Composition For Shaping Keratin Fibers Containing Starches Modified With Propylene Oxide
SCHWEINSBERG; MATTHIAS ; et al.
U.S. patent application number 13/453530 was filed with the patent office on 2012-12-27 for composition for shaping keratin fibers containing starches modified with propylene oxide. Invention is credited to Carine Dogan, Lydiane Huet, Thorsten Knappe, Ralf Roenisch, Mathias Schriefers, MATTHIAS SCHWEINSBERG.
| Application Number | 20120328532 13/453530 |
| Document ID | / |
| Family ID | 43648714 |
| Filed Date | 2012-12-27 |



View All Diagrams
| United States Patent Application | 20120328532 |
| Kind Code | A1 |
| SCHWEINSBERG; MATTHIAS ; et al. | December 27, 2012 |
COMPOSITION FOR SHAPING KERATIN FIBERS CONTAINING STARCHES MODIFIED WITH PROPYLENE OXIDE
Abstract
Cosmetic agents for temporarily reshaping keratin fibers, particularly human hair, containing in a cosmetic carrier at least one starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the starch modified with propylene oxide), wherein the agents achieve styling with a good degree of hold and elevated flexibility. The propylene oxide-modified starches are based on renewable raw materials. Here, effective styling agents for hair can be provided without having to rely on setting polymers obtained from fossil fuels.
| Inventors: | SCHWEINSBERG; MATTHIAS; (Hamburg, DE) ; Roenisch; Ralf; (Wuppertal, DE) ; Schriefers; Mathias; (Moenchengladbach, DE) ; Huet; Lydiane; (Strassburg, FR) ; Dogan; Carine; (Vigneux sur Seine, FR) ; Knappe; Thorsten; (Scenefeld, DE) |
| Family ID: | 43648714 |
| Appl. No.: | 13/453530 |
| Filed: | April 23, 2012 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| PCT/EP2010/065862 | Oct 21, 2010 | |||
| 13453530 | ||||
| Current U.S. Class: | 424/47 ; 424/70.13 |
| Current CPC Class: | A61K 8/608 20130101; A61K 8/046 20130101; A61K 8/732 20130101; A61K 8/8152 20130101; A61K 8/8158 20130101; A61Q 5/06 20130101; A61K 2800/5422 20130101; A61K 8/8147 20130101; A61K 2800/5424 20130101 |
| Class at Publication: | 424/47 ; 424/70.13 |
| International Class: | A61K 8/73 20060101 A61K008/73; A61Q 5/06 20060101 A61Q005/06 |
Foreign Application Data
| Date | Code | Application Number |
|---|---|---|
| Oct 22, 2009 | DE | 10 2009 045 925.1 |
| Oct 22, 2009 | DE | 10 2009 045 933.2 |
Claims
1. Cosmetic agent for temporarily reshaping keratin fibers comprising in a cosmetic carrier at least one starch modified with propylene oxide, wherein the modified starch has an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % based on weight of the modified starch.
2. Cosmetic agent according to claim 1, wherein the modified starch is gelatinized.
3. Cosmetic agent according to claim 1, wherein the modified starch is uncrosslinked.
4. Cosmetic agent according to claim 1, wherein the modified starch has an average molecular weight (weight-average) of 100 to 2,000 kDa.
5. Cosmetic agent according to claim 1, wherein, in a 43 wt. % solution in water, the modified starch has a viscosity from 150 to 1,500,000 mPas based on Brookfield viscometer with spindle #7 at 20.degree. C. and 20 rpm.
6. Cosmetic agent according to claim 1, wherein the modified starch is a tapioca starch modified with propylene oxide, a potato starch modified with propylene oxide, or a mixture thereof.
7. Cosmetic agent according to claim 1, wherein the modified starch is present in an amount of 0.01 wt. % to 40 wt. %, based on total weight of the agent.
8. Cosmetic agent according to claim 1 further comprising at least one polymer chosen from film-forming polymers and setting polymers.
9. Cosmetic agent according to claim 1 further comprising at least one compound of formula (II), HO--CH.sub.2--(CHOH).sub.n--CH.sub.2--OH (II) wherein n is an integer from 1 to 4.
10. Cosmetic agent according to claim 1 further comprising at least one propellant.
11. Method for temporarily reshaping keratin fibers comprising applying a cosmetic agent according to claim 1 onto the keratin fibers.
Description
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] The present application is a continuation of International Application No. PCT/EP2010/065862 filed 21 Oct. 2010, which claims priority to German Patent Application Nos. 10 2009 045 925.1 and 10 2009 045 933.2, both filed 22 Oct. 2009, each of which are incorporated herein by reference.
[0002] The present invention relates to cosmetic agents for temporarily reshaping keratin fibers, containing in a cosmetic carrier at least one starch modified with propylene oxide and having an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the starch modified with propylene oxide).
[0003] Agents for temporary shaping typically contain synthetic polymers as the shaping component. Preparations containing a dissolved or dispersed polymer can be applied to hair by propellant gases or by a pump mechanism. Hair gels and hair waxes, however, are generally not applied directly onto the hair but are rather distributed in the hair using a comb or the hands.
[0004] Synthetic polymers typically used in agents for temporary shaping are produced from appropriate synthetically obtainable monomers. These monomers are obtained from fossil substances such as petroleum by conversion into the corresponding polymer building blocks, inter alia with input of energy. In a more sustainable approach to nature as our habitat and to resources, it remains desirable to use only those cosmetic raw materials for cosmetic products which can be obtained from "renewable" raw materials using the least possible energy. However, synthetic polymers can only be reduced in quantity or substituted if the replacement provides the characteristics desired for the intended application and ensures that the keratin-containing fibres adequately maintain a stable shape.
[0005] Moreover, naturally based replacement polymers should maintain the bounce and silkiness of keratin-containing fibers which have been set in shape. Formation of polymer particles which are visible to the naked eye on keratin-containing fibers must be avoided. Furthermore, the keratin-containing fibers must not look dull, but should instead have a natural gloss.
[0006] The present invention provides a cosmetic composition with a shape-setting action which brings about improved or equivalent shape setting and does not exhibit the above-stated disadvantages. In so doing, it is intended to make little or, if possible, no use of synthetic polymers based on fossil raw materials.
[0007] The present invention accordingly firstly provides a cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, containing in a cosmetic carrier at least one starch modified with propylene oxide, wherein the starch has an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the starch modified with propylene oxide). These agents exhibit excellent parameters for use on hair. The starch can be incorporated into the agents by simple mixing virtually without input of heat at a temperature of at most 30.degree. C.
[0008] Keratin fibers according to the invention refer to furs, wool, feathers and particularly human hair.
[0009] Starch is a storage carbohydrate which is stored by many plants in the form of starch grains (granules) ranging in size from 1 to 200 .mu.m in various plant parts, for example, in tubers or roots, cereal seeds, fruits and in the medulla. Starch modified with propylene oxide which can be used according to the invention can be obtained from starch from potatoes, maize, rice, peas, acorns, chestnuts, barley, wheat, bananas, sago, millet, sorghum, oats, barley, rye, beans, sweet potato, arrowroot or manioc. Particularly pronounced effects according to the invention are achieved using tapioca starch modified with propylene oxide, potato starch modified with propylene oxide or mixtures of both of these starches. It is very particularly preferred to use potato starch modified with propylene oxide as the corresponding modified starch.
[0010] Starch belongs to the homoglycan family and is a polycondensation product of D-glucose. Starch consists of three structurally different polymers: d-glucopyranose, namely amylose, amylopectin and an "intermediate fraction". Higher plants contain 0 to 45 wt. % of amylose relative to dry solids.
[0011] The intermediate fraction, also referred to as "abnormal amylopectin", lies structurally between amylose and amylopectin. The quantities stated for amylopectin for the purposes of the present application include the intermediate fraction.
[0012] It is preferred for the starch modified with propylene oxide to have an amylose content of less than 25 wt. %, particularly less than 20 wt. %, based on total weight of the starch. It was found that, in order to achieve the effect according to the invention, a particularly suitable starch is one having 17 to 22 wt. % amylose and 78 to 83 wt. % amylopectin.
[0013] Amylose consists of predominantly linear .alpha.-1,4-glycosidically linked d-glucose M.sub.r 50,000-150,000. The resultant chains formation double helices in the starch.
[0014] In addition to the .alpha.-1,4 linkages described for amylose, amylopectin also contains from 4 to 6% of .alpha.-1,6 bonds as branch points. The average distance between the branch points amounts for instance to 12 to 17 glucose units. The molar mass of 10.sup.7 to 710.sup.8 corresponds to approx. 10.sup.5 glucose units, amylopectin accordingly being among the largest biopolymers. Said branches are distributed within the molecule in such a way that a cluster structure with relatively short side chains develops. Two of these side chains in each case form a double helix. Due to the numerous branch points, amylopectin is relatively readily soluble in water.
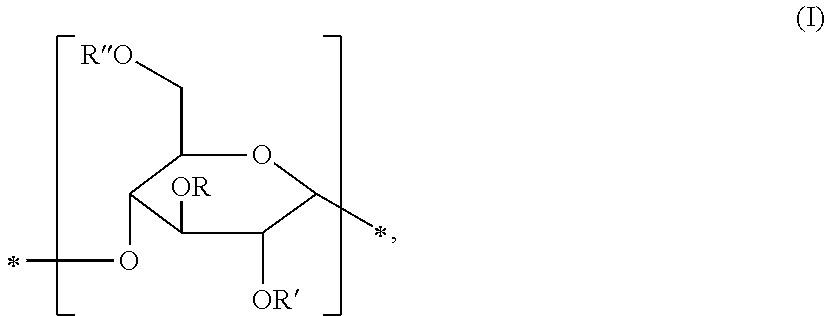
[0015] According to the invention, a starch modified with propylene oxide is taken to mean a reaction product of a starch with propylene oxide. Such a reaction product comprises at least one structural unit of formula (I),
##STR00001##
wherein at least one of R, R' or R'' is a group of the formula
##STR00002##
where n 0 and at most 2 of R, R', R'' are a hydrogen atom. A bond marked with the symbol * in the formulae of the present application corresponds to a free valence of the corresponding structural unit. Starches modified with propylene oxide are, for example, prepared by reacting a native starch with propylene oxide. Prior to modification with propylene oxide, the starch may have been subjected to various physical or chemical processes such as heat treatment, shearing or cleavage by thermal treatment, acid hydrolysis, oxidation, or enzymatic treatment, etc.
[0016] It is preferred for the starch modified with propylene oxide when used in the agent according to the invention not to be present as individual starch grains (granules). Accordingly, the starch grains are opened up, for example, by heat or shearing, and the corresponding polysaccharide molecules are released therefrom. The released polysaccharide molecules can be modified with propylene oxide after or before release.
[0017] In a preferred embodiment, the propoxylated starch is gelatinized. If an aqueous suspension of starch is heated or compressed at a critical temperature or pressure, tangential swelling of the bodies is observed accompanied by loss of birefringence, a modified X-ray structure and an abrupt increase in the viscosity of the solution. This phenomenon is known as gelatinization.
[0018] Starches according to the invention modified with propylene oxide are present in the agent in a molecular weight distribution. Molecular weight distribution is determined experimentally by gel filtration chromatography against dextran. One important feature of the invention is the weight-average molecular weight of the propylene oxide-modified starches present in the agent. This weight-average is an average molecular weight which takes account of the total weight of the molecules of different molecular weight, and not merely the number of molecules. The "weight fraction"
w.sub.i=(N.sub.iM.sub.i)/[.SIGMA.(N.sub.iM.sub.i)]
is first defined in order to calculate the weight average statistically. This indicates the proportion by weight of macromolecules in the sample consisting of i segments (e.g., monomer building blocks) of mass M.sub.i consist and occur N.sub.i times in the sample. The following equation accordingly applies to the weight-average molecular weight M.sub.w=.SIGMA.w.sub.iM.sub.i:
M.sub.w=[.SIGMA.(N.sub.iM.sup.2.sub.i)]/[.SIGMA.(N.sub.iM.sub.i)].
[0019] Particularly preferred agents are those having starches modified with propylene oxide and which have an average molecular weight (weight-average) of 100 to 2,000 kDa, particularly 500 to 1,800 kDa, very preferably 700 to 1,000 kDa.
[0020] It is particularly preferred for the starch modified with propylene oxide to be uncrosslinked. Crosslinking of starch modified with propylene oxide is present when the linear or branched polysaccharide macromolecules of the starch are covalently linked by a crosslinking agent to form a three-dimensional, insoluble polymer network which is then only swellable. Native starch is generally regarded as uncrosslinked and, should crosslinking be desired, would require artificial crosslinking by chemical synthesis. Such artificial crosslinking can be carried out with crosslinking agents such as epichlorohydrin. (Propylene oxide-modified) starches which do not exhibit such crosslinking are uncrosslinked.
[0021] In order to achieve a lower molecular weight, for example, 100 to 400 kDa or 200 to 300 kDa, the starches are subjected to mechanical cleavage, enzymatic cleavage (particularly with .alpha.-amylase, .beta.-amylase, glucoamylase or debranching enzymes), cleavage by acid hydrolysis (particularly with hydrochloric acid, sulfuric acid or phosphoric acid), thermal cleavage or reaction with oxidizing agents (such as periodate, hypochlorite, chromic acid, permanganate, nitrogen dioxide, hydrogen peroxide or organic percarboxylic acid, preferably with hydrogen peroxide). Kneaders, extruders, stator/rotor devices and/or stirrers are suitable for mechanically cleaving starch.
[0022] Oxidative cleavage using hydrogen peroxide is preferably suitable. To accomplish this, starch modified with propylene oxide is placed in water, heated to 50 to 70.degree. C., hydrogen peroxide is added, and the mixture stirred at 70 to 85.degree. C. for 2 to 5 hours.
[0023] The starch's propylene oxide content has an impact on styling hold and styling flexibility as well as on the stability of the cosmetic agent. It has surprisingly been found that ideal application parameters are obtained when the modified starch has, based on weight of the starch, a propylene oxide content of 4 to 6 wt. %. Propylene oxide content can, for example, be determined, once Hodges cleavage has been carried out, according to DIN EN 13268.
[0024] It has furthermore been found that cosmetic agents which are ideally suitable for the invention are those in which, in a 43 wt. % solution in water (i.e., a 43 wt. % aqueous solution), the modified starch exhibits a viscosity from 150 to 1,500,000 mPas (Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm). Ideally suitable propylene oxide-modified polysaccharides exhibit viscosities of 3,000 to 200,000 mPas, particularly 10,000 to 100,000 mPas, very preferably 40,000 to 70,000 mPas (measured under the above-stated conditions).
[0025] It is preferred for the cosmetic agent to contain the polysaccharide modified with propylene oxide in an amount of 0.01 wt. % to 40 wt. %, more preferably 0.5 wt. % to 10 wt. %, very preferably 2 wt. % to 6 wt. %, based on total weight of the agent.
[0026] Agents according to the invention preferably comprise foams or gels as the cosmetic carrier and therefore are preferably in the form of a foam or gel. Excellent setting can be achieved with the starches. Furthermore, gels produced with the modified starches additionally achieve superb transparency.
[0027] Very particularly preferred cosmetic agents according to the invention comply with at least one of the following embodiments A) to R):
[0028] A): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch).
[0029] B): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch).
[0030] C): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0031] D): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0032] E): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0033] F): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0034] G): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch).
[0035] H): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch).
[0036] I): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0037] J): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0038] K): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0039] L): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked tapioca starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0040] M): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa and a propylene oxide content of 4 to 6 wt. % (relative to the weight of the starch modified with propylene oxide).
[0041] N): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa and a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch).
[0042] O): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0043] P): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 50 to 2,500 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0044] Q): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 3,000 to 200,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0045] R): A cosmetic agent for temporarily reshaping keratin fibers, particularly human hair, comprising in a cosmetic carrier at least one uncrosslinked potato starch modified with propylene oxide, the starch having an average molecular weight (weight-average) of 700 to 1,000 kDa, a propylene oxide content of 4 to 6 wt. % (based on weight of the modified starch) and a viscosity of 10,000 to 100,000 mPas (in a 43 wt. % aqueous solution, Brookfield viscometer, spindle #7 at 20.degree. C. and 20 rpm).
[0046] For the above-stated embodiments, the preferred features of the agent according to the invention apply mutatis mutandis, as do in particular the quantities used.
[0047] In addition to starch modified with propylene oxide (particularly that modified with propylene oxide according to embodiments A) to R)), the cosmetic agent according to the invention preferably also contains at least one polymer chosen from film-forming polymers or setting polymers. In this way, it is possible, for example, to finely tune the reshaping result and conditioning of the fibers. These additional polymers differ from starches modified with propylene oxide and can have an anionic, amphoteric, nonionic, permanently cationic or temporarily cationic (preferably nonionic, permanently cationic or temporarily cationic) charge.
[0048] Polymers according to the invention refer to compounds synthesized from a plurality of molecules wherein one kind or a plurality of species of atoms or atomic groups ("constitutive units", "basic building blocks" or "repeat units") is repeatedly arranged adjacent one another and have a molecular weight of at least 10,000 g/mol. The polymers are obtained by polyreaction, which can proceed artificially (i.e., synthetically) or naturally.
[0049] Film-forming polymers refer to those polymers which, on drying, leave behind a continuous film on the skin, hair or nails. Such film formers can be used in a wide variety of cosmetic products, such as face masks, make-up, hair fixatives, hairsprays, hair gels, hair waxes, hair tonics, shampoos or nail polishes. Particular preference is given to those polymers having sufficient solubility in water, alcohol or water/alcohol mixtures. Thus, corresponding solutions can be produced which may be simply applied or further processed.
[0050] Film-forming polymers further refer to those polymers capable of, when applied in a 0.01 to 20 wt. % aqueous, alcoholic or aqueous-alcoholic solution, depositing a transparent polymer film on the hair.
[0051] Hair-setting polymers assist in holding or building up the volume and fullness of the overall hairstyle. These polymers are simultaneously also film-forming polymers and therefore generally typical substances for shaping hair treatment agents such as hair setting preparations, hair mousses, hair waxes, and hair sprays. Film formation in this respect can occur only at points and connect only a few fibres together.
[0052] Additional cationic film-forming and/or cationic setting polymers can be chosen from cationic, quaternized cellulose derivatives. Cationic, quaternized celluloses which are advantageous for the purposes of the invention are generally those having more than one permanent cationic charge in a side chain.
[0053] Among these, emphasis should be placed on those cationic cellulose derivatives produced by reaction of hydroxyethylcellulose with a dimethyldiallylammonium reactant (particularly dimethyldiallylammonium chloride), optionally in the presence of further reactants. Among these cationic celluloses, cationic celluloses which are particularly suitable are those with the INCI name Polyquaternium-4, distributed, for example, under the names Celquat.RTM. H 100, Celquat.RTM. L 200 by National Starch.
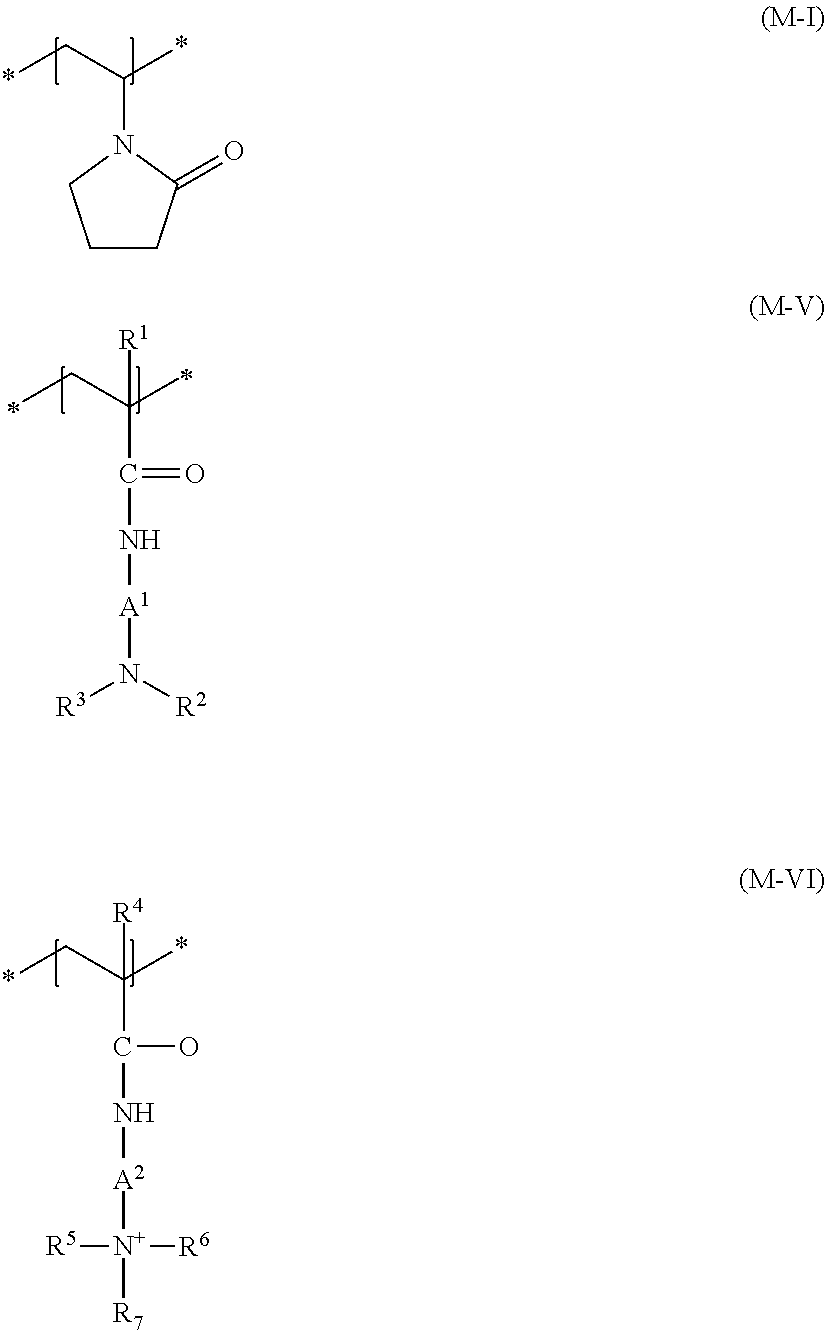
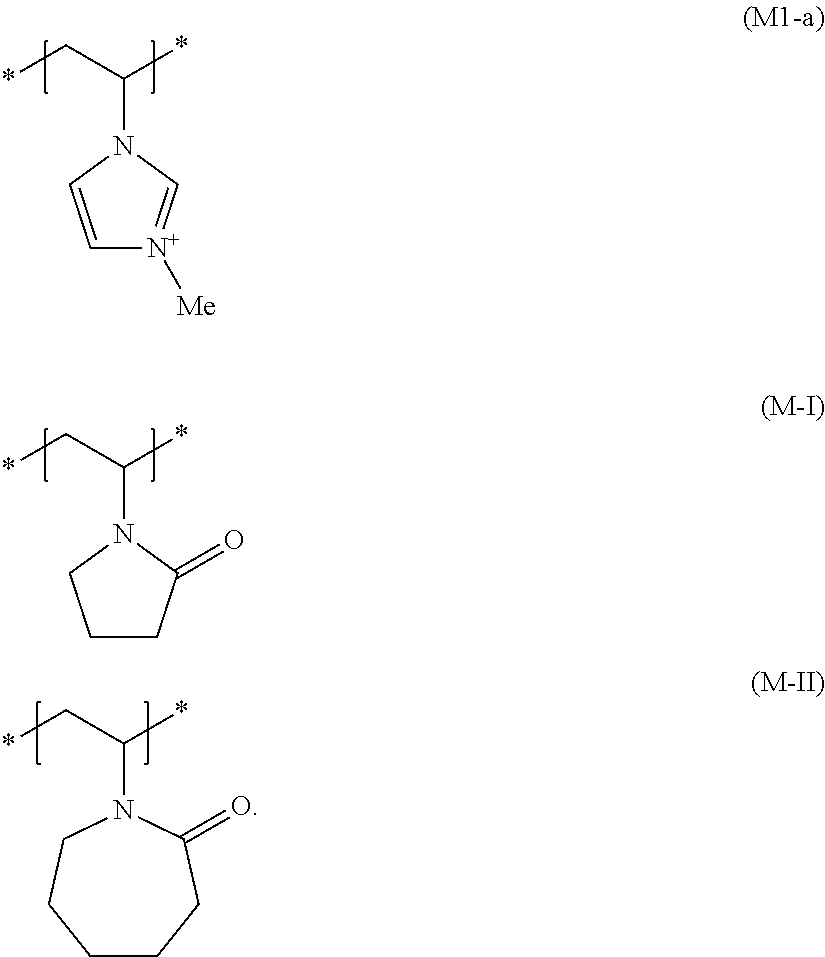
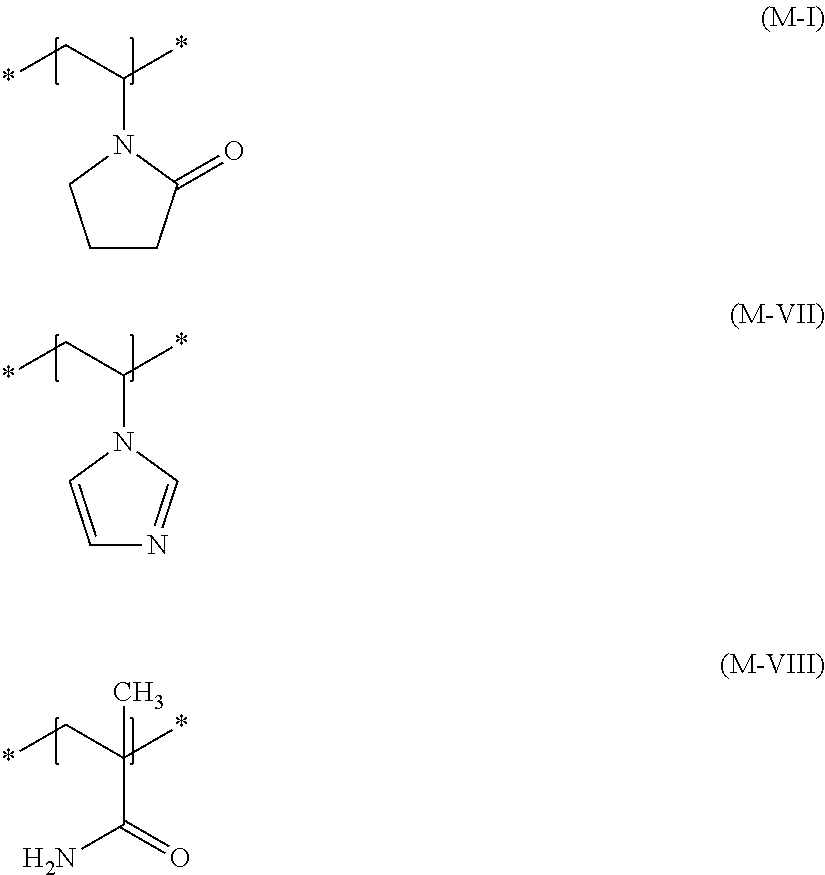
[0054] Additional cationic film-forming and/or cationic setting polymers which are suitable are those having at least one structural unit of formula (M-I) and at least one structural unit of the formula (M-VI), and optionally at least one structural unit of the formula (M-V)
##STR00003##
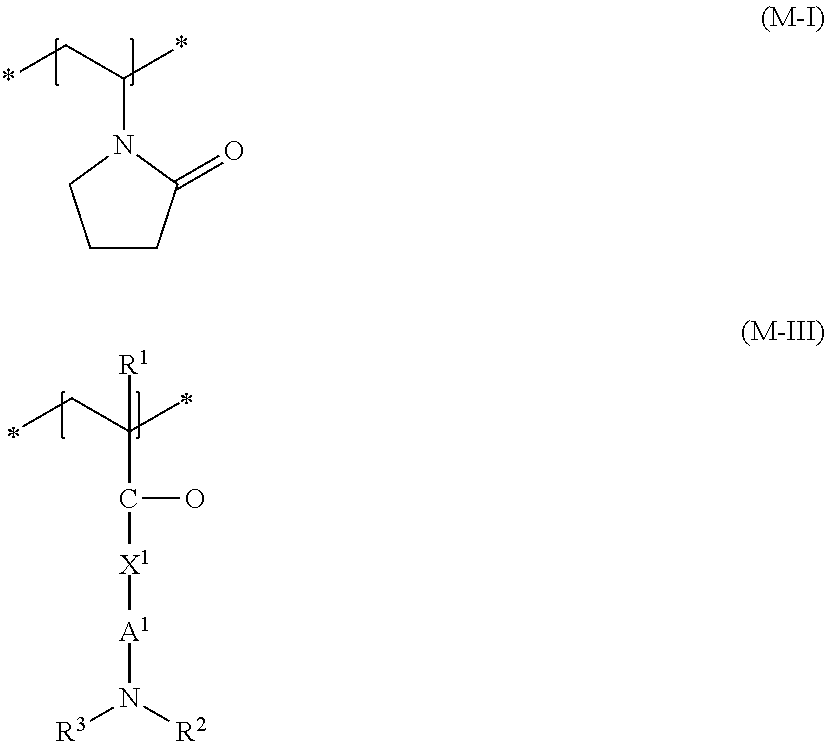
wherein R.sup.1 and R.sup.4 are, mutually independently, a hydrogen atom or a methyl group, A.sup.1 and A.sup.2 are, mutually independently, a 1,2-ethanediyl, 1,3-propanediyl or 1,4-butanediyl group, R.sup.2, R.sup.3, R.sup.5 and R.sup.6 are, mutually independently, a (C.sub.1 to C.sub.4) alkyl group, and R.sup.7 is a (C.sub.8 to C.sub.30) alkyl group.
[0055] The positive charge of monomer (M-VI) can be offset using any possible physiologically acceptable anions, such as chloride, bromide, hydrogensulfate, methylsulfate, ethylsulfate, tetrafluoroborate, phosphate, hydrogenphosphate, dihydrogenphosphate or p-toluenesulfonate, triflate. Suitable compounds are, for example, commercially available as [0056] copolymers of diethylsulfate-quaternized dimethylaminoethylmethacrylate methosulfate with vinylpyrrolidone with the INCI name Polyquaternium-11 under the names Gafquat.RTM. 440, Gafquat.RTM.734, Gafquat.RTM.755 (in each case ISP) and Luviquat PQ 11 PN (BASF SE), [0057] copolymers of methacryloylaminopropyllauryldimethylammonium chloride with vinylpyrrolidone and dimethylaminopropylmethacrylamide with the INCI name Polyquaternium-55 under the trade names, Styleze.RTM. W-10, Styleze.RTM. W-20 (ISP), [0058] copolymers of N-vinylpyrrolidone, N-vinylcaprolactam, N-(3-dimethylaminopropyl)-methacrylamide and 3-(methacryloyl)propyllauryldimethylammonium chloride (INCI name: Polyquaternium-69) under the trade name AquaStyle.RTM. 300 (28-32 wt. % active substance in ethanol-water mixture) (ISP).
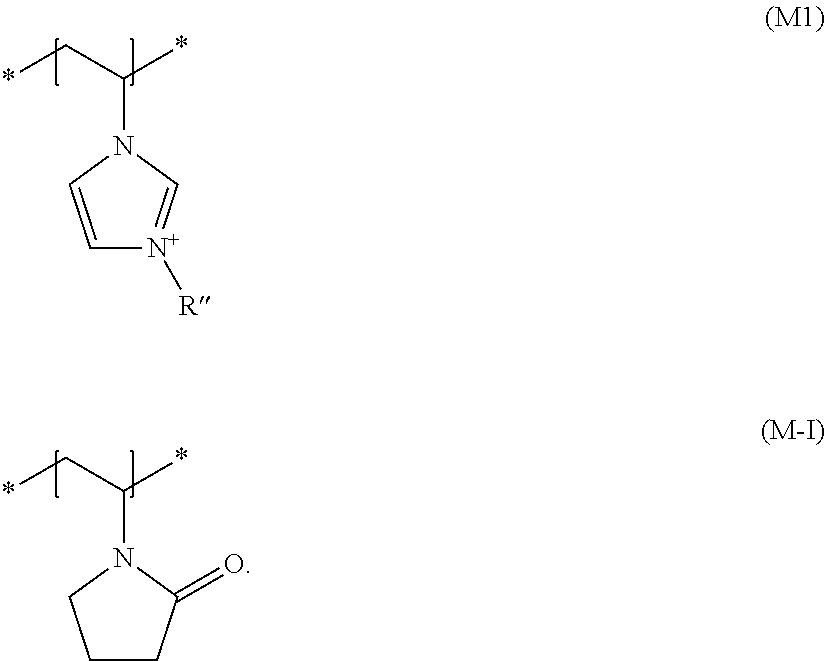
[0059] Additional film-forming and/or setting polymers chosen from cationic polymers having at least one structural unit comprising a permanently cationized nitrogen atom which may particularly be used in the invention are those cationic film-forming and/or cationic setting polymers having at least one structural element of the formula (M1)
##STR00004##
wherein R'' is a (C.sub.1 to C.sub.4) alkyl group, particularly a methyl group, and additionally have at least one further cationic and/or nonionic structural element.
[0060] The positive polymer charge of the component can be offset using any possible physiologically acceptable anions, such as chloride, bromide, hydrogensulfate, methylsulfate, ethylsulfate, tetrafluoroborate, phosphate, hydrogenphosphate, dihydrogenphosphate or p-toluenesulfonate, triflate.
[0061] It is preferred for the cosmetic agent according to the invention to additionally contain as the cationic film-forming and/or cationic setting polymer at least one copolymer (b1) which, in addition to at least one structural element of formula (M1), additionally contains a structural element of formula (M-I)
##STR00005##
wherein R'' is a (C.sub.1 to C.sub.4) alkyl group, particularly a methyl group.
[0062] The positive polymer charge of copolymers (b1) can be offset using any possible physiologically acceptable anions, such as chloride, bromide, hydrogensulfate, methylsulfate, ethylsulfate, tetrafluoroborate, phosphate, hydrogenphosphate, dihydrogenphosphate or p-toluenesulfonate, triflate.
[0063] Cationic film-forming and/or cationic setting polymers which are very particularly preferred as copolymers (b1) contain 10 to 30 mol %, preferably 15 to 25 mol % and particularly 20 mol % of structural units according to formula (M1) and 70 to 90 mol %, preferably 75 to 85 mol % and particularly 80 mol % of structural units according to formula (M-I).
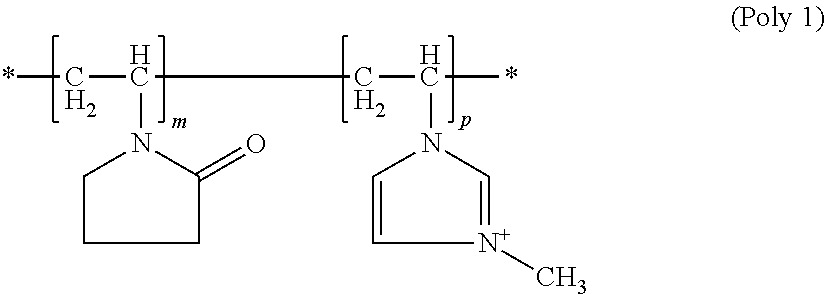
[0064] Here, it is particularly preferred for copolymers (b1) to contain, in addition to polymer units occurring from the incorporation of structural units according to formula (M1) and (M-I) into the copolymer, up to 5 wt. %, preferably at most 1 wt. %, of polymer units originating from the incorporation of other monomers. Copolymers (b1) are preferably exclusively synthesized from structural units of formula (M1) with R''=methyl, and (M-I) and can be described by the general formula (Poly1)
##STR00006##
wherein m and p vary depending on the molar mass of the polymer and are not intended to imply that the copolymers are block copolymers. Instead, structural units of formula (M1) and formula (M-I) can be randomly distributed in the molecule.
[0065] If a chloride ion is used to offset the positive charge of the polymer of formula (Polyl), such N-methylvinylimidazole/vinylpyrrolidone copolymers are designated according to INCI nomenclature as Polyquaternium-16 and are obtainable, for example, from BASF under the trade names Luviquat.RTM. Style, Luviquat.RTM. FC 370, Luviquat.RTM.FC 550, Luviquat.RTM.FC 905 and Luviquat.RTM.MQ 552.
[0066] If a methosulfate is used to offset the positive charge of the polymer of the formula (Poly1), such N-methylvinylimidazole/vinylpyrrolidone copolymers are designated according to INCI nomenclature as Polyquaternium-44 and are obtainable, for example, from BASF under the trade name Luviquat.RTM. UltraCare.
[0067] In addition to or instead of copolymer(s) (b1), cosmetic agents according to the invention can also contain copolymers (b2) which, based on copolymer (b1), comprise structural units of formula (M-II) as additional structural units
##STR00007##
[0068] Further cosmetic agents which are particularly preferred according to the invention contain as cationic film-forming and/or cationic setting polymer at least one copolymer (b2) having at least one structural unit according to formula (M1-a), at least one structural unit according to formula (M-I) and at least one structural unit according to formula (M-II)
##STR00008##
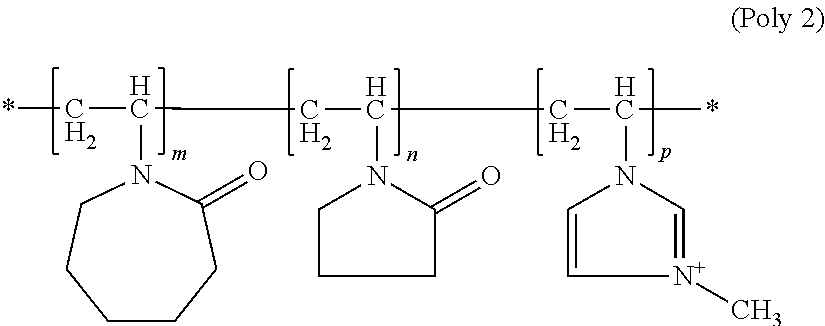
[0069] Here, it is particularly preferred for copolymers (b2) to contain, in addition to polymer units which arise from the incorporation of the stated structural units according to formula (M1-a), (MI) and (M-II) into the copolymer, up to 5 wt. %, preferably at most 1 wt. %, of polymer units originating from the incorporation of other monomers. Copolymers (b2) are preferably exclusively synthesized from structural units of the formulae (M1-a), (M-I) and (M-II) and can be described by the general formula (Poly2)
##STR00009##
wherein m, n and p vary depending on the molar mass of the polymer and are not intended to imply that the copolymers are block copolymers. Instead, structural units of the formulae may be present randomly distributed in the molecule.
[0070] The positive polymer charge of component (b2) can be offset using any possible physiologically acceptable anions, such as chloride, bromide, hydrogensulfate, methylsulfate, ethylsulfate, tetrafluoroborate, phosphate, hydrogenphosphate, dihydrogenphosphate or p-toluenesulfonate, triflate.
[0071] If a methosulfate is used to offset the positive charge of the polymer of the formula (Poly2), such N-methylvinylimidazole/vinylpyrrolidone/vinylcaprolactam copolymers are designated according to INCI nomenclature as Polyquaternium-46 and are obtainable, for example, from BASF under the trade name Luviquat.RTM. Hold.
[0072] Very particularly preferred copolymers (b2) contain 1 to 20 mol %, preferably 5 to 15 mol % and particularly 10 mol % of structural units according to formula (M1-a) and 30 to 50 mol %, preferably 35 to 45 mol % and particularly 40 mol % of structural units according to formula (I) and 40 to 60 mol %, preferably 45 to 55 mol % and particularly 60 mol % of structural units according to formula (M-II).
[0073] In addition to or instead of copolymer(s) (b1) and/or (b2), cosmetic agents according to the invention can also contain copolymers (b3) as a film-forming cationic and/or setting cationic polymer, wherein copolymers (b3) contain as structural units those of formulae (M1-a) and (I) together with further structural units from the group of vinylimidazole units and further structural units from the group of acrylamide and/or methacrylamide units.
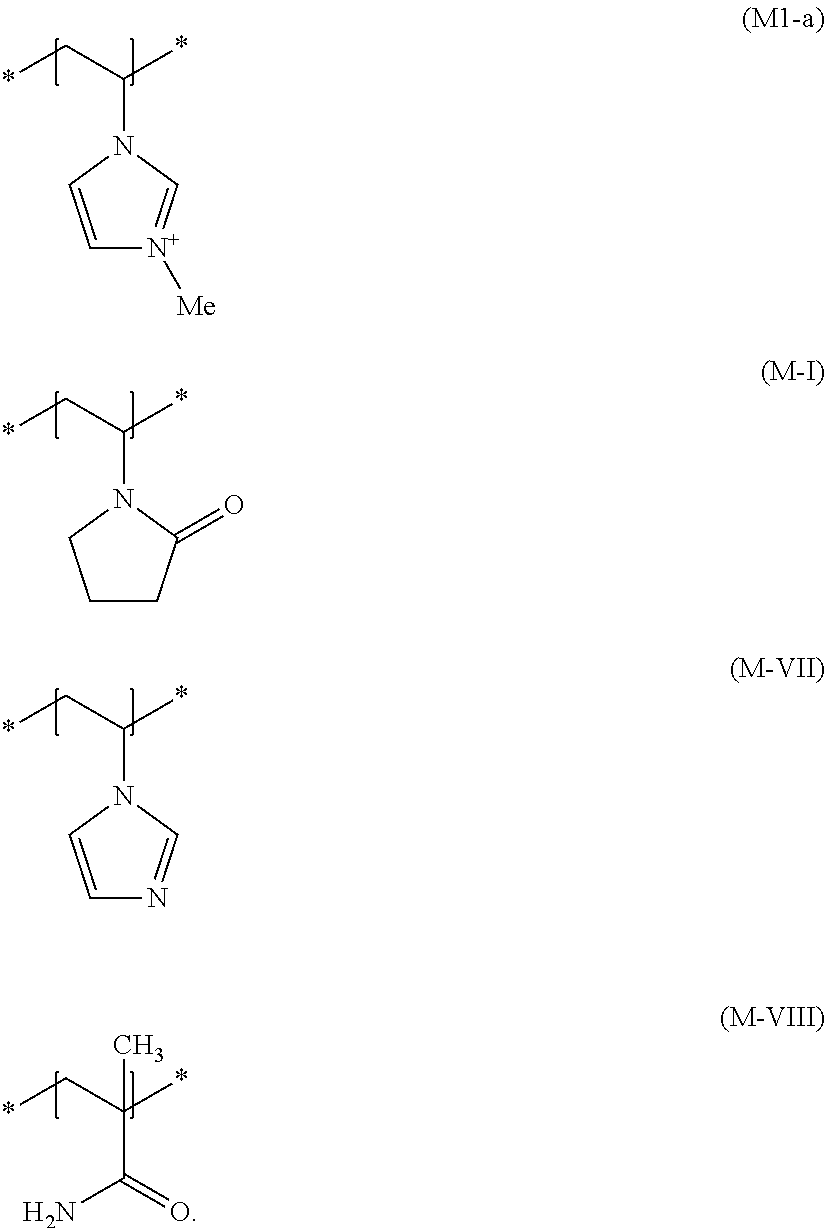
[0074] Further particularly preferred cosmetic agents contain as cationic film-forming and/or cationic setting polymer at least one copolymer (b3) having at least one structural unit according to formula (M1-a), at least one structural unit according to formula (M-I), at least one structural unit according to formula (M-VII), and at least one structural unit according to formula (M-VIII)
##STR00010##
[0075] Here, it is particularly preferred for copolymers (b3) to contain, in addition to polymer units arising from the incorporation of the structural units according to formulae (M1-a), (M-I), (M-VII) and (M-VIII) into the copolymer, up to 5 wt. %, preferably at most 1 wt. %, of polymer units originating from the incorporation of other monomers. Copolymers (b3) are preferably exclusively synthesized from structural units of formulae (M1-a), (M-I), (M-VII) and (M-VIII) and can be described by general formula (Poly3)
##STR00011##
wherein m, n, o and p vary depending on the molar mass of the polymer and are not intended to imply that the copolymers are block copolymers. Instead, structural units of formulae (M1-a), (M-I), (M-VII) and (M-VIII) can be present randomly distributed in the molecule.
[0076] The positive polymer charge of component (b2) can be offset using any possible physiologically acceptable anions, such as chloride, bromide, hydrogensulfate, methylsulfate, ethylsulfate, tetrafluoroborate, phosphate, hydrogenphosphate, dihydrogenphosphate or p-toluenesulfonate, triflate.
[0077] If a methosulfate is used to offset the positive charge of the polymer of the formula (Poly3), such N-methylvinylimidazole/vinylpyrrolidone/vinylimidazole/methacrylamide copolymers are designated according to INCI nomenclature as Polyquaternium-68 and are obtainable, for example, from BASF under the trade name Luviquat.RTM. Supreme.
[0078] Very particularly preferred copolymers (b3) contain 1 to 12 mol %, preferably 3 to 9 mol % and in particular 6 mol % of structural units according to formula (M1-a) and 45 to 65 mol %, preferably 50 to 60 mol % and particularly 55 mol % of structural units according to formula (M-I) and 1 to 20 mol %, preferably 5 to 15 mol % and particularly 10 mol % of structural units according to formula (M-VII) and 20 to 40 mol %, preferably 25 to 35 mol % and particularly 29 mol % of structural units according to formula (M-VIII).
[0079] Among additional film-forming cationic and/or setting polymers chosen from cationic polymers with at least one structural element of formula (M1), the following are considered preferred: [0080] vinylpyrrolidone/1-vinyl-3-methyl-1H-imidazolium chloride copolymers (such as that with the INCI name Polyquaternium-16 under the trade names Luviquat.RTM. Style, Luviquat.RTM. FC 370, Luviquat.RTM.FC 550, Luviquat.RTM.FC 905 and Luviquat.RTM. MQ 552 (BASF SE)), [0081] vinylpyrrolidone/1-vinyl-3-methyl-1H-imidazolium methylsulfate copolymers (such as that with the INCI name Polyquaternium-44 under the trade name Luviquat.RTM. Care (BASF SE)), [0082] vinylpyrrolidone/vinylcaprolactam/1-vinyl-3-methyl-1H-imidazolium terpolymer (such as that with the INCI name Polyquaternium-46 under the trade names Luviquat.RTM. Care or Luviquat.RTM. Hold (BASF SE)), [0083] vinylpyrrolidone/methacrylamide/vinylimidazole/1-vinyl-3-methyl-1H-imidaz- olium methylsulfate copolymer (such as that with the INCI name Polyquaternium-68 under the trade name Luviquat.RTM. Supreme (BASF SE)), and mixtures of these polymers.
[0084] In a preferred embodiment, cosmetic agents according to the invention contain as additional film-forming and/or setting polymer at least one film-forming nonionic and/or setting nonionic polymer.
[0085] According to the invention, a nonionic polymer refers to a polymer which, in a protic solvent under standard conditions, has substantially no structural units with permanently cationic or anionic groups which have to be offset by counterions to obtain electroneutrality. Cationic groups include quaternized ammonium groups but not protonated amines. Anionic groups include carboxylic and sulfonic acid groups.
[0086] Film-forming nonionic and/or setting nonionic polymers are present in the agent preferably in an amount of 0.1 wt. % to 20.0 wt. %, more preferably 0.2 wt. % to 15.0 wt. %, very preferably 0.5 wt. % to 5.0 wt. %, based on total weight of the cosmetic agent.
[0087] Film-forming nonionic and/or setting nonionic polymers are preferably chosen from at least one polymer from [0088] homopolymers and nonionic copolymers of N-vinylpyrrolidone, [0089] nonionic copolymers of isobutene, [0090] nonionic copolymers of maleic anhydride.
[0091] A preferred combination of film-forming nonionic and/or setting nonionic polymers is one comprising at least one nonionic copolymer of maleic anhydride and at least one polymer from homopolymers and nonionic copolymers of N-vinylpyrrolidone.
[0092] Suitable polyvinylpyrrolidones include commercial products such as Luviskol.RTM. K 90 or Luviskol.RTM. K 85 from BASF SE.
[0093] Suitable polyvinyl alcohols are distributed, for example, under the trade names Elvanol.RTM. by Du Pont or Vinol.RTM. 523/540 by Air Products.
[0094] Suitable polyvinyl acetate is distributed, for example, under the trade name Vinac.RTM. as an emulsion by Air Products.
[0095] Very particularly preferred agents according to the invention are those having as film-forming nonionic and/or setting nonionic polymer at least one polymer chosen from [0096] copolymers of maleic anhydride and methyl vinyl ether, [0097] polyvinylpyrrolidone, [0098] copolymers of N-vinylpyrrolidone and vinyl esters of carboxylic acids with 2 to 18 carbon atoms, particularly N-vinylpyrrolidone and vinyl acetate, [0099] copolymers of N-vinylpyrrolidone and N-vinylimidazole and methacrylamide, [0100] copolymers of N-vinylpyrrolidone and N-vinylimidazole and acrylamide, [0101] copolymers of N-vinylpyrrolidone with N,N-di(C.sub.1 to C.sub.4)-alkylamino-(C.sub.2 to C.sub.4)-alkylacrylamide, [0102] copolymers of N-vinylpyrrolidone with N,N-di(C.sub.1 to C.sub.4)-alkylamino-(C.sub.2 to C.sub.4)-alkylacrylamide, or mixtures of these polymers.
[0103] It is preferred for the molar ratio of structural units obtained from the monomer N-vinylpyrrolidone to structural units obtained from the monomer vinyl acetate of the polymer to be in a range from 20:80 to 80:20, particularly from 30:70 to 60:40.
[0104] Suitable copolymers of vinylpyrrolidone and vinyl acetate are obtainable, for example, under the trademark Luviskol.RTM. VA 37, Luviskol.RTM. VA 55, Luviskol.RTM. VA 64 and Luviskol.RTM. VA 73 from BASF SE.
[0105] Further preferred cosmetic agents according to the invention additionally contain as nonionic film-forming and/or nonionic setting polymer at least one copolymer (n1) having at least one structural unit according to formula (M-I), at least one structural unit according to formula (M-VII), and at least one structural unit according to formula (M-VIII)
##STR00012##
[0106] Here, it is particularly preferred for these copolymers to contain, in addition to polymer units arising from incorporation of structural units according to formulae (M1-a), (I), (VII) and (VIII) into the copolymer, up to 5 wt. %, preferably at most 1 wt. %, of polymer units originating from the incorporation of other monomers. Copolymers (n1) are preferably exclusively synthesized from structural units of formulae (M1-a), (I), (VII) and (VIII) and can be described by the general formula (Poly4)
##STR00013##
wherein m, n, o and p vary depending on the molar mass of the polymer and are not intended to imply that the copolymers are block copolymers. Instead, structural units of formulae (I), (VII) and (VIII) can be present randomly distributed in the molecule.
[0107] One particularly preferred polymer is chosen from polymers having the INCI name VP/Methacrylamide/Vinyl Imidazole Copolymer, obtainable, for example, under the trade name Luviset Clear from BASF SE.
[0108] Further suitable according to the invention are those pulverulent compositions additionally having at least one nonionic film-forming and/or nonionic setting polymer comprising at least one structural unit of formula (M-I) and at least one structural unit of formula (M-III)
##STR00014##
wherein R.sup.1 is a hydrogen atom or a methyl group, X.sup.1 is an oxygen atom or an NH group, A.sup.1 is a 1,2-ethanediyl, 1,3-propanediyl or 1,4-butanediyl group R.sup.2 and R.sup.3 are, mutually independently, a (C.sub.1 to C.sub.4) alkyl group.
[0109] It is particularly preferred for the above nonionic film-forming and/or nonionic setting polymer to be chosen from at least one polymer having at least one or a plurality of the following features:
R.sup.1 is a methyl group, X.sup.1 is an NH group, A.sup.1 is 1,2-ethanediyl or 1,3-propanediyl, R.sup.2 and R.sup.3 are, mutually independently, methyl or ethyl (particularly preferably methyl).
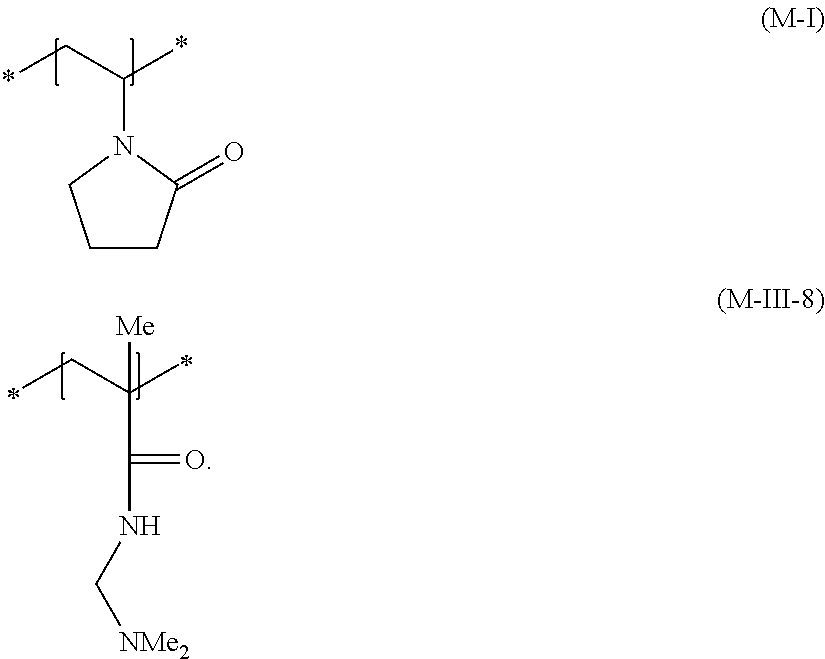
[0110] Additional nonionic film-forming and/or nonionic setting polymer of this embodiment is particularly preferably at least one polymer having at least one structural unit of formula (M-I) and at least one structural unit of formula (M-III-8),
##STR00015##
[0111] One very particularly preferred additional nonionic film-forming and/or nonionic setting polymer of this embodiment is a copolymer of N-vinylpyrrolidone and N,N-dimethylaminopropylmethacrylamide, sold, for example, with the INCI name VP/DMAPA Acrylates Copolymer, for example, under the tradename Styleze.RTM. CC 10 by ISP.
[0112] Preferred cosmetic agents according to one embodiment are those having at least one additional film-forming and/or setting polymer, provided that all these additional polymers are polysaccharide-based polymers. These additional polymers differ from the propylene oxide-modified polysaccharides. It is preferred that all further polymers of the cosmetic agent be chosen from xanthan, dehydroxanthan, alginate, guar gum, gum arabic, locust bean gum, starch, chitosan or mixtures thereof.
[0113] The additional film-forming and/or setting polymers are preferably present in an amount of 0.5 wt. % to 30 wt. %, particularly 2.5 wt. % to 20 wt. %, based on weight of the agent.
[0114] In order to improve the effects according to the invention, it is preferably suitable to add at least one compound of formula (II),
HO--CH.sub.2--(CHOH).sub.n--CH.sub.2--OH (II)
wherein n is an integer from 1 to 4.
[0115] Agents according to the invention are particularly effective if they contain glycerol and/or sorbitol as compounds of formula (II).
[0116] An input of compounds of formula (II) in an amount from 0.2 to 10 wt. %, particularly 0.5 to 7 wt. % has proven advantageous.
[0117] It is also preferred to use at least one nonionic surfactant. According to the invention, these surfactants can already have an emulsifying action.
[0118] Nonionic surfactants contain as hydrophilic group, for example, a polyol group, a polyalkylene glycol ether group or a combination of a polyol group and polyglycol ether group. Such compounds include [0119] addition products of 2 to 100 mol of ethylene oxide and/or 1 to 5 mol of propylene oxide onto linear or branched fatty alcohols with 8 to 30 carbon atoms, onto fatty acids with 8 to 30 carbon atoms and onto alkylphenols with 8 to 15 C atoms in the alkyl group, [0120] addition products of 2 to 20 units of glycerol onto linear or branched fatty alcohols with 8 to 30 carbon atoms in the alkyl group, onto linear or branched fatty acids with 8 to 30 carbon atoms in the alkyl group, such as those grades obtainable under the commercial names Dermofeel.RTM. G 10 LW (Straetmans Chemische Produkte), [0121] addition products, end group-terminated with a methyl or C.sub.2-C.sub.6 alkyl residue, of 2 to 50 mol of ethylene oxide and/or 1 to 5 mol of propylene oxide onto linear and branched fatty alcohols having 8 to 30 carbon atoms, onto fatty acids having 8 to 30 C atoms and onto alkylphenols having 8 to 15 C atoms in the alkyl group, such as the grades obtainable under the commercial names Dehydrol.RTM. LS, Dehydrol.RTM. LT (Cognis), [0122] C.sub.12-C.sub.30 fatty acid mono- and diesters of addition products of 1 to 30 mol of ethylene oxide onto glycerol, [0123] addition products of 5 to 60 mol of ethylene oxide onto castor oil and hardened castor oil, [0124] polyol fatty acid esters, such as the commercial product Hydagen.RTM. HSP (Cognis) or Sovermol grades (Cognis), [0125] alkoxylated triglycerides, [0126] alkoxylated fatty acid alkyl esters of the formula (E4-I)
[0126] R.sup.1CO-(OCH.sub.2CHR.sup.2).sub.wOR.sup.3 (E4-I) [0127] wherein R.sup.1CO is a linear or branched, saturated and/or unsaturated acyl residue having 6 to 22 carbon atoms, R.sup.2 is hydrogen or methyl, R.sup.3 is linear or branched alkyl residues having 1 to 4 carbon atoms and w is a number from 1 to 20, [0128] amine oxides, [0129] hydroxy mixed ethers described, for example, in German Patent Application No. 19738866, [0130] sorbitan fatty acid esters and addition products of ethylene oxide onto sorbitan fatty acid esters such as polysorbates, [0131] sugar fatty acid esters and addition products of ethylene oxide onto sugar fatty acid esters, [0132] addition products of ethylene oxide onto fatty acid alkanolamides and fatty amines, [0133] sugar surfactants of the alkyl and alkenyl oligoglycoside type of the formula (E4-II),
[0133] R.sup.4O-[G].sub.p (E4-II) [0134] wherein R.sup.4 is an alkyl or alkenyl residue having 4 to 22 carbon atoms, G is a sugar residue having 5 or 6 carbon atoms and p is a number from 1 to 10. They may be obtained according to relevant methods of preparative organic chemistry.
[0135] Suitable nonionic surfactants which are particularly preferred for use in the agent according to the invention are those chosen from [0136] addition products of 2 to 20 units of glycerol onto linear or branched fatty alcohols with 8 to 30 carbon atoms in the alkyl group, [0137] addition products of 2 to 20 units of glycerol onto linear or branched fatty acids with 8 to 30 carbon atoms in the alkyl group, [0138] sugar surfactants of the alkyl and alkenyl oligoglycoside type according to formula (E4-II) above, and mixtures of these surfactants.
[0139] Nonionic surfactants are preferably present in the agent according to the invention in an amount of 0.005 wt. % to 10 wt. %, particularly 0.01 to 2 wt. %, based on weight of the agent.
[0140] Agents according to the invention can also contain at least one plant extract. Typically, these extracts are produced by extraction of the entire plant. However, in individual cases it may be preferable to produce the extracts solely from the blossoms and/or leaves of the plant. Suitable plant extracts are obtained by extraction with organic solvents (e.g., ethanol, isopropanol, diethyl ether, petroleum ether, benzene, chloroform) or by steam distillation. According to the invention, preference is given to extracts from bamboo, linseed, water lily, green tea, oak bark, stinging nettle, witch hazel, hops, henna, chamomile, burdock root, horsetail, hawthorn, lime blossom, almond, Aloe vera, pine-needle, horse chestnut, sandalwood, juniper, coconut, mango, apricot, lime, wheat, kiwi fruit, melon, orange, grapefruit, sage, rosemary, birch, mallow, lady's smock, wild thyme, yarrow, thyme, melissa, restharrow, coltsfoot, marsh mallow, meristem, ginseng and ginger root. The additional plant extract is preferably present in the agent in an amount of 0.05 wt. % to 1.0 wt. %, particularly 0.1 wt. % to 0.5 wt. %, based on weight of the cosmetic agent.
[0141] It is preferred in particular if the agent according to the invention is formulated as a cream so that the cosmetic agent according to the invention additionally contains at least one oil phase.
[0142] An oil phase according to the invention refers to a phase which is liquid at 20.degree. C. and which, at 20.degree. C., dissolves in an amount of less than 1 g in 100 g of water.
[0143] The oil phase preferably has a viscosity of up to 1,000 mPas, (Brookfield, RVDV II+, 20.degree. C., 20 revolutions per minute, spindle no. 1).
[0144] In a preferred embodiment, the oil of the oil phase is chosen from at least one oil of [0145] plant oils, [0146] animal oils, [0147] ester oils, [0148] liquid fatty acids and/or the mono-, di- and trifatty acid esters of saturated and/or unsaturated linear and/or branched C.sub.6 to C.sub.22 fatty acids with glycerol.
[0149] Preferred vegetable oils are chosen from at least one of amaranth oil, sunflower oil, olive oil, soy oil, rapeseed oil, castor oil, sesame oil, almond oil, jojoba oil, orange oil, apricot kernel oil, macadamia nut oil, wheat germ oil, peach stone oil and the liquid fractions of coconut oil.
[0150] Preferred ester oils are chosen from esters of C.sub.6-C.sub.30 fatty acids with C.sub.2-C.sub.30 fatty alcohols. Monoesters of fatty acids with alcohols having 2 to 24 C atoms are preferred. Examples of fatty acid moieties used in the esters are caproic acid, caprylic acid, 2-ethylhexanoic acid, capric acid, lauric acid, isotridecanoic acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, isostearic acid, oleic acid, elaidic acid, petroselinic acid, linoleic acid, linolenic acid, elaeostearic acid, arachidic acid, gadoleic acid, behenic acid and erucic acid and the technical mixtures thereof, which are obtained, for example, on pressure splitting of natural fats and oils, on oxidation of aldehydes from Roelen's oxo synthesis or the dimerization of unsaturated fatty acids. Examples of fatty alcohol moieties in the ester oils are isopropyl alcohol, caproic alcohol, caprylic alcohol, 2-ethylhexyl alcohol, capric alcohol, lauryl alcohol, isotridecyl alcohol, myristyl alcohol, cetyl alcohol, palmoleyl alcohol, stearyl alcohol, isostearyl alcohol, oleyl alcohol, elaidyl alcohol, petroselinyl alcohol, linolyl alcohol, linolenyl alcohol, elaeostearyl alcohol, arachyl alcohol, gadoleyl alcohol, behenyl alcohol, erucyl alcohol and brassidyl alcohol and the technical mixtures thereof, which are obtained, for example, on high pressure hydrogenation of technical methyl esters based on fats and oils or aldehydes from Roelen's oxo synthesis and as a monomer fraction on the dimerization of unsaturated fatty alcohols. Particularly preferred substances according to the invention are isopropyl myristate (Rilanit.RTM. IPM), isononanoic acid C.sub.16-18 alkyl ester (Cetiol.RTM. SN), 2-ethylhexyl palmitate (Cegesoft.RTM. 24), stearic acid 2-ethylhexyl ester (Cetiol.RTM. 868), cetyl oleate, glycerol tricaprylate, coconut fatty alcohol caprinate/caprylate (Cetiol.RTM. LC), n-butyl stearate, oleyl erucate (Cetiol.RTM. J 600), isopropyl palmitate (Rilanit.RTM. IPP), oleyl oleate (Cetiol.RTM.), lauric acid hexyl ester (Cetiol.RTM. A), di-n-butyl adipate (Cetiol.RTM. B), myristyl myristate (Cetiol.RTM. MM), cetearyl isononanoate (Cetiol.RTM. SN), oleic acid decyl ester (Cetiol.RTM. V).
[0151] Mono-, di- and trifatty acid esters of saturated and/or unsaturated linear and/or branched fatty acids with glycerol which are preferably usable as oil in the oil phase are triglyceride esters of capric acid and caprylic acid (INCI name: Caprylic/Capric Triglyceride), for example, obtainable as a commercial product from Cognis under the name Myritol.RTM. 312.
[0152] The additional oil phase is preferably present in the agent according to the invention in an amount of 0.05 wt. % to 25 wt. %, particularly 0.1 wt. % to 20 wt. %, based on weight of the cosmetic agent.
[0153] Agents according to the invention which additionally contain at least one fatty substance are also suitable.
[0154] According to the invention, fatty substances are those compounds which, at 20.degree. C., are soluble in an amount of less than 1 g in 100 g of water.
[0155] The fatty substance is preferably chosen from candelilla wax, shea butter, carnauba wax, beeswax, coconut oil, C.sub.12 to C.sub.20 fatty acids (particularly palmitic acid, stearic acid).
[0156] The additional fatty substance is preferably present in the agent according to the invention in an amount of 0.05 wt. % to 35 wt. %, particularly of 1 wt. % to 20 wt. %, based on weight of the cosmetic agent.
[0157] Agents according to the invention contain their active ingredients in a cosmetic carrier, preferably in a hydrous cosmetic carrier, alcoholic cosmetic carrier or an aqueous-alcoholic cosmetic carrier. For temporarily reshaping hair, such carriers include lotions, water-in-oil emulsions, oil-in-water emulsions, creams, gels, foams, pomades, waxes or other preparations suitable for use on hair.
[0158] For the present invention, aqueous-alcoholic carriers include aqueous compositions containing 3 to 70 wt. % of a C.sub.1-C.sub.4 alcohol, particularly ethanol or isopropanol. The agents can also contain further organic solvents such as methoxybutanol, benzyl alcohol, diethylene glycol monoethyl ether, 1,2-propylene glycol or 1,3-propylene glycol. Any water-soluble organic solvents are preferred.
[0159] A cationic surfactant can be used as a conditioner. Here, preference is given to cationic surfactants such as quaternary ammonium compounds, ester quats and the amidoamines. Preferred quaternary ammonium compounds are ammonium halides, particularly chlorides and bromides such as alkyltrimethylammonium chlorides, dialkyldimethylammonium chlorides and trialkylmethylammonium chlorides (e.g., cetyltrimethylammonium chloride, stearyltrimethylammonium chloride, distearyldimethylammonium chloride, lauryldimethylammonium chloride, lauryldimethylbenzylammonium chloride and tricetylmethylammonium chloride), and the imidazolinium compounds known under the INCI names Quaternium-27 and Quaternium-83. Long alkyl chains of these surfactants preferably comprise 10 to 18 carbon atoms. Since, however, addition of surface-active substances can have a negative effect on the hydrophobic properties of hydrophobized silicon dioxide and thus on the stability of the cosmetic agent, the amount of conditioning surfactant has to be carefully matched to total composition. Preferably, no surfactant components are added.
[0160] Moreover, at least one vitamin, one provitamin, one vitamin precursor and/or of one of the derivatives thereof can be used as conditioner.
[0161] Preferred vitamins, provitamins and vitamin precursors according to the invention are those conventionally assigned to the groups A, B, C, E, F and H. Particularly preferred vitamins are those belonging to the B group or to the vitamin B complex, very particularly preferably vitamin B.sub.5 (pantothenic acid, panthenol and pantolactone).
[0162] A series of carboxylic acids are also suitable as conditioner.
[0163] Short-chain carboxylic acids in particular can be advantageous. For the purposes of the invention, short-chain carboxylic acids and the derivatives thereof are carboxylic acids which can be saturated or unsaturated and/or linear or branched or cyclic and/or aromatic and/or heterocyclic and have a molecular weight of less than 750. Preference may be given to saturated or unsaturated straight-chain or branched carboxylic acids with a chain length of from 1 to 16 C atoms in the chain, very particularly those with a chain length of from 1 to 12 C atoms.
[0164] Further suitable conditioners are protein hydrolysates and/or the derivatives thereof, use of protein hydrolysates of plant origin (e.g., soy, almond, pea, potato and wheat protein hydrolysates) being preferred. Such products are obtainable, for example, under the tradenames Gluadin.RTM. (Cognis), DiaMin.RTM. (Diamalt), Lexein.RTM. (Inolex), Hydrosoy.RTM. (Croda), Hydrolupin.RTM. (Croda), Hydrosesame.RTM. (Croda), Hydrotritium.RTM. (Croda) and Crotein.RTM. (Croda).
[0165] Although use of protein hydrolysates as such is preferred, amino acid mixtures obtained in other ways can also optionally be used in their place. It is also possible to use derivatives of protein hydrolysates, for example, in the form of the fatty acid condensation products thereof. Such products are distributed, for example, under the names Lamepon.RTM. (Cognis), Lexein.RTM. (Inolex), Crolastin.RTM. (Croda), Crosilk.RTM. (Croda) or Crotein.RTM. (Croda).
[0166] The teaching according to the invention comprises all isomeric forms, such as cis-trans isomers, diastereomers and chiral isomers.
[0167] According to the invention, it is also possible to use a mixture of a plurality of protein hydrolysates.
[0168] Furthermore, lipids and oil bodies are suitable as conditioners, for example, plant oils, liquid paraffin oils, isoparaffin oils, synthetic hydrocarbons and ester oils, enzymes and pearl extracts.
[0169] With addition of a UV filter, both the preparations themselves and the treated fibers can be protected from the harmful effects of UV radiation. It may therefore be advantageous to also add at least one UV filter to the cosmetic agent. Suitable UV filters are not subject to any general restrictions regarding structure and physical properties. Rather, any UV filters usable in the field of cosmetics whose absorption maximum is in the UVA (315-400 nm), the UVB (280-315 nm) or the UVC (<280 nm) range are suitable. UV filters with an absorption maximum in the UVB range, particularly from approx. 280 to approx. 300 nm, are particularly preferred.
[0170] Preferred UV filters according to the invention can be chosen from substituted benzophenones, p-aminobenzoic acid esters, diphenylacrylic acid esters, cinnamic acid esters, salicylic acid esters, benzimidazoles and o-aminobenzoic acid esters. Examples which may be mentioned here are 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid and the sodium salt thereof (benzophenone-4; Uvinul.RTM.MS 40; Uvasorb.RTM.S 5).
[0171] In one particular embodiment, the cosmetic agent contains one or more direct dyes. This makes it possible, when applying the composition, for the treated keratin fibers not only to be temporarily structured but also to be dyed at the same time. This may be particularly desirable when, for example, only temporary dyeing with conspicuous fashion colors is desired, which can be removed again from the keratin fibers simply by washing.
[0172] Cosmetic agents according to the invention can also contain alkalizing agents, conventionally alkali metal or alkaline earth metal hydroxides, ammonia or organic amines. Preferred alkalizing agents are monoethanolamine, monoisopropanolamine, 2-amino-2-methylpropanol, 2-amino-2-methyl-1,3-propanediol, 2-amino-2-ethyl-1,3-propanediol, 2-amino-2-methylbutanol and triethanolamine and alkali metal and alkaline earth metal hydroxides. In particular, monoethanolamine, triethanolamine and 2-amino-2-methylpropanol and 2-amino-2-methyl-1,3-propanediol are preferred in the context of this group. .omega.-Amino acids such as .omega.-aminocaproic acid can also be used as alkalizing agents.
[0173] The present invention secondly provides for use of a cosmetic agent of the first subject matter of the invention for temporarily reshaping and/or setting the shape of keratin fibers, particularly human hair.
[0174] The present invention thirdly provides a method for temporarily reshaping keratin fibers, particularly human hair, wherein a cosmetic agent of the first subject matter of the invention is applied onto the keratin fibres.
[0175] It is preferable if, after exposure to the cosmetic agent of the first subject matter of the invention, the keratin fibers are not rinsed and are left on the fibers.
[0176] The following Examples are intended to explain the subject matter of the present invention without limiting it in any way.
EXAMPLES
[0177] Unless stated otherwise, all quantities in this Examples section are stated in weight percent. The following formulations were prepared:
Example 1.1
"Hair Mousse"
TABLE-US-00001 [0178] Raw material E1[wt. %] E2[wt. %] Hydagen HCMF .sup.1 0.50 -- Polyquaternium-4 -- 0.30 Polyquaternium-11 -- 1.50 Lactic acid 0.28 -- Luviskol 60/40 W NP .sup.2 10.70 -- Nonionic starch modified with propylene oxide .sup.3 2.70 2.00 Sodium benzoate 0.30 -- D-Panthenol 0.15 0.15 Dow Corning 939 .sup.4 0.20 0.20 Dehyquart A CA .sup.5 1.00 1.00 PEG-40 Hydrogenated Castor Oil -- 0.30 Glycerol -- 0.15 Propane/butane 8.00 8.00 Water Ad 100 Ad 100 .sup.1 chitosan (80% deacetylated), molecular weight 50,000 to 1,000,000 g/mol, Cognis .sup.2 copolymer of N-vinylpyrrolidone and vinyl acetate, .sup.3 potato starch, propylene oxide content: 4 wt. % propylene oxide, viscosity: 64,000 mPa s, average molecular weight (weight-average): 800 kDa .sup.4 approximately 32-36% active substance, INCI name: Amodimethicone, Trideceth-12, Cetrimonium Chloride (Dow Corning) .sup.5 trimethylhexadecylammonium chloride (approximately 24-26% active substance; INCI name: Aqua (Water), Cetrimonium Chloride) (Cognis)
[0179] The respective formulation ingredients except for the propellant were mixed and the mixture introduced into an aerosol container satisfying the following industrial parameters: aluminum reservoir with valve product 522983 PV10697 from Precision (Deutsche Prazisions-Ventil GmbH). The aerosol can was appropriately sealed and the propellant introduced.
Example 1.2
"Hair Gels"
TABLE-US-00002 [0180] Raw material E4 E5 E6 E7 Disodium EDTA -- -- 0.05 -- 1,2-Propanediol 6.00 -- -- -- Starch modified with propylene 8.50 10.00 10.00 3.00 oxide .sup.6 Methylparaben 0.10 -- -- -- Aculyn 28 .sup.7 3.00 -- -- -- Aculyn 88 .sup.8 -- -- -- 1.80 Synthalen W 2000 .sup.9 -- -- -- 1.50 Polygel W 30 .sup.10 -- 3.00 -- -- Structure 2001 .sup.11 -- -- 5.00 -- PEG-40 Hydrogenated Castor Oil 0.40 -- -- 0.20 PPG-5-Ceteth-20 -- -- 0.50 -- Euxyl K320 .sup.12 -- -- -- 1.00 Luviskol K 90 .sup.13 -- -- 12.00 -- Luviskol K 85 .sup.14 -- 5.00 -- -- Polyethylene glycol 1500 2.00 -- -- -- Glycerol 10.00 2.75 2.00 -- D-Panthenol 0.15 -- 0.20 0.20 Neolone PE 0.30 0.60 0.60 Triethanolamine -- -- -- 0.80 Lactic acid -- 0.10 0.30 -- Uvinul P25 .sup.15 -- -- -- 0.05 Perfume 0.15 0.20 0.20 0.20 Water Ad 100 Ad 100 Ad 100 Ad 100 .sup.6 potato starch modified with 4.5 wt. % propylene oxide; average molecular weight 800 kDa, viscosity of a 43 wt. % solution in water 64,000 mPa s .sup.7 copolymer of (meth)acrylic acid, (meth)acrylic acid ester and beheneth-25 methacrylic acid ester (19-21 wt. % solids content in water; INCI name: Acrylates/Beheneth-25 Methacrylate Copolymer) (Rohm & Haas) .sup.8 copolymer of (meth)acrylic acid, (meth)acrylic acid ester and steareth-20 methacrylic acid ester (28-33 wt. % solids content in water; INCI name: Acrylates/Steareth-20 Methacrylate Copolymer) (Rohm & Haas) .sup.9 copolymer of (meth)acrylic acid, (meth)acrylic acid ester and palmeth-25 methacrylic acid ester (30-32 wt. % solids content in water; INCI name: Acrylates/Palmeth-25 Methacrylate Copolymer) (3 V Sigma) .sup.10 copolymer of (meth)acrylic acid, (meth)acrylic acid ester and palmeth-25 itaconic acid ester (30 wt. % solids content in water; INCI name: Acrylates/Palmeth-25 Itaconate Copolymer) (3 V Sigma) .sup.11 copolymer of (meth)acrylic acid, (meth)acrylic acid ester and steareth-20 itaconic acid ester (30 wt. % solids content in water; INCI name: Acrylates/Steareth-20 Itaconate Copolymer) (3 V Sigma) .sup.12 mixture of phenoxyethanol, methylparaben, ethylparaben, propylene glycol (Schulke & Mayr) .sup.13 polyvinylpyrrolidone (approx. 20% solids content in water; INCI name: PVP) (BASF) .sup.14 polyvinylpyrrolidone (approx. 20% solids content in water; INCI name: PVP) (BASF) .sup.15 4-aminobenzoic acid ethyl ester + 25 mol ethylene oxide (INCI name: PEG-25 PABA) (BASF SE)
2.0 Proof of Action
[0181] The following starches modified with propylene oxide were used:
TABLE-US-00003 TABLE 1 Propylene oxide-modified starches - Average molecular Viscosity range Name of the weight range of the of the modified modified starch modified starch (kDa) starch [mPa s] HPS A .sup.16 700-900 40,000-70,000 HPS B .sup.17 700-900 40,000-70,000 HPS C .sup.18 700-900 40,000-70,000 .sup.16 tapioca starch modified with 4.5-5.5 wt. % propylene oxide .sup.17 tapioca starch modified with 9.5-10.5 wt. % propylene oxide .sup.18 tapioca starch modified with 19-21 wt. % propylene oxide
[0182] Polymer solutions in water, each of 5 wt. % strength of the respective hydroxypropyl starches HPS A to C, of polyvinylpyrrolidone (PVP) (Luviskol.RTM. K 85, BASF SE) and of polyvinylpyrrolidone/vinyl acetate copolymer (PVP/VA) (Luviskol.RTM. 64 W NP, BASF SE) were produced. Curl retention measurements were then carried out on strands of hair treated therewith. Here, strands of hair were investigated according to the procedure described in 3.0 for determining High Humidity Curl Retention (HHCR).
[0183] The following results were obtained:
TABLE-US-00004 TABLE 2 High humidity curl retention (HHCR) - Polymer solution HHCR HPS A - 5 wt. % 90% HPS A - 5 wt. % 65% HPS A - 5 wt. % 75% PVP/VA - 5 wt. % 26% PVP - 5 wt. % 23%
[0184] The strands of hair treated with agents according to the invention exhibited hairstyle retention which was more resistant to atmospheric humidity. Better HHCR values and better styling hold were obtained when carrying out similar experiments with correspondingly modified potato starch.
3.0 Performance of High Humidity Curl Retention Measurement
[0185] Standardized strands of hair from Kerling (item no. 827560) of the "European Natural", color 6/0 type) of a length (L.sub.max) of 220 mm and a weight of 0.6 g were used. The strands were washed with a 12.5 wt. % sodium laureth sulfate solution by way of preparation. The strands of hair were dried overnight in a drying oven at 318 K.
[0186] 0.18 g of the compositions were applied onto a strand of hair and rubbed in. The strand was then wound onto a curler (Fripac-medis, diam. 7 mm, item no. D-1203) and dried overnight at room temperature.
[0187] The curlers were carefully removed and the strands hung up. The length of curls was in each case measured (L.sub.0) and the strands placed in a conditioning cabinet. They were stored there at 294 K and a relative atmospheric humidity of 85% over a period of 24 h, after which the length of the curls was remeasured (L.sub.t).
[0188] Five test strands per composition were correspondingly treated and measured.
[0189] High Humidity Curl Retention (HHCR) was calculated according to the following formula and the arithmetic mean of the HHCR values for the 5 test strands was determined for each composition:
HHCR = L max - L t L max - L 0 ##EQU00001##
* * * * *














XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.