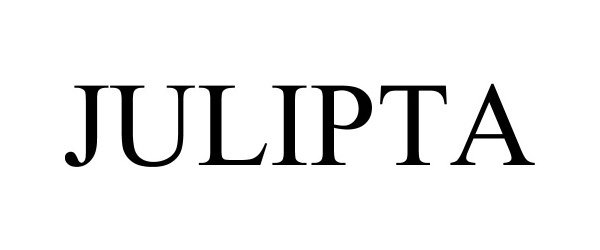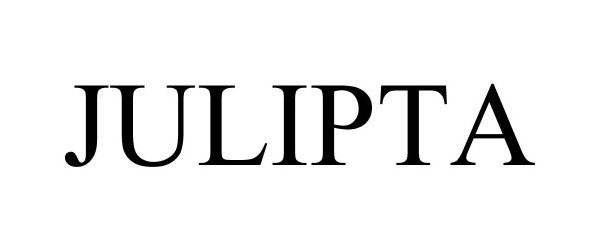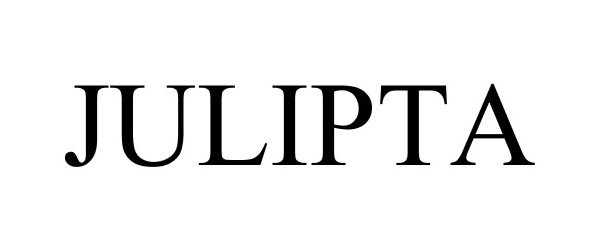JULIPTA
Allergan Sales, LLC
| Research |
|
| Serial Number | 87174410 |
| Mark Drawing Code | 4000: Illustration: Drawing with word(s)/letter(s)/number(s) in Block form |
| Law Office Assigned | M10 |
| Employee Name | ELLINGER FATHY, JESSIC |
Timeline
| 2016-09-16 | Application Filed |
| 2017-01-31 | Published for Opposition |
| 2019-09-24 | Location: INTENT TO USE SECTION |
| 2019-09-24 | Status: Live/Pending |
| 2019-09-25 | Transaction Date |
Trademark Applicants & Owners
| Owner: | |
| Address | 5 Giralda Farms Madison DE 07940 |
| Legal Entity Type | Limited Liability Company |
| Legal Entity State | DE |
Documents
Click the blue "Refresh" button to load certificates, specimines, application, and other documents.
Attorney of Record
Good, Services, and Codes
| International Codes: | 5 |
| U.S. Codes: | 006,018,044,046,051,052 |
| Type Code | Type |
|---|---|
| GS0051 | Oral, transdermal, injectable and intravaginal contraceptive preparations; intravaginal contraceptive sponge; Hormone replacement therapy preparations; Pharmaceutical preparations for the prevention of preterm birth or pregnancy; Pharmaceutical preparations for the treatment and prevention of urinary incontinence and overactive bladder; Pharmaceutical preparations for the treatment and prevention of iron deficiency and anemia; Pharmaceutical preparations for the treatment and prevention of osteoporosis; Pharmaceutical preparations for the treatment and prevention of endometriosis; Pharmaceutical preparations for the treatment and prevention of symptoms associated with uterine fibroids; Iron chelating pharmaceutical preparations; Fertility enhancing preparations; Pharmaceuticals and medicines for the treatment and prevention of sexual dysfunction; Pharmaceuticals and medicines affecting sensory organs; Pharmaceuticals and medicines affecting the central nervous system; Pharmaceuticals and medicines affecting urogenital organs; Pharmaceuticals and medicines affecting circulatory systems; Pharmaceuticals and medicines for the treatment of cardiovascular and blood pressure diseases and disorders; Pharmaceutical preparations for the treatment and prevention of digestive tract, gastrointestinal and dermatological diseases and disorders; Pharmaceuticals and medicines for the treatment and prevention of irritable bowel syndrome; Pharmaceutical preparations that support, encourage or promote bone strength or bone health or which are used in the treatment of bone disorders or bone diseases; Antibiotics; Antifungal preparations; Antivirals and Antiprotozoals; Pharmaceutical preparations for the prevention and treatment of bacterial infections; Pharmaceutical preparations for the prevention and treatment of diabetes and diabetic neuropathy; Pharmaceutical preparations for the prevention and treatment of pain and neuropathic pain; Pharmaceutical preparations for the prevention and treatment of neurological diseases and disorders; Pharmaceutical preparations for the prevention and treatment of neurodegenerative diseases and disorders; Pharmaceutical preparations for the prevention and treatment of peripheral nervous system diseases and disorders; Pharmaceutical preparations for the prevention and treatment of hypertension; Pharmaceutical preparations for the prevention and treatment of Alzheimer's disease, dementia, AIDS-related dementia, CNS, neurological disorders, neurodegenerative disorders, and depression; Pharmaceuticals, namely, neuroprotective agents; antidepressants; pharmaceuticals, namely, antipsychotics; coronary vasodilating agents; Pharmaceutical preparations for the treatment of hypothyroidism; Pharmaceutical preparations for skin care used in connection with anti-aging, the treatment of glabellar lines, facial wrinkles, asymmetries and defects and conditions of the human skin, facial aesthetic surgery, facial aesthetic reconstruction, breast aesthetics; topical antibiotics, anti-inflammatories, anti-infectives, anti-glaucoma eye drops and decongestant formulations; sterile ointments for medical use; ocular wetting solutions, artificial tears and formulations pharmaceutical preparations for the treatment of minor ocular inflammations and allergic conditions; Pharmaceutical preparations for the treatment of neurological disorders, muscle dystonias, smooth muscle disorders, autonomic nerve disorders, headaches, hyperhydrosis, sports injuries, cerebral palsy, spasms, tremors and pain; dermatological formulations, namely, medicated dry skin lotions and creams, acne medications and medicated skin lighteners; Pharmaceuticals and cosmeceuticals, namely, medicated skin cleansers, toners, moisturizers, skin rejuvenators, rehydrating creams, and lotions for the face and body |
Trademark Filing History
| Description | Date | Event Coding |
|---|---|---|
| NEW APPLICATION ENTERED IN TRAM | 2016-09-20 | 1 NWAP I:Incoming Correspondence |
| NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM | 2016-09-21 | 2 NWOS I:Incoming Correspondence |
| ASSIGNED TO EXAMINER | 2016-12-21 | 3 DOCK D:Assigned to Examiner |
| APPROVED FOR PUB - PRINCIPAL REGISTER | 2016-12-22 | 4 CNSA P: |
| NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | 2017-01-11 | 5 NONP E:E-Mail |
| PUBLISHED FOR OPPOSITION | 2017-01-31 | 6 PUBO A:Allowance for Publication |
| OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | 2017-01-31 | 7 NPUB E:E-Mail |
| TEAS POST PUBLICATION AMENDMENT RECEIVED | 2017-02-16 | 8 EPPA I:Incoming Correspondence |
| ASSIGNED TO PETITION STAFF | 2017-03-03 | 9 APET A:Allowance for Publication |
| CHANGES/CORRECTIONS AFTER PUB APPROVAL ENTERED | 2017-03-04 | 10 CHPB I:Incoming Correspondence |
| NOA E-MAILED - SOU REQUIRED FROM APPLICANT | 2017-03-28 | 11 NOAM E:E-Mail |
| TEAS EXTENSION RECEIVED | 2017-08-16 | 12 EEXT I:Incoming Correspondence |
| EXTENSION 1 FILED | 2017-08-16 | 13 EXT1 S: |
| EXTENSION 1 GRANTED | 2017-08-16 | 14 EX1G S: |
| NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | 2017-08-18 | 15 EXRA E:E-Mail |
| TEAS EXTENSION RECEIVED | 2018-01-30 | 16 EEXT I:Incoming Correspondence |
| EXTENSION 2 FILED | 2018-01-30 | 17 EXT2 S: |
| EXTENSION 2 GRANTED | 2018-01-30 | 18 EX2G S: |
| NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | 2018-02-01 | 19 EXRA E:E-Mail |
| TEAS EXTENSION RECEIVED | 2018-09-07 | 20 EEXT I:Incoming Correspondence |
| EXTENSION 3 FILED | 2018-09-07 | 21 EXT3 S: |
| EXTENSION 3 GRANTED | 2018-09-07 | 22 EX3G S: |
| NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | 2018-09-11 | 23 EXRA E:E-Mail |
| TEAS EXTENSION RECEIVED | 2019-03-01 | 24 EEXT I:Incoming Correspondence |
| EXTENSION 4 FILED | 2019-03-01 | 25 EXT4 S: |
| EXTENSION 4 GRANTED | 2019-03-01 | 26 EX4G S: |
| NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | 2019-03-05 | 27 EXRA E:E-Mail |
| TEAS EXTENSION RECEIVED | 2019-09-20 | 28 EEXT I:Incoming Correspondence |
| CASE ASSIGNED TO INTENT TO USE PARALEGAL | 2019-09-24 | 29 AITU A:Allowance for Publication |
| EXTENSION 5 FILED | 2019-09-20 | 30 EXT5 S: |
| EXTENSION 5 GRANTED | 2019-09-24 | 31 EX5G S: |
| NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | 2019-09-25 | 32 EXRA E:E-Mail |
| TEAS EXPRESS ABANDONMENT RECEIVED | 2020-04-29 | 33 EXAR I:Incoming Correspondence |
| ABANDONMENT - AFTER PUBLICATION | 2020-04-30 | 34 ABN5 O:Outgoing Correspondence |
| ABANDONMENT NOTICE E-MAILED - AFTER PUBLICATION | 2020-04-30 | 35 MAB5 E:E-Mail |
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.