Tailored Oils
Franklin; Scott ; et al.
U.S. patent application number 16/030741 was filed with the patent office on 2019-01-03 for tailored oils. The applicant listed for this patent is Corbion Biotech, Inc.. Invention is credited to Riyaz Bhat, Scott Franklin, Jeffrey L. Moseley, George Rudenko, Aravind Somanchi, Xinhua Zhao.
| Application Number | 20190002934 16/030741 |
| Document ID | / |
| Family ID | 51795776 |
| Filed Date | 2019-01-03 |










View All Diagrams
| United States Patent Application | 20190002934 |
| Kind Code | A1 |
| Franklin; Scott ; et al. | January 3, 2019 |
TAILORED OILS
Abstract
Recombinant DNA techniques are used to produce oleaginous recombinant cells that produce triglyceride oils having desired fatty acid profiles and regiospecific or stereospecific profiles. Genes manipulated include those encoding stearoyl-ACP desaturase, delta 12 fatty acid desaturase, acyl-ACP thioesterase, ketoacyl-ACP synthase, and lysophosphatidic acid acyltransferase. The oil produced can have enhanced oxidative or thermal stability, or can be useful as a frying oil, shortening, roll-in shortening, tempering fat, cocoa butter replacement, as a lubricant, or as a feedstock for various chemical processes. The fatty acid profile can be enriched in midchain profiles or the oil can be enriched in triglycerides of the saturated-unsaturated-saturated type.
| Inventors: | Franklin; Scott; (Woodside, CA) ; Somanchi; Aravind; (Redwood City, CA) ; Rudenko; George; (Mountain View, CA) ; Bhat; Riyaz; (South San Francisco, CA) ; Zhao; Xinhua; (Foster City, CA) ; Moseley; Jeffrey L.; (Redwood City, CA) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 51795776 | ||||||||||
| Appl. No.: | 16/030741 | ||||||||||
| Filed: | July 9, 2018 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 14506491 | Oct 3, 2014 | 10053715 | ||
| 16030741 | ||||
| 62023109 | Jul 10, 2014 | |||
| 61923327 | Jan 3, 2014 | |||
| 61895355 | Oct 24, 2013 | |||
| 61892399 | Oct 17, 2013 | |||
| 61887268 | Oct 4, 2013 | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C12P 7/64 20130101; C12Y 203/01041 20130101; C12P 7/6409 20130101; C11C 1/002 20130101; C11D 9/00 20130101; C12Y 301/02014 20130101 |
| International Class: | C12P 7/64 20060101 C12P007/64; C11D 9/00 20060101 C11D009/00; C11C 1/00 20060101 C11C001/00 |
Claims
1.-84. (canceled)
85. A recombinant cell of the genus Prototheca or Chlorella, said recombinant cell comprising a knockout or knockdown of an endogenous Fatty acyl-ACP thioesterase gene, and further comprising exogenous nucleic acids that encode lysophosphatidic acid acyltransferase (LPAAT).
86. The recombinant cell of claim 85, wherein said exogenous nucleic acids that encode LPAAT encodes an LPPAT having at least 90% amino acid sequence identity to an LPAAT selected from the group consisting of SEQ ID NOs: 16, 77, 78, 79 and 157.
87. The recombinant cell of claim 86, wherein said exogenous nucleic acids that encode LPAAT encodes an LPPAT having at least 95% amino acid sequence identity to an LPAAT selected from the group consisting of SEQ ID NOs: 16, 77, 78, 79 and 157.
88. The recombinant cell of claim of claim 86, wherein said exogenous nucleic acids encodes an LPAAT comprising the amino acid sequences of SEQ ID NO: 157.
89. The recombinant cell of claim of claim 86, wherein said exogenous nucleic acids encodes an LPAAT comprising the amino acid sequences of SEQ ID NOs: 17, 77, 78 or 79.
90. The recombinant cell of claim 89, wherein said exogenous nucleic acids that encode LPAAT are nucleic acids having at least 90% sequence identity to the nucleic acids selected from the group consisting of SEQ ID NOs: 18, 80, 81, 82, 83, 84 and 85.
91. The recombinant cell of claim 85, wherein said exogenous nucleic acids that encode LPAAT are nucleic acids having at least 95% sequence identity to the nucleic acids selected from the group consisting of SEQ ID NOs: 18, 80, 81, 82, 83, 84 and 85.
92. The recombinant cell of claim 85, wherein triglyceride oil produced by the recombinant cell is enriched in stearate-oleate-stearate (SOS) triglycerides.
93. The recombinant cell of claim 92, wherein the SOS triglyceride content comprises at least 50% of the triglyceride oils.
94. The recombinant cell of claim 93, wherein the SOS triglyceride content comprises at least 60% of the triglyceride oils.
95. The recombinant cell of claim 85, wherein triglyceride oil produced by the recombinant cell is enriched in mid-chain fatty acid.
96. The recombinant cell of claim 95, wherein the triglyceride oil is enriched in C12:0.
97. The method of claim 85, wherein said recombinant cell is of the genus Prototheca.
98. The method of claim 97, wherein said recombinant cell is a Prototheca moriformis cell.
99. The method of claim 92, wherein said recombinant cell is of the genus Prototheca.
100. The method of claim 99, wherein said recombinant cell is a Prototheca moriformis cell.
101. A method of producing oil, the method comprising the steps of: a. cultivating a recombinant cell of the genus Prototheca or Chlorella, said recombinant cell comprising a knockout or knockdown of an endogenous Fatty acyl-ACP thioesterase gene, and further comprising exogenous nucleic acids that encode lysophosphatidic acid acyltransferase (LPAAT); and b. isolating the oil from said recombinant cell.
102. The method of claim 101, wherein said recombinant cell is of the genus Prototheca.
103. The method of claim 102, wherein said recombinant cell is a Prototheca moriformis cell.
104. An oil produced by the steps of: a. cultivating a recombinant cell of the genus Prototheca or Chlorella, said recombinant cell comprising a knockout or knockdown of an endogenous Fatty acyl-ACP thioesterase gene, and further comprising exogenous nucleic acids that encode lysophosphatidic acid acyltransferase (LPAAT); and b. isolating the oil from said recombinant cell.
Description
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation of U.S. patent application Ser. No. 14/506,491, filed Oct. 3, 2014, which claims the benefit under 35 U.S.C. 119(e) of U.S. Provisional Patent Application Nos. 61/887,268, filed Oct. 4, 2013; 61/892,399, filed Oct. 17, 2013; 61/895,355, filed Oct. 24, 2013; 61/923,327, filed Jan. 3, 2014; and 62/023,109, filed Jul. 10, 2014. Each of these applications is incorporated herein by reference in its entirety for all purposes. This application includes subject matter related to that disclosed in U.S. Provisional Patent Application No. 62/023,112, entitled "Novel Ketoacyl ACP Synthase Genes and Uses Thereof," filed Jul. 10, 2014, which is also hereby incorporated by reference in its entirety for all purposes. In particular, Tables 1, 7 and 8 of 62/023,112, and the corresponding sequences identified therein, are hereby incorporated by reference.
REFERENCE TO A SEQUENCE LISTING
[0002] The instant application contains a Sequence Listing which has been submitted electronically in ASCII format and is hereby incorporated by reference in its entirety. Said ASCII copy, created on Aug. 28, 2017, is named SOLAP059US-SL.txt and is 599,266 bytes in size.
[0003] This application includes an electronic sequence listing in a file named "452572-Sequence2.txt", created on Jan. 20, 2015, and containing 551,031 bytes, which is hereby incorporated by reference in its entirety for all purposes.
FIELD OF THE INVENTION
[0004] Embodiments of the present invention relate to oils/fats, fuels, foods, and oleochemicals and their production from cultures of genetically engineered cells. Specific embodiments relate to oils with a high content of triglycerides bearing fatty acyl groups upon the glycerol backbone in particular regiospecific patterns, highly stable oils, oils with high levels of oleic or mid-chain fatty acids, and products produced from such oils.
BACKGROUND OF THE INVENTION
[0005] PCT Publications WO2008/151149, WO2010/06032, WO2011/150410, WO2011/150411, WO2012/061647, and WO2012/106560 disclose oils and methods for producing those oils in microbes, including microalgae. These publications also describe the use of such oils to make oleochemicals and fuels.
[0006] Tempering is a process of converting a fat into a desired polymorphic form by manipulation of the temperature of the fat or fat-containing substance, commonly used in chocolate making.
[0007] Certain enzymes of the fatty acyl-CoA elongation pathway function to extend the length of fatty acyl-CoA molecules. Elongase-complex enzymes extend fatty acyl-CoA molecules in 2 carbon additions, for example myristoyl-CoA to palmitoyl-CoA, stearoyl-CoA to arachidyl-CoA, or oleoyl-CoA to eicosanoyl-CoA, eicosanoyl-CoA to erucyl-CoA. In addition, elongase enzymes also extend acyl chain length in 2 carbon increments. KCS enzymes condense acyl-CoA molecules with two carbons from malonyl-CoA to form beta-ketoacyl-CoA. KCS and elongases may show specificity for condensing acyl substrates of particular carbon length, modification (such as hydroxylation), or degree of saturation. For example, the jojoba (Simmondsia chinensis) beta-ketoacyl-CoA synthase has been demonstrated to prefer monounsaturated and saturated C18- and C20-CoA substrates to elevate production of erucic acid in transgenic plants (Lassner et al., Plant Cell, 1996, Vol 8(2), pp. 281-292), whereas specific elongase enzymes of Trypanosoma brucei show preference for elongating short and midchain saturated CoA substrates (Lee et al., Cell, 2006, Vol 126(4), pp. 691-9).
[0008] The type II fatty acid biosynthetic pathway employs a series of reactions catalyzed by soluble proteins with intermediates shuttled between enzymes as thioesters of acyl carrier protein (ACP). By contrast, the type I fatty acid biosynthetic pathway uses a single, large multifunctional polypeptide.
[0009] The oleaginous, non-photosynthetic alga, Prototheca moriformis, stores copious amounts of triacylglyceride oil under conditions when the nutritional carbon supply is in excess, but cell division is inhibited due to limitation of other essential nutrients. Bulk biosynthesis of fatty acids with carbon chain lengths up to C18 occurs in the plastids; fatty acids are then exported to the endoplasmic reticulum where (if it occurs) elongation past C18 and incorporation into triacylglycerides (TAGs) is believed to occur. Lipids are stored in large cytoplasmic organelles called lipid bodies until environmental conditions change to favor growth, whereupon they are mobilized to provide energy and carbon molecules for anabolic metabolism.
SUMMARY OF THE INVENTION
[0010] In accordance with an embodiment, a method includes cultivating a recombinant cell, the cell [0011] (i) expressing an exogenous KASI or KASIV gene, optionally encoding a protein having at least 60, 65, 70, 75, 80, 85, 90, or 95% amino acid sequence identity to an enzyme encoded by any of SEQ ID NOs: 46-49, and at least one FATB acyl-ACP thioesterase gene optionally encoding a protein having at least 60, 65, 70, 75, 80, 85, 90, or 95% nucleic acid sequence identity to SEQ ID NOs: 11, 87, 89, 159, 162 or 163; [0012] (ii) expressing a gene encoding a FATA, FATB, KASI, KASII, LPAAT, SAD, or FAD2 under the control of a nitrogen-sensitive promoter having at least 60, 65, 70, 75, 80, 85, 90, or 95% sequence identity to any of SEQ ID NOs: 129 to 147; or [0013] (iii) having a knockout or knockdown of a SAD gene, a FAD2 gene, and a FATA gene, an overexpressing an exogenous C18-preferring FATA gene, an oleoyl-preferring LPAAT gene, and a KASII gene; and extracting oil from the cell.
[0014] In a related embodiment, the cell is of type (ii) and comprises at least a second acyl-ACP thioesterase, optionally encoding a protein having at least 60, 65, 70, 75, 80, 85, 90, or 95% nucleic acid sequence identity to any of SEQ ID NOS:: 11, 87, 89, 159, 162 or 163. The oil can have at least 30% C10:0 and at least 30% C12:0. The oil can have a viscosity of less than 30 cS and optionally of 25 cS.+-.20% at 40.degree. C. as measured by ASTM D445. The C10:0 and C12:0 fatty acids can be balanced to within 20%, 10% or 5%.
[0015] In a related embodiment, the cell is of type (iii) and the cell oil comprises at least 60% stearate-oleate-stearate (SOS). Optionally,the C18-preferring FATA gene encodes a protein with at least 60, 65, 70, 75, 80, 85, 90, or 95% amino acid identity to SEQ ID NO: 156, the LPAAT gene encodes a protein with at least 60, 65, 70, 75, 80, 85, 90, or 95% amino acid identity to SEQ ID NO: 157 and/or the KASII gene encodes a protein with at least 60, 65, 70, 75, 80, 85, 90, or 95% amino acid identity to SEQ ID NO 160 or 161.
[0016] Optionally, the cell is a microalga, optionally of Trebouxiophyceae, and optionally of the genus Prototheca.
[0017] In a related embodiment, there is an oil, soap, oleochemical, foodstuff, or other oil-derived product produced according to one of the aforementioned methods.
[0018] In accordance with an embodiment of the present invention, a method comprises cultivating an oleaginous recombinant cell. The cell comprises an exogenous gene encoding a palmitate ACP-desaturase enzyme active to produce an oil having a fatty acid profile characterized by a ratio of palmitoleic acid to palmitic acid of at least 0.1 and/or palmitoleic acid levels of 0.5% or more, as determined by FAME GC/FID analysis. Optionally, the cell is of an oleaginous recombinant eukaryotic microalga.
[0019] In related embodiments, the exogenous gene encodes a palmitoyl-ACP desaturase (PAD) having desaturating activity toward ACP-palmitate. Optionally, the exogenous PAD gene encodes a stearoyl-ACP desaturase variant having increased activity toward ACP-palmitate. The variant can be a L118W mutant. The gene can be in operable linkage with a promoter, plastid-targeting transit peptide, and 5'UTR active to express the gene product in a eukaryotic oleaginous microalga. The microalga can be of Trebouxiophyceae, and optionally of the genus Chlorella or Prototheca. Alternately, the microalga has 23 S rRNA with at least 65, 70, 75, 80, 85, 90 or 95% nucleotide sequence identity to SEQ ID NO: 76.
[0020] Optionally, the fatty acid profile is further characterized by less than 3.5% saturated fatty acids. Optionally, the cell is cultivated to at least 40% oil by dry cell weight. Optionally, the microalga further comprises a knockout or knockdown of an endogenous acyl-ACP thioesterase and/or an exogenous KASII gene. This may reduce the levels of saturated fatty acids in the oil. For example, the exogenous KASII gene can be inserted into the coding region of the endogenous acyl-ACP thioesterase. Optionally, the inserted KASII gene is inverted in orientation relative to the endogenous acyl-ACP thioesterase.
[0021] In any of these embodiments, the oil can be produced by heterotrophically cultivating the microalga on sucrose and the microalga comprises an exogenous invertase gene that allows it to metabolize the sucrose.
[0022] The oil may be recovered. The recovered oil may be used for frying or as an ingredient in a prepared food. The oil may have a microalga sterol profile. In a specific embodiment, the microalga sterol profile is characterized by an excess of ergosterol over .beta.-sitosterol and/or the presence of 22, 23-dihydrobrassicasterol, poriferasterol or clionasterol.
[0023] In another embodiment, a method comprises cultivating an oleaginous cell, optionally a microalga, so that the cell produces an oil with less than 10% palmitic acid, greater than Optionally the cell is a microalga with FAD and FATA knockouts and expresses an exogenous KASII gene.
[0024] In a related embodiment, a method comprises cultivating an oleaginous cell, optionally a microalga, so that the cell produces an oil with a fatty acid profile in which: the sum of lauric and myristic acids is at least 50%; total saturated fatty acids are at least 50% and levels of capric and lauric fatty acids are balanced to within 20%; or capric acid is at least 45% and lauric acid is at least 45%. In specific related embodiments the sum of lauric and myristic acids is at least 60%, 70% or 7%%. Optionally, the cell comprises an exogenous plant FATB gene.
[0025] Optionally, the cell comprises an exogenous exogenous KASI or KASIV gene.
[0026] The oil may be recovered. The recovered oil may be used for frying or as an ingredient in a prepared food. The oil may have a microalgal sterol profile. In a specific embodiment, the microalgal sterol profile is characterized by an excess of ergosterol over .beta.-sitosterol and/or the presence of 22, 23-dihydrobrassicasterol, poriferasterol or clionasterol. The oil can be used to make a foodstuff or chemical.
[0027] In another embodiment, a method comprises cultivating an oleaginous cell, optionally a microalga, so that the cell produces an oil with a fatty acid profile characterized by 10% or less linolenic acid and 20% or more linoleic acid. The cell can comprise an overexpressed KASII gene and a FAD gene replacement. Optionally, the cell comprises an exogenous gene encoding an oleate-specific acyl-ACP thioesterase or a knockout of one or more FATA alleles, together with an exogenous gene encoding an oleate-specific acyl-ACP thioesterase. The overexpression of the FAD gene can be by environmental control of a regulatable promoter. The oil can be recovered and used to produce a foodstuff or chemicals. The oil may comprise a microalgal sterol profile.
[0028] In another aspect, the present invention provides a method for producing a triglyceride oil, in which the method comprises: (a) cultivating an oleaginous cell under nitrogen-replete conditions, thereby increasing the number of cells, then; (b) cultivating the cells under nitrogen-poor conditions thereby causing the cells to accumulate triglycerides to at least 20% by dry cell weight; comprising a FADc (FAD2) allele, optionally a sole allele, under control of a promoter that is active under the nitrogen replete conditions and inactive under the nitrogen-starved conditions, the promoter retaining at least half of its activity at pH 5.0 as compared to pH 7.0; and (c) obtaining the oil, wherein the oil comprises reduced linoleic acid due to the downregulation of the FADc gene under the nitrogen-starved conditions.
[0029] In some embodiments, the cell is cultivated at a pH of less than 6.5 using sucrose in the presence of invertase. In some cases, the invertase is produced by the cell. In some cases, the invertase is produced from an exogenous gene expressed by the cell.
[0030] In some embodiments, the oil obtained has a fatty acid profile with less than 3%, 2%, 1%, or 0.5% linoleic acid.
[0031] In some embodiments, the cell further comprises a FADc knockout so as to amplify the change in linoleic acid. In some cases, the transcript level of FADc decreases by a factor of 10 or more between the nitrogen-replete and nitrogen-starved conditions.
[0032] In another aspect, the present invention provides a method for producing a triglyceride cell oil comprising cultivating a recombinant cell comprising an exogenous FATB gene and an exogenous KASI gene, wherein the expression of the KASI gene causes the oil to have a shorter chain distribution relative to a control cell with the FATB gene but without the KASI gene.
[0033] In another aspect, the present invention provides a recombinant cell comprising a FATB acyl-ACP thioesterase gene having at least 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 88% nucleotide identity to SEQ ID NOs: 90 or 91 or equivalent sequence due to the degeneracy of the genetic code, or encoding an enzyme having at least 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 88% amino acid identity to SEQ ID NOs: 90 or 91. In some embodiments, the cell produces triglycerides that are shifted in fatty acid profile due to expression of the FATB gene.
[0034] In an embodiment of the invention, there is a process for producing an oil. The process includes obtaining a cell oil from a genetically engineered microbe, optionally a microalga, and fractionating the cell oil to produce a stearin fraction. The stearin fraction can be characterized by a TAG profile having at least 70% SOS with no more than 4% trisaturates and an sn-2 profile characterized by least 90% oleate at the sn-2 position. Optionally, the microbe is a microalga comprising one or more of an overexpressed KASII gene, a SAD knockout or knockdown, or an exogenous C18-preferring FATA gene, an exogenous LPAAT, and a FAD2 knockout or knockdown. Optionally, the stearin fraction has a maximum heat-flow temperatures or DSC-derived SFC curve that is an essentially identical to the equivalent curve of Kokum butter. The fractionation can be a two step fractionation performed at a first temperature that removes 00S, optionally about 24.degree. C., and a second temperature that removes trisaturates, optionally about 29.degree. C.
[0035] In accordance with an embodiment of the invention a method produces a triglyceride oil characterized by a TAG profile. The method includes providing an oleaginous plastidic host cell overexpressing a KASII gene, an exogenous FATA gene and an exogenous LPAAT gene, cultivating the cell so as to produce the oil, and isolating the oil; the TAG profile has greater than 50% SOS an less than 10% trisaturates.
[0036] In related embodiments, the cell includes a knockdown or knockout of an endogenous SAD2 gene and/or knockdown or knockout of an endogenous FATA gene. The exogenous FATA gene can encode a functional FATA acyl-ACP thioesterase protein with at least 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% sequence identity to SEQ ID NO: 92. The exogenous LPAAT gene can encode a functional Lysophosphatidic acid acyltransferase protein with at least 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% sequence identity to SEQ ID NO: 93. Optionally, the host cell can be a microalga, optionally of Trebouxiophyceae, and optionally of the genus Chlorella or Prototheca, and optionally having 23S rRNA with at least 65, 70, 75, 80, 85, 90 or 95% nucleotide sequence identity to SEQ ID NO: 76.
[0037] In an embodiment, a recombinant microlagal host cell optionally of Trebouxiophyceae, and optionally of the genus Chlorella or Prototheca, and optionally having 23S rRNA with at least 65, 70, 75, 80, 85, 90 or 95% nucleotide sequence identity to SEQ ID NO: 76, expresses an exogenous FATA gene encodes a functional FATA acyl-ACP thioesterase protein with at least 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% sequence identity to SEQ ID NO: 92.
[0038] In an embodiment, a recombinant microlagal host cell optionally of Trebouxiophyceae, and optionally of the genus Chlorella or Prototheca, and optionally having 23S rRNA with at least 65, 70, 75, 80, 85, 90 or 95% nucleotide sequence identity to SEQ ID NO: 76, expresses an exogenous LPAAT gene encodes a functional Lysophosphatidic acid acyltransferase protein with at least 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% sequence identity to SEQ ID NO: 93.
[0039] These and other aspects and embodiments of the invention are described and/or exemplified in the accompanying drawings, a brief description of which immediately follows, the detailed description of the invention, and in the examples. Any or all of the features discussed above and throughout the application can be combined in various embodiments of the present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0040] FIGS. 1-14 show fatty acid profiles and melting curves of refined, bleached and deodorized oils from genetically engineered Prototheca moriformis strains, as discussed in Example 4;
[0041] FIG. 15 shows the stability of different oils as a function of antioxidant concentration, as discussed in Example 5;
[0042] FIG. 16 shows various properties of cell oils with very low levels of polyunsaturated fatty acids in accordance with an embodiment of the invention; and
[0043] FIG. 17 shows a plot of percent solid fat content for various oils as follows: (a) P. moriformis RBD oil without lipid pathway engineering; (b) Brazilian cocoa butter+25% milk fat; (c) three replicates of P. moriformis RBD oil from a strain expressing hairpin nucleic acids that reduce levels of a SAD allele thus reducing oleic acid and increasing stearic acid in the TAG profile; (d) P. moriformis RBD oil from a strain overexpressing an endogenous OTE (oleoyl acyl-ACP thioesterase, see Example 45); (e) Malaysian cocoa butter+25% milk fat; and (f) Malaysian cocoa butter. The cocoa butter and cocoa butter milk fat values are literature values (Bailey's Industrial Oils and Fat Products, 6.sup.th ed.).
[0044] FIG. 18 shows the results of thermal stability testing performed on methylated oil prepared from high-oleic (HO) and high-stability high-oleic (HSAO) triglyceride oils prepared from heterotrophically grown oleaginous microalgae, in comparison to a soya methyl ester control sample.
[0045] FIG. 19 shows various properties of high-oleic and high-stability high-oleic algal oils.
[0046] FIG. 20 shows TAG composition of Strain K-4, Strain AU and Strain AV oils from flask and fermenter biomass. La=laurate (C12:0), M=myristate (C14:0), P=palmitate (C16:0), Po=palmitoleate (C16:1), S=stearate (C18:0), O=oleate (C18:1), L=linoleate (C18:2), Ln=.alpha.-linolenate (C18:3), A=arachidate (C20:0), B=behenate (C22:0), Lg=lignocerate (C24:0), Hx=hexacosanoate (C26:0) S-S-S refers to the sum of TAGs in which all three fatty acids are saturated. In each block of bars, the strains are shown in the order illustrated at the bottom of the figure.
[0047] FIG. 21 shows TAG composition of Strain AW Strain AX and Strain AY oils from shake flask biomass. La=laurate (C12:0), M=myristate (C14:0), P=palmitate (C16:0), Po=palmitoleate (C16:1), S=stearate (C18:0), O=oleate (C18:1), L=linoleate (C18:2), Ln=.alpha.-linolenate (C18:3), A=arachidate (C20:0), B=behenate (C22:0), Lg=lignocerate (C24:0), Hx=hexacosanoate (C26:0). S-S-S refers to the sum of TAGs in which all three fatty acids are saturated. In each block of bars, the strains are shown in the order illustrated at the bottom of the figure.
[0048] FIG. 22 shows the fatty acid profile and solid fat content of a refined, bleached and deodorized myristate rich oil from a genetically engineered Prototheca moriformis strain as discussed in Example 52.
[0049] FIG. 23 shows the pairwise alignment of heterologous FAE proteins (SEQ ID NOS 165-171, respectively, in order of appearance) expressed in STRAIN Z.
[0050] FIG. 24 shows genetic modification of a microalgal strain to produced double knockouts of FAD2/FADc and FATA.
DETAILED DESCRIPTION OF THE INVENTION
I. DEFINITIONS
[0051] An "allele" refers to a copy of a gene where an organism has multiple similar or identical gene copies, even if on the same chromosome. An allele may encode the same or similar protein.
[0052] In connection with two fatty acids in a fatty acid profile, "balanced" shall mean that the two fatty acids are within a specified percentage of their mean area percent. Thus, for fatty acid a in x % abundance and fatty acid b in y % abundance, the fatty acids are "balanced to within z %" if |x-((x+y)/2)| and |y-((x+y)/2)| are .ltoreq.100(z).
[0053] A "cell oil" or "cell fat" shall mean a predominantly triglyceride oil obtained from an organism, where the oil has not undergone blending with another natural or synthetic oil, or fractionation so as to substantially alter the fatty acid profile of the triglyceride. In connection with an oil comprising triglycerides of a particular regiospecificity, the cell oil or cell fat has not been subjected to interesterification or other synthetic process to obtain that regiospecific triglyceride profile, rather the regiospecificity is produced naturally, by a cell or population of cells. For a cell oil produced by a cell, the sterol profile of oil is generally determined by the sterols produced by the cell, not by artificial reconstitution of the oil by adding sterols in order to mimic the cell oil. In connection with a cell oil or cell fat, and as used generally throughout the present disclosure, the terms oil and fat are used interchangeably, except where otherwise noted. Thus, an "oil" or a "fat" can be liquid, solid, or partially solid at room temperature, depending on the makeup of the substance and other conditions. Here, the term "fractionation" means removing material from the oil in a way that changes its fatty acid profile relative to the profile produced by the organism, however accomplished. The terms "cell oil" and "cell fat" encompass such oils obtained from an organism, where the oil has undergone minimal processing, including refining, bleaching and/or degumming, which does not substantially change its triglyceride profile. A cell oil can also be a "noninteresterified cell oil", which means that the cell oil has not undergone a process in which fatty acids have been redistributed in their acyl linkages to glycerol and remain essentially in the same configuration as when recovered from the organism.
[0054] "Exogenous gene" shall mean a nucleic acid that codes for the expression of an RNA and/or protein that has been introduced into a cell (e.g. by transformation/transfection), and is also referred to as a "transgene". A cell comprising an exogenous gene may be referred to as a recombinant cell, into which additional exogenous gene(s) may be introduced. The exogenous gene may be from a different species (and so heterologous), or from the same species (and so homologous), relative to the cell being transformed. Thus, an exogenous gene can include a homologous gene that occupies a different location in the genome of the cell or is under different control, relative to the endogenous copy of the gene. An exogenous gene may be present in more than one copy in the cell. An exogenous gene may be maintained in a cell as an insertion into the genome (nuclear or plastid) or as an episomal molecule.
[0055] "FADc", also referred to as "FAD2" is a gene encoding a delta-12 fatty acid desaturase.
[0056] "Fatty acids" shall mean free fatty acids, fatty acid salts, or fatty acyl moieties in a glycerolipid. It will be understood that fatty acyl groups of glycerolipids can be described in terms of the carboxylic acid or anion of a carboxylic acid that is produced when the triglyceride is hydrolyzed or saponified.
[0057] "Fixed carbon source" is a molecule(s) containing carbon, typically an organic molecule that is present at ambient temperature and pressure in solid or liquid form in a culture media that can be utilized by a microorganism cultured therein. Accordingly, carbon dioxide is not a fixed carbon source.
[0058] "In operable linkage" is a functional linkage between two nucleic acid sequences, such a control sequence (typically a promoter) and the linked sequence (typically a sequence that encodes a protein, also called a coding sequence). A promoter is in operable linkage with an exogenous gene if it can mediate transcription of the gene.
[0059] "Microalgae" are eukaryotic microbial organisms that contain a chloroplast or other plastid, and optionally that is capable of performing photosynthesis, or a prokaryotic microbial organism capable of performing photosynthesis. Microalgae include obligate photoautotrophs, which cannot metabolize a fixed carbon source as energy, as well as heterotrophs, which can live solely off of a fixed carbon source. Microalgae include unicellular organisms that separate from sister cells shortly after cell division, such as Chlamydomonas, as well as microbes such as, for example, Volvox, which is a simple multicellular photosynthetic microbe of two distinct cell types. Microalgae include cells such as Chlorella, Dunaliella, and Prototheca. Microalgae also include other microbial photosynthetic organisms that exhibit cell-cell adhesion, such as Agmenellum, Anabaena, and Pyrobotrys. Microalgae also include obligate heterotrophic microorganisms that have lost the ability to perform photosynthesis, such as certain dinoflagellate algae species and species of the genus Prototheca.
[0060] In connection with fatty acid length, "mid-chain" shall mean C8 to C16 fatty acids.
[0061] In connection with a recombinant cell, the term "knockdown" refers to a gene that has been partially suppressed (e.g., by about 1-95%) in terms of the production or activity of a protein encoded by the gene.
[0062] Also, in connection with a recombinant cell, the term "knockout" refers to a gene that has been completely or nearly completely (e.g., >95%) suppressed in terms of the production or activity of a protein encoded by the gene. Knockouts can be prepared by homologous recombination of a noncoding sequence into a coding sequence, gene deletion, mutation or other method.
[0063] An "oleaginous" cell is a cell capable of producing at least 20% lipid by dry cell weight, naturally or through recombinant or classical strain improvement. An "oleaginous microbe" or "oleaginous microorganism" is a microbe, including a microalga that is oleaginous (especially eukaryotic microalgae that store lipid). An oleaginous cell also encompasses a cell that has had some or all of its lipid or other content removed, and both live and dead cells.
[0064] An "ordered oil" or "ordered fat" is one that forms crystals that are primarily of a given polymorphic structure. For example, an ordered oil or ordered fat can have crystals that are greater than 50%, 60%, 70%, 80%, or 90% of the .beta. or .beta.' polymorphic form.
[0065] In connection with a cell oil, a "profile" is the distribution of particular species or triglycerides or fatty acyl groups within the oil. A "fatty acid profile" is the distribution of fatty acyl groups in the triglycerides of the oil without reference to attachment to a glycerol backbone. Fatty acid profiles are typically determined by conversion to a fatty acid methyl ester (FAME), followed by gas chromatography (GC) analysis with flame ionization detection (FID), as in Example 1. The fatty acid profile can be expressed as one or more percent of a fatty acid in the total fatty acid signal determined from the area under the curve for that fatty acid. FAME-GC-FID measurement approximate weight percentages of the fatty acids. A "sn-2 profile" is the distribution of fatty acids found at the sn-2 position of the triacylglycerides in the oil. A "regiospecific profile" is the distribution of triglycerides with reference to the positioning of acyl group attachment to the glycerol backbone without reference to stereospecificity. In other words, a regiospecific profile describes acyl group attachment at sn-1/3 vs. sn-2. Thus, in a regiospecific profile, POS (palmitate-oleate-stearate) and SOP (stearate-oleate-palmitate) are treated identically. A "stereospecific profile" describes the attachment of acyl groups at sn-1, sn-2 and sn-3. Unless otherwise indicated, triglycerides such as SOP and POS are to be considered equivalent. A "TAG profile" is the distribution of fatty acids found in the triglycerides with reference to connection to the glycerol backbone, but without reference to the regiospecific nature of the connections. Thus, in a TAG profile, the percent of SSO in the oil is the sum of SSO and SOS, while in a regiospecific profile, the percent of SSO is calculated without inclusion of SOS species in the oil. In contrast to the weight percentages of the FAME-GC-FID analysis, triglyceride percentages are typically given as mole percentages; that is the percent of a given TAG molecule in a TAG mixture.
[0066] The term "percent sequence identity," in the context of two or more amino acid or nucleic acid sequences, refers to two or more sequences or subsequences that are the same or have a specified percentage of amino acid residues or nucleotides that are the same, when compared and aligned for maximum correspondence, as measured using a sequence comparison algorithm or by visual inspection. For sequence comparison to determine percent nucleotide or amino acid identity, typically one sequence acts as a reference sequence, to which test sequences are compared. When using a sequence comparison algorithm, test and reference sequences are input into a computer, subsequence coordinates are designated, if necessary, and sequence algorithm program parameters are designated. The sequence comparison algorithm then calculates the percent sequence identity for the test sequence(s) relative to the reference sequence, based on the designated program parameters. Optimal alignment of sequences for comparison can be conducted using the NCBI BLAST software (ncbi.nlm.nih.gov/BLAST/) set to default parameters. For example, to compare two nucleic acid sequences, one may use blastn with the "BLAST 2 Sequences" tool Version 2.0.12 (Apr. 21, 2000) set at the following default parameters: Matrix: BLOSUM62; Reward for match: 1; Penalty for mismatch: -2; Open Gap: 5 and Extension Gap: 2 penalties; Gap.times.drop-off: 50; Expect: 10; Word Size: 11; Filter: on. For a pairwise comparison of two amino acid sequences, one may use the "BLAST 2 Sequences" tool Version 2.0.12 (Apr. 21, 2000) with blastp set, for example, at the following default parameters: Matrix: BLOSUM62; Open Gap: 11 and Extension Gap: 1 penalties; Gap.times.drop-off 50; Expect: 10; Word Size: 3; Filter: on.
[0067] "Recombinant" is a cell, nucleic acid, protein or vector that has been modified due to the introduction of an exogenous nucleic acid or the alteration of a native nucleic acid. Thus, e.g., recombinant cells can express genes that are not found within the native (non-recombinant) form of the cell or express native genes differently than those genes are expressed by a non-recombinant cell. Recombinant cells can, without limitation, include recombinant nucleic acids that encode for a gene product or for suppression elements such as mutations, knockouts, antisense, interfering RNA (RNAi) or dsRNA that reduce the levels of active gene product in a cell. A "recombinant nucleic acid" is a nucleic acid originally formed in vitro, in general, by the manipulation of nucleic acid, e.g., using polymerases, ligases, exonucleases, and endonucleases, using chemical synthesis, or otherwise is in a form not normally found in nature. Recombinant nucleic acids may be produced, for example, to place two or more nucleic acids in operable linkage. Thus, an isolated nucleic acid or an expression vector formed in vitro by ligating DNA molecules that are not normally joined in nature, are both considered recombinant for the purposes of this invention. Once a recombinant nucleic acid is made and introduced into a host cell or organism, it may replicate using the in vivo cellular machinery of the host cell; however, such nucleic acids, once produced recombinantly, although subsequently replicated intracellularly, are still considered recombinant for purposes of this invention. Similarly, a "recombinant protein" is a protein made using recombinant techniques, i.e., through the expression of a recombinant nucleic acid.
[0068] The terms "triglyceride", "triacylglyceride" and "TAG" are used interchangeably as is known in the art.
IL GENERAL
[0069] Illustrative embodiments of the present invention feature oleaginous cells that produce altered fatty acid profiles and/or altered regiospecific distribution of fatty acids in glycerolipids, and products produced from the cells. Examples of oleaginous cells include microbial cells having a type II fatty acid biosynthetic pathway, including plastidic oleaginous cells such as those of oleaginous algae and, where applicable, oil producing cells of higher plants including but not limited to commercial oilseed crops such as soy, corn, rapeseed/canola, cotton, flax, sunflower, safflower and peanut. Other specific examples of cells include heterotrophic or obligate heterotrophic microalgae of the phylum Chlorophtya, the class Trebouxiophytae, the order Chlorellales, or the family Chlorellacae. Examples of oleaginous microalgae and method of cultivation are also provided in Published PCT Patent Applications WO2008/151149, WO2010/06032, WO2011/150410, and WO2011/150411, including species of Chlorella and Prototheca, a genus comprising obligate heterotrophs. The oleaginous cells can be, for example, capable of producing 25, 30, 40, 50, 60, 70, 80, 85, or about 90% oil by cell weight, .+-.5%. Optionally, the oils produced can be low in highly unsaturated fatty acids such as DHA or EPA fatty acids. For example, the oils can comprise less than 5%, 2%, or 1% DHA and/or EPA. The above-mentioned publications also disclose methods for cultivating such cells and extracting oil, especially from microalgal cells; such methods are applicable to the cells disclosed herein and incorporated by reference for these teachings. When microalgal cells are used they can be cultivated autotrophically (unless an obligate heterotroph) or in the dark using a sugar (e.g., glucose, fructose and/or sucrose) In any of the embodiments described herein, the cells can be heterotrophic cells comprising an exogenous invertase gene so as to allow the cells to produce oil from a sucrose feedstock. Alternately, or in addition, the cells can metabolize xylose from cellulosic feedstocks. For example, the cells can be genetically engineered to express one or more xylose metabolism genes such as those encoding an active xylose transporter, a xylulose-5-phosphate transporter, a xylose isomerase, a xylulokinase, a xylitol dehydrogenase and a xylose reductase. See WO2012/154626, "GENETICALLY ENGINEERED MICROORGANISMS THAT METABOLIZE XYLOSE", published Nov. 15, 2012, including disclosure of genetically engineered Prototheca strains that utilize xylose.
[0070] The oleaginous cells may, optionally, be cultivated in a bioreactor/fermenter. For example, heterotrophic oleaginous microalgal cells can be cultivated on a sugar-containing nutrient broth. Optionally, cultivation can proceed in two stages: a seed stage and a lipid-production stage. In the seed stage, the number of cells is increased from s starter culture. Thus, the seeds stage typically includes a nutrient rich, nitrogen replete, media designed to encourage rapid cell division. After the seeds stage, the cells may be fed sugar under nutrient-limiting (e.g. nitrogen sparse) conditions so that the sugar will be converted into triglycerides. For example, the rate of cell division in the lipid-production stage can be decreased by 50%, 80% or more relative to the seed stage. Additionally, variation in the media between the seed stage and the lipid-production stage can induce the recombinant cell to express different lipid-synthesis genes and thereby alter the triglycerides being produced. For example, as discussed below, nitrogen and/or pH sensitive promoters can be placed in front of endogenous or exogenous genes. This is especially useful when an oil is to be produced in the lipid-production phase that does not support optimal growth of the cells in the seed stage. In an example below, a cell has a fatty acid desaturase with a pH sensitive promoter so than an oil that is low in linoleic acid is produced in the lipid production stage while an oil that has adequate linoleic acid for cell division is produced during the seed stage. The resulting low linoleic oil has exceptional oxidative stability.
[0071] The oleaginous cells express one or more exogenous genes encoding fatty acid biosynthesis enzymes. As a result, some embodiments feature cell oils that were not obtainable from a non-plant or non-seed oil, or not obtainable at all.
[0072] The oleaginous cells (optionally microalgal cells) can be improved via classical strain improvement techniques such as UV and/or chemical mutagenesis followed by screening or selection under environmental conditions, including selection on a chemical or biochemical toxin. For example the cells can be selected on a fatty acid synthesis inhibitor, a sugar metabolism inhibitor, or an herbicide. As a result of the selection, strains can be obtained with increased yield on sugar, increased oil production (e.g., as a percent of cell volume, dry weight, or liter of cell culture), or improved fatty acid or TAG profile.
[0073] For example, the cells can be selected on one or more of 1,2-Cyclohexanedione; 19-Norethindone acetate; 2,2-dichloropropionic acid; 2,4,5-trichlorophenoxyacetic acid; 2,4,5-trichlorophenoxyacetic acid, methyl ester; 2,4-dichlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid, butyl ester; 2,4-dichlorophenoxyacetic acid, isooctyl ester; 2,4-dichlorophenoxyacetic acid, methyl ester; 2,4-dichlorophenoxybutyric acid; 2,4-dichlorophenoxybutyric acid, methyl ester; 2,6-dichlorobenzonitrile; 2-deoxyglucose; 5-Tetradecyloxy-w-furoic acid; A-922500; acetochlor; alachlor; ametryn; amphotericin; atrazine; benfluralin; bensulide; bentazon; bromacil; bromoxynil; Cafenstrole; carbonyl cyanide m-chlorophenyl hydrazone (CCCP); carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP); cerulenin; chlorpropham; chlorsulfuron; clofibric acid; clopyralid; colchicine; cycloate; cyclohexamide; C75; DACTHAL (dimethyl tetrachloroterephthalate); dicamba; dichloroprop ((R)-2-(2,4-dichlorophenoxy)propanoic acid); Diflufenican; dihyrojasmonic acid, methyl ester; diquat; diuron; dimethylsulfoxide; Epigallocatechin gallate (EGCG); endothall; ethalfluralin; ethanol; ethofumesate; Fenoxaprop-p-ethyl; Fluazifop-p-Butyl; fluometuron; fomasefen; foramsulfuron; gibberellic acid; glufosinate ammonium; glyphosate; haloxyfop; hexazinone; imazaquin; isoxaben; Lipase inhibitor THL ((-)-Tetrahydrolipstatin); malonic acid; MCPA (2-methyl-4-chlorophenoxyacetic acid); MCPB (4-(4-chloro-o-tolyloxy)butyric acid); mesotrione; methyl dihydrojasmonate; metolachlor; metribuzin; Mildronate; molinate; naptalam; norharman; orlistat; oxadiazon; oxyfluorfen; paraquat; pendimethalin; pentachlorophenol; PF-04620110; phenethyl alcohol; phenmedipham; picloram; Platencin; Platensimycin; prometon; prometryn; pronamide; propachlor; propanil; propazine; pyrazon; Quizalofop-p-ethyl; s-ethyl dipropylthiocarbamate (EPTC); s,s,s-tributylphosphorotrithioate; salicylhydroxamic acid; sesamol; siduron; sodium methane arsenate; simazine; T-863 (DGAT inhibitor); tebuthiuron; terbacil; thiobencarb; tralkoxydim; triallate; triclopyr; triclosan; trifluralin; and vulpinic acid.
[0074] The oleaginous cells produce a storage oil, which is primarily triacylglyceride and may be stored in storage bodies of the cell. A raw oil may be obtained from the cells by disrupting the cells and isolating the oil. The raw oil may comprise sterols produced by the cells. WO2008/151149, WO2010/06032, WO2011/150410, and WO2011/1504 disclose heterotrophic cultivation and oil isolation techniques for oleaginous microalgae. For example, oil may be obtained by providing or cultivating, drying and pressing the cells. The oils produced may be refined, bleached and deodorized (RBD) as known in the art or as described in WO2010/120939. The raw or RBD oils may be used in a variety of food, chemical, and industrial products or processes. Even after such processing, the oil may retain a sterol profile characteristic of the source. Microalgal sterol profiles are disclosed below. See especially Section XII of this patent application. After recovery of the oil, a valuable residual biomass remains. Uses for the residual biomass include the production of paper, plastics, absorbents, adsorbents, drilling fluids, as animal feed, for human nutrition, or for fertilizer.
[0075] Where a fatty acid profile of a triglyceride (also referred to as a "triacylglyceride" or "TAG") cell oil is given here, it will be understood that this refers to a nonfractionated sample of the storage oil extracted from the cell analyzed under conditions in which phospholipids have been removed or with an analysis method that is substantially insensitive to the fatty acids of the phospholipids (e.g. using chromatography and mass spectrometry). The oil may be subjected to an RBD process to remove phospholipids, free fatty acids and odors yet have only minor or negligible changes to the fatty acid profile of the triglycerides in the oil. Because the cells are oleaginous, in some cases the storage oil will constitute the bulk of all the TAGs in the cell. Examples 1, 2, and 8 below give analytical methods for determining TAG fatty acid composition and regiospecific structure.
[0076] Broadly categorized, certain embodiments of the invention include (i) auxotrophs of particular fatty acids; (ii) cells that produce oils having low concentrations of polyunsaturated fatty acids, including cells that are auxotrophic for unsaturated fatty acids; (iii) cells producing oils having high concentrations of particular fatty acids due to expression of one or more exogenous genes encoding enzymes that transfer fatty acids to glycerol or a glycerol ester; (iv) cells producing regiospecific oils, (v) genetic constructs or cells encoding a newly discovered gene encoding an LPAAT enzyme from Cuphea PSR23 (see Example 43), (vi) cells producing low levels of saturated fatty acids and/or high levels of palmitoleic acid, (vii) cells producing erucic acid, and (viii) other inventions related to producing cell oils with altered profiles. The embodiments also encompass the oils made by such cells, the residual biomass from such cells after oil extraction, oleochemicals, fuels and food products made from the oils and methods of cultivating the cells.
[0077] In any of the embodiments below, the cells used are optionally cells having a type II fatty acid biosynthetic pathway such as microalgal cells including heterotrophic or obligate heterotrophic microalgal cells, including cells classified as Chlorophyta, Trebouxiophyceae , Chlorellales, Chlorellaceae, or Chlorophyceae, or cells engineered to have a type II fatty acid biosynthetic pathway using the tools of synthetic biology (i.e., transplanting the genetic machinery for a type II fatty acid biosynthesis into an organism lacking such a pathway). Use of a host cell with a type II pathway avoids the potential for non-interaction between an exogenous acyl-ACP thioesterase or other ACP-binding enzyme and the multienzyme complex of type I cellular machinery. In specific embodiments, the cell is of the species Prototheca moriformis, Prototheca krugani, Prototheca stagnora or Prototheca zopfii or has a 23S rRNA sequence with at least 65, 70, 75, 80, 85, 90 or 95% nucleotide identity SEQ ID NO: 76. By cultivating in the dark or using an obligate heterotroph, the cell oil produced can be low in chlorophyll or other colorants. For example, the cell oil can have less than 100, 50, 10, 5, 1, 0.0.5 ppm of chlorophyll without substantial purification.
[0078] The stable carbon isotope value 613C is an expression of the ratio of .sup.13C/.sup.12C relative to a standard (e.g. PDB, carbonite of fossil skeleton of Belemnite americana from Peedee formation of South Carolina). The stable carbon isotope value .delta.13C (.Salinity.) of the oils can be related to the 613C value of the feedstock used. In some embodiments the oils are derived from oleaginous organisms heterotrophically grown on sugar derived from a C4 plant such as corn or sugarcane. In some embodiments the 613C (.Salinity.) of the oil is from -10 to -17.Salinity. or from -13 to -16.Salinity..
[0079] In specific embodiments and examples discussed below, one or more fatty acid synthesis genes (e.g., encoding an acyl-ACP thioesterase, a keto-acyl ACP synthase, an LPAAT, a stearoyl ACP desaturase, or others described herein) is incorporated into a microalga. It has been found that for certain microalga, a plant fatty acid synthesis gene product is functional in the absence of the corresponding plant acyl carrier protein (ACP), even when the gene product is an enzyme, such as an acyl-ACP thioesterase, that requires binding of ACP to function. Thus, optionally, the microalgal cells can utilize such genes to make a desired oil without co-expression of the plant ACP gene.
[0080] For the various embodiments of recombinant cells comprising exogenous genes or combinations of genes, it is contemplated that substitution of those genes with genes having 60, 70, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% nucleic acid sequence identity can give similar results, as can substitution of genes encoding proteins having 60, 70, 80, 85, 90, 91, 92, 93, 94, 95, 95.5, 96, 96.5, 97, 97.5, 98, 98.5, 99 or 99.5% amino acid sequence identity. Likewise, for novel regulatory elements, it is contemplated that substitution of those nucleic acids with nucleic acids having 60, 70, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% nucleic acid can be efficacious. In the various embodiments, it will be understood that sequences that are not necessary for function (e.g. FLAG.RTM. tags or inserted restriction sites) can often be omitted in use or ignored in comparing genes, proteins and variants.
[0081] Although discovered using or exemplified with microalgae, the novel genes and gene combinations reported here can be used in higher plants using techniques that are well known in the art. For example, the use of exogenous lipid metabolism genes in higher plants is described in U.S. Pat. Nos. 6,028,247, 5,850,022, 5,639,790, 5,455,167, 5,512,482,and 5,298,421 disclose higher plants with exogenous acyl-ACP thioesterases. WO2009129582 and WO1995027791 disclose cloning of LPAAT in plants. FAD2 suppression in higher plants is taught in WO 2013112578, and WO 2008006171.
[0082] As described in Example 63, transcript profiling was used to discover promoters that modulate expression in response to low nitrogen conditions. The promoters are useful to selectively express various genes and to alter the fatty acid composition of microbial oils. In accordance with an embodiment, there are non-natural constructs comprising a heterologous promoter and a gene, wherein the promoter comprises at least 60, 65, 70, 75, 80, 85, 90, or 95% sequence identity to any of the promoters of Example 63 (e.g., SEQ ID NOs: 130-147) and the gene is differentially expressed under low vs. high nitrogen conditions. Optionally, the expression is less pH sensitive than for the AMT03 promoter. For example, the promoters can be placed in front of a FAD2 gene in a linoleic acid auxotroph to produce an oil with less than 5, 4, 3, 2, or 1% linoleic acid after culturing under high, then low nitrogen conditions.
III. FATTY ACID AUXOTROPHS/REDUCING FATTY ACID LEVELS TO GROWTH INHIBITORY CONDITIONS DURING AN OIL PRODUCTION PHASE
[0083] In an embodiment, the cell is genetically engineered so that all alleles of a lipid pathway gene are knocked out. Alternately, the amount or activity of the gene products of the alleles is knocked down so as to require supplementation with fatty acids. A first transformation construct can be generated bearing donor sequences homologous to one or more of the alleles of the gene. This first transformation construct may be introduced and selection methods followed to obtain an isolated strain characterized by one or more allelic disruptions. Alternatively, a first strain may be created that is engineered to express a selectable marker from an insertion into a first allele, thereby inactivating the first allele. This strain may be used as the host for still further genetic engineering to knockout or knockdown the remaining allele(s) of the lipid pathway gene (e.g., using a second selectable marker to disrupt a second allele). Complementation of the endogenous gene can be achieved through engineered expression of an additional transformation construct bearing the endogenous gene whose activity was originally ablated, or through the expression of a suitable heterologous gene. The expression of the complementing gene can either be regulated constitutively or through regulatable control, thereby allowing for tuning of expression to the desired level so as to permit growth or create an auxotrophic condition at will. In an embodiment, a population of the fatty acid auxotroph cells are used to screen or select for complementing genes; e.g., by transformation with particular gene candidates for exogenous fatty acid synthesis enzymes, or a nucleic acid library believed to contain such candidates.
[0084] Knockout of all alleles of the desired gene and complementation of the knocked-out gene need not be carried out sequentially. The disruption of an endogenous gene of interest and its complementation either by constitutive or inducible expression of a suitable complementing gene can be carried out in several ways. In one method, this can be achieved by co-transformation of suitable constructs, one disrupting the gene of interest and the second providing complementation at a suitable, alternative locus. In another method, ablation of the target gene can be effected through the direct replacement of the target gene by a suitable gene under control of an inducible promoter ("promoter hijacking"). In this way, expression of the targeted gene is now put under the control of a regulatable promoter. An additional approach is to replace the endogenous regulatory elements of a gene with an exogenous, inducible gene expression system. Under such a regime, the gene of interest can now be turned on or off depending upon the particular needs. A still further method is to create a first strain to express an exogenous gene capable of complementing the gene of interest, then to knockout out or knockdown all alleles of the gene of interest in this first strain. The approach of multiple allelic knockdown or knockout and complementation with exogenous genes may be used to alter the fatty acid profile, regiospecific profile, sn-2 profile, or the TAG profile of the engineered cell.
[0085] Where a regulatable promoter is used, the promoter can be pH-sensitive (e.g., amt03), nitrogen and pH sensitive (e.g., amt03), or nitrogen sensitive but pH-insensitive (e.g., newly discovered promoters of Example 63) or variants thereof comprising at least 60, 65, 70, 75, 80, 85, 90, 95, 96, 97, 98 or 99% sequence identity to any of the aforementioned promoters. In connection with a promoter, pH-insensitive means that the promoter is less sensitive than the amt03 promoter when environmental conditions are shifter from pH 6.8 to 5.0 (e.g., at least 5, 10, 15, or 20% less relative change in activity upon the pH-shift as compared to an equivalent cell with amt03 as the promoter).
[0086] In a specific embodiment, the recombinant cell comprises nucleic acids operable to reduce the activity of an endogenous acyl-ACP thioesterase; for example a FatA or FatB acyl-ACP thioesterase having a preference for hydrolyzing fatty acyl-ACP chains of length C18 (e.g., stearate (C18:0) or oleate (C18:1), or C8:0-C16:0 fatty acids. The activity of an endogenous acyl-ACP thioesterase may be reduced by knockout or knockdown approaches. Knockdown may be achieved, for example, through the use of one or more RNA hairpin constructs, by promoter hijacking (substitution of a lower activity or inducible promoter for the native promoter of an endogenous gene), or by a gene knockout combined with introduction of a similar or identical gene under the control of an inducible promoter. Example 34 describes the engineering of a Prototheca strain in which two alleles of the endogenous fatty acyl-ACP thioesterase (FATA1) have been knocked out. The activity of the Prototheca moriformis FATA1 was complemented by the expression of an exogenous FatA or FatB acyl-ACP thioesterase. Example 36 details the use of RNA hairpin constructs to reduce the expression of FATA in Prototheca, which resulted in an altered fatty acid profile having less palmitic acid and more oleic acid.
[0087] Accordingly, oleaginous cells, including those of organisms with a type II fatty acid biosynthetic pathway can have knockouts or knockdowns of acyl-ACP thioesterase-encoding alleles to such a degree as to eliminate or severely limit viability of the cells in the absence of fatty acid supplementation or genetic complementations. These strains can be used to select for transformants expressing acyl-ACP-thioesterase transgenes. Alternately, or in addition, the strains can be used to completely transplant exogenous acyl-ACP-thioesterases to give dramatically different fatty acid profiles of cell oils produced by such cells. For example, FATA expression can be completely or nearly completely eliminated and replaced with FATB genes that produce mid-chain fatty acids. Alternately, an organism with an endogenous FatA gene having specificity for palmitic acid (C16) relative to stearic or oleic acid (C18) can be replaced with an exogenous FatA gene having a greater relative specificity for stearic acid (C18:0) or replaced with an exogenous FatA gene having a greater relative specificity for oleic acid (C18:1). In certain specific embodiments, these transformants with double knockouts of an endogenous acyl-ACP thioesterase produce cell oils with more than 50, 60, 70, 80, or 90% caprylic, capric, lauric, myristic, or palmitic acid, or total fatty acids of chain length less than 18 carbons. Such cells may require supplementation with longer chain fatty acids such as stearic or oleic acid or switching of environmental conditions between growth permissive and restrictive states in the case of an inducible promoter regulating a FatA gene.
[0088] In an embodiment the oleaginous cells are cultured (e.g., in a bioreactor). The cells are fully auxotrophic or partially auxotrophic (i.e., lethality or synthetic sickness) with respect to one or more types of fatty acid. The cells are cultured with supplementation of the fatty acid(s) so as to increase the cell number, then allowing the cells to accumulate oil (e.g. to at least 40% by dry cell weight). Alternatively, the cells comprise a regulatable fatty acid synthesis gene that can be switched in activity based on environmental conditions and the environmental conditions during a first, cell division, phase favor production of the fatty acid and the environmental conditions during a second, oil accumulation, phase disfavor production of the fatty acid. In the case of an inducible gene, the regulation of the inducible gene can be mediated, without limitation, via environmental pH (for example, by using the AMT3 promoter as described in the Examples).
[0089] As a result of applying either of these supplementation or regulation methods, a cell oil may be obtained from the cell that has low amounts of one or more fatty acids essential for optimal cell propagation. Specific examples of oils that can be obtained include those low in stearic, linoleic and/or linolenic acids.
[0090] These cells and methods are illustrated in connection with low polyunsaturated oils in the section immediately below and in Example 6 (fatty acid desaturase auxotroph) in connection with oils low in polyunsaturated fatty acids and in Example 34 (acyl-ACP thioesterase auxotroph).
[0091] Likewise, fatty acid auxotrophs can be made in other fatty acid synthesis genes including those encoding a SAD, FAD, KASIII, KASI, KASII, KCS, elongase, GPAT, LPAAT, DGAT or AGPAT or PAP. These auxotrophs can also be used to select for complement genes or to eliminate native expression of these genes in favor of desired exogenous genes in order to alter the fatty acid profile, regiospecific profile, or TAG profile of cell oils produced by oleaginous cells.
[0092] Accordingly, in an embodiment of the invention, there is a method for producing an oil/fat. The method comprises cultivating a recombinant oleaginous cell in a growth phase under a first set of conditions that is permissive to cell division so as to increase the number of cells due to the presence of a fatty acid, cultivating the cell in an oil production phase under a second set of conditions that is restrictive to cell division but permissive to production of an oil that is depleted in the fatty acid, and extracting the oil from the cell, wherein the cell has a mutation or exogenous nucleic acids operable to suppress the activity of a fatty acid synthesis enzyme, the enzyme optionally being a stearoyl-ACP desaturase, delta 12 fatty acid desaturase, or a ketoacyl-ACP synthase. The oil produced by the cell can be depleted in the fatty acid by at least 50, 60, 70, 80, or 90%. The cell can be cultivated heterotrophically. The cell can be a microalgal cell cultivated heterotrophically or autotrophically and may produce at least 40, 50, 60, 70, 80, or 90% oil by dry cell weight.
IV. (A) LOW POLYUNSATURATED CELL OILS
[0093] In an embodiment of the present invention, the cell oil produced by the cell has very low levels of polyunsaturated fatty acids. As a result, the cell oil can have improved stability, including oxidative stability. The cell oil can be a liquid or solid at room temperature, or a blend of liquid and solid oils, including the regiospecific or stereospecific oils, high stearate oils, or high mid-chain oils described infra. Oxidative stability can be measured by the Rancimat method using the AOC S Cd 12b-92 standard test at a defined temperature. For example, the OSI (oxidative stability index) test may be run at temperatures between 110.degree. C. and 140.degree. C. The oil is produced by cultivating cells (e.g., any of the plastidic microbial cells mentioned above or elsewhere herein) that are genetically engineered to reduce the activity of one or more fatty acid desaturase. For example, the cells may be genetically engineered to reduce the activity of one or more fatty acyl .DELTA.12 desaturase(s) responsible for converting oleic acid (18:1) into linoleic acid (18:2) and/or one or more fatty acyl 415 desaturase(s) responsible for converting linoleic acid (18:2) into linolenic acid (18:3). Various methods may be used to inhibit the desaturase including knockout or mutation of one or more alleles of the gene encoding the desaturase in the coding or regulatory regions, inhibition of RNA transcription, or translation of the enzyme, including RNAi, siRNA, miRNA, dsRNA, antisense, and hairpin RNA techniques. Other techniques known in the art can also be used including introducing an exogenous gene that produces an inhibitory protein or other substance that is specific for the desaturase. In specific examples, a knockout of one fatty acyl .DELTA.12 desaturase allele is combined with RNA-level inhibition of a second allele.
[0094] In a specific embodiment, fatty acid desaturase (e.g., .DELTA.12 fatty acid desaturase) activity in the cell is reduced to such a degree that the cell is unable to be cultivated or is difficult to cultivate (e.g., the cell division rate is decreased more than 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 97 or 99%). Achieving such conditions may involve knockout, or effective suppression of the activity of multiple gene copies (e.g. 2, 3, 4 or more) of the desaturase or their gene products. A specific embodiment includes the cultivation in cell culture of a full or partial fatty acid auxotroph with supplementation of the fatty acid or a mixture of fatty acids so as to increase the cell number, then allowing the cells to accumulate oil (e.g. to at least 40% by cell weight). Alternatively, the cells comprise a regulatable fatty acid synthesis gene that can be switched in activity. For example, the regulation can be based on environmental conditions and the environmental conditions during a first, cell division, phase favor production of the fatty acid and the environmental conditions during a second, oil accumulation, phase disfavor production of the oil. For example, culture media pH and/or nitrogen levels can be used as an environmental control to switch expression of a lipid pathway gene to produce a state of high or low synthetic enzyme activity. Examples of such cells are described in Example 7.
[0095] In a specific embodiment, a cell is cultivated using a modulation of linoleic acid levels within the cell. In particular, the cell oil is produced by cultivating the cells under a first condition that is permissive to an increase in cell number due to the presence of linoleic acid and then cultivating the cells under a second condition that is characterized by linoleic acid starvation and thus is inhibitory to cell division, yet permissive of oil accumulation. For example, a seed culture of the cells may be produced in the presence of linoleic acid added to the culture medium. For example, the addition of linoleic acid to 0.25 g/L in the seed culture of a Prototheca strain deficient in linoleic acid production due to ablation of two alleles of a fatty acyl .DELTA.12 desaturase (i.e., a linoleic auxotroph) was sufficient to support cell division to a level comparable to that of wild type cells. Optionally, the linoleic acid can then be consumed by the cells, or otherwise removed or diluted. The cells are then switched into an oil producing phase (e.g., supplying sugar under nitrogen limiting conditions such as described in WO2010/063032). Surprisingly, oil production has been found to occur even in the absence of linoleic acid production or supplementation, as demonstrated in the obligate heterotroph oleaginous microalgae Prototheca but generally applicable to other oleaginous microalgae, microorganisms, or even multicellular organisms (e.g., cultured plant cells). Under these conditions, the oil content of the cell can increase to about 10, 20, 30, 40, 50, 60, 70, 80, 90%, or more by dry cell weight, while the oil produced can have polyunsaturated fatty acid (e.g.; linoleic+linolenic) profile with 5%, 4%, 3%, 2%, 1%, 0.5%, 0.3%, 0.2%, 0.1%, 0.05% or less, as a percent of total triacylglycerol fatty acids in the oil. For example, the oil content of the cell can be 50% or more by dry cell weight and the triglyceride of the oil produced less than 3% polyunsaturated fatty acids.
[0096] These oils can also be produced without the need (or reduced need) to supplement the culture with linoleic acid by using cell machinery to produce the linoleic acid during the cell division phase, but less or no linoleic acid in the lipid production phase. The linoleic-producing cell machinery may be regulatable so as to produce substantially less linoleic acid during the oil producing phase. The regulation may be via modulation of transcription of the desaturase gene(s) or modulation or modulation of production of an inhibitor substance (e.g., regulated production of hairpin RNA/RNAi). For example, the majority, and preferably all, of the fatty acid .DELTA.12 desaturase activity can be placed under a regulatable promoter regulated to express the desaturase in the cell division phase, but to be reduced or turned off during the oil accumulation phase. The regulation can be linked to a cell culture condition such as pH, and/or nitrogen level, as described in the examples herein, or other environmental condition. In practice, the condition may be manipulated by adding or removing a substance (e.g., protons via addition of acid or base) or by allowing the cells to consume a substance (e.g., nitrogen-supplying nutrients) to effect the desired switch in regulation of the desaturase activity.
[0097] Other genetic or non-genetic methods for regulating the desaturase activity can also be used. For example, an inhibitor of the desaturase can be added to the culture medium in a manner that is effective to inhibit polyunsaturated fatty acids from being produced during the oil production phase.
[0098] Accordingly, in a specific embodiment of the invention, there is a method comprising providing a recombinant cell having a regulatable delta 12 fatty acid desaturase gene, under control of a recombinant regulatory element via an environmental condition. The cell is cultivated under conditions that favor cell multiplication. Upon reaching a given cell density, the cell media is altered to switch the cells to lipid production mode by nutrient limitation (e.g. reduction of available nitrogen). During the lipid production phase, the environmental condition is such that the activity of the delta 12 fatty acid desaturase is downregulated. The cells are then harvested and, optionally, the oil extracted. Due to the low level of delta 12 fatty acid desaturase during the lipid production phase, the oil has less polyunsaturated fatty acids and has improved oxidative stability. Optionally the cells are cultivated heterotrophically and optionally microalgal cells.
[0099] Using one or more of these desaturase regulation methods, it is possible to obtain a cell oil that it is believed has been previously unobtainable, especially in large scale cultivation in a bioreactor (e.g., more than 1000 L). The oil can have polyunsaturated fatty acid levels that are 5%, 4%, 3%, 2%, 1%, 0.5%, 0.3%, 0.2%, or less, as an area percent of total triacylglycerol fatty acids in the oil.
[0100] One consequence of having such low levels of polyunsaturates is that oils are exceptionally stable to oxidation. Indeed, in some cases the oils may be more stable than any previously known cell cell oil. In specific embodiments, the oil is stable, without added antioxidants, at 110.degree. C. so that the inflection point in conductance is not yet reached by 10 hours, 15 hours, 20 hours, 30 hours, 40, hours, 50 hours, 60 hours, or 70 hours under conditions of the AOCS Cd 12b-92. Rancimat test, noting that for very stable oils, replenishment of water may be required in such a test due to evaporation that occurs with such long testing periods (see Example 5). For example the oil can have and OSI value of 40-50 hours or 41-46 hours at 110.degree. C. without added antioxidants. When antioxidants (suitable for foods or otherwise) are added, the OSI value measured may be further increased. For example, with added tocopherol (100ppm) and ascorbyl palmitate (500 ppm) or PANA and ascorbyl palmitate, such an oil can have an oxidative stability index (OSI value) at 110.degree. C. in excess 100 or 200 hours, as measured by the Rancimat test. In another example, 1050 ppm of mixed tocopherols and 500 pm of ascorbyl palmitate are added to an oil comprising less than 1% linoleic acid or less than 1% linoleic+linolenic acids; as a result, the oil is stable at 110.degree. C. for 1, 2, 3, 4, 5, 6, 7, 8, or 9, 10, 11, 12, 13, 14, 15, or 16, 20, 30, 40 or 50 days, 5 to 15 days, 6 to 14 days, 7 to 13 days, 8 to 12 days, 9 to 11 days, about 10 days, or about 20 days. The oil can also be stable at 130.degree. C. for 1, 2, 3, 4, 5, 6, 7, 8, or 9, 10, 11, 12, 13, 14, 15, or 16, 20, 30, 40 or 50 days, 5 to 15 days, 6 to 14 days, 7 to 13 days, 8 to 12 days, 9 to 11 days, about 10 days, or about 20 days. In a specific example, such an oil was found to be stable for greater than 100 hours (about 128 hours as observed). In a further embodiment, the OSI value of the cell oil without added antioxidants at 120.degree. C. is greater than 15 hours or 20 hours or is in the range of 10-15, 15-20, 20-25, or 25-50 hours, or 50-100 hours.
[0101] In an example, using these methods, the oil content of a microalgal cell is between 40 and about 85% by dry cell weight and the polyunsaturated fatty acids in the fatty acid profile of the oil is between 0.001% and 3% in the fatty acid profile of the oil and optionally yields a cell oil having an OSI induction time of at least 20 hours at 110.degree. C. without the addition of antioxidants. In yet another example, there is a cell oil produced by RBD treatment of a cell oil from an oleaginous cell, the oil comprises between 0.001% and 2% polyunsaturated fatty acids and has an OSI induction time exceeding 30 hours at 110C without the addition of antioxidants. In yet another example, there is a cell oil produced by RBD treatment of a cell oil from an oleaginous cell, the oil comprises between 0.001% and 1% polyunsaturated fatty acids and has an OSI induction time exceeding 30 hours at 110C without the addition of antioxidants.
[0102] In another specific embodiment there is an oil with reduced polyunsaturate levels produced by the above-described methods. The oil is combined with antioxidants such as PANA and ascorbyl palmitate. For example, it was found that when such an oil was combined with 0.5% PANA and 500ppm of ascorbyl palmitate the oil had an OSI value of about 5 days at 130.degree. C. or 21 days at 110.degree. C. These remarkable results suggest that not only is the oil exceptionally stable, but these two antioxidants are exceptionally potent stabilizers of triglyceride oils and the combination of these antioxidants may have general applicability including in producing stable biodegradable lubricants (e.g., jet engine lubricants). In specific embodiments, the genetic manipulation of fatty acyl .DELTA.12 desaturase results in a 2 to 30, or 5 to 25, or 10 to 20 fold increase in OSI (e.g., at 110.degree. C.) relative to a strain without the manipulation. The oil can be produced by suppressing desaturase activity in a cell, including as described above.
[0103] Antioxidants suitable for use with the oils of the present invention include alpha, delta, and gamma tocopherol (vitamin E), tocotrienol, ascorbic acid (vitamin C), glutathione, lipoic acid, uric acid, (3-carotene, lycopene, lutein, retinol (vitamin A), ubiquinol (coenzyme Q), melatonin, resveratrol, flavonoids, rosemary extract, propyl gallate (PG), tertiary butylhydroquinone (TBHQ), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT), N,N'-di-2-butyl-1,4-phenylenediamine,2,6-di-tert-butyl-4-methylphenol, 2,4-dimethyl-6-tert-butylphenol, 2,4-dimethyl-6-tert-butylphenol, 2,4-dimethyl-6-tert-butylphenol, 2,6-di-tert-butyl-4-methylphenol, 2,6-di-tert-butylphenol, and phenyl-alpha-naphthylamine (PANA).
[0104] In addition to the desaturase modifications, in a related embodiment other genetic modifications may be made to further tailor the properties of the oil, as described throughout, including introduction or substitution of acyl-ACP thioesterases having altered chain length specificity and/or overexpression of an endogenous or exogenous gene encoding a KAS, SAD, LPAAT, or DGAT gene. For example, a strain that produces elevated oleic levels may also produce low levels of polyunsaturates. Such genetic modifications can include increasing the activity of stearoyl-ACP desaturase (SAD) by introducing an exogenous SAD gene, increasing elongase activity by introducing an exogenous KASII gene, and/or knocking down or knocking out a FATA gene.
[0105] In a specific embodiment, a high oleic cell oil with low polyunsaturates may be produced. For example, the oil may have a fatty acid profile with greater than 60, 70, 80, 90, or 95% oleic acid and less than 5, 4, 3, 2, or 1% polyunsaturates. In related embodiments, a cell oil is produced by a cell having recombinant nucleic acids operable to decrease fatty acid .DELTA.12 desaturase activity and optionally fatty acid 415 desaturase so as to produce an oil having less than or equal to 3% polyunsaturated fatty acids with greater than 60% oleic acid, less than 2% polyunsaturated fatty acids and greater than 70% oleic acid, less than 1% polyunsaturated fatty acids and greater than 80% oleic acid, or less than 0.5% polyunsaturated fatty acids and greater than 90% oleic acid. It has been found that one way to increase oleic acid is to use recombinant nucleic acids operable to decrease expression of a FATA acyl-ACP thioesterase and optionally overexpress a KAS II gene; such a cell can produce an oil with greater than or equal to 75% oleic acid. Alternately, overexpression of KASII can be used without the FATA knockout or knockdown. Oleic acid levels can be further increased by reduction of delta 12 fatty acid desaturase activity using the methods above, thereby decreasing the amount of oleic acid the is converted into the unsaturates linoleic acid and linolenic acid. Thus, the oil produced can have a fatty acid profile with at least 75% oleic and at most 3%, 2%, 1%, or 0.5% linoleic acid. In a related example, the oil has between 80 to 95% oleic acid and about 0.001 to 2% linoleic acid, 0.01 to 2% linoleic acid, or 0.1 to 2% linoleic acid. In another related embodiment, an oil is produced by cultivating an oleaginous cell (e.g., a microalga) so that the microbe produces a cell oil with less than 10% palmitic acid, greater than 85% oleic acid, 1% or less polyunsaturated fatty acids, and less than 7% saturated fatty acids. See Example 58 in which such an oil is produced in a microalga with FAD and FATA knockouts plus expression of an exogenous KASII gene. Such oils will have a low freezing point, with excellent stability and are useful in foods, for frying, fuels, or in chemical applications. Further, these oils may exhibit a reduced propensity to change color over time. In an illustrative chemical application, the high oleic oil is used to produce a chemical. The oleic acid double bonds of the oleic acid groups of the triglycerides in the oil can be epoxidized or hydroxylated to make a polyol. The epoxidized or hydroxylated oil can be used in a variety of applications. One such application is the production of polyurethane (including polyurethane foam) via condensation of the hydroxylated triglyceride with an isocyanate, as has been practiced with hydroxylated soybean oil or castor oil. See, e.g. US2005/0239915, US2009/0176904, US2005/0176839, US2009/0270520, and U.S. Pat. No. 4,264,743 and Zlatanic, et al, Biomacromolecules 2002, 3, 1048-1056 (2002) for examples of hydroxylation and polyurethane condensation chemistries. Suitable hydroxyl forming reactions include epoxidation of one or more double bonds of a fatty acid followed by acid catalyzed epoxide ring opening with water (to form a diol), alcohol (to form a hydroxyl ether), or an acid (to form a hydroxyl ester). There are multiple advantages of using the high-oleic/low polyunsaturated oil in producing a bio-based polyurethane: (1) the shelf-life, color or odor, of polyurethane foams may be improved; (2) the reproducibility of the product may be improved due to lack of unwanted side reactions resulting from polyunsaturates; (3) a greater degree of hydroxylation reaction may occur due to lack of polyunsaturates and the structural characteristics of the polyurethane product can be improved accordingly.
[0106] The low-polyunsaturated or high-oleic/low-polyunsaturated oils described here may be advantageously used in chemical applications where yellowing is undesirable. For example, yellowing can be undesirable in paints or coatings made from the triglycerides fatty acids derived from the triglycerides. Yellowing may be caused by reactions involving polyunsaturated fatty acids and tocotrienols and/or tocopherols. Thus, producing the high-stability oil in an oleaginous microbe with low levels of tocotrienols can be advantageous in elevating high color stability a chemical composition made using the oil. In contrast to commonly used plant oils, through appropriate choice of oleaginous microbe, the cell oils of these embodiments can have tocopherols and tocotrienols levels of 1 g/L or less. In a specific embodiment, a cell oil has a fatty acid profile with less than 2% with polyunsaturated fatty acids and less than 1 g/L for tocopherols, tocotrienols or the sum of tocopherols and tocotrienols. In another specific embodiment, the cell oil has a fatty acid profile with less than 1% with polyunsaturated fatty acids and less than 0.5 g/L for tocopherols, tocotrienols or the sum of tocopherols and tocotrienols
[0107] Any of the high-stability (low-polyunsaturate) cell oils or derivatives thereof can be used to formulate foods, drugs, vitamins, nutraceuticals, personal care or other products, and are especially useful for oxidatively sensitive products. For example, the high-stability cell oil (e.g., less than or equal to 3%, 2% or 1% polyunsaturates) can be used to formulate a sunscreen (e.g. a composition having one or more of avobenzone, homosalate, octisalate, octocrylene or oxybenzone) or retinoid face cream with an increased shelf life due to the absence of free-radical reactions associated with polyunsaturated fatty acids. For example, the shelf-life can be increased in terms of color, odor, organoleptic properties or %active compound remaining after accelerated degradation for 4 weeks at 54.degree. C. The high stability oil can also be used as a lubricant with excellent high-temperature stability. In addition to stability, the oils can be biodegradable, which is a rare combination of properties.
[0108] In another related embodiment, the fatty acid profile of a cell oil is elevated in C8 to C16 fatty acids through additional genetic modification, e.g. through overexpression of a short-chain to mid chain preferring acyl-ACP thioesterase or other modifications described here. A low polyunsaturated oil in accordance with these embodiments can be used for various industrial, food, or consumer products, including those requiring improved oxidative stability. In food applications, the oils may be used for frying with extended life at high temperature, or extended shelf life.
[0109] Where the oil is used for frying, the high stability of the oil may allow for frying without the addition of antioxidant and/or defoamers (e.g. silicone). As a result of omitting defoamers, fried foods may absorb less oil. Where used in fuel applications, either as a triglyceride or processed into biodiesel or renewable diesel (see, e.g., WO2008/151149 WO2010/063032, and WO2011/150410), the high stability can promote storage for long periods, or allow use at elevated temperatures. For example, the fuel made from the high stability oil can be stored for use in a backup generator for more than a year or more than 5 years. The frying oil can have a smoke point of greater than 200.degree. C., and free fatty acids of less than 0.1% (either as a cell oil or after refining).
[0110] The low polyunsaturated oils may be blended with food oils, including structuring fats such as those that form beta or beta prime crystals, including those produced as described below. These oils can also be blended with liquid oils. If mixed with an oil having linoleic acid, such as corn oil, the linoleic acid level of the blend may approximate that of high oleic plant oils such as high oleic sunflower oils (e.g., about 80% oleic and 8% linoleic).
[0111] Blends of the low polyunsaturated cell oil can be interesterified with other oils. For example, the oil can be chemically or enzymatically interesterified. In a specific embodiment, a low polyunsaturated oil according to an embodiment of the invention has at least 10% oleic acid in its fatty acid profile and less than 5% polyunsaturates and is enzymatically interesterified with a high saturate oil (e.g. hydrogenated soybean oil or other oil with high stearate levels) using an enzyme that is specific for sn-1 and sn-2 triacylglycerol positions. The result is an oil that includes a stearate-oleate-stearate (SOS). Methods for interesterification are known in the art; see for example, "Enzymes in Lipid Modification," Uwe T. Bornschuer, ed., Wiley_VCH, 2000, ISBN 3-527-30176-3.
[0112] High stability oils can be used as spray oils. For example, dried fruits such as raisins can be sprayed with a high stability oil having less than 5, 4, 3, 2, or 1% polyunsaturates. As a result, the spray nozzle used will become clogged less frequently due to polymerization or oxidation product buildup in the nozzle that might otherwise result from the presence of polyunsaturates.
[0113] In a further embodiment, an oil that is high is SOS, such as those described below can be improved in stability by knockdown or regulation of delta 12 fatty acid desaturase.
[0114] Optionally, where the FADc promoter is regulated, it can be regulated with a promoter that is operable at low pH (e.g., one for which the level of transcription of FADc is reduced by less than half upon switching from cultivation at pH 7.0 to cultivation at pH 5.0). The promoter can be sensitive to cultivation under low nitrogen conditions such that the promoter is active under nitrogen replete conditions and inactive under nitrogen starved conditions. For example, the promoter may cause a reduction in FADc transcript levels of 5, 10, 15-fold or more upon nitrogen starvation. Because the promoter is operable at pH 5.0, more optimal invertase activity can be obtained. For example, the cell can be cultivated in the presence of invertase at a pH of less than 6.5, 6.0 or 5.5. The cell may have a FADc knockout to increase the relative gene-dosage of the regulated FADc. Optionally, the invertase is produced by the cell (natively or due to an exogenous invertase gene). Because the promoter is less active under nitrogen starved conditions, fatty acid production can proceed during the lipid production phase that would not allow for optimal cell proliferation in the cell proliferation stage. In particular, a low linoleic oil may be produced. The cell can be cultivated to an oil content of at least 20% lipid by dry cell weight. The oil may have a fatty acid profile having less than 5, 4, 3, 2, 1, or 0.5, 0.2, or 0.1% linoleic acid. Example 62 describes the discovery of such promoters.
IV. (B) HIGH 18:2/LOW 18:3 OILS OBTAINED USING FAD GENE REPLACEMENT
[0115] Surprisingly, while researching the production of low polyunsaturate oils as described above, an oil with high polyunsaturates but having a unique fatty acid profile was discovered. The discovery of this oil is described in Example 59. Thus, it is possible to use an oleaginous plastidic cell (e.g., microalgal) culture to produce an oil with a fatty acid profile characterized by 10% or less linolenic acid (C18:3) and 20% or more linoleic acid (C18:2). Such oils can be produced in an oleaginous microalga or other oleaginous plastidic cell by overexpression of a (endogenous or exogenous) KASII and gene replacement of FADc (also referred to as FAD2) and, if necessary based on the host cell, replacing native acyl-ACP thioesterase activity. In Example 58-59, an endogenous KASII was overexpressed and an endogenous FADc gene was placed under control of a pH-inducible promoter, although constitutive expression would also work. Interestingly, the oils were much higher in linoleic acid when the FADc was overexpressed in a linoleic acid auxotroph (e.g., a FADc double knockout). It is believed that this is due to the presence of a previously unrecognized gene-level regulatory system in microalgae that must be disabled in order to efficiently accumulate linoleic acid. In addition, two copies of the endogenous acyl-ACP thioesterase were knocked out and replaced with an oleate-specific plant acyl-ACP thioesterase. Under permissive pH conditions, an oil with 10% or less linolenic acid (C18:3) and 20% or more linoleic acid (C18:2). The oil can be extracted and used for various uses included in foodstuffs or chemicals. If the host cell is a microalga, the oil can comprise microalgal sterols. As with other embodiments, the host cell can be a microalga transformed to express an exogenous invertase, thus enable conversion of sucrose into the oil under conditions of heterotrophic cultivation.
[0116] In a specific embodiment, a host cell comprises a FADc knockdown, knockout, or FADc with a down-regulatable promoter combined with an exogenous KASII gene that expresses a protein having at least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99% amino acid identity to the protein encoded by the Prototheca moriformis KASII gene disclose in Example 58, and optionally expresses an acyl-ACP thioesterase gene producing an oleate-specific acyl-ACP thioesterase enzyme. Optionally, the cell can be an a plant cell, a microbial cell, or a microalgal cell.
V. CELLS WITH EXOGENOUS ACYLTRANSFERASES
[0117] In various embodiments of the present invention, one or more genes encoding an acyltransferase (an enzyme responsible for the condensation of a fatty acid with glycerol or a glycerol derivative to form an acylglyceride) can be introduced into an oleaginous cell (e.g., a plastidic microalgal cell) so as to alter the fatty acid composition of a cell oil produced by the cell. The genes may encode one or more of a glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidic acid acyltransferase (LPAAT), also known as 1-acylglycerol-3-phosphate acyltransferase (AGPAT), phosphatidic acid phosphatase (PAP), or diacylglycerol acyltransferase (DGAT) that transfers an acyl group to the sn-3 position of DAG, thereby producing a TAG.
[0118] Recombinant nucleic acids may be integrated into a plasmid or chromosome of the cell. Alternately, the gene encodes an enzyme of a lipid pathway that generates TAG precursor molecules through fatty acyl-CoA-independent routes separate from that above. Acyl-ACPs may be substrates for plastidial GPAT and LPAAT enzymes and/or mitochondrial GPAT and LPAAT enzymes. Among further enzymes capable of incorporating acyl groups (e.g., from membrane phospholipids) to produce TAGs is phospholipid diacylglycerol acyltransferase (PDAT). Still further acyltransferases, including lysophosphosphatidylcholine acyltransferase (LPCAT), lysophosphosphatidylserine acyltransferase (LPSAT), lysophosphosphatidylethanolamine acyltransferase (LPEAT), and lysophosphosphatidylinositol acyltransferase (LPIAT), are involved in phospholipid synthesis and remodeling that may impact triglyceride composition.
[0119] The exogenous gene can encode an acyltransferase enzyme having preferential specificity for transferring an acyl substrate comprising a specific number of carbon atoms and/or a specific degree of saturation is introduced into a oleaginous cell so as to produce an oil enriched in a given regiospecific triglyceride. For example, the coconut (Cocos nucifera) lysophosphatidic acid acyltransferase has been demonstrated to prefer C12:0-CoA substrates over other acyl-CoA substrates (Knutzon et al., Plant Physiology, Vol. 120, 1999, pp. 739-746), whereas the 1-acyl-sn-3-glycerol-3-phosphate acyltransferase of maturing safflower seeds shows preference for linoleoyl-CoA and oleoyl-CoA substrates over other acyl-CoA substrates, including stearoyl-CoA (Ichihara et al., European Journal of Biochemistry, Vol. 167, 1989, pp. 339-347). Furthermore, acyltransferase proteins may demonstrate preferential specificity for one or more short-chain, medium-chain, or long-chain acyl-CoA or acyl-ACP substrates, but the preference may only be encountered where a particular, e.g. medium-chain, acyl group is present in the sn-1 or sn-3 position of the lysophosphatidic acid donor substrate. As a result of the exogenous gene, a TAG oil can be produced by the cell in which a particular fatty acid is found at the sn-2 position in greater than 20, 30, 40, 50, 60, 70, 90, or 90% of the TAG molecules.
[0120] In some embodiments of the invention, the cell makes an oil rich in saturated-unsaturated-saturated (sat-unsat-sat) TAGs. Sat-unsat-sat TAGS include 1,3-dihexadecanoyl-2-(9Z-octadecenoyl)-glycerol (referred to as 1-palmitoyl-2-oleyl-glycero-3-palmitoyl), 1,3-dioctadecanoyl-2-(9Z-octadecenoyl)-glycerol (referred to as 1-stearoyl-2-oleyl-glycero-3-stearoyl), and 1-hexadecanoyl-2-(9Z-octadecenoyl)-3-octadecanoy-glycerol (referred to as 1-palmitoyl-2-oleyl-glycero-3-stearoyl). These molecules are more commonly referred to as POP, SOS, and POS, respectively, where `P` represents palmitic acid, `S` represents stearic acid, and `O` represents oleic acid. Further examples of saturated-unsaturated-saturated TAGs include MOM, LOL, MOL, COC and COL, where `M` represents myristic acid, `L` represents lauric acid, and `C` represents capric acid (C8:0). Trisaturates, triglycerides with three saturated fatty acyl groups, are commonly sought for use in food applications for their greater rate of crystallization than other types of triglycerides. Examples of trisaturates include PPM, PPP, LLL, SSS, CCC, PPS, PPL, PPM, LLP, and LLS. In addition, the regiospecific distribution of fatty acids in a TAG is an important determinant of the metabolic fate of dietary fat during digestion and absorption.
[0121] According to certain embodiments of the present invention, oleaginous cells are transformed with recombinant nucleic acids so as to produce cell oils that comprise an elevated amount of a specified regiospecific triglyceride, for example 1-acyl-2-oleyl-glycero-3-acyl, or 1-acyl-2-lauric-glycero-3-acyl where oleic or lauric acid respectively is at the sn-2 position, as a result of introduced recombinant nucleic acids. Alternately, caprylic, capric, myristic, or palmitic acid may be at the sn-2 position. The amount of the specified regiospecific triglyceride present in the cell oil may be increased by greater than 5%, greater than 10%, greater than 15%, greater than 20%, greater than 25%, greater than 30%, greater than 35%, greater than 40%, greater than 50%, greater than 60%, greater than 70%, greater than 80%, greater than 90%, greater than 100-500%, or greater than 500% than in the cell oil produced by the microorganism without the recombinant nucleic acids. As a result, the sn-2 profile of the cell triglyceride may have greater than 10, 20, 30, 40, 50, 60, 70, 80, or 90% of the particular fatty acid.
[0122] The identity of the acyl chains located at the distinct stereospecific or regiospecific positions in a glycerolipid can be evaluated through one or more analytical methods known in the art (see Luddy et al., J. Am. Oil Chem. Soc., 41, 693-696 (1964), Brockerhoff, J. Lipid Res., 6, 10-15 (1965), Angers and Aryl, J. Am. Oil Chem. Soc.,Vol. 76:4, (1999), Buchgraber et al., Eur. J. Lipid Sci. Technol., 106, 621-648 (2004)), or in accordance with Examples 1, 2, and 8 given below.
[0123] The positional distribution of fatty acids in a triglyceride molecule can be influenced by the substrate specificity of acyltransferases and by the concentration and type of available acyl moieties substrate pool. Nonlimiting examples of enzymes suitable for altering the regiospecificity of a triglyceride produced in a recombinant microorganism are listed in Tables 1-4. One of skill in the art may identify additional suitable proteins.
TABLE-US-00001 TABLE 1 Glycerol-3-phosphate acyltransferases and GenBank accession numbers. glycerol-3-phosphate Arabidopsis BAA00575 acyltransferase thaliana glycerol-3-phosphate Chlamydomonas EDP02129 acyltransferase reinhardtii glycerol-3-phosphate Chlamydomonas Q886Q7 acyltransferase reinhardtii acyl-(acyl-carrier-protein): Cucurbita BAB39688 glycerol-3-phosphate moschata acyltransferase glycerol-3-phosphate Elaeis AAF64066 acyltransferase guineensis glycerol-3-phosphate Garcina ABS86942 acyltransferase mangostana glycerol-3-phosphate Gossypium ADK23938 acyltransferase hirsutum glycerol-3-phosphate Jatropha ADV77219 acyltransferase curcas plastid glycerol-3- Jatropha ACR61638 phosphate acyltransferase curcas plastidial glycerol- Ricinus EEF43526 phosphate acyltransferase communis glycerol-3-phosphate Vica faba AAD05164 acyltransferase glycerol-3-phosphate Zea mays ACG45812 acyltransferase
[0124] Lysophosphatidic acid acyltransferases suitable for use with the microbes and methods of the invention include, without limitation, those listed in Table 2.
TABLE-US-00002 TABLE 2 Lysophosphatidic acid acyltransferases and GenBank accession numbers. 1-acyl-sn-glycerol-3- Arabidopsis AEE85783 phosphate acyltransferase thaliana 1-acyl-sn-glycerol-3- Brassica ABQ42862 phosphate acyltransferase juncea 1-acyl-sn-glycerol-3- Brassica ABM92334 phosphate acyltransferase juncea 1-acyl-sn-glycerol-3- Brassica CAB09138 phosphate acyltransferase napus lysophosphatidic acid Chlamydomonas EDP02300 acyltransferase reinhardtii lysophosphatidic acid Limnanthes AAC49185 acyltransferase alba 1-acyl-sn-glycerol-3- Limnanthes CAA88620 phosphate acyltransferase douglasii (putative) acyl-CoA:sn-1-acylglycerol- Limnanthes ABD62751 3-phosphate acyltransferase douglasii 1-acylglycerol-3-phosphate Limnanthes CAA58239 O-acyltransferase douglasii 1-acyl-sn-glycerol-3- Ricinus EEF39377 phosphate acyltransferase communis
[0125] Diacylglycerol acyltransferases suitable for use with the microbes and methods of the invention include, without limitation, those listed in Table 3.
TABLE-US-00003 TABLE 3 Diacylglycerol acyltransferases and GenBank accession numbers. diacylglycerol Arabidopsis CAB45373 acyltransferase thaliana diacylglycerol Brassica AAY40784 acyltransferase juncea putative diacylglycerol Elaeis AEQ94187 acyltransferase guineensis putative diacylglycerol Elaeis AEQ94186 acyltransferase guineensis acyl CoA:diacylglycerol Glycine AAT73629 acyltransferase max diacylglycerol Helianthus ABX61081 acyltransferase annus acyl-CoA:diacylglycerol Olea AAS01606 acyltransferase 1 europaea diacylglycerol Ricinus AAR11479 acyltransferase communis
[0126] Phospholipid diacylglycerol acyltransferases suitable for use with the microbes and methods of the invention include, without limitation, those listed in Table 4.
TABLE-US-00004 TABLE 4 Phospholipid diacylglycerol acyltransferases and GenBank accession numbers. phospholipid:diacylglycerol Arabidopsis AED91921 acyltransferase thaliana Putative phospholipid: Elaeis AEQ94116 diacylglycerol guineensis acyltransferase phospholipid:diacylglycerol Glycine XP_003541296 acyltransferase 1-like max phospholipid:diacylglycerol Jatropha AEZ56255 acyltransferase curcas phospholipid:diacylglycerol Ricinus ADK92410 acyltransferase communis phospholipid:diacylglycerol Ricinus AEW99982 acyltransferase communis
[0127] In an embodiment of the invention, known or novel LPAAT genes are transformed into the oleaginous cells so as to alter the fatty acid profile of triglycerides produced by those cells, most notably by altering the sn-2 profile of the triglycerides. For example, by virtue of expressing an exogenous active LPAAT in an oleaginous cell, the percent of unsaturated fatty acid at the sn-2 position is increased by 10, 20, 30, 40, 50, 60, 70, 80, 90% or more. For example, a cell may produce triglycerides with 30% unsaturates (which may be primarily 18:1 and 18:2 and 18:3 fatty acids) at the sn-2 position. In this example, introduction of the LPAAT activity increases the unsaturates at the sn-2 position by 20% so that 36% of the triglycerides comprise unsaturates at the sn-2 position. Alternately, an exogenous LPAAT can be used to increase mid-chain fatty acids including saturated mid-chains such as C8:0, C10:0, C12:0, C14:0 or C16:0 moieties at the sn-2 position. As a result, mid-chain levels in the overall fatty acid profile may be increased. Examples 43 and 44 describe altering the sn-2 and fatty acid profiles in an oleaginous microbe. As can be seen from those examples, the choice of LPAAT gene is important in that different LPAATs can cause a shift in the sn-2 and fatty acid profiles toward different acyl group chain-lengths or saturation levels. For example, the LPAAT of Example 43 increases C10-C14 fatty acids and the LPAAT of Example 44 causes an increase in C16 and C18 fatty acids. As in these examples, introduction of an exogenous LPAAT can be combined with introduction of exogenous acyl-ACP thioesterase. Combining a mid-chain preferring LPAAT and a mid-chain preferring FatB was found to give an additive effect; the fatty acid profile was shifted more toward the mid-chain fatty acids when both an exogenous LPAAT and FatB gene was present than when only an exogenous FatB gene was present. In a specific embodiment, the oil produced by a cell comprising an exogenous mid-chain specific LPAAT and (optionally) an exogenous FatB acyl-ACP thioesterase gene can have a fatty acid profile with 40, 50, 60, 70, 80% or more of C8:0, C10:0, C12:0, C14:0, or C16:0 fatty acids (separately or in sum).
[0128] Specific embodiments of the invention are a nucleic acid construct, a cell comprising the nucleic acid construct, a method of cultivating the cell to produce a triglyceride, and the triglyceride oil produced where the nucleic acid construct has a promoter operably linked to a novel LPAAT coding sequence. The coding sequence can have an initiation codon upstream and a termination codon downstream followed by a 3 UTR sequence. In a specific embodiment, the LPAAT gene has LPAAT activity and a coding sequence have at least 75, 80, 85, 90, 95, 96, 97, 98, or 99% sequence identity to any of the cDNAs of SEQ ID NOs: 80 to 85 or a functional fragment thereof including equivalent sequences by virtue of degeneracy of the genetic code. Introns can be inserted into the sequence as well. Alternately, the LPAAT gene codes for the amino acid sequence of SEQ ID NOs 77-79 or functional fragments thereof, or a protein having at least 75, 80, 85, 90, 95, 96, 97, 98, or 99% amino acid sequence identity . In addition to microalgae and other oleaginous cells, plants expressing the novel LPAAT as transgenes are expressly included in the embodiments and can be produced using known genetic engineering techniques.
VI. CELLS WITH EXOGENOUS ELONGASES OR ELONGASE COMPLEX ENZYMES
[0129] In various embodiments of the present invention, one or more genes encoding elongases or components of the fatty acyl-CoA elongation complex can be introduced into an oleaginous cell (e.g., a plastidic microalgal cell) so as to alter the fatty acid composition of the cell or of a cell oil produced by the cell. The genes may encode a beta-ketoacyl-CoA synthase (also referred to as 3-ketoacyl synthase, beta-ketoacyl synthase or KCS), a ketoacyl-CoA reductase, a hydroxyacyl-CoA dehydratase, enoyl-CoA reductase, or elongase. The enzymes encoded by these genes are active in the elongation of acyl-coA molecules liberated by acyl-ACP thioesterases. Recombinant nucleic acids may be integrated into a plasmid or chromosome of the cell. In a specific embodiment, the cell is of Chlorophyta, including heterotrophic cells such as those of the genus Prototheca.
[0130] Beta-Ketoacyl-CoA synthase and elongase enzymes suitable for use with the microbes and methods of the invention include, without limitation, those listed in Table 5.
TABLE-US-00005 TABLE 5 Beta-Ketoacyl-CoA synthases and elongases listed with GenBank accession numbers. Trypanosoma brucei elongase 3 (GenBank Accession No. AAX70673), Marchanita polymorpha (GenBank Accession No. AAP74370), Trypanosoma cruzi fatty acid elongase, putative (GenBank Accession No. EFZ33366), Nannochloropsis oculata fatty acid elongase (GenBank Accession No. ACV21066.1), Leishmania donovani fatty acid elongase, putative (GenBank Accession No. CBZ32733.1), Glycine max 3-ketoacyl-CoA synthase 11-like (GenBank Accession No. XP_003524525.1), Medicago truncatula beta-ketoacyl-CoA synthase (GenBank Accession No. XP_003609222), Zea mays fatty acid elongase (GenBank Accession No. ACG36525), Gossypium hirsutum beta- ketoacyl-CoA synthase (GenBank Accession No. ABV60087), Helianthus annuus beta-ketoacyl-CoA synthase (GenBank Accession No. ACC60973.1), Saccharomyces cerevisiae ELO1 (GenBank Accession No. P39540), Simmondsia chinensis beta- ketoacyl-CoA synthase (GenBank Accession No. AAC49186), Tropaeolum majus putative fatty acid elongase (GenBank Accession No. AAL99199, Brassica napus fatty acid elongase (GenBank Accession No. AAA96054)
[0131] In an embodiment of the invention, an exogenous gene encoding a beta-ketoacyl-CoA synthase or elongase enzyme having preferential specificity for elongating an acyl substrate comprising a specific number of carbon atoms and/or a specific degree of acyl chain saturation is introduced into a oleaginous cell so as to produce a cell or an oil enriched in fatty acids of specified chain length and/or saturation. Example 40 describes engineering of Prototheca strains in which exogenous fatty acid elongases with preferences for extending midchain fatty acyl-CoAs have been overexpressed to increase the concentration of stearate. Examples 42 and 54 describe engineering of Prototheca in which exogenous elongases or beta-ketoacyl-CoA synthases with preferences for extending monounsaturated and saturated C18- and C20-CoA substrates are overexpressed to increase the concentration of erucic acid.
[0132] In specific embodiments, the oleaginous cell produces an oil comprising greater than 0.5, 1, 2, 5, 10, 20, 30, 40, 50, 60 70, or 80% erucic and/or eicosenoic acid. Alternately, the cell produces an oil comprising 0.5-5, 5-10, 10-15, 15-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90-99% erucic or eicosenoic acid. The cell may comprise recombinant acids described above in connection with high-oleic oils with a further introduction of an exogenous beta-ketoacyl-CoA synthase that is active in elongating oleoyl-CoA. As a result of the expression of the exogenous beta-ketoacyl-CoA synthase, the natural production of erucic or eicosenoic acid by the cell can be increased by more than 2, 3, 4, 5, 10, 20, 30, 40, 50, 70, 100, 130, 170 or 200 fold. The high erucic and/or eicosenoic oil can also be a high stability oil; e.g., one comprising less than 5, 4, 3, 2, or 1% polyunsaturates and/or having the OSI values described in Section IV or this application and accompanying Examples. In a specific embodiment, the cell is a microalgal cell, optionally cultivated heterotrophically. As in the other embodiments, the oil/fat can be produced by genetic engineering of a plastidic cell, including heterotrophic microalgae of the phylum Chlorophyta, the class Trebouxiophytae, the order Chlorellales, or the family Chlorellacae. Preferably, the cell is oleaginous and capable of accumulating at least 40% oil by dry cell weight. The cell can be an obligate heterotroph, such as a species of Prototheca, including Prototheca moriformis or Prototheca zopfii.
[0133] In specific embodiments, an oleaginous microbial cell, optionally an oleaginous microalgal cell, optionally of the phylum Chlorophyta, the class Trebouxiophytae, the order Chlorellales, or the family Chlorellacae expresses an enzyme having 80, 85, 90, 95, 96, 97, 98, or 99% amino acid sequence identity to an enzyme of Table 5.
VII. REGIOSPECIFIC AND STEREOSPECIFIC OILS/FATS
[0134] In an embodiment, a recombinant cell produces a cell fat or oil having a given regiospecific makeup. As a result, the cell can produce triglyceride fats having a tendency to form crystals of a given polymorphic form; e.g., when heated to above melting temperature and then cooled to below melting temperature of the fat. For example, the fat may tend to form crystal polymorphs of the .beta. or .beta.' form (e.g., as determined by X-ray diffraction analysis), either with or without tempering. The fats may be ordered fats. In specific embodiments, the fat may directly from either .beta. or .beta.' crystals upon cooling; alternatively, the fat can proceed through a .beta. form to a .beta.' form. Such fats can be used as structuring, laminating or coating fats for food applications. The cell fats can be incorporated into candy, dark or white chocolate, chocolate flavored confections, ice cream, margarines or other spreads, cream fillings, pastries, or other food products. Optionally, the fats can be semi-solid (at room temperature) yet free of artificially produced trans-fatty acids. Such fats can also be useful in skin care and other consumer or industrial products.
[0135] As in the other embodiments, the fat can be produced by genetic engineering of a plastidic cell, including heterotrophic eukaryotic microalgae of the phylum Chlorophyta, the class Trebouxiophytae, the order Chlorellales, or the family Chlorellacae. Preferably, the cell is oleaginous and capable of accumulating at least 40% oil by dry cell weight. The cell can be an obligate heterotroph, such as a species of Prototheca, including Prototheca moriformis or Prototheca zopfii. The fats can also be produced in autotrophic algae or plants. Optionally, the cell is capable of using sucrose to produce oil and a recombinant invertase gene may be introduced to allow metabolism of sucrose, as described in PCT Publications WO2008/151149, WO2010/06032, WO2011/150410, WO2011/150411, and international patent application PCT/US12/23696. The invertase may be codon optimized and integrated into a chromosome of the cell, as may all of the genes mentioned here. It has been found that cultivated recombinant microalgae can produce hardstock fats at temperatures below the melting point of the hardstock fat. For example, Prototheca moriformis can be altered to heterotrophically produce triglyceride oil with greater than 50% stearic acid at temperatures in the range of 15 to 30.degree. C., wherein the oil freezes when held at 30.degree. C.
[0136] In an embodiment, the cell fat has at least 30, 40, 50, 60, 70, 80, or 90% fat of the general structure [saturated fatty acid (sn-1)-unsaturated fatty acid (sn-2)-saturated fatty acid (sn-3)]. This is denoted below as Sat-Unsat-Sat fat. In a specific embodiment, the saturated fatty acid in this structure is preferably stearate or palmitate and the unsaturated fatty acid is preferably oleate. As a result, the fat can form primarily .beta. or .beta.' polymorphic crystals, or a mixture of these, and have corresponding physical properties, including those desirable for use in foods or personal care products. For example, the fat can melt at mouth temperature for a food product or skin temperature for a cream, lotion or other personal care product (e.g., a melting temperature of 30 to 40, or 32 to 35.degree. C.). Optionally, the fats can have a 2 L or 3L lamellar structure (e.g., as determined by X-ray diffraction analysis). Optionally, the fat can form this polymorphic form without tempering.
[0137] In a specific related embodiment, a cell fat triglyceride has a high concentration of SOS (i.e. triglyceride with stearate at the terminal sn-1 and sn-3 positions, with oleate at the sn-2 position of the glycerol backbone). For example, the fat can have triglycerides comprising at least 50, 60, 70, 80 or 90% SOS. In an embodiment, the fat has triglyceride of at least 80% SOS. Optionally, at least 50, 60, 70, 80 or 90% of the sn-2 linked fatty acids are unsaturated fatty acids. In a specific embodiment, at least 95% of the sn-2 linked fatty acids are unsaturated fatty acids. In addition, the SSS (tri-stearate) level can be less than 20, 10 or 5% and/or the C20:0 fatty acid (arachidic acid) level may be less than 6%, and optionally greater than 1% (e.g., from 1 to 5%). For example, in a specific embodiment, a cell fat produced by a recombinant cell has at least 70% SOS triglyceride with at least 80% sn-2 unsaturated fatty acyl moieties. In another specific embodiment, a cell fat produced by a recombinant cell has TAGs with at least 80% SOS triglyceride and with at least 95% sn-2 unsaturated fatty acyl moieties. In yet another specific embodiment, a cell fat produced by a recombinant cell has TAGs with at least 80% SOS, with at least 95% sn-2 unsaturated fatty acyl moieties, and between 1 to 6% C20 fatty acids.
[0138] In yet another specific embodiment, the sum of the percent stearate and palmitate in the fatty acid profile of the cell fat is twice the percentage of oleate, .+-.10, 20, 30 or 40% [e.g., (% P+% S)/%O=2.0.+-.20%]. Optionally, the sn-2 profile of this fat is at least 40%, and preferably at least 50, 60, 70, or 80% oleate (at the sn-2 position). Also optionally, this fat may be at least 40, 50, 60, 70, 80, or 90% SOS. Optionally, the fat comprises between 1 to 6% C20 fatty acids.
[0139] In any of these embodiments, the high SatUnsatSat fat may tend to form .beta.' polymorphic crystals. Unlike previously available plant fats like cocoa butter, the SatUnsatSat fat produced by the cell may form .beta.' polymorphic crystals without tempering. In an embodiment, the polymorph forms upon heating to above melting temperature and cooling to less that the melting temperature for 3, 2, 1, or 0.5 hours. In a related embodiment, the polymorph forms upon heating to above 60.degree. C. and cooling to 10.degree. C. for 3, 2, 1, or 0.5 hours.
[0140] In various embodiments the fat forms polymorphs of the .beta. form, .beta.' form, or both, when heated above melting temperature and the cooled to below melting temperature, and optionally proceeding to at least 50% of polymorphic equilibrium within 5, 4, 3, 2, 1, 0.5 hours or less when heated to above melting temperature and then cooled at 10.degree. C. The fat may form .beta.' crystals at a rate faster than that of cocoa butter.
[0141] Optionally, any of these fats can have less than 2 mole % diacylglycerol, or less than 2 mole % mono and diacylglycerols, in sum.
[0142] In an embodiment, the fat may have a melting temperature of between 30-60.degree. C., 30-40.degree. C., 32 to 37.degree. C., 40 to 60.degree. C. or 45 to 55.degree. C. In another embodiment, the fat can have a solid fat content (SFC) of 40 to 50%, 15 to 25%, or less than 15% at 20.degree. C. and/or have an SFC of less than 15% at 35.degree. C.
[0143] The cell used to make the fat may include recombinant nucleic acids operable to modify the saturate to unsaturate ratio of the fatty acids in the cell triglyceride in order to favor the formation of SatUnsatSat fat. For example, a knock-out or knock-down of stearoyl-ACP desaturase (SAD) gene can be used to favor the formation of stearate over oleate or expression of an exogenous mid-chain-preferring acyl-ACP thioesterase gene can increase the levels mid-chain saturates. Alternately a gene encoding a SAD enzyme can be overexpressed to increase unsaturates.
[0144] In a specific embodiment, the cell has recombinant nucleic acids operable to elevate the level of stearate in the cell. As a result, the concentration of SOS may be increased. Example 9 demonstrates that the regiospecific profile of the recombinant microbe is enriched for the SatUnsatSat triglycerides POP, POS, and SOS as a result of overexpressing a Brassica napus C18:0-preferring thioesterase. An additional way to increase the stearate of a cell is to decrease oleate levels. For cells having high oleate levels (e.g., in excess of one half the stearate levels) one can also employ recombinant nucleic acids or classical genetic mutations operable to decrease oleate levels. For example, the cell can have a knockout, knockdown, or mutation in one or more FATA alleles, which encode an oleate liberating acyl-ACP thioesterase, and/or one or more alleles encoding a stearoyl ACP desaturase (SAD). Example 35 describes the inhibition of SAD2 gene product expression using hairpin RNA to produce a fatty acid profile of 37% stearate in Prototheca moriformis (UTEX 1435), whereas the wildtype strain produced less than 4% stearate, a more than 9-fold improvement. Moreover, while the strains of Example 35 are engineered to reduce SAD activity, sufficient SAD activity remains to produce enough oleate to make SOS, POP, and POS. See the TAG profiles of Example 47. In specific examples, one of multiple SAD encoding alleles may be knocked out and/or one or more alleles are downregulated using inhibition techniques such as antisense, RNAi, or siRNA, hairpin RNA or a combination thereof. In various embodiments, the cell can produce TAGs that have 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90 to about 100% stearate. In other embodiments, the cells can produce TAGs that are 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90 to about 100% SOS. Optionally, or in addition to genetic modification, stearoyl ACP desaturase can be inhibited chemically; e.g., by addition of sterculic acid to the cell culture during oil production.
[0145] Surprisingly, knockout of a single FATA allele has been found to increase the presence of C18 fatty acids produced in microalgae. By knocking out one allele, or otherwise suppressing the activity of the FATA gene product (e.g., using hairpin RNA), while also suppressing the activity of stearoyl-ACP desaturase (using techniques disclosed herein), stearate levels in the cell can be increased.
[0146] Another genetic modification to increase stearate levels includes increasing a ketoacyl ACP synthase (KAS) activity in the cell so as to increase the rate of stearate production. It has been found that in microalgae, increasing KASII activity is effective in increasing C18 synthesis and particularly effective in elevating stearate levels in cell triglyceride in combination with recombinant DNA effective in decreasing SAD activity. Recombinant nucleic acids operable to increase KASII (e.g., an exogenous KasII gene) can be also be combined with a knockout or knockdown of a FatA gene, or with knockouts or knockdowns of both a FatA gene and a SAD gene). Optionally, the KASII gene encodes a protein having at least 75, 80, 85, 90, 95, 96, 97, 98, or 99% amino acid identity to the KASII Prototheca moriformis (mature protein given in SEQ ID NO: 161), or any plant KASII gene disclosed herein (e.g., in Example 60) or known in the art including a microalgal KASII.
[0147] Optionally, the cell can include an exogenous stearate-liberating acyl-ACP thioesterase, either as a sole modification or in combination with one or more other stearate-increasing genetic modifications. For example the cell may be engineered to overexpress an acyl-ACP thioesterase with preference for cleaving C18:0-ACPs. Example 9 describes the expression of exogenous C18:0-preferring acyl-ACP thioesterases to increase stearate in the fatty acid profile of the microalgae, Prototheca moriformis (UTEX 1435), from about 3.7% to about 30.4% (over 8-fold). Example 41 provides additional examples of C18:0-preferring acyl-ACP thioesterases function to elevate C18:0 levels in Prototheca. Optionally, the stearate-preferring acyl-ACP thioesterase gene encodes an enzyme having at least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98 or 9% amino acid identity to the gene products of Example 9 or 41 (Seq ID NOS. 28, 65, 67, 69, 71, 73, or 75 omitting FLAG tags when present). Introduction of the acyl-ACP-thioesterase can be combined with a knockout or knockdown of one or more endogenous acyl-ACP thioesterase alleles. Introduction of the thioesterase can also be combined with overexpression of an elongase (KCS) or beta-ketoacyl-CoA synthase. In addition, one or more exogenous genes (e.g., encoding SAD or KASII) can be regulated via an environmental condition (e.g., by placement in operable linkage with a regulatable promoter). In a specific example, pH and/or nitrogen level is used to regulate an amt03 promoter. The environmental condition may then be modulated to tune the cell to produce the desired amount of stearate appearing in cell triglycerides (e.g., to twice the oleate concentration). As a result of these manipulations, the cell may exhibit an increase in stearate of at least 5, 10, 15, or 20 fold.
[0148] As a further modification, alone or in combination with the other stearate increasing modifications, the cell can comprise recombinant nucleic acids operable to express an elongase or a beta-ketoacyl-CoA synthase. For example, overexpression of a C18:0-preferring acyl-ACP thioesterases may be combined with overexpression of a midchain-extending elongase or KCS to increase the production of stearate in the recombinant cell. One or more of the exogenous genes (e.g., encoding a thioesterase, elongase, or KCS) can be regulated via an environmental condition (e.g., by placement in operable linkage with a regulatable promoter). In a specific example, pH and/or nitrogen level is used to regulate an amt03 promoter or any of the promoters of example 63 including those that are less pH-sensitive than amt03. The environmental condition may then be modulated to tune the cell to produce the desired amount of stearate appearing in cell triglycerides (e.g., to twice the oleate concentration). As a result of these manipulations, the cell may exhibit an increase in stearate of at least 5, 10, 15, or 20 fold. In addition to stearate, arachidic, behenic, lignoceric, and cerotic acids may also be produced.
[0149] In specific embodiments, due to the genetic manipulations of the cell to increase stearate levels, the ratio of stearate to oleate in the oil produced by the cell is 2:1.+-.30% (i.e., in the range of 1.4:1 to 2.6:1), 2:1.+-.20% or 2:1.+-.10%.
[0150] Alternately, the cell can be engineered to favor formation of SatUnsatSat where Sat is palmitate or a mixture of palmitate and stearate. In this case introduction of an exogenous palmitate liberating acyl-ACP thioesterase can promote palmitate formation. In this embodiment, the cell can produce triglycerides, that are at least 30, 40, 50, 60, 70, or 80% POP, or triglycerides in which the sum of POP, SOS, and POS is at least 30, 40, 50, 60, 70, 80, or 90% of cell triglycerides. In other related embodiments, the POS level is at least 30, 40, 50, 60, 70, 80, or 90% of the triglycerides produced by the cell.
[0151] In a specific embodiment, the melting temperature of the oil is similar to that of cocoa butter (about 30-32.degree. C.). The POP, POS and SOS levels can approximate cocoa butter at about 16, 38, and 23% respectively. For example, POP can be 16%.+-.20%, POS can be 38%.+-.20%, and SOS can be 23%.+-.20%. Or, POP can be 16%.+-.15%, POS can be 38%.+-.15%, an SOS can be 23%.+-.15%. Or, POP can be 16%.+-.10%, POS can be 38%.+-.10%, an SOS can be 23%.+-.10%.
[0152] As a result of the recombinant nucleic acids that increase stearate, a proportion of the fatty acid profile may be arachidic acid. For example, the fatty acid profile can be 0.01% to 5%, 0.1 to 4%, or 1 to 3% arachidic acid. Furthermore, the regiospecific profile may have 0.01% to 4%, 0.05% to 3%, or 0.07% to 2% AOS, or may have 0.01% to 4%, 0.05% to 3%, or 0.07% to 2% AOA. It is believed that AOS and AOA may reduce blooming and fat migration in confection comprising the fats of the present invention, among other potential benefits.
[0153] In addition to the manipulations designed to increase stearate and/or palmitate, and to modify the SatUnsatSat levels, the levels of polyunsaturates may be suppressed, including as described above by reducing delta 12 fatty acid desaturase activity (e.g., as encoded by a Fad gene) and optionally supplementing the growth medium or regulating FAD expression. It has been discovered that, in microalgae (as evidenced by work in Prototheca strains), polyunsaturates are preferentially added to the sn-2 position. Thus, to elevate the percent of triglycerides with oleate at the sn-2 position, production of linoleic acid by the cell may be suppressed. The techniques described herein, in connection with highly oxidatively stable oils, for inhibiting or ablating fatty acid desaturase (FAD) genes or gene products may be applied with good effect toward producing SatUnsatSat oils by reducing polyunsaturates at the sn-2 position. As an added benefit, such oils can have improved oxidatively stability. As also described herein, the fats may be produced in two stages with polyunsaturates supplied or produced by the cell in the first stage with a deficit of polyunsaturates during the fat producing stage. The fat produced may have a fatty acid profile having less than or equal to 15,10,7, 5, 4, 3, 2, 1, or 0.5% polyunsaturates. In a specific embodiment, the oil/fat produced by the cell has greater than 50% SatUnsatSat, and optionally greater than 50% SOS, yet has less than 3% polyunsaturates. Optionally, polyunsaturates can be approximated by the sum of linoleic and linolenic acid area% in the fatty acid profile.
[0154] In an embodiment, the cell fat is a Shea stearin substitute having 65% to 95% SOS and optionally 0.001 to 5% SSS. In a related embodiment, the fat has 65% to 95% SOS, 0.001 to 5% SSS, and optionally 0.1 to 8% arachidic acid containing triglycerides. In another related embodiment, the fat has 65% to 95% SOS and the sum of SSS and SSO is less than 10% or less than 5%.
[0155] The cell's regiospecific preference can be learned using the analytical method described below (Examples 1-2, 8). Despite balancing the saturates and unsaturates as describe above, it is possible that the cell enzymes do not place the unsaturated fatty acid at the sn-2 position. In this case, genetic manipulations can confer the desired regiospecificity by (i) reducing the activity of endogenous sn-2 specific acyl transferases (e.g., LPAAT) and/or (ii) introducing an exogenous LPAAT with the desired specificity (i.e., introduction of oleate at sn-2). Where an exogenous LPAAT is introduced, preferably the gene encoding the LPAAT is integrated into a host chromosome and is targeted to the endoplasmic reticulum. In some cases, the host cell may have both specific and non-specific LPAAT alleles and suppressing the activity of one of these alleles (e.g., with a gene knockout) will confer the desired specificity. For example, genes encoding the LPAATs of SEQ ID NO: 78 and SEQ ID NO: 79 or an LPAAT comprising at least 90, 95, 98, or 99% amino acid identity to either of these sequences, or a functional fragment thereof, can be used to add oleate to the sn-2 position in order to boost the levels of SatUnsatSat TAGs. The genes can have at least 80, 85, 90, 95, 96, 97, 98, or 99% nucleotide identity to any of SEQ ID NOs: 80 to 85 or equivalent sequences by virtue of the degeneracy of the genetic code. Alternatively, the proteins encoded by the genes can have at least 80, 85, 90, 95, 96, 97, 98, or 99% nucleotide identity to the gene products of any of SEQ ID NOs: 80 to 85. These genes can be manifest as recombinant nucleic acid constructs, vectors, chromosomes or host cells comprising these sequences or functional fragments thereof, which can be found by systematic deletion of nucleic acid from the sequences using known techniques. As a result of expression of the genes, the amount of sat-unsat-sat TAGs such as SOS, POS, POP, or triglycerides with C8 to C16 fatty acids at the sn-2 position can be increased in a host cell.
[0156] Among other discoveries, the above discussion and Examples below highlight certain pathways to obtain high Sat-Unsat-Sat oils in general and SOS oils in particular in microorganisms or in plants. Thus, it is possible that the use of genetic engineering techniques, optionally combined with classical mutagenesis and breeding, a microalga or higher plant can be produced with an increase in the amount of SatUnsatSat or SOS produced of at least 1.5-fold, 2-fold, 3-fold, 4-fold, 5-fold, or more relative to the starting strain. In another aspect, the SatUnsatSat or SOS concentration of a species for which the wild-type produces less than 20%, 30%, 40% or 50% SatUnsatSat or SOS can be increased so that the SatUnsatSat or SOS is increased to at least 30%, 40%, 50% or 60%, respectively. The key changes, relative to the starting or wild-type organism, are to increase the amount of stearate (e.g., by reducing the amount of oleate formed from stearate, e.g., by reducing SAD activity, and/or increasing the amount of palmitate that is converted to stearate by reducing the activity of FATA and/or increasing the activity of KASII) and by decreasing the amount of linoleate by reducing FAD2/FADc activity.
[0157] Optionally, the starting organism can have triacylglycerol (TAG) biosynthetic machineries which are predisposed toward the synthesis of TAG species in which oleate or unsaturated fatty acids, predominate at the sn-2 position. Many oilseed crops have this characteristic. It has been demonstrated that lysophosphatidic acyltransferases (LPAATs) play a critical role in determining the species of fatty acids which will ultimately be inserted at the sn-2 position. Indeed, manipulation, through heterologous gene expression, of LPAATs in higher plant seeds, can alter the species of fatty acid occupying the sn-2 position.
[0158] One approach to generating oils with significant levels of so-called structuring fats (typically comprised of the species SOS-stearate-oleate-stearate, POS-palmitate-oleate-stearate, or POP-palmitate-oleate-palmitate) in agriculturally important oilseeds and in algae, is through the manipulation of endogenous as well as heterologous LPAAT expression. Expression of LPAATs from seeds containing high levels of structuring fats, for example, would be one strategy to increase the level of structuring fats in an oil seed or oleaginous algae that normally contains only limited quantities of such fats.
[0159] An alternative or supplementary strategy, however, is to take advantage of the innate propensity of LPAATs in agriculturally important oilseeds (eg, safflower-Carthamus sp., sunflower-Helianthus sp., canola-Brassica sp., peanut-Arachis sp., soybean-Glycine sp., corn-Zea sp., olive-Olea sp., flax-Linum sp., palm-Elaeis sp. and cotton-Gossypium sp., see representative profiles in Table 5a below) and through either genetic engineering alone or a combination of genetic engineering and classical strain improvement (i.e. mutagenesis) selectively manipulate the species of fatty acids present in order to increase the levels of structuring fats. In the case of SOS, these manipulations are comprised of a series of discrete steps, which can be carried out independently. These include:
[0160] Increasing the level of stearate. This can be achieved, as we have demonstrated in microalgae here and others have shown in higher plants, through the expression of stearate specific FATA activities or down regulation of the endogenous SAD activity; e.g., through direct gene knockout, RNA silencing, or mutation, including classical strain improvement. Simply elevating stearate levels alone, by the above approaches,however, will not be optimal. For example, in the case of palm oil, the already high levels of palmitate, coupled with increased stearate levels, will likely overwhelm the existing LPAAT activity, leading to significant amounts of stearate and palmitate incorporation into tri-saturated fatty acids (SSS, PPP, SSP, PPS etc.). Hence, steps must be taken to control palmitate levels as well.
[0161] Palmitate levels must be minimized in order to create high SOS containing fats because palmitate, even with a high-functioning LPAAT, will occupy sn-1 or sn-3 positions that could be taken up by stearate, and, as outlined above, too many saturates will result in significant levels of tri-saturated TAG species. Palmitate levels can be lowered. for example, through down-regulation of endogenous FATA activity through mutation/classical strain improvement, gene knockouts or RNAi-mediated strategies, in instances wherein the endogenous FATA activity has significant palmitate activity. Alternatively, or in concert with the above, palmitate levels can be lowered through over expression of endogenous KASII activity or classical strain improvement efforts which manifest in the same effect, such that elongation from palmitate to stearate is enhanced. Simply lowering palmitate levels via the above methods may not be sufficient, however. Take again the example of palm oil. Reduction of palmitate and elevation of stearate via the previous methods would still leave significant levels of linoleic acid. The endogenous LPAAT activity in most higher plants species while they will preferentially insert oleate in the sn-2 position, will insert linoleic as the next most preferred species. As oleate levels decrease, linoleic will come to occupy the sn-2 position with increased frequency. TAG species with linoleic at the sn-2 position have poor structuring properties as the TAGs will tend to display much higher melting temperatures than what is desired in a structuring fat. Hence, increases in stearate and reductions in palmitate must in turn be balanced by reductions in levels of linoleic fatty acids.
[0162] In turn, levels of linoleic fatty acids must be minimized in order to create high SOS-containing fats because linoleate, even with a high functioning LPAAT will occupy sn-2 positions to the exclusion of oleate, creating liquid oils as opposed to the desired solid fat (at room temperature). Linoleate levels can be lowered, as we have demonstrated in microalgae and others have shown in plant oilseeds, through down regulation of endogenous FAD2 desaturases; e.g., through mutation/classical strain improvement, FAD2 knockouts or RNAi mediated down regulation of endogenous FAD2 activity. Accordingly, the linoleic acid level in the fatty acid profile can be reduced by at least 10, 20, 30, 40, 50, 100, 200, or 300%. For example, an RNAi construct with at least 70, 75, 80, 85, 90, 95, 96, 97, 98, or 99% identity to those disclosed herein can be used to downregulate FAD2.
[0163] Although one can choose a starting strain with such an sn-2 preference one can also introduce an exogenous LPAAT gene having a greater oleate preference, to further boost oleate at the sn-2 position and to further boost Sat-Unsat-Sat in the TAG profile. Optionally, one can replace one or more endogenous LPAAT alleles with the exogenous, more specific LPAAT.
[0164] The cell oils resulting from the SatUnsatSat/SOS producing organisms can be distinguished from conventional sources of SOS/POP/POS in that the sterol profile will be indicative of the host organism as distinguishable from the conventional source. Conventional sources of SOS/POP/POS include cocoa, shea, mango, sal, illipe, kokum, and allanblackia. See section XII of this disclosure for a discussion of microalgal sterols.
TABLE-US-00006 TABLE 5a The fatty acid profiles of some commercial oilseed strains. Common Food Oils* C12:0 C14:0 C16:0 C16:1 C18:0 C18:1 C18:2 C18:3 Corn oil <1.0 8.0-19.0 <0.5 0.5-4.0 19-50 38-65 <2.0 (Zea mays) Cottonseed oil <0.1 0.5-2.0 17-29 <1.5 1.0-4.0 13-44 40-63 0.1-2.1 (Gossypium barbadense) Canola <0.1 <0.2 <6.0 <1.0 <2.5 >50 <40 <14 (Brassica rapa, B. napus, B. juncea) Olive <0.1 6.5-20.0 .ltoreq.3.5 0.5-5.0 56-85 3.5-20.0 .ltoreq.1.2 (Olea europea) Peanut <0.1 <0.2 7.0-16.0 <1.0 1.3-6.5 35-72 13.0-43 <0.6 (Arachis hypogaea) Palm 0.5-5.9 32.0-47.0 2.0-8.0 34-44 7.2-12.0 (Elaeis guineensis) Safflower <0.1 <1.0 2.0-10.0 <0.5 1.0-10.0 7.0-16.0 72-81 <1.5 (Carthamus tinctorus) Sunflower <0.1 <0.5 3.0-10.0 <1.0 1.0-10.0 14-65 20-75 <0.5 (Helianthus annus) Soybean <0.1 <0.5 7.0-12.0 <0.5 2.0-5.5 19-30 48-65 5.0-10.0 (Glycine max) Solin-Flax <0.1 <0.5 2.0-9.0 <0.5 2.0-5.0 8.0-60 40-80 <5.0 (Linum usitatissimum) *Unless otherwise indicated, data taken from the U.S. Pharacopeia's Food and Chemicals Codex, 7th Ed. 2010-2011**
[0165] Accordingly, in an embodiment of the present invention, there is a method for increasing the amount of SOS in an oil (i.e. oil or fat) produced by a cell. The method comprises providing a cell and using classical and/or genetic engineering techniques (e.g., mutation, selection, strain-improvement, introduction of an exogenous gene and/or regulator element, or RNA-level modulation such as RNAi) to (i) increase the stearate in the oil, (ii) decrease the linoleate in the oil, and optionally (iii) increase the stereospecificity of the addition of oleate in the sn-2 position. The step of increasing the stearate can comprise decreasing desaturation by SAD (e.g., knockout, knockdown or use of regulatory elements) and increasing the conversion of palmitate to stearate (including overexpression of an endogenous or exogenous KASII and/or knockout or knockdown of FATA). Optionally, an exogenous FATA with greater stearate specificity then an endogenous FATA is expressed in the cell to increase stearate levels. Here, stearate-specificity of a FATA gene is a measure of the gene product's rate of cleavage of stearate over palmitate. The stearate-specific FATA gene insertion can be combined with a knockdown or knockout of the less-specific endogenous FATA gene. In this way, the ratio of stearate to palmitate can be increased, by 10%, 20%, 30%, 40%, 50%, 100% or more. The step of decreasing the linoleate can be via reduction of FADc/FAD2 activity including knockout and/or knockdown . The step of increasing the oleate at the sn-2 position can comprise expressing an exogenous oleate-preferring LPAAT such as an LPAAT having at least 75, 80, 85, 90, 85, 96, 97, 98, or 99% amino acid identity to an LPAAT disclosed herein.
[0166] In a specific embodiment, the cell (e.g, an oleaginous microalgal or other plastidic cell) produces an oil enriched in SOS (e.g., at least 50% SOS and in some cases 60% SOS). The cell is modified in at least four genes: (i) a .beta.-ketoacyl-ACP synthase II (KASII) is overexpressed, (ii) activity of an endogenous FATA acyl-ACP thioesterase is reduced (iii) a stearate-specific FATA acyl-ACP thioesterase is overexpressed, (iii) endogenous SAD activity is decreased, and (iv) endogenous FAD activity is decreased. Example 65 demonstrates this embodiment in a Prototheca moriformis microalga by disrupting the coding region of endogenous FATA and SAD2 through homologous recombination, overexpressing a .beta.-ketoacyl-ACP synthase II (KASII) gene, and activating FAD2 RNAi to decrease polyunsaturates.
[0167] In another specific embodiment, the cell (e.g, an oleaginous microalgal or other plastidic cell) produces an oil enriched in SOS (e.g., at least 50% SOS and in some cases 60% SOS). The cell is modified in at least four genes: (i) a .beta.-ketoacyl-ACP synthase II (KASII) is overexpressed, (ii) activity of an endogenous FATA acyl-ACP thioesterase is reduced (iii) a stearate-specific FATA acyl-ACP thioesterase is overexpressed, (iv) endogenous SAD activity is decreased, (v) endogenous FAD activity is decreased and (vi) an exogenous oleate-preferring LPAAT is expressed. See Examples 65 and 66. Optionally, these genes or regulatory elements have at least 75, 80, 85, 90, 85, 96, 97, 98, or 99% nucleic acid or amino acid identity to a gene or gene-product or regulatory element disclosed herein. Optionally, one or more of these genes is under control of a pH-sensitive or nitrogen-sensitive (pH-sensitive or pH-insensitive) promoter such as one having at least 75, 80, 85, 90, 85, 96, 97, 98, or 99% nucleic acid identity to one of those disclosed herein. Optionally, the cell oil is fractionated (see Example 64).
[0168] In an embodiment, fats produced by cells according to the invention are used to produce a confection, candy coating, or other food product. As a result, a food product like a chocolate or candy bar may have the "snap" (e.g., when broken) of a similar product produced using cocoa butter. The fat used may be in a beta polymorphic form or tend to a beta polymorphic form. In an embodiment, a method includes adding such a fat to a confection. Optionally, the fat can be a cocoa butter equivalent per EEC regulations, having greater than 65% SOS, less than 45% unsaturated fatty acid, less than 5% polyunsaturated fatty acids, less than 1% lauric acid, and less than 2% trans fatty acid. The fats can also be used as cocoa butter extenders, improvers, replacers, or anti-blooming agents, or as Shea butter replacers, including in food and personal care products. High SOS fats produced using the cells and methods disclosed here can be used in any application or formulation that calls for Shea butter or Shea fraction. However, unlike Shea butter, fats produced by the embodiments of the invention can have low amounts of unsaponifiables; e.g. less than 7, 5, 3, or 2% unsaponifiables. In addition, Shea butter tends to degrade quickly due to the presence of diacylglycerides whereas fats produced by the embodiments of the invention can have low amounts of diacylglycerides; e.g., less than 5, 4, 3, 2, 1, or 0.5% diacylglycerides.
[0169] In an embodiment of the invention there is a cell fat suitable as a shortening, and in particular, as a roll-in shortening. Thus, the shortening may be used to make pastries or other multi-laminate foods. The shortening can be produced using methods disclosed herein for producing engineered organisms and especially heterotrophic microalgae. In an embodiment, the shortening has a melting temperature of between 40 to 60.degree. C. and preferably between 45-55.degree. C. and can have a triglyceride profile with 15 to 20% medium chain fatty acids (C8 to C14), 45-50% long chain saturated fatty acids (C16 and higher), and 30-35% unsaturated fatty acids (preferably with more oleic than linoleic). The shortening may form .beta.' polymorphic crystals, optionally without passing through the .beta. polymorphic form. The shortening may be thixotropic. The shortening may have a solid fat content of less than 15% at 35.degree. C. In a specific embodiment, there is a cell oil suitable as a roll-in shortening produced by a recombinant microalga, where the oil has a yield stress between 400 and 700 or 500 and 600 Pa and a storage modulus of greater than 1.times.10.sup.5 Pa or 1.times.10.sup.6 Pa. (see Example 46)
[0170] A structured solid-liquid fat system can be produced using the structuring oils by blending them with an oil that is a liquid at room temperature (e.g., an oil high in tristearin or triolein). The blended system may be suitable for use in a food spread, mayonnaise, dressing, shortening; i.e. by forming an oil-water-oil emulsion. The structuring fats according to the embodiments described here, and especially those high in SOS, can be blended with other oils/fats to make a cocoa butter equivalent, replacer, or extender. For example, a cell fat having greater than 65% SOS can be blended with palm mid-fraction to make a cocoa butter equivalent.
[0171] In general, such high Sat-Unsat-Sat fats or fat systems can be used in a variety of other products including whipped toppings, margarines, spreads, salad dressings, baked goods (e.g. breads, cookies, crackers muffins, and pastries), cheeses, cream cheese, mayonnaise, etc.
[0172] In a specific embodiment, a Sat-Unsat-Sat fat described above is used to produce a margarine, spread, or the like. For example, a margarine can be made from the fat using any of the recipes or methods found in U.S. Pat. Nos. 7,118,773, 6,171,636, 4,447,462, 5,690,985, 5,888,575, 5,972,412, 6,171,636, or international patent publications WO9108677A1.
[0173] In an embodiment, a fat comprises a cell (e.g., from microalgal cells) fat optionally blended with another fat and is useful for producing a spread or margarine or other food product is produced by the genetically engineered cell and has glycerides derived from fatty acids which comprises: [0174] (a) at least 10 weight % of C18 to C24 saturated fatty acids, [0175] (b) which comprise stearic and/or arachidic and/or behenic and/or lignoceric acid and [0176] (c) oleic and/or linoleic acid, while [0177] (d) the ratio of saturated C18 acid/saturated (C20+C22+C24)-acids .gtoreq.1, preferably .gtoreq.5, more preferably .gtoreq.10, [0178] which glycerides contain: [0179] (e) .ltoreq.5 weight % of linolenic acid calculated on total fatty acid weight [0180] (f) .ltoreq.5 weight % of trans fatty acids calculated on total fatty acid weight [0181] (g) .ltoreq.75 weight %, preferably .ltoreq.60 weight % of oleic acid at the sn-2 position: which glycerides contain calculated on total glycerides weight [0182] (h) .ltoreq.8 weight % HOH+HHO triglycerides [0183] (i) .ltoreq.5 weight % of trisaturated triglycerides, and optionally one or more of the following properties: [0184] (j) a solid fat content of .gtoreq.10% at 10.degree. C. [0185] (k) a solid fat content .ltoreq.15% at 35.degree. C., [0186] (l) a solid fat content of .gtoreq.15% at 10.degree. C. and a solid fat content .ltoreq.25% at 35.degree. C., [0187] (m) the ratio of (HOH+HHO) and (HLH+HHL) triglycerides is >1, and preferably >2, [0188] where H stands for C18-C24 saturated fatty acid, 0 for oleic acid, and L for linoleic acid.
[0189] Optionally, the solid content of the fat (% SFC) is 11 to 30 at 10.degree. C., 4 to 15 at 20.degree. C., 0.5 to 8 at 30.degree. C., and 0 to 4 at 35.degree. C. Alternately, the % SFC of the fat is 20 to 45 at 10.degree. C., 14 to 25 at 20.degree. C., 2 to 12 at 30.degree. C., and 0 to 5 at 35.degree. C. In related embodiment, the % SFC of the fat is 30 to 60 at 10.degree. C., 20 to 55 at 20.degree. C., 5 to 35 at 30.degree. C., and 0 to 15 at 35.degree. C. The C12-C16 fatty acid content can be <15 weight %. The fat can have <5 weight % disaturated diglycerides.
[0190] In related embodiments there is a spread, margarine or other food product made with the cell oil or cell oil blend. For example, the cell fat can be used to make an edible W/O (water/oil) emulsion spread comprising 70-20 wt. % of an aqueous phase dispersed in 30-80 wt. % of a fat phase which fat phase is a mixture of 50-99 wt. % of a vegetable triglyceride oil A and 1-50 wt. % of a structuring triglyceride fat B, which fat consists of 5-100 wt. % of a hardstock fat C and up to 95 wt. % of a fat D, where at least 45 wt. % of the hardstock fat C triglycerides consist of SatOSat triglycerides and where Sat denotes a fatty acid residue with a saturated C18-C24 carbon chain and O denotes an oleic acid residue and with the proviso that any hardstock fat C which has been obtained by fractionation, hydrogenation, esterification or interesterification of the fat is excluded. The hardstock fat can be a cell fat produced by a cell according to the methods disclosed herein. Accordingly, the hardstock fat can be a fat having a regiospecific profile having at least 50, 60, 70, 80, or 90% SOS. The W/O emulsion can be prepared to methods known in the art including in U.S. Pat. No. 7,118,773.
[0191] In related embodiment, the cell also expresses an endogenous hydrolyase enzyme that produces ricinoleic acid. As a result, the oil (e.g., a liquid oil or structured fat) produced may be more easily emulsified into a margarine, spread, or other food product or non-food product. For example, the oil produced may be emulsified using no added emulsifiers or using lower amounts of such emulsifiers. The U.S. patent application Ser. No. 13/365,253 discloses methods for expressing such hydroxylases in microalgae and other cells. In specific embodiments, a cell oil comprises at least 1, 2, or 5% SRS, where S is stearate and R is ricinoleic acid.
[0192] In an alternate embodiment, a cell oil that is a cocoa butter mimetic as described above (or other high sat-unsat-sat oil such as a Shea or Kolum mimetic) can be fractionated to remove trisaturates (e.g., tristearin and tripalmitin, SSP, and PPS). For example, it has been found that microalgae engineered to decrease SAD activity to increase SOS concentration make an oil that can be fractionated to remove trisaturated. See Example 47 and example 64. In specific embodiments, the melting temperature of the fractionated cell oil is similar to that of cocoa butter (about 30-32.degree. C.). The POP, POS and SOS levels can approximate cocoa butter at about 16, 38, and 23% respectively. For example, POP can be 16%.+-.20%, POS can be 38%.+-.20%, an SOS can be 23%.+-.20%. Or, POP can be 16%.+-.15%, POS can be 38%.+-.15%, an SOS can be 23%.+-.15%. Or, POP can be 16%.+-.10%, POS can be 38%.+-.10%, an SOS can be 23%.+-.10%. In addition, the tristearin levels can be less than 5% of the triacylglycerides.
[0193] In an embodiment, a method comprises obtaining a cell oil obtained from a genetically engineered (e.g., microalga or other microbe) cell that produces a starting oil with a TAG profile having at least 40, 50, or 60% SOS. Optionally, the cell comprises one or more of an overexpressed KASII gene, a SAD knockout or knockdown, or an exogenous C18-preferring FATA gene, an exogenous LPAAT, and a FAD2 knockout or knockdown. The oil is fractionated by dry fractionation or solvent fractionation to give an enriched oil (stearin fraction) that is increased in SOS and decreased in trisaturates relative to the starting oil. The enriched oil can have at least 60%, 70% or 80% SOS with no more than 5%, 4%, 3%, 2% or 1% trisaturates. The enriched oil can have a sn-2 profile having 85, 90, 95% or more oleate at the sn-2 position. For example, thefractionated oil can comprise at least 60% SOS, no more than 5% trisaturates and at least 85% oleate at the sn-2 position. Alternatively, the oil can comprise at least 70% SOS, no more than 4% trisaturates and at least 90% oleate at the sn-2 position or 80% SOS, no more than 4% trisaturates and at least 95% oleate at the sn-2 position. Optionally, the oil has essentially identical maximum heat-flow temperatures and/or the DSC-derived SFC curves to Kokum butter. The stearin fraction can be obtained by dry fractionation, solvent fractionation, or a combination of these. Optionally, the process includes a 2-step dry fractionation at a first temperature and a second temperature. The first termperature can be higher or lower than the second temperature. In a specific embodiment, the first temperature is effective at removing 00S and the second temperature is effective in removing trisaturates. Optionally, the stearin fraction is washed with a solvent (e.g. acetone) to remove the 00S after treatment at the first temperature. Optionally, the first temperature is about 24.degree. C. and the second temperature is about 29.degree. C.
VIII. HIGH MID-CHAIN OILS
[0194] In an embodiment of the present invention, the cell has recombinant nucleic acids operable to elevate the level of midchain fatty acids (e.g., C8:0, C10:0, C12:0, C14:0, or C16:0 fatty acids) in the cell or in the oil of the cell. One way to increase the levels of midchain fatty acids in the cell or in the oil of the cell is to engineer a cell to express an exogenous acyl-ACP thioesterase that has activity towards midchain fatty acyl-ACP substrates (e.g., one encoded by a FatB gene), either as a sole modification or in combination with one or more other genetic modifications. An additional genetic modification to increase the level of midchain fatty acids in the cell or oil of the cell is the expression of an exogenous lysophosphatidic acid acyltransferase gene encoding an active lysophosphatidic acid acyltransferase (LPAAT) that catalyzes the transfer of a mid-chain fatty-acyl group to the sn-2 position of a substituted acylglyceroester. For example, the LPAAT gene can have 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99% amino acid sequence identity or have 75, 80, 85 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99% nucleic acid sequence identity (or equivalent sequence to degeneracy of the genetic code) to the mid-chain preferring LPAATs disclosed in Examples 43-44 (SEQ ID NOs 77, 78, 79, 81,82, 84, and 85). In a specific related embodiment, both an exogenous acyl-ACP thioesterase and LPAAT are stably expressed in the cell. In an embodiment, recombinant nucleic acids are introduced into an oleaginous cell (and especially into a plastidic microbial cell) that cause expression of an exogenous mid-chain-specific thioesterase and an exogenous LPAAT that catalyzes the transfer of a mid-chain fatty-acyl group to the sn-2 position of a substituted acylglyceroester. As a result, the cell can be made to increase the percent of a midchain fatty acid in the TAGs that it produces by 10, 20 30, 40, 50, 60, 70, 80, 90-fold, or more. Introduction of the exogenous LPAAT can increase midchain fatty acids at the sn-2 position by 1.2, 1.5, 1.7, 2, 3, 4 fold or more compared to introducing an exogenous mid-chain preferring acyl-ACP thioesterase alone. In an embodiment, the mid-chain fatty acid is greater than 30, 40, 50 60, 70, 80, or 90% of the TAG fatty acids produced by the cell. In various embodiments, the mid-chain fatty acid is lauric, myristic, or palmitic. Examples 3, 43, and 44 describe expression of plant LPAATs in microalgal cells with resulting alterations in fatty acid profiles. As in the examples, the cells can also express an exogenous acyl-ACP thioesterase (which can also be from a plant) with a preference for a given fatty acyl-ACP chain length. For example, a microalgal cell can comprise exogenous genes encoding a LPAAT and an acyl-ACP thioesterase that preferentially cleave C8, C10, C12, C14, C8-C12, or C8-C10 fatty acids. In a specific embodiment, such a cell is capable of producing a cell oil with a fatty acid profile comprising 10-20, 20-30, 30-40, 40-50, 50-60, 60-70, 70-80, 80-90, or 90-99%, >20%, >30%, >40%, >50%, >60%, >70%, >80% or >90% C8, C10, C12, C14, C8-C12, or C8-C10 fatty acids. Other LPAATs can preferentially cleave C16 or C18 fatty acids (see Example 44). Further genetic manipulation of the fatty acid desaturase pathway (e.g., as described infra) can increase the stability of the oils.
[0195] Any of these cell oils can be interesterified. Interesterification can, for example, be used to lower the melting temperature or pour-point of the oil. In a specific embodiment, the cell oil comprises at least 50% of the sum of caprylic and capric acids and may be interesterified to reduce the pour point and/or kinematic viscosity. Such an oil (cell or interesterified) can optionally be a high stability oil comprising, for example, less than 2% polyunsaturated fatty acids.
[0196] Alternately, or in addition to expression of an exogenous LPAAT, the cell may comprise recombinant nucleic acids that are operable to express an exogenous KASI or KASIV enzyme and optionally to decrease or eliminate the activity of a KASII, which is particularly advantageous when a mid-chain-preferring acyl-ACP thioesterase is expressed. Example 37 describes the engineering of Prototheca cells to overexpress KASI or KASIV enzymes in conjunction with a mid-chain preferring acyl-ACP thioesterase to generate strains in which production of C10-C12 fatty acids is about 59% of total fatty acids. Mid-chain production can also be increased by suppressing the activity of KASI and/or KASII (e.g., using a knockout or knockdown). Example 38 details the chromosomal knockout of different alleles of Prototheca moriformis (UTEX 1435) KASI in conjunction with overexpression of a mid-chain preferring acyl-ACP thioesterase to achieve fatty acid profiles that are about 76% or 84% C10-C14 fatty acids. Example 39 provides recombinant cells and oils characterized by elevated midchain fatty acids as a result of expression of KASI RNA hairpin polynucleotides. In addition to any of these modifications, unsaturated or polyunsaturated fatty acid production can be suppressed (e.g., by knockout or knockdown) of a SAD or FAD enzyme.
[0197] In a particular embodiment, a recombinant cell produces TAG having 40% lauric acid or more. In another related embodiment, a recombinant cell produces TAG having a fatty acid profile of 40% or more of myristic, caprylic, capric, or palmitic acid. For example, an oleaginous recombinant clorophyte cell can produce 40% lauric or myristic acid in an oil that makes up 40, 50, or 60% or more of the cell's dry weight.
[0198] In a specific embodiment, a recombinant cell comprises nucleic acids operable to express a product of an exogenous gene encoding a lysophosphatidic acid acyltransferase that catalyzes the transfer of a mid-chain fatty-acyl group to the sn-2 position of a substituted acylglyceroester and nucleic acids operable to express a product of an acyl-ACP thioesterase exogenous gene encoding an active acyl-ACP thioesterase that catalyzes the cleavage of mid-chain fatty acids from ACP. As a result, in one embodiment, the oil produced can be characterized by a fatty acid profile elevated in C10 and C12 fatty acids and reduced in C16, C18, and C18:1 fatty acids as a result of the recombinant nucleic acids. See Example 3, in which overexpression of a Cuphea wrightii acyl-ACP thioesterase and a Cocos nucifera LPAAT gene increased the percentage of C12 fatty acids from about 0.04% in the untransformed cells to about 46% and increased the percentage of C10 fatty acids from about 0.01% in the untransformed cells to about 11%. For example, the FATB gene can have 75, 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99% amino acid sequence identity or have 75, 80, 85 90, 91, 92, 93, 94, 95, 96, 97, 98 or 99% nucleic acid sequence identity (or equivalent sequence to degeneracy of the genetic code) to SEQ ID NOs 10 or 11. In related embodiments, the increase in midchain fatty acid production is greater than 70%, from 75-85%, from 70-90%, from 90-200%, from 200-300%, from 300-400%, from 400-500%, or greater than 500%.
[0199] Average chain length can also be reduced by overexpression of a C18-specific acyl-ACP thioesterase. Recombinant nucleic acids operable to overexpress a C18 or other acyl-ACP thioesterase may be used alone or in combination with the other constructs described here to further reduce average chain length. Among other uses, the oils produced can be used as cocoa-butter/milk fat substitute. See Example 45 and the discussion of FIG. 17. In an embodiment, one of the above described high mid-chain producing cells is further engineered to produce a low polyunsaturated oil by knocking out or knocking down one or more fatty acyl desaturases, as described above in section IV. Accordingly, the oil produced can have the high stability characteristic mentioned in that section or in corresponding Examples. In a specific embodiment, the cell produces an oil comprising greater than 30% midchain fatty acids and 5% or less polyunsaturates. In a related embodiment, the cell produces an oil comprising greater than 40% midchain fatty acids and 4% or less polyunsaturates. In a further related embodiment, the cell produces an oil comprising greater than 50% midchain fatty acids and 3% or less polyunsaturates.
[0200] In a specific embodiment, the cell produces an oil characterized by a fatty acid profile in which the sum of lauric and myristic acids is at least 50%, 60% , 70%, or 75%. This can be accomplished using the techniques of Examples 37-39, 43-44, 52, and 60-61. For example, Example 52 describes a method for producing an oil that has a fatty acid profile in which the sum of lauric and myristic acids is about 79% using a recombinant cell with an exogenous plant FATB acyl-ACP thioesterase.
[0201] In another specific embodiment, the cell produces a cell oil characterized by a fatty acid profile in which capric acid (C10:0) is at least 30% and lauric acid (C12:0) is at least 30%. For example, the absolute level of capric acid and lauric acid in the cell oil can be balanced to within 5, 10, 15, 20 or 30%. This can be accomplished using the techniques of Examples 37-39, 43-44, 52, and 60-61. As in Example 60, exogenous plant FATB and KASI (or KASIV) genes can be combined to give balanced levels of capric and lauric. Optionally, an endogenous KASI gene can be knocked out and replaced with an exogenous KASI. In addition, two or more exogenous FATB genes can be used do reach a desired fatty acid profile. In a specific embodiment, a microalgal cell expresses at least one and optionally at least two exogenous FATB genes and an exogenous KASI/KASIV gene and produces an extractable cell oil with at least 30% C10 and at least 30% C12 fatty acids. For example, the cell can express a FATB acyl-ACP thioesterase having at least 70, 75, 80, 85, 90 or 95% amino acid sequence identity to the Cuphea hookeriana FATB2 (SEQ ID NO: 158) and a beta-ketoacyl ACP synthase having at least 70, 75, 80, 85, 90 or 95% amino acid sequence identity to the Cuphea wrightii KASA1 (SEQ ID NO: 159, with alternate transit peptide). Further, a second exogenous FATB gene/enzyme can be expressed. The second FATB can have at least 70, 75, 80, 85, 90 or 95% amino acid sequence identity to the Cuphea wrightii FATB2 acyl-ACP thioesterase (SEQ ID NO: 11.) For these purposes, plastid targeted peptides can be aligned with or with out the plastid targeting transit peptides, which are less conserved and more easily replaceable than the remaining enzyme domain sequence.
[0202] In an embodiment, the cell produces an oil comprising greater than 75% saturated fatty acids. Optionally, the cell produces an oil comprising greater than 75% saturated fatty acids with less than 25% capric acid, less than 50% lauric acid, and less than 5% palmitic acid. In related embodiments, the oil comprises at least 80%, 95% or 90% saturated fatty acids. Example 60 describes the production of such oil by microalgae comprising multiple exogenous FATB genes and replacement of an endogenous KASI gene with exogenous KASI or KASIV genes from plants.
[0203] Examples 60 and 62 also shows that selection of FATB and KAS genes can give rise to an oil with at least 50% total saturates with capric and lauric acids balanced to within 20% (or even to within 15%, or 10%).
[0204] High-mid chain oils in general, and those produced by strains similar to those of Example 60 and 62 can possess low kinematic viscosity. For example, the oil can have a kinematic viscosity as measured using ASTM D445 at 40.degree. C. of 25 cS.+-.20%, 25 cS.+-.10%, or 25 cS.+-.5%. Likewise, the oil can have a kinematic viscosity according to ASTM D445 at 100.degree. C. of 5.4 cS.+-.20%, 5.4 cS.+-.10%, or 5.4 cS.+-.5%. The oil can have a viscosity index as measured using ASTM 2280 of 160.+-.20%, 160.+-.10%, or 160.+-.5%.
[0205] In a specific example, an oil prepared using a strain similar to those reported in Example 60, produced an oil with greater than 30% C10:0 and greater than 30% C12:0 fatty acids. The oil had a kinematic viscocity by ASTM 445 of 24.61 cSt at 40.degree. C. and 5.36 cSt at 100.degree. C. with a viscosity index (ASTM 2270) of 159. To make this oil, a Cuphea hookeriana FATB2 acyl-ACP thioesterase was expressed with a Cuphea wrightii KASA1 gene (with a P. moriformis SAD transit peptide) in Prototheca moriformis under control of the UAPA1 and AMT03 promoters, respectively. Neomycin resitance was used at the selection marker and the construct with incorporated in the KAS1-1 site. Accordingly, in an embodiment, a host cell comprises an exogenous gene that expresses a protein having at least 70, 75, 80, 85, 90, or 95% amino acid sequence identity to SEQ ID NO: 158 and also expresses a protein having at least 70, 75, 80, 85, 90, or 95% amino acid sequence identity to SEQ ID NO: 159. The cell produces an oil comprising at least 30% C10:0 and/or at least 30% C12:0 fatty acids. Optionally, a cell oil can be extracted from the cell that has a kinematic viscosity as measured using ASTM D445 at 40.degree. C. of less than 30 cSt.
[0206] The high mid-chain oils or fatty acids derived from hydrolysis of these oils may be particularly useful in food, fuel and oleochemical applications including the production of lubricants and surfactants. For example, fatty acids derived from the cells can be esterified, cracked, reduced to an aldehyde or alcohol, aminated, sulfated, sulfonated, or subjected to other chemical process known in the art.
[0207] In some embodiments, the cell oil is interesterified and the kinematic viscosity of the interesterified cell oil is less than 30, 20, 15, 10, 9, 8, 7, 6, 5, 4, 3, 2, or 1 centiStokes at 40.degree. C. In some embodiments, the kinematic viscosity is less than 3 centiStokes at 40.degree. C. In some embodiments, the pour point of an interesterified cell oil is less than, 5.degree. C., 0.degree. C., -10.degree. C., -12.degree. C., -15.degree. C., -20.degree. C., -25.degree. C., -30.degree. C., -35.degree. C., -40.degree. C., -45.degree. C., or -50.degree. C. In some embodiments, the pour point is less than -10.degree. C. In some embodiments, the pour point is less than -20.degree. C.
[0208] Example 53 describes the use of a plant FatB gene in algae to produce oils in microalgae with greater than 60% myristate. In an embodiment, a gene encoding a protein having at least 90, 95, 96, 97, 98, or 99% amino acid identity to SEQ ID NO:87 or SEQ ID NO:89 is used, optionally in combination with a mid-chain preferred LPAAT as described above.
[0209] As described in Example 62, we surprisingly discovered that the combination of a KASI gene with a FATB gene can shift the fatty acid profile of an oil produced by the cell in ways that neither gene can do on its own. Specifically, recombinant cells with exogenous plant myristate-preferring acyl-ACP thioesterases were discovered to shift their fatty acid profile to a greater percentage of laurate when a KASI gene was co-expressed. This is unexpected because KASI has an elongase activity yet the fatty acid profile was shifted to shorter chains. In other words, a cell expressing both the exogenous FATB and KASI gene produced an oil having a fatty acid profile that is shifted toward shorter fatty acid chains than a control cell with the FATB gene but without the KASI gene. Accordingly, an embodiment of the invention comprises constructing a recombinant cell or using the cell to make an oil, where the cell comprises an exogenous FATB with a given chain-length preference and a KASI gene, wherein the cell makes an oil with a shift in distribution toward shorter chains than is obtained without the KASI gene. Optionally, the FATB gene has a nucleic acid sequence that is at least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% identical (or an equivalent sequence by virtue of degeneracy of the genetic code) or has an amino acid sequence that is least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% identical to the CcFATB2-UcFATB2 FATB of Example 62 (SEQ ID NO: 162), the Cuphea wrightii FATB2 (SEQ ID NO: 11), Cuphea palustris FATB2 (SEQ ID NO: 87; SEQ ID NO: 89), Cuphea hyssopifolia FATB1 (SEQ ID NO: 163), Cuphea hyssopifolia FATB3 (SEQ ID NO: 164), or Cuphea hookeriana FATB2 (SEQ ID NO: 158). Optionally, the KASI or KASIV gene has a nucleic acid sequence that is at least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% identical (or an equivalent sequence by virtue of degeneracy of the genetic code) or has an amino acid sequence that is least 80, 85, 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99% identical to the Cuphea wrightii KASAI of Example 62 (SEQ ID NO: 159), the Cuphea hookeriana KASIV encoded by the sequence of SEQ ID NO:49, or the Cuphea pulch. KASIV encoded by SEQ ID NO: 48.
IX. HIGH OLEIC/PALMITIC OIL
[0210] In another embodiment, there is a high oleic oil with about 60% oleic acid, 25% palmitic acid and optionally 5% polyunsaturates or less. The high oleic oil can be produced using the methods disclosed in U.S. patent application Ser. No. 13/365,253, which is incorporated by reference in relevant part. For example, the cell can have nucleic acids operable to suppress an acyl-ACP thioesterase (e.g., knockout or knockdown of a gene encoding FATA) while also expressing a gene that increases KASII activity. The cell can have further modifications to inhibit expression of delta 12 fatty acid desaturase, including regulation of gene expression as described above. As a result, the polyunsaturates can be less than or equal to 5, 4, 3, 2, or 1 area%.
X. LOW SATURATE OIL
[0211] In an embodiment, a cell oil is produced from a recombinant cell. The oil produced has a fatty acid profile that has less that 4%, 3%, 2%, or 1% (area %), saturated fatty acids. In a specific embodiment, the oil has 0.1 to 3.5% saturated fatty acids. Certain of such oils can be used to produce a food with negligible amounts of saturated fatty acids. Optionally, these oils can have fatty acid profiles comprising at least 90% oleic acid or at least 90% oleic acid with at least 3% polyunsaturated fatty acids. In an embodiment, a cell oil produced by a recombinant cell comprises at least 90% oleic acid, at least 3% of the sum of linoleic and linolenic acid and has less than 3.5% saturated fatty acids. In a related embodiment, a cell oil produced by a recombinant cell comprises at least 90% oleic acid, at least 3% of the sum of linoleic and linolenic acid and has less than 3.5% saturated fatty acids, the majority of the saturated fatty acids being comprised of chain length 10 to 16. These oils may be produced by recombinant oleaginous cells including but not limited to those described here and in U.S. patent application Ser. No. 13/365,253. For example, overexpression of a KASII enzyme in a cell with a highly active SAD can produce a high oleic oil with less than or equal to 3.5% saturates. Optionally, an oleate-specific acyl-ACP thioesterase is also overexpressed and/or an endogenous thioesterase having a propensity to hydrolyze acyl chains of less than C18 knocked out or suppressed. The oleate-specific acyl-ACP thioesterase may be a transgene with low activity toward ACP-palmitate and ACP-stearate so that the ratio of oleic acid relative to the sum of palmitic acid and stearic acid in the fatty acid profile of the oil produced is greater than 3, 5, 7, or 10. Alternately, or in addition, a FATA gene may be knocked out or knocked down, as in Example 36 below. A FATA gene may be knocked out or knocked down and an exogenous KASII overexpressed. Another optional modification is to increase KASI and/or KASIII activity, which can further suppress the formation of shorter chain saturates. Optionally, one or more acyltransferases (e.g., an LPAAT) having specificity for transferring unsaturated fatty acyl moieties to a substituted glycerol is also overexpressed and/or an endogenous acyltransferase is knocked out or attenuated. An additional optional modification is to increase the activity of KCS enzymes having specificity for elongating unsaturated fatty acids and/or an endogenous KCS having specificity for elongating saturated fatty acids is knocked out or attenuated. Optionally, oleate is increased at the expense of linoleate production by knockout or knockdown of a delta 12 fatty acid desaturase; e.g., using the techniques of Section IV of this patent application. Optionally, the exogenous genes used can be plant genes; e.g., obtained from cDNA derived from mRNA found in oil seeds.
[0212] As described in Example 51, levels of saturated fats may also be reduced by introduction of an exogenous gene (e.g., a plant gene) that desaturates palmitic acid to palmitoleic acid. Examples of suitable genes for use in the oleaginous cells are found in the plants, including Macfadyena unguis (Cat's claw), Macadamia integrifolia (Macadamia nut) and Hippophae rhamnoides (sea buckthorn). Variant exogenous or endogenous SADs that desaturate palmitoyl-ACP can also be used and are further discussed in Example 51. Optionally, the PAD or SAD gene has at least 95% amino acid sequence identity to the gene product described in Example 51. This modification can be used alone, or in combination with oleate-increasing modifications such as those described immediately above, in section IX and in the Examples, including knockout or knockdown of one or more endogenous FATA alleles and/or overexpression of KASII. In one embodiment, an oleaginous cell such as an oleaginous microalgae has a combination of (i) a FATA knockout or knockdown with (ii) expression of an exogenous PAD gene (this could also be a variant SAD with PAD activity such as a L118W mutant or equivalent, see Examples 55-56) and/or a mutation in an endogenous SAD gene to give PAD activity. Such as cell may further comprise an overexpressed endogenous or exogenous KASII gene. In accordance with any of these embodiments of the invention, the oleaginous cell produces an oil having a fatty acid profile with 1-2, 2-3, 3-4, 5-6, 7-8, 9-10, 10-15, 15-20, 20-30, 30-40, 40-60, 60-70, 70-80, 80-90, or 90-100 area percent palmitoleic acid. In a specific embodiment, the cell produces greater than 50% oleic acid, greater than 1% palmitoleic acid, and 3.5 area% or less of saturated fatty acids. In another specific embodiment, a eukaryotic microalgal cell comprises an exogenous gene that desaturates palmitic acid to palmitoleic acid in operable linkage with regulatory elements operable in the microalgal cell. Due to expression and activity of the exogenous gene product, the cell produces a cell oil having a fatty acid profile in which the ratio of palmitoleic acid (C16:1) to palmitic acid (C16:0) is at least 0.05, 0.1 or 0.15, or 0.18. See Example 55 for examples of cells that produce such oils. Optionally, palmitoleic acid comprises 0.5% or more of the profile. Optionally, the cell oil comprises less than 3.5% saturated fatty acids.
[0213] In addition to the above genetic modifications, the low saturate oil can be a high-stability oil by virtue of low amounts of polyunsaturated fatty acids. Methods and characterizations of high-stability, low-polyunsaturated oils are described in the section above entitled Low Polyunsaturated Oils, including method to reduce the activity of endogenous .DELTA.12 fatty acid desaturase. In a specific embodiment, an oil is produced by a oleaginous microbial cell having a type II fatty acid synthetic pathway and has no more than 3.5% saturated fatty acids and also has no more than 3% polyunsaturated fatty acids. In another specific embodiment, the oil has no more than 3% saturated fatty acids and also has no more than 2% polyunsaturated fatty acids. In another specific embodiment, the oil has no more than 3% saturated fatty acids and also has no more than 1% polyunsaturated fatty acids. In another specific embodiment, a eukaryotic microalgal cell comprises an exogenous gene that desaturates palmitic acid to palmitoleic acid in operable linkage with regulatory elements operable in the microalgal cell. The cell further comprises a knockout or knockdown of a FAD gene. Due to the genetic modifications, the cell produces a cell oil having a fatty acid profile in which the ratio of palmitoleic acid (C16:1) to palmitic acid (C16:0) is greater than 0.1, with no more than 3% polyunsaturated fatty acids. Optionally, palmitoleic acid comprises 0.5% or more of the profile. Optionally, the cell oil comprises less than 3.5% saturated fatty acids.
[0214] The low saturate and low saturate/high stability oil can be blended with less expensive oils to reach a targeted saturated fatty acid level at less expense. For example, an oil with 1% saturated fat can be blended with an oil having 7% saturated fat (e.g. high-oleic sunflower oil) to give an oil having 3.5% or less saturated fat.
[0215] Oils produced according to embodiments of the present invention can be used in the transportation fuel, oleochemical, and/or food and cosmetic industries, among other applications. For example, transesterification of lipids can yield long-chain fatty acid esters useful as biodiesel. Other enzymatic and chemical processes can be tailored to yield fatty acids, aldehydes, alcohols, alkanes, and alkenes. In some applications, renewable diesel, jet fuel, or other hydrocarbon compounds are produced. The present disclosure also provides methods of cultivating microalgae for increased productivity and increased lipid yield, and/or for more cost-effective production of the compositions described herein. The methods described here allow for the production of oils from plastidic cell cultures at large scale; e.g., 1000, 10,000, 100,000 liters or more.
[0216] In an embodiment, an oil extracted from the cell has 3.5%, 3%, 2.5%, or 2% saturated fat or less and is incorporated into a food product. The finished food product has 3.5, 3, 2.5, or 2% saturated fat or less. For example, oils recovered from such recombinant microalgae can be used for frying oils or as an ingredient in a prepared food that is low in saturated fats. The oils can be used neat or blended with other oils so that the food has less than 0.5 g of saturated fat per serving, thus allowing a label stating zero saturated fat (per US regulation). In a specific embodiment, the oil has a fatty acid profile with at least 90% oleic acid, less than 3% saturated fat, and more oleic acid than linoleic acid.
[0217] As with the other oils disclosed in this patent application, the low-saturate oils described in this section, including those with increased levels palmitoleic acid, can have a microalgal sterol profile as described in Section XII of this application. For example, via expression of an exogenous PAD gene, an oil can be produced with a fatty acid profile characterized by a ratio of palmitoleic acid to palmitic acid of at least 0.1 and/or palmitoleic acid levels of 0.5% or more, as determined by FAME GC/FID analysis and a sterol profile characterized by an excess of ergosterol over .beta.-sitosterol and/or the presence of 22, 23-dihydrobrassicasterol, poriferasterol or clionasterol.
XI. COCOA BUTTER/MILK-FAT BLEND MIMETICS
[0218] In certain embodiments, the cell produces a cell oil that has a temperature-dependent solid fat content ("SFC-curve") that approximates a blend of cocoa butter and milk fat. Such oils may be used where the cocoa butter/milk fat blend could be used; for example, in chocolates other confections, ice cream or other frozen desserts, pastries, or dough, including for quickbreads, or other baked goods. The oils may inhibit blooming, enhance flavor, enhance texture, or reduce costs. In a specific example, the cell oil approximates. Accordingly, an embodiment of the invention is using a cell oil from a recombinant microalgal cell to replace a cocoa butter/milk fat blend in a recipe. In a related embodiment,
[0219] FIG. 17 shows a plot of % solid fat content for various oils as follows (a) P. moriformis RBD oil without lipid pathway engineering, (b) Brazilian cocoa butter+25% milk fat, (c) three replicates of P. moriformis RBD oil from a strain expressing hairpin nucleic acids that reduce levels of a SAD allele thus reducing oleic acid and increasing stearic acid in the TAG profile, (d) P. moriformis RBD oil from a strain overexpressing an endogenous OTE (oleoyl acyl-ACP thioesterase, see Example 45), (e) Malaysian cocoa butter+25% milk fat, and (f) Malaysian cocoa butter. The cocoa butter and cocoa butter milk fat values are literature values (Bailey's Industrial Oils and Fat Products, 6.sup.th ed.)
[0220] In an embodiment of the present invention, a cell oil that is similar in thermal properties to a 75% cocoa butter/25% milk fat blend is produced by a microalgal or other cell described above. The cell comprises recombinant nucleic acids operable to alter the fatty acid profile of triglycerides produced by the cell so as that the oil has a solid fat content (e.g., as determined by NMR) of 38%.+-.30% at 20.degree. C., 32%.+-.30% at 25.degree. C., 17%.+-.30% at 30.degree. C., and less than 5%.+-.30% at 35.degree. C. For the sake of clarity, .+-.10% refers to percent of the percent SFC (e.g., 30% of 5% SFC is 1.5% SFC so the range is 3.5 to 6.5% SFC at 35.degree. C.). In related embodiments, the oil has a solid fat content (e.g., as determined by NMR) of 38%.+-.20% at 20.degree. C., 32%.+-.20% at 25.degree. C., 17%.+-.20% at 30.degree. C., and less than 5%.+-.20% at 35.degree. C. or the oil has a solid fat content (e.g., as determined by NMR) of 38%.+-.10% at 20.degree. C., 32%.+-.10% at 25.degree. C., 17%.+-.10% at 30.degree. C., and less than 5%.+-.10% at 35.degree. C.
[0221] In a another embodiment a cell high oleic oil produced according to the methods of section IX or corresponding Examples, is converted into a structuring fat such as a cocoa butter equivalent, substitute, extender by enzymatic interesterification or transesterification with a source of saturated fatty acids (e.g. a hardstock fat or saturated fatty acid esters). For example, a 1,3-specific lipase can be used to add stearate, palmitate or both to a high oleic oil having greater than 80% oleic acid.
XII. MINOR OIL COMPONENTS
[0222] The oils produced according to the above methods in some cases are made using a microalgal host cell. As described above, the microalga can be, without limitation, fall in the classification of Chlorophyta, Trebouxiophyceae , Chlorellales, Chlorellaceae, or Chlorophyceae. It has been found that microalgae of Trebouxiophyceae can be distinguished from vegetable oils based on their sterol profiles. Oil produced by Chlorella protothecoides was found to produce sterols that appeared to be brassicasterol, ergosterol, campesterol, stigmasterol, and .beta.-sitosterol, when detected by GC-MS. However, it is believed that all sterols produced by Chlorella have C24.beta. stereochemistry. Thus, it is believed that the molecules detected as campesterol, stigmasterol, and .beta.-sitosterol, are actually 22,23-dihydrobrassicasterol, poriferasterol and clionasterol, respectively. Thus, the oils produced by the microalgae described above can be distinguished from plant oils by the presence of sterols with C24.beta. stereochemistry and the absence of C24.alpha. stereochemistry in the sterols present. For example, the oils produced may contain 22, 23-dihydrobrassicasterol while lacking campesterol; contain clionasterol, while lacking in .beta.-sitosterol, and/or contain poriferasterol while lacking stigmasterol. Alternately, or in addition, the oils may contain significant amounts of .DELTA..sup.7-poriferasterol.
[0223] In one embodiment, the oils provided herein are not vegetable oils. Vegetable oils are oils extracted from plants and plant seeds. Vegetable oils can be distinguished from the non-plant oils provided herein on the basis of their oil content. A variety of methods for analyzing the oil content can be employed to determine the source of the oil or whether adulteration of an oil provided herein with an oil of a different (e.g. plant) origin has occurred. The determination can be made on the basis of one or a combination of the analytical methods. These tests include but are not limited to analysis of one or more of free fatty acids, fatty acid profile, total triacylglycerol content, diacylglycerol content, peroxide values, spectroscopic properties (e.g. UV absorption), sterol profile, sterol degradation products, antioxidants (e.g. tocopherols), pigments (e.g. chlorophyll), d13C values and sensory analysis (e.g. taste, odor, and mouth feel). Many such tests have been standardized for commercial oils such as the Codex Alimentarius standards for edible fats and oils.
[0224] Sterol profile analysis is a particularly well-known method for determining the biological source of organic matter. Campesterol, b-sitosterol, and stigmasterol are common plant sterols, with b-sitosterol being a principle plant sterol. For example, b-sitosterol was found to be in greatest abundance in an analysis of certain seed oils, approximately 64% in corn, 29% in rapeseed, 64% in sunflower, 74% in cottonseed, 26% in soybean, and 79% in olive oil (Gul et al. J. Cell and Molecular Biology 5:71-79, 2006).
[0225] Oil isolated from Prototheca moriformis strain UTEX1435 were separately clarified (CL), refined and bleached (RB), or refined, bleached and deodorized (RBD) and were tested for sterol content according to the procedure described in JAOCS vol. 60, no. 8, August 1983. Results of the analysis are shown below (units in mg/100 g) in Table 5b.
TABLE-US-00007 TABLE 5b Sterol profiles of oils from UTEX 1435. Refined, Clari- Refined & bleached, & Sterol Crude fied bleached deodorized 1 Ergosterol 384 398 293 302 (56%) (55%) (50%) (50%) 2 5,22-cholestadien-24- 14.6 18.8 14 15.2 methyl-3-ol (2.1%) (2.6%) (2.4%) (2.5%) (Brassicasterol) 3 24-methylcholest-5- 10.7 11.9 10.9 10.8 en-3-ol (Campersterol (1.6%) (1.6%) (1.8%) (1.8%) or 22,23- dihydrobrassicasterol) 4 5,22-cholestadien-24- 57.7 59.2 46.8 49.9 ethyl-3-ol (Stigmaserol (8.4%) (8.2%) (7.9%) (8.3%) or poriferasterol) 5 24-ethylcholest-5-en- 9.64 9.92 9.26 10.2 3-ol (.beta.-Sitosterol or (1.4%) (1.4%) (1.6%) (1.7%) clionasterol) 6 Other sterols 209 221 216 213 Total sterols 685.64 718.82 589.96 601.1
[0226] These results show three striking features. First, ergosterol was found to be the most abundant of all the sterols, accounting for about 50% or more of the total sterols. The amount of ergosterol is greater than that of campesterol, .beta.-sitosterol, and stigmasterol combined. Ergosterol is steroid commonly found in fungus and not commonly found in plants, and its presence particularly in significant amounts serves as a useful marker for non-plant oils. Secondly, the oil was found to contain brassicasterol. With the exception of rapeseed oil, brassicasterol is not commonly found in plant based oils. Thirdly, less than 2% .beta.-sitosterol was found to be present. .beta.-sitosterol is a prominent plant sterol not commonly found in microalgae, and its presence particularly in significant amounts serves as a useful marker for oils of plant origin. In summary, Prototheca moriformis strain UTEX1435 has been found to contain both significant amounts of ergosterol and only trace amounts of .beta.-sitosterol as a percentage of total sterol content. Accordingly, the ratio of ergosterol:.beta.-sitosterol or in combination with the presence of brassicasterol can be used to distinguish this oil from plant oils.
[0227] In some embodiments, the oil content of an oil provided herein contains, as a percentage of total sterols, less than 20%, 15%, 10%, 5%, 4%, 3%, 2%, or 1% .beta.-sitosterol. In other embodiments the oil is free from .beta.-sitosterol. For any of the oils or cell-oils disclosed in this application, the oil can have the sterol profile of any column of Table 5b, above, with a sterol-by-sterol variation of 30%, 20%, 10% or less.
[0228] In some embodiments, the oil is free from one or more of .beta.-sitosterol, campesterol, or stigmasterol. In some embodiments the oil is free from .beta.-sitosterol, campesterol, and stigmasterol. In some embodiments the oil is free from campesterol. In some embodiments the oil is free from stigmasterol.
[0229] In some embodiments, the oil content of an oil provided herein comprises, as a percentage of total sterols, less than 20%, 15%, 10%, 5%, 4%, 3%, 2%, or 1% 24-ethylcholest-5-en-3-ol. In some embodiments, the 24-ethylcholest-5-en-3-ol is clionasterol. In some embodiments, the oil content of an oil provided herein comprises, as a percentage of total sterols, at least 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, or 10% clionasterol.
[0230] In some embodiments, the oil content of an oil provided herein contains, as a percentage of total sterols, less than 20%, 15%, 10%, 5%, 4%, 3%, 2%, or 1% 24-methylcholest-5-en-3-ol. In some embodiments, the 24-methylcholest-5-en-3-ol is 22, 23-dihydrobrassicasterol. In some embodiments, the oil content of an oil provided herein comprises, as a percentage of total sterols, at least 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, or 10% 22,23-dihydrobrassicasterol.
[0231] In some embodiments, the oil content of an oil provided herein contains, as a percentage of total sterols, less than 20%, 15%, 10%, 5%, 4%, 3%, 2%, or 1% 5, 22-cholestadien-24-ethyl-3-ol. In some embodiments, the 5, 22-cholestadien-24-ethyl-3-ol is poriferasterol. In some embodiments, the oil content of an oil provided herein comprises, as a percentage of total sterols, at least 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, or 10% poriferasterol.
[0232] In some embodiments, the oil content of an oil provided herein contains ergosterol or brassicasterol or a combination of the two. In some embodiments, the oil content contains, as a percentage of total sterols, at least 5%, 10%, 20%, 25%, 35%, 40%, 45%, 50%, 55%, 60%, or 65% ergosterol. In some embodiments, the oil content contains, as a percentage of total sterols, at least 25% ergosterol. In some embodiments, the oil content contains, as a percentage of total sterols, at least 40% ergosterol. In some embodiments, the oil content contains, as a percentage of total sterols, at least 5%, 10%, 20%, 25%, 35%, 40%, 45%, 50%, 55%, 60%, or 65% of a combination of ergosterol and brassicasterol.
[0233] In some embodiments, the oil content contains, as a percentage of total sterols, at least 1%, 2%, 3%, 4% or 5% brassicasterol. In some embodiments, the oil content contains, as a percentage of total sterols less than 10%, 9%, 8%, 7%, 6%, or 5% brassicasterol.
[0234] In some embodiments the ratio of ergosterol to brassicasterol is at least 5:1, 10:1, 15:1, or 20:1.
[0235] In some embodiments, the oil content contains, as a percentage of total sterols, at least 5%, 10%, 20%, 25%, 35%, 40%, 45%, 50%, 55%, 60%, or 65% ergosterol and less than 20%, 15%, 10%, 5%, 4%, 3%, 2%, or 1% .beta.-sitosterol. In some embodiments, the oil content contains, as a percentage of total sterols, at least 25% ergosterol and less than 5% .beta.-sitosterol. In some embodiments, the oil content further comprises brassicasterol.
[0236] Sterols contain from 27 to 29 carbon atoms (C27 to C29) and are found in all eukaryotes. Animals exclusively make C27 sterols as they lack the ability to further modify the C27 sterols to produce C28 and C29 sterols. Plants however are able to synthesize C28 and C29 sterols, and C28/C29 plant sterols are often referred to as phytosterols. The sterol profile of a given plant is high in C29 sterols, and the primary sterols in plants are typically the C29 sterols b-sitosterol and stigmasterol. In contrast, the sterol profile of non-plant organisms contain greater percentages of C27 and C28 sterols. For example the sterols in fungi and in many microalgae are principally C28 sterols. The sterol profile and particularly the striking predominance of C29 sterols over C28 sterols in plants has been exploited for determining the proportion of plant and marine matter in soil samples (Huang, Wen-Yen, Meinschein W. G., "Sterols as ecological indicators"; Geochimica et Cosmochimia Acta. Vol 43. pp 739-745).
[0237] In some embodiments the primary sterols in the microalgal oils provided herein are sterols other than b-sitosterol and stigmasterol. In some embodiments of the microalgal oils, C29 sterols make up less than 50%, 40%, 30%, 20%, 10%, or 5% by weight of the total sterol content.
[0238] In some embodiments the microalgal oils provided herein contain C28 sterols in excess of C29 sterols. In some embodiments of the microalgal oils, C28 sterols make up greater than 50%, 60%, 70%, 80%, 90%, or 95% by weight of the total sterol content. In some embodiments the C28 sterol is ergosterol. In some embodiments the C28 sterol is brassicasterol.
XIII. FUELS AND CHEMICALS
[0239] The oils discussed above alone or in combination are useful in the production of foods, fuels and chemicals (including plastics, foams, films, etc.). The oils, triglycerides, fatty acids from the oils may be subjected to C--H activation, hydroamino methylation, methoxy-carbonation, ozonolysis, enzymatic transformations, epoxidation, methylation, dimerization, thiolation, metathesis, hydro-alkylation, lactonization, or other chemical processes.
[0240] The oils can be converted to alkanes (e.g., renewable diesel) or esters (e.g., methyl or ethyl esters for biodisesel produced by transesterification). The alkanes or esters may be used as fuel, as solvents or lubricants, or as a chemical feedstock. Methods for production of renewable diesel and biodiesel are well established in the art. See, for example, WO2011/150411.
[0241] In a specific embodiment of the present invention, a high-oleic or high-oleic-high stability oil described above is esterified. For example, the oils can be transesterified with methanol to an oil that is rich in methyl oleate. As described in Example 49, such formulations have been found to compare favorably with methyl oleate from soybean oil.
[0242] In another specific example, the oil is converted to C36 diacids or products of C36 diacids. Fatty acids produced from the oil can be polymerized to give a composition rich in C36 dimer acids. In a specific example, high-oleic oil is split to give a high-oleic fatty acid material which is polymerized to give a composition rich in C36-dimer acids. Optionally , the oil is high oleic high stability oil (e.g., greater than 60% oleic acid with less than 3% polyunsaturates, greater than 70% oleic acid with less than 2% polyunsaturates, or greater than 80% oleic acid with less than 1% polyunsaturates). It is believed that using a high oleic, high stability, starting material will give lower amounts of cyclic products, which may be desirable in some cases. After hydrolyzing the oil, one obtains a high concentration of oleic acid. In the process of making dimer acids, a high oleic acid stream will convert to a "cleaner" C36 dimer acid and not produce trimers acids (C54) and other more complex cyclic by-products which are obtained due to presence of C18:2 and C18:3 acids. For example, the oil can be hydrolyzed to fatty acids and the fatty acids purified and dimerized at 250.degree. C. in the presence of montmorillonite clay. See SRI Natural Fatty Acid, March 2009. A product rich in C36 dimers of oleic acid is recovered.
##STR00001##
[0243] Further, the C36 dimer acids can be esterified and hydrogenated to give diols. The diols can be polymerized by catalytic dehydration. Polymers can also be produced by transesterification of dimerdiols with dimethyl carbonate.
[0244] For the production of fuel in accordance with the methods of the invention lipids produced by cells of the invention are harvested, or otherwise collected, by any convenient means. Lipids can be isolated by whole cell extraction. The cells are first disrupted, and then intracellular and cell membrane/cell wall-associated lipids as well as extracellular hydrocarbons can be separated from the cell mass, such as by use of centrifugation. Intracellular lipids produced in oleaginous cells are, in some embodiments, extracted after lysing the cells. Once extracted, the lipids are further refined to produce oils, fuels, or oleochemicals.
[0245] Various methods are available for separating lipids from cellular lysates. For example, lipids and lipid derivatives such as fatty aldehydes, fatty alcohols, and hydrocarbons such as alkanes can be extracted with a hydrophobic solvent such as hexane (see Frenz et al. 1989, Enzyme Microb. Technol., 11:717). Lipids and lipid derivatives can also be extracted using liquefaction (see for example Sawayama et al. 1999, Biomass and Bioenergy 17:33-39 and Inoue et al. 1993, Biomass Bioenergy 6(4):269-274); oil liquefaction (see for example Minowa et al. 1995, Fuel 74(12):1735-1738); and supercritical CO.sub.2 extraction (see for example Mendes et al. 2003, Inorganica Chimica Acta 356:328-334). Miao and Wu describe a protocol of the recovery of microalgal lipid from a culture of Chlorella protothecoides in which the cells were harvested by centrifugation, washed with distilled water and dried by freeze drying. The resulting cell powder was pulverized in a mortar and then extracted with n-hexane. Miao and Wu, Biosource Technology (2006) 97:841-846.
[0246] Lipids and lipid derivatives can be recovered by extraction with an organic solvent. In some cases, the preferred organic solvent is hexane. Typically, the organic solvent is added directly to the lysate without prior separation of the lysate components. In one embodiment, the lysate generated by one or more of the methods described above is contacted with an organic solvent for a period of time sufficient to allow the lipid and/or hydrocarbon components to form a solution with the organic solvent. In some cases, the solution can then be further refined to recover specific desired lipid or hydrocarbon components. Hexane extraction methods are well known in the art.
[0247] Lipids produced by cells in vivo, or enzymatically modified in vitro, as described herein can be optionally further processed by conventional means. The processing can include "cracking" to reduce the size, and thus increase the hydrogen:carbon ratio, of hydrocarbon molecules. Catalytic and thermal cracking methods are routinely used in hydrocarbon and triglyceride oil processing. Catalytic methods involve the use of a catalyst, such as a solid acid catalyst. The catalyst can be silica-alumina or a zeolite, which result in the heterolytic, or asymmetric, breakage of a carbon-carbon bond to result in a carbocation and a hydride anion. These reactive intermediates then undergo either rearrangement or hydride transfer with another hydrocarbon. The reactions can thus regenerate the intermediates to result in a self-propagating chain mechanism. Hydrocarbons can also be processed to reduce, optionally to zero, the number of carbon-carbon double, or triple, bonds therein. Hydrocarbons can also be processed to remove or eliminate a ring or cyclic structure therein. Hydrocarbons can also be processed to increase the hydrogen:carbon ratio. This can include the addition of hydrogen ("hydrogenation") and/or the "cracking" of hydrocarbons into smaller hydrocarbons.
[0248] Thermal methods involve the use of elevated temperature and pressure to reduce hydrocarbon size. An elevated temperature of about 800.degree. C. and pressure of about 700 kPa can be used. These conditions generate "light," a term that is sometimes used to refer to hydrogen-rich hydrocarbon molecules (as distinguished from photon flux), while also generating, by condensation, heavier hydrocarbon molecules which are relatively depleted of hydrogen. The methodology provides homolytic, or symmetrical, breakage and produces alkenes, which may be optionally enzymatically saturated as described above.
[0249] Catalytic and thermal methods are standard in plants for hydrocarbon processing and oil refining. Thus hydrocarbons produced by cells as described herein can be collected and processed or refined via conventional means. See Hillen et al. (Biotechnology and Bioengineering, Vol. XXIV:193-205 (1982)) for a report on hydrocracking of microalgae-produced hydrocarbons. In alternative embodiments, the fraction is treated with another catalyst, such as an organic compound, heat, and/or an inorganic compound. For processing of lipids into biodiesel, a transesterification process is used as described below in this Section.
[0250] Hydrocarbons produced via methods of the present invention are useful in a variety of industrial applications. For example, the production of linear alkylbenzene sulfonate (LAS), an anionic surfactant used in nearly all types of detergents and cleaning preparations, utilizes hydrocarbons generally comprising a chain of 10-14 carbon atoms. See, for example, U.S. Pat. Nos. 6,946,430; 5,506,201; 6,692,730; 6,268,517; 6,020,509; 6,140,302; 5,080,848; and 5,567,359. Surfactants, such as LAS, can be used in the manufacture of personal care compositions and detergents, such as those described in U.S. Pat. Nos. 5,942,479; 6,086,903; 5,833,999; 6,468,955; and 6,407,044.
[0251] Increasing interest is directed to the use of hydrocarbon components of biological origin in fuels, such as biodiesel, renewable diesel, and jet fuel, since renewable biological starting materials that may replace starting materials derived from fossil fuels are available, and the use thereof is desirable. There is an urgent need for methods for producing hydrocarbon components from biological materials. The present invention fulfills this need by providing methods for production of biodiesel, renewable diesel, and jet fuel using the lipids generated by the methods described herein as a biological material to produce biodiesel, renewable diesel, and jet fuel.
[0252] Traditional diesel fuels are petroleum distillates rich in paraffinic hydrocarbons. They have boiling ranges as broad as 370.degree. to 780.degree. F., which are suitable for combustion in a compression ignition engine, such as a diesel engine vehicle. The American Society of Testing and Materials (ASTM) establishes the grade of diesel according to the boiling range, along with allowable ranges of other fuel properties, such as cetane number, cloud point, flash point, viscosity, aniline point, sulfur content, water content, ash content, copper strip corrosion, and carbon residue. Technically, any hydrocarbon distillate material derived from biomass or otherwise that meets the appropriate ASTM specification can be defined as diesel fuel (ASTM D975), jet fuel (ASTM D1655), or as biodiesel if it is a fatty acid methyl ester (ASTM D6751).
[0253] After extraction, lipid and/or hydrocarbon components recovered from the microbial biomass described herein can be subjected to chemical treatment to manufacture a fuel for use in diesel vehicles and jet engines.
[0254] Biodiesel is a liquid which varies in color--between golden and dark brown--depending on the production feedstock. It is practically immiscible with water, has a high boiling point and low vapor pressure. Biodiesel refers to a diesel-equivalent processed fuel for use in diesel-engine vehicles. Biodiesel is biodegradable and non-toxic. An additional benefit of biodiesel over conventional diesel fuel is lower engine wear. Typically, biodiesel comprises C14-C18 alkyl esters. Various processes convert biomass or a lipid produced and isolated as described herein to diesel fuels. A preferred method to produce biodiesel is by transesterification of a lipid as described herein. A preferred alkyl ester for use as biodiesel is a methyl ester or ethyl ester.
[0255] Biodiesel produced by a method described herein can be used alone or blended with conventional diesel fuel at any concentration in most modern diesel-engine vehicles. When blended with conventional diesel fuel (petroleum diesel), biodiesel may be present from about 0.1% to about 99.9%. Much of the world uses a system known as the "B" factor to state the amount of biodiesel in any fuel mix. For example, fuel containing 20% biodiesel is labeled B20. Pure biodiesel is referred to as B100.
[0256] Biodiesel can be produced by transesterification of triglycerides contained in oil-rich biomass. Thus, in another aspect of the present invention a method for producing biodiesel is provided. In a preferred embodiment, the method for producing biodiesel comprises the steps of (a) cultivating a lipid-containing microorganism using methods disclosed herein (b) lysing a lipid-containing microorganism to produce a lysate, (c) isolating lipid from the lysed microorganism, and (d) transesterifying the lipid composition, whereby biodiesel is produced. Methods for growth of a microorganism, lysing a microorganism to produce a lysate, treating the lysate in a medium comprising an organic solvent to form a heterogeneous mixture and separating the treated lysate into a lipid composition have been described above and can also be used in the method of producing biodiesel. The lipid profile of the biodiesel is usually highly similar to the lipid profile of the feedstock oil.
[0257] Lipid compositions can be subjected to transesterification to yield long-chain fatty acid esters useful as biodiesel. Preferred transesterification reactions are outlined below and include base catalyzed transesterification and transesterification using recombinant lipases. In a base-catalyzed transesterification process, the triacylglycerides are reacted with an alcohol, such as methanol or ethanol, in the presence of an alkaline catalyst, typically potassium hydroxide. This reaction forms methyl or ethyl esters and glycerin (glycerol) as a byproduct.
[0258] Transesterification has also been carried out, as discussed above, using an enzyme, such as a lipase instead of a base. Lipase-catalyzed transesterification can be carried out, for example, at a temperature between the room temperature and 80.degree. C., and a mole ratio of the TAG to the lower alcohol of greater than 1:1, preferably about 3:1. Lipases suitable for use in transesterification include, but are not limited to, those listed in Table 9. Other examples of lipases useful for transesterification are found in, e.g., U.S. Pat. Nos. 4,798,793; 4,940,845 5,156,963; 5,342,768; 5,776,741 and WO89/01032. Such lipases include, but are not limited to, lipases produced by microorganisms of Rhizopus, Aspergillus, Candida, Mucor, Pseudomonas, Rhizomucor, Candida, and Humicola and pancreas lipase.
[0259] Subsequent processes may also be used if the biodiesel will be used in particularly cold temperatures. Such processes include winterization and fractionation. Both processes are designed to improve the cold flow and winter performance of the fuel by lowering the cloud point (the temperature at which the biodiesel starts to crystallize). There are several approaches to winterizing biodiesel. One approach is to blend the biodiesel with petroleum diesel. Another approach is to use additives that can lower the cloud point of biodiesel. Another approach is to remove saturated methyl esters indiscriminately by mixing in additives and allowing for the crystallization of saturates and then filtering out the crystals. Fractionation selectively separates methyl esters into individual components or fractions, allowing for the removal or inclusion of specific methyl esters. Fractionation methods include urea fractionation, solvent fractionation and thermal distillation.
[0260] Another valuable fuel provided by the methods of the present invention is renewable diesel, which comprises alkanes, such as C10:0, C12:0, C14:0, C16:0 and C18:0 and thus, are distinguishable from biodiesel. High quality renewable diesel conforms to the ASTM D975 standard. The lipids produced by the methods of the present invention can serve as feedstock to produce renewable diesel. Thus, in another aspect of the present invention, a method for producing renewable diesel is provided. Renewable diesel can be produced by at least three processes: hydrothermal processing (hydrotreating); hydroprocessing; and indirect liquefaction. These processes yield non-ester distillates. During these processes, triacylglycerides produced and isolated as described herein, are converted to alkanes.
[0261] In one embodiment, the method for producing renewable diesel comprises (a) cultivating a lipid-containing microorganism using methods disclosed herein (b) lysing the microorganism to produce a lysate, (c) isolating lipid from the lysed microorganism, and (d) deoxygenating and hydrotreating the lipid to produce an alkane, whereby renewable diesel is produced. Lipids suitable for manufacturing renewable diesel can be obtained via extraction from microbial biomass using an organic solvent such as hexane, or via other methods, such as those described in U.S. Pat. No. 5,928,696. Some suitable methods may include mechanical pressing and centrifuging.
[0262] In some methods, the microbial lipid is first cracked in conjunction with hydrotreating to reduce carbon chain length and saturate double bonds, respectively. The material is then isomerized, also in conjunction with hydrotreating. The naptha fraction can then be removed through distillation, followed by additional distillation to vaporize and distill components desired in the diesel fuel to meet an ASTM D975 standard while leaving components that are heavier than desired for meeting the D975 standard. Hydrotreating, hydrocracking, deoxygenation and isomerization methods of chemically modifying oils, including triglyceride oils, are well known in the art. See for example European patent applications EP1741768 (A1); EP1741767 (A1); EP1682466 (A1); EP1640437 (A1); EP1681337 (A1); EP1795576 (A1); and U.S. Pat. Nos. 7,238,277; 6,630,066; 6,596,155; 6,977,322; 7,041,866; 6,217,746; 5,885,440; 6,881,873.
[0263] In one embodiment of the method for producing renewable diesel, treating the lipid to produce an alkane is performed by hydrotreating of the lipid composition. In hydrothermal processing, typically, biomass is reacted in water at an elevated temperature and pressure to form oils and residual solids. Conversion temperatures are typically 300.degree. to 660.degree. F., with pressure sufficient to keep the water primarily as a liquid, 100 to 170 standard atmosphere (atm). Reaction times are on the order of 15 to 30 minutes. After the reaction is completed, the organics are separated from the water. Thereby a distillate suitable for diesel is produced.
[0264] In some methods of making renewable diesel, the first step of treating a triglyceride is hydroprocessing to saturate double bonds, followed by deoxygenation at elevated temperature in the presence of hydrogen and a catalyst. In some methods, hydrogenation and deoxygenation occur in the same reaction. In other methods deoxygenation occurs before hydrogenation. Isomerization is then optionally performed, also in the presence of hydrogen and a catalyst. Naphtha components are preferably removed through distillation. For examples, see U.S. Pat. No. 5,475,160 (hydrogenation of triglycerides); U.S. Pat. No. 5,091,116 (deoxygenation, hydrogenation and gas removal); U.S. Pat. No. 6,391,815 (hydrogenation); and U.S. Pat. No. 5,888,947 (isomerization).
[0265] One suitable method for the hydrogenation of triglycerides includes preparing an aqueous solution of copper, zinc, magnesium and lanthanum salts and another solution of alkali metal or preferably, ammonium carbonate. The two solutions may be heated to a temperature of about 20.degree. C. to about 85.degree. C. and metered together into a precipitation container at rates such that the pH in the precipitation container is maintained between 5.5 and 7.5 in order to form a catalyst. Additional water may be used either initially in the precipitation container or added concurrently with the salt solution and precipitation solution. The resulting precipitate may then be thoroughly washed, dried, calcined at about 300.degree. C. and activated in hydrogen at temperatures ranging from about 100.degree. C. to about 400.degree. C. One or more triglycerides may then be contacted and reacted with hydrogen in the presence of the above-described catalyst in a reactor. The reactor may be a trickle bed reactor, fixed bed gas-solid reactor, packed bubble column reactor, continuously stirred tank reactor, a slurry phase reactor, or any other suitable reactor type known in the art. The process may be carried out either batchwise or in continuous fashion. Reaction temperatures are typically in the range of from about 170.degree. C. to about 250.degree. C. while reaction pressures are typically in the range of from about 300 psig to about 2000 psig. Moreover, the molar ratio of hydrogen to triglyceride in the process of the present invention is typically in the range of from about 20:1 to about 700:1. The process is typically carried out at a weight hourly space velocity (WHSV) in the range of from about 0.1 hr.sup.-1 to about 5 hr.sup.-1. One skilled in the art will recognize that the time period required for reaction will vary according to the temperature used, the molar ratio of hydrogen to triglyceride, and the partial pressure of hydrogen. The products produced by the such hydrogenation processes include fatty alcohols, glycerol, traces of paraffins and unreacted triglycerides. These products are typically separated by conventional means such as, for example, distillation, extraction, filtration, crystallization, and the like.
[0266] Petroleum refiners use hydroprocessing to remove impurities by treating feeds with hydrogen. Hydroprocessing conversion temperatures are typically 300.degree. to 700.degree. F. Pressures are typically 40 to 100 atm. The reaction times are typically on the order of 10 to 60 minutes. Solid catalysts are employed to increase certain reaction rates, improve selectivity for certain products, and optimize hydrogen consumption.
[0267] Suitable methods for the deoxygenation of an oil includes heating an oil to a temperature in the range of from about 350.degree. F. to about 550.degree. F. and continuously contacting the heated oil with nitrogen under at least pressure ranging from about atmospheric to above for at least about 5 minutes.
[0268] Suitable methods for isomerization include using alkali isomerization and other oil isomerization known in the art.
[0269] Hydrotreating and hydroprocessing ultimately lead to a reduction in the molecular weight of the triglyceride feed. The triglyceride molecule is reduced to four hydrocarbon molecules under hydroprocessing conditions: a propane molecule and three heavier hydrocarbon molecules, typically in the C8 to C18 range.
[0270] Thus, in one embodiment, the product of one or more chemical reaction(s) performed on lipid compositions of the invention is an alkane mixture that comprises ASTM D975 renewable diesel. Production of hydrocarbons by microorganisms is reviewed by Metzger et al. Appl Microbiol Biotechnol (2005) 66: 486-496 and A Look Back at the U.S. Department of Energy's Aquatic Species Program: Biodiesel from Algae, NREL/TP-580-24190, John Sheehan, Terri Dunahay, John Benemann and Paul Roessler (1998).
[0271] The distillation properties of a diesel fuel is described in terms of T10-T90 (temperature at 10% and 90%, respectively, volume distilled). The T10-T90 of the material produced in Example 13 was 57.9.degree. C. Methods of hydrotreating, isomerization, and other covalent modification of oils disclosed herein, as well as methods of distillation and fractionation (such as cold filtration) disclosed herein, can be employed to generate renewable diesel compositions with other T10-T90 ranges, such as 20, 25, 30, 35, 40, 45, 50, 60 and 65.degree. C. using triglyceride oils produced according to the methods disclosed herein.
[0272] Methods of hydrotreating, isomerization, and other covalent modification of oils disclosed herein, as well as methods of distillation and fractionation (such as cold filtration) disclosed herein, can be employed to generate renewable diesel compositions with other T10 values, such as T10 between 180 and 295, between 190 and 270, between 210 and 250, between 225 and 245, and at least 290.
[0273] Methods of hydrotreating, isomerization, and other covalent modification of oils disclosed herein, as well as methods of distillation and fractionation (such as cold filtration) disclosed herein can be employed to generate renewable diesel compositions with certain T90 values, such as T90 between 280 and 380, between 290 and 360, between 300 and 350, between 310 and 340, and at least 290.
[0274] The FBP of the material produced in Example 13 was 300.degree. C. Methods of hydrotreating, isomerization, and other covalent modification of oils disclosed herein, as well as methods of distillation and fractionation (such as cold filtration) disclosed herein, can be employed to generate renewable diesel compositions with other FBP values, such as FBP between 290 and 400, between 300 and 385, between 310 and 370, between 315 and 360, and at least 300.
[0275] Other oils provided by the methods and compositions of the invention can be subjected to combinations of hydrotreating, isomerization, and other covalent modification including oils with lipid profiles including (a) at least 1%-5%, preferably at least 4%, C8-C14; (b) at least 0.25%-1%, preferably at least 0.3%, C8; (c) at least 1%-5%, preferably at least 2%, C10; (d) at least 1%-5%, preferably at least 2%, C12; and (3) at least 20%-40%, preferably at least 30% C8-C14.
[0276] A traditional ultra-low sulfur diesel can be produced from any form of biomass by a two-step process. First, the biomass is converted to a syngas, a gaseous mixture rich in hydrogen and carbon monoxide. Then, the syngas is catalytically converted to liquids. Typically, the production of liquids is accomplished using Fischer-Tropsch (FT) synthesis. This technology applies to coal, natural gas, and heavy oils. Thus, in yet another preferred embodiment of the method for producing renewable diesel, treating the lipid composition to produce an alkane is performed by indirect liquefaction of the lipid composition.
[0277] The present invention also provides methods to produce jet fuel. Jet fuel is clear to straw colored. The most common fuel is an unleaded/paraffin oil-based fuel classified as Aeroplane A-1, which is produced to an internationally standardized set of specifications. Jet fuel is a mixture of a large number of different hydrocarbons, possibly as many as a thousand or more. The range of their sizes (molecular weights or carbon numbers) is restricted by the requirements for the product, for example, freezing point or smoke point. Kerosene-type Aeroplane fuel (including Jet A and Jet A-1) has a carbon number distribution between about 8 and 16 carbon numbers. Wide-cut or naphtha-type Aeroplane fuel (including Jet B) typically has a carbon number distribution between about 5 and 15 carbons.
[0278] In one embodiment of the invention, a jet fuel is produced by blending algal fuels with existing jet fuel. The lipids produced by the methods of the present invention can serve as feedstock to produce jet fuel. Thus, in another aspect of the present invention, a method for producing jet fuel is provided. Herewith two methods for producing jet fuel from the lipids produced by the methods of the present invention are provided: fluid catalytic cracking (FCC); and hydrodeoxygenation (HDO).
[0279] Fluid Catalytic Cracking (FCC) is one method which is used to produce olefins, especially propylene from heavy crude fractions. The lipids produced by the method of the present invention can be converted to olefins. The process involves flowing the lipids produced through an FCC zone and collecting a product stream comprised of olefins, which is useful as a jet fuel. The lipids produced are contacted with a cracking catalyst at cracking conditions to provide a product stream comprising olefins and hydrocarbons useful as jet fuel.
[0280] In one embodiment, the method for producing jet fuel comprises (a) cultivating a lipid-containing microorganism using methods disclosed herein, (b) lysing the lipid-containing microorganism to produce a lysate, (c) isolating lipid from the lysate, and (d) treating the lipid composition, whereby jet fuel is produced. In one embodiment of the method for producing a jet fuel, the lipid composition can be flowed through a fluid catalytic cracking zone, which, in one embodiment, may comprise contacting the lipid composition with a cracking catalyst at cracking conditions to provide a product stream comprising C.sub.2-C.sub.5 olefins.
[0281] In certain embodiments of this method, it may be desirable to remove any contaminants that may be present in the lipid composition. Thus, prior to flowing the lipid composition through a fluid catalytic cracking zone, the lipid composition is pretreated. Pretreatment may involve contacting the lipid composition with an ion-exchange resin. The ion exchange resin is an acidic ion exchange resin, such as Amberlyst.TM.-15 and can be used as a bed in a reactor through which the lipid composition is flowed, either upflow or downflow. Other pretreatments may include mild acid washes by contacting the lipid composition with an acid, such as sulfuric, acetic, nitric, or hydrochloric acid. Contacting is done with a dilute acid solution usually at ambient temperature and atmospheric pressure.
[0282] The lipid composition, optionally pretreated, is flowed to an FCC zone where the hydrocarbonaceous components are cracked to olefins. Catalytic cracking is accomplished by contacting the lipid composition in a reaction zone with a catalyst composed of finely divided particulate material. The reaction is catalytic cracking, as opposed to hydrocracking, and is carried out in the absence of added hydrogen or the consumption of hydrogen. As the cracking reaction proceeds, substantial amounts of coke are deposited on the catalyst. The catalyst is regenerated at high temperatures by burning coke from the catalyst in a regeneration zone. Coke-containing catalyst, referred to herein as "coked catalyst", is continually transported from the reaction zone to the regeneration zone to be regenerated and replaced by essentially coke-free regenerated catalyst from the regeneration zone. Fluidization of the catalyst particles by various gaseous streams allows the transport of catalyst between the reaction zone and regeneration zone. Methods for cracking hydrocarbons, such as those of the lipid composition described herein, in a fluidized stream of catalyst, transporting catalyst between reaction and regeneration zones, and combusting coke in the regenerator are well known by those skilled in the art of FCC processes. Exemplary FCC applications and catalysts useful for cracking the lipid composition to produce C.sub.2-C.sub.5 olefins are described in U.S. Pat. Nos. 6,538,169, 7,288,685, which are incorporated in their entirety by reference.
[0283] Suitable FCC catalysts generally comprise at least two components that may or may not be on the same matrix. In some embodiments, both two components may be circulated throughout the entire reaction vessel. The first component generally includes any of the well-known catalysts that are used in the art of fluidized catalytic cracking, such as an active amorphous clay-type catalyst and/or a high activity, crystalline molecular sieve. Molecular sieve catalysts may be preferred over amorphous catalysts because of their much-improved selectivity to desired products. In some preferred embodiments, zeolites may be used as the molecular sieve in the FCC processes. Preferably, the first catalyst component comprises a large pore zeolite, such as a Y-type zeolite, an active alumina material, a binder material, comprising either silica or alumina and an inert filler such as kaolin.
[0284] In one embodiment, cracking the lipid composition of the present invention, takes place in the riser section or, alternatively, the lift section, of the FCC zone. The lipid composition is introduced into the riser by a nozzle resulting in the rapid vaporization of the lipid composition. Before contacting the catalyst, the lipid composition will ordinarily have a temperature of about 149.degree. C. to about 316.degree. C. (300.degree. F. to 600.degree. F.). The catalyst is flowed from a blending vessel to the riser where it contacts the lipid composition for a time of abort 2 seconds or less.
[0285] The blended catalyst and reacted lipid composition vapors are then discharged from the top of the riser through an outlet and separated into a cracked product vapor stream including olefins and a collection of catalyst particles covered with substantial quantities of coke and generally referred to as "coked catalyst." In an effort to minimize the contact time of the lipid composition and the catalyst which may promote further conversion of desired products to undesirable other products, any arrangement of separators such as a swirl arm arrangement can be used to remove coked catalyst from the product stream quickly. The separator, e.g. swirl arm separator, is located in an upper portion of a chamber with a stripping zone situated in the lower portion of the chamber. Catalyst separated by the swirl arm arrangement drops down into the stripping zone. The cracked product vapor stream comprising cracked hydrocarbons including light olefins and some catalyst exit the chamber via a conduit which is in communication with cyclones. The cyclones remove remaining catalyst particles from the product vapor stream to reduce particle concentrations to very low levels. The product vapor stream then exits the top of the separating vessel. Catalyst separated by the cyclones is returned to the separating vessel and then to the stripping zone. The stripping zone removes adsorbed hydrocarbons from the surface of the catalyst by counter-current contact with steam.
[0286] Low hydrocarbon partial pressure operates to favor the production of light olefins. Accordingly, the riser pressure is set at about 172 to 241 kPa (25 to 35 psia) with a hydrocarbon partial pressure of about 35 to 172 kPa (5 to 25 psia), with a preferred hydrocarbon partial pressure of about 69 to 138 kPa (10 to 20 psia). This relatively low partial pressure for hydrocarbon is achieved by using steam as a diluent to the extent that the diluent is 10 to 55 wt-% of lipid composition and preferably about 15 wt-% of lipid composition. Other diluents such as dry gas can be used to reach equivalent hydrocarbon partial pressures.
[0287] The temperature of the cracked stream at the riser outlet will be about 510.degree. C. to 621.degree. C. (950.degree. F. to 1150.degree. F.). However, riser outlet temperatures above 566.degree. C. (1050.degree. F.) make more dry gas and more olefins. Whereas, riser outlet temperatures below 566.degree. C. (1050.degree. F.) make less ethylene and propylene. Accordingly, it is preferred to run the FCC process at a preferred temperature of about 566.degree. C. to about 630.degree. C., preferred pressure of about 138 kPa to about 240 kPa (20 to 35 psia). Another condition for the process is the catalyst to lipid composition ratio which can vary from about 5 to about 20 and preferably from about 10 to about 15.
[0288] In one embodiment of the method for producing a jet fuel, the lipid composition is introduced into the lift section of an FCC reactor. The temperature in the lift section will be very hot and range from about 700.degree. C. (1292.degree. F.) to about 760.degree. C. (1400.degree. F.) with a catalyst to lipid composition ratio of about 100 to about 150. It is anticipated that introducing the lipid composition into the lift section will produce considerable amounts of propylene and ethylene.
[0289] In another embodiment of the method for producing a jet fuel using the lipid composition or the lipids produced as described herein, the structure of the lipid composition or the lipids is broken by a process referred to as hydrodeoxygenation (HDO). HDO means removal of oxygen by means of hydrogen, that is, oxygen is removed while breaking the structure of the material. Olefinic double bonds are hydrogenated and any sulfur and nitrogen compounds are removed. Sulfur removal is called hydrodesulphurization (HDS). Pretreatment and purity of the raw materials (lipid composition or the lipids) contribute to the service life of the catalyst.
[0290] Generally in the HDO/HDS step, hydrogen is mixed with the feed stock (lipid composition or the lipids) and then the mixture is passed through a catalyst bed as a co-current flow, either as a single phase or a two phase feed stock. After the HDO/MDS step, the product fraction is separated and passed to a separate isomerization reactor. An isomerization reactor for biological starting material is described in the literature (FI 100 248) as a co-current reactor.
[0291] The process for producing a fuel by hydrogenating a hydrocarbon feed, e.g., the lipid composition or the lipids herein, can also be performed by passing the lipid composition or the lipids as a co-current flow with hydrogen gas through a first hydrogenation zone, and thereafter the hydrocarbon effluent is further hydrogenated in a second hydrogenation zone by passing hydrogen gas to the second hydrogenation zone as a counter-current flow relative to the hydrocarbon effluent. Exemplary HDO applications and catalysts useful for cracking the lipid composition to produce C.sub.2-C.sub.5 olefins are described in U.S. Pat. No. 7,232,935, which is incorporated in its entirety by reference.
[0292] Typically, in the hydrodeoxygenation step, the structure of the biological component, such as the lipid composition or lipids herein, is decomposed, oxygen, nitrogen, phosphorus and sulfur compounds, and light hydrocarbons as gas are removed, and the olefinic bonds are hydrogenated. In the second step of the process, i.e. in the so-called isomerization step, isomerization is carried out for branching the hydrocarbon chain and improving the performance of the paraffin at low temperatures.
[0293] In the first step, i.e. HDO step, of the cracking process, hydrogen gas and the lipid composition or lipids herein which are to be hydrogenated are passed to a HDO catalyst bed system either as co-current or counter-current flows, said catalyst bed system comprising one or more catalyst bed(s), preferably 1-3 catalyst beds. The HDO step is typically operated in a co-current manner. In case of a HDO catalyst bed system comprising two or more catalyst beds, one or more of the beds may be operated using the counter-current flow principle. In the HDO step, the pressure varies between 20 and 150 bar, preferably between 50 and 100 bar, and the temperature varies between 200 and 500.degree. C., preferably in the range of 300-400.degree. C. In the HDO step, known hydrogenation catalysts containing metals from Group VII and/or VIB of the Periodic System may be used. Preferably, the hydrogenation catalysts are supported Pd, Pt, Ni, NiMo or a CoMo catalysts, the support being alumina and/or silica. Typically, NiMo/Al.sub.2O.sub.3 and CoMo/Al.sub.2O.sub.3 catalysts are used.
[0294] Prior to the HDO step, the lipid composition or lipids herein may optionally be treated by prehydrogenation under milder conditions thus avoiding side reactions of the double bonds. Such prehydrogenation is carried out in the presence of a prehydrogenation catalyst at temperatures of 50-400.degree. C. and at hydrogen pressures of 1-200 bar, preferably at a temperature between 150 and 250.degree. C. and at a hydrogen pressure between 10 and 100 bar. The catalyst may contain metals from Group VIII and/or VIB of the Periodic System. Preferably, the prehydrogenation catalyst is a supported Pd, Pt, Ni, NiMo or a CoMo catalyst, the support being alumina and/or silica.
[0295] A gaseous stream from the HDO step containing hydrogen is cooled and then carbon monoxide, carbon dioxide, nitrogen, phosphorus and sulfur compounds, gaseous light hydrocarbons and other impurities are removed therefrom. After compressing, the purified hydrogen or recycled hydrogen is returned back to the first catalyst bed and/or between the catalyst beds to make up for the withdrawn gas stream. Water is removed from the condensed liquid. The liquid is passed to the first catalyst bed or between the catalyst beds.
[0296] After the HDO step, the product is subjected to an isomerization step. It is substantial for the process that the impurities are removed as completely as possible before the hydrocarbons are contacted with the isomerization catalyst. The isomerization step comprises an optional stripping step, wherein the reaction product from the HDO step may be purified by stripping with water vapor or a suitable gas such as light hydrocarbon, nitrogen or hydrogen. The optional stripping step is carried out in counter-current manner in a unit upstream of the isomerization catalyst, wherein the gas and liquid are contacted with each other, or before the actual isomerization reactor in a separate stripping unit utilizing counter-current principle.
[0297] After the stripping step the hydrogen gas and the hydrogenated lipid composition or lipids herein, and optionally an n-paraffin mixture, are passed to a reactive isomerization unit comprising one or several catalyst bed(s). The catalyst beds of the isomerization step may operate either in co-current or counter-current manner.
[0298] It is important for the process that the counter-current flow principle is applied in the isomerization step. In the isomerization step this is done by carrying out either the optional stripping step or the isomerization reaction step or both in counter-current manner. In the isomerization step, the pressure varies in the range of 20-150 bar, preferably in the range of 20-100 bar, the temperature being between 200 and 500.degree. C., preferably between 300 and 400.degree. C. In the isomerization step, isomerization catalysts known in the art may be used. Suitable isomerization catalysts contain molecular sieve and/or a metal from Group VII and/or a carrier. Preferably, the isomerization catalyst contains SAPO-11 or SAPO41 or ZSM-22 or ZSM-23 or ferrierite and Pt, Pd or Ni and Al.sub.2O.sub.3 or SiO.sub.2. Typical isomerization catalysts are, for example, Pt/SAPO-11/Al.sub.2O.sub.3, Pt/ZSM-22/Al.sub.2O.sub.3, Pt/ZSM-23/Al.sub.2O.sub.3 and Pt/SAPO-11/SiO.sub.2. The isomerization step and the HDO step may be carried out in the same pressure vessel or in separate pressure vessels. Optional prehydrogenation may be carried out in a separate pressure vessel or in the same pressure vessel as the HDO and isomerization steps.
[0299] Thus, in one embodiment, the product of one or more chemical reactions is an alkane mixture that comprises HRJ-5. In another embodiment, the product of the one or more chemical reactions is an alkane mixture that comprises ASTM D1655 jet fuel. In some embodiments, the composition conforming to the specification of ASTM 1655 jet fuel has a sulfur content that is less than 10 ppm. In other embodiments, the composition conforming to the specification of ASTM 1655 jet fuel has a T10 value of the distillation curve of less than 205.degree. C. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a final boiling point (FBP) of less than 300.degree. C. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a flash point of at least 38.degree. C. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a density between 775K/M.sup.3 and 840K/M.sup.3. In yet another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a freezing point that is below -47.degree. C. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a net Heat of Combustion that is at least 42.8 MJ/K. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a hydrogen content that is at least 13.4 mass %. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has a thermal stability, as tested by quantitative gravimetric JFTOT at 260.degree. C., which is below 3 mm of Hg. In another embodiment, the composition conforming to the specification of ASTM 1655 jet fuel has an existent gum that is below 7 mg/dl.
[0300] Thus, the present invention discloses a variety of methods in which chemical modification of microalgal lipid is undertaken to yield products useful in a variety of industrial and other applications. Examples of processes for modifying oil produced by the methods disclosed herein include, but are not limited to, hydrolysis of the oil, hydroprocessing of the oil, and esterification of the oil. Other chemical modification of microalgal lipid include, without limitation, epoxidation, oxidation, hydrolysis, sulfations, sulfonation, ethoxylation, propoxylation, amidation, and saponification. The modification of the microalgal oil produces basic oleochemicals that can be further modified into selected derivative oleochemicals for a desired function. In a manner similar to that described above with reference to fuel producing processes, these chemical modifications can also be performed on oils generated from the microbial cultures described herein. Examples of basic oleochemicals include, but are not limited to, soaps, fatty acids, fatty esters, fatty alcohols, fatty nitrogen compounds including fatty amides, fatty acid methyl esters, and glycerol. Examples of derivative oleochemicals include, but are not limited to, fatty nitriles, esters, dimer acids, quats (including betaines), surfactants, fatty alkanolamides, fatty alcohol sulfates, resins, emulsifiers, fatty alcohols, olefins, drilling muds, polyols, polyurethanes, polyacrylates, rubber, candles, cosmetics, metallic soaps, soaps, alpha-sulphonated methyl esters, fatty alcohol sulfates, fatty alcohol ethoxylates, fatty alcohol ether sulfates, imidazolines, surfactants, detergents, esters, quats (including betaines), ozonolysis products, fatty amines, fatty alkanolamides, ethoxysulfates, monoglycerides, diglycerides, triglycerides (including medium chain triglycerides), lubricants, hydraulic fluids, greases, dielectric fluids, mold release agents, metal working fluids, heat transfer fluids, other functional fluids, industrial chemicals (e.g., cleaners, textile processing aids, plasticizers, stabilizers, additives), surface coatings, paints and lacquers, electrical wiring insulation, and higher alkanes. Other derivatives include fatty amidoamines, amidoamine carboxylates, amidoamine oxides, amidoamine oxide carboxylates, amidoamine esters, ethanolamine amides, sulfonates, amidoamine sulfonates, diamidoamine dioxides, sulfonated alkyl ester alkoxylates, betaines, quarternized diamidoamine betaines, and sulfobetaines.
[0301] Hydrolysis of the fatty acid constituents from the glycerolipids produced by the methods of the invention yields free fatty acids that can be derivatized to produce other useful chemicals. Hydrolysis occurs in the presence of water and a catalyst which may be either an acid or a base. The liberated free fatty acids can be derivatized to yield a variety of products, as reported in the following: U.S. Pat. No. 5,304,664 (Highly sulfated fatty acids); U.S. Pat. No. 7,262,158 (Cleansing compositions); U.S. Pat. No. 7,115,173 (Fabric softener compositions); U.S. Pat. No. 6,342,208 (Emulsions for treating skin); U.S. Pat. No. 7,264,886 (Water repellent compositions); U.S. Pat. No. 6,924,333 (Paint additives); U.S. Pat. No. 6,596,768 (Lipid-enriched ruminant feedstock); and U.S. Pat. No. 6,380,410 (Surfactants for detergents and cleaners).
[0302] In some methods, the first step of chemical modification may be hydroprocessing to saturate double bonds, followed by deoxygenation at elevated temperature in the presence of hydrogen and a catalyst. In other methods, hydrogenation and deoxygenation may occur in the same reaction. In still other methods deoxygenation occurs before hydrogenation. Isomerization may then be optionally performed, also in the presence of hydrogen and a catalyst. Finally, gases and naphtha components can be removed if desired. For example, see U.S. Pat. No. 5,475,160 (hydrogenation of triglycerides); U.S. Pat. No. 5,091,116 (deoxygenation, hydrogenation and gas removal); U.S. Pat. No. 6,391,815 (hydrogenation); and U.S. Pat. No. 5,888,947 (isomerization).
[0303] In some embodiments of the invention, the triglyceride oils are partially or completely deoxygenated. The deoxygenation reactions form desired products, including, but not limited to, fatty acids, fatty alcohols, polyols, ketones, and aldehydes. In general, without being limited by any particular theory, the deoxygenation reactions involve a combination of various different reaction pathways, including without limitation: hydrogenolysis, hydrogenation, consecutive hydrogenation-hydrogenolysis, consecutive hydrogenolysis-hydrogenation, and combined hydrogenation-hydrogenolysis reactions, resulting in at least the partial removal of oxygen from the fatty acid or fatty acid ester to produce reaction products, such as fatty alcohols, that can be easily converted to the desired chemicals by further processing. For example, in one embodiment, a fatty alcohol may be converted to olefins through FCC reaction or to higher alkanes through a condensation reaction.
[0304] One such chemical modification is hydrogenation, which is the addition of hydrogen to double bonds in the fatty acid constituents of glycerolipids or of free fatty acids. The hydrogenation process permits the transformation of liquid oils into semi-solid or solid fats, which may be more suitable for specific applications.
[0305] Hydrogenation of oil produced by the methods described herein can be performed in conjunction with one or more of the methods and/or materials provided herein, as reported in the following: U.S. Pat. No. 7,288,278 (Food additives or medicaments); U.S. Pat. No. 5,346,724 (Lubrication products); U.S. Pat. No. 5,475,160 (Fatty alcohols); U.S. Pat. No. 5,091,116 (Edible oils); U.S. Pat. No. 6,808,737 (Structural fats for margarine and spreads); U.S. Pat. No. 5,298,637 (Reduced-calorie fat substitutes); U.S. Pat. No. 6,391,815 (Hydrogenation catalyst and sulfur adsorbent); U.S. Pat. No. 5,233,099 and U.S. Pat. No. 5,233,100 (Fatty alcohols); U.S. Pat. No. 4,584,139 (Hydrogenation catalysts); U.S. Pat. No. 6,057,375 (Foam suppressing agents); and U.S. Pat. No. 7,118,773 (Edible emulsion spreads).
[0306] One skilled in the art will recognize that various processes may be used to hydrogenate carbohydrates. One suitable method includes contacting the carbohydrate with hydrogen or hydrogen mixed with a suitable gas and a catalyst under conditions sufficient in a hydrogenation reactor to form a hydrogenated product. The hydrogenation catalyst generally can include Cu, Re, Ni, Fe, Co, Ru, Pd, Rh, Pt, Os, Ir, and alloys or any combination thereof, either alone or with promoters such as W, Mo, Au, Ag, Cr, Zn, Mn, Sn, B, P, Bi, and alloys or any combination thereof. Other effective hydrogenation catalyst materials include either supported nickel or ruthenium modified with rhenium. In an embodiment, the hydrogenation catalyst also includes any one of the supports, depending on the desired functionality of the catalyst. The hydrogenation catalysts may be prepared by methods known to those of ordinary skill in the art.
[0307] In some embodiments the hydrogenation catalyst includes a supported Group VIII metal catalyst and a metal sponge material (e.g., a sponge nickel catalyst). Raney nickel provides an example of an activated sponge nickel catalyst suitable for use in this invention. In other embodiment, the hydrogenation reaction in the invention is performed using a catalyst comprising a nickel-rhenium catalyst or a tungsten-modified nickel catalyst. One example of a suitable catalyst for the hydrogenation reaction of the invention is a carbon-supported nickel-rhenium catalyst.
[0308] In an embodiment, a suitable Raney nickel catalyst may be prepared by treating an alloy of approximately equal amounts by weight of nickel and aluminum with an aqueous alkali solution, e.g., containing about 25 weight % of sodium hydroxide. The aluminum is selectively dissolved by the aqueous alkali solution resulting in a sponge shaped material comprising mostly nickel with minor amounts of aluminum. The initial alloy includes promoter metals (i.e., molybdenum or chromium) in the amount such that about 1 to 2 weight % remains in the formed sponge nickel catalyst. In another embodiment, the hydrogenation catalyst is prepared using a solution of ruthenium (III) nitrosylnitrate, ruthenium (III) chloride in water to impregnate a suitable support material. The solution is then dried to form a solid having a water content of less than about 1% by weight. The solid may then be reduced at atmospheric pressure in a hydrogen stream at 300.degree. C. (uncalcined) or 400.degree. C. (calcined) in a rotary ball furnace for 4 hours. After cooling and rendering the catalyst inert with nitrogen, 5% by volume of oxygen in nitrogen is passed over the catalyst for 2 hours.
[0309] In certain embodiments, the catalyst described includes a catalyst support. The catalyst support stabilizes and supports the catalyst. The type of catalyst support used depends on the chosen catalyst and the reaction conditions. Suitable supports for the invention include, but are not limited to, carbon, silica, silica-alumina, zirconia, titania, ceria, vanadia, nitride, boron nitride, heteropolyacids, hydroxyapatite, zinc oxide, chromia, zeolites, carbon nanotubes, carbon fullerene and any combination thereof.
[0310] The catalysts used in this invention can be prepared using conventional methods known to those in the art. Suitable methods may include, but are not limited to, incipient wetting, evaporative impregnation, chemical vapor deposition, wash-coating, magnetron sputtering techniques, and the like.
[0311] The conditions for which to carry out the hydrogenation reaction will vary based on the type of starting material and the desired products. One of ordinary skill in the art, with the benefit of this disclosure, will recognize the appropriate reaction conditions. In general, the hydrogenation reaction is conducted at temperatures of 80.degree. C. to 250.degree. C., and preferably at 90.degree. C. to 200.degree. C., and most preferably at 100.degree. C. to 150.degree. C. In some embodiments, the hydrogenation reaction is conducted at pressures from 500 KPa to 14000 KPa.
[0312] The hydrogen used in the hydrogenolysis reaction of the current invention may include external hydrogen, recycled hydrogen, in situ generated hydrogen, and any combination thereof. As used herein, the term "external hydrogen" refers to hydrogen that does not originate from the biomass reaction itself, but rather is added to the system from another source.
[0313] In some embodiments of the invention, it is desirable to convert the starting carbohydrate to a smaller molecule that will be more readily converted to desired higher hydrocarbons. One suitable method for this conversion is through a hydrogenolysis reaction. Various processes are known for performing hydrogenolysis of carbohydrates. One suitable method includes contacting a carbohydrate with hydrogen or hydrogen mixed with a suitable gas and a hydrogenolysis catalyst in a hydrogenolysis reactor under conditions sufficient to form a reaction product comprising smaller molecules or polyols. As used herein, the term "smaller molecules or polyols" includes any molecule that has a smaller molecular weight, which can include a smaller number of carbon atoms or oxygen atoms than the starting carbohydrate. In an embodiment, the reaction products include smaller molecules that include polyols and alcohols. Someone of ordinary skill in the art would be able to choose the appropriate method by which to carry out the hydrogenolysis reaction.
[0314] In some embodiments, a 5 and/or 6 carbon sugar or sugar alcohol may be converted to propylene glycol, ethylene glycol, and glycerol using a hydrogenolysis catalyst. The hydrogenolysis catalyst may include Cr, Mo, W, Re, Mn, Cu, Cd, Fe, Co, Ni, Pt, Pd, Rh, Ru, Ir, Os, and alloys or any combination thereof, either alone or with promoters such as Au, Ag, Cr, Zn, Mn, Sn, Bi, B, O, and alloys or any combination thereof. The hydrogenolysis catalyst may also include a carbonaceous pyropolymer catalyst containing transition metals (e.g., chromium, molybdenum, tungsten, rhenium, manganese, copper, cadmium) or Group VIII metals (e.g., iron, cobalt, nickel, platinum, palladium, rhodium, ruthenium, iridium, and osmium). In certain embodiments, the hydrogenolysis catalyst may include any of the above metals combined with an alkaline earth metal oxide or adhered to a catalytically active support. In certain embodiments, the catalyst described in the hydrogenolysis reaction may include a catalyst support as described above for the hydrogenation reaction.
[0315] The conditions for which to carry out the hydrogenolysis reaction will vary based on the type of starting material and the desired products. One of ordinary skill in the art, with the benefit of this disclosure, will recognize the appropriate conditions to use to carry out the reaction. In general, they hydrogenolysis reaction is conducted at temperatures of 110.degree. C. to 300.degree. C., and preferably at 170.degree. C. to 220.degree. C., and most preferably at 200.degree. C. to 225.degree. C. In some embodiments, the hydrogenolysis reaction is conducted under basic conditions, preferably at a pH of 8 to 13, and even more preferably at a pH of 10 to 12. In some embodiments, the hydrogenolysis reaction is conducted at pressures in a range between 60 KPa and 16500 KPa, and preferably in a range between 1700 KPa and 14000 KPa, and even more preferably between 4800 KPa and 11000 KPa.
[0316] The hydrogen used in the hydrogenolysis reaction of the current invention can include external hydrogen, recycled hydrogen, in situ generated hydrogen, and any combination thereof.
[0317] In some embodiments, the reaction products discussed above may be converted into higher hydrocarbons through a condensation reaction in a condensation reactor. In such embodiments, condensation of the reaction products occurs in the presence of a catalyst capable of forming higher hydrocarbons. While not intending to be limited by theory, it is believed that the production of higher hydrocarbons proceeds through a stepwise addition reaction including the formation of carbon-carbon, or carbon-oxygen bond. The resulting reaction products include any number of compounds containing these moieties, as described in more detail below.
[0318] In certain embodiments, suitable condensation catalysts include an acid catalyst, a base catalyst, or an acid/base catalyst. As used herein, the term "acid/base catalyst" refers to a catalyst that has both an acid and a base functionality. In some embodiments the condensation catalyst can include, without limitation, zeolites, carbides, nitrides, zirconia, alumina, silica, aluminosilicates, phosphates, titanium oxides, zinc oxides, vanadium oxides, lanthanum oxides, yttrium oxides, scandium oxides, magnesium oxides, cerium oxides, barium oxides, calcium oxides, hydroxides, heteropolyacids, inorganic acids, acid modified resins, base modified resins, and any combination thereof. In some embodiments, the condensation catalyst can also include a modifier. Suitable modifiers include La, Y, Sc, P, B, Bi, Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, and any combination thereof. In some embodiments, the condensation catalyst can also include a metal. Suitable metals include Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh, Pd, Ir, Re, Mn, Cr, Mo, W, Sn, Os, alloys, and any combination thereof.
[0319] In certain embodiments, the catalyst described in the condensation reaction may include a catalyst support as described above for the hydrogenation reaction. In certain embodiments, the condensation catalyst is self-supporting. As used herein, the term "self-supporting" means that the catalyst does not need another material to serve as support. In other embodiments, the condensation catalyst in used in conjunction with a separate support suitable for suspending the catalyst. In an embodiment, the condensation catalyst support is silica.
[0320] The conditions under which the condensation reaction occurs will vary based on the type of starting material and the desired products. One of ordinary skill in the art, with the benefit of this disclosure, will recognize the appropriate conditions to use to carry out the reaction. In some embodiments, the condensation reaction is carried out at a temperature at which the thermodynamics for the proposed reaction are favorable. The temperature for the condensation reaction will vary depending on the specific starting polyol or alcohol. In some embodiments, the temperature for the condensation reaction is in a range from 80.degree. C. to 500.degree. C., and preferably from 125.degree. C. to 450.degree. C., and most preferably from 125.degree. C. to 250.degree. C. In some embodiments, the condensation reaction is conducted at pressures in a range between 0 Kpa to 9000 KPa, and preferably in a range between 0 KPa and 7000 KPa, and even more preferably between 0 KPa and 5000 KPa.
[0321] The higher alkanes formed by the invention include, but are not limited to, branched or straight chain alkanes that have from 4 to 30 carbon atoms, branched or straight chain alkenes that have from 4 to 30 carbon atoms, cycloalkanes that have from 5 to 30 carbon atoms, cycloalkenes that have from 5 to 30 carbon atoms, aryls, fused aryls, alcohols, and ketones. Suitable alkanes include, but are not limited to, butane, pentane, pentene, 2-methylbutane, hexane, hexene, 2-methylpentane, 3-methylpentane, 2,2,-dimethylbutane, 2,3-dimethylbutane, heptane, heptene, octane, octene, 2,2,4-trimethylpentane, 2,3-dimethyl hexane, 2,3,4-trimethylpentane, 2,3-dimethylpentane, nonane, nonene, decane, decene, undecane, undecene, dodecane, dodecene, tridecane, tridecene, tetradecane, tetradecene, pentadecane, pentadecene, nonyldecane, nonyldecene, eicosane, eicosene, uneicosane, uneicosene, doeicosane, doeicosene, trieicosane, trieicosene, tetraeicosane, tetraeicosene, and isomers thereof. Some of these products may be suitable for use as fuels.
[0322] In some embodiments, the cycloalkanes and the cycloalkenes are unsubstituted. In other embodiments, the cycloalkanes and cycloalkenes are mono-substituted. In still other embodiments, the cycloalkanes and cycloalkenes are multi-substituted. In the embodiments comprising the substituted cycloalkanes and cycloalkenes, the substituted group includes, without limitation, a branched or straight chain alkyl having 1 to 12 carbon atoms, a branched or straight chain alkylene having 1 to 12 carbon atoms, a phenyl, and any combination thereof. Suitable cycloalkanes and cycloalkenes include, but are not limited to, cyclopentane, cyclopentene, cyclohexane, cyclohexene, methyl-cyclopentane, methyl-cyclopentene, ethyl-cyclopentane, ethyl-cyclopentene, ethyl-cyclohexane, ethyl-cyclohexene, isomers and any combination thereof.
[0323] In some embodiments, the aryls formed are unsubstituted. In another embodiment, the aryls formed are mono-substituted. In the embodiments comprising the substituted aryls, the substituted group includes, without limitation, a branched or straight chain alkyl having 1 to 12 carbon atoms, a branched or straight chain alkylene having 1 to 12 carbon atoms, a phenyl, and any combination thereof. Suitable aryls for the invention include, but are not limited to, benzene, toluene, xylene, ethyl benzene, para xylene, meta xylene, and any combination thereof.
[0324] The alcohols produced in the invention have from 4 to 30 carbon atoms. In some embodiments, the alcohols are cyclic. In other embodiments, the alcohols are branched. In another embodiment, the alcohols are straight chained. Suitable alcohols for the invention include, but are not limited to, butanol, pentanol, hexanol, heptanol, octanol, nonanol, decanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol, hexadecanol, heptyldecanol, octyldecanol, nonyldecanol, eicosanol, uneicosanol, doeicosanol, trieicosanol, tetraeicosanol, and isomers thereof.
[0325] The ketones produced in the invention have from 4 to 30 carbon atoms. In an embodiment, the ketones are cyclic. In another embodiment, the ketones are branched. In another embodiment, the ketones are straight chained. Suitable ketones for the invention include, but are not limited to, butanone, pentanone, hexanone, heptanone, octanone, nonanone, decanone, undecanone, dodecanone, tridecanone, tetradecanone, pentadecanone, hexadecanone, heptyldecanone, octyldecanone, nonyldecanone, eicosanone, uneicosanone, doeicosanone, trieicosanone, tetraeicosanone, and isomers thereof.
[0326] Another such chemical modification is interesterification. Naturally produced glycerolipids do not have a uniform distribution of fatty acid constituents. In the context of oils, interesterification refers to the exchange of acyl radicals between two esters of different glycerolipids. The interesterification process provides a mechanism by which the fatty acid constituents of a mixture of glycerolipids can be rearranged to modify the distribution pattern. Interesterification is a well-known chemical process, and generally comprises heating (to about 200.degree. C.) a mixture of oils for a period (e.g., 30 minutes) in the presence of a catalyst, such as an alkali metal or alkali metal alkylate (e.g., sodium methoxide). This process can be used to randomize the distribution pattern of the fatty acid constituents of an oil mixture, or can be directed to produce a desired distribution pattern. This method of chemical modification of lipids can be performed on materials provided herein, such as microbial biomass with a percentage of dry cell weight as lipid at least 20%.
[0327] Directed interesterification, in which a specific distribution pattern of fatty acids is sought, can be performed by maintaining the oil mixture at a temperature below the melting point of some TAGs which might occur. This results in selective crystallization of these TAGs, which effectively removes them from the reaction mixture as they crystallize. The process can be continued until most of the fatty acids in the oil have precipitated, for example. A directed interesterification process can be used, for example, to produce a product with a lower calorie content via the substitution of longer-chain fatty acids with shorter-chain counterparts. Directed interesterification can also be used to produce a product with a mixture of fats that can provide desired melting characteristics and structural features sought in food additives or products (e.g., margarine) without resorting to hydrogenation, which can produce unwanted trans isomers.
[0328] Interesterification of oils produced by the methods described herein can be performed in conjunction with one or more of the methods and/or materials, or to produce products, as reported in the following: U.S. Pat. No. 6,080,853 (Nondigestible fat substitutes); U.S. Pat. No. 4,288,378 (Peanut butter stabilizer); U.S. Pat. No. 5,391,383 (Edible spray oil); U.S. Pat. No. 6,022,577 (Edible fats for food products); U.S. Pat. No. 5,434,278 (Edible fats for food products); U.S. Pat. No. 5,268,192 (Low calorie nut products); U.S. Pat. No. 5,258,197 (Reduce calorie edible compositions); U.S. Pat. No. 4,335,156 (Edible fat product); U.S. Pat. No. 7,288,278 (Food additives or medicaments); U.S. Pat. No. 7,115,760 (Fractionation process); U.S. Pat. No. 6,808,737 (Structural fats); U.S. Pat. No. 5,888,947 (Engine lubricants); U.S. Pat. No. 5,686,131 (Edible oil mixtures); and U.S. Pat. No. 4,603,188 (Curable urethane compositions).
[0329] In one embodiment in accordance with the invention, transesterification of the oil, as described above, is followed by reaction of the transesterified product with polyol, as reported in U.S. Pat. No. 6,465,642, to produce polyol fatty acid polyesters. Such an esterification and separation process may comprise the steps as follows: reacting a lower alkyl ester with polyol in the presence of soap; removing residual soap from the product mixture; water-washing and drying the product mixture to remove impurities; bleaching the product mixture for refinement; separating at least a portion of the unreacted lower alkyl ester from the polyol fatty acid polyester in the product mixture; and recycling the separated unreacted lower alkyl ester.
[0330] Transesterification can also be performed on microbial biomass with short chain fatty acid esters, as reported in U.S. Pat. No. 6,278,006. In general, transesterification may be performed by adding a short chain fatty acid ester to an oil in the presence of a suitable catalyst and heating the mixture. In some embodiments, the oil comprises about 5% to about 90% of the reaction mixture by weight. In some embodiments, the short chain fatty acid esters can be about 10% to about 50% of the reaction mixture by weight. Non-limiting examples of catalysts include base catalysts, sodium methoxide, acid catalysts including inorganic acids such as sulfuric acid and acidified clays, organic acids such as methane sulfonic acid, benzenesulfonic acid, and toluenesulfonic acid, and acidic resins such as Amberlyst 15. Metals such as sodium and magnesium, and metal hydrides also are useful catalysts.
[0331] Another such chemical modification is hydroxylation, which involves the addition of water to a double bond resulting in saturation and the incorporation of a hydroxyl moiety. The hydroxylation process provides a mechanism for converting one or more fatty acid constituents of a glycerolipid to a hydroxy fatty acid. Hydroxylation can be performed, for example, via the method reported in U.S. Pat. No. 5,576,027. Hydroxylated fatty acids, including castor oil and its derivatives, are useful as components in several industrial applications, including food additives, surfactants, pigment wetting agents, defoaming agents, water proofing additives, plasticizing agents, cosmetic emulsifying and/or deodorant agents, as well as in electronics, pharmaceuticals, paints, inks, adhesives, and lubricants. One example of how the hydroxylation of a glyceride may be performed is as follows: fat may be heated, preferably to about 30-50.degree. C. combined with heptane and maintained at temperature for thirty minutes or more; acetic acid may then be added to the mixture followed by an aqueous solution of sulfuric acid followed by an aqueous hydrogen peroxide solution which is added in small increments to the mixture over one hour; after the aqueous hydrogen peroxide, the temperature may then be increased to at least about 60.degree. C. and stirred for at least six hours; after the stirring, the mixture is allowed to settle and a lower aqueous layer formed by the reaction may be removed while the upper heptane layer formed by the reaction may be washed with hot water having a temperature of about 60.degree. C.; the washed heptane layer may then be neutralized with an aqueous potassium hydroxide solution to a pH of about 5 to 7 and then removed by distillation under vacuum; the reaction product may then be dried under vacuum at 100.degree. C. and the dried product steam-deodorized under vacuum conditions and filtered at about 50.degree. to 60.degree. C. using diatomaceous earth.
[0332] Hydroxylation of microbial oils produced by the methods described herein can be performed in conjunction with one or more of the methods and/or materials, or to produce products, as reported in the following: U.S. Pat. No. 6,590,113 (Oil-based coatings and ink); U.S. Pat. No. 4,049,724 (Hydroxylation process); U.S. Pat. No. 6,113,971 (Olive oil butter); U.S. Pat. No. 4,992,189 (Lubricants and lube additives); U.S. Pat. No. 5,576,027 (Hydroxylated milk); and U.S. Pat. No. 6,869,597 (Cosmetics).
[0333] Hydroxylated glycerolipids can be converted to estolides. Estolides consist of a glycerolipid in which a hydroxylated fatty acid constituent has been esterified to another fatty acid molecule. Conversion of hydroxylated glycerolipids to estolides can be carried out by warming a mixture of glycerolipids and fatty acids and contacting the mixture with a mineral acid, as described by Isbell et al., JAOCS 71(2):169-174 (1994). Estolides are useful in a variety of applications, including without limitation those reported in the following: U.S. Pat. No. 7,196,124 (Elastomeric materials and floor coverings); U.S. Pat. No. 5,458,795 (Thickened oils for high-temperature applications); U.S. Pat. No. 5,451,332 (Fluids for industrial applications); U.S. Pat. No. 5,427,704 (Fuel additives); and U.S. Pat. No. 5,380,894 (Lubricants, greases, plasticizers, and printing inks).
[0334] Another such chemical modification is olefin metathesis. In olefin metathesis, a catalyst severs the alkylidene carbons in an alkene (olefin) and forms new alkenes by pairing each of them with different alkylidine carbons. The olefin metathesis reaction provides a mechanism for processes such as truncating unsaturated fatty acid alkyl chains at alkenes by ethenolysis, cross-linking fatty acids through alkene linkages by self-metathesis, and incorporating new functional groups on fatty acids by cross-metathesis with derivatized alkenes.
[0335] In conjunction with other reactions, such as transesterification and hydrogenation, olefin metathesis can transform unsaturated glycerolipids into diverse end products. These products include glycerolipid oligomers for waxes; short-chain glycerolipids for lubricants; homo- and hetero-bifunctional alkyl chains for chemicals and polymers; short-chain esters for biofuel; and short-chain hydrocarbons for jet fuel. Olefin metathesis can be performed on triacylglycerols and fatty acid derivatives, for example, using the catalysts and methods reported in U.S. Pat. No. 7,119,216, US Patent Pub. No. 2010/0160506, and U.S. Patent Pub. No. 2010/0145086.
[0336] Olefin metathesis of bio-oils generally comprises adding a solution of Ru catalyst at a loading of about 10 to 250 ppm under inert conditions to unsaturated fatty acid esters in the presence (cross-metathesis) or absence (self-metathesis) of other alkenes. The reactions are typically allowed to proceed from hours to days and ultimately yield a distribution of alkene products. One example of how olefin metathesis may be performed on a fatty acid derivative is as follows: A solution of the first generation Grubbs Catalyst (dichloro[2(1-methylethoxy-.alpha.-O)phenyl]methylene-.alpha.-C] (tricyclohexyl-phosphine) in toluene at a catalyst loading of 222 ppm may be added to a vessel containing degassed and dried methyl oleate. Then the vessel may be pressurized with about 60 psig of ethylene gas and maintained at or below about 30.degree. C. for 3 hours, whereby approximately a 50% yield of methyl 9-decenoate may be produced.
[0337] Olefin metathesis of oils produced by the methods described herein can be performed in conjunction with one or more of the methods and/or materials, or to produce products, as reported in the following: Patent App. PCT/US07/081427 (.alpha.-olefin fatty acids) and U.S. patent application Ser. No. 12/281,938 (petroleum creams), Ser. No. 12/281,931 (paintball gun capsules), Ser. No. 12/653,742 (plasticizers and lubricants), Ser. No. 12/422,096 (bifunctional organic compounds), and Ser. No. 11/795,052 (candle wax).
[0338] Other chemical reactions that can be performed on microbial oils include reacting triacylglycerols with a cyclopropanating agent to enhance fluidity and/or oxidative stability, as reported in U.S. Pat. No. 6,051,539; manufacturing of waxes from triacylglycerols, as reported in U.S. Pat. No. 6,770,104; and epoxidation of triacylglycerols, as reported in "The effect of fatty acid composition on the acrylation kinetics of epoxidized triacylglycerols", Journal of the American Oil Chemists' Society, 79:1, 59-63, (2001) and Free Radical Biology and Medicine, 37:1, 104-114 (2004).
[0339] The generation of oil-bearing microbial biomass for fuel and chemical products as described above results in the production of delipidated biomass meal. Delipidated meal is a byproduct of preparing algal oil and is useful as animal feed for farm animals, e.g., ruminants, poultry, swine and aquaculture. The resulting meal, although of reduced oil content, still contains high quality proteins, carbohydrates, fiber, ash, residual oil and other nutrients appropriate for an animal feed. Because the cells are predominantly lysed by the oil separation process, the delipidated meal is easily digestible by such animals. Delipidated meal can optionally be combined with other ingredients, such as grain, in an animal feed. Because delipidated meal has a powdery consistency, it can be pressed into pellets using an extruder or expander or another type of machine, which are commercially available.
[0340] The invention, having been described in detail above, is exemplified in the following examples, which are offered to illustrate, but not to limit, the claimed invention.
XIV. EXAMPLES
Example 1
Fatty Acid Analysis by Fatty Acid Methyl Ester Detection
[0341] Lipid samples were prepared from dried biomass. 20-40 mg of dried biomass was resuspended in 2 mL of 5% H.sub.2SO.sub.4 in MeOH, and 200 ul of toluene containing an appropriate amount of a suitable internal standard (C19:0) was added. The mixture was sonicated briefly to disperse the biomass, then heated at 70-75.degree. C. for 3.5 hours. 2 mL of heptane was added to extract the fatty acid methyl esters, followed by addition of 2 mL of 6% K.sub.2CO.sub.3 (aq) to neutralize the acid. The mixture was agitated vigorously, and a portion of the upper layer was transferred to a vial containing Na.sub.2SO.sub.4 (anhydrous) for gas chromatography analysis using standard FAME GC/FID (fatty acid methyl ester gas chromatography flame ionization detection) methods. Fatty acid profiles reported below were determined by this method.
Example 2
Triacylglyceride Purification from Oil and Methods for Triacylglyceride Lipase Digestion
[0342] The triacylglyceride (TAG) fraction of each oil sample was isolated by dissolving .about.10 mg of oil in dichloromethane and loading it onto a Bond-Elut aminopropyl solid-phase extraction cartridge (500 mg) preconditioned with heptane. TAGs were eluted with dicholoromethane-MeOH (1:1) into a collection tube, while polar lipids were retained on the column. The solvent was removed with a stream of nitrogen gas. Tris buffer and 2 mg porcine pancreatic lipase (Type II, Sigma, 100-400 units/mg) were added to the TAG fraction, followed by addition of bile salt and calcium chloride solutions. The porcine pancreatic lipase cleaves sn-1 and sn-3 fatty acids, thereby generating 2-monoacylglycerides and free fatty acids. This mixture was heated with agitation at 40.degree. C. for three minutes, cooled briefly, then quenched with 6 N HCl. The mixture was then extracted with diethyl ether and the ether layer was washed with water then dried over sodium sulfate. The solvent was removed with a stream of nitrogen. To isolate the monoacylglyceride (MAG) fraction, the residue was dissolved in heptane and loaded onto a second aminopropyl solid phase extraction cartridge pretreated with heptane. Residual TAGs were eluted with diethyl ether-dichloromethane-heptane (1:9:40), diacylglycerides (DAGs) were eluted with ethyl acetate-heptane (1:4), and MAGs were eluted from the cartridge with dichloromethane-methanol (2:1). The resulting MAG, DAG, and TAG fractions were then concentrated to dryness with a stream of nitrogen and subjected to routine direct transesterification method of GC/FID analysis as described in Example 1.
Example 3
Engineering Microorganisms for Fatty Acid and SN-2 Profiles Increased in Lauric Acid Through Exogenous LPAAT Expression
[0343] This example describes the use of recombinant polynucleotides that encode a C. nucifera 1-acyl-sn-glycerol-3-phosphate acyltransferase (Cn LPAAT) enzyme to engineer a microorganism in which the fatty acid profile and the sn-2 profile of the transformed microorganism has been enriched in lauric acid.
[0344] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain A, was initially transformed with the plasmid construct pSZ1283 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. pSZ1283, described in PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696 hereby incorporated by reference, comprised the coding sequence of the Cuphea wrightii FATB2 (CwTE2) thioesterase (SEQ ID NO: 10), 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4), to express the protein sequence given in SEQ ID NO: 3, under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. The CwTE2 protein coding sequence to express the protein sequence given in SEQ ID NO: 11, was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR. The protein coding regions of CwTE2 and suc2 were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/U52009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0345] Upon transformation of pSZ1283 into Strain A, positive clones were selected on agar plates with sucrose as the sole carbon source. Primary transformants were then clonally purified and a single transformant, Strain B, was selected for further genetic modification. This genetically engineered strain was transformed with plasmid construct pSZ2046 to interrupt the pLoop genomic locus of Strain B. Construct pSZ2046 comprised the coding sequence of the C. nucifera 1-acyl-sn-glycerol-3-phosphate acyltransferase (Cn LPAAT) enzyme (SEQ ID NO: 12), 5' (SEQ ID NO: 13) and 3' (SEQ ID NO: 14) homologous recombination targeting sequences (flanking the construct) to the pLoop genomic region for integration into the nuclear genome, and a neomycin resistance protein-coding sequence under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5), and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This NeoR expression cassette is listed as SEQ ID NO: 15 and served as a selectable marker. The Cn LPAAT protein coding sequence was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR. The protein coding regions of Cn LPAAT and NeoR were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. The amino acid sequence of Cn LPAAT is provided as SEQ ID NO: 16.
[0346] Upon transformation of pSZ2046 into Strain B, thereby generating Strain C, positive clones were selected on agar plates comprising G418 (Geneticin). Individual transformants were clonally purified and grown at pH 7.0 under conditions suitable for lipid production as detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass from each transformant and fatty acid profiles from these samples were analyzed using standard fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1.The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis UTEX 1435 (U1) grown on glucose as a sole carbon source, untransformed Strain B and five pSZ2046 positive transformants (Strain C, 1-5) are presented in Table 6.
TABLE-US-00008 TABLE 6 Effect of LPAAT expression on fatty acid profiles of transformed Prototheca moriformis (UTEX 1435) comprising a mid-chain preferring thioesterase. Area % Fatty Strain Strain Strain Strain Strain Strain acid U1 B C-1 C-2 C-3 C-4 C-5 C10:0 0.01 5.53 11.37 11.47 10.84 11.13 11.12 C12:0 0.04 31.04 46.63 46.47 45.84 45.80 45.67 C14:0 1.27 15.99 15.14 15.12 15.20 15.19 15.07 C16:0 27.20 12.49 7.05 7.03 7.30 7.20 7.19 C18:0 3.85 1.30 0.71 0.72 0.74 0.74 0.74 C18:1 58.70 24.39 10.26 10.41 10.95 11.31 11.45 C18:2 7.18 7.79 7.05 6.93 7.30 6.88 7.01 C10- 0.50 36.57 58.00 57.94 56.68 56.93 56.79 C12
[0347] As shown in Table 6, the fatty acid profile of Strain B expressing CwTE2 showed increased composition of C10:0, C12:0, and C14:0 fatty acids and a decrease in C16:0, C18:0, and C18:1 fatty acids relative to the fatty acid profile of the untransformed UTEX 1435 strain. The impact of additional genetic modification on the fatty acid profile of the transformed strains, namely the expression of CnLPAAT in Strain B, is a still further increase in the composition of C10:0 and C12:0 fatty acids, a still further decrease in C16:0, C18:0, and C18:1 fatty acids, but no significant effect on the C14:0 fatty acid composition. These data indicate that the CnLPAAT shows substrate preference in the context of a microbial host organism.
[0348] The untransformed P. moriformis Strain A is characterized by a fatty acid profile comprising less than 0.5% C12 fatty acids and less than 1% C10-C12 fatty acids. In contrast, the fatty acid profile of Strain B expressing a C. wrightii thioesterase comprised 31% C12:0 fatty acids, with C10-C12 fatty acids comprising greater than 36% of the total fatty acids. Further, fatty acid profiles of Strain C, expressing a higher plant thioesterase and a CnLPAAT enzyme, comprised between 45.67% and 46.63% C12:0 fatty acids, with C10-C12% fatty acids comprising between 71 and 73% of total fatty acids. The result of expressing an exogenous thioesterase was a 62-fold increase in the percentage of C12 fatty acid present in the engineered microbe. The result of expressing an exogenous thioesterase and exogenous LPAAT was a 92-fold increase in the percentage of C12 fatty acids present in the engineered microbe.
[0349] The TAG fraction of oil samples extracted from Strains A, B, and C were analyzed for the sn-2 profile of their triacylglycerides. The TAGs were extracted and processed as described in Example 2 and analyzed as in Examples 1 and 2. The fatty acid composition and the sn-2 profiles of the TAG fraction of oil extracted from Strains A, B, and C (expressed as Area % of total fatty acids) are presented in Table 7. Values not reported are indicated as "n.r."
TABLE-US-00009 TABLE 7 Effect of LPAAT expression on the fatty acid composition and the sn-2 profile of TAGs produced from transformed Prototheca moriformis (UTEX 1435) comprising a mid-chain preferring thioesterase. Strain Area Strain C % Strain A Strain B (pSZ1500 + fatty (untransformed) (pSZ1500) pSZ2046) acid FA sn-2 profile FA sn-2 profile FA sn-2 profile C10:0 n.r. n.r. 11.9 14.2 12.4 7.1 C12:0 n.r. n.r. 42.4 25 47.9 52.8 C14:0 1.0 0.6 12 10.4 13.9 9.1 C16:0 23.9 1.6 7.2 1.3 6.1 0.9 C18:0 3.7 0.3 n.r n.r. 0.8 0.3 C18:1 64.3 90.5 18.3 36.6 9.9 17.5 C18:2 4.5 5.8 5.8 10.8 6.5 10 C18:3 n.r. n.r. n.r. n.r. 1.1 1.6
[0350] As shown in Table 7, the fatty acid composition of triglycerides (TAGs) isolated from Strain B expressing CwTE2 was increased for C10:0, C12:0, and C14:0 fatty acids and decrease in C16:0 and C18:1 fatty acids relative to the fatty acid profile of TAGs isolated from untransformed Strain A. The impact of additional genetic modification on the fatty acid profile of the transformed strains, namely the expression of CnLPAAT, was a still further increase in the composition of C10:0 and C12:0 fatty acids, a still further decrease in C16:0, C18:0, and C18:1 fatty acids, but no significant effect on the C14:0 fatty acid composition. These data indicate that expression of the exogenous CnLPAAT improves the midchain fatty acid profile of transformed microbes.
[0351] The untransformed P. moriformis Strain A is characterized by an sn-2 profile of about 0.6% C14, about 1.6% C16:0, about 0.3% C18:0, about 90% C18:1, and about 5.8% C18:2. In contrast to Strain A, Strain B, expressing a C. wrightii thioesterase is characterized by an sn-2 profile that is higher in midchain fatty acids and lower in long chain fatty acids. C12 fatty acids comprised 25% of the sn-2 profile of Strain B. The impact of additional genetic modification on the sn-2 profile of the transformed strains, namely the expression of CnLPAAT, was still a further increase in C12 fatty acids (from 25% to 52.8%), a decrease in C18:1 fatty acids (from 36.6% to 17.5%), and a decrease in C10:0 fatty acids. (The sn-2 profile composition of C14:0 and C16:0 fatty acids was relatively similar for Strains B and C.)
[0352] These data demonstrate the utility and effectiveness of polynucleotides permitting exogenous LPAAT expression to alter the fatty acid profile of engineered microorganisms, and in particular in increasing the concentration of C10:0 and C12:0 fatty acids in microbial cells. These data further demonstrate the utility and effectiveness of polynucleotides permitting exogenous thioesterase and exogenous LPAAT expression to alter the sn-2 profile of TAGs produced by microbial cells, in particular in increasing the C12 composition of sn-2 profiles and decreasing the C18:1 composition of sn-2 profiles.
Example 4
Thermal Behavior of Oils Produced from Recombinant Microalgae
[0353] FIGS. 1-14 include fatty acid profiles and melting curves of refined, bleached and deodorized oils from genetically engineered Prototheca moriformis strains. In some cases, modifications of the melting curves are obtained via genetic engineering. For example, some of the oils produced have shallower or sharper melting transitions relative to control microalgal oils (i.e., those produced from strains lacking a given genetic modification) or relative to widely available plant oils. In addition, FIG. 12 shows scanning calorimetry for a high palmitic oil when tempered by holding at room temperature for several days (lower trace) and for the same oil after performing the first scan (upper trace). The scans ranged from -60.degree. C. to +50.degree. C. with a heating rate of 10.degree. C/minute. The differences between the two traces suggests that tempering of the oil caused a change in crystal structure within the oil.
[0354] Also of note, FIGS. 10 and 11 show stability testing of RBD-5 and RBD 6. Remarkably, RBD-6, an oil with less than 0.1% 18:2 and 18:3 fatty acids was substantially stable as measured by the oxidative stability index (AOCS Method Cd 12b-92) even after 36 hours of heating at 110.degree. C.
[0355] Table 8, below, gives details of the genetic engineering of the strains identified in FIGS. 1-13.
TABLE-US-00010 TABLE 8 Genetically engineered strains. RB Z Ulmus Americana thioesterase RBD-1 Cuphea wrightii FATB2 thioesterase driven by amt03 RBD-2 Ulmus americana thioesterase RBD-3 Native C. hookeriana C16:0-specific thioesterase with amt03 promoter RBD Y Ulmus Americana thioesterase with Btub promoter RBD X SAD2B knockout with native C wrightii FAT2B thioesterase, amt03 promoter RBD W SAD2B KO with Native C. wrightii FATB2 driven by amt03 at insertion site RBD-4 control strain RBD-5 FATA-1 knockout with Carthamus oleate sp. TE driven by amt03 promoter at insertion site RBD-6 FADc knockout with Carthamus tinctorius oleoyl thioesterase
Example 5
Characteristics of Processed Oil Produced from Engineered Microorganisms
[0356] Methods and effects of transforming Prototheca moriformis (UTEX 1435) with transformation vector pSZ1500 (SEQ ID NO: 17) have been previously described in PCT Application Nos. PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0357] A classically mutagenized (for higher oil production) derivative of Prototheca moriformis (UTEX 1435), Strain A, was transformed with pSZ1500 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. pSZ1500 comprised nucleotide sequence of the Carthamus tinctorius oleyl-thioesterase (CtOTE) gene, codon-optimized for expression in P. moriformis UTEX 1435. The pSZ1500 expression construct included 5' (SEQ ID NO: 18) and 3' (SEQ ID NO: 19) homologous recombination targeting sequences (flanking the construct) to the FADc genomic region for integration into the nuclear genome and a S. cerevisiae suc2 sucrose invertase coding region under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selection marker. The CtOTE coding region was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR, and the native transit peptide was replaced with the C. protothecoides stearoyl-ACP desaturase transit peptide (SEQ ID NO: 9). The protein coding regions of CtOTE and suc2 were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0358] Primary pSZ1500 transformants of Strain A were selected on agar plates containing sucrose as a sole carbon source, clonally purified, and a single engineered line, Strain D was selected for analysis. Strain D was grown as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Hexane extraction of the oil from the generated biomass was then performed using standard methods, and the resulting triglyceride oil was determined to be free of residual hexane. Other methods of extraction of oil from microalgae using an expeller press are described in PCT Application No. PCT/US2010/031108 and are hereby incorporated by reference.
[0359] Different lots of oil extracted from biomass of Strain D were refined, bleached, and deodorized using standard vegetable oil processing methods. These procedures generated oil samples RBD437, RBD469, RBD501, RBD 502, RBD503, and RBD529, which were subjected to analytical testing protocols according to methods defined through the American Oil Chemists' Society, the American Society for Testing and Materials, and the International Organization for Standardization. The results of these analyses are summarized below in Tables 9-14.
TABLE-US-00011 TABLE 9 Analytical results for oil sample RBD469. Method Number Test Description Results Units AOCS Ca 3a-46 Insoluble impurities <0.01 % AOCS Ca 5a-40 Free Fatty Acids (Oleic) 0.02 % AOCS Ca 5a-40 Acid Value 0.04 mg KOH/g AOCS CA 9f-57 Neutral oil 98.9 % D97 Cloud Point -15 deg C. D97 Pour Point -18 deg C. Karl Fischer Moisture 0.01 % AOCS Cc 13d-55 Chlorophyll <0.01 ppm (modified) Iodine Value 78.3 g I.sub.2/100 g AOCS Cd 8b-90 Peroxide Value 0.31 meq/kg ISO 6885 p-Anisidine Value 0.65 AOCS Cc 18-80 Dropping Melting point 6.2 deg C. (Mettler) AOCS Cd 11d-96 Tricylglicerides 98.6 % AOCS Cd 11d-96 Monoglyceride <0.01 % AOCS Cd 11d-96 Diglycerides 0.68 % AOCS Cd 20-91 Total Polar Compounds 2.62 % IUPAC, 2.507 and Oxidized & Polymerized 17.62 % 2.508 Triacylglycerides AOCS Cc 9b-55 Flash Point 244 deg C. AOCS Cc 9a-48 Smoke Point 232 deg C. AOCS Cd 12b-92 Oxidataive Stability Index 31.6 hours Rancimat (110.degree. C.) AOCS Ca 6a-40 Unsaponified Matter 2.28 %
[0360] RBD469 oil was analyzed for trace element content, solid fat content, and Lovibond color according to AOCS methods. Results of these analyses are presented below in Table 10, Table 10, and Table 11.
TABLE-US-00012 TABLE 10 ICP Elemental Analysis of RBD469 oil. Method Number Test Description Results in ppm AOCS Ca 20-99 and Phosphorus 1.09 AOCS Ca 17-01 Calcium 0.1 (modified) Magnesium 0.04 Iron <0.02 Sulfur 28.8 Copper <0.05 Potassium <0.50 Sodium <0.50 Silicon 0.51 Boron 0.06 Aluminum <0.20 Lead <0.20 Lithium <0.02 Nickel <0.20 Vanadium <0.05 Zinc <0.02 Arsenic <0.20 Mercury <0.20 Cadmium <0.03 Chromium <0.02 Manganese <0.05 Silver <0.05 Titanium <0.05 Selenium <0.50 UOP779 Chloride organic <1 UOP779 Chloride inorganic 7.24 AOCS Ba 4e-93 Nitrogen 6.7
TABLE-US-00013 TABLE 11 Solid Fat Content of RBD469 Oil Method Number Solid Fat Content Result AOCS Cd 12b-93 Solid Fat Content 10.degree. C. 0.13% AOCS Cd 12b-93 Solid Fat Content 15.degree. C. 0.13% AOCS Cd 12b-93 Solid Fat Content 20.degree. C. 0.28% AOCS Cd 12b-93 Solid Fat Content 25.degree. C. 0.14% AOCS Cd 12b-93 Solid Fat Content 30.degree. C. 0.08% AOCS Cd 12b-93 Solid Fat Content 35.degree. C. 0.25%
TABLE-US-00014 TABLE 12 Lovibond Color of RBD469 Oil Method Number Color Result Unit AOCS Cc 13j-97 red 2 Unit AOCS Cc 13j-97 yellow 27 Unit
[0361] RBD469 oil was subjected to transesterification to produce fatty acid methyl esters (FAMEs). The resulting FAME profile of RBD469 is shown in Table 12.
TABLE-US-00015 TABLE 13 FAME Profile of RBD469 Oil Fatty Acid Area % C10 0.01 C12:0 0.04 C14:0 0.64 C15:0 0.08 C16:0 8.17 C16:1 iso 0.39 C16:1 0.77 C17:0 0.08 C18:0 1.93 C18:1 85.88 C18:1 iso 0.05 C18:2 0.05 C20:0 0.3 C20:1 0.06 C20:1 0.44 C22:0 0.11 C23:0 0.03 C24:0 0.1 Total FAMEs Identified 99.13
[0362] The oil stability indexes (OSI) of 6 RBD oil samples without supplemented antioxidants and 3 RBD oil samples supplemented with antioxidants were analyzed according to the Oil Stability Index AOCS Method Cd 12b-92. Shown in Table 14 are the results of OSI AOCS Cd 12b-92 tests, conducted at 110.degree. C., performed using a Metrohm 873 Biodiesel Rancimat. Results, except where indicated with an asterisks (*), are the average of multiple OSI runs. Those samples not analyzed are indicated (NA).
TABLE-US-00016 TABLE 14 Oil Stability Index at 110.degree. C. of RBD oil samples with and without antioxidants. Antioxidant Antioxidant OSI (hours) for each RBD Sample added Concentration RBD437 RBD469 RBD502 RBD501 RBD503 RBD529 None 0 65.41 38.33 72.10 50.32 63.04 26.68 Tocopherol & 35 ppm/ 77.72 48.60 82.67 NA NA NA Ascorbyl 16.7 ppm Palmitate Tocopherol & 140 ppm/ 130.27 81.54* 211.49* NA NA NA Ascorbyl 66.7 ppm Palmitate Tocopherol & 1050 ppm/ >157* >144 242.5* NA NA NA Ascorbyl 500 ppm Palmitate Tocopherol 50 ppm NA 46.97 NA NA NA NA TBHQ 20 ppm 63.37 37.4 NA NA NA NA
[0363] The untransformed P. moriformis (UTEX 1435) acid profile comprises less than 60% C18:1 fatty acids and greater than 7% C18:2 fatty acids. In contrast, Strain D (comprising pSZ1500) exhibited fatty acid profiles with an increased composition of C18:1 fatty acids (to above 85%) and a decrease in C18:2 fatty acids (to less than 0.06%). Upon refining, bleaching, and degumming, RBD oils samples prepared from the oil made from strain E exhibited OSI values >26 hrs. With addition of antioxidants, the OSI of RBD oils prepared from oils of Strain D increased from 48.60 hours to greater than 242 hours. In other experiments, OSI values of over 400 hours were achieved. Additional properties of a low polyunsaturated oil according to embodiments of the invention are given in FIG. 16.
Example 6
Improving the Levels of Oleic Acid of Engineered Microbes Through Allelic Disruption of a Fatty Acid Desaturase and an Acyl-ACP Thioesterase
[0364] This example describes the use of a transformation vector to disrupt a FATA locus of a Prototheca moriformis strain previously engineered for high oleic acid and low linoleic acid production. The transformation cassette used in this example comprised a selectable marker and nucleotide sequences encoding a P. moriformis KASII enzyme to engineer microorganisms in which the fatty acid profile of the transformed microorganism has been altered for further increased oleic acid and lowered palmitic acid levels.
[0365] Strain D, described in Example 5 and in PCT/US2012/023696, is a classically mutagenized (for higher oil production) derivative of P. moriformis (UTEX 1435) subsequently transformed with the transformation construct pSZ1500 (SEQ ID NO: 17) according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. This strain was used as the host for transformation with construct pSZ2276 to increase expression of a KASII enzyme while concomitantly ablating an endogenous acyl-ACP thioesterase genetic locus to generate Strain E. The pSZ2276 transformation construct included 5' (SEQ ID NO: 20) and 3' (SEQ ID NO: 21) homologous recombination targeting sequences (flanking the construct) to the FATA1 genomic region for integration into the P. moriformis nuclear genome, an A. thaliana THIC protein coding region under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This AtTHIC expression cassette is listed as SEQ ID NO: 23 and served as a selection marker. The P. moriformis KASII protein coding region was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR, and the native transit peptide of the KASII enzyme was replaced with the C. protothecoides stearoyl-ACP desaturase transit peptide (SEQ ID NO: 9). The codon-optimized sequence of PmKASII (Prototheca moriformis KASII) comprising a C. protothecoides S106 stearoyl-ACP desaturase transit peptide is provided the sequence listings as SEQ ID NO: 24. SEQ ID NO: 25 provides the protein translation of SEQ ID NO: 24. The protein coding regions of PmKASII and suc2 were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/U52009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0366] Primary pSZ2276 transformants of Strain D were selected on agar plates lacking thiamine, clonally purified, and a single engineered line, strain E was selected for analysis. Strain E was cultivated under heterotrophic lipid production conditions at pH5.0 and pH7.0 as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass from each transformant and fatty acid profiles from these samples were analyzed using standard fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1. The fatty acid profiles (expressed as Area % of total fatty acids) from the transgenic line arising from transformation with pSZ2276 into Strain D are shown in Table 15.
TABLE-US-00017 TABLE 15 Fatty acid profiles of Prototheca moriformis (UTEX 1435) Strains A, D, and E engineered for increased oleic acid and lowered linoleic acid levels. Transformation Area % Fatty Acid Strain Construct(s) pH C16:0 C18:0 C18:1 C18:2 C20:1 Strain None pH 5 26.6 3.3 60.5 6.7 0.07 A Strain None pH 7 28.3 4.1 58 6.5 0.06 A Strain pSZ1500 pH 5 17 3.6 77.1 0.01 0.14 D Strain pSZ1500 pH 7 19.5 5.3 72.6 0.01 0.09 D Strain pSZ1500 + pH 5 4.1 2.36 88.5 0.04 3.1 E pSZ2276 Strain pSZ1500 + pH 7 2.1 7.8 87.9 0.01 0.5 E pSZ2276
[0367] As shown in Table 15, targeted interruption of FADc alleles with a CtOTE expression cassette impacted the fatty acid profiles of transformed microorganisms. Fatty acid profiles of Strain D (comprising the pSZ1500 transformation vector) showed increased composition of C18:1 fatty acids with a concomitant decrease in C16:0 and C18:2 fatty acids relative to Strain A. Subsequent transformation of Strain D with pSZ2276 to overexpress a P. moriformis (UTEX 1435) KASII protein while concomitantly ablating a FATA genetic locus (thereby generating Strain E) resulted in still further impact on the fatty acid profiles of the transformed microorganisms. Fatty acid profiles of Strain E showed increased composition of C18:1 fatty acids, with a further decrease in C16:0 fatty acids relative to Strains A and D. Propagation of Strain E in culture conditions at pH 7, to induce expression from the Amt03 promoter, resulted in a fatty acid profile that was higher in C18:0 and C18:1 fatty acids and lower in C16:0 fatty acids, relative to the same strain cultured at pH 5.
[0368] These data demonstrate the utility of multiple genetic modifications to impact the fatty acid profile of a host organism for increased levels of oleic acid with concomitant decreased levels of linoleic acid and palmitic acid. Further, this example illustrates the use of recombinant polynucleotides to target gene interruption of an endogenous FATA allele with a cassette comprising a pH-regulatable promoter to control expression of an exogenous KASII protein-coding region in order to alter the fatty acid profile of a host microbe.
Example 7
Conditional Expression of a Fatty Acid Desaturase
[0369] This example describes the use of a transformation vector to conditionally express a delta 12 fatty acid desaturase (FADs) in a Prototheca moriformis strain previously engineered for high oleic acid and very low linoleic acid production in both seed and lipid productivity stages of propagation. Very low linoleic acid levels in cell oils are sought for use in certain applications. However, absence of linoleic acid during cell division phase ("seed stage") of a host microbe is disadvantageous. Linoleic acid may be supplemented to the seed medium to hasten cell division and not added during lipid production, but this addition imposes unwanted costs. To overcome this challenge, a transformation cassette was constructed for regulated expression of a FAD2 enzyme such that levels of linoleic acids sufficient for cell division could be achieved and oil with very low levels of linoleic acids could be produced during the oil production phase of culture of a microorganism. The transformation cassette used in this example comprised a selectable marker, a pH-regulatable promoter, and nucleotide sequences encoding a P. moriformis FAD2 enzyme to engineer microorganisms in which the fatty acid profile of the transformed microorganism has been altered for increased oleic acid production and regulatable linoleic acid production.
[0370] Strain D, described in Examples 5, 6, and in PCT/US2012/023696, is a classically mutagenized (for higher oil production) derivative of P. moriformis (UTEX 1435) subsequently transformed with the transformation construct pSZ1500 (SEQ ID NO: 17) according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. This strain was used as the host for transformation with construct pSZ2413 to introduce a pH-driven promoter for regulation of a P. moriformis FAD2 enzyme. The pSZ2413 transformation construct included 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the P. moriformis nuclear genome, an A. thaliana THIC protein coding region under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This AtTHIC expression cassette is listed as SEQ ID NO: 23 and served as a selection marker. The P. moriformis FAD2 protein coding region was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR. The codon-optimized sequence of PmFAD2 is provided the sequence listings as SEQ ID NO: 26. SEQ ID NO: 27 provides the protein translation of SEQ ID NO: 26. The protein coding regions of PmFAD2 and suc2 were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0371] Primary pSZ2413 transformants of Strain D were selected on agar plates lacking thiamine, clonally purified, and isolates of the engineered line, Strain F were selected for analysis. These isolates were cultivated under heterotrophic lipid production conditions at pH7.0 (to activate expression of FAD2 from the PmAmt03 promoter) and at pH5.0, as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass from each transformant and fatty acid profiles from these samples were analyzed using standard fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1. The resulting profile of C18:2 fatty acids (expressed in Area %) from nine representative isolates of transgenic Strain F (F-1 through F-9) arising from transformation with pSZ2413 into Strain D are shown in Table 16.
TABLE-US-00018 TABLE 16 C18:2 fatty acid profiles of Prototheca moriformis (UTEX 1435) Strains A, D, and F. Area % C18:2 Strain Transformation Construct (s) pH 5.0 pH 7.0 A None 6.07 7.26 D pSZ1500 0.01 0.01 F-1 pSZ1500 + pSZ2413 0.37 5.29 F-2 pSZ1500 + pSZ2413 0.45 6.87 F-3 pSZ1500 + pSZ2413 0.50 6.79 F-4 pSZ1500 + pSZ2413 0.57 5.06 F-5 pSZ1500 + pSZ2413 0.57 7.58 F-6 pSZ1500 + pSZ2413 0.60 6.88 F-7 pSZ1500 + pSZ2413 0.62 6.52 F-8 pSZ1500 + pSZ2413 0.63 5.79 F-9 pSZ1500 + pSZ2413 0.77 4.53
[0372] As shown in Table 16 the impact of regulated expression of the PmFAD2 enzyme, effected though strain culture at different pH levels, is a clear increase in the composition of C18:2 fatty acids in the transformed microorganism. Linoleic acid comprises about 6% to about 7.3% of fatty acids of Strain A. In contrast, Strain D (comprising the pSZ1500 transformation vector to ablate both FAD2 alleles) is characterized by a fatty acid profile of 0.01% linoleic acid. Transformation of Strain D with pSZ2413 to generate Strain F results in a recombinant microbe in which the production of linoleic acid is regulated by the Amt03 promoter. Propagation of Strain F isolates in culture conditions at pH 7, to induce FAD2 expression from the Amt03 promoter, resulted in a fatty acid profile characterized by about 4.5% to about 7.5% linoleic acid. In contrast, propagation of Strain F isolates in culture conditions at pH 5 resulted in a fatty acid profile characterized by about 0.33 to about 0.77% linoleic acid.
[0373] These data demonstrate the utility of and effectiveness of recombinant polynucleotides permitting conditional expression of a FAD2 enzyme to alter the fatty acid profile of engineered microorganisms, and in particular in regulating the production of C18:2 fatty acids in microbial cells.
Example 8
Analysis of Regiospecific Profile
[0374] LC/MS TAG distribution analyses were carried out using a Shimadzu Nexera ultra high performance liquid chromatography system that included a SIL-30AC autosampler, two LC-30AD pumps, a DGU-20A5 in-line degasser, and a CTO-20A column oven, coupled to a Shimadzu LCMS 8030 triple quadrupole mass spectrometer equipped with an APCI source. Data was acquired using a Q3 scan of m/z 350-1050 at a scan speed of 1428 u/sec in positive ion mode with the CID gas (argon) pressure set to 230 kPa. The APCI, desolvation line, and heat block temperatures were set to 300, 250, and 200.degree. C., respectively, the flow rates of the nebulizing and drying gases were 3.0 L/min and 5.0 L/min, respectively, and the interface voltage was 4500 V. Oil samples were dissolved in dichloromethane-methanol (1:1) to a concentration of 5 mg/mL, and 0.8 .mu.L of sample was injected onto Shimadzu Shim-pack XR-ODS III (2.2 .mu.m, 2.0.times.200 mm) maintained at 30.degree. C. A linear gradient from 30% dichloromethane-2-propanol (1:1)/acetonitrile to 51% dichloromethane-2-propanol (1:1)/acetonitrile over 27 minutes at 0.48 mL/min was used for chromatographic separations.
Example 9
Engineering Microbes for Increased Production of SOS, POP, And POS Triacylglycerides
[0375] This example describes the use of recombinant polynucleotides that encode a C18:0-preferring Brassica napus thioesterase (BnOTE) enzyme to engineer a microorganism in which the triacylglyceride distribution of the transformed microorganism has been enriched in SOS, POS, and POP triacylglycerides.
[0376] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain A, was initially transformed with the plasmid construct pSZ1358 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. pSZ1358, described in PCT/US2012/023696, hereby incorporated by reference, comprised the coding sequence of the Brassica napus thioesterase (BnOTE) thioesterase (SEQ ID NO: 28), 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4), to express the protein sequence given in SEQ ID NO: 3, under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. The BnOTE protein coding sequence to express the protein sequence given in SEQ ID NO: 29, was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3'UTR. The protein coding regions of BnOTE and suc2 were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0377] Primary pSZ1358 transformants of Strain A were selected on agar plates containing sucrose as a sole carbon source, clonally purified, and single engineered line, Strain G was selected for analysis. Strain G was cultivated under heterotrophic lipid production conditions at pH7.0 (to activate expression of BnOTE from the PmAmt03 promoter) as described in PCT/U52009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Oil samples obtained from Strain A and Strain G were analyzed for fatty acid composition using methods described in Examples 1 and 2, and, using the methods described in Example 8, for the regiospecificity of triacylglycerides in the oil. Fatty acid profiles of TAGs isolated from Strain A and G are shown in Table 17. Table 18 presents the regiospecificity profile of POP, POS, and SOS TAGs present in oil samples from Strain A and G.
TABLE-US-00019 TABLE 17 Effect of BnOTE expression on the fatty acid composition and the sn-2 profile of TAGs produced from transformed Prototheca moriformis. Strain G Area % Strain A (pSZ1358) Fatty acid FA profile FA profile C10:0 n.r. 0.5 C12:0 n.r. 0.5 C14:0 1.0 1.3 C16:0 23.9 25.8 C18:0 3.7 30.4 C18:1 64.3 30.2 C18:2 4.5 8.8 C18:3 .alpha. n.r. 0.4
TABLE-US-00020 TABLE 18 Effect of BnOTE expression on the regiospecific profile of POP, POS, and SOS TAGs produced from transformed Prototheca moriformis. Strain A (untransformed) Strain G (pSZ1358) Cocoa Butter Area Normalized Area Normalized Area Normalized TAG % Area % % Area % % Area % POP 13.09 76.8 10.6 23.5 17.9 22.1 POS 3.51 20.5 21.0 46.6 39.2 48.4 SOS 0.45 2.6 13.5 29.9 23.9 29.5 total 17.05 100 45.0 100 81.1 100
[0378] As shown in Table 17, the fatty acid composition of TAGs isolated from Strain G expressing BnOTE was markedly increased for C18:0 fatty acids (from 3.7% to 30.4%) and decreased in C18:1 fatty acids (from 64.3% to 30.2%) relative to the fatty acid profile of TAGs isolated from untransformed Strain A. The fatty acid composition of TAGs isolated from Strain A was characterized by about 23.9% palmitic acid, 3.7% stearic acid, and 64.3% oleic acid, a ratio for P:S:O of about 6.5:1:17.4. In contrast, the fatty acid composition of TAGs isolated from Strain G was characterized by about 25.8% palmitic acid, 30.4% stearic acid, and 30.2% oleic acid, a ratio for P:O:S of about 1:1.18:1.17.
[0379] The impact of expression of a C18:0 preferring thioesterase on the regiospecific profile of POP, POS, and SOS TAGs of oils produced from the transformed microorganism was an increase in all three TAGs as a proportion of the total TAGs present in the oil. As shown in Table 18, the sum of POP+POS+SOS TAGs accounted for 45% of the TAGs produced by Strain G, whereas POP, POS, and SOS TAGs summed to only about 17% of TAGs produced in Strain A. The percentages of POP, POS and SOS of strain G are compared to Cocoa butter in Table 18. As can be seen, ratios of POP, POS and SOS of Strain G are very similar to the ratios observed in cocoa butter.
[0380] These data demonstrate the utility and effectiveness of polynucleotides permitting exogenous thioesterase expression to alter the fatty acid and regiospecific profiles of TAGs of engineered microorganisms, in particular to increase the distribution of POP, POS, and SOS TAGs.
Examples 10-33
Engineering of Microorganisms
[0381] Examples 10-33 below describe the engineering of various microorganisms in accordance with the present invention. To alter the fatty acid profile of a microorganism, microorganisms can be genetically modified wherein endogenous or exogenous lipid biosynthesis pathway enzymes are expressed, overexpressed, or attenuated. Steps to genetically engineer a microbe to alter its fatty acid profile as to the degree of fatty acid unsaturation and to decrease or increase fatty acid chain length comprise the design and construction of a transformation vector (e.g., a plasmid), transformation of the microbe with one or more vectors, selection of transformed microbes (transformants), growth of the transformed microbe, and analysis of the fatty acid profile of the lipids produced by the engineered microbe.
[0382] Transgenes that alter the fatty acid profiles of host organisms can be expressed in numerous eukaryotic microbes. Examples of expression of transgenes in eukaryotic microbes including Chlamydomonas reinhardtii, Chlorella elhpsoidea, Chlorella saccarophila, Chlorella vulgaris, Chlorella kessleri, Chlorella sorokiniana, Haematococcus pluvialis, Gonium pectorals, Volvox carteri, Dunaliella tertiolecta, Dunaliella viridis, Dunaliella salina, Closterium peracerosum-strigosum-littorale complex, Nannochloropsis sp., Thalassiosira pseudonana, Phaeodactylum tricornutum, Navicula saprophila, Cylindrotheca fusiformis, Cyclotella cryptica, Symbiodinium microadriacticum, Amphidinium sp., Chaetoceros sp., Mortierella alpina, and Yarrowia hpolytica can be found in the scientific literature. These expression techniques can be combined with the teachings of the present invention to produce engineered microorganisms with altered fatty acid profiles.
[0383] Transgenes that alter the fatty acid profiles of host organisms or alter the regiospecific distribution of glycerolipids produced by host organisms can also be expressed in numerous prokaryotic microbes. Examples of expression of transgenes in oleaginous microbes including Rhodococcus opacus can be found in the literature. These expression techniques can be combined with the teachings of the present invention to produce engineered microorganisms with altered fatty acid profiles.
TABLE-US-00021 TABLES 19A-D Codon preference listing. Haemato Amino Chlorella Chlorella Chlorella Chlorella Dunaliella Volvox coccus Acid Codon sorokiniana vulgaris ellipsoidea kessleri tertiolecta carteri pluvialis Ala GCG 0.20 0.25 0.15 0.14 0.09 0.25 0.21 Ala GCA 0.05 0.24 0.32 0.10 0.17 0.13 0.27 Ala GCT 0.12 0.16 0.26 0.18 0.31 0.26 0.17 Ala GCC 0.63 0.35 0.27 0.58 0.43 0.36 0.35 Arg AGG 0.03 0.09 0.10 0.09 0.26 0.08 0.14 Arg AGA 0.04 0.05 0.14 0.01 0.09 0.03 0.05 Arg CGG 0.06 0.19 0.09 0.06 0.06 0.17 0.15 Arg CGA 0.00 0.10 0.08 0.00 0.08 0.08 0.10 Arg CGT 0.06 0.09 0.37 0.14 0.12 0.22 0.13 Arg CGC 0.81 0.48 0.22 0.71 0.40 0.43 0.42 Asn AAT 0.04 0.16 0.43 0.06 0.27 0.23 0.21 Asn AAC 0.96 0.84 0.57 0.94 0.73 0.77 0.79 Asp GAT 0.13 0.25 0.47 0.12 0.40 0.35 0.27 Asp GAC 0.87 0.75 0.53 0.88 0.60 0.65 0.73 Cys TGT 0.06 0.13 0.43 0.09 0.20 0.17 0.27 Cys TGC 0.94 0.87 0.57 0.91 0.80 0.83 0.64 End TGA 0.00 0.72 0.14 0.14 0.36 0.24 0.70 End TAG 0.33 0.11 0.29 0.00 0.00 0.18 0.22 End TAA 0.67 0.17 4.00 0.86 0.64 0.59 0.09 Gln CAG 0.42 0.40 0.15 0.40 0.27 0.29 0.33 Gln CAA 0.04 0.04 0.21 0.40 0.27 0.07 0.10 Glu GAG 0.53 0.50 0.33 0.40 0.27 0.53 0.49 Glu GAA 0.02 0.06 0.31 0.40 0.27 0.11 0.07 Gly GGG 0.04 0.16 0.19 0.08 0.10 0.12 0.22 Gly GGA 0.02 0.11 0.13 0.07 0.13 0.12 0.11 Gly GGT 0.03 0.12 0.39 0.24 0.25 0.23 0.15 Gly GGC 0.91 0.61 0.29 0.96 0.51 0.53 0.52 His CAT 0.14 0.16 0.30 0.08 0.25 0.35 0.27 His CAC 0.86 0.84 0.70 0.93 0.75 0.65 0.73 Ile ATA 0.00 0.04 0.07 0.01 0.04 0.08 0.09 Ile ATT 0.15 0.30 0.63 0.29 0.31 0.35 0.29 Ile ATC 0.85 0.66 0.65 0.69 0.65 0.57 0.62 Leu TTG 0.03 0.07 0.03 0.05 0.14 0.14 0.16 Leu TTA 0.00 0.01 0.32 0.00 0.02 0.03 0.02 Leu CTG 0.72 0.61 0.34 0.61 0.60 0.45 0.53 Leu CTA 0.01 0.03 0.03 0.04 0.04 0.07 0.07 Leu CTT 0.04 0.08 0.16 0.06 0.06 0.14 0.09 Leu CTC 0.20 0.20 0.12 0.24 0.14 0.17 0.13 Lys AAG 0.98 0.94 0.54 0.98 0.90 0.90 0.84 Lys AAA 0.02 0.06 0.46 0.02 0.10 0.10 0.16 Met ATG 1.00 1.00 1.00 1.00 1.00 1.00 1.00 Phe TTT 0.28 0.32 0.42 0.31 0.24 0.27 0.35 Phe TTC 0.72 0.68 0.58 0.69 0.76 0.73 0.65 Pro CCG 0.18 0.31 0.09 0.07 0.04 0.34 0.15 Pro CCA 0.06 0.17 0.36 0.07 0.04 0.20 0.24 Pro CCT 0.10 0.14 0.25 0.17 0.04 0.19 0.29 Pro CCC 0.66 0.38 0.29 0.69 0.04 0.27 0.32 Ser AGT 0.03 0.04 0.14 0.02 0.08 0.08 0.07 Ser AGC 0.27 0.38 0.18 0.18 0.31 0.27 0.31 Ser TCG 0.12 0.14 0.08 0.10 0.02 0.19 0.10 Ser TCA 0.03 0.08 0.14 0.08 0.09 0.09 0.14 Ser TCT 0.09 0.11 0.26 0.18 0.19 0.14 0.13 Ser TCC 0.47 0.24 0.20 0.44 0.30 0.24 0.24 Thr ACG 0.11 0.20 0.13 0.05 0.12 0.27 0.19 Thr ACA 0.01 0.20 0.32 0.07 0.20 0.12 0.23 Thr ACT 0.12 0.13 0.29 0.12 0.24 0.20 0.18 Thr ACC 0.76 0.47 0.26 0.76 0.44 0.41 0.40 Trp TGG 1.00 1.00 1.00 1.00 1.00 1.00 1.00 Tyr TAT 0.07 0.15 0.43 0.27 0.28 0.24 0.19 Tyr TAC 0.93 0.85 0.57 0.73 0.72 0.76 0.81 Val GTG 0.71 0.54 0.37 0.60 0.54 0.46 0.62 Val GTA 0.00 0.05 0.25 0.03 0.09 0.07 0.09 Val GTT 0.11 0.14 0.24 0.09 0.14 0.17 0.09 Val GTC 0.18 0.27 0.14 0.28 0.23 0.30 0.21 Closterium peracerosum- strigosum- Amino littorale Dunaliella Dunaliella Gonium Phaeodactylum Chaetoceros Acid Codon complex viridis sauna pectorale tricornutum cornpressum Ala GCG 0.48 0.13 0.15 0.43 0.15 0.08 Ala GCA 0.10 0.27 0.20 0.09 0.10 0.37 Ala GCT 0.15 0.25 0.27 0.08 0.23 0.36 Ala GCC 0.26 0.35 0.39 0.41 0.52 0.18 Arg AGG 0.04 0.25 0.22 0.13 0.02 0.14 Arg AGA 0.00 0.06 0.05 0.00 0.04 0.29 Arg CGG 0.18 0.08 0.12 0.40 0.10 0.00 Arg CGA 0.00 0.06 0.06 0.05 0.12 0.19 Arg CGT 0.13 0.15 0.13 0.08 0.41 0.38 Arg CGC 0.64 0.39 0.43 0.35 0.31 0.00 Asn AAT 0.04 0.17 0.23 0.07 0.30 0.58 Asn AAC 0.96 0.83 0.77 0.93 0.65 0.42 Asp GAT 0.30 0.38 0.40 0.11 0.41 0.53 Asp GAC 0.70 0.62 0.60 0.89 0.59 0.47 Cys TGT 0.06 0.24 0.17 0.20 0.39 0.44 Cys TGC 0.94 0.76 0.83 0.90 0.61 0.56 End TGA 0.75 0.31 0.37 0.50 0.06 0.50 End TAG 0.00 0.15 0.14 0.00 0.13 0.00 End TAA 0.25 0.54 0.49 0.50 0.81 0.50 Gln CAG 0.53 0.36 0.32 0.31 0.23 0.16 Gln CAA 0.09 0.12 0.08 0.07 0.14 0.19 Glu GAG 0.31 0.44 0.51 0.56 0.21 0.28 Glu GAA 0.06 0.09 0.09 0.07 0.42 0.37 Gly GGG 0.31 0.14 0.10 0.18 0.08 0.12 Gly GGA 0.06 0.11 0.12 0.09 0.34 0.33 Gly GGT 0.09 0.22 0.22 0.07 0.30 0.39 Gly GGC 0.53 0.54 0.56 0.65 0.28 0.16 His CAT 0.33 0.25 0.25 0.43 0.28 0.84 His CAC 0.67 0.75 0.75 0.57 0.72 0.16 Ile ATA 0.03 0.03 0.03 0.07 0.03 0.12 Ile ATT 0.23 0.25 0.31 0.33 0.51 0.65 Ile ATC 0.74 0.72 0.66 0.59 0.46 0.23 Leu TTG 0.04 0.11 0.12 0.04 0.26 0.11 Leu TTA 0.00 0.01 0.01 0.00 0.02 0.14 Leu CTG 0.31 0.60 0.61 0.64 0.15 0.05 Leu CTA 0.01 0.05 0.04 0.01 0.05 0.08 Leu CTT 0.04 0.07 0.08 0.05 0.18 0.51 Leu CTC 0.60 0.16 0.14 0.26 0.34 0.11 Lys AAG 0.86 0.87 0.89 0.93 0.75 0.52 Lys AAA 0.14 0.13 0.11 0.07 0.25 0.48 Met ATG 1.00 1.00 1.00 1.00 1.00 1.00 Phe TTT 0.09 0.25 0.29 0.10 0.44 0.65 Phe TTC 0.91 0.75 0.71 0.90 0.56 0.35 Pro CCG 0.28 0.10 0.08 0.53 0.29 0.05 Pro CCA 0.15 0.10 0.17 0.09 0.12 0.45 Pro CCT 0.12 0.10 0.30 0.04 0.20 0.33 Pro CCC 0.44 0.10 0.45 0.34 0.40 0.17 Ser AGT 0.04 0.09 0.06 0.02 0.12 0.14 Ser AGC 0.05 0.31 0.32 0.20 0.12 0.07 Ser TCG 0.22 0.04 0.06 0.42 0.19 0.08 Ser TCA 0.16 0.08 0.10 0.09 0.06 0.31 Ser TCT 0.05 0.17 0.15 0.07 0.15 0.23 Ser TCC 0.47 0.31 0.30 0.20 0.35 0.18 Thr ACG 0.30 0.16 0.13 0.42 0.23 0.10 Thr ACA 0.06 0.21 0.18 0.03 0.13 0.38 Thr ACT 0.22 0.18 0.23 0.08 0.19 0.27 Thr ACC 0.42 0.46 0.46 0.47 0.45 0.25 Trp TGG 1.00 1.00 1.00 1.00 1.00 1.00 Tyr TAT 0.07 0.16 0.21 0.12 0.18 0.67 Tyr TAC 0.93 0.84 0.79 0.88 0.82 0.33 Val GTG 0.50 0.64 0.62 0.57 0.22 0.30 Val GTA 0.02 0.03 0.05 0.04 0.09 0.27 Val GTT 0.06 0.11 0.11 0.04 0.22 0.10 Val GTC 0.42 0.22 0.23 0.35 0.47 0.33 Symbio- Thal- Cylindro- Amphi- dinium Nanno- assiosira Amino theca dinium micro- chloro- Cyclotella Nayicula pseudo- C. Acid Codon fusiformis carterae adriacticum psis sp cryptica pelliculosa nana reinhardtii Ala GCG 0.07 0.17 0.22 0.24 0.11 0.00 0.11 0.35 Ala GCA 0.14 0.33 0.26 0.10 0.16 0.13 0.25 0.08 Ala GCT 0.35 0.29 0.20 0.17 0.45 0.44 0.33 0.13 Ala GCC 0.43 0.20 0.32 0.48 0.27 0.44 0.30 0.43 Arg AGG 0.09 0.15 0.27 0.00 0.09 0.05 0.18 0.05 Arg AGA 0.14 0.03 0.27 0.00 0.05 0.10 0.17 0.01 Arg CGG 0.06 0.08 0.09 0.00 0.04 0.05 0.06 0.20 Arg CGA 0.16 0.18 0.09 0.29 0.08 0.35 0.11 0.04 Arg CGT 0.34 0.18 0.09 0.14 0.47 0.20 0.34 0.09 Arg CGC 0.22 0.40 0.18 0.57 0.28 0.25 0.15 0.62 Asn AAT 0.42 0.37 0.21 0.00 0.25 0.47 0.43 0.09 Asn AAC 0.58 0.63 0.79 1.00 0.75 0.53 0.57 0.91 Asp GAT 0.54 0.54 0.50 0.20 0.52 0.20 0.56 0.14 Asp GAC 0.46 0.46 0.50 0.80 0.48 0.80 0.44 0.86 Cys TGT 0.44 0.75 0.50 0.00 0.29 0.10 0.54 0.10 Cys TGC 0.56 0.25 0.50 1.00 0.71 0.90 0.46 0.90 End TGA 0.13 0.50 1.00 0.00 0.10 0.00 0.31 0.27 End TAG 0.10 0.00 0.00 0.00 0.00 0.00 0.38 0.22 End TAA 0.77 0.50 0.00 1.00 0.90 1.00 0.31 0.52 Gln CAG 0.12 0.33 0.28 0.41 0.19 0.21 0.16 0.38 Gln CAA 0.25 0.15 0.17 0.00 0.17 0.28 0.19 0.04 Glu GAG 0.23 0.41 0.50 0.59 0.38 0.17 0.40 0.55 Glu GAA 0.39 0.10 0.06 0.00 0.26 0.34 0.26 0.03 Gly GGG 0.06 0.19 0.32 0.10 0.10 0.03 0.12 0.11 Gly GGA 0.47 0.10 0.12 0.05 0.45 0.28 0.51 0.06 Gly GGT 0.35 0.34 0.16 0.25 0.22 0.13 0.23 0.11 Gly GGC 0.12 0.37 0.40 0.60 0.24 0.56 0.14 0.72 His CAT 0.39 0.12 0.40 0.00 0.42 1.00 0.50 0.11 His CAC 0.61 0.88 0.60 1.00 0.58 0.00 0.50 0.89 Ile ATA 0.06 0.05 0.00 0.00 0.04 0.00 0.08 0.03 Ile ATT 0.42 0.53 0.38 0.14 0.53 0.73 0.38 0.22 Ile ATC 0.52 0.42 0.63 0.86 0.42 0.27 0.54 0.75 Leu TTG 0.26 0.35 0.39 0.22 0.20 0.16 0.29 0.04 Leu TTA 0.09 0.01 0.00 0.00 0.03 0.00 0.05 0.01 Leu CTG 0.09 0.22 0.39 0.09 0.06 0.12 0.08 0.73 Leu CTA 0.05 0.00 0.04 0.00 0.03 0.04 0.06 0.03 Leu CTT 0.37 0.31 0.13 0.04 0.39 0.36 0.20 0.05 Leu CTC 0.13 0.12 0.04 0.65 0.29 0.32 0.32 0.15 Lys AAG 0.60 0.93 0.85 1.00 0.70 0.83 0.76 0.95 Lys AAA 0.40 0.07 0.15 0.00 0.30 0.17 0.24 0.05 Met ATG 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 Phe TTT 0.37 0.21 0.25 0.20 0.31 0.78 0.38 0.16 Phe TTC 0.63 0.79 0.75 0.80 0.69 0.22 0.62 0.84 Pro CCG 0.11 0.14 0.18 0.08 0.10 0.21 0.16 0.33 Pro CCA 0.33 0.42 0.09 0.08 0.16 0.29 0.31 0.08 Pro CCT 0.32 0.22 0.41 0.25 0.35 0.21 0.31 0.13 Pro CCC 0.24 0.22 0.32 0.58 0.39 0.29 0.23 0.47 Ser AGT 0.12 0.13 0.09 0.00 0.09 0.13 0.18 0.04 Ser AGC 0.09 0.24 0.14 0.13 0.08 0.28 0.11 0.35 Ser TCG 0.13 0.03 0.05 0.00 0.15 0.25 0.17 0.25 Ser TCA 0.12 0.25 0.05 0.00 0.12 0.08 0.12 0.05 Ser TCT 0.30 0.16 0.23 0.13 0.39 0.25 0.23 0.07 Ser TCC 0.24 0.19 0.45 0.75 0.18 0.03 0.19 0.25 Thr ACG 0.09 0.14 0.10 0.28 0.10 0.18 0.21 0.30 Thr ACA 0.15 0.28 0.10 0.00 0.15 0.09 0.19 0.08 Thr ACT 0.39 0.12 0.10 0.17 0.33 0.41 0.28 0.10 Thr ACC 0.37 0.47 0.70 0.56 0.43 0.32 0.32 0.52 Trp TGG 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 Tyr TAT 0.38 0.32 0.20 0.00 0.38 0.20 0.39 0.10 Tyr TAC 0.62 0.68 0.80 1.00 0.62 0.80 0.61 0.90 Val GTG 0.11 0.65 0.67 0.31 0.16 0.18 0.29 0.67 Val GTA 0.06 0.05 0.00 0.00 0.09 0.09 0.16 0.03 Val GTT 0.38 0.08 0.11 0.15 0.42 0.09 0.28 0.07 Val GTC 0.46 0.21 0.22 0.54 0.33 0.64 0.27 0.22 Amino Yarrowia Mortierella Rhodococcus Acid Codon lipolytica alpina opacus Ala GCG 0.08 0.14 0.35 Ala GCA 0.11 0.12 0.14 Ala GCT 0.35 0.29 0.09 Ala GCC 0.46 0.45 0.43 Arg AGG 0.05 0.05 0.05 Arg AGA 0.13 0.06 0.02 Arg CGG 0.12 0.06 0.26 Arg CGA 0.52 0.09 0.12 Arg CGT 0.11 0.32 0.11 Arg CGC 0.07 0.42 0.44 Asn AAT 0.17 0.15 0.21 Asn AAC 0.83 0.85 0.79 Asp GAT 0.35 0.42 0.24 Asp GAC 0.65 0.58 0.76 Cys TGT 0.46 0.13 0.26 Cys TGC 0.54 0.87 0.74 End TGA 0.16 0.05 0.72 End TAG 0.38 0.25 0.17 End TAA 0.46 0.70 0.11 Gln CAG 0.33 0.36 0.28 Gln CAA 0.08 0.06 0.06 Glu GAG 0.44 0.49 0.45 Glu GAA 0.14 0.09 0.22 Gly GGG 0.05 0.03 0.18 Gly GGA 0.28 0.29 0.15 Gly GGT 0.32 0.32 0.20 Gly GGC 0.34 0.36 0.48 His CAT 0.34 0.27 0.20 His CAC 0.66 0.73 0.80 Ile ATA 0.03 0.01 0.05
Ile ATT 0.44 0.33 0.14 Ile ATC 0.53 0.66 0.81 Leu TTG 0.09 0.27 0.09 Leu TTA 0.02 0.00 0.01 Leu CTG 0.37 0.26 0.41 Leu CTA 0.05 0.02 0.03 Leu CTT 0.18 0.12 0.06 Leu CTC 0.29 0.32 0.40 Lys AAG 0.84 0.91 0.80 Lys AAA 0.16 0.09 0.20 Met ATG 1.00 1.00 1.00 Phe TTT 0.38 0.39 0.09 Phe TTC 0.62 0.61 0.91 Pro CCG 0.10 0.07 0.52 Pro CCA 0.10 0.08 0.09 Pro CCT 0.32 0.36 0.07 Pro CCC 0.47 0.49 0.32 Ser AGT 0.07 0.05 0.08 Ser AGC 0.11 0.14 0.23 Ser TCG 0.16 0.32 0.33 Ser TCA 0.08 0.08 0.07 Ser TCT 0.28 0.12 0.05 Ser TCC 0.30 0.29 0.24 Thr ACG 0.11 0.17 0.28 Thr ACA 0.14 0.10 0.11 Thr ACT 0.26 0.23 0.07 Thr ACC 0.49 0.49 0.53 Trp TGG 1.00 1.00 1.00 Tyr TAT 0.18 0.20 0.18 Tyr TAC 0.82 0.80 0.82 Val GTG 0.33 0.22 0.37 Val GTA 0.05 0.02 0.05 Val GTT 0.26 0.27 0.10 Val GTC 0.36 0.49 0.49
TABLE-US-00022 TABLE 20 Lipid biosynthesis pathway proteins 3-Ketoacyl ACP synthase Cuphea hookeriana 3-ketoacyl-ACP synthase (GenBank Acc. No. AAC68861.1), Cuphea wrightii beta-ketoacyl-ACP synthase II (GenBank Acc. No. AAB37271.1), Cuphea lanceolata beta-ketoacyl-ACP synthase IV (GenBank Acc. No. CAC59946.1), Cuphea wrightii beta-ketoacyl-ACP synthase II (GenBank Acc. No. AAB37270.1), Ricinus communis ketoacyl-ACP synthase (GenBank Acc. No. XP_002516228), Gossypium hirsutum ketoacyl- ACP synthase (GenBank Acc. No. ADK23940.1), Glycine max plastid 3-keto-acyl-ACP synthase II-A (GenBank Acc No. AAW88763.1), Elaeis guineensis beta-ketoacyl-ACP synthase II (GenBank Acc. No. AAF26738.2), Helianthus annuus plastid 3-keto-acyl-ACP synthase I (GenkBank Acc. No. ABM53471.1), Glycine max3-keto-acyl-ACP synthase I (GenBank Acc. No. NP_001238610.1), Helianthus annuus plastid 3-keto-acyl-ACP synthase II (GenBank Acc ABI18155.1), Brassica napus beta-ketoacyl-ACP synthetase 2 (GenBank Acc. No. AAF61739.1), Perilla frutescens beta-ketoacyl-ACP synthase II (GenBank Acc. No. AAC04692.1), Helianthus annus beta-ketoacyl-ACP synthase II (GenBank Accession No. ABI18155), Ricinus communis beta-ketoacyl-ACP synthase II (GenBank Accession No. AAA33872), Haematococcus pluvialis beta-ketoacyl acyl carrier protein synthase (GenBank Accession No. HM560033.1), Jatropha curcasbeta ketoacyl-ACP synthase I (GenBank Accession No. ABJ90468.1), Populus trichocarpa beta-ketoacyl-ACP synthase I (GenBank Accession No. XP_002303661.1), Coriandrum sativum beta-ketoacyl-ACP synthetase I (GenBank Accession No. AAK58535.1), Arabidopsis thaliana 3-oxoacyl-[acyl-carrier- protein] synthase I (GenBank Accession No. NP_001190479.1), Vitis vinifera 3-oxoacyl- [acyl-carrier-protein] synthase I (GenBank Accession No. XP_002272874.2) Fatty acyl-ACP Thioesterases Umbellularia californica fatty acyl-ACP thioesterase (GenBank Acc. No. AAC49001), Cinnamomum camphora fatty acyl-ACP thioesterase (GenBank Acc. No. Q39473), Umbellularia californica fatty acyl-ACP thioesterase (GenBank Acc. No. Q41635), Myristica fragrans fatty acyl-ACP thioesterase (GenBank Acc. No. AAB71729), Myristica fragrans fatty acyl-ACP thioesterase (GenBank Acc. No. AAB71730), Elaeis guineensis fatty acyl- ACP thioesterase (GenBank Acc. No. ABD83939), Elaeis guineensis fatty acyl-ACP thioesterase (GenBank Acc. No. AAD42220), Populus tomentosa fatty acyl-ACP thioesterase (GenBank Acc. No. ABC47311), Arabidopsis thaliana fatty acyl-ACP thioesterase (GenBank Acc. No. NP_172327), Arabidopsis thaliana fatty acyl-ACP thioesterase (GenBank Acc. No. CAA85387), Arabidopsis thaliana fatty acyl-ACP thioesterase (GenBank Acc. No. CAA85388), Gossypium hirsutum fatty acyl-ACP thioesterase (GenBank Acc. No. Q9SQI3), Cuphea lanceolata fatty acyl-ACP thioesterase (GenBank Acc. No. CAA54060), Cuphea hookeriana fatty acyl-ACP thioesterase (GenBank Acc. No. AAC72882), Cuphea calophylla subsp. mesostemon fatty acyl-ACP thioesterase (GenBank Acc. No. ABB71581), Cuphea lanceolata fatty acyl-ACP thioesterase (GenBank Acc. No. CAC19933), Elaeis guineensis fatty acyl-ACP thioesterase (GenBank Acc. No. AAL15645), Cuphea hookeriana fatty acyl- ACP thioesterase (GenBank Acc. No. Q39513), Gossypium hirsutum fatty acyl-ACP thioesterase (GenBank Acc. No. AAD01982), Vitis vinifera fatty acyl-ACP thioesterase (GenBank Acc. No. CAN81819), Garcinia mangostana fatty acyl-ACP thioesterase (GenBank Acc. No. AAB51525), Brassica juncea fatty acyl-ACP thioesterase (GenBank Acc. No. ABI18986), Madhuca longifolia fatty acyl-ACP thioesterase (GenBank Acc. No. AAX51637), Brassica napus fatty acyl-ACP thioesterase (GenBank Acc. No. ABH11710), Brassica napus fatty acyl-ACP thioesterase (GenBank Acc. No. CAA52070.1), Oryza sativa (indica cultivar-group) fatty acyl-ACP thioesterase (GenBank Acc. No. EAY86877), Oryza sativa (japonica cultivar-group) fatty acyl-ACP thioesterase (GenBank Acc. No. NP_001068400), Oryza sativa (indica cultivar-group) fatty acyl-ACP thioesterase (GenBank Acc. No. EAY99617), Cuphea hookeriana fatty acyl-ACP thioesterase (GenBank Acc. No. AAC49269), Ulmus Americana fatty acyl-ACP thioesterase (GenBank Acc. No. AAB71731), Cuphea lanceolata fatty acyl-ACP thioesterase (GenBank Acc. No. CAB60830), Cuphea palustris fatty acyl-ACP thioesterase (GenBank Acc. No. AAC49180), Iris germanica fatty acyl-ACP thioesterase (GenBank Acc. No. AAG43858, Iris germanica fatty acyl-ACP thioesterase (GenBank Acc. No. AAG43858.1), Cuphea palustris fatty acyl-ACP thioesterase (GenBank Acc. No. AAC49179), Myristica fragrans fatty acyl-ACP thioesterase (GenBank Acc. No. AAB71729), Myristica fragrans fatty acyl-ACP thioesterase (GenBank Acc. No. AAB717291.1), Cuphea hookeriana fatty acyl-ACP thioesterase GenBank Acc. No. U39834), Umbelluaria californica fatty acyl-ACP thioesterase (GenBank Acc. No. M94159), Cinnamomum camphora fatty acyl-ACP thioesterase (GenBank Acc. No. U31813), Ricinus communis fatty acyl-ACP thioesterase (GenBank Acc. No. ABS30422.1), Helianthus annuus acyl-ACP thioesterase (GenBank Accession No. AAL79361.1), Jatropha curcas acyl-ACP thioesterase (GenBank Accession No. ABX82799.3), Zea mays oleoyl-acyl carrier protein thioesterase, (GenBank Accession No. ACG40089.1), Haematococcus pluvialis fatty acyl- ACP thioesterase (GenBank Accession No. HM560034.1) Desaturase Enzymes Linum usitatissimum fatty acid desaturase 3C, (GenBank Acc. No. ADV92272.1), Ricinus communis omega-3 fatty acid desaturase, endoplasmic reticulum, putative, (GenBank Acc. No. EEF36775.1), Vernicia fordii omega-3 fatty acid desaturase, (GenBank Acc. No. AAF12821), Glycine max chloroplast omega 3 fatty acid desaturase isoform 2, (GenBank Acc. No. ACF19424.1), Prototheca moriformis FAD-D omega 3 desaturase (SEQ ID NO: 35), Prototheca moriformis linoleate desaturase (SEQ ID NO: 36), Carthamus tinctorius delta 12 desaturase, (GenBank Accession No. ADM48790.1), Gossypium hirsutum omega-6 desaturase, (GenBank Accession No. CAA71199.1), Glycine max microsomal desaturase (GenBank Accession No. BAD89862.1), Zea mays fatty acid desaturase (GenBank Accession No. ABF50053.1), Brassica napa linoleic acid desaturase (GenBank Accession No. AAA32994.1), Camelina sativa omega-3 desaturase (SEQ ID NO: 37), Prototheca moriformis delta 12 desaturase allele 2 (SEQ ID NO: 38, Camelina sativa omega-3 FAD7-1 (SEQ ID NO: 39), Helianthus annuus stearoyl-ACP desaturase, (GenBank Accession No. AAB65145.1), Ricinus communis stearoyl-ACP desaturase, (GenBank Accession No. AACG59946.1), Brassica juncea plastidic delta-9-stearoyl-ACP desaturase (GenBank Accession No. AAD40245.1), Glycine max stearoyl-ACP desaturase (GenBank Accession No. ACJ39209.1), Olea europaea stearoyl-ACP desaturase (GenBank Accession No. AAB67840.1), Vernicia fordii stearoyl-acyl-carrier protein desaturase, (GenBank Accession No. ADC32803.1), Descurainia sophia delta-12 fatty acid desaturase (GenBank Accession No. ABS86964.2), Euphorbia lagascae delta12-oleic acid desaturase (GenBank Acc. No. AAS57577.1), Chlorella vulgaris delta 12 fatty acid desaturase (GenBank Accession No. ACF98528), Chlorella vulgaris omega-3 fatty acid desaturase (GenBank Accession No. BAB78717), Haematococcus pluvialis omega-3 fatty acid desaturase (GenBank Accession No. HM560035.1), Haematococcus pluvialis stearoyl-ACP-desaturase GenBank Accession No. EF586860.1, Haematococcus pluvialis stearoyl-ACP-desaturase GenBank Accession No. EF523479.1 Oleate 12-hydroxylase Enzymes Ricinus communis oleate 12-hydroxylase (GenBank Acc. No. AAC49010.1), Physaria lindheimeri oleate 12-hydroxylase (GenBank Acc. No. ABQ01458.1), Physaria lindheimeri mutant bifunctional oleate 12-hydroxylase: desaturase (GenBank Acc. No. ACF17571.1), Physaria lindheimeri bifunctional oleate 12-hydroxylase: desaturase (GenBank Accession No. ACQ42234.1), Physaria lindheimeri bifunctional oleate 12- hydroxylase: desaturase (GenBank Acc. No. AAC32755.1), Arabidopsis lyrata subsp. Lyrata (GenBank Acc. No. XP_002884883.1) Glycerol-3-phosphate Enzymes Arabidopsis thaliana glycerol-3-phosphate acyltransferase BAA00575, Chlamydomonas reinhardtii glycerol-3-phosphate acyltransferase (GenBank Acc. No. EDP02129), Chlamydomonas reinhardtii glycerol-3-phosphate acyltransferase (GenBank Acc. No. Q886Q7), Cucurbita moschata acyl-(acyl-carrier-protein): glycerol-3-phosphate acyltransferase (GenBank Acc. No. BAB39688), Elaeis guineensis glycerol-3-phosphate acyltransferase, ((GenBank Acc. No. AAF64066), Garcina mangostana glycerol-3-phosphate acyltransferase (GenBank Acc. No. ABS86942), Gossypium hirsutum glycerol-3-phosphate acyltransferase (GenBank Acc. No. ADK23938), Jatropha curcas glycerol-3-phosphate acyltransferase (GenBank Acc. No. ADV77219), Jatropha curcas plastid glycerol-3- phosphate acyltransferase (GenBank Acc. No. ACR61638), Ricinus communis plastidial glycerol-phosphate acyltransferase (GenBank Acc. No. EEF43526), Vica faba glycerol-3- phosphate acyltransferase (GenBank Accession No. AAD05164), Zea mays glycerol-3- phosphate acyltransferase (GenBank Acc. No. ACG45812) Lysophosphatidic acid acyltransferase Enzymes Arabidopsis thaliana 1-acyl-sn-glycerol-3-phosphate acyltransferase (GenBank Accession No. AEE85783), Brassica juncea 1-acyl-sn-glycerol-3-phosphate acyltransferase (GenBank Accession No. ABQ42862), Brassica juncea 1-acyl-sn-glycerol-3-phosphate acyltransferase (GenBank Accession No. ABM92334), Brassica napus 1-acyl-sn-glycerol-3-phosphate acyltransferase (GenBank Accession No. CAB09138), Chlamydomonas reinhardtii lysophosphatidic acid acyltransferase (GenBank Accession No. EDP02300), Cocos nucifera lysophosphatidic acid acyltransferase (GenBank Acc. No. AAC49119), Limnanthes alba lysophosphatidic acid acyltransferase (GenBank Accession No. EDP02300), Limnanthes douglasii 1-acyl-sn-glycerol-3-phosphate acyltransferase (putative) (GenBank Accession No. CAA88620), Limnanthes douglasii acyl-CoA: sn-1-acylglycerol-3-phosphate acyltransferase (GenBank Accession No. ABD62751), Limnanthes douglasii 1-acylglycerol-3-phosphate O- acyltransferase (GenBank Accession No. CAA58239), Ricinus communis 1-acyl-sn-glycerol- 3-phosphate acyltransferase (GenBank Accession No. EEF39377) Diacylglycerol acyltransferase Enzymes Arabidopsis thaliana diacylglycerol acyltransferase (GenBank Acc. No. CAB45373),
Brassica juncea diacylglycerol acyltransferase (GenBank Acc. No. AAY40784), Elaeis guineensis putative diacylglycerol acyltransferase (GenBank Acc. No. AEQ94187), Elaeis guineensis putative diacylglycerol acyltransferase (GenBank Acc. No. AEQ94186), Glycine max acyl CoA: diacylglycerol acyltransferase (GenBank Acc. No. AAT73629), Helianthus annus diacylglycerol acyltransferase (GenBank Acc. No. ABX61081), Olea europaea acyl- CoA: diacylglycerol acyltransferase 1 (GenBank Acc. No. AAS01606), Ricinus communis diacylglycerol acyltransferase (GenBank Acc. No. AAR11479) Phospholipid diacylglycerol acyltransferase Enzymes Arabidopsis thaliana phospholipid: diacylglycerol acyltransferase (GenBank Acc. No. AED91921), Elaeis guineensis putative phospholipid: diacylglycerol acyltransferase (GenBank Acc. No. AEQ94116), Glycine max phospholipid: diacylglycerol acyltransferase 1-like (GenBank Acc. No. XP_003541296), Jatropha curcas phospholipid: diacylglycerol acyltransferase (GenBank Acc. No. AEZ56255), Ricinus communis phospholipid: diacylglycerol acyltransferase (GenBank Acc. No. ADK92410), Ricinus communis phospholipid: diacylglycerol acyltransferase (GenBank Acc. No. AEW99982)
Example 10
Engineering Chlorella Sorokiniana
[0384] Expression of recombinant genes in accordance with the present invention in Chlorella sorokiniana can be accomplished by modifying the methods and vectors taught by Dawson et al. as discussed herein. Briefly, Dawson et al., Current Microbiology Vol. 35 (1997) pp. 356-362, reported the stable nuclear transformation of Chlorella sorokiniana with plasmid DNA. Using the transformation method of microprojectile bombardment, Dawson introduced the plasmid pSV72-NRg, encoding the full Chlorella vulgaris nitrate reductase gene (NR, GenBank Accession No. U39931), into mutant Chlorella sorokiniana (NR-mutants). The NR-mutants are incapable of growth without the use of nitrate as a source of nitrogen. Nitrate reductase catalyzes the conversion of nitrate to nitrite. Prior to transformation, Chlorella sorokiniana NR-mutants were unable to grow beyond the microcolony stage on culture medium comprising nitrate (NO.sub.3.sup.-) as the sole nitrogen source. The expression of the Chlorella vulgaris NR gene product in NR-mutant Chlorella sorokiniana was used as a selectable marker to rescue the nitrate metabolism deficiency. Upon transformation with the pSV72-NRg plasmid, NR-mutant Chlorella sorokiniana stably expressing the Chlorella vulgaris NR gene product were obtained that were able to grow beyond the microcolony stage on agar plates comprising nitrate as the sole carbon source. Evaluation of the DNA of the stable transformants was performed by Southern analysis and evaluation of the RNA of the stable transformants was performed by RNase protection. Selection and maintenance of the transformed Chlorella sorokiniana (NR mutant) was performed on agar plates (pH 7.4) comprising 0.2 g/L MgSO.sub.4, 0.67 g/L KH.sub.2PO.sub.4, 3.5 g/L K.sub.2HPO.sub.4, 1.0 g/L Na.sub.3C.sub.6H.sub.5O.sub.7.H.sub.2O and 16.0 g/L agar, an appropriate nitrogen source (e.g., NO.sub.3), micronutrients, and a carbon source. Dawson also reported the propagation of Chlorella sorokiniana and Chlorella sorokiniana NR mutants in liquid culture medium. Dawson reported that the plasmid pSV72-NRg and the promoter and 3' UTR/terminator of the Chlorella vulgaris nitrate reductase gene were suitable to enable heterologous gene expression in Chlorella sorokiniana NR-mutants. Dawson also reported that expression of the Chlorella vulgaris nitrate reductase gene product was suitable for use as a selectable marker in Chlorella sorokiniana NR-mutants.
[0385] In an embodiment of the present invention, vector pSV72-NRg, comprising nucleotide sequence encoding the Chlorella vulgaris nitrate reductase (CvNR) gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Chlorella sorokiniana to reflect the codon bias inherent in nuclear genes of Chlorella sorokiniana in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the CvNR promoter upstream of the protein-coding sequence and operably linked to the CvNR 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chlorella sorokiniana genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Chlorella sorokiniana with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the CvNR gene product can be used as a selectable marker to rescue the nitrogen assimilation deficiency of Chlorella sorokiniana NR mutant strains and to select for Chlorella sorokiniana NR-mutants stably expressing the transformation vector. Growth media suitable for Chlorella sorokiniana lipid production include, but are not limited to 0.5 g/L KH.sub.2PO.sub.4, 0.5 g/L K.sub.2HPO.sub.4, 0.25 g/L MgSO.sub.4-7H2O, with supplemental micronutrients and the appropriate nitrogen and carbon sources (Patterson, Lipids Vol. 5:7 (1970), pp. 597-600). Evaluation of fatty acid profiles of Chlorella sorokiniana lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 11
Engineering Chlorella Vulgaris
[0386] Expression of recombinant genes in accordance with the present invention in Chlorella vulgaris can be accomplished by modifying the methods and vectors taught by Chow and Tung et al. as discussed herein. Briefly, Chow and Tung et al., Plant Cell Reports, Volume 18 (1999), pp. 778-780, reported the stable nuclear transformation of Chlorella vulgaris with plasmid DNA. Using the transformation method of electroporation, Chow and Tung introduced the plasmid pIG121-Hm (GenBank Accession No. AB489142) into Chlorella vulgaris. The nucleotide sequence of pIG121-Hm comprised sequence encoding a beta-glucuronidase (GUS) reporter gene product operably-linked to a CaMV 35S promoter upstream of the GUS protein-coding sequence and further operably linked to the 3' UTR/terminator of the nopaline synthase (nos) gene downstream of the GUS protein-coding sequence. The sequence of plasmid pIG121-Hm further comprised a hygromycin B antibiotic resistance cassette. This hygromycin B antibiotic resistance cassette comprised a CaMV 35S promoter operably linked to sequence encoding the hygromycin phosphotransferase (hpt, GenBank Accession No. BAH24259) gene product. Prior to transformation, Chlorella vulgaris was unable to be propagated in culture medium comprising 50 ug/ml hygromycin B. Upon transformation with the pIG121-Hm plasmid, transformants of Chlorella vulgaris were obtained that were propagated in culture medium comprising 50 ug/ml hygromycin B. The expression of the hpt gene product in Chlorella vulgaris enabled propagation of transformed Chlorella vulgaris in the presence of 50 ug/mL hygromycin B, thereby establishing the utility of the a hygromycin B resistance cassette as a selectable marker for use in Chlorella vulgaris. Detectable activity of the GUS reporter gene indicated that CaMV 35S promoter and nos 3'UTR are suitable for enabling heterologous gene expression in Chlorella vulgaris. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Selection and maintenance of transformed Chlorella vulgaris was performed on agar plates comprising YA medium (agar and 4 g/L yeast extract). The propagation of Chlorella vulgaris in liquid culture medium was conducted as discussed by Chow and Tung. Propagation of Chlorella vulgaris in media other than YA medium has been described (for examples, see Chader et al., Revue des Energies Renouvelabes, Volume 14 (2011), pp. 21-26 and Illman et al., Enzyme and Microbial Technology, Vol. 27 (2000), pp. 631-635). Chow and Tung reported that the plasmid pIG121-Hm, the CaMV 35S promoter, and the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator are suitable to enable heterologous gene expression in Chlorella vulgaris. In addition, Chow and Tung reported the hygromycin B resistance cassette was suitable for use as a selectable marker in Chlorella vulgaris. Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Chlorella vulgaris have been discussed in Chader et al., Revue des Energies Renouvelabes, Volume 14 (2011), pp. 21-26.
[0387] In an embodiment of the present invention, pIG121-Hm, comprising the nucleotide sequence encoding the hygromycin B gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Chlorella vulgaris to reflect the codon bias inherent in nuclear genes of Chlorella vulgaris in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the CaMV 35S promoter upstream of the protein-coding sequence and operably linked to the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chlorella vulgaris genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Chlorella vulgaris with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the hygromycin B resistance gene product can be used as a marker to select for Chlorella vulgaris transformed with the transformation vector on, but not limited to, agar medium comprising hygromycin. Growth media suitable for Chlorella vulgaris lipid production include, but are not limited to BG11 medium (0.04 g/L KH.sub.2PO.sub.4, 0.075 g/L CaCl.sub.2, 0.036 g/L citric acid, 0.006 g/L Ammonium Ferric Citrate, 1 mg/L EDTA, and 0.02 g/L Na.sub.2CO.sub.3) supplemented with trace metals, and optionally 1.5 g/L NaNO3. Additional media suitable for culturing Chlorella vulgaris for lipid production include, for example, Watanabe medium (comprising 1.5 g/L KNO.sub.3, 1.25 g/L KH.sub.2PO.sub.4, 1.25 g l.sup.-1 MgSO.sub.4.7H.sub.2O, 20 mg l.sup.-1 FeSO.sub.4.7H.sub.2O with micronutrients and low-nitrogen medium (comprising 203 mg/l (NH.sub.4).sub.2HPO.sub.4, 2.236 g/l KCl, 2.465 g/l MgSO.sub.4, 1.361 g/l KH.sub.2PO.sub.4 and 10 mg/l FeSO.sub.4) as reported by Illman et al., Enzyme and Microbial Technology, Vol. 27 (2000), pp. 631-635. Evaluation of fatty acid profiles of Chlorella vulgaris lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 12
Engineering Chlorella Ellipsoidea
[0388] Expression of recombinant genes in accordance with the present invention in Chlorella ellipsoidea can be accomplished by modifying the methods and vectors taught by Chen et al. as discussed herein. Briefly, Chen et al., Current Genetics, Vol. 39:5 (2001), pp. 365-370, reported the stable transformation of Chlorella ellipsoidea with plasmid DNA. Using the transformation method of electroporation, Chen introduced the plasmid pBinU.OMEGA.NP-1 into Chlorella ellipsoidea. The nucleotide sequence of pBinU.OMEGA.NP-1 comprised sequence encoding the neutrophil peptide-1 (NP-1) rabbit gene product operably linked to a Zea mays Ubiquitin (ubi1) gene promoter upstream of the NP-1 protein-coding region and operably linked to the 3' UTR/terminator of the nopaline synthase (nos) gene downstream of the NP-1 protein-coding region. The sequence of plasmid pBinU.OMEGA.NP-1 further comprised a G418 antibiotic resistance cassette. This G418 antibiotic resistance cassette comprised sequence encoding the aminoglycoside 3'-phosphotransferase (aph 3') gene product. The aph 3' gene product confers resistance to the antibiotic G418. Prior to transformation, Chlorella ellipsoidea was unable to be propagated in culture medium comprising 30 ug/mL G418. Upon transformation with the pBinU.OMEGA.NP-1 plasmid, transformants of Chlorella ellipsoidea were obtained that were propagated in selective culture medium comprising 30 ug/mL G418. The expression of the aph 3' gene product in Chlorella ellipsoidea enabled propagation of transformed Chlorella ellipsoidea in the presence of 30 ug/mL G418, thereby establishing the utility of the G418 antibiotic resistance cassette as selectable marker for use in Chlorella ellipsoidea. Detectable activity of the NP-1 gene product indicated that the ubi 1 promoter and nos 3' UTR are suitable for enabling heterologous gene expression in Chlorella ellipsoidea. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Selection and maintenance of the transformed Chlorella ellipsoidea was performed on Knop medium (comprising 0.2 g/L K.sub.2HPO.sub.4, 0.2 g/L MgSO.sub.4.7H.sub.2O, 0.12 g/L KCl, and 10 mg/L FeCl3, pH 6.0-8.0 supplemented with 0.1% yeast extract and 0.2% glucose) with 15 ug/mL G418 (for liquid cultures) or with 30 ug/mL G418 (for solid cultures comprising 1.8% agar). Propagation of Chlorella ellipsoidea in media other than Knop medium has been reported (see Cho et al., Fisheries Science, Vol. 73:5 (2007), pp. 1050-1056, Jarvis and Brown, Current Genetics, Vol. 19 (1991), pp. 317-321 and Kim et al., Marine Biotechnology, Vol. 4 (2002), pp. 63-'73). Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Chlorella ellipsoidea have been reported (see Jarvis and Brown and Kim et al., Marine Biotechnology, Vol. 4 (2002), pp. 63-73). Chen reported that the plasmid pBinU.OMEGA.NP-1, the ubil promoter, and the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator are suitable to enable exogenous gene expression in Chlorella ellipsoidea. In addition, Chen reported that the G418 resistance cassette encoded on pBinU.OMEGA.NP-1 was suitable for use as a selectable marker in Chlorella ellipsoidea.
[0389] In an embodiment of the present invention, vector pBinU.OMEGA.NP-1, comprising the nucleotide sequence encoding the aph 3' gene product, conferring resistance to G418, for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Chlorella ellipsoidea to reflect the codon bias inherent in nuclear genes of Chlorella ellipsoidea in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Zea mays ubil promoter upstream of the protein-coding sequence and operably linked to the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chlorella ellipsoidea genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Chlorella ellipsoidea with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the aph 3' gene product can be used as a marker to select for Chlorella ellipsoidea transformed with the transformation vector on, but not limited to, Knop agar medium comprising G418. Growth media suitable for Chlorella ellipsoidea lipid production include, but are not limited to, Knop medium and those culture medium reported by Jarvis and Brown and Kim et al. Evaluation of fatty acid profiles of Chlorella ellipsoidea lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 13
Engineering Chlorella Kessleri
[0390] Expression of recombinant genes in accordance with the present invention in Chlorella kessleri can be accomplished by modifying the methods and vectors taught by El-Sheekh et al. as discussed herein. Briefly, El-Sheekh et al., Biologia Plantarium, Vol. 42:2 (1999), pp. 209-216, reported the stable transformation of Chlorella kessleri with plasmid DNA. Using the transformation method of microprojectile bombardment, El-Sheekh introduced the plasmid pBI121 (GenBank Accession No. AF485783) into Chlorella kessleri. Plasmid pBI121 comprised a kanamycin/neomycin antibiotic resistance cassette. This kanamycin/neomycin antibiotic resistance cassette comprised the Agrobacterium tumefaciens nopaline synthase (nos) gene promoter, sequence encoding the neomycin phosphotransferase II (nptII) gene product (GenBank Accession No. AAL92039) for resistance to kanamycin and G418, and the 3' UTR/terminator of the Agrobacterium tumefaciens nopaline synthase (nos) gene. pBI121 further comprised sequence encoding a beta-glucuronidase (GUS) reporter gene product operably linked to a CaMV 35S promoter and operably linked to a 3' UTR/terminator of the nos gene. Prior to transformation, Chlorella kessleri was unable to be propagated in culture medium comprising 15 ug/L kanamycin. Upon transformation with the pBI121plasmid, transformants of Chlorella kessleri were obtained that were propagated in selective culture medium comprising 15 mg/L kanamycin. The express ion of the nptII gene product in Chlorella kessleri enabled propagation in the presence of 15 mg/L kanamycin, thereby establishing the utility of the kanamycin/neomycin antibiotic resistance cassette as selectable marker for use in Chlorella kessleri. Detectable activity of the GUS gene product indicated that the CaMV 35S promoter and nos 3' UTR are suitable for enabling heterologous gene expression in Chlorella kessleri. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. As reported by El-Sheekh, selection and maintenance of transformed Chlorella kessleri was conducted on semisolid agar plates comprising YEG medium (1% yeast extract, 1% glucose) and 15 mg/L kanamycin. El-Sheekh also reported the propagation of Chlorella kessleri in YEG liquid culture media. Additional media suitable for culturing Chlorella kessleri for lipid production are disclosed in Sato et al., BBA Molecular and Cell Biology of Lipids, Vol. 1633 (2003), pp. 27-34). El-Sheekh reported that the plasmid pBI121, the CaMV promoter, and the nopaline synthase gene 3'UTR/terminator are suitable to enable heterologous gene expression in Chlorella kessleri. In addition, El-Sheekh reported that the kanamycin/neomycin resistance cassette encoded on pBI121 was suitable for use as a selectable marker in Chlorella kessleri.
[0391] In an embodiment of the present invention, vector pBI121, comprising the nucleotide sequence encoding the kanamycin/neomycin resistance gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Chlorella kessleri to reflect the codon bias inherent in nuclear genes of Chlorella kessleri in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the CaMV 35S promoter upstream of the protein-coding sequence and operably linked to the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chlorella kessleri genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Chlorella kessleri with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the nptII gene product can be used as a marker to select for Chlorella kessleri transformed with the transformation vector on, but not limited to, YEG agar medium comprising kanamycin or neomycin. Growth media suitable for Chlorella kessleri lipid production include, but are not limited to, YEG medium, and those culture media reported by Sato et al. Evaluation of fatty acid profiles of Chlorella kessleri lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 14
Engineering Dunaliella Tertiolecta
[0392] Expression of recombinant genes in accordance with the present invention in Dunaliella tertiolecta can be accomplished by modifying the methods and vectors taught by Walker et al. as discussed herein. Briefly, Walker et al., Journal of Applied Phycology, Vol. 17 (2005), pp. 363-368, reported stable nuclear transformation of Dunaliella tertiolecta with plasmid DNA. Using the transformation method of electroporation, Walker introduced the plasmid pDbleFLAG1.2 into Dunaliella tertiolecta. pDbleFLAG1.2 comprised sequence encoding a bleomycin antibiotic resistance cassette, comprising sequence encoding the Streptoalloteichus hindustanus Bleomycin binding protein (ble), for resistance to the antibiotic phleomycin, operably linked to the promoter and 3' UTR of the Dunaliella tertiolecta ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene (rbcS1, GenBank Accession No. AY530155). Prior to transformation, Dunaliella tertiolecta was unable to be propagated in culture medium comprising 1 mg/L phleomycin. Upon transformation with the pDbleFLAG1.2 plasmid, transformants of Dunaliella tertiolecta were obtained that were propagated in selective culture medium comprising 1 mg/L phleomycin. The expression of the ble gene product in Dunaliella tertiolecta enabled propagation in the presence of 1 mg/L phleomycin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Dunaliella tertiolecta. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. As reported by Walker, selection and maintenance of transformed Dunaliella tertiolecta was conducted in Dunaliella medium (DM, as described by Provasoli et al., Archiv fur Mikrobiologie, Vol. 25 (1957), pp. 392-428) further comprising 4.5 g/L NaCl and 1 mg/L pheomycin. Additional media suitable for culturing Dunaliella tertiolecta for lipid production are discussed in Takagi et al., Journal of Bioscience and Bioengineering, Vol. 101:3 (2006), pp. 223-226 and in Massart and Hanston, Proceedings Venice 2010, Third International Symposium on Energy from Biomass and Waste. Walker reported that the plasmid pDbleFLAG1.2 and the promoter and 3' UTR of the Dunaliella tertiolecta ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene are suitable to enable heterologous expression in Dunaliella tertiolecta. In addition, Walker reported that the bleomycin resistance cassette encoded on pDbleFLAG1.2 was suitable for use as a selectable marker in Dunaliella tertiolecta.
[0393] In an embodiment of the present invention, vector pDbleFLAG1.2, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Dunaliella tertiolecta to reflect the codon bias inherent in nuclear genes of Dunaliella tertiolecta in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the rbcS1 promoter upstream of the protein-coding sequence and operably linked to the rbcS1 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Dunaliella tertiolecta genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Dunaliella tertiolecta with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the ble gene product can be used as a marker to select for Dunaliella tertiolecta transformed with the transformation vector on, but not limited to, DM medium comprising pheomycin. Growth medium suitable for Dunaliella tertiolecta lipid production include, but are not limited to DM medium and those culture media described by Takagi et al. and Massart and Hanston. Evaluation of fatty acid profiles of Dunaliella tertiolecta lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 15
Engineering Volvox Carteri
[0394] Expression of recombinant genes in accordance with the present invention in Volvox carteri can be accomplished by modifying the methods and vectors taught by Hallman and Rappel et al. as discussed herein. Briefly, Hallman and Rappel et al., The Plant Journal, Volume 17 (1999), pp. 99-109, reported the stable nuclear transformation of Volvox carteri with plasmid DNA. Using the transformation method of microprojectile bombardment, Hallman and Rappel introduced the pzeoE plasmid into Volvox carteri. The pzeoE plasmid comprised sequence encoding a bleomycin antibiotic resistance cassette, comprising sequence encoding the Streptoalloteichus hindustanus Bleomycin binding protein (ble), for resistance to the antibiotic zeocin, operably linked to and the promoter and 3' UTR of the Volvox carteri beta-tubulin gene (GenBank Accession No. L24547). Prior to transformation, Volvox carteri was unable to be propagated in culture medium comprising 1.5 ug/ml zeocin. Upon transformation with the pzeoE plasmid, transformants of Volvox carteri were obtained that were propagated in selective culture medium comprising greater than 20 ug/ml zeocin. The expression of the ble gene product in Volvox carteri enabled propagation in the presence of 20 ug/ml zeocin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Volvox carteri. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. As reported by Hallman and Rappel, selection and maintenance of transformed Volvox carteri was conducted in Volvox medium (VM, as described by Provasoli and Pintner, The Ecology of Algae, Special Publication No. 2 (1959), Tyron, C. A. and Hartman, R. T., eds., Pittsburgh: University of Pittsburgh, pp. 88-96) with 1 mg/L pheomycin. Media suitable for culturing Volvox carteri for lipid production are also discussed by Starr in Starr R, C., Dev Biol Suppl., Vol. 4 (1970), pp. 59-100). Hallman and Rappel reported that the plasmid pzeoE and the promoter and 3' UTR of the Volvox carteri beta-tubulin gene are suitable to enable heterologous expression in Volvox carteri. In addition, Hallman and Rappel reported that the bleomycin resistance cassette encoded on pzeoE was suitable for use as a selectable marker in Volvox carteri. Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Volvox carteri and suitable for use as selective markers Volvox carteri in have been reported (for instance see Hallamann and Sumper, Proceedings of the National Academy of Sciences, Vol. 91 (1994), pp 11562-11566 and Hallman and Wodniok, Plant Cell Reports, Volume 25 (2006), pp. 582-581).
[0395] In an embodiment of the present invention, vector pzeoE, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 19, each protein-coding sequence codon-optimized for expression in Volvox carteri to reflect the codon bias inherent in nuclear genes of Volvox carteri in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Volvox carteri beta-tubulin promoter upstream of the protein-coding sequence and operably linked to the Volvox carteri beta-tubulin 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Volvox carteri genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Volvox carteri genome (referenced in the publication by Prochnik et al., Science, Vol. 329:5988 (2010), pp223-226). Stable transformation of Volvox carteri with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the ble gene product can be used as a marker to select for Volvox carteri transformed with the transformation vector on, but not limited to, VM medium comprising zeocin. Growth medium suitable for Volvox carteri lipid production include, but are not limited to VM medium and those culture media discussed by Starr. Evaluation of fatty acid profiles of Volvox carteri lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 16
Engineering Haematococcus Pluvialis
[0396] Expression of recombinant genes in accordance with the present invention in Haematococcus pluvialis can be accomplished by modifying the methods and vectors taught by Steinbrenner and Sandmann et al. as discussed herein. Briefly, Steinbrenner and Sandmann et al., Applied and Environmental Microbiology, Vol. 72:12 (2006), pp. 7477-7484, reported the stable nuclear transformation of Haematococcus pluvialis with plasmid DNA. Using the transformation method of microprojectile bombardment, Steinbrenner introduced the plasmid pPlat-pds-L504R into Haematococcus pluvialis. The plasmid pPlat-pds-L504R comprised a norflurazon resistance cassette, which comprised the promoter, protein-coding sequence, and 3'UTR of the Haematococcus pluvialis phytoene desaturase gene (Pds, GenBank Accession No. AY781170), wherein the protein-coding sequence of Pds was modified at position 504 (thereby changing a leucine to an arginine) to encode a gene product (Pds-L504R) that confers resistance to the herbicide norflurazon. Prior to transformation with pPlat-pds-L504R, Haematococcus pluvialis was unable to propagate on medium comprising 5 uM norflurazon. Upon transformation with the pPlat-pds-L504R plasmid, transformants of Haematococcus pluvialis were obtained that were propagated in selective culture medium comprising 5 uM norflurazon. The expression of the Pds-L504R gene product in Haematococcus pluvialis enabled propagation in the presence of 5 uM norflurazon, thereby establishing the utility of the norflurazon herbicide resistance cassette as selectable marker for use in Haematococcus pluvialis. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. As reported by Steinbrenner, selection and maintenance of transformed Haematococcus pluvialis was conducted on agar plates comprising OHA medium (OHM (0.41 g/L KNO.sub.3, 0.03 g/L Na.sub.2HPO.sub.4, 0.246 g/L MgSO.sub.4.7H.sub.2O, 0.11 g/L CaCl.sub.2.2H.sub.2O, 2.62 mg/L Fe.sub.(III)citrate.times.H.sub.2O, 0.011 mg/L CoCl.sub.2.6H.sub.2O, 0.012 mg/L CuSO.sub.4.5H.sub.2O, 0.075 mg/L Cr.sub.2O.sub.3, 0.98 mg/L MnCl.sub.2.4H.sub.2O, 0.12 mg/L Na.sub.2MoO.sub.4.times.2H.sub.2O, 0.005 mg/L SeO.sub.2 and 25 mg/L biotin, 17.5 mg/L thiamine, and 15 mg/L vitamin B12), supplemented with 2.42 g/L Tris-acetate, and 5 mM norflurazon. Propagation of Haematococcus pluvialis in liquid culture was performed by Steinbrenner and Sandmann using basal medium (basal medium as described by Kobayashi et al., Applied and Environmental Microbiology, Vol. 59 (1993), pp. 867-873). Steinbrenner and Sandmann reported that the pPlat-pds-L504R plasmid and promoter and 3' UTR of the Haematococcus pluvialis phytoene desaturase gene are suitable to enable heterologous expression in Haematococcus pluvialis. In addition, Steinbrenner and Sandmann reported that the norflurazon resistance cassette encoded on pPlat-pds-L504R was suitable for use as a selectable marker in Haematococcus pluvialis. Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Haematococcus pluvialis have been reported (see Kathiresan et al., Journal of Phycology, Vol. 45 (2009), pp 642-649).
[0397] In an embodiment of the present invention, vector pPlat-pds-L504R, comprising the nucleotide sequence encoding the Pds-L504R gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Haematococcus pluvialis to reflect the codon bias inherent in nuclear genes of Haematococcus pluvialis in accordance with Tables 19 A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Haematococcus pluvialis pds gene promoter upstream of the protein-coding sequence and operably linked to the Haematococcus pluvialis pds gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Haematococcus pluvialis genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Haematococcus pluvialis with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the Pds-L504R gene product can be used as a marker to select for Haematococcus pluvialis transformed with the transformation vector on, but not limited to, OHA medium comprising norflurazon. Growth media suitable for Haematococcus pluvialis lipid production include, but are not limited to basal medium and those culture media described by Kobayashi et al., Kathiresan et al, and Gong and Chen, Journal of Applied Phycology, Vol. 9:5 (1997), pp. 437-444). Evaluation of fatty acid profiles of Haematococcus pluvialis lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 17
Engineering Closterium Peracerosum-Strigosum-Littorale Complex
[0398] Expression of recombinant genes in accordance with the present invention in Closterium peracerosum-strigosum-littorals complex can be accomplished by modifying the methods and vectors taught by Abe et al. as discussed herein. Briefly, Abe et al., Plant Cell Physiology, Vol. 52:9 (2011), pp. 1676-1685, reported the stable nuclear transformation of Closterium peracerosum-strigosum-littorals complex with plasmid DNA. Using the transformation methods of microprojectile bombardment, Abe introduced the plasmid pSA106 into Closterium peracerosum-strigosum-littorals complex. Plasmid pSA106 comprised a bleomycin resistance cassette, comprising sequence encoding the Streptoalloteichus hindustanus Bleomycin binding protein gene (ble, GenBank Accession No. CAA37050) operably linked to the promoter and 3' UTR of the Closterium peracerosum-strigosum-littorals complex Chlorophyll a/b-binding protein gene (CAB, GenBank Accession No. AB363403). Prior to transformation with pSA106, Closterium peracerosum-strigosum-littorals complex was unable to propagate on medium comprising 3 ug/ml phleomycin. Upon transformation with pSA106, transformants of Closterium peracerosum-strigosum-littorals complex were obtained that were propagated in selective culture medium comprising 3 ug/ml phleomycin. The expression of the ble gene product in Closterium peracerosum-strigosum-littorals complex enabled propagation in the presence of 3 ug/ml phleomycin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Closterium peracerosum-strigosum-littorals complex. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. As reported by Abe, selection and maintenance of transformed Closterium peracerosum-strigosum-littorals complex was conducted first in top agar with C medium (0.1 g/L KNO.sub.3, 0.015 g/L Ca(NO.sub.3).sub.2.4H2O, 0.05 g/L glycerophosphate-Na2, 0.04 g/L MgSO.sub.4.7H.sub.2O, 0.5 g/L Tris (hydroxylmethyl) aminomethane, trace minerals, biotin, vitamins B.sub.1 and B.sub.12) and then subsequently isolated to agar plates comprising C medium supplemented with phleomycin. As reported by Abe, propagation of Closterium peracerosum-strigosum-littorals complex in liquid culture was performed in C medium. Additional liquid culture medium suitable for propagation of Closterium peracerosum-strigosum-littorale complex are discussed by Sekimoto et al., DNA Research, 10:4 (2003), pp. 147-153. Abe reported that the pSA106 plasmid and promoter and 3' UTR of the Closterium peracerosum-strigosum-littorals complex CAB gene are suitable to enable heterologous gene expression in Closterium peracerosum-strigosum-littorals complex. In addition, Abe reported that the bleomycin resistance cassette encoded on pSA106 was suitable for use as a selectable marker in Closterium peracerosum-strigosum-littorals complex. Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Closterium peracerosum-strigosum-littorale complex have been reported (see Abe et al., Plant Cell Physiology, Vol. 49 (2008), pp. 625-632).
[0399] In an embodiment of the present invention, vector pSA106, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Closterium peracerosum-strigosum-littorale complex to reflect the codon bias inherent in nuclear genes of Closterium peracerosum-strigosum-littorale complex in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Closterium peracerosum-strigosum-littorale complex CAB gene promoter upstream of the protein-coding sequence and operably linked to the Closterium peracerosum-strigosum-littorale complex CAB gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Closterium peracerosum-strigosum-littorale complex genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Closterium peracerosum-strigosum-littorale complex with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the ble gene product can be used as a marker to select for Closterium peracerosum-strigosum-littorale complex transformed with the transformation vector on, but not limited to, C medium comprising phleomycin. Growth media suitable for Closterium peracerosum-strigosum-littorale complex lipid production include, but are not limited to C medium and those culture media reported by Abe et al. and Sekimoto et al. Evaluation of fatty acid profiles of Closterium peracerosum-strigosum-littorale complex lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 18
Engineering Dunaliella Viridis
[0400] Expression of recombinant genes in accordance with the present invention in Dunaliella viridis can be accomplished by modifying the methods and vectors taught by Sun et al. as discussed herein. Briefly, Sun et al., Gene, Vol. 377 (2006), pp. 140-149, reported the stable transformation of Dunaliella viridis with plasmid DNA. Using the transformation method of electroporation, Sun introduced the plasmid pDVNR, encoding the full Dunaliella viridis nitrate reductase gene into mutant Dunaliella viridis (Dunaliella viridis NR-mutants.) The NR-mutants are incapable of growth without the use of nitrate as a source of nitrogen. Nitrate reductase catalyzes the conversion of nitrate to nitrite. Prior to transformation, Dunaliella viridis NR-mutants were unable to propagate in culture medium comprising nitrate (NO.sub.3.sup.-1 ) as the sole nitrogen source. The expression of the Dunaliella viridis NR gene product in NR-mutant Dunaliella viridis was used as a selectable marker to rescue the nitrate metabolism deficiency. Upon transformation with the pDVNR plasmid, NR-mutant Dunaliella viridis stably expressing the Dunaliella viridis NR gene product were obtained that were able to grow on agar plates comprising nitrate as the sole carbon source. Evaluation of the DNA of the stable transformants was performed by Southern analysis. Selection and maintenance of the transformed Dunaliella viridis (NR mutant) was performed on agar plates comprising 5 mM KNO.sub.3. Sun also reported the propagation of Dunaliella viridis and Dunaliella viridis NR mutants in liquid culture medium. Additional media suitable for propagation of Dunaliella viridis are reported by Gordillo et al., Journal of Applied Phycology, Vol. 10:2 (1998), pp. 135-144 and by Moulton and Burford, Hydrobiologia, Vols. 204-205:1 (1990), pp. 401-408. Sun reported that the plasmid pDVNR and the promoter and 3' UTR/terminator of the Dunaliella viridis nitrate reductase gene were suitable to enable heterologous expression in Dunaliella viridis NR-mutants. Sun also reported that expression of the Dunaliella viridis nitrate reductase gene product was suitable for use as a selectable marker in Dunaliella viridis NR-mutants.
[0401] In an embodiment of the present invention, vector pDVNR, comprising the nucleotide sequence encoding the Dunaliella viridis nitrate reductase (DvNR) gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Dunaliella viridis to reflect the codon bias inherent in nuclear genes of Dunaliella viridis in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the DvNR promoter upstream of the protein-coding sequence and operably linked to the DvNR 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Dunaliella viridis genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Dunaliella viridis NR mutants with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the DvNR gene product can be used as a selectable marker to rescue the nitrogen assimilation deficiency of Dunaliella viridis NR mutant strains and to select for Dunaliella viridis NR-mutants stably expressing the transformation vector. Growth media suitable for Dunaliella viridis lipid production include, but are not limited to those discussed by Sun et al., Moulton and Burford, and Gordillo et al. Evaluation of fatty acid profiles of Dunaliella viridis lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 19
Engineering Dunaliella Salina
[0402] Expression of recombinant genes in accordance with the present invention in Dunaliella salina can be accomplished by modifying the methods and vectors taught by Geng et al. as discussed herein. Briefly, Geng et al., Journal of Applied Phycology, Vol. 15 (2003), pp. 451-456, reported the stable transformation of Dunaliella salina with plasmid DNA. Using the transformation method of electroporation, Geng introduced the pU.OMEGA.HBsAg-CAT plasmid into Dunaliella salina. pU.OMEGA.HBsAg-CAT comprises a hepatitis B surface antigen (HBsAG) expression cassette comprising sequence encoding the hepatitis B surface antigen operably linked to a Zea mays ubil promoter upstream of the HBsAG protein-coding region and operably linked to the 3'UTR/terminator of the Agrobacterium tumefaciens nopaline synthase gene (nos) downstream of the HBsAG protein-coding region. pU.OMEGA.HBsAg-CAT further comprised a chloramphenicol resistance cassette, comprising sequence encoding the chloramphenicol acetyltransferase (CAT) gene product, conferring resistance to the antibiotic chloramphenicol, operably linked to the simian virus 40 promoter and enhancer. Prior to transformation with pU.OMEGA.HBsAg-CAT, Dunaliella salina was unable to propagate on medium comprising 60 mg/L chloramphenicol. Upon transformation with the pU.OMEGA.HBsAg-CAT plasmid, transformants of Dunaliella salina were obtained that were propagated in selective culture medium comprising 60 mg/L chloramphenicol. The expression of the CAT gene product in Dunaliella salina enabled propagation in the presence of 60 mg/L chloramphenicol, thereby establishing the utility of the chloramphenicol resistance cassette as selectable marker for use in Dunaliella salina. Detectable activity of the HBsAg gene product indicated that ubil promoter and nos 3'UTR/terminator are suitable for enabling gene expression in Dunaliella salina. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Geng reported that selection and maintenance of the transformed Dunaliella salina was performed on agar plates comprising Johnson's medium (J1, described by Borowitzka and Borowitzka (eds), Micro-algal Biotechnology. Cambridge University Press, Cambridge, pp. 460-461) with 60 mg/L chloramphenicol. Liquid propagation of Dunaliella salina was performed by Geng in J1 medium with 60 mg/L chloramphenicol. Propagation of Dunaliella salina in media other than J1 medium has been discussed (see Feng et al., Mol. Bio. Reports, Vol. 36 (2009), pp. 1433-1439 and Borowitzka et al., Hydrobiologia, Vols. 116-117:1 (1984), pp. 115-121). Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Dunaliella salina have been reported by Feng et al. Geng reported that the plasmid pU.OMEGA.HBsAg-CAT, the ubil promoter, and the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator are suitable to enable exogenous gene expression in Dunaliella salina. In addition, Geng reported that the CAT resistance cassette encoded on pU.OMEGA.HBsAg-CAT was suitable for use as a selectable marker in Dunaliella salina.
[0403] In an embodiment of the present invention, vector pU.OMEGA.HBsAg-CAT, comprising the nucleotide sequence encoding the CAT gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Dunaliella salina to reflect the codon bias inherent in nuclear genes of Dunaliella salina in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the ubil promoter upstream of the protein-coding sequence and operably linked to the Agrobacterium tumefaciens nopaline synthase gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Dunaliella salina genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Dunaliella salina with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the CAT gene product can be used as a selectable marker to select for Dunaliella salina transformed with the transformation vector in, but not limited to, J1 medium comprising chloramphenicol. Growth medium suitable for Dunaliella salina lipid production include, but are not limited to J1 medium and those culture media described by Feng et al. and Borowitzka et al. Evaluation of fatty acid profiles of Dunaliella salina lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 20
Engineering Gonium Pectoral
[0404] Expression of recombinant genes in accordance with the present invention in Gonium pectoral can be accomplished by modifying the methods and vectors taught by Lerche and Hallman et al. as discussed herein. Briefly, Lerche and Hallman et al., BMC Biotechnology, Volume 9:64, 2009, reported the stable nuclear transformation of Gonium pectorale with plasmid DNA. Using the transformation method of microprojectile bombardment, Lerche introduced the plasmid pPmr3 into Gonium pectorale. Plasmid pPmr3 comprised a paromomycin resistance cassette, comprising a sequence encoding the aminoglycoside 3'-phosphotransferase (aphVIII) gene product (GenBank Accession No. AAB03856) of Streptomyces rimosus for resistance to the antibiotic paromomycin, operably linked to the Volvox carteri hsp70A-rbcS3 hybrid promoter upstream of the aphVIII protein-coding region and operably linked to the 3' UTR/terminator of the Volvox carteri rbcS3 gene downstream of the aphVIII protein-coding region. Prior to transformation with pPmr3, Gonium pectorale was unable to propagate on medium comprising 0.06 ug/ml paromomycin. Upon transformation with pPmr3, transformants of Gonium pectorale were obtained that were propagated in selective culture medium comprising 0.75 and greater ug/ml paromomycin. The expression of the aphVIII gene product in Gonium pectorale enabled propagation in the presence of 0.75 and greater ug/ml paromomycin, thereby establishing the utility of the paromomycin antibiotic resistance cassette as selectable marker for use in Gonium pectorals. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Lerche and Hallman reported that selection and maintenance of the transformed Gonium pectorale was performed in liquid Jaworski's medium (20 mg/L Ca(NO.sub.3).sub.2.4H.sub.2O, 12.4 mg/L KH.sub.2PO.sub.4, 50 mg/L MgSO.sub.4.7H.sub.2O, 15.9 mg/L NaHCO.sub.3, 2.25 mg/L EDTA-FeNa, 2.25 mg/L EDTA Na.sub.2, 2.48 g/L H.sub.3BO.sub.3, 1.39 g/L MnCl.sub.2.4H.sub.2O, 1 mg/L (NH.sub.4).sub.6MO.sub.7O.sub.24.4H.sub.2O, 0.04 mg/L vitamin B12, 0.04 mg/L Thiamine-HCl, 0.04 mg/L biotin, 80 mg/L NaNO.sub.3, 36 mg/L Na.sub.4HPO.sub.4.12H.sub.2O) with 1.0 ug/ml paromomycin. Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Gonium pectorale are further discussed by Lerche and Hallman. Lerche and Hallman reported that the plasmid pPmr3, Volvox carteri hsp70A-rbcS3 hybrid promoter, and the 3' UTR/terminator of the Volvox carteri rbcS3 gene are suitable to enable exogenous gene expression in Gonium pectorals. In addition, Lerche and Hallman reported that the paromomycin resistance cassette encoded pPmr3 was suitable for use as a selectable marker in Gonium pectorale
[0405] In an embodiment of the present invention, vector pPmr3, comprising the nucleotide sequence encoding the aphVIII gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Gonium pectorale to reflect the codon bias inherent in nuclear genes of Gonium pectorale in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Volvox carteri hsp70A-rbcS3 hybrid promoter upstream of the protein-coding sequence and operably linked to the Volvox carteri rbcS3 gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Gonium pectorale genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Gonium pectorale with the transformation vector can be achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the aphVIII gene product can be used as a selectable marker to select for Gonium pectorale transformed with the transformation vector in, but not limited to, Jaworski's medium comprising paromomycin. Growth media suitable for Gonium pectorale lipid production include Jaworski's medium and media reported by Stein, American Journal of Botany, Vol. 45:9 (1958), pp. 664-672. Evaluation of fatty acid profiles of Gonium pectorale lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 21
Engineering Phaeodactylum Tricornutum
[0406] Expression of recombinant genes in accordance with the present invention in Phaeodactylum tricornutum can be accomplished by modifying the methods and vectors taught by Apt et al. as discussed herein. Briefly, Apt et al., Molecular and General Genetics, Vol. 252 (1996), pp. 572-579, reported the stable nuclear transformation of Phaeodactylum tricornutum with vector DNA. Using the transformation technique of microprojectile bombardment, Apt introduced the plasmid pfcpA into Phaeodactylum tricornutum. Plasmid pfcpA comprised a bleomycin resistance cassette, comprising sequence encoding the Streptoalloteichus hindustanus Bleomycin binding protein (ble), for resistance to the antibiotics phleomycin and zeocin, operably linked to the promoter of the Phaeodactylum tricornutum fucoxanthin chlorophyll a binding protein gene (fcpA) upstream of the ble protein-coding region and operably linked to the 3' UTR/terminator of the Phaeodactylum tricornutum fcpA gene at the 3' region, or downstream of the ble protein-coding region. Prior to transformation with pfcpA, Phaeodactylum tricornutum was unable to propagate on medium comprising 50 ug/ml zeocin. Upon transformation with pfcpA, transformants of Phaeodactylum tricornutum were obtained that were propagated in selective culture medium comprising 50 ug/ml zeocin. The expression of the ble gene product in Phaeodactylum tricornutum enabled propagation in the presence of 50 ug/ml zeocin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Phaeodactylum tricornutum. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Apt reported that selection and maintenance of the transformed Phaeodactylum tricornutum was performed on agar plates comprising LDM medium (as reported by Starr and Zeikus, Journal of Phycology, Vol. 29, Supplement, (1993)) with 50 mg/L zeocin. Apt reported liquid propagation of Phaeodactylum tricornutum transformants in LDM medium with 50 mg/L zeocin. Propagation of Phaeodactylum tricornutum in medium other than LDM medium has been discussed (by Zaslayskaia et al., Science, Vol. 292 (2001), pp. 2073-2075, and by Radokovits et al., Metabolic Engineering, Vol. 13 (2011), pp. 89-95). Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Phaeodactylum tricornutum have been reported in the same report by Apt et al., by Zaslayskaia et al., and by Radokovits et al.). Apt reported that the plasmid pfcpA, and the Phaeodactylum tricornutum fcpA promoter and 3' UTR/terminator are suitable to enable exogenous gene expression in Phaeodactylum tricornutum. In addition, Apt reported that the bleomycin resistance cassette encoded on pfcpA was suitable for use as a selectable marker in Phaeodactylum tricornutum.
[0407] In an embodiment of the present invention, vector pfcpA, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Phaeodactylum tricornutum to reflect the codon bias inherent in nuclear genes of Phaeodactylum tricornutum in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Phaeodactylum tricornutum fcpA gene promoter upstream of the protein-coding sequence and operably linked to the Phaeodactylum tricornutum fcpA gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Phaeodactylum tricornutum genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Phaeodactylum tricornutum genome (referenced in the publication by Bowler et al., Nature, Vol. 456 (2008), pp. 239-244). Stable transformation of Phaeodactylum tricornutum with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the ble gene product can be used as a marker to select for Phaeodactylum tricornutum transformed with the transformation vector in, but not limited to, LDM medium comprising paromomycin. Growth medium suitable for Phaeodactylum tricornutum lipid production include, but are not limited to f/2 medium as reported by Radokovits et al. Evaluation of fatty acid profiles of Phaeodactylum tricornutum lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 22
Engineering Chaetoceros sp.
[0408] Expression of recombinant genes in accordance with the present invention in Chaetoceros sp. can be accomplished by modifying the methods and vectors taught by Yamaguchi et al. as discussed herein. Briefly, Yamaguchi et al., Phsycological Research, Vol. 59:2 (2011), pp. 113-119, reported the stable nuclear transformation of Chaetoceros sp. with plasmid DNA. Using the transformation method of microprojectile bombardment, Yamaguchi introduced the plasmid pTpfcp/nat into Chaetoceros sp. pTpfcp/nat comprised a nourseothricin resistance cassette, comprising sequence encoding the nourseothricin acetyltransferase (nat) gene product (GenBank Accession No. AAC60439) operably linked to the Thalassiosira pseudonana fucoxanthin chlorophyll a/c binding protein gene (fcp) promoter upstream of the nat protein-coding region and operably linked to the Thalassiosira pseudonana fcp gene 3' UTR/terminator at the 3' region (downstream of the nat protein coding-sequence). The nat gene product confers resistance to the antibiotic nourseothricin. Prior to transformation with pTpfcp/nat, Chaetoceros sp. was unable to propagate on medium comprising 500 ug/ml nourseothricin. Upon transformation with pTpfcp/nat, transformants of Chaetoceros sp. were obtained that were propagated in selective culture medium comprising 500 ug/ml nourseothricin. The expression of the nat gene product in Chaetoceros sp. enabled propagation in the presence of 500 ug/ml nourseothricin, thereby establishing the utility of the nourseothricin antibiotic resistance cassette as selectable marker for use in Chaetoceros sp. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Yamaguchi reported that selection and maintenance of the transformed Chaetoceros sp. was performed on agar plates comprising f/2 medium (as reported by Guilard, R. R., Culture of Phytoplankton for feeding marine invertebrates, In Culture of Marine Invertebrate Animals, Smith and Chanley (eds) 1975, Plenum Press, New York, pp. 26-60) with 500 ug/ml nourseothricin. Liquid propagation of Chaetoceros sp. transformants, as performed by Yamaguchi, was carried out in f/2 medium with 500 mg/L nourseothricin. Propagation of Chaetoceros sp. in additional culture medium has been reported (for example in Napolitano et al., Journal of the World Aquaculture Society, Vol. 21:2 (1990), pp. 122-130, and by Volkman et al., Journal of Experimental Marine Biology and Ecology, Vol. 128:3 (1989), pp. 219-240). Additional plasmids, promoters, 3'UTR/terminators, and selectable markers suitable for enabling heterologous gene expression in Chaetoceros sp. have been reported in the same report by Yamaguchi et al. Yamaguchi reported that the plasmid pTpfcp/nat, and the Thalassiosira pseudonana fcp promoter and 3' UTR/terminator are suitable to enable exogenous gene expression in Chaetoceros sp. In addition, Yamaguchi reported that the nourseothricin resistance cassette encoded on pTpfcp/nat was suitable for use as a selectable marker in Chaetoceros sp.
[0409] In an embodiment of the present invention, vector pTpfcp/nat, comprising the nucleotide sequence encoding the nat gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in the closely-related Chaetoceros compressum to reflect the codon bias inherent in nuclear genes of Chaetoceros compressum in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Thalassiosira pseudonana fcp gene promoter upstream of the protein-coding sequence and operably linked to the Thalassiosira pseudonana fcp gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chaetoceros sp. genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Chaetoceros sp. with the transformation vector is achieved through well-known transformation including microprojectile bombardment or other known methods. Activity of the nat gene product can be used as a selectable marker to select for Chaetoceros sp. transformed with the transformation vector in, but not limited to, f/2 agar medium comprising nourseothricin. Growth medium suitable for Chaetoceros sp. lipid production include, but are not limited to, f/2 medium, and those culture media discussed by Napolitano et al. and Volkman et al. Evaluation of fatty acid profiles of Chaetoceros sp lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 23
Engineering Cylindrotheca Fusiformis
[0410] Expression of recombinant genes in accordance with the present invention in Cylindrotheca fusiformis can be accomplished by modifying the methods and vectors taught by Poulsen and Kroger et al. as discussed herein. Briefly, Poulsen and Kroger et al., FEBS Journal, Vol. 272 (2005), pp. 3413-3423, reported the transformation of Cylindrotheca fusiformis with plasmid DNA. Using the transformation method of microprojectile bombardment, Poulsen and Kroger introduced the pCF-ble plasmid into Cylindrotheca fusiformis. Plasmid pCF-ble comprised a bleomycin resistance cassette, comprising sequence encoding the Streptoalloteichus hindustanus Bleomycin binding protein (ble), for resistance to the antibiotics zeocin and phleomycin, operably linked to the Cylindrotheca fusiformis fucozanthin chlorophyll a/c binding protein gene (fcpA, GenBank Accession No. AY125580) promoter upstream of the ble protein-coding region and operably linked to the Cylindrotheca fusiformis fcpA gene 3'UTR/terminator at the 3' region (down-stream of the ble protein-coding region). Prior to transformation with pCF-ble, Cylindrotheca fusiformis was unable to propagate on medium comprising 1 mg/ml zeocin. Upon transformation with pCF-ble, transformants of Cylindrotheca fusiformis were obtained that were propagated in selective culture medium comprising 1 mg/ml zeocin. The expression of the ble gene product in Cylindrotheca fusiformis enabled propagation in the presence of 1 mg/ml zeocin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Cylindrotheca fusiformis. Poulsen and Kroger reported that selection and maintenance of the transformed Cylindrotheca fusiformis was performed on agar plates comprising artificial seawater medium with 1 mg/ml zeocin. Poulsen and Kroger reported liquid propagation of Cylindrotheca fusiformis transformants in artificial seawater medium with 1 mg/ml zeocin. Propagation of Cylindrotheca fusiformis in additional culture medium has been discussed (for example in Liang et al., Journal of Applied Phycology, Vol. 17:1 (2005), pp. 61-65, and by Orcutt and Patterson, Lipids, Vol. 9:12 (1974), pp. 1000-1003). Additional plasmids, promoters, and 3'UTR/terminators for enabling heterologous gene expression in Chaetoceros sp. have been reported in the same report by Poulsen and Kroger. Poulsen and Kroger reported that the plasmid pCF-ble and the Cylindrotheca fusiformis fcp promoter and 3' UTR/terminator are suitable to enable exogenous gene expression in Cylindrotheca fusiformis. In addition, Poulsen and Kroger reported that the bleomycin resistance cassette encoded on pCF-ble was suitable for use as a selectable marker in Cylindrotheca fusiformis.
[0411] In an embodiment of the present invention, vector pCF-ble, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Cylindrotheca fusiformis to reflect the codon bias inherent in nuclear genes of Cylindrotheca fusiformis in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Cylindrotheca fusiformis fcp gene promoter upstream of the protein-coding sequence and operably linked to the Cylindrotheca fusiformis fcp gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Cylindrotheca fusiformis genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Cylindrotheca fusiformis with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the ble gene product can be used as a selectable marker to select for Cylindrotheca fusiformis transformed with the transformation vector in, but not limited to, artificial seawater agar medium comprising zeocin. Growth media suitable for Cylindrotheca fusiformis lipid production include, but are not limited to, artificial seawater and those media reported by Liang et al. and Orcutt and Patterson. Evaluation of fatty acid profiles of Cylindrotheca fusiformis lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 24
Engineering Amphidinium sp.
[0412] Expression of recombinant genes in accordance with the present invention in Amphidinium sp. can be accomplished by modifying the methods and vectors taught by ten Lohuis and Miller et al. as discussed herein. Briefly, ten Lohuis and Miller et al., The Plant Journal, Vol. 13:3 (1998), pp. 427-435, reported the stable transformation of Amphidinium sp. with plasmid DNA. Using the transformation technique of agitation in the presence of silicon carbide whiskers, ten Lohuis introduced the plasmid pMT NPT/GUS into Amphidinium sp. pMT NPT/GUS comprised a neomycin resistance cassette, comprising sequence encoding the neomycin phosphotransferase II (nptII) gene product (GenBank Accession No. AAL92039) operably linked to the Agrobacterium tumefaciens nopaline synthase (nos) gene promoter upstream, or 5' of the nptII protein-coding region and operably linked to the 3' UTR/terminator of the nos gene at the 3' region (down-stream of the nptII protein-coding region). The nptII gene product confers resistance to the antibiotic G418. The pMT NPT/GUS plasmid further comprised sequence encoding a beta-glucuronidase (GUS) reporter gene product operably-linked to a CaMV 35S promoter and further operably linked to the CaMV 35S 3' UTR/terminator. Prior to transformation with pMT NPT/GUS, Amphidinium sp. was unable to be propagated on medium comprising 3 mg/ml G418. Upon transformation with pMT NPT/GUS, transformants of Amphidinium sp. were obtained that were propagated in selective culture medium comprising 3 mg/ml G418. The expression of the nptII gene product in Amphidinium sp. enabled propagation in the presence of 3 mg/ml G418, thereby establishing the utility of the neomycin antibiotic resistance cassette as selectable marker for use in Amphidinium sp. Detectable activity of the GUS reporter gene indicated that CaMV 35S promoter and 3'UTR are suitable for enabling gene expression in Amphidinium sp. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. ten Lohuis and Miller reported liquid propagation of Amphidinium sp transformants in medium comprising seawater supplemented with F/2 enrichment solution (provided by the supplier Sigma) and 3 mg/ml G418 as well as selection and maintenance of Amphidinium sp. transformants on agar medium comprising seawater supplemented with F/2 enrichment solution and 3 mg/ml G418. Propagation of Amphidinium sp. in additional culture medium has been reported (for example in Mansour et al., Journal of Applied Phycology, Vol. 17:4 (2005) pp. 287-v300). An additional plasmid, comprising additional promoters, 3'UTR/terminators, and a selectable marker for enabling heterologous gene expression in Amphidinium sp. have been reported in the same report by ten Lohuis and Miller. ten Lohuis and Miller reported that the plasmid pMT NPT/GUS and the promoter and 3' UTR/terminator of the nos and CaMV 35S genes are suitable to enable exogenous gene expression in Amphidinium sp. In addition, ten Lohuis and Miller reported that the neomycin resistance cassette encoded on pMT NPT/GUS was suitable for use as a selectable marker in Amphidinium sp.
[0413] In an embodiment of the present invention, vector pMT NPT/GUS, comprising the nucleotide sequence encoding the nptII gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Amphidinium sp. to reflect the codon bias inherent in nuclear genes of the closely-related species, Amphidinium carterae in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Agrobacterium tumefaciens nopaline synthase (nos) gene promoter upstream of the protein-coding sequence and operably linked to the nos 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Amphidinium sp. genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Amphidinium sp. with the transformation vector is achieved through well-known transformation techniques including silicon fibre-mediated microinjection or other known methods. Activity of the nptII gene product can be used as a selectable marker to select for Amphidinium sp. transformed with the transformation vector in, but not limited to, seawater agar medium comprising G418. Growth media suitable for Amphidinium sp. lipid production include, but are not limited to, artificial seawater and those media reported by Mansour et al. and ten Lohuis and Miller. Evaluation of fatty acid profiles of Amphidinium sp. lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 25
Engineering Symbiodinium Microadriacticum
[0414] Expression of recombinant genes in accordance with the present invention in Symbiodinium microadriacticum can be accomplished by modifying the methods and vectors taught by ten Lohuis and Miller et al. as discussed herein. Briefly, ten Lohuis and Miller et al., The Plant Journal, Vol. 13:3 (1998), pp. 427-435, reported the stable transformation of Symbiodinium microadriacticum with plasmid DNA. Using the transformation technique of silicon fibre-mediated microinjection, ten Lohuis introduced the plasmid pMT NPT/GUS into Symbiodinium microadriacticum. pMT NPT/GUS comprised a neomycin resistance cassette, comprising sequence encoding the neomycin phosphotransferase II (nptII) gene product (GenBank Accession No. AAL92039) operably linked to the Agrobacterium tumefaciens nopaline synthase (nos) gene promoter upstream, or 5' of the nptII protein-coding region and operably linked to the 3' UTR/terminator of the nos gene at the 3' region (down-stream of the nptII protein-coding region). The nptII gene product confers resistance to the antibiotic G418. The pMT NPT/GUS plasmid further comprised sequence encoding a beta-glucuronidase (GUS) reporter gene product operably-linked to a CaMV 35S promoter and further operably linked to the CaMV 35S 3' UTR/terminator. Prior to transformation with pMT NPT/GUS, Symbiodinium microadriacticum was unable to be propagated on medium comprising 3 mg/ml G418. Upon transformation with pMT NPT/GUS, transformants of Symbiodinium microadriacticum were obtained that were propagated in selective culture medium comprising 3 mg/ml G418. The expression of the nptII gene product in Symbiodinium microadriacticum enabled propagation in the presence of 3 mg/ml G418, thereby establishing the utility of the neomycin antibiotic resistance cassette as selectable marker for use in Symbiodinium microadriacticum. Detectable activity of the GUS reporter gene indicated that CaMV 35S promoter and 3'UTR are suitable for enabling gene expression in Symbiodinium microadriacticum. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. ten Lohuis and Miller reported liquid propagation of Symbiodinium microadriacticum transformants in medium comprising seawater supplemented with F/2 enrichment solution (provided by the supplier Sigma) and 3 mg/ml G418 as well as selection and maintenance of Symbiodinium microadriacticum transformants on agar medium comprising seawater supplemented with F/2 enrichment solution and 3 mg/ml G418. Propagation of Symbiodinium microadriacticum in additional culture medium has been discussed (for example in Iglesias-Prieto et al., Proceedings of the National Academy of Sciences, Vol. 89:21 (1992) pp. 10302-10305). An additional plasmid, comprising additional promoters, 3'UTR/terminators, and a selectable marker for enabling heterologous gene expression in Symbiodinium microadriacticum have been discussed in the same report by ten Lohuis and Miller. ten Lohuis and Miller reported that the plasmid pMT NPT/GUS and the promoter and 3' UTR/terminator of the nos and CaMV 35S genes are suitable to enable exogenous gene expression in Symbiodinium microadriacticum. In addition, ten Lohuis and Miller reported that the neomycin resistance cassette encoded on pMT NPT/GUS was suitable for use as a selectable marker in Symbiodinium microadriacticum.
[0415] In an embodiment of the present invention, vector pMT NPT/GUS, comprising the nucleotide sequence encoding the nptII gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected Table 20, each protein-coding sequence codon-optimized for expression in Symbiodinium microadriacticum to reflect the codon bias inherent in nuclear genes of Symbiodinium microadriacticum in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Agrobacterium tumefaciens nopaline synthase (nos) gene promoter upstream of the protein-coding sequence and operably linked to the nos 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Symbiodinium microadriacticum genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Symbiodinium microadriacticum with the transformation vector is achieved through well-known transformation techniques including silicon fibre-mediated microinjection or other known methods. Activity of the nptII gene product can be used as a selectable marker to select for Symbiodinium microadriacticum transformed with the transformation vector in, but not limited to, seawater agar medium comprising G418. Growth media suitable for Symbiodinium microadriacticum lipid production include, but are not limited to, artificial seawater and those media reported by Iglesias-Prieto et al. and ten Lohuis and Miller. Evaluation of fatty acid profiles of Symbiodinium microadriacticum lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 26
Engineering Nannochloropsis sp.
[0416] Expression of recombinant genes in accordance with the present invention in Nannochloropsis sp. W2J3B can be accomplished by modifying the methods and vectors taught by Kilian et al. as discussed herein. Briefly, Kilian et al., Proceedings of the National Academy of Sciences, Vol. 108:52 (2011) pp. 21265-21269, reported the stable nuclear transformation of Nannochloropsis with a transformation construct. Using the transformation method of electroporation, Kilian introduced the transformation construct C2 into Nannochloropsis sp. W2J3B. The C2 transformation construct comprised a bleomycin resistance cassette, comprising the coding sequence for the Streptoalloteichus hindustanus Bleomycin binding protein (ble), for resistance to the antibiotics phleomycin and zeocin, operably linked to and the promoter of the Nannochloropsis sp. W2J3B violaxanthin/chlorophyll a-binding protein gene VCP2 upstream of the ble protein-coding region and operably linked to the 3'UTR/terminator of the Nannochloropsis sp. W2J3B violaxanthin/chlorophyll a-binding gene VCP1 downstream of the ble protein-coding region. Prior to transformation with C2, Nannochloropsis sp. W2J3B was unable to propagate on medium comprising 2 ug/ml zeocin. Upon transformation with C2, transformants of Nannochloropsis sp. W2J3B were obtained that were propagated in selective culture medium comprising 2 ug/ml zeocin. The expression of the ble gene product in Nannochloropsis sp. W2J3B enabled propagation in the presence of 2 ug/ml zeocin, thereby establishing the utility of the bleomycin antibiotic resistance cassette as selectable marker for use in Nannochloropsis. Evaluation of the genomic DNA of the stable transformants was performed by PCR. Kilian reported liquid propagation of Nannochloropsis sp. W2J3B transformants in F/2 medium (reported by Guilard and Ryther, Canadian Journal of Microbiology, Vol. 8 (1962), pp. 229-239) comprising fivefold levels of trace metals, vitamins, and phosphate solution, and further comprising 2 ug/ml zeocin. Kilian also reported selection and maintenance of Nannochloropsis sp. W2J3B transformants on agar F/2 medium comprising artificial seawater 2 mg/ml zeocin. Propagation of Nannochloropsis in additional culture medium has been discussed (for example in Chiu et al., Bioresour Technol., Vol. 100:2 (2009), pp. 833-838 and Pal et al., Applied Microbiology and Biotechnology, Vol. 90:4 (2011), pp. 1429-1441.). Additional transformation constructs, comprising additional promoters and 3'UTR/terminators for enabling heterologous gene expression in Nannochloropsis sp. W2J3B and selectable markers for selection of transformants have been described in the same report by Kilian. Kilian reported that the transformation construct C2 and the promoter of the Nannochloropsis sp. W2J3B violaxanthin/chlorophyll a-binding protein gene VCP2 and 3' UTR/terminator of the Nannochloropsis sp. W2J3B violaxanthin/chlorophyll a-binding protein gene VCP1 are suitable to enable exogenous gene expression in Nannochloropsis sp. W2J3B. In addition, Kilian reported that the bleomycin resistance cassette encoded on C2 was suitable for use as a selectable marker in Nannochloropsis sp. W2J3B.
[0417] In an embodiment of the present invention, transformation construct C2, comprising the nucleotide sequence encoding the ble gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Nannochloropsis sp. W2J3B to reflect the codon bias inherent in nuclear genes of Nannochloropsis sp. in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Nannochloropsis sp. W2J3B VCP2 gene promoter upstream of the protein-coding sequence and operably linked to the Nannochloropsis sp. W2J3B VCP1 gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Nannochloropsis sp. W2J3B genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Nannochloropsis sp. W2J3B with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the ble gene product can be used as a selectable marker to select for Nannochloropsis sp. W2J3B transformed with the transformation vector in, but not limited to, F/2 medium comprising zeocin. Growth media suitable for Nannochloropsis sp. W2J3B lipid production include, but are not limited to, F/2 medium and those media reported by Chiu et al. and Pal et al. Evaluation of fatty acid profiles of Nannochloropsis sp. W2J3B lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 27
Engineering Cyclotella Cryptica
[0418] Expression of recombinant genes in accordance with the present invention in Cyclotella cryptica can be accomplished by modifying the methods and vectors taught by Dunahay et al. as discussed herein. Briefly, Dunahay et al., Journal of Phycology, Vol. 31 (1995), pp. 1004-1012, reported the stable transformation of Cyclotella cryptica with plasmid DNA. Using the transformation method of microprojectile bombardment, Dunahay introduced the plasmid pACCNPT5.1 into Cyclotella cryptica. Plasmid pACCNPT5.1 comprised a neomycin resistance cassette, comprising the coding sequence of the neomycin phosphotransferase II (nptII) gene product operably linked to the promoter of the Cyclotella cryptica acetyl-CoA carboxylase (ACCase) gene (GenBank Accession No. L20784) upstream of the nptII coding-region and operably linked to the 3'UTR/terminator of the Cyclotella cryptica ACCase gene at the 3' region (downstream of the nptII coding-region). The nptII gene product confers resistance to the antibiotic G418. Prior to transformation with pACCNPT5.1, Cyclotella cryptica was unable to propagate on 50% artificial seawater medium comprising 100 ug/ml G418. Upon transformation with pACCNPT5.1, transformants of Cyclotella cryptica were obtained that were propagated in selective 50% artificial seawater medium comprising 100 ug/ml G418. The expression of the nptII gene product in Cyclotella cryptica enabled propagation in the presence of 100 ug/ml G418, thereby establishing the utility of the neomycin antibiotic resistance cassette as selectable marker for use in Cyclotella cryptica. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Dunahay reported liquid propagation of Cyclotella cryptica in artificial seawater medium (ASW, as discussed by Brown, L., Phycologia, Vol. 21 (1982), pp. 408-410) supplemented with 1.07 mM sodium silicate and with 100 ug/ml G418. Dunahay also reported selection and maintenance of Cyclotella cryptica transformants on agar plates comprising ASW medium with 100 ug/ml G418. Propagation of Cyclotella cryptica in additional culture medium has been discussed (for example in Sriharan et al., Applied Biochemistry and Biotechnology, Vol. 28-29:1 (1991), pp. 317-326 and Pahl et al., Journal of Bioscience and Bioengineering, Vol. 109:3 (2010), pp. 235-239). Dunahay reported that the plasmid pACCNPT5.1 and the promoter of the Cyclotella cryptica acetyl-CoA carboxylase (ACCase) gene are suitable to enable exogenous gene expression in Cyclotella cryptica. In addition, Dunahay reported that the neomycin resistance cassette encoded on pACCNPT5.1 was suitable for use as a selectable marker in Cyclotella cryptica.
[0419] In an embodiment of the present invention, vector pACCNPT5.1, comprising the nucleotide sequence encoding the nptII gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Cyclotella cryptica to reflect the codon bias inherent in nuclear genes of Cyclotella cryptica in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Cyclotella cryptica ACCase promoter upstream of the protein-coding sequence and operably linked to the Cyclotella cryptica ACCase 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Cyclotella cryptica genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Cyclotella cryptica with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the nptII gene product can be used as a marker to select for Cyclotella cryptica transformed with the transformation vector in, but not limited to, agar ASW medium comprising G418. Growth media suitable for Cyclotella cryptica lipid production include, but are not limited to, ASW medium and those media reported by Sriharan et al., 1991 and Pahl et al. Evaluation of fatty acid profiles of Cyclotella cryptica lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 28
Engineering Navicula Saprophila
[0420] Expression of recombinant genes in accordance with the present invention in Navicula saprophila can be accomplished by modifying the methods and vectors taught by Dunahay et al. as discussed herein. Briefly, Dunahay et al., Journal of Phycology, Vol. 31 (1995), pp. 1004-1012, reported the stable transformation of Navicula saprophila with plasmid DNA. Using the transformation method of microprojectile bombardment, Dunahay introduced the plasmid pACCNPT5.1 into Navicula saprophila. Plasmid pACCNPT5.1 comprised a neomycin resistance cassette, comprising the coding sequence of the neomycin phosphotransferase II (nptII) gene product operably linked to the promoter of the Cyclotella cryptica acetyl-CoA carboxylase (ACCase) gene (GenBank Accession No. L20784) upstream of the nptII coding-region and operably linked to the 3'UTR/terminator of the Cyclotella cryptica ACCase gene at the 3' region (downstream of the nptII coding-region). The nptII gene product confers resistance to the antibiotic G418. Prior to transformation with pACCNPT5.1, Navicula saprophila was unable to propagate on artificial seawater medium comprising 100 ug/ml G418. Upon transformation with pACCNPT5.1, transformants of Navicula saprophila were obtained that were propagated in selective artificial seawater medium comprising 100 ug/ml G418. The expression of the nptII gene product in Navicula saprophila enabled propagation in the presence of G418, thereby establishing the utility of the neomycin antibiotic resistance cassette as selectable marker for use in Navicula saprophila. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Dunahay reported liquid propagation of Navicula saprophila in artificial seawater medium (ASW, as discussed by Brown, L., Phycologia, Vol. 21 (1982), pp. 408-410) supplemented with 1.07 mM sodium silicate and with 100 ug/ml G418. Dunahay also reported selection and maintenance of Navicula saprophila transformants on agar plates comprising ASW medium with 100 ug/ml G418. Propagation of Navicula saprophila in additional culture medium has been discussed (for example in Tadros and Johansen, Journal of Phycology, Vol. 24:4 (1988), pp. 445-452 and Sriharan et al., Applied Biochemistry and Biotechnology, Vol. 20-21:1 (1989), pp. 281-291). Dunahay reported that the plasmid pACCNPT5.1 and the promoter of the Cyclotella cryptica acetyl-CoA carboxylase (ACCase) gene are suitable to enable exogenous gene expression in Navicula saprophila. In addition, Dunahay reported that the neomycin resistance cassette encoded on pACCNPT5.1 was suitable for use as a selectable marker in Navicula saprophila.
[0421] In an embodiment of the present invention, vector pACCNPT5.1, comprising the nucleotide sequence encoding the nptII gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Navicula saprophila to reflect the codon bias inherent in nuclear genes of the closely-related Navicula pelliculosa in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Cyclotella cryptica ACCase gene promoter upstream of the protein-coding sequence and operably linked to the Cyclotella cryptica ACCase gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Navicula saprophila genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. Stable transformation of Navicula saprophila with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the nptII gene product can be used as a selectable marker to select for Navicula saprophila transformed with the transformation vector in, but not limited to, agar ASW medium comprising G418. Growth media suitable for Navicula saprophila lipid production include, but are not limited to, ASW medium and those media reported by Sriharan et al. 1989 and Tadros and Johansen. Evaluation of fatty acid profiles of Navicula saprophila lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 29
Engineering Thalassiosira Pseudonana
[0422] Expression of recombinant genes in accordance with the present invention in Thalassiosira pseudonana can be accomplished by modifying the methods and vectors taught by Poulsen et al. as discussed herein. Briefly, Poulsen et al., Journal of Phycology, Vol. 42 (2006), pp. 1059-1065, reported the stable transformation of Thalassiosira pseudonana with plasmid DNA. Using the transformation method of microprojectile bombardment, Poulsen introduced the plasmid pTpfcp/nat in to Thalassiosira pseudonana. pTpfcp/nat comprised a nourseothricin resistance cassette, comprising sequence encoding the nourseothricin acetyltransferase (nat) gene product (GenBank Accession No. AAC60439) operably linked to the Thalassiosira pseudonana fucoxanthin chlorophyll a/c binding protein gene (fcp) promoter upstream of the nat protein-coding region and operably linked to the Thalassiosira pseudonana fcp gene 3' UTR/terminator at the 3' region (downstream of the nat protein coding-sequence). The nat gene product confers resistance to the antibiotic nourseothricin. Prior to transformation with pTpfcp/nat, Thalassiosira pseudonana was unable to propagate on medium comprising 10 ug/ml nourseothricin. Upon transformation with pTpfcp/nat, transformants of Thalassiosira pseudonana were obtained that were propagated in selective culture medium comprising 100 ug/ml nourseothricin. The expression of the nat gene product in Thalassiosira pseudonana enabled propagation in the presence of 100 ug/ml nourseothricin, thereby establishing the utility of the nourseothricin antibiotic resistance cassette as selectable marker for use in Thalassiosira pseudonana. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Poulsen reported that selection and maintenance of the transformed Thalassiosira pseudonana was performed in liquid culture comprising modified ESAW medium (as discussed by Harrison et al., Journal of Phycology, Vol. 16 (1980), pp. 28-35) with 100 ug/ml nourseothricin. Propagation of Thalassiosira pseudonana in additional culture medium has been discussed (for example in Volkman et al., Journal of Experimental Marine Biology and Ecology, Vol. 128:3 (1989), pp. 219-240). An additional plasmid, comprising additional selectable markers suitable for use in Thalassiosira pseudonana has been discussed in the same report by Poulsen. Poulsen reported that the plasmid pTpfcp/nat, and the Thalassiosira pseudonana fcp promoter and 3' UTR/terminator are suitable to enable exogenous gene expression in Thalassiosira pseudonana. In addition, Poulsen reported that the nourseothricin resistance cassette encoded on pTpfcp/nat was suitable for use as a selectable marker in Thalassiosira pseudonana.
[0423] In an embodiment of the present invention, vector pTpfcp/nat, comprising the nucleotide sequence encoding the nat gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Thalassiosira pseudonana to reflect the codon bias inherent in nuclear genes of Thalassiosira pseudonana in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Thalassiosira pseudonana fcp gene promoter upstream of the protein-coding sequence and operably linked to the Thalassiosira pseudonana fcp gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Thalassiosira pseudonana genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Thalassiosira pseudonana genome (referenced in the publication by Armbrust et al., Science, Vol. 306: 5693 (2004): pp. 79-86). Stable transformation of Thalassiosira pseudonana with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the nat gene product can be used as a marker to select for Thalassiosira pseudonana transformed with the transformation vector in but not limited to, ESAW agar medium comprising nourseothricin. Growth media suitable for Thalassiosira pseudonana lipid production include, but are not limited to, ESAW medium, and those culture media discussed by Volkman et al. and Harrison et al. Evaluation of fatty acid profiles of Thalassiosira pseudonana lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 30
Engineering Chlamydomonas Reinhardtii
[0424] Expression of recombinant genes in accordance with the present invention in Chlamydomonas reinhardtii can be accomplished by modifying the methods and vectors taught by Cerutti et al. as discussed herein. Briefly, Cerutti et al., Genetics, Vol. 145:1 (1997), pp. 97-110, reported the stable nuclear transformation of Chlamydomonas reinhardtii with a transformation vector. Using the transformation method of microprojectile bombardment, Cerutti introduced transformation construct P[1030] into Chlamydomonas reinhardtii. Construct P[1030] comprised a spectinomycin resistance cassette, comprising sequence encoding the aminoglucoside 3''-adenyltransferase (aadA) gene product operably linked to the Chlamydomonas reinhardtii ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene (RbcS2, GenBank Accession No. X04472) promoter upstream of the aadA protein-coding region and operably linked to the Chlamydomonas reinhardtii RbcS2 gene 3' UTR/terminator at the 3' region (downstream of the aadA protein coding-sequence). The aadA gene product confers resistance to the antibiotic spectinomycin. Prior to transformation with P[1030], Chlamydomonas reinhardtii was unable to propagate on medium comprising 90 ug/ml spectinomycin. Upon transformation with P[1030], transformants of Chlamydomonas reinhardtii were obtained that were propagated in selective culture medium comprising 90 ug/ml spectinomycin, thereby establishing the utility of the spectinomycin antibiotic resistance cassette as a selectable marker for use in Chlamydomonas reinhardtii. Evaluation of the genomic DNA of the stable transformants was performed by Southern analysis. Cerutti reported that selection and maintenance of the transformed Chlamydomonas reinhardtii was performed on agar plates comprising Tris-acetate-phosphate medium (TAP, as described by Harris, The Chlamydomonas Sourcebook, Academic Press, San Diego, 1989) with 90 ug/ml spectinomycin. Cerutti additionally reported propagation of Chlamydomonas reinhardtii in TAP liquid culture with 90 ug/ml spectinomycin. Propagation of Chlamydomonas reinhardtii in alternative culture medium has been discussed (for example in Dent et al., African Journal of Microbiology Research, Vol. 5:3 (2011), pp. 260-270 and Yantao et al., Biotechnology and Bioengineering, Vol. 107:2 (2010), pp. 258-268). Additional constructs, comprising additional selectable markers suitable for use in Chlamydomonas reinhardtii as well as numerous regulatory sequences, including promoters and 3' UTRs suitable for promoting heterologous gene expression in Chlamydomonas reinhardtii are known in the art and have been discussed (for a review, see Radakovits et al., Eukaryotic Cell, Vol. 9:4 (2010), pp. 486-501). Cerutti reported that the transformation vector P[1030] and the Chlamydomonas reinhardtii promoter and 3' UTR/terminator are suitable to enable exogenous gene expression in Chlamydomonas reinhardtii. In addition, Cerutti reported that the spectinomycin resistance cassette encoded on P[1030] was suitable for use as a selectable marker in Chlamydomonas reinhardtii.
[0425] In an embodiment of the present invention, vector P[1030], comprising the nucleotide sequence encoding the aadA gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Chlamydomonas reinhardtii to reflect the codon bias inherent in nuclear genes of Chlamydomonas reinhardtii in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Chlamydomonas reinhardtii RbcS2 promoter upstream of the protein-coding sequence and operably linked to the Chlamydomonas reinhardtii RbcS2 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Chlamydomonas reinhardtii genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic site of an endogenous lipid biosynthesis pathway gene. One skilled in the art can identify such homology regions within the sequence of the Chlamydomonas reinhardtii genome (referenced in the publication by Merchant et al., Science, Vol. 318:5848 (2007), pp. 245-250). Stable transformation of Chlamydomonas reinhardtii with the transformation vector is achieved through well-known transformation techniques including microprojectile bombardment or other known methods. Activity of the aadA gene product can be used as a marker to select for Chlamydomonas reinhardtii transformed with the transformation vector on, but not limited to, TAP agar medium comprising spectinomycin. Growth media suitable for Chlamydomonas reinhardtii lipid production include, but are not limited to, ESAW medium, and those culture media discussed by Yantao et al. and Dent et al. Evaluation of fatty acid profiles of Chlamydomonas reinhardtii lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 31
Engineering Yarrowia Lipolytica
[0426] Expression of recombinant genes in accordance with the present invention in Yarrowia lipolytica can be accomplished by modifying the methods and vectors taught by Fickers et al. as discussed herein. Briefly, Fickers et al., Journal of Microbiological Methods, Vol. 55 (2003), pp. 727-737, reported the stable nuclear transformation of Yarrowia lipolytica with plasmid DNA. Using a lithium acetate transformation method, Fickers introduced the plasmid JMP123 into Yarrowia lipolytica. Plasmid JMP123 comprised a hygromycin B resistance cassette, comprising sequence encoding the hygromycin B phosphotransferase gene product (hph), operably-linked to the Yarrowia lipolytica LIP2 gene promoter (GenBank Accession No. AJ012632) upstream of the hph protein-coding region and operably linked to the Yarrowia lipolytica LIP2 gene 3'UTR/terminator downstream of the hph protein-coding region. Prior to transformation with JMP123, Yarrowia lipolytica were unable to propagate on medium comprising 100 ug/ml hygromycin. Upon transformation with JMP123, transformed Yarrowia lipolytica were obtained that were able to propagate on medium comprising 100 ug/ml hygromycin, thereby establishing the hygromycin B antibiotic resistance cassette as a selectable marker for use in Yarrowia lipolytica. The nucleotide sequence provided on JMP123 of the promoter and 3'UTR/terminator of the Yarrowia lipolytica LIP2 gene served as donor sequences for homologous recombination of the hph coding sequence into the LIP2 locus. Evaluation of the genomic DNA of the stable transformants was performed by Southern. Fickers reported that selection and maintenance of the transformed Yarrowia lipolytica was performed on agar plates comprising standard YPD medium (Yeast Extract Peptone Dextrose) with 100 ug/ml hygromycin. Liquid culturing of transformed Yarrowia lipolytica was performed in YPD medium with hygromycin. Other media and techniques used for culturing Yarrowia lipolytica have been reported and numerous other plasmids, promoters, 3' UTRs, and selectable markers for use in Yarrowia lipolytica have been reported (for example see Pignede et al., Applied and Environmental Biology, Vol. 66:8 (2000), pp. 3283-3289, Chuang et al., New Biotechnology, Vol. 27:4 (2010), pp. 277-282, and Barth and Gaillardin, (1996), In: K, W. (Ed.), Nonconventional Yeasts in Biotechnology. Sprinter-Verlag, Berlin-Heidelber, pp. 313-388). Fickers reported that the transformation vector JMP 123 and the Yarrowia lipolytica LIP2 gene promoter and 3' UTR/terminator are suitable to enable heterologous gene expression in Yarrowia lipolytica. In addition, Fickers reported that the hygromycin resistance cassette encoded on JMP123 was suitable for use as a selectable marker in Yarrowia lipolytica.
[0427] In an embodiment of the present invention, vector JMP123, comprising the nucleotide sequence encoding the hph gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Yarrowia lipolytica to reflect the codon bias inherent in nuclear genes of Yarrowia lipolytica in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Yarrowia lipolytica LIP2 gene promoter upstream of the protein-coding sequence and operably linked to the Yarrowia lipolytica LIP2 gene 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Yarrowia lipolytica genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Yarrowia lipolytica genome (referenced in the publication by Dujun et al., Nature, Vol. 430 (2004), pp. 35-44). Stable transformation of Yarrowia lipolytica with the transformation vector is achieved through well-known transformation techniques including lithium acetate transformation or other known methods. Activity of the hph gene product can be used as a marker to select for Yarrowia lipolytica transformed with the transformation vector on, but not limited to, YPD medium comprising hygromycin. Growth media suitable for Yarrowia lipolytica lipid production include, but are not limited to, YPD medium, and those culture media described by Chuang et al. Evaluation of fatty acid profiles of Yarrowia lipolytica lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 32
Engineering Mortierella Alpine
[0428] Expression of recombinant genes in accordance with the present invention in Mortierella alpine can be accomplished by modifying the methods and vectors taught by Mackenzie et al. as discussed herein. Briefly, Mackenzie et al., Applied and Environmental Microbiology, Vol. 66 (2000), pp. 4655-4661, reported the stable nuclear transformation of Mortierella alpina with plasmid DNA. Using a protoplast transformation method, MacKenzie introduced the plasmid pD4 into Mortierella alpina. Plasmid pD4 comprised a hygromycin B resistance cassette, comprising sequence encoding the hygromycin B phosphotransferase gene product (hpt), operably-linked to the Mortierella alpina histone H4.1 gene promoter (GenBank Accession No. AJ249812) upstream of the hpt protein-coding region and operably linked to the Aspergillus nidulans N-(5'-phophoribosyl)anthranilate isomerase (trpC) gene 3'UTR/terminator downstream of the hpt protein-coding region. Prior to transformation with pD4, Mortierella alpina were unable to propagate on medium comprising 300 ug/ml hygromycin. Upon transformation with pD4, transformed Mortierella alpina were obtained that were propagated on medium comprising 300 ug/ml hygromycin, thereby establishing the hygromycin B antibiotic resistance cassette as a selectable marker for use in Mortierella alpina. Evaluation of the genomic DNA of the stable transformants was performed by Southern. Mackenzie reported that selection and maintenance of the transformed Mortierella alpina was performed on PDA (potato dextrose agar) medium comprising hygromycin. Liquid culturing of transformed Mortierella alpina by Mackenzie was performed in PDA medium or in S2GYE medium (comprising 5% glucose, 0.5% yeast extract, 0.18% NH.sub.4SO.sub.4, 0.02% MgSO.sub.4-7H.sub.2O, 0.0001% FeCl.sub.3-6H.sub.2O, 0.1%, trace elements, 10 mM K.sub.2HPO.sub.4--NaH.sub.2PO.sub.4), with hygromycin. Other media and techniques used for culturing Mortierella alpina have been reported and other plasmids, promoters, 3' UTRs, and selectable markers for use in Mortierella alpina have been reported (for example see Ando et al., Applied and Environmental Biology, Vol. 75:17 (2009) pp. 5529-35 and Lu et al., Applied Biochemistry and Biotechnology, Vol. 164:7 (2001), pp. 979-90). Mackenzie reported that the transformation vector pD4 and the Mortierella alpina histone H4.1 promoter and A. nidulans trpC gene 3' UTR/terminator are suitable to enable heterologous gene expression in Mortierella alpina. In addition, Mackenzie reported that the hygromycin resistance cassette encoded on pD4 was suitable for use as a selectable marker in Mortierella alpina.
[0429] In an embodiment of the present invention, vector pD4, comprising the nucleotide sequence encoding the hpt gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Mortierella alpina to reflect the codon bias inherent in nuclear genes of Mortierella alpina in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the Mortierella alpina histone H4.1 gene promoter upstream of the protein-coding sequence and operably linked to the A. nidulans trpC 3'UTR/terminator at the 3' region, or downstream, of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Mortierella alpina genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Mortierella alpina genome (referenced in the publication by Wang et al., PLOS One, Vol. 6:12 (2011)). Stable transformation of Mortierella alpina with the transformation vector is achieved through well-known transformation techniques including protoplast transformation or other known methods. Activity of the hpt gene product can be used as a marker to select for Mortierella alpina transformed with the transformation vector on, but not limited to, PDA medium comprising hygromycin. Growth media suitable for Mortierella alpina lipid production include, but are not limited to, S2GYE medium, and those culture media described by Lu et al. and Ando et al. Evaluation of fatty acid profiles of Mortierella alpina lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 33
Engineering Rhodococcus Opacus PD630
[0430] Expression of recombinant genes in accordance with the present invention in Rhodococcus opacus PD630 can be accomplished by modifying the methods and vectors taught by Kalscheuer et al. as discussed herein. Briefly, Kalscheuer et al., Applied and Environmental Microbiology, Vol. 52 (1999), pp. 508-515, reported the stable transformation of Rhodococcus opacus with plasmid DNA. Using the transformation method of electroporation, Kalscheuer introduced the plasmid pNC9501 into Rhodococcus opacus PD630. Plasmid pNC9501 comprised a thiostrepton resistance (thio.sup.r) cassette, comprising the full nucleotide sequence of the Streptomyces azureus 23S rRNA A1067 methyltransferase gene, including the gene's promoter and 3' terminator sequence. Prior to transformation with pNC9501, Rhodococcus opacus was unable to propagate on medium comprising 1 mg/ml thiostrepton. Upon transformation of Rhodococcus opacus PD630 with pNC9501, transformants were obtained that propagated on culture medium comprising 1 mg/ml thiostrepton, thereby establishing the use of the thiostrepton resistance cassette as a selectable marker in Rhodococcus opacus PD630. A second plasmid described by Kalscheuer, pAK68, comprised the resistance thio.sup.r cassette as well as the gene sequences of the Ralstonia eutropha beta-ketothiolase (phaB), acetoacetyl-CoA reductase (phaA), and poly3-hydroxyalkanoic acid synthase (phaC) genes for polyhydroxyalkanoate biosynthesis, driven by the lacZ promoter. Upon pAK68 transformation of a Rhodococcus opacus PD630 strain deficient in polyhydroxyalkanoate biosynthesis, transformed Rhodococcus opacus PD630 were obtained that produced higher amounts of polyhydroxyalkanoates than the untransformed strain. Detectable activity of the introduced Ralstonia eutropha phaB, phaA, and phaC enzymes indicted that the regulatory elements encoded on the pAK68 plasmid were suitable for heterologous gene expression in Rhodococcus opacus PD630. Kalscheuer reported that selection and maintenance of the transformed Rhodococcus opacus PD630 was performed on standard Luria Broth (LB) medium, nutrient broth (NB), or mineral salts medium (MSM) comprising thiostrepton. Other media and techniques used for culturing Rhodococcus opacus PD630 have been described (for example see Kurosawa et al., Journal of Biotechnology, Vol. 147:3-4 (2010), pp. 212-218 and Alverez et al., Applied Microbial and Biotechnology, Vol. 54:2 (2000), pp. 218-223). Kalscheuer reported that the transformation vectors pNC9501 and pAK68, the promoters of the Streptomyces azureus 23S rRNA A1067 methyltransferase gene and lacZ gene are suitable to enable heterologous gene expression in Rhodococcus opacus PD630. In addition, Kalscheuer reported that the thio.sup.r cassette encoded on pNC9501 and pAK68 was suitable for use as a selectable marker in Rhodococcus opacus PD630.
[0431] In an embodiment of the present invention, vector pNC9501, comprising the nucleotide sequence encoding the thio.sup.r gene product for use as a selectable marker, is constructed and modified to further comprise a lipid biosynthesis pathway expression cassette sequence, thereby creating a transformation vector. The lipid biosynthesis pathway expression cassette encodes one or more lipid biosynthesis pathway proteins selected from Table 20, each protein-coding sequence codon-optimized for expression in Rhodococcus opacus PD630 to reflect the codon bias inherent in nuclear genes of Rhodococcus opacus in accordance with Tables 19A-D. For each lipid biosynthesis pathway protein of Table 20, the codon-optimized gene sequence can individually be operably linked to the lacZ gene promoter upstream of the protein-coding sequence. The transformation construct may additionally comprise homology regions to the Rhodococcus opacus PD630 genome for targeted genomic integration of the transformation vector. Homology regions may be selected to disrupt one or more genomic sites of endogenous lipid biosynthesis pathway genes. One skilled in the art can identify such homology regions within the sequence of the Rhodococcus opacus PD630 genome (referenced in the publication by Holder et al., PLOS Genetics, Vol. 7:9 (2011). Transformation of Rhodococcus opacus PD630 with the transformation vector is achieved through well-known transformation techniques including electroporation or other known methods. Activity of the Streptomyces azureus 23S rRNA A1067 methyltransferase gene product can be used as a marker to select for Rhodococcus opacus PD630 transformed with the transformation vector on, but not limited to, LB medium comprising thiostrepton. Growth media suitable Rhodococcus opacus PD630 lipid production include, but are not limited to those culture media discussed by Kurosawa et al. and Alvarez et al. Evaluation of fatty acid profiles of Rhodococcus opacus PD630 lipids can be assessed through standard lipid extraction and analytical methods described herein.
Example 34
Engineering Microalgae for Fatty Acid Auxotrophy
[0432] Strain B of Example 3, Prototheca moriformis (UTEX 1435) engineered to express a Cuphea wrightii thioesterase (CwTE2), was used as the host organism for further genetic modification to knockout both endogenous thioesterase alleles, FATA1-1 and FATA1-2. Here, a first transformation construct was generated to integrate a neomycin expression cassette into Strain B at the FATA1-1 locus. This construct, pSZ2226, included 5' (SEQ ID NO: 30) and 3' (SEQ ID NO: 31) homologous recombination targeting sequences (flanking the construct) to the FATA1-1 locus of the nuclear genome and a neomycin resistance protein-coding sequence under the control of the C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and the Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This NeoR expression cassette is listed as SEQ ID NO: 15 and served as a selectable marker.
[0433] Upon transformation of pSZ2226 into Strain B, individual transformants were selected on agar plates comprising sucrose and G418. A single isolate, Strain H, was selected for further genetic modification. A second transformation construct, pSZ2236, was generated to integrate polynucleotides enabling expression of a thiamine selectable marker into Strain H at the FATA1-2 locus. pSZ2236 included 5' (SEQ ID NO: 32) and 3' (SEQ ID NO: 33) homologous recombination targeting sequences (flanking the construct) to the FATA1-2 genomic region for integration into the P. moriformis (UTEX 1435) nuclear genome and an A. thaliana THIC protein coding region under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This AtTHIC expression cassette is listed as SEQ ID NO: 23 and served as a selectable marker. Upon transformation of Strain H with pSZ2236 to generate Strain I, individual transformants, were selected on agar plates comprising free fatty acids. Strain I was able to propagate on agar plates and in medium lacking thiamine and supplemented with free fatty acids.
Example 35
Engineering Microorganisms for Increased Production of Stearic Acid
[0434] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain J, was transformed with the plasmid construct pSZ2281 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. pSZ2281 included polynucleotides encoding RNA hairpins (SAD2hpC, SEQ ID NO: 34) to down-regulate the expression of stearoyl-ACP desaturase, 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4), to express the protein sequence given in SEQ ID NO: 3, under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. The polynucleotide sequence encoding the SAD2hpC RNA hairpin was under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6).
[0435] Upon transformation of Strain J with construct pSZ2281, thereby generating Strain K, positive clones were selected on agar plates containing sucrose as a sole carbon source. Individual transformants were clonally purified and propagated under heterotrophic conditions suitable for lipid production as those detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass and analyzed using standard fatty acid methyl ester gas chromatography flame ionization detection methods as described in Example 1 (also see PCT/US2012/023696). The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis UTEX Strain J propagated on glucose as a sole carbon source and three representative isolates of Strain K, propagated on sucrose as a sole carbon source, are presented in Table 21.
TABLE-US-00023 TABLE 21 Fatty acid profiles of Prototheca moriformis (UTEX 1435) cells engineered to express a hairpin RNA construct targeting stearoyl ACP desaturase gene/gene products. Area % Fatty acid Strain J Strain K-1 Strain K-2 Strain K-3 Strain K-4 C8:0 0.02 C10:0 0.01 0.00 0.02 0.02 0.04 C12:0 0.03 0.05 0.05 0.05 0.08 C14:0 1.22 0.89 0.87 0.77 1.2 C16:0 26.75 29.23 28.96 27.55 28.06 C18:0 3.06 37.39 36.76 36.41 40.82 C18:1 59.62 23.90 24.76 26.92 22.02 C18:2 7.33 5.44 5.54 5.54 4.53 C18:3 0.14 C20:0 1.43
[0436] The data presented in Table 21 show a clear impact of the expression of SAD2 hairpin RNA construct on the C18:0 and C18:1 fatty acid profiles of the transformed organism. The fatty acid profiles of Strain K transformants comprising a SAD2 hairpin RNA construct demonstrated an increase in the percentage of saturated C18:0 fatty acids with a concomitant diminution of unsaturated C18:1 fatty acids. Fatty acid profiles of the untransformed strain comprise about 3% C18:0. Fatty acid profiles of the transformed strains comprise about 37% C18:0. These data illustrate the successful expression and use of polynucleotides enabling expression of a SAD RNA hairpin construct in Prototheca moriformis to alter the percentage of saturated fatty acids in the engineered host microbes, and in particular in increasing the concentration of C18:0 fatty acids and decreasing C18:1 fatty acids in microbial cells.
[0437] Also shown in Table 21, strain K-4 had a yet further elevated level of stearate. Strain K4 was created by inserting the construct of strains K1-K3 into the SAD2B locus. Thus, by knocking out one copy of the SAD gene and inhibiting the remaining copies at the RNA level, a further reduction in oleic acid and corresponding increase in stearate was obtained. Triglyceride analysis of RBD oil obtained from strain K4 showed about 12% POP, 27% POS and 18% SOS.
Example 36
Engineering Microorganisms for Increased Production of Oleic Acid Through Knockdown of an Endogenous Acyl-ACP Thioesterase
[0438] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain J, was transformed independently with each of the constructs pSZ2402-pSZ2407 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Each of the constructs pSZ2402-pSZ2407 included different polynucleotides encoding a hairpin RNA targeted against Prototheca moriformis FATA1 mRNA transcripts to down-regulate the expression of fatty acyl-ACP thioesterase, 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4) to express the protein sequence given in SEQ ID NO: 3 under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. Sequence listing identities for the polynucleotides corresponding to each hairpin are listed in Table 22. The polynucleotide sequence encoding each RNA hairpin was under the control of the C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6).
TABLE-US-00024 TABLE 22 Plasmid constructs used to transform Prototheca moriformis (UTEX 1435) Strain J. Plasmid construct Hairpin designation SEQ ID NO: pSZ2402 PmFATA-hpB SEQ ID NO: 40 pSZ2403 PmFATA-hpC SEQ ID NO: 41 pSZ2404 PmFATA-hpD SEQ ID NO: 42 pSZ2405 PmFATA-hpE SEQ ID NO: 43 pSZ2406 PmFATA-hpF SEQ ID NO: 44 pSZ2407 PmFATA-hpG SEQ ID NO: 45
[0439] Upon independent transformation of Strain J with each of the constructs listed in Table 22, positive clones were selected on agar plates containing sucrose as a sole carbon source. Individual transformants were clonally purified and propagated under heterotrophic conditions suitable for lipid production as those detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass and analyzed using standard fatty acid methyl ester gas chromatography flame ionization detection methods as described in Example 1 (also see PCT/US2012/023696). The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis (UTEX 1435) Strain J propagated on glucose as a sole carbon source and representative isolates of each transformation of Strain J, propagated on sucrose as a sole carbon source, are presented in Table 23.
TABLE-US-00025 TABLE 23 Fatty acid profiles of Prototheca moriformis (UTEX 1435) cells engineered to express hairpin RNA constructs targeting fatty acyl-ACP thioesterase gene/gene products. Area % Fatty Acid Construct C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Strain J untransformed 0 0.05 1.32 26.66 3.1 59.07 7.39 PmFATA-hpB 0.04 0.07 1.36 24.88 2.24 61.92 6.84 0 0.08 1.33 25.34 2.39 61.72 6.5 0 0.07 1.29 25.44 2.26 61.7 6.69 0 0.06 1.33 25.1 2.37 61.56 6.87 PmFATA-hpC 0 0.08 1.18 22.03 1.71 63.8 8.63 0 0.07 1.21 24.5 2.23 62.32 7.19 0 0.08 1.29 24.93 2.24 62.02 7.01 0.05 0.06 1.29 25.45 2.26 61.81 6.76 PmFATA-hpD 0 0.02 0.68 15.8 1.88 72.64 6.96 0 0.03 0.78 17.56 1.7 71.8 6.03 0 0.03 0.92 19.04 2.03 68.82 7.05 0 0.04 1.27 23.14 2.25 65.27 6.07 PmFATA-hpE 0 0.03 0.79 18.55 2.13 69.66 6.77 0 0.04 1.11 21.01 1.74 65.18 8.55 0 0.03 1.08 21.11 1.54 64.76 8.87 0 0.03 1.17 21.93 1.71 63.89 8.77 PmFATA-hpF 0.03 0.04 0.34 8.6 1.69 78.08 8.87 0 0.03 0.49 10.2 1.52 76.97 8.78 0 0.03 1 20.47 2.22 66.34 7.45 0 0.03 1.03 21.61 1.88 65.39 7.76 PmFATA-hpG 0 0.03 1.03 20.57 2.36 64.73 8.75 0 0.03 1.2 24.39 2.47 61.9 7.49 0 0.04 1.29 24.14 2.29 61.41 8.22
[0440] The data presented in Table 23 show a clear impact of the expression of FATA hairpin RNA constructs on the C18:0 and C18:1 fatty acid profiles of the transformed organism. The fatty acid profiles of Strain J transformants comprising a FATA hairpin RNA construct demonstrated an increase in the percentage of C18:1 fatty acids with a concomitant diminution of C16:0 and C18:0 fatty acids. Fatty acid profiles of the untransformed Strain J are about 26.66% C16:0, 3% C18:0, and about 59% C18:1 fatty acids. In contrast, the fatty acid profiles of the transformed strains comprise as low as 8.6% C16:0 and 1.54% C18:0 and greater than 78% C18:1 fatty acids.
[0441] These data illustrate the utility and successful use of polynucleotide FATA RNA hairpin constructs in Prototheca moriformis to alter the fatty acids profile of engineered microbes, and in particular in increasing the concentration of C18:1 fatty acids and decreasing C18:0 and C16:0 fatty acids in microbial cells.
Example 37
Engineering Microorganisms for Increased Production of Mid-Chain Fatty Acids Through KASI or KASIV Overexpression
[0442] This example describes the use of recombinant polynucleotides that encode KASI or KASIV enzymes to engineer microorganisms in which the fatty acid profiles of the transformed microorganisms have been enriched in lauric acid, C10:0, and total saturated fatty acids.
[0443] Each of the constructs pSZD1132, pSZD1133, pSZD1134, or pSZD1201 was used independently to transform Strain B of Example 3, Prototheca moriformis (UTEX 1435) engineered to express a Cuphea wrightii thioesterase (CwTE2), according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Each of the above constructs included different polynucleotides encoding a KASI or KASIV enzyme, 5' (SEQ ID NO: 13) and 3' (SEQ ID NO: 14) homologous recombination targeting sequences (flanking the construct) to the pLoop genomic region for integration into the nuclear genome, and a neomycin resistance protein-coding sequence under the control of the C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and the Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This NeoR expression cassette is listed as SEQ ID NO: 15 and served as a selectable marker. Sequence listing identities for the polynucleotides corresponding to each construct are listed in Table 20. The polynucleotide sequence encoding each KAS enzyme was under the control of the P. moriformis UTEX 1435 Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). The protein coding regions of the KAS enzymes and neomycin resistance gene were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/U52009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0444] Upon transformation of individual plasmids into Strain B, positive clones were selected on agar plates comprising G418. Individual transformants were clonally purified and grown on sucrose as a sole carbon source at pH 7.0 under conditions suitable for lipid production as detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass from each transformant and fatty acid profiles from these samples were analyzed using standard fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1. The fatty acid profiles (expressed as Area % of total fatty acids) of Strain B and four positive transformants of each of pSZ2046 (Strains M-P, 1-4) are presented in Table 24.
TABLE-US-00026 TABLE 24 Plasmid constructs used to transform Prototheca moriformis (UTEX 1435) Strain B. Plasmid KASI/KASIV Transit construct source peptide SEQ ID NO: pSZD1134 Cuphea wrightii Native SEQ ID NO: 46 GenBank Accession No. U67317 pSZD1201 Cuphea wrightii PmSAD SEQ ID NO: 47 pSZD1132 Cuphea pulcherrima Native SEQ ID NO: 48 GenBank Accession No. AAC68860 pSZD1133 Cuphea hookeriana Native SEQ ID NO: 49
TABLE-US-00027 TABLE 25 Fatty acid profiles of Prototheca moriformis (UTEX 1435) Strain B engineered for increased C10, lauric acid, and total saturated fatty acids. Fatty Acid (Area %) % Plasmid C10- Saturates/ construct(s) No. C10 C12 C14 C16 C18:0 C18:1 C18:2 C12 Total pSZ1283 7.89 35.49 16.58 11.5 1.09 19.64 6.49 43.38 72.55 pSZ1283, 1 14.94 43.97 12.19 7.56 0.72 14.11 5.31 58.91 79.38 pSZD1134 pSZ1283, 2 10.27 39.61 15.35 9.61 0.94 17.1 5.88 49.88 75.78 pSZD1134 pSZ1283, 3 11.69 41.83 15.21 8.77 0.83 15.04 5.40 53.52 78.33 pSZD1134 D1134-20 4 10.76 40.77 15.32 9.19 0.88 16.06 5.76 51.53 76.92 pSZ1283, 1 10.77 40.31 15.21 9.43 0.88 16.18 5.97 51.08 76.6 pSZD1132 pSZ1283, 2 9.19 37.03 15.02 10.52 1.00 19.63 6.29 46.22 72.76 pSZD1132 pSZ1283, 3 8.97 36.09 15.01 10.77 1.05 20.38 6.39 45.06 71.89 pSZD1132 pSZ1283, 4 9.51 38.12 14.96 9.96 0.94 18.93 6.32 47.63 73.49 pSZD1132 pSZ1283, 1 13.06 46.21 9.84 7.12 0.75 16.7 5.22 59.27 76.98 pSZD1201 pSZ1283, 2 11.02 43.91 13.01 7.78 0.86 16.53 5.77 54.93 76.58 pSZD1201 pSZ1283, 3 11.59 45.14 12.41 7.61 0.82 15.72 5.65 56.73 77.57 pSZD1201 pSZ1283, 4 10.66 41.32 13.74 8.75 0.68 18.64 5.21 51.98 75.15 pSZD1201 pSZ1283, 1 6.90 36.08 15.15 11.02 1.00 21.74 6.77 42.98 70.15 pSZD1133 pSZ1283, 2 7.01 35.88 15.01 10.75 1.07 22.02 6.93 42.89 69.72 pSZD1133 pSZ1283, 3 10.65 41.94 12.38 8.48 0.85 18.28 6.15 52.59 74.3 pSZD1133 pSZ1283, 4 10.23 41.88 12.58 8.52 0.82 18.48 6.22 52.11 74.03 pSZD1133
[0445] The data presented in Table 25 show a clear impact of the exogenous expression of KASI and KASIV enzymes on the C10:0 and C12 fatty acid profiles of the transformed organism. The fatty acid profiles of Strain B, expressing the Cuphea wrightii thioesterase alone, comprised about 8% C10:0 and about 35.5% C12:0, with saturated fatty acids accounting for 72.55% of total fatty acids. In contrast, transformants of Strain B engineered to additionally express a Cuphea wrightii KASI with a P. moriformis stearoyl ACP desaturase transit peptide were characterized by a fatty acid profile of about 13% C10:0 and about 46% C12:0. Saturated fatty acids accounted for as high as 77% in transformants of Strain B co-expressing the C. wrightii KASI fusion protein. Similarly, transformants of Strain B engineered to express the C. wrightii KASI with the enzyme's native transit peptide were characterized by a fatty acid profile of about 15% C10, about 44% C12, and about 79% saturated fatty acids. The fatty acid profiles or many transformants of Strain B expressing either Cuphea pulcherrima KASIV or Cuphea hookeriana KASIV also displayed elevated C10% and C12% levels, compared to the fatty acid profile of Strain B itself.
[0446] These data demonstrate the utility and effectiveness of polynucleotides enabling expression of KASI and KASIV constructs in Prototheca moriformis (UTEX 1435) to alter the percentage of saturated fatty acids in the engineered host microbes, and in particular in increasing the concentration of C10:0 and C12:0 fatty acids in microbial cells.
Example 38
Engineering Microorganisms for Increased Production of Mid-Chain Fatty Acids Through KASI Knockout
[0447] This example describes the use of recombinant polynucleotides that disrupt different KASI alleles to engineer microorganisms in which the fatty acid profiles of the transformed microorganisms have been enriched in C10:0 and midchain fatty acids.
[0448] Constructs pSZ2302 and pSZ2304 were used to independently transform Strain B of Example 3, Prototheca moriformis (UTEX 1435) engineered to express a Cuphea wrightii thioesterase (CwTE2), according to biolistic transformation methods as described in PCT/U52009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. pSZ2302 included 5' (SEQ ID NO: 50) and 3' (SEQ ID NO: 51) homologous recombination targeting sequences (flanking the construct) to the KAS1 allele 1 genomic region for integration into the P. moriformis nuclear genome, anA. thaliana THIC protein coding region under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). pSZ2304 included 5' (SEQ ID NO: 52) and 3' (SEQ ID NO: 53) homologous recombination targeting sequences (flanking the construct) to the KAS1 allele 2 genomic region for integration into the P. moriformis nuclear genome, anA. thaliana THIC protein coding region under the control of the C. protothecoides actin promoter/5'UTR (SEQ ID NO: 22) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6).This AtTHIC expression cassette is listed as SEQ ID NO: 23 and served as a selection marker. The protein coding region of AtTHIC was codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0449] Upon independent transformation pSZ2302 and pSZ2304 into Strain B, thereby generating Strain Q and R, positive clones were selected on agar plates comprising thiamine. Individual transformants were clonally purified and cultivated on sucrose as a sole carbon source at pH 5.0 or pH 7.0 under heterotrophic conditions suitable for lipid production as detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass from each transformant and fatty acid profiles from these samples were analyzed using fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1. The fatty acid profiles (expressed as Area % of total fatty acids) of Strain B and positive pSZ2302 (Strain Q, 1-5) and pSZ2304 (Strain R, 1-5) transformants are presented in Tables 26 and 27.
TABLE-US-00028 TABLE 26 Fatty acid profiles of Prototheca moriformis (UTEX 1435) Strains B, Q, and R engineered for increased midchain fatty acids, cultured at pH 5.0. Trans- Fatty Acid (Area %) formation C10- Strain plasmid(s) C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 C14 UTEX 1435 None 0.00 0.04 1.28 26.67 3.05 59.96 7.19 1.32 Strain B pSZ1283 0.01 0.09 1.09 21.60 2.21 65.15 7.94 1.19 Strain Q-1 pSZ1283, 0.08 1.21 7.52 38.71 1.38 38.32 8.75 8.81 pSZ2302 Strain Q-2 pSZ1283, 0.15 1.36 7.51 38.23 1.33 38.27 8.94 9.02 pSZ2302 Strain Q-3 pSZ1283, 0.16 1.43 7.49 38.88 1.30 37.58 8.73 9.08 pSZ2302 Strain Q-4 pSZ1283, 0.00 1.71 7.42 37.67 1.43 37.26 10.38 9.13 pSZ2302 Strain Q-5 pSZ1283, 0.13 1.21 7.36 38.81 1.31 38.07 8.71 8.7 pSZ2302 Strain R-1 pSZ1283, 0.19 1.78 8.47 40.11 1.34 33.46 9.98 10.44 pSZ2304 Strain R-2 pSZ1283, 0.90 8.00 7.78 28.96 1.15 30.26 17.14 16.68 pSZ2304 Strain R-3 pSZ1283, 0.26 3.58 7.77 34.98 1.56 32.86 14.60 11.61 pSZ2304 Strain R-4 pSZ1283, 1.64 13.50 7.61 21.38 0.90 36.13 14.73 22.75 pSZ2304 Strain R-5 pSZ1283, 1.03 9.63 7.56 25.61 1.00 31.70 18.23 18.22 pSZ2304
TABLE-US-00029 TABLE 27 Fatty acid profiles of Prototheca moriformis (UTEX 1435), Strains B, Q, and R engineered for increased midchain fatty acids, cultured at pH 7.0. Fatty Acid (Area %) Transformation C10- Strain plasmid(s) C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 C14 UTEX None 0.01 0.04 1.34 27.94 3.24 57.46 7.88 1.39 1435 Strain B pSZ1283 4.72 29.57 15.56 12.63 1.20 27.65 7.39 49.85 Strain Q-1 pSZ1283, pSZ2302 16.00 50.61 9.52 5.33 0.54 11.79 5.28 76.13 Strain Q-2 pSZ1283, pSZ2302 16.32 49.79 9.82 5.52 0.54 12.28 4.87 75.93 Strain Q-3 pSZ1283, pSZ2302 15.08 47.58 10.23 5.93 0.56 15.12 4.50 72.89 Strain Q-4 pSZ1283, pSZ2302 14.27 47.30 10.44 6.17 0.56 15.50 4.59 72.01 Strain Q-5 pSZ1283, pSZ2302 14.75 47.28 10.32 6.04 0.59 15.50 4.65 72.35 Strain R-1 pSZ1283, pSZ2304 21.25 55.42 7.97 3.65 0.00 5.46 5.66 84.64 Strain R-2 pSZ1283, pSZ2304 13.00 55.05 10.88 5.78 0.28 7.90 6.29 78.93 Strain R-3 pSZ1283, pSZ2304 12.89 53.15 11.11 6.13 0.00 9.87 6.13 77.15 Strain R-4 pSZ1283, pSZ2304 12.80 51.64 13.86 6.69 0.00 7.51 6.70 78.3 Strain R-5 pSZ1283, pSZ2304 16.61 51.42 9.84 5.27 0.33 11.15 4.79 77.87
[0450] The data presented in Tables 26 and 27 show a clear impact of disruption of different KASI alleles on the fatty acid profiles of the transformed organisms. When cultivated at pH 5.0, the fatty acid profiles of Prototheca moriformis (UTEX 1435) and Prototheca moriformis (UTEX 1435) Strain B, expressing a Cuphea wrightii FATB2 thioesterase under control of a pH regulatable promoter were very similar. These profiles were characterized by about 1% C14:0, about 21-26% C16:0, about 2-3% C18:0, about 60-65% C18:1, about 7% C18:2, with C10-C14 fatty acids comprising about 1.19-1.3% of total fatty acids. In contrast, when cultivated at pH 5.0, Strain B further engineered to disrupt KASI allele 1 (Strain Q) or KASI allele 2 (Strain R) demonstrated altered fatty acid profiles that were characterized by increased levels of C12, increased levels of C14, decreased levels of C18, and decreased levels of C18:1 fatty acids compared to Strain B or UTEX 1435. The fatty acid profiles of isolates of Strains Q and R differed in that Strain R (allele 2 knockout) isolates had generally greater C12s and lower C16s and C18:1s than Strain Q (allele 1 knockout).
[0451] When cultivated at pH 7.0, the fatty acid profile of Prototheca moriformis (UTEX 1435) is distinct from that Prototheca moriformis (UTEX 1435) Strain B expressing a Cuphea wrightii FATB2 thioesterase under control of a pH regulatable promoter. When cultured at pH 7.0, Strain B was characterized by a fatty acid profile elevated in C10, C12, and C14 fatty acids (these comprised about 50% of the total fatty acids). When cultured at pH 7.0, Strain Q and Strain R demonstrated fatty acid profiles with still further increases in C10, C12, and C14 fatty acids and still further decreases in C18:0 and C18:1 fatty acids relative to that of Strain B. Again, differences in fatty acid profiles between Strain Q and R were observed with the profile of Strain R comprising greater percentage levels of C12 and lower levels of C18:1 than that of Strain Q.
[0452] These data illustrate the successful expression and use of polynucleotides enabling expression of KASI and KASIV constructs in Prototheca moriformis to alter the percentage of saturated fatty acids in the engineered host microbes, and in particular in increasing the concentration of C10:0 and C12:0 fatty acids and decreasing the concentration of C18:0 and C18:1 fatty acids in microbial cells. In addition, the data here indicate the different KASI alleles can be disrupted to result in altered fatty acid profiles of the transformed organisms.
Example 39
Engineering Microorganisms for Increased Production of Mid-Chain Fatty Acids Through KASI Knockdown
[0453] This example describes the use of recombinant polynucleotides that encode RNA hairpins to attenuate a KASI enzyme to engineer a microorganism in which the fatty acid profile of the transformed microorganism has been enriched in midchain fatty acids.
[0454] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain S, was transformed independently with each of the constructs pSZ2482-pSZ2485 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Each of the constructs pSZ2482-pSZ2485 included different polynucleotides encoding hairpin RNAs targeted against Prototheca moriformis (UTEX 1435) KASI mRNA transcripts to down-regulate the expression of fatty acyl-ACP thioesterase, 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4) to express the protein sequence given in SEQ ID NO: 3 under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. Sequence listing identities for the polynucleotides corresponding to each KASI hairpin are listed in Table 28. The polynucleotide sequence encoding each RNA hairpin was under the control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). The protein coding region of the suc2 expression cassette was codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
TABLE-US-00030 TABLE 28 Plasmid constructs used to transform Prototheca moriformis (UTEX 1435) Strain S. Transformation construct Hairpin SEQ ID NO: pSZ2482 KASI hairpin B SEQ ID NO: 54 pSZ2483 KASI hairpin C SEQ ID NO: 55 pSZ2484 KASI hairpin D SEQ ID NO: 56 pSZ2485 KASI hairpin E SEQ ID NO: 57
[0455] Upon independent transformation of Strain S with each of the constructs listed in Table 28, positive clones were selected on agar plates containing sucrose as a sole carbon source. Individual transformants were clonally purified and propagated under heterotrophic conditions suitable for lipid production as those detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass and analyzed using fatty acid methyl ester gas chromatography flame ionization detection methods as described in Example 1 (also see PCT/US2012/023696). The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis UTEX 1435 propagated on glucose as a sole carbon source and four representative isolates of each transformation of Strain S, propagated on sucrose as a sole carbon source, are presented in Table 29.
TABLE-US-00031 TABLE 29 Fatty acid profiles of Prototheca moriformis (UTEX 1435) cells engineered to express hairpin RNA constructs targeting KASI gene/gene products. Fatty Acid (Area %) Strain Plasmid Number C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 UTEX 1435 none 1 0.00 0.04 1.45 27.97 3.18 58.35 6.78 0.60 Strain S pSZ2482 1 0.19 0.74 8.47 38.30 2.15 36.24 9.45 1.42 2 0.07 0.25 4.16 32.46 2.62 49.57 7.73 0.82 3 0.03 0.10 2.68 27.48 2.65 56.40 8.14 0.55 4 0.03 0.10 2.60 27.44 2.01 55.54 9.15 0.78 pSZ2483 1 0.00 0.06 1.94 30.58 1.55 53.26 9.31 0.76 2 0.20 0.05 1.76 28.01 2.31 56.61 8.70 0.60 3 0.00 0.06 1.60 24.38 2.65 58.25 9.93 1.15 4 0.00 0.04 1.56 26.65 2.96 60.06 6.92 0.52 pSZ2484 1 0.72 3.71 19.15 38.03 1.68 14.22 15.00 4.21 2 0.66 2.76 16.34 38.19 1.78 18.52 14.91 3.38 3 0.69 2.96 16.20 37.28 1.77 19.05 15.26 3.48 4 0.18 0.70 8.61 36.80 2.35 36.22 10.89 1.10 pSZ2485 1 0.00 0.04 1.41 25.34 3.16 60.12 7.78 0.48 2 0.03 0.04 1.41 23.85 2.19 61.23 8.75 0.67 3 0.00 0.04 1.41 24.41 2.23 60.64 8.69 0.67 4 0.00 0.04 1.41 24.51 2.16 60.85 8.91 0.66
[0456] The data presented in Table 29 show a clear impact of the expression of KAS hairpin RNA constructs on the fatty acid profiles of the transformed organisms. The fatty acid profiles of Strain S transformants comprising either pSZ2482 or pSZ2484 KASI hairpin RNA construct demonstrated an increase in the percentage of C10, C12, C14, and C16 fatty acids with a concomitant diminution of C18:0 and C18:1 fatty acids relative to the fatty acid profile of UTEX 1435.
[0457] These data illustrate the utility and successful use of polynucleotide KASI RNA hairpin constructs in Prototheca moriformis (UTEX 1435) to alter the fatty acids profile of engineered microbes, and in particular in increasing the concentration of midchain fatty acids and decreasing C18:0 and C18:1 fatty acids in microbial cells.
Example 40
Engineering Microorganisms for Increased Production of Stearic Acid Through Fatty Acid Elongase Overexpression
[0458] This example describes the use of recombinant polynucleotides that encode fatty acid elongases to engineer a microorganism in which the fatty acid profile of the transformed microorganism has been enriched in stearic acid, arachidic acid, and docosadienoic acid.
[0459] A classically mutagenized strain of Prototheca moriformis (UTEX 1435), Strain J, was transformed independently with each of the constructs pSZ2323, pSZ2324, or pSZ2328 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Each of the constructs included a protein coding region to overexpress an elongase, 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4) to express the protein sequence given in SEQ ID NO: 3 under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. Sequence listing identities for the polynucleotides corresponding to each elongase are listed in Table 30. The polynucleotide sequence encoding each elongase was under control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). The protein coding regions of the exogenous elongases and the suc2 expression cassette were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
TABLE-US-00032 TABLE 30 Plasmid constructs used to transform Prototheca moriformis (UTEX 1435) Strain J. Plasmid GenBank construct Elongase source Accession No. SEQ ID NO: pSZ2328 Marchantia polymorpha AAP74370 58, 59 pSZ2324 Trypanosoma brucei AAX70673 60, 61 pSZ2323 Saccharomyces cerevisiae P39540 62, 63
[0460] Upon independent transformation of Strain J with the constructs listed in Table 30, positive clones were selected on agar plates containing sucrose as a sole carbon source. Individual transformants were clonally purified and propagated under heterotrophic conditions suitable for lipid production as those detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass and analyzed using fatty acid methyl ester gas chromatography flame ionization detection methods as described in Example 1 (also see PCT/US2012/023696). The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis UTEX 1435 Strain J propagated on glucose as a sole carbon source and three representative isolates of each transformation of Strain J, propagated on sucrose as a sole carbon source are presented in Table 31.
TABLE-US-00033 TABLE 31 Fatty acid profiles of Prototheca moriformis (UTEX 1435) Strain J cells engineered to overexpress elongases. Fatty Acid Area % Plasmid C14: C16: C16: C18: C18: C18: C18: C20: C22: construct No. 0 0 1 0 1 2 3.alpha. 0 2n6 None 1 1.39 27.42 0.77 3.33 57.46 8.05 0.61 0.30 0.03 pSZ2328 1 1.25 19.23 0.85 8.26 57.54 9.34 0.79 0.73 0.94 pSZ2328 2 1.22 17.76 0.69 7.86 60.56 9.38 0.59 0.6 0.47 pSZ2328 3 1.26 18.37 0.92 7.83 58.77 10.01 0.72 0.64 0.52 pSZ2324 1 1.51 22.97 1.09 8.71 53.01 9.63 0.65 0.68 0.55 pSZ2324 2 1.29 20.6 0.92 7.53 56.97 9.92 0.73 0.64 0.43 pSZ2324 3 1.28 20.59 0.93 7.33 57.52 9.68 0.65 0.58 0.42 pSZ2323 1 1.65 27.27 0.67 3.56 56.68 8.72 0.33 0.36 0.00 pSZ2323 2 1.56 28.44 0.74 3.36 55.22 9.07 0.46 0.39 0.03 pSZ2323 3 1.64 28.7 0.75 3.34 55.29 8.59 0.49 0.36 0.02
[0461] The data presented in Table 31 show a clear impact of the expression of Marchantia polymorpha and Trypanosoma brucei enzymes on the C14, C16, C18:0, C20:0, and C22:2n6 fatty acid profiles of the transformed organisms. The fatty acid profile of untransformed Strain J was about 27.42% C16:0, about 3% C18:0, about 57.5% C18:1, about 0.3% C20:0 and about 0.03% C22:2n6 fatty acids. In contrast to that of Strain J, the fatty acid profiles of Strain J transformed with different plasmid constructs to express elongases comprised lower percentage levels of C16 and higher percentage levels of C18:0, C20:0, and C22:2n6 fatty acids. The result of overexpression of Marchantia polymorpha elongase was about a 2.5 fold increase in percentage levels of C18:0 fatty acids, a 2 fold increase in percentage levels of C20:0 fatty acids, and about a 15 to 30 fold increase in percentage levels of C22:2n6 fatty acids relative to the fatty acid profile of Strain J.
[0462] These data illustrate the successful use of polynucleotides encoding elongases for expression in Prototheca moriformis (UTEX 1435) to alter the fatty acid profile of engineered microbes, and in particular in increasing the concentration of C18:0, C20:0, and C22:2n6 fatty acids and decreasing C16:0 fatty acids in recombinant microbial cells.
Example 41
Engineering Microorganisms for Increased Production of Stearic Acid Through Acyl-ACP Thioesterase Overexpression
[0463] This example describes the use of recombinant polynucleotides that encode different C18:0-preferring acyl-ACP thioesterases to engineer microorganisms in which the fatty acid profiles of the transformed microorganisms have been enriched in stearic acid.
[0464] Classically mutagenized strains of Prototheca moriformis (UTEX 1435), Strain J or Strain A, were transformed independently with the constructs listed in Table 32 according to biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Each of the constructs included a protein coding region to overexpress a fatty acyl-ACP thioesterase with a C-terminal 3.times. FLAG.RTM. epitope tag, 5' (SEQ ID NO: 1) and 3' (SEQ ID NO: 2) homologous recombination targeting sequences (flanking the construct) to the 6S genomic region for integration into the nuclear genome, and a S. cerevisiae suc2 sucrose invertase coding region (SEQ ID NO: 4) to express the protein sequence given in SEQ ID NO: 3 under the control of C. reinhardtii .beta.-tubulin promoter/5'UTR (SEQ ID NO: 5) and Chlorella vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). This S. cerevisiae suc2 expression cassette is listed as SEQ ID NO: 7 and served as a selectable marker. Sequence listing identities for the polynucleotides corresponding to each thioesterase are listed in Table 32. The polynucleotide sequence encoding each thioesterase was under control of the P. moriformis Amt03 promoter/5'UTR (SEQ ID NO: 8) and C. vulgaris nitrate reductase 3' UTR (SEQ ID NO: 6). The protein coding regions of the exogenous thioesterases and the suc2 expression cassette were codon optimized to reflect the codon bias inherent in P. moriformis UTEX 1435 nuclear genes as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
TABLE-US-00034 TABLE 32 Plasmid constructs used to transform Prototheca moriformis (UTEX 1435) Strain A or Strain J. Acyl-ACP Thioesterase, Acyl-ACP Transit Plasmid GenBank Thioesterase Peptide construct Accession No. source source SEQ ID NO: pSZD581 FATA, Brassica napus native 64, 65 CAA52070 pSZD643 FATA, Brassica napus UTEX 66, 67 CAA52070 250 SAD pSZD645 FATA, C. tinctorius UTEX 68, 69 AAA33019 250 SAD pSZD644 FATA, Ricinis communis native 70, 71 ABS30422 pSZD1323 FATA, G. mangostana native 72, 73 AAB51523 pSZD1320 FATA Theobroma cacao native 74, 75
[0465] Upon independent transformation of Strain A or J with the constructs listed in Table 32, positive clones were selected on agar plates containing sucrose as a sole carbon source. Individual transformants were clonally purified and propagated under heterotrophic conditions suitable for lipid production as those detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples were prepared from dried biomass and analyzed using fatty acid methyl ester gas chromatography flame ionization detection methods as described in Example 1 (also see PCT/US2012/023696). The fatty acid profiles (expressed as Area % of total fatty acids) of P. moriformis UTEX 1435 Strain J propagated on glucose as a sole carbon source and representative isolates of each transformation of Strain J, propagated on sucrose as a sole carbon source are presented in Table 33.
TABLE-US-00035 TABLE 33 Fatty acid profiles of Prototheca moriformis (UTEX 1435) Strain J cells engineered to overexpress exogenous acyl-ACP thioesterase enzymes. Plasmid Fatty Acid Area % Strain construct No. C14:0 C16:0 C18:0 C18:1 C18:2 C18:3.alpha. A None 1 1.08 25.48 3.23 59.70 8.25 0.70 J None 1 1.41 27.33 3.38 57.07 8.15 0.64 A pSZD581 1 1.02 26.60 14.47 44.80 10.05 0.65 2 1.08 28.24 13.57 43.89 10.07 0.68 3 0.97 24.70 9.13 50.85 11.27 0.82 A pSZD643 1 1.39 26.97 16.21 44.10 8.43 0.83 2 1.37 27.91 11.15 48.31 8.40 0.78 A pSZD645 1 0.90 23.39 8.35 50.69 13.34 0.96 A pSZD644 1 1.67 19.70 4.40 59.15 12.32 1.01 J pSZD1323 1 1.33 23.26 9.28 53.42 10.35 0.69 2 1.47 26.84 7.36 52.78 9.29 0.64 3 1.43 26.31 6.05 54.45 9.37 0.66 J pSZD1320 1 1.30 24.76 3.84 60.90 6.96 0.55 2 1.36 26.30 3.27 58.19 8.66 0.48 3 1.39 25.51 3.18 58.78 8.85 0.45
[0466] The data presented in Table 33 show a clear impact of the expression of exogenous acyl-ACP enzymes on the fatty acid profiles of the transformed microorganisms. The fatty acid profiles of untransformed Strain A and J were about 25% C16:0, about 3.3% C18:0, about 57 to 60% C18:1. In contrast, the fatty acid profiles of Strain A transformed with different plasmid constructs to express acyl-ACP enzymes comprised greater percentage levels of C18:0 and lower percentage levels of C18:1 fatty acids than that of Strain A. Expression of FATA enzymes from B. napus, C. tinctorius, R. communis and G. mangostana in Strain A or J enabled the accumulation of stearate levels in the transformed organisms. The result of overexpression of a Brassica napus acyl-ACP thioesterase was about a 2 to 5 fold increase in the percentage levels of C18:0 fatty acids of the fatty acid profile of the transformed organisms relative to the fatty acid profile of Strain A. Fatty acid profiles of cells engineered to overexpress a G. mangostana acyl-ACP FATA thioesterase with a C. protothecoides SAD1 transit peptide were characterized by about a 2 to 3 fold increase in the percentage levels of C18:0 fatty acids of the fatty acid profile of the transformed organism relative to the fatty acid profile of Strain J.
[0467] These data illustrate the utility and effective use of polynucleotides encoding fatty acyl-ACP thioesterases for expression in Prototheca moriformis (UTEX 1435) to alter the fatty acid profile of engineered microbes, and in particular in increasing the concentration of C18:0 and decreasing C18:1 fatty acids in recombinant microbial cells.
Example 42
Engineering Microorganisms for Increased Production of Erucic Acid Through Elongase or Beta-Ketoacyl-COA Synthase Overexpression
[0468] In an embodiment of the present invention, a recombinant polynucleotide transformation vector operable to express an exogenous elongase or beta-ketoacyl-CoA synthase in an optionally plastidic oleaginous microbe is constructed and employed to transform Prototheca moriformis (UTEX 1435) according to the biolistic transformation methods as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696 to obtain a cell increased for production of erucic acid. The transformation vector includes a protein coding region to overexpress an elongase or beta-ketoacyl-CoA synthase such as those listed in Table 5, promoter and 3'UTR control sequences to regulate expression of the exogenous gene, 5' and 3' homologous recombination targeting sequences targeting the recombinant polynucleotides for integration into the P. moriformis (UTEX 1435) nuclear genome, and nucleotides operable to express a selectable marker. The protein-coding sequences of the transformation vector are codon-optimized for expression in P. moriformis (UTEX 1435) as described in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Recombinant polynucleotides encoding promoters, 3' UTRs, and selectable markers operable for expression in P. moriformis (UTEX 1435) are disclosed herein and in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696.
[0469] Upon transformation of the transformation vector into P. moriformis (UTEX 1435) or a classically-mutagenized strain of P. moriformis (UTEX 1435), positive clones are selected on agar plates. Individual transformants are clonally purified and cultivated under heterotrophic conditions suitable for lipid production as detailed in PCT/US2009/066141, PCT/US2009/066142, PCT/US2011/038463, PCT/US2011/038464, and PCT/US2012/023696. Lipid samples are prepared from dried biomass from each transformant and fatty acid profiles from these samples are analyzed using fatty acid methyl ester gas chromatography flame ionization (FAME GC/FID) detection methods as described in Example 1. As a result of these manipulations, the cell may exhibit an increase in erucic acid of at least 5, 10, 15, or 20 fold.
Example 43
Generation Of Capric, Lauric, and Myristic Acid Rich Oils in Strain UTEX1435 by the Expression of Cuphea PSR23 LPAATs
[0470] We tested the effect of expression of two 1-acyl-sn-glycerol-3-phosphate acyltransferases (LPAATs) in a previously described P. moriformis (UTEX 1435) transgenic strain, expressing the acyl ACP thioesterase (FATB2) from Cuphea wrightii. The LPAAT2 and LPAAT3 genes from Cuphea PSR23 (CuPSR23) were identified by analysis of a combination of CuPSR23 genomic sequences and transcriptomic sequences derived from seed RNAs. The two LPAATs have not been previously described. The genes were codon optimized to reflect UTEX 1435 codon usage. Transformations, cell culture, lipid production and fatty acid analysis were all carried out as previously described.
[0471] Increased capric, lauric, and myristic accumulation in strain B by the expression of the Cuphea PSR23 1-acyl-sn-glycerol-3-phosphate acyltransferases (LPAAT2 and LPAAT3) [pSZ2299 and pSZ2300, respectively]: In this example, transgenic strains were generated via transformation of strain B with the constructs pSZ2299 or pSZ2300, encoding CuPSR23 LPAAT2 and LPAAT3, respectively. The transgenic strains were selected for resistance to the antibiotic G418. Construct pSZ2299 can be written as pLOOP5'::CrTUB2:NeoR:CvNR::PmAMT3:CuPSR23LPAAT2-1:CvNR::pLOOP3'. Construct pSZ2300 can be written as pLOOP5'::CrTUB2:NeoR:CvNR::PmAMT3:CuPSR23LPAAT3-1:CvNR::pLOOP3'. The sequence of the transforming DNA (pSZ2299 and pSZ2300) is provided below. The relevant restriction sites in the construct from 5'-3', BspQI, KpnI, XbaI, Mfe I, BamHI, EcoRI, SpeI, XhoI, SacI, BspQI, respectively, are indicated in lowercase, bold, and underlined. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences at the 5' and 3' end of the construct represent genomic DNA from UTEX 1435 that target integration to the pLoop locus via homologous recombination. Proceeding in the 5' to 3' direction, the selection cassette has the C. reinhardtii .beta.-tubulin promoter driving expression of the NeoR gene (conferring resistance to G418) and the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. The promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for NeoR are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR is indicated by lowercase underlined text. The spacer region between the two cassettes is indicated by upper case text. The second cassette containing the codon optimized LPAAT2 gene (pSZ2299) or LPAAT3 gene (pSZ2300) from Cuphea PSR23 is driven by the Prototheca moriformis endogenous AMT3 promoter, and has the same Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. In this cassette, the AMT3 promoter in indicated by lowercase, boxed text. The initiator ATG and terminator TGA for the CuPSR23 LPAAT2 and LPAAT3 genes are indicated in uppercase italics, while the coding regions are indicated by lowercase italics. The 3' UTR is indicated by lowercase underlined text. The final constructs were sequenced to ensure correct reading frames and targeting sequences.
TABLE-US-00036 pSZ2299 Transforming Construct: (SEQ ID NO:90) gctcttccgctaacggaggtctgtcaccaaatggaccccgtctattgcgggaaaccacggcgatggcacgtttc- aaaacttgat gaaatacaatattcagtatgtcgcgggcggcgacggcggggagctgatgtcgcgctgggtattgcttaatcgcc- agcttcgccc ccgtcttggcgcgaggcgtgaacaagccgaccgatgtgcacgagcaaatcctgacactagaagggctgactcgc- ccggcacggc tgaattacacaggcttgcaaaaataccagaatttgcacgcaccgtattcgcggtattttgttggacagtgaata- gcgatgcggc aatggcttgtggcgttagaaggtgcgacgaaggtggtgccaccactgtgccagccagtcctggcggctcccagg- gccccgatca agagccaggacatccaaactacccacagcatcaacgccccggcctatactcgaaccccacttgcactctgcaat- ggtatgggaa ##STR00002## ccgcctgggtggagcgcctgttcggctacgactgggcccagcagaccatcggctgctccgacgccgccgtgttc- cgcctgtccg cccagggccgccccgtgctgttcgtgaagaccgacctgtccggcgccctgaacgagctgcaggacgaggccgcc- cgcctgtcct ggctggccaccaccggcgtgccctgcgccgccgtgctggacgtggtgaccgaggccggccgcgactggctgctg- ctgggcgagg tgcccggccaggacctgctgtcctcccacctggcccccgccgagaaggtgtccatcatggccgacgccatgcgc- cgcctgcaca ccctggaccccgccacctgccccttcgaccaccaggccaagcaccgcatcgagcgcgcccgcacccgcatggag- gccggcctgg tggaccaggacgacctggacgaggagcaccagggcctggcccccgccgagctgttcgcccgcctgaaggcccgc- atgcccgacg gcgaggacctggtggtgacccacggcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccggc- ttcatcgact gcggccgcctgggcgtggccgaccgctaccaggacatcgccctggccacccgcgacatcgccgaggagctgggc- ggcgagtggg ccgaccgcttcctggtgctgtacggcatcgccgcccccgactcccagcgcatcgccttctaccgcctgctggac- gagttcttcT GAcaattggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgcca- cacttgctgc cttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttg- cgagttgcta gctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgc- aacttatcta cgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccg- cctgtattct cctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatgga- ggatccCGCG TCTCGAACAGAGCGCGCAGAGGAACGCTGAAGGTCTCGCCTCTGTCGCACCTCAGCGCGGCATACACCACAATA- ACCACCTGAC GAATGCGCTTGGTTCTTCGTCCATTAGCGAAGCGTCCGGTTCACACACGTGCCACGTTGGCGAGGTGGCAGGTG- ACAATGATCG ##STR00003## cctgatcatcaacctgttccaggcgctgtgcttcgtcctgatccgccccctgtccaagaacgcctaccgccgca- tcaaccgcgt gttcgcggagctgctgctgtccgagctgctgtgcctgttcgactggtgggcgggcgcgaagctgaagctgttca- ccgaccccga gacgttccgcctgatgggcaaggagcacgccctggtcatcatcaaccacatgaccgagctggactggatggtgg- gctgggtgat gggccagcacttcggctgcctgggctccatcatctccgtcgccaagaagtccacgaagttcctgcccgtgctgg- gctggtccat gtggttctccgagtacctgtacctggagcgctcctgggccaaggacaagtccaccctgaagtcccacatcgagc- gcctgatcga ctaccccctgcccttctggctggtcatcttcgtcgagggcacccgcttcacgcgcacgaagctgctggcggccc- agcagtacgc ggtctcctccggcctgcccgtcccccgcaacgtcctgatcccccgcacgaagggcttcgtctcctgcgtgtccc- acatgcgctc cttcgtccccgcggtgtacgacgtcacggtggcgttccccaagacgtcccccccccccacgctgctgaacctgt- tcgagggcca gtccatcatgctgcacgtgcacatcaagcgccacgccatgaaggacctgcccgagtccgacgacgccgtcgcgg- agtggtgccg cgacaagttcgtcgagaaggacgccctgctggacaagcacaacgcggaggacacgttctccggccaggaggtgt- gccactccgg ctcccgccagctgaagtccctgctggtcgtgatctcctgggtcgtggtgacgacgttcggcgccctgaagttcc- tgcagtggtc ctcctggaagggcaaggcgttctccgccatcggcctgggcatcgtcaccctgctgatgcacgtgctgatcctgt- cctcccaggc cgagcgctccaaccccgccgaggtggcccaggccaagctgaagaccggcctgtccatctccaagaaggtgacgg- acaaggagaa cTGAttaattaactcgaggcagcagcaptcggatagtatcgacacactctggacgctggtcgtgtgatggactg- ttgccgccac acttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtac- gcgcttttgc gagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcat- cccaaccgca acttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtt- tgggctccgc ctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaac- acaaatggaa agcttgagctcagcggcgacggtcctgctaccgtacgacgttgggcacgcccatgaaagtttgtataccgagct- tgttgagcga actgcaagcgcggctcaaggatacttgaactcctggattgatatcggtccaataatggatggaaaatccgaacc- tcgtgcaaga actgagcaaacctcgttacatggatgcacagtcgccagtccaatgaacattgaagtgagcgaactgttcgcttc- ggtggcagta ctactcaaagaatgagctgctgttaaaaatgcactctcgttctctcaagtgagtggcagatgagtgctcacgcc- ttgcacttcg ctgcccgtgtcatgccctgcgccccaaaatttgaaaaaagggatgagattattgggcaatggacgacgtcgtcg- ctccgggagt caggaccggcggaaaataagaggcaacacactccgcttcttagctcttcg pSZ2300 Transforming Construct: (SEQ ID NO:91) gctcttccgctaacggaggtctgtcaccaaatggaccccgtctattgcgggaaaccacggcgatggcacgtttc- aaaacttgat gaaatacaatattcagtatgtcgcgggcggcgacggcggggagctgatgtcgcgctgggtattgcttaatcgcc- agcttcgccc ccgtcttggcgcgaggcgtgaacaagccgaccgatgtgcacgagcaaatcctgacactagaagggctgactcgc- ccggcacggc tgaattacacaggcttgcaaaaataccagaatttgcacgcaccgtattcgcggtattttgttggacagtgaata- gcgatgcggc aatggcttgtggcgttagaaggtgcgacgaaggtggtgccaccactgtgccagccagtcctggcggctcccagg- gccccgatca agagccaggacatccaaactacccacagcatcaacgccccggcctatactcgaaccccacttgcactctgcaat- ggtatgggaa ##STR00004## gccgcctgggtggagcgcctgttcggctacgactgggcccagcagaccatcggctgctccgacgccgccgtgtt- ccgcctgtcc gcccagggccgccccgtgctgttcgtgaagaccgacctgtccggcgccctgaacgagctgcaggacgaggccgc- ccgcctgtcc tggctggccaccaccggcgtgccctgcgccgccgtgctggacgtggtgaccgaggccggccgcgactggctgct- gctgggcgag gtgcccggccaggacctgctgtcctcccacctggcccccgccgagaaggtgtccatcatggccgacgccatgcg- ccgcctgcac accctggaccccgccacctgccccttcgaccaccaggccaagcaccgcatcgagcgcgcccgcacccgcatgga- ggccggcctg gtggaccaggacgacctggacgaggagcaccagggcctggcccccgccgagctgttcgcccgcctgaaggcccg- catgcccgac ggcgaggacctggtggtgacccacggcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccgg- cttcatcgac tgcggccgcctgggcgtggccgaccgctaccaggacatcgccctggccacccgcgacatcgccgaggagctggg- cggcgagtgg gccgaccgcttcctggtgctgtacggcatcgccgcccccgactcccagcgcatcgccttctaccgcctgctgga- cgagttcttc TGAcaattggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgcc- acacttgctg ccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgctttt- gcgagttgct agctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccg- caacttatct acgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctcc- gcctgtattc tcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatgg- aggatccCGC GTCTCGAACAGAGCGCGCAGAGGAACGCTGAAGGTCTCGCCTCTGTCGCACCTCAGCGCGGCATACACCACAAT- AACCACCTGA CGAATGCGCTTGGTTCTTCGTCCATTAGCGAAGCGTCCGGTTCACACACGTGCCACGTTGGCGAGGTGGCAGGT- GACAATGATC ##STR00005## tgatcgtcaacctggtgcaggccgtctgcttcgtcctgatccgccccctgtccaagaacacgtaccgccgcatc- aaccgcgtgg tcgcggagctgctgtggctggagctggtgtggctgatcgactggtgggcgggcgtgaagatcaaggtcttcacg- gaccacgaga cgttccacctgatgggcaaggagcacgccctggtcatctgcaaccacaagtccgacatcgactggctggtcggc- tgggtcctgg gccagcgctccggctgcctgggctccaccctggcggtcatgaagaagtcctccaagttcctgcccgtcctgggc- tggtccatgt ggttctccgagtacctgttcctggagcgctcctgggccaaggacgagatcacgctgaagtccggcctgaaccgc- ctgaaggact accccctgcccttctggctggcgctgttcgtggagggcacgcgcttcacccgcgcgaagctgctggcggcgcag- cagtacgccg cgtcctccggcctgcccgtgccccgcaacgtgctgatcccccgcacgaagggcttcgtgtcctccgtgtcccac- atgcgctcct tcgtgcccgcgatctacgacgtcaccgtggccatccccaagacgtcccccccccccacgctgatccgcatgttc- aagggccagt cctccgtgctgcacgtgcacctgaagcgccacctgatgaaggacctgcccgagtccgacgacgccgtcgcgcag- tggtgccgcg acatcttcgtggagaaggacgcgctgctggacaagcacaacgccgaggacaccttctccggccaggagctgcag- gagaccggcc gccccatcaagtccctgctggtcgtcatctcctgggccgtcctggaggtgttcggcgccgtcaagttcctgcag- tggtcctccc tgctgtcctcctggaagggcctggcgttctccggcatcggcctgggcgtgatcaccctgctgatgcacatcctg- atcctgttct cccagtccgagcgctccacccccgccaaggtggcccccgcgaagcccaagaacgagggcgagtcctccaagacc- gagatggaga
aggagaagTGAttaattaactcgaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtg- atggactgtt gccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatctt- gtgtgtacgc gcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatcccatccctcgtttcatatcg- cttgcatccc aaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagc- cttggtttgg gctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtggga- tgggaacaca aatggaaagcttgagctcagcggcgacggtcctgctaccgtacgacgttgggcacgcccatgaaagtttgtata- ccgagcttgt tgagcgaactgcaagcgcggctcaaggatacttgaactcctggattgatatcggtccaataatggatggaaaat- ccgaacctcg tgcaagaactgagcaaacctcgttacatggatgcacagtcgccagtccaatgaacattgaagtgagcgaactgt- tcgcttcggt ggcagtactactcaaagaatgagctgctgttaaaaatgcactctcgttctctcaagtgagtggcagatgagtgc- tcacgcctgc acttcgctgcccgtgtcatgccctgcgccccaaaatttgaaaaaagggatgagattattgggcaatggacgacg- tcgtcgctcc gggagtcaggaccggcggaaaataagaggcaacacactccgcttcttagctcttcg
[0472] To determine the impact of the CuPSR23 LPAAT2 and LPAAT3 genes on mid-chain fatty acid accumulation, the above constructs containing the codon optimized CuPSR23 LPAAT2 or LPAAT3 genes driven by the UTEX 1453 AMT3 promoter were transformed into strain B.
[0473] Primary transformants were clonally purified and grown under standard lipid production conditions at pH7.0 (all the strains require growth at pH 7.0 to allow for maximal expression of the CuPSR23 LPAAT2 or LPAAT3 gene driven by the pH-regulated AMT3 promoter). The resulting profiles from a set of representative clones arising from these transformations are shown in Table 34, below. D1520 represents clones of Strain B with CuPSR23 LPAAT2 and D1521 represents clones of Strain B with CuPSR23 LPAAT3.
TABLE-US-00037 TABLE 34 Fatty acid profiles of Strain B and representative transgenic lines transformed with pSZ2299 and pSZ2300 DNA. Sample C10: C12: C14: C16: C18: C18: C18: ID 0 0 0 0 0 1 2 Strain B 4.83 28.54 15.64 12.64 1.3 27.99 7.75 D1520-A 8.59 35.09 16.55 11.96 1.69 19.49 5.59 D1520-B 8.13 33.93 16.46 12.44 1.57 20.66 5.96 D1520-C 7.6 33.1 16.21 12.65 1.5 21.41 6.48 D1520-D 7.35 32.54 16.03 12.79 1.67 22.16 6.41 D1520-E 7.28 32.21 16.2 12.99 1.73 22.39 6.28 D1521-A 6.14 31.5 15.98 12.96 1.96 22.52 8 D1521-B 6.17 31.38 15.98 12.87 2.08 22.54 7.92 D1521-C 5.99 31.31 15.75 12.79 2.23 22.45 8.36 D1521-D 5.95 31.05 15.71 12.84 2.48 22.69 8.32 D1521-E 5.91 30.58 15.85 13.22 1.97 23.55 7.84
[0474] The transgenic CuPSR23 LPAAT2 strains (D1520A-E) show a significant increase in the accumulation of C10:0, C12:0, and C14:0 fatty acids with a concomitant decrease in C18:1 and C18:2. The transgenic CuPSR23 LPAAT3 strains (D1521A-E) show a significant increase in the accumulation of C10:0, C12:0, and C14:0 fatty acids with a concomitant decrease in C18:1. The expression of the CuPSR23 LPAAT in these transgenic lines appears to be directly responsible for the increased accumulation of mid-chain fatty acids in general, and especially laurates. While the transgenic lines show a shift from longer chain fatty acids (C16:0 and above) to mid-chain fatty acids, the shift is targeted predominantly to C10:0 and C12:0 fatty acids with a slight effect on C14:0 fatty acids. The data presented also show that co-expression of the LPAAT2 and LPAAT3 genes from Cuphea PSR23 and the FATB2 from C. wrightii (expressed in the strain Strain B) have an additive effect on the accumulation of C12:0 fatty acids.
[0475] Our results suggest that the LPAAT enzymes from Cuphea PSR23 are active in the algal strains derived from UTEX 1435. These results also demonstrate that the enzyme functions in conjunction with the heterologous FatB2 acyl-ACP thioesterase enzyme expressed in Strain B, which is derived from Cuphea wrightii.
Example 44
Alteration of Fatty Acid Levels in Strain UTEX1435 by the Expression of Cuphea PSR23 LPAATX in Combination with Cuphea Wrightii FATB2
[0476] Here we demonstrate the effect of expression of a 1-acyl-sn-glycerol-3-phosphate acyltransferase (LPAAT) in a previously described P. moriformis (UTEX 1435) transgenic strain, Strain B. As described above, Strain B is a transgenic strain expressing the acyl ACP thioesterase (FATB2) from Cuphea wrightii, which accumulates C12:0 fatty acids between 40 to 49%. Further to Example 43, a third CuPSR23 LPAAT, LPAATx, was identified by analysis of a combination of CuPSR23 genomic sequences and transcriptomic sequences derived from seed RNAs. Expression of a mid-chain specific LPAAT should thus increase the percentage of TAGs that have a capric acid (C10:0 fatty acid), lauric acid (C12:0 fatty acid), or myristic acid (C14:0 fatty acid) at the sn-2 position, and should consequently elevate the overall levels of these fatty acids. In Example 43, LPAAT2 and LPAAT3 were shown to increase caprate, laurate, and myristate accumulation in strain B. LPAATx was introduced into strain B to determine its effect on fatty acid levels in this strain. The LPAATx gene was codon optimized to reflect UTEX 1435 codon usage. Transformations, cell culture, lipid production and fatty acid analysis were all carried out as previously described.
[0477] Decreased caprate, laurate, and myristate accumulation and increased palmitate and stearate accumulation in strain Strain B by the expression of the Cuphea PSR23 1-acyl-sn-glycerol-3-phosphate acyltransferase (LPAATx) [pSZ2575]: In this example, transgenic strains were generated via transformation of strain B with the construct pSZ2575 encoding CuPSR23 LPAATx. The transgenic strains were selected for resistance to the antibiotic G418. Construct pSZ2575 can be written as pLOOP5'::CrTUB2:NeoR:CvNR::PmAMT3:CuPSR23LPAATx:CvNR ::pLOOP3'. The sequence of the transforming DNA is provided below (pSZ2575). The relevant restriction sites in the construct from 5'-3', BspQI, KpnI, XbaI, MfeI, BamHI, EcoRI, SpeI, XhoI, SacI, BspQ1, respectively, are indicated in lowercase, bold, and underlined. BspQ1 sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences at the 5' and 3' end of the construct represent genomic DNA from UTEX 1435 that target integration to the pLoop locus via homologous recombination. Proceeding in the 5' to 3' direction, the selection cassette has the C. reinhardtii, .beta.-tubulin promoter driving expression of the NeoR gene (conferring resistance to G418) and the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. The promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for NeoR are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR is indicated by lowercase underlined text. The spacer region between the two cassettes is indicated by upper case text. The second cassette containing the codon optimized LPAATx gene (pSZ2575) from Cuphea PSR23 is driven by the Prototheca moriformis endogenous AMT3 promoter, and has the same Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. In this cassette, the AMT3 promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for the CuPSR23 LPAATx genes are indicated in uppercase italics, while the coding region is indicated by lowercase italics. The 3' UTR is indicated by lowercase underlined text. The final construct was sequenced to ensure correct reading frame and targeting sequences.
[0478] 104961 pSZ2575 Transforming Construct
TABLE-US-00038 (SEQ ID NO: 92) gctcttccgctaacggaggtctgtcaccaaatggaccccgtctattgcgggaaaccacggcgatggcacgtttc- aaaacttgat gaaatacaatattcagtatgtcgcgggcggcgacggcggggagctgatgtcgcgctgggtattgcttaatcgcc- agcttcgcc cccgtcttggcgcgaggcgtgaacaagccgaccgatgtgcacgagcaaatcctgacactagaagggctgactcg- cccggca cggctgaattacacaggcttgcaaaaataccagaatttgcacgcaccgtattcgcggtattttgttggacagtg- aatagcgatg cggcaatggcttgtggcgttagaaggtgcgacgaaggtggtgccaccactgtgccagccagtcctggcggctcc- cagggccc cgatcaagagccaggacatccaaactacccacagcatcaacgccccggcctatactcgaaccccacttgcactc- tgcaatggt ##STR00006## ##STR00007## ##STR00008## ##STR00009## ##STR00010## cctgggtggagcgcctgttcggctacgactgggcccagcagaccatcggctgctccgacgccgccgtgttccgc- ctgtccgccca gggccgccccgtgctgttcgtgaagaccgacctgtccggcgccctgaacgagctgcaggacgaggccgcccgcc- tgtcctggct ggccaccaccggcgtgccctgcgccgccgtgctggacgtggtgaccgaggccggccgcgactggctgctgctgg- gcgaggtgc ccggccaggacctgctgtcctcccacctggcccccgccgagaaggtgtccatcatggccgacgccatgcgccgc- ctgcacaccc tggaccccgccacctgccccttcgaccaccaggccaagcaccgcatcgagcgcgcccgcacccgcatggaggcc- ggcctggtg gaccaggacgacctggacgaggagcaccagggcctggcccccgccgagctgttcgcccgcctgaaggcccgcat- gcccgacg gcgaggacctggtggtgacccacggcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccggc- ttcatcgactg cggccgcctgggcgtggccgaccgctaccaggacatcgccctggccacccgcgacatcgccgaggagctgggcg- gcgagtgg gccgaccgcttcctggtgctgtacggcatcgccgcccccgactcccagcgcatcgccttctaccgcctgctgga- cgagttcttcTG Acaattggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccac- acttgctgccttga cctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagt- tgctagctgcttgtgctattt gcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgt- cctgctatccctcagcg ctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaa- cctgtaaaccagcactg caatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatccCGCGTCTCGAACAGAGCGCGCA GAGGAACGCTGAAGGTCTCGCCTCTGTCGCACCTCAGCGCGGCATACACCACAA TAACCACCTGACGAATGCGCTTGGTTCTTCGTCCATTAGCGAAGCGTCCGGTTCA CACACGTGCCACGTTGGCGAGGTGGCAGGTGACAATGATCGGTGGAGCTGATGG ##STR00011## ##STR00012## ##STR00013## ##STR00014## ##STR00015## ##STR00016## ##STR00017## ##STR00018## ##STR00019## ##STR00020## ##STR00021## ##STR00022## ##STR00023## ctagtATGgagatccccccccactgcctgtgctccccctcccccgccccctcccagctgtactacaagaagaag- aagcacgcc atcctgcagacccagaccccctaccgctaccgcgtgtcccccacctgcttcgcccccccccgcctgcgcaagca- gcacccctacc ccctgcccgtgctgtgctaccccaagctgctgcacttctcccagccccgctaccccctggtgcgctcccacctg- gccgaggccggc gtggcctaccgccccggctacgagctgctgggcaagatccgcggcgtgtgcttctacgccgtgaccgccgccgt- ggccctgctgct gttccagtgcatgctgctgctgcaccccttcgtgctgctgttcgaccccttcccccgcaaggcccaccacacca- tcgccaagctgtg gtccatctgctccgtgtccctgttctacaagatccacatcaagggcctggagaacctgccccccccccactccc- ccgccgtgtacgt gtccaaccaccagtccttcctggacatctacaccctgctgaccctgggccgcaccttcaagttcatctccaaga- ccgagatcttcctg taccccatcatcggctgggccatgtacatgctgggcaccatccccctgaagcgcctggactcccgctcccagct- ggacaccctga agcgctgcatggacctgatcaagaagggcgcctccgtgttcttcttccccgagggcacccgctccaaggacggc- aagctgggcg ccttcaagaagggcgccttctccatcgccgccaagtccaaggtgcccgtggtgcccatcaccctgatcggcacc- ggcaagatcat gccccccggctccgagctgaccgtgaaccccggcaccgtgcaggtgatcatccacaagcccatcgagggctccg- acgccgagg ccatgtgcaacgaggcccgcgccaccatctcccactccctggacgacTGAttaattaactcgaggcagcagcap- tcggatagt atcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatcc- ctgccgcttttatcaaa cagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaatacc- acccccagcatccccttcc ctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgct- cctgctcactgcccctcgc acagcttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcac- gggaagtagtgggat gggaacacaaatggaaagcttgagctcagcggcgacggtcctgctaccgtacgacgttgggcacgcccatgaaa- gffigtatac cgagcttgttgagcgaactgcaagcgcggctcaaggatacttgaactcctggattgatatcggtccaataatgg- atggaaaat ccgaacctcgtgcaagaactgagcaaacctcgttacatggatgcacagtcgccagtccaatgaacattgaagtg- agcgaact gttcgcttcggtggcagtactactcaaagaatgagctgctgttaaaaatgcactctcgttctctcaagtgagtg- gcagatgagtg ctcacgccttgcacttcgctgcccgtgtcatgccctgcgccccaaaatttgaaaaaagggatgagattattggg- caatggacga cgtcgtcgctccgggagtcaggaccggcggaaaataagaggcaacacactccgcttcttagctcttcg
[0479] To determine the impact of the CuPSR23 LPAATx gene on fatty acid accumulation, the above construct containing the codon optimized CuPSR23 LPAATx gene driven by the UTEX 1453 AMT3 promoter was transformed into strain B.
[0480] Primary transformants were clonally purified and grown under low nitrogen conditions at pH7.0; the strains require growth at pH 7.0 to allow for maximal expression of the CuPSR23 LPAATx and CwFATB2 genes driven by the pH-regulated AMT3 promoter. The resulting profiles from a set of representative clones arising from these transformations are shown in Table 35, below. D1542 represents clones of Strain B with CuPSR23 LPAATx.
TABLE-US-00039 TABLE 35 Fatty acid profiles of Strain B and representative transgenic lines transformed with pSZ2575. Sample ID C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Strain 4.77 28.63 15.48 12.65 1.28 28.20 7.57 B D1542- 1.19 13.25 10.48 21.34 4.49 32.07 14.78 A D1542- 1.15 14.01 10.62 20.61 3.99 32.12 15.24 B D1542- 1.21 13.69 10.83 20.40 3.59 33.54 15.05 C D1542- 1.56 16.83 11.51 18.44 2.94 33.97 12.74 D D1542- 2.15 18.58 11.94 18.22 3.17 32.63 11.62 E
[0481] The transgenic CuPSR23 LPAATx strains (D1542A-E) show a significant decrease in the accumulation of C10:0, C12:0, and C14:0 fatty acids relative to the parent, Strain B, with a concomitant increase in C16:0, C18:0, C18:1 and C18:2. The expression of the CuPSR23 LPAATx gene in these transgenic lines appears to be directly responsible for the decreased accumulation of mid-chain fatty acids (C10-C14) and the increased accumulation of C16:0 and C18 fatty acids, with the most pronounced increase observed in palmitates (C16:0). The data presented also show that despite the expression of the midchain specific FATB2 from C. wrightii (present in Strain B), the expression of CuPSR23 LPAATx appears to favor incorporation of longer chain fatty acids into TAGs.
[0482] Our results suggest that the LPAATx enzyme from Cuphea PSR23 is active in the algal strains derived from UTEX 1435. Contrary to Cuphea PSR23 LPAAT2 and LPAAT3, which increase mid-chain fatty acid levels, CuPSR23 LPAATx leads to increased C16:0 and C18:0 levels. These results demonstrate that the different LPAATs derived from CuPSR23 (LPAAT2, LPAAT3, and LPAATx) exhibit different fatty acid specificities in Strain B as judged by their effects on overall fatty acid levels.
Example 45
Reduction in Chain Length of Fatty Acid Profile as a Result of Overexpressing an Endogenous Microalgal FATA Acyl-ACP Thioesterase
[0483] Here, we demonstrate that over expression of the Prototheca moriformis endogenous thioesterases FATA1 in UTEX1435 results in a clear diminution of cell triglyceride C18:0 and C18:1 acyl chains with an increase in C16:0, C14:0.
[0484] Constructs used for the over expression of the P. moriformis FATA1 gene (pSZ2422, pSZ2421): To over express the PmFATA1 in P. moriformis STRAIN J, a codon optimized PmFATA1 gene was been transformed into STRAIN J. The Saccharomyces cerevisiae invertase gene was utilized as the selectable marker to confer the ability of growing on sucrose media. The construct pSZ2422 that have been expressed in STRAIN J can be written as: 6SA:: CrTUB2-ScSUC2-CvNR3':PmAMT3-Pm FATA1 (opt)-CvNR3'::6SB, and the construct pSZ2421 can be written as 6SA:: CrTUB2-ScSUC2-CvNR3':PmAMT3-S106SAD TP-Pm FATA1 (opt)-CvNR3'::6SB.
[0485] The sequence of the transforming DNA is provided below. Relevant restriction sites in the construct pSZ2422 are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from STRAIN J that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene (conferring the ability of STRAIN J to metabolize sucrose) is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous amt03 promoter of P. moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the PmFATA1 are indicated by uppercase, bold italics, while the remainder of the gene is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the STRAIN J 6S genomic region indicated by bold, lowercase text.
[0486] Relevant restriction sites in the construct pSZ2421 are the same as pSZ2422. In pSZ2421, the PmFATA1 is fused to the Chlorella protothecoides S106 stearoyl-ACP desaturase transit peptide and the transit peptide is located between initiator ATG of PmFATA1 and the Asc I site.
[0487] Nucleotide sequence of transforming DNA contained in pSZ2422
TABLE-US-00040 (SEQ ID NO: 93) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtc gctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaaga- ggagcatga gggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgc- accgaggc cgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatga- attgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgctt cgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgca- gaccggtgag agccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcat- cggcctcggcc ##STR00024## ##STR00025## ##STR00026## ##STR00027## ##STR00028## cgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgacc- ccaacggcc tgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacg- cccttgttctg gggccacgccacgtccgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgact- ccggcgc cttctccggctccatggtggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagc- gctgcgtggcca tctggacctacaacaccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcacc- gagtaccaga agaaccccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaag- tggatcatgac cgcggccaagtcccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccg- cgttcgccaa cgagggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagt- cctactgggt gatgttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacg- gcacccacttcg aggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacacc- gacccgaccta cgggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgct- cctccatgtccc tcgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgag- ccgatcctg aacatcagcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgt- cgacctgtc caacagcaccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgt- tcgcggacctc tccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctcctt- cttcctggaccgc gggaacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagccctt- caagagcg agaacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggc- gacgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcgac aagttccaggtgcgcgaggtcaagTGAcaattggcagcagcaptcggatagtatcgacacactctggacgctgg- tcgtgtgat ggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgt- ttgatcttgtgtgtacgcg cttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcg- cttgcatcccaaccgcaac ttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttg- ggctccgcctgtattctcc tggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggagg- atcccgcgtctcg aacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccac- ctgacgaatgcg cttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatga- tcggtggagctgatg ##STR00029## ##STR00030## ##STR00031## ##STR00032## ##STR00033## ##STR00034## ##STR00035## ##STR00036## ##STR00037## ##STR00038## ##STR00039## ##STR00040## ##STR00041## ##STR00042## ##STR00043## ##STR00044## ##STR00045## ##STR00046## ##STR00047## ##STR00048## ##STR00049## ##STR00050## ##STR00051## ##STR00052## ##STR00053## ##STR00054## ##STR00055## ##STR00056## acttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtac- gcgatttgcgagttgctag ctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgca- acttatctacgctgtcctg ctatccctcagcgctgacctgacctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcc- tggtactgcaacctgt aaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaattaagagctc- ttgttttccagaa ggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcga- atttaaaagctt ggaatgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaa- acttgccgc tcaaaccgcgtacctctgattcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgag- cagtctgtaat tgcctcagaatgtggaatcatctgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgcca- ctcgtacag cagaccattatgctacctcacaatagttcataacagtgaccatatttctcgaagctccccaacgagcacctcca- tgctctgagtg gccaccccccggccctggtgcttgcggagggcaggtcaaccggcatggggctaccgaaatccccgaccggatcc- caccaccc ccgcgatgggaagaatctctccccgggatgtgggcccaccaccagcacaacctgctggcccaggcgagcgtcaa- accatacc acacaaatatccttggcatcggccctgaattccttctgccgctctgctacccggtgcttctgtccgaagcaggg- gttgctaggga tcgctccgagtccgcaaacccttgtcgcgtggcggggcttgttcgagcttgaagagc
[0488] To determine the impact on fatty acid profiles when the endogenous FATA1 gene have been over expressed in STRAIN J, both the P. moriformis FATA1 with native transit peptide and PmFATA1 fused to a Chlorella protothecoides SAD transit peptide were driven by the amt03 promoter and the resulting plasmids were transformed independently into STRAIN J.
[0489] Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at pH7.0 (all the plasmids require growth at pH 7.0 to allow for maximal PmFATA1 gene expression when driven by the pH regulated amt03 promoter). The resulting profiles from representative clones arising from transformations with pSZ2422 and pSZ2421 into STRAIN J are shown in the tables below.
[0490] In Table 36, below, the impact of over expressing native PmFATA1 is a clear diminution of C18:1 chain lengths with an increase in C16:0, C14:0, and possibly in C18:0. Considering the protein localization of processing, we also tried the PmFATA1 fused to a Chlorella protothecoides stearoyl-ACP desaturase transit peptide. Similar to the results we observed in the amt03-native PmFATA1 construct, the C16:0 and C14:0 levels are significantly higher than the parental strain J.
TABLE-US-00041 TABLE 36 Fatty acid profiles in Strain J and derivative transgenic lines transformed with pSZ2422 DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 pH 7; Strain J; T374; 7.69 55.00 4.92 24.94 5.19 D1377-7 96well pH 7; Strain J; T374; 6.39 54.11 5.85 25.91 5.76 D1377-13 96well pH 7; Strain J; T374; 6.57 53.55 4.68 27.18 5.74 D1377-14 96well pH 7; Strain J; T374; 5.29 49.93 4.24 30.76 7.27 D1377-16 96well pH 7; Strain J; T374; 4.76 49.10 4.75 32.36 6.77 D1377-9 96well pH 7; Strain J; T374; 4.28 46.06 5.14 35.87 6.69 D1377-19 96well Ctrl-pH 7; Strain J 1.42 27.63 3.31 57.20 8.00
TABLE-US-00042 TABLE 37 Fatty acid profiles in STRAIN J and derivative transgenic lines transformed with pSZ2421 DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 pH 7; STRAIN J; T374; 6.76 57.06 4.12 23.66 6.07 D1376-21 96well pH 7; STRAIN J; T374; 6.56 54.62 5.44 25.69 5.64 D1376-22 96well pH 7; STRAIN J; T374; 4.54 48.38 4.27 33.23 7.24 D1376-23 96well pH 7; STRAIN J; T374; 4.48 47.66 4.60 34.28 6.91 D1376-19 96well pH 7; STRAIN J; T374; 4.53 47.30 4.67 34.51 6.80 D1376-20 96well pH 7; STRAIN J; T374; 3.56 42.70 4.03 39.85 7.52 D1376-17 96well Ctrl-pH 7; STRAIN J 1.42 27.63 3.31 57.20 8.00
[0491] Thus, we conclude that percent myristic and lauric acid levels in the fatty acid profile of a microalgal cell can be increased by overexpression of a C18-preferring acyl-ACP thioesterase.
Example 46
Cell Oils Suitable for Use as Roll-In Shortenings
[0492] The nutritional and functional properties of edible fats have been traditionally associated with specific chemical compositions and crystallization conditions. Switching from one oil source to another is usually a difficult task since both the melting behavior and structure of the fat changes dramatically, leading to adverse changes in functionality. In recent history, we can recall the painful period when partially hydrogenated fats were replaced with palm oil and palm oil fractions. We examined how the yield stress, elastic modulus, polymorphism, microstructure and melting profile of two fats with vastly different chemical compositions can be matched. Oil A was produced from Prototheca moriformis cells expressing an exogenous invertase and an Ulmus americana acyl-ACP thioesterase with a Chlorella protothecoides plastid targeting sequence. Oil B was produced from Prototheca moriformis cells expressing an exogenous invertase and a Cuphea hookeriana acyl-ACP thioesterase. Oil A contained greater than 62% (w/w) medium chain fatty acids, or MCT (C8:0-C14:0), 23% (C16:0+C18:0) and 9% C18:1, while Oil B contained less than 2% C8:0-C14:0, 54% (C16:0+C18:0) and 29% C18:1. Oil A was thus a medium chain triglyceride rich fat, while Oil B resembled palm oil. Both oils had a solid fat content of .about.45% at 20.degree. C., and very similar SFC versus temperature profiles. DSC (dynamic scanning calorimetry) melting profiles showed two major peaks centered around .about.12-13.degree. C. and .about.28-35.degree. C. Both fats were in the beta-prime polymorphic form (as determined by X-ray diffraction) and displayed asymmetric, elongated crystallite morphology with characteristic features. The yield stresses and storage moduli (G') of Oil A and Oil B were 520-550 Pa, and 7.times.10.sup.6 Pa-1.8.times.10.sup.7 Pa, respectively. A yield stress in this region suggests a satisfactory plasticity, which combined with a high storage modulus makes for an ideal roll-in shortening. Thus, it is possible to alter the chemical composition of a food oil while retaining its lamination functionality.
[0493] Other suitable enzymes for use with the cells and methods of any of the above embodiments of the invention include those that have at least 70% amino acid identity with one of the proteins listed in the description above and that exhibit the corresponding desired enzymatic activity. In additional embodiments, the enzymatic activity is present in a sequence that has at least about 75%, at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% identity with one of the above described nucleic acid sequences, all of which are hereby incorporated by reference as if fully set forth.
Example 47
Fractionation to Remove Trisaturates from a Tailored Microbial Oil that is a Cocoa Butter Mimetic
[0494] A refined bleached and deodorized oil was obtained from Strain K4 (see Example 35). The oil was heated to 70.degree. C. and cooled at 0.5.degree. C. per min to 36.degree. C. and held at 36.degree. C. for 1 hour. An approximately 2.5 ml sample was then centrifuged at 36.degree. C. for 1 hour at 4300. A liquid supernatant was recovered and analyzed using lipase and mass spectrometry. The sample was found to be depleted in tristearin (SSS), SSP, and PPS. The triacylglycerols of the sample were found to be very similar to that of cocoa butter and the liquid supernatant was even closer to that of cocoa butter in terms of low amounts of trisaturates. Further fractionation experiments are described in Example 64.
TABLE-US-00043 TABLE 38 TAG profile of oil from the K4 strain before and after fractionation as compared to cocoa butter. fractionation upper TAG K4 oil layer (liquid) cocoa butter OOL (+?) 0.12 0.12 0.00 POL 0.23 0.31 0.33 PLP 2.41 3.38 1.58 MOP 0.93 1.25 0.00 PPM (+MMS) 0.42 0.29 0.00 OOO 0.23 0.34 0.00 SOL 0.36 0.47 0.32 OOP 0.95 1.42 2.44 PLS 5.66 7.90 2.90 POP (+MSO) 11.80 15.20 17.93 PPP + MPS 2.22 1.07 0.36 OOS 1.19 1.68 3.02 SLS (+PLA) 3.96 5.11 1.77 POS 27.22 32.80 40.25 PPS (+SSM) 6.47 1.52 0.49 MaOO 0.00 0.00 0.36 SLA 0.31 0.34 0.00 SOS (+POA) 17.84 22.50 24.93 SSP (+PPA) 9.24 0.96 0.63 SOA (+POB) 1.39 1.68 1.51 SSS (+PSA) 5.25 0.23 0.33 SOB + LgOP 0.38 0.44 0.27 SSA 0.41 0.00 0.00 SOLg 0.41 0.00 0.00 PSLg + ASB 0.26 0.00 0.00 SOHx 0.12 0.51 0.00 SSLg 0.21 0.14 0.15 SUM area % 100.00 99.67 99.57
Example 48
Production of High-Stearate Triglyceride Oil in an Oleaginous Cell by Overexpression of KASII, Knockout of One SAD Allele and Repression of a Second SAD Allele
[0495] The oleaginous, non-photosynthetic alga, Prototheca moriformis, stores copious amounts of triacylglyceride oil under conditions where the nutritional carbon supply is in excess, but cell division is inhibited due to limitation of other essential nutrients. Bulk biosynthesis of fatty acids with carbon chain lengths up to C18 occurs in the plastids; fatty acids are then exported to the endoplasmic reticulum where elongation past C18 and incorporation into triacylglycerides (TAGs) is believed to occur. Lipids are stored in large cytoplasmic organelles called lipid bodies until environmental conditions change to favor growth, whereupon they are rapidly mobilized to provide energy and carbon molecules for anabolic metabolism. Wild-type P. moriformis storage lipid is mainly comprised of .about.60% oleic (C18:1), .about.25-30% palmitic (C16:0), and .about.5-8% linoleic (C18:2) acids, with minor amounts of stearic (C18:0), myristic (C14:0), .alpha.-linolenic (C18:3.alpha.), and palmitoleic (C16:1) acids. This fatty acid profile results from the relative activities and substrate affinities of the enzymes of the endogenous fatty acid biosynthetic pathway. P. moriformis is amenable to manipulation of fatty acid and lipid biosynthesis using molecular genetic tools, enabling the production of oils with fatty acid profiles that are very different to the wild-type composition. Herein we describe strains where we have modified the expression of stearoyl-ACP desaturase (SAD) and .beta.-ketoacyl-ACP synthase II (KASII) genes in order to generate strains with up to 57% stearate and as little as 7% palmitate. We identify additional strains with up to 55% stearate and as low as 2.4% linoleate when we perform similar modifications in conjunction with down-regulating the expression of the FATA thioesterase and the FAD2 fatty acid desaturase genes.
[0496] Soluble SADs are plastid-localized, di-iron enzymes which catalyze the desaturation of acyl carrier protein (ACP)-bound stearate to oleate (C18:1 cis-.DELTA..sup.9). Previously, we have established that hairpin constructs targeting the SAD1 or SAD2 transcripts activate the cellular RNA interference (RNAi) machinery, down-regulating SAD activity and resulting in elevated levels of C18:0 in the storage lipid. SAD activity is also reduced in strains where we disrupt one of the two alleles of SAD2, encoding the major SADs that are expressed during storage lipid biosynthesis. The Fatty Acid Desaturase 2 (FAD2) gene encodes an endoplasmic reticulum membrane-associated desaturase that converts oleate to linoleate (C18:2 cis-.DELTA..sup.9, cis-.DELTA..sup.12). Hairpin RNAi constructs targeting FAD2 reduce linoleate levels to 1-2%. KASII is a fatty acid synthase which specifically catalyzes the condensation of malonyl-ACP with palmitoyl (C16:0)-ACP to form .beta.-keto-stearoyl-ACP. We have shown that overexpression of KASII in P. moriformis causes C16:0 levels to decrease with a concomitant increase in C18:1 abundance. In the examples below we demonstrate that by down-regulating SAD gene expression using RNAi, disrupting an allele of the SAD2 gene, and overexpressing the KASII fatty acid synthase, we generate strains capable of accumulating stearate in excess of 50% of the total fatty acids, and with SOS as the major TAG species. SOS levels increase up to 47% in strains which combine SAD2 and FAD2 down-regulation with KASII overexpression.
[0497] Constructs used for SAD2 knockout/RNAi in S1920: A DNA construct, pSZ2282, was made to simultaneously disrupt the SAD2-1 allele and express a SAD2 hairpin construct in Strain J. A Saccharomyces cerevisiae SUC2 gene, encoding sucrose invertase, which was codon-optimized for expression in P. moriformis, was utilized as a selectable marker for transformation. The sequence of the transforming DNA is provided immediately below. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, AscI, MfeI, BamHI, AvrII, EcoRV, EcoRI, SpeI, BamHI, HinDIII, and SacI, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the Chlamydomonas reinhardtii TUB2 promoter driving the expression of the Saccharomyces cerevisiae SUC2 gene (encoding sucrose hydrolyzing activity, thereby permitting the strain to grow on sucrose) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for SUC2 are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the Chlorella vulgaris nitrate reductase (NR) gene is indicated by small capitals, followed by a spacer region indicated by lowercase text. A second C. reinhardtii TUB2 promoter sequence, indicated by lowercase boxed text, drives expression of the SAD2 hairpin C sequence. The sense and antisense strands are indicated with uppercase, bold italics, and are separated by the P. moriformis .DELTA..sup.12-fatty acid desaturase (FAD2) intron and the first 10 bases of the FAD2 second exon (uppercase italics). A second C. vulgaris NR 3' UTR is indicated by small capitals.
TABLE-US-00044 Nucleotide sequence of the transforming DNA from pSZ2282: (SEQ ID NO: 94) gctcttcgggtcgccgcgctgcctcgcgtcccctggtggtgcgcgcggtcgccagcgaggccccgctgggcgtt- ccgccctcggtgca gcgcccctcccccgtggtctactccaagctggacaagcagcaccgcctgacgcccgagcgcctggagctggtgc- agagcatggggc agtttgcggaggagagggtgctgcccgtgctgcaccccgtggacaagctgtggcagccgcaggactttttgccc- gaccccgagtcgc ccgacttcgaggatcaggtggcggagctgcgcgcgcgcgccaaggacctgcccgacgagtactttgtggtgctg- gtgggggacatg atcacggaggaggcgctgccgacctacatggccatgctcaacacgctggacggcgtgcgcgacgacacgggcgc- ggccgaccacc cgtgggcgcgctggacgcggcagtgggtggccgaggagaaccggcacggcgacctgctgaacaagtactgctgg- ctgacggggc gcgtcaacatgcgggccgtggaggtgaccatcaacaacctgatcaagagcggcatgaacccgcagacggacaac- aacccttattt ##STR00057## ##STR00058## ##STR00059## ##STR00060## ##STR00061## gaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgt- ggtacgac gagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctg- gggccacg ccacgtccgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgcc- ttctccggc tccatggtggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggc- catctggacc tacaacaccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtacca- gaagaacc ccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtggatc- atgaccgcgg ccaagtcccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgcgttc- gccaacgag ggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtccta- ctgggtgat gttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggca- cccacttcgag gccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccga- cccgacctac gggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctc- ctccatgtccc tcgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgag- ccgatcct gaacatcagcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacg- tcgacctg tccaacagcaccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgt- gttcgcgga cctctccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcct- ccttcttcctgg accgcgggaacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccag- cccttca agagcgagaacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaac- gacggcga cgtcgtgtccaccaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtgg- acaacctgt tctacatcgacaagttccaggtgcgcgaggtcaagTGAcaattgGCAGCAGCAGCTCGGATAGTATCGACACAC- TCTGGAC GCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAA- CAGCCTCA GTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAG- CATCCCCT TCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCT- GCTCCTGCT CACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTG- CAATGCT GATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgcgtctcgaacagagcgcgcagaggaacgc- tgaaggt ctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgcttggttcttcgtccat- tagcgaagcgtccg gttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctgatggtcgaaacgttcacag- cctagggatatc ##STR00062## ##STR00063## ##STR00064## ##STR00065## ##STR00066## ##STR00067## ##STR00068## ##STR00069## TGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTGACCCCCCCGGCTACCCTCCCGGCACCTFCCAG ##STR00070## ##STR00071## ##STR00072## ##STR00073## CAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGA- CCTGTGA ATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGC- TGCTTGTG CTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTA- CGCTGTCCT GCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTC- TCCTGGTA CTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAAAGCTGg- agctc cagccacggcaacaccgcgcgccttgcggccgagcacggcgacaagaacctgagcaagatctgcgggctgatcg- ccagcgacga gggccggcacgagatcgcctacacgcgcatcgtggacgagttcttccgcctcgaccccgagggcgccgtcgccg- cctacgccaaca tgatgcgcaagcagatcaccatgcccgcgcacctcatggacgacatgggccacggcgaggccaacccgggccgc- aacctcttcgc cgacttctccgcggtcgccgagaagatcgacgtctacgacgccgaggactactgccgcatcctggagcacctca- acgcgcgctgga aggtggacgagcgccaggtcagcggccaggccgccgcggaccaggagtacgtcctgggcctgccccagcgcttc- cggaaactcgc cgagaagaccgccgccaagcgcaagcgcgtcgcgcgcaggcccgtcgccttctcctggatctccgggcgcgaga- tcatggtctagg gagcgacgagtgtgcgtgcggggctggcgggagtgggacgccctcctcgctcctctctgttctgaacggaacaa- tcggccaccccg cgctacgcgccacgcatcgagcaacgaagaaaaccccccgatgataggttgcggtggctgccgggatatagatc- cggccgcacat caaagggcccctccgccagagaagaagctcctttcccagcagactcctgaagagc
[0498] Identification and analysis of SAD2 knockout/knockdown strains: Construct D1283, derived from pSZ2282, was transformed into Strain J. Primary transformants were clonally purified and grown under standard lipid production conditions at pH 5. The resulting fatty acid profiles from representative clones arising from transformation with pSZ2282 into Strain J are summarized in Table 39, below. D1283 transformants accumulated up to .about.42% C18:0 at the expense of C18:1, indicating that SAD activity was significantly reduced in these strains.
TABLE-US-00045 TABLE 39 Fatty acid profiles of D1283 [pSZ2282] primary transformants, compared to the wild-type parental strain, Strain J. Strain J D1283-4 D1283-7 D1283-19 D1283-27 D1283-40 D1283-24 Fatty C12:0 0.04 0.05 0.06 0.07 0.06 0.04 0.05 Acid C14:0 1.31 0.92 1.07 1.01 1.08 1.03 0.96 Area % C16:0 26.68 28.23 29.21 27.24 27.67 27.02 27.07 C16:1 0.78 0.05 0.06 0.08 0.33 0.14 0.12 C17:0 0.11 0.12 0.15 0.10 0.10 0.12 0.13 C18:0 3.15 41.98 40.94 34.20 26.26 23.18 22.82 C18:l 59.30 19.37 18.17 26.87 34.77 38.74 39.38 C18:2 7.47 6.22 7.43 7.42 7.31 7.25 7.38 C18:3.alpha. 0.57 0.93 1.03 0.75 0.71 0.72 0.51 C20:0 0.32 1.81 1.67 1.75 1.35 1.36 1.23 C20:l 0.00 0.10 0.00 0.12 0.00 0.12 0.11 C22:0 0.05 0.17 0.13 0.20 0.16 0.16 0.15 C24:0 0.00 0.00 0.00 0.10 0.00 0.00 0.00 sum C18 70.49 68.5 67.57 69.24 69.05 69.89 70.09 saturates 31.66 73.28 73.23 64.67 56.68 52.91 52.41 unsaturates 68.12 26.67 26.69 35.24 43.12 46.97 47.50
[0499] In Table 39, Stearate (C18:0) levels greater than the wild-type level are highlighted with bold text.
[0500] The fatty acid profiles of transformants D1283-4 and -7 were determined to be stable after more than 30 generations of growth in the absence of selection (growth on sucrose). The performance of selected strains in shake flask assays was then evaluated, and the fatty acid profiles and lipid titers are presented in Table 40, below. Strain X had the highest level of C18:0 (.about.44%) and the best lipid titer (.about.26%) relative to the Strain J parent, and so was selected for further fermentation development.
TABLE-US-00046 TABLE 40 Fatty acid profiles and lipid titers of SAD2 knockout/knock- down strains derived from D1283 primary transformants, compared to the wild-type parental strain, Strain J. Primary T342; D1283-4 T342; D1283-7 Strain J S T U V W X Fatty C14:0 1.59 1.61 1.58 1.55 1.81 1.84 1.34 Acid C16:0 30.47 29.41 28.58 29.24 28.77 29.09 28.47 Area % C16:l 0.82 0.05 0.07 0.05 0.07 0.05 0.06 C17:0 0.10 0.30 0.29 0.28 0.46 0.37 0.19 C18:0 3.58 42.85 41.86 43.38 39.99 41.41 44.42 C18:l 56.96 13.52 15.55 13.49 13.57 12.98 15.64 C18:2 5.50 8.01 7.85 7.65 10.37 9.47 5.72 C18:3.alpha. 0.37 0.78 0.73 0.82 0.95 0.91 0.64 C20:0 0.22 2.06 2.11 2.11 1.98 1.98 2.32 C22:0 0.05 0.32 0.34 0.33 0.33 0.32 0.35 C24:0 0.03 0.43 0.42 0.44 0.49 0.49 0.37 lipid titer 100 12.3 12.6 13.6 6.2 8.2 25.9 (% parent)
[0501] In Table 40, Stearate (C18:0) levels greater than the wild-type level are highlighted with bold text.
[0502] We optimized the performance of Strain X in 7-L fermentations, and found that we could match the .about.44% C18:0 level obtained in shake flasks, with lipid productivities that were .about.45% of the wild-type parent. The fatty acid profiles and lipid titers of representative strain K-4 fermentations are summarized in Table 41, below. Fermentation of Strain X under optimal conditions yielded nearly 44% C18:0, which was similar to the stearate level that accumulated in shake flask assays. Strain X produced high C18:0 levels at both flask and 7-L scale and had acceptable lipid productivity in 7-L fermentations; consequently this strain was selected as a base strain for additional modifications aimed at increasing C18:0 accumulation.
TABLE-US-00047 TABLE 41 Fatty acid profiles and lipid titers of strain X, compared to a control transgenic strain Strain Y. Strain Strain Y K-4 K-4 K-4 Fermentation 110088F14 120489F5 120531F8 120580F1 Fatty Acid C14:0 1.47 1.18 1.15 1.27 Area % C16:0 25.66 28.68 28.38 28.35 C16:1 0.71 0.11 0.09 0.06 C18:0 3.16 41.63 42.40 43.67 C18:1 62.24 20.78 19.38 17.63 C18:2 5.90 5.06 5.38 5.58 C18:3.alpha. 0.16 0.24 0.25 0.25 C20:0 0.24 1.36 1.99 2.11 C22:0 0.05 0.19 0.28 0.31 C24:0 0.05 0.34 0.29 0.31 sum C18 71.46 67.71 67.41 67.13 saturates 30.63 73.38 74.49 76.02 unsaturates 69.01 26.19 25.10 23.52 total lipid (g/L) 930 383 539 475
[0503] In Table 41, Stearate (C18:0) levels greater than the control are highlighted with bold text. Strain Y contains S. cerevisiae SUC2, encoding sucrose invertase, integrated at the 6S locus, and has a fatty acid profile that is indistinguishable from the Strain J wild-type parent.
[0504] Constructs used for KASII overexpression in Strain K-4: DNA construct pSZ2734 was made to overexpress a codon-optimized P. moriformis KASII gene in Strain X. The neoR gene from transposon Tn5, conferring resistance to aminoglycoside antibiotics, was used as a selectable marker for transformation. The sequence of the transforming DNA is provided immediately below. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, XbaI, MfeI, BamHI, AvrII, EcoRV, SpeI, AscI, ClaI, BglII, AflII HinDIII and SacI, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the 6S locus. Proceeding in the 5' to 3' direction, the C. reinhardtii TUB2 promoter driving the expression of neoR (encoding aminoglycoside phosphotransferase activity, thereby permitting the strain to grow on G418) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for neoR are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the C. vulgaris NR gene is indicated by small capitals, followed by a spacer region indicated by lowercase text. The P. moriformis SAD2-2 promoter sequence, indicated by boxed text, drives expression of the codon-optimized P. moriformis KASII gene. The region encoding the KASII plastid targeting sequence is indicated by uppercase italics. The sequence that encodes the mature P. moriformis KASII polypeptide is indicated with bold, uppercase italics, while a 3.times. FLAG epitope encoding sequence is in bold, underlined, uppercase italics. A second C. vulgaris NR 3' UTR is indicated by small capitals.
TABLE-US-00048 Nucleotide sequence of the transforming DNA from pSZ2734: (SEQ ID NO: 95) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgct gatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagagga- gcatgagggagg actcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccgag- gccgcctccaact ggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggtgtatgaattgtacagaa- caaccacgagc cttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgaccct- cgctgccgccgctt ctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccgatct- gaggacagtcggg gaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgcagaccggtgagagccgacttgtt- gtgcgccacccccca caccacctcctcccagaccaattctgtcacctttttggcgaaggcatcggcctcggcctgcagagaggacagca- gtgcccagccgct ##STR00074## ##STR00075## ##STR00076## ##STR00077## tatcaATGatcgagcaggacggcctccacgccggctcccccgccgcctgggtggagcgcctgttcggctacgac- tgggcccag cagaccatcggctgctccgacgccgccgtgttccgcctgtccgcccagggccgccccgtgctgttcgtgaagac- cgacctgtccg gcgccctgaacgagctgcaggacgaggccgcccgcctgtcctggctggccaccaccggcgtgccctgcgccgcc- gtgctggac gtggtgaccgaggccggccgcgactggctgctgctgggcgaggtgcccggccaggacctgctgtcctcccacct- ggcccccgc cgagaaggtgtccatcatggccgacgccatgcgccgcctgcacaccctggaccccgccacctgccccttcgacc- accaggcca agcaccgcatcgagcgcgcccgcacccgcatggaggccggcctggtggaccaggacgacctggacgaggagcac- cagggc ctggcccccgccgagctgttcgcccgcctgaaggcccgcatgcccgacggcgaggacctggtggtgacccacgg- cgacgcctg cctgcccaacatcatggtggagaacggccgcttctccggcttcatcgactgcggccgcctgggcgtggccgacc- gctaccagg acatcgccctggccacccgcgacatcgccgaggagctgggcggcgagtgggccgaccgcttcctggtgctgtac- ggcatcgcc gcccccgactcccagcgcatcgccttctaccgcctgctggacgagttcttcTGAcaattgGCAGCAGCAGCTCG- GATAG TATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGT GAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAG TTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGC ATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCC CTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCA ATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgcgtctcgaacagagcgcgcaga ggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgctt- ggttcttcgtccat tagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctgatggt- cgaaacgttcac ##STR00078## ##STR00079## ##STR00080## ##STR00081## ##STR00082## ##STR00083## ##STR00084## ##STR00085## ##STR00086## ##STR00087## ##STR00088## ##STR00089## ##STR00090## ##STR00091## ##STR00092## ##STR00093## ##STR00094## ##STR00095## ##STR00096## ##STR00097## ##STR00098## ##STR00099## GCAGACCGCCCACCAGCGCCCCCCCACCGAGGGCCACTGCTFCGGCGCCCGCCTGCCCACCGCCTCCC ##STR00100## ##STR00101## ##STR00102## ##STR00103## ##STR00104## ##STR00105## ##STR00106## ##STR00107## ##STR00108## ##STR00109## ##STR00110## ##STR00111## ##STR00112## ##STR00113## ##STR00114## ##STR00115## ##STR00116## ##STR00117## ##STR00118## ##STR00119## ##STR00120## ##STR00121## TGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCC GCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTT GTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAA CTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTT GGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCAC GGGAAGTAGTGGGATGGGAACACAAATGGAaagcttaattaagagctcttgttttccagaaggagttgctcctt- gag cctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcgaatttaaaagcttggaa- tgttggttcgtgcgt ctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcg- tacctctgctttc gcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcagtctgtaattgcctcagaat- gtggaatcatctgcc ccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacc- tcacaatagttca taacagtgaccatatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggt- gcttgcggagggc aggtcaaccggcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctcccc- gggatgtgggcc caccaccagcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctga- attccttctgccg ctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgt- ggcggggcttgttc gagcttgaagagc
[0505] Overexpression of KASII in Strain X: Construct D1653 derived from pSZ2734 was transformed into Strain X as described previously. Primary transformants were clonally purified and grown under standard lipid production conditions at pH 5. The resulting fatty acid profiles from representative clones arising from transformation of Strain X with D1653 are summarized in Table 42, below. Overexpression of KASII in the SAD2 knockout/knock-down Strain K-4 background resulted in multiple strains accumulating over 50% C18:0 and with substantially reduced levels of C16:0. We also observed that KASII over-expressing lines had lower overall ratios of saturated to unsaturated fatty acids compared to Strain X.
TABLE-US-00049 TABLE 42 Fatty acid profiles of D1653 [pSZ2734] primary transformants, compared to the Strain X base strain and the wild-type parental strain, Strain J. D165 D165 D165 D165 D165 D165 D165 D165 D165 D165 D165 D165 Strain J K-4 3-89 3-10A 3-2B 3-5B 3-7A 3-75 3-90 3-9B 3-72 3-6B 3-82 3-66 Fatty C12:0 0.04 0.06 0.27 0.13 0.20 0.19 0.24 0.13 0.12 0.27 0.16 0.18 0.25 0.22 Acid C14:0 1.44 1.06 1.55 1.65 1.79 1.67 1.70 1.53 1.50 1.74 1.57 1.64 1.48 1.56 Area C16:0 29.23 29.83 8.16 11.45 10.68 10.11 9.27 11.14 11.08 9.40 9.78 9.95 8.12 8.65 % C16:1 0.88 0.10 0.04 0.00 0.00 0.00 0.00 0.04 0.04 0.00 0.04 0.00 0.05 0.06 C18:0 2.97 40.17 54.25 53.87 53.61 53.46 53.32 53.32 53.15 52.43 52.20 51.23 50.52 50.02 C18:1 58.07 20.15 23.52 22.12 22.20 23.48 24.02 22.73 23.45 23.94 25.21 26.07 28.00 28.29 C18:2 6.25 5.25 6.75 6.05 6.42 6.25 6.56 6.19 5.96 6.88 6.28 6.31 6.59 6.31 C18:3.alpha. 0.50 0.68 0.79 0.88 0.78 0.79 0.79 0.85 0.82 0.86 0.78 0.78 0.78 0.83 C20:0 0.22 1.88 3.21 2.81 3.01 2.91 3.02 2.86 2.77 3.21 2.74 2.80 2.87 2.80 C20:1 0.02 0.07 0.19 0.21 0.34 0.27 0.28 0.12 0.11 0.41 0.14 0.30 0.28 0.26 C22:0 0.05 0.26 0.41 0.34 0.40 0.37 0.37 0.36 0.35 0.42 0.36 0.37 0.36 0.37 C24:0 0.04 0.27 0.49 0.38 0.42 0.41 0.45 0.38 0.36 0.46 0.39 0.37 0.41 0.41 sum C18 67.78 66.24 85.31 82.92 83.01 83.98 84.69 83.09 83.38 84.11 84.47 84.39 85.89 85.45 saturates 33.97 73.52 68.34 70.63 70.11 69.12 68.37 69.72 69.33 67.93 67.20 unsaturates 65.71 26.23 31.29 29.26 29.74 30.79 31.65 29.93 30.38 32.09 32.45
[0506] In Table 42, Stearate (C18:0) levels greater than the wild-type level are highlighted with bold text. Palmitate (C16:0) levels lower than Strain X or J are highlighted with bold. For three strains the ratio of saturated to unsaturated fatty acids is <2:1; these are highlighted with bold, italicized text.
[0507] Stable lines were isolated from the primary transformants shown in Table 42. The fatty acid profiles and lipid titers of shake flask cultures are presented in Table 43, below. The strains accumulated up to 55% C18:0, with as low as 7% C16:0, with comparable lipid titers to the Strain X parent. The saturates:unsaturates ratios were substantially reduced compared to Strain X. Strains AU and AV were selected for evaluation in 3-L high-density fermentations.
TABLE-US-00050 TABLE 43 Shake flask assays of strains derived from D1653, expressing KASII, driven by the PmSAD2-2 promoter, targeted to the 6S locus. 1653- 1653- 1653- 1653- D165 Primary 6B 9B 10A 72 3-89 Strain K-4 5664 AU BM BN BO BP BQ BR AV BS Fatty 10:0 .02 .04 .08 .09 .12 .06 .06 .08 .09 .12 .12 .12 Acid 12:0 .04 .09 .28 .29 .35 .20 .20 .23 .26 .32 .32 .33 Area 14:0 .42 .12 .81 .66 .73 .75 .72 .50 .61 .38 .43 .38 % 16:0 5.59 8.56 .39 .61 .44 .98 0.11 .26 .95 .81 .21 .63 16:1 .03 .10 .06 .05 .06 .06 .06 .04 .04 .03 .03 .03 18:0 .60 0.13 7.60 2.47 5.12 0.25 9.73 4.56 4.01 2.96 3.68 2.12 18:1 2.08 0.74 7.78 3.93 1.31 5.37 5.70 2.86 2.87 4.37 3.99 5.17 18:2 .16 .83 .98 .52 .72 .55 .64 .20 .24 .11 .83 .04 18:3.alpha. .40 .89 .21 .22 .49 .17 .07 .20 .29 .28 .24 .31 20:0 .18 .82 .62 .93 .75 .65 .66 .97 .72 .43 .10 .59 20:1 .04 .13 .37 .36 .39 .34 .34 .35 .34 .48 .41 .47 20:1 .07 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 20:1 .15 .08 .11 .09 .11 .10 .10 .09 .10 .12 .10 .12 22:0 .02 .20 .28 .30 .24 .29 .28 .30 .27 .32 .29 .35 24:0 .00 .03 .16 .29 .00 .03 .15 .16 .02 .05 .04 .07 Sum C18 1.23 7.58 4.57 5.13 5.63 4.34 4.13 5.81 5.40 6.71 6.73 6.63 Saturates 9.86 1.97 8.74 8.05 7.90 Unsaturates 9.91 7.76 1.07 1.73 1.87
[0508] In Table 43, Strain X is the parent strain; Strain J is the wild-type base strain. Stearate (C18:0) levels at least two-fold higher than in the wild-type strain are highlighted in bold. Palmitate levels that are less than in Strain J and Strain K-4 are highlighted bold. Bold italics indicate that the saturates:unsaturates ratio is <2:1.
[0509] The fatty acid profiles and performance metrics of strains AU and AV are detailed in Table 44, below. The fatty acid profile of the parent strain X, grown under the same fermentation conditions, is presented for comparison. The strains that over-express KASII accumulate about 11% more C18:0 than the strain K-4 parent. C16:0 is reduced to 7-9%, and levels of unsaturated fatty acids increase by 4-5%. The lipid titers of Strain AU and AV were comparable to K-4, indicating that KASII over-expression did not have deleterious effects on lipid production.
TABLE-US-00051 TABLE 44 End point fatty acid profiles of biomass from strain X, AU and AV fermentations. Strain K-4 AU AV Fermentation 120580F1 130097F3 130098F4 pH 5 5 5 C14:0 1.27 1.50 1.35 C16:0 28.35 8.88 7.33 C16:1 0.06 0.02 0.03 C18:0 43.67 56.88 57.24 C18:1 17.63 21.57 21.66 C18:2 5.58 6.06 6.94 C18:3.alpha. 0.25 0.29 0.22 C20:0 2.11 3.28 3.46 C22:0 0.31 0.40 0.40 C24:0 0.31 0.37 0.40 sum C18 67.13 84.80 86.06 saturates 76.02 71.31 70.18 unsaturates 23.52 27.94 28.85 total lipid (g/L) 475 529 418
[0510] The fermentations were cultured for 6 days using a fed-batch process. The Strain X fatty acid profile from fermentation 120580F1 was presented in Table 41, and is shown again in Table 44 for comparison with Strains AU and AV. All fermentations were carried out at 32.degree. C., pH 5, 30% dissolved oxygen (DO), 300 mM nitrogen [N], and 557.5 .mu.M iron. The sugar source was 70% sucrose (S70). Stearate (C18:0) levels higher than in the wild-type strain are indicated with bold. Palmitate (C16:0) levels that are less than in the wild-type are highlighted bold.
[0511] Lab scale oils were prepared from biomass derived from the shake flasks and fermentations described above. The TAG compositions of these oils were determined by LC/MS. SOS is the major TAG species in both Strain AU and AV, ranging from 33-35% in the biomass from shake flasks, and reaching 37% in the high-density fermentation biomass. The major palmitate-containing TAGs are substantially reduced, and trisaturate levels are less than half of those observed in Strain X oils. These results demonstrate that KASII over-expression in a high-stearate background significantly improves SOS accumulation, and reduces the accumulation of trisaturated TAGs.
[0512] Constructs used for FATA-1 disruption, KASII over-expression and FAD2 RNAi in Strain J: A DNA construct, pSZ2419, was made to simultaneously disrupt the FATA-1 allele, over-express P. moriformis KASII and express a FAD2 hairpin construct in Strain J. A version of the S. cerevisiae SUC2 gene, encoding sucrose invertase, which was codon-optimized for expression in P. moriformis, was utilized as a selectable marker for transformation. The sequence of the transforming DNA is provided immediately below. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, AscI, MfeI, BamHI, AvrII, EcoRV, EcoRI, SpeI, AscI, ClaI, BglII, AflII, HinDIII, SacI, SpeI, and XhoI, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the FATA-1 locus. Proceeding in the 5' to 3' direction, the C. reinhardtii TUB2 promoter driving the expression of the S. cerevisiae SUC2 gene (encoding sucrose hydrolyzing activity, thereby permitting the strain to grow on sucrose) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for SUC2 are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the C. vulgaris nitrate reductase (NR) gene is indicated by small capitals, followed by a spacer region indicated by lowercase text. The P. moriformis AMT3 promoter, indicated by lowercase boxed text, drives expression of the P. moriformis KASII gene. The region encoding the plastid targeting peptide from Chlorella protothecoides SAD1 is indicated by uppercase italics. The sequence that encodes the mature P. moriformis KASII polypeptide is indicated with bold, uppercase italics, while a 3.times. FLAG epitope encoding sequence is in bold, underlined, uppercase italics. A second C. vulgaris NR 3' UTR is indicated by small capitals. A second C. reinhardtii TUB2 promoter sequence, indicated by lowercase boxed text, drives expression of the P. moriformis FAD2 hairpin A sequence. The sense and antisense strands are indicated with uppercase, bold italics, and are separated by the FAD2 intron and the first 10 bases of the FAD2 second exon (uppercase italics). A third C. vulgaris NR 3' UTR is indicated by small capitals, followed by a second spacer region that is indicated by lowercase text.
TABLE-US-00052 Nucleotide sequence of the transforming DNA from pSZ2419: (SEQ ID NO: 96) gctcttcggagtcactgtgccactgagttcgactggtagctgaatggagtcgctgctccactaaacgaattgtc- agcaccgccagcc ggccgaggacccgagtcatagcgagggtagtagcgcgccatggcaccgaccagcctgcttgccagtactggcgt- ctcttccgcttct ctgtggtcctctgcgcgctccagcgcgtgcgcttttccggtggatcatgcggtccgtggcgcaccgcagcggcc- gctgcccatgcagc gccgctgcttccgaacagtggcggtcagggccgcacccgcggtagccgtccgtccggaacccgcccaagagttt- tgggagcagctt gagccctgcaagatggcggaggacaagcgcatcttcctggaggagcaccggtgcgtggaggtccggggctgacc- ggccgtcgcat tcaacgtaatcaatcgcatgatgatcagaggacacgaagtcttggtggcggtggccagaaacactgtccattgc- aagggcataggg atgcgttccttcacctctcatttctcatttctgaatccctccctgctcactctttctcctcctccttcccgttc- acgcagcattcggggtacc ##STR00122## ##STR00123## ##STR00124## ##STR00125## ggccggcttcgccgccaagatcagcgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcaccc- ccaacaagg gctggatgaacgaccccaacggcctgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaac- ccgaacg acaccgtctgggggacgcccttgttctggggccacgccacgtccgacgacctgaccaactgggaggaccagccc- atcgccatc gccccgaagcgcaacgactccggcgccttctccggctccatggtggtggactacaacaacacctccggcttctt- caacgacacc atcgacccgcgccagcgctgcgtggccatctggacctacaacaccccggagtccgaggagcagtacatctccta- cagcctgga cggcggctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactccacccagttccgcgacccga- aggtcttctg gtacgagccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccg- acgacctga agtcctggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgatc- gaggtcccc accgagcaggaccccagcaagtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctc- cttcaaccag tacttcgtcggcagcttcaacggcacccacttcgaggccttcgacaaccagtcccgcgtggtggacttcggcaa- ggactactac gccctgcagaccttcttcaacaccgacccgacctacgggagcgccctgggcatcgcgtgggcctccaactggga- gtactccgcc ttcgtgcccaccaacccctggcgctcctccatgtccctcgtgcgcaagttctccctcaacaccgagtaccaggc- caacccggaga cggagctgatcaacctgaaggccgagccgatcctgaacatcagcaacgccggcccctggagccggttcgccacc- aacaccac gttgacgaaggccaacagctacaacgtcgacctgtccaacagcaccggcaccctggagttcgagctggtgtacg- ccgtcaac accacccagacgatctccaagtccgtgttcgcggacctctccctctggttcaagggcctggaggaccccgagga- gtacctccgc atgggcttcgaggtgtccgcgtcctccttcttcctggaccgcgggaacagcaaggtgaagttcgtgaaggagaa- cccctacttc accaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaaggtgtacggctt- gctggacc agaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccgggaac- gccctgggc tccgtgaacatgacgacgggggtggacaacctgttctacatcgacaagttccaggtgcgcgaggtcaagTGAca- attgGCA GCAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACT TGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGT ACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTC GTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTG CTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCT GTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgc gtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaat- aaccacctgacg aatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtga- caatgatcggtgg ##STR00126## ##STR00127## ##STR00128## ##STR00129## ##STR00130## ##STR00131## ##STR00132## ##STR00133## ##STR00134## ##STR00135## ##STR00136## ##STR00137## ##STR00138## TTTCTCGGCGTFCAATGCCCGCTGCGGCGACCTGCGTCGCTCGGCGGGCTCCGGGCCCCGGCGCCCA ##STR00139## ##STR00140## ##STR00141## ##STR00142## ##STR00143## ##STR00144## ##STR00145## ##STR00146## ##STR00147## ##STR00148## ##STR00149## ##STR00150## ##STR00151## ##STR00152## ##STR00153## ##STR00154## ##STR00155## ##STR00156## ##STR00157## ##STR00158## ##STR00159## ##STR00160## GTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAG CCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAAT ACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTC CTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGC CTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGA ##STR00161## ##STR00162## ##STR00163## ##STR00164## ##STR00165## ##STR00166## ##STR00167## ##STR00168## GCCCGTATTGAGGTCCTGGTGGCGCGCATGGAGGAGAAGGCGCCTGTCCCGCTGACCCCCCCGGCT ##STR00169## ##STR00170## ##STR00171## ##STR00172## ##STR00173## GCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTT GATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCA TCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAG CGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGT ACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATG GAaagctgtattgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccg- ctctaattgtggagg gggttcgaagacagggtggttggctggatggggaaacgctggtcgcgggattcgatcctgctgcttatatcctc- cctggaagcacac ccacgactctgaagaagaaaacgtgcacacacacaacccaaccggccgaatatttgcttccttatcccgggtcc- aagagagactgc gatgcccccctcaatcagcatcctcctccctgccgcttcaatcttccctgcttgcctgcgcccgcggtgcgccg- tctgcccgcccagtc agtcactcctgcacaggccccttgtgcgcagtgctcctgtaccctttaccgctccttccattctgcgaggcccc- ctattgaatgtattcg ttgcctgtgtggccaagcgggctgctgggcgcgccgccgtcgggcagtgctcggcgactttggcggaagccgat- tgttcttctgtaag ccacgcgcttgctgctttgggaagagaagggggggggtactgaatggatgaggaggagaaggaggggtattggt- attatctgagtt gggtgaagagc
[0513] Identification and analysis of FATA-1 knockout, KASII over-expression and FAD2 RNAi strains: Construct D1358, derived from pSZ2419, was transformed into Strain J as described previously. Primary transformants were clonally purified and grown under standard lipid production conditions at pH 5. The resulting fatty acid profiles from representative clones arising from transformation of Strain J with D1358 are summarized in Table 45, below. The P. moriformis AMT3 promoter is repressed at pH 5 so the observed phenotypes did not reflect over-expression of P. moriformis KASII. Nevertheless, we observed that multiple strains had substantially reduced levels of C16:0 and 10-15% increases in C18:1, suggesting that the construct had disrupted the FATA-1 target gene, increasing the amount of palmitoyl-ACP available for extension by endogenous KASII. One line, D1358-13, was selected for further analysis. D1358-13 accumulated .about.17% C16:0, .about.75% C18:1 and less than 2% C18:2, indicating that we had successfully integrated at FATA-1 and down-regulated activity of the FAD2 .DELTA..sup.12-desaturase in this strain.
TABLE-US-00053 TABLE 45 Fatty acid profiles of D1358 [pSZ2419] primary transformants, compared to the wild-type parental strain, Strain J. Strain J D1358-13 D1358-19 D1358-11 D1358-9 D1358-30 D1358-28 D1358-6 D1358-8 D1358-10 D1358-3 Fatty C12:0 0.05 0.08 0.06 0.08 0.06 0.07 0.07 0.09 0.07 0.08 0.10 Acid C14:0 1.32 0.79 0.83 0.85 0.87 0.84 0.91 0.86 0.89 0.92 0.60 Area C16:0 26.66 17.43 18.84 20.03 16.27 18.4 19.1 18.18 15.6 16.42 11.24 % C16:1 0.84 0.74 0.79 0.97 0.60 0.77 1.17 0.75 0.56 0.61 0.57 C18:0 3.10 2.87 2.97 2.36 3.20 2.67 2.10 2.82 3.22 3.19 2.30 C18:1 59.07 74.78 69.54 68.78 71.48 69.55 69.02 68.93 70.44 69.64 75.27 C18:2 7.39 1.97 5.47 5.61 6.22 6.31 6.42 6.8 7.68 7.78 8.51 C18:3 .alpha. 0.55 0.23 0.59 0.51 0.26 0.39 0.46 0.38 0.24 0.27 0.24 C20:0 0.24 0.22 0.20 0.13 0.32 0.20 0.03 0.20 0.33 0.31 0.22 C20:1 0.11 0.40 0.29 0.37 0.23 0.33 0.33 0.39 0.36 0.27 0.40 C22:0 0.11 0.09 0.08 0.07 0.09 0.08 0.08 0.08 0.09 0.11 0.11 sum C18 70.11 79.85 78.57 77.26 81.16 78.92 78.00 78.93 81.58 80.88 86.32 saturates 31.48 21.48 22.98 23.52 20.81 22.26 22.29 22.23 20.20 21.03 14.57 unsaturates 67.96 78.12 76.68 76.24 78.79 77.35 77.4 77.25 79.28 78.57 84.99
[0514] In Table 45, Oleate (C18:1) levels greater than the wild-type level are highlighted with bold text. Palmitate (C16:0) levels less than the wild-type are highlighted with bold text. Levels of linoleate (C18:2) reduced by 1% or more compared to the Strain J parent are highlighted with bold text.
[0515] The fatty acid profiles of strains derived from transformant D1358-13 were determined to be stable after more than 60 generations of growth in the absence of selection (growth on sucrose). The performance of selected strains in shake flask assays was then evaluated, and the fatty acid profiles and lipid titers are presented in Table 46, below. Flask experiments were performed at pH 7, enabling activation of the PmAMT3 promoter driving expression of the KASII transgene. The combination of KASII over-expression and FATA-1 knockout leads to further reductions in palmitate levels and enhanced oleate accumulation compared to the phenotypes observed at pH 5 (Table 45). With more than 82% C18:1, less than 11% C16:0, less than 2% C18:2 and .about.83% of the wild-type lipid titer, Strain AA was determined to be the most appropriate strain from this set to serve as a host strain for subsequent modifications to elevate stearate levels. DNA blot analysis showed that S5003 has a simple insertion of construct D1358 [pSZ2419] at the FATA-1 locus.
TABLE-US-00054 TABLE 46 Fatty acid profiles and lipid titers of FATA-1 knockout, KASII over- expressing, FAD2 RNAi lines derived from D1358-13 primary transformants, compared to the wild-type parental strain, Strain J. Primary T389; D1358-13 Strain J AA AB AC AD AE AF AG AH AI AJ AK AL AM Fatty C12:0 0.05 0.08 0.09 0.11 0.19 0.11 0.14 0.10 0.12 0.08 0.11 0.09 0.20 0.20 Acid C14:0 1.34 0.96 0.98 1.03 1.04 0.96 1.02 0.98 1.03 0.98 1.01 1.00 1.03 1.02 Area C16:0 29.69 10.72 10.47 8.90 6.99 9.53 9.27 10.13 8.99 10.76 9.58 10.00 6.64 6.38 % C16:1 0.88 0.42 0.39 0.31 0.29 0.39 0.37 0.41 0.32 0.40 0.35 0.35 0.27 0.27 C18:0 2.78 2.92 3.00 3.16 2.71 2.88 2.85 2.91 3.21 3.03 3.10 3.20 2.77 2.71 C18:1 58.45 82.08 82.24 83.66 85.49 83.28 83.38 82.57 83.51 82.12 83.10 82.63 85.88 86.13 C18:2 5.83 1.89 1.88 1.80 2.01 1.83 1.89 1.89 1.77 1.73 1.75 1.76 1.94 1.96 C18:3.alpha. 0.42 0.23 0.23 0.25 0.35 0.27 0.29 0.27 0.25 0.22 0.24 0.23 0.34 0.36 C20:0 0.17 0.15 0.16 0.17 0.15 0.15 0.16 0.16 0.17 0.14 0.16 0.16 0.15 0.15 C20:1 0.05 0.23 0.24 0.27 0.36 0.28 0.29 0.26 0.27 0.21 0.25 0.24 0.38 0.39 sum C18 67.48 87.12 87.35 88.87 90.56 88.26 88.41 87.64 88.74 87.10 88.19 87.82 90.93 91.16 saturates 34.03 14.83 14.70 13.37 11.08 13.63 13.44 14.28 13.52 14.99 13.96 14.45 10.79 10.46 unsaturates 65.63 84.85 84.98 86.29 88.50 86.05 86.22 85.40 86.12 84.68 85.69 85.21 88.81 89.11 lipid titer 100.0 82.8 81.1 72.8 54.4 68.3 63.7 70.6 72.2 106.9 76.5 77.5 56.7 54.6 (% parent)
[0516] In Table 46, Stearate (C18:1) levels greater than the wild-type level are highlighted with bold text. Palmitate (C16:0) levels lower than the wild-type are highlighted with bold text. Linoleate (C18:2) levels that are lower than the wild-type are indicated with bold text.
[0517] Constructs used for SAD2 knockout/RNAi in S5003: Two DNA constructs, pSZ2283 and pSZ2697, were made to simultaneously disrupt the SAD2-1 allele and express a SAD2 hairpin construct in Strain AA. In each construct, the neoR gene from transposon Tn5, conferring resistance to aminoglycoside antibiotics, was used as a selectable marker for transformation. The sequence of the transforming DNA derived from pSZ2283 is provided immediately below. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, XbaI, MfeI, BamHI, AvrII, EcoRV, EcoRI, SpeI, BamHI, HinDIII, and SacI, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the Chlamydomonas reinhardtii TUB2 promoter driving the expression of neoR (encoding aminoglycoside phosphotransferase activity, thereby permitting the strain to grow on G418) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for neoR are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the C. vulgaris NR gene is indicated by small capitals, followed by a spacer region indicated by lowercase text. A second C. reinhardtii TUB2 promoter sequence, indicated by lowercase boxed text, drives expression of the SAD2 hairpin C sequence. The sense and antisense strands are indicated with uppercase, bold italics, and are separated by the P. moriformis FAD2 intron and the first 10 bases of the FAD2 second exon (uppercase italics). A second C. vulgaris NR 3' UTR is indicated by small capitals.
TABLE-US-00055 Nucleotide sequence of the transforming DNA from pSZ2283: (SEQ ID NO: 97) gctcttcgggtcgccgcgctgcctcgcgtcccctggtggtgcgcgcggtcgccagcgaggccccgctgggcgtt- ccgccctcggtgca gcgcccctcccccgtggtctactccaagctggacaagcagcaccgcctgacgcccgagcgcctggagctggtgc- agagcatggggc agtttgcggaggagagggtgctgcccgtgctgcaccccgtggacaagctgtggcagccgcaggactttttgccc- gaccccgagtcgc ccgacttcgaggatcaggtggcggagctgcgcgcgcgcgccaaggacctgcccgacgagtactttgtggtgctg- gtgggggacatg atcacggaggaggcgctgccgacctacatggccatgctcaacacgctggacggcgtgcgcgacgacacgggcgc- ggccgaccacc cgtgggcgcgctggacgcggcagtgggtggccgaggagaaccggcacggcgacctgctgaacaagtactgctgg- ctgacggggc gcgtcaacatgcgggccgtggaggtgaccatcaacaacctgatcaagagcggcatgaacccgcagacggacaac- aacccttattt ##STR00174## ##STR00175## ##STR00176## ##STR00177## ##STR00178## ggctacgactgggcccagcagaccatcggctgctccgacgccgccgtgttccgcctgtccgcccagggccgccc- cgtgctgttc gtgaagaccgacctgtccggcgccctgaacgagctgcaggacgaggccgcccgcctgtcctggctggccaccac- cggcgtgc cctgcgccgccgtgctggacgtggtgaccgaggccggccgcgactggctgctgctgggcgaggtgcccggccag- gacctgct gtcctcccacctggcccccgccgagaaggtgtccatcatggccgacgccatgcgccgcctgcacaccctggacc- ccgccacctg ccccttcgaccaccaggccaagcaccgcatcgagcgcgcccgcacccgcatggaggccggcctggtggaccagg- acgacctg gacgaggagcaccagggcctggcccccgccgagctgttcgcccgcctgaaggcccgcatgcccgacggcgagga- cctggtg gtgacccacggcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccggcttcatcgactgcgg- ccgcctgggc gtggccgaccgctaccaggacatcgccctggccacccgcgacatcgccgaggagctgggcggcgagtgggccga- ccgcttcc tggtgctgtacggcatcgccgcccccgactcccagcgcatcgccttctaccgcctgctggacgagttcttcTGA- caattgGCAG CAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTT GCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGT ACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTC GTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTG CTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCT GTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgc gtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaat- aaccacctgacg aatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtga- caatgatcggtgg ##STR00179## ##STR00180## ##STR00181## ##STR00182## ##STR00183## ##STR00184## ##STR00185## ##STR00186## GTTTTGGTTGCCCGTATCGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTGACC ##STR00187## ##STR00188## ##STR00189## ##STR00190## ##STR00191## GTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAAC AGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGA ATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTG TCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCG CCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGG ATGGGAACACAAATGGAaagctggagctccagccacggcaacaccgcgcgccttgcggccgagcacggcgacaa- gaacc tgagcaagatctgcgggctgatcgccagcgacgagggccggcacgagatcgcctacacgcgcatcgtggacgag- ttcttccgcctc gaccccgagggcgccgtcgccgcctacgccaacatgatgcgcaagcagatcaccatgcccgcgcacctcatgga- cgacatgggcc acggcgaggccaacccgggccgcaacctcttcgccgacttctccgcggtcgccgagaagatcgacgtctacgac- gccgaggactac tgccgcatcctggagcacctcaacgcgcgctggaaggtggacgagcgccaggtcagcggccaggccgccgcgga- ccaggagtac gtcctgggcctgccccagcgcttccggaaactcgccgagaagaccgccgccaagcgcaagcgcgtcgcgcgcag- gcccgtcgcctt ctcctggatctccgggcgcgagatcatggtctagggagcgacgagtgtgcgtgcggggctggcgggagtgggac- gccctcctcgct cctctctgttctgaacggaacaatcggccaccccgcgctacgcgccacgcatcgagcaacgaagaaaacccccc- gatgataggttg cggtggctgccgggatatagatccggccgcacatcaaagggcccctccgccagagaagaagctcctttcccagc- agactcctgaag agc
[0518] The sequence of the transforming DNA derived from pSZ2697 is provided immediately below. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' NsiI, SpeI, BamHI, HinDIII, SacII, EcoRV, KpnI, XbaI, MfeI, BamHI, AvrII, EcoRV, EcoRI and XbaI, respectively. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the SAD2 hairpin C sense and antisense strands are indicated with uppercase, bold italics, and are separated by the P. moriformis FAD2 intron and the first 10 bases of the FAD2 second exon (uppercase italics). The 3' UTR of the C. vulgaris NR gene is indicated by small capitals. The Chlorella sorokiniana Glutamate Dehydrogenase (GDH) promoter, driving the expression of neoR (encoding aminoglycoside phosphotransferase activity, thereby permitting the strain to grow on G418) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for neoR are indicated by uppercase italics, while the coding region is indicated with lowercase italics. A second C. vulgaris NR 3' UTR is indicated by small capitals, followed by a spacer region indicated by lowercase text.
TABLE-US-00056 Nucleotide sequence of the transforming DNA from pSZ2697: (SEQ ID NO: 98) atgcatgccggtcaccacccgcatgctcgtactacagcgcacgcaccgcttcgtgatccaccgggtgaacgtag- tcctcgacggaa acatctggttcgggcctcctgcttgcactcccgcccatgccgacaacctttctgctgttaccacgacccacaat- gcaacgcgacacga ccgtgtgggactgatcggttcactgcacctgcatgcaattgtcacaagcgcttactccaattgtattcgtttgt- tttctgggagcagttg ctcgaccgcccgcgtcccgcaggcagcgatgacgtgtgcgtggcctgggtgtttcgtcgaaaggccagcaaccc- taaatcgcaggc gatccggagattgggatctgatccgagtttggaccagatccgccccgatgcggcacgggaactgcatcgactcg- gcgcggaaccca gctttcgtaaatgccagattggtgtccgatacctggatttgccatcagcgaaacaagacttcagcagcgagcgt- atttggcgggcgt gctaccagggttgcatacattgcccatttctgtctggaccgctttactggcgcagagggtgagttgatggggtt- ggcaggcatcgaaa cgcgcgtgcatggtgtgcgtgtctgttttcggctgcacgaattcaatagtcggatgggcgacggtagaattggg- tgtggcgctcgcgt gcatgcctcgccccgtcgggtgtcatgaccgggactggaatcccccctcgcgaccatcttgctaacgctcccga- ctctcccgactagt ##STR00192## ##STR00193## ##STR00194## ##STR00195## GCCCGTATCGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTGACCCCCCCGGCT ##STR00196## ##STR00197## ##STR00198## ##STR00199## ##STR00200## GACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAG TGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACC CCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTA TCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATT CTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAA ##STR00201## ##STR00202## ##STR00203## ##STR00204## ##STR00205## ##STR00206## ##STR00207## ##STR00208## ##STR00209## ##STR00210## ##STR00211## ##STR00212## ##STR00213## ##STR00214## ##STR00215## ##STR00216## tagaatatcaATGatcgagcaggacggcctccacgccggctcccccgccgcctgggtggagcgcctgttcggct- acgactggg cccagcagaccatcggctgctccgacgccgccgtgttccgcctgtccgcccagggccgccccgtgctgttcgtg- aagaccgacct gtccggcgccctgaacgagctgcaggacgaggccgcccgcctgtcctggctggccaccaccggcgtgccctgcg- ccgccgtgc tggacgtggtgaccgaggccggccgcgactggctgctgctgggcgaggtgcccggccaggacctgctgtcctcc- cacctggcc cccgccgagaaggtgtccatcatggccgacgccatgcgccgcctgcacaccctggaccccgccacctgcccctt- cgaccaccag gccaagcaccgcatcgagcgcgcccgcacccgcatggaggccggcctggtggaccaggacgacctggacgagga- gcacca gggcctggcccccgccgagctgttcgcccgcctgaaggcccgcatgcccgacggcgaggacctggtggtgaccc- acggcgac gcctgcctgcccaacatcatggtggagaacggccgcttctccggcttcatcgactgcggccgcctgggcgtggc- cgaccgctac caggacatcgccctggccacccgcgacatcgccgaggagctgggcggcgagtgggccgaccgcttcctggtgct- gtacggca tcgccgcccccgactcccagcgcatcgccttctaccgcctgctggacgagttcttcTGAcaattgGCAGCAGCA- GCTCGGA TAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACC TGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGC GAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCT TGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTG CCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACT GCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgcgtctcgaacagagcgcg cagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgc- gcttggttcttcg tccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctg- atggtcgaaacg ttcacagcctagggatatcgaattccgggtcgccgcgctgcctcgcgtcccctggtggtgcgcgcggtcgccag- cgaggccccgctg ggcgttccgccctcggtgcagcgcccctcccccgtggtctactccaagctggacaagcagcaccgcctgacgcc- cgagcgcctgga gctggtgcagagcatggggcagtttgcggaggagagggtgctgcccgtgctgcaccccgtggacaagctgtggc- agccgcaggac tttttgcccgaccccgagtcgcccgacttcgaggatcaggtggcggagctgcgcgcgcgcgccaaggacctgcc- cgacgagtacttt gtggtgctggtgggggacatgatcacggaggaggcgctgccgacctacatggccatgctcaacacgctggacgg- cgtgcgcgacg acacgggcgcggccgaccacccgtgggcgcgctggacgcggcagtgggtggccgaggagaaccggcacggcgac- ctgctgaaca agtactgctggctgacggggcgcgtcaacatgcgggccgtggaggtgaccatcaacaacctgatcaagagcggc- atgaacccgca gacggacaacaacccttatttggggttcgtctacacctccttccaggagcgcgccaccaagtatctaga
[0519] Identification and analysis of SAD2 knockout/knockdown strains in the S5003 background: Constructs D1639, derived from pSZ2697, and D1682, derived from pSZ2283, were transformed into Strain AA as described previously. Primary transformants were clonally purified and grown under standard lipid production conditions at pH 7. The resulting fatty acid profiles from representative clones arising from transformation are summarized in Table 47, below. D1639 transformants accumulated up to 56% C18:0, and D1682 transformants accumulated a maximum of about 35% C18:0. Most of the increases in stearate came at the expense of C18:1, indicating that SAD activity was significantly reduced by the SAD2 knockout/RNAi constructs in these strains. C16:0 levels varied from 6% to 14%; C18:2 ranged from 2-5%. Most strains maintained the low C16:0 and C18:2 phenotypes of the Strain AA parent. These fatty acid profiles demonstrate that down-regulating SAD2 expression using knockout/RNAi constructs, in a background with disrupted FATA-1, KASII over-expression and FAD2 RNAi, produces strains with high C18:0, low C16:0 and low C18:2 phenotypes. These strains will be useful for production of high stability, high stearate, high oleic oils, and oils which have high SOS content.
TABLE-US-00057 TABLE 47 Fatty acid profiles of D1639 [pSZ2697] and D1682 [pSZ2283] primary transformants, compared to the wild-type strain, Strain J, and the Strain AA parental base strain. Strain J AA D1682-4 D1682-17 D1682-7 D1682-6 D1639-2 D1639-5 D1639-10 D1639-19 Fatty C12:0 0.04 0.11 0.14 0.10 0.32 0.31 0.00 0.19 0.17 0.00 Acid C14:0 1.29 0.98 1.03 0.94 1.11 1.15 1.64 1.39 1.61 1.02 Area C16:0 27.50 7.75 8.68 10.41 5.70 5.96 7.54 9.90 14.39 12.02 % C16:1 0.71 0.30 0.06 0.07 0.07 0.10 0.00 0.00 0.00 0.00 C18:0 3.28 3.60 35.46 29.92 24.66 22.30 55.96 53.38 43.46 37.30 C18:1 57.80 84.14 48.39 52.49 61.04 63.60 23.70 26.79 32.93 42.81 C18:2 7.90 2.09 2.37 2.36 3.03 2.88 5.09 3.50 3.22 2.79 C18:3 .alpha. 0.57 0.32 0.50 0.65 0.66 0.58 1.59 0.98 1.01 0.85 C20:0 0.28 0.23 2.07 1.87 1.75 1.51 3.04 2.73 2.29 2.22 C20:1 0.18 0.35 0.54 0.49 0.78 0.83 0.37 0.33 0.30 0.40 C22:0 0.06 0.02 0.27 0.27 0.23 0.20 0.43 0.36 0.29 0.29 C24:0 0.09 0.02 0.33 0.26 0.34 0.26 0.64 0.45 0.32 0.31 sum C18 69.55 90.14 86.72 85.42 89.39 89.36 86.34 84.65 80.62 83.75 saturates 32.54 12.70 47.98 43.77 34.11 31.69 69.25 68.40 62.53 53.16 unsaturates 67.16 87.21 51.86 56.06 65.58 67.99 30.75 31.60 37.46 46.85
[0520] In Table 47, Stearate (C18:0) levels greater than the wild-type level are highlighted with bold text. Oleate (C18:1) levels that are higher than in the wild-type are indicated with bold text. Palmitate (C16:0) levels less than the wild-type level are highlighted with bold. Reduced levels of linoleate (C18:2) compared to the wild-type are highlighted with bold text.
[0521] Stable lines were isolated from a number of D1639 and D1682 transformants. Shake flask assays were carried out to evaluate the performance of four lines derived from D1639-5. Fatty acid profiles and relative lipid titers from the biomass are shown in Table 48, below.
TABLE-US-00058 TABLE 48 Shake flask assays of strains derived from D1639-5, expressing SAD2hpC, driven by the CrTUB2 promoter, targeted to the SAD2-1 locus. Primary T530; D1639-5 Strain J AA AW AX AY BL Fatty C10:0 0.01 0.00 0.07 0.08 0.05 0.04 Acid C12:0 0.02 0.11 0.19 0.22 0.25 0.23 Area % C14:0 1.52 1.10 1.35 1.32 1.30 1.43 C16:0 31.61 9.59 9.28 8.44 7.74 9.46 C16:1 1.04 0.34 0.03 0.02 0.01 0.01 C17:0 0.10 0.11 0.10 0.10 0.10 0.09 C18:0 2.98 4.36 53.01 53.52 55.32 52.09 C18:1 54.81 80.84 27.26 27.52 27.42 28.06 C18:2 6.88 2.42 3.55 3.52 2.38 3.45 C18:3.alpha. 0.53 0.33 0.97 1.03 0.82 1.06 C20:0 0.26 0.31 2.88 2.94 3.15 2.72 C20:1 0.05 0.34 0.38 0.38 0.40 0.37 C22:0 0.03 0.06 0.36 0.37 0.39 0.35 C24:0 0.07 0.08 0.53 0.54 0.53 0.60 sum C18 65.19 87.95 84.79 85.58 85.94 84.66 saturates 36.59 15.70 67.76 67.52 68.82 66.99 unsaturates 63.30 84.26 32.19 32.46 31.02 32.95 % wild-type 100.0 70.3 34.8 33.7 31.4 35.3 lipid titer
[0522] In Table 48, Strain AA is the parent strain; Strain J is the wild-type base strain. Stearate (C18:0) levels higher than in the wild-type strain are indicated with bold. Bold text indicates the increased level of oleate (C18:1) in Strain AA compared to the wild-type. Palmitate (C16:0) levels that are less than in the wild-type are highlighted bold. Linoleate (C18:2) levels that are less than in the wild-type are indicated with bold.
[0523] Lab scale oils were prepared from biomass collected from the Strain AW, AX and AY shake flasks. The TAG compositions of these oils were determined by LC/MS, and are shown in FIG. 21. SOS accumulation ranged from 42-47% in these strains. POS was the next most abundant TAG, at 16-17%. Linoleate-containing TAGs were reduced by more than 50% compared to the Strain AU and AV oils, described above. Strain AW,AX, and AY oils contained 12-13% trisaturated TAGs (S-S-S), similar to the amounts that accumulated in the Strain AU and AX oils. Modulation of SAD activity during oil production to prevent overproduction of saturated fatty acids may help to reduce accumulation of trisaturates.
Example 49
Properties of Methyl Oleate from High Oleic Microalgal Oils
[0524] Esterified oils high in methyl oleate are useful in a variety of applications such as cleaning and lubrication of machinery. For some of these applications, high thermal stability is desired. Thermal stability testing was performed on methylated oil prepared from high-oleic and high-stability-high oleic triglyceride oils prepared from heterotrophically grown oleaginous microalgae as described above. The oils were bleached and deodorized prior to methylation. Commercially available soya methyl ester was used as a control.
[0525] High Oleic (HO) oil was prepared from a high oil-yielding strain of Prototheca moriformis transformed with a plasmid that can be described as FatA_Btub:inv:nr::amt03-CwTE2:nr_FatA1. This plasmid was designed to homologously recombine in the FATA1 chromosomal site, thus ablating a FATA acyl-ACP thioesterase chromosomal allele, while expressing an exogenous acyl-ACP thioesterase from Cuphea wrightii (CwTE2, SEQ ID NO: 11) under control of the pH-regulatable amt3 promoter. The CwTE2 gene can be downregulated by cultivation at pH 5 during oil production to further elevate oleate production. Sucrose invertase was also expressed as a selection marker and to allow for cultivation of the strain on sucrose as a sole carbon source. The 3' UTR sequences are from the Chlorella vulgaris nitrate reductase gene. The resulting HO strain is denoted Stain Q. The fatty acid profile of the oil produced by Strain Q is listed below in Table 49.
TABLE-US-00059 TABLE 49 Fatty acid profile of high oleic oil from Strain Q. Fatty Acid Area % C10 0.01 C12:0 0.03 C14:0 0.43 C15:0 0.03 C16:0 7.27 C16:1 iso 0.81 C16:1 0.689 C17:0 0.06 C18:0 1.198 C18:1 80.15 C18:1 iso 0.08 C18:2 8.38 C18:3 ALPHA 0.25 C20:0 0.02 C20:1 0.38 C22:0 0.04 C24:0 0.03
[0526] A high-stability-high-oleic oil (HSAO) was also prepared from a high oil-yielding strain of Prototheca moriformis transformed with a plasmid that can be described as FADc5'_0 Btub:inv:nr::btub-CpSAD_CtOTE:nr_FADc3'. The resulting strain (Strain R) expresses sucrose invertase as a selectable marker and to allow for cultivation on sucrose as a sole carbon source. In addition, a FAD allele (encoding fatty acid desaturase responsible for the conversion of oleate to linoleate) is disrupted and an oleate-specific acyl-ACP thioesterase (Carthamus tinctorius OTE, see example 5) fused to the transit peptide from the SAD gene of Chlorella protothecoides is expressed under control of the beta tubulin promoter. The 3' UTR sequences are from the Chlorella vulgaris nitrate reductase gene. The fatty acid profile of the oil produced by Strain R after heterotrophic cultivation is listed below in Table 50. The fatty acid profile has greater than 85% oleate yet almost none of the major polyunsaturates, linoleic and linolenic acids.
TABLE-US-00060 TABLE 50 Fatty acid profile of high oleic oil from Strain R. Fatty Acid Area % C10 0.02 C12:0 0.07 C14:0 0.09 C15:0 0.05 C16:0 7.28 C16:1 0.70 C17:0 0.08 C18:0 2.15 C18:1 86.32 C20:0 0.30 C20:1 0.46 C22:0 0.08 C23:0 0.01 C24:0 0.06
[0527] The HO and HSAO oils were methylated by known biodiesel production techniques to make methyl-HO and methyl-HSAO esters. These methyl esters where then subjection to thermal testing according to the following procedure: [0528] 1. Prepare equipment as shown in FIG. 1. [0529] 2. Add 1 litre of water to test vessel and bring to an active boil on the hotplate. [0530] 3. To each test product add 50ppm Cobalt (0.083 g of 6% Cobalt Napthenate in 100.0 gram sample) and mix thoroughly. [0531] 4. Weigh out, in a watch glass, 7.0 g of 100% cotton gauze, (#50 Cheese Cloth). [0532] 5. Evenly distribute 14.0 g of test product, as prepared in step 3, onto the gauze. [0533] 6. Place thermocouple (thermometer) through the center of #15 stopper. Wrap cotton around the thermocouple. [0534] 7. Place wrapped cotton into 24 mesh wire frame cylinder so that it occupies the upper 41/2 inches. [0535] 8. Position cylinder with wrapped gauze into the 1 L tall form beaker. Secure the beaker in the boiling water and begin recording the temperature increase with time. [0536] 9. Continue monitoring the temperature for 2 hours or until a 10 degree temperature drop in observed. [0537] 10. Plot temperature vs time on a graph. [0538] 11. Any sample which shows a temperature exceeding 100 degrees C. in 1 hour or 200 degrees C. in 2 hours should be regarded as a dangerous oxidation risk or one that is likely to spontaneously combust.
[0539] Results: The HO and HSAO methyl ester did not exhibit auto-oxidation as evidenced by a temperature rise. The control soya methyl ester sample did exhibit the potential for auto-oxidation. The time-temperature profiles are shown in FIG. 18.
[0540] In addition, methylated fatty acid from oil produced by Strain Q was found to have the following characteristics: [0541] Flash Point (ASTM D93) of 182.degree. C. [0542] Non-VOC [0543] Kauri Butanol value (ASTM D1133) of 53.5 [0544] Viscosity at 40.degree. C. (ASTM D445) of 4.57 mm2/s [0545] Acid Number (ASTM D664) of 0.17 mg KOH/g [0546] Boiling range distribution (ASTM D2887) 325-362.degree. C.
Example 50
Further Properties of High Oleic (HO) and High-Stability-High-Oleic (HSAO) Microalgal Oils
[0547] The high oleic oil and the high-stability high-oleic algal oils can have the properties shown in FIG. 19 or these values .+-.20% for the measured parameters.
[0548] In one experiment, HSAO microalgal oil showed 512 hour stability measured by OSI at 110.degree. C. (estimated using 130.degree. C. data) with antioxidants of 0.5% phenyl-alpha-naphthylamine (PANA) and 500 ppm ascorbyl palmitate (AP).
Example 51
Production of Low Saturate Oil by Conversion of Palmitic to Palmitoleate
[0549] As described in the examples above, genetic manipulation of microalgae can decrease saturated fat levels, especially by increasing the production of oleic acid. However, in some cases, the acyl-ACP thioesterases expressed in the oleaginous cell liberate more than desirable amounts of palmitate. Here, we describe methods for converting palmitate (16:0) to palmitoleate (16:1) by overexpressing a palmitoyl-ACP desaturase (PAD) gene. The PAD gene can be obtained from natural sources such as Macfadyena unguis (Cat's claw), Macadamia integrifolia (Macadamia nut), Hippophae rhamnoides (sea buckthorn), or by creating a PAD via mutation of a stearoyl-ACP desaturase to have 16:1 activity. The Macfadyena unguis desaturase is denoted (MuPAD).
[0550] A high-oil-producing strain of Prototheca moriformis (Strain Z) is biolistically transformed with plasmid DNA constructs with a PAD gene. For example, one of the high oleic strains described in the Examples 6, 36, or 49 can further comprise an exogenous PAD gene. The constructs comprises sucrose invertase as a selectable marker and either the MuPAD or a SAD gene (e.g., Olea europaea stearoyl-ACP desaturase, GenBank Accession No. AAB67840.1) having the L118W mutation to shift substrate-specificity toward palmitate. See Cahoon, et al., Plant Physiol (1998) 117:593-598. Both the amt3 and beta tubulin (Btub) promoters are used. In addition, the native transit peptide of a plant PAD gene can be swapped with one known to be effective in microalgae (e.g., the transit peptide from the Chlorella vularis SAD gene).
[0551] The PAD gene can be expressed in a variety of strains including those with a FATA knockout or knockdown and/or a KASII knockin to produce high-oleic oil. Optionally, these strains can also produce high-stability (low polyunsaturate) oil by virtue of a FAD (delta 12 fatty acid desaturase) knockout, knockdown, or by placing FAD expression under control of a regulatable promoter and producing oil under conditions that downregulate FAD. In addition, useful base strains for the introduction of PAD gene activities might also include strains possessing KASII knockouts, and FATA Knockins, whereby levels of C16:0 palmitate are elevated.
[0552] As a result, lower levels of palmitic acid are found in the fatty acid profile of the microalgal oil as this is converted into cis-palmitoleic and cis-vaccenic acids. In some cases the total area percent of saturated fatty acids is less than equal to 3.5%, 3% or 2.5%.
[0553] Constructs for over expression of Macfadyena unguis C16:0 desaturase (MuPAD) follow:
[0554] 1) pSZ3142: 6S::CrTUB2:ScSUC2:CvNR::PmAMT3:CpSADtp:MuPAD:CvNR::6S
[0555] Relevant Restriction Sites in the Construct pSZ3142 6S::CrTUB2:ScSUC2:CvNR::PmAMT3:CpSADtp:MuPAD:CvNR::6S are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene (conferring the ability of Strain Z to metabolize sucrose) is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous amt03 promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the MuPAD are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The Chlorella protothecoides S106 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00061 Nucleotide sequence of transforming DNA contained in pSZ3142: (SEQ ID NO: 99) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgct gatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagagga- gcatgagggag gactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccga- ggccgcctccaa ctggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacag- aacaaccacg agccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgac- cctcgctgccgcc gcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccg- atctgaggacagt cggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgcagaccggtgagagccgact- tgttgtgcgccac cccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcatcggcctcggcctgcagagagg- acagcagtgccc ##STR00217## ##STR00218## ##STR00219## ##STR00220## ##STR00221## gacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgtggtacg- acgagaag gacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctggggcca- cgccacgtc cgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccg- gctccatgg tggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctgg- acctacaaca ccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaac- cccgtgctg gccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtggatcatgaccgc- ggccaagtc ccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgcgttcgccaacg- agggcttcc tcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtcctactgggtg- atgttcatct ccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggcacccacttc- gaggccttcga caaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccgacccgacct- acgggagcg ccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctcctccatg- tccctcgtgcgc aagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagccgatcct- gaacatca gcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctg- tccaacag caccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcgg- acctctccctc tggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttcttcct- ggaccgcggg aacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaa- gagcgag aacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggcga- cgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcga ##STR00222## tggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtg- tttgatcttgtgtgtac gcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcata- tcgcttgcatcccaacc gcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttg- gtttgggctccgcctgt attctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaa- atggaggatccc gcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccaca- ataaccacctga cgaatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggt- gacaatgatcggt ##STR00223## ##STR00224## ##STR00225## ##STR00226## ##STR00227## ##STR00228## ##STR00229## ##STR00230## ##STR00231## ##STR00232## ##STR00233## ##STR00234## ##STR00235## ##STR00236## ##STR00237## agatctcttaaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgcc- gccacacttgctgc cttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttg- cgagttgctagctgctt gtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttat- ctacgctgtcctgcta tccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcct- ggtactgcaacctgta aaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaattaagagctct- tgttttccagaa ggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcga- atttaaaagcttgg aatgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaac- ttgccgctcaaa ccgcgtacctctgctttcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcagt- ctgtaattgcctca gaatgtggaatcatctgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtac- agcagaccatt atgctacctcacaatagttcataacagtgaccatatttctcgaagctccccaacgagcacctccatgctctgag- tggccaccccccg gccctggtgcttgcggagggcaggtcaaccggcatggggctaccgaaatccccgaccggatcccaccacccccg- cgatgggaag aatctctccccgggatgtgggcccaccaccagcacaacctgctggcccaggcgagcgtcaaaccataccacaca- aatatccttgg catcggccctgaattccttctgccgctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgct- ccgagtccgcaaa cccttgtcgcgtggcggggcttgttcgagcttgaagagc
[0556] 2) pSZ3145: 6S::CrTUB2:ScSUC2:CvNR::PmAMT3:MuPAD:CvNR::6S
[0557] Relevant restriction sites in the construct pSZ3145 6S::CrTUB2:ScSUC2:CvNR::PmAMT3: MuPAD:CvNR::6S are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene (conferring the ability of Strain Z to metabolize sucrose) is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous amt03 promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the MuPAD are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00062 Nucleotide sequence of transforming DNA contained in pSZ3145: (SEQ ID NO: 100) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgct gatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagagga- gcatgagggag gactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccga- ggccgcctccaa ctggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacag- aacaaccacg agccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgac- ccctcgctgccgcc gcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccg- atctgaggacagt cggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgcagaccggtgagagccgact- tgttgtgcgccac cccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcatcggcctcggcctgcagagagg- acagcagtgccc ##STR00238## ##STR00239## ##STR00240## ##STR00241## ##STR00242## gacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgtggtacg- acgagaag gacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctggggcca- cgccacgtc cgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccg- gctccatgg tggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctgg- acctacaaca ccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaac- cccgtgctg gccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtggatcatgaccgc- ggccaagtc ccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgcgttcgccaacg- agggcttcc tcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtcctactgggtg- atgttcatct ccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggcacccacttc- gaggccttcga caaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccgacccgacct- acgggagcg ccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctcctccatg- tccctcgtgcgc aagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagccgatcct- gaacatca gcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctg- tccaacag caccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcgg- acctctccctc tggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttcttcct- ggaccgcggg aacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaa- gagcgag aacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggcga- cgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcga ##STR00243## tggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtg- tttgatcttgtgtgtac gcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcata- tcgcttgcatcccaacc gcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttg- gtttgggctccgcctgt attctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaa- atggaggatccc gcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccaca- ataaccacctga cgaatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggt- gacaatgatcggt ##STR00244## ##STR00245## ##STR00246## ##STR00247## ##STR00248## ##STR00249## ##STR00250## ##STR00251## ##STR00252## ##STR00253## ##STR00254## ##STR00255## ##STR00256## ##STR00257## ##STR00258## cagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgcct- tgacctgtgaatat ccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgct- tgtgctatttgcgaata ccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgcta- tccctcagcgctgctc ctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgta- aaccagcactgcaat gctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaattaagagctcttgttttccagaagga- gttgctccttga gcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcgaatttaaaagcttgga- atgttggttcgtgc gtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccg- cgtacctctgct ttcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcaghtctgtaattgcctca- gaatgtggaatcatc tgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtacagccagaccattatg- ctacctcacaata gttcataacagtgaccatatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggcc- ctggtgcttgcgg agggcaggtcaaccggcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctc- tccccgggat gtgggcccaccaccagcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcg- gccctgaattc cttctgccgctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaaccc- ttgtcgcgtggcg gggcttgttcgagcttgaagagc
[0558] 3) pSZ3137: 6S::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp:MuPAD:CvNR::6S
[0559] Relevant restriction sites in the construct pSZ3137 6S::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp:MuPAD:CvNR::6S are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene (conferring the ability of Strain Z to metabolize sucrose) is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by C. reinhardtii (3-tubulin promoter, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the MuPAD are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The Chlorella protothecoides S106 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00063 Nucleotide sequence of transforming DNA contained in pSZ3137: (SEQ ID NO: 101) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgct gatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagagga- gcatgagggag gactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccga- ggccgcctccaa ctggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacag- aacaaccacg agccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgac- cctcgctgccgcc gcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccg- atctgaggacagt cggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgcagaccggtgagagccgact- tgttgtgcgccac cccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcatcggcctcggcctgcagagagg- acagcagtgccc ##STR00259## ##STR00260## ##STR00261## ##STR00262## ##STR00263## gacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgtggtacg- acgagaag gacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctggggcca- cgccacgtc cgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccg- gctccatgg tggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctgg- acctacaaca ccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaac- cccgtgctg gccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtggatcatgaccgc- ggccaagtc ccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgcgttcgccaacg- agggcttcc tcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtcctactgggtg- atgttcatct ccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggcacccacttc- gaggccttcga caaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccgacccgacct- acgggagcg ccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctcctccatg- tccctcgtgcgc aagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagccgatcct- gaacatca gcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctg- tccaacag caccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcgg- acctctccctc tggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttcttcct- ggaccgcggg aacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaa- gagcgag aacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggcga- cgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcga ##STR00264## tggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtg- tttgatcttgtgtgtac gcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcata- tcgcttgcatcccaacc gcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttg- gtttgggctccgcctgt attctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaa- atggaggatccc gcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccaca- ataaccacctga cgaatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggt- gacaatgatcggt ##STR00265## ##STR00266## ##STR00267## ##STR00268## ##STR00269## ##STR00270## agatctcttaaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgcc- gccacacttgctgc cttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttg- cgagttgctagctgctt gtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttat- ctacgctgtcctgcta tccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcct- ggtactgcaacctgta aaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaattaagagctct- tgttttccagaa ggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcga- atttaaaagcttgg aatgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaac- ttgccgctcaaa ccgcgtacctctgctttcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcagt- ctgtaattgcctca gaatgtggaatcatctgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtac- agcagaccatt atgctacctcacaatagttcataacagtgaccatatttctcgaagctccccaacgagcacctccatgctctgag- tggccaccccccg gccctggtgcttgcggagggcaggtcaaccggcatggggctaccgaaatccccgaccggatcccaccacccccg- cgatgggaag aatctctccccgggatgtgggcccaccaccagcacaacctgctggcccaggcgagcgtcaaaccataccacaca- aatatccttgg catcggccctgaattccttctgccgctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgct- ccgagtccgcaaa cccttgtcgcgtggcggggcttgttcgagcttgaagagc
Example 52
Myristate Rich Oil Produced by Overexpressing a Cuphea Palustris Thioesterase
[0560] Here, we demonstrate that over expression of a Cuphea palustris thioesterase (Cpal FATB2, accession AAC49180) in UTEX1435 results in a large increase in C14:0 production (over 60% of the fatty acid profile).
[0561] Constructs used for the overexpression of the Cpal FATB2 gene were codon optimized for expression in P. moriformis as described herein. Cuphea palustris FATB2 is a C14 preferring thioesterase. Two constructs, both encoding the Cpal FATB2 gene, were prepared. The first construct, pSZ2479, can be written as 6SA::CrTUB2-ScSUC2-CvNR:PmAMT3-CpSAD1tpExt-CpalFATB2ExtA-CvNR::6SB. The FatB2 coding sequence is given as SEQ ID NO: 86 and the amino acid sequence is given as SEQ ID NO: 87. The second construct, pSZ2480 can be written as 6SA::CrTUB2-ScSUC2-CvNR:PmAMT3-CpSAD1tpExt-CpalFATB2FLAG ExtA-CvNR::6SB. The nucleic acid sequence and amino acid sequence are given as SEQ ID NO: 88 and SEQ ID NO: 89.
[0562] P. moriformis transformed with pSZ2480 produced high levels of myristic acid. The myristate content was 65.70 percent. This is a very large increase when compared to the myristate content of the wild-type oil produced by the base strain, which has a myristate content of approximately 1%.
[0563] The fatty acid profile of the high myristate strain is shown in the Table 51 below.
TABLE-US-00064 TABLE 51 Fatty acid profile of high myristate strain. Fatty Acid % C10:0 0.04 C12:0 1.19 C14:0 65.7 C16:0 13.55 C18:0 0.57 C18:1 12.2 C18:2 5.13 C20:0 0.05 C22:0 0.01 C24:0 0.01
Example 53
Production of Eicosenoic and Erucic Fatty Acids
[0564] In this example we demonstrate that expression of heterologous fatty acid elongase (FAE), also known as 3-ketoacyl-CoA synthase (KCS), genes from Cramble abyssinica (CaFAE, Accession No: AY793549), Lunaria annua (LaFAE, ACJ61777), and Cardamine graeca (CgFAE, ACJ61778) leads to production of very long chain monounsaturated fatty acids such as eicosenoic (20:1.sup..DELTA.11) and erucic (22:1.sup..DELTA.13) acids in classically mutagenized derivative of UTEX 1435, Strain Z. On the other hand a putative FAE gene from Tropaeolum majus (TmFAE, ABD77097) and two FAE genes from Brassica napus (BnFAE1, AAA96054 and BnFAE2, AAT65206), while resulting in modest increase in eicosenoic (20:1.sup..DELTA.11), produced no detectable erucic acid in STRAIN Z. Interestingly the unsaturated fatty acid profile obtained with heterologous expression of BnFAE1 in STRAIN Z resulted in noticeable increase in Docosadienoic acid (22:2n6). All the genes were codon optimized to reflect UTEX 1435 codon usage. These results suggest that CaFAE, LaFAE or CgFAE genes encode condensing enzymes involved in the biosynthesis of very long-chain utilizing monounsaturated and saturated acyl substrates, with specific capability for improving the eicosenoic and erucic acid content.
[0565] Construct used for the expression of the Cramble abyssinica fatty acid elongase (CaFAE) in P. moriformis (UTEX 1435 strain Z)-[IpSZ3070]: In this example STRAIN Z strains, transformed with the construct pSZ3070, were generated, which express sucrose invertase (allowing for their selection and growth on medium containing sucrose) and C. abyssinica FAE gene. Construct pSZ3070 introduced for expression in STRAIN Z can be written as 6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-CaFAE-Cvnr::6S.
[0566] The sequence of the transforming DNA is provided below. Relevant restriction sites in the construct are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, XbaI, MfeI, BamHI, EcoRI, SpeI, AfIII, SacI, BspQI, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from STRAIN Z that permit targeted integration at the 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the Saccharomyces cerevisiae SUC2 gene (encoding sucrose hydrolyzing activity, thereby permitting the strain to grow on sucrose) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for SUC2 are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The Chlorella vulgaris nitrate reductase (NR) gene 3' UTR is indicated by lowercase underlined text followed by an endogenous AMT3 promoter of P. moriformis, indicated by boxed italicized text. The Initiator ATG and terminator TGA codons of the CaFAE are indicated by uppercase, bold italics, while the remainder of the gene is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the STRAIN Z 6S genomic region indicated by bold, lowercase text. The final construct was sequenced to ensure correct reading frames and targeting sequences.
TABLE-US-00065 Nucleotide sequence of transforming DNA contained in plasmid pSZ3070: (SEQ ID NO: 102) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgctgatgt ccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagaggagcatg- agggaggactcctggt ccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccgaggccgcctcc- aactggtcctccagca gccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacagaacaacgagccttg- tctaggcagaa tccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgaccctcgctgccgccgcttc- tcccgcacgcttctttcca gcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccgatctgaggacagtcggggaactct- gatcagtctaaacccc cttgcgcgttagtgttgccatcctttgcagaccggtgagagccgacttgttgtgcgccaccccccacaccacct- cctcccagaccaattctgt ##STR00271## ##STR00272## ##STR00273## ##STR00274## ##STR00275## atgacgaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacgg- cctgtggtacgacgag aaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctgggg- ccacgccacgtccgacg acctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccggctcc- atggtggtggactacaa caacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctggacctacaacaccc- cggagtccgaggagcagt acatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactcc- acccagttccgcgacccg aaggtcttctggtacgagccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagat- ctactcctccgacgacctg aagtcctggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgat- cgaggtccccaccgagca ggaccccagcaagtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctccttcaacc- agtacttcgtcggcagcttc aacggcacccacttcgaggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgca- gaccttcttcaacaccgac ccgacctacgggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaaccc- ctggcgctcctccatgtcc ctcgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccga- gccgatcctgaacatca gcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctg- tccaacagcaccggca ccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcggacctctcc- ctctggttcaagggcctgga ggaccccgaggagtacctccgcatgggcttcgaggtgtccgctcctccttcttcctggaccgcgggaacagcaa- ggtgaagttcgtgaagga gaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactaca- aggtgtacggcttgctgg accagaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccggg- aacgccctgggctccgtg ##STR00276## gtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatat- ccctgccgcttttatcaaacagcctc agtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccaccccca- gcatccccttccctcgtttcatatcgc ttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccc- tcgcacagccttggtttgggctccgcc tgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaaca- caaatggaggatcccgcgtctc gaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataacca- cctgacgaatgcgcttggtt cttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtgg- agctgatggtcgaaacgttcac ##STR00277## ##STR00278## ##STR00279## ##STR00280## ##STR00281## ##STR00282## ##STR00283## ##STR00284## ##STR00285## ##STR00286## ##STR00287## ##STR00288## ##STR00289## ggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttat- caaacagcctcagtgtgtttgatcttg tgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcg- tttcatatcgcttgcatcccaaccgca acttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtt- tgggctccgcctgtattctcctggtac tgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaa- ttaagagctcttgttttccaga aggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggggttcg- aatttaaaagcttggaatg ttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaacttgc- cgctcaaaccgcgtacc tctgctttcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcagtctgtaattg- cctcagaatgtggaatcatc tgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgc- tacctcacaatagttca taacagtgaccatatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggt- gcttgcggagggcaggt caaccggcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccggga- tgtgggcccaccacc agcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattcctt- ctgccgctctgctacccg gtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggcggggcttgt- tcgagcttgaagagc
[0567] Constructs used for the expression of the FAE genes from higher plants in STRAIN Z: In addition to the CaFAE gene (pSZ3070), LaFAE (pSZ3071) from Lunaria annua, CgFAE (pSZ3072) from Cardamine graeca, TmFAE (pSZ3067) Tropaeolum majus and BnFAE1 (pSZ3068) and BnFAE2 (pSZ3069) genes from Brassica napus have been constructed for expression in STRAIN Z. These constructs can be described as: [0568] pSZ3071--6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-LaFAE-Cvnr::6S [0569] pSZ3072--6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-CgFAE-Cvnr::6S [0570] pSZ3067--6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-TmFAE-Cvnr::6S [0571] pSZ3068--6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-BnFAE1-Cvnr::6S [0572] pSZ3069--6S::CrTUB2-ScSUC2-Cvnr:PmAmt03-BnFAE2-Cvnr::6S
[0573] All these constructs have the same vector backbone; selectable marker, promoters, and 3' utr as pSZ3070, differing only in the respective FAE genes. Relevant restriction sites in these constructs are also the same as in pSZ3070. The sequences of LaFAE, CgFAE, TmFAE, BnFAE1 and BnFAE2 are shown below. Relevant restriction sites as bold text including SpeI and AfIII are shown 5'-3' respectively.
[0574] To determine their impact on fatty acid profiles, the above constructs containing various heterologous FAE genes, driven by the PmAMT3 promoter, were transformed independently into STRAIN Z.
[0575] Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at pH7.0 (all the plasmids require growth at pH 7.0 to allow for maximal FAE gene expression when driven by the pH regulated PmAMT03 promoter). The resulting profiles from a set of representative clones arising from transformations with pSZ3070, pSZ3071, pSZ3072, pSZ3067, pSZ3068 and pSZ3069 into STRAIN Z are shown in Tables 52-57, respectively, below.
[0576] All the transgenic STRAIN Z strains expressing heterologous FAE genes show an increased accumulation of C20:1 and C22:1 fatty acid (see Tables 52-57). The increase in eicosenoic (20:1.sup..DELTA.11) and erucic (22:1.sup..DELTA.13) acids levels over the wildtype is consistently higher than the wildtype levels. Additionally, the unsaturated fatty acid profile obtained with heterologous expression of BnFAE1 in STRAIN Z resulted in noticeable increase in Docosadienoic acid (C22:2n6). Protein alignment of aforementioned FAE expressed in STRAIN Z is shown in FIG. 23.
TABLE-US-00066 TABLE 52 Unsaturated fatty acid profile in STRAIN Z and representative derivative transgenic lines transformed with pSZ3070 (CaFAE) DNA. Sample ID C18:1 C18:2 C18:3a C20:1 C22:1 C22:2n6 C22:5 STRAIN Z; T588; 51.49 9.13 0.65 4.35 1.24 0.11 0.00 D1828-20 STRAIN Z; T588; 55.59 7.65 0.50 3.78 0.85 0.00 0.13 D1828-23 STRAIN Z; T588; 54.70 7.64 0.50 3.44 0.85 0.09 0.00 D1828-43 STRAIN Z; T588; 52.43 7.89 0.59 2.72 0.73 0.00 0.00 D1828-12 STRAIN Z; T588; 56.02 7.12 0.52 3.04 0.63 0.10 0.11 D1828-19 Cntrl STRAIN Z 57.99 6.62 0.56 0.19 0.00 0.06 0.05 pH7 Cntrl STRAIN Z 57.70 7.08 0.54 0.11 0.00 0.05 0.05 pH5
TABLE-US-00067 TABLE 53 Unsaturated fatty acid profile in STRAIN Z and representative derivative transgenic lines transformed with pSZ3071 (LaFAE) DNA. Sample ID C18:1 C18:2 C18:3 a C20:1 C22:1 C22:2n6 C22:5 STRAIN Z; T588; 54.66 7.04 0.52 1.82 0.84 0.12 0.09 D1829-36 STRAIN Z; T588; 56.27 6.72 0.51 1.70 0.72 0.09 0.00 D1829-24 STRAIN Z; T588; 56.65 8.36 0.54 2.04 0.67 0.00 0.00 D1829-11 STRAIN Z; T588; 55.57 7.71 0.53 0.10 0.66 0.00 0.00 D1829-35 STRAIN Z; T588; 56.03 7.06 0.54 1.54 0.51 0.06 0.08 D1829-42 Cntrl STRAIN Z 57.70 7.08 0.54 0.11 0.00 0.06 0.05 pH7 Cntrl STRAIN Z 57.99 6.62 0.56 0.19 0.00 0.05 0.05 pH5
TABLE-US-00068 TABLE 54 Unsaturated fatty acid profile in STRAIN Z and representative derivative transgenic lines transformed with pSZ3072 (CgFAE) DNA. Sample ID C18:1 C18:2 C18:3 a C20:1 C22:1 C22:2n6 C22:5 STRAIN Z; T588; 57.74 7.79 0.52 1.61 0.25 0.11 0.05 D1830-47 STRAIN Z; T588; 58.06 7.39 0.55 1.64 0.22 0.07 0.06 D1830-16 STRAIN Z; T588; 57.77 6.86 0.51 1.34 0.19 0.09 0.00 D1830-12 STRAIN Z; T588; 58.45 7.54 0.49 1.65 0.19 0.06 0.00 D1830-37 STRAIN Z; T588; 57.10 7.28 0.56 1.43 0.19 0.07 0.00 D1830-44 Cntrl STRAIN Z 57.70 7.08 0.54 0.11 0.00 0.06 0.05 pH7 Cntrl STRAIN Z 57.99 6.62 0.56 0.19 0.00 0.05 0.05 pH5
TABLE-US-00069 TABLE 55 Unsaturated fatty acid profile in Strain AR and representative derivative transgenic lines transformed with pSZ3070 (TmFAE) DNA. No detectable Erucic (22:1) acid peaks were reported for these transgenic lines. Sample ID C18:1 C18:2 C18:3 a C20:1 C22:2n6 C22:5 STRAIN Z; T588; 59.97 7.44 0.56 0.57 0.00 0.00 D1825-47 STRAIN Z; T588; 58.77 7.16 0.51 0.50 0.09 0.11 D1825-35 STRAIN Z; T588; 60.40 7.82 0.47 0.44 0.07 0.07 D1825-27 STRAIN Z; T588; 58.07 7.32 0.54 0.41 0.05 0.05 D1825-14 STRAIN Z; T588; 58.66 7.74 0.46 0.39 0.08 0.00 D1825-40 Cntrl STRAIN Z 57.99 6.62 0.56 0.19 0.05 0.05 pH7 Cntrl STRAIN Z 57.70 7.08 0.54 0.11 0.06 0.05 pH5
TABLE-US-00070 TABLE 56 Unsaturated fatty acid profile in STRAIN Z and representative derivative transgenic lines transformed with pSZ3068 (BnFAE1) DNA. No detectable Erucic (22:1) acid peaks were reported for these transgenic lines. Sample ID C18:1 C18:2 C18:3 a C20:1 C22:2n6 C22:5 STRAIN Z; T588; 59.82 7.88 0.55 0.32 0.17 0.10 D1826-30 STRAIN Z; T588; 59.32 8.02 0.58 0.27 0.18 0.07 D1826-23 STRAIN Z; T588; 59.63 7.49 0.55 0.27 0.19 0.08 D1826-45 STRAIN Z; T588; 59.35 7.78 0.57 0.26 0.23 0.00 D1826-24 STRAIN Z; T588; 59.14 7.61 0.57 0.25 0.22 0.05 D1826-34 Cntrl STRAIN Z pH 7 57.81 7.15 0.59 0.19 0.04 0.06 Cntrl STRAIN Z pH 5 58.23 6.70 0.58 0.18 0.05 0.06
TABLE-US-00071 TABLE 57 Unsaturated fatty acid profile in STRAIN Z and representative derivative transgenic lines transformed with pSZ3069 (BnFAE2) DNA. No detectable Erucic (22:1) acid peaks were reported for these transgenic lines. Sample ID C18:1 C18:2 C18:3 a C20:1 C22:2n6 C22:5 STRAIN Z; T588; 60.59 8.20 0.57 0.34 0.00 0.07 D1827-6 STRAIN Z; T588; 59.62 6.44 0.52 0.30 0.07 0.00 D1827-42 STRAIN Z; T588; 59.71 7.99 0.59 0.30 0.06 0.00 D1827-48 STRAIN Z; T588; 60.66 8.21 0.59 0.29 0.04 0.00 D1827-43 STRAIN Z; T588; 60.26 7.99 0.57 0.28 0.04 0.00 D1827-3 Cntrl STRAIN Z pH 7 57.81 7.15 0.59 0.19 0.04 0.06 Cntrl STRAIN Z pH 5 58.23 6.70 0.58 0.18 0.05 0.06
Example 54
Elevating Total Unsaturated Fatty Acids Level by Expressing Heterologous Desaturase Genes
[0577] One of the approaches to generate a "zero SAT FAT" (e.g., total unsaturated fatty acids target at 97% or more/less than or equal to 3% saturated fat) strain is to express desaturase genes in a high oleic strain such as Strain N, which we found to produce about 85% C18:1 with total un-saturates around 93% in multiple fermentation runs. We investigated if the total saturates will be further diminished by expressing desaturase genes in Strain N.
[0578] In the examples below, we demonstrated the ability to reduce stearic and palmitic levels in wild type strain UTEX1435 by over expression of heterologous stearoyl-ACP desaturase genes, including desaturases from Olea europaea, Ricinus communis, and Chlorella protothecoides.
[0579] Construct used for the expression of the Olea europaea stearoyl-ACP desaturase: To introduce the O. europaea stearoyl-ACP desaturase (Accession No: AAB67840.1) into UTEX1435, Strain A, the Saccharomyces cerevisiae invertase gene was utilized as the selectable marker to confer the ability of growing on sucrose media. The construct that has been expressed in UTEX1435, Strain A can be written as 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp:OeSAD:CvNR::65B and is termed pSZ1377.
[0580] Relevant restriction sites in the construct pSZ1377 are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the second C. reinhardtii .beta.-tubulin promoter driving the expression of the OeSAD, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the OeSAD are indicated by uppercase, bold italics, while the remainder of the stearoyl-ACP desaturase coding region is indicated by bold italics. The Chlorella protothecoides stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00072 Nucleotide sequence of transforming DNA contained in pSZ 1377: (SEQ ID NO: 108) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgctgatgtcca tcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagaggagcatgagg- gaggactcctggtccaggg tcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccgaggccgcctccaactgg- tcctccagcagccgcagtcg ccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacagaacaaccacgagccttgtctaggc- agaatccctaccagtcat ggctttacctggatgacggcctgcgaacagctgtccagcgaccctcgctgccgccgcttctcccgcacgcttct- ttccagcaccgtgatggcgcg agccagcgccgcacgctggcgctgcgcttcgccgatctgaggacagtcggggaactctgatcagtctaaacccc- cttgcgcgttagtgttgcca tcctttgcagaccggtgagagccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtc- acctttttggcgaaggcatcgg ##STR00290## ##STR00291## ##STR00292## ##STR00293## ccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgtggtacgacgagaagg- acgccaagtggcacc tgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctggggccacgccacgtccgacgac- ctgaccaactgggagg accagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccggctccatggtggtggactacaac- aacacctccggcttcttc aacgacaccatcgacccgcgccagcgctgcgtggccatctggacctacaacaccccggagtccgaggagcagta- catctcctacagcctg gacggcggctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactccacccagttccgcgaccc- gaaggtcttctggtacg agccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccgacgac- ctgaagtcctggaagc tggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccacc- gagcaggaccccagca agtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtc- ggcagcttcaacggcaccc acttcgaggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttc- aacaccgacccgacctac gggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctc- ctccatgtccctcgtgcgc aagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagccgatcct- gaacatcagcaacgc cggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctgtccaaca- gcaccggcaccctgga gttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcggacctctccctctggt- tcaagggcctggaggacc ccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttcttcctggaccgcgggaacagcaaggtg- aagttcgtgaaggaga acccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaag- gtgtacggcttgctgga ccagaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccggga- acgccctgggctccgtg ##STR00294## agtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaata- tccctgccgcttttatcaaacag cctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacc- cccagcatccccttccctcgtttcat atcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcact- gcccctcgcacagccttggtttgg gctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtggga- tgggaacacaaatggaggat cccgcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacacc- acaataaccacctgacgaa tgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgaca- atgatcggtggagctgatggtc ##STR00295## ##STR00296## ##STR00297## ##STR00298## ##STR00299## gctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaa- cagcctcagtgtgtttgatcttgtg tgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtt- tcatatcgcttgcatcccaaccgca acttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtt- tgggctccgcctgtattctcctggt actgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagctt- aattaagagctcttgttttc cagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggaggggg- ttcgaatttaaaagcttggaa tgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggaccatcagctccaaaaaactt- gccgctcaaaccgcgtacct ctgctttcgcgcaatctgccctgttgaaatcgccaccacattcatattgtgacgcttgagcagtctgtaattgc- ctcagaatgtggaatcatctgc cccctgtgcgagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctac- ctcacaatagttcataaca gtgaccatatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgcttg- cggagggcaggtcaaccggc atggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccgggatgtgggcc- caccaccagcacaacctgc tggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattccttctgccgctctgc- taccccggtgcttctgtccgaa gcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggcggggcttgttcgagcttgaagagc
[0581] Construct used for the expression of the Ricinus communis stearoyl-ACP desaturase: To introduce the Ricinus communis stearoyl-ACP desaturase (Accession No: AAA74692.1) into UTEX1435, Strain A, the Saccharomyces cerevisiae invertase gene was utilized as the selectable marker to confer the ability of growing on sucrose media. The construct that has been expressed in UTEX1435, Strain A can be written as 6SA::CrTUB2:ScSUC2:CvNR::PmAMT03:CpSADtp:RcSAD:CvNR::6SB and is termed pSZ1454.
[0582] Relevant restriction sites in the construct pSZ1454 are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA that permit targeted integration at 6s nuclear chromosomal locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the endogenous AMT03 promoter driving the expression of the RcSAD, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the RcSAD are indicated by uppercase, bold italics, while the remainder of the stearoyl-ACP desaturase coding region is indicated by bold italics. The Chlorella protothecoides stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00073 Nucleotide sequence of transforming DNA contained in pSZ1454: (SEQ ID NO: 109) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtc gctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaaga- ggagcatga gggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgc- accgaggc cgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatga- attgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgctt cgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgca- gaccggtgag agccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcat- cggcctcggcc ##STR00300## ##STR00301## ##STR00302## ##STR00303## ##STR00304## cgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgacc- ccaacggcc tgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacg- cccttgttctg gggccacgccacgtccgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgact- ccggcgc cttctccggctccatggtggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagc- gctgcgtggcca tctggacctacaacaccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcacc- gagtaccaga agaaccccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaag- tggatcatgac cgcggccaagtcccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccg- cgttcgccaa cgagggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagt- cctactgggt gatgttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacg- gcacccacttcg aggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacacc- gacccgaccta cgggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgct- cctccatgtccc tcgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgag- ccgatcctg aacatcagcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgt- cgacctgtc caacagcaccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgt- tcgcggacctc tccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctcctt- cttcctggaccgc gggaacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagccctt- caagagcg agaacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggc- gacgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcgac ##STR00305## ggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgt- ttgatcttgtgtgtacgcg cttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcg- cttgcatcccaaccgcaac ttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttg- ggctccgcctgtattctcc tggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggagg- atcccgcgtctcg aacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccac- ctgacgaatgcg cttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatga- tcggtggagctgatg ##STR00306## ##STR00307## ##STR00308## ##STR00309## ##STR00310## ##STR00311## ##STR00312## ##STR00313## ##STR00314## ##STR00315## ##STR00316## ##STR00317## ##STR00318## ##STR00319## actgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgttt- gatcttgtgtgtacgcgctt ttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgctt- gcatcccaaccgcaactta tctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggc- tccgcctgtattctcctg gtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagc- ttaattaagagct cttgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattg- tggagggggttc gaatttaaaagcttggaatgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggacc- atcagctcca aaaaacttgccgctcaaaccgcgtacctctgctttcgcgcaatctgccctgttgaaatcgccaccacattcata- ttgtgacgcttg agcagtctgtaattgcctcagaatgtggaatcatctgccccctgtgcgagcccatgccaggcatgtcgcgggcg- aggacaccc gccactcgtacagcagaccattatgctacctcacaatagttcataacagtgaccatatttctcgaagctcccca- acgagcacct ccatgctctgagtggccaccccccggccctggtgcttgcggagggcaggtcaaccggcatggggctaccgaaat- ccccgacc ggatcccaccacccccgcgatgggaagaatctctccccgggatgtgggcccaccaccagcacaacctgctggcc- caggcga gcgtcaaaccataccacacaaatatccttggcatcggccctgaattccttctgccgctctgctacccggtgctt- ctgtccgaagc aggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggcggggcttgttcgagcttgaagagc
[0583] Construct used for the expression of the Chlorella protothecoides stearoyl-ACP desaturase: To introduce the Chlorella protothecoides stearoyl-ACP desaturase into UTEX1435, Strain Z, the Saccharomyces cerevisiae invertase gene was utilized as the selectable marker to confer the ability of growing on sucrose media. The construct that has been expressed in UTEX1435, Strain Z can be written as 6SA::CrTUB2:ScSUC2:CvNR::PmAMT03:CpSAD1:CvNR::6SB and is termed pSZ3144.
[0584] Relevant restriction sites in the construct pSZ3144 are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA that permit targeted integration at 6s locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii -tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the endogenous AMT03 promoter driving the expression of the CpSAD1, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the CpSAD1 are indicated by uppercase, bold italics, while the remainder of the stearoyl-ACP desaturase coding region is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6S genomic region indicated by bold, lowercase text.
TABLE-US-00074 Nucleotide sequence of transforming DNA contained in pSZ3144: (SEQ ID NO: 110) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtcgct gatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaagagga- gcatgagggag gactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgcaccga- ggccgcctccaa ctggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatgaattgtacag- aacaaccacg agccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagctgtccagcgac- cctcgctgccgcc gcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctggcgctgcgcttcgccg- atctgaggacagt cggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgcagaccggtgagagccgact- tgttgtgcgccac cccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcatcggcctcggcctgcagagagg- acagcagtgccc ##STR00320## ##STR00321## ##STR00322## ##STR00323## ##STR00324## gacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcctgtggtacg- acgagaag gacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttgttctggggcca- cgccacgtc cgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccggcgccttctccg- gctccatgg tggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctgg- acctacaaca ccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaac- cccgtgctg gccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtggatcatgaccgc- ggccaagtc ccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgcgttcgccaacg- agggcttcc tcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtcctactgggtg- atgttcatct ccatcaaccccggcgcccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggcacccactt- cgaggccttcga caaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccgacccgacct- acgggagcg ccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctcctccatg- tccctcgtgcgc aagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagccgatcct- gaacatca gcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctg- tccaacag caccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttcgcgg- acctctccctc tggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttcttcct- ggaccgcggg aacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttcaa- gagcgag aacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggcga- cgtcgtgtcc accaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgtt- ctacatcga ##STR00325## tggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtg- tttgatcttgtgtgtac gcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcata- tcgcttgcatcccaacc gcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttg- gtttgggctccgcctgt attctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaa- atggaggatccc gcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccaca- ataaccacctga cgaatgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggt- gacaatgatcggt ##STR00326## ##STR00327## ##STR00328## ##STR00329## ##STR00330## ##STR00331## ##STR00332## ##STR00333## ##STR00334## ##STR00335## ##STR00336## ##STR00337## ##STR00338## ##STR00339## ##STR00340## ccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttg- tgtgtacgcgcttttgc gagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcat- cccaaccgcaacttat ctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggct- ccgcctgtattctcctg gtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagc- ttaattaagag ctcttgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaat- tgtggagggggttcg aatttaaaagcttggaatgttggttcgtgcgtctggaacaagcccagacttgttgctcactgggaaaaggacca- tcagctccaaaa aacttgccgctcaaaccgcgtacctctgctttcgcgcaatctgccctgttgaaatcgccaccacattcatattg- tgacgcttgagcag tctgtaattgcctcagaatgtggaatcatctgccccctgtgcgagcccatgccaggcatgtcgcgggcgaggac- acccgccactcg tacagcagaccattatgctacctcacaatagttcataacagtgaccatatttctcgaagctccccaacgagcac- ctccatgctctga gtggccaccccccggccctggtgcttgcggagggcaggtcaaccggcatggggctaccgaaatccccgaccgga- tcccaccaccc ccgcgatgggaagaatctctccccgggatgtgggcccaccaccagcacaacctgctggcccaggcgagcgtcaa- accataccac acaaatatccttggcatcggccctgaattccttctgccgctctgctacccggtgcttctgtccgaagcaggggt- tgctagggatcgct ccgagtccgcaaacccttgtcgcgtggcggggcttgttcgagcttgaagagc
[0585] Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at either pH5.0 or pH7.0, depending on the promoters that drive the expression of the desaturase genes. Transgenic lines arising from the transformations with pSZ1377 (D583) were assayed in (low-nitrogen) lipid production media at pH5.0, because of the nature of the promoters and the fact that P. moriformis produces more lipid at pH5.0. Transgenic lines generated from the transformation of pSZ1454 (D648) and pSZ3144 (D1923) are assayed at pH 7.0 to allow for maximal desaturase gene expression when driven by the pH regulated PmAMT3 promoter. The resulting profiles from representative clones arising from transformations with D583, D648, and D1923 are shown in Tables 58, 59 and 60, respectively, below. The result of expression of OeSAD and CpSAD1 genes is a clear diminution of C18:0 chain lengths with an increase in C18:1. Also we noticed that there is a subtle increase in the level of C16:1, indicating these stearoyl-ACP desaturases may have broad specificity. The transformants resulted from the expression of RcSAD gene also diminishes in the level of C18:0, and elevation in C16:1. Notably, C16:1 could be increased from under 1% to over 1.5% or over 2%. However, there is also a drop in the level of C18:1 fatty acid and increase in C18:2, which may be caused by the growth defect of these transgenic lines.
TABLE-US-00075 TABLE 58 Lipid profile of representative clones arising from transformation with D583 (pSZ1377) DNA Sample ID C16:0 C16:1 C18:0 C18:1 C18:2 D583-25 19.20 1.53 1.15 64.08 11.76 D583-10 21.86 0.99 1.77 61.43 11.42 D583-3 21.94 0.95 1.85 62.22 10.53 D583-33 20.76 0.95 1.85 61.76 12.17 D583-26 20.18 0.92 1.89 62.56 11.97 D583-1 21.28 0.95 1.90 62.63 10.94 S1331 25.48 0.71 3.23 59.70 8.25
TABLE-US-00076 TABLE 59 Lipid profile of representative clones arising from transformation with D648 (pSZ1454) DNA Sample ID C16:0 C16:1 C18:0 C18:1 C18:2 D648-9 26.92 2.30 1.12 54.27 11.30 D648-28 26.54 2.50 1.32 52.58 12.90 D648-15 29.47 1.68 1.48 51.74 11.48 D648-12 27.39 1.41 1.66 54.45 11.58 D648-43 29.74 1.52 1.68 52.59 10.85 D648-7 26.98 1.62 1.69 54.51 11.39 S1331-pH 7 25.86 0.96 2.84 58.33 9.16
TABLE-US-00077 TABLE 60 Lipid profile of representative clones arising from transformation with D1923 (pSZ3144) DNA. Sample ID C14:0 C14.1 C16:0 C16:1 C18:0 C18:1 C18:2 Block 2; E2; pH7; 1.46 0.11 20.74 2.54 0.86 63.99 9.03 STRAIN Z; T613; D1923-2 Block 2; G12; pH7; 1.52 0.10 25.20 1.97 1.67 61.10 7.38 STRAIN Z; T613; D1923-36 Block 2; E8; pH7; 1.48 0.09 26.41 1.78 1.54 60.54 7.01 STRAIN Z; T613; D1923-8 Block 2; F3; 1.50 0.07 25.87 1.75 1.62 61.25 6.94 pH7STRAIN Z; T613; D1923-15 Block 2; F9; pH7; 1.47 0.07 27.02 1.73 1.84 60.15 6.55 STRAIN Z; T613; D1923-21 Block 2; F4; pH7; 1.44 0.07 24.30 1.71 1.41 62.79 7.29 STRAIN Z; T613; D1923-16 pH7 STRAIN Z 1.47 0.00 28.25 0.82 3.16 58.27 6.72
Example 55
Generation of Palmitoleic Acid by Introducing Mutated (L118W) Stearoyl-ACP Desaturases
[0586] To generate lower total saturates (Zero SAT FAT) strains, we have introduced both putative stearoyl-ACP desaturases (SAD) and palmitoyl-ACP desaturase (PAD) genes into Prototheca moriformis. We found that a single amino acid substitution (L118W) in P. moriformis SAD2-1 and Olea europaea SAD resulted in an increase in desaturation of palmitate moieties in the triglycerides produced by the cell. Oils with fatty acid profiles of over 5% palmitoleic acid were produced in the resulting transgenic lines. Therefore, these mutated SADs could be very useful to elevate palmitoleic as a route to lower total saturates, or to obtain palmitoleic acid containing oils. Oils with over 2, 3, 4, and 5 area% palmitoleic were obtained.
[0587] The Saccharomyces cerevisiae invertase gene (Accession no: NP 012104) was utilized as the selectable marker to introduce the Prototheca moriformis stearoyl-ACP desaturase PmSAD2-1 (L118W) and Olea europaea stearoyl-ACP desaturase OeSAD (L118W) into 6S nuclear chromosomal locus of P. moriformis strain Z by homologous recombination using previously described biolistic transformation methods.
[0588] The constructs that have we used to transform Strain Z can be written as: [0589] 1)6SA::CrTUB2:ScSUC2:CvNR::PmUAPA1: PmSAD2-1(L118W)-CvNR::6SB (pSZ3305, D2066) [0590] 2) 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2: PmSAD2-1(L118W)-CvNR::6SB (pSZ3299, D2060) [0591] 3) 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp-OeSAD (L118W)-CvNR::6SB (pSZ3298, D2059)
[0592] Construct pSZ3305: 6SA::CrTUB2:ScSUC2:CvNR::PmUAPA1: PmSAD2-1(L118W)-CvNR::6SB The sequence of the pSZ3305 transforming DNA is provided below. Relevant restriction sites in pSZ3305 6SA::CrTUB2:ScSUC2:CvNR::PmUAPA1: PmSAD2-1(L118W)-CvNR::6SB are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Asc I, Mfe I, EcoRV, SpeI, AscI, ClaI, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent 6SA genomic DNA that permit targeted integration at 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the P. moriformis UAPA1 promoter, indicated by boxed italics text. The initiator ATG and terminator TGA codons of the PmSAD2-1 (L118W) are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6SB genomic region indicated by bold, lowercase text.
TABLE-US-00078 Nucleotide sequence of transforming DNA contained in pSZ3305: (SEQ ID NO: 111) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcgtc gctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaaga- ggagcatga gggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgccgc- accgaggc cgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatga- attgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgctt cgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgca- gaccggtgag agccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggcat- cggcctcggcc ##STR00341## ##STR00342## ##STR00343## ##STR00344## ##STR00345## cctccatgacgaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgacccc- aacggcctg tggtacgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcc- cttgttctgg ggccacgccacgtccgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactc- cggcgcct tctccggctccatggtggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgc- tgcgtggccatc tggacctacaacaccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccga- gtaccagaa gaaccccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagt- ggatcatgacc gcggccaagtcccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccgc- gttcgccaac gagggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaagtc- ctactgggtg atgttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacgg- cacccacttcga ggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacaccg- acccgacctac gggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccctggcgctc- ctccatgtccct cgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacctgaaggccgagc- cgatcctga acatcagcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtc- gacctgtcc aacagcaccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgtt- cgcggacctct ccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtccgcgtcctccttc- ttcctggaccgcg ggaacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcgtgaacaaccagcccttc- aagagcga gaacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctgtacttcaacgacggcg- acgtcgtgtcca ccaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgttc- tacatcgaca ##STR00346## gactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtt- tgatcttgtgtgtacgcgc ttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgc- ttgcatcccaaccgcaactt atctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttggg- ctccgcctgtattctcct ggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggagga- tcccgcgtctcg aacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccac- ctgacgaatgcg cttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatga- tcggtggagctgatg ##STR00347## ##STR00348## ##STR00349## ##STR00350## ##STR00351## ##STR00352## ##STR00353## ##STR00354## ##STR00355## ##STR00356## ##STR00357## ##STR00358## ##STR00359## tcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccc- tgccgcttttatcaaac agcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaatacca- cccccagcatccccttccct cgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcc- tgctcactgcccctcgca cagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcac- gggaagtagtgggatg ggaacacaaatggaaagcttaattaagagctcttgttttccagaaggagttgctccttgagcctttcattctca- gcctcgataacctc caaagccgctctaattgtggagggggttcgaatttaaaagcttggaatgttggttcgtgcgtctggaacaagcc- cagacttgtt gctcactgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcgtacctctgctttcgcgcaatct- gccctgttga aatcgccaccacattcatattgtgacgcttgagcagtctgtaattgcctcagaatgtggaatcatctgccccct- gtgcgagccca tgccaggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacctcacaatagttcata- acagtgacc atatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgcttgcggagg- gcaggtcaac cggcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccgggatgtg- ggcccacc accagcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattc- cttctgccg ctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgt- ggcggggctt gttcgagcttgaagagc
[0593] Construct pSZ3299: 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2: PmSAD2-1(L118W)-CvNR::6SB The sequence of the pSZ3299 transforming DNA is provided in Sequence 56-2. Relevant restriction sites in pSZ3299 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2:PmSAD2-1(L118W)-CvNR::65B are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, XbaI, Mfe I, EcoRV, SpeI, AscI, ClaI, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent 6SA genomic DNA that permit targeted integration at 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the C. reinhardtii .beta.-tubulin promoter, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the PmSAD2-1 (L118W) are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6SB genomic region indicated by bold, lowercase text.
TABLE-US-00079 Nucleotide sequence of transforming DNA contained in pSZ3299: (SEQ ID NO: 112) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcg tcgctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaa- gaggagcat gagggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgcc- gcaccgagg ccgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatg- aattgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgct tcgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgc- agaccggtg agagccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggc- atcggcctc ##STR00360## ggccggcttcgccgccaagatcagcgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcaccc- ccaacaagg gctggatgaacgaccccaacggcctgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaac- ccgaacgac accgtctgggggacgcccttgttctggggccacgccacgtccgacgacctgaccaactgggaggaccagcccat- cgccatcgc cccgaagcgcaacgactccggcgccttctccggctccatggtggtggactacaacaacacctccggcttcttca- acgacacca tcgacccgcgccagcgctgcgtggccatctggacctacaacaccccggagtccgaggagcagtacatctcctac- agcctggac ggcggctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactccacccagttccgcgacccgaa- ggtcttctg gtacgagccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccg- acgacctga agtcctggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgatc- gaggtcccc accgagcaggaccccagcaagtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctc- cttcaacca gtacttcgtcggcagcttcaacggcacccacttcgaggccttcgacaaccagtcccgcgtggtggacttcggca- aggactact acgccctgcagaccttcttcaacaccgacccgacctacgggagcgccctgggcatcgcgtgggcctccaactgg- gagtactcc gccttcgtgcccaccaacccctggcgctcctccatgtccctcgtgcgcaagttctccctcaacaccgagtacca- ggccaaccc ggagacggagctgatcaacctgaaggccgagccgatcctgaacatcagcaacgccggcccctggagccggttcg- ccaccaaca ccacgttgacgaaggccaacagctacaacgtcgacctgtccaacagcaccggcaccctggagttcgagctggtg- tacgccgtc aacaccacccagacgatctccaagtccgtgttcgcggacctctccctctggttcaagggcctggaggaccccga- ggagtacct ccgcatgggcttcgaggtgtccgcgtcctccttcttcctggaccgcgggaacagcaaggtgaagttcgtgaagg- agaacccct acttcaccaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaaggtgtac- ggcttgctg gaccagaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccgg- gaacgccct gggctccgtgaacatgacgacgggggtggacaacctgttctacatcgacaagttccaggtgcgcgaggtcaagT- GAcaattgg cagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctg- ccttgacct gtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgc- tagctgctt gtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttat- ctacgctgt cctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgta- ttctcctgg tactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatc- ccgcgtctc gaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataacca- cctgacgaa tgcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgaca- atgatcggt ##STR00361## cgtggccgctcctggccgacgcgctgcctctcgtcctctggtggtgcacgccgtggcctccgaggctcctctgg- gcgtgcctc cctccgtgcagcgcccttctcccgtggtgtactccaagctggacaagcagcaccgcctgacgcctgagcgcctg- gagctggtg cagtccatgggccagttcgccgaggagcgcgtgctgcccgtgctgcaccccgtggacaagctgtggcagcccca- ggacttcct gcccgaccccgagtcccccgacttcgaggaccaggtggccgagctgcgcgcccgcgccaaggacctgcccgacg- agtacttcg tggtgctggtgggcgacatgatcaccgaggaggccctgcccacctacatggccatgctgaacacctgggacggc- gtgcgcgac gacaccggcgccgccgaccacccctgggcccgctggacccgccagtgggtggccgaggagaaccgccacggcga- cctgctgaa caagtactgctggctgaccggccgcgtgaacatgcgcgccgtggaggtgaccatcaacaacctgatcaagtccg- gcatgaacc cccagaccgacaacaacccctacctgggcttcgtgtacacctccttccaggagcgcgccaccaagtactcccac- ggcaacacc gcccgcctggccgccgagcacggcgacaagggcctgtccaagatctgcggcctgatcgcctccgacgagggccg- ccacgagat cgcctacacccgcatcgtggacgagttcttccgcctggaccccgagggcgccgtggccgcctacgccaacatga- tgcgcaagc agatcaccatgcccgcccacctgatggacgacatgggccacggcgaggccaaccccggccgcaacctgttcgcc- gacttctcc gccgtggccgagaagatcgacgtgtacgacgccgaggactactgccgcatcctggagcacctgaacgcccgctg- gaaggtgga cgagcgccaggtgtccggccaggccgccgccgaccaggagtacgtgctgggcctgccccagcgcttccgcaagc- tggccgaga agaccgccgccaagcgcaagcgcgtggcccgccgccccgtggccttctcctggatctccggccgcgagatcatg- gtgTGAatc gatagatctcttaaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgtt- gccgccaca cttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacg- cgcttttgc gagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcat- cccaaccgc aacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggt- ttgggctcc gcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatggga- acacaaatg gaaagcttaattaagagctcttgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacc- tccaaagcc gctctaattgtggagggggttcgaatttaaaagcttggaatgttggttcgtgcgtctggaacaagcccagactt- gttgctcac tgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcgtacctctgctttcgcgcaatctgccctg- ttgaaatcg ccaccacattcatattgtgacgcttgagcagtctgtaattgcctcagaatgtggaatcatctgccccctgtgcg- agcccatgc caggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacctcacaatagttcataaca- gtgaccata tttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgcttgcggagggca- ggtcaaccg gcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccgggatgtggg- cccaccacc agcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattcctt- ctgccgctc tgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggc- ggggcttgt tcgagcttgaagagc
[0594] Construct pSZ3298: 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp-OeSAD(L118W)-CvNR::6SB The sequence of the pSZ3299 transforming DNA is provided below. Relevant restriction sites in the construct pSZ3298 6SA::CrTUB2:ScSUC2:CvNR::CrTUB2:CpSADtp-OeSAD(L118W)-CvNR::6SB are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, XbaI, Mfe I, EcoRV, SpeI, AscI, ClaI, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent 6SA genomic DNA that permit targeted integration at 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the C. reinhardtii .beta.-tubulin promoter, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the OeSAD (L118W) are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The Chlorella protothecoides S106 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6SB genomic region indicated by bold, lowercase text.
TABLE-US-00080 Nucleotide sequence of transforming DNA contained in pSZ3298: (SEQ ID NO: 113) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcg tcgctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaa- gaggagcat gagggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgcc- gcaccgagg ccgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatg- aattgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgct tcgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgc- agaccggtg agagccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggc- atcggcctc ##STR00362## ggccggcttcgccgccaagatcagcgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcaccc- ccaacaagg gctggatgaacgaccccaacggcctgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaac- ccgaacgac accgtctgggggacgcccttgttctggggccacgccacgtccgacgacctgaccaactgggaggaccagcccat- cgccatcgc cccgaagcgcaacgactccggcgccttctccggctccatggtggtggactacaacaacacctccggcttcttca- acgacacca tcgacccgcgccagcgctgcgtggccatctggacctacaacaccccggagtccgaggagcagtacatctcctac- agcctggac ggcggctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactccacccagttccgcgacccgaa- ggtcttctg gtacgagccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccg- acgacctga agtcctggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgatc- gaggtcccc accgagcaggaccccagcaagtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctc- cttcaacca gtacttcgtcggcagcttcaacggcacccacttcgaggccttcgacaaccagtcccgcgtggtggacttcggca- aggactact acgccctgcagaccttcttcaacaccgacccgacctacgggagcgccctgggcatcgcgtgggcctccaactgg- gagtactcc gccttcgtgcccaccaacccctggcgctcctccatgtccctcgtgcgcaagttctccctcaacaccgagtacca- ggccaaccc ggagacggagctgatcaacctgaaggccgagccgatcctgaacatcagcaacgccggcccctggagccggttcg- ccaccaaca ccacgttgacgaaggccaacagctacaacgtcgacctgtccaacagcaccggcaccctggagttcgagctggtg- tacgccgtc aacaccacccagacgatctccaagtccgtgttcgcggacctctccctctggttcaagggcctggaggaccccga- ggagtacct ccgcatgggcttcgaggtgtccgcgtcctccttcttcctggaccgcgggaacagcaaggtgaagttcgtgaagg- agaacccct acttcaccaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaaggtgtac- ggcttgctg gaccagaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccgg- gaacgccct gggctccgtgaacatgacgacgggggtggacaacctgttctacatcgacaagttccaggtgcgcgaggtcaagT- GAcaattgg cagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctg- ccttgacct gtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgc- tagctgctt gtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttat- ctacgctgt cctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgta- ttctcctgg tactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatc- ccgcgtctc gaacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataacca- cctgacgaa ##STR00363## tcgctcggcgggctccgggccccggcgcccagcgaggcccctccccgtgcgcgggcgcgccgaggtgcacgtgc- aggtgaccc actccctggcccccgagaagcgcgagatcttcaactccctgaacaactgggcccaggagaacatcctggtgctg- ctgaaggac gtggacaagtgctggcagccctccgacttcctgcccgactccgcctccgagggcttcgacgagcaggtgatgga- gctgcgcaa gcgctgcaaggagatccccgacgactacttcatcgtgctggtgggcgacatgatcaccgaggaggccctgccca- cctaccaga ccatgctgaacacctgggacggcgtgcgcgacgagaccggcgcctccctgaccccctgggccatctggacccgc- gcctggacc gccgaggagaaccgccacggcgacctgctgaacaagtacctgtacctgtccggccgcgtggacatgaagcagat- cgagaagac catccagtacctgatcggctccggcatggacccccgcaccgagaacaacccctacctgggcttcatctacacct- ccttccagg agcgcgccaccttcatctcccacggcaacaccgcccgcctggccaaggagcacggcgacctgaagctggcccag- atctgcggc atcatcgccgccgacgagaagcgccacgagaccgcctacaccaagatcgtggagaagctgttcgagatcgaccc- cgacggcac cgtgctggccctggccgacatgatgcgcaagaaggtgtccatgcccgcccacctgatgtacgacggccaggacg- acaacctgt tcgagaacttctcctccgtggcccagcgcctgggcgtgtacaccgccaaggactacgccgacatcctggagttc- ctggtgggc cgctgggacatcgagaagctgaccggcctgtccggcgagggccgcaaggcccaggactacgtgtgcaccctgcc- cccccgcat ccgccgcctggaggagcgcgcccagtcccgcgtgaagaaggcctccgccacccccttctcctggatcttcggcc- gcgagatca acctgatggactacaaggaccacgacggcgactacaaggaccacgacatcgactacaaggacgacgacgacaag- TGAatcgat agatctcttaaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgcc- gccacactt gctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgc- ttttgcgag ttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatccc- aaccgcaac ttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttg- ggctccgcc tgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaaca- caaatggaa agcttaattaagagctcttgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacctcc- aaagccgct ctaattgtggagggggttcgaatttaaaagcttggaatgttggttcgtgcgtctggaacaagcccagacttgtt- gctcactgg gaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcgtacctctgctttcgcgcaatctgccctgttg- aaatcgcca ccacattcatattgtgacgcttgagcagtctgtaattgcctcagaatgtggaatcatctgccccctgtgcgagc- ccatgccag gcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacctcacaatagttcataacagtg- accatattt ctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgcttgcggagggcaggt- caaccggca tggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccgggatgtgggccc- accaccagc acaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattccttctg- ccgctctgc tacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggcggg- gcttgttcg agcttgaagagc
[0595] Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at pH5.0. The resulting profiles from representative clones arising from transformations with pSZ3305, pSZ3299 and pSZ3298 into Strain Z are shown in Tables 61-63 respectively. Thus, introductions of such mutations or genes can increase levels of palmitoleic acid and decrease levels of saturation in the fatty acid profiles of oils produced by recombinant microalgae. Oils were obtained with C16:1/C16:0 ratios of at least 0.1, 0.15, and 0.18.
TABLE-US-00081 TABLE 61 Fatty acid profiles in Strain Z and derivative transgenic lines transformed with pSZ3305 (D2066). C16:1: C16:0 Sample ID C14:0 C16:0 C16:1 C18:0 C18:1 C18:2 ratio pH5; T657; 1.27 24.73 4.55 3.63 58.62 5.84 0.18 D2066-29 pH5; T657; 1.27 22.89 3.94 3.17 60.69 6.61 0.17 D2066-16 pH5; T657; 1.33 25.47 3.07 3.58 59.32 5.86 0.12 D2066-36 pH5; T657; 1.28 22.48 2.42 3.66 61.65 7.02 0.11 D2066-19 pH5;; T657; 1.29 26.25 2.26 3.99 59.27 5.50 0.09 D2066-12 pH5; T657; 1.33 24.49 2.26 3.24 61.42 6.01 0.09 D2066-21 pH5; Strain Z 1.40 27.70 0.89 3.91 57.34 7.05 0.03 (200:1)
TABLE-US-00082 TABLE 62 Fatty acid profiles in Strain Z and derivative transgenic lines transformed with pSZ3299 (D2060). C16:1: C16:0 Sample ID C14:0 C16:0 C16:1 C18:0 C18:1 C18:2 ratio pH5; T655; D2060-9 1.35 24.67 2.73 3.21 60.34 6.22 0.11 pH5; T655; D2060-23 1.52 30.05 2.64 1.65 55.38 7.03 0.09 pH5; T655; D2060-21 1.29 23.54 2.43 2.94 62.25 6.18 0.10 pH5; T655; D2060-2 1.29 24.30 2.22 2.57 62.09 6.28 0.09 pH5; T655; D2060-12 1.37 27.67 1.90 2.84 59.69 5.41 0.07 pH5; T655; D2060-14 1.41 25.01 1.62 2.47 61.30 6.96 0.06 pH5 Strain Z 1.40 27.89 0.87 3.25 57.84 7.19 0.03
TABLE-US-00083 TABLE 63 Fatty acid profiles in Strain Z and derivative transgenic lines transformed with pSZ3298 (D2059). C16:1: C16:0 Sample ID C14:0 C16:0 C16:1 C18:0 C18:1 C18:2 ratio pH5; T655; D2059-21 1.09 25.44 5.04 1.86 54.78 10.44 0.19 pH5; T655; D2059-19 1.28 23.11 2.71 2.19 60.66 8.64 0.12 pH5; T655; D2059-4 1.68 28.19 1.61 2.54 58.39 6.37 0.06 pH5; T655; D2059-23 1.37 23.25 1.45 2.92 62.15 7.44 0.06 pH5; T655; D2059-1 1.38 23.34 1.28 2.68 62.31 7.62 0.05 pH5 Strain Z 1.40 27.89 0.87 3.25 57.84 7.19 0.03
Example 56
Down Regulation of FATA and Over Expression of the Prototheca Moriformis KETO-Acyl-ACP Synthase II (PMKASII) Gene
[0596] A transgenic P. moriformis line was created with downregulation of an endogenous FATA1 gene combined with overexpression of an endogenous KASII gene. The resulting strain produced a triglyceride-rich oil that was enriched in oleate.
[0597] In the example below, we have followed up on previous work demonstrating that triacylglycerols in algae can be significantly enriched in levels of oleate (C18:1) utilizing molecular genetic approaches, such as down regulating endogenous FATA1 (a single FATA allele) and over-expression of endogenous KASII activity. In this example, we focus our efforts on combining these approaches into a single transgenic line. Constructs that disrupt a single copy of the FATA1 allele while simultaneously overexpressing the P. moriformis KASII gene (PmKASII). were introduced into a high oleic Prototheca moriformis Strain AO. Strain AO was derived from a high 18:1 producing mutant derived from UTEX 1435 using classical mutagenesis techniques. One of the resulting strains, termed Strain AP, produced an oil with a fatty acid profile having 85% C18:1 with total un-saturates around 93% in multiple fermentation runs. The strain AP also had high lipid productivity.
[0598] The Saccharomyces cerevisiae invertase gene (Accession no: NP 012104) was utilized as the selectable marker to introduce the PmKASII into the FATA1 nuclear chromosomal locus of P. moriformis strain AO by homologous recombination using biolistic transformation. To investigate the KASII activity when driven by different promoters, PmKASII was fused to several promoters: PmUAPA1, PmLDH1, and PmAMT3. Note that the integration constructs are all designed as reverse orientation to the FATA1 gene; this was found to give a greater likelihood of stable invertase expression. Therefore, the constructs that have been expressed in Strain AH can be written as: [0599] 1)FATA1 3'::CrTUB2:ScSUC2:CvNR::PmUAPA1:PmKASII-CvNR::FATA1 5' (pSZ2533) [0600] 2) FATA1 3'::CrTUB2:ScSUC2:CvNR::PmLDH1:PmKASII-CvNR::FATA1 5' (pSZ2532) [0601] 3) FATA1 3'::CrTUB2:ScSUC2:CvNR::PmAMT3:PmKASII-CvNR::FATA1 5' (pSZ2750)
[0602] Strain AP is one of the transformants generated from pSZ2533. Relevant restriction sites in the construct pSZ2533 FATA13'::CrTUB2:ScSUC2:CvNR::PmUAPA1:PmKASII-CvNR::FATA1 5' are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Asc I, Mfe I, EcoRV, SpeI, AscI, ClaI, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent FATA1 3' genomic DNA that permit targeted integration at FATA1 locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii .beta.-tubulin promoter driving the expression of the yeast sucrose invertase gene is indicated by boxed text. The initiator ATG and terminator TGA for invertase are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the P. moriformis UAPA1 promoter, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the PmKASII are indicated by uppercase, bold italics, while the remainder of the coding region is indicated by bold italics. The Chlorella protothecoides S106 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the FATA1 5' genomic region indicated by bold, lowercase text.
TABLE-US-00084 Nucleotide sequence of transforming DNA contained in pSZ2533: (SEQ ID NO: 114) gctcttcacccaactcagataataccaatacccctccttctcctcctcatccattcagtacccccccccttctc- ttcccaaag cagcaagcgcgtggcttacagaagaacaatcggcttccgccaaagtcgccgagcactgcccgacggcggcgcgc- ccagcagcc cgcttggccacacaggcaacgaatacattcaatagggggcctcgcagaatggaaggagcggtaaagggtacagg- agcactgcg cacaaggggcctgtgcaggagtgactgactgggcgggcagacggcgcaccgcgggcgcaggcaagcagggaaga- ttgaagcgg cagggaggaggatgctgattgaggggggcatcgcagtctctcttggacccgggataaggaagcaaatattcggc- cggttgggt tgtgtgtgtgcacgttttcttcttcagagtcgtgggtgtgcttccagggaggatataagcagcaggatcgaatc- ccgcgacca ##STR00364## catgacgaacgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacg- gcctgtggt acgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgggggacgcccttg- ttctggggc cacgccacgtccgacgacctgaccaactgggaggaccagcccatcgccatcgccccgaagcgcaacgactccgg- cgccttctc cggctccatggtggtggactacaacaacacctccggcttcttcaacgacaccatcgacccgcgccagcgctgcg- tggccatct ggacctacaacaccccggagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgag- taccagaag aaccccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccagaagtg- gatcatgac cgcggccaagtcccaggactacaagatcgagatctactcctccgacgacctgaagtcctggaagctggagtccg- cgttcgcca acgagggcttcctcggctaccagtacgagtgccccggcctgatcgaggtccccaccgagcaggaccccagcaag- tcctactgg gtgatgttcatctccatcaaccccggcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaa- cggcaccca cttcgaggccttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttca- acaccgacc cgacctacgggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgcccaccaacccc- tggcgctcc tccatgtccctcgtgcgcaagttctccctcaacaccgagtaccaggccaacccggagacggagctgatcaacct- gaaggccga gccgatcctgaacatcagcaacgccggcccctggagccggttcgccaccaacaccacgttgacgaaggccaaca- gctacaacg tcgacctgtccaacagcaccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctcc- aagtccgtg ttcgcggacctctccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggtgtc- cgcgtcctc cttcttcctggaccgcgggaacagcaaggtgaagttcgtgaaggagaacccctacttcaccaaccgcatgagcg- tgaacaacc agcccttcaagagcgagaacgacctgtcctactacaaggtgtacggcttgctggaccagaacatcctggagctg- tacttcaac gacggcgacgtcgtgtccaccaacacctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgac- gggggtgga caacctgttctacatcgacaagttccaggtgcgcgaggtcaagTGAcaattggcagcagcagctcggatagtat- cgacacact ctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgctttt- atcaaacag cctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacc- cccagcatc cccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgc- tcctgctcc tgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagc- actgcaatg ctgatgcacgggaagtagtgggatgggaacacaaatggaggatcccgcgtctcgaacagagcgcgcagaggaac- gctgaaggt ctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgcttggttcttcgtccat- tagcgaagc gtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctgatggtcgaaacgtt- cacagccta ##STR00365## gggctccgggccccggcgcccagcgaggcccctccccgtgcgcgggcgcgccgccgccgccgccgacgccaacc- ccgcccgcc ccgagcgccgcgtggtgatcaccggccagggcgtggtgacctccctgggccagaccatcgagcagttctactcc- tccctgctg gagggcgtgtccggcatctcccagatccagaagttcgacaccaccggctacaccaccaccatcgccggcgagat- caagtccct gcagctggacccctacgtgcccaagcgctgggccaagcgcgtggacgacgtgatcaagtacgtgtacatcgccg- gcaagcagg ccctggagtccgccggcctgcccatcgaggccgccggcctggccggcgccggcctggaccccgccctgtgcggc- gtgctgatc ggcaccgccatggccggcatgacctccttcgccgccggcgtggaggccctgacccgcggcggcgtgcgcaagat- gaacccctt ctgcatccccttctccatctccaacatgggcggcgccatgctggccatggacatcggcttcatgggccccaact- actccatct ccaccgcctgcgccaccggcaactactgcatcctgggcgccgccgaccacatccgccgcggcgacgccaacgtg- atgctggcc ggcggcgccgacgccgccatcatcccctccggcatcggcggcttcatcgcctgcaaggccctgtccaagcgcaa- cgacgagcc cgagcgcgcctcccgcccctgggacgccgaccgcgacggcttcgtgatgggcgagggcgccggcgtgctggtgc- tggaggagc tggagcacgccaagcgccgcggcgccaccatcctggccgagctggtgggcggcgccgccacctccgacgcccac- cacatgacc gagcccgacccccagggccgcggcgtgcgcctgtgcctggagcgcgccctggagcgcgcccgcctggcccccga- gcgcgtggg ctacgtgaacgcccacggcacctccacccccgccggcgacgtggccgagtaccgcgccatccgcgccgtgatcc- cccaggact ccctgcgcatcaactccaccaagtccatgatcggccacctgctgggcggcgccggcgccgtggaggccgtggcc- gccatccag gccctgcgcaccggctggctgcaccccaacctgaacctggagaaccccgcccccggcgtggaccccgtggtgct- ggtgggccc ccgcaaggagcgcgccgaggacctggacgtggtgctgtccaactccttcggcttcggcggccacaactcctgcg- tgatcttcc gcaagtacgacgagatggactacaaggaccacgacggcgactacaaggaccacgacatcgactacaaggacgac- gacgacaag TGAatcgatagatctcttaaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatgg- actgttgcc gccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtg- tgtacgcgc ttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgc- ttgcatccc aaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagc- cttggtttg ggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtggg- atgggaaca caaatggaaagcttaattaagagcttgttttccagaaggagttgctccttgagcctttcattctcagcctcgat- aacctccaa agccgctctaattgtggagggggttcgaaccgaatgctgcgtgaacgggaaggaggaggagaaagagtgagcag- ggagggatt cgtgtcctctgatcatcatgcgattgattacgttgaatgcgacggccggtcagccccggacctccacgcaccgc- agaaatgag aaatgagaggtgaaggaacgcatccctatgcccttgcaatggacagtgtttctggccaccgccaccaagacttg- tgctcctcc aggaagatgcgcttgtcctccgccatcttgcagggctcaagctgctcccaaaactcttgggcgggttccggacg- gacggctac cgcgggtgcggccctgaccgccactgttcggaagcagcggcgctgcatgggcagcggccgctgcggtgcgccac- ggaccgcat gatccaccggaaaagcgcacgcgctggagcgcgcagaggaccacagagaagcggaagagacgccagtactggca- agcaggctg gtcggtgccatggcgcgctactaccctcgctatgactcgggtcctcggccggctggcggtgctgacaattcgtt- tagtggagc agcgactccattcagctaccagtcgaactcagtggcacagtgactccgctcttc
[0603] In addition to the construct pSZ2533, we also investigated the PmKASII activity when the KASII gene driven by other promoters, including PmLDH1, and PmAMT3. The plasmid pSZ2532 can be written as FATA1 3'::CrTUB2:ScSUC2:CvNR::PmLDH1:PmKASII-CvNR::FATA1 5', while the plasmid pSZ2750 can be written as FATA1 3'::CrTUB2:ScSUC2: CvNR::PmAMT3:PmKASII-CvNR::FATA1 5'. Since the sequences of these two plasmids are the same as pSZ2533 except for the promoter that drives the PmKASII, the following sequences only show the sequence of the PmLDH1 and PmAMT3 promoters.
TABLE-US-00085 Nucleotide sequence of PmLDH1 promoter that drive the expression of PmKASII in pSZ2532: (SEQ ID NO: 115) ##STR00366## Nucleotide sequence of PmAMT3 promoter that drive the expression of PmKASII in pSZ2750: (SEQ ID NO: 116) ##STR00367##
[0604] Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at either pH5.0 or pH7.0, depend on the promoters that driven the expression of the PmKASII gene. Transgenic lines arising from the transformations with pSZ2533 (D1636) and pSZ2532 (D1637) were assayed in lipid production media at pH5.0, because of the nature of the promoters and the fact that P. moriformis produces more lipid at pH5.0. Transgenic lines generated from the transformation of pSZ2750 (D1684) were assayed at pH 7.0 to allow for maximal PmKASII gene expression when driven by the pH regulated PmAMT3 promoter. The resulting profiles from representative clones arising from transformations with D1636 (pSZ2533), D1637 (pSZ2532), and D1684 (pSZ2750) are shown in Tables 64-66, respectively.
[0605] The impact of FATA1 knock-out and simultaneously overexpressing the P. moriformis KASII gene is a clear diminution of C16:0 chain lengths with a significant increase in C18:1. At pH5.0, it appears that PmUAPA1 is stronger than PmLDH1, the palmitate level in D1636 transformants is close to 3%, while none of the transformants in D1637 go below 7% at the same condition.
TABLE-US-00086 TABLE 64 Lipid profile of representative clones arising from transformation with D1636 (pSZ2533) DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 pH 5; T523; D1636-3 0.53 3.31 6.15 79.89 7.19 pH 5; T523; D1636-4 0.48 3.54 5.34 80.78 6.92 pH 5; T523; D1636-5 0.48 3.59 5.41 81.37 6.55 pH 5; T523; D1636-12 0.61 3.59 3.67 80.52 8.93 pH 5; T523; D1636-13 0.55 3.80 4.88 81.83 6.61 pH 5; T523; D1636-21 0.54 4.18 2.82 82.26 8.17 pH 5; Strain AO 0.89 17.28 2.69 70.53 6.86
TABLE-US-00087 TABLE 65 Lipid profile of representative clones arising from transformation with D1637 (pSZ2532) DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 pH 5; T523; D1637-6 0.46 7.64 3.43 80.08 6.33 pH 5; T523; D1637-12 0.66 8.49 1.90 77.06 9.59 pH 5; T523; D1637-13 0.47 8.59 3.18 79.39 6.54 pH 5; T523; D1637-15 0.60 9.60 2.51 76.41 8.85 pH 5; T523; D1637-7 0.61 11.16 2.21 75.82 8.04 pH 5; T523; D1637-8 0.93 11.29 3.61 74.84 6.61 pH 5; Strain AO 0.89 17.28 2.69 70.53 6.86
TABLE-US-00088 TABLE 66 Lipid profile of representative clones arising from transformation with D1684 (pSZ2750) DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 pH 7; T532; D1684-14 0.55 5.04 4.90 78.88 8.19 pH 7; T532; D1684-23 0.58 5.80 4.98 77.51 8.69 pH 7; T532; D1684-1 0.59 6.37 4.99 77.47 8.03 pH 7; T532; D1684-24 0.55 6.37 4.83 77.98 7.73 pH 7; T532; D1684-11 0.61 6.61 4.88 76.14 8.96 pH 7; T532; D1684-16 0.57 6.61 5.01 77.74 7.83 pH 7; Strain AO 0.84 20.12 3.52 66.86 6.77
Example 57
Generation of a High-Oleic High-Stability (HOHS) Oil-Producing Strain
[0606] Strain AP of Example 56 produces oil with about 85% oleic acid with total un-saturates around 93%. Here we show that that that the oxidative stability of the high-oleic oil can be improved by knock-down of a delta 12 fatty acid desaturase, thereby reducing linoleic acid production in the oleaginous cell.
[0607] We expressed a hairpin-RNA-producing construct in Strain AP targeting an endogenous FAD gene, PmFAD2. The resulting strains, including Strain AQ, produce >90% C18:1 and <1% C18:2 in fermenters. Most importantly, Strain AQ retains the same level of lipid productivity and sucrose hydrolyzing ability as its parental strain, Strain AP.
[0608] Generation of high oleic high stability oil producing strain AQ: Construct used for down regulating PmFAD2. To generate a strain that produces oil with high oxidative stability, the hairpin PmFAD2 was introduced into AP for down regulating PmFAD2 expression. Strain AQ is a stable line generated from the transformation of pSZ3372 DNA (6SA::PmHXT1:ScarMEL1:CvNR::CrTUB2: Hairpin PmFAD2:CvNR::6SB) into Strain AP. In this construct, the Saccharomyces carlbergensis MEL1 gene was utilized as the selectable marker to introduce the Hairpin PmFAD2 into the 6S nuclear chromosomal locus of P. moriformis strain AQ by homologous recombination using previously described transformation methods (biolistics).
[0609] The sequence of the pSZ3372 transforming DNA is provided below. Relevant restriction sites in pSZ3372 are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, SpeI, Mfe I, BamHI, EcoRV, SpeI, XhoI, SacI, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent 6SA genomic DNA that permits targeted integration at 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the P. moriformis HXT1 promoter driving the expression of the S. carlbergensis MEL1 gene is indicated by boxed text. The initiator ATG and terminator TGA for ScarMEL1 are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by the C. reinhardtii .beta.-tubulin promoter, indicated by boxed italics text. The hairpin PmFAD2 cassette includes the P. moriformis FAD2 exon1 (indicated by italics underlined text), the intron of PmFAD2 (italics lowercase text), and followed by the inverted PmFAD2 exon1 (indicated by italics underlined text). The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the 6SB genomic region indicated by bold, lowercase text.
TABLE-US-00089 Nucleotide sequence of transforming DNA contained in pSZ3372: (SEQ ID NO: 117) gctcttcgccgccgccactcctgctcgagcgcgcccgcgcgtgcgccgccagcgccttggccttttcgccgcgc- tcgtgcgcg tcgctgatgtccatcaccaggtccatgaggtctgccttgcgccggctgagccactgcttcgtccgggcggccaa- gaggagcat gagggaggactcctggtccagggtcctgacgtggtcgcggctctgggagcgggccagcatcatctggctctgcc- gcaccgagg ccgcctccaactggtcctccagcagccgcagtcgccgccgaccctggcagaggaagacaggtgaggggggtatg- aattgtaca gaacaaccacgagccttgtctaggcagaatccctaccagtcatggctttacctggatgacggcctgcgaacagc- tgtccagcg accctcgctgccgccgcttctcccgcacgcttctttccagcaccgtgatggcgcgagccagcgccgcacgctgg- cgctgcgct tcgccgatctgaggacagtcggggaactctgatcagtctaaacccccttgcgcgttagtgttgccatcctttgc- agaccggtg agagccgacttgttgtgcgccaccccccacaccacctcctcccagaccaattctgtcacctttttggcgaaggc- atcggcctc ##STR00368## ccctgaagggcgtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatgggctgggacaac- tggaacacg ttcgcctgcgacgtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctgaaggacat- gggctacaa gtacatcatcctggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgagcagaagt- tccccaacg gcatgggccacgtcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcgagtac- acgtgcgcc ggctaccccggctccctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacctgaa- gtacgacaa ctgctacaacaagggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccctga- acaagacgg gccgccccatcttctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcgaac- tcctggcgc atgtccggcgacgtcacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactg- caagtacgc cggcttccactgctccatcatgaacatcctgaacaaggccgcccccatgggccagaacgcgggcgtcggcggct- ggaacgacc tggacaacctggaggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatg- gtgaagtcc cccctgatcatcggcgcgaacgtgaacaacctgaaggcctcctcctactccatctactcccaggcgtccgtcat- cgccatcaa ccaggactccaacggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccagg- gcgagatcc agatgtggtccggccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgcccc- atgaacacg accctggaggagatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacct- gtgggcgaa ccgcgtcgacaactccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgcca- ccgagcagt cctacaaggacggcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcg- atcctgaac acgaccgtccccgcccacggcatcgcgttctaccgcctgcgcccctcctccTGAcaattggcagcagcagctcg- gatagtatc gacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctg- ccgctttta tcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcga- ataccaccc ccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctc- agcgctgct cctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgt- aaaccagca ctgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatcccgcgtctcgaacagagcgcgc- agaggaacg ctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgcttggttct- tcgtccatt agcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctgatggtc- gaaacgttc ##STR00369## cactgtttcgagcgctcggcgcttcgtagcagcatgtacctggcctttgacatcgcggtcatgtccctgctcta- cgtcgcgtc gacgtacatcgaccctgcaccggtgcctacgtgggtcaagtacggcatcatgtggccgctctactggttcttcc- aggtgtgtt tgagggttttggttgcccgtattgaggtcctggtggcgcgcatggaggagaaggcgcctgtcccgctgaccccc- ccggctacc ctcccggcaccttccagggcgcgtacgggaagaaccagtagagcggccacatgatgccgtacttgacccacgta- ggcaccggt gcagggtcgatgtacgtcgacgcgacgtagagcagggacatgaccgcgatgtcaaaggccaggtacatgctgct- acgaagcgc cgagcgctcgaaacagtgcgcggggatggccttgcgcagcgtcccgatcgtgaacggaggcttctccacaggct- gcctgttcg tcttgatagccatctcgaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggac- tgttgccgc cacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtg- tacgcgctt ttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgctt- gcatcccaa ccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagcct- tggtttggg ctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggat- gggaacaca aatggaaagctgtagagctcttgttttccagaaggagttgctccttgagcctttcattctcagcctcgataacc- tccaaagcc gctctaattgtggagggggttcgaatttaaaagcttggaatgttggttcgtgcgtctggaacaagcccagactt- gttgctcac tgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcgtacctctgctttcgcgcaatctgccctg- ttgaaatcg ccaccacattcatattgtgacgcttgagcagtctgtaattgcctcagaatgtggaatcatctgccccctgtgcg- agcccatgc caggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacctcacaatagttcataaca- gtgaccata tttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgcttgcggagggca- ggtcaaccg gcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccgggatgtggg- cccaccacc agcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccctgaattcctt- ctgccgctc tgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaacccttgtcgcgtggc- ggggcttgt tcgagcttgaagagc
[0610] We introduced the hairpin PmFAD2 construct into strain AP. Transgenic lines arising from the transformations with pSZ3372 (D2082) were assayed in lipid production media at pH5.0, the resulting profiles from representative clones are shown in Table 67. Among more than 400 transformants we had screened, the strain AQ was isolated from the transformant D2082.1, which produced <1% C18:2 during the initial profile screening. Thus, this strain can be used to produce a triglyceride oil that is both high in oleic acid and low in polyunsaturates. Due to the low polyunsaturate levels, the oil is expected to have a high oxidative stability when tested via the AOCS Cd 12b-92 method (see Section IV of this patent application and corresponding examples).
TABLE-US-00090 TABLE 67 Lipid profile of representative clones arising from transformation with D2082 (pSZ3372) DNA. Sample ID C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. SAP_pH 5.0_glucose_day 5-T658; D2082-1 4.42 3.80 89.36 0.65 0.10 StrainAP_pH 5.0_glucose_day 5-T658; D2082-87 3.77 4.01 88.70 1.52 0.19 StrainAP_pH 5.0_glucose_day 5-T658; D2082-93 5.14 3.58 87.63 1.65 0.19 StrainAP_pH 5.0_glucose_day 5-T658; D2082-78 3.74 2.40 89.69 1.97 0.23 StrainAP_pH 5.0_glucose_day 5 4.10 3.77 83.55 6.41 0.40
Example 58
Generating High Oleic "Zero" Linoleic Strains by Knock-Out Prototheca Moriformis (PM) FAD2 AND FATA Genes and Over-Expression of PMKASII Gene
[0611] Triacylglycerols in microalgae can be significantly enriched in levels of oleate (C18:1) utilizing molecular genetic approaches, such as down regulating endogenous FATA1 and FADc genes and over-expression of endogenous KASII activity. In this example, we focus our efforts on combining these approaches into a single transgenic line. Constructs that disrupt a single copy of the FATA1 allele while simultaneously overexpressing the Prototheca moriformis KASII gene were introduced into different .DELTA.fad2 lines, termed Strain R and Strain D (see genealogy in FIG. 24). The resulting strains, such as Strain AS and Strain AZ produces around 90% C18:1 with <0.05% C18:2.
[0612] Strain D and Strain R are .DELTA.fad2 lines that produce oils comprised of 0% C18:2, and between 76% to 87% C18:1, depending upon whether they are grown in shake flasks or high cell density fermentations, respectively. To further elevate oleate levels in Strain D and Strain R, constructs that disrupt a single copy of the FATA1 allele while simultaneously overexpressing the P. moriformis KAS II gene were introduced in Strain D/Strain R via particle bombardment.
[0613] Construct to knock out FATA genes and over expression of PmKASII in S2530 background. Relevant restriction sites in the construct FATA1::CpACT-AtThic-nr:AMT03-S106SAD-PmKASII-nr::FATA1 (termed pSZ2276) are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR I, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UTEX1435 that permit targeted integration at FATA1 gene via homologous recombination. Proceeding in the 5' to 3' direction, the actin gene promoter from UTEX 250 driving the expression of the Arabidopsis thaliana THIC gene is indicated by the boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous AMT03 promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the P. moriformis KASII gene are indicated by uppercase, bold italics, while the remainder of the PmKASII coding region is indicated by bold italics. The Chlorella protothecoides UTEX 250 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX1435 FATA1 genomic region indicated by bold, lowercase text.
TABLE-US-00091 Nucleotide sequence of transforming DNA contained in pSZ2276: (SEQ ID NO: 118) gctcttcggagtcactgtgccactgagttcgactggtagctgaatggagtcgctgctccactaaacgaattgtc- agcaccgcc agccggccgaggacccgagtcatagcgagggtagtagcgcgccatggcaccgaccagcctgcttgccagtactg- gcgtctctt ccgcttctctgtggtcctctgcgcgctccagcgcgtgcgcttttccggtggatcatgcggtccgtggcgcaccg- cagcggccg ctgcccatgcagcgccgctgcttccgaacagtggcggtcagggccgcacccgcggtagccgtccgtccggaacc- cgcccaaga gttttgggagcagcttgagccctgcaagatggcggaggacaagcgcatcttcctggaggagcaccggtgcgtgg- aggtccggg gctgaccggccgtcgcattcaacgtaatcaatcgcatgatgatcagaggacacgaagtcttggtggcggtggcc- agaaacact gtccattgcaagggcatagggatgcgttccttcacctctcatttctcatttctgaatccctccctgctcactct- ttctcctcc ##STR00370## tggtctgcaacaacaagaaccactccgcccgccccaagctgcccaactcctccctgctgcccggcttcgacgtg- gtggtccag gccgcggccacccgcttcaagaaggagacgacgaccacccgcgccacgctgacgttcgacccccccacgaccaa- ctccgagcg cgccaagcagcgcaagcacaccatcgacccctcctcccccgacttccagcccatcccctccttcgaggagtgct- tccccaagt ccacgaaggagcacaaggaggtggtgcacgaggagtccggccacgtcctgaaggtgcccttccgccgcgtgcac- ctgtccggc ggcgagcccgccttcgacaactacgacacgtccggcccccagaacgtcaacgcccacatcggcctggcgaagct- gcgcaagga gtggatcgaccgccgcgagaagctgggcacgccccgctacacgcagatgtactacgcgaagcagggcatcatca- cggaggaga tgctgtactgcgcgacgcgcgagaagctggaccccgagttcgtccgctccgaggtcgcgcggggccgcgccatc- atcccctcc aacaagaagcacctggagctggagcccatgatcgtgggccgcaagttcctggtgaaggtgaacgcgaacatcgg- caactccgc cgtggcctcctccatcgaggaggaggtctacaaggtgcagtgggccaccatgtggggcgccgacaccatcatgg- acctgtcca cgggccgccacatccacgagacgcgcgagtggatcctgcgcaactccgcggtccccgtgggcaccgtccccatc- taccaggcg ctggagaaggtggacggcatcgcggagaacctgaactgggaggtgttccgcgagacgctgatcgagcaggccga- gcagggcgt ggactacttcacgatccacgcgggcgtgctgctgcgctacatccccctgaccgccaagcgcctgacgggcatcg- tgtcccgcg gcggctccatccacgcgaagtggtgcctggcctaccacaaggagaacttcgcctacgagcactgggacgacatc- ctggacatc tgcaaccagtacgacgtcgccctgtccatcggcgacggcctgcgccccggctccatctacgacgccaacgacac- ggcccagtt cgccgagctgctgacccagggcgagctgacgcgccgcgcgtgggagaaggacgtgcaggtgatgaacgagggcc- ccggccacg tgcccatgcacaagatccccgagaacatgcagaagcagctggagtggtgcaacgaggcgcccttctacaccctg- ggccccctg acgaccgacatcgcgcccggctacgaccacatcacctccgccatcggcgcggccaacatcggcgccctgggcac- cgccctgct gtgctacgtgacgcccaaggagcacctgggcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctaca- agatcgccg cccacgcggccgacctggccaagcagcacccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttc- gagttccgc tggatggaccagttcgcgctgtccctggaccccatgacggcgatgtccttccacgacgagacgctgcccgcgga- cggcgcgaa ggtcgcccacttctgctccatgtgcggccccaagttctgctccatgaagatcacggaggacatccgcaagtacg- ccgaggaga acggctacggctccgccgaggaggccatccgccagggcatggacgccatgtccgaggagttcaacatcgccaag- aagacgatc tccggcgagcagcacggcgaggtcggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTG- Acaattggc agcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgc- cttgacctg tgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgattttgcgagttgct- agctgcttg tgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatc- tacgctgtc ctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtat- tctcctggt actgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatcc- cgcgtctcg aacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccac- ctgacgaat gcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaa- tgatcggtg ##STR00371## gcccgcctgcccaccgcctcccgccgcgccgtgcgccgcgcctggtcccgcatcgcccgcgggcgcgccgccgc- cgccgccga cgccaaccccgcccgccccgagcgccgcgtggtgatcaccggccagggcgtggtgacctccctgggccagacca- tcgagcagt tctactcctccctgctggagggcgtgtccggcatctcccagatccagaagttcgacaccaccggctacaccacc- accatcgcc ggcgagatcaagtccctgcagctggacccctacgtgcccaagcgctgggccaagcgcgtggacgacgtgatcaa- gtacgtgta catcgccggcaagcaggccctggagtccgccggcctgcccatcgaggccgccggcctggccggcgccggcctgg- accccgccc tgtgcggcgtgctgatcggcaccgccatggccggcatgacctccttcgccgccggcgtggaggccctgacccgc- ggcggcgtg cgcaagatgaaccccttctgcatccccttctccatctccaacatgggcggcgccatgctggccatggacatcgg- cttcatggg ccccaactactccatctccaccgcctgcgccaccggcaactactgcatcctgggcgccgccgaccacatccgcc- gcggcgacg ccaacgtgatgctggccggcggcgccgacgccgccatcatcccctccggcatcggcggcttcatcgcctgcaag- gccctgtcc aagcgcaacgacgagcccgagcgcgcctcccgcccctgggacgccgaccgcgacggcttcgtgatgggcgaggg- cgccggcgt gctggtgctggaggagctggagcacgccaagcgccgcggcgccaccatcctggccgagctggtgggcggcgccg- ccacctccg acgcccaccacatgaccgagcccgacccccagggccgcggcgtgcgcctgtgcctggagcgcgccctggagcgc- gcccgcctg gcccccgagcgcgtgggctacgtgaacgcccacggcacctccacccccgccggcgacgtggccgagtaccgcgc- catccgcgc cgtgatcccccaggactccctgcgcatcaactccaccaagtccatgatcggccacctgctgggcggcgccggcg- ccgtggagg ccgtggccgccatccaggccctgcgcaccggctggctgcaccccaacctgaacctggagaaccccgcccccggc- gtggacccc gtggtgctggtgggcccccgcaaggagcgcgccgaggacctggacgtggtgctgtccaactccttcggcttcgg- cggccacaa ctcctgcgtgatcttccgcaagtacgacgagatggactacaaggaccacgacggcgactacaaggaccacgaca- tcgactaca aggacgacgacgacaagTGAatcgatagatctcttaaggcagcagcagctcggatagtatcgacacactctgga- cgctggtcg tgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctca- gtgtgtttg atcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatcccctt- ccctcgttt catatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctc- actgcccct cgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgat- gcacgggaa gtagtgggatgggaacacaaatggaaagcttaattaagagctcttgttttccagaaggagttgctccttgagcc- tttcattct cagcctcgataacctccaaagccgctctaattgtggagggggttcgaagacagggtggttggctggatggggaa- acgctggtc gcgggattcgatcctgctgcttatatcctccctggaagcacacccacgactctgaagaagaaaacgtgcacaca- cacaaccca accggccgaatatttgcttccttatcccgggtccaagagagactgcgatgcccccctcaatcagcatcctcctc- cctgccgct tcaatcttccctgcttgcctgcgcccgcggtgcgccgtctgcccgcccagtcagtcactcctgcacaggcccct- tgtgcgcag tgctcctgtaccctttaccgctccttccattctgcgaggccccctattgaatgtattcgttgcctgtgtggcca- agcgggctg ctgggcgcgccgccgtcgggcagtgctcggcgactttggcggaagccgattgttcttctgtaagccacgcgctt- gctgctttg ggaagagaagggggggggtactgaatggatgaggaggagaaggaggggtattggtattatctgagttgggtgaa- gagc
[0614] Construct to knock out FATA genes and over expression of PmKASII in S2532 background. Relevant restriction sites in the construct FATA1::CpACT-AtThic-nr:PmUAPA1-S106SAD-PmKASII-nr::FATA1 (termed pSZ2441) are indicated in lowercase, bold and underlining and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, BamH I, EcoR V, Spe I, Asc I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UTEX1435 that permit targeted integration at FATA1 gene via homologous recombination. Proceeding in the 5' to 3' direction, the actin gene promoter from UTEX 250 driving the expression of the A. thaliana THIC gene is indicated by the boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous UAPA1 promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the P. moriformis KASII gene are indicated by uppercase, bold italics, while the remainder of the PmKASII coding region is indicated by bold italics. The Chlorella protothecoides UTEX 250 stearoyl-ACP desaturase transit peptide is located between initiator ATG and the Asc I site. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX1435 FATA1 genomic region indicated by bold, lowercase text.
TABLE-US-00092 Nucleotide sequence of transforming DNA contained in pSZ2441: (SEQ ID NO: 119) gctcttcggagtcactgtgccactgagttcgactggtagctgaatggagtcgctgctccactaaacgaattgtc- agcaccgcc agccggccgaggacccgagtcatagcgagggtagtagcgcgccatggcaccgaccagcctgcttgccagtactg- gcgtctctt ccgcttctctgtggtcctctgcgcgctccagcgcgtgcgcttttccggtggatcatgcggtccgtggcgcaccg- cagcggccg ctgcccatgcagcgccgctgcttccgaacagtggcggtcagggccgcacccgcggtagccgtccgtccggaacc- cgcccaaga gttttgggagcagcttgagccctgcaagatggcggaggacaagcgcatcttcctggaggagcaccggtgcgtgg- aggtccggg gctgaccggccgtcgcattcaacgtaatcaatcgcatgatgatcagaggacacgaagtcttggtggcggtggcc- agaaacact gtccattgcaagggcatagggatgcgttccttcacctctcatttctcatttctgaatccctccctgctcactct- ttctcctcc ##STR00372## tggtctgcaacaacaagaaccactccgcccgccccaagctgcccaactcctccctgctgcccggcttcgacgtg- gtggtccag gccgcggccacccgcttcaagaaggagacgacgaccacccgcgccacgctgacgttcgacccccccacgaccaa- ctccgagcg cgccaagcagcgcaagcacaccatcgacccctcctcccccgacttccagcccatcccctccttcgaggagtgct- tccccaagt ccacgaaggagcacaaggaggtggtgcacgaggagtccggccacgtcctgaaggtgcccttccgccgcgtgcac- ctgtccggc ggcgagcccgccttcgacaactacgacacgtccggcccccagaacgtcaacgcccacatcggcctggcgaagct- gcgcaagga gtggatcgaccgccgcgagaagctgggcacgccccgctacacgcagatgtactacgcgaagcagggcatcatca- cggaggaga tgctgtactgcgcgacgcgcgagaagctggaccccgagttcgtccgctccgaggtcgcgcggggccgcgccatc- atcccctcc aacaagaagcacctggagctggagcccatgatcgtgggccgcaagttcctggtgaaggtgaacgcgaacatcgg- caactccgc cgtggcctcctccatcgaggaggaggtctacaaggtgcagtgggccaccatgtggggcgccgacaccatcatgg- acctgtcca cgggccgccacatccacgagacgcgcgagtggatcctgcgcaactccgcggtccccgtgggcaccgtccccatc- taccaggcg ctggagaaggtggacggcatcgcggagaacctgaactgggaggtgttccgcgagacgctgatcgagcaggccga- gcagggcgt ggactacttcacgatccacgcgggcgtgctgctgcgctacatccccctgaccgccaagcgcctgacgggcatcg- tgtcccgcg gcggctccatccacgcgaagtggtgcctggcctaccacaaggagaacttcgcctacgagcactgggacgacatc- ctggacatc tgcaaccagtacgacgtcgccctgtccatcggcgacggcctgcgccccggctccatctacgacgccaacgacac- ggcccagtt cgccgagctgctgacccagggcgagctgacgcgccgcgcgtgggagaaggacgtgcaggtgatgaacgagggcc- ccggccacg tgcccatgcacaagatccccgagaacatgcagaagcagctggagtggtgcaacgaggcgcccttctacaccctg- ggccccctg acgaccgacatcgcgcccggctacgaccacatcacctccgccatcggcgcggccaacatcggcgccctgggcac- cgccctgct gtgctacgtgacgcccaaggagcacctgggcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctaca- agatcgccg cccacgcggccgacctggccaagcagcacccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttc- gagttccgc tggatggaccagttcgcgctgtccctggaccccatgacggcgatgtccttccacgacgagacgctgcccgcgga- cggcgcgaa ggtcgcccacttctgctccatgtgcggccccaagttctgctccatgaagatcacggaggacatccgcaagtacg- ccgaggaga acggctacggctccgccgaggaggccatccgccagggcatggacgccatgtccgaggagttcaacatcgccaag- aagacgatc tccggcgagcagcacggcgaggtcggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTG- Acaattggc agcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgc- cttgacctg tgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgct- agctgcttg tgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatc- tacgctgtc ctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtat- tctcctggt actgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatcc- cgcgtctcg aacagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccac- ctgacgaat gcgcttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaa- tgatcggtg ##STR00373## tgcggcgacctgcgtcgctcggcgggctccgggccccggcgcccagcgaggcccctccccgtgcgcgggcgcgc- cgccgccgc cgccgacgccaaccccgcccgccccgagcgccgcgtggtgatcaccggccagggcgtggtgacctccctgggcc- agaccatcg agcagttctactcctccctgctggagggcgtgtccggcatctcccagatccagaagttcgacaccaccggctac- accaccacc atcgccggcgagatcaagtccctgcagctggacccctacgtgcccaagcgctgggccaagcgcgtggacgacgt- gatcaagta cgtgtacatcgccggcaagcaggccctggagtccgccggcctgcccatcgaggccgccggcctggccggcgccg- gcctggacc ccgccctgtgcggcgtgctgatcggcaccgccatggccggcatgacctccttcgccgccggcgtggaggccctg- acccgcggc ggcgtgcgcaagatgaaccccttctgcatccccttctccatctccaacatgggcggcgccatgctggccatgga- catcggctt catgggccccaactactccatctccaccgcctgcgccaccggcaactactgcatcctgggcgccgccgaccaca- tccgccgcg gcgacgccaacgtgatgctggccggcggcgccgacgccgccatcatcccctccggcatcggcggcttcatcgcc- tgcaaggcc ctgtccaagcgcaacgacgagcccgagcgcgcctcccgcccctgggacgccgaccgcgacggcttcgtgatggg- cgagggcgc cggcgtgctggtgctggaggagctggagcacgccaagcgccgcggcgccaccatcctggccgagctggtgggcg- gcgccgcca cctccgacgcccaccacatgaccgagcccgacccccagggccgcggcgtgcgcctgtgcctggagcgcgccctg- gagcgcgcc cgcctggcccccgagcgcgtgggctacgtgaacgcccacggcacctccacccccgccggcgacgtggccgagta- ccgcgccat ccgcgccgtgatcccccaggactccctgcgcatcaactccaccaagtccatgatcggccacctgctgggcggcg- ccggcgccg tggaggccgtggccgccatccaggccctgcgcaccggctggctgcaccccaacctgaacctggagaaccccgcc- cccggcgtg gaccccgtggtgctggtgggcccccgcaaggagcgcgccgaggacctggacgtggtgctgtccaactccttcgg- cttcggcgg ccacaactcctgcgtgatcttccgcaagtacgacgagatggactacaaggaccacgacggcgactacaaggacc- acgacatcg actacaaggacgacgacgacaagTGAatcgatagatctcttaaggcagcagcagctcggatagtatcgacacac- tctggacgc tggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaaca- gcctcagtg tgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcat- ccccttccc tcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctc- ctgctcact gcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaat- gctgatgca cgggaagtagtgggatgggaacacaaatggaaagcttaattaagagctcttgttttccagaaggagttgctcct- tgagccttt cattctcagcctcgataacctccaaagccgctctaattgtggagggggttcgaagacagggtggttggctggat- ggggaaacg ctggtcgcgggattcgatcctgctgcttatatcctccctggaagcacacccacgactctgaagaagaaaacgtg- cacacacac aacccaaccggccgaatatttgcttccttatcccgggtccaagagagactgcgatgcccccctcaatcagcatc- ctcctccct gccgcttcaatcttccctgcttgcctgcgcccgcggtgcgccgtctgcccgcccagtcagtcactcctgcacag- gccccttgt gcgcagtgctcctgtaccctttaccgctccttccattctgcgaggccccctattgaatgtattcgttgcctgtg- tggccaagc gggctgctgggcgcgccgccgtcgggcagtgctcggcgactttggcggaagccgattgttcttctgtaagccac- gcgcttgct gctttgggaagagaagggggggggtactgaatggatgaggaggagaaggaggggtattggtattatctgagttg- ggtgaagag c
[0615] Southern blot analysis of Strain AS and Strain AZ indicated that both are PmFATA double knock-out mutants. Since the PmFAD2 disruption cassettes contain a Carthamus tinctorius putative oleoyl-specific ACP-thioesterase (CtOTE), the absence of the endogenous FATA genes seems to be fully complemented by the expression of the CtOTE.
[0616] To determine the impact of FATA1 inactivation and over expression of PmKASII gene on lipid composition in .DELTA.fad2 lines Strain D/Strain R, the primary transformants of D1266/Strain D and D1415/Strain R were clonally purified and grown under standard lipid production conditions at both pH5.0 and pH7.0. The resulting profiles from the transgenic line arising from transformation with pSZ2276 into Strain D are shown in Table 68, and transgenic lines arising from transformation with pSZ2441 into Strain R are shown in Table 69.
[0617] As can be seen from Table 68, in Strain AZ at pH7.0, the combination of full activity of PmKASII driven by AMT03 and FATA1 knock results in very low levels of C16:0 (2%). Meanwhile, the Carthamus tinctorius thioesterase is also activated since it is also driven by AMT03 promoter. We observe 7.8% C18:0 when Strain AZ is cultivated at pH7. At pH5.0, decrease of the C16:0 level is largely contributed by the FATA1 inactivation, although PmKASII can be partially activated since we run the seed culture at pH6.8. The stearic level of Strain AZ is low at pH5.0 due to the low expression of the C. tinctorius TE. Overall, the oleic levels of Strain AZ exceed 85% (around 88%) at both pH7.0 and pH5.0.
TABLE-US-00093 TABLE 68 Fatty acid profiles in S1331, S2530 and S4266 at both pH 5.0 and pH 7.0 Strains C16:0 C18:0 C18:1 C18:2 C20:1 Strain A_pH 5 26.6 3.3 60.5 6.7 0.07 Strain A_pH 7 28.3 4.1 58 6.5 0.06 Strain D_pH 5 17 3.6 77.1 0.01 0.14 Strain D_pH 7 19.5 5.3 72.6 0.01 0.09 Strain AZ_pH 5 4.1 2.36 88.5 0.04 3.1 Strain AZ_pH 7 2.1 7.8 87.9 0.01 0.5
[0618] In the transgenic line Strain AS, both CrTUB2 and PmUAPA1 promoters are pH unbiased, hence, as reported in Table 69, the lipid profile at pH5.0 and pH7.0 are essentially same. Relative to Strain AZ, Strain AS produces much less stearic acid. Although the palmitic level in Strain AS is bit higher than that in Strain AZ, the oleic level in Strain AS is above 90%, which is the highest level we observed in the shake flask experiment.
TABLE-US-00094 TABLE 69 Fatty acid profiles in S1331, S2532 and S5204 at both pH 5.0 and pH 7.0 Strains C16:0 C18:0 C18:1 C18:2 Strain A_pH 5 26.6 3.3 60.5 6.7 Strain A_pH 7 28.3 4.1 58 6.5 Strain R_pH 5 23.3 2.1 72.1 0.01 Strain R_pH 7 23.4 2.3 71.9 0.01 Strain AS_pH 5 5.5 1.4 91.5 0.01 Strain AS_pH 7 5.6 1.6 91.3 0.01
Example 59
Complementation of FAD2 and FATA Knockout and KASII Overexpression Generates a Unique Oil with High C18-2 and Low C18-3 Levels
[0619] As described in Example 58, Strain AS was generated by knocking both copies of PmFATA1 in a Prototheca moriformis strain while simultaneously overexpressing PmKASII gene into a .DELTA.fad2 line (Strain R). Strain R is a FAD2 (also known as FADc) knockout strain generated by insertion of a oleate-specific C. tinctorius acyl-ACP thioesterase (GenBank Accession No: AAA33019.1) into a high-lipid producing strain derived from UTEX 1435, under the control of CrTUB2 promoter at the FAD2 locus. Strain AS and its parent, Strain R, have a disrupted endogenous PmFAD2-1 gene resulting in no .DELTA.12 specific desaturase activity manifested as 0% C18:2 (linoleic acid) levels in both nitrogen-rich seed and nitrogen-poor lipid production conditions. Lack of C18:2 in Stain AS (and its parent Strain R) resulted in growth defects which could be partially mitigated by exogenous addition of linoleic acid in the seed stage. However, for industrial applications, exogenous addition of linoleic acid is expensive. Complementation of Strain R (and a second .DELTA.fad2 strain) with PmFAD2-1 restored C18:2 levels back to wild type levels and also resulted in rescued growth characteristics during seed and lipid production without any linoleic supplementation.
[0620] In the present example we demonstrate that: [0621] In trans expression of fatty acid desaturase-2 gene from Prototheca moriformis (PmFad2-1) under the control of a pH inducible PmAMT3 promoter results in functional complementation of PmFAD2-1 with restored growth and C18:2 levels in .DELTA.fad2, .DELTA.fata1 strain AS; [0622] Complementation of Strain AS is conditional/inducible and occurs at pH 7.0 when the AMT3 promoter is actively driving the expression of PmFAD2-1 as opposed to pH 5.0 when the AMT3 promoter is inactive; and [0623] Over expression of PmFAD2-1 at pH 7.0 results in strains with >20% C18:2 levels. The fatty acid profile of these high C18:2 strains mimic canola oil closely except that the new oil has 5 fold less C18:3 than the canola oil (10%). The elevated C18:2 levels are seen only in strains derived from Strain AS overexpressing PmFAD2-1 since overexpression of the same gene in wild-type (i.e., non-engineered) control Strain Z does not result in higher C18:2 levels.
[0624] Construct used for the expression of the Prototheca moriformis fatty acid desaturase 2 (PmFAD2-1) in .DELTA.fad2 strains Strain AS and Strain Z--[pSZ2721]. .DELTA.fad2 .DELTA.fata1 Strain AS and Strain Z were transformed with the construct pSZ2721. The sequence of the transforming DNA is provided below. Relevant restriction sites in the construct pSZ2721 (6S::CpACT-ScMEL1-CvNR::PmAMT3-PmFAD2-1-CvNR::6S) are indicated in lowercase, underlined and bold, and are from 5'-3' BspQ 1, KpnI, Xba I, Mfe I, BamH I, EcoR I, Spe I, Cla I, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UEX 1435 that permits targeted integration of PmFAD2-1 at the 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the actin (ACT) gene promoter from UTEX 250 driving the expression of the Saccharomyces cerevisiae MEL1 gene is indicated by the boxed text. The initiator ATG and terminator TGA for ScMEL1 are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an endogenous AMT03 promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the PmFAD2-1 are indicated by uppercase, bold italics, while the remainder of the gene is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX 1435 6S genomic region indicated by bold, lowercase text. The final construct was sequenced to ensure correct reading frames and targeting sequences.
TABLE-US-00095 Nucleotide sequence of transforming DNA contained in plasmid pSZ2721: (SEQ ID NO: 120) gctcttcggagtcactgtgccactgagttcgactggtagctgaatggagtcgctgctccactaaacgaattgtc- agcaccgcc agccggccgaggacccgagtcatagcgagggtagtagcgcgccatggcaccgaccagcctgcttgccagtactg- gcgtctctt ccgcttctctgtggtcctctgcgcgctccagcgcgtgcgcttttccggtggatcatgcggtccgtggcgcaccg- cagcggccg ctgcccatgcagcgccgctgcttccgaacagtggcggtcagggccgcacccgcggtagccgtccgtccggaacc- cgcccaaga gttttgggagcagcttgagccctgcaagatggcggaggacaagcgcatcttcctggaggagcaccggtgcgtgg- aggtccggg gctgaccggccgtcgcattcaacgtaatcaatcgcatgatgatcagaggacacgaagtcttggtggcggtggcc- agaaacact gtccattgcaagggcatagggatgcgttccttcacctctcatttctcatttctgaatccctccctgctcactct- ttctcctcc ##STR00374## ccctgaagggcgtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatgggctgggacaac- tggaacacg ttcgcctgcgacgtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctgaaggacat- gggctacaa gtacatcatcctggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgagcagaagt- tccccaacg gcatgggccacgtcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcgagtac- acgtgcgcc ggctaccccggctccctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacctgaa- gtacgacaa ctgctacaacaagggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccctga- acaagacgg gccgccccatcttctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcgaac- tcctggcgc atgtccggcgacgtcacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactg- caagtacgc cggcttccactgctccatcatgaacatcctgaacaaggccgccccatgggccagaacgcgggcgtcggcggctg- gaacgacct ggacaacctggaggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatgg- tgaagtccc ccctgatcatcggcgcgaacgtgaacaacctgaaggcctcctcctactccatctactcccaggcgtccgtcatc- gccatcaac caggactccaacggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccaggg- cgagatcca gatgtggtccggccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgcccca- tgaacacga ccctggaggagatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacctg- tgggcgaac cgcgtcgacaactccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgccac- cgagcagtc ctacaaggacggcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcga- tcctgaaca cgaccgtccccgcccacggcatcgcgttctaccgcctgcgcccctcctccTGAcaattggcagcagcagctcgg- atagtatcg acacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgc- cgcttttat caaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaa- taccacccc cagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctca- gcgctgctc ctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgta- aaccagcac tgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatcccgcgtctcgaacagagcgcgca- gaggaacg ctgaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgct- tggttcttc gtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagct- gatggtcga ##STR00375## gcgcaaggccatccccgcccactgcttcgagcgctccgccctgcgctcctccatgtacctggccttcgacatcg- ccgtgatgt ccctgctgtacgtggcctccacctacatcgaccccgcccccgtgcccacctgggtgaagtacggcgtgatgtgg- cccctgtac tggttcttccagggcgccttcggcaccggcgtgtgggtgtgcgcccacgagtgcggccaccaggccttctcctc- ctcccaggc catcaacgacggcgtgggcctggtgttccactccctgctgctggtgccctactactcctggaagcactcccacc- gccgccacc actccaacaccggctgcctggacaaggacgaggtgttcgtgcccccccaccgcgccgtggcccacgagggcctg- gagtgggag gagtggctgcccatccgcatgggcaaggtgctggtgaccctgaccctgggctggcccctgtacctgatgttcaa- cgtggcctc ccgcccctacccccgcttcgccaaccacttcgacccctggtcccccatcttctccaagcgcgagcgcatcgagg- tggtgatct ccgacctggccctggtggccgtgctgtccggcctgtccgtgctgggccgcaccatgggctgggcctggctggtg- aagacctac gtggtgccctacctgatcgtgaacatgtggctggtgctgatcaccctgctgcagcacacccaccccgccctgcc- ccactactt cgagaaggactgggactggctgcgcggcgccatggccaccgtggaccgctccatgggcccccccttcatggaca- acatcctgc accacatctccgacacccacgtgctgcaccacctgttctccaccatcccccactaccacgccgaggaggcctcc- gccgccatc cgccccatcctgggcaagtactaccagtccgactcccgctgggtgggccgcgccctgtgggaggactggcgcga- ctgccgcta cgtggtgcccgacgcccccgaggacgactccgccctgtggttccacaagTAGatcgatagatctcttaaggcag- cagcagctc ggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtg- aatatccct gccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtg- ctatttgcg aataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcct- gctatccct cagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtac- tgcaacctg taaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaaagcttaattaagagct- cttgttttc cagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggaggggg- ttcgaagac agggtggttggctggatggggaaacgctgctggtcgcgggattcgatcctgctgcttatatcctccctggaagc- acacccacg actctgaagaagaaaacgtgcacacacacaacccaaccggccgaatatttgcttccttatcccgggtccaagag- agactgcga tgcccccctcaatcagcatcctcctccctgccgcttcaatcttccctgcttgcctgcgcccgcggtgcgccgtc- tgcccgccc agtcagtcactcctgcacaggccccttgtgcgcagtgctcctgtaccctttaccgctccttccattctgcgagg- ccccctatt gaatgtattcgttgcctgtgtggccaagcgggctgctgggcgcgccgccgtcgggcagtgctcggcgactttgg- cggaagccg attgttcttctgtaagccacgcgcttgctgctttgggaagagaagggggggggtactgaatggatgaggaggag- aaggagggg tattggtattatctgagttgggtgaagagc
[0625] To determine its impact on growth and fatty acid profiles, the above construct was transformed independently into a .DELTA.fad2 .DELTA.fata1 Strain AS or wild type Strain Z. Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at pH7.0 (AMT3 promoter active) and pH5.0 (AMT3 promoter inactive) for Strain AS transformants or at pH7.0 for Strain Z transformants. The resulting profiles from a set of representative clones arising from transformations are shown in Tables 70-73 respectively.
[0626] Expression of endogenous PmFad2-1 driven by AMT3 promoter at pH 7.0, in Strain AS resulted in .DELTA.12 specific desaturase activity with complete restoration of C18:2 fatty acid levels of the base strain A (Table 70). No such .DELTA.12 specific desaturase activity and thus no significant C18:2 restoration is detected when the lipid production is run at pH 5.0 when the AMT3 promoter is inactive (Table 71).
[0627] Interestingly, lipid production in complemented Strain AS strains at pH 7.0 results in several strains with 2 fold or more increase in C18:2 levels. The resulting strains produce an oil profile that is similar to Canola oil except that the new oil has less C18:3 levels than the commercially available canola oil (Table 72). The increase in C18:2 is not seen in wild type (Strain Z) strains transformed with the same AMT3 driven PmFAD2-1.
[0628] While we have seen other strains with high C18:2 levels, all of them were associated with growth defects in seed as well as lipid production media. Here, however, we have been able to increase the C18:2 levels in a targeted manner without any detrimental effect on the growth of resulting strains. While .DELTA.fad2 strain R and .DELTA.fad2 .DELTA.fata1 strain AS grow very poorly and hardly reach an OD750 of 10-20 in 42 hours, complemented Strain AS (D1673) lines grow very rapidly in the same time span and reach OD750 between 50-80.
[0629] Thus, it can be seen that we were able to produce cell oils with fatty acid profiles of less than 10% linolenic acid yet >20% linoleic acid (indeed we achieved <2% linolenic acid and >20% linoleic acid). It is surprising that C18:2 levels are elevated only in Strain AS, which has almost 90% C18:1 levels as compared to Strain Z with only 57% C18:1 levels, suggests excess availability of substrate C18:1 in the ER is a key to boost C18:2 levels. Since Prototheca has evolved to utilize C18:1 onto TAGS very efficiently, in wild type situations most likely the substrate leaves the ER very rapidly before being further desaturated by FAD2 enzymes. This limitation may be overcome in strains like Strain AS with very high C18:1 levels that likely stays available for desaturation by PmFAD2-1.
TABLE-US-00096 TABLE 70 Fatty acid profile in representative complemented (D1673) and parent Strain AS lines at pH 7.0 transformed with pSZ2721 (PmFAD2-1) DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3a AS; T533; D1673-16; pH 7.0 0.49 6.33 2.44 66.53 21.36 1.38 AS; T533; D1673-17; pH 7.0 0.44 6.02 2.25 68.97 19.53 1.36 AS; T533; D1673-02; pH 7.0 0.38 5.92 2.30 71.01 17.77 1.30 AS; T533; D1673-03; pH 7.0 0.38 5.83 2.31 71.31 17.45 1.29 AS; T533; D1673-10; pH 7.0 0.38 5.63 2.21 71.72 17.37 1.23 AS; pH 7.0 0.30 5.59 1.63 90.88 0.10 0.00 AT; pH 7.0 1.34 27.99 3.54 55.48 9.07 0.79
TABLE-US-00097 TABLE 71 Fatty acid profile in same representative complemented (D1673) and parent Strain AS lines at pH 5.0 transformed with pSZ2721 (PmFAD2-1) DNA. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3a AS; T533; D1673-16; 0.47 5.16 1.76 90.94 0.06 0.18 pH 5.0 AS; T533; D1673-17; 0.45 4.97 1.72 91.32 0.05 0.00 pH 5.0 AS; T533; D1673-02; 0.46 5.20 1.75 90.94 0.05 0.18 pH 5.0 AS; T533; D1673-03; 0.41 4.93 1.65 89.92 1.56 0.16 pH 5.0 AS; T533; D1673-10; 0.45 4.97 1.69 89.96 1.35 0.16 pH 5.0 AS; pH 5.0 0.39 5.67 1.36 91.13 0.00 0.00 AT; pH 5.0 1.03 24.69 3.30 63.47 5.80 0.38
TABLE-US-00098 TABLE 72 Fatty acid profile of a stable D1673 line along with base strain Z and Canola oil. Sample ID C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. C20:1 pH 5 Strain Z 27.54 3.29 57.91 7.17 0.59 0.10 pH 7 Strain Z 27.92 3.09 58.30 6.71 0.59 0.07 pH 7; AS; T533; D1673.5.2-1 4.43 1.31 70.32 20.30 1.72 0.75 pH 7; AS; T533; D1673.5.2-2 4.55 1.26 67.53 22.17 1.82 1.22 pH 7; AS; T533; D1673.5.2-3 4.34 1.29 69.51 20.78 1.65 1.01 pH 7; AS; T533; D1673.5.2-4 4.81 1.26 68.08 21.53 1.77 1.06 pH 7; AS; T533; D1673.5.2-5 4.61 1.30 68.02 21.57 1.74 1.17 pH 7; AS; T533; D1673.5.2-6 4.36 1.30 68.88 21.16 1.68 1.10 pH 7; AS; T533; D1673.5.2-7 4.38 1.28 69.30 21.08 1.70 0.97 pH 7; AS; T533; D1673.5.2-8 4.87 1.27 68.44 20.87 1.83 1.14 Canola Oil 4.00 2.00 62.00 22.00 10.00 1.00
TABLE-US-00099 TABLE 73 Fatty acid profile in Strain Z at pH 5.0 and pH 7.0 and representative derivative transgenic lines at pH 7.0 transformed with pSZ2721 (PmFAD2-1) DNA. The lines are sorted by C18:2 levels. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3a Z; T573; D1791-23; pH 7.0 1.45 29.96 3.28 54.72 7.99 0.66 Z; T573; D1791-6; pH 7.0 1.73 30.25 2.48 55.01 7.74 0.69 Z; T573; D1791-17; pH 7.0 1.41 29.00 3.42 55.77 7.64 0.68 Z; T573; D1791-14; pH 7.0 1.48 29.82 3.45 55.22 7.56 0.67 Z; T573; D1791-8; pH 7.0 2.30 37.15 2.54 47.62 7.44 0.67 Z; T573; D1791-2; pH 7.0 1.38 29.29 3.45 56.10 7.12 0.63 Z; T573; D1791-10; pH 7.0 1.46 29.30 3.39 56.16 7.11 0.60 Z; T573; D1791-5; pH 7.0 1.45 29.45 3.36 56.15 7.02 0.61 Z; T573; D1791-11; pH 7.0 1.43 29.52 3.44 55.99 7.01 0.60 Z; T573; D1791-13; pH 7.0 1.41 28.96 3.46 56.47 7.01 0.62 Z; pH 7.0 1.41 27.76 3.45 57.71 7.17 0.58 Z; pH 5.0 1.49 28.19 3.27 58.04 6.65 0.57
Example 60
Combinatorial Expression of Mid-Chain Thioesterases and Ketoacyl Synthases to Generate Oils with Highly Elevated and Balanced C10:0 AND C12:0 Fatty Acid Levels
[0630] In this example we describe two molecular approaches to generate oils with highly elevated and balanced C10:0 and C12:0 fatty acids in a classically mutagenized high-oil-yielding derivative of UTEX 1435, Strain BA. Resulting transgenic strains co-express two distinct mid-chain specific thioesterases, the broad specificity C10:0-C14:0 Cuphea wrightii FATB2 thioesterase (expressed in Stain BA), and predominantly C10:0--specific Cuphea hookeriana FATB2 thioesterase (part of incoming vectors). In addition, D1550 transformants express C. wrightii KASIV elongase gene integrated at a neutral genomic site, Thi4b, (vector pSZ2424), while D1681 transformants--C. wrightii KASAI elongase as a part of an endogenous KASI disruption cassette (vector pSZ2746). The use of different KASI activities of plant origin in combination with the exogenous thioesterases resulted in a significant increase in overall C10-C12 levels as well as improved C10:0 specificity of the C. hookeriana thioesterase. The best strain synthesized about 85% total C10:0-C12:0 fatty acids with balanced levels of about 42% C10:0 and ca. 44% C12:0 fatty acids, respectively, less than 4% C14:0, and less than 1.5% C8:0. The results show that selection of FATB and KAS genes can give rise to an oil with at least 50% total saturates with capric and lauric acids balanced to within 20% (or even to within 15%, or 10%).
[0631] Relevant restriction sites in pSZ2424 are indicated in lowercase, bold and underlining text and are 5'-3' Pme I, Kpn I, Xba I, Mfe I, Eco RI, Spe I, Xho I, Hind III, SnaBI, Spe I, Asc I, Xho I, Eco RI, Sac I, BspQ I, respectively. Pme I and BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UTEX1435 that permit targeted integration at Thi4b locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii B-tubulin promoter driving the expression of the neomycin phosphotransferase gene (NeoR, conferring the ability of cells to grow on G418) is indicated by boxed text. The initiator ATG and terminator TGA for NeoR are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text. Next is the Amt03 promoter of Prototheca moriformis indicated by boxed lowercase text driving the expression of Cuphea hookeriana KASIV gene (ChKASIV) indicated in lowercase italics. The initiator ATG and terminator TGA for ChKASIV are indicated by uppercase, bold italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text. Next is the Amt03 promoter of Prototheca moriformis indicated by boxed lowercase text driving the expression of Cuphea hookeriana FATB2 gene (ChFATB2) fused to plastid transit peptide sequence derived from Prototheca moriformis FAD gene indicated in lowercase italics. The initiator ATG and terminator TGA for ChFATB2 are indicated by uppercase, bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX1435 Thi4b flanking sequence.
TABLE-US-00100 Nucleotide sequence of transforming DNA contained in pSZ2424: (SEQ ID NO: 121) gtttaaacccctcaactgcgacgctgggaaccttctccgggcaggcgatgtgcgtgggtttgcctccttggcac- ggctctacacc ttcgagtacgccatgaggcggtgatggctgtggctgtgccccacttcgtccagggacggcaagtccatcatctg- catgcttggt gcgacgctacagcagtccctctgcagcagaggagcacgactttggccatttcacgcactcgagtgtacacaatt- catttttctta aagtaaatgactgctgattgaccagatgctgtaacgctgatttcgctccagatcgcacagtcacagattgcgac- catgttgctg cgtctgaaaatctggattccgaattcgaccctggcgctccatccatgcaacagatggcgacacttgttacaatt- cctgtcgccca tcggcatggagcaggtccacttagatccccgatcacccacgcgcatctcgctaatagtcattcattcgtgtctt- cgatcaaagtc aggtgagtatgcatggatcttggttgacgatgcggtatgggtttgcgccgctgactgcagggtctgtccaaggc- aagccaaccc agctcctctcctcgacaatactctcgcagacaaagccagccacttgccatccagattgccaataaactcaatca- tggcttctgtc atgccatccatgggtctgatgaatggtcacgctcgtgtcctgaccgttccccagcctctggcgtcccctgcccc- gcccaccagcc ##STR00376## gactgggcccagcagaccatcggctgctccgacgccgccgtgttccgcctgtccgcccagggccgccccgtgct- gttcgtgaaga ccgacctgtccggcgccctgaacgagctgcaggacgaggccgcccgcctgtcctggctggccaccaccggcgtg- ccctgcgcc gccgtgctggacgtggtgaccgaggccggccgcgactggctgctgctgggcgaggtgcccggccaggacctgct- gtcctcccac ctggcccccgccgagaaggtgtccatcatggccgacgccatgcgccgcctgcacaccctggaccccgccacctg- ccccttcgac caccaggccaagcaccgcatcgagcgcgcccgcacccgcatggaggccggcctggtggaccaggacgacctgga- cgagga gcaccagggcctggcccccgccgagctgttcgcccgcctgaaggcccgcatgcccgacggcgaggacctggtgg- tgacccacg gcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccggcttcatcgactgcggccgcctgggc- gtggccgaccg ctaccaggacatcgccctggccacccgcgacatcgccgaggagctgggcggcgagtgggccgaccgcttcctgg- tgctgtacg gcatcgccgcccccgactcccagcgcatcgccttctaccgcctgctggacgagttcttcTGAcaattggcagca- gcagtcggat agtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaata- tccctgccgcttttatca aacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgctatttgcgaataccaccc- ccagcatcccctt ccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctg- ctcctgctcactgcccctc gcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatg- cacgggaagtagtgg ##STR00377## ##STR00378## tgcacctggctggtcgccgcgtgcatgcccacctccagcgacaacgacccccgctcgctgtcccacaagcgcct- gcgcctgagc cgccgccgccgcaccctgagctcgcactgctccctgcgcggcagcaccttccagtgcctggacccctgcaacca- gcagcgcttcc tgggcgacaacggcttcgcgtcgctgttcggctccaagcccctgcgcagcaaccgcggccacctgcgcctgggc- cgcacctcgc actccggcgaggtgatggccgtcgcgatgcagcccgcccaggaggtgagcaccaacaagaagcccgcgaccaag- cagcgcc gcgtggtcgtgaccggcatgggcgtcgtgacccccctgggccacgaccccgacgtgtattataacaacctgctg- gacggcatctc gggcatctccgagatcgagaacttcgactgcagccagttccccacccgcatcgccggcgagatcaagtcgttct- ccaccgacggc tgggtcgcgcccaagttcagcgagcgcatggacaagttcatgctgtatatgctgaccgccggcaagaaggcgct- ggccgacggc ggcatcaccgaggacgcgatgaaggagctgaacaagcgcaagtgcggcgtgctgatcggctcgggcctgggcgg- catgaag gtcttctccgacagcatcgaggccctgcgcacctcgtataagaagatctcccccttctgcgtgcccttcagcac- caccaacatgggc tcggcgatcctggcgatggacctgggctggatgggccccaactattccatcagcaccgcgtgcgccacctcgaa- cttctgcatcct gaacgcggccaaccacatcatcaagggcgaggcggacatgatgctgtgcggcggctccgacgccgcggtgctgc- ccgtcggc ctgggcggcttcgtggcctgccgcgcgctgagccagcgcaacaacgaccccaccaaggcctcgcgcccctggga- ctccaaccg cgacggcttcgtcatgggcgagggcgcgggcgtgctgctgctggaggagctggagcacgccaagaagcgcggcg- cgaccatc tatgccgagttcctgggcggcagcttcacctgcgacgcgtatcacatgaccgagccccaccccgagggcgccgg- cgtcatcctgt gcatcgagaaggcgctggcccagtcgggcgtgtcccgcgaggacgtgaactatatcaacgcgcacgccaccagc- acccccgc gggcgacatcaaggagtatcaggccctggcgcactgcttcggccagaactcggagctgcgcgtcaactccacca- agagcatga tcggccacctgctgggcggcgccggcggcgtggaggcggtcgccgtggtccaggcgatccgcaccggctggatc- caccccaac atcaacctggaggaccccgacgagggcgtggacgccaagctgctggtcggccccaagaaggagaagctgaaggt- gaaggtc ggcctgtcgaactccttcggcttcggcggccacaacagctcgatcctgttcgcgccctgcaacTGActcgaggc- agcagcagct cggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgt- gaatatccctgccgctt ttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgatttgcgagttgctagagcttgtgctatttgcg- aataccacccccagcatc cccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgc- tcctgctcctgctcactgc ccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgc- tgatgcacgggaagta ##STR00379## ##STR00380## gcctccgttcacgatcgggacgctgcgcaaggccatccccgcgcactgtttcgagcgctcggcgcttcgtgggc- gcgcccagctg cccgactggagccgcctgctgaccgccatcaccaccgtgttcgtgaagtccaagcgccccgacatgcacgaccg- caagtccaa gcgccccgacatgctggtggacagcttcggcctggagtccaccgtgcaggacggcctggtgttccgccagtcct- tctccatccgct cctacgagatcggcaccgaccgcaccgccagcatcgagaccctgatgaaccacctgcaggagacctccctgaac- cactgcaa gagcaccggcatcctgctggacggcttcggccgcaccctggagatgtgcaagcgcgacctgatctgggtggtga- ttaagatgca gatcaaggtgaaccgctaccccgcctggggcgacaccgtggagatcaacacccgcttcagccgcctgggcaaga- tcggcatgg gccgcgactggctgatctccgactgcaacaccggcgagatcctggtgcgcgccaccagcgcctacgccatgatg- aaccagaag acccgccgcctgtccaagctgccctacgaggtgcaccaggagatcgtgcccctgttcgtggacagccccgtgat- cgaggactccg acctgaaggtgcacaagttcaaggtgaagaccggcgacagcatccagaagggcctgacccccggctggaacgac- ctggacgt gaaccagcacgtgtccaacgtgaagtacatcggctggatcctggagagcatgcccaccgaggtgctggagaccc- aggagctgt gctccctggccctggagtaccgccgcgagtgcggccgcgactccgtgctggagagcgtgaccgccatggacccc- agcaaggtg ggcgtgcgctcccagtaccagcacctgctgcgcctggaggacggcaccgccatcgtgaacggcgccaccgagtg- gcgcccca agaacgccggcgccaacggcgccatctccaccggcaagaccagcaacggcaactccgtgtccatggactacaag- gaccacg acggcgactacaaggaccacgacatcgactacaaggacgacgacgacaagTGActcgaggcagcagcagctcgg- atagta tcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccc- tgccgcttttatcaaac agcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaatacca- cccccagcatccccttccct cgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcc- tgctcactgcccctcgca cagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcac- gggaagtagtgggatg ggaacacaaatggaaagctgtagaattctccagagctccagcgccatgccacgccttttgatggcttcaagtac- gataacggtgt tggattgtgcgtttgttgcgtagtgtgcatggcttagaataatgcagttggatttcttgctcacggcaatgtcg- gcttgtccgcag gttcaaccccatttcggagtctcaggtcagccgcgcaatgaccagccgctacttcaaggacttgcacgacaacg- ccgaggtga gctatgtttaggccttgagtgaaaattgtcgtcgaagcatattcgcgctccgcgatagcatccaagcaaaatgt- caagtgcgttc cgatttgcgtccgcaggtcgatgttgtgatcgtcggtgccggatccgccggtctgtcctgcgcttacgagctga- ccaagcacccc gacgtccgggtacgcgagctgagattcgattggacataaactgaaaatgaaatcttttggagaaatgtaagggt- ctcaagcgg tgctcgattgcaagaaattggtcgtcccccactccgcaggtcgccatcatcgagcagggcgttgcacctggtgg- cggcgcctg gctggggggacagctgttctcggccatgtgtgtacgtagaagggtggatttcggatggtttcgttgcacagctg- tttgtcaatga tttgtcttagactattgccgatgtttctaaatgttttaggagctatgatatgtctgcaggcgactgaagagc
[0632] Relevant restriction sites in pSZ2746 are indicated in lowercase, bold and underlining text and are 5'-3' BspQ 1, Kpn I, Xba I, Mfe I, Hind III, AscI, Spe I, Xho I, Eco RI, Nde I, Sna BI, Xho I, Hind III, Sac I, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UTEX1435 that permit targeted integration (and knockout) at the KASI locus via homologous recombination. Proceeding in the 5' to 3' direction, the C. reinhardtii B-tubulin promoter driving the expression of the neomycin phosphotransferase gene (NeoR, conferring the ability of cells to grow on G418) is indicated by boxed text. The initiator ATG and terminator TGA for NeoR are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text. Next is the UAPA1 promoter of Prototheca moriformis indicated by boxed lowercase text driving the expression of Cuphea hookeriana FATB2 gene (ChFATB2) fused to plastid transit peptide sequence derived from Prototheca moriformis FAD gene indicated in lowercase italics. The initiator ATG and terminator TGA for ChFATB2 are indicated by uppercase, bold italics. The B. braunii cd191 3'UTR is indicated by lowercase underlined text. Next is the Amt03 promoter of Prototheca moriformis indicated by boxed lowercase text driving the expression of Cuphea wrightii KASAI gene indicated by lowercase italics fused to Prototheca moriformis SAD1 plastid transit peptide sequence. The C. wrightii KASAI sequence is in lowercase italics and is delineated by initiator ATG and terminator TGA. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX1435 KASI flanking sequence.
TABLE-US-00101 Nucleotide sequence of transforming DNA contained in pSZ2746: (SEQ ID NO: 122) gctcttcgctcaccgcgtgaattgctgtcccaaacgtaagcatcatcgtggctcggtcacgcgatcctggatcc- ggggatccta gaccgctggtggagagcgctgccgtcggattggtggcaagtaagattgcgcaggttggcgaagggagagaccaa- aaccgga ggctggaagcgggcacaacatcgtattattgcgtatagtagagcagtggcagtcgcatttcgaggtccgcaacg- gatctcgca agctcgctacgctcacagtaggagaaaggggaccactgcccctgccagaatggtcgcgaccctctccctcgccg- gccccgcct gcaacacgcagtgcgtatccggcaagcgggctgtcgccttcaaccgcccccatgttggcgtccgggctcgatca- ggtgcgctg aggggggtttggtgtgcccgcgcctctgggcccgtgtcggccgtgcggacgtggggccctgggcagtggatcag- cagggtttg cgtgcaaatgcctataccggcgattgaatagcgatgaacgggatacggttgcgctcactccatgcccatgcgac- cccgtttctg ##STR00381## ggctcccccgccgcctgggtggagcgcctgttcggctacgactgggcccagcagaccatcggctgctccgacgc- cgccgtgttcc gcctgtccgcccagggccgccccgtgctgttcgtgaagaccgacctgtccggcgccctgaacgagctgcaggac- gaggccgcc cgcctgtcctggctggccaccaccggcgtgccctgcgccgccgtgctggacgtggtgaccgaggccggccgcga- ctggctgctg ctgggcgaggtgcccggccaggacctgctgtcctcccacctggcccccgccgagaaggtgtccatcatggccga- cgccatgcgc cgcctgcacaccctggaccccgccacctgccccttcgaccaccaggccaagcaccgcatcgagcgcgcccgcac- ccgcatgga ggccggcctggtggaccaggacgacctggacgaggagcaccagggcctggcccccgccgagctgttcgcccgcc- tgaaggcc cgcatgcccgacggcgaggacctggtggtgacccacggcgacgcctgcctgcccaacatcatggtggagaacgg- ccgcttctcc ggcttcatcgactgcggccgcctgggcgtggccgaccgctaccaggacatcgccctggccacccgcgacatcgc- cgaggagct gggcggcgagtgggccgaccgcttcctggtgctgtacggcatcgccgcccccgactcccagcgcatcgccttct- accgcctgctg gacgagttcttcTGAcaattggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtgatgg- actgttgccgcc acacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgt- acgcgcttttgcgagttgct agctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccg- caacttatctacgctgtcc tgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgcctgtatt- ctcctggtactgcaacct ##STR00382## ##STR00383## gggacgctgcgcaaggccatccccgcgcactgtttcgagcgctcggcgcttcgtgggcgcgcccagctgcccga- ctggagccgc ctgctgaccgccatcaccaccgtgttcgtgaagtccaagcgccccgacatgcacgaccgcaagtccaagcgccc- cgacatgctg gtggacagcttcggcctggagtccaccgtgcaggacggcctggtgttccgccagtccttctccatccgctccta- cgagatcggcac cgaccgcaccgccagcatcgagaccctgatgaaccacctgcaggagacctccctgaaccactgcaagagcaccg- gcatcctgc tggacggcttcggccgcaccctggagatgtgcaagcgcgacctgatctgggtggtgattaagatgcagatcaag- gtgaaccgcta ccccgcctggggcgacaccgtggagatcaacacccgcttcagccgcctgggcaagatcggcatgggccgcgact- ggctgatctc cgactgcaacaccggcgagatcctggtgcgcgccaccagcgcctacgccatgatgaaccagaagacccgccgcc- tgtccaag ctgccctacgaggtgcaccaggagatcgtgcccctgttcgtggacagccccgtgatcgaggactccgacctgaa- ggtgcacaag ttcaaggtgaagaccggcgacagcatccagaagggcctgacccccggctggaacgacctggacgtgaaccagca- cgtgtcca acgtgaagtacatcggctggatcctggagagcatgcccaccgaggtgctggagacccaggagctgtgctccctg- gccctggagt accgccgcgagtgcggccgcgactccgtgctggagagcgtgaccgccatggaccccagcaaggtgggcgtgcgc- tcccagtac cagcacctgctgcgcctggaggacggcaccgccatcgtgaacggcgccaccgagtggcgccccaagaacgccgg- cgccaac ggcgccatctccaccggcaagaccagcaacggcaactccgtgtccatggactacaaggaccacgacggcgacta- caaggacc acgacatcgactacaaggacgacgacgacaagTGActcgagagcgtccagcgtgtgggatgaagggtgcgatgg- aacggg gctgccgccccccctctgggcatctagctctgcaccgcacgccaggaagcccaagccaggccccgtcacactcc- ctcgctgaagtg ttccccccctgccccacactcatccaggtatcaacgccatcatgttctacgtccccgtcatcttcaactccctg- gggagcgggcgccgc ##STR00384## ##STR00385## atgtcggcgtgccccgcgatgactggcagggcccctggggcacgtcgctccggacggccagtcgccacccgcct- gaggtacgta ttccagtgcctggtggccagctgcatcgacccctgcgaccagtaccgcagcagcgccagcctgagcttcctggg- cgacaacggct tcgccagcctgttcggcagcaagcccttcatgagcaaccgcggccaccgccgcctgcgccgcgccagccacagc- ggcgaggc catggccgtggccctgcagcccgcccaggaggccggcaccaagaagaagcccgtgatcaagcagcgccgcgtgg- tggtgacc ggcatgggcgtggtgacccccctgggccacgagcccgacgtgttctacaacaacctgctggacggcgtgagcgg- catcagcga gatcgagaccttcgactgcacccagttccccacccgcatcgccggcgagatcaagagcttcagcaccgacggct- gggtggcccc caagctgagcaagcgcatggacaagttcatgctgtacctgctgaccgccggcaagaaggccctggccgacggcg- gcatcaccg acgaggtgatgaaggagctggacaagcgcaagtgcggcgtgctgatcggcagcggcatgggcggcatgaaggtg- ttcaacga cgccatcgaggccctgcgcgtgagctacaagaagatgaaccccttctgcgtgcccttcgccaccaccaacatgg- gcagcgccat gctggccatggacctgggctggatgggccccaactacagcatcagcaccgcctgcgccaccagcaacttctgca- tcctgaacgc cgccaaccacatcatccgcggcgaggccgacatgatgctgtgcggcggcagcgacgccgtgatcatccccatcg- gcctgggcg gcttcgtggcctgccgcgccctgagccagcgcaacagcgaccccaccaaggccagccgcccctgggacagcaac- cgcgacg gcttcgtgatgggcgagggcgccggcgtgctgctgctggaggagctggagcacgccaagaagcgcggcgccacc- atctacgcc gagttcctgggcggcagcttcacctgcgacgcctaccacatgaccgagccccaccccgagggcgccggcgtgat- cctgtgcatc gagaaggccctggcccaggccggcgtgagcaaggaggacgtgaactacatcaacgcccacgccaccagcaccag- cgccgg cgacatcaaggagtaccaggccctggcccgctgcttcggccagaacagcgagctgcgcgtgaacagcaccaaga- gcatgatc ggccacctgctgggcgccgccggcggcgtggaggccgtgaccgtggtgcaggccatccgcaccggctggattca- ccccaacct gaacctggaggaccccgacaaggccgtggacgccaagctgctggtgggccccaagaaggagcgcctgaacgtga- aggtggg cctgagcaacagcttcggcttcggcggccacaacagcagcatcctgttcgccccctgcaacgtgTGActcgagg- cagcagcag ctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacct- gtgaatatccctgccg cttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctat- ttgcgaataccacccccagc atccccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgc- tgctcctgctcctgctcact gcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaat- gctgatgcacgggaa gtagtgggatgggaacacaaatggaaagcttgagctccacctgcatccgcctggcgctcgaggacgccggcgtc- tcgcccgac gaggtcaactacgtcaacgcgcacgccacctccaccctggtgggcgacaaggccgaggtgcgcgcggtcaagtc- ggtctttg gcgacatgaagggcatcaagatgaacgccaccaagtccatgatcgggcactgcctgggcgccgccggcggcatg- gaggccg tcgccacgctcatggccatccgcaccggctgggtgcaccccaccatcaaccacgacaaccccatcgccgaggtc- gacggcct ggacgtcgtcgccaacgccaaggcccagcacaaaatcaacgtcgccatctccaactccttcggcttcggcgggc- acaactcc gtcgtcgcctttgcgcccttccgcgagtaggcggagcgagcgcgcttggctgaggagggaggcggggtgcgagc- cctttggct gcgcgcgatactctccccgcacgagcagactccacgcgcctgaatctacttgtcaacgagcaaccgtgtgtttt- gtccgtggcc attcttattatttctccgactgtggccgtactctgtttggctgtgcaagcaccgaagagcc
[0633] Fatty acids profiles from representative shake flask cultures of stable lines derived from D1550 transformants are shown in Table 74. Two independent genetic lineages yielded strains with high and balanced levels of C10-C12:0 fatty acids.
TABLE-US-00102 TABLE 74 Fatty acid profiles in S5050 and derivative transgenic lines generated after transformation with pSZ2424 DNA. Fatty Acid (area %) Strain C8:0 C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Total Saturates Strain BA 0.32 14.96 43.84 16.60 10.49 0.54 9.64 2.49 86.75 D1550-29.C4.A2 4.02 32.82 40.98 7.37 5.06 0.40 5.61 2.37 90.65 D1550-29.C4.A3 4.50 33.93 40.23 7.09 4.91 0.36 5.38 2.30 91.02 D1550-29.C4.A4 3.57 34.31 41.04 6.86 4.90 0.36 4.98 2.56 91.04 D1550-29.C4.A5 4.66 34.23 39.68 6.96 4.90 0.36 5.55 2.32 90.79 D1550-29.C6.E2 3.59 35.44 40.49 6.32 4.74 0.34 4.94 2.63 90.92 D1550-29.C6.E3 3.60 35.55 40.90 6.33 4.67 0.34 4.66 2.52 91.39 D1550-29.C6.E4 BB 3.97 35.85 40.23 6.26 4.65 0.34 4.83 2.51 91.30 D1550-29.C6.E5 4.02 35.19 39.89 6.59 4.79 0.34 5.12 2.60 90.82 D1550-29-1.14 3.30 39.62 40.04 5.16 4.04 0.30 3.49 2.67 92.46 D1550-29-1.2 3.12 39.50 40.22 5.13 3.86 0.29 3.42 2.82 92.12 D1550-29-1.12 3.26 39.36 39.91 5.13 4.15 0.30 3.73 2.77 92.11 D1550-29-1.17 3.25 39.21 40.21 5.22 4.11 0.30 3.70 2.67 92.30 D1550-29-1.39 4.12 38.44 39.23 5.83 4.25 0.30 3.96 2.46 92.17 D1550-29-1.35 BC 3.60 38.06 39.79 5.89 4.35 0.29 3.98 2.58 91.98 D1550-29-1.7 3.15 39.18 40.04 5.24 4.05 0.32 3.68 2.88 91.98 D1550-29-1.1 2.87 38.29 40.76 5.20 4.20 0.31 3.79 2.86 91.63
[0634] Next, we analyzed the performance of D1681 strains that were constructed using the KASI replacement strategy. Interestingly, unlike D1550 transformants, the D1681 strains demonstrated greater variability in fatty acid profiles (Table 75). In addition, the D1681 derived lines had lower C8:0 levels than what we observed in the D1550 derived transgenic lines suggesting a direct role of C. wrightii KASAI in improving C10:0 specificity of C. hookeriana FATB2 thioesterase.
TABLE-US-00103 TABLE 75 Fatty acid profiles in Strain BA and derivative transgenic lines generated after transformation with pSZ2746 DNA. Fatty Acid (area %) Total Strain C8:0 C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Saturates Strain BA 0.89 13.17 40.52 17.53 11.60 0.59 11.38 2.95 84.30 D1681.3.7-2 BD 1.44 31.83 44.97 6.52 4.83 0.30 6.53 2.45 89.89 D1681.3.7-10 1.84 31.41 43.64 6.90 5.15 0.31 7.08 2.46 89.25 D1681.3.7-12 BE 1.85 31.64 43.50 6.76 5.08 0.31 7.16 2.49 89.14 D1681.3.4-1 1.29 31.61 45.92 6.65 4.49 0.29 6.20 2.46 90.25 D1681.3.4-6 BF 1.48 32.26 45.11 6.55 4.56 0.29 6.23 2.41 90.25 D1681.3.4-9 1.42 31.22 45.40 6.93 4.69 0.30 6.49 2.47 89.96 D1681.3.8-1 1.35 27.72 44.72 8.78 5.97 0.37 7.36 2.51 88.91 D1681.3.8-4 1.44 27.51 44.34 8.72 6.05 0.36 7.84 2.51 88.42 D1681-2.1-37 0.64 34.80 47.17 4.84 3.81 0.31 4.37 2.43 91.57 D1681-2.1-34 0.62 35.26 47.07 4.77 3.77 0.30 4.22 2.36 91.79 D1681-2.1-28 BG 0.64 35.99 46.80 4.65 3.68 0.29 4.02 2.34 92.05 D1681-2.1-12 0.67 34.78 47.21 4.94 3.79 0.34 4.30 2.35 91.73 D1681.2.4-1.3 BH 0.57 33.95 47.73 4.93 3.39 0.03 0.24 3.96 92.60 D1681.2.4-1.4 0.56 36.71 47.55 4.86 3.26 0.03 0.24 3.61 92.97 D1681.2.4-1.12 BI 1.89 34.72 44.70 6.32 4.03 0.02 0.27 5.18 91.68 D1681.2.4-1.2 1.73 36.43 44.25 5.57 4.09 0.03 0.32 4.55 92.10
[0635] Eight strains representing D1550 and D1681 families (from Tables 74-75) were subsequently evaluated in high cell density fermentations as shown in Table 76. Fermentations resulted in oils with a slightly improved mid-chain profile or the balance of C10-C12:0 fatty acid levels compared to the lab scale fermentation. Strain BE evaluated in two independent fermentations demonstrated superior profile reaching 85.2% C10-C12:0 fatty acid levels, 3.5% C14:0 levels, and ca. 1.2% C8:0 fatty acid levels, and accumulated over 92% total saturates.
TABLE-US-00104 TABLE 76 End-point fatty acid profiles in D1550 and D1681 derivative transgenic lines subjected to high cell density fermentation. Strain BB BC BG BH BI BF BE Run 130067 130196 130197 130291 130292 130253 130246 PF13029 Fatty C8:0 5.3 4.62 4.62 0.54 1.53 1.59 2.1 1.19 Acid C10:0 36.19 36.16 36.16 33.24 40 40.46 40.94 41.59 Profile C12:0 39.07 38.77 38.77 47.65 43.09 42.39 41.25 43.6 (Area %) C14:0 5.31 5.18 5.18 5 4.62 4.42 4.2 3.49 C16:0 3.72 3.9 3.9 3.4 2.83 2.63 2.8 2.28 C18:0 0.24 0.28 0.28 0.27 0.22 0.32 0.28 0.15 C18:1 6.12 6.79 6.79 5.88 4.95 5.34 5.8 4.76 C18:2 2.43 2.76 2.76 2.49 1.95 2.05 1.89 1.96 C10-C14 80.57 80.11 80.11 85.89 87.71 87.27 86.39 88.68 C10-C12 75.26 74.93 74.93 80.89 83.09 82.85 82.19 85.19 Total Saturates 89.83 88.91 88.91 90.1 92.29 91.81 91.57 92.3
Example 61
Tag Regiospecificity in UTEX1435 by Expression of Cuphea PSR23 LPAAT2 and LPAAT3 Genes
[0636] In Example 43, we demonstrated that the expression of 2 different 1-acyl-sn-glycerol-3-phosphate acyltransferases (LPAATs), the LPAAT2 and LPAAT3 genes from Cuphea PSR23 (CuPSR23) in the UTEX1435 derivative strain S2014 resulted in elevation of C10:0, C12:0 and C14:0 fatty acids levels. In this example we provide evidence that Cuphea PSR23 LPAAT2 exhibits high specificity towards incorporating C10:0 fatty acids at sn-2 position in TAGs. The Cuphea PSR23 LPAAT3 specifically incorporates C18:2 fatty acids at sn-2 position in TAGs.
[0637] Composition and properties of Prototheca moriformis (UTEX 1435) transgenic strain B, transforming vectors pSZ2299 and pSZ2300 that express CuPSR23 LPAAT2 and LPAAT3 genes, respectively, and their sequences were described previously.
[0638] To determine the impact of Cuphea PSR23 LPAAT genes on the resulting fatty acid profiles we have taken advantage of Strain B which synthesizes both mid chain and long chain fatty acids at relatively high levels. As shown in Table 77, the expression of the LPAAT2 gene (D1520) in Strain B resulted in increased C10-C12:0 levels (up to 12% in the best strain, D1520.3-7) suggesting that this LPAAT is specific for mid chain fatty acids. Alternatively, expression of the LPAAT3 gene resulted in a relatively modest increase, (up to 5% in the best strain, D1521.28-7) indicating it has little or no impact on mid-chain levels.
TABLE-US-00105 TABLE 77 Fatty acid profiles of Strain B and representative transgenic lines transformed with pSZ2299 (D1520) and pSZ2300 (D1521) DNA. Fatty Acid (area %) C10: C12: C14: C16: C18: C18: C18: C10- Total Strain C8:0 0 0 0 0 0 1 2 C12 Saturates Strain B 0.09 4.95 29.02 15.59 12.55 1.27 27.93 7.60 33.97 63.47 D1520.8-6 0.00 6.71 31.15 15.80 13.04 1.42 24.32 6.56 37.86 68.12 D1520.13-4 0.00 6.58 30.96 16.14 13.34 1.25 24.32 6.27 37.54 68.27 D1520.19-4 0.00 7.53 32.94 16.64 12.63 1.17 21.96 6.11 40.47 70.91 D1520.3-7 0.06 9.44 36.26 16.71 11.44 1.28 18.41 5.59 45.70 75.19 D1521.13-8 0.00 6.21 33.13 16.70 12.30 1.18 20.84 8.70 39.34 69.52 D1521.18-2 0.00 5.87 31.91 16.46 12.60 1.22 22.14 8.59 37.78 68.06 D1521.24-8 0.00 5.75 31.47 16.13 12.60 1.42 23.31 8.22 37.22 67.37 D1521.28-7 0.00 6.28 32.82 16.33 12.27 1.43 21.98 7.91 39.10 69.13
[0639] To determine if expression of the Cuphea PSR23 LPAAT genes affected regiospecificity of fatty acids at the sn-2 position, we analyzed TAGs from representative D1520 and D1521 strains utilizing the porcine pancreatic lipase method. See Example 2. As demonstrated in Table 78, the Cuphea PSR23 LPAAT2 gene shows remarkable specificity towards C10:0 fatty acids and appears to incorporate 50% more C10:0 fatty acids into the sn-2 position. The Cuphea PSR23 LPAAT3 gene appears to act exclusively on C18:2 fatty acids, resulting in redistribution of C18:2 fatty acids onto sn-2 position. Accordingly, microbial triglyceride oils with sn-2 profiles of greater than 15% or 20% C10:0 or C18:2 fatty acids are obtainable by introduction of an exogenous LPAAT gene having corresponding specificity.
TABLE-US-00106 TABLE 78 TAG and sn-2 fatty acid profiles in oils of parental S2014 strain and the progeny strains expressing Cuphea PSR23 LPAAT2 (BJ) and LPAAT3 (BK) genes. Strain Strain B Strain BI (D1520.3-7) Strain BK (D1521.13-8) Analysis TAG Profile sn-2 Profile TAG Profile sn-2 Profile TAG Profile sn-2 Profile Fatty C8:0 0 0 0.1 0 0 0 Acid C10:0 12 14.2 11 24.9 6.21 6.3 (area C12:0 42.8 25.1 40.5 24.3 33.13 19.5 %) C4:0 12.1 10.4 16.3 10 16.7 11.8 C6:0 7.3 1.3 10.2 1.4 12.3 3 C8:0 0.7 0.2 0.9 0.6 1.18 0.5 C8:1 18.5 36.8 15.4 29.2 20.84 36.3 C8:2 5.8 10.9 4.9 8.7 8.7 20.9 C18:3a 0.6 0.8 0.4 0.8 0.48 1.2 C10-C14 66.9 49.7 67.8 59.2 56.0 37.6 C10-C12 54.8 39.3 51.5 49.2 39.3 25.8
Example 62
Introduction of Heterologous Thioesterases into a Heterologous KAS-Expressing Prototheca Moriformis Strain
[0640] Here we demonstrate that heterologous fatty acyl-ACP thioesterases exhibit altered thioesterase specificity when combined with a heterologous plant KASI gene, Cuphea wrightii .beta.-ketoacyl-ACP synthase (KAS), CwKASA1, in P. moriformis (UTEX 1435) transgenic strain, S5818. S5818 is a transgenic strain expressing a thioesterase chimera from Cinnamomum camphora and Umbellularia californica, CcFATB2-UcFATB2 chimera B, at the 6S locus and additionally expressing the Cuphea wrightii KAS, CwKASA1, at the pLOOP locus. The addition of the CcFATB2-UcFATB2 chimera B and CwKASA1 genes leads to an S5818 fatty acid profile with 45% C12:0 and 14% C14:0. Five different constructs encoding thioesterases that were previously shown to exhibit predominantly C14:0 thioesterase activity and with less pronounced C12:0 thioesterase activity in P. moriformis were introduced into S5818 in an effort to increase C14:0 and C12:0 levels in this background. However, introduction of the five different C14:0 thioesterases into S5818 led to unexpected but significant increases in C12:0 fatty acid levels (>50% overall) with only modest increases in C14:0 fatty acid levels (<20% overall). This result suggests that the KASI-FATB thioesterase combination exhibits a unique activity not displayed when either gene is introduced separately. The results demonstrate that combination of heterologous KAS genes with heterologous thioesterases in oleaginous cells can be used to produce fatty acid profiles not exhibited by introduction of either gene alone. Furthermore, introduction of heterologous KASs may be an important and fruitful approach for revealing novel specificities of additional heterologous thioesterases.
[0641] Strain S5818 generation. S5818 was created by two successive transformations. The UTEX1435 base strain, S3150 (Strain Z above), was transformed with pSZ2448 (6 SA::CrTUB2-ScSUC2-CvNR:PmAMT3-CpSAD1tpExt-CcFATB2-UcFATB2-chimeraB-ExtA-C- vNR::6SB), encoding the CcFATB2-UcFATB2 chimera B thioesterase targeting the 6S locus, to yield strain S4954. S4954 produces .about.32% C12:0 and .about.16% C14:0 fatty acid levels (Table 62-1). S4954 was subsequently transformed with pSZ2229 (pLOOP::CrTUB2-NeoR-CvNR:PmAMT3-PmSADtp_CwKASAI-CvNR::pLOOP), encoding the C. wrightii KASA1 gene targeting the pLOOP locus, to yield strain S5818. S5818 produces .about.45% C12:0 and .about.14% C14:0 fatty acid levels (Table 79).
TABLE-US-00107 TABLE 79 Fatty acid profiles of S3150, S4954, and S5818. Sample ID C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Strain Z 0 0.05 1.49 28.83 3.24 57.87 6.27 S4954 0.17 31.52 16.39 9.81 1.19 32.14 7.19 S5818 0.34 45.16 13.77 8.54 0.81 24.63 5.38
[0642] Identification of C14:0 thioesterases. In an effort to increase C14:0 fatty acid levels, and to a lesser degree C12:0 fatty acid levels, several thioesterases that were found to exhibit C14:0 and C12:0 thioesterase activity in P. moriformis were cloned into vectors for introduction into S5818. The Cuphea hyssopifolia thioesterase ChsFATB3 was discovered by us as part of efforts to identify novel thioesterases by sequencing the mature, plant oilseeds of C. hyssopifolia. Although C. hyssopifolia seeds exhibit .about.84% C12:0 and .about.5% C14:0 fatty acid levels, the ChsFATB3 thioesterase we identified exhibits strong C14:0 thioesterase activity when expressed in S3150 (up to .about.34% C14:0). A version of ChsFATB3 in which we optimized the putative plastid-targeting transit peptide, named pSADD1tp_trimmed:ChsFATB3, similarly exhibited strong C14:0 thioesterase activity (.about.33% C14:0; Table 80).
TABLE-US-00108 TABLE 80 Fatty acid profiles of Cuphea hyssopifolia seeds and S3150 with introduction of ChsFATB3 or CpSAD1tp_trimmed:ChsFATB3. Sample ID C8:0 C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Cuphea hyssopifolia seeds 0.24 6.53 83.69 5.13 1.10 0.12 0.00 1.74 S3150 0.00 0.00 0.05 1.49 28.83 3.24 57.87 6.27 S3150 + ChsFATB3 (T537; D1701-48) 0.00 0.00 8.09 33.66 26.46 1.57 23.75 5.3 S3150 + CpSAD1tp_trimmed:ChsFATB3 0.00 0.14 7.25 33.32 27.04 1.57 24.37 5.12 (T580; D1813-8)
[0643] Similarly, we also identified the Cuphea heterophylla thioesterase ChtFATB1a as part of our efforts to identify novel thioesterases by sequencing the mature, plant oil seeds of C. heterophylla. Although C. heterophylla seeds exhibit .about.44% C10:0, .about.40% C12:0 fatty acid levels, and only .about.4% C14:0, the transit peptide optimized version of the ChtFATB1a thioesterase we identified, CpSAD1tp_trimmed:ChtFATB1a, exhibits strong C14:0 thioesterase activity when expressed in S3150 (up to .about.35% C14:0; Table 81).
TABLE-US-00109 TABLE 81 Fatty acid profiles of Cuphea heterophylla seeds and S3150 with introduction of CpSAD1tp_trimmed:ChtFATB1a. Sample ID C8:0 C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 Cuphea heterophylla seeds 3.50 44.27 40.04 4.26 1.22 0.25 2.21 3.56 S3150 0.00 0.00 0.05 1.49 28.83 3.24 57.87 6.27 S3150 + CpSAD1tp_trimmed:ChtFATB1a 0.00 0.15 13.18 35.16 24.1 1.19 18.87 6.02 (T580; D1811-44)
[0644] A published Cuphea palustris C14:0 thioesterase, CpalFATB2, was also introduced into S5818 (vide infra).
[0645] Introduction of C14:0 thioesterases into S5818. Five constructs were generated using C14:0 thioesterases for introduction into S5818 (Table 82).
TABLE-US-00110 TABLE 82 Constructs engineered for introduction into S5818. D# pSZ# Construct D2104 pSZ3390 DAO1b::PmHXT1-ScarMel1-CvNR:PmUAPA1noSacI-CpSAD1tpExt-CpaIFATB2FLAGExtA-C- vNR::DAO1b D2202 pSZ3493 DAO1b5.sup.1::PmHXT1-ScarMEL1-CvNR:PmAMT3-ChsFATB3-CvNR::DAO1b3.sup.1 D2203 pSZ3494 DAO1b5.sup.1::PmHXT1-ScarMEL1-CvNR:PmAMT3-CpSAD1tp_trimmed:ChsFATB3-CvNR:- :DAO1b3.sup.1 D2204 pSZ3495 DAO1b5.sup.1::PmHXT1-ScarMEL1-CvNR:PmAMT3-CpSAD1tp_trimmed:ChtFATB1a-CvNR- ::DAO1b3.sup.1 D2235 pSZ3531 THI4A::PmHXT1-ScarMel1 - CpEF1a:PmUAPA1noSacI-CpSAD1tpExt-CpaIFATB2FLAGExtA-CvNR::THI4A
[0646] pSZ3390 and pSZ3531 introduce the CpalFATB2 thioesterase gene into the DAO1b and THI4A loci, respectively, under the control of the pH5-responsive UAPA1 promoter. pSZ3493, pSZ3494, and pSZ3495 introduce ChsFATB3, CpSAD1tp_trimmed:ChsFATB3, and CpSAD1tp trimmed:ChtFATB1a, respectively, into the DAO1b locus under the control of the pH7-responsive AMT3 promoter. Transgenic strains were selected for the ability to grow on melibiose. Cell culture, lipid production, and fatty acid analysis were all carried out as previously described. The transforming DNA for pSZ3390, pSZ3493, pSZ3494, pSZ3495, and pSZ3531 are provided below.
[0647] pSZ3390: pSZ3390 can be written as DAO1b:: PmHXT1-ScarMel1-CvNR:PmUAPA1noSacI-CpSAD1tpExt-CpalFATB2FLAGExtA-CvNR::DO- A1b. The relevant restriction sites in the construct from 5'-3', BspQI, KpnI, SpeI, SnaBI, XhoI, EcoRI, SpeI, HindIII, SacI, BspQI, respectively, are indicated in lowercase, bold, and underlined. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences at the 5' and 3' end of the construct represent genomic DNA from UTEX 1435 that target integration to the DAO1b locus via homologous recombination. Proceeding in the 5' to 3' direction, the selection cassette has the P. moriformis HXT1 promoter driving the expression of the S. carlbergensis MEL1 gene (conferring the ability to grow on melibiose) and the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. The promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for ScarMEL1 are indicated by bold, uppercase italics, while the coding region is indicated with lowercase italics. The 3'UTR is indicated by lowercase, underlined text. The second cassette containing the CpSAD1tpExt-CpalFATB2FLAGExtA gene, fused to the heterologous Chlorella protothecoides SAD1 plastid-targeting transit peptide, is driven by the P. moriformis UAPA1 pH5-responsive promoter and has the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. In this cassette, the UAPA1 promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for the CpSAD1tpExt-CpalFATB2FLAGExtA gene are indicated in bold, uppercase italics, while the coding region is indicated by lowercase italics. The 3' UTR is indicated by lowercase, underlined text.
TABLE-US-00111 pSZ3390 transforming construct: (SEQ ID NO: 123) gaagagcGCCCAATGTTTAAACagcccgcaccctcgttgatctgggagccctgcgcagccccttaaatcatctc- ag tcaggtttctgtgttcaactgagcctaaagggctttcgtcatgcgcacgagcacacgtatatcggccacgcagt- ttctcaaaagc ggtagaacagttcgcgagccctcgtaggtcgaaaacttgcgccagtactattaaattaaattaattgatcgaac- gagacgcga aacttttgcagaatgccaccgagtttgcccagagaatgggagtggcgccattcaccatccgcctgtgcccggct- tgattcgccg agacgatggacggcgagaccagggagcggcttgcgagccccgagccggtagcaggaacaatgatcgacaatctt- cctgtcc aattactggcaaccattagaaagagccggagcgcgttgaaagtctgcaatcgagtaatttttcgatacgtcggg- cctgctgaa ccctaaggctccggactttgtttaaggcgatccaagatgcacgcggccccaggcacgtatctcaagcacaaacc- ccagcctta gtttcgagactttgggagatagcgaccgatatctagtttggcattttgtatattaattacctcaagcaatggag- cgctctgatgcg gtgcagcgtcggctgcagcacctggcagtggcgctagggtcgccctatcgctcggaacctggtcagctggctcc- cgcctcctgc ##STR00386## ctccctgaagggcgtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatgggctgggaca- actggaacac gttcgcctgcgacgtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctgaaggaca- tgggctacaa gtacatcatcctggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgagcagaagt- tccccaacggc atgggccacgtcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcgagtacac- gtgcgccggcta ccccggctccctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacctgaagtacg- acaactgct acaacaagggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccctgaacaag- acgggccg ccccatcttctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcgaactcct- ggcgcatgtccgg cgacgtcacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactgcaagtacg- ccggcttcc actgctccatcatgaacatcctgaacaaggccgcccccatgggccagaacgcgggcgtcggcggctggaacgac- ctggacaa cctggaggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatggtgaagt- cccccctgat catcggcgcgaacgtgaacaacctgaaggcctcctcctactccatctactcccaggcgtccgtcatcgccatca- accaggactcc aacggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccagggcgagatcca- gatgtggtc cggccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgccccatgaacacga- ccctggagg agatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacctgtgggcgaac- cgcgtcgacaa ctccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgccaccgagcagtcct- acaaggacg gcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcgatcctgaacacg- accgtccccg cccacggcatcgcgttctaccgcctgcgcccctcctccTGAtacgtactcgaggcagcagcagtcggatagtat- cgacacactc tggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgctttta- tcaaacagcctcagtgt gtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatc- cccttccctcgtttcatatc gcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcc- cctcgcacagccttggtt tgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtg- ggatgggaacacaa ##STR00387## ##STR00388## ctcggcgttcaatgcccgctgcggcgacctgcgtcgctcggcgggctccgggccccggcgcccagcgaggcccc- tccccgtgcg cgctgccatcgccagcgaggtccccgtggccaccacctccccccgggcgcaccccaaggcgaacggcagcgcgg- tgtcgctg aagtcgggctccctggagacccaggaggacaagacgagcagctcgtccccccccccccgcacgttcatcaacca- gctgcccgt gtggagcatgctgctgtcggcggtgaccacggtcttcggcgtggccgagaagcagtggcccatgctggaccgca- agtccaagcg ccccgacatgctggtcgagcccctgggcgtggaccgcatcgtctacgacggcgtgagcttccgccagtcgttct- ccatccgcagct acgagatcggcgccgaccgcaccgcctcgatcgagacgctgatgaacatgttccaggagacctccctgaaccac- tgcaagatc atcggcctgctgaacgacggcttcggccgcacgcccgagatgtgcaagcgcgacctgatctgggtcgtgaccaa- gatgcagatc gaggtgaaccgctaccccacgtggggcgacaccatcgaggtcaacacgtgggtgagcgcctcgggcaagcacgg- catgggcc gcgactggctgatctccgactgccacaccggcgagatcctgatccgcgcgacgagcgtctgggcgatgatgaac- cagaagacc cgccgcctgtcgaagatcccctacgaggtgcgccaggagatcgagccccagttcgtcgactccgcccccgtgat- cgtggacgac cgcaagttccacaagctggacctgaagacgggcgacagcatctgcaacggcctgaccccccgctggacggacct- ggacgtga accagcacgtcaacaacgtgaagtacatcggctggatcctgcagtcggtccccaccgaggtgttcgagacgcag- gagctgtgcg gcctgaccctggagtaccgccgcgagtgcggccgcgactccgtgctggagagcgtcacggccatggacccctcg- aaggaggg cgaccgctccctgtaccagcacctgctgcgcctggaggacggcgcggacatcgtgaagggccgcaccgagtggc- gccccaag aacgccggcgccaagggcgccatcctgacgggcaagaccagcaacggcaactcgatctccatggactacaagga- ccacgac ggcgactacaaggaccacgacatcgactacaaggacgacgacgacaagTGAaagcttgcagcagcagctcggat- agtatcg acacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgc- cgcttttatcaaacagc ctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccaccc- ccagcatccccttccctcgt ttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgc- tcactgcccctcgcacag ccttggtttgggctcccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgg- gaagtagtgggatgg gaacacaaatggaaagctggagctcagcgtctgcgtgttgggagctggagtcgtgggcttgacgacggcgctgc- agctgttgca ggatgtgcctggcgtgcgcgttcacgtcgtggctgagaaatatggcgacgaaacgttgacggctggggccggcg- ggctgtgg atgccatacgcattgggtacgcggccattggatgggattgataggcttatggagggataatagagtttttgccg- gatccaacgc atgtggatgcggtatcccggtgggctgaaagtgtggaaggatagtgcattggctattcacatgcactgcccacc- ccttttggca ggaaatgtgccggcatcgttggtgcaccgatggggaaaatcgacgttcgaccactacatgaagatttatacgtc- tgaagatgc agcgactgcgggtgcgaaacggatgacggtttggtcgtgtatgtcacagcatgtgctggatcttgcgggctaac- tccccctgcc acggcccattgcaggtgtcatgttgactggagggtacgacctttcgtccgtcaaattcccagaggaggacccgc- tctgggccg acattgtgcccactgaagagc
[0648] pSZ3493, pSZ3494, and pSZ3495: pSZ3493 can be written as DOA1b5'::PmHXT1-ScarMEL1-CvNR:PmAMT3-ChsFATB3-CvNR::DOA1b3'. pSZ3494 can be written as DOA1b5'::PmHXT1-ScarMEL1-CvNR:PmAMT3-CpSAD1tp trimmed:ChsFATB3-CvNR::DOA1b3'. pSZ3495 can be written as DOA1b5'::PmHXT1-ScarMEL1-CvNR:PmAMT3-CpSAD1tp_trimmed: ChtFATB1a-CvNR::DOA1b3'. The sequences of the three constructs differ only in the sequence of the thioesterase gene. The full transforming sequence for pSZ3493 is displayed in SEQ ID NO:124. The sequences of the CpSAD1tp_trimmed: ChsFATB3 and CpSAD1tp_trimmed:ChtFATBla genes alone, which take the place of ChsFATB3 from pSZ3493 in the pSZ3494 and pSZ3495 sequences, are displayed in SEQ ID NOs:125 and 126, respectively, along with flanking restriction sites.
[0649] The relevant restriction sites in the pSZ3493 construct from 5'-3', BspQI, KpnI, SpeI, SnaBI, XhoI, EcoRI, SpeI, XhoI, SacI, BspQI, respectively, are indicated in lowercase, bold, and underlined. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences at the 5' and 3' end of the construct represent genomic DNA from UTEX 1435 that target integration to the DAO1b locus via homologous recombination. Proceeding in the 5' to 3' direction, the selection cassette has the P. moriformis HXT1 promoter driving the expression of the S. carlbergensis MEL1 gene (conferring the ability to grow on melibiose) and the Chlorella vulgaris Nitrate reductase (NR) gene 3' UTR. The promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for ScarMEL1 are indicated by bold, uppercase italics, while the coding region is indicated with lowercase italics. The 3'UTR is indicated by lowercase, underlined text. The second cassette is comprised of the ChsFATB3 gene driven by the P. moriformis AMT3 pH7-responsive promoter and with the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. In this cassette, the AMT3 promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for the ChsFATB3 gene are indicated in bold, uppercase italics, while the coding region is indicated by lowercase italics. The 3' UTR is indicated by lowercase, underlined text.
TABLE-US-00112 pSZ3493 transforming construct: (SEQ ID NO: 124) gaagagcGCCCAATGTTTAAACagcccgcaccctcgttgatctgggagccctgcgcagccccttaaatcatctc- ag tcaggtttctgtgttcaactgagcctaaagggctttcgtcatgcgcacgagcacacgtatatcggccacgcagt- ttctcaaaagc ggtagaacagttcgcgagccctcgtaggtcgaaaacttgcgccagtactattaaattaaattaattgatcgaac- gagacgcga aacttttgcagaatgccaccgagtttgcccagagaatgggagtggcgccattcaccatccgcctgtgcccggct- tgattcgccg agacgatggacggcgagaccagggagcggcttgcgagccccgagccggtagcaggaacaatgatcgacaatctt- cctgtcc aattactggcaaccattagaaagagccggagcgcgttgaaagtctgcaatcgagtaatttttcgatacgtcggg- cctgctgaa ccctaaggctccggactttgtttaaggcgatccaagatgcacgcggccccaggcacgtatctcaagcacaaacc- ccagcctta gtttcgagactttgggagatagcgaccgatatctagtttggcattttgtatattaattacctcaagcaatggag- cgctctgatgcg gtgcagcgtcggctgcagcacctggcagtggcgctagggtcgccctatcgctcggaacctggtcagctggctcc- cgcctcctgc ##STR00389## ctccctgaagggcgtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatgggctgggaca- actggaacac gttcgcctgcgacgtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctgaaggaca- tgggctacaa gtacatcatcctggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgagcagaagt- tccccaacggc atgggccacgtcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcgagtacac- gtgcgccggcta ccccggctccctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacctgaagtacg- acaactgct acaacaagggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccctgaacaag- acgggccg ccccatcttctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcgaactcct- ggcgcatgtccgg cgacgtcacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactgcaagtacg- ccggcttcc actgctccatcatgaacatcctgaacaaggccgcccccatgggccagaacgcgggcgtcggcggctggaacgac- ctggacaa cctggaggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatggtgaagt- cccccctgat catcggcgcgaacgtgaacaacctgaaggcctcctcctactccatctactcccaggcgtccgtcatcgccatca- accaggactcc aacggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccagggcgagatcca- gatgtggtc cggccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgccccatgaacacga- ccctggagg agatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacctgtgggcgaac- cgcgtcgacaa ctccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgccaccgagcagtcct- acaaggacg gcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcgatcctgaacacg- accgtccccg cccacggcatcgcgttctaccgcctgcgcccctcctccTGAtacgtactcgaggcagcagcagctcggatagta- tcgacacactc tggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgctttta- tcaaacagcctcagtgt gtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatc- cccttccctcgtttcatatc gcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcc- cctcgcacagccttggtt Igggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtg- ggatgggaacacaa ##STR00390## ##STR00391## cggcacctcccccaagcccggcaagttcggcaactggcccacctccctgtccgtgcccttcaagtccaagtcca- accacaacgg cggcttccaggtgaaggccaacgcctccgcccgccccaaggccaacggctccgccgtgtccctgaagtccggct- ccctggacac ccaggaggacacctcctcctcctcctcccccccccgcaccttcatcaaccagctgcccgactggtccatgctgc- tgtccgccatcac caccgtgttcgtggccgccgagaagcagtggaccatgctggaccgcaagtccaagcgccccgacatgctgatgg- accccttcgg cgtggaccgcgtggtgcaggacggcgccgtgttccgccagtccttctccatccgctcctacgagatcggcgccg- accgcaccgcc tccatcgagaccctgatgaacatcttccaggagacctccctgaaccactgcaagtccatcggcctgctgaacga- cggcttcggcc gcacccccgagatgtgcaagcgcgacctgatctgggtggtgaccaagatgcacgtggaggtgaaccgctacccc- acctggggc gacaccatcgaggtgaacacctgggtgtccgagtccggcaagaccggcatgggccgcgactggctgatctccga- ctgccacac cggcgagatcctgatccgcgccacctccatgtgcgccatgatgaaccagaagacccgccgcttctccaagttcc- cctacgaggtg cgccaggagctggccccccacttcgtggactccgcccccgtgatcgaggactaccagaagctgcacaagctgga- cgtgaagac cggcgactccatctgcaacggcctgaccccccgctggaacgacctggacgtgaaccagcacgtgaacaacgtga- agtacatcg gctggatcctggagtccgtgcccaccgaggtgttcgagacccaggagctgtgcggcctgaccctggagtaccgc- cgcgagtgcg gccgcgactccgtgctggagtccgtgaccgccatggacccctccaaggagggcgaccgctccctgtaccagcac- ctgctgcgcc tggaggacggcgccgacatcgccaagggccgcaccaagtggcgccccaagaacgccggcaccaacggcgccatc- tccaccg gcaagacctccaacggcaactccatctccatggactacaaggaccacgacggcgactacaaggaccacgacatc- gactacaa ggacgacgacgacaagTGActcgaggcagcagcagctcggatagtatcgacacactctggacgctggtcgtgtg- atggactgtt gccgccacacttgctgccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatctt- gtgtgtacgcgcttttgcg agttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatc- ccaaccgcaacttatctac gctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagccttggtttgggctccgc- ctgtattctcctggtact gcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaAAGCTGTAT- AGGG ATAACAGGGTAATgagctcagcgtctgcgtgttgggagctggagtcgtgggcttgacgacggcgctgcagctgt- tg caggatgtgcctggcgtgcgcgttcacgtcgtggctgagaaatatggcgacgaaacgttgacggctggggccgg- cgggctgt ggatgccatacgcattgggtacgcggccattggatgggattgataggcttatggagggataatagagtttttgc- cggatccaac gcatgtggatgcggtatcccggtgggctgaaagtgtggaaggatagtgcattggctattcacatgcactgccca- ccccttttgg caggaaatgtgccggcatcgttggtgcaccgatggggaaaatcgacgttcgaccactacatgaagatttatacg- tctgaagat gcagcgactgcgggtgcgaaacggatgacggtttggtcgtgtatgtcacagcatgtgctggatcttgcgggcta- actccccctg ccacggcccattgcaggtgtcatgttgactggagggtacgacctttcgtccgtcaaattcccagaggaggaccc- gctctgggcc gacattgtgcccactgaagagc CpSAD1tp_trimmed:ChsFATB3 (from pSZ3494): (SEQ ID NO: 125) actagtAACAATGgccaccgcctccaccttctccgccttcaacgcccgctgcggcgacctgcgccgctccgccg- gctccgg cccccgccgccccgcccgccccctgcccgtgcgcgccgccatcaacgcctccgcccgccccaaggccaacggct- ccgccgtgt ccctgaagtccggctccctggacacccaggaggacacctcctcctcctcctcccccccccgcaccttcatcaac- cagctgcccgac tggtccatgctgctgtccgccatcaccaccgtgttcgtggccgccgagaagcagtggaccatgctggaccgcaa- gtccaagcgcc ccgacatgctgatggaccccttcggcgtggaccgcgtggtgcaggacggcgccgtgttccgccagtccttctcc- atccgctcctac gagatcggcgccgaccgcaccgcctccatcgagaccctgatgaacatcttccaggagacctccctgaaccactg- caagtccatc ggcctgctgaacgacggcttcggccgcacccccgagatgtgcaagcgcgacctgatctgggtggtgaccaagat- gcacgtgga ggtgaaccgctaccccacctggggcgacaccatcgaggtgaacacctgggtgtccgagtccggcaagaccggca- tgggccgc gactggctgatctccgactgccacaccggcgagatcctgatccgcgccacctccatgtgcgccatgatgaacca- gaagacccgc cgcttctccaagttcccctacgaggtgcgccaggagctggccccccacttcgtggactccgcccccgtgatcga- ggactaccaga agctgcacaagctggacgtgaagaccggcgactccatctgcaacggcctgaccccccgctggaacgacctggac- gtgaacca gcacgtgaacaacgtgaagtacatcggctggatcctggagtccgtgcccaccgaggtgttcgagacccaggagc- tgtgcggcct gaccctggagtaccgccgcgagtgcggccgcgactccgtgctggagtccgtgaccgccatggacccctccaagg- agggcgacc gctccctgtaccagcacctgctgcgcctggaggacggcgccgacatcgccaagggccgcaccaagtggcgcccc- aagaacgc cggcaccaacggcgccatctccaccggcaagacctccaacggcaactccatctccatggactacaaggaccacg- acggcgact acaaggaccacgacatcgactacaaggacgacgacgacaagTGActcgag CpSAD1tp_trimmed:ChtFATB1a (from pSZ3495): (SEQ ID NO: 126) actagtAACAATGgccaccgcctccaccttctccgccttcaacgcccgctgcggcgacctgcgccgctccgccg- gctccgg cccccgccgccccgcccgccccctgcccgtgcgcgccgccatcaacgcctccgcccaccccaaggccaacggct- ccgccgtga acctgaagtccggctccctggagacccaggaggacacctcctcctcctccccccccccccgcaccttcatcaag- cagctgcccga ctggggcatgctgctgtccaagatcaccaccgtgttcggcgccgccgagcgccagtggaagcgccccggcatgc- tggtggagcc cttcggcgtggaccgcatcttccaggacggcgtgttcttccgccagtccttctccatccgctcctacgagatcg- gcgccgaccgcac cgcctccatcgagaccctgatgaacatcttccaggagacctccctgaaccactgcaagtccatcggcctgctga- acgacggcttc
ggccgcacccccgagatgtgcaagcgcgacctgatctgggtggtgaccaagatccaggtggaggtgaaccgcta- ccccacctg gggcgacaccatcgaggtgaacacctgggtgtccgagtccggcaagaacggcatgggccgcgactggctgatct- ccgactgcc gcaccggcgagatcctgatccgcgccacctccgtgtgggccatgatgaaccgcaagacccgccgcctgtccaag- ttcccctacg aggtgcgccaggagatcgccccccacttcgtggactccgcccccgtgatcgaggacgacaagaagctgcacaag- ctggacgtg aagaccggcgactccatccgcaagggcctgaccccccgctggaacgacctggacgtgaaccagcacgtgaacaa- cgtgaagt acatcggctggatcctgaagtccgtgcccgccgaggtgttcgagacccaggagctgtgcggcgtgaccctggag- taccgccgcg agtgcggccgcgactccgtgctggagtccgtgaccgccatggacaccgccaaggagggcgaccgctccctgtac- cagcacctg ctgcgcctggaggacggcgccgacatcaccatcggccgcaccgagtggcgccccaagaacgccggcgccaacgg- cgccatct ccaccggcaagacctccaacgagaactccgtgtccatggactacaaggaccacgacggcgactacaaggaccac- gacatcga ctacaaggacgacgacgacaagTGActcgag
[0650] pSZ3531: pSZ3531 can be written as THI4A::PmHXT1-ScarMel1-CpEF1a:PmUAPA1noSacI-CpSAD1tpExt-CpalFATB2FLAGExtA- -CvNR::THI4A. The relevant restriction sites in the construct from 5'-3', BspQI, KpnI, SpeI, SnaBI, EcoRV, EcoRI, SpeI, HindIII, SacI, BspQI, respectively, are indicated in lowercase, bold, and underlined. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences at the 5' and 3' end of the construct represent genomic DNA from UTEX 1435 that target integration to the THI4A locus via homologous recombination. Proceeding in the 5' to 3' direction, the selection cassette has the P. moriformis HXT1 promoter driving the expression of the S. carlbergensis MEL1 gene (conferring the ability to grow on melibiose) and the Chlorella protothecoides EF1A gene 3' UTR. The promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for ScarMEL1 are indicated by bold, uppercase italics, while the coding region is indicated with lowercase italics. The 3'UTR is indicated by lowercase, underlined text. The second cassette containing the CpSAD1tpExt-CpalFATB2FLAGExtA gene, fused to the heterologous Chlorella protothecoides SAD1 plastid-targeting transit peptide, is driven by the P. moriformis UAPA1 pH5-responsive promoter and has the Chlorella vulgaris Nitrate Reductase (NR) gene 3' UTR. In this cassette, the UAPA1 promoter is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for the CpSAD1tpExt-CpalFATB2FLAGExtA gene are indicated in bold, uppercase italics, while the coding region is indicated by lowercase italics. The 3' UTR is indicated by lowercase, underlined text.
TABLE-US-00113 pSZ3531 transforming construct: (SEQ ID NO: 127) gaagagcGCCCAATGTTTAAACCcctcaactgcgacgctgggaaccttctccgggcaggcgatgtgcgtgggtt- t gcctccttggcacggctctacaccgtcgagtacgccatgaggcggtgatggctgtgtcggttgccacttcgtcc- agagacggca agtcgtccatcctctgcgtgtgtggcgcgacgctgcagcagtccctctgcagcagatgagcgtgactttggcca- tttcacgcact cgagtgtacacaatccatttttcttaaagcaaatgactgctgattgaccagatactgtaacgctgatttcgctc- cagatcgcaca gatagcgaccatgttgctgcgtctgaaaatctggattccgaattcgaccctggcgctccatccatgcaacagat- ggcgacactt gttacaattcctgtcacccatcggcatggagcaggtccacttagattcccgatcacccacgcacatctcgctaa- tagtcattcgtt cgtgtcttcgatcaatctcaagtgagtgtgcatggatcttggttgacgatgcggtatgggtttgcgccgctggc- tgcagggtctg cccaaggcaagctaacccagctcctctccccgacaatactctcgcaggcaaagccggtcacttgccttccagat- tgccaataa actcaattatggcctctgtcatgccatccatgggtctgatgaatggtcacgctcgtgtcctgaccgttccccag- cctctggcgtcc ##STR00392## ##STR00393## ggcctgggcctgacgccccagatgggctgggacaactggaacacgttcgcctgcgacgtctccgagcagctgct- gctggacacg gccgaccgcatctccgacctgggcctgaaggacatgggctacaagtacatcatcctggacgactgctggtcctc- cggccgcgact ccgacggcttcctggtcgccgacgagcagaagttccccaacggcatgggccacgtcgccgaccacctgcacaac- aactccttcc tgttcggcatgtactcctccgcgggcgagtacacgtgcgccggctaccccggctccctgggccgcgaggaggag- gacgcccagt tcttcgcgaacaaccgcgtggactacctgaagtacgacaactgctacaacaagggccagttcggcacgcccgag- atctcctacc accgctacaaggccatgtccgacgccctgaacaagacgggccgccccatcttctactccctgtgcaactggggc- caggacctga ccttctactggggctccggcatcgcgaactcctggcgcatgtccggcgacgtcacggcggagttcacgcgcccc- gactcccgctg cccctgcgacggcgacgagtacgactgcaagtacgccggcttccactgctccatcatgaacatcctgaacaagg- ccgcccccat gggccagaacgcgggcgtcggcggctggaacgacctggacaacctggaggtcggcgtcggcaacctgacggacg- acgagg agaaggcgcacttctccatgtgggccatggtgaagtcccccctgatcatcggcgcgaacgtgaacaacctgaag- gcctcctccta ctccatctactcccaggcgtccgtcatcgccatcaaccaggactccaacggcatccccgccacgcgcgtctggc- gctactacgtgt ccgacacggacgagtacggccagggcgagatccagatgtggtccggccccctggacaacggcgaccaggtcgtg- gcgctgct gaacggcggctccgtgtcccgccccatgaacacgaccctggaggagatcttcttcgactccaacctgggctcca- agaagctgac ctccacctgggacatctacgacctgtgggcgaaccgcgtcgacaactccacggcgtccgccatcctgggccgca- acaagaccg ccaccggcatcctgtacaacgccaccgagcagtcctacaaggacggcctgtccaagaacgacacccgcctgttc- ggccagaag atcggctccctgtcccccaacgcgatcctgaacacgaccgtccccgcccacggcatcgcgttctaccgcctgcg- cccctcctccT GATACAACTTATtacgtaacggagcgtcgtgcgggagggagtgtgccgagcggggagtcccggtctgtgcgagg- ccc ggcagctgacgctggcgagccgtacgccccgagggtccccctcccctgcaccctcttccccttccctctgacgg- ccgcgcctgttct ##STR00394## ##STR00395## catccactttctcggcgttcaatgcccgctgcggcgacctgcgtcgctcggcgggctccgggccccggcgccca- gcgaggcccct ccccgtgcgcgctgccatcgccagcgaggtccccgtggccaccacctccccccgggcgcaccccaaggcgaacg- gcagcgcg gtgtcgctgaagtcgggctccctggagacccaggaggacaagacgagcagctcgtccccccccccccgcacgtt- catcaacca gctgcccgtgtggagcatgctgctgtcggcggtgaccacggtcttcggcgtggccgagaagcagtggcccatgc- tggaccgcaa gtccaagcgccccgacatgctggtcgagcccctgggcgtggaccgcatcgtctacgacggcgtgagcttccgcc- agtcgttctcc atccgcagctacgagatcggcgccgaccgcaccgcctcgatcgagacgctgatgaacatgttccaggagacctc- cctgaaccac tgcaagatcatcggcctgctgaacgacggcttcggccgcacgcccgagatgtgcaagcgcgacctgatctgggt- cgtgaccaag atgcagatcgaggtgaaccgctaccccacgtggggcgacaccatcgaggtcaacacgtgggtgagcgcctcggg- caagcacg gcatgggccgcgactggctgatctccgactgccacaccggcgagatcctgatccgcgcgacgagcgtctgggcg- atgatgaacc agaagacccgccgcctgtcgaagatcccctacgaggtgcgccaggagatcgagccccagttcgtcgactccgcc- cccgtgatcg tggacgaccgcaagttccacaagctggacctgaagacgggcgacagcatctgcaacggcctgaccccccgctgg- acggacct ggacgtgaaccagcacgtcaacaacgtgaagtacatcggctggatcctgcagtcggtccccaccgaggtgttcg- agacgcagg agctgtgcggcctgaccctggagtaccgccgcgagtgcggccgcgactccgtgctggagagcgtcacggccatg- gacccctcg aaggagggcgaccgctccctgtaccagcacctgctgcgcctggaggacggcgcggacatcgtgaagggccgcac- cgagtggc gccccaagaacgccggcgccaagggcgccatcctgacgggcaagaccagcaacggcaactcgatctccatggac- tacaagg accacgacggcgactacaaggaccacgacatcgactacaaggacgacgacgacaagTGAaagcttgcagcagca- gctcgaagcttgcagcagcagctcg gatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtga- atatccctgccgctttt atcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcg- aataccacccccagcatcc ccttccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgct- cctgctcctgctcactgcc cctcgcacagccttggtttgggctcccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgc- tgatgcacgggaagta gtgggatgggaacacaaatggaanctggagctccagcgccatgccacgccattgatggcttcaagtacgattac- ggtgttgg attgtgtgtttgttgcgtagtgtgcatggtttagaataatacacttgatttcttgctcacggcaatctcggctt- gtccgcaggttcaa ccccatttcggagtctcaggtcagccgcgcaatgaccagccgctacttcaaggacttgcacgacaacgccgagg- tgagctatg tttaggacttgattggaaattgtcgtcgacgcatattcgcgctccgcgacagcacccaagcaaaatgtcaagtg- cgttccgattt gcgtccgcaggtcgatgttgtgatcgtcggcgccggatccgccggtctgtcctgcgcttacgagctgaccaagc- accctgacgt ccgggtacgcgagctgagattcgattagacataaattgaagattaaacccgtagaaaaatttgatggtcgcgaa- actgtgctc gattgcaagaaattgatcgtcctccactccgcaggtcgccatcatcgagcagggcgttgctcccggcggcggcg- cctggctgg ggggacagctgttctcggccatgtgtgtacgtagaaggatgaatttcagctggttttcgttgcacagctgtttg- tgcatgatttgtt tcagactattgttgaatgtttttagatttcttaggatgcatgatttgtctgcatgcgactgaagagc
[0651] Increased C12:0 levels in strain S5818 by the expression of heterologous "C14:0-specific" thioesterases. In an effort to increase C14:0 fatty acid levels in S5818, several thioesterases that had previously displayed pronounced C14:0 thioesterase activity in P. moriformis were transformed into the S5818 background. Contrary to our expectations, we observed marked increases in C12:0 levels with decreases or only marginal increases in C14:0 levels. For example, introduction of the ChsFATB3 thioesterase (which leads to an increase in C14:0 levels of up to 34% in S3150) into S5818 causes C12:0 levels to rise to .about.77% (.DELTA.=+32% C12:0) and C14:0 levels to drop to .about.7% (.DELTA.=-7%). In addition, introduction of CpalFATB2 into S5818 at the DAO1b locus causes C12:0 levels to rise to .about.64% (.DELTA.=+19%) and C14:0 levels to drop to .about.12% (.DELTA.=-2%). The results for the top five transformants for each of the five constructs are displayed in Table 83.
[0652] Of note, S5818 expresses the C. wrightii KASAI gene from the pLOOP locus. As C. wrightii produces seed oil with 62% C12:0, we believe it likely that the CwKASA1 gene has evolved to be specific for production of C12:0 fatty acids when combined with C. wrightii thioesterases. Indeed, C. wrightii FATB2 encodes a thioesterase that exhibits C12:0 activity when introduced into P. moriformis. Thus, it is possible that the "C14:0" thioesterase genes identified in our transcriptome sequencing, namely ChsFATB3 and ChtFATB1a, exhibit C14:0 activity only when in combination with the P. moriformis endogenous KASI gene. These results further extend to CpalFATB2, which has been repeatedly shown to increase C14:0 levels in P. moriformis (data not shown). However, when ChsFATB3, ChtFATB1a, and CpalFATB2 are combined with a KASI gene from a Cuphea species that produces high C12:0 fatty acids, such as CwKASA1 from Cuphea wrightii, then a C12:0 activity of these thioesterases is revealed/exhibited. It should be further noted that C. hyssopifolia and C. heterophylla produce only low levels of C14:0 in oilseeds (5% and 4%, respectively) while producing relatively high levels of C12:0 (84% and 40%, respectively). Since the ChsFATB3 and ChtFATB1a thioesterases were identified from RNAs expressed in mature oil seeds, it is possible that these thioesterases indeed exhibited C12:0 activity in Cuphea seeds, significantly contributing to the high levels of C12:0 found therein.
[0653] Our results indicate that the combination of thioesterase and KAS is likely to be extremely important in determining the specificity of the thioesterase-KAS machinery in generating midchain fatty acids. Furthermore, introduction of heterologous KASs may be an important and fruitful approach for revealing novel specificities of additional heterologous thioesterases.
TABLE-US-00114 TABLE 83 Fatty acid profiles for the top 5 transformants for each of the pSZ3493, pSZ3494, pSZ3495, pSZ3390, and pSZ3531 constructs upon introduction into S5818. Sample ID pSZ#; construct Strain # C10:0 C12:0 C14:0 C16:0 C18:0 C18:1 C18:2 N/A S5818 0.34 45.16 13.77 8.54 0.81 24.63 5.38 pSZ3493; ChsFATB3 Block 6; C6; pH 7; S5818; T678; D2202-30 0.76 76.58 7.49 3.76 0.32 6.58 3.77 Block 6; D6; pH 7; S5818; T678; D2202-42 0.61 62.82 13.92 6.75 0.37 10.76 3.88 Block 6; D11; pH 7; S5818; T678; D2202-47 4.60 55.99 9.26 5.90 0.44 18.32 4.40 Block 6; A9; pH 7; S5818; T678; D2202-9 0.47 53.94 17.59 8.51 0.44 13.70 4.33 Block 6; D3; pH 7; S5818; T678; D2202-39 0.43 53.94 15.62 8.04 0.43 15.99 4.45 pSZ3494; Block 2; B8; pH 7; S5818; T678; D2203-20 0.43 56.76 14.15 7.60 0.56 14.23 5.09 CpSAD1tp_trimmed: Block 2; C1; pH 7; S5818; T678; D2203-25 0.46 54.82 17.08 7.81 0.46 13.67 4.85 ChsFATB3 Block 2; D1; pH 7; S5818; T678; D2203-37 0.43 54.47 11.51 8.14 0.95 18.58 4.99 Block 2; D7; pH 7; S5818; T678; D2203-43 0.43 52.86 18.70 8.91 0.58 13.18 4.45 Block 2; C11; pH 7; S5818; T678; D2203-35 0.44 52.81 19.54 8.87 0.54 12.57 4.29 pSZ3495; Block 2; G10; pH 7; S5818; T678; D2204-34 0.58 55.18 19.86 7.72 0.60 10.88 4.29 CpSAD1tp_trimmed: Block 2; H7; pH 7; S5818; T678; D2204-43 0.68 54.79 20.14 7.78 0.56 10.99 4.18 ChtFATB1a Block 2; H5; pH 7; S5818; T678; D2204-41 0.60 54.69 20.38 7.39 0.55 11.50 4.13 Block 2; G8; pH 7; S5818; T678; D2204-32 0.66 54.26 20.39 7.69 0.55 11.45 4.26 Block 2; F6; pH 7; S5818; T678; D2204-18 0.67 54.23 20.04 7.60 0.56 11.80 4.23 pSZ3390; CpSAD1tpExt- Block 4; A5; pH 7; S5818; T674; D2104-5 0.58 63.83 12.10 5.89 0.55 10.46 5.44 CpalFATB2FLAGExtA Block 4; A12; pH 7; S5818; T674; D2104-12 0.48 61.92 16.15 5.87 0.50 9.86 4.41 (DAO1b) Block 4; B9; pH 7; S5818; T674; D2104-21 0.41 54.31 18.41 7.26 0.51 13.80 4.49 Block 4; B5; pH 7; S5818; T674; D2104-17 0.37 53.56 16.54 7.25 0.58 16.34 4.42 Block 4; B11; pH 7; S5818; T674; D2104-23 0.41 52.44 17.99 7.60 0.54 15.38 4.66 pSZ3531; CpSAD1tpExt- Block 5B; A8; pH 7; S5818; T684; D2235-8 0.52 59.36 15.70 6.93 0.45 11.41 4.63 CpalFATB2FLAGExtA Block 5B; A12; pH 7; S5818; T684; D2235-12 0.44 55.60 16.98 6.98 0.53 14.21 4.59 (THI4A) Block 5B; B11; pH 7; S5818; T684; D2235-23 0.36 49.58 17.43 8.72 0.57 17.44 4.62 Block 5B; A4; pH 7; S5818; T684; D2235-4 0.35 49.43 18.63 8.22 0.62 17.29 4.54 Block 5B; A11; pH 7; S5818; T684; D2235-11 0.36 48.92 15.93 7.84 0.68 20.38 4.96
Example 63
A Suite of Regulatable Promoters to Conditionally Control Gene Expression Levels in Oleaginous Cells in Synchrony with Lipid Production
[0654] S5204 was generated by knocking out both copies of FATA1 in Prototheca moriformis (PmFATA1) while simultaneously overexpressing the endogenous PmKASII gene in a .DELTA.fad2 line, S2532. S2532 itself is a FAD2 (also known as FADc) double knockout strain that was previously generated by insertion of C. tinctorius ACP thioesterase (Accession No: AAA33019.1) into S1331, under the control of CrTUB2 promoter at the FAD2 locus. S5204 and its parent S2532 have a disrupted endogenous PmFAD2-1 gene resulting in no .DELTA.12 specific desaturase activity manifested as 0% C18:2 (linoleic acid) levels in both seed and lipid production stages. Lack of any C18:2 in S5204 (and its parent S2532) results in growth defects which can be partially mitigated by exogenous addition of linoleic acid in the seed stage. For industrial applications of a zero linoleic oil however, exogenous addition of linoleic acid entails additional cost. We have previously shown that complementation of S5204 (and other .DELTA.fad2 strains S2530 and S2532) with pH inducible AMT03p driven PmFAD2-1 restores C18:2 to wild-type levels at pH 7.0 and also results in rescued growth characteristics during seed stage without any linoleic supplementation. Additionally when the seed from pH 7.0 grown complemented lines is subsequently transferred into low-nitrogen lipid production flasks with pH adjusted to 5.0 (to control AMT03p driven FAD2 protein levels), the resulting final oil profile matches the parent S5204 or S2532 profile with zero linoleic levels but with rescued growth and productivity metrics. Thus in essence with AMT03p driven FAD2-1 we have developed a pH regulatable strain that potentially could be used to generate oils with varying linoleic levels depending on the desired application.
[0655] Prototheca moriformis undergoes rapid cell division during the first 24-30 hrs in fermenters before nitrogen runs out in the media and the cells switch to storing lipids. This initial cell division and growth in fermenters is critical for the overall strain productivity and, as reported above, FAD2 protein is crucial for sustaining vigorous growth characteristic of a particular strain. However when first generation, single insertion, genetically clean, PmFAD2-1 complemented strains (S4694 and S4695) were run in 7 L fermenters at pH 5.0 (with seed grown at pH 7.0), they did not perform on par with the original parent base strain (S1331) in terms of productivity. Western data suggested that AMT03p promoter driving PmFAD2-1 (as measured by FAD2 protein levels) is severely down regulated between 0-30 hrs in fermenters irrespective of fermenter pH (5.0 or 7.0). Work on fermentation conditions (batched vs unbatched/limited initial N, pH shift from 7 to 5 at different time points during production phase) suggested that initial batching (and excess amounts) of nitrogen during early lipid production was the likely cause of AMT03p promoter down regulation in fermenters. Indeed, this initial repression in AMT03 can be directly seen in transcript time-course during fermentation. A significant depression of Amt03 expression was observed early in the run, which corresponds directly with NH4 levels in the fermenter.
[0656] When the fermentations were performed with limited N, we were able to partially rescue the AMT03p promoter activity and while per cell productivity of S4694/S4695 was on par with the parent S1331, the overall productivity still lagged behind. These results suggest that a suboptimal or inactive AMT03p promoter and thus limitation of FAD2 protein in early fermentation stages inhibits any complemented strains from attaining their full growth potential and overall productivity. Here we identify new, improved promoter that allow differential gene activity during high-nitrogen growth and low-nitrogen lipid production phases.
[0657] In particular, we observed that: [0658] In trans expression of the fatty acid desaturase-2 gene from Prototheca moriformis (PmFad2-1) under the control of down regulated promoter elements identified using a transcriptome based bioinformatics approach results in functional complementation of PmFAD2-1 with restored growth in .DELTA.fad2, .DELTA.fata1 strain S5204. [0659] Complementation of S5204 manifested in a robust growth phenotype only occurs in seed and early fermentation stages when the new promoter elements are actively driving the expression of PmFAD2-1. [0660] Once the cells enter the active lipid production phase (around the time when N runs out in the fermenter), the newly identified promoters are down regulated resulting in no additional FAD2 protein and the final oil profile of the complemented lines is same as the parent S5204 albeit with better growth characteristics. [0661] These strains should potentially mitigate the problems that were encountered with AMT03p driven FAD2 in earlier complemented strains. [0662] Importantly, we have identified down-regulatable promoters of varying strengths, some of which are relatively strong in the beginning with low-to-moderate levels provided during the remainder of the run. Thus depending on phenotype these promoters can be selected for fine-tuning the desired levels of transgenes.
[0663] Bioinformatics Methods: RNA was prepared from cells taken from 8 time points during a typical fermenter run. RNA was polyA-selected for run on an Illumina HiSeq. Illumina paired-end data (100 bp reads.times.2, .about.600 bp fragment size) was collected and processed for read quality using FastQC [www.bioinformatics.babraham.ac.uk/projects/fastqc/]. Reads were run through a custom read-processing pipeline that de-duplicates, quality-trims, and length-trims reads.
[0664] Transcripts were assembled from Illumina paired-end reads using Oases/velvet [Velvet: algorithms for de novo short read assembly using de Bruijn graphs. D. R. Zerbino and E. Birney. Genome Research 18:821-829] and assessed by N50 and other metrics. The transcripts from all 8 time points were further collapsed using CD-Hit. [Limin Fu, Beifang Niu, Zhengwei Zhu, Sitao Wu and Weizhong Li, CD-HIT: accelerated for clustering the next generation sequencing data. Bioinformatics, (2012), 28 (23): 3150-3152. doi: 10.1093/bioinformatics/bts565; Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences", Weizhong Li & Adam Godzik Bioinformatics, (2006) 22:1658-9].
[0665] These transcripts were used as the base (reference assembly) for expression-level analysis. Reads from the 8 time points were analyzed using RSEM which provides raw read counts as well as a normalized value provided in Transcripts Per Million (TPM). [Li, Bo & Dewey, Colin N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome, BioMed Central: The Open Access Publisher. Retrieved at Oct. 10, 2012, from the website temoa: Open Educational Resources (OER) Portal at www.temoa.info/node/441614] The TPM was used to determine expression levels. Genes previously identified in screens for strong promoters were also used to gauge which levels should be considered as significantly high or low. This data was loaded into a Postgres database and visualized with Spotfire, along with integrated data that includes gene function and other characteristics such as categorization based on expression profile. This enabled rapid and targeted analysis of genes with significant changes in expression.
[0666] The promoters for genes, which we selected, were mapped onto a high-quality reference genome for S376 (our reference Prototheca moriformis strain). Briefly, PacBio long reads (.about.2 kb) were error-corrected by high-quality PacBio CCS reads (.about.600 bp) and assembled using the Allora assembler in SMRTPipe [pacbiodevnet.com]. This reference genome, in conjunction with transcriptome read mapping, was used to annotate the precise gene structures, promoter and UTR locations, and promoter elements within the region of interest, which then guided further sequencing and promoter element selection.
[0667] The criteria used for identifying new promoter elements were: [0668] 1. Reasonable expression (e.g., >500, <100, or <50 transcripts per million [TPM]) of a downstream gene in seed and early lipid production stages (T0-T30 hrs) [0669] 2. Severe down regulation of the gene above (e.g., >5-fold. 10-fold, or 15-fold) when the nitrogen gets depleted in the fermenters. [0670] 3. pH neutrality of the promoter elements (e.g., less than a 2-fold change in TPM on going from pH 5.0 top 7.0 in cultivation conditions), or at least effective operation under pH5 conditions.
[0671] Using the above described criteria we identified several potentially down regulated promoter elements that were eventually used to drive PmFAD2-1 expression in S5204. A range of promoters was chosen that included some that started as being weak promoters and went down to extremely low levels, through those that started quite high and dropped only to moderately low levels. This was done because it was unclear a priori how much expression would be needed for FAD2 early on to support robust growth, and how little FAD2 would be required during the lipid production phase in order to achieve the zero linoleic phenotype.
[0672] The promoter elements that were selected for screening and their allelic forms were named after their downstream gene and are as follows: [0673] 1. Carbamoyl phosphate synthase (PmCPS1p and PmCPS2p) [0674] 2. Dipthine synthase (PmDPS1p and PmDPS2p) [0675] 3. Inorganic pyrophosphatase (PmIPP1p) [0676] 4. Adenosylhomocysteinase (PmAHC1p and PmAHC2p) [0677] 5. Peptidyl-prolyl cis-trans isomerase (PmPPI1p and PmPPI2p) [0678] 6. GMP Synthetase (PmGMPS1p and PmGMPS2p) [0679] 7. Glutamate Synthase (PmGSp) [0680] 8. Citrate Synthase (PmCS1p and PmCS2p) [0681] 9. Gamma Glutamyl Hydrolase (PmGGH1p) [0682] 10. Acetohydroxyacid Isomerase (PmAHI1p and PmAHI2p) [0683] 11. Cysteine Endopeptidase (PmCEP1p) [0684] 12. Fatty acid desaturase 2 (PmFAD2-1p and PmFad2-2p) [CONTROL]
[0685] The transcript profile of two representative genes viz. PmIPP (Inorganic Pyrophosphatase) and PmAHC, (Adenosylhomocysteinase) start off very strong (4000-5000 TPM) but once the cells enter active lipid production their levels fall off very quickly. While the transcript levels of PmIPP drop off to nearly 0 TPM, the levels of PmAHC drop to around 250 TPM and then stay steady for the rest of the fermentation. All the other promoters (based on their downstream gene transcript levels) showed similar downward expression profiles.
[0686] The elements were PCR amplified and wherever possible promoters from allelic genes were identified, cloned and named accordingly e.g. the promoter elements for 2 genes of Carbamoyl phosphate synthase were named PmCPS1p and PmCPS2p. As a comparator promoter elements from PmFAD2-1 and PmFAD2-2 were also amplified and used to drive PmFAD2-1 gene. While, in the present example, we used FAD2-1 expression and hence C18:2 levels to interrogate the newly identified down regulated promoters, in principle these promoter elements can be used to down regulate any gene of interest.
[0687] Construct used for the expression of the Prototheca moriformis fatty acid desaturase 2 (PmFAD2-1) under the expression of PmCPS1p in dfad2 strains S5204--[pSZ3377]: The .DELTA.fad2 .DELTA.fata1 S5204 strain was transformed with the construct pSZ3377. The sequence of the transforming DNA is provided below. Relevant restriction sites in the construct pSZ3377 (6S::PmHXT1p-ScMEL1-CvNR::PmCPS1p-PmFAD2-1-CvNR::6S) are indicated in lowercase, underlined and bold, and are from 5'-3' BspQ 1, KpnI, SpeI, SnaBI, EcoRV, SpeI, AflII, SacI, BspQ I, respectively. BspQI sites delimit the 5' and 3' ends of the transforming DNA. Bold, lowercase sequences represent genomic DNA from UTEX 1435 that permits targeted integration of the transforming DNA at the 6S locus via homologous recombination. Proceeding in the 5' to 3' direction, the Hexose transporter (HXT1) gene promoter from UTEX 1435 driving the expression of the Saccharomyces cerevisiae Melibiase (ScMEL1) gene is indicated by the boxed text. The initiator ATG and terminator TGA for ScMEL1 are indicated by uppercase, bold italics while the coding region is indicated in lowercase italics. The Chlorella vulgaris nitrate reductase 3' UTR is indicated by lowercase underlined text followed by an UTEX 1435 CPS1p promoter of Prototheca moriformis, indicated by boxed italics text. The Initiator ATG and terminator TGA codons of the PmFAD2-1 are indicated by uppercase, bold italics, while the remainder of the gene is indicated by bold italics. The C. vulgaris nitrate reductase 3' UTR is again indicated by lowercase underlined text followed by the UTEX 1435 6S genomic region indicated by bold, lowercase text. The final construct was sequenced to ensure correct reading frames and targeting sequences.
TABLE-US-00115 Nucleotide sequence of transforming DNA contained in plasmid pSZ3377: (SEQ ID NO: 128) gctcttcggagtcactgtgccactgagttcgactggtagctgaatggagtcgctgctccactaaacgaattgtc- agcaccgcca gccggccgaggacccgagtcatagcgagggtagtagcgcgccatggcaccgaccagcctgcttgccagtactgg- cgtctcttc cgcttctctgtggtcctctgcgcgctccagcgcgtgcgcttttccggtggatcatgcggtccgtggcgcaccgc- agcggccgctg cccatgcagcgccgctgcttccgaacagtggcggtcagggccgcacccgcggtagccgtccgtccggaacccgc- ccaagagt tttgggagcagcttgagccctgcaagatggcggaggacaagcgcatcttcctggaggagcaccggtgcgtggag- gtccgggg ctgaccggccgtcgcattcaacgtaatcaatcgcatgatgatcagaggacacgaagtcttggtggcggtggcca- gaaacact gtccattgcaagggcatagggatgcgttccttcacctctcatttctcatttctgaatccctccctgctcactct- ttctcctcctccttc ##STR00396## gcctgcatctccctgaagggcgtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatggg- ctgggacaact ggaacacgttcgcctgcgacgtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctg- aaggacatgg gctacaagtacatcatcctggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgag- cagaagttccc caacggcatgggccacgtcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcg- agtacacgtgc gccggctaccccggctccctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacct- gaagtacga caactgctacaacaagggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccc- tgaacaaga cgggccgccccatcttctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcg- aactcctggcgc atgtccggcgacgtcacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactg- caagtacgc cggcttccactgctccatcatgaacatcctgaacaaggccgcccccatgggccagaacgcgggcgtcggcggct- ggaacgacct ggacaacctggaggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatgg- tgaagtccc ccctgatcatcggcgcgaacgtgaacaacctgaaggcctcctcctactccatctactcccaggcgtccgtcatc- gccatcaaccag gactccaacggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccagggcga- gatccagat gtggtccggccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgccccatga- acacgaccct ggaggagatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacctgtggg- cgaaccgcgtc gacaactccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgccaccgagca- gtcctacaa ggacggcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcgatcctga- acacgaccgt ccccgcccacggcatcgcgttctaccgcctgcgcccctcctccTGAtacgtagcagcagcagtcggatagtatc- gacacactct ggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgccgcttttat- caaacagcctcagtgtg tttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatcc- ccttccctcgtttcatatcgc ttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccc- tcgcacagccttggtttg ggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgcacgggaagtagtggg- atgggaacacaaat ##STR00397## cagcccgtggagaagccccccttcaccatcggcaccctgcgcaaggccatccccgcccactgcttcgagcgctc- cgccctgcgct cctccatgtacctggccttcgacatcgccgtgatgtccctgctgtacgtggcctccacctacatcgaccccgcc- cccgtgcccacctg ggtgaagtacggcgtgatgtggcccctgtactggttcttccagggcgccttcggcaccggcgtgtgggtgtgcg- cccacgagtgcg gccaccaggccttctcctcctcccaggccatcaacgacggcgtgggcctggtgttccactccctgctgctggtg- ccctactactcctg gaagcactcccaccgccgccaccactccaacaccggctgcctggacaaggacgaggtgttcgtgcccccccacc- gcgccgtgg cccacgagggcctggagtgggaggagtggctgcccatccgcatgggcaaggtgctggtgaccctgaccctgggc- tggcccctgt acctgatgttcaacgtggcctcccgcccctacccccgcttcgccaaccacttcgacccctggtcccccatcttc- tccaagcgcgagc gcatcgaggtggtgatctccgacctggccctggtggccgtgctgtccggcctgtccgtgctgggccgcaccatg- ggctgggcctgg ctggtgaagacctacgtggtgccctacctgatcgtgaacatgtggctggtgctgatcaccctgctgcagcacac- ccaccccgccct gccccactacttcgagaaggactgggactggctgcgcggcgccatggccaccgtggaccgctccatgggccccc- ccttcatgga caacatcctgcaccacatctccgacacccacgtgctgcaccacctgttctccaccatcccccactaccacgccg- aggaggcctcc gccgccatccgccccatcctgggcaagtactaccagtccgactcccgctgggtgggccgcgccctgtgggagga- ctggcgcgac tgccgctacgtggtgcccgacgcccccgaggacgactccgccctgtggttccacaagTAGatcgatcttaaggc- agcagcagct cggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgt- gaatatccctgccgctt ttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgcttgtgctatttg- cgaataccacccccagcatc ccatccctcgtttcatatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgct- cctgctcctgctcactgc ccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgc- tgatgcacgggaagta gtgggatgggaacacaaatggaaagcttaattaagagctcttgttttccagaaggagttgctccttgagccttt- cattctcagcctcg ataacctccaaagccgctctaattgtggagggggttcgaatttaaaagcttggaatgttggttcgtgcgtctgg- aacaagccca gacttgttgctcactgggaaaaggaccatcagctccaaaaaacttgccgctcaaaccgcgtacctctgctttcg- cgcaatctgc cctgttgaaatcgccaccacattcatattgtgacgcttgagcagtctgtaattgcctcagaatgtggaatcatc- tgccccctgtgc gagcccatgccaggcatgtcgcgggcgaggacacccgccactcgtacagcagaccattatgctacctcacaata- gttcataac agtgaccatatttctcgaagctccccaacgagcacctccatgctctgagtggccaccccccggccctggtgctt- gcggagggca ggtcaaccggcatggggctaccgaaatccccgaccggatcccaccacccccgcgatgggaagaatctctccccg- ggatgtgg gcccaccaccagcacaacctgctggcccaggcgagcgtcaaaccataccacacaaatatccttggcatcggccc- tgaattcct tctgccgctctgctacccggtgcttctgtccgaagcaggggttgctagggatcgctccgagtccgcaaaccctt- gtcgcgtggcg gggcttgttcgagcttgaagagc
[0688] The recombination between C. vulgaris nitrate reductase 3' UTR's in the construct pSZ3377 results in multiple copies of PmFAD2-1 in transgenic lines which would then manifest most likely as higher C18:2 levels at the end of fermentation. Since the goal was to create a strain with 0% terminal C18:2, we took precautions to avoid this recombination. In another version of the above plasmid ScMEL1 gene was followed by Chlorella protothecoides (UTEX 250) elongation factor 1a (CpEF1a) 3' UTR instead of C. vulgaris 3' UTR. The sequence of C. protothecoides (UTEX 250) elongation factor 1a (CpEF1a) 3' UTR used in construct pSZ3384 and other constructs with this 3' UTR (described below) is shown below. Plasmid pSZ3384 could be written as 6S::PmHXT1p-ScMEL1-CpEF1a::PmCPS1p-PmFAD2-1-CvNR::6S.
[0689] Nucleotide sequence of Chlorella protothecoides (UTEX 250) elongation factor 1a (CpEF1a) 3' UTR in pSZ3384:
TABLE-US-00116 (SEQ ID NO: 129) tacaacttattacgtaacggagcgtcgtgcgggagggagtgtgccgagcg gggagtcccggtctgtgcgaggcccggcagctgacgctggcgagccgtac gccccgagggtccccctcccctgcaccctcttccccttccctctgacggc cgcgcctgttcttgcatgttcagcgacgaggatatc
[0690] The C. protothecoides (UTEX 250) elongation factor 1a 3' UTR sequence is flanked by restriction sites SnaBI on 5' and EcoRV on 3' ends shown in lowercase bold underlined text. Note that the plasmids containing CpEF1a 3' UTR (pSZ3384 and others described below) after ScMEL1 stop codon contains 10 extra nucleotides before the 5' SnaBI site. These nucleotides are not present in the plasmids that contain C. vulgaris nitrate reductase 3' UTR after the S. ScMEL1 stop codon.
[0691] In addition to plasmids pSZ3377 and pSZ3384 expressing either a recombinative CvNR-Promoter-PmFAD2-1-CvNR or non-recombinative CpEF1a-Promoter-PmFAD2-1-CvNR expression unit described above, plasmids using other promoter elements mentioned above were constructed for expression in S5204. These constructs along with their transformation identifiers (D #) can be described as:
TABLE-US-00117 Plasmid ID D # Description pSZ3378 D2090 6SA::pPmHXT1-ScarIMEL1-CvNR:PmCPS2p-PmFad2-1-CvNR::6SB pSZ3385 D2097 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmCPS2p-PmFad2-1-CvNR::6SB pSZ3379 D2091 6SA::pPmHXT1-ScarIMEL1-CvNR:PmDPS1p-PmFad2-1-CvNR::6SB pSZ3386 D2098 6SA::pPmHXT1)-ScarIMEL1-CpEF1a:PmDPS1p-PmFad2-1-CvNR::6SB pSZ3380 D2092 6SA::pPmHXT1-ScarIMEL1-CvNR:PmDPS2p-PmFad2-1-CvNR::6SB pSZ3387 D2099 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmDPS2p-PmFad2-1-CvNR::6SB pSZ3480 D2259 6SA::pPmHXT1-ScarIMEL1-CvNR:PmIPP1p-PmFad2-1-CvNR::6SB pSZ3481 D2260 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmIPP1p-PmFad2-1-CvNR::6SB pSZ3509 D2434 6SA::pPmHXT1-ScarIMEL1-CvNR:PmAHC1p-PmFad2-1-CvNR::6SB pSZ3516 D2266 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmAHC1p-PmFad2-1-CvNR::6SB pSZ3510 D2435 6SA::pPmHXT1-ScarIMEL1-CvNR:PmAHC2p-PmFad2-1-CvNR::6SB pSZ3513 D2263 6SA::pPmHXT1-ScarIMEL1-CvNR:PmPPI1p-PmFad2-1-CvNR::6SB pSZ3689 D2440 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmPPI1p-PmFad2-1-CvNR::6SB pSZ3514 D2264 6SA::pPmHXT1-ScarIMEL1-CvNR:PmPPI2p-PmFad2-1-CvNR::6SB pSZ3518 D2268 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmPPI2p-PmFad2-1-CvNR::6SB pSZ3515 D2265 6SA::pPmHXT1-ScarIMEL1-CvNR:PmGMPS1p-PmFad2-1-CvNR::6SB pSZ3519 D2269 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmGMPS1p-PmFad2-1-CvNR::6SB pSZ3520 D2270 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmGMPS2p-PmFad2-1-CvNR::6SB pSZ3684 D2436 6SA::pPmHXT1-ScarIMEL1-CvNR:PmCS1p-PmFad2-1-CvNR::6SB pSZ3686 D2438 6SA::pPmHXT1-ScarIMEL1-CpEF1A:PmCS1p-PmFad2-1-CvNR::6SB pSZ3685 D2437 6SA::pPmHXT1-ScarIMEL1-CvNR:PmCS2p-PmFad2-1-CvNR::6SB pSZ3688 D2439 6SA::pPmHXT1-ScarIMEL1-CvNR:PmGGHp-PmFad2-1-CvNR::6SB pSZ3511 D2261 6SA::pPmHXT1-ScarIMEL1-CvNR:PmAHI2p-PmFad2-1-CvNR::6SB pSZ3517 D2267 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmAHI1p-PmFad2-1-CvNR::6SB pSZ3512 D2262 6SA::pPmHXT1-ScarIMEL1-CvNR:PmCEP1p-PmFad2-1-CvNR::6SB pSZ3375 D2087 6SA::pPmHXT1-ScarIMEL1-CvNR:PmFAD2-1p-PmFad2-1-CvNR::6SB pSZ3382 D2094 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmFAD2-1p-PmFad2-1-CvNR::6SB pSZ3376 D2088 6SA::pPmHXT1-ScarIMEL1-CvNR:PmFAD2-2p-PmFad2-1-CvNR::6SB pSZ3383 D2095 6SA::pPmHXT1-ScarIMEL1-CpEF1a:PmFAD2-2p-PmFad2-1-CvNR::6SB
[0692] The above constructs are the same as pSZ3377 or pSZ3384 except for the promoter element that drives PmFAD2-1. The sequences of different promoter elements used in the above constructs are shown below.
TABLE-US-00118 Nucleotide sequence of Carbamoyl phosphate synthase allele 2 promoter contained in plasmid pSZ3378 and pSZ3385 (PmCPS2p promoter sequence): (SEQ ID NO: 130) ##STR00398## Nucleotide sequence of Dipthine synthase allele 1 promoter contained in plasmid pSZ3379 and pSZ3386 (PmDPS1p promoter sequence): (SEQ ID NO: 131) ##STR00399## Nucleotide sequence of Dipthine synthase allele 2 promoter contained in plasmid pSZ3380 and pSZ3387 (PmDPS2p promoter sequence): (SEQ ID NO: 132) ##STR00400## Nucleotide sequence of Inorganic pyrophosphatase allele 1 promoter contained in plasmid pSZ3480 and pSZ3481 (PmIPP1p promoter sequence): (SEQ ID NO:133) ##STR00401## ##STR00402## Nucleotide sequence of Adenosylhomocysteinase allele 1 promoter contained in plasmid pSZ3509 and pSZ3516 (PmAHC1p promoter sequence): (SEQ ID NO: 134) ##STR00403## Nucleotide sequence of Adenosylhomocysteinase allele 2 promoter contained in plasmid pSZ3510 (PmAHC2p promoter sequence): (SEQ ID NO: 135) ##STR00404## Nucleotide sequence of Peptidyl-prolyl cis-trans isomerase allele 1 promoter contained in plasmid pSZ3513 and pSZ3689 (PmPPI1p promoter sequence): (SEQ ID NO: 136) ##STR00405## Nucleotide sequence of Peptidyl-prolyl cis-trans isomerase allele 2 promoter contained in plasmid pSZ3514 and pSZ3518 (PmPPI2p promoter sequence): (SEQ ID NO: 137) ##STR00406## Nucleotide sequence of GMP Synthetase allele 1 promoter contained in plasmid pSZ3515 and pSZ3519 (PmGMPS1p promoter sequence): (SEQ ID NO: 138) ##STR00407## Nucleotide sequence of GMP Synthetase allele 2 promoter contained in plasmid pSZ3520 (PmGMPS2p promoter sequence): (SEQ ID NO: 139) ##STR00408## Nucleotide sequence of Citrate synthase allele 1 promoter contained in plasmid pSZ3684 and pSZ3686 (PmCS1p promoter sequence): (SEQ ID NO: 140) ##STR00409## ##STR00410## Nucleotide sequence of Citrate synthase allele 2 promoter contained in plasmid pSZ3685 (PmCS2p promoter sequence): (SEQ ID NO: 141) ##STR00411## ##STR00412## Nucleotide sequence of Gamma Glutamyl Hydrolase allele 1 promoter contained in plasmid pSZ3688 (PmGGH1p promoter sequence): (SEQ ID NO: 142) ##STR00413## Nucleotide sequence of Acetohydroxyacid Isomerase allele 1 promoter contained in plasmid pSZ3517 (PmAHI1p promoter sequence): (SEQ ID NO: 143) ##STR00414## Nucleotide sequence of Acetohydroxyacid Isomerase allele 2 promoter contained in plasmid pSZ3511 (PmAHI2p promoter sequence): (SEQ ID NO: 144) ##STR00415## Nucleotide sequence of Cysteine Endopeptidase allele 1 promoter contained in plasmid pSZ3512 (PmCEP1 promoter sequence): (SEQ ID NO: 145) ##STR00416## Nucleotide sequence of Fatty acid desaturase 2 allele 1 promoter contained in plasmid pSZ3375 and 3382 (PmFAD2-1 promoter sequence): (SEQ ID NO: 146) ##STR00417## Nucleotide sequence of Fatty acid desaturase 2 allele 2 promoter contained in plasmid pSZ3376 and 3383 (PmFAD2-2 promoter sequence): (SEQ ID NO: 147) ##STR00418##
[0693] To determine their impact on growth and fatty acid profiles, the above-described constructs were independently transformed into a .DELTA.fad2 .DELTA.fata1 strain S5204. Primary transformants were clonally purified and grown under standard lipid production conditions at pH5.0 or at pH7.0. The resulting profiles from a set of representative clones arising from transformations are shown in Tables 84-114.
TABLE-US-00119 TABLE 84 Fatty acid profile in some representative complemented (D2087) and parent S5204 lines transformed with pSZ3375 DNA containing PmFAD2-1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH 7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH 5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH 7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH 5; S5204 0.39 5.67 1.36 91.13 0 0 pH 7; S5204; T665; D2087-22 0.38 4.43 1.78 83.93 7.58 0.81 pH 7; S5204; T665; D2087-16 0.41 4.92 1.94 83.21 7.55 0.84 pH 7; S5204; T665; D2087-17 0.40 4.82 1.78 83.51 7.52 0.79 pH 7; S5204; T665; D2087-26 1.30 8.06 2.54 79.03 7.30 0.82 pH 7; S5204; T665; D2087-29 1.13 7.88 2.45 79.48 7.26 0.79
TABLE-US-00120 TABLE 85 Fatty acid profile in some representative complemented (D) and parent S5204 lines transformed with pSZ3382 DNA containing PmFAD2-1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH 7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH 5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH 7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH 5; S5204 0.39 5.67 1.36 91.13 0 0 pH 7; S5204; T672; D2094-5 0.49 5.76 2.95 83.39 5.08 0.84 pH 7; S5204; T672; D2094-25 0.35 5.01 2.41 85.10 5.09 0.64 pH 7; S5204; T672; D2094-13 0.33 5.07 2.30 84.89 5.30 0.69 pH 7; S5204; T672; D2094-11 0.38 4.33 1.78 85.63 5.31 0.85 pH 7; S5204; T672; D2094-8 0.35 5.29 2.32 84.59 5.34 0.66
TABLE-US-00121 TABLE 86 Fatty acid profile in some representative complemented (D2088) and parent S5204 lines transformed with pSZ3376 DNA containing PmFAD2-2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH 7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH 5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH 7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH 5; S5204 0.39 5.67 1.36 91.13 0 0 pH 7; S5204; T665; D2088-16 1.11 8.18 2.92 78.13 6.96 0.87 pH 7; S5204; T665; D2088-20 1.06 7.78 2.95 78.65 6.95 0.84 pH 7; S5204; T665; D2088-29 0.91 7.13 2.87 79.63 6.93 0.78 pH 7; S5204; T665; D2088-6 1.18 8.29 2.98 77.90 6.91 0.88 pH 7; S5204; T665; D2088-18 1.10 7.98 3.09 78.42 6.78 0.81
TABLE-US-00122 TABLE 87 Fatty acid profile in some representative complemented (D) and parent S5204 lines transformed with pSZ3383 DNA containing PmFAD2-2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH7; S5204; T673; 0.30 5.43 2.45 85.10 4.62 0.68 D2095-47 pH7; S5204; T673; 0.38 5.16 2.48 84.46 5.41 0.68 D2095-14 pH7; S5204; T673; 0.43 4.60 2.54 84.82 5.47 0.58 D2095-16 pH7; S5204; T673; 0.34 5.41 2.57 84.21 5.49 0.66 D2095-6 pH7; S5204; T673; 0.42 5.30 2.49 83.97 5.57 0.68 D2095-39
TABLE-US-00123 TABLE 88 Fatty acid profile in representative complemented (D2089) and parent S5204 lines transformed with pSZ3377 DNA containing PmCPS1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T672; 0.35 4.73 2.29 88.94 1.79 0.39 D2089-40 pH7; S5204; T672; 0.51 4.85 2.96 87.55 2.05 0.41 D2089-2 pH7; S5204; T672; 0.56 5.00 3.04 87.24 2.07 0.36 D2089-14 pH7; S5204; T672; 0.38 5.04 2.39 88.02 2.39 0.44 D2089-7 pH7; S5204; T672; 0.38 5.00 2.37 87.93 2.42 0.43 D2089-18
TABLE-US-00124 TABLE 89 Fatty acid profile in some representative complemented (D2096) and parent S5204 lines transformed with pSZ3384 DNA containing PmCPS1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T673; 0.33 4.18 1.10 92.91 0.00 0.00 D2096-6 pH7; S5204; T673; 0.36 4.14 1.33 92.42 0.34 0.12 D2096-12 pH7; S5204; T673; 0.32 4.35 1.64 92.12 0.35 0.14 D2096-14 pH7; S5204; T673; 0.50 6.44 0.95 89.81 0.46 0.32 D2096-8 pH7; S5204; T673; 0.29 3.93 1.79 91.19 1.34 0.37 D2096-1
TABLE-US-00125 TABLE 90 Fatty acid profile in some representative complemented (D2090) and parent S5204 lines transformed with pSZ3378 DNA containing PmCPS2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T672; 0.33 4.73 1.84 91.24 0.00 0.00 D2090-5 pH7; S5204; T672; 0.42 4.99 2.01 91.06 0.00 0.00 D2090-29 pH7; S5204; T672; 0.43 4.31 1.87 90.44 0.78 0.16 D2090-22 pH7; S5204; T672; 0.32 3.77 2.43 89.72 1.68 0.35 D2090-1 pH7; S5204; T672; 0.49 5.01 1.97 88.48 1.84 0.38 D2090-32
TABLE-US-00126 TABLE 91 Fatty acid profile in some representative complemented (D2097) and parent S5204 lines transformed with pSZ3385 DNA containing PmCPS2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH5; S5204; T680; 0.50 5.73 1.97 87.12 2.61 0.76 D2097-1 pH5; S5204; T680; 0.75 8.20 2.46 85.73 0.89 0.53 D2097-2
TABLE-US-00127 TABLE 92 Fatty acid profile in some representative complemented (D2091) and parent S5204 lines transformed with pSZ3379 DNA containing PmDPS1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T672; 1.42 4.39 2.32 89.87 0.00 0.00 D2091-4 pH7; S5204; T672; 0.27 4.79 2.24 90.94 0.00 0.00 D2091-14 pH7; S5204; T672; 0.30 5.26 2.20 90.73 0.00 0.00 D2091-15 pH7; S5204; T672; 0.31 4.51 1.77 91.65 0.00 0.00 D2091-19 pH7; S5204; T672; 0.31 5.36 2.24 90.67 0.00 0.00 D2091-46
TABLE-US-00128 TABLE 93 Fatty acid profile in some representative complemented (D2098) and parent S5204 lines transformed with pSZ3386 DNA containing PmDPS1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T680; 0.34 4.89 1.56 92.08 0.00 0.00 D2098-39 pH7; S5204; T680; 0.30 4.31 1.61 92.34 0.30 0.00 D2098-7 pH7; S5204; T680; 0.33 3.89 1.58 92.65 0.36 0.00 D2098-3 pH7; S5204; T680; 0.32 4.18 1.64 92.34 0.36 0.11 D2098-25 pH7; S5204; T680; 0.32 4.36 1.50 92.10 0.37 0.12 D2098-13
TABLE-US-00129 TABLE 94 Fatty acid profile in some representative complemented (D2092) and parent S5204 lines transformed with pSZ3380 DNA containing PmDPS2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T672; 0.29 5.13 1.59 92.16 0.00 0.00 D2092-35 pH7; S5204; T672; 0.37 4.66 1.75 91.71 0.19 0.05 D2092-29 pH7; S5204; T672; 0.24 3.47 1.84 93.19 0.43 0.11 D2092-15 pH7; S5204; T672; 0.25 3.50 1.82 93.16 0.44 0.09 D2092-21 pH7; S5204; T672; 0.28 3.18 1.50 93.59 0.52 0.12 D2092-16
TABLE-US-00130 TABLE 95 Fatty acid profile in some representative complemented (D2099) and parent S5204 lines transformed with pSZ3387 DNA containing PmDPS2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH7; S5204; T680; 0.31 4.02 1.46 93.07 0.00 0.00 D2099-20 pH7; S5204; T680; 0.28 4.67 1.50 92.38 0.00 0.00 D2099-24 pH7; S5204; T680; 0.40 4.07 1.22 93.26 0.00 0.00 D2099-27 pH7; S5204; T680; 0.32 4.59 1.57 92.40 0.00 0.00 D2099-30 pH7; S5204; T680; 0.30 4.56 1.54 92.49 0.00 0.00 D2099-35
TABLE-US-00131 TABLE 96 Fatty acid profile in some representative complemented (D2259) and parent S5204 lines transformed with pSZ3480 DNA containing PmIPP1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH5; S5204; T711; 0.36 5.27 2.19 89.32 1.51 0.51 D2259-43 pH5; S5204; T711; 0.35 4.88 2.17 86.34 4.41 0.70 D2259-22 pH5; S5204; T711; 0.35 4.82 2.18 86.32 4.45 0.69 D2259-28 pH5; S5204; T711; 0.33 4.90 2.08 86.33 4.49 0.74 D2259-21 pH5; S5204; T711; 0.50 5.97 2.14 84.67 4.49 0.74 D2259-36
TABLE-US-00132 TABLE 97 Fatty acid profile in some representative complemented (D2260) and parent S5204 lines transformed with pSZ3481 DNA containing PmIPP1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.10 0.00 pH5; S5204 0.39 5.67 1.36 91.13 0.00 0.00 pH5; S5204; T711; 0.36 4.96 2.10 89.46 1.55 0.49 D2260-32 pH5; S5204; T711; 0.33 4.83 1.99 89.40 1.63 0.58 D2260-10 pH5; S5204; T711; 0.34 4.83 2.16 89.39 1.64 0.49 D2260-2 pH5; S5204; T711; 0.37 4.81 2.11 89.51 1.69 0.26 D2260-30 pH5; S5204; T711; 0.33 4.91 2.17 89.73 1.72 0.16 D2260-41
TABLE-US-00133 TABLE 98 Fatty acid profile in some representative complemented (D2434) and parent S5204 lines transformed with pSZ3509 DNA containing PmAHC1p driving PmFAD2-1. Sample ID C14.0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.33 4.45 1.55 81.55 8.51 1.38 T768; D2434-32 pH5; S5204; 0.62 7.27 1.58 78.65 9.44 1.49 T768; D2434-27 pH5; S5204; 0.38 5.81 1.79 79.63 10.01 1.18 T768; D2434-4 pH5; S5204; 0.5 5.93 1.5 78.7 10.25 1.56 T768; D2434-23 pH5; S5204; 0.51 6.08 1.6 78.79 10.25 1.36 T768; D2434-43
TABLE-US-00134 TABLE 99 Fatty acid profile in some representative complemented (D2266) and parent S5204 lines transformed with pSZ3516 DNA containing PmAHC1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; T718; 0.32 5.41 1.94 91.26 0.11 0.00 D2266-46 pH5; S5204; T718; 0.36 5.33 1.90 91.17 0.17 0.00 D2266-36 pH5; S5204; T718; 0.37 4.96 2.13 90.82 0.41 0.00 D2266-35 pH5; S5204; T718; 0.38 5.33 2.10 90.31 0.44 0.31 D2266-41 pH5; S5204; T718; 0.36 5.15 2.23 90.55 0.48 0.31 D2266-5
TABLE-US-00135 TABLE 100 Fatty acid profile in some representative complemented (D2435) and parent S5204 lines transformed with pSZ3510 DNA containing PmAHC2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; T768; 0.35 6.09 1.90 78.52 11.01 1.18 D2435-37 pH5; S5204; T768; 0.43 5.90 1.97 78.74 10.97 1.20 D2435-3 pH5; S5204; T768; 0.40 6.01 1.89 79.00 10.97 1.14 D2435-20 pH5; S5204; T768; 0.39 6.11 1.89 78.26 10.84 1.24 D2435-13 pH5; S5204; T768; 0.46 6.02 1.97 79.48 10.46 1.19 D2435-34
TABLE-US-00136 TABLE 101 Fatty acid profile in some representative complemented (D2263) and parent S5204 lines transformed with pSZ3513 DNA containing PmPPI1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; T718; 0.75 9.44 1.98 87.09 0.00 0.00 D2263-13 pH5; S5204; T718; 0.58 7.72 1.64 89.26 0.00 0.00 D2263-14 pH5; S5204; T718; 0.62 7.92 1.56 89.25 0.00 0.00 D2263-19 pH5; S5204; T718; 0.42 7.39 1.70 89.28 0.00 0.00 D2263-26 pH5; S5204; T718; 0.58 7.32 1.30 90.07 0.00 0.00 D2263-29
TABLE-US-00137 TABLE 102 Fatty acid profile in some representative complemented (D2440) and parent S5204 lines transformed with pSZ3689 DNA containing PmPPI1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.31 6.24 1.41 90.42 0.17 0.05 T770; D2440-23 pH5; S5204; 0.23 4.69 1.41 91.72 0.17 0.00 T770; D2440-32 pH5; S5204; 0.30 6.31 1.49 90.21 0.17 0.00 T770; D2440-38 pH5; S5204; 0.30 6.33 1.38 90.29 0.18 0.05 T770; D2440-7 pH5; S5204; 0.29 6.38 1.36 90.39 0.18 0.05 T770; D2440-36 pH5; S5204; 0.34 5.63 1.15 91.15 0.19 0.05 T770; D2440-8
TABLE-US-00138 TABLE 103 Fatty acid profile in some representative complemented (D2264) and parent S5204 lines transformed with pSZ3514 DNA containing PmPPI2p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH7; S6207; 0.49 6.15 1.61 90.82 0.00 0.00 T718; D2264-1 pH7; S6207; 0.38 5.36 1.51 91.58 0.00 0.00 T718; D2264-6 pH7; S6207; 0.45 6.09 1.46 91.10 0.00 0.00 T718; D2264-29 pH7; S6207; 0.40 5.42 2.28 89.86 0.90 0.00 T718; D2264-4 pH7; S6207; 0.40 5.37 2.02 90.18 1.04 0.00 T718; D2264-7
TABLE-US-00139 TABLE 104 Fatty acid profile in some representative complemented (D2268) and parent S5204 lines transformed with pSZ3518 DNA containing PmPPI2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.39 6.43 1.78 90.49 0.00 0.00 T720; D2268-1 pH5; S5204; 0.38 6.49 1.74 90.38 0.00 0.00 T720; D2268-2 pH5; S5204; 0.38 6.56 1.74 90.27 0.00 0.00 T720; D2268-3 pH5; S5204; 0.45 5.73 1.52 91.75 0.00 0.00 T720; D2268-4 pH5; S5204; 0.38 6.58 1.81 90.79 0.00 0.00 T720; D2268-5
TABLE-US-00140 TABLE 105 Fatty acid profile in some representative complemented (D2265) and parent S5204 lines transformed with pSZ3515 DNA containing PmGMPS1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.46 7.02 1.71 90.06 0.00 0.00 T718; D2265-16 pH5; S5204; 0.00 7.90 1.90 89.27 0.00 0.00 T718; D2265-43 pH5; S5204; 0.46 5.53 1.68 91.28 0.35 0.00 T718; D2265-14 pH5; S5204; 0.39 6.17 1.75 90.44 0.42 0.00 T718; D2265-4 pH5; S5204; 0.49 5.87 1.77 90.51 0.45 0.00 T718; D2265-9
TABLE-US-00141 TABLE 106 Fatty acid profile in some representative complemented (D2269) and parent S5204 lines transformed with pSZ3519 DNA containing PmGMPS1p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.38 6.73 1.68 90.24 0.00 0.00 T720; D2269-1 pH5; S5204; 0.36 6.76 1.71 90.17 0.00 0.00 T720; D2269-3 pH5; S5204; 0.42 6.57 1.71 90.32 0.00 0.00 T720; D2269-4 pH5; S5204; 0.59 8.81 1.93 87.97 0.00 0.00 T720; D2269-5 pH5; S5204; 0.50 7.29 1.73 89.29 0.00 0.00 T720; D2269-6
TABLE-US-00142 TABLE 107 Fatty acid profile in some representative complemented (D2270) and parent S5204 lines transformed with pSZ3520 DNA containing PmGMPS2p driving PmFAD2-1. Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.37 6.80 1.74 90.18 0.00 0.00 T720; D2270-1 pH5; S5204; 0.46 6.76 1.83 89.90 0.00 0.00 T720; D2270-2 pH5; S5204; 0.41 6.69 1.70 90.22 0.00 0.00 T720; D2270-3 pH5; S5204; 0.43 7.44 1.72 89.31 0.00 0.00 T720; D2270-4 pH5; S5204; 0.44 6.98 1.78 89.79 0.00 0.00 T720; D2270-5
TABLE-US-00143 TABLE 108 Fatty acid profile in some representative complemented (D2436) and parent S5204 lines transformed with pSZ3684 DNA containing PmCS1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 7.59 1.57 88.88 0.18 0.00 0.00 T768; D2436-48 pH5; S5204; 6.37 1.50 85.00 3.97 1.04 0.00 T768; D2436-1 pH5; S5204; 9.40 1.86 81.13 4.11 1.21 0.00 T768; D2436-16 pH5; S5204; 6.07 1.77 84.78 4.26 0.94 0.00 T768; D2436-8 pH5; S5204; 5.97 1.62 85.28 4.50 0.98 0.00 T768; D2436-32
TABLE-US-00144 TABLE 109 Fatty acid profile in some representative complemented (D2438) and parent S5204 lines transformed with pSZ3686 DNA containing PmCS1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.50 5.96 1.69 89.87 1.30 0.00 T770; D2438-7 pH5; S5204; 0.41 6.05 1.86 87.88 2.46 0.00 T770; D2438-11 pH5; S5204; 0.41 5.75 1.93 88.35 2.50 0.00 T770; D2438-9 pH5; S5204; 0.45 6.18 1.85 87.86 2.59 0.00 T770; D2438-15 pH5; S5204; 0.40 5.92 1.97 87.80 2.59 0.00 T770; D2438-37
TABLE-US-00145 TABLE 110 Fatty acid profile in some representative complemented (D2437) and parent S5204 lines transformed with pSZ3685 DNA containing PmCSCp driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.00 4.83 1.98 90.43 1.17 0.53 T768; D2437-15 pH5; S5204; 0.45 6.03 1.81 88.69 1.88 0.31 T768; D2437-35 pH5; S5204; 0.39 4.96 2.00 88.58 3.24 0.00 T768; D2437-17 pH5; S5204; 0.90 9.55 2.07 82.29 3.37 1.24 T768; D2437-26 pH5; S5204; 0.53 10.76 1.55 79.62 4.46 1.12 T768; D2437-8
TABLE-US-00146 TABLE 111 Fatty acid profile in some representative complemented (D2439) and parent S5204 lines transformed with pSZ3688 DNA containing PmGGHp driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.31 6.79 1.47 89.97 0.00 0.00 T770; D2439-11 pH5; S5204; 0.27 4.19 0.94 92.91 0.08 0.00 T770; D2439-22 pH5; S5204; 0.39 6.02 1.26 90.91 0.16 0.00 T770; D2439-12 pH5; S5204; 0.64 6.50 1.10 89.53 0.20 0.00 T770; D2439-34 pH5; S5204; 0.33 5.25 1.45 89.98 1.08 0.51 T770; D2439-32
TABLE-US-00147 TABLE 112 Fatty acid profile in some representative complemented (D2261) and parent S5204 lines transformed with pSZ3511 DNA containing PmAHI2p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.45 5.06 2.02 89.35 1.73 0.63 T711; D2261-35 pH5; S5204; 0.46 5.12 2.19 88.92 2.16 0.19 T711; D2261-8 pH5; S5204; 0.37 5.12 2.15 88.62 2.30 0.45 T711; D2261-43 pH5; S5204; 0.42 5.27 2.14 88.23 2.39 0.30 T711; D2261-2 pH5; S5204; 0.41 5.14 2.23 88.44 2.39 0.45 T711; D2261-24
TABLE-US-00148 TABLE 113 Fatty acid profile in some representative complemented (D2267) and parent S5204 lines transformed with pSZ3517 DNA containing PmAHI1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.34 4.87 2.11 90.00 1.20 0.39 T720; D2267-3 pH5; S5204; 0.37 5.00 2.14 89.50 1.46 0.49 T720; D2267-20 pH5; S5204; 0.34 4.90 2.08 89.75 1.67 0.36 T720; D2267-36 pH5; S5204; 0.37 4.95 2.14 89.77 1.69 0.00 T720; D2267-15 pH5; S5204; 0.35 4.85 2.12 89.71 1.72 0.32 T720; D2267-2
TABLE-US-00149 TABLE 114 Fatty acid profile in some representative complemented (D2262) and parent S5204 lines transformed with pSZ3512 DNA containing PmCEP1p driving PmFAD2-1. C18:3 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 .alpha. pH7; S3150 1.71 29.58 3.13 56.53 6.43 0.68 pH5; S3150 1.56 27.70 2.98 59.49 5.95 0.53 pH7; S5204 0.30 5.59 1.63 90.88 0.1 0 pH5; S5204 0.39 5.67 1.36 91.13 0 0 pH5; S5204; 0.48 5.50 2.08 90.58 0.35 0.00 T711; D2262-3 pH5; S5204; 0.39 5.20 2.17 89.90 1.08 0.37 T711; D2262-33 pH5; S5204; 0.34 5.08 1.93 89.69 1.34 0.37 T711; D2262-24 pH5; S5204; 0.40 4.89 2.19 89.88 1.45 0.27 T711; D2262-32 pH5; S5204; 0.39 4.95 2.75 89.30 1.47 0.27 T711; D2262-34
[0694] Combined baseline expression of endogenous PmFAD2-1 and PmFAD2-2 in wild type Prototheca strains (like S3150, S1920 or S1331) manifests as 5-7% C18:2. S5204 overexpresses PmKASII which results in the elongation of C16:0 to C18:0. This increased pool of C18:0 is eventually desaturated by PmSAD2 resulting in elevated C18:1 levels. Additionally disruption of the both copies of PmFAD2 (viz. PmFAD2-1 and PmFAD2-2) in S5204 prevents further desaturation of C18:1 into C18:2 and results in a unique high oleic oil (C18:1) with 0% linoleic acid (C18:2). However as mentioned above any strain with 0% C18:2 grows very poorly and requires exogenous addition of linoleic acid to sustain growth/productivity. Complementation of a strain like S5204 with inducible PmAMT03p driven PmFAD2-1 can rescue the growth phenotype while preserving the terminal high C18:1 with 0% C18:2 levels. However data suggests that PmAMT03 shuts off in the early stages of fermentation thus severely compromising the ability of any complemented strain to achieve its full growth and productivity potential. The goal of this work was to identify promoter elements that would allow the complemented strains to grow efficiently in early stages of fermentation (TO-T30 hrs; irrespective of excess batched N in the fermenters) and then effectively shut off once the cells enter active lipid production (when N in the media gets depleted) so that the complemented strains would still finish with very high C18:1 and 0% C18:2 levels. As a comparator we also complemented S5204 with PmFAD2-1 being driven by either PmFAd2-1p or PmFAD2-2p promoter elements.
[0695] Complementation of S5204 with PmFAD2-1 driven by either PmFAD2-1p or PmFAD2-2p promoter elements results in complete restoration of the C18:2 levels using vectors either designed to amplify PmFAD2-1 copy number (e.g. pSZ3375 or pSZ3376) or the ones where PmFAD2-1 copy number is restricted to one (pSZ3382 or pSZ3383). Copy number of the PmFAD2-1 in these strains seems to have very marginal effect on the terminal C18:2 levels.
[0696] On the other hand expression of PmFAD2-1 driven by any of new promoter elements results in marked decrease in terminal C18:2 levels. The representative profiles from various strains expressing new promoters driving FAD2-1 are shown in Tables 84-114. This reduction in C18:2 levels is even more pronounced in strains where the copy number of PmFAD2-1 is limited to one. Promoter elements like PmDPS1 (D2091 & D2098), PmDPS2 (D2092 & D2099), PmPPI 1 (D2263 & D2440), PmPPI2 (D2264 & D2268), PmGMPS1 (D2265 & D2269), PmGMPS2 (D2270) resulted in strains with 0% or less than 0.5% terminal C18:2 levels in both single or multiple copy PmFAD2-1 versions. The rest of the promoters resulted in terminal C18:2 levels that ranged between 1-5%. One unexpected result was the data from PmAHC1p and PmAHC2p driving PmFAD2-1 in D2434 and D2435. Both these promoters resulted in very high levels of C18:2 (9-20%) in multiple copy FAD2-1 versions. The levels of terminal C18:2 in single copy version in D2266 was more in line with the transcriptomic data suggesting that PmAHC promoter activity and the corresponding PmAHC transcription is severely downregulated when cells are actively producing lipid in depleted nitrogen environment. A quick look at the transcriptome revealed that the initial transcription of PmAHC is very high (4000-5500 TPM) which then suddenly drops down to 250 TPM. Thus it is conceivable that in strains with multiple copies on PmFAD2-1 (D2434 and D2435), the massive amount of PmFAD2-1 protein produced earlier in the fermentation lingers and results in high C18:2 levels. In single copy PmFAD2-1 strains this is not the case and thus we do not see elevated C18:2 levels in D2266.
[0697] In complemented strains with 0% terminal C18:2 levels, the key question was whether they were complemented in the first place. In order to ascertain that, representative strains along with parent S5204 and previously AMT03p driven PmFAD2-1 complemented S2532 (viz S4695) strains were grown in seed medium in 96 well blocks. The cultures were seeded at 0.1 OD units per ml and the OD750 was checked at different time points. Compared to S5204, which grew very poorly, only S4695 and newly complemented strains grew to any meaningful OD's at 20 and 44 hrs (Table 115) demonstrating that the promoters identified above are active early on and switch off once cells enter the active lipid production phase.
TABLE-US-00150 TABLE 115 Growth characteristics of .DELTA.fad2 .DELTA.fata1 strain S5204, S4695 and representative complemented S5204 lines in seed medium sorted by OD750 at 44 hrs. Note that in 1 ml 96 well blocks after initial rapid division and growth, cells stop growing efficiently because of lack of nutrients, aeration etc. OD750 OD750 OD750 Sample ID C14:0 C16:0 C18:0 C18:1 C18:2 C18:3.alpha. @20 hrs @44 hrs @68 hrs S5204 0.162 7.914 10.93 S5204 0.224 6.854 9.256 S4695 1.456 29.032 32.766 pH7; S5204; T672; D2091-46 0.31 5.36 2.24 90.67 0.00 0.00 1.38 33.644 33.226 pH5; S5204; T720; D2268-1 0.39 6.43 1.78 90.49 0.00 0.00 0.75 32.782 31.624 S5204; 1720; D2270-47 0.39 6.69 1.81 90.05 0.00 0.00 1.204 32.752 31.602 pH5; S5204; T720; D2270-39 0.39 6.87 1.81 89.94 0.00 0.00 1.012 32.552 33.138 pH7; S5204; T680; D2099-35 0.30 4.56 1.54 92.49 0.00 0.00 0.48 32.088 31.92 pH5; S5204; T720; D2270-44 0.51 6.85 1.74 90.06 0.00 0.00 1.468 31.802 30.61 pH5; S5204; T720; D2270-41 0.00 7.85 1.65 89.18 0.00 0.00 1.576 31.35 30.69 pH5; S5204; T720; D2270-17 0.46 6.78 1.71 90.24 0.00 0.00 1.79 30.732 24.768 pH7; S5204; T680; D2099-30 0.32 4.59 1.57 92.40 0.00 0.00 0.59 30.166 34.64 pH5; S5204; T720; D2268-40 0.42 6.66 1.86 90.02 0.00 0.00 0.764 29.62 29 pH5; S5204; T720; D2270-23 0.39 6.52 1.72 90.35 0.00 0.00 1.334 29.604 27.518 pH5; S5204; T720; D2270-42 0.61 6.59 1.53 90.28 0.00 0.00 2.042 28.986 32.184 pH7; S5204; T672; D2090-5 0.33 4.73 1.84 91.24 0.00 0.00 1.326 28.976 35.508 pH7; S5204; T672; D2091-15 0.30 5.26 2.20 90.73 0.00 0.00 0.826 28.824 32.848 pH7; S5204; T680; D2099-20 0.31 4.02 1.46 93.07 0.00 0.00 1.31 28.732 26.61 pH5; S5204; T720; D2269-19 0.42 6.51 1.61 90.43 0.00 0.00 1.278 28.65 31.362 pH5; S5204; T720; D2269-29 0.43 7.36 1.72 89.35 0.00 0.00 1.342 28.376 28.66 pH5; S5204; T720; D2270-19 0.39 6.81 1.75 90.05 0.00 0.00 2.142 28.376 25.934 pH5; S5204; T720; D2270-43 0.80 7.64 1.66 88.93 0.00 0.00 1.896 28.174 32.376 pH5; S5204; T720; D2270-46 0.45 6.75 1.72 90.02 0.00 0.00 1.644 28.122 30.464 pH5; S5204; T720; D2268-3 0.38 6.56 1.74 90.27 0.00 0.00 0.926 28.114 31.552 pH5; S5204; T720; D2268-12 0.00 5.68 1.84 91.53 0.00 0.00 1.414 28.106 30.644 pH5; S5204; T720; D2269-37 0.54 7.12 1.75 89.80 0.00 0.00 1.268 28.078 30.014 pH5; S5204; T720; D2270-31 0.46 6.94 1.74 89.71 0.00 0.00 1.224 28.064 29.344 pH5; S5204; T720; D2270-48 0.00 7.21 1.87 90.16 0.00 0.00 1.352 28 28.21 pH5; S5204; T720; D2269-8 0.33 6.67 1.64 90.34 0.00 0.00 0.96 27.912 27.564 pH5; S5204; T720; D2268-32 0.44 6.59 1.85 90.11 0.00 0.00 0.78 27.834 31.952 pH5; S5204; T720; D2269-47 0.42 6.83 1.82 89.85 0.00 0.00 1.17 27.76 29.648 pH7; S5204; T672; D2091-19 0.31 4.51 1.77 91.65 0.00 0.00 1.568 27.682 25.828 pH5; S5204; T720; D2270-38 0.39 6.65 1.83 90.11 0.00 0.00 1.74 27.606 31.104 pH5; S5204; T720; D2268-2 0.38 6.49 1.74 90.38 0.00 0.00 0.95 27.564 32.254 pH5; S5204; T720; D2269-35 0.38 7.04 1.68 89.82 0.00 0.00 1.19 27.482 29.186 pH5; S5204; T720; D2269-20 0.36 7.01 1.73 89.86 0.00 0.00 0.966 27.47 28.284 pH5; S5204; T720; D2269-13 0.39 6.76 1.89 89.98 0.00 0.00 0.936 27.39 33.464 pH7; S5204; T680; D2099-24 0.28 4.67 1.50 92.38 0.00 0.00 0.8 27.28 27.35 pH5; S5204; T720; D2268-11 0.38 6.56 1.85 90.56 0.00 0.00 1.136 27.254 32.508 pH5; S5204; T720; D2270-3 0.41 6.69 1.70 90.22 0.00 0.00 0.872 27.214 30.23 pH5; S5204; T720; D2269-33 0.39 6.36 1.67 90.59 0.00 0.00 0.956 27.194 30.568 pH5; S5204; T720; D2268-10 0.45 6.93 1.70 90.16 0.00 0.00 0.612 27.126 31.616 pH5; S5204; T720; D2269-43 0.36 6.55 1.84 90.25 0.00 0.00 0.998 27.086 29.618 pH5; S5204; T720; D2270-1 0.37 6.80 1.74 90.18 0.00 0.00 2.428 27.004 31.044 pH5; S5204; T720; D2268-4 0.45 5.73 1.52 91.75 0.00 0.00 0.736 26.948 28.796 pH5; S5204; T720; D2270-9 0.38 6.88 1.74 90.22 0.00 0.00 2.68 26.944 29.92 pH5; S5204; T720; D2269-26 0.41 6.85 1.68 90.03 0.00 0.00 0.896 26.794 31.31 pH5; S5204; T720; D2270-24 0.39 6.51 1.78 90.33 0.00 0.00 1.51 26.682 27.486 pH5; S5204; T720; D2269-18 0.41 7.04 1.71 89.83 0.00 0.00 1.024 26.58 29.794 pH5; S5204; T720; D2269-32 0.38 6.81 1.72 90.06 0.00 0.00 1.214 26.48 29.478 pH5; S5204; T720; D2268-31 0.33 6.68 1.76 90.20 0.00 0.00 0.808 26.432 31.294 pH5; S5204; T720; D2269-7 0.29 5.33 1.69 91.59 0.00 0.00 1.1 26.41 28.754 pH5; S5204; T720; D2268-6 0.39 6.62 1.70 90.28 0.00 0.00 0.626 26.372 30.822 pH7; S5204; T680; D2099-27 0.40 4.07 1.22 93.26 0.00 0.00 0.936 26.116 29.75 pH5; S5204; T720; D2269-39 0.48 6.88 1.82 89.67 0.00 0.00 2.218 26.106 30.8 pH5; S5204; T720; D2269-12 0.35 6.39 1.80 90.47 0.00 0.00 1.18 26.032 28.19 pH5; S5204; T720; D2269-42 0.39 6.99 1.67 89.91 0.00 0.00 2.132 25.924 27.854 pH5; S5204; T720; D2268-8 0.56 6.77 1.49 90.20 0.00 0.00 0.96 25.702 29.788 pH5; S5204; T720; D2270-37 0.44 7.33 1.71 89.69 0.00 0.00 0.916 25.612 34.034 pH5; S5204; T720; D2270-40 0.00 9.30 1.62 88.12 0.00 0.00 2.072 25.552 29.474 pH5; S5204; T720; D2270-14 0.43 7.40 1.71 89.73 0.00 0.00 1.916 25.526 27.908 pH5; S5204; T720; D2269-21 0.40 6.69 1.69 89.99 0.00 0.00 0.826 25.396 29 pH5; S5204; T718; D2265-16 0.46 7.02 1.71 90.06 0.00 0.00 0.9 25.332 32.018 pH5; S5204; T720; D2270-15 0.40 6.90 1.68 90.32 0.00 0.00 1.594 25.32 26.794 pH5; S5204; T720; D2269-40 0.00 7.00 1.66 90.15 0.00 0.00 1.804 25.286 29.468 pH5; S5204; T720; D2268-5 0.38 6.58 1.81 90.79 0.00 0.00 0.678 25.156 33.066 pH5; S5204; T720; D2270-18 0.45 6.20 1.45 91.09 0.00 0.00 2.646 25.126 27.536 pH5; S5204; T720; D2269-25 0.44 7.02 1.69 89.91 0.00 0.00 0.868 25.018 32.104 pH5; S5204; T720; D2269-30 0.45 6.77 1.78 90.00 0.00 0.00 0.718 24.978 29.868 pH5; S5204; T720; D2270-25 0.31 6.82 1.68 90.09 0.00 0.00 2.32 24.814 36.024 pH5; S5204; T720; D2270-21 0.52 7.23 1.70 89.99 0.00 0.00 1.92 24.58 25.398 pH5; S5204; T720; D2269-38 0.00 7.45 1.50 90.19 0.00 0.00 1.494 24.578 30.178 pH5; S5204; T720; D2268-9 0.48 5.94 1.51 90.83 0.00 0.00 0.73 24.344 30.83 pH5; S5204; T720; D2268-37 0.44 6.35 1.84 90.31 0.00 0.00 0.548 24.306 32.848 pH5; S5204; T720; D2269-28 0.41 7.12 1.66 89.73 0.00 0.00 0.808 24.288 31.27 pH5; S5204; T720; D2270-5 0.44 6.98 1.78 89.79 0.00 0.00 2.328 24.14 30.186 pH5; S5204; T720; D2269-23 0.44 6.99 1.71 89.43 0.00 0.00 0.876 24.076 29.494 pH5; S5204; T720; D2269-9 0.38 6.84 1.71 90.32 0.00 0.00 0.806 24 26.844 pH5; S5204; T720; D2269-24 0.55 7.31 1.71 89.68 0.00 0.00 1.09 23.97 29.642 pH5; S5204; T720; D2270-35 0.36 6.58 1.72 90.38 0.00 0.00 1.554 23.71 28.868 pH5; S5204; T720; D2269-15 0.00 5.69 1.36 91.86 0.00 0.00 1.246 23.584 28.196 pH5; S5204; T720; D2270-28 0.39 7.15 1.82 89.92 0.00 0.00 1.648 23.486 30.858 pH7; S5204; T680; D2098-39 0.34 4.89 1.56 92.08 0.00 0.00 1.08 23.46 31.888 pH5; S5204; T720; D2269-27 0.33 6.87 1.68 89.98 0.00 0.00 1.3 23.262 33.112 pH5; S5204; T718; D2265-43 0.00 7.90 1.90 89.27 0.00 0.00 0.832 23.23 30.052 pH5; S5204; T720; D2270-30 0.41 pH5; S5204; T720; D2269-22 0.39 7.12 1.72 89.63 0.00 0.00 1.08 22.634 27.532 pH5; S5204; T718; D2263-30 0.54 7.58 1.57 89.47 0.00 0.00 0.71 22.564 29.996 pH7; S5204; T672; D2091-47 0.32 5.22 2.23 90.45 0.00 0.00 0.938 22.486 32.046 pH5; S5204; T720; D2269-1 0.38 6.73 1.68 90.24 0.00 0.00 1.154 22.48 29.994 pH7; S5204; T673; D2096-6 0.33 4.18 1.10 92.91 0.00 0.00 0.91 22.446 28.714 pH5; S5204; T720; D2270-33 0.40 6.95 1.76 89.89 0.00 0.00 2.28 22.408 29.656 pH5; S5204; T718; D2263-14 0.58 7.72 1.64 89.26 0.00 0.00 0.306 22.35 32.294 pH5; S5204; T720; D2270-34 0.36 6.75 1.77 90.10 0.00 0.00 2.398 22.3 28.958 pH7; S5204; T672; D2090-29 0.42 4.99 2.01 91.06 0.00 0.00 1.16 22.112 30.376 pH5; S5204; T720; D2269-14 0.00 7.86 1.80 89.57 0.00 0.00 0.574 21.802 31.558 pH5; S5204; T718; D2263-29 0.58 7.32 1.30 90.07 0.00 0.00 0.418 21.746 30.426 pH5; S5204; T718; D2263-19 0.62 7.92 1.56 89.25 0.00 0.00 0.574 21.692 29.514 pH5; S5204; T720; D2269-10 0.39 6.82 1.70 90.05 0.00 0.00 1.104 21.622 25.264 pH5; S5204; T720; D2269-4 0.42 6.57 1.71 90.32 0.00 0.00 1.082 21.466 29.698 pH5; S5204; T720; D2270-4 0.43 7.44 1.72 89.31 0.00 0.00 1.758 21.446 32.656 pH5; S5204; T720; D2269-34 0.00 6.69 1.78 90.64 0.00 0.00 0.946 21.438 28.538 pH5; S5204; T720; D2270-16 0.39 7.08 1.71 89.70 0.00 0.00 1.592 21.422 27.72 pH5; S5204; T718; D2263-26 0.42 7.39 1.70 89.28 0.00 0.00 0.514 21.328 29.746 pH5; S5204; T720; D2269-3 0.36 6.76 1.71 90.17 0.00 0.00 0.668 21.242 29.74 pH5; S5204; T720; D2270-22 0.35 6.77 1.67 90.15 0.00 0.00 1.194 21.026 25.084 pH5; S5204; T720; D2270-26 0.41 6.81 1.82 89.66 0.00 0.00 1.606 20.948 32.142 pH5; S5204; T720; D2270-10 0.46 6.98 1.80 90.03 0.00 0.00 0.792 20.728 28.264 pH5; S5204; T720; D2269-16 0.51 6.17 1.50 90.64 0.00 0.00 0.922 20.502 30.132 pH5; S5204; T720; D2270-8 0.50 6.95 1.42 90.34 0.00 0.00 2.252 20.486 28.34 pH5; S5204; T720; D2270-2 0.46 6.76 1.83 89.90 0.00 0.00 0.97 20.366 31.758 pH5; S5204; T720; D2269-36 0.00 7.43 1.66 89.88 0.00 0.00 0.754 20.006 29.648 pH5; S5204; T720; D2269-31 0.72 9.29 1.86 86.92 0.00 0.00 2.062 19.002 27.61 pH5; S5204; T720; D2269-44 0.00 9.45 1.58 88.16 0.00 0.00 1.378 18.576 22.52 pH7; S5204; T672; D2091-14 0.27 4.79 2.24 90.94 0.00 0.00 0.93 18.1 30.434 pH5; S5204; T720; D2270-32 0.40 7.14 1.74 89.63 0.00 0.00 1.668 17.966 27.06 pH5; S5204; T720; D2270-11 0.82 9.24 1.93 87.35 0.00 0.00 1.178 15.998 28.196 pH5; S5204; T720; D2269-48 0.72 9.05 2.14 88.08 0.00 0.00 1.172 14.694 25.384 pH5; S5204; T720; D2269-17 0.66 9.08 2.12 87.12 0.00 0.00 0.84 14.488 25.886 pH5; S5204; T720; D2270-20 0.62 8.35 1.97 88.43 0.00 0.00 1.37 14.168 23.794 pH5; S5204; T718; D2263-13 0.75 9.44 1.98 87.09 0.00 0.00 0.64 13.854 29.466 pH5; S5204; T720; D2269-46 0.43 6.87 1.71 89.81 0.00 0.00 0.646 10.452 31.464 pH5; S5204; T720; D2269-5 0.59 8.81 1.93 87.97 0.00 0.00 0.654 9.37 25.786 pH7; S5204; T672; D2091-4 1.42 4.39 2.32 89.87 0.00 0.00 0.686 8.182 16.454 pH5; S5204; T720; D2269-6 0.50 7.29 1.73 89.29 0.00 0.00 0.79 7.978 21.346
pH5; S5204; T720; D2270-45 0.00 9.16 1.65 88.19 0.00 0.00 0.464 3.448 16.796 Blank 0 0 0
[0698] It is contemplated that these promoters, or variants thereof, discovered here can be used to regulate a fatty acid synthesis gene (e.g., any of the FATA, FATB, SAD, FAD2, KASI/IV, KASII, LPAAT or KCS genes disclosed herein) or other gene or gene-suppression element expressed in a cell including a microalgal cell. Variants can have for example 60, 65, 70, 75, 80, 85, 90, 95, 96, 97, 98, 99% or greater identity to the sequences disclosed here.
Example 64
Fractionation of a High SOS Oil to Increase SOS Concentration and Reduce Trisaturates
[0699] Microalgal oil was fractionated using dry fractionation and solvent fractionation techniques. The starting material was an oil that was high in SOS triglycerides. The oil was produced from Prototheca moriformis strain S7566, in which a the endogenous KASII gene was inserted into (and thereby knocking out) a SADII locus; additionally, the C18-preferring FATA1 gene from Garcinia mangostana was inserted and a FADII hairpin RNA was produced; as described above. After cultivation and extracted, the oil was refined, bleached and deodorized. The fatty acid profile of the oil is given in Table 115. The SOS TAG area% was about 62%. During the RBD processing, the total trisaturates (i.e. triglycerides with three fully saturated acyl chains such as SSS, PSS, PPS, PPP, etc.) in the oil decreased from 5.1% to 1.2%.
TABLE-US-00151 TABLE 116 Fatty acid profile of clarified oil from strain 7566. Strain S7566 Fatty Acid Area % C14:0 0.49 C16:0 3.12 C18:0 54.77 C18:l 35.88 C18:2 2.16 C18:3 .alpha. 0.23 C20:0 1.64 C22:0 0.19 C24:0 0.11 sum C18 93.05 saturates 60.69 unsaturates 38.55
[0700] The oil was fractionated using solvent (acetone or hexane) and dry fractionation. Acetone fractionation (1:1 oil-solvent, w/w; crystallization at 5.degree. C.) gave excellent recovery of an SOS-enriched stearin fraction, with relatively little SOS in the olein fraction. SOS was at 77%, with total trisaturates <1% for the stearin fraction.
[0701] Hexane fractionation(1:1 oil-solvent, w/w; crystallization at 5.degree. C.) gave a higher level (85%) of SOS, but also gave higher trisaturates (1.6%). Thus, using a single-step solvent fractionation, oils with over 75% SOS and less than 2% trisaturates were obtainable.
[0702] Dry fractionation was also successful in enriching SOS and decreasing trisaturates. The general approach was to remove trisaturates by crystallization at a higher temperature, then removing 00S at a lower temperature. The reverse order was also tried and yielded a superior result. It was also found that rinsing the SOS-enriched ("stearin") fraction with acetone helped in removing the olein fraction.
[0703] In one test, the oil was crystallized at 24.degree. C. and the stearin fraction was rinsed with acetone. Analysis showed that 00S levels decreased. The stearin fraction was heated and allowed to cool and crystallize overnight at 29.degree. C. The resulting liquid oil was separated from the crystallized trisaturates to afford a product with 84% SOS and <0.5% total trisaturates. Lipase-based sn-2 profile analysis of revealed that over 96% of that position was occupied by unsaturated fatty acids (93.3% oleate, 3.2% linolate, and 0.2% linolenate), while only 2.2% stearate was located there.
[0704] The DSC heating curve thermogram and DSC-derived solid fat content curve of the two step dry fractionated oil was compared to those of kokum butter. The two oils have essentially identical maximum heat-flow temperatures and the DSC-derived SFC curves are super-imposable. The oil could be expected to behave functionally similarly to kokum butter.
Example 65
Production of Microbial Oil with Over 60% SOS Content
[0705] Here, we demonstrate in the microalga Prototheca moriformis, that by disrupting an allele of the SAD2 gene, overexpressing KASII, knocking out endogenous FATA-1, overexpressing a more stearate-specific FATA (GarmFATA1 from Garcinia mangostana) relative to the endogenous FATA and activating FAD2 RNAi, we generate strains capable of accumulating over 60% SOS, useful as a structuring fat.
[0706] To reduce SAD activity, Strain S3150 was transformed with DNA constructs designed to recombine in the SAD2-1 and SAD2-2 alleles and express the selectable marker, Arabidopsis thialiana THIC (AtTHIC, codon-optimized for expression in P. moriformis). THIC encodes 4-amino-5-hydroxymethyl-2-methylpyrimidine synthase, thereby allowing growth in the absence of added thiamine. Transformants were selected in the absence of exogenous thiamine.
[0707] To make the SAD2-1 ablation construct pSZ2601, the Arabidopsis thaliana THIC gene (AtTHIC, codon-optimized for expression in P. moriformis), was utilized as a selectable marker for transformation. The sequence of the transforming DNA is shown in SEQ ID NO:148. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, PmeI, KpnI, XbaI, MfeI, SacI, BspQI and PmeI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the Chlorella protothecoides ACT promoter (CpACT) driving the expression of the AtTHIC gene (encoding 4-amino-5-hydroxymethyl-2-methylpyrimidine synthase activity, thereby permitting the strain to grow in the absence of exogenous thiamine) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the Chlorella vulgaris nitrate reductase (CvNR) gene is indicated by small capitals.
TABLE-US-00152 Nucleotide sequence of the transforming DNA from pSZ2601: (SEQ ID NO: 148) gaagagcgcccaatgtttaaacGCCGGTCACCACCCGCATGCTCGTACTACAGCGCACGCACCGCTTCGTG ATCCACCGGGTGAACGTAGTCCTCGACGGAAACATCTGGTTCGGGCCTCCTGCTTGCACTCCCGCCC ATGCCGACAACCTTTCTGCTGTTACCACGACCCACAATGCAACGCGACACGACCGTGTGGGACTGAT CGGTTCACTGCACCTGCATGCAATTGTCACAAGCGCTTACTCCAATTGTATTCGTTTGTTTTCTGGGA GCAGTTGCTCGACCGCCCGCGTCCCGCAGGCAGCGATGACGTGTGCGTGGCCTGGGTGTTTCGTCG AAAGGCCAGCAACCCTAAATCGCAGGCGATCCGGAGATTGGGATCTGATCCGAGTTTGGACCAGAT CCGCCCCGATGCGGCACGGGAACTGCATCGACTCGGCGCGGAACCCAGCTTTCGTAAATGCCAGAT TGGTGTCCGATACCTGGATTTGCCATCAGCGAAACAAGACTTCAGCAGCGAGCGTATTTGGCGGGC GTGCTACCAGGGTTGCATACATTGCCCATTTCTGTCTGGACCGCTTTACTGGCGCAGAGGGTGAGTT GATGGGGTTGGCAGGCATCGAAACGCGCGTGCATGGTGTGCGTGTCTGTTTTCGGCTGCACGAATT CAATAGTCGGATGGGCGACGGTAGAATTGGGTGTGGCGCTCGCGTGCATGCCTCGCCCCGTCGGGT GTCATGACCGGGACTGGAATCCCCCCTCGCGACCATCTTGCTAACGCTCCCGACTCTCCCGACCGCG ##STR00419## ##STR00420## ##STR00421## ##STR00422## ##STR00423## ##STR00424## ##STR00425## ##STR00426## ##STR00427## ##STR00428## ##STR00429## ##STR00430## ##STR00431## ##STR00432## gcccaactcctccctgctgcccggcttcgacgtggtggtccaggccgcggccacccgcttcaagaaggagacga- cgaccaccc gcgccacgctgacgttcgacccccccacgaccaactccgagcgcgccaagcagcgcaagcacaccatcgacccc- tcctccccc gacttccagcccatcccctccttcgaggagtgcttccccaagtccacgaaggagcacaaggaggtggtgcacga- ggagtccg gccacgtcctgaaggtgcccttccgccgcgtgcacctgtccggcggcgagcccgccttcgacaactacgacacg- tccggccccc agaacgtcaacgcccacatcggcctggcgaagctgcgcaaggagtggatcgaccgccgcgagaagctgggcacg- ccccgct acacgcagatgtactacgcgaagcagggcatcatcacggaggagatgctgtactgcgcgacgcgcgagaagctg- gaccccg agttcgtccgctccgaggtcgcgcggggccgcgccatcatcccctccaacaagaagcacctggagctggagccc- atgatcgtg ggccgcaagttcctggtgaaggtgaacgcgaacatcggcaactccgccgtggcctcctccatcgaggaggaggt- ctacaagg tgcagtgggccaccatgtggggcgccgacaccatcatggacctgtccacgggccgccacatccacgagacgcgc- gagtggat cctgcgcaactccgcggtccccgtgggcaccgtccccatctaccaggcgctggagaaggtggacggcatcgcgg- agaacctg aactgggaggtgttccgcgagacgctgatcgagcaggccgagcagggcgtggactacttcacgatccacgcggg- cgtgctgc tgcgctacatccccctgaccgccaagcgcctgacgggcatcgtgtcccgcggcggctccatccacgcgaagtgg- tgcctggcct accacaaggagaacttcgcctacgagcactgggacgacatcctggacatctgcaaccagtacgacgtcgccctg- tccatcggc gacggcctgcgccccggctccatctacgacgccaacgacacggcccagttcgccgagctgctgacccagggcga- gctgacgc gccgcgcgtgggagaaggacgtgcaggtgatgaacgagggccccggccacgtgcccatgcacaagatccccgag- aacatg cagaagcagctggagtggtgcaacgaggcgcccttctacaccctgggccccctgacgaccgacatcgcgcccgg- ctacgacc acatcacctccgccatcggcgcggccaacatcggcgccctgggcaccgccctgctgtgctacgtgacgcccaag- gagcacctg ggcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctacaagatcgccgcccacgcggccgacctggc- caagcag cacccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttcgagttccgctggatggaccagttcgc- gctgtccctg gaccccatgacggcgatgtccttccacgacgagacgctgcccgcggacggcgcgaaggtcgcccacttctgctc- catgtgcgg ccccaagttctgctccatgaagatcacggaggacatccgcaagtacgccgaggagaacggctacggctccgccg- aggaggc catccgccagggcatggacgccatgtccgaggagttcaacatcgccaagaagacgatctccggcgagcagcacg- gcgaggt cggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTGAcaattgGCAGCAGCAGCTCGGA- TAGTATC GACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTG- CCGCTTT TATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGC- GAATACCA CCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCC- TCAGCGCT GCTCCTG CTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAAC- CTGTAAACC AGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAGGATCGTAgagctcTAGGGAGCG- A CGAGTGTGCGTGCGGGGCTGGCGGGAGTGGGACGCCCTCCTCGCTCCTCTCTGTTCTGAACGGAAC AATCGGCCACCCCGCGCTACGCGCCACGCATCGAGCAACGAAGAAAACCCCCCGATGATAGGTTGC GGTGGCTGCCGGGATATAGATCCGGCCGCACATCAAAGGGCCCCTCCGCCAGAGAAGAAGCTCCTT TCCCAGCAGACTCCTTCTGCTGCCAAAACACTTCTCTGTCCACAGCAACACCAAAGGATGAACAGATC AACTTGCGTCTCCGCGTAGCTTCCTCGGCTAGCGTGCTTGCAACAGGTCCCTGCACTATTATCTTCCT GCTTTCCTCTGAATTATGCGGCAGGCGAGCGCTCGCTCTGGCGAGCGCTCCTTCGCGCCGCCCTCGC TGATCGAGTGTACAGTCAATGAATGGTCCTGGGCGAAGAACGAGGGAATTTGTGGGTAAAACAAG CATCGTCTCTCAGGCCCCGGCGCAGTGGCCGTTAAAGTCCAAGACCGTGACCAGGCAGCGCAGCGC GTCCGTGTGCGGGCCCTGCCTGGCGGCTCGGCGTGCCAGGCTCGAGAGCAGCTCCCTCAGGTCGCC TTGGACGGCCTCTGCGAGGCCGGTGAGGGCCTGCAGGAGCGCCTCGAGCGTGGCAGTGGCGGTCG TATCCGGGTCGCCGGTCACCGCCTGCGACTCGCCATCCgaagagcgtttaaac
[0708] The sequence of the transforming DNA from the SAD2-1 disruption construct, pSZ2607, is shown below in SEQ ID NO:149. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' PmeI, KpnI, XbaI, MfeI, SacI, BspQI and PmeI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the Chlorella protothecoides ACT promoter (CpACT) driving the expression of the AtTHIC gene (encoding 4-amino-5-hydroxymethyl-2-methylpyrimidine synthase activity, thereby permitting the strain to grow in the absence of exogenous thiamine) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the Chlorella vulgaris nitrate reductase (CvNR) gene is indicated by small capitals.
TABLE-US-00153 Nucleotide sequence of the transforming DNA from pSZ2607: (SEQ ID NO: 149) gtttaaacGCCGGTCACCACCCGCATGCTCGTACTACAGCGCACGCACCGCTTCGTGATCCACCGGGTG AACGTAGTCCTCGACGGAAACATCTGGTTCGGGCCTCCTGCTTGCACTCCCGCCCATGCCGACAACC TTTCTGCTGTTACCACGACCCACAATGCAACGCGACACGACCGTGTGGGACTGATCGGTTCACTGCA CCTGCATGCAATTGTCACAAGCGCTTACTCCAATTGTATTCGTTTGTTTTCTGGGAGCAGTTGCTCGA CCGCCCGCGTCCCGCAGGCAGCGATGACGTGTGCGTGGCCTGGGTGTTTCGTCGAAAGGCCAGCAA CCCTAAATCGCAGGCGATCCGGAGATTGGGATCTGATCCGAGTTTGGACCAGATCCGCCCCGATGC GGCACGGGAACTGCATCGACTCGGCGCGGAACCCAGCTTTCGTAAATGCCAGATTGGTGTCCGATA CCTGGATTTGCCATCAGCGAAACAAGACTTCAGCAGCGAGCGTATTTGGCGGGCGTGCTACCAGGG TTGCATACATTGCCCATTTCTGTCTGGACCGCTTTACTGGCGCAGAGGGTGAGTTGATGGGGTTGGC AGGCATCGAAACGCGCGTGCATGGTGTGCGTGTCTGTTTTCGGCTGCACGAATTCAATAGTCGGAT GGGCGACGGTAGAATTGGGTGTGGCGCTCGCGTGCATGCCTCGCCCCGTCGGGTGTCATGACCGG GACTGGAATCCCCCCTCGCGACCATCTTGCTAACGCTCCCGACTCTCCCGACCGCGCGCAGGATAGA ##STR00433## ##STR00434## ##STR00435## ##STR00436## ##STR00437## ##STR00438## ##STR00439## ##STR00440## ##STR00441## ##STR00442## ##STR00443## ##STR00444## ##STR00445## ##STR00446## gccgcgtccgtccactgcaccctgatgtccgtggtctgcaacaacaagaaccactccgcccgccccaagctgcc- caactcctccc tgctgcccggcttcgacgtggtggtccaggccgcggccacccgcttcaagaaggagacgacgaccacccgcgcc- acgctgac gttcgacccccccacgaccaactccgagcgcgccaagcagcgcaagcacaccatcgacccctcctcccccgact- tccagcccat cccctccttcgaggagtgcttccccaagtccacgaaggagcacaaggaggtggtgcacgaggagtccggccacg- tcctgaag gtgcccttccgccgcgtgcacctgtccggcggcgagcccgccttcgacaactacgacacgtccggcccccagaa- cgtcaacgcc cacatcggcctggcgaagctgcgcaaggagtggatcgaccgccgcgagaagctgggcacgccccgctacacgca- gatgtac tacgcgaagcagggcatcatcacggaggagatgctgtactgcgcgacgcgcgagaagctggaccccgagttcgt- ccgctccg aggtcgcgcggggccgcgccatcatcccctccaacaagaagcacctggagctggagcccatgatcgtgggccgc- aagttcct ggtgaaggtgaacgcgaacatcggcaactccgccgtggcctcctccatcgaggaggaggtctacaaggtgcagt- gggccac catgtggggcgccgacaccatcatggacctgtccacgggccgccacatccacgagacgcgcgagtggatcctgc- gcaactcc gcggtccccgtgggcaccgtccccatctaccaggcgctggagaaggtggacggcatcgcggagaacctgaactg- ggaggtg ttccgcgagacgctgatcgagcaggccgagcagggcgtggactacttcacgatccacgcgggcgtgctgctgcg- ctacatccc cctgaccgccaagcgcctgacgggcatcgtgtcccgcggcggctccatccacgcgaagtggtgcctggcctacc- acaaggag aacttcgcctacgagcactgggacgacatcctggacatctgcaaccagtacgacgtcgccctgtccatcggcga- cggcctgcg ccccggctccatctacgacgccaacgacacggcccagttcgccgagctgctgacccagggcgagctgacgcgcc- gcgcgtgg gagaaggacgtgcaggtgatgaacgagggccccggccacgtgcccatgcacaagatccccgagaacatgcagaa- gcagct ggagtggtgcaacgaggcgcccttctacaccctgggccccctgacgaccgacatcgcgcccggctacgaccaca- tcacctccg ccatcggcgcggccaacatcggcgccctgggcaccgccctgctgtgctacgtgacgcccaaggagcacctgggc- ctgcccaac cgcgacgacgtgaaggcgggcgtcatcgcctacaagatcgccgcccacgcggccgacctggccaagcagcaccc- ccacgccc aggcgtgggacgacgcgctgtccaaggcgcgcttcgagttccgctggatggaccagttcgcgctgtccctggac- cccatgacg gcgatgtccttccacgacgagacgctgcccgcggacggcgcgaaggtcgcccacttctgctccatgtgcggccc- caagttctgc tccatgaagatcacggaggacatccgcaagtacgccgaggagaacggctacggctccgccgaggaggccatccg- ccaggg catggacgccatgtccgaggagttcaacatcgccaagaagacgatctccggcgagcagcacggcgaggtcggcg- gcgagat ctacctgcccgagtcctacgtcaaggccgcgcagaagTGAcaattgGCAGCAGCAGCTCGGATAGTATCGACAC- ACTCTGG ACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCA- AACAGCCT CAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCC- AGCATCCC CTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTC- CTGCTCCTG CTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCAC- TGCAATGC TGATGCACGGGAAGTAGTGGGATGGGAAcAcAAATGGAGGATCGTAgagctcCAGCCACGGCAACACCGCGCG CCTTGCGGCCGAGCACGGCGACAAGAACCTGAGCAAGATCTGCGGGCTGATCGCCAGCGACGAGG GCCGGCACGAGATCGCCTACACGCGCATCGTGGACGAGTTCTTCCGCCTCGACCCCGAGGGCGCCG TCGCCGCCTACGCCAACATGATGCGCAAGCAGATCACCATGCCCGCGCACCTCATGGACGACATGG GCCACGGCGAGGCCAACCCGGGCCGCAACCTCTTCGCCGACTTCTCCGCGGTCGCCGAGAAGATCG ACGTCTACGACGCCGAGGACTACTGCCGCATCCTGGAGCACCTCAACGCGCGCTGGAAGGTGGACG AGCGCCAGGTCAGCGGCCAGGCCGCCGCGGACCAGGAGTACGTCCTGGGCCTGCCCCAGCGCTTCC GGAAACTCGCCGAGAAGACCGCCGCCAAGCGCAAGCGCGTCGCGCGCAGGCCCGTCGCCTTCTCCT GGATCTCCGGGCGCGAGATCATGGTCTAGGGAGCGACGAGTGTGCGTGCGGGGCTGGCGGGAGT GGGACGCCCTCCTCGCTCCTCTCTGTTCTGAACGGAACAATCGGCCACCCCGCGCTACGCGCCACGC ATCGAGCAACGAAGAAAACCCCCCGATGATAGGTTGCGGTGGCTGCCGGGATATAGATCCGGCCGC ACATCAAAGGGCCCCTCCGCCAGAGAAGAAGCTCCTTTCCCAGCAGACTCCTgaagagcgtttaaac
[0709] The sequence of the transforming DNA from the SAD2-2 disruption construct,pSZ2622, is shown below in SEQ ID NO:150. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, PmeI, KpnI, XbaI, MfeI, SacI, BspQI and PmeI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. Proceeding in the 5' to 3' direction, the Chlorella protothecoides ACT promoter (CpACT) driving the expression of the AtTHIC gene (encoding 4-amino-5-hydroxymethyl-2-methylpyrimidine synthase activity, thereby permitting the strain to grow in the absence of exogenous thiamine) is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the Chlorella vulgaris nitrate reductase (CvNR) gene is indicated by small capitals.
TABLE-US-00154 Nucleotide sequence of the transforming DNA from pSZ2622: (SEQ ID NO: 150) gaagagcgcccaatgtttaaacGCCGGTCACCATCCGCATGCTCATATTACAGCGCACGCACCGCTTCGTGA TCCACCGGGTGAACGTAGTCCTCGACGGAAACATCTGGCTCGGGCCTCGTGCTGGCACTCCCTCCCA TGCCGACAACCTTTCTGCTGTCACCACGACCCACGATGCAACGCGACACGACCCGGTGGGACTGATC GGTTCACTGCACCTGCATGCAATTGTCACAAGCGCATACTCCAATCGTATCCGTTTGATTTCTGTGAA AACTCGCTCGACCGCCCGCGTCCCGCAGGCAGCGATGACGTGTGCGTGACCTGGGTGTTTCGTCGA AAGGCCAGCAACCCCAAATCGCAGGCGATCCGGAGATTGGGATCTGATCCGAGCTTGGACCAGATC CCCCACGATGCGGCACGGGAACTGCATCGACTCGGCGCGGAACCCAGCTTTCGTAAATGCCAGATT GGTGTCCGATACCTTGATTTGCCATCAGCGAAACAAGACTTCAGCAGCGAGCGTATTTGGCGGGCG TGCTACCAGGGTTGCATACATTGCCCATTTCTGTCTGGACCGCTTTACCGGCGCAGAGGGTGAGTTG ATGGGGTTGGCAGGCATCGAAACGCGCGTGCATGGTGTGTGTGTCTGTTTTCGGCTGCACAATTTCA ATAGTCGGATGGGCGACGGTAGAATTGGGTGTTGCGCTCGCGTGCATGCCTCGCCCCGTCGGGTGT CATGACCGGGACTGGAATCCCCCCTCGCGACCCTCCTGCTAACGCTCCCGACTCTCCCGCCCGCGCG ##STR00447## ##STR00448## ##STR00449## ##STR00450## ##STR00451## ##STR00452## ##STR00453## ##STR00454## ##STR00455## ##STR00456## ##STR00457## ##STR00458## ##STR00459## ##STR00460## ##STR00461## cccaactcctccctgctgcccggcttcgacgtggtggtccaggccgcggccacccgcttcaagaaggagacgac- gaccacccg cgccacgctgacgttcgacccccccacgaccaactccgagcgcgccaagcagcgcaagcacaccatcgacccct- cctcccccg acttccagcccatcccctccttcgaggagtgcttccccaagtccacgaaggagcacaaggaggtggtgcacgag- gagtccggc cacgtcctgaaggtgcccttccgccgcgtgcacctgtccggcggcgagcccgccttcgacaactacgacacgtc- cggcccccag aacgtcaacgcccacatcggcctggcgaagctgcgcaaggagtggatcgaccgccgcgagaagctgggcacgcc- ccgctac acgcagatgtactacgcgaagcagggcatcatcacggaggagatgctgtactgcgcgacgcgcgagaagctgga- ccccga gttcgtccgctccgaggtcgcgcggggccgcgccatcatcccctccaacaagaagcacctggagctggagccca- tgatcgtgg gccgcaagttcctggtgaaggtgaacgcgaacatcggcaactccgccgtggcctcctccatcgaggaggaggtc- tacaaggt gcagtgggccaccatgtggggcgccgacaccatcatggacctgtccacgggccgccacatccacgagacgcgcg- agtggatc ctgcgcaactccgcggtccccgtgggcaccgtccccatctaccaggcgctggagaaggtggacggcatcgcgga- gaacctga actgggaggtgttccgcgagacgctgatcgagcaggccgagcagggcgtggactacttcacgatccacgcgggc- gtgctgct gcgctacatccccctgaccgccaagcgcctgacgggcatcgtgtcccgcggcggctccatccacgcgaagtggt- gcctggccta ccacaaggagaacttcgcctacgagcactgggacgacatcctggacatctgcaaccagtacgacgtcgccctgt- ccatcggcg acggcctgcgccccggctccatctacgacgccaacgacacggcccagttcgccgagctgctgacccagggcgag- ctgacgcg ccgcgcgtgggagaaggacgtgcaggtgatgaacgagggccccggccacgtgcccatgcacaagatccccgaga- acatgc agaagcagctggagtggtgcaacgaggcgcccttctacaccctgggccccctgacgaccgacatcgcgcccggc- tacgacca catcacctccgccatcggcgcggccaacatcggcgccctgggcaccgccctgctgtgctacgtgacgcccaagg- agcacctgg gcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctacaagatcgccgcccacgcggccgacctggcc- aagcagc acccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttcgagttccgctggatggaccagttcgcg- ctgtccctg gaccccatgacggcgatgtccttccacgacgagacgctgcccgcggacggcgcgaaggtcgcccacttctgctc- catgtgcgg ccccaagttctgctccatgaagatcacggaggacatccgcaagtacgccgaggagaacggctacggctccgccg- aggaggc catccgccagggcatggacgccatgtccgaggagttcaacatcgccaagaagacgatctccggcgagcagcacg- gcgaggt cggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTGAcaattgGCAGCAGCAGCTCGGA- TAGTATC GACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTG- CCGCTTT TATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGC- GAATACCA CCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCC- CTCAGCGCT GCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACC- TGTAAACC AGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAGGATCGTAgagctcCAGCCACGG- C AACACCGCGCGCCTGGCGGCCGAGCACGGCGACAAGGGCCTGAGCAAGATCTGCGGGCTGATCGC CAGCGACGAGGGCCGGCACGAGATCGCCTACACGCGCATCGTGGACGAGTTCTTCCGCCTCGACCC CGAGGGCGCCGTCGCCGCCTACGCCAACATGATGCGCAAGCAGATCACCATGCCCGCGCACCTCAT GGACGACATGGGCCACGGCGAGGCCAACCCGGGCCGCAACCTCTTCGCCGACTTCTCCGCCGTCGC CGAGAAGATCGACGTCTACGACGCCGAGGACTACTGCCGCATCCTGGAGCACCTCAACGCGCGCTG GAAGGTGGACGAGCGCCAGGTCAGCGGCCAGGCCGCCGCGGACCAGGAGTACGTTCTGGGCCTGC CCCAGCGCTTCCGGAAACTCGCCGAGAAGACCGCCGCCAAGCGCAAGCGCGTCGCGCGCAGGCCC GTCGCCTTCTCCTGGATCTCCGGACGCGAGATTATGGTCTAGGGAGGTACGAGCGCGCGCGAGGGA TTGGTGGGAGTGGGACGCGCTCGTCGCTCCTTTCTATTCTGAAGGGAAGATTGGCCACCCCGCTCCA CGCGCCACGCATCGAGCAACGAAGAAAACCCCCCGATGATAGGTTGCAGTGGCTGCCGAGATATAG ATCCGGCTGCACGTCAAAGGGCCCCTCGGCCAGAGAAGAAGCTCTTTTCCCAGCGACCGCAGACTCC Tgaagagcgtttaaac
[0710] Constructs D1557, D1565 and D1566, derived from pSZ2601, pSZ2607 and pSZ2622, respectively, were transformed into S3150 as described previously. Primary transformants were clonally purified and grown under low-nitrogen lipid production conditions at pH 5. The resulting fatty acid profiles from representative clones are summarized in Table 117. SAD2-1 disruption strains derived from D1557 and D1565 transformants accumulated up to 13.4% C18:0 at the expense of C18:1, indicating that SAD activity was significantly reduced in these strains. C18:0 levels only increased to 8.5% in SAD2-2 disruption strains, suggesting that the expression or activity of SAD2-2 was lower than that of SAD2-1. We also observed that C20:0 levels increased up to 1.1% in strains with elevated C18:0, demonstrating that C18:0 was an effective primer for fatty acid elongation reactions in the endoplasmic reticulum (ER).
TABLE-US-00155 TABLE 117 Fatty acid profiles from representative clones. Strain S3150 D1557-2 D1557-3 D1565-10 D1565-3 D1565-8 D1566-5 D1566-6 D1566-1 Fatty C14:0 1.30 1.14 1.20 1.08 1.12 1.11 1.18 1.12 1.21 Acid C16:0 28.71 29.32 29.74 28.84 29.34 29.11 29.21 29.13 28.46 Area C16:1 0.76 0.21 0.23 0.21 0.21 0.21 0.32 0.31 0.31 % C17:0 0.12 0.14 0.15 0.15 0.14 0.14 0.14 0.16 0.14 C18:0 2.93 13.42 11.92 14.29 14.14 14.04 8.47 8.47 7.68 C18:1 58.08 46.29 47.65 45.75 45.31 45.69 51.29 51.33 53.38 C18:2 6.81 7.15 6.96 7.09 7.18 7.19 7.25 7.34 6.92 C18:3 .alpha. 0.59 0.69 0.63 0.72 0.72 0.73 0.71 0.73 0.62 C20:0 0.24 0.93 0.84 1.10 1.09 1.04 0.75 0.77 0.63 C22:0 0.05 0.16 0.15 0.19 0.19 0.18 0.14 0.14 0.11 C24:0 0.06 0.16 0.16 0.20 0.20 0.20 0.17 0.17 0.14 sum C18 68.40 67.55 67.16 67.85 67.35 67.65 67.72 67.87 68.60 saturates 33.49 45.35 44.24 45.94 46.32 45.93 40.18 40.04 38.48 unsaturates 66.52 54.62 55.76 54.04 53.68 54.07 59.83 59.97 61.50
[0711] In order to increase C18:0 accumulation at the expense of C16:0 we generated DNA constructs which simultaneously ablated SAD2-1 and over-expressed a codon-optimized version of the endogenous .beta.-ketoacyl-ACP synthase II (PmKASII) gene. The sequence of the transforming DNA from the SAD2-1 ablation, PmKASII over-expression construct, pSZ2624, is shown below in SEQ ID NO:151. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' PmeI, SpeI, AscI, ClaI, SacI, AvrII, EcoRV, AflII, KpnI, XbaI, MfeI, BamHI, BspQI and PmeI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the SAD2-1 locus. The SAD2-1 5' integration flank contained the endogeneous SAD2-1 promoter, enabling the in situ activation of the PmKASII gene. Proceeding in the 5' to 3' direction, the region encoding the PmKASII plastid targeting sequence is indicated by lowercase, underlined italics. The sequence that encodes the mature PmKASII polypeptide is indicated with lowercase italics, while a 3.times. FLAG epitope encoding sequence is in bold italics. The initiator ATG and terminator TGA for PmKASII-FLAG are indicated by uppercase italics. Two spacer regions are represented by lowercase text. The CpACT promoter driving the expression of the AtTHIC gene is indicated by lowercase, boxed text. The initiator ATG and terminator TGA for AtTHIC are indicated by uppercase italics, while the coding region is indicated with lowercase italics. The 3' UTR of the Chlorella vulgaris nitrate reductase (CvNR) gene is indicated by small capitals.
TABLE-US-00156 Nucleotide sequence of the transforming DNA from pSZ2624: (SEQ ID NO: 151) gtttaaacGCCGGTCACCACCCGCATGCTCGTACTACAGCGCACGCACCGCTTCGTGATCCACCGGGTG AACGTAGTCCTCGACGGAAACATCTGGTTCGGGCCTCCTGCTTGCACTCCCGCCCATGCCGACAACC TTTCTGCTGTTACCACGACCCACAATGCAACGCGACACGACCGTGTGGGACTGATCGGTTCACTGCA CCTGCATGCAATTGTCACAAGCGCTTACTCCAATTGTATTCGTTTGTTTTCTGGGAGCAGTTGCTCGA CCGCCCGCGTCCCGCAGGCAGCGATGACGTGTGCGTGGCCTGGGTGTTTCGTCGAAAGGCCAGCAA CCCTAAATCGCAGGCGATCCGGAGATTGGGATCTGATCCGAGTTTGGACCAGATCCGCCCCGATGC GGCACGGGAACTGCATCGACTCGGCGCGGAACCCAGCTTTCGTAAATGCCAGATTGGTGTCCGATA CCTGGATTTGCCATCAGCGAAACAAGACTTCAGCAGCGAGCGTATTTGGCGGGCGTGCTACCAGGG TTGCATACATTGCCCATTTCTGTCTGGACCGCTTTACTGGCGCAGAGGGTGAGTTGATGGGGTTGGC AGGCATCGAAACGCGCGTGCATGGTGTGCGTGTCTGTTTTCGGCTGCACGAATTCAATAGTCGGAT GGGCGACGGTAGAATTGGGTGTGGCGCTCGCGTGCATGCCTCGCCCCGTCGGGTGTCATGACCGG GACTGGAATCCCCCCTCGCGACCATCTTGCTAACGCTCCCGACTCTCCCGACCGCGCGCAGGATAGA CTCTTGTTCAACCAATCGACAactagtATGcagaccgcccaccagcgcccccccaccgagggccactgcttcgg- cgcc cgcctgcccaccgcctcccgccgcgccgtgcgccgcgcctggtcccgcatcgcccgcgggcgcgccgccgccgc- cgccgacgcc aaccccgcccgccccgagcgccgcgtggtgatcaccggccagggcgtggtgacctccctgggccagaccatcga- gcagttcta ctcctccctgctggagggcgtgtccggcatctcccagatccagaagttcgacaccaccggctacaccaccacca- tcgccggcga gatcaagtccctgcagctggacccctacgtgcccaagcgctgggccaagcgcgtggacgacgtgatcaagtacg- tgtacatcg ccggcaagcaggccctggagtccgccggcctgcccatcgaggccgccggcctggccggcgccggcctggacccc- gccctgtgc ggcgtgctgatcggcaccgccatggccggcatgacctccttcgccgccggcgtggaggccctgacccgcggcgg- cgtgcgcaa gatgaaccccttctgcatccccttctccatctccaacatgggcggcgccatgctggccatggacatcggcttca- tgggccccaact actccatctccaccgcctgcgccaccggcaactactgcatcctgggcgccgccgaccacatccgccgcggcgac- gccaacgtga tgctggccggcggcgccgacgccgccatcatcccctccggcatcggcggcttcatcgcctgcaaggccctgtcc- aagcgcaacg acgagcccgagcgcgcctcccgcccctgggacgccgaccgcgacggcttcgtgatgggcgagggcgccggcgtg- ctggtgct ggaggagctggagcacgccaagcgccgcggcgccaccatcctggccgagctggtgggcggcgccgccacctccg- acgccca ccacatgaccgagcccgacccccagggccgcggcgtgcgcctgtgcctggagcgcgccctggagcgcgcccgcc- tggcccccg agcgcgtgggctacgtgaacgcccacggcacctccacccccgccggcgacgtggccgagtaccgcgccatccgc- gccgtgatc ccccaggactccctgcgcatcaactccaccaagtccatgatcggccacctgctgggcggcgccggcgccgtgga- ggccgtggc cgccatccaggccctgcgcaccggctggctgcaccccaacctgaacctggagaaccccgcccccggcgtggacc- ccgtggtgc tggtgggcccccgcaaggagcgcgccgaggacctggacgtggtgctgtccaactccttcggcttcggcggccac- aactcctgc gtgatcttccgcaagtacgacgagatggactacaaggaccacgacggcgactacaaggaccacgacatcgacta- caagg acgacgacgacaagTGAatcgatAGATCTCTTAAGGCAGCAGCAGCTCGGATAGTATCGACACACTCTGGACGC- TGGTC GTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTC- AGTGTGTT TGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCC- TTCCCTCG TTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTG- CTCACTGCC CCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCT- GATGCAC GGGAAGTAGTGGGATGGGAACACAAATGGAAAGCTTAATTAAgagctccgcgtctcgaacagagcgcgcagagg- aacgctg aaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacctgacgaatgcgcttggttcttcg- tccattagcgaagc gtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatgatcggtggagctgatggtcgaacgttc- acagcctaggt gatatccatcttaaggatctaagtaagattcgaagcgctcgaccgtgccggacggactgcagccccatgtcgta- gtgaccgccaat gtaagtgggctggcgtttccctgtacgtgagtcaacgtcactgcacgcgcaccaccctctcgaccggcaggacc- aggcatcgcgag ##STR00462## ##STR00463## ##STR00464## ##STR00465## ##STR00466## ##STR00467## ##STR00468## ##STR00469## ##STR00470## ##STR00471## ##STR00472## ##STR00473## ##STR00474## ##STR00475## ##STR00476## ccaagctgcccaactcctccctgctgcccggcttcgacgtggtggtccaggccgcggccacccgcttcaagaag- gagacgacg accacccgcgccacgctgacgttcgacccccccacgaccaactccgagcgcgccaagcagcgcaagcacaccat- cgacccctc ctcccccgacttccagcccatcccctccttcgaggagtgcttccccaagtccacgaaggagcacaaggaggtgg- tgcacgagg agtccggccacgtcctgaaggtgcccttccgccgcgtgcacctgtccggcggcgagcccgccttcgacaactac- gacacgtccg gcccccagaacgtcaacgcccacatcggcctggcgaagctgcgcaaggagtggatcgaccgccgcgagaagctg- ggcacgc cccgctacacgcagatgtactacgcgaagcagggcatcatcacggaggagatgctgtactgcgcgacgcgcgag- aagctgg accccgagttcgtccgctccgaggtcgcgcggggccgcgccatcatcccctccaacaagaagcacctggagctg- gagcccatg atcgtgggccgcaagttcctggtgaaggtgaacgcgaacatcggcaactccgccgtggcctcctccatcgagga- ggaggtct acaaggtgcagtgggccaccatgtggggcgccgacaccatcatggacctgtccacgggccgccacatccacgag- acgcgcg agtggatcctgcgcaactccgcggtccccgtgggcaccgtccccatctaccaggcgctggagaaggtggacggc- atcgcgga gaacctgaactgggaggtgttccgcgagacgctgatcgagcaggccgagcagggcgtggactacttcacgatcc- acgcggg cgtgctgctgcgctacatccccctgaccgccaagcgcctgacgggcatcgtgtcccgcggcggctccatccacg- cgaagtggtg cctggcctaccacaaggagaacttcgcctacgagcactgggacgacatcctggacatctgcaaccagtacgacg- tcgccctgt ccatcggcgacggcctgcgccccggctccatctacgacgccaacgacacggcccagttcgccgagctgctgacc- cagggcga gctgacgcgccgcgcgtgggagaaggacgtgcaggtgatgaacgagggccccggccacgtgcccatgcacaaga- tccccg agaacatgcagaagcagctggagtggtgcaacgaggcgcccttctacaccctgggccccctgacgaccgacatc- gcgcccgg ctacgaccacatcacctccgccatcggcgcggccaacatcggcgccctgggcaccgccctgctgtgctacgtga- cgcccaagg agcacctgggcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctacaagatcgccgcccacgcggcc- gacctggc caagcagcacccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttcgagttccgctggatggacc- agttcgcg ctgtccctggaccccatgacggcgatgtccttccacgacgagacgctgcccgcggacggcgcgaaggtcgccca- cttctgctcc atgtgcggccccaagttctgctccatgaagatcacggaggacatccgcaagtacgccgaggagaacggctacgg- ctccgccg aggaggccatccgccagggcatggacgccatgtccgaggagttcaacatcgccaagaagacgatctccggcgag- cagcacg gcgaggtcggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTGAcaattgACGGAGCGT- CGTGCG GGAGGGAGTGTGCCGAGCGGGGAGTCCCGGTCTGTGCGAGGCCCGGCAGCTGACGCTGGCGAGCCGTACGCCCC- GAG GGICCCCCTCCCCTGCACCCICTICCCCTICCCTCTGACGGCCGCGCCTGITCTTGCATGITCAGCGACggatc- cTAGGGA GCGACGAGTGTGCGTGCGGGGCTGGCGGGAGTGGGACGCCCTCCTCGCTCCTCTCTGTTCTGAACG GAACAATCGGCCACCCCGCGCTACGCGCCACGCATCGAGCAACGAAGAAAACCCCCCGATGATAGG TTGCGGTGGCTGCCGGGATATAGATCCGGCCGCACATCAAAGGGCCCCTCCGCCAGAGAAGAAGCT CCTTTCCCAGCAGACTCCTTCTGCTGCCAAAACACTTCTCTGTCCACAGCAACACCAAAGGATGAACA GATCAACTTGCGTCTCCGCGTAGCTTCCTCGGCTAGCGTGCTTGCAACAGGTCCCTGCACTATTATCT TCCTGCTTTCCTCTGAATTATGCGGCAGGCGAGCGCTCGCTCTGGCGAGCGCTCCTTCGCGCCGCCCT CGCTGATCGAGTGTACAGTCAATGAATGGTCCTGGGCGAAGAACGAGGGAATTTGTGGGTAAAACA AGCATCGTCTCTCAGGCCCCGGCGCAGTGGCCGTTAAAGTCCAAGACCGTGACCAGGCAGCGCAGC GCGTCCGTGTGCGGGCCCTGCCTGGCGGCTCGGCGTGCCAGGCTCGAGAGCAGCTCCCTCAGGTCG CCTTGGACGGCCTCTGCGAGGCCGGTGAGGGCCTGCAGGAGCGCCTCGAGCGTGGCAGTGGCGGT CGTATCCGGGTCGCCGGTCACCGCCTGCGACTCGCCATCCgaagagcgtttaaac
[0712] Using the methods of this example, by overexpressing KASII, and Garcinia mangostana FATA, and by reducing expression of endogenous SAD, FAD2, and FATA, we produced a strain of P. moriformis that produced and oil with greater than 55% SOS with Sat-O-Sat (where O is oleate and Sat is any saturated fatty acid) of about 70-75% and trisaturatated TAGs of less than 6.5%.
Example 66
Combining KASII, FATA and LPAAT Transgenes to Produce an Oil High in SOS
[0713] In Prototheca moriformis, we overexpressed the P. moriformis KASII, knocked out an endogenous SAD2 allele, knocked out the endogenous FATA allele, and overexpressed both a LPAAT from Brassica napus and a FATA gene from Garcinia mangostana ("GarmFAT1"). The resulting strain produced an oil with over 55% SOS, over 70% Sat-O-Sat, and less than 8% trisaturated TAGs.
[0714] A base strain was transformed with a linearized plasmid with flanking regions designed for homologous recombination at the SAD2 site. As in examples above, the construct ablated SAD2 and overexpressed P. moriformis KASII. A ThiC selection marker was used. This strain was further transformed with a construct designed to overexpress GarmFATA1 with a P. moriformis SASD1 plastid targeting peptide via homologous recombination at the 6S chromosomal site using invertase as a selection marker. The resulting strain, produced oil with about 62% stearate, 6% palmitate, 5% linoleate, 45% SOS and 20% trisaturates.
[0715] The sequence of the transforming DNA from the GarmFATA1 expression construct (pSZ3204) is shown below in SEQ ID NO:152. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, XbaI, MfeI, BamHI, AvrII, EcoRV, SpeI, AscI, ClaI, AflII, SacI and BspQI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the 6S locus. Proceeding in the 5' to 3' direction, the CrTUB2 promoter driving the expression of Saccharomyces cerevisiae SUC2 (ScSUC2) gene, enabling strains to utilize exogenous sucrose, is indicated by lowercase, boxed text. The initiator ATG and terminator TGA of ScSUC2 are indicated by uppercase italics, while the coding region is represented by lowercase italics. The 3' UTR of the CvNR gene is indicated by small capitals. A spacer region is represented by lowercase text. The P. moriformis SAD2-2 (PmSAD2-2) promoter driving the expression of the chimeric CpSAD1tp_GarmFATA1 FLAG gene is indicated by lowercase, boxed text. The initiator ATG and terminator TGA are indicated by uppercase italics; the sequence encoding CpSAD1tp is represented by lowercase, underlined italics; the sequence encoding the GarmFATA1 mature polypeptide is indicated by lowercase italics; and the 3.times. FLAG epitope tag is represented by uppercase, bold italics. A second CvNR 3' UTR is indicated by small capitals.
TABLE-US-00157 Nucleotide sequence of the transforming DNA from pSZ3204: (SEQ ID NO: 152) gctcttcGCCGCCGCCACTCCTGCTCGAGCGCGCCCGCGCGTGCGCCGCCAGCGCCTTGGCCTTTTCGC CGCGCTCGTGCGCGTCGCTGATGTCCATCACCAGGTCCATGAGGTCTGCCTTGCGCCGGCTGAGCCA CTGCTTCGTCCGGGCGGCCAAGAGGAGCATGAGGGAGGACTCCTGGTCCAGGGTCCTGACGTGGT CGCGGCTCTGGGAGCGGGCCAGCATCATCTGGCTCTGCCGCACCGAGGCCGCCTCCAACTGGTCCT CCAGCAGCCGCAGTCGCCGCCGACCCTGGCAGAGGAAGACAGGTGAGGGGGGTATGAATTGTACA GAACAACCACGAGCCTTGTCTAGGCAGAATCCCTACCAGTCATGGCTTTACCTGGATGACGGCCTGC GAACAGCTGTCCAGCGACCCTCGCTGCCGCCGCTTCTCCCGCACGCTTCTTTCCAGCACCGTGATGGC GCGAGCCAGCGCCGCACGCTGGCGCTGCGCTTCGCCGATCTGAGGACAGTCGGGGAACTCTGATCA GTCTAAACCCCCTTGCGCGTTAGTGTTGCCATCCTTTGCAGACCGGTGAGAGCCGACTTGTTGTGCG CCACCCCCCACACCACCTCCTCCCAGACCAATTCTGTCACCTTTTTGGCGAAGGCATCGGCCTCGGCC ##STR00477## ##STR00478## ##STR00479## ##STR00480## ##STR00481## gcttcgccgccaagatcagcgcctccatgacgaacgagacgtccgaccgccccctggtgcacttcacccccaac- aagggctgg atgaacgaccccaacggcctgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaa- cgacacc gtctgggggacgcccttgttctggggccacgccacgtccgacgacctgaccaactgggaggaccagcccatcgc- catcgcccc gaagcgcaacgactccggcgccttctccggctccatggtggtggactacaacaacacctccggcttcttcaacg- acaccatcga cccgcgccagcgctgcgtggccatctggacctacaacaccccggagtccgaggagcagtacatctcctacagcc- tggacggcg gctacaccttcaccgagtaccagaagaaccccgtgctggccgccaactccacccagttccgcgacccgaaggtc- ttctggtacg agccctcccagaagtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccgacgac- ctgaagtcc tggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcctgatcgaggt- ccccaccga gcaggaccccagcaagtcctactgggtgatgttcatctccatcaaccccggcgccccggccggcggctccttca- accagtacttc gtcggcagcttcaacggcacccacttcgaggccttcgacaaccagtcccgcgtggtggacttcggcaaggacta- ctacgccctg cagaccttcttcaacaccgacccgacctacgggagcgccctgggcatcgcgtgggcctccaactgggagtactc- cgccttcgtg cccaccaacccctggcgctcctccatgtccctcgtgcgcaagttctccctcaacaccgagtaccaggccaaccc- ggagacggag ctgatcaacctgaaggccgagccgatcctgaacatcagcaacgccggcccctggagccggttcgccaccaacac- cacgttgac gaaggccaacagctacaacgtcgacctgtccaacagcaccggcaccctggagttcgagctggtgtacgccgtca- acaccacc cagacgatctccaagtccgtgttcgcggacctctccctctggttcaagggcctggaggaccccgaggagtacct- ccgcatgggc ttcgaggtgtccgcgtcctccttcttcctggaccgcgggaacagcaaggtgaagttcgtgaaggagaaccccta- cttcaccaac cgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaaggtgtacggcttgctgga- ccagaaca tcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacacctacttcatgaccaccgggaacgccctg- ggctccgtga acatgacgacgggggtggacaacctgttctacatcgacaagttccaggtgcgcgaggtcaagTGAcaattgGCA- GCAGCAG CTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCT- GTGAATA TCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGC- TTGTGCTA TTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGC- TGTCCTGCT ATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCC- TGGTACTG CAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAggatcccgcg- tctcga acagagcgcgcagaggaacgctgaaggtctcgcctctgtcgcacctcagcgcggcatacaccacaataaccacc- tgacgaatgcg cttggttcttcgtccattagcgaagcgtccggttcacacacgtgccacgttggcgaggtggcaggtgacaatga- tcggtggagctgat ##STR00482## ##STR00483## ##STR00484## ##STR00485## ##STR00486## ##STR00487## ##STR00488## ##STR00489## ##STR00490## ##STR00491## ##STR00492## ##STR00493## ##STR00494## ##STR00495## ##STR00496## ##STR00497## ##STR00498## actagtATGgccaccgcatccactttctcggcgttcaatgcccgctgcggcgacctgcgtcgctcggcgggctc- cgggccccgg cgcccagcgaggcccctccccgtgcgcgggcgcgccatccccccccgcatcatcgtggtgtcctcctcctcctc- caaggtgaaccc cctgaagaccgaggccgtggtgtcctccggcctggccgaccgcctgcgcctgggctccctgaccgaggacggcc- tgtcctaca aggagaagttcatcgtgcgctgctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacctg- ctgcagg aggtgggctgcaaccacgcccagtccgtgggctactccaccggcggcttctccaccacccccaccatgcgcaag- ctgcgcctga tctgggtgaccgcccgcatgcacatcgagatctacaagtaccccgcctggtccgacgtggtggagatcgagtcc- tggggccag ggcgagggcaagatcggcacccgccgcgactggatcctgcgcgactacgccaccggccaggtgatcggccgcgc- cacctcca agtgggtgatgatgaaccaggacacccgccgcctgcagaaggtggacgtggacgtgcgcgacgagtacctggtg- cactgcc cccgcgagctgcgcctggccttccccgaggagaacaactcctccctgaagaagatctccaagctggaggacccc- tcccagtac tccaagctgggcctggtgccccgccgcgccgacctggacatgaaccagcacgtgaacaacgtgacctacatcgg- ctgggtgct ggagtccatgccccaggagatcatcgacacccacgagctgcagaccatcaccctggactaccgccgcgagtgcc- agcacgac gacgtggtggactccctgacctcccccgagccctccgaggacgccgaggccgtgttcaaccacaacggcaccaa- cggctccgc caacgtgtccgccaacgaccacggctgccgcaacttcctgcacctgctgcgcctgtccggcaacggcctggaga- tcaaccgcg ##STR00499## ##STR00500## CGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCT- GCCGCTT TTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTG- CGAATACC ACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATC- CCTCAGCGC TGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAAC- CTGTAAAC CAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAAcAcAAATGGAaagcttaattaagagctcTTGT- TTTCC AGAAGGAGTTGCTCCTTGAGCCTTTCATTCTCAGCCTCGATAACCTCCAAAGCCGCTCTAATTGTGGA GGGGGTTCGAATTTAAAAGCTTGGAATGTTGGTTCGTGCGTCTGGAACAAGCCCAGACTTGTTGCTC ACTGGGAAAAGGACCATCAGCTCCAAAAAACTTGCCGCTCAAACCGCGTACCTCTGCTTTCGCGCAA TCTGCCCTGTTGAAATCGCCACCACATTCATATTGTGACGCTTGAGCAGTCTGTAATTGCCTCAGAAT GTGGAATCATCTGCCCCCTGTGCGAGCCCATGCCAGGCATGTCGCGGGCGAGGACACCCGCCACTC GTACAGCAGACCATTATGCTACCTCACAATAGTTCATAACAGTGACCATATTTCTCGAAGCTCCCCAA CGAGCACCTCCATGCTCTGAGTGGCCACCCCCCGGCCCTGGTGCTTGCGGAGGGCAGGTCAACCGG CATGGGGCTACCGAAATCCCCGACCGGATCCCACCACCCCCGCGATGGGAAGAATCTCTCCCCGGG ATGTGGGCCCACCACCAGCACAACCTGCTGGCCCAGGCGAGCGTCAAACCATACCACACAAATATCC TTGGCATCGGCCCTGAATTCCTTCTGCCGCTCTGCTACCCGGTGCTTCTGTCCGAAGCAGGGGTTGCT AGGGATCGCTCCGAGTCCGCAAACCCTTGTCGCGTGGCGGGGCTTGTTCGAGCTTgaagagc
[0716] The resulting strain was further transformed with a construct designed to recombine at (and thereby disrupt) the endogenous FATA and also express the LPAAT from B. napus under control of the UAPA1 promoter and using alpha galactosidase as a selectable marker with selection on melbiose. The resulting strain showed increased production of SOS (about 57-60%) and Sat-O-Sat (about 70-76%) and lower amounts of trisaturates (4.8 to 7.6%).
[0717] Strains were generated in the high-C18:0 S6573 background in which we maximized SOS production and minimized the formation of trisaturated TAGs by targeting both the Brassica napus LPAT2(Bnl.13) gene and the PmFAD2hpA RNAi construct to the FATA-1 locus. The sequence of the transforming DNA from the PmFAD2hpA expression construct pSZ4164 is shown below in SEQ ID NO:153. Relevant restriction sites are indicated in lowercase, bold, and are from 5'-3' BspQI, KpnI, SpeI, SnaBI, BamHI, NdeI, NsiI, AflII, EcoRI, SpeI, BsiWI, XhoI, SacI and BspQI. Underlined sequences at the 5' and 3' flanks of the construct represent genomic DNA from P. moriformis that enable targeted integration of the transforming DNA via homologous recombination at the FATA-1 locus. Proceeding in the 5' to 3' direction, the PmHXT1 promoter driving the expression of Saccharomyces carlbergensis MEL1 (ScarMEL1) gene, enabling strains to utilize exogenous melibiose, is indicated by lowercase, boxed text. The initiator ATG and terminator TGA of ScarMEL1 are indicated by uppercase italics, while the coding region is represented by lowercase italics. The 3' UTR of the P. moriformis PGK gene is indicated by small capitals. A spacer region is represented by lowercase text. The P. moriformis UAPA1 promoter driving the expression of the BnLPAT2(Bnl.13) gene is indicated by lowercase, boxed text. The initiator ATG and terminator TGA are indicated by uppercase italics; the sequence encoding BnLPAT2(Bnl.13) is represented by lowercase, underlined italics. The 3' UTR of the CvNR gene is indicated by small capitals. A second spacer region is represented by lowercase text. The C. reinhardtii CrTUB2 promoter driving the expression of the PmFAD2hpA hairpin sequence is indicated by lowercase, boxed text. The FAD2 exon 1 sequence in the forward orientation is indicated with lowercase italics; the FAD2 intron 1 sequence is represented with lowercase, bold italics; a short linker region is indicated with lowercase text, and the FAD2 exon 1 sequence in the reverse orientation is indicated with lowercase, underlined italics. A second CvNR 3' UTR is indicated by small capitals.
TABLE-US-00158 Nucleotide sequence of the transforming DNA from pSZ4164: (SEQ ID NO: 153) gctcttcCCAACTCAGATAATACCAATACCCCTCCTTCTCCTCCTCATCCATTCAGTACCCCCCCCCTTCTC TTCCCAAAGCAGCAAGCGCGTGGCTTACAGAAGAACAATCGGCTTCCGCCAAAGTCGCCGAGCACT GCCCGACGGCGGCGCGCCCAGCAGCCCGCTTGGCCACACAGGCAACGAATACATTCAATAGGGGG CCTCGCAGAATGGAAGGAGCGGTAAAGGGTACAGGAGCACTGCGCACAAGGGGCCTGTGCAGGA GTGACTGACTGGGCGGGCAGACGGCGCACCGCGGGCGCAGGCAAGCAGGGAAGATTGAAGCGGC AGGGAGGAGGATGCTGATTGAGGGGGGCATCGCAGTCTCTCTTGGACCCGGGATAAGGAAGCAAA TATTCGGCCGGTTGGGTTGTGTGTGTGCACGTTTTCTTCTTCAGAGTCGTGGGTGTGCTTCCAGGGA GGATATAAGCAGCAGGATCGAATCCCGCGACCAGCGTTTCCCCATCCAGCCAACCACCCTGTCggtac ##STR00501## ##STR00502## ##STR00503## ##STR00504## ##STR00505## ##STR00506## ##STR00507## ##STR00508## ##STR00509## ##STR00510## gtgttcggcgtctccccctcctacaacggcctgggcctgacgccccagatgggctgggacaactggaacacgtt- cgcctgcgac gtctccgagcagctgctgctggacacggccgaccgcatctccgacctgggcctgaaggacaatgggctacaagt- acatcatcct ggacgactgctggtcctccggccgcgactccgacggcttcctggtcgccgacgagcagaagttccccaacggca- tgggccacg tcgccgaccacctgcacaacaactccttcctgttcggcatgtactcctccgcgggcgagtacacgtgcgccggc- taccccggctc cctgggccgcgaggaggaggacgcccagttcttcgcgaacaaccgcgtggactacctgaatacgacaactgcta- caacaa gggccagttcggcacgcccgagatctcctaccaccgctacaaggccatgtccgacgccctgaacaagacgggcc- gccccatct tctactccctgtgcaactggggccaggacctgaccttctactggggctccggcatcgcgaactcctggcgcatg- tccggcgacgt cacggcggagttcacgcgccccgactcccgctgcccctgcgacggcgacgagtacgactgcaagtacgccggct- tccactgctc catcatgaacatcctgaacaaggccgcccccatgggccagaacgcgggcgtcggcggctggaacgacctggaca- acctgga ggtcggcgtcggcaacctgacggacgacgaggagaaggcgcacttctccatgtgggccatggtgaagtcccccc- tgatcatc ggcgcgaacgtgaacaacctgaaggcctcctcctactccatctgctcccaggcgtccgtcatcgccatcaacca- ggactccaac ggcatccccgccacgcgcgtctggcgctactacgtgtccgacacggacgagtacggccagggcgagatccagat- gtggtccg gccccctggacaacggcgaccaggtcgtggcgctgctgaacggcggctccgtgtcccgccccatgaacacgacc- ctggagga gatcttcttcgactccaacctgggctccaagaagctgacctccacctgggacatctacgacctgtgggcgaacc- gcgtcgacaa ctcccacggcgtccgccatcctgggccgcaacaagaccgccaccggcatcctgtacaacgccaccgagcagtcc- tacaaggac ggcctgtccaagaacgacacccgcctgttcggccagaagatcggctccctgtcccccaacgcgatcctgaacac- gaccgtcccc gcccacggcatcgcgttctaccgcctgcgcccctcctccTGAtacaacttattacgtaTTCTGACCGGCGCTGA- TGTGGCGCGG ACGCCGTCGTACTCTTTCAGACTTTACTCTTGAGGAATTGAACCTTTCTCGCTTGCTGGCATGTAAACATTGGC- GCAATTAA TTGTGTGATGAAGAAAGGGTGGCACAAGATGGATCGCGAATGTACGAGATCGACAACGATGGTGATTGTTATGA- GGGG CCAAACCTGGCTCAATCTTGTCGCATGTCCGGCGCAATGTGATCCAGCGGCGTGACTCTCGCAACCTGGTAGTG- TGTGGCG CACCGGGTCGCTTTGATTAAAACTGATCGCATTGCCATCCCGTCAACTCACAAGCCTACTCTAGCTCCCATTGC- GCACTCGG GCGCCCGGCTCGATCAATGTTCTGAGCGGAGGGCGAAGCGTCAGGAAATCGTCTCGGCAGCTGGAAGCGCATGG- AATGC GGAGCGGAGATCGAATCAggatcccgcgtctcgaacagagcgcgcagaggaacgctgaaggtctcgcctctgtc- gcacctcagc gcggcatacaccacaataaccacctgacgaatgcgcttggttcttcgtccattagcgaagcgtccggttcacac- acgtgccacgttg ##STR00511## ##STR00512## ##STR00513## ##STR00514## ##STR00515## ##STR00516## ##STR00517## ##STR00518## ##STR00519## ##STR00520## ##STR00521## ##STR00522## ##STR00523## ctgctgcaggccatctgctacgtgctgatccgccccctgtccaagaacacctaccgcaagatcaaccgcgtggt- ggccgagacc ctgtggctggagctggtgtggatcgtggactggtgggccggcgtgaagatccaggtgttcgccgacaacgagac- cttcaacc gcatgggcaaggagcacgccctggtggtgtgcaaccaccgctccgacatcgactggctggtgggctggatcctg- gcccagcg ctccggctgcctgggctccgccctggccgtgatgaagaagtcctccaagttcctgcccgtgatcggctggtcca- tgtggttctccg agtacctgttcctggagcgcaactgggccaaggacgagtccaccctgaagtccggcctgcagcgcctgaacgac- ttcccccgc cccttctggctggccctgttcgtggagggcacccgcttcaccgaggccaagctgaaggccgcccaggagtacgc- cgcctcctcc gagctgcccgtgccccgcaacgtgctgatcccccgcaccaagggcttcgtgtccgccgtgtccaacatgcgctc- cttcgtgcccg ccatctacgacatgaccgtggccatccccaagacctcccccccccccaccatgctgcgcctgttcaagggccag- ccctccgtggt gcacgtgcacatcaagtgccactccatgaaggacctgcccgagtccgacgacgccatcgcccagtggtgccgcg- accagttcg tggccaaggacgccctgctggacaagcacatcgccgccgacaccttccccggccagcaggagcagaacatcggc- cgccccat caagtccctggccgtggtgctgtcctggtcctgcctgctgatcctgggcgccatgaagttcctgactggtccaa- cctgttctcctc ctggaagggcatcgccttctccgccctgggcctgggcatcatcaccctgtgcatgcagatcctgatccgctcct- cccagtccgag cgctccacccccgccaggtggtgcccgccaagcccaaggacaaccacaacgactcggctcctcctcccagaccg- aggtgga gaagcagaagTGAatgcatGCAGCAGCAGCTCGGATGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACT- GTTG CCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTG- TGTGTACG CGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATAT- CGCTGCAT CCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCAC- AGCCTTGG TTTGGGCTCCGCCTGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAG- TGGGAT GGGAACACAAATGGActtaaggatctaagtaagattcgaagcgctcgaccgtgccggacggactgcagccccat- gtcgtagtga ccgccaatgtaagtgggctggcgtttccctgtacgtgagtcaacgtcactgcacgcgcaccaccctctcgaccg- gcaggaccaggca tcgcgagatacagcgcgagccagacacggagtgccgagctatgcgcacgctccaactagatatcatgtggatga- tgagcatgaatt ##STR00524## ##STR00525## ##STR00526## ##STR00527## gtggagaagcctccgttcacgatcgggacgctgcgcaaggccatccccgcgcactgtttcgagcgctcggcgct- tcgtagcag catgtacctggcctttgacatcgcggtcatgtccctgctctacgtcgcgtcgacgtacatcgaccctgcaccgg- tgcctacgtggg ##STR00528## ##STR00529## agtagagcggccacatgatgccgtacttgacccacgtaggcaccggtgcagggtcgatatacgtcgacacgaca- tagggca gggacatgaccgcgatgtcaaaggccaggtacatgctgctacgaagcgccgagcactcgaaacggtacgcgggg- atggcct tgcgcagcgtcccgatcgtgaacggaggcttctccacaggctgcctgttcgtcttgatagccatctcgagGCAG- CAGCAGCTCG GATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGA- ATATCCC TGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGT- GCTATTTG CGAATACCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTC- CTGCTATCC CTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGT- ACTGCAAC CTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAAAGCTGTAgagctc- ttgtttt ccagaaggagttgctccttgagcctttcattctcagcctcgataacctccaaagccgctctaattgtggagggg- gttcgaaCCGAA TGCTGCGTGAACGGGAAGGAGGAGGAGAAAGAGTGAGCAGGGAGGGATTCAGAAATGAGAAATG AGAGGTGAAGGAACGCATCCCTATGCCCTTGCAATGGACAGTGTTTCTGGCCACCGCCACCAAGACT TCGTGTCCTCTGATCATCATGCGATTGATTACGTTGAATGCGACGGCCGGTCAGCCCCGGACCTCCA CGCACCGGTGCTCCTCCAGGAAGATGCGCTTGTCCTCCGCCATCTTGCAGGGCTCAAGCTGCTCCCA AAACTCTTGGGCGGGTTCCGGACGGACGGCTACCGCGGGTGCGGCCCTGACCGCCACTGTTCGGAA GCAGCGGCGCTGCATGGGCAGCGGCCGCTGCGGTGCGCCACGGACCGCATGATCCACCGGAAAAG CGCACGCGCTGGAGCGCGCAGAGGACCACAGAGAAGCGGAAGAGACGCCAGTACTGGCAAGCAG GCTGGTCGGTGCCATGGCGCGCTACTACCCTCGCTATGACTCGGGTCCTCGGCCGGCTGGCGGTGCT GACAATTCGTTTAGTGGAGCAGCGACTCCATTCAGCTACCAGTCGAACTCAGTGGCACAGTGACTcc gctcttc
[0718] The described embodiments of the invention are intended to be merely exemplary and numerous variations and modifications will be apparent to those skilled in the art. All such variations and modifications are intended to be within the scope of the present invention. For example, the various triglyceride oils can be tailored in for a mixture of midchain and long chain fatty acids in order to adjust parameters such as polarity, solvency, and foam-height of the oils or chemicals made from the oils. In addition, where a knockout of a gene is called for, an equivalent result may be reached using knockdown techniques including mutation and expression of inhibitory substances such as RNAi or antisense.
TABLE-US-00159 SEQUENCE LISTING SEQ ID NO: 1 6S 5' genomic donor sequence GCTCTTCGCCGCCGCCACTCCTGCTCGAGCGCGCCCGCGCGTGCGCCGCCAGCGCCTTGGCCTTTTCG CCGCGCTCGTGCGCGTCGCTGATGTCCATCACCAGGTCCATGAGGTCTGCCTTGCGCCGGCTGAGCCA CTGCTTCGTCCGGGCGGCCAAGAGGAGCATGAGGGAGGACTCCTGGTCCAGGGTCCTGACGTGGTCGC GGCTCTGGGAGCGGGCCAGCATCATCTGGCTCTGCCGCACCGAGGCCGCCTCCAACTGGTCCTCCAGC AGCCGCAGTCGCCGCCGACCCTGGCAGAGGAAGACAGGTGAGGGGGGTATGAATTGTACAGAACAACC ACGAGCCTTGTCTAGGCAGAATCCCTACCAGTCATGGCTTTACCTGGATGACGGCCTGCGAACAGCTG TCCAGCGACCCTCGCTGCCGCCGCTTCTCCCGCACGCTTCTTTCCAGCACCGTGATGGCGCGAGCCAG CGCCGCACGCTGGCGCTGCGCTTCGCCGATCTGAGGACAGTCGGGGAACTCTGATCAGTCTAAACCCC CTTGCGCGTTAGTGTTGCCATCCTTTGCAGACCGGTGAGAGCCGACTTGTTGTGCGCCACCCCCCACA CCACCTCCTCCCAGACCAATTCTGTCACCTTTTTGGCGAAGGCATCGGCCTCGGCCTGCAGAGAGGAC AGCAGTGCCCAGCCGCTGGGGGTTGGCGGATGCACGCTCAGGTACC SEQ ID NO: 2 6S 3' genomic donor sequence GAGCTCCTTGTTTTCCAGAAGGAGTTGCTCCTTGAGCCTTTCATTCTCAGCCTCGATAACCTCCAAAG CCGCTCTAATTGTGGAGGGGGTTCGAATTTAAAAGCTTGGAATGTTGGTTCGTGCGTCTGGAACAAGC CCAGACTTGTTGCTCACTGGGAAAAGGACCATCAGCTCCAAAAAACTTGCCGCTCAAACCGCGTACCT CTGCTTTCGCGCAATCTGCCCTGTTGAAATCGCCACCACATTCATATTGTGACGCTTGAGCAGTCTGT AATTGCCTCAGAATGTGGAATCATCTGCCCCCTGTGCGAGCCCATGCCAGGCATGTCGCGGGCGAGGA CACCCGCCACTCGTACAGCAGACCATTATGCTACCTCACAATAGTTCATAACAGTGACCATATTTCTC GAAGCTCCCCAACGAGCACCTCCATGCTCTGAGTGGCCACCCCCCGGCCCTGGTGCTTGCGGAGGGCA GGTCAACCGGCATGGGGCTACCGAAATCCCCGACCGGATCCCACCACCCCCGCGATGGGAAGAATCTC TCCCCGGGATGTGGGCCCACCACCAGCACAACCTGCTGGCCCAGGCGAGCGTCAAACCATACCACACA AATATCCTTGGCATCGGCCCTGAATTCCTTCTGCCGCTCTGCTACCCGGTGCTTCTGTCCGAAGCAGG GGTTGCTAGGGATCGCTCCGAGTCCGCAAACCCTTGTCGCGTGGCGGGGCTTGTTCGAGCTTGAAGAG C SEQ ID NO: 3 S. cereviseae invertase protein sequence MLLQAFLFLLAGFAAKISASMTNETSDRPLVHFTPNKGWMNDPNGLWYDEKDAKWHLYFQYNPNDTVW GTPLFWGHATSDDLTNWEDQPIAIAPKRNDSGAFSGSMVVDYNNTSGFFNDTIDPRQRCVAIWTYNTP ESEEQYISYSLDGGYTFTEYQKNPVLAANSTQFRDPKVFWYEPSQKWIMTAAKSQDYKIETYSSDDLK SWKLESAFANEGFLGYQYECPGLIEVPTEQDPSKSYWVMFISINPGAPAGGSFNQYFVGSFNGTHFEA FDNQSRVVDFGKDYYALQTFFNTDPTYGSALGIAWASNWEYSAFVPTNPWRSSMSLVRKFSLNTEYQA NPETELINLKAEPILNISNAGPWSRFATNTTLTKANSYNVDLSNSTGTLEFELVYAVNTTQTISKSVF ADLSLWFKGLEDPEEYLRMGFEVSASSFFLDRGNSKVKFVKENPYFTNRMSVNNQPFKSENDLSYYKV YGLLDQNILELYFNDGDVVSTNTYFMTTGNALGSVNMTTGVDNLFYIDKFQVREVK SEQ ID NO: 4 S. cereviseae invertase protein coding sequence codon optimized for expression in P. moriformis (UTEX 1435) ATGctgctgcaggccttcctgttcctgctggccggcttcgccgccaagatcagcgcctccatgacgaa cgagacgtccgaccgccccctggtgcacttcacccccaacaagggctggatgaacgaccccaacggcc tgtggtacgacgagaaggacgccaagtggcacctgtacttccagtacaacccgaacgacaccgtctgg gggacgcccttgttctggggccacgccacgtccgacgacctgaccaactgggaggaccagcccatcgc catcgccccgaagcgcaacgactccggcgccttctccggctccatggtggtggactacaacaacacct ccggcttcttcaacgacaccatcgacccgcgccagcgctgcgtggccatctggacctacaacaccccg gagtccgaggagcagtacatctcctacagcctggacggcggctacaccttcaccgagtaccagaagaa ccccgtgctggccgccaactccacccagttccgcgacccgaaggtcttctggtacgagccctcccaga agtggatcatgaccgcggccaagtcccaggactacaagatcgagatctactcctccgacgacctgaag tcctggaagctggagtccgcgttcgccaacgagggcttcctcggctaccagtacgagtgccccggcct gatcgaggtccccaccgagcaggaccccagcaagtcctactgggtgatgttcatctccatcaaccccg gcgccccggccggcggctccttcaaccagtacttcgtcggcagcttcaacggcacccacttcgaggcc ttcgacaaccagtcccgcgtggtggacttcggcaaggactactacgccctgcagaccttcttcaacac cgacccgacctacgggagcgccctgggcatcgcgtgggcctccaactgggagtactccgccttcgtgc ccaccaacccctggcgctcctccatgtccctcgtgcgcaagttctccctcaacaccgagtaccaggcc aacccggagacggagctgatcaacctgaaggccgagccgatcctgaacatcagcaacgccggcccctg gagccggttcgccaccaacaccacgttgacgaaggccaacagctacaacgtcgacctgtccaacagca ccggcaccctggagttcgagctggtgtacgccgtcaacaccacccagacgatctccaagtccgtgttc gcggacctctccctctggttcaagggcctggaggaccccgaggagtacctccgcatgggcttcgaggt gtccgcgtcctccttcttcctggaccgcgggaacagcaaggtgaagttcgtgaaggagaacccctact tcaccaaccgcatgagcgtgaacaaccagcccttcaagagcgagaacgacctgtcctactacaaggtg tacggcttgctggaccagaacatcctggagctgtacttcaacgacggcgacgtcgtgtccaccaacac ctacttcatgaccaccgggaacgccctgggctccgtgaacatgacgacgggggtggacaacctgttct acatcgacaagttccaggtgcgcgaggtcaagTGA SEQ ID NO: 5 Chlamydomonas reinhardtii TUB2 (B-tub) promoter/5' UTR CTTTCTTGCGCTATGACACTTCCAGCAAAAGGTAGGGCGGGCTGCGAGACGGCTTCCCGGCGCTGCAT GCAACACCGATGATGCTTCGACCCCCCGAAGCTCCTTCGGGGCTGCATGGGCGCTCCGATGCCGCTCC AGGGCGAGCGCTGTTTAAATAGCCAGGCCCCCGATTGCAAAGACATTATAGCGAGCTACCAAAGCCAT ATTCAAACACCTAGATCACTACCACTTCTACACAGGCCACTCGAGCTTGTGATCGCACTCCGCTAAGG GGGCGCCTCTTCCTCTTCGTTTCAGTCACAACCCGCAAAC SEQ ID NO: 6 Chlorella vulgaris nitrate reductase 3'UTR GCAGCAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCACA CTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTGTG TGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTTCC CTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTGCT CCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTGCA ACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAAAGCTT SEQ ID NO: 7 Nucleotide sequence of the codon-optimized expression cassette of S. cerevisiae suc2 gene with C. reinhardtii .beta.-tubulin promoter/5'UTR and C. vulgaris nitrate reductase 3' UTR CTTTCTTGCGCTATGACACTTCCAGCAAAAGGTAGGGCGGGCTGCGAGACGGCTTCCCGGCGCTGCAT GCAACACCGATGATGCTTCGACCCCCCGAAGCTCCTTCGGGGCTGCATGGGCGCTCCGATGCCGCTCC AGGGCGAGCGCTGTTTAAATAGCCAGGCCCCCGATTGCAAAGACATTATAGCGAGCTACCAAAGCCAT ATTCAAACACCTAGATCACTACCACTTCTACACAGGCCACTCGAGCTTGTGATCGCACTCCGCTAAGG GGGCGCCTCTTCCTCTTCGTTTCAGTCACAACCCGCAAACGGCGCGCCATGCTGCTGCAGGCCTTCCT GTTCCTGCTGGCCGGCTTCGCCGCCAAGATCAGCGCCTCCATGACGAACGAGACGTCCGACCGCCCCC TGGTGCACTTCACCCCCAACAAGGGCTGGATGAACGACCCCAACGGCCTGTGGTACGACGAGAAGGAC GCCAAGTGGCACCTGTACTTCCAGTACAACCCGAACGACACCGTCTGGGGGACGCCCTTGTTCTGGGG CCACGCCACGTCCGACGACCTGACCAACTGGGAGGACCAGCCCATCGCCATCGCCCCGAAGCGCAACG ACTCCGGCGCCTTCTCCGGCTCCATGGTGGTGGACTACAACAACACCTCCGGCTTCTTCAACGACACC ATCGACCCGCGCCAGCGCTGCGTGGCCATCTGGACCTACAACACCCCGGAGTCCGAGGAGCAGTACAT CTCCTACAGCCTGGACGGCGGCTACACCTTCACCGAGTACCAGAAGAACCCCGTGCTGGCCGCCAACT CCACCCAGTTCCGCGACCCGAAGGTCTTCTGGTACGAGCCCTCCCAGAAGTGGATCATGACCGCGGCC AAGTCCCAGGACTACAAGATCGAGATCTACTCCTCCGACGACCTGAAGTCCTGGAAGCTGGAGTCCGC GTTCGCCAACGAGGGCTTCCTCGGCTACCAGTACGAGTGCCCCGGCCTGATCGAGGTCCCCACCGAGC AGGACCCCAGCAAGTCCTACTGGGTGATGTTCATCTCCATCAACCCCGGCGCCCCGGCCGGCGGCTCC TTCAACCAGTACTTCGTCGGCAGCTTCAACGGCACCCACTTCGAGGCCTTCGACAACCAGTCCCGCGT GGTGGACTTCGGCAAGGACTACTACGCCCTGCAGACCTTCTTCAACACCGACCCGACCTACGGGAGCG CCCTGGGCATCGCGTGGGCCTCCAACTGGGAGTACTCCGCCTTCGTGCCCACCAACCCCTGGCGCTCC TCCATGTCCCTCGTGCGCAAGTTCTCCCTCAACACCGAGTACCAGGCCAACCCGGAGACGGAGCTGAT CAACCTGAAGGCCGAGCCGATCCTGAACATCAGCAACGCCGGCCCCTGGAGCCGGTTCGCCACCAACA CCACGTTGACGAAGGCCAACAGCTACAACGTCGACCTGTCCAACAGCACCGGCACCCTGGAGTTCGAG CTGGTGTACGCCGTCAACACCACCCAGACGATCTCCAAGTCCGTGTTCGCGGACCTCTCCCTCTGGTT CAAGGGCCTGGAGGACCCCGAGGAGTACCTCCGCATGGGCTTCGAGGTGTCCGCGTCCTCCTTCTTCC TGGACCGCGGGAACAGCAAGGTGAAGTTCGTGAAGGAGAACCCCTACTTCACCAACCGCATGAGCGTG AACAACCAGCCCTTCAAGAGCGAGAACGACCTGTCCTACTACAAGGTGTACGGCTTGCTGGACCAGAA CATCCTGGAGCTGTACTTCAACGACGGCGACGTCGTGTCCACCAACACCTACTTCATGACCACCGGGA ACGCCCTGGGCTCCGTGAACATGACGACGGGGGTGGACAACCTGTTCTACATCGACAAGTTCCAGGTG CGCGAGGTCAAGTGACAATTGGCAGCAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTG TGATGGACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGC CTCAGTGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATA CCACCCCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTC CTGCTATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGC CIGTATTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGAT GGGAACACAAATGGAGGATCC SEQ ID NO: 8 Prototheca moriformis (UTEX 435) Amt03 promoter GGCCGACAGGACGCGCGTCAAAGGTGCTGGTCGTGTATGCCCTGGCCGGCAGGTCGTTGCTGCTGCTG GTTAGTGATTCCGCAACCCTGATTTTGGCGTCTTATTTTGGCGTGGCAAACGCTGGCGCCCGCGAGCC GGGCCGGCGGCGATGCGGTGCCCCACGGCTGCCGGAATCCAAGGGAGGCAAGAGCGCCCGGGTCAGTT GAAGGGCTTTACGCGCAAGGTACAGCCGCTCCTGCAAGGCTGCGTGGTGGAATTGGACGTGCAGGTCC TGCTGAAGTTCCTCCACCGCCTCACCAGCGGACAAAGCACCGGTGTATCAGGTCCGTGTCATCCACTC TAAAGAGCTCGACTACGACCTACTGATGGCCCTAGATTCTTCATCAAAAACGCCTGAGACACTTGCCC AGGATTGAAACTCCCTGAAGGGACCACCAGGGGCCCTGAGTTGTTCCTTCCCCCCGTGGCGAGCTGCC AGCCAGGCTGTACCTGTGATCGAGGCTGGCGGGAAAATAGGCTTCGTGTGCTCAGGTCATGGGAGGTG CAGGACAGCTCATGAAACGCCAACAATCGCACAATTCATGTCAAGCTAATCAGCTATTTCCTCTTCAC GAGCTGTAATTGTCCCAAAATTCTGGTCTACCGGGGGTGATCCTTCGTGTACGGGCCCTTCCCTCAAC CCTAGGTATGCGCGCATGCGGTCGCCGCGCAACTCGCGCGAGGGCCGAGGGTTTGGGACGGGCCGTCC CGAAATGCAGTTGCACCCGGATGCGTGGCACCTTTTTTGCGATAATTTATGCAATGGACTGCTCTGCA AAATTCTGGCTCTGTCGCCAACCCTAGGATCAGCGGCGTAGGATTTCGTAATCATTCGTCCTGATGGG GAGCTACCGACTACCCTAATATCAGCCCGACTGCCTGACGCCAGCGTCCACTTTTGTGCACACATTCC ATTCGTGCCCAAGACATTTCATTGTGGTGCGAAGCGTCCCCAGTTACGCTCACCTGTTTCCCGACCTC CTTACTGTTCTGTCGACAGAGCGGGCCCACAGGCCGGTCGCAGCC SEQ ID NO: 9 Chlorella protothecoides (UTEX 250) stearoyl ACP desaturase transit peptide cDNA sequence codon optimized for expression in P. moriformis. ACTAGTATGGCCACCGCATCCACTTTCTCGGCGTTCAATGCCCGCTGCGGCGACCTGCGTCGCTCGGC GGGCTCCGGGCCCCGGCGCCCAGCGAGGCCCCTCCCCGTGCGCGGGCGCGCC SEQ ID NO: 10 Cuphea wrightii FatB2 thioesterase nucleic acid sequence; Gen Bank Accession No. U56104 ATGGTGGTGGCCGCCGCCGCCAGCAGCGCCTTCTTCCCCGTGCCCGCCCCCCGCCCCACCCCCAAGCC CGGCAAGTTCGGCAACTGGCCCAGCAGCCTGAGCCAGCCCTTCAAGCCCAAGAGCAACCCCAACGGCC GCTTCCAGGTGAAGGCCAACGTGAGCCCCCACGGGCGCGCCCCCAAGGCCAACGGCAGCGCCGTGAGC CTGAAGTCCGGCAGCCTGAACACCCTGGAGGACCCCCCCAGCAGCCCCCCCCCCCGCACCTTCCTGAA CCAGCTGCCCGACTGGAGCCGCCTGCGCACCGCCATCACCACCGTGTTCGTGGCCGCCGAGAAGCAGT TCACCCGCCTGGACCGCAAGAGCAAGCGCCCCGACATGCTGGTGGACTGGTTCGGCAGCGAGACCATC GTGCAGGACGGCCTGGTGTTCCGCGAGCGCTTCAGCATCCGCAGCTACGAGATCGGCGCCGACCGCAC CGCCAGCATCGAGACCCTGATGAACCACCTGCAGGACACCAGCCTGAACCACTGCAAGAGCGTGGGCC TGCTGAACGACGGCTTCGGCCGCACCCCCGAGATGTGCACCCGCGACCTGATCTGGGTGCTGACCAAG ATGCAGATCGTGGTGAACCGCTACCCCACCTGGGGCGACACCGTGGAGATCAACAGCTGGTTCAGCCA GAGCGGCAAGATCGGCATGGGCCGCGAGTGGCTGATCAGCGACTGCAACACCGGCGAGATCCTGGTGC GCGCCACCAGCGCCTGGGCCATGATGAACCAGAAGACCCGCCGCTTCAGCAAGCTGCCCTGCGAGGTG CGCCAGGAGATCGCCCCCCACTTCGTGGACGCCCCCCCCGTGATCGAGGACAACGACCGCAAGCTGCA CAAGTTCGACGTGAAGACCGGCGACAGCATCTGCAAGGGCCTGACCCCCGGCTGGAACGACTTCGACG TGAACCAGCACGTGAGCAACGTGAAGTACATCGGCTGGATTCTGGAGAGCATGCCCACCGAGGTGCTG GAGACCCAGGAGCTGTGCAGCCTGACCCTGGAGTACCGCCGCGAGTGCGGCCGCGAGAGCGTGGTGGA GAGCGTGACCAGCATGAACCCCAGCAAGGTGGGCGACCGCAGCCAGTACCAGCACCTGCTGCGCCTGG AGGACGGCGCCGACATCATGAAGGGCCGCACCGAGTGGCGCCCCAAGAACGCCGGCACCAACCGCGCC ATCAGCACCTGA SEQ ID NO: 11 Cuphea wrightii FatB2 thioesterase amino acid sequence; Gen Bank Accession No. U56104 MVVAAAASSAFFPVPAPRPTPKPGKFGNWPSSLSQPFKPKSNPNGRFQVKANVSPHPKANGSAVSLKS GSLNTLEDPPSSPPPRTFLNQLPDWSRLRTAITTVFVAAEKQFTRLDRKSKRPDMLVDWFGSETIVQD GLVFRERFSIRSYEIGADRTASIETLMNHLQDTSLNHCKSVGLLNDGFGRTPEMCTRDLIWVLTKMQI VVNRYPTWGDIVEINSWFSQSGKIGMGREWLISDCNTGEILVRATSAWAMMNQKTRRESKLPCEVRQE IAPHFVDAPPVIEDNDRKLHKFDVKTGDSICKGLTPGWNDFDVNQHVSNVKYIGWILESMPTEVLETQ ELCSLTLEYRRECGRESVVESVTSMNPSKVGDRSQYQHLLRLEDGADIMKGRTEWRPKNAGTNRAIST SEQ ID NO: 12 Codon-optimized coding region of Cocus nucifera C12:0-preferring LPAAT from pSZ2046 ATGGACGCCTCCGGCGCCTCCTCCTTCCTGCGCGGCCGCTGCCTGGAGTCCTGCTTCAAGGCCTCCTT CGGCTACGTAATGTCCCAGCCCAAGGACGCCGCCGGCCAGCCCTCCCGCCGCCCCGCCGACGCCGACG ACTTCGTGGACGACGACCGCTGGATCACCGTGATCCTGTCCGTGGTGCGCATCGCCGCCTGCTTCCTG TCCATGATGGTGACCACCATCGTGTGGAACATGATCATGCTGATCCTGCTGCCCTGGCCCTACGCCCG CATCCGCCAGGGCAACCTGTACGGCCACGTGACCGGCCGCATGCTGATGTGGATTCTGGGCAACCCCA TCACCATCGAGGGCTCCGAGTTCTCCAACACCCGCGCCATCTACATCTGCAACCACGCCTCCCTGGTG GACATCTTCCTGATCATGTGGCTGATCCCCAAGGGCACCGTGACCATCGCCAAGAAGGAGATCATCTG GTATCCCCTGTTCGGCCAGCTGTACGTGCTGGCCAACCACCAGCGCATCGACCGCTCCAACCCCTCCG CCGCCATCGAGTCCATCAAGGAGGTGGCCCGCGCCGTGGTGAAGAAGAACCTGTCCCTGATCATCTTC CCCGAGGGCACCCGCTCCAAGACCGGCCGCCTGCTGCCCTTCAAGAAGGGCTTCATCCACATCGCCCT CCAGACCCGCCTGCCCATCGTGCCGATGGTGCTGACCGGCACCCACCTGGCCTGGCGCAAGAACTCCC TGCGCGTGCGCCCCGCCCCCATCACCGTGAAGTACTTCTCCCCCATCAAGACCGACGACTGGGAGGAG GAGAAGATCAACCACTACGTGGAGATGATCCACGCCCTGTACGTGGACCACCTGCCCGAGTCCCAGAA GCCCCTGGTGTCCAAGGGCCGCGACGCCTCCGGCCGCTCCAACTCCTGA SEQ ID NO: 13 pLoop 5' genomic donor sequence gctcttcgctaacggaggtctgtcaccaaatggaccccgtctattgcgggaaaccacggcgatggcac gtttcaaaacttgatgaaatacaatattcagtatgtcgcgggcggcgacggcggggagctgatgtcgc gctgggtattgcttaatcgccagcttcgcccccgtcttggcgcgaggcgtgaacaagccgaccgatgt gcacgagcaaatcctgacactagaagggctgactcgcccggcacggctgaattacacaggcttgcaaa aataccagaatttgcacgcaccgtattcgcggtattttgttggacagtgaatagcgatgcggcaatgg cttgtggcgttagaaggtgcgacgaaggtggtgccaccactgtgccagccagtcctggcggctcccag ggccccgatcaagagccaggacatccaaactacccacagcatcaacgccccggcctatactcgaaccc cacttgcactctgcaatggtatgggaaccacggggcagtcttgtgtgggtcgcgcctatcgcggtcgg cgaagaccgggaaggtacc SEQ ID NO: 14 pLoop 3' genomic donor sequence gagctcagcggcgacggtcctgctaccgtacgacgttgggcacgcccatgaaagtttgtataccgagc ttgttgagcgaactgcaagcgcggctcaaggatacttgaactcctggattgatatcggtccaataatg gatggaaaatccgaacctcgtgcaagaactgagcaaacctcgttacatggatgcacagtcgccagtcc aatgaacattgaagtgagcgaactgttcgcttcggtggcagtactactcaaagaatgagctgctgtta aaaatgcactctcgttctctcaagtgagtggcagatgagtgctcacgccttgcacttcgctgcccgtg tcatgccctgcgccccaaaatttgaaaaaagggatgagattattgggcaatggacgacgtcgtcgctc cgggagtcaggaccggcggaaaataagaggcaacacactccgcttcttagctcttcc SEQ ID NO: 15 NeoR expression cassette including C. reinhardtii p-tubulin promoter/5'UTR and C. vulgaris nitrate reductase 3' UTR ##STR00530## gcctccacgccggctcccccgccgcctgggtggagcgcctgttcggctacgactgggcccagcagacc atcggctgctccgacgccgccgtgttccgcctgtccgcccagggccgccccgtgctgttcgtgaagac cgacctgtccggcgccctgaacgagctgcaggacgaggccgcccgcctgtcctggctggccaccaccg gcgtgccctgcgccgccgtgctggacgtggtgaccgaggccggccgcgactggctgctgctgggcgag gtgcccggccaggacctgctgtcctcccacctggcccccgccgagaaggtgtccatcatggccgacgc catgcgccgcctgcacaccctggaccccgccacctgccccttcgaccaccaggccaagcaccgcatcg agcgcgcccgcacccgcatggaggccggcctggtggaccaggacgacctggacgaggagcaccagggc ctggcccccgccgagctgttcgcccgcctgaaggcccgcatgcccgacggcgaggacctggtggtgac ccacggcgacgcctgcctgcccaacatcatggtggagaacggccgcttctccggcttcatcgactgcg gccgcctgggcgtggccgaccgctaccaggacatcgccctggccacccgcgacatcgccgaggagctg ggcggcgagtgggccgaccgcttcctggtgctgtacggcatcgccgcccccgactcccagcgcatcgc cttctaccgcctgctggacgagttcttcTGAcaattggcagcagcagctoggatagtatcgacacact ctggacgctggtcgtgtgatggactgttgccgccacacttgctgccttgacctgtgaatatccctgcc gcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgcgcttttgcgagttgctagctgctt gtgctatttgcgaataccacccccagcatccccttccctcgtttcatatcgcttgcatcccaaccgca acttatctacgctgtcctgctatccctcagcgctgctcctgctcctgctcactgcccctcgcacagcc ttggtttgggctccgcctgtattctcctggtactgcaacctgtaaaccagcactgcaatgctgatgca cgggaagtagtgggatgggaacacaaatggaggatcc
SEQ ID NO: 16 Cocos nucifera 1-acyl-sn-glycerol-3-phosphate acyltransferase (LPAAT) MDASGASSFLRGRCLESCFKASFGYVMSQPKDAAGQPSRRPADADDFVDDDRWITVILSV VRIAACFLSMMVITIVWNMIMLILLPWPYARIRQGNLYGHVTGRMLMWILGNPITIEGSE FSNTRAIYICNHASLVDIFLIMMLIPKGIVTIAKKEIIWYPLFGQLYVLANHQRIDRSNP SAAIESIKEVARAVVKKNLSLIIFPEGTRSKTGRLLPFKKGFIHIALQTRLPIVPMVLTG THLAWRKNSLRVRPAPITVKYFSPIKTDDWEEEKINHYVEMIHALYVDHLPESQKPLVSK GRDASGRSNS SEQ ID NO: 17 pSZ1500 GGGCTGGTCTGAATCCTTCAGGCGGGTGTTACCCGAGAAAGAAAGGGTGCCGATTTCAAAGCAGACCC ATGTGCCGGGCCCTGTGGCCTGTGTTGGCGCCTATGTAGTCACCCCCCCTCACCCAATTGTCGCCAGT TTGCGCACTCCATAAACTCAAAACAGCAGCTTCTGAGCTGCGCTGTTCAAGAACACCTCTGGGGTTTG CTCACCCGCGAGGTCGACGCCCAGCATGGCTATCAAGACGAACAGGCAGCCTGTGGAGAAGCCTCCGT TCACGATCGGGACGCTGCGCAAGGCCATCCCCGCGCACTGTTTCGAGCGCTCGGCGCTTCGTAGCAGC ATGTACCTGGCCTTTGACATCGCGGTCATGTCCCTGCTCTACGTCGCGTCGACGTACATCGACCCTGC ACCGGTGCCTACGTGGGTCAAGTACGGCATCATGTGGCCGCTCTACTGGTTCTTCCAGGTGTGTTTGA GGGTTTTGGTTGCCCGTATTGAGGTCCTGGTGGCGCGCATGGAGGAGAAGGCGCCTGTCCCGCTGACC CCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCCTTCGGCACGGGTGTCTGGGTGTGCGCGCACGAG TGCGGCCACCAGGCCTTTTCCTCCAGCCAGGCCATCAACGACGGCGTGGGCCTGGTGTTCCACAGCCT GCTGCTGGTGCCCTACTACTCCTGGAAGCACTCGCACCGGGTACCCTTTCTTGCGCTATGACACTTCC AGCAAAAGGTAGGGCGGGCTGCGAGACGGCTTCCCGGCGCTGCATGCAACACCGATGATGCTTCGACC CCCCGAAGCTCCTTCGGGGCTGCATGGGCGCTCCGATGCCGCTCCAGGGCGAGCGCTGTTTAAATAGC CAGGCCCCCGATTGCAAAGACATTATAGCGAGCTACCAAAGCCATATTCAAACACCTAGATCACTACC ACTTCTACACAGGCCACTCGAGCTTGTGATCGCACTCCGCTAAGGGGGCGCCTCTTCCTCTTCGTTTC AGTCACAACCCGCAAACTCTAGAATATCAATGCTGCTGCAGGCCTTCCTGTTCCTGCTGGCCGGCTTC GCCGCCAAGATCAGCGCCTCCATGACGAACGAGACGTCCGACCGCCCCCTGGTGCACTTCACCCCCAA CAAGGGCTGGATGAACGACCCCAACGGCCTGTGGTACGACGAGAAGGACGCCAAGTGGCACCTGTACT TCCAGTACAACCCGAACGACACCGTCTGGGGGACGCCCTTGTTCTGGGGCCACGCCACGTCCGACGAC CTGACCAACTGGGAGGACCAGCCCATCGCCATCGCCCCGAAGCGCAACGACTCCGGCGCCTTCTCCGG CTCCATGGTGGTGGACTACAACAACACCTCCGGCTTCTTCAACGACACCATCGACCCGCGCCAGCGCT GCGTGGCCATCTGGACCTACAACACCCCGGAGTCCGAGGAGCAGTACATCTCCTACAGCCTGGACGGC GGCTACACCTTCACCGAGTACCAGAAGAACCCCGTGCTGGCCGCCAACTCCACCCAGTTCCGCGACCC GAAGGTCTTCTGGTACGAGCCCTCCCAGAAGTGGATCATGACCGCGGCCAAGTCCCAGGACTACAAGA TCGAGATCTACTCCTCCGACGACCTGAAGTCCTGGAAGCTGGAGTCCGCGTTCGCCAACGAGGGCTTC CTCGGCTACCAGTACGAGTGCCCCGGCCTGATCGAGGTCCCCACCGAGCAGGACCCCAGCAAGTCCTA CTGGGTGATGTTCATCTCCATCAACCCCGGCGCCCCGGCCGGCGGCTCCTTCAACCAGTACTTCGTCG GCAGCTTCAACGGCACCCACTTCGAGGCCTTCGACAACCAGTCCCGCGTGGTGGACTTCGGCAAGGAC TACTACGCCCTGCAGACCTTCTTCAACACCGACCCGACCTACGGGAGCGCCCTGGGCATCGCGTGGGC CTCCAACTGGGAGTACTCCGCCTTCGTGCCCACCAACCCCTGGCGCTCCTCCATGTCCCTCGTGCGCA AGTTCTCCCTCAACACCGAGTACCAGGCCAACCCGGAGACGGAGCTGATCAACCTGAAGGCCGAGCCG ATCCTGAACATCAGCAACGCCGGCCCCTGGAGCCGGTTCGCCACCAACACCACGTTGACGAAGGCCAA CAGCTACAACGTCGACCTGTCCAACAGCACCGGCACCCTGGAGTTCGAGCTGGTGTACGCCGTCAACA CCACCCAGACGATCTCCAAGTCCGTGTTCGCGGACCTCTCCCTCTGGTTCAAGGGCCTGGAGGACCCC GAGGAGTACCTCCGCATGGGCTTCGAGGTGTCCGCGTCCTCCTTCTTCCTGGACCGCGGGAACAGCAA GGTGAAGTTCGTGAAGGAGAACCCCTACTTCACCAACCGCATGAGCGTGAACAACCAGCCCTTCAAGA GCGAGAACGACCTGTCCTACTACAAGGTGTACGGCTTGCTGGACCAGAACATCCTGGAGCTGTACTTC AACGACGGCGACGTCGTGTCCACCAACACCTACTTCATGACCACCGGGAACGCCCTGGGCTCCGTGAA CATGACGACGGGGGTGGACAACCTGTTCTACATCGACAAGTTCCAGGTGCGCGAGGTCAAGTGACAAT TGGCAGCAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATGGACTGTTGCCGCCA CACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAGTGTGTTTGATCTTG TGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACCCCCAGCATCCCCTT CCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCTATCCCTCAGCGCTG CTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTATTCTCCTGGTACTG CAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAACACAAATGGAGGAT CCCGCGTCTCGAACAGAGCGCGCAGAGGAACGCTGAAGGTCTCGCCTCTGTCGCACCTCAGCGCGGCA TACACCACAATAACCACCTGACGAATGCGCTTGGTTCTTCGTCCATTAGCGAAGCGTCCGGTTCACAC ACGTGCCACGTTGGCGAGGTGGCAGGTGACAATGATCGGTGGAGCTGATGGTCGAAACGTTCACAGCC TAGGGATATCGAATTCGGCCGACAGGACGCGCGTCAAAGGTGCTGGTCGTGTATGCCCTGGCCGGCAG GTCGTTGCTGCTGCTGGTTAGTGATTCCGCAACCCTGATTTTGGCGTCTTATTTTGGCGTGGCAAACG CTGGCGCCCGCGAGCCGGGCCGGCGGCGATGCGGTGCCCCACGGCTGCCGGAATCCAAGGGAGGCAAG AGCGCCCGGGTCAGTTGAAGGGCTTTACGCGCAAGGTACAGCCGCTCCTGCAAGGCTGCGTGGTGGAA TTGGACGTGCAGGTCCTGCTGAAGTTCCTCCACCGCCTCACCAGCGGACAAAGCACCGGTGTATCAGG TCCGTGTCATCCACTCTAAAGAACTCGACTACGACCTACTGATGGCCCTAGATTCTTCATCAAAAACG CCTGAGACACTTGCCCAGGATTGAAACTCCCTGAAGGGACCACCAGGGGCCCTGAGTTGTTCCTTCCC CCCGTGGCGAGCTGCCAGCCAGGCTGTACCTGTGATCGAGGCTGGCGGGAAAATAGGCTTCGTGTGCT CAGGTCATGGGAGGTGCAGGACAGCTCATGAAACGCCAACAATCGCACAATTCATGTCAAGCTAATCA GCTATTTCCTCTTCACGAGCTGTAATTGTCCCAAAATTCTGGTCTACCGGGGGTGATCCTTCGTGTAC GGGCCCTTCCCTCAACCCTAGGTATGCGCGCATGCGGTCGCCGCGCAACTCGCGCGAGGGCCGAGGGT TTGGGACGGGCCGTCCCGAAATGCAGTTGCACCCGGATGCGTGGCACCTTTTTTGCGATAATTTATGC AATGGACTGCTCTGCAAAATTCTGGCTCTGTCGCCAACCCTAGGATCAGCGGCGTAGGATTTCGTAAT CATTCGTCCTGATGGGGAGCTACCGACTACCCTAATATCAGCCCGACTGCCTGACGCCAGCGTCCACT TTIGTGCACACATTCCATTCGTGCCCAAGACATTTCATTGTGGTGCGAAGCGTCCCCAGTTACGCTCA CCTGTTTCCCGACCTCCTTACTGTTCTGTCGACAGAGCGGGCCCACAGGCCGGTCGCAGCCACTAGTA TGGCCACCGCATCCACTTTCTCGGCGTTCAATGCCCGCTGCGGCGACCTGCGTCGCTCGGCGGGCTCC GGGCCCCGGCGCCCAGCGAGGCCCCTCCCCGTGCGCGGGCGCGCCGCCACCGGCGAGCAGCCCTCCGG CGTGGCCTCCCTGCGCGAGGCCGACAAGGAGAAGTCCCTGGGCAACCGCCTGCGCCTGGGCTCCCTGA CCGAGGACGGCCTGTCCTACAAGGAGAAGTTCGTGATCCGCTGCTACGAGGTGGGCATCAACAAGACC GCCACCATCGAGACCATCGCCAACCTGCTGCAGGAGGTGGGCGGCAACCACGCCCAGGGCGTGGGCTT CTCCACCGACGGCTTCGCCACCACCACCACCATGCGCAAGCTGCACCTGATCTGGGTGACCGCCCGCA TGCACATCGAGATCTACCGCTACCCCGCCTGGTCCGACGTGATCGAGATCGAGACCTGGGTGCAGGGC GAGGGCAAGGTGGGCACCCGCCGCGACTGGATCCTGAAGGACTACGCCAACGGCGAGGTGATCGGCCG CGCCACCTCCAAGTGGGTGATGATGAACGAGGACACCCGCCGCCTGCAGAAGGTGTCCGACGACGTGC GCGAGGAGTACCTGGTGTTCTGCCCCCGCACCCTGCGCCTGGCCTTCCCCGAGGAGAACAACAACTCC ATGAAGAAGATCCCCAAGCTGGAGGACCCCGCCGAGTACTCCCGCCTGGGCCTGGTGCCCCGCCGCTC CGACCTGGACATGAACAAGCACGTGAACAACGTGACCTACATCGGCTGGGCCCTGGAGTCCATCCCCC CCGAGATCATCGACACCCACGAGCTGCAGGCCATCACCCTGGACTACCGCCGCGAGTGCCAGCGCGAC GACATCGTGGACTCCCTGACCTCCCGCGAGCCCCTGGGCAACGCCGCCGGCGTGAAGTTCAAGGAGAT CAACGGCTCCGTGTCCCCCAAGAAGGACGAGCAGGACCTGTCCCGCTTCATGCACCTGCTGCGCTCCG CCGGCTCCGGCCTGGAGATCAACCGCTGCCGCACCGAGTGGCGCAAGAAGCCCGCCAAGCGCATGGAC TACAAGGACCACGACGGCGACTACAAGGACCACGACATCGACTACAAGGACGACGACGACAAGTGAAT CGATAGATCTCTTAAGGCAGCAGCAGCTCGGATAGTATCGACACACTCTGGACGCTGGTCGTGTGATG GACTGTTGCCGCCACACTTGCTGCCTTGACCTGTGAATATCCCTGCCGCTTTTATCAAACAGCCTCAG TGTGTTTGATCTTGTGTGTACGCGCTTTTGCGAGTTGCTAGCTGCTTGTGCTATTTGCGAATACCACC CCCAGCATCCCCTTCCCTCGTTTCATATCGCTTGCATCCCAACCGCAACTTATCTACGCTGTCCTGCT ATCCCTCAGCGCTGCTCCTGCTCCTGCTCACTGCCCCTCGCACAGCCTTGGTTTGGGCTCCGCCTGTA TTCTCCTGGTACTGCAACCTGTAAACCAGCACTGCAATGCTGATGCACGGGAAGTAGTGGGATGGGAA CACAAATGGAAAGCTTAATTAAGAGCTCCCGCCACCACTCCAACACGGGGTGCCTGGACAAGGACGAG GTGTTTGTGCCGCCGCACCGCGCAGTGGCGCACGAGGGCCTGGAGTGGGAGGAGTGGCTGCCCATCCG CATGGGCAAGGTGCTGGTCACCCTGACCCTGGGCTGGCCGCTGTACCTCATGTTCAACGTCGCCTCGC GGCCGTACCCGCGCTTCGCCAACCACTTTGACCCGTGGTCGCCCATCTTCAGCAAGCGCGAGCGCATC GAGGTGGTCATCTCCGACCTGGCGCTGGTGGCGGTGCTCAGCGGGCTCAGCGTGCTGGGCCGCACCAT GGGCTGGGCCTGGCTGGTCAAGACCTACGTGGTGCCCTACCTGATCGTGAACATGTGGCTCGTGCTCA TCACGCTGCTCCAGCACACGCACCCGGCGCTGCCGCACTACTTCGAGAAGGACTGGGACTGGCTGCGC GGCGCCATGGCCACCGTGGACCGCTCCATGGGCCCGCCCTTCATGGACAACATCCTGCACCACATCTC CGACACCCACGTGCTGCACCACCTCTTCAGCACCATCCCGCACTACCACGCCGAGGAGGCCTCCGCCG CCATCAGGCCCATCCTGGGCAAGTACTACCAGTCCGACAGCCGCTGGGTCGGCCGCGCCCTGTGGGAG GACTGGCGCGACTGCCGCTACGTCGTCCCGGACGCGCCCGAGGACGACTCCGCGCTCTGGTTCCACAA GTGAGTGAGTGA SEQ ID NO: 18 5' FADc genomic region donor DNA GGGCTGGTCTGAATCCTTCAGGCGGGTGTTACCCGAGAAAGAAAGGGTGCCGATTTCAAAGCAGACCC ATGTGCCGGGCCCTGTGGCCTGTGTTGGCGCCTATGTAGTCACCCCCCCTCACCCAATTGTCGCCAGT TTGCGCACTCCATAAACTCAAAACAGCAGCTTCTGAGCTGCGCTGTTCAAGAACACCTCTGGGGTTTG CTCACCCGCGAGGTCGACGCCCAGCATGGCTATCAAGACGAACAGGCAGCCTGTGGAGAAGCCTCCGT TCACGATCGGGACGCTGCGCAAGGCCATCCCCGCGCACTGTTTCGAGCGCTCGGCGCTTCGTAGCAGC ATGTACCTGGCCTTTGACATCGCGGTCATGTCCCTGCTCTACGTCGCGTCGACGTACATCGACCCTGC ACCGGTGCCTACGTGGGTCAAGTACGGCATCATGTGGCCGCTCTACTGGTTCTTCCAGGTGTGTTTGA GGGTTTTGGTTGCCCGTATTGAGGTCCTGGTGGCGCGCATGGAGGAGAAGGCGCCTGTCCCGCTGACC CCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCCTTCGGCACGGGTGTCTGGGTGTGCGCGCACGAG TGCGGCCACCAGGCCTTTTCCTCCAGCCAGGCCATCAACGACGGCGTGGGCCTGGTGTTCCACAGCCT GCTGCTGGTGCCCTACTACTCCTGGAAGCACTCGCACCG SEQ ID NO: 19 3' FADc genomic region donor DNA CCGCCACCACTCCAACACGGGGTGCCTGGACAAGGACGAGGTGTTTGTGCCGCCGCACCGCGCAGTGG CGCACGAGGGCCTGGAGTGGGAGGAGTGGCTGCCCATCCGCATGGGCAAGGTGCTGGTCACCCTGACC CIGGGCTGGCCGCTGTACCTCATGTTCAACGTCGCCTCGCGGCCGTACCCGCGCTTCGCCAACCACTT TGACCCGTGGTCGCCCATCTTCAGCAAGCGCGAGCGCATCGAGGTGGTCATCTCCGACCTGGCGCTGG TGGCGGTGCTCAGCGGGCTCAGCGTGCTGGGCCGCACCATGGGCTGGGCCTGGCTGGTCAAGACCTAC GTGGTGCCCTACCTGATCGTGAACATGTGGCTCGTGCTCATCACGCTGCTCCAGCACACGCACCCGGC GCTGCCGCACTACTTCGAGAAGGACTGGGACTGGCTGCGCGGCGCCATGGCCACCGTGGACCGCTCCA TGGGCCCGCCCTTCATGGACAACATCCTGCACCACATCTCCGACACCCACGTGCTGCACCACCTCTTC AGCACCATCCCGCACTACCACGCCGAGGAGGCCTCCGCCGCCATCAGGCCCATCCTGGGCAAGTACTA CCAGTCCGACAGCCGCTGGGTCGGCCGCGCCCTGTGGGAGGACTGGCGCGACTGCCGCTACGTCGTCC CGGACGCGCCCGAGGACGACTCCGCGCTCTGGTTCCACAAGTGAGTGAGTGA SEQ ID NO: 20 5' donor DNA sequence of Prototheca moriformis FATA1 knockout homologous recombination targeting construct GCTCTTCGGAGTCACTGTGCCACTGAGTTCGACTGGTAGCTGAATGGAGTCGCTGCTCCACTAAACGA ATTGTCAGCACCGCCAGCCGGCCGAGGACCCGAGTCATAGCGAGGGTAGTAGCGCGCCATGGCACCGA CCAGCCTGCTTGCCAGTACTGGCGTCTCTTCCGCTTCTCTGTGGTCCTCTGCGCGCTCCAGCGCGTGC GCTTTTCCGGTGGATCATGCGGTCCGTGGCGCACCGCAGCGGCCGCTGCCCATGCAGCGCCGCTGCTT CCGAACAGTGGCGGTCAGGGCCGCACCCGCGGTAGCCGTCCGTCCGGAACCCGCCCAAGAGTTTTGGG AGCAGCTTGAGCCCTGCAAGATGGCGGAGGACAAGCGCATCTTCCTGGAGGAGCACCGGTGCGTGGAG GTCCGGGGCTGACCGGCCGTCGCATTCAACGTAATCAATCGCATGATGATCAGAGGACACGAAGTCTT GGTGGCGGTGGCCAGAAACACTGTCCATTGCAAGGGCATAGGGATGCGTTCCTTCACCTCTCATTTCT CATTTCTGAATCCCTCCCTGCTCACTCTTTCTCCTCCTCCTTCCCGTTCACGCAGCATTCGGGGTACC SEQ ID NO: 21 3' donor DNA sequence of Prototheca moriformis FATA1 knockout homologous recombination targeting construct GACAGGGTGGTTGGCTGGATGGGGAAACGCTGGTCGCGGGATTCGATCCTGCTGCTTATATCCICCCT GGAAGCACACCCACGACTCTGAAGAAGAAAACGTGCACACACACAACCCAACCGGCCGAATATTTGCT TCCTTATCCCGGGTCCAAGAGAGACTGCGATGCCCCCCTCAATCAGCATCCTCCTCCCTGCCGCTTCA ATCTTCCCTGCTTGCCTGCGCCCGCGGTGCGCCGTCTGCCCGCCCAGTCAGTCACTCCTGCACAGGCC CCTTGTGCGCAGTGCTCCTGTACCCTTTACCGCTCCTTCCATTCTGCGAGGCCCCCTATTGAATGTAT TCGTTGCCTGTGTGGCCAAGCGGGCTGCTGGGCGCGCCGCCGTCGGGCAGTGCTCGGCGACTTTGGCG GAAGCCGATTGTTCTTCTGTAAGCCACGCGCTTGCTGCTTTGGGAAGAGAAGGGGGGGGGTACTGAAT GGATGAGGAGGAGAAGGAGGGGTATTGGTATTATCTGAGTTGGGTGAAGAGC SEQ ID NO: 22 Chlorella protothecoides actin promoter/5'UTR agtttaggtccagcgtccgtggggggggacgggctgggagcttgggccgggaagggcaagacgatgca gtccctctggggagtcacagccgactgtgtgtgttgcactgtgcggcccgcagcactcacacgcaaaa tgcctggccgacaggcaggccctgtccagtgcaacatccacggtccctctcatcaggctcaccttgct cattgacataacggaatgcgtaccgctctttcagatctgtccatccagagaggggagcaggctcccca ccgacgctgtcaaacttgcttcctgcccaaccgaaaacattattgtttgagggggggggggggggggc agattgcatggcgggatatctcgtgaggaacatcactgggacactgtggaacacagtgagtgcagtat gcagagcatgtatgctaggggtcagcgcaggaagggggcctttcccagtctcccatgccactgcaccg tatccacgactcaccaggaccagcttcttgatcggcttccgctcccgtggacaccagtgtgtagcctc tggactccaggtatgcgtgcaccgcaaaggccagccgatcgtgccgattcctggggtggaggatatga gtcagccaacttggggctcagagtgcacactggggcacgatacgaaacaacatctacaccgtgtcctc catgctgacacaccacagcttcgctccacctgaatgtgggcgcatgggcccgaatcacagccaatgtc gctgctgccataatgtgatccagaccctctccgcccagatgccgagcggatcgtgggcgctgaataga ttcctgtttcgatcactgtttgggtcctttccttttcgtctcggatgcgcgtctcgaaacaggctgcg tcgggctttcggatcccttttgctccctccgtcaccatcctgcgcgcgggcaagttgcttgaccctgg gctgtaccagggttggagggtattaccgcgtcaggccattcccagcccggattcaattcaaagtctgg gccaccaccctccgccgctctgtctgatcactccacattcgtgcatacactacgttcaagtcctgatc caggcgtgtctcgggacaaggtgtgcttgagtttgaatctcaaggacccactccagcacagctgctgg ttgaccccgccctcgcaa SEQ ID NO: 23 AtTHIC expression cassette comprising Chlorella protothecoides actin promoter/5'UTR, Arabidopsis thaliana THIC protein coding sequence codon-optimized for expression in Prototheca moriformis, and Chlorella vulgaris nitrate reductase 3' UTR agtttaggtccagcgtccgtggggggggacgggctgggagcttgggccgggaagggcaagacgatgca gtccctctggggagtcacagccgactgtgtgtgttgcactgtgcggcccgcagcactcacacgcaaaa tgcctggccgacaggcaggccctgtccagtgcaacatccacggtccctctcatcaggctcaccttgct cattgacataacggaatgcgtaccgctctttcagatctgtccatccagagaggggagcaggctcccca ccgacgctgtcaaacttgcttcctgcccaaccgaaaacattattgtttgagggggggggggggggggc agattgcatggcgggatatctcgtgaggaacatcactgggacactgtggaacacagtgagtgcagtat gcagagcatgtatgctaggggtcagcgcaggaagggggcctttcccagtctcccatgccactgcaccg tatccacgactcaccaggaccagcttcttgatcggcttccgctcccgtggacaccagtgtgtagcctc tggactccaggtatgcgtgcaccgcaaaggccagccgatcgtgccgattcctggggtggaggatatga gtcagccaacttggggctcagagtgcacactggggcacgatacgaaacaacatctacaccgtgtcctc catgctgacacaccacagcttcgctccacctgaatgtgggcgcatgggcccgaatcacagccaatgtc gctgctgccataatgtgatccagaccctctccgcccagatgccgagcggatcgtgggcgctgaataga ttcctgtttcgatcactgtttgggtcctttccttttcgtctcggatgcgcgtctcgaaacaggctgcg tcgggctttcggatcccttttgctccctccgtcaccatcctgcgcgcgggcaagttgcttgaccctgg gctgtaccagggttggagggtattaccgcgtcaggccattcccagcccggattcaattcaaagtctgg gccaccaccctccgccgctctgtctgatcactccacattcgtgcatacactacgttcaagtcctgatc caggcgtgtctcgggacaaggtgtgcttgagtttgaatctcaaggacccactccagcacagctgctgg ttgaccccgccctcgcaatctagaATGgccgcgtccgtccactgcaccctgatgtccgtggtctgcaa caacaagaaccactccgcccgccccaagctgcccaactcctccctgctgcccggcttcgacgtggtgg tccaggccgcggccacccgcttcaagaaggagacgacgaccacccgcgccacgctgacgttcgacccc cccacgaccaactccgagcgcgccaagcagcgcaagcacaccatcgacccctcctcccccgacttcca gcccatcccctccttcgaggagtgcttccccaagtccacgaaggagcacaaggaggtggtgcacgagg agtccggccacgtcctgaaggtgcccttccgccgcgtgcacctgtccggcggcgagcccgccttcgac aactacgacacgtccggcccccagaacgtcaacgcccacatcggcctggcgaagctgcgcaaggagtg gatcgaccgccgcgagaagctgggcacgccccgctacacgcagatgtactacgcgaagcagggcatca tcacggaggagatgctgtactgcgcgacgcgcgagaagctggaccccgagttcgtccgctccgaggtc gcgcggggccgcgccatcatcccctccaacaagaagcacctggagctggagcccatgatcgtgggccg caagttcctggtgaaggtgaacgcgaacatcggcaactccgccgtggcctcctccatcgaggaggagg tctacaaggtgcagtgggccaccatgtggggcgccgacaccatcatggacctgtccacgggccgccac atccacgagacgcgcgagtggatcctgcgcaactccgcggtccccgtgggcaccgtccccatctacca ggcgctggagaaggtggacggcatcgcggagaacctgaactgggaggtgttccgcgagacgctgatcg agcaggccgagcagggcgtggactacttcacgatccacgcgggcgtgctgctgcgctacatccccctg accgccaagcgcctgacgggcatcgtgtcccgcggcggctccatccacgcgaagtggtgcctggccta ccacaaggagaacttcgcctacgagcactgggacgacatcctggacatctgcaaccagtacgacgtcg ccctgtccatcggcgacggcctgcgccccggctccatctacgacgccaacgacacggcccagttcgcc gagctgctgacccagggcgagctgacgcgccgcgcgtgggagaaggacgtgcaggtgatgaacgaggg ccccggccacgtgcccatgcacaagatccccgagaacatgcagaagcagctggagtggtgcaacgagg cgcccttctacaccctgggccccctgacgaccgacatcgcgcccggctacgaccacatcacctccgcc atcggcgcggccaacatcggcgccctgggcaccgccctgctgtgctacgtgacgcccaaggagcacct gggcctgcccaaccgcgacgacgtgaaggcgggcgtcatcgcctacaagatcgccgcccacgcggccg acctggccaagcagcacccccacgcccaggcgtgggacgacgcgctgtccaaggcgcgcttcgagttc cgctggatggaccagttcgcgctgtccctggaccccatgacggcgatgtccttccacgacgagacgct gcccgcggacggcgcgaaggtcgcccacttctgctccatgtgcggccccaagttctgctccatgaaga tcacggaggacatccgcaagtacgccgaggagaacggctacggctccgccgaggaggccatccgccag ggcatggacgccatgtccgaggagttcaacatcgccaagaagacgatctccggcgagcagcacggcga ggtcggcggcgagatctacctgcccgagtcctacgtcaaggccgcgcagaagTGAcaattggcagcag cagctcggatagtatcgacacactctggacgctggtcgtgtgatggactgttgccgccacacttgctg ccttgacctgtgaatatccctgccgcttttatcaaacagcctcagtgtgtttgatcttgtgtgtacgc gcttttgcgagttgctagctgcttgtgctatttgcgaataccacccccagcatccccttccctcgttt catatcgcttgcatcccaaccgcaacttatctacgctgtcctgctatccctcagcgctgctcctgctc ctgctcactgcccctcgcacagccttggtttgggctccgcctgtattctcctggtactgcaacctgta aaccagcactgcaatgctgatgcacgggaagtagtgggatgggaacacaaatggaggatcc SEQ ID NO: 24 PmKASII (Prototheca moriformis KASII) comprising a C. protothecoides S106 stearoyl-ACP desaturase transit peptide ATGgccaccgcatccactttctcggcgttoaatgcccgctgcggcgacctgcgtcgctcggcgggctc cgggccccggcgcccagcgaggcccctccccgtgcgcgggcgcgccgccgccgccgccgacgccaacc ccgcccgccccgagcgccgcgtggtgatcaccggccagggcgtggtgacctccctgggccagaccatc
gagcagttctactcctccctgctggagggcgtgtccggcatctcccagatccagaagttcgacaccac cggctacaccaccaccatcgccggcgagatcaagtccctgcagctggacccctacgtgcccaagcgct gggccaagcgcgtggacgacgtgatcaagtacgtgtacatcgccggcaagcaggccctggagtccgcc ggcctgcccatcgaggccgccggcctggccggcgccggcctggaccccgccctgtgcggcgtgctgat cggcaccgccatggccggcatgacctccttcgccgccggcgtggaggccctgacccgcggcggcgtgc gcaagatgaaccccttctgcatccccttctccatctccaacatgggcggcgccatgctggccatggac atcggcttcatgggccccaactactccatctccaccgcctgcgccaccggcaactactgcatcctggg cgccgccgaccacatccgccgcggcgacgccaacgtgatgctggccggcggcgccgacgccgccatca tcccctccggcatcggcggcttcatcgcctgcaaggccctgtccaagcgcaacgacgagcccgagcgc gcctcccgcccctgggacgccgaccgcgacggcttcgtgatgggcgagggcgccggcgtgctggtgct ggaggagctggagcacgccaagcgccgcggcgccaccatcctggccgagctggtgggcggcgccgcca cctccgacgcccaccacatgaccgagcccgacccccagggccgcggcgtgcgcctgtgcctggagcgc gccctggagcgcgcccgcctggcccccgagcgcgtgggctacgtgaacgcccacggcacctccacccc cgccggcgacgtggccgagtaccgcgccatccgcgccgtgatcccccaggactccctgcgcatcaact ccaccaagtccatgatcggccacctgctgggcggcgccggcgccgtggaggccgtggccgccatccag gccctgcgcaccggctggctgcaccccaacctgaacctggagaaccccgcccccggcgtggaccccgt ggtgctggtgggcccccgcaaggagcgcgccgaggacctggacgtggtgctgtccaactccttcggct tcggcggccacaactcctgcgtgatcttccgcaagtacgacgagatggactacaaggaccacgacggc gactacaaggaccacgacatcgactacaaggacgacgacgacaagTGA SEQ ID NO: 25 PmKASII (Prototheca moriformis KASII) comprising a C. protothecoides S106 stearoylACP desaturase transit peptide MATASTESAFNARCGDLRRSAGSGPRRPARPLPVRGRAAAAADANPARPERRVVITGQGVVISLGQII EQFYSSLLEGVSGISQIQKFDTTGYTTTIAGEIKSLQLDPYVPKRWAKRVDDVIKYVYIAGKQALESA GLPIEAAGLAGAGLDPALCGVLIGTAMAGMTSFAAGVEALTRGGVRKMNPFCIPFSISNMGGAMLAMD IGFMGPNYSISTACATGNYCILGAADHIRRGDANVMLAGGADAAIIPSGIGGFIACKALSKRNDEPER ASRPWDADRDGFVMGEGAGVLVLEELEHAKRRGATILAELVGGAATSDAHHMTEPDPQGRGVRLCLER ALERARLAPERVGYVNAHGTSTPAGDVAEYRAIRAVIPQDSLRINSTKSMIGHLLGGAGAVEAVAAIQ ALRTGWLHPNLNLENPAPGVDPVVLVGPRKERAEDLDVVLSNSFGFGGHNSCVIFRKYDEMDYKDHDG DYKDHDIDYKDDDDK SEQ ID NO: 26 Codon-optimized Prototheca moriformis (UTEX 1435) FAD2 protein- coding sequence ATGgccatcaagaccaaccgccagcccgtggagaagccccccttcaccatcggcaccctgcgcaaggc catccccgcccactgcttcgagcgctccgccctgcgctcctccatgtacctggccttcgacatcgccg tgatgtccctgctgtacgtggcctccacctacatcgaccccgcccccgtgcccacctgggtgaagtac ggcgtgatgtggcccctgtactggttcttccagggcgccttcggcaccggcgtgtgggtgtgcgccca cgagtgcggccaccaggccttctcctcctcccaggccatcaacgacggcgtgggcctggtgttccact ccctgctgctggtgccctactactcctggaagcactcccaccgccgccaccactccaacaccggctgc ctggacaaggacgaggtgttcgtgcccccccaccgcgccgtggcccacgagggcctggagtgggagga gtggctgcccatccgcatgggcaaggtgctggtgaccctgaccctgggctggcccctgtacctgatgt tcaacgtggcctcccgcccctacccccgcttcgccaaccacttcgacccctggtcccccatcttctcc aagcgcgagcgcatcgaggtggtgatctccgacctggccctggtggccgtgctgtccggcctgtccgt gctgggccgcaccatgggctgggcctggctggtgaagacctacgtggtgccctacctgatcgtgaaca tgtggctggtgctgatcaccctgctgcagcacacccaccccgccctgccccactacttcgagaaggac tgggactggctgcgcggcgccatggccaccgtggaccgctccatgggcccccccttcatggacaacat cctgcaccacatctccgacacccacgtgctgcaccacctgttctccaccatcccccactaccacgccg aggaggcctccgccgccatccgccccatcctgggcaagtactaccagtccgactcccgctgggtgggc cgcgccctgtgggaggactggcgcgactgccgctacgtggtgcccgacgcccccgaggacgactccgc cctgtggttccacaagTAG SEQ ID NO: 27 Amino acid sequence of Prototheca moriformis FAD2 MAIKTNRQPVEKPPFTIGILRKAIPAHCFERSALRSSMYLAFDIAVMSLLYVASTYIDPAPVPIWVKY GVMWPLYWFFQGAFGTGVWVCAHECGHQAFSSSQAINDGVGLVFHSLLLVPYYSWKHSHRRHHSNTGC LDKDEVFVPPHRAVAHEGLEWEEWLPIRMGKVLVTLTLGWPLYLMFNVASRPYPRFANHFDPWSPIFS KRERIEVVISDLALVAVLSGLSVLGRTMGWAWLVKTYVVPYLIVNMWLVLITLLQHTHPALPHYFEKD WDWLRGAMATVDRSMGPPFMDNILHHISDTHVLHHLFSTIPHYHAEEASAAIRPILGKYYQSDSRWVG RALWEDWRDCRYVVPDAPEDDSALWFHK SEQ ID NO: 28 Codon-optimized coding region of Brassica napus C18:0-preferring thioesterase from pSZ1358 ACTAGTATGCTGAAGCTGTCCTGCAACGTGACCAACAACCTGCACACCTTCTCCTTCTTCTCCGACTC CTCCCTGTTCATCCCCGTGAACCGCCGCACCATCGCCGTGTCCTCCGGGCGCGCCTCCCAGCTGCGCA AGCCCGCCCTGGACCCCCTGCGCGCCGTGATCTCCGCCGACCAGGGCTCCATCTCCCCCGTGAACTCC TGCACCCCCGCCGACCGCCTGCGCGCCGGCCGCCTGATGGAGGACGGCTACTCCTACAAGGAGAAGTT CATCGTGCGCTCCTACGAGGTGGGCATCAACAAGACCGCCACCGTGGAGACCATCGCCAACCTGCTGC AGGAGGTGGCCTGCAACCACGTGCAGAAGTGCGGCTTCTCCACCGACGGCTTCGCCACCACCCTGACC ATGCGCAAGCTGCACCTGATCTGGGTGACCGCCCGCATGCACATCGAGATCTACAAGTACCCCGCCTG GTCCGACGTGGTGGAGATCGAGACCTGGTGCCAGTCCGAGGGCCGCATCGGCACCCGCCGCGACTGGA TCCTGCGCGACTCCGCCACCAACGAGGTGATCGGCCGCGCCACCTCCAAGTGGGTGATGATGAACCAG GACACCCGCCGCCTGCAGCGCGTGACCGACGAGGTGCGCGACGAGTACCTGGTGTTCTGCCCCCGCGA GCCCCGCCTGGCCTTCCCCGAGGAGAACAACTCCTCCCTGAAGAAGATCCCCAAGCTGGAGGACCCCG CCCAGTACTCCATGCTGGAGCTGAAGCCCCGCCGCGCCGACCTGGACATGAACCAGCACGTGAACAAC GTGACCTACATCGGCTGGGTGCTGGAGTCCATCCCCCAGGAGATCATCGACACCCACGAGCTGCAGGT GATCACCCTGGACTACCGCCGCGAGTGCCAGCAGGACGACATCGTGGACTCCCTGACCACCTCCGAGA TCCCCGACGACCCCATCTCCAAGTTCACCGGCACCAACGGCTCCGCCATGTCCTCCATCCAGGGCCAC AACGAGTCCCAGTTCCTGCACATGCTGCGCCTGTCCGAGAACGGCCAGGAGATCAACCGCGGCCGCAC CCAGTGGCGCAAGAAGTCCTCCCGCATGGACTACAAGGACCACGACGGCGACTACAAGGACCACGACA TCGACTACAAGGACGACGACGACAAGTGAATCGAT SEQ ID NO: 29 Amino acid sequence of Brassica napus C18:0-preferring thioesterase (Accession No. CAA52070.1) MLKLSCNVTNNLHTFSFFSDSSLFIPVNRRTIAVSSSQLRKPALDPLRAVISADQGSISPVNSCTPAD RLRAGRLMEDGYSYKEKFIVRSYEVGINKTATVETIANLLQEVACNHVQKCGESIDGFATTLIMRKLH LIWVTARMHIETYKYPAWSDVVEIETWCQSEGRIGIRRDWILRDSATNEVIGRATSKWVMMNQDTRRL QRVTDEVRDEYLVFCPREPRLAFPEENNSSLKKIPKLEDPAQYSMLELKPRRADLDMNQHVNNVTYIG WVLESIPQEIIDTHELQVITLDYRRECQQDDIVDSLTTSEIPDDPISKFTGTNGSAMSSIQGHNESQF LHMLRLSENGQEINRGRTQWRKKSSR SEQ ID NO: 30 Prototheca moriformis FATA1 allele 1 5' homology donor region GGAGTCACTGTGCCACTGAGTTCGACTGGTAGCTGAATGGAGTCGCTGCTCCACTAAACGAATTGTCA GCACCGCCAGCCGGCCGAGGACCCGAGTCATAGCGAGGGTAGTAGCGCGCCATGGCACCGACCAGCCT GCTTGCCAGTACTGGCGTCTCTTCCGCTTCTCTGTGGTCCTCTGCGCGCTCCAGCGCGTGCGCTTTTC CGGTGGATCATGCGGICCGTGGCGCACCGCAGCGGCCGCTGCCCATGCAGCGCCGCTGCTTCCGAACA GTGGCGGTCAGGGCCGCACCCGCGGTAGCCGTCCGTCCGGAACCCGCCCAAGAGTTTTGGGAGCAGCT TGAGCCCTGCAAGATGGCGGAGGACAAGCGCATCTTCCTGGAGGAGCACCGGTGCGTGGAGGTCCGGG GCTGACCGGCCGTCGCATTCAACGTAATCAATCGCATGATGATCAGAGGACACGAAGTCTTGGTGGCG GTGGCCAGAAACACTGTCCATTGCAAGGGCATAGGGATGCGTTCCTTCACCTCTCATTTCTCATTTCT GAATCCCTCCCTGCTCACTCTTTCTCCTCCTCCTTCCCGTTCACGCAGCATTCGG SEQ ID NO: 31 Prototheca moriformis FATA1 allele 1 3' homology donor region GACAGGGTGGTTGGCTGGATGGGGAAACGCTGGTCGCGGGATTCGATCCTGCTGCTTATATCCTCCCT GGAAGCACACCCACGACTCTGAAGAAGAAAACGTGCACACACACAACCCAACCGGCCGAATATTTGCT TCCTTATCCCGGGTCCAAGAGAGACTGCGATGCCCCCCTCAATCAGCATCCTCCTCCCTGCCGCTTCA ATCTTCCCTGCTTGCCTGCGCCCGCGGTGCGCCGTCTGCCCGCCCAGTCAGTCACTCCTGCACAGGCC CCTTGTGCGCAGTGCTCCTGTACCCTTTACCGCTCCTTCCATTCTGCGAGGCCCCCTATTGAATGTAT TCGTTGCCTGTGTGGCCAAGCGGGCTGCTGGGCGCGCCGCCGTCGGGCAGTGCTCGGCGACTTTGGCG GAAGCCGATTGTTCTTCTGTAAGCCACGCGCTTGCTGCTTTGGGAAGAGAAGGGGGGGGGTACTGAAT GGATGAGGAGGAGAAGGAGGGGTATTGGTATTATCTGAGTTGGGT SEQ ID NO: 32 Prototheca moriformis FATA1 allele 2 5' homology donor region AATGGAGTCGCTGCTCCACTAATCGAATTGTCAGCACCGCCAGCCGGCCGAGGACCCGAGTCATAGCG AGGGTAGTAGCGCGCCATGGCACCGACCAGCCTGCTTGCCCGTACTGGCGTCTCTTCCGCTTCTCTGT GCTCCTCTACGCGCTCCGGCGCGTGCGCTTTTCCGGTGGATCATGCGGTCCGTGGCGCACCGCAGCGG CCGCTGCCCATGCAGCGCCGCTGCTTCCGAACAGTGGCTGTCAGGGCCGCACCCGCAGTAGCCGTCCG TCCGGAACCCGCCCAAGAGTTTTGGGAGCAGCTTGAGCCCTGCAAGATGGCGGAGGACAAGCGCATCT TCCTGGAGGAGCACCGGTGCGCGGAGGTCCGGGGCTGACCGGCCGTCGCATTCAACGTAATCAATCGC ATGATGATCACAGGACGCGACGTCTTGGTGGCGGTGGCCAGGGACACTGCCCATTGCACAGGCATAGG AATGCGTTCCTTCTCATTTCTCAGTTTTCTGAGCCCCTCCCTCTTCACTCTTTCTCCTCCTCCTCCCC TCTCACGCAGCATTCGTGG SEQ ID NO: 33 Prototheca moriformis FATA1 allele 2 3' homology donor region CACTAGTATCGATTTCGAACAGAGGAGAGGGTGGCTGGTAGTTGCGGGATGGCTGGTCGCCCGTCGAT CCTGCTGCTGCTATTGTCTCCTCCTGCACAAGCCCACCCACGACTCCGAAGAAGAAGAAGAAAACGCG CACACACACAACCCAACCGGCCGAATATTTGCTTCCTTATCCCGGGTCCAAGAGAGACGGCGATGCCC CCCTCAATCAGCCTCCTCCTCCCTGCCGCTCCAATCTTCCCTGCTTGCATGCGCCCGCGAGAGGCTGT CTGCGCGCCCCGTCAGTCACTCCCCGTGCAGACGCCTCGTGCTCGGTGCTCCTGTATCCTTTACCGCT CCTTTCATTCTGCGAGGCCCCCTGTTGAATGTATTCGTTGCCTGTGTGGCCAAGCGCGCTGCTGGGCG CGCCGCCGTCGGGCGGTGCTCGGCGACTCTGGCGGAAGCCGGTTGTTCTTCTGTAAGCCACGCGCTTG CTGCTTTTGGAAAAGAGGGGGGTTTACTGAATGGAGGAGGAGCAGGATAATTGGTAGTATCTGAGTTG TTG SEQ ID NO: 34 SAD2 hairpin C actagtGCGCTGGACGCGGCAGTGGGTGGCCGAGGAGAACCGGCACGGCGACCTGCTGAACAAGTACT GTTGGCTGACGGGGCGCGTCAACATGCGGGCCGTGGAGGTGACCATCAACAACCTGATCAAGAGCGGC ATGAACCCGCAGACGGACAACAACCCTTACTTGGGCTTCGTCTACACCTCCTTCCAGGAGCGCGCGAC CAAGTACAGCCACGGCAACACCGCGCGCCTTGCGGCCGAGCAGTGTGTTTGAGGGTTTTGGTTGCCCG TATCGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTGACCCCCCCGGCTACCCTCC CGGCACCTTCCAGGGCGCGTACGggatccTGCTCGGCCGCAAGGCGCGCGGTGTTGCCGTGGCTGTAC TTGGTCGCGCGCTCCTGGAAGGAGGTGTAGACGAAGCCCAAGTAAGGGTTGTTGTCCGTCTGCGGGTT CATGCCGCTCTTGATCAGGTTGTTGATGGTCACCTCCACGGCCCGCATGTTGACGCGCCCCGTCAGCC AACAGTACTTGTTCAGCAGGTCGCCGTGCCGGTTCTCCTCGGCCACCCACTGCCGCGTCCAGCGCaag ctt SEQ ID NO: 35 Prototheca moriformis FAD-D omega 3 desaturase MSIQFALRAAYIKGICQRLSGRGAALGLSRDWIPGWILPRCWPASAAATAPPRARHQERAIHLTSGRR RHSALASDADERALPSNAPGLVMASQANYFRVRLLPEQEEGELESWSPNVRHTTLLCKPRAMLSKLQM RVMVGDRVIVTAIDPVNMTVHAPPFDPLPATRFLVAGEAADMDITVVLNKADLVPEEESAALAQEVAS WGPVVLTSTLTGRGLQELERQLGSTTAVLAGPSGAGKSSIINALARAARERPSDASVSNVPEEQVVGE DGRALANPPPFTLADIRNAIPKDCFRKSAAKSLAYLGDLSITGMAVLAYKINSPWLMPLYWFAQGTMF WALFVVGHDCGHQSFSTSKRLNDALAWLGALAAGTWTWALGVLPMLNLYLAPYVWLLVTYLHHHGPSD PREEMPWYRGREWSYMRGGLTTIDRDYGLFNKVHHDIGTHVVHH SEQ ID NO: 36 MFWALFVVGHDCGHQSFSTSKRLNDAVGLFVHSTIGVPYHGWRISHRTHHNNHGHVENDESWYPPTES GLKAMTDMGRQGRFHFPSMLFVYPFYLFWRSPGKTGSHFSPATDLFALWEAPLIRTSNACQLAWLGAL AAGTWALGVLPMLNLYLAPYVISVAWLDLVTYLHHHGPSDPREEMPWYRGREWSYMRGGLTTIDRDYG LFNKVHHDIGTHVVHHLFPQIPHYNLCRATKAAKKVLGPYYREPERCPLGLLPVHLLAPLLRSLGQDH FVDDAGSVLFYRRAEGINPWIQKLLPWLGGARRGADAQRDAAQ SEQ ID NO: 37 Camelina sativa omega-3 EAD7-2 MANLVLSECGIRPLPRIYTTPRSNFVSNNNKPIFKFRPFTSYKTSSSPLACSRDGFGKNWSLNVSVPL TTTTPIVDESPLKEEEEEKQRFDPGAPPPFNLADIRAAIPKHCWVKNPWKSMSYVLRDVAIVFALAAG ASYLNNWIVWPLYWLAQGTMFWALFVLGHDCGHGSFSNNPRLNNVVGHLLHSSILVPYHGWRISHRTH HQNHGHVENDESWHPMSEKIYQSLDKPTRFFRFTLPLVMLAYPFYLWARSPGKKGSHYHPESDLFLPK EKTDVLTSTACWTAMAALLICLNFVVGPVQMLKLYGIPYWINVMWLDFVTYLHHHGHEDKLPWYRGKE WSYLRGGLTTLDRDYGVINNIHHDIGTHVIHHLFPQIPHYHLVEATEAVKPVLGKYYREPDKSGPLPL HLLGILAKSIKEDHYVSDEGDVVYYKADPNMYGEIKVGAD SEQ ID NO: 38 Prototheca moriformis delta 12 desaturase allele 2 MAIKTNRQPVEKPPFTIGILRKAIPAHCFERSALRSSMYLAFDIAVMSLLYVASTYIDPAPVPIWVKY GIMWPLYWFFQGAFGTGVWVCAHECGHQAFSSSQAINDGVGLVFHSLLLVPYYSWKHSHRRHHSNTGC LDKDEVFVPPHRAVAHEGLEWEEWLPIRMGKVLVTLTLGWPLYLMFNVASRPYPRFANHFDPWSPIFS KRERIEVVISDLALVAVLSGLSVLGRTMGWAWLVKTYVVPYMIVNMWLVLITLLQHTHPALPHYFEKD WDWLRGAMATVDRSMGPPFMDSILHHISDTHVLHHLFSTIPHYHAEEASAAIRPILGKYYQSDSRWVG RALWEDWRDCRYVVPDAPEDDSALWFHK SEQ ID NO: 39 Camelina sativa omega-3 FAD7-1 MANLVLSECGIRPLPRIYTTPRSNFVSNNNKPIFKFRPLTSYKTSSPLFCSRDGFGRNWSLNVSVPLA TTTPIVDESPLEEEEEEEKQRFDPGAPPPFNLADIRAAIPKHCWVKNPWKSMSYVLRDVAIVFALAAG AAYLNNWIVWPLYWLAQGTMFWALFVLGHDCGHGSFSNNPRLNNVVGHLLHSSILVPYHGWRISHRTH HQNHGHVENDESWHPMSEKIYQSLDKPTRFFRFTLPLVMLAYPFYLWARSPGKKGSHYHPESDLFLPK EKTDVLTSTACWTAMAALLICLNFVVGPVQMLKLYGIPYWINVMWLDFVTYLHHHGHEDKLPWYRGKE WSYLRGGLTTLDRDYGVINNIHHDIGTHVIHHLFPQIPHYHLVEATEAVKPVLGKYYREPDKSGPLPL HLLGILAKSIKEDHYVSDEGDVVYYKADPNMYGEIKVGAD SEQ ID NO: 40 PmFATA-hpB actagtCATTCGGGGCAACGAGGTGGGCCCCICGCAGCGGCTGACGATCACGGCGGTGGCCAACATCC TGCAGGAGGCGGCGGGCAACCACGCGGTGGCCATGTGGGGCCGGAGCGTGTGTTTGAGGGTTTTGGTT GCCCGTATTGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTGACCCCCCCGGCTAC CCTCCCGGCACCTTCCAGGGCGCGTACGggatccGCTCCGGCCCCACATGGCCACCGCGTGGTTGCCC GCCGCCTCCTGCAGGATGTTGGCCACCGCCGTGATCGTCAGCCGCTGCGAGGGGCCCACCTCGTTGCC CCGAATGaagctt SEQ ID NO: 41 PmFATA-hpC actagtGGAGGGTTTCGCGACGGACCCGGAGCTGCAGGAGGCGGGTCTCATCTTTGTGATGACGCGCA TGCAGATCCAGATGTACCGCTACCCGCGCTGGGGCGACCTGATGCAGGTGGAGACCTGGTTCCAGAGT GTGTTTGAGGGTTTTGGTTGCCCGTATTGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCC CGCTGACCCCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCGTACGggatccTCTGGAACCAGGTCT CCACCTGCATCAGGTCGCCCCAGCGCGGGTAGCGGTACATCTGGATCTGCATGCGCGTCATCACAAAG ATGAGACCCGCCTCCTGCAGCTCCGGGTCCGTCGCGAAACCCTCCaagctt SEQ ID NO: 42 PmFATA-hpD actagtCGGCGGGCAAGCTGGGCGCGCAGCGCGAGTGGGTGCTGCGCGACAAGCTGACCGGCGAGGCG CTGGGCGCGGCCACCTCGAGCTGGGTCATGATCAACATCCGCACGCGCCGGCCGTGCCGCATGCCGGG TGTGTTTGAGGGTTTTGGTTGCCCGTATCGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTC CCGCTGACCCCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCGTACGggatccCCGGCATGCGGCAC GGCCGGCGCGTGCGGATGTTGATCATGACCCAGCTCGAGGTGGCCGCGCCCAGCGCCTCGCCGGTCAG CTTGTCGCGCAGCACCCACTCGCGCTGCGCGCCCAGCTTGCCCGCCGaagctt SEQ ID NO: 43 PmFATA-hpE actagtGTCCGCGTCAAGTCGGCCTTCTTCGCGCGCGAGCCGCCGCGCCTGGCGCTGCCGCCCGCGGT CACGCGTGCCAAGCTGCCCAACATCGCGACGCCGGCGCCGCTGCGCGGGCACCGCCAGGTCGCGCGCC GCACCGACATGGACATGAACGGGCACGTGAACAACGTGGCCTACCTGGCCTGGTGCCTGGAGTGTGTT TGAGGGTTTTGGTTGCCCGTATTGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCTG ACCCCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCGTACGggatccTCCAGGCACCAGGCCAGGTA GGCCACGTTGTTCACGTGCCCGTTCATGTCCATGTCGGTGCGGCGCGCGACCTGGCGGTGCCCGCGCA GCGGCGCCGGCGTCGCGATGTTGGGCAGCTTGGCACGCGTGACCGCGGGCGGCAGCGCCAGGCGCGGC GGCTCGCGCGCGAAGAAGGCCGACTTGACGCGGACaagott SEQ ID NO: 44 PmFATA-hpF actagtCCGTGCCCGAGCACGTCTTCAGCGACTACCACCTCTACCAGATGGAGATCGACTTCAAGGCC GAGTGCCACGCGGGCGACGTCATCTCCTCCCAGGCCGAGCAGATCCCGCCCCAGGAGGCGCTCACGCA CAACGGCGCCGGCCGCAACCCCTCCTGCTTCGTCCATAGCATTCTGCGCGCCGAGACCGAGCGTGTGT TTGAGGGTTTTGGTTGCCCGTATCGAGGTCCTGGTGGCGCGCATGGGGGAGAAGGCGCCTGTCCCGCT GACCCCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCGTACGggatccGCTCGGTCTCGGCGCGCAG AATGCTATGGACGAAGCAGGAGGGGTTGCGGCCGGCGCCGTTGTGCGTGAGCGCCTCCTGGGGCGGGA TCTGCTCGGCCTGGGAGGAGATGACGTCGCCCGCGTGGCACTCGGCCTTGAAGTCGATCTCCATCTGG TAGAGGTGGTAGTCGCTGAAGACGTGCTCGGGCACGGaagctt SEQ ID NO: 45 PmFATA-hpG
actagtTCGTCCGCGCGCGAACCACATGGTCGGCCCCCATCGACGCGCCCGCCGCCAAGCCGCCCAAG GCGAGCCACTGAGGACAGGGTGGTTGGCTGGATGGGGAAACGCTGGTCGCGGGATTCGATCCTGCTGC TTATATCCTCGTGTGTTTGAGGGTTTTGGTTGCCCGTATTGAGGTCCTGGTGGCGCGCATGGGGGAGA AGGCGCCTGTCCCGCTGACCCCCCCGGCTACCCTCCCGGCACCTTCCAGGGCGCGTACGggatccGAG GATATAAGCAGCAGGATCGAATCCCGCGACCAGCGTTTCCCCATCCAGCCAACCACCCTGTCCTCAGT GGCTCGCCTTGGGCGGCTTGGCGGCGGGCGCGTCGATGGGGGCCGACCATGTGGTTCGCGCGCGGACG Aaagctt SEQ ID NO: 46 Codon-optimized Cuphea wrightii KASAI ATGGCCGCCGCCGCCAGCATGGTGGCCAGCCCCTTCTGCACCTGGCTGGTGGCCAGCTGCATGAGCAC CAGCTTCGACAACGACCCCCGCAGCCCCAGCGTGAAGCGCTTCCCCCGCCGCAAGCGCGTGCTGAGCC AGCGCGGCAGCACCTACGTATTCCAGTGCCTGGTGGCCAGCTGCATCGACCCCTGCGACCAGTACCGC AGCAGCGCCAGCCTGAGCTTCCTGGGCGACAACGGCTTCGCCAGCCTGTTCGGCAGCAAGCCCTTCAT GAGCAACCGCGGCCACCGCCGCCTGCGCCGCGCCAGCCACAGCGGCGAGGCCATGGCCGIGGCCCTGC AGCCCGCCCAGGAGGCCGGCACCAAGAAGAAGCCCGTGATCAAGCAGCGCCGCGTGGTGGTGACCGGC ATGGGCGTGGTGACCCCCCTGGGCCACGAGCCCGACGTGTTCTACAACAACCTGCTGGACGGCGTGAG CGGCATCAGCGAGATCGAGACCTTCGACTGCACCCAGTTCCCCACCCGCATCGCCGGCGAGATCAAGA GCTTCAGCACCGACGGCTGGGTGGCCCCCAAGCTGAGCAAGCGCATGGACAAGTTCATGCTGTACCTG CTGACCGCCGGCAAGAAGGCCCTGGCCGACGGCGGCATCACCGACGAGGTGATGAAGGAGCTGGACAA GCGCAAGTGCGGCGTGCTGATCGGCAGCGGCATGGGCGGCATGAAGGTGTTCAACGACGCCATCGAGG CCCTGCGCGTGAGCTACAAGAAGATGAACCCCTTCTGCGTGCCCTTCGCCACCACCAACATGGGCAGC GCCATGCTGGCCATGGACCTGGGCTGGATGGGCCCCAACTACAGCATCAGCACCGCCTGCGCCACCAG CAACTTCTGCATCCTGAACGCCGCCAACCACATCATCCGCGGCGAGGCCGACATGATGCTGTGCGGCG GCAGCGACGCCGTGATCATCCCCATCGGCCTGGGCGGCTTCGTGGCCTGCCGCGCCCTGAGCCAGCGC AACAGCGACCCCACCAAGGCCAGCCGCCCCTGGGACAGCAACCGCGACGGCTTCGTGATGGGCGAGGG CGCCGGCGTGCTGCTGCTGGAGGAGCTGGAGCACGCCAAGAAGCGCGGCGCCACCATCTACGCCGAGT TCCTGGGCGGCAGCTTCACCTGCGACGCCTACCACATGACCGAGCCCCACCCCGAGGGCGCCGGCGTG ATCCTGTGCATCGAGAAGGCCCTGGCCCAGGCCGGCGTGAGCAAGGAGGACGTGAACTACATCAACGC CCACGCCACCAGCACCAGCGCCGGCGACATCAAGGAGTACCAGGCCCTGGCCCGCTGCTTCGGCCAGA ACAGCGAGCTGCGCGTGAACAGCACCAAGAGCATGATCGGCCACCTGCTGGGCGCCGCCGGCGGCGTG GAGGCCGTGACCGTGGTGCAGGCCATCCGCACCGGCTGGATTCACCCCAACCTGAACCTGGAGGACCC CGACAAGGCCGTGGACGCCAAGCTGCTGGTGGGCCCCAAGAAGGAGCGCCTGAACGTGAAGGTGGGCC TGAGCAACAGCTTCGGCTTCGGCGGCCACAACAGCAGCATCCTGTTCGCCCCCTGCAACGTGTGA SEQ ID NO: 47 Codon-optimized Cuphea wrightii KASAI with P. moriformis SAD transit peptide ATGGGCCGCGGTGTCTCCCTTCCCCGGCCCAGGGTCGCGGTGCGCGCCCAGTCGGCGAGTCAGGTTTT GGAGAGCTGTATTCCAGTGCCTGGTGGCCAGCTGCATCGACCCCTGCGACCAGTACCGCAGCAGCGCC AGCCTGAGCTTCCTGGGCGACAACGGCTTCGCCAGCCTGTTCGGCAGCAAGCCCTTCATGAGCAACCG CGGCCACCGCCGCCTGCGCCGCGCCAGCCACAGCGGCGAGGCCATGGCCGTGGCCCTGCAGCCCGCCC AGGAGGCCGGCACCAAGAAGAAGCCCGTGATCAAGCAGCGCCGCGTGGTGGTGACCGGCATGGGCGTG GTGACCCCCCTGGGCCACGAGCCCGACGTGTTCTACAACAACCTGCTGGACGGCGTGAGCGGCATCAG CGAGATCGAGACCTTCGACTGCACCCAGTTCCCCACCCGCATCGCCGGCGAGATCAAGAGCTTCAGCA CCGACGGCTGGGTGGCCCCCAAGCTGAGCAAGCGCATGGACAAGTTCATGCTGTACCTGCTGACCGCC GGCAAGAAGGCCCTGGCCGACGGCGGCATCACCGACGAGGTGATGAAGGAGCTGGACAAGCGCAAGTG CGGCGTGCTGATCGGCAGCGGCATGGGCGGCATGAAGGTGTTCAACGACGCCATCGAGGCCCTGCGCG TGAGCTACAAGAAGATGAACCCCTTCTGCGTGCCCTTCGCCACCACCAACATGGGCAGCGCCATGCTG GCCATGGACCTGGGCTGGATGGGCCCCAACTACAGCATCAGCACCGCCTGCGCCACCAGCAACTTCTG CATCCTGAACGCCGCCAACCACATCATCCGCGGCGAGGCCGACATGATGCTGTGCGGCGGCAGCGACG CCGTGATCATCCCCATCGGCCTGGGCGGCTTCGTGGCCTGCCGCGCCCTGAGCCAGCGCAACAGCGAC CCCACCAAGGCCAGCCGCCCCTGGGACAGCAACCGCGACGGCTTCGTGATGGGCGAGGGCGCCGGCGT GCTGCTGCTGGAGGAGCTGGAGCACGCCAAGAAGCGCGGCGCCACCATCTACGCCGAGTTCCTGGGCG GCAGCTTCACCTGCGACGCCTACCACATGACCGAGCCCCACCCCGAGGGCGCCGGCGTGATCCTGTGC ATCGAGAAGGCCCTGGCCCAGGCCGGCGTGAGCAAGGAGGACGTGAACTACATCAACGCCCACGCCAC CAGCACCAGCGCCGGCGACATCAAGGAGTACCAGGCCCTGGCCCGCTGCTTCGGCCAGAACAGCGAGC TGCGCGTGAACAGCACCAAGAGCATGATCGGCCACCTGCTGGGCGCCGCCGGCGGCGTGGAGGCCGTG ACCGTGGTGCAGGCCATCCGCACCGGCTGGATTCACCCCAACCTGAACCTGGAGGACCCCGACAAGGC CGTGGACGCCAAGCTGCTGGTGGGCCCCAAGAAGGAGCGCCTGAACGTGAAGGTGGGCCTGAGCAACA GCTTCGGCTTCGGCGGCCACAACAGCAGCATCCTGTTCGCCCCCTGCAACGTGTGA SEQ ID NO: 48 Codon-optimized Cuphea pulcherrima KASIV ATGCCCGCGGCCAGCTCGCTGCTGGCGTCCCCCCTGTGCACCTGGCTGCTGGCCGCGTGCATGAGCAC CTCGTTCCACCCCTCCGACCCCCTGCCCCCCAGCATCTCGTCCCCCCGCCGCCGCCTGAGCCGCCGCC GCATCCTGTCGCAGTGCGCCCCCCTGCCCTCCGCGAGCTCGGCCCTGCGCGGCTCCAGCTTCCACACC CTGGTGACCTCGTATCTGGCGTGCTTCGAGCCCTGCCACGACTATTATACCAGCGCCTCCCTGTTCGG CTCGCGCCCCATCCGCACCACCCGCCGCCACCGCCGCCTGAACCGCGCGAGCCCCICGCGCGAGGCGA TGGCGGTCGCCCTGCAGCCCGAGCAGGAGGTGACCACCAAGAAGAAGCCCTCCATCAAGCAGCGCCGC GTCGTGGTCACCGGCATGGGCGTGGTCACCCCCCTGGGCCACGACCCCGACGTGTTCTATAACAACCT GCTGGACGGCACCAGCGGCATCTCGGAGATCGAGACCTTCGACTGCGCGCAGTTCCCCACCCGCATCG CCGGCGAGATCAAGTCCTTCAGCACCGACGGCTGGGTCGCGCCCAAGCTGTCGAAGCGCATGGACAAG TTCATGCTGTATATGCTGACCGCCGGCAAGAAGGCGCTGACCGACGGCGGCATCACCGAGGACGTGAT GAAGGAGCTGGACAAGCGCAAGTGCGGCGTCCTGATCGGCTCCGCGATGGGCGGCATGAAGGTGTTCA ACGACGCGATCGAGGCCCTGCGCATCAGCTATAAGAAGATGAACCCCTTCTGCGTGCCCTTCGCGACC ACCAACATGGGCTCGGCCATGCTGGCGATGGACCTGGGCTGGATGGGCCCCAACTATTCCATCAGCAC CGCCTGCGCGACCTCGAACTTCTGCATCATGAACGCGGCCAACCACATCATCCGCGGCGAGGCGGACG TCATGCTGTGCGGCGGCTCCGACGCCGTGATCATCCCCATCGGCATGGGCGGCTTCGTCGCGTGCCGC GCCCTGAGCCAGCGCAACTCGGACCCCACCAAGGCGTCCCGCCCCTGGGACAGCAACCGCGACGGCTT CGTGATGGGCGAGGGCGCCGGCGTCCTGCTGCTGGAGGAGCTGGAGCACGCGAAGAAGCGCGGCGCCA CCATCTATGCGGAGTTCCTGGGCGGCTCGTTCACCTGCGACGCCTATCACATGACCGAGCCCCACCCC GACGGCGCCGGCGTGATCCTGTGCATCGAGAAGGCGCTGGCCCAGTCCGGCGTCAGCCGCGAGGACGT GAACTATATCAACGCGCACGCCACCTCGACCCCCGCGGGCGACATCAAGGAGTATCAGGCCCTGATCC ACTGCTTCGGCCAGAACCGCGAGCTGAAGGTCAACTCCACCAAGAGCATGATCGGCCACCTGCTGGGC GCGGCGGGCGGCGTGGAGGCGGTCTCGGTGGTCCAGGCCATCCGCACCGGCTGGATCCACCCCAACAT CAACCTGGAGAACCCCGACGAGGGCGTGGACACCAAGCTGCTGGTGGGCCCCAAGAAGGAGCGCCTGA ACGTCAAGGTGGGCCTGTCCAACAGCTTCGGCTTCGGCGGCCACAACTCGTCCATCCTGTTCGCGCCC TATATCTGA SEQ ID NO: 49 Codon-optimized Cuphea hookeriana KASIV ATGGTGGCCGCCGCCGCCTCCAGCGCCTTCTTCCCCGTGCCCGCCCCCGGCGCCTCCCCCAAGCCCGG CAAGTTCGGCAACTGGCCCTCCAGCCTGAGCCCCTCCTTCAAGCCCAAGTCCATCCCCAACGGCGGCT TCCAGGTGAAGGCCAACGACAGCGCCCACCCCAAGGCCAACGGCTCCGCCGTGAGCCTGAAGAGCGGC AGCCTGAACACCCAGGAGGACACCTCCTCCAGCCCCCCCCCCCGCACCTTCCTGCACCAGCTGCCCGA CTGGAGCCGCCTGCTGACCGCCATCACCACCGTGTTCGTGAAGTCCAAGCGCCCCGACATGCACGACC GCAAGTCCAAGCGCCCCGACATGCTGGTGGACAGCTTCGGCCTGGAGTCCACCGTGCAGGACGGCCTG GTGTTCCGCCAGTCCTTCTCCATCCGCTCCTACGAGATCGGCACCGACCGCACCGCCAGCATCGAGAC CCTGATGAACCACCTGCAGGAGACCTCCCTGAACCACTGCAAGAGCACCGGCATCCTGCTGGACGGCT TCGGCCGCACCCTGGAGATGTGCAAGCGCGACCTGATCTGGGTGGTGATCAAGATGCAGATCAAGGTG AACCGCTACCCCGCCTGGGGCGACACCGTGGAGATCAACACCCGCTTCAGCCGCCTGGGCAAGATCGG CATGGGCCGCGACTGGCTGATCTCCGACTGCAACACCGGCGAGATCCTGGTGCGCGCCACCAGCGCCT ACGCCATGATGAACCAGAAGACCCGCCGCCTGTCCAAGCTGCCCTACGAGGTGCACCAGGAGATCGTG CCCCTGTTCGTGGACAGCCCCGTGATCGAGGACTCCGACCTGAAGGTGCACAAGTTCAAGGTGAAGAC CGGCGACAGCATCCAGAAGGGCCTGACCCCCGGCTGGAACGACCTGGACGTGAACCAGCACGTGTCCA ACGTGAAGTACATCGGCTGGATCCTGGAGAGCATGCCCACCGAGGTGCTGGAGACCCAGGAGCTGTGC TCCCTGGCCCTGGAGTACCGCCGCGAGTGCGGCCGCGACTCCGTGCTGGAGAGCGTGACCGCCATGGA CCCCAGCAAGGTGGGCGTGCGCTCCCAGTACCAGCACCTGCTGCGCCTGGAGGACGGCACCGCCATCG TGAACGGCGCCACCGAGTGGCGCCCCAAGAACGCCGGCGCCAACGGCGCCATCTCCACCGGCAAGACC AGCAACGGCAACTCCGTGTCCATGTGA SEQ ID NO: 50 Prototheca moriformis (UTEX 1435) KAS1 allele 1 5' donor sequence gctcttcctcaccgcgtgaattgctgtcccaaacgtaagcatcatcgtggctcggtcacgcgatcctg gatccggggatcctagaccgctggtggagagcgctgccgtcggattggtggcaagtaagattgcgcag gttggcgaagggagagaccaaaaccggaggctggaagcgggcacaacatcgtattattgcgtatagta gagcagtggcagtcgcatttcgaggtccgcaacggatctcgcaagctcgctacgctcacagtaggaga aaggggaccactgcccctgccagaatggtcgcgaccctctccctcgccggccccgcctgcaacacgca gtgcgtatccggcaagcgggctgtcgccttcaaccgcccccatgttggcgtccgggctcgatcaggtg cgctgaggggggtttggtgtgcccgcgcctctgggcccgtgtcggccgtgcggacgtggggccctggg cagtggatcagcagggtttgcgtgcaaatgcctataccggcgattgaatagcgatgaacgggatacgg ttgcgctcactccatgcccatgcgaccccgtttctgtccgccagccgtggtcgcccgggctgcgaagc gggaccccacccagcgcattgtgatcaccggaatgggcgtgggtacc SEQ ID NO: 51 Prototheca moriformis (UTEX 1435) KAS1 allele 1 3' donor sequence gagctccacctgcatccgcctggcgctcgaggacgccggcgtctcgcccgacgaggtcaactacgtca acgcgcacgccacctccaccctggtgggcgacaaggccgaggtgcgcgcggtcaagtcggtctttggc gacatgaagggcatcaagatgaacgccaccaagtccatgatcgggcactgcctgggcgccgccggcgg catggaggccgtcgccacgctcatggccatccgcaccggctgggtgcaccccaccatcaaccacgaca accccatcgccgaggtcgacggcctggacgtcgtcgccaacgccaaggcccagcacaaaatcaacgtc gccatctccaactccttcggcttcggcgggcacaactccgtcgtcgcctttgcgcccttccgcgagta ggcggagcgagcgcgcttggctgaggagggaggcggggtgcgagccctttggctgcgcgcgatactct ccccgcacgagcagactccacgcgcctgaatctacttgtcaacgagcaaccgtgtgttttgtccgtgg ccattcttattatttctccgactgtggccgtactctgtttggctgtgcaagcaccgaagagcc SEQ ID NO: 52 Prototheca moriformis (UTEX 1435) KAS1 allele 2 5' donor sequence gctcttcgcgcaagctcgctacgctcacagtaggagataggggaccactgcccctgccagaatggtcg cgaccctgtccctcgccggccccgcctgcaacacgcagtgcgtatccagcaagcgggttgtcgccttc aaccgcccccatgttggcgtccgggctcgatcaggtgcgctgaggggggtttggtgggcccgcgcctc tgggcccgtgtcggccgtgcggacgtggggcccggggtagtggatcagcaggggttgcatgcaaatgc ctataccggcgattgaatagcgatgaacgggatacggttgcgctcactccatgcccatgcgaccccgt ttctgtccgccagccgtggtcgcccgagctgcgaagcgggaccccacccagcgcattgtgatcaccgg aatgggcgtggcctccgtgtttggcaacgatgtcgagaccttttacgacaagcttctggaaggaacga gcggcgtggacctgatttccaggtgcgtaggtccttggatgaatgcgtctaggttgcgaggtgactgg ccaggaagcagcaggcttggggtttggtgttctgatttctggtaatttgaggtttcattataagattc tgtacggtcttgtttcggggtacc SEQ ID NO: 53 Prototheca moriformis (UTEX 1435) KAS1 allele 2 3' donor sequence gagctccacctgcatccgcctggcgctcgaggacgccggcgtctcgcccgacgaggtcaactacgtca acgcgcacgccacctccaccctggtgggcgacaaggccgaggtgcgcgcggtcaagtcggtctttggc gacatgaagggcatcaagatgaacgccaccaagtccatgatcgggcactgcctgggcgccgccggcgg catggaggccgtcgccacgctcatggccatccgcaccggctgggtgcaccccaccatcaaccacgaca accccatcgccgaggtcgacggcctggacgtcgtcgccaacgccaaggcccagcacaaaatcaacgtc gccatctccaactccttcggcttcggcgggcacaactccgtcgtcgcctttgcgcccttccgcgagta ggcggagcgagcgcgcttggctgaggagggaggcggggtgcgagccctttggctgcgcgcgatactct ccccgcacgagcagactccacgcgcctgaatctacttgtcaacgagcaaccgtgtgttttgtccgtgg ccattcttattatttctccgactgtggccgtactctgtttggctgtgcaagcaccgaagagcc SEQ ID NO: 54 Prototheca moriformis (UTEX 1435) KSI-hairpin B actagtcaTGGTCGCCCGGGCTGCGAAGCGGGACCCCACCCAGCGCATTGTGATCACCGGAATGGGCG TGGCCTCCGTGTTTGGCAACGATGTCGAGACCTTTTACAgtgtgtttgagggttttggttgcccgtat tgaggtcctggtggcgcgcatggaggagaaggcgcctgtcccgctgacccccccggctaccctcccgg caccttccagggcgcgtacgggatccTGTAAAAGGTCTCGACATCGTTGCCAAACACGGAGGCCACGC CCATTCCGGTGATCACAATGCGCTGGGTGGGGTCCCGCTTCGCAGCCCGGGCGACCAaagctt SEQ ID NO: 55 Prototheca moriformis (UTEX 1435) KSI-hairpin C actagtcaTTGACATCTCCGAGTTCCCGACCAAGTTTGCGGCGCAGATCACCGGCTTCTCCGTGGAGG ACTGCGTGGACAAGAAGAACGCGCGGCGGTACGACGACGCGCTGTCGTACGCGATGGTGGCCTCCAAG AAGGCCCTGCGCCAGGCGGGACTGGAGAAGGACAAGTGCCCCGAGGGCTACGGAGgtgtgtttgaggg ttttggttgcccgtattgaggtcctggtggcgcgcatggaggagaaggcgcctgtcccgctgaccccc ccggctaccctcccggcaccttccagggcgcgtacgggatccCTCCGTAGCCCTCGGGGCACTTGTCC TTCTCCAGTCCCGCCTGGCGCAGGGCCTTCTTGGAGGCCACCATCGCGTACGACAGCGCGTCGTCGTA CCGCCGCGCGTTCTTCTTGTCCACGCAGTCCTCCACGGAGAAGCCGGTGATCTGCGCCGCAAACTTGG TCGGGAACTCGGAGATGTCAAaagctt SEQ ID NO: 56 Prototheca moriformis (UTEX 1435) KSI-hairpin D actagtcaTGGGCGTGAGCACCTGCATCCGCCTGGCGCTCGAGGACGCCGGCGTCTCGCCCGACGAGG TCAACTACGTCAACGCGCACGCCACCTCCACCCTGGTGGGCGACAAGGCCGAGGTGCGCGCGGTCAAG TCGGTCTTTGGCGACATGAAGGGCATCAAGATgtgtgtttgagggttttggttgcccgtattgaggtc ctggtggcgcgcatggaggagaaggcgcctgtcccgctgacccccccggctaccctcccggcaccttc cagggcgcgtacgggatccATCTTGATGCCCTTCATGTCGCCAAAGACCGACTTGACCGCGCGCACCT CGGCCTTGTCGCCCACCAGGGTGGAGGTGGCGTGCGCGTTGACGTAGTTGACCTCGTCGGGCGAGACG CCGGCGTCCTCGAGCGCCAGGCGGATGCAGGTGCTCACGCCCAaagctt SEQ ID NO: 57 Prototheca moriformis (UTEX 1435) KSI-hairpin E actagtcaCAACCATCAACCACGACAACCCCATCGCCGAGGTCGACGGCCTGGACGTCGTCGCCAACG CCAAGGCCCAGCACAAAATCAACGTCGCCATCTCCAACTCCTTCGgtgtgtttgagggttttggttgc ccgtattgaggtcctggtggcgcgcatggaggagaaggcgcctgtcccgctgacccccccggctaccc tcccggcaccttccagggcgcgtacgggatccCGAAGGAGTTGGAGATGGCGACGTTGATTTTGTGCT GGGCCTTGGCGTTGGCGACGACGTCCAGGCCGTCGACCTCGGCGATGGGGTTGTCGTGGTTGATGGTa agctt SEQ ID NO: 58 Codon optimized M. polymorpha FAE3 (GenBank Accession No. AAP74370) ATGgactcccgcgcccagaaccgcgacggcggcgaggacgtgaagcaggagctgctgtccgccggcga cgacggcaaggtgccctgccccaccgtggccatcggcatccgccagcgcctgcccgacttcctgcagt ccgtgaacatgaagtacgtgaagctgggctaccactacctgatcacccacgccatgttcctgctgacc ctgcccgccttcttcctggtggccgccgagatcggccgcctgggccacgagcgcatctaccgcgagct gtggacccacctgcacctgaacctggtgtccatcatggcctgctcctccgccctggtggccggcgcca ccctgtacttcatgtcccgcccccgccccgtgtacctggtggagttcgcctgctaccgccccgacgag cgcctgaaggtgtccaaggacttcttcctggacatgtcccgccgcaccggcctgttctcctcctcctc catggacttccagaccaagatcacccagcgctccggcctgggcgacgagacctacctgccccccgcca tcctggcctccccccccaacccctgcatgcgcgaggcccgcgaggaggccgccatggtgatgttcggc gccctggacgagctgttcgagcagaccggcgtgaagcccaaggagatcggcgtgctggtggtgaactg ctccctgttcaaccccaccccctccatgtccgccatgatcgtgaaccactaccacatgcgcggcaaca tcaagtccctgaacctgggcggcatgggctgctccgccggcctgatctccatcgacctggcccgcgac ctgctgcaggtgcacggcaacacctacgccgtggtggtgtccaccgagaacatcaccctgaactggta cttcggcgacgaccgctccaagctgatgtccaactgcatcttccgcatgggcggcgccgccgtgctgc tgtccaacaagcgccgcgagcgccgccgcgccaagtacgagctgctgcacaccgtgcgcacccacaag ggcgccgacgacaagtgcttccgctgcgtgtaccaggaggaggactccaccggctccctgggcgtgtc cctgtcccgcgagctgatggccgtggccggcaacgccctgaaggccaacatcaccaccctgggccccc tggtgctgcccctgtccgagcagatcctgttcttcgcctccctggtggcccgcaagttcctgaacatg aagatgaagccctacatccccgacttcaagctggccttcgagcacttctgcatccacgccggcggccg cgccgtgctggacgagctggagaagaacctggacctgaccgagtggcacatggagccctcccgcatga ccctgtaccgcttcggcaacacctcctcctcctccctgtggtacgagctggcctacaccgaggcccag ggccgcgtgaagcgcggcgaccgcctgtggcagatcgccttcggctccggcttcaagtgcaactccgc cgtgtggcgcgcgctgcgcaccgtgaagccccccgtgaacaacgcctggtccgacgtgatcgaccgct tccccgtgaagctgccccagttcTGA SEQ ID NO: 59 M. polymorpha FAE3 (GenBank Accession No. AAP74370) MDSRAQNRDGGEDVKQELLSAGDDGKVPCPTVAIGIRQRLPDFLQSVNMKYVKLGYHYLITHAMFLLT LPAFFLVAAEIGRLGHERIYRELWTHLHLNLVSIMACSSALVAGATLYFMSRPRPVYLVEFACYRPDE RLKVSKDFFLDMSRRTGLFSSSSMDFQTKITQRSGLGDETYLPPAILASPPNPCMREAREEAAMVMFG ALDELFEQTGVKPKEIGVLVVNCSLFNPIPSMSAMIVNHYHMRGNIKSLNLGGMGCSAGLISIDLARD LLQVHGNIYAVVVSTENITLNWYFGDDRSKLMSNCIFRMGGAAVLLSNKRRERRRAKYELLHIVRTHK GADDKCFRCVYQEEDSIGSLGVSLSRELMAVAGNALKANITTLGPLVLPLSEQILFFASLVARKFLNM KMKPYIPDFKLAFEHFCIHAGGRAVLDELEKNLDLTEWHMEPSRMTLYRFGNISSSSLWYELAYTEAQ GRVKRGDRLWQIAFGSGFKCNSAVWRALRIVKPPVNNAWSDVIDRFPVKLPQF SEQ ID NO: 60 Trypanosoma brucei ELO3 (GenBank Accession No. AAX70673) ##STR00531## gtggatgctggaccacccctccgtgccctacatcgccggcgtgatgtacctgatcctggtgctgtacg tgcccaagtccatcatggcctcccagccccccctgaacctgcgcgccgccaacatcgtgtggaacctg ttcctgaccctgttctccatgtgcggcgcctactacaccgtgccctacctggtgaaggccttcatgaa ccccgagatcgtgatggccgcctccggcatcaagctggacgccaacacctcccccatcatcacccact ccggcttctacaccaccacctgcgccctggccgactccttctacttcaacggcgacgtgggcttctgg gtggccctgttcgccctgtccaagatccccgagatgatcgacaccgccttcctggtgttccagaagaa gcccgtgatcttcctgcactggtaccaccacctgaccgtgatgctgttctgctggttcgcctacgtgc agaagatctcctccggcctgtggttcgcctccatgaactactccgtgcactccatcatgtacctgtac tacttcgtgtgcgcctgcggccaccgccgcctggtgcgccccttcgcccccatcatcaccttcgtgca gatcttccagatggtggtgggcaccatcgtggtgtgctacacctacaccgtgaagcacgtgctgggcc
gctcctgcaccgtgaccgacttctccctgcacaccggcctggtgatgtacgtgtcctacctgctgctg ttctcccagctgttctaccgctcctacctgtccccccgcgacaaggcctccatcccccacgtggccgc ##STR00532## SEQ ID NO: 61 Trypanosoma brucei ELO3 (GenBank Accession No. AAX70673) MYPTHRDLILNNYSDIYRSPTCHYHTWHTLIHTPINELLFPNLPRECDFGYDIPYFRGQIDVFDGWSM IHFTSSNWCIPITVCLCYIMMIAGLKKYMGPRDGGRAPIQAKNYIIAWNLFLSFFSFAGVYYTVPYHL FDPENGLFAQGFYSTVCNNGAYYGNGNVGFFVWLFIYSKIFELVDIFFLLIRKNPVIFLHWYHHLTVL LYCWHAYSVRIGIGIWFATMNYSVHSVMYLYFAMTQYGPSTKKFAKKFSKFITTIQILQMVVGIIVTF AAMLYVTFDVPCYTSLANSVLGLMMYASYFVLFVQLYVSHYVSPKHVKQE SEQ ID NO: 62 Codon optimized Saccharomyces cerevisiae ELO1 (GenBank Accession No. P39540) ##STR00533## cttcttcaacatctacctgtgggactacttcaaccgcgccgtgggctgggccaccgccggccgcttcc agcccaaggacttcgagttcaccgtgggcaagcagcccctgtccgagccccgccccgtgctgctgttc atcgccatgtactacgtggtgatcttcggcggccgctccctggtgaagtcctgcaagcccctgaagct gcgcttcatctcccaggtgcacaacctgatgctgacctccgtgtccttcctgtggctgatcctgatgg tggagcagatgctgcccatcgtgtaccgccacggcctgtacttcgccgtgtgcaacgtggagtcctgg acccagcccatggagaccctgtactacctgaactacatgaccaagttcgtggagttcgccgacaccgt gctgatggtgctgaagcaccgcaagctgaccttcctgcacacctaccaccacggcgccaccgccctgc tgtgctacaaccagctggtgggctacaccgccgtgacctgggtgcccgtgaccctgaacctggccgtg cacgtgctgatgtactggtactacttcctgtccgcctccggcatccgcgtgtggtggaaggcctgggt gacccgcctgcagatcgtgcagttcatgctggacctgatcgtggtgtactacgtgctgtaccagaaga tcgtggccgcctacttcaagaacgcctgcaccccccagtgcgaggactgcctgggctccatgaccgcc atcgccgccggcgccgccatcctgacctcctacctgttcctgttcatctccttctacatcgaggtgta ##STR00534## SEQ ID NO: 63 Saccharomyces cerevisiae ELO1 (GenBank Accession No. P39540) MVSDWKNFCLEKASRFRPTIDRPFFNIYLWDYFNRAVGWATAGRFQPKDFEFTVGKQPLSEPRPVLLF IAMYYVVIFGGRSLVKSCKPLKLRFISQVHNLMLTSVSFLWLILMVEQMLPIVYRHGLYFAVCNVESW TQPMETLYYLNYMTKFVEFADTVLMVLKHRKLTFLHTYHHGATALLCYNQLVGYTAVTWVPVTLNLAV HVLMYWYYFLSASGIRVWWKAWVTRLQIVQFMLDLIVVYYVLYQKIVAAYFKNACTPQCEDCLGSMTA IAAGAAILTSYLFLFISFYIEVYKRGSASGKKKINKNN SEQ ID NO: 64 Codon optimized Brassica napus acyl-ACP thioesterase (GenBank Accession No. CAA52070) with 3X FLAG Tag ATGctgaagctgtcctgcaacgtgaccaacaacctgcacaccttctccttcttctccgactcctccct gttcatccccgtgaaccgccgcaccatcgccgtgtcctccgggcgcgcctcccagctgcgcaagcccg ccctggaccccctgcgcgccgtgatctccgccgaccagggctccatctcccccgtgaactcctgcacc cccgccgaccgcctgcgcgccggccgcctgatggaggacggctactcctacaaggagaagttcatcgt gcgctcctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacctgctgcaggagg tggcctgcaaccacgtgcagaagtgcggcttctccaccgacggcttcgccaccaccctgaccatgcgc aagctgcacctgatctgggtgaccgcccgcatgcacatcgagatctacaagtaccccgcctggtccga cgtggtggagatcgagacctggtgccagtccgagggccgcatcggcacccgccgcgactggatcctgc gcgactccgccaccaacgaggtgatcggccgcgccacctccaagtgggtgatgatgaaccaggacacc cgccgcctgcagcgcgtgaccgacgaggtgcgcgacgagtacctggtgttctgcccccgcgagccccg cctggccttccccgaggagaacaactcctccctgaagaagatccccaagctggaggaccccgcccagt actccatgctggagctgaagccccgccgcgccgacctggacatgaaccagcacgtgaacaacgtgacc tacatcggctgggtgctggagtccatcccccaggagatcatcgacacccacgagctgcaggtgatcac cctggactaccgccgcgagtgccagcaggacgacatcgtggactccctgaccacctccgagatccccg acgaccccatctccaagttcaccggcaccaacggctccgccatgtcctccatccagggccacaacgag tcccagttcctgcacatgctgcgcctgtccgagaacggccaggagatcaaccgcggccgcacccagtg gcgcaagaagtoctoccgcATGGACTACAAGGACCACGACGGCGACTACAAGGACCACGACATCGACT ACAAGGACGACGACGACAAGTGA SEQ ID NO: 65 Brassica napus acyl-ACP thioesterase (Genbank Accession No. CAA52070) with 3X FLAG Tag (bold) ##STR00535## PADRLRAGRLMEDGYSYKEKFIVRSYEVGINKTATVETIANLLQEVACNHVQKCGFSTDGFATTLTMR KLHLIWVTARMHIETYKYPAWSDVVEIETWCQSEGRIGTRRDWILRDSATNEVIGRATSKWVMMNQDT RRLQRVTDEVRDEYLVFCPREPRLAFFEENNSSLKKIPKLEDPAQYSMLELKPRRADLDMNQHVNNVT YIGWVLESIPQEIIDTHELQVITLDYRRECQQDDIVDSLTTSEIPDDPISKFTGTNGSAMSSIQGHNE SQFLHMLRLSENGQEINRGRTQWRKKSSRMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 66 Codon optimized Brassica napus acyl-ACP thioesterase (GenBank Accession No. CAA52070), with UTEX 250 stearoyl-ACP desaturase (SAD) chloroplast transit peptide and 3X FLAG Tag ATGgccaccgcatccactttctcggcgttcaatgcccgctgcggcgacctgcgtcgctcggcgggctc cgggccccggcgcccagcgaggcccctccccgtgcgcgggcgcgcctcccagctgcgcaagcccgccc tggaccccctgcgcgccgtgatctccgccgaccagggctccatctcccccgtgaactcctgcaccccc gccgaccgcctgcgcgccggccgcctgatggaggacggctactcctacaaggagaagttcatcgtgcg ctcctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacctgctgcaggaggtgg cctgcaaccacgtgcagaagtgcggcttctccaccgacggcttcgccaccaccctgaccatgcgcaag ctgcacctgatctgggtgaccgcccgcatgcacatcgagatctacaagtaccccgcctggtccgacgt ggtggagatcgagacctggtgccagtccgagggccgcatcggcacccgccgcgactggatcctgcgcg actccgccaccaacgaggtgatcggccgcgccacctccaagtgggtgatgatgaaccaggacacccgc cgcctgcagcgcgtgaccgacgaggtgcgcgacgagtacctggtgttctgcccccgcgagccccgcct ggccttccccgaggagaacaactcctccctgaagaagatccccaagctggaggaccccgcccagtact ccatgctggagctgaagccccgccgcgccgacctggacatgaaccagcacgtgaacaacgtgacctac atcggctgggtgctggagtccatcccccaggagatcatcgacacccacgagctgcaggtgatcaccct ggactaccgccgcgagtgccagcaggacgacatcgtggactccctgaccacctccgagatccccgacg accccatctccaagttcaccggcaccaacggctccgccatgtcctccatccagggccacaacgagtcc cagttcctgcacatgctgcgcctgtccgagaacggccaggagatcaaccgcggccgcacccagtggcg caagaagtoctoccgcATGGACTACAAGGACCACGACGGCGACTACAAGGACCACGACATCGACTACA AGGACGACGACGACAAGTGA SEQ ID NO: 67 Brassica napus acyl-ACP thioesterase (GenBank Accession No. CAA52070) with UTEX 250 stearoyl-ACP desaturase (SAD) chloroplast transit peptide and 3X FLAG .RTM. Tag ##STR00536## ADRLRAGRLMEDGYSYKEKFIVRSYEVGINKTATVETIANLLQEVACNHVQKCGFSTDGFATTLTMRK LHLIWVTARMHIETYKYPAWSDVVEIETWCQSEGRIGIRRDWILRDSATNEVIGRATSKWVMMNQDTR RLQRVTDEVRDEYLVFCPREPRLAFPEENNSSLKKIPKLEDPAQYSMLELKPRRADLDMNQHVNNVTY IGWVLESIPQEIIDTHELQVITLDYRRECQQDDIVDSLTTSEIPDDPISKFTGTNGSAMSSIQGHNES QFLHMLRLSENGQEINRGRTQWRKKSSRMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 68 Codon optimized C. tinctorius FATA (GenBank Accession No. AAA33019) with UTEX 250 stearoyl-ACP desaturase (SAD) chloroplast transit peptide and 3X FLAG .RTM. Tag ##STR00537## gcgtggcctccctgcgcgaggccgacaaggagaagtccctgggcaaccgcctgcgcctgggctccctg accgaggacggcctgtcctacaaggagaagttcgtgatccgctgctacgaggtgggcatcaacaagac cgccaccatcgagaccatcgccaacctgctgcaggaggtgggcggcaaccacgcccagggcgtgggct tctccaccgacggcttcgccaccaccaccaccatgcgcaagctgcacctgatctgggtgaccgcccgc atgcacatcgagatctaccgctaccccgcctggtccgacgtgatcgagatcgagacctgggtgcaggg cgagggcaaggtgggcacccgccgcgactggatcctgaaggactacgccaacggcgaggtgatcggcc gcgccacctccaagtgggtgatgatgaacgaggacacccgccgcctgcagaaggtgtccgacgacgtg cgcgaggagtacctggtgttctgcccccgcaccctgcgcctggccttccccgaggagaacaacaactc catgaagaagatccccaagctggaggaccccgccgagtactcccgcctgggcctggtgccccgccgct ccgacctggacatgaacaagcacgtgaacaacgtgacctacatcggctgggccctggagtccatcccc cccgagatcatcgacacccacgagctgcaggccatcaccctggactaccgccgcgagtgccagcgcga cgacatcgtggactccctgacctcccgcgagcccctgggcaacgccgccggcgtgaagttcaaggaga tcaacggctccgtgtcccccaagaaggacgagcaggacctgtcccgcttcatgcacctgctgcgctcc gccggctccggcctggagatcaaccgctgccgcaccgagtggcgcaagaagcccgccaagcgcATGGA ##STR00538## SEQ ID NO: 69 C. tinctorius FATA (GenBank Accession No. AAA33019) with UTEX 250 stearoyl-ACP desaturase (SAD) chloroplast transit peptide ##STR00539## TEDGLSYKEKFVIRCYEVGINKTATIETIANLLQEVGGNHAQGVGFSTDGFATTTTMRKLHLIWVTAR MHIETYRYPAWSDVIEIETWVQGEGKVGTRRDWILKDYANGEVIGRATSKWVMMNEDTRRLQKVSDDV REEYLVFCPRTLRLAFPEENNNSMKKIPKLEDPAEYSRLGLVPRRSDLDMNKHVNNVTYIGWALESIP PEIIDTHELQAITLDYRRECQRDDIVDSLTSREPLGNAAGVKFKEINGSVSPKKDEQDLSRFMHLLRS AGSGLEINRCRIEWRKKPAKRMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 70 Codon optimized R. communis FATA (Genbank Accession No. ABS30422) with a 3xFLAG epitope tag ##STR00540## gacccacttcaacaaccgcccctacttcacccgccgcccctccatccccaccttcttctcctccaaga actcctccgcctccctgcaggccgtggtgtccgacatctcctccgtggagtccgccgcctgcgactcc ctggccaaccgcctgcgcctgggcaagctgaccgaggacggcttctcctacaaggagaagttcatcgt ggggcgcgcccgctcctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacctgc tgcaggaggtgggctgcaaccacgcccagtccgtgggcttctccaccgacggcttcgccaccaccacc tccatgcgcaagatgcacctgatctgggtgaccgcccgcatgcacatcgagatctacaagtaccccgc ctggtccgacgtggtggaggtggagacctggtgccagtccgagggccgcatcggcacccgccgcgact ggatcctgaccgactacgccaccggccagatcatcggccgcgccacctccaagtgggtgatgatgaac caggacacccgccgcctgcagaaggtgaccgacgacgtgcgcgaggagtacctggtgttctgcccccg cgagctgcgcctggccttccccgaggagaacaaccgctcctccaagaagatctccaagctggaggacc ccgcccagtactccaagctgggcctggtgccccgccgcgccgacctggacatgaaccagcacgtgaac aacgtgacctacatcggctgggtgctggagtccatcccccaggagatcatcgacacccacgagctgca gaccatcaccctggactaccgccgcgagtgccagcacgacgacatcgtggactccctgacctccgtgg agccctccgagaacctggaggccgtgtccgagctgcgcggcaccaacggctccgccaccaccaccgcc ggcgacgaggactgccgcaacttcctgcacctgctgcgcctgtccggcgacggcctggagatcaaccg cggccgcaccgagtggcgcaagaagtccgcccgcATGGACTACAAGGACCACGACGGCGACTACAAGG ##STR00541## SEQ ID NO: 71 R. communis FATA (Genbank Accession No. AB530422) with a 3xFLAG .RTM. epitope tag ##STR00542## SMRKMHLIWVTARMHIETYKYPAWSDVVEVETWCQSEGRIGIRRDWILTDYATGQIIGRATSKWVMMN QDTRRLQKVTDDVREEYLVFCPRELRLAFFEENNRSSKKISKLEDPAQYSKLGLVPRRADLDMNQHVN NVTYIGWVLESIPQEIIDTHELQTITLDYRRECQHDDIVDSLTSVEPSENLEAVSELRGTNGSATTTA GDEDCRNFLHLLRLSGDGLEINRGRIEWRKKSARMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 72 Codon optimized G. mangostana FATA1 (GenBank Accession No. AAB51523) with 3X FLAG .RTM. epitope tag ##STR00543## ccccccccgcatcatcgtggtgtcctcctcctcctccaaggtgaaccccctgaagaccgaggccgtgg tgtcctccggcctggccgaccgcctgcgcctgggctccctgaccgaggacggcctgtcctacaaggag aagttcatcgtgcgctgctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacct gctgcaggaggtgggctgcaaccacgcccagtccgtgggctactccaccggcggcttctccaccaccc ccaccatgcgcaagctgcgcctgatctgggtgaccgcccgcatgcacatcgagatctacaagtacccc gcctggtccgacgtggtggagatcgagtcctggggccagggcgagggcaagatcggcacccgccgcga ctggatcctgcgcgactacgccaccggccaggtgatcggccgcgccacctccaagtgggtgatgatga accaggacacccgccgcctgcagaaggtggacgtggacgtgcgcgacgagtacctggtgcactgcccc cgcgagctgcgcctggccttccccgaggagaacaactcctccctgaagaagatctccaagctggagga cccctcccagtactccaagctgggcctggtgccccgccgcgccgacctggacatgaaccagcacgtga acaacgtgacctacatcggctgggtgctggagtccatgccccaggagatcatcgacacccacgagctg cagaccatcaccctggactaccgccgcgagtgccagcacgacgacgtggtggactccctgacctcccc cgagccctccgaggacgccgaggccgtgttcaaccacaacggcaccaacggctccgccaacgtgtccg ccaacgaccacggctgccgcaacttcctgcacctgctgcgcctgtccggcaacggcctggagatcaac cgcggccgcaccgagtggcgcaagaagcccacccgcATGGACTACAAGGACCACGACGGCGACTACAA ##STR00544## SEQ ID NO: 73 G. mangostana FATA1 (GenBank Accession No. AAB51523) with 3X FLAG .RTM. epitope tag MLKLSSSRSPLARIPTRPRPNSIPPRIIVVSSSSSKVNPLKTEAVVSSGLADRLRLGSLTEDGLSYKE KFIVRCYEVGINKTATVETIANLLQEVGCNHAQSVGYSIGGESTIPTMRKLRLIWVTARMHIETYKYP AWSDVVEIESWGQGEGKIGIRRDWILRDYATGQVIGRATSKWVMMNQDTRRLQKVDVDVRDEYLVHCP RELRLAFPEENNSSLKKISKLEDPSQYSKLGLVPRRADLDMNQHVNNVTYIGWVLESMPQEIIDTHEL QTITLDYRRECQHDDVVDSLTSPEPSEDAEAVFNHNGTNGSANVSANDHGCRNFLHLLRLSGNGLEIN RGRTEWRKKFTRMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 74 Codon optimized Theobroma cacao FATA1 with 3X FLAG .RTM. epitope tag ##STR00545## cccccccgcccccttctccttccgctggcgcacccccgtggtggtgtcctgctccccctcctcccgcc ccaacctgtcccccctgcaggtggtgctgtccggccagcagcaggccggcatggagctggtggagtcc ggctccggctccctggccgaccgcctgcgcctgggctccctgaccgaggacggcctgtcctacaagga gaagttcatcgtgcgctgctacgaggtgggcatcaacaagaccgccaccgtggagaccatcgccaacc tgctgcaggaggtgggctgcaaccacgcccagtccgtgggctactccaccgacggcttcgccaccacc cgcaccatgcgcaagctgcacctgatctgggtgaccgcccgcatgcacatcgagatctacaagtaccc cgcctggtccgacgtgatcgagatcgagacctggtgccagtccgagggccgcatcggcacccgccgcg actggatcctgaaggacttcggcaccggcgaggtgatcggccgcgccacctccaagtgggtgatgatg aaccaggacacccgccgcctgcagaaggtgtccgacgacgtgcgcgaggagtacctggtgttctgccc ccgcgagctgcgcctggccttccccgaggagaacaacaactccctgaagaagatcgccaagctggacg actccttccagtactcccgcctgggcctgatgccccgccgcgccgacctggacatgaaccagcacgtg aacaacgtgacctacatcggctgggtgctggagtccatgccccaggagatcatcgacacccacgagct gcagaccatcaccctggactaccgccgcgagtgccagcaggacgacgtggtggactccctgacctccc ccgagcaggtggagggcaccgagaaggtgtccgccatccacggcaccaacggctccgccgccgcccgc gaggacaagcaggactgccgccagttcctgcacctgctgcgcctgtcctccgacggccaggagatcaa ccgcggccgcaccgagtggcgcaagaagcccgcccgcATGGACTACAAGGACCACGACGGCGACTACA ##STR00546## SEQ ID NO: 75 Theobroma cacao FATA1 with 3X FLAG .RTM. epitope tag MLKLSSCNVTDQRQALAQCRFLAPPAPFSFRWRTPVVVSCSPSSRPNLSPLQVVLSGQQQAGMELVES GSGSLADRLRLGSLTEDGLSYKEKFIVRCYEVGINKTATVETIANLLQEVGCNHAQSVGYSTDGFATT RTMRKLHLIWVTARMHIETYKYPAWSDVIEIETWCQSEGRIGTRRDWILKDFGTGEVIGRATSKWVMM NQDTRRLQKVSDDVREEYLVFCPRELRLAFPEENNNSLKKIAKLDDSFQYSRLGLMPRRADLDMNQHV NNVTYIGWVLESMPQEIIDTHELQTITLDYRRECQQDDVVDSLTSPEQVEGTEKVSAIHGTNGSAAAR EDKQDCRQFLHLLRLSSDGQEINRGRTEWRKKPARMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 76 23S rRNA for UTEX 1439, UTEX 1441, UTEX 1435, UTEX 1437 Prototheca moriformis TGTTGAAGAATGAGCCGGCGACTTAAAATAAATGGCAGGCTAAGAGAATTAATAACTCGAAACCTAAG CGAAAGCAAGTCTTAATAGGGCGCTAATTTAACAAAACATTAAATAAAATCTAAAGTCATTTATTTTA GACCCGAACCTGAGTGATCTAACCATGGTCAGGATGAAACTTGGGTGACACCAAGTGGAAGTCCGAAC CGACCGATGTTGAAAAATCGGCGGATGAACTGTGGTTAGTGGTGAAATACCAGTCGAACTCAGAGCTA GCTGGTTCTCCCCGAAATGCGTTGAGGCGCAGCAATATATCTCGTCTATCTAGGGGTAAAGCACTGTT TCGGTGCGGGCTATGAAAATGGTACCAAATCGTGGCAAACTCTGAATACTAGAAATGACGATATATTA GTGAGACTATGGGGGATAAGCTCCATAGTCGAGAGGGAAACAGCCCAGACCACCAGTTAAGGCCCCAA AATGATAATGAAGTGGTAAAGGAGGTGAAAATGCAAATACAACCAGGAGGTTGGCTTAGAAGCAGCCA TCCTTTAAAGAGTGCGTAATAGCTCACTG SEQ ID NO: 77 Cu P5R23 LPAAT2-1 MAIAAAAVIFLFGLIFFASGLIINLFQALCFVLIRPLSKNAYRRINRVFAELLLSELLCLFDWWAGAK LKLFTDPETFRLMGKEHALVIINHMTELDWMVGWVMGQHFGCLGSIISVAKKSTKFLPVLGWSMWFSE YLYLERSWAKDKSTLKSHIERLIDYPLPFWLVIFVEGTRFTRTKLLAAQQYAVSSGLPVPRNVLIPRT KGFVSCVSHMRSFVPAVYDVTVAFPKTSPPPTLLNLFEGQSIMLHVHIKRHAMKDLPESDDAVAEWCR DKFVEKDALLDKHNAEDTFSGQEVCHSGSRQLKSLLVVISWVVVTTFGALKFLQWSSWKGKAFSAIGL GIVTLLMHVLILSSQAERSNPAEVAQAKLKTGLSISKKVTDKEN SEQ ID NO: 78 CuPSR23 LPAAT3-1
MAIAAAAVIVPLSLLFFVSGLIVNLVQAVCFVLIRPLSKNTYRRINRVVAELLWLELVWLIDWWAGVK IKVFTDHETFHLMGKEHALVICNHKSDIDWLVGWVLGQRSGCLGSTLAVMKKSSKFLPVLGWSMWFSE YLFLERSWAKDEITLKSGLNRLKDYPLPFWLALFVEGTRFTRAKLLAAQQYAASSGLPVPRNVLIPRT KGFVSSVSHMRSFVPAIYDVIVAIPKTSPPPTLIRMFKGQSSVLHVHLKRHLMKDLPESDDAVAQWCR DIFVEKDALLDKHNAEDTFSGQELQETGRPIKSLLVVISWAVLEVFGAVKFLQWSSLLSSWKGLAFSG IGLGVITLLMHILILFSQSERSTPAKVAPAKPKNEGESSKTEMEKEK SEQ ID NO: 79 Amino acid sequence for CuPSR23 LPPATx MEIPPHCLCSPSPAPSQLYYKKKKHAILQTQTPYRYRVSPTCFAPPRLRKQHPYPLPVLCYPKLLHFS QPRYPLVRSHLAEAGVAYRPGYELLGKIRGVCFYAVTAAVALLLFQCMLLLHPFVLLFDPFPRKAHHT IAKLWSICSVSLFYKIHIKGLENLPPPHSPAVYVSNHQSFLDIYTLLTLGRTFKFISKTEIFLYPIIG WAMYMLGTIPLKRLDSRSQLDTLKRCMDLIKKGASVFFFPEGTRSKDGKLGAFKKGAFSIAAKSKVPV VPITLIGTGKIMPPGSELTVNPGTVQVIIHKPIEGSDAEAMCNEARATISHSLDD SEQ ID NO: 80 cDNA sequence for CuPSR23 LPAATx coding region ATGGAGATCCCGCCTCACTGTCTCTGTTCGCCTTCGCCTGCGCCTTCGCAATTGTATTACAAGAAGAA GAAGCATGCCATTCTCCAAACTCAAACTCCCTATAGATATAGAGTTTCCCCGACATGCTTTGCCCCCC CCCGATTGAGGAAGCAGCATCCTTACCCTCTCCCTGTCCTCTGCTATCCAAAACTCCTCCACTTCAGC CAGCCTAGGTACCCTCTGGTTAGATCTCATTTGGCTGAAGCTGGTGTTGCTTATCGTCCAGGATACGA ATTATTAGGAAAAATAAGGGGAGTGTGTTTCTATGCTGTCACTGCTGCCGTTGCCTTGCTTCTATTTC AGTGCATGCTCCTCCTCCATCCCTTTGTGCTCCTCTTCGATCCATTTCCAAGAAAGGCTCACCATACC ATCGCCAAACTCTGGTCTATCTGCTCTGTTTCTCTTTTTTACAAGATTCACATCAAGGGTTTGGAAAA TCTTCCCCCACCCCACTCTCCTGCCGTCTATGTCTCTAATCATCAGAGTTTTCTCGACATCTATACTC TCCTCACTCTCGGTAGAACCTTCAAGTTCATCAGCAAGACTGAGATCTTTCTCTATCCAATTATCGGT TGGGCCATGTATATGTTGGGTACCATTCCTCTCAAGCGGTTGGACAGCAGAAGCCAATTGGACACTCT TAAGCGATGTATGGATCTCATCAAGAAGGGAGCATCCGTCTTTTTCTTCCCAGAGGGAACACGAAGTA AAGATGGGAAACTGGGTGCTTTCAAGAAAGGTGCATTCAGCATCGCAGCAAAAAGCAAGGTTCCTGTT GTGCCGATCACCCTTATTGGAACTGGCAAGATTATGCCACCTGGGAGCGAACTTACTGTCAATCCAGG AACTGTGCAAGTAATCATACATAAACCTATCGAAGGAAGTGATGCAGAAGCAATGTGCAATGAAGCTA GAGCCACGATTTCTCACTCACTTGATGATTAA SEQ ID NO: 81 cDNA sequence for CuPSR23 LPAAT 2-1 coding region ATGGCGATTGCAGCGGCAGCTGTCATCTTCCTCTTCGGCCTTATCTTCTTCGCCTCCGGCCTCATAAT CAATCTCTTCCAGGCGCTTTGCTTTGTCCTTATTCGGCCTCTTTCGAAAAACGCCTACMGGAGAATAA ACAGAGTTTTTGCAGAATTGTTGTTGTCGGAGCTTTTATGCCTATTCGATTGGTGGGCTGGTGCTAAG CTCAAATTATTTACCGACCCTGAAACCTTTCGCCTTATGGGCAAGGAACATGCTCTTGTCATAATTAA TCACATGACTGAACTTGACTGGATGGTTGGATGGGTTATGGGTCAGCATTTTGGTTGCCTTGGGAGCA TAATATCTGTTGCGAAGAAATCAACAAAATTTCTTCCGGTATTGGGGTGGTCAATGTGGTTTTCAGAG TACCTATATCTTGAGAGAAGCTGGGCCAAGGATAAAAGTACATTAAAGTCACATATCGAGAGGCTGAT AGACTACCCCCTGCCCTTCTGGTTGGTAATTTTTGTGGAAGGAACTCGGTTTACTCGGACAAAACTCT TGGCAGCCCAGCAGTATGCTGTCTCATCTGGGCTACCAGTGCCGAGAAATGTTTTGATCCCACGTACT AAGGGTTTTGTTTCATGTGTAAGTCACATGCGATCATTTGTTCCAGCAGTATATGATGTCACAGTGGC ATTCCCTAAGACTTCACCTCCACCAACGTTGCTAAATCTTTTCGAGGGTCAGTCCATAATGCTTCACG TTCACATCAAGCGACATGCAATGAAAGATTTACCAGAATCCGATGATGCAGTAGCAGAGTGGTGTAGA GACAAATTTGTGGAAAAGGATGCTTTGTTGGACAAGCATAATGCTGAGGACACTTTCAGTGGTCAAGA AGTTTGTCATAGCGGCAGCCGCCAGTTAAAGTCTCTTCTGGTGGTAATATCTTGGGTGGTTGTAACAA CATTTGGGGCTCTAAAGTTCCTTCAGTGGTCATCATGGAAGGGGAAAGCATTTTCAGCTATCGGGCTG GGCATCGTCACTCTACTTATGCACGTATTGATTCTATCCTCACAAGCAGAGCGGTCTAACCCTGCGGA GGTGGCACAGGCAAAGCTAAAGACCGGGTTGTCGATCTCAAAGAAGGTAACGGACAAGGAAAACTAG SEQ ID NO: 82 cDNA sequence for CuPSR23 LPAAx 3-1 coding region ATGGCGATTGCTGCGGCAGCTGTCATCGTCCCGCTCAGCCTCCTCTTCTTCGTCTCCGGCCTCATCGT CAATCTCGTACAGGCAGTTTGCTTTGTACTGATTAGGCCTCTGTCGAAAAACACTTACAGAAGAATAA ACAGAGTGGTTGCAGAATTGTTGTGGTTGGAGTTGGTATGGCTGATTGATTGGTGGGCTGGTGTCAAG ATAAAAGTATTCACGGATCATGAAACCTTTCACCTTATGGGCAAAGAACATGCTCTTGTCATTTGTAA TCACAAGAGTGACATAGACTGGCTGGTTGGGTGGGTTCTGGGACAGCGGTCAGGTTGCCTTGGAAGCA CATTAGCTGTTATGAAGAAATCATCAAAGTTTCTCCCGGTATTAGGGTGGTCAATGTGGTTCTCAGAG TATCTATTCCTTGAAAGAAGCTGGGCCAAGGATGAAATTACATTAAAGTCAGGTTTGAATAGGCTGAA AGACTATCCCTTACCCTTCTGGTTGGCACTTTTTGTGGAAGGAACTCGGTTCACTCGAGCAAAACTCT TGGCAGCCCAGCAGTATGCTGCCTCTTCGGGGCTACCTGTGCCGAGAAATGTTCTGATCCCGCGTACT AAGGGTTTTGTTTCTTCTGTGAGTCACATGCGATCATTTGTTCCAGCCATATATGATGTTACAGTGGC AATCCCAAAGACGTCACCTCCACCAACATTGATAAGAATGTTCAAGGGACAGTCCTCAGTGCTTCACG TCCACCTCAAGCGACACCTAATGAAAGATTTACCTGAATCAGATGATGCTGTTGCTCAGTGGTGCAGA GATATATTCGTCGAGAAGGATGCTTTGTTGGATAAGCATAATGCTGAGGACACTTTCAGTGGCCAAGA ACTTCAAGAAACTGGCCGCCCAATAAAGTCTCTTCTGGTTGTAATCTCTTGGGCGGTGTTGGAGGTAT TTGGAGCTGTGAAGTTTCTTCAATGGTCATCGCTGTTATCATCATGGAAGGGACTTGCATTTTCGGGA ATAGGACTGGGTGTCATCACGCTACTCATGCACATACTGATTTTATTCTCACAATCCGAGCGGTCTAC CCCTGCAAAAGTGGCACCAGCAAAGCCAAAGAATGAGGGAGAGTCCTCCAAGACGGAAATGGAAAAGG AAAAGTAG SEQ ID NO: 83 cDNA sequence for CuPSR23 LPAATx coding region codon optimized for Prototheca moriformis ATGgagatccccccccactgcctgtgctccccctcccccgccccctcccagctgtactacaagaagaa gaagcacgccatcctgcagacccagaccccctaccgctaccgcgtgtcccccacctgcttcgcccccc cccgcctgcgcaagcagcacccctaccccctgcccgtgctgtgctaccccaagctgctgcacttctcc cagccccgctaccccctggtgcgctcccacctggccgaggccggcgtggcctaccgccccggctacga gctgctgggcaagatccgcggcgtgtgcttctacgccgtgaccgccgccgtggccctgctgctgttcc agtgcatgctgctgctgcaccccttcgtgctgctgttcgaccccttcccccgcaaggcccaccacacc atcgccaagctgtggtccatctgctccgtgtccctgttctacaagatccacatcaagggcctggagaa cctgccccccccccactcccccgccgtgtacgtgtccaaccaccagtccttcctggacatctacaccc tgctgaccctgggccgcaccttcaagttcatctccaagaccgagatcttcctgtaccccatcatcggc tgggccatgtacatgctgggcaccatccccctgaagcgcctggactcccgctcccagctggacaccct gaagcgctgcatggacctgatcaagaagggcgcctccgtgttcttcttccccgagggcacccgctcca aggacggcaagctgggcgccttcaagaagggcgccttctccatcgccgccaagtccaaggtgcccgtg gtgcccatcaccctgatcggcaccggcaagatcatgccccccggctccgagctgaccgtgaaccccgg caccgtgcaggtgatcatccacaagcccatcgagggctccgacgccgaggccatgtgcaacgaggccc gcgccaccatctcccactccctggacgacTGA SEQ ID NO: 84 cDNA sequence for CuPSR23 LPAAT 2-1 coding region codon optimized for Prototheca moriformis ATGgcgatcgcggccgcggcggtgatcttcctgttcggcctgatcttcttcgcctccggcctgatcat caacctgttccaggcgctgtgcttcgtcctgatccgccccctgtccaagaacgcctaccgccgcatca accgcgtgttcgcggagctgctgctgtccgagctgctgtgcctgttcgactggtgggcgggcgcgaag ctgaagctgttcaccgaccccgagacgttccgcctgatgggcaaggagcacgccctggtcatcatcaa ccacatgaccgagctggactggatggtgggctgggtgatgggccagcacttcggctgcctgggctcca tcatctccgtcgccaagaagtccacgaagttcctgcccgtgctgggctggtccatgtggttctccgag tacctgtacctggagcgctcctgggccaaggacaagtccaccctgaagtcccacatcgagcgcctgat cgactaccccctgcccttctggctggtcatcttcgtcgagggcacccgcttcacgcgcacgaagctgc tggcggcccagcagtacgcggtctcctccggcctgcccgtcccccgcaacgtcctgatcccccgcacg aagggcttcgtctcctgcgtgtcccacatgcgctccttcgtccccgcggtgtacgacgtcacggtggc gttccccaagacgtcccccccccccacgctgctgaacctgttcgagggccagtccatcatgctgcacg tgcacatcaagcgccacgccatgaaggacctgcccgagtccgacgacgccgtcgcggagtggtgccgc gacaagttcgtcgagaaggacgccctgctggacaagcacaacgcggaggacacgttctccggccagga ggtgtgccactccggctcccgccagctgaagtccctgctggtcgtgatctcctgggtcgtggtgacga cgttcggcgccctgaagttcctgcagtggtcctcctggaagggcaaggcgttctccgccatcggcctg ggcatcgtcaccctgctgatgcacgtgctgatcctgtcctcccaggccgagcgctccaaccccgccga ggtggcccaggccaagctgaagaccggcctgtccatctccaagaaggtgacggacaaggagaacTGA SEQ ID NO: 85 cDNA sequence for CuPSR23 LPAAx 3-1 coding region codon optimized for Prototheca moriformis ATGgccatcgcggcggccgcggtgatcgtgcccctgtccctgctgttcttcgtgtccggcctgatcgt caacctggtgcaggccgtctgcttcgtcctgatccgccccctgtccaagaacacgtaccgccgcatca accgcgtggtcgcggagctgctgtggctggagctggtgtggctgatcgactggtgggcgggcgtgaag atcaaggtcttcacggaccacgagacgttccacctgatgggcaaggagcacgccctggtcatctgcaa ccacaagtccgacatcgactggctggtcggctgggtcctgggccagcgctccggctgcctgggctcca ccctggcggtcatgaagaagtcctccaagttcctgcccgtcctgggctggtccatgtggttctccgag tacctgttcctggagcgctcctgggccaaggacgagatcacgctgaagtccggcctgaaccgcctgaa ggactaccccctgcccttctggctggcgctgttcgtggagggcacgcgcttcacccgcgcgaagctgc tggcggcgcagcagtacgccgcgtcctccggcctgcccgtgccccgcaacgtgctgatcccccgcacg aagggcttcgtgtcctccgtgtcccacatgcgctccttcgtgcccgcgatctacgacgtcaccgtggc catccccaagacgtcccccccccccacgctgatccgcatgttcaagggccagtcctccgtgctgcacg tgcacctgaagcgccacctgatgaaggacctgcccgagtccgacgacgccgtcgcgcagtggtgccgc gacatcttcgtggagaaggacgcgctgctggacaagcacaacgccgaggacaccttctccggccagga gctgcaggagaccggccgccccatcaagtccctgctggtcgtcatctcctgggccgtcctggaggtgt tcggcgccgtcaagttcctgcagtggtcctccctgctgtcctcctggaagggcctggcgttctccggc atcggcctgggcgtgatcaccctgctgatgcacatcctgatcctgttctcccagtccgagcgctccac ccccgccaaggtggcccccgcgaagcccaagaacgagggcgagtcctccaagaccgagatggagaagg agaagTGA SEQ ID NO: 86 Nucleic acid sequence encoding 14:0-ACP thioesterase, Cuphea palustris (Cpal FATB2, accession AAC49180) containing an extended heterologous transit peptide from C. protothecoides and a 41 amino acid N-terminal extension derived from the native Cpal FATB2 sequence in construct D1481 [pSZ2479] GCGCACCCCAAGGCGAACGGCAGCGCGGTGTCGCTGAAGTCGGGCTCCCIGGAGACCCAGGAGGACAA GACGAGCAGCTCGTCCCCCCCCCCCCGCACGTTCATCAACCAGCTGCCCGTGTGGAGCATGCTGCTGT CGGCGGTGACCACGGTCTTCGGCGTGGCCGAGAAGCAGTGGCCCATGCTGGACCGCAAGTCCAAGCGC CCCGACATGCTGGTCGAGCCCCTGGGCGTGGACCGCATCGTCTACGACGGCGTGAGCTTCCGCCAGTC GTTCTCCATCCGCAGCTACGAGATCGGCGCCGACCGCACCGCCTCGATCGAGACGCTGATGAACATGT TCCAGGAGACCTCCCTGAACCACTGCAAGATCATCGGCCTGCTGAACGACGGCTTCGGCCGCACGCCC GAGATGTGCAAGCGCGACCTGATCTGGGTCGTGACCAAGATGCAGATCGAGGTGAACCGCTACCCCAC GTGGGGCGACACCATCGAGGTCAACACGTGGGTGAGCGCCTCGGGCAAGCACGGCATGGGCCGCGACT GGCTGATCTCCGACTGCCACACCGGCGAGATCCTGATCCGCGCGACGAGCGTCTGGGCGATGATGAAC CAGAAGACCCGCCGCCTGTCGAAGATCCCCTACGAGGTGCGCCAGGAGATCGAGCCCCAGTTCGTCGA CTCCGCCCCCGTGATCGTGGACGACCGCAAGTTCCACAAGCTGGACCTGAAGACGGGCGACAGCATCT GCAACGGCCTGACCCCCCGCTGGACGGACCTGGACGTGAACCAGCACGTCAACAACGTGAAGTACATC GGCTGGATCCTGCAGTCGGTCCCCACCGAGGTGTTCGAGACGCAGGAGCTGTGCGGCCTGACCCTGGA GTACCGCCGCGAGTGCGGCCGCGACTCCGTGCTGGAGAGCGTCACGGCCATGGACCCCTCGAAGGAGG GCGACCGCTCCCTGTACCAGCACCTGCTGCGCCTGGAGGACGGCGCGGACATCGTGAAGGGCCGCACC GAGTGGCGCCCCAAGAACGCCGGCGCCAAGGGCGCCATCCTGACGGGCAAGACCAGCAACGGCAACTC GATCTCCTGA SEQ ID NO: 87 Amino acid sequence of 14:0-ACP thioesterase, Cuphea palustris (Cpal FATB2, accession AAC49180) containing an extended heterologous transit peptide from C. protothecoides and a 41 amino acid N- terminal extension derived from the native Cpal FATB2 sequence encoded by construct D1481 [pSZ2479] AHPKANGSAVSLKSGSLETQEDKTSSSSPPPRTFINQLPVWSMLLSAVTTVFGVAEKQWP MLDRKSKRPDMLVEPLGVDRIVYDGVSFRQSFSIRSYEIGADRTASIETLMNMFQETSLN HCKIIGLLNDGFGRTPEMCKRDLIWVVTKMQIEVNRYPTWGDTIEVNTWVSASGKHGMGR DWLISDCHTGEILIRATSVMAMMNQKTRRLSKIPYEVRQEIEPQFVDSAPVIVDDRKFHK LDLKTGDSICNGLTPRWTDLDVNQHVNNVKYIGWILQSVPTEVFETQELCGLTLEYRREC GRDSVLESVTAMDPSKEGDRSLYQHLLRLEDGADIVKGRTEWRPKNAGAKGAILTGKTSN GNSIS SEQ ID No: 88 Nucleic acid sequence encoding 14:0-ACP thioesterase, Cuphea palustris (Cpal FATB2, accession AAC49180) containing an extended heterologous transit peptide from C. protothecoides, a 41 amino acid N-terminal extension derived from the native Cpal FATB2 sequence, and a C-terminal FLAG epitope tag in construct D1482 [pSZ2480] GCGCACCCCAAGGCGAACGGCAGCGCGGTGTCGCTGAAGTCGGGCTCCCTGGAGACCCAGGAGGACAA GACGAGCAGCTCGTCCCCCCCCCCCCGCACGTTCATCAACCAGCTGCCCGTGTGGAGCATGCTGCTGT CGGCGGTGACCACGGTCTTCGGCGTGGCCGAGAAGCAGTGGCCCATGCTGGACCGCAAGTCCAAGCGC CCCGACATGCTGGTCGAGCCCCTGGGCGTGGACCGCATCGTCTACGACGGCGTGAGCTTCCGCCAGTC GTTCTCCATCCGCAGCTACGAGATCGGCGCCGACCGCACCGCCTCGATCGAGACGCTGATGAACATGT TCCAGGAGACCTCCCTGAACCACTGCAAGATCATCGGCCTGCTGAACGACGGCTTCGGCCGCACGCCC GAGATGTGCAAGCGCGACCTGATCTGGGTCGTGACCAAGATGCAGATCGAGGTGAACCGCTACCCCAC GTGGGGCGACACCATCGAGGTCAACACGTGGGTGAGCGCCTCGGGCAAGCACGGCATGGGCCGCGACT GGCTGATCTCCGACTGCCACACCGGCGAGATCCTGATCCGCGCGACGAGCGTCTGGGCGATGATGAAC CAGAAGACCCGCCGCCTGTCGAAGATCCCCTACGAGGTGCGCCAGGAGATCGAGCCCCAGTTCGTCGA CTCCGCCCCCGTGATCGTGGACGACCGCAAGTTCCACAAGCTGGACCTGAAGACGGGCGACAGCATCT GCAACGGCCTGACCCCCCGCTGGACGGACCTGGACGTGAACCAGCACGTCAACAACGTGAAGTACATC GGCTGGATCCTGCAGTCGGTCCCCACCGAGGTGTTCGAGACGCAGGAGCTGTGCGGCCTGACCCTGGA GTACCGCCGCGAGTGCGGCCGCGACTCCGTGCTGGAGAGCGTCACGGCCATGGACCCCTCGAAGGAGG GCGACCGCTCCCTGTACCAGCACCTGCTGCGCCTGGAGGACGGCGCGGACATCGTGAAGGGCCGCACC GAGTGGCGCCCCAAGAACGCCGGCGCCAAGGGCGCCATCCTGACGGGCAAGACCAGCAACGGCAACTC GATCTCCatggactacaaggaccacgacggcgactacaaggaccacgacatcgactacaaggacgacg acgacaagtga SEQ ID NO: 89 Amino acid sequence of 14:0-ACP thioesterase, Cuphea palustris (Cpal FATB2, accession AAC49180) containing an extended heterologous transit peptide from C. protothecoides, a 41 amino acid N-terminal extension derived from the native Cpal FATB2 sequence, and a C- terminal FLAG epitope tag encoded by construct D1482 [pSZ2480] AHPKANGSAVSLKSGSLETQEDKTSSSSPPPRTFINQLPVWSMLLSAVTTVFGVAEKQWP MLDRKSKRPDMLVEPLGVDRIVYDGVSFRQSFSIRSYEIGADRTASIETLMNMFQETSLN HCKIIGLLNDGFGRTPEMCKRDLIWVVTKMQIEVNRYPTWGDTIEVNTWVSASGKHGMGR DWLISDCHTGEILIRATSVMAMMNQKTRRLSKIPYEVRQEIEPQFVDSAPVIVDDRKFHK LDLKTGDSICNGLTPRWTDLDVNQHVNNVKYIGWILQSVPTEVFETQELCGLTLEYRREC GRDSVLESVTAMDPSKEGDRSLYQHLLRLEDGADIVKGRTEWRPKNAGAKGAILTGKTSN GNSISMDYKDHDGDYKDHDIDYKDDDDK SEQ ID NO: 154 Cuphea hyssopifolia FATB3 coding region, codon optimized for Prototheca moriformis gtggccgccgaggcctcctccgccctgttctccgtgcgcacccccggcacctcccccaagcccggcaa gttcggcaactggcccacctccctgtccgtgcccttcaagtccaagtccaaccacaacggcggcttcc aggtgaaggccaacgcctccgcccgccccaaggccaacggctccgccgtgtccctgaagtccggctcc ctggacacccaggaggacacctcctcctcctcctcccccccccgcaccttcatcaaccagctgcccga ctggtccatgctgctgtccgccatcaccaccgtgttcgtggccgccgagaagcagtggaccatgctgg accgcaagtccaagcgccccgacatgctgatggaccccttcggcgtggaccgcgtggtgcaggacggc gccgtgttccgccagtccttctccatccgctcctacgagatcggcgccgaccgcaccgcctccatcga gaccctgatgaacatcttccaggagacctccctgaaccactgcaagtccatcggcctgctgaacgacg gcttcggccgcacccccgagatgtgcaagcgcgacctgatctgggtggtgaccaagatgcacgtggag gtgaaccgctaccccacctggggcgacaccatcgaggtgaacacctgggtgtccgagtccggcaagac cggcatgggccgcgactggctgatctccgactgccacaccggcgagatcctgatccgcgccacctcca tgtgcgccatgatgaaccagaagacccgccgcttctccaagttcccctacgaggtgcgccaggagctg gccccccacttcgtggactccgcccccgtgatcgaggactaccagaagctgcacaagctggacgtgaa gaccggcgactccatctgcaacggcctgaccccccgctggaacgacctggacgtgaaccagcacgtga acaacgtgaagtacatcggctggatcctggagtccgtgcccaccgaggtgttcgagacccaggagctg tgcggcctgaccctggagtaccgccgcgagtgcggccgcgactccgtgctggagtccgtgaccgccat ggacccctccaaggagggcgaccgctccctgtaccagcacctgctgcgcctggaggacggcgccgaca tcgccaagggccgcaccaagtggcgccccaagaacgccggcaccaacggcgccatctccaccggcaag acctccaacggcaactccatctccatggactacaaggaccacgacggcgactacaaggaccacgacat cgactacaaggacgacgacgacaag SEQ ID NO: 155 Cuphea hyssopifolia FATB1 coding region, codon optimized for Prototheca moriformis gccaccgcctccaccttctccgccttcaacgcccgctgcggcgacctgcgccgctccgccggctccgg cccccgccgccccgcccgccccctgcccgtgcgcgccgccatcaacgcctccgcccaccccaaggcca acggctccgccgtgaacctgaagtccggctccctggagacccaggaggacacctcctcctcctccccc coccoccgcaccttcatcaagcagctgcccgactggggcatgctgctgtccaagatcaccaccgtgtt cggcgccgccgagcgccagtggaagcgcccoggcatgctggtggagccottoggcgtggaccgcatct tccaggacggcgtgttottccgccagtocttctccatccgctcctacgagatcggcgccgaccgcacc gcctccatcgagaccctgatgaacatcttccaggagacctocctgaaccactgcaagtccatcggcct gctgaacgacggcttoggccgcaccoccgagatgtgcaagcgcgacctgatctgggtggtgaccaaga tccaggtggaggtgaaccgctaccccacctggggcgacaccatcgaggtgaacacctgggtgtccgag tcoggcaagaacggcatgggccgcgactggctgatctccgactgccgcaccggcgagatcctgatccg
cgccacctccgtgtgggccatgatgaaccgcaagacccgccgcctgtccaagttccoctacgaggtgc gccaggagatcgccocccacttcgtggactccgccoccgtgatcgaggacgacaagaagctgcacaag ctggacgtgaagaccggcgactccatccgcaagggcctgaccocccgctggaacgacctggacgtgaa ccagcacgtgaacaacgtgaagtacatoggctggatcctgaagtccgtgcccgccgaggtgttcgaga cccaggagctgtgoggcgtgaccctggagtaccgccgcgagtgoggccgcgactccgtgctggagtcc gtgaccgccatggacaccgccaaggagggcgaccgctocctgtaccagcacctgctgcgcctggagga cggcgccgacatcaccatoggccgcaccgagtggcgccocaagaacgccggcgccaacggcgccatct ccaccggcaagacctccaacgagaactccgtgtccatggactacaaggaccacgacggcgactacaag gaccacgacatcgactacaaggacgacgacgacaag SEQ ID NO: 156 Garcinia mangostana FATA1 CDS MLKLSSSRSPLARIPTRPRPNSIPPRIIVVSSSSSKVNPLKTEAVVSSGLADRLRLGSLTEDGLSYKEKF IVRCYEVGINKTATVETIANLLQEVGCNHAQSVGYSIGGFSTIPTMRKLRLIWVTARMHIEIYKYPAWSD VVEIESWGQGEGKIGIRRDWILRDYATGQVIGRATSKWVMMNQDTRRLQKVDVDVRDEYLVHCPRELRLA FPEENNSSLKKISKLEDPSQYSKLGLVPRRADLDMNQHVNNVIYIGWVLESMPQEIIDTHELQIITLDYR RECQHDDVVDSLTSPEPSEDAEAVFNHNGINGSANVSANDHGCRNFLHLLRLSGNGLEINRGRIEWRKKP TR SEQ ID NO: 157 Brassic napus LPAAT CDS MAMAAAVIVPLGILFFISGLVVNLLQAVCYVLVRPMSKNTYRKINRVVAETLWLELVWIVDWWAGVKIQV FADDETFNRMGKEHALVVCNHRSDIDWLVGWILAQRSGCLGSALAVMKKSSKFLPVIGWSMWFSEYLFLE RNWAKDESTLQSGLQRLNDFPRPFWLALFVEGTRFTEAKLKAAQEYAASSELPVPRNVLIPRIKGFVSAV SNMRSFVPAIYDMTVAIPKTSPPPTMLRLFKGQPSVVHVHIKCHSMKDLPEPEDEIAQWCRDQFVAKDAL LDKHIAADTFPGQKEQNIGRPIKSLAVVVSWACLLTLGAMKFLHWSNLFSSWKGIALSAFGLGIITLCMQ ILIRSSQSERSTPAKVAPAKPKDNHQSGPSSQTEVEEKQK SEQ ID NO: 158 Cuphea hookeriana FATB2 CDS MVAAAASSAFFPVPAPGASPKPGKEGNWPSSLSPSFKPKSIPNGGFQVKANDSAHPKANGSAVSLKSGSL NIQEDISSSPPPRTFLHQLPDWSRLLTAITIVFVKSKRPDMHDRKSKRPDMLVDSFGLESTVQDGLVFRQ SFSIRSYEIGTDRTASIETLMNHLQETSLNHCKSTGILLDGFGRTLEMCKRDLIWVVIKMQIKVNRYPAW GDIVEINTRFSRLGKIGMGRDWLISDCNTGEILVRATSAYAMMNQKTRRLSKLPYEVHQEIVPLFVDSPV IEDSDLKVHKEKVKIGDSIQKGLIPGWNDLDVNQHVSNVKYIGWILESMPTEVLETQELCSLALEYRREC GRDSVLESVTAMDPSKVGVRSQYQHLLRLEDGTAIVNGATEWRPKNAGANGAISIGKISNGNSVS SEQ ID NO: 159 Cuphea wrightii KASAI CDS with P moriformis SAD transit peptide (underlined) MASAAFTMSACPAMTGRAPGARRSGRPVATRLRYVFQCLVASCIDPCDQYRSSASLSFLGDNGFASLF GSKPFMSNRGHRRLRRASHSGEAMAVALQPAQEAGIKKKPVIKQRRVVVTGMGVVTPLGHEPDVFYNN LLDGVSGISEIETEDCTQFPTRIAGEIKSFSIDGWVAPKLSKRMDKFMLYLLTAGKKALADGGITDEV MKELDKRKCGVLIGSGMGGMKVFNDAIEALRVSYKKMNPFCVPFATTNMGSAMLAMDLGWMGPNYSIS TACATSNFCILNAANHIIRGEADMMLCGGSDAVIIPIGLGGFVACRALSQRNSDPIKASRPWDSNRDG FVMGEGAGVLLLEELEHAKKRGATIYAEFLGGSFTCDAYHMTEPHPEGAGVILCIEKALAQAGVSKED VNYINAHATSTSAGDIKEYQALARCFGQNSELRVNSTKSMIGHLLGAAGGVEAVTVVQAIRTGWIHPN LNLEDPDKAVDAKLLVGPKKERLNVKVGLSNSFGEGGHNSSILFAPCNV SEQ ID NO: 160 Native Protheca moriformis KASII amino acid sequence (native transit peptide is underlined) MQTAHQRPPTEGHCFGARLPTASRRAVRRAWSRIARAAAAADANPARPERRVVITGQGVVTSLGQTIE QFYSSLLEGVSGISQIQKFDTTGYTTTIAGEIKSLQLDPYVPKRWAKRVDDVIKYVYIAGKQALESAG LPIEAAGLAGAGLDPALCGVLIGTAMAGMTSFAAGVEALTRGGVRKMNPFCIPFSISNMGGAMLAMDI GFMGPNYSISTACAIGNYCILGAADHIRRGDANVMLAGGADAAIIPSGIGGFIACKALSKRNDEPERA SRPWDADRDGFVMGEGAGVLVLEELEHAKRRGATILAELVGGAATSDAHHMTEPDPQGRGVRLCLERA LERARLAPERVGYVNAHGTSTPAGDVAEYRAIRAVIPQDSLRINSTKSMIGHLLGGAGAVEAVAAIQA LRTGWLHPNLNLENPAPGVDPVVLVGPRKERAEDLDVVLSNSFGEGGHNSCVIERKYDE SEQ ID NO: 161 Mature native Protheca moriformis KASII amino acid sequence (native transit peptide is underlined) AAAAADANPARPERRVVITGQGVVISLGQIIEQFYSSLLEGVSGISQIQKFDTTGYTTTIAGEIKSLQ LDPYVPKRWAKRVDDVIKYVYIAGKQALESAGLPIEAAGLAGAGLDPALCGVLIGTAMAGMTSFAAGV EALTRGGVRKMNPFCIPFSISNMGGAMLAMDIGFMGPNYSISTACAIGNYCILGAADHIRRGDANVML AGGADAAIIPSGIGGFIACKALSKRNDEPERASRPWDADRDGFVMGEGAGVLVLEELEHAKRRGATIL AELVGGAATSDAHHMTEPDPQGRGVRLCLERALERARLAPERVGYVNAHGTSTPAGDVAEYRAIRAVI PQDSLRINSTKSMIGHLLGGAGAVEAVAAIQALRIGWLHPNLNLENPAPGVDPVVLVGPRKERAEDLD VVLSNSFGFGGHNSCVIFRKYDE SEQ ID NO: 162 CcFATB2-UcFATB2 chimeric FATB PDWSMLFAVITTIFSAAEKQWTNLEWKPKPNPPQLLDDHFGPHGLVERRTFAIRSYEVGP DRSTSIVAVMNHLQEAALNHAKSVGILGDGFGTTLEMSKRDLIWVVRRTHVAVERYPTWG DIVEVECWIGASGNNGMRRDFLVRDCKTGEILTRCTSLSVLMNTRIRRLSTIPDEVRGEI GPAFIDNVAVKDDEIKKLQKLNDSTADYIQGGLIPRWNDLDVNQHVNNLKYVAWVFETVP DSIFESHHISSFTLEYRRECTRDSVLRSLTTVSGGSSEAGLVCDHLLQLEGGSEVLRART EWRPKLTDSFRGISVIPAEPRV SEQ ID NO: 163 Cuphea hyssopifolia FATB1 MVATNAAAFSAYTFFLTSPTHGYSSKRLADTQNGYPGTSLKSKSTPPPAAAAARNGALPLLASICKCP KKADGSMQLDSSLVEGFQFYIRSYEVGADQTVSIQTVLNYLQEAAINHVQSAGYFGDSFGATPEMTKR NLIWVITKMQVLVDRYPAWGDVVQVDTWICSSGKNSMQRDWFVRDLKTGDIITRASSVWVLMNRLTRK LSKIPEAVLEEAKLFVMNTAPTVDDNRKLPKLDGSSADYVLSGLTPRWSDLDMNQHVNNVKYIAWILE SVPQSIPETHKLSAITVEYRRECGKNSVLQSLTNVSGDGITCGNSIIECHHLLQLETGPEILLARTEW ISKEPGFRGAPIQAEKVYNNK* SEQ ID NO: 164 Cuphea hyssopifolia FATB3 MVAAEASSALFSVRTPGTSPKPGKFGNWPTSLSVPFKSKSNHNGGFQVKANASARPKANGSAVSLKSG SLDTQEDTSSSSSPPRTFINQLPDWSMLLSAITTVFVAAEKQWTMLDRKSKRPDMLMDPFGVDRVVQD GAVFRQSFSIRSYEIGADRTASIETLMNIFQETSLNHCKSIGLLNDGFGRTPEMCKRDLIWVVTKMHV EVNRYPTWGDTIEVNTWVSESGKTGMGRDWLISDCHTGEILIRATSMCAMMNQKTRRESKFPYEVRQE LAPHFVDSAPVIEDYQKLHKLDVKTGDSICNGLTPRWNDLDVNQHVNNVKYIGWILESVPTEVFETQE LCGLTLEYRRECGRDSVLESVTAMDPSKEGDRSLYQHLLRLEDGADIAKGRTKWRPKNAGTNGAISTG KTSNGNSIS*
Sequence CWU 1
1
1711726DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 1gctcttcgcc gccgccactc ctgctcgagc
gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga
tgtccatcac caggtccatg aggtctgcct 120tgcgccggct gagccactgc
ttcgtccggg cggccaagag gagcatgagg gaggactcct 180ggtccagggt
cctgacgtgg tcgcggctct gggagcgggc cagcatcatc tggctctgcc
240gcaccgaggc cgcctccaac tggtcctcca gcagccgcag tcgccgccga
ccctggcaga 300ggaagacagg tgaggggggt atgaattgta cagaacaacc
acgagccttg tctaggcaga 360atccctacca gtcatggctt tacctggatg
acggcctgcg aacagctgtc cagcgaccct 420cgctgccgcc gcttctcccg
cacgcttctt tccagcaccg tgatggcgcg agccagcgcc 480gcacgctggc
gctgcgcttc gccgatctga ggacagtcgg ggaactctga tcagtctaaa
540cccccttgcg cgttagtgtt gccatccttt gcagaccggt gagagccgac
ttgttgtgcg 600ccacccccca caccacctcc tcccagacca attctgtcac
ctttttggcg aaggcatcgg 660cctcggcctg cagagaggac agcagtgccc
agccgctggg ggttggcgga tgcacgctca 720ggtacc 7262749DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
2gagctccttg ttttccagaa ggagttgctc cttgagcctt tcattctcag cctcgataac
60ctccaaagcc gctctaattg tggagggggt tcgaatttaa aagcttggaa tgttggttcg
120tgcgtctgga acaagcccag acttgttgct cactgggaaa aggaccatca
gctccaaaaa 180acttgccgct caaaccgcgt acctctgctt tcgcgcaatc
tgccctgttg aaatcgccac 240cacattcata ttgtgacgct tgagcagtct
gtaattgcct cagaatgtgg aatcatctgc 300cccctgtgcg agcccatgcc
aggcatgtcg cgggcgagga cacccgccac tcgtacagca 360gaccattatg
ctacctcaca atagttcata acagtgacca tatttctcga agctccccaa
420cgagcacctc catgctctga gtggccaccc cccggccctg gtgcttgcgg
agggcaggtc 480aaccggcatg gggctaccga aatccccgac cggatcccac
cacccccgcg atgggaagaa 540tctctccccg ggatgtgggc ccaccaccag
cacaacctgc tggcccaggc gagcgtcaaa 600ccataccaca caaatatcct
tggcatcggc cctgaattcc ttctgccgct ctgctacccg 660gtgcttctgt
ccgaagcagg ggttgctagg gatcgctccg agtccgcaaa cccttgtcgc
720gtggcggggc ttgttcgagc ttgaagagc 7493532PRTSaccharomyces
cerevisiae 3Met Leu Leu Gln Ala Phe Leu Phe Leu Leu Ala Gly Phe Ala
Ala Lys 1 5 10 15 Ile Ser Ala Ser Met Thr Asn Glu Thr Ser Asp Arg
Pro Leu Val His 20 25 30 Phe Thr Pro Asn Lys Gly Trp Met Asn Asp
Pro Asn Gly Leu Trp Tyr 35 40 45 Asp Glu Lys Asp Ala Lys Trp His
Leu Tyr Phe Gln Tyr Asn Pro Asn 50 55 60 Asp Thr Val Trp Gly Thr
Pro Leu Phe Trp Gly His Ala Thr Ser Asp 65 70 75 80 Asp Leu Thr Asn
Trp Glu Asp Gln Pro Ile Ala Ile Ala Pro Lys Arg 85 90 95 Asn Asp
Ser Gly Ala Phe Ser Gly Ser Met Val Val Asp Tyr Asn Asn 100 105 110
Thr Ser Gly Phe Phe Asn Asp Thr Ile Asp Pro Arg Gln Arg Cys Val 115
120 125 Ala Ile Trp Thr Tyr Asn Thr Pro Glu Ser Glu Glu Gln Tyr Ile
Ser 130 135 140 Tyr Ser Leu Asp Gly Gly Tyr Thr Phe Thr Glu Tyr Gln
Lys Asn Pro 145 150 155 160 Val Leu Ala Ala Asn Ser Thr Gln Phe Arg
Asp Pro Lys Val Phe Trp 165 170 175 Tyr Glu Pro Ser Gln Lys Trp Ile
Met Thr Ala Ala Lys Ser Gln Asp 180 185 190 Tyr Lys Ile Glu Ile Tyr
Ser Ser Asp Asp Leu Lys Ser Trp Lys Leu 195 200 205 Glu Ser Ala Phe
Ala Asn Glu Gly Phe Leu Gly Tyr Gln Tyr Glu Cys 210 215 220 Pro Gly
Leu Ile Glu Val Pro Thr Glu Gln Asp Pro Ser Lys Ser Tyr 225 230 235
240 Trp Val Met Phe Ile Ser Ile Asn Pro Gly Ala Pro Ala Gly Gly Ser
245 250 255 Phe Asn Gln Tyr Phe Val Gly Ser Phe Asn Gly Thr His Phe
Glu Ala 260 265 270 Phe Asp Asn Gln Ser Arg Val Val Asp Phe Gly Lys
Asp Tyr Tyr Ala 275 280 285 Leu Gln Thr Phe Phe Asn Thr Asp Pro Thr
Tyr Gly Ser Ala Leu Gly 290 295 300 Ile Ala Trp Ala Ser Asn Trp Glu
Tyr Ser Ala Phe Val Pro Thr Asn 305 310 315 320 Pro Trp Arg Ser Ser
Met Ser Leu Val Arg Lys Phe Ser Leu Asn Thr 325 330 335 Glu Tyr Gln
Ala Asn Pro Glu Thr Glu Leu Ile Asn Leu Lys Ala Glu 340 345 350 Pro
Ile Leu Asn Ile Ser Asn Ala Gly Pro Trp Ser Arg Phe Ala Thr 355 360
365 Asn Thr Thr Leu Thr Lys Ala Asn Ser Tyr Asn Val Asp Leu Ser Asn
370 375 380 Ser Thr Gly Thr Leu Glu Phe Glu Leu Val Tyr Ala Val Asn
Thr Thr 385 390 395 400 Gln Thr Ile Ser Lys Ser Val Phe Ala Asp Leu
Ser Leu Trp Phe Lys 405 410 415 Gly Leu Glu Asp Pro Glu Glu Tyr Leu
Arg Met Gly Phe Glu Val Ser 420 425 430 Ala Ser Ser Phe Phe Leu Asp
Arg Gly Asn Ser Lys Val Lys Phe Val 435 440 445 Lys Glu Asn Pro Tyr
Phe Thr Asn Arg Met Ser Val Asn Asn Gln Pro 450 455 460 Phe Lys Ser
Glu Asn Asp Leu Ser Tyr Tyr Lys Val Tyr Gly Leu Leu 465 470 475 480
Asp Gln Asn Ile Leu Glu Leu Tyr Phe Asn Asp Gly Asp Val Val Ser 485
490 495 Thr Asn Thr Tyr Phe Met Thr Thr Gly Asn Ala Leu Gly Ser Val
Asn 500 505 510 Met Thr Thr Gly Val Asp Asn Leu Phe Tyr Ile Asp Lys
Phe Gln Val 515 520 525 Arg Glu Val Lys 530 41599DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
4atgctgctgc aggccttcct gttcctgctg gccggcttcg ccgccaagat cagcgcctcc
60atgacgaacg agacgtccga ccgccccctg gtgcacttca cccccaacaa gggctggatg
120aacgacccca acggcctgtg gtacgacgag aaggacgcca agtggcacct
gtacttccag 180tacaacccga acgacaccgt ctgggggacg cccttgttct
ggggccacgc cacgtccgac 240gacctgacca actgggagga ccagcccatc
gccatcgccc cgaagcgcaa cgactccggc 300gccttctccg gctccatggt
ggtggactac aacaacacct ccggcttctt caacgacacc 360atcgacccgc
gccagcgctg cgtggccatc tggacctaca acaccccgga gtccgaggag
420cagtacatct cctacagcct ggacggcggc tacaccttca ccgagtacca
gaagaacccc 480gtgctggccg ccaactccac ccagttccgc gacccgaagg
tcttctggta cgagccctcc 540cagaagtgga tcatgaccgc ggccaagtcc
caggactaca agatcgagat ctactcctcc 600gacgacctga agtcctggaa
gctggagtcc gcgttcgcca acgagggctt cctcggctac 660cagtacgagt
gccccggcct gatcgaggtc cccaccgagc aggaccccag caagtcctac
720tgggtgatgt tcatctccat caaccccggc gccccggccg gcggctcctt
caaccagtac 780ttcgtcggca gcttcaacgg cacccacttc gaggccttcg
acaaccagtc ccgcgtggtg 840gacttcggca aggactacta cgccctgcag
accttcttca acaccgaccc gacctacggg 900agcgccctgg gcatcgcgtg
ggcctccaac tgggagtact ccgccttcgt gcccaccaac 960ccctggcgct
cctccatgtc cctcgtgcgc aagttctccc tcaacaccga gtaccaggcc
1020aacccggaga cggagctgat caacctgaag gccgagccga tcctgaacat
cagcaacgcc 1080ggcccctgga gccggttcgc caccaacacc acgttgacga
aggccaacag ctacaacgtc 1140gacctgtcca acagcaccgg caccctggag
ttcgagctgg tgtacgccgt caacaccacc 1200cagacgatct ccaagtccgt
gttcgcggac ctctccctct ggttcaaggg cctggaggac 1260cccgaggagt
acctccgcat gggcttcgag gtgtccgcgt cctccttctt cctggaccgc
1320gggaacagca aggtgaagtt cgtgaaggag aacccctact tcaccaaccg
catgagcgtg 1380aacaaccagc ccttcaagag cgagaacgac ctgtcctact
acaaggtgta cggcttgctg 1440gaccagaaca tcctggagct gtacttcaac
gacggcgacg tcgtgtccac caacacctac 1500ttcatgacca ccgggaacgc
cctgggctcc gtgaacatga cgacgggggt ggacaacctg 1560ttctacatcg
acaagttcca ggtgcgcgag gtcaagtga 15995312DNAChlamydomonas
reinhardtii 5ctttcttgcg ctatgacact tccagcaaaa ggtagggcgg gctgcgagac
ggcttcccgg 60cgctgcatgc aacaccgatg atgcttcgac cccccgaagc tccttcgggg
ctgcatgggc 120gctccgatgc cgctccaggg cgagcgctgt ttaaatagcc
aggcccccga ttgcaaagac 180attatagcga gctaccaaag ccatattcaa
acacctagat cactaccact tctacacagg 240ccactcgagc ttgtgatcgc
actccgctaa gggggcgcct cttcctcttc gtttcagtca 300caacccgcaa ac
3126408DNAChlorella vulgaris 6gcagcagcag ctcggatagt atcgacacac
tctggacgct ggtcgtgtga tggactgttg 60ccgccacact tgctgccttg acctgtgaat
atccctgccg cttttatcaa acagcctcag 120tgtgtttgat cttgtgtgta
cgcgcttttg cgagttgcta gctgcttgtg ctatttgcga 180ataccacccc
cagcatcccc ttccctcgtt tcatatcgct tgcatcccaa ccgcaactta
240tctacgctgt cctgctatcc ctcagcgctg ctcctgctcc tgctcactgc
ccctcgcaca 300gccttggttt gggctccgcc tgtattctcc tggtactgca
acctgtaaac cagcactgca 360atgctgatgc acgggaagta gtgggatggg
aacacaaatg gaaagctt 40872333DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 7ctttcttgcg ctatgacact
tccagcaaaa ggtagggcgg gctgcgagac ggcttcccgg 60cgctgcatgc aacaccgatg
atgcttcgac cccccgaagc tccttcgggg ctgcatgggc 120gctccgatgc
cgctccaggg cgagcgctgt ttaaatagcc aggcccccga ttgcaaagac
180attatagcga gctaccaaag ccatattcaa acacctagat cactaccact
tctacacagg 240ccactcgagc ttgtgatcgc actccgctaa gggggcgcct
cttcctcttc gtttcagtca 300caacccgcaa acggcgcgcc atgctgctgc
aggccttcct gttcctgctg gccggcttcg 360ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg gtgcacttca 420cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag aaggacgcca
480agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
cccttgttct 540ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc gccatcgccc 600cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac aacaacacct 660ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc tggacctaca 720acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc tacaccttca
780ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
gacccgaagg 840tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc caggactaca 900agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc gcgttcgcca 960acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc cccaccgagc 1020aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc gccccggccg
1080gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
gaggccttcg 1140acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag accttcttca 1200acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac tgggagtact 1260ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc aagttctccc 1320tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag gccgagccga
1380tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
acgttgacga 1440aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag ttcgagctgg 1500tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac ctctccctct 1560ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag gtgtccgcgt 1620cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag aacccctact
1680tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
ctgtcctact 1740acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac gacggcgacg 1800tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc gtgaacatga 1860cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag gtcaagtgac 1920aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg tgtgatggac
1980tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
atcaaacagc 2040ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc ttgtgctatt 2100tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat cccaaccgca 2160acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc actgcccctc 2220gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg taaaccagca
2280ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga tcc
233381065DNAPrototheca moriformis 8ggccgacagg acgcgcgtca aaggtgctgg
tcgtgtatgc cctggccggc aggtcgttgc 60tgctgctggt tagtgattcc gcaaccctga
ttttggcgtc ttattttggc gtggcaaacg 120ctggcgcccg cgagccgggc
cggcggcgat gcggtgcccc acggctgccg gaatccaagg 180gaggcaagag
cgcccgggtc agttgaaggg ctttacgcgc aaggtacagc cgctcctgca
240aggctgcgtg gtggaattgg acgtgcaggt cctgctgaag ttcctccacc
gcctcaccag 300cggacaaagc accggtgtat caggtccgtg tcatccactc
taaagagctc gactacgacc 360tactgatggc cctagattct tcatcaaaaa
cgcctgagac acttgcccag gattgaaact 420ccctgaaggg accaccaggg
gccctgagtt gttccttccc cccgtggcga gctgccagcc 480aggctgtacc
tgtgatcgag gctggcggga aaataggctt cgtgtgctca ggtcatggga
540ggtgcaggac agctcatgaa acgccaacaa tcgcacaatt catgtcaagc
taatcagcta 600tttcctcttc acgagctgta attgtcccaa aattctggtc
taccgggggt gatccttcgt 660gtacgggccc ttccctcaac cctaggtatg
cgcgcatgcg gtcgccgcgc aactcgcgcg 720agggccgagg gtttgggacg
ggccgtcccg aaatgcagtt gcacccggat gcgtggcacc 780ttttttgcga
taatttatgc aatggactgc tctgcaaaat tctggctctg tcgccaaccc
840taggatcagc ggcgtaggat ttcgtaatca ttcgtcctga tggggagcta
ccgactaccc 900taatatcagc ccgactgcct gacgccagcg tccacttttg
tgcacacatt ccattcgtgc 960ccaagacatt tcattgtggt gcgaagcgtc
cccagttacg ctcacctgtt tcccgacctc 1020cttactgttc tgtcgacaga
gcgggcccac aggccggtcg cagcc 10659120DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
9actagtatgg ccaccgcatc cactttctcg gcgttcaatg cccgctgcgg cgacctgcgt
60cgctcggcgg gctccgggcc ccggcgccca gcgaggcccc tccccgtgcg cgggcgcgcc
120101236DNACuphea wrightii 10atggtggtgg ccgccgccgc cagcagcgcc
ttcttccccg tgcccgcccc ccgccccacc 60cccaagcccg gcaagttcgg caactggccc
agcagcctga gccagccctt caagcccaag 120agcaacccca acggccgctt
ccaggtgaag gccaacgtga gcccccacgg gcgcgccccc 180aaggccaacg
gcagcgccgt gagcctgaag tccggcagcc tgaacaccct ggaggacccc
240cccagcagcc cccccccccg caccttcctg aaccagctgc ccgactggag
ccgcctgcgc 300accgccatca ccaccgtgtt cgtggccgcc gagaagcagt
tcacccgcct ggaccgcaag 360agcaagcgcc ccgacatgct ggtggactgg
ttcggcagcg agaccatcgt gcaggacggc 420ctggtgttcc gcgagcgctt
cagcatccgc agctacgaga tcggcgccga ccgcaccgcc 480agcatcgaga
ccctgatgaa ccacctgcag gacaccagcc tgaaccactg caagagcgtg
540ggcctgctga acgacggctt cggccgcacc cccgagatgt gcacccgcga
cctgatctgg 600gtgctgacca agatgcagat cgtggtgaac cgctacccca
cctggggcga caccgtggag 660atcaacagct ggttcagcca gagcggcaag
atcggcatgg gccgcgagtg gctgatcagc 720gactgcaaca ccggcgagat
cctggtgcgc gccaccagcg cctgggccat gatgaaccag 780aagacccgcc
gcttcagcaa gctgccctgc gaggtgcgcc aggagatcgc cccccacttc
840gtggacgccc cccccgtgat cgaggacaac gaccgcaagc tgcacaagtt
cgacgtgaag 900accggcgaca gcatctgcaa gggcctgacc cccggctgga
acgacttcga cgtgaaccag 960cacgtgagca acgtgaagta catcggctgg
attctggaga gcatgcccac cgaggtgctg 1020gagacccagg agctgtgcag
cctgaccctg gagtaccgcc gcgagtgcgg ccgcgagagc 1080gtggtggaga
gcgtgaccag catgaacccc agcaaggtgg gcgaccgcag ccagtaccag
1140cacctgctgc gcctggagga cggcgccgac atcatgaagg gccgcaccga
gtggcgcccc 1200aagaacgccg gcaccaaccg cgccatcagc acctga
123611408PRTCuphea wrightii 11Met Val Val Ala Ala Ala Ala Ser Ser
Ala Phe Phe Pro Val Pro Ala 1 5 10 15 Pro Arg Pro Thr Pro Lys Pro
Gly Lys Phe Gly Asn Trp Pro Ser Ser 20 25 30 Leu Ser Gln Pro Phe
Lys Pro Lys Ser Asn Pro Asn Gly Arg Phe Gln 35 40 45 Val Lys Ala
Asn Val Ser Pro His Pro Lys Ala Asn Gly Ser Ala Val 50 55 60 Ser
Leu Lys Ser Gly Ser Leu Asn Thr Leu Glu Asp Pro Pro Ser Ser 65 70
75 80 Pro Pro Pro Arg Thr Phe Leu Asn Gln Leu Pro Asp Trp Ser Arg
Leu 85 90 95 Arg Thr Ala Ile Thr Thr Val Phe Val Ala Ala Glu Lys
Gln Phe Thr 100 105 110 Arg Leu Asp Arg Lys Ser Lys Arg Pro Asp Met
Leu Val Asp Trp Phe 115 120 125 Gly Ser Glu Thr Ile Val Gln Asp Gly
Leu Val Phe Arg Glu Arg Phe 130 135 140 Ser Ile Arg Ser Tyr Glu Ile
Gly Ala Asp Arg Thr Ala Ser Ile Glu 145 150 155 160 Thr Leu Met Asn
His Leu Gln Asp Thr Ser Leu Asn His Cys Lys Ser 165 170 175 Val Gly
Leu Leu Asn Asp Gly Phe Gly Arg Thr Pro Glu Met Cys Thr 180 185 190
Arg Asp Leu Ile Trp Val Leu Thr Lys Met Gln Ile Val Val Asn Arg 195
200 205 Tyr Pro Thr Trp Gly Asp Thr Val Glu Ile Asn Ser Trp Phe Ser
Gln 210 215 220 Ser Gly Lys Ile Gly Met Gly Arg Glu Trp Leu Ile Ser
Asp Cys Asn 225 230 235 240 Thr Gly Glu Ile Leu Val Arg Ala Thr Ser
Ala Trp Ala Met Met Asn 245 250 255 Gln Lys Thr Arg Arg Phe Ser Lys
Leu Pro Cys Glu Val Arg Gln Glu 260 265 270 Ile Ala Pro His Phe Val
Asp Ala Pro Pro Val Ile Glu Asp Asn Asp 275 280 285 Arg Lys Leu His
Lys Phe Asp Val Lys Thr Gly Asp Ser Ile Cys Lys 290 295 300 Gly Leu
Thr Pro Gly Trp Asn Asp Phe Asp Val Asn Gln His Val Ser 305 310 315
320 Asn Val Lys Tyr Ile Gly Trp Ile Leu Glu Ser Met Pro Thr Glu Val
325 330 335 Leu Glu Thr Gln Glu Leu Cys Ser Leu Thr Leu Glu Tyr Arg
Arg Glu 340
345 350 Cys Gly Arg Glu Ser Val Val Glu Ser Val Thr Ser Met Asn Pro
Ser 355 360 365 Lys Val Gly Asp Arg Ser Gln Tyr Gln His Leu Leu Arg
Leu Glu Asp 370 375 380 Gly Ala Asp Ile Met Lys Gly Arg Thr Glu Trp
Arg Pro Lys Asn Ala 385 390 395 400 Gly Thr Asn Arg Ala Ile Ser Thr
405 12933DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 12atggacgcct ccggcgcctc ctccttcctg
cgcggccgct gcctggagtc ctgcttcaag 60gcctccttcg gctacgtaat gtcccagccc
aaggacgccg ccggccagcc ctcccgccgc 120cccgccgacg ccgacgactt
cgtggacgac gaccgctgga tcaccgtgat cctgtccgtg 180gtgcgcatcg
ccgcctgctt cctgtccatg atggtgacca ccatcgtgtg gaacatgatc
240atgctgatcc tgctgccctg gccctacgcc cgcatccgcc agggcaacct
gtacggccac 300gtgaccggcc gcatgctgat gtggattctg ggcaacccca
tcaccatcga gggctccgag 360ttctccaaca cccgcgccat ctacatctgc
aaccacgcct ccctggtgga catcttcctg 420atcatgtggc tgatccccaa
gggcaccgtg accatcgcca agaaggagat catctggtat 480cccctgttcg
gccagctgta cgtgctggcc aaccaccagc gcatcgaccg ctccaacccc
540tccgccgcca tcgagtccat caaggaggtg gcccgcgccg tggtgaagaa
gaacctgtcc 600ctgatcatct tccccgaggg cacccgctcc aagaccggcc
gcctgctgcc cttcaagaag 660ggcttcatcc acatcgccct ccagacccgc
ctgcccatcg tgccgatggt gctgaccggc 720acccacctgg cctggcgcaa
gaactccctg cgcgtgcgcc ccgcccccat caccgtgaag 780tacttctccc
ccatcaagac cgacgactgg gaggaggaga agatcaacca ctacgtggag
840atgatccacg ccctgtacgt ggaccacctg cccgagtccc agaagcccct
ggtgtccaag 900ggccgcgacg cctccggccg ctccaactcc tga
93313563DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 13gctcttcgct aacggaggtc tgtcaccaaa
tggaccccgt ctattgcggg aaaccacggc 60gatggcacgt ttcaaaactt gatgaaatac
aatattcagt atgtcgcggg cggcgacggc 120ggggagctga tgtcgcgctg
ggtattgctt aatcgccagc ttcgcccccg tcttggcgcg 180aggcgtgaac
aagccgaccg atgtgcacga gcaaatcctg acactagaag ggctgactcg
240cccggcacgg ctgaattaca caggcttgca aaaataccag aatttgcacg
caccgtattc 300gcggtatttt gttggacagt gaatagcgat gcggcaatgg
cttgtggcgt tagaaggtgc 360gacgaaggtg gtgccaccac tgtgccagcc
agtcctggcg gctcccaggg ccccgatcaa 420gagccaggac atccaaacta
cccacagcat caacgccccg gcctatactc gaaccccact 480tgcactctgc
aatggtatgg gaaccacggg gcagtcttgt gtgggtcgcg cctatcgcgg
540tcggcgaaga ccgggaaggt acc 56314465DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
14gagctcagcg gcgacggtcc tgctaccgta cgacgttggg cacgcccatg aaagtttgta
60taccgagctt gttgagcgaa ctgcaagcgc ggctcaagga tacttgaact cctggattga
120tatcggtcca ataatggatg gaaaatccga acctcgtgca agaactgagc
aaacctcgtt 180acatggatgc acagtcgcca gtccaatgaa cattgaagtg
agcgaactgt tcgcttcggt 240ggcagtacta ctcaaagaat gagctgctgt
taaaaatgca ctctcgttct ctcaagtgag 300tggcagatga gtgctcacgc
cttgcacttc gctgcccgtg tcatgccctg cgccccaaaa 360tttgaaaaaa
gggatgagat tattgggcaa tggacgacgt cgtcgctccg ggagtcagga
420ccggcggaaa ataagaggca acacactccg cttcttagct cttcc
465151533DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 15ctttcttgcg ctatgacact tccagcaaaa
ggtagggcgg gctgcgagac ggcttcccgg 60cgctgcatgc aacaccgatg atgcttcgac
cccccgaagc tccttcgggg ctgcatgggc 120gctccgatgc cgctccaggg
cgagcgctgt ttaaatagcc aggcccccga ttgcaaagac 180attatagcga
gctaccaaag ccatattcaa acacctagat cactaccact tctacacagg
240ccactcgagc ttgtgatcgc actccgctaa gggggcgcct cttcctcttc
gtttcagtca 300caacccgcaa actctagaat atcaatgatc gagcaggacg
gcctccacgc cggctccccc 360gccgcctggg tggagcgcct gttcggctac
gactgggccc agcagaccat cggctgctcc 420gacgccgccg tgttccgcct
gtccgcccag ggccgccccg tgctgttcgt gaagaccgac 480ctgtccggcg
ccctgaacga gctgcaggac gaggccgccc gcctgtcctg gctggccacc
540accggcgtgc cctgcgccgc cgtgctggac gtggtgaccg aggccggccg
cgactggctg 600ctgctgggcg aggtgcccgg ccaggacctg ctgtcctccc
acctggcccc cgccgagaag 660gtgtccatca tggccgacgc catgcgccgc
ctgcacaccc tggaccccgc cacctgcccc 720ttcgaccacc aggccaagca
ccgcatcgag cgcgcccgca cccgcatgga ggccggcctg 780gtggaccagg
acgacctgga cgaggagcac cagggcctgg cccccgccga gctgttcgcc
840cgcctgaagg cccgcatgcc cgacggcgag gacctggtgg tgacccacgg
cgacgcctgc 900ctgcccaaca tcatggtgga gaacggccgc ttctccggct
tcatcgactg cggccgcctg 960ggcgtggccg accgctacca ggacatcgcc
ctggccaccc gcgacatcgc cgaggagctg 1020ggcggcgagt gggccgaccg
cttcctggtg ctgtacggca tcgccgcccc cgactcccag 1080cgcatcgcct
tctaccgcct gctggacgag ttcttctgac aattggcagc agcagctcgg
1140atagtatcga cacactctgg acgctggtcg tgtgatggac tgttgccgcc
acacttgctg 1200ccttgacctg tgaatatccc tgccgctttt atcaaacagc
ctcagtgtgt ttgatcttgt 1260gtgtacgcgc ttttgcgagt tgctagctgc
ttgtgctatt tgcgaatacc acccccagca 1320tccccttccc tcgtttcata
tcgcttgcat cccaaccgca acttatctac gctgtcctgc 1380tatccctcag
cgctgctcct gctcctgctc actgcccctc gcacagcctt ggtttgggct
1440ccgcctgtat tctcctggta ctgcaacctg taaaccagca ctgcaatgct
gatgcacggg 1500aagtagtggg atgggaacac aaatggagga tcc
153316310PRTCocos nucifera 16Met Asp Ala Ser Gly Ala Ser Ser Phe
Leu Arg Gly Arg Cys Leu Glu 1 5 10 15 Ser Cys Phe Lys Ala Ser Phe
Gly Tyr Val Met Ser Gln Pro Lys Asp 20 25 30 Ala Ala Gly Gln Pro
Ser Arg Arg Pro Ala Asp Ala Asp Asp Phe Val 35 40 45 Asp Asp Asp
Arg Trp Ile Thr Val Ile Leu Ser Val Val Arg Ile Ala 50 55 60 Ala
Cys Phe Leu Ser Met Met Val Thr Thr Ile Val Trp Asn Met Ile 65 70
75 80 Met Leu Ile Leu Leu Pro Trp Pro Tyr Ala Arg Ile Arg Gln Gly
Asn 85 90 95 Leu Tyr Gly His Val Thr Gly Arg Met Leu Met Trp Ile
Leu Gly Asn 100 105 110 Pro Ile Thr Ile Glu Gly Ser Glu Phe Ser Asn
Thr Arg Ala Ile Tyr 115 120 125 Ile Cys Asn His Ala Ser Leu Val Asp
Ile Phe Leu Ile Met Trp Leu 130 135 140 Ile Pro Lys Gly Thr Val Thr
Ile Ala Lys Lys Glu Ile Ile Trp Tyr 145 150 155 160 Pro Leu Phe Gly
Gln Leu Tyr Val Leu Ala Asn His Gln Arg Ile Asp 165 170 175 Arg Ser
Asn Pro Ser Ala Ala Ile Glu Ser Ile Lys Glu Val Ala Arg 180 185 190
Ala Val Val Lys Lys Asn Leu Ser Leu Ile Ile Phe Pro Glu Gly Thr 195
200 205 Arg Ser Lys Thr Gly Arg Leu Leu Pro Phe Lys Lys Gly Phe Ile
His 210 215 220 Ile Ala Leu Gln Thr Arg Leu Pro Ile Val Pro Met Val
Leu Thr Gly 225 230 235 240 Thr His Leu Ala Trp Arg Lys Asn Ser Leu
Arg Val Arg Pro Ala Pro 245 250 255 Ile Thr Val Lys Tyr Phe Ser Pro
Ile Lys Thr Asp Asp Trp Glu Glu 260 265 270 Glu Lys Ile Asn His Tyr
Val Glu Met Ile His Ala Leu Tyr Val Asp 275 280 285 His Leu Pro Glu
Ser Gln Lys Pro Leu Val Ser Lys Gly Arg Asp Ala 290 295 300 Ser Gly
Arg Ser Asn Ser 305 310 176676DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 17gggctggtct
gaatccttca ggcgggtgtt acccgagaaa gaaagggtgc cgatttcaaa 60gcagacccat
gtgccgggcc ctgtggcctg tgttggcgcc tatgtagtca ccccccctca
120cccaattgtc gccagtttgc gcactccata aactcaaaac agcagcttct
gagctgcgct 180gttcaagaac acctctgggg tttgctcacc cgcgaggtcg
acgcccagca tggctatcaa 240gacgaacagg cagcctgtgg agaagcctcc
gttcacgatc gggacgctgc gcaaggccat 300ccccgcgcac tgtttcgagc
gctcggcgct tcgtagcagc atgtacctgg cctttgacat 360cgcggtcatg
tccctgctct acgtcgcgtc gacgtacatc gaccctgcac cggtgcctac
420gtgggtcaag tacggcatca tgtggccgct ctactggttc ttccaggtgt
gtttgagggt 480tttggttgcc cgtattgagg tcctggtggc gcgcatggag
gagaaggcgc ctgtcccgct 540gacccccccg gctaccctcc cggcaccttc
cagggcgcct tcggcacggg tgtctgggtg 600tgcgcgcacg agtgcggcca
ccaggccttt tcctccagcc aggccatcaa cgacggcgtg 660ggcctggtgt
tccacagcct gctgctggtg ccctactact cctggaagca ctcgcaccgg
720gtaccctttc ttgcgctatg acacttccag caaaaggtag ggcgggctgc
gagacggctt 780cccggcgctg catgcaacac cgatgatgct tcgacccccc
gaagctcctt cggggctgca 840tgggcgctcc gatgccgctc cagggcgagc
gctgtttaaa tagccaggcc cccgattgca 900aagacattat agcgagctac
caaagccata ttcaaacacc tagatcacta ccacttctac 960acaggccact
cgagcttgtg atcgcactcc gctaaggggg cgcctcttcc tcttcgtttc
1020agtcacaacc cgcaaactct agaatatcaa tgctgctgca ggccttcctg
ttcctgctgg 1080ccggcttcgc cgccaagatc agcgcctcca tgacgaacga
gacgtccgac cgccccctgg 1140tgcacttcac ccccaacaag ggctggatga
acgaccccaa cggcctgtgg tacgacgaga 1200aggacgccaa gtggcacctg
tacttccagt acaacccgaa cgacaccgtc tgggggacgc 1260ccttgttctg
gggccacgcc acgtccgacg acctgaccaa ctgggaggac cagcccatcg
1320ccatcgcccc gaagcgcaac gactccggcg ccttctccgg ctccatggtg
gtggactaca 1380acaacacctc cggcttcttc aacgacacca tcgacccgcg
ccagcgctgc gtggccatct 1440ggacctacaa caccccggag tccgaggagc
agtacatctc ctacagcctg gacggcggct 1500acaccttcac cgagtaccag
aagaaccccg tgctggccgc caactccacc cagttccgcg 1560acccgaaggt
cttctggtac gagccctccc agaagtggat catgaccgcg gccaagtccc
1620aggactacaa gatcgagatc tactcctccg acgacctgaa gtcctggaag
ctggagtccg 1680cgttcgccaa cgagggcttc ctcggctacc agtacgagtg
ccccggcctg atcgaggtcc 1740ccaccgagca ggaccccagc aagtcctact
gggtgatgtt catctccatc aaccccggcg 1800ccccggccgg cggctccttc
aaccagtact tcgtcggcag cttcaacggc acccacttcg 1860aggccttcga
caaccagtcc cgcgtggtgg acttcggcaa ggactactac gccctgcaga
1920ccttcttcaa caccgacccg acctacggga gcgccctggg catcgcgtgg
gcctccaact 1980gggagtactc cgccttcgtg cccaccaacc cctggcgctc
ctccatgtcc ctcgtgcgca 2040agttctccct caacaccgag taccaggcca
acccggagac ggagctgatc aacctgaagg 2100ccgagccgat cctgaacatc
agcaacgccg gcccctggag ccggttcgcc accaacacca 2160cgttgacgaa
ggccaacagc tacaacgtcg acctgtccaa cagcaccggc accctggagt
2220tcgagctggt gtacgccgtc aacaccaccc agacgatctc caagtccgtg
ttcgcggacc 2280tctccctctg gttcaagggc ctggaggacc ccgaggagta
cctccgcatg ggcttcgagg 2340tgtccgcgtc ctccttcttc ctggaccgcg
ggaacagcaa ggtgaagttc gtgaaggaga 2400acccctactt caccaaccgc
atgagcgtga acaaccagcc cttcaagagc gagaacgacc 2460tgtcctacta
caaggtgtac ggcttgctgg accagaacat cctggagctg tacttcaacg
2520acggcgacgt cgtgtccacc aacacctact tcatgaccac cgggaacgcc
ctgggctccg 2580tgaacatgac gacgggggtg gacaacctgt tctacatcga
caagttccag gtgcgcgagg 2640tcaagtgaca attggcagca gcagctcgga
tagtatcgac acactctgga cgctggtcgt 2700gtgatggact gttgccgcca
cacttgctgc cttgacctgt gaatatccct gccgctttta 2760tcaaacagcc
tcagtgtgtt tgatcttgtg tgtacgcgct tttgcgagtt gctagctgct
2820tgtgctattt gcgaatacca cccccagcat ccccttccct cgtttcatat
cgcttgcatc 2880ccaaccgcaa cttatctacg ctgtcctgct atccctcagc
gctgctcctg ctcctgctca 2940ctgcccctcg cacagccttg gtttgggctc
cgcctgtatt ctcctggtac tgcaacctgt 3000aaaccagcac tgcaatgctg
atgcacggga agtagtggga tgggaacaca aatggaggat 3060cccgcgtctc
gaacagagcg cgcagaggaa cgctgaaggt ctcgcctctg tcgcacctca
3120gcgcggcata caccacaata accacctgac gaatgcgctt ggttcttcgt
ccattagcga 3180agcgtccggt tcacacacgt gccacgttgg cgaggtggca
ggtgacaatg atcggtggag 3240ctgatggtcg aaacgttcac agcctaggga
tatcgaattc ggccgacagg acgcgcgtca 3300aaggtgctgg tcgtgtatgc
cctggccggc aggtcgttgc tgctgctggt tagtgattcc 3360gcaaccctga
ttttggcgtc ttattttggc gtggcaaacg ctggcgcccg cgagccgggc
3420cggcggcgat gcggtgcccc acggctgccg gaatccaagg gaggcaagag
cgcccgggtc 3480agttgaaggg ctttacgcgc aaggtacagc cgctcctgca
aggctgcgtg gtggaattgg 3540acgtgcaggt cctgctgaag ttcctccacc
gcctcaccag cggacaaagc accggtgtat 3600caggtccgtg tcatccactc
taaagaactc gactacgacc tactgatggc cctagattct 3660tcatcaaaaa
cgcctgagac acttgcccag gattgaaact ccctgaaggg accaccaggg
3720gccctgagtt gttccttccc cccgtggcga gctgccagcc aggctgtacc
tgtgatcgag 3780gctggcggga aaataggctt cgtgtgctca ggtcatggga
ggtgcaggac agctcatgaa 3840acgccaacaa tcgcacaatt catgtcaagc
taatcagcta tttcctcttc acgagctgta 3900attgtcccaa aattctggtc
taccgggggt gatccttcgt gtacgggccc ttccctcaac 3960cctaggtatg
cgcgcatgcg gtcgccgcgc aactcgcgcg agggccgagg gtttgggacg
4020ggccgtcccg aaatgcagtt gcacccggat gcgtggcacc ttttttgcga
taatttatgc 4080aatggactgc tctgcaaaat tctggctctg tcgccaaccc
taggatcagc ggcgtaggat 4140ttcgtaatca ttcgtcctga tggggagcta
ccgactaccc taatatcagc ccgactgcct 4200gacgccagcg tccacttttg
tgcacacatt ccattcgtgc ccaagacatt tcattgtggt 4260gcgaagcgtc
cccagttacg ctcacctgtt tcccgacctc cttactgttc tgtcgacaga
4320gcgggcccac aggccggtcg cagccactag tatggccacc gcatccactt
tctcggcgtt 4380caatgcccgc tgcggcgacc tgcgtcgctc ggcgggctcc
gggccccggc gcccagcgag 4440gcccctcccc gtgcgcgggc gcgccgccac
cggcgagcag ccctccggcg tggcctccct 4500gcgcgaggcc gacaaggaga
agtccctggg caaccgcctg cgcctgggct ccctgaccga 4560ggacggcctg
tcctacaagg agaagttcgt gatccgctgc tacgaggtgg gcatcaacaa
4620gaccgccacc atcgagacca tcgccaacct gctgcaggag gtgggcggca
accacgccca 4680gggcgtgggc ttctccaccg acggcttcgc caccaccacc
accatgcgca agctgcacct 4740gatctgggtg accgcccgca tgcacatcga
gatctaccgc taccccgcct ggtccgacgt 4800gatcgagatc gagacctggg
tgcagggcga gggcaaggtg ggcacccgcc gcgactggat 4860cctgaaggac
tacgccaacg gcgaggtgat cggccgcgcc acctccaagt gggtgatgat
4920gaacgaggac acccgccgcc tgcagaaggt gtccgacgac gtgcgcgagg
agtacctggt 4980gttctgcccc cgcaccctgc gcctggcctt ccccgaggag
aacaacaact ccatgaagaa 5040gatccccaag ctggaggacc ccgccgagta
ctcccgcctg ggcctggtgc cccgccgctc 5100cgacctggac atgaacaagc
acgtgaacaa cgtgacctac atcggctggg ccctggagtc 5160catccccccc
gagatcatcg acacccacga gctgcaggcc atcaccctgg actaccgccg
5220cgagtgccag cgcgacgaca tcgtggactc cctgacctcc cgcgagcccc
tgggcaacgc 5280cgccggcgtg aagttcaagg agatcaacgg ctccgtgtcc
cccaagaagg acgagcagga 5340cctgtcccgc ttcatgcacc tgctgcgctc
cgccggctcc ggcctggaga tcaaccgctg 5400ccgcaccgag tggcgcaaga
agcccgccaa gcgcatggac tacaaggacc acgacggcga 5460ctacaaggac
cacgacatcg actacaagga cgacgacgac aagtgaatcg atagatctct
5520taaggcagca gcagctcgga tagtatcgac acactctgga cgctggtcgt
gtgatggact 5580gttgccgcca cacttgctgc cttgacctgt gaatatccct
gccgctttta tcaaacagcc 5640tcagtgtgtt tgatcttgtg tgtacgcgct
tttgcgagtt gctagctgct tgtgctattt 5700gcgaatacca cccccagcat
ccccttccct cgtttcatat cgcttgcatc ccaaccgcaa 5760cttatctacg
ctgtcctgct atccctcagc gctgctcctg ctcctgctca ctgcccctcg
5820cacagccttg gtttgggctc cgcctgtatt ctcctggtac tgcaacctgt
aaaccagcac 5880tgcaatgctg atgcacggga agtagtggga tgggaacaca
aatggaaagc ttaattaaga 5940gctcccgcca ccactccaac acggggtgcc
tggacaagga cgaggtgttt gtgccgccgc 6000accgcgcagt ggcgcacgag
ggcctggagt gggaggagtg gctgcccatc cgcatgggca 6060aggtgctggt
caccctgacc ctgggctggc cgctgtacct catgttcaac gtcgcctcgc
6120ggccgtaccc gcgcttcgcc aaccactttg acccgtggtc gcccatcttc
agcaagcgcg 6180agcgcatcga ggtggtcatc tccgacctgg cgctggtggc
ggtgctcagc gggctcagcg 6240tgctgggccg caccatgggc tgggcctggc
tggtcaagac ctacgtggtg ccctacctga 6300tcgtgaacat gtggctcgtg
ctcatcacgc tgctccagca cacgcacccg gcgctgccgc 6360actacttcga
gaaggactgg gactggctgc gcggcgccat ggccaccgtg gaccgctcca
6420tgggcccgcc cttcatggac aacatcctgc accacatctc cgacacccac
gtgctgcacc 6480acctcttcag caccatcccg cactaccacg ccgaggaggc
ctccgccgcc atcaggccca 6540tcctgggcaa gtactaccag tccgacagcc
gctgggtcgg ccgcgccctg tgggaggact 6600ggcgcgactg ccgctacgtc
gtcccggacg cgcccgagga cgactccgcg ctctggttcc 6660acaagtgagt gagtga
667618719DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 18gggctggtct gaatccttca ggcgggtgtt
acccgagaaa gaaagggtgc cgatttcaaa 60gcagacccat gtgccgggcc ctgtggcctg
tgttggcgcc tatgtagtca ccccccctca 120cccaattgtc gccagtttgc
gcactccata aactcaaaac agcagcttct gagctgcgct 180gttcaagaac
acctctgggg tttgctcacc cgcgaggtcg acgcccagca tggctatcaa
240gacgaacagg cagcctgtgg agaagcctcc gttcacgatc gggacgctgc
gcaaggccat 300ccccgcgcac tgtttcgagc gctcggcgct tcgtagcagc
atgtacctgg cctttgacat 360cgcggtcatg tccctgctct acgtcgcgtc
gacgtacatc gaccctgcac cggtgcctac 420gtgggtcaag tacggcatca
tgtggccgct ctactggttc ttccaggtgt gtttgagggt 480tttggttgcc
cgtattgagg tcctggtggc gcgcatggag gagaaggcgc ctgtcccgct
540gacccccccg gctaccctcc cggcaccttc cagggcgcct tcggcacggg
tgtctgggtg 600tgcgcgcacg agtgcggcca ccaggccttt tcctccagcc
aggccatcaa cgacggcgtg 660ggcctggtgt tccacagcct gctgctggtg
ccctactact cctggaagca ctcgcaccg 71919732DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
19ccgccaccac tccaacacgg ggtgcctgga caaggacgag gtgtttgtgc cgccgcaccg
60cgcagtggcg cacgagggcc tggagtggga ggagtggctg cccatccgca tgggcaaggt
120gctggtcacc ctgaccctgg gctggccgct gtacctcatg ttcaacgtcg
cctcgcggcc 180gtacccgcgc ttcgccaacc actttgaccc gtggtcgccc
atcttcagca agcgcgagcg 240catcgaggtg gtcatctccg acctggcgct
ggtggcggtg ctcagcgggc tcagcgtgct 300gggccgcacc atgggctggg
cctggctggt caagacctac gtggtgccct acctgatcgt 360gaacatgtgg
ctcgtgctca tcacgctgct ccagcacacg cacccggcgc tgccgcacta
420cttcgagaag gactgggact ggctgcgcgg cgccatggcc accgtggacc
gctccatggg 480cccgcccttc atggacaaca tcctgcacca catctccgac
acccacgtgc tgcaccacct 540cttcagcacc atcccgcact accacgccga
ggaggcctcc gccgccatca ggcccatcct 600gggcaagtac taccagtccg
acagccgctg ggtcggccgc gccctgtggg aggactggcg 660cgactgccgc
tacgtcgtcc cggacgcgcc cgaggacgac tccgcgctct ggttccacaa
720gtgagtgagt ga 73220612DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 20gctcttcgga
gtcactgtgc cactgagttc gactggtagc tgaatggagt cgctgctcca 60ctaaacgaat
tgtcagcacc gccagccggc
cgaggacccg agtcatagcg agggtagtag 120cgcgccatgg caccgaccag
cctgcttgcc agtactggcg tctcttccgc ttctctgtgg 180tcctctgcgc
gctccagcgc gtgcgctttt ccggtggatc atgcggtccg tggcgcaccg
240cagcggccgc tgcccatgca gcgccgctgc ttccgaacag tggcggtcag
ggccgcaccc 300gcggtagccg tccgtccgga acccgcccaa gagttttggg
agcagcttga gccctgcaag 360atggcggagg acaagcgcat cttcctggag
gagcaccggt gcgtggaggt ccggggctga 420ccggccgtcg cattcaacgt
aatcaatcgc atgatgatca gaggacacga agtcttggtg 480gcggtggcca
gaaacactgt ccattgcaag ggcataggga tgcgttcctt cacctctcat
540ttctcatttc tgaatccctc cctgctcact ctttctcctc ctccttcccg
ttcacgcagc 600attcggggta cc 61221528DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
21gacagggtgg ttggctggat ggggaaacgc tggtcgcggg attcgatcct gctgcttata
60tcctccctgg aagcacaccc acgactctga agaagaaaac gtgcacacac acaacccaac
120cggccgaata tttgcttcct tatcccgggt ccaagagaga ctgcgatgcc
cccctcaatc 180agcatcctcc tccctgccgc ttcaatcttc cctgcttgcc
tgcgcccgcg gtgcgccgtc 240tgcccgccca gtcagtcact cctgcacagg
ccccttgtgc gcagtgctcc tgtacccttt 300accgctcctt ccattctgcg
aggcccccta ttgaatgtat tcgttgcctg tgtggccaag 360cgggctgctg
ggcgcgccgc cgtcgggcag tgctcggcga ctttggcgga agccgattgt
420tcttctgtaa gccacgcgct tgctgctttg ggaagagaag ggggggggta
ctgaatggat 480gaggaggaga aggaggggta ttggtattat ctgagttggg tgaagagc
528221174DNAChlorella protothecoides 22agtttaggtc cagcgtccgt
ggggggggac gggctgggag cttgggccgg gaagggcaag 60acgatgcagt ccctctgggg
agtcacagcc gactgtgtgt gttgcactgt gcggcccgca 120gcactcacac
gcaaaatgcc tggccgacag gcaggccctg tccagtgcaa catccacggt
180ccctctcatc aggctcacct tgctcattga cataacggaa tgcgtaccgc
tctttcagat 240ctgtccatcc agagagggga gcaggctccc caccgacgct
gtcaaacttg cttcctgccc 300aaccgaaaac attattgttt gagggggggg
gggggggggc agattgcatg gcgggatatc 360tcgtgaggaa catcactggg
acactgtgga acacagtgag tgcagtatgc agagcatgta 420tgctaggggt
cagcgcagga agggggcctt tcccagtctc ccatgccact gcaccgtatc
480cacgactcac caggaccagc ttcttgatcg gcttccgctc ccgtggacac
cagtgtgtag 540cctctggact ccaggtatgc gtgcaccgca aaggccagcc
gatcgtgccg attcctgggg 600tggaggatat gagtcagcca acttggggct
cagagtgcac actggggcac gatacgaaac 660aacatctaca ccgtgtcctc
catgctgaca caccacagct tcgctccacc tgaatgtggg 720cgcatgggcc
cgaatcacag ccaatgtcgc tgctgccata atgtgatcca gaccctctcc
780gcccagatgc cgagcggatc gtgggcgctg aatagattcc tgtttcgatc
actgtttggg 840tcctttcctt ttcgtctcgg atgcgcgtct cgaaacaggc
tgcgtcgggc tttcggatcc 900cttttgctcc ctccgtcacc atcctgcgcg
cgggcaagtt gcttgaccct gggctgtacc 960agggttggag ggtattaccg
cgtcaggcca ttcccagccc ggattcaatt caaagtctgg 1020gccaccaccc
tccgccgctc tgtctgatca ctccacattc gtgcatacac tacgttcaag
1080tcctgatcca ggcgtgtctc gggacaaggt gtgcttgagt ttgaatctca
aggacccact 1140ccagcacagc tgctggttga ccccgccctc gcaa
1174233529DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 23agtttaggtc cagcgtccgt ggggggggac
gggctgggag cttgggccgg gaagggcaag 60acgatgcagt ccctctgggg agtcacagcc
gactgtgtgt gttgcactgt gcggcccgca 120gcactcacac gcaaaatgcc
tggccgacag gcaggccctg tccagtgcaa catccacggt 180ccctctcatc
aggctcacct tgctcattga cataacggaa tgcgtaccgc tctttcagat
240ctgtccatcc agagagggga gcaggctccc caccgacgct gtcaaacttg
cttcctgccc 300aaccgaaaac attattgttt gagggggggg gggggggggc
agattgcatg gcgggatatc 360tcgtgaggaa catcactggg acactgtgga
acacagtgag tgcagtatgc agagcatgta 420tgctaggggt cagcgcagga
agggggcctt tcccagtctc ccatgccact gcaccgtatc 480cacgactcac
caggaccagc ttcttgatcg gcttccgctc ccgtggacac cagtgtgtag
540cctctggact ccaggtatgc gtgcaccgca aaggccagcc gatcgtgccg
attcctgggg 600tggaggatat gagtcagcca acttggggct cagagtgcac
actggggcac gatacgaaac 660aacatctaca ccgtgtcctc catgctgaca
caccacagct tcgctccacc tgaatgtggg 720cgcatgggcc cgaatcacag
ccaatgtcgc tgctgccata atgtgatcca gaccctctcc 780gcccagatgc
cgagcggatc gtgggcgctg aatagattcc tgtttcgatc actgtttggg
840tcctttcctt ttcgtctcgg atgcgcgtct cgaaacaggc tgcgtcgggc
tttcggatcc 900cttttgctcc ctccgtcacc atcctgcgcg cgggcaagtt
gcttgaccct gggctgtacc 960agggttggag ggtattaccg cgtcaggcca
ttcccagccc ggattcaatt caaagtctgg 1020gccaccaccc tccgccgctc
tgtctgatca ctccacattc gtgcatacac tacgttcaag 1080tcctgatcca
ggcgtgtctc gggacaaggt gtgcttgagt ttgaatctca aggacccact
1140ccagcacagc tgctggttga ccccgccctc gcaatctaga atggccgcgt
ccgtccactg 1200caccctgatg tccgtggtct gcaacaacaa gaaccactcc
gcccgcccca agctgcccaa 1260ctcctccctg ctgcccggct tcgacgtggt
ggtccaggcc gcggccaccc gcttcaagaa 1320ggagacgacg accacccgcg
ccacgctgac gttcgacccc cccacgacca actccgagcg 1380cgccaagcag
cgcaagcaca ccatcgaccc ctcctccccc gacttccagc ccatcccctc
1440cttcgaggag tgcttcccca agtccacgaa ggagcacaag gaggtggtgc
acgaggagtc 1500cggccacgtc ctgaaggtgc ccttccgccg cgtgcacctg
tccggcggcg agcccgcctt 1560cgacaactac gacacgtccg gcccccagaa
cgtcaacgcc cacatcggcc tggcgaagct 1620gcgcaaggag tggatcgacc
gccgcgagaa gctgggcacg ccccgctaca cgcagatgta 1680ctacgcgaag
cagggcatca tcacggagga gatgctgtac tgcgcgacgc gcgagaagct
1740ggaccccgag ttcgtccgct ccgaggtcgc gcggggccgc gccatcatcc
cctccaacaa 1800gaagcacctg gagctggagc ccatgatcgt gggccgcaag
ttcctggtga aggtgaacgc 1860gaacatcggc aactccgccg tggcctcctc
catcgaggag gaggtctaca aggtgcagtg 1920ggccaccatg tggggcgccg
acaccatcat ggacctgtcc acgggccgcc acatccacga 1980gacgcgcgag
tggatcctgc gcaactccgc ggtccccgtg ggcaccgtcc ccatctacca
2040ggcgctggag aaggtggacg gcatcgcgga gaacctgaac tgggaggtgt
tccgcgagac 2100gctgatcgag caggccgagc agggcgtgga ctacttcacg
atccacgcgg gcgtgctgct 2160gcgctacatc cccctgaccg ccaagcgcct
gacgggcatc gtgtcccgcg gcggctccat 2220ccacgcgaag tggtgcctgg
cctaccacaa ggagaacttc gcctacgagc actgggacga 2280catcctggac
atctgcaacc agtacgacgt cgccctgtcc atcggcgacg gcctgcgccc
2340cggctccatc tacgacgcca acgacacggc ccagttcgcc gagctgctga
cccagggcga 2400gctgacgcgc cgcgcgtggg agaaggacgt gcaggtgatg
aacgagggcc ccggccacgt 2460gcccatgcac aagatccccg agaacatgca
gaagcagctg gagtggtgca acgaggcgcc 2520cttctacacc ctgggccccc
tgacgaccga catcgcgccc ggctacgacc acatcacctc 2580cgccatcggc
gcggccaaca tcggcgccct gggcaccgcc ctgctgtgct acgtgacgcc
2640caaggagcac ctgggcctgc ccaaccgcga cgacgtgaag gcgggcgtca
tcgcctacaa 2700gatcgccgcc cacgcggccg acctggccaa gcagcacccc
cacgcccagg cgtgggacga 2760cgcgctgtcc aaggcgcgct tcgagttccg
ctggatggac cagttcgcgc tgtccctgga 2820ccccatgacg gcgatgtcct
tccacgacga gacgctgccc gcggacggcg cgaaggtcgc 2880ccacttctgc
tccatgtgcg gccccaagtt ctgctccatg aagatcacgg aggacatccg
2940caagtacgcc gaggagaacg gctacggctc cgccgaggag gccatccgcc
agggcatgga 3000cgccatgtcc gaggagttca acatcgccaa gaagacgatc
tccggcgagc agcacggcga 3060ggtcggcggc gagatctacc tgcccgagtc
ctacgtcaag gccgcgcaga agtgacaatt 3120ggcagcagca gctcggatag
tatcgacaca ctctggacgc tggtcgtgtg atggactgtt 3180gccgccacac
ttgctgcctt gacctgtgaa tatccctgcc gcttttatca aacagcctca
3240gtgtgtttga tcttgtgtgt acgcgctttt gcgagttgct agctgcttgt
gctatttgcg 3300aataccaccc ccagcatccc cttccctcgt ttcatatcgc
ttgcatccca accgcaactt 3360atctacgctg tcctgctatc cctcagcgct
gctcctgctc ctgctcactg cccctcgcac 3420agccttggtt tgggctccgc
ctgtattctc ctggtactgc aacctgtaaa ccagcactgc 3480aatgctgatg
cacgggaagt agtgggatgg gaacacaaat ggaggatcc 3529241476DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
24atggccaccg catccacttt ctcggcgttc aatgcccgct gcggcgacct gcgtcgctcg
60gcgggctccg ggccccggcg cccagcgagg cccctccccg tgcgcgggcg cgccgccgcc
120gccgccgacg ccaaccccgc ccgccccgag cgccgcgtgg tgatcaccgg
ccagggcgtg 180gtgacctccc tgggccagac catcgagcag ttctactcct
ccctgctgga gggcgtgtcc 240ggcatctccc agatccagaa gttcgacacc
accggctaca ccaccaccat cgccggcgag 300atcaagtccc tgcagctgga
cccctacgtg cccaagcgct gggccaagcg cgtggacgac 360gtgatcaagt
acgtgtacat cgccggcaag caggccctgg agtccgccgg cctgcccatc
420gaggccgccg gcctggccgg cgccggcctg gaccccgccc tgtgcggcgt
gctgatcggc 480accgccatgg ccggcatgac ctccttcgcc gccggcgtgg
aggccctgac ccgcggcggc 540gtgcgcaaga tgaacccctt ctgcatcccc
ttctccatct ccaacatggg cggcgccatg 600ctggccatgg acatcggctt
catgggcccc aactactcca tctccaccgc ctgcgccacc 660ggcaactact
gcatcctggg cgccgccgac cacatccgcc gcggcgacgc caacgtgatg
720ctggccggcg gcgccgacgc cgccatcatc ccctccggca tcggcggctt
catcgcctgc 780aaggccctgt ccaagcgcaa cgacgagccc gagcgcgcct
cccgcccctg ggacgccgac 840cgcgacggct tcgtgatggg cgagggcgcc
ggcgtgctgg tgctggagga gctggagcac 900gccaagcgcc gcggcgccac
catcctggcc gagctggtgg gcggcgccgc cacctccgac 960gcccaccaca
tgaccgagcc cgacccccag ggccgcggcg tgcgcctgtg cctggagcgc
1020gccctggagc gcgcccgcct ggcccccgag cgcgtgggct acgtgaacgc
ccacggcacc 1080tccacccccg ccggcgacgt ggccgagtac cgcgccatcc
gcgccgtgat cccccaggac 1140tccctgcgca tcaactccac caagtccatg
atcggccacc tgctgggcgg cgccggcgcc 1200gtggaggccg tggccgccat
ccaggccctg cgcaccggct ggctgcaccc caacctgaac 1260ctggagaacc
ccgcccccgg cgtggacccc gtggtgctgg tgggcccccg caaggagcgc
1320gccgaggacc tggacgtggt gctgtccaac tccttcggct tcggcggcca
caactcctgc 1380gtgatcttcc gcaagtacga cgagatggac tacaaggacc
acgacggcga ctacaaggac 1440cacgacatcg actacaagga cgacgacgac aagtga
147625491PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 25Met Ala Thr Ala Ser Thr Phe Ser Ala Phe Asn
Ala Arg Cys Gly Asp 1 5 10 15 Leu Arg Arg Ser Ala Gly Ser Gly Pro
Arg Arg Pro Ala Arg Pro Leu 20 25 30 Pro Val Arg Gly Arg Ala Ala
Ala Ala Ala Asp Ala Asn Pro Ala Arg 35 40 45 Pro Glu Arg Arg Val
Val Ile Thr Gly Gln Gly Val Val Thr Ser Leu 50 55 60 Gly Gln Thr
Ile Glu Gln Phe Tyr Ser Ser Leu Leu Glu Gly Val Ser 65 70 75 80 Gly
Ile Ser Gln Ile Gln Lys Phe Asp Thr Thr Gly Tyr Thr Thr Thr 85 90
95 Ile Ala Gly Glu Ile Lys Ser Leu Gln Leu Asp Pro Tyr Val Pro Lys
100 105 110 Arg Trp Ala Lys Arg Val Asp Asp Val Ile Lys Tyr Val Tyr
Ile Ala 115 120 125 Gly Lys Gln Ala Leu Glu Ser Ala Gly Leu Pro Ile
Glu Ala Ala Gly 130 135 140 Leu Ala Gly Ala Gly Leu Asp Pro Ala Leu
Cys Gly Val Leu Ile Gly 145 150 155 160 Thr Ala Met Ala Gly Met Thr
Ser Phe Ala Ala Gly Val Glu Ala Leu 165 170 175 Thr Arg Gly Gly Val
Arg Lys Met Asn Pro Phe Cys Ile Pro Phe Ser 180 185 190 Ile Ser Asn
Met Gly Gly Ala Met Leu Ala Met Asp Ile Gly Phe Met 195 200 205 Gly
Pro Asn Tyr Ser Ile Ser Thr Ala Cys Ala Thr Gly Asn Tyr Cys 210 215
220 Ile Leu Gly Ala Ala Asp His Ile Arg Arg Gly Asp Ala Asn Val Met
225 230 235 240 Leu Ala Gly Gly Ala Asp Ala Ala Ile Ile Pro Ser Gly
Ile Gly Gly 245 250 255 Phe Ile Ala Cys Lys Ala Leu Ser Lys Arg Asn
Asp Glu Pro Glu Arg 260 265 270 Ala Ser Arg Pro Trp Asp Ala Asp Arg
Asp Gly Phe Val Met Gly Glu 275 280 285 Gly Ala Gly Val Leu Val Leu
Glu Glu Leu Glu His Ala Lys Arg Arg 290 295 300 Gly Ala Thr Ile Leu
Ala Glu Leu Val Gly Gly Ala Ala Thr Ser Asp 305 310 315 320 Ala His
His Met Thr Glu Pro Asp Pro Gln Gly Arg Gly Val Arg Leu 325 330 335
Cys Leu Glu Arg Ala Leu Glu Arg Ala Arg Leu Ala Pro Glu Arg Val 340
345 350 Gly Tyr Val Asn Ala His Gly Thr Ser Thr Pro Ala Gly Asp Val
Ala 355 360 365 Glu Tyr Arg Ala Ile Arg Ala Val Ile Pro Gln Asp Ser
Leu Arg Ile 370 375 380 Asn Ser Thr Lys Ser Met Ile Gly His Leu Leu
Gly Gly Ala Gly Ala 385 390 395 400 Val Glu Ala Val Ala Ala Ile Gln
Ala Leu Arg Thr Gly Trp Leu His 405 410 415 Pro Asn Leu Asn Leu Glu
Asn Pro Ala Pro Gly Val Asp Pro Val Val 420 425 430 Leu Val Gly Pro
Arg Lys Glu Arg Ala Glu Asp Leu Asp Val Val Leu 435 440 445 Ser Asn
Ser Phe Gly Phe Gly Gly His Asn Ser Cys Val Ile Phe Arg 450 455 460
Lys Tyr Asp Glu Met Asp Tyr Lys Asp His Asp Gly Asp Tyr Lys Asp 465
470 475 480 His Asp Ile Asp Tyr Lys Asp Asp Asp Asp Lys 485 490
261107DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 26atggccatca agaccaaccg ccagcccgtg
gagaagcccc ccttcaccat cggcaccctg 60cgcaaggcca tccccgccca ctgcttcgag
cgctccgccc tgcgctcctc catgtacctg 120gccttcgaca tcgccgtgat
gtccctgctg tacgtggcct ccacctacat cgaccccgcc 180cccgtgccca
cctgggtgaa gtacggcgtg atgtggcccc tgtactggtt cttccagggc
240gccttcggca ccggcgtgtg ggtgtgcgcc cacgagtgcg gccaccaggc
cttctcctcc 300tcccaggcca tcaacgacgg cgtgggcctg gtgttccact
ccctgctgct ggtgccctac 360tactcctgga agcactccca ccgccgccac
cactccaaca ccggctgcct ggacaaggac 420gaggtgttcg tgccccccca
ccgcgccgtg gcccacgagg gcctggagtg ggaggagtgg 480ctgcccatcc
gcatgggcaa ggtgctggtg accctgaccc tgggctggcc cctgtacctg
540atgttcaacg tggcctcccg cccctacccc cgcttcgcca accacttcga
cccctggtcc 600cccatcttct ccaagcgcga gcgcatcgag gtggtgatct
ccgacctggc cctggtggcc 660gtgctgtccg gcctgtccgt gctgggccgc
accatgggct gggcctggct ggtgaagacc 720tacgtggtgc cctacctgat
cgtgaacatg tggctggtgc tgatcaccct gctgcagcac 780acccaccccg
ccctgcccca ctacttcgag aaggactggg actggctgcg cggcgccatg
840gccaccgtgg accgctccat gggccccccc ttcatggaca acatcctgca
ccacatctcc 900gacacccacg tgctgcacca cctgttctcc accatccccc
actaccacgc cgaggaggcc 960tccgccgcca tccgccccat cctgggcaag
tactaccagt ccgactcccg ctgggtgggc 1020cgcgccctgt gggaggactg
gcgcgactgc cgctacgtgg tgcccgacgc ccccgaggac 1080gactccgccc
tgtggttcca caagtag 110727368PRTPrototheca moriformis 27Met Ala Ile
Lys Thr Asn Arg Gln Pro Val Glu Lys Pro Pro Phe Thr 1 5 10 15 Ile
Gly Thr Leu Arg Lys Ala Ile Pro Ala His Cys Phe Glu Arg Ser 20 25
30 Ala Leu Arg Ser Ser Met Tyr Leu Ala Phe Asp Ile Ala Val Met Ser
35 40 45 Leu Leu Tyr Val Ala Ser Thr Tyr Ile Asp Pro Ala Pro Val
Pro Thr 50 55 60 Trp Val Lys Tyr Gly Val Met Trp Pro Leu Tyr Trp
Phe Phe Gln Gly 65 70 75 80 Ala Phe Gly Thr Gly Val Trp Val Cys Ala
His Glu Cys Gly His Gln 85 90 95 Ala Phe Ser Ser Ser Gln Ala Ile
Asn Asp Gly Val Gly Leu Val Phe 100 105 110 His Ser Leu Leu Leu Val
Pro Tyr Tyr Ser Trp Lys His Ser His Arg 115 120 125 Arg His His Ser
Asn Thr Gly Cys Leu Asp Lys Asp Glu Val Phe Val 130 135 140 Pro Pro
His Arg Ala Val Ala His Glu Gly Leu Glu Trp Glu Glu Trp 145 150 155
160 Leu Pro Ile Arg Met Gly Lys Val Leu Val Thr Leu Thr Leu Gly Trp
165 170 175 Pro Leu Tyr Leu Met Phe Asn Val Ala Ser Arg Pro Tyr Pro
Arg Phe 180 185 190 Ala Asn His Phe Asp Pro Trp Ser Pro Ile Phe Ser
Lys Arg Glu Arg 195 200 205 Ile Glu Val Val Ile Ser Asp Leu Ala Leu
Val Ala Val Leu Ser Gly 210 215 220 Leu Ser Val Leu Gly Arg Thr Met
Gly Trp Ala Trp Leu Val Lys Thr 225 230 235 240 Tyr Val Val Pro Tyr
Leu Ile Val Asn Met Trp Leu Val Leu Ile Thr 245 250 255 Leu Leu Gln
His Thr His Pro Ala Leu Pro His Tyr Phe Glu Lys Asp 260 265 270 Trp
Asp Trp Leu Arg Gly Ala Met Ala Thr Val Asp Arg Ser Met Gly 275 280
285 Pro Pro Phe Met Asp Asn Ile Leu His His Ile Ser Asp Thr His Val
290 295 300 Leu His His Leu Phe Ser Thr Ile Pro His Tyr His Ala Glu
Glu Ala 305 310 315 320 Ser Ala Ala Ile Arg Pro Ile Leu Gly Lys Tyr
Tyr Gln Ser Asp Ser 325 330 335 Arg Trp Val Gly Arg Ala Leu Trp Glu
Asp Trp Arg Asp Cys Arg Tyr 340 345 350 Val Val Pro Asp Ala Pro Glu
Asp Asp Ser Ala Leu Trp Phe His Lys 355 360 365 281191DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
28actagtatgc tgaagctgtc ctgcaacgtg accaacaacc tgcacacctt ctccttcttc
60tccgactcct ccctgttcat ccccgtgaac cgccgcacca tcgccgtgtc ctccgggcgc
120gcctcccagc tgcgcaagcc cgccctggac cccctgcgcg ccgtgatctc
cgccgaccag 180ggctccatct cccccgtgaa ctcctgcacc cccgccgacc
gcctgcgcgc cggccgcctg 240atggaggacg gctactccta caaggagaag
ttcatcgtgc gctcctacga ggtgggcatc 300aacaagaccg ccaccgtgga
gaccatcgcc aacctgctgc aggaggtggc ctgcaaccac 360gtgcagaagt
gcggcttctc
caccgacggc ttcgccacca ccctgaccat gcgcaagctg 420cacctgatct
gggtgaccgc ccgcatgcac atcgagatct acaagtaccc cgcctggtcc
480gacgtggtgg agatcgagac ctggtgccag tccgagggcc gcatcggcac
ccgccgcgac 540tggatcctgc gcgactccgc caccaacgag gtgatcggcc
gcgccacctc caagtgggtg 600atgatgaacc aggacacccg ccgcctgcag
cgcgtgaccg acgaggtgcg cgacgagtac 660ctggtgttct gcccccgcga
gccccgcctg gccttccccg aggagaacaa ctcctccctg 720aagaagatcc
ccaagctgga ggaccccgcc cagtactcca tgctggagct gaagccccgc
780cgcgccgacc tggacatgaa ccagcacgtg aacaacgtga cctacatcgg
ctgggtgctg 840gagtccatcc cccaggagat catcgacacc cacgagctgc
aggtgatcac cctggactac 900cgccgcgagt gccagcagga cgacatcgtg
gactccctga ccacctccga gatccccgac 960gaccccatct ccaagttcac
cggcaccaac ggctccgcca tgtcctccat ccagggccac 1020aacgagtccc
agttcctgca catgctgcgc ctgtccgaga acggccagga gatcaaccgc
1080ggccgcaccc agtggcgcaa gaagtcctcc cgcatggact acaaggacca
cgacggcgac 1140tacaaggacc acgacatcga ctacaaggac gacgacgaca
agtgaatcga t 119129366PRTBrassica napus 29Met Leu Lys Leu Ser Cys
Asn Val Thr Asn Asn Leu His Thr Phe Ser 1 5 10 15 Phe Phe Ser Asp
Ser Ser Leu Phe Ile Pro Val Asn Arg Arg Thr Ile 20 25 30 Ala Val
Ser Ser Ser Gln Leu Arg Lys Pro Ala Leu Asp Pro Leu Arg 35 40 45
Ala Val Ile Ser Ala Asp Gln Gly Ser Ile Ser Pro Val Asn Ser Cys 50
55 60 Thr Pro Ala Asp Arg Leu Arg Ala Gly Arg Leu Met Glu Asp Gly
Tyr 65 70 75 80 Ser Tyr Lys Glu Lys Phe Ile Val Arg Ser Tyr Glu Val
Gly Ile Asn 85 90 95 Lys Thr Ala Thr Val Glu Thr Ile Ala Asn Leu
Leu Gln Glu Val Ala 100 105 110 Cys Asn His Val Gln Lys Cys Gly Phe
Ser Thr Asp Gly Phe Ala Thr 115 120 125 Thr Leu Thr Met Arg Lys Leu
His Leu Ile Trp Val Thr Ala Arg Met 130 135 140 His Ile Glu Ile Tyr
Lys Tyr Pro Ala Trp Ser Asp Val Val Glu Ile 145 150 155 160 Glu Thr
Trp Cys Gln Ser Glu Gly Arg Ile Gly Thr Arg Arg Asp Trp 165 170 175
Ile Leu Arg Asp Ser Ala Thr Asn Glu Val Ile Gly Arg Ala Thr Ser 180
185 190 Lys Trp Val Met Met Asn Gln Asp Thr Arg Arg Leu Gln Arg Val
Thr 195 200 205 Asp Glu Val Arg Asp Glu Tyr Leu Val Phe Cys Pro Arg
Glu Pro Arg 210 215 220 Leu Ala Phe Pro Glu Glu Asn Asn Ser Ser Leu
Lys Lys Ile Pro Lys 225 230 235 240 Leu Glu Asp Pro Ala Gln Tyr Ser
Met Leu Glu Leu Lys Pro Arg Arg 245 250 255 Ala Asp Leu Asp Met Asn
Gln His Val Asn Asn Val Thr Tyr Ile Gly 260 265 270 Trp Val Leu Glu
Ser Ile Pro Gln Glu Ile Ile Asp Thr His Glu Leu 275 280 285 Gln Val
Ile Thr Leu Asp Tyr Arg Arg Glu Cys Gln Gln Asp Asp Ile 290 295 300
Val Asp Ser Leu Thr Thr Ser Glu Ile Pro Asp Asp Pro Ile Ser Lys 305
310 315 320 Phe Thr Gly Thr Asn Gly Ser Ala Met Ser Ser Ile Gln Gly
His Asn 325 330 335 Glu Ser Gln Phe Leu His Met Leu Arg Leu Ser Glu
Asn Gly Gln Glu 340 345 350 Ile Asn Arg Gly Arg Thr Gln Trp Arg Lys
Lys Ser Ser Arg 355 360 365 30599DNAPrototheca moriformis
30ggagtcactg tgccactgag ttcgactggt agctgaatgg agtcgctgct ccactaaacg
60aattgtcagc accgccagcc ggccgaggac ccgagtcata gcgagggtag tagcgcgcca
120tggcaccgac cagcctgctt gccagtactg gcgtctcttc cgcttctctg
tggtcctctg 180cgcgctccag cgcgtgcgct tttccggtgg atcatgcggt
ccgtggcgca ccgcagcggc 240cgctgcccat gcagcgccgc tgcttccgaa
cagtggcggt cagggccgca cccgcggtag 300ccgtccgtcc ggaacccgcc
caagagtttt gggagcagct tgagccctgc aagatggcgg 360aggacaagcg
catcttcctg gaggagcacc ggtgcgtgga ggtccggggc tgaccggccg
420tcgcattcaa cgtaatcaat cgcatgatga tcagaggaca cgaagtcttg
gtggcggtgg 480ccagaaacac tgtccattgc aagggcatag ggatgcgttc
cttcacctct catttctcat 540ttctgaatcc ctccctgctc actctttctc
ctcctccttc ccgttcacgc agcattcgg 59931521DNAPrototheca moriformis
31gacagggtgg ttggctggat ggggaaacgc tggtcgcggg attcgatcct gctgcttata
60tcctccctgg aagcacaccc acgactctga agaagaaaac gtgcacacac acaacccaac
120cggccgaata tttgcttcct tatcccgggt ccaagagaga ctgcgatgcc
cccctcaatc 180agcatcctcc tccctgccgc ttcaatcttc cctgcttgcc
tgcgcccgcg gtgcgccgtc 240tgcccgccca gtcagtcact cctgcacagg
ccccttgtgc gcagtgctcc tgtacccttt 300accgctcctt ccattctgcg
aggcccccta ttgaatgtat tcgttgcctg tgtggccaag 360cgggctgctg
ggcgcgccgc cgtcgggcag tgctcggcga ctttggcgga agccgattgt
420tcttctgtaa gccacgcgct tgctgctttg ggaagagaag ggggggggta
ctgaatggat 480gaggaggaga aggaggggta ttggtattat ctgagttggg t
52132563DNAPrototheca moriformis 32aatggagtcg ctgctccact aatcgaattg
tcagcaccgc cagccggccg aggacccgag 60tcatagcgag ggtagtagcg cgccatggca
ccgaccagcc tgcttgcccg tactggcgtc 120tcttccgctt ctctgtgctc
ctctacgcgc tccggcgcgt gcgcttttcc ggtggatcat 180gcggtccgtg
gcgcaccgca gcggccgctg cccatgcagc gccgctgctt ccgaacagtg
240gctgtcaggg ccgcacccgc agtagccgtc cgtccggaac ccgcccaaga
gttttgggag 300cagcttgagc cctgcaagat ggcggaggac aagcgcatct
tcctggagga gcaccggtgc 360gcggaggtcc ggggctgacc ggccgtcgca
ttcaacgtaa tcaatcgcat gatgatcaca 420ggacgcgacg tcttggtggc
ggtggccagg gacactgccc attgcacagg cataggaatg 480cgttccttct
catttctcag ttttctgagc ccctccctct tcactctttc tcctcctcct
540cccctctcac gcagcattcg tgg 56333547DNAPrototheca moriformis
33cactagtatc gatttcgaac agaggagagg gtggctggta gttgcgggat ggctggtcgc
60ccgtcgatcc tgctgctgct attgtctcct cctgcacaag cccacccacg actccgaaga
120agaagaagaa aacgcgcaca cacacaaccc aaccggccga atatttgctt
ccttatcccg 180ggtccaagag agacggcgat gcccccctca atcagcctcc
tcctccctgc cgctccaatc 240ttccctgctt gcatgcgccc gcgagaggct
gtctgcgcgc cccgtcagtc actccccgtg 300cagacgcctc gtgctcggtg
ctcctgtatc ctttaccgct cctttcattc tgcgaggccc 360cctgttgaat
gtattcgttg cctgtgtggc caagcgcgct gctgggcgcg ccgccgtcgg
420gcggtgctcg gcgactctgg cggaagccgg ttgttcttct gtaagccacg
cgcttgctgc 480ttttggaaaa gaggggggtt tactgaatgg aggaggagca
ggataattgg tagtatctga 540gttgttg 54734615DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
34actagtgcgc tggacgcggc agtgggtggc cgaggagaac cggcacggcg acctgctgaa
60caagtactgt tggctgacgg ggcgcgtcaa catgcgggcc gtggaggtga ccatcaacaa
120cctgatcaag agcggcatga acccgcagac ggacaacaac ccttacttgg
gcttcgtcta 180cacctccttc caggagcgcg cgaccaagta cagccacggc
aacaccgcgc gccttgcggc 240cgagcagtgt gtttgagggt tttggttgcc
cgtatcgagg tcctggtggc gcgcatgggg 300gagaaggcgc ctgtcccgct
gacccccccg gctaccctcc cggcaccttc cagggcgcgt 360acgggatcct
gctcggccgc aaggcgcgcg gtgttgccgt ggctgtactt ggtcgcgcgc
420tcctggaagg aggtgtagac gaagcccaag taagggttgt tgtccgtctg
cgggttcatg 480ccgctcttga tcaggttgtt gatggtcacc tccacggccc
gcatgttgac gcgccccgtc 540agccaacagt acttgttcag caggtcgccg
tgccggttct cctcggccac ccactgccgc 600gtccagcgca agctt
61535452PRTPrototheca moriformis 35Met Ser Ile Gln Phe Ala Leu Arg
Ala Ala Tyr Ile Lys Gly Thr Cys 1 5 10 15 Gln Arg Leu Ser Gly Arg
Gly Ala Ala Leu Gly Leu Ser Arg Asp Trp 20 25 30 Thr Pro Gly Trp
Thr Leu Pro Arg Cys Trp Pro Ala Ser Ala Ala Ala 35 40 45 Thr Ala
Pro Pro Arg Ala Arg His Gln Glu Arg Ala Ile His Leu Thr 50 55 60
Ser Gly Arg Arg Arg His Ser Ala Leu Ala Ser Asp Ala Asp Glu Arg 65
70 75 80 Ala Leu Pro Ser Asn Ala Pro Gly Leu Val Met Ala Ser Gln
Ala Asn 85 90 95 Tyr Phe Arg Val Arg Leu Leu Pro Glu Gln Glu Glu
Gly Glu Leu Glu 100 105 110 Ser Trp Ser Pro Asn Val Arg His Thr Thr
Leu Leu Cys Lys Pro Arg 115 120 125 Ala Met Leu Ser Lys Leu Gln Met
Arg Val Met Val Gly Asp Arg Val 130 135 140 Ile Val Thr Ala Ile Asp
Pro Val Asn Met Thr Val His Ala Pro Pro 145 150 155 160 Phe Asp Pro
Leu Pro Ala Thr Arg Phe Leu Val Ala Gly Glu Ala Ala 165 170 175 Asp
Met Asp Ile Thr Val Val Leu Asn Lys Ala Asp Leu Val Pro Glu 180 185
190 Glu Glu Ser Ala Ala Leu Ala Gln Glu Val Ala Ser Trp Gly Pro Val
195 200 205 Val Leu Thr Ser Thr Leu Thr Gly Arg Gly Leu Gln Glu Leu
Glu Arg 210 215 220 Gln Leu Gly Ser Thr Thr Ala Val Leu Ala Gly Pro
Ser Gly Ala Gly 225 230 235 240 Lys Ser Ser Ile Ile Asn Ala Leu Ala
Arg Ala Ala Arg Glu Arg Pro 245 250 255 Ser Asp Ala Ser Val Ser Asn
Val Pro Glu Glu Gln Val Val Gly Glu 260 265 270 Asp Gly Arg Ala Leu
Ala Asn Pro Pro Pro Phe Thr Leu Ala Asp Ile 275 280 285 Arg Asn Ala
Ile Pro Lys Asp Cys Phe Arg Lys Ser Ala Ala Lys Ser 290 295 300 Leu
Ala Tyr Leu Gly Asp Leu Ser Ile Thr Gly Met Ala Val Leu Ala 305 310
315 320 Tyr Lys Ile Asn Ser Pro Trp Leu Trp Pro Leu Tyr Trp Phe Ala
Gln 325 330 335 Gly Thr Met Phe Trp Ala Leu Phe Val Val Gly His Asp
Cys Gly His 340 345 350 Gln Ser Phe Ser Thr Ser Lys Arg Leu Asn Asp
Ala Leu Ala Trp Leu 355 360 365 Gly Ala Leu Ala Ala Gly Thr Trp Thr
Trp Ala Leu Gly Val Leu Pro 370 375 380 Met Leu Asn Leu Tyr Leu Ala
Pro Tyr Val Trp Leu Leu Val Thr Tyr 385 390 395 400 Leu His His His
Gly Pro Ser Asp Pro Arg Glu Glu Met Pro Trp Tyr 405 410 415 Arg Gly
Arg Glu Trp Ser Tyr Met Arg Gly Gly Leu Thr Thr Ile Asp 420 425 430
Arg Asp Tyr Gly Leu Phe Asn Lys Val His His Asp Ile Gly Thr His 435
440 445 Val Val His His 450 36315PRTArtificial SequenceDescription
of Artificial Sequence Synthetic polypeptide 36Met Phe Trp Ala Leu
Phe Val Val Gly His Asp Cys Gly His Gln Ser 1 5 10 15 Phe Ser Thr
Ser Lys Arg Leu Asn Asp Ala Val Gly Leu Phe Val His 20 25 30 Ser
Ile Ile Gly Val Pro Tyr His Gly Trp Arg Ile Ser His Arg Thr 35 40
45 His His Asn Asn His Gly His Val Glu Asn Asp Glu Ser Trp Tyr Pro
50 55 60 Pro Thr Glu Ser Gly Leu Lys Ala Met Thr Asp Met Gly Arg
Gln Gly 65 70 75 80 Arg Phe His Phe Pro Ser Met Leu Phe Val Tyr Pro
Phe Tyr Leu Phe 85 90 95 Trp Arg Ser Pro Gly Lys Thr Gly Ser His
Phe Ser Pro Ala Thr Asp 100 105 110 Leu Phe Ala Leu Trp Glu Ala Pro
Leu Ile Arg Thr Ser Asn Ala Cys 115 120 125 Gln Leu Ala Trp Leu Gly
Ala Leu Ala Ala Gly Thr Trp Ala Leu Gly 130 135 140 Val Leu Pro Met
Leu Asn Leu Tyr Leu Ala Pro Tyr Val Ile Ser Val 145 150 155 160 Ala
Trp Leu Asp Leu Val Thr Tyr Leu His His His Gly Pro Ser Asp 165 170
175 Pro Arg Glu Glu Met Pro Trp Tyr Arg Gly Arg Glu Trp Ser Tyr Met
180 185 190 Arg Gly Gly Leu Thr Thr Ile Asp Arg Asp Tyr Gly Leu Phe
Asn Lys 195 200 205 Val His His Asp Ile Gly Thr His Val Val His His
Leu Phe Pro Gln 210 215 220 Ile Pro His Tyr Asn Leu Cys Arg Ala Thr
Lys Ala Ala Lys Lys Val 225 230 235 240 Leu Gly Pro Tyr Tyr Arg Glu
Pro Glu Arg Cys Pro Leu Gly Leu Leu 245 250 255 Pro Val His Leu Leu
Ala Pro Leu Leu Arg Ser Leu Gly Gln Asp His 260 265 270 Phe Val Asp
Asp Ala Gly Ser Val Leu Phe Tyr Arg Arg Ala Glu Gly 275 280 285 Ile
Asn Pro Trp Ile Gln Lys Leu Leu Pro Trp Leu Gly Gly Ala Arg 290 295
300 Arg Gly Ala Asp Ala Gln Arg Asp Ala Ala Gln 305 310 315
37448PRTCamelina sativa 37Met Ala Asn Leu Val Leu Ser Glu Cys Gly
Ile Arg Pro Leu Pro Arg 1 5 10 15 Ile Tyr Thr Thr Pro Arg Ser Asn
Phe Val Ser Asn Asn Asn Lys Pro 20 25 30 Ile Phe Lys Phe Arg Pro
Phe Thr Ser Tyr Lys Thr Ser Ser Ser Pro 35 40 45 Leu Ala Cys Ser
Arg Asp Gly Phe Gly Lys Asn Trp Ser Leu Asn Val 50 55 60 Ser Val
Pro Leu Thr Thr Thr Thr Pro Ile Val Asp Glu Ser Pro Leu 65 70 75 80
Lys Glu Glu Glu Glu Glu Lys Gln Arg Phe Asp Pro Gly Ala Pro Pro 85
90 95 Pro Phe Asn Leu Ala Asp Ile Arg Ala Ala Ile Pro Lys His Cys
Trp 100 105 110 Val Lys Asn Pro Trp Lys Ser Met Ser Tyr Val Leu Arg
Asp Val Ala 115 120 125 Ile Val Phe Ala Leu Ala Ala Gly Ala Ser Tyr
Leu Asn Asn Trp Ile 130 135 140 Val Trp Pro Leu Tyr Trp Leu Ala Gln
Gly Thr Met Phe Trp Ala Leu 145 150 155 160 Phe Val Leu Gly His Asp
Cys Gly His Gly Ser Phe Ser Asn Asn Pro 165 170 175 Arg Leu Asn Asn
Val Val Gly His Leu Leu His Ser Ser Ile Leu Val 180 185 190 Pro Tyr
His Gly Trp Arg Ile Ser His Arg Thr His His Gln Asn His 195 200 205
Gly His Val Glu Asn Asp Glu Ser Trp His Pro Met Ser Glu Lys Ile 210
215 220 Tyr Gln Ser Leu Asp Lys Pro Thr Arg Phe Phe Arg Phe Thr Leu
Pro 225 230 235 240 Leu Val Met Leu Ala Tyr Pro Phe Tyr Leu Trp Ala
Arg Ser Pro Gly 245 250 255 Lys Lys Gly Ser His Tyr His Pro Glu Ser
Asp Leu Phe Leu Pro Lys 260 265 270 Glu Lys Thr Asp Val Leu Thr Ser
Thr Ala Cys Trp Thr Ala Met Ala 275 280 285 Ala Leu Leu Ile Cys Leu
Asn Phe Val Val Gly Pro Val Gln Met Leu 290 295 300 Lys Leu Tyr Gly
Ile Pro Tyr Trp Ile Asn Val Met Trp Leu Asp Phe 305 310 315 320 Val
Thr Tyr Leu His His His Gly His Glu Asp Lys Leu Pro Trp Tyr 325 330
335 Arg Gly Lys Glu Trp Ser Tyr Leu Arg Gly Gly Leu Thr Thr Leu Asp
340 345 350 Arg Asp Tyr Gly Val Ile Asn Asn Ile His His Asp Ile Gly
Thr His 355 360 365 Val Ile His His Leu Phe Pro Gln Ile Pro His Tyr
His Leu Val Glu 370 375 380 Ala Thr Glu Ala Val Lys Pro Val Leu Gly
Lys Tyr Tyr Arg Glu Pro 385 390 395 400 Asp Lys Ser Gly Pro Leu Pro
Leu His Leu Leu Gly Ile Leu Ala Lys 405 410 415 Ser Ile Lys Glu Asp
His Tyr Val Ser Asp Glu Gly Asp Val Val Tyr 420 425 430 Tyr Lys Ala
Asp Pro Asn Met Tyr Gly Glu Ile Lys Val Gly Ala Asp 435 440 445
38368PRTPrototheca moriformis 38Met Ala Ile Lys Thr Asn Arg Gln Pro
Val Glu Lys Pro Pro Phe Thr 1 5 10 15 Ile Gly Thr Leu Arg Lys Ala
Ile Pro Ala His Cys Phe Glu Arg Ser 20 25 30 Ala Leu Arg Ser Ser
Met Tyr Leu Ala Phe Asp Ile Ala Val Met Ser 35 40 45 Leu Leu Tyr
Val Ala Ser Thr Tyr Ile Asp Pro Ala Pro Val Pro Thr 50 55 60 Trp
Val Lys Tyr Gly Ile Met Trp Pro Leu Tyr Trp Phe Phe Gln Gly 65 70
75
80 Ala Phe Gly Thr Gly Val Trp Val Cys Ala His Glu Cys Gly His Gln
85 90 95 Ala Phe Ser Ser Ser Gln Ala Ile Asn Asp Gly Val Gly Leu
Val Phe 100 105 110 His Ser Leu Leu Leu Val Pro Tyr Tyr Ser Trp Lys
His Ser His Arg 115 120 125 Arg His His Ser Asn Thr Gly Cys Leu Asp
Lys Asp Glu Val Phe Val 130 135 140 Pro Pro His Arg Ala Val Ala His
Glu Gly Leu Glu Trp Glu Glu Trp 145 150 155 160 Leu Pro Ile Arg Met
Gly Lys Val Leu Val Thr Leu Thr Leu Gly Trp 165 170 175 Pro Leu Tyr
Leu Met Phe Asn Val Ala Ser Arg Pro Tyr Pro Arg Phe 180 185 190 Ala
Asn His Phe Asp Pro Trp Ser Pro Ile Phe Ser Lys Arg Glu Arg 195 200
205 Ile Glu Val Val Ile Ser Asp Leu Ala Leu Val Ala Val Leu Ser Gly
210 215 220 Leu Ser Val Leu Gly Arg Thr Met Gly Trp Ala Trp Leu Val
Lys Thr 225 230 235 240 Tyr Val Val Pro Tyr Met Ile Val Asn Met Trp
Leu Val Leu Ile Thr 245 250 255 Leu Leu Gln His Thr His Pro Ala Leu
Pro His Tyr Phe Glu Lys Asp 260 265 270 Trp Asp Trp Leu Arg Gly Ala
Met Ala Thr Val Asp Arg Ser Met Gly 275 280 285 Pro Pro Phe Met Asp
Ser Ile Leu His His Ile Ser Asp Thr His Val 290 295 300 Leu His His
Leu Phe Ser Thr Ile Pro His Tyr His Ala Glu Glu Ala 305 310 315 320
Ser Ala Ala Ile Arg Pro Ile Leu Gly Lys Tyr Tyr Gln Ser Asp Ser 325
330 335 Arg Trp Val Gly Arg Ala Leu Trp Glu Asp Trp Arg Asp Cys Arg
Tyr 340 345 350 Val Val Pro Asp Ala Pro Glu Asp Asp Ser Ala Leu Trp
Phe His Lys 355 360 365 39448PRTCamelina sativa 39Met Ala Asn Leu
Val Leu Ser Glu Cys Gly Ile Arg Pro Leu Pro Arg 1 5 10 15 Ile Tyr
Thr Thr Pro Arg Ser Asn Phe Val Ser Asn Asn Asn Lys Pro 20 25 30
Ile Phe Lys Phe Arg Pro Leu Thr Ser Tyr Lys Thr Ser Ser Pro Leu 35
40 45 Phe Cys Ser Arg Asp Gly Phe Gly Arg Asn Trp Ser Leu Asn Val
Ser 50 55 60 Val Pro Leu Ala Thr Thr Thr Pro Ile Val Asp Glu Ser
Pro Leu Glu 65 70 75 80 Glu Glu Glu Glu Glu Glu Lys Gln Arg Phe Asp
Pro Gly Ala Pro Pro 85 90 95 Pro Phe Asn Leu Ala Asp Ile Arg Ala
Ala Ile Pro Lys His Cys Trp 100 105 110 Val Lys Asn Pro Trp Lys Ser
Met Ser Tyr Val Leu Arg Asp Val Ala 115 120 125 Ile Val Phe Ala Leu
Ala Ala Gly Ala Ala Tyr Leu Asn Asn Trp Ile 130 135 140 Val Trp Pro
Leu Tyr Trp Leu Ala Gln Gly Thr Met Phe Trp Ala Leu 145 150 155 160
Phe Val Leu Gly His Asp Cys Gly His Gly Ser Phe Ser Asn Asn Pro 165
170 175 Arg Leu Asn Asn Val Val Gly His Leu Leu His Ser Ser Ile Leu
Val 180 185 190 Pro Tyr His Gly Trp Arg Ile Ser His Arg Thr His His
Gln Asn His 195 200 205 Gly His Val Glu Asn Asp Glu Ser Trp His Pro
Met Ser Glu Lys Ile 210 215 220 Tyr Gln Ser Leu Asp Lys Pro Thr Arg
Phe Phe Arg Phe Thr Leu Pro 225 230 235 240 Leu Val Met Leu Ala Tyr
Pro Phe Tyr Leu Trp Ala Arg Ser Pro Gly 245 250 255 Lys Lys Gly Ser
His Tyr His Pro Glu Ser Asp Leu Phe Leu Pro Lys 260 265 270 Glu Lys
Thr Asp Val Leu Thr Ser Thr Ala Cys Trp Thr Ala Met Ala 275 280 285
Ala Leu Leu Ile Cys Leu Asn Phe Val Val Gly Pro Val Gln Met Leu 290
295 300 Lys Leu Tyr Gly Ile Pro Tyr Trp Ile Asn Val Met Trp Leu Asp
Phe 305 310 315 320 Val Thr Tyr Leu His His His Gly His Glu Asp Lys
Leu Pro Trp Tyr 325 330 335 Arg Gly Lys Glu Trp Ser Tyr Leu Arg Gly
Gly Leu Thr Thr Leu Asp 340 345 350 Arg Asp Tyr Gly Val Ile Asn Asn
Ile His His Asp Ile Gly Thr His 355 360 365 Val Ile His His Leu Phe
Pro Gln Ile Pro His Tyr His Leu Val Glu 370 375 380 Ala Thr Glu Ala
Val Lys Pro Val Leu Gly Lys Tyr Tyr Arg Glu Pro 385 390 395 400 Asp
Lys Ser Gly Pro Leu Pro Leu His Leu Leu Gly Ile Leu Ala Lys 405 410
415 Ser Ile Lys Glu Asp His Tyr Val Ser Asp Glu Gly Asp Val Val Tyr
420 425 430 Tyr Lys Ala Asp Pro Asn Met Tyr Gly Glu Ile Lys Val Gly
Ala Asp 435 440 445 40353DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 40actagtcatt
cggggcaacg aggtgggccc ctcgcagcgg ctgacgatca cggcggtggc 60caacatcctg
caggaggcgg cgggcaacca cgcggtggcc atgtggggcc ggagcgtgtg
120tttgagggtt ttggttgccc gtattgaggt cctggtggcg cgcatggggg
agaaggcgcc 180tgtcccgctg acccccccgg ctaccctccc ggcaccttcc
agggcgcgta cgggatccgc 240tccggcccca catggccacc gcgtggttgc
ccgccgcctc ctgcaggatg ttggccaccg 300ccgtgatcgt cagccgctgc
gaggggccca cctcgttgcc ccgaatgaag ctt 35341391DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
41actagtggag ggtttcgcga cggacccgga gctgcaggag gcgggtctca tctttgtgat
60gacgcgcatg cagatccaga tgtaccgcta cccgcgctgg ggcgacctga tgcaggtgga
120gacctggttc cagagtgtgt ttgagggttt tggttgcccg tattgaggtc
ctggtggcgc 180gcatggggga gaaggcgcct gtcccgctga cccccccggc
taccctcccg gcaccttcca 240gggcgcgtac gggatcctct ggaaccaggt
ctccacctgc atcaggtcgc cccagcgcgg 300gtagcggtac atctggatct
gcatgcgcgt catcacaaag atgagacccg cctcctgcag 360ctccgggtcc
gtcgcgaaac cctccaagct t 39142393DNAArtificial SequenceDescription
of Artificial Sequence Synthetic polynucleotide 42actagtcggc
gggcaagctg ggcgcgcagc gcgagtgggt gctgcgcgac aagctgaccg 60gcgaggcgct
gggcgcggcc acctcgagct gggtcatgat caacatccgc acgcgccggc
120cgtgccgcat gccgggtgtg tttgagggtt ttggttgccc gtatcgaggt
cctggtggcg 180cgcatggggg agaaggcgcc tgtcccgctg acccccccgg
ctaccctccc ggcaccttcc 240agggcgcgta cgggatcccc ggcatgcggc
acggccggcg cgtgcggatg ttgatcatga 300cccagctcga ggtggccgcg
cccagcgcct cgccggtcag cttgtcgcgc agcacccact 360cgcgctgcgc
gcccagcttg cccgccgaag ctt 39343517DNAArtificial SequenceDescription
of Artificial Sequence Synthetic polynucleotide 43actagtgtcc
gcgtcaagtc ggccttcttc gcgcgcgagc cgccgcgcct ggcgctgccg 60cccgcggtca
cgcgtgccaa gctgcccaac atcgcgacgc cggcgccgct gcgcgggcac
120cgccaggtcg cgcgccgcac cgacatggac atgaacgggc acgtgaacaa
cgtggcctac 180ctggcctggt gcctggagtg tgtttgaggg ttttggttgc
ccgtattgag gtcctggtgg 240cgcgcatggg ggagaaggcg cctgtcccgc
tgaccccccc ggctaccctc ccggcacctt 300ccagggcgcg tacgggatcc
tccaggcacc aggccaggta ggccacgttg ttcacgtgcc 360cgttcatgtc
catgtcggtg cggcgcgcga cctggcggtg cccgcgcagc ggcgccggcg
420tcgcgatgtt gggcagcttg gcacgcgtga ccgcgggcgg cagcgccagg
cgcggcggct 480cgcgcgcgaa gaaggccgac ttgacgcgga caagctt
51744519DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 44actagtccgt gcccgagcac gtcttcagcg
actaccacct ctaccagatg gagatcgact 60tcaaggccga gtgccacgcg ggcgacgtca
tctcctccca ggccgagcag atcccgcccc 120aggaggcgct cacgcacaac
ggcgccggcc gcaacccctc ctgcttcgtc catagcattc 180tgcgcgccga
gaccgagcgt gtgtttgagg gttttggttg cccgtatcga ggtcctggtg
240gcgcgcatgg gggagaaggc gcctgtcccg ctgacccccc cggctaccct
cccggcacct 300tccagggcgc gtacgggatc cgctcggtct cggcgcgcag
aatgctatgg acgaagcagg 360aggggttgcg gccggcgccg ttgtgcgtga
gcgcctcctg gggcgggatc tgctcggcct 420gggaggagat gacgtcgccc
gcgtggcact cggccttgaa gtcgatctcc atctggtaga 480ggtggtagtc
gctgaagacg tgctcgggca cggaagctt 51945415DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
45actagttcgt ccgcgcgcga accacatggt cggcccccat cgacgcgccc gccgccaagc
60cgcccaaggc gagccactga ggacagggtg gttggctgga tggggaaacg ctggtcgcgg
120gattcgatcc tgctgcttat atcctcgtgt gtttgagggt tttggttgcc
cgtattgagg 180tcctggtggc gcgcatgggg gagaaggcgc ctgtcccgct
gacccccccg gctaccctcc 240cggcaccttc cagggcgcgt acgggatccg
aggatataag cagcaggatc gaatcccgcg 300accagcgttt ccccatccag
ccaaccaccc tgtcctcagt ggctcgcctt gggcggcttg 360gcggcgggcg
cgtcgatggg ggccgaccat gtggttcgcg cgcggacgaa agctt
415461629DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 46atggccgccg ccgccagcat ggtggccagc
cccttctgca cctggctggt ggccagctgc 60atgagcacca gcttcgacaa cgacccccgc
agccccagcg tgaagcgctt cccccgccgc 120aagcgcgtgc tgagccagcg
cggcagcacc tacgtattcc agtgcctggt ggccagctgc 180atcgacccct
gcgaccagta ccgcagcagc gccagcctga gcttcctggg cgacaacggc
240ttcgccagcc tgttcggcag caagcccttc atgagcaacc gcggccaccg
ccgcctgcgc 300cgcgccagcc acagcggcga ggccatggcc gtggccctgc
agcccgccca ggaggccggc 360accaagaaga agcccgtgat caagcagcgc
cgcgtggtgg tgaccggcat gggcgtggtg 420acccccctgg gccacgagcc
cgacgtgttc tacaacaacc tgctggacgg cgtgagcggc 480atcagcgaga
tcgagacctt cgactgcacc cagttcccca cccgcatcgc cggcgagatc
540aagagcttca gcaccgacgg ctgggtggcc cccaagctga gcaagcgcat
ggacaagttc 600atgctgtacc tgctgaccgc cggcaagaag gccctggccg
acggcggcat caccgacgag 660gtgatgaagg agctggacaa gcgcaagtgc
ggcgtgctga tcggcagcgg catgggcggc 720atgaaggtgt tcaacgacgc
catcgaggcc ctgcgcgtga gctacaagaa gatgaacccc 780ttctgcgtgc
ccttcgccac caccaacatg ggcagcgcca tgctggccat ggacctgggc
840tggatgggcc ccaactacag catcagcacc gcctgcgcca ccagcaactt
ctgcatcctg 900aacgccgcca accacatcat ccgcggcgag gccgacatga
tgctgtgcgg cggcagcgac 960gccgtgatca tccccatcgg cctgggcggc
ttcgtggcct gccgcgccct gagccagcgc 1020aacagcgacc ccaccaaggc
cagccgcccc tgggacagca accgcgacgg cttcgtgatg 1080ggcgagggcg
ccggcgtgct gctgctggag gagctggagc acgccaagaa gcgcggcgcc
1140accatctacg ccgagttcct gggcggcagc ttcacctgcg acgcctacca
catgaccgag 1200ccccaccccg agggcgccgg cgtgatcctg tgcatcgaga
aggccctggc ccaggccggc 1260gtgagcaagg aggacgtgaa ctacatcaac
gcccacgcca ccagcaccag cgccggcgac 1320atcaaggagt accaggccct
ggcccgctgc ttcggccaga acagcgagct gcgcgtgaac 1380agcaccaaga
gcatgatcgg ccacctgctg ggcgccgccg gcggcgtgga ggccgtgacc
1440gtggtgcagg ccatccgcac cggctggatt caccccaacc tgaacctgga
ggaccccgac 1500aaggccgtgg acgccaagct gctggtgggc cccaagaagg
agcgcctgaa cgtgaaggtg 1560ggcctgagca acagcttcgg cttcggcggc
cacaacagca gcatcctgtt cgccccctgc 1620aacgtgtga
1629471552DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 47atgggccgcg gtgtctccct tccccggccc
agggtcgcgg tgcgcgccca gtcggcgagt 60caggttttgg agagctgtat tccagtgcct
ggtggccagc tgcatcgacc cctgcgacca 120gtaccgcagc agcgccagcc
tgagcttcct gggcgacaac ggcttcgcca gcctgttcgg 180cagcaagccc
ttcatgagca accgcggcca ccgccgcctg cgccgcgcca gccacagcgg
240cgaggccatg gccgtggccc tgcagcccgc ccaggaggcc ggcaccaaga
agaagcccgt 300gatcaagcag cgccgcgtgg tggtgaccgg catgggcgtg
gtgacccccc tgggccacga 360gcccgacgtg ttctacaaca acctgctgga
cggcgtgagc ggcatcagcg agatcgagac 420cttcgactgc acccagttcc
ccacccgcat cgccggcgag atcaagagct tcagcaccga 480cggctgggtg
gcccccaagc tgagcaagcg catggacaag ttcatgctgt acctgctgac
540cgccggcaag aaggccctgg ccgacggcgg catcaccgac gaggtgatga
aggagctgga 600caagcgcaag tgcggcgtgc tgatcggcag cggcatgggc
ggcatgaagg tgttcaacga 660cgccatcgag gccctgcgcg tgagctacaa
gaagatgaac cccttctgcg tgcccttcgc 720caccaccaac atgggcagcg
ccatgctggc catggacctg ggctggatgg gccccaacta 780cagcatcagc
accgcctgcg ccaccagcaa cttctgcatc ctgaacgccg ccaaccacat
840catccgcggc gaggccgaca tgatgctgtg cggcggcagc gacgccgtga
tcatccccat 900cggcctgggc ggcttcgtgg cctgccgcgc cctgagccag
cgcaacagcg accccaccaa 960ggccagccgc ccctgggaca gcaaccgcga
cggcttcgtg atgggcgagg gcgccggcgt 1020gctgctgctg gaggagctgg
agcacgccaa gaagcgcggc gccaccatct acgccgagtt 1080cctgggcggc
agcttcacct gcgacgccta ccacatgacc gagccccacc ccgagggcgc
1140cggcgtgatc ctgtgcatcg agaaggccct ggcccaggcc ggcgtgagca
aggaggacgt 1200gaactacatc aacgcccacg ccaccagcac cagcgccggc
gacatcaagg agtaccaggc 1260cctggcccgc tgcttcggcc agaacagcga
gctgcgcgtg aacagcacca agagcatgat 1320cggccacctg ctgggcgccg
ccggcggcgt ggaggccgtg accgtggtgc aggccatccg 1380caccggctgg
attcacccca acctgaacct ggaggacccc gacaaggccg tggacgccaa
1440gctgctggtg ggccccaaga aggagcgcct gaacgtgaag gtgggcctga
gcaacagctt 1500cggcttcggc ggccacaaca gcagcatcct gttcgccccc
tgcaacgtgt ga 1552481641DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 48atgcccgcgg
ccagctcgct gctggcgtcc cccctgtgca cctggctgct ggccgcgtgc 60atgagcacct
cgttccaccc ctccgacccc ctgcccccca gcatctcgtc cccccgccgc
120cgcctgagcc gccgccgcat cctgtcgcag tgcgcccccc tgccctccgc
gagctcggcc 180ctgcgcggct ccagcttcca caccctggtg acctcgtatc
tggcgtgctt cgagccctgc 240cacgactatt ataccagcgc ctccctgttc
ggctcgcgcc ccatccgcac cacccgccgc 300caccgccgcc tgaaccgcgc
gagcccctcg cgcgaggcga tggcggtcgc cctgcagccc 360gagcaggagg
tgaccaccaa gaagaagccc tccatcaagc agcgccgcgt cgtggtcacc
420ggcatgggcg tggtcacccc cctgggccac gaccccgacg tgttctataa
caacctgctg 480gacggcacca gcggcatctc ggagatcgag accttcgact
gcgcgcagtt ccccacccgc 540atcgccggcg agatcaagtc cttcagcacc
gacggctggg tcgcgcccaa gctgtcgaag 600cgcatggaca agttcatgct
gtatatgctg accgccggca agaaggcgct gaccgacggc 660ggcatcaccg
aggacgtgat gaaggagctg gacaagcgca agtgcggcgt cctgatcggc
720tccgcgatgg gcggcatgaa ggtgttcaac gacgcgatcg aggccctgcg
catcagctat 780aagaagatga accccttctg cgtgcccttc gcgaccacca
acatgggctc ggccatgctg 840gcgatggacc tgggctggat gggccccaac
tattccatca gcaccgcctg cgcgacctcg 900aacttctgca tcatgaacgc
ggccaaccac atcatccgcg gcgaggcgga cgtcatgctg 960tgcggcggct
ccgacgccgt gatcatcccc atcggcatgg gcggcttcgt cgcgtgccgc
1020gccctgagcc agcgcaactc ggaccccacc aaggcgtccc gcccctggga
cagcaaccgc 1080gacggcttcg tgatgggcga gggcgccggc gtcctgctgc
tggaggagct ggagcacgcg 1140aagaagcgcg gcgccaccat ctatgcggag
ttcctgggcg gctcgttcac ctgcgacgcc 1200tatcacatga ccgagcccca
ccccgacggc gccggcgtga tcctgtgcat cgagaaggcg 1260ctggcccagt
ccggcgtcag ccgcgaggac gtgaactata tcaacgcgca cgccacctcg
1320acccccgcgg gcgacatcaa ggagtatcag gccctgatcc actgcttcgg
ccagaaccgc 1380gagctgaagg tcaactccac caagagcatg atcggccacc
tgctgggcgc ggcgggcggc 1440gtggaggcgg tctcggtggt ccaggccatc
cgcaccggct ggatccaccc caacatcaac 1500ctggagaacc ccgacgaggg
cgtggacacc aagctgctgg tgggccccaa gaaggagcgc 1560ctgaacgtca
aggtgggcct gtccaacagc ttcggcttcg gcggccacaa ctcgtccatc
1620ctgttcgcgc cctatatctg a 1641491251DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
49atggtggccg ccgccgcctc cagcgccttc ttccccgtgc ccgcccccgg cgcctccccc
60aagcccggca agttcggcaa ctggccctcc agcctgagcc cctccttcaa gcccaagtcc
120atccccaacg gcggcttcca ggtgaaggcc aacgacagcg cccaccccaa
ggccaacggc 180tccgccgtga gcctgaagag cggcagcctg aacacccagg
aggacacctc ctccagcccc 240cccccccgca ccttcctgca ccagctgccc
gactggagcc gcctgctgac cgccatcacc 300accgtgttcg tgaagtccaa
gcgccccgac atgcacgacc gcaagtccaa gcgccccgac 360atgctggtgg
acagcttcgg cctggagtcc accgtgcagg acggcctggt gttccgccag
420tccttctcca tccgctccta cgagatcggc accgaccgca ccgccagcat
cgagaccctg 480atgaaccacc tgcaggagac ctccctgaac cactgcaaga
gcaccggcat cctgctggac 540ggcttcggcc gcaccctgga gatgtgcaag
cgcgacctga tctgggtggt gatcaagatg 600cagatcaagg tgaaccgcta
ccccgcctgg ggcgacaccg tggagatcaa cacccgcttc 660agccgcctgg
gcaagatcgg catgggccgc gactggctga tctccgactg caacaccggc
720gagatcctgg tgcgcgccac cagcgcctac gccatgatga accagaagac
ccgccgcctg 780tccaagctgc cctacgaggt gcaccaggag atcgtgcccc
tgttcgtgga cagccccgtg 840atcgaggact ccgacctgaa ggtgcacaag
ttcaaggtga agaccggcga cagcatccag 900aagggcctga cccccggctg
gaacgacctg gacgtgaacc agcacgtgtc caacgtgaag 960tacatcggct
ggatcctgga gagcatgccc accgaggtgc tggagaccca ggagctgtgc
1020tccctggccc tggagtaccg ccgcgagtgc ggccgcgact ccgtgctgga
gagcgtgacc 1080gccatggacc ccagcaaggt gggcgtgcgc tcccagtacc
agcacctgct gcgcctggag 1140gacggcaccg ccatcgtgaa cggcgccacc
gagtggcgcc ccaagaacgc cggcgccaac 1200ggcgccatct ccaccggcaa
gaccagcaac ggcaactccg tgtccatgtg a 125150659DNAPrototheca
moriformis 50gctcttcctc accgcgtgaa ttgctgtccc aaacgtaagc atcatcgtgg
ctcggtcacg 60cgatcctgga tccggggatc ctagaccgct ggtggagagc gctgccgtcg
gattggtggc 120aagtaagatt gcgcaggttg gcgaagggag agaccaaaac
cggaggctgg aagcgggcac 180aacatcgtat tattgcgtat agtagagcag
tggcagtcgc atttcgaggt ccgcaacgga 240tctcgcaagc tcgctacgct
cacagtagga gaaaggggac cactgcccct gccagaatgg 300tcgcgaccct
ctccctcgcc ggccccgcct gcaacacgca gtgcgtatcc ggcaagcggg
360ctgtcgcctt caaccgcccc catgttggcg tccgggctcg atcaggtgcg
ctgagggggg 420tttggtgtgc ccgcgcctct gggcccgtgt
cggccgtgcg gacgtggggc cctgggcagt 480ggatcagcag ggtttgcgtg
caaatgccta taccggcgat tgaatagcga tgaacgggat 540acggttgcgc
tcactccatg cccatgcgac cccgtttctg tccgccagcc gtggtcgccc
600gggctgcgaa gcgggacccc acccagcgca ttgtgatcac cggaatgggc gtgggtacc
65951607DNAPrototheca moriformis 51gagctccacc tgcatccgcc tggcgctcga
ggacgccggc gtctcgcccg acgaggtcaa 60ctacgtcaac gcgcacgcca cctccaccct
ggtgggcgac aaggccgagg tgcgcgcggt 120caagtcggtc tttggcgaca
tgaagggcat caagatgaac gccaccaagt ccatgatcgg 180gcactgcctg
ggcgccgccg gcggcatgga ggccgtcgcc acgctcatgg ccatccgcac
240cggctgggtg caccccacca tcaaccacga caaccccatc gccgaggtcg
acggcctgga 300cgtcgtcgcc aacgccaagg cccagcacaa aatcaacgtc
gccatctcca actccttcgg 360cttcggcggg cacaactccg tcgtcgcctt
tgcgcccttc cgcgagtagg cggagcgagc 420gcgcttggct gaggagggag
gcggggtgcg agccctttgg ctgcgcgcga tactctcccc 480gcacgagcag
actccacgcg cctgaatcta cttgtcaacg agcaaccgtg tgttttgtcc
540gtggccattc ttattatttc tccgactgtg gccgtactct gtttggctgt
gcaagcaccg 600aagagcc 60752636DNAPrototheca moriformis 52gctcttcgcg
caagctcgct acgctcacag taggagatag gggaccactg cccctgccag 60aatggtcgcg
accctgtccc tcgccggccc cgcctgcaac acgcagtgcg tatccagcaa
120gcgggttgtc gccttcaacc gcccccatgt tggcgtccgg gctcgatcag
gtgcgctgag 180gggggtttgg tgggcccgcg cctctgggcc cgtgtcggcc
gtgcggacgt ggggcccggg 240gtagtggatc agcaggggtt gcatgcaaat
gcctataccg gcgattgaat agcgatgaac 300gggatacggt tgcgctcact
ccatgcccat gcgaccccgt ttctgtccgc cagccgtggt 360cgcccgagct
gcgaagcggg accccaccca gcgcattgtg atcaccggaa tgggcgtggc
420ctccgtgttt ggcaacgatg tcgagacctt ttacgacaag cttctggaag
gaacgagcgg 480cgtggacctg atttccaggt gcgtaggtcc ttggatgaat
gcgtctaggt tgcgaggtga 540ctggccagga agcagcaggc ttggggtttg
gtgttctgat ttctggtaat ttgaggtttc 600attataagat tctgtacggt
cttgtttcgg ggtacc 63653607DNAPrototheca moriformis 53gagctccacc
tgcatccgcc tggcgctcga ggacgccggc gtctcgcccg acgaggtcaa 60ctacgtcaac
gcgcacgcca cctccaccct ggtgggcgac aaggccgagg tgcgcgcggt
120caagtcggtc tttggcgaca tgaagggcat caagatgaac gccaccaagt
ccatgatcgg 180gcactgcctg ggcgccgccg gcggcatgga ggccgtcgcc
acgctcatgg ccatccgcac 240cggctgggtg caccccacca tcaaccacga
caaccccatc gccgaggtcg acggcctgga 300cgtcgtcgcc aacgccaagg
cccagcacaa aatcaacgtc gccatctcca actccttcgg 360cttcggcggg
cacaactccg tcgtcgcctt tgcgcccttc cgcgagtagg cggagcgagc
420gcgcttggct gaggagggag gcggggtgcg agccctttgg ctgcgcgcga
tactctcccc 480gcacgagcag actccacgcg cctgaatcta cttgtcaacg
agcaaccgtg tgttttgtcc 540gtggccattc ttattatttc tccgactgtg
gccgtactct gtttggctgt gcaagcaccg 600aagagcc 60754335DNAPrototheca
moriformis 54actagtcatg gtcgcccggg ctgcgaagcg ggaccccacc cagcgcattg
tgatcaccgg 60aatgggcgtg gcctccgtgt ttggcaacga tgtcgagacc ttttacagtg
tgtttgaggg 120ttttggttgc ccgtattgag gtcctggtgg cgcgcatgga
ggagaaggcg cctgtcccgc 180tgaccccccc ggctaccctc ccggcacctt
ccagggcgcg tacgggatcc tgtaaaaggt 240ctcgacatcg ttgccaaaca
cggaggccac gcccattccg gtgatcacaa tgcgctgggt 300ggggtcccgc
ttcgcagccc gggcgaccaa agctt 33555503DNAPrototheca moriformis
55actagtcatt gacatctccg agttcccgac caagtttgcg gcgcagatca ccggcttctc
60cgtggaggac tgcgtggaca agaagaacgc gcggcggtac gacgacgcgc tgtcgtacgc
120gatggtggcc tccaagaagg ccctgcgcca ggcgggactg gagaaggaca
agtgccccga 180gggctacgga ggtgtgtttg agggttttgg ttgcccgtat
tgaggtcctg gtggcgcgca 240tggaggagaa ggcgcctgtc ccgctgaccc
ccccggctac cctcccggca ccttccaggg 300cgcgtacggg atccctccgt
agccctcggg gcacttgtcc ttctccagtc ccgcctggcg 360cagggccttc
ttggaggcca ccatcgcgta cgacagcgcg tcgtcgtacc gccgcgcgtt
420cttcttgtcc acgcagtcct ccacggagaa gccggtgatc tgcgccgcaa
acttggtcgg 480gaactcggag atgtcaaaag ctt 50356457DNAPrototheca
moriformis 56actagtcatg ggcgtgagca cctgcatccg cctggcgctc gaggacgccg
gcgtctcgcc 60cgacgaggtc aactacgtca acgcgcacgc cacctccacc ctggtgggcg
acaaggccga 120ggtgcgcgcg gtcaagtcgg tctttggcga catgaagggc
atcaagatgt gtgtttgagg 180gttttggttg cccgtattga ggtcctggtg
gcgcgcatgg aggagaaggc gcctgtcccg 240ctgacccccc cggctaccct
cccggcacct tccagggcgc gtacgggatc catcttgatg 300cccttcatgt
cgccaaagac cgacttgacc gcgcgcacct cggccttgtc gcccaccagg
360gtggaggtgg cgtgcgcgtt gacgtagttg acctcgtcgg gcgagacgcc
ggcgtcctcg 420agcgccaggc ggatgcaggt gctcacgccc aaagctt
45757345DNAPrototheca moriformis 57actagtcaca accatcaacc acgacaaccc
catcgccgag gtcgacggcc tggacgtcgt 60cgccaacgcc aaggcccagc acaaaatcaa
cgtcgccatc tccaactcct tcggtgtgtt 120tgagggtttt ggttgcccgt
attgaggtcc tggtggcgcg catggaggag aaggcgcctg 180tcccgctgac
ccccccggct accctcccgg caccttccag ggcgcgtacg ggatcccgaa
240ggagttggag atggcgacgt tgattttgtg ctgggccttg gcgttggcga
cgacgtccag 300gccgtcgacc tcggcgatgg ggttgtcgtg gttgatggta agctt
345581590DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 58atggactccc gcgcccagaa ccgcgacggc
ggcgaggacg tgaagcagga gctgctgtcc 60gccggcgacg acggcaaggt gccctgcccc
accgtggcca tcggcatccg ccagcgcctg 120cccgacttcc tgcagtccgt
gaacatgaag tacgtgaagc tgggctacca ctacctgatc 180acccacgcca
tgttcctgct gaccctgccc gccttcttcc tggtggccgc cgagatcggc
240cgcctgggcc acgagcgcat ctaccgcgag ctgtggaccc acctgcacct
gaacctggtg 300tccatcatgg cctgctcctc cgccctggtg gccggcgcca
ccctgtactt catgtcccgc 360ccccgccccg tgtacctggt ggagttcgcc
tgctaccgcc ccgacgagcg cctgaaggtg 420tccaaggact tcttcctgga
catgtcccgc cgcaccggcc tgttctcctc ctcctccatg 480gacttccaga
ccaagatcac ccagcgctcc ggcctgggcg acgagaccta cctgcccccc
540gccatcctgg cctccccccc caacccctgc atgcgcgagg cccgcgagga
ggccgccatg 600gtgatgttcg gcgccctgga cgagctgttc gagcagaccg
gcgtgaagcc caaggagatc 660ggcgtgctgg tggtgaactg ctccctgttc
aaccccaccc cctccatgtc cgccatgatc 720gtgaaccact accacatgcg
cggcaacatc aagtccctga acctgggcgg catgggctgc 780tccgccggcc
tgatctccat cgacctggcc cgcgacctgc tgcaggtgca cggcaacacc
840tacgccgtgg tggtgtccac cgagaacatc accctgaact ggtacttcgg
cgacgaccgc 900tccaagctga tgtccaactg catcttccgc atgggcggcg
ccgccgtgct gctgtccaac 960aagcgccgcg agcgccgccg cgccaagtac
gagctgctgc acaccgtgcg cacccacaag 1020ggcgccgacg acaagtgctt
ccgctgcgtg taccaggagg aggactccac cggctccctg 1080ggcgtgtccc
tgtcccgcga gctgatggcc gtggccggca acgccctgaa ggccaacatc
1140accaccctgg gccccctggt gctgcccctg tccgagcaga tcctgttctt
cgcctccctg 1200gtggcccgca agttcctgaa catgaagatg aagccctaca
tccccgactt caagctggcc 1260ttcgagcact tctgcatcca cgccggcggc
cgcgccgtgc tggacgagct ggagaagaac 1320ctggacctga ccgagtggca
catggagccc tcccgcatga ccctgtaccg cttcggcaac 1380acctcctcct
cctccctgtg gtacgagctg gcctacaccg aggcccaggg ccgcgtgaag
1440cgcggcgacc gcctgtggca gatcgccttc ggctccggct tcaagtgcaa
ctccgccgtg 1500tggcgcgcgc tgcgcaccgt gaagcccccc gtgaacaacg
cctggtccga cgtgatcgac 1560cgcttccccg tgaagctgcc ccagttctga
159059529PRTMetrosideros polymorpha 59Met Asp Ser Arg Ala Gln Asn
Arg Asp Gly Gly Glu Asp Val Lys Gln 1 5 10 15 Glu Leu Leu Ser Ala
Gly Asp Asp Gly Lys Val Pro Cys Pro Thr Val 20 25 30 Ala Ile Gly
Ile Arg Gln Arg Leu Pro Asp Phe Leu Gln Ser Val Asn 35 40 45 Met
Lys Tyr Val Lys Leu Gly Tyr His Tyr Leu Ile Thr His Ala Met 50 55
60 Phe Leu Leu Thr Leu Pro Ala Phe Phe Leu Val Ala Ala Glu Ile Gly
65 70 75 80 Arg Leu Gly His Glu Arg Ile Tyr Arg Glu Leu Trp Thr His
Leu His 85 90 95 Leu Asn Leu Val Ser Ile Met Ala Cys Ser Ser Ala
Leu Val Ala Gly 100 105 110 Ala Thr Leu Tyr Phe Met Ser Arg Pro Arg
Pro Val Tyr Leu Val Glu 115 120 125 Phe Ala Cys Tyr Arg Pro Asp Glu
Arg Leu Lys Val Ser Lys Asp Phe 130 135 140 Phe Leu Asp Met Ser Arg
Arg Thr Gly Leu Phe Ser Ser Ser Ser Met 145 150 155 160 Asp Phe Gln
Thr Lys Ile Thr Gln Arg Ser Gly Leu Gly Asp Glu Thr 165 170 175 Tyr
Leu Pro Pro Ala Ile Leu Ala Ser Pro Pro Asn Pro Cys Met Arg 180 185
190 Glu Ala Arg Glu Glu Ala Ala Met Val Met Phe Gly Ala Leu Asp Glu
195 200 205 Leu Phe Glu Gln Thr Gly Val Lys Pro Lys Glu Ile Gly Val
Leu Val 210 215 220 Val Asn Cys Ser Leu Phe Asn Pro Thr Pro Ser Met
Ser Ala Met Ile 225 230 235 240 Val Asn His Tyr His Met Arg Gly Asn
Ile Lys Ser Leu Asn Leu Gly 245 250 255 Gly Met Gly Cys Ser Ala Gly
Leu Ile Ser Ile Asp Leu Ala Arg Asp 260 265 270 Leu Leu Gln Val His
Gly Asn Thr Tyr Ala Val Val Val Ser Thr Glu 275 280 285 Asn Ile Thr
Leu Asn Trp Tyr Phe Gly Asp Asp Arg Ser Lys Leu Met 290 295 300 Ser
Asn Cys Ile Phe Arg Met Gly Gly Ala Ala Val Leu Leu Ser Asn 305 310
315 320 Lys Arg Arg Glu Arg Arg Arg Ala Lys Tyr Glu Leu Leu His Thr
Val 325 330 335 Arg Thr His Lys Gly Ala Asp Asp Lys Cys Phe Arg Cys
Val Tyr Gln 340 345 350 Glu Glu Asp Ser Thr Gly Ser Leu Gly Val Ser
Leu Ser Arg Glu Leu 355 360 365 Met Ala Val Ala Gly Asn Ala Leu Lys
Ala Asn Ile Thr Thr Leu Gly 370 375 380 Pro Leu Val Leu Pro Leu Ser
Glu Gln Ile Leu Phe Phe Ala Ser Leu 385 390 395 400 Val Ala Arg Lys
Phe Leu Asn Met Lys Met Lys Pro Tyr Ile Pro Asp 405 410 415 Phe Lys
Leu Ala Phe Glu His Phe Cys Ile His Ala Gly Gly Arg Ala 420 425 430
Val Leu Asp Glu Leu Glu Lys Asn Leu Asp Leu Thr Glu Trp His Met 435
440 445 Glu Pro Ser Arg Met Thr Leu Tyr Arg Phe Gly Asn Thr Ser Ser
Ser 450 455 460 Ser Leu Trp Tyr Glu Leu Ala Tyr Thr Glu Ala Gln Gly
Arg Val Lys 465 470 475 480 Arg Gly Asp Arg Leu Trp Gln Ile Ala Phe
Gly Ser Gly Phe Lys Cys 485 490 495 Asn Ser Ala Val Trp Arg Ala Leu
Arg Thr Val Lys Pro Pro Val Asn 500 505 510 Asn Ala Trp Ser Asp Val
Ile Asp Arg Phe Pro Val Lys Leu Pro Gln 515 520 525 Phe
60906DNATrypanosoma brucei 60atgctgatga acttcggcgg ctcctacgac
gcctacatca acaacttcca gggcaccttc 60ctggccgagt ggatgctgga ccacccctcc
gtgccctaca tcgccggcgt gatgtacctg 120atcctggtgc tgtacgtgcc
caagtccatc atggcctccc agccccccct gaacctgcgc 180gccgccaaca
tcgtgtggaa cctgttcctg accctgttct ccatgtgcgg cgcctactac
240accgtgccct acctggtgaa ggccttcatg aaccccgaga tcgtgatggc
cgcctccggc 300atcaagctgg acgccaacac ctcccccatc atcacccact
ccggcttcta caccaccacc 360tgcgccctgg ccgactcctt ctacttcaac
ggcgacgtgg gcttctgggt ggccctgttc 420gccctgtcca agatccccga
gatgatcgac accgccttcc tggtgttcca gaagaagccc 480gtgatcttcc
tgcactggta ccaccacctg accgtgatgc tgttctgctg gttcgcctac
540gtgcagaaga tctcctccgg cctgtggttc gcctccatga actactccgt
gcactccatc 600atgtacctgt actacttcgt gtgcgcctgc ggccaccgcc
gcctggtgcg ccccttcgcc 660cccatcatca ccttcgtgca gatcttccag
atggtggtgg gcaccatcgt ggtgtgctac 720acctacaccg tgaagcacgt
gctgggccgc tcctgcaccg tgaccgactt ctccctgcac 780accggcctgg
tgatgtacgt gtcctacctg ctgctgttct cccagctgtt ctaccgctcc
840tacctgtccc cccgcgacaa ggcctccatc ccccacgtgg ccgccgagat
caagaagaag 900gagtga 90661322PRTTrypanosoma brucei 61Met Tyr Pro
Thr His Arg Asp Leu Ile Leu Asn Asn Tyr Ser Asp Ile 1 5 10 15 Tyr
Arg Ser Pro Thr Cys His Tyr His Thr Trp His Thr Leu Ile His 20 25
30 Thr Pro Ile Asn Glu Leu Leu Phe Pro Asn Leu Pro Arg Glu Cys Asp
35 40 45 Phe Gly Tyr Asp Ile Pro Tyr Phe Arg Gly Gln Ile Asp Val
Phe Asp 50 55 60 Gly Trp Ser Met Ile His Phe Thr Ser Ser Asn Trp
Cys Ile Pro Ile 65 70 75 80 Thr Val Cys Leu Cys Tyr Ile Met Met Ile
Ala Gly Leu Lys Lys Tyr 85 90 95 Met Gly Pro Arg Asp Gly Gly Arg
Ala Pro Ile Gln Ala Lys Asn Tyr 100 105 110 Ile Ile Ala Trp Asn Leu
Phe Leu Ser Phe Phe Ser Phe Ala Gly Val 115 120 125 Tyr Tyr Thr Val
Pro Tyr His Leu Phe Asp Pro Glu Asn Gly Leu Phe 130 135 140 Ala Gln
Gly Phe Tyr Ser Thr Val Cys Asn Asn Gly Ala Tyr Tyr Gly 145 150 155
160 Asn Gly Asn Val Gly Phe Phe Val Trp Leu Phe Ile Tyr Ser Lys Ile
165 170 175 Phe Glu Leu Val Asp Thr Phe Phe Leu Leu Ile Arg Lys Asn
Pro Val 180 185 190 Ile Phe Leu His Trp Tyr His His Leu Thr Val Leu
Leu Tyr Cys Trp 195 200 205 His Ala Tyr Ser Val Arg Ile Gly Thr Gly
Ile Trp Phe Ala Thr Met 210 215 220 Asn Tyr Ser Val His Ser Val Met
Tyr Leu Tyr Phe Ala Met Thr Gln 225 230 235 240 Tyr Gly Pro Ser Thr
Lys Lys Phe Ala Lys Lys Phe Ser Lys Phe Ile 245 250 255 Thr Thr Ile
Gln Ile Leu Gln Met Val Val Gly Ile Ile Val Thr Phe 260 265 270 Ala
Ala Met Leu Tyr Val Thr Phe Asp Val Pro Cys Tyr Thr Ser Leu 275 280
285 Ala Asn Ser Val Leu Gly Leu Met Met Tyr Ala Ser Tyr Phe Val Leu
290 295 300 Phe Val Gln Leu Tyr Val Ser His Tyr Val Ser Pro Lys His
Val Lys 305 310 315 320 Gln Glu 62933DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
62atggtgtccg actggaagaa cttctgcctg gagaaggcct cccgcttccg ccccaccatc
60gaccgcccct tcttcaacat ctacctgtgg gactacttca accgcgccgt gggctgggcc
120accgccggcc gcttccagcc caaggacttc gagttcaccg tgggcaagca
gcccctgtcc 180gagccccgcc ccgtgctgct gttcatcgcc atgtactacg
tggtgatctt cggcggccgc 240tccctggtga agtcctgcaa gcccctgaag
ctgcgcttca tctcccaggt gcacaacctg 300atgctgacct ccgtgtcctt
cctgtggctg atcctgatgg tggagcagat gctgcccatc 360gtgtaccgcc
acggcctgta cttcgccgtg tgcaacgtgg agtcctggac ccagcccatg
420gagaccctgt actacctgaa ctacatgacc aagttcgtgg agttcgccga
caccgtgctg 480atggtgctga agcaccgcaa gctgaccttc ctgcacacct
accaccacgg cgccaccgcc 540ctgctgtgct acaaccagct ggtgggctac
accgccgtga cctgggtgcc cgtgaccctg 600aacctggccg tgcacgtgct
gatgtactgg tactacttcc tgtccgcctc cggcatccgc 660gtgtggtgga
aggcctgggt gacccgcctg cagatcgtgc agttcatgct ggacctgatc
720gtggtgtact acgtgctgta ccagaagatc gtggccgcct acttcaagaa
cgcctgcacc 780ccccagtgcg aggactgcct gggctccatg accgccatcg
ccgccggcgc cgccatcctg 840acctcctacc tgttcctgtt catctccttc
tacatcgagg tgtacaagcg cggctccgcc 900tccggcaaga agaagatcaa
caagaacaac tga 93363310PRTSaccharomyces cerevisiae 63Met Val Ser
Asp Trp Lys Asn Phe Cys Leu Glu Lys Ala Ser Arg Phe 1 5 10 15 Arg
Pro Thr Ile Asp Arg Pro Phe Phe Asn Ile Tyr Leu Trp Asp Tyr 20 25
30 Phe Asn Arg Ala Val Gly Trp Ala Thr Ala Gly Arg Phe Gln Pro Lys
35 40 45 Asp Phe Glu Phe Thr Val Gly Lys Gln Pro Leu Ser Glu Pro
Arg Pro 50 55 60 Val Leu Leu Phe Ile Ala Met Tyr Tyr Val Val Ile
Phe Gly Gly Arg 65 70 75 80 Ser Leu Val Lys Ser Cys Lys Pro Leu Lys
Leu Arg Phe Ile Ser Gln 85 90 95 Val His Asn Leu Met Leu Thr Ser
Val Ser Phe Leu Trp Leu Ile Leu 100 105 110 Met Val Glu Gln Met Leu
Pro Ile Val Tyr Arg His Gly Leu Tyr Phe 115 120 125 Ala Val Cys Asn
Val Glu Ser Trp Thr Gln Pro Met Glu Thr Leu Tyr 130 135 140 Tyr Leu
Asn Tyr Met Thr Lys Phe Val Glu Phe Ala Asp Thr Val Leu 145 150 155
160 Met Val Leu Lys His Arg Lys Leu Thr Phe Leu His Thr Tyr His His
165 170 175 Gly Ala Thr Ala Leu Leu Cys Tyr Asn Gln Leu Val Gly Tyr
Thr Ala 180 185 190 Val Thr Trp Val Pro Val Thr Leu Asn Leu Ala Val
His Val Leu Met 195 200 205 Tyr Trp Tyr Tyr Phe Leu Ser Ala Ser Gly
Ile Arg Val Trp Trp Lys 210 215 220 Ala Trp Val Thr Arg Leu Gln Ile
Val Gln Phe Met Leu Asp Leu Ile 225 230
235 240 Val Val Tyr Tyr Val Leu Tyr Gln Lys Ile Val Ala Ala Tyr Phe
Lys 245 250 255 Asn Ala Cys Thr Pro Gln Cys Glu Asp Cys Leu Gly Ser
Met Thr Ala 260 265 270 Ile Ala Ala Gly Ala Ala Ile Leu Thr Ser Tyr
Leu Phe Leu Phe Ile 275 280 285 Ser Phe Tyr Ile Glu Val Tyr Lys Arg
Gly Ser Ala Ser Gly Lys Lys 290 295 300 Lys Ile Asn Lys Asn Asn 305
310 641179DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 64atgctgaagc tgtcctgcaa cgtgaccaac
aacctgcaca ccttctcctt cttctccgac 60tcctccctgt tcatccccgt gaaccgccgc
accatcgccg tgtcctccgg gcgcgcctcc 120cagctgcgca agcccgccct
ggaccccctg cgcgccgtga tctccgccga ccagggctcc 180atctcccccg
tgaactcctg cacccccgcc gaccgcctgc gcgccggccg cctgatggag
240gacggctact cctacaagga gaagttcatc gtgcgctcct acgaggtggg
catcaacaag 300accgccaccg tggagaccat cgccaacctg ctgcaggagg
tggcctgcaa ccacgtgcag 360aagtgcggct tctccaccga cggcttcgcc
accaccctga ccatgcgcaa gctgcacctg 420atctgggtga ccgcccgcat
gcacatcgag atctacaagt accccgcctg gtccgacgtg 480gtggagatcg
agacctggtg ccagtccgag ggccgcatcg gcacccgccg cgactggatc
540ctgcgcgact ccgccaccaa cgaggtgatc ggccgcgcca cctccaagtg
ggtgatgatg 600aaccaggaca cccgccgcct gcagcgcgtg accgacgagg
tgcgcgacga gtacctggtg 660ttctgccccc gcgagccccg cctggccttc
cccgaggaga acaactcctc cctgaagaag 720atccccaagc tggaggaccc
cgcccagtac tccatgctgg agctgaagcc ccgccgcgcc 780gacctggaca
tgaaccagca cgtgaacaac gtgacctaca tcggctgggt gctggagtcc
840atcccccagg agatcatcga cacccacgag ctgcaggtga tcaccctgga
ctaccgccgc 900gagtgccagc aggacgacat cgtggactcc ctgaccacct
ccgagatccc cgacgacccc 960atctccaagt tcaccggcac caacggctcc
gccatgtcct ccatccaggg ccacaacgag 1020tcccagttcc tgcacatgct
gcgcctgtcc gagaacggcc aggagatcaa ccgcggccgc 1080acccagtggc
gcaagaagtc ctcccgcatg gactacaagg accacgacgg cgactacaag
1140gaccacgaca tcgactacaa ggacgacgac gacaagtga
117965392PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 65Met Leu Lys Leu Ser Cys Asn Val Thr Asn Asn
Leu His Thr Phe Ser 1 5 10 15 Phe Phe Ser Asp Ser Ser Leu Phe Ile
Pro Val Asn Arg Arg Thr Ile 20 25 30 Ala Val Ser Ser Gly Arg Ala
Ser Gln Leu Arg Lys Pro Ala Leu Asp 35 40 45 Pro Leu Arg Ala Val
Ile Ser Ala Asp Gln Gly Ser Ile Ser Pro Val 50 55 60 Asn Ser Cys
Thr Pro Ala Asp Arg Leu Arg Ala Gly Arg Leu Met Glu 65 70 75 80 Asp
Gly Tyr Ser Tyr Lys Glu Lys Phe Ile Val Arg Ser Tyr Glu Val 85 90
95 Gly Ile Asn Lys Thr Ala Thr Val Glu Thr Ile Ala Asn Leu Leu Gln
100 105 110 Glu Val Ala Cys Asn His Val Gln Lys Cys Gly Phe Ser Thr
Asp Gly 115 120 125 Phe Ala Thr Thr Leu Thr Met Arg Lys Leu His Leu
Ile Trp Val Thr 130 135 140 Ala Arg Met His Ile Glu Ile Tyr Lys Tyr
Pro Ala Trp Ser Asp Val 145 150 155 160 Val Glu Ile Glu Thr Trp Cys
Gln Ser Glu Gly Arg Ile Gly Thr Arg 165 170 175 Arg Asp Trp Ile Leu
Arg Asp Ser Ala Thr Asn Glu Val Ile Gly Arg 180 185 190 Ala Thr Ser
Lys Trp Val Met Met Asn Gln Asp Thr Arg Arg Leu Gln 195 200 205 Arg
Val Thr Asp Glu Val Arg Asp Glu Tyr Leu Val Phe Cys Pro Arg 210 215
220 Glu Pro Arg Leu Ala Phe Pro Glu Glu Asn Asn Ser Ser Leu Lys Lys
225 230 235 240 Ile Pro Lys Leu Glu Asp Pro Ala Gln Tyr Ser Met Leu
Glu Leu Lys 245 250 255 Pro Arg Arg Ala Asp Leu Asp Met Asn Gln His
Val Asn Asn Val Thr 260 265 270 Tyr Ile Gly Trp Val Leu Glu Ser Ile
Pro Gln Glu Ile Ile Asp Thr 275 280 285 His Glu Leu Gln Val Ile Thr
Leu Asp Tyr Arg Arg Glu Cys Gln Gln 290 295 300 Asp Asp Ile Val Asp
Ser Leu Thr Thr Ser Glu Ile Pro Asp Asp Pro 305 310 315 320 Ile Ser
Lys Phe Thr Gly Thr Asn Gly Ser Ala Met Ser Ser Ile Gln 325 330 335
Gly His Asn Glu Ser Gln Phe Leu His Met Leu Arg Leu Ser Glu Asn 340
345 350 Gly Gln Glu Ile Asn Arg Gly Arg Thr Gln Trp Arg Lys Lys Ser
Ser 355 360 365 Arg Met Asp Tyr Lys Asp His Asp Gly Asp Tyr Lys Asp
His Asp Ile 370 375 380 Asp Tyr Lys Asp Asp Asp Asp Lys 385 390
661176DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 66atggccaccg catccacttt ctcggcgttc
aatgcccgct gcggcgacct gcgtcgctcg 60gcgggctccg ggccccggcg cccagcgagg
cccctccccg tgcgcgggcg cgcctcccag 120ctgcgcaagc ccgccctgga
ccccctgcgc gccgtgatct ccgccgacca gggctccatc 180tcccccgtga
actcctgcac ccccgccgac cgcctgcgcg ccggccgcct gatggaggac
240ggctactcct acaaggagaa gttcatcgtg cgctcctacg aggtgggcat
caacaagacc 300gccaccgtgg agaccatcgc caacctgctg caggaggtgg
cctgcaacca cgtgcagaag 360tgcggcttct ccaccgacgg cttcgccacc
accctgacca tgcgcaagct gcacctgatc 420tgggtgaccg cccgcatgca
catcgagatc tacaagtacc ccgcctggtc cgacgtggtg 480gagatcgaga
cctggtgcca gtccgagggc cgcatcggca cccgccgcga ctggatcctg
540cgcgactccg ccaccaacga ggtgatcggc cgcgccacct ccaagtgggt
gatgatgaac 600caggacaccc gccgcctgca gcgcgtgacc gacgaggtgc
gcgacgagta cctggtgttc 660tgcccccgcg agccccgcct ggccttcccc
gaggagaaca actcctccct gaagaagatc 720cccaagctgg aggaccccgc
ccagtactcc atgctggagc tgaagccccg ccgcgccgac 780ctggacatga
accagcacgt gaacaacgtg acctacatcg gctgggtgct ggagtccatc
840ccccaggaga tcatcgacac ccacgagctg caggtgatca ccctggacta
ccgccgcgag 900tgccagcagg acgacatcgt ggactccctg accacctccg
agatccccga cgaccccatc 960tccaagttca ccggcaccaa cggctccgcc
atgtcctcca tccagggcca caacgagtcc 1020cagttcctgc acatgctgcg
cctgtccgag aacggccagg agatcaaccg cggccgcacc 1080cagtggcgca
agaagtcctc ccgcatggac tacaaggacc acgacggcga ctacaaggac
1140cacgacatcg actacaagga cgacgacgac aagtga 117667391PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
67Met Ala Thr Ala Ser Thr Phe Ser Ala Phe Asn Ala Arg Cys Gly Asp 1
5 10 15 Leu Arg Arg Ser Ala Gly Ser Gly Pro Arg Arg Pro Ala Arg Pro
Leu 20 25 30 Pro Val Arg Gly Arg Ala Ser Gln Leu Arg Lys Pro Ala
Leu Asp Pro 35 40 45 Leu Arg Ala Val Ile Ser Ala Asp Gln Gly Ser
Ile Ser Pro Val Asn 50 55 60 Ser Cys Thr Pro Ala Asp Arg Leu Arg
Ala Gly Arg Leu Met Glu Asp 65 70 75 80 Gly Tyr Ser Tyr Lys Glu Lys
Phe Ile Val Arg Ser Tyr Glu Val Gly 85 90 95 Ile Asn Lys Thr Ala
Thr Val Glu Thr Ile Ala Asn Leu Leu Gln Glu 100 105 110 Val Ala Cys
Asn His Val Gln Lys Cys Gly Phe Ser Thr Asp Gly Phe 115 120 125 Ala
Thr Thr Leu Thr Met Arg Lys Leu His Leu Ile Trp Val Thr Ala 130 135
140 Arg Met His Ile Glu Ile Tyr Lys Tyr Pro Ala Trp Ser Asp Val Val
145 150 155 160 Glu Ile Glu Thr Trp Cys Gln Ser Glu Gly Arg Ile Gly
Thr Arg Arg 165 170 175 Asp Trp Ile Leu Arg Asp Ser Ala Thr Asn Glu
Val Ile Gly Arg Ala 180 185 190 Thr Ser Lys Trp Val Met Met Asn Gln
Asp Thr Arg Arg Leu Gln Arg 195 200 205 Val Thr Asp Glu Val Arg Asp
Glu Tyr Leu Val Phe Cys Pro Arg Glu 210 215 220 Pro Arg Leu Ala Phe
Pro Glu Glu Asn Asn Ser Ser Leu Lys Lys Ile 225 230 235 240 Pro Lys
Leu Glu Asp Pro Ala Gln Tyr Ser Met Leu Glu Leu Lys Pro 245 250 255
Arg Arg Ala Asp Leu Asp Met Asn Gln His Val Asn Asn Val Thr Tyr 260
265 270 Ile Gly Trp Val Leu Glu Ser Ile Pro Gln Glu Ile Ile Asp Thr
His 275 280 285 Glu Leu Gln Val Ile Thr Leu Asp Tyr Arg Arg Glu Cys
Gln Gln Asp 290 295 300 Asp Ile Val Asp Ser Leu Thr Thr Ser Glu Ile
Pro Asp Asp Pro Ile 305 310 315 320 Ser Lys Phe Thr Gly Thr Asn Gly
Ser Ala Met Ser Ser Ile Gln Gly 325 330 335 His Asn Glu Ser Gln Phe
Leu His Met Leu Arg Leu Ser Glu Asn Gly 340 345 350 Gln Glu Ile Asn
Arg Gly Arg Thr Gln Trp Arg Lys Lys Ser Ser Arg 355 360 365 Met Asp
Tyr Lys Asp His Asp Gly Asp Tyr Lys Asp His Asp Ile Asp 370 375 380
Tyr Lys Asp Asp Asp Asp Lys 385 390 681155DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
68atggccaccg catccacttt ctcggcgttc aatgcccgct gcggcgacct gcgtcgctcg
60gcgggctccg ggccccggcg cccagcgagg cccctccccg tgcgcgggcg cgccgccacc
120ggcgagcagc cctccggcgt ggcctccctg cgcgaggccg acaaggagaa
gtccctgggc 180aaccgcctgc gcctgggctc cctgaccgag gacggcctgt
cctacaagga gaagttcgtg 240atccgctgct acgaggtggg catcaacaag
accgccacca tcgagaccat cgccaacctg 300ctgcaggagg tgggcggcaa
ccacgcccag ggcgtgggct tctccaccga cggcttcgcc 360accaccacca
ccatgcgcaa gctgcacctg atctgggtga ccgcccgcat gcacatcgag
420atctaccgct accccgcctg gtccgacgtg atcgagatcg agacctgggt
gcagggcgag 480ggcaaggtgg gcacccgccg cgactggatc ctgaaggact
acgccaacgg cgaggtgatc 540ggccgcgcca cctccaagtg ggtgatgatg
aacgaggaca cccgccgcct gcagaaggtg 600tccgacgacg tgcgcgagga
gtacctggtg ttctgccccc gcaccctgcg cctggccttc 660cccgaggaga
acaacaactc catgaagaag atccccaagc tggaggaccc cgccgagtac
720tcccgcctgg gcctggtgcc ccgccgctcc gacctggaca tgaacaagca
cgtgaacaac 780gtgacctaca tcggctgggc cctggagtcc atcccccccg
agatcatcga cacccacgag 840ctgcaggcca tcaccctgga ctaccgccgc
gagtgccagc gcgacgacat cgtggactcc 900ctgacctccc gcgagcccct
gggcaacgcc gccggcgtga agttcaagga gatcaacggc 960tccgtgtccc
ccaagaagga cgagcaggac ctgtcccgct tcatgcacct gctgcgctcc
1020gccggctccg gcctggagat caaccgctgc cgcaccgagt ggcgcaagaa
gcccgccaag 1080cgcatggact acaaggacca cgacggcgac tacaaggacc
acgacatcga ctacaaggac 1140gacgacgaca agtga 115569384PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
69Met Ala Thr Ala Ser Thr Phe Ser Ala Phe Asn Ala Arg Cys Gly Asp 1
5 10 15 Leu Arg Arg Ser Ala Gly Ser Gly Pro Arg Arg Pro Ala Arg Pro
Leu 20 25 30 Pro Val Arg Gly Arg Ala Ala Thr Gly Glu Gln Pro Ser
Gly Val Ala 35 40 45 Ser Leu Arg Glu Ala Asp Lys Glu Lys Ser Leu
Gly Asn Arg Leu Arg 50 55 60 Leu Gly Ser Leu Thr Glu Asp Gly Leu
Ser Tyr Lys Glu Lys Phe Val 65 70 75 80 Ile Arg Cys Tyr Glu Val Gly
Ile Asn Lys Thr Ala Thr Ile Glu Thr 85 90 95 Ile Ala Asn Leu Leu
Gln Glu Val Gly Gly Asn His Ala Gln Gly Val 100 105 110 Gly Phe Ser
Thr Asp Gly Phe Ala Thr Thr Thr Thr Met Arg Lys Leu 115 120 125 His
Leu Ile Trp Val Thr Ala Arg Met His Ile Glu Ile Tyr Arg Tyr 130 135
140 Pro Ala Trp Ser Asp Val Ile Glu Ile Glu Thr Trp Val Gln Gly Glu
145 150 155 160 Gly Lys Val Gly Thr Arg Arg Asp Trp Ile Leu Lys Asp
Tyr Ala Asn 165 170 175 Gly Glu Val Ile Gly Arg Ala Thr Ser Lys Trp
Val Met Met Asn Glu 180 185 190 Asp Thr Arg Arg Leu Gln Lys Val Ser
Asp Asp Val Arg Glu Glu Tyr 195 200 205 Leu Val Phe Cys Pro Arg Thr
Leu Arg Leu Ala Phe Pro Glu Glu Asn 210 215 220 Asn Asn Ser Met Lys
Lys Ile Pro Lys Leu Glu Asp Pro Ala Glu Tyr 225 230 235 240 Ser Arg
Leu Gly Leu Val Pro Arg Arg Ser Asp Leu Asp Met Asn Lys 245 250 255
His Val Asn Asn Val Thr Tyr Ile Gly Trp Ala Leu Glu Ser Ile Pro 260
265 270 Pro Glu Ile Ile Asp Thr His Glu Leu Gln Ala Ile Thr Leu Asp
Tyr 275 280 285 Arg Arg Glu Cys Gln Arg Asp Asp Ile Val Asp Ser Leu
Thr Ser Arg 290 295 300 Glu Pro Leu Gly Asn Ala Ala Gly Val Lys Phe
Lys Glu Ile Asn Gly 305 310 315 320 Ser Val Ser Pro Lys Lys Asp Glu
Gln Asp Leu Ser Arg Phe Met His 325 330 335 Leu Leu Arg Ser Ala Gly
Ser Gly Leu Glu Ile Asn Arg Cys Arg Thr 340 345 350 Glu Trp Arg Lys
Lys Pro Ala Lys Arg Met Asp Tyr Lys Asp His Asp 355 360 365 Gly Asp
Tyr Lys Asp His Asp Ile Asp Tyr Lys Asp Asp Asp Asp Lys 370 375 380
701194DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 70atgctgaagg tgccctgctg caacgccacc
gaccccatcc agtccctgtc ctcccagtgc 60cgcttcctga cccacttcaa caaccgcccc
tacttcaccc gccgcccctc catccccacc 120ttcttctcct ccaagaactc
ctccgcctcc ctgcaggccg tggtgtccga catctcctcc 180gtggagtccg
ccgcctgcga ctccctggcc aaccgcctgc gcctgggcaa gctgaccgag
240gacggcttct cctacaagga gaagttcatc gtggggcgcg cccgctccta
cgaggtgggc 300atcaacaaga ccgccaccgt ggagaccatc gccaacctgc
tgcaggaggt gggctgcaac 360cacgcccagt ccgtgggctt ctccaccgac
ggcttcgcca ccaccacctc catgcgcaag 420atgcacctga tctgggtgac
cgcccgcatg cacatcgaga tctacaagta ccccgcctgg 480tccgacgtgg
tggaggtgga gacctggtgc cagtccgagg gccgcatcgg cacccgccgc
540gactggatcc tgaccgacta cgccaccggc cagatcatcg gccgcgccac
ctccaagtgg 600gtgatgatga accaggacac ccgccgcctg cagaaggtga
ccgacgacgt gcgcgaggag 660tacctggtgt tctgcccccg cgagctgcgc
ctggccttcc ccgaggagaa caaccgctcc 720tccaagaaga tctccaagct
ggaggacccc gcccagtact ccaagctggg cctggtgccc 780cgccgcgccg
acctggacat gaaccagcac gtgaacaacg tgacctacat cggctgggtg
840ctggagtcca tcccccagga gatcatcgac acccacgagc tgcagaccat
caccctggac 900taccgccgcg agtgccagca cgacgacatc gtggactccc
tgacctccgt ggagccctcc 960gagaacctgg aggccgtgtc cgagctgcgc
ggcaccaacg gctccgccac caccaccgcc 1020ggcgacgagg actgccgcaa
cttcctgcac ctgctgcgcc tgtccggcga cggcctggag 1080atcaaccgcg
gccgcaccga gtggcgcaag aagtccgccc gcatggacta caaggaccac
1140gacggcgact acaaggacca cgacatcgac tacaaggacg acgacgacaa gtga
119471397PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 71Met Leu Lys Val Pro Cys Cys Asn Ala Thr Asp
Pro Ile Gln Ser Leu 1 5 10 15 Ser Ser Gln Cys Arg Phe Leu Thr His
Phe Asn Asn Arg Pro Tyr Phe 20 25 30 Thr Arg Arg Pro Ser Ile Pro
Thr Phe Phe Ser Ser Lys Asn Ser Ser 35 40 45 Ala Ser Leu Gln Ala
Val Val Ser Asp Ile Ser Ser Val Glu Ser Ala 50 55 60 Ala Cys Asp
Ser Leu Ala Asn Arg Leu Arg Leu Gly Lys Leu Thr Glu 65 70 75 80 Asp
Gly Phe Ser Tyr Lys Glu Lys Phe Ile Val Gly Arg Ala Arg Ser 85 90
95 Tyr Glu Val Gly Ile Asn Lys Thr Ala Thr Val Glu Thr Ile Ala Asn
100 105 110 Leu Leu Gln Glu Val Gly Cys Asn His Ala Gln Ser Val Gly
Phe Ser 115 120 125 Thr Asp Gly Phe Ala Thr Thr Thr Ser Met Arg Lys
Met His Leu Ile 130 135 140 Trp Val Thr Ala Arg Met His Ile Glu Ile
Tyr Lys Tyr Pro Ala Trp 145 150 155 160 Ser Asp Val Val Glu Val Glu
Thr Trp Cys Gln Ser Glu Gly Arg Ile 165 170 175 Gly Thr Arg Arg Asp
Trp Ile Leu Thr Asp Tyr Ala Thr Gly Gln Ile 180 185 190 Ile Gly Arg
Ala Thr Ser Lys Trp Val Met Met Asn Gln Asp Thr Arg 195 200 205 Arg
Leu Gln Lys Val Thr Asp Asp Val Arg Glu Glu Tyr Leu Val Phe 210 215
220 Cys Pro Arg Glu Leu Arg Leu Ala Phe Pro Glu Glu
Asn Asn Arg Ser 225 230 235 240 Ser Lys Lys Ile Ser Lys Leu Glu Asp
Pro Ala Gln Tyr Ser Lys Leu 245 250 255 Gly Leu Val Pro Arg Arg Ala
Asp Leu Asp Met Asn Gln His Val Asn 260 265 270 Asn Val Thr Tyr Ile
Gly Trp Val Leu Glu Ser Ile Pro Gln Glu Ile 275 280 285 Ile Asp Thr
His Glu Leu Gln Thr Ile Thr Leu Asp Tyr Arg Arg Glu 290 295 300 Cys
Gln His Asp Asp Ile Val Asp Ser Leu Thr Ser Val Glu Pro Ser 305 310
315 320 Glu Asn Leu Glu Ala Val Ser Glu Leu Arg Gly Thr Asn Gly Ser
Ala 325 330 335 Thr Thr Thr Ala Gly Asp Glu Asp Cys Arg Asn Phe Leu
His Leu Leu 340 345 350 Arg Leu Ser Gly Asp Gly Leu Glu Ile Asn Arg
Gly Arg Thr Glu Trp 355 360 365 Arg Lys Lys Ser Ala Arg Met Asp Tyr
Lys Asp His Asp Gly Asp Tyr 370 375 380 Lys Asp His Asp Ile Asp Tyr
Lys Asp Asp Asp Asp Lys 385 390 395 721128DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
72atgctgaagc tgtcctcctc ccgctccccc ctggcccgca tccccacccg cccccgcccc
60aactccatcc ccccccgcat catcgtggtg tcctcctcct cctccaaggt gaaccccctg
120aagaccgagg ccgtggtgtc ctccggcctg gccgaccgcc tgcgcctggg
ctccctgacc 180gaggacggcc tgtcctacaa ggagaagttc atcgtgcgct
gctacgaggt gggcatcaac 240aagaccgcca ccgtggagac catcgccaac
ctgctgcagg aggtgggctg caaccacgcc 300cagtccgtgg gctactccac
cggcggcttc tccaccaccc ccaccatgcg caagctgcgc 360ctgatctggg
tgaccgcccg catgcacatc gagatctaca agtaccccgc ctggtccgac
420gtggtggaga tcgagtcctg gggccagggc gagggcaaga tcggcacccg
ccgcgactgg 480atcctgcgcg actacgccac cggccaggtg atcggccgcg
ccacctccaa gtgggtgatg 540atgaaccagg acacccgccg cctgcagaag
gtggacgtgg acgtgcgcga cgagtacctg 600gtgcactgcc cccgcgagct
gcgcctggcc ttccccgagg agaacaactc ctccctgaag 660aagatctcca
agctggagga cccctcccag tactccaagc tgggcctggt gccccgccgc
720gccgacctgg acatgaacca gcacgtgaac aacgtgacct acatcggctg
ggtgctggag 780tccatgcccc aggagatcat cgacacccac gagctgcaga
ccatcaccct ggactaccgc 840cgcgagtgcc agcacgacga cgtggtggac
tccctgacct cccccgagcc ctccgaggac 900gccgaggccg tgttcaacca
caacggcacc aacggctccg ccaacgtgtc cgccaacgac 960cacggctgcc
gcaacttcct gcacctgctg cgcctgtccg gcaacggcct ggagatcaac
1020cgcggccgca ccgagtggcg caagaagccc acccgcatgg actacaagga
ccacgacggc 1080gactacaagg accacgacat cgactacaag gacgacgacg acaagtga
112873375PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 73Met Leu Lys Leu Ser Ser Ser Arg Ser Pro Leu
Ala Arg Ile Pro Thr 1 5 10 15 Arg Pro Arg Pro Asn Ser Ile Pro Pro
Arg Ile Ile Val Val Ser Ser 20 25 30 Ser Ser Ser Lys Val Asn Pro
Leu Lys Thr Glu Ala Val Val Ser Ser 35 40 45 Gly Leu Ala Asp Arg
Leu Arg Leu Gly Ser Leu Thr Glu Asp Gly Leu 50 55 60 Ser Tyr Lys
Glu Lys Phe Ile Val Arg Cys Tyr Glu Val Gly Ile Asn 65 70 75 80 Lys
Thr Ala Thr Val Glu Thr Ile Ala Asn Leu Leu Gln Glu Val Gly 85 90
95 Cys Asn His Ala Gln Ser Val Gly Tyr Ser Thr Gly Gly Phe Ser Thr
100 105 110 Thr Pro Thr Met Arg Lys Leu Arg Leu Ile Trp Val Thr Ala
Arg Met 115 120 125 His Ile Glu Ile Tyr Lys Tyr Pro Ala Trp Ser Asp
Val Val Glu Ile 130 135 140 Glu Ser Trp Gly Gln Gly Glu Gly Lys Ile
Gly Thr Arg Arg Asp Trp 145 150 155 160 Ile Leu Arg Asp Tyr Ala Thr
Gly Gln Val Ile Gly Arg Ala Thr Ser 165 170 175 Lys Trp Val Met Met
Asn Gln Asp Thr Arg Arg Leu Gln Lys Val Asp 180 185 190 Val Asp Val
Arg Asp Glu Tyr Leu Val His Cys Pro Arg Glu Leu Arg 195 200 205 Leu
Ala Phe Pro Glu Glu Asn Asn Ser Ser Leu Lys Lys Ile Ser Lys 210 215
220 Leu Glu Asp Pro Ser Gln Tyr Ser Lys Leu Gly Leu Val Pro Arg Arg
225 230 235 240 Ala Asp Leu Asp Met Asn Gln His Val Asn Asn Val Thr
Tyr Ile Gly 245 250 255 Trp Val Leu Glu Ser Met Pro Gln Glu Ile Ile
Asp Thr His Glu Leu 260 265 270 Gln Thr Ile Thr Leu Asp Tyr Arg Arg
Glu Cys Gln His Asp Asp Val 275 280 285 Val Asp Ser Leu Thr Ser Pro
Glu Pro Ser Glu Asp Ala Glu Ala Val 290 295 300 Phe Asn His Asn Gly
Thr Asn Gly Ser Ala Asn Val Ser Ala Asn Asp 305 310 315 320 His Gly
Cys Arg Asn Phe Leu His Leu Leu Arg Leu Ser Gly Asn Gly 325 330 335
Leu Glu Ile Asn Arg Gly Arg Thr Glu Trp Arg Lys Lys Pro Thr Arg 340
345 350 Met Asp Tyr Lys Asp His Asp Gly Asp Tyr Lys Asp His Asp Ile
Asp 355 360 365 Tyr Lys Asp Asp Asp Asp Lys 370 375
741197DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 74atgctgaagc tgtcctcctg caacgtgacc
gaccagcgcc aggccctggc ccagtgccgc 60ttcctggccc cccccgcccc cttctccttc
cgctggcgca cccccgtggt ggtgtcctgc 120tccccctcct cccgccccaa
cctgtccccc ctgcaggtgg tgctgtccgg ccagcagcag 180gccggcatgg
agctggtgga gtccggctcc ggctccctgg ccgaccgcct gcgcctgggc
240tccctgaccg aggacggcct gtcctacaag gagaagttca tcgtgcgctg
ctacgaggtg 300ggcatcaaca agaccgccac cgtggagacc atcgccaacc
tgctgcagga ggtgggctgc 360aaccacgccc agtccgtggg ctactccacc
gacggcttcg ccaccacccg caccatgcgc 420aagctgcacc tgatctgggt
gaccgcccgc atgcacatcg agatctacaa gtaccccgcc 480tggtccgacg
tgatcgagat cgagacctgg tgccagtccg agggccgcat cggcacccgc
540cgcgactgga tcctgaagga cttcggcacc ggcgaggtga tcggccgcgc
cacctccaag 600tgggtgatga tgaaccagga cacccgccgc ctgcagaagg
tgtccgacga cgtgcgcgag 660gagtacctgg tgttctgccc ccgcgagctg
cgcctggcct tccccgagga gaacaacaac 720tccctgaaga agatcgccaa
gctggacgac tccttccagt actcccgcct gggcctgatg 780ccccgccgcg
ccgacctgga catgaaccag cacgtgaaca acgtgaccta catcggctgg
840gtgctggagt ccatgcccca ggagatcatc gacacccacg agctgcagac
catcaccctg 900gactaccgcc gcgagtgcca gcaggacgac gtggtggact
ccctgacctc ccccgagcag 960gtggagggca ccgagaaggt gtccgccatc
cacggcacca acggctccgc cgccgcccgc 1020gaggacaagc aggactgccg
ccagttcctg cacctgctgc gcctgtcctc cgacggccag 1080gagatcaacc
gcggccgcac cgagtggcgc aagaagcccg cccgcatgga ctacaaggac
1140cacgacggcg actacaagga ccacgacatc gactacaagg acgacgacga caagtga
119775398PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 75Met Leu Lys Leu Ser Ser Cys Asn Val Thr Asp
Gln Arg Gln Ala Leu 1 5 10 15 Ala Gln Cys Arg Phe Leu Ala Pro Pro
Ala Pro Phe Ser Phe Arg Trp 20 25 30 Arg Thr Pro Val Val Val Ser
Cys Ser Pro Ser Ser Arg Pro Asn Leu 35 40 45 Ser Pro Leu Gln Val
Val Leu Ser Gly Gln Gln Gln Ala Gly Met Glu 50 55 60 Leu Val Glu
Ser Gly Ser Gly Ser Leu Ala Asp Arg Leu Arg Leu Gly 65 70 75 80 Ser
Leu Thr Glu Asp Gly Leu Ser Tyr Lys Glu Lys Phe Ile Val Arg 85 90
95 Cys Tyr Glu Val Gly Ile Asn Lys Thr Ala Thr Val Glu Thr Ile Ala
100 105 110 Asn Leu Leu Gln Glu Val Gly Cys Asn His Ala Gln Ser Val
Gly Tyr 115 120 125 Ser Thr Asp Gly Phe Ala Thr Thr Arg Thr Met Arg
Lys Leu His Leu 130 135 140 Ile Trp Val Thr Ala Arg Met His Ile Glu
Ile Tyr Lys Tyr Pro Ala 145 150 155 160 Trp Ser Asp Val Ile Glu Ile
Glu Thr Trp Cys Gln Ser Glu Gly Arg 165 170 175 Ile Gly Thr Arg Arg
Asp Trp Ile Leu Lys Asp Phe Gly Thr Gly Glu 180 185 190 Val Ile Gly
Arg Ala Thr Ser Lys Trp Val Met Met Asn Gln Asp Thr 195 200 205 Arg
Arg Leu Gln Lys Val Ser Asp Asp Val Arg Glu Glu Tyr Leu Val 210 215
220 Phe Cys Pro Arg Glu Leu Arg Leu Ala Phe Pro Glu Glu Asn Asn Asn
225 230 235 240 Ser Leu Lys Lys Ile Ala Lys Leu Asp Asp Ser Phe Gln
Tyr Ser Arg 245 250 255 Leu Gly Leu Met Pro Arg Arg Ala Asp Leu Asp
Met Asn Gln His Val 260 265 270 Asn Asn Val Thr Tyr Ile Gly Trp Val
Leu Glu Ser Met Pro Gln Glu 275 280 285 Ile Ile Asp Thr His Glu Leu
Gln Thr Ile Thr Leu Asp Tyr Arg Arg 290 295 300 Glu Cys Gln Gln Asp
Asp Val Val Asp Ser Leu Thr Ser Pro Glu Gln 305 310 315 320 Val Glu
Gly Thr Glu Lys Val Ser Ala Ile His Gly Thr Asn Gly Ser 325 330 335
Ala Ala Ala Arg Glu Asp Lys Gln Asp Cys Arg Gln Phe Leu His Leu 340
345 350 Leu Arg Leu Ser Ser Asp Gly Gln Glu Ile Asn Arg Gly Arg Thr
Glu 355 360 365 Trp Arg Lys Lys Pro Ala Arg Met Asp Tyr Lys Asp His
Asp Gly Asp 370 375 380 Tyr Lys Asp His Asp Ile Asp Tyr Lys Asp Asp
Asp Asp Lys 385 390 395 76573DNAPrototheca moriformis 76tgttgaagaa
tgagccggcg acttaaaata aatggcaggc taagagaatt aataactcga 60aacctaagcg
aaagcaagtc ttaatagggc gctaatttaa caaaacatta aataaaatct
120aaagtcattt attttagacc cgaacctgag tgatctaacc atggtcagga
tgaaacttgg 180gtgacaccaa gtggaagtcc gaaccgaccg atgttgaaaa
atcggcggat gaactgtggt 240tagtggtgaa ataccagtcg aactcagagc
tagctggttc tccccgaaat gcgttgaggc 300gcagcaatat atctcgtcta
tctaggggta aagcactgtt tcggtgcggg ctatgaaaat 360ggtaccaaat
cgtggcaaac tctgaatact agaaatgacg atatattagt gagactatgg
420gggataagct ccatagtcga gagggaaaca gcccagacca ccagttaagg
ccccaaaatg 480ataatgaagt ggtaaaggag gtgaaaatgc aaatacaacc
aggaggttgg cttagaagca 540gccatccttt aaagagtgcg taatagctca ctg
57377384PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 77Met Ala Ile Ala Ala Ala Ala Val Ile Phe Leu
Phe Gly Leu Ile Phe 1 5 10 15 Phe Ala Ser Gly Leu Ile Ile Asn Leu
Phe Gln Ala Leu Cys Phe Val 20 25 30 Leu Ile Arg Pro Leu Ser Lys
Asn Ala Tyr Arg Arg Ile Asn Arg Val 35 40 45 Phe Ala Glu Leu Leu
Leu Ser Glu Leu Leu Cys Leu Phe Asp Trp Trp 50 55 60 Ala Gly Ala
Lys Leu Lys Leu Phe Thr Asp Pro Glu Thr Phe Arg Leu 65 70 75 80 Met
Gly Lys Glu His Ala Leu Val Ile Ile Asn His Met Thr Glu Leu 85 90
95 Asp Trp Met Val Gly Trp Val Met Gly Gln His Phe Gly Cys Leu Gly
100 105 110 Ser Ile Ile Ser Val Ala Lys Lys Ser Thr Lys Phe Leu Pro
Val Leu 115 120 125 Gly Trp Ser Met Trp Phe Ser Glu Tyr Leu Tyr Leu
Glu Arg Ser Trp 130 135 140 Ala Lys Asp Lys Ser Thr Leu Lys Ser His
Ile Glu Arg Leu Ile Asp 145 150 155 160 Tyr Pro Leu Pro Phe Trp Leu
Val Ile Phe Val Glu Gly Thr Arg Phe 165 170 175 Thr Arg Thr Lys Leu
Leu Ala Ala Gln Gln Tyr Ala Val Ser Ser Gly 180 185 190 Leu Pro Val
Pro Arg Asn Val Leu Ile Pro Arg Thr Lys Gly Phe Val 195 200 205 Ser
Cys Val Ser His Met Arg Ser Phe Val Pro Ala Val Tyr Asp Val 210 215
220 Thr Val Ala Phe Pro Lys Thr Ser Pro Pro Pro Thr Leu Leu Asn Leu
225 230 235 240 Phe Glu Gly Gln Ser Ile Met Leu His Val His Ile Lys
Arg His Ala 245 250 255 Met Lys Asp Leu Pro Glu Ser Asp Asp Ala Val
Ala Glu Trp Cys Arg 260 265 270 Asp Lys Phe Val Glu Lys Asp Ala Leu
Leu Asp Lys His Asn Ala Glu 275 280 285 Asp Thr Phe Ser Gly Gln Glu
Val Cys His Ser Gly Ser Arg Gln Leu 290 295 300 Lys Ser Leu Leu Val
Val Ile Ser Trp Val Val Val Thr Thr Phe Gly 305 310 315 320 Ala Leu
Lys Phe Leu Gln Trp Ser Ser Trp Lys Gly Lys Ala Phe Ser 325 330 335
Ala Ile Gly Leu Gly Ile Val Thr Leu Leu Met His Val Leu Ile Leu 340
345 350 Ser Ser Gln Ala Glu Arg Ser Asn Pro Ala Glu Val Ala Gln Ala
Lys 355 360 365 Leu Lys Thr Gly Leu Ser Ile Ser Lys Lys Val Thr Asp
Lys Glu Asn 370 375 380 78387PRTArtificial SequenceDescription of
Artificial Sequence Synthetic polypeptide 78Met Ala Ile Ala Ala Ala
Ala Val Ile Val Pro Leu Ser Leu Leu Phe 1 5 10 15 Phe Val Ser Gly
Leu Ile Val Asn Leu Val Gln Ala Val Cys Phe Val 20 25 30 Leu Ile
Arg Pro Leu Ser Lys Asn Thr Tyr Arg Arg Ile Asn Arg Val 35 40 45
Val Ala Glu Leu Leu Trp Leu Glu Leu Val Trp Leu Ile Asp Trp Trp 50
55 60 Ala Gly Val Lys Ile Lys Val Phe Thr Asp His Glu Thr Phe His
Leu 65 70 75 80 Met Gly Lys Glu His Ala Leu Val Ile Cys Asn His Lys
Ser Asp Ile 85 90 95 Asp Trp Leu Val Gly Trp Val Leu Gly Gln Arg
Ser Gly Cys Leu Gly 100 105 110 Ser Thr Leu Ala Val Met Lys Lys Ser
Ser Lys Phe Leu Pro Val Leu 115 120 125 Gly Trp Ser Met Trp Phe Ser
Glu Tyr Leu Phe Leu Glu Arg Ser Trp 130 135 140 Ala Lys Asp Glu Ile
Thr Leu Lys Ser Gly Leu Asn Arg Leu Lys Asp 145 150 155 160 Tyr Pro
Leu Pro Phe Trp Leu Ala Leu Phe Val Glu Gly Thr Arg Phe 165 170 175
Thr Arg Ala Lys Leu Leu Ala Ala Gln Gln Tyr Ala Ala Ser Ser Gly 180
185 190 Leu Pro Val Pro Arg Asn Val Leu Ile Pro Arg Thr Lys Gly Phe
Val 195 200 205 Ser Ser Val Ser His Met Arg Ser Phe Val Pro Ala Ile
Tyr Asp Val 210 215 220 Thr Val Ala Ile Pro Lys Thr Ser Pro Pro Pro
Thr Leu Ile Arg Met 225 230 235 240 Phe Lys Gly Gln Ser Ser Val Leu
His Val His Leu Lys Arg His Leu 245 250 255 Met Lys Asp Leu Pro Glu
Ser Asp Asp Ala Val Ala Gln Trp Cys Arg 260 265 270 Asp Ile Phe Val
Glu Lys Asp Ala Leu Leu Asp Lys His Asn Ala Glu 275 280 285 Asp Thr
Phe Ser Gly Gln Glu Leu Gln Glu Thr Gly Arg Pro Ile Lys 290 295 300
Ser Leu Leu Val Val Ile Ser Trp Ala Val Leu Glu Val Phe Gly Ala 305
310 315 320 Val Lys Phe Leu Gln Trp Ser Ser Leu Leu Ser Ser Trp Lys
Gly Leu 325 330 335 Ala Phe Ser Gly Ile Gly Leu Gly Val Ile Thr Leu
Leu Met His Ile 340 345 350 Leu Ile Leu Phe Ser Gln Ser Glu Arg Ser
Thr Pro Ala Lys Val Ala 355 360 365 Pro Ala Lys Pro Lys Asn Glu Gly
Glu Ser Ser Lys Thr Glu Met Glu 370 375 380 Lys Glu Lys 385
79327PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 79Met Glu Ile Pro Pro His Cys Leu Cys Ser Pro
Ser Pro Ala Pro Ser 1 5 10 15 Gln Leu Tyr Tyr Lys Lys Lys Lys His
Ala Ile Leu Gln Thr Gln Thr 20 25 30 Pro Tyr Arg Tyr Arg Val Ser
Pro Thr Cys Phe Ala Pro Pro Arg Leu 35 40 45 Arg Lys Gln His Pro
Tyr Pro
Leu Pro Val Leu Cys Tyr Pro Lys Leu 50 55 60 Leu His Phe Ser Gln
Pro Arg Tyr Pro Leu Val Arg Ser His Leu Ala 65 70 75 80 Glu Ala Gly
Val Ala Tyr Arg Pro Gly Tyr Glu Leu Leu Gly Lys Ile 85 90 95 Arg
Gly Val Cys Phe Tyr Ala Val Thr Ala Ala Val Ala Leu Leu Leu 100 105
110 Phe Gln Cys Met Leu Leu Leu His Pro Phe Val Leu Leu Phe Asp Pro
115 120 125 Phe Pro Arg Lys Ala His His Thr Ile Ala Lys Leu Trp Ser
Ile Cys 130 135 140 Ser Val Ser Leu Phe Tyr Lys Ile His Ile Lys Gly
Leu Glu Asn Leu 145 150 155 160 Pro Pro Pro His Ser Pro Ala Val Tyr
Val Ser Asn His Gln Ser Phe 165 170 175 Leu Asp Ile Tyr Thr Leu Leu
Thr Leu Gly Arg Thr Phe Lys Phe Ile 180 185 190 Ser Lys Thr Glu Ile
Phe Leu Tyr Pro Ile Ile Gly Trp Ala Met Tyr 195 200 205 Met Leu Gly
Thr Ile Pro Leu Lys Arg Leu Asp Ser Arg Ser Gln Leu 210 215 220 Asp
Thr Leu Lys Arg Cys Met Asp Leu Ile Lys Lys Gly Ala Ser Val 225 230
235 240 Phe Phe Phe Pro Glu Gly Thr Arg Ser Lys Asp Gly Lys Leu Gly
Ala 245 250 255 Phe Lys Lys Gly Ala Phe Ser Ile Ala Ala Lys Ser Lys
Val Pro Val 260 265 270 Val Pro Ile Thr Leu Ile Gly Thr Gly Lys Ile
Met Pro Pro Gly Ser 275 280 285 Glu Leu Thr Val Asn Pro Gly Thr Val
Gln Val Ile Ile His Lys Pro 290 295 300 Ile Glu Gly Ser Asp Ala Glu
Ala Met Cys Asn Glu Ala Arg Ala Thr 305 310 315 320 Ile Ser His Ser
Leu Asp Asp 325 80984DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 80atggagatcc
cgcctcactg tctctgttcg ccttcgcctg cgccttcgca attgtattac 60aagaagaaga
agcatgccat tctccaaact caaactccct atagatatag agtttccccg
120acatgctttg cccccccccg attgaggaag cagcatcctt accctctccc
tgtcctctgc 180tatccaaaac tcctccactt cagccagcct aggtaccctc
tggttagatc tcatttggct 240gaagctggtg ttgcttatcg tccaggatac
gaattattag gaaaaataag gggagtgtgt 300ttctatgctg tcactgctgc
cgttgccttg cttctatttc agtgcatgct cctcctccat 360ccctttgtgc
tcctcttcga tccatttcca agaaaggctc accataccat cgccaaactc
420tggtctatct gctctgtttc tcttttttac aagattcaca tcaagggttt
ggaaaatctt 480cccccacccc actctcctgc cgtctatgtc tctaatcatc
agagttttct cgacatctat 540actctcctca ctctcggtag aaccttcaag
ttcatcagca agactgagat ctttctctat 600ccaattatcg gttgggccat
gtatatgttg ggtaccattc ctctcaagcg gttggacagc 660agaagccaat
tggacactct taagcgatgt atggatctca tcaagaaggg agcatccgtc
720tttttcttcc cagagggaac acgaagtaaa gatgggaaac tgggtgcttt
caagaaaggt 780gcattcagca tcgcagcaaa aagcaaggtt cctgttgtgc
cgatcaccct tattggaact 840ggcaagatta tgccacctgg gagcgaactt
actgtcaatc caggaactgt gcaagtaatc 900atacataaac ctatcgaagg
aagtgatgca gaagcaatgt gcaatgaagc tagagccacg 960atttctcact
cacttgatga ttaa 984811155DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 81atggcgattg
cagcggcagc tgtcatcttc ctcttcggcc ttatcttctt cgcctccggc 60ctcataatca
atctcttcca ggcgctttgc tttgtcctta ttcggcctct ttcgaaaaac
120gcctacmgga gaataaacag agtttttgca gaattgttgt tgtcggagct
tttatgccta 180ttcgattggt gggctggtgc taagctcaaa ttatttaccg
accctgaaac ctttcgcctt 240atgggcaagg aacatgctct tgtcataatt
aatcacatga ctgaacttga ctggatggtt 300ggatgggtta tgggtcagca
ttttggttgc cttgggagca taatatctgt tgcgaagaaa 360tcaacaaaat
ttcttccggt attggggtgg tcaatgtggt tttcagagta cctatatctt
420gagagaagct gggccaagga taaaagtaca ttaaagtcac atatcgagag
gctgatagac 480taccccctgc ccttctggtt ggtaattttt gtggaaggaa
ctcggtttac tcggacaaaa 540ctcttggcag cccagcagta tgctgtctca
tctgggctac cagtgccgag aaatgttttg 600atcccacgta ctaagggttt
tgtttcatgt gtaagtcaca tgcgatcatt tgttccagca 660gtatatgatg
tcacagtggc attccctaag acttcacctc caccaacgtt gctaaatctt
720ttcgagggtc agtccataat gcttcacgtt cacatcaagc gacatgcaat
gaaagattta 780ccagaatccg atgatgcagt agcagagtgg tgtagagaca
aatttgtgga aaaggatgct 840ttgttggaca agcataatgc tgaggacact
ttcagtggtc aagaagtttg tcatagcggc 900agccgccagt taaagtctct
tctggtggta atatcttggg tggttgtaac aacatttggg 960gctctaaagt
tccttcagtg gtcatcatgg aaggggaaag cattttcagc tatcgggctg
1020ggcatcgtca ctctacttat gcacgtattg attctatcct cacaagcaga
gcggtctaac 1080cctgcggagg tggcacaggc aaagctaaag accgggttgt
cgatctcaaa gaaggtaacg 1140gacaaggaaa actag 1155821164DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
82atggcgattg ctgcggcagc tgtcatcgtc ccgctcagcc tcctcttctt cgtctccggc
60ctcatcgtca atctcgtaca ggcagtttgc tttgtactga ttaggcctct gtcgaaaaac
120acttacagaa gaataaacag agtggttgca gaattgttgt ggttggagtt
ggtatggctg 180attgattggt gggctggtgt caagataaaa gtattcacgg
atcatgaaac ctttcacctt 240atgggcaaag aacatgctct tgtcatttgt
aatcacaaga gtgacataga ctggctggtt 300gggtgggttc tgggacagcg
gtcaggttgc cttggaagca cattagctgt tatgaagaaa 360tcatcaaagt
ttctcccggt attagggtgg tcaatgtggt tctcagagta tctattcctt
420gaaagaagct gggccaagga tgaaattaca ttaaagtcag gtttgaatag
gctgaaagac 480tatcccttac ccttctggtt ggcacttttt gtggaaggaa
ctcggttcac tcgagcaaaa 540ctcttggcag cccagcagta tgctgcctct
tcggggctac ctgtgccgag aaatgttctg 600atcccgcgta ctaagggttt
tgtttcttct gtgagtcaca tgcgatcatt tgttccagcc 660atatatgatg
ttacagtggc aatcccaaag acgtcacctc caccaacatt gataagaatg
720ttcaagggac agtcctcagt gcttcacgtc cacctcaagc gacacctaat
gaaagattta 780cctgaatcag atgatgctgt tgctcagtgg tgcagagata
tattcgtcga gaaggatgct 840ttgttggata agcataatgc tgaggacact
ttcagtggcc aagaacttca agaaactggc 900cgcccaataa agtctcttct
ggttgtaatc tcttgggcgg tgttggaggt atttggagct 960gtgaagtttc
ttcaatggtc atcgctgtta tcatcatgga agggacttgc attttcggga
1020ataggactgg gtgtcatcac gctactcatg cacatactga ttttattctc
acaatccgag 1080cggtctaccc ctgcaaaagt ggcaccagca aagccaaaga
atgagggaga gtcctccaag 1140acggaaatgg aaaaggaaaa gtag
116483984DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 83atggagatcc ccccccactg cctgtgctcc
ccctcccccg ccccctccca gctgtactac 60aagaagaaga agcacgccat cctgcagacc
cagaccccct accgctaccg cgtgtccccc 120acctgcttcg cccccccccg
cctgcgcaag cagcacccct accccctgcc cgtgctgtgc 180taccccaagc
tgctgcactt ctcccagccc cgctaccccc tggtgcgctc ccacctggcc
240gaggccggcg tggcctaccg ccccggctac gagctgctgg gcaagatccg
cggcgtgtgc 300ttctacgccg tgaccgccgc cgtggccctg ctgctgttcc
agtgcatgct gctgctgcac 360cccttcgtgc tgctgttcga ccccttcccc
cgcaaggccc accacaccat cgccaagctg 420tggtccatct gctccgtgtc
cctgttctac aagatccaca tcaagggcct ggagaacctg 480cccccccccc
actcccccgc cgtgtacgtg tccaaccacc agtccttcct ggacatctac
540accctgctga ccctgggccg caccttcaag ttcatctcca agaccgagat
cttcctgtac 600cccatcatcg gctgggccat gtacatgctg ggcaccatcc
ccctgaagcg cctggactcc 660cgctcccagc tggacaccct gaagcgctgc
atggacctga tcaagaaggg cgcctccgtg 720ttcttcttcc ccgagggcac
ccgctccaag gacggcaagc tgggcgcctt caagaagggc 780gccttctcca
tcgccgccaa gtccaaggtg cccgtggtgc ccatcaccct gatcggcacc
840ggcaagatca tgccccccgg ctccgagctg accgtgaacc ccggcaccgt
gcaggtgatc 900atccacaagc ccatcgaggg ctccgacgcc gaggccatgt
gcaacgaggc ccgcgccacc 960atctcccact ccctggacga ctga
984841155DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 84atggcgatcg cggccgcggc ggtgatcttc
ctgttcggcc tgatcttctt cgcctccggc 60ctgatcatca acctgttcca ggcgctgtgc
ttcgtcctga tccgccccct gtccaagaac 120gcctaccgcc gcatcaaccg
cgtgttcgcg gagctgctgc tgtccgagct gctgtgcctg 180ttcgactggt
gggcgggcgc gaagctgaag ctgttcaccg accccgagac gttccgcctg
240atgggcaagg agcacgccct ggtcatcatc aaccacatga ccgagctgga
ctggatggtg 300ggctgggtga tgggccagca cttcggctgc ctgggctcca
tcatctccgt cgccaagaag 360tccacgaagt tcctgcccgt gctgggctgg
tccatgtggt tctccgagta cctgtacctg 420gagcgctcct gggccaagga
caagtccacc ctgaagtccc acatcgagcg cctgatcgac 480taccccctgc
ccttctggct ggtcatcttc gtcgagggca cccgcttcac gcgcacgaag
540ctgctggcgg cccagcagta cgcggtctcc tccggcctgc ccgtcccccg
caacgtcctg 600atcccccgca cgaagggctt cgtctcctgc gtgtcccaca
tgcgctcctt cgtccccgcg 660gtgtacgacg tcacggtggc gttccccaag
acgtcccccc cccccacgct gctgaacctg 720ttcgagggcc agtccatcat
gctgcacgtg cacatcaagc gccacgccat gaaggacctg 780cccgagtccg
acgacgccgt cgcggagtgg tgccgcgaca agttcgtcga gaaggacgcc
840ctgctggaca agcacaacgc ggaggacacg ttctccggcc aggaggtgtg
ccactccggc 900tcccgccagc tgaagtccct gctggtcgtg atctcctggg
tcgtggtgac gacgttcggc 960gccctgaagt tcctgcagtg gtcctcctgg
aagggcaagg cgttctccgc catcggcctg 1020ggcatcgtca ccctgctgat
gcacgtgctg atcctgtcct cccaggccga gcgctccaac 1080cccgccgagg
tggcccaggc caagctgaag accggcctgt ccatctccaa gaaggtgacg
1140gacaaggaga actga 1155851164DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 85atggccatcg
cggcggccgc ggtgatcgtg cccctgtccc tgctgttctt cgtgtccggc 60ctgatcgtca
acctggtgca ggccgtctgc ttcgtcctga tccgccccct gtccaagaac
120acgtaccgcc gcatcaaccg cgtggtcgcg gagctgctgt ggctggagct
ggtgtggctg 180atcgactggt gggcgggcgt gaagatcaag gtcttcacgg
accacgagac gttccacctg 240atgggcaagg agcacgccct ggtcatctgc
aaccacaagt ccgacatcga ctggctggtc 300ggctgggtcc tgggccagcg
ctccggctgc ctgggctcca ccctggcggt catgaagaag 360tcctccaagt
tcctgcccgt cctgggctgg tccatgtggt tctccgagta cctgttcctg
420gagcgctcct gggccaagga cgagatcacg ctgaagtccg gcctgaaccg
cctgaaggac 480taccccctgc ccttctggct ggcgctgttc gtggagggca
cgcgcttcac ccgcgcgaag 540ctgctggcgg cgcagcagta cgccgcgtcc
tccggcctgc ccgtgccccg caacgtgctg 600atcccccgca cgaagggctt
cgtgtcctcc gtgtcccaca tgcgctcctt cgtgcccgcg 660atctacgacg
tcaccgtggc catccccaag acgtcccccc cccccacgct gatccgcatg
720ttcaagggcc agtcctccgt gctgcacgtg cacctgaagc gccacctgat
gaaggacctg 780cccgagtccg acgacgccgt cgcgcagtgg tgccgcgaca
tcttcgtgga gaaggacgcg 840ctgctggaca agcacaacgc cgaggacacc
ttctccggcc aggagctgca ggagaccggc 900cgccccatca agtccctgct
ggtcgtcatc tcctgggccg tcctggaggt gttcggcgcc 960gtcaagttcc
tgcagtggtc ctccctgctg tcctcctgga agggcctggc gttctccggc
1020atcggcctgg gcgtgatcac cctgctgatg cacatcctga tcctgttctc
ccagtccgag 1080cgctccaccc ccgccaaggt ggcccccgcg aagcccaaga
acgagggcga gtcctccaag 1140accgagatgg agaaggagaa gtga
1164861098DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 86gcgcacccca aggcgaacgg cagcgcggtg
tcgctgaagt cgggctccct ggagacccag 60gaggacaaga cgagcagctc gtcccccccc
ccccgcacgt tcatcaacca gctgcccgtg 120tggagcatgc tgctgtcggc
ggtgaccacg gtcttcggcg tggccgagaa gcagtggccc 180atgctggacc
gcaagtccaa gcgccccgac atgctggtcg agcccctggg cgtggaccgc
240atcgtctacg acggcgtgag cttccgccag tcgttctcca tccgcagcta
cgagatcggc 300gccgaccgca ccgcctcgat cgagacgctg atgaacatgt
tccaggagac ctccctgaac 360cactgcaaga tcatcggcct gctgaacgac
ggcttcggcc gcacgcccga gatgtgcaag 420cgcgacctga tctgggtcgt
gaccaagatg cagatcgagg tgaaccgcta ccccacgtgg 480ggcgacacca
tcgaggtcaa cacgtgggtg agcgcctcgg gcaagcacgg catgggccgc
540gactggctga tctccgactg ccacaccggc gagatcctga tccgcgcgac
gagcgtctgg 600gcgatgatga accagaagac ccgccgcctg tcgaagatcc
cctacgaggt gcgccaggag 660atcgagcccc agttcgtcga ctccgccccc
gtgatcgtgg acgaccgcaa gttccacaag 720ctggacctga agacgggcga
cagcatctgc aacggcctga ccccccgctg gacggacctg 780gacgtgaacc
agcacgtcaa caacgtgaag tacatcggct ggatcctgca gtcggtcccc
840accgaggtgt tcgagacgca ggagctgtgc ggcctgaccc tggagtaccg
ccgcgagtgc 900ggccgcgact ccgtgctgga gagcgtcacg gccatggacc
cctcgaagga gggcgaccgc 960tccctgtacc agcacctgct gcgcctggag
gacggcgcgg acatcgtgaa gggccgcacc 1020gagtggcgcc ccaagaacgc
cggcgccaag ggcgccatcc tgacgggcaa gaccagcaac 1080ggcaactcga tctcctga
109887365PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 87Ala His Pro Lys Ala Asn Gly Ser Ala Val Ser
Leu Lys Ser Gly Ser 1 5 10 15 Leu Glu Thr Gln Glu Asp Lys Thr Ser
Ser Ser Ser Pro Pro Pro Arg 20 25 30 Thr Phe Ile Asn Gln Leu Pro
Val Trp Ser Met Leu Leu Ser Ala Val 35 40 45 Thr Thr Val Phe Gly
Val Ala Glu Lys Gln Trp Pro Met Leu Asp Arg 50 55 60 Lys Ser Lys
Arg Pro Asp Met Leu Val Glu Pro Leu Gly Val Asp Arg 65 70 75 80 Ile
Val Tyr Asp Gly Val Ser Phe Arg Gln Ser Phe Ser Ile Arg Ser 85 90
95 Tyr Glu Ile Gly Ala Asp Arg Thr Ala Ser Ile Glu Thr Leu Met Asn
100 105 110 Met Phe Gln Glu Thr Ser Leu Asn His Cys Lys Ile Ile Gly
Leu Leu 115 120 125 Asn Asp Gly Phe Gly Arg Thr Pro Glu Met Cys Lys
Arg Asp Leu Ile 130 135 140 Trp Val Val Thr Lys Met Gln Ile Glu Val
Asn Arg Tyr Pro Thr Trp 145 150 155 160 Gly Asp Thr Ile Glu Val Asn
Thr Trp Val Ser Ala Ser Gly Lys His 165 170 175 Gly Met Gly Arg Asp
Trp Leu Ile Ser Asp Cys His Thr Gly Glu Ile 180 185 190 Leu Ile Arg
Ala Thr Ser Val Trp Ala Met Met Asn Gln Lys Thr Arg 195 200 205 Arg
Leu Ser Lys Ile Pro Tyr Glu Val Arg Gln Glu Ile Glu Pro Gln 210 215
220 Phe Val Asp Ser Ala Pro Val Ile Val Asp Asp Arg Lys Phe His Lys
225 230 235 240 Leu Asp Leu Lys Thr Gly Asp Ser Ile Cys Asn Gly Leu
Thr Pro Arg 245 250 255 Trp Thr Asp Leu Asp Val Asn Gln His Val Asn
Asn Val Lys Tyr Ile 260 265 270 Gly Trp Ile Leu Gln Ser Val Pro Thr
Glu Val Phe Glu Thr Gln Glu 275 280 285 Leu Cys Gly Leu Thr Leu Glu
Tyr Arg Arg Glu Cys Gly Arg Asp Ser 290 295 300 Val Leu Glu Ser Val
Thr Ala Met Asp Pro Ser Lys Glu Gly Asp Arg 305 310 315 320 Ser Leu
Tyr Gln His Leu Leu Arg Leu Glu Asp Gly Ala Asp Ile Val 325 330 335
Lys Gly Arg Thr Glu Trp Arg Pro Lys Asn Ala Gly Ala Lys Gly Ala 340
345 350 Ile Leu Thr Gly Lys Thr Ser Asn Gly Asn Ser Ile Ser 355 360
365 881167DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 88gcgcacccca aggcgaacgg cagcgcggtg
tcgctgaagt cgggctccct ggagacccag 60gaggacaaga cgagcagctc gtcccccccc
ccccgcacgt tcatcaacca gctgcccgtg 120tggagcatgc tgctgtcggc
ggtgaccacg gtcttcggcg tggccgagaa gcagtggccc 180atgctggacc
gcaagtccaa gcgccccgac atgctggtcg agcccctggg cgtggaccgc
240atcgtctacg acggcgtgag cttccgccag tcgttctcca tccgcagcta
cgagatcggc 300gccgaccgca ccgcctcgat cgagacgctg atgaacatgt
tccaggagac ctccctgaac 360cactgcaaga tcatcggcct gctgaacgac
ggcttcggcc gcacgcccga gatgtgcaag 420cgcgacctga tctgggtcgt
gaccaagatg cagatcgagg tgaaccgcta ccccacgtgg 480ggcgacacca
tcgaggtcaa cacgtgggtg agcgcctcgg gcaagcacgg catgggccgc
540gactggctga tctccgactg ccacaccggc gagatcctga tccgcgcgac
gagcgtctgg 600gcgatgatga accagaagac ccgccgcctg tcgaagatcc
cctacgaggt gcgccaggag 660atcgagcccc agttcgtcga ctccgccccc
gtgatcgtgg acgaccgcaa gttccacaag 720ctggacctga agacgggcga
cagcatctgc aacggcctga ccccccgctg gacggacctg 780gacgtgaacc
agcacgtcaa caacgtgaag tacatcggct ggatcctgca gtcggtcccc
840accgaggtgt tcgagacgca ggagctgtgc ggcctgaccc tggagtaccg
ccgcgagtgc 900ggccgcgact ccgtgctgga gagcgtcacg gccatggacc
cctcgaagga gggcgaccgc 960tccctgtacc agcacctgct gcgcctggag
gacggcgcgg acatcgtgaa gggccgcacc 1020gagtggcgcc ccaagaacgc
cggcgccaag ggcgccatcc tgacgggcaa gaccagcaac 1080ggcaactcga
tctccatgga ctacaaggac cacgacggcg actacaagga ccacgacatc
1140gactacaagg acgacgacga caagtga 116789388PRTArtificial
SequenceDescription of Artificial Sequence Synthetic polypeptide
89Ala His Pro Lys Ala Asn Gly Ser Ala Val Ser Leu Lys Ser Gly Ser 1
5 10 15 Leu Glu Thr Gln Glu Asp Lys Thr Ser Ser Ser Ser Pro Pro Pro
Arg 20 25 30 Thr Phe Ile Asn Gln Leu Pro Val Trp Ser Met Leu Leu
Ser Ala Val 35 40 45 Thr Thr Val Phe Gly Val Ala Glu Lys Gln Trp
Pro Met Leu Asp Arg 50 55 60 Lys Ser Lys Arg Pro Asp Met Leu Val
Glu Pro Leu Gly Val Asp Arg 65 70 75 80 Ile Val Tyr Asp Gly Val Ser
Phe Arg Gln Ser Phe Ser Ile Arg Ser 85 90 95 Tyr Glu Ile Gly Ala
Asp Arg Thr Ala Ser Ile Glu Thr Leu Met Asn 100 105 110 Met Phe Gln
Glu Thr Ser Leu Asn His Cys Lys Ile Ile Gly Leu Leu 115 120 125
Asn
Asp Gly Phe Gly Arg Thr Pro Glu Met Cys Lys Arg Asp Leu Ile 130 135
140 Trp Val Val Thr Lys Met Gln Ile Glu Val Asn Arg Tyr Pro Thr Trp
145 150 155 160 Gly Asp Thr Ile Glu Val Asn Thr Trp Val Ser Ala Ser
Gly Lys His 165 170 175 Gly Met Gly Arg Asp Trp Leu Ile Ser Asp Cys
His Thr Gly Glu Ile 180 185 190 Leu Ile Arg Ala Thr Ser Val Trp Ala
Met Met Asn Gln Lys Thr Arg 195 200 205 Arg Leu Ser Lys Ile Pro Tyr
Glu Val Arg Gln Glu Ile Glu Pro Gln 210 215 220 Phe Val Asp Ser Ala
Pro Val Ile Val Asp Asp Arg Lys Phe His Lys 225 230 235 240 Leu Asp
Leu Lys Thr Gly Asp Ser Ile Cys Asn Gly Leu Thr Pro Arg 245 250 255
Trp Thr Asp Leu Asp Val Asn Gln His Val Asn Asn Val Lys Tyr Ile 260
265 270 Gly Trp Ile Leu Gln Ser Val Pro Thr Glu Val Phe Glu Thr Gln
Glu 275 280 285 Leu Cys Gly Leu Thr Leu Glu Tyr Arg Arg Glu Cys Gly
Arg Asp Ser 290 295 300 Val Leu Glu Ser Val Thr Ala Met Asp Pro Ser
Lys Glu Gly Asp Arg 305 310 315 320 Ser Leu Tyr Gln His Leu Leu Arg
Leu Glu Asp Gly Ala Asp Ile Val 325 330 335 Lys Gly Arg Thr Glu Trp
Arg Pro Lys Asn Ala Gly Ala Lys Gly Ala 340 345 350 Ile Leu Thr Gly
Lys Thr Ser Asn Gly Asn Ser Ile Ser Met Asp Tyr 355 360 365 Lys Asp
His Asp Gly Asp Tyr Lys Asp His Asp Ile Asp Tyr Lys Asp 370 375 380
Asp Asp Asp Lys 385 905428DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 90gctcttccgc
taacggaggt ctgtcaccaa atggaccccg tctattgcgg gaaaccacgg 60cgatggcacg
tttcaaaact tgatgaaata caatattcag tatgtcgcgg gcggcgacgg
120cggggagctg atgtcgcgct gggtattgct taatcgccag cttcgccccc
gtcttggcgc 180gaggcgtgaa caagccgacc gatgtgcacg agcaaatcct
gacactagaa gggctgactc 240gcccggcacg gctgaattac acaggcttgc
aaaaatacca gaatttgcac gcaccgtatt 300cgcggtattt tgttggacag
tgaatagcga tgcggcaatg gcttgtggcg ttagaaggtg 360cgacgaaggt
ggtgccacca ctgtgccagc cagtcctggc ggctcccagg gccccgatca
420agagccagga catccaaact acccacagca tcaacgcccc ggcctatact
cgaaccccac 480ttgcactctg caatggtatg ggaaccacgg ggcagtcttg
tgtgggtcgc gcctatcgcg 540gtcggcgaag accgggaagg taccctttct
tgcgctatga cacttccagc aaaaggtagg 600gcgggctgcg agacggcttc
ccggcgctgc atgcaacacc gatgatgctt cgaccccccg 660aagctccttc
ggggctgcat gggcgctccg atgccgctcc agggcgagcg ctgtttaaat
720agccaggccc ccgattgcaa agacattata gcgagctacc aaagccatat
tcaaacacct 780agatcactac cacttctaca caggccactc gagcttgtga
tcgcactccg ctaagggggc 840gcctcttcct cttcgtttca gtcacaaccc
gcaaactcta gaatatcaat gatcgagcag 900gacggcctcc acgccggctc
ccccgccgcc tgggtggagc gcctgttcgg ctacgactgg 960gcccagcaga
ccatcggctg ctccgacgcc gccgtgttcc gcctgtccgc ccagggccgc
1020cccgtgctgt tcgtgaagac cgacctgtcc ggcgccctga acgagctgca
ggacgaggcc 1080gcccgcctgt cctggctggc caccaccggc gtgccctgcg
ccgccgtgct ggacgtggtg 1140accgaggccg gccgcgactg gctgctgctg
ggcgaggtgc ccggccagga cctgctgtcc 1200tcccacctgg cccccgccga
gaaggtgtcc atcatggccg acgccatgcg ccgcctgcac 1260accctggacc
ccgccacctg ccccttcgac caccaggcca agcaccgcat cgagcgcgcc
1320cgcacccgca tggaggccgg cctggtggac caggacgacc tggacgagga
gcaccagggc 1380ctggcccccg ccgagctgtt cgcccgcctg aaggcccgca
tgcccgacgg cgaggacctg 1440gtggtgaccc acggcgacgc ctgcctgccc
aacatcatgg tggagaacgg ccgcttctcc 1500ggcttcatcg actgcggccg
cctgggcgtg gccgaccgct accaggacat cgccctggcc 1560acccgcgaca
tcgccgagga gctgggcggc gagtgggccg accgcttcct ggtgctgtac
1620ggcatcgccg cccccgactc ccagcgcatc gccttctacc gcctgctgga
cgagttcttc 1680tgacaattgg cagcagcagc tcggatagta tcgacacact
ctggacgctg gtcgtgtgat 1740ggactgttgc cgccacactt gctgccttga
cctgtgaata tccctgccgc ttttatcaaa 1800cagcctcagt gtgtttgatc
ttgtgtgtac gcgcttttgc gagttgctag ctgcttgtgc 1860tatttgcgaa
taccaccccc agcatcccct tccctcgttt catatcgctt gcatcccaac
1920cgcaacttat ctacgctgtc ctgctatccc tcagcgctgc tcctgctcct
gctcactgcc 1980cctcgcacag ccttggtttg ggctccgcct gtattctcct
ggtactgcaa cctgtaaacc 2040agcactgcaa tgctgatgca cgggaagtag
tgggatggga acacaaatgg aggatcccgc 2100gtctcgaaca gagcgcgcag
aggaacgctg aaggtctcgc ctctgtcgca cctcagcgcg 2160gcatacacca
caataaccac ctgacgaatg cgcttggttc ttcgtccatt agcgaagcgt
2220ccggttcaca cacgtgccac gttggcgagg tggcaggtga caatgatcgg
tggagctgat 2280ggtcgaaacg ttcacagcct agggatatcg aattcggccg
acaggacgcg cgtcaaaggt 2340gctggtcgtg tatgccctgg ccggcaggtc
gttgctgctg ctggttagtg attccgcaac 2400cctgattttg gcgtcttatt
ttggcgtggc aaacgctggc gcccgcgagc cgggccggcg 2460gcgatgcggt
gccccacggc tgccggaatc caagggaggc aagagcgccc gggtcagttg
2520aagggcttta cgcgcaaggt acagccgctc ctgcaaggct gcgtggtgga
attggacgtg 2580caggtcctgc tgaagttcct ccaccgcctc accagcggac
aaagcaccgg tgtatcaggt 2640ccgtgtcatc cactctaaag agctcgacta
cgacctactg atggccctag attcttcatc 2700aaaaacgcct gagacacttg
cccaggattg aaactccctg aagggaccac caggggccct 2760gagttgttcc
ttccccccgt ggcgagctgc cagccaggct gtacctgtga tcgaggctgg
2820cgggaaaata ggcttcgtgt gctcaggtca tgggaggtgc aggacagctc
atgaaacgcc 2880aacaatcgca caattcatgt caagctaatc agctatttcc
tcttcacgag ctgtaattgt 2940cccaaaattc tggtctaccg ggggtgatcc
ttcgtgtacg ggcccttccc tcaaccctag 3000gtatgcgcgc atgcggtcgc
cgcgcaactc gcgcgagggc cgagggtttg ggacgggccg 3060tcccgaaatg
cagttgcacc cggatgcgtg gcaccttttt tgcgataatt tatgcaatgg
3120actgctctgc aaaattctgg ctctgtcgcc aaccctagga tcagcggcgt
aggatttcgt 3180aatcattcgt cctgatgggg agctaccgac taccctaata
tcagcccgac tgcctgacgc 3240cagcgtccac ttttgtgcac acattccatt
cgtgcccaag acatttcatt gtggtgcgaa 3300gcgtccccag ttacgctcac
ctgtttcccg acctccttac tgttctgtcg acagagcggg 3360cccacaggcc
ggtcgcagcc actagtatgg cgatcgcggc cgcggcggtg atcttcctgt
3420tcggcctgat cttcttcgcc tccggcctga tcatcaacct gttccaggcg
ctgtgcttcg 3480tcctgatccg ccccctgtcc aagaacgcct accgccgcat
caaccgcgtg ttcgcggagc 3540tgctgctgtc cgagctgctg tgcctgttcg
actggtgggc gggcgcgaag ctgaagctgt 3600tcaccgaccc cgagacgttc
cgcctgatgg gcaaggagca cgccctggtc atcatcaacc 3660acatgaccga
gctggactgg atggtgggct gggtgatggg ccagcacttc ggctgcctgg
3720gctccatcat ctccgtcgcc aagaagtcca cgaagttcct gcccgtgctg
ggctggtcca 3780tgtggttctc cgagtacctg tacctggagc gctcctgggc
caaggacaag tccaccctga 3840agtcccacat cgagcgcctg atcgactacc
ccctgccctt ctggctggtc atcttcgtcg 3900agggcacccg cttcacgcgc
acgaagctgc tggcggccca gcagtacgcg gtctcctccg 3960gcctgcccgt
cccccgcaac gtcctgatcc cccgcacgaa gggcttcgtc tcctgcgtgt
4020cccacatgcg ctccttcgtc cccgcggtgt acgacgtcac ggtggcgttc
cccaagacgt 4080cccccccccc cacgctgctg aacctgttcg agggccagtc
catcatgctg cacgtgcaca 4140tcaagcgcca cgccatgaag gacctgcccg
agtccgacga cgccgtcgcg gagtggtgcc 4200gcgacaagtt cgtcgagaag
gacgccctgc tggacaagca caacgcggag gacacgttct 4260ccggccagga
ggtgtgccac tccggctccc gccagctgaa gtccctgctg gtcgtgatct
4320cctgggtcgt ggtgacgacg ttcggcgccc tgaagttcct gcagtggtcc
tcctggaagg 4380gcaaggcgtt ctccgccatc ggcctgggca tcgtcaccct
gctgatgcac gtgctgatcc 4440tgtcctccca ggccgagcgc tccaaccccg
ccgaggtggc ccaggccaag ctgaagaccg 4500gcctgtccat ctccaagaag
gtgacggaca aggagaactg attaattaac tcgaggcagc 4560agcagctcgg
atagtatcga cacactctgg acgctggtcg tgtgatggac tgttgccgcc
4620acacttgctg ccttgacctg tgaatatccc tgccgctttt atcaaacagc
ctcagtgtgt 4680ttgatcttgt gtgtacgcgc ttttgcgagt tgctagctgc
ttgtgctatt tgcgaatacc 4740acccccagca tccccttccc tcgtttcata
tcgcttgcat cccaaccgca acttatctac 4800gctgtcctgc tatccctcag
cgctgctcct gctcctgctc actgcccctc gcacagcctt 4860ggtttgggct
ccgcctgtat tctcctggta ctgcaacctg taaaccagca ctgcaatgct
4920gatgcacggg aagtagtggg atgggaacac aaatggaaag cttgagctca
gcggcgacgg 4980tcctgctacc gtacgacgtt gggcacgccc atgaaagttt
gtataccgag cttgttgagc 5040gaactgcaag cgcggctcaa ggatacttga
actcctggat tgatatcggt ccaataatgg 5100atggaaaatc cgaacctcgt
gcaagaactg agcaaacctc gttacatgga tgcacagtcg 5160ccagtccaat
gaacattgaa gtgagcgaac tgttcgcttc ggtggcagta ctactcaaag
5220aatgagctgc tgttaaaaat gcactctcgt tctctcaagt gagtggcaga
tgagtgctca 5280cgccttgcac ttcgctgccc gtgtcatgcc ctgcgcccca
aaatttgaaa aaagggatga 5340gattattggg caatggacga cgtcgtcgct
ccgggagtca ggaccggcgg aaaataagag 5400gcaacacact ccgcttctta gctcttcg
5428915436DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 91gctcttccgc taacggaggt ctgtcaccaa
atggaccccg tctattgcgg gaaaccacgg 60cgatggcacg tttcaaaact tgatgaaata
caatattcag tatgtcgcgg gcggcgacgg 120cggggagctg atgtcgcgct
gggtattgct taatcgccag cttcgccccc gtcttggcgc 180gaggcgtgaa
caagccgacc gatgtgcacg agcaaatcct gacactagaa gggctgactc
240gcccggcacg gctgaattac acaggcttgc aaaaatacca gaatttgcac
gcaccgtatt 300cgcggtattt tgttggacag tgaatagcga tgcggcaatg
gcttgtggcg ttagaaggtg 360cgacgaaggt ggtgccacca ctgtgccagc
cagtcctggc ggctcccagg gccccgatca 420agagccagga catccaaact
acccacagca tcaacgcccc ggcctatact cgaaccccac 480ttgcactctg
caatggtatg ggaaccacgg ggcagtcttg tgtgggtcgc gcctatcgcg
540gtcggcgaag accgggaagg taccctttct tgcgctatga cacttccagc
aaaaggtagg 600gcgggctgcg agacggcttc ccggcgctgc atgcaacacc
gatgatgctt cgaccccccg 660aagctccttc ggggctgcat gggcgctccg
atgccgctcc agggcgagcg ctgtttaaat 720agccaggccc ccgattgcaa
agacattata gcgagctacc aaagccatat tcaaacacct 780agatcactac
cacttctaca caggccactc gagcttgtga tcgcactccg ctaagggggc
840gcctcttcct cttcgtttca gtcacaaccc gcaaactcta gaatatcaat
gatcgagcag 900gacggcctcc acgccggctc ccccgccgcc tgggtggagc
gcctgttcgg ctacgactgg 960gcccagcaga ccatcggctg ctccgacgcc
gccgtgttcc gcctgtccgc ccagggccgc 1020cccgtgctgt tcgtgaagac
cgacctgtcc ggcgccctga acgagctgca ggacgaggcc 1080gcccgcctgt
cctggctggc caccaccggc gtgccctgcg ccgccgtgct ggacgtggtg
1140accgaggccg gccgcgactg gctgctgctg ggcgaggtgc ccggccagga
cctgctgtcc 1200tcccacctgg cccccgccga gaaggtgtcc atcatggccg
acgccatgcg ccgcctgcac 1260accctggacc ccgccacctg ccccttcgac
caccaggcca agcaccgcat cgagcgcgcc 1320cgcacccgca tggaggccgg
cctggtggac caggacgacc tggacgagga gcaccagggc 1380ctggcccccg
ccgagctgtt cgcccgcctg aaggcccgca tgcccgacgg cgaggacctg
1440gtggtgaccc acggcgacgc ctgcctgccc aacatcatgg tggagaacgg
ccgcttctcc 1500ggcttcatcg actgcggccg cctgggcgtg gccgaccgct
accaggacat cgccctggcc 1560acccgcgaca tcgccgagga gctgggcggc
gagtgggccg accgcttcct ggtgctgtac 1620ggcatcgccg cccccgactc
ccagcgcatc gccttctacc gcctgctgga cgagttcttc 1680tgacaattgg
cagcagcagc tcggatagta tcgacacact ctggacgctg gtcgtgtgat
1740ggactgttgc cgccacactt gctgccttga cctgtgaata tccctgccgc
ttttatcaaa 1800cagcctcagt gtgtttgatc ttgtgtgtac gcgcttttgc
gagttgctag ctgcttgtgc 1860tatttgcgaa taccaccccc agcatcccct
tccctcgttt catatcgctt gcatcccaac 1920cgcaacttat ctacgctgtc
ctgctatccc tcagcgctgc tcctgctcct gctcactgcc 1980cctcgcacag
ccttggtttg ggctccgcct gtattctcct ggtactgcaa cctgtaaacc
2040agcactgcaa tgctgatgca cgggaagtag tgggatggga acacaaatgg
aggatcccgc 2100gtctcgaaca gagcgcgcag aggaacgctg aaggtctcgc
ctctgtcgca cctcagcgcg 2160gcatacacca caataaccac ctgacgaatg
cgcttggttc ttcgtccatt agcgaagcgt 2220ccggttcaca cacgtgccac
gttggcgagg tggcaggtga caatgatcgg tggagctgat 2280ggtcgaaacg
ttcacagcct agggatatcg aattcggccg acaggacgcg cgtcaaaggt
2340gctggtcgtg tatgccctgg ccggcaggtc gttgctgctg ctggttagtg
attccgcaac 2400cctgattttg gcgtcttatt ttggcgtggc aaacgctggc
gcccgcgagc cgggccggcg 2460gcgatgcggt gccccacggc tgccggaatc
caagggaggc aagagcgccc gggtcagttg 2520aagggcttta cgcgcaaggt
acagccgctc ctgcaaggct gcgtggtgga attggacgtg 2580caggtcctgc
tgaagttcct ccaccgcctc accagcggac aaagcaccgg tgtatcaggt
2640ccgtgtcatc cactctaaag agctcgacta cgacctactg atggccctag
attcttcatc 2700aaaaacgcct gagacacttg cccaggattg aaactccctg
aagggaccac caggggccct 2760gagttgttcc ttccccccgt ggcgagctgc
cagccaggct gtacctgtga tcgaggctgg 2820cgggaaaata ggcttcgtgt
gctcaggtca tgggaggtgc aggacagctc atgaaacgcc 2880aacaatcgca
caattcatgt caagctaatc agctatttcc tcttcacgag ctgtaattgt
2940cccaaaattc tggtctaccg ggggtgatcc ttcgtgtacg ggcccttccc
tcaaccctag 3000gtatgcgcgc atgcggtcgc cgcgcaactc gcgcgagggc
cgagggtttg ggacgggccg 3060tcccgaaatg cagttgcacc cggatgcgtg
gcaccttttt tgcgataatt tatgcaatgg 3120actgctctgc aaaattctgg
ctctgtcgcc aaccctagga tcagcggcgt aggatttcgt 3180aatcattcgt
cctgatgggg agctaccgac taccctaata tcagcccgac tgcctgacgc
3240cagcgtccac ttttgtgcac acattccatt cgtgcccaag acatttcatt
gtggtgcgaa 3300gcgtccccag ttacgctcac ctgtttcccg acctccttac
tgttctgtcg acagagcggg 3360cccacaggcc ggtcgcagcc actagtatgg
ccatcgcggc ggccgcggtg atcgtgcccc 3420tgtccctgct gttcttcgtg
tccggcctga tcgtcaacct ggtgcaggcc gtctgcttcg 3480tcctgatccg
ccccctgtcc aagaacacgt accgccgcat caaccgcgtg gtcgcggagc
3540tgctgtggct ggagctggtg tggctgatcg actggtgggc gggcgtgaag
atcaaggtct 3600tcacggacca cgagacgttc cacctgatgg gcaaggagca
cgccctggtc atctgcaacc 3660acaagtccga catcgactgg ctggtcggct
gggtcctggg ccagcgctcc ggctgcctgg 3720gctccaccct ggcggtcatg
aagaagtcct ccaagttcct gcccgtcctg ggctggtcca 3780tgtggttctc
cgagtacctg ttcctggagc gctcctgggc caaggacgag atcacgctga
3840agtccggcct gaaccgcctg aaggactacc ccctgccctt ctggctggcg
ctgttcgtgg 3900agggcacgcg cttcacccgc gcgaagctgc tggcggcgca
gcagtacgcc gcgtcctccg 3960gcctgcccgt gccccgcaac gtgctgatcc
cccgcacgaa gggcttcgtg tcctccgtgt 4020cccacatgcg ctccttcgtg
cccgcgatct acgacgtcac cgtggccatc cccaagacgt 4080cccccccccc
cacgctgatc cgcatgttca agggccagtc ctccgtgctg cacgtgcacc
4140tgaagcgcca cctgatgaag gacctgcccg agtccgacga cgccgtcgcg
cagtggtgcc 4200gcgacatctt cgtggagaag gacgcgctgc tggacaagca
caacgccgag gacaccttct 4260ccggccagga gctgcaggag accggccgcc
ccatcaagtc cctgctggtc gtcatctcct 4320gggccgtcct ggaggtgttc
ggcgccgtca agttcctgca gtggtcctcc ctgctgtcct 4380cctggaaggg
cctggcgttc tccggcatcg gcctgggcgt gatcaccctg ctgatgcaca
4440tcctgatcct gttctcccag tccgagcgct ccacccccgc caaggtggcc
cccgcgaagc 4500ccaagaacga gggcgagtcc tccaagaccg agatggagaa
ggagaagtga ttaattaact 4560cgaggcagca gcagctcgga tagtatcgac
acactctgga cgctggtcgt gtgatggact 4620gttgccgcca cacttgctgc
cttgacctgt gaatatccct gccgctttta tcaaacagcc 4680tcagtgtgtt
tgatcttgtg tgtacgcgct tttgcgagtt gctagctgct tgtgctattt
4740gcgaatacca cccccagcat ccccttccct cgtttcatat cgcttgcatc
ccaaccgcaa 4800cttatctacg ctgtcctgct atccctcagc gctgctcctg
ctcctgctca ctgcccctcg 4860cacagccttg gtttgggctc cgcctgtatt
ctcctggtac tgcaacctgt aaaccagcac 4920tgcaatgctg atgcacggga
agtagtggga tgggaacaca aatggaaagc ttgagctcag 4980cggcgacggt
cctgctaccg tacgacgttg ggcacgccca tgaaagtttg tataccgagc
5040ttgttgagcg aactgcaagc gcggctcaag gatacttgaa ctcctggatt
gatatcggtc 5100caataatgga tggaaaatcc gaacctcgtg caagaactga
gcaaacctcg ttacatggat 5160gcacagtcgc cagtccaatg aacattgaag
tgagcgaact gttcgcttcg gtggcagtac 5220tactcaaaga atgagctgct
gttaaaaatg cactctcgtt ctctcaagtg agtggcagat 5280gagtgctcac
gcctgcactt cgctgcccgt gtcatgccct gcgccccaaa atttgaaaaa
5340agggatgaga ttattgggca atggacgacg tcgtcgctcc gggagtcagg
accggcggaa 5400aataagaggc aacacactcc gcttcttagc tcttcg
5436925257DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 92gctcttccgc taacggaggt ctgtcaccaa
atggaccccg tctattgcgg gaaaccacgg 60cgatggcacg tttcaaaact tgatgaaata
caatattcag tatgtcgcgg gcggcgacgg 120cggggagctg atgtcgcgct
gggtattgct taatcgccag cttcgccccc gtcttggcgc 180gaggcgtgaa
caagccgacc gatgtgcacg agcaaatcct gacactagaa gggctgactc
240gcccggcacg gctgaattac acaggcttgc aaaaatacca gaatttgcac
gcaccgtatt 300cgcggtattt tgttggacag tgaatagcga tgcggcaatg
gcttgtggcg ttagaaggtg 360cgacgaaggt ggtgccacca ctgtgccagc
cagtcctggc ggctcccagg gccccgatca 420agagccagga catccaaact
acccacagca tcaacgcccc ggcctatact cgaaccccac 480ttgcactctg
caatggtatg ggaaccacgg ggcagtcttg tgtgggtcgc gcctatcgcg
540gtcggcgaag accgggaagg taccctttct tgcgctatga cacttccagc
aaaaggtagg 600gcgggctgcg agacggcttc ccggcgctgc atgcaacacc
gatgatgctt cgaccccccg 660aagctccttc ggggctgcat gggcgctccg
atgccgctcc agggcgagcg ctgtttaaat 720agccaggccc ccgattgcaa
agacattata gcgagctacc aaagccatat tcaaacacct 780agatcactac
cacttctaca caggccactc gagcttgtga tcgcactccg ctaagggggc
840gcctcttcct cttcgtttca gtcacaaccc gcaaactcta gaatatcaat
gatcgagcag 900gacggcctcc acgccggctc ccccgccgcc tgggtggagc
gcctgttcgg ctacgactgg 960gcccagcaga ccatcggctg ctccgacgcc
gccgtgttcc gcctgtccgc ccagggccgc 1020cccgtgctgt tcgtgaagac
cgacctgtcc ggcgccctga acgagctgca ggacgaggcc 1080gcccgcctgt
cctggctggc caccaccggc gtgccctgcg ccgccgtgct ggacgtggtg
1140accgaggccg gccgcgactg gctgctgctg ggcgaggtgc ccggccagga
cctgctgtcc 1200tcccacctgg cccccgccga gaaggtgtcc atcatggccg
acgccatgcg ccgcctgcac 1260accctggacc ccgccacctg ccccttcgac
caccaggcca agcaccgcat cgagcgcgcc 1320cgcacccgca tggaggccgg
cctggtggac caggacgacc tggacgagga gcaccagggc 1380ctggcccccg
ccgagctgtt cgcccgcctg aaggcccgca tgcccgacgg cgaggacctg
1440gtggtgaccc acggcgacgc ctgcctgccc aacatcatgg tggagaacgg
ccgcttctcc 1500ggcttcatcg actgcggccg cctgggcgtg gccgaccgct
accaggacat cgccctggcc 1560acccgcgaca tcgccgagga gctgggcggc
gagtgggccg accgcttcct ggtgctgtac 1620ggcatcgccg cccccgactc
ccagcgcatc gccttctacc gcctgctgga cgagttcttc 1680tgacaattgg
cagcagcagc tcggatagta tcgacacact ctggacgctg gtcgtgtgat
1740ggactgttgc cgccacactt gctgccttga cctgtgaata tccctgccgc
ttttatcaaa 1800cagcctcagt gtgtttgatc ttgtgtgtac gcgcttttgc
gagttgctag ctgcttgtgc 1860tatttgcgaa taccaccccc agcatcccct
tccctcgttt catatcgctt gcatcccaac 1920cgcaacttat ctacgctgtc
ctgctatccc tcagcgctgc tcctgctcct gctcactgcc 1980cctcgcacag
ccttggtttg ggctccgcct gtattctcct ggtactgcaa cctgtaaacc
2040agcactgcaa tgctgatgca cgggaagtag tgggatggga acacaaatgg
aggatcccgc 2100gtctcgaaca gagcgcgcag aggaacgctg aaggtctcgc
ctctgtcgca cctcagcgcg 2160gcatacacca caataaccac ctgacgaatg
cgcttggttc ttcgtccatt agcgaagcgt 2220ccggttcaca cacgtgccac
gttggcgagg tggcaggtga caatgatcgg tggagctgat 2280ggtcgaaacg
ttcacagcct agggatatcg aattcggccg acaggacgcg cgtcaaaggt
2340gctggtcgtg tatgccctgg ccggcaggtc gttgctgctg ctggttagtg
attccgcaac 2400cctgattttg gcgtcttatt ttggcgtggc aaacgctggc
gcccgcgagc cgggccggcg 2460gcgatgcggt gccccacggc tgccggaatc
caagggaggc aagagcgccc gggtcagttg 2520aagggcttta cgcgcaaggt
acagccgctc ctgcaaggct gcgtggtgga attggacgtg 2580caggtcctgc
tgaagttcct ccaccgcctc accagcggac aaagcaccgg tgtatcaggt
2640ccgtgtcatc cactctaaag agctcgacta cgacctactg atggccctag
attcttcatc 2700aaaaacgcct gagacacttg cccaggattg aaactccctg
aagggaccac caggggccct 2760gagttgttcc ttccccccgt ggcgagctgc
cagccaggct gtacctgtga tcgaggctgg 2820cgggaaaata ggcttcgtgt
gctcaggtca tgggaggtgc aggacagctc atgaaacgcc 2880aacaatcgca
caattcatgt caagctaatc agctatttcc tcttcacgag ctgtaattgt
2940cccaaaattc tggtctaccg ggggtgatcc ttcgtgtacg ggcccttccc
tcaaccctag 3000gtatgcgcgc atgcggtcgc cgcgcaactc gcgcgagggc
cgagggtttg ggacgggccg 3060tcccgaaatg cagttgcacc cggatgcgtg
gcaccttttt tgcgataatt tatgcaatgg 3120actgctctgc aaaattctgg
ctctgtcgcc aaccctagga tcagcggcgt aggatttcgt 3180aatcattcgt
cctgatgggg agctaccgac taccctaata tcagcccgac tgcctgacgc
3240cagcgtccac ttttgtgcac acattccatt cgtgcccaag acatttcatt
gtggtgcgaa 3300gcgtccccag ttacgctcac ctgtttcccg acctccttac
tgttctgtcg acagagcggg 3360cccacaggcc ggtcgcagcc actagtatgg
agatcccccc ccactgcctg tgctccccct 3420cccccgcccc ctcccagctg
tactacaaga agaagaagca cgccatcctg cagacccaga 3480ccccctaccg
ctaccgcgtg tcccccacct gcttcgcccc cccccgcctg cgcaagcagc
3540acccctaccc cctgcccgtg ctgtgctacc ccaagctgct gcacttctcc
cagccccgct 3600accccctggt gcgctcccac ctggccgagg ccggcgtggc
ctaccgcccc ggctacgagc 3660tgctgggcaa gatccgcggc gtgtgcttct
acgccgtgac cgccgccgtg gccctgctgc 3720tgttccagtg catgctgctg
ctgcacccct tcgtgctgct gttcgacccc ttcccccgca 3780aggcccacca
caccatcgcc aagctgtggt ccatctgctc cgtgtccctg ttctacaaga
3840tccacatcaa gggcctggag aacctgcccc ccccccactc ccccgccgtg
tacgtgtcca 3900accaccagtc cttcctggac atctacaccc tgctgaccct
gggccgcacc ttcaagttca 3960tctccaagac cgagatcttc ctgtacccca
tcatcggctg ggccatgtac atgctgggca 4020ccatccccct gaagcgcctg
gactcccgct cccagctgga caccctgaag cgctgcatgg 4080acctgatcaa
gaagggcgcc tccgtgttct tcttccccga gggcacccgc tccaaggacg
4140gcaagctggg cgccttcaag aagggcgcct tctccatcgc cgccaagtcc
aaggtgcccg 4200tggtgcccat caccctgatc ggcaccggca agatcatgcc
ccccggctcc gagctgaccg 4260tgaaccccgg caccgtgcag gtgatcatcc
acaagcccat cgagggctcc gacgccgagg 4320ccatgtgcaa cgaggcccgc
gccaccatct cccactccct ggacgactga ttaattaact 4380cgaggcagca
gcagctcgga tagtatcgac acactctgga cgctggtcgt gtgatggact
4440gttgccgcca cacttgctgc cttgacctgt gaatatccct gccgctttta
tcaaacagcc 4500tcagtgtgtt tgatcttgtg tgtacgcgct tttgcgagtt
gctagctgct tgtgctattt 4560gcgaatacca cccccagcat ccccttccct
cgtttcatat cgcttgcatc ccaaccgcaa 4620cttatctacg ctgtcctgct
atccctcagc gctgctcctg ctcctgctca ctgcccctcg 4680cacagccttg
gtttgggctc cgcctgtatt ctcctggtac tgcaacctgt aaaccagcac
4740tgcaatgctg atgcacggga agtagtggga tgggaacaca aatggaaagc
ttgagctcag 4800cggcgacggt cctgctaccg tacgacgttg ggcacgccca
tgaaagtttg tataccgagc 4860ttgttgagcg aactgcaagc gcggctcaag
gatacttgaa ctcctggatt gatatcggtc 4920caataatgga tggaaaatcc
gaacctcgtg caagaactga gcaaacctcg ttacatggat 4980gcacagtcgc
cagtccaatg aacattgaag tgagcgaact gttcgcttcg gtggcagtac
5040tactcaaaga atgagctgct gttaaaaatg cactctcgtt ctctcaagtg
agtggcagat 5100gagtgctcac gccttgcact tcgctgcccg tgtcatgccc
tgcgccccaa aatttgaaaa 5160aagggatgag attattgggc aatggacgac
gtcgtcgctc cgggagtcag gaccggcgga 5220aaataagagg caacacactc
cgcttcttag ctcttcg 5257936714DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 93gctcttcgcc
gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc
gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg aggtctgcct
120tgcgccggct gagccactgc ttcgtccggg cggccaagag gagcatgagg
gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct gggagcgggc
cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac tggtcctcca
gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg tgaggggggt
atgaattgta cagaacaacc acgagccttg tctaggcaga 360atccctacca
gtcatggctt tacctggatg acggcctgcg aacagctgtc cagcgaccct
420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg tgatggcgcg
agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga ggacagtcgg
ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt gccatccttt
gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca caccacctcc
tcccagacca attctgtcac ctttttggcg aaggcatcgg 660cctcggcctg
cagagaggac agcagtgccc agccgctggg ggttggcgga tgcacgctca
720ggtacccttt cttgcgctat gacacttcca gcaaaaggta gggcgggctg
cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc ttcgaccccc
cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct ccagggcgag
cgctgtttaa atagccaggc ccccgattgc 900aaagacatta tagcgagcta
ccaaagccat attcaaacac ctagatcact accacttcta 960cacaggccac
tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc ctcttcgttt
1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc aggccttcct
gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc atgacgaacg
agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa gggctggatg
aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca agtggcacct
gtacttccag tacaacccga acgacaccgt ctgggggacg 1260cccttgttct
ggggccacgc cacgtccgac gacctgacca actgggagga ccagcccatc
1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg gctccatggt
ggtggactac 1380aacaacacct ccggcttctt caacgacacc atcgacccgc
gccagcgctg cgtggccatc 1440tggacctaca acaccccgga gtccgaggag
cagtacatct cctacagcct ggacggcggc 1500tacaccttca ccgagtacca
gaagaacccc gtgctggccg ccaactccac ccagttccgc 1560gacccgaagg
tcttctggta cgagccctcc cagaagtgga tcatgaccgc ggccaagtcc
1620caggactaca agatcgagat ctactcctcc gacgacctga agtcctggaa
gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac cagtacgagt
gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag caagtcctac
tgggtgatgt tcatctccat caaccccggc 1800gccccggccg gcggctcctt
caaccagtac ttcgtcggca gcttcaacgg cacccacttc 1860gaggccttcg
acaaccagtc ccgcgtggtg gacttcggca aggactacta cgccctgcag
1920accttcttca acaccgaccc gacctacggg agcgccctgg gcatcgcgtg
ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac ccctggcgct
cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga gtaccaggcc
aacccggaga cggagctgat caacctgaag 2100gccgagccga tcctgaacat
cagcaacgcc ggcccctgga gccggttcgc caccaacacc 2160acgttgacga
aggccaacag ctacaacgtc gacctgtcca acagcaccgg caccctggag
2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct ccaagtccgt
gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac cccgaggagt
acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt cctggaccgc
gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact tcaccaaccg
catgagcgtg aacaaccagc ccttcaagag cgagaacgac 2460ctgtcctact
acaaggtgta cggcttgctg gaccagaaca tcctggagct gtacttcaac
2520gacggcgacg tcgtgtccac caacacctac ttcatgacca ccgggaacgc
cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg ttctacatcg
acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc agcagctcgg
atagtatcga cacactctgg acgctggtcg 2700tgtgatggac tgttgccgcc
acacttgctg ccttgacctg tgaatatccc tgccgctttt 2760atcaaacagc
ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt tgctagctgc
2820ttgtgctatt tgcgaatacc acccccagca tccccttccc tcgtttcata
tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc tatccctcag
cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt ggtttgggct
ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca ctgcaatgct
gatgcacggg aagtagtggg atgggaacac aaatggagga 3060tcccgcgtct
cgaacagagc gcgcagagga acgctgaagg tctcgcctct gtcgcacctc
3120agcgcggcat acaccacaat aaccacctga cgaatgcgct tggttcttcg
tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg gcgaggtggc
aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca cagcctaggg
atatcgaatt cggccgacag gacgcgcgtc 3300aaaggtgctg gtcgtgtatg
ccctggccgg caggtcgttg ctgctgctgg ttagtgattc 3360cgcaaccctg
attttggcgt cttattttgg cgtggcaaac gctggcgccc gcgagccggg
3420ccggcggcga tgcggtgccc cacggctgcc ggaatccaag ggaggcaaga
gcgcccgggt 3480cagttgaagg gctttacgcg caaggtacag ccgctcctgc
aaggctgcgt ggtggaattg 3540gacgtgcagg tcctgctgaa gttcctccac
cgcctcacca gcggacaaag caccggtgta 3600tcaggtccgt gtcatccact
ctaaagaact cgactacgac ctactgatgg ccctagattc 3660ttcatcaaaa
acgcctgaga cacttgccca ggattgaaac tccctgaagg gaccaccagg
3720ggccctgagt tgttccttcc ccccgtggcg agctgccagc caggctgtac
ctgtgatcga 3780ggctggcggg aaaataggct tcgtgtgctc aggtcatggg
aggtgcagga cagctcatga 3840aacgccaaca atcgcacaat tcatgtcaag
ctaatcagct atttcctctt cacgagctgt 3900aattgtccca aaattctggt
ctaccggggg tgatccttcg tgtacgggcc cttccctcaa 3960ccctaggtat
gcgcgcatgc ggtcgccgcg caactcgcgc gagggccgag ggtttgggac
4020gggccgtccc gaaatgcagt tgcacccgga tgcgtggcac cttttttgcg
ataatttatg 4080caatggactg ctctgcaaaa ttctggctct gtcgccaacc
ctaggatcag cggcgtagga 4140tttcgtaatc attcgtcctg atggggagct
accgactacc ctaatatcag cccgactgcc 4200tgacgccagc gtccactttt
gtgcacacat tccattcgtg cccaagacat ttcattgtgg 4260tgcgaagcgt
ccccagttac gctcacctgt ttcccgacct ccttactgtt ctgtcgacag
4320agcgggccca caggccggtc gcagccacta gtatggcccc cacctccctg
ctggcctcca 4380ccggcgtgtc ctccgcctcc ctgtggtcct ccgcccgctc
ctccgcctgc gccttccccg 4440tggaccacgc cgtgcgcggc gccccccagc
gccccctgcc catgcagcgc cgctgcttcc 4500gcaccgtggc cgtgcgcggg
cgcgccgccg cccccgccgt ggccgtgcgc cccgagcccg 4560cccaggagtt
ctgggagcag ctggagccct gcaagatggc cgaggacaag cgcatcttcc
4620tggaggagca ccgcatccgc ggcaacgagg tgggcccctc ccagcgcctg
accatcaccg 4680ccgtggccaa catcctgcag gaggccgccg gcaaccacgc
cgtggccatg tggggccgct 4740cctccgaggg cttcgccacc gaccccgagc
tgcaggaggc cggcctgatc ttcgtgatga 4800cccgcatgca gatccagatg
taccgctacc cccgctgggg cgacctgatg caggtggaga 4860cctggttcca
gaccgccggc aagctgggcg cccagcgcga gtgggtgctg cgcgacaagc
4920tgaccggcga ggccctgggc gccgccacct cctcctgggt gatgatcaac
atccgcaccc 4980gccgcccctg ccgcatgccc gagctggtgc gcgtgaagtc
cgccttcttc gcccgcgagc 5040ccccccgcct ggccctgccc cccgccgtga
cccgcgccaa gctgcccaac atcgccaccc 5100ccgcccccct gcgcggccac
cgccaggtgg cccgccgcac cgacatggac atgaacggcc 5160acgtgaacaa
cgtggcctac ctggcctggt gcctggaggc cgtgcccgag cacgtgttct
5220ccgactacca cctgtaccag atggagatcg acttcaaggc cgagtgccac
gccggcgacg 5280tgatctcctc ccaggccgag cagatccccc cccaggaggc
cctgacccac aacggcgccg 5340gccgcaaccc ctcctgcttc gtgcactcca
tcctgcgcgc cgagaccgag ctggtgcgcg 5400cccgcaccac ctggtccgcc
cccatcgacg cccccgccgc caagcccccc aaggcctccc 5460acatggacta
caaggaccac gacggcgact acaaggacca cgacatcgac tacaaggacg
5520acgacgacaa gtgaatcgat agatctctta aggcagcagc agctcggata
gtatcgacac 5580actctggacg ctggtcgtgt gatggactgt tgccgccaca
cttgctgcct tgacctgtga 5640atatccctgc cgcttttatc aaacagcctc
agtgtgtttg atcttgtgtg tacgcgcttt 5700tgcgagttgc tagctgcttg
tgctatttgc gaataccacc cccagcatcc ccttccctcg 5760tttcatatcg
cttgcatccc aaccgcaact tatctacgct gtcctgctat ccctcagcgc
5820tgctcctgct cctgctcact gcccctcgca cagccttggt ttgggctccg
cctgtattct 5880cctggtactg caacctgtaa accagcactg caatgctgat
gcacgggaag tagtgggatg 5940ggaacacaaa tggaaagctt aattaagagc
tcttgttttc cagaaggagt tgctccttga 6000gcctttcatt ctcagcctcg
ataacctcca aagccgctct aattgtggag ggggttcgaa 6060tttaaaagct
tggaatgttg gttcgtgcgt ctggaacaag cccagacttg ttgctcactg
6120ggaaaaggac catcagctcc aaaaaacttg ccgctcaaac cgcgtacctc
tgctttcgcg 6180caatctgccc tgttgaaatc gccaccacat tcatattgtg
acgcttgagc agtctgtaat 6240tgcctcagaa tgtggaatca tctgccccct
gtgcgagccc atgccaggca tgtcgcgggc 6300gaggacaccc gccactcgta
cagcagacca ttatgctacc tcacaatagt tcataacagt 6360gaccatattt
ctcgaagctc cccaacgagc acctccatgc tctgagtggc caccccccgg
6420ccctggtgct tgcggagggc aggtcaaccg gcatggggct accgaaatcc
ccgaccggat 6480cccaccaccc ccgcgatggg aagaatctct ccccgggatg
tgggcccacc accagcacaa 6540cctgctggcc caggcgagcg tcaaaccata
ccacacaaat atccttggca tcggccctga 6600attccttctg ccgctctgct
acccggtgct tctgtccgaa gcaggggttg ctagggatcg 6660ctccgagtcc
gcaaaccctt gtcgcgtggc ggggcttgtt cgagcttgaa gagc
6714945279DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 94gctcttcggg tcgccgcgct gcctcgcgtc
ccctggtggt gcgcgcggtc gccagcgagg 60ccccgctggg cgttccgccc tcggtgcagc
gcccctcccc cgtggtctac tccaagctgg 120acaagcagca ccgcctgacg
cccgagcgcc tggagctggt gcagagcatg gggcagtttg 180cggaggagag
ggtgctgccc gtgctgcacc ccgtggacaa gctgtggcag ccgcaggact
240ttttgcccga ccccgagtcg cccgacttcg aggatcaggt ggcggagctg
cgcgcgcgcg 300ccaaggacct gcccgacgag tactttgtgg tgctggtggg
ggacatgatc acggaggagg 360cgctgccgac ctacatggcc atgctcaaca
cgctggacgg cgtgcgcgac gacacgggcg 420cggccgacca cccgtgggcg
cgctggacgc ggcagtgggt ggccgaggag aaccggcacg 480gcgacctgct
gaacaagtac tgctggctga cggggcgcgt caacatgcgg gccgtggagg
540tgaccatcaa caacctgatc aagagcggca tgaacccgca gacggacaac
aacccttatt 600tggggttcgt ctacacctcc ttccaggagc gcgccaccaa
gtaggtaccc tttcttgcgc 660tatgacactt ccagcaaaag gtagggcggg
ctgcgagacg gcttcccggc gctgcatgca 720acaccgatga tgcttcgacc
ccccgaagct ccttcggggc tgcatgggcg ctccgatgcc 780gctccagggc
gagcgctgtt taaatagcca ggcccccgat tgcaaagaca ttatagcgag
840ctaccaaagc catattcaaa cacctagatc actaccactt ctacacaggc
cactcgagct 900tgtgatcgca ctccgctaag ggggcgcctc ttcctcttcg
tttcagtcac aacccgcaaa 960cggcgcgcca tgctgctgca ggccttcctg
ttcctgctgg ccggcttcgc cgccaagatc 1020agcgcctcca tgacgaacga
gacgtccgac cgccccctgg tgcacttcac ccccaacaag 1080ggctggatga
acgaccccaa cggcctgtgg tacgacgaga aggacgccaa gtggcacctg
1140tacttccagt acaacccgaa cgacaccgtc tgggggacgc ccttgttctg
gggccacgcc 1200acgtccgacg acctgaccaa ctgggaggac cagcccatcg
ccatcgcccc gaagcgcaac 1260gactccggcg ccttctccgg ctccatggtg
gtggactaca acaacacctc cggcttcttc 1320aacgacacca tcgacccgcg
ccagcgctgc gtggccatct ggacctacaa caccccggag 1380tccgaggagc
agtacatctc ctacagcctg gacggcggct acaccttcac cgagtaccag
1440aagaaccccg tgctggccgc caactccacc cagttccgcg acccgaaggt
cttctggtac 1500gagccctccc agaagtggat catgaccgcg gccaagtccc
aggactacaa gatcgagatc 1560tactcctccg acgacctgaa gtcctggaag
ctggagtccg cgttcgccaa cgagggcttc 1620ctcggctacc agtacgagtg
ccccggcctg atcgaggtcc ccaccgagca ggaccccagc 1680aagtcctact
gggtgatgtt catctccatc aaccccggcg ccccggccgg cggctccttc
1740aaccagtact tcgtcggcag cttcaacggc acccacttcg aggccttcga
caaccagtcc 1800cgcgtggtgg acttcggcaa ggactactac gccctgcaga
ccttcttcaa caccgacccg 1860acctacggga gcgccctggg catcgcgtgg
gcctccaact gggagtactc cgccttcgtg 1920cccaccaacc cctggcgctc
ctccatgtcc ctcgtgcgca agttctccct caacaccgag 1980taccaggcca
acccggagac ggagctgatc aacctgaagg ccgagccgat cctgaacatc
2040agcaacgccg gcccctggag ccggttcgcc accaacacca cgttgacgaa
ggccaacagc 2100tacaacgtcg acctgtccaa cagcaccggc accctggagt
tcgagctggt gtacgccgtc 2160aacaccaccc agacgatctc caagtccgtg
ttcgcggacc tctccctctg gttcaagggc 2220ctggaggacc ccgaggagta
cctccgcatg ggcttcgagg tgtccgcgtc ctccttcttc 2280ctggaccgcg
ggaacagcaa ggtgaagttc gtgaaggaga acccctactt caccaaccgc
2340atgagcgtga acaaccagcc cttcaagagc gagaacgacc tgtcctacta
caaggtgtac 2400ggcttgctgg accagaacat cctggagctg tacttcaacg
acggcgacgt cgtgtccacc 2460aacacctact tcatgaccac cgggaacgcc
ctgggctccg tgaacatgac gacgggggtg 2520gacaacctgt tctacatcga
caagttccag gtgcgcgagg tcaagtgaca attggcagca 2580gcagctcgga
tagtatcgac acactctgga cgctggtcgt gtgatggact gttgccgcca
2640cacttgctgc cttgacctgt gaatatccct gccgctttta tcaaacagcc
tcagtgtgtt 2700tgatcttgtg tgtacgcgct tttgcgagtt gctagctgct
tgtgctattt gcgaatacca 2760cccccagcat ccccttccct cgtttcatat
cgcttgcatc ccaaccgcaa cttatctacg 2820ctgtcctgct atccctcagc
gctgctcctg ctcctgctca ctgcccctcg cacagccttg 2880gtttgggctc
cgcctgtatt ctcctggtac tgcaacctgt aaaccagcac tgcaatgctg
2940atgcacggga agtagtggga tgggaacaca aatggaggat cccgcgtctc
gaacagagcg 3000cgcagaggaa cgctgaaggt ctcgcctctg tcgcacctca
gcgcggcata caccacaata 3060accacctgac gaatgcgctt ggttcttcgt
ccattagcga agcgtccggt tcacacacgt 3120gccacgttgg cgaggtggca
ggtgacaatg atcggtggag ctgatggtcg aaacgttcac 3180agcctaggga
tatcgaattc ctttcttgcg ctatgacact tccagcaaaa ggtagggcgg
3240gctgcgagac ggcttcccgg cgctgcatgc aacaccgatg atgcttcgac
cccccgaagc 3300tccttcgggg ctgcatgggc gctccgatgc cgctccaggg
cgagcgctgt ttaaatagcc 3360aggcccccga ttgcaaagac attatagcga
gctaccaaag ccatattcaa acacctagat 3420cactaccact tctacacagg
ccactcgagc ttgtgatcgc actccgctaa gggggcgcct 3480cttcctcttc
gtttcagtca caacccgcaa acactagtgc gctggacgcg gcagtgggtg
3540gccgaggaga accggcacgg cgacctgctg aacaagtact gttggctgac
ggggcgcgtc 3600aacatgcggg ccgtggaggt gaccatcaac aacctgatca
agagcggcat gaacccgcag 3660acggacaaca acccttactt gggcttcgtc
tacacctcct tccaggagcg cgcgaccaag 3720tacagccacg gcaacaccgc
gcgccttgcg gccgagcagt gtgtttgagg gttttggttg 3780cccgtatcga
ggtcctggtg gcgcgcatgg gggagaaggc gcctgtcccg ctgacccccc
3840cggctaccct cccggcacct tccagggcgc gtacgggatc ctgctcggcc
gcaaggcgcg 3900cggtgttgcc gtggctgtac ttggtcgcgc gctcctggaa
ggaggtgtag acgaagccca 3960agtaagggtt gttgtccgtc tgcgggttca
tgccgctctt gatcaggttg ttgatggtca 4020cctccacggc ccgcatgttg
acgcgccccg tcagccaaca gtacttgttc agcaggtcgc 4080cgtgccggtt
ctcctcggcc acccactgcc gcgtccagcg caagcttgca gcagcagctc
4140ggatagtatc gacacactct ggacgctggt cgtgtgatgg actgttgccg
ccacacttgc 4200tgccttgacc tgtgaatatc cctgccgctt ttatcaaaca
gcctcagtgt gtttgatctt 4260gtgtgtacgc gcttttgcga gttgctagct
gcttgtgcta tttgcgaata ccacccccag 4320catccccttc cctcgtttca
tatcgcttgc atcccaaccg caacttatct acgctgtcct 4380gctatccctc
agcgctgctc ctgctcctgc tcactgcccc tcgcacagcc ttggtttggg
4440ctccgcctgt attctcctgg tactgcaacc tgtaaaccag cactgcaatg
ctgatgcacg 4500ggaagtagtg ggatgggaac acaaatggaa agctggagct
ccagccacgg caacaccgcg 4560cgccttgcgg ccgagcacgg cgacaagaac
ctgagcaaga tctgcgggct gatcgccagc 4620gacgagggcc ggcacgagat
cgcctacacg cgcatcgtgg acgagttctt ccgcctcgac 4680cccgagggcg
ccgtcgccgc ctacgccaac atgatgcgca agcagatcac catgcccgcg
4740cacctcatgg acgacatggg ccacggcgag gccaacccgg gccgcaacct
cttcgccgac 4800ttctccgcgg tcgccgagaa gatcgacgtc tacgacgccg
aggactactg ccgcatcctg 4860gagcacctca acgcgcgctg gaaggtggac
gagcgccagg tcagcggcca ggccgccgcg 4920gaccaggagt acgtcctggg
cctgccccag cgcttccgga aactcgccga gaagaccgcc 4980gccaagcgca
agcgcgtcgc gcgcaggccc gtcgccttct cctggatctc cgggcgcgag
5040atcatggtct agggagcgac gagtgtgcgt
gcggggctgg cgggagtggg acgccctcct 5100cgctcctctc tgttctgaac
ggaacaatcg gccaccccgc gctacgcgcc acgcatcgag 5160caacgaagaa
aaccccccga tgataggttg cggtggctgc cgggatatag atccggccgc
5220acatcaaagg gcccctccgc cagagaagaa gctcctttcc cagcagactc
ctgaagagc 5279956580DNAArtificial SequenceDescription of Artificial
Sequence Synthetic polynucleotide 95gctcttcgcc gccgccactc
ctgctcgagc gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc gcgctcgtgc
gcgtcgctga tgtccatcac caggtccatg aggtctgcct 120tgcgccggct
gagccactgc ttcgtccggg cggccaagag gagcatgagg gaggactcct
180ggtccagggt cctgacgtgg tcgcggctct gggagcgggc cagcatcatc
tggctctgcc 240gcaccgaggc cgcctccaac tggtcctcca gcagccgcag
tcgccgccga ccctggcaga 300ggaagacagg tgaggggtgt atgaattgta
cagaacaacc acgagccttg tctaggcaga 360atccctacca gtcatggctt
tacctggatg acggcctgcg aacagctgtc cagcgaccct 420cgctgccgcc
gcttctcccg cacgcttctt tccagcaccg tgatggcgcg agccagcgcc
480gcacgctggc gctgcgcttc gccgatctga ggacagtcgg ggaactctga
tcagtctaaa 540cccccttgcg cgttagtgtt gccatccttt gcagaccggt
gagagccgac ttgttgtgcg 600ccacccccca caccacctcc tcccagacca
attctgtcac ctttttggcg aaggcatcgg 660cctcggcctg cagagaggac
agcagtgccc agccgctggg ggttggcgga tgcacgctca 720ggtacccttt
cttgcgctat gacacttcca gcaaaaggta gggcgggctg cgagacggct
780tcccggcgct gcatgcaaca ccgatgatgc ttcgaccccc cgaagctcct
tcggggctgc 840atgggcgctc cgatgccgct ccagggcgag cgctgtttaa
atagccaggc ccccgattgc 900aaagacatta tagcgagcta ccaaagccat
attcaaacac ctagatcact accacttcta 960cacaggccac tcgagcttgt
gatcgcactc cgctaagggg gcgcctcttc ctcttcgttt 1020cagtcacaac
ccgcaaactc tagaatatca atgatcgagc aggacggcct ccacgccggc
1080tcccccgccg cctgggtgga gcgcctgttc ggctacgact gggcccagca
gaccatcggc 1140tgctccgacg ccgccgtgtt ccgcctgtcc gcccagggcc
gccccgtgct gttcgtgaag 1200accgacctgt ccggcgccct gaacgagctg
caggacgagg ccgcccgcct gtcctggctg 1260gccaccaccg gcgtgccctg
cgccgccgtg ctggacgtgg tgaccgaggc cggccgcgac 1320tggctgctgc
tgggcgaggt gcccggccag gacctgctgt cctcccacct ggcccccgcc
1380gagaaggtgt ccatcatggc cgacgccatg cgccgcctgc acaccctgga
ccccgccacc 1440tgccccttcg accaccaggc caagcaccgc atcgagcgcg
cccgcacccg catggaggcc 1500ggcctggtgg accaggacga cctggacgag
gagcaccagg gcctggcccc cgccgagctg 1560ttcgcccgcc tgaaggcccg
catgcccgac ggcgaggacc tggtggtgac ccacggcgac 1620gcctgcctgc
ccaacatcat ggtggagaac ggccgcttct ccggcttcat cgactgcggc
1680cgcctgggcg tggccgaccg ctaccaggac atcgccctgg ccacccgcga
catcgccgag 1740gagctgggcg gcgagtgggc cgaccgcttc ctggtgctgt
acggcatcgc cgcccccgac 1800tcccagcgca tcgccttcta ccgcctgctg
gacgagttct tctgacaatt ggcagcagca 1860gctcggatag tatcgacaca
ctctggacgc tggtcgtgtg atggactgtt gccgccacac 1920ttgctgcctt
gacctgtgaa tatccctgcc gcttttatca aacagcctca gtgtgtttga
1980tcttgtgtgt acgcgctttt gcgagttgct agctgcttgt gctatttgcg
aataccaccc 2040ccagcatccc cttccctcgt ttcatatcgc ttgcatccca
accgcaactt atctacgctg 2100tcctgctatc cctcagcgct gctcctgctc
ctgctcactg cccctcgcac agccttggtt 2160tgggctccgc ctgtattctc
ctggtactgc aacctgtaaa ccagcactgc aatgctgatg 2220cacgggaagt
agtgggatgg gaacacaaat ggaggatccc gcgtctcgaa cagagcgcgc
2280agaggaacgc tgaaggtctc gcctctgtcg cacctcagcg cggcatacac
cacaataacc 2340acctgacgaa tgcgcttggt tcttcgtcca ttagcgaagc
gtccggttca cacacgtgcc 2400acgttggcga ggtggcaggt gacaatgatc
ggtggagctg atggtcgaaa cgttcacagc 2460ctagggatat cctgaagaat
gggaggcagg tgttgttgat tatgagtgtg taaaagaaag 2520gggtagagag
ccgtcctcag atccgactac tatgcaggta gccgctcgcc catgcccgcc
2580tggctgaata ttgatgcatg cccatcaagg caggcaggca tttctgtgca
cgcaccaagc 2640ccacaatctt ccacaacaca cagcatgtac caacgcacgc
gtaaaagttg gggtgctgcc 2700agtgcgtcat gccaggcatg atgtgctcct
gcacatccgc catgatctcc tccatcgtct 2760cgggtgtttc cggcgcctgg
tccgggagcc gttccgccag atacccagac gccacctccg 2820acctcacggg
gtacttttcg agcgtctgcc ggtagtcgac gatcgcgtcc accatggagt
2880agccgaggcg ccggaactgg cgtgacggag ggaggagagg gaggagagag
aggggggggg 2940ggggggggga tgattacacg ccagtctcac aacgcatgca
agacccgttt gattatgagt 3000acaatcatgc actactagat ggatgagcgc
caggcataag gcacaccgac gttgatggca 3060tgagcaactc ccgcatcata
tttcctattg tcctcacgcc aagccggtca ccatccgcat 3120gctcatatta
cagcgcacgc accgcttcgt gatccaccgg gtgaacgtag tcctcgacgg
3180aaacatctgg ctcgggcctc gtgctggcac tccctcccat gccgacaacc
tttctgctgt 3240caccacgacc cacgatgcaa cgcgacacga cccggtggga
ctgatcggtt cactgcacct 3300gcatgcaatt gtcacaagcg catactccaa
tcgtatccgt ttgatttctg tgaaaactcg 3360ctcgaccgcc cgcgtcccgc
aggcagcgat gacgtgtgcg tgacctgggt gtttcgtcga 3420aaggccagca
accccaaatc gcaggcgatc cggagattgg gatctgatcc gagcttggac
3480cagatccccc acgatgcggc acgggaactg catcgactcg gcgcggaacc
cagctttcgt 3540aaatgccaga ttggtgtccg ataccttgat ttgccatcag
cgaaacaaga cttcagcagc 3600gagcgtattt ggcgggcgtg ctaccagggt
tgcatacatt gcccatttct gtctggaccg 3660ctttaccggc gcagagggtg
agttgatggg gttggcaggc atcgaaacgc gcgtgcatgg 3720tgtgtgtgtc
tgttttcggc tgcacaattt caatagtcgg atgggcgacg gtagaattgg
3780gtgttgcgct cgcgtgcatg cctcgccccg tcgggtgtca tgaccgggac
tggaatcccc 3840cctcgcgacc ctcctgctaa cgctcccgac tctcccgccc
gcgcgcagga tagactctag 3900ttcaaccaat cgacaactag tatgcagacc
gcccaccagc gcccccccac cgagggccac 3960tgcttcggcg cccgcctgcc
caccgcctcc cgccgcgccg tgcgccgcgc ctggtcccgc 4020atcgcccgcg
ggcgcgccgc cgccgccgcc gacgccaacc ccgcccgccc cgagcgccgc
4080gtggtgatca ccggccaggg cgtggtgacc tccctgggcc agaccatcga
gcagttctac 4140tcctccctgc tggagggcgt gtccggcatc tcccagatcc
agaagttcga caccaccggc 4200tacaccacca ccatcgccgg cgagatcaag
tccctgcagc tggaccccta cgtgcccaag 4260cgctgggcca agcgcgtgga
cgacgtgatc aagtacgtgt acatcgccgg caagcaggcc 4320ctggagtccg
ccggcctgcc catcgaggcc gccggcctgg ccggcgccgg cctggacccc
4380gccctgtgcg gcgtgctgat cggcaccgcc atggccggca tgacctcctt
cgccgccggc 4440gtggaggccc tgacccgcgg cggcgtgcgc aagatgaacc
ccttctgcat ccccttctcc 4500atctccaaca tgggcggcgc catgctggcc
atggacatcg gcttcatggg ccccaactac 4560tccatctcca ccgcctgcgc
caccggcaac tactgcatcc tgggcgccgc cgaccacatc 4620cgccgcggcg
acgccaacgt gatgctggcc ggcggcgccg acgccgccat catcccctcc
4680ggcatcggcg gcttcatcgc ctgcaaggcc ctgtccaagc gcaacgacga
gcccgagcgc 4740gcctcccgcc cctgggacgc cgaccgcgac ggcttcgtga
tgggcgaggg cgccggcgtg 4800ctggtgctgg aggagctgga gcacgccaag
cgccgcggcg ccaccatcct ggccgagctg 4860gtgggcggcg ccgccacctc
cgacgcccac cacatgaccg agcccgaccc ccagggccgc 4920ggcgtgcgcc
tgtgcctgga gcgcgccctg gagcgcgccc gcctggcccc cgagcgcgtg
4980ggctacgtga acgcccacgg cacctccacc cccgccggcg acgtggccga
gtaccgcgcc 5040atccgcgccg tgatccccca ggactccctg cgcatcaact
ccaccaagtc catgatcggc 5100cacctgctgg gcggcgccgg cgccgtggag
gccgtggccg ccatccaggc cctgcgcacc 5160ggctggctgc accccaacct
gaacctggag aaccccgccc ccggcgtgga ccccgtggtg 5220ctggtgggcc
cccgcaagga gcgcgccgag gacctggacg tggtgctgtc caactccttc
5280ggcttcggcg gccacaactc ctgcgtgatc ttccgcaagt acgacgagat
ggactacaag 5340gaccacgacg gcgactacaa ggaccacgac atcgactaca
aggacgacga cgacaagtga 5400atcgatagat ctcttaaggc agcagcagct
cggatagtat cgacacactc tggacgctgg 5460tcgtgtgatg gactgttgcc
gccacacttg ctgccttgac ctgtgaatat ccctgccgct 5520tttatcaaac
agcctcagtg tgtttgatct tgtgtgtacg cgcttttgcg agttgctagc
5580tgcttgtgct atttgcgaat accaccccca gcatcccctt ccctcgtttc
atatcgcttg 5640catcccaacc gcaacttatc tacgctgtcc tgctatccct
cagcgctgct cctgctcctg 5700ctcactgccc ctcgcacagc cttggtttgg
gctccgcctg tattctcctg gtactgcaac 5760ctgtaaacca gcactgcaat
gctgatgcac gggaagtagt gggatgggaa cacaaatgga 5820aagcttaatt
aagagctctt gttttccaga aggagttgct ccttgagcct ttcattctca
5880gcctcgataa cctccaaagc cgctctaatt gtggaggggg ttcgaattta
aaagcttgga 5940atgttggttc gtgcgtctgg aacaagccca gacttgttgc
tcactgggaa aaggaccatc 6000agctccaaaa aacttgccgc tcaaaccgcg
tacctctgct ttcgcgcaat ctgccctgtt 6060gaaatcgcca ccacattcat
attgtgacgc ttgagcagtc tgtaattgcc tcagaatgtg 6120gaatcatctg
ccccctgtgc gagcccatgc caggcatgtc gcgggcgagg acacccgcca
6180ctcgtacagc agaccattat gctacctcac aatagttcat aacagtgacc
atatttctcg 6240aagctcccca acgagcacct ccatgctctg agtggccacc
ccccggccct ggtgcttgcg 6300gagggcaggt caaccggcat ggggctaccg
aaatccccga ccggatccca ccacccccgc 6360gatgggaaga atctctcccc
gggatgtggg cccaccacca gcacaacctg ctggcccagg 6420cgagcgtcaa
accataccac acaaatatcc ttggcatcgg ccctgaattc cttctgccgc
6480tctgctaccc ggtgcttctg tccgaagcag gggttgctag ggatcgctcc
gagtccgcaa 6540acccttgtcg cgtggcgggg cttgttcgag cttgaagagc
6580968087DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 96gctcttcgga gtcactgtgc cactgagttc
gactggtagc tgaatggagt cgctgctcca 60ctaaacgaat tgtcagcacc gccagccggc
cgaggacccg agtcatagcg agggtagtag 120cgcgccatgg caccgaccag
cctgcttgcc agtactggcg tctcttccgc ttctctgtgg 180tcctctgcgc
gctccagcgc gtgcgctttt ccggtggatc atgcggtccg tggcgcaccg
240cagcggccgc tgcccatgca gcgccgctgc ttccgaacag tggcggtcag
ggccgcaccc 300gcggtagccg tccgtccgga acccgcccaa gagttttggg
agcagcttga gccctgcaag 360atggcggagg acaagcgcat cttcctggag
gagcaccggt gcgtggaggt ccggggctga 420ccggccgtcg cattcaacgt
aatcaatcgc atgatgatca gaggacacga agtcttggtg 480gcggtggcca
gaaacactgt ccattgcaag ggcataggga tgcgttcctt cacctctcat
540ttctcatttc tgaatccctc cctgctcact ctttctcctc ctccttcccg
ttcacgcagc 600attcggggta ccctttcttg cgctatgaca cttccagcaa
aaggtagggc gggctgcgag 660acggcttccc ggcgctgcat gcaacaccga
tgatgcttcg accccccgaa gctccttcgg 720ggctgcatgg gcgctccgat
gccgctccag ggcgagcgct gtttaaatag ccaggccccc 780gattgcaaag
acattatagc gagctaccaa agccatattc aaacacctag atcactacca
840cttctacaca ggccactcga gcttgtgatc gcactccgct aagggggcgc
ctcttcctct 900tcgtttcagt cacaacccgc aaacggcgcg ccatgctgct
gcaggccttc ctgttcctgc 960tggccggctt cgccgccaag atcagcgcct
ccatgacgaa cgagacgtcc gaccgccccc 1020tggtgcactt cacccccaac
aagggctgga tgaacgaccc caacggcctg tggtacgacg 1080agaaggacgc
caagtggcac ctgtacttcc agtacaaccc gaacgacacc gtctggggga
1140cgcccttgtt ctggggccac gccacgtccg acgacctgac caactgggag
gaccagccca 1200tcgccatcgc cccgaagcgc aacgactccg gcgccttctc
cggctccatg gtggtggact 1260acaacaacac ctccggcttc ttcaacgaca
ccatcgaccc gcgccagcgc tgcgtggcca 1320tctggaccta caacaccccg
gagtccgagg agcagtacat ctcctacagc ctggacggcg 1380gctacacctt
caccgagtac cagaagaacc ccgtgctggc cgccaactcc acccagttcc
1440gcgacccgaa ggtcttctgg tacgagccct cccagaagtg gatcatgacc
gcggccaagt 1500cccaggacta caagatcgag atctactcct ccgacgacct
gaagtcctgg aagctggagt 1560ccgcgttcgc caacgagggc ttcctcggct
accagtacga gtgccccggc ctgatcgagg 1620tccccaccga gcaggacccc
agcaagtcct actgggtgat gttcatctcc atcaaccccg 1680gcgccccggc
cggcggctcc ttcaaccagt acttcgtcgg cagcttcaac ggcacccact
1740tcgaggcctt cgacaaccag tcccgcgtgg tggacttcgg caaggactac
tacgccctgc 1800agaccttctt caacaccgac ccgacctacg ggagcgccct
gggcatcgcg tgggcctcca 1860actgggagta ctccgccttc gtgcccacca
acccctggcg ctcctccatg tccctcgtgc 1920gcaagttctc cctcaacacc
gagtaccagg ccaacccgga gacggagctg atcaacctga 1980aggccgagcc
gatcctgaac atcagcaacg ccggcccctg gagccggttc gccaccaaca
2040ccacgttgac gaaggccaac agctacaacg tcgacctgtc caacagcacc
ggcaccctgg 2100agttcgagct ggtgtacgcc gtcaacacca cccagacgat
ctccaagtcc gtgttcgcgg 2160acctctccct ctggttcaag ggcctggagg
accccgagga gtacctccgc atgggcttcg 2220aggtgtccgc gtcctccttc
ttcctggacc gcgggaacag caaggtgaag ttcgtgaagg 2280agaaccccta
cttcaccaac cgcatgagcg tgaacaacca gcccttcaag agcgagaacg
2340acctgtccta ctacaaggtg tacggcttgc tggaccagaa catcctggag
ctgtacttca 2400acgacggcga cgtcgtgtcc accaacacct acttcatgac
caccgggaac gccctgggct 2460ccgtgaacat gacgacgggg gtggacaacc
tgttctacat cgacaagttc caggtgcgcg 2520aggtcaagtg acaattggca
gcagcagctc ggatagtatc gacacactct ggacgctggt 2580cgtgtgatgg
actgttgccg ccacacttgc tgccttgacc tgtgaatatc cctgccgctt
2640ttatcaaaca gcctcagtgt gtttgatctt gtgtgtacgc gcttttgcga
gttgctagct 2700gcttgtgcta tttgcgaata ccacccccag catccccttc
cctcgtttca tatcgcttgc 2760atcccaaccg caacttatct acgctgtcct
gctatccctc agcgctgctc ctgctcctgc 2820tcactgcccc tcgcacagcc
ttggtttggg ctccgcctgt attctcctgg tactgcaacc 2880tgtaaaccag
cactgcaatg ctgatgcacg ggaagtagtg ggatgggaac acaaatggag
2940gatcccgcgt ctcgaacaga gcgcgcagag gaacgctgaa ggtctcgcct
ctgtcgcacc 3000tcagcgcggc atacaccaca ataaccacct gacgaatgcg
cttggttctt cgtccattag 3060cgaagcgtcc ggttcacaca cgtgccacgt
tggcgaggtg gcaggtgaca atgatcggtg 3120gagctgatgg tcgaaacgtt
cacagcctag ggatatcgaa ttcggccgac aggacgcgcg 3180tcaaaggtgc
tggtcgtgta tgccctggcc ggcaggtcgt tgctgctgct ggttagtgat
3240tccgcaaccc tgattttggc gtcttatttt ggcgtggcaa acgctggcgc
ccgcgagccg 3300ggccggcggc gatgcggtgc cccacggctg ccggaatcca
agggaggcaa gagcgcccgg 3360gtcagttgaa gggctttacg cgcaaggtac
agccgctcct gcaaggctgc gtggtggaat 3420tggacgtgca ggtcctgctg
aagttcctcc accgcctcac cagcggacaa agcaccggtg 3480tatcaggtcc
gtgtcatcca ctctaaagaa ctcgactacg acctactgat ggccctagat
3540tcttcatcaa aaacgcctga gacacttgcc caggattgaa actccctgaa
gggaccacca 3600ggggccctga gttgttcctt ccccccgtgg cgagctgcca
gccaggctgt acctgtgatc 3660gaggctggcg ggaaaatagg cttcgtgtgc
tcaggtcatg ggaggtgcag gacagctcat 3720gaaacgccaa caatcgcaca
attcatgtca agctaatcag ctatttcctc ttcacgagct 3780gtaattgtcc
caaaattctg gtctaccggg ggtgatcctt cgtgtacggg cccttccctc
3840aaccctaggt atgcgcgcat gcggtcgccg cgcaactcgc gcgagggccg
agggtttggg 3900acgggccgtc ccgaaatgca gttgcacccg gatgcgtggc
accttttttg cgataattta 3960tgcaatggac tgctctgcaa aattctggct
ctgtcgccaa ccctaggatc agcggcgtag 4020gatttcgtaa tcattcgtcc
tgatggggag ctaccgacta ccctaatatc agcccgactg 4080cctgacgcca
gcgtccactt ttgtgcacac attccattcg tgcccaagac atttcattgt
4140ggtgcgaagc gtccccagtt acgctcacct gtttcccgac ctccttactg
ttctgtcgac 4200agagcgggcc cacaggccgg tcgcagccac tagtatggcc
accgcatcca ctttctcggc 4260gttcaatgcc cgctgcggcg acctgcgtcg
ctcggcgggc tccgggcccc ggcgcccagc 4320gaggcccctc cccgtgcgcg
ggcgcgccgc cgccgccgcc gacgccaacc ccgcccgccc 4380cgagcgccgc
gtggtgatca ccggccaggg cgtggtgacc tccctgggcc agaccatcga
4440gcagttctac tcctccctgc tggagggcgt gtccggcatc tcccagatcc
agaagttcga 4500caccaccggc tacaccacca ccatcgccgg cgagatcaag
tccctgcagc tggaccccta 4560cgtgcccaag cgctgggcca agcgcgtgga
cgacgtgatc aagtacgtgt acatcgccgg 4620caagcaggcc ctggagtccg
ccggcctgcc catcgaggcc gccggcctgg ccggcgccgg 4680cctggacccc
gccctgtgcg gcgtgctgat cggcaccgcc atggccggca tgacctcctt
4740cgccgccggc gtggaggccc tgacccgcgg cggcgtgcgc aagatgaacc
ccttctgcat 4800ccccttctcc atctccaaca tgggcggcgc catgctggcc
atggacatcg gcttcatggg 4860ccccaactac tccatctcca ccgcctgcgc
caccggcaac tactgcatcc tgggcgccgc 4920cgaccacatc cgccgcggcg
acgccaacgt gatgctggcc ggcggcgccg acgccgccat 4980catcccctcc
ggcatcggcg gcttcatcgc ctgcaaggcc ctgtccaagc gcaacgacga
5040gcccgagcgc gcctcccgcc cctgggacgc cgaccgcgac ggcttcgtga
tgggcgaggg 5100cgccggcgtg ctggtgctgg aggagctgga gcacgccaag
cgccgcggcg ccaccatcct 5160ggccgagctg gtgggcggcg ccgccacctc
cgacgcccac cacatgaccg agcccgaccc 5220ccagggccgc ggcgtgcgcc
tgtgcctgga gcgcgccctg gagcgcgccc gcctggcccc 5280cgagcgcgtg
ggctacgtga acgcccacgg cacctccacc cccgccggcg acgtggccga
5340gtaccgcgcc atccgcgccg tgatccccca ggactccctg cgcatcaact
ccaccaagtc 5400catgatcggc cacctgctgg gcggcgccgg cgccgtggag
gccgtggccg ccatccaggc 5460cctgcgcacc ggctggctgc accccaacct
gaacctggag aaccccgccc ccggcgtgga 5520ccccgtggtg ctggtgggcc
cccgcaagga gcgcgccgag gacctggacg tggtgctgtc 5580caactccttc
ggcttcggcg gccacaactc ctgcgtgatc ttccgcaagt acgacgagat
5640ggactacaag gaccacgacg gcgactacaa ggaccacgac atcgactaca
aggacgacga 5700cgacaagtga atcgatagat ctcttaaggc agcagcagct
cggatagtat cgacacactc 5760tggacgctgg tcgtgtgatg gactgttgcc
gccacacttg ctgccttgac ctgtgaatat 5820ccctgccgct tttatcaaac
agcctcagtg tgtttgatct tgtgtgtacg cgcttttgcg 5880agttgctagc
tgcttgtgct atttgcgaat accaccccca gcatcccctt ccctcgtttc
5940atatcgcttg catcccaacc gcaacttatc tacgctgtcc tgctatccct
cagcgctgct 6000cctgctcctg ctcactgccc ctcgcacagc cttggtttgg
gctccgcctg tattctcctg 6060gtactgcaac ctgtaaacca gcactgcaat
gctgatgcac gggaagtagt gggatgggaa 6120cacaaatgga aagcttaatt
aagagctcct ttcttgcgct atgacacttc cagcaaaagg 6180tagggcgggc
tgcgagacgg cttcccggcg ctgcatgcaa caccgatgat gcttcgaccc
6240cccgaagctc cttcggggct gcatgggcgc tccgatgccg ctccagggcg
agcgctgttt 6300aaatagccag gcccccgatt gcaaagacat tatagcgagc
taccaaagcc atattcaaac 6360acctagatca ctaccacttc tacacaggcc
actcgagctt gtgatcgcac tccgctaagg 6420gggcgcctct tcctcttcgt
ttcagtcaca acccgcaaac actagtatgg ctatcaagac 6480gaacaggcag
cctgtggaga agcctccgtt cacgatcggg acgctgcgca aggccatccc
6540cgcgcactgt ttcgagcgct cggcgcttcg tagcagcatg tacctggcct
ttgacatcgc 6600ggtcatgtcc ctgctctacg tcgcgtcgac gtacatcgac
cctgcaccgg tgcctacgtg 6660ggtcaagtac ggcatcatgt ggccgctcta
ctggttcttc caggtgtgtt tgagggtttt 6720ggttgcccgt attgaggtcc
tggtggcgcg catggaggag aaggcgcctg tcccgctgac 6780ccccccggct
accctcccgg caccttccag ggcgcgtacg ggaagaacca gtagagcggc
6840cacatgatgc cgtacttgac ccacgtaggc accggtgcag ggtcgatgta
cgtcgacgcg 6900acgtagagca gggacatgac cgcgatgtca aaggccaggt
acatgctgct acgaagcgcc 6960gagcgctcga aacagtgcgc ggggatggcc
ttgcgcagcg tcccgatcgt gaacggaggc 7020ttctccacag gctgcctgtt
cgtcttgata gccatctcga ggcagcagca gctcggatag 7080tatcgacaca
ctctggacgc tggtcgtgtg atggactgtt gccgccacac ttgctgcctt
7140gacctgtgaa tatccctgcc gcttttatca aacagcctca gtgtgtttga
tcttgtgtgt 7200acgcgctttt gcgagttgct agctgcttgt gctatttgcg
aataccaccc ccagcatccc 7260cttccctcgt ttcatatcgc ttgcatccca
accgcaactt atctacgctg tcctgctatc 7320cctcagcgct gctcctgctc
ctgctcactg cccctcgcac agccttggtt tgggctccgc 7380ctgtattctc
ctggtactgc aacctgtaaa ccagcactgc aatgctgatg cacgggaagt
7440agtgggatgg gaacacaaat ggaaagctgt attgttttcc agaaggagtt
gctccttgag 7500cctttcattc tcagcctcga taacctccaa agccgctcta
attgtggagg gggttcgaag 7560acagggtggt tggctggatg gggaaacgct
ggtcgcggga ttcgatcctg ctgcttatat 7620cctccctgga agcacaccca
cgactctgaa gaagaaaacg tgcacacaca caacccaacc 7680ggccgaatat
ttgcttcctt atcccgggtc caagagagac tgcgatgccc ccctcaatca
7740gcatcctcct ccctgccgct tcaatcttcc ctgcttgcct gcgcccgcgg
tgcgccgtct 7800gcccgcccag tcagtcactc ctgcacaggc cccttgtgcg
cagtgctcct gtacccttta 7860ccgctccttc cattctgcga ggccccctat
tgaatgtatt cgttgcctgt gtggccaagc 7920gggctgctgg gcgcgccgcc
gtcgggcagt gctcggcgac tttggcggaa gccgattgtt 7980cttctgtaag
ccacgcgctt gctgctttgg gaagagaagg gggggggtac tgaatggatg
8040aggaggagaa ggaggggtat tggtattatc
tgagttgggt gaagagc 8087974479DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 97gctcttcggg
tcgccgcgct gcctcgcgtc ccctggtggt gcgcgcggtc gccagcgagg 60ccccgctggg
cgttccgccc tcggtgcagc gcccctcccc cgtggtctac tccaagctgg
120acaagcagca ccgcctgacg cccgagcgcc tggagctggt gcagagcatg
gggcagtttg 180cggaggagag ggtgctgccc gtgctgcacc ccgtggacaa
gctgtggcag ccgcaggact 240ttttgcccga ccccgagtcg cccgacttcg
aggatcaggt ggcggagctg cgcgcgcgcg 300ccaaggacct gcccgacgag
tactttgtgg tgctggtggg ggacatgatc acggaggagg 360cgctgccgac
ctacatggcc atgctcaaca cgctggacgg cgtgcgcgac gacacgggcg
420cggccgacca cccgtgggcg cgctggacgc ggcagtgggt ggccgaggag
aaccggcacg 480gcgacctgct gaacaagtac tgctggctga cggggcgcgt
caacatgcgg gccgtggagg 540tgaccatcaa caacctgatc aagagcggca
tgaacccgca gacggacaac aacccttatt 600tggggttcgt ctacacctcc
ttccaggagc gcgccaccaa gtaggtaccc tttcttgcgc 660tatgacactt
ccagcaaaag gtagggcggg ctgcgagacg gcttcccggc gctgcatgca
720acaccgatga tgcttcgacc ccccgaagct ccttcggggc tgcatgggcg
ctccgatgcc 780gctccagggc gagcgctgtt taaatagcca ggcccccgat
tgcaaagaca ttatagcgag 840ctaccaaagc catattcaaa cacctagatc
actaccactt ctacacaggc cactcgagct 900tgtgatcgca ctccgctaag
ggggcgcctc ttcctcttcg tttcagtcac aacccgcaaa 960ctctagaata
tcaatgatcg agcaggacgg cctccacgcc ggctcccccg ccgcctgggt
1020ggagcgcctg ttcggctacg actgggccca gcagaccatc ggctgctccg
acgccgccgt 1080gttccgcctg tccgcccagg gccgccccgt gctgttcgtg
aagaccgacc tgtccggcgc 1140cctgaacgag ctgcaggacg aggccgcccg
cctgtcctgg ctggccacca ccggcgtgcc 1200ctgcgccgcc gtgctggacg
tggtgaccga ggccggccgc gactggctgc tgctgggcga 1260ggtgcccggc
caggacctgc tgtcctccca cctggccccc gccgagaagg tgtccatcat
1320ggccgacgcc atgcgccgcc tgcacaccct ggaccccgcc acctgcccct
tcgaccacca 1380ggccaagcac cgcatcgagc gcgcccgcac ccgcatggag
gccggcctgg tggaccagga 1440cgacctggac gaggagcacc agggcctggc
ccccgccgag ctgttcgccc gcctgaaggc 1500ccgcatgccc gacggcgagg
acctggtggt gacccacggc gacgcctgcc tgcccaacat 1560catggtggag
aacggccgct tctccggctt catcgactgc ggccgcctgg gcgtggccga
1620ccgctaccag gacatcgccc tggccacccg cgacatcgcc gaggagctgg
gcggcgagtg 1680ggccgaccgc ttcctggtgc tgtacggcat cgccgccccc
gactcccagc gcatcgcctt 1740ctaccgcctg ctggacgagt tcttctgaca
attggcagca gcagctcgga tagtatcgac 1800acactctgga cgctggtcgt
gtgatggact gttgccgcca cacttgctgc cttgacctgt 1860gaatatccct
gccgctttta tcaaacagcc tcagtgtgtt tgatcttgtg tgtacgcgct
1920tttgcgagtt gctagctgct tgtgctattt gcgaatacca cccccagcat
ccccttccct 1980cgtttcatat cgcttgcatc ccaaccgcaa cttatctacg
ctgtcctgct atccctcagc 2040gctgctcctg ctcctgctca ctgcccctcg
cacagccttg gtttgggctc cgcctgtatt 2100ctcctggtac tgcaacctgt
aaaccagcac tgcaatgctg atgcacggga agtagtggga 2160tgggaacaca
aatggaggat cccgcgtctc gaacagagcg cgcagaggaa cgctgaaggt
2220ctcgcctctg tcgcacctca gcgcggcata caccacaata accacctgac
gaatgcgctt 2280ggttcttcgt ccattagcga agcgtccggt tcacacacgt
gccacgttgg cgaggtggca 2340ggtgacaatg atcggtggag ctgatggtcg
aaacgttcac agcctaggga tatcgaattc 2400ctttcttgcg ctatgacact
tccagcaaaa ggtagggcgg gctgcgagac ggcttcccgg 2460cgctgcatgc
aacaccgatg atgcttcgac cccccgaagc tccttcgggg ctgcatgggc
2520gctccgatgc cgctccaggg cgagcgctgt ttaaatagcc aggcccccga
ttgcaaagac 2580attatagcga gctaccaaag ccatattcaa acacctagat
cactaccact tctacacagg 2640ccactcgagc ttgtgatcgc actccgctaa
gggggcgcct cttcctcttc gtttcagtca 2700caacccgcaa acactagtgc
gctggacgcg gcagtgggtg gccgaggaga accggcacgg 2760cgacctgctg
aacaagtact gttggctgac ggggcgcgtc aacatgcggg ccgtggaggt
2820gaccatcaac aacctgatca agagcggcat gaacccgcag acggacaaca
acccttactt 2880gggcttcgtc tacacctcct tccaggagcg cgcgaccaag
tacagccacg gcaacaccgc 2940gcgccttgcg gccgagcagt gtgtttgagg
gttttggttg cccgtatcga ggtcctggtg 3000gcgcgcatgg gggagaaggc
gcctgtcccg ctgacccccc cggctaccct cccggcacct 3060tccagggcgc
gtacgggatc ctgctcggcc gcaaggcgcg cggtgttgcc gtggctgtac
3120ttggtcgcgc gctcctggaa ggaggtgtag acgaagccca agtaagggtt
gttgtccgtc 3180tgcgggttca tgccgctctt gatcaggttg ttgatggtca
cctccacggc ccgcatgttg 3240acgcgccccg tcagccaaca gtacttgttc
agcaggtcgc cgtgccggtt ctcctcggcc 3300acccactgcc gcgtccagcg
caagcttgca gcagcagctc ggatagtatc gacacactct 3360ggacgctggt
cgtgtgatgg actgttgccg ccacacttgc tgccttgacc tgtgaatatc
3420cctgccgctt ttatcaaaca gcctcagtgt gtttgatctt gtgtgtacgc
gcttttgcga 3480gttgctagct gcttgtgcta tttgcgaata ccacccccag
catccccttc cctcgtttca 3540tatcgcttgc atcccaaccg caacttatct
acgctgtcct gctatccctc agcgctgctc 3600ctgctcctgc tcactgcccc
tcgcacagcc ttggtttggg ctccgcctgt attctcctgg 3660tactgcaacc
tgtaaaccag cactgcaatg ctgatgcacg ggaagtagtg ggatgggaac
3720acaaatggaa agctggagct ccagccacgg caacaccgcg cgccttgcgg
ccgagcacgg 3780cgacaagaac ctgagcaaga tctgcgggct gatcgccagc
gacgagggcc ggcacgagat 3840cgcctacacg cgcatcgtgg acgagttctt
ccgcctcgac cccgagggcg ccgtcgccgc 3900ctacgccaac atgatgcgca
agcagatcac catgcccgcg cacctcatgg acgacatggg 3960ccacggcgag
gccaacccgg gccgcaacct cttcgccgac ttctccgcgg tcgccgagaa
4020gatcgacgtc tacgacgccg aggactactg ccgcatcctg gagcacctca
acgcgcgctg 4080gaaggtggac gagcgccagg tcagcggcca ggccgccgcg
gaccaggagt acgtcctggg 4140cctgccccag cgcttccgga aactcgccga
gaagaccgcc gccaagcgca agcgcgtcgc 4200gcgcaggccc gtcgccttct
cctggatctc cgggcgcgag atcatggtct agggagcgac 4260gagtgtgcgt
gcggggctgg cgggagtggg acgccctcct cgctcctctc tgttctgaac
4320ggaacaatcg gccaccccgc gctacgcgcc acgcatcgag caacgaagaa
aaccccccga 4380tgataggttg cggtggctgc cgggatatag atccggccgc
acatcaaagg gcccctccgc 4440cagagaagaa gctcctttcc cagcagactc
ctgaagagc 4479985243DNAArtificial SequenceDescription of Artificial
Sequence Synthetic polynucleotide 98atgcatgccg gtcaccaccc
gcatgctcgt actacagcgc acgcaccgct tcgtgatcca 60ccgggtgaac gtagtcctcg
acggaaacat ctggttcggg cctcctgctt gcactcccgc 120ccatgccgac
aacctttctg ctgttaccac gacccacaat gcaacgcgac acgaccgtgt
180gggactgatc ggttcactgc acctgcatgc aattgtcaca agcgcttact
ccaattgtat 240tcgtttgttt tctgggagca gttgctcgac cgcccgcgtc
ccgcaggcag cgatgacgtg 300tgcgtggcct gggtgtttcg tcgaaaggcc
agcaacccta aatcgcaggc gatccggaga 360ttgggatctg atccgagttt
ggaccagatc cgccccgatg cggcacggga actgcatcga 420ctcggcgcgg
aacccagctt tcgtaaatgc cagattggtg tccgatacct ggatttgcca
480tcagcgaaac aagacttcag cagcgagcgt atttggcggg cgtgctacca
gggttgcata 540cattgcccat ttctgtctgg accgctttac tggcgcagag
ggtgagttga tggggttggc 600aggcatcgaa acgcgcgtgc atggtgtgcg
tgtctgtttt cggctgcacg aattcaatag 660tcggatgggc gacggtagaa
ttgggtgtgg cgctcgcgtg catgcctcgc cccgtcgggt 720gtcatgaccg
ggactggaat cccccctcgc gaccatcttg ctaacgctcc cgactctccc
780gactagtgcg ctggacgcgg cagtgggtgg ccgaggagaa ccggcacggc
gacctgctga 840acaagtactg ttggctgacg gggcgcgtca acatgcgggc
cgtggaggtg accatcaaca 900acctgatcaa gagcggcatg aacccgcaga
cggacaacaa cccttacttg ggcttcgtct 960acacctcctt ccaggagcgc
gcgaccaagt acagccacgg caacaccgcg cgccttgcgg 1020ccgagcagtg
tgtttgaggg ttttggttgc ccgtatcgag gtcctggtgg cgcgcatggg
1080ggagaaggcg cctgtcccgc tgaccccccc ggctaccctc ccggcacctt
ccagggcgcg 1140tacgggatcc tgctcggccg caaggcgcgc ggtgttgccg
tggctgtact tggtcgcgcg 1200ctcctggaag gaggtgtaga cgaagcccaa
gtaagggttg ttgtccgtct gcgggttcat 1260gccgctcttg atcaggttgt
tgatggtcac ctccacggcc cgcatgttga cgcgccccgt 1320cagccaacag
tacttgttca gcaggtcgcc gtgccggttc tcctcggcca cccactgccg
1380cgtccagcgc aagcttgcag cagcagctcg gatagtatcg acacactctg
gacgctggtc 1440gtgtgatgga ctgttgccgc cacacttgct gccttgacct
gtgaatatcc ctgccgcttt 1500tatcaaacag cctcagtgtg tttgatcttg
tgtgtacgcg cttttgcgag ttgctagctg 1560cttgtgctat ttgcgaatac
cacccccagc atccccttcc ctcgtttcat atcgcttgca 1620tcccaaccgc
aacttatcta cgctgtcctg ctatccctca gcgctgctcc tgctcctgct
1680cactgcccct cgcacagcct tggtttgggc tccgcctgta ttctcctggt
actgcaacct 1740gtaaaccagc actgcaatgc tgatgcacgg gaagtagtgg
gatgggaaca caaatggaaa 1800gctggagctc aaagatatca acttaattaa
ccaaggtacc cgcctgcaac gcaagggcag 1860ccacagccgc tcccacccgc
cgctgaaccg acacgtgctt gggcgcctgc cgcctgcctg 1920ccgcatgctt
gtgctggtga ggctgggcag tgctgccatg ctgattgagg cttggttcat
1980cgggtggaag cttatgtgtg tgctgggctt gcatgccggg caatgcgcat
ggtggcaaga 2040gggcggcagc acttgctgga gctgccgcgg tgcctccagg
tggttcaatc gcggcagcca 2100gagggatttc agatgatcgc gcgtacaggt
tgagcagcag tgtcagcaaa ggtagcagtt 2160tgccagaatg atcggttcag
ctgttaatca atgccagcaa gagaaggggt caagtgcaaa 2220cacgggcatg
ccacagcacg ggcaccgggg agtggaatgg caccaccaag tgtgtgcgag
2280ccagcatcgc cgcctggctg tttcagctac aacggcagga gtcatccaac
gtaaccatga 2340gctgatcaac actgcaatca tcgggcgggc gtgatgcaag
catgcctggc gaagacacat 2400ggtgtgcgga tgctgccggc tgctgcctgc
tgcgcacgcc gttgagttgg cagcaggctc 2460agccatgcac tggatggcag
ctgggctgcc actgcaatgt ggtggatagg atgcaagtgg 2520agcgaatacc
aaaccctctg gctgcttgct gggttgcatg gcatcgcacc atcagcagga
2580gcgcatgcga agggactggc cccatgcacg ccatgccaaa ccggagcgca
ccgagtgtcc 2640acactgtcac caggcccgca agctttgcag aaccatgctc
atggacgcat gtagcgctga 2700cgtcccttga cggcgctcct ctcgggtgtg
ggaaacgcaa tgcagcacag gcagcagagg 2760cggcggcagc agagcggcgg
cagcagcggc gggggccacc cttcttgcgg ggtcgcgccc 2820cagccagcgg
tgatgcgctg atcccaaacg agttcacatt catttgcatg cctggagaag
2880cgaggctggg gcctttgggc tggtgcagcc cgcaatggaa tgcgggaccg
ccaggctagc 2940agcaaaggcg cctcccctac tccgcatcga tgttccatag
tgcattggac tgcatttggg 3000tggggcggcc ggctgtttct ttcgtgttgc
aaaacgcgcc agctcagcaa cctgtcccgt 3060gggtcccccg tgccgatgaa
atcgtgtgca cgccgatcag ctgattgccc ggctcgcgaa 3120gtaggcgccc
tcctttctgc tcgccctctc tccgtcccgc ctctagaata tcaatgatcg
3180agcaggacgg cctccacgcc ggctcccccg ccgcctgggt ggagcgcctg
ttcggctacg 3240actgggccca gcagaccatc ggctgctccg acgccgccgt
gttccgcctg tccgcccagg 3300gccgccccgt gctgttcgtg aagaccgacc
tgtccggcgc cctgaacgag ctgcaggacg 3360aggccgcccg cctgtcctgg
ctggccacca ccggcgtgcc ctgcgccgcc gtgctggacg 3420tggtgaccga
ggccggccgc gactggctgc tgctgggcga ggtgcccggc caggacctgc
3480tgtcctccca cctggccccc gccgagaagg tgtccatcat ggccgacgcc
atgcgccgcc 3540tgcacaccct ggaccccgcc acctgcccct tcgaccacca
ggccaagcac cgcatcgagc 3600gcgcccgcac ccgcatggag gccggcctgg
tggaccagga cgacctggac gaggagcacc 3660agggcctggc ccccgccgag
ctgttcgccc gcctgaaggc ccgcatgccc gacggcgagg 3720acctggtggt
gacccacggc gacgcctgcc tgcccaacat catggtggag aacggccgct
3780tctccggctt catcgactgc ggccgcctgg gcgtggccga ccgctaccag
gacatcgccc 3840tggccacccg cgacatcgcc gaggagctgg gcggcgagtg
ggccgaccgc ttcctggtgc 3900tgtacggcat cgccgccccc gactcccagc
gcatcgcctt ctaccgcctg ctggacgagt 3960tcttctgaca attggcagca
gcagctcgga tagtatcgac acactctgga cgctggtcgt 4020gtgatggact
gttgccgcca cacttgctgc cttgacctgt gaatatccct gccgctttta
4080tcaaacagcc tcagtgtgtt tgatcttgtg tgtacgcgct tttgcgagtt
gctagctgct 4140tgtgctattt gcgaatacca cccccagcat ccccttccct
cgtttcatat cgcttgcatc 4200ccaaccgcaa cttatctacg ctgtcctgct
atccctcagc gctgctcctg ctcctgctca 4260ctgcccctcg cacagccttg
gtttgggctc cgcctgtatt ctcctggtac tgcaacctgt 4320aaaccagcac
tgcaatgctg atgcacggga agtagtggga tgggaacaca aatggaggat
4380cccgcgtctc gaacagagcg cgcagaggaa cgctgaaggt ctcgcctctg
tcgcacctca 4440gcgcggcata caccacaata accacctgac gaatgcgctt
ggttcttcgt ccattagcga 4500agcgtccggt tcacacacgt gccacgttgg
cgaggtggca ggtgacaatg atcggtggag 4560ctgatggtcg aaacgttcac
agcctaggga tatcgaattc cgggtcgccg cgctgcctcg 4620cgtcccctgg
tggtgcgcgc ggtcgccagc gaggccccgc tgggcgttcc gccctcggtg
4680cagcgcccct cccccgtggt ctactccaag ctggacaagc agcaccgcct
gacgcccgag 4740cgcctggagc tggtgcagag catggggcag tttgcggagg
agagggtgct gcccgtgctg 4800caccccgtgg acaagctgtg gcagccgcag
gactttttgc ccgaccccga gtcgcccgac 4860ttcgaggatc aggtggcgga
gctgcgcgcg cgcgccaagg acctgcccga cgagtacttt 4920gtggtgctgg
tgggggacat gatcacggag gaggcgctgc cgacctacat ggccatgctc
4980aacacgctgg acggcgtgcg cgacgacacg ggcgcggccg accacccgtg
ggcgcgctgg 5040acgcggcagt gggtggccga ggagaaccgg cacggcgacc
tgctgaacaa gtactgctgg 5100ctgacggggc gcgtcaacat gcgggccgtg
gaggtgacca tcaacaacct gatcaagagc 5160ggcatgaacc cgcagacgga
caacaaccct tatttggggt tcgtctacac ctccttccag 5220gagcgcgcca
ccaagtatct aga 5243996804DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 99gctcttcgcc
gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc
gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg aggtctgcct
120tgcgccggct gagccactgc ttcgtccggg cggccaagag gagcatgagg
gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct gggagcgggc
cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac tggtcctcca
gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg tgaggggggt
atgaattgta cagaacaacc acgagccttg tctaggcaga 360atccctacca
gtcatggctt tacctggatg acggcctgcg aacagctgtc cagcgaccct
420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg tgatggcgcg
agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga ggacagtcgg
ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt gccatccttt
gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca caccacctcc
tcccagacca attctgtcac ctttttggcg aaggcatcgg 660cctcggcctg
cagagaggac agcagtgccc agccgctggg ggttggcgga tgcacgctca
720ggtacccttt cttgcgctat gacacttcca gcaaaaggta gggcgggctg
cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc ttcgaccccc
cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct ccagggcgag
cgctgtttaa atagccaggc ccccgattgc 900aaagacatta tagcgagcta
ccaaagccat attcaaacac ctagatcact accacttcta 960cacaggccac
tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc ctcttcgttt
1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc aggccttcct
gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc atgacgaacg
agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa gggctggatg
aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca agtggcacct
gtacttccag tacaacccga acgacaccgt ctgggggacg 1260cccttgttct
ggggccacgc cacgtccgac gacctgacca actgggagga ccagcccatc
1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg gctccatggt
ggtggactac 1380aacaacacct ccggcttctt caacgacacc atcgacccgc
gccagcgctg cgtggccatc 1440tggacctaca acaccccgga gtccgaggag
cagtacatct cctacagcct ggacggcggc 1500tacaccttca ccgagtacca
gaagaacccc gtgctggccg ccaactccac ccagttccgc 1560gacccgaagg
tcttctggta cgagccctcc cagaagtgga tcatgaccgc ggccaagtcc
1620caggactaca agatcgagat ctactcctcc gacgacctga agtcctggaa
gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac cagtacgagt
gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag caagtcctac
tgggtgatgt tcatctccat caaccccggc 1800gccccggccg gcggctcctt
caaccagtac ttcgtcggca gcttcaacgg cacccacttc 1860gaggccttcg
acaaccagtc ccgcgtggtg gacttcggca aggactacta cgccctgcag
1920accttcttca acaccgaccc gacctacggg agcgccctgg gcatcgcgtg
ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac ccctggcgct
cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga gtaccaggcc
aacccggaga cggagctgat caacctgaag 2100gccgagccga tcctgaacat
cagcaacgcc ggcccctgga gccggttcgc caccaacacc 2160acgttgacga
aggccaacag ctacaacgtc gacctgtcca acagcaccgg caccctggag
2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct ccaagtccgt
gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac cccgaggagt
acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt cctggaccgc
gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact tcaccaaccg
catgagcgtg aacaaccagc ccttcaagag cgagaacgac 2460ctgtcctact
acaaggtgta cggcttgctg gaccagaaca tcctggagct gtacttcaac
2520gacggcgacg tcgtgtccac caacacctac ttcatgacca ccgggaacgc
cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg ttctacatcg
acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc agcagctcgg
atagtatcga cacactctgg acgctggtcg 2700tgtgatggac tgttgccgcc
acacttgctg ccttgacctg tgaatatccc tgccgctttt 2760atcaaacagc
ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt tgctagctgc
2820ttgtgctatt tgcgaatacc acccccagca tccccttccc tcgtttcata
tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc tatccctcag
cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt ggtttgggct
ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca ctgcaatgct
gatgcacggg aagtagtggg atgggaacac aaatggagga 3060tcccgcgtct
cgaacagagc gcgcagagga acgctgaagg tctcgcctct gtcgcacctc
3120agcgcggcat acaccacaat aaccacctga cgaatgcgct tggttcttcg
tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg gcgaggtggc
aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca cagcctaggg
atatcgaatt cggccgacag gacgcgcgtc 3300aaaggtgctg gtcgtgtatg
ccctggccgg caggtcgttg ctgctgctgg ttagtgattc 3360cgcaaccctg
attttggcgt cttattttgg cgtggcaaac gctggcgccc gcgagccggg
3420ccggcggcga tgcggtgccc cacggctgcc ggaatccaag ggaggcaaga
gcgcccgggt 3480cagttgaagg gctttacgcg caaggtacag ccgctcctgc
aaggctgcgt ggtggaattg 3540gacgtgcagg tcctgctgaa gttcctccac
cgcctcacca gcggacaaag caccggtgta 3600tcaggtccgt gtcatccact
ctaaagaact cgactacgac ctactgatgg ccctagattc 3660ttcatcaaaa
acgcctgaga cacttgccca ggattgaaac tccctgaagg gaccaccagg
3720ggccctgagt tgttccttcc ccccgtggcg agctgccagc caggctgtac
ctgtgatcga 3780ggctggcggg aaaataggct tcgtgtgctc aggtcatggg
aggtgcagga cagctcatga 3840aacgccaaca atcgcacaat tcatgtcaag
ctaatcagct atttcctctt cacgagctgt 3900aattgtccca aaattctggt
ctaccggggg tgatccttcg tgtacgggcc cttccctcaa 3960ccctaggtat
gcgcgcatgc ggtcgccgcg caactcgcgc gagggccgag ggtttgggac
4020gggccgtccc gaaatgcagt tgcacccgga tgcgtggcac cttttttgcg
ataatttatg 4080caatggactg ctctgcaaaa ttctggctct gtcgccaacc
ctaggatcag cggcgtagga 4140tttcgtaatc attcgtcctg atggggagct
accgactacc ctaatatcag cccgactgcc 4200tgacgccagc gtccactttt
gtgcacacat tccattcgtg cccaagacat ttcattgtgg 4260tgcgaagcgt
ccccagttac gctcacctgt ttcccgacct ccttactgtt ctgtcgacag
4320agcgggccca caggccggtc gcagccacta gtatggccac cgcatccact
ttctcggcgt 4380tcaatgcccg ctgcggcgac ctgcgtcgct cggcgggctc
cgggccccgg cgcccagcga 4440ggcccctccc cgtgcgcggg cgcgccgcca
ccctgcgctc cggcctgcgc gacgtggaga 4500ccgtgaagaa gaccttctcc
cccgcccgcg aggtgcacgt gcaggtgacc cactccatgg 4560ccccccagaa
gatcgagatc ttcaaggcca tggaggactg ggccgagaac aacatcctgg
4620tgcacctgaa gaacgtggag aagtgccccc agccccagga cttcctgccc
gaccccgcct 4680ccgacgagtt ccacgaccag atcaaggagc tgcgcgagcg
cgccaaggag atccccgacg 4740actacttcgt ggtgctggtg ggcgacatga
tcaccgagga ggccctgccc acctaccaga 4800ccatgctgaa cacctgggac
ggcgtgcgcg acgagaccgg cgcctccccc acctcctggg 4860ccatctggac
ccgcgcctgg accgccgagg agaaccgcca cggcgacccc ctgaacaagt
4920acctgtacct gtccggccgc gtggacatga agcagatcga gaagaccatc
cagtacctga
4980tcggctccgg catggacccc cgcaccgaga actcccccta cctgggcttc
atctacacct 5040ccttccagga gcgcgccacc ttcatctccc acggcaacac
cgcccgcctg gcccgcgacc 5100acggcgactt caagctggcc cagatctgcg
gcaccatcgc ctccgacgag aagcgccacg 5160agaccgccta caccaagatc
gtggagaagc tgttcgagat cgaccccgac ggcaccgtgc 5220tggccttcgg
cgacatgatg aagaagaaga tctccatgcc cgaccacttc atgtacgacg
5280gccgcgacga caacctgttc gaccacttct cctccgtggc ccagcgcctg
ggcgtgtaca 5340ccgccaagga ctacgccgac atcctggagc acctggtggg
ccgctggaag gtggagaagc 5400tgaccggcct gtccgccgag ggccagaagg
cccaggacta cgtgtgcggc ctgccccccc 5460gcatccgccg cctggaggag
cgcgcccaga tccgcgccaa gcaggccccc cgcctgccct 5520tctcctggat
ctacgaccgc gaggtgcagc tgatggacta caaggaccac gacggcgact
5580acaaggacca cgacatcgac tacaaggacg acgacgacaa gtgaatcgat
agatctctta 5640aggcagcagc agctcggata gtatcgacac actctggacg
ctggtcgtgt gatggactgt 5700tgccgccaca cttgctgcct tgacctgtga
atatccctgc cgcttttatc aaacagcctc 5760agtgtgtttg atcttgtgtg
tacgcgcttt tgcgagttgc tagctgcttg tgctatttgc 5820gaataccacc
cccagcatcc ccttccctcg tttcatatcg cttgcatccc aaccgcaact
5880tatctacgct gtcctgctat ccctcagcgc tgctcctgct cctgctcact
gcccctcgca 5940cagccttggt ttgggctccg cctgtattct cctggtactg
caacctgtaa accagcactg 6000caatgctgat gcacgggaag tagtgggatg
ggaacacaaa tggaaagctt aattaagagc 6060tcttgttttc cagaaggagt
tgctccttga gcctttcatt ctcagcctcg ataacctcca 6120aagccgctct
aattgtggag ggggttcgaa tttaaaagct tggaatgttg gttcgtgcgt
6180ctggaacaag cccagacttg ttgctcactg ggaaaaggac catcagctcc
aaaaaacttg 6240ccgctcaaac cgcgtacctc tgctttcgcg caatctgccc
tgttgaaatc gccaccacat 6300tcatattgtg acgcttgagc agtctgtaat
tgcctcagaa tgtggaatca tctgccccct 6360gtgcgagccc atgccaggca
tgtcgcgggc gaggacaccc gccactcgta cagcagacca 6420ttatgctacc
tcacaatagt tcataacagt gaccatattt ctcgaagctc cccaacgagc
6480acctccatgc tctgagtggc caccccccgg ccctggtgct tgcggagggc
aggtcaaccg 6540gcatggggct accgaaatcc ccgaccggat cccaccaccc
ccgcgatggg aagaatctct 6600ccccgggatg tgggcccacc accagcacaa
cctgctggcc caggcgagcg tcaaaccata 6660ccacacaaat atccttggca
tcggccctga attccttctg ccgctctgct acccggtgct 6720tctgtccgaa
gcaggggttg ctagggatcg ctccgagtcc gcaaaccctt gtcgcgtggc
6780ggggcttgtt cgagcttgaa gagc 68041006792DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
100gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga
3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg tctcgcctct
gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga cgaatgcgct
tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg
gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca
cagcctaggg atatcgaatt cggccgacag gacgcgcgtc 3300aaaggtgctg
gtcgtgtatg ccctggccgg caggtcgttg ctgctgctgg ttagtgattc
3360cgcaaccctg attttggcgt cttattttgg cgtggcaaac gctggcgccc
gcgagccggg 3420ccggcggcga tgcggtgccc cacggctgcc ggaatccaag
ggaggcaaga gcgcccgggt 3480cagttgaagg gctttacgcg caaggtacag
ccgctcctgc aaggctgcgt ggtggaattg 3540gacgtgcagg tcctgctgaa
gttcctccac cgcctcacca gcggacaaag caccggtgta 3600tcaggtccgt
gtcatccact ctaaagaact cgactacgac ctactgatgg ccctagattc
3660ttcatcaaaa acgcctgaga cacttgccca ggattgaaac tccctgaagg
gaccaccagg 3720ggccctgagt tgttccttcc ccccgtggcg agctgccagc
caggctgtac ctgtgatcga 3780ggctggcggg aaaataggct tcgtgtgctc
aggtcatggg aggtgcagga cagctcatga 3840aacgccaaca atcgcacaat
tcatgtcaag ctaatcagct atttcctctt cacgagctgt 3900aattgtccca
aaattctggt ctaccggggg tgatccttcg tgtacgggcc cttccctcaa
3960ccctaggtat gcgcgcatgc ggtcgccgcg caactcgcgc gagggccgag
ggtttgggac 4020gggccgtccc gaaatgcagt tgcacccgga tgcgtggcac
cttttttgcg ataatttatg 4080caatggactg ctctgcaaaa ttctggctct
gtcgccaacc ctaggatcag cggcgtagga 4140tttcgtaatc attcgtcctg
atggggagct accgactacc ctaatatcag cccgactgcc 4200tgacgccagc
gtccactttt gtgcacacat tccattcgtg cccaagacat ttcattgtgg
4260tgcgaagcgt ccccagttac gctcacctgt ttcccgacct ccttactgtt
ctgtcgacag 4320agcgggccca caggccggtc gcagccacta gtatggccct
gaagctgaac gccatcaact 4380tccagtcccc caagtgctcc tccttcggcc
tgccccccgt ggtgtccctg cgctccccca 4440agctgtccgt ggccgccacc
ctgcgctccg gcctgcgcga cgtggagacc gtgaagaaga 4500ccttctcccc
cgcccgcgag gtgcacgtgc aggtgaccca ctccatggcc ccccagaaga
4560tcgagatctt caaggccatg gaggactggg ccgagaacaa catcctggtg
cacctgaaga 4620acgtggagaa gtgcccccag ccccaggact tcctgcccga
ccccgcctcc gacgagttcc 4680acgaccagat caaggagctg cgcgagcgcg
ccaaggagat ccccgacgac tacttcgtgg 4740tgctggtggg cgacatgatc
accgaggagg ccctgcccac ctaccagacc atgctgaaca 4800cctgggacgg
cgtgcgcgac gagaccggcg cctcccccac ctcctgggcc atctggaccc
4860gcgcctggac cgccgaggag aaccgccacg gcgaccccct gaacaagtac
ctgtacctgt 4920ccggccgcgt ggacatgaag cagatcgaga agaccatcca
gtacctgatc ggctccggca 4980tggacccccg caccgagaac tccccctacc
tgggcttcat ctacacctcc ttccaggagc 5040gcgccacctt catctcccac
ggcaacaccg cccgcctggc ccgcgaccac ggcgacttca 5100agctggccca
gatctgcggc accatcgcct ccgacgagaa gcgccacgag accgcctaca
5160ccaagatcgt ggagaagctg ttcgagatcg accccgacgg caccgtgctg
gccttcggcg 5220acatgatgaa gaagaagatc tccatgcccg accacttcat
gtacgacggc cgcgacgaca 5280acctgttcga ccacttctcc tccgtggccc
agcgcctggg cgtgtacacc gccaaggact 5340acgccgacat cctggagcac
ctggtgggcc gctggaaggt ggagaagctg accggcctgt 5400ccgccgaggg
ccagaaggcc caggactacg tgtgcggcct gcccccccgc atccgccgcc
5460tggaggagcg cgcccagatc cgcgccaagc aggccccccg cctgcccttc
tcctggatct 5520acgaccgcga ggtgcagctg atggactaca aggaccacga
cggcgactac aaggaccacg 5580acatcgacta caaggacgac gacgacaagt
gaatcgatag atctcttaag gcagcagcag 5640ctcggatagt atcgacacac
tctggacgct ggtcgtgtga tggactgttg ccgccacact 5700tgctgccttg
acctgtgaat atccctgccg cttttatcaa acagcctcag tgtgtttgat
5760cttgtgtgta cgcgcttttg cgagttgcta gctgcttgtg ctatttgcga
ataccacccc 5820cagcatcccc ttccctcgtt tcatatcgct tgcatcccaa
ccgcaactta tctacgctgt 5880cctgctatcc ctcagcgctg ctcctgctcc
tgctcactgc ccctcgcaca gccttggttt 5940gggctccgcc tgtattctcc
tggtactgca acctgtaaac cagcactgca atgctgatgc 6000acgggaagta
gtgggatggg aacacaaatg gaaagcttaa ttaagagctc ttgttttcca
6060gaaggagttg ctccttgagc ctttcattct cagcctcgat aacctccaaa
gccgctctaa 6120ttgtggaggg ggttcgaatt taaaagcttg gaatgttggt
tcgtgcgtct ggaacaagcc 6180cagacttgtt gctcactggg aaaaggacca
tcagctccaa aaaacttgcc gctcaaaccg 6240cgtacctctg ctttcgcgca
atctgccctg ttgaaatcgc caccacattc atattgtgac 6300gcttgagcag
tctgtaattg cctcagaatg tggaatcatc tgccccctgt gcgagcccat
6360gccaggcatg tcgcgggcga ggacacccgc cactcgtaca gcagaccatt
atgctacctc 6420acaatagttc ataacagtga ccatatttct cgaagctccc
caacgagcac ctccatgctc 6480tgagtggcca ccccccggcc ctggtgcttg
cggagggcag gtcaaccggc atggggctac 6540cgaaatcccc gaccggatcc
caccaccccc gcgatgggaa gaatctctcc ccgggatgtg 6600ggcccaccac
cagcacaacc tgctggccca ggcgagcgtc aaaccatacc acacaaatat
6660ccttggcatc ggccctgaat tccttctgcc gctctgctac ccggtgcttc
tgtccgaagc 6720aggggttgct agggatcgct ccgagtccgc aaacccttgt
cgcgtggcgg ggcttgttcg 6780agcttgaaga gc 67921016051DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
101gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga
3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg tctcgcctct
gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga cgaatgcgct
tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg
gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca
cagcctaggg atatcgaatt cctttcttgc gctatgacac 3300ttccagcaaa
aggtagggcg ggctgcgaga cggcttcccg gcgctgcatg caacaccgat
3360gatgcttcga ccccccgaag ctccttcggg gctgcatggg cgctccgatg
ccgctccagg 3420gcgagcgctg tttaaatagc caggcccccg attgcaaaga
cattatagcg agctaccaaa 3480gccatattca aacacctaga tcactaccac
ttctacacag gccactcgag cttgtgatcg 3540cactccgcta agggggcgcc
tcttcctctt cgtttcagtc acaacccgca aacactagta 3600tggccaccgc
atccactttc tcggcgttca atgcccgctg cggcgacctg cgtcgctcgg
3660cgggctccgg gccccggcgc ccagcgaggc ccctccccgt gcgcgggcgc
gccgccaccc 3720tgcgctccgg cctgcgcgac gtggagaccg tgaagaagac
cttctccccc gcccgcgagg 3780tgcacgtgca ggtgacccac tccatggccc
cccagaagat cgagatcttc aaggccatgg 3840aggactgggc cgagaacaac
atcctggtgc acctgaagaa cgtggagaag tgcccccagc 3900cccaggactt
cctgcccgac cccgcctccg acgagttcca cgaccagatc aaggagctgc
3960gcgagcgcgc caaggagatc cccgacgact acttcgtggt gctggtgggc
gacatgatca 4020ccgaggaggc cctgcccacc taccagacca tgctgaacac
ctgggacggc gtgcgcgacg 4080agaccggcgc ctcccccacc tcctgggcca
tctggacccg cgcctggacc gccgaggaga 4140accgccacgg cgaccccctg
aacaagtacc tgtacctgtc cggccgcgtg gacatgaagc 4200agatcgagaa
gaccatccag tacctgatcg gctccggcat ggacccccgc accgagaact
4260ccccctacct gggcttcatc tacacctcct tccaggagcg cgccaccttc
atctcccacg 4320gcaacaccgc ccgcctggcc cgcgaccacg gcgacttcaa
gctggcccag atctgcggca 4380ccatcgcctc cgacgagaag cgccacgaga
ccgcctacac caagatcgtg gagaagctgt 4440tcgagatcga ccccgacggc
accgtgctgg ccttcggcga catgatgaag aagaagatct 4500ccatgcccga
ccacttcatg tacgacggcc gcgacgacaa cctgttcgac cacttctcct
4560ccgtggccca gcgcctgggc gtgtacaccg ccaaggacta cgccgacatc
ctggagcacc 4620tggtgggccg ctggaaggtg gagaagctga ccggcctgtc
cgccgagggc cagaaggccc 4680aggactacgt gtgcggcctg cccccccgca
tccgccgcct ggaggagcgc gcccagatcc 4740gcgccaagca ggccccccgc
ctgcccttct cctggatcta cgaccgcgag gtgcagctga 4800tggactacaa
ggaccacgac ggcgactaca aggaccacga catcgactac aaggacgacg
4860acgacaagtg aatcgataga tctcttaagg cagcagcagc tcggatagta
tcgacacact 4920ctggacgctg gtcgtgtgat ggactgttgc cgccacactt
gctgccttga cctgtgaata 4980tccctgccgc ttttatcaaa cagcctcagt
gtgtttgatc ttgtgtgtac gcgcttttgc 5040gagttgctag ctgcttgtgc
tatttgcgaa taccaccccc agcatcccct tccctcgttt 5100catatcgctt
gcatcccaac cgcaacttat ctacgctgtc ctgctatccc tcagcgctgc
5160tcctgctcct gctcactgcc cctcgcacag ccttggtttg ggctccgcct
gtattctcct 5220ggtactgcaa cctgtaaacc agcactgcaa tgctgatgca
cgggaagtag tgggatggga 5280acacaaatgg aaagcttaat taagagctct
tgttttccag aaggagttgc tccttgagcc 5340tttcattctc agcctcgata
acctccaaag ccgctctaat tgtggagggg gttcgaattt 5400aaaagcttgg
aatgttggtt cgtgcgtctg gaacaagccc agacttgttg ctcactggga
5460aaaggaccat cagctccaaa aaacttgccg ctcaaaccgc gtacctctgc
tttcgcgcaa 5520tctgccctgt tgaaatcgcc accacattca tattgtgacg
cttgagcagt ctgtaattgc 5580ctcagaatgt ggaatcatct gccccctgtg
cgagcccatg ccaggcatgt cgcgggcgag 5640gacacccgcc actcgtacag
cagaccatta tgctacctca caatagttca taacagtgac 5700catatttctc
gaagctcccc aacgagcacc tccatgctct gagtggccac cccccggccc
5760tggtgcttgc ggagggcagg tcaaccggca tggggctacc gaaatccccg
accggatccc 5820accacccccg cgatgggaag aatctctccc cgggatgtgg
gcccaccacc agcacaacct 5880gctggcccag gcgagcgtca aaccatacca
cacaaatatc cttggcatcg gccctgaatt 5940ccttctgccg ctctgctacc
cggtgcttct gtccgaagca ggggttgcta gggatcgctc 6000cgagtccgca
aacccttgtc gcgtggcggg gcttgttcga gcttgaagag c
60511027041DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 102gctcttcgcc gccgccactc ctgctcgagc
gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga
tgtccatcac caggtccatg aggtctgcct 120tgcgccggct gagccactgc
ttcgtccggg cggccaagag gagcatgagg gaggactcct 180ggtccagggt
cctgacgtgg tcgcggctct gggagcgggc cagcatcatc tggctctgcc
240gcaccgaggc cgcctccaac tggtcctcca gcagccgcag tcgccgccga
ccctggcaga 300ggaagacagg tgaggggggt atgaattgta cagaacaacc
acgagccttg tctaggcaga 360atccctacca gtcatggctt tacctggatg
acggcctgcg aacagctgtc cagcgaccct 420cgctgccgcc gcttctcccg
cacgcttctt tccagcaccg tgatggcgcg agccagcgcc 480gcacgctggc
gctgcgcttc gccgatctga ggacagtcgg ggaactctga tcagtctaaa
540cccccttgcg cgttagtgtt gccatccttt gcagaccggt gagagccgac
ttgttgtgcg 600ccacccccca caccacctcc tcccagacca attctgtcac
ctttttggcg aaggcatcgg 660cctcggcctg cagagaggac agcagtgccc
agccgctggg ggttggcgga tgcacgctca 720ggtacccttt cttgcgctat
gacacttcca gcaaaaggta gggcgggctg cgagacggct 780tcccggcgct
gcatgcaaca ccgatgatgc ttcgaccccc cgaagctcct tcggggctgc
840atgggcgctc cgatgccgct ccagggcgag cgctgtttaa atagccaggc
ccccgattgc 900aaagacatta tagcgagcta ccaaagccat attcaaacac
ctagatcact accacttcta 960cacaggccac tcgagcttgt gatcgcactc
cgctaagggg gcgcctcttc ctcttcgttt 1020cagtcacaac ccgcaaactc
tagaatatca atgctgctgc aggccttcct gttcctgctg 1080gccggcttcg
ccgccaagat cagcgcctcc atgacgaacg agacgtccga ccgccccctg
1140gtgcacttca cccccaacaa gggctggatg aacgacccca acggcctgtg
gtacgacgag 1200aaggacgcca agtggcacct gtacttccag tacaacccga
acgacaccgt ctgggggacg 1260cccttgttct ggggccacgc cacgtccgac
gacctgacca actgggagga ccagcccatc 1320gccatcgccc cgaagcgcaa
cgactccggc gccttctccg gctccatggt ggtggactac 1380aacaacacct
ccggcttctt caacgacacc atcgacccgc gccagcgctg cgtggccatc
1440tggacctaca acaccccgga gtccgaggag cagtacatct cctacagcct
ggacggcggc 1500tacaccttca ccgagtacca gaagaacccc gtgctggccg
ccaactccac ccagttccgc 1560gacccgaagg tcttctggta cgagccctcc
cagaagtgga tcatgaccgc ggccaagtcc 1620caggactaca agatcgagat
ctactcctcc gacgacctga agtcctggaa gctggagtcc 1680gcgttcgcca
acgagggctt cctcggctac cagtacgagt gccccggcct gatcgaggtc
1740cccaccgagc aggaccccag caagtcctac tgggtgatgt tcatctccat
caaccccggc 1800gccccggccg gcggctcctt caaccagtac ttcgtcggca
gcttcaacgg cacccacttc 1860gaggccttcg acaaccagtc ccgcgtggtg
gacttcggca aggactacta cgccctgcag 1920accttcttca acaccgaccc
gacctacggg agcgccctgg gcatcgcgtg ggcctccaac 1980tgggagtact
ccgccttcgt gcccaccaac ccctggcgct cctccatgtc cctcgtgcgc
2040aagttctccc tcaacaccga gtaccaggcc aacccggaga cggagctgat
caacctgaag 2100gccgagccga tcctgaacat cagcaacgcc ggcccctgga
gccggttcgc caccaacacc 2160acgttgacga aggccaacag ctacaacgtc
gacctgtcca acagcaccgg caccctggag 2220ttcgagctgg tgtacgccgt
caacaccacc cagacgatct ccaagtccgt gttcgcggac 2280ctctccctct
ggttcaaggg cctggaggac cccgaggagt acctccgcat gggcttcgag
2340gtgtccgcgt cctccttctt cctggaccgc gggaacagca aggtgaagtt
cgtgaaggag 2400aacccctact tcaccaaccg catgagcgtg aacaaccagc
ccttcaagag cgagaacgac 2460ctgtcctact acaaggtgta cggcttgctg
gaccagaaca tcctggagct gtacttcaac 2520gacggcgacg tcgtgtccac
caacacctac ttcatgacca ccgggaacgc cctgggctcc 2580gtgaacatga
cgacgggggt ggacaacctg ttctacatcg acaagttcca ggtgcgcgag
2640gtcaagtgac aattggcagc agcagctcgg atagtatcga cacactctgg
acgctggtcg 2700tgtgatggac tgttgccgcc acacttgctg ccttgacctg
tgaatatccc tgccgctttt 2760atcaaacagc ctcagtgtgt ttgatcttgt
gtgtacgcgc ttttgcgagt tgctagctgc 2820ttgtgctatt tgcgaatacc
acccccagca tccccttccc tcgtttcata tcgcttgcat 2880cccaaccgca
acttatctac gctgtcctgc tatccctcag cgctgctcct gctcctgctc
2940actgcccctc gcacagcctt ggtttgggct ccgcctgtat tctcctggta
ctgcaacctg 3000taaaccagca ctgcaatgct gatgcacggg aagtagtggg
atgggaacac aaatggagga 3060tcccgcgtct cgaacagagc gcgcagagga
acgctgaagg tctcgcctct gtcgcacctc 3120agcgcggcat acaccacaat
aaccacctga cgaatgcgct tggttcttcg tccattagcg 3180aagcgtccgg
ttcacacacg tgccacgttg gcgaggtggc aggtgacaat gatcggtgga
3240gctgatggtc gaaacgttca cagcctaggg atatcgaatt cggccgacag
gacgcgcgtc 3300aaaggtgctg gtcgtgtatg ccctggccgg caggtcgttg
ctgctgctgg ttagtgattc 3360cgcaaccctg attttggcgt cttattttgg
cgtggcaaac gctggcgccc gcgagccggg 3420ccggcggcga tgcggtgccc
cacggctgcc ggaatccaag ggaggcaaga gcgcccgggt 3480cagttgaagg
gctttacgcg caaggtacag ccgctcctgc aaggctgcgt ggtggaattg
3540gacgtgcagg tcctgctgaa gttcctccac cgcctcacca gcggacaaag
caccggtgta 3600tcaggtccgt gtcatccact ctaaagaact cgactacgac
ctactgatgg ccctagattc 3660ttcatcaaaa acgcctgaga cacttgccca
ggattgaaac tccctgaagg gaccaccagg 3720ggccctgagt tgttccttcc
ccccgtggcg agctgccagc caggctgtac ctgtgatcga 3780ggctggcggg
aaaataggct tcgtgtgctc aggtcatggg aggtgcagga cagctcatga
3840aacgccaaca atcgcacaat tcatgtcaag ctaatcagct atttcctctt
cacgagctgt 3900aattgtccca aaattctggt ctaccggggg tgatccttcg
tgtacgggcc cttccctcaa 3960ccctaggtat gcgcgcatgc ggtcgccgcg
caactcgcgc gagggccgag ggtttgggac 4020gggccgtccc gaaatgcagt
tgcacccgga tgcgtggcac cttttttgcg ataatttatg 4080caatggactg
ctctgcaaaa ttctggctct gtcgccaacc ctaggatcag cggcgtagga
4140tttcgtaatc attcgtcctg atggggagct accgactacc ctaatatcag
cccgactgcc 4200tgacgccagc gtccactttt gtgcacacat tccattcgtg
cccaagacat ttcattgtgg 4260tgcgaagcgt ccccagttac gctcacctgt
ttcccgacct ccttactgtt ctgtcgacag 4320agcgggccca caggccggtc
gcagccacta gtatgacctc catcaacgtg aagctgctgt 4380accactacgt
gatcaccaac ctgttcaacc tgtgcttctt ccccctgacc gccatcgtgg
4440ccggcaaggc ctcccgcctg accatcgacg acctgcacca cctgtactac
tcctacctgc 4500agcacaacgt gatcaccatc gcccccctgt tcgccttcac
cgtgttcggc tccatcctgt 4560acatcgtgac ccgccccaag cccgtgtacc
tggtggagta ctcctgctac ctgcccccca 4620cccagtgccg ctcctccatc
tccaaggtga tggacatctt ctaccaggtg cgcaaggccg 4680accccttccg
caacggcacc tgcgacgact cctcctggct ggacttcctg cgcaagatcc
4740aggagcgctc cggcctgggc gacgagaccc acggccccga gggcctgctg
caggtgcccc 4800cccgcaagac cttcgccgcc gcccgcgagg agaccgagca
ggtgatcgtg ggcgccctga 4860agaacctgtt cgagaacacc aaggtgaacc
ccaaggacat cggcatcctg gtggtgaact 4920cctccatgtt caaccccacc
ccctccctgt ccgccatggt ggtgaacacc ttcaagctgc 4980gctccaacgt
gcgctccttc aacctgggcg gcatgggctg ctccgccggc gtgatcgcca
5040tcgacctggc caaggacctg ctgcacgtgc acaagaacac ctacgccctg
gtggtgtcca 5100ccgagaacat cacctacaac atctacgccg gcgacaaccg
ctccatgatg gtgtccaact 5160gcctgttccg cgtgggcggc gccgccatcc
tgctgtccaa caagccccgc gaccgccgcc 5220gctccaagta cgagctggtg
cacaccgtgc gcacccacac cggcgccgac gacaagtcct 5280tccgctgcgt
gcagcagggc gacgacgaga acggcaagac cggcgtgtcc ctgtccaagg
5340acatcaccga ggtggccggc cgcaccgtga agaagaacat cgccaccctg
ggccccctga 5400tcctgcccct gtccgagaag ctgctgttct tcgtgacctt
catggccaag aagctgttca 5460aggacaaggt gaagcactac tacgtgcccg
acttcaagct ggccatcgac cacttctgca 5520tccacgccgg cggccgcgcc
gtgatcgacg tgctggagaa gaacctgggc ctggccccca 5580tcgacgtgga
ggcctcccgc tccaccctgc accgcttcgg caacacctcc tcctcctcca
5640tctggtacga gctggcctac atcgaggcca agggccgcat gaagaagggc
aacaaggtgt 5700ggcagatcgc cctgggctcc ggcttcaagt gcaactccgc
cgtgtgggtg gccctgtcca 5760acgtgaaggc ctccaccaac tccccctggg
agcactgcat cgaccgctac cccgtgaaga 5820tcgactccga ctccgccaag
tccgagaccc gcgcccagaa cggccgctcc tgacttaagg 5880cagcagcagc
tcggatagta tcgacacact ctggacgctg gtcgtgtgat ggactgttgc
5940cgccacactt gctgccttga cctgtgaata tccctgccgc ttttatcaaa
cagcctcagt 6000gtgtttgatc ttgtgtgtac gcgcttttgc gagttgctag
ctgcttgtgc tatttgcgaa 6060taccaccccc agcatcccct tccctcgttt
catatcgctt gcatcccaac cgcaacttat 6120ctacgctgtc ctgctatccc
tcagcgctgc tcctgctcct gctcactgcc cctcgcacag 6180ccttggtttg
ggctccgcct gtattctcct ggtactgcaa cctgtaaacc agcactgcaa
6240tgctgatgca cgggaagtag tgggatggga acacaaatgg aaagcttaat
taagagctct 6300tgttttccag aaggagttgc tccttgagcc tttcattctc
agcctcgata acctccaaag 6360ccgctctaat tgtggagggg gttcgaattt
aaaagcttgg aatgttggtt cgtgcgtctg 6420gaacaagccc agacttgttg
ctcactggga aaaggaccat cagctccaaa aaacttgccg 6480ctcaaaccgc
gtacctctgc tttcgcgcaa tctgccctgt tgaaatcgcc accacattca
6540tattgtgacg cttgagcagt ctgtaattgc ctcagaatgt ggaatcatct
gccccctgtg 6600cgagcccatg ccaggcatgt cgcgggcgag gacacccgcc
actcgtacag cagaccatta 6660tgctacctca caatagttca taacagtgac
catatttctc gaagctcccc aacgagcacc 6720tccatgctct gagtggccac
cccccggccc tggtgcttgc ggagggcagg tcaaccggca 6780tggggctacc
gaaatccccg accggatccc accacccccg cgatgggaag aatctctccc
6840cgggatgtgg gcccaccacc agcacaacct gctggcccag gcgagcgtca
aaccatacca 6900cacaaatatc cttggcatcg gccctgaatt ccttctgccg
ctctgctacc cggtgcttct 6960gtccgaagca ggggttgcta gggatcgctc
cgagtccgca aacccttgtc gcgtggcggg 7020gcttgttcga gcttgaagag c
70411031530DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 103actagtatga cctccatcaa cgtgaagctg
ctgtaccact acgtgatcac caacttcttc 60aacctgtgct tcttccccct gaccgccatc
ctggccggca aggcctcccg cctgaccacc 120aacgacctgc accacttcta
ctcctacctg cagcacaacc tgatcaccct gaccctgctg 180ttcgccttca
ccgtgttcgg ctccgtgctg tacttcgtga cccgccccaa gcccgtgtac
240ctggtggact actcctgcta cctgcccccc cagcacctgt ccgccggcat
ctccaagacc 300atggagatct tctaccagat ccgcaagtcc gaccccctgc
gcaacgtggc cctggacgac 360tcctcctccc tggacttcct gcgcaagatc
caggagcgct ccggcctggg cgacgagacc 420tacggccccg agggcctgtt
cgagatcccc ccccgcaaga acctggcctc cgcccgcgag 480gagaccgagc
aggtgatcaa cggcgccctg aagaacctgt tcgagaacac caaggtgaac
540cccaaggaga tcggcatcct ggtggtgaac tcctccatgt tcaaccccac
cccctccctg 600tccgccatgg tggtgaacac cttcaagctg cgctccaaca
tcaagtcctt caacctgggc 660ggcatgggct gctccgccgg cgtgatcgcc
atcgacctgg ccaaggacct gctgcacgtg 720cacaagaaca cctacgccct
ggtggtgtcc accgagaaca tcacccagaa catctacacc 780ggcgacaacc
gctccatgat ggtgtccaac tgcctgttcc gcgtgggcgg cgccgccatc
840ctgctgtcca acaagcccgg cgaccgccgc cgctccaagt accgcctggc
ccacaccgtg 900cgcacccaca ccggcgccga cgacaagtcc ttcggctgcg
tgcgccagga ggaggacgac 960tccggcaaga ccggcgtgtc cctgtccaag
gacatcaccg gcgtggccgg catcaccgtg 1020cagaagaaca tcaccaccct
gggccccctg gtgctgcccc tgtccgagaa gatcctgttc 1080gtggtgacct
tcgtggccaa gaagctgctg aaggacaaga tcaagcacta ctacgtgccc
1140gacttcaagc tggccgtgga ccacttctgc atccacgccg gcggccgcgc
cgtgatcgac 1200gtgctggaga agaacctggg cctgtccccc atcgacgtgg
aggcctcccg ctccaccctg 1260caccgcttcg gcaacacctc ctcctcctcc
atctggtacg agctggccta catcgaggcc 1320aagggccgca tgaagaaggg
caacaaggcc tggcagatcg ccgtgggctc cggcttcaag 1380tgcaactccg
ccgtgtgggt ggccctgcgc aacgtgaagg cctccgccaa ctccccctgg
1440gagcactgca tccacaagta ccccgtgcag atgtactccg gctcctccaa
gtccgagacc 1500cgcgcccaga acggccgctc ctgacttaag
15301041533DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 104actagtatga cctccatcaa cgtgaagctg
ctgtaccact acgtgctgac caacttcttc 60aacctgtgcc tgttccccct gaccgccttc
cccgccggca aggcctccca gctgaccacc 120aacgacctgc accacctgta
ctcctacctg caccacaacc tgatcaccgt gaccctgctg 180ttcgccttca
ccgtgttcgg ctccatcctg tacatcgtga cccgccccaa gcccgtgtac
240ctggtggact actcctgcta cctgcccccc cgccacctgt cctgcggcat
ctcccgcgtg 300atggagatct tctacgagat ccgcaagtcc gacccctccc
gcgaggtgcc cttcgacgac 360ccctcctccc tggagttcct gcgcaagatc
caggagcgct ccggcctggg cgacgagacc 420tacggccccc agggcctggt
gcacgacatg cccctgcgca tgaacttcgc cgccgcccgc 480gaggagaccg
agcaggtgat caacggcgcc ctggagaagc tgttcgagaa caccaaggtg
540aacccccgcg agatcggcat cctggtggtg aactcctcca tgttcaaccc
caccccctcc 600ctgtccgcca tggtggtgaa caccttcaag ctgcgctcca
acatcaagtc cttctccctg 660ggcggcatgg gctgctccgc cggcatcatc
gccatcgacc tggccaagga cctgctgcac 720gtgcacaaga acacctacgc
cctggtggtg tccaccgaga acatcaccca ctccacctac 780accggcgaca
accgctccat gatggtgtcc aactgcctgt tccgcatggg cggcgccgcc
840atcctgctgt ccaacaaggc cggcgaccgc cgccgctcca agtacaagct
ggcccacacc 900gtgcgcaccc acaccggcgc cgacgaccag tccttccgct
gcgtgcgcca ggaggacgac 960gaccgcggca agatcggcgt gtgcctgtcc
aaggacatca ccgccgtggc cggcaagacc 1020gtgaccaaga acatcgccac
cctgggcccc ctggtgctgc ccctgtccga gaagttcctg 1080tacgtggtgt
ccctgatggc caagaagctg ttcaagaaca agatcaagca cacctacgtg
1140cccgacttca agctggccat cgaccacttc tgcatccacg ccggcggccg
cgccgtgatc 1200gacgtgctgg agaagaacct ggccctgtcc cccgtggacg
tggaggcctc ccgctccacc 1260ctgcaccgct tcggcaacac ctcctcctcc
tccatctggt acgagctggc ctacatcgag 1320gccaagggcc gcatgaagaa
gggcaacaag gtgtggcaga tcgccatcgg ctccggcttc 1380aagtgcaact
ccgccgtgtg ggtggccctg tgcaacgtga agccctccgt gaactccccc
1440tgggagcact gcatcgaccg ctaccccgtg gagatcaact acggctcctc
caagtccgag 1500acccgcgccc agaacggccg ctcctgactt aag
15331051524DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 105actagtatgt ccggcaccaa ggccacctcc
gtgtccgtgc ccctgcccga cttcaagcag 60tccgtgaacc tgaagtacgt gaagctgggc
taccactact ccatcaccca cgccatgtac 120ctgttcctga cccccctgct
gctgatcatg tccgcccaga tctccacctt ctccatccag 180gacttccacc
acctgtacaa ccacctgatc ctgcacaacc tgtcctccct gatcctgtgc
240atcgccctgc tgctgttcgt gctgaccctg tacttcctga cccgccccac
ccccgtgtac 300ctgctgaact tctcctgcta caagcccgac gccatccaca
agtgcgaccg ccgccgcttc 360atggacacca tccgcggcat gggcacctac
accgaggaga acatcgagtt ccagcgcaag 420gtgctggagc gctccggcat
cggcgagtcc tcctacctgc cccccaccgt gttcaagatc 480cccccccgcg
tgtacgacgc cgaggagcgc gccgaggccg agatgctgat gttcggcgcc
540gtggacggcc tgttcgagaa gatctccgtg aagcccaacc agatcggcgt
gctggtggtg 600aactgcggcc tgttcaaccc catcccctcc ctgtcctcca
tgatcgtgaa ccgctacaag 660atgcgcggca acgtgttctc ctacaacctg
ggcggcatgg gctgctccgc cggcgtgatc 720tccatcgacc tggccaagga
cctgctgcag gtgcgcccca actcctacgc cctggtggtg 780tccctggagt
gcatctccaa gaacctgtac ctgggcgagc agcgctccat gctggtgtcc
840aactgcctgt tccgcatggg cggcgccgcc atcctgctgt ccaacaagat
gtccgaccgc 900tggcgctcca agtaccgcct ggtgcacacc gtgcgcaccc
acaagggcac cgaggacaac 960tgcttctcct gcgtgacccg caaggaggac
tccgacggca agatcggcat ctccctgtcc 1020aagaacctga tggccgtggc
cggcgacgcc ctgaagacca acatcaccac cctgggcccc 1080ctggtgctgc
ccatgtccga gcagctgctg ttcttcgcca ccctggtggg caagaaggtg
1140ttcaagatga agctgcagcc ctacatcccc gacttcaagc tggccttcga
gcacttctgc 1200atccacgccg gcggccgcgc cgtgctggac gagctggaga
agaacctgaa gctgtcctcc 1260tggcacatgg agccctcccg catgtccctg
taccgcttcg gcaacacctc ctcctcctcc 1320ctgtggtacg agctggccta
ctccgaggcc aagggccgca tcaagaaggg cgaccgcgtg 1380tggcagatcg
ccttcggctc cggcttcaag tgcaactccg ccgtgtggaa ggccctgcgc
1440aacgtgaacc ccgccgagga gaagaacccc tggatggacg agatccacct
gttccccgtg 1500gaggtgcccc tgaactgact taag 15241061530DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
106actagtatga cctccatcaa cgtgaagctg ctgtaccact acgtgatcac
caacctgttc 60aacctgtgct tcttccccct gaccgccatc gtggccggca aggcctacct
gaccatcgac 120gacctgcacc acctgtacta ctcctacctg cagcacaacc
tgatcaccat cgcccccctg 180ctggccttca ccgtgttcgg ctccgtgctg
tacatcgcca cccgccccaa gcccgtgtac 240ctggtggagt actcctgcta
cctgcccccc acccactgcc gctcctccat ctccaaggtg 300atggacatct
tcttccaggt gcgcaaggcc gacccctccc gcaacggcac ctgcgacgac
360tcctcctggc tggacttcct gcgcaagatc caggagcgct ccggcctggg
cgacgagacc 420cacggccccg agggcctgct gcaggtgccc ccccgcaaga
ccttcgcccg cgcccgcgag 480gagaccgagc aggtgatcat cggcgccctg
gagaacctgt tcaagaacac caacgtgaac 540cccaaggaca tcggcatcct
ggtggtgaac tcctccatgt tcaaccccac cccctccctg 600tccgccatgg
tggtgaacac cttcaagctg cgctccaacg tgcgctcctt caacctgggc
660ggcatgggct gctccgccgg cgtgatcgcc atcgacctgg ccaaggacct
gctgcacgtg 720cacaagaaca cctacgccct ggtggtgtcc accgagaaca
tcacctacaa catctacgcc 780ggcgacaacc gctccatgat ggtgtccaac
tgcctgttcc gcgtgggcgg cgccgccatc 840ctgctgtcca acaagccccg
cgaccgccgc cgctccaagt acgagctggt gcacaccgtg 900cgcacccaca
ccggcgccga cgacaagtcc ttccgctgcg tgcagcaggg cgacgacgag
960aacggccaga ccggcgtgtc cctgtccaag gacatcaccg acgtggccgg
ccgcaccgtg 1020aagaagaaca tcgccaccct gggccccctg atcctgcccc
tgtccgagaa gctgctgttc 1080ttcgtgacct tcatgggcaa gaagctgttc
aaggacgaga tcaagcacta ctacgtgccc 1140gacttcaagc tggccatcga
ccacttctgc atccacgccg gcggcaaggc cgtgatcgac 1200gtgctggaga
agaacctggg cctggccccc atcgacgtgg aggcctcccg ctccaccctg
1260caccgcttcg gcaacacctc ctcctcctcc atctggtacg agctggccta
catcgagccc 1320aagggccgca tgaagaaggg caacaaggtg tggcagatcg
ccctgggctc cggcttcaag 1380tgcaactccg ccgtgtgggt ggccctgaac
aacgtgaagg cctccaccaa ctccccctgg 1440gagcactgca tcgaccgcta
ccccgtgaag atcgactccg actccggcaa gtccgagacc 1500cgcgtgccca
acggccgctc ctgacttaag 15301071599DNAArtificial SequenceDescription
of Artificial Sequence Synthetic polynucleotide 107actagtatgg
agcgcaccaa ctccatcgag atggaccagg agcgcctgac cgccgagatg 60gccttcaagg
actcctcctc cgccgtgatc cgcatccgcc gccgcctgcc cgacttcctg
120acctccgtga agctgaagta cgtgaagctg ggcctgcaca actccttcaa
cttcaccacc 180ttcctgttcc tgctgatcat cctgcccctg accggcaccg
tgctggtgca gctgaccggc 240ctgaccttcg agaccttctc cgagctgtgg
tacaaccacg ccgcccagct ggacggcgtg 300acccgcctgg cctgcctggt
gtccctgtgc ttcgtgctga tcatctacgt gaccaaccgc 360tccaagcccg
tgtacctggt ggacttctcc tgctacaagc ccgaggacga gcgcaagatg
420tccgtggact ccttcctgaa gatgaccgag cagaacggcg ccttcaccga
cgacaccgtg 480cagttccagc agcgcatctc caaccgcgcc ggcctgggcg
acgagaccta cctgccccgc 540ggcatcacct ccaccccccc caagctgaac
atgtccgagg cccgcgccga ggccgaggcc 600gtgatgttcg gcgccctgga
ctccctgttc gagaagaccg gcatcaagcc cgccgaggtg 660ggcatcctga
tcgtgtcctg ctccctgttc aaccccaccc cctccctgtc cgccatgatc
720gtgaaccact acaagatgcg cgaggacatc aagtcctaca acctgggcgg
catgggctgc 780tccgccggcc tgatctccat cgacctggcc aacaacctgc
tgaaggccaa ccccaactcc 840tacgccgtgg tggtgtccac cgagaacatc
accctgaact ggtacttcgg caacgaccgc 900tccatgctgc tgtgcaactg
catcttccgc atgggcggcg ccgccatcct gctgtccaac 960cgccgccagg
accgctccaa gtccaagtac gagctggtga acgtggtgcg cacccacaag
1020ggctccgacg acaagaacta caactgcgtg taccagaagg aggacgagcg
cggcaccatc 1080ggcgtgtccc tggcccgcga gctgatgtcc gtggccggcg
acgccctgaa gaccaacatc 1140accaccctgg gccccatggt gctgcccctg
tccggccagc tgatgttctc cgtgtccctg 1200gtgaagcgca agctgctgaa
gctgaaggtg aagccctaca tccccgactt caagctggcc 1260ttcgagcact
tctgcatcca cgccggcggc cgcgccgtgc tggacgaggt gcagaagaac
1320ctggacctgg aggactggca catggagccc tcccgcatga ccctgcaccg
cttcggcaac 1380acctcctcct cctccctgtg gtacgagatg gcctacaccg
aggccaaggg ccgcgtgaag 1440gccggcgacc gcctgtggca gatcgccttc
ggctccggct tcaagtgcaa ctccgccgtg 1500tggaaggccc tgcgcgtggt
gtccaccgag gagctgaccg gcaacgcctg ggccggctcc 1560atcgagaact
accccgtgaa gatcgtgcag tgacttaag 15991085988DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
108gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga
3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg tctcgcctct
gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga cgaatgcgct
tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg
gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca
cagcctaggg atatcgaatt cctttcttgc gctatgacac 3300ttccagcaaa
aggtagggcg ggctgcgaga cggcttcccg gcgctgcatg caacaccgat
3360gatgcttcga ccccccgaag ctccttcggg gctgcatggg cgctccgatg
ccgctccagg 3420gcgagcgctg tttaaatagc caggcccccg attgcaaaga
cattatagcg agctaccaaa 3480gccatattca aacacctaga tcactaccac
ttctacacag gccactcgag cttgtgatcg 3540cactccgcta agggggcgcc
tcttcctctt cgtttcagtc acaacccgca aacactagta 3600tggccaccgc
atccactttc tcggcgttca atgcccgctg cggcgacctg cgtcgctcgg
3660cgggctccgg gccccggcgc ccagcgaggc ccctccccgt gcgcgggcgc
gccgaggtgc 3720acgtgcaggt gacccactcc ctggcccccg agaagcgcga
gatcttcaac tccctgaaca 3780actgggccca ggagaacatc ctggtgctgc
tgaaggacgt ggacaagtgc tggcagccct 3840ccgacttcct gcccgactcc
gcctccgagg gcttcgacga gcaggtgatg gagctgcgca 3900agcgctgcaa
ggagatcccc gacgactact tcatcgtgct ggtgggcgac atgatcaccg
3960aggaggccct gcccacctac cagaccatgc tgaacaccct ggacggcgtg
cgcgacgaga 4020ccggcgcctc cctgaccccc tgggccatct ggacccgcgc
ctggaccgcc gaggagaacc 4080gccacggcga cctgctgaac aagtacctgt
acctgtccgg ccgcgtggac atgaagcaga 4140tcgagaagac catccagtac
ctgatcggct ccggcatgga cccccgcacc gagaacaacc 4200cctacctggg
cttcatctac acctccttcc aggagcgcgc caccttcatc tcccacggca
4260acaccgcccg cctggccaag gagcacggcg acctgaagct ggcccagatc
tgcggcatca 4320tcgccgccga cgagaagcgc cacgagaccg cctacaccaa
gatcgtggag aagctgttcg 4380agatcgaccc cgacggcacc gtgctggccc
tggccgacat gatgcgcaag aaggtgtcca 4440tgcccgccca cctgatgtac
gacggccagg acgacaacct gttcgagaac ttctcctccg 4500tggcccagcg
cctgggcgtg tacaccgcca aggactacgc cgacatcctg gagttcctgg
4560tgggccgctg ggacatcgag aagctgaccg gcctgtccgg cgagggccgc
aaggcccagg 4620actacgtgtg caccctgccc ccccgcatcc gccgcctgga
ggagcgcgcc cagtcccgcg 4680tgaagaaggc ctccgccacc cccttctcct
ggatcttcgg ccgcgagatc aacctgatgg 4740actacaagga ccacgacggc
gactacaagg accacgacat cgactacaag gacgacgacg 4800acaagtgaat
cgatagatct cttaaggcag cagcagctcg gatagtatcg acacactctg
4860gacgctggtc gtgtgatgga ctgttgccgc cacacttgct gccttgacct
gtgaatatcc 4920ctgccgcttt tatcaaacag cctcagtgtg tttgatcttg
tgtgtacgcg cttttgcgag 4980ttgctagctg cttgtgctat ttgcgaatac
cacccccagc atccccttcc ctcgtttcat 5040atcgcttgca tcccaaccgc
aacttatcta cgctgtcctg ctatccctca gcgctgctcc 5100tgctcctgct
cactgcccct cgcacagcct tggtttgggc tccgcctgta ttctcctggt
5160actgcaacct gtaaaccagc actgcaatgc tgatgcacgg gaagtagtgg
gatgggaaca 5220caaatggaaa gcttaattaa gagctcttgt tttccagaag
gagttgctcc ttgagccttt 5280cattctcagc ctcgataacc tccaaagccg
ctctaattgt ggagggggtt cgaatttaaa 5340agcttggaat gttggttcgt
gcgtctggaa caagcccaga cttgttgctc actgggaaaa 5400ggaccatcag
ctccaaaaaa cttgccgctc aaaccgcgta cctctgcttt cgcgcaatct
5460gccctgttga aatcgccacc acattcatat tgtgacgctt gagcagtctg
taattgcctc 5520agaatgtgga atcatctgcc ccctgtgcga gcccatgcca
ggcatgtcgc gggcgaggac 5580acccgccact cgtacagcag accattatgc
tacctcacaa tagttcataa cagtgaccat 5640atttctcgaa gctccccaac
gagcacctcc atgctctgag tggccacccc ccggccctgg 5700tgcttgcgga
gggcaggtca accggcatgg ggctaccgaa atccccgacc ggatcccacc
5760acccccgcga tgggaagaat ctctccccgg gatgtgggcc caccaccagc
acaacctgct 5820ggcccaggcg agcgtcaaac cataccacac aaatatcctt
ggcatcggcc ctgaattcct 5880tctgccgctc tgctacccgg tgcttctgtc
cgaagcaggg gttgctaggg atcgctccga 5940gtccgcaaac ccttgtcgcg
tggcggggct tgttcgagct tgaagagc 59881096807DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
109gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga
3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg tctcgcctct
gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga cgaatgcgct
tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg
gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca
cagcctaggg atatcgaatt cggccgacag gacgcgcgtc 3300aaaggtgctg
gtcgtgtatg ccctggccgg caggtcgttg ctgctgctgg ttagtgattc
3360cgcaaccctg attttggcgt cttattttgg cgtggcaaac gctggcgccc
gcgagccggg 3420ccggcggcga tgcggtgccc cacggctgcc ggaatccaag
ggaggcaaga gcgcccgggt 3480cagttgaagg gctttacgcg caaggtacag
ccgctcctgc aaggctgcgt ggtggaattg 3540gacgtgcagg tcctgctgaa
gttcctccac cgcctcacca gcggacaaag caccggtgta 3600tcaggtccgt
gtcatccact ctaaagaact cgactacgac ctactgatgg ccctagattc
3660ttcatcaaaa acgcctgaga cacttgccca ggattgaaac tccctgaagg
gaccaccagg 3720ggccctgagt tgttccttcc ccccgtggcg agctgccagc
caggctgtac ctgtgatcga 3780ggctggcggg aaaataggct tcgtgtgctc
aggtcatggg aggtgcagga cagctcatga 3840aacgccaaca atcgcacaat
tcatgtcaag ctaatcagct atttcctctt cacgagctgt 3900aattgtccca
aaattctggt ctaccggggg tgatccttcg tgtacgggcc cttccctcaa
3960ccctaggtat gcgcgcatgc ggtcgccgcg caactcgcgc gagggccgag
ggtttgggac 4020gggccgtccc gaaatgcagt tgcacccgga tgcgtggcac
cttttttgcg ataatttatg 4080caatggactg ctctgcaaaa ttctggctct
gtcgccaacc ctaggatcag cggcgtagga 4140tttcgtaatc attcgtcctg
atggggagct accgactacc ctaatatcag cccgactgcc 4200tgacgccagc
gtccactttt gtgcacacat tccattcgtg cccaagacat ttcattgtgg
4260tgcgaagcgt ccccagttac gctcacctgt ttcccgacct ccttactgtt
ctgtcgacag 4320agcgggccca caggccggtc gcagccacta gtatggccac
cgcatccact ttctcggcgt 4380tcaatgcccg ctgcggcgac ctgcgtcgct
cggcgggctc cgggccccgg cgcccagcga 4440ggcccctccc cgtgcgcggg
cgcgccgcct ccaccctgaa gtccggctcc aaggaggtgg 4500agaacctgaa
gaagcccttc atgccccccc gcgaggtgca cgtgcaggtg acccactcca
4560tgccccccca gaagatcgag atcttcaagt ccctggacaa ctgggccgag
gagaacatcc 4620tggtgcacct gaagcccgtg gagaagtgct ggcagcccca
ggacttcctg cccgaccccg 4680cctccgacgg cttcgacgag caggtgcgcg
agctgcgcga gcgcgccaag gagatccccg 4740acgactactt cgtggtgctg
gtgggcgaca tgatcaccga ggaggccctg cccacctacc 4800agaccatgct
gaacaccctg gacggcgtgc gcgacgagac cggcgcctcc cccacctcct
4860gggccatctg gacccgcgcc tggaccgccg aggagaaccg ccacggcgac
ctgctgaaca 4920agtacctgta cctgtccggc cgcgtggaca tgcgccagat
cgagaagacc atccagtacc 4980tgatcggctc cggcatggac ccccgcaccg
agaactcccc ctacctgggc ttcatctaca 5040cctccttcca ggagcgcgcc
accttcatct cccacggcaa caccgcccgc caggccaagg 5100agcacggcga
catcaagctg gcccagatct gcggcaccat cgccgccgac gagaagcgcc
5160acgagaccgc ctacaccaag atcgtggaga agctgttcga gatcgacccc
gacggcaccg 5220tgctggcctt cgccgacatg atgcgcaaga agatctccat
gcccgcccac ctgatgtacg 5280acggccgcga cgacaacctg ttcgaccact
tctccgccgt ggcccagcgc ctgggcgtgt 5340acaccgccaa ggactacgcc
gacatcctgg agttcctggt gggccgctgg aaggtggaca 5400agctgaccgg
cctgtccgcc gagggccaga aggcccagga ctacgtgtgc cgcctgcccc
5460cccgcatccg ccgcctggag gagcgcgccc agggccgcgc caaggaggcc
cccaccatgc 5520ccttctcctg gatcttcgac cgccaggtga agctgatgga
ctacaaggac cacgacggcg 5580actacaagga ccacgacatc gactacaagg
acgacgacga caagtgaatc gatagatctc 5640ttaaggcagc agcagctcgg
atagtatcga cacactctgg acgctggtcg tgtgatggac 5700tgttgccgcc
acacttgctg ccttgacctg tgaatatccc tgccgctttt atcaaacagc
5760ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt tgctagctgc
ttgtgctatt 5820tgcgaatacc acccccagca tccccttccc tcgtttcata
tcgcttgcat cccaaccgca 5880acttatctac gctgtcctgc tatccctcag
cgctgctcct gctcctgctc actgcccctc 5940gcacagcctt ggtttgggct
ccgcctgtat tctcctggta ctgcaacctg taaaccagca 6000ctgcaatgct
gatgcacggg aagtagtggg atgggaacac aaatggaaag cttaattaag
6060agctcttgtt ttccagaagg agttgctcct tgagcctttc attctcagcc
tcgataacct 6120ccaaagccgc tctaattgtg gagggggttc gaatttaaaa
gcttggaatg ttggttcgtg 6180cgtctggaac aagcccagac ttgttgctca
ctgggaaaag gaccatcagc tccaaaaaac 6240ttgccgctca aaccgcgtac
ctctgctttc gcgcaatctg ccctgttgaa atcgccacca 6300cattcatatt
gtgacgcttg agcagtctgt aattgcctca gaatgtggaa tcatctgccc
6360cctgtgcgag cccatgccag gcatgtcgcg ggcgaggaca cccgccactc
gtacagcaga 6420ccattatgct acctcacaat agttcataac agtgaccata
tttctcgaag ctccccaacg 6480agcacctcca tgctctgagt ggccaccccc
cggccctggt gcttgcggag ggcaggtcaa 6540ccggcatggg gctaccgaaa
tccccgaccg gatcccacca cccccgcgat gggaagaatc 6600tctccccggg
atgtgggccc accaccagca caacctgctg gcccaggcga gcgtcaaacc
6660ataccacaca aatatccttg gcatcggccc tgaattcctt ctgccgctct
gctacccggt 6720gcttctgtcc gaagcagggg ttgctaggga tcgctccgag
tccgcaaacc cttgtcgcgt 6780ggcggggctt gttcgagctt gaagagc
68071106744DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 110gctcttcgcc gccgccactc ctgctcgagc
gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga
tgtccatcac caggtccatg aggtctgcct 120tgcgccggct gagccactgc
ttcgtccggg cggccaagag gagcatgagg gaggactcct 180ggtccagggt
cctgacgtgg tcgcggctct gggagcgggc cagcatcatc tggctctgcc
240gcaccgaggc cgcctccaac tggtcctcca gcagccgcag tcgccgccga
ccctggcaga 300ggaagacagg tgaggggggt atgaattgta cagaacaacc
acgagccttg tctaggcaga 360atccctacca gtcatggctt tacctggatg
acggcctgcg aacagctgtc cagcgaccct 420cgctgccgcc gcttctcccg
cacgcttctt tccagcaccg tgatggcgcg agccagcgcc 480gcacgctggc
gctgcgcttc gccgatctga ggacagtcgg ggaactctga tcagtctaaa
540cccccttgcg cgttagtgtt gccatccttt gcagaccggt gagagccgac
ttgttgtgcg 600ccacccccca caccacctcc tcccagacca attctgtcac
ctttttggcg aaggcatcgg 660cctcggcctg cagagaggac agcagtgccc
agccgctggg ggttggcgga tgcacgctca 720ggtacccttt cttgcgctat
gacacttcca gcaaaaggta gggcgggctg cgagacggct 780tcccggcgct
gcatgcaaca ccgatgatgc ttcgaccccc cgaagctcct tcggggctgc
840atgggcgctc cgatgccgct ccagggcgag cgctgtttaa atagccaggc
ccccgattgc 900aaagacatta tagcgagcta ccaaagccat attcaaacac
ctagatcact accacttcta 960cacaggccac tcgagcttgt gatcgcactc
cgctaagggg gcgcctcttc ctcttcgttt 1020cagtcacaac ccgcaaactc
tagaatatca atgctgctgc aggccttcct gttcctgctg 1080gccggcttcg
ccgccaagat cagcgcctcc atgacgaacg agacgtccga ccgccccctg
1140gtgcacttca cccccaacaa gggctggatg aacgacccca acggcctgtg
gtacgacgag 1200aaggacgcca agtggcacct gtacttccag tacaacccga
acgacaccgt ctgggggacg 1260cccttgttct ggggccacgc cacgtccgac
gacctgacca actgggagga ccagcccatc 1320gccatcgccc cgaagcgcaa
cgactccggc gccttctccg gctccatggt ggtggactac 1380aacaacacct
ccggcttctt caacgacacc atcgacccgc gccagcgctg cgtggccatc
1440tggacctaca acaccccgga gtccgaggag cagtacatct cctacagcct
ggacggcggc 1500tacaccttca ccgagtacca gaagaacccc gtgctggccg
ccaactccac ccagttccgc 1560gacccgaagg tcttctggta cgagccctcc
cagaagtgga tcatgaccgc ggccaagtcc 1620caggactaca agatcgagat
ctactcctcc
gacgacctga agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt
cctcggctac cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc
aggaccccag caagtcctac tgggtgatgt tcatctccat caaccccggc
1800gccccggccg gcggctcctt caaccagtac ttcgtcggca gcttcaacgg
cacccacttc 1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca
aggactacta cgccctgcag 1920accttcttca acaccgaccc gacctacggg
agcgccctgg gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt
gcccaccaac ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc
tcaacaccga gtaccaggcc aacccggaga cggagctgat caacctgaag
2100gccgagccga tcctgaacat cagcaacgcc ggcccctgga gccggttcgc
caccaacacc 2160acgttgacga aggccaacag ctacaacgtc gacctgtcca
acagcaccgg caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc
cagacgatct ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg
cctggaggac cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt
cctccttctt cctggaccgc gggaacagca aggtgaagtt cgtgaaggag
2400aacccctact tcaccaaccg catgagcgtg aacaaccagc ccttcaagag
cgagaacgac 2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca
tcctggagct gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac
ttcatgacca ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt
ggacaacctg ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac
aattggcagc agcagctcgg atagtatcga cacactctgg acgctggtcg
2700tgtgatggac tgttgccgcc acacttgctg ccttgacctg tgaatatccc
tgccgctttt 2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc
ttttgcgagt tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca
tccccttccc tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac
gctgtcctgc tatccctcag cgctgctcct gctcctgctc 2940actgcccctc
gcacagcctt ggtttgggct ccgcctgtat tctcctggta ctgcaacctg
3000taaaccagca ctgcaatgct gatgcacggg aagtagtggg atgggaacac
aaatggagga 3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg
tctcgcctct gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga
cgaatgcgct tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg
tgccacgttg gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc
gaaacgttca cagcctaggg atatcgaatt cggccgacag gacgcgcgtc
3300aaaggtgctg gtcgtgtatg ccctggccgg caggtcgttg ctgctgctgg
ttagtgattc 3360cgcaaccctg attttggcgt cttattttgg cgtggcaaac
gctggcgccc gcgagccggg 3420ccggcggcga tgcggtgccc cacggctgcc
ggaatccaag ggaggcaaga gcgcccgggt 3480cagttgaagg gctttacgcg
caaggtacag ccgctcctgc aaggctgcgt ggtggaattg 3540gacgtgcagg
tcctgctgaa gttcctccac cgcctcacca gcggacaaag caccggtgta
3600tcaggtccgt gtcatccact ctaaagaact cgactacgac ctactgatgg
ccctagattc 3660ttcatcaaaa acgcctgaga cacttgccca ggattgaaac
tccctgaagg gaccaccagg 3720ggccctgagt tgttccttcc ccccgtggcg
agctgccagc caggctgtac ctgtgatcga 3780ggctggcggg aaaataggct
tcgtgtgctc aggtcatggg aggtgcagga cagctcatga 3840aacgccaaca
atcgcacaat tcatgtcaag ctaatcagct atttcctctt cacgagctgt
3900aattgtccca aaattctggt ctaccggggg tgatccttcg tgtacgggcc
cttccctcaa 3960ccctaggtat gcgcgcatgc ggtcgccgcg caactcgcgc
gagggccgag ggtttgggac 4020gggccgtccc gaaatgcagt tgcacccgga
tgcgtggcac cttttttgcg ataatttatg 4080caatggactg ctctgcaaaa
ttctggctct gtcgccaacc ctaggatcag cggcgtagga 4140tttcgtaatc
attcgtcctg atggggagct accgactacc ctaatatcag cccgactgcc
4200tgacgccagc gtccactttt gtgcacacat tccattcgtg cccaagacat
ttcattgtgg 4260tgcgaagcgt ccccagttac gctcacctgt ttcccgacct
ccttactgtt ctgtcgacag 4320agcgggccca caggccggtc gcagccacta
gtatggccac cgcctccacc ttctccgcct 4380tcaacgcccg ctgcggcgac
ctgcgccgct ccgccggctc cggcccccgc cgccccgccc 4440gccccctgcc
cgtgcgcgcc gccatcgcct ccgaggtgcc cgtggccacc acctcccccc
4500gccccggccc caccgtgtac tccaagctgg acaaggccca caccctgacc
cccgagcgca 4560tggagctgat caacggcatg tccgccttcg ccgaggagcg
catcctgccc gtgctgcagc 4620ccgtggagaa gctgtggcag ccccaggacc
tgctgcccga ccccgagtcc cccgacttcc 4680tggaccaggt ggccgagctg
cgcgcccgcg ccgccaacgt gcccgacgac tacttcgtgg 4740tgctggtggg
cgacatgatc accgaggagg ccctgcccac ctacatggcc atgctgaaca
4800ccctggacgg cgtgcgcgac gagaccggcg ccgccgacca cccctggggc
cgctggaccc 4860gccagtgggt ggccgaggag aaccgccacg gcgacctgct
gaacaagtac tgctggctga 4920ccggccgcgt gaacatgaag gccatcgagg
tgaccatcca gaacctgatc ggctccggca 4980tgaaccccaa gaccgagaac
aacccctacc tgggcttcgt gtacacctcc ttccaggagc 5040gcgccaccaa
gtactcccac ggcaacaccg cccgcctggc cgcccagtac ggcgacgcca
5100ccctgtccaa ggtgtgcggc gtgatcgccg ccgacgaggg ccgccacgag
atcgcctaca 5160cccgcatcgt ggaggagttc ttccgcctgg accccgaggg
cgccatgtcc gcctacgccg 5220acatgatgcg caagcagatc accatgcccg
cccacctgat ggacgaccag cagcacggca 5280cccgcaacac cggccgcaac
ctgttcgccg acttctccgc cgtgaccgag aagctggacg 5340tgtacgacgc
cgaggactac tgcaagatcc tggagcacct gaactcccgc tggaagatcg
5400ccgaccgcac cgtgtccggc gacgccggcg ccgaccagga gtacgtgctg
cgcctgccct 5460cccgcttccg caagctggcc gagaagtccg ccgccaagcg
cgccaagacc aagcccaagc 5520ccgtggcctt ctcctggctg tccggccgcg
aggtgatggt gtgaatcgat agatctctta 5580aggcagcagc agctcggata
gtatcgacac actctggacg ctggtcgtgt gatggactgt 5640tgccgccaca
cttgctgcct tgacctgtga atatccctgc cgcttttatc aaacagcctc
5700agtgtgtttg atcttgtgtg tacgcgcttt tgcgagttgc tagctgcttg
tgctatttgc 5760gaataccacc cccagcatcc ccttccctcg tttcatatcg
cttgcatccc aaccgcaact 5820tatctacgct gtcctgctat ccctcagcgc
tgctcctgct cctgctcact gcccctcgca 5880cagccttggt ttgggctccg
cctgtattct cctggtactg caacctgtaa accagcactg 5940caatgctgat
gcacgggaag tagtgggatg ggaacacaaa tggaaagctt aattaagagc
6000tcttgttttc cagaaggagt tgctccttga gcctttcatt ctcagcctcg
ataacctcca 6060aagccgctct aattgtggag ggggttcgaa tttaaaagct
tggaatgttg gttcgtgcgt 6120ctggaacaag cccagacttg ttgctcactg
ggaaaaggac catcagctcc aaaaaacttg 6180ccgctcaaac cgcgtacctc
tgctttcgcg caatctgccc tgttgaaatc gccaccacat 6240tcatattgtg
acgcttgagc agtctgtaat tgcctcagaa tgtggaatca tctgccccct
6300gtgcgagccc atgccaggca tgtcgcgggc gaggacaccc gccactcgta
cagcagacca 6360ttatgctacc tcacaatagt tcataacagt gaccatattt
ctcgaagctc cccaacgagc 6420acctccatgc tctgagtggc caccccccgg
ccctggtgct tgcggagggc aggtcaaccg 6480gcatggggct accgaaatcc
ccgaccggat cccaccaccc ccgcgatggg aagaatctct 6540ccccgggatg
tgggcccacc accagcacaa cctgctggcc caggcgagcg tcaaaccata
6600ccacacaaat atccttggca tcggccctga attccttctg ccgctctgct
acccggtgct 6660tctgtccgaa gcaggggttg ctagggatcg ctccgagtcc
gcaaaccctt gtcgcgtggc 6720ggggcttgtt cgagcttgaa gagc
67441116667DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 111gctcttcgcc gccgccactc ctgctcgagc
gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga
tgtccatcac caggtccatg aggtctgcct 120tgcgccggct gagccactgc
ttcgtccggg cggccaagag gagcatgagg gaggactcct 180ggtccagggt
cctgacgtgg tcgcggctct gggagcgggc cagcatcatc tggctctgcc
240gcaccgaggc cgcctccaac tggtcctcca gcagccgcag tcgccgccga
ccctggcaga 300ggaagacagg tgaggggggt atgaattgta cagaacaacc
acgagccttg tctaggcaga 360atccctacca gtcatggctt tacctggatg
acggcctgcg aacagctgtc cagcgaccct 420cgctgccgcc gcttctcccg
cacgcttctt tccagcaccg tgatggcgcg agccagcgcc 480gcacgctggc
gctgcgcttc gccgatctga ggacagtcgg ggaactctga tcagtctaaa
540cccccttgcg cgttagtgtt gccatccttt gcagaccggt gagagccgac
ttgttgtgcg 600ccacccccca caccacctcc tcccagacca attctgtcac
ctttttggcg aaggcatcgg 660cctcggcctg cagagaggac agcagtgccc
agccgctggg ggttggcgga tgcacgctca 720ggtacccttt cttgcgctat
gacacttcca gcaaaaggta gggcgggctg cgagacggct 780tcccggcgct
gcatgcaaca ccgatgatgc ttcgaccccc cgaagctcct tcggggctgc
840atgggcgctc cgatgccgct ccagggcgag cgctgtttaa atagccaggc
ccccgattgc 900aaagacatta tagcgagcta ccaaagccat attcaaacac
ctagatcact accacttcta 960cacaggccac tcgagcttgt gatcgcactc
cgctaagggg gcgcctcttc ctcttcgttt 1020cagtcacaac ccgcaaacgg
cgcgccatgc tgctgcaggc cttcctgttc ctgctggccg 1080gcttcgccgc
caagatcagc gcctccatga cgaacgagac gtccgaccgc cccctggtgc
1140acttcacccc caacaagggc tggatgaacg accccaacgg cctgtggtac
gacgagaagg 1200acgccaagtg gcacctgtac ttccagtaca acccgaacga
caccgtctgg gggacgccct 1260tgttctgggg ccacgccacg tccgacgacc
tgaccaactg ggaggaccag cccatcgcca 1320tcgccccgaa gcgcaacgac
tccggcgcct tctccggctc catggtggtg gactacaaca 1380acacctccgg
cttcttcaac gacaccatcg acccgcgcca gcgctgcgtg gccatctgga
1440cctacaacac cccggagtcc gaggagcagt acatctccta cagcctggac
ggcggctaca 1500ccttcaccga gtaccagaag aaccccgtgc tggccgccaa
ctccacccag ttccgcgacc 1560cgaaggtctt ctggtacgag ccctcccaga
agtggatcat gaccgcggcc aagtcccagg 1620actacaagat cgagatctac
tcctccgacg acctgaagtc ctggaagctg gagtccgcgt 1680tcgccaacga
gggcttcctc ggctaccagt acgagtgccc cggcctgatc gaggtcccca
1740ccgagcagga ccccagcaag tcctactggg tgatgttcat ctccatcaac
cccggcgccc 1800cggccggcgg ctccttcaac cagtacttcg tcggcagctt
caacggcacc cacttcgagg 1860ccttcgacaa ccagtcccgc gtggtggact
tcggcaagga ctactacgcc ctgcagacct 1920tcttcaacac cgacccgacc
tacgggagcg ccctgggcat cgcgtgggcc tccaactggg 1980agtactccgc
cttcgtgccc accaacccct ggcgctcctc catgtccctc gtgcgcaagt
2040tctccctcaa caccgagtac caggccaacc cggagacgga gctgatcaac
ctgaaggccg 2100agccgatcct gaacatcagc aacgccggcc cctggagccg
gttcgccacc aacaccacgt 2160tgacgaaggc caacagctac aacgtcgacc
tgtccaacag caccggcacc ctggagttcg 2220agctggtgta cgccgtcaac
accacccaga cgatctccaa gtccgtgttc gcggacctct 2280ccctctggtt
caagggcctg gaggaccccg aggagtacct ccgcatgggc ttcgaggtgt
2340ccgcgtcctc cttcttcctg gaccgcggga acagcaaggt gaagttcgtg
aaggagaacc 2400cctacttcac caaccgcatg agcgtgaaca accagccctt
caagagcgag aacgacctgt 2460cctactacaa ggtgtacggc ttgctggacc
agaacatcct ggagctgtac ttcaacgacg 2520gcgacgtcgt gtccaccaac
acctacttca tgaccaccgg gaacgccctg ggctccgtga 2580acatgacgac
gggggtggac aacctgttct acatcgacaa gttccaggtg cgcgaggtca
2640agtgacaatt ggcagcagca gctcggatag tatcgacaca ctctggacgc
tggtcgtgtg 2700atggactgtt gccgccacac ttgctgcctt gacctgtgaa
tatccctgcc gcttttatca 2760aacagcctca gtgtgtttga tcttgtgtgt
acgcgctttt gcgagttgct agctgcttgt 2820gctatttgcg aataccaccc
ccagcatccc cttccctcgt ttcatatcgc ttgcatccca 2880accgcaactt
atctacgctg tcctgctatc cctcagcgct gctcctgctc ctgctcactg
2940cccctcgcac agccttggtt tgggctccgc ctgtattctc ctggtactgc
aacctgtaaa 3000ccagcactgc aatgctgatg cacgggaagt agtgggatgg
gaacacaaat ggaggatccc 3060gcgtctcgaa cagagcgcgc agaggaacgc
tgaaggtctc gcctctgtcg cacctcagcg 3120cggcatacac cacaataacc
acctgacgaa tgcgcttggt tcttcgtcca ttagcgaagc 3180gtccggttca
cacacgtgcc acgttggcga ggtggcaggt gacaatgatc ggtggagctg
3240atggtcgaaa cgttcacagc ctagggatat catagcgact gctacccccc
gaccatgtgc 3300cgaggcagaa attatataca agaagcagat cgcaattagg
cacatcgctt tgcattatcc 3360acacactatt catcgctgct gcggcaaggc
tgcagagtgt atttttgtgg cccaggagct 3420gagtccgaag tcgacgcgac
gagcggcgca ggatccgacc cctagacgag ctctgtcatt 3480ttccaagcac
gcagctaaat gcgctgagac cgggtctaaa tcatccgaaa agtgtcaaaa
3540tggccgattg ggttcgccta ggacaatgcg ctgcggattc gctcgagtcc
gctgccggcc 3600aaaaggcggt ggtacaggaa ggcgcacggg gccaaccctg
cgaagccggg ggcccgaacg 3660ccgaccgccg gccttcgatc tcgggtgtcc
ccctcgtcaa tttcctctct cgggtgcagc 3720cacgaaagtc gtgacgcagg
tcacgaaatc cggttacgaa aaacgcaggt cttcgcaaaa 3780acgtgagggt
ttcgcgtctc gccctagcta ttcgtatcgc cgggtcagac ccacgtgcag
3840aaaagccctt gaataacccg ggaccgtggt taccgcgccg cctgcaccag
ggggcttata 3900taagcccaca ccacacctgt ctcaccacgc atttctccaa
ctcgcgactt ttcggaagaa 3960attgttatcc acctagtata gactgccacc
tgcaggacct tgtgtcttgc agtttgtatt 4020ggtcccggcc gtcgagctcg
acagatctgg gctagggttg gcctggccgc tcggcactcc 4080cctttagccg
cgcgcatccg cgttccagag gtgcgattcg gtgtgtggag cattgtcatg
4140cgcttgtggg ggtcgttccg tgcgcggcgg gtccgccatg ggcgccgacc
tgggccctag 4200ggtttgtttt cgggccaagc gagcccctct cacctcgtcg
cccccccgca ttccctctct 4260cttgcagcca ctagtatggc ctccgctgtg
accttcgcct gcgctcctcc tcgcaggcgc 4320gccggtgccg tggccgctcc
tggccgacgc gctgcctctc gtcctctggt ggtgcacgcc 4380gtggcctccg
aggctcctct gggcgtgcct ccctccgtgc agcgcccttc tcccgtggtg
4440tactccaagc tggacaagca gcaccgcctg acgcctgagc gcctggagct
ggtgcagtcc 4500atgggccagt tcgccgagga gcgcgtgctg cccgtgctgc
accccgtgga caagctgtgg 4560cagccccagg acttcctgcc cgaccccgag
tcccccgact tcgaggacca ggtggccgag 4620ctgcgcgccc gcgccaagga
cctgcccgac gagtacttcg tggtgctggt gggcgacatg 4680atcaccgagg
aggccctgcc cacctacatg gccatgctga acacctggga cggcgtgcgc
4740gacgacaccg gcgccgccga ccacccctgg gcccgctgga cccgccagtg
ggtggccgag 4800gagaaccgcc acggcgacct gctgaacaag tactgctggc
tgaccggccg cgtgaacatg 4860cgcgccgtgg aggtgaccat caacaacctg
atcaagtccg gcatgaaccc ccagaccgac 4920aacaacccct acctgggctt
cgtgtacacc tccttccagg agcgcgccac caagtactcc 4980cacggcaaca
ccgcccgcct ggccgccgag cacggcgaca agggcctgtc caagatctgc
5040ggcctgatcg cctccgacga gggccgccac gagatcgcct acacccgcat
cgtggacgag 5100ttcttccgcc tggaccccga gggcgccgtg gccgcctacg
ccaacatgat gcgcaagcag 5160atcaccatgc ccgcccacct gatggacgac
atgggccacg gcgaggccaa ccccggccgc 5220aacctgttcg ccgacttctc
cgccgtggcc gagaagatcg acgtgtacga cgccgaggac 5280tactgccgca
tcctggagca cctgaacgcc cgctggaagg tggacgagcg ccaggtgtcc
5340ggccaggccg ccgccgacca ggagtacgtg ctgggcctgc cccagcgctt
ccgcaagctg 5400gccgagaaga ccgccgccaa gcgcaagcgc gtggcccgcc
gccccgtggc cttctcctgg 5460atctccggcc gcgagatcat ggtgtgaatc
gatagatctc ttaaggcagc agcagctcgg 5520atagtatcga cacactctgg
acgctggtcg tgtgatggac tgttgccgcc acacttgctg 5580ccttgacctg
tgaatatccc tgccgctttt atcaaacagc ctcagtgtgt ttgatcttgt
5640gtgtacgcgc ttttgcgagt tgctagctgc ttgtgctatt tgcgaatacc
acccccagca 5700tccccttccc tcgtttcata tcgcttgcat cccaaccgca
acttatctac gctgtcctgc 5760tatccctcag cgctgctcct gctcctgctc
actgcccctc gcacagcctt ggtttgggct 5820ccgcctgtat tctcctggta
ctgcaacctg taaaccagca ctgcaatgct gatgcacggg 5880aagtagtggg
atgggaacac aaatggaaag cttaattaag agctcttgtt ttccagaagg
5940agttgctcct tgagcctttc attctcagcc tcgataacct ccaaagccgc
tctaattgtg 6000gagggggttc gaatttaaaa gcttggaatg ttggttcgtg
cgtctggaac aagcccagac 6060ttgttgctca ctgggaaaag gaccatcagc
tccaaaaaac ttgccgctca aaccgcgtac 6120ctctgctttc gcgcaatctg
ccctgttgaa atcgccacca cattcatatt gtgacgcttg 6180agcagtctgt
aattgcctca gaatgtggaa tcatctgccc cctgtgcgag cccatgccag
6240gcatgtcgcg ggcgaggaca cccgccactc gtacagcaga ccattatgct
acctcacaat 6300agttcataac agtgaccata tttctcgaag ctccccaacg
agcacctcca tgctctgagt 6360ggccaccccc cggccctggt gcttgcggag
ggcaggtcaa ccggcatggg gctaccgaaa 6420tccccgaccg gatcccacca
cccccgcgat gggaagaatc tctccccggg atgtgggccc 6480accaccagca
caacctgctg gcccaggcga gcgtcaaacc ataccacaca aatatccttg
6540gcatcggccc tgaattcctt ctgccgctct gctacccggt gcttctgtcc
gaagcagggg 6600ttgctaggga tcgctccgag tccgcaaacc cttgtcgcgt
ggcggggctt gttcgagctt 6660gaagagc 66671125991DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
112gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg
aagtagtggg atgggaacac aaatggagga 3060tcccgcgtct cgaacagagc
gcgcagagga acgctgaagg tctcgcctct gtcgcacctc 3120agcgcggcat
acaccacaat aaccacctga cgaatgcgct tggttcttcg tccattagcg
3180aagcgtccgg ttcacacacg tgccacgttg gcgaggtggc aggtgacaat
gatcggtgga 3240gctgatggtc gaaacgttca cagcctaggg atatcgaatt
cctttcttgc gctatgacac 3300ttccagcaaa aggtagggcg ggctgcgaga
cggcttcccg gcgctgcatg caacaccgat 3360gatgcttcga ccccccgaag
ctccttcggg gctgcatggg cgctccgatg ccgctccagg 3420gcgagcgctg
tttaaatagc caggcccccg attgcaaaga cattatagcg agctaccaaa
3480gccatattca aacacctaga tcactaccac ttctacacag gccactcgag
cttgtgatcg 3540cactccgcta agggggcgcc tcttcctctt cgtttcagtc
acaacccgca aacactagta 3600tggcctccgc tgtgaccttc gcctgcgctc
ctcctcgcag gcgcgccggt gccgtggccg 3660ctcctggccg acgcgctgcc
tctcgtcctc tggtggtgca cgccgtggcc tccgaggctc 3720ctctgggcgt
gcctccctcc gtgcagcgcc cttctcccgt ggtgtactcc aagctggaca
3780agcagcaccg cctgacgcct gagcgcctgg agctggtgca gtccatgggc
cagttcgccg 3840aggagcgcgt gctgcccgtg ctgcaccccg tggacaagct
gtggcagccc caggacttcc 3900tgcccgaccc cgagtccccc gacttcgagg
accaggtggc cgagctgcgc gcccgcgcca 3960aggacctgcc cgacgagtac
ttcgtggtgc tggtgggcga catgatcacc gaggaggccc 4020tgcccaccta
catggccatg ctgaacacct gggacggcgt gcgcgacgac accggcgccg
4080ccgaccaccc ctgggcccgc tggacccgcc agtgggtggc cgaggagaac
cgccacggcg 4140acctgctgaa caagtactgc tggctgaccg gccgcgtgaa
catgcgcgcc gtggaggtga 4200ccatcaacaa cctgatcaag tccggcatga
acccccagac cgacaacaac ccctacctgg 4260gcttcgtgta cacctccttc
caggagcgcg ccaccaagta ctcccacggc aacaccgccc 4320gcctggccgc
cgagcacggc gacaagggcc tgtccaagat ctgcggcctg atcgcctccg
4380acgagggccg ccacgagatc gcctacaccc gcatcgtgga cgagttcttc
cgcctggacc 4440ccgagggcgc cgtggccgcc tacgccaaca tgatgcgcaa
gcagatcacc atgcccgccc 4500acctgatgga cgacatgggc cacggcgagg
ccaaccccgg ccgcaacctg ttcgccgact 4560tctccgccgt ggccgagaag
atcgacgtgt acgacgccga ggactactgc cgcatcctgg 4620agcacctgaa
cgcccgctgg aaggtggacg agcgccaggt gtccggccag gccgccgccg
4680accaggagta cgtgctgggc ctgccccagc gcttccgcaa gctggccgag
aagaccgccg 4740ccaagcgcaa gcgcgtggcc cgccgccccg tggccttctc
ctggatctcc ggccgcgaga 4800tcatggtgtg aatcgataga tctcttaagg
cagcagcagc tcggatagta tcgacacact 4860ctggacgctg gtcgtgtgat
ggactgttgc cgccacactt gctgccttga cctgtgaata 4920tccctgccgc
ttttatcaaa cagcctcagt gtgtttgatc ttgtgtgtac gcgcttttgc
4980gagttgctag ctgcttgtgc tatttgcgaa taccaccccc agcatcccct
tccctcgttt 5040catatcgctt gcatcccaac cgcaacttat ctacgctgtc
ctgctatccc tcagcgctgc 5100tcctgctcct gctcactgcc cctcgcacag
ccttggtttg ggctccgcct gtattctcct 5160ggtactgcaa cctgtaaacc
agcactgcaa tgctgatgca cgggaagtag tgggatggga 5220acacaaatgg
aaagcttaat taagagctct tgttttccag aaggagttgc tccttgagcc
5280tttcattctc agcctcgata acctccaaag ccgctctaat tgtggagggg
gttcgaattt 5340aaaagcttgg aatgttggtt cgtgcgtctg gaacaagccc
agacttgttg ctcactggga 5400aaaggaccat cagctccaaa aaacttgccg
ctcaaaccgc gtacctctgc tttcgcgcaa 5460tctgccctgt tgaaatcgcc
accacattca tattgtgacg cttgagcagt ctgtaattgc 5520ctcagaatgt
ggaatcatct gccccctgtg cgagcccatg ccaggcatgt cgcgggcgag
5580gacacccgcc actcgtacag cagaccatta tgctacctca caatagttca
taacagtgac 5640catatttctc gaagctcccc aacgagcacc tccatgctct
gagtggccac cccccggccc 5700tggtgcttgc ggagggcagg tcaaccggca
tggggctacc gaaatccccg accggatccc 5760accacccccg cgatgggaag
aatctctccc cgggatgtgg gcccaccacc agcacaacct 5820gctggcccag
gcgagcgtca aaccatacca cacaaatatc cttggcatcg gccctgaatt
5880ccttctgccg ctctgctacc cggtgcttct gtccgaagca ggggttgcta
gggatcgctc 5940cgagtccgca aacccttgtc gcgtggcggg gcttgttcga
gcttgaagag c 59911135988DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 113gctcttcgcc
gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc agcgccttgg 60ccttttcgcc
gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg aggtctgcct
120tgcgccggct gagccactgc ttcgtccggg cggccaagag gagcatgagg
gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct gggagcgggc
cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac tggtcctcca
gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg tgaggggggt
atgaattgta cagaacaacc acgagccttg tctaggcaga 360atccctacca
gtcatggctt tacctggatg acggcctgcg aacagctgtc cagcgaccct
420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg tgatggcgcg
agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga ggacagtcgg
ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt gccatccttt
gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca caccacctcc
tcccagacca attctgtcac ctttttggcg aaggcatcgg 660cctcggcctg
cagagaggac agcagtgccc agccgctggg ggttggcgga tgcacgctca
720ggtacccttt cttgcgctat gacacttcca gcaaaaggta gggcgggctg
cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc ttcgaccccc
cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct ccagggcgag
cgctgtttaa atagccaggc ccccgattgc 900aaagacatta tagcgagcta
ccaaagccat attcaaacac ctagatcact accacttcta 960cacaggccac
tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc ctcttcgttt
1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc aggccttcct
gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc atgacgaacg
agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa gggctggatg
aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca agtggcacct
gtacttccag tacaacccga acgacaccgt ctgggggacg 1260cccttgttct
ggggccacgc cacgtccgac gacctgacca actgggagga ccagcccatc
1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg gctccatggt
ggtggactac 1380aacaacacct ccggcttctt caacgacacc atcgacccgc
gccagcgctg cgtggccatc 1440tggacctaca acaccccgga gtccgaggag
cagtacatct cctacagcct ggacggcggc 1500tacaccttca ccgagtacca
gaagaacccc gtgctggccg ccaactccac ccagttccgc 1560gacccgaagg
tcttctggta cgagccctcc cagaagtgga tcatgaccgc ggccaagtcc
1620caggactaca agatcgagat ctactcctcc gacgacctga agtcctggaa
gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac cagtacgagt
gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag caagtcctac
tgggtgatgt tcatctccat caaccccggc 1800gccccggccg gcggctcctt
caaccagtac ttcgtcggca gcttcaacgg cacccacttc 1860gaggccttcg
acaaccagtc ccgcgtggtg gacttcggca aggactacta cgccctgcag
1920accttcttca acaccgaccc gacctacggg agcgccctgg gcatcgcgtg
ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac ccctggcgct
cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga gtaccaggcc
aacccggaga cggagctgat caacctgaag 2100gccgagccga tcctgaacat
cagcaacgcc ggcccctgga gccggttcgc caccaacacc 2160acgttgacga
aggccaacag ctacaacgtc gacctgtcca acagcaccgg caccctggag
2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct ccaagtccgt
gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac cccgaggagt
acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt cctggaccgc
gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact tcaccaaccg
catgagcgtg aacaaccagc ccttcaagag cgagaacgac 2460ctgtcctact
acaaggtgta cggcttgctg gaccagaaca tcctggagct gtacttcaac
2520gacggcgacg tcgtgtccac caacacctac ttcatgacca ccgggaacgc
cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg ttctacatcg
acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc agcagctcgg
atagtatcga cacactctgg acgctggtcg 2700tgtgatggac tgttgccgcc
acacttgctg ccttgacctg tgaatatccc tgccgctttt 2760atcaaacagc
ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt tgctagctgc
2820ttgtgctatt tgcgaatacc acccccagca tccccttccc tcgtttcata
tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc tatccctcag
cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt ggtttgggct
ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca ctgcaatgct
gatgcacggg aagtagtggg atgggaacac aaatggagga 3060tcccgcgtct
cgaacagagc gcgcagagga acgctgaagg tctcgcctct gtcgcacctc
3120agcgcggcat acaccacaat aaccacctga cgaatgcgct tggttcttcg
tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg gcgaggtggc
aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca cagcctaggg
atatcgaatt cctttcttgc gctatgacac 3300ttccagcaaa aggtagggcg
ggctgcgaga cggcttcccg gcgctgcatg caacaccgat 3360gatgcttcga
ccccccgaag ctccttcggg gctgcatggg cgctccgatg ccgctccagg
3420gcgagcgctg tttaaatagc caggcccccg attgcaaaga cattatagcg
agctaccaaa 3480gccatattca aacacctaga tcactaccac ttctacacag
gccactcgag cttgtgatcg 3540cactccgcta agggggcgcc tcttcctctt
cgtttcagtc acaacccgca aacactagta 3600tggccaccgc atccactttc
tcggcgttca atgcccgctg cggcgacctg cgtcgctcgg 3660cgggctccgg
gccccggcgc ccagcgaggc ccctccccgt gcgcgggcgc gccgaggtgc
3720acgtgcaggt gacccactcc ctggcccccg agaagcgcga gatcttcaac
tccctgaaca 3780actgggccca ggagaacatc ctggtgctgc tgaaggacgt
ggacaagtgc tggcagccct 3840ccgacttcct gcccgactcc gcctccgagg
gcttcgacga gcaggtgatg gagctgcgca 3900agcgctgcaa ggagatcccc
gacgactact tcatcgtgct ggtgggcgac atgatcaccg 3960aggaggccct
gcccacctac cagaccatgc tgaacacctg ggacggcgtg cgcgacgaga
4020ccggcgcctc cctgaccccc tgggccatct ggacccgcgc ctggaccgcc
gaggagaacc 4080gccacggcga cctgctgaac aagtacctgt acctgtccgg
ccgcgtggac atgaagcaga 4140tcgagaagac catccagtac ctgatcggct
ccggcatgga cccccgcacc gagaacaacc 4200cctacctggg cttcatctac
acctccttcc aggagcgcgc caccttcatc tcccacggca 4260acaccgcccg
cctggccaag gagcacggcg acctgaagct ggcccagatc tgcggcatca
4320tcgccgccga cgagaagcgc cacgagaccg cctacaccaa gatcgtggag
aagctgttcg 4380agatcgaccc cgacggcacc gtgctggccc tggccgacat
gatgcgcaag aaggtgtcca 4440tgcccgccca cctgatgtac gacggccagg
acgacaacct gttcgagaac ttctcctccg 4500tggcccagcg cctgggcgtg
tacaccgcca aggactacgc cgacatcctg gagttcctgg 4560tgggccgctg
ggacatcgag aagctgaccg gcctgtccgg cgagggccgc aaggcccagg
4620actacgtgtg caccctgccc ccccgcatcc gccgcctgga ggagcgcgcc
cagtcccgcg 4680tgaagaaggc ctccgccacc cccttctcct ggatcttcgg
ccgcgagatc aacctgatgg 4740actacaagga ccacgacggc gactacaagg
accacgacat cgactacaag gacgacgacg 4800acaagtgaat cgatagatct
cttaaggcag cagcagctcg gatagtatcg acacactctg 4860gacgctggtc
gtgtgatgga ctgttgccgc cacacttgct gccttgacct gtgaatatcc
4920ctgccgcttt tatcaaacag cctcagtgtg tttgatcttg tgtgtacgcg
cttttgcgag 4980ttgctagctg cttgtgctat ttgcgaatac cacccccagc
atccccttcc ctcgtttcat 5040atcgcttgca tcccaaccgc aacttatcta
cgctgtcctg ctatccctca gcgctgctcc 5100tgctcctgct cactgcccct
cgcacagcct tggtttgggc tccgcctgta ttctcctggt 5160actgcaacct
gtaaaccagc actgcaatgc tgatgcacgg gaagtagtgg gatgggaaca
5220caaatggaaa gcttaattaa gagctcttgt tttccagaag gagttgctcc
ttgagccttt 5280cattctcagc ctcgataacc tccaaagccg ctctaattgt
ggagggggtt cgaatttaaa 5340agcttggaat gttggttcgt gcgtctggaa
caagcccaga cttgttgctc actgggaaaa 5400ggaccatcag ctccaaaaaa
cttgccgctc aaaccgcgta cctctgcttt cgcgcaatct 5460gccctgttga
aatcgccacc acattcatat tgtgacgctt gagcagtctg taattgcctc
5520agaatgtgga atcatctgcc ccctgtgcga gcccatgcca ggcatgtcgc
gggcgaggac 5580acccgccact cgtacagcag accattatgc tacctcacaa
tagttcataa cagtgaccat 5640atttctcgaa gctccccaac gagcacctcc
atgctctgag tggccacccc ccggccctgg 5700tgcttgcgga gggcaggtca
accggcatgg ggctaccgaa atccccgacc ggatcccacc 5760acccccgcga
tgggaagaat ctctccccgg gatgtgggcc caccaccagc acaacctgct
5820ggcccaggcg agcgtcaaac cataccacac aaatatcctt ggcatcggcc
ctgaattcct 5880tctgccgctc tgctacccgg tgcttctgtc cgaagcaggg
gttgctaggg atcgctccga 5940gtccgcaaac ccttgtcgcg tggcggggct
tgttcgagct tgaagagc 59881146696DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 114gctcttcacc
caactcagat aataccaata cccctccttc tcctcctcat ccattcagta 60ccccccccct
tctcttccca aagcagcaag cgcgtggctt acagaagaac aatcggcttc
120cgccaaagtc gccgagcact gcccgacggc ggcgcgccca gcagcccgct
tggccacaca 180ggcaacgaat acattcaata gggggcctcg cagaatggaa
ggagcggtaa agggtacagg 240agcactgcgc acaaggggcc tgtgcaggag
tgactgactg ggcgggcaga cggcgcaccg 300cgggcgcagg caagcaggga
agattgaagc ggcagggagg aggatgctga ttgagggggg 360catcgcagtc
tctcttggac ccgggataag gaagcaaata ttcggccggt tgggttgtgt
420gtgtgcacgt tttcttcttc agagtcgtgg gtgtgcttcc agggaggata
taagcagcag 480gatcgaatcc cgcgaccagc gtttccccat ccagccaacc
accctgtcgg taccctttct 540tgcgctatga cacttccagc aaaaggtagg
gcgggctgcg agacggcttc ccggcgctgc 600atgcaacacc gatgatgctt
cgaccccccg aagctccttc ggggctgcat gggcgctccg 660atgccgctcc
agggcgagcg ctgtttaaat agccaggccc ccgattgcaa agacattata
720gcgagctacc aaagccatat tcaaacacct agatcactac cacttctaca
caggccactc 780gagcttgtga tcgcactccg ctaagggggc gcctcttcct
cttcgtttca gtcacaaccc 840gcaaacggcg cgccatgctg ctgcaggcct
tcctgttcct gctggccggc ttcgccgcca 900agatcagcgc ctccatgacg
aacgagacgt ccgaccgccc cctggtgcac ttcaccccca 960acaagggctg
gatgaacgac cccaacggcc tgtggtacga cgagaaggac gccaagtggc
1020acctgtactt ccagtacaac ccgaacgaca ccgtctgggg gacgcccttg
ttctggggcc 1080acgccacgtc cgacgacctg accaactggg aggaccagcc
catcgccatc gccccgaagc 1140gcaacgactc cggcgccttc tccggctcca
tggtggtgga ctacaacaac acctccggct 1200tcttcaacga caccatcgac
ccgcgccagc gctgcgtggc catctggacc tacaacaccc 1260cggagtccga
ggagcagtac atctcctaca gcctggacgg cggctacacc ttcaccgagt
1320accagaagaa ccccgtgctg gccgccaact ccacccagtt ccgcgacccg
aaggtcttct 1380ggtacgagcc ctcccagaag tggatcatga ccgcggccaa
gtcccaggac tacaagatcg 1440agatctactc ctccgacgac ctgaagtcct
ggaagctgga gtccgcgttc gccaacgagg 1500gcttcctcgg ctaccagtac
gagtgccccg gcctgatcga ggtccccacc gagcaggacc 1560ccagcaagtc
ctactgggtg atgttcatct ccatcaaccc cggcgccccg gccggcggct
1620ccttcaacca gtacttcgtc ggcagcttca acggcaccca cttcgaggcc
ttcgacaacc 1680agtcccgcgt ggtggacttc ggcaaggact actacgccct
gcagaccttc ttcaacaccg 1740acccgaccta cgggagcgcc ctgggcatcg
cgtgggcctc caactgggag tactccgcct 1800tcgtgcccac caacccctgg
cgctcctcca tgtccctcgt gcgcaagttc tccctcaaca 1860ccgagtacca
ggccaacccg gagacggagc tgatcaacct gaaggccgag ccgatcctga
1920acatcagcaa cgccggcccc tggagccggt tcgccaccaa caccacgttg
acgaaggcca 1980acagctacaa cgtcgacctg tccaacagca ccggcaccct
ggagttcgag ctggtgtacg 2040ccgtcaacac cacccagacg atctccaagt
ccgtgttcgc ggacctctcc ctctggttca 2100agggcctgga ggaccccgag
gagtacctcc gcatgggctt cgaggtgtcc gcgtcctcct 2160tcttcctgga
ccgcgggaac agcaaggtga agttcgtgaa ggagaacccc tacttcacca
2220accgcatgag cgtgaacaac cagcccttca agagcgagaa cgacctgtcc
tactacaagg 2280tgtacggctt gctggaccag aacatcctgg agctgtactt
caacgacggc gacgtcgtgt 2340ccaccaacac ctacttcatg accaccggga
acgccctggg ctccgtgaac atgacgacgg 2400gggtggacaa cctgttctac
atcgacaagt tccaggtgcg cgaggtcaag tgacaattgg 2460cagcagcagc
tcggatagta tcgacacact ctggacgctg gtcgtgtgat ggactgttgc
2520cgccacactt gctgccttga cctgtgaata tccctgccgc ttttatcaaa
cagcctcagt 2580gtgtttgatc ttgtgtgtac gcgcttttgc gagttgctag
ctgcttgtgc tatttgcgaa 2640taccaccccc agcatcccct tccctcgttt
catatcgctt gcatcccaac cgcaacttat 2700ctacgctgtc ctgctatccc
tcagcgctgc tcctgctcct gctcactgcc cctcgcacag 2760ccttggtttg
ggctccgcct gtattctcct ggtactgcaa cctgtaaacc agcactgcaa
2820tgctgatgca cgggaagtag tgggatggga acacaaatgg aggatcccgc
gtctcgaaca 2880gagcgcgcag aggaacgctg aaggtctcgc ctctgtcgca
cctcagcgcg gcatacacca 2940caataaccac ctgacgaatg cgcttggttc
ttcgtccatt agcgaagcgt ccggttcaca 3000cacgtgccac gttggcgagg
tggcaggtga caatgatcgg tggagctgat ggtcgaaacg 3060ttcacagcct
agggatatca tagcgactgc taccccccga ccatgtgccg aggcagaaat
3120tatatacaag aagcagatcg caattaggca catcgctttg cattatccac
acactattca 3180tcgctgctgc ggcaaggctg cagagtgtat ttttgtggcc
caggagctga gtccgaagtc 3240gacgcgacga gcggcgcagg atccgacccc
tagacgagct ctgtcatttt ccaagcacgc 3300agctaaatgc gctgagaccg
ggtctaaatc atccgaaaag tgtcaaaatg gccgattggg 3360ttcgcctagg
acaatgcgct gcggattcgc tcgagtccgc tgccggccaa aaggcggtgg
3420tacaggaagg cgcacggggc caaccctgcg aagccggggg cccgaacgcc
gaccgccggc 3480cttcgatctc gggtgtcccc ctcgtcaatt tcctctctcg
ggtgcagcca cgaaagtcgt 3540gacgcaggtc acgaaatccg gttacgaaaa
acgcaggtct tcgcaaaaac gtgagggttt 3600cgcgtctcgc cctagctatt
cgtatcgccg ggtcagaccc acgtgcagaa aagcccttga 3660ataacccggg
accgtggtta ccgcgccgcc tgcaccaggg ggcttatata agcccacacc
3720acacctgtct caccacgcat ttctccaact cgcgactttt cggaagaaat
tgttatccac 3780ctagtataga ctgccacctg caggaccttg tgtcttgcag
tttgtattgg tcccggccgt 3840cgagctcgac agatctgggc tagggttggc
ctggccgctc ggcactcccc tttagccgcg 3900cgcatccgcg ttccagaggt
gcgattcggt gtgtggagca ttgtcatgcg cttgtggggg 3960tcgttccgtg
cgcggcgggt ccgccatggg cgccgacctg ggccctaggg tttgttttcg
4020ggccaagcga gcccctctca cctcgtcgcc cccccgcatt ccctctctct
tgcagccttg 4080ccactagtat ggccaccgca tccactttct cggcgttcaa
tgcccgctgc ggcgacctgc 4140gtcgctcggc gggctccggg ccccggcgcc
cagcgaggcc cctccccgtg cgcgggcgcg 4200ccgccgccgc cgccgacgcc
aaccccgccc gccccgagcg ccgcgtggtg atcaccggcc 4260agggcgtggt
gacctccctg ggccagacca tcgagcagtt ctactcctcc ctgctggagg
4320gcgtgtccgg catctcccag atccagaagt tcgacaccac cggctacacc
accaccatcg 4380ccggcgagat caagtccctg cagctggacc cctacgtgcc
caagcgctgg gccaagcgcg 4440tggacgacgt gatcaagtac gtgtacatcg
ccggcaagca ggccctggag tccgccggcc 4500tgcccatcga ggccgccggc
ctggccggcg ccggcctgga ccccgccctg tgcggcgtgc 4560tgatcggcac
cgccatggcc ggcatgacct ccttcgccgc cggcgtggag gccctgaccc
4620gcggcggcgt gcgcaagatg aaccccttct gcatcccctt ctccatctcc
aacatgggcg 4680gcgccatgct ggccatggac atcggcttca tgggccccaa
ctactccatc tccaccgcct 4740gcgccaccgg caactactgc atcctgggcg
ccgccgacca catccgccgc ggcgacgcca 4800acgtgatgct ggccggcggc
gccgacgccg ccatcatccc ctccggcatc ggcggcttca 4860tcgcctgcaa
ggccctgtcc aagcgcaacg acgagcccga gcgcgcctcc cgcccctggg
4920acgccgaccg cgacggcttc gtgatgggcg agggcgccgg cgtgctggtg
ctggaggagc 4980tggagcacgc caagcgccgc ggcgccacca tcctggccga
gctggtgggc ggcgccgcca 5040cctccgacgc ccaccacatg accgagcccg
acccccaggg ccgcggcgtg cgcctgtgcc 5100tggagcgcgc cctggagcgc
gcccgcctgg cccccgagcg cgtgggctac gtgaacgccc 5160acggcacctc
cacccccgcc ggcgacgtgg ccgagtaccg cgccatccgc gccgtgatcc
5220cccaggactc cctgcgcatc aactccacca agtccatgat cggccacctg
ctgggcggcg 5280ccggcgccgt ggaggccgtg gccgccatcc aggccctgcg
caccggctgg ctgcacccca 5340acctgaacct ggagaacccc gcccccggcg
tggaccccgt ggtgctggtg ggcccccgca 5400aggagcgcgc cgaggacctg
gacgtggtgc tgtccaactc cttcggcttc ggcggccaca 5460actcctgcgt
gatcttccgc aagtacgacg agatggacta caaggaccac gacggcgact
5520acaaggacca cgacatcgac tacaaggacg acgacgacaa gtgaatcgat
agatctctta 5580aggcagcagc agctcggata gtatcgacac actctggacg
ctggtcgtgt gatggactgt 5640tgccgccaca cttgctgcct tgacctgtga
atatccctgc cgcttttatc aaacagcctc 5700agtgtgtttg atcttgtgtg
tacgcgcttt tgcgagttgc tagctgcttg tgctatttgc 5760gaataccacc
cccagcatcc ccttccctcg tttcatatcg cttgcatccc aaccgcaact
5820tatctacgct gtcctgctat ccctcagcgc tgctcctgct cctgctcact
gcccctcgca 5880cagccttggt ttgggctccg cctgtattct
cctggtactg caacctgtaa accagcactg 5940caatgctgat gcacgggaag
tagtgggatg ggaacacaaa tggaaagctt aattaagagc 6000tcttgttttc
cagaaggagt tgctccttga gcctttcatt ctcagcctcg ataacctcca
6060aagccgctct aattgtggag ggggttcgaa ccgaatgctg cgtgaacggg
aaggaggagg 6120agaaagagtg agcagggagg gattcagaaa tgagaaatga
gaggtgaagg aacgcatccc 6180tatgcccttg caatggacag tgtttctggc
caccgccacc aagacttcgt gtcctctgat 6240catcatgcga ttgattacgt
tgaatgcgac ggccggtcag ccccggacct ccacgcaccg 6300gtgctcctcc
aggaagatgc gcttgtcctc cgccatcttg cagggctcaa gctgctccca
6360aaactcttgg gcgggttccg gacggacggc taccgcgggt gcggccctga
ccgccactgt 6420tcggaagcag cggcgctgca tgggcagcgg ccgctgcggt
gcgccacgga ccgcatgatc 6480caccggaaaa gcgcacgcgc tggagcgcgc
agaggaccac agagaagcgg aagagacgcc 6540agtactggca agcaggctgg
tcggtgccat ggcgcgctac taccctcgct atgactcggg 6600tcctcggccg
gctggcggtg ctgacaattc gtttagtgga gcagcgactc cattcagcta
6660ccagtcgaac tcagtggcac agtgactccg ctcttc 6696115993DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
115gatatctccc tccgtctctg cactctggcg cccctcctcc gtctcgtgga
ctgacggacg 60agagtctggg cgccgctttt ctatccacac cgccctttcc gcatcgaaga
caccacccat 120cgtgccgcca ggtcttcccc aatcacccgc cctgtggtcc
tctctcccag ccgtgtttgg 180tcgctgcgtc cacatttttc cattcgtgcc
ccacgatcct cgcccatctt ggcgccttgg 240ataggcaccc ttttttcagc
acgccctggt gtgtagcaca acctgacctc tctctaccgc 300atcgcctccc
tcccacacct cagttgactc cctcgtcgca cgttgcaccc gcaagctccc
360catttcatcc tattgacaat cgcacactgt acatgtatgc tcattatttt
gcaaaaaaac 420agggggtcgg ttcactcctg gcagacgacg cggtgctgcc
gcgcgccgct gaggcggcgt 480cgcgacggca acacccatcg caccgcacgt
cgacgagtca acccaccctg ctcaacggtg 540atctccccat cgcgacaccc
cccgtgaccg tactatgtgc gtccatacgc aacatgaaaa 600ggaccttggt
ccccggaggc ggcgagctcg taatcccgag gttggccccg cttccgctgg
660acacccatcg catcttccgg ctcgcccgct gtcgagcaag cgccctcgtg
cgcgcaaccc 720ttgtggtgcc tgcccgcaga gccgggcata aaggcgagca
ccacacccga accagtccaa 780tttgctttct gcattcactc accaactttt
acatccacac atcgtactac cacacctgcc 840cagtcgggtt tgatttctat
tgcaaaggtg cgggggggtt ggcgcactgc gtgggttgtg 900cagccggccg
ccgcggctgt acccagcgat caggtagctt gggctgtatc ttctcaagca
960ttaccttgtc ctgggcgtag gtttgccact agt 9931161083DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
116gatatcgaat tcggccgaca ggacgcgcgt caaaggtgct ggtcgtgtat
gccctggccg 60gcaggtcgtt gctgctgctg gttagtgatt ccgcaaccct gattttggcg
tcttattttg 120gcgtggcaaa cgctggcgcc cgcgagccgg gccggcggcg
atgcggtgcc ccacggctgc 180cggaatccaa gggaggcaag agcgcccggg
tcagttgaag ggctttacgc gcaaggtaca 240gccgctcctg caaggctgcg
tggtggaatt ggacgtgcag gtcctgctga agttcctcca 300ccgcctcacc
agcggacaaa gcaccggtgt atcaggtccg tgtcatccac tctaaagaac
360tcgactacga cctactgatg gccctagatt cttcatcaaa aacgcctgag
acacttgccc 420aggattgaaa ctccctgaag ggaccaccag gggccctgag
ttgttccttc cccccgtggc 480gagctgccag ccaggctgta cctgtgatcg
aggctggcgg gaaaataggc ttcgtgtgct 540caggtcatgg gaggtgcagg
acagctcatg aaacgccaac aatcgcacaa ttcatgtcaa 600gctaatcagc
tatttcctct tcacgagctg taattgtccc aaaattctgg tctaccgggg
660gtgatccttc gtgtacgggc ccttccctca accctaggta tgcgcgcatg
cggtcgccgc 720gcaactcgcg cgagggccga gggtttggga cgggccgtcc
cgaaatgcag ttgcacccgg 780atgcgtggca ccttttttgc gataatttat
gcaatggact gctctgcaaa attctggctc 840tgtcgccaac cctaggatca
gcggcgtagg atttcgtaat cattcgtcct gatggggagc 900taccgactac
cctaatatca gcccgactgc ctgacgccag cgtccacttt tgtgcacaca
960ttccattcgt gcccaagaca tttcattgtg gtgcgaagcg tccccagtta
cgctcacctg 1020tttcccgacc tccttactgt tctgtcgaca gagcgggccc
acaggccggt cgcagccact 1080agt 10831175662DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
117gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtaccgcgg tgagaatcga aaatgcatcg tttctaggtt
cggagacggt caattccctg 780ctccggcgaa tctgtcggtc aagctggcca
gtggacaatg ttgctatggc agcccgcgca 840catgggcctc ccgacgcggc
catcaggagc ccaaacagcg tgtcagggta tgtgaaactc 900aagaggtccc
tgctgggcac tccggcccca ctccgggggc gggacgccag gcattcgcgg
960tcggtcccgc gcgacgagcg aaatgatgat tcggttacga gaccaggacg
tcgtcgaggt 1020cgagaggcag cctcggacac gtctcgctag ggcaacgccc
cgagtccccg cgagggccgt 1080aaacattgtt tctgggtgtc ggagtgggca
ttttgggccc gatccaatcg cctcatgccg 1140ctctcgtctg gtcctcacgt
tcgcgtacgg cctggatccc ggaaagggcg gatgcacgtg 1200gtgttgcccc
gccattggcg cccacgtttc aaagtccccg gccagaaatg cacaggaccg
1260gcccggctcg cacaggccat gctgaacgcc cagatttcga cagcaacacc
atctagaata 1320atcgcaacca tccgcgtttt gaacgaaacg aaacggcgct
gtttagcatg tttccgacat 1380cgtgggggcc gaagcatgct ccggggggag
gaaagcgtgg cacagcggta gcccattctg 1440tgccacacgc cgacgaggac
caatccccgg catcagcctt catcgacggc tgcgccgcac 1500atataaagcc
ggacgcctaa ccggtttcgt ggttatgact agtatgttcg cgttctactt
1560cctgacggcc tgcatctccc tgaagggcgt gttcggcgtc tccccctcct
acaacggcct 1620gggcctgacg ccccagatgg gctgggacaa ctggaacacg
ttcgcctgcg acgtctccga 1680gcagctgctg ctggacacgg ccgaccgcat
ctccgacctg ggcctgaagg acatgggcta 1740caagtacatc atcctggacg
actgctggtc ctccggccgc gactccgacg gcttcctggt 1800cgccgacgag
cagaagttcc ccaacggcat gggccacgtc gccgaccacc tgcacaacaa
1860ctccttcctg ttcggcatgt actcctccgc gggcgagtac acgtgcgccg
gctaccccgg 1920ctccctgggc cgcgaggagg aggacgccca gttcttcgcg
aacaaccgcg tggactacct 1980gaagtacgac aactgctaca acaagggcca
gttcggcacg cccgagatct cctaccaccg 2040ctacaaggcc atgtccgacg
ccctgaacaa gacgggccgc cccatcttct actccctgtg 2100caactggggc
caggacctga ccttctactg gggctccggc atcgcgaact cctggcgcat
2160gtccggcgac gtcacggcgg agttcacgcg ccccgactcc cgctgcccct
gcgacggcga 2220cgagtacgac tgcaagtacg ccggcttcca ctgctccatc
atgaacatcc tgaacaaggc 2280cgcccccatg ggccagaacg cgggcgtcgg
cggctggaac gacctggaca acctggaggt 2340cggcgtcggc aacctgacgg
acgacgagga gaaggcgcac ttctccatgt gggccatggt 2400gaagtccccc
ctgatcatcg gcgcgaacgt gaacaacctg aaggcctcct cctactccat
2460ctactcccag gcgtccgtca tcgccatcaa ccaggactcc aacggcatcc
ccgccacgcg 2520cgtctggcgc tactacgtgt ccgacacgga cgagtacggc
cagggcgaga tccagatgtg 2580gtccggcccc ctggacaacg gcgaccaggt
cgtggcgctg ctgaacggcg gctccgtgtc 2640ccgccccatg aacacgaccc
tggaggagat cttcttcgac tccaacctgg gctccaagaa 2700gctgacctcc
acctgggaca tctacgacct gtgggcgaac cgcgtcgaca actccacggc
2760gtccgccatc ctgggccgca acaagaccgc caccggcatc ctgtacaacg
ccaccgagca 2820gtcctacaag gacggcctgt ccaagaacga cacccgcctg
ttcggccaga agatcggctc 2880cctgtccccc aacgcgatcc tgaacacgac
cgtccccgcc cacggcatcg cgttctaccg 2940cctgcgcccc tcctcctgac
aattggcagc agcagctcgg atagtatcga cacactctgg 3000acgctggtcg
tgtgatggac tgttgccgcc acacttgctg ccttgacctg tgaatatccc
3060tgccgctttt atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc
ttttgcgagt 3120tgctagctgc ttgtgctatt tgcgaatacc acccccagca
tccccttccc tcgtttcata 3180tcgcttgcat cccaaccgca acttatctac
gctgtcctgc tatccctcag cgctgctcct 3240gctcctgctc actgcccctc
gcacagcctt ggtttgggct ccgcctgtat tctcctggta 3300ctgcaacctg
taaaccagca ctgcaatgct gatgcacggg aagtagtggg atgggaacac
3360aaatggagga tcccgcgtct cgaacagagc gcgcagagga acgctgaagg
tctcgcctct 3420gtcgcacctc agcgcggcat acaccacaat aaccacctga
cgaatgcgct tggttcttcg 3480tccattagcg aagcgtccgg ttcacacacg
tgccacgttg gcgaggtggc aggtgacaat 3540gatcggtgga gctgatggtc
gaaacgttca cagcctaggg atatcgaatt cctttcttgc 3600gctatgacac
ttccagcaaa aggtagggcg ggctgcgaga cggcttcccg gcgctgcatg
3660caacaccgat gatgcttcga ccccccgaag ctccttcggg gctgcatggg
cgctccgatg 3720ccgctccagg gcgagcgctg tttaaatagc caggcccccg
attgcaaaga cattatagcg 3780agctaccaaa gccatattca aacacctaga
tcactaccac ttctacacag gccactcgag 3840cttgtgatcg cactccgcta
agggggcgcc tcttcctctt cgtttcagtc acaacccgca 3900aacactagta
tggctatcaa gacgaacagg cagcctgtgg agaagcctcc gttcacgatc
3960gggacgctgc gcaaggccat ccccgcgcac tgtttcgagc gctcggcgct
tcgtagcagc 4020atgtacctgg cctttgacat cgcggtcatg tccctgctct
acgtcgcgtc gacgtacatc 4080gaccctgcac cggtgcctac gtgggtcaag
tacggcatca tgtggccgct ctactggttc 4140ttccaggtgt gtttgagggt
tttggttgcc cgtattgagg tcctggtggc gcgcatggag 4200gagaaggcgc
ctgtcccgct gacccccccg gctaccctcc cggcaccttc cagggcgcgt
4260acgggaagaa ccagtagagc ggccacatga tgccgtactt gacccacgta
ggcaccggtg 4320cagggtcgat gtacgtcgac gcgacgtaga gcagggacat
gaccgcgatg tcaaaggcca 4380ggtacatgct gctacgaagc gccgagcgct
cgaaacagtg cgcggggatg gccttgcgca 4440gcgtcccgat cgtgaacgga
ggcttctcca caggctgcct gttcgtcttg atagccatct 4500cgaggcagca
gcagctcgga tagtatcgac acactctgga cgctggtcgt gtgatggact
4560gttgccgcca cacttgctgc cttgacctgt gaatatccct gccgctttta
tcaaacagcc 4620tcagtgtgtt tgatcttgtg tgtacgcgct tttgcgagtt
gctagctgct tgtgctattt 4680gcgaatacca cccccagcat ccccttccct
cgtttcatat cgcttgcatc ccaaccgcaa 4740cttatctacg ctgtcctgct
atccctcagc gctgctcctg ctcctgctca ctgcccctcg 4800cacagccttg
gtttgggctc cgcctgtatt ctcctggtac tgcaacctgt aaaccagcac
4860tgcaatgctg atgcacggga agtagtggga tgggaacaca aatggaaagc
tgtagagctc 4920ttgttttcca gaaggagttg ctccttgagc ctttcattct
cagcctcgat aacctccaaa 4980gccgctctaa ttgtggaggg ggttcgaatt
taaaagcttg gaatgttggt tcgtgcgtct 5040ggaacaagcc cagacttgtt
gctcactggg aaaaggacca tcagctccaa aaaacttgcc 5100gctcaaaccg
cgtacctctg ctttcgcgca atctgccctg ttgaaatcgc caccacattc
5160atattgtgac gcttgagcag tctgtaattg cctcagaatg tggaatcatc
tgccccctgt 5220gcgagcccat gccaggcatg tcgcgggcga ggacacccgc
cactcgtaca gcagaccatt 5280atgctacctc acaatagttc ataacagtga
ccatatttct cgaagctccc caacgagcac 5340ctccatgctc tgagtggcca
ccccccggcc ctggtgcttg cggagggcag gtcaaccggc 5400atggggctac
cgaaatcccc gaccggatcc caccaccccc gcgatgggaa gaatctctcc
5460ccgggatgtg ggcccaccac cagcacaacc tgctggccca ggcgagcgtc
aaaccatacc 5520acacaaatat ccttggcatc ggccctgaat tccttctgcc
gctctgctac ccggtgcttc 5580tgtccgaagc aggggttgct agggatcgct
ccgagtccgc aaacccttgt cgcgtggcgg 5640ggcttgttcg agcttgaaga gc
56621187963DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 118gctcttcgga gtcactgtgc cactgagttc
gactggtagc tgaatggagt cgctgctcca 60ctaaacgaat tgtcagcacc gccagccggc
cgaggacccg agtcatagcg agggtagtag 120cgcgccatgg caccgaccag
cctgcttgcc agtactggcg tctcttccgc ttctctgtgg 180tcctctgcgc
gctccagcgc gtgcgctttt ccggtggatc atgcggtccg tggcgcaccg
240cagcggccgc tgcccatgca gcgccgctgc ttccgaacag tggcggtcag
ggccgcaccc 300gcggtagccg tccgtccgga acccgcccaa gagttttggg
agcagcttga gccctgcaag 360atggcggagg acaagcgcat cttcctggag
gagcaccggt gcgtggaggt ccggggctga 420ccggccgtcg cattcaacgt
aatcaatcgc atgatgatca gaggacacga agtcttggtg 480gcggtggcca
gaaacactgt ccattgcaag ggcataggga tgcgttcctt cacctctcat
540ttctcatttc tgaatccctc cctgctcact ctttctcctc ctccttcccg
ttcacgcagc 600attcggggta ccagtttagg tccagcgtcc gtgggggggg
acgggctggg agcttgggcc 660gggaagggca agacgatgca gtccctctgg
ggagtcacag ccgactgtgt gtgttgcact 720gtgcggcccg cagcactcac
acgcaaaatg cctggccgac aggcaggccc tgtccagtgc 780aacatccacg
gtccctctca tcaggctcac cttgctcatt gacataacgg aatgcgtacc
840gctctttcag atctgtccat ccagagaggg gagcaggctc cccaccgacg
ctgtcaaact 900tgcttcctgc ccaaccgaaa acattattgt ttgagggggg
gggggggggg gcagattgca 960tggcgggata tctcgtgagg aacatcactg
ggacactgtg gaacacagtg agtgcagtat 1020gcagagcatg tatgctaggg
gtcagcgcag gaagggggcc tttcccagtc tcccatgcca 1080ctgcaccgta
tccacgactc accaggacca gcttcttgat cggcttccgc tcccgtggac
1140accagtgtgt agcctctgga ctccaggtat gcgtgcaccg caaaggccag
ccgatcgtgc 1200cgattcctgg ggtggaggat atgagtcagc caacttgggg
ctcagagtgc acactggggc 1260acgatacgaa acaacatcta caccgtgtcc
tccatgctga cacaccacag cttcgctcca 1320cctgaatgtg ggcgcatggg
cccgaatcac agccaatgtc gctgctgcca taatgtgatc 1380cagaccctct
ccgcccagat gccgagcgga tcgtgggcgc tgaatagatt cctgtttcga
1440tcactgtttg ggtcctttcc ttttcgtctc ggatgcgcgt ctcgaaacag
gctgcgtcgg 1500gctttcggat cccttttgct ccctccgtca ccatcctgcg
cgcgggcaag ttgcttgacc 1560ctgggctgta ccagggttgg agggtattac
cgcgtcaggc cattcccagc ccggattcaa 1620ttcaaagtct gggccaccac
cctccgccgc tctgtctgat cactccacat tcgtgcatac 1680actacgttca
agtcctgatc caggcgtgtc tcgggacaag gtgtgcttga gtttgaatct
1740caaggaccca ctccagcaca gctgctggtt gaccccgccc tcgcaatcta
gaatggccgc 1800gtccgtccac tgcaccctga tgtccgtggt ctgcaacaac
aagaaccact ccgcccgccc 1860caagctgccc aactcctccc tgctgcccgg
cttcgacgtg gtggtccagg ccgcggccac 1920ccgcttcaag aaggagacga
cgaccacccg cgccacgctg acgttcgacc cccccacgac 1980caactccgag
cgcgccaagc agcgcaagca caccatcgac ccctcctccc ccgacttcca
2040gcccatcccc tccttcgagg agtgcttccc caagtccacg aaggagcaca
aggaggtggt 2100gcacgaggag tccggccacg tcctgaaggt gcccttccgc
cgcgtgcacc tgtccggcgg 2160cgagcccgcc ttcgacaact acgacacgtc
cggcccccag aacgtcaacg cccacatcgg 2220cctggcgaag ctgcgcaagg
agtggatcga ccgccgcgag aagctgggca cgccccgcta 2280cacgcagatg
tactacgcga agcagggcat catcacggag gagatgctgt actgcgcgac
2340gcgcgagaag ctggaccccg agttcgtccg ctccgaggtc gcgcggggcc
gcgccatcat 2400cccctccaac aagaagcacc tggagctgga gcccatgatc
gtgggccgca agttcctggt 2460gaaggtgaac gcgaacatcg gcaactccgc
cgtggcctcc tccatcgagg aggaggtcta 2520caaggtgcag tgggccacca
tgtggggcgc cgacaccatc atggacctgt ccacgggccg 2580ccacatccac
gagacgcgcg agtggatcct gcgcaactcc gcggtccccg tgggcaccgt
2640ccccatctac caggcgctgg agaaggtgga cggcatcgcg gagaacctga
actgggaggt 2700gttccgcgag acgctgatcg agcaggccga gcagggcgtg
gactacttca cgatccacgc 2760gggcgtgctg ctgcgctaca tccccctgac
cgccaagcgc ctgacgggca tcgtgtcccg 2820cggcggctcc atccacgcga
agtggtgcct ggcctaccac aaggagaact tcgcctacga 2880gcactgggac
gacatcctgg acatctgcaa ccagtacgac gtcgccctgt ccatcggcga
2940cggcctgcgc cccggctcca tctacgacgc caacgacacg gcccagttcg
ccgagctgct 3000gacccagggc gagctgacgc gccgcgcgtg ggagaaggac
gtgcaggtga tgaacgaggg 3060ccccggccac gtgcccatgc acaagatccc
cgagaacatg cagaagcagc tggagtggtg 3120caacgaggcg cccttctaca
ccctgggccc cctgacgacc gacatcgcgc ccggctacga 3180ccacatcacc
tccgccatcg gcgcggccaa catcggcgcc ctgggcaccg ccctgctgtg
3240ctacgtgacg cccaaggagc acctgggcct gcccaaccgc gacgacgtga
aggcgggcgt 3300catcgcctac aagatcgccg cccacgcggc cgacctggcc
aagcagcacc cccacgccca 3360ggcgtgggac gacgcgctgt ccaaggcgcg
cttcgagttc cgctggatgg accagttcgc 3420gctgtccctg gaccccatga
cggcgatgtc cttccacgac gagacgctgc ccgcggacgg 3480cgcgaaggtc
gcccacttct gctccatgtg cggccccaag ttctgctcca tgaagatcac
3540ggaggacatc cgcaagtacg ccgaggagaa cggctacggc tccgccgagg
aggccatccg 3600ccagggcatg gacgccatgt ccgaggagtt caacatcgcc
aagaagacga tctccggcga 3660gcagcacggc gaggtcggcg gcgagatcta
cctgcccgag tcctacgtca aggccgcgca 3720gaagtgacaa ttggcagcag
cagctcggat agtatcgaca cactctggac gctggtcgtg 3780tgatggactg
ttgccgccac acttgctgcc ttgacctgtg aatatccctg ccgcttttat
3840caaacagcct cagtgtgttt gatcttgtgt gtacgcgctt ttgcgagttg
ctagctgctt 3900gtgctatttg cgaataccac ccccagcatc cccttccctc
gtttcatatc gcttgcatcc 3960caaccgcaac ttatctacgc tgtcctgcta
tccctcagcg ctgctcctgc tcctgctcac 4020tgcccctcgc acagccttgg
tttgggctcc gcctgtattc tcctggtact gcaacctgta 4080aaccagcact
gcaatgctga tgcacgggaa gtagtgggat gggaacacaa atggaggatc
4140ccgcgtctcg aacagagcgc gcagaggaac gctgaaggtc tcgcctctgt
cgcacctcag 4200cgcggcatac accacaataa ccacctgacg aatgcgcttg
gttcttcgtc cattagcgaa 4260gcgtccggtt cacacacgtg ccacgttggc
gaggtggcag gtgacaatga tcggtggagc 4320tgatggtcga aacgttcaca
gcctagggat atcgaattcg gccgacagga cgcgcgtcaa 4380aggtgctggt
cgtgtatgcc ctggccggca ggtcgttgct gctgctggtt agtgattccg
4440caaccctgat tttggcgtct tattttggcg tggcaaacgc tggcgcccgc
gagccgggcc 4500ggcggcgatg cggtgcccca cggctgccgg aatccaaggg
aggcaagagc gcccgggtca 4560gttgaagggc tttacgcgca aggtacagcc
gctcctgcaa ggctgcgtgg tggaattgga 4620cgtgcaggtc ctgctgaagt
tcctccaccg cctcaccagc ggacaaagca ccggtgtatc 4680aggtccgtgt
catccactct aaagaactcg actacgacct actgatggcc ctagattctt
4740catcaaaaac gcctgagaca cttgcccagg attgaaactc cctgaaggga
ccaccagggg 4800ccctgagttg ttccttcccc ccgtggcgag ctgccagcca
ggctgtacct gtgatcgagg 4860ctggcgggaa aataggcttc gtgtgctcag
gtcatgggag gtgcaggaca gctcatgaaa 4920cgccaacaat cgcacaattc
atgtcaagct aatcagctat ttcctcttca cgagctgtaa 4980ttgtcccaaa
attctggtct accgggggtg atccttcgtg tacgggccct tccctcaacc
5040ctaggtatgc gcgcatgcgg tcgccgcgca actcgcgcga gggccgaggg
tttgggacgg 5100gccgtcccga aatgcagttg cacccggatg cgtggcacct
tttttgcgat aatttatgca 5160atggactgct ctgcaaaatt ctggctctgt
cgccaaccct aggatcagcg gcgtaggatt 5220tcgtaatcat tcgtcctgat
ggggagctac cgactaccct aatatcagcc cgactgcctg 5280acgccagcgt
ccacttttgt gcacacattc cattcgtgcc caagacattt cattgtggtg
5340cgaagcgtcc ccagttacgc tcacctgttt cccgacctcc ttactgttct
gtcgacagag 5400cgggcccaca ggccggtcgc agccactagt atgcagaccg
cccaccagcg cccccccacc 5460gagggccact gcttcggcgc ccgcctgccc
accgcctccc gccgcgccgt gcgccgcgcc 5520tggtcccgca tcgcccgcgg
gcgcgccgcc gccgccgccg acgccaaccc cgcccgcccc 5580gagcgccgcg
tggtgatcac cggccagggc gtggtgacct ccctgggcca gaccatcgag
5640cagttctact cctccctgct ggagggcgtg tccggcatct cccagatcca
gaagttcgac 5700accaccggct acaccaccac catcgccggc gagatcaagt
ccctgcagct ggacccctac 5760gtgcccaagc gctgggccaa gcgcgtggac
gacgtgatca agtacgtgta catcgccggc 5820aagcaggccc tggagtccgc
cggcctgccc atcgaggccg ccggcctggc cggcgccggc 5880ctggaccccg
ccctgtgcgg cgtgctgatc ggcaccgcca tggccggcat gacctccttc
5940gccgccggcg tggaggccct gacccgcggc ggcgtgcgca agatgaaccc
cttctgcatc 6000cccttctcca tctccaacat gggcggcgcc
atgctggcca tggacatcgg cttcatgggc 6060cccaactact ccatctccac
cgcctgcgcc accggcaact actgcatcct gggcgccgcc 6120gaccacatcc
gccgcggcga cgccaacgtg atgctggccg gcggcgccga cgccgccatc
6180atcccctccg gcatcggcgg cttcatcgcc tgcaaggccc tgtccaagcg
caacgacgag 6240cccgagcgcg cctcccgccc ctgggacgcc gaccgcgacg
gcttcgtgat gggcgagggc 6300gccggcgtgc tggtgctgga ggagctggag
cacgccaagc gccgcggcgc caccatcctg 6360gccgagctgg tgggcggcgc
cgccacctcc gacgcccacc acatgaccga gcccgacccc 6420cagggccgcg
gcgtgcgcct gtgcctggag cgcgccctgg agcgcgcccg cctggccccc
6480gagcgcgtgg gctacgtgaa cgcccacggc acctccaccc ccgccggcga
cgtggccgag 6540taccgcgcca tccgcgccgt gatcccccag gactccctgc
gcatcaactc caccaagtcc 6600atgatcggcc acctgctggg cggcgccggc
gccgtggagg ccgtggccgc catccaggcc 6660ctgcgcaccg gctggctgca
ccccaacctg aacctggaga accccgcccc cggcgtggac 6720cccgtggtgc
tggtgggccc ccgcaaggag cgcgccgagg acctggacgt ggtgctgtcc
6780aactccttcg gcttcggcgg ccacaactcc tgcgtgatct tccgcaagta
cgacgagatg 6840gactacaagg accacgacgg cgactacaag gaccacgaca
tcgactacaa ggacgacgac 6900gacaagtgaa tcgatagatc tcttaaggca
gcagcagctc ggatagtatc gacacactct 6960ggacgctggt cgtgtgatgg
actgttgccg ccacacttgc tgccttgacc tgtgaatatc 7020cctgccgctt
ttatcaaaca gcctcagtgt gtttgatctt gtgtgtacgc gcttttgcga
7080gttgctagct gcttgtgcta tttgcgaata ccacccccag catccccttc
cctcgtttca 7140tatcgcttgc atcccaaccg caacttatct acgctgtcct
gctatccctc agcgctgctc 7200ctgctcctgc tcactgcccc tcgcacagcc
ttggtttggg ctccgcctgt attctcctgg 7260tactgcaacc tgtaaaccag
cactgcaatg ctgatgcacg ggaagtagtg ggatgggaac 7320acaaatggaa
agcttaatta agagctcttg ttttccagaa ggagttgctc cttgagcctt
7380tcattctcag cctcgataac ctccaaagcc gctctaattg tggagggggt
tcgaagacag 7440ggtggttggc tggatgggga aacgctggtc gcgggattcg
atcctgctgc ttatatcctc 7500cctggaagca cacccacgac tctgaagaag
aaaacgtgca cacacacaac ccaaccggcc 7560gaatatttgc ttccttatcc
cgggtccaag agagactgcg atgcccccct caatcagcat 7620cctcctccct
gccgcttcaa tcttccctgc ttgcctgcgc ccgcggtgcg ccgtctgccc
7680gcccagtcag tcactcctgc acaggcccct tgtgcgcagt gctcctgtac
cctttaccgc 7740tccttccatt ctgcgaggcc ccctattgaa tgtattcgtt
gcctgtgtgg ccaagcgggc 7800tgctgggcgc gccgccgtcg ggcagtgctc
ggcgactttg gcggaagccg attgttcttc 7860tgtaagccac gcgcttgctg
ctttgggaag agaagggggg gggtactgaa tggatgagga 7920ggagaaggag
gggtattggt attatctgag ttgggtgaag agc 79631197887DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
119gctcttcgga gtcactgtgc cactgagttc gactggtagc tgaatggagt
cgctgctcca 60ctaaacgaat tgtcagcacc gccagccggc cgaggacccg agtcatagcg
agggtagtag 120cgcgccatgg caccgaccag cctgcttgcc agtactggcg
tctcttccgc ttctctgtgg 180tcctctgcgc gctccagcgc gtgcgctttt
ccggtggatc atgcggtccg tggcgcaccg 240cagcggccgc tgcccatgca
gcgccgctgc ttccgaacag tggcggtcag ggccgcaccc 300gcggtagccg
tccgtccgga acccgcccaa gagttttggg agcagcttga gccctgcaag
360atggcggagg acaagcgcat cttcctggag gagcaccggt gcgtggaggt
ccggggctga 420ccggccgtcg cattcaacgt aatcaatcgc atgatgatca
gaggacacga agtcttggtg 480gcggtggcca gaaacactgt ccattgcaag
ggcataggga tgcgttcctt cacctctcat 540ttctcatttc tgaatccctc
cctgctcact ctttctcctc ctccttcccg ttcacgcagc 600attcggggta
ccagtttagg tccagcgtcc gtgggggggg acgggctggg agcttgggcc
660gggaagggca agacgatgca gtccctctgg ggagtcacag ccgactgtgt
gtgttgcact 720gtgcggcccg cagcactcac acgcaaaatg cctggccgac
aggcaggccc tgtccagtgc 780aacatccacg gtccctctca tcaggctcac
cttgctcatt gacataacgg aatgcgtacc 840gctctttcag atctgtccat
ccagagaggg gagcaggctc cccaccgacg ctgtcaaact 900tgcttcctgc
ccaaccgaaa acattattgt ttgagggggg gggggggggg gcagattgca
960tggcgggata tctcgtgagg aacatcactg ggacactgtg gaacacagtg
agtgcagtat 1020gcagagcatg tatgctaggg gtcagcgcag gaagggggcc
tttcccagtc tcccatgcca 1080ctgcaccgta tccacgactc accaggacca
gcttcttgat cggcttccgc tcccgtggac 1140accagtgtgt agcctctgga
ctccaggtat gcgtgcaccg caaaggccag ccgatcgtgc 1200cgattcctgg
ggtggaggat atgagtcagc caacttgggg ctcagagtgc acactggggc
1260acgatacgaa acaacatcta caccgtgtcc tccatgctga cacaccacag
cttcgctcca 1320cctgaatgtg ggcgcatggg cccgaatcac agccaatgtc
gctgctgcca taatgtgatc 1380cagaccctct ccgcccagat gccgagcgga
tcgtgggcgc tgaatagatt cctgtttcga 1440tcactgtttg ggtcctttcc
ttttcgtctc ggatgcgcgt ctcgaaacag gctgcgtcgg 1500gctttcggat
cccttttgct ccctccgtca ccatcctgcg cgcgggcaag ttgcttgacc
1560ctgggctgta ccagggttgg agggtattac cgcgtcaggc cattcccagc
ccggattcaa 1620ttcaaagtct gggccaccac cctccgccgc tctgtctgat
cactccacat tcgtgcatac 1680actacgttca agtcctgatc caggcgtgtc
tcgggacaag gtgtgcttga gtttgaatct 1740caaggaccca ctccagcaca
gctgctggtt gaccccgccc tcgcaatcta gaatggccgc 1800gtccgtccac
tgcaccctga tgtccgtggt ctgcaacaac aagaaccact ccgcccgccc
1860caagctgccc aactcctccc tgctgcccgg cttcgacgtg gtggtccagg
ccgcggccac 1920ccgcttcaag aaggagacga cgaccacccg cgccacgctg
acgttcgacc cccccacgac 1980caactccgag cgcgccaagc agcgcaagca
caccatcgac ccctcctccc ccgacttcca 2040gcccatcccc tccttcgagg
agtgcttccc caagtccacg aaggagcaca aggaggtggt 2100gcacgaggag
tccggccacg tcctgaaggt gcccttccgc cgcgtgcacc tgtccggcgg
2160cgagcccgcc ttcgacaact acgacacgtc cggcccccag aacgtcaacg
cccacatcgg 2220cctggcgaag ctgcgcaagg agtggatcga ccgccgcgag
aagctgggca cgccccgcta 2280cacgcagatg tactacgcga agcagggcat
catcacggag gagatgctgt actgcgcgac 2340gcgcgagaag ctggaccccg
agttcgtccg ctccgaggtc gcgcggggcc gcgccatcat 2400cccctccaac
aagaagcacc tggagctgga gcccatgatc gtgggccgca agttcctggt
2460gaaggtgaac gcgaacatcg gcaactccgc cgtggcctcc tccatcgagg
aggaggtcta 2520caaggtgcag tgggccacca tgtggggcgc cgacaccatc
atggacctgt ccacgggccg 2580ccacatccac gagacgcgcg agtggatcct
gcgcaactcc gcggtccccg tgggcaccgt 2640ccccatctac caggcgctgg
agaaggtgga cggcatcgcg gagaacctga actgggaggt 2700gttccgcgag
acgctgatcg agcaggccga gcagggcgtg gactacttca cgatccacgc
2760gggcgtgctg ctgcgctaca tccccctgac cgccaagcgc ctgacgggca
tcgtgtcccg 2820cggcggctcc atccacgcga agtggtgcct ggcctaccac
aaggagaact tcgcctacga 2880gcactgggac gacatcctgg acatctgcaa
ccagtacgac gtcgccctgt ccatcggcga 2940cggcctgcgc cccggctcca
tctacgacgc caacgacacg gcccagttcg ccgagctgct 3000gacccagggc
gagctgacgc gccgcgcgtg ggagaaggac gtgcaggtga tgaacgaggg
3060ccccggccac gtgcccatgc acaagatccc cgagaacatg cagaagcagc
tggagtggtg 3120caacgaggcg cccttctaca ccctgggccc cctgacgacc
gacatcgcgc ccggctacga 3180ccacatcacc tccgccatcg gcgcggccaa
catcggcgcc ctgggcaccg ccctgctgtg 3240ctacgtgacg cccaaggagc
acctgggcct gcccaaccgc gacgacgtga aggcgggcgt 3300catcgcctac
aagatcgccg cccacgcggc cgacctggcc aagcagcacc cccacgccca
3360ggcgtgggac gacgcgctgt ccaaggcgcg cttcgagttc cgctggatgg
accagttcgc 3420gctgtccctg gaccccatga cggcgatgtc cttccacgac
gagacgctgc ccgcggacgg 3480cgcgaaggtc gcccacttct gctccatgtg
cggccccaag ttctgctcca tgaagatcac 3540ggaggacatc cgcaagtacg
ccgaggagaa cggctacggc tccgccgagg aggccatccg 3600ccagggcatg
gacgccatgt ccgaggagtt caacatcgcc aagaagacga tctccggcga
3660gcagcacggc gaggtcggcg gcgagatcta cctgcccgag tcctacgtca
aggccgcgca 3720gaagtgacaa ttggcagcag cagctcggat agtatcgaca
cactctggac gctggtcgtg 3780tgatggactg ttgccgccac acttgctgcc
ttgacctgtg aatatccctg ccgcttttat 3840caaacagcct cagtgtgttt
gatcttgtgt gtacgcgctt ttgcgagttg ctagctgctt 3900gtgctatttg
cgaataccac ccccagcatc cccttccctc gtttcatatc gcttgcatcc
3960caaccgcaac ttatctacgc tgtcctgcta tccctcagcg ctgctcctgc
tcctgctcac 4020tgcccctcgc acagccttgg tttgggctcc gcctgtattc
tcctggtact gcaacctgta 4080aaccagcact gcaatgctga tgcacgggaa
gtagtgggat gggaacacaa atggaggatc 4140ccgcgtctcg aacagagcgc
gcagaggaac gctgaaggtc tcgcctctgt cgcacctcag 4200cgcggcatac
accacaataa ccacctgacg aatgcgcttg gttcttcgtc cattagcgaa
4260gcgtccggtt cacacacgtg ccacgttggc gaggtggcag gtgacaatga
tcggtggagc 4320tgatggtcga aacgttcaca gcctagggat atcatagcga
ctgctacccc ccgaccatgt 4380gccgaggcag aaattatata caagaagcag
atcgcaatta ggcacatcgc tttgcattat 4440ccacacacta ttcatcgctg
ctgcggcaag gctgcagagt gtatttttgt ggcccaggag 4500ctgagtccga
agtcgacgcg acgagcggcg caggatccga cccctagacg agctctgtca
4560ttttccaagc acgcagctaa atgcgctgag accgggtcta aatcatccga
aaagtgtcaa 4620aatggccgat tgggttcgcc taggacaatg cgctgcggat
tcgctcgagt ccgctgccgg 4680ccaaaaggcg gtggtacagg aaggcgcacg
gggccaaccc tgcgaagccg ggggcccgaa 4740cgccgaccgc cggccttcga
tctcgggtgt ccccctcgtc aatttcctct ctcgggtgca 4800gccacgaaag
tcgtgacgca ggtcacgaaa tccggttacg aaaaacgcag gtcttcgcaa
4860aaacgtgagg gtttcgcgtc tcgccctagc tattcgtatc gccgggtcag
acccacgtgc 4920agaaaagccc ttgaataacc cgggaccgtg gttaccgcgc
cgcctgcacc agggggctta 4980tataagccca caccacacct gtctcaccac
gcatttctcc aactcgcgac ttttcggaag 5040aaattgttat ccacctagta
tagactgcca cctgcaggac cttgtgtctt gcagtttgta 5100ttggtcccgg
ccgtcgagct cgacagatct gggctagggt tggcctggcc gctcggcact
5160cccctttagc cgcgcgcatc cgcgttccag aggtgcgatt cggtgtgtgg
agcattgtca 5220tgcgcttgtg ggggtcgttc cgtgcgcggc gggtccgcca
tgggcgccga cctgggccct 5280agggtttgtt ttcgggccaa gcgagcccct
ctcacctcgt cgcccccccg cattccctct 5340ctcttgcagc cactagtatg
gccaccgcat ccactttctc ggcgttcaat gcccgctgcg 5400gcgacctgcg
tcgctcggcg ggctccgggc cccggcgccc agcgaggccc ctccccgtgc
5460gcgggcgcgc cgccgccgcc gccgacgcca accccgcccg ccccgagcgc
cgcgtggtga 5520tcaccggcca gggcgtggtg acctccctgg gccagaccat
cgagcagttc tactcctccc 5580tgctggaggg cgtgtccggc atctcccaga
tccagaagtt cgacaccacc ggctacacca 5640ccaccatcgc cggcgagatc
aagtccctgc agctggaccc ctacgtgccc aagcgctggg 5700ccaagcgcgt
ggacgacgtg atcaagtacg tgtacatcgc cggcaagcag gccctggagt
5760ccgccggcct gcccatcgag gccgccggcc tggccggcgc cggcctggac
cccgccctgt 5820gcggcgtgct gatcggcacc gccatggccg gcatgacctc
cttcgccgcc ggcgtggagg 5880ccctgacccg cggcggcgtg cgcaagatga
accccttctg catccccttc tccatctcca 5940acatgggcgg cgccatgctg
gccatggaca tcggcttcat gggccccaac tactccatct 6000ccaccgcctg
cgccaccggc aactactgca tcctgggcgc cgccgaccac atccgccgcg
6060gcgacgccaa cgtgatgctg gccggcggcg ccgacgccgc catcatcccc
tccggcatcg 6120gcggcttcat cgcctgcaag gccctgtcca agcgcaacga
cgagcccgag cgcgcctccc 6180gcccctggga cgccgaccgc gacggcttcg
tgatgggcga gggcgccggc gtgctggtgc 6240tggaggagct ggagcacgcc
aagcgccgcg gcgccaccat cctggccgag ctggtgggcg 6300gcgccgccac
ctccgacgcc caccacatga ccgagcccga cccccagggc cgcggcgtgc
6360gcctgtgcct ggagcgcgcc ctggagcgcg cccgcctggc ccccgagcgc
gtgggctacg 6420tgaacgccca cggcacctcc acccccgccg gcgacgtggc
cgagtaccgc gccatccgcg 6480ccgtgatccc ccaggactcc ctgcgcatca
actccaccaa gtccatgatc ggccacctgc 6540tgggcggcgc cggcgccgtg
gaggccgtgg ccgccatcca ggccctgcgc accggctggc 6600tgcaccccaa
cctgaacctg gagaaccccg cccccggcgt ggaccccgtg gtgctggtgg
6660gcccccgcaa ggagcgcgcc gaggacctgg acgtggtgct gtccaactcc
ttcggcttcg 6720gcggccacaa ctcctgcgtg atcttccgca agtacgacga
gatggactac aaggaccacg 6780acggcgacta caaggaccac gacatcgact
acaaggacga cgacgacaag tgaatcgata 6840gatctcttaa ggcagcagca
gctcggatag tatcgacaca ctctggacgc tggtcgtgtg 6900atggactgtt
gccgccacac ttgctgcctt gacctgtgaa tatccctgcc gcttttatca
6960aacagcctca gtgtgtttga tcttgtgtgt acgcgctttt gcgagttgct
agctgcttgt 7020gctatttgcg aataccaccc ccagcatccc cttccctcgt
ttcatatcgc ttgcatccca 7080accgcaactt atctacgctg tcctgctatc
cctcagcgct gctcctgctc ctgctcactg 7140cccctcgcac agccttggtt
tgggctccgc ctgtattctc ctggtactgc aacctgtaaa 7200ccagcactgc
aatgctgatg cacgggaagt agtgggatgg gaacacaaat ggaaagctta
7260attaagagct cttgttttcc agaaggagtt gctccttgag cctttcattc
tcagcctcga 7320taacctccaa agccgctcta attgtggagg gggttcgaag
acagggtggt tggctggatg 7380gggaaacgct ggtcgcggga ttcgatcctg
ctgcttatat cctccctgga agcacaccca 7440cgactctgaa gaagaaaacg
tgcacacaca caacccaacc ggccgaatat ttgcttcctt 7500atcccgggtc
caagagagac tgcgatgccc ccctcaatca gcatcctcct ccctgccgct
7560tcaatcttcc ctgcttgcct gcgcccgcgg tgcgccgtct gcccgcccag
tcagtcactc 7620ctgcacaggc cccttgtgcg cagtgctcct gtacccttta
ccgctccttc cattctgcga 7680ggccccctat tgaatgtatt cgttgcctgt
gtggccaagc gggctgctgg gcgcgccgcc 7740gtcgggcagt gctcggcgac
tttggcggaa gccgattgtt cttctgtaag ccacgcgctt 7800gctgctttgg
gaagagaagg gggggggtac tgaatggatg aggaggagaa ggaggggtat
7860tggtattatc tgagttgggt gaagagc 78871207072DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
120gctcttcgga gtcactgtgc cactgagttc gactggtagc tgaatggagt
cgctgctcca 60ctaaacgaat tgtcagcacc gccagccggc cgaggacccg agtcatagcg
agggtagtag 120cgcgccatgg caccgaccag cctgcttgcc agtactggcg
tctcttccgc ttctctgtgg 180tcctctgcgc gctccagcgc gtgcgctttt
ccggtggatc atgcggtccg tggcgcaccg 240cagcggccgc tgcccatgca
gcgccgctgc ttccgaacag tggcggtcag ggccgcaccc 300gcggtagccg
tccgtccgga acccgcccaa gagttttggg agcagcttga gccctgcaag
360atggcggagg acaagcgcat cttcctggag gagcaccggt gcgtggaggt
ccggggctga 420ccggccgtcg cattcaacgt aatcaatcgc atgatgatca
gaggacacga agtcttggtg 480gcggtggcca gaaacactgt ccattgcaag
ggcataggga tgcgttcctt cacctctcat 540ttctcatttc tgaatccctc
cctgctcact ctttctcctc ctccttcccg ttcacgcagc 600attcggggta
ccagtttagg tccagcgtcc gtgggggggg acgggctggg agcttgggcc
660gggaagggca agacgatgca gtccctctgg ggagtcacag ccgactgtgt
gtgttgcact 720gtgcggcccg cagcactcac acgcaaaatg cctggccgac
aggcaggccc tgtccagtgc 780aacatccacg gtccctctca tcaggctcac
cttgctcatt gacataacgg aatgcgtacc 840gctctttcag atctgtccat
ccagagaggg gagcaggctc cccaccgacg ctgtcaaact 900tgcttcctgc
ccaaccgaaa acattattgt ttgagggggg gggggggggg gcagattgca
960tggcgggata tctcgtgagg aacatcactg ggacactgtg gaacacagtg
agtgcagtat 1020gcagagcatg tatgctaggg gtcagcgcag gaagggggcc
tttcccagtc tcccatgcca 1080ctgcaccgta tccacgactc accaggacca
gcttcttgat cggcttccgc tcccgtggac 1140accagtgtgt agcctctgga
ctccaggtat gcgtgcaccg caaaggccag ccgatcgtgc 1200cgattcctgg
ggtggaggat atgagtcagc caacttgggg ctcagagtgc acactggggc
1260acgatacgaa acaacatcta caccgtgtcc tccatgctga cacaccacag
cttcgctcca 1320cctgaatgtg ggcgcatggg cccgaatcac agccaatgtc
gctgctgcca taatgtgatc 1380cagaccctct ccgcccagat gccgagcgga
tcgtgggcgc tgaatagatt cctgtttcga 1440tcactgtttg ggtcctttcc
ttttcgtctc ggatgcgcgt ctcgaaacag gctgcgtcgg 1500gctttcggat
cccttttgct ccctccgtca ccatcctgcg cgcgggcaag ttgcttgacc
1560ctgggctgta ccagggttgg agggtattac cgcgtcaggc cattcccagc
ccggattcaa 1620ttcaaagtct gggccaccac cctccgccgc tctgtctgat
cactccacat tcgtgcatac 1680actacgttca agtcctgatc caggcgtgtc
tcgggacaag gtgtgcttga gtttgaatct 1740caaggaccca ctccagcaca
gctgctggtt gaccccgccc tcgcaatcta gaatgttcgc 1800gttctacttc
ctgacggcct gcatctccct gaagggcgtg ttcggcgtct ccccctccta
1860caacggcctg ggcctgacgc cccagatggg ctgggacaac tggaacacgt
tcgcctgcga 1920cgtctccgag cagctgctgc tggacacggc cgaccgcatc
tccgacctgg gcctgaagga 1980catgggctac aagtacatca tcctggacga
ctgctggtcc tccggccgcg actccgacgg 2040cttcctggtc gccgacgagc
agaagttccc caacggcatg ggccacgtcg ccgaccacct 2100gcacaacaac
tccttcctgt tcggcatgta ctcctccgcg ggcgagtaca cgtgcgccgg
2160ctaccccggc tccctgggcc gcgaggagga ggacgcccag ttcttcgcga
acaaccgcgt 2220ggactacctg aagtacgaca actgctacaa caagggccag
ttcggcacgc ccgagatctc 2280ctaccaccgc tacaaggcca tgtccgacgc
cctgaacaag acgggccgcc ccatcttcta 2340ctccctgtgc aactggggcc
aggacctgac cttctactgg ggctccggca tcgcgaactc 2400ctggcgcatg
tccggcgacg tcacggcgga gttcacgcgc cccgactccc gctgcccctg
2460cgacggcgac gagtacgact gcaagtacgc cggcttccac tgctccatca
tgaacatcct 2520gaacaaggcc gcccccatgg gccagaacgc gggcgtcggc
ggctggaacg acctggacaa 2580cctggaggtc ggcgtcggca acctgacgga
cgacgaggag aaggcgcact tctccatgtg 2640ggccatggtg aagtcccccc
tgatcatcgg cgcgaacgtg aacaacctga aggcctcctc 2700ctactccatc
tactcccagg cgtccgtcat cgccatcaac caggactcca acggcatccc
2760cgccacgcgc gtctggcgct actacgtgtc cgacacggac gagtacggcc
agggcgagat 2820ccagatgtgg tccggccccc tggacaacgg cgaccaggtc
gtggcgctgc tgaacggcgg 2880ctccgtgtcc cgccccatga acacgaccct
ggaggagatc ttcttcgact ccaacctggg 2940ctccaagaag ctgacctcca
cctgggacat ctacgacctg tgggcgaacc gcgtcgacaa 3000ctccacggcg
tccgccatcc tgggccgcaa caagaccgcc accggcatcc tgtacaacgc
3060caccgagcag tcctacaagg acggcctgtc caagaacgac acccgcctgt
tcggccagaa 3120gatcggctcc ctgtccccca acgcgatcct gaacacgacc
gtccccgccc acggcatcgc 3180gttctaccgc ctgcgcccct cctcctgaca
attggcagca gcagctcgga tagtatcgac 3240acactctgga cgctggtcgt
gtgatggact gttgccgcca cacttgctgc cttgacctgt 3300gaatatccct
gccgctttta tcaaacagcc tcagtgtgtt tgatcttgtg tgtacgcgct
3360tttgcgagtt gctagctgct tgtgctattt gcgaatacca cccccagcat
ccccttccct 3420cgtttcatat cgcttgcatc ccaaccgcaa cttatctacg
ctgtcctgct atccctcagc 3480gctgctcctg ctcctgctca ctgcccctcg
cacagccttg gtttgggctc cgcctgtatt 3540ctcctggtac tgcaacctgt
aaaccagcac tgcaatgctg atgcacggga agtagtggga 3600tgggaacaca
aatggaggat cccgcgtctc gaacagagcg cgcagaggaa cgctgaaggt
3660ctcgcctctg tcgcacctca gcgcggcata caccacaata accacctgac
gaatgcgctt 3720ggttcttcgt ccattagcga agcgtccggt tcacacacgt
gccacgttgg cgaggtggca 3780ggtgacaatg atcggtggag ctgatggtcg
aaacgttcac agcctaggga tatcgaattc 3840ggccgacagg acgcgcgtca
aaggtgctgg tcgtgtatgc cctggccggc aggtcgttgc 3900tgctgctggt
tagtgattcc gcaaccctga ttttggcgtc ttattttggc gtggcaaacg
3960ctggcgcccg cgagccgggc cggcggcgat gcggtgcccc acggctgccg
gaatccaagg 4020gaggcaagag cgcccgggtc agttgaaggg ctttacgcgc
aaggtacagc cgctcctgca 4080aggctgcgtg gtggaattgg acgtgcaggt
cctgctgaag ttcctccacc gcctcaccag 4140cggacaaagc accggtgtat
caggtccgtg tcatccactc taaagaactc gactacgacc 4200tactgatggc
cctagattct tcatcaaaaa cgcctgagac acttgcccag gattgaaact
4260ccctgaaggg accaccaggg gccctgagtt gttccttccc cccgtggcga
gctgccagcc 4320aggctgtacc tgtgatcgag gctggcggga aaataggctt
cgtgtgctca ggtcatggga 4380ggtgcaggac agctcatgaa acgccaacaa
tcgcacaatt catgtcaagc taatcagcta 4440tttcctcttc acgagctgta
attgtcccaa aattctggtc taccgggggt gatccttcgt 4500gtacgggccc
ttccctcaac cctaggtatg cgcgcatgcg gtcgccgcgc aactcgcgcg
4560agggccgagg gtttgggacg ggccgtcccg aaatgcagtt gcacccggat
gcgtggcacc 4620ttttttgcga taatttatgc aatggactgc tctgcaaaat
tctggctctg tcgccaaccc 4680taggatcagc ggcgtaggat ttcgtaatca
ttcgtcctga tggggagcta ccgactaccc 4740taatatcagc ccgactgcct
gacgccagcg tccacttttg tgcacacatt ccattcgtgc 4800ccaagacatt
tcattgtggt gcgaagcgtc cccagttacg ctcacctgtt tcccgacctc
4860cttactgttc tgtcgacaga gcgggcccac aggccggtcg cagccactag
tatggccatc 4920aagaccaacc gccagcccgt ggagaagccc cccttcacca
tcggcaccct gcgcaaggcc 4980atccccgccc actgcttcga gcgctccgcc
ctgcgctcct ccatgtacct ggccttcgac 5040atcgccgtga tgtccctgct
gtacgtggcc tccacctaca tcgaccccgc ccccgtgccc 5100acctgggtga
agtacggcgt gatgtggccc ctgtactggt tcttccaggg cgccttcggc
5160accggcgtgt gggtgtgcgc ccacgagtgc ggccaccagg ccttctcctc
ctcccaggcc 5220atcaacgacg gcgtgggcct ggtgttccac tccctgctgc
tggtgcccta ctactcctgg 5280aagcactccc accgccgcca ccactccaac
accggctgcc tggacaagga cgaggtgttc 5340gtgccccccc accgcgccgt
ggcccacgag ggcctggagt gggaggagtg gctgcccatc 5400cgcatgggca
aggtgctggt gaccctgacc ctgggctggc ccctgtacct gatgttcaac
5460gtggcctccc gcccctaccc ccgcttcgcc aaccacttcg acccctggtc
ccccatcttc 5520tccaagcgcg agcgcatcga ggtggtgatc tccgacctgg
ccctggtggc cgtgctgtcc 5580ggcctgtccg tgctgggccg caccatgggc
tgggcctggc tggtgaagac ctacgtggtg 5640ccctacctga tcgtgaacat
gtggctggtg ctgatcaccc tgctgcagca cacccacccc 5700gccctgcccc
actacttcga gaaggactgg gactggctgc gcggcgccat ggccaccgtg
5760gaccgctcca tgggcccccc cttcatggac aacatcctgc accacatctc
cgacacccac 5820gtgctgcacc acctgttctc caccatcccc cactaccacg
ccgaggaggc ctccgccgcc 5880atccgcccca tcctgggcaa gtactaccag
tccgactccc gctgggtggg ccgcgccctg 5940tgggaggact ggcgcgactg
ccgctacgtg gtgcccgacg cccccgagga cgactccgcc 6000ctgtggttcc
acaagtagat cgatagatct cttaaggcag cagcagctcg gatagtatcg
6060acacactctg gacgctggtc gtgtgatgga ctgttgccgc cacacttgct
gccttgacct 6120gtgaatatcc ctgccgcttt tatcaaacag cctcagtgtg
tttgatcttg tgtgtacgcg 6180cttttgcgag ttgctagctg cttgtgctat
ttgcgaatac cacccccagc atccccttcc 6240ctcgtttcat atcgcttgca
tcccaaccgc aacttatcta cgctgtcctg ctatccctca 6300gcgctgctcc
tgctcctgct cactgcccct cgcacagcct tggtttgggc tccgcctgta
6360ttctcctggt actgcaacct gtaaaccagc actgcaatgc tgatgcacgg
gaagtagtgg 6420gatgggaaca caaatggaaa gcttaattaa gagctcttgt
tttccagaag gagttgctcc 6480ttgagccttt cattctcagc ctcgataacc
tccaaagccg ctctaattgt ggagggggtt 6540cgaagacagg gtggttggct
ggatggggaa acgctggtcg cgggattcga tcctgctgct 6600tatatcctcc
ctggaagcac acccacgact ctgaagaaga aaacgtgcac acacacaacc
6660caaccggccg aatatttgct tccttatccc gggtccaaga gagactgcga
tgcccccctc 6720aatcagcatc ctcctccctg ccgcttcaat cttccctgct
tgcctgcgcc cgcggtgcgc 6780cgtctgcccg cccagtcagt cactcctgca
caggcccctt gtgcgcagtg ctcctgtacc 6840ctttaccgct ccttccattc
tgcgaggccc cctattgaat gtattcgttg cctgtgtggc 6900caagcgggct
gctgggcgcg ccgccgtcgg gcagtgctcg gcgactttgg cggaagccga
6960ttgttcttct gtaagccacg cgcttgctgc tttgggaaga gaaggggggg
ggtactgaat 7020ggatgaggag gagaaggagg ggtattggta ttatctgagt
tgggtgaaga gc 70721218834DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 121gtttaaaccc
ctcaactgcg acgctgggaa ccttctccgg gcaggcgatg tgcgtgggtt 60tgcctccttg
gcacggctct acaccttcga gtacgccatg aggcggtgat ggctgtggct
120gtgccccact tcgtccaggg acggcaagtc catcatctgc atgcttggtg
cgacgctaca 180gcagtccctc tgcagcagag gagcacgact ttggccattt
cacgcactcg agtgtacaca 240attcattttt cttaaagtaa atgactgctg
attgaccaga tgctgtaacg ctgatttcgc 300tccagatcgc acagtcacag
attgcgacca tgttgctgcg tctgaaaatc tggattccga 360attcgaccct
ggcgctccat ccatgcaaca gatggcgaca cttgttacaa ttcctgtcgc
420ccatcggcat ggagcaggtc cacttagatc cccgatcacc cacgcgcatc
tcgctaatag 480tcattcattc gtgtcttcga tcaaagtcag gtgagtatgc
atggatcttg gttgacgatg 540cggtatgggt ttgcgccgct gactgcaggg
tctgtccaag gcaagccaac ccagctcctc 600tcctcgacaa tactctcgca
gacaaagcca gccacttgcc atccagattg ccaataaact 660caatcatggc
ttctgtcatg ccatccatgg gtctgatgaa tggtcacgct cgtgtcctga
720ccgttcccca gcctctggcg tcccctgccc cgcccaccag cccacgccgc
gcggcagtcg 780ctgccaaggc tgtctcggag gtaccctttc ttgcgctatg
acacttccag caaaaggtag 840ggcgggctgc gagacggctt cccggcgctg
catgcaacac cgatgatgct tcgacccccc 900gaagctcctt cggggctgca
tgggcgctcc gatgccgctc cagggcgagc gctgtttaaa 960tagccaggcc
cccgattgca aagacattat agcgagctac caaagccata ttcaaacacc
1020tagatcacta ccacttctac acaggccact cgagcttgtg atcgcactcc
gctaaggggg 1080cgcctcttcc tcttcgtttc agtcacaacc cgcaaactct
agaatatcaa tgatcgagca 1140ggacggcctc cacgccggct cccccgccgc
ctgggtggag cgcctgttcg gctacgactg 1200ggcccagcag accatcggct
gctccgacgc cgccgtgttc cgcctgtccg cccagggccg 1260ccccgtgctg
ttcgtgaaga ccgacctgtc cggcgccctg aacgagctgc aggacgaggc
1320cgcccgcctg tcctggctgg ccaccaccgg cgtgccctgc gccgccgtgc
tggacgtggt 1380gaccgaggcc ggccgcgact ggctgctgct gggcgaggtg
cccggccagg acctgctgtc 1440ctcccacctg gcccccgccg agaaggtgtc
catcatggcc gacgccatgc gccgcctgca 1500caccctggac cccgccacct
gccccttcga ccaccaggcc aagcaccgca tcgagcgcgc 1560ccgcacccgc
atggaggccg gcctggtgga ccaggacgac ctggacgagg agcaccaggg
1620cctggccccc gccgagctgt tcgcccgcct gaaggcccgc atgcccgacg
gcgaggacct 1680ggtggtgacc cacggcgacg cctgcctgcc caacatcatg
gtggagaacg gccgcttctc 1740cggcttcatc gactgcggcc gcctgggcgt
ggccgaccgc taccaggaca tcgccctggc 1800cacccgcgac atcgccgagg
agctgggcgg cgagtgggcc gaccgcttcc tggtgctgta 1860cggcatcgcc
gcccccgact cccagcgcat cgccttctac cgcctgctgg acgagttctt
1920ctgacaattg gcagcagcag ctcggatagt atcgacacac tctggacgct
ggtcgtgtga 1980tggactgttg ccgccacact tgctgccttg acctgtgaat
atccctgccg cttttatcaa 2040acagcctcag tgtgtttgat cttgtgtgta
cgcgcttttg cgagttgcta gctgcttgtg 2100ctatttgcga ataccacccc
cagcatcccc ttccctcgtt tcatatcgct tgcatcccaa 2160ccgcaactta
tctacgctgt cctgctatcc ctcagcgctg ctcctgctcc tgctcactgc
2220ccctcgcaca gccttggttt gggctccgcc tgtattctcc tggtactgca
acctgtaaac 2280cagcactgca atgctgatgc acgggaagta gtgggatggg
aacacaaatg gaaagctgta 2340tagggataag aattcggccg acaggacgcg
cgtcaaaggt gctggtcgtg tatgccctgg 2400ccggcaggtc gttgctgctg
ctggttagtg attccgcaac cctgattttg gcgtcttatt 2460ttggcgtggc
aaacgctggc gcccgcgagc cgggccggcg gcgatgcggt gccccacggc
2520tgccggaatc caagggaggc aagagcgccc gggtcagttg aagggcttta
cgcgcaaggt 2580acagccgctc ctgcaaggct gcgtggtgga attggacgtg
caggtcctgc tgaagttcct 2640ccaccgcctc accagcggac aaagcaccgg
tgtatcaggt ccgtgtcatc cactctaaag 2700aactcgacta cgacctactg
atggccctag attcttcatc aaaaacgcct gagacacttg 2760cccaggattg
aaactccctg aagggaccac caggggccct gagttgttcc ttccccccgt
2820ggcgagctgc cagccaggct gtacctgtga tcgaggctgg cgggaaaata
ggcttcgtgt 2880gctcaggtca tgggaggtgc aggacagctc atgaaacgcc
aacaatcgca caattcatgt 2940caagctaatc agctatttcc tcttcacgag
ctgtaattgt cccaaaattc tggtctaccg 3000ggggtgatcc ttcgtgtacg
ggcccttccc tcaaccctag gtatgcgcgc atgcggtcgc 3060cgcgcaactc
gcgcgagggc cgagggtttg ggacgggccg tcccgaaatg cagttgcacc
3120cggatgcgtg gcaccttttt tgcgataatt tatgcaatgg actgctctgc
aaaattctgg 3180ctctgtcgcc aaccctagga tcagcggcgt aggatttcgt
aatcattcgt cctgatgggg 3240agctaccgac taccctaata tcagcccgac
tgcctgacgc cagcgtccac ttttgtgcac 3300acattccatt cgtgcccaag
acatttcatt gtggtgcgaa gcgtccccag ttacgctcac 3360ctgtttcccg
acctccttac tgttctgtcg acagagcggg cccacaggcc ggtcgcagcc
3420actagtgcga ccgccagctg catggtggcg tcgcccttct gcacctggct
ggtcgccgcg 3480tgcatgccca cctccagcga caacgacccc cgctcgctgt
cccacaagcg cctgcgcctg 3540agccgccgcc gccgcaccct gagctcgcac
tgctccctgc gcggcagcac cttccagtgc 3600ctggacccct gcaaccagca
gcgcttcctg ggcgacaacg gcttcgcgtc gctgttcggc 3660tccaagcccc
tgcgcagcaa ccgcggccac ctgcgcctgg gccgcacctc gcactccggc
3720gaggtgatgg ccgtcgcgat gcagcccgcc caggaggtga gcaccaacaa
gaagcccgcg 3780accaagcagc gccgcgtggt cgtgaccggc atgggcgtcg
tgacccccct gggccacgac 3840cccgacgtgt attataacaa cctgctggac
ggcatctcgg gcatctccga gatcgagaac 3900ttcgactgca gccagttccc
cacccgcatc gccggcgaga tcaagtcgtt ctccaccgac 3960ggctgggtcg
cgcccaagtt cagcgagcgc atggacaagt tcatgctgta tatgctgacc
4020gccggcaaga aggcgctggc cgacggcggc atcaccgagg acgcgatgaa
ggagctgaac 4080aagcgcaagt gcggcgtgct gatcggctcg ggcctgggcg
gcatgaaggt cttctccgac 4140agcatcgagg ccctgcgcac ctcgtataag
aagatctccc ccttctgcgt gcccttcagc 4200accaccaaca tgggctcggc
gatcctggcg atggacctgg gctggatggg ccccaactat 4260tccatcagca
ccgcgtgcgc cacctcgaac ttctgcatcc tgaacgcggc caaccacatc
4320atcaagggcg aggcggacat gatgctgtgc ggcggctccg acgccgcggt
gctgcccgtc 4380ggcctgggcg gcttcgtggc ctgccgcgcg ctgagccagc
gcaacaacga ccccaccaag 4440gcctcgcgcc cctgggactc caaccgcgac
ggcttcgtca tgggcgaggg cgcgggcgtg 4500ctgctgctgg aggagctgga
gcacgccaag aagcgcggcg cgaccatcta tgccgagttc 4560ctgggcggca
gcttcacctg cgacgcgtat cacatgaccg agccccaccc cgagggcgcc
4620ggcgtcatcc tgtgcatcga gaaggcgctg gcccagtcgg gcgtgtcccg
cgaggacgtg 4680aactatatca acgcgcacgc caccagcacc cccgcgggcg
acatcaagga gtatcaggcc 4740ctggcgcact gcttcggcca gaactcggag
ctgcgcgtca actccaccaa gagcatgatc 4800ggccacctgc tgggcggcgc
cggcggcgtg gaggcggtcg ccgtggtcca ggcgatccgc 4860accggctgga
tccaccccaa catcaacctg gaggaccccg acgagggcgt ggacgccaag
4920ctgctggtcg gccccaagaa ggagaagctg aaggtgaagg tcggcctgtc
gaactccttc 4980ggcttcggcg gccacaacag ctcgatcctg ttcgcgccct
gcaactgact cgaggcagca 5040gcagctcgga tagtatcgac acactctgga
cgctggtcgt gtgatggact gttgccgcca 5100cacttgctgc cttgacctgt
gaatatccct gccgctttta tcaaacagcc tcagtgtgtt 5160tgatcttgtg
tgtacgcgct tttgcgagtt gctagctgct tgtgctattt gcgaatacca
5220cccccagcat ccccttccct cgtttcatat cgcttgcatc ccaaccgcaa
cttatctacg 5280ctgtcctgct atccctcagc gctgctcctg ctcctgctca
ctgcccctcg cacagccttg 5340gtttgggctc cgcctgtatt ctcctggtac
tgcaacctgt aaaccagcac tgcaatgctg 5400atgcacggga agtagtggga
tgggaacaca aatggaaagc ttcacatacg taggccgaca 5460ggacgcgcgt
caaaggtgct ggtcgtgtat gccctggccg gcaggtcgtt gctgctgctg
5520gttagtgatt ccgcaaccct gattttggcg tcttattttg gcgtggcaaa
cgctggcgcc 5580cgcgagccgg gccggcggcg atgcggtgcc ccacggctgc
cggaatccaa gggaggcaag 5640agcgcccggg tcagttgaag ggctttacgc
gcaaggtaca gccgctcctg caaggctgcg 5700tggtggaatt ggacgtgcag
gtcctgctga agttcctcca ccgcctcacc agcggacaaa 5760gcaccggtgt
atcaggtccg tgtcatccac tctaaagaac tcgactacga cctactgatg
5820gccctagatt cttcatcaaa aacgcctgag acacttgccc aggattgaaa
ctccctgaag 5880ggaccaccag gggccctgag ttgttccttc cccccgtggc
gagctgccag ccaggctgta 5940cctgtgatcg aggctggcgg gaaaataggc
ttcgtgtgct caggtcatgg gaggtgcagg 6000acagctcatg aaacgccaac
aatcgcacaa ttcatgtcaa gctaatcagc tatttcctct 6060tcacgagctg
taattgtccc aaaattctgg tctaccgggg gtgatccttc gtgtacgggc
6120ccttccctca accctaggta tgcgcgcatg cggtcgccgc gcaactcgcg
cgagggccga 6180gggtttggga cgggccgtcc cgaaatgcag ttgcacccgg
atgcgtggca ccttttttgc 6240gataatttat gcaatggact gctctgcaaa
attctggctc tgtcgccaac cctaggatca 6300gcggcgtagg atttcgtaat
cattcgtcct gatggggagc taccgactac cctaatatca 6360gcccgactgc
ctgacgccag cgtccacttt tgtgcacaca ttccattcgt gcccaagaca
6420tttcattgtg gtgcgaagcg tccccagtta cgctcacctg tttcccgacc
tccttactgt 6480tctgtcgaca gagcgggccc acaggccggt cgcagccact
agtatggcta tcaagacgaa 6540caggcagcct gtggagaagc ctccgttcac
gatcgggacg ctgcgcaagg ccatccccgc 6600gcactgtttc gagcgctcgg
cgcttcgtgg gcgcgcccag ctgcccgact ggagccgcct 6660gctgaccgcc
atcaccaccg tgttcgtgaa gtccaagcgc cccgacatgc acgaccgcaa
6720gtccaagcgc cccgacatgc tggtggacag cttcggcctg gagtccaccg
tgcaggacgg 6780cctggtgttc cgccagtcct tctccatccg ctcctacgag
atcggcaccg accgcaccgc 6840cagcatcgag accctgatga accacctgca
ggagacctcc ctgaaccact gcaagagcac 6900cggcatcctg ctggacggct
tcggccgcac cctggagatg tgcaagcgcg acctgatctg 6960ggtggtgatt
aagatgcaga tcaaggtgaa ccgctacccc gcctggggcg acaccgtgga
7020gatcaacacc cgcttcagcc gcctgggcaa gatcggcatg ggccgcgact
ggctgatctc 7080cgactgcaac accggcgaga tcctggtgcg cgccaccagc
gcctacgcca tgatgaacca 7140gaagacccgc cgcctgtcca agctgcccta
cgaggtgcac caggagatcg tgcccctgtt 7200cgtggacagc cccgtgatcg
aggactccga cctgaaggtg cacaagttca aggtgaagac 7260cggcgacagc
atccagaagg gcctgacccc cggctggaac gacctggacg tgaaccagca
7320cgtgtccaac gtgaagtaca tcggctggat cctggagagc atgcccaccg
aggtgctgga 7380gacccaggag ctgtgctccc tggccctgga gtaccgccgc
gagtgcggcc gcgactccgt 7440gctggagagc gtgaccgcca tggaccccag
caaggtgggc gtgcgctccc agtaccagca 7500cctgctgcgc ctggaggacg
gcaccgccat cgtgaacggc gccaccgagt ggcgccccaa 7560gaacgccggc
gccaacggcg ccatctccac cggcaagacc agcaacggca actccgtgtc
7620catggactac aaggaccacg acggcgacta caaggaccac gacatcgact
acaaggacga 7680cgacgacaag tgactcgagg cagcagcagc tcggatagta
tcgacacact ctggacgctg 7740gtcgtgtgat ggactgttgc cgccacactt
gctgccttga cctgtgaata tccctgccgc 7800ttttatcaaa cagcctcagt
gtgtttgatc ttgtgtgtac gcgcttttgc gagttgctag 7860ctgcttgtgc
tatttgcgaa taccaccccc agcatcccct tccctcgttt catatcgctt
7920gcatcccaac cgcaacttat ctacgctgtc ctgctatccc tcagcgctgc
tcctgctcct 7980gctcactgcc cctcgcacag ccttggtttg ggctccgcct
gtattctcct ggtactgcaa 8040cctgtaaacc agcactgcaa tgctgatgca
cgggaagtag tgggatggga acacaaatgg 8100aaagctgtag aattctccag
agctccagcg ccatgccacg ccttttgatg gcttcaagta 8160cgataacggt
gttggattgt gcgtttgttg cgtagtgtgc atggcttaga ataatgcagt
8220tggatttctt gctcacggca atgtcggctt gtccgcaggt tcaaccccat
ttcggagtct 8280caggtcagcc gcgcaatgac cagccgctac ttcaaggact
tgcacgacaa cgccgaggtg 8340agctatgttt aggccttgag tgaaaattgt
cgtcgaagca tattcgcgct ccgcgatagc 8400atccaagcaa aatgtcaagt
gcgttccgat ttgcgtccgc aggtcgatgt tgtgatcgtc 8460ggtgccggat
ccgccggtct gtcctgcgct tacgagctga ccaagcaccc cgacgtccgg
8520gtacgcgagc tgagattcga ttggacataa actgaaaatg aaatcttttg
gagaaatgta 8580agggtctcaa gcggtgctcg attgcaagaa attggtcgtc
ccccactccg caggtcgcca 8640tcatcgagca gggcgttgca cctggtggcg
gcgcctggct ggggggacag ctgttctcgg 8700ccatgtgtgt acgtagaagg
gtggatttcg gatggtttcg ttgcacagct gtttgtcaat 8760gatttgtctt
agactattgc cgatgtttct aaatgtttta ggagctatga tatgtctgca
8820ggcgactgaa gagc 88341228329DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 122gctcttcgct
caccgcgtga attgctgtcc caaacgtaag catcatcgtg gctcggtcac 60gcgatcctgg
atccggggat cctagaccgc tggtggagag cgctgccgtc ggattggtgg
120caagtaagat tgcgcaggtt ggcgaaggga gagaccaaaa ccggaggctg
gaagcgggca 180caacatcgta ttattgcgta tagtagagca gtggcagtcg
catttcgagg tccgcaacgg 240atctcgcaag ctcgctacgc tcacagtagg
agaaagggga ccactgcccc tgccagaatg 300gtcgcgaccc tctccctcgc
cggccccgcc tgcaacacgc agtgcgtatc cggcaagcgg 360gctgtcgcct
tcaaccgccc ccatgttggc gtccgggctc gatcaggtgc gctgaggggg
420gtttggtgtg cccgcgcctc tgggcccgtg tcggccgtgc ggacgtgggg
ccctgggcag 480tggatcagca gggtttgcgt gcaaatgcct ataccggcga
ttgaatagcg atgaacggga 540tacggttgcg ctcactccat gcccatgcga
ccccgtttct gtccgccagc cgtggtcgcc 600cgggctgcga agcgggaccc
cacccagcgc attgtgatca ccggaatggg cgtggggtac 660cctttcttgc
gctatgacac ttccagcaaa aggtagggcg ggctgcgaga cggcttcccg
720gcgctgcatg caacaccgat gatgcttcga ccccccgaag ctccttcggg
gctgcatggg 780cgctccgatg ccgctccagg gcgagcgctg tttaaatagc
caggcccccg attgcaaaga 840cattatagcg agctaccaaa gccatattca
aacacctaga tcactaccac ttctacacag 900gccactcgag cttgtgatcg
cactccgcta agggggcgcc tcttcctctt cgtttcagtc 960acaacccgca
aactctagaa tatcaatgat cgagcaggac ggcctccacg ccggctcccc
1020cgccgcctgg gtggagcgcc tgttcggcta cgactgggcc cagcagacca
tcggctgctc 1080cgacgccgcc gtgttccgcc tgtccgccca gggccgcccc
gtgctgttcg tgaagaccga 1140cctgtccggc gccctgaacg agctgcagga
cgaggccgcc cgcctgtcct ggctggccac 1200caccggcgtg ccctgcgccg
ccgtgctgga cgtggtgacc gaggccggcc gcgactggct 1260gctgctgggc
gaggtgcccg gccaggacct gctgtcctcc cacctggccc ccgccgagaa
1320ggtgtccatc atggccgacg ccatgcgccg cctgcacacc ctggaccccg
ccacctgccc 1380cttcgaccac caggccaagc accgcatcga gcgcgcccgc
acccgcatgg aggccggcct 1440ggtggaccag gacgacctgg acgaggagca
ccagggcctg gcccccgccg agctgttcgc 1500ccgcctgaag gcccgcatgc
ccgacggcga ggacctggtg gtgacccacg gcgacgcctg 1560cctgcccaac
atcatggtgg agaacggccg cttctccggc ttcatcgact gcggccgcct
1620gggcgtggcc gaccgctacc aggacatcgc cctggccacc cgcgacatcg
ccgaggagct 1680gggcggcgag tgggccgacc gcttcctggt gctgtacggc
atcgccgccc ccgactccca 1740gcgcatcgcc ttctaccgcc tgctggacga
gttcttctga caattggcag cagcagctcg 1800gatagtatcg acacactctg
gacgctggtc gtgtgatgga ctgttgccgc cacacttgct 1860gccttgacct
gtgaatatcc ctgccgcttt tatcaaacag cctcagtgtg tttgatcttg
1920tgtgtacgcg cttttgcgag ttgctagctg cttgtgctat ttgcgaatac
cacccccagc 1980atccccttcc ctcgtttcat atcgcttgca tcccaaccgc
aacttatcta cgctgtcctg 2040ctatccctca gcgctgctcc tgctcctgct
cactgcccct cgcacagcct tggtttgggc 2100tccgcctgta ttctcctggt
actgcaacct gtaaaccagc actgcaatgc tgatgcacgg 2160gaagtagtgg
gatgggaaca caaatggaaa gctgtatagg gataaaagct tatagcgact
2220gctacccccc gaccatgtgc cgaggcagaa attatataca agaagcagat
cgcaattagg 2280cacatcgctt tgcattatcc acacactatt catcgctgct
gcggcaaggc tgcagagtgt 2340atttttgtgg cccaggagct gagtccgaag
tcgacgcgac gagcggcgca ggatccgacc 2400cctagacgag cactgtcatt
ttccaagcac gcagctaaat gcgctgagac cgggtctaaa 2460tcatccgaaa
agtgtcaaaa tggccgattg ggttcgccta ggacaatgcg ctgcggattc
2520gctcgagtcc gctgccggcc aaaaggcggt ggtacaggaa ggcgcacggg
gccaaccctg 2580cgaagccggg ggcccgaacg ccgaccgccg gccttcgatc
tcgggtgtcc ccctcgtcaa 2640tttcctctct cgggtgcagc cacgaaagtc
gtgacgcagg tcacgaaatc cggttacgaa 2700aaacgcaggt cttcgcaaaa
acgtgagggt ttcgcgtctc gccctagcta ttcgtatcgc 2760cgggtcagac
ccacgtgcag aaaagccctt gaataacccg ggaccgtggt taccgcgccg
2820cctgcaccag ggggcttata taagcccaca ccacacctgt ctcaccacgc
atttctccaa 2880ctcgcgactt ttcggaagaa attgttatcc acctagtata
gactgccacc tgcaggacct 2940tgtgtcttgc agtttgtatt ggtcccggcc
gtcgagcacg acagatctgg gctagggttg 3000gcctggccgc tcggcactcc
cctttagccg cgcgcatccg cgttccagag gtgcgattcg 3060gtgtgtggag
cattgtcatg cgcttgtggg ggtcgttccg tgcgcggcgg gtccgccatg
3120ggcgccgacc tgggccctag ggtttgtttt cgggccaagc gagcccctct
cacctcgtcg 3180cccccccgca ttccctctct cttgcagcca ctagtatggc
tatcaagacg aacaggcagc 3240ctgtggagaa gcctccgttc acgatcggga
cgctgcgcaa ggccatcccc gcgcactgtt 3300tcgagcgctc ggcgcttcgt
gggcgcgccc agctgcccga ctggagccgc ctgctgaccg 3360ccatcaccac
cgtgttcgtg aagtccaagc gccccgacat gcacgaccgc aagtccaagc
3420gccccgacat gctggtggac agcttcggcc tggagtccac cgtgcaggac
ggcctggtgt 3480tccgccagtc cttctccatc cgctcctacg agatcggcac
cgaccgcacc gccagcatcg 3540agaccctgat gaaccacctg caggagacct
ccctgaacca ctgcaagagc accggcatcc 3600tgctggacgg cttcggccgc
accctggaga tgtgcaagcg cgacctgatc tgggtggtga 3660ttaagatgca
gatcaaggtg aaccgctacc ccgcctgggg cgacaccgtg gagatcaaca
3720cccgcttcag ccgcctgggc aagatcggca tgggccgcga ctggctgatc
tccgactgca 3780acaccggcga gatcctggtg cgcgccacca gcgcctacgc
catgatgaac cagaagaccc 3840gccgcctgtc caagctgccc tacgaggtgc
accaggagat cgtgcccctg ttcgtggaca 3900gccccgtgat cgaggactcc
gacctgaagg
tgcacaagtt caaggtgaag accggcgaca 3960gcatccagaa gggcctgacc
cccggctgga acgacctgga cgtgaaccag cacgtgtcca 4020acgtgaagta
catcggctgg atcctggaga gcatgcccac cgaggtgctg gagacccagg
4080agctgtgctc cctggccctg gagtaccgcc gcgagtgcgg ccgcgactcc
gtgctggaga 4140gcgtgaccgc catggacccc agcaaggtgg gcgtgcgctc
ccagtaccag cacctgctgc 4200gcctggagga cggcaccgcc atcgtgaacg
gcgccaccga gtggcgcccc aagaacgccg 4260gcgccaacgg cgccatctcc
accggcaaga ccagcaacgg caactccgtg tccatggact 4320acaaggacca
cgacggcgac tacaaggacc acgacatcga ctacaaggac gacgacgaca
4380agtgactcga gagcgtccag cgtgtgggat gaagggtgcg atggaacggg
gctgccgccc 4440cccctctggg catctagctc tgcaccgcac gccaggaagc
ccaagccagg ccccgtcaca 4500ctccctcgct gaagtgttcc ccccctgccc
cacactcatc caggtatcaa cgccatcatg 4560ttctacgtcc ccgtcatctt
caactccctg gggagcgggc gccgcgcgtc gctgctgaac 4620accatcatca
tcaacgccgt caactttgtt aattaagaat tcggccgaca ggacgcgcgt
4680caaaggtgct ggtcgtgtat gccctggccg gcaggtcgtt gctgctgctg
gttagtgatt 4740ccgcaaccct gattttggcg tcttattttg gcgtggcaaa
cgctggcgcc cgcgagccgg 4800gccggcggcg atgcggtgcc ccacggctgc
cggaatccaa gggaggcaag agcgcccggg 4860tcagttgaag ggctttacgc
gcaaggtaca gccgctcctg caaggctgcg tggtggaatt 4920ggacgtgcag
gtcctgctga agttcctcca ccgcctcacc agcggacaaa gcaccggtgt
4980atcaggtccg tgtcatccac tctaaagaac tcgactacga cctactgatg
gccctagatt 5040cttcatcaaa aacgcctgag acacttgccc aggattgaaa
ctccctgaag ggaccaccag 5100gggccctgag ttgttccttc cccccgtggc
gagctgccag ccaggctgta cctgtgatcg 5160aggctggcgg gaaaataggc
ttcgtgtgct caggtcatgg gaggtgcagg acagctcatg 5220aaacgccaac
aatcgcacaa ttcatgtcaa gctaatcagc tatttcctct tcacgagctg
5280taattgtccc aaaattctgg tctaccgggg gtgatccttc gtgtacgggc
ccttccctca 5340accctaggta tgcgcgcatg cggtcgccgc gcaactcgcg
cgagggccga gggtttggga 5400cgggccgtcc cgaaatgcag ttgcacccgg
atgcgtggca ccttttttgc gataatttat 5460gcaatggact gctctgcaaa
attctggctc tgtcgccaac cctaggatca gcggcgtagg 5520atttcgtaat
cattcgtcct gatggggagc taccgactac cctaatatca gcccgactgc
5580ctgacgccag cgtccacttt tgtgcacaca ttccattcgt gcccaagaca
tttcattgtg 5640gtgcgaagcg tccccagtta cgctcacctg tttcccgacc
tccttactgt tctgtcgaca 5700gagcgggccc acaggccggt cgcagcccat
atggcttccg cggcattcac catgtcggcg 5760tgccccgcga tgactggcag
ggcccctggg gcacgtcgct ccggacggcc agtcgccacc 5820cgcctgaggt
acgtattcca gtgcctggtg gccagctgca tcgacccctg cgaccagtac
5880cgcagcagcg ccagcctgag cttcctgggc gacaacggct tcgccagcct
gttcggcagc 5940aagcccttca tgagcaaccg cggccaccgc cgcctgcgcc
gcgccagcca cagcggcgag 6000gccatggccg tggccctgca gcccgcccag
gaggccggca ccaagaagaa gcccgtgatc 6060aagcagcgcc gcgtggtggt
gaccggcatg ggcgtggtga cccccctggg ccacgagccc 6120gacgtgttct
acaacaacct gctggacggc gtgagcggca tcagcgagat cgagaccttc
6180gactgcaccc agttccccac ccgcatcgcc ggcgagatca agagcttcag
caccgacggc 6240tgggtggccc ccaagctgag caagcgcatg gacaagttca
tgctgtacct gctgaccgcc 6300ggcaagaagg ccctggccga cggcggcatc
accgacgagg tgatgaagga gctggacaag 6360cgcaagtgcg gcgtgctgat
cggcagcggc atgggcggca tgaaggtgtt caacgacgcc 6420atcgaggccc
tgcgcgtgag ctacaagaag atgaacccct tctgcgtgcc cttcgccacc
6480accaacatgg gcagcgccat gctggccatg gacctgggct ggatgggccc
caactacagc 6540atcagcaccg cctgcgccac cagcaacttc tgcatcctga
acgccgccaa ccacatcatc 6600cgcggcgagg ccgacatgat gctgtgcggc
ggcagcgacg ccgtgatcat ccccatcggc 6660ctgggcggct tcgtggcctg
ccgcgccctg agccagcgca acagcgaccc caccaaggcc 6720agccgcccct
gggacagcaa ccgcgacggc ttcgtgatgg gcgagggcgc cggcgtgctg
6780ctgctggagg agctggagca cgccaagaag cgcggcgcca ccatctacgc
cgagttcctg 6840ggcggcagct tcacctgcga cgcctaccac atgaccgagc
cccaccccga gggcgccggc 6900gtgatcctgt gcatcgagaa ggccctggcc
caggccggcg tgagcaagga ggacgtgaac 6960tacatcaacg cccacgccac
cagcaccagc gccggcgaca tcaaggagta ccaggccctg 7020gcccgctgct
tcggccagaa cagcgagctg cgcgtgaaca gcaccaagag catgatcggc
7080cacctgctgg gcgccgccgg cggcgtggag gccgtgaccg tggtgcaggc
catccgcacc 7140ggctggattc accccaacct gaacctggag gaccccgaca
aggccgtgga cgccaagctg 7200ctggtgggcc ccaagaagga gcgcctgaac
gtgaaggtgg gcctgagcaa cagcttcggc 7260ttcggcggcc acaacagcag
catcctgttc gccccctgca acgtgtgact cgaggcagca 7320gcagctcgga
tagtatcgac acactctgga cgctggtcgt gtgatggact gttgccgcca
7380cacttgctgc cttgacctgt gaatatccct gccgctttta tcaaacagcc
tcagtgtgtt 7440tgatcttgtg tgtacgcgct tttgcgagtt gctagctgct
tgtgctattt gcgaatacca 7500cccccagcat ccccttccct cgtttcatat
cgcttgcatc ccaaccgcaa cttatctacg 7560ctgtcctgct atccctcagc
gctgctcctg ctcctgctca ctgcccctcg cacagccttg 7620gtttgggctc
cgcctgtatt ctcctggtac tgcaacctgt aaaccagcac tgcaatgctg
7680atgcacggga agtagtggga tgggaacaca aatggaaagc ttgagctcca
cctgcatccg 7740cctggcgctc gaggacgccg gcgtctcgcc cgacgaggtc
aactacgtca acgcgcacgc 7800cacctccacc ctggtgggcg acaaggccga
ggtgcgcgcg gtcaagtcgg tctttggcga 7860catgaagggc atcaagatga
acgccaccaa gtccatgatc gggcactgcc tgggcgccgc 7920cggcggcatg
gaggccgtcg ccacgctcat ggccatccgc accggctggg tgcaccccac
7980catcaaccac gacaacccca tcgccgaggt cgacggcctg gacgtcgtcg
ccaacgccaa 8040ggcccagcac aaaatcaacg tcgccatctc caactccttc
ggcttcggcg ggcacaactc 8100cgtcgtcgcc tttgcgccct tccgcgagta
ggcggagcga gcgcgcttgg ctgaggaggg 8160aggcggggtg cgagcccttt
ggctgcgcgc gatactctcc ccgcacgagc agactccacg 8220cgcctgaatc
tacttgtcaa cgagcaaccg tgtgttttgt ccgtggccat tcttattatt
8280tctccgactg tggccgtact ctgtttggct gtgcaagcac cgaagagcc
83291236750DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 123gaagagcgcc caatgtttaa acagcccgca
ccctcgttga tctgggagcc ctgcgcagcc 60ccttaaatca tctcagtcag gtttctgtgt
tcaactgagc ctaaagggct ttcgtcatgc 120gcacgagcac acgtatatcg
gccacgcagt ttctcaaaag cggtagaaca gttcgcgagc 180cctcgtaggt
cgaaaacttg cgccagtact attaaattaa attaattgat cgaacgagac
240gcgaaacttt tgcagaatgc caccgagttt gcccagagaa tgggagtggc
gccattcacc 300atccgcctgt gcccggcttg attcgccgag acgatggacg
gcgagaccag ggagcggctt 360gcgagccccg agccggtagc aggaacaatg
atcgacaatc ttcctgtcca attactggca 420accattagaa agagccggag
cgcgttgaaa gtctgcaatc gagtaatttt tcgatacgtc 480gggcctgctg
aaccctaagg ctccggactt tgtttaaggc gatccaagat gcacgcggcc
540ccaggcacgt atctcaagca caaaccccag ccttagtttc gagactttgg
gagatagcga 600ccgatatcta gtttggcatt ttgtatatta attacctcaa
gcaatggagc gctctgatgc 660ggtgcagcgt cggctgcagc acctggcagt
ggcgctaggg tcgccctatc gctcggaacc 720tggtcagctg gctcccgcct
cctgctcagc ctcttccggt accgcggtga gaatcgaaaa 780tgcatcgttt
ctaggttcgg agacggtcaa ttccctgctc cggcgaatct gtcggtcaag
840ctggccagtg gacaatgttg ctatggcagc ccgcgcacat gggcctcccg
acgcggccat 900caggagccca aacagcgtgt cagggtatgt gaaactcaag
aggtccctgc tgggcactcc 960ggccccactc cgggggcggg acgccaggca
ttcgcggtcg gtcccgcgcg acgagcgaaa 1020tgatgattcg gttacgagac
caggacgtcg tcgaggtcga gaggcagcct cggacacgtc 1080tcgctagggc
aacgccccga gtccccgcga gggccgtaaa cattgtttct gggtgtcgga
1140gtgggcattt tgggcccgat ccaatcgcct catgccgctc tcgtctggtc
ctcacgttcg 1200cgtacggcct ggatcccgga aagggcggat gcacgtggtg
ttgccccgcc attggcgccc 1260acgtttcaaa gtccccggcc agaaatgcac
aggaccggcc cggctcgcac aggccatgct 1320gaacgcccag atttcgacag
caacaccatc tagaataatc gcaaccatcc gcgttttgaa 1380cgaaacgaaa
cggcgctgtt tagcatgttt ccgacatcgt gggggccgaa gcatgctccg
1440gggggaggaa agcgtggcac agcggtagcc cattctgtgc cacacgccga
cgaggaccaa 1500tccccggcat cagccttcat cgacggctgc gccgcacata
taaagccgga cgcctaaccg 1560gtttcgtggt tatgactagt atgttcgcgt
tctacttcct gacggcctgc atctccctga 1620agggcgtgtt cggcgtctcc
ccctcctaca acggcctggg cctgacgccc cagatgggct 1680gggacaactg
gaacacgttc gcctgcgacg tctccgagca gctgctgctg gacacggccg
1740accgcatctc cgacctgggc ctgaaggaca tgggctacaa gtacatcatc
ctggacgact 1800gctggtcctc cggccgcgac tccgacggct tcctggtcgc
cgacgagcag aagttcccca 1860acggcatggg ccacgtcgcc gaccacctgc
acaacaactc cttcctgttc ggcatgtact 1920cctccgcggg cgagtacacg
tgcgccggct accccggctc cctgggccgc gaggaggagg 1980acgcccagtt
cttcgcgaac aaccgcgtgg actacctgaa gtacgacaac tgctacaaca
2040agggccagtt cggcacgccc gagatctcct accaccgcta caaggccatg
tccgacgccc 2100tgaacaagac gggccgcccc atcttctact ccctgtgcaa
ctggggccag gacctgacct 2160tctactgggg ctccggcatc gcgaactcct
ggcgcatgtc cggcgacgtc acggcggagt 2220tcacgcgccc cgactcccgc
tgcccctgcg acggcgacga gtacgactgc aagtacgccg 2280gcttccactg
ctccatcatg aacatcctga acaaggccgc ccccatgggc cagaacgcgg
2340gcgtcggcgg ctggaacgac ctggacaacc tggaggtcgg cgtcggcaac
ctgacggacg 2400acgaggagaa ggcgcacttc tccatgtggg ccatggtgaa
gtcccccctg atcatcggcg 2460cgaacgtgaa caacctgaag gcctcctcct
actccatcta ctcccaggcg tccgtcatcg 2520ccatcaacca ggactccaac
ggcatccccg ccacgcgcgt ctggcgctac tacgtgtccg 2580acacggacga
gtacggccag ggcgagatcc agatgtggtc cggccccctg gacaacggcg
2640accaggtcgt ggcgctgctg aacggcggct ccgtgtcccg ccccatgaac
acgaccctgg 2700aggagatctt cttcgactcc aacctgggct ccaagaagct
gacctccacc tgggacatct 2760acgacctgtg ggcgaaccgc gtcgacaact
ccacggcgtc cgccatcctg ggccgcaaca 2820agaccgccac cggcatcctg
tacaacgcca ccgagcagtc ctacaaggac ggcctgtcca 2880agaacgacac
ccgcctgttc ggccagaaga tcggctccct gtcccccaac gcgatcctga
2940acacgaccgt ccccgcccac ggcatcgcgt tctaccgcct gcgcccctcc
tcctgatacg 3000tactcgaggc agcagcagct cggatagtat cgacacactc
tggacgctgg tcgtgtgatg 3060gactgttgcc gccacacttg ctgccttgac
ctgtgaatat ccctgccgct tttatcaaac 3120agcctcagtg tgtttgatct
tgtgtgtacg cgcttttgcg agttgctagc tgcttgtgct 3180atttgcgaat
accaccccca gcatcccctt ccctcgtttc atatcgcttg catcccaacc
3240gcaacttatc tacgctgtcc tgctatccct cagcgctgct cctgctcctg
ctcactgccc 3300ctcgcacagc cttggtttgg gctccgcctg tattctcctg
gtactgcaac ctgtaaacca 3360gcactgcaat gctgatgcac gggaagtagt
gggatgggaa cacaaatgga aagctgtaga 3420attcatagcg actgctaccc
cccgaccatg tgccgaggca gaaattatat acaagaagca 3480gatcgcaatt
aggcacatcg ctttgcatta tccacacact attcatcgct gctgcggcaa
3540ggctgcagag tgtatttttg tggcccagga gctgagtccg aagtcgacgc
gacgagcggc 3600gcaggatccg acccctagac gagcactgtc attttccaag
cacgcagcta aatgcgctga 3660gaccgggtct aaatcatccg aaaagtgtca
aaatggccga ttgggttcgc ctaggacaat 3720gcgctgcgga ttcgctcgag
tccgctgccg gccaaaaggc ggtggtacag gaaggcgcac 3780ggggccaacc
ctgcgaagcc gggggcccga acgccgaccg ccggccttcg atctcgggtg
3840tccccctcgt caatttcctc tctcgggtgc agccacgaaa gtcgtgacgc
aggtcacgaa 3900atccggttac gaaaaacgca ggtcttcgca aaaacgtgag
ggtttcgcgt ctcgccctag 3960ctattcgtat cgccgggtca gacccacgtg
cagaaaagcc cttgaataac ccgggaccgt 4020ggttaccgcg ccgcctgcac
cagggggctt atataagccc acaccacacc tgtctcacca 4080cgcatttctc
caactcgcga cttttcggaa gaaattgtta tccacctagt atagactgcc
4140acctgcagga ccttgtgtct tgcagtttgt attggtcccg gccgtcgagc
acgacagatc 4200tgggctaggg ttggcctggc cgctcggcac tcccctttag
ccgcgcgcat ccgcgttcca 4260gaggtgcgat tcggtgtgtg gagcattgtc
atgcgcttgt gggggtcgtt ccgtgcgcgg 4320cgggtccgcc atgggcgccg
acctgggccc tagggtttgt tttcgggcca agcgagcccc 4380tctcacctcg
tcgccccccc gcattccctc tctcttgcag ccactagtaa caatggccac
4440cgcatccact ttctcggcgt tcaatgcccg ctgcggcgac ctgcgtcgct
cggcgggctc 4500cgggccccgg cgcccagcga ggcccctccc cgtgcgcgct
gccatcgcca gcgaggtccc 4560cgtggccacc acctcccccc gggcgcaccc
caaggcgaac ggcagcgcgg tgtcgctgaa 4620gtcgggctcc ctggagaccc
aggaggacaa gacgagcagc tcgtcccccc ccccccgcac 4680gttcatcaac
cagctgcccg tgtggagcat gctgctgtcg gcggtgacca cggtcttcgg
4740cgtggccgag aagcagtggc ccatgctgga ccgcaagtcc aagcgccccg
acatgctggt 4800cgagcccctg ggcgtggacc gcatcgtcta cgacggcgtg
agcttccgcc agtcgttctc 4860catccgcagc tacgagatcg gcgccgaccg
caccgcctcg atcgagacgc tgatgaacat 4920gttccaggag acctccctga
accactgcaa gatcatcggc ctgctgaacg acggcttcgg 4980ccgcacgccc
gagatgtgca agcgcgacct gatctgggtc gtgaccaaga tgcagatcga
5040ggtgaaccgc taccccacgt ggggcgacac catcgaggtc aacacgtggg
tgagcgcctc 5100gggcaagcac ggcatgggcc gcgactggct gatctccgac
tgccacaccg gcgagatcct 5160gatccgcgcg acgagcgtct gggcgatgat
gaaccagaag acccgccgcc tgtcgaagat 5220cccctacgag gtgcgccagg
agatcgagcc ccagttcgtc gactccgccc ccgtgatcgt 5280ggacgaccgc
aagttccaca agctggacct gaagacgggc gacagcatct gcaacggcct
5340gaccccccgc tggacggacc tggacgtgaa ccagcacgtc aacaacgtga
agtacatcgg 5400ctggatcctg cagtcggtcc ccaccgaggt gttcgagacg
caggagctgt gcggcctgac 5460cctggagtac cgccgcgagt gcggccgcga
ctccgtgctg gagagcgtca cggccatgga 5520cccctcgaag gagggcgacc
gctccctgta ccagcacctg ctgcgcctgg aggacggcgc 5580ggacatcgtg
aagggccgca ccgagtggcg ccccaagaac gccggcgcca agggcgccat
5640cctgacgggc aagaccagca acggcaactc gatctccatg gactacaagg
accacgacgg 5700cgactacaag gaccacgaca tcgactacaa ggacgacgac
gacaagtgaa agcttgcagc 5760agcagctcgg atagtatcga cacactctgg
acgctggtcg tgtgatggac tgttgccgcc 5820acacttgctg ccttgacctg
tgaatatccc tgccgctttt atcaaacagc ctcagtgtgt 5880ttgatcttgt
gtgtacgcgc ttttgcgagt tgctagctgc ttgtgctatt tgcgaatacc
5940acccccagca tccccttccc tcgtttcata tcgcttgcat cccaaccgca
acttatctac 6000gctgtcctgc tatccctcag cgctgctcct gctcctgctc
actgcccctc gcacagcctt 6060ggtttgggct cccgcctgta ttctcctggt
actgcaacct gtaaaccagc actgcaatgc 6120tgatgcacgg gaagtagtgg
gatgggaaca caaatggaaa gctggagctc agcgtctgcg 6180tgttgggagc
tggagtcgtg ggcttgacga cggcgctgca gctgttgcag gatgtgcctg
6240gcgtgcgcgt tcacgtcgtg gctgagaaat atggcgacga aacgttgacg
gctggggccg 6300gcgggctgtg gatgccatac gcattgggta cgcggccatt
ggatgggatt gataggctta 6360tggagggata atagagtttt tgccggatcc
aacgcatgtg gatgcggtat cccggtgggc 6420tgaaagtgtg gaaggatagt
gcattggcta ttcacatgca ctgcccaccc cttttggcag 6480gaaatgtgcc
ggcatcgttg gtgcaccgat ggggaaaatc gacgttcgac cactacatga
6540agatttatac gtctgaagat gcagcgactg cgggtgcgaa acggatgacg
gtttggtcgt 6600gtatgtcaca gcatgtgctg gatcttgcgg gctaactccc
cctgccacgg cccattgcag 6660gtgtcatgtt gactggaggg tacgaccttt
cgtccgtcaa attcccagag gaggacccgc 6720tctgggccga cattgtgccc
actgaagagc 67501246838DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 124gaagagcgcc
caatgtttaa acagcccgca ccctcgttga tctgggagcc ctgcgcagcc 60ccttaaatca
tctcagtcag gtttctgtgt tcaactgagc ctaaagggct ttcgtcatgc
120gcacgagcac acgtatatcg gccacgcagt ttctcaaaag cggtagaaca
gttcgcgagc 180cctcgtaggt cgaaaacttg cgccagtact attaaattaa
attaattgat cgaacgagac 240gcgaaacttt tgcagaatgc caccgagttt
gcccagagaa tgggagtggc gccattcacc 300atccgcctgt gcccggcttg
attcgccgag acgatggacg gcgagaccag ggagcggctt 360gcgagccccg
agccggtagc aggaacaatg atcgacaatc ttcctgtcca attactggca
420accattagaa agagccggag cgcgttgaaa gtctgcaatc gagtaatttt
tcgatacgtc 480gggcctgctg aaccctaagg ctccggactt tgtttaaggc
gatccaagat gcacgcggcc 540ccaggcacgt atctcaagca caaaccccag
ccttagtttc gagactttgg gagatagcga 600ccgatatcta gtttggcatt
ttgtatatta attacctcaa gcaatggagc gctctgatgc 660ggtgcagcgt
cggctgcagc acctggcagt ggcgctaggg tcgccctatc gctcggaacc
720tggtcagctg gctcccgcct cctgctcagc ctcttccggt accgcggtga
gaatcgaaaa 780tgcatcgttt ctaggttcgg agacggtcaa ttccctgctc
cggcgaatct gtcggtcaag 840ctggccagtg gacaatgttg ctatggcagc
ccgcgcacat gggcctcccg acgcggccat 900caggagccca aacagcgtgt
cagggtatgt gaaactcaag aggtccctgc tgggcactcc 960ggccccactc
cgggggcggg acgccaggca ttcgcggtcg gtcccgcgcg acgagcgaaa
1020tgatgattcg gttacgagac caggacgtcg tcgaggtcga gaggcagcct
cggacacgtc 1080tcgctagggc aacgccccga gtccccgcga gggccgtaaa
cattgtttct gggtgtcgga 1140gtgggcattt tgggcccgat ccaatcgcct
catgccgctc tcgtctggtc ctcacgttcg 1200cgtacggcct ggatcccgga
aagggcggat gcacgtggtg ttgccccgcc attggcgccc 1260acgtttcaaa
gtccccggcc agaaatgcac aggaccggcc cggctcgcac aggccatgct
1320gaacgcccag atttcgacag caacaccatc tagaataatc gcaaccatcc
gcgttttgaa 1380cgaaacgaaa cggcgctgtt tagcatgttt ccgacatcgt
gggggccgaa gcatgctccg 1440gggggaggaa agcgtggcac agcggtagcc
cattctgtgc cacacgccga cgaggaccaa 1500tccccggcat cagccttcat
cgacggctgc gccgcacata taaagccgga cgcctaaccg 1560gtttcgtggt
tatgactagt atgttcgcgt tctacttcct gacggcctgc atctccctga
1620agggcgtgtt cggcgtctcc ccctcctaca acggcctggg cctgacgccc
cagatgggct 1680gggacaactg gaacacgttc gcctgcgacg tctccgagca
gctgctgctg gacacggccg 1740accgcatctc cgacctgggc ctgaaggaca
tgggctacaa gtacatcatc ctggacgact 1800gctggtcctc cggccgcgac
tccgacggct tcctggtcgc cgacgagcag aagttcccca 1860acggcatggg
ccacgtcgcc gaccacctgc acaacaactc cttcctgttc ggcatgtact
1920cctccgcggg cgagtacacg tgcgccggct accccggctc cctgggccgc
gaggaggagg 1980acgcccagtt cttcgcgaac aaccgcgtgg actacctgaa
gtacgacaac tgctacaaca 2040agggccagtt cggcacgccc gagatctcct
accaccgcta caaggccatg tccgacgccc 2100tgaacaagac gggccgcccc
atcttctact ccctgtgcaa ctggggccag gacctgacct 2160tctactgggg
ctccggcatc gcgaactcct ggcgcatgtc cggcgacgtc acggcggagt
2220tcacgcgccc cgactcccgc tgcccctgcg acggcgacga gtacgactgc
aagtacgccg 2280gcttccactg ctccatcatg aacatcctga acaaggccgc
ccccatgggc cagaacgcgg 2340gcgtcggcgg ctggaacgac ctggacaacc
tggaggtcgg cgtcggcaac ctgacggacg 2400acgaggagaa ggcgcacttc
tccatgtggg ccatggtgaa gtcccccctg atcatcggcg 2460cgaacgtgaa
caacctgaag gcctcctcct actccatcta ctcccaggcg tccgtcatcg
2520ccatcaacca ggactccaac ggcatccccg ccacgcgcgt ctggcgctac
tacgtgtccg 2580acacggacga gtacggccag ggcgagatcc agatgtggtc
cggccccctg gacaacggcg 2640accaggtcgt ggcgctgctg aacggcggct
ccgtgtcccg ccccatgaac acgaccctgg 2700aggagatctt cttcgactcc
aacctgggct ccaagaagct gacctccacc tgggacatct 2760acgacctgtg
ggcgaaccgc gtcgacaact ccacggcgtc cgccatcctg ggccgcaaca
2820agaccgccac cggcatcctg tacaacgcca ccgagcagtc ctacaaggac
ggcctgtcca 2880agaacgacac ccgcctgttc ggccagaaga tcggctccct
gtcccccaac gcgatcctga 2940acacgaccgt ccccgcccac ggcatcgcgt
tctaccgcct gcgcccctcc tcctgatacg 3000tactcgaggc agcagcagct
cggatagtat cgacacactc tggacgctgg tcgtgtgatg 3060gactgttgcc
gccacacttg ctgccttgac ctgtgaatat ccctgccgct tttatcaaac
3120agcctcagtg tgtttgatct tgtgtgtacg cgcttttgcg agttgctagc
tgcttgtgct 3180atttgcgaat accaccccca gcatcccctt ccctcgtttc
atatcgcttg catcccaacc 3240gcaacttatc tacgctgtcc tgctatccct
cagcgctgct cctgctcctg ctcactgccc 3300ctcgcacagc cttggtttgg
gctccgcctg tattctcctg gtactgcaac ctgtaaacca 3360gcactgcaat
gctgatgcac gggaagtagt gggatgggaa cacaaatgga aagctgtaga
3420attcggccga caggacgcgc gtcaaaggtg ctggtcgtgt atgccctggc
cggcaggtcg 3480ttgctgctgc tggttagtga ttccgcaacc ctgattttgg
cgtcttattt tggcgtggca 3540aacgctggcg cccgcgagcc gggccggcgg
cgatgcggtg ccccacggct gccggaatcc 3600aagggaggca agagcgcccg
ggtcagttga agggctttac gcgcaaggta cagccgctcc 3660tgcaaggctg
cgtggtggaa ttggacgtgc
aggtcctgct gaagttcctc caccgcctca 3720ccagcggaca aagcaccggt
gtatcaggtc cgtgtcatcc actctaaaga actcgactac 3780gacctactga
tggccctaga ttcttcatca aaaacgcctg agacacttgc ccaggattga
3840aactccctga agggaccacc aggggccctg agttgttcct tccccccgtg
gcgagctgcc 3900agccaggctg tacctgtgat cgaggctggc gggaaaatag
gcttcgtgtg ctcaggtcat 3960gggaggtgca ggacagctca tgaaacgcca
acaatcgcac aattcatgtc aagctaatca 4020gctatttcct cttcacgagc
tgtaattgtc ccaaaattct ggtctaccgg gggtgatcct 4080tcgtgtacgg
gcccttccct caaccctagg tatgcgcgca tgcggtcgcc gcgcaactcg
4140cgcgagggcc gagggtttgg gacgggccgt cccgaaatgc agttgcaccc
ggatgcgtgg 4200cacctttttt gcgataattt atgcaatgga ctgctctgca
aaattctggc tctgtcgcca 4260accctaggat cagcggcgta ggatttcgta
atcattcgtc ctgatgggga gctaccgact 4320accctaatat cagcccgact
gcctgacgcc agcgtccact tttgtgcaca cattccattc 4380gtgcccaaga
catttcattg tggtgcgaag cgtccccagt tacgctcacc tgtttcccga
4440cctccttact gttctgtcga cagagcgggc ccacaggccg gtcgcagcca
ctagtatggt 4500ggccgccgag gcctcctccg ccctgttctc cgtgcgcacc
cccggcacct cccccaagcc 4560cggcaagttc ggcaactggc ccacctccct
gtccgtgccc ttcaagtcca agtccaacca 4620caacggcggc ttccaggtga
aggccaacgc ctccgcccgc cccaaggcca acggctccgc 4680cgtgtccctg
aagtccggct ccctggacac ccaggaggac acctcctcct cctcctcccc
4740cccccgcacc ttcatcaacc agctgcccga ctggtccatg ctgctgtccg
ccatcaccac 4800cgtgttcgtg gccgccgaga agcagtggac catgctggac
cgcaagtcca agcgccccga 4860catgctgatg gaccccttcg gcgtggaccg
cgtggtgcag gacggcgccg tgttccgcca 4920gtccttctcc atccgctcct
acgagatcgg cgccgaccgc accgcctcca tcgagaccct 4980gatgaacatc
ttccaggaga cctccctgaa ccactgcaag tccatcggcc tgctgaacga
5040cggcttcggc cgcacccccg agatgtgcaa gcgcgacctg atctgggtgg
tgaccaagat 5100gcacgtggag gtgaaccgct accccacctg gggcgacacc
atcgaggtga acacctgggt 5160gtccgagtcc ggcaagaccg gcatgggccg
cgactggctg atctccgact gccacaccgg 5220cgagatcctg atccgcgcca
cctccatgtg cgccatgatg aaccagaaga cccgccgctt 5280ctccaagttc
ccctacgagg tgcgccagga gctggccccc cacttcgtgg actccgcccc
5340cgtgatcgag gactaccaga agctgcacaa gctggacgtg aagaccggcg
actccatctg 5400caacggcctg accccccgct ggaacgacct ggacgtgaac
cagcacgtga acaacgtgaa 5460gtacatcggc tggatcctgg agtccgtgcc
caccgaggtg ttcgagaccc aggagctgtg 5520cggcctgacc ctggagtacc
gccgcgagtg cggccgcgac tccgtgctgg agtccgtgac 5580cgccatggac
ccctccaagg agggcgaccg ctccctgtac cagcacctgc tgcgcctgga
5640ggacggcgcc gacatcgcca agggccgcac caagtggcgc cccaagaacg
ccggcaccaa 5700cggcgccatc tccaccggca agacctccaa cggcaactcc
atctccatgg actacaagga 5760ccacgacggc gactacaagg accacgacat
cgactacaag gacgacgacg acaagtgact 5820cgaggcagca gcagctcgga
tagtatcgac acactctgga cgctggtcgt gtgatggact 5880gttgccgcca
cacttgctgc cttgacctgt gaatatccct gccgctttta tcaaacagcc
5940tcagtgtgtt tgatcttgtg tgtacgcgct tttgcgagtt gctagctgct
tgtgctattt 6000gcgaatacca cccccagcat ccccttccct cgtttcatat
cgcttgcatc ccaaccgcaa 6060cttatctacg ctgtcctgct atccctcagc
gctgctcctg ctcctgctca ctgcccctcg 6120cacagccttg gtttgggctc
cgcctgtatt ctcctggtac tgcaacctgt aaaccagcac 6180tgcaatgctg
atgcacggga agtagtggga tgggaacaca aatggaaagc tgtataggga
6240taacagggta atgagctcag cgtctgcgtg ttgggagctg gagtcgtggg
cttgacgacg 6300gcgctgcagc tgttgcagga tgtgcctggc gtgcgcgttc
acgtcgtggc tgagaaatat 6360ggcgacgaaa cgttgacggc tggggccggc
gggctgtgga tgccatacgc attgggtacg 6420cggccattgg atgggattga
taggcttatg gagggataat agagtttttg ccggatccaa 6480cgcatgtgga
tgcggtatcc cggtgggctg aaagtgtgga aggatagtgc attggctatt
6540cacatgcact gcccacccct tttggcagga aatgtgccgg catcgttggt
gcaccgatgg 6600ggaaaatcga cgttcgacca ctacatgaag atttatacgt
ctgaagatgc agcgactgcg 6660ggtgcgaaac ggatgacggt ttggtcgtgt
atgtcacagc atgtgctgga tcttgcgggc 6720taactccccc tgccacggcc
cattgcaggt gtcatgttga ctggagggta cgacctttcg 6780tccgtcaaat
tcccagagga ggacccgctc tgggccgaca ttgtgcccac tgaagagc
68381251303DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 125actagtaaca atggccaccg cctccacctt
ctccgccttc aacgcccgct gcggcgacct 60gcgccgctcc gccggctccg gcccccgccg
ccccgcccgc cccctgcccg tgcgcgccgc 120catcaacgcc tccgcccgcc
ccaaggccaa cggctccgcc gtgtccctga agtccggctc 180cctggacacc
caggaggaca cctcctcctc ctcctccccc ccccgcacct tcatcaacca
240gctgcccgac tggtccatgc tgctgtccgc catcaccacc gtgttcgtgg
ccgccgagaa 300gcagtggacc atgctggacc gcaagtccaa gcgccccgac
atgctgatgg accccttcgg 360cgtggaccgc gtggtgcagg acggcgccgt
gttccgccag tccttctcca tccgctccta 420cgagatcggc gccgaccgca
ccgcctccat cgagaccctg atgaacatct tccaggagac 480ctccctgaac
cactgcaagt ccatcggcct gctgaacgac ggcttcggcc gcacccccga
540gatgtgcaag cgcgacctga tctgggtggt gaccaagatg cacgtggagg
tgaaccgcta 600ccccacctgg ggcgacacca tcgaggtgaa cacctgggtg
tccgagtccg gcaagaccgg 660catgggccgc gactggctga tctccgactg
ccacaccggc gagatcctga tccgcgccac 720ctccatgtgc gccatgatga
accagaagac ccgccgcttc tccaagttcc cctacgaggt 780gcgccaggag
ctggcccccc acttcgtgga ctccgccccc gtgatcgagg actaccagaa
840gctgcacaag ctggacgtga agaccggcga ctccatctgc aacggcctga
ccccccgctg 900gaacgacctg gacgtgaacc agcacgtgaa caacgtgaag
tacatcggct ggatcctgga 960gtccgtgccc accgaggtgt tcgagaccca
ggagctgtgc ggcctgaccc tggagtaccg 1020ccgcgagtgc ggccgcgact
ccgtgctgga gtccgtgacc gccatggacc cctccaagga 1080gggcgaccgc
tccctgtacc agcacctgct gcgcctggag gacggcgccg acatcgccaa
1140gggccgcacc aagtggcgcc ccaagaacgc cggcaccaac ggcgccatct
ccaccggcaa 1200gacctccaac ggcaactcca tctccatgga ctacaaggac
cacgacggcg actacaagga 1260ccacgacatc gactacaagg acgacgacga
caagtgactc gag 13031261282DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 126actagtaaca
atggccaccg cctccacctt ctccgccttc aacgcccgct gcggcgacct 60gcgccgctcc
gccggctccg gcccccgccg ccccgcccgc cccctgcccg tgcgcgccgc
120catcaacgcc tccgcccacc ccaaggccaa cggctccgcc gtgaacctga
agtccggctc 180cctggagacc caggaggaca cctcctcctc ctcccccccc
ccccgcacct tcatcaagca 240gctgcccgac tggggcatgc tgctgtccaa
gatcaccacc gtgttcggcg ccgccgagcg 300ccagtggaag cgccccggca
tgctggtgga gcccttcggc gtggaccgca tcttccagga 360cggcgtgttc
ttccgccagt ccttctccat ccgctcctac gagatcggcg ccgaccgcac
420cgcctccatc gagaccctga tgaacatctt ccaggagacc tccctgaacc
actgcaagtc 480catcggcctg ctgaacgacg gcttcggccg cacccccgag
atgtgcaagc gcgacctgat 540ctgggtggtg accaagatcc aggtggaggt
gaaccgctac cccacctggg gcgacaccat 600cgaggtgaac acctgggtgt
ccgagtccgg caagaacggc atgggccgcg actggctgat 660ctccgactgc
cgcaccggcg agatcctgat ccgcgccacc tccgtgtggg ccatgatgaa
720ccgcaagacc cgccgcctgt ccaagttccc ctacgaggtg cgccaggaga
tcgcccccca 780cttcgtggac tccgcccccg tgatcgagga cgacaagaag
ctgcacaagc tggacgtgaa 840gaccggcgac tccatccgca agggcctgac
cccccgctgg aacgacctgg acgtgaacca 900gcacgtgaac aacgtgaagt
acatcggctg gatcctgaag tccgtgcccg ccgaggtgtt 960cgagacccag
gagctgtgcg gcgtgaccct ggagtaccgc cgcgagtgcg gccgcgactc
1020cgtgctggag tccgtgaccg ccatggacac cgccaaggag ggcgaccgct
ccctgtacca 1080gcacctgctg cgcctggagg acggcgccga catcaccatc
ggccgcaccg agtggcgccc 1140caagaacgcc ggcgccaacg gcgccatctc
caccggcaag acctccaacg agaactccgt 1200gtccatggac tacaaggacc
acgacggcga ctacaaggac cacgacatcg actacaagga 1260cgacgacgac
aagtgactcg ag 12821276696DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 127gaagagcgcc
caatgtttaa acccctcaac tgcgacgctg ggaaccttct ccgggcaggc 60gatgtgcgtg
ggtttgcctc cttggcacgg ctctacaccg tcgagtacgc catgaggcgg
120tgatggctgt gtcggttgcc acttcgtcca gagacggcaa gtcgtccatc
ctctgcgtgt 180gtggcgcgac gctgcagcag tccctctgca gcagatgagc
gtgactttgg ccatttcacg 240cactcgagtg tacacaatcc atttttctta
aagcaaatga ctgctgattg accagatact 300gtaacgctga tttcgctcca
gatcgcacag atagcgacca tgttgctgcg tctgaaaatc 360tggattccga
attcgaccct ggcgctccat ccatgcaaca gatggcgaca cttgttacaa
420ttcctgtcac ccatcggcat ggagcaggtc cacttagatt cccgatcacc
cacgcacatc 480tcgctaatag tcattcgttc gtgtcttcga tcaatctcaa
gtgagtgtgc atggatcttg 540gttgacgatg cggtatgggt ttgcgccgct
ggctgcaggg tctgcccaag gcaagctaac 600ccagctcctc tccccgacaa
tactctcgca ggcaaagccg gtcacttgcc ttccagattg 660ccaataaact
caattatggc ctctgtcatg ccatccatgg gtctgatgaa tggtcacgct
720cgtgtcctga ccgttcccca gcctctggcg tcccctgccc cgcccaccag
cccacgccgc 780gcggcagtcg ctgccaaggc tgtctcggag gtaccgcggt
gagaatcgaa aatgcatcgt 840ttctaggttc ggagacggtc aattccctgc
tccggcgaat ctgtcggtca agctggccag 900tggacaatgt tgctatggca
gcccgcgcac atgggcctcc cgacgcggcc atcaggagcc 960caaacagcgt
gtcagggtat gtgaaactca agaggtccct gctgggcact ccggccccac
1020tccgggggcg ggacgccagg cattcgcggt cggtcccgcg cgacgagcga
aatgatgatt 1080cggttacgag accaggacgt cgtcgaggtc gagaggcagc
ctcggacacg tctcgctagg 1140gcaacgcccc gagtccccgc gagggccgta
aacattgttt ctgggtgtcg gagtgggcat 1200tttgggcccg atccaatcgc
ctcatgccgc tctcgtctgg tcctcacgtt cgcgtacggc 1260ctggatcccg
gaaagggcgg atgcacgtgg tgttgccccg ccattggcgc ccacgtttca
1320aagtccccgg ccagaaatgc acaggaccgg cccggctcgc acaggccatg
ctgaacgccc 1380agatttcgac agcaacacca tctagaataa tcgcaaccat
ccgcgttttg aacgaaacga 1440aacggcgctg tttagcatgt ttccgacatc
gtgggggccg aagcatgctc cggggggagg 1500aaagcgtggc acagcggtag
cccattctgt gccacacgcc gacgaggacc aatccccggc 1560atcagccttc
atcgacggct gcgccgcaca tataaagccg gacgcctaac cggtttcgtg
1620gttatgacta gtatgttcgc gttctacttc ctgacggcct gcatctccct
gaagggcgtg 1680ttcggcgtct ccccctccta caacggcctg ggcctgacgc
cccagatggg ctgggacaac 1740tggaacacgt tcgcctgcga cgtctccgag
cagctgctgc tggacacggc cgaccgcatc 1800tccgacctgg gcctgaagga
catgggctac aagtacatca tcctggacga ctgctggtcc 1860tccggccgcg
actccgacgg cttcctggtc gccgacgagc agaagttccc caacggcatg
1920ggccacgtcg ccgaccacct gcacaacaac tccttcctgt tcggcatgta
ctcctccgcg 1980ggcgagtaca cgtgcgccgg ctaccccggc tccctgggcc
gcgaggagga ggacgcccag 2040ttcttcgcga acaaccgcgt ggactacctg
aagtacgaca actgctacaa caagggccag 2100ttcggcacgc ccgagatctc
ctaccaccgc tacaaggcca tgtccgacgc cctgaacaag 2160acgggccgcc
ccatcttcta ctccctgtgc aactggggcc aggacctgac cttctactgg
2220ggctccggca tcgcgaactc ctggcgcatg tccggcgacg tcacggcgga
gttcacgcgc 2280cccgactccc gctgcccctg cgacggcgac gagtacgact
gcaagtacgc cggcttccac 2340tgctccatca tgaacatcct gaacaaggcc
gcccccatgg gccagaacgc gggcgtcggc 2400ggctggaacg acctggacaa
cctggaggtc ggcgtcggca acctgacgga cgacgaggag 2460aaggcgcact
tctccatgtg ggccatggtg aagtcccccc tgatcatcgg cgcgaacgtg
2520aacaacctga aggcctcctc ctactccatc tactcccagg cgtccgtcat
cgccatcaac 2580caggactcca acggcatccc cgccacgcgc gtctggcgct
actacgtgtc cgacacggac 2640gagtacggcc agggcgagat ccagatgtgg
tccggccccc tggacaacgg cgaccaggtc 2700gtggcgctgc tgaacggcgg
ctccgtgtcc cgccccatga acacgaccct ggaggagatc 2760ttcttcgact
ccaacctggg ctccaagaag ctgacctcca cctgggacat ctacgacctg
2820tgggcgaacc gcgtcgacaa ctccacggcg tccgccatcc tgggccgcaa
caagaccgcc 2880accggcatcc tgtacaacgc caccgagcag tcctacaagg
acggcctgtc caagaacgac 2940acccgcctgt tcggccagaa gatcggctcc
ctgtccccca acgcgatcct gaacacgacc 3000gtccccgccc acggcatcgc
gttctaccgc ctgcgcccct cctcctgata caacttatta 3060cgtaacggag
cgtcgtgcgg gagggagtgt gccgagcggg gagtcccggt ctgtgcgagg
3120cccggcagct gacgctggcg agccgtacgc cccgagggtc cccctcccct
gcaccctctt 3180ccccttccct ctgacggccg cgcctgttct tgcatgttca
gcgacgagga tatcgaattc 3240atagcgactg ctaccccccg accatgtgcc
gaggcagaaa ttatatacaa gaagcagatc 3300gcaattaggc acatcgcttt
gcattatcca cacactattc atcgctgctg cggcaaggct 3360gcagagtgta
tttttgtggc ccaggagctg agtccgaagt cgacgcgacg agcggcgcag
3420gatccgaccc ctagacgagc actgtcattt tccaagcacg cagctaaatg
cgctgagacc 3480gggtctaaat catccgaaaa gtgtcaaaat ggccgattgg
gttcgcctag gacaatgcgc 3540tgcggattcg ctcgagtccg ctgccggcca
aaaggcggtg gtacaggaag gcgcacgggg 3600ccaaccctgc gaagccgggg
gcccgaacgc cgaccgccgg ccttcgatct cgggtgtccc 3660cctcgtcaat
ttcctctctc gggtgcagcc acgaaagtcg tgacgcaggt cacgaaatcc
3720ggttacgaaa aacgcaggtc ttcgcaaaaa cgtgagggtt tcgcgtctcg
ccctagctat 3780tcgtatcgcc gggtcagacc cacgtgcaga aaagcccttg
aataacccgg gaccgtggtt 3840accgcgccgc ctgcaccagg gggcttatat
aagcccacac cacacctgtc tcaccacgca 3900tttctccaac tcgcgacttt
tcggaagaaa ttgttatcca cctagtatag actgccacct 3960gcaggacctt
gtgtcttgca gtttgtattg gtcccggccg tcgagcacga cagatctggg
4020ctagggttgg cctggccgct cggcactccc ctttagccgc gcgcatccgc
gttccagagg 4080tgcgattcgg tgtgtggagc attgtcatgc gcttgtgggg
gtcgttccgt gcgcggcggg 4140tccgccatgg gcgccgacct gggccctagg
gtttgttttc gggccaagcg agcccctctc 4200acctcgtcgc ccccccgcat
tccctctctc ttgcagccac tagtaacaat ggccaccgca 4260tccactttct
cggcgttcaa tgcccgctgc ggcgacctgc gtcgctcggc gggctccggg
4320ccccggcgcc cagcgaggcc cctccccgtg cgcgctgcca tcgccagcga
ggtccccgtg 4380gccaccacct ccccccgggc gcaccccaag gcgaacggca
gcgcggtgtc gctgaagtcg 4440ggctccctgg agacccagga ggacaagacg
agcagctcgt cccccccccc ccgcacgttc 4500atcaaccagc tgcccgtgtg
gagcatgctg ctgtcggcgg tgaccacggt cttcggcgtg 4560gccgagaagc
agtggcccat gctggaccgc aagtccaagc gccccgacat gctggtcgag
4620cccctgggcg tggaccgcat cgtctacgac ggcgtgagct tccgccagtc
gttctccatc 4680cgcagctacg agatcggcgc cgaccgcacc gcctcgatcg
agacgctgat gaacatgttc 4740caggagacct ccctgaacca ctgcaagatc
atcggcctgc tgaacgacgg cttcggccgc 4800acgcccgaga tgtgcaagcg
cgacctgatc tgggtcgtga ccaagatgca gatcgaggtg 4860aaccgctacc
ccacgtgggg cgacaccatc gaggtcaaca cgtgggtgag cgcctcgggc
4920aagcacggca tgggccgcga ctggctgatc tccgactgcc acaccggcga
gatcctgatc 4980cgcgcgacga gcgtctgggc gatgatgaac cagaagaccc
gccgcctgtc gaagatcccc 5040tacgaggtgc gccaggagat cgagccccag
ttcgtcgact ccgcccccgt gatcgtggac 5100gaccgcaagt tccacaagct
ggacctgaag acgggcgaca gcatctgcaa cggcctgacc 5160ccccgctgga
cggacctgga cgtgaaccag cacgtcaaca acgtgaagta catcggctgg
5220atcctgcagt cggtccccac cgaggtgttc gagacgcagg agctgtgcgg
cctgaccctg 5280gagtaccgcc gcgagtgcgg ccgcgactcc gtgctggaga
gcgtcacggc catggacccc 5340tcgaaggagg gcgaccgctc cctgtaccag
cacctgctgc gcctggagga cggcgcggac 5400atcgtgaagg gccgcaccga
gtggcgcccc aagaacgccg gcgccaaggg cgccatcctg 5460acgggcaaga
ccagcaacgg caactcgatc tccatggact acaaggacca cgacggcgac
5520tacaaggacc acgacatcga ctacaaggac gacgacgaca agtgaaagct
tgcagcagca 5580gctcggatag tatcgacaca ctctggacgc tggtcgtgtg
atggactgtt gccgccacac 5640ttgctgcctt gacctgtgaa tatccctgcc
gcttttatca aacagcctca gtgtgtttga 5700tcttgtgtgt acgcgctttt
gcgagttgct agctgcttgt gctatttgcg aataccaccc 5760ccagcatccc
cttccctcgt ttcatatcgc ttgcatccca accgcaactt atctacgctg
5820tcctgctatc cctcagcgct gctcctgctc ctgctcactg cccctcgcac
agccttggtt 5880tgggctcccg cctgtattct cctggtactg caacctgtaa
accagcactg caatgctgat 5940gcacgggaag tagtgggatg ggaacacaaa
tggaaagctg gagctccagc gccatgccac 6000gccctttgat ggcttcaagt
acgattacgg tgttggattg tgtgtttgtt gcgtagtgtg 6060catggtttag
aataatacac ttgatttctt gctcacggca atctcggctt gtccgcaggt
6120tcaaccccat ttcggagtct caggtcagcc gcgcaatgac cagccgctac
ttcaaggact 6180tgcacgacaa cgccgaggtg agctatgttt aggacttgat
tggaaattgt cgtcgacgca 6240tattcgcgct ccgcgacagc acccaagcaa
aatgtcaagt gcgttccgat ttgcgtccgc 6300aggtcgatgt tgtgatcgtc
ggcgccggat ccgccggtct gtcctgcgct tacgagctga 6360ccaagcaccc
tgacgtccgg gtacgcgagc tgagattcga ttagacataa attgaagatt
6420aaacccgtag aaaaatttga tggtcgcgaa actgtgctcg attgcaagaa
attgatcgtc 6480ctccactccg caggtcgcca tcatcgagca gggcgttgct
cccggcggcg gcgcctggct 6540ggggggacag ctgttctcgg ccatgtgtgt
acgtagaagg atgaatttca gctggttttc 6600gttgcacagc tgtttgtgca
tgatttgttt cagactattg ttgaatgttt ttagatttct 6660taggatgcat
gatttgtctg catgcgactg aagagc 66961285851DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
128gctcttcgga gtcactgtgc cactgagttc gactggtagc tgaatggagt
cgctgctcca 60ctaaacgaat tgtcagcacc gccagccggc cgaggacccg agtcatagcg
agggtagtag 120cgcgccatgg caccgaccag cctgcttgcc agtactggcg
tctcttccgc ttctctgtgg 180tcctctgcgc gctccagcgc gtgcgctttt
ccggtggatc atgcggtccg tggcgcaccg 240cagcggccgc tgcccatgca
gcgccgctgc ttccgaacag tggcggtcag ggccgcaccc 300gcggtagccg
tccgtccgga acccgcccaa gagttttggg agcagcttga gccctgcaag
360atggcggagg acaagcgcat cttcctggag gagcaccggt gcgtggaggt
ccggggctga 420ccggccgtcg cattcaacgt aatcaatcgc atgatgatca
gaggacacga agtcttggtg 480gcggtggcca gaaacactgt ccattgcaag
ggcataggga tgcgttcctt cacctctcat 540ttctcatttc tgaatccctc
cctgctcact ctttctcctc ctccttcccg ttcacgcagc 600attcggggta
ccgcggtgag aatcgaaaat gcatcgtttc taggttcgga gacggtcaat
660tccctgctcc ggcgaatctg tcggtcaagc tggccagtgg acaatgttgc
tatggcagcc 720cgcgcacatg ggcctcccga cgcggccatc aggagcccaa
acagcgtgtc agggtatgtg 780aaactcaaga ggtccctgct gggcactccg
gccccactcc gggggcggga cgccaggcat 840tcgcggtcgg tcccgcgcga
cgagcgaaat gatgattcgg ttacgagacc aggacgtcgt 900cgaggtcgag
aggcagcctc ggacacgtct cgctagggca acgccccgag tccccgcgag
960ggccgtaaac attgtttctg ggtgtcggag tgggcatttt gggcccgatc
caatcgcctc 1020atgccgctct cgtctggtcc tcacgttcgc gtacggcctg
gatcccggaa agggcggatg 1080cacgtggtgt tgccccgcca ttggcgccca
cgtttcaaag tccccggcca gaaatgcaca 1140ggaccggccc ggctcgcaca
ggccatgctg aacgcccaga tttcgacagc aacaccatct 1200agaataatcg
caaccatccg cgttttgaac gaaacgaaac ggcgctgttt agcatgtttc
1260cgacatcgtg ggggccgaag catgctccgg ggggaggaaa gcgtggcaca
gcggtagccc 1320attctgtgcc acacgccgac gaggaccaat ccccggcatc
agccttcatc gacggctgcg 1380ccgcacatat aaagccggac gcctaaccgg
tttcgtggtt atgactagta tgttcgcgtt 1440ctacttcctg acggcctgca
tctccctgaa gggcgtgttc ggcgtctccc cctcctacaa 1500cggcctgggc
ctgacgcccc agatgggctg ggacaactgg aacacgttcg cctgcgacgt
1560ctccgagcag ctgctgctgg acacggccga ccgcatctcc gacctgggcc
tgaaggacat 1620gggctacaag tacatcatcc tggacgactg ctggtcctcc
ggccgcgact ccgacggctt 1680cctggtcgcc gacgagcaga agttccccaa
cggcatgggc cacgtcgccg accacctgca 1740caacaactcc ttcctgttcg
gcatgtactc ctccgcgggc gagtacacgt gcgccggcta 1800ccccggctcc
ctgggccgcg aggaggagga cgcccagttc ttcgcgaaca accgcgtgga
1860ctacctgaag tacgacaact gctacaacaa gggccagttc ggcacgcccg
agatctccta 1920ccaccgctac aaggccatgt ccgacgccct gaacaagacg
ggccgcccca tcttctactc 1980cctgtgcaac tggggccagg acctgacctt
ctactggggc tccggcatcg cgaactcctg 2040gcgcatgtcc ggcgacgtca
cggcggagtt cacgcgcccc gactcccgct gcccctgcga 2100cggcgacgag
tacgactgca agtacgccgg cttccactgc tccatcatga acatcctgaa
2160caaggccgcc cccatgggcc agaacgcggg
cgtcggcggc tggaacgacc tggacaacct 2220ggaggtcggc gtcggcaacc
tgacggacga cgaggagaag gcgcacttct ccatgtgggc 2280catggtgaag
tcccccctga tcatcggcgc gaacgtgaac aacctgaagg cctcctccta
2340ctccatctac tcccaggcgt ccgtcatcgc catcaaccag gactccaacg
gcatccccgc 2400cacgcgcgtc tggcgctact acgtgtccga cacggacgag
tacggccagg gcgagatcca 2460gatgtggtcc ggccccctgg acaacggcga
ccaggtcgtg gcgctgctga acggcggctc 2520cgtgtcccgc cccatgaaca
cgaccctgga ggagatcttc ttcgactcca acctgggctc 2580caagaagctg
acctccacct gggacatcta cgacctgtgg gcgaaccgcg tcgacaactc
2640cacggcgtcc gccatcctgg gccgcaacaa gaccgccacc ggcatcctgt
acaacgccac 2700cgagcagtcc tacaaggacg gcctgtccaa gaacgacacc
cgcctgttcg gccagaagat 2760cggctccctg tcccccaacg cgatcctgaa
cacgaccgtc cccgcccacg gcatcgcgtt 2820ctaccgcctg cgcccctcct
cctgatacgt agcagcagca gctcggatag tatcgacaca 2880ctctggacgc
tggtcgtgtg atggactgtt gccgccacac ttgctgcctt gacctgtgaa
2940tatccctgcc gcttttatca aacagcctca gtgtgtttga tcttgtgtgt
acgcgctttt 3000gcgagttgct agctgcttgt gctatttgcg aataccaccc
ccagcatccc cttccctcgt 3060ttcatatcgc ttgcatccca accgcaactt
atctacgctg tcctgctatc cctcagcgct 3120gctcctgctc ctgctcactg
cccctcgcac agccttggtt tgggctccgc ctgtattctc 3180ctggtactgc
aacctgtaaa ccagcactgc aatgctgatg cacgggaagt agtgggatgg
3240gaacacaaat ggagatatcg cgaggggtct gcctgggcca gccgctccct
ctaaacacgg 3300gacgcgtggt ccaattcggg cttcgggacc ctttggcggt
ttgaacgcca gggatggggc 3360gcccgcgagc ctggggaccc cggcaacggc
ttccccagag cctgccttgc aatctcgcgc 3420gtcctctccc tcagcacgtg
gcggttccac gtgtggtcgg gcttcccgga ctagctcgcg 3480tcgtgaccta
gcttaatgaa cccagccggg cctgtagcac cgcctaagag gttttgatta
3540tttcattata ccaatctatt cgccactagt atggccatca agaccaaccg
ccagcccgtg 3600gagaagcccc ccttcaccat cggcaccctg cgcaaggcca
tccccgccca ctgcttcgag 3660cgctccgccc tgcgctcctc catgtacctg
gccttcgaca tcgccgtgat gtccctgctg 3720tacgtggcct ccacctacat
cgaccccgcc cccgtgccca cctgggtgaa gtacggcgtg 3780atgtggcccc
tgtactggtt cttccagggc gccttcggca ccggcgtgtg ggtgtgcgcc
3840cacgagtgcg gccaccaggc cttctcctcc tcccaggcca tcaacgacgg
cgtgggcctg 3900gtgttccact ccctgctgct ggtgccctac tactcctgga
agcactccca ccgccgccac 3960cactccaaca ccggctgcct ggacaaggac
gaggtgttcg tgccccccca ccgcgccgtg 4020gcccacgagg gcctggagtg
ggaggagtgg ctgcccatcc gcatgggcaa ggtgctggtg 4080accctgaccc
tgggctggcc cctgtacctg atgttcaacg tggcctcccg cccctacccc
4140cgcttcgcca accacttcga cccctggtcc cccatcttct ccaagcgcga
gcgcatcgag 4200gtggtgatct ccgacctggc cctggtggcc gtgctgtccg
gcctgtccgt gctgggccgc 4260accatgggct gggcctggct ggtgaagacc
tacgtggtgc cctacctgat cgtgaacatg 4320tggctggtgc tgatcaccct
gctgcagcac acccaccccg ccctgcccca ctacttcgag 4380aaggactggg
actggctgcg cggcgccatg gccaccgtgg accgctccat gggccccccc
4440ttcatggaca acatcctgca ccacatctcc gacacccacg tgctgcacca
cctgttctcc 4500accatccccc actaccacgc cgaggaggcc tccgccgcca
tccgccccat cctgggcaag 4560tactaccagt ccgactcccg ctgggtgggc
cgcgccctgt gggaggactg gcgcgactgc 4620cgctacgtgg tgcccgacgc
ccccgaggac gactccgccc tgtggttcca caagtagatc 4680gatcttaagg
cagcagcagc tcggatagta tcgacacact ctggacgctg gtcgtgtgat
4740ggactgttgc cgccacactt gctgccttga cctgtgaata tccctgccgc
ttttatcaaa 4800cagcctcagt gtgtttgatc ttgtgtgtac gcgcttttgc
gagttgctag ctgcttgtgc 4860tatttgcgaa taccaccccc agcatcccct
tccctcgttt catatcgctt gcatcccaac 4920cgcaacttat ctacgctgtc
ctgctatccc tcagcgctgc tcctgctcct gctcactgcc 4980cctcgcacag
ccttggtttg ggctccgcct gtattctcct ggtactgcaa cctgtaaacc
5040agcactgcaa tgctgatgca cgggaagtag tgggatggga acacaaatgg
aaagcttaat 5100taagagctct tgttttccag aaggagttgc tccttgagcc
tttcattctc agcctcgata 5160acctccaaag ccgctctaat tgtggagggg
gttcgaattt aaaagcttgg aatgttggtt 5220cgtgcgtctg gaacaagccc
agacttgttg ctcactggga aaaggaccat cagctccaaa 5280aaacttgccg
ctcaaaccgc gtacctctgc tttcgcgcaa tctgccctgt tgaaatcgcc
5340accacattca tattgtgacg cttgagcagt ctgtaattgc ctcagaatgt
ggaatcatct 5400gccccctgtg cgagcccatg ccaggcatgt cgcgggcgag
gacacccgcc actcgtacag 5460cagaccatta tgctacctca caatagttca
taacagtgac catatttctc gaagctcccc 5520aacgagcacc tccatgctct
gagtggccac cccccggccc tggtgcttgc ggagggcagg 5580tcaaccggca
tggggctacc gaaatccccg accggatccc accacccccg cgatgggaag
5640aatctctccc cgggatgtgg gcccaccacc agcacaacct gctggcccag
gcgagcgtca 5700aaccatacca cacaaatatc cttggcatcg gccctgaatt
ccttctgccg ctctgctacc 5760cggtgcttct gtccgaagca ggggttgcta
gggatcgctc cgagtccgca aacccttgtc 5820gcgtggcggg gcttgttcga
gcttgaagag c 5851129186DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 129tacaacttat
tacgtaacgg agcgtcgtgc gggagggagt gtgccgagcg gggagtcccg 60gtctgtgcga
ggcccggcag ctgacgctgg cgagccgtac gccccgaggg tccccctccc
120ctgcaccctc ttccccttcc ctctgacggc cgcgcctgtt cttgcatgtt
cagcgacgag 180gatatc 186130305DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 130gcgaggggtc
tgcctgggcc agccgctccc tctgaacacg ggacgcgtgg tccaattcgg 60gcttcgggac
cctttggcgg tttgaacgcc tgggagaggg cgcccgcgag cctggggacc
120ccggcaacgg cttccccaga gcctgccttg caatctcgcg cgtcctctcc
ctcagcacgt 180ggcggttcca cgtgtggtcg ggcgtcccgg actagctcac
gtcgtgacct agcttaatga 240acccagccgg gcctgcagca ccaccttaga
ggttttgatt atttgattag accaatctat 300tcacc 305131305DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
131ggcgaataga ttggtataat gaaataatca aaacctctta ggcggtgcta
caggcccggc 60tgggttcatt aagctaggtc acgacgcgag ctagtccggg aagcccgacc
acacgtggaa 120ccgccacgtg ctgagggaga ggacgcgcga gattgcaagg
caggctctgg ggaagccgtt 180gccggggtcc ccaggctcgc gggcgcccca
tccctggcgt tcaaaccgcc aaagggtccc 240gaagcccgaa ttggaccacg
cgtcccgtgt ttagagggag cggctggccc aggcagaccc 300ctcgc
305132305DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 132ggtgaataga ttggtctaat caaataatca
aaacctctaa ggtggtgctg caggcccggc 60tgggttcatt aagctaggtc acgacgtgag
ctagtccggg acgcccgacc acacgtggaa 120ccgccacgtg ctgagggaga
ggacgcgcga gattgcaagg caggctctgg ggaagccgtt 180gccggggtcc
ccaggctcgc gggcgccctc tcccaggcgt tcaaaccgcc aaagggtccc
240gaagcccgaa ttggaccacg cgtcccgtgt tcagagggag cggctggccc
aggcagaccc 300ctcgc 3051331322DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 133gtgatgggtt
ctttagacga tccagcccag gatcatgtgt tgcccacatg gagcctatcc 60acgctggcct
agaaggcaag cacatttcaa ggtgaaccca cgtccatgga gcgatggcgc
120caatatctcg cctctagacc aagcggttct caccccaact gcgtcatttg
tatgtatggc 180tgcaaagttg tcggtacgat agaggccgcc aacctggcgg
cgagggcgag gagctggttg 240ccgatctgtg cccaagcatg tgtcggagct
cggctgtctc ggcagcgagc tcctgtgcaa 300ggggcttgca tcgagaatgt
caggcgatag acactgcacg ttggggacac ggaggtgccc 360ctgtggcgtg
tcctggatgc cctcgggtcc gtcgcgagaa gctctggcga ccagcacccg
420gccacaaccg cagcaggcgt tcacccacaa gaatcttcca gatcgtgatg
cgcatgtatc 480gtgacacgat tggcgaggtc cgcaggacgc acacggactc
gtccactcat cagaactggt 540cagggcaccc atctgcgtcc cttttcagga
accacccacc gctgccaggc accttcgcca 600gcggcggact ccacacagag
aatgccttgc tgtgagagac catggccggc aagtgctgtc 660ggatctgccc
gcatacggtc agtccccagc acaaggaagc caagagtaca ggctgttggt
720gtcgatggag gagtggccgt tcccacaagt agtgagcggc agctgctcaa
cggcttcccc 780ctgttcatct tggcaaagcc agtgacttcc tacaagtatg
tgatgcagat cggcactgca 840atctgtcggc atgcgtacag aacatcggct
cgccagggca gcgttgctcg ctctggatga 900gctgcttggg aggaatcatc
ggcacacgcc cgtgccgtgc ccgcgccccg cgcccgtcgg 960gaaaggcccc
cggttaggac actgccgcgt cagccagtcg tgggatcgat cggacgtggc
1020gaatcctcgc ccggacaccc tcatcacacc ccacatttcc ctgcaagcaa
tcttgccgac 1080aaaatagtca agatccattg ggtttaggga acacgtgcga
gactgggcag ctgtatctgt 1140ccttgccccg cgtcaaattc ctgggcgtga
cgcagtcaca ggagaatcta ttagaccctg 1200gacttgcagc tcagtcatgg
gcgtgagtgg ctaaagcacc taggtcaggc gagtaccgcc 1260ccttccccag
gattcactct tctgcgattg acgttgagcc tgcatcgggc tgcttcgtca 1320cc
1322134841DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 134tcggagctaa agcagagact ggacaagact
tgcgttcgca tactggtgac acagaatagc 60tcccatctat tcatacgcct ttgggaaaag
gaacgagcct tgtggcctct gcattgctgc 120ctgctttgag gccgaggacg
gtgcgggacg ctcagatcca tcagcgatcg ccccaccctc 180agagcacctc
cgatccaagg caatactatc aggcaaagtt tccaaattca aacattccaa
240aatcacgcca gggactggat cacacacgca gatcagcgcc gttttgctct
ttgcctacgg 300gcgactgtgc cacttgtcga cccctggtga cgggagggac
cacgcctgcg gttggcatcc 360acttcgacgg acccagggac ggtttctcat
gccaaacctg agatttgagc acccagatga 420gcacattatg cgttttagga
tgcctgagca gcgggcgtgc aggaatctgg tctcgccaga 480ttcaccgaag
atgcgcccat cggagcgagg cgagggcttt gtgaccacgc aaggcagtgt
540gaggcaaaca catagggaca cctgcgtctt tcaatgcaca gacatctatg
gtgcccatgt 600atataaaatg ggctacttct gagtcaaacc aacgcaaact
gcgctatggc aaggccggcc 660aaggttggaa tcccggtctg tctggatttg
agtttgtggg ggctatcacg tgacaatccc 720tgggattggg cggcagcagc
gcacggcctg ggtggcaatg gcgcactaat actgctgaaa 780gcacggctct
gcatcccttt ctcttgacct gcgattggtc cttttcgcaa gcgtgatcat 840c
841135841DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 135tcggagctaa agcagaaact gaacaagact
tgcgttcgca tacttgtgac actgaatagg 60ttcaatctat tcatacgcct ttgggaaact
gaacgagcct tgtggcctct gcattgctgc 120ctgctttgag gccgaggacg
gcgcggaacg cacagatcca tcagcgatcg ccccaccctc 180agagtacatc
cgatccaagg caatactatc aggcaaagtt tccaaattca aacattccaa
240aattacgtca gggactggat cacacacgca gatcagcgcc gttttgctct
ttgcctacgg 300gcgactgtgc cacttgtcga cgcctggtga cgggagggac
cacgcctgcg gttggcatcc 360acttcgacgg acccagggac ggtctcacat
gccaaacctg agatttgagc accaagatga 420gcacattatg cgtttttgga
tgcctgagca gcgggcgtgc aggaatctgg tctcgccaga 480ttcaccgaag
atgcggccat cggagcgagg cgagggctgt gtggccacgc caggcagtgt
540gaggcaaaca cacagggaca tctgcttctt tcgatgcaca gacatctatg
ttgcccgtgc 600atataaaatg ggctacttct gaatcaaacc aacgcaaact
tcgctatggc aaggccggcc 660aaggttggaa tcccggtctg tctggatttg
agtttgtggg ggctatcacg tgacaatccc 720tgggattggg cggcagcagc
gcacggcctg gatggcaatg gcgcactaat actgctgaaa 780gcacggctct
gcatcccttt ctcttgacct gcgattggtc cttttcgcaa gcgtgatcat 840c
841136512DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 136caccgatcac tccgtcgccg cccaagagaa
atcaacctcg atggagggcg aggtggatca 60gaggtattgg ttatcgttcg ttcttagtct
caatcaatcg tacaccttgc agttgcccga 120gtttctccac acatacagca
cctcccgctc ccagcccatt cgagcgaccc aatccgggcg 180atcccagcga
tcgtcgtcgc ttcagtgctg accggtggaa agcaggagat ctcgggcgag
240caggaccaca tccagcccag gatcttcgac tggctcagag ctgaccctca
cgcggcacag 300caaaagtagc acgcacgcgt tatgcaaact ggttacaacc
tgtccaacag tgttgcgacg 360ttgactggct acattgtctg tctgtcgcga
gtgcgcctgg gcccttacgg tgggacactg 420gaactccgcc ccgagtcgaa
cacctagggc gacgcccgca gcttggcatg acagctctcc 480ttgtgttcta
aataccttgc gcgtgtggga ga 512137516DNAArtificial SequenceDescription
of Artificial Sequence Synthetic polynucleotide 137atccaccgat
cactccgtcg ccgcccaaga gaattcaacc tcgatggagg gcaaggtgga 60tcagaggtat
tggttatcgt tcgctattag tctcaatcaa tcgtgcacct tgcagttgct
120cgagtttctc cacacataca gcacctcccg ctcccagccc attcgagcga
cccaatccgg 180gcgatcccag cgatcgtcgt cgcttcagtg ctgaccggtg
gaaagcagga gatctcgggc 240gagcaggacc acatccagca caggatcttc
gactggctca gagctgaccc tcacgcggca 300cagcaaaagt agcccgcacg
cgttatgcaa acaggttaca acctgtccaa cactgttgcg 360acgttgactg
gctacattgt ctgtctgtcg cgagtacgcc tggaccctta cggtgggaca
420ctggaactcc gccccgagtc gaacacctag ggcgacgccc gcagcttggc
atgacagctc 480tccttgtatt ctaaatacct cgcgcgtgtg ggagaa
516138335DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 138atgatgcgcg tgtacgacta tcaaggaaga
aagaggactt aatttcttac cttctaacca 60ccatattctt tttgctggat gcttgctcgt
ctcgatgaca attgtgaacc tcttgtgtga 120ccctgaccct gctgcaaggc
tctccgaccg cacgcaaggc gcagccggcg cgtccggagg 180cgatcggatc
caatccagtc gtcctcccgc agcccgggca cgtttgccca tgcaggccct
240tccacaccgc tcaagagact cccgaacacc gcccactcgg cactcgcttc
ggctgccgag 300tgcgcgtttg agtttgccct gccacagaag acacc
335139335DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 139atgatgcgcg tgtacgacta tcaaggaaga
aagaggactt aatttcttac cttctaacca 60ccatattctt tttgctggat gcttgctcgt
ctcgatgaca attgtgaacc tcttgtgtga 120ccctgaccct gctgcaaggc
tctccgaccg cacgcaaggc gcagccggcg cgtccggagg 180cgatcggatc
caatccagtc gtcctcccgc agcccgggca cgtttgccca tgcaggccct
240tccacaccgc tcaagagact cccgaacacc gcccactcgg cactcgcttc
ggctgccgag 300tgcgcgtttg agtttgccct gccacaggag acatc
3351401097DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 140cccgggcgag ctgtacgcct acggagcgag
gcctggtgtg accgttgcga tctcgccagc 60agacgtcgcg gagcctcgtc ccaaaggccc
tttctgatcg agcttgtcgt ccactggacg 120ctttaagttg cgcgcgcgat
gggataaccg agctgatctg cactcagatt ttggtttgtt 180ttcgcgcatg
gtgcagcgag gggaggtact acgctggggt acgagatcct ccggattccc
240agaccgtgtt gccggcattt acccggtcat cgccagcgat tcgggacgac
aaggccttat 300cctgtgctga gacgctcgag cacgtttata aaattgtggg
taccgcggta tgcacagcgt 360tcaacacgcg ccacgccgaa attggttggt
gggggagcac gtatgggact gacgtatggc 420cagcagcgaa cactcaccga
acaagtgcca atgtatacct tgcatcaatg atgctccggc 480agcttcgatt
gactgtctcg aaaaagtgtg agcaagcaga tcatgtggcc gctctgtcgc
540gcagcacctg acgcattcga cacccacggc aatgcccagg ccagggaata
gagagtaaga 600caactcccat tgttcagcaa aacattgcac tgcagtgcct
tcacaactat acaatgaatg 660ggagggaata tgggctctgc atgggacagc
ttagctggga cattcggcta ctgaacaaga 720aaaccccacg agaaccaatt
ggcgaaacct gccgggagga ggtgatcgtt tctgtaaatg 780gcttacgcat
tcccccccgg cggctcacga ggggtgtggt gaaccctgcc agctgatcaa
840gtgcttgctg acgtcggcca gggaggtgta tgtgattggg ccgtggggcg
tgagttatcc 900taccgccgga cccgcgaagt cacatgacga atggccgtgc
gggatgacga gagcacgact 960cgctctttct tcgccggccc ggcttcatgg
aggacaataa taaagggtgg ccaccggcaa 1020cagccctcca tacctgaacc
gattccagac ccaaacctct tgaattttga gggatccagt 1080tcaccggtat agtcacg
10971411105DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 141atccccgggc gagctgtacg cctacggagc
gaggcctggt gtgaccgttg cgatctcgcc 60agcagacgtc gcggagcctc gtcccaaagg
ccctttctga tcgagcttgt cgtccactgg 120acgctttaag ttgcgcgcgc
gatgggataa ccgagctgat ctgcactcag attttggttt 180gttttcgcgc
atggtgcagc gaggggaggt actacgctgg ggtacgagat cctccggatt
240cccagaccgt gttgccggca tttacccggt catcgccagc gattcgggac
gacaaggcct 300tatcctgtgc tgagacgctc gagcacgttt ataaaattgt
ggtcaccgtg gtacgcacag 360cgtccaacac gcgccacgcc gaaattcgtt
ggtgggggag cacgtatcgg actgacgtat 420ggccagcagc gaacactcac
caaacaggtg ccaatgtata gcttgcatca atgatgctct 480ggcagcttcg
attgactgtc tcgaaaaagt gtgtgcaaac agattatgtg gccgctctgt
540ggccgcgcag cacctgacgc actcgacacc cacggcaatg cccaggccaa
ggaacagaga 600gtaagacaac tcccattgtt cagtaaaaca ttgcactgca
gtgccttcac aaacatacaa 660cgaatgggag ggaatatggg cttcgaatgg
gacagcttag ctgggacatt cggttactga 720acaagaaaac cccacgagaa
ccaactggcg aaacctgccg ggaggaggtg atcgtttttg 780taaatggctt
acgcattccc cccccggcgg ctcacggggg gtgtggtgaa ccctgccagc
840tgatcaagtg cttgctgacg tcggccaggg aggtgtatgt gatttggccg
tggggcgtga 900gttatcctac cgccggaccc gcgaagtcac atgacgaatg
gccgtgcggg atgacgagag 960cagggctcgc tctttcttcg ccggcccggc
ttcatggagg acaataataa agggtggcca 1020ccggcaacag ccctccatac
ctgaaccgat tccagaccca aacctcttga attttgaggg 1080atccagttca
ccggtatagt cacga 1105142754DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 142gcgagtggtt
ttgctgccgg gaagggagtg gggagcgtcg agcgagggac gcggcgctcg 60aggcgcacgt
cgtctgtcaa cgcgcgcggc cctcgcggcc cgcggcccca cccagctcta
120atcatcgaaa actaagaggc tccacacgcc tgtcgtagaa tgcatgggat
tcgccagtag 180accacgatct gcgccgaaga agctggtcta cccgacgttt
tttgttgctc ctttattctg 240aatgatatga agatagtgtg cgcagtgcca
cgcataggca tcaggagcaa gggaggacgg 300gtcaacttga aagaaccaaa
ccatccatcc gagaaatgcg catcatcttt gtagtaccat 360caaacgcctt
ggccaatgtc ttctgcatgg acaacacaac ctgctcctgg ccacacggtc
420gacttggagc gccccatgcg cccaggtcgc cacgacccgc ggcccagcgc
gcggcgattc 480gcctcacgag atcccggcgg acccggcacg cccgcgggcc
gacggtgcgc ttggcgatgc 540tgctcattaa cccacggccg tcacccgatc
cacatgctct ttttcaacac atccacattg 600gaatagagct ctaccagggt
gagtactgca ttctttgggg ctgggaggac cccactcgac 660acctggtcct
tcatcggccg aaagcccgaa cctgagcgct tccccgcccc gttcctcatc
720cccgactttc cgatggccca ttgcagtttc aaac 754143318DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
143atctgggtgg aggactggga gtaagatgta aggatattaa ttaaacattc
tagtttgttg 60atggcacaac agtcaatgca tttcagtcgt cttgctcctt ataacctatg
cgtgtgccat 120cgccggccat gcacctgtgg cgtggtaccg accatcgggg
agaggcccga gattcggagg 180tacctcccgc cctgggcgag cccttcacgt
gacggcacaa gtcccttgca tcggcccgcg 240agcacggaat acagagcccc
gtgcccccca cgggccctca catcatccac tccattgttc 300ttgccacacc gatcagca
318144316DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 144tgggtggagg actgggaaga agatgtaagg
atatcaattt aacattctag tttgttgatg 60gcacaacagt cactgaatac cgggcgtctg
gctgctaaaa tagccggagc gtgtgccatc 120gccggccatg catctgtggc
gtggtaccga ccatcaggga gaggcccgag attcggaggt 180acctcccgcc
ctgggcgagc ccttcacgtg
acggcacaag tcccttgcat cggcccgcga 240gcacggaata cagagccccg
tgctccccac gggccctcac atcatccact ccattgttct 300tgccacaccg atcagc
316145350DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 145ataacgaggc acaatgatcg atatttctat
cgaacaactg tatttagccc tgtacgtacc 60ccgctcttgg gccagcccgt ccgtgcttgc
cttcggaaaa ttgcatggcg cctcatgcaa 120actcgcgctc tcacagcaga
tctcgcccag ctcccgggag agcaatcgcg ggtggggccc 180ggggcgaatc
caggacgcgc cccgcggggc cgctccactc gccagggcca atgggcggct
240tatagtcctg gcatgggctc tgcatgcaca gtatcgcagt ttgggcgagg
tgttgccccc 300gcgatttcga atacgcgacg cccggtactc gtgcgagaac
agggttcttg 350146818DNAArtificial SequenceDescription of Artificial
Sequence Synthetic polynucleotide 146atcgcgatgg tgcgcactcg
tgcgcaatga atatggggtc acgcggtgga cgaacgcgga 60gggggcctgg ccgaatctag
gcttgcattc ctcagatcac tttctgccgg cggtccgggg 120tttgcgcgtc
gcgcaacgct ccgtctccct agccgctgcg caccgcgcgt gcgacgcgaa
180ggtcattttc cagaacaacg accatggctt gtcttagcga tcgctcgaat
gactgctagt 240gagtcgtacg ctcgacccag tcgctcgcag gagaacgcgg
caactgccga gcttcggctt 300gccagtcgtg actcgtatgt gatcaggaat
cattggcatt ggtagcatta taattcggct 360tccgcgctgt ttatgggcat
ggcaatgtct catgcagtcg accttagtca accaattctg 420ggtggccagc
tccgggcgac cgggctccgt gtcgccgggc accacctcct gccatgagta
480acagggccgc cctctcctcc cgacgttggc ccactgaata ccgtgtcttg
gggccctaca 540tgatgggctg cctagtcggg cgggacgcgc aactgcccgc
gcaatctggg acgtggtctg 600aatcctccag gcgggtttcc ccgagaaaga
aagggtgccg atttcaaagc agagccatgt 660gccgggccct gtggcctgtg
ttggcgccta tgtagtcacc ccccctcacc caattgtcgc 720cagtttgcgc
aatccataaa ctcaaaactg cagcttctga gctgcgctgt tcaagaacac
780ctctggggtt tgctcacccg cgaggtcgac gcccagca 818147819DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
147atcacgatgg tgcgcattcg tgcaaagtga atatggggtc acgcggtgga
cgaacgcgga 60gggggcatga ccgaatctag gctcgcattc ctcagatcac ttcatgccgg
cggtccgggg 120tttgcgcgtc gcgcaaggct acgtctccct agccgctgcg
caccacgcgt gcgacgcgga 180ggccatcttc cggagcaacg accatggatt
gtcttagcga tcgcacgaat gagtgctagt 240gagtcgtacg ctcgacccag
tcgctcgcag gagaaggcgg cagctgccga gcttcggctt 300accagtcgtg
actcgtatgt gatcaggaat cattggcatt ggtagcatta taattcggct
360tccgcgctgc gtatgggcat ggcaatgtct catgcagtcg atcttagtca
accaattttg 420ggtggccagg tccgggcgac cgggctccgt gtcgccgggc
accacctcct gccaggagta 480gcagggccgc cctctcgtcc cgacgttggc
ccactgaata ccgtggcttc gagccctaca 540tgatgggctg cctagtcggg
cgggacgcgc aactgcccgc gcgatctggg ggctggtctg 600aatccttcag
gcgggtgtta cccgagaaag aaagggtgcc gatttcaaag cagacccatg
660tgccgggccc tgtggcctgt gttggcgcct atgtagtcac cccccctcac
ccaattgtcg 720ccagtttgcg cactccataa actcaaaaca gcagcttctg
agctgcgctg ttcaagaaca 780cctctggggt ttgctcaccc gcgaggtcga cgcccagca
8191485104DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 148gaagagcgcc caatgtttaa acgccggtca
ccacccgcat gctcgtacta cagcgcacgc 60accgcttcgt gatccaccgg gtgaacgtag
tcctcgacgg aaacatctgg ttcgggcctc 120ctgcttgcac tcccgcccat
gccgacaacc tttctgctgt taccacgacc cacaatgcaa 180cgcgacacga
ccgtgtggga ctgatcggtt cactgcacct gcatgcaatt gtcacaagcg
240cttactccaa ttgtattcgt ttgttttctg ggagcagttg ctcgaccgcc
cgcgtcccgc 300aggcagcgat gacgtgtgcg tggcctgggt gtttcgtcga
aaggccagca accctaaatc 360gcaggcgatc cggagattgg gatctgatcc
gagtttggac cagatccgcc ccgatgcggc 420acgggaactg catcgactcg
gcgcggaacc cagctttcgt aaatgccaga ttggtgtccg 480atacctggat
ttgccatcag cgaaacaaga cttcagcagc gagcgtattt ggcgggcgtg
540ctaccagggt tgcatacatt gcccatttct gtctggaccg ctttactggc
gcagagggtg 600agttgatggg gttggcaggc atcgaaacgc gcgtgcatgg
tgtgcgtgtc tgttttcggc 660tgcacgaatt caatagtcgg atgggcgacg
gtagaattgg gtgtggcgct cgcgtgcatg 720cctcgccccg tcgggtgtca
tgaccgggac tggaatcccc cctcgcgacc atcttgctaa 780cgctcccgac
tctcccgacc gcgcgcagga tagactcttg ttcaaccaat cgacaggtac
840cagtttaggt ccagcgtccg tgggggggga cgggctggga gcttgggccg
ggaagggcaa 900gacgatgcag tccctctggg gagtcacagc cgactgtgtg
tgttgcactg tgcggcccgc 960agcactcaca cgcaaaatgc ctggccgaca
ggcaggccct gtccagtgca acatccacgg 1020tccctctcat caggctcacc
ttgctcattg acataacgga atgcgtaccg ctctttcaga 1080tctgtccatc
cagagagggg agcaggctcc ccaccgacgc tgtcaaactt gcttcctgcc
1140caaccgaaaa cattattgtt tgaggggggg gggggggggg cagattgcat
ggcgggatat 1200ctcgtgagga acatcactgg gacactgtgg aacacagtga
gtgcagtatg cagagcatgt 1260atgctagggg tcagcgcagg aagggggcct
ttcccagtct cccatgccac tgcaccgtat 1320ccacgactca ccaggaccag
cttcttgatc ggcttccgct cccgtggaca ccagtgtgta 1380gcctctggac
tccaggtatg cgtgcaccgc aaaggccagc cgatcgtgcc gattcctggg
1440tggaggatat gagtcagcca acttggggct cagagtgcac actggggcac
gatacgaaac 1500aacatctaca ccgtgtcctc catgctgaca caccacagct
tcgctccacc tgaatgtggg 1560cgcatgggcc cgaatcacag ccaatgtcgc
tgctgccata atgtgatcca gaccctctcc 1620gcccagatgc cgagcggatc
gtgggcgctg aatagattcc tgtttcgatc actgtttggg 1680tcctttcctt
ttcgtctcgg atgcgcgtct cgaaacaggc tgcgtcgggc tttcggatcc
1740cttttgctcc ctccgtcacc atcctgcgcg cgggcaagtt gcttgaccct
gggctgatac 1800cagggttgga gggtattacc gcgtcaggcc attcccagcc
cggattcaat tcaaagtctg 1860ggccaccacc ctccgccgct ctgtctgatc
actccacatt cgtgcataca ctacgttcaa 1920gtcctgatcc aggcgtgtct
cgggacaagg tgtgcttgag tttgaatctc aaggacccac 1980tccagcacag
ctgctggttg accccgccct cgcaatctag aatggccgcg tccgtccact
2040gcaccctgat gtccgtggtc tgcaacaaca agaaccactc cgcccgcccc
aagctgccca 2100actcctccct gctgcccggc ttcgacgtgg tggtccaggc
cgcggccacc cgcttcaaga 2160aggagacgac gaccacccgc gccacgctga
cgttcgaccc ccccacgacc aactccgagc 2220gcgccaagca gcgcaagcac
accatcgacc cctcctcccc cgacttccag cccatcccct 2280ccttcgagga
gtgcttcccc aagtccacga aggagcacaa ggaggtggtg cacgaggagt
2340ccggccacgt cctgaaggtg cccttccgcc gcgtgcacct gtccggcggc
gagcccgcct 2400tcgacaacta cgacacgtcc ggcccccaga acgtcaacgc
ccacatcggc ctggcgaagc 2460tgcgcaagga gtggatcgac cgccgcgaga
agctgggcac gccccgctac acgcagatgt 2520actacgcgaa gcagggcatc
atcacggagg agatgctgta ctgcgcgacg cgcgagaagc 2580tggaccccga
gttcgtccgc tccgaggtcg cgcggggccg cgccatcatc ccctccaaca
2640agaagcacct ggagctggag cccatgatcg tgggccgcaa gttcctggtg
aaggtgaacg 2700cgaacatcgg caactccgcc gtggcctcct ccatcgagga
ggaggtctac aaggtgcagt 2760gggccaccat gtggggcgcc gacaccatca
tggacctgtc cacgggccgc cacatccacg 2820agacgcgcga gtggatcctg
cgcaactccg cggtccccgt gggcaccgtc cccatctacc 2880aggcgctgga
gaaggtggac ggcatcgcgg agaacctgaa ctgggaggtg ttccgcgaga
2940cgctgatcga gcaggccgag cagggcgtgg actacttcac gatccacgcg
ggcgtgctgc 3000tgcgctacat ccccctgacc gccaagcgcc tgacgggcat
cgtgtcccgc ggcggctcca 3060tccacgcgaa gtggtgcctg gcctaccaca
aggagaactt cgcctacgag cactgggacg 3120acatcctgga catctgcaac
cagtacgacg tcgccctgtc catcggcgac ggcctgcgcc 3180ccggctccat
ctacgacgcc aacgacacgg cccagttcgc cgagctgctg acccagggcg
3240agctgacgcg ccgcgcgtgg gagaaggacg tgcaggtgat gaacgagggc
cccggccacg 3300tgcccatgca caagatcccc gagaacatgc agaagcagct
ggagtggtgc aacgaggcgc 3360ccttctacac cctgggcccc ctgacgaccg
acatcgcgcc cggctacgac cacatcacct 3420ccgccatcgg cgcggccaac
atcggcgccc tgggcaccgc cctgctgtgc tacgtgacgc 3480ccaaggagca
cctgggcctg cccaaccgcg acgacgtgaa ggcgggcgtc atcgcctaca
3540agatcgccgc ccacgcggcc gacctggcca agcagcaccc ccacgcccag
gcgtgggacg 3600acgcgctgtc caaggcgcgc ttcgagttcc gctggatgga
ccagttcgcg ctgtccctgg 3660accccatgac ggcgatgtcc ttccacgacg
agacgctgcc cgcggacggc gcgaaggtcg 3720cccacttctg ctccatgtgc
ggccccaagt tctgctccat gaagatcacg gaggacatcc 3780gcaagtacgc
cgaggagaac ggctacggct ccgccgagga ggccatccgc cagggcatgg
3840acgccatgtc cgaggagttc aacatcgcca agaagacgat ctccggcgag
cagcacggcg 3900aggtcggcgg cgagatctac ctgcccgagt cctacgtcaa
ggccgcgcag aagtgacaat 3960tggcagcagc agctcggata gtatcgacac
actctggacg ctggtcgtgt gatggactgt 4020tgccgccaca cttgctgcct
tgacctgtga atatccctgc cgcttttatc aaacagcctc 4080agtgtgtttg
atcttgtgtg tacgcgcttt tgcgagttgc tagctgcttg tgctatttgc
4140gaataccacc cccagcatcc ccttccctcg tttcatatcg cttgcatccc
aaccgcaact 4200tatctacgct gtcctgctat ccctcagcgc tgctcctgct
cctgctcact gcccctcgca 4260cagccttggt ttgggctccg cctgtattct
cctggtactg caacctgtaa accagcactg 4320caatgctgat gcacgggaag
tagtgggatg ggaacacaaa tggaggatcg tagagctcta 4380gggagcgacg
agtgtgcgtg cggggctggc gggagtggga cgccctcctc gctcctctct
4440gttctgaacg gaacaatcgg ccaccccgcg ctacgcgcca cgcatcgagc
aacgaagaaa 4500accccccgat gataggttgc ggtggctgcc gggatataga
tccggccgca catcaaaggg 4560cccctccgcc agagaagaag ctcctttccc
agcagactcc ttctgctgcc aaaacacttc 4620tctgtccaca gcaacaccaa
aggatgaaca gatcaacttg cgtctccgcg tagcttcctc 4680ggctagcgtg
cttgcaacag gtccctgcac tattatcttc ctgctttcct ctgaattatg
4740cggcaggcga gcgctcgctc tggcgagcgc tccttcgcgc cgccctcgct
gatcgagtgt 4800acagtcaatg aatggtcctg ggcgaagaac gagggaattt
gtgggtaaaa caagcatcgt 4860ctctcaggcc ccggcgcagt ggccgttaaa
gtccaagacc gtgaccaggc agcgcagcgc 4920gtccgtgtgc gggccctgcc
tggcggctcg gcgtgccagg ctcgagagca gctccctcag 4980gtcgccttgg
acggcctctg cgaggccggt gagggcctgc aggagcgcct cgagcgtggc
5040agtggcggtc gtatccgggt cgccggtcac cgcctgcgac tcgccatccg
aagagcgttt 5100aaac 51041495110DNAArtificial SequenceDescription of
Artificial Sequence Synthetic polynucleotide 149gtttaaacgc
cggtcaccac ccgcatgctc gtactacagc gcacgcaccg cttcgtgatc 60caccgggtga
acgtagtcct cgacggaaac atctggttcg ggcctcctgc ttgcactccc
120gcccatgccg acaacctttc tgctgttacc acgacccaca atgcaacgcg
acacgaccgt 180gtgggactga tcggttcact gcacctgcat gcaattgtca
caagcgctta ctccaattgt 240attcgtttgt tttctgggag cagttgctcg
accgcccgcg tcccgcaggc agcgatgacg 300tgtgcgtggc ctgggtgttt
cgtcgaaagg ccagcaaccc taaatcgcag gcgatccgga 360gattgggatc
tgatccgagt ttggaccaga tccgccccga tgcggcacgg gaactgcatc
420gactcggcgc ggaacccagc tttcgtaaat gccagattgg tgtccgatac
ctggatttgc 480catcagcgaa acaagacttc agcagcgagc gtatttggcg
ggcgtgctac cagggttgca 540tacattgccc atttctgtct ggaccgcttt
actggcgcag agggtgagtt gatggggttg 600gcaggcatcg aaacgcgcgt
gcatggtgtg cgtgtctgtt ttcggctgca cgaattcaat 660agtcggatgg
gcgacggtag aattgggtgt ggcgctcgcg tgcatgcctc gccccgtcgg
720gtgtcatgac cgggactgga atcccccctc gcgaccatct tgctaacgct
cccgactctc 780ccgaccgcgc gcaggataga ctcttgttca accaatcgac
aggtaccagt ttaggtccag 840cgtccgtggg gggggacggg ctgggagctt
gggccgggaa gggcaagacg atgcagtccc 900tctggggagt cacagccgac
tgtgtgtgtt gcactgtgcg gcccgcagca ctcacacgca 960aaatgcctgg
ccgacaggca ggccctgtcc agtgcaacat ccacggtccc tctcatcagg
1020ctcaccttgc tcattgacat aacggaatgc gtaccgctct ttcagatctg
tccatccaga 1080gaggggagca ggctccccac cgacgctgtc aaacttgctt
cctgcccaac cgaaaacatt 1140attgtttgag gggggggggg ggggggcaga
ttgcatggcg ggatatctcg tgaggaacat 1200cactgggaca ctgtggaaca
cagtgagtgc agtatgcaga gcatgtatgc taggggtcag 1260cgcaggaagg
gggcctttcc cagtctccca tgccactgca ccgtatccac gactcaccag
1320gaccagcttc ttgatcggct tccgctcccg tggacaccag tgtgtagcct
ctggactcca 1380ggtatgcgtg caccgcaaag gccagccgat cgtgccgatt
cctgggtgga ggatatgagt 1440cagccaactt ggggctcaga gtgcacactg
gggcacgata cgaaacaaca tctacaccgt 1500gtcctccatg ctgacacacc
acagcttcgc tccacctgaa tgtgggcgca tgggcccgaa 1560tcacagccaa
tgtcgctgct gccataatgt gatccagacc ctctccgccc agatgccgag
1620cggatcgtgg gcgctgaata gattcctgtt tcgatcactg tttgggtcct
ttccttttcg 1680tctcggatgc gcgtctcgaa acaggctgcg tcgggctttc
ggatcccttt tgctccctcc 1740gtcaccatcc tgcgcgcggg caagttgctt
gaccctgggc tgataccagg gttggagggt 1800attaccgcgt caggccattc
ccagcccgga ttcaattcaa agtctgggcc accaccctcc 1860gccgctctgt
ctgatcactc cacattcgtg catacactac gttcaagtcc tgatccaggc
1920gtgtctcggg acaaggtgtg cttgagtttg aatctcaagg acccactcca
gcacagctgc 1980tggttgaccc cgccctcgca atctagaatg gccgcgtccg
tccactgcac cctgatgtcc 2040gtggtctgca acaacaagaa ccactccgcc
cgccccaagc tgcccaactc ctccctgctg 2100cccggcttcg acgtggtggt
ccaggccgcg gccacccgct tcaagaagga gacgacgacc 2160acccgcgcca
cgctgacgtt cgaccccccc acgaccaact ccgagcgcgc caagcagcgc
2220aagcacacca tcgacccctc ctcccccgac ttccagccca tcccctcctt
cgaggagtgc 2280ttccccaagt ccacgaagga gcacaaggag gtggtgcacg
aggagtccgg ccacgtcctg 2340aaggtgccct tccgccgcgt gcacctgtcc
ggcggcgagc ccgccttcga caactacgac 2400acgtccggcc cccagaacgt
caacgcccac atcggcctgg cgaagctgcg caaggagtgg 2460atcgaccgcc
gcgagaagct gggcacgccc cgctacacgc agatgtacta cgcgaagcag
2520ggcatcatca cggaggagat gctgtactgc gcgacgcgcg agaagctgga
ccccgagttc 2580gtccgctccg aggtcgcgcg gggccgcgcc atcatcccct
ccaacaagaa gcacctggag 2640ctggagccca tgatcgtggg ccgcaagttc
ctggtgaagg tgaacgcgaa catcggcaac 2700tccgccgtgg cctcctccat
cgaggaggag gtctacaagg tgcagtgggc caccatgtgg 2760ggcgccgaca
ccatcatgga cctgtccacg ggccgccaca tccacgagac gcgcgagtgg
2820atcctgcgca actccgcggt ccccgtgggc accgtcccca tctaccaggc
gctggagaag 2880gtggacggca tcgcggagaa cctgaactgg gaggtgttcc
gcgagacgct gatcgagcag 2940gccgagcagg gcgtggacta cttcacgatc
cacgcgggcg tgctgctgcg ctacatcccc 3000ctgaccgcca agcgcctgac
gggcatcgtg tcccgcggcg gctccatcca cgcgaagtgg 3060tgcctggcct
accacaagga gaacttcgcc tacgagcact gggacgacat cctggacatc
3120tgcaaccagt acgacgtcgc cctgtccatc ggcgacggcc tgcgccccgg
ctccatctac 3180gacgccaacg acacggccca gttcgccgag ctgctgaccc
agggcgagct gacgcgccgc 3240gcgtgggaga aggacgtgca ggtgatgaac
gagggccccg gccacgtgcc catgcacaag 3300atccccgaga acatgcagaa
gcagctggag tggtgcaacg aggcgccctt ctacaccctg 3360ggccccctga
cgaccgacat cgcgcccggc tacgaccaca tcacctccgc catcggcgcg
3420gccaacatcg gcgccctggg caccgccctg ctgtgctacg tgacgcccaa
ggagcacctg 3480ggcctgccca accgcgacga cgtgaaggcg ggcgtcatcg
cctacaagat cgccgcccac 3540gcggccgacc tggccaagca gcacccccac
gcccaggcgt gggacgacgc gctgtccaag 3600gcgcgcttcg agttccgctg
gatggaccag ttcgcgctgt ccctggaccc catgacggcg 3660atgtccttcc
acgacgagac gctgcccgcg gacggcgcga aggtcgccca cttctgctcc
3720atgtgcggcc ccaagttctg ctccatgaag atcacggagg acatccgcaa
gtacgccgag 3780gagaacggct acggctccgc cgaggaggcc atccgccagg
gcatggacgc catgtccgag 3840gagttcaaca tcgccaagaa gacgatctcc
ggcgagcagc acggcgaggt cggcggcgag 3900atctacctgc ccgagtccta
cgtcaaggcc gcgcagaagt gacaattggc agcagcagct 3960cggatagtat
cgacacactc tggacgctgg tcgtgtgatg gactgttgcc gccacacttg
4020ctgccttgac ctgtgaatat ccctgccgct tttatcaaac agcctcagtg
tgtttgatct 4080tgtgtgtacg cgcttttgcg agttgctagc tgcttgtgct
atttgcgaat accaccccca 4140gcatcccctt ccctcgtttc atatcgcttg
catcccaacc gcaacttatc tacgctgtcc 4200tgctatccct cagcgctgct
cctgctcctg ctcactgccc ctcgcacagc cttggtttgg 4260gctccgcctg
tattctcctg gtactgcaac ctgtaaacca gcactgcaat gctgatgcac
4320gggaagtagt gggatgggaa cacaaatgga ggatcgtaga gctccagcca
cggcaacacc 4380gcgcgccttg cggccgagca cggcgacaag aacctgagca
agatctgcgg gctgatcgcc 4440agcgacgagg gccggcacga gatcgcctac
acgcgcatcg tggacgagtt cttccgcctc 4500gaccccgagg gcgccgtcgc
cgcctacgcc aacatgatgc gcaagcagat caccatgccc 4560gcgcacctca
tggacgacat gggccacggc gaggccaacc cgggccgcaa cctcttcgcc
4620gacttctccg cggtcgccga gaagatcgac gtctacgacg ccgaggacta
ctgccgcatc 4680ctggagcacc tcaacgcgcg ctggaaggtg gacgagcgcc
aggtcagcgg ccaggccgcc 4740gcggaccagg agtacgtcct gggcctgccc
cagcgcttcc ggaaactcgc cgagaagacc 4800gccgccaagc gcaagcgcgt
cgcgcgcagg cccgtcgcct tctcctggat ctccgggcgc 4860gagatcatgg
tctagggagc gacgagtgtg cgtgcggggc tggcgggagt gggacgccct
4920cctcgctcct ctctgttctg aacggaacaa tcggccaccc cgcgctacgc
gccacgcatc 4980gagcaacgaa gaaaaccccc cgatgatagg ttgcggtggc
tgccgggata tagatccggc 5040cgcacatcaa agggcccctc cgccagagaa
gaagctcctt tcccagcaga ctcctgaaga 5100gcgtttaaac
51101505129DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 150gaagagcgcc caatgtttaa acgccggtca
ccatccgcat gctcatatta cagcgcacgc 60accgcttcgt gatccaccgg gtgaacgtag
tcctcgacgg aaacatctgg ctcgggcctc 120gtgctggcac tccctcccat
gccgacaacc tttctgctgt caccacgacc cacgatgcaa 180cgcgacacga
cccggtggga ctgatcggtt cactgcacct gcatgcaatt gtcacaagcg
240catactccaa tcgtatccgt ttgatttctg tgaaaactcg ctcgaccgcc
cgcgtcccgc 300aggcagcgat gacgtgtgcg tgacctgggt gtttcgtcga
aaggccagca accccaaatc 360gcaggcgatc cggagattgg gatctgatcc
gagcttggac cagatccccc acgatgcggc 420acgggaactg catcgactcg
gcgcggaacc cagctttcgt aaatgccaga ttggtgtccg 480ataccttgat
ttgccatcag cgaaacaaga cttcagcagc gagcgtattt ggcgggcgtg
540ctaccagggt tgcatacatt gcccatttct gtctggaccg ctttaccggc
gcagagggtg 600agttgatggg gttggcaggc atcgaaacgc gcgtgcatgg
tgtgtgtgtc tgttttcggc 660tgcacaattt caatagtcgg atgggcgacg
gtagaattgg gtgttgcgct cgcgtgcatg 720cctcgccccg tcgggtgtca
tgaccgggac tggaatcccc cctcgcgacc ctcctgctaa 780cgctcccgac
tctcccgccc gcgcgcagga tagactctag ttcaaccaat cgacaggtac
840cagtttaggt ccagcgtccg tgggggggga cgggctggga gcttgggccg
ggaagggcaa 900gacgatgcag tccctctggg gagtcacagc cgactgtgtg
tgttgcactg tgcggcccgc 960agcactcaca cgcaaaatgc ctggccgaca
ggcaggccct gtccagtgca acatccacgg 1020tccctctcat caggctcacc
ttgctcattg acataacgga atgcgtaccg ctctttcaga 1080tctgtccatc
cagagagggg agcaggctcc ccaccgacgc tgtcaaactt gcttcctgcc
1140caaccgaaaa cattattgtt tgaggggggg gggggggggg cagattgcat
ggcgggatat 1200ctcgtgagga acatcactgg gacactgtgg aacacagtga
gtgcagtatg cagagcatgt 1260atgctagggg tcagcgcagg aagggggcct
ttcccagtct cccatgccac tgcaccgtat 1320ccacgactca ccaggaccag
cttcttgatc ggcttccgct cccgtggaca ccagtgtgta 1380gcctctggac
tccaggtatg cgtgcaccgc aaaggccagc cgatcgtgcc gattcctggg
1440tggaggatat gagtcagcca acttggggct cagagtgcac actggggcac
gatacgaaac 1500aacatctaca ccgtgtcctc catgctgaca caccacagct
tcgctccacc tgaatgtggg 1560cgcatgggcc cgaatcacag ccaatgtcgc
tgctgccata atgtgatcca gaccctctcc 1620gcccagatgc cgagcggatc
gtgggcgctg aatagattcc tgtttcgatc actgtttggg 1680tcctttcctt
ttcgtctcgg atgcgcgtct cgaaacaggc tgcgtcgggc tttcggatcc
1740cttttgctcc ctccgtcacc atcctgcgcg cgggcaagtt gcttgaccct
gggctgatac 1800cagggttgga gggtattacc gcgtcaggcc attcccagcc
cggattcaat tcaaagtctg 1860ggccaccacc ctccgccgct ctgtctgatc
actccacatt cgtgcataca ctacgttcaa 1920gtcctgatcc aggcgtgtct
cgggacaagg tgtgcttgag tttgaatctc aaggacccac 1980tccagcacag
ctgctggttg accccgccct
cgcaatctag aatggccgcg tccgtccact 2040gcaccctgat gtccgtggtc
tgcaacaaca agaaccactc cgcccgcccc aagctgccca 2100actcctccct
gctgcccggc ttcgacgtgg tggtccaggc cgcggccacc cgcttcaaga
2160aggagacgac gaccacccgc gccacgctga cgttcgaccc ccccacgacc
aactccgagc 2220gcgccaagca gcgcaagcac accatcgacc cctcctcccc
cgacttccag cccatcccct 2280ccttcgagga gtgcttcccc aagtccacga
aggagcacaa ggaggtggtg cacgaggagt 2340ccggccacgt cctgaaggtg
cccttccgcc gcgtgcacct gtccggcggc gagcccgcct 2400tcgacaacta
cgacacgtcc ggcccccaga acgtcaacgc ccacatcggc ctggcgaagc
2460tgcgcaagga gtggatcgac cgccgcgaga agctgggcac gccccgctac
acgcagatgt 2520actacgcgaa gcagggcatc atcacggagg agatgctgta
ctgcgcgacg cgcgagaagc 2580tggaccccga gttcgtccgc tccgaggtcg
cgcggggccg cgccatcatc ccctccaaca 2640agaagcacct ggagctggag
cccatgatcg tgggccgcaa gttcctggtg aaggtgaacg 2700cgaacatcgg
caactccgcc gtggcctcct ccatcgagga ggaggtctac aaggtgcagt
2760gggccaccat gtggggcgcc gacaccatca tggacctgtc cacgggccgc
cacatccacg 2820agacgcgcga gtggatcctg cgcaactccg cggtccccgt
gggcaccgtc cccatctacc 2880aggcgctgga gaaggtggac ggcatcgcgg
agaacctgaa ctgggaggtg ttccgcgaga 2940cgctgatcga gcaggccgag
cagggcgtgg actacttcac gatccacgcg ggcgtgctgc 3000tgcgctacat
ccccctgacc gccaagcgcc tgacgggcat cgtgtcccgc ggcggctcca
3060tccacgcgaa gtggtgcctg gcctaccaca aggagaactt cgcctacgag
cactgggacg 3120acatcctgga catctgcaac cagtacgacg tcgccctgtc
catcggcgac ggcctgcgcc 3180ccggctccat ctacgacgcc aacgacacgg
cccagttcgc cgagctgctg acccagggcg 3240agctgacgcg ccgcgcgtgg
gagaaggacg tgcaggtgat gaacgagggc cccggccacg 3300tgcccatgca
caagatcccc gagaacatgc agaagcagct ggagtggtgc aacgaggcgc
3360ccttctacac cctgggcccc ctgacgaccg acatcgcgcc cggctacgac
cacatcacct 3420ccgccatcgg cgcggccaac atcggcgccc tgggcaccgc
cctgctgtgc tacgtgacgc 3480ccaaggagca cctgggcctg cccaaccgcg
acgacgtgaa ggcgggcgtc atcgcctaca 3540agatcgccgc ccacgcggcc
gacctggcca agcagcaccc ccacgcccag gcgtgggacg 3600acgcgctgtc
caaggcgcgc ttcgagttcc gctggatgga ccagttcgcg ctgtccctgg
3660accccatgac ggcgatgtcc ttccacgacg agacgctgcc cgcggacggc
gcgaaggtcg 3720cccacttctg ctccatgtgc ggccccaagt tctgctccat
gaagatcacg gaggacatcc 3780gcaagtacgc cgaggagaac ggctacggct
ccgccgagga ggccatccgc cagggcatgg 3840acgccatgtc cgaggagttc
aacatcgcca agaagacgat ctccggcgag cagcacggcg 3900aggtcggcgg
cgagatctac ctgcccgagt cctacgtcaa ggccgcgcag aagtgacaat
3960tggcagcagc agctcggata gtatcgacac actctggacg ctggtcgtgt
gatggactgt 4020tgccgccaca cttgctgcct tgacctgtga atatccctgc
cgcttttatc aaacagcctc 4080agtgtgtttg atcttgtgtg tacgcgcttt
tgcgagttgc tagctgcttg tgctatttgc 4140gaataccacc cccagcatcc
ccttccctcg tttcatatcg cttgcatccc aaccgcaact 4200tatctacgct
gtcctgctat ccctcagcgc tgctcctgct cctgctcact gcccctcgca
4260cagccttggt ttgggctccg cctgtattct cctggtactg caacctgtaa
accagcactg 4320caatgctgat gcacgggaag tagtgggatg ggaacacaaa
tggaggatcg tagagctcca 4380gccacggcaa caccgcgcgc ctggcggccg
agcacggcga caagggcctg agcaagatct 4440gcgggctgat cgccagcgac
gagggccggc acgagatcgc ctacacgcgc atcgtggacg 4500agttcttccg
cctcgacccc gagggcgccg tcgccgccta cgccaacatg atgcgcaagc
4560agatcaccat gcccgcgcac ctcatggacg acatgggcca cggcgaggcc
aacccgggcc 4620gcaacctctt cgccgacttc tccgccgtcg ccgagaagat
cgacgtctac gacgccgagg 4680actactgccg catcctggag cacctcaacg
cgcgctggaa ggtggacgag cgccaggtca 4740gcggccaggc cgccgcggac
caggagtacg ttctgggcct gccccagcgc ttccggaaac 4800tcgccgagaa
gaccgccgcc aagcgcaagc gcgtcgcgcg caggcccgtc gccttctcct
4860ggatctccgg acgcgagatt atggtctagg gaggtacgag cgcgcgcgag
ggattggtgg 4920gagtgggacg cgctcgtcgc tcctttctat tctgaaggga
agattggcca ccccgctcca 4980cgcgccacgc atcgagcaac gaagaaaacc
ccccgatgat aggttgcagt ggctgccgag 5040atatagatcc ggctgcacgt
caaagggccc ctcggccaga gaagaagctc ttttcccagc 5100gaccgcagac
tcctgaagag cgtttaaac 51291517194DNAArtificial SequenceDescription
of Artificial Sequence Synthetic polynucleotide 151gtttaaacgc
cggtcaccac ccgcatgctc gtactacagc gcacgcaccg cttcgtgatc 60caccgggtga
acgtagtcct cgacggaaac atctggttcg ggcctcctgc ttgcactccc
120gcccatgccg acaacctttc tgctgttacc acgacccaca atgcaacgcg
acacgaccgt 180gtgggactga tcggttcact gcacctgcat gcaattgtca
caagcgctta ctccaattgt 240attcgtttgt tttctgggag cagttgctcg
accgcccgcg tcccgcaggc agcgatgacg 300tgtgcgtggc ctgggtgttt
cgtcgaaagg ccagcaaccc taaatcgcag gcgatccgga 360gattgggatc
tgatccgagt ttggaccaga tccgccccga tgcggcacgg gaactgcatc
420gactcggcgc ggaacccagc tttcgtaaat gccagattgg tgtccgatac
ctggatttgc 480catcagcgaa acaagacttc agcagcgagc gtatttggcg
ggcgtgctac cagggttgca 540tacattgccc atttctgtct ggaccgcttt
actggcgcag agggtgagtt gatggggttg 600gcaggcatcg aaacgcgcgt
gcatggtgtg cgtgtctgtt ttcggctgca cgaattcaat 660agtcggatgg
gcgacggtag aattgggtgt ggcgctcgcg tgcatgcctc gccccgtcgg
720gtgtcatgac cgggactgga atcccccctc gcgaccatct tgctaacgct
cccgactctc 780ccgaccgcgc gcaggataga ctcttgttca accaatcgac
aactagtatg cagaccgccc 840accagcgccc ccccaccgag ggccactgct
tcggcgcccg cctgcccacc gcctcccgcc 900gcgccgtgcg ccgcgcctgg
tcccgcatcg cccgcgggcg cgccgccgcc gccgccgacg 960ccaaccccgc
ccgccccgag cgccgcgtgg tgatcaccgg ccagggcgtg gtgacctccc
1020tgggccagac catcgagcag ttctactcct ccctgctgga gggcgtgtcc
ggcatctccc 1080agatccagaa gttcgacacc accggctaca ccaccaccat
cgccggcgag atcaagtccc 1140tgcagctgga cccctacgtg cccaagcgct
gggccaagcg cgtggacgac gtgatcaagt 1200acgtgtacat cgccggcaag
caggccctgg agtccgccgg cctgcccatc gaggccgccg 1260gcctggccgg
cgccggcctg gaccccgccc tgtgcggcgt gctgatcggc accgccatgg
1320ccggcatgac ctccttcgcc gccggcgtgg aggccctgac ccgcggcggc
gtgcgcaaga 1380tgaacccctt ctgcatcccc ttctccatct ccaacatggg
cggcgccatg ctggccatgg 1440acatcggctt catgggcccc aactactcca
tctccaccgc ctgcgccacc ggcaactact 1500gcatcctggg cgccgccgac
cacatccgcc gcggcgacgc caacgtgatg ctggccggcg 1560gcgccgacgc
cgccatcatc ccctccggca tcggcggctt catcgcctgc aaggccctgt
1620ccaagcgcaa cgacgagccc gagcgcgcct cccgcccctg ggacgccgac
cgcgacggct 1680tcgtgatggg cgagggcgcc ggcgtgctgg tgctggagga
gctggagcac gccaagcgcc 1740gcggcgccac catcctggcc gagctggtgg
gcggcgccgc cacctccgac gcccaccaca 1800tgaccgagcc cgacccccag
ggccgcggcg tgcgcctgtg cctggagcgc gccctggagc 1860gcgcccgcct
ggcccccgag cgcgtgggct acgtgaacgc ccacggcacc tccacccccg
1920ccggcgacgt ggccgagtac cgcgccatcc gcgccgtgat cccccaggac
tccctgcgca 1980tcaactccac caagtccatg atcggccacc tgctgggcgg
cgccggcgcc gtggaggccg 2040tggccgccat ccaggccctg cgcaccggct
ggctgcaccc caacctgaac ctggagaacc 2100ccgcccccgg cgtggacccc
gtggtgctgg tgggcccccg caaggagcgc gccgaggacc 2160tggacgtggt
gctgtccaac tccttcggct tcggcggcca caactcctgc gtgatcttcc
2220gcaagtacga cgagatggac tacaaggacc acgacggcga ctacaaggac
cacgacatcg 2280actacaagga cgacgacgac aagtgaatcg atagatctct
taaggcagca gcagctcgga 2340tagtatcgac acactctgga cgctggtcgt
gtgatggact gttgccgcca cacttgctgc 2400cttgacctgt gaatatccct
gccgctttta tcaaacagcc tcagtgtgtt tgatcttgtg 2460tgtacgcgct
tttgcgagtt gctagctgct tgtgctattt gcgaatacca cccccagcat
2520ccccttccct cgtttcatat cgcttgcatc ccaaccgcaa cttatctacg
ctgtcctgct 2580atccctcagc gctgctcctg ctcctgctca ctgcccctcg
cacagccttg gtttgggctc 2640cgcctgtatt ctcctggtac tgcaacctgt
aaaccagcac tgcaatgctg atgcacggga 2700agtagtggga tgggaacaca
aatggaaagc ttaattaaga gctccgcgtc tcgaacagag 2760cgcgcagagg
aacgctgaag gtctcgcctc tgtcgcacct cagcgcggca tacaccacaa
2820taaccacctg acgaatgcgc ttggttcttc gtccattagc gaagcgtccg
gttcacacac 2880gtgccacgtt ggcgaggtgg caggtgacaa tgatcggtgg
agctgatggt cgaaacgttc 2940acagcctagg tgatatccat cttaaggatc
taagtaagat tcgaagcgct cgaccgtgcc 3000ggacggactg cagccccatg
tcgtagtgac cgccaatgta agtgggctgg cgtttccctg 3060tacgtgagtc
aacgtcactg cacgcgcacc accctctcga ccggcaggac caggcatcgc
3120gagatacagc gcgagccaga cacggagtgc cgagctatgc gcacgctcca
actaggtacc 3180agtttaggtc cagcgtccgt ggggggggac gggctgggag
cttgggccgg gaagggcaag 3240acgatgcagt ccctctgggg agtcacagcc
gactgtgtgt gttgcactgt gcggcccgca 3300gcactcacac gcaaaatgcc
tggccgacag gcaggccctg tccagtgcaa catccacggt 3360ccctctcatc
aggctcacct tgctcattga cataacggaa tgcgtaccgc tctttcagat
3420ctgtccatcc agagagggga gcaggctccc caccgacgct gtcaaacttg
cttcctgccc 3480aaccgaaaac attattgttt gagggggggg gggggggggc
agattgcatg gcgggatatc 3540tcgtgaggaa catcactggg acactgtgga
acacagtgag tgcagtatgc agagcatgta 3600tgctaggggt cagcgcagga
agggggcctt tcccagtctc ccatgccact gcaccgtatc 3660cacgactcac
caggaccagc ttcttgatcg gcttccgctc ccgtggacac cagtgtgtag
3720cctctggact ccaggtatgc gtgcaccgca aaggccagcc gatcgtgccg
attcctgggt 3780ggaggatatg agtcagccaa cttggggctc agagtgcaca
ctggggcacg atacgaaaca 3840acatctacac cgtgtcctcc atgctgacac
accacagctt cgctccacct gaatgtgggc 3900gcatgggccc gaatcacagc
caatgtcgct gctgccataa tgtgatccag accctctccg 3960cccagatgcc
gagcggatcg tgggcgctga atagattcct gtttcgatca ctgtttgggt
4020cctttccttt tcgtctcgga tgcgcgtctc gaaacaggct gcgtcgggct
ttcggatccc 4080ttttgctccc tccgtcacca tcctgcgcgc gggcaagttg
cttgaccctg ggctgatacc 4140agggttggag ggtattaccg cgtcaggcca
ttcccagccc ggattcaatt caaagtctgg 4200gccaccaccc tccgccgctc
tgtctgatca ctccacattc gtgcatacac tacgttcaag 4260tcctgatcca
ggcgtgtctc gggacaaggt gtgcttgagt ttgaatctca aggacccact
4320ccagcacagc tgctggttga ccccgccctc gcaatctaga atggccgcgt
ccgtccactg 4380caccctgatg tccgtggtct gcaacaacaa gaaccactcc
gcccgcccca agctgcccaa 4440ctcctccctg ctgcccggct tcgacgtggt
ggtccaggcc gcggccaccc gcttcaagaa 4500ggagacgacg accacccgcg
ccacgctgac gttcgacccc cccacgacca actccgagcg 4560cgccaagcag
cgcaagcaca ccatcgaccc ctcctccccc gacttccagc ccatcccctc
4620cttcgaggag tgcttcccca agtccacgaa ggagcacaag gaggtggtgc
acgaggagtc 4680cggccacgtc ctgaaggtgc ccttccgccg cgtgcacctg
tccggcggcg agcccgcctt 4740cgacaactac gacacgtccg gcccccagaa
cgtcaacgcc cacatcggcc tggcgaagct 4800gcgcaaggag tggatcgacc
gccgcgagaa gctgggcacg ccccgctaca cgcagatgta 4860ctacgcgaag
cagggcatca tcacggagga gatgctgtac tgcgcgacgc gcgagaagct
4920ggaccccgag ttcgtccgct ccgaggtcgc gcggggccgc gccatcatcc
cctccaacaa 4980gaagcacctg gagctggagc ccatgatcgt gggccgcaag
ttcctggtga aggtgaacgc 5040gaacatcggc aactccgccg tggcctcctc
catcgaggag gaggtctaca aggtgcagtg 5100ggccaccatg tggggcgccg
acaccatcat ggacctgtcc acgggccgcc acatccacga 5160gacgcgcgag
tggatcctgc gcaactccgc ggtccccgtg ggcaccgtcc ccatctacca
5220ggcgctggag aaggtggacg gcatcgcgga gaacctgaac tgggaggtgt
tccgcgagac 5280gctgatcgag caggccgagc agggcgtgga ctacttcacg
atccacgcgg gcgtgctgct 5340gcgctacatc cccctgaccg ccaagcgcct
gacgggcatc gtgtcccgcg gcggctccat 5400ccacgcgaag tggtgcctgg
cctaccacaa ggagaacttc gcctacgagc actgggacga 5460catcctggac
atctgcaacc agtacgacgt cgccctgtcc atcggcgacg gcctgcgccc
5520cggctccatc tacgacgcca acgacacggc ccagttcgcc gagctgctga
cccagggcga 5580gctgacgcgc cgcgcgtggg agaaggacgt gcaggtgatg
aacgagggcc ccggccacgt 5640gcccatgcac aagatccccg agaacatgca
gaagcagctg gagtggtgca acgaggcgcc 5700cttctacacc ctgggccccc
tgacgaccga catcgcgccc ggctacgacc acatcacctc 5760cgccatcggc
gcggccaaca tcggcgccct gggcaccgcc ctgctgtgct acgtgacgcc
5820caaggagcac ctgggcctgc ccaaccgcga cgacgtgaag gcgggcgtca
tcgcctacaa 5880gatcgccgcc cacgcggccg acctggccaa gcagcacccc
cacgcccagg cgtgggacga 5940cgcgctgtcc aaggcgcgct tcgagttccg
ctggatggac cagttcgcgc tgtccctgga 6000ccccatgacg gcgatgtcct
tccacgacga gacgctgccc gcggacggcg cgaaggtcgc 6060ccacttctgc
tccatgtgcg gccccaagtt ctgctccatg aagatcacgg aggacatccg
6120caagtacgcc gaggagaacg gctacggctc cgccgaggag gccatccgcc
agggcatgga 6180cgccatgtcc gaggagttca acatcgccaa gaagacgatc
tccggcgagc agcacggcga 6240ggtcggcggc gagatctacc tgcccgagtc
ctacgtcaag gccgcgcaga agtgacaatt 6300gacggagcgt cgtgcgggag
ggagtgtgcc gagcggggag tcccggtctg tgcgaggccc 6360ggcagctgac
gctggcgagc cgtacgcccc gagggtcccc ctcccctgca ccctcttccc
6420cttccctctg acggccgcgc ctgttcttgc atgttcagcg acggatccta
gggagcgacg 6480agtgtgcgtg cggggctggc gggagtggga cgccctcctc
gctcctctct gttctgaacg 6540gaacaatcgg ccaccccgcg ctacgcgcca
cgcatcgagc aacgaagaaa accccccgat 6600gataggttgc ggtggctgcc
gggatataga tccggccgca catcaaaggg cccctccgcc 6660agagaagaag
ctcctttccc agcagactcc ttctgctgcc aaaacacttc tctgtccaca
6720gcaacaccaa aggatgaaca gatcaacttg cgtctccgcg tagcttcctc
ggctagcgtg 6780cttgcaacag gtccctgcac tattatcttc ctgctttcct
ctgaattatg cggcaggcga 6840gcgctcgctc tggcgagcgc tccttcgcgc
cgccctcgct gatcgagtgt acagtcaatg 6900aatggtcctg ggcgaagaac
gagggaattt gtgggtaaaa caagcatcgt ctctcaggcc 6960ccggcgcagt
ggccgttaaa gtccaagacc gtgaccaggc agcgcagcgc gtccgtgtgc
7020gggccctgcc tggcggctcg gcgtgccagg ctcgagagca gctccctcag
gtcgccttgg 7080acggcctctg cgaggccggt gagggcctgc aggagcgcct
cgagcgtggc agtggcggtc 7140gtatccgggt cgccggtcac cgcctgcgac
tcgccatccg aagagcgttt aaac 71941527081DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
152gctcttcgcc gccgccactc ctgctcgagc gcgcccgcgc gtgcgccgcc
agcgccttgg 60ccttttcgcc gcgctcgtgc gcgtcgctga tgtccatcac caggtccatg
aggtctgcct 120tgcgccggct gagccactgc ttcgtccggg cggccaagag
gagcatgagg gaggactcct 180ggtccagggt cctgacgtgg tcgcggctct
gggagcgggc cagcatcatc tggctctgcc 240gcaccgaggc cgcctccaac
tggtcctcca gcagccgcag tcgccgccga ccctggcaga 300ggaagacagg
tgaggggggt atgaattgta cagaacaacc acgagccttg tctaggcaga
360atccctacca gtcatggctt tacctggatg acggcctgcg aacagctgtc
cagcgaccct 420cgctgccgcc gcttctcccg cacgcttctt tccagcaccg
tgatggcgcg agccagcgcc 480gcacgctggc gctgcgcttc gccgatctga
ggacagtcgg ggaactctga tcagtctaaa 540cccccttgcg cgttagtgtt
gccatccttt gcagaccggt gagagccgac ttgttgtgcg 600ccacccccca
caccacctcc tcccagacca attctgtcac ctttttggcg aaggcatcgg
660cctcggcctg cagagaggac agcagtgccc agccgctggg ggttggcgga
tgcacgctca 720ggtacccttt cttgcgctat gacacttcca gcaaaaggta
gggcgggctg cgagacggct 780tcccggcgct gcatgcaaca ccgatgatgc
ttcgaccccc cgaagctcct tcggggctgc 840atgggcgctc cgatgccgct
ccagggcgag cgctgtttaa atagccaggc ccccgattgc 900aaagacatta
tagcgagcta ccaaagccat attcaaacac ctagatcact accacttcta
960cacaggccac tcgagcttgt gatcgcactc cgctaagggg gcgcctcttc
ctcttcgttt 1020cagtcacaac ccgcaaactc tagaatatca atgctgctgc
aggccttcct gttcctgctg 1080gccggcttcg ccgccaagat cagcgcctcc
atgacgaacg agacgtccga ccgccccctg 1140gtgcacttca cccccaacaa
gggctggatg aacgacccca acggcctgtg gtacgacgag 1200aaggacgcca
agtggcacct gtacttccag tacaacccga acgacaccgt ctgggggacg
1260cccttgttct ggggccacgc cacgtccgac gacctgacca actgggagga
ccagcccatc 1320gccatcgccc cgaagcgcaa cgactccggc gccttctccg
gctccatggt ggtggactac 1380aacaacacct ccggcttctt caacgacacc
atcgacccgc gccagcgctg cgtggccatc 1440tggacctaca acaccccgga
gtccgaggag cagtacatct cctacagcct ggacggcggc 1500tacaccttca
ccgagtacca gaagaacccc gtgctggccg ccaactccac ccagttccgc
1560gacccgaagg tcttctggta cgagccctcc cagaagtgga tcatgaccgc
ggccaagtcc 1620caggactaca agatcgagat ctactcctcc gacgacctga
agtcctggaa gctggagtcc 1680gcgttcgcca acgagggctt cctcggctac
cagtacgagt gccccggcct gatcgaggtc 1740cccaccgagc aggaccccag
caagtcctac tgggtgatgt tcatctccat caaccccggc 1800gccccggccg
gcggctcctt caaccagtac ttcgtcggca gcttcaacgg cacccacttc
1860gaggccttcg acaaccagtc ccgcgtggtg gacttcggca aggactacta
cgccctgcag 1920accttcttca acaccgaccc gacctacggg agcgccctgg
gcatcgcgtg ggcctccaac 1980tgggagtact ccgccttcgt gcccaccaac
ccctggcgct cctccatgtc cctcgtgcgc 2040aagttctccc tcaacaccga
gtaccaggcc aacccggaga cggagctgat caacctgaag 2100gccgagccga
tcctgaacat cagcaacgcc ggcccctgga gccggttcgc caccaacacc
2160acgttgacga aggccaacag ctacaacgtc gacctgtcca acagcaccgg
caccctggag 2220ttcgagctgg tgtacgccgt caacaccacc cagacgatct
ccaagtccgt gttcgcggac 2280ctctccctct ggttcaaggg cctggaggac
cccgaggagt acctccgcat gggcttcgag 2340gtgtccgcgt cctccttctt
cctggaccgc gggaacagca aggtgaagtt cgtgaaggag 2400aacccctact
tcaccaaccg catgagcgtg aacaaccagc ccttcaagag cgagaacgac
2460ctgtcctact acaaggtgta cggcttgctg gaccagaaca tcctggagct
gtacttcaac 2520gacggcgacg tcgtgtccac caacacctac ttcatgacca
ccgggaacgc cctgggctcc 2580gtgaacatga cgacgggggt ggacaacctg
ttctacatcg acaagttcca ggtgcgcgag 2640gtcaagtgac aattggcagc
agcagctcgg atagtatcga cacactctgg acgctggtcg 2700tgtgatggac
tgttgccgcc acacttgctg ccttgacctg tgaatatccc tgccgctttt
2760atcaaacagc ctcagtgtgt ttgatcttgt gtgtacgcgc ttttgcgagt
tgctagctgc 2820ttgtgctatt tgcgaatacc acccccagca tccccttccc
tcgtttcata tcgcttgcat 2880cccaaccgca acttatctac gctgtcctgc
tatccctcag cgctgctcct gctcctgctc 2940actgcccctc gcacagcctt
ggtttgggct ccgcctgtat tctcctggta ctgcaacctg 3000taaaccagca
ctgcaatgct gatgcacggg aagtagtggg atgggaacac aaatggagga
3060tcccgcgtct cgaacagagc gcgcagagga acgctgaagg tctcgcctct
gtcgcacctc 3120agcgcggcat acaccacaat aaccacctga cgaatgcgct
tggttcttcg tccattagcg 3180aagcgtccgg ttcacacacg tgccacgttg
gcgaggtggc aggtgacaat gatcggtgga 3240gctgatggtc gaaacgttca
cagcctaggg atatcctgaa gaatgggagg caggtgttgt 3300tgattatgag
tgtgtaaaag aaaggggtag agagccgtcc tcagatccga ctactatgca
3360ggtagccgct cgcccatgcc cgcctggctg aatattgatg catgcccatc
aaggcaggca 3420ggcatttctg tgcacgcacc aagcccacaa tcttccacaa
cacacagcat gtaccaacgc 3480acgcgtaaaa gttggggtgc tgccagtgcg
tcatgccagg catgatgtgc tcctgcacat 3540ccgccatgat ctcctccatc
gtctcgggtg tttccggcgc ctggtccggg agccgttccg 3600ccagataccc
agacgccacc tccgacctca cggggtactt ttcgagcgtc tgccggtagt
3660cgacgatcgc gtccaccatg gagtagccga ggcgccggaa ctggcgtgac
ggagggagga 3720gagggaggag agagaggggg gggggggggg gggatgatta
cacgccagtc tcacaacgca 3780tgcaagaccc gtttgattat gagtacaatc
atgcactact agatggatga gcgccaggca 3840taaggcacac cgacgttgat
ggcatgagca actcccgcat catatttcct attgtcctca 3900cgccaagccg
gtcaccatcc gcatgctcat attacagcgc acgcaccgct tcgtgatcca
3960ccgggtgaac gtagtcctcg acggaaacat ctggctcggg cctcgtgctg
gcactccctc 4020ccatgccgac aacctttctg ctgtcaccac gacccacgat
gcaacgcgac acgacccggt 4080gggactgatc ggttcactgc acctgcatgc
aattgtcaca agcgcatact ccaatcgtat 4140ccgtttgatt tctgtgaaaa
ctcgctcgac cgcccgcgtc ccgcaggcag cgatgacgtg 4200tgcgtgacct
gggtgtttcg tcgaaaggcc agcaacccca aatcgcaggc gatccggaga
4260ttgggatctg atccgagctt ggaccagatc ccccacgatg cggcacggga
actgcatcga 4320ctcggcgcgg aacccagctt tcgtaaatgc cagattggtg
tccgatacct tgatttgcca 4380tcagcgaaac aagacttcag cagcgagcgt
atttggcggg cgtgctacca gggttgcata 4440cattgcccat ttctgtctgg
accgctttac cggcgcagag ggtgagttga tggggttggc 4500aggcatcgaa
acgcgcgtgc atggtgtgtg
tgtctgtttt cggctgcaca atttcaatag 4560tcggatgggc gacggtagaa
ttgggtgttg cgctcgcgtg catgcctcgc cccgtcgggt 4620gtcatgaccg
ggactggaat cccccctcgc gaccctcctg ctaacgctcc cgactctccc
4680gcccgcgcgc aggatagact ctagttcaac caatcgacaa ctagtatggc
caccgcatcc 4740actttctcgg cgttcaatgc ccgctgcggc gacctgcgtc
gctcggcggg ctccgggccc 4800cggcgcccag cgaggcccct ccccgtgcgc
gggcgcgcca tccccccccg catcatcgtg 4860gtgtcctcct cctcctccaa
ggtgaacccc ctgaagaccg aggccgtggt gtcctccggc 4920ctggccgacc
gcctgcgcct gggctccctg accgaggacg gcctgtccta caaggagaag
4980ttcatcgtgc gctgctacga ggtgggcatc aacaagaccg ccaccgtgga
gaccatcgcc 5040aacctgctgc aggaggtggg ctgcaaccac gcccagtccg
tgggctactc caccggcggc 5100ttctccacca cccccaccat gcgcaagctg
cgcctgatct gggtgaccgc ccgcatgcac 5160atcgagatct acaagtaccc
cgcctggtcc gacgtggtgg agatcgagtc ctggggccag 5220ggcgagggca
agatcggcac ccgccgcgac tggatcctgc gcgactacgc caccggccag
5280gtgatcggcc gcgccacctc caagtgggtg atgatgaacc aggacacccg
ccgcctgcag 5340aaggtggacg tggacgtgcg cgacgagtac ctggtgcact
gcccccgcga gctgcgcctg 5400gccttccccg aggagaacaa ctcctccctg
aagaagatct ccaagctgga ggacccctcc 5460cagtactcca agctgggcct
ggtgccccgc cgcgccgacc tggacatgaa ccagcacgtg 5520aacaacgtga
cctacatcgg ctgggtgctg gagtccatgc cccaggagat catcgacacc
5580cacgagctgc agaccatcac cctggactac cgccgcgagt gccagcacga
cgacgtggtg 5640gactccctga cctcccccga gccctccgag gacgccgagg
ccgtgttcaa ccacaacggc 5700accaacggct ccgccaacgt gtccgccaac
gaccacggct gccgcaactt cctgcacctg 5760ctgcgcctgt ccggcaacgg
cctggagatc aaccgcggcc gcaccgagtg gcgcaagaag 5820cccacccgca
tggactacaa ggaccacgac ggcgactaca aggaccacga catcgactac
5880aaggacgacg acgacaagtg aatcgataga tctcttaagg cagcagcagc
tcggatagta 5940tcgacacact ctggacgctg gtcgtgtgat ggactgttgc
cgccacactt gctgccttga 6000cctgtgaata tccctgccgc ttttatcaaa
cagcctcagt gtgtttgatc ttgtgtgtac 6060gcgcttttgc gagttgctag
ctgcttgtgc tatttgcgaa taccaccccc agcatcccct 6120tccctcgttt
catatcgctt gcatcccaac cgcaacttat ctacgctgtc ctgctatccc
6180tcagcgctgc tcctgctcct gctcactgcc cctcgcacag ccttggtttg
ggctccgcct 6240gtattctcct ggtactgcaa cctgtaaacc agcactgcaa
tgctgatgca cgggaagtag 6300tgggatggga acacaaatgg aaagcttaat
taagagctct tgttttccag aaggagttgc 6360tccttgagcc tttcattctc
agcctcgata acctccaaag ccgctctaat tgtggagggg 6420gttcgaattt
aaaagcttgg aatgttggtt cgtgcgtctg gaacaagccc agacttgttg
6480ctcactggga aaaggaccat cagctccaaa aaacttgccg ctcaaaccgc
gtacctctgc 6540tttcgcgcaa tctgccctgt tgaaatcgcc accacattca
tattgtgacg cttgagcagt 6600ctgtaattgc ctcagaatgt ggaatcatct
gccccctgtg cgagcccatg ccaggcatgt 6660cgcgggcgag gacacccgcc
actcgtacag cagaccatta tgctacctca caatagttca 6720taacagtgac
catatttctc gaagctcccc aacgagcacc tccatgctct gagtggccac
6780cccccggccc tggtgcttgc ggagggcagg tcaaccggca tggggctacc
gaaatccccg 6840accggatccc accacccccg cgatgggaag aatctctccc
cgggatgtgg gcccaccacc 6900agcacaacct gctggcccag gcgagcgtca
aaccatacca cacaaatatc cttggcatcg 6960gccctgaatt ccttctgccg
ctctgctacc cggtgcttct gtccgaagca ggggttgcta 7020gggatcgctc
cgagtccgca aacccttgtc gcgtggcggg gcttgttcga gcttgaagag 7080c
70811538286DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 153gctcttccca actcagataa taccaatacc
cctccttctc ctcctcatcc attcagtacc 60cccccccttc tcttcccaaa gcagcaagcg
cgtggcttac agaagaacaa tcggcttccg 120ccaaagtcgc cgagcactgc
ccgacggcgg cgcgcccagc agcccgcttg gccacacagg 180caacgaatac
attcaatagg gggcctcgca gaatggaagg agcggtaaag ggtacaggag
240cactgcgcac aaggggcctg tgcaggagtg actgactggg cgggcagacg
gcgcaccgcg 300ggcgcaggca agcagggaag attgaagcgg cagggaggag
gatgctgatt gaggggggca 360tcgcagtctc tcttggaccc gggataagga
agcaaatatt cggccggttg ggttgtgtgt 420gtgcacgttt tcttcttcag
agtcgtgggt gtgcttccag ggaggatata agcagcagga 480tcgaatcccg
cgaccagcgt ttccccatcc agccaaccac cctgtcggta ccgcggtgag
540aatcgaaaat gcatcgtttc taggttcgga gacggtcaat tccctgctcc
ggcgaatctg 600tcggtcaagc tggccagtgg acaatgttgc tatggcagcc
cgcgcacatg ggcctcccga 660cgcggccatc aggagcccaa acagcgtgtc
agggtatgtg aaactcaaga ggtccctgct 720gggcactccg gccccactcc
gggggcggga cgccaggcat tcgcggtcgg tcccgcgcga 780cgagcgaaat
gatgattcgg ttacgagacc aggacgtcgt cgaggtcgag aggcagcctc
840ggacacgtct cgctagggca acgccccgag tccccgcgag ggccgtaaac
attgtttctg 900ggtgtcggag tgggcatttt gggcccgatc caatcgcctc
atgccgctct cgtctggtcc 960tcacgttcgc gtacggcctg gatcccggaa
agggcggatg cacgtggtgt tgccccgcca 1020ttggcgccca cgtttcaaag
tccccggcca gaaatgcaca ggaccggccc ggctcgcaca 1080ggccatgctg
aacgcccaga tttcgacagc aacaccatct agaataatcg caaccatccg
1140cgttttgaac gaaacgaaac ggcgctgttt agcatgtttc cgacatcgtg
ggggccgaag 1200catgctccgg ggggaggaaa gcgtggcaca gcggtagccc
attctgtgcc acacgccgac 1260gaggaccaat ccccggcatc agccttcatc
gacggctgcg ccgcacatat aaagccggac 1320gcctaaccgg tttcgtggtt
atgactagta tgttcgcgtt ctacttcctg acggcctgca 1380tctccctgaa
gggcgtgttc ggcgtctccc cctcctacaa cggcctgggc ctgacgcccc
1440agatgggctg ggacaactgg aacacgttcg cctgcgacgt ctccgagcag
ctgctgctgg 1500acacggccga ccgcatctcc gacctgggcc tgaaggacat
gggctacaag tacatcatcc 1560tggacgactg ctggtcctcc ggccgcgact
ccgacggctt cctggtcgcc gacgagcaga 1620agttccccaa cggcatgggc
cacgtcgccg accacctgca caacaactcc ttcctgttcg 1680gcatgtactc
ctccgcgggc gagtacacgt gcgccggcta ccccggctcc ctgggccgcg
1740aggaggagga cgcccagttc ttcgcgaaca accgcgtgga ctacctgaag
tacgacaact 1800gctacaacaa gggccagttc ggcacgcccg agatctccta
ccaccgctac aaggccatgt 1860ccgacgccct gaacaagacg ggccgcccca
tcttctactc cctgtgcaac tggggccagg 1920acctgacctt ctactggggc
tccggcatcg cgaactcctg gcgcatgtcc ggcgacgtca 1980cggcggagtt
cacgcgcccc gactcccgct gcccctgcga cggcgacgag tacgactgca
2040agtacgccgg cttccactgc tccatcatga acatcctgaa caaggccgcc
cccatgggcc 2100agaacgcggg cgtcggcggc tggaacgacc tggacaacct
ggaggtcggc gtcggcaacc 2160tgacggacga cgaggagaag gcgcacttct
ccatgtgggc catggtgaag tcccccctga 2220tcatcggcgc gaacgtgaac
aacctgaagg cctcctccta ctccatctac tcccaggcgt 2280ccgtcatcgc
catcaaccag gactccaacg gcatccccgc cacgcgcgtc tggcgctact
2340acgtgtccga cacggacgag tacggccagg gcgagatcca gatgtggtcc
ggccccctgg 2400acaacggcga ccaggtcgtg gcgctgctga acggcggctc
cgtgtcccgc cccatgaaca 2460cgaccctgga ggagatcttc ttcgactcca
acctgggctc caagaagctg acctccacct 2520gggacatcta cgacctgtgg
gcgaaccgcg tcgacaactc cacggcgtcc gccatcctgg 2580gccgcaacaa
gaccgccacc ggcatcctgt acaacgccac cgagcagtcc tacaaggacg
2640gcctgtccaa gaacgacacc cgcctgttcg gccagaagat cggctccctg
tcccccaacg 2700cgatcctgaa cacgaccgtc cccgcccacg gcatcgcgtt
ctaccgcctg cgcccctcct 2760cctgatacaa cttattacgt attctgaccg
gcgctgatgt ggcgcggacg ccgtcgtact 2820ctttcagact ttactcttga
ggaattgaac ctttctcgct tgctggcatg taaacattgg 2880cgcaattaat
tgtgtgatga agaaagggtg gcacaagatg gatcgcgaat gtacgagatc
2940gacaacgatg gtgattgtta tgaggggcca aacctggctc aatcttgtcg
catgtccggc 3000gcaatgtgat ccagcggcgt gactctcgca acctggtagt
gtgtgcgcac cgggtcgctt 3060tgattaaaac tgatcgcatt gccatcccgt
caactcacaa gcctactcta gctcccattg 3120cgcactcggg cgcccggctc
gatcaatgtt ctgagcggag ggcgaagcgt caggaaatcg 3180tctcggcagc
tggaagcgca tggaatgcgg agcggagatc gaatcaggat cccgcgtctc
3240gaacagagcg cgcagaggaa cgctgaaggt ctcgcctctg tcgcacctca
gcgcggcata 3300caccacaata accacctgac gaatgcgctt ggttcttcgt
ccattagcga agcgtccggt 3360tcacacacgt gccacgttgg cgaggtggca
ggtgacaatg atcggtggag ctgatggtcg 3420aaacgttcac agcctagcat
agcgactgct accccccgac catgtgccga ggcagaaatt 3480atatacaaga
agcagatcgc aattaggcac atcgctttgc attatccaca cactattcat
3540cgctgctgcg gcaaggctgc agagtgtatt tttgtggccc aggagctgag
tccgaagtcg 3600acgcgacgag cggcgcagga tccgacccct agacgagctc
tgtcattttc caagcacgca 3660gctaaatgcg ctgagaccgg gtctaaatca
tccgaaaagt gtcaaaatgg ccgattgggt 3720tcgcctagga caatgcgctg
cggattcgct cgagtccgct gccggccaaa aggcggtggt 3780acaggaaggc
gcacggggcc aaccctgcga agccgggggc ccgaacgccg accgccggcc
3840ttcgatctcg ggtgtccccc tcgtcaattt cctctctcgg gtgcagccac
gaaagtcgtg 3900acgcaggtca cgaaatccgg ttacgaaaaa cgcaggtctt
cgcaaaaacg tgagggtttc 3960gcgtctcgcc ctagctattc gtatcgccgg
gtcagaccca cgtgcagaaa agcccttgaa 4020taacccggga ccgtggttac
cgcgccgcct gcaccagggg gcttatataa gcccacacca 4080cacctgtctc
accacgcatt tctccaactc gcgacttttc ggaagaaatt gttatccacc
4140tagtatagac tgccacctgc aggaccttgt gtcttgcagt ttgtattggt
cccggccgtc 4200gagctcgaca gatctgggct agggttggcc tggccgctcg
gcactcccct ttagccgcgc 4260gcatccgcgt tccagaggtg cgattcggtg
tgtggagcat tgtcatgcgc ttgtgggggt 4320cgttccgtgc gcggcgggtc
cgccatgggc gccgacctgg gccctagggt ttgttttcgg 4380gccaagcgag
cccctctcac ctcgtcgccc ccccgcattc cctctctctt gcagcccata
4440tggccatggc cgccgccgtg atcgtgcccc tgggcatcct gttcttcatc
tccggcctgg 4500tggtgaacct gctgcaggcc atctgctacg tgctgatccg
ccccctgtcc aagaacacct 4560accgcaagat caaccgcgtg gtggccgaga
ccctgtggct ggagctggtg tggatcgtgg 4620actggtgggc cggcgtgaag
atccaggtgt tcgccgacaa cgagaccttc aaccgcatgg 4680gcaaggagca
cgccctggtg gtgtgcaacc accgctccga catcgactgg ctggtgggct
4740ggatcctggc ccagcgctcc ggctgcctgg gctccgccct ggccgtgatg
aagaagtcct 4800ccaagttcct gcccgtgatc ggctggtcca tgtggttctc
cgagtacctg ttcctggagc 4860gcaactgggc caaggacgag tccaccctga
agtccggcct gcagcgcctg aacgacttcc 4920cccgcccctt ctggctggcc
ctgttcgtgg agggcacccg cttcaccgag gccaagctga 4980aggccgccca
ggagtacgcc gcctcctccg agctgcccgt gccccgcaac gtgctgatcc
5040cccgcaccaa gggcttcgtg tccgccgtgt ccaacatgcg ctccttcgtg
cccgccatct 5100acgacatgac cgtggccatc cccaagacct cccccccccc
caccatgctg cgcctgttca 5160agggccagcc ctccgtggtg cacgtgcaca
tcaagtgcca ctccatgaag gacctgcccg 5220agtccgacga cgccatcgcc
cagtggtgcc gcgaccagtt cgtggccaag gacgccctgc 5280tggacaagca
catcgccgcc gacaccttcc ccggccagca ggagcagaac atcggccgcc
5340ccatcaagtc cctggccgtg gtgctgtcct ggtcctgcct gctgatcctg
ggcgccatga 5400agttcctgca ctggtccaac ctgttctcct cctggaaggg
catcgccttc tccgccctgg 5460gcctgggcat catcaccctg tgcatgcaga
tcctgatccg ctcctcccag tccgagcgct 5520ccacccccgc caaggtggtg
cccgccaagc ccaaggacaa ccacaacgac tccggctcct 5580cctcccagac
cgaggtggag aagcagaagt gaatgcatgc agcagcagct cggatagtat
5640cgacacactc tggacgctgg tcgtgtgatg gactgttgcc gccacacttg
ctgccttgac 5700ctgtgaatat ccctgccgct tttatcaaac agcctcagtg
tgtttgatct tgtgtgtacg 5760cgcttttgcg agttgctagc tgcttgtgct
atttgcgaat accaccccca gcatcccctt 5820ccctcgtttc atatcgcttg
catcccaacc gcaacttatc tacgctgtcc tgctatccct 5880cagcgctgct
cctgctcctg ctcactgccc ctcgcacagc cttggtttgg gctccgcctg
5940tattctcctg gtactgcaac ctgtaaacca gcactgcaat gctgatgcac
gggaagtagt 6000gggatgggaa cacaaatgga cttaaggatc taagtaagat
tcgaagcgct cgaccgtgcc 6060ggacggactg cagccccatg tcgtagtgac
cgccaatgta agtgggctgg cgtttccctg 6120tacgtgagtc aacgtcactg
cacgcgcacc accctctcga ccggcaggac caggcatcgc 6180gagatacagc
gcgagccaga cacggagtgc cgagctatgc gcacgctcca actagatatc
6240atgtggatga tgagcatgaa ttcctttctt gcgctatgac acttccagca
aaaggtaggg 6300cgggctgcga gacggcttcc cggcgctgca tgcaacaccg
atgatgcttc gaccccccga 6360agctccttcg gggctgcatg ggcgctccga
tgccgctcca gggcgagcgc tgtttaaata 6420gccaggcccc cgattgcaaa
gacattatag cgagctacca aagccatatt caaacaccta 6480gatcactacc
acttctacac aggccactcg agcttgtgat cgcactccgc taagggggcg
6540cctcttcctc ttcgtttcag tcacaacccg caaacactag tatggctatc
aagacgaaca 6600ggcagcctgt ggagaagcct ccgttcacga tcgggacgct
gcgcaaggcc atccccgcgc 6660actgtttcga gcgctcggcg cttcgtagca
gcatgtacct ggcctttgac atcgcggtca 6720tgtccctgct ctacgtcgcg
tcgacgtaca tcgaccctgc accggtgcct acgtgggtca 6780agtacggcat
catgtggccg ctctactggt tcttccaggt gtgtttgagg gttttggttg
6840cccgtattga ggtcctggtg gcgcgcatgg aggagaaggc gcctgtcccg
ctgacccccc 6900cggctaccct cccggcacct tccagggcgc gtacgggaag
aaccagtaga gcggccacat 6960gatgccgtac ttgacccacg taggcaccgg
tgcagggtcg atgtacgtcg acgcgacgta 7020gagcagggac atgaccgcga
tgtcaaaggc caggtacatg ctgctacgaa gcgccgagcg 7080ctcgaaacag
tgcgcgggga tggccttgcg cagcgtcccg atcgtgaacg gaggcttctc
7140cacaggctgc ctgttcgtct tgatagccat ctcgaggcag cagcagctcg
gatagtatcg 7200acacactctg gacgctggtc gtgtgatgga ctgttgccgc
cacacttgct gccttgacct 7260gtgaatatcc ctgccgcttt tatcaaacag
cctcagtgtg tttgatcttg tgtgtacgcg 7320cttttgcgag ttgctagctg
cttgtgctat ttgcgaatac cacccccagc atccccttcc 7380ctcgtttcat
atcgcttgca tcccaaccgc aacttatcta cgctgtcctg ctatccctca
7440gcgctgctcc tgctcctgct cactgcccct cgcacagcct tggtttgggc
tccgcctgta 7500ttctcctggt actgcaacct gtaaaccagc actgcaatgc
tgatgcacgg gaagtagtgg 7560gatgggaaca caaatggaaa gctgtagagc
tcttgttttc cagaaggagt tgctccttga 7620gcctttcatt ctcagcctcg
ataacctcca aagccgctct aattgtggag ggggttcgaa 7680ccgaatgctg
cgtgaacggg aaggaggagg agaaagagtg agcagggagg gattcagaaa
7740tgagaaatga gaggtgaagg aacgcatccc tatgcccttg caatggacag
tgtttctggc 7800caccgccacc aagacttcgt gtcctctgat catcatgcga
ttgattacgt tgaatgcgac 7860ggccggtcag ccccggacct ccacgcaccg
gtgctcctcc aggaagatgc gcttgtcctc 7920cgccatcttg cagggctcaa
gctgctccca aaactcttgg gcgggttccg gacggacggc 7980taccgcgggt
gcggccctga ccgccactgt tcggaagcag cggcgctgca tgggcagcgg
8040ccgctgcggt gcgccacgga ccgcatgatc caccggaaaa gcgcacgcgc
tggagcgcgc 8100agaggaccac agagaagcgg aagagacgcc agtactggca
agcaggctgg tcggtgccat 8160ggcgcgctac taccctcgct atgactcggg
tcctcggccg gctggcggtg ctgacaattc 8220gtttagtgga gcagcgactc
cattcagcta ccagtcgaac tcagtggcac agtgactccg 8280ctcttc
82861541317DNAArtificial SequenceDescription of Artificial Sequence
Synthetic polynucleotide 154gtggccgccg aggcctcctc cgccctgttc
tccgtgcgca cccccggcac ctcccccaag 60cccggcaagt tcggcaactg gcccacctcc
ctgtccgtgc ccttcaagtc caagtccaac 120cacaacggcg gcttccaggt
gaaggccaac gcctccgccc gccccaaggc caacggctcc 180gccgtgtccc
tgaagtccgg ctccctggac acccaggagg acacctcctc ctcctcctcc
240cccccccgca ccttcatcaa ccagctgccc gactggtcca tgctgctgtc
cgccatcacc 300accgtgttcg tggccgccga gaagcagtgg accatgctgg
accgcaagtc caagcgcccc 360gacatgctga tggacccctt cggcgtggac
cgcgtggtgc aggacggcgc cgtgttccgc 420cagtccttct ccatccgctc
ctacgagatc ggcgccgacc gcaccgcctc catcgagacc 480ctgatgaaca
tcttccagga gacctccctg aaccactgca agtccatcgg cctgctgaac
540gacggcttcg gccgcacccc cgagatgtgc aagcgcgacc tgatctgggt
ggtgaccaag 600atgcacgtgg aggtgaaccg ctaccccacc tggggcgaca
ccatcgaggt gaacacctgg 660gtgtccgagt ccggcaagac cggcatgggc
cgcgactggc tgatctccga ctgccacacc 720ggcgagatcc tgatccgcgc
cacctccatg tgcgccatga tgaaccagaa gacccgccgc 780ttctccaagt
tcccctacga ggtgcgccag gagctggccc cccacttcgt ggactccgcc
840cccgtgatcg aggactacca gaagctgcac aagctggacg tgaagaccgg
cgactccatc 900tgcaacggcc tgaccccccg ctggaacgac ctggacgtga
accagcacgt gaacaacgtg 960aagtacatcg gctggatcct ggagtccgtg
cccaccgagg tgttcgagac ccaggagctg 1020tgcggcctga ccctggagta
ccgccgcgag tgcggccgcg actccgtgct ggagtccgtg 1080accgccatgg
acccctccaa ggagggcgac cgctccctgt accagcacct gctgcgcctg
1140gaggacggcg ccgacatcgc caagggccgc accaagtggc gccccaagaa
cgccggcacc 1200aacggcgcca tctccaccgg caagacctcc aacggcaact
ccatctccat ggactacaag 1260gaccacgacg gcgactacaa ggaccacgac
atcgactaca aggacgacga cgacaag 13171551260DNAArtificial
SequenceDescription of Artificial Sequence Synthetic polynucleotide
155gccaccgcct ccaccttctc cgccttcaac gcccgctgcg gcgacctgcg
ccgctccgcc 60ggctccggcc cccgccgccc cgcccgcccc ctgcccgtgc gcgccgccat
caacgcctcc 120gcccacccca aggccaacgg ctccgccgtg aacctgaagt
ccggctccct ggagacccag 180gaggacacct cctcctcctc cccccccccc
cgcaccttca tcaagcagct gcccgactgg 240ggcatgctgc tgtccaagat
caccaccgtg ttcggcgccg ccgagcgcca gtggaagcgc 300cccggcatgc
tggtggagcc cttcggcgtg gaccgcatct tccaggacgg cgtgttcttc
360cgccagtcct tctccatccg ctcctacgag atcggcgccg accgcaccgc
ctccatcgag 420accctgatga acatcttcca ggagacctcc ctgaaccact
gcaagtccat cggcctgctg 480aacgacggct tcggccgcac ccccgagatg
tgcaagcgcg acctgatctg ggtggtgacc 540aagatccagg tggaggtgaa
ccgctacccc acctggggcg acaccatcga ggtgaacacc 600tgggtgtccg
agtccggcaa gaacggcatg ggccgcgact ggctgatctc cgactgccgc
660accggcgaga tcctgatccg cgccacctcc gtgtgggcca tgatgaaccg
caagacccgc 720cgcctgtcca agttccccta cgaggtgcgc caggagatcg
ccccccactt cgtggactcc 780gcccccgtga tcgaggacga caagaagctg
cacaagctgg acgtgaagac cggcgactcc 840atccgcaagg gcctgacccc
ccgctggaac gacctggacg tgaaccagca cgtgaacaac 900gtgaagtaca
tcggctggat cctgaagtcc gtgcccgccg aggtgttcga gacccaggag
960ctgtgcggcg tgaccctgga gtaccgccgc gagtgcggcc gcgactccgt
gctggagtcc 1020gtgaccgcca tggacaccgc caaggagggc gaccgctccc
tgtaccagca cctgctgcgc 1080ctggaggacg gcgccgacat caccatcggc
cgcaccgagt ggcgccccaa gaacgccggc 1140gccaacggcg ccatctccac
cggcaagacc tccaacgaga actccgtgtc catggactac 1200aaggaccacg
acggcgacta caaggaccac gacatcgact acaaggacga cgacgacaag
1260156352PRTGarcinia mangostana 156Met Leu Lys Leu Ser Ser Ser Arg
Ser Pro Leu Ala Arg Ile Pro Thr 1 5 10 15 Arg Pro Arg Pro Asn Ser
Ile Pro Pro Arg Ile Ile Val Val Ser Ser 20 25 30 Ser Ser Ser Lys
Val Asn Pro Leu Lys Thr Glu Ala Val Val Ser Ser 35 40 45 Gly Leu
Ala Asp Arg Leu Arg Leu Gly Ser Leu Thr Glu Asp Gly Leu 50 55 60
Ser Tyr Lys Glu Lys Phe Ile Val Arg Cys Tyr Glu Val Gly Ile Asn 65
70 75 80 Lys Thr Ala Thr Val Glu Thr Ile Ala Asn Leu Leu Gln Glu
Val Gly 85 90 95 Cys Asn His Ala Gln Ser Val Gly Tyr Ser Thr Gly
Gly Phe Ser Thr 100 105 110 Thr Pro Thr Met Arg Lys Leu Arg Leu Ile
Trp Val Thr Ala Arg Met 115 120 125 His Ile Glu Ile Tyr Lys Tyr Pro
Ala Trp Ser Asp Val Val Glu Ile 130 135 140 Glu Ser Trp Gly Gln Gly
Glu Gly Lys Ile Gly Thr Arg Arg Asp Trp 145 150 155 160 Ile Leu Arg
Asp Tyr Ala Thr Gly Gln Val Ile Gly Arg Ala Thr Ser 165 170 175 Lys
Trp Val Met Met Asn Gln Asp Thr Arg Arg Leu Gln Lys Val Asp 180
185 190 Val Asp Val Arg Asp Glu Tyr Leu Val His Cys Pro Arg Glu Leu
Arg 195 200 205 Leu Ala Phe Pro Glu Glu Asn Asn Ser Ser Leu Lys Lys
Ile Ser Lys 210 215 220 Leu Glu Asp Pro Ser Gln Tyr Ser Lys Leu Gly
Leu Val Pro Arg Arg 225 230 235 240 Ala Asp Leu Asp Met Asn Gln His
Val Asn Asn Val Thr Tyr Ile Gly 245 250 255 Trp Val Leu Glu Ser Met
Pro Gln Glu Ile Ile Asp Thr His Glu Leu 260 265 270 Gln Thr Ile Thr
Leu Asp Tyr Arg Arg Glu Cys Gln His Asp Asp Val 275 280 285 Val Asp
Ser Leu Thr Ser Pro Glu Pro Ser Glu Asp Ala Glu Ala Val 290 295 300
Phe Asn His Asn Gly Thr Asn Gly Ser Ala Asn Val Ser Ala Asn Asp 305
310 315 320 His Gly Cys Arg Asn Phe Leu His Leu Leu Arg Leu Ser Gly
Asn Gly 325 330 335 Leu Glu Ile Asn Arg Gly Arg Thr Glu Trp Arg Lys
Lys Pro Thr Arg 340 345 350 157390PRTBrassic napus 157Met Ala Met
Ala Ala Ala Val Ile Val Pro Leu Gly Ile Leu Phe Phe 1 5 10 15 Ile
Ser Gly Leu Val Val Asn Leu Leu Gln Ala Val Cys Tyr Val Leu 20 25
30 Val Arg Pro Met Ser Lys Asn Thr Tyr Arg Lys Ile Asn Arg Val Val
35 40 45 Ala Glu Thr Leu Trp Leu Glu Leu Val Trp Ile Val Asp Trp
Trp Ala 50 55 60 Gly Val Lys Ile Gln Val Phe Ala Asp Asp Glu Thr
Phe Asn Arg Met 65 70 75 80 Gly Lys Glu His Ala Leu Val Val Cys Asn
His Arg Ser Asp Ile Asp 85 90 95 Trp Leu Val Gly Trp Ile Leu Ala
Gln Arg Ser Gly Cys Leu Gly Ser 100 105 110 Ala Leu Ala Val Met Lys
Lys Ser Ser Lys Phe Leu Pro Val Ile Gly 115 120 125 Trp Ser Met Trp
Phe Ser Glu Tyr Leu Phe Leu Glu Arg Asn Trp Ala 130 135 140 Lys Asp
Glu Ser Thr Leu Gln Ser Gly Leu Gln Arg Leu Asn Asp Phe 145 150 155
160 Pro Arg Pro Phe Trp Leu Ala Leu Phe Val Glu Gly Thr Arg Phe Thr
165 170 175 Glu Ala Lys Leu Lys Ala Ala Gln Glu Tyr Ala Ala Ser Ser
Glu Leu 180 185 190 Pro Val Pro Arg Asn Val Leu Ile Pro Arg Thr Lys
Gly Phe Val Ser 195 200 205 Ala Val Ser Asn Met Arg Ser Phe Val Pro
Ala Ile Tyr Asp Met Thr 210 215 220 Val Ala Ile Pro Lys Thr Ser Pro
Pro Pro Thr Met Leu Arg Leu Phe 225 230 235 240 Lys Gly Gln Pro Ser
Val Val His Val His Ile Lys Cys His Ser Met 245 250 255 Lys Asp Leu
Pro Glu Pro Glu Asp Glu Ile Ala Gln Trp Cys Arg Asp 260 265 270 Gln
Phe Val Ala Lys Asp Ala Leu Leu Asp Lys His Ile Ala Ala Asp 275 280
285 Thr Phe Pro Gly Gln Lys Glu Gln Asn Ile Gly Arg Pro Ile Lys Ser
290 295 300 Leu Ala Val Val Val Ser Trp Ala Cys Leu Leu Thr Leu Gly
Ala Met 305 310 315 320 Lys Phe Leu His Trp Ser Asn Leu Phe Ser Ser
Trp Lys Gly Ile Ala 325 330 335 Leu Ser Ala Phe Gly Leu Gly Ile Ile
Thr Leu Cys Met Gln Ile Leu 340 345 350 Ile Arg Ser Ser Gln Ser Glu
Arg Ser Thr Pro Ala Lys Val Ala Pro 355 360 365 Ala Lys Pro Lys Asp
Asn His Gln Ser Gly Pro Ser Ser Gln Thr Glu 370 375 380 Val Glu Glu
Lys Gln Lys 385 390 158415PRTCuphea hookeriana 158Met Val Ala Ala
Ala Ala Ser Ser Ala Phe Phe Pro Val Pro Ala Pro 1 5 10 15 Gly Ala
Ser Pro Lys Pro Gly Lys Phe Gly Asn Trp Pro Ser Ser Leu 20 25 30
Ser Pro Ser Phe Lys Pro Lys Ser Ile Pro Asn Gly Gly Phe Gln Val 35
40 45 Lys Ala Asn Asp Ser Ala His Pro Lys Ala Asn Gly Ser Ala Val
Ser 50 55 60 Leu Lys Ser Gly Ser Leu Asn Thr Gln Glu Asp Thr Ser
Ser Ser Pro 65 70 75 80 Pro Pro Arg Thr Phe Leu His Gln Leu Pro Asp
Trp Ser Arg Leu Leu 85 90 95 Thr Ala Ile Thr Thr Val Phe Val Lys
Ser Lys Arg Pro Asp Met His 100 105 110 Asp Arg Lys Ser Lys Arg Pro
Asp Met Leu Val Asp Ser Phe Gly Leu 115 120 125 Glu Ser Thr Val Gln
Asp Gly Leu Val Phe Arg Gln Ser Phe Ser Ile 130 135 140 Arg Ser Tyr
Glu Ile Gly Thr Asp Arg Thr Ala Ser Ile Glu Thr Leu 145 150 155 160
Met Asn His Leu Gln Glu Thr Ser Leu Asn His Cys Lys Ser Thr Gly 165
170 175 Ile Leu Leu Asp Gly Phe Gly Arg Thr Leu Glu Met Cys Lys Arg
Asp 180 185 190 Leu Ile Trp Val Val Ile Lys Met Gln Ile Lys Val Asn
Arg Tyr Pro 195 200 205 Ala Trp Gly Asp Thr Val Glu Ile Asn Thr Arg
Phe Ser Arg Leu Gly 210 215 220 Lys Ile Gly Met Gly Arg Asp Trp Leu
Ile Ser Asp Cys Asn Thr Gly 225 230 235 240 Glu Ile Leu Val Arg Ala
Thr Ser Ala Tyr Ala Met Met Asn Gln Lys 245 250 255 Thr Arg Arg Leu
Ser Lys Leu Pro Tyr Glu Val His Gln Glu Ile Val 260 265 270 Pro Leu
Phe Val Asp Ser Pro Val Ile Glu Asp Ser Asp Leu Lys Val 275 280 285
His Lys Phe Lys Val Lys Thr Gly Asp Ser Ile Gln Lys Gly Leu Thr 290
295 300 Pro Gly Trp Asn Asp Leu Asp Val Asn Gln His Val Ser Asn Val
Lys 305 310 315 320 Tyr Ile Gly Trp Ile Leu Glu Ser Met Pro Thr Glu
Val Leu Glu Thr 325 330 335 Gln Glu Leu Cys Ser Leu Ala Leu Glu Tyr
Arg Arg Glu Cys Gly Arg 340 345 350 Asp Ser Val Leu Glu Ser Val Thr
Ala Met Asp Pro Ser Lys Val Gly 355 360 365 Val Arg Ser Gln Tyr Gln
His Leu Leu Arg Leu Glu Asp Gly Thr Ala 370 375 380 Ile Val Asn Gly
Ala Thr Glu Trp Arg Pro Lys Asn Ala Gly Ala Asn 385 390 395 400 Gly
Ala Ile Ser Thr Gly Lys Thr Ser Asn Gly Asn Ser Val Ser 405 410 415
159525PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 159Met Ala Ser Ala Ala Phe Thr Met Ser Ala
Cys Pro Ala Met Thr Gly 1 5 10 15 Arg Ala Pro Gly Ala Arg Arg Ser
Gly Arg Pro Val Ala Thr Arg Leu 20 25 30 Arg Tyr Val Phe Gln Cys
Leu Val Ala Ser Cys Ile Asp Pro Cys Asp 35 40 45 Gln Tyr Arg Ser
Ser Ala Ser Leu Ser Phe Leu Gly Asp Asn Gly Phe 50 55 60 Ala Ser
Leu Phe Gly Ser Lys Pro Phe Met Ser Asn Arg Gly His Arg 65 70 75 80
Arg Leu Arg Arg Ala Ser His Ser Gly Glu Ala Met Ala Val Ala Leu 85
90 95 Gln Pro Ala Gln Glu Ala Gly Thr Lys Lys Lys Pro Val Ile Lys
Gln 100 105 110 Arg Arg Val Val Val Thr Gly Met Gly Val Val Thr Pro
Leu Gly His 115 120 125 Glu Pro Asp Val Phe Tyr Asn Asn Leu Leu Asp
Gly Val Ser Gly Ile 130 135 140 Ser Glu Ile Glu Thr Phe Asp Cys Thr
Gln Phe Pro Thr Arg Ile Ala 145 150 155 160 Gly Glu Ile Lys Ser Phe
Ser Thr Asp Gly Trp Val Ala Pro Lys Leu 165 170 175 Ser Lys Arg Met
Asp Lys Phe Met Leu Tyr Leu Leu Thr Ala Gly Lys 180 185 190 Lys Ala
Leu Ala Asp Gly Gly Ile Thr Asp Glu Val Met Lys Glu Leu 195 200 205
Asp Lys Arg Lys Cys Gly Val Leu Ile Gly Ser Gly Met Gly Gly Met 210
215 220 Lys Val Phe Asn Asp Ala Ile Glu Ala Leu Arg Val Ser Tyr Lys
Lys 225 230 235 240 Met Asn Pro Phe Cys Val Pro Phe Ala Thr Thr Asn
Met Gly Ser Ala 245 250 255 Met Leu Ala Met Asp Leu Gly Trp Met Gly
Pro Asn Tyr Ser Ile Ser 260 265 270 Thr Ala Cys Ala Thr Ser Asn Phe
Cys Ile Leu Asn Ala Ala Asn His 275 280 285 Ile Ile Arg Gly Glu Ala
Asp Met Met Leu Cys Gly Gly Ser Asp Ala 290 295 300 Val Ile Ile Pro
Ile Gly Leu Gly Gly Phe Val Ala Cys Arg Ala Leu 305 310 315 320 Ser
Gln Arg Asn Ser Asp Pro Thr Lys Ala Ser Arg Pro Trp Asp Ser 325 330
335 Asn Arg Asp Gly Phe Val Met Gly Glu Gly Ala Gly Val Leu Leu Leu
340 345 350 Glu Glu Leu Glu His Ala Lys Lys Arg Gly Ala Thr Ile Tyr
Ala Glu 355 360 365 Phe Leu Gly Gly Ser Phe Thr Cys Asp Ala Tyr His
Met Thr Glu Pro 370 375 380 His Pro Glu Gly Ala Gly Val Ile Leu Cys
Ile Glu Lys Ala Leu Ala 385 390 395 400 Gln Ala Gly Val Ser Lys Glu
Asp Val Asn Tyr Ile Asn Ala His Ala 405 410 415 Thr Ser Thr Ser Ala
Gly Asp Ile Lys Glu Tyr Gln Ala Leu Ala Arg 420 425 430 Cys Phe Gly
Gln Asn Ser Glu Leu Arg Val Asn Ser Thr Lys Ser Met 435 440 445 Ile
Gly His Leu Leu Gly Ala Ala Gly Gly Val Glu Ala Val Thr Val 450 455
460 Val Gln Ala Ile Arg Thr Gly Trp Ile His Pro Asn Leu Asn Leu Glu
465 470 475 480 Asp Pro Asp Lys Ala Val Asp Ala Lys Leu Leu Val Gly
Pro Lys Lys 485 490 495 Glu Arg Leu Asn Val Lys Val Gly Leu Ser Asn
Ser Phe Gly Phe Gly 500 505 510 Gly His Asn Ser Ser Ile Leu Phe Ala
Pro Cys Asn Val 515 520 525 160467PRTPrototheca moriformis 160Met
Gln Thr Ala His Gln Arg Pro Pro Thr Glu Gly His Cys Phe Gly 1 5 10
15 Ala Arg Leu Pro Thr Ala Ser Arg Arg Ala Val Arg Arg Ala Trp Ser
20 25 30 Arg Ile Ala Arg Ala Ala Ala Ala Ala Asp Ala Asn Pro Ala
Arg Pro 35 40 45 Glu Arg Arg Val Val Ile Thr Gly Gln Gly Val Val
Thr Ser Leu Gly 50 55 60 Gln Thr Ile Glu Gln Phe Tyr Ser Ser Leu
Leu Glu Gly Val Ser Gly 65 70 75 80 Ile Ser Gln Ile Gln Lys Phe Asp
Thr Thr Gly Tyr Thr Thr Thr Ile 85 90 95 Ala Gly Glu Ile Lys Ser
Leu Gln Leu Asp Pro Tyr Val Pro Lys Arg 100 105 110 Trp Ala Lys Arg
Val Asp Asp Val Ile Lys Tyr Val Tyr Ile Ala Gly 115 120 125 Lys Gln
Ala Leu Glu Ser Ala Gly Leu Pro Ile Glu Ala Ala Gly Leu 130 135 140
Ala Gly Ala Gly Leu Asp Pro Ala Leu Cys Gly Val Leu Ile Gly Thr 145
150 155 160 Ala Met Ala Gly Met Thr Ser Phe Ala Ala Gly Val Glu Ala
Leu Thr 165 170 175 Arg Gly Gly Val Arg Lys Met Asn Pro Phe Cys Ile
Pro Phe Ser Ile 180 185 190 Ser Asn Met Gly Gly Ala Met Leu Ala Met
Asp Ile Gly Phe Met Gly 195 200 205 Pro Asn Tyr Ser Ile Ser Thr Ala
Cys Ala Thr Gly Asn Tyr Cys Ile 210 215 220 Leu Gly Ala Ala Asp His
Ile Arg Arg Gly Asp Ala Asn Val Met Leu 225 230 235 240 Ala Gly Gly
Ala Asp Ala Ala Ile Ile Pro Ser Gly Ile Gly Gly Phe 245 250 255 Ile
Ala Cys Lys Ala Leu Ser Lys Arg Asn Asp Glu Pro Glu Arg Ala 260 265
270 Ser Arg Pro Trp Asp Ala Asp Arg Asp Gly Phe Val Met Gly Glu Gly
275 280 285 Ala Gly Val Leu Val Leu Glu Glu Leu Glu His Ala Lys Arg
Arg Gly 290 295 300 Ala Thr Ile Leu Ala Glu Leu Val Gly Gly Ala Ala
Thr Ser Asp Ala 305 310 315 320 His His Met Thr Glu Pro Asp Pro Gln
Gly Arg Gly Val Arg Leu Cys 325 330 335 Leu Glu Arg Ala Leu Glu Arg
Ala Arg Leu Ala Pro Glu Arg Val Gly 340 345 350 Tyr Val Asn Ala His
Gly Thr Ser Thr Pro Ala Gly Asp Val Ala Glu 355 360 365 Tyr Arg Ala
Ile Arg Ala Val Ile Pro Gln Asp Ser Leu Arg Ile Asn 370 375 380 Ser
Thr Lys Ser Met Ile Gly His Leu Leu Gly Gly Ala Gly Ala Val 385 390
395 400 Glu Ala Val Ala Ala Ile Gln Ala Leu Arg Thr Gly Trp Leu His
Pro 405 410 415 Asn Leu Asn Leu Glu Asn Pro Ala Pro Gly Val Asp Pro
Val Val Leu 420 425 430 Val Gly Pro Arg Lys Glu Arg Ala Glu Asp Leu
Asp Val Val Leu Ser 435 440 445 Asn Ser Phe Gly Phe Gly Gly His Asn
Ser Cys Val Ile Phe Arg Lys 450 455 460 Tyr Asp Glu 465
161431PRTPrototheca moriformis 161Ala Ala Ala Ala Ala Asp Ala Asn
Pro Ala Arg Pro Glu Arg Arg Val 1 5 10 15 Val Ile Thr Gly Gln Gly
Val Val Thr Ser Leu Gly Gln Thr Ile Glu 20 25 30 Gln Phe Tyr Ser
Ser Leu Leu Glu Gly Val Ser Gly Ile Ser Gln Ile 35 40 45 Gln Lys
Phe Asp Thr Thr Gly Tyr Thr Thr Thr Ile Ala Gly Glu Ile 50 55 60
Lys Ser Leu Gln Leu Asp Pro Tyr Val Pro Lys Arg Trp Ala Lys Arg 65
70 75 80 Val Asp Asp Val Ile Lys Tyr Val Tyr Ile Ala Gly Lys Gln
Ala Leu 85 90 95 Glu Ser Ala Gly Leu Pro Ile Glu Ala Ala Gly Leu
Ala Gly Ala Gly 100 105 110 Leu Asp Pro Ala Leu Cys Gly Val Leu Ile
Gly Thr Ala Met Ala Gly 115 120 125 Met Thr Ser Phe Ala Ala Gly Val
Glu Ala Leu Thr Arg Gly Gly Val 130 135 140 Arg Lys Met Asn Pro Phe
Cys Ile Pro Phe Ser Ile Ser Asn Met Gly 145 150 155 160 Gly Ala Met
Leu Ala Met Asp Ile Gly Phe Met Gly Pro Asn Tyr Ser 165 170 175 Ile
Ser Thr Ala Cys Ala Thr Gly Asn Tyr Cys Ile Leu Gly Ala Ala 180 185
190 Asp His Ile Arg Arg Gly Asp Ala Asn Val Met Leu Ala Gly Gly Ala
195 200 205 Asp Ala Ala Ile Ile Pro Ser Gly Ile Gly Gly Phe Ile Ala
Cys Lys 210 215 220 Ala Leu Ser Lys Arg Asn Asp Glu Pro Glu Arg Ala
Ser Arg Pro Trp 225 230 235 240 Asp Ala Asp Arg Asp Gly Phe Val Met
Gly Glu Gly Ala Gly Val Leu 245 250 255 Val Leu Glu Glu Leu Glu His
Ala Lys Arg Arg Gly Ala Thr Ile Leu 260 265 270 Ala Glu Leu Val Gly
Gly Ala Ala Thr Ser Asp Ala His His Met Thr 275 280 285 Glu Pro Asp
Pro Gln Gly Arg Gly Val Arg Leu Cys Leu Glu Arg Ala 290 295 300 Leu
Glu Arg Ala Arg Leu Ala Pro Glu Arg Val Gly Tyr Val Asn Ala 305
310
315 320 His Gly Thr Ser Thr Pro Ala Gly Asp Val Ala Glu Tyr Arg Ala
Ile 325 330 335 Arg Ala Val Ile Pro Gln Asp Ser Leu Arg Ile Asn Ser
Thr Lys Ser 340 345 350 Met Ile Gly His Leu Leu Gly Gly Ala Gly Ala
Val Glu Ala Val Ala 355 360 365 Ala Ile Gln Ala Leu Arg Thr Gly Trp
Leu His Pro Asn Leu Asn Leu 370 375 380 Glu Asn Pro Ala Pro Gly Val
Asp Pro Val Val Leu Val Gly Pro Arg 385 390 395 400 Lys Glu Arg Ala
Glu Asp Leu Asp Val Val Leu Ser Asn Ser Phe Gly 405 410 415 Phe Gly
Gly His Asn Ser Cys Val Ile Phe Arg Lys Tyr Asp Glu 420 425 430
162322PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptide 162Pro Asp Trp Ser Met Leu Phe Ala Val Ile
Thr Thr Ile Phe Ser Ala 1 5 10 15 Ala Glu Lys Gln Trp Thr Asn Leu
Glu Trp Lys Pro Lys Pro Asn Pro 20 25 30 Pro Gln Leu Leu Asp Asp
His Phe Gly Pro His Gly Leu Val Phe Arg 35 40 45 Arg Thr Phe Ala
Ile Arg Ser Tyr Glu Val Gly Pro Asp Arg Ser Thr 50 55 60 Ser Ile
Val Ala Val Met Asn His Leu Gln Glu Ala Ala Leu Asn His 65 70 75 80
Ala Lys Ser Val Gly Ile Leu Gly Asp Gly Phe Gly Thr Thr Leu Glu 85
90 95 Met Ser Lys Arg Asp Leu Ile Trp Val Val Arg Arg Thr His Val
Ala 100 105 110 Val Glu Arg Tyr Pro Thr Trp Gly Asp Thr Val Glu Val
Glu Cys Trp 115 120 125 Ile Gly Ala Ser Gly Asn Asn Gly Met Arg Arg
Asp Phe Leu Val Arg 130 135 140 Asp Cys Lys Thr Gly Glu Ile Leu Thr
Arg Cys Thr Ser Leu Ser Val 145 150 155 160 Leu Met Asn Thr Arg Thr
Arg Arg Leu Ser Thr Ile Pro Asp Glu Val 165 170 175 Arg Gly Glu Ile
Gly Pro Ala Phe Ile Asp Asn Val Ala Val Lys Asp 180 185 190 Asp Glu
Ile Lys Lys Leu Gln Lys Leu Asn Asp Ser Thr Ala Asp Tyr 195 200 205
Ile Gln Gly Gly Leu Thr Pro Arg Trp Asn Asp Leu Asp Val Asn Gln 210
215 220 His Val Asn Asn Leu Lys Tyr Val Ala Trp Val Phe Glu Thr Val
Pro 225 230 235 240 Asp Ser Ile Phe Glu Ser His His Ile Ser Ser Phe
Thr Leu Glu Tyr 245 250 255 Arg Arg Glu Cys Thr Arg Asp Ser Val Leu
Arg Ser Leu Thr Thr Val 260 265 270 Ser Gly Gly Ser Ser Glu Ala Gly
Leu Val Cys Asp His Leu Leu Gln 275 280 285 Leu Glu Gly Gly Ser Glu
Val Leu Arg Ala Arg Thr Glu Trp Arg Pro 290 295 300 Lys Leu Thr Asp
Ser Phe Arg Gly Ile Ser Val Ile Pro Ala Glu Pro 305 310 315 320 Arg
Val 163361PRTCuphea hyssopifolia 163Met Val Ala Thr Asn Ala Ala Ala
Phe Ser Ala Tyr Thr Phe Phe Leu 1 5 10 15 Thr Ser Pro Thr His Gly
Tyr Ser Ser Lys Arg Leu Ala Asp Thr Gln 20 25 30 Asn Gly Tyr Pro
Gly Thr Ser Leu Lys Ser Lys Ser Thr Pro Pro Pro 35 40 45 Ala Ala
Ala Ala Ala Arg Asn Gly Ala Leu Pro Leu Leu Ala Ser Ile 50 55 60
Cys Lys Cys Pro Lys Lys Ala Asp Gly Ser Met Gln Leu Asp Ser Ser 65
70 75 80 Leu Val Phe Gly Phe Gln Phe Tyr Ile Arg Ser Tyr Glu Val
Gly Ala 85 90 95 Asp Gln Thr Val Ser Ile Gln Thr Val Leu Asn Tyr
Leu Gln Glu Ala 100 105 110 Ala Ile Asn His Val Gln Ser Ala Gly Tyr
Phe Gly Asp Ser Phe Gly 115 120 125 Ala Thr Pro Glu Met Thr Lys Arg
Asn Leu Ile Trp Val Ile Thr Lys 130 135 140 Met Gln Val Leu Val Asp
Arg Tyr Pro Ala Trp Gly Asp Val Val Gln 145 150 155 160 Val Asp Thr
Trp Thr Cys Ser Ser Gly Lys Asn Ser Met Gln Arg Asp 165 170 175 Trp
Phe Val Arg Asp Leu Lys Thr Gly Asp Ile Ile Thr Arg Ala Ser 180 185
190 Ser Val Trp Val Leu Met Asn Arg Leu Thr Arg Lys Leu Ser Lys Ile
195 200 205 Pro Glu Ala Val Leu Glu Glu Ala Lys Leu Phe Val Met Asn
Thr Ala 210 215 220 Pro Thr Val Asp Asp Asn Arg Lys Leu Pro Lys Leu
Asp Gly Ser Ser 225 230 235 240 Ala Asp Tyr Val Leu Ser Gly Leu Thr
Pro Arg Trp Ser Asp Leu Asp 245 250 255 Met Asn Gln His Val Asn Asn
Val Lys Tyr Ile Ala Trp Ile Leu Glu 260 265 270 Ser Val Pro Gln Ser
Ile Pro Glu Thr His Lys Leu Ser Ala Ile Thr 275 280 285 Val Glu Tyr
Arg Arg Glu Cys Gly Lys Asn Ser Val Leu Gln Ser Leu 290 295 300 Thr
Asn Val Ser Gly Asp Gly Ile Thr Cys Gly Asn Ser Ile Ile Glu 305 310
315 320 Cys His His Leu Leu Gln Leu Glu Thr Gly Pro Glu Ile Leu Leu
Ala 325 330 335 Arg Thr Glu Trp Ile Ser Lys Glu Pro Gly Phe Arg Gly
Ala Pro Ile 340 345 350 Gln Ala Glu Lys Val Tyr Asn Asn Lys 355 360
164417PRTCuphea hyssopifolia 164Met Val Ala Ala Glu Ala Ser Ser Ala
Leu Phe Ser Val Arg Thr Pro 1 5 10 15 Gly Thr Ser Pro Lys Pro Gly
Lys Phe Gly Asn Trp Pro Thr Ser Leu 20 25 30 Ser Val Pro Phe Lys
Ser Lys Ser Asn His Asn Gly Gly Phe Gln Val 35 40 45 Lys Ala Asn
Ala Ser Ala Arg Pro Lys Ala Asn Gly Ser Ala Val Ser 50 55 60 Leu
Lys Ser Gly Ser Leu Asp Thr Gln Glu Asp Thr Ser Ser Ser Ser 65 70
75 80 Ser Pro Pro Arg Thr Phe Ile Asn Gln Leu Pro Asp Trp Ser Met
Leu 85 90 95 Leu Ser Ala Ile Thr Thr Val Phe Val Ala Ala Glu Lys
Gln Trp Thr 100 105 110 Met Leu Asp Arg Lys Ser Lys Arg Pro Asp Met
Leu Met Asp Pro Phe 115 120 125 Gly Val Asp Arg Val Val Gln Asp Gly
Ala Val Phe Arg Gln Ser Phe 130 135 140 Ser Ile Arg Ser Tyr Glu Ile
Gly Ala Asp Arg Thr Ala Ser Ile Glu 145 150 155 160 Thr Leu Met Asn
Ile Phe Gln Glu Thr Ser Leu Asn His Cys Lys Ser 165 170 175 Ile Gly
Leu Leu Asn Asp Gly Phe Gly Arg Thr Pro Glu Met Cys Lys 180 185 190
Arg Asp Leu Ile Trp Val Val Thr Lys Met His Val Glu Val Asn Arg 195
200 205 Tyr Pro Thr Trp Gly Asp Thr Ile Glu Val Asn Thr Trp Val Ser
Glu 210 215 220 Ser Gly Lys Thr Gly Met Gly Arg Asp Trp Leu Ile Ser
Asp Cys His 225 230 235 240 Thr Gly Glu Ile Leu Ile Arg Ala Thr Ser
Met Cys Ala Met Met Asn 245 250 255 Gln Lys Thr Arg Arg Phe Ser Lys
Phe Pro Tyr Glu Val Arg Gln Glu 260 265 270 Leu Ala Pro His Phe Val
Asp Ser Ala Pro Val Ile Glu Asp Tyr Gln 275 280 285 Lys Leu His Lys
Leu Asp Val Lys Thr Gly Asp Ser Ile Cys Asn Gly 290 295 300 Leu Thr
Pro Arg Trp Asn Asp Leu Asp Val Asn Gln His Val Asn Asn 305 310 315
320 Val Lys Tyr Ile Gly Trp Ile Leu Glu Ser Val Pro Thr Glu Val Phe
325 330 335 Glu Thr Gln Glu Leu Cys Gly Leu Thr Leu Glu Tyr Arg Arg
Glu Cys 340 345 350 Gly Arg Asp Ser Val Leu Glu Ser Val Thr Ala Met
Asp Pro Ser Lys 355 360 365 Glu Gly Asp Arg Ser Leu Tyr Gln His Leu
Leu Arg Leu Glu Asp Gly 370 375 380 Ala Asp Ile Ala Lys Gly Arg Thr
Lys Trp Arg Pro Lys Asn Ala Gly 385 390 395 400 Thr Asn Gly Ala Ile
Ser Thr Gly Lys Thr Ser Asn Gly Asn Ser Ile 405 410 415 Ser
165542PRTArtificial SequenceDescription of Artificial Sequence
Synthetic polypeptideMOD_RES(22)..(22)Any amino
acidMOD_RES(24)..(33)Any amino acidMOD_RES(92)..(92)Ile or
LeuMOD_RES(93)..(93)Any amino acidMOD_RES(128)..(130)Any amino
acidMOD_RES(142)..(142)Any amino acidMOD_RES(145)..(145)Any amino
acidMOD_RES(148)..(148)Any amino acidMOD_RES(153)..(153)Any amino
acidMOD_RES(158)..(158)Any amino acidMOD_RES(182)..(182)Any amino
acidMOD_RES(184)..(184)Any amino acidMOD_RES(192)..(192)Any amino
acidMOD_RES(202)..(202)Any amino acidMOD_RES(206)..(206)Any amino
acidMOD_RES(249)..(249)Any amino acidMOD_RES(295)..(295)Any amino
acidMOD_RES(310)..(310)Any amino acidMOD_RES(322)..(322)Any amino
acidMOD_RES(350)..(350)Any amino acidMOD_RES(352)..(353)Any amino
acidMOD_RES(356)..(356)Any amino acidMOD_RES(359)..(359)Any amino
acidMOD_RES(373)..(373)Any amino acidMOD_RES(380)..(380)Any amino
acidMOD_RES(399)..(400)Any amino acidMOD_RES(490)..(490)Any amino
acidMOD_RES(529)..(529)Any amino acid 165Met Glu Arg Thr Asn Ser
Ile Glu Met Asp Gln Glu Arg Leu Thr Ala 1 5 10 15 Glu Met Ala Phe
Lys Xaa Ser Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa 20 25 30 Xaa Leu
Pro Asp Phe Met Thr Ser Ile Asn Val Lys Leu Leu Tyr His 35 40 45
Tyr Val Ile Thr Asn Leu Phe Asn Leu Cys Phe Phe Pro Leu Thr Ala 50
55 60 Ile Val Ala Gly Lys Ala Ser Arg Leu Thr Ile Asx Asp Leu His
His 65 70 75 80 Leu Tyr Ser Tyr Leu Gln His Asn Leu Ile Thr Xaa Xaa
Leu Leu Phe 85 90 95 Ala Phe Thr Val Phe Gly Ser Ile Leu Tyr Ile
Val Thr Arg Pro Lys 100 105 110 Pro Val Tyr Leu Val Asp Tyr Ser Cys
Tyr Leu Pro Pro Thr His Xaa 115 120 125 Xaa Xaa Ser Ile Ser Lys Val
Met Asp Ile Phe Tyr Gln Xaa Arg Lys 130 135 140 Xaa Asp Pro Xaa Arg
Asn Gly Thr Xaa Asp Asp Ser Ser Xaa Leu Asp 145 150 155 160 Phe Leu
Arg Lys Ile Gln Glu Arg Ser Gly Leu Gly Asp Glu Thr Tyr 165 170 175
Gly Pro Glu Gly Leu Xaa Gln Xaa Pro Pro Arg Lys Asn Phe Ala Xaa 180
185 190 Ala Arg Glu Glu Thr Glu Gln Val Ile Xaa Gly Ala Leu Xaa Asn
Leu 195 200 205 Phe Glu Asn Thr Lys Val Asn Pro Lys Glu Ile Gly Ile
Leu Val Val 210 215 220 Asn Ser Ser Met Phe Asn Pro Thr Pro Ser Leu
Ser Ala Met Val Val 225 230 235 240 Asn Thr Phe Lys Leu Arg Ser Asn
Xaa Lys Ser Phe Asn Leu Gly Gly 245 250 255 Met Gly Cys Ser Ala Gly
Val Ile Ala Ile Asp Leu Ala Lys Asp Leu 260 265 270 Leu His Val His
Lys Asn Thr Tyr Ala Leu Val Val Ser Thr Glu Asn 275 280 285 Ile Thr
Tyr Asn Ile Tyr Xaa Gly Asp Asn Arg Ser Met Met Val Ser 290 295 300
Asn Cys Leu Phe Arg Xaa Gly Gly Ala Ala Ile Leu Leu Ser Asn Lys 305
310 315 320 Pro Xaa Asp Arg Arg Arg Ser Lys Tyr Glu Leu Val His Thr
Val Arg 325 330 335 Thr His Thr Gly Ala Asp Asp Lys Ser Phe Arg Cys
Val Xaa Gln Xaa 340 345 350 Xaa Asp Glu Xaa Gly Lys Xaa Gly Val Ser
Leu Ser Lys Asp Ile Thr 355 360 365 Ala Val Ala Gly Xaa Thr Val Lys
Lys Asn Ile Xaa Thr Leu Gly Pro 370 375 380 Leu Val Leu Pro Leu Ser
Glu Lys Leu Leu Phe Phe Val Thr Xaa Xaa 385 390 395 400 Ala Lys Lys
Leu Phe Lys Asp Lys Ile Lys His Tyr Tyr Val Pro Asp 405 410 415 Phe
Lys Leu Ala Ile Asp His Phe Cys Ile His Ala Gly Gly Arg Ala 420 425
430 Val Ile Asp Val Leu Glu Lys Asn Leu Gly Leu Ser Pro Ile Asp Val
435 440 445 Glu Ala Ser Arg Ser Thr Leu His Arg Phe Gly Asn Thr Ser
Ser Ser 450 455 460 Ser Ile Trp Tyr Glu Leu Ala Tyr Ile Glu Ala Lys
Gly Arg Met Lys 465 470 475 480 Lys Gly Asn Lys Val Trp Gln Ile Ala
Xaa Gly Ser Gly Phe Lys Cys 485 490 495 Asn Ser Ala Val Trp Val Ala
Leu Arg Asn Val Lys Ala Ser Thr Asn 500 505 510 Ser Pro Trp Glu His
Cys Ile Asp Arg Tyr Pro Val Lys Ile Asp Ser 515 520 525 Xaa Ser Ser
Lys Ser Glu Thr Arg Ala Gln Asn Gly Arg Ser 530 535 540
166505PRTBrassica napus 166Met Thr Ser Ile Asn Val Lys Leu Leu Tyr
His Tyr Val Ile Thr Asn 1 5 10 15 Leu Phe Asn Leu Cys Phe Phe Pro
Leu Thr Ala Ile Val Ala Gly Lys 20 25 30 Ala Tyr Leu Thr Ile Asp
Asp Leu His His Leu Tyr Tyr Ser Tyr Leu 35 40 45 Gln His Asn Leu
Ile Thr Ile Ala Pro Leu Leu Ala Phe Thr Val Phe 50 55 60 Gly Ser
Val Leu Tyr Ile Ala Thr Arg Pro Lys Pro Val Tyr Leu Val 65 70 75 80
Glu Tyr Ser Cys Tyr Leu Pro Pro Thr His Cys Arg Ser Ser Ile Ser 85
90 95 Lys Val Met Asp Ile Phe Phe Gln Val Arg Lys Ala Asp Pro Ser
Arg 100 105 110 Asn Gly Thr Cys Asp Asp Ser Ser Trp Leu Asp Phe Leu
Arg Lys Ile 115 120 125 Gln Glu Arg Ser Gly Leu Gly Asp Glu Thr His
Gly Pro Glu Gly Leu 130 135 140 Leu Gln Val Pro Pro Arg Lys Thr Phe
Ala Arg Ala Arg Glu Glu Thr 145 150 155 160 Glu Gln Val Ile Ile Gly
Ala Leu Glu Asn Leu Phe Lys Asn Thr Asn 165 170 175 Val Asn Pro Lys
Asp Ile Gly Ile Leu Val Val Asn Ser Ser Met Phe 180 185 190 Asn Pro
Thr Pro Ser Leu Ser Ala Met Val Val Asn Thr Phe Lys Leu 195 200 205
Arg Ser Asn Val Arg Ser Phe Asn Leu Gly Gly Met Gly Cys Ser Ala 210
215 220 Gly Val Ile Ala Ile Asp Leu Ala Lys Asp Leu Leu His Val His
Lys 225 230 235 240 Asn Thr Tyr Ala Leu Val Val Ser Thr Glu Asn Ile
Thr Tyr Asn Ile 245 250 255 Tyr Ala Gly Asp Asn Arg Ser Met Met Val
Ser Asn Cys Leu Phe Arg 260 265 270 Val Gly Gly Ala Ala Ile Leu Leu
Ser Asn Lys Pro Arg Asp Arg Arg 275 280 285 Arg Ser Lys Tyr Glu Leu
Val His Thr Val Arg Thr His Thr Gly Ala 290 295 300 Asp Asp Lys Ser
Phe Arg Cys Val Gln Gln Gly Asp Asp Glu Asn Gly 305 310 315 320 Gln
Thr Gly Val Ser Leu Ser Lys Asp Ile Thr Asp Val Ala Gly Arg 325 330
335 Thr Val Lys Lys Asn Ile Ala Thr Leu Gly Pro Leu Ile Leu Pro Leu
340 345 350 Ser Glu Lys Leu Leu Phe Phe Val Thr Phe Met Gly Lys Lys
Leu Phe 355 360 365 Lys Asp Glu Ile Lys His Tyr Tyr Val Pro Asp Phe
Lys Leu Ala Ile 370 375 380 Asp His Phe Cys Ile His Ala Gly Gly Lys
Ala Val Ile Asp Val Leu 385
390 395 400 Glu Lys Asn Leu Gly Leu Ala Pro Ile Asp Val Glu Ala Ser
Arg Ser 405 410 415 Thr Leu His Arg Phe Gly Asn Thr Ser Ser Ser Ser
Ile Trp Tyr Glu 420 425 430 Leu Ala Tyr Ile Glu Pro Lys Gly Arg Met
Lys Lys Gly Asn Lys Val 435 440 445 Trp Gln Ile Ala Leu Gly Ser Gly
Phe Lys Cys Asn Ser Ala Val Trp 450 455 460 Val Ala Leu Asn Asn Val
Lys Ala Ser Thr Asn Ser Pro Trp Glu His 465 470 475 480 Cys Ile Asp
Arg Tyr Pro Val Lys Ile Asp Ser Asp Ser Gly Lys Ser 485 490 495 Glu
Thr Arg Val Pro Asn Gly Arg Ser 500 505 167528PRTBrassica napus
167Met Glu Arg Thr Asn Ser Ile Glu Met Asp Gln Glu Arg Leu Thr Ala
1 5 10 15 Glu Met Ala Phe Lys Asp Ser Ser Ser Ala Val Ile Arg Ile
Arg Arg 20 25 30 Arg Leu Pro Asp Phe Leu Thr Ser Val Lys Leu Lys
Tyr Val Lys Leu 35 40 45 Gly Leu His Asn Ser Phe Asn Phe Thr Thr
Phe Leu Phe Leu Leu Ile 50 55 60 Ile Leu Pro Leu Thr Gly Thr Val
Leu Val Gln Leu Thr Gly Leu Thr 65 70 75 80 Phe Glu Thr Phe Ser Glu
Leu Trp Tyr Asn His Ala Ala Gln Leu Asp 85 90 95 Gly Val Thr Arg
Leu Ala Cys Leu Val Ser Leu Cys Phe Val Leu Ile 100 105 110 Ile Tyr
Val Thr Asn Arg Ser Lys Pro Val Tyr Leu Val Asp Phe Ser 115 120 125
Cys Tyr Lys Pro Glu Asp Glu Arg Lys Met Ser Val Asp Ser Phe Leu 130
135 140 Lys Met Thr Glu Gln Asn Gly Ala Phe Thr Asp Asp Thr Val Gln
Phe 145 150 155 160 Gln Gln Arg Ile Ser Asn Arg Ala Gly Leu Gly Asp
Glu Thr Tyr Leu 165 170 175 Pro Arg Gly Ile Thr Ser Thr Pro Pro Lys
Leu Asn Met Ser Glu Ala 180 185 190 Arg Ala Glu Ala Glu Ala Val Met
Phe Gly Ala Leu Asp Ser Leu Phe 195 200 205 Glu Lys Thr Gly Ile Lys
Pro Ala Glu Val Gly Ile Leu Ile Val Ser 210 215 220 Cys Ser Leu Phe
Asn Pro Thr Pro Ser Leu Ser Ala Met Ile Val Asn 225 230 235 240 His
Tyr Lys Met Arg Glu Asp Ile Lys Ser Tyr Asn Leu Gly Gly Met 245 250
255 Gly Cys Ser Ala Gly Leu Ile Ser Ile Asp Leu Ala Asn Asn Leu Leu
260 265 270 Lys Ala Asn Pro Asn Ser Tyr Ala Val Val Val Ser Thr Glu
Asn Ile 275 280 285 Thr Leu Asn Trp Tyr Phe Gly Asn Asp Arg Ser Met
Leu Leu Cys Asn 290 295 300 Cys Ile Phe Arg Met Gly Gly Ala Ala Ile
Leu Leu Ser Asn Arg Arg 305 310 315 320 Gln Asp Arg Ser Lys Ser Lys
Tyr Glu Leu Val Asn Val Val Arg Thr 325 330 335 His Lys Gly Ser Asp
Asp Lys Asn Tyr Asn Cys Val Tyr Gln Lys Glu 340 345 350 Asp Glu Arg
Gly Thr Ile Gly Val Ser Leu Ala Arg Glu Leu Met Ser 355 360 365 Val
Ala Gly Asp Ala Leu Lys Thr Asn Ile Thr Thr Leu Gly Pro Met 370 375
380 Val Leu Pro Leu Ser Gly Gln Leu Met Phe Ser Val Ser Leu Val Lys
385 390 395 400 Arg Lys Leu Leu Lys Leu Lys Val Lys Pro Tyr Ile Pro
Asp Phe Lys 405 410 415 Leu Ala Phe Glu His Phe Cys Ile His Ala Gly
Gly Arg Ala Val Leu 420 425 430 Asp Glu Val Gln Lys Asn Leu Asp Leu
Glu Asp Trp His Met Glu Pro 435 440 445 Ser Arg Met Thr Leu His Arg
Phe Gly Asn Thr Ser Ser Ser Ser Leu 450 455 460 Trp Tyr Glu Met Ala
Tyr Thr Glu Ala Lys Gly Arg Val Lys Ala Gly 465 470 475 480 Asp Arg
Leu Trp Gln Ile Ala Phe Gly Ser Gly Phe Lys Cys Asn Ser 485 490 495
Ala Val Trp Lys Ala Leu Arg Val Val Ser Thr Glu Glu Leu Thr Gly 500
505 510 Asn Ala Trp Ala Gly Ser Ile Glu Asn Tyr Pro Val Lys Ile Val
Gln 515 520 525 168506PRTCrambe abyssinica 168Met Thr Ser Ile Asn
Val Lys Leu Leu Tyr His Tyr Val Ile Thr Asn 1 5 10 15 Leu Phe Asn
Leu Cys Phe Phe Pro Leu Thr Ala Ile Val Ala Gly Lys 20 25 30 Ala
Ser Arg Leu Thr Ile Asp Asp Leu His His Leu Tyr Tyr Ser Tyr 35 40
45 Leu Gln His Asn Val Ile Thr Ile Ala Pro Leu Phe Ala Phe Thr Val
50 55 60 Phe Gly Ser Ile Leu Tyr Ile Val Thr Arg Pro Lys Pro Val
Tyr Leu 65 70 75 80 Val Glu Tyr Ser Cys Tyr Leu Pro Pro Thr Gln Cys
Arg Ser Ser Ile 85 90 95 Ser Lys Val Met Asp Ile Phe Tyr Gln Val
Arg Lys Ala Asp Pro Phe 100 105 110 Arg Asn Gly Thr Cys Asp Asp Ser
Ser Trp Leu Asp Phe Leu Arg Lys 115 120 125 Ile Gln Glu Arg Ser Gly
Leu Gly Asp Glu Thr His Gly Pro Glu Gly 130 135 140 Leu Leu Gln Val
Pro Pro Arg Lys Thr Phe Ala Ala Ala Arg Glu Glu 145 150 155 160 Thr
Glu Gln Val Ile Val Gly Ala Leu Lys Asn Leu Phe Glu Asn Thr 165 170
175 Lys Val Asn Pro Lys Asp Ile Gly Ile Leu Val Val Asn Ser Ser Met
180 185 190 Phe Asn Pro Thr Pro Ser Leu Ser Ala Met Val Val Asn Thr
Phe Lys 195 200 205 Leu Arg Ser Asn Val Arg Ser Phe Asn Leu Gly Gly
Met Gly Cys Ser 210 215 220 Ala Gly Val Ile Ala Ile Asp Leu Ala Lys
Asp Leu Leu His Val His 225 230 235 240 Lys Asn Thr Tyr Ala Leu Val
Val Ser Thr Glu Asn Ile Thr Tyr Asn 245 250 255 Ile Tyr Ala Gly Asp
Asn Arg Ser Met Met Val Ser Asn Cys Leu Phe 260 265 270 Arg Val Gly
Gly Ala Ala Ile Leu Leu Ser Asn Lys Pro Arg Asp Arg 275 280 285 Arg
Arg Ser Lys Tyr Glu Leu Val His Thr Val Arg Thr His Thr Gly 290 295
300 Ala Asp Asp Lys Ser Phe Arg Cys Val Gln Gln Gly Asp Asp Glu Asn
305 310 315 320 Gly Lys Thr Gly Val Ser Leu Ser Lys Asp Ile Thr Glu
Val Ala Gly 325 330 335 Arg Thr Val Lys Lys Asn Ile Ala Thr Leu Gly
Pro Leu Ile Leu Pro 340 345 350 Leu Ser Glu Lys Leu Leu Phe Phe Val
Thr Phe Met Ala Lys Lys Leu 355 360 365 Phe Lys Asp Lys Val Lys His
Tyr Tyr Val Pro Asp Phe Lys Leu Ala 370 375 380 Ile Asp His Phe Cys
Ile His Ala Gly Gly Arg Ala Val Ile Asp Val 385 390 395 400 Leu Glu
Lys Asn Leu Gly Leu Ala Pro Ile Asp Val Glu Ala Ser Arg 405 410 415
Ser Thr Leu His Arg Phe Gly Asn Thr Ser Ser Ser Ser Ile Trp Tyr 420
425 430 Glu Leu Ala Tyr Ile Glu Ala Lys Gly Arg Met Lys Lys Gly Asn
Lys 435 440 445 Val Trp Gln Ile Ala Leu Gly Ser Gly Phe Lys Cys Asn
Ser Ala Val 450 455 460 Trp Val Ala Leu Ser Asn Val Lys Ala Ser Thr
Asn Ser Pro Trp Glu 465 470 475 480 His Cys Ile Asp Arg Tyr Pro Val
Lys Ile Asp Ser Asp Ser Ala Lys 485 490 495 Ser Glu Thr Arg Ala Gln
Asn Gly Arg Ser 500 505 169506PRTCardamine graeca 169Met Thr Ser
Ile Asn Val Lys Leu Leu Tyr His Tyr Val Leu Thr Asn 1 5 10 15 Phe
Phe Asn Leu Cys Leu Phe Pro Leu Thr Ala Phe Pro Ala Gly Lys 20 25
30 Ala Ser Gln Leu Thr Thr Asn Asp Leu His His Leu Tyr Ser Tyr Leu
35 40 45 His His Asn Leu Ile Thr Val Thr Leu Leu Phe Ala Phe Thr
Val Phe 50 55 60 Gly Ser Ile Leu Tyr Ile Val Thr Arg Pro Lys Pro
Val Tyr Leu Val 65 70 75 80 Asp Tyr Ser Cys Tyr Leu Pro Pro Arg His
Leu Ser Cys Gly Ile Ser 85 90 95 Arg Val Met Glu Ile Phe Tyr Glu
Ile Arg Lys Ser Asp Pro Ser Arg 100 105 110 Glu Val Pro Phe Asp Asp
Pro Ser Ser Leu Glu Phe Leu Arg Lys Ile 115 120 125 Gln Glu Arg Ser
Gly Leu Gly Asp Glu Thr Tyr Gly Pro Gln Gly Leu 130 135 140 Val His
Asp Met Pro Leu Arg Met Asn Phe Ala Ala Ala Arg Glu Glu 145 150 155
160 Thr Glu Gln Val Ile Asn Gly Ala Leu Glu Lys Leu Phe Glu Asn Thr
165 170 175 Lys Val Asn Pro Arg Glu Ile Gly Ile Leu Val Val Asn Ser
Ser Met 180 185 190 Phe Asn Pro Thr Pro Ser Leu Ser Ala Met Val Val
Asn Thr Phe Lys 195 200 205 Leu Arg Ser Asn Ile Lys Ser Phe Ser Leu
Gly Gly Met Gly Cys Ser 210 215 220 Ala Gly Ile Ile Ala Ile Asp Leu
Ala Lys Asp Leu Leu His Val His 225 230 235 240 Lys Asn Thr Tyr Ala
Leu Val Val Ser Thr Glu Asn Ile Thr His Ser 245 250 255 Thr Tyr Thr
Gly Asp Asn Arg Ser Met Met Val Ser Asn Cys Leu Phe 260 265 270 Arg
Met Gly Gly Ala Ala Ile Leu Leu Ser Asn Lys Ala Gly Asp Arg 275 280
285 Arg Arg Ser Lys Tyr Lys Leu Ala His Thr Val Arg Thr His Thr Gly
290 295 300 Ala Asp Asp Gln Ser Phe Arg Cys Val Arg Gln Glu Asp Asp
Asp Arg 305 310 315 320 Gly Lys Ile Gly Val Cys Leu Ser Lys Asp Ile
Thr Ala Val Ala Gly 325 330 335 Lys Thr Val Thr Lys Asn Ile Ala Thr
Leu Gly Pro Leu Val Leu Pro 340 345 350 Leu Ser Glu Lys Phe Leu Tyr
Val Val Ser Leu Met Ala Lys Lys Leu 355 360 365 Phe Lys Asn Lys Ile
Lys His Thr Tyr Val Pro Asp Phe Lys Leu Ala 370 375 380 Ile Asp His
Phe Cys Ile His Ala Gly Gly Arg Ala Val Ile Asp Val 385 390 395 400
Leu Glu Lys Asn Leu Ala Leu Ser Pro Val Asp Val Glu Ala Ser Arg 405
410 415 Ser Thr Leu His Arg Phe Gly Asn Thr Ser Ser Ser Ser Ile Trp
Tyr 420 425 430 Glu Leu Ala Tyr Ile Glu Ala Lys Gly Arg Met Lys Lys
Gly Asn Lys 435 440 445 Val Trp Gln Ile Ala Ile Gly Ser Gly Phe Lys
Cys Asn Ser Ala Val 450 455 460 Trp Val Ala Leu Cys Asn Val Lys Pro
Ser Val Asn Ser Pro Trp Glu 465 470 475 480 His Cys Ile Asp Arg Tyr
Pro Val Glu Ile Asn Tyr Gly Ser Ser Lys 485 490 495 Ser Glu Thr Arg
Ala Gln Asn Gly Arg Ser 500 505 170505PRTLunaria annua 170Met Thr
Ser Ile Asn Val Lys Leu Leu Tyr His Tyr Val Ile Thr Asn 1 5 10 15
Phe Phe Asn Leu Cys Phe Phe Pro Leu Thr Ala Ile Leu Ala Gly Lys 20
25 30 Ala Ser Arg Leu Thr Thr Asn Asp Leu His His Phe Tyr Ser Tyr
Leu 35 40 45 Gln His Asn Leu Ile Thr Leu Thr Leu Leu Phe Ala Phe
Thr Val Phe 50 55 60 Gly Ser Val Leu Tyr Phe Val Thr Arg Pro Lys
Pro Val Tyr Leu Val 65 70 75 80 Asp Tyr Ser Cys Tyr Leu Pro Pro Gln
His Leu Ser Ala Gly Ile Ser 85 90 95 Lys Thr Met Glu Ile Phe Tyr
Gln Ile Arg Lys Ser Asp Pro Leu Arg 100 105 110 Asn Val Ala Leu Asp
Asp Ser Ser Ser Leu Asp Phe Leu Arg Lys Ile 115 120 125 Gln Glu Arg
Ser Gly Leu Gly Asp Glu Thr Tyr Gly Pro Glu Gly Leu 130 135 140 Phe
Glu Ile Pro Pro Arg Lys Asn Leu Ala Ser Ala Arg Glu Glu Thr 145 150
155 160 Glu Gln Val Ile Asn Gly Ala Leu Lys Asn Leu Phe Glu Asn Thr
Lys 165 170 175 Val Asn Pro Lys Glu Ile Gly Ile Leu Val Val Asn Ser
Ser Met Phe 180 185 190 Asn Pro Thr Pro Ser Leu Ser Ala Met Val Val
Asn Thr Phe Lys Leu 195 200 205 Arg Ser Asn Ile Lys Ser Phe Asn Leu
Gly Gly Met Gly Cys Ser Ala 210 215 220 Gly Val Ile Ala Ile Asp Leu
Ala Lys Asp Leu Leu His Val His Lys 225 230 235 240 Asn Thr Tyr Ala
Leu Val Val Ser Thr Glu Asn Ile Thr Gln Asn Ile 245 250 255 Tyr Thr
Gly Asp Asn Arg Ser Met Met Val Ser Asn Cys Leu Phe Arg 260 265 270
Val Gly Gly Ala Ala Ile Leu Leu Ser Asn Lys Pro Gly Asp Arg Arg 275
280 285 Arg Ser Lys Tyr Arg Leu Ala His Thr Val Arg Thr His Thr Gly
Ala 290 295 300 Asp Asp Lys Ser Phe Gly Cys Val Arg Gln Glu Glu Asp
Asp Ser Gly 305 310 315 320 Lys Thr Gly Val Ser Leu Ser Lys Asp Ile
Thr Gly Val Ala Gly Ile 325 330 335 Thr Val Gln Lys Asn Ile Thr Thr
Leu Gly Pro Leu Val Leu Pro Leu 340 345 350 Ser Glu Lys Ile Leu Phe
Val Val Thr Phe Val Ala Lys Lys Leu Leu 355 360 365 Lys Asp Lys Ile
Lys His Tyr Tyr Val Pro Asp Phe Lys Leu Ala Val 370 375 380 Asp His
Phe Cys Ile His Ala Gly Gly Arg Ala Val Ile Asp Val Leu 385 390 395
400 Glu Lys Asn Leu Gly Leu Ser Pro Ile Asp Val Glu Ala Ser Arg Ser
405 410 415 Thr Leu His Arg Phe Gly Asn Thr Ser Ser Ser Ser Ile Trp
Tyr Glu 420 425 430 Leu Ala Tyr Ile Glu Ala Lys Gly Arg Met Lys Lys
Gly Asn Lys Ala 435 440 445 Trp Gln Ile Ala Val Gly Ser Gly Phe Lys
Cys Asn Ser Ala Val Trp 450 455 460 Val Ala Leu Arg Asn Val Lys Ala
Ser Ala Asn Ser Pro Trp Glu His 465 470 475 480 Cys Ile His Lys Tyr
Pro Val Gln Met Tyr Ser Gly Ser Ser Lys Ser 485 490 495 Glu Thr Arg
Ala Gln Asn Gly Arg Ser 500 505 171503PRTTropaeolum majus 171Met
Ser Gly Thr Lys Ala Thr Ser Val Ser Val Pro Leu Pro Asp Phe 1 5 10
15 Lys Gln Ser Val Asn Leu Lys Tyr Val Lys Leu Gly Tyr His Tyr Ser
20 25 30 Ile Thr His Ala Met Tyr Leu Phe Leu Thr Pro Leu Leu Leu
Ile Met 35 40 45 Ser Ala Gln Ile Ser Thr Phe Ser Ile Gln Asp Phe
His His Leu Tyr 50 55 60 Asn His Leu Ile Leu His Asn Leu Ser Ser
Leu Ile Leu Cys Ile Ala 65 70 75 80 Leu Leu Leu Phe Val Leu Thr Leu
Tyr Phe Leu Thr Arg Pro Thr Pro 85 90 95 Val Tyr Leu Leu Asn Phe
Ser Cys Tyr Lys Pro Asp Ala Ile His Lys 100 105 110 Cys Asp Arg Arg
Arg Phe Met Asp Thr Ile Arg Gly Met Gly Thr Tyr 115 120 125 Thr Glu
Glu Asn Ile Glu Phe Gln
Arg Lys Val Leu Glu Arg Ser Gly 130 135 140 Ile Gly Glu Ser Ser Tyr
Leu Pro Pro Thr Val Phe Lys Ile Pro Pro 145 150 155 160 Arg Val Tyr
Asp Ala Glu Glu Arg Ala Glu Ala Glu Met Leu Met Phe 165 170 175 Gly
Ala Val Asp Gly Leu Phe Glu Lys Ile Ser Val Lys Pro Asn Gln 180 185
190 Ile Gly Val Leu Val Val Asn Cys Gly Leu Phe Asn Pro Ile Pro Ser
195 200 205 Leu Ser Ser Met Ile Val Asn Arg Tyr Lys Met Arg Gly Asn
Val Phe 210 215 220 Ser Tyr Asn Leu Gly Gly Met Gly Cys Ser Ala Gly
Val Ile Ser Ile 225 230 235 240 Asp Leu Ala Lys Asp Leu Leu Gln Val
Arg Pro Asn Ser Tyr Ala Leu 245 250 255 Val Val Ser Leu Glu Cys Ile
Ser Lys Asn Leu Tyr Leu Gly Glu Gln 260 265 270 Arg Ser Met Leu Val
Ser Asn Cys Leu Phe Arg Met Gly Gly Ala Ala 275 280 285 Ile Leu Leu
Ser Asn Lys Met Ser Asp Arg Trp Arg Ser Lys Tyr Arg 290 295 300 Leu
Val His Thr Val Arg Thr His Lys Gly Thr Glu Asp Asn Cys Phe 305 310
315 320 Ser Cys Val Thr Arg Lys Glu Asp Ser Asp Gly Lys Ile Gly Ile
Ser 325 330 335 Leu Ser Lys Asn Leu Met Ala Val Ala Gly Asp Ala Leu
Lys Thr Asn 340 345 350 Ile Thr Thr Leu Gly Pro Leu Val Leu Pro Met
Ser Glu Gln Leu Leu 355 360 365 Phe Phe Ala Thr Leu Val Gly Lys Lys
Val Phe Lys Met Lys Leu Gln 370 375 380 Pro Tyr Ile Pro Asp Phe Lys
Leu Ala Phe Glu His Phe Cys Ile His 385 390 395 400 Ala Gly Gly Arg
Ala Val Leu Asp Glu Leu Glu Lys Asn Leu Lys Leu 405 410 415 Ser Ser
Trp His Met Glu Pro Ser Arg Met Ser Leu Tyr Arg Phe Gly 420 425 430
Asn Thr Ser Ser Ser Ser Leu Trp Tyr Glu Leu Ala Tyr Ser Glu Ala 435
440 445 Lys Gly Arg Ile Lys Lys Gly Asp Arg Val Trp Gln Ile Ala Phe
Gly 450 455 460 Ser Gly Phe Lys Cys Asn Ser Ala Val Trp Lys Ala Leu
Arg Asn Val 465 470 475 480 Asn Pro Ala Glu Glu Lys Asn Pro Trp Met
Asp Glu Ile His Leu Phe 485 490 495 Pro Val Glu Val Pro Leu Asn
500
References









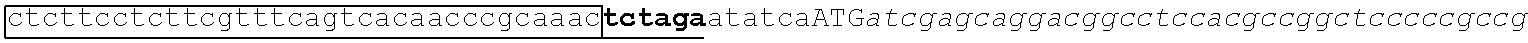

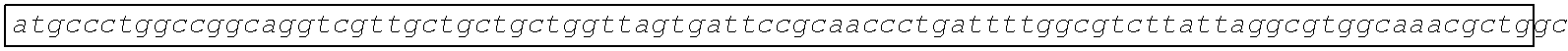





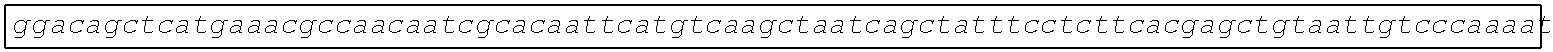





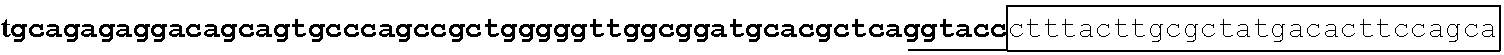






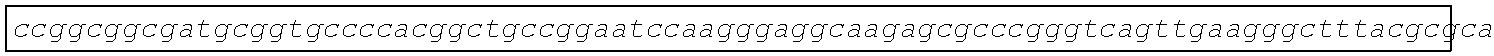






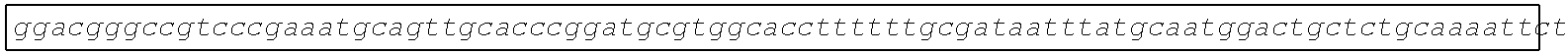






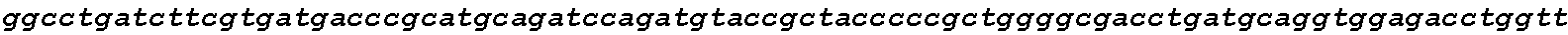




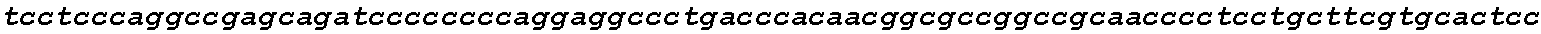






















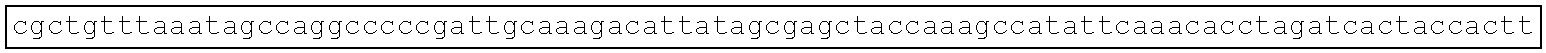












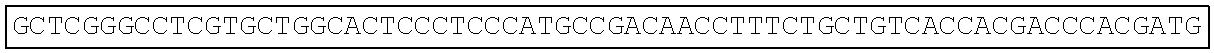
















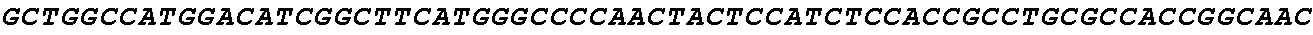










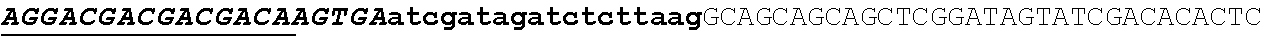




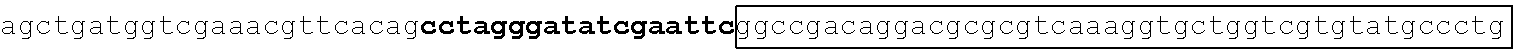


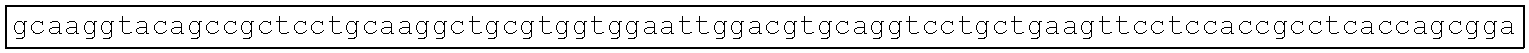













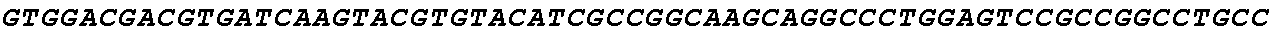


















































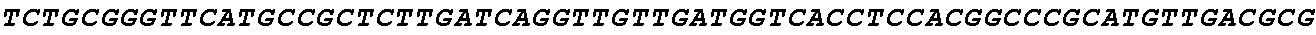


































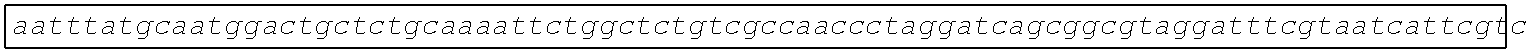


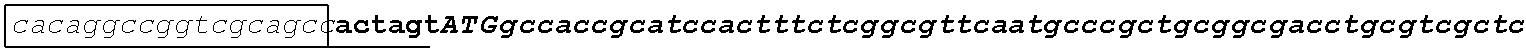






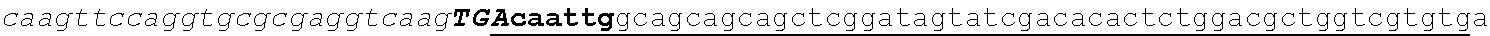




















































































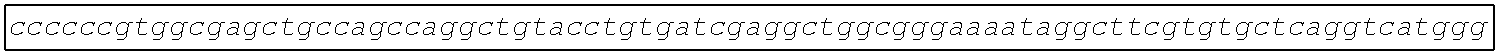


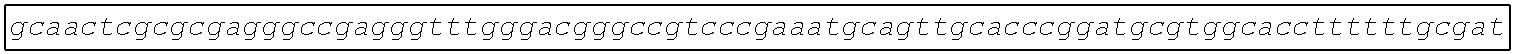









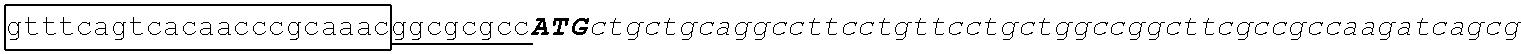

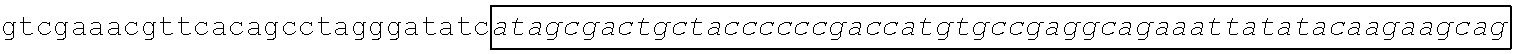





































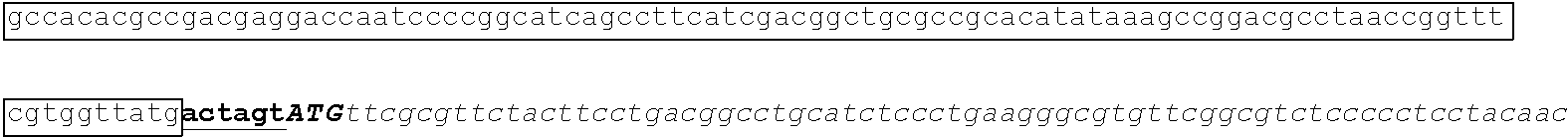








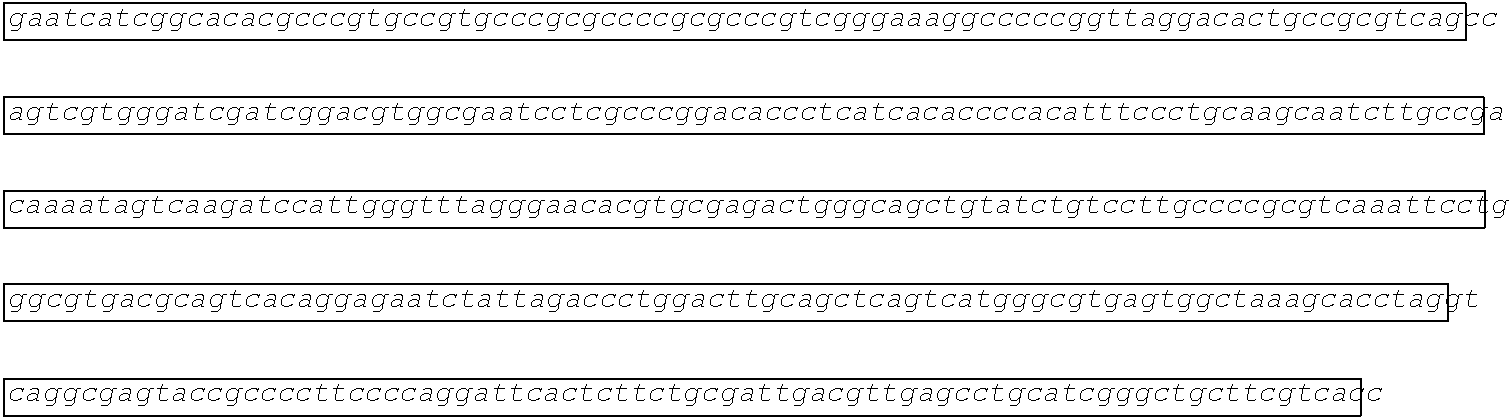
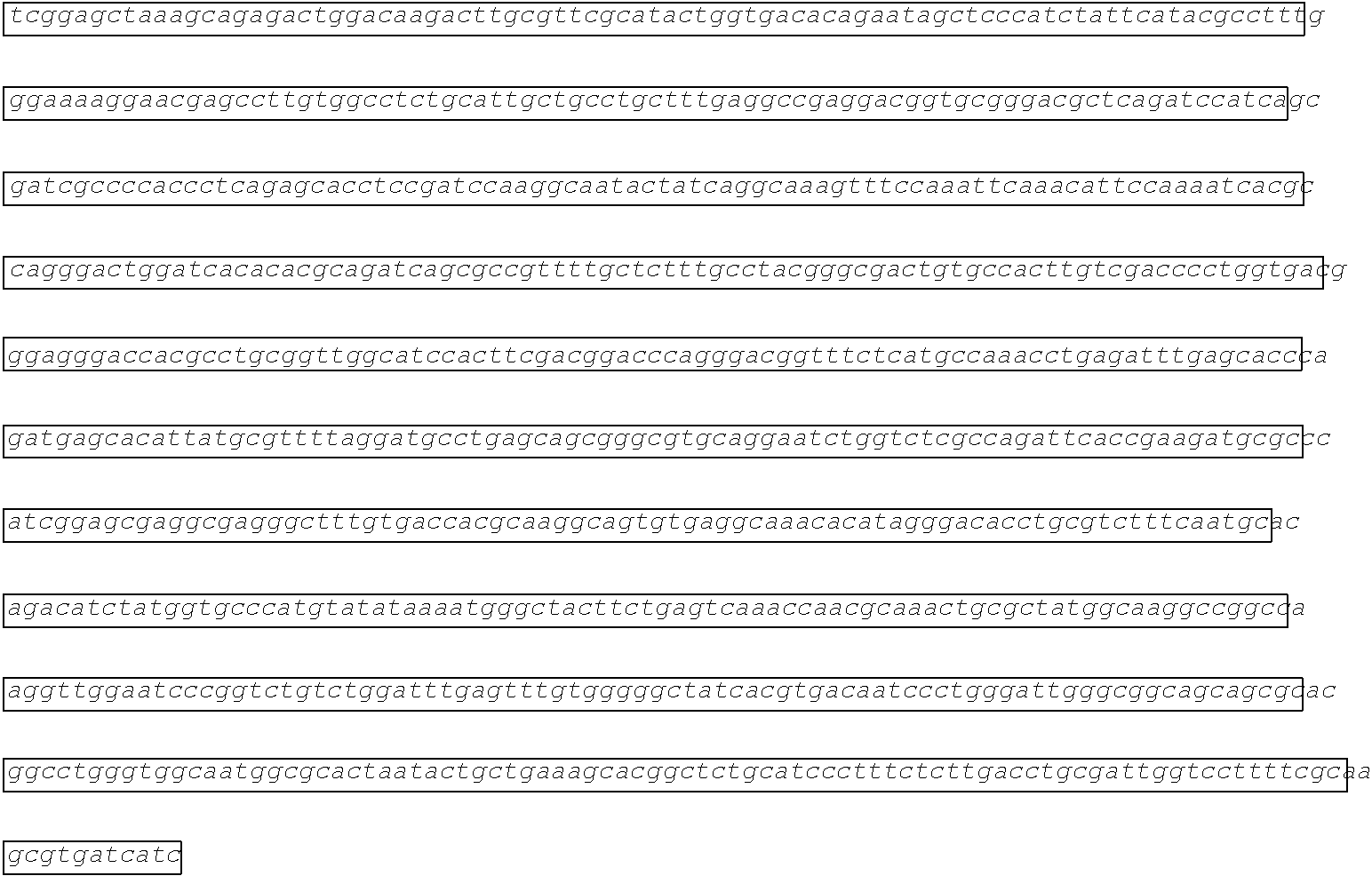



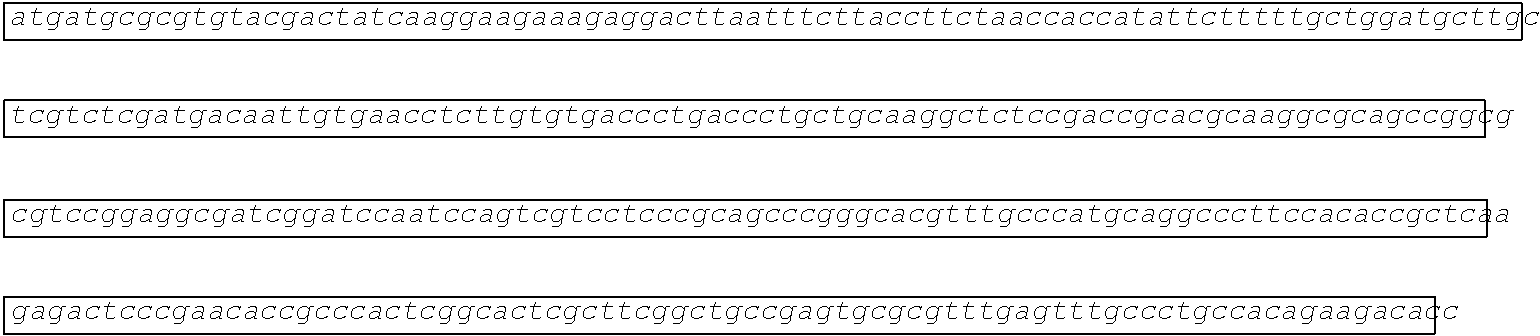









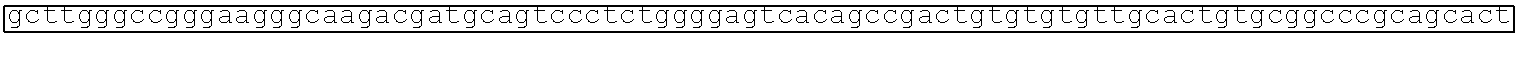


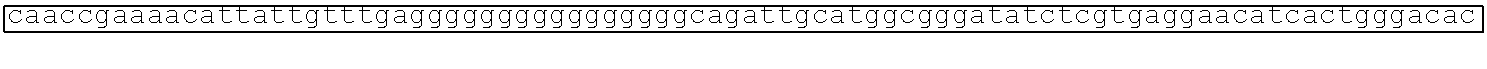


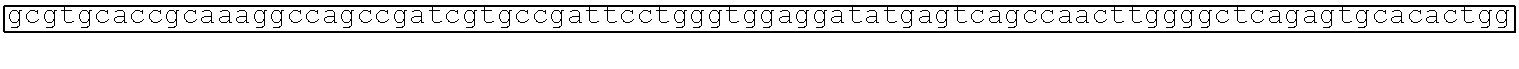

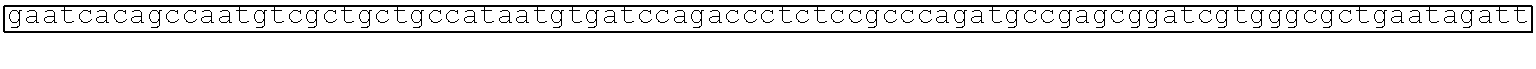


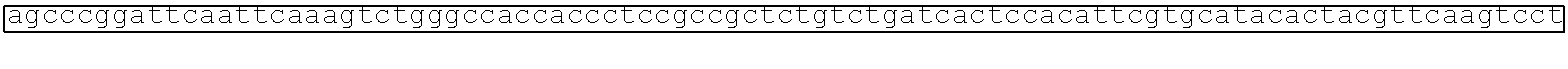



















































































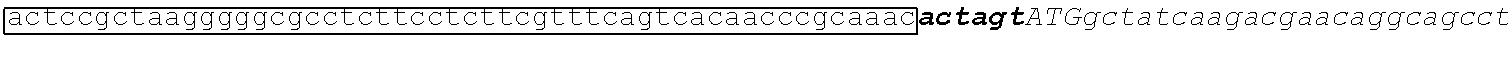





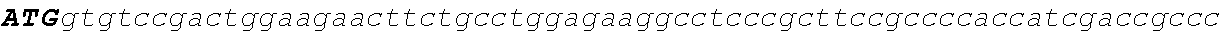





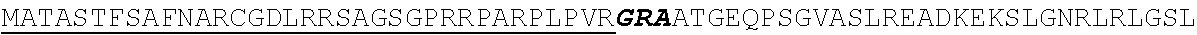

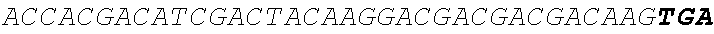


D00001

D00002

D00003
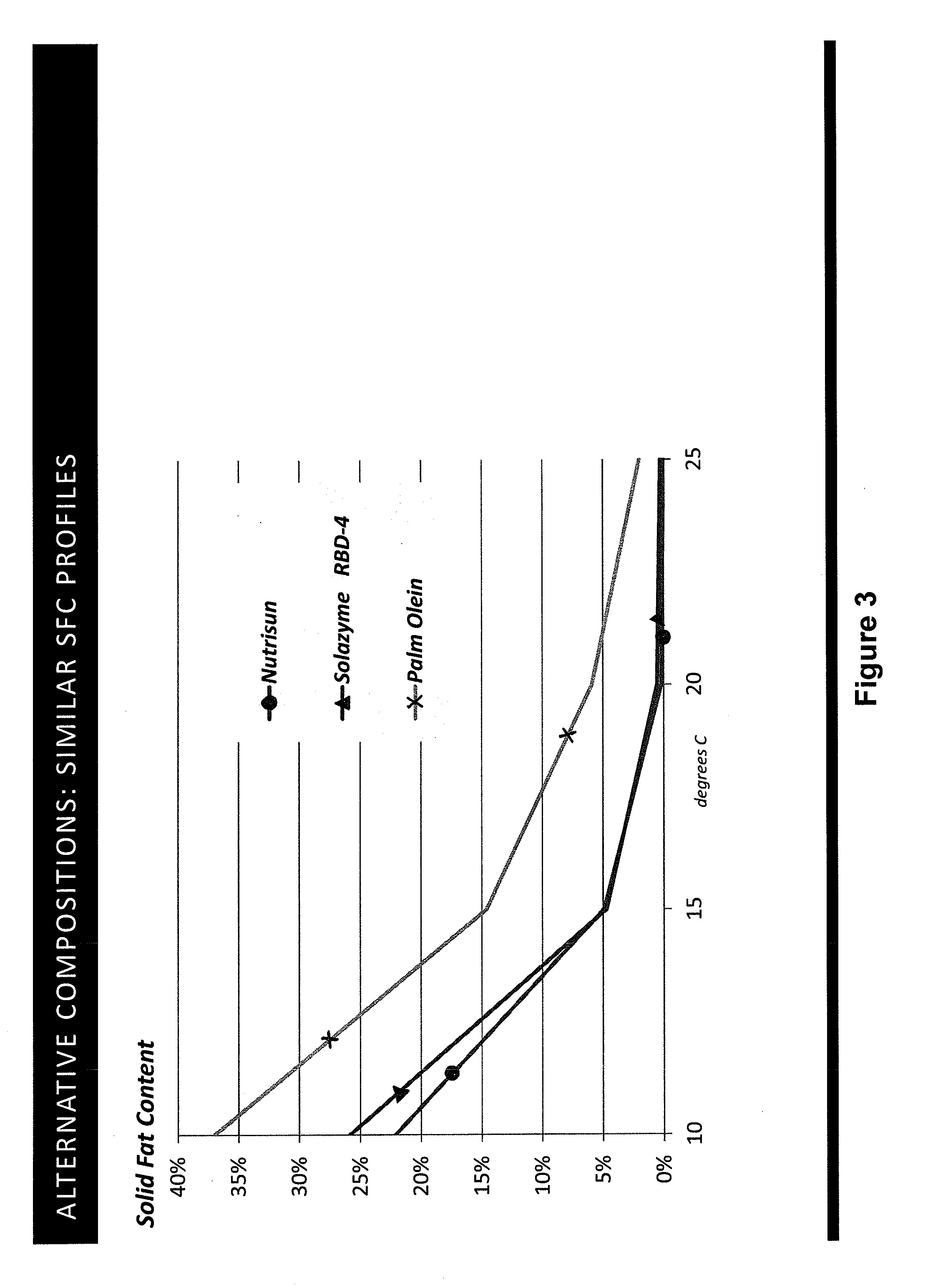
D00004

D00005

D00006

D00007
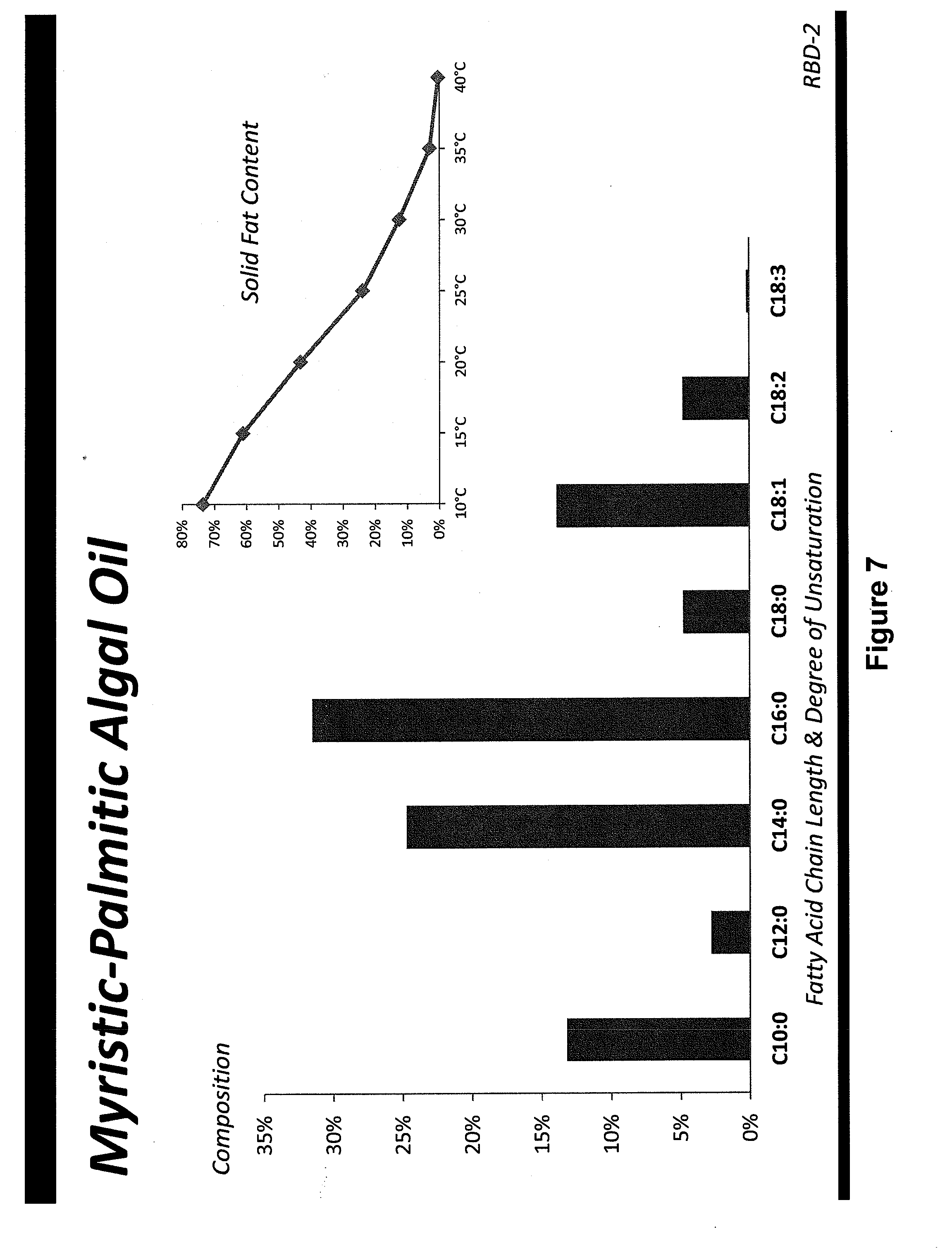
D00008
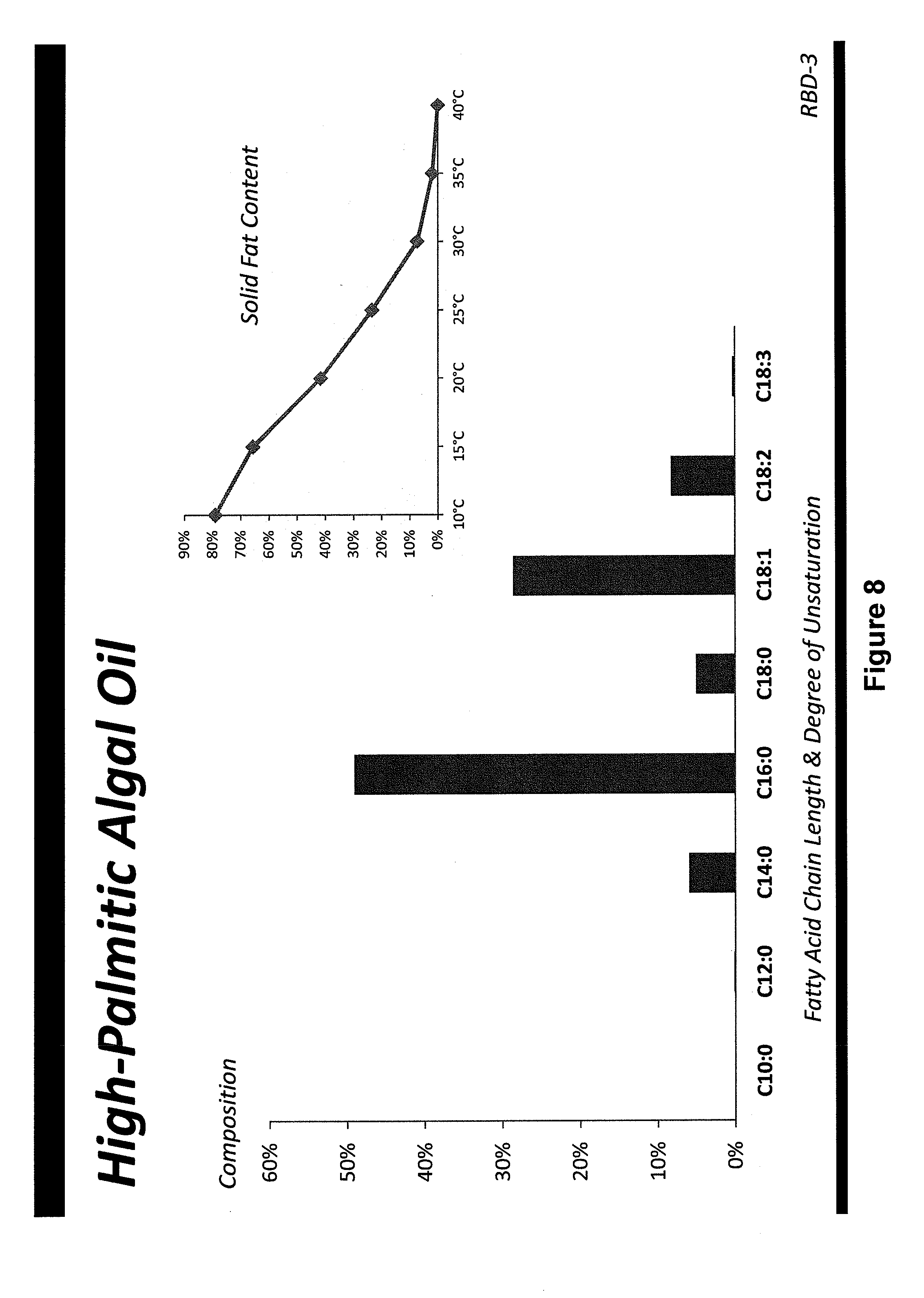
D00009

D00010
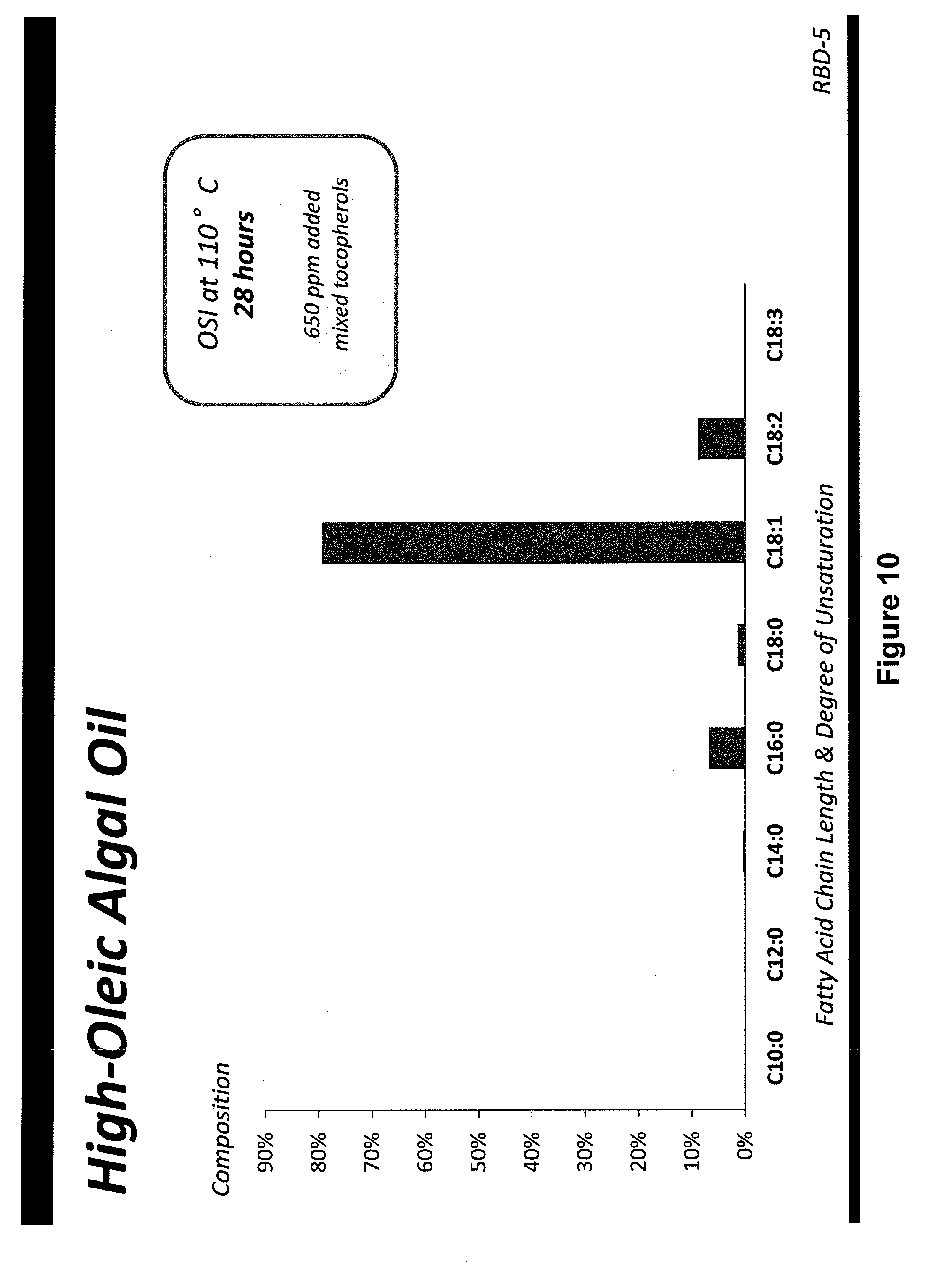
D00011

D00012

D00013

D00014

D00015

D00016

D00017

D00018

D00019

D00020
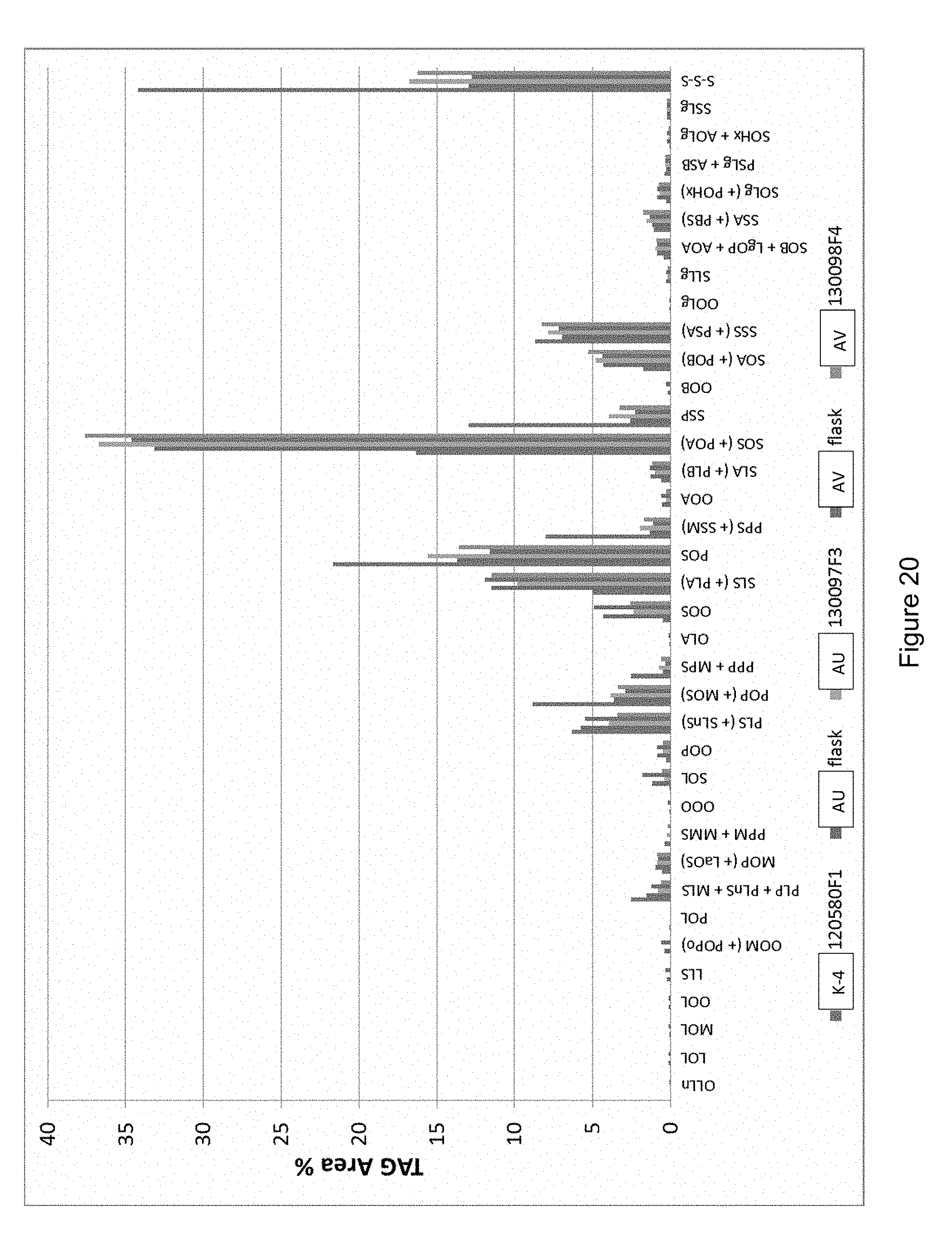
D00021

D00022

D00023

D00024

P00001

P00002

P00003

P00004

P00005
P00006

P00007

P00008
P00009

P00010

P00011

P00012
P00013

P00014
P00015

P00016

P00017
P00018

P00019

P00020

P00021

P00022

P00023

P00024

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.