Antibodies Against G-csfr And Uses Thereof
Nash; Andrew Donald ; et al.
U.S. patent application number 16/111323 was filed with the patent office on 2019-02-21 for antibodies against g-csfr and uses thereof. This patent application is currently assigned to CSL LIMITED. The applicant listed for this patent is CSL LIMITED. Invention is credited to Arna Elizabeth Andrews, Manuel Baca, Felicity Meredith Dunlop, Kirsten Mae Edwards, Matthew Philip Hardy, Andrew Donald Nash, Con Panousis.
| Application Number | 20190055313 16/111323 |
| Document ID | / |
| Family ID | 47353852 |
| Filed Date | 2019-02-21 |


| United States Patent Application | 20190055313 |
| Kind Code | A1 |
| Nash; Andrew Donald ; et al. | February 21, 2019 |
ANTIBODIES AGAINST G-CSFR AND USES THEREOF
Abstract
The present disclosure provides proteins comprising antigen binding domains of antibodies that bind to human granulocyte-colony stimulating factor receptor.
| Inventors: | Nash; Andrew Donald; (Victoria, AU) ; Andrews; Arna Elizabeth; (Victoria, AU) ; Baca; Manuel; (Gaithersburg, MD) ; Edwards; Kirsten Mae; (Victoria, AU) ; Hardy; Matthew Philip; (Victoria, AU) ; Panousis; Con; (Victoria, AU) ; Dunlop; Felicity Meredith; (Victoria, AU) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | CSL LIMITED Victoria AU |
||||||||||
| Family ID: | 47353852 | ||||||||||
| Appl. No.: | 16/111323 | ||||||||||
| Filed: | August 24, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 15490318 | Apr 18, 2017 | |||
| 16111323 | ||||
| 14886176 | Oct 19, 2015 | |||
| 15490318 | ||||
| 13495539 | Jun 13, 2012 | 9193793 | ||
| 14886176 | ||||
| 61496351 | Jun 13, 2011 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 37/00 20180101; A61P 7/00 20180101; A61P 37/06 20180101; A61P 29/00 20180101; A61P 11/00 20180101; C07K 2317/76 20130101; A61K 2039/545 20130101; A61K 2039/505 20130101; C07K 16/2866 20130101; C07K 2317/21 20130101; C07K 2317/34 20130101; C07K 2317/73 20130101; A61P 35/00 20180101; C07K 2317/33 20130101; A61P 19/02 20180101; A61P 25/00 20180101; C07K 2317/55 20130101; C07K 2317/92 20130101 |
| International Class: | C07K 16/28 20060101 C07K016/28 |
Claims
1. A protein comprising an antigen binding site of an antibody, wherein the antigen binding site binds to human granulocyte-colony stimulating factor receptor (hG-CSFR) and neutralizes granulocyte-colony stimulating factor (G-CSF) signaling, and wherein the protein inhibits growth of colony forming units-granulocytes (CFU-G) from CD34.sup.+ bone marrow cells grown in the presence of G-CSF with an IC.sub.50 of at least about 0.2 nM.
2. The protein of claim 1, wherein the antigen binding site binds to both human and cynomolgus monkey granulocyte-colony stimulating factor receptor (G-CSFR) with a similar affinity and neutralizes granulocyte-colony stimulating factor (G-CSF) signaling.
3. The protein of claim 1 comprising at least a heavy chain variable region (V.sub.H) and a light chain variable region (V.sub.L), wherein the V.sub.H and V.sub.L bind to form a Fv comprising an antigen binding domain.
4. The protein of claim 3, wherein the V.sub.H and the V.sub.L are in a single polypeptide chain or wherein the V.sub.L and V.sub.H are in separate polypeptide chains.
5. The protein of claim 4, wherein if the V.sub.H and V.sub.1 are in the same polypeptide chain, the protein is: (i) a single chain Fv fragment (scFv); (ii) a dimeric scFv (di-scFv); or (iii) at least one of (i) and/or (ii) linked to a constant region of an antibody, a Fc or a heavy chain constant domain (C.sub.H) 2 and/or C.sub.H3; or if the V.sub.H and V.sub.L are in separate polypeptide chains, the protein is: (i) a diabody; (ii) a triabody; (iii) a tetrabody; (iv) a Fab; (v) a F(ab').sub.2; (vi) a Fv; (vii) an antibody; or (viii) one of (i) to (vi) linked to a constant region of an antibody, a Fc or a heavy chain constant domain (C.sub.H) 2 and/or C.sub.H3.
6. The protein of claim 1 which is chimeric, de-immunized, humanized, human or primatized.
7. The protein of claim 1, which inhibits G-CSF-induced proliferation of a BaF3 cell expressing hG-CSFR with an IC.sub.50 of at least about 0.5 nM.
8. The protein of claim 1, which binds to a polypeptide comprising amino acids 1 to 311 of SEQ ID NO: 1 expressed as a fusion with an antibody Fc region with an affinity of at least about 0.5 nM, wherein affinity is determined in an assay in which the polypeptide immobilized and the protein or antibody contacted to the immobilized polypeptide.
9. The protein of claim 1, which binds to hG-CSFR expressed on the surface of a cell at an affinity of at least about 1 nM.
10. The protein of claim 1 conjugated to another compound.
11. A composition comprising the protein of claim 1 and a pharmaceutically acceptable carrier.
12. A method for treating or preventing a granulocyte-colony stimulating factor (G-CSF)-mediated condition in a subject, the method comprising administering the protein of claim 1 to the subject.
13. The method of claim 12 comprising administering an amount of the protein or antibody sufficient to reduce the number of neutrophils in a subject without inducing neutropenia.
14. The method of claim 13, wherein the amount of the protein is between 0.05 mg/kg and 30 mg/kg.
Description
RELATED APPLICATION
[0001] This application is a continuation U.S. patent application Ser. No. 14/886,176, filed Oct. 19, 2015, which is a continuation of Ser. No. 13/495,539, filed Jun. 13, 2012, which claims the benefit of priority of U.S. Provisional Application No. 61/496,351, filed Jun. 13, 2011, the entire contents of which are incorporated herein by reference.
SEQUENCE LISTING
[0002] The present application is filed together with a Sequence Listing in electronic form. The entire contents of the Sequence Listing are hereby incorporated by reference.
FIELD
[0003] The present disclosure relates to antibodies that bind to granulocyte-colony stimulating factor receptor (G-CSFR) receptor and uses thereof, e.g., in therapy.
BACKGROUND
[0004] Granulocyte colony-stimulating factor (G-CSF) is a major regulator of granulocyte production. G-CSF is produced by bone marrow stromal cells, endothelial cells, macrophages, and fibroblasts, and production is induced by inflammatory stimuli. G-CSF acts through the G-CSF receptor (G-CSFR), which is expressed on early myeloid progenitors, mature neutrophils, monocytes/macrophages, T and B lymphocytes and endothelial cells. Mice deficient in G-CSF or the G-CSFR exhibit marked neutropenia, demonstrating the importance of G-CSF in steady-state granulopoiesis. However, G-CSF appears to be dispensable for emergency granulopoiesis, e.g., in response to an infection. G-CSF increases the production and release of neutrophils, mobilizes hematopoietic stem and progenitor cell, and modulates the differentiation, lifespan, and effector functions of mature neutrophils. G-CSF may also exert effects on macrophages, including expansion of monocyte/macrophage exacerbates rheumatoid arthritis (RA), murine collagen-induced arthritis (CIA) and a passive transfer model of CIA in rats. G-CSF has been found in the serum and synovial fluid of RA patients. Furthermore, interleukin (IL)-1 and tumor necrosis factor .alpha. (TNF.alpha.), which are found at increased levels in patients suffering from RA, induce the production of G-CSF by human synovial fibroblasts and chondrocytes. Mice deficient in G-CSF are resistant to the induction of acute and chronic inflammatory arthritis.
[0005] G-CSF has also been shown to play a role in multiple sclerosis (MS). For example, G-CSF enhances adhesion of an auto-reactive T cell line model of MS to extracellular matrix as effectively as interferon .gamma. and TNF.alpha., which are known to exacerbate MS symptoms. Moreover, G-CSF deficient mice are resistant to development of experimental autoimmune encephalomyelitis (EAE).
[0006] G-CSF and G-CSFR have also been tied to cancer, with studies showing that this signaling pathway contributes to chemotherapy resistance, growth, survival, invasiveness and metastasis of various cancers. Moreover, G-CSF has been shown to induce to angiogenesis, a process important in the development of solid tumors.
[0007] It will be clear to the skilled person from the foregoing, that there is a need in the art for reagents that reduce the signaling of G-CSF through the G-CSFR. Exemplary agents will be suitable for use as therapeutics, e.g., to treat or prevent a G-CSF-mediated condition.
SUMMARY
[0008] The present inventors have produced a class of proteins comprising antibody binding sites (e.g., Fabs and antibodies) that bind to human G-CSFR (hG-CSFR) and potently neutralize G-CSF signaling, e.g., prevent formation of granulocytes from CD34.sup.+ bone marrow cells and/or prevent cell proliferation in response to G-CSF and/or reduce or prevent neutrophilia induced by administration of G-CSF. A class of proteins identified by the inventors also cross-react cynomolgus monkey G-CSFR (cynoG-CSFR), which facilitates pre-clinical studies with the proteins. A class of proteins identified by the inventors bind to hG-CSFR with high affinity. A class of proteins identified by the inventors are human antibodies, which are suitable for treatment of a variety of conditions.
[0009] The present disclosure provides a protein comprising an antigen binding site of an antibody, wherein the antigen binding site binds to hG-CSFR and neutralizes G-CSF signaling, and wherein the protein inhibits growth of colony forming units-granulocytes (CFU-G) from CD34.sup.+ bone marrow cells grown in the presence of G-CSF with an IC.sub.50 of at least about 0.2 nM. For example, the IC.sub.50 is 0.1 nM or less, for example, 0.09 nM or less, or 0.08 nM or less, or 0.07 nM or less, or 0.06 nM or less or 0.05 nM or less. In one example, the IC.sub.50 is 0.04 nM or less. In another example, the IC.sub.50 is 0.02 nM or less. Methods for assessing IC.sub.50 of a protein in such an assay are described herein. For example, the IC.sub.50 is determined in the presence of 10 ng/ml of hG-CSF.
[0010] In one example, the IC.sub.50 is determined by culturing CD34.sup.+ bone marrow cells in the presence of 10 ng/ml stem cell factor and 10 ng/ml hG-CSF. For example, the cells are grown in semi-solid cell culture medium. In one example, the CFU-G are enumerated after 14 days of culture.
[0011] The present disclosure additionally or alternatively provides a protein comprising an antigen binding site of an antibody, wherein the antigen binding site binds to both human and cynomolgus monkey G-CSFR with a similar affinity and neutralizes G-CSF signaling. Such proteins are advantageous since they facilitate pre-clinical studies in non-human mammals.
[0012] In one example, the affinity of the protein is determined using a biosensor, e.g., by surface plasmon resonance. For example, the ligand binding region or soluble hG-CSFR or soluble cynoG-CSFR or hG-CSFR-Fc or cyno-G-CSFR-Fc is immobilized and the affinity of the protein of the disclosure is determined.
[0013] The present disclosure additionally provides a provides a protein comprising an antigen binding site of an antibody, wherein the antigen binding site binds specifically to the same epitope iii hG-CSFR as that bound by C1.2 (comprising a heavy chain variable region (V.sub.H) comprising a sequence set forth in SEQ ID NO: 2 and a light chain variable region (V.sub.L) comprising a sequence set forth in SEQ ID NO: 3) or C1.2G (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 5).
[0014] The present disclosure additionally or alternatively provides a protein comprising an antigen binding site of an antibody, wherein (i) the protein competitively inhibits binding of C1.2 (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 2 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 3) or C1.2G (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 5) to hG-CSFR; (ii) the protein neutralizes G-CSF signaling; and (iii) the level of binding of the protein to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for any one of:
[0015] (a) the arginine at position 287 of SEQ ID NO:1;
[0016] (b) the histidine at position 237 of SEQ ID NO:1;
[0017] (c) the methionine at position 198 of SEQ ID NO:1;
[0018] (d) the tyrosine at position 172 of SEQ ID NO:1;
[0019] (e) the leucine at position 171 of SEQ ID NO:1; or
[0020] (f) the leucine at position 111 of SEQ ID NO:1
is lower than the level of binding of the protein to a polypeptide of SEQ ID NO: 1.
[0021] The present disclosure additionally or alternatively provides a protein comprising an antigen binding site of an antibody, wherein (i) the protein competitively inhibits binding of C1.2 (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 2 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 3) or C1.2G (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 5) to hG-CSFR; (ii) the protein neutralizes G-CSF signaling; and (iii) preferentially binds to a polypeptide of SEQ ID NO: 1 relative to its ability to bind to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for any one of:
[0022] (a) the arginine at position 287 of SEQ ID NO:1;
[0023] (b) the histidine at position 237 of SEQ ID NO:1;
[0024] (c) the methionine at position 198 of SEQ ID NO:1;
[0025] (d) the tyrosine at position 172 of SEQ ID NO:1;
[0026] (e) the leucine at position 171 of SEQ ID NO:1; or
[0027] (f) the leucine at position 111 of SEQ ID NO:1.
[0028] The present disclosure additionally or alternatively provides a protein comprising an antigen binding site of an antibody, wherein (i) the protein binds to hG-CSFR; (ii) the protein neutralizes G-CSF signaling; and (iii) the level of binding of the protein to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for any one of:
[0029] (a) the arginine at position 287 of SEQ ID NO:1
[0030] (b) the histidine at position 237 of SEQ ID NO:1;
[0031] (c) the methionine at position 198 of SEQ ID NO:1;
[0032] (d) the tyrosine at position 172 of SEQ ID NO:1;
[0033] (e) the leucine at position 171 of SEQ ID NO:1; or
[0034] (f) the leucine at position 111 of SEQ ID NO:1
is lower than the level of binding of the protein to a polypeptide of SEQ ID NO: 1.
[0035] The present disclosure additionally or alternatively provides a protein comprising an antigen binding site of an antibody, wherein (i) the protein binds to hG-CSFR; (ii) the protein neutralizes G-CSF signaling; and (iii) preferentially binds to a polypeptide of SEQ ID NO: 1 relative to its ability to bind to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for any one of
[0036] (a) the arginine at position 287 of SEQ ID NO:1
[0037] (b) the histidine at position 237 of SEQ ID NO:1;
[0038] (c) the methionine at position 198 of SEQ ID NO:1;
[0039] (d) the tyrosine at position 172 of SEQ ID NO:1;
[0040] (e) the leucine at position 171 of SEQ ID NO:1; or
[0041] (f) the leucine at position 111 of SEQ ID NO:1.
[0042] In one example, the level of binding of the protein to the polypeptide comprising the alanine substitution is reduced by at least about 10 fold or 20 fold or 50 fold or 100 fold or 150 fold or 200 fold compared to the binding of the protein to the polypeptide of SEQ ID NO: 1. Preferably, the level of binding of the protein to the polypeptide comprising the alanine substitution is reduced by at least about 50 fold. Preferably, the level of binding of the protein to the polypeptide comprising the alanine substitution is reduced by at least about 60 fold.
[0043] In one example, the antigen binding site of the protein does not detectably bind to a polypeptide of SEQ ID NO:1 in which an alanine is substituted for the arginine at position 287 of SEQ ID NO: 1.
[0044] In one example, the level of binding is assessed using a biosensor, e.g., by surface plasmon resonance. For example, the protein is immobilized and the level of binding to a polypeptide of SEQ ID NO:1 or to a form of the polypeptide comprising an alanine substitution is determined.
[0045] Additional forms of a polypeptide comprising the amino acids of SEQ ID NO: 1 with or without other substitutions bound or not significantly bound or not detectably bound by a protein of the present disclosure are described herein and are to be taken to apply mutatis mutandis to the present examples of the disclosure.
[0046] In one example, the antigen binding site cross-reacts with:
(i) a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the lysine at position 167 of SEQ ID NO: 1; and/or (ii) a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the histidine at position 168 of SEQ ID NO: 1.
[0047] In one example, the antigen binding site additionally cross-reacts with a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the leucine at position 169 of SEQ ID NO: 1
[0048] In one example, the protein competitively inhibits the binding of C1.2 (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 2 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 3) or C1.2G (comprising a V.sub.H comprising a sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising a sequence set forth in SEQ ID NO: 5) to one or more of:
(i) a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the lysine at a position 167 of SEQ ID NO: 1; and/or (ii) a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the histidine at position 168 of SEQ ID NO: 1.
[0049] In one example, a protein described herein according to any example binds to an epitope comprising residues within one or two or three or four regions selected from 111-115, 170-176, 218-234 and/or 286-300 of SEQ ID NO: 1.
[0050] In one example, upon binding of a protein described herein according to any example to a polypeptide of SEQ ID NO:1 and cleavage using protelolytic enzymes remains bound to one or two or three or four peptides comprising or consisting of amino acids 111-115 of SEQ ID NO:1 or amino acids 170-176 of SEQ ID NO: 1 or amino acids 218-234 of SEQ ID NO: 1 or amino acids 286-300 of SEQ ID NO: 1.
[0051] In one example, the protein binds to a conformational epitope.
[0052] The present disclosure additionally or alternatively provides a protein that binds to hG-CSFR and neutralizes G-CSF signaling, the protein comprising at least one of:
(i) a V.sub.H comprising a complementarity determining region (CDR) 1 comprising a sequence set forth in SEQ ID NO: 6, a CDR2 comprising a sequence set forth in SEQ ID NO: 7 and a CDR3 comprising a sequence at least about 55% identity to the sequence set forth in SEQ ID NO: 8; (ii) a V.sub.H comprising a sequence at least about 80%, such as 85% or 90% or 91% or 92% or 93% or 94% or 95% or 96% or 97% or 98% or 99% identical to a sequence set forth in SEQ ID NO: 2 and/or 4; (iii) a V.sub.L comprising a CDR1 comprising a sequence set forth in SEQ ID NO: 9, a CDR2 comprising a sequence set forth in SEQ ID NO: 10 and a CDR3 comprising a sequence at least about 33% identity to the sequence set forth in SEQ ID NO: 11; and (iv) a V.sub.L comprising a sequence at least about 80%, such as 85% or 90% or 91% or 92% or 93% or 94% or 95% or 96% or 97% or 98% or 99% identical to a sequence set forth in SEQ ID NO: 3 and/or 5.
[0053] Such a protein can comprise any one or more of the functional activities described herein, e.g., preferential binding to a polypeptide of SEQ ID NO:1 relative to the level of binding of a polypeptide of SEQ ID NO:1 in which an alanine is substituted for any one of:
[0054] (a) the arginine at position 287 of SEQ ID NO:1
[0055] (b) the histidine at position 237 of SEQ ID NO:1;
[0056] (c) the methionine at position 198 of SEQ ID NO:1;
[0057] (d) the tyrosine at position 172 of SEQ ID NO: 1;
[0058] (e) the leucine at position 171 of SEQ ID NO:1; or
[0059] (f) the leucine at position 111 of SEQ ID NO:1.
[0060] In one example, the percentage identity at (ii) is at least about 95%.
[0061] In one example, the percentage identity at (iv) is at least about 94%.
[0062] In one example, differences between the recited sequence and the protein are substitutions.
[0063] The skilled artisan will be capable of determining sites for mutations to a protein of the disclosure, e.g., within a framework region of a variable region containing protein. Moreover, the inventors have identified numerous sites in a V.sub.H CDR3 and a V.sub.L CDR3 that can be mutated as well as numerous mutations that maintain activity of a protein of the disclosure. For example a mutation, e.g., a substitution is within one or more (e.g., 2 or 3 or 4) of the four C-terminal residues of HCDR3 and/or one or more (e.g., 2 or 3 or 4 or 5 or 6) of the N-terminal or C-terminal residues of LCDR3. In one example, the N-terminal five amino acids of V.sub.H CDR3 are LGELG. In one example, the three N-terminal amino acids of V.sub.L CDR3 are QQS and/or the three C-terminal amino acids of V.sub.L CDR3 are PLT.
[0064] In one example, the V.sub.H comprises a CDR3 comprising a sequence LGELGX.sub.1X.sub.2X.sub.3X.sub.4, wherein:
X.sub.1 is selected from the group consisting of tryptophan, glutamine, methionine, serine, phenylalanine, glutamic acid and histidine and/or is a neutral amino acid, such as tryptophan, glutamine or methionine, for example, the amino acid is tryptophan; X.sub.2 is an amino acid selected from the group consisting of phenylalanine, tyrosine, methionine, serine, glycine and isoleucine, for example is phenylalanine, tyrosine, methionine or serine, for example, the amino acid is phenylalanine; X.sub.3 is an amino acid selected from the group consisting of aspartic acid, methionine, glutamine, serine, leucine, valine, arginine and histidine, for example, is proline, glutamic acid, alanine, leucine, phenylalanine or tyrosine, for example, the amino acid is aspartic acid; and X.sub.4 is any amino acid or an amino acid selected from the group consisting of proline, glutamic acid, alanine, leucine, phenylalanine, tyrosine, threonine, asparagine, aspartic acid, serine, glycine, arginine, and lysine, for example, the amino acid is proline.
[0065] In one example, the V.sub.L comprises a CDR3 comprising a sequence X.sub.1X.sub.2X.sub.3X.sub.4X.sub.5X.sub.6X.sub.7X.sub.8X.sub.9, wherein:
X.sub.1 is an amino acid selected from the group consisting of glutamine, glutamic acid, histidine, alanine and serine and/or is a hydrophilic amino acid, such as glutamine or glutamic acid, for example, the amino acid is glutamine; X.sub.2 is an amino acid selected from the group consisting of glutamine, valine, phenylalanine, asparagine and glutamic acid, for example, the amino acid is glutamine; X.sub.3 is an amino acid selected from the group consisting of serine and glycine, for example, the amino acid is serine; X.sub.4 is an amino acid selected from the group consisting of tryptophan, methionine, phenylalanine, tyrosine, isoleucine and leucine, for example, the amino acid is tryptophan or tyrosine; X.sub.5 is any amino acid or an amino acid selected from the group consisting of glutamic acid, methionine, glutamine, tryptophan, serine, valine, asparagine, glycine, alanine, arganine, histidine, tyrosine, lysine and threonine, for example, the amino acid is serine; X.sub.6 is an amino acid selected from the group consisting of tyrosine, methionine, isoleucine and threonine, for example, the amino acid is methionine, tyrosine or threonine; X.sub.7 is an amino acid selected from the group consisting of proline, alanine, histidine, glycine and lysine, for example the amino acid is proline; X.sub.8 is an amino acid selected from the group consisting of leucine, glutamine, methionine, alanine, phenylalanine, isoleucine, lysine, histidine and glycine, for example, the amino acid is leucine; X.sub.9 is any amino acid or an amino acid selected from the group consisting of threonine, phenylalanine, tyrosine, methionine, lysine, serine, histidine, proline, tryptophan, isoleucine, glutamine, glycine and valine, for example, the amino acid is threonine.
[0066] The present disclosure additionally or alternatively provides a protein (e.g., an antibody) that binds to hG-CSFR and neutralizes G-CSF signaling, the protein comprising at least one variable region of an antibody selected from the group consisting of:
(i) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 2; (ii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 3; (iii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4; (iv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 5; (v) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 14; (vi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 15; (vii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 16; (viii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 17; (ix) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 18; (x) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 19; (xi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 20; (xii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 21; (xiii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 22; (xiv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 23; (xv) a V.sub.1 comprising an amino acid sequence set forth in SEQ ID NO: 24; (xvi) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 25; (xvii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 26; (xviii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 27; (xix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 28; (xx) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 29; (xxi) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 30; (xxii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 31; (xxiii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 32; (xxiv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 33; (xxv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 34; (xxvi) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 35; (xxxii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 36; (xxviii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 37; (xxix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 38; (xxx) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 39; (xxxi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 40; (xxxii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 41; (xxxiii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 42; (xxxiv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 43; (xxxv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 44; (xxxvi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 45; (xxxvii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 46; (xxxviii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 47; (xxix) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 48; (xl) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 49; (xli) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 50; (xlii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 51; (xliii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 52; (xliv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 53; (xlv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 54; (xlvi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 55; (xlvii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 56; (xlviii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 57; (xlix) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 58; (l) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 59; (li) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 60; (lii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 61; (liii) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 62; and (liv) a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 63.
[0067] In one example, a protein described herein comprises at least a V.sub.H and a V.sub.L, wherein the V.sub.H and V.sub.L bind to form a Fv comprising an antigen binding domain. The skilled artisan will understand that the antigen binding domain comprises the binding site of the antibody.
[0068] In one example, the V.sub.H and the V.sub.L are in a single polypeptide chain. For example, the protein is: [0069] (i) a single chain Fv fragment (scFv); [0070] (ii) a dimeric scFv (di-scFv); or [0071] (iii) at least one of (i) and/or (ii) linked to a constant region of an antibody, Fc or a heavy chain constant domain (C.sub.H) 2 and/or C.sub.H3.
[0072] In one example, the V.sub.L and V.sub.H are in separate polypeptide chains.
[0073] For example, the protein is: [0074] (i) a diabody; [0075] (ii) a triabody; [0076] (iii) a tetrabody; [0077] (iv) a Fab; [0078] (v) a F(ab').sub.2; [0079] (vi) a Fv; or [0080] (vii) one of (i) to (vi) linked to a constant region of an antibody, Fc or a heavy chain constant domain (C.sub.H) 2 and/or C.sub.H3.
[0081] The foregoing proteins (described in the previous two lists) can also be referred to as antigen binding domains of antibodies.
[0082] In one example, the protein is an antibody. In one example, the antibody is a naked antibody.
[0083] In one example, a protein is chimeric, de-immunized, humanized, human or primatized.
[0084] In one example, the protein or antibody is human.
[0085] The present disclosure additionally or alternatively provides an antibody that binds to hG-CSFR and neutralizes G-CSF signaling, the antibody comprising:
(1) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 2 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 3; (ii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 5; (iii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 15 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 14; (iv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 16; (v) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 17; (vi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 18; (vii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 20 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 19; (viii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 21; (ix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 22; (x) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 24 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 23; (ix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 25; (x) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 26; (xi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 28 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 27; (xii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a comprising an amino acid sequence set forth in SEQ ID NO: 29; (xiii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 31 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 30; (xiv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 33 and a comprising an amino acid sequence set forth in SEQ ID NO: 32; (xv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 34; (xvi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 36 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 35; (xvii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 38 and a V.sub.L comprising an amino acid sequence set forth in SEQ II) NO: 37; (xviii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 40 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 39; (xix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 42 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 41; (xx) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 43; (xxi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 45 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 44; (xxii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 46; (xxiii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.1 comprising an amino acid sequence set forth in SEQ ID NO: 47; (xxiv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 49 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 48; (xxv) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 51 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 50; (xxvi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 53 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 52; (xxvii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 54; (xxviii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 55 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 5; (xxix) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 57 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 56; (xxx) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 59 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 58; (xxxi) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 61 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 60; (xxxii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 62; (xxxiii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 63; and (xxxix) a V.sub.H comprising three CDRs of a V.sub.H set forth in any one or more of (i) to (xxxiii) and a V.sub.L comprising three CDRs of a V.sub.L set forth in any one or more of (i) to (xxxiii).
[0086] Sequences of exemplary V.sub.H and V.sub.L are described in Table 3, wherein the recited V.sub.H or V.sub.L CDR3 sequence is substituted for the corresponding sequence in the V.sub.H or V.sub.L of C1.2 or C1.2G as described herein.
[0087] In one example, the present disclosure provides an antibody that binds to hG-CSFR and neutralizes G-CSF signaling, the antibody comprising:
(i) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 2 and a V.sub.L comprising an amino acid sequence set forth in SEQ ID NO: 3; or (ii) a V.sub.H comprising an amino acid sequence set forth in SEQ ID NO: 4 and a V.sub.L comprising art amino acid sequence set forth in SEQ ID NO: 5.
[0088] The present disclosure additionally or alternatively provides an antibody comprising a heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 64 and a light chain comprising an amino acid sequence set forth in SEQ ID NO: 65. In one example, the antibody binds to hG-CSFR and neutralizes G-CSF signaling.
[0089] The present disclosure additionally or alternatively provides an antibody comprising a heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 68 and a light chain comprising an amino acid sequence set forth in SEQ ID NO: 65. In one example, the antibody binds to hG-CSFR and neutralizes G-CSF signaling.
[0090] The present disclosure additionally or alternatively provides an antibody comprising one heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 64 and one heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 68 and two light chains comprising an amino acid sequence set forth in SEQ ID NO: 65. In one example, the antibody binds to hG-CSFR and neutralizes G-CSF signaling.
[0091] Reference herein to a protein or antibody that "binds to" hG-CSFR provides literal support for a protein or antibody that "binds specifically to" hG-CSFR.
[0092] In one example, a protein or antibody described herein does not significantly bind to mouse G-CSFR and/or does not detectably bind to mouse G-CSFR.
[0093] In one example, a protein or antibody described herein according to any example competitively inhibits binding of C1.2 and/or C1.2G to hG-CSFR or a cell expressing same or SEQ ID NO: 1 or a soluble hG-CSFR (e.g., comprising aminoacids 1-311 of SEQ ID NO:1 fused to a Fc region of an antibody).
[0094] In one example, a protein or antibody described herein binds to a ligand binding region of hG-CSFR and a ligand binding region of cynoG-CSFR with similar affinity. In one example, the protein binds to soluble hG-CSFR and soluble cynoG-CSFR with similar affinity. In one example, the protein binds to a polypeptide comprising SEQ ID NO: 1 and to a polypeptide comprising SEQ ID NO: 67 with similar affinity. In one example, the protein binds to hG-CSFR-Fc and cynoG-CSFR-Fc as described herein with similar affinity. In one example, the affinity is at least about 2 nM, for example, at least about 1.5 nM, such as at least about 1.2 nM, 1.1 nM or 1 nM. In one example, the 0.5 nM, such as, at least about 0.46 nM or 0.45 nM or 0.40 nM or 0.39 nM. In another example, the affinity is at least about 0.1 nM, such as at least about 0.09 nM, for example, at least about 0.08 nM. In one example, the level of binding is assessed using a biosensor, e.g., by surface plasmon resonance. For example, the ligand binding region or soluble hG-CSFR or soluble cynoG-CSFR or hG-CSFR-Fc or cyno-G-CSFR-Fc is immobilized and the level of binding to a protein of the disclosure is determined.
[0095] In another example, the protein of the disclosure is immobilized on, for example, a biosensor and the level of binding of the ligand binding region or soluble hG-CSFR or soluble cynoG-CSFR or hG-CSFR-Fc or cyno-G-CSFR-Fc is determined. For example, the level of binding to the extracellular domain of hG-CSFR or cynoG-CSFR is determined. In accordance with this example, the affinity of the protein for the extracellular domain of cynoG-CSFR is at least about 1 nM, such as at least about 0.9 nM, for example, at least about 0.75 nM. For example, the affinity is at least about 0.7 nM, such as at least about 0.6 nM, for example, at least about 0.5 nM. In one example, the affinity is about 0.5 nM. Alternatively, or additionally, the affinity of the protein for the extracellular domain of hG-CSFR is at least about 7 nM or 6 nM or 5 nM, such as at least about 4 nM, for example, at least about 3 nM, e.g., at least about 2.5 nM. For example, the affinity is at least about 2.4 or 2.5 nM.
[0096] The present disclosure also provides antigen binding domains or antigen binding fragments of the foregoing antibodies.
[0097] In one example, a protein or antibody as described herein comprises a constant region of an IgG4 antibody or a stabilized constant region of an IgG4 antibody. In one example, the protein or antibody comprises an IgG4 constant region with a proline at position 241 (according to the numbering system of Kabat (Kabat et al., Sequences of Proteins of Immunological Interest Washington D.C. United States Department of Health and Human Services, 1987 and/or 1991)).
[0098] The C-terminal lysine of the heavy chain constant region of a whole antibody of the disclosure may be removed, for example, during production or purification of the antibody, or by recombinantly engineering the nucleic acid encoding a heavy chain of the antibody. Accordingly, whole antibodies may comprise antibody populations with all C-terminal lysine residues removed, antibody populations with no C-terminal lysine residues removed, and antibody populations having a mixture of antibodies with and without the C-terminal lysine residue. In some examples, the antibody populations may additionally comprise antibodies in which the C-terminal lysine residue is removed in one of the heavy chain constant regions. Similarly, a composition of whole antibodies may comprise the same or a similar mix of antibody populations with or without the C-terminal lysine residue.
[0099] In one example, the stabilized constant region comprises a sequence from position 119 to position 445 of SEQ ID NO: 64. In one example, the stabilized constant region comprises a sequence from position 119 to position 444 of SEQ ID NO: 68. In one example a protein or antibody as described herein or a composition of a protein or antibody as described herein, comprises a heavy chain constant region, including a stabilized heavy chain constant region, comprising a mixture of sequences fully or partially with or without the C-terminal lysine residue.
[0100] In one example, an antibody of the disclosure comprises a V.sub.H disclosed herein linked or fused to an IgG4 constant region or stabilized IgG4 constant region (e.g., as discussed above) and the V.sub.L is linked to or fused to a kappa light chain constant region.
[0101] The present disclosure also provides a protein or antibody which inhibits G-CSF-induced proliferation of a BaF3 cell expressing hG-CSFR with an IC.sub.50 of at least about 6 nM. For example, the IC.sub.50 is 5.9 nM or less. In another example, the IC.sub.50 is 2 nM or less or 1 nM or less or 0.7 nM or less or 0.6 nM or less or 0.5 nM or less. In one example, the IC.sub.50 is determined by culturing BaF3 cells (e.g. about 2.times.10.sup.4 cells) in the presence of about 0.5 ng/ml hG-CSF, e.g., for about 48 hours. In one example, the proliferation of the BaF3 cells is determined by measuring 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction.
[0102] The present disclosure also provides a protein or antibody which inhibits G-CSF-induced proliferation of a BaF3 cell expressing hG-CSFR with an IC.sub.50 of at least about 10 .mu.g/ml. For example, the IC.sub.50 is 5 .mu.g/ml or less. In another example, the IC.sub.50 is 3 .mu.g/ml or less or 2 .mu.g/ml or less or 1 .mu.g/ml or less. In one example, the IC.sub.50 is about 0.8 .mu.g/ml. In one example, the IC.sub.50 is determined by culturing BaF3 cells (e.g. about 1.times.10.sup.4 cells) in the presence of about 10 ng/ml hG-CSF, e.g., for about 48 hours. In one example, the proliferation of the BaF3 cells is determined by measuring .sup.3H-thymidine incorporation.
[0103] In one example, a protein or antibody of the disclosure binds to a soluble hG-CSFR comprising amino acid 1-311 of SEQ II) NO: 1 expressed as a fusion with an antibody Fc region (hG-CSFR-Fc) with an affinity of at least about 1.5 nM. For example, the affinity is at least about 0.5 nM or 0.4 nM or 0.35 nM or 0.33 nM. In one example, the affinity of the protein is determined using a biosensor, e.g., by surface plasmon resonance. For example, the hG-CSFR-Fc is immobilized and the affinity of the protein of the disclosure is determined.
[0104] In one example, a protein or antibody of the disclosure binds to hG-CSFR expressed on the surface of a cell at an affinity of at least about 1 nM, for example, at least about 0.5 nM, such as, at least 0.4 nM, for example, at least 0.3 nM, such as, at least 0.2 nM.
[0105] In one example, a protein as described herein according to any example is capable of reducing the number of neutrophils in circulation when or if administered to a cynomolgus monkey. For example, the protein reduces the number of neutrophils in circulation when or if administered to a cynomolgus monkey at a dose of between 0.05 m/kg-30 mg/kg, preferably between 0.1 mg/kg-10 mg/kg, e.g., administered at a dose of 0.1 mg/kg or 1 mg/kg or 2 mg/kg or 5 mg/kg or 10 mg/kg. For example, the protein reduces the number of neutrophils in circulation when or if administered following administration of G-CSF or filgrastim or a PEGylated form thereof, e.g., when or if the protein is administered about 12 hours after administration of G-CSF or filgrastim or a PEGylated form thereof. In one example, the reduction is a 2 fold or 3 fold reduction. In one example, the neutrophils are reduced about 10-24 hours, e.g., about 12 hours following administration.
[0106] In one example, a protein or antibody as described herein is isolated and/or recombinant.
[0107] In one example, a protein or antibody of the disclosure is conjugated to another compound, for example, a detectable label or a compound that extends the half-life of the protein or antibody, such as polyethylene glycol or an albumin binding protein.
[0108] The present disclosure also provides a nucleic acid encoding the protein or antibody of the present disclosure.
[0109] In one example, such a nucleic acid is included in an expression construct in which the nucleic acid is operably linked to a promoter. Such an expression construct can be in a vector, e.g., a plasmid.
[0110] In examples of the disclosure directed to single polypeptide chain proteins, the expression construct may comprise a promoter linked to a nucleic acid encoding that polypeptide chain.
[0111] In examples directed to multiple polypeptide chains that form a protein, an expression construct comprises a nucleic acid encoding a polypeptide comprising, e.g., a V.sub.H operably linked to a promoter and a nucleic acid encoding a polypeptide comprising, e.g., a V.sub.L operably linked to a promoter.
[0112] In another example, the expression construct is a bicistronic expression construct, e.g., comprising the following operably linked components in 5' to 3' order:
(i) a promoter (ii) a nucleic acid encoding a first polypeptide; (iii) an internal ribosome entry site; and (iv) a nucleic acid encoding a second polypeptide, wherein the first polypeptide comprises a V.sub.H and the second polypeptide comprises a V.sub.L, or vice versa.
[0113] The present disclosure also contemplates separate expression constructs one of which encodes a first polypeptide comprising a V.sub.H and another of which encodes a second polypeptide comprising a V.sub.L. For example, the present disclosure also provides a composition comprising:
(i) a first expression construct comprising a nucleic acid encoding a polypeptide comprising a V.sub.H operably linked to a promoter; and (ii) a second expression construct comprising a nucleic acid encoding a polypeptide comprising a V.sub.L operably linked to a promoter.
[0114] The present disclosure also provides an isolated or recombinant cell expressing a protein of the disclosure.
[0115] In one example, the cell comprises the expression construct of the disclosure or:
(i) a first expression construct comprising a nucleic acid encoding a polypeptide comprising a V.sub.H operably linked to a promoter; and (ii) a second expression construct comprising a nucleic acid encoding a polypeptide comprising a V.sub.L operably linked to a promoter.
[0116] Examples of cells of the present disclosure include bacterial cells, yeast cells, insect cells or mammalian cells.
[0117] The present disclosure additionally provides methods for producing a protein or antibody of the disclosure. For example, such a method involves maintaining the expression construct(s) of the disclosure under conditions sufficient for the protein to be produced.
[0118] In one example, a method for producing a protein, or antibody of the disclosure comprises culturing the cell of the disclosure under conditions sufficient for the protein or antibody to be produced and, optionally, secreted.
[0119] In one example, the method for producing a protein of the disclosure additionally comprises isolating the protein or antibody and, optionally, formulating the protein or antibody into a pharmaceutical composition.
[0120] The present disclosure additionally provides a composition comprising a protein or antibody of the disclosure and a pharmaceutically acceptable carrier.
[0121] The present disclosure additionally provides a composition comprising:
(i) an antibody comprising a heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 64 and a light chain comprising an amino acid sequence set forth in SEQ ID NO: 65; and (ii) (a) an antibody comprising a heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 64 and a light chain comprising an amino acid sequence set forth in SEQ ID NO: 65; and/or [0122] (b) an antibody comprising one heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 64 and one heavy chain comprising an amino acid sequence set forth in SEQ ID NO: 68 and two light chains comprising an amino acid sequence set forth in SEQ ID NO: 65, and, optionally, a pharmaceutically acceptable carrier.
[0123] The present disclosure also provides a method for treating or preventing a G-CSF-mediated condition in a subject, the method comprising administering the protein, antibody or composition of the disclosure. In this regard, a protein, antibody or composition can be used to prevent a relapse of a condition, and this is considered preventing the condition.
[0124] In one example, the G-CSF-mediated condition is an autoimmune disease, an inflammatory disease or cancer. For example, the autoimmune disease or the inflammatory disease is arthritis, multiple sclerosis, pulmonary inflammation or chronic obstructive pulmonary disease.
[0125] In one example, the method comprises administering an amount of the protein or antibody sufficient to reduce the number of neutrophils in a subject without inducing neutropenia.
[0126] The present disclosure alternatively or additionally provides a method for reducing the number of neutrophils in a subject without inducing neutropenia, the method comprising administering a protein comprising an antigen binding site of an antibody that binds (or specifically binds) to hG-CSFR to the subject. An exemplary protein is an antibody or comprises an antigen binding domain thereof (e.g., a V.sub.H and/or a V.sub.L) or is an antigen binding fragment thereof. Exemplary proteins and antibodies are described herein.
[0127] In one example, a method described herein comprises administering an amount of the protein or antibody sufficient to reduce the number of neutrophils in a subject without inducing moderate neutropenia.
[0128] In one example, a method described herein comprises administering an amount of the protein or antibody sufficient to reduce the number of neutrophils in a subject without inducing severe neutropenia.
[0129] In one example, a method described herein comprises administering between about 0.05 mg/kg and 30 mg/kg of the protein or antibody. For example, the method comprising administering between 0.1 mg/kg and 10 mg/kg or between 0.2 mg/kg and 5 mg/kg of the protein or antibody. In one example, the method comprises administering about 0.5-2.0 mg/kg of the protein or antibody.
[0130] The present disclosure also provides for use of a protein or antibody as described herein in any example in medicine.
[0131] The present disclosure also provides for use of a protein or antibody as described herein according to any example in the manufacture of a medicament to treat a G-CSF-mediated condition.
[0132] The present disclosure also provides a method for localizing and/or detecting and/or diagnosing and/or prognosing G-CSF-mediated condition associated with a cell expressing G-CSFR, the method comprising detecting in vivo a protein or antibody as described herein bound to the G-CSFR expressing cell, if present, wherein the protein or antibody is conjugated to a detectable tag.
[0133] In one example, the method additionally comprises administering the protein to the subject.
[0134] The present disclosure also provides a method for detecting G-CSFR or a cell expressing same in a sample, the method comprising contacting the sample with a protein or antibody as described herein according to any example such that a complex forms and detecting the complex, wherein detection of the complex is indicative of G-CSFR or a cell expressing same in the sample.
[0135] The present disclosure also provides a method for diagnosing or prognosing a G-CSF-mediated condition, the method comprising performing a method as described herein according to any example to detect G-CSFR or a cell expressing same, wherein detection of the G-CSFR or cell expressing same is diagnostic or prognostic of the condition.
[0136] The present disclosure also provides a kit comprising a protein or antibody as described herein according to any example packaged with instructions for use in a method as described herein.
KEY TO SEQUENCE LISTING
[0137] SEQ ID NO: 1--amino acids 25-335 of Homo sapiens G-CSFB (hG-CSFR) with a C-terminal polyhistidine tag
SEQ ID NO: 2--V.sub.H of C1.2
SEQ ID NO: 3--V.sub.L of C1.2
SEQ ID NO: 4--V.sub.H of C1.2G
SEQ ID NO: 5--V.sub.L of C1.2G
SEQ ID NO: 6--HCDR1 of C1.2
SEQ ID NO: 7--HCDR2 of C1.2
SEQ ID NO: 8--HCDR3 of C1.2
SEQ ID NO: 9--LCDR1 of C1.2
SEQ ID NO: 10--LCDR2 of C1.2
SEQ ID NO:11 LCDR3 of C1.2
[0138] SEQ ID NO: 12--consensus sequence of HCDR3 of C1.2 SEQ ID NO: 13--consensus sequence of LCDR3 of C1.2 SEQ ID NO: 14--V.sub.L of antibody 987 SEQ ID NO: 15--V.sub.H of antibody 987 SEQ ID NO: 16--V.sub.L of antibody 95 SEQ ID NO: 17--V.sub.L of antibody 79 SEQ ID NO: 18--V.sub.L of antibody 83 SEQ ID NO: 19--V.sub.L of antibody 1003 SEQ ID NO: 20--of antibody 1003 SEQ ID NO: 21--V.sub.L of antibody 44 SEQ ID NO: 22--V.sub.L of antibody 97 SEQ ID NO: 23--V.sub.L of antibody 986 SEQ ID NO: 24--V.sub.H of antibody 986 SEQ ID NO: 25--V.sub.L of antibody 56 SEQ ID NO: 26--V.sub.L of antibody 77 SEQ ID NO: 27--V.sub.L of antibody 54 SEQ ID NO: 28--V.sub.H of antibody 54 SEQ ID NO: 29--V.sub.L of antibody 802 SEQ ID NO: 30--V.sub.L of antibody 967 SEQ ID NO: 31--V.sub.H of antibody 967 SEQ ID NO: 32--V.sub.L of antibody 989 SEQ ID NO: 33--V.sub.H of antibody 989 SEQ ID NO: 34--V.sub.L of antibody 63 SEQ ID NO: 35--V.sub.L of antibody 1002 SEQ ID NO: 36--V.sub.H of antibody 1002 SEQ ID NO: 37--V.sub.L of antibody 994 SEQ ID NO: 38--V.sub.H of antibody 994 SEQ ID NO: 39--V.sub.L of antibody 969 SEQ ID NO: 40--V.sub.H of antibody 969 SEQ ID NO: 41--V.sub.L of antibody 1000 SEQ ID NO: 42--V.sub.H of antibody 1000 SEQ ID NO: 43--V.sub.L of antibody 94 SEQ ID NO: 44--V.sub.L of antibody 975 SEQ ID NO: 45--V.sub.H of antibody 975 SEQ ID NO: 46--V.sub.L of antibody 75 SEQ ID NO: 47--V.sub.L of antibody 814 SEQ ID NO: 48--V.sub.L of antibody 973 SEQ ID NO: 49--V.sub.H of antibody 973 SEQ ID NO: 50--V.sub.L of antibody 977 SEQ ID NO: 51--V.sub.H of antibody 977 SEQ ID NO: 52--V.sub.L of antibody 984 SEQ ID NO: 53--V.sub.H of antibody 984 SEQ ID NO: 54--V.sub.L of antibody 61 SEQ ID NO: 55--V.sub.H of antibody 852 SEQ ID NO: 56--V.sub.L of antibody 996 SEQ ID NO: 57--V.sub.H of antibody 996 SEQ ID NO: 58--V.sub.L of antibody 43 SEQ ID NO: 59--V.sub.H of antibody 43 SEQ ID NO: 60--V.sub.L of antibody 999 SEQ ID NO: 61--V.sub.H of antibody 999 SEQ ID NO: 62--V.sub.L of antibody 870 SEQ ID NO: 63--V.sub.1 of antibody 877 SEQ ID NO: 64--Heavy chain of C1.2G with stabilized IgG4 constant region SEQ ID NO: 65--Light chain of C1.2G with kappa constant region SEQ ID NO: 66--sequence of exemplary h-GCSFR SEQ ID NO: 67--polypeptide comprising Ig and CRH domains of Macaca fascicularis G-CSFR (cynoG-CSFR) with a C-terminal polyhistidine tag SEQ ID NO: 68--Heavy chain of C1.2G with stabilized IgG4 constant region and lacking C-terminal lysine.
BRIEF DESCRIPTION OF THE DRAWINGS
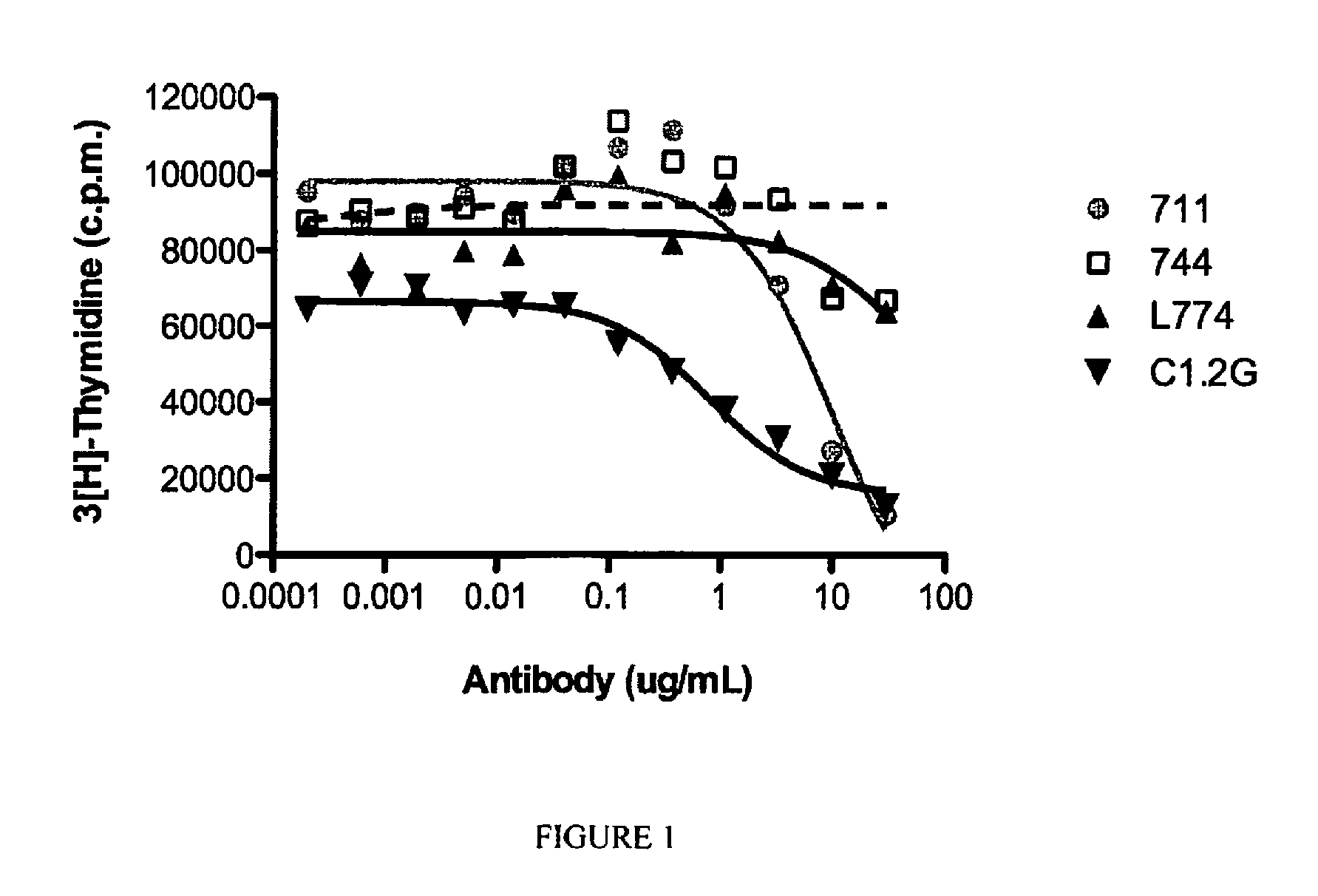
[0139] FIG. 1 is a graphical representation showing inhibition of G-CSF-mediated proliferation of BaF3 cells by increasing concentrations of various anti-G-CSFR antibodies. The relative IC.sub.50 values for each antibody were; 10.1 .mu.g/mL for mAb711, 37.4 .mu.g/ml for mAb 774, 0.8 .mu.g/mL for C1.2G and was not determinable for mAb 744.
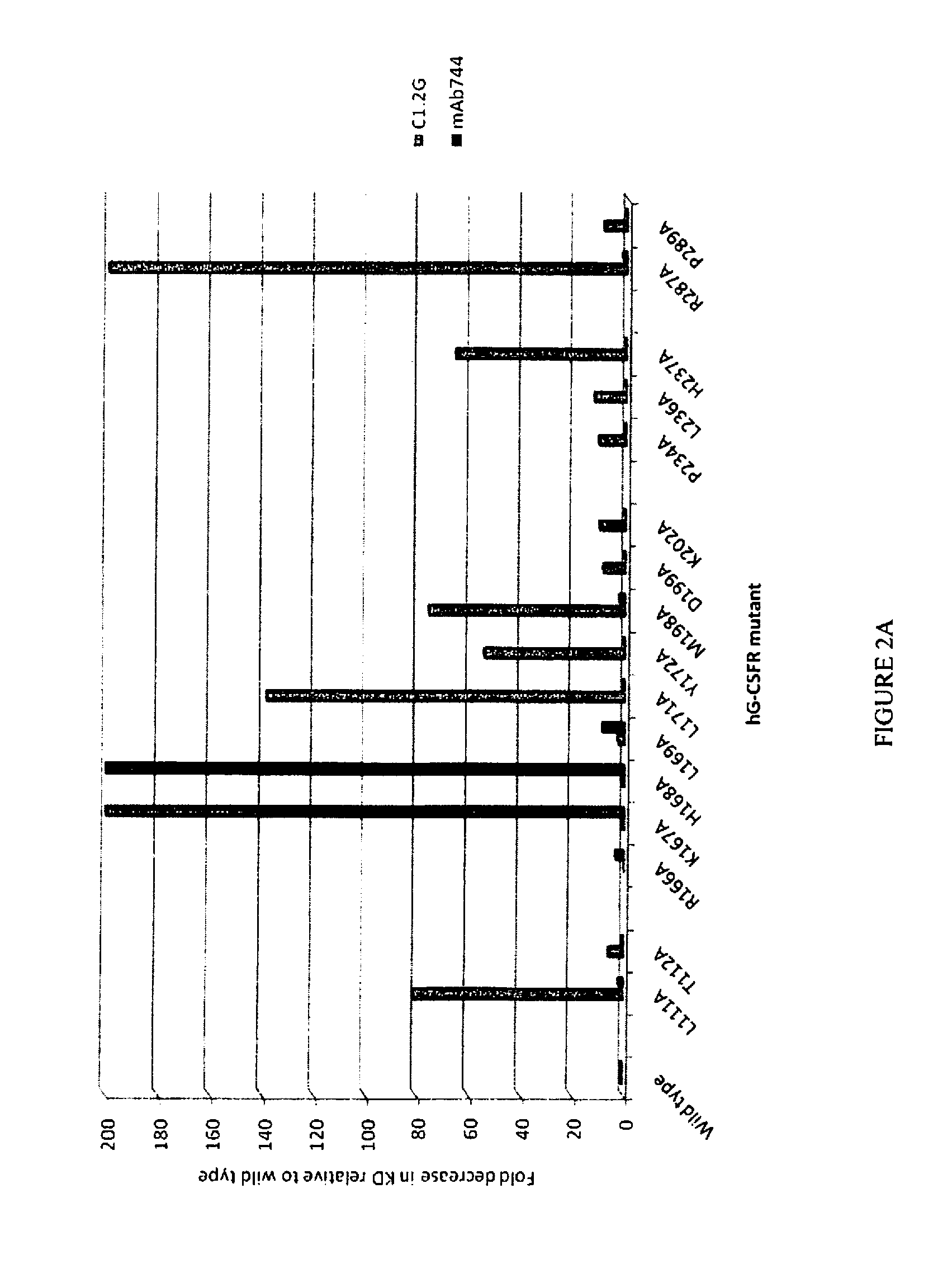
[0140] FIG. 2A is a graphical representation showing the relative binding of C1.2G and mAb744 to a series of alanine point mutants of SEQ ID NO:1 compared to their binding to SEQ ID NO:1 (positions of mutations are indicated with reference to SEQ ID NO: 1). The fold decrease in K.sub.D of the antibody for the mutant receptor compared to SEQ ID NO: 1 is depicted.
[0141] FIG. 2B is a graphical representation showing the relative binding of C1.2G, mAb744 and mAb774 to a series of alanine point mutants of SEQ ID NO:1 compared to their binding to SEQ ID NO:1 (positions of mutations are indicated with reference to SEQ ID NO: 1). The fold decrease in K.sub.D of the antibody for the mutant receptor compared to SEQ ID NO:1 is depicted.
[0142] FIG. 3 is a graphical representation showing results of an assay in which pegylated G-CSF was administered to cynomolgus monkeys and one day later C1.2 was administered. The number of neutrophils per .mu.L blood was assessed.
DETAILED DESCRIPTION
General
[0143] Throughout this specification, unless specifically stated otherwise or the context requires otherwise, reference to a single step, composition of matter, group of steps or group of compositions of matter shall be taken to encompass one and a plurality (i.e. one or more) of those steps, compositions of matter, groups of steps or groups of compositions of matter.
[0144] Those skilled in the art will appreciate that the present disclosure is susceptible to variations and modifications other than those specifically described. It is to be understood that the disclosure includes all such variations and modifications. The disclosure also includes all of the steps, features, compositions and compounds referred to or indicated in this specification, individually or collectively, and any and all combinations or any two or more of said steps or features.
[0145] The present disclosure is not to be limited in scope by the specific examples described herein, which are intended for the purpose of exemplification only. Functionally-equivalent products, compositions and methods are clearly within the scope of the present disclosure.
[0146] Any example of the present disclosure herein shall be taken to apply mutatis mutandis to any other example of the disclosure unless specifically stated otherwise.
[0147] Unless specifically defined otherwise, all technical and scientific terms used herein shall be taken to have the same meaning as commonly understood by one of ordinary skill in the art (for example, in cell culture, molecular genetics, immunology, immunohistochemistry, protein chemistry, and biochemistry).
[0148] Unless otherwise indicated, the recombinant protein, cell culture, and immunological techniques utilized in the present disclosure are standard procedures, well known to those skilled in the art. Such techniques are described and explained throughout the literature in sources such as, J. Perbal, A Practical Guide to Molecular Cloning, John Wiley and Sons (1984), J. Sambrook et al. Molecular Cloning: A Laboratory Manual, Cold Spring Harbour Laboratory Press (1989), T. A. Brown (editor), Essential Molecular Biology: A Practical Approach, Volumes 1 and 2, IRL Press (1991), D. M. Glover and B. D. Hames (editors), DNA Cloning: A Practical Approach, Volumes 1-4, IRL Press (1995 and 1996), and F. M. Ausubel et al. (editors), Current Protocols in Molecular Biology, Greene Pub. Associates and Wiley-Interscience (1988, including all updates until present), Ed Harlow and David Lane (editors) Antibodies: A Laboratory Manual, Cold Spring Harbour Laboratory, (1988), and J. E. Coligan et al. (editors) Current Protocols in Immunology, John Wiley & Sons (including all updates until present).
[0149] The description and definitions of variable regions and parts thereof, immunoglobulins, antibodies and fragments thereof herein may be further clarified by the discussion in Kabat Sequences of Proteins of Immunological Interest, National Institutes of Health, Bethesda, Md., 1987 and 1991, Bork et al., J Mol. Biol. 242, 309-320, 1994, Chothia and Lesk J. Mol. Biol. 196:901-917, 1987, Chothia et al. Nature 342, 877-883, 1989 and/or or Al-Lazikani et al., J Mol Biol 273, 927-948, 1997.
[0150] The term "and/or", e.g., "X and/or Y" shall be understood to mean either "X and Y" or "X or Y" and shall be taken to provide explicit support for both meanings or for either meaning.
[0151] Throughout this specification the word "comprise", or variations such as "comprises" or "comprising", will be understood to imply the inclusion of a stated element, integer or step, or group of elements, integers or steps, but not the exclusion of any other element, integer or step, or group of elements, integers or steps.
[0152] As used herein the term "derived from" shall be taken to indicate that a specified integer may be obtained from a particular source albeit not necessarily directly from that source.
Selected Definitions
[0153] For the purposes of nomenclature only and not limitation an exemplary sequence of a human G-CSFR is set out in NCBI Reference Sequence: NP_000751.1 (and set out in SEQ ID NO: 66). The sequence of cynomolgus monkey G-CSFR can be determined using sequences provided herein and/or in publically available databases and/or determined using standard techniques (e.g., as described in Ausubel et al., (editors), Current Protocols in Molecular Biology, Greene Pub. Associates and Wiley-Interscience (1988, including all updates until present) or Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press (1989)) Reference to human G-CSFR may be abbreviated to hG-CSFR and reference to cynomolgus monkey G-CSFR may be abbreviated to cynoG-CSFR. Reference to soluble G-CSFR refers to polypeptides comprising the ligand binding region of G-CSFR. The Ig and CRH domains of the G-CSFR are involved in ligand binding and receptor dimerization (Layton et al., J. Biol Chem., 272: 29735-29741, 1997 and Fukunaga et al, EMBO J. 10: 2855-2865, 1991). Soluble forms of G-CSFR comprising these portions of the receptor have been used in various studies of the receptor and mutation of the free cysteines at positions 78, 163, and 228 of the receptor assists in expression and isolation of the soluble receptor polypeptide (Mine et al., Biochem., 43: 2458-2464 2004) without affecting ligand binding. In the present studies soluble forms of the receptor comprising amino acids 25-335 of hG-CSFR with mutations C78A, C163S and C228S were used (e.g. SEQ ID NO:1) and the corresponding segment of cynoG-CSFR with the cysteine mutations was used (e.g., SEQ ID NO 67) for studies on the cynomolgus monkey receptor. Various point mutations of the soluble receptor of SEQ ID NO:1 and SEQ ID NO: 67 have also been utilized. Reference to hG-CSFR-Fc means the polypeptide of SEQ ID NO:1 wherein the C-terminal polyhistidine tag has been replaced with an Fc sequence (e.g., a polypeptide comprising amino acids 1-311 of SEQ ID NO: 1 fused to an Fc). cynoG-CSFR-Fc means the corresponding segment of cynoG-CSFR with an Fc sequence attached to its C-terminus (e.g., a polypeptide comprising amino acids 1-311 of SEQ ID NO: 67 fused to an Fc). The inventors have shown that antibodies and proteins comprising antigen binding sites thereof (e.g., Fab) bind to wild type hG-CSF polypeptides and to these mutant proteins with highly similar affinity. Accordingly, studies using the mutant proteins are a model of studies using hG-CSFR and/or cynoG-CSFR.
[0154] Reference herein to G-CSF includes native forms of G-CSF, mutant forms thereof, e.g., filgrastim and pegylated forms of G-CSF or filgrastim. This term also encompasses mutant forms of G-CSF retaining activity to bind to G-CSFR (e.g., hG-CSFR) and induce signaling.
[0155] The term "isolated protein" or "isolated polypeptide" is a protein or polypeptide that by virtue of its origin or source of derivation is not associated with naturally-associated components that accompany it in its native state; is substantially free of other proteins from the same source. A protein may be rendered substantially free of naturally associated components or substantially purified by isolation, using protein purification techniques known in the art. By "substantially purified" is meant the protein is substantially free of contaminating agents, e.g., at least about 70% or 75% or 80% or 85% or 90% or 95% or 96% or 97% or 98% or 99% free of contaminating agents.
[0156] The term "recombinant" shall be understood to mean the product of artificial genetic recombination. Accordingly, in the context of a recombinant protein comprising an antibody antigen binding domain, this term does not encompass an antibody naturally-occurring within a subject's body that is the product of natural recombination that occurs during B cell maturation. However, if such an antibody is isolated, it is to be considered an isolated protein comprising an antibody antigen binding domain. Similarly, if nucleic acid encoding the protein is isolated and expressed using recombinant means, the resulting protein is a recombinant protein comprising an antibody antigen binding domain. A recombinant protein also encompasses a protein expressed by artificial recombinant means when it is within a cell, tissue or subject, e.g., in which it is expressed.
[0157] The term "protein" shall be taken to include a single polypeptide chain, i.e., a series of contiguous amino acids linked by peptide bonds or a series of polypeptide chains covalently or non-covalently linked to one another (i.e., a polypeptide complex). For example, the series of polypeptide chains can be covalently linked using a suitable chemical or a disulphide bond. Examples of non-covalent bonds include hydrogen bonds, ionic bonds, Van der Waals forces, and hydrophobic interactions.
[0158] The term "polypeptide" or "polypeptide chain" will be understood from the foregoing paragraph to mean a series of contiguous amino acids linked by peptide bonds.
[0159] As used herein, the term "antigen binding site" shall be taken to mean a structure formed by a protein that is capable of binding or specifically binding to an antigen. The antigen binding site need not be a series of contiguous amino acids, or even amino acids in a single polypeptide chain. For example, in a Fv produced from two different polypeptide chains the antigen binding site is made up of a series of amino acids of a V.sub.L and a V.sub.H that interact with the antigen and that are generally, however not always in the one or more of the CDRs in each variable region. In some examples, an antigen binding site is a V.sub.H or a V.sub.L or a Fv.
[0160] The skilled artisan will be aware that an "antibody" is generally considered to be a protein that comprises a variable region made up of a plurality of polypeptide chains, e.g., a polypeptide comprising a V.sub.L and a polypeptide comprising a V.sub.H. An antibody also generally comprises constant domains, some of which can be arranged into a constant region, which includes a constant fragment or fragment crystallizable (Fc), in the case of a heavy chain. A V.sub.H and a V.sub.L interact to form a Fv comprising an antigen binding region that is capable of specifically binding to one or a few closely related antigens. Generally, a light chain from mammals is either a .kappa. light chain or a .lamda. light chain and a heavy chain from mammals is .alpha., .delta., .epsilon., .gamma., or .mu.. Antibodies can be of any type (e.g., IgG, IgE, IgM, IgD, IgA, and IgY), class (e.g., IgG.sub.1, IgG.sub.2, IgG.sub.3, IgG.sub.4, IgA.sub.1 and IgA.sub.2) or subclass. The term "antibody" also encompasses humanized antibodies, primatized antibodies, human antibodies and chimeric antibodies.
[0161] The terms "full-length antibody," "intact antibody" or "whole antibody" are used interchangeably to refer to an antibody in its substantially intact form, as opposed to an antigen binding fragment of an antibody. Specifically, whole antibodies include those with heavy and light chains including an Fc region. The constant domains may be wild-type sequence constant domains (e.g., human wild-type sequence constant domains) or amino acid sequence variants thereof.
[0162] As used herein, "variable region" refers to the portions of the light and/or heavy chains of an antibody as defined herein that is capable of specifically binding to an antigen and includes amino acid sequences of complementarity determining regions (CDRs); i.e., CDR1, CDR2, and CDR3, and framework regions (FRs). Exemplary variable regions comprise three or four FRs (e.g., FR1, FR2, FR3 and optionally FR4) together with three CDRs. In the case of a protein derived from an IgNAR, the protein may lack a CDR2. V.sub.H refers to the variable region of the heavy chain. V.sub.L refers to the variable region of the light chain.
[0163] As used herein, the term "complementarity determining regions" (syn. CDRs; i.e., CDR1, CDR2, and CDR3) refers to the amino acid residues of an antibody variable region the presence of which are necessary for antigen binding. Each variable region typically has three CDR regions identified as CDR1, CDR2 and CDR3. The amino acid positions assigned to CDRs and FRs can be defined according to Kabat Sequences of Proteins of Immunological Interest, National Institutes of Health, Bethesda, Md., 1987 and 1991 or other numbering systems in the performance of this disclosure, e.g., the canonical numbering system of Chothia and Lesk J. Mol Biol. 196: 901-917, 1987; Chothia et al. Nature 342, 877-883, 1989; and/or Al-Lazikani et al., J Mol Biol 273: 927-948, 1997; the IMGT numbering system of Lefranc et al., Devel. And Compar. Immunol., 27: 55-77, 2003; or the AHO numbering system of Honnegher and Plukthun J. Mol. Biol., 309: 657-670, 2001. For example, according to the numbering system of Kabat, V.sub.H framework regions (FRs) and CDRs are positioned as follows: residues 1-30 (FR1), 31-35 (CDR1), 36-49 (FR2), 50-65 (CDR2), 66-94 (FR3), 95-102 (CDR3) and 103-113 (FR4). According to the numbering system of Kabat, V.sub.L FRs and CDRs are positioned as follows: residues 1-23 (FR1), 24-34 (CDR1), 35-49 (FR2), 50-56 (CDR2), 57-88 (FR3), 89-97 (CDR3) and 98-107 (FR4). The present disclosure is not limited to FRs and CDRs as defined by the Kabat numbering system, but includes all numbering systems, including those discussed above. In one example, reference herein to a CDR (or a FR) is in respect of those regions according to the Kabat numbering system.
[0164] "Framework regions" (FRs) are those variable region residues other than the CDR residues.
[0165] As used herein, the term "Fv" shall be taken to mean any protein, whether comprised of multiple polypeptides or a single polypeptide, in which a V.sub.L and a V.sub.H associate and form a complex having an antigen binding site, i.e., capable of specifically binding to an antigen. The V.sub.H and the V.sub.L which form the antigen binding site can be in a single polypeptide chain or in different polypeptide chains. Furthermore, an Fv of the disclosure (as well as any protein of the disclosure) may have multiple antigen binding sites which may or may not bind the same antigen. This term shall be understood to encompass fragments directly derived from an antibody as well as proteins corresponding to such a fragment produced using recombinant means. In some examples, the V.sub.H is not linked to a heavy chain constant domain (C.sub.H) 1 and/or the V.sub.L is not linked to a light chain constant domain (C.sub.L). Exemplary Fv containing polypeptides or proteins include a Fab fragment, a Fab' fragment, a F(ab') fragment, a scFv, a diabody, a triabody, a tetrabody or higher order complex, or any of the foregoing linked to a constant region or domain thereof, e.g., C.sub.H2 or C.sub.H3 domain, e.g., a minibody. A "Fab fragment" consists of a monovalent antigen-binding fragment of an immunoglobulin, and can be produced by digestion of a whole antibody with the enzyme papain, to yield a fragment consisting of an intact light chain and a portion of a heavy chain or can be produced using recombinant means. A "Fab' fragment" of an antibody can be obtained by treating a whole antibody with pepsin, followed by reduction, to yield a molecule consisting of an intact light chain and a portion of a heavy chain comprising a V.sub.H and a single constant domain. Two Fab' fragments are obtained per antibody treated in this manner. A Fab' fragment can also be produced by recombinant means. A "F(ab')2 fragment" of an antibody consists of a dimer of two Fab' fragments held together by two disulfide bonds, and is obtained by treating a whole antibody molecule with the enzyme pepsin, without subsequent reduction. A "Fab.sub.2" fragment is a recombinant fragment comprising two Fab fragments linked using, for example a leucine zipper or a C.sub.H3 domain. A "single chain Fv" or "scFv" is a recombinant molecule containing the variable region fragment (Fv) of an antibody in which the variable region of the light chain and the variable region of the heavy chain are covalently linked by a suitable, flexible polypeptide
[0166] As used herein, the term "binds" in reference to the interaction of a protein or an antigen binding site thereof with an antigen means that the interaction is dependent upon the presence of a particular structure (e.g., an antigenic determinant or epitope) or a the antigen. For example, an antibody recognizes and binds to a specific protein structure rather than to proteins generally. If an antibody binds to epitope "A", the presence of a molecule containing epitope "A" (or free, unlabeled "A"), in a reaction containing labeled "A" and the protein, will reduce the amount of labeled "A" bound to the antibody.
[0167] As used herein, the term "specifically binds" or "binds specifically" shall be taken to mean that a protein of the disclosure reacts or associates more frequently, more rapidly, with greater duration and/or with greater affinity with a particular antigen or cell expressing same than it does with alternative antigens or cells. For example, a protein binds to G-CSFR (e.g., hG-CSFR) with materially greater affinity (e.g., 20 fold or 40 fold or 60 fold or 80 fold to 100 fold or 150 fold or 200 fold) than it does to other cytokine receptor or to antigens commonly recognized by polyreactive natural antibodies (i.e., by naturally occurring antibodies known to bind a variety of antigens naturally found in humans). In an example of the present disclosure, a protein that "specifically binds" to one form of hG-CSFR or a polypeptide comprising a region thereof (e.g., the ligand binding domain of hG-GCSFR) or a polypeptide comprising amino acids 1-311 of SEQ ID NO: 1 with an affinity at least 20 fold or 40 fold or 60 fold or 80 fold or 100 fold or 150 fold or 200 fold greater than it does to a mutant form of hG-CSFR or a polypeptide comprising a region thereof (e.g., a mutant form of the ligand binding domain of h-GCSFR) or a mutant form of SEQ ID NO:1 comprising an alanine substituted for the native arginine at position 287. Additional exemplary changes to SEQ ID NO: 1 and their effect on binding are described herein. Generally, but not necessarily, reference to binding means specific binding, and each term shall be understood to provide explicit support for the other term.
[0168] As used herein, the term "does not detectably bind" shall be understood to mean that a protein, e.g., an antibody, binds to a candidate antigen at a level less than 10%, or 8% or 6% or 5% above background. The background can be the level of binding signal detected in the absence of the protein and/or in the presence of a negative control protein (e.g., an isotype control antibody) and/or the level of binding detected in the presence of a negative control antigen. The level of binding is detected using biosensor analysis (e.g. Biacore) in which the protein is immobilized and contacted with an antigen.
[0169] As used herein, the term "does not significantly bind" shall be understood to mean that the level of binding of a protein of the disclosure to a polypeptide is not statistically significantly higher than background, e.g., the level of binding signal detected in the absence of the protein and/or in the presence of a negative control protein (e.g., an isotype control antibody) and/or the level of binding detected in the presence of a negative control polypeptide. The level of binding is detected using biosensor analysis (e.g. Biacore) in which the protein is immobilized and contacted with an antigen.
[0170] As used herein, phrases referring to "reduced binding" or "binding being at a lower level" in relation to an antigen will be understood to mean that an antibody binds to an antigen (e.g., an alanine point mutant of SEQ ID NO:1 at any one of positions 287, 237, 198, 172, 171 or 111) with an affinity at least about 20 fold or 40 fold or 60 fold less than a control epitope or antigen (e.g. SEQ ID NO:1). For example, a protein of the present disclosure can bind to a polypeptide of SEQ ID NO:1 in which an alanine is substituted for the histidine at position 237 at a level 20 fold or 40 fold or 60 fold less than it binds to a polypeptide of SEQ ID NO:1. Preferably, the protein binds at a level 20 fold less, more preferably 40 fold less, still more preferably 60 fold less.
[0171] A protein or antibody may be considered to "preferentially bind" to a polypeptide if it binds that polypeptide with a dissociation constant (K.sub.D) that is less than the protein's or antibody's K.sub.D for another polypeptide. In one example, a protein or antibody is considered to preferentially bind to a polypeptide if it binds the polypeptide with an affinity (i.e., K.sub.D) that is at least about 20 fold or 40 fold or 60 fold or 80 fold or 100 fold or 120 fold or 140 fold or 160 fold more than the protein's or antibody's K.sub.D for another polypeptide.
[0172] As used herein, the term "similar affinity" will be understood to mean that a protein of the present disclosure binds to two antigens (e.g., the ligand binding domain of G-CSFR from humans and from cynomolgus monkeys) with affinities that are within about 5 fold or less of one another, e.g., within about 4, 3, 2, or 1 fold of one another, such as, within about 0.5 fold of one another or the levels of binding are substantially identical, e.g., when the affinity is assessed by immobilizing the two antigens (e.g., the ligand binding domain of G-CSFR or extracellular domains from humans and from cynomolgus monkeys) and contacting the immobilized proteins with a protein of the disclosure.
[0173] For the purposes of clarification and as will be apparent to the skilled artisan based on the exemplified subject matter herein, reference to "affinity" in this specification is a reference to K.sub.D of a protein or antibody.
[0174] For the purposes of clarification and as will be apparent to the skilled artisan based on the description herein, reference to an "affinity of at least about" will be understood to mean that the affinity (or K.sub.D) is equal to the recited value or higher (i.e., the value recited as the affinity is lower), i.e., an affinity of 2 nM is greater than an affinity of 3 nM. Stated another way, this term could be "an affinity of X or less", wherein X is a value recited herein.
[0175] An "IC.sub.50 of at least about" will be understood to mean that the IC.sub.50 is equal to the recited value or greater (i.e., the value recited as the IC.sub.50 is lower), i.e., an IC.sub.50 of 2 nM is greater than an IC.sub.50 of 3 nM. Stated another way, this term could be "an IC.sub.50 of X or less", wherein X is a value recited herein.
[0176] As used herein, the term "epitope" (syn. "antigenic determinant") shall be understood to mean a region of hG-CSFR to which a protein comprising an antigen binding site of an antibody binds. This term is not necessarily limited to the specific residues or structure to which the protein makes contact. For example, this term includes the region spanning amino acids contacted by the protein and/or 5-10 or 2-5 or 1-3 amino acids outside of this region. In some examples, the epitope comprises a series of discontinuous amino acids that are positioned close to one another when hG-CSFR is folded, i.e., a "conformational epitope". For example, a conformational epitope comprises amino acids in one or more or two or more or all of the regions corresponding to 111-115, 170-176, 218-234 and/or 286-300 of SEQ ID NO: 1. The skilled artisan will also be aware that the term "epitope" is not limited to peptides or polypeptides. For example, the term "epitope" includes chemically active surface groupings of molecules such as sugar side chains, phosphoryl side chains, or sulfonyl side chains, and, in certain examples, may have specific three dimensional structural characteristics, and/or specific charge characteristics.
[0177] The term "competitively inhibits" shall be understood to mean that a protein of the disclosure (or an antigen binding site thereof) reduces or prevents binding of a recited antibody or protein to G-CSFR, e.g., to hG-CSFR. This may be due to the protein (or antigen binding site) and antibody binding to the same or an overlapping epitope. It will be apparent from the foregoing that the protein need not completely inhibit binding of the antibody, rather it need only reduce binding by a statistically significant amount, for example, by at least about 10% or 20% or 30% or 40% or 50% or 60% or 70% or 80% or 90% or 95%. Preferably, the protein reduces binding of the antibody by at least about 30%, more preferably by at least about 50%, more preferably, by at least about 70%, still more preferably by at least about 75%, even more preferably, by at least about 80% or 85% and even more preferably, by at least about 90%. Methods for determining competitive inhibition of binding are known in the art and/or described herein. For example, the antibody is exposed to G-CSFR either in the presence or absence of the protein. If less antibody binds in the presence of the protein than in the absence of the protein, the protein is considered to competitively inhibit binding of the antibody. In one example, the competitive inhibition is not due to steric hindrance.
[0178] "Overlapping" in the context of two epitopes shall be taken to mean that two epitopes share a sufficient number of amino acid residues to permit a protein (or antigen binding site thereof) that binds to one epitope to competitively inhibit the binding of a protein (or antigen binding site) that binds to the other epitope. For example, the "overlapping" epitopes share at least 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 20 amino acids.
[0179] As used herein, the term "neutralize" shall be taken to mean that a protein is capable of blocking, reducing or preventing G-CSF-mediated signaling in a cell through the G-CSFR. Methods for determining neutralization are known in the art and/or described herein.
[0180] As used herein, the term "condition" refers to a disruption of or interference with normal function, and is not to be limited to any specific condition, and will include diseases or disorders.
[0181] As used herein, a "G-CSF-associated condition" refers to any condition that is caused by or associated with neutrophils, an excess of G-CSF or cells expressing G-CSFR or with administration of G-CSF. The skilled artisan will be readily able to determine such conditions. In this regard, in some examples the condition is an inflammatory condition, an autoimmune condition or cancer (including metastasis).
[0182] As used herein, the terms "preventing", "prevent" or "prevention" include administering a protein of the disclosure to thereby stop or hinder the development of at least one symptom of a condition. This term also encompasses treatment of a subject in remission to prevent or hinder relapse. For example, a subject suffering from relapsing-remitting multiple sclerosis is treated during remission to thereby prevent a relapse.
[0183] As used herein, the terms "treating", "treat" or "treatment" include administering a protein described herein to thereby reduce or eliminate at least one symptom of a specified disease or condition.
[0184] As used herein, the term "neutropenia" will be understood to encompass mild neutropenia (1000<=ANC<1500), moderate neutropenia (500<=ANC<1000) and Severe neutropenia (ANC<500) (absolute neutrophil count (ANC) measured in cells per microliter of blood).
[0185] As used herein, the term "subject" shall be taken to mean any animal including humans, for example a mammal. Exemplary subjects include but are not limited to humans and non-human primates. For example, the subject is a human.
Antibodies
[0186] In one example, a protein as described herein according to any example is an antibody.
[0187] Methods for generating antibodies are known in the art and/or described in Harlow and Lane (editors) Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, (1988). Generally, in such methods G-CSFR (e.g., hG-CSFR) or a region thereof (e.g., an extracellular domain) or immunogenic fragment or epitope thereof or a cell expressing and displaying same (i.e., an immunogen), optionally formulated with any suitable or desired carrier, adjuvant, or pharmaceutically acceptable excipient, is administered to a non-human animal, for example, a mouse, chicken, rat, rabbit, guinea pig, dog, horse, cow, goat or pig. The immunogen may be administered intranasally, intramuscularly, sub-cutaneously, intravenously, intradermally, intraperitoneally, or by other known route.
[0188] The production of polyclonal antibodies may be monitored by sampling blood of the immunized animal at various points following immunization. One or more further immunizations may be given, if required to achieve a desired antibody titer. The process of boosting and titering is repeated until a suitable titer is achieved. When a desired level of immunogenicity is obtained, the immunized animal is bled and the serum isolated and stored, and/or the animal is used to generate monoclonal antibodies (mabs).
[0189] Monoclonal antibodies are one exemplary form of antibody contemplated by the present disclosure. The term "monoclonal antibody" or "mAb" refers to a homogeneous antibody population capable of binding to the same antigen(s), for example, to the same epitope within the antigen. This term is not intended to be limited as regards to the source of the antibody or the manner in which it is made.
[0190] For the production of mAbs any one of a number of known techniques may be used, such as, for example, the procedure exemplified in U.S. Pat. No. 4,196,265 or Harlow and Lane (1988), supra.
[0191] For example, a suitable animal is immunized with an immunogen under conditions sufficient to stimulate antibody producing cells. Rodents such as rabbits, mice and rats are exemplary animals. Mice genetically-engineered to express human antibodies and, for example, do not express murine antibodies, can also be used to generate an antibody of the present disclosure (e.g., as described in WO2002/066630).
[0192] Following immunization, somatic cells with the potential for producing antibodies, specifically B lymphocytes (B cells), are selected for use in the mAb generating protocol. These cells may be obtained from biopsies of spleens, tonsils or lymph nodes, or from a peripheral blood sample. The B cells from the immunized animal are then fused with cells of an immortal myeloma cell, generally derived from the same species as the animal that was immunized with the immunogen.
[0193] Hybrids are amplified by culture in a selective medium comprising an agent that blocks the de novo synthesis of nucleotides in the tissue culture media. Exemplary agents are aminopterin, methotrexate and azaserine.
[0194] The amplified hybridomas are subjected to a functional selection for antibody specificity and/or titer, such as, for example, by flow cytometry and/or immunohistochemstry and/or immunoassay (e.g. radioimmunoassay, enzyme immunoassay, cytotoxicity assay, plaque assay, dot immunoassay, and the like).
[0195] Alternatively, ABL-MYC technology (NeoClone, Madison Wis. 53713, USA) is used to produce cell lines secreting MAbs (e.g., as described in Largaespada et al. J. Immunol. Methods. 197: 85-95, 1996).
[0196] Antibodies can also be produced or isolated by screening a display library, e.g., a phage display library, e.g., as described in U.S. Pat. No. 6,300,064 and/or U.S. Pat. No. 5,885,793. For example, the present inventors have isolated fully human antibodies from a phage display library.
[0197] As described herein, some proteins of the present disclosure that bind hG-CSFR cross-react with cynoG-CSFR and/or bind to some mutant forms of hG-CSFR or polypeptides comprising regions of hG-CSFR that have been mutated and/or not others and/or bind to a specific epitope within hG-CSFR. These characteristics can be used in the generation of an antibody or a protein comprising a binding site thereof.
[0198] For example, a phage display library is screened with a polypeptide comprising the ligand binding domain of hG-CSFR to identify proteins that bind thereto. Mutant forms of the ligand binding domain of hG-CSFR (e.g., an alanine point mutant of SEQ ID NO:1 at position 287) to which the protein is not to detectably bind are then used to remove cross-reactive proteins and mutant forms of the ligand binding domain of hG-CSFR or regions thereof (e.g., an alanine point mutant of SEQ ID NO:1 at position 168) to which the protein is to bind are used to isolate proteins that are correctly cross-reactive. A screening process for immunization of a non-human mammal can also be devised based on the foregoing.
[0199] In another example, a phage display library is screened or an animal is immunized with a polypeptide comprising the ligand binding domain of cynoG-CSFR and identified proteins and/or antibodies are screened to identify those that are cross-reactive with hG-CSFR and/or the ligand binding domain thereof.
[0200] In a further example, a G-CSFR or a ligand binding domain thereof (optionally a mutant form to which C1.2 or C1.2G binds) is contacted with C1.2 or C1.2G. A phage display library is then brought into contact with the G-CSFR or the ligand binding domain and phage expressing proteins that can compete with C1.2 or C1.2G for binding selected.
[0201] In a still further example, a chimeric protein comprising, e.g., a mouse G-CSFR in which an epitope of interest from a hG-CSFR is substituted for the corresponding mouse sequence. This chimeric protein is then used to immunize mice (which are less likely to induce an immune response against the mouse protein) and/or to screen a phage display library. The resulting antibodies/proteins are then screened to identify those that bind to hG-CSFR (particularly at the epitope of interest) and not mouse G-CSFR.
[0202] The antibody of the present disclosure may be a synthetic antibody. For example, the antibody is a chimeric antibody, a humanized antibody, a human antibody or a de-immunized antibody.
Chimeric Antibodies
[0203] In one example, an antibody described herein is a chimeric antibody. The term "chimeric antibody" refers to antibodies in which a portion of the heavy and/or light chain is identical with or homologous to corresponding sequences in antibodies derived from a particular species (e.g., murine, such as mouse) or belonging to a particular antibody class or subclass, while the remainder of the chain(s) is identical with or homologous to corresponding sequences in antibodies derived from another species (e.g., primate, such as human) or belonging to another antibody class or subclass. Typically chimeric antibodies utilize rodent or rabbit variable regions and human constant regions, in order to produce an antibody with predominantly human domains. Methods for producing chimeric antibodies are described in, e.g., U.S. Pat. No. 4,816,567; and U.S. Pat. No. 5,807,715.
Humanized and Human Antibodies
[0204] The antibodies of the present disclosure may be humanized or human.
[0205] The term "humanized antibody" shall be understood to refer to a subclass of chimeric antibodies having an antigen binding site or variable region derived from an antibody from a non-human species and the remaining antibody structure based upon the structure and/or sequence of a human antibody. In a humanized antibody, the antigen-binding site generally comprises the complementarity determining regions (CDRs) from the non-human antibody grafted onto appropriate FRs in the variable regions of a human antibody and the remaining regions from a human antibody. Antigen binding sites may be wild-type (i.e., identical to those of the non-human antibody) or modified by one or more amino acid substitutions. In some instances, FR residues of the human antibody are replaced by corresponding non-human residues.
[0206] Methods for humanizing non-human antibodies or parts thereof (e.g., variable regions) are known in the art. Humanization can be performed following the method of U.S. Pat. No. 5,225,539, or U.S. Pat. No. 5,585,089. Other methods for humanizing an antibody are not excluded.
[0207] The term "human antibody" as used herein refers to antibodies having variable regions (e.g. V.sub.H, V.sub.L) and, optionally constant regions derived from or corresponding to sequences found in humans, e.g. in the human germline or somatic cells. The "human" antibodies can include amino acid residues not encoded by human sequences, e.g. mutations introduced by random or site directed mutations in vitro (in particular mutations which involve conservative substitutions or mutations in a small number of residues of the antibody, e.g. in 1, 2, 3, 4, 5 or 6 of the residues of the antibody, e.g. in 1, 2, 3, 4, 5 or 6 of the residues making up one or more of the CDRs of the antibody). These "human antibodies" do not actually need to be produced by a human, rather, they can be produced using recombinant means and/or isolated from a transgenic animal (e.g., mouse) comprising nucleic acid encoding human antibody constant and/or variable regions (e.g., as described above). Human antibodies can be produced using various techniques known in the art, including phage display libraries (e.g., as described in U.S. Pat. No. 5,885,793).
[0208] Human antibodies which recognize a selected epitope can also be generated using a technique referred to as "guided selection." In this approach a selected non-human monoclonal antibody, e.g., a mouse antibody, is used to guide the selection of a completely human antibody recognizing the same epitope (e.g., as described in U.S. Pat. No. 5,565,332).
[0209] Exemplary human antibodies are described herein and include C1.2 and C1.2G and/or variable regions thereof. These human antibodies provide an advantage of reduced immunogenicity in a human compared to non-human antibodies.
Antibody Binding Domain Containing Proteins
Single-Domain Antibodies
[0210] In some examples, a protein of the disclosure is or comprises a single-domain antibody (which is used interchangeably with the term "domain antibody" or "dAb"). A single-domain antibody is a single polypeptide chain comprising all or a portion of the heavy chain variable region of an antibody. In certain examples, a single-domain antibody is a human single-domain antibody (Domantis, Inc., Waltham, Mass.; see, e.g., U.S. Pat. No. 6,248,516).
Diabodies, Triabodies, Tetrabodies
[0211] In some examples, a protein of the disclosure is or comprises a diabody, triabody, tetrabody or higher order protein complex such as those described in WO98/044001 and/or WO94/007921.
[0212] For example, a diabody is a protein comprising two associated polypeptide chains, each polypeptide chain comprising the structure V.sub.L-X-V.sub.H or V.sub.H-X-V.sub.L, wherein V.sub.L is an antibody light chain variable region, V.sub.H is an antibody heavy chain variable region, X is a linker comprising insufficient residues to permit the V.sub.H and V.sub.L in a single polypeptide chain to associate (or form an Fv) or is absent, and wherein the V.sub.H of one polypeptide chain binds to a V.sub.L of the other polypeptide chain to form an antigen binding site, i.e., to form a Fv molecule capable of specifically binding to one or more antigens. The V.sub.L and V.sub.H can be the same in each polypeptide chain or the V.sub.L and V.sub.H can be different in each polypeptide chain so as to form a bispecific diabody (i.e., comprising two Fvs having different specificity).
Single Chain Fv (scFv)
[0213] The skilled artisan will be aware that scFvs comprise V.sub.H and V.sub.L regions in a single polypeptide chain and a polypeptide linker between the V.sub.H and V.sub.L which enables the scFv to form the desired structure for antigen binding (i.e., for the V.sub.H and V.sub.L of the single polypeptide chain to associate with one another to form a Fv). For example, the linker comprises in excess of 12 amino acid residues with (Gly.sub.4Ser).sub.3 being one of the more favored linkers for a scFv.
[0214] The present disclosure also contemplates a disulfide stabilized Fv (or diFv or dsFv), in which a single cysteine residue is introduced into a FR of and a FR of V.sub.L and the cysteine residues linked by a disulfide bond to yield a stable Fv.
[0215] Alternatively, or in addition, the present disclosure encompasses a dimeric scFv, i.e., a protein comprising two scFv molecules linked by a non-covalent or covalent linkage, e.g., by a leucine zipper domain (e.g., derived from Fos or Jun). Alternatively, two scFvs are linked by a peptide linker of sufficient length to permit both scFvs to form and to bind to an antigen, e.g., as described in US20060263367.
Heavy Chain Antibodies
[0216] Heavy chain antibodies differ structurally from many other forms of antibodies, in so far as they comprise a heavy chain, but do not comprise a light chain.
[0217] Accordingly, these antibodies are also referred to as "heavy chain only antibodies". Heavy chain antibodies are found in, for example, camelids and cartilaginous fish (also called IgNAR).
[0218] The variable regions present in naturally occurring heavy chain antibodies are generally referred to as "V.sub.HH domains" in camelid antibodies and V-NAR in IgNAR, in order to distinguish them from the heavy chain variable regions that are present in conventional 4-chain antibodies (which are referred to as "V.sub.H domains") and from the light chain variable regions that are present in conventional 4-chain antibodies (which are referred to as "V.sub.L domains").
[0219] A general description of heavy chain antibodies from camelids and the variable regions thereof and methods for their production and/or isolation and/or use is found inter alia in the following references WO94/04678, WO97/49805 and WO 97/49805.
[0220] A general description of heavy chain antibodies from cartilaginous fish and the variable regions thereof and methods for their production and/or isolation and/or use is found inter alia in WO2005/118629.
Other Antibodies and Antibody Fragments
[0221] The present disclosure also contemplates other antibodies and antibody fragments, such as:
(i) "key and hole" bispecific proteins as described in U.S. Pat. No. 5,731,168; (ii) heteroconjugate proteins, e.g., as described in U.S. Pat. No. 4,676,980; (iii) heteroconjugate proteins produced using a chemical cross-linker, e.g., as described in U.S. Pat. No. 4,676,980; and (iv) Fab.sub.3 (e.g., as described in EP19930302894).
De-Immunized Antibodies and Proteins
[0222] The present disclosure also contemplates a de-immunized antibody or protein. De-immunized antibodies and proteins have one or more epitopes, e.g., B cell epitopes or T cell epitopes removed (i.e., mutated) to thereby reduce the likelihood that a mammal will raise an immune response against the antibody or protein. Methods for producing de-immunized antibodies and proteins are known in the art and described, for example, in WO00/34317, WO2004/108158 and WO2004/064724.
[0223] Methods for introducing suitable mutations and expressing and assaying the resulting protein will be apparent to the skilled artisan based on the description herein.
Mutations to Proteins
[0224] The present disclosure also contemplates mutant forms of a protein of the disclosure. In this regard, data presented herein indicate sites within a CDR of a protein of the disclosure that can be changed in addition to exemplary changes that can be made. The skilled person will understand that changes can additionally or alternatively be made within a FR of a variable region containing protein without inhibiting or significantly reducing its function in the context of the present disclosure.
[0225] For example, such a mutant protein comprises one or more conservative amino acid substitutions compared to a sequence set forth herein. In some examples, the protein comprises 30 or fewer or 20 or fewer or 10 or fewer, e.g., 9 or 8 or 7 or 6 or 5 or 4 or 3 or 2 conservative amino acid substitutions. A "conservative amino acid substitution" is one in which the amino acid residue is replaced with an amino acid residue having a similar side chain and/or hydropathicity and/or hydrophilicity.
[0226] In one example, a mutant protein has only, or not more than, one or two or three or four or five or six conservative amino acid changes when compared to a naturally occurring protein. Details of conservative amino acid changes are provided below. As the skilled person would be aware, e.g., from the disclosure herein, such minor changes can reasonably be predicted not to alter the activity of the protein.
[0227] Families of amino acid residues having similar side chains have been defined in the art, including basic side chains (e.g., lysine, arginine, histidine), acidic side chains (e.g., aspartic acid, glutamic acid), uncharged polar side chains (e.g., glycine, asparagine, glutamine, serine, threonine, tyrosine, cysteine), nonpolar side chains (e.g., alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine, tryptophan), .beta.-branched side chains (e.g., threonine, valine, isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine, tryptophan, histidine).
[0228] The present disclosure also contemplates non-conservative amino acid changes (e.g., substitutions) in a protein of the present disclosure, e.g., in a CDR, such as CDR3. For example, the present inventors have identified several non-conservative amino acid substitutions that can be made while retaining an activity of a protein of the disclosure. In one example, the protein comprises fewer than 6 or 5 or 4 or 3 or 2 or 1 non-conservative amino acid substitutions, e.g., in a CDR3, such as in a CDR3.
[0229] The present disclosure also contemplates one or more insertions or deletions compared to a sequence set forth herein. In some examples, the protein comprises 10 or fewer, e.g., 9 or 8 or 7 or 6 or 5 or 4 or 3 or 2 insertions and/or deletions.
Constant Regions
[0230] The present disclosure encompasses proteins and/or antibodies described herein comprising a constant region of an antibody. This includes antigen binding fragments of an antibody fused to a Fc.
[0231] Sequences of constant regions useful for producing the proteins of the present disclosure may be obtained from a number of different sources. In some examples, the constant region or portion thereof of the protein is derived from a human antibody. The constant region or portion thereof may be derived from any antibody class, including IgM, IgG, IgD, IgA and IgE, and any antibody isotype, including IgG1, IgG2, IgG3 and IgG4. In one example, the constant region is human isotype IgG4 or a stabilized IgG4 constant region.
[0232] In one example, the Fc region of the constant region has a reduced ability to induce effector function, e.g., compared to a native or wild-type human IgG1 or IgG3 Fc region. In one example, the effector function is antibody-dependent cell-mediated cytotoxicity (ADCC) and/or antibody-dependent cell-mediated phagocytosis (ADCP) and/or complement-dependent cytotoxicity (CDC). Methods for assessing the level of effector function of an Fc region containing protein are known in the art and/or described herein.
[0233] In one example, the Fc region is an IgG4 Fc region (i.e., from an IgG4 constant region), e.g., a human IgG4 Fc region. Sequences of suitable IgG4 Fc regions will be apparent to the skilled person and/or available in publically available databases (e.g., available from National Center for Biotechnology Information).
[0234] In one example, the constant region is a stabilized IgG4 constant region. The term "stabilized IgG4 constant region" will be understood to mean an IgG4 constant region that has been modified to reduce Fab arm exchange or the propensity to undergo Fab arm exchange or formation of a half-antibody or a propensity to form a half antibody. "Fab arm exchange" refers to a type of protein modification for human IgG4, in which an IgG4 heavy chain and attached light chain (half-molecule) is swapped for a heavy-light chain pair from another IgG4 molecule. Thus, IgG4 molecules may acquire two distinct Fab arms recognizing two distinct antigens (resulting in bispecific molecules). Fab arm exchange occurs naturally in vivo and can be induced in vitro by purified blood cells or reducing agents such as reduced glutathione. A "half antibody" forms when an IgG4 antibody dissociates to form two molecules each containing a single heavy chain and a single light chain.
[0235] In one example, a stabilized IgG4 constant region comprises a praline at position 241 of the hinge region according to the system of Kabat (Kabat et al., Sequences of Proteins of Immunological Interest Washington D.C. United States Department of Health and Human Services, 1987 and/or 1991). This position corresponds to position 228 of the hinge region according to the EU numbering system (Kabat et al., Sequences of Proteins of Immunological Interest Washington D.C. United States Department of Health and Human Services, 2001 and Edelman et al., Proc. Natl. Acad. USA, 63, 78-85, 1969). In human IgG4, this residue is generally a serine. Following substitution of the serine for proline, the IgG4 hinge region comprises a sequence CPPC. In this regard, the skilled person will be aware that the "hinge region" is a proline-rich portion of an antibody heavy chain constant region that links the Fc and Fab regions that confers mobility on the two Fab arms of an antibody. The hinge region includes cysteine residues which are involved in inter-heavy chain disulfide bonds. It is generally defined as stretching from Glu226 to Pro243 of human IgG1 according to the numbering system of Kabat. Hinge regions of other IgG isotypes may be aligned with the IgG1 sequence by placing the first and last cysteine residues forming inter-heavy chain disulphide (S--S) bonds in the same positions (see for example WO2010/080538).
[0236] Additional examples of stabilized IgG4 antibodies are antibodies in which arginine at position 409 in a heavy chain constant region of human IgG4 (according to the EU numbering system) is substituted with lysine, threonine, methionine, or leucine (e.g., as described in WO2006/033386). The Fc region of the constant region may additionally or alternatively comprise a residue selected from the group consisting of: alanine, valine, glycine, isoleucine and leucine at the position corresponding to 405 (according to the EU numbering system). Optionally, the hinge region comprises a proline at position 241 (i.e., a CPPC sequence) (as described above).
[0237] In another example, the Fc region is a region modified to have reduced effector function, i.e., a "non-immunostimulatory Fc region". For example, the Fc region is an IgG1 Fc region comprising a substitution at one or more positions selected from the group consisting of 268, 309, 330 and 331. In another example, the Fc region is an IgG1 Fc region comprising one or more of the following changes E233P, L234V, L235A and deletion of G236 and/or one or more of the following changes A327G, A330S and P331S (Armour et al., Eur J Immunol. 29:2613-2624, 1999; Shields et al., J Biol Chem. 276(9):6591-604, 2001). Additional examples of non-immunostimulatory Fc regions are described, for example, in Dall'Acqua et al. J Immunol. 177: 1129-1138 2006; and/or Hezareh J Virol; 75: 12161-12168, 2001).
[0238] In another example, the Fc region is a chimeric Fc region, e.g., comprising at least one C.sub.H2 domain from an IgG4 antibody and at least one C.sub.H3 domain from an IgG1 antibody, wherein the Fc region comprises a substitution at one or more amino acid positions selected from the group consisting of 240, 262, 264, 266, 297, 299, 307, 309, 323, 399, 409 and 427 (EU numbering) (e.g., as described in WO2010/085682). Exemplary substitutions include 240F, 262L, 264T, 266F, 297Q, 299A, 299K, 307P, 309K, 309M, 309P, 323F, 399S, and 427F.
Additional Modifications
[0239] The present disclosure also contemplates additional modifications to an antibody.
[0240] For example, the antibody comprises one or more amino acid substitutions that increase the half-life of the protein. For example, the antibody comprises a Fc region comprising one or more amino acid substitutions that increase the affinity of the Fc region for the neonatal Fc region (FcRn). For example, the Fc region has increased affinity for FcRn at lower pH, e.g., about pH 6.0, to facilitate Fc/FcRn binding in an endosome. In one example, the Fc region has increased affinity for FcRn at about pH 6 compared to its affinity at about pH 7.4, which facilitates the re-release of Fc into blood following cellular recycling. These amino acid substitutions are useful for extending the half life of a protein, by reducing clearance from the blood.
[0241] Exemplary amino acid substitutions include T250Q and/or M428L or 1252A, T254S and T266F or M252Y, S254T and T256E or H433K and N434F according to the EU numbering system. Additional or alternative amino acid substitutions are described, for example, in US20070135620 or U.S. Pat. No. 7,083,784.
Protein Production
[0242] In one example, a protein described herein according to any example is produced by culturing a hybridoma under conditions sufficient to produce the protein, e.g., as described herein and/or as is known in the art.
Recombinant Expression
[0243] In another example, a protein described herein according to any example is recombinant.
[0244] In the case of a recombinant protein, nucleic acid encoding same can be cloned into expression constructs or vectors, which are then transfected into host cells, such as E. coli cells, yeast cells, insect cells, or mammalian cells, such as simian COS cells, Chinese Hamster Ovary (CHO) cells, human embryonic kidney (HEK) cells, or myeloma cells that do not otherwise produce the protein. Exemplary cells used for expressing a protein are CHO cells, myeloma cells or HEK cells. Molecular cloning techniques to achieve these ends are known in the art and described, for example in Ausubel et al., (editors), Current Protocols in Molecular Biology, Greene Pub. Associates and Wiley-Interscience (1988, including all updates until present) or Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press (1989). A wide variety of cloning and in vitro amplification methods are suitable for the construction of recombinant nucleic acids. Methods of producing recombinant antibodies are also known in the art, see, e.g., U.S. Pat. No. 4,816,567 or U.S. Pat. No. 5,530,101.
[0245] Following isolation, the nucleic acid is inserted operably linked to a promoter in an expression construct or expression vector for further cloning (amplification of the DNA) or for expression in a cell-free system or in cells.
[0246] As used herein, the term "promoter" is to be taken in its broadest context and includes the transcriptional regulatory sequences of a genomic gene, including the TATA box or initiator element, which is required for accurate transcription initiation, with or without additional regulatory elements (e.g., upstream activating sequences, transcription factor binding sites, enhancers and silencers) that alter expression of a nucleic acid, e.g., in response to a developmental and/or external stimulus, or in a tissue specific manner. In the present context, the term "promoter" is also used to describe a recombinant, synthetic or fusion nucleic acid, or derivative which confers, activates or enhances the expression of a nucleic acid to which it is operably linked. Exemplary promoters can contain additional copies of one or more specific regulatory elements to further enhance expression and/or alter the spatial expression and/or temporal expression of said nucleic acid.
[0247] As used herein, the term "operably linked to" means positioning a promoter relative to a nucleic acid such that expression of the nucleic acid is controlled by the promoter.
[0248] Many vectors for expression in cells are available. The vector components generally include, but are not limited to, one or more of the following: a signal sequence, a sequence encoding a protein (e.g., derived from the information provided herein), an enhancer element, a promoter, and a transcription termination sequence. The skilled artisan will be aware of suitable sequences for expression of a protein. Exemplary signal sequences include prokaryotic secretion signals (e.g., pelB, alkaline phosphatase, penicillinase, Ipp, or heat-stable enterotoxin II), yeast secretion signals (e.g., invertase leader, at factor leader, or acid phosphatase leader) or mammalian secretion signals (e.g., herpes simplex gD signal).
[0249] Exemplary promoters active in mammalian cells include cytomegalovirus immediate early promoter (CMV-IE), human elongation factor 1-.alpha. promoter (EF1), small nuclear RNA promoters (U1a and U1b), .alpha.-myosin heavy chain promoter, Simian virus 40 promoter (SV40), Rous sarcoma virus promoter (RSV), Adenovirus major late promoter, .beta.-actin promoter; hybrid regulatory element comprising a CMV enhancer/.beta.-actin promoter or an immunoglobulin promoter or active fragment thereof. Examples of useful mammalian host cell lines are monkey kidney CV1 line transformed by SV40 (COS-7, ATCC CRL 1651); human embryonic kidney line (293 or 293 cells subcloned for growth in suspension culture; baby hamster kidney cells (BHK, ATCC CCL 10); or Chinese hamster ovary cells (CHO).
[0250] Typical promoters suitable for expression in yeast cells such as for example a yeast cell selected from the group comprising Pichia pastoris, Saccharomyces cerevisiae and S. pombe, include, hut are not limited to, the ADH1 promoter, the GAL1 promoter, the GAL4 promoter, the CUP1 promoter, the PHO5 promoter, the nmt promoter, the RPR1 promoter, or the TEF1 promoter.
[0251] Means for introducing the isolated nucleic acid or expression construct comprising same into a cell for expression are known to those skilled in the art. The technique used for a given cell depends on the known successful techniques. Means for introducing recombinant DNA into cells include microinjection, transfection mediated by DEAE-dextran, transfection mediated by liposomes such as by using lipofectamine (Gibco, MD, USA) and/or cellfectin (Gibco, MD, USA), PEG-mediated DNA uptake, electroporation and microparticle bombardment such as by using DNA-coated tungsten or gold particles (Agracetus Inc., WI, USA) amongst others.
[0252] The host cells used to produce the protein may be cultured in a variety of media, depending on the cell type used. Commercially available media such as Ham's F10 (Sigma), Minimal Essential Medium ((MEM), (Sigma), RPMI-1640 (Sigma), and Dulbecco's Modified Eagle's Medium ((DMEM), Sigma) are suitable for culturing mammalian cells. Media for culturing other cell types discussed herein are known in the art.
Isolation of Proteins
[0253] Methods for isolating a protein are known in the art and/or described herein.
[0254] Where a protein is secreted into culture medium, supernatants from such expression systems can be first concentrated using a commercially available protein concentration filter, for example, an Amicon or Millipore Pellicon ultrafiltration unit. A protease inhibitor such as PMSF may be included in any of the foregoing steps to inhibit proteolysis and antibiotics may be included to prevent the growth of adventitious contaminants. Alternatively, or additionally, supernatants can be filtered and/or separated from cells expressing the protein, e.g., using continuous centrifugation.
[0255] The protein prepared from the cells can be purified using, for example, ion exchange, hydroxyapatite chromatography, hydrophobic interaction chromatography, gel electrophoresis, dialysis, affinity chromatography (e.g., protein A affinity chromatography or protein G chromatography), or any combination of the foregoing. These methods are known in the art and described, for example in WO99/57134 or Ed Harlow and David Lane (editors) Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, (1988).
[0256] The skilled artisan will also be aware that a protein can be modified to include a tag to facilitate purification or detection, e.g., a poly-histidine tag, e.g., a hexa-histidine tag, or a influenza virus hemagglutinin (HA) tag, or a Simian Virus 5 (V5) tag, or a FLAG tag, or a glutathione S-transferase (GST) tag. The resulting protein is then purified using methods known in the art, such as, affinity purification. For example, a protein comprising a hexa-his tag is purified by contacting a sample comprising the protein with nickel-nitrilotriacetic acid (Ni-NTA) that specifically binds a hexa-his tag immobilized on a solid or semi-solid support, washing the sample to remove unbound protein, and subsequently eluting the bound protein. Alternatively, or in addition a ligand or antibody that binds to a tag is used in an affinity purification method.
Assaying Activity of a Protein
Binding to G-CSFR and Mutants Thereof
[0257] It will be apparent to the skilled artisan from the disclosure herein that some proteins of the present disclosure bind to the ligand binding domain of hG-CSFR and to specific mutant forms of the ligand binding domain of hG-CSFR (e.g., SEQ ID NO:1 without or with certain point mutations) and/or bind to both human and cynomolgus monkey G-CSFR. Methods for assessing binding to a protein are known in the art, e.g., as described in Scopes (In: Protein purification: principles and practice, Third Edition, Springer Verlag, 1994). Such a method generally involves labeling the protein and contacting it with immobilized antigen. Following washing to remove non-specific bound protein, the amount of label and, as a consequence, bound protein is detected. Of course, the protein can be immobilized and the antigen labeled. Panning-type assays can also be used. Alternatively, or additionally, surface plasmon resonance assays can be used.
[0258] The assays described above can also be used to detect the level of binding of a protein to hG-CSFR or a ligand binding domain thereof (e.g., SEQ ID NO: 1) or mutant form thereof.
[0259] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the lysine at position 167 of SEQ ID NO: 1 and/or in which an alanine is substituted for the histidine at position 168 of SEQ ID NO: 1 at substantially the same level (e.g., within 10% or 5% or 1%) as it binds to SEQ ID NO: 1.
[0260] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the arginine at position 287 of SEQ ID NO:1 at a level at least about 100 fold or 150 fold or 160 fold or 200 fold lower than it binds to a polypeptide of SEQ ID NO: 1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the arginine at position 287 of SEQ ID NO: 1 at a level at least about 160 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0261] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO:1 in which an alanine is substituted for the histidine at position 237 of SEQ ID NO: 1 at a level at least about 20 fold or 40 fold or 50 fold or 60 fold lower than it binds to a polypeptide of SEQ ID NO: 1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the histidine at position 237 of SEQ ID NO: 1 at a level at least about 50 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0262] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the methionine at position 198 of SEQ ID NO: 1 at a level at least about 20 fold or 40 fold or 60 fold or 70 fold lower than it binds to a polypeptide of SEQ ID NO: 1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the methionine at position 198 of SEQ ID NO:1 at a level at least about 40 fold lower than it binds to a polypeptide of SEQ ID NO:1.
[0263] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the tyrosine at position 172 of SEQ ID NO:1 at a level at least about 20 fold or 30 fold or 40 fold lower than it binds to a polypeptide of SEQ ID NO: 1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the tyrosine at position 172 of SEQ ID NO: 1 at a level at least about 40 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0264] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO:1 in which an alanine is substituted for the leucine at position 171 of SEQ ID NO: 1 at a level at least about 100 fold or 120 fold or 130 fold or 140 fold lower than it binds to a polypeptide of SEQ ID NO:1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO:1 in which an alanine is substituted for the leucine at position 171 of SEQ ID NO: 1 at a level at least about 140 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0265] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the leucine at a position 111 of SEQ ID NO:1 at a level at least about 20 fold or 40 fold or 60 fold or 70 fold lower than it binds to a polypeptide of SEQ ID NO: 1. In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the leucine at a position 111 of SEQ ID NO: 1 at a level at least about 60 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0266] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the histidine at position 168 of SEQ ID NO:1 at a level no more than 5 fold or 4 fold or 3 fold or 2 fold or 1 fold lower than it binds to a polypeptide of SEQ ID NO:1.
[0267] In one example, a protein of the present disclosure binds to a polypeptide of SEQ ID NO: 1 in which an alanine is substituted for the lysine at position 167 of SEQ ID NO: 1 at a level no more than 5 fold or 4 fold or 3 fold or 2 fold or 1 fold lower than it binds to a polypeptide of SEQ ID NO: 1.
[0268] The level of binding is conveniently determined using a biosensor.
[0269] The present disclosure contemplates any combination of the foregoing characteristics. In one example, a protein described herein has all of the binding characteristics set forth in the preceding seven paragraphs.
Epitope Mapping
[0270] In another example, the epitope bound by a protein described herein is mapped. Epitope mapping methods will be apparent to the skilled artisan. For example, a series of overlapping peptides spanning the hG-CSFR sequence or a region thereof comprising an epitope of interest, e.g., peptides comprising 10.15 amino acids are produced. The protein is then contacted to each peptide and the peptide(s) to which it binds determined. This permits determination of peptide(s) comprising the epitope to which the protein binds. If multiple non-contiguous peptides are bound by the protein, the protein may bind a conformational epitope.
[0271] Alternatively, or in addition, amino acid residues within hG-CSFR are mutated. e.g., by alanine scanning mutagenesis, and mutations that reduce or prevent protein binding are determined. Any mutation that reduces or prevents binding of the protein is likely to be within the epitope bound by the protein.
[0272] A further method is exemplified herein, and involves binding hG-CSFR or a region thereof to an immobilized protein of the present disclosure and digesting the resulting complex with proteases. Peptide that remains bound to the immobilized protein are then isolated and analyzed, e.g., using mass spectrometry, to determine their sequence.
[0273] A further method involves converting hydrogens in hG-CSFR or a region thereof to deutrons and binding the resulting protein to an immobilized protein of the present disclosure. The deutrons are then converted back to hydrogen, the hG-CSFR or region thereof isolated, digested with enzymes and analyzed, e.g., using mass spectrometry to identify those regions comprising deutrons, which would have been protected from conversion to hydrogen by the binding of a protein described herein.
[0274] Optionally, the dissociation constant (Kd) of a protein for hG-CSFR or an epitope thereof is determined. The "Kd" or "Kd value" for a hG-CSFR binding protein is in one example measured by a radiolabeled or fluorescently-labeled hG-CSFR binding assay. This assay equilibrates the protein with a minimal concentration of labeled G-CSFR in the presence of a titration series of unlabeled hG-CSFR. Following washing to remove unbound hG-CSFR, the amount of label is determined, which is indicative of the Kd of the protein.
[0275] According to another example the Kd or Kd value is measured by using surface plasmon resonance assays, e.g., using BIAcore surface plasmon resonance (BIAcore, Inc., Piscataway, N.J.) with immobilized hG-CSFR or a region thereof.
[0276] In some examples, proteins having a similar Kd or a higher Kd than C1.2 or C1.2G are selected, because they are likely to compete for binding to hG-CSFR.
Determining Competitive Binding
[0277] Assays for determining a protein that competitively inhibits binding of monoclonal antibody C1.2 or C1.2G will be apparent to the skilled artisan. For example, C1.2 or C1.2G is conjugated to a detectable label, e.g., a fluorescent label or a radioactive label. The labeled antibody and the test protein are then mixed and contacted with hG-CSFR or a region thereof (e.g., a polypeptide comprising SEQ ID NO: 1) or a cell expressing same. The level of labeled C1.2 or C1.2G is then determined and compared to the level determined when the labeled antibody is contacted with the hG-CSFR, region or cells in the absence of the protein. If the level of labeled C1.2 or C1.2G is reduced in the presence of the test protein compared to the absence of the protein, the protein is considered to competitively inhibit binding of C1.2 or C1.2G to hG-CSFR.
[0278] Optionally, the test protein is conjugated to different label to C1.2 or C1.2G. This alternate labeling permits detection of the level of binding of the test protein to hG-CSFR or the region thereof or the cell.
[0279] In another example, the protein is permitted to bind to hG-CSFR or a region thereof (e.g., a polypeptide comprising SEQ ID NO: 1) or a cell expressing same prior to contacting the hG-CSFR, region or cell with C1.2 or C1.2G. A reduction in the amount of bound C1.2 or C1.2G in the presence of the protein compared to in the absence of the protein indicates that the protein competitively inhibits C1.2 or C1.2G binding to hG-CSFR. A reciprocal assay can also be performed using labeled protein and first allowing C1.2 or C1.2G to bind to G-CSFR. In this case, a reduced amount of labeled protein bound to hG-CSFR in the presence of C1.2 or C1.2G compared to in the absence of C1.2 or C1.2G indicates that the protein competitively inhibits binding of C1.2 or C1.2G to hG-CSFR.
[0280] Any of the foregoing assays can be performed with a mutant form of hG-CSFR and/or SEQ ID NO:1 and/or a ligand binding region of hG-CSFR to which C1.2 or C1.2G binds, e.g., as described herein.
Determining Neutralization
[0281] In some examples of the present disclosure, a protein is capable of neutralizing hG-CSFR signaling.
[0282] Various assays are known in the art for assessing the ability of a protein to neutralize signaling of a ligand through a receptor.
[0283] In one example, the protein reduces or prevents G-CSF binding to the hG-CSFR. These assays can be performed as a competitive binding assay as described herein using labeled G-CSF and/or labeled protein.
[0284] In another example, the protein reduces formation of CFU-G when CD34.sup.+ bone marrow cells are cultured in the presence of G-CSF. In such assays, CD34.sup.+ bone marrow cells are cultured in a semi-solid cell culture medium in the presence of G-CSF (e.g., about 10 ng/ml cell culture medium) and, optionally stem cell factor (e.g., about 10 ng/ml cell culture medium) in the presence or absence of a test protein. After a sufficient time for granulocyte clones (CFU-G) to form, the number of clones or colonies is determined. A reduction in the number of colonies in the presence of the protein compared to in the absence of the protein indicates that the protein neutralizes G-CSF signaling. By testing multiple concentrations of the protein an IC.sub.50 is determined, i.e., a concentration at which 50% of the maximum inhibition of CFU-G formation occurs. In one example, the IC.sub.50 is 0.2 nM or less, such as 0.1 nM or less, for example, 0.09 nM or less, or 0.08 nM or less, or 0.07 nM or less, or 0.06 nM or less or 0.05 nM or less. In one example, the IC.sub.50 is 0.04 nM or less. In another example, the IC.sub.50 is 0.02 nM or less. The foregoing IC.sub.50s relate to any CFU-G assay described herein.
[0285] In a further example, the protein reduces proliferation of cells (e.g., BaF3 cells) expressing hG-CSFR which are cultured in the presence of G-CSF. Cells are cultured in the presence of G-CSF (e.g., 0.5 ng/ml) and the presence or absence of a test protein. Methods for assessing cell proliferation are known in the art and include, for example, MTT reduction and thymidine incorporation. A protein that reduces the level of proliferation compared to the level observed in the absence of the protein is considered to neutralize G-CSF signaling. By testing multiple concentrations of the protein an IC.sub.50 is determined, i.e., a concentration at which 50% of the maximum inhibition of cell proliferation occurs. In one example, the IC.sub.50 is 6 nM or less, such as 5.9 nM or less. In another example, the IC.sub.50 is 2 nM or less or 1 nM or less or 0.7 nM or cell or 0.6 nM or less or 0.5 nM or less. The foregoing IC.sub.50s relate to any cell proliferation assay described herein.
[0286] In a further example, the protein reduces mobilization of hematopoietic stem cells and/or endothelial progenitor cells in vivo following G-CSF administration and/or reduces the number of neutrophils in vivo, e.g., following G-CSF administration (however this is not essential). For example, the protein is administered to a subject, optionally before, at the time of or after administration of G-CSF or a modified form thereof (e.g., PEGylated G-CSF or filgrastim). The number of hematopoietic stem cells (e.g., expressing CD34 and/or Thy1) and/or endothelial progenitor cells (e.g., expressing CD34 and VEGFR2) and/or neutrophils (identified morphologically and/or expressing e.g., CD10, CD14, CD31 and/or CD88) is assessed. A protein that reduces the level of the cell(s) compared to the level observed in the absence of the protein is considered to neutralize G-CSF signaling. In one example, the protein reduces the number of neutrophils without inducing neutropenia.
[0287] Other methods for assessing neutralization of G-CSF signaling are contemplated by the present disclosure.
Determining Effector Function
[0288] As discussed herein, some proteins of the present disclosure have reduced effector function. Methods for assessing ADCC activity are known in the art.
[0289] In one example, the level of ADCC activity is assessed using a .sup.51Cr release assay, an europium release assay or a .sup.35S release assay. In each of these assays, cells expressing G-CSFR are cultured with one or more of the recited compounds for a time and under conditions sufficient for the compound to be taken up by the cell. In the case of a .sup.35S release assay, cells expressing hG-CSFR can be cultured with .sup.35S-labeled methionine and/or cysteine for a time sufficient for the labeled amino acids to be incorporated into newly synthesized proteins. Cells are then cultured in the presence or absence of the protein and in the presence of immune effector cells, e.g., peripheral blood mononuclear cells (PBMC) and/or NK cells. The amount of .sup.51Cr, europium and/or .sup.35S in cell culture medium is then detected, and little or no change in the presence of the protein compared to in the absence of protein (or a reduced level of the compound compared to the level observed in the presence of an anti-hG-CSFR antibody comprising a human IgG1 Fc) indicates that the protein has reduced effector function. Exemplary publications disclosing assays for assessing the level of ADCC induced by a protein include Hellstrom, et al. Proc. Natl Acad. Sci. USA 83:7059-7063, 1986 and Bruggemann, et al., J. Exp. Med. 166:1351-1361, 1987.
[0290] Other assays for assessing the level of ADCC induced by a protein include ACTI.TM. nonradioactive cytotoxicity assay for flow cytometry (CellTechnology, Inc. CA, USA) or CytoTox 96.RTM. non-radioactive cytotoxicity assay (Promega, WI, USA).
[0291] C1q binding assays may also be carried out to confirm that the protein is able to bind C1q and may induce CDC. To assess complement activation, a CDC assay may be performed (see, for example, Gazzano-Santoro et al, J. Immunol. Methods 202: 163, 1996.
Determining Half Life
[0292] Some proteins encompassed by the present disclosure have an improved half-life, e.g., are modified to extend their half-life compared to proteins that are unmodified. Methods for determining a protein with an improved half-life will be apparent to the skilled person. For example, the ability of a protein to bind to a neonatal Fc receptor (FcRn) is assessed. In this regard, increased binding affinity for FcRn increased the serum half-life of the molecule (see for example, Kim et al., Eur J Immunol., 24:2429, 1994).
[0293] The half-life of a protein of the disclosure can also be measured by pharmacokinetic studies, e.g., according to the method described by Kim et al, Eur J of Immunol 24:542, 1994. According to this method radiolabeled protein is injected intravenously into mice and its plasma concentration is periodically measured as a function of time, for example at 3 minutes to 72 hours after the injection. The clearance curve thus obtained should be biphasic, that is, an alpha phase and beta phase. For the determination of the in vivo half-life of the protein, the clearance rate in beta-phase is calculated and compared with that of the wild type or unmodified protein.
Assessing Therapeutic Efficacy
[0294] Assays for assessing therapeutic efficacy are described hereinabove in relation to determining neutralization by a protein.
[0295] In another example, the efficacy of a protein to treat a condition is assessed using an in vivo assay.
[0296] For example, the protein is tested in an animal model of arthritis. Exemplary models include a SKG strain of mouse (Sakaguchi et al., Nature, 426: 454-460), rat type II collagen arthritis model, mouse type II collagen arthritis model or antigen induced arthritis models in several species (Bendele J Musculoskel Neuron Interact; 1(4):377-385, 2001). In these assays, arthritis is induced and the ability of the protein to reduce one or more symptoms of arthritis, e.g., joint inflammation and/or markers of inflammation in synovial fluid is assessed. A protein that reduces a symptom of arthritis is considered useful for treating this condition or a G-CSF-mediated condition (e.g., a G-CSF-mediated inflammatory condition).
[0297] The protein can also or alternatively be tested in a model of COPD, e.g., in which a non-human mammal (e.g., a rodent, such as, a mouse) is exposed to cigarette smoke. Following exposure, the mammal is administered a protein and the level of lung inflammation and/or the number of neutrophils in the lung is assessed or estimated using standard techniques. A protein that reduces lung inflammation and/or the number of neutrophils is considered useful for treating lung inflammation or COPD or a G-CSF-mediated condition (e.g., a G-CSF-mediated inflammatory condition, such as a G-CSF-mediated inflammatory lung condition).
[0298] Proteins described herein can also tested in in vivo models of inflammatory neurological disease. Exemplary models include EAE models in which a mouse or rat is immunized with a myelin sheath protein or peptide derived therefrom (e.g., MOG, MBP or PLP) and an immune response is generated against the protein thereby inducing a model of MS. Alternatively, T cells that are immunoreactive with a myelin sheath protein are introduced into mice or rats to induce EAE. Exemplary EAE models are reviewed in, for example Tsunoda and Fujinami, J Neuropathol Exp Neurol. 55:673-686, 1996.
[0299] Other models of MS include transgenic animals expressing T cell receptors specific for a myelin protein, e.g., MOG, MBP or PLP. Exemplary models are described, for example, in Bettelli et al., JEM 197:1073-1081, 2003; Illes et al., Proc. Natl. Acad. Sci. USA, 101: 11749-11754, 2004; or Rossi et al., J. Biomolecular Screening, 12: 481-489, 2007; or are commercially available, e.g., from Jackson Laboratories USA (e.g. mice 2D2 having transgenic T cell receptors reactive with MOG).
[0300] In a further example, a protein described herein according to any example is tested in a model of uveitis. Models of uveitis include those induced by immunizing a non-human mammal with a protein such as retinal arrestin, recoverin or rhodopsin or administration of bacterial endotoxin to eye. Exemplary models of uveitis are described, for example, in Caspi, Drug Discovery Today, 3: 3-9, 2006.
[0301] A protein of the disclosure can also be tested in models of angiogenesis, e.g., Iris Pharma Inc's models of ocular angiogenesis, or an alginate encapsulated tumor cell model, and/or by assessing the ability of a cancer cell to metastasize in a subject.
Conditions to be Treated
[0302] The present disclosure contemplates treatment or prevention of any condition that is caused by or exacerbated by G-CSF in a subject. In one example, the condition is an autoimmune or inflammatory condition.
[0303] In one example, the inflammatory or autoimmune condition is an inflammatory joint condition, such as, inflammatory arthritis, rheumatoid arthritis or idiopathic arthritis, e.g., juvenile idiopathic arthritis. In one example, the condition is rheumatoid arthritis.
[0304] In one example, the inflammatory or autoimmune condition is an inflammatory eye condition. For example, the condition is uveitis.
[0305] In one example, the inflammatory or autoimmune condition is an inflammatory lung condition, such as, a pulmonary disease associated with neutrophil infiltration, e.g., COPD. In one example, the condition is COPD.
[0306] In one example, the inflammatory or autoimmune condition is an inflammatory neurological condition, such as, Devices disease, a viral infection in the brain or multiple sclerosis. In one example, the condition is multiple sclerosis, which includes chronic progressive multiple sclerosis or relapsing-remitting multiple sclerosis.
[0307] In another example, the condition is cancer (including angiogenesis thereof) or metastasis thereof.
[0308] In one example, the subject is resistant to, does not adequately respond to, or is unsuitable for treatment with another compound used to treat the condition. For example, the subject suffering from an autoimmune or inflammatory condition is resistant to, does not adequately respond to, or is unsuitable for treatment with a corticosteroid and/or an immunosuppressant and/or cyclophosphamide and and/or methotrexate and/or an anti-TNF antibody or soluble TNF receptor and/or an anti-CD20 antibody and/or an anti-IL6 antibody and/or an anti-CD22 antibody.
Compositions
[0309] In some examples, a protein as described herein can be administered orally, parenterally, by inhalation spray, adsorption, absorption, topically, rectally, nasally, bucally, vaginally, intraventricularly, via an implanted reservoir in dosage formulations containing conventional non-toxic pharmaceutically-acceptable carriers, or by any other convenient dosage form. The term "parenteral" as used herein includes subcutaneous, intravenous, intramuscular, intraperitoneal, intrathecal, intraventricular, intrasternal, and intracranial injection or infusion techniques.
[0310] Methods for preparing a protein into a suitable fawn for administration to a subject (e.g. a pharmaceutical composition) are known in the art and include, for example, methods as described in Remington's Pharmaceutical Sciences (18th ed., Mack Publishing Co., Easton, Pa., 1990) and U.S. Pharmacopeia: National Formulary (Mack Publishing Company, Easton, Pa., 1984).
[0311] The pharmaceutical compositions of this disclosure are particularly useful for parenteral administration, such as intravenous administration or administration into a body cavity or lumen of an organ or joint. The compositions for administration will commonly comprise a solution of protein dissolved in a pharmaceutically acceptable carrier, for example an aqueous carrier. A variety of aqueous carriers can be used, e.g., buffered saline and the like. The compositions may contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions such as pH adjusting and buffering agents, toxicity adjusting agents and the like, for example, sodium acetate, sodium chloride, potassium chloride, calcium chloride, sodium lactate and the like. The concentration of proteins of the present disclosure in these formulations can vary widely, and will be selected primarily based on fluid volumes, viscosities, body weight and the like in accordance with the particular mode of administration selected and the patient's needs. Exemplary carriers include water, saline, Ringer's solution, dextrose solution, and 5% human serum albumin. Nonaqueous vehicles such as mixed oils and ethyl oleate may also be used. Liposomes may also be used as carriers. The vehicles may contain minor amounts of additives that enhance isotonicity and chemical stability, e.g., buffers and preservatives.
[0312] Upon formulation, proteins of the present disclosure will be administered in a manner compatible with the dosage formulation and in such amount as is therapeutically/prophylactically effective. Formulations are easily administered in a variety of dosage forms, such as the type of injectable solutions described above, but other pharmaceutically acceptable forms are also contemplated, e.g., tablets, pills, capsules or other solids for oral administration, suppositories, pessaries, nasal solutions or sprays, aerosols, inhalants, liposomal forms and the like. Pharmaceutical "slow release" capsules or compositions may also be used. Slow release formulations are generally designed to give a constant drug level over an extended period and may be used to deliver compounds of the present disclosure.
[0313] WO2002/080967 describes compositions and methods for administering aerosolized compositions comprising antibodies for the treatment of, e.g., asthma, which are also suitable for administration of a protein of the present disclosure.
Combination Therapies
[0314] In one example, a protein of the present disclosure is administered in combination with another compound useful for treating a disease or condition described herein, either as combined or additional treatment steps or as additional components of a therapeutic formulation.
[0315] For example, the other compound is an anti-inflammatory compound. Alternatively, or additionally, the other compound is an immunosuppressant. Alternatively, or additionally, the other compound is a corticosteroid, such as prednisone and/or prednisolone. Alternatively, or additionally, the other compound is methotrexate. Alternatively, or additionally, the other compound is cyclophosphamide. Alternatively, or additionally, the other compound is mycophenolate mofetil. Alternatively, or additionally, the other compound is an anti-CD20 antibody (e.g., rituximab or ofatumumab). Alternatively, or additionally, the other compound is an anti-CD22 antibody (e.g., epratuzumab). Alternatively, or additionally, the other compound is an anti-TNF antibody (e.g., infliximab or adalimumab or golimuinab) or soluble TNF receptor (e.g., etanercept). Alternatively, or additionally, the other compound is a CTLA-4 antagonist (e.g., abatacept, CTLA4-Ig). Alternatively, or additionally, the other compound is an anti-IL-6 antibody. Alternatively, or additionally, the other compound is a BLys antagonist, such as an anti-BLys antibody (e.g., belimumab).
[0316] In another example, the other compound is a chemotherapy drug or other drug used for treating cancer.
[0317] In another example, the protein described herein is administered before or after radiotherapy for the treatment of cancer.
Dosages and Timing of Administration
[0318] Suitable dosages of proteins of the present disclosure will vary depending on the specific protein, the condition to be treated and/or the subject being treated. It is within the ability of a skilled physician to determine a suitable dosage, e.g., by commencing with a sub-optimal dosage and incrementally modifying the dosage to determine an optimal or useful dosage. Alternatively, to determine an appropriate dosage for treatment/prophylaxis, data from the cell culture assays or animal studies are used, wherein a suitable dose is within a range of circulating concentrations that include the ED.sub.50 of the active compound with little or no toxicity. The dosage may vary within this range depending upon the dosage form employed and the route of administration utilized. A therapeutically/prophylactically effective dose can be estimated initially from cell culture assays. A dose may be formulated in animal models to achieve a circulating plasma concentration range that includes the IC.sub.50 (i.e., the concentration of the compound which achieves a half-maximal inhibition of symptoms) as determined in cell culture. Such information can be used to more accurately determine useful doses in humans. Levels in plasma maybe measured, for example, by high performance liquid chromatography.
[0319] In some examples, a method of the present disclosure comprises administering a prophylactically or therapeutically effective amount of a protein described herein.
[0320] The term "therapeutically effective amount" is the quantity which, when administered to a subject in need of treatment, improves the prognosis and/or state of the subject and/or that reduces or inhibits one or more symptoms of a clinical condition described herein to a level that is below that observed and accepted as clinically diagnostic or clinically characteristic of that condition. The amount to be administered to a subject will depend on the particular characteristics of the condition to be treated, the type and stage of condition being treated, the mode of administration, and the characteristics of the subject, such as general health, other diseases, age, sex, genotype, and body weight. A person skilled in the art will be able to determine appropriate dosages depending on these and other factors. Accordingly, this term is not to be construed to limit the present disclosure to a specific quantity, e.g., weight or amount of protein(s), rather the present disclosure encompasses any amount of the protein(s) sufficient to achieve the stated result in a subject. In one example, a therapeutically effective amount of the protein does not induce neutropenia.
[0321] As used herein, the term "prophylactically effective amount" shall be taken to mean a sufficient quantity of a protein to prevent or inhibit or delay the onset of one or more detectable symptoms of a clinical condition. The skilled artisan will be aware that such an amount will vary depending on, for example, the specific protein(s) administered and/or the particular subject and/or the type or severity or level of condition and/or predisposition (genetic or otherwise) to the condition. Accordingly, this term is not to be construed to limit the present disclosure to a specific quantity, e.g., weight or amount of protein(s), rather the present disclosure encompasses any amount of the protein(s) sufficient to achieve the stated result in a subject. In one example, a prophylactically effective amount of the protein does not induce neutropenia.
[0322] For in vivo administration of the proteins described herein, normal dosage amounts may vary from about 10 ng/kg up to about 100 mg/kg of an individual's body weight or more per day. Exemplary dosages and ranges thereof are described herein. For repeated administrations over several days or longer, depending on the severity of the disease or disorder to be treated, the treatment can be sustained until a desired suppression of symptoms is achieved.
[0323] In some examples, the protein is administered at an initial (or loading) dose of between about 1 mg/kg to about 30 mg/kg, such as from about 1 mg/kg to about 10 mg/kg, or about 1 mg/kg or about 2 mg/kg or 5 mg/kg. The protein can then be administered at a lower maintenance dose of between about 0.01 mg/kg to about 2 mg/kg, such as from about 0.05 mg/kg to about 1 mg/kg, for example, from about 0.1 mg/kg to about 1 mg/kg, such as about 0.1 mg/kg or 0.5 mg/kg or 1 mg/kg. The maintenance doses may be administered every 7-30 days, such as, every 10-15 days, for example, every 10 or 11 or 12 or 13 or 14 or 15 days.
[0324] In some examples, the protein is administered at a dose of between about 0.01 mg/kg to about 50 mg/kg, such as between about 0.05 mg/kg to about 30 mg/kg, for example, between about 0.1 mg/kg to about 20 mg/kg, for example, between about 0.1 mg/kg to about 10 mg/kg, such as between about 0.1 mg/kg to about 2 mg/kg. For example, the protein is administered at a dose of between about 0.01 mg/kg to about 5 mg/kg, such as from about 0.1 mg/kg to about 2 mg/kg, such as about 0.2 mg/kg or 0.3 mg/kg or 0.5 mg/kg or 1 mg/kg or 1.5 mg/kg (e.g., without a higher loading dose or a lower maintenance dose). In some examples, numerous doses are administered, e.g., every 7-30 days, such as, every 10-22 days, for example, every 10-15 days, for example, every 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 days. For example, the protein is administered every 7 days or every 14 days or every 21 days.
[0325] In some examples, at the time of commencing therapy, the mammal is administered the protein on no more than 7 consecutive days or 6 consecutive days or 5 consecutive days or 4 consecutive days.
[0326] In the case of a mammal that is not adequately responding to treatment, multiple doses in a week may be administered. Alternatively, or in addition, increasing doses may be administered.
[0327] In another example, for mammals experiencing an adverse reaction, the initial (or loading) dose may be split over numerous days in one week or over numerous consecutive days.
[0328] Administration of a protein according to the methods of the present disclosure can be continuous or intermittent, depending, for example, on the recipient's physiological condition, whether the purpose of the administration is therapeutic or prophylactic, and other factors known to skilled practitioners. The administration of a protein may be essentially continuous over a preselected period of time or may be in a series of spaced doses, e.g., either during or after development of a condition.
NON-LIMITING EXAMPLES
Methods
[0329] The Ig and CRH domains of the G-CSFR are involved in ligand binding and receptor dimerization (Layton et al., J. Biol Chem, 272: 29735-29741, 1997 and Fukunaga et al, EMBO J. 10: 2855-2865 1991). Soluble forms of G-CSFR (with either a C-terminal polyhistidine tag or an Fc sequence) comprising these portions of the receptor have been used in various studies of the receptor, and mutation of the free cysteines at positions 78, 163, and 228 of the receptor assists in expression and isolation of the soluble receptor polypeptide (Mine et al., Biochem., 43: 2458-2464, 2004) without affecting ligand binding. In the present studies soluble forms of the receptor comprising amino acids 25-335 of hG-CSFR with mutations C78A, C163S and C228S were generally used (e.g. SEQ ID NO:1) and the corresponding segment of cynoG-CSFR with the cysteine mutations was generally used for studies on the cynomolgus monkey receptor. Various point mutations of the soluble receptor of SEQ ID NO:1 have also been utilized. Reference to hG-CSFR-Fc means the polypeptide of SEQ ID NO:1 wherein the C-terminal polyhistidine tag has been replaced with an Fc sequence. cynoG-CSFR-Fc means the corresponding segment of cynoG-CSFR with an Fc sequence attached to its C-terminal. In some instance the corresponding extracellular domains of the wild type receptor have been used, and in these instances it is specifically noted. The inventors have shown that antibodies and proteins comprising antigen binding sites thereof (e.g., Fab) bind to wild type hG-CSF polypeptides and to these mutant proteins with highly similar affinity. Accordingly, studies using the mutant proteins are a model of studies using wild type hG-CSFR.
Identification of Fabs from Phage Display Library
[0330] A phage display library was screened for clones binding to hG-CSFR which eluted upon addition of G-CSF ligand. Fabs were assessed for their ability to bind hG-CSFR, compete G-CSF binding, and cynomolgus G-CSFR cross-reactivity and some clones reformatted as IgG4 antibodies. Potency was then tested through neutralization of G-CSF mediated proliferation in a BaF3 cell line stably transfected with hG-CSFR (described below) and to inhibit CFU-G formation in the presence of G-CSF.
Mammalian Expression Vector Construction for IgG Expression
[0331] Mammalian expression vectors were constructed using standard molecular biology techniques by cloning the entire light chain (variable and constant domains) and the variable region of the heavy chain from the selected phage-derived Fab constructs.
Cell Culture and Transient Transfection
[0332] Serum-free suspension adapted 293-T cells were obtained from Genechoice Inc. Cells were cultured in FreeStyle.TM. Expression Medium (Invitrogen) supplemented with penicillin/streptomycin/fungizone reagent (Invitrogen). Prior to transfection the cells were maintained at 37.degree. C. in humidified incubators with an atmosphere of 8% CO.sub.2.
[0333] The Transient transfection of the mammalian expression vectors using 293-T cells was performed using 293fectin transfection reagent (Invitrogen) according to the manufacturer's instructions. The cell culture supernatants were harvested after 5 days incubation by centrifugation at 2500 rpm and were then passed through a 0.45 .mu.M filter (Nalgene) prior to purification using standard methods for IgG purification.
Control Antibodies
[0334] Murine monoclonal antibodies 711, 744 and 774 (Layton et al., Growth Factors, 14: 117-130, 1997) were used as control mouse antibodies.
Affinity Measurements of Fabs
[0335] To measure affinity of Fab for G-CSFR or G-CSFR-Fc, Fabs were expressed in E. coli and affinity measured using a Biacore 2000.
Measurement of Binding Kinetics for mAbs
[0336] Anti-human (Goat anti-human IgG (gamma) mouse adsorbed, Invitrogen, Cat No. H10500) or anti mouse Fc specific antibody (Jackson Immuno Research Labs inc. Cat No. 515-005-071) was chemically immobilized on a CM-5 sensor surface using amine coupling chemistry.
[0337] The immobilized antibodies were then used to capture anti hG-CSFR mAbs from solution. Soluble hG-CSFR proteins (as described in the methods section) were then injected over captured mAb at various concentrations. mAbs were captured for 180 seconds at 0.3 .mu.g/ml. Soluble hG-CSFR at 0, 1.25, 2.5, 5, 10, 20 and 40 nM (in duplicate) was injected for 10 minutes and dissociation was monitored for 30 minutes. Responses from a reference flow cell (in which mAb was not captured, but otherwise treated identically), were subtracted. The responses from a blank injection were then subtracted from the resultant sensorgrams.
[0338] The final corrected responses were fitted using non-linear regression to a model describing 1:1 kinetics, including a term for mass transport limitation. The Rmax value was fitted locally, to account for slight deviations in the level of mAb captured. Equilibrium dissociation constant (KD) was determined.
Kinetic Analysis of Fab C1.2, 5D11, 711 and 744
[0339] Fab fragments were generated by papain digestion where 3 mg of antibody was digested (1:500) with pre-activated papain for 40 minutes as per instructions using a papain digestion kit (Sigma, USA). Resultant Fab was purified, by adsorption, away from residual Fc and undigested antibody using protein A purification (mAbSelect, GE, Sweden).
[0340] Duplicate biosensor analysis of the Fab were performed using a Biacore 2000 (GE, Sweden) with a doubling dilution of Fab antibodies (100 nM to 0.39 nM in 0.1 mg/ml BSA) at a flow rate of 30 .mu.l/min. Binding (100 .mu.l) was monitored to respective flow cells containing control immobilised blank, immobilized cyno G-CSFR-Fc, immobilized human G-CSFR-Fc and immobilized human G-CSFR. Receptor proteins (20 .mu.g per ml in 20 mM Sodium Acetate pH 4.5) were previously immobilized to a CM5 chip using a NHS/EDC coupling kit as per the manufacturer's instructions (Biacore GE, Sweden). Target immobilization values were set at 700, 700 and 500 resonance units for cyno G-CSFR-Fc, hG-CSFR-Fc and hG-CSFR, respectively. Chip immobilisation was quenched with 50 mM ethanolamine pH 8.0. Dissociation of surface binding was monitored for 1000 seconds prior to desorption of the remaining complex using 50 mM Phosphoric acid. Reference binding was then subtracted from the control channel and kinetics generated using biaevaluation software on the Biacore 2000.
[0341] Duplicate biosensor analysis of the antibodies c1.2 and 5D11 in IgG4 fomat were performed using a Biacore 2000 with a doubling dilution of the antibodies (312 nM to 2.4 nM in 0.1 mg/ml BSA) at a flow rate of 30 ul/min. Binding (100 .mu.l) was monitored to respective flow cells containing control immobilized blank, immobilized cyno-G-CSFR-Fc, immobilized hG-CSFR-Fc and immobilized hG-CSFR. Receptor proteins (20 ug per ml in 20 mM Sodium Acetate pH 4.5) were previously immobilized to a CM5 chip using a NHS/EDC coupling kit as per the manufacturer's instructions (Biacore GE, Sweden). Target immobilization values were set at 700, 700 and 500 resonance units for cyno-G-CSFR-Fc, hG-CSFR-Fc and hG-CSFR, respectively. Chip immobilization was quenched with 50 mM ethanolamine pH 8.0. Dissociation of surface binding was monitored for 1000 seconds prior to desorption of the remaining complex using 50 mM Phosphoric acid. Reference binding was then subtracted from the control channel and kinetics generated using biaevaluation software on the Biacore 2000.
BIAcore mAb Kinetics of Affinity Matured C1.2G Antibodies
[0342] Anti human (Goat anti Human IgG (gamma) mouse adsorbed, Invitrogen, Cat No. 1110500) was chemically immobilised on a CM-5 sensor surface using amine coupling chemistry and then used to capture the C1.2G affinity matured anti hG-CSFR mAbs at 1 mg/ml for 3 mins. Soluble hG-CSFR was then injected over the captured mAb at 0, 10 and 40 nM. Soluble hG-CSFR was injected for 5 minutes and dissociation was monitored for 30 minutes. Responses from a reference flow cell (in which mAb was not captured, but otherwise treated identically), were subtracted. The responses from a blank injection were then subtracted from the resultant sensorgrams.
[0343] The final corrected responses were fitted using non-linear regression to a model describing 1:1 kinetics, including a term for mass transport limitation. The Rmax value was fitted locally, to account for slight deviations in the level of mAb captured. Association rate (ka), dissociation rate (kd) and equilibrium dissociation constant (K.sub.D) were determined.
hG-CSFR/BaF3 Proliferation. Bioassay--MTT Reduction
[0344] BaF3 cells expressing hG-CSFR were obtained from the Ludwig Institute Melbourne. To assess the inhibition of G-CSF mediated proliferation by anti hG-CSFR antibodies, serial dilutions of antibody were added to 2.times.10.sup.4 cells/well in DME medium with 5% FCS and 0.5 ng/ml hGCSF in 96 well plates and incubated for 48 hours at 37.degree. C., 10% CO.sub.2. Cell proliferation was determined by MTT reduction and measured by absorbance at 490 nM.
hG-CSFR/BaF3 Proliferation Bioassay--3H-Thymidine Incorporation
[0345] BAF/3 cells engineered to express human G-CSFR and which proliferate in response to human G-CSF were used to measure the ability of various monoclonal antibodies to neutralize the activity of G-CSF. Cells were plated at 1.times.10.sup.4 cells in 96 well plates in RPMI/105FCS in the presence of 10 ng/mL human G-CSF and increasing concentrations of of various anti-G-CSFR monoclonal antibodies for 48 hours at 37.degree. C. Cells were pulsed with .sup.3H-thymidine for the last 6 hours of culture before being harvested onto glass fibre filters and the level of radioactive thymidine incorporated into DNA determined by liquid scintillation counting.
Human CFU-G Progenitor Bioassay
[0346] CD34.sup.+ bone marrow cells were incubated in semi-solid medium in the presence of 10 ng/ml stem cell factor, 10 ng/ml hG-CSF and titrating concentrations of test antibody. CFU-G were enumerated after 14 days of culture.
Epitope Comparison--Competition Binding
[0347] This design of this experiment is built on the premise that for 2 antibodies to be capable of simultaneously binding a single molecule, the epitopes of those 2 antibodies must be different.
[0348] Soluble G-CSFR of SEQ ID NO:1 was captured from solution by a surface immobilized antibody. A second antibody was then injected over the complex. Responses from a reference flow cell (in which soluble hG-CSFR was not captured, but otherwise treated identically), were subtracted. Binding of the second antibody indicates that the epitopes of the 2 antibodies differ.
[0349] Responses measured at the end of the antibody binding phase were divided by the response at the end of the hG-CSFR capture phase, to correct antibody binding level for the amount of hG-CSFR captured. These capture corrected responses were then used to compare the binding of each antibody to hG-CSFR in the presence of the other antibodies.
[0350] Antibodies C1.2, 5D11, 711 and 744 were chemically immobilized on a CM-5 sensor surface using amine coupling chemistry. Soluble hG-CSFR was captured at 100 nM for 180 seconds. Each of the antibodies was then injected in duplicate over captured hG-CSFR at 100 nM for 180 seconds. A blank injection of buffer only was also performed.
Epitope Mapping of C1.2G, 711, 744 and 774
[0351] A series of alanine point mutations of SEQ ID NO:1 were generated, expressed in HEK293 cells and then purified. The binding affinity of these mutants for antibodies C1.2, 744 and 774 was measured, and compared to that of SEQ ID NO:1. If a mutation resulted in a change of affinity by more than a factor of 2 from that of SEQ ID NO:1, that residue was deemed to contribute to the binding interaction, and thus is likely to be in or near the epitope. A third mAb (711) with an epitope separate and distinct to C1.2, 744 and 774, was included as a control in order to account for any major structural changes brought about by the mutations.
[0352] Anti human (Goat anti Human IgG (gamma) mouse adsorbed, Invitrogen, Cat No. H10500) or anti mouse Fc specific antibody (Jackson Immuno Research Labs inc. Cat No. 515-005-071) was chemically immobilized on a CM-5 sensor surface using amine coupling chemistry. The immobilized antibodies were then used to capture anti hG-CSFR mAbs from solution. Wild-type hG-CSFR ligand binding domain (SEQ ID NO:1) and each alanine point mutant were then injected over captured mAbs at various concentrations. Responses from a reference flow cell (in which mAb was not captured, but otherwise treated identically), were subtracted. The responses from a blank injection were then subtracted from the resultant sensorgrams,
[0353] The final corrected responses were fitted using non-linear regression to a model describing 1:1 kinetics, including a term for mass transport limitation. The Rmax value was fitted locally, to account for slight deviations in the level of mAb captured. Association rate (ka), dissociation rate (kd) and equilibrium dissociation constant (KD) were determined.
[0354] C1.2 germline mAb was captured at 0.3 ug/ml for 180 sec, 711 at 1 .mu.g/ml for 180 sec, and 744 and 774 at 5 .mu.g/ml for 180 sec.
[0355] For C1.2 and 744 kinetics, WT hG-CSFR and each ala mutant were injected at 0, 2, 10, 50 and 250 nM for 300 sec and dissociation monitored for a further 1800 sec.
[0356] For antibody 774 kinetics, WT hG-CSFR and each ala mutant were injected at 0, 2, 10, 50 and 250 nM for 300 sec and dissociation monitored for a further 600 sec.
[0357] For antibody 711 kinetics, WT hG-CSFR and each ala mutant were injected at 0 and 100 nM for 180 sec and dissociation monitored for a further 180 sec.
Example 1: Fully Human Anti-hG-CSFR Antibodies are Potent Inhibitors G-CSF Signaling
[0358] Using affinity measurements and the BaF3 proliferation assay described above, antibodies 711 and 744 were assessed for affinity to hG-CSFR and G-CSF neutralization assays. Antibody 711 was found to bind to hG-CSFR-Fc fusion (based on SEQ ID NO:1 as discussed in the methods) with an affinity greater than antibody 744 (K.sub.D of 0.86 nM and 8.7 nM, respectively). Using the MTT-based bioassay described above, antibody 711 was also found to more potently inhibit G-CSF-mediated cell proliferation than antibody 744 (IC.sub.50 (nM G-CSF) of 8.8 nM and 2.4 nM, respectively).
[0359] Using the .sup.3H-thymidine incorporation assay, antibody 711 was found to inhibit G-CSF-mediated cell proliferation with an IC.sub.50 of 10.1 .mu.g/ml; antibody 774 was found to inhibit G-CSF-mediated cell proliferation with an IC.sub.50 of 37.4 .mu.g/ml and the IC.sub.50 for antibody 744 was not determinable (see FIG. 1).
[0360] Human antibodies isolated from a phage display library (antibodies C1.2 and 5D11) and mouse monoclonal antibody 711 were assessed for their ability to inhibit G-CSF-mediated proliferation of BaF3 cells using the bioassay described above. Results showed that antibody C1.2 inhibited BaF3 proliferation with an IC.sub.50 of 0.5 nM; antibody 5D11 inhibited proliferation an IC.sub.50 of 5.9 nM; and 711 inhibited proliferation with an IC.sub.50 of 3.4 nM.
[0361] The antibodies were also assessed for their ability to reduce or inhibit CFU-G formation by CD34.sup.+ bone marrow cells in the presence of G-CSF as described above. Results showed that antibody C1.2 inhibited CFU-G formation with an IC.sub.50 of 0.016 nM; antibody 5D11 inhibited CFU-G formation with an IC.sub.50 of 0.039 nM; and 711 inhibited CFU-G formation with an IC.sub.50 of 0.411 nM.
[0362] Based on these assays, the human antibodies C1.2 and 5D11 are more potent than 711 in inhibiting CFU-G formation and C1.2 is the most potent antibody in both bioassays.
Example 2: Affinity of Antibodies for Human G-CSFR and Cynomolgus Monkey G-CSFR
[0363] Affinities of Fabs and IgG4 forms of 5D11 and C1.2 and Fabs of 711 and 744 were also assessed to determine their affinities for the ligand binding domain of hG-CSFR(SEQ ID NO:1), hG-CSFR-Fc and cynomolgus monkey G-CSFR-Fc (based on SEQ ID NO:1 as discussed in the methods). In these assays, the regions of G-CSFR were immobilized and binding of the indicated antibody or fragment to the immobilized polypeptide determined as described in more detail in the general methods (Section entitled "Kinetic analysis of Fab C1.2, 5D11, 711 and 744"). Results are shown in Table 1.
TABLE-US-00001 TABLE 1 Affinities of 5D11 and C1.2 for human and cynomolgus monkey G-CSFR Chip Immobilised with: Antibody/Fab hG-CSFR hG-CSFR-Fc cyno G-CSFR-Fc 5D11 Fab 1.30 nM 1.20 nM 0.42 nM C1.2 Fab 0.37 nM 0.33 nM 0.39 nM 5D11 IgG4 65 pM 61 pM 37 pM C1.2 IgG4 27 pM 77 pM 54 pM
[0364] These data show that 5D11 and C1.2 have high affinities for hG-CSFR and that these affinities are improved when the Fabs are expressed as complete IgG4 antibodies. Moreover, the affinities for hG-CSFR and cynoG-CSFR for 5D11 or C1.2 are similar. C1.2 has higher affinity for hG-CSFR than 5D11.
[0365] The Fab of antibody 711 was shown to have an affinity for hG-CSFR-Fc of 0.86 nM and for cynoG-CSFR of 1.8 .mu.M. The Fab of antibody 744 was shown to have an affinity for hG-CSFR-Fc of 8.7 nM and for cynoG-CSFR of >10 .mu.M. Thus, these Fabs have much poorer affinity for cynoG-CSFR than they do for hG-CSFR.
[0366] Reciprocal assays were also performed (i.e., in which antibodies were immobilized and binding of wild type h-GCSFR or wild type cynoG-CSFR to the immobilized antibody was determined in accordance with the general methods (Section entitled "Measurement of Binding Kinetics for mAbs"). Representative results are shown in Table 2.
TABLE-US-00002 TABLE 2 Representative affinities of antibodies C1.2G, 5D11, 711, 744 and 774 for wild type hG-CSFR or wild type cynoG-CSFR. Binding to wt Binding to wt Captured Antibody hG-CSFR (K.sub.D) cyno G-CSFR (K.sub.D) C1.2G (human IgG4) 1.4 nM 494 pM 5D11 (human IgG4) 6.09 nM 477 pM mAb 711 (mIgG1) 1.69 nM 97 nM mAb744 (mIgG2a) 7.82 nM Negligible binding mAb774 (mIgG1) 23.4 nM Negligible binding
[0367] These data shown that C1.2G and 5D11 bind to wild type cynoG-CSFR with higher affinities than 711, 744 and 774. Moreover, the affinity of C1.2G for wild type hG-CSFR and wild type cynoG-CSFR are within about 3 fold of one another and the affinity of 5D11 for wild type hG-CSFR and wild type cynoG-CSFR are within about 13 fold of one another.
Example 3: Germlining of C1.2
[0368] To minimize potential immunogenicity, the variable region framework of C1.2 was changed to match that of the closest human germline framework. This required a single change in the framework of the heavy chain and five changes in the light chain and resulted in a V.sub.H with a sequence set forth in SEQ ID NO: 4 and a V.sub.L with a sequence set forth in SEQ ID NO: 5. Affinity of the germlined antibody (C1.2G) for G-CSFR was similar to C1.2 (k.sub.a 9.54.times.10.sup.4.+-.5.5.times.10.sup.3; k.sub.d 1.31.times.10.sup.-4.+-.2.6.times.10.sup.-6; K.sub.D 1.37.+-.0.07 (N=8)). Affinity of C1.2G for hG-CSFR expressed on the cell surface of BaF3 cells was shown to be 257 pM.
[0369] Using the .sup.3H-thymidine incorporation assay described above, antibody C1.2G was found to inhibit G-CSF-mediated cell proliferation with an IC.sub.50 of 0.8 .mu.g/ml (FIG. 1).
[0370] Reformatting of this antibody into a stabilized IgG4 produced an antibody comprising a heavy chain with a sequence set forth in SEQ ID NO: 64 and a light chain with a sequence set forth in SEQ ID NO: 65.
[0371] When expressed in CHO cells a lysine variant of the antibody was observed, i.e., in which one or both of the heavy chains lacked a C-terminal lysine residue (i.e., thus comprising a sequence set forth in SEQ ID NO: 64).
Example 4: Epitope Mapping
Competition Binding
[0372] Published data has shown that mAb711 and mAb744 bound to different domains of the hG-CSFR. Competition binding experiments showed that mAb711 but not mAb744 was able to bind to the hG-CSFR subsequent to the binding of C1.2 Ab suggesting that C1.2 binds to a similar region of the receptor as mAb744 but a different region to that of mAb711.
Epitope Excision to Identify Peptides Involved in C1.2 Binding
[0373] Epitope excision followed by mass spectrometry analysis was used to identify four peptides of the hG-CSFR that were involved in the binding of C1.2. In this method, the hG-CSFR protein is first bound to an immobilized C1.2 antibody and then digested with proteolytic enzymes. The bound peptides are then identified by MALDI and Electrospray mass spectrometry. The four peptides identified by this approach mapped to positions 111-115, 170-176, 218-234 and/or 286-300 of hG-CSFR (SEQ ID NO: 1).
Binding of C1.2 and mAb744 to hG-CSFR Region Mutants
[0374] Published data (Tamada et al Proc Natl Acad Sci USA. 103:3135-3140, 2006; Aritomi et al Acta Crystallogr D Biol Crystallogr; 56:751-753 1999) has identified surface residues on the hG-CSFR. A number of these residues located within the four peptides identified by epitope excision experiments were substituted by alanine and the resulting mutant forms of a region of hG-CSFR were expressed and purified. The binding of C1.2 and mAb744 to each of these mutants was assessed and the key residues involved in binding identified. Results are shown in FIG. 2A. Alanine substitution of residues K167 and H168 resulted in a complete loss of binding by mAb744 whilst binding of C1.2 was unaffected. In contrast, alanine substitution of residue R287 resulted in a complete loss of binding by C1.2 with no effect on mAb744 binding. Other residues that significantly reduced the binding of C1.2 were L111, L171, Y172, M198 and H237. MAb744 bound these mutants at a similar affinity to that of the wild type receptor.
[0375] The above assay was repeated with the the same antibodies together with mAb774/ As shown in FIG. 2B, alanine substitution of residues K167, H168 and L169 resulted in a complete loss of binding by mAb774 whilst binding of C1.2 was unaffected. As with mAb 744, alanine substitution of residue L111, Y172, H237 and R287 had little or no effect on mAb774 binding.
Example 5: Affinity Maturation of C1.2G
[0376] Affinity maturation was performed by mutating residues in HCDR3 and/or LCDR3 of C1.2G and screening for Fabs that bound to hG-CSFR. Libraries of mutant antibodies were panned using biotinylated hG-CSFR-Fc recombinant protein, either at a constant concentration over several panning rounds or at reducing concentrations.
[0377] At the completion of panning, a number of phage clones were selected from each enriched library and sequenced. Unique clones were then selected based on sequence and reformatted into fully human IgG4/kappa antibodies for binding analysis to hG-CSFR using Biacore (following the general methods in the Section entitled "Measurement of Binding Kinetics for mAbs") and, in some cases ability to inhibit G-CSF-mediated proliferation of BaF3 cells. The reformatted antibodies with improved affinities as compared to the parental C1.2G mAb are listed in Table 3.
TABLE-US-00003 TABLE 3 Characteristics of affinity matured antibodies LCDR3 HCDR3 IC50 mAb Sequence Sequence.sup.3 K.sub.D (M) (nM) C1.2G-987 IQYPQM.sup.1 LGQSSA 4.46E-11 0.88 C1.2G-95 WEYPLV.sup.1 wt 5.78E-11 0.26 C1.2G-79 QVSWEY.sup.2 wt 6.13E-11 0.31 C1.2G-83 WMYALF.sup.1 wt 6.60E-11 0.21 C1.2G-1003 WHYPLT.sup.1 LGSGST 6.82E-11 0.26 C1.2G-44 YSYPQK.sup.1 wt 7.33E-11 0.39 C1.2G-97 FMYPLY.sup.1 wt 9.06E-11 0.21 C1.2G-986 YAYPQQ.sup.1 LGFFQE 9.11E-11 0.28 C1.2G-56 YMYPIK.sup.1 wt 9.93E-11 0.22 C1.2G-77 EQGWNY.sup.2 wt 1.07E-10 0.23 C1.2G-54 MWMPMG.sup.1 LGMFLE 1.10E-10 0.50 C1.2G-802 HFSMQY.sup.2 wt 1.11E-10 0.26 C1.2G-967 WAYGLS.sup.1 LGMYDL 1.34E-10 0.29 C1.2G-989 FYYPFY.sup.1 LGQYMF 1.44E-10 0.34 C1.2G-63 ANSWGY.sup.2 wt 1.61E-10 0.26 C1.2G-1002 WTYGQT.sup.1 LGMYMN 1.67E-10 0.18 C1.2G-994 LEYPQM.sup.1 LGQFMD 1.70E-10 0.49 C1.2G-969 FQYAQH.sup.1 LGQYQF 1.81E-10 0.41 C1.2G-1000 WMYAHM.sup.1 LGQMMY 1.82E-10 0.34 C1.2G-94 WVYPAW.sup.1 wt 1.89E-10 ND C1.2G-975 WQIKLK.sup.1 LGQSML 2.09E-10 ND C1.2G-75 EESMNY.sup.2 wt 2.11E-10 ND C1.2G-814 SQSMEY.sup.2 wt 2.21E-10 ND C1.2G-973 FKYPMT.sup.1 LGQMVY 2.30E-10 ND C1.2G-977 WVYHLP.sup.1 LGEIRE 2.46E-10 ND C1.2G-984 IEYPAH.sup.1 LGMMQS 2.50E-1O ND C1.2G-61 QQGMWM.sup.2 wt 2.57E-10 ND C1.2G-852 wt LGHSLA 2.92E-10 ND C1.2G-996 MWMPIF.sup.1 LGQYMG 3.36E-10 ND C1.2G-43 IGYPGS.sup.1 LGQFMR 3.36E-10 ND C1.2G-999 WEYAMF.sup.1 LGMFHK 3.56E-10 ND C1.2G-870 WMYHKI.sup.1 wt 3.67E-10 ND C1.2G-877 FRYPFY.sup.1 wt 5.08E-10 ND C1.2G wt wt 6.33E-10 0.32 .sup.1Sequence is preceded by the sequence QQS .sup.2Sequence is followed by the sequence PLT .sup.3Sequence (other than wt) is preceded by the sequence LGE wt--sequence of CDR3 from C1.2G ND--not determined
Example 6: C1.2G Reduces Neutrophil Levels without Inducing Neutropenia
[0378] Cynomolgus monkeys were administered pegylated G-CSF and C1.2G (10 mg/kg) administered 12 hours later. As shown in FIG. 3, C1.2 significantly reduced the level of neutrophils compared to control animals, however did not induce neutropenia.
[0379] Similar experiments dosing cynomolgus monkeys with between 0.1 to 10 mg/kg of C1.2G 2 hours prior to administration with G-CSF also reduced the level of neutrophils compared to control animals, however did not induce neutropenia.
Sequence CWU 1
1
681319PRTartificialamino acids 25-335 of Homo sapiens G-CSFR
(hG-CSFR) with a C-terminal polyhistidine tag 1Glu Cys Gly His Ile
Ser Val Ser Ala Pro Ile Val His Leu Gly Asp 1 5 10 15 Pro Ile Thr
Ala Ser Cys Ile Ile Lys Gln Asn Cys Ser His Leu Asp 20 25 30 Pro
Glu Pro Gln Ile Leu Trp Arg Leu Gly Ala Glu Leu Gln Pro Gly 35 40
45 Gly Arg Gln Gln Arg Leu Ser Asp Gly Thr Gln Glu Ser Ile Ile Thr
50 55 60 Leu Pro His Leu Asn His Thr Gln Ala Phe Leu Ser Cys Ala
Leu Asn 65 70 75 80 Trp Gly Asn Ser Leu Gln Ile Leu Asp Gln Val Glu
Leu Arg Ala Gly 85 90 95 Tyr Pro Pro Ala Ile Pro His Asn Leu Ser
Cys Leu Met Asn Leu Thr 100 105 110 Thr Ser Ser Leu Ile Cys Gln Trp
Glu Pro Gly Pro Glu Thr His Leu 115 120 125 Pro Thr Ser Phe Thr Leu
Lys Ser Phe Lys Ser Arg Gly Asn Cys Gln 130 135 140 Thr Gln Gly Asp
Ser Ile Leu Asp Cys Val Pro Lys Asp Gly Gln Ser 145 150 155 160 His
Cys Ser Ile Pro Arg Lys His Leu Leu Leu Tyr Gln Asn Met Gly 165 170
175 Ile Trp Val Gln Ala Glu Asn Ala Leu Gly Thr Ser Met Ser Pro Gln
180 185 190 Leu Cys Leu Asp Pro Met Asp Val Val Lys Leu Glu Pro Pro
Met Leu 195 200 205 Arg Thr Met Asp Pro Ser Pro Glu Ala Ala Pro Pro
Gln Ala Gly Cys 210 215 220 Leu Gln Leu Ser Trp Glu Pro Trp Gln Pro
Gly Leu His Ile Asn Gln 225 230 235 240 Lys Cys Glu Leu Arg His Lys
Pro Gln Arg Gly Glu Ala Ser Trp Ala 245 250 255 Leu Val Gly Pro Leu
Pro Leu Glu Ala Leu Gln Tyr Glu Leu Cys Gly 260 265 270 Leu Leu Pro
Ala Thr Ala Tyr Thr Leu Gln Ile Arg Cys Ile Arg Trp 275 280 285 Pro
Leu Pro Gly His Trp Ser Asp Trp Ser Pro Ser Leu Glu Leu Arg 290 295
300 Thr Thr Glu Arg Ala Pro Thr His His His His His His His His 305
310 315 2118PRTartificialVH of C1.2 2Glu Val Gln Leu Leu Glu Ser
Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu Ser
Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met Gly
Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45 Ser
Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50 55
60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr
Tyr Cys 85 90 95 Ala Met Leu Gly Glu Leu Gly Trp Phe Asp Pro Trp
Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
3107PRTartificialVL of C1.2 3Asp Ile Gln Met Thr Gln Ser Pro Ser
Ala Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile Thr Cys
Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp Tyr Gln
Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr Tyr Ala
Ser Asn Leu Gln Asn Gly Ile Pro Ser Arg Phe Ser Gly 50 55 60 Ser
Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro 65 70
75 80 Glu Asp Phe Ala Thr Tyr His Cys Gln Gln Ser Tyr Ser Thr Pro
Leu 85 90 95 Thr Phe Gly Gly Gly Thr Asn Val Glu Ile Arg 100 105
4118PRTartificialVH of C1.2G 4Glu Val Gln Leu Leu Glu Ser Gly Gly
Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu Ser Cys Ala
Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met Gly Trp Val
Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45 Ser Ser Ile
Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50 55 60 Lys
Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr 65 70
75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr
Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Trp Phe Asp Pro Trp Gly
Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
5107PRTartificialVL of C1.2G 5Asp Ile Gln Met Thr Gln Ser Pro Ser
Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile Thr Cys
Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp Tyr Gln
Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr Tyr Ala
Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55 60 Ser
Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro 65 70
75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Tyr Ser Thr Pro
Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100 105
65PRTartificialHCDR1 of C1.2 6Leu Tyr Trp Met Gly 1 5
717PRTartificialC1.2 HCDR2 7Ser Ile Ser Ser Ser Gly Gly Val Thr Pro
Tyr Ala Asp Ser Val Lys 1 5 10 15 Gly 89PRTartificialC1.2 HCDR3
8Leu Gly Glu Leu Gly Trp Phe Asp Pro 1 5 911PRTartificialC1.2 LCDR1
9Arg Ala Ser Gln Gly Ile Ser Ser Tyr Leu Asn 1 5 10
106PRTartificialC1.2 LCDR2 10Ala Ser Asn Leu Gln Asn 1 5
119PRTartificialC1.2 LCDR3 11Gln Gln Ser Tyr Ser Thr Pro Leu Thr 1
5 128PRTartificialconsensus sequence of HCDR3 of
C1.2VARIANT(5)..(5)X is an amino acid selected from the group
consisting of tryptophan, glutamine, methionine, serine,
phenylalanine, glutamic acid and histidineVARIANT(6)..(6)X iis an
amino acid selected from the group consisting of phenylalanine,
tyrosine, methionine, serine, glycine and
isoleucineVARIANT(7)..(7)X is an amino acid selected from the group
consisting of aspartic acid, methionine, glutamine, serine,
leucine, valine, arginine and histidineVARIANT(8)..(8)X is an amino
acid selected from the group consisting of proline gltuamic acid,
alanine, leucine, phenylalanine, tyronis, threonine, asparagine,
aspartic acid, serine , glycine, arginine, lysine 12Leu Gly Glu Leu
Xaa Xaa Xaa Xaa 1 5 139PRTartificialconsensus sequence of LCDR3 of
C1.2VARIANT(1)..(1)X is an amino acid selected from the group
consisting of glutamine, glutamic acid, histidine, alanine or
serineVARIANT(2)..(2)X is an amino acid selected from the group
consisting of glutamine, valine, phenylalanine, asparagine and
glutamic acidVARIANT(3)..(3)X ian amino acid selected from the
group consisting of serine or glycineVARIANT(4)..(4)X is an amino
acid selected from the group consisting of tryptophan, methionine,
phenylalanine, tyrosine, isoleucine and leucineVARIANT(5)..(5)X is
an amino acid selected from the group consisting of glutamic acid,
methionine, glutamine, tryptophan, serine, valine, asparagine,
glycine, alanine, arganine, histidine, tyrosine, lysine or
threonineVARIANT(6)..(6)X is an amino acid selected from the group
consisting of tyrosine, methionine, isoleucine or
threonineVARIANT(7)..(7)X is an amino acid selected from the group
consisting of proline, alanine, histidine, glycine and
lysineVARIANT(8)..(8)X is an amino acid selected from the group
consisting of leucine, glutamine, methionine, alanine,
phenylalanine, isoleucine, lysine, histidine and
glycineVARIANT(9)..(9)X is an amino acid selected from the group
consisting of threonine, phenylalanine, tyrosine, methionine,
lysine, serine, histidine, proline, tryptophan, isoleucine,
glutamine, glycine and valine 13Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 14107PRTartificialVL of antibody 987 14Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Ile Gln
Tyr Pro Gln 85 90 95 Met Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 15118PRTartificialVH of antibody 987 15Glu Val Gln Leu Leu
Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg
Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp
Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val
50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr
Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Ser Ser
Ala Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
16107PRTartificialVL of antibody 95 16Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Glu Tyr
Pro Leu 85 90 95 Val Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 17107PRTartificialVL of antibody 79 17Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Val Ser Trp Glu
Tyr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 18107PRTartificialVL of antibody 83 18Asp Ile Gln Met Thr
Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val
Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu
Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40
45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly
50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu
Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp
Met Tyr Ala Leu 85 90 95 Phe Phe Gly Gly Gly Thr Lys Val Glu Ile
Lys 100 105 19107PRTartificialVL of antibody 1003 19Asp Ile Gln Met
Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg
Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30
Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35
40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser
Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser
Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser
Trp His Tyr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu
Ile Lys 100 105 20118PRTartificialVH of antibody 1003 20Glu Val Gln
Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser
Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25
30 Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp
Ser Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys
Asn Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Ser
Gly Ser Thr Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser
115 21107PRTartificialVL of antibody 44 21Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Tyr Ser
Tyr Pro Gln 85 90 95 Lys Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 22107PRTartificialVL of antibody 97 22Asp Ile Gln Met Thr
Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val
Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu
Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40
45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly
50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu
Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Phe
Met Tyr Pro Leu 85 90 95 Tyr Phe Gly Gly Gly Thr Lys Val Glu Ile
Lys 100 105 23107PRTartificialVL of antibody 986 23Asp Ile Gln Met
Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg
Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30
Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35
40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser
Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser
Leu Gln Pro 65
70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Tyr Ala Tyr
Pro Gln 85 90 95 Gln Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 24118PRTartificialVH of antibody 986 24Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Phe Phe Gln Glu
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
25107PRTartificialVL of antibody 56 25Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Tyr Met Tyr
Pro Ile 85 90 95 Lys Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 26107PRTartificialVL of antibody 77 26Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Glu Gln Gly Trp Asn
Tyr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 27107PRTartificialVL of antibody 54 27Asp Ile Gln Met Thr
Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val
Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu
Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40
45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly
50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu
Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Met
Trp Met Pro Met 85 90 95 Gly Phe Gly Gly Gly Thr Lys Val Glu Ile
Lys 100 105 28118PRTartificialVH of antibody 54 28Glu Val Gln Leu
Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30
Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35
40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser
Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn
Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Met Phe
Leu Glu Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
29107PRTartificialVL of antibody 802 29Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys His Phe Ser Met Gln Tyr
Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 30107PRTartificialVL of antibody 967 30Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Ala
Tyr Gly Leu 85 90 95 Ser Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 31118PRTartificialVH of antibody 967 31Glu Val Gln Leu Leu
Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg
Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp
Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val
50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr
Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Met Tyr Asp
Leu Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
32107PRTartificialVL of antibody 989 32Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Phe Tyr Tyr
Pro Phe 85 90 95 Tyr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 33118PRTartificialVH of antibody 989 33Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Tyr Met Phe
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
34107PRTartificialVL of antibody 63 34Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Ala Asn Ser Trp Gly Tyr
Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 35107PRTartificialVL of antibody 1002 35Asp Ile Gln Met Thr Gln
Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr
Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn
Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45
Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50
55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln
Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Thr
Tyr Gly Gln 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 36118PRTartificialVH of antibody 1002 36Glu Val Gln Leu Leu
Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg
Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp
Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val
50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr
Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Met Tyr Met
Asn Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
37107PRTartificialVL of antibody 994 37Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Leu Glu Tyr
Pro Gln 85 90 95 Met Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 38118PRTartificialVH of antibody 994 38Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Phe Met Asp
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
39107PRTartificialVL of antibody 969 39Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Phe Gln Tyr
Ala Gln 85 90 95 His Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 40118PRTartificialVH of antibody 969 40Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Tyr Gln Phe
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
41107PRTartificialVL of antibody 1000 41Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Met Tyr
Ala His 85 90 95 Met Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 42118PRTartificialVH of antibody 1000 42Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Met Met Tyr
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
43107PRTartificialVL of antibody 94 43Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu
Ile 35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile
Ser Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln
Gln Ser Trp Val Tyr Pro Ala 85 90 95 Trp Phe Gly Gly Gly Thr Lys
Val Glu Ile Lys 100 105 44107PRTartificialVL of antibody 975 44Asp
Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10
15 Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr
20 25 30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu
Leu Ile 35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser
Arg Phe Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr
Ile Ser Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys
Gln Gln Ser Trp Gln Ile Lys Leu 85 90 95 Lys Phe Gly Gly Gly Thr
Lys Val Glu Ile Lys 100 105 45118PRTartificialVH of antibody 975
45Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1
5 10 15 Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu
Tyr 20 25 30 Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro
Tyr Ala Asp Ser Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp
Asn Ser Lys Asn Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg
Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu
Leu Gly Gln Ser Met Leu Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr
Val Ser Ser 115 46107PRTartificialVL of antibody 75 46Asp Ile Gln
Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp
Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25
30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile
35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe
Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Glu Glu
Ser Met Asn Tyr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val
Glu Ile Lys 100 105 47107PRTartificialVL of antibody 814 47Asp Ile
Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15
Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20
25 30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu
Ile 35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile
Ser Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Ser
Gln Ser Met Glu Tyr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys
Val Glu Ile Lys 100 105 48107PRTartificialVL of antibody 973 48Asp
Ile Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10
15 Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr
20 25 30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu
Leu Ile 35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser
Arg Phe Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr
Ile Ser Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys
Gln Gln Ser Phe Lys Tyr Pro Met 85 90 95 Thr Phe Gly Gly Gly Thr
Lys Val Glu Ile Lys 100 105 49118PRTartificialVH of antibody 973
49Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1
5 10 15 Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu
Tyr 20 25 30 Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu
Glu Trp Val 35 40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro
Tyr Ala Asp Ser Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp
Asn Ser Lys Asn Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg
Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu
Leu Gly Gln Met Val Tyr Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr
Val Ser Ser 115 50107PRTartificialVL of antibody 977 50Asp Ile Gln
Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp
Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25
30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile
35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe
Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln
Ser Trp Val Tyr His Leu 85 90 95 Pro Phe Gly Gly Gly Thr Lys Val
Glu Ile Lys 100 105 51118PRTartificialVH of antibody 977 51Glu Val
Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20
25 30 Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp
Val 35 40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala
Asp Ser Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser
Lys Asn Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu
Asp Thr Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly
Glu Ile Arg Glu Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser
Ser 115 52107PRTartificialVL of antibody 984 52Asp Ile Gln Met Thr
Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val
Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu
Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40
45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly
50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu
Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Ile
Glu Tyr Pro Ala 85 90 95 His Phe Gly Gly Gly Thr Lys Val Glu Ile
Lys 100 105 53118PRTartificialVH of antibody 984 53Glu Val Gln Leu
Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu
Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30
Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35
40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser
Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn
Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr
Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Met Met
Gln Ser Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
54108PRTartificialVL of antibody 61 54Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Gly Met Trp Met
Thr Pro 85 90 95 Leu Thr Phe Gly Gly Gly Thr Lys Val Glu Ile Lys
100 105 55118PRTartificialVH of antibody 852 55Glu Val Gln Leu Leu
Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg
Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp
Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40
45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val
50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr
Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala
Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly His Ser Leu
Ala Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
56107PRTartificialVL of antibody 996 56Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Met Trp Met
Pro Ile 85 90 95 Phe Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 57118PRTartificialVH of antibody 996 57Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Tyr Met Gly
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
58107PRTartificialVL of antibody 43 58Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Ile Gly Tyr
Pro Gly 85 90 95 Ser Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 59118PRTartificialVH of antibody 43 59Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Gln Phe Met Arg
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
60107PRTartificialVL of antibody 999 60Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Glu Tyr
Ala Met 85 90 95 Phe Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 61118PRTartificialVH of antibody 999 61Glu Val Gln Leu Leu Glu
Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu
Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met
Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45
Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50
55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu
Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val
Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Met Phe His Lys
Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser 115
62107PRTartificialVL of antibody 870 62Asp Ile Gln Met Thr Gln Ser
Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg Val Thr Ile
Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30 Leu Asn Trp
Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35 40 45 Tyr
Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser Gly 50 55
60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser Trp Met Tyr
His Lys 85 90 95 Ile Phe Gly Gly Gly Thr Lys Val Glu Ile Lys 100
105 63107PRTartificialVL of antibody 877 63Asp Ile
Gln Met Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15
Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20
25 30 Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu
Ile 35 40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg
Phe Ser Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile
Ser Ser Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln
Gln Ser Phe Arg Tyr Pro Phe 85 90 95 Tyr Phe Gly Gly Gly Thr Lys
Val Glu Ile Lys 100 105 64445PRTartificialC1.2G heavy chain IgG4
with S241P mutation 64Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu
Val Gln Pro Gly Gly 1 5 10 15 Ser Leu Arg Leu Ser Cys Ala Ala Ser
Gly Phe Thr Phe Ser Leu Tyr 20 25 30 Trp Met Gly Trp Val Arg Gln
Ala Pro Gly Lys Gly Leu Glu Trp Val 35 40 45 Ser Ser Ile Ser Ser
Ser Gly Gly Val Thr Pro Tyr Ala Asp Ser Val 50 55 60 Lys Gly Arg
Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr 65 70 75 80 Leu
Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys 85 90
95 Ala Lys Leu Gly Glu Leu Gly Trp Phe Asp Pro Trp Gly Gln Gly Thr
100 105 110 Leu Val Thr Val Ser Ser Ala Ser Thr Lys Gly Pro Ser Val
Phe Pro 115 120 125 Leu Ala Pro Cys Ser Arg Ser Thr Ser Glu Ser Thr
Ala Ala Leu Gly 130 135 140 Cys Leu Val Lys Asp Tyr Phe Pro Glu Pro
Val Thr Val Ser Trp Asn 145 150 155 160 Ser Gly Ala Leu Thr Ser Gly
Val His Thr Phe Pro Ala Val Leu Gln 165 170 175 Ser Ser Gly Leu Tyr
Ser Leu Ser Ser Val Val Thr Val Pro Ser Ser 180 185 190 Ser Leu Gly
Thr Lys Thr Tyr Thr Cys Asn Val Asp His Lys Pro Ser 195 200 205 Asn
Thr Lys Val Asp Lys Arg Val Glu Ser Lys Tyr Gly Pro Pro Cys 210 215
220 Pro Pro Cys Pro Ala Pro Glu Phe Leu Gly Gly Pro Ser Val Phe Leu
225 230 235 240 Phe Pro Pro Lys Pro Lys Asp Thr Leu Met Ile Ser Arg
Thr Pro Glu 245 250 255 Val Thr Cys Val Val Val Asp Val Ser Gln Glu
Asp Pro Glu Val Gln 260 265 270 Phe Asn Trp Tyr Val Asp Gly Val Glu
Val His Asn Ala Lys Thr Lys 275 280 285 Pro Arg Glu Glu Gln Phe Asn
Ser Thr Tyr Arg Val Val Ser Val Leu 290 295 300 Thr Val Leu His Gln
Asp Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys 305 310 315 320 Val Ser
Asn Lys Gly Leu Pro Ser Ser Ile Glu Lys Thr Ile Ser Lys 325 330 335
Ala Lys Gly Gln Pro Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser 340
345 350 Gln Glu Glu Met Thr Lys Asn Gln Val Ser Leu Thr Cys Leu Val
Lys 355 360 365 Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu Ser
Asn Gly Gln 370 375 380 Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro Val
Leu Asp Ser Asp Gly 385 390 395 400 Ser Phe Phe Leu Tyr Ser Arg Leu
Thr Val Asp Lys Ser Arg Trp Gln 405 410 415 Glu Gly Asn Val Phe Ser
Cys Ser Val Met His Glu Ala Leu His Asn 420 425 430 His Tyr Thr Gln
Lys Ser Leu Ser Leu Ser Leu Gly Lys 435 440 445
65214PRTartificialC1.2G with kappa light chain 65Asp Ile Gln Met
Thr Gln Ser Pro Ser Ser Leu Ser Ala Ser Val Gly 1 5 10 15 Asp Arg
Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Ser Tyr 20 25 30
Leu Asn Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile 35
40 45 Tyr Tyr Ala Ser Asn Leu Gln Asn Gly Val Pro Ser Arg Phe Ser
Gly 50 55 60 Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser
Leu Gln Pro 65 70 75 80 Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ser
Tyr Ser Thr Pro Leu 85 90 95 Thr Phe Gly Gly Gly Thr Lys Val Glu
Ile Lys Arg Thr Val Ala Ala 100 105 110 Pro Ser Val Phe Ile Phe Pro
Pro Ser Asp Glu Gln Leu Lys Ser Gly 115 120 125 Thr Ala Ser Val Val
Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala 130 135 140 Lys Val Gln
Trp Lys Val Asp Asn Ala Leu Gln Ser Gly Asn Ser Gln 145 150 155 160
Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser 165
170 175 Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val
Tyr 180 185 190 Ala Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val
Thr Lys Ser 195 200 205 Phe Asn Arg Gly Glu Cys 210 66836PRTHomo
sapiens 66Met Ala Arg Leu Gly Asn Cys Ser Leu Thr Trp Ala Ala Leu
Ile Ile 1 5 10 15 Leu Leu Leu Pro Gly Ser Leu Glu Glu Cys Gly His
Ile Ser Val Ser 20 25 30 Ala Pro Ile Val His Leu Gly Asp Pro Ile
Thr Ala Ser Cys Ile Ile 35 40 45 Lys Gln Asn Cys Ser His Leu Asp
Pro Glu Pro Gln Ile Leu Trp Arg 50 55 60 Leu Gly Ala Glu Leu Gln
Pro Gly Gly Arg Gln Gln Arg Leu Ser Asp 65 70 75 80 Gly Thr Gln Glu
Ser Ile Ile Thr Leu Pro His Leu Asn His Thr Gln 85 90 95 Ala Phe
Leu Ser Cys Cys Leu Asn Trp Gly Asn Ser Leu Gln Ile Leu 100 105 110
Asp Gln Val Glu Leu Arg Ala Gly Tyr Pro Pro Ala Ile Pro His Asn 115
120 125 Leu Ser Cys Leu Met Asn Leu Thr Thr Ser Ser Leu Ile Cys Gln
Trp 130 135 140 Glu Pro Gly Pro Glu Thr His Leu Pro Thr Ser Phe Thr
Leu Lys Ser 145 150 155 160 Phe Lys Ser Arg Gly Asn Cys Gln Thr Gln
Gly Asp Ser Ile Leu Asp 165 170 175 Cys Val Pro Lys Asp Gly Gln Ser
His Cys Cys Ile Pro Arg Lys His 180 185 190 Leu Leu Leu Tyr Gln Asn
Met Gly Ile Trp Val Gln Ala Glu Asn Ala 195 200 205 Leu Gly Thr Ser
Met Ser Pro Gln Leu Cys Leu Asp Pro Met Asp Val 210 215 220 Val Lys
Leu Glu Pro Pro Met Leu Arg Thr Met Asp Pro Ser Pro Glu 225 230 235
240 Ala Ala Pro Pro Gln Ala Gly Cys Leu Gln Leu Cys Trp Glu Pro Trp
245 250 255 Gln Pro Gly Leu His Ile Asn Gln Lys Cys Glu Leu Arg His
Lys Pro 260 265 270 Gln Arg Gly Glu Ala Ser Trp Ala Leu Val Gly Pro
Leu Pro Leu Glu 275 280 285 Ala Leu Gln Tyr Glu Leu Cys Gly Leu Leu
Pro Ala Thr Ala Tyr Thr 290 295 300 Leu Gln Ile Arg Cys Ile Arg Trp
Pro Leu Pro Gly His Trp Ser Asp 305 310 315 320 Trp Ser Pro Ser Leu
Glu Leu Arg Thr Thr Glu Arg Ala Pro Thr Val 325 330 335 Arg Leu Asp
Thr Trp Trp Arg Gln Arg Gln Leu Asp Pro Arg Thr Val 340 345 350 Gln
Leu Phe Trp Lys Pro Val Pro Leu Glu Glu Asp Ser Gly Arg Ile 355 360
365 Gln Gly Tyr Val Val Ser Trp Arg Pro Ser Gly Gln Ala Gly Ala Ile
370 375 380 Leu Pro Leu Cys Asn Thr Thr Glu Leu Ser Cys Thr Phe His
Leu Pro 385 390 395 400 Ser Glu Ala Gln Glu Val Ala Leu Val Ala Tyr
Asn Ser Ala Gly Thr 405 410 415 Ser Arg Pro Thr Pro Val Val Phe Ser
Glu Ser Arg Gly Pro Ala Leu 420 425 430 Thr Arg Leu His Ala Met Ala
Arg Asp Pro His Ser Leu Trp Val Gly 435 440 445 Trp Glu Pro Pro Asn
Pro Trp Pro Gln Gly Tyr Val Ile Glu Trp Gly 450 455 460 Leu Gly Pro
Pro Ser Ala Ser Asn Ser Asn Lys Thr Trp Arg Met Glu 465 470 475 480
Gln Asn Gly Arg Ala Thr Gly Phe Leu Leu Lys Glu Asn Ile Arg Pro 485
490 495 Phe Gln Leu Tyr Glu Ile Ile Val Thr Pro Leu Tyr Gln Asp Thr
Met 500 505 510 Gly Pro Ser Gln His Val Tyr Ala Tyr Ser Gln Glu Met
Ala Pro Ser 515 520 525 His Ala Pro Glu Leu His Leu Lys His Ile Gly
Lys Thr Trp Ala Gln 530 535 540 Leu Glu Trp Val Pro Glu Pro Pro Glu
Leu Gly Lys Ser Pro Leu Thr 545 550 555 560 His Tyr Thr Ile Phe Trp
Thr Asn Ala Gln Asn Gln Ser Phe Ser Ala 565 570 575 Ile Leu Asn Ala
Ser Ser Arg Gly Phe Val Leu His Gly Leu Glu Pro 580 585 590 Ala Ser
Leu Tyr His Ile His Leu Met Ala Ala Ser Gln Ala Gly Ala 595 600 605
Thr Asn Ser Thr Val Leu Thr Leu Met Thr Leu Thr Pro Glu Gly Ser 610
615 620 Glu Leu His Ile Ile Leu Gly Leu Phe Gly Leu Leu Leu Leu Leu
Thr 625 630 635 640 Cys Leu Cys Gly Thr Ala Trp Leu Cys Cys Ser Pro
Asn Arg Lys Asn 645 650 655 Pro Leu Trp Pro Ser Val Pro Asp Pro Ala
His Ser Ser Leu Gly Ser 660 665 670 Trp Val Pro Thr Ile Met Glu Glu
Asp Ala Phe Gln Leu Pro Gly Leu 675 680 685 Gly Thr Pro Pro Ile Thr
Lys Leu Thr Val Leu Glu Glu Asp Glu Lys 690 695 700 Lys Pro Val Pro
Trp Glu Ser His Asn Ser Ser Glu Thr Cys Gly Leu 705 710 715 720 Pro
Thr Leu Val Gln Thr Tyr Val Leu Gln Gly Asp Pro Arg Ala Val 725 730
735 Ser Thr Gln Pro Gln Ser Gln Ser Gly Thr Ser Asp Gln Val Leu Tyr
740 745 750 Gly Gln Leu Leu Gly Ser Pro Thr Ser Pro Gly Pro Gly His
Tyr Leu 755 760 765 Arg Cys Asp Ser Thr Gln Pro Leu Leu Ala Gly Leu
Thr Pro Ser Pro 770 775 780 Lys Ser Tyr Glu Asn Leu Trp Phe Gln Ala
Ser Pro Leu Gly Thr Leu 785 790 795 800 Val Thr Pro Ala Pro Ser Gln
Glu Asp Asp Cys Val Phe Gly Pro Leu 805 810 815 Leu Asn Phe Pro Leu
Leu Gln Gly Ile Arg Val His Gly Met Glu Ala 820 825 830 Leu Gly Ser
Phe 835 67319PRTartificialIg and CRH domains of Macaca fascicularis
G-CSFR (cynoG-CSFR) with a C-terminal polyhistidine tag 67Glu Cys
Gly His Ile Ser Val Ser Ala Pro Ile Val His Leu Gly Asp 1 5 10 15
Pro Ile Thr Ala Ser Cys Ile Ile Lys Gln Asn Cys Ser His Leu Asp 20
25 30 Leu Glu Pro Gln Ile Leu Trp Arg Leu Gly Ala Glu Leu Gln Pro
Gly 35 40 45 Gly Arg Gln Gln Arg Leu Ser Asp Gly Ser Gln Gln Ser
Thr Ile Thr 50 55 60 Leu Pro His Leu Asn His Thr Arg Ala Phe Leu
Ser Cys Ala Leu Asn 65 70 75 80 Trp Gly Asn Ser Leu Gln Ile Leu Asp
Gln Val Glu Leu Arg Ala Gly 85 90 95 Tyr Pro Pro Ala Val Pro Arg
Asn Leu Ser Cys Leu Met Asn Leu Thr 100 105 110 Thr Ser Ser Leu Ile
Cys Gln Trp Glu Pro Gly Pro Glu Thr His Leu 115 120 125 Pro Thr Ser
Phe Thr Leu Lys Ser Phe Lys Ser Arg Gly Asn Cys Gln 130 135 140 Thr
Gln Gly Asp Ser Ile Met Asp Cys Val Pro Glu Asp Gly Gln Ser 145 150
155 160 His Cys Ser Ile Pro Arg Arg His Leu Leu Leu Tyr Gln Asn Met
Gly 165 170 175 Ile Trp Val Gln Ala Glu Asn Ala Leu Gly Thr Ser Met
Ser Pro Gln 180 185 190 Leu Cys Leu Glu Pro Met Asp Val Val Lys Leu
Glu Pro Pro Met Leu 195 200 205 Arg Thr Met Asp Pro Ser Pro Glu Ala
Ala Pro Pro Gln Ala Gly Cys 210 215 220 Leu Gln Leu Ser Trp Glu Pro
Trp Gln Pro Ala Leu His Ile Asn Gln 225 230 235 240 Lys Cys Glu Leu
Arg His Lys Pro Gln Ser Gly Glu Ala Ser Trp Ala 245 250 255 Leu Val
Gly Pro Leu Pro Leu Glu Ala Leu Arg Tyr Glu Leu Cys Gly 260 265 270
Leu Leu Pro Ala Thr Ala Tyr Thr Leu Gln Ile Arg Cys Ile Arg Trp 275
280 285 Pro Leu Pro Gly His Trp Ser Asn Trp Ser Pro Ser Leu Glu Leu
Arg 290 295 300 Thr Thr Glu Arg Ala Pro Thr His His His His His His
His His 305 310 315 68444PRTartificialC1.2G heavy chain IgG4 with
S241P mutation and lacking C-terminal lysine residue 68Glu Val Gln
Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly 1 5 10 15 Ser
Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Leu Tyr 20 25
30 Trp Met Gly Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45 Ser Ser Ile Ser Ser Ser Gly Gly Val Thr Pro Tyr Ala Asp
Ser Val 50 55 60 Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys
Asn Thr Leu Tyr 65 70 75 80 Leu Gln Met Asn Ser Leu Arg Ala Glu Asp
Thr Ala Val Tyr Tyr Cys 85 90 95 Ala Lys Leu Gly Glu Leu Gly Trp
Phe Asp Pro Trp Gly Gln Gly Thr 100 105 110 Leu Val Thr Val Ser Ser
Ala Ser Thr Lys Gly Pro Ser Val Phe Pro 115 120 125 Leu Ala Pro Cys
Ser Arg Ser Thr Ser Glu Ser Thr Ala Ala Leu Gly 130 135 140 Cys Leu
Val Lys Asp Tyr Phe Pro Glu Pro Val Thr Val Ser Trp Asn 145 150 155
160 Ser Gly Ala Leu Thr Ser Gly Val His Thr Phe Pro Ala Val Leu Gln
165 170 175 Ser Ser Gly Leu Tyr Ser Leu Ser Ser Val Val Thr Val Pro
Ser Ser 180 185 190 Ser Leu Gly Thr Lys Thr Tyr Thr Cys Asn Val Asp
His Lys Pro Ser 195 200 205 Asn Thr Lys Val Asp Lys Arg Val Glu Ser
Lys Tyr Gly Pro Pro Cys 210 215 220 Pro Pro Cys Pro Ala Pro Glu Phe
Leu Gly Gly Pro Ser Val Phe Leu 225 230 235 240 Phe Pro Pro Lys Pro
Lys Asp Thr Leu Met Ile Ser Arg Thr Pro Glu 245 250 255 Val Thr Cys
Val Val Val Asp Val Ser Gln Glu Asp Pro Glu Val Gln 260 265 270 Phe
Asn Trp Tyr Val Asp Gly Val Glu Val His Asn Ala Lys Thr Lys 275 280
285 Pro Arg Glu Glu Gln Phe Asn Ser Thr Tyr Arg Val Val Ser Val Leu
290 295 300 Thr Val Leu His Gln Asp Trp Leu Asn Gly Lys Glu Tyr Lys
Cys Lys 305 310 315 320 Val Ser Asn Lys Gly Leu Pro Ser Ser Ile Glu
Lys Thr Ile Ser Lys 325 330
335 Ala Lys Gly Gln Pro Arg Glu Pro Gln Val Tyr Thr Leu Pro Pro Ser
340 345 350 Gln Glu Glu Met Thr Lys Asn Gln Val Ser Leu Thr Cys Leu
Val Lys 355 360 365 Gly Phe Tyr Pro Ser Asp Ile Ala Val Glu Trp Glu
Ser Asn Gly Gln 370 375 380 Pro Glu Asn Asn Tyr Lys Thr Thr Pro Pro
Val Leu Asp Ser Asp Gly 385 390 395 400 Ser Phe Phe Leu Tyr Ser Arg
Leu Thr Val Asp Lys Ser Arg Trp Gln 405 410 415 Glu Gly Asn Val Phe
Ser Cys Ser Val Met His Glu Ala Leu His Asn 420 425 430 His Tyr Thr
Gln Lys Ser Leu Ser Leu Ser Leu Gly 435 440
D00001

D00002

D00003

D00004

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.