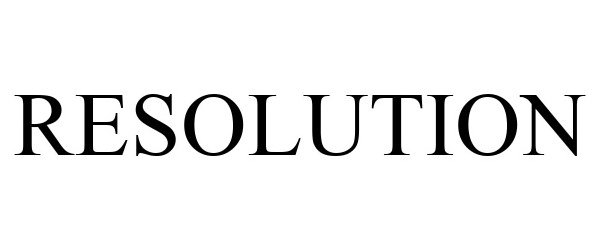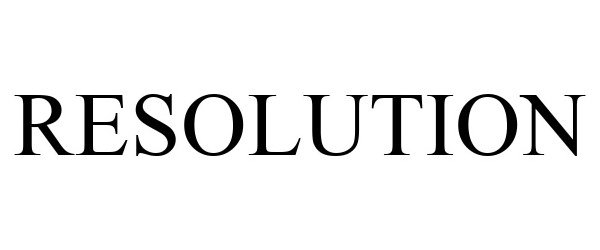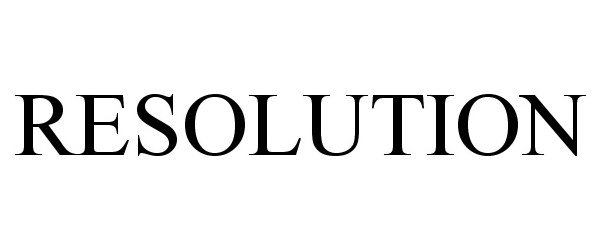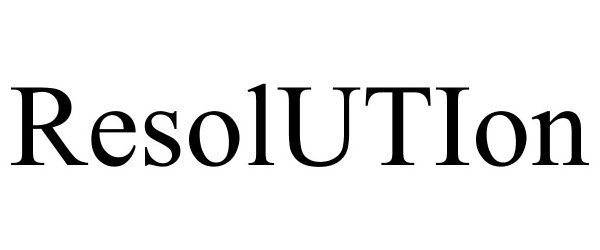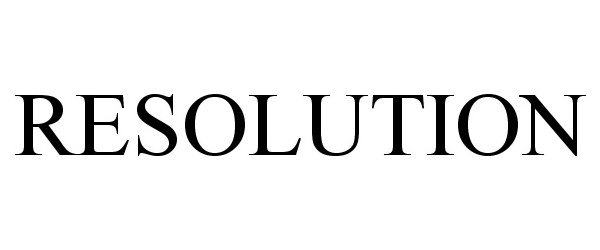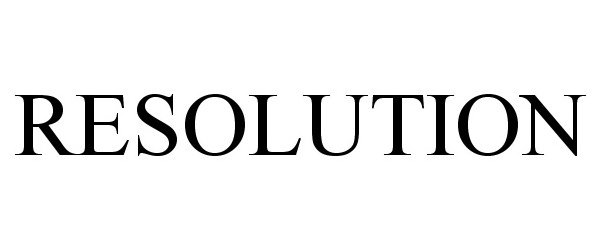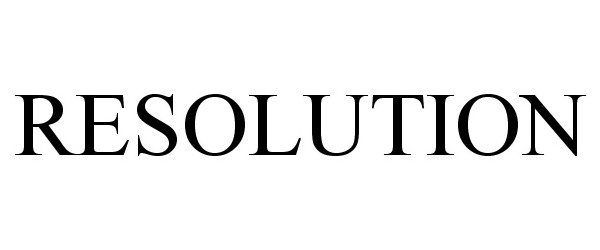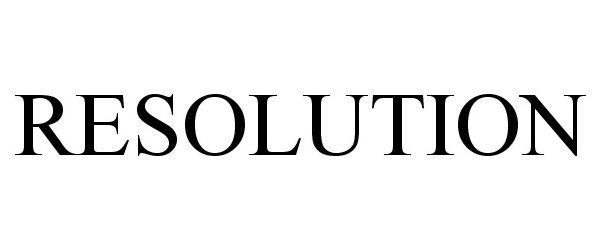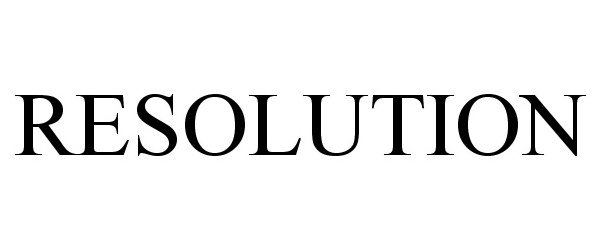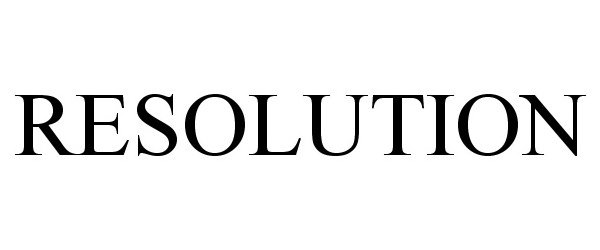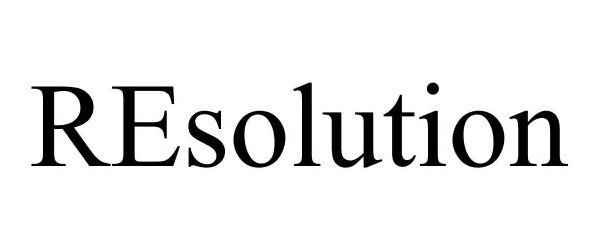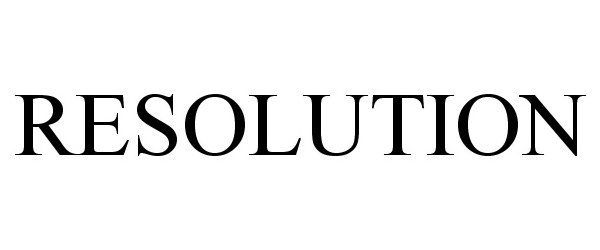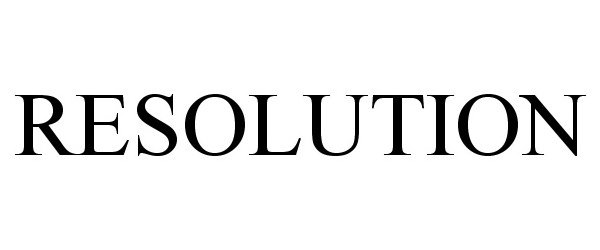IC 001. US 001 005 006 010 026 046. G & S: Chemicals preparations for scientific purposes; chemicals for use in research; chemicals, enzymes, DNA and RNA for research use; cells for scientific purposes; molecular biology research tools in the nature of biological, chemical and biochemical reagents for research use; biological preparations for cell therapy
IC 005. US 005 006 018 044 046 051 052. G & S: Pharmaceutical preparations for the treatment of hematologic diseases, immune response disorders, neurological disorders, respiratory diseases, ophthalmological conditions, metabolic diseases, genetic conditions, cancer, infectious diseases and cardiovascular diseases; medical and veterinary preparations for therapeutic purposes for the treatment of hematologic diseases, immune response disorders, neurological disorders, respiratory diseases, ophthalmological conditions, metabolic diseases, genetic conditions, cancer, infectious diseases and cardiovascular diseases; biological, chemical and biochemical reagents for medical use; cells for medical or veterinary use; pharmaceutical and biopharmaceutical preparations for modifying human or animal cells for medical or therapeutic purposes; pharmaceutical and biopharmaceutical preparations of cells from humans or animals which have been adapted for medical or therapeutic purposes
IC 040. US 100 103 106. G & S: Treatment and processing of biological materials; custom manufacture of therapeutic drugs, namely, custom manufacture of cells and cell therapies
IC 042. US 100 101. G & S: Scientific and technological services and research and design relating thereto, namely, scientific research in the field of in the fields of haematology, immunology, neurology, respiratory diseases, ophthalmology, metabolic conditions, genetic conditions, oncology, immuno-oncology, infectious diseases and cardiovascular diseases; industrial research services, in the field of cell therapies and gene therapies; chemical and biological analysis and research services; scientific research in human and animal cell therapy; scientific research into preparations for manipulating and adapting human and animal cells; design and development of new products for others; scientific laboratory services, namely, manipulation of human and animal cells for therapeutic purposes ; advisory and consultancy relating to all the aforesaid services
IC 044. US 100 101. G & S: Medical and veterinary research services including the manipulation of biological reagents to be used for medical or therapeutic purposes; medical and veterinary research services including the manipulation of cells within a laboratory or research facility to enable the cells to be used for medical or therapeutic purposes; medical services; veterinary services; medical services for the treatment of conditions of the human or animal body; medical services in the nature of extraction of human and animal cells from patients; medical services, namely, reinsertion of cells into human and animal patients; providing advice and consultancy in the field of medical and veterinary therapies
| International Codes: | 1 |
| U.S. Codes: | 001,005,006,010,026,046 |
| International Codes: | 5 |
| U.S. Codes: | 005,006,018,044,046,051,052 |
| International Codes: | 40 |
| U.S. Codes: | 100,103,106 |
| International Codes: | 42 |
| U.S. Codes: | 100,101 |
| International Codes: | 44 |
| U.S. Codes: | 100,101 |
| Type Code | Type |
|---|
| GS0011 | Chemicals for scientific purposes; chemicals for use in research; chemicals, enzymes, DNA and RNA for research use; cells for scientific purposes; molecular biology research tools in the nature of biological, chemical and biochemical reagents for research use; Pharmaceutical preparations; medical and veterinary preparations for therapeutic purposes; biological, chemical and biochemical reagents for medical use; cells for medical or veterinary use; preparations for cell therapy; preparations for modifying human or animal cells for medical or therapeutic purposes; preparations of cells from humans or animals which have been adapted for medical or therapeutic purposes; Treatment and processing of biological materials; custom manufacture of therapeutic drugs, including custom manufacture of cells and cell therapies; information, advisory and consultancy services in relation to all of the aforesaid services; Scientific and technological services and research and design relating thereto; industrial analysis and research services; medical and veterinary research services including the manipulation of biological reagents to be used for medical or therapeutic purposes; chemical and biological analysis and research services; medical and veterinary research services including the manipulation of cells within a laboratory or research facility to enable the cells to be used for medical or therapeutic purposes; scientific research in human and animal cell therapy; scientific research into preparations for manipulating and adapting human and animal cells; design and development of new products (for others); scientific laboratory services; advisory and consultancy relating to all the aforesaid services; Medical services; veterinary services; medical services for the treatment of conditions of the human or animal body; services in the extraction of human and animal cells from patients; manipulation of human and animal cells for therapeutic purposes; reinsertion of cells into human and animal patients; providing advice and consultancy in the field of medical and veterinary therapies |
| GS0401 | Treatment and processing of biological materials; custom manufacture of therapeutic drugs, namely, custom manufacture of cells and cell therapies |