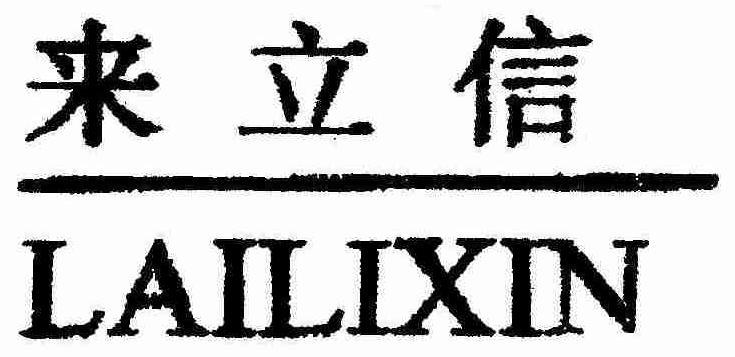LAILIXIN
Zhejiang Medicine Co. Ltd. Xinchang Pharmaceutical Factory
Mark For: LAILIXIN™ trademark registration is intended to cover the categories of
| Research |
|
| Serial Number | 76682427 |
| Mark Literal Elements | LAILIXIN |
| Mark Drawing Type | 4 - STANDARD CHARACTER MARK |
| Mark Type | TRADEMARK |
| Register | PRINCIPAL |
| Current Location | TMO LAW OFFICE 112 - EXAMINING ATTORNEY ASSIGNED 2011-10-20 |
| Basis | 1(b) |
| Class Status | ACTIVE |
| Primary US Classes |
|
| Primary International Class |
|
| Filed Use | No |
| Current Use | No |
| Intent To Use | Yes |
| Filed ITU | No |
| 44D Filed | No |
| 44E Current | No |
| 66A Current | No |
| Current Basis | No |
| No Basis | Yes |
| Attorney Name | Jack Matalon |
| Law Office Assigned | M30 |
| Employee Name | SPRUILL, DARRYL M |
Timeline
| 2007-10-01 | Application Filed |
| 2008-07-11 | Date of First Use |
| 2008-09-09 | Published |
| 2008-09-09 | Published for Opposition |
| 2009-10-27 | Date of Use In Commerce |
| 2011-09-14 | Abandon |
| 2011-10-20 | Location: TMO LAW OFFICE 112 - EXAMINING ATTORNEY ASSIGNED |
| 2011-10-20 | Status: Abandoned because the applicant failed to respond or filed a late response to an Office action. To view all documents in this fi |
| 2011-10-20 | Status: Dead/Abandoned |
| 2018-07-08 | Transaction Date |
Trademark Parties (Applicants & Owners)
| Party: | |
| Address | 59 East Huancheng Rd. Xinchang Zhejiang CHINA 312500 |
| Legal Entity Type | Corporation |
| Legal Entity State | CHINA |
Documents
Attorney of Record
Good, Services, and Codes
(ABANDONED)
IC 005. US 006 018 044 046 051 052. G & S: Full line of bulk pharmaceuticals, pharmaceutical intermediates, pharmaceutical preparations, antibiotics for animals and humans, animal feed supplements, human food supplements, herbal supplements for animals and humans, mineral supplements for animals and humans, dietary supplements for animals and humans, and vitamins for animals and humans in the form of caplets, capsules, creams, drops, douches, foams, gel-tabs, gels, inhalants, injectables, lotions, ointments, pastilles, powders, rinses, shampoos, solutions, spansules, sponges, sprays, suppositories, syrups and tablets including Ace-inhibitors; Adrenergic agonists; Adrenergic blockers; Anabolics; Analgesics, narcotic and non-narcotic analgesics; Anesthetics; Antianorexics; Antacids; Anthelmintics; Antiacnes; Antiallergics; Antianginals; Antiarthritics and antirheumatics; Antiasthmatics; Antibacterials; Antibiotics; Anticoagulants; Anticonvulsants; Antidepressants; Antidiabetics; Antidiarrheals; Antidiuretics; Antiemetics; Antifungals; Antiglaucomas; Antihistaminics; Antihypertensives; Antihyperthyroids; Antihypotensives; Anti-inflammatories; Anti-itch creams, ointments and sprays; Antimalarials; Antimigraines; Antimuscarinics; Antineoplastics; Antioxidants; Antiparkinsonians; Antiprotozoals; Antipruritics; Antipsoriatics; Antipsychotics; Antiseborrheics; Antiseptics; Antispasmodics; Antisyphilitics; Antithrombotics; Antitussives; Antiulceratives; Antivirals; Anxiolyties; Astringents; Bacteriostats for medicinal, dental and veterinary use; Bath salts for medical purposes; Beta-blockers; Biocides; Biological and chemical reagents for medical or veterinary use; Bronchodilators; Burn-relief medications; Calcium channel blockers; Cardiotonics; nutritional additives for use in dietary supplements, namely, carotenoids; Chemotherapeutics; Choleretics; Cholesterol reducers; Cholinergics; Cholinesterase inhibitors; Cleansing solutions for medical use; Clinical medical reagents; CNS stimulants; Contraceptives; Decongestants; Dermatologicals; Diagnostic preparations for medical purposes; Dietary and nutritional supplements; Diuretics; Dopamine receptor agonists; Enzyme cofactors; Estrogens; Expectorants; Food supplements; Gastric and pancreatic preparations; Germicides; Glucosidase inhibitors; Gonad-stimulating principles; Hematinics; Hematopoietics; Hemostatics; Hepatoprotectants; Herbal products, namely, aromatherapy packs containing herbs used for relief from headaches, insomnia and sinus discomfort; Homeopathic supplements; Immunomodulators; Immunosuppressants. Laxatives; Medicinal herbal extracts; Mineral nutritional supplements; Mineralocorticoids; Mixed vitamin preparations; Mucolytics; Multi-vitamin preparations; Muscle relaxants; Mydriatics; Neutraceuticals for use as dietary supplements; Nootropics; Nutritional additives to foodstuffs for animals, for medical purposes; Nutritional additives for medical purposes for use in foods and dietary supplements for human consumption; Plant extracts for medical, veterinary and pharmaceutical purposes; Processed food adapted for medical purposes; Progestogens; Prolactin inhibitors; Prostaglandins and prostaglandin analogs; dietary supplements; Respiratory stimulants; Reverse transcriptase inhibitors; Sedatives; Seratonin receptor agonists and antagonists; Soy protein for use as a nutritional ingredient in various powdered and ready-to-drink beverages; medicated topical skin protectants for medical use; Vasodilators; Vasoprotectants; Vitamin and mineral preparations for use as ingredients in the food and pharmaceutical industry; Yeast or yeast extracts for medical, veterinary or pharmaceutical purposes. FIRST USE: 20080711. FIRST USE IN COMMERCE: 20091027
| International Codes: | 5 |
| U.S. Codes: | 006,018,044,046,051,052 |
| Type Code | Type |
|---|---|
| GS0051 | Full line of bulk pharmaceuticals, pharmaceutical intermediates, pharmaceutical preparations, antibiotics for animals and humans, animal feed supplements, human food supplements, herbal supplements for animals and humans, mineral supplements for animals and humans, dietary supplements for animals and humans, and vitamins for animals and humans in the form of caplets, capsules, creams, drops, douches, foams, gel-tabs, gels, inhalants, injectables, lotions, ointments, pastilles, powders, rinses, shampoos, solutions, spansules, sponges, sprays, suppositories, syrups and tablets including Ace-inhibitors; Adrenergic agonists; Adrenergic blockers; Anabolics; Analgesics, narcotic and non-narcotic analgesics; Anesthetics; Antianorexics; Antacids; Anthelmintics; Antiacnes; Antiallergics; Antianginals; Antiarthritics and antirheumatics; Antiasthmatics; Antibacterials; Antibiotics; Anticoagulants; Anticonvulsants; Antidepressants; Antidiabetics; Antidiarrheals; Antidiuretics; Antiemetics; Antifungals; Antiglaucomas; Antihistaminics; Antihypertensives; Antihyperthyroids; Antihypotensives; Anti-inflammatories; Anti-itch creams, ointments and sprays; Antimalarials; Antimigraines; Antimuscarinics; Antineoplastics; Antioxidants; Antiparkinsonians; Antiprotozoals; Antipruritics; Antipsoriatics; Antipsychotics; Antiseborrheics; Antiseptics; Antispasmodics; Antisyphilitics; Antithrombotics; Antitussives; Antiulceratives; Antivirals; Anxiolyties; Astringents; Bacteriostats for medicinal, dental and veterinary use; Bath salts for medical purposes; Beta-blockers; Biocides; Biological and chemical reagents for medical or veterinary use; Bronchodilators; Burn-relief medications; Calcium channel blockers; Cardiotonics; nutritional additives for use in dietary supplements, namely, carotenoids; Chemotherapeutics; Choleretics; Cholesterol reducers; Cholinergics; Cholinesterase inhibitors; Cleansing solutions for medical use; Clinical medical reagents; CNS stimulants; Contraceptives; Decongestants; Dermatologicals; Diagnostic preparations for medical purposes; Dietary and nutritional supplements; Diuretics; Dopamine receptor agonists; Enzyme cofactors; Estrogens; Expectorants; Food supplements; Gastric and pancreatic preparations; Germicides; Glucosidase inhibitors; Gonad-stimulating principles; Hematinics; Hematopoietics; Hemostatics; Hepatoprotectants; Herbal products, namely, aromatherapy packs containing herbs used for relief from headaches, insomnia and sinus discomfort; Homeopathic supplements; Immunomodulators; Immunosuppressants; Laxatives; Medicinal herbal extracts; Mineral nutritional supplements; Mineralocorticoids; Mixed vitamin preparations; Mucolytics; Multi-vitamin preparations; Muscle relaxants; Mydriatics; Neutraceuticals for use as dietary supplements; Nootropics; Nutritional additives to foodstuffs for animals, for medical purposes; Nutritional additives for medical purposes for use in foods and dietary supplements for human consumption; Plant extracts for medical, veterinary and pharmaceutical purposes; Processed food adapted for medical purposes; Progestogens; Prolactin inhibitors; Prostaglandins and prostaglandin analogs; dietary supplements; Respiratory stimulants; Reverse transcriptase inhibitors; Sedatives; Seratonin receptor agonists and antagonists; Soy protein for use as a nutritional ingredient in various powdered and ready-to-drink beverages; medicated topical skin protectants for medical use; Vasodilators; Vasoprotectants; Vitamin and mineral preparations for use as ingredients in the food and pharmaceutical industry; Yeast or yeast extracts for medical, veterinary or pharmaceutical purposes |
Trademark Filing History
| Description | Date | Proceeding Number |
|---|---|---|
| ABANDONMENT NOTICE MAILED - FAILURE TO RESPOND | 2011-10-21 | |
| ABANDONMENT - FAILURE TO RESPOND OR LATE RESPONSE | 2011-10-20 | |
| FINAL REFUSAL MAILED | 2011-03-14 | |
| SU - FINAL REFUSAL - WRITTEN | 2011-03-13 | 76731 |
| NON-FINAL ACTION MAILED | 2010-08-12 | |
| SU - NON-FINAL ACTION - WRITTEN | 2010-08-11 | 76731 |
| ASSIGNED TO EXAMINER | 2010-07-21 | 76731 |
| CORRESPONDENCE RECEIVED IN LAW OFFICE | 2010-07-20 | 73296 |
| AMENDMENT FROM APPLICANT ENTERED | 2010-07-20 | 73296 |
| PAPER RECEIVED | 2010-07-19 | |
| NON-FINAL ACTION MAILED | 2010-07-01 | |
| SU - NON-FINAL ACTION - WRITTEN | 2010-06-30 | 81876 |
| STATEMENT OF USE PROCESSING COMPLETE | 2010-06-08 | 76873 |
| CASE ASSIGNED TO INTENT TO USE PARALEGAL | 2010-06-05 | 76873 |
| USE AMENDMENT FILED | 2010-05-19 | 76873 |
| TEAS VOLUNTARY AMENDMENT RECEIVED | 2010-05-19 | |
| TEAS STATEMENT OF USE RECEIVED | 2010-05-19 | |
| TEAS EXTENSION RECEIVED | 2009-12-01 | |
| SOU EXTENSION 2 GRANTED | 2009-12-01 | 98765 |
| SOU EXTENSION 2 FILED | 2009-12-01 | 98765 |
| TEAS EXTENSION RECEIVED | 2009-04-28 | |
| SOU EXTENSION 1 GRANTED | 2009-04-28 | 98765 |
| SOU EXTENSION 1 FILED | 2009-04-28 | 98765 |
| NOA MAILED - SOU REQUIRED FROM APPLICANT | 2008-12-02 | |
| PUBLISHED FOR OPPOSITION | 2008-09-09 | |
| NOTICE OF PUBLICATION | 2008-08-20 | |
| LAW OFFICE PUBLICATION REVIEW COMPLETED | 2008-08-05 | 73296 |
| APPROVED FOR PUB - PRINCIPAL REGISTER | 2008-07-30 | |
| EXAMINERS AMENDMENT MAILED | 2008-07-14 | |
| EXAMINERS AMENDMENT -WRITTEN | 2008-07-14 | 81876 |
| EXAMINER'S AMENDMENT ENTERED | 2008-07-14 | 88888 |
| EXAMINERS AMENDMENT MAILED | 2008-07-09 | |
| EXAMINERS AMENDMENT -WRITTEN | 2008-07-09 | 81876 |
| EXAMINER'S AMENDMENT ENTERED | 2008-07-09 | 88888 |
| ASSIGNED TO EXAMINER | 2008-07-02 | 81876 |
| CORRESPONDENCE RECEIVED IN LAW OFFICE | 2008-06-16 | 73296 |
| AMENDMENT FROM APPLICANT ENTERED | 2008-06-16 | 73296 |
| ASSIGNED TO LIE | 2008-06-11 | 73296 |
| FAX RECEIVED | 2008-06-10 | |
| NON-FINAL ACTION WRITTEN | 2008-01-10 | 74309 |
| NON-FINAL ACTION MAILED | 2008-01-10 | |
| ASSIGNED TO EXAMINER | 2008-01-07 | 74309 |
| APPLICATION FILING RECEIPT MAILED | 2007-10-13 | |
| NEW APPLICATION ENTERED IN TRAM | 2007-10-09 |
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.