Biofilm Treatment of Composite Materials Containing Mycelium
Schaak; Damen Donald ; et al.
U.S. patent application number 14/745042 was filed with the patent office on 2015-12-31 for biofilm treatment of composite materials containing mycelium. The applicant listed for this patent is Matthew James Lucht, Damen Donald Schaak. Invention is credited to Matthew James Lucht, Damen Donald Schaak.
| Application Number | 20150376565 14/745042 |
| Document ID | / |
| Family ID | 54929854 |
| Filed Date | 2015-12-31 |


| United States Patent Application | 20150376565 |
| Kind Code | A1 |
| Schaak; Damen Donald ; et al. | December 31, 2015 |
Biofilm Treatment of Composite Materials Containing Mycelium
Abstract
The process provides a biofilm including and not limited to cellulose produced by bacteria that can be used as a bio-resin and as a surface application for myceliated and non-myceliated biomaterials. In one embodiment, the process comprises the steps of obtaining an agricultural substrate; and cohabitating a selected bacteria with a selected fungus in the agricultural substrate for a period of time to allow the bacteria to grow alongside the fungus and to excrete a biofilm from the bacteria into the substrate to provide bio-resin like strengthening compounds to the agricultural substrate.
| Inventors: | Schaak; Damen Donald; (Troy, NY) ; Lucht; Matthew James; (Troy, NY) | ||||||||||
| Applicant: |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family ID: | 54929854 | ||||||||||
| Appl. No.: | 14/745042 | ||||||||||
| Filed: | June 19, 2015 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 62017315 | Jun 26, 2014 | |||
| Current U.S. Class: | 435/252.4 |
| Current CPC Class: | C12N 1/20 20130101; C12N 1/14 20130101 |
| International Class: | C12N 1/20 20060101 C12N001/20; C12N 1/14 20060101 C12N001/14 |
Claims
1. A process comprising the steps of obtaining an agricultural substrate; and cohabitating a selected bacteria with a selected fungus in said agricultural substrate for a period of time to allow said bacteria to grow alongside said fungus and to excrete a biofilm from said bacteria into said substrate to provide bio-resin like strengthening compounds to said agricultural substrate.
2. A process as set forth in claim 1 wherein said bacteria is a Bacillus subtilis species.
3. A process as set forth in claim 2 wherein said fungus is Ganoderma.
4. A process as set forth in claim 1 wherein said agricultural substrate is in the form of discrete particles and said fungus grows a network of interconnected mycelia cells extending through and around discrete particles of the substrate to bond said discrete particles together into a self-supporting composite material.
5. A process of growing a biofilm producing bacteria into a myceliated substrate comprising the steps of inoculating a Lysogeny Broth (LB) with one colony of Bacillus subtilis sp. for a period of time to grow the Bacillus subtilis sp into a culture; passaging the culture into a LB media and continuing incubation until the culture reaches mid log phase; co-inoculating an agricultural substrate with a fungal species and said mid log phase culture using less than 20% fungal v/v inoculum, and 1 ml of mid log phase bacteria culture per 6 grams of dry agricultural substrate; and thereafter incubating the co-inoculated agricultural substrate for a period of from 2 to 10 days to obtain a self-supporting composite material.
6. A process as set forth in claim 5 further comprising the steps of hot pressing the material to bond all components therein.
7. A process as set forth in claim 6 wherein said steps of hot pressing takes place for a period of 10 minutes at 400.degree. F. under 350 psi of platen pressure.
8. A process for growing microbial cellulose in vitro for application to biomaterial surfaces comprising the steps of inoculating 200 ml of buffered S&H medium with one colony of microbial cellulose producing bacteria Acetobacter xylinus to form a culture; incubating said culture at 30.degree. C. for a time period of from 24 to 96 hours to form a cellulose biofilm thereon; thereafter harvesting the cellulose biofilm; and applying the cellulose biofilm to the surface of a biomaterial surface on an agricultural substrate to form a bio-film like skin thereon.
9. A process as set forth in claim 8 wherein said agricultural substrate is myceliated with a network of interconnected mycelia cells extending through and around discrete particles of the substrate to bond said discrete particles together into a self-supporting composite material.
10. A process of growing a biofilm in vitro for application within a biomaterial inoculating 200 ml of LB media with a colony of biofilm producing bacteria Bacillus subtilis sp. bacteria to form a culture; incubating said culture at 37.degree. C. until a biofilm is formed; harvesting the biofilm by one of direct biofilm extraction and harvesting of the entirety of the culture; mixing the harvested biofilm into one of a myceliated agricultural substrate and a non-myceliated agricultural substrate to form a biomaterial; and hot pressing the biomaterial to fully bond all components therein.
11. A process of producing a bacterial antimicrobial in situ comprising the steps of inoculating 100 ml LB media with one colony of Streptomycin natalensis to form a culture; growing the culture at 37.degree. C. to mid log phase; co-inoculating 600 g agricultural substrate with both bacteria and fungi species using 100 ml mid log bacteria culture and <20% fungal inoculum; incubating the co-inoculated material at room temperature for 6 days; and heating the incubated co-inoculated material to inactive microbial growth by desiccation.
12. A process as set forth in claim 11 wherein said bacteria is S.natalensis and said fungi is Ganoderma.
13. A process of producing a bacterial antimicrobial in vitro comprising the steps of inoculating 100 ml LB media with one colony of Streptomycin alboniger to form a culture; growing the culture at 37.degree. C. to mid log phase; filtering the Streptomycin alboniger out of the culture to reduce the culture to spent media spiked with antimicrobial compounds; adding 100 ml of the antimicrobial supernatant to 600 g agricultural substrate and incubating the substrate for 3 hours at room temperature; inoculating the substrate with <20% fungi (Ganoderma) and incubate for 6 days at room temperature; and terminating microbial growth in the incubated substrate by desiccation.
14. A process comprising the steps of obtaining an agricultural substrate; and inoculating said agricultural substrate with a selected bacteria grown to mid log phase and incubating the inoculated agricultural substrate for a period of time to allow said bacteria to excrete a biofilm from said bacteria into said substrate to provide bio-resin like strengthening compounds to said agricultural substrate.
15. A process as set forth in claim 14 wherein said bacteria is Bacillus subtilis and is inoculated into said agricultural substrate at a rate of 1 milliliter of bacterial culture to 6 grams dry of agricultural substrate.
Description
[0001] This invention claims priority of Provisional Patent Application 62/017,315 filed Jun. 26, 2014.
[0002] This invention relates to a process that provides a biofilm including and not limited to cellulose produced by bacteria that can be used as a bio-resin and as a surface application for myceliated and non-myceliated biomaterials. More particularly, this invention relates to a biofilm treatment of myceliated biomaterials.
BACKGROUND OF THE INVENTION
[0003] Biofilms are comprised of excreted protein, DNA, and polysaccharides that tend to form a complex matrix consisting of organic and nonorganic materials. These biofilms contain the cells that produced them providing the cells with a film or slim to live on. [Karatan, E., Watnick, P. (June 2009). "Signals, Regulatory Networks, and Materials That Build and Break Bacterial Biofilms"].
[0004] The production of biofilms can provide bacterium an anchor or platform to grow from and serve as a protective barrier from the environment. Polysaccharides typically encapsulate the biofilms providing a bound matrix of living and nonliving organic matter. [Hall-Stoodley L, Costerton J W, Stoodley P (February 2004). "Bacterial biofilms: from the natural environment to infectious diseases"]
[0005] Biofilms can provide the bacteria a sanctuary from antibiotics, desiccation, and nutritional stress. Some biofilms produced from bacteria are composed of single polysaccharides like microbial cellulose.
[0006] As is known, U.S. patent application Ser. No. 12/001,556, filed Dec. 12, 2007, describes various techniques for making a biomaterial composed of a substrate of discrete particles and a network of interconnected mycelia cells extending through and around the discrete particles and bonding discrete particles together.
[0007] It is an object of the invention to provide improvements to the methods of making biomaterials.
[0008] It is another object of the invention to provide improvements to the methods of making myceliated biomaterials and non-myceliated biomaterials.
[0009] It is another object of the invention to utilize bacterial biofilms to provide biomaterial materials such as described in U.S. patent application Ser. No. 12/001,556 with an added resin matrix and surface layer.
[0010] Briefly, the invention provides a process by which biofilms including and not limited to cellulose produced by bacteria can be used as a bio-resin and as a surface application for myceliated and non-myceliated biomaterials.
[0011] In particular, the process comprises the steps of obtaining an agricultural substrate; and cohabitating a selected bacteria with a selected fungus in the agricultural substrate for a period of time to allow the bacteria to grow alongside the fungus and to excrete a biofilm from the bacteria into the substrate to provide bio-resin like strengthening compounds to the agricultural substrate.
[0012] In accordance with the techniques described in U.S. patent application Ser. No. 12/001,556, the fungus grows a network of interconnected mycelia cells extending through and around discrete particles of the substrate to bond the discrete particles together into a self-supporting composite material.
[0013] In one embodiment, the bacteria will be cohabitated with a selected fungus in agricultural substrates (AS), e.g. corn stalks. During this cohabitation period, the bacteria will grow alongside the fungal strains and excrete biofilms into the substrate providing bio-resin like strengthening compounds to the self-supporting composite material.
[0014] In another embodiment, the bacteria may be grown in vitro, thus producing a biofilm, which would then be harvested and applied to the AS at various stages of the growth process and in some instances encapsulating the material in a biofilm like skin. These in vitro applications would be applied to both the internal and external surfaces of the biomaterials. Some bacterial strains will be genetically engineered to optimize biofilm quality, excretion levels, and induction. In this embodiment, the biofilm may be applied to myceliated substrates, i.e. a substrate wherein the fungus grows a network of interconnected mycelia cells extending through and around discrete particles of the substrate, or the biofilm may be applied to non-myceliated substrates.
[0015] Utilizing genetic engineering techniques, inducible controlling sequences may be inserted into the bacterial genome to regulate biofilm production and various other biofilm components. Inducible gene expression will be regulated by photoreceptors, temperature signaling, small molecules, constitutively expressed promoters, or through knocking out genes.
[0016] Agricultural substrates are vulnerable to unwanted microbial bio burden that can cause the growing material to become contaminated. These contaminated materials fail to grow properly thus reducing product yields and performance. Here, the antimicrobial properties of some bacteria species are to be harnessed. Both bacteria and fungi species will be cohabitated together in the selected agricultural substrates (e.g. corn stalks). In this particular application, the bacteria will excrete antimicrobial compounds that reduce the competition between unwanted microbes and the selected fungal species. This strategy will enhance the ability of the fungus to resist external bio burden commonly growing throughout the selected agricultural substrates. Molecular genetic techniques are also utilized to reprogram the bacteria strains to overexpress antimicrobials both in vitro, and in situ.
[0017] These and other objects of the invention will become more apparent from the following detailed description taken in conjunction with the accompanying drawings wherein:
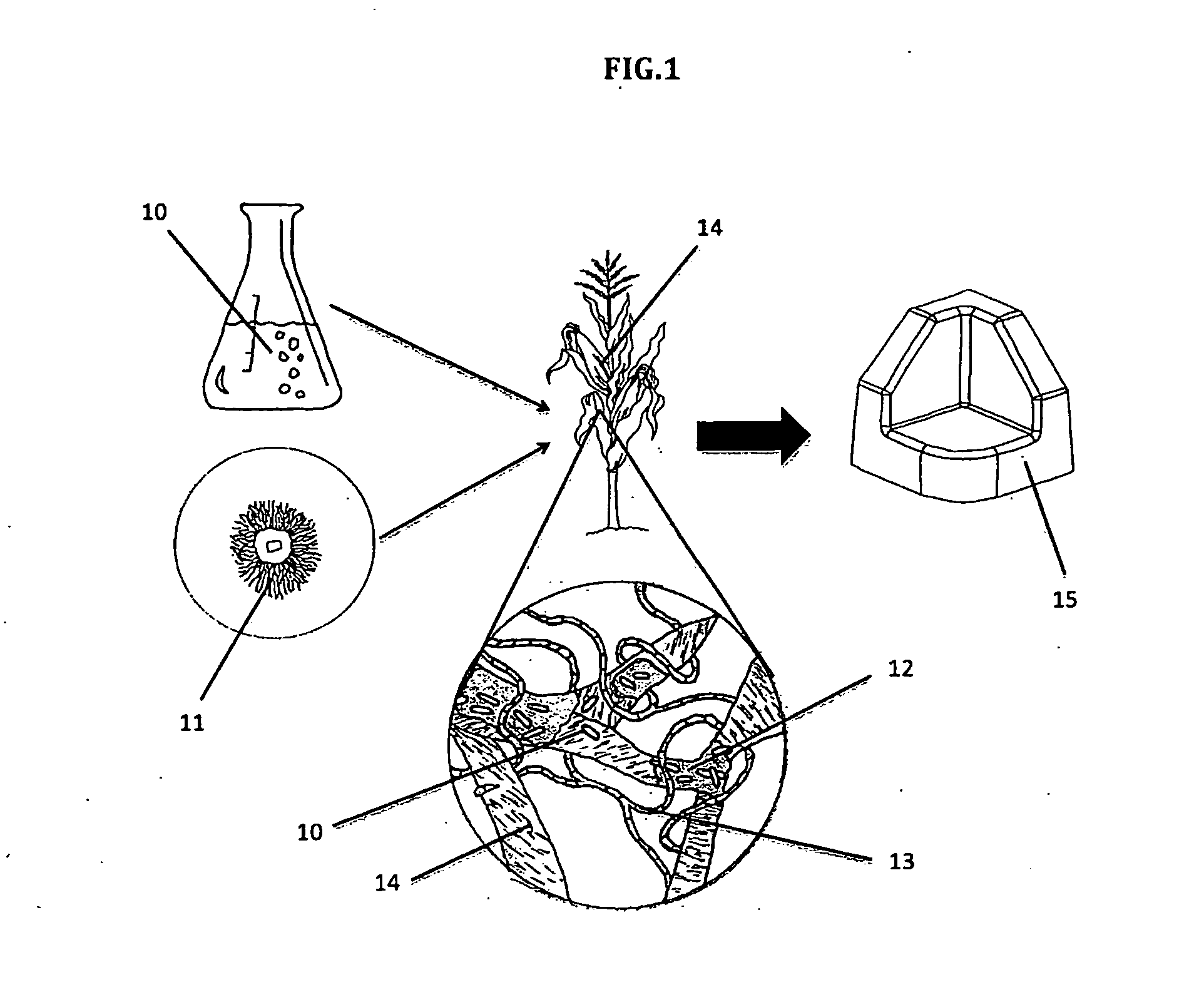
[0018] FIG. 1 illustrates a schematic of the steps of a process in accordance with the invention;
[0019] FIG. 2 illustrates photoimages of a flask containing a biofilm produced in vitro in accordance with the invention; and
[0020] FIG. 3 illustrates a view of a microbial cellulose film being harvested from a dish in accordance with the invention.
[0021] Referring to FIG. 1, in accordance with one embodiment of the process of the invention, a liquid medium of a bacteria culture (a Bacillus subtilis species) is first prepared, for example, in a flask 10, along with the preparation of fungal mycelium, for example, on an agar plate 11.
[0022] Thereafter the bacteria culture and fungal mycelium are applied to an agricultural substrate, for example, corn stalks.
[0023] As illustrated, during incubation of the agricultural substrate, the bacteria produces a biofilm (bioresin) 12 while the fungal mycelium grows hyphae 13 that grow to form a network of interconnected mycelia cells extending through and around discrete particles 14 of the agricultural substrate to bond the discrete particles together into a self-supporting composite material, i.e. the finished product 15.
[0024] As indicted, the finished product 15 is a biomaterial, i.e. a molded block, that can be used for protective packaging.
[0025] FIG. 1 represents the ability of a fungus and bacteria strains to cohabitate.
Process Steps for Growing Biofilms Grown In Situ:
[0026] a. Inoculate the media that has specificity to the bacteria being cultured with one colony of bacteria. Grow the bacterial culture until the culture reaches mid log phase. [0027] In this step, the bacteria strain was inoculated into lysogeny broth (LB) and grown to optimal cell density. The quantities of the LB medium are dependent on the bacterial strain used and the quantity of agricultural substrate the LB is inoculated into. For Bacillus subtilis (bacteria strain), the agricultural substrate was inoculated at 1:6 (1 milliliter of bacterial culture: 6 grams dry agricultural substrate). [0028] b. Add bacterial culture grown to mid log phase to either myceliated or non-myceliated agricultural substrate (AS). If preparing "myceliated AS", co-inoculate the AS with the bacteria culture and one of the selected fungal species. If "non-myceliated AS" is prepared, the AS will only be inoculated with the bacterial culture. The biofilm produced by the bacteria will be the sole microbial bioresin in the substrate. [0029] In this step, the agricultural substrate was simultaneously inoculated with both Ganoderma (fungus) and a Bacillus subtilis species (bacteria) [0030] c. Incubate the biomaterial until the AS is fully colonized with the added bacteria and/or fungal mycelium. [0031] In this step, the biomaterial was incubated for 6 days [0032] d. Terminate growth of materials by method of desiccation. [0033] In this step, the biomaterial was dried to terminate microbial growth. [0034] Referring to FIG. 2, wherein like reference characters indicate like parts as above, a process for producing a bacterial biofilm in vitro employs a flask 15, into which a biofilm excreting bacteria 16 is placed.
Process Steps for Growing Biofilms Grown In Vitro:
[0034] [0035] a. Inoculate medium with bacteria (as above) [0036] b. Incubate culture at optimal conditions for the desired time [0037] c. Harvest the biofilm (17) [0038] d. Apply the biofilm to the myceliated or non-myceliated substrate [0039] e. Either continue to grow out materials or terminate growth.
Applications
I. Bacterial Biofilms Produced In Situ.
[0039] [0040] a. As a means of binding together discrete lignocellulose particles (both as a sole microbial component and in combination with other bacteria and fungi). Growing the biofilm-producing bacteria directly into the myceliated and non-myceliated biomaterial (in situ) to provide the cohabitated substrate with the addition of a biofilm that will act as both a resin for strength and particle bonding. [0041] b. As a surface application for altering physical properties and aesthetics. Once the material has finished growing, the biofilm will have been excreted throughout the material (internal and external surfaces). The extent to which the biofilm coats the external surfaces of the finished material is dependent on the bacteria species used during cohabitation and the resultant biofilm produced. [0042] c. As a nutrition source for cohabitating fungi. The organic material accumulated throughout the production of the biofilm may also become a source of nutrition for other bacteria and fungal species growing in the substrate.
II. Bacterial Biofilms Produced In Vitro.
[0042] [0043] a. As a means of binding together discrete lignocellulose particles (both as a sole microbial component, and in combination with other bacteria and fungi). [0044] b. As a surface application for altering physical properties and aesthetics. [0045] c. As a nutrition source for cohabitating fungi. The organic material accumulated throughout the production of the biofilm may also become a source of nutrition for other bacteria and fungal species growing in the substrate.
III. Genetically Engineered Biofilm Producing Bacteria Strains
[0045] [0046] a. Genetically modified biofilm dependent genes will allow for optimal levels of biofilm production. This will be done through bacterial controlling sequences specifically engineered for our species of bacteria and their associated biofilms. [0047] b. Provide the capability to induce biofilm production at selected time points during material growth. This will be done through bacterial controlling sequences, which will be regulated through photoreceptors, temperature signaling, small molecules or constitutive promoters.
IV. Bacterial Antimicrobials Produced In Situ
[0047] [0048] a. As a means to cohabitate both fungi and bacteria species together with the purpose of reducing the background bio burden residing in our agricultural substrates throughout the materials growth process.
V. Bacterial Antimicrobials Produced In Vitro
[0048] [0049] a. Culture antimicrobial producing bacteria in vitro (liquid media). Use the antimicrobial spiked culture as a bio burden treatment to agricultural substrates.
VI. Genetically Engineered Antimicrobial Producing Bacteria
[0049] [0050] a. Through genetic modifications, reprogram the cohabitating bacteria to express or overexpress antimicrobials in both in vitro and in situ paradigms.
[0051] The following are specific examples of the process for making a biofilm.
Example 1
Grow Biofilm-Producing Bacteria into Myceliated Substrate
[0052] a. Inoculate 25 ml Lysogeny Broth (LB) with one colony of Bacillus subtilis sp. (bacteria), grow overnight at 37.degree. C. [0053] a. Passage the culture (1:10) into 250 ml LB media, and continue incubation until the culture reaches mid log phase. [0054] b. Co-inoculate the AS with both fungal and bacterial species using <20% fungal v/v inoculum, and 1 ml of log phase bacteria culture per 6 grams of dry AS. [0055] c. Incubate the co-inoculated AS for 2-10 days. [0056] d. Hot press the material (10 minutes at 400.degree. F., held under 350 psi of platen pressure.) to bond all components if applicable to material performance. [0057] e. Terminate growth of material
Example 2
Grow Microbial Cellulose in Vitro and Apply to Biomaterial Surfaces
[0057] [0058] a. Inoculate 200 ml of a common liquid medium used to culture bacteria, such as a buffered S&H medium, with one colony of microbial cellulose producing bacteria Acetobacter xylinus. [0059] b. Incubate culture at 30.degree. C. for 24-96 hours to form a biofilm thereon. [0060] c. Harvest the cellulose biofilm 17 (FIG. 3) [0061] d. Apply the cellulose to the surface of partially or fully myceliated AS thereby forming a bio-film like skin. [0062] e. Allow the biomaterial to grow into the cellulose if further biofilm integration is required for material performance. [0063] f. Terminate growth of material
Example 3
Grow Microbial Biofilm In Vitro and Apply within Biomaterials
[0063] [0064] a. Inoculate 200 ml of LB media with colony of biofilm producing bacteria Bacillus subtilis sp. bacteria [0065] b. Incubate culture at 37.degree. C. until the biofilm has reached optimal qualities and quantities (24-96 hours) [0066] c. Harvest the biofilm by either direct biofilm extraction (FIG. 3), or for other biofilms that remain homogenous in the media, the entirety of the culture will be harvested. [0067] d. Mix the harvested biofilm into myceliated or non-myceliated AS. [0068] e. Hot press the material to fully bond all components. [0069] f. Terminate the growth of material.
Example 4
Bacterial Antimicrobials Produced In Situ
[0069] [0070] a. Inoculate 100 ml LB media with one colony of Streptomycin natalensis. Grow culture at 37.degree. C. to mid log phase. [0071] b. Co-inoculate 600 g AS with both bacteria (S.natalensis) and fungi (Ganoderma) species using 100 ml mid log bacteria culture, and <20% fungal inoculum. [0072] c. Incubate co-inoculated material at room temperature for 6 days. [0073] d. Heat inactive microbial growth by desiccation. [0074] e. Material will have enhanced resistance to bio burden throughout the incubation process resulting in limited loss of product due to contaminated material.
Example 5
Bacterial Antimicrobials Produced In Vitro
[0074] [0075] a. Inoculate 100 ml LB media with one colony of Streptomycin alboniger. Grow culture at 37.degree. C. to mid log phase. [0076] b. Filter the bacteria out of the culture using 0.2 um filters thus reducing the culture to spent media spiked with antimicrobial compounds (may also retain the bacteria if co-habitation of the antimicrobial producing bacteria and fungus is needed in next steps). [0077] c. Add 100 ml of the antimicrobial supernatant to 600 g AS, and incubate the treated AS for 3 hours at room temperature. [0078] d. Inoculate the treated AS with <20% Fungi (Ganoderma), and incubate for 6 days at room temperature. [0079] e. Terminate all microbial growth by desiccation.
[0080] In all of the processes described above, the inoculated agricultural substrates may be placed in molds of predetermined shape in order to produce products having a shape corresponding to the shape of the interior of the mold.
[0081] The invention thus provides a process of making a bacterial biofilm that can be used as a bio-resin and as a surface application for myceliated and non-myceliated biomaterials.
* * * * *
D00001

D00002

D00003

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.