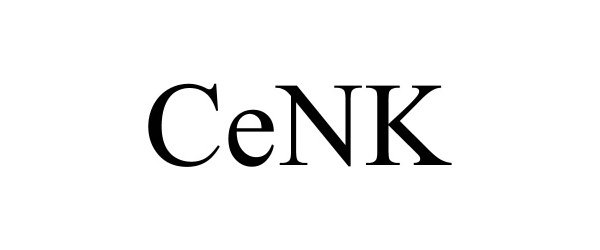IC 001. US 001 005 006 010 026 046. G & S: Cells for scientific, laboratory, medical, clinical, therapeutic, and diagnostic research; cell-derived preparations, namely, natural killer cell-derived preparations, for scientific, laboratory, medical, clinical, therapeutic, and diagnostic research; Kits comprised of cells, reagents and related instructions, for clinical, medicinal, and therapeutic research purposes; kits comprised of cell-derived preparations, namely, natural killer cell-derived preparations, reagents and related instructions, for clinical, medicinal, and therapeutic research purposes
IC 005. US 006 018 044 046 051 052. G & S: Cells for clinical, medical, veterinary, and therapeutic purposes; cell-derived preparations, namely, natural killer cell-derived preparations, for clinical, medical, veterinary, and therapeutic purposes; therapeutic products derived from cells, namely, natural killer cells, for clinical, medical, veterinary, and therapeutic purposes; therapeutic products derived from cell-derived preparations, namely, natural killer cell-derived preparations, for clinical, medical, veterinary, and therapeutic purposes; Kits comprised of cells, reagents and related instructions, for clinical, medicinal, veterinary, and therapeutic purposes; kits comprised of cell-derived preparations, namely, natural killer cell-derived preparations, reagents and related instructions, for clinical, medicinal, veterinary, and therapeutic purposes
IC 042. US 100 101. G & S: Research in the field of cancer; product development in the fields of science, medicine, pharmaceuticals, veterinary medicine, therapeutics and research related thereto
IC 044. US 100 101. G & S: Providing information relating to clinical, medicinal, and therapeutic properties of cells and cell-derived preparations
| International Codes: | 1 |
| U.S. Codes: | 001,005,006,010,026,046 |
| International Codes: | 5 |
| U.S. Codes: | 006,018,044,046,051,052 |
| International Codes: | 42 |
| U.S. Codes: | 100,101 |
| International Codes: | 44 |
| U.S. Codes: | 100,101 |
| Type Code | Type |
|---|
| GS0011 | Cells for scientific, laboratory, medical, clinical, therapeutic, and diagnostic research; cell-derived preparations, namely, natural killer cell-derived preparations, for scientific, laboratory, medical, clinical, therapeutic, and diagnostic research; Kits comprised of cells, reagents and related instructions, for clinical, medicinal, and therapeutic research purposes; kits comprised of cell-derived preparations, namely, natural killer cell-derived preparations, reagents and related instructions, for clinical, medicinal, and therapeutic research purposes |
| GS0051 | Cells for clinical, medical, veterinary, and therapeutic purposes; cell-derived preparations, namely, natural killer cell-derived preparations, for clinical, medical, veterinary, and therapeutic purposes; therapeutic products derived from cells, namely, natural killer cells, for clinical, medical, veterinary, and therapeutic purposes; therapeutic products derived from cell-derived preparations, namely, natural killer cell-derived preparations, for clinical, medical, veterinary, and therapeutic purposes; Kits comprised of cells, reagents and related instructions, for clinical, medicinal, veterinary, and therapeutic purposes; kits comprised of cell-derived preparations, namely, natural killer cell-derived preparations, reagents and related instructions, for clinical, medicinal, veterinary, and therapeutic purposes |