sodium iodide; and subsequently 670 mg (7.16 mmol) of 2-chloroacetamide were added. The reaction mixture was heated in a microwave system at 100.degree. C. for 10 hours. The reaction mixture was quenched with water and basified with 1 N sodium hydroxide solution. The aqueous phase was extracted 3.times. with dichloromethane. The combined organic extracts were dried with magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 624 mg of the title compound.
[0656] ESI-MS [M+H.sup.+]=217.20
47.2 Preparation of 2-(2',3'-dihydro-1 .sup.1H-spiro[cyclopropane-1,4'-quinolin]-1'-yl)ethan-amine
[0657] To 624 mg (2.89 mmol) of 2-(2',3'-dihydro-1 .sup.1H-spiro[cyclopropane-1,4'-quinolin]-1'-yl)acetamide in 2 ml of THF were added 5.77 ml (11.54 mmol) of 2 molar borane dimethylsulfide THF solution and subsequently heated for 9 hours at 60.degree. C. in a microwave system. To the reaction mixture were added 2 ml of methanol, acidified with 2 molar hydrochloric acid and heated for 15 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethyl acetate and extracted twice with 1 molar hydrochloric acid. The combined aqueous phases were set to pH 10 with sodium hydroxide solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 299 mg of the title compound.
[0658] ESI-MS [M+H.sup.+]=203.20
47.3 Preparation of 1,2,3,4,6,7-Hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-cyclopr- opane]
[0659] A solution of 299.6 mg (1.48 mmol) of 2-(2',3'-dihydro-1 .sup.1H-spiro[cyclopropane-1,4'-quinolin]-1'-yl)ethanamine in 3 ml of ethanol was treated with 110 .mu.l (1.48 mmol) of 37% aqueous formaldehyde solution and 126 .mu.l (1.63 mmol) of trifluoroacetic acid. The reaction mixture was stirred overnight at room temperature. Water was added and the reaction mixture was basified using 2 N aqueous sodium hydroxide solution. The aqueous phase was extracted three times with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 131 mg of the title compound.
[0660] ESI-MS [M+H.sup.+]=215.20
[0661] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.90 (d, 1H), 6.70 (m, 1H), 6.55 (d, 1H), 3.75 (s, 2H), 3.25 (m, 2H), 3.00 (m, 2H), 2.90 (m, 2H), 1.60 (m, 2H), 0.95 (s, 2H), 0.80 (s, 2H).
Example 48
4-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'- -cyclopropane](Compound of the Formula I.s, I.t or I.u in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-281 of Table B)
[0662] The title compound was prepared in analogy to example 47, using however 2',3'-dihydro-1 .sup.1H-spiro[cyclopropane-1,4'-quinoline] and 2-bromopropanamide.
[0663] ESI-MS [M+H+]=229.20
[0664] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.85 (d, 1H), 6.65 (m, 1H), 6.55 (d, 1H), 3.80 (d, 1H), 3.50 (d, 1H), 3.25 (m, 3H), 2.85 (d, 1H), 2.70 (d, 1H), 1.70 (m, 1H), 1.60 (m, 1H), 1.05 (m, 1H), 0.90 (m, 3H), 0.85 (m, 1H), 0.75 (m, 2H).
Example 49
11-Fluoro-8,8-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]qu- inoline (Compound of the Formula I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-2 of Table B)
[0665] The title compound was prepared in analogy to example 47, using however 7-fluoro-4,4-dimethyl-1,2,3,4-tetrahydroquinoline and 2-bromoacetamide.
[0666] ESI-MS [M+H.sup.+]=235.20
[0667] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.15 (dd, 1H), 6.55 (dd, 1H), 3.80 (s, 2H), 3.20 (m, 2H), 3.10 (m, 2H), 2.90 (m, 2H), 1.60 (m, 2H), 1.20 (s, 6H).
Example 50
10-Methoxy-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]q- uinoline, Trifluoroacetic Acid (Compound of the Formula I.h in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-7 of Table B)
[0668] The title compound was prepared in analogy to example 1, using however 7-methoxy-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate and 3-methylbut-2-enoyl chloride.
[0669] ESI-MS [M+H.sup.+]=247.20
[0670] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.75 (bs, 2H), 6.95 (s, 1H), 6.85 (s, 1H), 4.15 (m, 2H), 3.70 (s, 3H), 3.20 (m, 2H), 3.15 (m, 4H), 1.65 (m, 2H), 1.25 (s, 6H).
Example 51
4,8,8-Trimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline 2,2,2-trifluoroacetate (Compound of the Formula I.s, I.t or I.u in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-1 of Table B)
[0671] The title compound was prepared in analogy to example 47, using however 4,4-dimethyl-1,2,3,4-tetrahydroquinoline and 2-bromopropanamide.
[0672] ESI-MS [M+H.sup.+]=231.20
[0673] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 9.90 (bs, 1H), 9.20 (bs, 1H); 7.35 (d, 1H), 7.10 (d, 1H), 6.90 (m, 1H), 4.30 (d, 1H), 4.05 (d, 1H), 3.45 (m, 1H), 3.25 (m, 3H), 3.15 (m, 1H), 1.70 (m, 2H), 1.30 (m, 9H).
Example 52
4-Ethyl-8,8-dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quin- oline (Compound of the Formula I.v, I.w or I.x in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-1 of Table B)
[0674] The title compound was prepared in analogy to example 47, using however 4,4-dimethyl-1,2,3,4-tetrahydroquinoline and 2-bromobutanamide.
[0675] ESI-MS [M+H.sup.+]=245.20
[0676] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.15 (d, 1H), 6.85 (d, 1H), 6.70 (m, 1H), 3.85 (d, 1H), 3.50 (d, 1H), 3.20 (m, 2H), 3.00 (d, 1H), 2.95 (m, 1H), 2.75 (d, 1H), 1.60 (m, 2H), 1.40 (m, 2H), 1.25 (s, 3H), 1.30 (s, 3H), 0.80 (m, 3H).
Example 53
6,8,8-Trimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline (Compound of the Formula I.y, I.z or I.Zz in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-1 of Table B)
[0677] The title compound was prepared in analogy to example 47, using however 2,4,4-trimethyl-1,2,3,4-tetrahydroquinoline and 2-chloroacetamide.
[0678] ESI-MS [M+H.sup.+]=231.20
[0679] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.05 (d, 1H), 6.90 (d, 1H), 6.65 (m, 1H), 3.85 (d, 1H), 3.45 (d, 1H), 3.30 (m, 3H), 3.00 (m, 1H), 2.70 (m, 1H), 1.65 (m, 1H), 1.40 (m, 1H), 1.25 (s, 3H), 1.15 (s, 6H).
Example 54
One Enantiomer of 8-ethyl-8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]-quinol- ine, Trifluoroacetic Acid (Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-41 of Table B)
[0680] The title compound was prepared in analogy to example 1, using however tert-butyl 2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate and (Z)-3-methylpent-2-enoyl chloride.
[0681] ESI-MS [M+H.sup.+]=231.20
[0682] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.90 (bs, 1H), 8.65 (bs, 1H), 7.30 (d, 1H), 7.15 (d, 1H), 6.90 (m, 1H), 4.20 (m, 1H), 4.10 (m, 1H), 3.25 (m, 6H), 1.85 (m, 1H), 1.70 (m, 1H), 1.60 (m, 1H), 1.45 (m, 1H), 1.20 (s, 3H), 0.70 (m, 3H).
Example 55
Other Enantiomer of 8-ethyl-8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoli- ne, Trifluoroacetic Acid (Enantiomer of the Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-41 of Table B)
[0683] The title compound was prepared in analogy to example 1, using however tert-butyl 2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate and (Z)-3-methylpent-2-enoyl chloride.
[0684] ESI-MS [M+H.sup.+]=231.10
[0685] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 8.90 (bs, 1H), 8.65 (bs, 1H), 7.30 (d, 1H), 7.15 (d, 1H), 6.90 (m, 1H), 4.20 (m, 1H), 4.10 (m, 1H), 3.20 (m, 6H), 1.85 (m, 1H), 1.70 (m, 1H), 1.60 (m, 1H), 1.45 (m, 1H), 1.20 (s, 3H), 0.70 (m, 3H).
Example 56
4-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'- -cyclobutane](Compound of the Formula I.s, I.t or I.u in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-321 of Table B)
56.1 Preparation of 2-cyclobutylidene-N-phenylacetamide
[0686] 3.35 g (36 mmol) of aniline were dissolved in 60 ml of dichloromethane and treated with 13.83 ml (79 mmol) of N-ethyl-N-isopropylpropan-2-amine. Within 10 min 19.80 ml of a 1 molar solution of 2-cyclobutylideneacetyl chloride in dichloromethane was added at 0.degree. C. and the solution was stirred overnight at room temperature. The reaction mixture was poured on ice water and extracted 2.times. with dichloromethane. The combined organic phases were washed once with saturated sodium chloride solution, dried with magnesium sulfate and concentrated in vacuo. The crude oil was dissolved in dichloromethane and treated with ether till crystallization, yielding 5.5 g of the title compound.
[0687] ESI-MS [M+H.sup.+]=188.10
56.2 Preparation of 1'H-spiro[cyclobutane-1,4'-quinolin]-2'(3'H)-one
[0688] A solution of 2.0 g (10.68 mmol) of 2-cyclobutylidene-N-phenylacetamide in 214 ml of toluene was irradiated with a 700 W mercury lamp in a flow reactor (FEP-tubing, pyrex filter) with a flow rate of 1 ml per min at a temperature range of 0.degree. C. to 20.degree. C. with a residence time of 5 to 7 min. The reaction mixture was concentrated in vacuo and the residue was purified by column chromatography on silica (eluent: 0-55% ethyl acetate in cyclohexane) to yield 940 mg of the title compound.
[0689] ESI-MS [M+H.sup.+]=188.10
56.3 Preparation of 2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinoline]
[0690] To 850 mg (4.54 mmol) of 1'H-spiro[cyclobutane-1,4'-quinolin]-2'(3'H)-one in 5 ml of THF were added 9.08 ml (9.08 mmol) of 1 molar borane dimethylsulfide THF solution and subsequently heated for 30 min at 80.degree. C. in a microwave system. The reaction mixture was acidified with 6 molar hydrochloric acid solution and treated with ethyl acetate. The organic phase was extracted twice with 1 molar hydrochloric acid solution. The combined aqueous phases were set to pH 10 with sodium bicarbonate and extracted 3.times. with ethyl acetate. The combined organic phases were dried and concentrated in vacuo to yield 565 mg of the crude title compound.
[0691] ESI-MS [M+H.sup.+]=174.10
56.4 Preparation of 2-(2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)propanamide
[0692] ESI-MS [M+H.sup.+]=245.20
[0693] A solution of 300 mg (1.73 mmol) of 2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinoline] in 10 ml dimethylformamide was treated with 69 mg (1.73 mmol) of sodium hydride (60% in mineral oil) and stirred for 15 min at 50.degree. C., then transferred to a microwave vial and stirred for 5 min at 70.degree. C. in a microwave system. Subsequently 790 mg (5.19 mmol) of 2-bromoacetamide were added and the reaction mixture was heated in a microwave system at 100.degree. C. for 7 hours. Additional 263 mg (1.73 mmol) of 2-bromoacetamide were added and the reaction mixture was heated in a microwave system at 100.degree. C. for 2 hours. The reaction mixture was quenched with ice water, acidified to pH=5 and extracted with ether. The aqueous phase was basified with sodium bicarbonate solution and extracted 3.times. with dichloromethane. The combined organic extracts were dried with magnesium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-55% ethyl acetate in heptane) to yield 258 mg of the title compound.
56.5 Preparation of 2-(2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)propan-1-amin- e
[0694] To 258 mg (0.348 mmol) of 2-(2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)propanamide in 2 ml THF were added 1.05 ml (2.10 mmol) of 2 molar borane dimethylsulfide THF solution and subsequently heated for 6 hours at 60.degree. C. in a microwave system. Subsequently 348 .mu.l (0.70 mmol) of 2 molar borane dimethylsulfide THF solution was added to the reaction mixture and heated for 2 hours at 60.degree. C. in a microwave system. Subsequently 697 .mu.l (1.39 mmol) of 2 molar borane dimethylsulfide THF solution was added to the reaction mixture and heated for 3 hours at 60.degree. C. in a microwave system. To the reaction mixture was added 2 ml of methanol, acidified with 2 molar hydrochloric acid and heated for 5 minutes at 60.degree. C. in a microwave system. The reaction mixture was diluted with ethyl acetate and extracted twice with 1 molar hydrochloric acid. The combined aqueous phases were set to pH 10 with sodium bicarbonate solution and extracted 3.times. with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-20% methanol in dichloromethane) to yield 90 mg of the title compound.
[0695] ESI-MS [M+H.sup.+]=231.20
[0696] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.35 (d, 1H), 6.95 (dd, 1H), 6.70 (d, 1H), 6.55 (dd, 1H), 3.80 (m, 1H), 3.05 (m, 1H), 3.00 (m, 1H), 2.70 (m, 1H), 2.55 (m, 1H), 2.35 (m, 1H), 2.25 (m, 1H), 2.00 (m, 1H), 1.90 (m, 5H), 1.05 (d, 3H).
56.6 Preparation of 4-methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]-quinoline-8,- 1'-cyclobutane]
[0697] A solution of 90 mg (0.313 mmol) of 2-(2',3'-dihydro-1'H-spiro[cyclobutane-1,4'-quinolin]-1'-yl)propan-1-amin- e in 2 ml of ethanol was treated with 23 .mu.l (0.313 mmol) of 37% aqueous formaldehyde solution and 26 .mu.l (0.344 mmol) of trifluoroacetic acid. The reaction mixture was stirred overnight at room temperature. Further 12 .mu.l (0.156 mmol) of 37% aqueous formaldehyde solution and 12 .mu.l (0.156 mmol) of trifluoroacetic acid were added to the reaction mixture and stirring was continued overnight at room temperature. The reaction mixture was concentrated in vacuo and to the residue was added aqueous sodium bicarbonate solution. The aqueous phase was extracted twice with dichloromethane. The combined organic phases were dried and concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-10% methanol in dichloromethane) to yield 38 mg of the title compound.
[0698] ESI-MS [M+H.sup.+]=243.20
[0699] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 7.35 (d, 1H), 6.90 (d, 1H), 6.75 (d, 1H), 3.80 (m, 1H), 3.40 (m, 1H), 3.20 (m, 2H), 3.10 (m, 1H), 2.80 (m, 1H), 2.70 (m, 1H), 2.40 (m, 1H), 2.15 (m, 1H), 2.00 (m, 4H), 1.85 (m, 1H), 1.70 (m, 1H), 0.80 (s, 3H).
Example 57
1-Fluoro-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline, Trifluoroacetic Acid (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-866 of Table A)
57.1 Preparation of methyl pyrrolidine-2-carboxylate
[0700] To a solution of pyrrolidine-2-carboxylic acid (75 g, 651 mmol) in methanol (750 ml), SOCl.sub.2 (250 ml) was added dropwise at 0.degree. C., and the reaction mixture was stirred for 16 hrs at 20.degree. C. One additional vial was set up in the same way. Both reaction mixtures were combined and concentrated under reduced pressure to give the title compound (90 g, 543 mmol, yield 50%).
[0701] .sup.1H NMR (400 MHz, DMSO-d.sub.6): .delta. [ppm]: 9.83 (s, 1H), 4.32 (t, J=7.6 Hz, 1H), 3.72 (s, 3H), 3.21-3.15 (m, 2H), 2.22-2.20 (m, 1H), 1.96-1.87 (m, 3H)
57.2 Preparation of methyl 1-(2-fluoro-6-nitro-phenyl)-pyrrolidine-2-carboxylate
[0702] To a mixture of methyl pyrrolidine-2-carboxylate (17.18 g, 104 mmol) in acetonitril (500 mL), triethylamine (26.3 mL, 189 mmol) and 1,2-difluoro-3-nitrobenzene (15 g, 94 mmol) were added at 20.degree. C., and the reaction mixture was stirred for 12 h at 80.degree. C. Then the mixture was cooled to 20.degree. C., concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel (petrol ether:ethyl acetate=20:1 to 10:1) to give the title compound (20 g, 74.6 mmol, yield 79%).
[0703] .sup.1H NMR (400 MHz, CDCl.sub.3): .delta. [ppm]: 7.36 (d, J=8 Hz, 1H), 7.15-7.11 (m, 1H), 6.99-6.96 (m, 1H), 4.35-4.31 (m, 1H), 3.55 (s, 3H), 3.52-3.49 (m, 1H), 3.06-3.04 (m, 1H), 2.29-2.26 (m, 1H), 2.06-1.89 (m, 3H)
57.3 Preparation of 9-fluoro-2,3,3a,5,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-4-one
[0704] To a solution of methyl 1-(2-fluoro-6-nitro-phenyl)-pyrrolidine-2-carboxylate (10 g, 37.3 mmol) in methanol (200 ml), Pd/C (3.97 g, 37.3 mmol) was added under Ar protection, then the reaction mixture was stirred for 12 h at 20.degree. C. at 15 psi under H.sub.2 atmosphere. One additional vial was set up as described above. The two reaction mixtures were combined, filtered, and the filtrate was concentrated under reduced pressure to give the title compound (10 g, 48.5 mmol, yield 65%).
[0705] .sup.1H NMR (400 MHz, CDCl.sub.3): .delta. [ppm]: 9.33 (s, 1H), 6.73-6.63 (m, 2H), 6.55-6.53 (m, 1H), 3.89-3.86 (m, 1H), 3.72-3.68 (m, 1H), 3.40-3.39 (m, 1H), 2.30-2.22 (m, 2H), 1.92-1.84 (m, 2H)
57.4 Preparation of 9-fluoro-1,2,3,3a,4,5,5a,6-octahydropyrrolo[1,2-a]quinoxaline
[0706] To a solution of 9-fluoro-2,3,3a,5,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-4-one (10 g, 48.5 mmol) in THF (200 ml), a solution of BH.sub.3.THF (12.5 g, 145 mmol) was added at 0.degree. C., then the reaction solution was stirred 12 h at 70.degree. C. After cooling to 0.degree. C., methanol (100 ml) was added dropwise at 0.degree. C., and the reaction solution was stirred for 30 minutes at 20.degree. C., concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel (petrol ether:ethyl acetate=20:1 to 10:1) to give the title compound (5 g, 26 mmol, yield 53.6%).
[0707] .sup.1H NMR (400 MHz, CDCl.sub.3): .delta. [ppm]: 6.54-6.49 (m, 1H), 6.40-6.37 (m, 1H), 6.28 (d, J=8 Hz, 1H), 3.92 (s, 1H), 3.78-3.76 (m, 1H), 3.34-3.20 (m, 1H), 3.20-3.17 (m, 1H), 2.99-2.97 (m, 1H), 2.66-2.61 (m, 1H), 2.13-2.11 (m, 1H), 1.86-1.78 (m, 2H), 1.50-1.47 (m, 1H)
57.5 Preparation of 2-(9-fluoro-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)acet- amide
[0708] To a solution of 9-fluoro-1,2,3,3a,4,5,5a,6-octahydropyrrolo[1,2-a]quinoxaline (5.6 g, 29.1 mmol), diisopropylethyl amine (30.5 mL, 175 mmol) in dimethylformamide (DMF) (100 ml), 2-bromoacetamide (16.08 g, 117 mmol) was added and the reaction solution was stirred 12 h at 100.degree. C. After cooling to 20.degree. C., water (300 ml) was added, and the mixture was extracted with ethyl acetate (3.times.200 ml); the organic layer was dried over anhydrous Na.sub.2SO.sub.4, concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel (dichloromethane: methanol=100:1 to 50:1) to give the title compound (3 g, 12.03 mmol, yield 41.3%).
[0709] LCMS (ESI+): nm/z 250 (M+H).sup.+
57.6 Preparation of 2-(9-fluoro-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)etha- namine
[0710] To a solution of 2-(9-fluoro-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)acet- amide (3 g, 12.03 mmol) in THF (20 ml), a solution of BH.sub.3.THF (3.1 g, 36.1 mmol) was added at 0.degree. C., then the reaction solution was stirred for 12 h at 70.degree. C. After cooling to 0.degree. C., methanol (100 ml) was added at 0.degree. C., and the reaction solution was stirred for 30 minutes at 20.degree. C. Then the solution was concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel (petrol ether/ethyl acetate=20:1 to 10:1) to give title compound (1.3 g, 5.52 mmol, yield 45.9%).
[0711] .sup.1H NMR (400 MHz, CD.sub.3OD): .delta. [ppm]: 6.63-6.59 (m, 1H), 6.48 (d, J=8.4 Hz, 1H), 6.34-6.29 (m, 1H), 3.73-3.70 (m, 1H), 3.34-3.25 (m, 3H), 3.11-3.08 (m, 1H), 2.84-2.79 (m, 3H), 2.64-2.61 (m, 1H), 2.13-2.09 (m, 1H), 1.80-1.76 (m, 2H), 1.55-1.51 (m, 1H)
57.7 Preparation of 1-fluoro-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0712] To a solution of 2-(9-fluoro-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)etha- namine (1.1 g, 4.67 mmol) and formaldehyde (2.106 g, 70.1 mmol) in ethanol (20 ml), trifluoroacetic acid (TFA) (2.88 mL, 37.4 mmol) was added, and the reaction solution was stirred for 12 h at 90.degree. C. After cooling to 40.degree. C., the solution was concentrated under reduced pressure, and the residue was purified by prep-HPLC to give the title compound as TFA salt (1.087 g, yield 94%).
Prep-HPLC Method:
[0713] Instrument: Shimadzu LC-20AP preparative HPLC
Column: Luna(2) C18 250*50 mm i.d. 10 u
[0714] Mobile phase: A for H.sub.2O (0.09% TFAl) and B for CH.sub.3CN Gradient: B from 0% to 20% in 20 min Flow rate: 80 ml/min
Wavelength: 220 &254 nm
[0715] Injection amount: 1.1 g per injection
[0716] .sup.1H NMR (400 MHz, CD.sub.3OD): .delta. [ppm]: 6.79-6.71 (m, 2H), 4.23 (s, 2H), 4.01-3.96 (m, 1H), 3.36-3.32 (m, 3H), 3.29-3.27 (m, 2H), 3.15-3.12 (m, 2H), 2.49-2.43 (m, 1H), 2.29-2.21 (m, 1H), 1.95-1.90 (m, 2H), 1.58-1.54 (m, 1H)
Example 58
1-Bromo-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1- ,2-a]quinoxaline (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-868 of Table A)
58.1 Preparation of 1-(2-bromo-6-nitro-phenyl)-pyrrolidine-2-carboxylate
[0717] A mixture of 1-bromo-2-fluoro-3-nitrobenzene (60 g, 273 mmol), methyl pyrrolidine-2-carboxylate (54.2 g, 327 mmol; see example 57.1) and triethylamine (83 g, 818 mmol) was heated at 70.degree. C. for 16 h. Then the mixture was cooled, diluted with ethyl acetate (1000 ml), washed with 2N HCl (500 ml), aqueous K.sub.2CO.sub.3 (300 ml) and brine (300 ml) sequentially, and the aqueous phase was extracted with ethyl acetate (500 ml) again. The ethyl acetate layer was dried over Na.sub.2SO.sub.4, filtered, concentrated, and the residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=50:1 to 10:1) to afford the title compound (66 g, yield 74%) as yellow solid.
[0718] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.80-7.78 (m, 1H), 7.64-7.62 (m, 1H), 7.19 (t, J=8.2 Hz, 1H), 4.26-4.16 (m, 1H), 3.70-3.65 (m, 1H), 3.59 (s, 3H), 3.36-3.23 (m, 1H), 2.40-2.23 (m, 2H), 2.15-2.04 (m, 2H)
58.2 Preparation of 9-bromo-2,3,3a,5,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-4-one
[0719] To a solution of 1-(2-bromo-6-nitro-phenyl)-pyrrolidine-2-carboxylate (45 g, 137 mmol) in methanol (225 ml) was added a solution of NH.sub.4Cl (65.8 g, 1230 mmol) in water (225 ml) and Fe powder (53.4 g, 957 mmol) at 23.degree. C., and the resulting mixture was heated at 85.degree. C. for 1.5 h. Then the reaction was cooled, diluted with methanol (1000 ml), and the resulting mixture was stirred for 10 minutes, filtered, and the filter cake was washed with methanol (500 ml). The filtrate was concentrated under reduced pressure, the residue was put into water, extracted with ethyl acetate (3.times.800 ml), the extracts were washed with brine (400 ml), dried over Na.sub.2SO.sub.4, filtered, and concentrated to afford the title compound (33 g, yield 90%) as a light yellow solid.
[0720] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 9.78 (s, 1H), 7.27-7.17 (m, 1H), 6.91-6.75 (m, 2H), 4.10-3.90 (m, 2H), 3.09-3.06 (m, 1H), 2.86-2.68 (m, 1H), 2.37-2.20 (m, 1H), 2.04-2.02 (m, 1H), 1.88-1.68 (m, 1H)
58.3 Preparation of 9-bromo-1,2,3,3a,4,5,5a,6-octahydropyrrolo[1,2-a]quinoxaline
[0721] To a solution of 9-bromo-2,3,3a,5,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-4-one (24 g, 90 mmol) in THF (360 ml) was added dropwise BH.sub.3.DMS (50 ml, 500 mmol, 10 M in dimethylsulfide (DMS)) at 23.degree. C., and the resulting solution was stirred at 70.degree. C. for 2 h. The reaction was then cooled, and quenched with methanol. One additional reaction was set up and quenched as described above. The two resulting mixtures were combined, concentrated to about one third of volume, 6N HCl (250 mL) was added, and the resulting solution was heated at 70.degree. C. for 20 minutes. Then the solution was cooled, adjusted to pH 9 by addition of saturated aqueous K.sub.2CO.sub.3, extracted with ethyl acetate (3.times.400 mL), the extracts were washed with brine, dried over Na.sub.2SO.sub.4, concentrated to give the residue, which was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=20:1 to 5:1) to afford the title compound (40 g, yield 88%) as a white solid.
[0722] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 6.93-6.91 (m, 1H), 6.66 (t, J=7.7 Hz, 1H), 6.54-6.51 (m, 1H), 4.24-4.20 (m, 1H), 4.08 (s, 1H), 3.37-3.35 (m, 1H), 3.14-.311 (m, 1H), 2.89-2.76 (m, 1H), 2.76-2.62 (m, 1H), 2.37-2.22 (m, 1H), 1.96-1.93 (m, 1H), 1.89-1.77 (m, 1H), 1.75-1.63 (m, 1H).
58.4 Preparation of 2-(9-bromo-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)aceta- mide
[0723] A mixture of 9-bromo-1,2,3,3a,4,5,5a,6-octahydropyrrolo[1,2-a]quinoxaline (20 g, 79 mmol), 2-bromoacetamide (32.7 g, 237 mmol), and DIEA (51.1 g, 395 mmol) in DMF (200 mL) was stirred at 100.degree. C. for 15 h. The reaction was cooled, diluted with water (600 ml), and extracted with ethyl acetate (3.times.400 ml). The organic layers were washed with brine (200 ml), dried over Na.sub.2SO.sub.4 and filtered, concentrated to give a residue, which was recrystallized from ethyl acetate to afford the title compound (22 g, yield 90%) as a light yellow solid.
[0724] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.04 (d, J=7.9 Hz, 1H), 6.75 (t, J=7.9 Hz, 1H), 6.55 (d, J=7.9 Hz, 1H), 6.39 (s, 1H), 5.69 (s, 1H), 4.28-4.25 (m, 1H), 3.96-3.75 (m, 2H), 3.55-3.42 (m, 1H), 3.13-3.04 (m, 1H), 3.03-2.91 (m, 1H), 2.77-2.65 (m, 1H), 2.39-2.23 (m, 1H), 2.04-1.90 (m, 1H), 1.90-1.78 (m, 1H), 1.71-1.67 (m, 1H)
58.5 Preparation of 2-(9-bromo-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)ethan- amine
[0725] To a solution of 2-(9-bromo-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)aceta- mide (11 g, 35.5 mmol) in THF (165 ml) was added dropwise BH.sub.3.DMS (18 ml, 180 mmol, 10 M in DMS) at 23.degree. C., and the resulting solution was heated at 70.degree. C. for 4 h. The reaction was then quenched with methanol. One additional reaction was set up and quenched as described above. The two resulting mixtures were combined, concentrated to about one third of volume, 6N HCl (200 mL) was added, and the resulting solution was heated at 70.degree. C. for 20 minutes. The solution was then cooled, adjusted to pH 9 by addition of saturated aqueous K.sub.2CO.sub.3, and extracted with ethyl acetate (3.times.300 m). The extracts were washed with brine, dried over Na.sub.2SO.sub.4 and concentrated to give a residue, which was purified by column chromatography on silica gel (eluted with dichloromethane/methanol=60:1 to 15:1) to afford the title compound (15 g, yield 71%) as a light yellow oil.
[0726] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 6.94-6.87 (m, 1H), 6.78-6.67 (m, 2H), 4.25-4.21 (m, 1H), 3.51-3.40 (m, 1H), 3.39-3.28 (m, 2H), 3.07-3.05 (m, 1H), 3.02-2.84 (m, 3H), 2.67-2.57 (m, 1H), 2.29-2.27 (m, 1H), 2.00-1.87 (m, 1H), 1.87-1.74 (m, 1H), 1.71-1.60 (m, 1H), 1.26 (s, 2H)
58.6 Preparation of 1-bromo-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0727] To a solution of 2-(9-bromo-2,3,3a,4,5a,6-hexahydro-1H-pyrrolo[1,2-a]quinoxalin-5-yl)ethan- amine (13 g, 43.9 mmol) in ethanol (390 ml) was added formaldehyde (7.1 g, 88 mmol, 37% aqueous) and TFA (15 g, 132 mmol), and the resulting solution was heated at 80.degree. C. for 2 h. Then the reaction was concentrated, the residue was dissolved in ethyl acetate (300 ml), and washed with saturated aqueous K.sub.2CO.sub.3 (150 ml), and brine (100 ml). The ethyl acetate layer was dried over Na.sub.2SO.sub.4, filtered, and concentrated to afford the title compound (13 g, crude) as a yellow solid.
[0728] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.17-7.08 (m, 1H), 6.69-6.60 (m, 1H), 4.40-4.27 (m, 1H), 3.96-3.78 (m, 2H), 3.24-3.14 (m, 1H), 3.13-3.02 (m, 2H), 3.01-2.88 (m, 3H), 2.79-2.70 (m, 1H), 2.66-2.58 (m, 1H), 2.36-2.28 (m, 1H), 2.23 (s, 1H), 1.99-1.88 (m, 1H), 1.83-1.71 (m, 1H), 1.71-1.61 (m, 1H)
[0729] Analytical Method: The gradient was 1-90% B in 3.4 min, 90-100% B in 0.45 min, 100-1% B in 0.01 min, and then held at 1% B for 0.65 min (0.8 mL/min flow rate). Mobile phase A was 0.0375% TFA in water, mobile phase B was 0.018% TFA in acetonitril. The column used for the chromatography was a 2.1.times.50 mm Venusil XBP-C18 column (5 m particles). Detection methods are diode array (DAD) and evaporative light scattering (ELSD) detection as well as positive/negative electrospray ionization.).
Example 59
1-Methyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline, Trifluoroacetic Acid (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-869 of Table A)
59.1 Preparation of boc-protected 1-bromo-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0730] To a solution of 1-bromo-5,6,7,9,9a, 10,1 1,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (13 g, 42.2 mmol) in dichloromethane (DCM) (260 ml) were added triethylamine (6.4 g, 63.3 mmol) and a solution of Boc.sub.2O (10.1 g, 46.4 mmol) in DCM (20 ml) at 0.degree. C., then it was allowed to warm to 23.degree. C. and stirred for 16 h. The reaction was diluted with DCM (250 ml), washed with 2 N HCl (200 ml), saturated aqueous K.sub.2CO.sub.3 (150 ml) and brine (150 ml), and the water phase was extracted with DCM (200 mL) again. The combined organic layers were dried over Na.sub.2SO.sub.4, filtered, and concentrated, and the residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=30:1 to 4:1) to give the boc-protected compound (6 g, yield 35%, 2 steps).
[0731] LCMS (ESI+): m/z 408 (M+H).sup.+
[0732] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: 7.11 (d, J=7.50 Hz, 1H), 6.73 (d, J=7.94 Hz, 1H), 4.67-4.50 (m, 1H), 4.36-4.24 (m, 1H), 4.18-4.01 (m, 1H), 3.91 (d, J=11.91 Hz, 1H), 3.27 (s, 1H), 3.20-2.90 (m, 4H), 2.76-2.55 (m, 2H), 2.27-2.25 (m, 1H), 1.97-1.66 (m, 3H), 1.46 (m, 9H)
59.2 Preparation of boc-protected 1-methyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0733] To a mixture of boc-protected 1-bromo-5,6,7,9,9a,10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[- 1,2-a]quinoxaline (500 mg, 1.2 mmol), tricyclohexylphosphine (34.3 mg, 0.12 mmol), and Pd(OAc).sub.2 (13.7 mg, 0.06 mmol) in toluene (20 ml) under N.sub.2 atmosphere was added methylboronic acid (366 mg, 6.1 mmol), K.sub.3PO.sub.4 (1.04 g, 4.9 mmol) and Water (2 mL). The reaction mixture was heated at 100.degree. C. for 16 h. After cooling, the reaction mixture was diluted with ethyl acetate (100 ml) and washed with brine. The organic layer was dried over Na.sub.2SO.sub.4, filtered and concentrated. The residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=25:1 to 4:1) to afford the title compound (0.35 g, yield 73%) as white solid.
[0734] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 6.95-6.49 (m, 2H), 4.81-4.48 (m, 1H), 4.20-3.68 (m, 3H), 3.52-3.17 (m, 2H), 3.11-2.81 (m, 3H), 2.78-2.51 (m, 2H), 2.32 (s, 3H), 2.01-1.86 (m, 1H), 1.86-1.71 (m, 1H), 1.71-1.60 (m, 1H), 1.44 (s, 9H)
59.3 Preparation of 1-methyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0735] To a solution of boc-protected 1-methyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (350 mg, 0.9 mmol) in DCM (6 ml) was added TFA (3 ml), and the resulting solution was stirred at 20.degree. C. for 5 h. The reaction was then concentrated and the residue was purified by Prep-HPLC and lyophilized to afford the title compound (242 mg, yield 92%, TFA salt) as a light gray solid.
[0736] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: 7.12 (d, J=7.5 Hz, 1H), 6.97-6.86 (m, 1H), 4.34-4.22 (m, 2H), 4.16-4.11 (m, 1H), 3.69-3.66 (m, 1H), 3.54-3.43 (m, 1H), 3.39-3.33 (m, 2H), 3.30-3.23 (m, 2H), 3.10-2.97 (m, 2H), 2.43 (s, 3H), 2.41-2.31 (m, 1H), 2.13-1.98 (m, 2H), 1.95-1.85 (m, 1H)
Example 60
1-Cyclopropyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline, Trifluoroacetic Acid (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-873 of Table A)
[0737] The compound was prepared in analogy to example 59.
[0738] LCMS (ESI+): m/z 270 (M+H).sup.+
[0739] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: 7.10 (d, J=7.9 Hz, 1H), 6.67 (d, J=7.9 Hz, 1H), 4.41-4.38 (m, 1H), 4.26 (s, 2H), 3.67-3.66 (m, 1H), 3.52-3.42 (m, 1H), 3.39-3.33 (m, 2H), 3.29-3.21 (m, 2H), 3.15-3.09 (m, 1H), 3.00-2.87 (m, 1H), 2.46-2.31 (m, 1H), 2.17-1.94 (m, 3H), 1.93-1.82 (m, 1H), 1.20-1.17 (m, 1H), 1.07-0.93 (m, 2H), 0.69-0.54 (m, 1H).
Example 61
1-Cyclobutyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-874 of Table A)
61.1 Preparation of boc-protected 1-(1-hydroxy-cyclobut-1-yl)-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0740] To a solution of boc-protected 1-bromo-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (800 mg, 1.96 mmol) in THF (16 ml) was added dropwise n-BuLi (1.6 ml, 3.92 mmol, 2.5 M in hexane) at -70.degree. C. with stirring under N.sub.2 atmosphere, and the resulting solution was stirred at -70.degree. C. for 1 hour. A solution of cyclobutanone (275 mg, 3.92 mmol) in THF (0.5 ml) was added dropwise at -70.degree. C., and the reaction solution was stirred for 2 h at same temperature. Then it was allowed to warm slowly to 20.degree. C. and stirred for 16 h. The reaction was quenched by saturated aqueous NH.sub.4Cl, extracted with ethyl acetate (2.times.100 ml), the organic layers were dried over Na.sub.2SO.sub.4, concentrated, and the residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=10:1 to 2:1) to afford the title compound (0.5 g, yield 64%) as white solid.
[0741] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.07-6.90 (m, 2H), 4.64-4.48 (m, 1H), 4.23 (d, J=14.1 Hz, 1H), 3.86 (d, J=11.5 Hz, 1H), 3.68-3.67 (m, 1H), 3.52-3.31 (m, 1H), 3.25-3.11 (m, 2H), 3.10-2.94 (m, 2H), 2.94-2.76 (m, 2H), 2.60-2.47 (m, 2H), 2.46-2.33 (m, 2H), 2.29-2.16 (m, 1H), 2.01-1.82 (m, 3H), 1.82-1.68 (m, 1H), 1.68-1.54 (m, 1H), 1.41 (s, 9H).
61.2 Preparation of 1-cyclobutyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0742] To a solution of boc-protected 1-(1-hydroxy-cyclobut-1-yl)-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (380 mg, 0.95 mmol) in DCM (3 ml) was added triethylsilane (1.66 g, 14.3 mmol), and the resulting solution was stirred for 0.5 h. Then TFA (10 ml) was added, and the resulting solution was stirred at 60.degree. C. overnight. The solvent was removed under reduced pressure. The residue was dissolved in 3 N HCl (50 ml), and washed with DCM (3.times.50 ml). The aqueous phase was adjusted to pH 9 by addition of saturated aqueous K.sub.2CO.sub.3, the resulting mixture was extracted with DCM (2.times.100 ml), the DCM layers were washed with brine (30 ml), dried over Na.sub.2SO.sub.4, filtered, and concentrated to afford the title compound (160 mg, yield 60%) as a gray solid.
[0743] LCMS (ESI+): m/z 284(M+H)
[0744] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: 6.91 (d, J=7.9 Hz, 1H), 6.79 (d, J=7.5 Hz, 1H), 3.89-3.71 (m, 3H), 3.69-3.60 (m, 1H), 3.17-3.05 (m, 1H), 3.05-2.84 (m, 5H), 2.68-2.48 (m, 2H), 2.48-2.36 (m, 1H), 2.33-2.18 (m, 2H), 2.17-1.93 (m, 3H), 1.93-1.74 (m, 3H), 1.68-1.54 (m, 1H)
Example 62
1-Cyclopentyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline, Trifluoroacetic Acid (Compound of the Formula I.a in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-875 of Table A)
62.1 Preparation of boc-protected 1-(1-hydroxy-cyclopent-1-yl)-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0745] To a solution of boc-protected 1-bromo-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (100 mg, 0.25 mmol) in THF (2 ml) was added dropwise n-BuLi (0.2 ml, 0.5 mmol, 2.5 M in hexane) at -70.degree. C. with stirring under N.sub.2 atmosphere, and the resulting solution was stirred at -70.degree. C. for 1 hour. A solution of cyclopentanone (40 mg, 0.5 mmol) in THF (0.2 ml) was added dropwise at -70.degree. C., and the reaction solution was stirred for 8 h at same temperature. It was then allowed to warm slowly to 20.degree. C. and stirred for 16 h. The reaction was quenched by saturated aqueous NH.sub.4Cl, extracted with ethyl acetate (2.times.50 ml), the organic layers were dried over Na.sub.2SO.sub.4, concentrated, and the residue was purified by Prep-TLC (petrol ether/ethyl acetate=2:1) to afford the title compound (16 mg, yield 22%) as white solid.
[0746] .sup.1H NMR (400 MHz; DMSO-d.sub.6 (T=273+80 K)): .delta. [ppm]: 7.50 (s, 1H), 6.81-6.75 (m, 1H), 6.74-6.68 (m, 1H), 4.46-4.24 (m, 2H), 3.75-3.66 (m, 2H), 3.42-3.26 (m, 2H), 3.24-3.12 (m, 2H), 2.93 (d, J=9.7 Hz, 1H), 2.63-2.51 (m, 2H), 2.15-2.03 (m, 4H), 1.99-1.90 (m, 1H), 1.83-1.82 (m, 5H), 1.72-1.55 (m, 3H), 1.34 (s, 9H)
62.2 Preparation of boc-protected 1-cyclopent-1-enyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0747] A mixture of boc-protected 1-(1-hydroxy-cyclopent-1-yl)-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (100 mg, 0.25 mmol), 2-(cyclopent-1-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (95 mg, 0.49 mmol), Pd(PPh.sub.3).sub.2Cl.sub.2 (12 mg, 0.017 mmol) and Cs.sub.2CO.sub.3 (160 mg, 0.49 mmol) in dioxane (2 ml) and water (0.6 ml) was placed in a microwave tube under N.sub.2 atmosphere. The reaction mixture was heated at 100 C for 1 hour in microwave reactor. Six additional reactions were set up as described above. All seven reaction mixtures were combined, was put into water, extracted with ethyl acetate (2.times.100 ml); the extracts were washed with brine, dried over Na.sub.2SO.sub.4, and concentrated to give crude product, which was purified by column chromatography on silica gel (eleted with petrol ether/ethyl acetate=25:1 to 5:1) to afford the title compound (400 mg, yield 58%) as light yellow solid.
[0748] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta.[ppm]: 6.93-6.68 (m, 2H), 5.94-5.78 (m, 1H), 4.81-4.52 (m, 1H), 4.15 (d, J=13.7 Hz, 1H), 4.08-3.78 (m, 1H), 3.71-3.58 (m, 1H), 3.45-3.17 (m, 2H), 3.15-2.85 (m, 4H), 2.69-2.41 (m, 4H), 2.38-2.18 (m, 2H), 2.02-1.96 (m, 2H), 1.86-1.66 (m, 2H), 1.59-1.51 (m, 1H), 1.44 (s, 9H)
62.3 Preparation of boc-protected 1-cyclopentyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0749] A mixture of 1-cyclopent-1-enyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (0.3 g, 0.76 mmol), and Pd/C (0.09 g, 5%) in methanol (20 ml) was stirred at 20.degree. C. under a H.sub.2 balloon for 16 h. Then the reaction was filtered, and concentrated to afford the title compound (0.29 g, yield 96%) as light yellow solid.
[0750] LCMS (ESI+): m/z 398 (M+H).sup.+
62.4 Preparation of 1-cyclopentyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline
[0751] To a solution of boc-protected 1-cyclopentyl-5,6,7,9,9a, 10,11,12-octahydro-4H-[1,4]diazepino[1,7,6-de]pyrrolo[1,2-a]quinoxaline (0.29 g, 0.74 mmol) in DCM (6 ml) was added TFA (3 ml), and the resulting solution was stirred for 3 h at 25.degree. C. The reaction was concentrated and lyophilized to afford the title compound (351 mg, yield 90%, TFA salt) as brown oil.
[0752] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta.[ppm]: 7.34 (d, J=8.4 Hz, 1H), 7.13 (d, J=7.9 Hz, 1H), 4.37-4.25 (m, 2H), 4.24-4.14 (m, 1H), 3.96-3.87 (m, 1H), 3.62-3.52 (m, 1H), 3.44-3.32 (m, 4H), 3.30-3.19 (m, 3H), 2.48-2.35 (m, 1H), 2.25-2.04 (m, 4H), 2.04-1.85 (m, 3H), 1.85-1.70 (m, 2H), 1.70-1.56 (m, 2H)
Example 63
8-Methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-241 of Table B)
63.1 Preparation of boc-protected ethyl 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoate
[0753] To a solution of 4-boc-protected 2,3,4,5-tetrahydro-1H-1,4-benzodiazepine (7 g, 28.2 mmol) in acetic acid (14 ml) was added ethyl acrylate (14.1 g, 141 mmol) and the reaction mixture was heated at 100.degree. C. for 16 hrs. Then it was cooled, concentrated, and the residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=25:1 to 5:1) to give the title compound (5 g, yield 51%) as light yellow oil.
[0754] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.32-7.14 (m, 2H), 7.03-6.88 (m, 2H), 4.43-4.26 (m, 2H), 4.20-4.08 (m, 2H), 3.64-3.51 (m, 4H), 3.10-2.99 (m, 2H), 2.63-2.53 (m, 2H), 1.49-1.38 (m, 9H), 1.30-1.19 (m, 3H)
63.2 Preparation of boc-protected 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoic Acid
[0755] To a solution of boc-protected ethyl 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoate (7.5 g, 21.5 mmol) in THF (70 mL), H.sub.2O (50 mL) and methanol (23 ml) was added a solution of NaOH (2.6 g, 64.6 mmol) in H.sub.2O (20 mL) at 5.degree. C., and the resulting solution was stirred at 25.degree. C. for 3 h. Then the solution was cooled to 5.degree. C., adjusted to pH 3 by addition of 1N HCl, and the resulting mixture was extracted with ethyl acetate three times. The ethyl acetate layers were combined, dried over Na.sub.2SO.sub.4, filtered, and concentrated to give the title compound (6.7 g, yield 97%) as yellow oil.
[0756] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: .delta. 7.23-7.06 (m, 2H), 6.98-6.85 (m, 2H), 4.39-4.21 (m, 2H), 3.65-3.41 (m, 4H), 3.05-2.91 (m, 2H), 2.56 (t, J=6.6 Hz, 2H), 1.40-1.31 (m, 9H).
63.3 Preparation of boc-protected 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoyl Chloride
[0757] To a solution of boc-protected 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoic acid (6.5 g, 20.3 mmol) in dichloromethane (300 ml) were added dimethylformamide (0.15 g, 2 mmol) and dropwise a solution of (COCl).sub.2 (7.7 g, 60.9 mmol) in dichloromethane (30 ml) at 0.degree. C. The the reaction was stirred for 1 hour at 25.degree. C., and concentrated under reduced pressure below 30.degree. C. to give the title compound as a residue which was used directly in the next step without further purification.
63.4 Preparation of boc-protected 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one
[0758] To a solution of boc-protected 3-(2,3,4,5-tetrahydro-1,4-benzodiazepin-1-yl)propanoyl chloride (6.6 g, crude, .about.20 mmol) in dichloroethane (400 ml) was added A1Cl.sub.3 (10.4 g, 78 mmol) in portions at 0-5.degree. C., then the resulting mixture was stirred at 65.degree. C. for 7 hrs. The reaction was quenched with water (100 ml), adjusted to pH 9 by addition of aqueous saturated K.sub.2CO.sub.3, then Boc.sub.2O (6.4 g, 29.2 mmol) was added, and the resulting mixture was stirred for 2 hrs. The mixture was extracted with dichloromethane twice, the organic phase was washed with brine, dried over Na.sub.2SO.sub.4, concentrated, and the residue was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=10:1 to 2:1) to give the title compound (4.2 g, yield 71%) as yellow solid.
[0759] .sup.1H NMR (400 MHz, CDCl.sub.3): .delta. [ppm]: 7.85 (d, J=7.5 Hz, 1H), 7.40-7.20 (m, 1H), 6.83 (t, J=7.5 Hz, 1H), 4.58-4.40 (m, 2H), 3.74 (d, J=3.5 Hz, 2H), 3.60-3.50 (m, 2H), 3.44-3.37 (m, 2H), 2.72-2.62 (m, 2H), 1.47-1.29 (m, 9H).
63.5 Preparation of 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one
[0760] To a solution of boc-protected 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one (500 mg, 1.7 mmol) in dichloromethane (10 ml) was added trifluoroacetic acid (5 ml), and then the resulting mixture was stirred at 25.degree. C. for 3 hrs. This was concentrated and lyophilized to give the title compound (500 mg, yield 100%, trifluoroacetic acid salt) as yellow solid.
[0761] .sup.1H NMR (400 MHz; CD.sub.3OD): .delta. [ppm]: 7.92-790 (m, 1H), 7.49 (d, J=6.6 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 4.40 (s, 2H), 3.71-3.63 (m, 4H), 3.55-3.47 (m, 2H), 2.75-2.67 (m, 2H).
63.6 Preparation of Cbz-protected 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one
[0762] To a solution of 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one (2 g, 6.92 mmol) (TFA salt) in DCM (30 ml) were added triethylamine (1.4 g, 13.8 mmol) and a solution of CbzCl (Cbz=benzyloxycarbonyl; 1.5 g, 9 mmol) in DCM (5 mL) at 0-5.degree. C., then the reaction mixture was stirred at 25.degree. C. for 2 hrs. Then it was diluted with DCM (100 ml), washed with aqueous K.sub.2CO.sub.3 (50 mL) and brine (50 ml); the water phase was extracted with DCM again, the DCM layer was dried over Na.sub.2SO.sub.4, filtered, and concentrated under reduced pressure to give the title compound (1.5 g, yield 64%) as brown oil.
[0763] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.79 (d, J=7.6 Hz, 1H), 7.30-7.11 (m, 6H), 6.76-6.72 (m, 1H), 5.04-5.01 (m, 2H), 4.55-4.48 (m, 2H), 3.75-3.74 (m, 2H), 3.48-3.38 (m, 4H), 2.60-2.55 (m, 2H)
63.7 Preparation of Cbz-protected 8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol
[0764] To a solution of Cbz-protected 1,2,3,4,6,7-hexahydro[1,4]diazepino[6,7,1-ij]-quinolin-8-one (1.5 g, 4.5 mmol) in THF (45 ml) was added dropwise methyllithium (3.7 ml, 5.8 mmol, 1.6 M in ether) at -70.degree. C., and the resulting solution was stirred at -70.degree. C. for 2 h. Then it was quenched with aqueous NH.sub.4Cl (20 ml), extracted with ethyl acetate (50 ml) three times, the ethyl acetate layers were washed with brine (30 ml), dried over Na.sub.2SO.sub.4, filtered, concentrated, and the residue was purified by column chromatography on silica gel (eluted with DCM/methanol=150:1 to 50:1) to give the title compound (1 g, yield 64%) as light yellow oil.
[0765] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.47 (d, J=7.5 Hz, 1H), 7.39-7.25 (m, 5H), 7.25-6.99 (m, 1H), 6.93-6.80 (m, 1H), 5.18-5.02 (m, 2H), 4.62-4.35 (m, 2H), 3.79 s, 1H), 3.73-3.59 (m, 1H), 3.45-3.34 (m, 1H), 3.29-3.15 (m, 3H), 2.00-1.89 (m, 2H), 1.59 (s, 3H)
63.8 Preparation of 8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol
[0766] A mixture of Cbz-protected 8-hydroxy-8-methyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quino- line (0.5 g, 1.42 mmol), NH.sub.3--H.sub.2O (1 ml) and Pd/C (168 mg, 10%) in THF (30 ml) was stirred under a H.sub.2 balloon for 2 h. Then it was filtered, concentrated, and the residue was purified by Prep-HPC (basic method) and lyophilized to give the title compound (214 mg, yield 69%) as white solid.
[0767] LCMS (ESI+): m/z 219 (M+H).sup.+
[0768] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.44 (d, J=7.5 Hz, 1H), 7.01 (d, J=7.1 Hz, 1H), 6.83 (t, J=7.5 Hz, 1H), 3.92-3.81 (m, 2H), 3.43-3.32 (m, 1H), 3.29-3.17 (m, 1H), 3.14-3.00 (m, 4H), 1.99-1.91 (m, 2H), 1.59 (s, 3H)
Example 64
8-Methoxy-8-methyl-2,3,4,6,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinol- ine (Compound of the Formula I.g, I.h or I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-201 of Table B)
64.1 Preparation of Cbz-protected 8-methoxy-8-methyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quino- line
[0769] To a solution of Cbz-protected 8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol (0.5 mg, 1.42 mmol; see example 63.2) in DMF (10 ml) was added NaH (113 mg, 2.84 mmol) in portions at 0.about.5.degree. C., and then the resulting mixture was stirred at 25.degree. C. for 0.5 hrs. A solution of MeI (403 mg, 2.84 mmol) in DMF (2 ml) was added dropwise at 0-5.degree. C., then the reaction mixture was stirred at 25.degree. C. for 16 h. It was quenched with water (30 ml), and extracted with ethyl acetate (60 ml) twice. The ethyl acetate layer was washed with brine, dried over Na.sub.2SO.sub.4, and concentrated to give a residue, which was purified by column chromatography on silica gel (eluted with petrol ether/ethyl acetate=15:1 to 4:1) to give the title compound (200 mg, yield 39%) as light yellow oil.
[0770] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.41-7.24 (m, 6H), 7.23-7.01 (m, 1H), 6.93-6.79 (m, 1H), 5.12 (s, 2H), 4.64-4.49 (m, 1H), 4.44-4.34 (m, 1H), 3.88-3.75 (m, 1H), 3.69-3.58 (m, 1H), 3.40-3.26 (m, 2H), 3.23-3.12 (m, 2H), 3.08 (s, 3H), 2.28-2.19 (m, 1H), 1.72-1.63 (m, 1H), 1.53 (s, 3H)
64.2 Preparation of 8-methoxy-8-methyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quino- line
[0771] A mixture of Cbz-protected 8-methoxy-8-methyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quino- line (160 mg, 0.44 mmol), NH.sub.3--H.sub.2O (0.5 ml) and Pd/C (50 mg, 10%) in THF (20 ml) was stirred under a H.sub.2 balloon at 25.degree. C. for 2 hrs. Then it was filtered, concentrated, and the residue was purified by Prep-HPC (basic method) and lyophilized to give the title compound (55 mg, yield 54%) as yellow oil.
[0772] LCMS (ESI+): m/z 233(M+H).sup.+
[0773] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.30-7.27 (m, 1H), 7.02 (d, J=7.1 Hz, 1H), 6.83 (t, J=7.5 Hz, 1H), 3.94-3.84 (m, 2H), 3.38-3.24 (m, 2H), 3.16-3.01 (m, 7H), 2.27-2.22 (m, 1H), 1.69-1.63 (m, 1H), 1.54 (s, 3H)
Example 65
2,8-Dimethyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-o- l
[0774] A mixture of Cbz-protected 8-methyl-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinolin-8-ol (50 mg, 0.14 mmol; see example 63.2), formaldehyde (21 mg, 0.28 mmol, 40% in water) and Pd/C (17 mg, 10%) in methanol (5 ml) was stirred under a H.sub.2 balloon for 6 hrs. Then it was filtered, concentrated, and the residue was purified by Prep-HPC (basic method) and lyophilized to give the title compound (12 mg, yield 36%) as light yellow solid.
[0775] .sup.1H NMR (400 MHz; CDCl.sub.3): .delta. [ppm]: 7.44-7.42 (m, 1H), 7.07-7.00 (m, 1H), 6.83 (t, J=7.5 Hz, 1H), 3.71-3.57 (m, 2H), 3.40-3.31 (m, 1H), 3.25-3.17 (m, 1H), 3.17-3.04 (m, 2H), 2.82 (t, J=5.1 Hz, 2H), 2.37 (s, 3H), 1.94 (t, J=6.0 Hz, 2H), 1.58 (s, 3H)
Example 66
11-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane], 2,2,2-trifluoroacetic Acid (Compound of the Formula I.i in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-325 of Table B)
[0776] The title compound was prepared in analogy to example 1, using however tert-butyl 6-methyl-2,3,4,5-tetrahydro-1H-1,4-benzodiazepine-4-carboxylate and 2-cyclobutylidene-acetic acid as starting materials.
[0777] ESI-MS [M+H.sup.+]=243.20
[0778] .sup.1H NMR (DMSO-d.sub.6, 500 MHz) [ppm]: 7.28 (d, J=7.9 Hz, 1H), 6.71 (d, J=7.9 Hz, 1H), 3.74 (s, 2H), 3.10-3.03 (m, 2H), 2.99-2.94 (m, 2H), 2.90-2.84 (m, 2H), 2.34-2.25 (m, 2H), 2.22 (s, 3H), 2.09-1.96 (m, 1H), 1.92-1.83 (m, 5H).
Example 67
9-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'- -cyclobutane], Trifluoroacetic Acid (Compound of the Formula I.g in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-325 in Table B)
[0779] The intermediate tert-butyl 9-chloro-3,4,6,7-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-c- yclobutane]-2(1H)-carboxylate was prepared in analogy to example 1, steps 1.1 to 1.3 using tert-butyl 8-chloro-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate and 2-cyclobutylideneacetyl chloride as starting materials.
67.1 Preparation of tert-butyl 9-methyl-3,4,6,7-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-c- yclobutane]-2(1H)-carboxylate
[0780] 75 mg (0.21 mmol) of tert-butyl 9-chloro-3,4,6,7-tetrahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'-c- yclobutane]-2(1H)-carboxylate were dissolved in 0.5 ml of tetrahydrofurane, 0.5 ml toluene and 5 .mu.l water. To this solution 35 .mu.l (0.25 mmol) trimethylboroxine, 0.2 mg (0.4 .mu.mol) of dicyclohexyl(2',4',6'-triisopropyl-[1,1'-biphenyl]-2yl)phosphine and 65.8 mg (0.31 mmol) of potassium phosphate were added and the solution was degassed with Argon. 0.1 mg (0.4 .mu.mol) of palladium(II)acetate were added and the reaction mixture was heated at 100.degree. C. for 10 minutes in a microwave unit. The reaction mixture was concentrated in vacuo. The residue was purified by column chromatography on silica (eluent: 0-30% methanol in dichloromethane) to yield 40 mg of the title compound.
[0781] ESI-MS [M+H.sup.+]=343.20
67.2 Preparation of 9-methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]-quinoline-8,- 1'-cyclobutane], Trifluoroacetic Acid
[0782] To 40 mg (0.12 mmol) of tert-butyl 9-methyl-3,4,6,7-tetrahydrospiro[[1,4]diazepino-[6,7,1-ij]quinoline-8,1'-- cyclobutane]-2(1H)-carboxylate in 3 ml dichloromethane were added 90 .mu.l (1.2 mmol) of trifluoroacetic acid at 0.degree. C. The solution was stirred for 2 h at room temperature. The reaction mixture was concentrated in vacuo. The residue was purified by preparative HPLC to yield 4.6 mg of the title compound.
[0783] ESI-MS [M+H.sup.+]=243.20
[0784] .sup.1H NMR (CDCl.sub.3, 500 MHz): .delta. [ppm]: 9.15 (bs, 2H), 6.95 (d, 1H), 6.80 (d, 1H), 4.15 (m, 2H), 3.30 (m, 2H), 3.25 (m, 2H), 3.10 (m, 2H), 2.85 (m, 2H), 2.70 (s, 3H), 2.20 (m, 2H), 2.00 (m, 2H), 1.85 (m, 2H).
Example 68
4,5,6,7,9,9a,9b,10,10a, 11-Decahydrocyclopropa[3,4]pyrrolo[1,2-a][1,4]diazepino[1,7,6-de]quinoxal- ine
##STR00024##
[0786] The title compound was prepared in analogy to example 44 using however 1-fluoro-2-nitrobenzene and ethyl 3-azabicyclo[3.1.0]hexane-2-carboxylate as starting materials.
[0787] ESI-MS [M+H+]=242.20
[0788] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.65 (m, 1H), 6.35 (d, 1H), 6.30 (d, 1H), 3.65 (m, 1H), 3.60 (m, 1H), 3.35 (m, 4H), 3.30 (m, 1H), 2.90 (m, 3H), 2.55 (m, 1H), 1.65 (m, 1H), 1.60 (m, 1H), 0.50 (m, 1H), 0.15 (m, 1H).
Example 69
1-Ethyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-289 in Table A)
[0789] The title compound was prepared in analogy to example 33, using however acetaldehyde instead of cyclobutanone.
[0790] ESI-MS [M+H.sup.+]=218.20
[0791] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.70 (m, 1H), 6.60 (d, 1H), 6.40 (d, 1H), 3.65 (s, 2H), 3.35 (m, 2H), 3.15 (m, 2H), 3.10 (m, 2H), 2.90 (m, 2H), 2.80 (m, 2H), 1.05 (t, 3H).
Example 70
1-Propyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline (Compound of the Formula I.a, I.b or I.c in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-325 in Table A)
[0792] The title compound was prepared in analogy to example 33, using however propionaldehyde instead of cyclobutanone.
[0793] ESI-MS [M+H.sup.+]=232.20
[0794] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.70 (m, 1H), 6.60 (d, 1H), 6.40 (d, 1H), 3.65 (s, 2H), 3.25 (m, 2H), 3.15 (m, 4H), 2.90 (m, 2H), 2.80 (m, 2H), 1.50 (m, 2H), 0.90 (t, 3H).
Example 71
1-Cyclobutyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]qui- noxaline (Compound of the Formula I.Gg, I.Hh or I.II in which the Combination of R.sup.5A, R.sup.5B, R.sup.6 and R.sup.9a is as in Row a-433 in Table a)
[0795] The title compound was prepared in analogy to example 33, using tert-butyl 5-methyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-- carboxylate and cyclobutanone. tert-Butyl 5-methyl-2,3,5,6-tetrahydro-1H-[1,4]diazepino[1,7,6-de]quinoxaline-7(8H)-- carboxylate was prepared in analogy to example 30 however using 2-bromopropaneamide instead of 2-chloroacetamide and in analogy to example 32.
[0796] ESI-MS [M+H.sup.+]=258.20
[0797] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.65 (m, 1H), 6.55 (d, 1H), 6.45 (d, 1H), 3.90 (m, 1H), 3.80 (m, 1H), 3.55 (m, 1H), 3.30-3.15 (m, 3H), 3.10 (m, 1H), 2.95 (m, 1H), 2.85 (m, 1H), 2.75 (m, 1H), 2.20 (m, 1H), 2.15 (m, 1H), 2.05 (m, 2H), 1.65 (m, 2H), 0.9 (d, 3H).
Example 72
5-Methyl-1-(oxetan-3-yl)-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]- quinoxaline (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-541 in Table A)
[0798] The title compound was prepared in analogy to example 71, using however oxetane-3-one instead of cyclobutanone.
[0799] ESI-MS [M+H.sup.+]=260.20
[0800] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.60 (m, 1H), 6.55 (d, 1H), 6.05 (d, 1H), 4.80 (m, 1H), 4.75 (m, 1H), 4.65 (m, 1H), 4.60 (m, 1H), 4.45 (m, 1H), 3.80 (d, 1H), 3.50 (d, 1H), 3.35 (m, 2H), 3.15 (m, 2H), 2.90 (m, 1H), 2.85 (m, 1H), 2.75 (m, 1H), 0.9 (d, 3H).
Example 73
1-Ethyl-5-methyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxal- ine (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-289 in Table A)
[0801] The title compound was prepared in analogy to example 71, using however acetaldehyde instead of cyclobutanone.
[0802] ESI-MS [M+H.sup.+]=232.20
[0803] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.80 (m, 1H), 6.65 (d, 1H), 6.50 (d, 1H), 3.95 (d, 1H), 3.80 (d, 1H), 3.45-3.05 (m, 8H), 2.85 (m, 1H), 1.15 (t, 3H), 1.00 (d, 3H).
Example 74
5-Methyl-1-propyl-2,3,5,6,7,8-hexahydro-1H-[1,4]diazepino[1,7,6-de]quinoxa- line (Compound of the Formula I.Gg, I.Hh or I.ii in which the Combination of R.sup.5a, R.sup.5b, R.sup.6 and R.sup.9a is as in Row A-325 in Table A)
[0804] The title compound was prepared in analogy to example 71, using however propionaldehyde instead of cyclobutanone.
[0805] ESI-MS [M+H.sup.+]=246.20
[0806] .sup.1H NMR (DMSO-d.sub.6, 500 MHz): .delta. [ppm]: 6.80 (m, 1H), 6.60 (d, 1H), 6.50 (d, 1H), 3.95 (d, 1H), 3.80 (d, 1H), 3.30 (m, 4H), 3.20 (m, 2H), 3.10 (m, 1H), 3.00 (m, 1H), 2.80 (m, 1H), 1.60 (m, 2H), 0.95 (d, 3H), 0.90 (t, 3H).
Example 75
9-Chloro-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1'- -cyclobutane], Trifluoroacetic Acid (Compound of the Formula I.g in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-323 in Table B)
[0807] The title compound was prepared in analogy to example 1 using tert-butyl 8-chloro-2,3-dihydro-1H-benzo[e][1,4]diazepine-4(5H)-carboxylate and 2-cyclobutylideneacetyl chloride as starting materials.
[0808] ESI-MS [M+H.sup.+]=236.10
[0809] .sup.1H NMR (CDCl.sub.3, 500 MHz): .delta. [ppm]: 9.60 (bs, 2H), 7.00 (m, 2H), 4.15 (s, 2H), 3.25 (m, 6H), 3.10 (m, 2H), 2.15 (m, 2H), 2.05 (m, 2H), 1.75 (m, 2H).
Example 76
10-Methyl-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane], Trifluoroacetic Acid (Compound of the Formula I.h in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-325 in Table B)
[0810] ESI-MS [M+H.sup.+]=243.20
[0811] .sup.1H NMR (CDCl.sub.3, 500 MHz): .delta. [ppm]: 9.35 (bs, 2H), 7.40 (s, 1H), 6.90 (s, 1H), 4.15 (m, 2H), 3.35 (m, 2H), 3.25 (m, 2H), 3.15 (m, 2H), 2.40 (m, 2H), 2.30 (s, 3H), 2.15-1.90 (m, 6H).
Example 77
10-Chloro-1,2,3,4,6,7-hexahydrospiro[[1,4]diazepino[6,7,1-ij]quinoline-8,1- '-cyclobutane], Trifluoroacetic Acid (Compound of the Formula I.h in which the Combination of R.sup.5a, R.sup.5b, R.sup.7, R.sup.8 and R.sup.9a is as in Row B-323 in Table B)
[0812] ESI-MS [M+H.sup.+]=236.10
[0813] .sup.1H NMR (CDCl.sub.3, 500 MHz): .delta. [ppm]: 9.65 (bs, 2H), 7.55 (s, 1H), 7.05 (s, 1H), 4.15 (s, 2H), 3.30 (m, 4H), 3.20 (m, 2H), 2.35 (m, 2H), 2.15-1.90 (m, 6H).
II. Biological Tests
Functional Activity
[0814] The functional activity of compounds of formula I was assayed by incubation with U2OS_HTR.sub.2C--.beta.-Arrestin cells (DiscoverX, 93-0289C3) to induce beta-arrestin2 recruitment to the 5-HT.sub.2C receptor. The agonist-induced recruitment and proximity of the receptor and beta-arrestin2 leads to complementation and formation of active (3-galactosidase. The enzyme complementation results in enzyme activity, which is measured following the termination of the agonist incubation using DiscoveRx's detection reagent, which contains a chemiluminescent substrate which produces a high intensity signal. Cells were plated and a medium-change to a 1% serum containing medium was performed 24 h later. The next day, test compounds were added and incubated for 1.5 h before addition of detection reagent.
[0815] The response produced was measured and compared with the response produced by 10 [mu]M 5-HT or the maximal effect induced by 5-HT (defined as 100%) to which it was expressed as a percentage response (relative efficacy). Dose response curves were constructed using Graphpad Prism (Graph Software Inc.) or using in house adapted software using a 4 parameter dose response model with variable slope (fit=(Bottom+(Top-Bottom)/(1+10 ((LogEC50-x)*HillSlope))res=(y-fit)). Results are compiled in the table below.
TABLE-US-00003 TABLE Potency (EC50) in # functional assay % efficacy 1 ++ 71 6 ++ 63 8 ++ 40 9 +++ 61 16 + 55 17 ++ 84 19 ++ 25 22 ++ 69 25 ++ 90 26 +++ 107 27 + 87 28 ++ 55 33 + 78 35 ++ 112 36 + 31 37 + 21 40 ++ 131 41 + 75 42 +++ 163 43 + 59 44 ++ 55 45 ++ 77 46 + 71 47 ++ 59 48 ++ 105 49 + 78 51 ++ 102 54 + 24 55 + 26 56 +++ 124 57 + 35 59 ++ 51 66 + 53 67 +++ 116 68 ++ 76 69 + 54 70 ++ 63 76 +++ 98 Potency (EC50): + from 200 nM to <1 .mu.M ++ from 20 nM to <200 nM +++ <20 nM
* * * * *













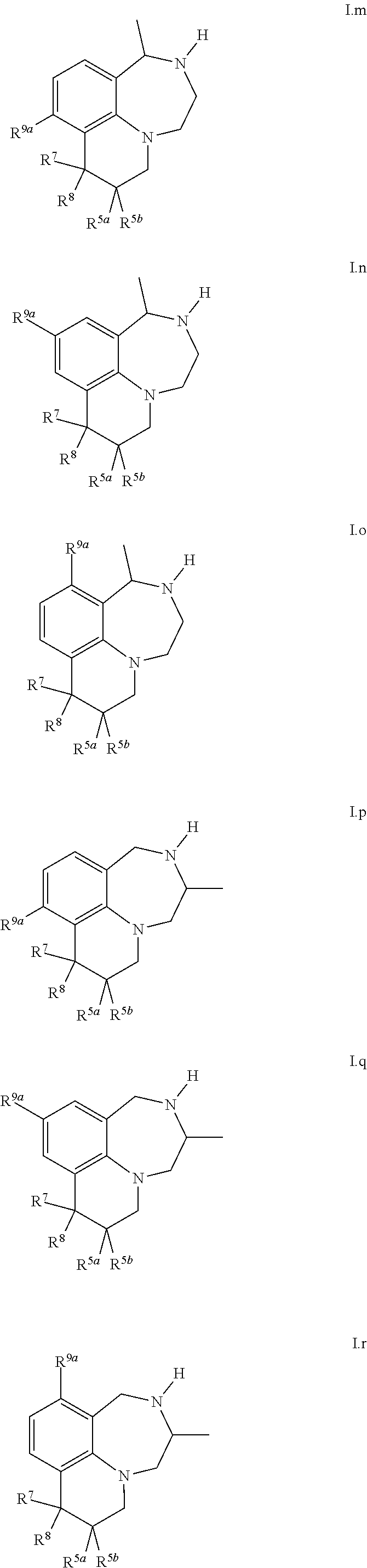


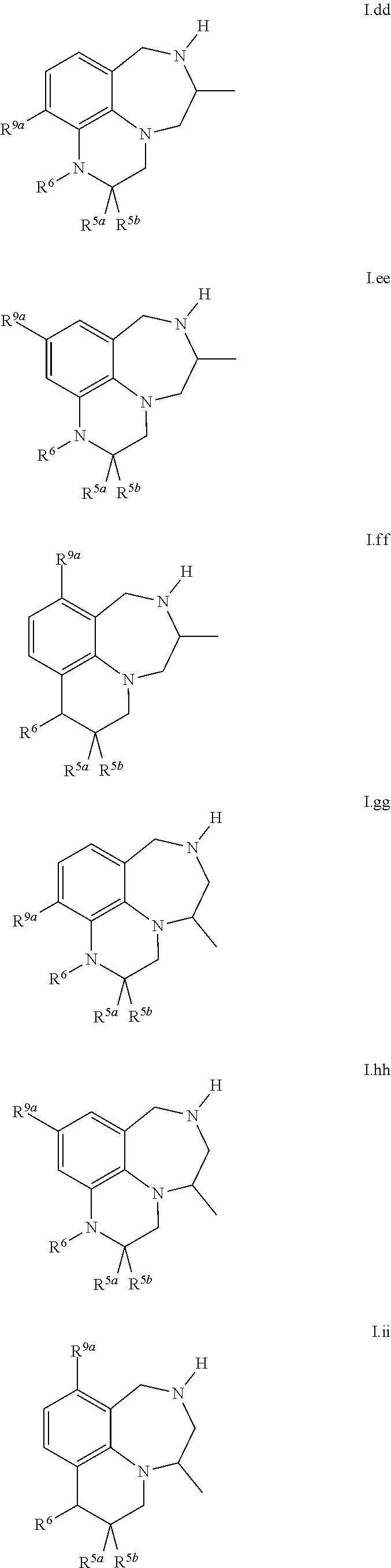


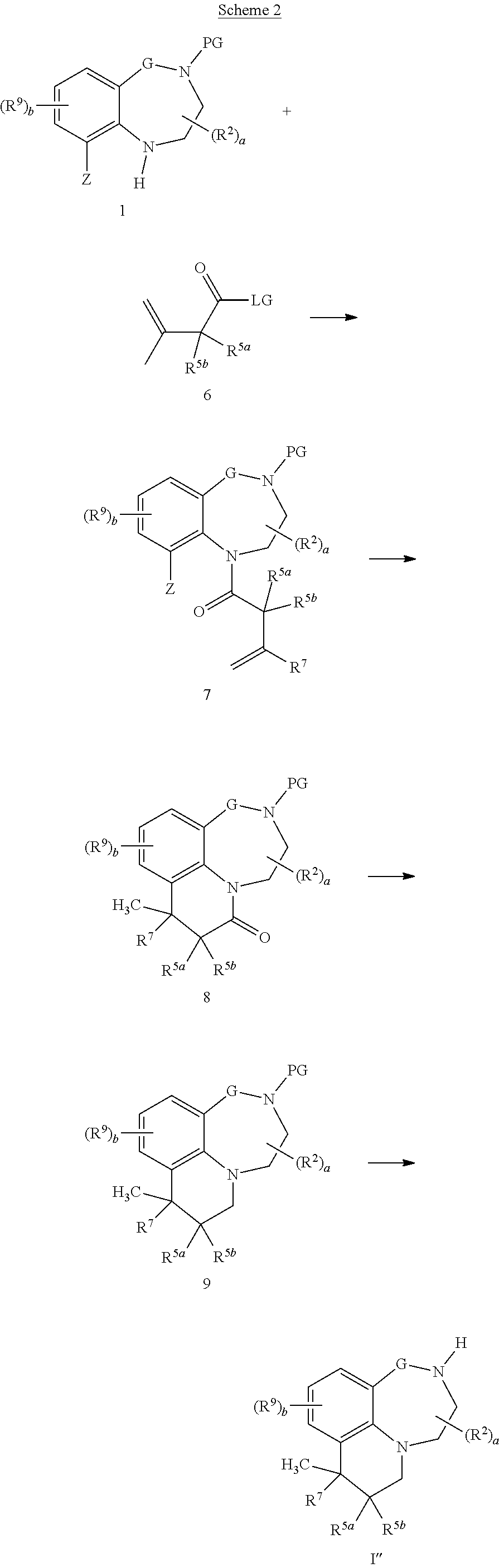
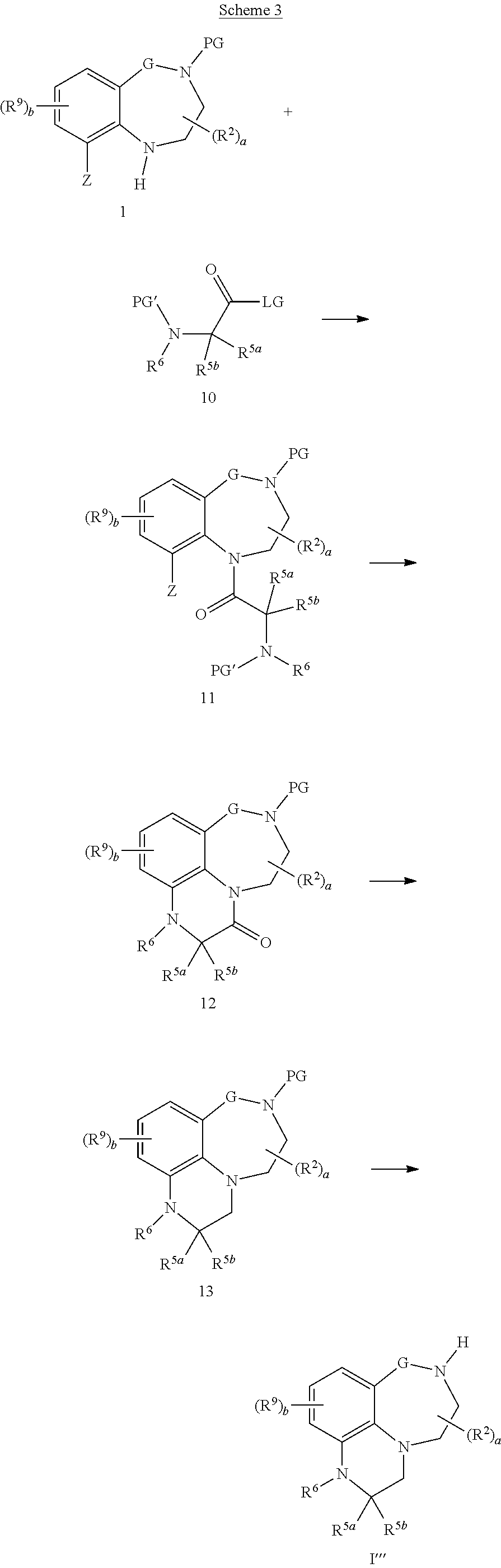

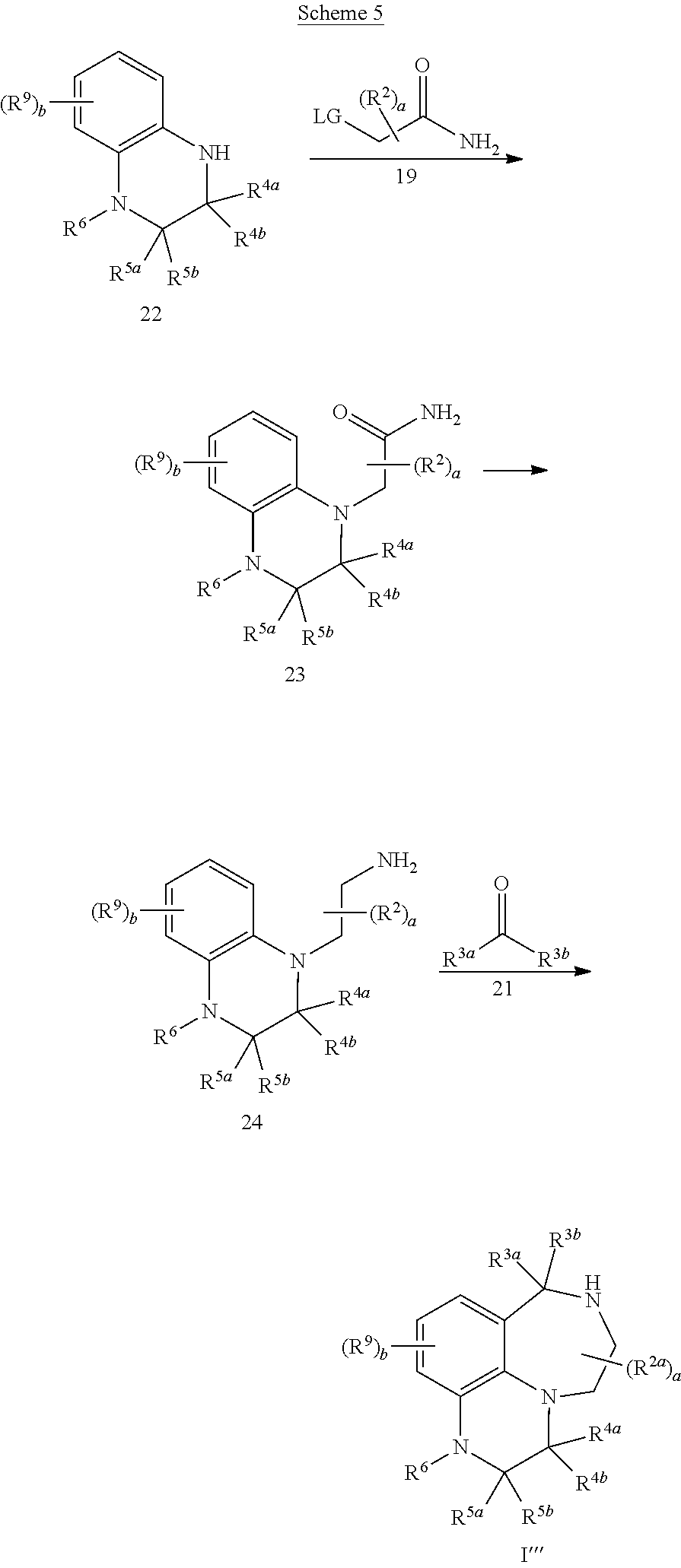
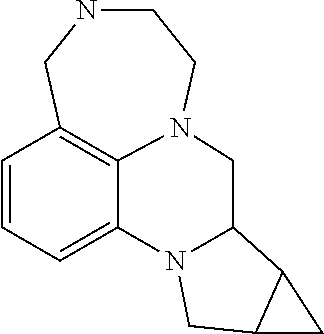
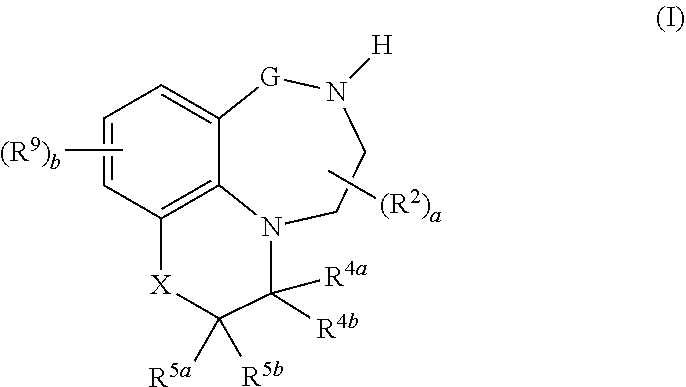
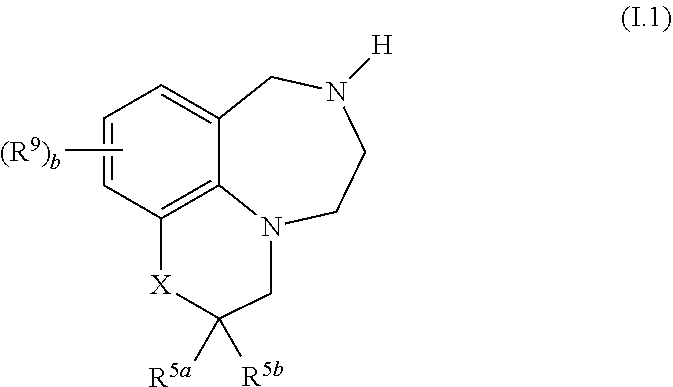


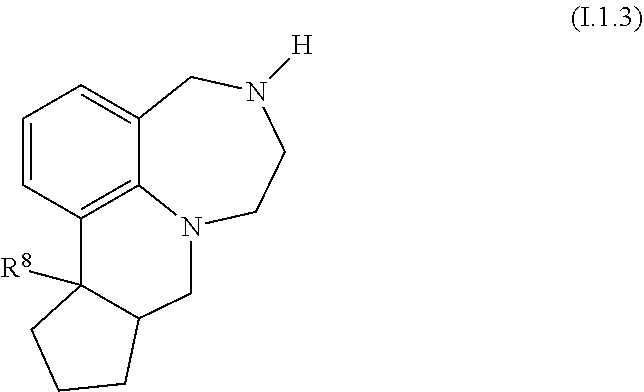

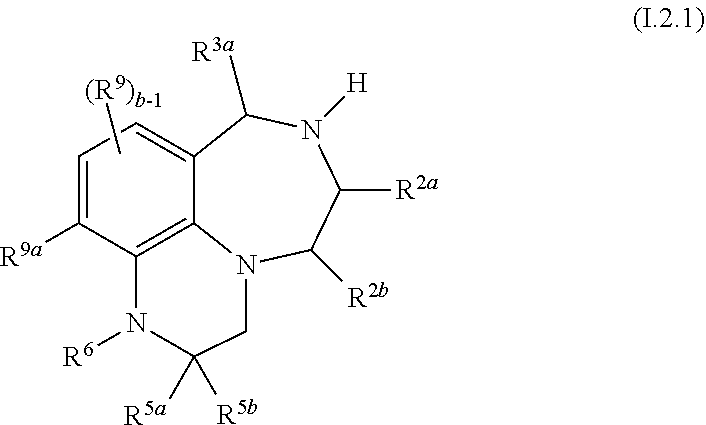
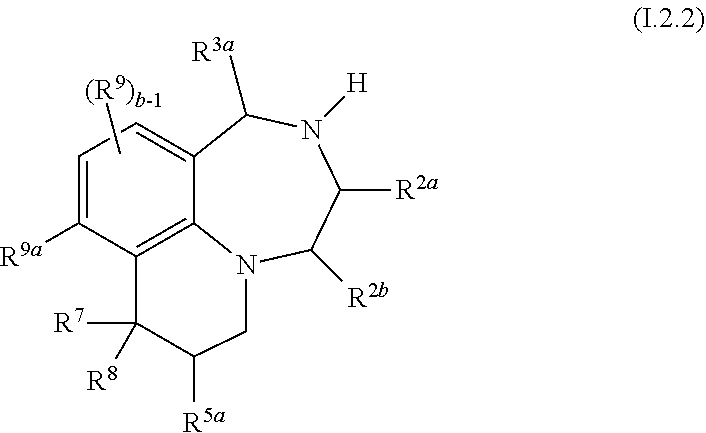
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.