Genetically Engineered Bacterium For Treatment Of Breast Cancer, Method For Constructing The Bacterium, And Applications Thereof
Lin; Yan ; et al.
U.S. patent application number 14/766558 was filed with the patent office on 2015-12-31 for genetically engineered bacterium for treatment of breast cancer, method for constructing the bacterium, and applications thereof. This patent application is currently assigned to Nanjing Sinogen Biotech & Pharmaceutical Inc.. The applicant listed for this patent is NANJING SINOGEN BIOTECH & PHARMACEUTICAL INC.. Invention is credited to Fanghong Li, Xiaoxi Li, Yan Lin, Pengli Yu, Allan Zhao, Sujin Zhou.
| Application Number | 20150376593 14/766558 |
| Document ID | / |
| Family ID | 48544949 |
| Filed Date | 2015-12-31 |
| United States Patent Application | 20150376593 |
| Kind Code | A1 |
| Lin; Yan ; et al. | December 31, 2015 |
GENETICALLY ENGINEERED BACTERIUM FOR TREATMENT OF BREAST CANCER, METHOD FOR CONSTRUCTING THE BACTERIUM, AND APPLICATIONS THEREOF
Abstract
The current invention discloses a genetically engineered bacterium used for the treatment of breast cancer. The said bacterium is attenuated Salmonella typhimurium VNP20009 with cloned L-methioninase gene. The method for constructing this genetically engineered bacterium and the application thereof are also disclosed herein. In the current invention, our biologic drug for the treatment of breast cancer is a type of safe, non-toxic new drug with anti-tumor activity. It can highly express methioninase through recombinant DNA technology using attenuated Salmonella typhimurium VNP20009 as a carrier, which has a strong anti-tumor activity and can meet the needs. The preparation method is simple and easy to operate, showing good application prospect.
| Inventors: | Lin; Yan; (Nanjing, Jiangsu, CN) ; Zhou; Sujin; (Nanjing, Jiangsu, CN) ; Zhao; Allan; (Nanjing, Jiangsu, CN) ; Li; Xiaoxi; (Nanjing, Jiangsu, CN) ; Yu; Pengli; (Nanjing, Jiangsu, CN) ; Li; Fanghong; (Nanjing, Jiangsu, CN) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assignee: | Nanjing Sinogen Biotech &
Pharmaceutical Inc. Nanjing, Jiangsu CN |
||||||||||
| Family ID: | 48544949 | ||||||||||
| Appl. No.: | 14/766558 | ||||||||||
| Filed: | February 27, 2014 | ||||||||||
| PCT Filed: | February 27, 2014 | ||||||||||
| PCT NO: | PCT/CN2014/072652 | ||||||||||
| 371 Date: | August 7, 2015 |
| Current U.S. Class: | 424/93.2 ; 435/471 |
| Current CPC Class: | C07K 14/195 20130101; C12Y 404/01011 20130101; Y02A 50/481 20180101; A61K 48/005 20130101; A61K 38/00 20130101; C12N 9/88 20130101; C12N 15/74 20130101; Y02A 50/30 20180101; A61P 35/00 20180101 |
| International Class: | C12N 9/88 20060101 C12N009/88; C12N 15/74 20060101 C12N015/74 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Feb 28, 2013 | CN | 201310062253.7 |
Claims
1. An application of a genetically engineered bacterium for the treatment of breast cancer, wherein the genetically engineered bacterium is attenuated Salmonella typhimurium VNP20009 with a cloned L-methioninase gene.
2. A method for constructing the genetically engineered bacterium for breast cancer according to claim 1, wherein the L-methioninase gene is subcloned into pUC57 plasmid, and then subcloned into pSVSPORT plasmid through Kpn I and Hind III restriction sites to obtain pSVSPORT-L-methioninase expression plasmid, which then is transformed via electropolaration into attenuated Salmonella typhimurium VNP20009, to obtain the genetically engineered bacterium.
3. The method for constructing the genetically engineered bacterium for the treatment of breast cancer according to claim 2, wherein conditions for the electroporation are as follows: voltage 2400 V, resistor 400 .OMEGA., capacitor 25 .mu.F, time constant 4 ms.
4. The application of genetically engineered bacterium according to claim 1 in preparing drugs for treatment of breast cancer.
Description
TECHNICAL FIELD
[0001] The current invention relates to drugs for the treatment of cancers, in particular, to the construction and applications of the genetically engineered bacterium in preparing the drugs for the treatment of breast cancer.
BACKGROUND OF THE INVENTION
[0002] Breast cancer is one of the common malignancies of women, and its incidence is in the first place among female cancer patients. Currently the primary treatment procedures for breast cancer include surgery, radiotherapy, chemotherapy, and hormone therapy, etc. Chemotherapy is always playing an important role in the comprehensive treatment of breast cancer due to the sensitivity of breast cancer to anti-cancer drugs Traditional drugs for chemotherapy in breast cancer mainly include doxorubicin, cyclophosphamide, 5-fluorouracil, etc. Although these drugs have been widely used in the treatment of breast cancer, their therapeutic efficacy is limited due to the toxicity and resistance of patients. In recent years, targeted therapy is used clinically, with the primary drugs like Herceptin, tyrosine kinase inhibitor, lapatinib, pertuzumab monoclonal antibody, bevacizumab, flavopiridol, etc. However, due to patient's tolerance, physical properties of drugs like instability and solubility and the targets, targeted therapy only applies to a subgroup of patients. With the advances in bacterial- and viral-based gene therapy and genetic engineering technology, mounting studies have focused on bacterial treatment of tumors since the middle 1990s. Results have shown that Salmonella typhimurium can inhibit the growth of tumor cells in mice in a targeted and efficient manner.
[0003] Salmonella is a group of Gram-negative, invasive intracellular facultative anaerobes parasitized in human and animal intestinal tracts. VNP20009 is an attenuated Salmonella typhimurium strain with the deletion of msb B and pur I genes. It is genetically stable and sensitive to antibiotics. The msb B protein is necessary for the lipid acylation to endotoxin, and the lipid acylation at A-terminal cannot be achieved when deleted, lowering the toxicity. The pur I protein is involved in purine metabolism, deletion of this gene leads to dependence of exogenous adenine when culturing the bacteria. These gene manipulations in VNP20009 also lower the production of tumor necrosis factor (TNF), thereby reducing the inflammatory response. Consequently, the low pathogenicity improves the safety of its clinical usage. VNP20009 has been widely used in cancer research, which can influence the growth of a variety of solid tumor models of mice, including melanoma, lung cancer, colon cancer, breast cancer, renal cancer and prostate cancer. VNP20009, as a vector of gene therapy, has the ability to accumulate in the tumor site in a highly targeted fashion. Researchers have found in the mouse models carrying a variety of solid tumors that the quantity of VNP20009 in tumors is 200-1000 times as high as that in non-cancerous major organs, such as the liver. It uses a more complex set of mechanisms to target tumors. VNP20009 can preferentially accumulate and multiply under the hypoxic and necrotic conditions in the tumor tissue. At the same time, the bacteria multiply significantly faster in the tumor tissues than in the normal tissues, making it possible for the attenuated Salmonella to be a new type of anti-tumor agent and the vector of targeted gene therapy. Potential mechanisms for the effect of a slow tumor growth by VNP20009 may include the follows: 1) Breakdown of nutrients necessary for tumor growth by the bacteria, e.g., the enzymes produced by bacteria such as asparaginase, can deplete essential amino acids for tumor growth; 2) Stimulation of local toxin secretion or tumor necrosis factor a to tumor microenvironment can negatively influence the tumor angiogenesis. In addition, the non-specific inflammatory reaction at the bacterial growth site can activate anti-tumor T cells. Studies have shown that although attenuated Salmonella VNP2009 is an ideal carrier for gene therapy which can be applied safely with high allowable dose, its application independently has no strong anti-tumor effect and a further combination with other drugs is needed.
[0004] Tumor cells require adequate nutrition in order to maintain its high rate of reproduction. In addition to carbohydrates, the need for methionine (Met), glutamine, and arginine is particularly high. Previous studies have established that Met-dependency is a common feature of most tumor cells, such as breast cancer, lung cancer, colon cancer, kidney cancer, bladder cancer, melanoma, glioma, etc. High Met-dependency does not exist in normal cells. Both in vivo and in vitro experiments have confirmed that dietary intervention with methionine deficiency can delay the proliferation of tumor cells. However, long-term deficiency of Met can cause malnutrition, metabolic disorders, and aggravate tumor growth due to a long-term DNA hypomethylation. Thus, by specifically degrading Met to methylselenol, a-ketobutyrate and ammonia through L-methioninase and lowering the level of methionine in vivo, we will be able to effectively inhibit the growth of tumor cells or even degrade them. Experiments in animal models have confirmed that intraperitoneal injection of methioninase can inhibit the growth of Yoshida sarcoma and lung tumor in nude mice. In previous clinical trials, four patients with breast cancer, lung cancer, kidney cancer and lymphoma received methioninase injection once every 24 h. Methioninase could significantly reduce the methionine content in plasma. However, since methioninase is not natively expressed in mammalians, exogenous administration often causes the immunological response.
SUMMARY OF THE INVENTION
[0005] The first technical know-how in the current invention is to provide a genetically engineered bacterium for effective treatment of breast cancer. The strain is safe and non-toxic with anti-tumor activity and it can meet the clinical needs.
[0006] The second technical know-how in the current invention is to provide the method for constructing the above genetically engineered bacterium.
[0007] The final technical know-how in the current invention is to provide the application of the above genetically engineered bacterium.
[0008] To reach such goal, the current invention deployed the technical schemes as follows:
[0009] A genetically engineered bacterium for the treatment of breast cancer, and the said genetically engineered bacterium is attenuated Salmonella typhimurium VNP20009 with cloned L-methioninase gene.
[0010] Wherein the said VNP20009 contains pSVSPORT plasmid and the said L-methioninase gene is cloned on pSVSPORT plasmid.
[0011] The method for the construction of the genetically engineered bacterium for the treatment of breast cancer is as follows: the L-methioninase gene is subcloned into pUC57 plasmid, and then subcloned into pSVSPORT plasmid through Kpn I and Hind III restriction sites to obtain pSVSPORT-L-methioninase expression plasmid, which then is transformed into attenuated Salmonella typhimurium VNP20009, to obtain the genetically engineered bacterium.
[0012] Wherein the said electroporation condition is as follows: voltage 2400 V, resistor 400 .OMEGA., capacitor 25 .mu.F, time constant 4 ms.
[0013] The application of above genetically engineered bacterium in preparing drugs for treatment of breast cancer.
[0014] The current invention provides a genetically engineered tumor-targeting bacterium. It has tumor targeting and can continuously express L-methioninase in tumor tissues, which then consume methionine and a series of other nutrients, and depletes the tumor cells of nutrition, causing slow growth. Besides, the strain possibly activates caspase-3 apoptosis signaling pathway, leading to the death of the host tumor cells. Therefore, it can be used as the drug for the treatment of breast cancer.
[0015] Beneficial effects: compared with prior technology, our drug used for the treatment of breast cancer is a new, safe, non-toxic biological drug with anti-tumor activity, which can highly express methioninase through recombinant DNA technology using attenuated Salmonella typhimurium VNP20009 as a carrier. It can meet the needs with a strong anti-tumor activity. The preparation method is simple and easy to operate, showing good application prospect.
BRIEF DESCRIPTION OF THE DRAWINGS
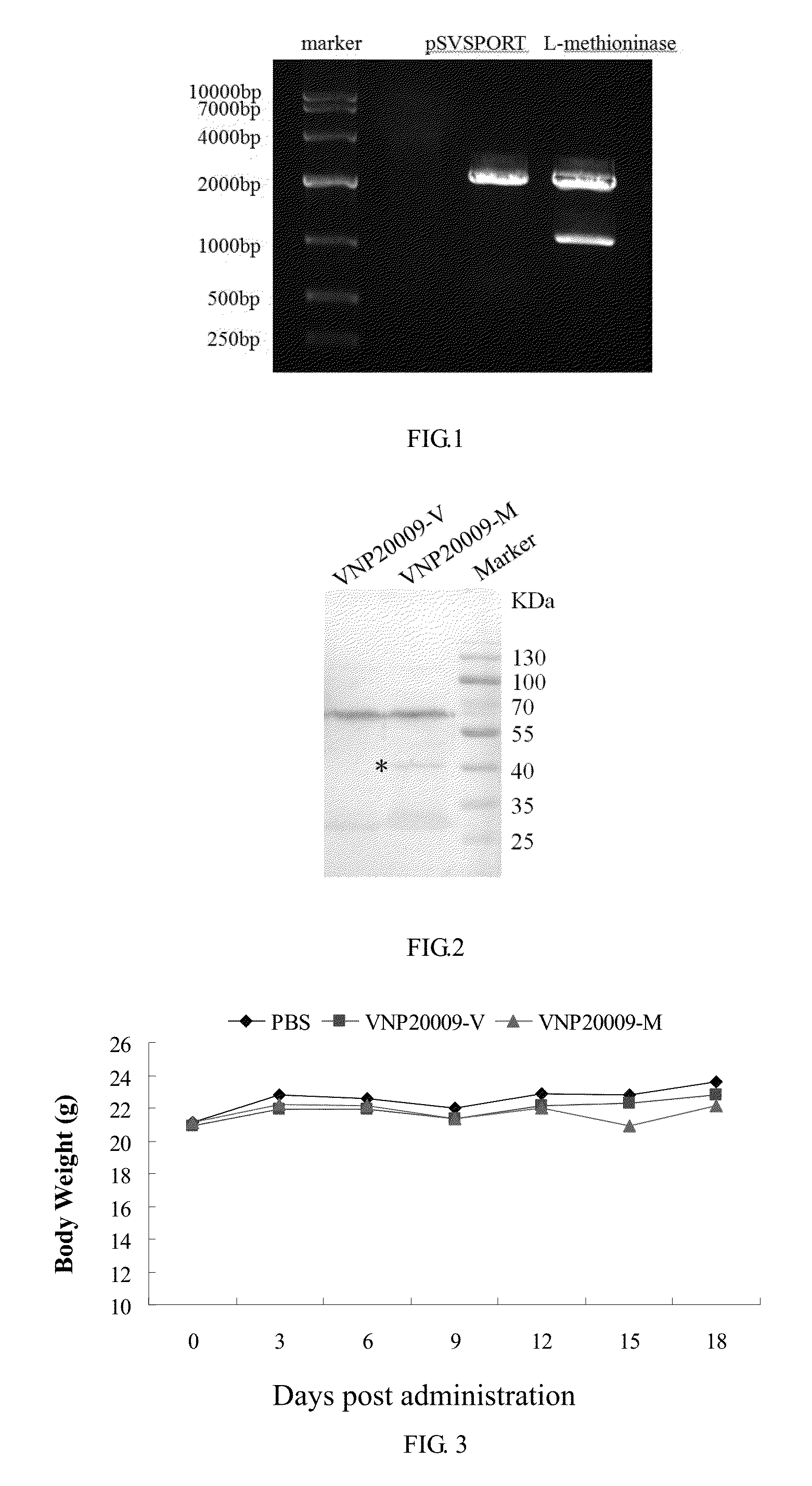
[0016] FIG. 1 shows 1% agarose gel electrophoresis by plasmid pSVSPORT-L-methioninase following restriction enzyme digestion.
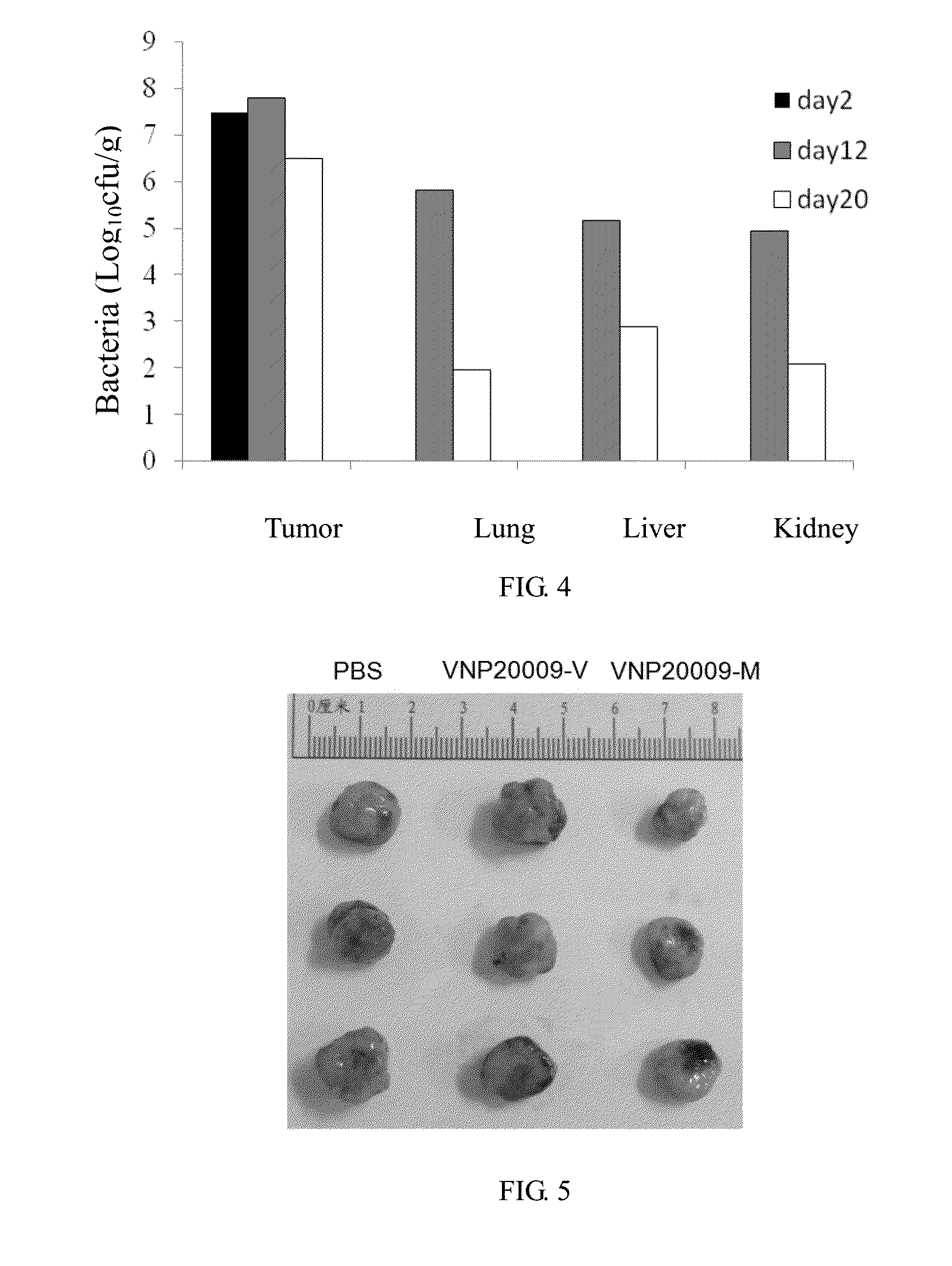
[0017] FIG. 2 shows methioninase expression identification by Western blot.
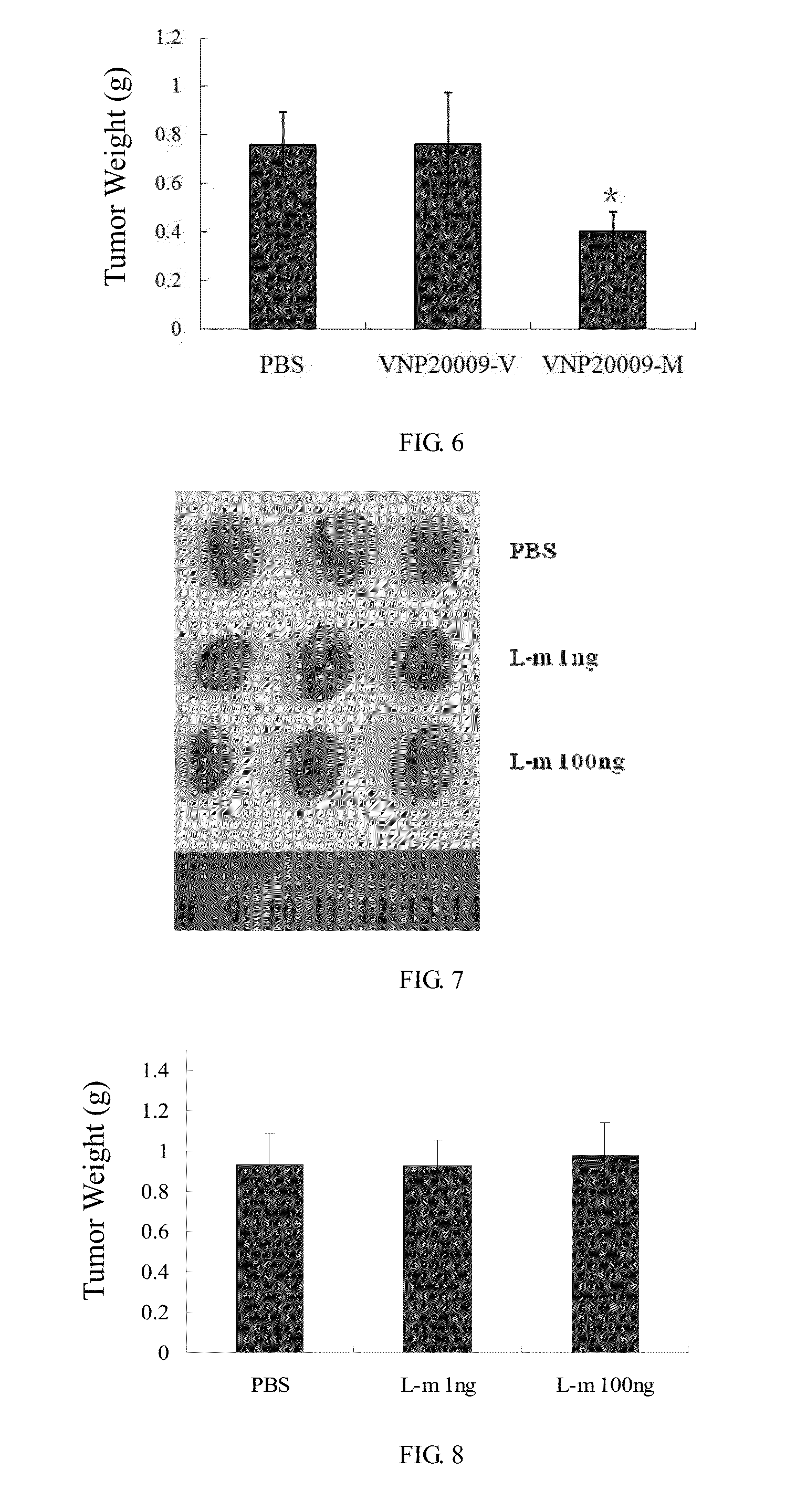
[0018] FIG. 3 shows the influence of Salmonella injection on the body weight of nude mice.
[0019] FIG. 4 shows the results of Salmonella distribution following intratumoral injection in nude mice.
[0020] FIG. 5 shows the tumor size 2 weeks after administration of Salmonella.
[0021] FIG. 6 shows the tumor weight 2 weeks after administration of Salmonella.
[0022] FIG. 7 shows the tumor size 2 weeks after administration of L-methioninase.
[0023] FIG. 8 shows the tumor weight 2 weeks after administration of L-methioninase.
DETAILED DESCRIPTION OF THE EMBODIMENT
[0024] The invention is described herein in connection with drawings and certain specific embodiments. However, to the extent that the following detailed description is specific to a particular embodiment or a particular use, this is intended to be illustrative only and is not to be construed as limiting the scope of the invention.
Example 1: Construction of Genetically Engineered Bacterium
(1) Construction of Plasmids Expressing L-Methioninase Gene
[0025] Firstly, the L-methioninase (GenBank: L43133.1) is synthesized and subcloned into pUC57 plasmid (GenScript Corporation), then subcloned into plasmid pSVSPORT (Invitrogen) through Kpn I and Hind III restriction sites, to get pSVSPORT-L-methioninase expressing plasmid. The specific procedures are as follows:
[0026] Double enzyme digestion of plasmid pSVSPORT with Kpn I and Hind III: 2 .mu.g plasmid DNA, 3 .mu.L 10.times. buffer, 1.5 .mu.L Kpn I, 1.5 .mu.L Hind III. Add ddH.sub.2O to 30 .mu.L and incubate at 37.degree. C. for 3 h, and then separate the digests by 1% agarose gel electrophoresis, to cut out DNA bands with the size of 4.1 kb, and then purify DNA using the gel recovery and purification kit.
[0027] The DNA fragments in L-methioninase coding region obtained by gene synthesis are subcloned into plasmid pUC57 (GenScript Corporation). Perform restriction digests as follows: 3 .mu.g plasmid DNA, 3 .mu.L 10.times. buffer, 1.5 .mu.L Kpn I, 1.5 .mu.L Hind III. Add ddH.sub.2O to 30 .mu.L and incubate at 37.degree. C. for 3 h. Then separate the digests by 1% agarose gel electrophoresis. We cut out DNA bands with the size of 1.2 kb, and then purify DNA using a gel recovery and purification kit.
[0028] The pSVSPORT (Kpn I/Hind III) is ligated to DNA fragment of L-methioninase coding region (Kpn I/Hind III). Add 2 .mu.L vector, 6 .mu.L inserted fragment, 1 .mu.L T4 DNA ligase in the ligation reaction, and incubate at 16.degree. C. for 16 h.
[0029] The ligation product is transformed to competent cells of E.coli DH5.alpha. (Takara). Use one tube 50 .mu.L of DH5.alpha. competent cells and place on ice until thawing. Add 5 .mu.L of the above ligation product to the DH5.alpha. and mix them gently, and then incubate on ice for 30 min; after heat shock at 42.degree. C. for 60 s, cold shock on ice for 2 min; add 500 .mu.L of LB without antibiotic and culture at 37.degree. C. with shaking for 1 h; spin tube at 4000 rpm for 5 min; remove all but 100 .mu.L of LB and resuspend pellet with pipette tip. Place suspensions on LB plate containing ampicillin, and then incubate at 37.degree. C. for 16 h.
[0030] When clones grow out, pick up the monoclonal colonies into 3 mL LB containing ampicillin, culture at 37.degree. C. with shaking for 1 h. Extract the plasmid DNA from cultures and identify by Kpn I and Hind III restriction analysis. DNA bands of 4.1 and 1.2 kb are measured in positive clones, as shown in FIG. 1. Then the positive clone is sent for sequencing to confirm the identity of the insert fragment.
(2) Construct VNP20009-L-Methioninase Strain
[0031] The plasmid pSVSPORT and pSVSPORT-L-methioninase are electroporate into VNP20009 strain (YS1646), named VNP20009-V and VNP20009-M respectively. The specific construction procedures are as follows:
[0032] Place competent bacteria VNP20009 on ice. After thawing, transfer it to a pre-cooled electroporation cuvette and add 24 plasmid, slightly mix them, then incubate on ice for 1 min. Put the cuvette into electroporation apparatus seted to 2400 V, 400 .OMEGA., 25 .mu.F and 4 ms. After pulse, immediately add 1 mL SOC medium to the cuvette and mix gently. Culture at 37.degree. C. with shaking for 1 h, centrifuge at 4000 rpm for 5 min and remove all but 100 .mu.L of LB and resuspend pellet with pipette tip. Plate the electroporation mixture on LB plate containing ampicillin, and then incubate at 37.degree. C. for 16 h. After VNP20009-V and VNP20009-M are cultured with LB, extract the plasmid and identification by restriction digestion.
[0033] Extract proteins from 1.times.10.sup.8 Salmonella and separate by 10% SDS-PAGE electrophoresis, transfer to PVDF membranes in an ice bath. The membranes are blocked by incubation in BSA at room temperature for 1 h. After three 5-min washes in TBST, the membranes are incubated at 4.degree. C. overnight with rabbit antibody against L-methioninase (1:1000). After three 5-min washes in TBST, the membranes are incubated with horseradish peroxide-conjugated anti-rabbit secondary antibodies (1:10000) for 1 hr at room temperature. After three 5-min washes in TBST, the protein bands are visualized using enhanced chemiluminescence (ECL) reagents. The results are shown in FIG. 2. There is a specific band at about 43 kD molecular weight, suggesting compared with that of VNP20009 and VNP20009-V, L-methioninase expression of VNP20009-M is significantly increased.
Example 2: The Anti-Tumor Effect of VNP20009-L-Methioninase Strain
[0034] 1. Culture breast cancer cell MDA-MB-231 using MEM medium containing 10% fetal bovine serum and inoculate 2.times.10.sup.6 cells on the right armpit of nude mice. Observe the state of mice every 2 to 3 days and measure the tumor size using a vernier caliper (volume=0.52 .times.length .times.width.sup.2). When the tumor size reaches 0.1.about.0.2 cm.sup.3 , tumor-bearing mice are randomized: PBS, VNP20009-V and VNP20009-M groups.
[0035] 2. Culture VNP20009-V and VNP20009-M with LB-O. When OD.apprxeq.0.6, collect the thallus and re-suspend it in PBS. Mice are administered by intratumoral injection at a dose of 2.times.10.sup.6CFU each, while the control group are administered with the same volume of PBS. After administration, observe the activities, eating Patterns and body weight of nude mice, results are shown in FIG. 3. After bacterial injection, the body weight of mice is not affected; moreover, the feeding and feces of nude mice have no abnormalities, indicating that VNP20009-V and VNP20009-M have no obvious toxicity to nude mice.
[0036] 3. After administration, on day 2, 12, 20, take major tissues of nude mice, to grind and homogenize with PBS and culture them on LB plates overnight after gradient dilution. Results are shown in FIG. 4--the quantitative colony count results of tissue homogenate. After two day of intratumoral bacteria injection, the bacteria count in the tumor tissue is 3.times.10.sup.7 CFU/g, while no bacteria is detected in liver, kidney, etc. Twelve days later, the count of bacteria in the tumor tissue is 6.3.times.10.sup.7 CFU/g, while that in the liver is 1.5.times.10.sup.5 CFU/g, to reach a ratio about 400:1. Twenty days later, the ratio of bacteria between the tumor tissue and other tissues is about 4000:1.about.35000:1, indicating that VNP20009 has a well targeting ability to this kind of breast tumor.
[0037] 4. Measure the length and width of the tumor every 2-3 days, calculate the tumor volume and plot the tumor volume curve of nude mice. Two weeks after administration, there is a significant difference in the tumor size between the control and experiment group. Randomly take three mice from each group, strip the tumor of the nude mice, weigh it and take photos. The results are shown in FIG. 5 and FIG. 6, after administration of Salmonella VNP20009-M, the tumor grows slowly, the tumor volume and weight is about 1/2 of that in the PBS and VNP20009-V group, but there is no significant difference between VNP20009-V and PBS group, suggesting that VNP20009 with high expression of L-methioninase has significant inhibitory effect on the tumors of breast cancer.
[0038] 5. The procedures are the same as those in 1. Tumor-bearing nude mice are divided into three groups and administered with PBS, L-methioninase 1 ng/mouse, L-methioninase 100 ng/mouse by intratumoral injection. Two weeks later, tumors are stripped, weighed and photographed. Results are shown in FIGS. 7,8. There is no significant difference in tumor size and weight among the three groups. The L-methioninase level in L-methioninase ing/mouse is equivalent to that contained in 2.times.10.sup.6 CFU VNP20009-M. Thus, the administration of equal or even 100-fold dose of L-methioninase shows no significant anti-tumor effects. This indicates that with the L-methioninase depletion or degradation, a single administration does not function, while the continuous high-expression of L-methioninase using VNP20009 as the carrier can make up this drawback, showing significant anti-tumor effects.
* * * * *
D00001

D00002

D00003

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.