Patient support
Ghodsi , et al. November 17, 2
U.S. patent number D901,940 [Application Number D/664,890] was granted by the patent office on 2020-11-17 for patient support. This patent grant is currently assigned to Stryker Corporation. The grantee listed for this patent is Stryker Corporation. Invention is credited to Seyed Behrad Ghodsi, Justin Jon Raymond.
| United States Patent | D901,940 |
| Ghodsi , et al. | November 17, 2020 |
Patient support
Claims
CLAIM The ornamental design for a patient support, as shown and described.
| Inventors: | Ghodsi; Seyed Behrad (Portage, MI), Raymond; Justin Jon (Jackson, MI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | Stryker Corporation (Kalamazoo,
MI) |
||||||||||
| Appl. No.: | D/664,890 | ||||||||||
| Filed: | September 28, 2018 |
| Current U.S. Class: | D6/601; D24/184 |
| Current International Class: | 0609 |
| Field of Search: | ;D6/595,596,601,604,718.26 ;D30/118 ;D23/277 ;D21/809 ;D24/183,184 |
References Cited [Referenced By]
U.S. Patent Documents
| 1235669 | August 1917 | Eggers |
| 3254348 | June 1966 | Addario |
| D207081 | February 1967 | Katzfey et al. |
| 3940811 | March 1976 | Tomikawa et al. |
| D246816 | January 1978 | Tam et al. |
| 4336621 | June 1982 | Schwartz et al. |
| D275322 | August 1984 | Nakao et al. |
| 4605582 | August 1986 | Sias et al. |
| D285512 | September 1986 | Bool |
| 4665573 | May 1987 | Fiore |
| 4947500 | August 1990 | Seiler |
| 4964183 | October 1990 | LaForce, Jr. |
| 5007124 | April 1991 | Raburn et al. |
| 5109559 | May 1992 | West |
| 5179742 | January 1993 | Oberle |
| 5180619 | January 1993 | Landi et al. |
| 5280657 | January 1994 | Stagg |
| D353446 | December 1994 | Wheeler |
| D354876 | January 1995 | Pace |
| 5482355 | January 1996 | Franzen, Jr. |
| 5491854 | February 1996 | Music |
| 5611096 | March 1997 | Bartlett et al. |
| 5617595 | April 1997 | Landi et al. |
| 5630238 | May 1997 | Weismiller et al. |
| 5794289 | August 1998 | Wortman et al. |
| D408539 | April 1999 | Simpkins |
| D419495 | January 2000 | Muhanna |
| 6115861 | September 2000 | Reeder et al. |
| 6269504 | August 2001 | Romano et al. |
| 6286166 | September 2001 | Henley et al. |
| 6367106 | April 2002 | Gronsman |
| D461900 | August 2002 | Siepmann et al. |
| 6430766 | August 2002 | Henley et al. |
| D483088 | December 2003 | Zheng |
| 6658676 | December 2003 | Persson et al. |
| D486344 | February 2004 | Ku |
| 6687937 | February 2004 | Harker |
| D487373 | March 2004 | Ku |
| 6701556 | March 2004 | Romano et al. |
| 6701558 | March 2004 | VanSteenburg |
| 6718584 | April 2004 | Rabaiotti et al. |
| 6735799 | May 2004 | Ellis et al. |
| 6829796 | December 2004 | Salvatini et al. |
| D501112 | January 2005 | Kasatshko |
| D502350 | March 2005 | O'Reagan |
| 7060213 | June 2006 | Pearce |
| D524086 | July 2006 | Garrigues |
| 7191480 | March 2007 | Romano et al. |
| 7191482 | March 2007 | Romano et al. |
| D551484 | September 2007 | Kasatshko |
| D552738 | October 2007 | Helfet |
| D555250 | November 2007 | Wells |
| D574960 | August 2008 | Parrish |
| 7461425 | December 2008 | Chambers et al. |
| 7464425 | December 2008 | Chambers et al. |
| 7469432 | December 2008 | Chambers |
| 7469436 | December 2008 | Meyer et al. |
| 7480953 | January 2009 | Romano et al. |
| D604421 | November 2009 | Albrecht et al. |
| 7617555 | November 2009 | Romano et al. |
| 7641623 | January 2010 | Biondo et al. |
| 7648392 | January 2010 | Chambers et al. |
| 7666341 | February 2010 | Pearce |
| 7685664 | March 2010 | Stolpmann et al. |
| 7712164 | May 2010 | Chambers |
| 7712171 | May 2010 | Butler |
| 7802332 | September 2010 | Kummer et al. |
| 7886386 | February 2011 | Balonick et al. |
| 7904976 | March 2011 | Hakamiun et al. |
| 7914611 | March 2011 | Vrzalik et al. |
| 7966680 | June 2011 | Romano et al. |
| 8069514 | December 2011 | Poulos et al. |
| D652665 | January 2012 | Bamford |
| 8090478 | January 2012 | Skinner et al. |
| 8108957 | February 2012 | Richards et al. |
| 8117701 | February 2012 | Bobey et al. |
| 8118920 | February 2012 | Vrzalik et al. |
| 8196241 | June 2012 | Balonick et al. |
| 8201292 | June 2012 | Dionne et al. |
| 8332979 | December 2012 | Flick et al. |
| D674499 | January 2013 | Palm |
| 8372182 | February 2013 | Vrzalik et al. |
| 8397326 | March 2013 | Lafleche et al. |
| 8413277 | April 2013 | Davis et al. |
| 8434748 | May 2013 | Pearce et al. |
| 8437876 | May 2013 | Receveur et al. |
| 8490233 | July 2013 | Essers |
| 8572778 | November 2013 | Newkirk et al. |
| 8601620 | December 2013 | Romano et al. |
| 8620477 | December 2013 | Skinner et al. |
| 8628067 | January 2014 | Pearce et al. |
| 8661588 | March 2014 | Leach |
| 8689373 | April 2014 | Caines |
| 8712591 | April 2014 | Receveur |
| 8745788 | June 2014 | Bhai |
| 8844079 | September 2014 | Skinner et al. |
| 8845562 | September 2014 | Receveur et al. |
| 8918930 | December 2014 | Stroh et al. |
| 8919750 | December 2014 | Pearce et al. |
| D723839 | March 2015 | Bond et al. |
| 9009892 | April 2015 | Lachenbruch et al. |
| 9049943 | June 2015 | Caminade et al. |
| D735876 | August 2015 | Wolff |
| 9107511 | August 2015 | Skinner et al. |
| D745682 | December 2015 | Ziegler |
| 9320664 | April 2016 | Newkirk et al. |
| 9358168 | June 2016 | Williamson et al. |
| 9414977 | August 2016 | Ponsi et al. |
| 9462893 | October 2016 | Romano et al. |
| 9526348 | December 2016 | Richards et al. |
| 9526349 | December 2016 | Lafleche et al. |
| 9549619 | January 2017 | Hashiba |
| 9655457 | May 2017 | Meyer et al. |
| 9707141 | July 2017 | Bobey et al. |
| 9737153 | August 2017 | Chaffee |
| 9782312 | October 2017 | Brubaker |
| 9849051 | December 2017 | Newkirk et al. |
| 9907408 | March 2018 | Vrzalik et al. |
| 9907718 | March 2018 | Weitzel et al. |
| 9913770 | March 2018 | Lachenbruch et al. |
| D816237 | April 2018 | Roberts |
| 9943172 | April 2018 | Lachenbruch et al. |
| D839982 | February 2019 | Berenson |
| D855817 | August 2019 | Kessler |
| D877915 | March 2020 | Ghodsi |
| D879966 | March 2020 | Ghodsi |
| D888964 | June 2020 | Ghodsi |
| 2005/0034230 | February 2005 | Weedling et al. |
| 2006/0005314 | January 2006 | Lee |
| 2006/0021133 | February 2006 | Davis |
| 2007/0143928 | June 2007 | Biggie et al. |
| 2008/0040860 | February 2008 | Price et al. |
| 2009/0013470 | January 2009 | Richards et al. |
| 2010/0076356 | March 2010 | Biondo et al. |
| 2010/0212087 | August 2010 | Leib et al. |
| 2011/0163885 | July 2011 | Poulos et al. |
| 2013/0014324 | January 2013 | Receveur et al. |
| 2013/0231596 | September 2013 | Hornbach et al. |
| 2014/0031730 | January 2014 | Hornbach et al. |
| 2015/0074914 | March 2015 | Caminade |
| 2015/0164720 | June 2015 | Gibson et al. |
| 2016/0157631 | June 2016 | Milnes et al. |
| 2016/0184154 | June 2016 | Lafleche et al. |
| 2016/0326674 | November 2016 | Goijarts et al. |
| 2016/0361215 | December 2016 | Gibson et al. |
| 2017/0027791 | February 2017 | McKnight et al. |
| 2017/0151113 | June 2017 | Lachenbruch et al. |
| 2017/0251824 | September 2017 | Pearce |
| 2017/0251825 | September 2017 | Pearce |
| 2017/0254379 | September 2017 | Whatcott |
| 2018/0104123 | April 2018 | Newkirk et al. |
| 2018/0369038 | December 2018 | Bhimavarapu et al. |
| 2019/0201262 | July 2019 | Galer |
| 2020/0100965 | April 2020 | Lafleche |
| 2020/0100967 | April 2020 | Galer |
| 9507679 | Mar 1995 | WO | |||
| 02065877 | Aug 2002 | WO | |||
| 2013086197 | Jun 2013 | WO | |||
Other References
|
Stryker Medical IsoTour Patient Support Surface (on-line), no date available. Retrieved from Internet Feb. 11, 2020, URL: https://competition.adessignaward.com/gooddesign.php?ID=75618 (6 pages). cited by examiner . Direct Healthcare Services Ltd., "Dyna-Form Low Air Loss System User Manual", Version 01, Mar. 1, 2013, 16 pages. cited by applicant . Drive, "Med Aire Plus 8'' Alternating Pressure and Low Air Loss Mattress Replacement System Operator's Manual", Item #14029, Control Unit 14029XP), Rev. 4, Mar. 15, 2016, 20 pages. cited by applicant . English language abstract and machine-assisted English translation for CN 2251310 extracted from espacenet.com database on Feb. 15, 2019, 5 pages. cited by applicant . Gaymar Industries Inc., "Aire Twin Alternating Pressure and Low-Loss Therapy Mattress Replacement Systems ATC80, ATM500, ATM800, ATW5000, ATW8000 Operator's Manual", May 2005, 8 pages. cited by applicant . Gaymar Industries Inc., Auto Aire Select Dynamic Low-Air Loss Therapy With Alternating Pressure and Active Sensor Technology Ref C2500MES Control Unit, Ref M2500S Series Mattress, Ref X3580S Operator's Manual Rev. B, Sep. 2010, 42 pages. cited by applicant . Gaymar Industries Inc., "Auto Aire Select Dynamic Low-Air Loss Therapy With Alternating Pressure and Active Sensor Technology Ref C2500MS Control Unit, Ref M2500S Series Mattress, Ref Auto Aire Select Safety Mattress Service Manual", Rev. B, Sep. 2010, 14 pages. cited by applicant . Gaymar Industries Inc., "Clini-Dyne Rotational Therapy System CLP2000 Control Unit, CLM Series Mattress Operator's Manual", Rev. A, Oct. 2010, 8 pages. cited by applicant . Gaymar Industries Inc., "Instructions for Use Sof Care Mattress Series", Rev. A, May 2010, 12 pages. cited by applicant . Gaymar Industries Inc., "O2 Zoned Multi-Functional Portable Rotation System P4000, P4001, C4000 Series Control Unit, M4000 Series Mattress Operator's Manual", Rev. B, Oct. 2010, 12 pages. cited by applicant . Gaymar Industries Inc., "Sof Care Bed Cushions Instructions for Use", Sep. 1993, 2 pages. cited by applicant . Gaymar Industries Inc., "Sof Matt Low Air Loss Mattress System Operator's Manual/Service Manual", Oct. 2010, 40 pages. cited by applicant . Gaymar Industries Inc., "Symmetric Aire Mattress Ref SYM3000 Series Instructions for Use", Apr. 2012, 3 pages. cited by applicant . Hill-Rom, "300 Wound Surface Prevention and Treatment Surfaces Brochure", Rev. 2, May 18, 2010, 2 pages. cited by applicant . Hill-Rom, "Hill-Rom P500 Therapy Surface Product No. P005723 and P005787 User Manual", Rev. 3, 2010, 54 pages. cited by applicant . Hill-Rom, Synergy Air Elite Low Air Loss Therapy Brochure, Rev. 2, Dec. 30, 2008, 4 pages. cited by applicant . Hill-Rom, "Synergy Air Elite Low Air Loss Therapy Webpage", https://homecare.hill-rom.com/en/durable-medical-equipment/therapy-mattre- sses/therapy-mattresses/synergy-air-elite-low-air-loss-therapy/, 2019, 2 pages. cited by applicant . Invacare Corporation, "MicroAir MA90 Series-Owner's Operator and Maintenance Manual", 2008, 28 pages. cited by applicant . Kap Medical, "Digital Alternating Pressure True LAL System Brochure", 2013, 2 pages. cited by applicant . Molnlycke Health Care AB, "Turning and Positioning Webpage", http://molnlycke.us/products-solutions/turning-and-positioning-system/ formerly known as http://sundancesolutions.com/, 2016, 2 pages. cited by applicant . Sage Products, "Pressure Injury and Safe Patient Handling Solutions--Prevalon Mobile Air Transfer Systems; Prevalon Turn & Position Systems; Prevalon Heel Protectors; Prevalon Seated Positioning System Brochure", 2017, 20 pages. cited by applicant . Sage Products, "Prevalon AirTap Patient Repositioning System Operator's Manual", 2016, 10 pages. cited by applicant . Sage Products, "Prevalon AirTap XXL Patient Repositioning System Brochure", 2018, 4 pages. cited by applicant . Sage Products, "Prevalon Standard Turn and Position System Instructions for Use", 2016, 1 page. cited by applicant . Sage Products, "Prevalon Turn and Position System 2.0 Instructions for Use", 2016, 1 page. cited by applicant . Sage Products, "Sage Product Catalog--Pressure Injury Prevention and Safe Patient Handling", 2018, 4 pages. cited by applicant . Stryker Medical, "Arise 1000 EX Low Air-Loss Bariatric Surface Specification Sheet", Rev. B, 2008, 2 pages. cited by applicant . Stryker Medical, "Arise 1000EX Low Air Loss Therapy Mattress Model 2236 Operations/Maintenance Manual", Rev. B, May 2009, 30 pages. cited by applicant . Stryker Medical, "ComfortGel SE Support Surface Ref 1805 Operations Manual", Rev. C, Sep. 2016, 18 pages. cited by applicant . Stryker Medical, "ComfortGel Support Surface Ref 2850 Operations Manual", Rev. A, Aug. 2017, 22 pages. cited by applicant . Stryker Medical, "ComfortGel Support Surface Specification Sheet", Rev. E6, Dec. 2011, 2 pages. cited by applicant . Stryker Medical, "EOLE DC Powered Support Surface Ref 2871 Operations/Maintenance Manual", Dec. 2017, 288 pages. cited by applicant . Stryker Medical, "EOLE Powered Support Surface Ref 2870 Operations/Maintenance Manual", Dec. 2017, 288 pages. cited by applicant . Stryker Medical, "IsoAir Ref 2940 Service Manual", Rev. 1.0, Nov. 2016, 144 pages. cited by applicant . Stryker Medical, "IsoFlex LAL Support Surface Specification Sheet", Rev. D.3, 2016, 2 pages. cited by applicant . Stryker Medical, "IsoFlex SE Support Surface Ref 1806 Operations Manual", Rev. C, Sep. 2016, 20 pages. cited by applicant . Stryker Medical, "IsoGel Air Support Surface Ref 2860 Operations Manual", Rev. F, May 2015, 25 pages. cited by applicant . Stryker Medical, "IsoGel LAL Support Surface Ref 2860 Operations/Maintenance Manual", Rev. C, Aug. 2017, 28 pages. cited by applicant . Stryker Medical, "IsoGel Support Surface Dimensional Gel Technology Specification Sheet", Dec. 2013, 2 pages. cited by applicant . Stryker Medical, "Isolibrium Support Surface Ref 2971 Operations/Maintenance Manual", Rev. B, Oct. 2014, 202 pages. cited by applicant . Stryker Medical, "Isolibrium Support Surface Ref 2971 Operations/Maintenance Manual", Rev. C, May 2014, 200 pages. cited by applicant . Stryker Medical, "Isolibrium Support Surface Specification Sheet", Rev. E4, Nov. 2013, 2 pages. cited by applicant . Stryker Medical, "Isolibrium Support Surface Version 4.0, Ref 2971, Ref 2972 Maintenance Manual", Rev. B, May 2017, 88 pages. cited by applicant . Stryker Medical, "Isolibrium Support Surface Version 4.0, Ref 2971, Ref 2972 Maintenance Manual", Rev. C, May 2017, 88 pages. cited by applicant . Stryker Medical, "Mattress Assembly p. 2235-100-001--Bariatric Bed Model 2230", Rev. A, 2008, 2 pages. cited by applicant . Stryker Medical, "Position Pro Therapy Mattress Model 2920 Operations/Maintenance Manual", Rev. A, Feb. 2007, 55 pages. cited by applicant . Stryker Medical, "PositionPro Patient Repositioning Support Surface-Standalone with Pendant or Integrated with FL27 InTouch CC Model Beds Ref 2920 Maintenance Manual", Rev. A, Oct. 2016, 56 pages. cited by applicant . Stryker Medical, "PositionPro Patient Repositioning Support Surface-Standalone with Pendant or Integrated with FL27 InTouch CC Model Beds Ref 2920 Maintenance Manual", Rev. G, Oct. 2016, 42 pages. cited by applicant . Stryker Medical, "Pressure Management--Dynamic Mattress System 2500 Operations/Maintenance Manual", published at least prior to Sep. 2018, 17 pages. cited by applicant . Stryker Medical, "Pressure Management--Dynamic Mattress System 2500 Operations/Maintenance Manual", published at least prior to Sep. 2018, 23 pages. cited by applicant . Stryker Medical, "ProForm Non-Powered Support Surface Model 2710 Operations/Maintenance Manual", Rev. G, Sep. 14, 2015, 134 pages. cited by applicant . Stryker Medical, "Save Simply Brochure", 2018, 2 pages. cited by applicant . Stryker Medical, "Sof Care DuoGard Overlay Brochure", 2017, 2 pages. cited by applicant . Stryker Medical, SofCare Stretcher Overlay Ref 2780-400-000, Ref 2780-500-000 Operations Manual, Rev. B, Dec. 2014, 2 pages. cited by applicant . Stryker Medical, "SPR Plus Low Air Loss Overlay 3-Layer Technology Specification Sheet", Rev. D.2, Aug. 2011, 2 pages. cited by applicant . Stryker Medical, "SPR Plus Overlay Ref 2790-100-000 Operations Manual", Rev. B, Dec. 2014, 2 pages. cited by applicant . Stryker Medical, "Stryker Air I, Stryker Air II Low Air Loss Therapy Mattress (LAL), Model 2236 Operations/Maintenance Manual", Rev. B, May 2009, 30 pages. cited by applicant . Stryker Medical, "Stryker IsoAir Ref 2940 Operation/Maintenance Manual", Rev. 4.00, Dec. 2015, 52 pages. cited by applicant . Stryker Medical, "Ultra Comfort SE Support Surface Ref. 1703; Ref. 1704 Operations Manual", Rev. D, May 2017, 40 pages. cited by applicant . Stryker Medical, "XPRT Pulmonary Therapy & Wound Care Mattress Model 2950 Operations/Maintenance Manual", Rev. C, Aug. 2007, 114 pages. cited by applicant . Stryker Medical, "XPRT Therapeutic Support Surface Ref 2950 Operations/Maintenance Manual", Rev. B, Jun. 2012, 468 pages. cited by applicant . Stryker Medical, XPRT Therapy Mattress Model 2950 Operations Manual, Rev. A, Jul. 2006, 98 pages. cited by applicant . Stryker Medical, "XPRT Therapy Mattress Model 2950 Operations/Maintenance Manual", Rev. A, May 2006, 98 pages. cited by applicant . Stryker Medical, "XPRT Therapy Mattress Model 2950 Operations/Maintenance Manual", Rev. H, Jun. 2006, 98 pages. cited by applicant . Stryker Medical,"DuoGard Overlay Ref 2780-300-000 Operations Manual", Rev. B, Dec. 2014, 1 page. cited by applicant . Design U.S. Appl. No. 29/664,884, filed Sep. 28, 2018. cited by applicant . Design U.S. Appl. No. 29/664,888, filed Sep. 28, 2018. cited by applicant . Design U.S. Appl. No. 29/664,886, filed Sep. 28, 2018. cited by applicant. |
Primary Examiner: Barnes; Kimberly
Attorney, Agent or Firm: Howard & Howard Attorneys PLLC
Description
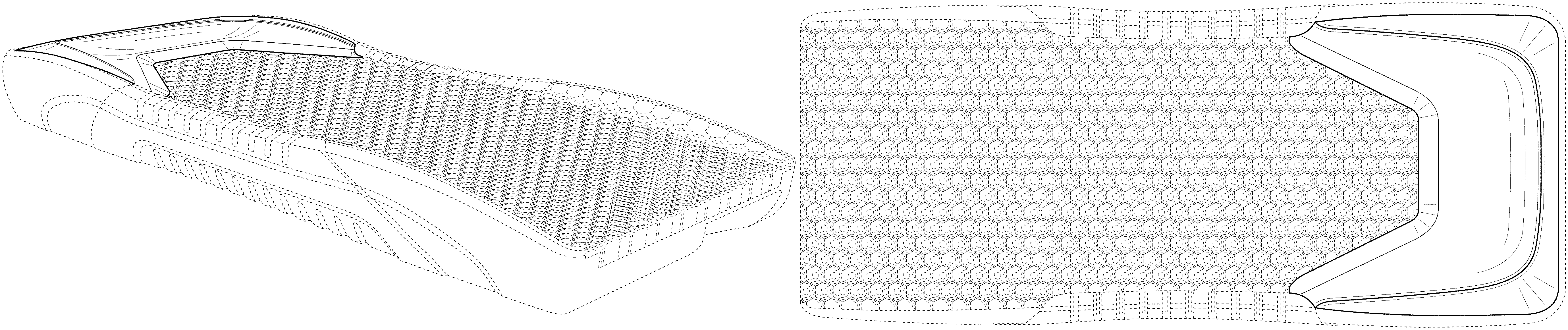
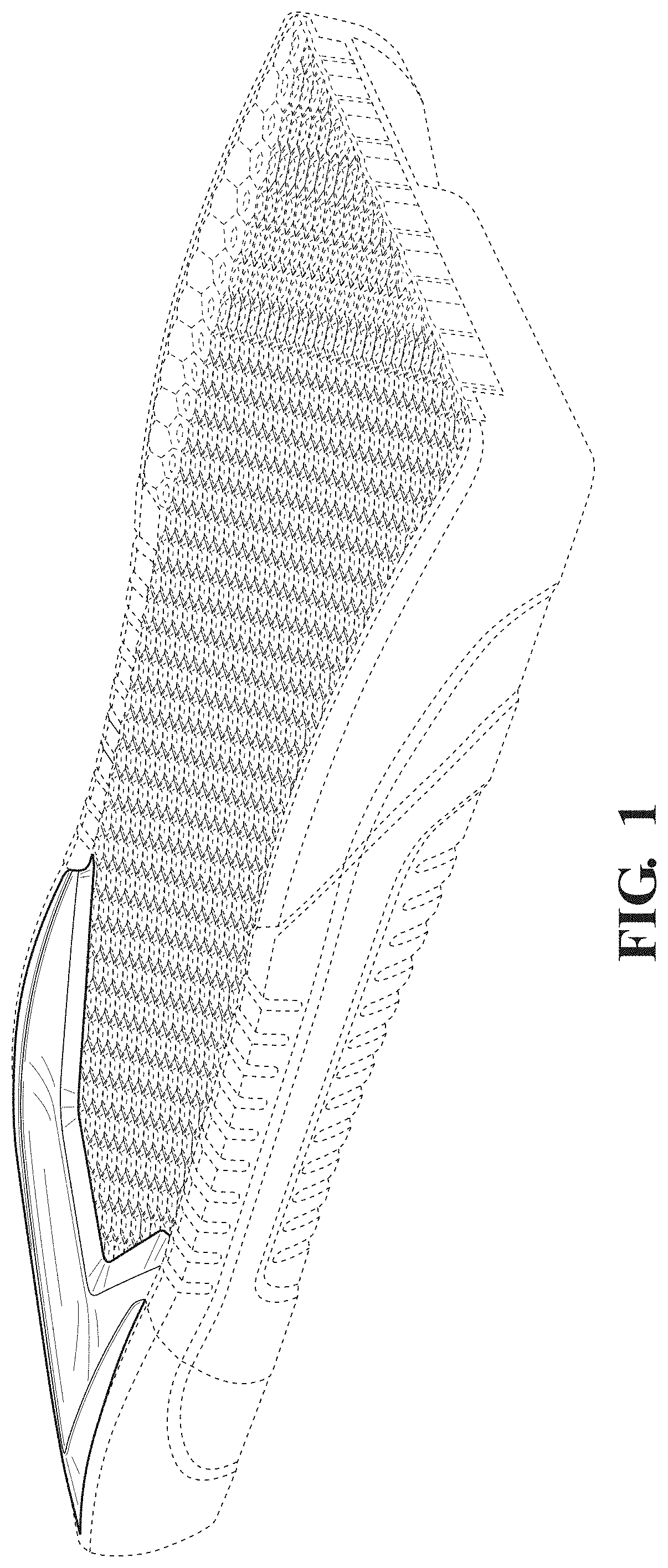
FIG. 1 is a top front, left side, perspective view of a patient support showing our new design.
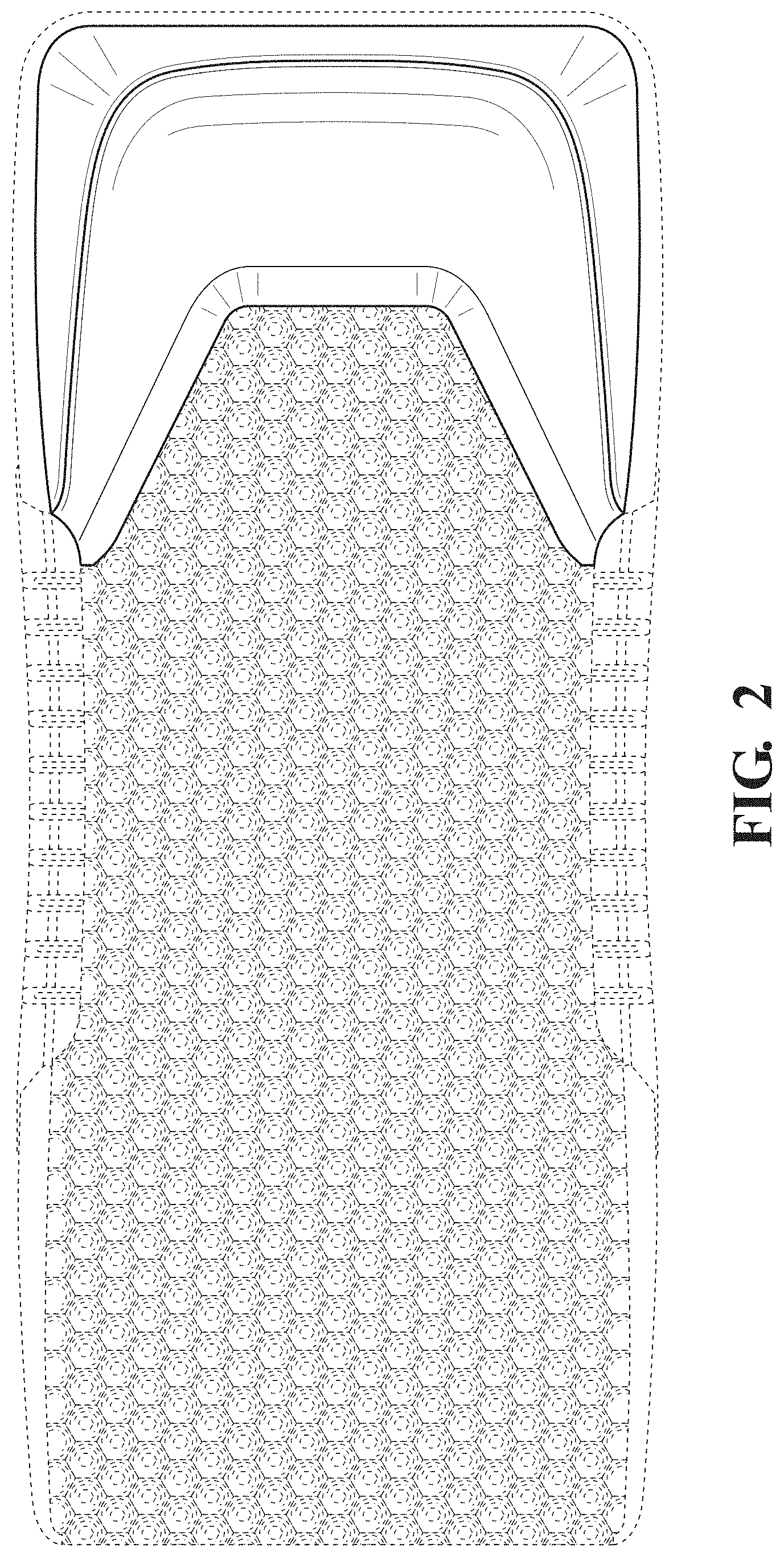
FIG. 2 is a top view thereof.
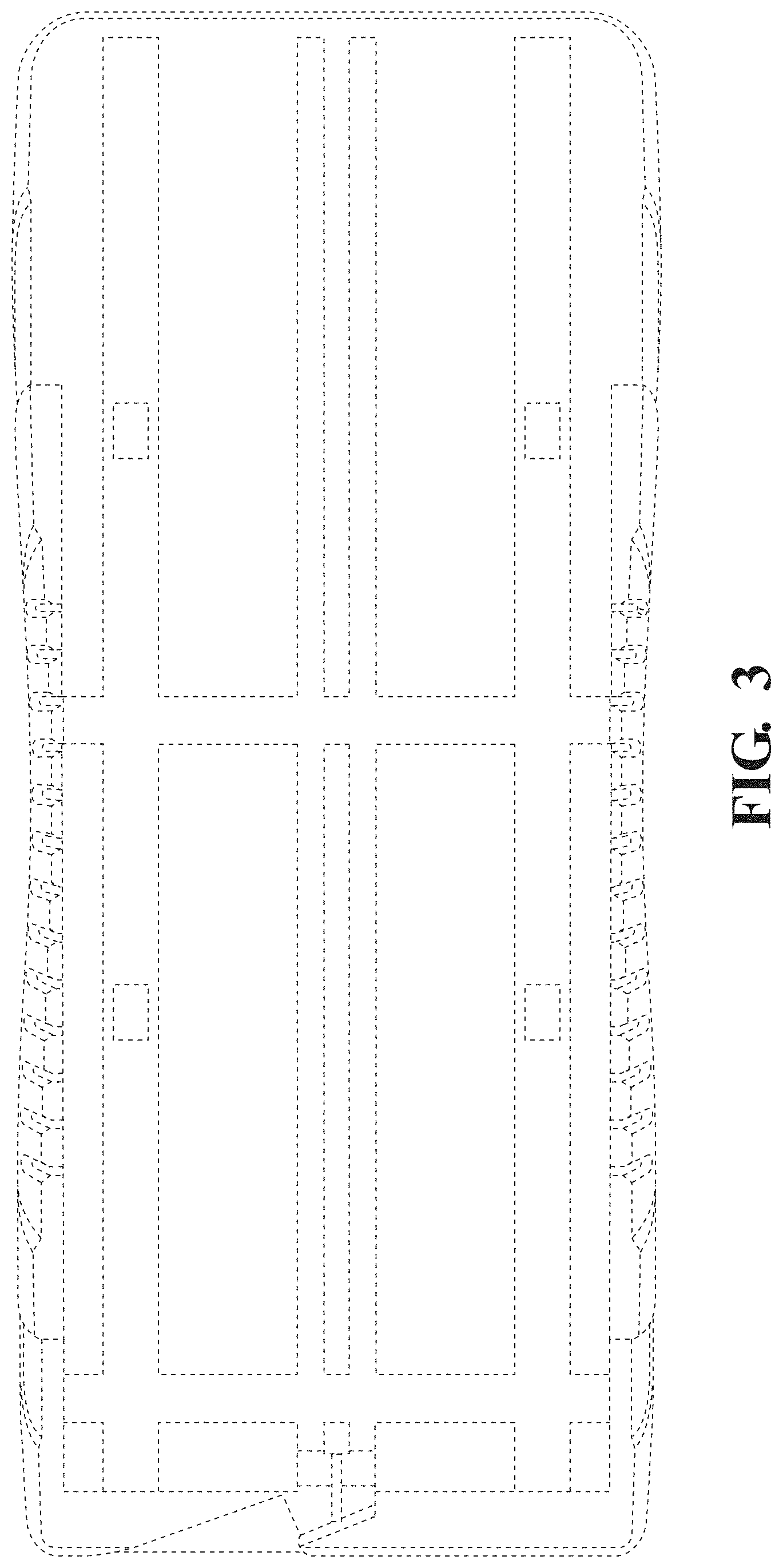
FIG. 3 is a bottom view thereof.
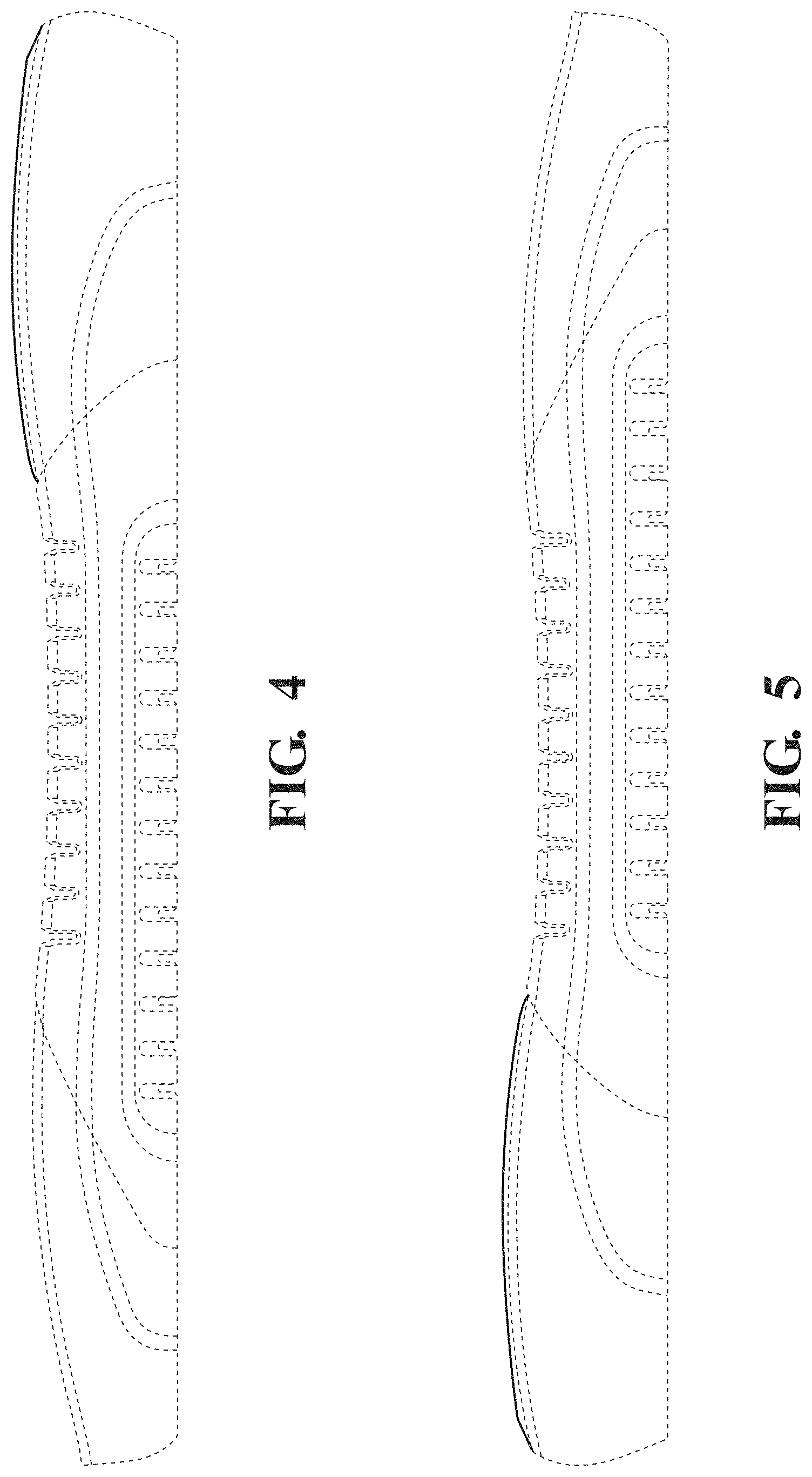
FIG. 4 is a right side view thereof.
FIG. 5 is a left side view thereof.
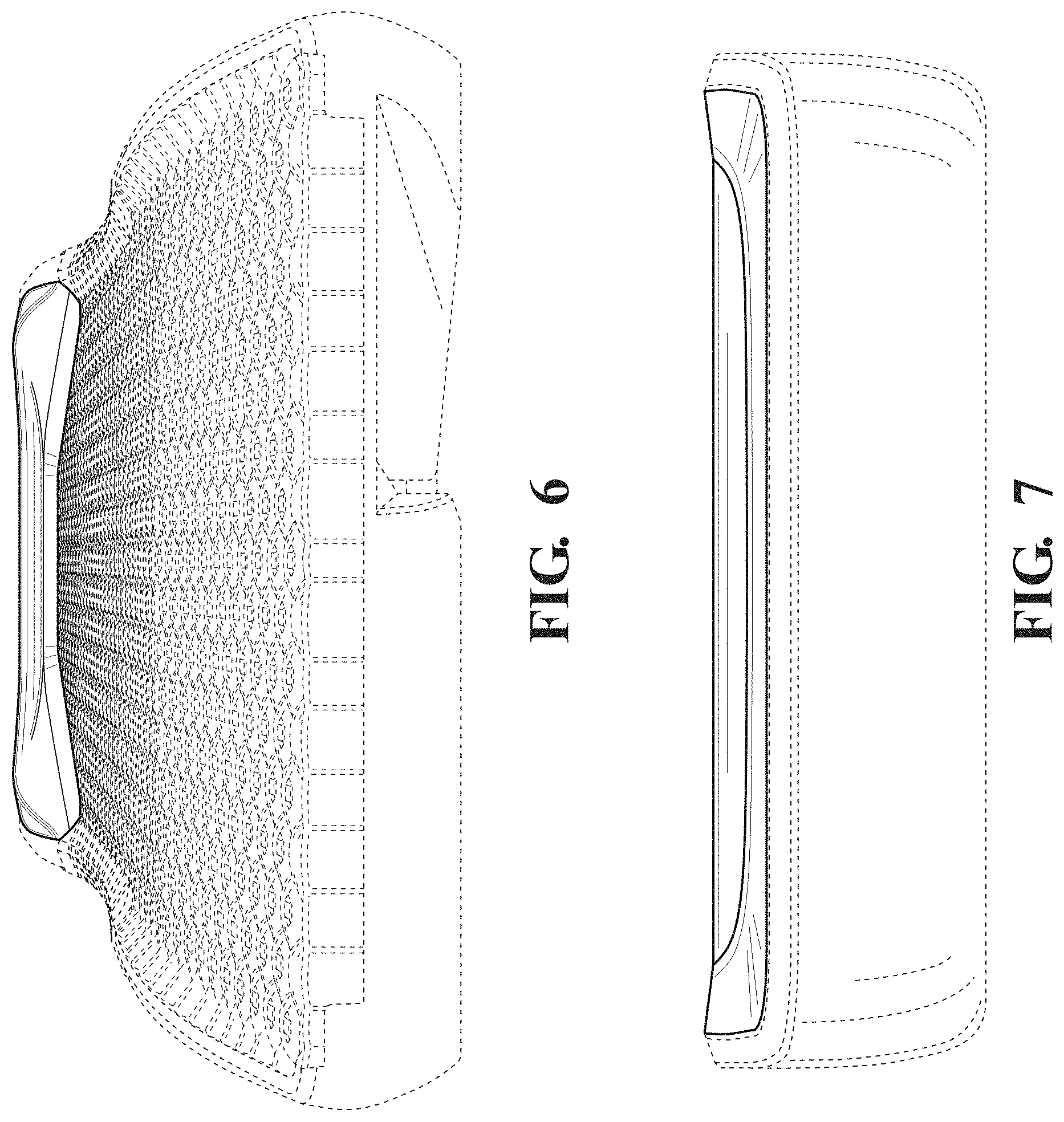
FIG. 6 is a top front, perspective view thereof; and,
FIG. 7 is a rear view thereof.
The broken lines in the drawings illustrate portions of the patient support that form no part of the claimed design.
* * * * *
References
D00000

D00001

D00002

D00003

D00004

D00005

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.