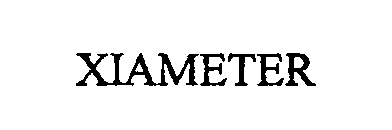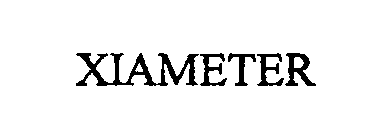(ABANDONED)
IC 001. US 001 005 006 010 026 046. G & S: Silicone used in the manufacture of chemicals, fluids, resins, and plastics used in the medical and pharmaceutical field; silicones for medical and pharmaceutical purposes, namely, silicones for use in the manufacture of skin care products; silicones for medical purposes, namely, silicones for use in the manufacture of non-adherent dressings, burn treatment gauzes, cavity wound dressings, skin barriers, and burn masks; silicone fluids, namely, antifoams and antifoam silicone emulsions; silicone fluids, namely, silicone dispersions for use with cutting edges, needles, and biomedical devices; chemicals used in industry, namely, silicone-based topical excipients for use in the manufacture of drug delivery, skin care products, and pharmaceutical products; liquid silicone rubbers for use in the manufacture of O-rings, stoppers, and closures in medical devices; liquid silicone rubbers for use in the manufacture of medical device implants; silicone-containing pourable elastomer resins for use in the manufacture of drug matrixes for pharmaceutical applications; silicone-containing elastomer resins for use in the manufacture of medical prostheses and pressure cushions; silicone resins for use in the manufacture of pharmaceutical and biopharmaceutical tubing; silicones for use in the manufacture of dental hygiene products and dental impressions; silicone-based adhesives for use in assembling and sealing medical device components in the manufacture of medical devices; silicone-based adhesives for use in encapsulating and sealing electrical components in the manufacture of medical devices; Silicone used in the manufacture of chemicals, fluids, resins, and plastics used in the implantation of medical devices in the body(ABANDONED)
IC 005. US 006 018 044 046 051 052. G & S: Silicones for medical and pharmaceutical purposes, namely, silicone-containing chemicals, fluids, resins, and plastics for use in the treatment of scars; silicones for medical and pharmaceutical purposes, namely, silicone-containing chemicals, fluids, resins, and plastics for use in the treatment of burns; silicones for medical and pharmaceutical purposes, namely, silicone-containing chemicals, fluids, resins, and plastics for use in the treatment of wounds; silicone-based medical adhesives for use in the attachment of wound dressings, prosthetics, transdermal drug delivery systems, and scar therapy medical devices to the body; silicone-based medical adhesives for pharmaceutical topical or transdermal applications(ABANDONED)
IC 010. US 026 039 044. G & S: Silicone elastomer coatings sold as integral components of a wide variety of implantable medical devices; silicone-based molded tubing assemblies for biopharmaceutical and pharmaceutical fluid transfer; silicone-based medical grade tubing for medical and pharmaceutical drug administration purposes(ABANDONED)
IC 017. US 001 005 012 013 035 050. G & S: Liquid rubbers for medical applications, namely, liquid silicone rubbers for use in fabric coating for medical devices; Liquid rubbers for medical applications, namely liquid silicone rubbers for use in mesh coating for medical devices
| International Codes: | 1 |
| U.S. Codes: | 001,005,006,010,026,046 |
| International Codes: | 5 |
| U.S. Codes: | 006,018,044,046,051,052 |
| International Codes: | 10 |
| U.S. Codes: | 026,039,044 |
| International Codes: | 17 |
| U.S. Codes: | 001,005,012,013,035,050 |
| Type Code | Type |
|---|
| GS0011 | Silicone used in the manufacture of chemicals, fluids, resins, and plastics used in the medical and pharmaceutical field; silicones for medical and pharmaceutical purposes, namely, silicones for use in the manufacture of skin care products; silicones for medical purposes, namely, silicones for use in the manufacture of non-adherent dressings, burn treatment gauzes, cavity wound dressings, skin barriers, and burn masks; silicone fluids, namely, antifoams and antifoam silicone emulsions; silicone fluids, namely, silicone dispersions for use with cutting edges, needles, and biomedical devices; chemicals used in industry, namely, silicone-based topical excipients for use in the manufacture of drug delivery, skin care products, and pharmaceutical products; liquid silicone rubbers for use in the manufacture of O-rings, stoppers, and closures in medical devices; liquid silicone rubbers for use in the manufacture of medical device implants; silicone-containing pourable elastomer resins for use in the manufacture of drug matrixes for pharmaceutical applications; silicone-containing elastomer resins for use in the manufacture of medical prostheses and pressure cushions; silicone resins for use in the manufacture of pharmaceutical and biopharmaceutical tubing; silicones for use in the manufacture of dental hygiene products and dental impressions; silicone-based adhesives for use in assembling and sealing medical device components in the manufacture of medical devices; silicone-based adhesives for use in encapsulating and sealing electrical components in the manufacture of medical devices; Silicone used in the manufacture of chemicals, fluids, resins, and plastics used in the implantation of medical devices in the body |