Transcutaneous electrical nerve stimulation (TENS) device
Cryan , et al. J
U.S. patent number D837,394 [Application Number D/610,296] was granted by the patent office on 2019-01-01 for transcutaneous electrical nerve stimulation (tens) device. This patent grant is currently assigned to Neurometrix, Inc.. The grantee listed for this patent is Neurometrix, Inc.. Invention is credited to Bonniejean Boettcher, Marc Cryan, Tyler Grossman.



| United States Patent | D837,394 |
| Cryan , et al. | January 1, 2019 |
Transcutaneous electrical nerve stimulation (TENS) device
Claims
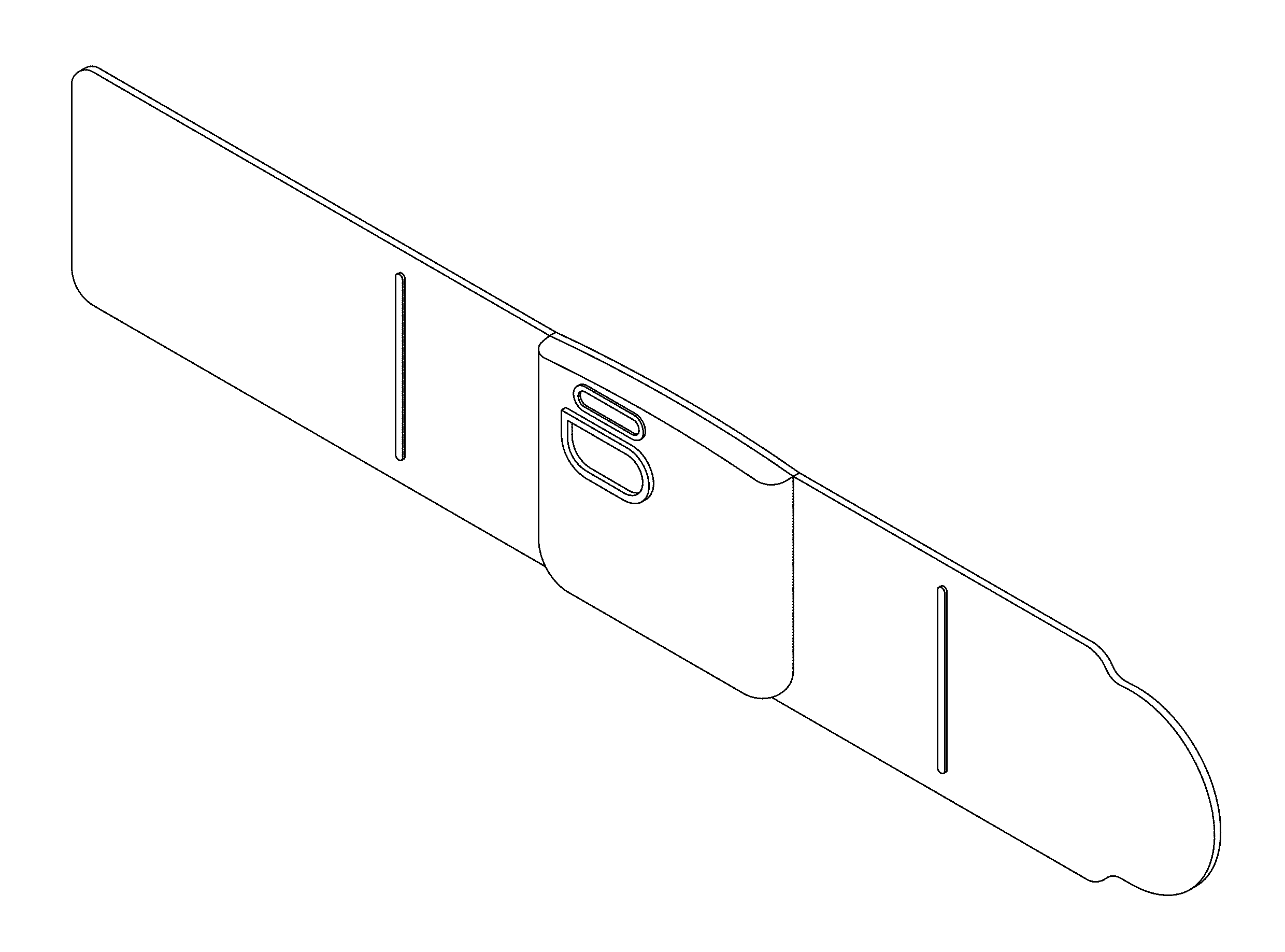
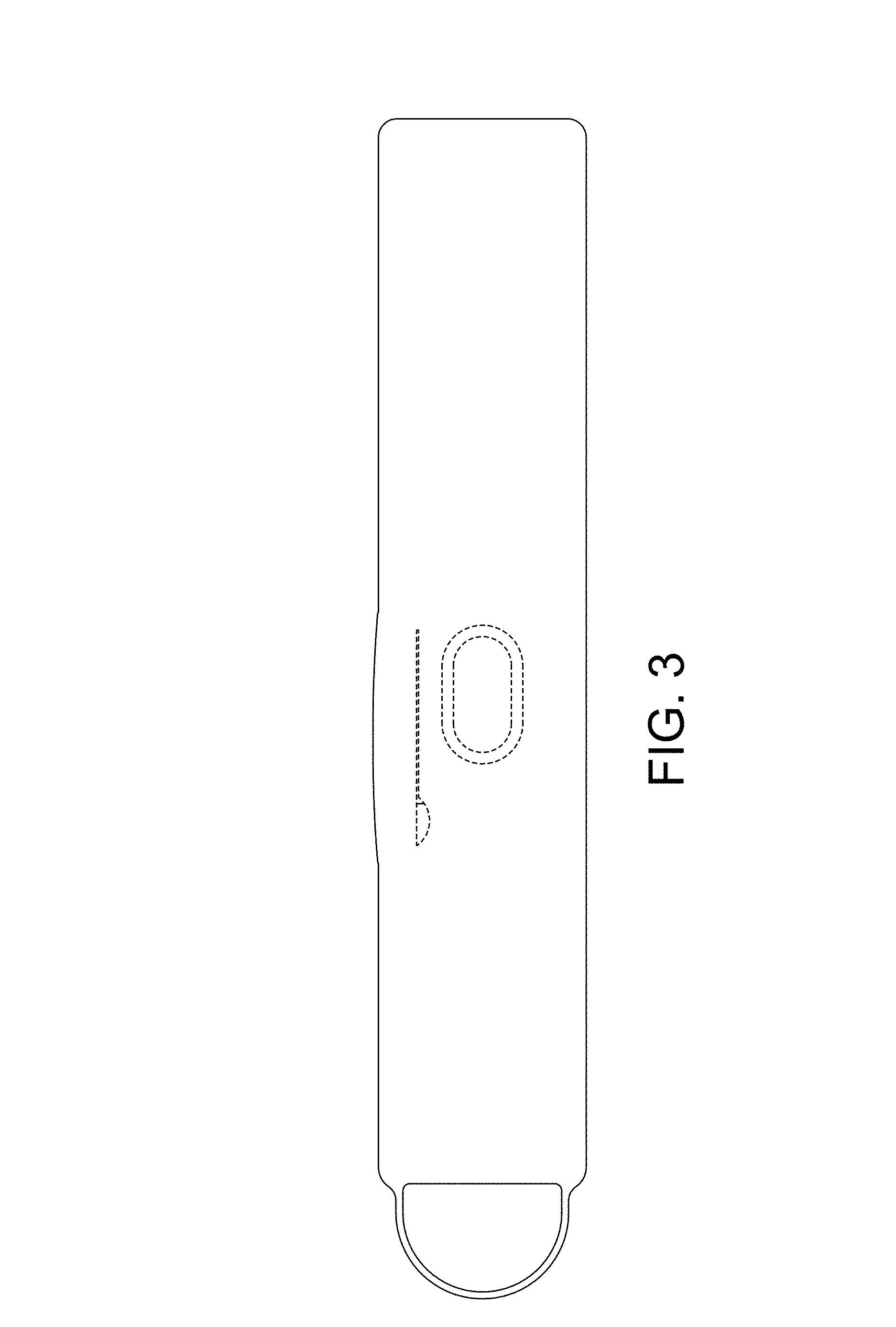
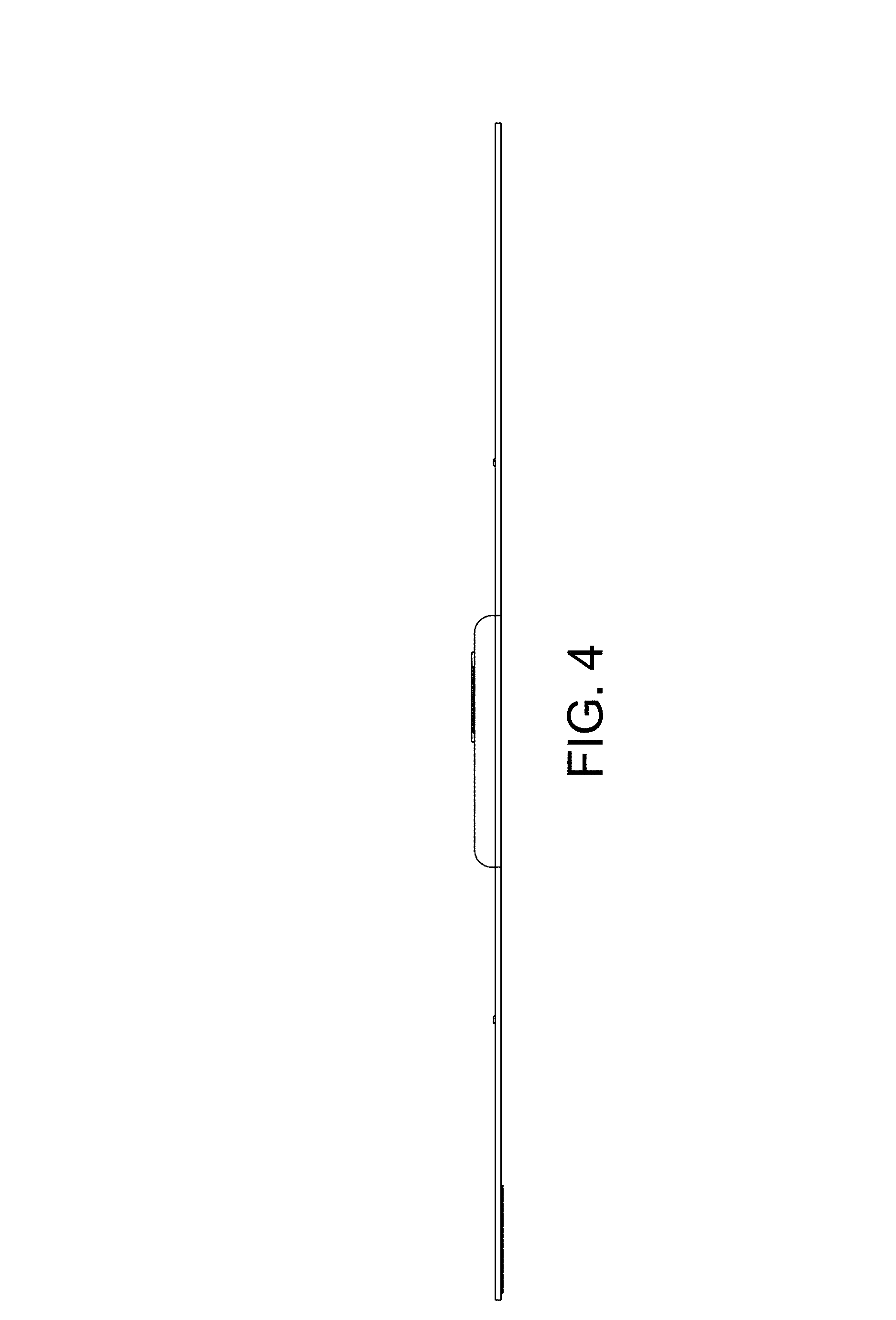
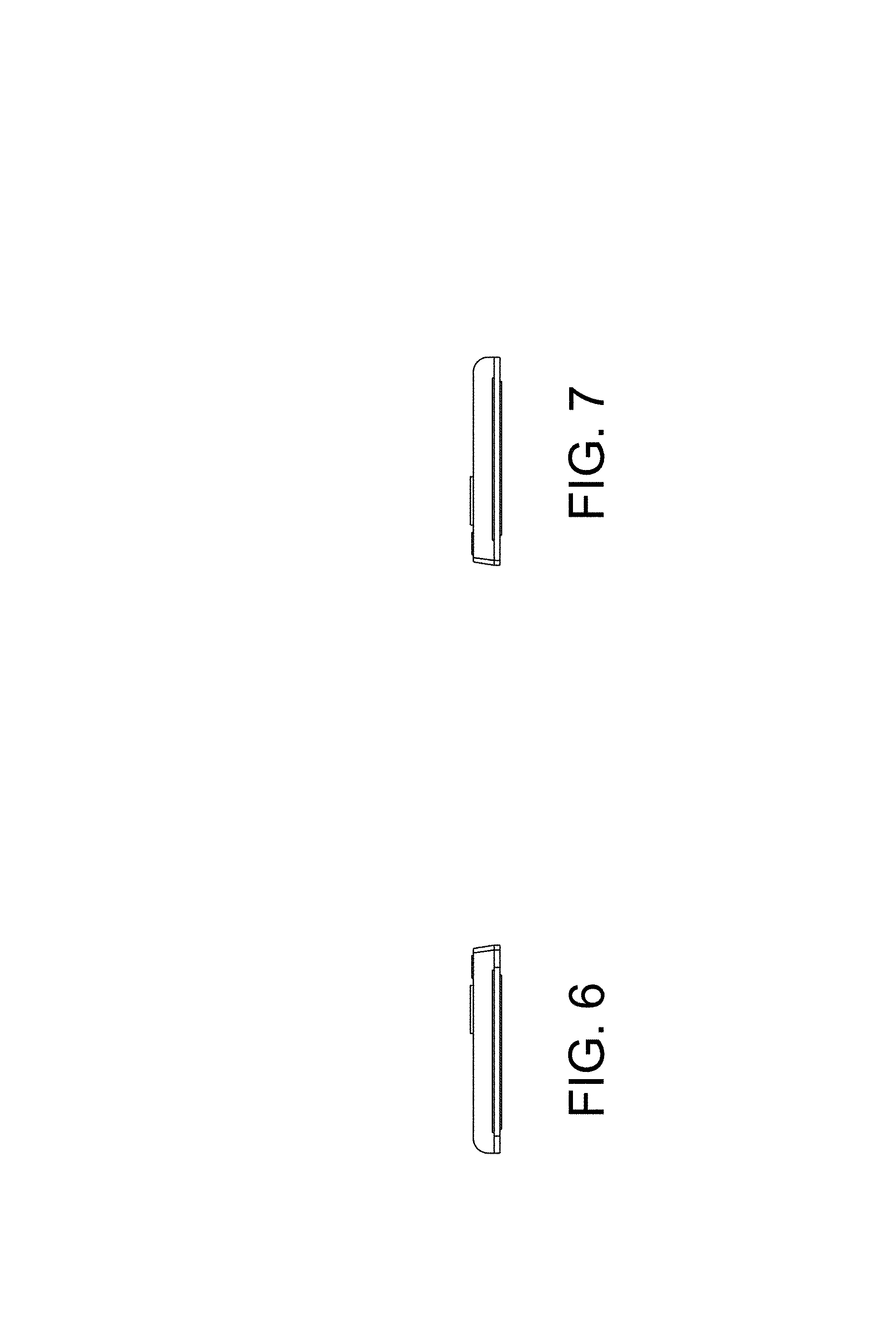
CLAIM The ornamental design for a transcutaneous electrical nerve stimulation (TENS) device, as shown and described.
| Inventors: | Cryan; Marc (Maynard, MA), Grossman; Tyler (St. Petersburg, FL), Boettcher; Bonniejean (Maynard, MA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | Neurometrix, Inc. (Waltham,
MA) |
||||||||||
| Appl. No.: | D/610,296 | ||||||||||
| Filed: | July 11, 2017 |
| Current U.S. Class: | D24/200 |
| Current International Class: | 2403 |
| Field of Search: | ;D24/171,185,186,187,188,200,201,206,207,209,215 ;D29/101.3,101.5 ;D3/205,299 ;D21/683 ;128/95.1,96.1,99.1,845,846,112.1 ;602/2,5,6,41 ;607/1,48 |
References Cited [Referenced By]
U.S. Patent Documents
| 2327874 | August 1943 | Jong |
| D243417 | February 1977 | Allen et al. |
| 4033356 | July 1977 | Hara |
| 4121573 | October 1978 | Crovella et al. |
| D255938 | July 1980 | Hawke et al. |
| 4419998 | December 1983 | Heath |
| 4503863 | March 1985 | Katims |
| 4605010 | August 1986 | McEwen |
| 4630483 | October 1986 | Engdahl |
| 4738250 | April 1988 | Fulkerson et al. |
| D299746 | February 1989 | Guldalian, Jr. |
| 5010896 | April 1991 | Westbrook |
| 5063929 | November 1991 | Bartelt et al. |
| 5125100 | June 1992 | Katznelson |
| 5169384 | December 1992 | Bosniak et al. |
| 5327902 | July 1994 | Lemmen |
| 5350414 | September 1994 | Kolen |
| 5487759 | January 1996 | Bastyr et al. |
| 5562718 | October 1996 | Palermo |
| 5755750 | May 1998 | Petruska et al. |
| 5797902 | August 1998 | Netherly |
| 5806522 | September 1998 | Katims |
| 5851191 | December 1998 | Gozani |
| D407822 | April 1999 | Davis et al. |
| 5948000 | September 1999 | Larsen et al. |
| 5991355 | November 1999 | Dhalke |
| 6132386 | October 2000 | Gozani et al. |
| 6141587 | October 2000 | Mower |
| 6146335 | November 2000 | Gozani |
| 6161044 | December 2000 | Silverstone |
| 6266558 | July 2001 | Gozani et al. |
| 6298255 | October 2001 | Cordero et al. |
| 6312392 | November 2001 | Herzon |
| 6430450 | August 2002 | Bach-y-Rita et al. |
| 6456884 | September 2002 | Kenney |
| D475138 | May 2003 | Baura et al. |
| 6662051 | December 2003 | Eraker et al. |
| D484984 | January 2004 | Takizawa |
| D534871 | January 2007 | Larsen |
| 7459984 | December 2008 | Wang et al. |
| D584414 | January 2009 | Lash |
| D598114 | August 2009 | Cryan |
| D600352 | September 2009 | Cryan |
| D609353 | February 2010 | Cryan |
| 7668598 | February 2010 | Horregraven et al. |
| 7720548 | May 2010 | King |
| 7725193 | May 2010 | Chu |
| 7760428 | July 2010 | Sieckmann |
| D625016 | October 2010 | Potts |
| 7844325 | November 2010 | Takehara |
| 7917201 | March 2011 | Gozani et al. |
| D638131 | May 2011 | Buckels et al. |
| 8108049 | January 2012 | King |
| 8121702 | February 2012 | King |
| 8131374 | March 2012 | Moore et al. |
| D669186 | October 2012 | Gozani |
| D669187 | October 2012 | Gozani |
| 8320988 | November 2012 | Axelgaard |
| 8421642 | April 2013 | Mcintosh et al. |
| D704848 | May 2014 | Thomas |
| D712052 | August 2014 | Thomas |
| D713049 | September 2014 | Shah |
| 8825175 | September 2014 | King |
| 8862238 | October 2014 | Rahimi et al. |
| 8948876 | February 2015 | Gozani et al. |
| 9168375 | October 2015 | Ratlimi et al. |
| 9173581 | November 2015 | Boettcher et al. |
| D745975 | December 2015 | Igaue |
| 9220431 | December 2015 | Holzhacker |
| D746987 | January 2016 | Okuda et al. |
| D754355 | April 2016 | Ganapathy et al. |
| D760395 | June 2016 | Barbaric et al. |
| 9452287 | September 2016 | Rosenbluth et al. |
| 9474898 | October 2016 | Gozani et al. |
| D775361 | December 2016 | Vosch |
| 9656070 | May 2017 | Gozani et al. |
| 9675801 | June 2017 | Kong et al. |
| 9730606 | August 2017 | Bianchi |
| 9731126 | August 2017 | Ferree et al. |
| D798170 | September 2017 | Toth |
| 9827420 | November 2017 | Ferree et al. |
| D810311 | February 2018 | Chen |
| D810952 | February 2018 | Hsu |
| 2002/0010497 | January 2002 | Merfeld et al. |
| 2002/0173828 | November 2002 | Gozani et al. |
| 2003/0023192 | January 2003 | Foxlin |
| 2003/0035506 | February 2003 | Tybinkowski et al. |
| 2003/0074037 | April 2003 | Moore et al. |
| 2003/0093006 | May 2003 | Wells et al. |
| 2003/0114892 | June 2003 | Nathan et al. |
| 2003/0208246 | November 2003 | Kotlik et al. |
| 2004/0017895 | January 2004 | Suzuki et al. |
| 2004/0231772 | November 2004 | Leonard et al. |
| 2005/0059903 | March 2005 | Izumi |
| 2005/0080463 | April 2005 | Stahmann et al. |
| 2005/0083527 | April 2005 | Flaherty et al. |
| 2005/0234525 | October 2005 | Phillips |
| 2006/0020291 | January 2006 | Gozani et al. |
| 2006/0052788 | March 2006 | Thelon et al. |
| 2006/0085049 | April 2006 | Cory et al. |
| 2006/0095088 | May 2006 | De Ridder |
| 2006/0173507 | August 2006 | Mrva et al. |
| 2006/0190057 | August 2006 | Reese |
| 2007/0041507 | February 2007 | Kendall et al. |
| 2007/0060922 | March 2007 | Dreyfuss |
| 2007/0129771 | June 2007 | Kurtz et al. |
| 2007/0149892 | June 2007 | Guldalian |
| 2007/0185409 | August 2007 | Wu et al. |
| 2007/0219441 | September 2007 | Carlin et al. |
| 2007/0276449 | November 2007 | Gunter et al. |
| 2008/0077192 | March 2008 | Harry et al. |
| 2008/0146980 | June 2008 | Rousso et al. |
| 2008/0147146 | June 2008 | Wahlgren et al. |
| 2008/0288026 | November 2008 | Cross et al. |
| 2008/0306400 | December 2008 | Takehara |
| 2008/0312551 | December 2008 | Fadem |
| 2008/0312709 | December 2008 | Volpe et al. |
| 2009/0030476 | January 2009 | Hargrove |
| 2009/0105795 | April 2009 | Minogue et al. |
| 2009/0112214 | April 2009 | Philippon et al. |
| 2009/0131993 | May 2009 | Rousso et al. |
| 2009/0209840 | August 2009 | Axelgaard |
| 2009/0240303 | September 2009 | Wahlstrand et al. |
| 2009/0264789 | October 2009 | Molnar et al. |
| 2009/0270947 | October 2009 | Stone et al. |
| 2009/0326604 | December 2009 | Tyler et al. |
| 2010/0004715 | January 2010 | Fahey |
| 2010/0042180 | February 2010 | Mueller et al. |
| 2010/0057149 | March 2010 | Fahey |
| 2010/0087903 | April 2010 | Van Herk et al. |
| 2010/0094103 | April 2010 | Kaplan et al. |
| 2010/0114257 | May 2010 | Torgerson |
| 2010/0128851 | May 2010 | Bailey et al. |
| 2010/0131028 | May 2010 | Hsu et al. |
| 2010/0198124 | August 2010 | Bhugra |
| 2010/0217349 | August 2010 | Fahey |
| 2010/0241464 | September 2010 | Amigo et al. |
| 2011/0066209 | March 2011 | Bodlaender et al. |
| 2011/0106214 | May 2011 | Carbunaru et al. |
| 2011/0224665 | September 2011 | Crosby et al. |
| 2011/0257468 | October 2011 | Oser et al. |
| 2011/0264171 | October 2011 | Torgerson |
| 2011/0276107 | November 2011 | Simon et al. |
| 2011/0282164 | November 2011 | Yang et al. |
| 2012/0010680 | January 2012 | Wei et al. |
| 2012/0016259 | January 2012 | Odderson |
| 2012/0108998 | May 2012 | Molnar et al. |
| 2012/0226186 | September 2012 | Baars et al. |
| 2013/0096641 | April 2013 | Strother et al. |
| 2013/0158627 | June 2013 | Gozani et al. |
| 2013/0197341 | August 2013 | Grob |
| 2013/0317333 | November 2013 | Yang |
| 2014/0039450 | February 2014 | Green et al. |
| 2014/0107729 | April 2014 | Sumners et al. |
| 2014/0163444 | June 2014 | Ingvarsson et al. |
| 2014/0206976 | July 2014 | Thompson |
| 2014/0276549 | September 2014 | Osorio |
| 2014/0296934 | October 2014 | Gozani et al. |
| 2014/0296935 | October 2014 | Ferree et al. |
| 2014/0309709 | October 2014 | Gozani et al. |
| 2014/0336730 | November 2014 | Simon et al. |
| 2014/0379045 | December 2014 | Rahimi et al. |
| 2015/0038873 | February 2015 | Boettcher et al. |
| 2015/0045853 | February 2015 | Alataris et al. |
| 2015/0148865 | May 2015 | Gozani et al. |
| 2015/0174402 | June 2015 | Thomas et al. |
| 2015/0238094 | August 2015 | Lai |
| 2015/0306387 | October 2015 | Kong et al. |
| 2015/0321000 | November 2015 | Rosenbluth et al. |
| 2015/0328467 | November 2015 | Demers et al. |
| 2015/0335288 | November 2015 | Toth et al. |
| 2016/0120425 | May 2016 | Boettcher et al. |
| 2016/0271413 | September 2016 | Vallejo et al. |
| 2017/0036015 | February 2017 | Gozani et al. |
| 2017/0056643 | March 2017 | Herb et al. |
| 2017/0188872 | July 2017 | Hughes |
| 2017/0209693 | July 2017 | An |
| 2017/0224990 | August 2017 | Goldwasser |
| 2017/0312515 | November 2017 | Ferree et al. |
| 2017/0368345 | December 2017 | Kong |
| 2018/0015285 | January 2018 | Gozani et al. |
| 2018/0028808 | February 2018 | Ferree et al. |
| 1919139 | Feb 2007 | CN | |||
| 101626804 | Jan 2010 | CN | |||
| 102355847 | Feb 2012 | CN | |||
| 102740919 | Feb 2012 | CN | |||
| 102010052710 | May 2012 | DE | |||
| 60-41851 | Mar 1985 | JP | |||
| S60-194933 | Oct 1985 | JP | |||
| 61-171943 | Oct 1986 | JP | |||
| 4-347140 | Dec 1992 | JP | |||
| 9-117453 | May 1997 | JP | |||
| 2000-167067 | Jun 2000 | JP | |||
| 2005-34402 | Feb 2005 | JP | |||
| 2005-81068 | Mar 2005 | JP | |||
| 2006-68300 | Mar 2006 | JP | |||
| 4185846 | Sep 2008 | JP | |||
| WO 97/42999 | Nov 1997 | WO | |||
| WO 99/64105 | Dec 1999 | WO | |||
| WO 00/09999 | Feb 2000 | WO | |||
| WO 2003/051453 | Jun 2003 | WO | |||
| WO 2004/078132 | Sep 2004 | WO | |||
| WO 2007/061746 | May 2007 | WO | |||
| WO 2008/079757 | Jul 2008 | WO | |||
| WO 2008/088985 | Jul 2008 | WO | |||
| WO 2011/075179 | Jun 2011 | WO | |||
| WO 2011/137193 | Nov 2011 | WO | |||
| WO 2012/037527 | Mar 2012 | WO | |||
| WO 2012/116407 | Sep 2012 | WO | |||
| WO 2013/074809 | May 2013 | WO | |||
| WO 2014/161000 | Oct 2014 | WO | |||
| WO 2014/172381 | Oct 2014 | WO | |||
| WO 2016/111863 | Jul 2016 | WO | |||
Other References
|
Ancoli-Israel, S. et al., The Role of Actigraphy in the Study of Sleep and Circadian Rhythms, Sleep, 2003, 26(3), p. 342-392. cited by applicant . Barbarisi, Manlio et al., Pregabalin and Transcutaneous Electrical Nerve Stimulation for Postherpetic Neuralgia Treatment, The Clinical Journal of Pain, Sep. 2010;26(7):567-572. cited by applicant . Bjordal JM et al., Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain, European Journal of Pain, 2003, vol. 7(2): 181-188. cited by applicant . Bloodworth DM et al., Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. American Journal of Physical Medicine & Rehabilitation, 2004, vol. 83(8): 584-591. cited by applicant . Chandran P et al., Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration, Pain, 2003, vol. 102: 195-201. cited by applicant . Chen CC et al, A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants, Clinical Physiology and Functioning Imaging, 2010, vol. 30(4): 260-268. cited by applicant . Chen CC et al., An investigation into the effects of frequency-modulated transcutaneous electrical nerve stimulation (TENS) on experimentally-induced pressure pain in healthy human participants, The Journal of Pain, 2009, vol. 10(10): 1029-1037. cited by applicant . Chen CC et al., Differential frequency effects of strong nonpainful transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in healthy human participants, The Clinical Journal of Pain, 2011, vol. 27(5): 434-441. cited by applicant . Chen CC et al., Does the pulse frequency of transcutaneous electrical nerve stimulation (TENS) influence hypoalgesia? A systematic review of studies using experimental pain and healthy human participants, Physiotherapy, 2008, vol. 94: 11-20. cited by applicant . Claydon LS et al., Dose-specific effects of transcutaneous electrical nerve stimulation on experimental pain, Clinical Journal of Pain, 2011, vol. 27(7): 635-647. cited by applicant . Cole, R.J. et al., Automatic Sleep/Wake Identification From Wrist Activity, Sleep, 1992, 15(5), p. 461-469. cited by applicant . Cruccu G. et al., EFNS guidelines on neurostimulation therapy for neuropathic pain, European Journal of Neurology, 2007, vol. 14: 952-970. cited by applicant . Davies Hto et al., Diminishing returns or appropriate treatment strategy?--an analysis of short-term outcomes after pain clinic treatment, Pain, 1997, vol. 70: 203-208. cited by applicant . Desantana JM et al., Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain, Curr Rheumatol Rep. 2008, vol. 10(6): 492-499. cited by applicant . Dubinsky RM et al, Assessment: Efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): Report of the therapeutics and technology assessment subcommittee of the american academy of neurology, Neurology, 2010, vol. 74: 173-176. cited by applicant . Fary RE et al., Monophasic electrical stimulation produces high rates of adverse skin reactions in healthy subjects, Physiotherapy Theory and Practice, 2011, vol. 27(3): 246-251. cited by applicant . Fishbain, David A. et al. Does Pain Mediate the Pain Interference with Sleep Problem in Chronic Pain? Findings from Studies for Management of Diabetic Peripheral Neuropathic Pain with Duloxetine, Journal of Pain Symptom Management, Dec. 2008;36(6):639-647. cited by applicant . Fishbain, David A. et al., Transcutaneous Electrical Nerve Stimulation (TENS) Treatment Outcome in Long-Term Users, The Clinical Journal of Pain, Sep. 1996;12(3):201-214. cited by applicant . Food and Drug Administration, Draft Guidance for Industry and Staff: Class II Special Controls Guidance Document: Transcutaneous Electrical Nerve Stimulator for Pain Relief, Apr. 5, 2010. cited by applicant . Garrison DW et al., Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS), Pain, 1994, vol. 58: 309-315. cited by applicant . Gilron, I. et al., Chronobiological Characteristics of Neuropathic Pain: Clinical Predictors of Diurnal Pain Rhythmicity, The Clinical Journal of Pain, 2013. cited by applicant . Hori, T. et al., Skin Potential Activities and Their Regional Differences During Normal Sleep in Humans, The Japanese Journal of Physiology, 1970, vol. 20, p. 657-671. cited by applicant . Jelinek HF et al., Electric pulse frequency and magnitude of perceived sensation during electrocutaneous forearm stimulation, Arch Phys Med Rehabil, 2010, vol. 91; 1372-1382. cited by applicant . Jin DM et al., Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials, Diabetes Research and Clinical Practice, 2010, vol. 89: 10-15. cited by applicant . Johnson MI et al., Analgesic effects of different frequencies of transcutaneous electrical nerve stimulation on cold-induced pain in normal subjects, Pain, 1989, vol. 39: 231-236. cited by applicant . Johnson MI et al., Transcutaneous Electrical Nerve Stimulation (TENS) and TENS-like devices: do they provide pain relief?, Pain Reviews, 2001, vol. 8: 7-44. cited by applicant . Johnson MI et al., Transcutaneous electrical nerve stimulation for the management of painful conditions; focus on neuropathic pain, Expert Review of Neurotherapeutics, 2011, vol. 11(5): 735-753. cited by applicant . Johnson, M.I. et al., An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain. Mar. 1991;44(3):221-229. cited by applicant . Kaczmarek, Kurt A. et al., Electrotactile and Vibrotactile Displays for Sensory Substitution Systems. IEEE Trans. Biomed. Eng. Jan. 1991;38 (1):1-16. cited by applicant . Kantor G et al., The effects of selected stimulus waveforms on pulse and phase characteristics at sensory and motor thresholds, Physical Therapy, 1994, vol. 74(10): 951-962. cited by applicant . Keller, Thierry et al., Electrodes for transcutaneous (surface) electrical stimulation. J. Automatic Control; University of Belgrade. 2008;18(2):35-45. cited by applicant . Koumans, A. J. R. et al., Electrodermal Levels and Fluctuations During Normal Sleep, Psychophysiology, 1968, 5(3), p. 300-306. cited by applicant . Kripke, D.F. et al., Wrist Actigraphic Scoring for Sleep Laboratory Patients: Algorithm Development, Journal of Sleep Research, 2010, 19(4), p. 612-619. cited by applicant . Law PPW et al., Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis, J Rehabil Med, 2004, vol. 36: 220-225. cited by applicant . Leonard G et al., Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone, Pain, 2010, vol. 151: 215-219. cited by applicant . Levy et al., A comparison of two methods for measuring thermal thresholds in diabetic neuropathy, Journal of Neurology, Neurosurgery, and Psychiatry, 1989, vol. 52: 1072-1077. cited by applicant . Lykken, D.T., Properties of Electrodes Used in Electrodermal Measurement, J. Comp. Physiol. Psychol. Oct. 1959;52:629-634. cited by applicant . Lykken, D.T., Square-Wave Analysis of Skin Impedance. Psychophysiology. Sep. 1970;7(2):262-275. cited by applicant . Melzack R et al., Pain mechanisms: A New Theory, Science, 1965, vol. 150(3699): 971-979. cited by applicant . Moran F et al., Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity, The Journal of Pain, 2011, vol. 12(8): 929-935. cited by applicant . Oosterhof, Jan et al., Outcome of transcutaneous electrical nerve stimulation in chronic pain: short-term results of a double-blind, randomised, placebo-controlled trial. J. Headache Pain. Sep. 2006;7 (4):196-205. cited by applicant . Oosterhof, Jan et al., The long-term outcome of transcutaneous electrical nerve stimulation in the treatment for patients with chronic pain: a randomized, placebo-controlled trial. Pain Pract. Sep. 2012;12(7):513-522. cited by applicant . Pantaleao MA et al., Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia, The Journal of Pain, 2011, vol. 12(5): 581-590. cited by applicant . Paquet, J. et al., Wake Detection Capacity of Actigraphy During Sleep, Sleep, 2007, 30(10), p. 1362-1369. cited by applicant . Pieber K et al., Electrotherapy for the treatment of painful diabetic peripheral neuropathy: a review, Journal of Rehabilitation Medicine, 2010, vol. 42: 289-295. cited by applicant . Raskin, J. et al., A Double-Blind, Randomized Multicenter Trial Comparing Duloxetine with Placebo in the Management of Diabetic Peripheral Neuropathic Pain, Pain Medicine, 2005, 6(5), p. 346-356. cited by applicant . Sadeh, A., The Role and Validity of Actigraphy in Sleep Medicine: An Update, Sleep Medicine Reviews, 2011, vol. 15, p. 259-267. cited by applicant . Sadosky, A. et al., Burden of Illness Associated with Painful Diabetic Peripheral Neuropathy Among Adults Seeking Treatment in the US: Results from a Retrospective Chart Review and Cross-Sectional Survey, Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 2013, vol. 6, p. 79-92. cited by applicant . Scherder, E. J. A. et al., Transcutaneous Electrical Nerve Stimulation (TENS) improves the Rest-Activity Rhythm in Midstage Alzheimer's Disease, Behavioral Brain Research, 1999. vol. 101, p. 105-107. cited by applicant . Tryon, W. W., Issues of Validity in Actigraphic Sleep Assessment, Sleep, 2004, 27(1), p. 158-165. cited by applicant . Tsai, Y. et al., Impact of Subjective Sleep Quality on Glycemic Control in Type 2 Diabetes Mellitus, Family Practice, 2012, vol. 29, p. 30-35. cited by applicant . Van Boxtel, A., Skin resistance during square-wave electrical pulses of 1 to 10 mA. Med. Biol. Eng. Comput, Nov. 1977;15(6):679-687. cited by applicant . Van Someren, E. J. W. et al., Gravitational Artefact in Frequency Spectra of Movement Acceleration: Implications for Actigraphy in Young and Elderly Subjects. Journal of Neuroscience Methods, 1996, vol. 65, p. 55-62. cited by applicant . Webster, J. B. et al., An Activity-Based Sleep Monitor System for Ambulatory Use, Sleep, 1982, 5(4), p. 389-399. cited by applicant . Zelman, D. C. et al., Sleep Impairment in Patients With Painful Diabetic Peripheral Neuropathy, The Clinical Journal of Pain, 2006, 22(8), p. 681-685. cited by applicant . Aurora, R. et al., The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults an Update for 2012: Practice Parameters with an Evidence-Based Systematic Review and Meta-Analyses, Sleep, 2012, vol. 35, No. 8, p. 1039-1062. cited by applicant . Bonnet, M, et al., Recording and Scoring Leg Movements, Sleep, 1993, vol. 16, No. 8, p. 748-759. cited by applicant . Boyle, J. et al., Randomized, Placebo-Controlled Comparison of Amitriptyline, Duloxeline, and Pregabalin in Patients With Chronic Diabetic Peripheral Neuropathic Pain, Diabetes Care, 2012, vol. 35, p. 2451-2458. cited by applicant . Kovacevic-Ristanovic, R. et al., Nonpharmacologic Treatment of Periodic Leg Movements in Sleep, Arch. Phys. Med. Rehabil., 1991, vol. 72, p. 385-389. cited by applicant . Lopes, L. et al., Restless Legs Syndrome and Quality of Sleep in Type 2 Diabetes, Diabetes Care, 2005, vol. 28, No. 11, p. 2633-2636. cited by applicant . Nightingale, S., The neuropathic pain market. Nature Reviews, 2012, vol. 11, p. 101-102. cited by applicant . Zucconi, M. et al., The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG), Sleep Medicine, 2005, vol. 7, p. 175-183. cited by applicant . Dailey D.L. et al., Transcutaneous Electrical Nerve Stimulation Reduces Pain, Fatigue and Hyperalgesia while Restoring Central Inhibition in Primary Fibromyalgia, Pain, Nov. 2013, vol. 154, No. 11, pp. 2554-2562. cited by applicant. |
Primary Examiner: Laymon; Wan
Assistant Examiner: Samuel; Clint A
Description
FIG. 1 is a perspective view of the transcutaneous electrical nerve stimulation (TENS) device;
FIG. 2 is a front view of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 1;
FIG. 3 is a rear view of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 1;
FIG. 4 is a side view, in elevation, of one side of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 2;
FIG. 5 is a side view, in elevation, of the other side of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 2;
FIG. 6 is an end view, in elevation, of one end of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 2; and,
FIG. 7 is an end view, in elevation, of the other end of the transcutaneous electrical nerve stimulation (TENS) device, taken from the frame of reference of FIG. 2.
The broken lines are included for the purpose of illustrating unclaimed portions of the transcutaneous electrical nerve stimulation (TENS) device and form no part of the claimed design.
* * * * *
D00000

D00001

D00002

D00003

D00004

D00005

D00006

XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.