Method for preparing compound and method for preparing polymer employing the same
Ho , et al.
U.S. patent number 10,723,841 [Application Number 16/047,674] was granted by the patent office on 2020-07-28 for method for preparing compound and method for preparing polymer employing the same. This patent grant is currently assigned to INDUSTRIAL TECHNOLOGY RESEARCH. The grantee listed for this patent is INDUSTRIAL TECHNOLOGY RESEARCH INSTITUTE. Invention is credited to Yih-Her Chang, Chien-Ming Chen, Cheng-Hsing Fan, Po-Hsien Ho, Hsin-Ching Kao, Chih-Hsiang Lin, Feng-Jen Tsai.

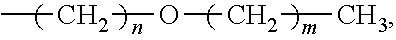







View All Diagrams
| United States Patent | 10,723,841 |
| Ho , et al. | July 28, 2020 |
Method for preparing compound and method for preparing polymer employing the same
Abstract
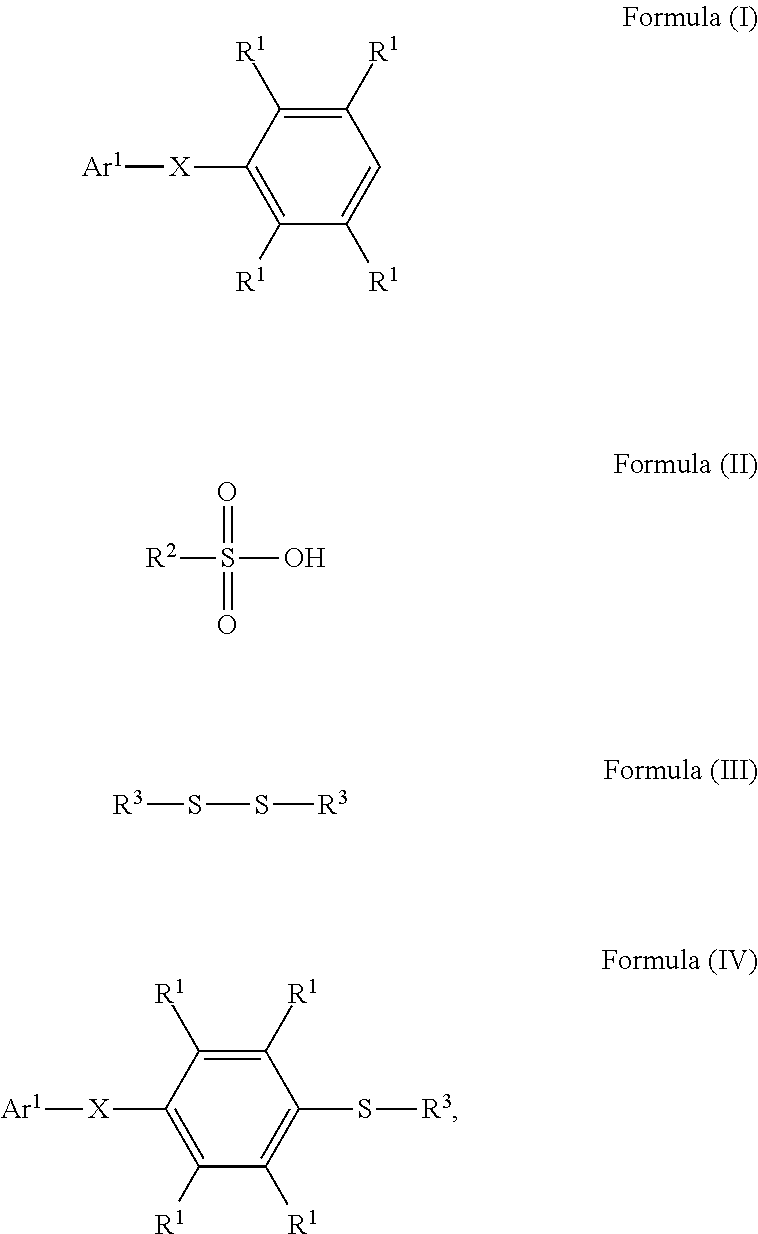
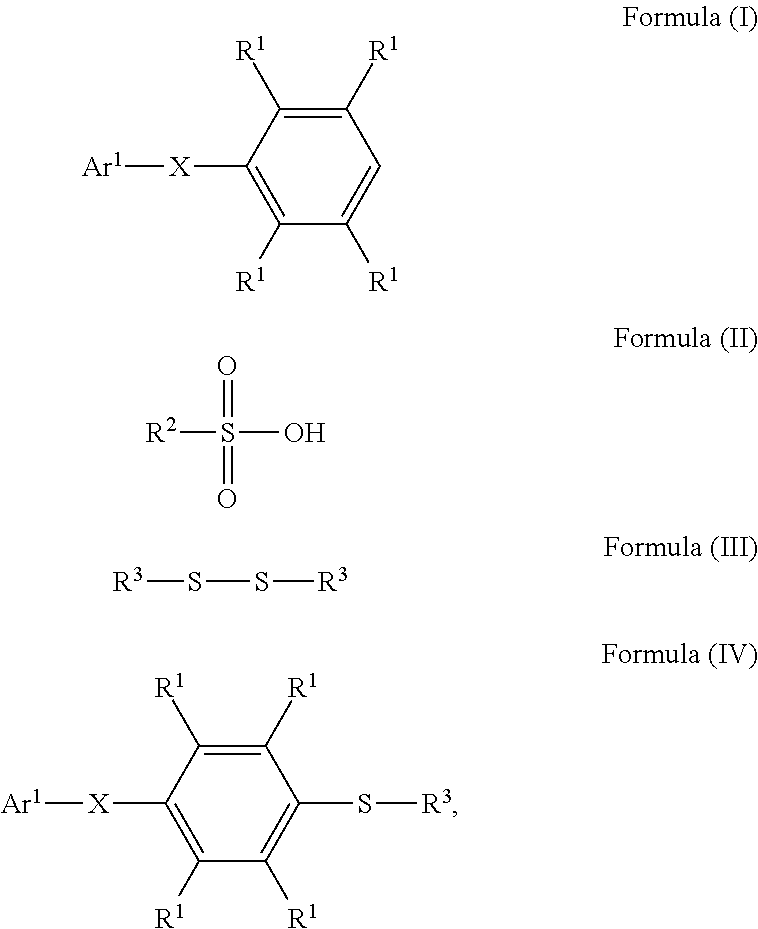
A method for preparing a compound and a method for preparing a polymer employing the same are provided. The method for preparing a compound includes reacting a compound having a structure represented by Formula (I) with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II) to obtain a compound having a structure represented by Formula (IV) ##STR00001## wherein Ar.sup.1 is substituted or unsubstituted aryl group; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
| Inventors: | Ho; Po-Hsien (Taipei, TW), Lin; Chih-Hsiang (Taipei, TW), Tsai; Feng-Jen (Taipei, TW), Fan; Cheng-Hsing (Tainan, TW), Chang; Yih-Her (Baoshan Township, TW), Kao; Hsin-Ching (Baoshan Township, TW), Chen; Chien-Ming (Taoyuan, TW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Applicant: |
|
||||||||||
| Assignee: | INDUSTRIAL TECHNOLOGY RESEARCH
(Hsinchu, TW) |
||||||||||
| Family ID: | 63244384 | ||||||||||
| Appl. No.: | 16/047,674 | ||||||||||
| Filed: | July 27, 2018 |
Prior Publication Data
| Document Identifier | Publication Date | |
|---|---|---|
| US 20190040202 A1 | Feb 7, 2019 | |
Related U.S. Patent Documents
| Application Number | Filing Date | Patent Number | Issue Date | ||
|---|---|---|---|---|---|
| 62539681 | Aug 1, 2017 | ||||
Foreign Application Priority Data
| Dec 27, 2017 [TW] | 106145978 A | |||
| Current U.S. Class: | 1/1 |
| Current CPC Class: | C08G 75/02 (20130101); C08G 75/029 (20130101); C08G 75/20 (20130101); C07C 319/20 (20130101); C07C 319/14 (20130101); C07C 315/02 (20130101); C08G 75/23 (20130101); C08G 75/0272 (20130101); C07C 315/00 (20130101); C07C 317/22 (20130101); C07C 323/00 (20130101); C08G 75/0227 (20130101); C08G 75/0236 (20130101); C08G 75/025 (20130101); C07C 319/20 (20130101); C07C 321/30 (20130101); C07C 319/14 (20130101); C07C 323/20 (20130101); C07C 315/02 (20130101); C07C 317/22 (20130101) |
| Current International Class: | C08G 75/0236 (20160101); C08G 75/02 (20160101); C08G 75/23 (20060101); C08G 75/029 (20160101); C08G 75/0227 (20160101); C07C 315/02 (20060101); C07C 319/14 (20060101); C08G 75/025 (20160101); C08G 75/20 (20160101); C07C 323/00 (20060101); C07C 317/22 (20060101); C07C 319/20 (20060101); C07C 315/00 (20060101) |
References Cited [Referenced By]
U.S. Patent Documents
| 3634355 | January 1972 | Barr et al. |
| 8143453 | March 2012 | Reddy et al. |
| 9045598 | June 2015 | El-Toufaili et al. |
| 2003/0004302 | January 2003 | Okamoto et al. |
| 2015/0065677 | March 2015 | El-Toufaili et al. |
| 101033294 | Sep 2007 | CN | |||
| 101092479 | Dec 2007 | CN | |||
| 101774953 | Jul 2010 | CN | |||
| 102516139 | Jun 2012 | CN | |||
| 105492498 | Apr 2016 | CN | |||
| 3190139 | Jul 2017 | EP | |||
| 3190140 | Jul 2017 | EP | |||
| 3190141 | Jul 2017 | EP | |||
| 3190142 | Jul 2017 | EP | |||
| 3190143 | Jul 2017 | EP | |||
| 7-304872 | Nov 1995 | JP | |||
| 8-245558 | Sep 1996 | JP | |||
| 2016-147955 | Aug 2016 | JP | |||
| 2017-125014 | Jul 2017 | JP | |||
| 2017-125190 | Jul 2017 | JP | |||
| 2017-160185 | Sep 2017 | JP | |||
| 2017-197712 | Nov 2017 | JP | |||
| 2017-197713 | Nov 2017 | JP | |||
| 201512249 | Apr 2015 | TW | |||
Other References
|
Dizman, C., et al, "Recent advances in the preparation of functionalized polysulfones," Polymer International, 2013, vol. 62, p. 991-1007. cited by applicant . Matsumoto, K., et al, "Synthesis of poly(ether sulfone)s by self-polycondensation of AB-type monomers," Polymer Journal, 2013, vol. 45, pp. 909-914. cited by applicant . Tsuchida, E., et al, "Synthesis of High Molecular Weight Poly(phenylene sulfide) by Oxidative Polymerization via Poly(sulfonium cation) from Methyl Phenyl Sulfoxide," Macromolecules, 1993, vol. 26, pp. 7144-7148. cited by applicant . Tsuchida, E., et al, "Synthetic Route to Poly(sulfonyl-1,4-phenylenethio-1,4-phenylene) via a Poly(sulfonium cation)," Macromolecules, 1993. vol. 26, pp. 7389-7390. cited by applicant . Yokozawa, T., et al, "Chain-growth polycondensation: The living polymerization process in polycondensation," Progress in Polymer Science, Jan. 1, 2007, vol. 32, pp. 147-172. cited by applicant . Taiwanese Notice of Allowance and Search Report, dated Dec. 25, 2018, for Taiwanese Application No. 106145978. cited by applicant . Cogolli et al., "Nucleophilic Aromatic Substitution Reactions of Unactivated Aryl Halides With Thiolate Ions in Hexamethylphosphoramide", The Journal of Organic Chemistry, vol. 44, No. 15, Jul. 1, 1979, pp. 2642-2646. cited by applicant . Ding et al., "Preparation of Poly(thioarylene)s From Cyclic Disulfide Oligomers", Macromolecules, American Chemical Society, vol. 30, No. 9, May 5, 1997 (abstract published Apr. 1997), pp. 2527-2531. cited by applicant . Extended European Search Report dated Jan. 4, 2019, for corresponding European Application No. 18186311.9. cited by applicant . Gabler et al., "Neue Polyphenylensulfone Reaktionen An Festen Polymeren", Chimia International Journal for Chemistry, vol. 28, No. 9. Sep. 1974, pp. 567-575. cited by applicant . Miller et al., "Reactions of Diaryl Disulfides with Active, Nonnucleophilic Alkylating Agents", Journal of Organic Chemistry, vol. 36, No. 11, Jun. 1, 1971, pp. 1513-1519. cited by applicant . Tsuchida et al., "Polymerization at Diphenyl Disulfide by the S--S Bond Cleavage With a Lewis Acid: A Novel Preparation Route to Poly(p-phenylene sulfide)", Macromolecules, vol. 23, No. 8, Apr. 16, 1990 pp. 2101-2106. cited by applicant . Japanese Office Action dated Jun. 25, 2019, for corresponding Japanese Application No. 2018-142980, with English translation. cited by applicant . Jilek et al., "Potential metabolites of the neuroleptic agents belonging to the 8-(methylthio)-10-piperazino-10,11-dihydrodibenzo[b,f]thiepin series; Synthesis of 2-hydroxy and 3-hydroxy derivatives", Collection Czechoslovak Chem. Commun., vol. 50, 1985, pp. 2179-2190. cited by applicant. |
Primary Examiner: Fang; Shane
Attorney, Agent or Firm: Birch, Stewart, Kolasch & Birch, LLP
Parent Case Text
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No. 62/539,681, filed on Aug. 1, 2017, which is hereby incorporated by reference herein.
The application is based on, and claims priority from, Taiwan Application Serial Number 106145978, filed on Dec. 27, 2017, the disclosure of which is hereby incorporated by reference herein in its entirety.
Claims
What is claimed is:
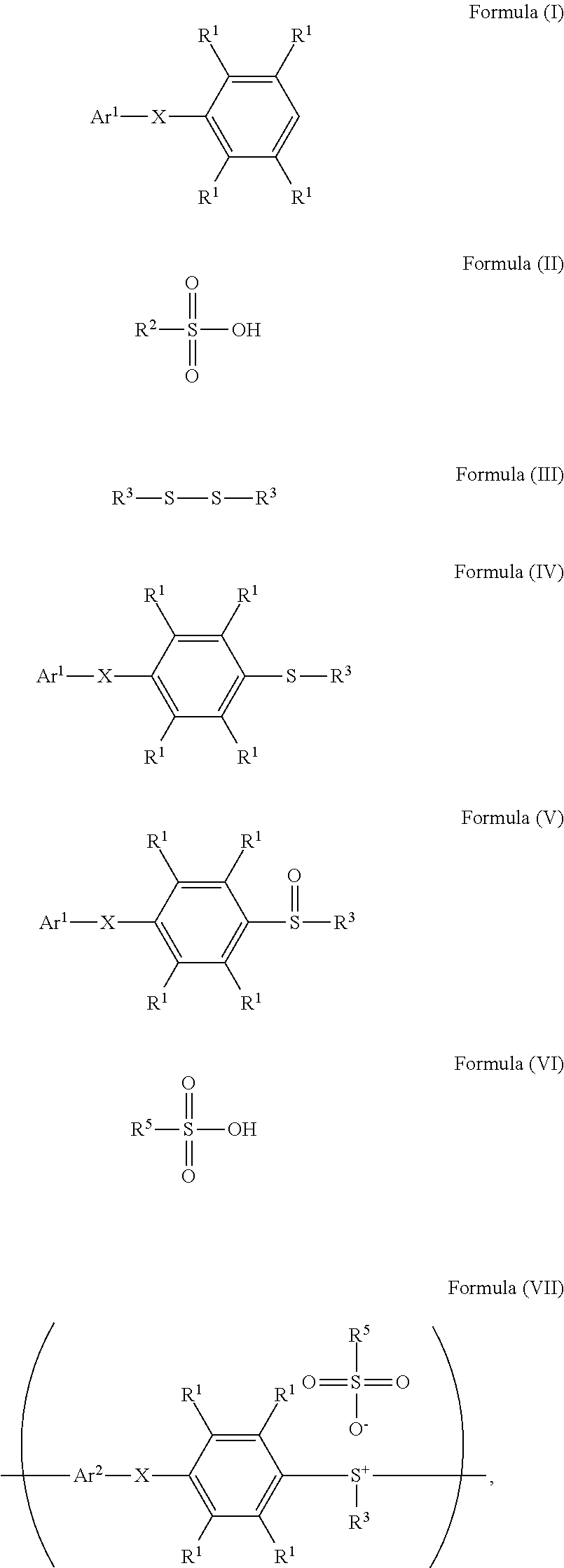
1. A method for preparing a compound, comprising: reacting a compound having a structure represented by Formula (I) with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV) ##STR00047## wherein Ar.sup.1 is substituted or unsubstituted aryl group; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
2. The method as claimed in claim 1, wherein Ar.sup.1 is substituted or unsubstituted phenyl group, biphenyl group, naphthyl group, thienyl group, indolyl group, phenanthrenyl group, indenyl group, anthracenyl group, or fluorenylene group.
3. The method as claimed in claim 1, wherein R.sup.1 is independently hydrogen, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
4. The method as claimed in claim 1, wherein the compound having the structure of Formula (I) is ##STR00048## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group.
5. The method as claimed in claim 1, wherein the compound having the structure of Formula (II) is sulfuric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, or a combination thereof.
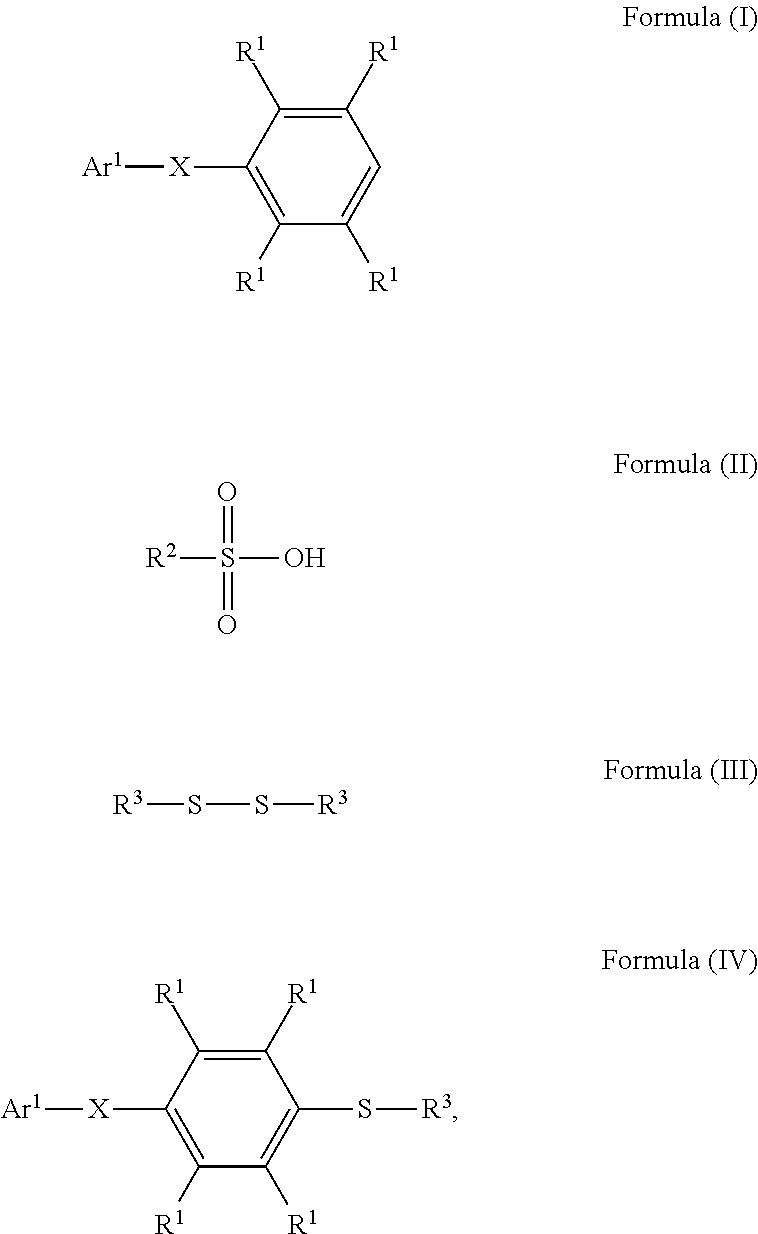
6. The method f as claimed in claim 1, wherein R.sup.3 is independently methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, hexyl group, cyclopentyl group, cyclohexyl group, cycloheptyl group, cyclooctyl group, or ##STR00049## wherein 1.ltoreq.n.ltoreq.5, 0.ltoreq.m.ltoreq.4, and 1.ltoreq.n+m.ltoreq.5.
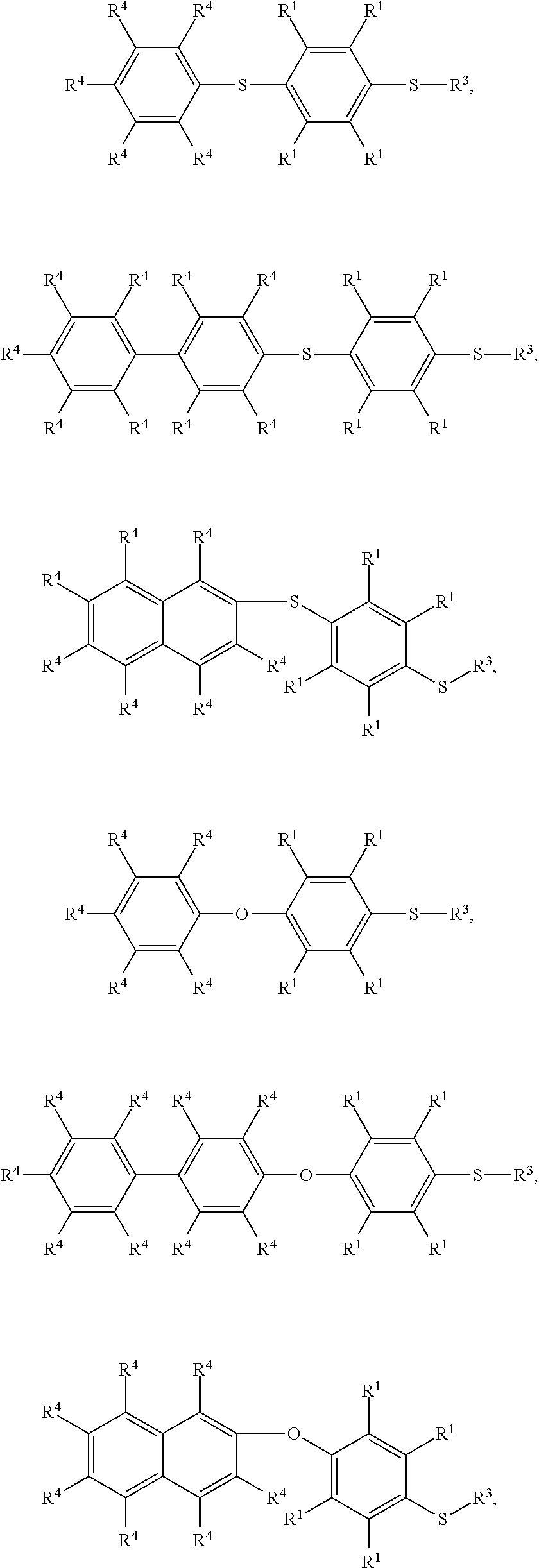
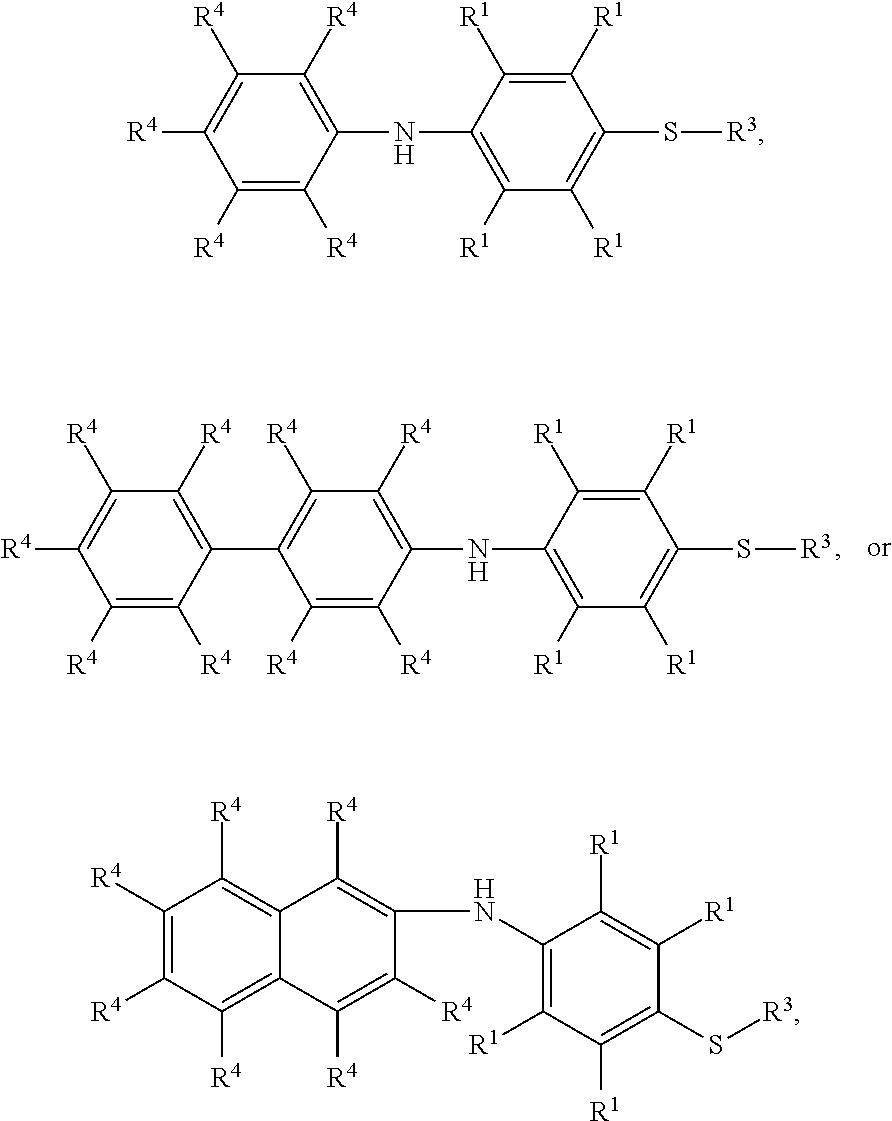
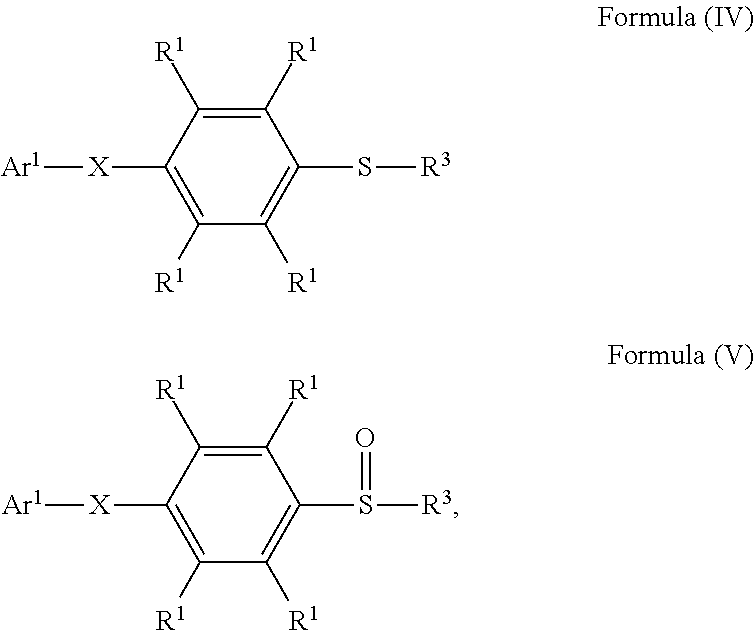
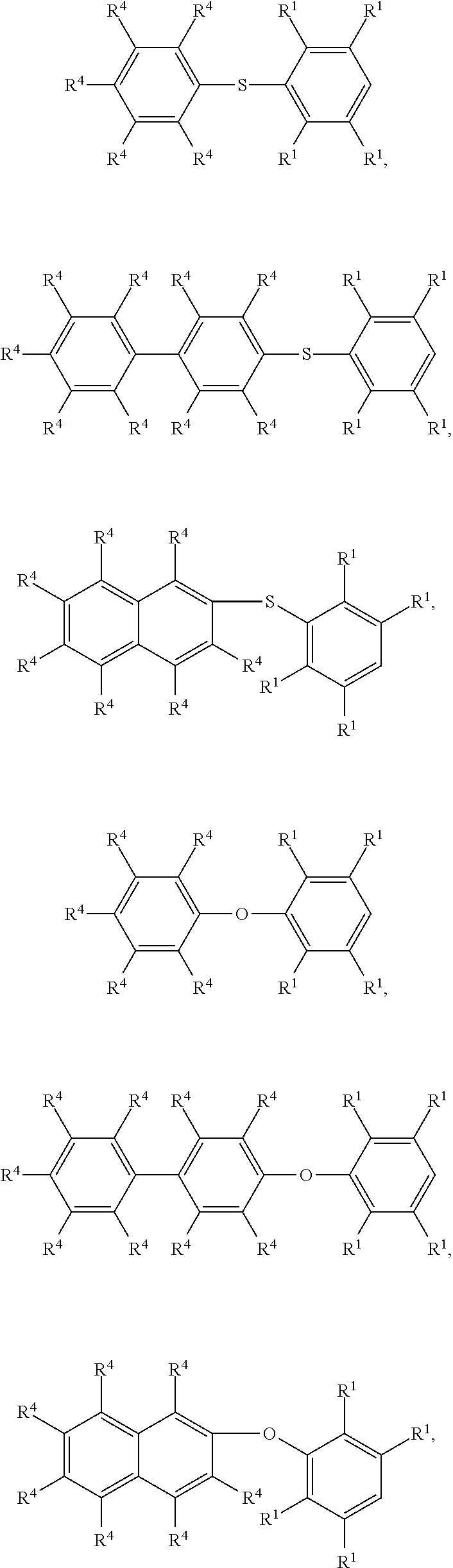
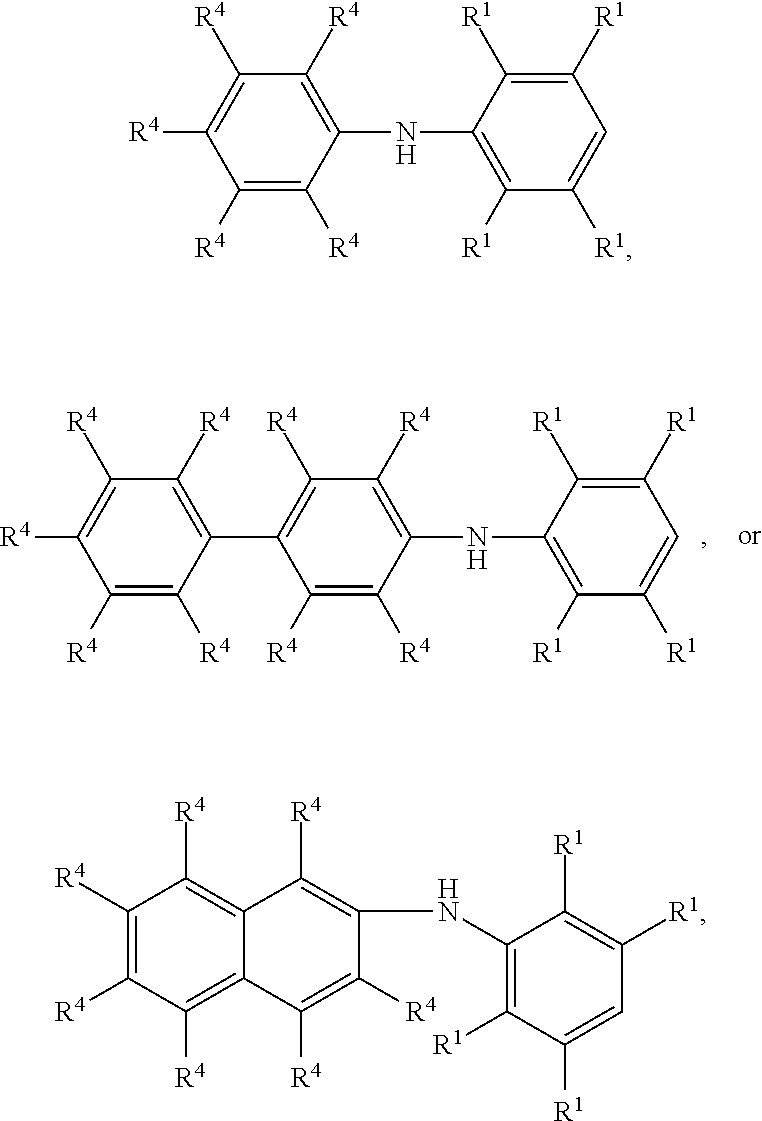
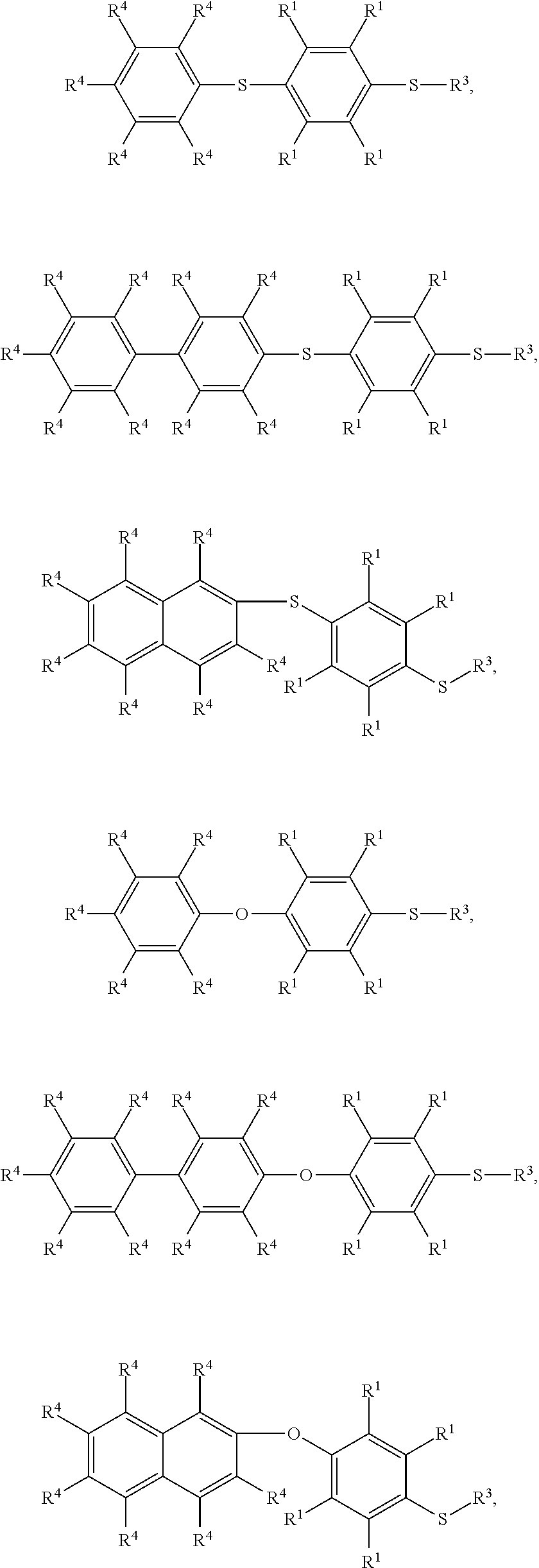
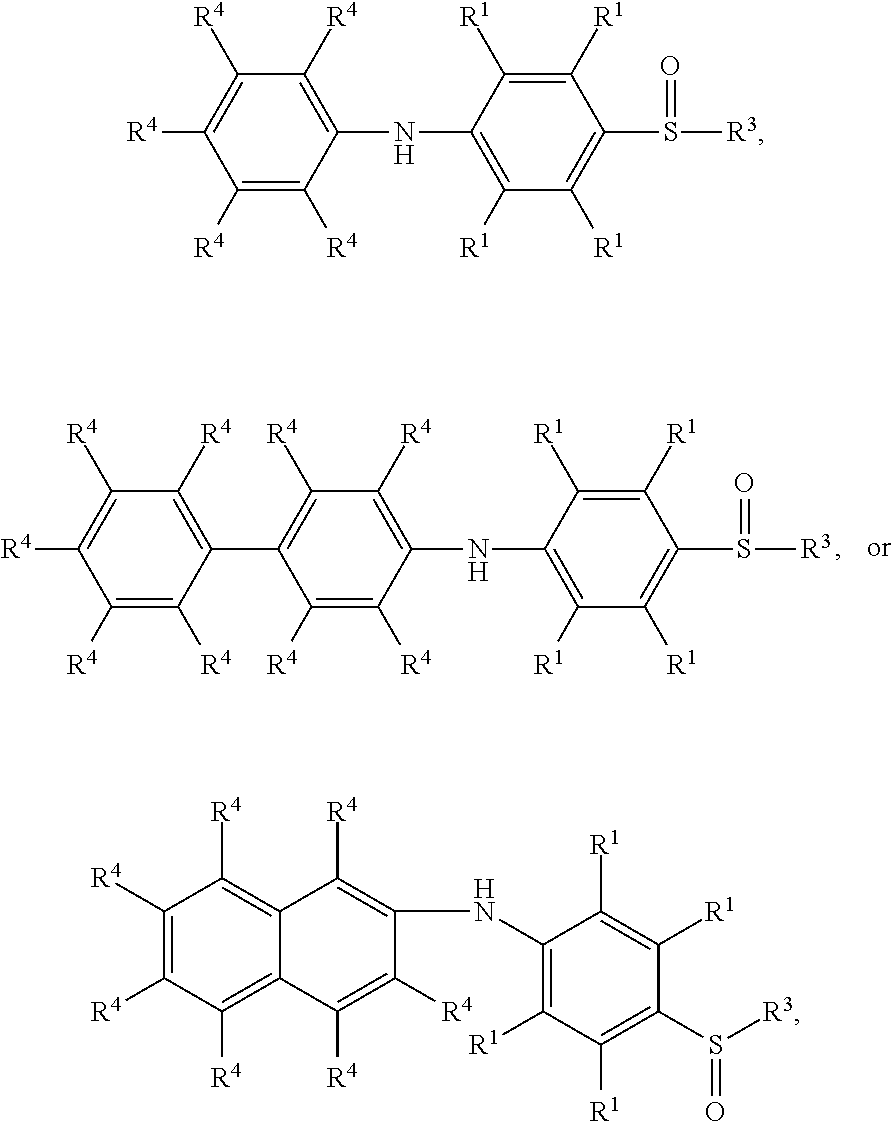
7. The method as claimed in claim 1, wherein the compound having the structure of Formula (IV) is ##STR00050## ##STR00051## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
8. The method as claimed in claim 1, further comprising: reacting the compound having the structure represented by Formula (IV) with a compound (A), obtaining a compound having the structure represented by Formula (V) ##STR00052## wherein the compound (A) is nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof; Ar.sup.1 is substituted or unsubstituted; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
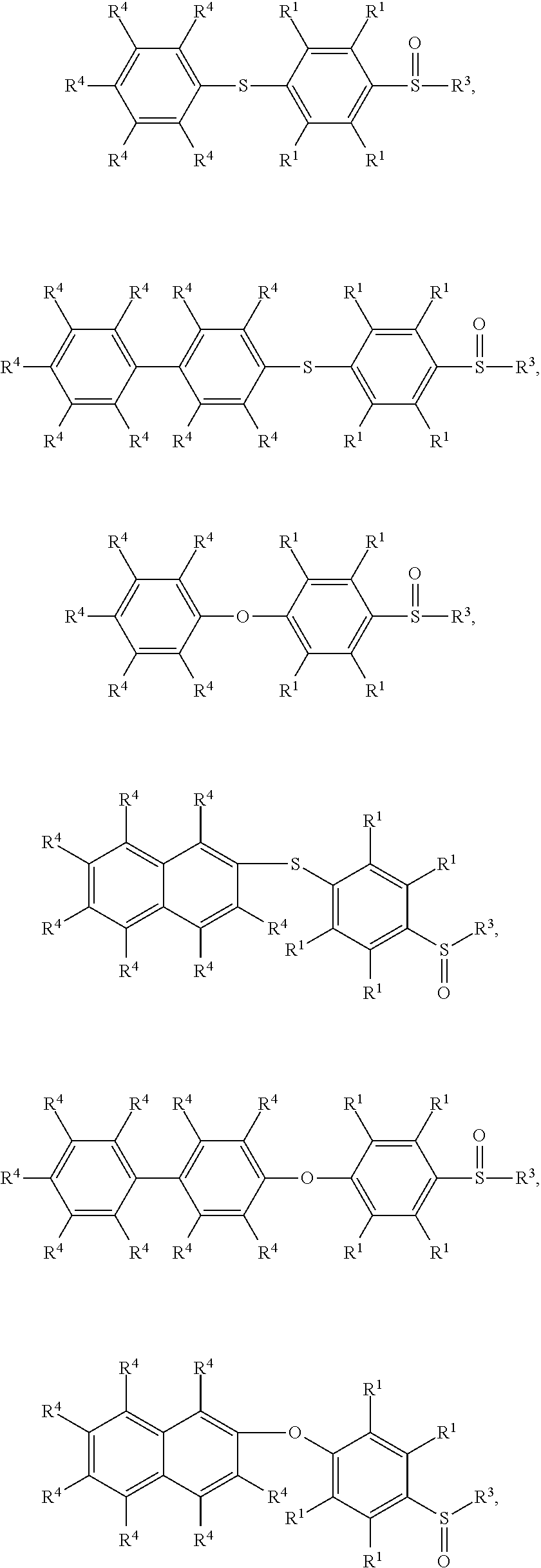
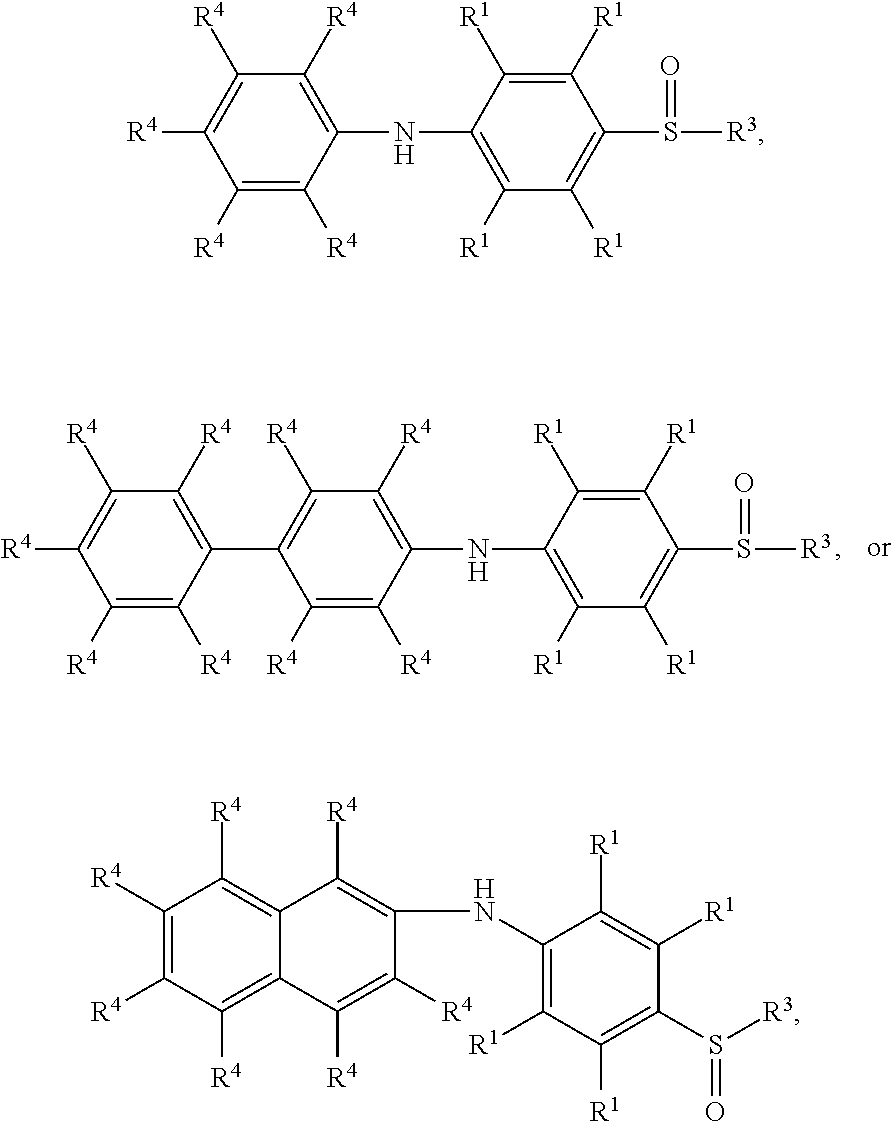
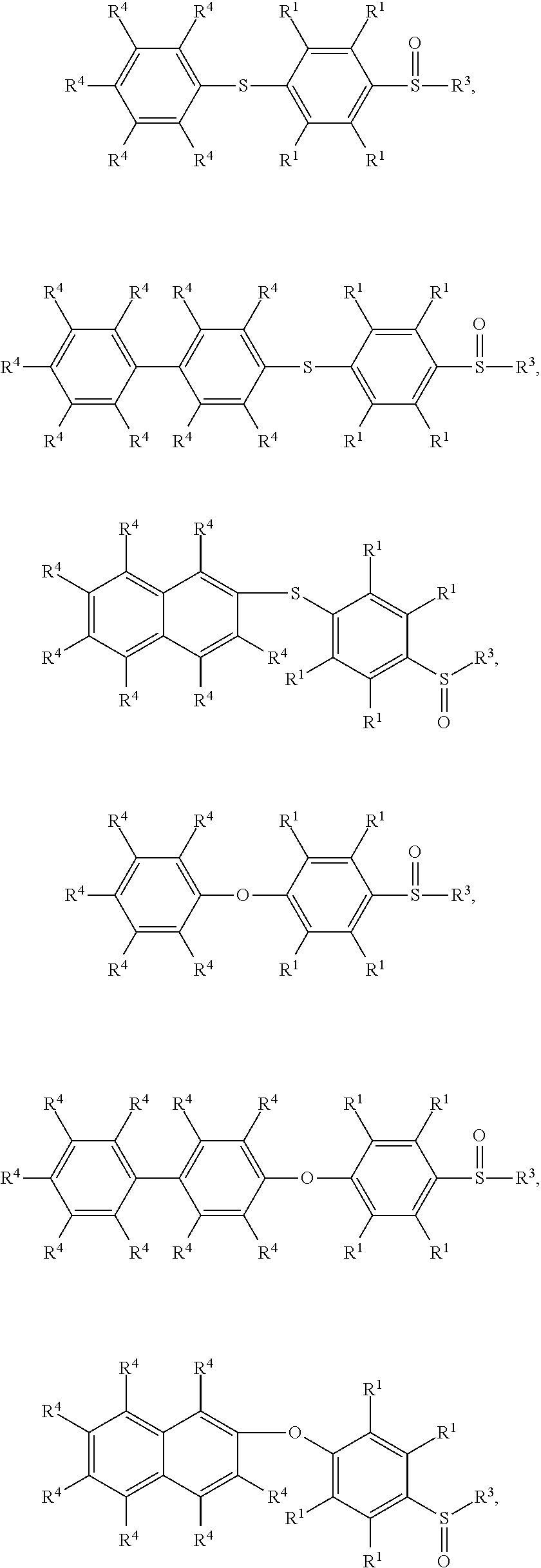
9. The method as claimed in claim 8, wherein the compound having the structure of Formula (V) is ##STR00053## ##STR00054## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
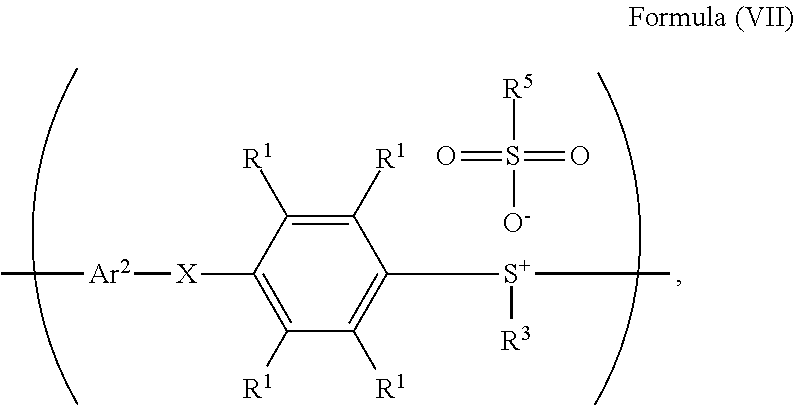
10. A method for preparing a polymer, comprising: reacting a compound having a structure represented by Formula (I) with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV); reacting the compound having the structure represented by Formula (IV) with a compound (A), obtaining a compound having the structure represented by Formula (V), wherein the compound (A) is nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof; and reacting the compound having the structure represented by Formula (V) with a compound having a structure represented by Formula (VI), obtaining a polymer having a repeat unit represented by Formula (VII) ##STR00055## wherein Ar.sup.1 is substituted or unsubstituted aryl group; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
11. The method as claimed in claim 10, wherein Ar.sup.1 is substituted or unsubstituted phenyl group, biphenyl group, naphthyl group, thienyl group, indolyl group, phenanthrenyl group, indenyl group, anthracenyl group, or fluorenylene group.
12. The method as claimed in claim 10, wherein R.sup.1 is independently hydrogen, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
13. The method as claimed in claim 10, wherein the compound having the structure of Formula (I) is ##STR00056## ##STR00057## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group.
14. The method as claimed in claim 10, wherein the compound having the structure of Formula (II) is sulfuric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid or a combination thereof.
15. The method as claimed in claim 10, wherein R.sup.3 is independently methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, hexyl group, cyclopentyl group, cyclohexyl group, cycloheptyl group, cyclooctyl group, or ##STR00058## wherein 1.ltoreq.n.ltoreq.5, 0.ltoreq.m.ltoreq.4, and 1.ltoreq.n+m.ltoreq.5.
16. The method as claimed in claim 10, wherein the compound having the structure of Formula (IV) is ##STR00059## ##STR00060## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
17. The method as claimed in claim 10, wherein the compound having the structure of Formula (V) is ##STR00061## ##STR00062## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.4 is independently hydrogen or C.sub.1-6 alkyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
18. The method as claimed in claim 10, wherein the compound having the structure of Formula (VI) is sulfuric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, or a combination thereof.
19. The method as claimed in claim 10, wherein Ar.sup.2 is substituted or unsubstituted phenylene group, biphenylene group, naphthylene group, thienylene group, indolylene group, phenanthrenylene group, indenylene group, anthracenylene group, or fluorenylene group.
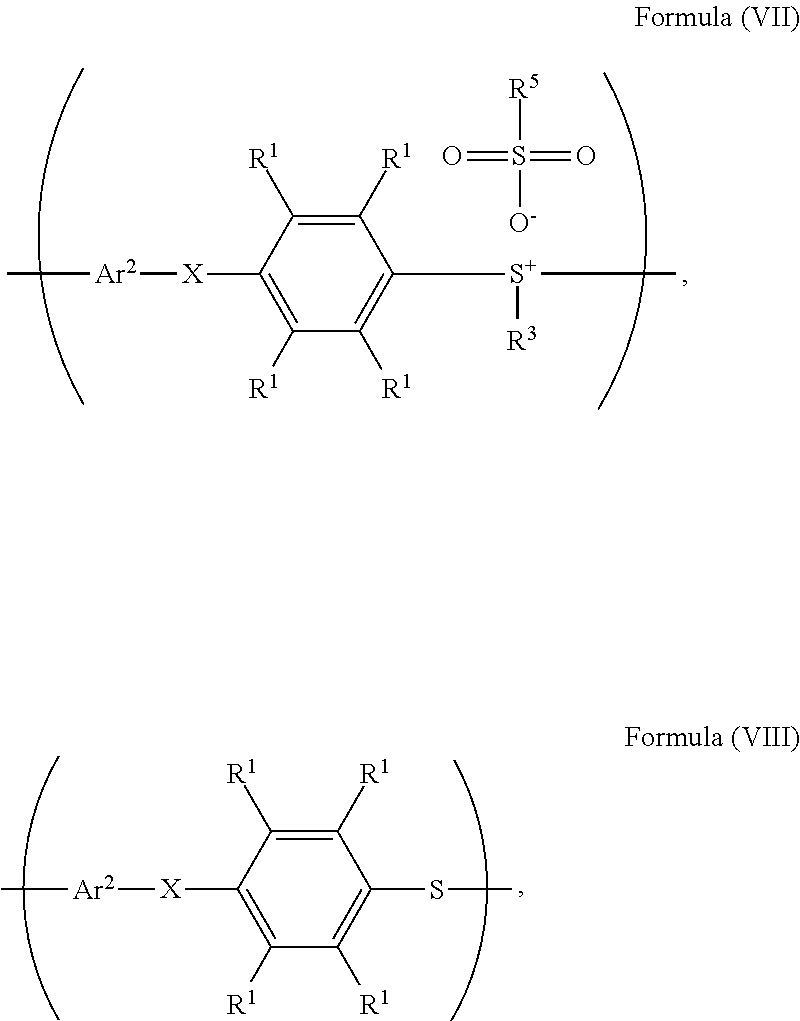
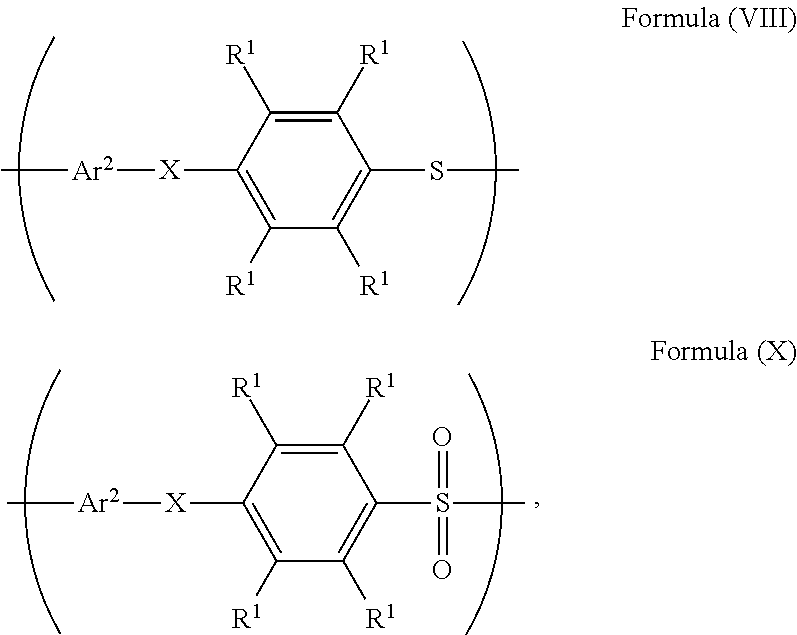
20. The method as claimed in claim 10, further comprising: reacting a nucleophile with the polymer having the repeat unit represented by Formula (VII), obtaining a polymer having a repeat unit represented by Formula (VIII) ##STR00063## wherein X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
21. The method as claimed in claim 20, wherein the nucleophile is pyridine, 4-methylpyridine, triethylamine, potassium chloride, methanol, ethanol, dimethylformamide, dimethylacetamide, N-methylpyrrolidone, or a combination thereof.
22. The method as claimed in claim 20, when X of the polymer having the repeat unit represented by Formula (VIII) is --O-- or --NH--, further comprising: reacting the polymer having the repeat unit represented by Formula (VIII) with hydrogen peroxide, obtaining a polymer having a repeat unit represented by Formula (X) ##STR00064## wherein X is --O-- or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
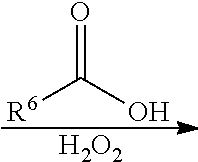
23. The method as claimed in claim 20, wherein a polymer having the repeat unit represented by Formula (VIII) is reacted with hydrogen peroxide in the presence of a compound having a structure represented by Formula (IX) ##STR00065## wherein R.sup.6 is C.sub.1-6 alkyl group.
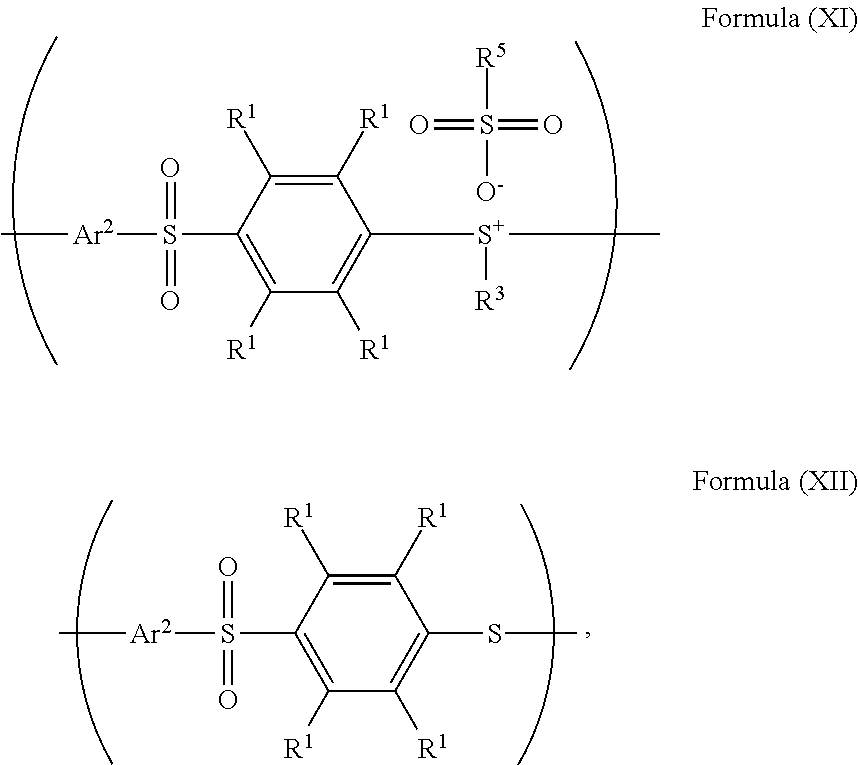
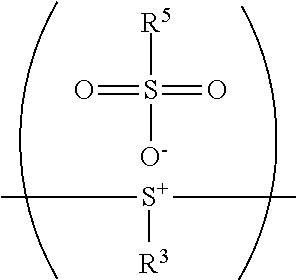
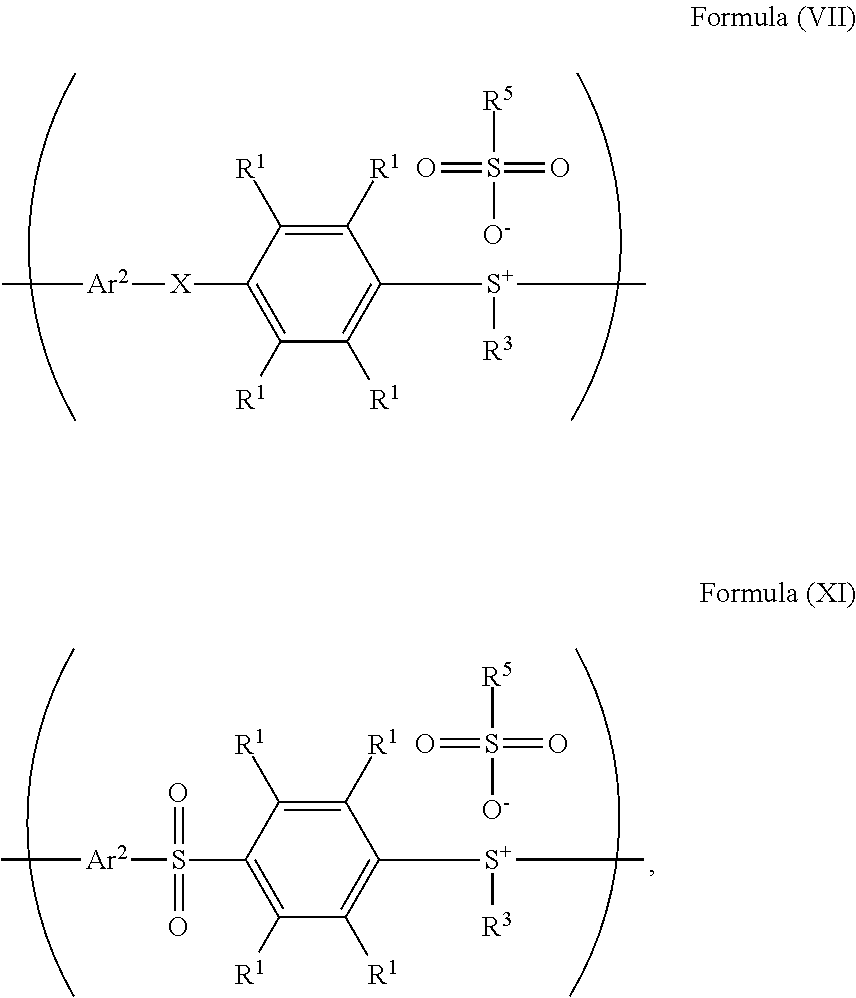
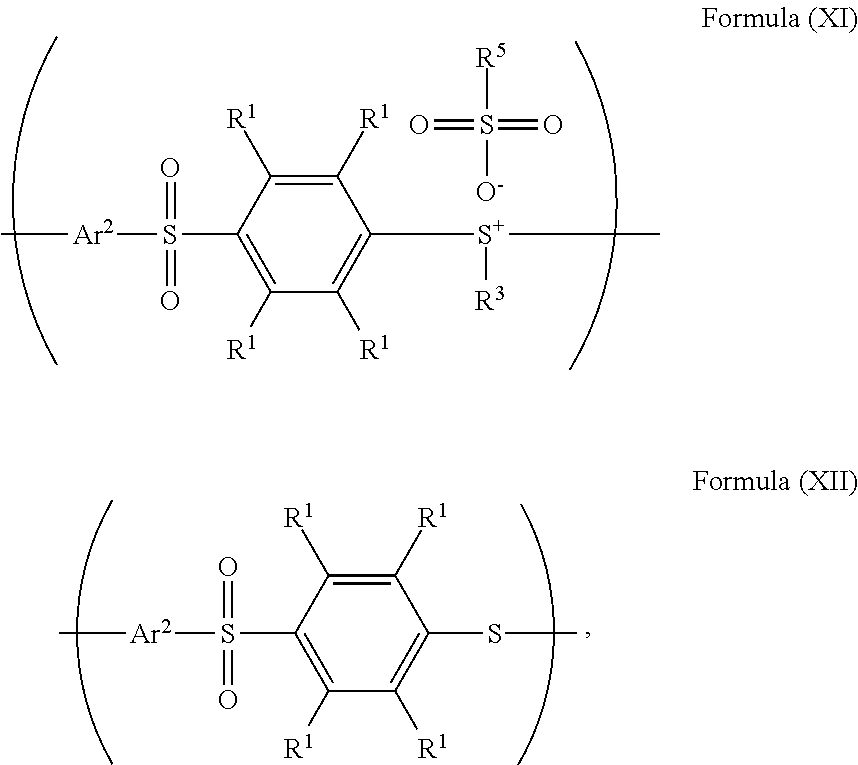
24. The method as claimed in claim 10, when X of the polymer having the repeat unit represented by Formula (VII) is --S--, further comprising: reacting the polymer having the repeat unit represented by Formula (VII) with hydrogen peroxide, obtaining a polymer having a repeat unit represented by Formula (XI) ##STR00066## wherein X is --S--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
25. The method as claimed in claim 24, wherein the polymer having the repeat unit represented by Formula (VII) is reacted with hydrogen peroxide in the presence of a compound having a structure represented by Formula (IX) ##STR00067## wherein R.sup.6 is C.sub.1-6 alkyl group.
26. The method as claimed in claim 24 further comprising: reacting a nucleophile with the polymer having the repeat unit represented by Formula (XI), obtaining a polymer having a repeat unit represented by Formula (XII) ##STR00068## wherein R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
27. The method as claimed in claim 26, wherein the nucleophile is pyridine, 4-methylpyridine, triethylamine, potassium chloride, methanol, ethanol, dimethylformamide, dimethylacetamide, N-methylpyrrolidone, or a combination thereof.
Description
TECHNICAL FIELD
The technical field relates to a method for preparing a compound and a method for preparing a polymer.
BACKGROUND
Polyarylene sulfide (PAS) (or polythioether sulfone (PTES)) is a material with good physical characteristics such as thermal resistance, chemical resistance, flame resistance, non-toxicity, and electrical insulation characteristics. Thus, polyarylene sulfide (PAS) (or polythioether sulfone (PTES)) can be used in computer accessories and auto accessories; as a coating for parts that come into contact with corrosive chemicals; and as industrial fibers having chemical resistance.
However, conventional methods for preparing polyarylene sulfide (PAS), polythioether sulfone (PTES), or monomers thereof are halogen-containing processes that, in principle, results in a low yield and produces unrecyclable halogen-containing byproducts that can cause environmental pollution.
SUMMARY
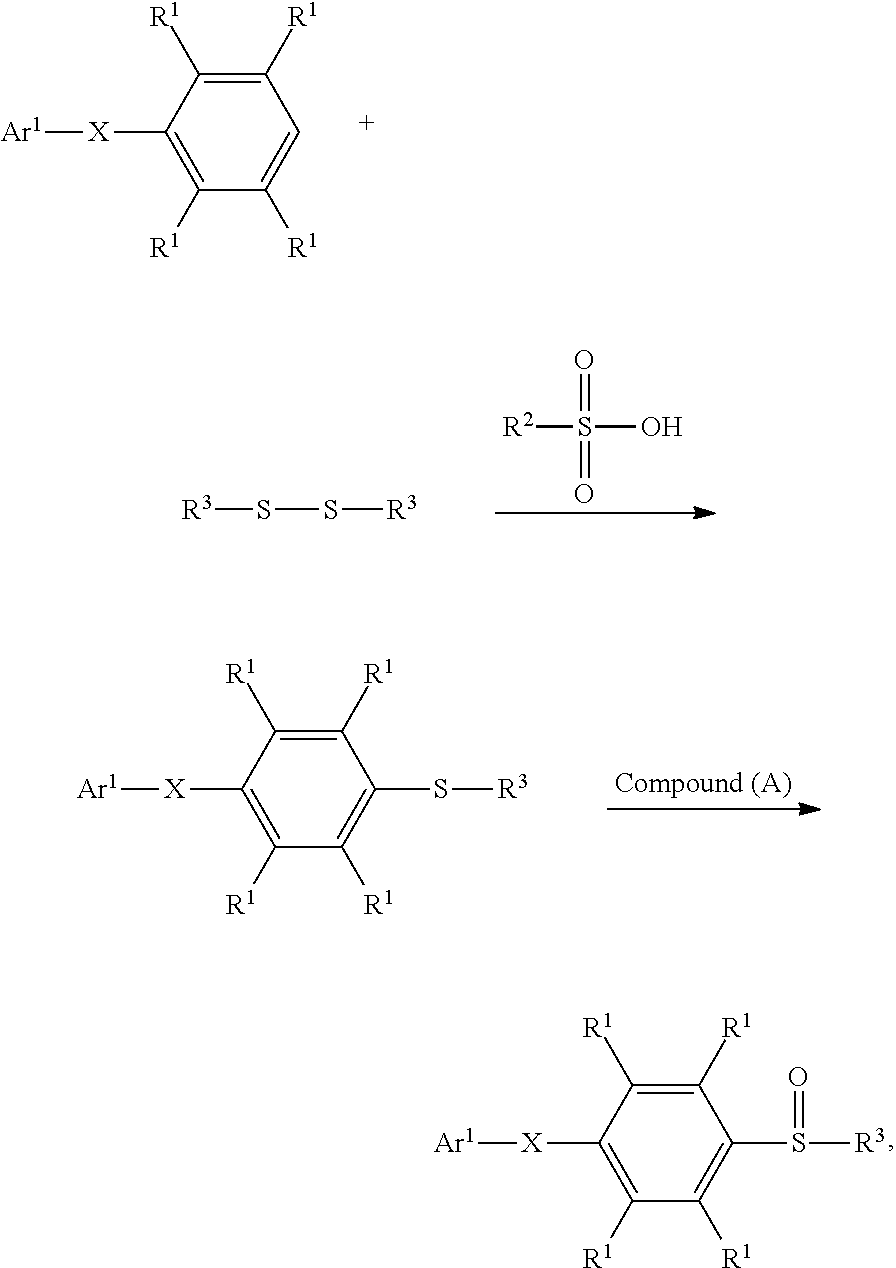
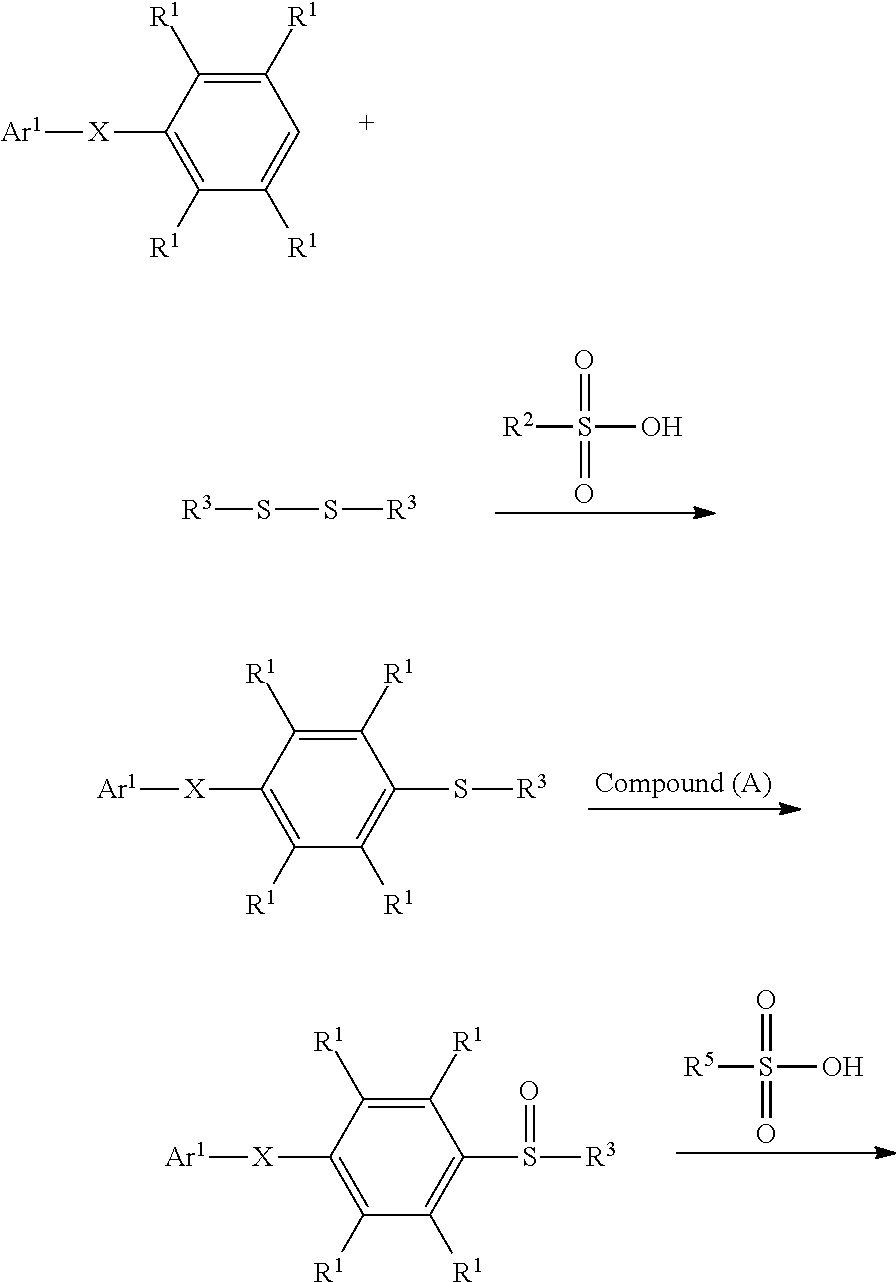
According to embodiments of the disclosure, the disclosure provides a method for preparing a compound. The method includes the following steps. A compound having a structure represented by Formula (I) is reacted with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV)
##STR00002## wherein Ar.sup.1 is substituted or unsubstituted aryl group; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group.
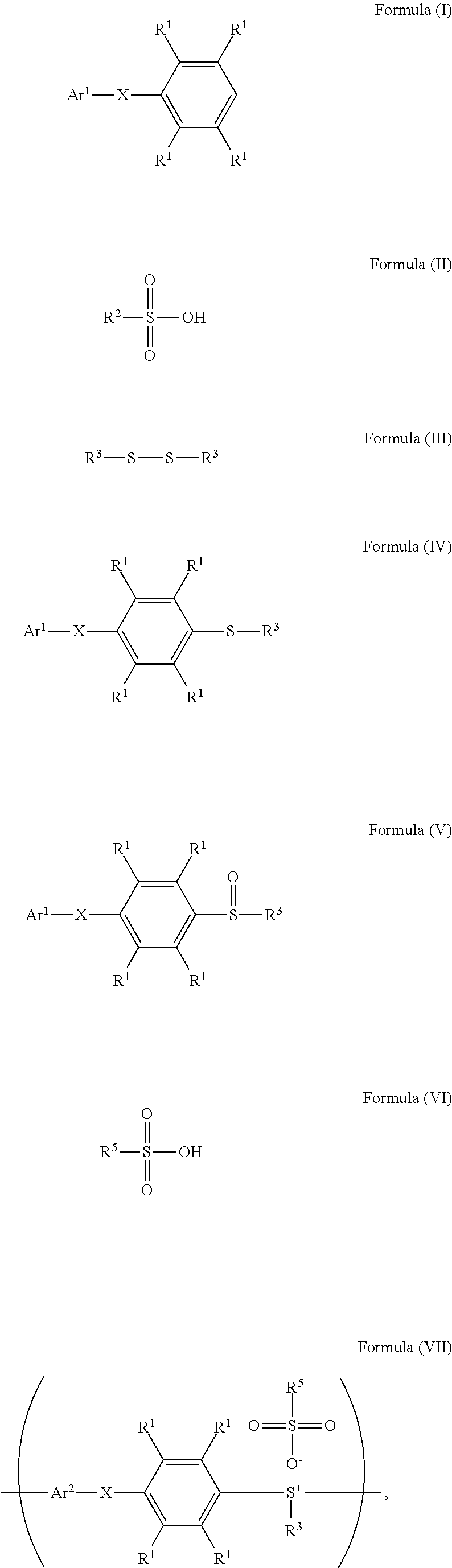
According to embodiments of the disclosure, the disclosure also provides a method for preparing a polymer. The method includes the following steps. A compound having a structure represented by Formula (I) is reacted with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV). The compound having the structure represented by Formula (IV) is reacted with a compound (A), obtaining a compound having the structure represented by Formula (V). The compound (A) is nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof. The compound having the structure represented by Formula (V) is reacted with a compound having a structure represented by Formula (VI), obtaining a polymer having a repeat unit represented by Formula (VII)
##STR00003## wherein Ar.sup.1 is substituted or unsubstituted aryl group; X is --O--, --S--, or --NH--; R.sup.1 is independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; R.sup.3 is independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical.
A detailed description is given in the following embodiments with reference to the accompanying drawings.
DETAILED DESCRIPTION
The disclosure provides a method for preparing a compound, wherein the starting materials or catalysts of the method for preparing a compound are halogen-free compounds. Thus, no halogen-containing side product is formed. In addition, there is no halogen-containing compound remained in the obtained result. The method for preparing a compound of the disclosure does not include an additional step for removing a halogen-containing side product or residual halogen-containing compound, thereby reducing preparation cost and increasing product yield. Thus, a halogen-free monomer, which can be used in a subsequent polymerization, is obtained.
Furthermore, the disclosure also provides a method for preparing a polymer (such as polyether sulfone (PES) or polythioether sulfone (PTES). The starting materials of the method for preparing the monomer of the polymer and the method for preparing a polymer are halogen-free compounds. Thus, no halogen-containing side product is formed. In addition, there is no halogen-containing compound remained in the obtained result. The method for preparing a polymer of the disclosure does not include an additional step for removing a halogen-containing side product or residual halogen-containing compound, thereby reducing preparation cost and increasing product yield. Thus, a halogen-free polymer is obtained. Furthermore, the method for preparing a polymer of the disclosure includes subjecting a monomer to an electrophilic polymerization and then performing an oxidation after polymerization. Therefore, the obtained polymer has an increased molecular weight and a reduced polydispersity index (PDI).
According to embodiments of the disclosure, the disclosure provides a method for preparing a compound, wherein the compound can serve as a monomer for a subsequent polymerization (such as polyether sulfone (PES) polymerization or polythioether sulfone (PTES) polymerization). The method for preparing a compound includes: reacting a compound having a structure represented by Formula (I) with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV)
##STR00004## wherein Ar.sup.1 can be substituted or unsubstituted aryl group; X can be --O--, --S--, or --NH--; R.sup.1 can be independently hydrogen or C.sub.1-6 alkyl group; R.sup.2 can be hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and R.sup.3 can be independently C.sub.1-6 alkyl group, C.sub.5-8 cycloalkyl group, or C.sub.2-6 alkoxyalkyl group. Herein, the substituted aryl group of the disclosure means that at least one hydrogen atom bonded to carbon atoms of the aryl group can be replaced with C.sub.1-6 alkyl group.
According to embodiments of the disclosure, wherein Ar.sup.1 can be substituted or unsubstituted phenyl group, biphenyl group, naphthyl group, thienyl group, indolyl group, phenanthrenyl group, indenyl group, anthracenyl group, or fluorenylene group. In particular, the substituted phenyl group, substituted biphenyl group, substituted naphthyl group, substituted thienyl group, substituted indolyl group, substituted phenanthrenyl group, substituted indenyl group, substituted anthracenyl group, or substituted fluorenylene group means that at least one hydrogen atom bonded to carbon atoms of the aforementioned group can be replaced with C.sub.1-6 alkyl group.
According to embodiments of the disclosure, C.sub.1-6 alkyl group can be linear or branched alkyl group, such as methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
According to embodiments of the disclosure, R.sup.1 can be independently hydrogen, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
According to embodiments of the disclosure, R.sup.2 can be hydroxyl group, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, hexyl group, phenyl group, or tolyl group.
According to embodiments of the disclosure, R.sup.3 can be independently methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, hexyl group, cyclopentyl group, cyclohexyl group, cycloheptyl group, cyclooctyl group, or
##STR00005## wherein 1.ltoreq.n.ltoreq.5, 0.ltoreq.m.ltoreq.4, and 1.ltoreq.n+m.ltoreq.5.
According to embodiments of the disclosure, The compound having the structure of Formula (I) can be
##STR00006## ##STR00007## wherein R.sup.1 has the same definition as above; and R.sup.4 can be independently hydrogen or C.sub.1-6 alkyl group.
According to embodiments of the disclosure, R.sup.4 can be independently hydrogen, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
According to embodiments of the disclosure, the compound having the structure of Formula (II) can be sulfuric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, or a combination thereof.
According to embodiments of the disclosure, the compound having the structure of Formula (III) can be
##STR00008##
According to embodiments of the disclosure, the disclosure provides a method for preparing the compound having the structure of Formula (IV), wherein the compound having the structure of Formula (IV) can be
##STR00009## wherein R.sup.1, R.sup.3, and R.sup.4 have the same definition as above.
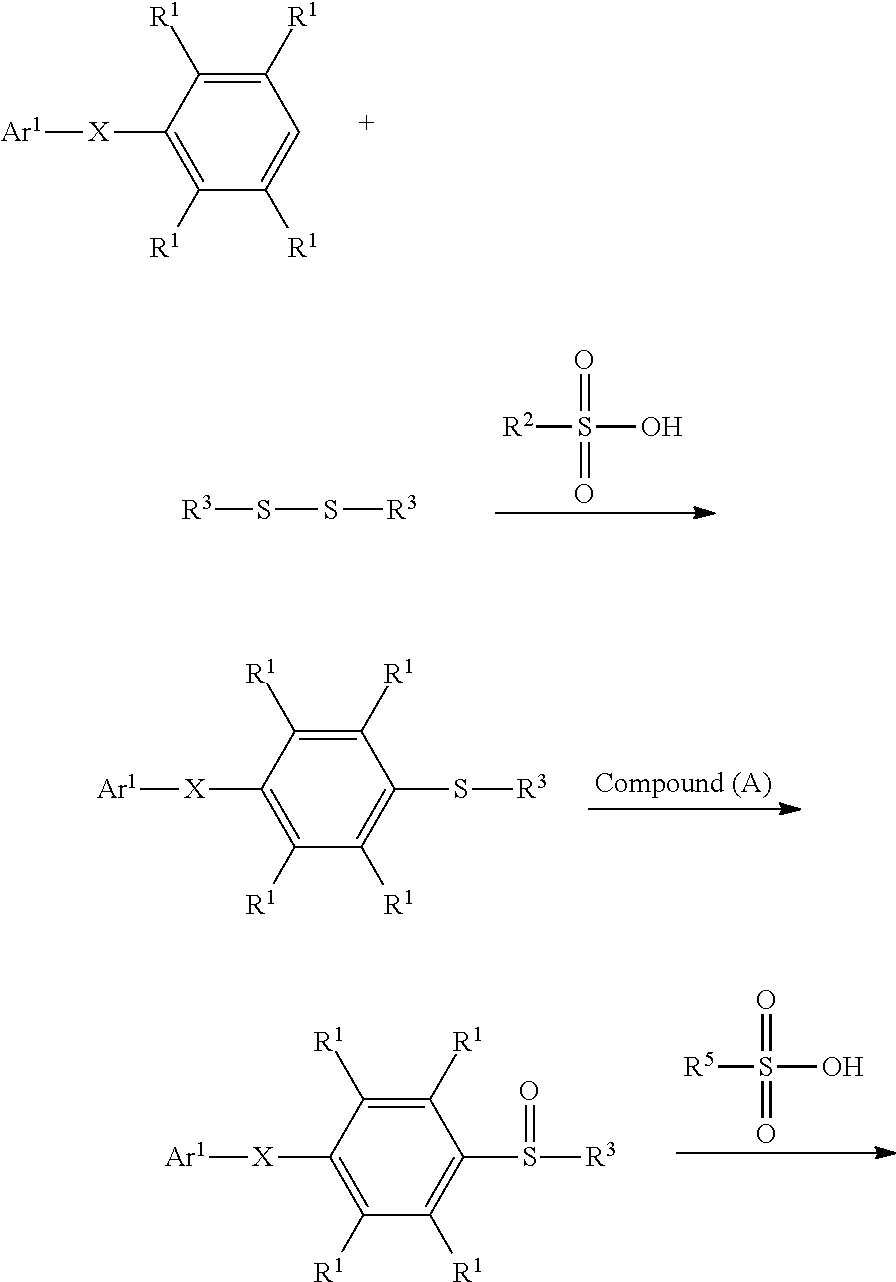
According to embodiments of the disclosure, the method for preparing the compound having the structure of Formula (IV) of the disclosure can include dissolving the compound having the structure of Formula (I) and the compound having the structure of Formula (II) in a solvent, obtaining a mixture. Next, the compound having the structure of Formula (III) is added into the mixture to undergo a reaction, obtaining a compound having a structure represented by Formula (IV). The synthesis pathway of the above reaction is as follows:
##STR00010## wherein Ar.sup.1, X, R.sup.1, R.sup.2, and R.sup.3 have the same definition as above. Herein, the solvent can be any solvent (such as halogen-free organic solvent) which can be used to dissolve the compound having the structure of Formula (I) and the compound having the structure of Formula (II). Furthermore, a halogen-containing organic solvent, which is easily removed after the reaction is complete and would not be active in the desired reaction, can also serve as the solvent of the above reaction. According to embodiments of the disclosure, the solvent can be an aprotic solvent. The solvent, for example, can include acetonitrile, linear or cyclic alkane (such as propane, butane, or cyclohexane), haloalkane (dichloromethane, trichloromethane, or dichloroethane). Furthermore, the reaction can be performed in the absence of a solvent.
According to embodiments of the disclosure, in the method for preparing the compound of the disclosure, the molar ratio of the compound having the structure of Formula (II) to the compound having the structure of Formula (I) can be from about 0.5 to 5; Furthermore, in the method for preparing the compound of the disclosure, The molar ratio of the compound having the structure of Formula (I) to the compound having the structure of Formula (III) can be from about 1 to 20, such as from about 1 to 3, or from about 1 to 10.
According to some embodiments of the disclosure, the method for preparing the compound of the disclosure, after preparing the compound having the structure of Formula (IV), further includes reacting the compound having the structure represented by Formula (IV) with a compound (A), obtaining a compound having the structure represented by Formula (V)
##STR00011## wherein the compound (A) can be nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof; and Ar.sup.1, X, and R.sup.3 has the same definition as above.
According to embodiments of the disclosure, the disclosure provides a method for preparing the compound having the structure of Formula (V), wherein the compound having the structure of Formula (V) can be
##STR00012## wherein R.sup.1, R.sup.3, and R.sup.4 have the same definition as above.
According to embodiments of the disclosure, the method for preparing the compound having the structure of Formula (V) of the disclosure can include dissolving the compound having the structure of Formula (I) and the compound having the structure of Formula (II) in a first solvent, obtaining a mixture. Next, the compound having the structure of Formula (III) is added into the mixture to undergo a reaction, obtaining a compound having a structure represented by Formula (IV). Next, the compound having the structure of Formula (IV) is dissolved in a second solvent, and the compound (A) is added to undergo a reaction, obtaining a compound having the structure represented by Formula (V). The synthesis pathway of the above reaction is as follows:
##STR00013## wherein Ar.sup.1, X, R.sup.1, R.sup.2 and R.sup.3 have the same definition as above. Herein, when X is S, the S atom bonded to R.sup.3 group can be selectively oxidized in comparison with X since the S atom bonded to R.sup.3 group exhibits a relatively low steric hindrance and has a suitable oxidation potential for oxidation.
According to embodiments of the disclosure, the first solvent can be any solvent which can be used to dissolve the compound having the structure of Formula (I) and the compound having the structure of Formula (II). The second solvent can be any solvent which can be used to dissolve the compound having the structure of Formula (IV). According to embodiments of the disclosure, a halogen-free organic solvent can serve as the first solvent or the second solvent. Furthermore, a halogen-containing organic solvent, which is easily removed after the reaction is complete and would not be active in the desired reaction, can also serve as the solvent of the above reaction. According to embodiments of the disclosure, the first solvent or the second solvent can be an aprotic solvent. The solvent, for example, can include acetonitrile, linear or cyclic alkane (such as propane, butane, or cyclohexane), or haloalkane (dichloromethane, trichloromethane, or dichloroethane). Furthermore, the reaction can be performed in the absence of a solvent. The molar ratio of the compound having the structure of Formula (IV) to the compound (A) can be from about 0.8 to 30.
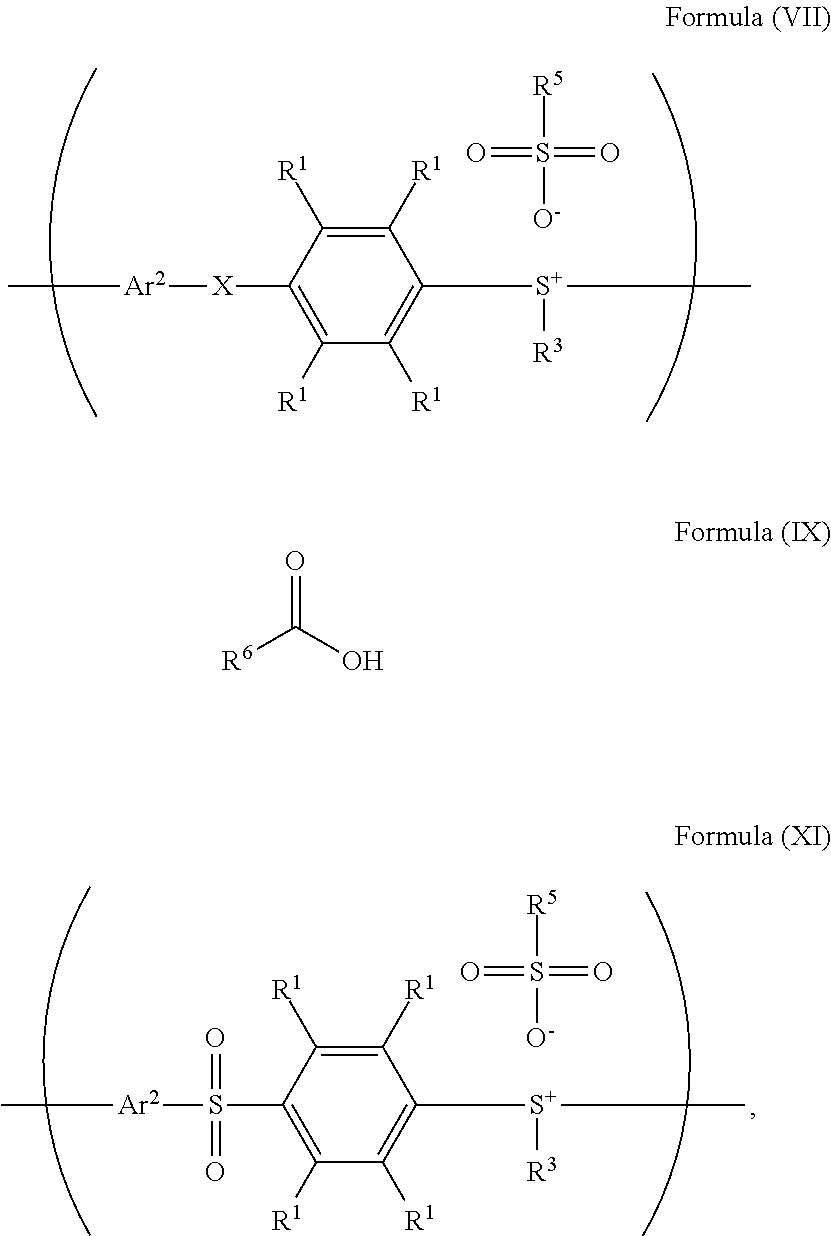
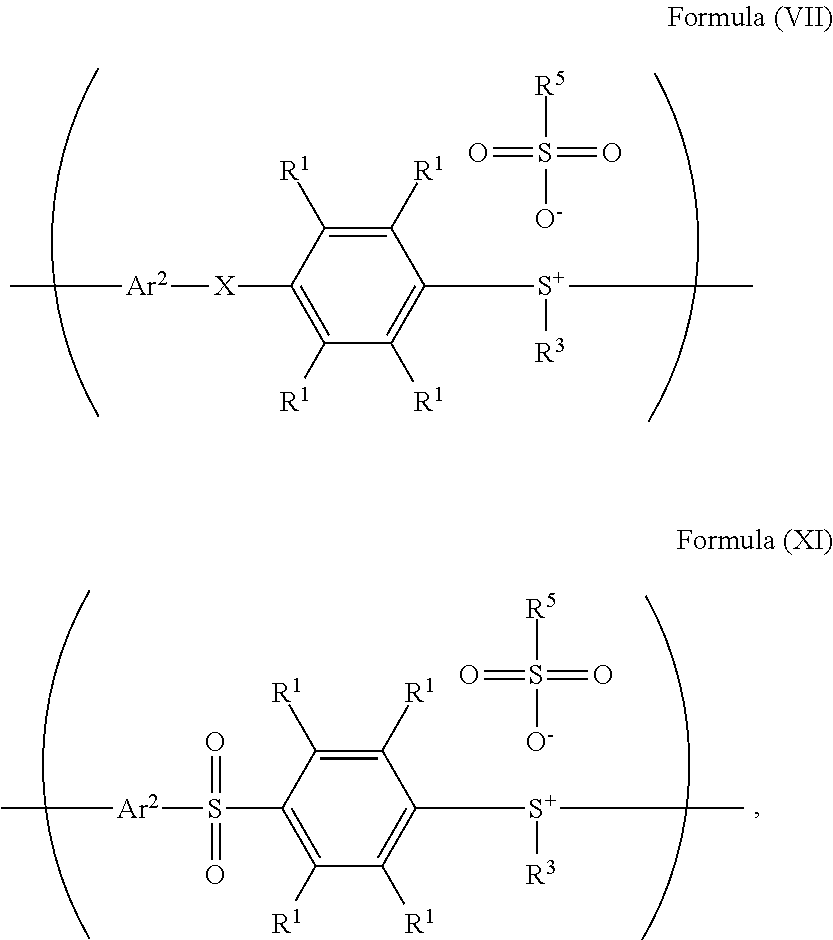
According to embodiments of the disclosure, the disclosure provides a method for preparing a polymer. The method for preparing a polymer includes the following steps. First, a compound having a structure represented by Formula (I) is reacted with a compound having a structure represented by Formula (III) in the presence of a compound having a structure represented by Formula (II), obtaining a compound having a structure represented by Formula (IV). Next, the compound having the structure represented by Formula (IV) is reacted with a compound (A), obtaining a compound having the structure represented by Formula (V), wherein the compound (A) is nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof. Next, the compound having the structure represented by Formula (V) is reacted with a compound having a structure represented by Formula (VI), obtaining a polymer having a repeat unit represented by Formula (VII)
##STR00014## wherein Ar.sup.1, X, R.sup.1, R.sup.2, and R.sup.3 has the same definition as above; R.sup.5 is hydroxyl group, C.sub.1-6 alkyl group, phenyl group, or tolyl group; and Ar.sup.2 is substituted or unsubstituted aryl diradical. The method for preparing a polymer of the disclosure can be used to prepare a polymer having a number average molecular weight greater than or equal to 1,000. It should be noted that the method for preparing a polymer of the disclosure is particularly suitable for preparing a polymer having a great number average molecular weight (such as greater than or equal to 80,000) and a narrow polydispersity index (PDI) (such as less than or equal to 2). According to embodiments of the disclosure, the method for preparing a polymer of the disclosure is particularly suitable for preparing a polymer having a number average molecular weight from 80,000 to 500,000 and a polydispersity index (PDI) from 1 to 2. According to some embodiments of the disclosure, the method for preparing a polymer of the disclosure is particularly suitable for preparing a polymer having a number average molecular weight from 80,000 to 200,000 and polydispersity index (PDI) from 1.4 to 2.
According to embodiments of the disclosure, R.sup.5 can be hydroxyl, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, hexyl group, phenyl group, or tolyl group.
According to embodiments of the disclosure, the substituted aryl diradical of the disclosure means that at least one hydrogen atom bonded to carbon atoms of the aryl diradical can be replaced with C.sub.1-6 alkyl group.
According to embodiments of the disclosure, Ar.sup.2 can be substituted or unsubstituted phenylene group, biphenylene group, naphthylene group, thienylene group, indolylene group, phenanthrenylene group, indenylene group, anthracenylene group, or fluorenylene group. In particular, the substituted phenylene group, substituted biphenylene group, substituted naphthylene group, substituted thienylene group, substituted indolylene group, substituted phenanthrenylene group, substituted indenylene group, substituted anthracenylene group, or substituted fluorenylene group means that at least one hydrogen atom bonded to carbon atoms of the aforementioned group can be replaced with C.sub.1-6 alkyl group.
According to embodiments of the disclosure, in the method for preparing the polymer of the disclosure, the molar ratio of the compound having the structure of Formula (II) to the compound having the structure of Formula (I) can be from about 0.5 to 5. Furthermore, in the method for preparing the compound of the disclosure, the molar ratio of the compound having the structure of Formula (I) to the compound having the structure of Formula (III) can be from about 1 to 20, such as from about 1 to 3, or from about 1 to 10. The molar ratio of the compound having the structure of Formula (IV) to the compound (A) (such as nitric acid, sulfuric acid, acetic acid, hydrogen peroxide, or a combination thereof) can be from about 0.8 to 30; and the molar ratio of the compound having the structure of Formula (V) to the compound having the structure of Formula (VI) can be from about 0.8 to 20, such as from about 1.2 to 5. Furthermore, the compound having the structure of Formula (VI) can serve as a reactant for reacting with the compound having the structure of Formula (V), and the excessive compound having the structure of Formula (VI) can also serve as the reaction solvent.
According to embodiments of the disclosure, the compound having the structure of Formula (VI) can be sulfuric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, trifluoromethanesulfonic acid or a combination thereof. Furthermore, according to embodiments of the disclosure, the compound having the structure of Formula (II) and the compound having the structure of Formula (VI) can be the same or different.
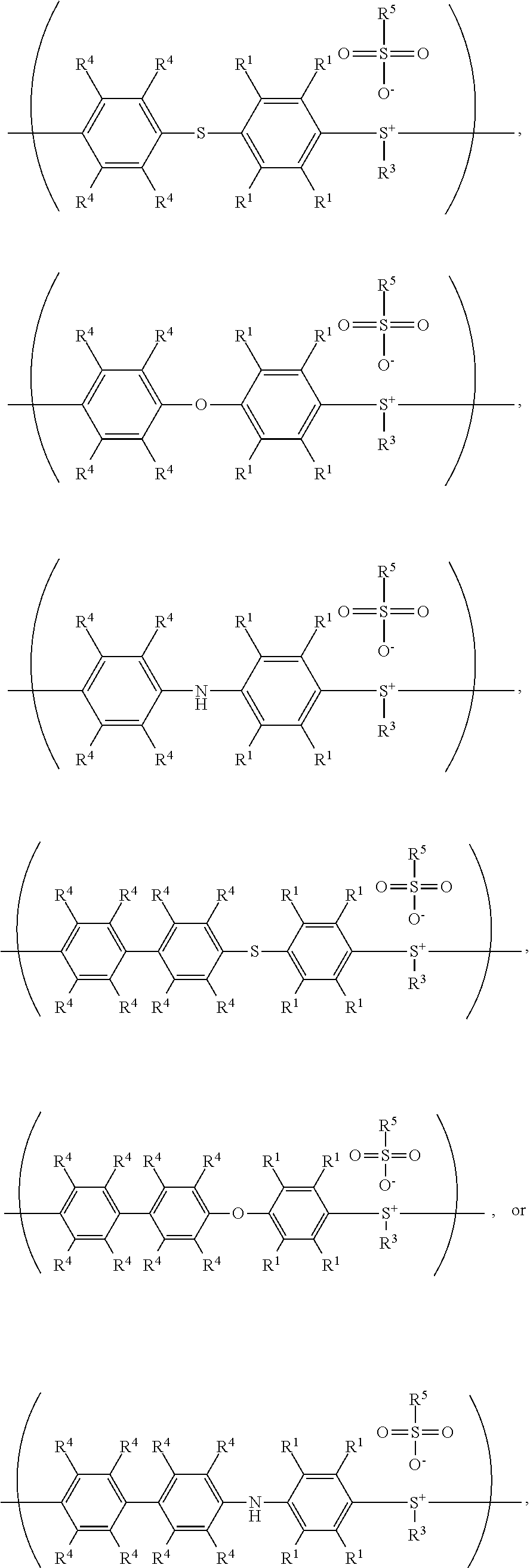
According to embodiments of the disclosure, the method for preparing the polymer of the disclosure can be used to prepare the polymer having a repeat unit represented by Formula (VII). For example, the repeat unit represented by Formula (VII) can be
##STR00015## wherein R.sup.1, R.sup.3, R.sup.4, and R.sup.5 have the same definition as above.
According to embodiments of the disclosure, the method for preparing the polymer having a repeat unit represented by Formula (VII) of the disclosure can include dissolving the compound having the structure of Formula (I) and the compound having the structure of Formula (II) in a first solvent, obtaining a mixture. Next, the compound having the structure of Formula (III) is added into the mixture to undergo a reaction, obtaining a compound having a structure represented by Formula (IV). Next, the compound having the structure of Formula (IV) is dissolved in a second solvent, the compound (A) is added to undergo a reaction, obtaining a compound having the structure represented by Formula (V). Next, the compound having the structure represented by Formula (V) is reacted with a compound having a structure represented by Formula (VI), obtaining a polymer having a repeat unit represented by Formula (VII). The synthesis pathway for preparing the above polymer is as follows:
##STR00016## polymer having a repeat unit represented by Formula (VII)
##STR00017## wherein Ar.sup.1, Ar.sup.2, X, R.sup.1, R.sup.2, R.sup.3, and R.sup.5 have the same definition as above.
According to embodiments of the disclosure, after preparing the polymer having the repeat unit represented by Formula (VII), the method for preparing the polymer of the disclosure further includes reacting a nucleophile with the polymer having the repeat unit represented by Formula (VII), obtaining a polymer having a repeat unit represented by Formula (VIII)
##STR00018## wherein Ar.sup.2, X, R.sup.1, R.sup.3, and R.sup.5 have the same definition as above. The synthesis pathway of the above method for preparing the polymer having the repeat unit represented by Formula (VIII) is as follows:
##STR00019## polymer baying a repeat unit represented by Formula (VII)
##STR00020## polymer having a repeat unit represented by Formula (VIII)
##STR00021## wherein Ar.sup.1, Ar.sup.2, X, R.sup.1, R.sup.2, R.sup.3 and R.sup.5 have the same definition as above.
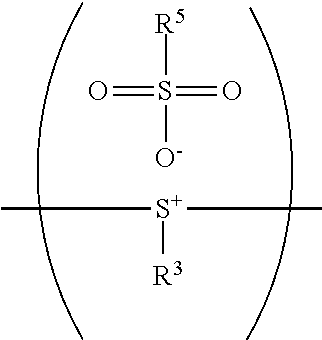
According to embodiments of the disclosure, the nucleophile can be substituted or unsubstituted pyridine or derivatives thereof (such as pyridine or 4-methylpyridine), amine (such as triethylamine), halogenated salt (such as potassium chloride), alcohol (such as methanol or ethanol), amide (such as dimethylformamide, dimethylacetamide, or N-methylpyrrolidone), or a combination thereof. The equivalent ratio of the nucleophile to the moiety represented by
##STR00022## of the polymer having the repeat unit represented by Formula (VII) can be from 1 to 10. According to embodiments of the disclosure, the nucleophile and the polymer having the repeat unit represented by Formula (VII) can optionally be dissolved into an organic solvent before undergoing the reaction.
According to embodiments of the disclosure, when X of the repeat unit represented by Formula (VIII) is --O-- or --NH-- (i.e. the repeat unit represented by Formula (VIII) is
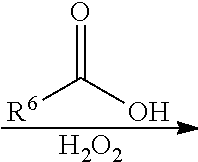
##STR00023## wherein Ar.sup.2, and R.sup.1 have the same definition as above), the method for preparing the polymer of the disclosure can further include, after obtaining the polymer having the repeat unit represented by Formula (VIII), reacting the polymer having the repeat unit represented by Formula (VIII) with hydrogen peroxide (H.sub.2O.sub.2), obtaining a polymer having a repeat unit represented by Formula (X). Alternatively, the polymer having the repeat unit represented by Formula (VIII) can be reacted with hydrogen peroxide in the presence of the compound having a structure represented by Formula (IX), obtaining the polymer having the repeat unit represented by Formula (X)
##STR00024## wherein X can be --O-- or --NH--; R.sup.6 is C.sub.1-6 alkyl group; and Ar.sup.2 and R.sup.1 have the same definition as above. The synthesis pathway of the above method for preparing the polymer having the repeat unit represented by Formula (X) is as follows:
##STR00025## polymer having a repeat unit represented by Formula (VII)
##STR00026## polymer having a repeat unit represented by Formula (VIII)
##STR00027## polymer having a repeat unit represented by Formula (X)
##STR00028## wherein X can be --O-- or --NH--; and Ar.sup.1, Ar.sup.2, R.sup.1, R.sup.2, R.sup.3, R.sup.5 and R.sup.6 have the same definition as above.
According to other embodiments of the disclosure, in the method for preparing the polymer having the repeat unit represented by Formula (X) of the disclosure, the polymer having the repeat unit represented by Formula (VIII), the compound having the structure of Formula (IX), and hydrogen peroxide can be dissolved into a solvent before undergoing the reaction. For example, the solvent can be amide-type solvent or sulfoxide-type solvent.
According to embodiments of the disclosure, R.sup.6 can be independently methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, t-butyl group, sec-butyl group, isobutyl group, pentyl group, or hexyl group.
According to embodiments of the disclosure, when X of the repeat unit represented by Formula (VII) is --S-- (i.e. the repeat unit represented by Formula (VII) is
##STR00029## wherein Ar.sup.2, R.sup.1, R.sup.3, and R.sup.5 have the same definition as above), the method for preparing the polymer of the disclosure, after obtaining the polymer having the repeat unit represented by Formula (VII), can further include reacting the polymer having the repeat unit represented by Formula (VII) with hydrogen peroxide, obtaining a polymer having a repeat unit represented by Formula (XI). Alternatively, the polymer having the repeat unit represented by Formula (VII) is reacted with hydrogen peroxide in the presence of the compound having the structure of Formula (IX), obtaining the polymer having the repeat unit represented by Formula (XI).
##STR00030## wherein X can be --S--; and Ar.sup.2, R.sup.1, R.sup.3, R.sup.5 and R.sup.6 have the same definition as above. The synthesis pathway of the above method for preparing the polymer having the repeat unit represented by Formula (XI) is as follows:
##STR00031## polymer having a repeat unit represented by Formula (VII)
##STR00032## polymer having a repeat unit represented by Formula (XI)
##STR00033## wherein X can be --S--; and Ar.sup.1, Ar.sup.2, R.sup.1, R.sup.2, R.sup.3, R.sup.5 and R.sup.6 have the same definition as above.
According to other embodiments of the disclosure, in the method for preparing the polymer having a repeat unit represented by Formula (XI) of the disclosure, the polymer having the repeat unit represented by Formula (VII) and hydrogen peroxide can be dissolved into an organic solvent and then the mixture is reacted with the compound having the structure represented by Formula (IX) to undergo the reaction. For example, the solvent can be nitrile-type solvent, amide-type solvent, or sulfoxide-type solvent. Furthermore, the polymer having the repeat unit represented by Formula (VII), the compound having the structure represented by Formula (IX), and hydrogen peroxide can be dissolved into an organic solvent before undergoing the reaction.
According to embodiments of the disclosure, after preparing the polymer having a repeat unit represented by Formula (XI), the method for preparing the polymer of the disclosure can further include reacting a nucleophile with the polymer having the repeat unit represented by Formula (XI), obtaining a polymer having a repeat unit represented by Formula (XII)
##STR00034## wherein Ar.sup.2, R.sup.1, R.sup.3, and R.sup.5 have the same definition as above. The synthesis pathway of the above method for preparing the polymer having the repeat unit represented by Formula (XII) is as follows:
##STR00035## polymer having a repeat unit represented by Formula (VII)
##STR00036## polymer having a repeat unit represented by Formula (XI)
##STR00037## polymer having a repeat unit represented by Formula (XII)
##STR00038## wherein X is --S--; and Ar.sup.1, Ar.sup.2, R.sup.1, R.sup.2, R.sup.3, R.sup.5, and R.sup.6 have the same definition as above.
According to embodiments of the disclosure, the nucleophile can be substituted or unsubstituted pyridine or derivatives thereof (such as pyridine or 4-methylpyridine), amine (such as triethylamine), halogenated salt (such as potassium chloride), alcohol (such as methanol or ethanol), amide (such as dimethylformamide, dimethylacetamide, or N-methylpyrrolidone), or a combination thereof. The equivalent ratio of the nucleophile to the moiety represented by
##STR00039## of the polymer having the repeat unit represented by Formula (XI) can be from 1 to 10. According to embodiments of the disclosure, the nucleophile and the polymer having the repeat unit represented by Formula (XI) can optionally be dissolved into an organic solvent before undergoing the reaction.
Below, exemplary embodiments will be described in detail so as to be easily realized by a person having ordinary knowledge in the art. The inventive concept may be embodied in various forms without being limited to the exemplary embodiments set forth herein. Descriptions of well-known parts are omitted for clarity.
Example 1
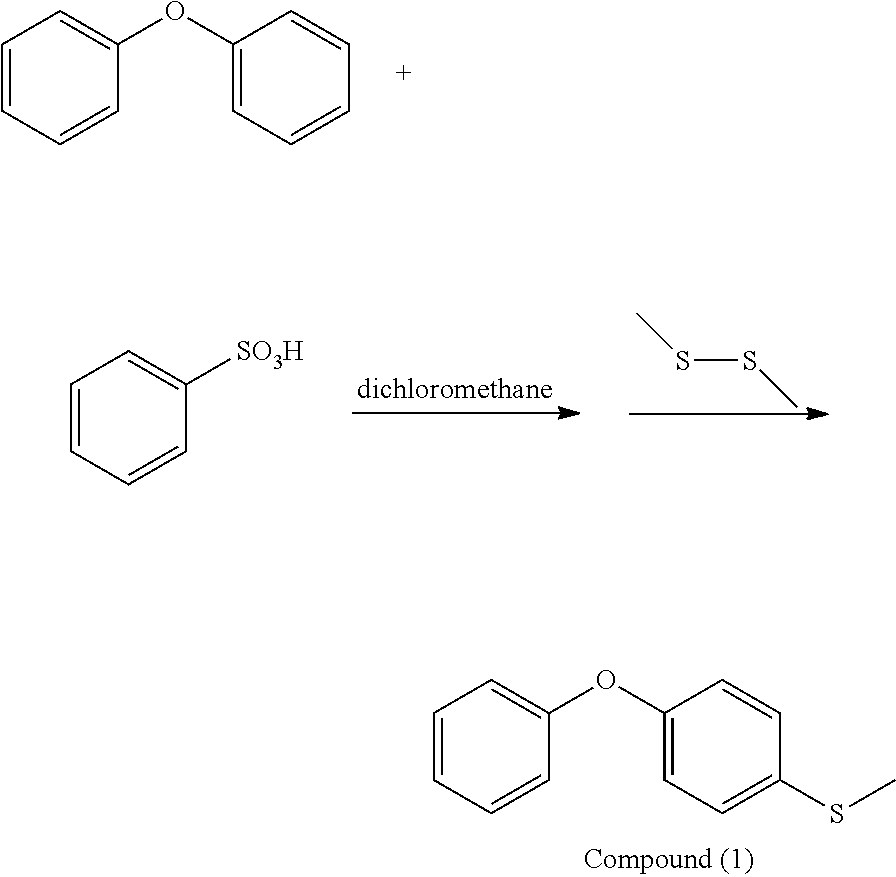
5 g of diphenyl ether, 11.7 g of benzenesulfonic acid, and 50 ml of dichloromethane were added into a reaction bottle under nitrogen atmosphere, and then cooled to 15.degree. C. Next, 5.54 g of 1,2-dimethyldisulfane was added into the reaction bottle. After reacting at 15.degree. C. for 20 hr, the result was mixed with 50 ml of sodium hydroxide aqueous solution (the weight ratio of sodium hydroxide to water is 1:10). After stirring for 0.5 hr, the result was extracted three times using dichloromethane and water as the extraction solvent. Next, an organic phase was separated and dried, obtaining Compound (1). The synthesis pathway of the above reaction was as follows:
##STR00040##
Compound (1) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, CDCl.sub.3): 2.50 (--CH.sub.3, 3H, s), 7.00 (phenyl, 4H, m), 7.14 (phenyl, 1H, t), 7.32-7.41 (phenyl, 4H, m).
Example 2
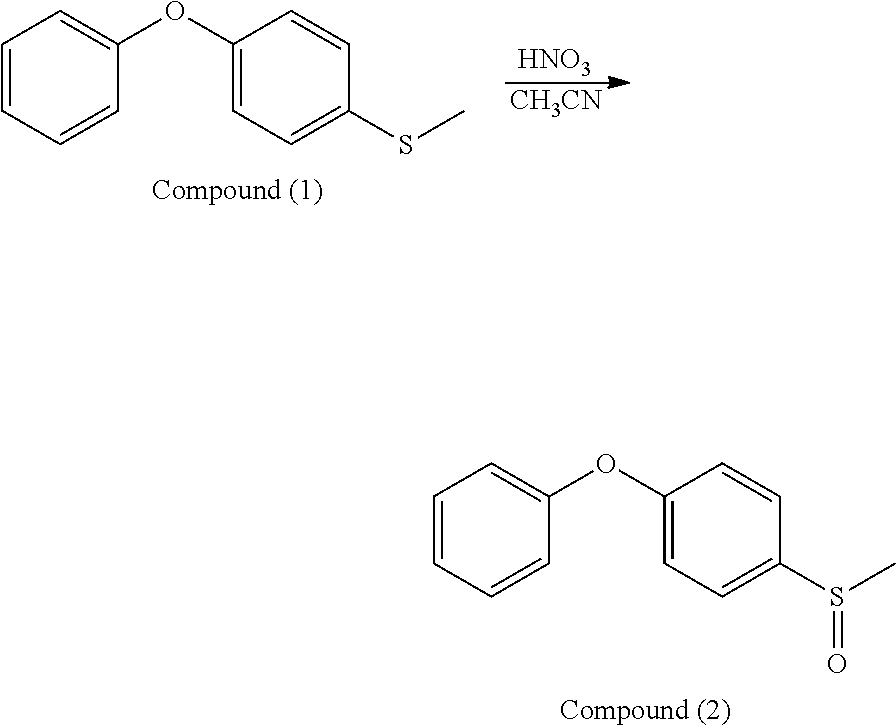
0.73 g of Compound (1), 12 ml of nitric acid aqueous solution (with a concentration of 20%), and 4 ml of acetonitrile were added into a reaction bottle. After stirring at room temperature for 4 hr, 10 ml of sodium hydroxide aqueous solution (with a concentration of 3%) was added into the reaction bottle. Next, after the reaction was complete, the result was separated and dried, obtaining Compound (2) (orange powder). The synthesis pathway of the above reaction was as follows:
##STR00041##
Compound (2) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, CD.sub.3CO): 2.71 (--CH.sub.3, 3H, s), 7.10-7.25 (phenyl, 5H, m), 7.44-7.48 (phenyl, 2H, t), 7.70-7.72 (phenyl, 2H, d).
Example 3
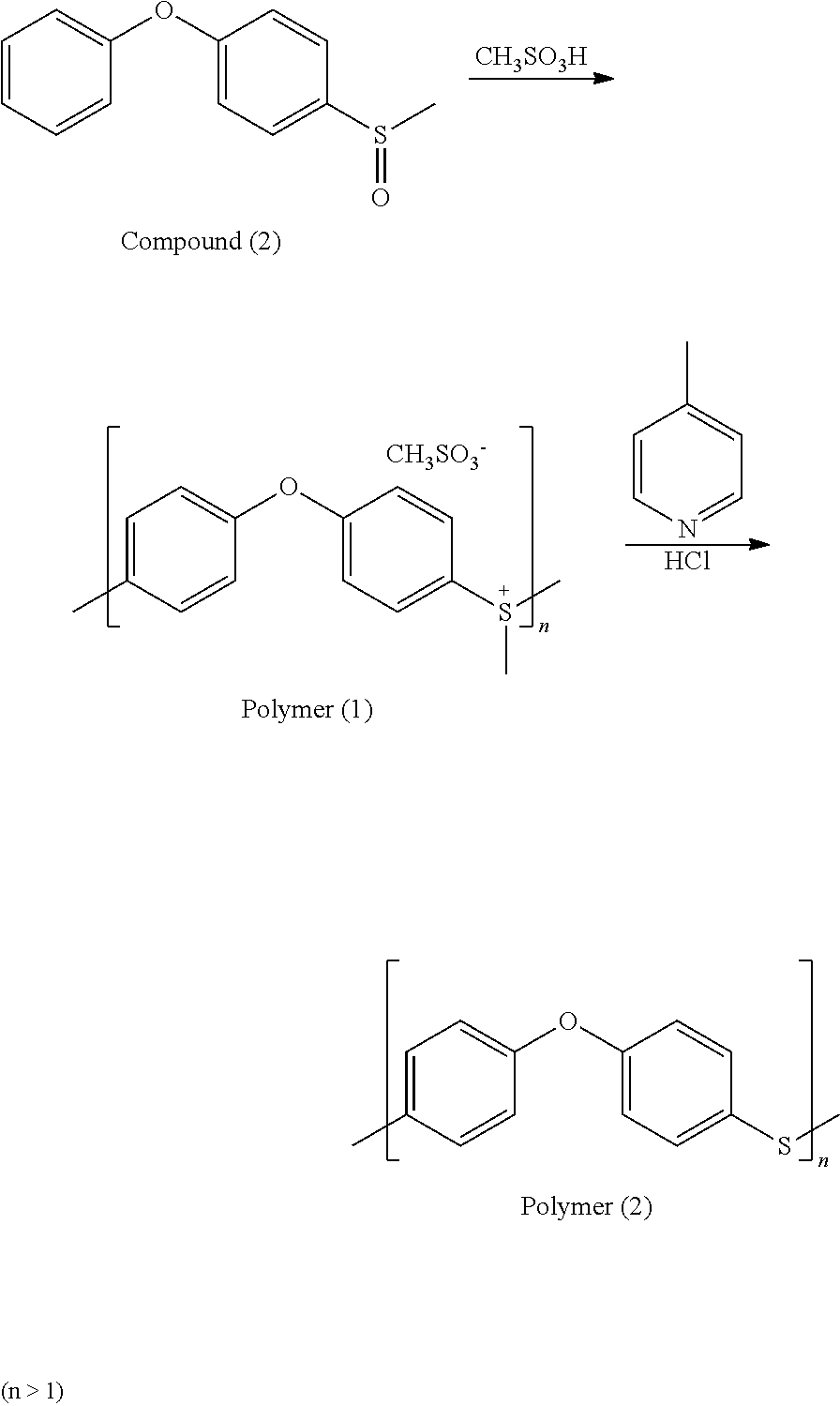
0.65 g of Compound (2) was added into a reaction bottle. Next, 3 ml of methanesulfonic acid (CH.sub.3SO.sub.3H) was added into the reaction bottle placed in an ice bath. After reacting for 1 hr, the reaction bottle was raised to room temperature and then reacted at room temperature for 20 hr, obtaining a solution including Polymer (1). Next, the solution including Polymer (1) was added into 100 ml of ethyl ether and stirred for 30 min. Next, 6 ml of 4-methylpyridine was added under nitrogen atmosphere and then the result was stirred at 100.degree. C. for 4-6 hr. After the reaction was complete, the result was added into 100 ml of hydrochloric acid solution (with a concentration of 10%) and then stirred for 10 min. After concentrating, Polymer (2) was obtained. The synthesis pathway of the above reaction was as follows:
##STR00042##
Polymer (2) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, (CD.sub.3).sub.2SO): 7.04 (phenyl, d), 7.36 (phenyl, d).
Example 4
0.2 g of Polymer (2), 10 ml of acetic acid, 0.9 g of hydrogen peroxide solution (with a concentration of 30%) and 2 ml of dimethylacetamide (DMAc) were added into a reaction bottle, and the reaction bottle was stirred at 80.degree. C. for 6 hr. Next, the result was concentrated, obtaining Polymer (3). The synthesis pathway of the above reaction was as follows:
##STR00043##
Polymer (3) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, (CD.sub.3).sub.2SO): 7.28 (phenyl, d), 7.99 (phenyl, d). Next, Polymer (3) was analyzed by Fourier-transform infrared (FT-IR) spectroscopy, and the result shows that the strong absorption peaks are 1483 cm.sup.-1 (characteristic vibration frequency of benzene ring), 1575 cm.sup.-1 (characteristic vibration frequency of benzene ring), 1295 cm.sup.-1 (asymmetry vibration frequency of S.dbd.O), 1318 cm.sup.-1 (asymmetry vibration frequency of S.dbd.O), and 1145 cm.sup.-1 (symmetry vibration frequency of S.dbd.O). The properties of Polymer (3) were measured by a differential scanning calorimetry (DSC), and the result shows Polymer (3) has a glass transition temperature (Tg) of about 210.degree. C. Polymer (3) was analyzed by gel permeation chromatography (GPC), and the results show Polymer (3) has a weight average molecular weight (Mw) of about 128,287, a number average molecular weight (Mn) of about 85,435, and a polydispersity index (PDI) of about 1.5.
Example 5
5.58 g of diphenyl sulfide and 5.64 g of 1,2-dimethyldisulfane were added into a reaction bottle, and then 50 ml of dichloromethane as solvent was added into the reaction bottle. Next, 11.7 g of benzenesulfonic acid was added into the reaction bottle. After reacting at 15.degree. C. for 44 hr, the result was extracted three times using 150 ml of n-hexane dichloromethane and water as the extraction solvent. Next, an organic phase was separated, dried, and purified by column chromatography, obtaining Compound (3). The synthesis pathway of the above reaction was as follows:
##STR00044##
Compound (3) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, CDCl.sub.3): 2.50 (--CH.sub.3, 3H, s), 7.21-7.34 (phenyl, 9H, m).
Example 6
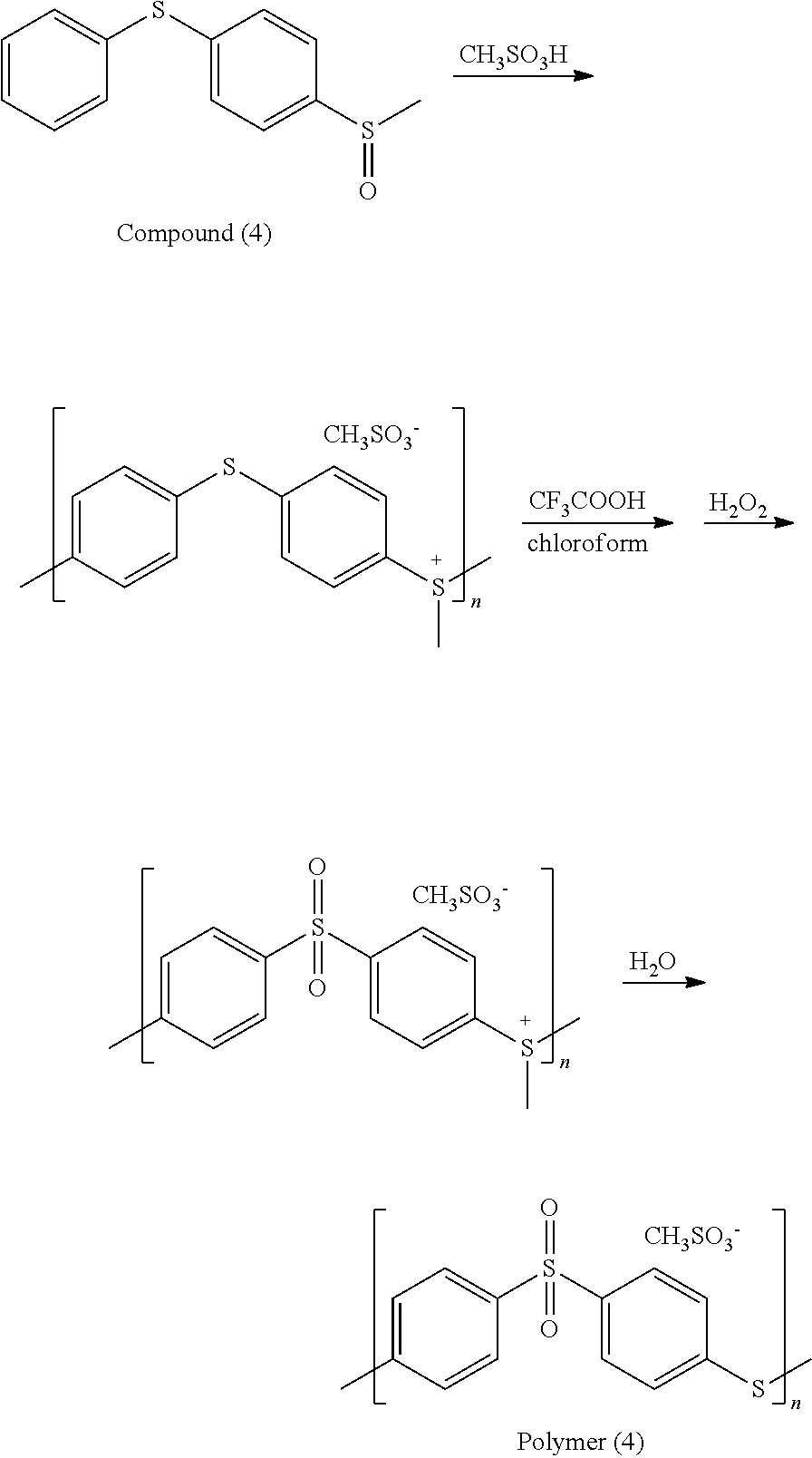
5.07 g of Compound (3) was added into a reaction bottle, and then 20 ml of acetonitrile as solvent was added into the reaction bottle. Next, 60 ml of nitric acid aqueous solution (with a concentration of 20%) was added into the reaction bottle. After reacting at room temperature for 4 hr, 12 g of sodium hydroxide was added into the reaction bottle to neutralize the solution. The result was extracted three times using 150 ml of dichloromethane as the extraction solvent. Next, an organic phase was separated and dried, obtaining Compound (4). The synthesis pathway of the above reaction was as follows:
##STR00045##
Compound (4) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, CDCl.sub.3): 2.73 (--CH.sub.3, 3H, s), 7.34-7.49 (phenyl, 9H, m).
Example 7
3 g of Compound (4) was added into a reaction bottle, and 10 ml of methanesulfonic acid was added into the reaction bottle at 15.degree. C. After reacting for 20 hr, 50 ml of water was added into the reaction bottle to cause a precipitation. The precipitate was separated and dried, obtaining a white solid. The white solid was dissolved in a solvent including 46 ml of chloroform and 46 ml of trifluoroacetic acid. Next, 4.14 g of hydrogen peroxide aqueous solution (with a concentration of 30%) was added and then the result was reacted at 60.degree. C. for 5 hr. After the reaction was complete, the result was mixed with a water to cause a precipitation. The precipitate was separated and dried, obtaining Polymer (4). The synthesis pathway of the above reaction was as follows:
##STR00046##
Polymer (4) was analyzed by nuclear magnetic resonance (NMR) spectroscopy and the result is as follows: .sup.1H NMR (400 MHz, ppm, d.sup.6-DMSO): 7.56 (phenyl, 4H, s), 7.93 (phenyl, 4H, s). The properties of Polymer (4) were measured by a differential scanning calorimetry (DSC), and the result shows Polymer (4) has a glass transition temperature (Tg) of about 222.degree. C.
Accordingly, the disclosure provides a method for preparing a compound, wherein the starting material or catalyst of the method for preparing a compound is a halogen-free compound. Thus, no halogen-containing side product is formed. In addition, there is no halogen-containing compound remained in the obtained result. The method for preparing a compound of the disclosure does not include an additional step for removing a halogen-containing side product or residual halogen-containing compound, thereby reducing preparation cost and increasing product yield. Thus, a halogen-free monomer, which can be used in a subsequent polymerization, is obtained. Furthermore, the disclosure also provides a method for preparing a polymer (such as polyether sulfone (PES) or polythioether sulfone (PTES)). Since the method for preparing a polymer includes subjecting a monomer to an electrophilic polymerization in an acidic environment and then performing an oxidation after polymerization, the obtained polymer exhibits increased molecular weight and relatively low polydispersity index (PDI).
It will be clear that various modifications and variations can be made to the disclosed methods and materials. It is intended that the specification and examples be considered as exemplary only, with the true scope of the disclosure being indicated by the following claims and their equivalents.
* * * * *
C00001

C00002

C00003

C00004

C00005
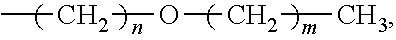
C00006

C00007

C00008

C00009

C00010

C00011

C00012

C00013
C00014
C00015
C00016

C00017
C00018
C00019
C00020

C00021

C00022
C00023

C00024

C00025
C00026

C00027

C00028

C00029

C00030
C00031

C00032
C00033
C00034

C00035

C00036
C00037
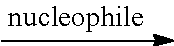
C00038
C00039
C00040
C00041
C00042
C00043

C00044

C00045

C00046
C00047
C00048

C00049
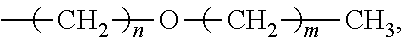
C00050
C00051
C00052
C00053
C00054
C00055

C00056
C00057
C00058

C00059
C00060

C00061
C00062
C00063

C00064
C00065

C00066
C00067

C00068
XML
uspto.report is an independent third-party trademark research tool that is not affiliated, endorsed, or sponsored by the United States Patent and Trademark Office (USPTO) or any other governmental organization. The information provided by uspto.report is based on publicly available data at the time of writing and is intended for informational purposes only.
While we strive to provide accurate and up-to-date information, we do not guarantee the accuracy, completeness, reliability, or suitability of the information displayed on this site. The use of this site is at your own risk. Any reliance you place on such information is therefore strictly at your own risk.
All official trademark data, including owner information, should be verified by visiting the official USPTO website at www.uspto.gov. This site is not intended to replace professional legal advice and should not be used as a substitute for consulting with a legal professional who is knowledgeable about trademark law.