Adeno-Associated Virus Virions for Treatment of Epilepsy
Muramatsu; Shin-ichi ; et al.
U.S. patent application number 16/069370 was filed with the patent office on 2019-01-24 for adeno-associated virus virions for treatment of epilepsy. This patent application is currently assigned to Jichi Medical University. The applicant listed for this patent is Gene Therapy Research Institution Co., Ltd., Jichi Medical University. Invention is credited to Shin-ichi Muramatsu, Keiji Oguro, Kuniko Shimazaki.
| Application Number | 20190022251 16/069370 |
| Document ID | / |
| Family ID | 59311265 |
| Filed Date | 2019-01-24 |





| United States Patent Application | 20190022251 |
| Kind Code | A1 |
| Muramatsu; Shin-ichi ; et al. | January 24, 2019 |
Adeno-Associated Virus Virions for Treatment of Epilepsy
Abstract
Provided is a novel gene therapy means for neurological diseases including epilepsy. The present invention provides: a recombinant adeno-associated virus vector for use in the treatment of neurological diseases including epilepsy, which comprises a polynucleotide encoding a protein capable of improving the excitation-inhibiting function of an inhibitory synapse in vivo, preferably neuroligin-2 protein; a pharmaceutical composition comprising said recombinant vector; and others. The present invention also provides a method for treating a disease such as epilepsy using the recombinant vector.
| Inventors: | Muramatsu; Shin-ichi; (Tochigi, JP) ; Oguro; Keiji; (Tochigi, JP) ; Shimazaki; Kuniko; (Tochigi, JP) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | Jichi Medical University Tochigi JP Gene Therapy Research Institution Co., Ltd. Kawasaki JP |
||||||||||
| Family ID: | 59311265 | ||||||||||
| Appl. No.: | 16/069370 | ||||||||||
| Filed: | January 13, 2017 | ||||||||||
| PCT Filed: | January 13, 2017 | ||||||||||
| PCT NO: | PCT/JP2017/001048 | ||||||||||
| 371 Date: | July 11, 2018 |
| Current U.S. Class: | 1/1 |
| Current CPC Class: | A61P 25/28 20180101; C12N 2750/14122 20130101; A61K 48/0075 20130101; C12N 2750/14143 20130101; C07K 14/705 20130101; A61K 48/0058 20130101; A61K 45/06 20130101; A61P 25/08 20180101; A61K 38/177 20130101; A61K 35/761 20130101; A61K 48/005 20130101 |
| International Class: | A61K 48/00 20060101 A61K048/00; A61K 35/761 20060101 A61K035/761; A61K 45/06 20060101 A61K045/06 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Jan 15, 2016 | JP | 2016-006191 |
Claims
1. A recombinant adeno-associated virus vector, wherein the virus vector comprises a polynucleotide encoding a protein for improving an excitation-inhibiting function of inhibitory synapses in a living subject, and is used for treatment of a disease selected from the group consisting of epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, and developmental disorders (attention deficit hyperactivity disorder and Asperger's syndrome), wherein the polynucleotide comprises a nucleotide sequence encoding a neuroligin 2 protein which comprises the amino acid sequence of SEQ ID NO: 2, 4 or 6, or an amino acid sequence having about 90% or more identity with said amino acid sequence and capable of binding to neurexin, and wherein the recombinant adeno-associated virus vector comprises: a protein having a variant amino acid sequence in which tyrosine at position 445 in the amino acid sequence of a wild-type AAV1 capsid protein is substituted with phenylalanine; a protein having a variant amino acid sequence in which tyrosine at position 445 in the amino acid sequence of a wild-type AAV2 capsid protein is substituted with phenylalanine; or a protein having a variant amino acid sequence in which tyrosine at position 446 in the amino acid sequence of a wild-type AAV9 capsid protein is substituted with phenylalanine.
2. (canceled)
3. The recombinant adeno-associated virus vector according to claim 1, wherein the disease is epilepsy.
4. (canceled)
5. The recombinant adeno-associated virus vector according to claim 1, wherein the polynucleotide comprises a promoter sequence selected from the group consisting of a synapsin I promoter sequence, a myelin basic protein promoter sequence, a neuron specific enolase promoter sequence, a calcium/calmodulin-dependent protein kinase II (CMKII) promoter sequence, a tubulin .alpha.1 promoter sequence, a platelet-derived growth factor .beta. chain promoter sequence, a glial fibrillary acidic protein (GFAP) promoter sequence, a L7 promoter (cerebellar Purkinje cell specific promoter) sequence, a glial fibrillary acidic protein (hGfa2) promoter sequence, and a glutamate receptor delta 2 promoter (cerebellar Purkinje cell specific promoter) sequence, and a glutamic acid decarboxylase (GAD65/GAD67) promoter sequence.
6. The recombinant adeno-associated virus vector according to claim 1, wherein the polynucleotide comprises an inverted terminal repeat (ITR) selected from the group consisting of AAV1, AAV2, AAV3, AAV4, AAV8, and AAV9.
7. The recombinant adeno-associated virus vector according to claim 1, wherein the polynucleotide further comprises a polynucleotide for inhibiting the excitation of excitatory synapses.
8. A pharmaceutical composition, comprising the recombinant adeno-associated virus recombinant vector according to claim 1.
9. The pharmaceutical composition according to claim 8, which is administered intracerebrally.
10. The pharmaceutical composition according to claim 8, which is administered intrathecally.
11. The pharmaceutical composition according to claim 8, which is administered peripherally.
12. The pharmaceutical composition according to claim 8, which is used in combination with a chemotherapeutic agent for a neuropsychiatric disease.
Description
TECHNICAL FIELD
[0001] The present invention relates to genetically recombinant adeno-associated virus (rAAV) virions for neural diseases. More specifically, the present invention relates to rAAV for treatment of neuropsychiatric diseases such as epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, and developmental disorders (attention deficit hyperactivity disorder and Asperger's syndrome).
BACKGROUND ART
[0002] Epilepsy is classified into partial convulsions that are seizures generated partially in body parts as a result of occurrence of the abnormal excitation of neurons in the brain at relatively limited sites, and generalized seizures (e.g., tonic-clonic seizures) that are generalized convulsions generated because the abnormally excited neurons in the brain affect the cortex entirely. A generalized seizure causes the loss of consciousness, and is a disease that causes no convulsion, but may cause impaired consciousness alone or abnormal psychiatric symptoms (e.g, psychomotor seizures). Current general therapeutic approaches are mainly pharmacotherapies using antiepileptic drugs (e.g., phenytoin, carbamazepine, and valproic acid). Surgical treatment is also performed for intractable epilepsy cases.
[0003] About 30% of epilepsy cases is accounted for by intractable diseases, the seizures of which are not suppressed by drug treatment. In the case of inner temporal lobe epilepsy, surgical treatment such as temporal lobectomy may be effective. However, the excision of bilateral hippocampi causes a decrease in memory retention, so that if seizure foci are located in bilateral hippocampi, such a case is not a candidate for surgery. Moreover, the number of patients with intractable epilepsy such as childhood epileptic encephalopathy (e.g., West syndrome) having unknown seizure foci is estimated to be about 100,000 in Japan, and no curable therapy exists for such patients.
[0004] To treat such intractable epilepsy, a method for treating each disease by a gene therapy targeting nerve cells has also been examined. As a means (vector) for delivering a therapeutic gene to nerve cells, a means of using a recombinant adeno-associated virus (rAAV) is known in the art. Examples of such rAAV include those disclosed in International Publications WO2012/057363, WO2008/124724, WO2003/093479, and the like.
[0005] Means for adjusting neural activity targeting synapse-associated proteins have been studied. For example, the document of Kohl, C et al. (Non Patent Literature 1) discloses that the direct administration of an rAAV vector expressing neuroligin 2 (NLGN2) that is a synapse localized protein of nerve cells to hippocampi to overexpress NLGN2, results in altered social behavior and inhibitory synaptic transmission, but does not describe any specific treatment of the disease. The document of Moe et al. (Non Patent Literature 2) discloses that epilepsy symptoms are alleviated by treatment of epilepsy using an rAAV vector expressing neuropeptide Y. Moreover, the document of Fang et al. (Non Patent Literature 3) discloses that epilepsy symptoms are alleviated as a result of direct administration of an rAAV vector expressing the antisense of neuroligin 1 (NLGN1) that is a synapse localized protein of nerve cells to hippocampi.
PRIOR ART DOCUMENTS
Patent Literatures
[0006] Patent Literature 1: WO 2012/057363 [0007] Patent Literature 2: WO 2008/124724 [0008] Patent Literature 3: WO 2003/093479
Non Patent Literatures
[0008] [0009] Non Patent Literature 1: Kohl, C. et al., PLOS ONE, 2013 February, vol. 8, e56871 [0010] Non Patent Literature 2: Moe', F. M. et al., JASPER'S BASIC MECHANISMS OF THE EPILEPSY, 2012, [0011] Non Patent Literature 3: Kullmann, D. M. et al., Nature Reviews Neurology, 2014, vol. 10, page 300-304 [0012] Non Patent Literature 4: Fang et al., Mol. Neurobiol. 2014 Nov. 27 [Epub]
SUMMARY OF INVENTION
Technical Problem
[0013] A novel medicine for treating epilepsy by gene transfer is required.
[0014] Furthermore, such a medicine is desired to be advantageous in actual medication such that it has fewer side effects, and can be more simply administered, for example.
Solution to Problem
[0015] As a result of intensive studies to establish a gene therapy for epilepsy, the inventors of the present application have discovered that through preparation of a recombinant adeno-associated virus vector comprising a polynucleotide encoding neuroligin 2 that is a protein for improving the excitation-inhibiting capability of inhibitory synapses, and administration of the vector to a living subject, epilepsy symptoms are improved, and thus have completed the invention of the present application.
[0016] Specifically, the present application provides a recombinant adeno-associated virus (rAAV) vector as described below for treatment of the following nerve cell-related diseases such as epilepsy, and a pharmaceutical composition comprising the vector, for example.
{1} A recombinant adeno-associated virus vector, comprising a polynucleotide encoding a protein for improving an excitation-inhibiting function of inhibitory synapses in a living subject, which is used for treatment of a disease selected from the group consisting of epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, and developmental disorders (attention deficit hyperactivity disorder and Asperger's syndrome). {2} The recombinant adeno-associated virus vector according to {1}, wherein the polynucleotide comprises a nucleotide sequence encoding a neuroligin 2 protein which comprises the amino acid sequence of SEQ ID NO: 2, 4 or 6, or an amino acid sequence having about 90% or more identity with said amino acid sequence and binding to neurexin. {3} The recombinant adeno-associated virus vector according to {1} or {2}, wherein the disease is epilepsy. {4} The adeno-associated virus recombinant vector according to any one of {1} to {3}, wherein the recombinant adeno-associated virus vector comprises: a protein having a variant amino acid sequence in which tyrosine at position 445 in the amino acid sequence of the wild-type AAV1 capsid protein is substituted with phenylalanine; a protein having a variant amino acid sequence in which tyrosine at position 445 in the amino acid sequence of the wild-type AAV2 capsid protein is substituted with phenylalanine; or a protein having a variant amino acid sequence in which tyrosine at position 446 in the amino acid sequence of the wild-type AAV9 capsid protein is substituted with phenylalanine. {5} The recombinant adeno-associated virus vector according to any one of {1} to {4}, wherein the above polynucleotide comprises a promoter sequence selected from the group consisting of a synapsin I promoter sequence, a myelin basic protein promoter sequence, a neuron specific enolase promoter sequence, a calcium/calmodulin-dependent protein kinase II (CMKII) promoter sequence, a tubulin .alpha.I promoter sequence, a platelet-derived growth factor .beta. chain promoter sequence, a glial fibrillary acidic protein (GFAP) promoter sequence, a L7 promoter (cerebellar Purkinje cell specific promoter) sequence, a glial fibrillary acidic protein (hGfa2) promoter sequence, and a glutamate receptor delta 2 promoter (cerebellar Purkinje cell specific promoter) sequence, and a glutamic acid decarboxylase (GAD65/GAD67) promoter sequence. {6} The recombinant adeno-associated virus vector according to any one of {1} to {5}, wherein the above polynucleotide comprises an inverted terminal repeat (ITR) selected from the group consisting of AAV1, AAV2, AAV3, AAV4, AAV8, and AAV9. {7} The recombinant adeno-associated virus vector according to any one of {1} to {6}, wherein the above polynucleotide further comprises a polynucleotide for inhibiting the excitation of excitatory synapses. {8} A pharmaceutical composition, comprising the recombinant adeno-associated virus vector according to any one of {1} to {7}. {9} The pharmaceutical composition according to {8}, which is administered intracerebrally. {10} The pharmaceutical composition according to {8}, which is administered intrathecally. {11} The pharmaceutical composition according to {8}, which is administered peripherally. {12} The pharmaceutical composition according to any one of {8} to {11}, which is used in combination with a chemotherapeutic agent for a neuropsychiatric disease. {13} A method for treatment of a disease selected from the group consisting of epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, and developmental disorders (attention deficit hyperactivity disorder and Asperger's syndrome), comprising administering to the living subject a recombinant adeno-associated virus vector that comprises a polynucleotide having a nucleotide sequence encoding a protein for improving the excitation-inhibiting function of inhibitory synapses in a living subject. {14} The treatment method according to {13}, which is combined with a chemotherapy.
Advantageous Effects of Invention
[0017] Enhancement of the functions of a synaptic inhibitory system by a gene therapy according to the invention of the present application is useful as a method for treatment of epilepsy. The composition of the invention of the present application can be expected to be effective for even patients with intractable epilepsy having unspecified seizure foci, such as childhood epileptic encephalopathy (e.g., West syndrome).
BRIEF DESCRIPTION OF DRAWINGS
[0018] FIG. 1a shows the result of confirming the expression of the recombinant neuroligin 2 protein in a mouse to which vascular-administration-type rAAV was administered by the FLAG antibody staining of tissue sections. FIG. 1b shows the result of detecting the expression of the recombinant neuroligin 2 in a mouse to which a control was administered.
[0019] FIG. 2 shows the results of measuring epileptic seizure frequencies of mice subjected to 3 types of intracardiac administration (intracardiac administration of vascular-administration-type rAAV expressing NLGN2, vascular-administration-type rAAV expressing GFP protein, and physiological saline) at ages in weeks plotted on the horizontal axis.
[0020] FIG. 3 shows the results of seizure duration of mice subjected to the above 3 types of intracardiac administration.
[0021] FIG. 4 shows the results of seizure intensity of mice subjected to the above 3 types of intracardiac administration.
[0022] FIG. 5 shows the results of aggregating the results of seizure duration.times.seizure intensity in FIG. 3 and FIG. 4.
[0023] FIG. 6 shows the results of thresholds when mice subjected to the above 3 types of administration were subjected to electric stimulation at each age in weeks.
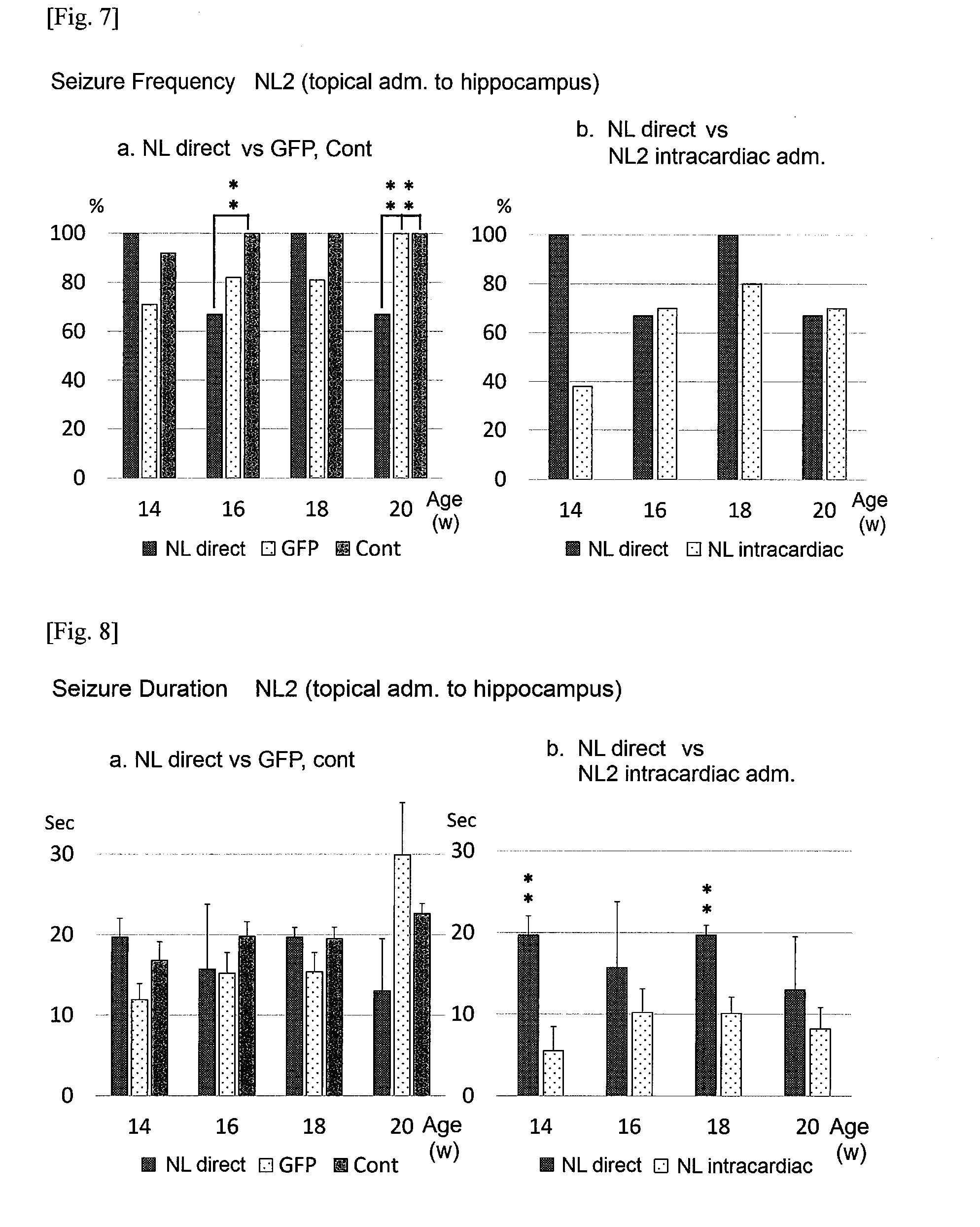
[0024] FIG. 7a shows the results of measuring the seizure frequencies of epilepsy of mice to which vascular-administration-type rAAV expressing NLGN2, vascular-administration-type rAAV expressing GFP protein, and physiological saline were topically administered to hippocampi at ages in weeks indicated on the horizontal axis (FIG. 7a), as well as the results of the same of mice to which vascular-administration-type rAAV expressing NLGN2, and vascular-administration-type rAAV expressing GFP protein were intracardially administered at ages in weeks plotted on the horizontal axis (FIG. 7b).
[0025] FIG. 8 shows the results of measuring seizure duration of mice in FIG. 7.
[0026] FIG. 9 shows the results of measuring seizure intensity of mice in FIG. 7.
[0027] FIG. 10 shows the aggregated results of seizure duration.times.seizure intensity of mice in FIG. 7.
[0028] FIG. 11 shows thresholds of electric stimulation measured at each age in weeks of mice in FIG. 7.
DESCRIPTION OF EMBODIMENTS
[0029] In this application, a recombinant adeno-associated virus vector is provided for treatment of a disease selected from the group consisting of epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, developmental disorders (attention deficit hyperactivity disorder, and Asperger's syndrome), which comprises a polynucleotide encoding a protein for improving the excitation-inhibiting function of inhibitory synapses in a living subject.
1. Excitation Control in Excitatory Synapse and Inhibitory Synapse
[0030] In this application, the term "synapse(s)" refers to junctional complexes between a synaptic knob formed of each swollen axonal terminal of nerve cells, and its target neuron or myocyte. In a living subject, excitatory synapses for transmitting excitation and inhibitory synapses for inhibiting the excitation transmission are present. Moreover, most synapses are chemical synapses (slow signal transduction) that are mediated by transmission of chemical substances. Another type of synapses includes electric synapses exhibiting quick response in terms of time, but are uncommonly observed in the central nervous system of a mature mammal.
[0031] In excitatory synapses, amino acids such as glutamic acid, aspartic acid, cysteic acid, and homocysteic acid function as transmitters, excitatory postsynaptic potential (EPSP) is generated, and then when the electric potential exceeds a threshold, excitation (impulse) transmission is performed. On the other hand, in inhibitory synapses, amino acids such as .gamma.-aminobutyric acid (GABA), glycine, taurine, alanine, cystathionine, and serine function as transmitters, and then inhibitory postsynaptic potential (IPSP) is generated, which is considered to suppress the impulse of postsynaptic neurons or makes the generation thereof difficult. EPSP and IPSP include fast EPSP or fast IPSP exhibiting a rapid time course (the entire time course is within 100 milliseconds) and slow EPSP or slow IPSP exhibiting extremely slow time course lasting for tens of seconds to tens of minutes. Here, in rapid IPSP, GABA associated with a GABA.sub.A receptor (Cl.sup.-) channel and glycine associated with a glycine receptor (Cl.sup.-) channel are known to act. As slow IPSP transmitters, GABA.sub.B acting through a GABA.sub.B receptor as well as acetylcholine and catecholamine are known to act.
[0032] Examples of a protein for improving the excitation-inhibiting function of inhibitory synapses include neuroligin 2 and neurexin involved in synapse stabilization, GABA receptor, glutaminedecarboxylase (GAD) involved in GABA biosynthesis, Na.sup.+ channel protein and Cl.sup.- channel protein involved in glycine transport, neuropeptide Y, gephyrin that is a scaffold protein, SLITRK3 that is a transmembrane protein and involved in inhibitory synapse formation, and PTPRD that is receptor-type tyrosine phosphatase binding to SLITRK3. A polynucleotide contained in the vector of the present invention comprises preferably a nucleotide sequence encoding a neuroligin 2 protein (SEQ ID NO: 2, 4 or 6) as a protein for improving the excitation-inhibiting function of inhibitory synapses.
[0033] The term "neuroligin (NLGN)" refers to a membrane protein family existing in a postsynaptic membrane, and is generally classified into neuroligins 1 to 4. Each of these neuroligins specifically binds to a cell adhesion molecule neurexin (Neurexin: NRXN) protein of a presynaptic membrane in order to connect a synapse preterminal and a postsynaptic site. Neuroligin 1 is located in excitatory synapses, and is considered to mediate excitatory synaptic transmission. On the other hand, neuroligin 2 is located in inhibitory synapses, and is considered to mediate inhibitory synaptic transmission. Moreover, neuroligin 3 is expressed in both excitatory synapses and inhibitory synapses, heart, pancreas, and the like, and neuroligin 4 is expressed in heart, liver and the like.
[0034] Neurexin proteins that are binding partners of neuroligins are generally classified into neurexin 1.alpha. to 3.alpha. and 1.beta. to 3.beta.. Herein, .alpha. neurexins and .beta. neurexins are long-chain proteins and short-chain proteins, respectively, which are generated from the same gene by the action of different promoters. Neuroligin 2 to be used in the invention of the present application functionally binds to neurexin 1.alpha..
[0035] A known amino acid sequence can be used as the amino acid sequence of the neuroligin 2 protein to be used in the invention of the present application. Examples of such amino acid sequence include Genbank Accession No. AAM46111 (human), EDL12455 (mouse), and EDM04903 (rat). Examples of such proteins of other animal species that can be used herein include proteins derived from mammals such as monkey, dog, pig, cattle and horse. The amino acid sequences of human, mouse and rat neuroligin 2 proteins are represented by SEQ ID NO: 2, 4 and 6, respectively.
[0036] Furthermore, examples of the neuroligin 2 protein to be used in the invention of the present application include a protein that has an amino acid sequence having about 90% or more, 91% or more, 92% or more, 93% or more, 94% or more, 95% or more, 96% or more, 97% or more, 98% or more, 99% or more, 99.1% or more, 99.2% or more, 99.3% or more, 99.4% or more, 99.5% or more, 99.6% or more, 99.7% or more, 99.8% or more, or 99.9% or more identity with the amino acid sequence of SEQ ID NO: 2, 4 or 6, and is capable of binding to a neurexin 1.alpha. protein under physiological conditions. The larger numerical values are generally more preferred. In addition, the amino acid sequence of human neuroligin 2 and that of mouse neuroligin 2 share 98% or more identity, and the amino acid sequence of human neuroligin 2 and that of rat neuroligin 2 share 91% or more identity. In the present invention, the phrase "a variant protein functions to a degree equivalent to that of the original protein" (for example, a protein exhibits binding ability equivalent to that of the original protein) means that, for example, the specific activity ranges from about 0.01 to 100, preferably ranges from about 0.5 to 20, and more preferably ranges from about 0.5 to 2, but examples thereof are not limited thereto.
[0037] Furthermore, examples of the neuroligin 2 protein to be used in the invention of the present application include a protein comprising the amino acid sequence of SEQ ID NO: 2, 4 or 6 or an amino acid sequence that has the above identity with the amino acid sequence of SEQ ID NO: 2, 4 or 6, in which one or more amino acids are deleted, substituted, inserted and/or added, and is capable of binding to the neurexin 1.alpha. protein under physiological conditions. Among the above amino acid deletion, substitution, insertion and addition, two or more types thereof may take place simultaneously. An example of such a protein is a protein comprising an amino acid sequence that is prepared from the amino acid sequence of SEQ ID NO: 2, 4 or 6 by deletion, substitution, insertion and/or addition of, for example, 1 to 50, 1 to 40, 1 to 39, 1 to 38, 1 to 37, 1 to 36, 1 to 35, 1 to 34, 1 to 33, 1 to 32, 1 to 31, 1 to 30, 1 to 29, 1 to 28, 1 to 27, 1 to 26, 1 to 25, 1 to 24, 1 to 23, 1 to 22, 1 to 21, 1 to 20, 1 to 19, 1 to 18, 1 to 17, 1 to 16, 1 to 15, 1 to 14, 1 to 13, 1 to 12, 1 to 11, 1 to 10, 1 to 9 (1 to several), 1 to 8, 1 to 7, 1 to 6, 1 to 5, 1 to 4, 1 to 3, 1 to 2, or one amino acid residue, and is capable of binding to a neurexin a protein under physiological conditions. The smaller number of the above amino acid residues to be deleted, substituted, inserted and/or added are generally more preferred.
[0038] Examples of amino acid residues in the protein (polypeptide) of the present invention, which can be substituted with each other, are as described below. Amino acid residues included in the same group can be substituted with each other.
[0039] Group A: leucine, isoleucine, norleucine, valine, norvaline, alanine, 2-aminobutanoic acid, methionine, o-methylserine, t-butylglycine, t-butylalanine, and cyclohexylalanine;
[0040] Group B: aspartic acid, glutamic acid, isoaspartic acid, isoglutamic acid, 2-aminoadipic acid, and 2-aminosuberic acid;
[0041] Group C: asparagine, and glutamine;
[0042] Group D: lysine, arginine, ornithine, 2,4-diaminobutanoic acid, and 2,3-diaminopropionic acid;
[0043] Group E: proline, 3-hydroxyproline, and 4-hydroxyproline;
[0044] Group F: serine, threonine, and homoserine; and
[0045] Group G: phenylalanine, and tyrosine.
A neuroligin protein in which an amino acid residue(s) is substituted can be prepared according to a method known by persons skilled in the art, such as a general genetic engineering technique. Such genetic engineering procedures can be referred to, for example, Molecular Cloning 3rd Edition, J. Sambrook et al., Cold Spring Harbor Lab. Press. 2001, Current Protocols in Molecular Biology, John Wiley & Sons 1987-1997.
[0046] Furthermore, examples of a polynucleotide that is preferably used in the invention of the present application include a polynucleotide having the polynucleotide sequence of SEQ ID NO: 1, 3 or 5, in which 1 or more (for example, 1 to 50, 1 to 40, 1 to 30, 1 to 25, 1 to 20, 1 to 15, 1 to 10, 1 to 9 (1 to several), 1 to 8, 1 to 7, 1 to 6, 1 to 5, 1 to 4, 1 to 3, 1 to 2, and 1) nucleotides are deleted, substituted, inserted and/or added, and encoding the protein that comprises the amino acid sequence of SEQ ID NO: 2, 4 or 6, or a protein that comprises an amino acid sequence prepared from the amino acid sequence of SEQ ID NO: 2, 4 or 6 by deletion, substitution, insertion and/or addition of one or more amino acids as described above, and is capable of binding to a neurexin 1.alpha.. Among these deletion, substitution, insertion and addition, two or more types thereof may be contained in combination simultaneously. A smaller number of the above nucleotides to be deleted, substituted, inserted and/or added are generally more preferred. Moreover, examples of a preferable polynucleotide in the invention of the present application include a polynucleotide which is hybridizable under stringent hybridization conditions to SEQ ID NO: 7, 9 or 11 or its complementary sequence and encodes the amino acid sequence of SEQ ID NO: 2, 4 or 6, and a polynucleotide encoding a protein which comprises an amino acid sequence prepared from the amino acid sequence of SEQ ID NO: 2, 4 or 6 by deletion, substitution, insertion and/or addition of one or more amino acids as described above, and is capable of binding to a neurexin.
[0047] Hybridization can be performed by well-known methods or methods modified therefrom, for example, methods described in Molecular Cloning (3rd Edition, J. Sambrook et al., Cold Spring Harbor Lab. Press. 2001), etc. When commercially-available libraries are used, hybridization may be performed in accordance with the methods described in instructions provided by manufacturers, etc. As used herein, the term "stringent conditions" may be any of low stringent conditions, moderate stringent conditions and high stringent conditions. The term "low stringent conditions" refers to conditions of, for example, 5.times.SSC, 5.times.Denhardt's solution, 0.5% SDS, and 50% formamide at 32.degree. C. The term "moderate stringent conditions" refers to conditions of, for example, 5.times.SSC, 5.times. Denhardt's solution, 0.5% SDS, and 50% formamide at 42.degree. C. The term "high stringent conditions" refers to conditions of, for example, 5.times.SSC, 5.times. Denhardt's solution, 0.5% SDS, and 50% formamide at 50.degree. C. Under these conditions, it can be expected that DNA with higher homology is obtained efficiently at higher temperatures. Multiple factors are involved in hybridization stringency including temperature, probe concentration, probe length, ionic strength, time, salt concentration and others, but persons skilled in the art can appropriately select these factors to achieve similar stringency.
[0048] Examples of such a hybridizable polynucleotide include polynucleotides having, e.g., 70% or more, 80% or more, 90% or more, 91% or more, 92% or more, 93% or more, 94% or more, 95% or more, 96% or more, 97% or more, 98% or more, 99% or more, 99.1% or more, 99.2% or more, 99.3% or more, 99.4% or more, 99.5% or more, 99.6% or more, 99.7% or more, 99.8% or more, 99.9% or more identity with the nucleotide sequence of SEQ ID NO: 7, 9 or 11, as calculated by using default parameters under a homology search software, such as FASTA and BLAST. In general, the larger numerical value of the above homology is more preferred.
[0049] The identity or homology between amino acid sequences or polynucleotide sequences can be determined using the algorithm BLAST by Karlin and Altschul (Proc. Natl. Acad. Sci. USA, 87: 2264-2268, 1990; Proc. Natl Acad. Sci. U.S.A., 90: 5873, 1993). Programs called BLASTN and BLASTX based on the BLAST algorithm have been developed (Altschul S. F. et al., J. Mol. Biol. 215: 403, 1990). When a nucleotide sequence is analyzed using BLASTN, the parameters are, for example, score=100 and word length=12. When an amino acid sequence is analyzed using BLASTX, the parameters are, for example, score=50 and word length=3. When BLAST and Gapped BLAST programs are used, default parameters for each of the programs are employed.
2. Target Disease in the Present Invention
[0050] The invention of the present application provides an rAAV vector useful for treatment of a disease selected from the group consisting of epilepsy, schizophrenia, autism spectrum disorder, mental retardation, anxiety, manic-depressive psychosis, migraine, phobic and compulsive symptoms, drug addiction, Angelman syndrome, dyskinesia, dystonia, Alzheimer's disease, developmental disorders (attention deficit hyperactivity disorder, and Asperger's syndrome), and particularly epilepsy.
[0051] Epilepsy refers to pathological conditions in which excessive, synchronous discharging of cerebral nerve cells results in repeated clinical seizures falling in the identical type in one individual (e.g., generalized tonic-clonic seizure, absence seizure, seizure with auditory hallucination, and tonic seizure of a part of extremities). According to the classification of International League Against Epilepsy (ILAE) in 1981, clinical seizures are divided into partial seizures (simple partial seizure and complex partial seizure), generalized seizures (absence seizure, myoclonus seizure, tonic-clonic seizure, atonic seizure), and common variable seizures. Furthermore, according to the "Classification of epilepsy, epilepsy syndrome, and related seizure disorders" of ILAE in 1989, epilepsy is classified into localization-related epilepsy (sub-classified into age-related, symptomatic, cryptogenic epilepsy), generalized epilepsy (sub-classified into idiopathic, cryptogenic or symptomatic epilepsy), cases that cannot be determined to be focal or generalized epilepsy, and special syndrome (e.g., febrile convulsion).
[0052] An example of epilepsy with a brief seizure in flexion, which begins at infancy (around 1 year old) as a cardinal sign is West syndrome (or spasmus nutans). This disease forms a series of momentary tonic seizures by which the patient bends his/her upper part of the body and head part forward continuously. There are various causes of West syndrome, and the causes including congenital brain malformation, neurocutaneous syndrome such as tuberous sclerosis, and inborn errors of metabolism such as vitamin B6 deficiency are known. West syndrome is often accompanied by mental retardation, and generally evolves as the patient grows into generalized epilepsy mainly associated with generalized tonic-clonic seizure (grand mal epilepsy), or other types of epilepsy, such as Lennox syndrome, temporal lobe epilepsy, and the like. The rAAV vector of the present invention can have a therapeutic effect against West syndrome.
3. Recombinant Adeno-Associated Virus (rAAV) Vector of the Present Invention
[0053] In the invention of the present application, as a vector for delivering a gene to be used for controlling synaptic functions to nervous system cells, a recombinant adeno-associated virus vector (herein also referred to as "vascular-administration-type vector") described in WO 2012/057363, which is capable of efficiently delivering genes to nerve cells also through peripheral administration, or a vector described in WO 2008/124724 etc., can be used, for example. The rAAV vector of the present invention can pass through the blood-brain barrier of a living subject, and thus is capable of introducing a therapeutic gene of interest to nervous system cells of the brain, the spinal cord or the like of a patient by an administration means for delivery to the brain through the blood-brain barrier, such as by peripheral administration to the patient. Moreover, the vector can also be administered intrathecally or directly to a target site in the brain.
[0054] The rAAV vector of the present invention can be prepared from preferably natural adeno-associated virus type 1 (AAV1), type 2 (AAV2), type 3 (AAV3), type 4 (AAV4), type 5 (AAV5), type 6 (AAV6), type 7 (AAV7), type 8 (AAV8), type 9 (AAV9) or the like, but examples thereof are not limited thereto. The nucleotide sequences of these adeno-associated viral genomes are known and can be referred to the nucleotide sequences of GenBank accession numbers: AF063497.1 (AAV1), AF043303 (AAV2), NC_001729 (AAV3), NC_001829.1 (AAV4), NC_006152.1 (AAV5), AF028704.1 (AAV6), NC_006260.1 (AAV7), NC_006261.1 (AAV8), and AY530579 (AAV9), respectively. Among them, the types 2, 3, 5 and 9 are human-derived. According to the present invention, it is particularly preferred to use the capsid protein (VP1, VP2, VP3 or the like) derived from AAV1, AAV2 or AAV9. Among human-derived AAVs, AAV1 and AAV9 were reported to have comparatively high multiplicity of infection on nerve cells (Taymans, et al., Hum Gene Ther 18:195-206, 2007, etc.).
[0055] A capsid protein to be contained in the rAAV vector used in the present invention is preferably a variant protein, as described in WO2012/057363, WO2008/124724 or the like, which has an amino acid sequence in which at least one tyrosine is substituted with another amino acid such as phenylalanine as compared with the wild-type amino acid sequence. Examples thereof include a variant protein having the amino acid sequence (SEQ ID NO: 9) formed by substitution of tyrosine at position 445 with phenylalanine from the amino acid sequence of a wild-type AAV1 capsid protein, a variant protein having an amino acid sequence (SEQ ID NO: 10) in which the tyrosine residue at position 444 in the amino acid sequence of a wild-type AAV2 capsid protein is substituted with the phenylalanine residue, and a variant protein having the amino acid sequence (SEQ ID NO: 11) in which the tyrosine residue at position 446 in the amino acid sequence of a wild-type AAV9 capsid protein is substituted with the phenylalanine residue (WO2012/057363 and WO 2008/124724). Such a capsid protein has a function of forming a capsomere solely or in combination with the other capsid protein members (for example, VP2 and VP3). Moreover, a polynucleotide comprising a therapeutic gene of interest to be delivered to nervous system cells is packaged in the capsomere.
[0056] When the rAAV vector of the present invention is administered into the blood stream, the rAAV vector can pass through the blood-brain barrier of a living subject including an adult and a fetus. In the present invention, examples of nervous system cells as targets of gene transfer include, at least nerve cells contained in the central nervous system such as the brain and the spinal cord, and examples of the cells may further include neuroglial cells, microglial cells, astrocytes, oligodendrocytes, ependymocytes, and cerebrovascular endothelial cells. The percentage of nerve cells in nervous system cells to which a gene is transferred is preferably, 70% or more, 80% or more, 85% or more, 90% or more, 91% or more, 92% or more, 93% or more, 94% or more, 95% or more, 96% or more, 97% or more, 98% or more, 99% or more, 99.1% or more, 99.2% or more, 99.3% or more, 99.4% or more, 99.5% or more, 99.6% or more, 99.7% or more, 99.8% or more, 99.9% or more, or 100%.
[0057] The Rep protein used in the present invention may have the same amino acid sequence identity described above, and may contain deletion, substitution, insertion and/or addition of the same number of amino acid residues described above, as long as it has known functions to the same degree such as a function of recognizing an ITR sequence and replicating the genome depending on the sequence, a function of recruiting and packaging a wild-type AAV genome (or rAAV genome) into a viral vector, and a function of forming the rAAV vector of the present invention. Examples of the range of functionally equivalent degrees include ranges described in description concerning the above specific activity. In the present invention, preferably, a Rep protein derived from known AAV3 is used.
[0058] A polynucleotide encoding the Rep protein used in the present invention may have the same number of identity described above or may contain deletion, substitution, insertion and/or addition of nucleotides in the same number described above, as long as it encodes a Rep protein having known functions to the same degree, such as a function of recognizing an ITR sequence and replicating the genome depending on the sequence, a function of recruiting and packaging a wild-type AAV genome (or rAAV genome) into a viral vector, and a function of forming the rAAV vector of the present invention. Examples of the range of functionally equivalent degrees include ranges described in description concerning the above specific activity. In the present invention, preferably, a rep protein derived from AAV3 or AAV2 is used.
[0059] In an embodiment of the present invention, capsid protein VP1's (VP1, VP2 and/or VP3) encoded by an internal region of the above wild-type AAV genome, and the Rep protein are used through incorporation of a polynucleotide encoding them into an AAV helper plasmid. The capsid proteins (VP1, VP2 and/or VP3) and the Rep protein used in the present invention may be incorporated into one, two, three or more types of plasmid, if necessary. In certain cases, one or more types of these capsid proteins and Rep protein may be contained in the AAV genome. In the present invention, preferably, the capsid proteins (VP1, VP2 and/or VP3) and the Rep protein are all encoded by one type of polynucleotide and provided in the form of an AAV helper plasmid.
[0060] A polynucleotide to be packaged in the rAAV vector of the present invention (referred to as the polynucleotide) can be prepared by substituting a polynucleotide of the internal region (specifically, one of or both the rep gene and the cap gene) located between ITR on the 5' side and that on the 3' side of the wild-type genome with a gene cassette containing a polynucleotide encoding a protein of interest (therapeutic gene), a promoter sequence for transcription of the polynucleotide, and the like. Preferably, ITR on the 5' side and that on the 3' side are located at the 5' end and the 3' end of the AAV genome, respectively. Preferably, the rAAV genome of the present invention includes 5'-ITR and 3'-ITR contained in AAV1, AAV2, AAV3, AAV4, AAV8 or AAV9 genome. In general, since an ITR portion easily takes a sequence wherein the complementary sequence is replaced (flip and flop structure), and the 5' to 3' direction may be reversed in the ITR contained in the rAAV genome of the present invention. In the rAAV genome of the present invention, the length of the polynucleotide which is replaced by the internal region (i.e., therapeutic gene) is preferably similar to the length of the original polynucleotide from a practical viewpoint. Specifically, it is preferred that the rAAV genome of the present invention has almost the same size as 5 kb, which is the full length of the wild type genome, for example, about 2 kb to 6 kb, preferably about 4 kb to 6 kb. Except for the length of a transcription regulatory region including a promoter, polyadenylation, etc. (assuming that the length is e.g., about 1 kb to 1.5 kb), the size of a therapeutic gene to be incorporated into the rAAV genome of the present invention preferably ranges from about 0.01 kb to 3.7 kb, more preferably, about 0.01 kb to 2.5 kb, and further preferably, about 0.01 kb to 2 kb, in length, but not limited thereto.
[0061] In general, a polynucleotide to be packaged in a recombinant adeno-associated virus vector may take times (several days) until the therapeutic protein of interest is expressed, when the genome is single-stranded. In such a case, a therapeutic gene to be introduced may be designed to be an sc (self-complementary) type in order to exhibit an effect within a shorter time period. Details about this procedure is described in Foust K D, et al. (Nat Biotechnol. 2009 January; 27(1):59-65), for example. The polynucleotide packaged in the rAAV vector of the present invention may be a non-sc type or a sc type.
[0062] In an embodiment, the rAAV vector of the present invention comprises a polynucleotide (i.e., such a polynucleotide is packaged) comprising, preferably, a nerve cell-specific promoter sequence and a therapeutic gene operably linked to the promoter sequence. As the promoter sequence to be used in the present invention, a nerve cell-specific promoter sequence is derived from nerve cells, neuroglial cells, oligodendrocytes, cerebrovascular endothelial cells, microglial cells, or ventricular epithelial cells, for example, but the examples thereof are not limited thereto. Specific examples of such promoter sequence include, but are not limited to, a synapsin I promoter sequence, a myelin basic protein promoter sequence, a neuron specific enolase promoter sequence, a glial fibrillary acidic protein promoter sequence, a L7 promoter (cerebellar Purkinje cell specific promoter) sequence, a glutamate receptor delta 2 promoter (cerebellar Purkinje cell specific promoter) sequence, a glial fibrillary acidic protein (hGfa2) promoter sequence, and a glutamic acid decarboxylase (GAD65/GAD67) promoter sequence. Moreover, in the rAAV vector of the present invention, promoter sequences such as a calcium/calmodulin-dependent protein kinase II (CMKII) promoter sequence, a tubulin .alpha.I promoter sequence, a platelet-derived growth factor .beta. chain promoter sequence, and the like can also be used. The above promoter sequences may be used independently or in optional combination of two or more thereof. In addition, the above promoter sequences may be strong promoter sequences that are generally used, such as a CMV promoter and a CAG promoter. Examples of particularly preferable promoter sequences in the present invention include a synapsin I promoter sequence, a myelin basic protein promoter sequence, a L7 promoter (cerebellar Purkinje cell specific promoter) sequence, and a glutamate receptor delta 2 promoter (cerebellar Purkinje cell specific promoter). Furthermore, known sequences such as an enhancer sequence which assists in transcription of mRNA, translation into a protein, etc., a Kozak sequence, an appropriate polyadenylation signal sequence, etc may also be contained.
[0063] A therapeutic gene of interest to be incorporated into the rAAV genome of the present invention is delivered with high efficiency to nerve cells and then integrated into the genome of the cells. When the rAAV vector of the present invention is used, the therapeutic gene can be transferred to about 10 times more, about 20 times or more, about 30 times or more, about 40 times or more or about 50 times or more of the nerve cell as compared with a conventional rAAV vector. The number of nerve cells carrying the gene transferred thereto can be determined, e.g., by preparing an rAAV vector for packaging the rAAV vector genome with any marker gene incorporated therein, administering the rAAV vector to an animal to be tested, and then measuring the number of nervous system cells expressing the marker gene (or marker protein) incorporated in the rAAV vector genome. The marker gene to be used herein is selected from known genes. Examples of such marker gene include a LacZ gene, a green fluorescence protein (GFP) gene, and a light emitting protein gene (e.g., firefly luciferase).
4. Other Therapeutic Genes
[0064] As other means or additional means for improving the excitation-inhibiting function of inhibitory synapses, for example, a means of enhancing the expression of neurexin 1.alpha. that is a binding partner of neuroligin 2, and a means of improving the intracellular signal transduction of neuroligin 2 can be expected. Alternatively, as such other means or additional means, a means of lowering the functions of excitatory synapses, such as suppressing the expression of a protein involving the operation of excitatory synapses, which is specifically a means of reducing the number of neuroligin 1 by using the antisense of neuroligin 1 (Non Patent Literature 4: Fang et al., Mol. Neurobiol. 2014 November) can also be useful.
[0065] The rAAV vector of the present invention may express different proteins for controlling synaptic functions. Examples of such different proteins include neutralizing antibodies against proteins and receptors existing on synaptic membranes (including antigen-binding sites, Fab, Fab2, single-chain antibody (scFv), etc.). Examples of the classes of these antibodies include IgG, IgM, IgA, IgD, and IgE.
[0066] For example, for inhibition of the functions of excitatory synapses, a therapeutic gene to be incorporated into the rAAV genome of the present invention may be a polynucleotide for modifying (for example, disrupting or lowering) a function of a target endogenous gene, or a polynucleotide for changing (for example, lowering) an expression level of an endogenous protein, such as an antisense molecule, a ribozyme, interfering RNA (iRNA), and micro RNA (miRNA). For example, in order to effectively inhibit the expression of a target gene by using an antisense sequence, preferably, the length of an antisense nucleic acid is 10 or more nucleotides, 15 or more nucleotides, 20 or more nucleotides, or 100 or more nucleotides, or even more preferably 500 or more nucleotides. In general, the length of an antisense nucleic acid to be used is shorter than 5 kb, and is preferably shorter than 2.5 kb.
[0067] By using a ribozyme, the mRNA encoding a protein of interest can be specifically cleaved to decrease the expression of the protein. For the design of such a ribozyme, reference may be made to various known publications (see e.g., FEBS Lett. 228: 228, 1988; FEBS Lett. 239: 285, 1988; Nucl. Acids. Res. 17: 7059, 1989; Nature 323: 349, 1986, etc.).
[0068] The term "RNAi" refers to a phenomenon that, when a double-stranded RNA with a sequence identical or similar to a target gene sequence is introduced into cells, expression of both a target foreign gene introduced and the target endogenous gene is decreased. Examples of RNA used herein include double-stranded RNA of 21 to 25 nucleotides in length that triggers RNA interference, such as dsRNA (double strand RNA), siRNA (small interfering RNA), shRNA (short hairpin RNA) or miRNA (microRNA). These RNAs can be locally delivered to a desired site by a delivery system using liposomes, or a vector that generates the double-stranded RNA described above can be used for local expression thereof. Methods for preparing or using such double-stranded RNA (dsRNA, siRNA, shRNA or miRNA) are known from many publications (see, e.g., National Publication of International Patent Application No. 2002-516062, U.S. Patent No. 2002/086356A, Nature Genetics, 24(2), 180-183, 2000 February).
[0069] To use these other therapeutic genes, for example, a known internal ribosome entry site (IRES) sequence is allowed to intervene in a polynucleotide contained in the vector of the present invention. When the rAAV genome of the present invention is a non-sc type, it is possible to select promoters with more varied lengths and genes of interest, and also a plurality of genes of interest. A polynucleotide to be packaged in the rAAV vector of the present invention has a full length of preferably about 5 kb or less (about 4.7 kb or less when an ITR region is excluded).
5. Preparation of the rAAV Vector of the Present Invention
[0070] A general method can be employed as a method for preparing the rAAV vector of the present invention. For example, the method may comprise a step of transfecting a cultured cell with: (a) a first polynucleotide encoding a capsid protein (generally referred to as an AAV helper plasmid), and (b) a second polynucleotide (carrying a therapeutic gene of interest) to be packaged in the rAAV vector of the present invention; and may further comprise a step of transfecting the cultured cell with (c) a plasmid encoding an adenovirus-derived factor, also referred to as an adenovirus (AdV) helper plasmid, or a step of infecting cultured cells with an adenovirus. The method can also comprise a step of culturing the transfected cultured cell and a step of collecting the recombinant adeno-associated virus vector from the culture supernatant. Furthermore, (d) an example of a method for preparing the rAAV vector of the present invention includes a method for producing an rAAV in a large scale by preparing baculoviruses containing the above polynucleotides (a) and (b), respectively, and then infecting insect cells, Sf9 or the like with the viruses. This method is already known and also used in Examples of the Description.
[0071] A nucleotide encoding the capsid protein of the present invention in the first polynucleotide (a) is preferably operably bound to a known promoter sequence that is operable in cultured cells. As such a promoter sequence, for example, a cytomegalovirus (CMV) promoter, an EF-1.alpha. promoter, an SV40 promoter, and the like can be appropriately used. Furthermore, the first polynucleotide can comprise a known enhancer sequence, a Kozak sequence, a polyA addition signal sequence and the like, as appropriate.
[0072] The second polynucleotide (b) comprises a therapeutic gene at a position where it is operable with a nervous system cell-specific promoter. Furthermore, the second polynucleotide can comprise a known enhancer sequence, a Kozak sequence, a polyA addition signal sequence, and the like as appropriate. The first polynucleotide can further comprise a cloning site, which can be cleaved by various known restriction enzymes, and is located downstream from the nervous system cell-specific promoter sequence. A multicloning site containing a plurality of restriction enzyme recognition sites is more preferred. Persons skilled in the art may incorporate a therapeutic gene of interest downstream of the nervous system cell-specific promoter, in accordance with known genetic engineering procedures. For such genetic engineering procedures, see, e.g., Molecular Cloning 3rd Edition, J. Sambrook et al., Cold Spring Harbor Lab: Press. 2001, etc.
[0073] In preparation of the rAAV vector of the present invention, a helper virus plasmid (e.g., adenovirus, herpes virus or vaccinia) is used and can be introduced into cultured cells simultaneously with the above first and second polynucleotides. Preferably, the preparation method of the present invention further comprises a step of introducing an adenovirus (AdV) helper plasmid. In the present invention, preferably, AdV helper is derived from a virus of the same species as that of cultured cells. For example, when human cultured cells 293T are used, a human AdV-derived helper virus vector can be used. As such an AdV helper vector, a commercially available AAV Helper-Free System (Agilent Technologies, catalog No. 240071) can be used, for example.
[0074] In preparation of the rAAV vector of the present invention, examples of a method for transfecting cultured cells with the above one or more types of plasmid, which can be used herein, include various known methods such as the calcium phosphate method, lipofection method, and electroporation method, etc. Such methods are described in, e.g., Molecular Cloning 3rd Ed., Current Protocols in Molecular Biology, John Wiley & Sons 1987-1997, etc.
6. Pharmaceutical Composition Containing the rAAV Vector of the Present Invention
[0075] The rAAV vector of the present invention can comprise genes useful for treatment of neurological disorders, particularly, diseases relating to protein dysfunction in synapses (e.g., schizophrenia and autism spectrum disorder). The rAAV vector comprising these genes can be administered intravascularly to pass through the blood-brain barrier, and thus can be incorporated into nerve cells of the brain, the spinal cord, and the retina. The rAAV vector comprising such a therapeutic gene can be contained in the pharmaceutical composition of the present invention. As such therapeutic genes, for example, polynucleotides encoding the above-mentioned antibodies, neurotrophic factor (NGF), growth factor (HGF), acidic fibroblast growth factor (aFGF), miRNA and the like can be selected. It can be expected to treat neurological disorder through peripheral administration of such rAAV vector to a test subject.
[0076] The active ingredient of the pharmaceutical composition of the present invention may be formulated solely or in combination therein, and can also be provided as a pharmaceutical preparation by formulation with a pharmaceutically acceptable carrier or an additive for a pharmaceutical preparation. In this case, the active ingredient of the present invention may be contained in an amount e.g., 0.1 to 99.9 wt % in the preparation.
[0077] Examples of the pharmaceutically acceptable carriers or additives that can be used include excipients, disintegrants, disintegration aids, binders, lubricants, coating agents, dyes, diluents, dissolution agents, dissolution aids, isotonic agents, pH regulators, stabilizers, etc. For oral administration, excipients that are generally used in the art, such as microcrystalline cellulose, sodium citrate, calcium carbonate, disintegrants such as starch and alginic acid, granulation binders such as polyvinylpyrrolidone, and lubricants can be used in combination. When aqueous suspensions and/or elixirs are desired for oral administration, the active ingredient may be used in combination with various sweeteners or corrigents, coloring agents or dyes, and, if necessary, emulsifying and/or suspending agents as well, together with diluents such as water, ethanol, propylene glycol, glycerin, etc. and combinations thereof.
[0078] Examples of the pharmaceutical preparations suitable for oral administration can include powders, tablets, capsules, fine granules, granules, liquids or syrups, etc. Examples of the pharmaceutical preparations suitable for parenteral administration can include injections, intrathecal injections, suppositories, etc. For parenteral administration, solutions of the active ingredient of the present invention dissolved in either sesame or peanut oil or in aqueous propylene glycol solution may be employed. The aqueous solutions should be appropriately buffered (preferably pH 8 or higher) as necessary; it is first necessary to render the liquid diluent isotonic. As such a liquid diluent, physiological saline can be used. The thus prepared aqueous solutions are suitable for intravenous injection. On the other hand, the oily solutions are suitable for intra-articular injection, intra-muscular injection and subcutaneous injection. The preparation of all these solutions under sterile conditions can be readily accomplished by standard pharmaceutical techniques well known to those skilled in the art. Furthermore, the active ingredient of the present invention can also be administered topically to the skin, etc. In this case, topical administration is desirably performed by way of creams, jellies, pastes, ointments and the like, in accordance with standard pharmaceutical practice.
[0079] The dose of the pharmaceutical composition of the present invention is not particularly limited, and an appropriate dose can be chosen depending on various conditions such as type of disease, age and symptoms of the patient, administration route, therapeutic goal, presence or absence of concurrent drugs, etc. The dose of the pharmaceutical composition of the present invention is, but not limited to, for example, 1 to 5,000 mg, and preferably 10 to 1,000 mg per day for an adult (e.g., body weight of 60 kg). Such daily dose may be administered in 2 to 4 divided doses per day. When vg (vector genome) is used as a dosage unit, the dose can be selected from, but not limited to, e.g., the range from 10.sup.9 to 10.sup.14 vg, preferably, 10.sup.10 to 10.sup.13 vg, and more preferably, 10.sup.10 to 10.sup.12 vg per kg body weight.
7. Administration of the rAAV Vector of the Present Invention
[0080] The rAAV of the present invention is capable of passing through the blood-brain barrier of a living subject (including incomplete fetal and newborn blood-brain barriers, and established adult blood-brain barriers) and thus capable of delivering genes in the rAAV to nervous system cells of the brain, the spinal cord, and the like through peripheral administration to a living subject (including adults and fetuses or newborns). Furthermore, the rAAV vector to be used in the present invention can target nerve cells contained in an adult's brain, spinal cord, and the like through peripheral administration. As used herein, the term "peripheral administration" refers to administration routes which those skilled in the art usually understand as peripheral administration, including intravenous administration, intraarterial administration, intraperitoneal administration, intracardiac administration, intramuscular administration, and umbilical intravascular administration (e.g., the target is a fetus), and so on. Furthermore, an administration method, which involves using a fluid other than blood that is fluidly communicated with the brain, such as intrathecal administration, can also be used for the rAAV vector of the present invention. In another embodiment, the rAAV vector of the present invention can also be locally administered to a target site within the brain, such as hippocampi. For example, when the rAAV of the present invention is administered via intrathecal administration into a spinal fluid, or via peripheral administration into blood, a means for administration simpler than intraparenchymal administration can be provided.
8. Kit for Preparation of the rAAV Vector of the Present Invention
[0081] In another embodiment, the present invention provides a kit for preparing the rAAV of the present invention. Such a kit can contain, for example, (a) a first polynucleotide for expression of capsid protein VP1 or the like, and (b) a second polynucleotide to be packaged in the rAAV vector. For example, the first polynucleotide comprises a polynucleotide encoding the amino acids of SEQ ID NO. For example, the second polynucleotide may or may not comprise a therapeutic gene of interest, but can preferably comprise various restriction enzyme cleavage sites for incorporation of such a therapeutic gene of interest.
[0082] The kit for preparing the rAAV vector of the present invention can further contain any component described herein (e.g., an AdV helper.). The kit of the present invention may further include instructions describing the protocols for preparation of the rAAV vector using the kit of the present invention.
9. Chemotherapeutic Agent to be Used in Combination with the rAAV of the Present Invention
[0083] The rAAV vector according to the invention of the present application can also be used in combination with an existing chemotherapeutic agent. Examples of such a chemotherapeutic agent include phenytoin, carbamazepine, valproic acid, topiramate, lamotrigine, rufinamide, phenobarbital, diazepam, clonazepam, ethosuximide, zonisamide, gabapentin, levetiracetam, midazolam, clobazam, and propofol. For example, after administration of the rAAV of the invention of the present application, a significant reduction in the dose of the above chemotherapeutic agent can be expected.
10. Determination of Therapeutic Effects
[0084] The therapeutic effects of the rAAV vector of the present invention can be determined using a known means for determining if excitation can be inhibited by the therapeutic effects. Examples of such a known means include, but are not limited to, analysis of behavior levels, analysis of the pharmacodynamics of labeled transmitters (e.g., GABA), measurement of excitatory postsynaptic potential and inhibitory postsynaptic potential, measurement of changes in threshold of epilepsy induced by medicines or electric stimulation, brain wave, optical topography, and positron emission tomography (PET).
11. Terms Used in the Description
[0085] The meaning indicated by each term as used herein is as described below. Terms not particularly described herein are intended to refer to meanings that are normally understood by persons skilled in the art.
[0086] As used herein, the terms "virus or viral vector", "virus virion," and "virus or viral particles" are interchangeably used, unless otherwise indicated.
[0087] As used herein, the term "nervous system" refers to an organ system made up of nerve tissues. As used herein, the term "nervous system cells" include at least nerve cells included in the central nervous system including brains, spinal cords, etc. and may further include neuroglial cells, microglial cells, astrocytes, oligodendrocytes, ependymocytes, cerebrovascular endothelial cells, etc.
[0088] As used herein, the term "polynucleotide" is interchangeably used with "nucleic acid," "gene" or "nucleic acid molecule," which is intended to mean a nucleotide polymer. As used herein, the term "nucleotide sequence" is used interchangeably with "nucleic acid sequence" or "base sequence," which is represented by a sequence of deoxyribonucleotides (abbreviated as A, G, C, and T). For example, the "polynucleotide comprising the nucleotide sequence of SEQ ID NO: 1 or a fragment thereof" is intended to mean a polynucleotide comprising a sequence shown by the respective deoxynucleotides A, G, C and/or T of SEQ ID NO: 1, or a fragment thereof.
[0089] Each of "virus or viral genome" and "polynucleotide" according to the present invention may exist in the form of a DNA (e.g., cDNA or genomic DNA), respectively, and may also be in the form of an RNA (e.g., mRNA). Each of the viral genome and the polynucleotide as used herein may be a double-stranded or single-stranded DNA. Single-stranded DNA or RNA may be a coding strand (also known as a sense strand) or a non-coding strand (also known as an anti-sense strand). Regarding the explanation herein for placing a promoter, a gene of interest, polyadenylation signal, etc. in the gene, which are encoded by the rAAV genome, if the rAAV genome is a sense strand, the strand itself is described and if it is an antisense strand, its complementary strand is described, unless otherwise specified.
[0090] As used herein, the terms "protein" and "polypeptide" are interchangeably used and intended to mean a polymer of amino acids. The polypeptide as used herein is represented in accordance with conventional peptide designation, in which the N-terminus (amino terminus) is on the left hand and the C-terminus (carboxyl terminus) on the right hand. The partial peptide in the polypeptide of the present invention (as used herein, may briefly be referred to as the partial peptide of the present invention) includes a partial peptide of the polypeptide of the present invention described above, preferably having the same properties as those of the above polypeptide of the present invention.
[0091] As used herein, the term "plasmid" refers to various known gene elements, for example, a plasmid, a phage, a transposon, a cosmid, a chromosome, etc. The plasmid can be replicated in a particular host and transport gene sequences between cells. As used herein, the plasmid contains various known nucleotides (DNA, RNA, PNA and a mixture thereof) and may be a single strand or a double strand, and preferably a double strand. As used herein, the term "rAAV vector plasmid" is intended to include a double strand formed by rAAV vector genome and its complementary strand, unless otherwise specified. The plasmid used in the present invention may be linear or circular.
[0092] As use herein, the term "packaging" refers to the events including preparation of single-stranded viral genomes, assembly of coat (capsid) proteins, enclosure of viral genome within a capsid (encapsidation), and the like. When an appropriate plasmid vector (normally, a plurality of plasmids) is introduced into a cell line that allows packaging under appropriate conditions, recombinant viral particles (i.e., virus virions, viral vectors) are constructed and secreted into the culture.
EXAMPLES
[0093] The present invention is described below in more detail by referring to Examples, but the scope of the invention should not be limited to the following Examples.
Experimental Outline
[0094] Intracardiac administration of rAAV-Neuroligin2 (rAAV-NL2) at 6 weeks of age For:-Seizure frequency-Duration-Intensity-Duration.times.Intensity-Changes in threshold for electric stimulation
Intracerebral Expression of Neuroligin2-Carrying Intravascular-Administration-Type rAAV
[0095] Reports concerning the gene therapy for epilepsy using model animals are found here and there, but they all involve performing topical administration stereotactically. For application to a human patient, administration methods without invasive procedures are desired. This time, the inventors of the present application prepared an intravascular-administration-type adeno-associated virus (rAAV) vector, administered the vector to EL mice naturally developing epilepsy (Suzuki, Proc. Jpn. Acad., Ser.B89 (2013)), and then observed the condition of intracerebral expression and the presence or the absence of an effect of suppressing seizure.
Experimental Materials and Methods
[0096] Recombinant Adeno-Associated Virus (rAAV) Vector
[0097] The vector to be used in the Examples is previously disclosed AAV9/3 (having tyrosine mutation (Y446.fwdarw.F) introduced into AAV9 capsid, and ITR of AAV3) carrying a Synapsin I promoter (WO 2012/057363). To differentiate from endogenous Neuroligin 2 (NL2), an rAAV vector expressing neuroligin2 with the N-terminus, to which a FLAG tag (DDDDK) sequence had been bound, was prepared (AAV9/3-Syn1-FLAG (DDDDK)-NL2), and then administered to subject animals.
[0098] Administration to Animal
[0099] EL mice (6 weeks of age, male, body weight: 22-32 g) were used.
[0100] Under 2-4% sevoflurane anesthesia, inracardiac injection of AAV9/3-Syn1-FLAG (DDDDK)-NL2 was performed at 4.1.times.10.sup.13 vector genome/ml.times.0.1 ml/mouse (NL2 inracardiac injection group n=10). A group to which AAV9/3-Syn1-AcGFP-WPRE was administered via intracardiac injection at 2.3.times.10.sup.13 vg/ml.times.0.1 ml/mouse (n=17), and a group to which only physiological saline was administered at 0.1 ml/mouse (n=14) were designated as control groups. Also, for comparison with topical administration, a group of mice to which AAV9/3-Syn1-FLAG (DDDDK)-NL 2 was injected to the bilateral hippocampal CA3 region (0.5 mm anterior to and 3.0 mm lateral to bregma, and 2.0 mm from the brain surface) at 4.1.times.10.sup.13 vg/ml.times.0.005 ml (n=3) was designated as the topical administration group.
TABLE-US-00001 TABLE 1 Number of Administration site Titer vg/mouse mice rAAV-NL2 Intracardiac 4.1 .times. 10.sup.12 10 rAAV-GFP Intracardiac 2.3 .times. 10.sup.12 17 Physiological saline Intracardiac -- 14 rAAV-NL2 Bilateral hippocampi 2.0 .times. 10.sup.11 3 rAAV-GFP Bilateral hippocampi 1.1 .times. 10.sup.11 3 Physiological saline Bilateral hippocampi -- 3
[0101] Evaluation by Angular Acceleratory Stimulation
[0102] After administration of the vector and the like, each of the mice was rotated with the tail held for predetermined times (8 rotations) every week until 22 weeks of age, and the behavior of each of the mice was video-recorded. Thereafter, the presence or the absence of seizures, duration when a mouse had developed a seizure, and the intensity thereof were observed on video.
Seizure intensity was scored as follows:
[0103] 1 point: no seizure;
[0104] 2 points: only raised the tail or only shook the body;
[0105] 3 points: developed a clear seizure, but kept the posture without falling down; and
[0106] 4 points: developed a severe seizure and could not keep the posture and fell down sideways.
Seizure duration was scored as follows:
[0107] 1 point: no seizure;
[0108] 2 points: 1-10 seconds;
[0109] 3 points: 11-20 seconds;
[0110] 4 points: 21-30 seconds;
[0111] 5 points: 31-60 seconds; and
[0112] 6 points: 61 seconds or longer.
[0113] The seizure incidence, the mean seizure duration, the mean seizure intensity, and the mean seizure duration.times.intensity of each group were evaluated every week.
[0114] Evaluation by Electric Stimulation
[0115] At 5 (before administration of the vector), 12, 18, 22 weeks of age, electrodes were placed on both ears of a mouse, electric stimulation (the following parameters were used in Neuropack S1 (NIHON KOHDEN): duration: 1 ms, interval: 50 ms, 10 train, strength: max 50 mA every 0 mA to 5 mA) was applied to induce an epileptic seizure, and then the seizure threshold was measured. If no seizure was induced by 50 mA, the seizure threshold was determined to be 60 mA for evaluation. For only the topical administration group of mice, this procedure was performed at 5, 12, and 22 weeks of age.
[0116] Statistical Processing was Evaluated by the Following Tests.
[0117] Seizure incidence: Fisher's exact test
[0118] Other: Welch's t test
[0119] Histological Analysis
[0120] Preparation of brain specimen: Each mouse was deeply anesthetized with pentobarbial, and then 4% paraformaldehyde-containing 0.1M phosphate buffer (pH7.4) was injected through the left ventricle for perfusion fixation. After fixation, the brain was dissected out, immersion fixed in a fixative for a half day, transferred into 15% sucrose-containing 0.1M phosphate buffer (pH 7.4), and then stored in a refrigerator until a histochemical experiment.
[0121] rAAV9-GFP Expression and Cell Identification
[0122] 40-.mu.m sagittal sections were prepared using a cryo-microtome and then GFP expression was identified at each brain site using a fluorescence microscope. GFP-expressing cells were identified by double staining with the following markers.
[0123] Nerve cells: NeuN or MAP2; glial cells: GFAP; and inhibitory interneurons: Parvalbumin
[0124] rAAV9-NL2 Expression and Cell Identification
[0125] In a manner similar to that in identification of GFP, 40-.mu.m sagittal sections were prepared, and the transgene NL2 expression was identified by FLAG (DDDDK) antibody staining. FLAG antibody was purchased from Abcam plc., and the expression was identified under a fluorescence microscope by image processing using a Alexafluor 488 conjugated secondary antibody.
Experimental Results and Discussion
[0126] Hippocampal slices (specimens) were subjected to measurement of changes in intracellular Ca.sup.2+ influx upon ischaemic loading based on changes in fluorescence of rhod 2-AM (DOJINDO catalog No.: R002). As compared with DDY mice, EL mice exhibited, in the CA3 region, significant increases in intracellular Ca.sup.2+ influx induced in hippocampi by ischaemiac loading, suggesting the vulnerability of the inhibitory system in the CA3 region. Next, the expression of intervening cells of an excitation inhibition system in EL hippocampi was histologically examined by immunostaining with the parvalbumin antibody (catalog No.: LS-C39101). No significant difference was found between EL mice and DDY mice in terms of the number of parvalbumin-positive cells in each hippocampal region, suggesting possible changes at the synaptic level. Based on the above results, an rAAV vector expressing a gene of an inhibitory system synapse-related molecule was prepared, the vector was administered to EL via stereotactic hippocampal injection and intravascular injection, and then intracerebral distribution was histologically observed by staining using a FLAG antibody (FIG. 1a, and FIG. 1b). As shown in FIG. 1a, FLAG-tagged NLGN2 was broadly expressed in hippocampal and cerebral cortical nerve cells as a result of intravascular administration of the vector the present invention, so that successful gene delivery by rAAV could be confirmed.
[0127] Subsequently, the intravascular injection group was observed for the presence or the absence of the effect of suppressing epileptic seizure (FIG. 2 to FIG. 6). A group to which a green fluorescent protein EGFP expression AAV vector was administered, and a group to which physiological saline was administered were designated as control groups. The effect of suppressing epilepsy was evaluated for each attribute; that is, frequency of seizure development, intensity, duration, and seizure intensity.times.duration of a mouse at each age in weeks. In addition, significant differences were observed at positions marked with "*" and "**" in the figures. The group to which NLGN2 had been administered suppressed seizures more significantly than the control groups, and exhibited no change in threshold for electric stimulation (FIG. 2 to FIG. 6). No change in threshold for electric stimulation suggested that the initiation of the operation of inhibitory synapses remained unchanged from the time before introduction and suggested the low side effect.
[0128] Expression of the gene of interest was observed in hippocampal neurons of the hippocampal injection group, and in whole brain neurons including hippocampal neurons of the intravascular administration group (the results not shown). Compared with the control groups, the hippocampal injection group, specifically, the group to which NLGN2 had been administered exhibited a significant difference in seizure frequency in some cases, but exhibited overall no significant difference in the effect (FIG. 7 to FIG. 11). In the figures, significant differences were observed for those indicated with "*" and "**". Moreover, compared with the intracardiac administration groups, the intracardiac administration groups were generally observed to tend to exhibit the higher effects on suppressing epilepsy than the other groups in any attribute of seizure frequency, seizure duration and seizure intensity.
[0129] The target molecules were supplied to whole brain neurons by the intravascular-administration-type AAV vector. The excitation inhibitory effect of said molecules is capable of suppressing epileptic seizures without changing the threshold for electric stimulation, suggesting a possibility of a non-invasive epilepsy gene therapy as a more advantageous therapeutic method.
INDUSTRIAL APPLICABILITY
[0130] The use of the rAAV vector of the present invention can be expected to treat (e.g., alleviation, improvement, and repair) genetic malfunctions in nervous system cells (including congenital and acquired malfunctions).
SEQUENCE LISTING FREE TEXT
[0131] SEQ ID NO: 1: human neuroligin2 nucleotide sequence SEQ ID NO: 2: human neuroligin2 amino acid sequence SEQ ID NO: 3: mouse neuroligin2 nucleotide sequence SEQ ID NO: 4: mouse neuroligin2 amino acid sequence SEQ ID NO: 5: rat neuroligin2 nucleotide sequence SEQ ID NO: 6: rat neuroligin2 amino acid sequence SEQ ID NO: 7: Flag-tagged mouse neuroligin2 nucleotide sequence SEQ ID NO: 8: Flag-tagged mouse neuroligin2 amino acid sequence SEQ ID NO: 9: AAV1 capsid protein Y445F variant amino acid sequence SEQ ID NO: 10: AAV2 capsid protein Y444F variant amino acid sequence SEQ ID NO: 11: AAV9 capsid protein Y446F variant amino acid sequence
Sequence CWU 1
1
1112505DNAHomo sapiensCDS(1)..(2505) 1atg tgg ctc ctg gcg ctg tgt
ctg gtg ggg ctg gcg ggg gct caa cgc 48Met Trp Leu Leu Ala Leu Cys
Leu Val Gly Leu Ala Gly Ala Gln Arg 1 5 10 15 ggg gga ggg ggt ccc
ggc ggc ggc gcc ccg ggc ggc ccc ggc ctg ggc 96Gly Gly Gly Gly Pro
Gly Gly Gly Ala Pro Gly Gly Pro Gly Leu Gly 20 25 30 ctc ggc agc
ctc ggc gag gag cgc ttc ccg gtg gtg aac acg gcc tac 144Leu Gly Ser
Leu Gly Glu Glu Arg Phe Pro Val Val Asn Thr Ala Tyr 35 40 45 ggg
cga gtg cgc ggt gtg cgg cgc gag ctc aac aac gag atc ctg ggc 192Gly
Arg Val Arg Gly Val Arg Arg Glu Leu Asn Asn Glu Ile Leu Gly 50 55
60 ccc gtc gtg cag ttc ttg ggc gtg ccc tac gcc acg ccg ccc ctg ggc
240Pro Val Val Gln Phe Leu Gly Val Pro Tyr Ala Thr Pro Pro Leu Gly
65 70 75 80 gcc cgc cgc ttc cag ccg cct gag gcg ccc gcc tcg tgg ccc
ggc gtg 288Ala Arg Arg Phe Gln Pro Pro Glu Ala Pro Ala Ser Trp Pro
Gly Val 85 90 95 cgc aac gcc acc acc ctg ccg ccc gcc tgc ccg cag
aac ctg cac ggg 336Arg Asn Ala Thr Thr Leu Pro Pro Ala Cys Pro Gln
Asn Leu His Gly 100 105 110 gcg ctg ccc gcc atc atg ctg cct gtg tgg
ttc acc gac aac ttg gag 384Ala Leu Pro Ala Ile Met Leu Pro Val Trp
Phe Thr Asp Asn Leu Glu 115 120 125 gcg gcc gcc acc tac gtg cag aac
cag agc gag gac tgc ctg tac ctc 432Ala Ala Ala Thr Tyr Val Gln Asn
Gln Ser Glu Asp Cys Leu Tyr Leu 130 135 140 aac ctc tac gtg ccc acc
gag gac ggt ccg ctc aca aaa aaa cgt gac 480Asn Leu Tyr Val Pro Thr
Glu Asp Gly Pro Leu Thr Lys Lys Arg Asp 145 150 155 160 gag gcg acg
ctc aat ccg cca gac aca gat atc cgt gac cct ggg aag 528Glu Ala Thr
Leu Asn Pro Pro Asp Thr Asp Ile Arg Asp Pro Gly Lys 165 170 175 aag
cct gtg atg ctg ttt ctc cat ggc ggc tcc tac atg gag ggg acc 576Lys
Pro Val Met Leu Phe Leu His Gly Gly Ser Tyr Met Glu Gly Thr 180 185
190 gga aac atg ttc gat ggc tca gtc ctg gct gcc tat ggc aac gtc att
624Gly Asn Met Phe Asp Gly Ser Val Leu Ala Ala Tyr Gly Asn Val Ile
195 200 205 gta gcc acg ctc aac tac cgt ctt ggg gtg ctc ggt ttt ctc
agc acc 672Val Ala Thr Leu Asn Tyr Arg Leu Gly Val Leu Gly Phe Leu
Ser Thr 210 215 220 ggg gac cag gct gca aaa ggc aac tat ggg ctc ctg
gac cag atc cag 720Gly Asp Gln Ala Ala Lys Gly Asn Tyr Gly Leu Leu
Asp Gln Ile Gln 225 230 235 240 gcc ctg cgc tgg ctc agt gaa aac atc
gcc cac ttt ggg ggc gac ccc 768Ala Leu Arg Trp Leu Ser Glu Asn Ile
Ala His Phe Gly Gly Asp Pro 245 250 255 gag cgt atc acc atc ttt ggt
tcc ggg gca ggg gcc tcc tgc gtc aac 816Glu Arg Ile Thr Ile Phe Gly
Ser Gly Ala Gly Ala Ser Cys Val Asn 260 265 270 ctt ctg atc ctc tcc
cac cat tca gaa ggg ctg ttc cag aag gcc atc 864Leu Leu Ile Leu Ser
His His Ser Glu Gly Leu Phe Gln Lys Ala Ile 275 280 285 gcc cag agt
ggc acc gcc att tcc agc tgg tct gtc aac tac cag ccg 912Ala Gln Ser
Gly Thr Ala Ile Ser Ser Trp Ser Val Asn Tyr Gln Pro 290 295 300 ctc
aag tac acg cgg ctg ctg gca gcc aag gtg ggc tgt gac cga gag 960Leu
Lys Tyr Thr Arg Leu Leu Ala Ala Lys Val Gly Cys Asp Arg Glu 305 310
315 320 gac agc gct gaa gct gtg gag tgt ctg cgc cgg aag ccc tcc cgg
gag 1008Asp Ser Ala Glu Ala Val Glu Cys Leu Arg Arg Lys Pro Ser Arg
Glu 325 330 335 ctg gtg gac cag gac gtg cag cct gcc cgc tac cac atc
gcc ttt ggg 1056Leu Val Asp Gln Asp Val Gln Pro Ala Arg Tyr His Ile
Ala Phe Gly 340 345 350 ccc gtg gtg gat ggc gac gtg gtc ccc gat gac
cct gag atc ctc atg 1104Pro Val Val Asp Gly Asp Val Val Pro Asp Asp
Pro Glu Ile Leu Met 355 360 365 cag cag gga gaa ttc ctc aac tac gac
atg ctc atc ggc gtc aac cag 1152Gln Gln Gly Glu Phe Leu Asn Tyr Asp
Met Leu Ile Gly Val Asn Gln 370 375 380 gga gag ggc ctc aag ttc gtg
gag gac tct gca gag agc gag gac ggt 1200Gly Glu Gly Leu Lys Phe Val
Glu Asp Ser Ala Glu Ser Glu Asp Gly 385 390 395 400 gtg tct gcc agc
gcc ttt gac ttc act gtc tcc aac ttt gtg gac aac 1248Val Ser Ala Ser
Ala Phe Asp Phe Thr Val Ser Asn Phe Val Asp Asn 405 410 415 ctg tat
ggc tac ccg gaa ggc aag gat gtg ctt cgg gag acc atc aag 1296Leu Tyr
Gly Tyr Pro Glu Gly Lys Asp Val Leu Arg Glu Thr Ile Lys 420 425 430
ttt atg tac aca gac tgg gcc gac cgg gac aat ggc gaa atg cgc cgc
1344Phe Met Tyr Thr Asp Trp Ala Asp Arg Asp Asn Gly Glu Met Arg Arg
435 440 445 aaa acc ctg ctg gcg ctc ttt act gac cac caa tgg gtg gca
cca gct 1392Lys Thr Leu Leu Ala Leu Phe Thr Asp His Gln Trp Val Ala
Pro Ala 450 455 460 gtg gcc act gcc aag ctg cac gcc gac tac cag tct
ccc gtc tac ttt 1440Val Ala Thr Ala Lys Leu His Ala Asp Tyr Gln Ser
Pro Val Tyr Phe 465 470 475 480 tac acc ttc tac cac cac tgc cag gcg
gag ggc cgg cct gag tgg gca 1488Tyr Thr Phe Tyr His His Cys Gln Ala
Glu Gly Arg Pro Glu Trp Ala 485 490 495 gat gcg gcg cac ggg gat gaa
ctg ccc tat gtc ttt ggc gtg ccc atg 1536Asp Ala Ala His Gly Asp Glu
Leu Pro Tyr Val Phe Gly Val Pro Met 500 505 510 gtg ggt gcc acc gac
ctc ttc ccc tgt aac ttc tcc aag aat gac gtc 1584Val Gly Ala Thr Asp
Leu Phe Pro Cys Asn Phe Ser Lys Asn Asp Val 515 520 525 atg ctc agt
gcc gtg gtc atg acc tac tgg acc aac ttc gcc aag act 1632Met Leu Ser
Ala Val Val Met Thr Tyr Trp Thr Asn Phe Ala Lys Thr 530 535 540 ggg
gac ccc aac cag ccg gtg ccg cag gat acc aag ttc atc cac acc 1680Gly
Asp Pro Asn Gln Pro Val Pro Gln Asp Thr Lys Phe Ile His Thr 545 550
555 560 aag ccc aat cgc ttc gag gag gtg gtg tgg agc aaa ttc aac agc
aag 1728Lys Pro Asn Arg Phe Glu Glu Val Val Trp Ser Lys Phe Asn Ser
Lys 565 570 575 gag aag cag tat ctg cac ata ggc ctg aag cca cgc gtg
cgt gac aac 1776Glu Lys Gln Tyr Leu His Ile Gly Leu Lys Pro Arg Val
Arg Asp Asn 580 585 590 tac cgc gcc aac aag gtg gcc ttc tgg ctg gag
ctc gtg ccc cac ctg 1824Tyr Arg Ala Asn Lys Val Ala Phe Trp Leu Glu
Leu Val Pro His Leu 595 600 605 cac aac ctg cac acg gag ctc ttc acc
acc acc acg cgc ctg cct ccc 1872His Asn Leu His Thr Glu Leu Phe Thr
Thr Thr Thr Arg Leu Pro Pro 610 615 620 tac gcc acg cgc tgg ccg cct
cgt ccc ccc gct ggc gcc ccg ggc aca 1920Tyr Ala Thr Arg Trp Pro Pro
Arg Pro Pro Ala Gly Ala Pro Gly Thr 625 630 635 640 cgc cgg ccc ccg
ccg cct gcc acc ctg cct ccc gag ccc gag ccc gag 1968Arg Arg Pro Pro
Pro Pro Ala Thr Leu Pro Pro Glu Pro Glu Pro Glu 645 650 655 ccc ggc
cca agg gcc tat gac cgc ttc ccc ggg gac tca cgg gac tac 2016Pro Gly
Pro Arg Ala Tyr Asp Arg Phe Pro Gly Asp Ser Arg Asp Tyr 660 665 670
tcc acg gag ctg agc gtc acc gtg gcc gtg ggt gcc tcc ctc ctc ttc
2064Ser Thr Glu Leu Ser Val Thr Val Ala Val Gly Ala Ser Leu Leu Phe
675 680 685 ctc aac atc ctg gcc ttt gct gcc ctc tac tac aag cgg gac
cgg cgg 2112Leu Asn Ile Leu Ala Phe Ala Ala Leu Tyr Tyr Lys Arg Asp
Arg Arg 690 695 700 cag gag ctg cgg tgc agg cgg ctt agc cca cct ggc
ggc tca ggc tct 2160Gln Glu Leu Arg Cys Arg Arg Leu Ser Pro Pro Gly
Gly Ser Gly Ser 705 710 715 720 ggc gtg cct ggt ggg ggc ccc ctg ctc
ccc gcc gcg ggc cgt gag ctg 2208Gly Val Pro Gly Gly Gly Pro Leu Leu
Pro Ala Ala Gly Arg Glu Leu 725 730 735 cca cca gag gag gag ctg gtg
tca ctg cag ctg aag cgg ggt ggt ggc 2256Pro Pro Glu Glu Glu Leu Val
Ser Leu Gln Leu Lys Arg Gly Gly Gly 740 745 750 gtc ggg gcg gac cct
gcc gag gct ctg cgc cct gcc tgc ccg ccc gac 2304Val Gly Ala Asp Pro
Ala Glu Ala Leu Arg Pro Ala Cys Pro Pro Asp 755 760 765 tac acc ctg
gcc ctg cgc cgg gca ccg gac gat gtg cct ctc ttg gcc 2352Tyr Thr Leu
Ala Leu Arg Arg Ala Pro Asp Asp Val Pro Leu Leu Ala 770 775 780 ccc
ggg gcc ctg acc ctg ctg ccc agt ggc ctg ggg cca ccg cca ccc 2400Pro
Gly Ala Leu Thr Leu Leu Pro Ser Gly Leu Gly Pro Pro Pro Pro 785 790
795 800 cca ccg ccc ccc tcc ctt cat ccc ttc ggg ccc ttc ccc ccg ccc
cct 2448Pro Pro Pro Pro Ser Leu His Pro Phe Gly Pro Phe Pro Pro Pro
Pro 805 810 815 ccc acc gcc acc agc cac aac aac acg cta ccc cac ccc
cac tcc acc 2496Pro Thr Ala Thr Ser His Asn Asn Thr Leu Pro His Pro
His Ser Thr 820 825 830 act cgg gta 2505Thr Arg Val 835 2835PRTHomo
sapiens 2Met Trp Leu Leu Ala Leu Cys Leu Val Gly Leu Ala Gly Ala
Gln Arg 1 5 10 15 Gly Gly Gly Gly Pro Gly Gly Gly Ala Pro Gly Gly
Pro Gly Leu Gly 20 25 30 Leu Gly Ser Leu Gly Glu Glu Arg Phe Pro
Val Val Asn Thr Ala Tyr 35 40 45 Gly Arg Val Arg Gly Val Arg Arg
Glu Leu Asn Asn Glu Ile Leu Gly 50 55 60 Pro Val Val Gln Phe Leu
Gly Val Pro Tyr Ala Thr Pro Pro Leu Gly 65 70 75 80 Ala Arg Arg Phe
Gln Pro Pro Glu Ala Pro Ala Ser Trp Pro Gly Val 85 90 95 Arg Asn
Ala Thr Thr Leu Pro Pro Ala Cys Pro Gln Asn Leu His Gly 100 105 110
Ala Leu Pro Ala Ile Met Leu Pro Val Trp Phe Thr Asp Asn Leu Glu 115
120 125 Ala Ala Ala Thr Tyr Val Gln Asn Gln Ser Glu Asp Cys Leu Tyr
Leu 130 135 140 Asn Leu Tyr Val Pro Thr Glu Asp Gly Pro Leu Thr Lys
Lys Arg Asp 145 150 155 160 Glu Ala Thr Leu Asn Pro Pro Asp Thr Asp
Ile Arg Asp Pro Gly Lys 165 170 175 Lys Pro Val Met Leu Phe Leu His
Gly Gly Ser Tyr Met Glu Gly Thr 180 185 190 Gly Asn Met Phe Asp Gly
Ser Val Leu Ala Ala Tyr Gly Asn Val Ile 195 200 205 Val Ala Thr Leu
Asn Tyr Arg Leu Gly Val Leu Gly Phe Leu Ser Thr 210 215 220 Gly Asp
Gln Ala Ala Lys Gly Asn Tyr Gly Leu Leu Asp Gln Ile Gln 225 230 235
240 Ala Leu Arg Trp Leu Ser Glu Asn Ile Ala His Phe Gly Gly Asp Pro
245 250 255 Glu Arg Ile Thr Ile Phe Gly Ser Gly Ala Gly Ala Ser Cys
Val Asn 260 265 270 Leu Leu Ile Leu Ser His His Ser Glu Gly Leu Phe
Gln Lys Ala Ile 275 280 285 Ala Gln Ser Gly Thr Ala Ile Ser Ser Trp
Ser Val Asn Tyr Gln Pro 290 295 300 Leu Lys Tyr Thr Arg Leu Leu Ala
Ala Lys Val Gly Cys Asp Arg Glu 305 310 315 320 Asp Ser Ala Glu Ala
Val Glu Cys Leu Arg Arg Lys Pro Ser Arg Glu 325 330 335 Leu Val Asp
Gln Asp Val Gln Pro Ala Arg Tyr His Ile Ala Phe Gly 340 345 350 Pro
Val Val Asp Gly Asp Val Val Pro Asp Asp Pro Glu Ile Leu Met 355 360
365 Gln Gln Gly Glu Phe Leu Asn Tyr Asp Met Leu Ile Gly Val Asn Gln
370 375 380 Gly Glu Gly Leu Lys Phe Val Glu Asp Ser Ala Glu Ser Glu
Asp Gly 385 390 395 400 Val Ser Ala Ser Ala Phe Asp Phe Thr Val Ser
Asn Phe Val Asp Asn 405 410 415 Leu Tyr Gly Tyr Pro Glu Gly Lys Asp
Val Leu Arg Glu Thr Ile Lys 420 425 430 Phe Met Tyr Thr Asp Trp Ala
Asp Arg Asp Asn Gly Glu Met Arg Arg 435 440 445 Lys Thr Leu Leu Ala
Leu Phe Thr Asp His Gln Trp Val Ala Pro Ala 450 455 460 Val Ala Thr
Ala Lys Leu His Ala Asp Tyr Gln Ser Pro Val Tyr Phe 465 470 475 480
Tyr Thr Phe Tyr His His Cys Gln Ala Glu Gly Arg Pro Glu Trp Ala 485
490 495 Asp Ala Ala His Gly Asp Glu Leu Pro Tyr Val Phe Gly Val Pro
Met 500 505 510 Val Gly Ala Thr Asp Leu Phe Pro Cys Asn Phe Ser Lys
Asn Asp Val 515 520 525 Met Leu Ser Ala Val Val Met Thr Tyr Trp Thr
Asn Phe Ala Lys Thr 530 535 540 Gly Asp Pro Asn Gln Pro Val Pro Gln
Asp Thr Lys Phe Ile His Thr 545 550 555 560 Lys Pro Asn Arg Phe Glu
Glu Val Val Trp Ser Lys Phe Asn Ser Lys 565 570 575 Glu Lys Gln Tyr
Leu His Ile Gly Leu Lys Pro Arg Val Arg Asp Asn 580 585 590 Tyr Arg
Ala Asn Lys Val Ala Phe Trp Leu Glu Leu Val Pro His Leu 595 600 605
His Asn Leu His Thr Glu Leu Phe Thr Thr Thr Thr Arg Leu Pro Pro 610
615 620 Tyr Ala Thr Arg Trp Pro Pro Arg Pro Pro Ala Gly Ala Pro Gly
Thr 625 630 635 640 Arg Arg Pro Pro Pro Pro Ala Thr Leu Pro Pro Glu
Pro Glu Pro Glu 645 650 655 Pro Gly Pro Arg Ala Tyr Asp Arg Phe Pro
Gly Asp Ser Arg Asp Tyr 660 665 670 Ser Thr Glu Leu Ser Val Thr Val
Ala Val Gly Ala Ser Leu Leu Phe 675 680 685 Leu Asn Ile Leu Ala Phe
Ala Ala Leu Tyr Tyr Lys Arg Asp Arg Arg 690 695 700 Gln Glu Leu Arg
Cys Arg Arg Leu Ser Pro Pro Gly Gly Ser Gly Ser 705 710 715 720 Gly
Val Pro Gly Gly Gly Pro Leu Leu Pro Ala Ala Gly Arg Glu Leu 725 730
735 Pro Pro Glu Glu Glu Leu Val Ser Leu Gln Leu Lys Arg Gly Gly Gly
740 745 750 Val Gly Ala Asp Pro Ala Glu Ala Leu Arg Pro Ala Cys Pro
Pro Asp 755 760 765 Tyr Thr Leu Ala Leu Arg Arg Ala Pro Asp Asp Val
Pro Leu Leu Ala 770 775 780 Pro Gly Ala Leu Thr Leu Leu Pro Ser Gly
Leu Gly Pro Pro Pro Pro 785 790 795 800 Pro Pro Pro Pro Ser Leu His
Pro Phe Gly Pro Phe Pro Pro Pro Pro 805 810 815 Pro Thr Ala Thr Ser
His Asn Asn Thr Leu Pro His Pro His Ser Thr 820 825 830 Thr Arg Val
835 32508DNAMus musculusCDS(1)..(2508) 3atg tgg ctc ctg gcg ttg
tgt ctg gtg ggg ctg gct ggg gct caa cgg 48Met Trp Leu Leu Ala Leu
Cys Leu Val Gly Leu Ala Gly Ala Gln Arg 1 5 10 15 gga gga ggg ggt
ccc ggc ggc ggc gcc ccg ggc ggc cca ggc ctg ggc 96Gly Gly Gly Gly
Pro Gly Gly Gly Ala Pro Gly Gly Pro Gly Leu Gly 20 25 30 ctc ggc
agc ctc ggg gag gag cgc ttc ccc gtg gtg aac aca gcc tac 144Leu Gly
Ser Leu Gly Glu Glu Arg Phe Pro Val Val Asn Thr Ala Tyr 35 40 45
ggg cga gtg cgc ggt gtg cgg cgc gag ctc aac aac gag atc ctg ggc
192Gly Arg Val Arg Gly Val Arg Arg Glu Leu Asn Asn Glu Ile Leu Gly
50 55 60 ccg gtc gtg cag ttc ttg ggc gtg ccc tac gcc acg ccg ccc
ttg ggc 240Pro Val Val Gln Phe Leu Gly Val Pro Tyr Ala Thr Pro Pro
Leu Gly 65 70 75 80 gcc cgc cgc ttc cag ccg cct gag gca cct gcc tcg
tgg ccc ggc gtg 288Ala Arg Arg Phe Gln Pro Pro Glu Ala Pro Ala Ser
Trp Pro Gly Val 85 90 95 cgc aac gcc acc acc ctg ccg ccc gcc tgc
ccg cag aac ctg cac ggg 336Arg Asn Ala Thr Thr Leu Pro Pro Ala Cys
Pro Gln Asn Leu His Gly 100 105 110 gcc ctg ccg gcc atc atg ctg cct
gtg tgg ttc acc gac aac ttg gag 384Ala Leu Pro Ala Ile Met Leu Pro
Val Trp Phe Thr Asp Asn Leu Glu 115 120 125 gcg gcc gcc acc tac gtg
cag aac cag agc gag gac tgc ctg tac ctc 432Ala Ala Ala Thr Tyr Val
Gln Asn Gln Ser Glu Asp Cys Leu Tyr Leu 130 135 140 aac ctc tac gtg
ccc act gag gac ggt ccg ctc aca aaa aaa cgt gac 480Asn Leu Tyr Val
Pro Thr Glu Asp Gly Pro Leu Thr Lys Lys Arg Asp 145 150 155 160 gag
gcg acg ctc aat ccg cca gac aca gat atc cgg gac tct ggg aag 528Glu
Ala Thr Leu Asn Pro Pro Asp Thr Asp Ile Arg Asp Ser Gly Lys 165 170
175 aaa ccg gtc atg ctg ttt cta cac ggc ggc tcc tac atg gaa ggc acc
576Lys Pro Val Met Leu Phe Leu His Gly Gly Ser Tyr Met Glu Gly Thr
180 185 190 ggg aac atg ttt gac ggc tca gtc ctg gct gcc tat ggc aat
gtc atc 624Gly Asn Met Phe Asp Gly Ser Val Leu Ala Ala Tyr Gly Asn
Val Ile 195 200 205 gta gtc aca ctc aac tac cgt ctt ggg gtg ctc ggt
ttt ctc agc act 672Val Val Thr Leu Asn Tyr Arg Leu Gly Val Leu Gly
Phe Leu Ser Thr 210 215 220 ggt gac cag gct gca aaa ggc aac tat ggg
ctc ctg gac cag atc cag 720Gly Asp Gln Ala Ala Lys Gly Asn Tyr Gly
Leu Leu Asp Gln Ile Gln 225 230 235 240 gcc ctg cgc tgg ctc agt gaa
aac att gcc cac ttt gga ggt gac cct 768Ala Leu Arg Trp Leu Ser Glu
Asn Ile Ala His Phe Gly Gly Asp Pro 245 250 255 gaa cgc atc act atc
ttt ggg tct ggt gca ggg gcc tcc tgt gtc aac 816Glu Arg Ile Thr Ile
Phe Gly Ser Gly Ala Gly Ala Ser Cys Val Asn 260 265 270 ttg ctg atc
ctt tcc cac cac tca gaa gga ctg ttc cag aag gcc att 864Leu Leu Ile
Leu Ser His His Ser Glu Gly Leu Phe Gln Lys Ala Ile 275 280 285 gct
caa agt ggt act gcc att tcc agc tgg tct gtc aac tac cag ccg 912Ala
Gln Ser Gly Thr Ala Ile Ser Ser Trp Ser Val Asn Tyr Gln Pro 290 295
300 ctc aag tac acg cgg ctg ctg gcg gcc aaa gtg ggc tgt gac cga gaa
960Leu Lys Tyr Thr Arg Leu Leu Ala Ala Lys Val Gly Cys Asp Arg Glu
305 310 315 320 gac agc act gaa gct gtg gag tgt ctg cgc cgg aag tct
tcc cgg gag 1008Asp Ser Thr Glu Ala Val Glu Cys Leu Arg Arg Lys Ser
Ser Arg Glu 325 330 335 cta gtg gac cag gat gta cag cct gcc cgc tac
cac att gcc ttt ggg 1056Leu Val Asp Gln Asp Val Gln Pro Ala Arg Tyr
His Ile Ala Phe Gly 340 345 350 cct gtg gtg gac ggc gac gta gtc cct
gat gac ccc gag atc ctc atg 1104Pro Val Val Asp Gly Asp Val Val Pro
Asp Asp Pro Glu Ile Leu Met 355 360 365 caa cag ggg gaa ttc ctc aac
tac gac atg ctc att ggt gtc aac cag 1152Gln Gln Gly Glu Phe Leu Asn
Tyr Asp Met Leu Ile Gly Val Asn Gln 370 375 380 gga gag ggt ctc aag
ttc gtg gag gac tct gca gag agt gag gac ggt 1200Gly Glu Gly Leu Lys
Phe Val Glu Asp Ser Ala Glu Ser Glu Asp Gly 385 390 395 400 gtg tct
gcc agc gcc ttt gac ttc acc gtc tcc aac ttt gtg gac aac 1248Val Ser
Ala Ser Ala Phe Asp Phe Thr Val Ser Asn Phe Val Asp Asn 405 410 415
ttg tac ggg tac cca gaa ggc aag gac gtg ctt cga gag acc atc aag
1296Leu Tyr Gly Tyr Pro Glu Gly Lys Asp Val Leu Arg Glu Thr Ile Lys
420 425 430 ttc atg tac acg gac tgg gct gac agg gac aat ggc gag atg
cgg cgg 1344Phe Met Tyr Thr Asp Trp Ala Asp Arg Asp Asn Gly Glu Met
Arg Arg 435 440 445 aag acc ctg ctg gcg ctc ttt acc gac cac cag tgg
gtc gcc ccg gct 1392Lys Thr Leu Leu Ala Leu Phe Thr Asp His Gln Trp
Val Ala Pro Ala 450 455 460 gtg gcc acc gcc aag ctg cat gcc gac tac
cag tcc ccc gtc tac ttt 1440Val Ala Thr Ala Lys Leu His Ala Asp Tyr
Gln Ser Pro Val Tyr Phe 465 470 475 480 tac act ttc tac cac cac tgc
cag gca gag ggc cgg cca gag tgg gca 1488Tyr Thr Phe Tyr His His Cys
Gln Ala Glu Gly Arg Pro Glu Trp Ala 485 490 495 gac gca gcg cac ggg
gac gag ctg ccc tac gtc ttt ggt gtc ccc atg 1536Asp Ala Ala His Gly
Asp Glu Leu Pro Tyr Val Phe Gly Val Pro Met 500 505 510 gtg ggc gcc
act gac ctc ttc ccc tgc aac ttc tcc aag aac gat gtc 1584Val Gly Ala
Thr Asp Leu Phe Pro Cys Asn Phe Ser Lys Asn Asp Val 515 520 525 atg
ctc agc gca gta gtc atg acc tat tgg acc aac ttc gcc aag act 1632Met
Leu Ser Ala Val Val Met Thr Tyr Trp Thr Asn Phe Ala Lys Thr 530 535
540 ggt gac ccc aac cag cct gtg cca cag gac acc aag ttc atc cac acc
1680Gly Asp Pro Asn Gln Pro Val Pro Gln Asp Thr Lys Phe Ile His Thr
545 550 555 560 aag ccc aac cgc ttt gaa gag gta gtg tgg agc aag ttc
aac agc aag 1728Lys Pro Asn Arg Phe Glu Glu Val Val Trp Ser Lys Phe
Asn Ser Lys 565 570 575 gaa aag cag tat ctg cac ata ggc ttg aaa cca
cgc gtg cgc gac aac 1776Glu Lys Gln Tyr Leu His Ile Gly Leu Lys Pro
Arg Val Arg Asp Asn 580 585 590 tac cgt gcc aac aag gtg gcc ttc tgg
ctg gag ctc gtg ccc cac ctg 1824Tyr Arg Ala Asn Lys Val Ala Phe Trp
Leu Glu Leu Val Pro His Leu 595 600 605 cac aac ctg cac aca gag ctc
ttc acc acc acc act cgc ctg cct ccc 1872His Asn Leu His Thr Glu Leu
Phe Thr Thr Thr Thr Arg Leu Pro Pro 610 615 620 tat gcc aca cgc tgg
cca cct cgc aca cct ggt cct ggc act tcc ggc 1920Tyr Ala Thr Arg Trp
Pro Pro Arg Thr Pro Gly Pro Gly Thr Ser Gly 625 630 635 640 aca cgc
cgt cct ccc cca cct gcc act ctg cca cct gag tct gat att 1968Thr Arg
Arg Pro Pro Pro Pro Ala Thr Leu Pro Pro Glu Ser Asp Ile 645 650 655
gac cta ggc cca agg gcc tat gac cgc ttc ccc ggt gac tcg agg gac
2016Asp Leu Gly Pro Arg Ala Tyr Asp Arg Phe Pro Gly Asp Ser Arg Asp
660 665 670 tac tcc acg gag cta agc gtg act gtg gca gtg ggt gcc tcc
ctc ctc 2064Tyr Ser Thr Glu Leu Ser Val Thr Val Ala Val Gly Ala Ser
Leu Leu 675 680 685 ttc ctc aac atc ctt gcc ttt gcc gcc ctc tat tac
aag cgg gac cgg 2112Phe Leu Asn Ile Leu Ala Phe Ala Ala Leu Tyr Tyr
Lys Arg Asp Arg 690 695 700 cgc cag gag ctg cgg tgc cgg agg ctt agc
cca cca gga ggc tca ggc 2160Arg Gln Glu Leu Arg Cys Arg Arg Leu Ser
Pro Pro Gly Gly Ser Gly 705 710 715 720 tca ggt gtg cct ggt ggg ggc
ccc ctg ctt ccc act gct ggc cgt gag 2208Ser Gly Val Pro Gly Gly Gly
Pro Leu Leu Pro Thr Ala Gly Arg Glu 725 730 735 cta ccc ccg gag gag
gag cta gta tcg ctg cag ctg aag cgg ggt ggt 2256Leu Pro Pro Glu Glu
Glu Leu Val Ser Leu Gln Leu Lys Arg Gly Gly 740 745 750 ggt gtt ggg
gcg gac cct gct gag gcc ctg cgc cct gcc tgt cca ccc 2304Gly Val Gly
Ala Asp Pro Ala Glu Ala Leu Arg Pro Ala Cys Pro Pro 755 760 765 gac
tat acc ctg gcc ttg cgc cgg gca ccg gac gat gtg cct ctc ttg 2352Asp
Tyr Thr Leu Ala Leu Arg Arg Ala Pro Asp Asp Val Pro Leu Leu 770 775
780 gcc ccc ggg gcc cta acc ctg ctg cct agt ggc ctg ggg ccc ccg ccc
2400Ala Pro Gly Ala Leu Thr Leu Leu Pro Ser Gly Leu Gly Pro Pro Pro
785 790 795 800 ccg ccc cca ccc cct tct ctc cat ccc ttt ggg ccc ttc
cca ccc cca 2448Pro Pro Pro Pro Pro Ser Leu His Pro Phe Gly Pro Phe
Pro Pro Pro 805 810 815 ccc cct act gct acc agc cac aac aac acg cta
ccc cat ccc cac tcc 2496Pro Pro Thr Ala Thr Ser His Asn Asn Thr Leu
Pro His Pro His Ser 820 825 830 acc act cgg gta 2508Thr Thr Arg Val
835 4836PRTMus musculus 4Met Trp Leu Leu Ala Leu Cys Leu Val Gly
Leu Ala Gly Ala Gln Arg 1 5 10 15 Gly Gly Gly Gly Pro Gly Gly Gly
Ala Pro Gly Gly Pro Gly Leu Gly 20 25 30 Leu Gly Ser Leu Gly Glu
Glu Arg Phe Pro Val Val Asn Thr Ala Tyr 35 40 45 Gly Arg Val Arg
Gly Val Arg Arg Glu Leu Asn Asn Glu Ile Leu Gly 50 55 60 Pro Val
Val Gln Phe Leu Gly Val Pro Tyr Ala Thr Pro Pro Leu Gly 65 70 75 80
Ala Arg Arg Phe Gln Pro Pro Glu Ala Pro Ala Ser Trp Pro Gly Val 85
90 95 Arg Asn Ala Thr Thr Leu Pro Pro Ala Cys Pro Gln Asn Leu His
Gly 100 105 110 Ala Leu Pro Ala Ile Met Leu Pro Val Trp Phe Thr Asp
Asn Leu Glu 115 120 125 Ala Ala Ala Thr Tyr Val Gln Asn Gln Ser Glu
Asp Cys Leu Tyr Leu 130 135 140 Asn Leu Tyr Val Pro Thr Glu Asp Gly
Pro Leu Thr Lys Lys Arg Asp 145 150 155 160 Glu Ala Thr Leu Asn Pro
Pro Asp Thr Asp Ile Arg Asp Ser Gly Lys 165 170 175 Lys Pro Val Met
Leu Phe Leu His Gly Gly Ser Tyr Met Glu Gly Thr 180 185 190 Gly Asn
Met Phe Asp Gly Ser Val Leu Ala Ala Tyr Gly Asn Val Ile 195 200 205
Val Val Thr Leu Asn Tyr Arg Leu Gly Val Leu Gly Phe Leu Ser Thr 210
215 220 Gly Asp Gln Ala Ala Lys Gly Asn Tyr Gly Leu Leu Asp Gln Ile
Gln 225 230 235 240 Ala Leu Arg Trp Leu Ser Glu Asn Ile Ala His Phe
Gly Gly Asp Pro 245 250 255 Glu Arg Ile Thr Ile Phe Gly Ser Gly Ala
Gly Ala Ser Cys Val Asn 260 265 270 Leu Leu Ile Leu Ser His His Ser
Glu Gly Leu Phe Gln Lys Ala Ile 275 280 285 Ala Gln Ser Gly Thr Ala
Ile Ser Ser Trp Ser Val Asn Tyr Gln Pro 290 295 300 Leu Lys Tyr Thr
Arg Leu Leu Ala Ala Lys Val Gly Cys Asp Arg Glu 305 310 315 320 Asp
Ser Thr Glu Ala Val Glu Cys Leu Arg Arg Lys Ser Ser Arg Glu 325 330
335 Leu Val Asp Gln Asp Val Gln Pro Ala Arg Tyr His Ile Ala Phe Gly
340 345 350 Pro Val Val Asp Gly Asp Val Val Pro Asp Asp Pro Glu Ile
Leu Met 355 360 365 Gln Gln Gly Glu Phe Leu Asn Tyr Asp Met Leu Ile
Gly Val Asn Gln 370 375 380 Gly Glu Gly Leu Lys Phe Val Glu Asp Ser
Ala Glu Ser Glu Asp Gly 385 390 395 400 Val Ser Ala Ser Ala Phe Asp
Phe Thr Val Ser Asn Phe Val Asp Asn 405 410 415 Leu Tyr Gly Tyr Pro
Glu Gly Lys Asp Val Leu Arg Glu Thr Ile Lys 420 425 430 Phe Met Tyr
Thr Asp Trp Ala Asp Arg Asp Asn Gly Glu Met Arg Arg 435 440 445 Lys
Thr Leu Leu Ala Leu Phe Thr Asp His Gln Trp Val Ala Pro Ala 450 455
460 Val Ala Thr Ala Lys Leu His Ala Asp Tyr Gln Ser Pro Val Tyr Phe
465 470 475 480 Tyr Thr Phe Tyr His His Cys Gln Ala Glu Gly Arg Pro
Glu Trp Ala 485 490 495 Asp Ala Ala His Gly Asp Glu Leu Pro Tyr Val
Phe Gly Val Pro Met 500 505 510 Val Gly Ala Thr Asp Leu Phe Pro Cys
Asn Phe Ser Lys Asn Asp Val 515 520 525 Met Leu Ser Ala Val Val Met
Thr Tyr Trp Thr Asn Phe Ala Lys Thr 530 535 540 Gly Asp Pro Asn Gln
Pro Val Pro Gln Asp Thr Lys Phe Ile His Thr 545 550 555 560 Lys Pro
Asn Arg Phe Glu Glu Val Val Trp Ser Lys Phe Asn Ser Lys 565 570 575
Glu Lys Gln Tyr Leu His Ile Gly Leu Lys Pro Arg Val Arg Asp Asn 580
585 590 Tyr Arg Ala Asn Lys Val Ala Phe Trp Leu Glu Leu Val Pro His
Leu 595 600 605 His Asn Leu His Thr Glu Leu Phe Thr Thr Thr Thr Arg
Leu Pro Pro 610 615 620 Tyr Ala Thr Arg Trp Pro Pro Arg Thr Pro Gly
Pro Gly Thr Ser Gly 625 630 635 640 Thr Arg Arg Pro Pro Pro Pro Ala
Thr Leu Pro Pro Glu Ser Asp Ile 645 650 655 Asp Leu Gly Pro Arg Ala
Tyr Asp Arg Phe Pro Gly Asp Ser Arg Asp 660 665 670 Tyr Ser Thr Glu
Leu Ser Val Thr Val Ala Val Gly Ala Ser Leu Leu 675 680 685 Phe Leu
Asn Ile Leu Ala Phe Ala Ala Leu Tyr Tyr Lys Arg Asp Arg 690 695 700
Arg Gln Glu Leu Arg Cys Arg Arg Leu Ser Pro Pro Gly Gly Ser Gly 705
710 715 720 Ser Gly Val Pro Gly Gly Gly Pro Leu Leu Pro Thr Ala Gly
Arg Glu 725 730 735 Leu Pro Pro Glu Glu Glu Leu Val Ser Leu Gln Leu
Lys Arg Gly Gly 740 745 750 Gly Val Gly Ala Asp Pro Ala Glu Ala Leu
Arg Pro Ala Cys Pro Pro 755 760 765 Asp Tyr Thr Leu Ala Leu Arg Arg
Ala Pro Asp Asp Val Pro Leu Leu 770 775 780 Ala Pro Gly Ala Leu Thr
Leu Leu Pro Ser Gly Leu Gly Pro Pro Pro 785 790 795 800 Pro Pro Pro
Pro Pro Ser Leu His Pro Phe Gly Pro Phe Pro Pro Pro 805 810 815 Pro
Pro Thr Ala Thr Ser His Asn Asn Thr Leu Pro His Pro His Ser 820 825
830 Thr Thr Arg Val 835 52508DNARattus norvegicusCDS(1)..(2508)
5atg tgg ctc ctg gcg ttg tgt ctg gtg ggg ctg gct ggg gct caa cgg
48Met Trp Leu
Leu Ala Leu Cys Leu Val Gly Leu Ala Gly Ala Gln Arg 1 5 10 15 gga
gga ggg ggt ccc ggc ggc ggc gcc ccg ggc ggc cca ggc ctg ggc 96Gly
Gly Gly Gly Pro Gly Gly Gly Ala Pro Gly Gly Pro Gly Leu Gly 20 25
30 ctc ggc agc ctc ggg gag gag cgc ttc ccg gtg gtg aac aca gcc tac
144Leu Gly Ser Leu Gly Glu Glu Arg Phe Pro Val Val Asn Thr Ala Tyr
35 40 45 ggg cga gtg cgc ggt gtg cgg cgc gag ctc aac aac gag atc
ctg ggc 192Gly Arg Val Arg Gly Val Arg Arg Glu Leu Asn Asn Glu Ile
Leu Gly 50 55 60 ccg gtc gtg cag ttc ttg ggc gtg ccc tac gcc acg
ccg ccc ttg ggc 240Pro Val Val Gln Phe Leu Gly Val Pro Tyr Ala Thr
Pro Pro Leu Gly 65 70 75 80 gcc cgc cgc ttc cag ccg cct gag gca cct
gcc tcg tgg ccc ggc gtg 288Ala Arg Arg Phe Gln Pro Pro Glu Ala Pro
Ala Ser Trp Pro Gly Val 85 90 95 cgc aac gcc acc acc ctg ccg ccc
gcc tgc ccg cag aac ctg cac ggg 336Arg Asn Ala Thr Thr Leu Pro Pro
Ala Cys Pro Gln Asn Leu His Gly 100 105 110 gcc ctg ccg gcc atc atg
ctg cct gtg tgg ttc acc gac aac ttg gag 384Ala Leu Pro Ala Ile Met
Leu Pro Val Trp Phe Thr Asp Asn Leu Glu 115 120 125 gcg gcc gcc acc
tac gtg cag aac cag agc gag gac tgc ctg tac ctc 432Ala Ala Ala Thr
Tyr Val Gln Asn Gln Ser Glu Asp Cys Leu Tyr Leu 130 135 140 aac ctc
tac gtg ccc act gag gac ggt ccg ctc aca aaa aaa cgt gac 480Asn Leu
Tyr Val Pro Thr Glu Asp Gly Pro Leu Thr Lys Lys Arg Asp 145 150 155
160 gag gcg acg ctc aat ccg cca gac aca gat atc cgg gac tct ggg aag
528Glu Ala Thr Leu Asn Pro Pro Asp Thr Asp Ile Arg Asp Ser Gly Lys
165 170 175 aaa cca gtc atg ctg ttt cta cac ggc ggc tcc tac atg gag
ggc acc 576Lys Pro Val Met Leu Phe Leu His Gly Gly Ser Tyr Met Glu
Gly Thr 180 185 190 ggg aac atg ttc gac ggc tca gtc ctg gct gcc tat
ggc aat gtc atc 624Gly Asn Met Phe Asp Gly Ser Val Leu Ala Ala Tyr
Gly Asn Val Ile 195 200 205 gta gcc aca ctc aac tac cgt ctt ggg gtg
ctc ggc ttt ctc agc act 672Val Ala Thr Leu Asn Tyr Arg Leu Gly Val
Leu Gly Phe Leu Ser Thr 210 215 220 ggt gac cag gct gca aaa ggc aac
tac ggg ctc ctg gac cag atc cag 720Gly Asp Gln Ala Ala Lys Gly Asn
Tyr Gly Leu Leu Asp Gln Ile Gln 225 230 235 240 gcc ctg cgc tgg ctc
agt gaa aac att gcc cac ttt ggc ggt gac cct 768Ala Leu Arg Trp Leu
Ser Glu Asn Ile Ala His Phe Gly Gly Asp Pro 245 250 255 gaa cgc atc
acc atc ttt ggg tct ggt gcg ggg gcc tcc tgt gtc aac 816Glu Arg Ile
Thr Ile Phe Gly Ser Gly Ala Gly Ala Ser Cys Val Asn 260 265 270 ttg
ctg atc ctc tcc cac cat tca gaa ggg ctg ttc cag aag gcc att 864Leu
Leu Ile Leu Ser His His Ser Glu Gly Leu Phe Gln Lys Ala Ile 275 280
285 gct cag agt ggc act gcc att tcc agc tgg tct gtc aac tac cag ccg
912Ala Gln Ser Gly Thr Ala Ile Ser Ser Trp Ser Val Asn Tyr Gln Pro
290 295 300 ctc aag tac acg cgg ctg ctg gca gcc aaa gtg ggc tgt gac
cga gag 960Leu Lys Tyr Thr Arg Leu Leu Ala Ala Lys Val Gly Cys Asp
Arg Glu 305 310 315 320 gac agc acg gaa gct gtg gaa tgt cta cgc cgg
aag tct tcc cgg gag 1008Asp Ser Thr Glu Ala Val Glu Cys Leu Arg Arg
Lys Ser Ser Arg Glu 325 330 335 cta gta gac cag gat gtg cag cct gcc
cgc tac cac att gcc ttt ggg 1056Leu Val Asp Gln Asp Val Gln Pro Ala
Arg Tyr His Ile Ala Phe Gly 340 345 350 cct gtg gtg gac ggc gat gta
gtc cct gat gac cct gag atc ctc atg 1104Pro Val Val Asp Gly Asp Val
Val Pro Asp Asp Pro Glu Ile Leu Met 355 360 365 caa cag gga gaa ttc
ctc aac tac gac atg ctc att ggc gtc aac cag 1152Gln Gln Gly Glu Phe
Leu Asn Tyr Asp Met Leu Ile Gly Val Asn Gln 370 375 380 gga gag ggt
ctc aag ttc gtg gag gac tct gca gag agt gag gac ggg 1200Gly Glu Gly
Leu Lys Phe Val Glu Asp Ser Ala Glu Ser Glu Asp Gly 385 390 395 400
gtg tct gcc agc gcc ttt gac ttc act gtc tcc aac ttt gtg gac aac
1248Val Ser Ala Ser Ala Phe Asp Phe Thr Val Ser Asn Phe Val Asp Asn
405 410 415 ttg tat ggg tat cca gag ggc aag gat gtg ctt cga gag acc
atc aag 1296Leu Tyr Gly Tyr Pro Glu Gly Lys Asp Val Leu Arg Glu Thr
Ile Lys 420 425 430 ttc atg tac acg gac tgg gct gac cgg gac aat ggc
gag atg cgg cgt 1344Phe Met Tyr Thr Asp Trp Ala Asp Arg Asp Asn Gly
Glu Met Arg Arg 435 440 445 aag acc ctg ttg gca ctc ttt act gac cac
cag tgg gta gcc cca gct 1392Lys Thr Leu Leu Ala Leu Phe Thr Asp His
Gln Trp Val Ala Pro Ala 450 455 460 gtg gcc act gcc aag cta cat gct
gac tac cag tcc cct gtc tac ttt 1440Val Ala Thr Ala Lys Leu His Ala
Asp Tyr Gln Ser Pro Val Tyr Phe 465 470 475 480 tac act ttt tac cac
cac tgc cag gct gag ggc cgg cca gag tgg gca 1488Tyr Thr Phe Tyr His
His Cys Gln Ala Glu Gly Arg Pro Glu Trp Ala 485 490 495 gat gca gcg
cat ggg gat gag ctg ccc tac gtc ttt ggt gtg ccc atg 1536Asp Ala Ala
His Gly Asp Glu Leu Pro Tyr Val Phe Gly Val Pro Met 500 505 510 gtg
ggc gcc acc gac ctc ttc ccc tgc aac ttc tcc aag aat gat gtc 1584Val
Gly Ala Thr Asp Leu Phe Pro Cys Asn Phe Ser Lys Asn Asp Val 515 520
525 atg ctc agc gca gta gtc atg acc tac tgg acc aac ttc gcc aag act
1632Met Leu Ser Ala Val Val Met Thr Tyr Trp Thr Asn Phe Ala Lys Thr
530 535 540 ggt gac ccc aac cag cct gtg cca cag gac acc aag ttc atc
cac acc 1680Gly Asp Pro Asn Gln Pro Val Pro Gln Asp Thr Lys Phe Ile
His Thr 545 550 555 560 aag ccc aac cgc ttt gaa gag gtg gta tgg agc
aag ttc aac agc aag 1728Lys Pro Asn Arg Phe Glu Glu Val Val Trp Ser
Lys Phe Asn Ser Lys 565 570 575 gaa aag cag tac ctg cac ata ggc ttg
aaa cca cgc gtg cgt gac aac 1776Glu Lys Gln Tyr Leu His Ile Gly Leu
Lys Pro Arg Val Arg Asp Asn 580 585 590 tac cgt gcc aac aag gtg gcc
ttc tgg ctg gag ctc gtg ccc cac ctg 1824Tyr Arg Ala Asn Lys Val Ala
Phe Trp Leu Glu Leu Val Pro His Leu 595 600 605 cac aac ctg cac aca
gag ctc ttc acc acc acc act cgc ctg cct ccc 1872His Asn Leu His Thr
Glu Leu Phe Thr Thr Thr Thr Arg Leu Pro Pro 610 615 620 tac gcc aca
cgc tgg cca cct cgc aca ccg ggt cct ggc act tcc ggc 1920Tyr Ala Thr
Arg Trp Pro Pro Arg Thr Pro Gly Pro Gly Thr Ser Gly 625 630 635 640
aca cgc cgt cct ccc cca ccc gcc act ctg cca cct gag tct gat att
1968Thr Arg Arg Pro Pro Pro Pro Ala Thr Leu Pro Pro Glu Ser Asp Ile
645 650 655 gac ctg ggc cca agg gcc tat gac cgc ttc ccc ggt gac tcg
agg gac 2016Asp Leu Gly Pro Arg Ala Tyr Asp Arg Phe Pro Gly Asp Ser
Arg Asp 660 665 670 tac tcc acg gag cta agc gtg act gta gca gtg ggt
gcc tcc ctc ctc 2064Tyr Ser Thr Glu Leu Ser Val Thr Val Ala Val Gly
Ala Ser Leu Leu 675 680 685 ttc ctc aac atc ctt gcc ttt gcc gcc ctc
tac tac aag cgg gac cgg 2112Phe Leu Asn Ile Leu Ala Phe Ala Ala Leu
Tyr Tyr Lys Arg Asp Arg 690 695 700 cgc cag gag ctg cgg tgc agg cgg
ctt agc cca cca gga ggc tca ggc 2160Arg Gln Glu Leu Arg Cys Arg Arg
Leu Ser Pro Pro Gly Gly Ser Gly 705 710 715 720 tca ggt gtg cct ggt
ggg ggc ccc ttg ctt ccc act gct ggc cgt gag 2208Ser Gly Val Pro Gly
Gly Gly Pro Leu Leu Pro Thr Ala Gly Arg Glu 725 730 735 cta ccc cct
gag gag gag ctg gta tcg ctg cag ctg aag cgg ggt ggt 2256Leu Pro Pro
Glu Glu Glu Leu Val Ser Leu Gln Leu Lys Arg Gly Gly 740 745 750 ggc
gtt ggg gcg gac cct gct gag gcc ctg cgc cct gcc tgt cca ccc 2304Gly
Val Gly Ala Asp Pro Ala Glu Ala Leu Arg Pro Ala Cys Pro Pro 755 760
765 gac tat acc ctg gcc ttg cgc cgg gca ccg gac gat gtg cct ctc ttg
2352Asp Tyr Thr Leu Ala Leu Arg Arg Ala Pro Asp Asp Val Pro Leu Leu
770 775 780 gcc ccg ggg gcc ctg acc ctg ctg ccc agt ggc ctg ggg ccc
ccg ccc 2400Ala Pro Gly Ala Leu Thr Leu Leu Pro Ser Gly Leu Gly Pro
Pro Pro 785 790 795 800 cca ccc cca cct cct tct ctc cat ccc ttt ggg
ccc ttc cca cca cca 2448Pro Pro Pro Pro Pro Ser Leu His Pro Phe Gly
Pro Phe Pro Pro Pro 805 810 815 ccc cct act gct acc agc cac aac aac
acg cta ccc cat ccc cac tcc 2496Pro Pro Thr Ala Thr Ser His Asn Asn
Thr Leu Pro His Pro His Ser 820 825 830 acc act cgg gta 2508Thr Thr
Arg Val 835 6836PRTRattus norvegicus 6Met Trp Leu Leu Ala Leu Cys
Leu Val Gly Leu Ala Gly Ala Gln Arg 1 5 10 15 Gly Gly Gly Gly Pro
Gly Gly Gly Ala Pro Gly Gly Pro Gly Leu Gly 20 25 30 Leu Gly Ser
Leu Gly Glu Glu Arg Phe Pro Val Val Asn Thr Ala Tyr 35 40 45 Gly
Arg Val Arg Gly Val Arg Arg Glu Leu Asn Asn Glu Ile Leu Gly 50 55
60 Pro Val Val Gln Phe Leu Gly Val Pro Tyr Ala Thr Pro Pro Leu Gly
65 70 75 80 Ala Arg Arg Phe Gln Pro Pro Glu Ala Pro Ala Ser Trp Pro
Gly Val 85 90 95 Arg Asn Ala Thr Thr Leu Pro Pro Ala Cys Pro Gln
Asn Leu His Gly 100 105 110 Ala Leu Pro Ala Ile Met Leu Pro Val Trp
Phe Thr Asp Asn Leu Glu 115 120 125 Ala Ala Ala Thr Tyr Val Gln Asn
Gln Ser Glu Asp Cys Leu Tyr Leu 130 135 140 Asn Leu Tyr Val Pro Thr
Glu Asp Gly Pro Leu Thr Lys Lys Arg Asp 145 150 155 160 Glu Ala Thr
Leu Asn Pro Pro Asp Thr Asp Ile Arg Asp Ser Gly Lys 165 170 175 Lys
Pro Val Met Leu Phe Leu His Gly Gly Ser Tyr Met Glu Gly Thr 180 185
190 Gly Asn Met Phe Asp Gly Ser Val Leu Ala Ala Tyr Gly Asn Val Ile
195 200 205 Val Ala Thr Leu Asn Tyr Arg Leu Gly Val Leu Gly Phe Leu
Ser Thr 210 215 220 Gly Asp Gln Ala Ala Lys Gly Asn Tyr Gly Leu Leu
Asp Gln Ile Gln 225 230 235 240 Ala Leu Arg Trp Leu Ser Glu Asn Ile
Ala His Phe Gly Gly Asp Pro 245 250 255 Glu Arg Ile Thr Ile Phe Gly
Ser Gly Ala Gly Ala Ser Cys Val Asn 260 265 270 Leu Leu Ile Leu Ser
His His Ser Glu Gly Leu Phe Gln Lys Ala Ile 275 280 285 Ala Gln Ser
Gly Thr Ala Ile Ser Ser Trp Ser Val Asn Tyr Gln Pro 290 295 300 Leu
Lys Tyr Thr Arg Leu Leu Ala Ala Lys Val Gly Cys Asp Arg Glu 305 310
315 320 Asp Ser Thr Glu Ala Val Glu Cys Leu Arg Arg Lys Ser Ser Arg
Glu 325 330 335 Leu Val Asp Gln Asp Val Gln Pro Ala Arg Tyr His Ile
Ala Phe Gly 340 345 350 Pro Val Val Asp Gly Asp Val Val Pro Asp Asp
Pro Glu Ile Leu Met 355 360 365 Gln Gln Gly Glu Phe Leu Asn Tyr Asp
Met Leu Ile Gly Val Asn Gln 370 375 380 Gly Glu Gly Leu Lys Phe Val
Glu Asp Ser Ala Glu Ser Glu Asp Gly 385 390 395 400 Val Ser Ala Ser
Ala Phe Asp Phe Thr Val Ser Asn Phe Val Asp Asn 405 410 415 Leu Tyr
Gly Tyr Pro Glu Gly Lys Asp Val Leu Arg Glu Thr Ile Lys 420 425 430
Phe Met Tyr Thr Asp Trp Ala Asp Arg Asp Asn Gly Glu Met Arg Arg 435
440 445 Lys Thr Leu Leu Ala Leu Phe Thr Asp His Gln Trp Val Ala Pro
Ala 450 455 460 Val Ala Thr Ala Lys Leu His Ala Asp Tyr Gln Ser Pro
Val Tyr Phe 465 470 475 480 Tyr Thr Phe Tyr His His Cys Gln Ala Glu
Gly Arg Pro Glu Trp Ala 485 490 495 Asp Ala Ala His Gly Asp Glu Leu
Pro Tyr Val Phe Gly Val Pro Met 500 505 510 Val Gly Ala Thr Asp Leu
Phe Pro Cys Asn Phe Ser Lys Asn Asp Val 515 520 525 Met Leu Ser Ala
Val Val Met Thr Tyr Trp Thr Asn Phe Ala Lys Thr 530 535 540 Gly Asp
Pro Asn Gln Pro Val Pro Gln Asp Thr Lys Phe Ile His Thr 545 550 555
560 Lys Pro Asn Arg Phe Glu Glu Val Val Trp Ser Lys Phe Asn Ser Lys
565 570 575 Glu Lys Gln Tyr Leu His Ile Gly Leu Lys Pro Arg Val Arg
Asp Asn 580 585 590 Tyr Arg Ala Asn Lys Val Ala Phe Trp Leu Glu Leu
Val Pro His Leu 595 600 605 His Asn Leu His Thr Glu Leu Phe Thr Thr
Thr Thr Arg Leu Pro Pro 610 615 620 Tyr Ala Thr Arg Trp Pro Pro Arg
Thr Pro Gly Pro Gly Thr Ser Gly 625 630 635 640 Thr Arg Arg Pro Pro
Pro Pro Ala Thr Leu Pro Pro Glu Ser Asp Ile 645 650 655 Asp Leu Gly
Pro Arg Ala Tyr Asp Arg Phe Pro Gly Asp Ser Arg Asp 660 665 670 Tyr
Ser Thr Glu Leu Ser Val Thr Val Ala Val Gly Ala Ser Leu Leu 675 680
685 Phe Leu Asn Ile Leu Ala Phe Ala Ala Leu Tyr Tyr Lys Arg Asp Arg
690 695 700 Arg Gln Glu Leu Arg Cys Arg Arg Leu Ser Pro Pro Gly Gly
Ser Gly 705 710 715 720 Ser Gly Val Pro Gly Gly Gly Pro Leu Leu Pro
Thr Ala Gly Arg Glu 725 730 735 Leu Pro Pro Glu Glu Glu Leu Val Ser
Leu Gln Leu Lys Arg Gly Gly 740 745 750 Gly Val Gly Ala Asp Pro Ala
Glu Ala Leu Arg Pro Ala Cys Pro Pro 755 760 765 Asp Tyr Thr Leu Ala
Leu Arg Arg Ala Pro Asp Asp Val Pro Leu Leu 770 775 780 Ala Pro Gly
Ala Leu Thr Leu Leu Pro Ser Gly Leu Gly Pro Pro Pro 785 790 795 800
Pro Pro Pro Pro Pro Ser Leu His Pro Phe Gly Pro Phe Pro Pro Pro 805
810 815 Pro Pro Thr Ala Thr Ser His Asn Asn Thr Leu Pro His Pro His
Ser 820 825 830 Thr Thr Arg Val 835 72532DNAArtificial SequenceFlag
tag + mouse neuroligin2CDS(1)..(2532)misc_feature(1)..(27)Flag
tagmisc_feature(28)..(2532)mouse neuroligin2 7atg gat tac aag gac
gac gat
gac aag tgg ctc ctg gcg ttg tgt ctg 48Met Asp Tyr Lys Asp Asp Asp
Asp Lys Trp Leu Leu Ala Leu Cys Leu 1 5 10 15 gtg ggg ctg gct ggg
gct caa cgg gga gga ggg ggt ccc ggc ggc ggc 96Val Gly Leu Ala Gly
Ala Gln Arg Gly Gly Gly Gly Pro Gly Gly Gly 20 25 30 gcc ccg ggc
ggc cca ggc ctg ggc ctc ggc agc ctc ggg gag gag cgc 144Ala Pro Gly
Gly Pro Gly Leu Gly Leu Gly Ser Leu Gly Glu Glu Arg 35 40 45 ttc
ccc gtg gtg aac aca gcc tac ggg cga gtg cgc ggt gtg cgg cgc 192Phe
Pro Val Val Asn Thr Ala Tyr Gly Arg Val Arg Gly Val Arg Arg 50 55
60 gag ctc aac aac gag atc ctg ggc ccg gtc gtg cag ttc ttg ggc gtg
240Glu Leu Asn Asn Glu Ile Leu Gly Pro Val Val Gln Phe Leu Gly Val
65 70 75 80 ccc tac gcc acg ccg ccc ttg ggc gcc cgc cgc ttc cag ccg
cct gag 288Pro Tyr Ala Thr Pro Pro Leu Gly Ala Arg Arg Phe Gln Pro
Pro Glu 85 90 95 gca cct gcc tcg tgg ccc ggc gtg cgc aac gcc acc
acc ctg ccg ccc 336Ala Pro Ala Ser Trp Pro Gly Val Arg Asn Ala Thr
Thr Leu Pro Pro 100 105 110 gcc tgc ccg cag aac ctg cac ggg gcc ctg
ccg gcc atc atg ctg cct 384Ala Cys Pro Gln Asn Leu His Gly Ala Leu
Pro Ala Ile Met Leu Pro 115 120 125 gtg tgg ttc acc gac aac ttg gag
gcg gcc gcc acc tac gtg cag aac 432Val Trp Phe Thr Asp Asn Leu Glu
Ala Ala Ala Thr Tyr Val Gln Asn 130 135 140 cag agc gag gac tgc ctg
tac ctc aac ctc tac gtg ccc act gag gac 480Gln Ser Glu Asp Cys Leu
Tyr Leu Asn Leu Tyr Val Pro Thr Glu Asp 145 150 155 160 ggt ccg ctc
aca aaa aaa cgt gac gag gcg acg ctc aat ccg cca gac 528Gly Pro Leu
Thr Lys Lys Arg Asp Glu Ala Thr Leu Asn Pro Pro Asp 165 170 175 aca
gat atc cgg gac tct ggg aag aaa ccg gtc atg ctg ttt cta cac 576Thr
Asp Ile Arg Asp Ser Gly Lys Lys Pro Val Met Leu Phe Leu His 180 185
190 ggc ggc tcc tac atg gaa ggc acc ggg aac atg ttt gac ggc tca gtc
624Gly Gly Ser Tyr Met Glu Gly Thr Gly Asn Met Phe Asp Gly Ser Val
195 200 205 ctg gct gcc tat ggc aat gtc atc gta gtc aca ctc aac tac
cgt ctt 672Leu Ala Ala Tyr Gly Asn Val Ile Val Val Thr Leu Asn Tyr
Arg Leu 210 215 220 ggg gtg ctc ggt ttt ctc agc act ggt gac cag gct
gca aaa ggc aac 720Gly Val Leu Gly Phe Leu Ser Thr Gly Asp Gln Ala
Ala Lys Gly Asn 225 230 235 240 tat ggg ctc ctg gac cag atc cag gcc
ctg cgc tgg ctc agt gaa aac 768Tyr Gly Leu Leu Asp Gln Ile Gln Ala
Leu Arg Trp Leu Ser Glu Asn 245 250 255 att gcc cac ttt gga ggt gac
cct gaa cgc atc act atc ttt ggg tct 816Ile Ala His Phe Gly Gly Asp
Pro Glu Arg Ile Thr Ile Phe Gly Ser 260 265 270 ggt gca ggg gcc tcc
tgt gtc aac ttg ctg atc ctt tcc cac cac tca 864Gly Ala Gly Ala Ser
Cys Val Asn Leu Leu Ile Leu Ser His His Ser 275 280 285 gaa gga ctg
ttc cag aag gcc att gct caa agt ggt act gcc att tcc 912Glu Gly Leu
Phe Gln Lys Ala Ile Ala Gln Ser Gly Thr Ala Ile Ser 290 295 300 agc
tgg tct gtc aac tac cag ccg ctc aag tac acg cgg ctg ctg gcg 960Ser
Trp Ser Val Asn Tyr Gln Pro Leu Lys Tyr Thr Arg Leu Leu Ala 305 310
315 320 gcc aaa gtg ggc tgt gac cga gaa gac agc act gaa gct gtg gag
tgt 1008Ala Lys Val Gly Cys Asp Arg Glu Asp Ser Thr Glu Ala Val Glu
Cys 325 330 335 ctg cgc cgg aag tct tcc cgg gag cta gtg gac cag gat
gta cag cct 1056Leu Arg Arg Lys Ser Ser Arg Glu Leu Val Asp Gln Asp
Val Gln Pro 340 345 350 gcc cgc tac cac att gcc ttt ggg cct gtg gtg
gac ggc gac gta gtc 1104Ala Arg Tyr His Ile Ala Phe Gly Pro Val Val
Asp Gly Asp Val Val 355 360 365 cct gat gac ccc gag atc ctc atg caa
cag ggg gaa ttc ctc aac tac 1152Pro Asp Asp Pro Glu Ile Leu Met Gln
Gln Gly Glu Phe Leu Asn Tyr 370 375 380 gac atg ctc att ggt gtc aac
cag gga gag ggt ctc aag ttc gtg gag 1200Asp Met Leu Ile Gly Val Asn
Gln Gly Glu Gly Leu Lys Phe Val Glu 385 390 395 400 gac tct gca gag
agt gag gac ggt gtg tct gcc agc gcc ttt gac ttc 1248Asp Ser Ala Glu
Ser Glu Asp Gly Val Ser Ala Ser Ala Phe Asp Phe 405 410 415 acc gtc
tcc aac ttt gtg gac aac ttg tac ggg tac cca gaa ggc aag 1296Thr Val
Ser Asn Phe Val Asp Asn Leu Tyr Gly Tyr Pro Glu Gly Lys 420 425 430
gac gtg ctt cga gag acc atc aag ttc atg tac acg gac tgg gct gac
1344Asp Val Leu Arg Glu Thr Ile Lys Phe Met Tyr Thr Asp Trp Ala Asp
435 440 445 agg gac aat ggc gag atg cgg cgg aag acc ctg ctg gcg ctc
ttt acc 1392Arg Asp Asn Gly Glu Met Arg Arg Lys Thr Leu Leu Ala Leu
Phe Thr 450 455 460 gac cac cag tgg gtc gcc ccg gct gtg gcc acc gcc
aag ctg cat gcc 1440Asp His Gln Trp Val Ala Pro Ala Val Ala Thr Ala
Lys Leu His Ala 465 470 475 480 gac tac cag tcc ccc gtc tac ttt tac
act ttc tac cac cac tgc cag 1488Asp Tyr Gln Ser Pro Val Tyr Phe Tyr
Thr Phe Tyr His His Cys Gln 485 490 495 gca gag ggc cgg cca gag tgg
gca gac gca gcg cac ggg gac gag ctg 1536Ala Glu Gly Arg Pro Glu Trp
Ala Asp Ala Ala His Gly Asp Glu Leu 500 505 510 ccc tac gtc ttt ggt
gtc ccc atg gtg ggc gcc act gac ctc ttc ccc 1584Pro Tyr Val Phe Gly
Val Pro Met Val Gly Ala Thr Asp Leu Phe Pro 515 520 525 tgc aac ttc
tcc aag aac gat gtc atg ctc agc gca gta gtc atg acc 1632Cys Asn Phe
Ser Lys Asn Asp Val Met Leu Ser Ala Val Val Met Thr 530 535 540 tat
tgg acc aac ttc gcc aag act ggt gac ccc aac cag cct gtg cca 1680Tyr
Trp Thr Asn Phe Ala Lys Thr Gly Asp Pro Asn Gln Pro Val Pro 545 550
555 560 cag gac acc aag ttc atc cac acc aag ccc aac cgc ttt gaa gag
gta 1728Gln Asp Thr Lys Phe Ile His Thr Lys Pro Asn Arg Phe Glu Glu
Val 565 570 575 gtg tgg agc aag ttc aac agc aag gaa aag cag tat ctg
cac ata ggc 1776Val Trp Ser Lys Phe Asn Ser Lys Glu Lys Gln Tyr Leu
His Ile Gly 580 585 590 ttg aaa cca cgc gtg cgc gac aac tac cgt gcc
aac aag gtg gcc ttc 1824Leu Lys Pro Arg Val Arg Asp Asn Tyr Arg Ala
Asn Lys Val Ala Phe 595 600 605 tgg ctg gag ctc gtg ccc cac ctg cac
aac ctg cac aca gag ctc ttc 1872Trp Leu Glu Leu Val Pro His Leu His
Asn Leu His Thr Glu Leu Phe 610 615 620 acc acc acc act cgc ctg cct
ccc tat gcc aca cgc tgg cca cct cgc 1920Thr Thr Thr Thr Arg Leu Pro
Pro Tyr Ala Thr Arg Trp Pro Pro Arg 625 630 635 640 aca cct ggt cct
ggc act tcc ggc aca cgc cgt cct ccc cca cct gcc 1968Thr Pro Gly Pro
Gly Thr Ser Gly Thr Arg Arg Pro Pro Pro Pro Ala 645 650 655 act ctg
cca cct gag tct gat att gac cta ggc cca agg gcc tat gac 2016Thr Leu
Pro Pro Glu Ser Asp Ile Asp Leu Gly Pro Arg Ala Tyr Asp 660 665 670
cgc ttc ccc ggt gac tcg agg gac tac tcc acg gag cta agc gtg act
2064Arg Phe Pro Gly Asp Ser Arg Asp Tyr Ser Thr Glu Leu Ser Val Thr
675 680 685 gtg gca gtg ggt gcc tcc ctc ctc ttc ctc aac atc ctt gcc
ttt gcc 2112Val Ala Val Gly Ala Ser Leu Leu Phe Leu Asn Ile Leu Ala
Phe Ala 690 695 700 gcc ctc tat tac aag cgg gac cgg cgc cag gag ctg
cgg tgc cgg agg 2160Ala Leu Tyr Tyr Lys Arg Asp Arg Arg Gln Glu Leu
Arg Cys Arg Arg 705 710 715 720 ctt agc cca cca gga ggc tca ggc tca
ggt gtg cct ggt ggg ggc ccc 2208Leu Ser Pro Pro Gly Gly Ser Gly Ser
Gly Val Pro Gly Gly Gly Pro 725 730 735 ctg ctt ccc act gct ggc cgt
gag cta ccc ccg gag gag gag cta gta 2256Leu Leu Pro Thr Ala Gly Arg
Glu Leu Pro Pro Glu Glu Glu Leu Val 740 745 750 tcg ctg cag ctg aag
cgg ggt ggt ggt gtt ggg gcg gac cct gct gag 2304Ser Leu Gln Leu Lys
Arg Gly Gly Gly Val Gly Ala Asp Pro Ala Glu 755 760 765 gcc ctg cgc
cct gcc tgt cca ccc gac tat acc ctg gcc ttg cgc cgg 2352Ala Leu Arg
Pro Ala Cys Pro Pro Asp Tyr Thr Leu Ala Leu Arg Arg 770 775 780 gca
ccg gac gat gtg cct ctc ttg gcc ccc ggg gcc cta acc ctg ctg 2400Ala
Pro Asp Asp Val Pro Leu Leu Ala Pro Gly Ala Leu Thr Leu Leu 785 790
795 800 cct agt ggc ctg ggg ccc ccg ccc ccg ccc cca ccc cct tct ctc
cat 2448Pro Ser Gly Leu Gly Pro Pro Pro Pro Pro Pro Pro Pro Ser Leu
His 805 810 815 ccc ttt ggg ccc ttc cca ccc cca ccc cct act gct acc
agc cac aac 2496Pro Phe Gly Pro Phe Pro Pro Pro Pro Pro Thr Ala Thr
Ser His Asn 820 825 830 aac acg cta ccc cat ccc cac tcc acc act cgg
gta 2532Asn Thr Leu Pro His Pro His Ser Thr Thr Arg Val 835 840
8844PRTArtificial SequenceSynthetic Construct 8Met Asp Tyr Lys Asp
Asp Asp Asp Lys Trp Leu Leu Ala Leu Cys Leu 1 5 10 15 Val Gly Leu
Ala Gly Ala Gln Arg Gly Gly Gly Gly Pro Gly Gly Gly 20 25 30 Ala
Pro Gly Gly Pro Gly Leu Gly Leu Gly Ser Leu Gly Glu Glu Arg 35 40
45 Phe Pro Val Val Asn Thr Ala Tyr Gly Arg Val Arg Gly Val Arg Arg
50 55 60 Glu Leu Asn Asn Glu Ile Leu Gly Pro Val Val Gln Phe Leu
Gly Val 65 70 75 80 Pro Tyr Ala Thr Pro Pro Leu Gly Ala Arg Arg Phe
Gln Pro Pro Glu 85 90 95 Ala Pro Ala Ser Trp Pro Gly Val Arg Asn
Ala Thr Thr Leu Pro Pro 100 105 110 Ala Cys Pro Gln Asn Leu His Gly
Ala Leu Pro Ala Ile Met Leu Pro 115 120 125 Val Trp Phe Thr Asp Asn
Leu Glu Ala Ala Ala Thr Tyr Val Gln Asn 130 135 140 Gln Ser Glu Asp
Cys Leu Tyr Leu Asn Leu Tyr Val Pro Thr Glu Asp 145 150 155 160 Gly
Pro Leu Thr Lys Lys Arg Asp Glu Ala Thr Leu Asn Pro Pro Asp 165 170
175 Thr Asp Ile Arg Asp Ser Gly Lys Lys Pro Val Met Leu Phe Leu His
180 185 190 Gly Gly Ser Tyr Met Glu Gly Thr Gly Asn Met Phe Asp Gly
Ser Val 195 200 205 Leu Ala Ala Tyr Gly Asn Val Ile Val Val Thr Leu
Asn Tyr Arg Leu 210 215 220 Gly Val Leu Gly Phe Leu Ser Thr Gly Asp
Gln Ala Ala Lys Gly Asn 225 230 235 240 Tyr Gly Leu Leu Asp Gln Ile
Gln Ala Leu Arg Trp Leu Ser Glu Asn 245 250 255 Ile Ala His Phe Gly
Gly Asp Pro Glu Arg Ile Thr Ile Phe Gly Ser 260 265 270 Gly Ala Gly
Ala Ser Cys Val Asn Leu Leu Ile Leu Ser His His Ser 275 280 285 Glu
Gly Leu Phe Gln Lys Ala Ile Ala Gln Ser Gly Thr Ala Ile Ser 290 295
300 Ser Trp Ser Val Asn Tyr Gln Pro Leu Lys Tyr Thr Arg Leu Leu Ala
305 310 315 320 Ala Lys Val Gly Cys Asp Arg Glu Asp Ser Thr Glu Ala
Val Glu Cys 325 330 335 Leu Arg Arg Lys Ser Ser Arg Glu Leu Val Asp
Gln Asp Val Gln Pro 340 345 350 Ala Arg Tyr His Ile Ala Phe Gly Pro
Val Val Asp Gly Asp Val Val 355 360 365 Pro Asp Asp Pro Glu Ile Leu
Met Gln Gln Gly Glu Phe Leu Asn Tyr 370 375 380 Asp Met Leu Ile Gly
Val Asn Gln Gly Glu Gly Leu Lys Phe Val Glu 385 390 395 400 Asp Ser
Ala Glu Ser Glu Asp Gly Val Ser Ala Ser Ala Phe Asp Phe 405 410 415
Thr Val Ser Asn Phe Val Asp Asn Leu Tyr Gly Tyr Pro Glu Gly Lys 420
425 430 Asp Val Leu Arg Glu Thr Ile Lys Phe Met Tyr Thr Asp Trp Ala
Asp 435 440 445 Arg Asp Asn Gly Glu Met Arg Arg Lys Thr Leu Leu Ala
Leu Phe Thr 450 455 460 Asp His Gln Trp Val Ala Pro Ala Val Ala Thr
Ala Lys Leu His Ala 465 470 475 480 Asp Tyr Gln Ser Pro Val Tyr Phe
Tyr Thr Phe Tyr His His Cys Gln 485 490 495 Ala Glu Gly Arg Pro Glu
Trp Ala Asp Ala Ala His Gly Asp Glu Leu 500 505 510 Pro Tyr Val Phe
Gly Val Pro Met Val Gly Ala Thr Asp Leu Phe Pro 515 520 525 Cys Asn
Phe Ser Lys Asn Asp Val Met Leu Ser Ala Val Val Met Thr 530 535 540
Tyr Trp Thr Asn Phe Ala Lys Thr Gly Asp Pro Asn Gln Pro Val Pro 545
550 555 560 Gln Asp Thr Lys Phe Ile His Thr Lys Pro Asn Arg Phe Glu
Glu Val 565 570 575 Val Trp Ser Lys Phe Asn Ser Lys Glu Lys Gln Tyr
Leu His Ile Gly 580 585 590 Leu Lys Pro Arg Val Arg Asp Asn Tyr Arg
Ala Asn Lys Val Ala Phe 595 600 605 Trp Leu Glu Leu Val Pro His Leu
His Asn Leu His Thr Glu Leu Phe 610 615 620 Thr Thr Thr Thr Arg Leu
Pro Pro Tyr Ala Thr Arg Trp Pro Pro Arg 625 630 635 640 Thr Pro Gly
Pro Gly Thr Ser Gly Thr Arg Arg Pro Pro Pro Pro Ala 645 650 655 Thr
Leu Pro Pro Glu Ser Asp Ile Asp Leu Gly Pro Arg Ala Tyr Asp 660 665
670 Arg Phe Pro Gly Asp Ser Arg Asp Tyr Ser Thr Glu Leu Ser Val Thr
675 680 685 Val Ala Val Gly Ala Ser Leu Leu Phe Leu Asn Ile Leu Ala
Phe Ala 690 695 700 Ala Leu Tyr Tyr Lys Arg Asp Arg Arg Gln Glu Leu
Arg Cys Arg Arg 705 710 715 720 Leu Ser Pro Pro Gly Gly Ser Gly Ser
Gly Val Pro Gly Gly Gly Pro 725 730 735 Leu Leu Pro Thr Ala Gly Arg
Glu Leu Pro Pro Glu Glu Glu Leu Val 740 745 750 Ser Leu Gln Leu Lys
Arg Gly Gly Gly Val Gly Ala Asp Pro Ala Glu 755 760 765 Ala Leu Arg
Pro Ala Cys Pro Pro Asp Tyr Thr Leu Ala Leu Arg Arg 770 775 780 Ala
Pro Asp Asp Val Pro Leu Leu Ala Pro Gly Ala Leu Thr Leu Leu 785 790
795 800 Pro Ser Gly Leu Gly Pro Pro Pro Pro Pro Pro Pro Pro Ser Leu
His 805 810 815 Pro Phe Gly Pro Phe Pro Pro Pro Pro Pro Thr Ala Thr
Ser His Asn 820 825 830 Asn Thr Leu Pro His Pro His Ser Thr Thr Arg
Val 835 840 9736PRTArtificial SequenceSynthetic
Construct 9Met Ala Ala Asp Gly Tyr Leu Pro Asp Trp Leu Glu Asp Asn
Leu Ser 1 5 10 15 Glu Gly Ile Arg Glu Trp Trp Asp Leu Lys Pro Gly
Ala Pro Lys Pro 20 25 30 Lys Ala Asn Gln Gln Lys Gln Asp Asp Gly
Arg Gly Leu Val Leu Pro 35 40 45 Gly Tyr Lys Tyr Leu Gly Pro Phe
Asn Gly Leu Asp Lys Gly Glu Pro 50 55 60 Val Asn Ala Ala Asp Ala
Ala Ala Leu Glu His Asp Lys Ala Tyr Asp 65 70 75 80 Gln Gln Leu Lys
Ala Gly Asp Asn Pro Tyr Leu Arg Tyr Asn His Ala 85 90 95 Asp Ala
Glu Phe Gln Glu Arg Leu Gln Glu Asp Thr Ser Phe Gly Gly 100 105 110
Asn Leu Gly Arg Ala Val Phe Gln Ala Lys Lys Arg Val Leu Glu Pro 115
120 125 Leu Gly Leu Val Glu Glu Gly Ala Lys Thr Ala Pro Gly Lys Lys
Arg 130 135 140 Pro Val Glu Gln Ser Pro Gln Glu Pro Asp Ser Ser Ser
Gly Ile Gly 145 150 155 160 Lys Thr Gly Gln Gln Pro Ala Lys Lys Arg
Leu Asn Phe Gly Gln Thr 165 170 175 Gly Asp Ser Glu Ser Val Pro Asp
Pro Gln Pro Leu Gly Glu Pro Pro 180 185 190 Ala Thr Pro Ala Ala Val
Gly Pro Thr Thr Met Ala Ser Gly Gly Gly 195 200 205 Ala Pro Met Ala
Asp Asn Asn Glu Gly Ala Asp Gly Val Gly Asn Ala 210 215 220 Ser Gly
Asn Trp His Cys Asp Ser Thr Trp Leu Gly Asp Arg Val Ile 225 230 235
240 Thr Thr Ser Thr Arg Thr Trp Ala Leu Pro Thr Tyr Asn Asn His Leu
245 250 255 Tyr Lys Gln Ile Ser Ser Ala Ser Thr Gly Ala Ser Asn Asp
Asn His 260 265 270 Tyr Phe Gly Tyr Ser Thr Pro Trp Gly Tyr Phe Asp
Phe Asn Arg Phe 275 280 285 His Cys His Phe Ser Pro Arg Asp Trp Gln
Arg Leu Ile Asn Asn Asn 290 295 300 Trp Gly Phe Arg Pro Lys Arg Leu
Asn Phe Lys Leu Phe Asn Ile Gln 305 310 315 320 Val Lys Glu Val Thr
Thr Asn Asp Gly Val Thr Thr Ile Ala Asn Asn 325 330 335 Leu Thr Ser
Thr Val Gln Val Phe Ser Asp Ser Glu Tyr Gln Leu Pro 340 345 350 Tyr
Val Leu Gly Ser Ala His Gln Gly Cys Leu Pro Pro Phe Pro Ala 355 360
365 Asp Val Phe Met Ile Pro Gln Tyr Gly Tyr Leu Thr Leu Asn Asn Gly
370 375 380 Ser Gln Ala Val Gly Arg Ser Ser Phe Tyr Cys Leu Glu Tyr
Phe Pro 385 390 395 400 Ser Gln Met Leu Arg Thr Gly Asn Asn Phe Thr
Phe Ser Tyr Thr Phe 405 410 415 Glu Glu Val Pro Phe His Ser Ser Tyr
Ala His Ser Gln Ser Leu Asp 420 425 430 Arg Leu Met Asn Pro Leu Ile
Asp Gln Tyr Leu Tyr Phe Leu Asn Arg 435 440 445 Thr Gln Asn Gln Ser
Gly Ser Ala Gln Asn Lys Asp Leu Leu Phe Ser 450 455 460 Arg Gly Ser
Pro Ala Gly Met Ser Val Gln Pro Lys Asn Trp Leu Pro 465 470 475 480
Gly Pro Cys Tyr Arg Gln Gln Arg Val Ser Lys Thr Lys Thr Asp Asn 485
490 495 Asn Asn Ser Asn Phe Thr Trp Thr Gly Ala Ser Lys Tyr Asn Leu
Asn 500 505 510 Gly Arg Glu Ser Ile Ile Asn Pro Gly Thr Ala Met Ala
Ser His Lys 515 520 525 Asp Asp Glu Asp Lys Phe Phe Pro Met Ser Gly
Val Met Ile Phe Gly 530 535 540 Lys Glu Ser Ala Gly Ala Ser Asn Thr
Ala Leu Asp Asn Val Met Ile 545 550 555 560 Thr Asp Glu Glu Glu Ile
Lys Ala Thr Asn Pro Val Ala Thr Glu Arg 565 570 575 Phe Gly Thr Val
Ala Val Asn Phe Gln Ser Ser Ser Thr Asp Pro Ala 580 585 590 Thr Gly
Asp Val His Ala Met Gly Ala Leu Pro Gly Met Val Trp Gln 595 600 605
Asp Arg Asp Val Tyr Leu Gln Gly Pro Ile Trp Ala Lys Ile Pro His 610
615 620 Thr Asp Gly His Phe His Pro Ser Pro Leu Met Gly Gly Phe Gly
Leu 625 630 635 640 Lys Asn Pro Pro Pro Gln Ile Leu Ile Lys Asn Thr
Pro Val Pro Ala 645 650 655 Asn Pro Pro Ala Glu Phe Ser Ala Thr Lys
Phe Ala Ser Phe Ile Thr 660 665 670 Gln Tyr Ser Thr Gly Gln Val Ser
Val Glu Ile Glu Trp Glu Leu Gln 675 680 685 Lys Glu Asn Ser Lys Arg
Trp Asn Pro Glu Val Gln Tyr Thr Ser Asn 690 695 700 Tyr Ala Lys Ser
Ala Asn Val Asp Phe Thr Val Asp Asn Asn Gly Leu 705 710 715 720 Tyr
Thr Glu Pro Arg Pro Ile Gly Thr Arg Tyr Leu Thr Arg Pro Leu 725 730
735 10735PRTArtificial SequenceSynthetic Construct 10Met Ala Ala
Asp Gly Tyr Leu Pro Asp Trp Leu Glu Asp Thr Leu Ser 1 5 10 15 Glu
Gly Ile Arg Gln Trp Trp Lys Leu Lys Pro Gly Pro Pro Pro Pro 20 25
30 Lys Pro Ala Glu Arg His Lys Asp Asp Ser Arg Gly Leu Val Leu Pro
35 40 45 Gly Tyr Lys Tyr Leu Gly Pro Phe Asn Gly Leu Asp Lys Gly
Glu Pro 50 55 60 Val Asn Glu Ala Asp Ala Ala Ala Leu Glu His Asp
Lys Ala Tyr Asp 65 70 75 80 Arg Gln Leu Asp Ser Gly Asp Asn Pro Tyr
Leu Lys Tyr Asn His Ala 85 90 95 Asp Ala Glu Phe Gln Glu Arg Leu
Lys Glu Asp Thr Ser Phe Gly Gly 100 105 110 Asn Leu Gly Arg Ala Val
Phe Gln Ala Lys Lys Arg Val Leu Glu Pro 115 120 125 Leu Gly Leu Val
Glu Glu Pro Val Lys Thr Ala Pro Gly Lys Lys Arg 130 135 140 Pro Val
Glu His Ser Pro Val Glu Pro Asp Ser Ser Ser Gly Thr Gly 145 150 155
160 Lys Ala Gly Gln Gln Pro Ala Arg Lys Arg Leu Asn Phe Gly Gln Thr
165 170 175 Gly Asp Ala Asp Ser Val Pro Asp Pro Gln Pro Leu Gly Gln
Pro Pro 180 185 190 Ala Ala Pro Ser Gly Leu Gly Thr Asn Thr Met Ala
Thr Gly Ser Gly 195 200 205 Ala Pro Met Ala Asp Asn Asn Glu Gly Ala
Asp Gly Val Gly Asn Ser 210 215 220 Ser Gly Asn Trp His Cys Asp Ser
Thr Trp Met Gly Asp Arg Val Ile 225 230 235 240 Thr Thr Ser Thr Arg
Thr Trp Ala Leu Pro Thr Tyr Asn Asn His Leu 245 250 255 Tyr Lys Gln
Ile Ser Ser Gln Ser Gly Ala Ser Asn Asp Asn His Tyr 260 265 270 Phe
Gly Tyr Ser Thr Pro Trp Gly Tyr Phe Asp Phe Asn Arg Phe His 275 280
285 Cys His Phe Ser Pro Arg Asp Trp Gln Arg Leu Ile Asn Asn Asn Trp
290 295 300 Gly Phe Arg Pro Lys Arg Leu Asn Phe Lys Leu Phe Asn Ile
Gln Val 305 310 315 320 Lys Glu Val Thr Gln Asn Asp Gly Thr Thr Thr
Ile Ala Asn Asn Leu 325 330 335 Thr Ser Thr Val Gln Val Phe Thr Asp
Ser Glu Tyr Gln Leu Pro Tyr 340 345 350 Val Leu Gly Ser Ala His Gln
Gly Cys Leu Pro Pro Phe Pro Ala Asp 355 360 365 Val Phe Met Val Pro
Gln Tyr Gly Tyr Leu Thr Leu Asn Asn Gly Ser 370 375 380 Gln Ala Val
Gly Arg Ser Ser Phe Tyr Cys Leu Glu Tyr Phe Pro Ser 385 390 395 400
Gln Met Leu Arg Thr Gly Asn Asn Phe Thr Phe Ser Tyr Thr Phe Glu 405
410 415 Asp Val Pro Phe His Ser Ser Tyr Ala His Ser Gln Ser Leu Asp
Arg 420 425 430 Leu Met Asn Pro Leu Ile Asp Gln Tyr Leu Tyr Phe Leu
Ser Arg Thr 435 440 445 Asn Thr Pro Ser Gly Thr Thr Thr Gln Ser Arg
Leu Gln Phe Ser Gln 450 455 460 Ala Gly Ala Ser Asp Ile Arg Asp Gln
Ser Arg Asn Trp Leu Pro Gly 465 470 475 480 Pro Cys Tyr Arg Gln Gln
Arg Val Ser Lys Thr Ser Ala Asp Asn Asn 485 490 495 Asn Ser Glu Tyr
Ser Trp Thr Gly Ala Thr Lys Tyr His Leu Asn Gly 500 505 510 Arg Asp
Ser Leu Val Asn Pro Gly Pro Ala Met Ala Ser His Lys Asp 515 520 525
Asp Glu Glu Lys Phe Phe Pro Gln Ser Gly Val Leu Ile Phe Gly Lys 530
535 540 Gln Gly Ser Glu Lys Thr Asn Val Asp Ile Glu Lys Val Met Ile
Thr 545 550 555 560 Asp Glu Glu Glu Ile Arg Thr Thr Asn Pro Val Ala
Thr Glu Gln Tyr 565 570 575 Gly Ser Val Ser Thr Asn Leu Gln Arg Gly
Asn Arg Gln Ala Ala Thr 580 585 590 Ala Asp Val Asn Thr Gln Gly Val
Leu Pro Gly Met Val Trp Gln Asp 595 600 605 Arg Asp Val Tyr Leu Gln
Gly Pro Ile Trp Ala Lys Ile Pro His Thr 610 615 620 Asp Gly His Phe
His Pro Ser Pro Leu Met Gly Gly Phe Gly Leu Lys 625 630 635 640 His
Pro Pro Pro Gln Ile Leu Ile Lys Asn Thr Pro Val Pro Ala Asn 645 650
655 Pro Ser Thr Thr Phe Ser Ala Ala Lys Phe Ala Ser Phe Ile Thr Gln
660 665 670 Tyr Ser Thr Gly Gln Val Ser Val Glu Ile Glu Trp Glu Leu
Gln Lys 675 680 685 Glu Asn Ser Lys Arg Trp Asn Pro Glu Ile Gln Tyr
Thr Ser Asn Tyr 690 695 700 Asn Lys Ser Val Asn Val Asp Phe Thr Val
Asp Thr Asn Gly Val Tyr 705 710 715 720 Ser Glu Pro Arg Pro Ile Gly
Thr Arg Tyr Leu Thr Arg Asn Leu 725 730 735 11736PRTArtificial
SequenceSynthetic Construct 11Met Ala Ala Asp Gly Tyr Leu Pro Asp
Trp Leu Glu Asp Asn Leu Ser 1 5 10 15 Glu Gly Ile Arg Glu Trp Trp
Ala Leu Lys Pro Gly Ala Pro Gln Pro 20 25 30 Lys Ala Asn Gln Gln
His Gln Asp Asn Ala Arg Gly Leu Val Leu Pro 35 40 45 Gly Tyr Lys
Tyr Leu Gly Pro Gly Asn Gly Leu Asp Lys Gly Glu Pro 50 55 60 Val
Asn Ala Ala Asp Ala Ala Ala Leu Glu His Asp Lys Ala Tyr Asp 65 70
75 80 Gln Gln Leu Lys Ala Gly Asp Asn Pro Tyr Leu Lys Tyr Asn His
Ala 85 90 95 Asp Ala Glu Phe Gln Glu Arg Leu Lys Glu Asp Thr Ser
Phe Gly Gly 100 105 110 Asn Leu Gly Arg Ala Val Phe Gln Ala Lys Lys
Arg Leu Leu Glu Pro 115 120 125 Leu Gly Leu Val Glu Glu Ala Ala Lys
Thr Ala Pro Gly Lys Lys Arg 130 135 140 Pro Val Glu Gln Ser Pro Gln
Glu Pro Asp Ser Ser Ala Gly Ile Gly 145 150 155 160 Lys Ser Gly Ala
Gln Pro Ala Lys Lys Arg Leu Asn Phe Gly Gln Thr 165 170 175 Gly Asp
Thr Glu Ser Val Pro Asp Pro Gln Pro Ile Gly Glu Pro Pro 180 185 190
Ala Ala Pro Ser Gly Val Gly Ser Leu Thr Met Ala Ser Gly Gly Gly 195
200 205 Ala Pro Val Ala Asp Asn Asn Glu Gly Ala Asp Gly Val Gly Ser
Ser 210 215 220 Ser Gly Asn Trp His Cys Asp Ser Gln Trp Leu Gly Asp
Arg Val Ile 225 230 235 240 Thr Thr Ser Thr Arg Thr Trp Ala Leu Pro
Thr Tyr Asn Asn His Leu 245 250 255 Tyr Lys Gln Ile Ser Asn Ser Thr
Ser Gly Gly Ser Ser Asn Asp Asn 260 265 270 Ala Tyr Phe Gly Tyr Ser
Thr Pro Trp Gly Tyr Phe Asp Phe Asn Arg 275 280 285 Phe His Cys His
Phe Ser Pro Arg Asp Trp Gln Arg Leu Ile Asn Asn 290 295 300 Asn Trp
Gly Phe Arg Pro Lys Arg Leu Asn Phe Lys Leu Phe Asn Ile 305 310 315
320 Gln Val Lys Glu Val Thr Asp Asn Asn Gly Val Lys Thr Ile Ala Asn
325 330 335 Asn Leu Thr Ser Thr Val Gln Val Phe Thr Asp Ser Asp Tyr
Gln Leu 340 345 350 Pro Tyr Val Leu Gly Ser Ala His Glu Gly Cys Leu
Pro Pro Phe Pro 355 360 365 Ala Asp Val Phe Met Ile Pro Gln Tyr Gly
Tyr Leu Thr Leu Asn Asp 370 375 380 Gly Ser Gln Ala Val Gly Arg Ser
Ser Phe Tyr Cys Leu Glu Tyr Phe 385 390 395 400 Pro Ser Gln Met Leu
Arg Thr Gly Asn Asn Phe Gln Phe Ser Tyr Glu 405 410 415 Phe Glu Asn
Val Pro Phe His Ser Ser Tyr Ala His Ser Gln Ser Leu 420 425 430 Asp
Arg Leu Met Asn Pro Leu Ile Asp Gln Tyr Leu Tyr Phe Leu Ser 435 440
445 Lys Thr Ile Asn Gly Ser Gly Gln Asn Gln Gln Thr Leu Lys Phe Ser
450 455 460 Val Ala Gly Pro Ser Asn Met Ala Val Gln Gly Arg Asn Tyr
Ile Pro 465 470 475 480 Gly Pro Ser Tyr Arg Gln Gln Arg Val Ser Thr
Thr Val Thr Gln Asn 485 490 495 Asn Asn Ser Glu Phe Ala Trp Pro Gly
Ala Ser Ser Trp Ala Leu Asn 500 505 510 Gly Arg Asn Ser Leu Met Asn
Pro Gly Pro Ala Met Ala Ser His Lys 515 520 525 Glu Gly Glu Asp Arg
Phe Phe Pro Leu Ser Gly Ser Leu Ile Phe Gly 530 535 540 Lys Gln Gly
Thr Gly Arg Asp Asn Val Asp Ala Asp Lys Val Met Ile 545 550 555 560
Thr Asn Glu Glu Glu Ile Lys Thr Thr Asn Pro Val Ala Thr Glu Ser 565
570 575 Tyr Gly Gln Val Ala Thr Asn His Gln Ser Ala Gln Ala Gln Ala
Gln 580 585 590 Thr Gly Trp Val Gln Asn Gln Gly Ile Leu Pro Gly Met
Val Trp Gln 595 600 605 Asp Arg Asp Val Tyr Leu Gln Gly Pro Ile Trp
Ala Lys Ile Pro His 610 615 620 Thr Asp Gly Asn Phe His Pro Ser Pro
Leu Met Gly Gly Phe Gly Met 625 630 635 640 Lys His Pro Pro Pro Gln
Ile Leu Ile Lys Asn Thr Pro Val Pro Ala 645 650 655 Asp Pro Pro Thr
Ala Phe Asn Lys Asp Lys Leu Asn Ser Phe Ile Thr 660 665 670 Gln Tyr
Ser Thr Gly Gln Val Ser Val Glu Ile Glu Trp Glu Leu Gln 675 680 685
Lys Glu Asn Ser Lys Arg Trp Asn Pro Glu Ile Gln Tyr Thr Ser Asn 690
695 700 Tyr Tyr Lys Ser Asn Asn Val Glu Phe Ala Val Asn Thr Glu Gly
Val 705 710 715 720 Tyr Ser Glu Pro Arg Pro Ile Gly Thr Arg Tyr Leu
Thr Arg Asn Leu 725 730 735
D00001

D00002

D00003

D00004

D00005

D00006

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.