Method of Inducing and/or Enhancing an Immune Response to Tumor Antigens
Berinstein; Neil ; et al.
U.S. patent application number 13/196704 was filed with the patent office on 2011-12-29 for method of inducing and/or enhancing an immune response to tumor antigens. Invention is credited to Brian Barber, Neil Berinstein, Philippe Moingeon, James Tartaglia.
| Application Number | 20110319480 13/196704 |
| Document ID | / |
| Family ID | 26857297 |
| Filed Date | 2011-12-29 |









| United States Patent Application | 20110319480 |
| Kind Code | A1 |
| Berinstein; Neil ; et al. | December 29, 2011 |
Method of Inducing and/or Enhancing an Immune Response to Tumor Antigens
Abstract
An improved method of inducing and/or enhancing an immune response to a tumor antigen is disclosed. The method involves administering the tumor antigen. nucleic acid coding therefor, vectors and/or cells comprising said nucleic acid or vaccines comprising the aforementioned to a lymphatic site.
| Inventors: | Berinstein; Neil; (Tornoto, CA) ; Tartaglia; James; (Schenectady, NY) ; Moingeon; Philippe; (Pommiers, FR) ; Barber; Brian; (Mississauga, CA) |
| Family ID: | 26857297 |
| Appl. No.: | 13/196704 |
| Filed: | August 2, 2011 |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | ||
|---|---|---|---|---|
| 09693754 | Oct 20, 2000 | 8017590 | ||
| 13196704 | ||||
| 60223325 | Aug 7, 2000 | |||
| 60160879 | Oct 22, 1999 | |||
| Current U.S. Class: | 514/44R ; 435/320.1; 536/23.5 |
| Current CPC Class: | A61K 39/001192 20180801; C12N 9/6494 20130101; A61K 39/001186 20180801; C12Y 304/24011 20130101; A61K 39/00 20130101; C12N 2710/24043 20130101; A61K 39/001191 20180801; A61K 39/001151 20180801; A61K 39/001156 20180801; A61K 39/00117 20180801; A61K 39/001106 20180801; A61K 39/001195 20180801; A61P 31/14 20180101; A61K 39/0011 20130101; A61P 37/04 20180101; A61K 39/001188 20180801; C12N 15/86 20130101; A61K 39/001184 20180801; A61K 2039/53 20130101; A61K 39/001182 20180801; A61K 39/001194 20180801; C07K 14/4748 20130101; A61P 35/00 20180101; C12N 2710/24071 20130101; A61P 37/02 20180101 |
| Class at Publication: | 514/44.R ; 536/23.5; 435/320.1 |
| International Class: | A61K 31/711 20060101 A61K031/711; C12N 15/63 20060101 C12N015/63; A61P 35/00 20060101 A61P035/00; C12N 15/12 20060101 C12N015/12 |
Claims
1-19. (canceled)
20. An isolated nucleic acid encoding SEQ ID NO.: 110.
21. The isolated nucleic acid sequence of claim 20 that is SEQ ID NO.: 111.
22. An expression vector comprising the nucleic acid of claim 20.
23. An expression vector comprising the nucleic acid of claim 21.
24. The expression vector of claim 22 wherein the vector is a bacterial or a viral vector.
25. The expression vector of claim 24 wherein the viral vector is selected from the group consisting of adenovirus, alphavirus, lenti virus, and poxvirus.
26. The expression vector of claim 25 wherein poxvirus is selected from the group consisting of vaccinia, fowlpox, avipox, orthopox, canary pox, swinepox, TROVAC, NYV AC, ALVAC(1), ALVAC(2), MVA, Wyeth and Poxvac-TC.
27. The expression vector of claim 25 wherein the poxvirus selected from the group consisting of NY VAC, ALVAC, and ALVAC:(2).
28. The expression vector of claim 23 wherein the vector is a bacterial or a viral vector.
29. The expression vector of claim 28 wherein the viral vector is selected from the group consisting of adenovirus, alphavirus, lentivirus, and poxvirus.
30. The expression vector of claim 29 wherein poxvirus is selected from the group consisting of vaccinia, NYVAC, avipox, canarypox, ALVAC. ALVAC(2), fowlpox, and TROVAC.
31. The expression vector of claim 30 wherein the poxvirus selected from the group consisting of NY VAC, ALVAC, and ALVAC(2).
32. A composition comprising the isolated nucleic acid of claim 20 and a pharmaceutically acceptable vehicle.
33. A composition comprising the isolated nucleic acid of claim 21 and a pharmaceutically acceptable vehicle.
34. A composition comprising an expression vector of claim 22 and a pharmaceutically acceptable carrier.
35. A composition comprising an expression vector of claim 23 and a pharmaceutically acceptable carrier.
36. A composition comprising an expression vector of claim 24 and a pharmaceutically acceptable carrier.
37. A composition comprising an expression vector of claim 25 and a pharmaceutically acceptable carrier.
38. A composition comprising an expression vector of claim 26 and a pharmaceutically acceptable carrier.
39. A composition comprising an expression vector of claim 27 and a pharmaceutically acceptable carrier.
40. A composition comprising an expression vector of claim 28 and a pharmaceutically acceptable carrier.
41. A composition comprising an expression vector of claim 29 and a pharmaceutically acceptable carrier.
42. A composition comprising; an expression vector of claim 30 and a pharmaceutically acceptable carrier.
43. A composition comprising an expression vector of claim 31 and a pharmaceutically acceptable carrier,
Description
FIELD OF THE INVENTION
[0001] The present invention relates to methods for inducing and/or enhancing immune responses to tumor antigens.
BACKGROUND OF THE INVENTION
[0002] Using immunological approaches to cancer therapy has been difficult as tumor cells are self-derived and therefore not as immunogenic as exogenous agents such as bacteria and viruses. As a result, the prospects of cancer immunotherapy rely upon the identification of tumor associated antigens ('TAA'') which can be recognized by the immune system. Specifically, target antigens eliciting T cell-mediated responses are of critical interest. This comes from evidence that cytotoxic T lymphocytes (CTLs) can induce tumor regression both in animal models (Kast W. et al (1989) Cell 59:6035; Greendberg P. (1991) Adv. Immunol. 49:281) and in humans (Boon T. et al. (1994) Annu. Rev. Immunol. 12:337). To date, many tumor associated antigens have been identified. These include the antigens MAGE, BAGE, GAGE, RAGE, gp100, MART-1/Melan-A, tyrosinase, carcinoembryonic antigen (CEA) as well as many others (Horig and Kaufman (1999) Clinical Immunology 92:211-223). Some of these tumor associated antigens are discussed below,
[0003] The first human tumor associated antigen characterized was identified from a melanoma. This antigen (originally designated MAGE 1) was identified using CTLs isolated from an HLA A1+melanoma patient to screen HLA A1 target cells transfected with tumor DNA (van der Bruggen P. (1991) Science, 254:1643; these tumor associated antigens are now designated MAGE-Al, MAGE-A2, etc). Interestingly, MAGE 1 was found to belong to a family of at least 12 closely related genes located on the X chromosome (de Plaen, E. et al. (1994) Immunogenetics 40:360). The nucleic acid sequence of the 11 additional MAGE genes share 65-85% identity with that of MAGE-1 (de Smet, C. et al. (1994) Immunogenetics 39:121). Both MAGE 1 and 3 are present in normal tissues, but expressed only in the testis (de Plaen, E. et al. (1994) Supra; de Smet, C. et al. (1994) Supra; Takahashi, K. et al. (1995) Cancer Res. 55:3478; Chyomey, P. et al. (1995) Immunogenetics 43:97). These initial results have subsequently been extended with the identification of new gene families (i.e. RAGE, BAGE, GAGE), all of which are typically not expressed in normal tissues (except testis) but expressed in a variety of tumor types.
[0004] Human carcinoembryonic antigen (CEA) is a 180 kD glycoprotein expressed on the majority of colon, rectal, stomach and pancreatic tumors (Muaro et al. (1985) Cancer Res. 45:5769), some 50% of breast carcinomas (Steward et al. (1974) Cancer 33:1246) and 70% of lung carcinomas (Vincent, R.G. and Chu, T.M. (1978) J. Thor. Cardiovas. Surg. 66:320). CEA was first described as a cancer specific fetal antigen in adenocarcinoma of the human digestive tract in 1965 (Gold, P. and Freeman, S.O. (1965) Exp. Med. 121:439). Since that time, CEA has been characterized as a cell surface antigen produced in excess in nearly all solid tumors of the human gastrointestinal tract. The gene for the human CEA protein has been cloned (Oikawa et at (1987) Biochim. Biophys. Res. 142:511-518; European Application No. EP 0346710). CEA is also expressed in fetal gut tissue and to a lesser extent on normal colon epithelium. The immunogenicity of CEA has been ambiguous, with several studies reporting the presence of anti-CEA antibodies in patients (Gold et al. (1973) Nature New Biology 239:60; Pompecki, R. (1980) Eur. J. Cancer 16:973; Ura et al. (1985) Cancer Lett.25:283; Fuchs et al. (1988) Cancer Immuno!. Immunother. 26:180) while other studies have not (LoGerfo et al. (1972) Int. J. Cancer 9:344; MacSween, J. M. (1975) Int J. Cancer 15:246; Chester K. A. and Begent, H. J. (1984) Clin. Exp. Immunot 58:685).
[0005] Gp100 is normally found in melanosomes and expressed in melanocytes, retinal cells, and other neural crest derivatives. The function of gp100 is currently unknown. By mass spectrometry, three immunodominant HLA-A2 binding gp100 epitopes have been identified: 89-154 (amino acids 154-162), g9-209 (amino acids 209-217); and g9-280 (amino acids 280-288). Notably, two of these epitopes (as peptides) have been synthetically altered so as to induce a more vigorous immune response in the original T cell clone: the threonine at position 2 in gp-209 was changed to a methionine, and the alanine residue at position 9 in gp-280 was changed to a valine. These changes increase the binding affinity of the epitope-peptides to the HLA-A2 molecule without changing the intrinsic natural epitopes recognized by the T cell receptor (TCR). Rosenberg and colleagues (NIH) have already successfully immunized melanoma patients with one of these modified peptides and have reported achieving objective clinical responses in some patients.
[0006] Despite significant advances that have been made with respect to immunological approaches to cancer treatment, there is still a need in the art to improve cancer immunotherapies.
SUMMARY OF THE INVENTION
[0007] The present invention relates to improved methods for inducing and/or enhancing an immune response to a tumor antigen.
[0008] The present inventors have found that administering the tumor antigen or nucleic acid coding therefor directly into a lymphatic site (such as a lymph node) induces and/or significantly enhances the immune response to the tumor antigen and/or breaks tolerance to the tumor antigen, both which have been a major challenge in previous methods of cancer immunotherapy.
[0009] Accordingly, one aspect the present invention provides a method for inducing and/or enhancing an immune response in an animal to a tumor antigen comprising administering an effective amount of a tumor antigen, nucleic acid coding therefor, vector or cell comprising said nucleic acid, or vaccine comprising the aforementioned to a lymphatic site in the animal.
[0010] In another aspect, the present invention provides a method for breaking immune tolerance to a tumor antigen in an animal comprising administering an effective amount of a tumor antigen, nucleic acid coding therefor, vector or cell comprising said nucleic acid, or vaccine comprising the aforementioned to a lymphatic site in the animal.
[0011] Other features and advantages of the present invention will become apparent from the following detailed description. It should be understood, however, that the detailed description and the specific examples while indicating preferred embodiments of the invention are given by way of illustration only, since various changes and modifications within the spirit and scope of the invention will become apparent to those skilled in the art from this detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The invention will now be described in relation to the drawings in which:
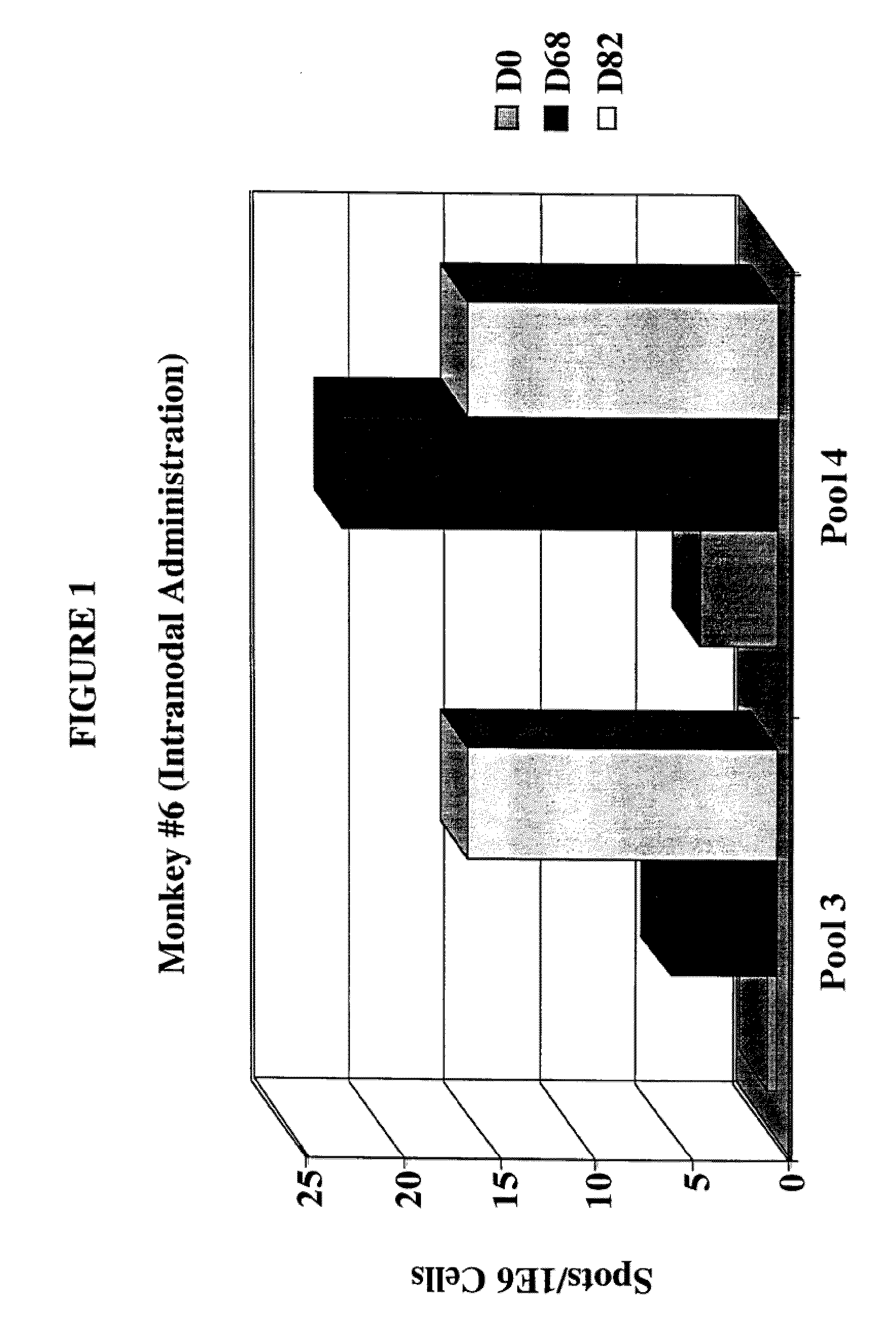
[0013] FIG. 1 is a bar graph showing the results of an IFN-y-ELISPOT analysis of an animal receiving an intranodal injection of the tumor antigen.
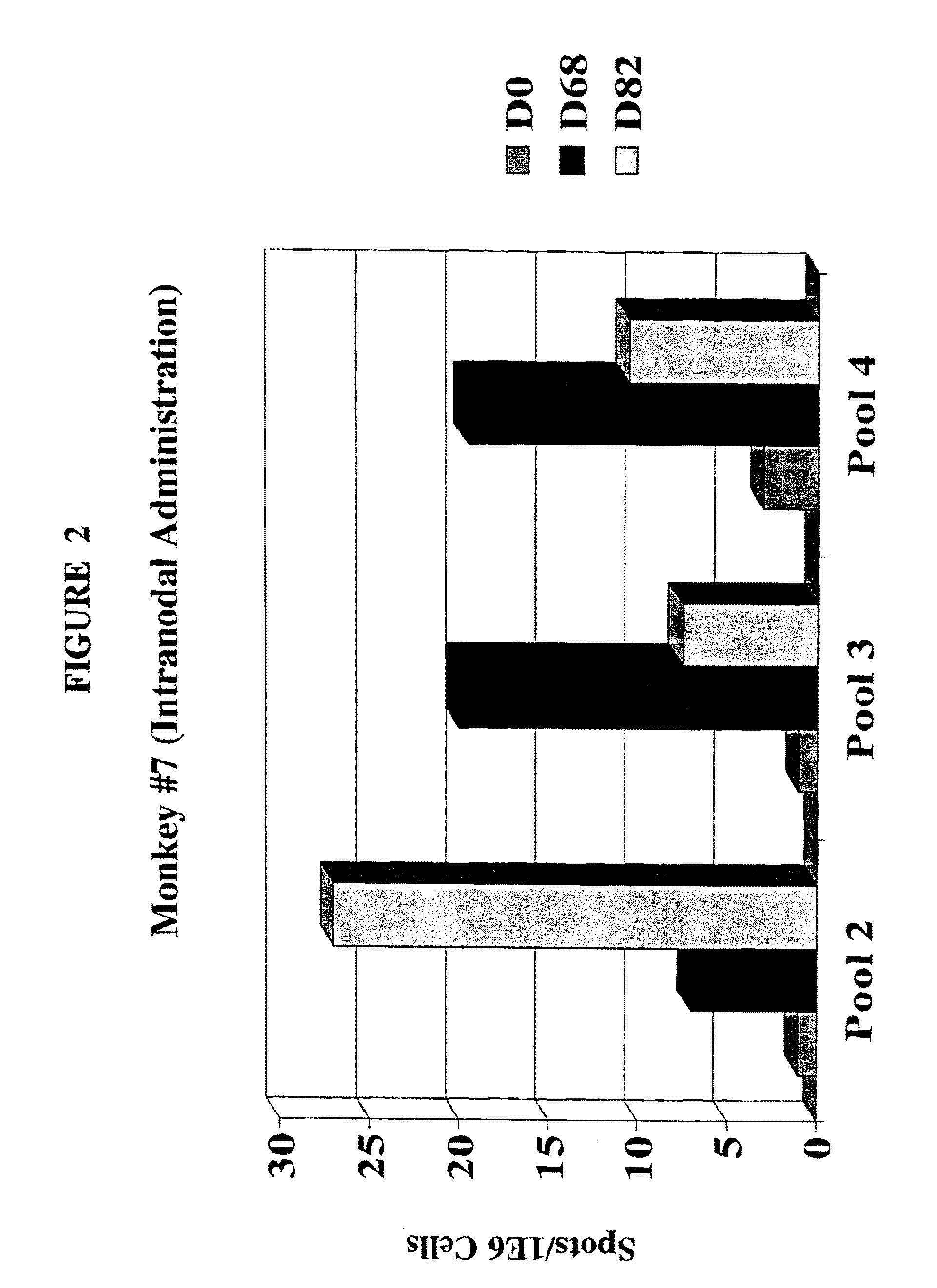
[0014] FIG. 2 is a bar graph showing the results of an IFN-y-ELISPOT analysis of an animal receiving an intranodal injection of the tumor antigen.
[0015] FIG. 3 is a bar graph showing the results of an IFN-y-ELISPOT analysis of an animal receiving a subcutaneous injection of the tumor antigen.
[0016] FIG. 4 is a bar graph showing the results of an IFN-y-ELISPOT analysis of an animal receiving a subcutaneous injection of the tumor antigen.
[0017] FIG. 5 is a graph showing the antibody response after a regiment of intranodal (group 2) and subcutaneous (group 3) administration of ALVAC-modified gp100/modified gp100 peptide immunogens.
[0018] FIG. 6 is the nucleic acid sequence of a modified gp1OOM cDNA (SEQ.ID.NO.:109).
[0019] FIG. 7 is the deduced amino acid sequence of the modified gp1OOM protein (SEQ.ID.NO.:110).
[0020] FIG. 8 is the nucleic acid and amino acid sequence of a modified. CEA (SEQ.ID.NOS.: 111 and 112).
DETAILED DESCRIPTION OF THE INVENTION
[0021] As hereinbefore mentioned, the present invention relates to an improved method for inducing and/or enhancing the immune response to a tumor antigen. Accordingly,, the present invention provides a method for inducing and/or enhancing an immune response in an animal to a tumor antigen comprising administering an effective amount of a tumor antigen, a nucleic acid sequence encoding a tumor antigen, a vector or cell comprising the nucleic acid sequence, or a vaccine comprising the tumor antigen, the nucleic acid sequence encoding the tumor antigen, or a vector comprising the nucleic acid sequence encoding the tumor antigen to a lymphatic site in the animal.
[0022] The term "inducing and/or enhancing an immune response" means that the method evokes and/or enhances any response of the animal's immune system.
[0023] "Immune response" is defined as any response of the immune system, for example, of either a cell-mediated (i.e. cytotoxic T-lymphocyte mediated) or humoral (i.e. antibody mediated) nature. These immune responses can be assessed by a number of in vivo or in vitro assays well known to one skilled in the art including, but not limited to, antibody assays (for example ELISA assays) antigen specific cytotoxicity assays, production of cytokines (for example ELISPOT assays), regression of tumors expressing the tumor antigens, inhibition of cancer cells expressing the tumor antigens, etc..
[0024] The term "lymphatic site" means a site in the body that is associated with the lymphatic system including lymphatic organs, tissues, cells, nodes or glands such as spleen, thymus, tonsils, Peyer's patches, bone marrow, lymphocytes, thoracic duct as well as all of the lymph nodes of the body.
[0025] The term "animal" as used herein includes all members of the animal kingdom and is preferably human.
[0026] The term "effective amount" as used herein means an amount effective, at dosages and for periods .of time necessary to achieve the desired results.
[0027] The term "tumor antigen" as used herein includes both tumor associated antigens (TAAs) and tumor specific antigens (TSAs). A tumor associated antigen means an antigen that is expressed on the surface of a tumor cell in higher amounts than is observed on normal cells or an antigen that is expressed on normal cells during fetal development. A tumor specific antigen is an antigen that is unique to tumor cells and is not expressed on normal cells. The term tumor antigen includes TAAs or TSAs that have been already identified and those that have yet to be identified and includes fragments, epitopes and any and all modifications to the tumor antigens.
[0028] The tumor associated antigen can be any tumor associated antigen including, but not limited to, gp100 (Kawakami et al., J. Immuno!. 154:3961-3968 (1995); Cox et al., Science, 264:716-719 (1994)), MART - 1/MeIan A (Kawakami et al., J. Exp. Med., 180:347-352 (1994); Castelli et al., J. Exp. Med., 181:363-368 (1995)), gp75 (TRP-1) (Wang et al., J. Exp. Med., 186:1131-1140 (1996)), and Tyrosinase (Wolfel et al., Eur. J. Immunol., 24:759-764 (1994); Topalian et at., J. Exp. Med., 183:1965-1971 (1996)` melanoma proteoglycan (Helistrom et al., J. Immunol., 130:1467-1 (1983); Ross et al., Arch. Biochem Biophys., 225:370-383 (1983)); mor-specific, widely shared antigens, for example: antigens of MAGE family, for example, MAGE-1, 2,3,4,6, and 12 (Van der Bruggen et al., Science, 254:1643-1647 (1991); Rogner et al., Genomics, 29:729-731 (1995)), antigens of BAGE family (Boel et al., Immunity, 2:167-175 (1995)), antigens of GAGE family, for example, GAGE-1,2 (Van den Eynde et al., J. Exp. Med., 182:689-698 (1995)), antigens of RAGE family, for example, RAGE-1 (Gaugler et at., Immunogenetics, 44:323-330 (1996)), N-acetylglucosaminyltransferase-V (Guilloux et at, J. Exp. Med., 183:1173-1183 (1996)), and p15 (Robbins et al., J. Immunol.154:5944-5950 (1995)); tumor specific mutated antigens; mutated .beta.-catenin (Robbins et al., J. Exp. Med., 183:1185-1192 (1996)), mutated MUM-1 (Coulie et al., Proc. Natl. Acad. Sci. USA, 92:7976-7980 (1995)), and mutated cyclin dependent kinases-4 (CDK4) (Wolfel et al., Science, 269:1281-1284 (1995)); mutated oncogene products: p21 ras (Fossum et at., Int. J. Cancer, 56:40-45 (1994)), BCR-abl (Bocchia et al., Blood, 85:2680-2684 (1995)), p53 (Theobald et al., Proc. Natl. Acad. Sci. USA, 92:11993-11997 (1995)), and p185 HER2/neu (Fisk et al., J. Exp. Med., 181:2109-2117 (1995)); Peoples et al., Proc. Nat!. Acad. Sci., USA, 92:432-436 (1995)); mutated epidermal growth factor receptor (EGFR) (Fujimoto et al., Eur. J. Gynecol. Onca, 16:40-47 (1995)); Harris et al., Breast Cancer Res. Treat, 29:1-2 (1994)); carcinoembryonic antigens (CEA) (Kwong et al., J. Natl. Cancer Inst., 85:982-990 (1995)); carcinoma associated mutated mucins, for example, MUC-1 gene products (Jerome et al., J. lmmunol., 151:1654-1662 (1993), loannides et al., J. Immunol., 151:3693-3703 (1993), Takahashi et al., J. Immunol., 153:2102-2109 (1994)); EBNA gene products of EBV, for example, EBNA-1 gene product (Rickinson et al., Cancer Surveys, 13:53-80 (1992)); E7, E6 proteins of human papillomavirus (Ressing et al., J. Immunol, 154:5934-5943 (1995)); prostate specific antigens (PSA) (Xue et al., The Prostate, 30:73-78 (1997)); prostate specific membrane antigen (PSMA) (Israeli, et al., Cancer Res., 54:1807-1811 (1994)); PCTA-1 (Sue et al., Proc. Nat!. Acad. Sci. USA, 93:7252-7257 (1996)); idiotypic epitopes or antigens, for example, immunoglobulin idiotypes or T cell receptor idiotypes, (Chen et al., J. Immunol., 153:4775-4787 (1994); Syrengelas et al., Nat. Med., 2:1038-1040 (1996)); KSA (US Patent # 5348887); NY-ESO-1 (WO 98/14464).
[0029] Also included are modified tumor antigens and/or epitope/peptides derived therefrom (both unmodified and modified). Examples include, but are not limited to, modified and unmodified epitope/peptides derived from gp100 (WO 98/02598; WO 95/29193; WO 97/34613; WO 98/33810; CEA (WO 99/19478; S. Zaremba et al. (1997) Cancer Research 57:4570-7; K.T. Tsang et al. (1995) J. Int. Cancer Inst. 87:982-90); MART-1 (WO 98/58951, WO 98/02538; D. Valmeri et al. (2000) J. Immunol. 164:1125-31); p53 (M. Eura et al. (2000) Clinical Cancer Research 6:979-86); TRP-1 and TRP-2 (WO 97/29195); tyrosinase (WO 96/21734; WO 97/11669; WO 97/34613; WO 98/33810; WO 95/23234; WO 97/26535); KSA (WO 97/15597); PSA (WO 96/40754); NY-ESO 1 (WO 99/18206); HER2/neu (U.S. Pat. No. 5869445); MAGE family related (L. Heidecker et al. (2000) J. Immunol. 164:6041-5; WO 95/04542; WO 95/25530; WO 95/25739; WO 96/26214; WO 97/31017; WO 98/10780).
[0030] In a preferred embodiment, the tumor-associated antigen is gp100, a modified gp100 or a fragment thereof. In particular, the inventors have prepared a modified gp100 peptide termed gp1OOM which has the nucleic acid sequence shown in FIG. 6 (SEQ.ID.NO.:109) and the deduced amino acid sequence shown in FIG. 7 (SEQ.ID.NO.:110). The inventors have shown that the intranodal injection of a recombinant avipox virus comprising a nucleic acid coding for fragments of the modified gp100 (comprising modified epitopes 209(2M) (IMDQVPFSY, SEQ.ID.NO.:1) and 290(9V) (YLEPGPVTV, SEQ.ID.NO.:2)) followed by modified epitope/peptide boosts induced both a humoral and cell mediated response that was several times higher than when the same anitgens were administered subcutaneously. The experimental details and results are discussed in Example 1.
[0031] In another embodiment, the tumor-associated antigen is carcinoembryonic antigen (CEA), a modified CEA or a fragment thereof. The nucleic acid sequence of a modified CEA antigen is shown in FIG. 8 and SEQ.ID.NO.:111. The corresponding amino acid sequence is shown in FIG. 8 and SEQ.ID.NO.:112. Preferably, the modified CEA antigen comprises the sequence YLSGADLNL, SEQ.ID.NO.:113.
[0032] Additional embodiments of the invention encompass nucleic acid sequences comprising sequences encoding the tumor antigens and fragments or modified forms thereof as hereinbefore described. The term "nucleic acid sequence" refers to a sequence of nucleotide or nucleoside monomers consisting of naturally occurring bases, sugars and intersugar (backbone) linkages. The term also includes modified or substituted sequences comprising non-naturally occurring monomers or portions thereof, which function similarly. The nucleic acid sequences of the present invention may be ribonucleic (RNA) or deoxyribonucleic acids (DNA) and may contain naturally occurring bases including adenine, guanine, cytosine, thymidine and uracil. The sequences may also contain modified bases such as xanthine, hypoxanthine, 2-aminoadenine, 6-methyl, 2-propyl, and other alkyl adenines, 5-halo uracil, 5-halo cytosine, 6-aza uracil, 6-aza cytosine and 6-aza thymine, pseudo uracil, 4-thiouracil, 8-halo adenine, 8-amino adenine, 8-thiol adenine, 8-thio-alkyl adenines, 8-hydroxyl adenine and other 8-substituted adenines, 8-halo guanines, 8-amino guanine, 8-thiol guanine, 8-thioalkyl guanines, 8-hydroxyl guanine and other 8-substituted guanines, other aza and deaza uracils, thymidines, cytosines, adenines, or guanines, 5-trifluoromethyl uracil and 5-trifluoro cytosine.
[0033] The nucleic acid sequences encoding the tumor antigens of the invention include,' but are not limited to, viral nucleic acid(s), plasmid(s), bacterial DNA, naked/free DNA and RNA. The nucleic acids encompass both single and double stranded forms. As such, these nucleic acids comprise the relevant base sequences coding for the aforementioned tumor antigens. For purposes of definitiveness, the "relevant base sequences coding for the aforementioned polypeptides" further encompass complementary nucleic acid sequences. As such, embodiments of the invention encompass nucleic acid sequences per se encoding for the aforementioned tumor antigens, or recombinant nucleic acids into which has been inserted said nucleic acids coding for tumor antigens (as described below).
[0034] Bacterial DNA useful in recombinant nucleic acid embodiments of the invention are known to those of ordinary skill in the art. Sources of bacterial DNA include, for example, Shigella, Salmonella, Vibrio cholerae, Lactobacillus, Bacille Calmette Guerin (BCG), and Streptococcus. In bacterial DNA embodiments of the invention, nucleic acid of the invention may be inserted into the bacterial genome, can remain in a free state, or be carried on a plasmid.
[0035] Viral recombinant nucleic acid embodiments of the invention may be derived from a poxvirus or other virus such as adenovirus or alphavirus. Preferably the viral nucleic acid is incapable of integration in recipient animal cells. The elements for expression from said nucleic acid may include a promoter suitable for expression in recipient animal cells.
[0036] Specific vial recombinant nucleic acid embodiments of the invention encompass (but are not limited to) poxviral, alphaviral, and adenoviral nucleic acid. Poxviral nucleic acid may be selected from the group consisting of avipox, orthopox, and suipox nucleic acid. Particular embodiments encompass poxviral nucleic acid selected from vaccinia, fowlpox, canary pox and swinepox; specific examples include TROVAC, NYVAC, ALVAC, MVA Wyeth and Poxvac-TC (described in more detail below).
[0037] It is further contemplated that recombinant nucleic acids of this invention may further comprise nucleic acid sequences encoding at least one member chosen from the group consisting of cytokines, lymphokines, and co-stimulatory molecules. Examples include (but are not limited to) interleukin 2, interleukin 12, interleukin 6, interferon gamma, tumor necrosis factor Alpha, GM-CSF, B7.1, B7.2, ICAM-1, LFA-3, and Cd72.
[0038] Standard techniques of molecular biology for preparing and purifying nucleic acids well known to those skilled in the art can be used in the preparation of the recombinant nucleic acid aspects of the invention (for example, as taught in Current Protocols in Molecular Biology, F. M. Ausubel et al. (Eds.), John Wiley and Sons, Inc, N.Y., U.S.A. (1998), Chpts. 1, 2 and 4; Molecular Cloning: A Laboratory Manual (2.sup.nd Ed.), J. Sambrook, E. F. Fritsch and T. Maniatis (Eds.), Cold Spring Harbor Laboratory Press, N.Y., U.S.A. (1989), Chpts. 1, 2, 3 and 7).
[0039] Aspects of this invention further encompass vectors comprising the aforementioned nucleic acids. In certain embodiments, said vectors may be recombinant viruses or bacteria (as described below).
[0040] Adenovirus vectors and methods for their construction have been described (e.g. U.S. Pat. Nos. 5994132, 5932210, 6057158 and Published PCT Applications WO 9817783, WO 9744475, WO 9961034, WO 9950292, WO 9927101, WO 9720575, WO 9640955, WO 9630534-all of which are herein incorporated by reference). Alphavirus vectors have also been described in the art and can be used in embodiments of this invention (e.g. U.S. Pat. Nos. 5792462, 5739026, 5843723, 5789245, and Published PCT Applications WO 9210578, WO 9527044, WO 9531565, WO 9815636-all of which are herein incorporated by reference), as have lentivirus vectors (e.g. U.S. Patent Nos. 6013516, 5994136 and Published PCT Applications WO 9817816, WO 9712622, WO 9817815, WO 9839463, WO 9846083, WO 9915641, WO 9919501, WO 9930742,WO 9931251, WO 9851810, WO 0000600-all of which are herein incorporated by reference). Poxvirus vectors that can be used include, for example, avipox, orthopox or suipox poxvirus (as described in U.S. Pat. Nos. 5364773, 4603112, 5762938, 5378457, 5494807, 5505941, 5756103, 5833975 and 5990091-all of which are herein incorporated by reference). Poxvirus vectors comprising a nucleic acid coding for a tumor antigen can be obtained by homologous recombination as is known to one skilled in the art. As such, the nucleic acid coding for the tumor antigen is inserted into the viral genome under appropriate conditions for expression in mammalian cells (as described below).
[0041] In one embodiment of the invention the poxvirus vector is ALVAC (1) or ALVAC (2) (both of which have been derived from canarypox virus). ALVAC (1) (or ALVAC (2)) does not productively replicate in non-avian hosts, a characteristic thought to improve its safety profile. ALVAC (1) is an attenuated canarypox virus-based vector that was a plaque-cloned derivative of the licensed canarypox vaccine, Kanapox (Tartaglia et al. (1992) Virology 188:217; U.S. Pat. Nos. 5505941, 5756103 and 5833975-all of which are incorporated herein by reference). ALVAC (1) has some general properties which are the same as some general properties of Kanapox. ALVAC-based recombinant viruses expressing extrinsic immunogens have also been demonstrated efficacious as vaccine vectors (Tartaglia et al, In AIDS Research Reviews (vol. 3) Koff W., Wong-Staol F. and Kenedy R.C. (eds.), Marcel Dekker NY, pp. 361-378 (1993a); Tartaglia,,J. et al. (1993b) J. Virol. 67:2370). For instance, mice immunized with an ALVAC (1) recombinant expressing the rabies virus glycoprotein were protected from lethal challenge with rabies virus (Tartaglia, J. et al., (1992) supra) demonstrating the potential for ALVAC (1) as a vaccine vector. ALVAC-based recombinants have also proven efficacious in dogs challenged with canine distemper virus (Taylor, J. et al. (1992) Virology 187:321) and rabies virus (Perkus, M. E. et al., In Combined Vaccines and Simultaneous Administration: Current Issues and Perspective, Annals of the New York Academy of Sciences (1994)), in cats challenged with feline leukemia virus (Tartaglia, J. et al., (1993b) supra), and in horses challenged with equine influenza virus (Taylor, J. et al., In Proceedings of the Third International Symposium on Avian Influenza, Univ. of Wisconsin-Madison, Madison, Wisconsin, pp. 331-335 (1993)).
[0042] ALVAC (2) is a second-generation ALVAC vector in which vaccinia transcription elements E3L and K3L have been inserted within the C6 locus (U.S. Pat. No. 5990091, incorporated herein by reference). The E3L encodes a protein capable of specifically binding to dsRNA. The K3L ORF has significant homology to El F-2. Within ALVAC (2) the E3L gene is under the transcriptional control of its natural promoter, whereas K3L has been placed under the control of the early!late vaccine H6 promoter. The E3L and K3L genes act to inhibit PKR activity in cells infected with ALVAC (II), allowing enhancement of the level and persistence of foreign gene expression.
[0043] Additional viral vectors encompass natural host-restricted poxviruses. Fowlpox virus (FPV) is the prototypic virus of the Avipox genus of the Poxvirus family. Replication of avipox viruses is limited to avian species (Matthews, R.E.F. (1982) Intervirology 17:42) and there are no reports in the literature of avipox virus causing a productive infection in any non-avian species including man. This host restriction provides an inherent safety barrier to transmission of the virus to other species and makes use of avipox virus based vectors in veterinary and human applications an attractive proposition.
[0044] FPV has been used advantageously as a vector expressing immunogens from poultry pathogens. The hemagglutinin protein of a virulent avian influenza virus was expressed in an FPV recombinant. After inoculation of the recombinant into chickens and turkeys, an immune response was induced which was protective against either a homologous or a heterologous virulent influenza virus challenge (Taylor, J. et al. (1988) Vaccine 6: 504). FPV recombinants expressing the surface glycoproteins of Newcastle Disease Virus have also been developed (Taylor, J. et al. (1990) J. Virol. 64:1441; Edbauer, C. et al. (1990) Virology 179:901; U.S. Patent No.
[0045] 5766599-incorporated herein by reference).
[0046] A highly attenuated strain of vaccinia, designated MVA, has also been used as a vector for poxvirus-based vaccines. Use of MVA is described in U.S. Pat. No. 5,185,146.
[0047] Other attenuated poxvirus vectors have been prepared via genetic modification to wild type strains of vaccinia. The NYVAC vector, for example, is derived by deletion of specific virulence and host-range genes from the Copenhagen strain of vaccinia (Tartaglia, J. et al. (1992), supra; U.S. Pat. Nos. 5364773 and 5494807-incorporated herein by reference) and has proven useful as a recombinant vector in eliciting a protective immune response against expressed foreign antigens.
[0048] Recombinant viruses can be constructed by processes known to those skilled in the art (for example, as previously described for vaccinia and `avipox viruses; U.S. Pat. Nos. 4769330; 4722848; 4603112; 5110587; and 5174993-all of which are incorporated herein by reference).
[0049] In further embodiments of the invention, live and/or attenuated bacteria may also be used as vectors. For example, non-toxicogenic Vibrio cholerae mutant strains may be useful as bacterial vectors in embodiments of this invention; as described in U.S. Pat. No. 4,882,278 (disclosing a strain in which a substantial amount of the coding sequence of each of the two ctxA alleles has been deleted so that no functional cholera toxin is produced), WO 92/11354 (strain in which the irgA locus is inactivated by mutation; this mutation can be combined in a single strain with ctxA mutations), and WO 94/1533 (deletion mutant lacking functional ctxA and attRS1 DNA sequences). These strains can be genetically engineered to express heterologous antigens, as described in WO 94/19482. (All of the aforementioned issued patent/patent applications are incorporated herein by reference.)
[0050] Attenuated Salmonella typhimurium strains, genetically engineered for recombinant expression of heterologous antigens and their use as oral immunogens are described, for example, in WO 92/11361.
[0051] As noted, those skilled in the art will readily recognize that other bacterial strains useful as bacterial vectors in embodiments of this invention include (but are not limited to) Shigella flexneri, Streptococcus gordonii, and Bacille Calmette Guerin (as described in WO 88/6626, WO 90/0594, WO 91/13157, WO 92/1796, and WO 92/21376; all of which are incorporated herein by reference). In bacterial vector embodiments of this invention, a nucleic acid coding for a tumor antigen may be inserted into the bacterial genome, can remain in a free state, or be carried on a plasmid.
[0052] It is further contemplated that the invention encompasses vectors which comprise nucleic acids coding for at least one member from the group consisting of cytokines, lymphokines and immunostimulatory molecules. Said nucleic acid sequences can be contiguous with sequences coding for the tumor antigen or encoded on distinct nucleic acids.
[0053] Cells comprising the aforementioned tumor antigens, nucleic acids coding therefor, and/or vectors encompass further embodiments of the invention. These cells encompass any potential cell into which the aforementioned tumor antigen, nucleic acid, and/or vector might be introduced and/or transfected and/or infected (for example, bacteria, COS cells, Vero cells, chick embryo fibroblasts, tumor cells, antigen presenting cells, dendritic cells, etc.). The choice of process for the introduction and/or transfection and/or infection into cells is dependant upon the intrinsic nature of the introduced agent (i.e. free DNA, plasmid, recombinant virus), as will be known to one skilled in the art (for example, as taught in Current Protocols in Molecular Biology, F.M. Ausubel et al. (Eds.), John Wiley and Sons, Inc., N.Y., U.S.A. (1998), Chpt. 9; Molecular Cloning: A Laboratory Manual (2nd Ed.), J. Sambrook, E.F. Fritsch and T. Maniatis (Eds.), Cold Spring Harbor Laboratory Press, N.Y., U.S.A. (1989), Chpts. 1, 2, 3 and 16).
[0054] Further embodiments of the invention encompass vaccines comprising the tumor antigens and/or nucleic acids coding therefor and/or vectors and/or cells previously described.
[0055] The vaccine of the invention comprising the tumor antigen may be a multivalent vaccine and additionally contain several peptides, epitopes or fragments of a particular tumor antigen or contain peptides related to other tumor antigens and/or infectious agents in a prophylactically or therapeutically effective manner. Multivalent vaccines against cancers may contain a number of individual TM's, or immunogenic fragments thereof, alone or in combinations which are effective to modulate an immune response to cancer.
[0056] A vaccine of the invention may contain a nucleic acid molecule encoding a tumor antigen of the invention. Such vaccines are referred to as nucleic acid vaccines but are also termed genetic vaccines, polynucleotide vaccines or DNA vaccines, all of which are within the scope of the present invention. In such an embodiment, the tumor antigen is produced in vivo in the host animal. Additional embodiments if the invention encompass vectors (i.e. bacteria, recombinant viruses) comprising the aforementioned nucleic acids.
[0057] The present invention also contemplates mixtures of the tumor antigens, nucleic acids coding therefor, vectors comprising said nucleic acids, cells and/or vaccines comprising the aforementioned, and at least one member selected from the group consisting of cytokines, lymphokines, immunostimulatory molecules, and nucleic acids coding therefor. Additional embodiments of this invention further encompass pharmaceutical corn positions comprising the aforementioned tumor antigens, nucleic acids coding therefor, vectors, cells, vaccines or mixtures for administration to subjects in a biologically compatible form suitable for administration in vivo. By "biologically compatible form suitable for administration in vivo" is meant a form of the substance to be administered in which any toxic effects are outweighed by the therapeutic effects. Administration of a therapeutically active amount of the pharmaceutical compositions of the present invention, or an "effective amount", is defined as an amount effective at dosages and for periods of time, necessary to achieve the desired result of eliciting an immune response in an animal. A therapeutically effective amount of a substance may vary according to factors such as the disease state/health, age, sex, and weight of the recipient, and the inherent ability of the particular tumor antigen, nucleic acid coding therefor, vector, cell, or vaccine to elicit a desired immune response. Dosage regima may be adjusted to provide the optimum therapeutic response. For example, several divided doses may be administered daily, or at periodic intervals, and/or the dose may be proportionally reduced as indicated by the exigencies of circumstances.
[0058] The pharmaceutical compositions described herein can be prepared by per se known methods for the preparation of pharmaceutically acceptable compositions which can be administered to animals such that an effective quantity of the active substance (i.e. tumor antigen, nucleic acid, recombinant virus, vaccine) is combined in a mixture with a pharmaceutically acceptable vehicle. Suitable vehicles are described, for example, in "Handbook of Pharmaceutical Additives" (compiled by Michael and Irene Ash, Gower Publishing Limited, Aldershot, England (1995)). On this basis, the compositions include, albeit not exclusively, solutions of the substances in association with one or more pharmaceutically acceptable vehicles or diluents, and may be contained in buffered solutions with a suitable pH and/or be iso-osmotic with physiological fluids. In this regard, reference can be made to U.S. Pat. No. 5,843,456. These compositions may further comprise an adjuvant (as described below).
[0059] Further embodiments of the invention encompass methods of inhibiting a tumor antigen expressing cancer cell in a patient comprising administering to said patient an effective amount of a tumor antigen, nucleic acid coding therefor, vector, cell, or vaccine of the invention. Patients with solid tumors expressing tumor antigens include (but are not limited to) those suffering from colon cancer, lung cancer, pancreas cancer, endometrial cancer, breast cancer, thyroid cancer, melanoma, oral cancer, laryngeal cancer, seminoma, hepatocellular cancer, bile duct cancer, squamous cell carcinoma, and prostate cancer. As such, methods of treating patients with cancer per se encompassing the aforementioned methods of inducing an immune response and/or inhibiting a tumor antigen expressing cell are contemplated aspects/embodiments of the invention.
[0060] As mentioned previously, an animal may be immunized with a tumor antigen, nucleic acid coding therefore, vector, cell or vaccine of the invention by administering the aforementioned to a lymphatic site. The administration can be achieved in a single dose or repeated at intervals. The appropriate dosage is dependant on various parameters understood by the skilled artisans, such as the immunogen itself (i.e. polypeptide vs. nucleic acid (and more specifically type thereof)), the route of administration and the condition of the animal to be vaccinated (weight, age and the like).
[0061] As previously noted, nucleic acids (in particular plasmids and/or free/naked DNA and/or RNA coding for the tumor antigen of the invention) can be administered to an animal for purposes of inducing/eliciting an immune response (for example, US Patent No. 5589466; McDonnell and Askari, NEJM 334:42-45 (1996); Kowalczyk and Ertl, Cell Mol. Life Sci. 55:751-770 (1999)). Typically, this nucleic acid is a form that is unable to replicate in the target animal's cell and unable to integrate in said animal's genome. The DNA/RNA molecule encoding the tumor antigen is also typically placed under the control of a promoter suitable for expression in the animal's cell. The promoter can function ubiquitously or tissue-specifically. Examples of non-tissue specific promoters include the early Cytomegalovirus (CMV) promoter (described in U.S. Pat. No. 4,168,062) and the Rous Sarcoma Virus promoter. The desmin promoter is tissue-specific and drives expression in muscle cells. More generally, useful vectors have been described (i.e., WO 94/21797).
[0062] For administration of nucleic acids coding for a tumor antigen, said nucleic acid can encode a precursor or mature form of the polypeptide/protein. When it encodes a precursor form, the precursor form can be homologous or heterologous. In the latter case, a eucaryotic leader sequence can be used, such as the leader sequence of the tissue-type plasminogen factor (tPA).
[0063] For use as an immunogen, a nucleic acid of the invention can be formulated according to various methods known to a skilled artisan. First, a nucleic acid can be used in a naked/free form, free of any delivery vehicles (such as anionic liposomes, cationic lipids, microparticles, (e.g., gold microparticles), precipitating agents (e.g., calcium phosphate) or any other transfection-facilitating agent. In this case the nucleic acid can be simply diluted in a physiologically acceptable solution (such as sterile saline or sterile buffered saline) with or without a carrier. When present, the carrier preferably is isotonic, hypotonic, or weakly hypertonic, and has a relatively low ionic strength (such as provided by a sucrose solution (e.g., a solution containing 20% sucrose)).
[0064] Alternatively, a nucleic acid can be associated with agents that assist in cellular uptake. It can be, i.e., (i) complemented with a chemical agent that modifies the cellular permeability (such as bupivacaine; see, for example, WO 94/16737), (ii) encapsulated into liposomes, or (iii) associated with cationic lipids or silica, gold, or tungsten microparticles.
[0065] Cationic lipids are well known in the art and are commonly used for gene delivery. Such lipids include' Lipofectin (also known as DOTMA (N41-(2,3-dioleyloxy)propyl}-N,N,N-trimethylammonium chloride), DOTAP (1,2-bis(oleyloxy)-3-(trimethylammonio) propane). DDAB (dimethyldioctadecyl-ammonium bromide), DOGS (dioctadecylamidologlycyl spermine) and cholesterol derivatives such as DC-Chol (3 beta-(N-(N',N'-dimethyl aminomethane)-carbamoyl) cholesterol). A description of these cationic lipids can be found in EP 187702, WO 90/11092, U.S. Pat. No. 5283185, WO 91/15501, WO 95/26356, and U.S. Pat. No. 5527928. Cationic lipids for gene delivery are preferably used in association with a neutral lipid such as DOPE (dioleyl phosphatidylethanolamine) as, for example, described in WO 90/11092.
[0066] Other transfection-facilitating compounds can be added to a formulation containing cationic liposomes. A number of them are described in, for example, WO 93/18759, WO 93/19768, WO 94/25608, and WO 95/2397. They include, for example, spermine derivatives useful for facilitating the transport of DNA through the nuclear membrane (see, for example, WO 93/18759) and membrane-permeabilizing compounds such as GALA, Gramicidine S, and cationic bile salts (see, for example, WO 93/19768).
[0067] Gold or tungsten microparticles can also be used for nucleic acid delivery (as described in WO 91/359 and WO 93/17706). In this case, the microparticle-coated polynucleotides can be injected via intradermal or intraepidermal routes using a needleless injection device ("gene gun"; such as those described, for example, in U.S. Pat. No. 4,945,050, U.S. Pat. No. 5,015,580, and WO 94/24263).
[0068] Anionic and neutral liposomes are also well-known in the art (see, for example, Liposomes: A Practical Approach, RPC New Ed, IRL Press (1990), for a detailed description of methods for making liposomes) and are useful for delivering a large range of products, including nucleic acids.
[0069] Particular embodiments of the aforementioned methods (i.e. to induce/elicit immune responses) encompass prime-boost protocols for the administration of immunogens of the invention. More specifically, these protocols encompass (but are not limited to) a "priming" step with a particular/distinct form of immunogen (i.e. nucleic acid (for example, plasmid, bacterial/viral/free or naked)) coding for tumor antigen, or vector (i.e. recombinant virus, bacteria) comprising said nucleic acid) followed by at least one "boosting" step encompassing the administration of an alternate (i.e. distinct from that used to "prime") form of the tumor antigen (i.e. protein or fragment thereof (for example, epitope/peptide), nucleic acid coding for the tumor antigen (or fragment thereof), or vector comprising said nucleic acid). Examples of "prime-boost" methodologies are known to those skilled in the art (as taught, for example, in PCT published applications WO 98/58956, WO 98/56919, WO 97/39771). One advantage of said protocols is the potential to circumvent the problem of generating neutralizing immune responses to vectors per se (i.e. recombinant viruses) wherein is inserted/incorporated nucleic acids encoding the immunogen or fragments thereof (see for example, R.M. Conry et al. (2000) Clin. Cancer Res. 6:34-41).
[0070] As is well known to those of ordinary skill in the art, the ability of an immunogen to induce/elicit an immune response can be improved if, regardless of administration formulation (i.e. recombinant virus, nucleic acid, polypeptide), said immunogen is co-administered with an adjuvant. Adjuvants are described and discussed in "Vaccine Design-the Subunit and Adjuvant Approach" (edited by Powell and Newman, Plenum Press, New York, U.S.A., pp. 61-79 and 141-228 (1995)). Adjuvants typically enhance the immunogenicity of an immunogen but are not necessarily immunogenic in and of themselves. Adjuvants may act by retaining the immunogen locally near the site of administration to produce a depot effect facilitating a slow, sustained release of immunizing agent to cells of the immune system. Adjuvants can also attract cells of the immune system to an immunogen depot and stimulate such cells to elicit immune responses. As such, embodiments of this invention encompass compositions further comprising adjuvants.
[0071] Desirable characteristics of ideal adjuvants include:
[0072] 1) lack of toxicity; 2) ability to stimulate a long-lasting immune response; 3) simplicity of manufacture and stability in long-term storage; 4) ability to elicit both cellular and humoral responses to antigens administered by various routes, if required; 5) synergy with other adjuvants; 6) capability of selectively interacting with populations of antigen presenting cells (APC); 7) ability to specifically elicit appropriate TH1 or TH2 cell-specific immune responses; and 8) ability to selectively increase appropriate antibody isotype levels (for example, IgA) against antigens/immunogens.
[0073] However, many adjuvants are toxic and can cause undesirable side effects, thus making them unsuitable for use in humans and many animals. For example, some adjuvants may induce granulomas, acute and chronic inflammations (i.e. Freund's complete adjuvant (FCA)), cytolysis (i.e. saponins and pluronic polymers) and pyrogenicity, arthritis and anterior uveitis (i.e. muramyl dipeptide (MDP) and lipopolysaccharide (LPS)). Indeed, only aluminum hydroxide and aluminum phosphate (collectively commonly referred to as alum) are routinely used as adjuvants in human and veterinary vaccines. The efficacy of alum in increasing antibody responses to diphtheria and tetanus toxoids is well established. Notwithstanding, it does have limitations. For example, alum is ineffective for influenza vaccination and inconsistently elicits a cell mediated immune response with other immunogens. The antibodies elicited by alum- adjuvanted antigens are mainly of the IgG1 isotype in the mouse, which may not be optimal for protection in vaccination contexts.
[0074] Adjuvants may be characterized as "intrinsic" or "extrinsic". Intrinsic adjuvants (such as lipopolysaccharides) are integral and normal components of agents which in themselves are used as vaccines (i.e. killed or attenuated bacteria). Extrinsic adjuvants are typically nonintegral immunomodulators generally linked to antigens in a noncovalent manner, and are formulated to enhance the host immune response.
[0075] In embodiments of the invention, adjuvants can be at least one member chosen from the group consisting of cytokines, lymphokines, and co-stimulatory molecules. Examples include (but are not limited to) interleukin 2, interleukin 12, interleukin 6, interferon gamma, tumor necrosis factor alpha, GM-CSF, B7.1, B7.2, ICAM-1, LFA-3, and CD72. Particular embodiments specifically encompass the use of GM-CSF as an adjuvant (as taught, for example, in U.S. Pat. Nos. 5679356, 5904920, 5637483, 5759535, 5254534, European Patent Application EP 211684, and published PCT document WO 97/28816 - all of which are herein incorporated by reference).
[0076] A variety of potent extrinsic adjuvants have been described. These include (but are not limited to) saponins complexed to membrane protein antigens (immune stimulating complexes), pluronic polymers with mineral oil, killed mycobacteria and mineral oil, Freund's complete adjuvant, bacterial products such as muramyl dipeptide (MDP) and lipopolysaccharide (LPS), as well as lipid A, and liposomes.
[0077] The use of saponins per se as adjuvants is also well known (Lacaille-Dubois, M. and Wagner, H. (1996) Phytomedicine 2:363). For example, Quil A (derived from the bark of the South American tree Quillaja Saponaria Molina) and fractions thereof has been extensively described (i.e. U.S. Pat. No. 5057540; Kensil, C.R. (1996) Crit Rev Ther Drug Carrier Syst. 12:1; and European Patent EP 362279). The haemolytic saponins QS21 and QS17 (HPLC purified fractions of Quil A) have been described as potent systemic adjuvants (U.S. Pat. No. 5057540; European Patent EP 362279). Also described in these references is the use of QS7 (a non-haemolytic fraction of Quil-A) which acts as a potent adjuvant for systemic vaccines. Use of QS21 is further described in Kensil et al. ((1991) J. lmmunol 146:431). Combinations of QS21 and polysorbate or cyclodextrin are also known (WO 9910008). Particulate adjuvant systems comprising fractions of Quil A (such as QS21 and QS7) are described in WO 9633739 and WO 9611711.
[0078] Another preferred adjuvant/immunostimulant is an immunostimulatory oligonucleotide containing unmethylated CpG dinucleotides ("CpG"). CpG is an abbreviation for cytosine-guanosine dinucleotide motifs present in DNA. CpG is known in the art as being an adjuvant when administered by both systemic and mucosal routes (WO 9602555; European Patent EP 468520; Davies et al. (1998) J. Immunol. 160:87; McCluskie and Davis (1998) J. Immunol. 161:4463). In a number of studies, synthetic oligonucleotides derived from BCG gene sequences have also been shown to be capable of inducing immunostimulatory effects (both in vitro and in vivo; Krieg, (1995) Nature 374:546). Detailed analyses of immunostimulatory oligonucleotide sequences has demonstrated that the CG motif must be in a certain sequence context, and that such sequences are common in bacterial DNA but are rare in vertebrate DNA. (For example, the immunostimulatory sequence is often: purine, purine, C, G, pyrimidine, pyrimidine, wherein the CG motif is not methylated; however other unmethylated CpG sequences are known to be immunostimulatory and a s such may also be used in the present invention.) As will be evident to one of normal skill in the art, said CG motifs/sequences can be incorporated into nucleic acids of the invention per se, or reside on distinct nucleic acids.
[0079] A variety of other adjuvants are taught in the art, and as such are encompassed by embodiments of this invention. U.S. Pat. No. 4,855,283 granted to Lockhoff et al. (incorporated herein by reference) teaches glycolipid analogues and their use as adjuvants. These include N-glycosylamides, N-glycosylureas and N-glycosylcarbamates, each of which is substituted in the sugar residue by an amino acid, as immuno-modulators or adjuvants. Furthermore, Lockhoff et al. ((1991) Chem. Int. Ed. Engl. 30:1611) have reported that N-glycolipid analogs displaying structural similarities to the naturally-occurring glycolipids (such as glycophospholipids and glycoglycerolipids) are ,also capable of eliciting strong immune responses in both herpes simplex virus vaccine and pseudorabies virus vaccine.
[0080] U.S. Pat. No. 4,258,029 granted to Moloney (incorporated herein by reference) teaches that octadecyl tyrosine hydrochloride (OTH) functions as an adjuvant when complexed with tetanus toxoid and formalin inactivated type I, II and Ill poliomyelitis virus vaccine. Nixon-George et al. ((1990) J. lmmunol. 14:4798) have also reported that octadecyl esters of aromatic amino acids complexed with a recombinant hepatitis B surface antigen enhanced the host immune responses against hepatitis B virus.
[0081] Adjuvant compounds may also be chosen from the polymers of acrylic or methacrylic acid and the copolymers of maleic anhydride and alkenyl derivative. Adjuvant compounds are the polymers of acrylic or methacrylic acid which are cross-linked, especially with polyalkenyl ethers of sugars or polyalcohols. These compounds are known by the term carbomer (Pharmeuropa Vol. 8, No. 2, Jun. 1996). Preferably, a solution of adjuvant according to the invention, especially of carbomer, is prepared in distilled water, preferably in the presence of sodium chloride, the solution obtained being at acidic pH. This stock solution is diluted by adding it to the desired quantity (for obtaining the desired final concentration), or a substantial part thereof, of water charged with NaCl, preferably physiological saline (NaCL 9 g/I) all at once in several portions with concomitant or subsequent neutralization (pH 7.3 to 7.4), preferably with NaOH. This solution at physiological pH will be used as it is for mixing with the immunizing agent; said mixture being amenable to storage in the freeze-dried, liquid or frozen form.
[0082] Persons skilled in the art can also refer to U.S. Pat. No. 2,909,462(incorporated herein by reference) which describes adjuvants encompassing acrylic polymers cross-linked with a polyhydroxylated compound having at least 3 hydroxyl groups (preferably not more than 8), the hydrogen atoms of the at least three hydroxyls being replaced by unsaturated aliphatic radicals having at least 2 carbon atoms. The preferred radicals are those containing from 2 to 4 carbon atoms (e.g. vinyls, allyls and other ethylenically unsaturated groups). The unsaturated radicals may themselves contain other substituents, such as methyl. The products sold under the name Carbopol (BF Goodrich, Ohio, USA) are particularly appropriate. They are cross-linked with allyl sucrose or with allyl pentaerythritol. Among them, there may be mentioned Carbopol (for example, 974P, 934P and 971P). Among the copolymers of maleic anhydride and alkenyl derivative, the copolymers EMA (Monsanto; which are copolymers of maleic anhydride and ethylene, linear or cross-linked, (for example cross-linked with divinyl ether)) are preferred. Reference may be made to J. Fields et al. ((1960) Nature 186: 778) for a further description of these chemicals (incorporated (herein by reference).
[0083] In further aspects of this invention, adjuvants useful for parenteral administration of immunizing agent include aluminum compounds (such as aluminum hydroxide, aluminum phosphate, and aluminum hydroxy phosphate; but might also be a salt of calcium, iron or zinc, or may be an insoluble suspension of acylated tyrosine, or acylated sugars, cationically or anionically derivatised polysaccharides, or polyphosphazenes). The antigen can be precipitated with, or adsorbed onto, the aluminum compound according to standard protocols well known to those skilled in the art.
[0084] Other adjuvants encompassed by embodiments of this invention include lipid A (in particular 3-de-O-acylated monophosphoryl lipid A (3D-MPL). 3D-MPL is a well known adjuvant manufactured by Ribi Immunochem, Montana. It is often supplied chemically as a mixture of 3-de-O-acylated monophosphoryl lipid A with 4, 5, or 6 acylated chains. It can be prepared by the methods taught in GB 2122204B. A preferred form of 3D-MPL is in the form of a particulate formulation having a particle size less than 0.2 .mu.m in diameter (European Patent EP 689454).
[0085] Adjuvants for mucosal immunization may include bacterial toxins (e.g., the cholera toxin (CT), the E. coli heat-labile toxin (LT), the Clostridium difficile toxin A and the pertussis toxin (PT), or combinations, subunits, toxoids, or mutants thereof). For example, a purified preparation of native cholera toxin subunit B (CTB) can be of use. Fragments, homologs, derivatives, and fusion to any of these toxins are also suitable, provided that they retain adjuvant activity. A mutant having reduced toxicity may be used. Mutants have been described (e.g., in WO 95/17211 (Arg-7-Lys CT mutant), WO 96/6627 (Arg-192-Gly LT mutant), and WO 95/34323 (Arg-9-Lys and Glu-129-Gly PT mutant)). Additional LT mutants include, for example Ser-63-Lys, Ala-69-Gly, Glu-110-Asp, and Glu-112-Asp mutants. Other adjuvants (such as a bacterial monophosphoryl lipid A (MPLA)) of various sources (e.g., E. coli, Salmonella minnesota, Salmonella typhimurium, or Shigella flexneri) can also be used in the mucosal administration of immunizing agents.
[0086] Adjuvants useful for both mucosal and parenteral immunization include polyphosphazene (for example, WO 95/2415), DC-chol (3 b-(N-(N',N'-dimethyl aminomethane)-carbamoyl) cholesterol (for example, U.S. Pat. No. 5,283,185 and WO 96/14831) and QS-21 (for example, WO 88/9336).
[0087] Adjuvants/immunostimulants as described herein may be formulated together with carriers, such as for example liposomes, oil in water emulsions, and/or metallic salts including aluminum salts (such as aluminum hydroxide). For example, 3D-MPL may be formulated with aluminum hydroxide (as discussed in EP 689454) or oil in water emulsions (as discussed in WO 9517210); QS21 may be advantageously formulated with cholesterol containing liposomes (as discussed in WO 9633739), in oil water emulsions (as discussed in WO 9517210) or alum (as discussed in
[0088] WO 9815287). When formulated into vaccines, immunostimulatory oligonucleotides (i.e. CpGs) are generally administered in free solution together with free antigen (as discussed in WO 9602555; McCluskie and Davis (1998) Supra), covalently conjugated to an antigen (as discussed in WO 9816247), or formulated with a carrier such as aluminum hydroxide or alum (as discussed in Davies et al. Supra; Brazolot-Millan et at (1998) Proc. Natl. Acad. Sci. 95:15553).
[0089] Combinations of adjuvants/immunostimulants are also within the scope of this invention. For example, a combination of a monophosphoryl lipid A and a saponin derivative (as described in WO 9400153, VVO 9517210, WO 9633739, WO 9856414, WO 9912565, WO 9911214) can be used, or more particularly the combination of QS21 and 3D-MPL (as described in WO 9400153). A combination of an immunostimulatory oligonucleotide and a saponin (such as QS21), or a combination of monophosphoryl lipid A (preferably 3D-MPL) in combination with an aluminum salt also form a potent adjuvant for use in the present invention.
[0090] The following non-limiting example is illustrative of the present invention:
[0091] EXAMPLES
[0092] Example 1
[0093] This example compares the intranodal injection with subcutaneous injection of a representative tumor antigen (modified gp100).
[0094] Methods and Experimental Design
[0095] Test System
[0096] Cynomolgus monkeys (Macaca fascicularis) purpose bred animals. Supplier: Siconbrec "Simian Conservation Breeding & Research Center Inc.", Fema Building, 44 Gil Puyat Avenue Makati, Metro Manila, Philippines. Number of animals in the study: 12 (6 males and 6 females). Age at initiation of treatment: 26 to 38 months.
[0097] Body weight range at initiation of treatment (day -1):
[0098] males: 1.73 to 2.34 kg
[0099] females: 1.71 to 2.65 kg.
[0100] Animal Husbandry
[0101] Housing: one air-conditioned room;
[0102] temperature: 19 to 25.degree. C. (target range),
[0103] relative humidity: >40%
[0104] air changes: minimum 8 air changes per hour,
[0105] lighting cycle: 12 hours light (artificial)/12 hours dark.
[0106] Caging: animals were housed singly in stainless steel mesh cages (approximately 540 x 810 x 760 mm).
[0107] Diet: expanded complete commercial primate diet (Mazuri diet, Special Diet Services Ltd. Witham, Essex, CMB, 3AD, Great Britain) analyzed for chemical and bacterial contaminants. Quantity distributed: 100g diet/animallday. In addition, animals received fruit daily (apple or banana) Animals were fasted for at least 16 hours before blood sampling for clinical laboratory investigations and before necropsy.
[0108] Water: drinking water ad libitum (via bottles).
[0109] Contaminants: no known contaminants were present in diet or water at levels which might have interfered with achieving the objective of the study.
[0110] Pre-Treatment Procedures
[0111] Animal health procedure: all animals received a clinical examination for ill-health on arrival and a veterinary clinical examination during the acclimatization period.
[0112] Acclimatization period: at least 3 weeks between animal arrival and start of treatment.
[0113] Experimental Design
[0114] Allocation to treatment groups was performed during the acclimatization period using a random allocation procedure based on body weight classes.
[0115] Animals were assigned to the treatment groups shown in Table 1. The dose levels administered were shown in Table 2.
[0116] Administration of the Test/Control Articles
[0117] Group 1 and 2 Animals
[0118] Method of administration: injection in the left inguinal lymph node. Animals were lightly anaesthetized before each administration by an intramuscular injection of ketmine hydrochloride (Imalgene.RTM. 500 - Merial, Lyon, France). The same lymph node was injected on each occasion (left side). Each injection was followed by a local disinfection with iodine (Vetedine.RTM.- Vetoquinol, Lure, France).
[0119] Group 3
[0120] Route: subcutaneous.
[0121] Method of administration: bolus injection using a sterile syringe and needle introduced subcutaneously. Four injection sites were used followed by a local disinfection with iodine (Vetedine.RTM.- Vetoquinol, Lure, France). Animals were also lightly anaesthetized before each administration by an intramuscular injection of ketamine hydrochloride (Imalgene.RTM. 500 - Merial, Lyon, France) in order to be under the same conditions as groups 1 and 2 animals. Four injection sites in the dorsal cervicaVinterscapular regions were used as shown in Table 3.
[0122] ELISPOT Analysis
[0123] An ELISPOT assay was used in order to assess the cell mediated immune response generated in the monkeys in the various treatment groups. In particular, an ELISPOT IFNy assay was used in order to measure IFNy production from T lymphocytes obtained from the monkeys in response to gp100 antigens.
[0124] Materials and Methods
[0125] Plates: MILLIPORE Multiscreen HA plate / MAHA 545.10 (96 wells).
[0126] Capture antibodies: MABTECH monoclonal anti-IFNy antibodies/G-Z4 1 mg/mL. Detection antibodies: MABTECH monoclonal anti-IFN.sub.y antibodies/7-B6-1-biotin 1 mg/ml.
[0127] Enzyme: SIGMA, Extravidin-PA conjuate/E2636
[0128] Substrate: BIORAD, NBT/BCIP - Alkaline phosphatase conjugate substrate kit/ref: 170-64 32. Coating
[0129] Place 1004 per well of capture antibodies at 1 pg/mL diluted at 1/1000 in carbonate bicarbonate buffer 0.1M pH 9.6 into the multiwell plate. Incubate overnight at 4.degree. C. Wash 4 times in 1X PBS. Saturation
[0130] Place 200 .mu.L per well of RPMI supplemented with 10% FCS, non essential amino acids, pyruvate, Hepes buffer and Peni-Strepto. Incubate 2 hours at 37.degree. C. Test
[0131] Cells from the immunized animals are tested against (a) medium alone; (b) pooled peptides at a concentration of 1 mg/mL; and (c) a non specific stimulus (PMA-lono). The pooled peptides used in this Example to stimulate IFN-.gamma., production were derived from gp100 and are illustrated in Tables 4 to 7. The final volume of each sample is 200 .mu.l.. Incubate 20 hours at 37.degree. C.
[0132] Wash 4 times in 1X PBS and 0.05% Tween 20.
[0133] Detection
[0134] Place 100 .mu.L per well of detection antibodies at 1 .mu.g/mL diluted in 1/1000 IX PBS, 1% BSA and 0.05% Tween 20. Incubate 2 hours at room temperature. Wash 4 times in 1X PBS and 0.05% Tween 20. Reaction
[0135] Place 100 .mu.l per well of Extravidin-PA conjugate diluted 1/6000 in 1x PBS, 1% BSA and 0.05% Tween 20. Incubate 45 minutes at room temperature. Wash 4 times in 1X PBS and 0.05% Tween 20. Substrate Addition
[0136] Place 100 .mu.l per well of substrate previously prepared. For example, for 1 plate, prepare: 9.6 mL of distilled water, 0.4 mL of 25X buffer, 0.1 mL of solution A (NBT) and 0.1 ml of solution B (BCIP). Incubate 30-45 minutes at room temperature. Wash in distilled water. Dry and transfer to a plastic film. The number of spots are counted using a Zeiss image analyzer. Each zo spot corresponds to an individual IFN-y secreting T cell.
[0137] Results
[0138] The animals that tested positive on the ELISPOT analysis are shown in FIGS. 1-4. Overall, the results demonstrate that of the animals tested, 2 out of 2 (i.e. 100%) of the animals that received the intranodal administration of the gp100 antigen, and 2 out of 4 (i.e. 50%) of the animals that received the subcutaneous administration of the gp100 antigen had a positive cell mediated immune response.
[0139] ELISA Analysis
[0140] The ELISA was performed utilizing standard methodology known in the art. Briefly, the human gp100 ("hgp100"; produced in Baculovirus) was diluted in coating buffer (carbonate-bicarbonate, pH9.6) and added to 96 wells at 0.5ug/well. Plates were placed at 4.degree. C. overnight. Plates were then washed and blocking buffer (phosphate buffered saline/0.5% Tween 20/1.0% BSA, pH7.2) was added for 2 hours at 37.degree. C. The plates were then washed and the sera was diluted in dilution buffer (phosphate buffered saline/0.5 .degree. o Tween 20/ 0.1 BSA, pH7.2). For this study, monkey sera was diluted to 1:800 and "7" serial 3 fold dilutions were done for each sample tested. The human sera controls were diluted to 1:50 in dilution buffer and "7" serial 2 fold dilutions were performed. Each dilution was done in duplicate. The plates were incubated a further 2 hours at 37.degree. C. The plates were washed and the horse radish peroxidase (HRP)-conjugated anti-human secondary antibody (anti-human Ig whole antibody from sheep (Amersham Life Science, NA933)) diluted 1:100 in dilution buffer was added to the wells and incubated for 1 hour at 37.degree. C. The plates were washed and OPD (o-phenylenediamine dihydrochloride) substrate with H.sub.2O.sub.2 in substrate buffer (50mM phosphate/25mM citrate, pH 7.2) was added to the wells. For a kinetics ELISA, the plate was read repeatedly (2 minute intervals for 15 minutes) unstopped (without "stop" buffer). Plates were read at 450nm.
[0141] Results
[0142] The results of the above experiment are presented in Table 8 and in FIG. 5. The animals of group 2 received intranodal injections of ALVAC(2)-gp100(mod) followed by boosts with the modified gp100 peptides 209(2M) and 290(9V); the animals in group 3 received a subcutaneous injection of the ALVAC(2) construct followed by peptide boosts; the animals in group 1 received intranodal injections of saline as a control.
[0143] As can be seen from FIG. 5, intranodal injection of the antigens induced a humoral response that was much greater than when the antigen was injected subcutaneously.
[0144] In summary, the results of this Example demonstrate that intranodal injection of a tumor antigen induces both a humoral and cell mediated response that is much greater than when the tumor antigen is injected by the conventional subcutaneous route of administration.
[0145] While the present invention has been described with reference to what are presently considered to be the preferred examples, it is to be understood that the invention is not limited to the disclosed examples. To the contrary, the invention is intended to cover various modifications and equivalent arrangements included within the spirit and scope of the appended claims.
[0146] All publications, patents and patent applications are herein incorporated by reference in their entirety to the same extent as if each individual publication, patent or patent application was specifically and individually indicated to be incorporated by reference in its entirety.
TABLE-US-00001 TABLE 1 Route of Number Group admin- of number istration Treatment days and compound administered Animals 1 Intranodal Saline (NaCl 0.9%): days 28, 42, 56 4 Then 70, 71, 72, 73, 74 Then 84, 85, 86, 87 and 88 2 Intranodal ALVAC(2)-gp100 mod: days 28, 42, 56 4 *mgp100 peptides: days 70, 71, 72, 73, 74 Then 84, 85, 86, 87 and 88 3 Subcuta- Saline (NaCl 0.9%): day 1 4 neous ALVAC(2)-gp100 mod: days 28, 42, 56 *mgp100 peptides: days 70 and 84 *209(2M)-IMDQVPFSY; 290(9V) YLEPGPVTV *Group 1 animals (control) received the control article (saline for injection (NaCl 0.9%)). *Group 3 animals received the control article (saline for injection (NaCl 0.9%)) on day 1 only.
TABLE-US-00002 TABLE 2 Group Dose volume Number Dose level (ml/administration) 1 Saline (NaCl 0.9%): 0 0.250 2 Dose: 0.25 .times. 10.sup.7.4 CCID 50 0.250 ALVAC (2)-gp100 mod: 0.25 10.sup.7.4 CCID50 Dose: 200 .mu.g (Total) of peptides IMDQVPFSY (209(2M)), 0.2 and YLEPGPVTV (290(9V)) (100 .mu.g each) 3 Saline (NaCl 0.9%) 0.250 ALVAC(2)-gp100 mod: 0.25 10.sup.7.4 CCID 50 0.250 Dose: 200 .mu.g (Total) of peptides IMDQVPFSY (209(2M)) 0.2 and YLEPGPVTV (290(9V)) (100 .mu.g each)
TABLE-US-00003 TABLE 3 Days Sites used 1 and 28 lower left 42 upper left 56 upper right 70 lower left 84 lower right
TABLE-US-00004 TABLE 4 Peptide Pool #1 Peptide Sequence SEQ. ID. NO. 1329 HLAVIGALLAVGATK SEQ. ID. NO. 3 1330 GALLAVGATKVPRNQ SEQ. ID. NO. 4 1331 VGATKVPRNQDWLGV SEQ. ID. NO. 5 1332 VPRNQDWLGVSRQLR SEQ. ID. NO. 6 1333 DWLGVSRQLRTKAWN SEQ. ID. NO. 7 1334 SRQLRTKAWNRQLYP SEQ. ID. NO. 8 1335 TKAWNRQLYPEWTEA SEQ. ID. NO. 9 1336 RQLYPEWTEAQRLDC SEQ. ID. NO. 10 1337 EWTEAQRLDCWRGGQ SEQ. ID. NO. 11 1338 QRLDCWRGGQVSLKV SEQ. ID. NO. 12 1339 WRGGQVSLKVSNDGP SEQ. ID. NO. 13 1340 VSLKVSNDGPTLIGA SEQ. ID. NO. 14 1344 IALNFPGSQKVLPDG SEQ. ID. NO. 15 1345 PGSQKVLPDGQVIWV SEQ. ID. NO. 16 1346 VLPDGQVIWVNNTII SEQ. ID. NO. 17 1347 QVIWVNNTIINGSQV SEQ. ID. NO. 18 1348 NNTIINGSQVWGGQP SEQ. ID. NO. 19 1349 NGSQVWGGQPVYPQE SEQ. ID. NO. 20 1350 WGGQPVYPQETDDAC SEQ. ID. NO. 21 1351 VYPQETDDACIFPDG SEQ. ID. NO. 22 1352 TDDACIFPDGGPCPS SEQ. ID. NO. 23 1353 IFPDGGPCPSGSWSQ SEQ. ID. NO. 24 1355 GSWSQKRSFVYVWKT SEQ. ID. NO. 25 1356 KRSFVYVWKTWGQYW SEQ. ID. NO. 26 1357 YVWKTWGQYWQVLGG SEQ. ID. NO. 27 1358 WGQYWQVLGGPVSGL SEQ. ID. NO. 28 1359 QVLGGPVSGLSIGTG SEQ. ID. NO. 29
TABLE-US-00005 TABLE 5 Peptide Pool #2 Peptide Sequence SEQ. ID. NO. 1360 PVSGLSIGTGRAMLG SEQ. ID. NO. 30 1361 SIGTGRAMLGTHTME SEQ. ID. NO. 31 1362 RAMLGTHTMEVTVYH SEQ. ID. NO. 32 1363 THTMEVTVYHRRGSR SEQ. ID. NO. 33 1364 VTVYHRRGSRSYVPL SEQ. ID. NO. 34 1365 RRGSRSYVPLAHSSS SEQ. ID. NO. 35 1366 SYVPLAHSSSAFTIT SEQ. ID. NO. 36 1368 AFTITDQVPFSVSVS SEQ. ID. NO. 37 1369 DQVPFSVSVSQLRAL SEQ. ID. NO. 38 1370 SVSVSQLRALDGGNK SEQ. ID. NO. 39 1372 DGGNKHFLRNQPLTF SEQ. ID. NO. 40 1373 HFLRNQPLTFALQLH SEQ. ID. NO. 41 1374 QPLTFALQLHDPSGY SEQ. ID. NO. 42 1375 ALQLHDPSGYLAEAD SEQ. ID. NO. 43 1379 DFGDSSGTLISRALV SEQ. ID. NO. 44 1380 STGLISRALVVTHTY SEQ. ID. NO. 45 1381 SRALVVTHTYLEPGP SEQ. ID. NO. 46 1382 VTHTYLEPGPVTAQV SEQ. ID. NO. 47 1383 LEPGPVTAQVVLQAA SEQ. ID. NO. 48 1384 VTAQVVLQAAIPLTS SEQ. ID. NO. 49 1385 VLQAAIPLTSCGSSP SEQ. ID. NO. 50 1386 IPLTSCGSSPVPGTT SEQ. ID. NO. 51 1388 VPGTTDGHRPTAEAP SEQ. ID. NO. 52 1389 DGHRPTAEAPNTTAG SEQ. ID. NO. 53 1390 TAEAPNTTAGQVPTT SEQ. ID. NO. 54 1392 QVPTTEVVGTTPGQA SEQ. ID. NO. 55 1393 EVVGTTPGQAPTAEP SEQ. ID. NO. 56
TABLE-US-00006 TABLE 6 Peptide Pool #3 Peptide Sequence SEQ. ID. NO. 1394 TPGQAPTAEPSGTTS SEQ. ID. NO. 57 1395 PTAEPSGTTSVQVPT SEQ. ID. NO. 58 1396 SGTTSVQVPTTEVIS SEQ. ID. NO. 59 1397 VQVPTTEVISTAPVQ SEQ. ID. NO. 60 1398 TEVISTAPVQMPTAE SEQ. ID. NO. 61 1399 TAPVQMPTAESTGMT SEQ. ID. NO. 62 1400 MPTAESTGMTPEKVP SEQ. ID. NO. 63 1401 STGMTPEKVPVSEVM SEQ. ID. NO. 64 1402 PEKVPVSEVMGTTLA SEQ. ID. NO. 65 1403 VSEVMGTTLAEMSTP SEQ. ID. NO. 66 1404 GTTLAEMSTPEATGM SEQ. ID. NO. 67 1405 EMSTPEATGMTPAEV SEQ. ID. NO. 68 1408 SIVVLSGTTAAQVTT SEQ. ID. NO. 69 1409 SGTTAAQVTTTEWVE SEQ. ID. NO. 70 1410 AQVTTTEWVETTARE SEQ. ID. NO. 71 1411 TEWVETTARELPIPE SEQ. ID. NO. 72 1412 TTARELPIPEPEGPD SEQ. ID. NO. 73 1413 LPIPEPEGPDASSIM SEQ. ID. NO. 74 1414 PEGPDASSIMSTESI SEQ. ID. NO. 75 1415 ASSIMSTESITGSLG SEQ. ID. NO. 76 1416 STESITGSLGPLLDG SEQ. ID. NO. 77 1417 TGSLGPLLDGTATLR SEQ. ID. NO. 78 1418 PLLDGTATLRLVKRQ SEQ. ID. NO. 79 1419 TATLRLVKRQVPLDC SEQ. ID. NO. 80 1420 LVKRQVPLDCVLYRY SEQ. ID. NO. 81 1421 VPLDCVLYRYGSFSV SEQ. ID. NO. 82 1422 VLYRYGSFSVTLDIV SEQ. ID. NO. 83
TABLE-US-00007 TABLE 7 Peptide Pool #4 Peptide Sequence SEQ. ID. NO. 1424 TLDIVQGIESAEILQ SEQ. ID. NO. 84 1425 QGIESAEILQAVPSG SEQ. ID. NO. 85 1426 AEILQAVPSGEGDAF SEQ. ID. NO. 86 1427 AVPSGEGDAFELTVS SEQ. ID. NO. 87 1428 EGDAFELTVSCQGGL SEQ. ID. NO. 88 1429 ELTVSCQGGLPKEAC SEQ. ID. NO. 89 1430 CQGGLPKEACMEISS SEQ. ID. NO. 90 1431 PKEACMEISSPGCQP SEQ. ID. NO. 91 1432 MEISSPGCQPPAQRL SEQ. ID. NO. 92 1434 PAQRLCQPVLPSPAC SEQ. ID. NO. 93 1435 CQPVLPSPACQLVLH SEQ. ID. NO. 94 1436 PSPACQLVLHQILKG SEQ. ID. NO. 95 1437 QLVLHQILKGGSGTY SEQ. ID. NO. 96 1441 LADTNSLAVVSTQLI SEQ. ID. NO. 97 1442 SLAVVSTQLIMPGQE SEQ. ID. NO. 98 1443 STQLIMPGQEAGLGQ SEQ. ID. NO. 99 1444 MPGQEAGLGQVPLIV SEQ. ID. NO. 100 1445 AGLGQVPLIVGILLV SEQ. ID. NO. 101 1448 LMAVVLASLIYRRRL SEQ. ID. NO. 102 1450 YRRRLMKQDFSVPQL SEQ. ID. NO. 103 1451 MKQDFSVPQLPHSSS SEQ. ID. NO. 104 1452 SVPQLPHSSSHWLRL SEQ. ID. NO. 105 1453 PHSSSHWLRLPRIFC SEQ. ID. NO. 106 1454 HWLRLPRIFCSCPIG SEQ. ID. NO. 107 1455 PRIFCSCPIGENSPL SEQ. ID. NO. 108
TABLE-US-00008 TABLE 8 DAY (mOD/min) Monkey # 0 57 68 96 1 3 5 2 2 2 4 6 12 10 3 7 6 10 8 4 7 6 8 8 5 5 9 20 15 6 11 8 10 12 7 11 23 51 30 8 7 30 70 22 9 1 7 5 3 10 2 6 6 4 11 3 7 14 8 12 6 9 15 6
Sequence CWU 1
1
11319PRTHomo sapiens 1Ile Met Asp Gln Val Pro Phe Ser Tyr1
529PRTHomo sapiens 2Tyr Leu Glu Pro Gly Pro Val Thr Val1
5315PRTHomo sapiens 3His Leu Ala Val Ile Gly Ala Leu Leu Ala Val
Gly Ala Thr Lys1 5 10 15415PRTHomo sapiens 4Gly Ala Leu Leu Ala Val
Gly Ala Thr Lys Val Pro Arg Asn Gln1 5 10 15514PRTHomo sapiens 5Val
Gly Ala Thr Lys Val Pro Arg Asn Asp Trp Leu Gly Val1 5 10615PRTHomo
sapiens 6Val Pro Arg Asn Gln Asp Trp Leu Gly Val Ser Arg Gln Leu
Arg1 5 10 15715PRTHomo sapiens 7Asp Trp Leu Gly Val Ser Arg Gln Leu
Arg Thr Lys Ala Trp Asn1 5 10 15815PRTHomo sapiens 8Ser Arg Gln Leu
Arg Thr Lys Ala Trp Asn Arg Gln Leu Tyr Pro1 5 10 15915PRTHomo
sapiens 9Thr Lys Ala Trp Asn Arg Gln Leu Tyr Pro Glu Trp Thr Glu
Ala1 5 10 151015PRTHomo sapiens 10Arg Gln Leu Tyr Pro Glu Trp Thr
Glu Ala Gln Arg Leu Asp Cys1 5 10 151115PRTHomo sapiens 11Glu Trp
Thr Glu Ala Gln Arg Leu Asp Cys Trp Arg Gly Gly Gln1 5 10
151215PRTHomo sapiens 12Gln Arg Leu Asp Cys Trp Arg Gly Gly Gln Val
Ser Leu Lys Val1 5 10 151315PRTHomo sapiens 13Trp Arg Gly Gly Gln
Val Ser Leu Lys Val Ser Asn Asp Gly Pro1 5 10 151415PRTHomo sapiens
14Val Ser Leu Lys Val Ser Asn Asp Gly Pro Thr Leu Ile Gly Ala1 5 10
151515PRTHomo sapiens 15Ile Ala Leu Asn Phe Pro Gly Ser Gln Lys Val
Leu Pro Asp Gly1 5 10 151615PRTHomo sapiens 16Pro Gly Ser Gln Lys
Val Leu Pro Asp Gly Gln Val Ile Trp Val1 5 10 151715PRTHomo sapiens
17Val Leu Pro Asp Gly Gln Val Ile Trp Val Asn Asn Thr Ile Ile1 5 10
151815PRTHomo sapiens 18Gln Val Ile Trp Val Asn Asn Thr Ile Ile Asn
Gly Ser Gln Val1 5 10 151915PRTHomo sapiens 19Asn Asn Thr Ile Ile
Asn Gly Ser Gln Val Trp Gly Gly Gln Pro1 5 10 152015PRTHomo sapiens
20Asn Gly Ser Gln Val Trp Gly Gly Gln Pro Val Tyr Pro Gln Glu1 5 10
152115PRTHomo sapiens 21Trp Gly Gly Gln Pro Val Tyr Pro Gln Glu Thr
Asp Asp Ala Cys1 5 10 152215PRTHomo sapiens 22Val Tyr Pro Gln Glu
Thr Asp Asp Ala Cys Ile Phe Pro Asp Gly1 5 10 152315PRTHomo sapiens
23Thr Asp Asp Ala Cys Ile Phe Pro Asp Gly Gly Pro Cys Pro Ser1 5 10
152415PRTHomo sapiens 24Ile Phe Pro Asp Gly Gly Pro Cys Pro Ser Gly
Ser Trp Ser Gln1 5 10 152515PRTHomo sapiens 25Gly Ser Trp Ser Gln
Lys Arg Ser Phe Val Tyr Val Trp Lys Thr1 5 10 152615PRTHomo sapiens
26Lys Arg Ser Phe Val Tyr Val Trp Lys Thr Trp Gly Gln Tyr Trp1 5 10
152715PRTHomo sapiens 27Tyr Val Trp Lys Thr Trp Gly Gln Tyr Trp Gln
Val Leu Gly Gly1 5 10 152815PRTHomo sapiens 28Trp Gly Gln Tyr Trp
Gln Val Leu Gly Gly Pro Val Ser Gly Leu1 5 10 152915PRTHomo sapiens
29Gln Val Leu Gly Gly Pro Val Ser Gly Leu Ser Ile Gly Thr Gly1 5 10
153015PRTHomo sapiens 30Pro Val Ser Gly Leu Ser Ile Gly Thr Gly Arg
Ala Met Leu Gly1 5 10 153115PRTHomo sapiens 31Ser Ile Gly Thr Gly
Arg Ala Met Leu Gly Thr His Thr Met Glu1 5 10 153215PRTHomo sapiens
32Arg Ala Met Leu Gly Thr His Thr Met Glu Val Thr Val Tyr His1 5 10
153315PRTHomo sapiens 33Thr His Thr Met Glu Val Thr Val Tyr His Arg
Arg Gly Ser Arg1 5 10 153415PRTHomo sapiens 34Val Thr Val Tyr His
Arg Arg Gly Ser Arg Ser Tyr Val Pro Leu1 5 10 153515PRTHomo sapiens
35Arg Arg Gly Ser Arg Ser Tyr Val Pro Leu Ala His Ser Ser Ser1 5 10
153615PRTHomo sapiens 36Ser Tyr Val Pro Leu Ala His Ser Ser Ser Ala
Phe Thr Ile Thr1 5 10 153715PRTHomo sapiens 37Ala Phe Thr Ile Thr
Asp Gln Val Pro Phe Ser Val Ser Val Ser1 5 10 153815PRTHomo sapiens
38Asp Gln Val Pro Phe Ser Val Ser Val Ser Gln Leu Arg Ala Leu1 5 10
153915PRTHomo sapiens 39Ser Val Ser Val Ser Gln Leu Arg Ala Leu Asp
Gly Gly Asn Lys1 5 10 154015PRTHomo sapiens 40Asp Gly Gly Asn Lys
His Phe Leu Arg Asn Gln Pro Leu Thr Phe1 5 10 154115PRTHomo sapiens
41His Phe Leu Arg Asn Gln Pro Leu Thr Phe Ala Leu Gln Leu His1 5 10
154215PRTHomo sapiens 42Gln Pro Leu Thr Phe Ala Leu Gln Leu His Asp
Pro Ser Gly Tyr1 5 10 154315PRTHomo sapiens 43Ala Leu Gln Leu His
Asp Pro Ser Gly Tyr Leu Ala Glu Ala Asp1 5 10 154415PRTHomo sapiens
44Asp Phe Gly Asp Ser Ser Gly Thr Leu Ile Ser Arg Ala Leu Val1 5 10
154515PRTHomo sapiens 45Ser Thr Gly Leu Ile Ser Arg Ala Leu Val Val
Thr His Thr Tyr1 5 10 154615PRTHomo sapiens 46Ser Arg Ala Leu Val
Val Thr His Thr Tyr Leu Glu Pro Gly Pro1 5 10 154715PRTHomo sapiens
47Val Thr His Thr Tyr Leu Glu Pro Gly Pro Val Thr Ala Gln Val1 5 10
154815PRTHomo sapiens 48Leu Glu Pro Gly Pro Val Thr Ala Gln Val Val
Leu Gln Ala Ala1 5 10 154915PRTHomo sapiens 49Val Thr Ala Gln Val
Val Leu Gln Ala Ala Ile Pro Leu Thr Ser1 5 10 155015PRTHomo sapiens
50Val Leu Gln Ala Ala Ile Pro Leu Thr Ser Cys Gly Ser Ser Pro1 5 10
155115PRTHomo sapiens 51Ile Pro Leu Thr Ser Cys Gly Ser Ser Pro Val
Pro Gly Thr Thr1 5 10 155215PRTHomo sapiens 52Val Pro Gly Thr Thr
Asp Gly His Arg Pro Thr Ala Glu Ala Pro1 5 10 155315PRTHomo sapiens
53Asp Gly His Arg Pro Thr Ala Glu Ala Pro Asn Thr Thr Ala Gly1 5 10
155415PRTHomo sapiens 54Thr Ala Glu Ala Pro Asn Thr Thr Ala Gly Gln
Val Pro Thr Thr1 5 10 155515PRTHomo sapiens 55Gln Val Pro Thr Thr
Glu Val Val Gly Thr Thr Pro Gly Gln Ala1 5 10 155615PRTHomo sapiens
56Glu Val Val Gly Thr Thr Pro Gly Gln Ala Pro Thr Ala Glu Pro1 5 10
155715PRTHomo sapiens 57Thr Pro Gly Gln Ala Pro Thr Ala Glu Pro Ser
Gly Thr Thr Ser1 5 10 155815PRTHomo sapiens 58Pro Thr Ala Glu Pro
Ser Gly Thr Thr Ser Val Gln Val Pro Thr1 5 10 155915PRTHomo sapiens
59Ser Gly Thr Thr Ser Val Gln Val Pro Thr Thr Glu Val Ile Ser1 5 10
156015PRTHomo sapiens 60Val Gln Val Pro Thr Thr Glu Val Ile Ser Thr
Ala Pro Val Gln1 5 10 156115PRTHomo sapiens 61Thr Glu Val Ile Ser
Thr Ala Pro Val Gln Met Pro Thr Ala Glu1 5 10 156215PRTHomo sapiens
62Thr Ala Pro Val Gln Met Pro Thr Ala Glu Ser Thr Gly Met Thr1 5 10
156315PRTHomo sapiens 63Met Pro Thr Ala Glu Ser Thr Gly Met Thr Pro
Glu Lys Val Pro1 5 10 156415PRTHomo sapiens 64Ser Thr Gly Met Thr
Pro Glu Lys Val Pro Val Ser Glu Val Met1 5 10 156515PRTHomo sapiens
65Pro Glu Lys Val Pro Val Ser Glu Val Met Gly Thr Thr Leu Ala1 5 10
156615PRTHomo sapiens 66Val Ser Glu Val Met Gly Thr Thr Leu Ala Glu
Met Ser Thr Pro1 5 10 156715PRTHomo sapiens 67Gly Thr Thr Leu Ala
Glu Met Ser Thr Pro Glu Ala Thr Gly Met1 5 10 156815PRTHomo sapiens
68Glu Met Ser Thr Pro Glu Ala Thr Gly Met Thr Pro Ala Glu Val1 5 10
156915PRTHomo sapiens 69Ser Ile Val Val Leu Ser Gly Thr Thr Ala Ala
Gln Val Thr Thr1 5 10 157015PRTHomo sapiens 70Ser Gly Thr Thr Ala
Ala Gln Val Thr Thr Thr Glu Trp Val Glu1 5 10 157115PRTHomo sapiens
71Ala Gln Val Thr Thr Thr Glu Trp Val Glu Thr Thr Ala Arg Glu1 5 10
157215PRTHomo sapiens 72Thr Glu Trp Val Glu Thr Thr Ala Arg Glu Leu
Pro Ile Pro Glu1 5 10 157315PRTHomo sapiens 73Thr Thr Ala Arg Glu
Leu Pro Ile Pro Glu Pro Glu Gly Pro Asp1 5 10 157415PRTHomo sapiens
74Leu Pro Ile Pro Glu Pro Glu Gly Pro Asp Ala Ser Ser Ile Met1 5 10
157515PRTHomo sapiens 75Pro Glu Gly Pro Asp Ala Ser Ser Ile Met Ser
Thr Glu Ser Ile1 5 10 157615PRTHomo sapiens 76Ala Ser Ser Ile Met
Ser Thr Glu Ser Ile Thr Gly Ser Leu Gly1 5 10 157715PRTHomo sapiens
77Ser Thr Glu Ser Ile Thr Gly Ser Leu Gly Pro Leu Leu Asp Gly1 5 10
157815PRTHomo sapiens 78Thr Gly Ser Leu Gly Pro Leu Leu Asp Gly Thr
Ala Thr Leu Arg1 5 10 157915PRTHomo sapiens 79Pro Leu Leu Asp Gly
Thr Ala Thr Leu Arg Leu Val Lys Arg Gln1 5 10 158015PRTHomo sapiens
80Thr Ala Thr Leu Arg Leu Val Lys Arg Gln Val Pro Leu Asp Cys1 5 10
158115PRTHomo sapiens 81Leu Val Lys Arg Gln Val Pro Leu Asp Cys Val
Leu Tyr Arg Tyr1 5 10 158215PRTHomo sapiens 82Val Pro Leu Asp Cys
Val Leu Tyr Arg Tyr Gly Ser Phe Ser Val1 5 10 158315PRTHomo sapiens
83Val Leu Tyr Arg Tyr Gly Ser Phe Ser Val Thr Leu Asp Ile Val1 5 10
158415PRTHomo sapiens 84Thr Leu Asp Ile Val Gln Gly Ile Glu Ser Ala
Glu Ile Leu Gln1 5 10 158515PRTHomo sapiens 85Gln Gly Ile Glu Ser
Ala Glu Ile Leu Gln Ala Val Pro Ser Gly1 5 10 158615PRTHomo sapiens
86Ala Glu Ile Leu Gln Ala Val Pro Ser Gly Glu Gly Asp Ala Phe1 5 10
158715PRTHomo sapiens 87Ala Val Pro Ser Gly Glu Gly Asp Ala Phe Glu
Leu Thr Val Ser1 5 10 158815PRTHomo sapiens 88Glu Gly Asp Ala Phe
Glu Leu Thr Val Ser Cys Gln Gly Gly Leu1 5 10 158915PRTHomo sapiens
89Glu Leu Thr Val Ser Cys Gln Gly Gly Leu Pro Lys Glu Ala Cys1 5 10
159015PRTHomo sapiens 90Cys Gln Gly Gly Leu Pro Lys Glu Ala Cys Met
Glu Ile Ser Ser1 5 10 159115PRTHomo sapiens 91Pro Lys Glu Ala Cys
Met Glu Ile Ser Ser Pro Gly Cys Gln Pro1 5 10 159215PRTHomo sapiens
92Met Glu Ile Ser Ser Pro Gly Cys Gln Pro Pro Ala Gln Arg Leu1 5 10
159315PRTHomo sapiens 93Pro Ala Gln Arg Leu Cys Gln Pro Val Leu Pro
Ser Pro Ala Cys1 5 10 159415PRTHomo sapiens 94Cys Gln Pro Val Leu
Pro Ser Pro Ala Cys Gln Leu Val Leu His1 5 10 159515PRTHomo sapiens
95Pro Ser Pro Ala Cys Gln Leu Val Leu His Gln Ile Leu Lys Gly1 5 10
159615PRTHomo sapiens 96Gln Leu Val Leu His Gln Ile Leu Lys Gly Gly
Ser Gly Thr Tyr1 5 10 159715PRTHomo sapiens 97Leu Ala Asp Thr Asn
Ser Leu Ala Val Val Ser Thr Gln Leu Ile1 5 10 159815PRTHomo sapiens
98Ser Leu Ala Val Val Ser Thr Gln Leu Ile Met Pro Gly Gln Glu1 5 10
159915PRTHomo sapiens 99Ser Thr Gln Leu Ile Met Pro Gly Gln Glu Ala
Gly Leu Gly Gln1 5 10 1510015PRTHomo sapiens 100Met Pro Gly Gln Glu
Ala Gly Leu Gly Gln Val Pro Leu Ile Val1 5 10 1510115PRTHomo
sapiens 101Ala Gly Leu Gly Gln Val Pro Leu Ile Val Gly Ile Leu Leu
Val1 5 10 1510215PRTHomo sapiens 102Leu Met Ala Val Val Leu Ala Ser
Leu Ile Tyr Arg Arg Arg Leu1 5 10 1510315PRTHomo sapiens 103Tyr Arg
Arg Arg Leu Met Lys Gln Asp Phe Ser Val Pro Gln Leu1 5 10
1510415PRTHomo sapiens 104Met Lys Gln Asp Phe Ser Val Pro Gln Leu
Pro His Ser Ser Ser1 5 10 1510515PRTHomo sapiens 105Ser Val Pro Gln
Leu Pro His Ser Ser Ser His Trp Leu Arg Leu1 5 10 1510615PRTHomo
sapiens 106Pro His Ser Ser Ser His Trp Leu Arg Leu Pro Arg Ile Phe
Cys1 5 10 1510715PRTHomo sapiens 107His Trp Leu Arg Leu Pro Arg Ile
Phe Cys Ser Cys Pro Ile Gly1 5 10 1510815PRTHomo sapiens 108Pro Arg
Ile Phe Cys Ser Cys Pro Ile Gly Glu Asn Ser Pro Leu1 5 10
151091986DNAArtificial SequenceHomo sapiens 109atg gat ctg gtg cta
aaa aga tgc ctt ctt cat ttg gct gtg ata ggt 48Met Asp Leu Val Leu
Lys Arg Cys Leu Leu His Leu Ala Val Ile Gly1 5 10 15gct ttg ctg gct
gtg ggg gct aca aaa gta ccc aga aac cag gac tgg 96Ala Leu Leu Ala
Val Gly Ala Thr Lys Val Pro Arg Asn Gln Asp Trp 20 25 30ctt ggt gtc
tca agg caa ctc aga acc aaa gcc tgg aac agg cag ctg 144Leu Gly Val
Ser Arg Gln Leu Arg Thr Lys Ala Trp Asn Arg Gln Leu 35 40 45tat cca
gag tgg aca gaa gcc cag aga ctt gac tgc tgg aga ggt ggt 192Tyr Pro
Glu Trp Thr Glu Ala Gln Arg Leu Asp Cys Trp Arg Gly Gly 50 55 60caa
gtg tcc ctc aag gtc agt aat gat ggg cct aca ctg att ggt gca 240Gln
Val Ser Leu Lys Val Ser Asn Asp Gly Pro Thr Leu Ile Gly Ala65 70 75
80aat gcc tcc ttc tct att gcc ttg aac ttc cct gga agc caa aag gta
288Asn Ala Ser Phe Ser Ile Ala Leu Asn Phe Pro Gly Ser Gln Lys Val
85 90 95ttg cca gat ggg cag gtt atc tgg gtc aac aat acc atc atc aat
ggg 336Leu Pro Asp Gly Gln Val Ile Trp Val Asn Asn Thr Ile Ile Asn
Gly 100 105 110agc cag gtg tgg gga gga cag cca gtg tat ccc cag gaa
act gac gat 384Ser Gln Val Trp Gly Gly Gln Pro Val Tyr Pro Gln Glu
Thr Asp Asp 115 120 125gcc tgc atc ttc cct gat ggt gga cct tgc cca
tct ggc tct tgg tct 432Ala Cys Ile Phe Pro Asp Gly Gly Pro Cys Pro
Ser Gly Ser Trp Ser 130 135 140cag aag aga agc ttt gtt tat gtc tgg
aag acc tgg ggc caa tac tgg 480Gln Lys Arg Ser Phe Val Tyr Val Trp
Lys Thr Trp Gly Gln Tyr Trp145 150 155 160caa gtt cta ggg ggc cca
gtg tct ggg ctg agc att ggg aca ggc agg 528Gln Val Leu Gly Gly Pro
Val Ser Gly Leu Ser Ile Gly Thr Gly Arg 165 170 175gca atg ctg ggc
aca cac acg atg gaa gtg act gtc tac cat cgc cgg 576Ala Met Leu Gly
Thr His Thr Met Glu Val Thr Val Tyr His Arg Arg 180 185 190gga tcc
cgg agc tat gtg cct ctt gct cat tcc agc tca gcc ttc acc 624Gly Ser
Arg Ser Tyr Val Pro Leu Ala His Ser Ser Ser Ala Phe Thr 195 200
205att atg gac cag gtg cct ttc tcc gtg agc gtg tcc cag ttg cgg gcc
672Ile Met Asp Gln Val Pro Phe Ser Val Ser Val Ser Gln Leu Arg Ala
210 215 220ttg gat gga ggg aac aag cac ttc ctg aga aat cag cct ctg
acc ttt
720Leu Asp Gly Gly Asn Lys His Phe Leu Arg Asn Gln Pro Leu Thr
Phe225 230 235 240gcc ctc cag ctc cat gac ccc agt ggc tat ctg gct
gaa gct gac ctc 768Ala Leu Gln Leu His Asp Pro Ser Gly Tyr Leu Ala
Glu Ala Asp Leu 245 250 255tcc tac acc tgg gac ttt gga gac agt agt
gga acc ctg atc tct cgg 816Ser Tyr Thr Trp Asp Phe Gly Asp Ser Ser
Gly Thr Leu Ile Ser Arg 260 265 270gca ctt gtg gtc act cat act tac
ctg gag cct ggc cca gtc act gtt 864Ala Leu Val Val Thr His Thr Tyr
Leu Glu Pro Gly Pro Val Thr Val 275 280 285cag gtg gtc ctg cag gct
gcc att cct ctc acc tcc tgt ggc tcc tcc 912Gln Val Val Leu Gln Ala
Ala Ile Pro Leu Thr Ser Cys Gly Ser Ser 290 295 300cca gtt cca ggc
acc aca gat ggg cac agg cca act gca gag gcc cct 960Pro Val Pro Gly
Thr Thr Asp Gly His Arg Pro Thr Ala Glu Ala Pro305 310 315 320aac
acc aca gct ggc caa gtg cct act aca gaa gtt gtg ggt act aca 1008Asn
Thr Thr Ala Gly Gln Val Pro Thr Thr Glu Val Val Gly Thr Thr 325 330
335cct ggt cag gcg cca act gca gag ccc tct gga acc aca tct gtg cag
1056Pro Gly Gln Ala Pro Thr Ala Glu Pro Ser Gly Thr Thr Ser Val Gln
340 345 350gtg cca acc act gaa gtc ata agc act gca cct gtg cag atg
cca act 1104Val Pro Thr Thr Glu Val Ile Ser Thr Ala Pro Val Gln Met
Pro Thr 355 360 365gca gag agc aca ggt atg aca cct gag aag gtg cca
gtt tca gag gtc 1152Ala Glu Ser Thr Gly Met Thr Pro Glu Lys Val Pro
Val Ser Glu Val 370 375 380atg ggt acc aca ctg gca gag atg tca act
cca gag gct aca ggt atg 1200Met Gly Thr Thr Leu Ala Glu Met Ser Thr
Pro Glu Ala Thr Gly Met385 390 395 400aca cct gca gag gta tca att
gtg gtg ctt tct gga acc aca gct gca 1248Thr Pro Ala Glu Val Ser Ile
Val Val Leu Ser Gly Thr Thr Ala Ala 405 410 415cag gta aca act aca
gag tgg gtg gag acc aca gct aga gag cta cct 1296Gln Val Thr Thr Thr
Glu Trp Val Glu Thr Thr Ala Arg Glu Leu Pro 420 425 430atc cct gag
cct gaa ggt cca gat gcc agc tca atc atg tct acg gaa 1344Ile Pro Glu
Pro Glu Gly Pro Asp Ala Ser Ser Ile Met Ser Thr Glu 435 440 445agt
att aca ggt tcc ctg ggc ccc ctg ctg gat ggt aca gcc acc tta 1392Ser
Ile Thr Gly Ser Leu Gly Pro Leu Leu Asp Gly Thr Ala Thr Leu 450 455
460agg ctg gtg aag aga caa gtc ccc ctg gat tgt gtt ctg tat cga tat
1440Arg Leu Val Lys Arg Gln Val Pro Leu Asp Cys Val Leu Tyr Arg
Tyr465 470 475 480ggt tcc ttt tcc gtc acc ctg gac att gtc cag ggt
att gaa agt gcc 1488Gly Ser Phe Ser Val Thr Leu Asp Ile Val Gln Gly
Ile Glu Ser Ala 485 490 495gag atc ctg cag gct gtg ccg tcc ggt gag
ggg gat gca ttt gag ctg 1536Glu Ile Leu Gln Ala Val Pro Ser Gly Glu
Gly Asp Ala Phe Glu Leu 500 505 510act gtg tcc tgc caa ggc ggg ctg
ccc aag gaa gcc tgc atg gag atc 1584Thr Val Ser Cys Gln Gly Gly Leu
Pro Lys Glu Ala Cys Met Glu Ile 515 520 525tca tcg cca ggg tgc cag
ccc cct gcc cag cgg ctg tgc cag cct gtg 1632Ser Ser Pro Gly Cys Gln
Pro Pro Ala Gln Arg Leu Cys Gln Pro Val 530 535 540cta ccc agc cca
gcc tgc cag ctg gtt ctg cac cag ata ctg aag ggt 1680Leu Pro Ser Pro
Ala Cys Gln Leu Val Leu His Gln Ile Leu Lys Gly545 550 555 560ggc
tcg ggg aca tac tgc ctc aat gtg tct ctg gct gat acc aac agc 1728Gly
Ser Gly Thr Tyr Cys Leu Asn Val Ser Leu Ala Asp Thr Asn Ser 565 570
575ctg gca gtg gtc agc acc cag ctt atc atg cct ggt caa gaa gca ggc
1776Leu Ala Val Val Ser Thr Gln Leu Ile Met Pro Gly Gln Glu Ala Gly
580 585 590ctt ggg cag gtt ccg ctg atc gtg ggc atc ttg ctg gtg ttg
atg gct 1824Leu Gly Gln Val Pro Leu Ile Val Gly Ile Leu Leu Val Leu
Met Ala 595 600 605gtg gtc ctt gca tct ctg ata tat agg cgc aga ctt
atg aag caa gac 1872Val Val Leu Ala Ser Leu Ile Tyr Arg Arg Arg Leu
Met Lys Gln Asp 610 615 620ttc tcc gta ccc cag ttg cca cat agc agc
agt cac tgg ctg cgt cta 1920Phe Ser Val Pro Gln Leu Pro His Ser Ser
Ser His Trp Leu Arg Leu625 630 635 640ccc cgc atc ttc tgc tct tgt
ccc att ggt gag aac agc ccc ctc ctc 1968Pro Arg Ile Phe Cys Ser Cys
Pro Ile Gly Glu Asn Ser Pro Leu Leu 645 650 655agt ggg cag cag gtc
tga 1986Ser Gly Gln Gln Val 660110661PRTArtificial SequenceHomo
sapiens 110Met Asp Leu Val Leu Lys Arg Cys Leu Leu His Leu Ala Val
Ile Gly1 5 10 15Ala Leu Leu Ala Val Gly Ala Thr Lys Val Pro Arg Asn
Gln Asp Trp 20 25 30Leu Gly Val Ser Arg Gln Leu Arg Thr Lys Ala Trp
Asn Arg Gln Leu 35 40 45Tyr Pro Glu Trp Thr Glu Ala Gln Arg Leu Asp
Cys Trp Arg Gly Gly 50 55 60Gln Val Ser Leu Lys Val Ser Asn Asp Gly
Pro Thr Leu Ile Gly Ala65 70 75 80Asn Ala Ser Phe Ser Ile Ala Leu
Asn Phe Pro Gly Ser Gln Lys Val 85 90 95Leu Pro Asp Gly Gln Val Ile
Trp Val Asn Asn Thr Ile Ile Asn Gly 100 105 110Ser Gln Val Trp Gly
Gly Gln Pro Val Tyr Pro Gln Glu Thr Asp Asp 115 120 125Ala Cys Ile
Phe Pro Asp Gly Gly Pro Cys Pro Ser Gly Ser Trp Ser 130 135 140Gln
Lys Arg Ser Phe Val Tyr Val Trp Lys Thr Trp Gly Gln Tyr Trp145 150
155 160Gln Val Leu Gly Gly Pro Val Ser Gly Leu Ser Ile Gly Thr Gly
Arg 165 170 175Ala Met Leu Gly Thr His Thr Met Glu Val Thr Val Tyr
His Arg Arg 180 185 190Gly Ser Arg Ser Tyr Val Pro Leu Ala His Ser
Ser Ser Ala Phe Thr 195 200 205Ile Met Asp Gln Val Pro Phe Ser Val
Ser Val Ser Gln Leu Arg Ala 210 215 220Leu Asp Gly Gly Asn Lys His
Phe Leu Arg Asn Gln Pro Leu Thr Phe225 230 235 240Ala Leu Gln Leu
His Asp Pro Ser Gly Tyr Leu Ala Glu Ala Asp Leu 245 250 255Ser Tyr
Thr Trp Asp Phe Gly Asp Ser Ser Gly Thr Leu Ile Ser Arg 260 265
270Ala Leu Val Val Thr His Thr Tyr Leu Glu Pro Gly Pro Val Thr Val
275 280 285Gln Val Val Leu Gln Ala Ala Ile Pro Leu Thr Ser Cys Gly
Ser Ser 290 295 300Pro Val Pro Gly Thr Thr Asp Gly His Arg Pro Thr
Ala Glu Ala Pro305 310 315 320Asn Thr Thr Ala Gly Gln Val Pro Thr
Thr Glu Val Val Gly Thr Thr 325 330 335Pro Gly Gln Ala Pro Thr Ala
Glu Pro Ser Gly Thr Thr Ser Val Gln 340 345 350Val Pro Thr Thr Glu
Val Ile Ser Thr Ala Pro Val Gln Met Pro Thr 355 360 365Ala Glu Ser
Thr Gly Met Thr Pro Glu Lys Val Pro Val Ser Glu Val 370 375 380Met
Gly Thr Thr Leu Ala Glu Met Ser Thr Pro Glu Ala Thr Gly Met385 390
395 400Thr Pro Ala Glu Val Ser Ile Val Val Leu Ser Gly Thr Thr Ala
Ala 405 410 415Gln Val Thr Thr Thr Glu Trp Val Glu Thr Thr Ala Arg
Glu Leu Pro 420 425 430Ile Pro Glu Pro Glu Gly Pro Asp Ala Ser Ser
Ile Met Ser Thr Glu 435 440 445Ser Ile Thr Gly Ser Leu Gly Pro Leu
Leu Asp Gly Thr Ala Thr Leu 450 455 460Arg Leu Val Lys Arg Gln Val
Pro Leu Asp Cys Val Leu Tyr Arg Tyr465 470 475 480Gly Ser Phe Ser
Val Thr Leu Asp Ile Val Gln Gly Ile Glu Ser Ala 485 490 495Glu Ile
Leu Gln Ala Val Pro Ser Gly Glu Gly Asp Ala Phe Glu Leu 500 505
510Thr Val Ser Cys Gln Gly Gly Leu Pro Lys Glu Ala Cys Met Glu Ile
515 520 525Ser Ser Pro Gly Cys Gln Pro Pro Ala Gln Arg Leu Cys Gln
Pro Val 530 535 540Leu Pro Ser Pro Ala Cys Gln Leu Val Leu His Gln
Ile Leu Lys Gly545 550 555 560Gly Ser Gly Thr Tyr Cys Leu Asn Val
Ser Leu Ala Asp Thr Asn Ser 565 570 575Leu Ala Val Val Ser Thr Gln
Leu Ile Met Pro Gly Gln Glu Ala Gly 580 585 590Leu Gly Gln Val Pro
Leu Ile Val Gly Ile Leu Leu Val Leu Met Ala 595 600 605Val Val Leu
Ala Ser Leu Ile Tyr Arg Arg Arg Leu Met Lys Gln Asp 610 615 620Phe
Ser Val Pro Gln Leu Pro His Ser Ser Ser His Trp Leu Arg Leu625 630
635 640Pro Arg Ile Phe Cys Ser Cys Pro Ile Gly Glu Asn Ser Pro Leu
Leu 645 650 655Ser Gly Gln Gln Val 6601112106DNAArtificial
SequenceHomo sapiens 111atg gag tct ccc tcg gcc cct ccc cac aga tgg
tgc atc ccc tgg cag 48Met Glu Ser Pro Ser Ala Pro Pro His Arg Trp
Cys Ile Pro Trp Gln1 5 10 15agg ctc ctg ctc aca gcc tca ctt cta acc
ttc tgg aac ccg ccc acc 96Arg Leu Leu Leu Thr Ala Ser Leu Leu Thr
Phe Trp Asn Pro Pro Thr 20 25 30act gcc aag ctc act att gaa tcc acg
ccg ttc aat gtc gca gag ggg 144Thr Ala Lys Leu Thr Ile Glu Ser Thr
Pro Phe Asn Val Ala Glu Gly 35 40 45aag gag gtg ctt cta ctt gtc cac
aat ctg ccc cag cat ctt ttt ggc 192Lys Glu Val Leu Leu Leu Val His
Asn Leu Pro Gln His Leu Phe Gly 50 55 60tac agc tgg tac aaa ggt gaa
aga gtg gat ggc aac cgt caa att ata 240Tyr Ser Trp Tyr Lys Gly Glu
Arg Val Asp Gly Asn Arg Gln Ile Ile65 70 75 80gga tat gta ata gga
act caa caa gct acc cca ggg ccc gca tac agt 288Gly Tyr Val Ile Gly
Thr Gln Gln Ala Thr Pro Gly Pro Ala Tyr Ser 85 90 95ggt cga gag ata
ata tac ccc aat gca tcc ctg ctg atc cag aac atc 336Gly Arg Glu Ile
Ile Tyr Pro Asn Ala Ser Leu Leu Ile Gln Asn Ile 100 105 110atc cag
aat gac aca gga ttc tac acc cta cac gtc ata aag tca gat 384Ile Gln
Asn Asp Thr Gly Phe Tyr Thr Leu His Val Ile Lys Ser Asp 115 120
125ctt gtg aat gaa gaa gca act ggc cag ttc cgg gta tac ccg gag ctg
432Leu Val Asn Glu Glu Ala Thr Gly Gln Phe Arg Val Tyr Pro Glu Leu
130 135 140ccc aag ccc tcc atc tcc agc aac aac tcc aaa ccc gtg gag
gac aag 480Pro Lys Pro Ser Ile Ser Ser Asn Asn Ser Lys Pro Val Glu
Asp Lys145 150 155 160gat gct gtg gcc ttc acc tgt gaa cct gag act
cag gac gca acc tac 528Asp Ala Val Ala Phe Thr Cys Glu Pro Glu Thr
Gln Asp Ala Thr Tyr 165 170 175ctg tgg tgg gta aac aat cag agc ctc
ccg gtc agt ccc agg ctg cag 576Leu Trp Trp Val Asn Asn Gln Ser Leu
Pro Val Ser Pro Arg Leu Gln 180 185 190ctg tcc aat ggc aac agg acc
ctc act cta ttc aat gtc aca aga aat 624Leu Ser Asn Gly Asn Arg Thr
Leu Thr Leu Phe Asn Val Thr Arg Asn 195 200 205gac aca gca agc tac
aaa tgt gaa acc cag aac cca gtg agt gcc agg 672Asp Thr Ala Ser Tyr
Lys Cys Glu Thr Gln Asn Pro Val Ser Ala Arg 210 215 220cgc agt gat
tca gtc atc ctg aat gtc ctc tat ggc ccg gat gcc ccc 720Arg Ser Asp
Ser Val Ile Leu Asn Val Leu Tyr Gly Pro Asp Ala Pro225 230 235
240acc att tcc cct cta aac aca tct tac aga tca ggg gaa aat ctg aac
768Thr Ile Ser Pro Leu Asn Thr Ser Tyr Arg Ser Gly Glu Asn Leu Asn
245 250 255ctc tcc tgc cac gca gcc tct aac cca cct gca cag tac tct
tgg ttt 816Leu Ser Cys His Ala Ala Ser Asn Pro Pro Ala Gln Tyr Ser
Trp Phe 260 265 270gtc aat ggg act ttc cag caa tcc acc caa gag ctc
ttt atc ccc aac 864Val Asn Gly Thr Phe Gln Gln Ser Thr Gln Glu Leu
Phe Ile Pro Asn 275 280 285atc act gtg aat aat agt gga tcc tat acg
tgc caa gcc cat aac tca 912Ile Thr Val Asn Asn Ser Gly Ser Tyr Thr
Cys Gln Ala His Asn Ser 290 295 300gac act ggc ctc aat agg acc aca
gtc acg acg atc aca gtc tat gag 960Asp Thr Gly Leu Asn Arg Thr Thr
Val Thr Thr Ile Thr Val Tyr Glu305 310 315 320cca ccc aaa ccc ttc
atc acc agc aac aac tcc aac ccc gtg gag gat 1008Pro Pro Lys Pro Phe
Ile Thr Ser Asn Asn Ser Asn Pro Val Glu Asp 325 330 335gag gat gct
gta gcc tta acc tgt gaa cct gag att cag aac aca acc 1056Glu Asp Ala
Val Ala Leu Thr Cys Glu Pro Glu Ile Gln Asn Thr Thr 340 345 350tac
ctg tgg tgg gta aat aat cag agc ctc ccg gtc agt ccc agg ctg 1104Tyr
Leu Trp Trp Val Asn Asn Gln Ser Leu Pro Val Ser Pro Arg Leu 355 360
365cag ctg tcc aat gac aac agg acc ctc act cta ctc agt gtc aca agg
1152Gln Leu Ser Asn Asp Asn Arg Thr Leu Thr Leu Leu Ser Val Thr Arg
370 375 380aat gat gta gga ccc tat gag tgt gga atc cag aac gaa tta
agt gtt 1200Asn Asp Val Gly Pro Tyr Glu Cys Gly Ile Gln Asn Glu Leu
Ser Val385 390 395 400gac cac agc gac cca gtc atc ctg aat gtc ctc
tat ggc cca gac gac 1248Asp His Ser Asp Pro Val Ile Leu Asn Val Leu
Tyr Gly Pro Asp Asp 405 410 415ccc acc att tcc ccc tca tac acc tat
tac cgt cca ggg gtg aac ctc 1296Pro Thr Ile Ser Pro Ser Tyr Thr Tyr
Tyr Arg Pro Gly Val Asn Leu 420 425 430agc ctc tcc tgc cat gca gcc
tct aac cca cct gca cag tat tct tgg 1344Ser Leu Ser Cys His Ala Ala
Ser Asn Pro Pro Ala Gln Tyr Ser Trp 435 440 445ctg att gat ggg aac
atc cag caa cac aca caa gag ctc ttt atc tcc 1392Leu Ile Asp Gly Asn
Ile Gln Gln His Thr Gln Glu Leu Phe Ile Ser 450 455 460aac atc act
gag aag aac agc gga ctc tat acc tgc cag gcc aat aac 1440Asn Ile Thr
Glu Lys Asn Ser Gly Leu Tyr Thr Cys Gln Ala Asn Asn465 470 475
480tca gcc agt ggc cac agc agg act aca gtc aag aca atc aca gtc tct
1488Ser Ala Ser Gly His Ser Arg Thr Thr Val Lys Thr Ile Thr Val Ser
485 490 495gcg gag ctg ccc aag ccc tcc atc tcc agc aac aac tcc aaa
ccc gtg 1536Ala Glu Leu Pro Lys Pro Ser Ile Ser Ser Asn Asn Ser Lys
Pro Val 500 505 510gag gac aag gat gct gtg gcc ttc acc tgt gaa cct
gag gct cag aac 1584Glu Asp Lys Asp Ala Val Ala Phe Thr Cys Glu Pro
Glu Ala Gln Asn 515 520 525aca acc tac ctg tgg tgg gta aat ggt cag
agc ctc cca gtc agt ccc 1632Thr Thr Tyr Leu Trp Trp Val Asn Gly Gln
Ser Leu Pro Val Ser Pro 530 535 540agg ctg cag ctg tcc aat ggc aac
agg acc ctc act cta ttc aat gtc 1680Arg Leu Gln Leu Ser Asn Gly Asn
Arg Thr Leu Thr Leu Phe Asn Val545 550 555 560aca aga aat gac gca
aga gcc tat gta tgt gga atc cag aac tca gtg 1728Thr Arg Asn Asp Ala
Arg Ala Tyr Val Cys Gly Ile Gln Asn Ser Val 565 570 575agt gca aac
cgc agt gac cca gtc acc ctg gat gtc ctc tat ggg ccg 1776Ser Ala Asn
Arg Ser Asp Pro Val Thr Leu Asp Val Leu Tyr Gly Pro 580 585 590gac
acc ccc atc att tcc ccc cca gac tcg tct tac ctt tcg gga gcg 1824Asp
Thr Pro Ile Ile Ser Pro Pro Asp Ser Ser Tyr Leu Ser Gly Ala 595 600
605gac ctc aac ctc tcc tgc cac tcg gcc tct aac cca tcc ccg cag tat
1872Asp Leu Asn Leu Ser Cys His Ser Ala Ser Asn Pro Ser Pro Gln Tyr
610 615 620tct tgg cgt atc aat ggg ata ccg cag caa cac aca caa gtt
ctc ttt 1920Ser Trp Arg Ile Asn Gly Ile Pro Gln Gln His Thr Gln Val
Leu Phe625 630 635 640atc gcc aaa atc acg cca aat aat aac ggg acc
tat gcc tgt ttt gtc 1968Ile Ala Lys Ile Thr Pro Asn Asn Asn Gly Thr
Tyr Ala Cys Phe Val 645 650 655tct aac ttg gct act ggc cgc aat aat
tcc ata gtc aag agc atc aca 2016Ser Asn Leu Ala Thr Gly Arg Asn Asn
Ser Ile Val Lys Ser Ile Thr 660 665 670gtc tct gca tct gga act tct
cct ggt ctc tca gct ggg gcc act gtc 2064Val Ser Ala Ser Gly Thr Ser
Pro Gly Leu Ser Ala Gly Ala Thr Val
675 680 685ggc atc atg att gga gtg ctg gtt ggg gtt gct ctg ata tag
2106Gly Ile Met Ile Gly Val Leu Val Gly Val Ala Leu Ile 690 695
700112701PRTArtificial SequenceHomo sapiens 112Met Glu Ser Pro Ser
Ala Pro Pro His Arg Trp Cys Ile Pro Trp Gln1 5 10 15Arg Leu Leu Leu
Thr Ala Ser Leu Leu Thr Phe Trp Asn Pro Pro Thr 20 25 30Thr Ala Lys
Leu Thr Ile Glu Ser Thr Pro Phe Asn Val Ala Glu Gly 35 40 45Lys Glu
Val Leu Leu Leu Val His Asn Leu Pro Gln His Leu Phe Gly 50 55 60Tyr
Ser Trp Tyr Lys Gly Glu Arg Val Asp Gly Asn Arg Gln Ile Ile65 70 75
80Gly Tyr Val Ile Gly Thr Gln Gln Ala Thr Pro Gly Pro Ala Tyr Ser
85 90 95Gly Arg Glu Ile Ile Tyr Pro Asn Ala Ser Leu Leu Ile Gln Asn
Ile 100 105 110Ile Gln Asn Asp Thr Gly Phe Tyr Thr Leu His Val Ile
Lys Ser Asp 115 120 125Leu Val Asn Glu Glu Ala Thr Gly Gln Phe Arg
Val Tyr Pro Glu Leu 130 135 140Pro Lys Pro Ser Ile Ser Ser Asn Asn
Ser Lys Pro Val Glu Asp Lys145 150 155 160Asp Ala Val Ala Phe Thr
Cys Glu Pro Glu Thr Gln Asp Ala Thr Tyr 165 170 175Leu Trp Trp Val
Asn Asn Gln Ser Leu Pro Val Ser Pro Arg Leu Gln 180 185 190Leu Ser
Asn Gly Asn Arg Thr Leu Thr Leu Phe Asn Val Thr Arg Asn 195 200
205Asp Thr Ala Ser Tyr Lys Cys Glu Thr Gln Asn Pro Val Ser Ala Arg
210 215 220Arg Ser Asp Ser Val Ile Leu Asn Val Leu Tyr Gly Pro Asp
Ala Pro225 230 235 240Thr Ile Ser Pro Leu Asn Thr Ser Tyr Arg Ser
Gly Glu Asn Leu Asn 245 250 255Leu Ser Cys His Ala Ala Ser Asn Pro
Pro Ala Gln Tyr Ser Trp Phe 260 265 270Val Asn Gly Thr Phe Gln Gln
Ser Thr Gln Glu Leu Phe Ile Pro Asn 275 280 285Ile Thr Val Asn Asn
Ser Gly Ser Tyr Thr Cys Gln Ala His Asn Ser 290 295 300Asp Thr Gly
Leu Asn Arg Thr Thr Val Thr Thr Ile Thr Val Tyr Glu305 310 315
320Pro Pro Lys Pro Phe Ile Thr Ser Asn Asn Ser Asn Pro Val Glu Asp
325 330 335Glu Asp Ala Val Ala Leu Thr Cys Glu Pro Glu Ile Gln Asn
Thr Thr 340 345 350Tyr Leu Trp Trp Val Asn Asn Gln Ser Leu Pro Val
Ser Pro Arg Leu 355 360 365Gln Leu Ser Asn Asp Asn Arg Thr Leu Thr
Leu Leu Ser Val Thr Arg 370 375 380Asn Asp Val Gly Pro Tyr Glu Cys
Gly Ile Gln Asn Glu Leu Ser Val385 390 395 400Asp His Ser Asp Pro
Val Ile Leu Asn Val Leu Tyr Gly Pro Asp Asp 405 410 415Pro Thr Ile
Ser Pro Ser Tyr Thr Tyr Tyr Arg Pro Gly Val Asn Leu 420 425 430Ser
Leu Ser Cys His Ala Ala Ser Asn Pro Pro Ala Gln Tyr Ser Trp 435 440
445Leu Ile Asp Gly Asn Ile Gln Gln His Thr Gln Glu Leu Phe Ile Ser
450 455 460Asn Ile Thr Glu Lys Asn Ser Gly Leu Tyr Thr Cys Gln Ala
Asn Asn465 470 475 480Ser Ala Ser Gly His Ser Arg Thr Thr Val Lys
Thr Ile Thr Val Ser 485 490 495Ala Glu Leu Pro Lys Pro Ser Ile Ser
Ser Asn Asn Ser Lys Pro Val 500 505 510Glu Asp Lys Asp Ala Val Ala
Phe Thr Cys Glu Pro Glu Ala Gln Asn 515 520 525Thr Thr Tyr Leu Trp
Trp Val Asn Gly Gln Ser Leu Pro Val Ser Pro 530 535 540Arg Leu Gln
Leu Ser Asn Gly Asn Arg Thr Leu Thr Leu Phe Asn Val545 550 555
560Thr Arg Asn Asp Ala Arg Ala Tyr Val Cys Gly Ile Gln Asn Ser Val
565 570 575Ser Ala Asn Arg Ser Asp Pro Val Thr Leu Asp Val Leu Tyr
Gly Pro 580 585 590Asp Thr Pro Ile Ile Ser Pro Pro Asp Ser Ser Tyr
Leu Ser Gly Ala 595 600 605Asp Leu Asn Leu Ser Cys His Ser Ala Ser
Asn Pro Ser Pro Gln Tyr 610 615 620Ser Trp Arg Ile Asn Gly Ile Pro
Gln Gln His Thr Gln Val Leu Phe625 630 635 640Ile Ala Lys Ile Thr
Pro Asn Asn Asn Gly Thr Tyr Ala Cys Phe Val 645 650 655Ser Asn Leu
Ala Thr Gly Arg Asn Asn Ser Ile Val Lys Ser Ile Thr 660 665 670Val
Ser Ala Ser Gly Thr Ser Pro Gly Leu Ser Ala Gly Ala Thr Val 675 680
685Gly Ile Met Ile Gly Val Leu Val Gly Val Ala Leu Ile 690 695
7001139PRTArtificial SequenceHomo sapiens 113Tyr Leu Ser Gly Ala
Asp Leu Asn Leu1 5
D00001

D00002

D00003

D00004

D00005

D00006

D00007

D00008

D00009

D00010

D00011

S00001
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.