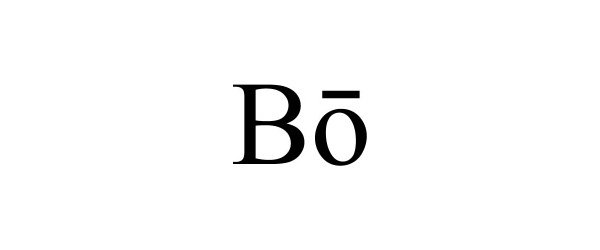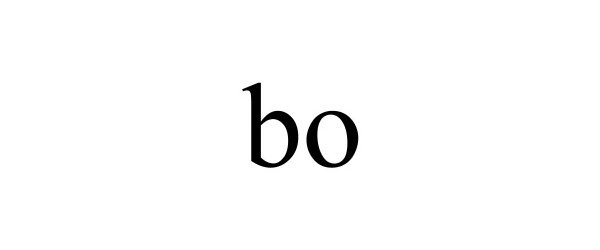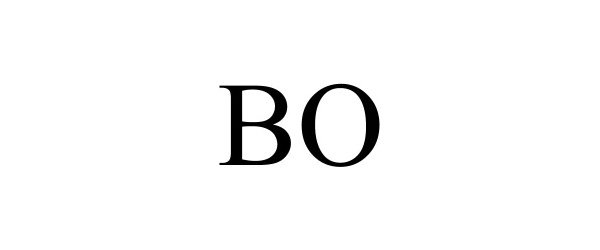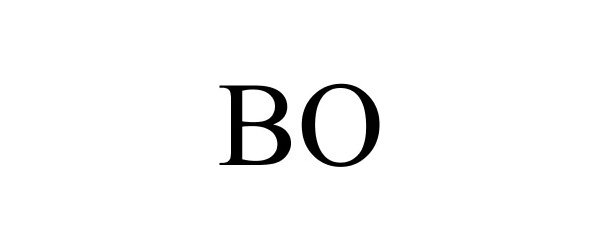| GS0011 | Cell culture media for laboratory use; Cell culture media for scientific and research use; Biological preparation for use in cell cultures, other than for medical or veterinary use; Cell culture reagents for scientific and research use; Fetal animal serums; Cell growth supplements; Scientific materials for culturing cells; Fetal animal serums, cell growth supplements, and support media for the production of in-vitro fertilization (IVF) embryos; Biochemicals for in vitro and in vivo scientific and research use; Cell culture reagents for laboratory use; Animal serums for scientific or research use; Animals serum for use with cell culture media used to grow cells in scientific or laboratory research; Animal serums for use as a supplement to cell culture media used to grow cells in scientific or laboratory research; Animal serums for use with cell culture media used to grow cells in medical research; Animal serums for use as a supplement to cell culture media used to grow cells in medical research; Cultures of cell media for scientific or research use; Chemical reagents for use in biotechnology, other than for medical or veterinary use; Nutritive agents for processing living cells; Cell growth media for growing cells, tissue culture media, cytokines, antibodies, and reagents for medical research use; Serums for scientific and research use, namely, animal serums used for cell culturing; Biological preparations for scientific and research use; Culture media for cultivating animal cells; Culture fluids for cultivating animal cells; Cell culture media for medical use; Biological preparations for use in cell cultures for medical or veterinary use; Cell culture reagents for medical use; Serums for medical or veterinary use, namely, animal serums used for cell culturing; Serums for medical use, namely, cell culturing; Animal serums for medical or veterinary use; Medical, scientific, and cell culture reagents; Genetically and otherwise modified cells for medical or clinical use; Living cells for medical use; Diagnostic preparations for medical and veterinary purposes; Cell growth media for growing cells, tissue culture media, cytokines, antibodies, and reagents for medical, and diagnostic or clinical use; Tissue culture media growth factors in the nature of cell growth, cell growth media, and nutritional supplements for in vitro culturing, development, maintenance, expansion, assay or manipulation of cells, all for medical or veterinary use; Biological preparations for medical or veterinary use; Biochemicals for in vitro and in vivo medical use; Cell culture reagents for medical or veterinary use; Animals serum for use with cell culture media used to grow cells for medical or veterinary use; Animal serums for use as a supplement to cell culture media used to grow cells for medical or veterinary use; Animal serums for use with cell culture media used to grow cells in medical diagnostics, clinical diagnostics, or veterinary diagnostics; Cultures of cell media for medical or veterinary use; Chemical reagents for use in biotechnology for medical or veterinary use; Cell growth media for growing cells, tissue culture media, cytokines, antibodies, and reagents for medical or veterinary use; Biological preparations for medical or veterinary use |
| GS0051 | Cell culture reagents for medical use; serums for medical use, in the field of cell culturing; serums for medical or veterinary use, namely, animal serums used for growing cell culturing; animal serums for medical or veterinary use; genetically and otherwise modified cells for medical or clinical use; living cells for medical use; diagnostic preparations for medical and veterinary purposes; cell growth media for growing cells, tissue culture media, cytokines, antibodies, and reagents for medical, and diagnostic or clinical use; tissue culture media growth factors in the nature of cell growth, cell growth media, and nutritional supplements for in vitro culturing, development, maintenance, expansion, assay or manipulation of cells, all for medical or veterinary use; biological preparations for medical or veterinary use; biochemicals for in vitro and in vivo medical use; cell culture reagents for medical or veterinary use; animals serum for use with cell culture media used to grow cells for medical or veterinary use; animal serums for use as a supplement to cell culture media used to grow cells for medical or veterinary use; animal serums for use with cell culture media used to grow cells in medical diagnostics, clinical diagnostics, or veterinary diagnostics; cultures of cell media for medical or veterinary use; chemical reagents for use in biotechnology for medical or veterinary use; cell growth media for growing cells, tissue culture media, cytokines, antibodies, and reagents for medical or veterinary use; biological preparations for medical or veterinary use |