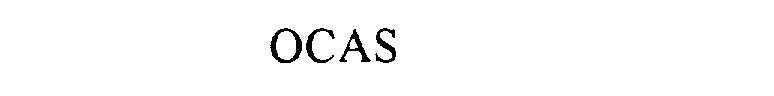OCAS
Astellas Pharma Inc.
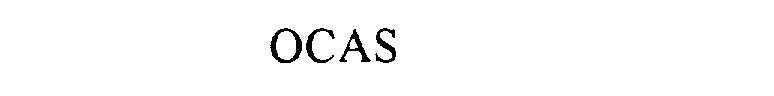
Mark For: OCAS® trademark registration is intended to cover the categories of
| Research |
|
| Serial Number | 76148225 |
| Registration Number | 3403943 |
| Mark Literal Elements | OCAS |
| Mark Drawing Type | 1 - TYPESET WORD(S) /LETTER(S) /NUMBER(S) |
| Mark Type | TRADEMARK. SERVICE MARK |
| Standard Character Claim | No |
| Register | PRINCIPAL |
| Current Location | PUBLICATION AND ISSUE SECTION 2008-04-01 |
| Basis | 44(e) |
| Class Status | SECTION 8 - CANCEL |
| Primary US Classes |
|
| Primary International Class |
|
| Filed Use | No |
| Current Use | No |
| Intent To Use | No |
| Filed ITU | Yes |
| 44D Filed | No |
| 44E Current | Yes |
| 66A Current | No |
| Current Basis | No |
| No Basis | No |
| Cancellation Code | 2 |
| Domestic Representative | Hanson Bridgett Marcus Vlahos & Rudy, LLP |
| Attorney Name | Barbara L. Friedman |
| Attorney Docket Number | 26914.1 |
| Law Office Assigned | L10 |
| Employee Name | PEREZ, STEVEN M |
Timeline
| 2000-10-16 | Application Filed |
| 2008-01-15 | Published |
| 2008-01-15 | Published for Opposition |
| 2008-04-01 | Location: PUBLICATION AND ISSUE SECTION |
| 2008-04-01 | Trademark Registered |
| 2014-11-07 | Cancelled |
| 2014-11-07 | Status: Dead/Cancelled |
| 2014-11-07 | Status: Registration cancelled because registrant did not file an acceptable declaration under Section 8. To view all documents in this |
| 2018-07-08 | Transaction Date |
Trademark Parties (Applicants & Owners)
| Party: | |
| Address | 3-11, NIHONBASHI-HONCHO 2-CHOME CHUO-KU, TOKYO JAPAN |
| Legal Entity Type | Corporation |
| Legal Entity State | JAPAN |
| Party: | |
| Address | Stanford Research Park 1050 Arastradero Road Palo Alto DE 94304 |
| Legal Entity Type | |
| Legal Entity State | JP |
*multiple parties listed, check assignment documents for ownership information
Documents
Attorney of Record
Good, Services, and Codes
(CANCELLED)
IC 005. US 006 018 044 046 051 052. G & S: CONTROLLED RELEASE DELIVERY FORMULATION SOLD AS AN INTEGRAL COMPONENT OF ORAL PHARMACEUTICAL PREPARATIONS PROVIDING CONTINUING DRUG RELEASE AND ABSORPTION FOR A PROLONGED PERIOD OF TIME FOR THE TREATMENT OF DISEASE OR HEALTH-RELATED CONDITIONS INVOLVING BLOOD AND BLOOD-FORMING ORGANS, THE CARDIAC CIRCULATORY SYSTEMS, THE PERIPHERAL VASCULAR CIRCULATORY SYSTEM, THE ENDOCRINE SYSTEM, THE GENITOURINARY SYSTEM, THE IMMUNE SYSTEM, INFECTIOUS AND PARASIT
IC CONDITIONS, THE UPPER AND LOWER DIGESTIVE SYSTEM, MENTAL DISORDERS, METABOL
IC DISORDERS, MULTI-SYSTEM AND ILL DEFINED CONDITIONS, MUSCULOSKELETAL SYSTEMS AND CONNECTIVE TISSUE, NEOPLASMS, THE NERVOUS SYSTEM, NUTRITIONAL DISORDERS, PREGNANCY, THE RESPIRATORY SYSTEM, SENSORY ORGAN CONDITIONS, SKIN AND SUBCUTANEOUS CONDITIONS(CANCELLED)
IC 040. US 100 103 106. G & S: MANUFACTURE OF ORAL CONTROLLED- RELEASE PHARMACEUTICAL PRODUCTS TO THE ORDER OR SPECIFICATION OF OTHERS
| International Codes: | 5 |
| U.S. Codes: | 006,018,044,046,051,052 |
| International Codes: | 40 |
| U.S. Codes: | 100,103,106 |
| Type Code | Type |
|---|---|
| GS0051 | CONTROLLED RELEASE DELIVERY FORMULATION SOLD AS AN INTEGRAL COMPONENT OF ORAL PHARMACEUTICAL PREPARATIONS PROVIDING CONTINUING DRUG RELEASE AND ABSORPTION FOR A PROLONGED PERIOD OF TIME FOR THE TREATMENT OF DISEASE OR HEALTH-RELATED CONDITIONS INVOLVING BLOOD AND BLOOD-FORMING ORGANS, THE CARDIAC CIRCULATORY SYSTEMS, THE PERIPHERAL VASCULAR CIRCULATORY SYSTEM, THE ENDOCRINE SYSTEM, THE GENITOURINARY SYSTEM, THE IMMUNE SYSTEM, INFECTIOUS AND PARASITIC CONDITIONS, THE UPPER AND LOWER DIGESTIVE SYSTEM, MENTAL DISORDERS, METABOLIC DISORDERS, MULTI-SYSTEM AND ILL DEFINED CONDITIONS, MUSCULOSKELETAL SYSTEMS AND CONNECTIVE TISSUE, NEOPLASMS, THE NERVOUS SYSTEM, NUTRITIONAL DISORDERS, PREGNANCY, THE RESPIRATORY SYSTEM, SENSORY ORGAN CONDITIONS, SKIN AND SUBCUTANEOUS CONDITIONS |
Trademark Filing History
| Description | Date | Proceeding Number |
|---|---|---|
| CANCELLED SEC. 8 (6-YR) | 2014-11-07 | |
| REGISTERED-PRINCIPAL REGISTER | 2008-04-01 | |
| TEAS CHANGE OF CORRESPONDENCE RECEIVED | 2008-03-11 | |
| PUBLISHED FOR OPPOSITION | 2008-01-15 | |
| NOTICE OF PUBLICATION | 2007-12-26 | |
| LAW OFFICE PUBLICATION REVIEW COMPLETED | 2007-12-12 | 78288 |
| APPROVED FOR PUB - PRINCIPAL REGISTER | 2007-12-11 | |
| ASSIGNMENT OF OWNERSHIP NOT UPDATED AUTOMATICALLY | 2007-10-24 | |
| ASSIGNMENT OF OWNERSHIP NOT UPDATED AUTOMATICALLY | 2007-08-06 | |
| TEAS/EMAIL CORRESPONDENCE ENTERED | 2007-08-02 | 78288 |
| CORRESPONDENCE RECEIVED IN LAW OFFICE | 2007-08-02 | 78288 |
| ASSIGNED TO LIE | 2007-08-01 | 78288 |
| TEAS RESPONSE TO OFFICE ACTION RECEIVED | 2007-07-17 | |
| TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED | 2007-05-04 | |
| ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED | 2007-05-04 | |
| PREVIOUS ALLOWANCE COUNT WITHDRAWN | 2007-02-08 | |
| NON-FINAL ACTION WRITTEN | 2007-02-08 | 74301 |
| NON-FINAL ACTION E-MAILED | 2007-02-08 | 6325 |
| ASSIGNED TO EXAMINER | 2007-02-08 | 74290 |
| ATTORNEY REVIEW COMPLETED | 2007-02-07 | 74301 |
| ASSIGNED TO EXAMINER | 2007-01-23 | 74301 |
| TEAS CHANGE OF CORRESPONDENCE RECEIVED | 2006-12-27 | |
| ATTORNEY REVIEW/DECISION ON AMENDMENT REQUIRED | 2006-11-21 | |
| AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP | 2006-10-04 | |
| 1(B) BASIS DELETED; NEW BASIS REQUESTED | 2006-09-15 | 67832 |
| PETITION TO DIRECTOR - CHANGE BASIS - GRANTED | 2006-08-30 | 67832 |
| PETITION TO DIRECTOR - CHANGE BASIS - RECEIVED | 2006-08-07 | 67832 |
| PAPER RECEIVED | 2006-08-07 | |
| AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP | 2006-04-05 | |
| TEAS CHANGE OF CORRESPONDENCE RECEIVED | 2006-03-30 | |
| ASSIGNED TO EXAMINER | 2006-02-17 | 74301 |
| NOTICE OF ALLOWANCE CANCELLED | 2006-01-31 | 67832 |
| TEAS EXTENSION RECEIVED | 2006-01-30 | |
| SOU EXTENSION 5 GRANTED | 2006-01-30 | 98765 |
| SOU EXTENSION 5 FILED | 2006-01-30 | 98765 |
| SOU EXTENSION 4 GRANTED | 2005-08-12 | 61813 |
| TEAS EXTENSION RECEIVED | 2005-08-05 | |
| SOU EXTENSION 4 FILED | 2005-08-05 | 61813 |
| SOU EXTENSION 3 GRANTED | 2005-02-23 | 71427 |
| TEAS EXTENSION RECEIVED | 2005-02-02 | |
| SOU EXTENSION 3 FILED | 2005-02-02 | 71427 |
| TEAS CHANGE OF CORRESPONDENCE RECEIVED | 2004-10-20 | |
| SOU EXTENSION 2 GRANTED | 2004-08-31 | |
| CASE FILE IN TICRS | 2004-08-18 | |
| PAPER RECEIVED | 2004-08-02 | |
| SOU EXTENSION 2 FILED | 2004-07-30 | |
| SOU EXTENSION 1 GRANTED | 2004-02-22 | |
| SOU EXTENSION 1 FILED | 2004-02-05 | |
| PAPER RECEIVED | 2004-02-05 | |
| NOA MAILED - SOU REQUIRED FROM APPLICANT | 2003-08-05 | |
| PUBLISHED FOR OPPOSITION | 2003-05-13 | |
| NOTICE OF PUBLICATION | 2003-04-23 | |
| APPROVED FOR PUB - PRINCIPAL REGISTER | 2002-02-11 | |
| CORRESPONDENCE RECEIVED IN LAW OFFICE | 2001-08-20 | |
| NON-FINAL ACTION MAILED | 2001-03-13 | |
| ASSIGNED TO EXAMINER | 2001-03-03 | 74290 |
| ASSIGNED TO EXAMINER | 2001-02-21 | 67443 |
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.